- UNC Chapel Hill

New Trial Highlights Incremental Progress Towards a Cure for HIV-1
February 13, 2024
By Kendall Daniels

CHAPEL HILL, N.C. – Antiretroviral therapies (ART) stop HIV replication in its tracks, allowing people with HIV to live relatively normal lives. However, despite these treatments, some HIV still lingers inside cells in a dormant state known as “latency.” If ART is discontinued, HIV will awaken from its dormant state, begin to replicate, and cause acquired immunodeficiency syndrome (AIDS). To create a cure, researchers have been attempting to drive HIV out of latency and target it for destruction.
A new clinical trial led by Cynthia Gay, MD, MPH , associate professor of infectious diseases, David Margolis, MD , the Sarah Kenan Distinguished Professor of Medicine, Microbiology & Immunology, and Epidemiology, and other clinicians and researchers at the UNC School of Medicine suggests that a combination of the drug vorinostat and immunotherapy can coax HIV-infected cells out of latency and attack them.
The immunotherapy was provided by a team led by Catherine Bollard, MD, at the George Washington University, who took white blood cells from the study participants and expanded them in the laboratory, augmenting the cells’ ability to attack HIV-infected cells, before re-infusion at UNC.
Their results, published in the Journal of Infectious Diseases , showed a small dent on the latent reservoir, demonstrating that there is more work to be done in the field.
“We did show that this approach can reduce the reservoir, but the reductions were not nearly large enough, and statistically speaking were what we call a “trend” but not highly statistically significant,” said David Margolis, MD , director of the HIV Cure Center and senior author on the paper. “We need to create better approaches to flush out the virus and attack it when it comes out. We need to keep chipping away at the reservoir until there’s nothing there.”
Waking up Latent HIV in Our Genes

DNA inside cell nuclei is kept in a tightly packed space by chromosomes, which act as highly organized storage facilities. When you unfurl a chromosome, you’ll find loop-de-loop-like fibers called chromatin. If you keep unfurling, you’ll see long strands of DNA wrapped around scaffold proteins known as histones, like beads on a string. Finally, when the unfurling is complete, you will see the iconic DNA double helix.
Vorinostat works by inhibiting a lock-like enzyme called histone deacetylase. By stopping this mechanism, tiny doors within the chromatin fibers unlock and open up, effectively “waking up” latent HIV from its slumber and making it vulnerable to an immune system attack. As a result, a tiny blip of HIV expression shows up on very sensitive molecular assays.
But the effects of vorinostat are short lived, only lasting a day per dose. For this reason, Margolis and other researchers are trying to find safe and effective ways to administer the drug and keep the chromatin channels open for longer periods of time.
Attacking Exposed HIV Reservoirs
For the study, six participants were given multiple doses of vorinostat. Researchers then extracted immune cells from the participants and expanded the cells that knew how to attack HIV-infected cells.
This immunotherapy method, which has been successful against other viruses such as Epstein-Barr virus and cytomegalovirus, involves giving participants back their expanded immune cells in the hopes that these cells will further multiply in number and launch an all-out attack on the newly exposed HIV-infected cells.
However, in the first part of this study, only one of the six participants saw a drop in their HIV reservoir levels. To test whether the result was simply random or something more, researchers gave three participants their usual dose of vorinostat, but introduced five times the amount of engineered immune cells. All three of the participants had a slight decline in their reservoirs.
But, statistically speaking, the results were not large enough to be definitive.
“This is not the result we wanted, but it is research that needed to be done,” said Margolis. “We are working on improving both latency reversal and clearance of infected cells, and we hope to do more studies as soon as we can, using newer and better approaches.”
A Dedicated Cohort
Many of the participants in the study have been working with Margolis’s research team for years, sacrificing their own time and blood for research efforts. Their long-term partnership and commitment have been essential for data collection. The data, which follows the size of the viral reservoir in these people over years prior to this study, makes the small changes found more compelling.
“People living with HIV come in a couple of times a year, and we measure residual traces of virus in their blood cells, which doesn’t have any immediate benefit to them,” said Margolis. “It’s a very altruistic action and we couldn’t make any progress without their help.”
Media contact: Kendall Daniels , Communications Specialist, UNC Health | UNC School of Medicine
Filed Under:
More from Newsroom
- Researchers Wrestle with Accuracy of AI Technology Used to Create New Drug Candidates
- Jiang Receives $4.46-million NIMH Grant
- University Libraries New Website Launch on May 14
July 24, 2023

Emory’s Gavegnano Group demonstrates ability to remove key barrier to an HIV cure.
The results of a novel study presented by Emory researchers during the International AIDS Society (IAS) Conference in Brisbane, Australia, have revealed exciting findings in the pursuit of an HIV cure. The study, led by Monica Reece, a PhD candidate in Emory’s Microbiology and Genetics Program, and directed by Christina Gavegnano, PhD, demonstrates the potential of Jak inhibitors, specifically ruxolitinib, to significantly decay the viral reservoir in people with HIV, offering a novel pathway toward long-term remission or a cure.
The HIV viral reservoir, essentially a small number of immune cells containing dormant virus integrated into the genomes of individuals who have suppressed viral replication with HIV treatment, has posed a major impediment to achieving an HIV cure. These cells are completely undetectable by the immune system because the virus is dormant. But as soon as treatment stops, the virus reactivates.
“The barrier to an HIV cure is that the virus hides inside the DNA of cells,” says Gavegnano, director of the Gavegnano Drug Discovery Program and senior author on the study. “The brass ring is an agent that can eliminate these‘reservoir cells,’ which would ultimately eliminate HIV from a person’s body.”
While Gavegnano and her Emory colleagues have shown that Jak inhibitors (Janus kinase inhibitors) could reverse the immune dysfunction caused by HIV since their discovery in 2010, questions about their impact on the HIV reservoir and the exact mechanism contributing to the immunologic improvements have remained unanswered, until now.
The data presented at IAS represented secondary results from a Phase 2a clinical trial centered on investigating ruxolitinib’s effects on viral reservoirs in people with HIV during a five-week regimen, specifically in a subset of individuals with high viral reservoir levels at baseline.
The study measured integrated proviral DNA, which is the genetic material of a virus as incorporated into, and able to replicate with, the genome of a host cell, and examined changes in total, intact only, and defective proviral DNA copies over time. Based on a linear model of decay, the researchers estimated an astonishing 99.99% clearance of the peripheral HIV-1 reservoir in less than three years. These data provide optimism for the use of Jak inhibitors as a backbone for cure-based eradication strategies in the battle against HIV.
Reece, lead author of the study says, “These data suggest that our Jak inhibitors can not only reverse the immune dysfunction that prevents HIV-1 cure, but also significantly decay the reservoir in people living with HIV. Collectively our trial demonstrates a mechanism by which ruxolitinib, or other Jak inhibitors such as baricitinib, also extensively studied by our group, decay the reservoir, which underscores potential for cure-based therapies.”
The profound impact of Ruxolitinib treatment was not limited to reservoir reduction. The study also shed light on several significant biomarkers that were altered by the drug primarily related to:
- Immune activation: Ruxolitinib exhibited the potential to modulate immune activation, which is crucial in controlling viral replication and maintaining immune health in individuals with HIV.
- Cell survival: Ruxolitinib demonstrated the ability to impact cell survival, influencing the lifespan of reservoir cells and potentially limiting viral reservoir longevity.
- Immune dysregulation: The study identified ruxolitinib’s impact on immune dysregulation, offering hope for mitigating the chronic inflammation and immune dysfunction often observed in individuals with HIV.
It is important to note that the study focused on the peripheral viral reservoir and may not fully represent the entire viral reservoir within the body, including sanctuary sites where HIV can persist despite treatment.
Regardless, the findings from Emory University’s study offer hope and renewed enthusiasm for efforts to unravel the complexities of HIV persistence and ultimately find a cure.
“These data are valuable because they show that Jak inhibitors can contribute to a long-term cure strategy for HIV, but they can also be used to slow the inflammatory process caused by other infectious diseases,” says Vincent Marconi, MD, professor of medicine and global health at Emory University School of Medicine.
Marconi, who led the initial phase 2a trial, has already been investigating the efficacy of Jak inhibitors, like ruxolitinib and baricitinib, in patients with acute COVID and now long COVID. He continues, “using an anti-inflammatory drug to treat the effects of a virus could be revolutionary.”
In addition to the data presented by Reece and Gavegnano, another presentation at IAS has shown how ruxolitinib administered to a patient following a stem cell transplant led to an undetectable viral load 20 months after stopping antiretroviral therapy, highlighting the different mechanisms in which these class of drugs could be valuable in HIV care and treatment.
Further research and clinical trials will be needed to fully understand the effects of Jak inhibitor use in HIV and other immune-suppressing conditions. Emory researchers have an extensive history of working with Jak inhibitors. Gavegnano and researcher Raymond Schinazi are listed on the issued patents as sole inventors, and they, alongside their co-investigators, have built a roadmap for tackling a variety of immunosuppressive viruses with these drugs.
Gavegnano emphasizes, “The safety and efficacy outcomes we observed in this study provide a strong foundation for further research on cure-based interventions containing a Jak inhibitor, and we hope to bring this therapy one step closer to helping people living with HIV.”
- News Releases
- School of Medicine
- School of Medicine's Department of Medicine
- Woodruff Health Sciences Center
- Graduate School
- Infectious Diseases
- Global Health
- Health Sciences Research
- GDBBS Program
- GDBBS: Microbiology and Molecular Genetics
Recent News
Download emory news photo.
By downloading Emory news media, you agree to the following terms of use:
Creative Commons Attribution-NoDerivatives 4.0 International Public License
By exercising the Licensed Rights (defined below), You accept and agree to be bound by the terms and conditions of this Creative Commons Attribution-NoDerivatives 4.0 International Public License ("Public License"). To the extent this Public License may be interpreted as a contract, You are granted the Licensed Rights in consideration of Your acceptance of these terms and conditions, and the Licensor grants You such rights in consideration of benefits the Licensor receives from making the Licensed Material available under these terms and conditions.
Section 1 – Definitions.
- Adapted Material means material subject to Copyright and Similar Rights that is derived from or based upon the Licensed Material and in which the Licensed Material is translated, altered, arranged, transformed, or otherwise modified in a manner requiring permission under the Copyright and Similar Rights held by the Licensor. For purposes of this Public License, where the Licensed Material is a musical work, performance, or sound recording, Adapted Material is always produced where the Licensed Material is synched in timed relation with a moving image.
- Copyright and Similar Rights means copyright and/or similar rights closely related to copyright including, without limitation, performance, broadcast, sound recording, and Sui Generis Database Rights, without regard to how the rights are labeled or categorized. For purposes of this Public License, the rights specified in Section 2(b)(1)-(2) are not Copyright and Similar Rights.
- Effective Technological Measures means those measures that, in the absence of proper authority, may not be circumvented under laws fulfilling obligations under Article 11 of the WIPO Copyright Treaty adopted on December 20, 1996, and/or similar international agreements.
- Exceptions and Limitations means fair use, fair dealing, and/or any other exception or limitation to Copyright and Similar Rights that applies to Your use of the Licensed Material.
- Licensed Material means the artistic or literary work, database, or other material to which the Licensor applied this Public License.
- Licensed Rights means the rights granted to You subject to the terms and conditions of this Public License, which are limited to all Copyright and Similar Rights that apply to Your use of the Licensed Material and that the Licensor has authority to license.
- Licensor means the individual(s) or entity(ies) granting rights under this Public License.
- Share means to provide material to the public by any means or process that requires permission under the Licensed Rights, such as reproduction, public display, public performance, distribution, dissemination, communication, or importation, and to make material available to the public including in ways that members of the public may access the material from a place and at a time individually chosen by them.
- Sui Generis Database Rights means rights other than copyright resulting from Directive 96/9/EC of the European Parliament and of the Council of 11 March 1996 on the legal protection of databases, as amended and/or succeeded, as well as other essentially equivalent rights anywhere in the world.
- You means the individual or entity exercising the Licensed Rights under this Public License. Your has a corresponding meaning.
Section 2 – Scope.
- reproduce and Share the Licensed Material, in whole or in part; and
- produce and reproduce, but not Share, Adapted Material.
- Exceptions and Limitations . For the avoidance of doubt, where Exceptions and Limitations apply to Your use, this Public License does not apply, and You do not need to comply with its terms and conditions.
- Term . The term of this Public License is specified in Section 6(a) .
- Media and formats; technical modifications allowed . The Licensor authorizes You to exercise the Licensed Rights in all media and formats whether now known or hereafter created, and to make technical modifications necessary to do so. The Licensor waives and/or agrees not to assert any right or authority to forbid You from making technical modifications necessary to exercise the Licensed Rights, including technical modifications necessary to circumvent Effective Technological Measures. For purposes of this Public License, simply making modifications authorized by this Section 2(a)(4) never produces Adapted Material.
- Offer from the Licensor – Licensed Material . Every recipient of the Licensed Material automatically receives an offer from the Licensor to exercise the Licensed Rights under the terms and conditions of this Public License.
- No downstream restrictions . You may not offer or impose any additional or different terms or conditions on, or apply any Effective Technological Measures to, the Licensed Material if doing so restricts exercise of the Licensed Rights by any recipient of the Licensed Material.
- No endorsement . Nothing in this Public License constitutes or may be construed as permission to assert or imply that You are, or that Your use of the Licensed Material is, connected with, or sponsored, endorsed, or granted official status by, the Licensor or others designated to receive attribution as provided in Section 3(a)(1)(A)(i) .
Other rights .
- Moral rights, such as the right of integrity, are not licensed under this Public License, nor are publicity, privacy, and/or other similar personality rights; however, to the extent possible, the Licensor waives and/or agrees not to assert any such rights held by the Licensor to the limited extent necessary to allow You to exercise the Licensed Rights, but not otherwise.
- Patent and trademark rights are not licensed under this Public License.
- To the extent possible, the Licensor waives any right to collect royalties from You for the exercise of the Licensed Rights, whether directly or through a collecting society under any voluntary or waivable statutory or compulsory licensing scheme. In all other cases the Licensor expressly reserves any right to collect such royalties.
Section 3 – License Conditions.
Your exercise of the Licensed Rights is expressly made subject to the following conditions.
Attribution .
If You Share the Licensed Material, You must:
- identification of the creator(s) of the Licensed Material and any others designated to receive attribution, in any reasonable manner requested by the Licensor (including by pseudonym if designated);
- a copyright notice;
- a notice that refers to this Public License;
- a notice that refers to the disclaimer of warranties;
- a URI or hyperlink to the Licensed Material to the extent reasonably practicable;
- indicate if You modified the Licensed Material and retain an indication of any previous modifications; and
- indicate the Licensed Material is licensed under this Public License, and include the text of, or the URI or hyperlink to, this Public License.
- You may satisfy the conditions in Section 3(a)(1) in any reasonable manner based on the medium, means, and context in which You Share the Licensed Material. For example, it may be reasonable to satisfy the conditions by providing a URI or hyperlink to a resource that includes the required information.
- If requested by the Licensor, You must remove any of the information required by Section 3(a)(1)(A) to the extent reasonably practicable.
Section 4 – Sui Generis Database Rights.
Where the Licensed Rights include Sui Generis Database Rights that apply to Your use of the Licensed Material:
- for the avoidance of doubt, Section 2(a)(1) grants You the right to extract, reuse, reproduce, and Share all or a substantial portion of the contents of the database, provided You do not Share Adapted Material;
- if You include all or a substantial portion of the database contents in a database in which You have Sui Generis Database Rights, then the database in which You have Sui Generis Database Rights (but not its individual contents) is Adapted Material; and
- You must comply with the conditions in Section 3(a) if You Share all or a substantial portion of the contents of the database.
Section 5 – Disclaimer of Warranties and Limitation of Liability.
- Unless otherwise separately undertaken by the Licensor, to the extent possible, the Licensor offers the Licensed Material as-is and as-available, and makes no representations or warranties of any kind concerning the Licensed Material, whether express, implied, statutory, or other. This includes, without limitation, warranties of title, merchantability, fitness for a particular purpose, non-infringement, absence of latent or other defects, accuracy, or the presence or absence of errors, whether or not known or discoverable. Where disclaimers of warranties are not allowed in full or in part, this disclaimer may not apply to You.
- To the extent possible, in no event will the Licensor be liable to You on any legal theory (including, without limitation, negligence) or otherwise for any direct, special, indirect, incidental, consequential, punitive, exemplary, or other losses, costs, expenses, or damages arising out of this Public License or use of the Licensed Material, even if the Licensor has been advised of the possibility of such losses, costs, expenses, or damages. Where a limitation of liability is not allowed in full or in part, this limitation may not apply to You.
- The disclaimer of warranties and limitation of liability provided above shall be interpreted in a manner that, to the extent possible, most closely approximates an absolute disclaimer and waiver of all liability.
Section 6 – Term and Termination.
- This Public License applies for the term of the Copyright and Similar Rights licensed here. However, if You fail to comply with this Public License, then Your rights under this Public License terminate automatically.
Where Your right to use the Licensed Material has terminated under Section 6(a) , it reinstates:
- automatically as of the date the violation is cured, provided it is cured within 30 days of Your discovery of the violation; or
- upon express reinstatement by the Licensor.
- For the avoidance of doubt, the Licensor may also offer the Licensed Material under separate terms or conditions or stop distributing the Licensed Material at any time; however, doing so will not terminate this Public License.
- Sections 1 , 5 , 6 , 7 , and 8 survive termination of this Public License.
Section 7 – Other Terms and Conditions.
- The Licensor shall not be bound by any additional or different terms or conditions communicated by You unless expressly agreed.
- Any arrangements, understandings, or agreements regarding the Licensed Material not stated herein are separate from and independent of the terms and conditions of this Public License.
Section 8 – Interpretation.
- For the avoidance of doubt, this Public License does not, and shall not be interpreted to, reduce, limit, restrict, or impose conditions on any use of the Licensed Material that could lawfully be made without permission under this Public License.
- To the extent possible, if any provision of this Public License is deemed unenforceable, it shall be automatically reformed to the minimum extent necessary to make it enforceable. If the provision cannot be reformed, it shall be severed from this Public License without affecting the enforceability of the remaining terms and conditions.
- No term or condition of this Public License will be waived and no failure to comply consented to unless expressly agreed to by the Licensor.
- Nothing in this Public License constitutes or may be interpreted as a limitation upon, or waiver of, any privileges and immunities that apply to the Licensor or You, including from the legal processes of any jurisdiction or authority.
Creative Commons is not a party to its public licenses. Notwithstanding, Creative Commons may elect to apply one of its public licenses to material it publishes and in those instances will be considered the “Licensor.” The text of the Creative Commons public licenses is dedicated to the public domain under the CC0 Public Domain Dedication . Except for the limited purpose of indicating that material is shared under a Creative Commons public license or as otherwise permitted by the Creative Commons policies published at creativecommons.org/policies , Creative Commons does not authorize the use of the trademark “Creative Commons” or any other trademark or logo of Creative Commons without its prior written consent including, without limitation, in connection with any unauthorized modifications to any of its public licenses or any other arrangements, understandings, or agreements concerning use of licensed material. For the avoidance of doubt, this paragraph does not form part of the public licenses.

An official website of the United States government
Here's how you know
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
Office of AIDS Research
- HIV Policy and Research
- NIH Strategic Plan for HIV and HIV-Related Research
- NIH HIV Research Budget
- NIH HIV Research Priority Areas
- Reduce the Incidence of HIV
- Develop Next-Generation HIV Therapies
Research Toward HIV Cure
- Address HIV-Associated Comorbidities, Coinfections, & Complications
- Cross-Cutting Areas

Viral latency and sanctuaries
Latent HIV reservoirs—small amounts of HIV that persist in people taking ART—present a significant challenge to finding a cure for HIV. Latent reservoirs remain in people with HIV when HIV becomes part of the body’s DNA in infected cells. Additionally, reservoirs of HIV can be found in certain “sanctuary” sites in the body that allow the virus to hide and be protected from both the immune system and ART. To cure HIV, the NIH supports studies to develop novel approaches and treatments that target these HIV reservoirs.
Sustained viral remission and viral eradication

Current science suggests that the path to an HIV cure involves first achieving sustained viral remission without ART. This is called sustained ART-free viral remission or a functional cure. For sustained ART-free viral remission, infectious virus must remain undetectable by sensitive testing methods for a long time without treatment. One research aim will be to prolong the time between treatments to be measured eventually not in weeks, but in months or even years. The NIH supports research into treatments leading to sustained ART-free viral remission . New cure-inducing treatments must be as safe, effective, and available for widespread use as are current-day ART regimens.
Viral eradication—eliminating the virus entirely—is the more challenging, longer-term goal.
Research Strategies
The NIH supports research to better understand how the HIV reservoir forms, persists, and reactivates, as well as investigations to develop new cure treatment strategies targeting HIV reservoirs.
A range of biomarkers and techniques, including single-cell and imaging technologies, are being studied to determine how to identify and describe the HIV reservoir. These techniques also are being used to better understand mechanisms of viral reactivation from latently infected cells.
Experimental treatments in development include therapeutic vaccines, genetically engineered immune cells that are resistant to HIV infection, drugs that reactivate latent HIV to make the virus visible to the immune system, cure-inducing immunotherapies, and interventions to permanently silence HIV in infected cells.
The search for an HIV cure involves important behavioral and social processes that complement the domains of biomedicine. BSSR in HIV cure research is focused on important aspects such as: counseling and support interventions to address the psychosocial needs and concerns of study participants related to analytical treatment interruptions (ATIs); risk reduction in the course of ATI study participation; motivation, acceptability, and decision‐making processes of potential study participants; how cure affects the identity and social position of people with HIV; and the scalability of a proven cure strategy in the context of further advances in HIV prevention and treatment.

The NIH is leveraging resources toward an HIV cure through several public-private partnerships. NIH small business awards enable companies to help foster a diverse pipeline of experimental treatments in development. The combined support of government, industry, and nongovernmental foundations is fostering the expansion of a talented scientific workforce dedicated to advancing HIV cure research.
OAR scientist Dr. Paul Sato coordinates Research Toward an HIV Cure .
This page last reviewed on September 8, 2022
After decades of failures, researchers have renewed hopes for an effective HIV vaccine
The world needs an HIV vaccine if it ever hopes to beat a virus that still infects over 1 million people a year and contributes to hundreds of thousands of deaths.
Despite 20 years of failures in major HIV vaccine trials — four this decade alone — researchers say recent scientific advances have likely, hopefully, put them on the right track to develop a highly effective vaccine against the insidious virus.
But probably not until the 2030s.
“An effective vaccine is really the only way to provide long-term immunity against HIV, and that’s what we need,” Dr. Julie McElrath, the director of the vaccine and infectious disease division at the Fred Hutchinson Cancer Center in Seattle, said Monday at the Conference on Retroviruses and Opportunistic Infections in Denver.
All current HIV vaccine action is in the laboratory, animal studies or very early human trials.
Researchers at the retrovirus conference presented favorable results from two HIV vaccine studies. One found that a modification to the simian version of HIV spurred production of what are known as broadly neutralizing antibodies against the virus in monkeys. Another showed promise in the effort to coax the immune system’s B cells to make the powerful antibodies in humans.
“These trials illustrate as a proof of concept that we can train the immune system. But we need to further optimize it and test it in clinical trials,” Karlijn van der Straten, a Ph.D. student at the Academic Medical Center at Amsterdam University, who presented the human study, said at a news conference Monday.
Still, the scrappy scientists in this field face a towering challenge. HIV is perhaps the most complex pathogen ever known.
“The whole field has learned from the past,” said William Schief, who leads Moderna’s HIV vaccine efforts. “We’ve learned strategies that don’t work.”
The cost has already been immense. Nearly $17 billion was spent worldwide on HIV -vaccine research from 2000 to 2021. Nearly $1 billion more is spent annually, according to the Joint United Nations Program on HIV/AIDS and the nonprofit HIV group AVAC.
“Maintaining the funding for HIV vaccines right now is really important,” said Dr. Nina Russell, who directs HIV research at the Bill & Melinda Gates Foundation. She pointed to the field’s own “progress and the excitement” and to how “HIV vaccine science and scientists continue to drive innovation and science that benefits other infectious diseases and global health in general.”
Case in point: Covid. Thanks to HIV research, the mRNA vaccine technology was already available in 2020 to speed a coronavirus vaccine to market.
Why the HIV vaccine efficacy trials failed
In strong contrast to Covid, the HIV vaccine endeavor has spanned four decades. Only one of the nine HIV vaccine trials have shown efficacy: a trial conducted in Thailand and published in 2009 that reported a modest 31% reduction in HIV risk.
HIV vaccine researchers subsequently spent years seeking to retool and improve that vaccine strategy, leading to a series of trials that launched in the late 2010s — only to fail.
Researchers have concluded those latest trials were doomed because, aside from prompting an anti-HIV response based in immune cells, they only drove the immune system to produce what are known as non-neutralizing antibodies. Those weapons just weren’t strong enough for such a fearsome foe.
Preventing HIV through vaccination remains a daunting challenge because the immune system doesn’t naturally mount an effective defense against the virus, as it does with so many other vaccine-preventable infections, including Covid. An HIV vaccine must coax from the body a supercharged immune response with no natural equivalent.
That path to victory is based on a crucial caveat: A small proportion of people with HIV do produce what are known as broadly neutralizing antibodies against the virus. They attack HIV in multiple ways and can neutralize a swath of variants of the virus.
Those antibodies don’t do much apparent good for people who develop them naturally, because they typically don’t arise until years into infection. HIV establishes a permanent reservoir in the body within about a week after infection, one that their immune response can’t eliminate. So HIV-positive people with such antibodies still require antiretroviral treatment to remain healthy.
Researchers believe that broadly neutralizing antibodies could prevent HIV from ever seeding an infection, provided the defense was ready in advance of exposure. A pair of major efficacy trials, published in 2021 , demonstrated that infusions of cloned versions of one such antibody did, indeed, protect people who were exposed to certain HIV strains that are susceptible to that antibody.
However, globally, those particular strains of the virus comprise only a small subset of all circulating HIV. That means researchers can’t simply prompt a vaccine to produce that one antibody and expect it to be effective. Importantly, from this study they got a sense of what antibody level would be required to prevent infection.
It’s a high benchmark, but at least investigators now have a clearer sense of the challenge before them.
Also frustrating the HIV vaccine quest is that the virus mutates like mad. Whatever spot on the surface of the virus that antibodies target might be prone to change through mutation, thus allowing the virus to evade their attack. Consequently, researchers search for targets on the virus’ surface that aren’t highly subject to mutation.
Experts also believe warding off the mutation threat will require targeting multiple sites on the virus. So researchers are seeking to develop a portfolio of immune system prompts that would spur production of an array of broadly neutralizing antibodies.
Prompting the development of such antibodies requires a complex, step-by step process of coaxing the infection-fighting B cells, getting them to multiply and then guiding their maturation into potent broadly neutralizing antibody-producing factories.
HIV vaccine development ‘in a better place’
Dr. Carl Dieffenbach, the head of the AIDS division at the National Institute of Allergy and Infectious Diseases, said numerous recent technological advances — including mRNA, better animal models of HIV infection and high-tech imaging technology — have improved researchers’ precision in designing, and speed in producing, new proteins to spur anti-HIV immune responses.
Global collaboration among major players is also flourishing, researchers said. There are several early-stage human clinical trials of HIV-vaccine components underway.
Three mRNA- based early human trials of such components have been launched since 2022. Among them, they have been led or otherwise funded by the global vaccine research nonprofit group IAVI, Fred Hutch, Moderna, Scripps Research, the Gates Foundation, the National Institutes of Health, the U.S. Agency for International Development, and university teams. More such trials are in the works.
On Friday, Science magazine reported concerning recent findings that among the three mRNA trials, a substantial proportion of participants — 7% to 18%, IAVI said in a statement — experienced skin-related symptoms following injections, including hives, itching and welts.
IAVI said in its statement that it and partners are investigating the HIV trials’ skin-related outcomes, most of which were “mild or moderate and managed with simple allergy medications.”
Researchers have shown success in one of those mRNA trials in executing a particular step in the B-cell cultivation process.
That vaccine component also generated “helper” CD4 cells primed to combat HIV. The immune cells are expected to operate like an orchestra conductor for the immune system, coordinating a response by sending instructions to B cells and scaling up other facets of an assault on HIV.
A complementary strategy under investigation seeks to promote the development of “killer” CD8 cells that might be primed to kill off any immune cells that the antibodies failed to save from infection.
Crucially, investigators believe they are now much better able to discern top vaccine component candidates from the duds. They plan to spend the coming years developing such components so that when they do assemble the most promising among them into a multi-pronged vaccine, they can be much more confident of ultimate success in a trial.
“An HIV vaccine could end HIV,” McElrath said at the Denver conference. “So I say, ‘Let’s just get on with it.”
Dr. Mark Feinberg, president and CEO of IAVI, suggested that the first trial to test effectiveness of the vaccine might not launch until 2030 or later.
Even so, he was bullish.
“The field of HIV vaccine development is in a better place now than it’s ever been,” he said.
Benjamin Ryan is independent journalist specializing in science and LGBTQ coverage. He contributes to NBC News, The New York Times, The Guardian and Thomson Reuters Foundation and has also written for The Washington Post, The Nation, The Atlantic and New York.
- Skip to main content
- Keyboard shortcuts for audio player
The FDA has approved a new drug in the fight against AIDS
Jason Beaubien
The Food and Drug Administration has approved the first injectable medication for HIV prevention. Health advocates say it could be a game changer in protecting people against AIDS
Copyright © 2021 NPR. All rights reserved. Visit our website terms of use and permissions pages at www.npr.org for further information.
NPR transcripts are created on a rush deadline by an NPR contractor. This text may not be in its final form and may be updated or revised in the future. Accuracy and availability may vary. The authoritative record of NPR’s programming is the audio record.
- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
News releases.
News Release
Monday, March 14, 2022
NIH launches clinical trial of three mRNA HIV vaccines
Phase 1 study is among first to examine mRNA technology for HIV.

The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has launched a Phase 1 clinical trial evaluating three experimental HIV vaccines based on a messenger RNA (mRNA) platform—a technology used in several approved COVID-19 vaccines. NIAID is sponsoring the study, called HVTN 302, and the NIAID-funded HIV Vaccine Trials Network (HVTN), based at Fred Hutchinson Cancer Research Center in Seattle, is conducting the trial.
“Finding an HIV vaccine has proven to be a daunting scientific challenge,” said Anthony S. Fauci, M.D. NIAID director. “With the success of safe and highly effective COVID-19 vaccines, we have an exciting opportunity to learn whether mRNA technology can achieve similar results against HIV infection.”
An mRNA vaccine works by delivering a piece of genetic material that instructs the body to make a protein fragment of a target pathogen (such as a virus), which the immune system recognizes and remembers, so it can mount a substantial response if later exposed to that pathogen. The HVTN 302 study will examine whether the following three experimental HIV mRNA vaccines are safe and can induce an immune response: 1) BG505 MD39.3 mRNA, 2) BG505 MD39.3 gp151 mRNA, and 3) BG505 MD39.3 gp151 CD4KO mRNA. Each investigational vaccine candidate is designed to present the spike protein found on the surface of HIV that facilitates entry into human cells. Each of the experimental vaccines encodes for different but highly related, stabilized proteins. None of the three vaccine candidates can cause HIV infection.
The specific mRNA sequences contained in the vaccines were designed and developed by investigators at the NIAID-funded Scripps Consortium for HIV/AIDS Vaccine Development (CHAVD) at the Scripps Research Institute and the Bill & Melinda Gates Foundation-funded IAVI Neutralizing Antibody Center at Scripps, in collaboration with scientists at Cambridge, Massachusetts-based Moderna, Inc. Moderna manufactured the investigational vaccines through a NIAID-supported contract.
Led by principal investigators Jesse Clark, M.D., of the University of California Los Angeles, and Sharon Riddler, M.D., of the University of Pittsburgh, the HVTN 302 study will enroll up to 108 adults ages 18 to 55 years at 11 sites in: Birmingham, Alabama; Boston; Los Angeles; New York City; Philadelphia; Pittsburgh; Rochester, New York and Seattle. Each participant will be randomly assigned to one of six groups each receiving three vaccinations of one of the experimental vaccines. The first three groups (18 participants each), called Group A, will receive intramuscular injections of 100 micrograms (mcg) of their assigned vaccine candidate at the initial visit, at month two and again at month six. Participants in Group A will be evaluated two weeks after initial vaccination to ensure safety criteria have been met. If so, the remaining three groups of 18 participants each (Group B) will be vaccinated with 250 mcg of the assigned investigational vaccine, followed by injections two and six months after the initial vaccination.
Safety and immune responses will be examined via blood and lymph node fine-needle aspiration samples taken at specified timepoints throughout the trial. Clinical staff will closely monitor participant safety throughout the study. The clinical trial is expected to be completed by July 2023.
More information about the HVTN 302 study is available on ClinicalTrials.gov using the identifier NCT05217641 .
NIAID conducts and supports research—at NIH, throughout the United States, and worldwide—to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing, and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website .
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov .
NIH…Turning Discovery Into Health ®
Connect with Us
- More Social Media from NIH

New Insights into HIV Latent Cells Yield Possible Cure Targets

Scanning electron micrograph of an HIV-infected H9 T cell
Photo: NIAID
Scientists from NIAID’s Vaccine Research Center and colleagues used cutting-edge technology to reveal new insights into cellular reservoirs of HIV. These new insights could have big implications toward an HIV cure.
An enhanced understanding of the HIV-infected memory CD4+ T cells that persist over decades in individuals taking antiretroviral therapy has been a long-time goal of HIV cure researchers. However, technology limitations have made it difficult to isolate or analyze these individual cells. As a result, scientists have been unable to determine whether the cells possess distinctive attributes that HIV-cure-directed therapies may exploit.
At the International AIDS Conference in Montreal, Dr. Eli Boritz, chief of the virus persistence and dynamics section in the VRC Laboratory of Immunology, described NIAID’s longstanding collaboration with a bioengineering research group at the University of California, San Francisco.
The researchers developed a custom microfluidic sorting technology—Focused Interrogation of Cells by Nucleic Acid Detection and Sequencing (FIND-Seq). This technology defines gene expression patterns from rare cells harboring latent HIV, allowing messenger RNA capture and virus DNA detection to be performed sequentially while maintaining segregation among cells.
The scientists applied the FIND-Seq technology to blood cells from 6 people with HIV who had begun taking ART while chronically infected and who had experienced more than 1 year of viral suppression. They found clear differences between the HIV-infected CD4+ T cells and their uninfected counterparts, including gene expression patterns linked to the suppression of multiple steps in the HIV lifecycle and to cell survival and proliferation.
The scientists maintain these results indicate that the HIV-infected memory CD4+ T-cell reservoir is a distinctive cell population that may be uniquely susceptible to specific targeted therapies. In this regard, the study reinforces recent interest among scientists in improving upon HIV cure strategies that are based on latency reversal by incorporating drugs that relieve blocks at multiple HIV lifecycle steps, and by combining these with agents that potentiate physiologic cell death.
- China Mainland
- Saudi Arabia
- Türkiye
- United Arab Emirates
- South Africa
- Australia and New Zealand
- Belgium and Luxembourg
- Czech Republic & Slovakia
- Netherlands
- Poland & The Baltics
- Switzerland
- United Kingdom
Press Releases
February 21, 2023
Gilead Presents New Data From HIV Cure Research Program and Collaborations Exploring Novel Investigational Combinations and Strategies
– Innovative Investigational Approaches Include Targeting of the HIV Viral Reservoir and Enhancing Immune Response in the Absence of Antiretroviral Therapy –
– Findings Support Continued Evaluation of Novel Strategies including Broadly Neutralizing HIV Antibodies, Vaccine Candidates, and TLR Agonists –
FOSTER CITY, Calif.--(BUSINESS WIRE)-- Gilead Sciences, Inc. (Nasdaq: GILD) today announced results from four collaborative studies evaluating novel investigational combinations and strategies with the potential to target the HIV viral reservoir or enhance immune response to maintain virologic control in the absence of antiretroviral therapy (ART). These latest findings represent an ongoing multi-pronged approach in Gilead’s HIV cure research program. The data were presented at the Conference on Retroviruses and Opportunistic Infections (CROI) 2023 in Seattle.
Findings from the HIV cure research program include results from three studies evaluating strategies to maintain virologic control in the absence of ART. Results from the Phase 2a TITAN trial show that dual treatment with the broadly neutralizing HIV antibodies (bNAbs; 3BNC117 and 10-1074) led to a significant delay in viral rebound. A Phase 1/2 proof-of-concept study conducted by the University of California San Francisco, with support from Gilead Sciences and amfAR, The Foundation for AIDS Research, provides evidence that combination immunotherapy consisting of a vaccine, an immune modulator, and bNAbs (10-1074 and VRC07-523LS) may provide virologic control. A separate AELIX-003 Phase 2 trial showed that a combination of a vaccine and an immune modulator induced a strong T cell response.
“The results from the TITAN study exemplify the progress that research partnerships can bring in the pursuit of a cure for HIV and demonstrate the potential of combination strategies to play a critical role in that effort,” said Ole Søgaard, MD, Professor in the Department of Infectious Diseases at Aarhus University in Denmark. “Additional clinical research into the potential of bNAbs is warranted and may help in the discovery of novel approaches that transform HIV management for patients.”
A fourth pre-clinical study in a macaque model conducted in collaboration with Gritstone bio, Inc. showed that simian immunodeficiency virus (SIV) ChAd and samRNA vaccines in combination with immune modulators induced a strong immune response.
“The insights generated by the studies presented at CROI this year are advancing scientific knowledge on potential paths to a cure for HIV and expanding global understanding of what role broadly neutralizing antibodies, vaccines, and immune modulators may play in the future of HIV for people living with the virus,” said Devi SenGupta, Executive Director, HIV Clinical Development, Gilead Sciences. “Gilead will continue exploring novel combination strategies in our pursuit to help end the HIV epidemic for everyone, everywhere.”
Curing HIV remains the ultimate aspiration of Gilead’s HIV research and development efforts. Gilead has a comprehensive cure research and development program that is advancing with speed and conviction. As Gilead progresses further with testing investigational curative regimens, the company’s partnerships and collaborations are more important than ever in this complex effort. Gilead aims to ensure its research and development efforts contribute to the entire scientific community’s search for a cure. Gilead’s work to develop a cure for HIV is one part of the company’s larger role in the global efforts to end the HIV epidemic and part of its focus on person-centric innovation.
HIV cure research studies presented at CROI include:
Lefitolimod, vesatolimod, teropavimab, zinlirvimab, and the other experimental compounds noted are investigational and are not approved by the U.S. Food and Drug Administration or any other regulatory authority. Their safety and efficacy have not been established.
There is no cure for HIV infection or AIDS.
The TITAN trial (NCT03837756) was funded in part through the Gilead Cure Grants Program. For more information, please visit: https://clinicaltrials.gov/ct2/show/NCT03837756 .
About Gilead Sciences’ HIV Cure Research Program
Curing HIV is a formidable challenge, but one that remains the focus and ultimate aspiration of Gilead’s HIV research and development efforts. We are mapping the path forward to a cure for HIV through close collaborations with industry, academic and community partners to ensure our research efforts can contribute to the entire scientific community’s search for a cure. Together with our partners, we are committed to helping end the epidemic by driving the next generation of cure strategies that will transform care and improve outcomes for all people living with HIV.
About Gilead Sciences
Gilead Sciences, Inc. is a biopharmaceutical company that has pursued and achieved breakthroughs in medicine for more than three decades, with the goal of creating a healthier world for all people. The company is committed to advancing innovative medicines to prevent and treat life-threatening diseases and address unmet needs in virology, oncology and inflammation.
For 35 years, Gilead has been a leading innovator in the field of HIV, driving advances in treatment, prevention and cure research. Gilead researchers have developed 12 HIV medications , including the first single-tablet regimen to treat HIV, the first antiretroviral for pre-exposure prophylaxis (PrEP) to reduce the risk of acquiring HIV infection, and the first long-acting injectable HIV treatment medication administered twice-yearly. Our advances in medical research have helped to transform HIV into a treatable, preventable, chronic condition for millions of people.
Gilead is committed to continued scientific innovation to provide solutions for the evolving needs of people affected by HIV around the world. Through partnerships and collaborations, the company also aims to improve education, expand access and address barriers to care, with the goal of ending the HIV epidemic for everyone, everywhere. Gilead was recognized as the number one philanthropic funder of HIV-related programs in a report released by Funders Concerned About AIDS.
Gilead operates in more than 35 countries worldwide, with headquarters in Foster City, California.
Forward-Looking Statements
This press release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including Gilead’s ability to initiate, progress or complete clinical trials or studies within currently anticipated timelines or at all, and the possibility of unfavorable results from ongoing or additional clinical trials or studies, including those involving and other bNAbs, and experimental compounds of our partner; uncertainties relating to regulatory applications and related filing and approval timelines, and the risk that any regulatory approvals, if granted, may be subject to significant limitations on use; and any assumptions underlying any of the foregoing. These and other risks, uncertainties and factors are described in detail in Gilead’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2022, as filed with the U.S. Securities and Exchange Commission. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. The reader is cautioned that any such forward-looking statements are not guarantees of future performance and involve risks and uncertainties, and is cautioned not to place undue reliance on these forward-looking statements. All forward-looking statements are based on information currently available to Gilead, and Gilead assumes no obligation and disclaims any intent to update any such forward-looking statements.
Gilead and the Gilead logo are registered trademarks of Gilead Sciences, Inc., or its related companies. All other trademarks are the property of their respective owner(s).
For more information about Gilead, please visit the company’s website at www.gilead.com , follow Gilead on Twitter ( @Gilead Sciences ) and LinkedIn , or call Gilead Public Affairs at 1-800-GILEAD-5 or 1-650-574-3000.
Jacquie Ross, Investors [email protected]
Meaghan Smith, Media [email protected]
Intersititial body copy

- May 18, 2024 | Discover How MIT’s SuperLimbs Help Astronauts Stand Tall on the Moon
- May 18, 2024 | Toxic Downpour: “Forever Chemicals” Rain on All Five Great Lakes
- May 18, 2024 | New UCLA Research Reveals That Practice Significantly Rewires the Brain
- May 18, 2024 | Scientists Discover Potential Opioid Replacement for Back Pain
- May 18, 2024 | Termites on Tour: How Climate Change Is Bringing Pests to Your Doorstep
New Research Paves Way for Potential Universal HIV Cure
By Oregon Health & Science University June 16, 2023
A research team from Oregon Health & Science University (OHSU) has shed light on the mechanisms by which stem cell transplants can cure HIV, a breakthrough that brings us closer to a universal cure for AIDS. The study found that two non-human primates were cured of a form of HIV after stem cell transplantation, revealing that two factors must be present for a cure – the donor stem cells attacking the HIV-infected cells and preventing the virus from infecting the new cells.
New research unveils initial insights into the mechanisms by which stem cell transplantation can eliminate the virus responsible for AIDS.
New findings from Oregon Health & Science University help explain how at least five individuals have been cured of HIV following stem cell transplants. This research paves the way toward the potential development of a widespread remedy for the virus that results in AIDS, currently affecting approximately 38 million people globally.
The study, which was published in the journal Immunity , sheds light on how two nonhuman primates were successfully treated for the monkey form of HIV through stem cell transplants. It further reveals that a cure can only be achieved when two specific conditions coincide and present the sequential process in which HIV is eradicated from the body. These findings provide valuable insights that can guide efforts to extend this curative strategy to a larger population.
“Five patients have already demonstrated that HIV can be cured,” said the study’s lead researcher, Jonah Sacha, Ph.D., a professor at OHSU’s Oregon National Primate Research Center and Vaccine and Gene Therapy Institute.
“This study is helping us home in on the mechanisms involved in making that cure happen,” Sacha continued. “We hope our discoveries will help to make this cure work for anyone, and ideally through a single injection instead of a stem cell transplant.”
The first known case of HIV being cured through a stem cell transplant was reported in 2009. A man who was living with HIV was also diagnosed with acute myeloid leukemia, a type of cancer, and underwent a stem cell transplant in Berlin, Germany. Stem cell transplants, which are also called bone marrow transplants, are used to treat some forms of cancer. Known as the Berlin patient, he received donated stem cells from someone with a mutated CCR5 gene, which normally codes for a receptor on the surface of white blood cells that HIV uses to infect new cells. A CCR5 mutation makes it difficult for the virus to infect cells and can make people resistant to HIV. Since the Berlin patient, four more people have been similarly cured.
This study was conducted with a species of nonhuman primate known as Mauritian cynomolgus macaques, which the research team previously demonstrated can successfully receive stem cell transplants. While all of the study’s eight subjects had HIV, four of them underwent a transplant with stem cells from HIV-negative donors, and the other half served as the study’s controls and went without transplants.
Of the four that received transplants, two were cured of HIV after successfully being treated for graft-versus-host disease, which is commonly associated with stem cell transplants.
Other researchers have tried to cure nonhuman primates of HIV using similar methods, but this study marks the first time that HIV-cured research animals have survived long-term. Both remain alive and HIV-free today, about four years after transplantation. Sacha attributes their survival to exceptional care from Oregon National Primate Research Center veterinarians and the support of two study coauthors, OHSU clinicians who care for people who undergo stem cell transplants: Richard T. Maziarz, M.D., and Gabrielle Meyers, M.D.
“These results highlight the power of linking human clinical studies with pre-clinical macaque experiments to answer questions that would be almost impossible to do otherwise, as well as demonstrate a path forward to curing human disease,” said Maziarz, a professor of medicine in the OHSU School of Medicine and medical director of the adult blood and marrow stem cell transplant and cellular therapy programs in the OHSU Knight Cancer Institute.
The how behind the cure
Although Sacha said it was gratifying to confirm stem cell transplantation cured the nonhuman primates, he and his fellow scientists also wanted to understand how it worked. While evaluating samples from the subjects, the scientists determined there were two different, but equally important, ways they beat HIV.
First, the transplanted donor stem cells helped kill the recipients’ HIV-infected cells by recognizing them as foreign invaders and attacking them, similar to the process of graft-versus-leukemia that can cure people of cancer.
Second, in the two subjects that were not cured, the virus managed to jump into the transplanted donor cells. A subsequent experiment verified that HIV was able to infect the donor cells while they were attacking HIV. This led the researchers to determine that stopping HIV from using the CCR5 receptor to infect donor cells is also needed for a cure to occur.
The researchers also discovered that HIV was cleared from the subjects’ bodies in a series of steps. First, the scientists saw that HIV was no longer detectable in the blood circulating in their arms and legs. Next, they couldn’t find HIV in lymph nodes, or lumps of immune tissue that contain white blood cells and fight infection. Lymph nodes in the limbs were the first to be HIV-free, followed by lymph nodes in the abdomen.
The step-wise fashion by which the scientists observed HIV being cleared could help physicians as they evaluate the effectiveness of potential HIV cures. For example, clinicians could focus on analyzing blood collected from both peripheral veins and lymph nodes. This knowledge may also help explain why some patients who have received transplants initially have appeared to be cured, but HIV was later detected. Sacha hypothesizes that those patients may have had a small reservoir of HIV in their abdominal lymph nodes that enabled the virus to persist and spread again throughout the body.
Sacha and colleagues continue to study the two nonhuman primates cured of HIV. Next, they plan to dig deeper into their immune responses, including identifying all of the specific immune cells involved and which specific cells or molecules were targeted by the immune system.
Reference: “Allogeneic immunity clears latent virus following allogeneic stem cell transplantation in SIV-infected ART-suppressed macaques” by Helen L. Wu, Kathleen Busman-Sahay, Whitney C. Weber, Courtney M. Waytashek, Carla D. Boyle, Katherine B. Bateman, Jason S. Reed, Joseph M. Hwang, Christine Shriver-Munsch, Tonya Swanson, Mina Northrup, Kimberly Armantrout, Heidi Price, Mitch Robertson-LeVay, Samantha Uttke, Mithra R. Kumar, Emily J. Fray, Sol Taylor-Brill, Stephen Bondoc, Rebecca Agnor, Stephanie L. Junell, Alfred W. Legasse, Cassandra Moats, Rachele M. Bochart, Joseph Sciurba, Benjamin N. Bimber, Michelle N. Sullivan, Brandy Dozier, Rhonda P. MacAllister, Theodore R. Hobbs, Lauren D. Martin, Angela Panoskaltsis-Mortari, Lois M.A. Colgin, Robert F. Siliciano, Janet D. Siliciano, Jacob D. Estes, Jeremy V. Smedley, Michael K. Axthelm, Gabrielle Meyers, Richard T. Maziarz, Benjamin J. Burwitz, Jeffrey J. Stanton and Jonah B. Sacha, 25 May 2023, Immunity . DOI: 10.1016/j.immuni.2023.04.019
The study was funded by the National Institutes of Health , the Foundation for AIDS Research, and the Foundation for AIDS Immune Research.
More on SciTechDaily
New Method To Cure HIV Yields Long-Term Successful Results
Hearts From Donors Who Used Illicit Drugs or Died Due to an Overdose Are Safe for Transplant
The first ‘functional hiv cure’ in an infant.
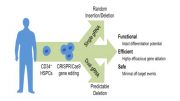
New Gene-Editing Technique Could Prove to be an Effective Technique for Blocking HIV
New Study: Gene Therapy Can Effectively Eliminate HIV Infection

New Technique Could Increase the Number of Usable Donated Hearts by 30%
Tragic Drop in Life-Saving Organ Transplants Amid COVID-19 Outbreak
Engineered Stem Cells Suppress HIV in Living Tissues
Be the first to comment on "new research paves way for potential universal hiv cure", leave a comment cancel reply.
Email address is optional. If provided, your email will not be published or shared.
Save my name, email, and website in this browser for the next time I comment.
To revisit this article, visit My Profile, then View saved stories .
- Backchannel
- Newsletters
- WIRED Insider
- WIRED Consulting
Emily Mullin
There’s New Hope for an HIV Vaccine
Since it was first identified in 1983, HIV has infected more than 85 million people and caused some 40 million deaths worldwide.
While medication known as pre-exposure prophylaxis , or PrEP, can significantly reduce the risk of getting HIV, it has to be taken every day to be effective. A vaccine to provide lasting protection has eluded researchers for decades. Now, there may finally be a viable strategy for making one.
An experimental vaccine developed at Duke University triggered an elusive type of broadly neutralizing antibody in a small group of people enrolled in a 2019 clinical trial. The findings were published today in the scientific journal Cell .
“This is one of the most pivotal studies in the HIV vaccine field to date,” says Glenda Gray, an HIV expert and the president and CEO of the South African Medical Research Council, who was not involved in the study.
A few years ago, a team from Scripps Research and the International AIDS Vaccine Initiative (IAVI) showed that it was possible to stimulate the precursor cells needed to make these rare antibodies in people. The Duke study goes a step further to generate these antibodies, albeit at low levels.
“This is a scientific feat and gives the field great hope that one can construct an HIV vaccine regimen that directs the immune response along a path that is required for protection,” Gray says.
Vaccines work by training the immune system to recognize a virus or other pathogen. They introduce something that looks like the virus—a piece of it, for example, or a weakened version of it—and by doing so, spur the body’s B cells into producing protective antibodies against it. Those antibodies stick around so that when a person later encounters the real virus, the immune system remembers and is poised to attack.
While researchers were able to produce Covid-19 vaccines in a matter of months, creating a vaccine against HIV has proven much more challenging. The problem is the unique nature of the virus. HIV mutates rapidly, meaning it can quickly outmaneuver immune defenses. It also integrates into the human genome within a few days of exposure, hiding out from the immune system.
“Parts of the virus look like our own cells, and we don’t like to make antibodies against our own selves,” says Barton Haynes, director of the Duke Human Vaccine Institute and one of the authors on the paper.
The particular antibodies that researchers are interested in are known as broadly neutralizing antibodies, which can recognize and block different versions of the virus. Because of HIV’s shape-shifting nature, there are two main types of HIV and each has several strains. An effective vaccine will need to target many of them.
Some HIV-infected individuals generate broadly neutralizing antibodies, although it often takes years of living with HIV to do so, Haynes says. Even then, people don’t make enough of them to fight off the virus. These special antibodies are made by unusual B cells that are loaded with mutations they’ve acquired over time in reaction to the virus changing inside the body. “These are weird antibodies,” Haynes says. “The body doesn’t make them easily.”

Will Knight

Steven Levy

Scott Gilbertson

Brendan I. Koerner
Haynes and his colleagues aimed to speed up that process in healthy, HIV-negative people. Their vaccine uses synthetic molecules that mimic a part of HIV’s outer coat, or envelope, called the membrane proximal external region. This area remains stable even as the virus mutates. Antibodies against this region can block many circulating strains of HIV.
The trial enrolled 20 healthy participants who were HIV-negative. Of those, 15 people received two of four planned doses of the investigational vaccine, and five received three doses. The trial was halted when one participant experienced an allergic reaction that was not life-threatening. The team found that the reaction was likely due to an additive in the vaccine, which they plan to remove in future testing.
Still, they found that two doses of the vaccine were enough to induce low levels of broadly neutralizing antibodies within a few weeks. Notably, B cells seemed to remain in a state of development to allow them to continue acquiring mutations, so they could evolve along with the virus. Researchers tested the antibodies on HIV samples in the lab and found that they were able to neutralize between 15 and 35 percent of them.
Jeffrey Laurence, a scientific consultant at the Foundation for AIDS Research (amfAR) and a professor of medicine at Weill Cornell Medical College, says the findings represent a step forward, but that challenges remain. “It outlines a path for vaccine development, but there’s a lot of work that needs to be done,” he says.
For one, he says, a vaccine would need to generate antibody levels that are significantly higher and able to neutralize with greater efficacy. He also says a one-dose vaccine would be ideal. “If you’re ever going to have a vaccine that’s helpful to the world, you’re going to need one dose,” he says.
Targeting more regions of the virus envelope could produce a more robust response. Haynes says the next step is designing a vaccine with at least three components, all aimed at distinct regions of the virus. The goal is to guide the B cells to become much stronger neutralizers, Haynes says. “We’re going to move forward and build on what we have learned.”
You Might Also Like …
In your inbox: Will Knight's Fast Forward explores advances in AI
He emptied a crypto exchange onto a thumb drive —then disappeared
The real-time deepfake romance scams have arrived
Boomergasms are booming
Heading outdoors? Here are the best sleeping bags for every adventure

Maggie Chen
Beth Mole, Ars Technica

Matt Reynolds
Max G. Levy

Personalize Your Experience
Log in or create an account for a personalized experience based on your selected interests.
Already have an account? Log In
Free standard shipping is valid on orders of $45 or more (after promotions and discounts are applied, regular shipping rates do not qualify as part of the $45 or more) shipped to US addresses only. Not valid on previous purchases or when combined with any other promotional offers.
Register for an enhanced, personalized experience.
Receive free access to exclusive content, a personalized homepage based on your interests, and a weekly newsletter with topics of your choice.
Home / Innovation & Research / The innovative research behind HIV/AIDS treatment
The innovative research behind HIV/AIDS treatment
Please login to bookmark.

It’s been 40 years since the release of the first scientific report describing acquired immune deficiency syndrome (AIDS). Thanks to innovative research, scientists learned how the HIV virus that causes AIDS replicates and how the immune system responds to the virus. Today, many people with HIV take just one pill a day to suppress the virus, and treatment is continuing to evolve.
In this video, Dr. Stacey Rizza , Mayo Clinic infectious disease physician and HIV researcher, explains how dedicated innovative science contributed to where we are today and what scientists are working on for the future.
What did the early research find?
Because of truly dedicated innovative science, within a few years, the scientific community figured out that AIDS was due to HIV. It then took a few years to figure out how to test for that virus. Several years later, the scientific community was able to quantitate how much virus was in a person’s blood. During all this time, truly innovative research into how the virus replicates and how the immune system responds to the virus allowed bio pharmacy companies to develop what we call anti-retroviral drugs or medications to slow down the viral replication. How has medication to treat HIV evolved?
The first drug approved for HIV was in 1987, which was AZT (now known as zidovudine). At that time, it was the fastest drug ever approved by the FDA (Food and Drug Administration) and started the fast-track mechanism through the FDA.
Then several other drugs within that same class were approved in the early 1990s. In late 1995, very early 1996, the first HIV protease inhibitors were approved. At that point, it was possible to combine three different medications from two different classes and completely suppress the HIV replication.
In the last 20 years, we’ve gone from people taking multiple medicines with lots of side effects to many of my patients with HIV now take a single pill a day. That’s a combination of medicines coformulated into one pill a day that’s extremely well-tolerated and completely suppresses their virus. We know it does not eliminate the virus. If they were to stop taking that medicine, the virus would come back. But we now have a handful of people in the world who have been what we called functionally cured of HIV, meaning they’ve gone through some research protocols that eliminated the reservoir of HIV in their body.
The new drugs are so effective in people who have fully suppressed virus that many only need to use two medications to maintain HIV treatment and control. New research is investigating ways to deliver the medications differently, such as a shot that lasts several months, or maybe someday even implantable medication delivery mechanisms so that people don’t have to take the pill every day. It is very exciting that HIV therapy is moving that direction.
Why isn’t there a cure for HIV?
The reason why it is so difficult to cure HIV is that once HIV infects a person’s body, it integrates into the host genome of several cell types. Those cells then hide in any of the lymphoid tissue, such as the lymph nodes, the liver and the spleen. And they lay there as what we call “latent” or “hiding”, as long as the person is on HIV therapy. Anytime a virus does leave a cell, it gets taken care of by HIV therapy. But if the infected individual stops the HIV therapy, that latent virus will come back. To cure HIV, you have to eliminate those hiding viruses in the cells or that latent viral reservoir, which is the term. There are many ways you can approach eliminating the reservoir.
Where is the research now?
One of the more popular ways that have been investigated is something called — and there are many different terms for it — “prime, shock, and kill” or “kick, and kill”, which is essentially giving medications that first wake the virus up from latency and then find ways to make the cells that have the virus susceptible to dying. When the virus is awake, and the cell is susceptible to dying, it kills itself but does not kill any other cells in the body.
Essentially, it specifically targets the HIV-infected cells and eliminates them without hurting anything else. This new science is exciting. It’s getting closer and closer to understanding how to do this effectively. And if you can do that with oral medications rather than fancy therapies like gene therapy or bone marrow transplant, it’s scalable to large parts of the world, and you can touch millions of people that way. That’s where the area of research is on how to make those hiding cells wake up, how to make them sensitive to die, and how to target just the HIV-infected cell.
Will we see a vaccine for HIV?
HIV has been a very hard vaccine to develop. In the world of viruses, vaccines fall into one of three buckets. They fall into the bucket where they respond to antibodies induced by the vaccine, and the vaccines are outstanding. Such viruses include polio, mumps, and lucky for us, SARS-CoV-2. Then we have the second category, like the influenza vaccine, which is about 60% effective. It certainly saves lives and makes a difference, but it’s not perfect. And then we have the third bucket, which quite frankly is the vast majority of viruses that infect humans. And HIV is in that category, where simply forming an antibody to the virus is not adequate to prevent infection. You have to do very sophisticated engineering to induce T cell effects, as well as innate effects and antibody effects. Even then, sometimes it’s very hard to decide what is the part of the virus to target. After decades, and billions of dollars of research, we’re still not there for HIV. There have been many approaches of how to do this science. Many different scientific delivery mechanisms, many different areas of the viruses targeted, many different parts of the immune system targeted, and so far, none of them have been effective at preventing HIV infection.
What needs to happen next?
We still need to slow down the number of people getting infected through good public health measures and good education to stop the HIV epidemic. We still need to get more people who are infected on therapy.
We know we can do it with public health measures. But we also need to find out more about how we eliminate that reservoir and get people cured of the virus in a simple and effective way so that we can cure more people. And the last major hurdle we have is to develop an effective vaccine. We still don’t have a vaccine that can prevent infection, a preventive vaccine, or a therapeutic vaccine where you give it to people who already have the virus that can help them control the infection. A huge amount of research has happened, but we’re still not there yet.
This article originally appeared on Mayo Clinic News Network.

Relevant reading
The Nurses of Mayo Clinic
Spanning more than a century of dedication and service, this hardcover book presents the spirit of nursing at Mayo Clinic. Set against the background of national and world events and mirroring the remarkable progress of medicine, also included are many letters and photographs that are made public for the first…

Discover more Innovation & Research content from articles, podcasts, to videos.
You May Also Enjoy

Privacy Policy
We've made some updates to our Privacy Policy. Please take a moment to review.
A trial HIV vaccine triggered elusive and essential antibodies in humans
Finding points the way toward a successful vaccine that elicits broadly neutralizing antibodies.
An HIV vaccine candidate developed at the Duke Human Vaccine Institute triggered low levels of an elusive type of broadly neutralizing HIV antibodies among a small group of people enrolled in a 2019 clinical trial.
The finding, reported May 17 in the journal Cell , not only provides proof that a vaccine can elicit these antibodies to fight diverse strains of HIV, but that it can also initiate the process within weeks, setting in motion an essential immune response.
The vaccine candidate targets an area on the HIV-1 outer envelope called the membrane proximal external region (MPER), which remains stable even as the virus mutates. Antibodies against this stable region in the HIV outer coat can block infection by many different circulating strains of HIV.
"This work is a major step forward as it shows the feasibility of inducing antibodies with immunizations that neutralize the most difficult strains of HIV," said senior author Barton F. Haynes, M.D., director of the Duke Human Vaccine Institute (DHVI). "Our next steps are to induce more potent neutralizing antibodies against other sites on HIV to prevent virus escape. We are not there yet, but the way forward is now much clearer."
The research team analyzed data from a phase 1 clinical trial of a vaccine candidate developed by Haynes and S. Munir Alam, Ph.D., at DHVI.
Twenty healthy, HIV-negative people enrolled in the trial. Fifteen participants received two of four planned doses of the investigational vaccine, and five received three doses.
After just two immunizations, the vaccine had a 95% serum response rate and a 100% blood CD4+ T-cell response rate -- two key measurements that demonstrated strong immune activation. Most of the serum responses mapped to the portion of the virus targeted by the vaccine.
Importantly, broadly neutralizing antibodies were induced after just two doses.
The trial was halted when one participant experienced a non-life-threatening allergic reaction, similar to rare incidents reported with COVID-19 vaccinations. The team investigated the cause of the event, which was likely from an additive.
"To get a broadly neutralizing antibody, a series of events needs to happen, and it typically takes several years post-infection," said lead author Wilton Williams, Ph.D., associate professor in Duke's Department of Surgery and member of DHVI. "The challenge has always been to recreate the necessary events in a shorter space of time using a vaccine. It was very exciting to see that, with this vaccine molecule, we could actually get neutralizing antibodies to emerge within weeks."
Other features of the vaccine were also promising, most notably how the crucial immune cells remained in a state of development that allowed them to continue acquiring mutations, so they could evolve along with the ever-changing virus.
The researchers said there is more work to be done to create a more robust response, and to target more regions of the virus envelope. A successful HIV vaccine will likely have at least three components, all aimed at distinct regions of the virus.
"Ultimately, we will need to hit all the sites on the envelope that are vulnerable so that the virus cannot escape," Haynes said. "But this study demonstrates that broadly neutralizing antibodies can indeed be induced in humans by vaccination. Now that we know that induction is possible, we can replicate what we have done here with immunogens that target the other vulnerable sites on the virus envelope."
In addition to Haynes and Williams, study authors include S. Munir Alam, Gilad Ofek, Nathaniel Erdmann, David Montefiori, Michael S. Seaman, Kshitij Wagh, Bette Korber, Robert J. Edwards, Katayoun Mansouri, Amanda Eaton, Derek W. Cain, Mitchell Martin, Robert Parks, Maggie Barr, Andrew Foulger, Kara Anasti, Parth Patel, Salam Sammour, Ruth J. Parsons, Xiao Huang, Jared Lindenberger, Susan Fetics, Katarzyna Janowska, Aurelie Niyongabo, Benjamin M. Janus, Anagh Astavans, Christopher B. Fox, Ipsita Mohanty, Tyler Evangelous, Yue Chen, Madison Berry, Helene Kirshner, Elizabeth Van Itallie, Kevin Saunders, Kevin Wiehe, Kristen W. Cohen, M. Juliana McElrath, Lawrence Corey, Priyamvada Acharya, Stephen R. Walsh, and Lindsey R. Baden.
- HIV and AIDS
- Infectious Diseases
- Bird Flu Research
- Microbes and More
- Antiretroviral drug
- Flu vaccine
- MMR vaccine
Story Source:
Materials provided by Duke University Medical Center . Note: Content may be edited for style and length.
Journal Reference :
- Wilton B. Williams, S. Munir Alam, Gilad Ofek, Nathaniel Erdmann, David C. Montefiori, Michael S. Seaman, Kshitij Wagh, Bette Korber, Robert J. Edwards, Katayoun Mansouri, Amanda Eaton, Derek W. Cain, Mitchell Martin, JongIn Hwang, Aria Arus-Altuz, Xiaozhi Lu, Fangping Cai, Nolan Jamieson, Robert Parks, Maggie Barr, Andrew Foulger, Kara Anasti, Parth Patel, Salam Sammour, Ruth J. Parsons, Xiao Huang, Jared Lindenberger, Susan Fetics, Katarzyna Janowska, Aurelie Niyongabo, Benjamin M. Janus, Anagh Astavans, Christopher B. Fox, Ipsita Mohanty, Tyler Evangelous, Yue Chen, Madison Berry, Helene Kirshner, Elizabeth Van Itallie, Kevin O. Saunders, Kevin Wiehe, Kristen W. Cohen, M. Juliana McElrath, Lawrence Corey, Priyamvada Acharya, Stephen R. Walsh, Lindsey R. Baden, Barton F. Haynes. Vaccine induction of heterologous HIV-1-neutralizing antibody B cell lineages in humans . Cell , 2024; DOI: 10.1016/j.cell.2024.04.033
Cite This Page :
Explore More
- High-Efficiency Photonic Integrated Circuit
- Life Expectancy May Increase by 5 Years by 2050
- Toward a Successful Vaccine for HIV
- Highly Efficient Thermoelectric Materials
- Toward Human Brain Gene Therapy
- Whale Families Learn Each Other's Vocal Style
- AI Can Answer Complex Physics Questions
- Otters Use Tools to Survive a Changing World
- Monogamy in Mice: Newly Evolved Type of Cell
- Sustainable Electronics, Doped With Air
Trending Topics
Strange & offbeat.
- What Are HIV and AIDS?
- How Is HIV Transmitted?
- Who Is at Risk for HIV?
- Symptoms of HIV
- U.S. Statistics
- Impact on Racial and Ethnic Minorities
- Global Statistics
- HIV and AIDS Timeline
- In Memoriam
- Supporting Someone Living with HIV
- Standing Up to Stigma
- Getting Involved
- HIV Treatment as Prevention
- Pre-exposure Prophylaxis (PrEP)
- Post-exposure Prophylaxis (PEP)
- Preventing Sexual Transmission of HIV
- Alcohol and HIV Risk
- Substance Use and HIV Risk
- Preventing Perinatal Transmission of HIV
- HIV Vaccines
- Long-acting HIV Prevention Tools
- Microbicides
- Who Should Get Tested?
- HIV Testing Locations
- HIV Testing Overview
- Understanding Your HIV Test Results
- Living with HIV
- Talking About Your HIV Status
- Locate an HIV Care Provider
- Types of Providers
- Take Charge of Your Care
- What to Expect at Your First HIV Care Visit
- Making Care Work for You
- Seeing Your Health Care Provider
- HIV Lab Tests and Results
- Returning to Care
- HIV Treatment Overview
- Viral Suppression and Undetectable Viral Load
- Taking Your HIV Medicine as Prescribed
- Tips on Taking Your HIV Medication Every Day
- Paying for HIV Care and Treatment
- Other Health Issues of Special Concern for People Living with HIV
- Alcohol and Drug Use
- Coronavirus (COVID-19) and People with HIV
- Hepatitis B & C
- Vaccines and People with HIV
- Flu and People with HIV
- Mental Health
- Mpox and People with HIV
- Opportunistic Infections
- Sexually Transmitted Infections
- Syphilis and People with HIV
- HIV and Women's Health Issues
- Aging with HIV
- Emergencies and Disasters and HIV
- Employment and Health
- Exercise and Physical Activity
- Food Safety and Nutrition
- Housing and Health
- Traveling Outside the U.S.
- Civil Rights
- Workplace Rights
- Limits on Confidentiality
- National HIV/AIDS Strategy (2022-2025)
- Implementing the National HIV/AIDS Strategy
- Prior National HIV/AIDS Strategies (2010-2021)
- Key Strategies
- Priority Jurisdictions
- HHS Agencies Involved
- Learn More About EHE
- Ready, Set, PrEP
- Ready, Set, PrEP Pharmacies
- Ready, Set, PrEP Resources
- AHEAD: America’s HIV Epidemic Analysis Dashboard
- HIV Prevention Activities
- HIV Testing Activities
- HIV Care and Treatment Activities
- HIV Research Activities
- Activities Combating HIV Stigma and Discrimination
- The Affordable Care Act and HIV/AIDS
- HIV Care Continuum
- Syringe Services Programs
- Finding Federal Funding for HIV Programs
- Fund Activities
- The Fund in Action
- About PACHA
- Members & Staff
- Subcommittees
- Prior PACHA Meetings and Recommendations
- I Am a Work of Art Campaign
- Awareness Campaigns
- Global HIV/AIDS Overview
- U.S. Government Global HIV/AIDS Activities
- U.S. Government Global-Domestic Bidirectional HIV Work
- Global HIV/AIDS Organizations
- National Black HIV/AIDS Awareness Day February 7
- HIV Is Not A Crime Awareness Day February 28
- National Women and Girls HIV/AIDS Awareness Day March 10
- National Native HIV/AIDS Awareness Day March 20
- National Youth HIV & AIDS Awareness Day April 10
- HIV Vaccine Awareness Day May 18
- National Asian & Pacific Islander HIV/AIDS Awareness Day May 19
- HIV Long-Term Survivors Awareness Day June 5
- National HIV Testing Day June 27
- Zero HIV Stigma July 21
- Southern HIV/AIDS Awareness Day August 20
- National Faith HIV/AIDS Awareness Day August 27
- National African Immigrants and Refugee HIV/AIDS and Hepatitis Awareness Day September 9
- National HIV/AIDS and Aging Awareness Day September 18
- National Gay Men's HIV/AIDS Awareness Day September 27
- National Latinx AIDS Awareness Day October 15
- World AIDS Day December 1
- Event Planning Guide
- U.S. Conference on HIV/AIDS (USCHA)
- National Ryan White Conference on HIV Care & Treatment
- AIDS 2020 (23rd International AIDS Conference Virtual)
Want to stay abreast of changes in prevention, care, treatment or research or other public health arenas that affect our collective response to the HIV epidemic? Or are you new to this field?
HIV.gov curates learning opportunities for you, and the people you serve and collaborate with.
Stay up to date with the webinars, Twitter chats, conferences and more in this section.
HIV Declines Among Young People and Drives Overall Decrease in New HIV Infections
- Share on Facebook
- Share on Twitter
- Share on LinkedIn
- Share on Email

Cross-posted from CDC NCHHSTP Newsroom
Increased investments, innovation, and focus on equity are vitally needed to end the HIV epidemic
Estimated annual new HIV infections were 12% lower in 2021 compared to 2017—dropping from about 36,500 infections to about 32,100—according to new CDC data published today. The decline was driven by a 34% decrease in new infections among 13- to 24-year-olds, mostly among gay and bisexual males. HIV prevention efforts must go further and progress must be faster, however, for gains to reach populations equitably and for national goals to end the HIV epidemic to be reached.
According to CDC’s latest estimates, annual HIV infections dropped from 9,300 in 2017 to 6,100 in 2021 among 13- to 24-year-olds. Declines among young gay and bisexual males (who account for roughly 80% of new infections in this age group) drove the trend, falling from an estimated 7,400 infections to about 4,900 during the timeframe.
“Our nation’s HIV prevention efforts continue to move in the right direction,” said CDC Director Rochelle P. Walensky, M.D., M.P.H. “Longstanding factors, such as systemic inequities, social and economic marginalization and residential segregation, however, stand between highly effective HIV treatment and prevention and people who could benefit from them. Efforts must be accelerated and strengthened for progress to reach all groups faster and equitably.”
Data suggest that improved reach of HIV testing, treatment, and pre-exposure prophylaxis (PrEP) has contributed to progress in HIV prevention among young gay and bisexual males.

“In prevention, patience is not a virtue,” said Jonathan Mermin, M.D., M.P.H., Director of CDC’s National Center for HIV, Viral Hepatitis, STD, and TB Prevention. “Decreasing HIV incidence among youth, including young gay and bisexual males, shows us what is possible. But ending the HIV epidemic and achieving equity requires we expand this progress to all.”
HIV PrEP and treatment outcomes improve, though longstanding factors limit gains among Black people and Hispanic/Latino people
The decline in annual HIV infections among young gay and bisexual males was not even across all racial and ethnic groups. Declines were lower among young Black/African American (subsequently, Black) and 13- to 24-year-old Hispanic/Latino gay and bisexual males than young White gay and bisexual males, suggesting that HIV prevention and treatment are not reaching everyone in this group equitably—and reflecting broader disparities that hinder HIV prevention.

Among key HIV prevention indicators, the greatest improvement was in the number of people taking PrEP to prevent HIV. In 2021, about 30% of the 1.2 million people who could benefit from PrEP were prescribed it—a notable improvement compared to about 13% prescribed PrEP in 2017. However, although most people who could benefit from PrEP are Black or Hispanic/Latino people, estimates suggest relatively few Black people or Hispanic/Latino people were prescribed PrEP in 2021.

There were also small increases in other key prevention measures, but not at the pace needed to reach national goals. More people with HIV were aware of their status in 2021 than 2017, with an uptick from 86% to 87%. Though data aren’t directly comparable due to reporting differences, the portion of people with diagnosed HIV who were virally suppressed due to effective treatment was slightly higher in 2021 than in 2017, up from 63% to 66%. Viral suppression was lower among Black people and Hispanic/Latino people than White people.

Deeply entrenched social determinants of health continue to drive these disparities and their outcomes. Most new HIV infections in 2021 were among gay and bisexual men, the majority of whom were Black or Hispanic/Latino. About one-fifth of new HIV infections in 2021 were among women, and over half of those were among Black women.

“At least three people in the U.S. get HIV every hour—at a time when we have more effective prevention and treatment options than ever before," said Robyn Neblett Fanfair, M.D., M.P.H., Acting Director of CDC’s Division of HIV Prevention. “These tools must reach deep into communities and be delivered faster to expand progress from some groups to all groups.”
To realize this goal and end the HIV epidemic, we must:
- Increase investments in proven HIV prevention programs through the Ending the HIV Epidemic in the U.S. (EHE) initiative.
- Maximize innovations by expanding HIV self-testing and growing the number and type of settings to reach people with HIV services, like STI clinics.
- Center equity in every aspect of our work so that HIV prevention interventions better reach people who are disproportionately affected by HIV.
Tools to end the HIV epidemic in the U.S. are available now, but our nation will not succeed until they equitably reach the people who need them to stay healthy.
Additional Data (CDC estimates)
- As of 2021, 1.2 million people in the United States have HIV, and that 87% of them had received a diagnosis. This means that 1 of 8 people with HIV in the United States do not know they have it.
- About two-thirds of new HIV infections in 2021 were among gay and bisexual men. By age, most (9,100) of those were among 25- to 34-year-olds; followed by 13- to 24-year-olds (4,900); and 35- to 44-year-olds (4,000).
- 16,700 in the South
- 6,600 in the West
- 4,400 in the Northeast
- 4,400 in the Midwest
- 51,878 of 468,540
- 64,018 of 312,820
- 234,318 of 300,650
- 15,779 of 131,180
- The United States made remarkable strides in eliminating perinatal HIV between 2010 and 2019. In 2021, the downward trend continued with 0.6 cases of perinatal HIV per 100,000 live births, down from the 2010 rate of 1.9 cases per 100,000 live births.
The U.S. Department of Health and Human Services coordinates the federal Ending the HIV Epidemic in the U.S. (EHE) initiative, which aims to reduce new HIV infections by 90% from 2017 levels by 2030. Early challenges, including resource allocation and the arrival of the COVID-19 pandemic in 2020 (the first year of EHE implementation nationwide) have hindered desired pace toward U.S. goals. The FY24 President’s Budget requested $850 million in funding across CDC, HRSA, IHS, and NIH to support continued EHE scale-up and implementation. This represents a $277 million (48%) increase over the FY23 enacted funding level. The President’s Budget also proposed $237 million for a national PrEP program. These are critical resources to help propel the nation’s HIV prevention efforts forward.
HIV Surveillance Supplemental Report: Estimated HIV Incidence in the United States, 2017-2021 https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-28-no-3/index.html
Monitoring Selected National HIV Prevention and Care Objectives by Using HIV Surveillance Data, U.S. and 6 Dependent Areas, 2021 https://www.cdc.gov/hiv/library/reports/hiv-surveillance/vol-28-no-4/index.html
U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES
CDC works 24/7 protecting America’s health, safety, and security. Whether disease start at home or abroad, are curable or preventable, chronic or acute, or from human activity or deliberate attack, CDC responds to America’s most pressing health threats. CDC is headquartered in Atlanta and has experts located throughout the United States and the world.
Related HIV.gov Blogs
- CDC Centers for Disease Control & Prevention
- Gay & Bisexual Men Gay & Bisexual Men
- Surveillance Public health surveillance
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- ADVERTISEMENT FEATURE Advertiser retains sole responsibility for the content of this article
HIV: Progress and future challenges in treatment, prevention and cure
- Diana Brainard ,
- Tomas Cihlar ,
- Romas Geleziunas &
- Devi SenGupta
- Diana Brainard
- Tomas Cihlar
- Romas Geleziunas
Produced by
Advances in care over the last 30 years have helped transform HIV from a fatal disease into a chronic, manageable condition for many people. 1 Innovations in treatment and access are at the core of this progress. The advent of antiretroviral therapy (ART), once-daily single tablet regimens (STRs) with three medications combined into one pill, and many subsequent improvements in STRs combining efficacy and long-term safety represent some of the most significant milestones that have helped bring radical change in the outlook for people living with HIV.
Progress in treatment has been transformative, but significant gaps in care still remain. HIV continues to be a major global public health issue. In the United States, the South accounts for seven of the top ten states and nine of the top ten metropolitan areas with the highest rates of new infections. This is pointed evidence that the epidemic is ongoing for some communities. 2 , 3 Globally, it’s estimated that more than 36 million people are living with HIV with acute challenges faced by Sub-Saharan Africa and many low- and middle-income countries. 4 With the goal of reducing the individual and societal burden of HIV, prevention of new infections is as important as treating those who are living with HIV.
At Gilead, we believe that anyone living with or at risk of HIV should benefit from the latest treatment innovations and have access to medicines. For almost 30 years, we have been at the forefront of the fight against HIV by leading advances in treatment, prevention and access. Our experience, dedication, and infrastructure are helping us move forward as we continue to focus on and make major investments in further expanding the options for HIV treatment and prevention. Equally as important, our close connections with the field, built over decades of collaborative research and development, enable us to help advance preclinical and clinical programs with the goal of achieving a functional cure for HIV.
Reducing Pill Burden with Single Tablet Regimens
In the early days of the HIV epidemic, high pill burden made successful adherence to treatment challenging. 5 Selective non-adherence to a regimen increases the likelihood of developing resistance, which could limit future treatment options. 6 STRs have revolutionized care by simplifying dosing and their impact has been profound and lasting. Today, clinical treatment guidelines from the US Department of Health and Human Services (DHHS) include certain STRs as recommended initial treatment regimen options for most people living with HIV. According to DHHS, factors that should guide the selection of a treatment regimen include virologic efficacy, toxicity, pill burden, dosing frequency, drug-drug interaction potential, resistance test results, comorbid conditions, access, and cost. 7
Despite the progress made in improving the efficacy and safety of HIV treatments, there are people with severely limited treatment options due to resistance to multiple ART classes. 6 They rely on complex treatment regimens, sometimes involving injectable medications in combination with multiple pills several times a day. 8 This type of complexity further increases the chance of treatment failure. Thus, we continue to strive to develop new agents that are active against resistant variants of HIV and target novel mechanisms of action, in order to provide simpler and more efficacious treatment options to all people living with HIV, irrespective of their prior treatment history. Our goal is to continue pursuing further ART innovations that enable STRs to reach beyond first and second line therapy options.
Researching Care with Long-Acting ART
Efforts to continue enhancing care will be critical to further curbing the HIV epidemic. Long-acting formulations with the potential to be dosed weekly, monthly, or even less frequently may help facilitate treatment adherence, a strong predictor of treatment success and health outcomes. 9 To become suitable for long-acting administration, new agents need to fit criteria different from those applied to daily oral ART. Novel regimens must match the high efficacy and safety observed with currently approved HIV treatments and do so with administration that is ideally minimally painful and can be performed outside a doctor’s office.
Long-acting antiretrovirals have already been identified among integrase and reverse transcriptase inhibitors, but additional drug targets are needed to help realize the full potential of long-acting ART for a range of patients. 10 Capsid, a multimeric shell present in infectious viral particles, is essential for virus replication as it plays crucial roles in both early and late stages of the viral life cycle. HIV capsid inhibitors represent a potential new class of antiretrovirals with high in vitro potency and very slow metabolic clearance leading to a prolonged half-life in in-vivo preclinical models. 11 Importantly, treatment failure due to circulating or archived pre-existing resistance is unlikely in the setting of novel therapeutic targets. Optimized combinations of multiple genetically engineered broadly neutralizing anti-HIV antibodies might also represent a new class of agents with long-acting potential. 12
Pioneering Prevention Options
Prevention methods and practices are essential tools in the fight against HIV. The innovation of pre-exposure prophylaxis (PrEP), an approach that involves daily antiretroviral medication in combination with safer sex practices to reduce the chance of acquiring HIV in individuals at risk for HIV, has been widely accepted by community advocates and is included in World Health Organization (WHO) and US Centers for Disease Control (CDC) clinical guidelines as part of a comprehensive prevention strategy. 13 , 14 Data are emerging to suggest the rate of new HIV infections can be dramatically reduced when PrEP and Treatment as Prevention (TasP) approaches are broadly adopted. 15
Since the introduction of PrEP as a prevention strategy, there have been ongoing initiatives across industry, government and community to help drive successful implementation, ranging from building awareness and improving persistence among communities disproportionately impacted by HIV to encouraging appropriate use through daily adherence. Despite an increase in PrEP uptake over the last five years among men who have sex with men, use among women has remained relatively low. 16 , 17 The reasons are likely multifactorial; however, providing additional PrEP treatment options, such as long-acting agents, could have an impact on engaging women at risk for HIV. 18 In this context, innovations in long-acting ART have the potential to be extended from treatment to prevention.
Continuing Innovation in Treatment and Prevention Access
Today’s access challenges will require continued evaluation and innovation. Diligent and responsive programs that regularly refine their approach to access scale-up will have the most success.
In the US, this requires industry leadership and close coordination with community partners as well as federal, state, and local policymakers. Early stage diagnosis and treatment initiation, connection to healthcare, and community support are key factors. Gilead’s FOCUS (Frontlines of Communities in the US) program was created in 2010 with the goal to develop and share screening, diagnosis and linkage to care best practices with hospitals, community health centers and community-based organizations in accordance with guidelines recommended by the CDC, US Preventative Services Task Force (USPSTF), and state and local public health departments. Through September 2018, FOCUS partners have conducted more than 5.1 million HIV tests from 261 participating organizations in 83 cities and counties.
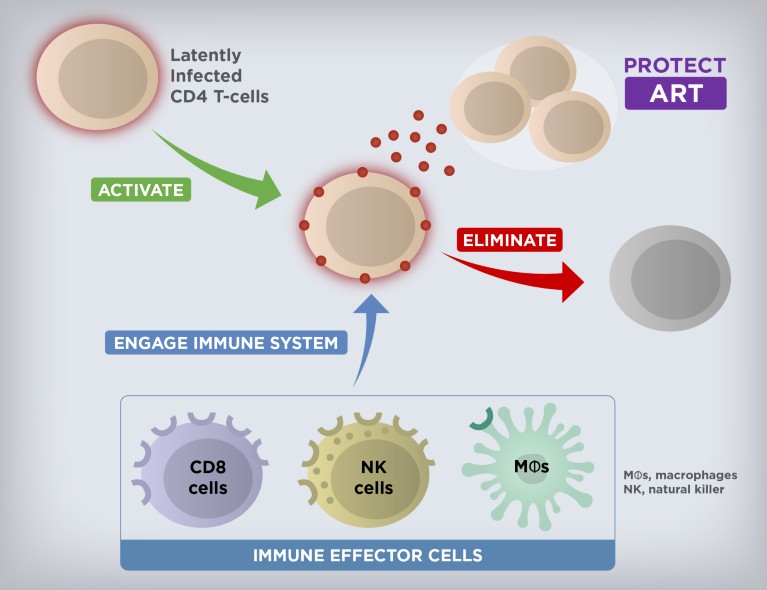
Cure Strategies to Eliminate HIV Reservoirs. Current research is exploring the potential of combination investigational therapies to induce expression of latent HIV and in parallel stimulate and engage host immune functions to specifically target and eliminate the infected cells from the activated reservoir.
Globally, the need is greatest in resource-limited countries with low HIV awareness and lack of education about treatment and prevention options. Since 2006, Gilead has entered into voluntary licensing agreements with generic manufacturers in low- and middle-income countries that grant them the rights to produce and sell high-quality, low-cost generic versions of Gilead medicines. Today, these agreements have expanded access in 116 countries and resulted in an 85 percent reduction in local prices due to manufacturer competition. We also partner with NGOs, governments, and academic institutions to raise awareness, address barriers to treatment access, reduce stigma and deliver frontline services. For example, in Africa, our work with the PEPFAR’s DREAMS initiative provides PrEP medications to young women who are most vulnerable to HIV.

Cure as the Ultimate Goal
While still elusive, cure remains the ultimate long-term goal for Gilead’s HIV research and development efforts. It is well established that the latent HIV reservoir is the main obstacle in achieving cure. 19 While the total size of the HIV reservoir varies among people living with HIV, the latently infected cells are rare (approximately 1 to 100 per million resting CD4+ T cells in the blood). 20 The reservoir is also anatomically heterogeneous and persistent, due to the residence of latent HIV primarily in long-lived memory CD4+ T cells. 21 The quiescent nature of these memory CD4+ T cells makes the reservoir essentially invisible to the immune system and not susceptible to ART. However, if ART is stopped, the virus quickly rebounds, leading to spreading HIV replication and expansion of the reservoir in the vast majority of individuals. 22
A multi-pronged approach will likely be needed to achieve the goal of curing HIV. The discovery and development of latency-reversing agents, immune modulators, and genetically engineered effector antibodies, as well as therapeutic vaccines with a potential for combination therapy to activate and eliminate the persistent reservoir are a current area of focus (Figure 1). Initial results from testing a combination of a TLR7 agonist with a broadly neutralizing antibody or a therapeutic vaccine in non-human primates infected with a chimeric simian-human immunodeficiency virus or simian immunodeficiency virus, respectively, have been a source of cautious optimism 20 , 22 and helped advance several investigational agents into early-stage clinical studies. As we progress further with testing investigational curative regimens, our partnerships and collaborations are more important than ever in this complex effort.
Extraordinary progress has been made in HIV since the epidemic emerged 37 years ago, but significant challenges still remain ahead of us. Gilead is strongly committed to driving the next generation of treatment, prevention, and cure strategy innovations that will continue changing the trajectory of the HIV epidemic by transforming care and improving overall outcomes for all people living with or at risk of HIV.
Barré-Sinoussi F, Ross AL, Delfraissy J. Nature Reviews Microbiology . 2013; 11 (12): 877-883.
Article PubMed Google Scholar
Centers for Disease Control (CDC). Atlas Plus. HIV Diagnoses 2016 https://gis.cdc.gov/grasp/nchhstpatlas/tables.html
CDC. 2016 HIV Surveillance Report, 2016; vol. 28. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2016-vol-28.pdf
Global Health Observatory (GHO) Data: HIV/AIDS (2018). http://www.who.int/gho/hiv/en/
Nachega JB, et al. Clinical Infectious Diseases . 2014; 58 (9): 1297–1307.
Bangsberg DR. The Journal of Infectious Diseases . 2008; 197 (3): S272-278.
Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Department of Health and Human Services. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf
Iacob SA & Iacob DG. Ibalizumab. Frontiers in Microbiology . 2017; 8 : 2323.
Barnhart M. Global Health: Science and Practice . 2017; 5 (2): 182–187.
Article Google Scholar
Landovitz RJ, Kofron R, McCauley M. Current Opinion in HIV and AIDS . 2016; 11 (1): 122–128
Carnes CK, Sheehan JH, Aiken C. Current Opinion in HIV and AIDS. 2018; 13 (4): 359–365.
Mendoza P, et al. Nature. 2018; 561 (7724): 479-484
World Health Organization. Policy brief on oral pre-exposure prophylaxis of HIV infection (PrEP). http://apps.who.int/iris/bitstream/handle/10665/197906/WHO_HIV_2015.48_eng.pdf;jsessionid=220ED2F6B4B466872D5082D71FA61E29?sequence=1
Preexposure Prophylaxis for the Prevention of HIV Infection in the United States - 2017 Update. A Clinical Practice Guideline. Department of Health and Human Services. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2017.pdf
San Francisco Department of Public Health: Population Health Division. HIV Epidemiology Annual Report 2017. https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/AnnualReport2017-Green-20180904-Web.pdf
Kamitani E, et al. AIDS . 2018.
PubMed Google Scholar
Aaron E, et al. AIDS Patient Care STDS. 2018; 32 (1): 16-23.
amfAR. Long-Acting HIV Treatment and Prevention Are Coming: Preparing for Potential Game Changers. https://www.amfar.org/uploadedFiles/_amfarorg/Articles/On_The_Hill/2018/overview.pdf.
Borducchi EN, et al. Nature . 2018; 1.
Google Scholar
Ho YC, et al. Cell. 2013; 155 (3), 540–551
Estes JD, et al. Nature Medicine . 2017; 23 (11): 1271–1276
Borducchi EN, et al. Nature . 2016; 540 : 284-287.
Download references
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- How It Spreads
- Living with HIV
- HIV Awareness Days
- HIV in the United States
- Resource Library
- Occupational Exposure
- Connect With Us
- HIV Nexus Home
- HIV Public Health Partners
- Ending the HIV Epidemic in the U.S. (EHE)
- Let's Stop HIV Together
Fast Facts: HIV in the United States
At a glance.
HIV remains a persistent problem in the United States. In the United States, estimated HIV infections decreased 12% overall from 2017 to 2021. Learn more about HIV trends in the United States.
HIV affects some groups more than others. Social and structural issues—such as HIV stigma, homophobia, discrimination, poverty, and limited access to high-quality health care—influence health outcomes and continue to drive inequities.
HIV incidence
HIV incidence refers to the estimated number of new HIV infections in a given year.
Estimated HIV infections in the US by transmission category, 2021*
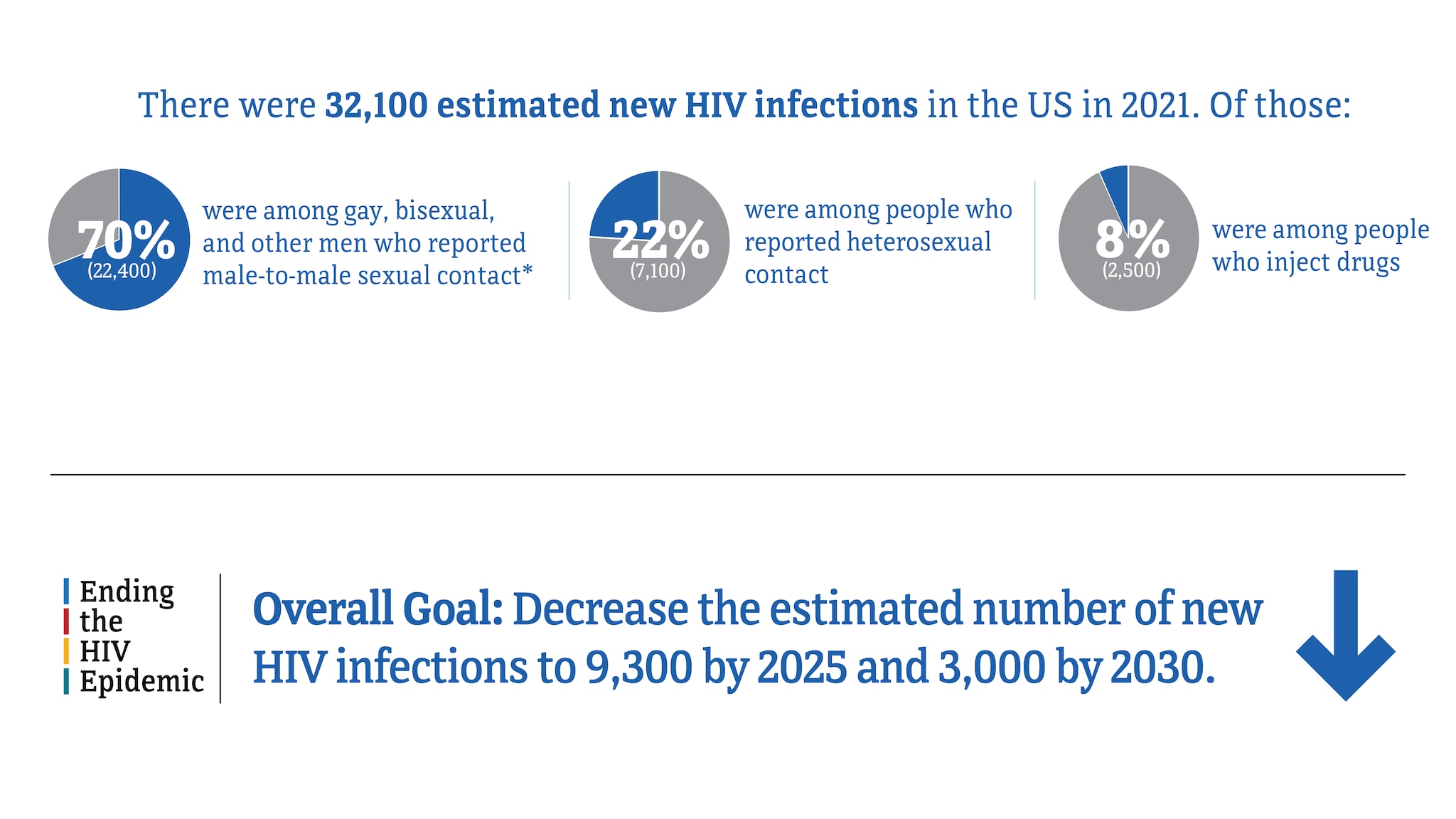
* Includes infections attributed to male-to-male sexual contact and injection drug use (men who reported both risk factors).
Source: CDC. Estimated HIV incidence and prevalence in the United States, 2017–2021. HIV Surveillance Supplemental Report , 2023; 28(3).
HIV infections among gay, bisexual, and other men who reported male-to-male sexual contact
In 2021, gay, bisexual, and other men who reported male-to-male sexual contact accounted for 70% (22,400) of the 32,100 estimated new HIV infections and 86% of estimated infections among all men.
Estimated HIV infections among gay and bisexual men in the US, 2017-2021*
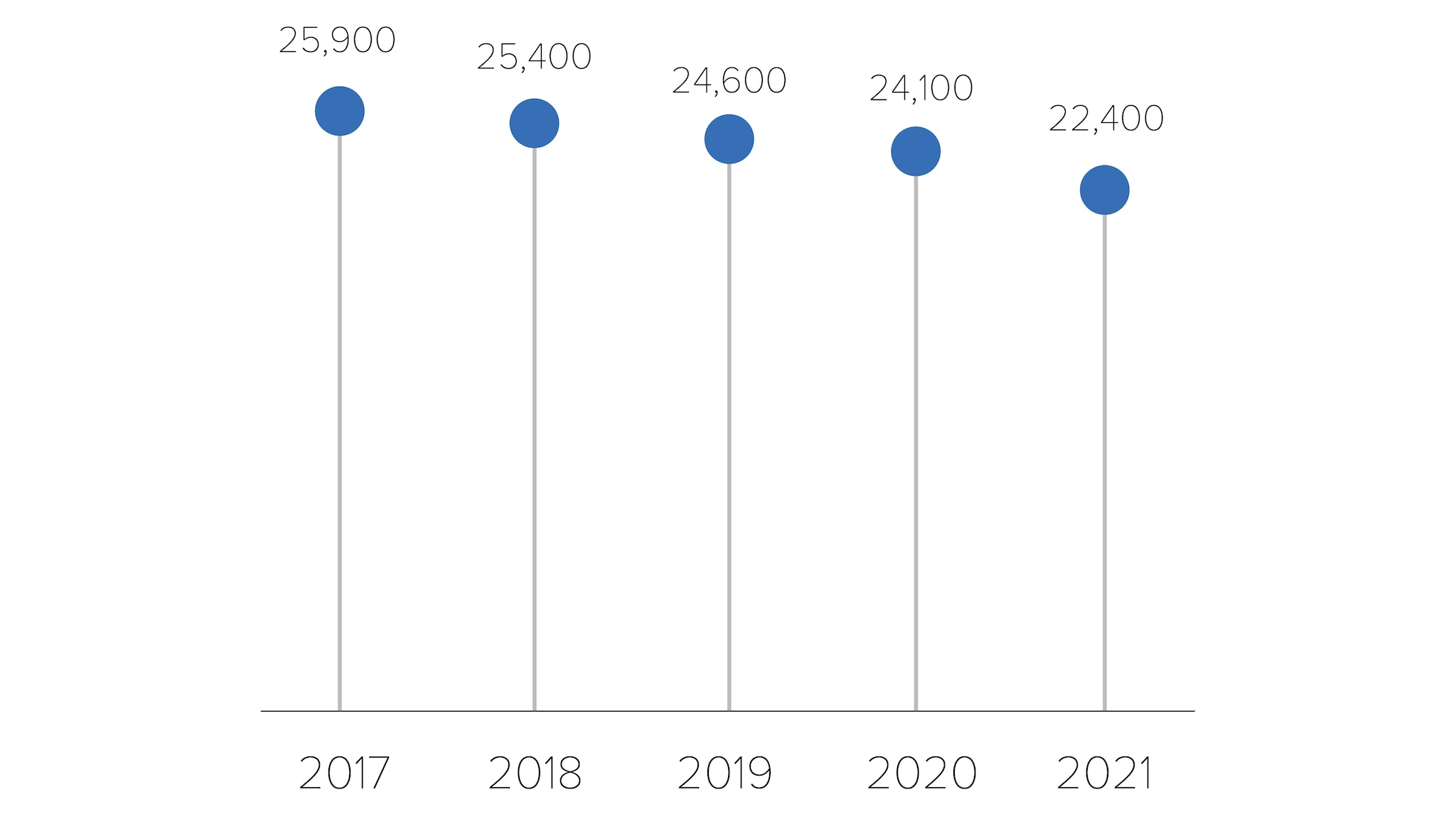
* Among people aged 13 and older.
Source: CDC. Estimated HIV incidence and prevalence in the United States, 2017–2021. HIV Surveillance Supplemental Report , 2023; 28(3).
HIV infections among people who reported heterosexual contact
In 2021, people reporting heterosexual contact accounted for 22% (7,100) of the 32,100 estimated new HIV infections.
- Men reporting heterosexual contact accounted for 6% (2,000) of estimated new HIV infections.
- Women reporting heterosexual contact accounted for 16% (5,100) of estimated new HIV infections.
Estimated HIV infections among people who reported heterosexual contact in the US, 2017-2021*
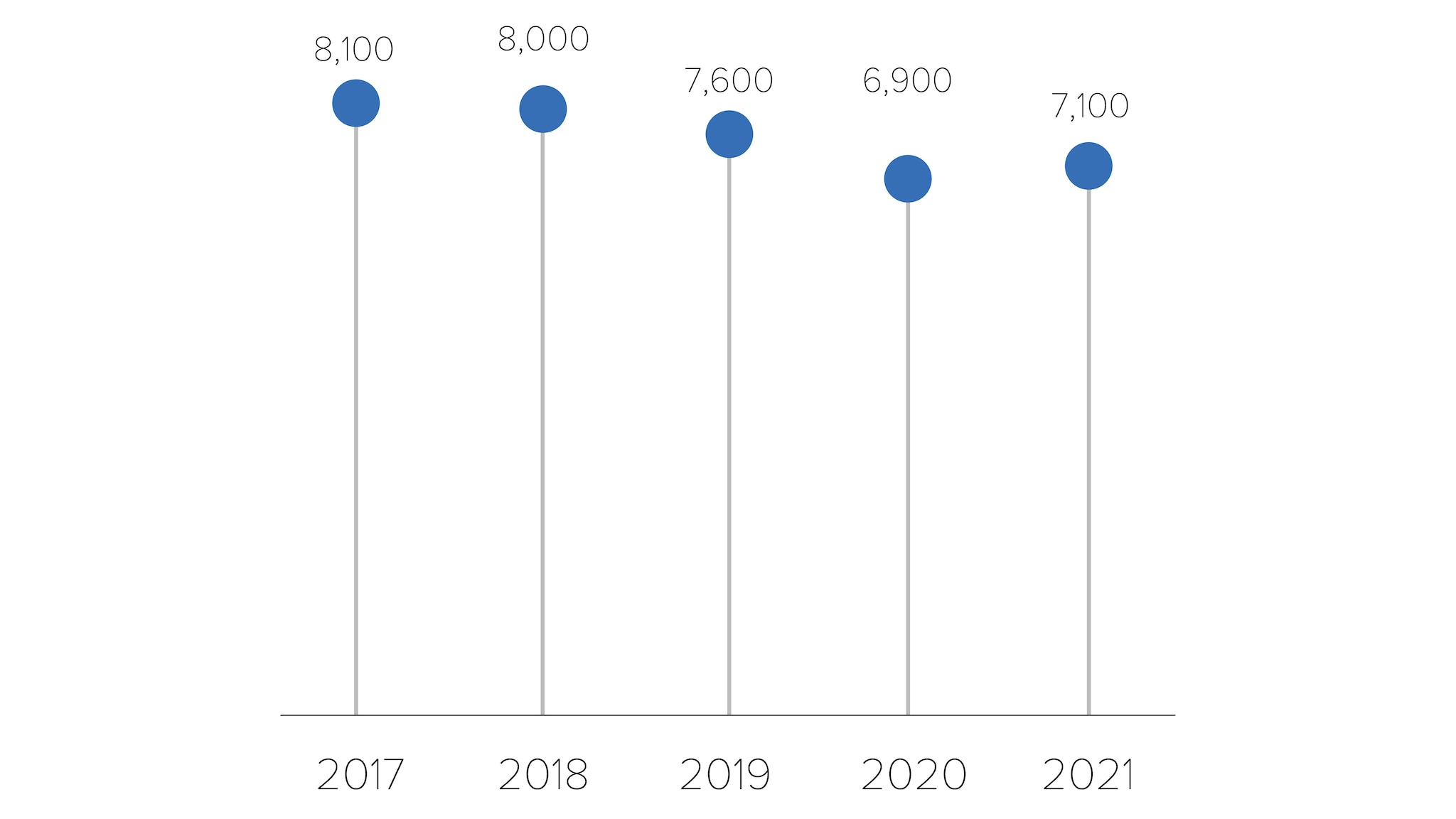
HIV infections among people who inject drugs (PWID)
In 2021, PWID accounted for 8% (2,500) of the 32,100 estimated new HIV infections.
- Men who inject drugs accounted for 4% (1,400) of estimated new HIV infections.
- Women who inject drugs accounted for 3% (1,100) of estimated new HIV infections.
Estimated HIV infections among people who inject drugs (PWID) in the US, 2017-2021*
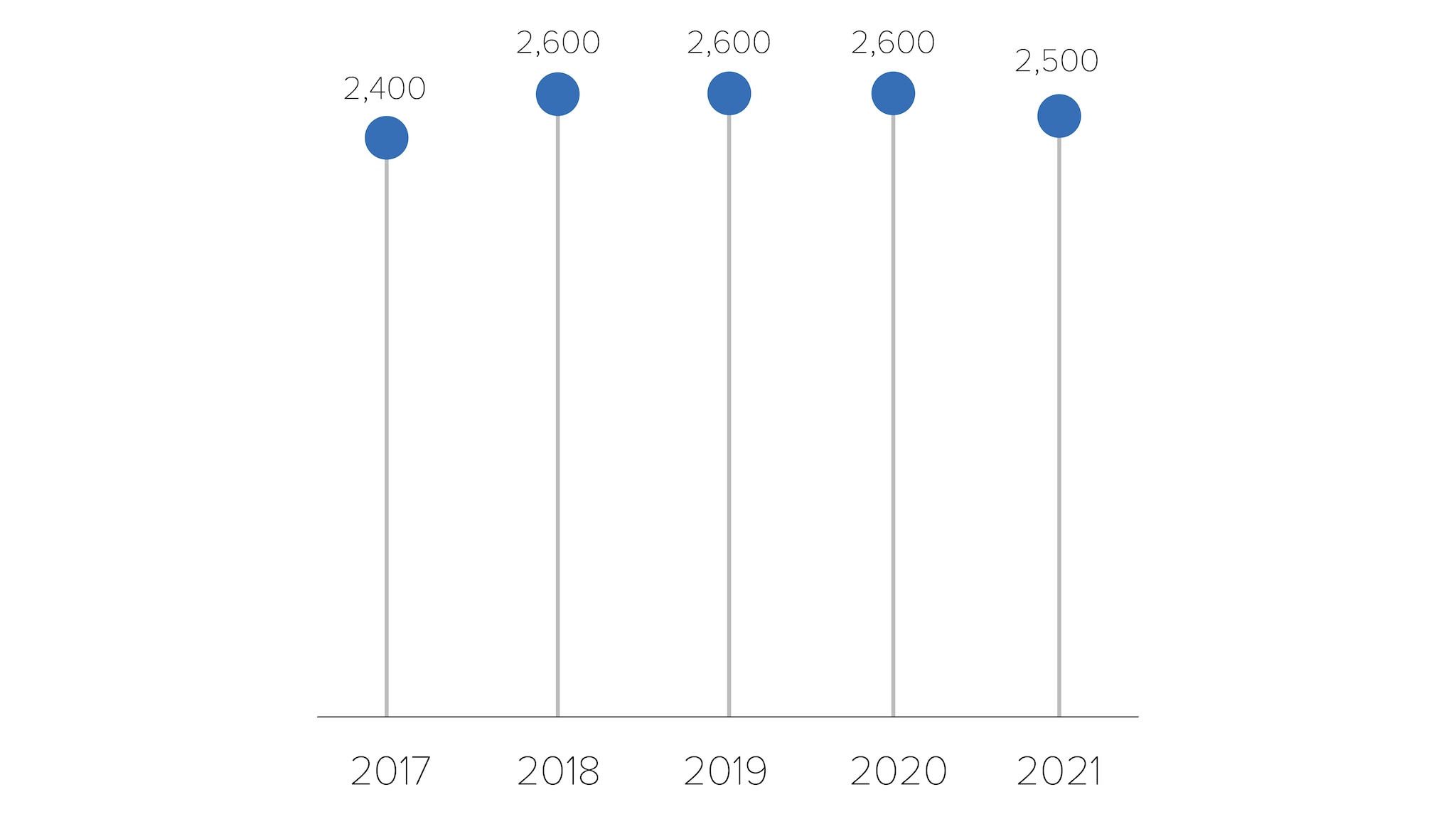
HIV infections by region
In 2021, the South accounted for more than half (52%) of the 32,100 estimated new HIV infections.
Estimated HIV infections in the US by region, 2021*
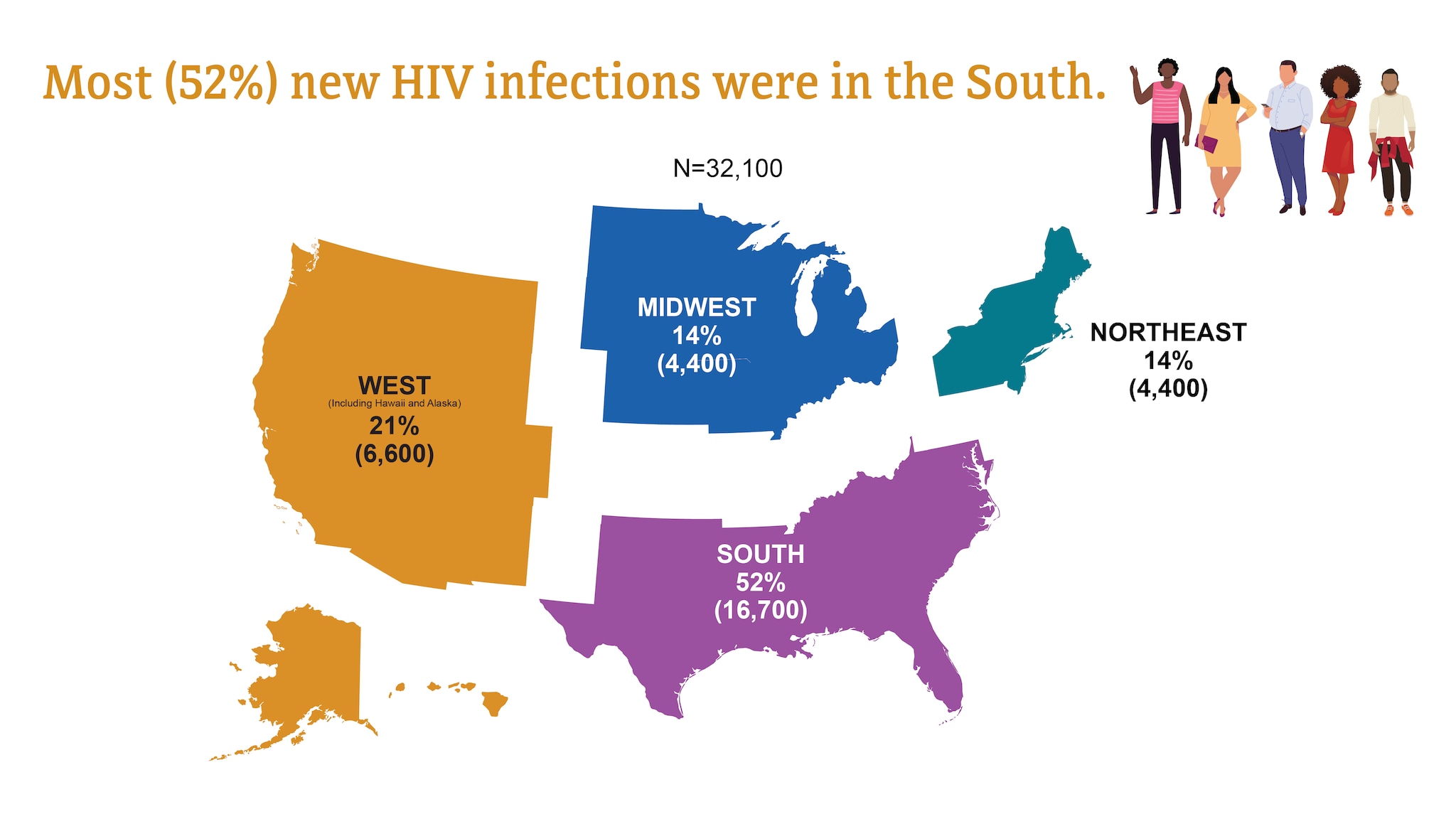
HIV diagnoses
HIV diagnoses refers to the number of people who received an HIV diagnosis during a given year.
HIV diagnoses in the US and dependent areas by transmission category, 2021*
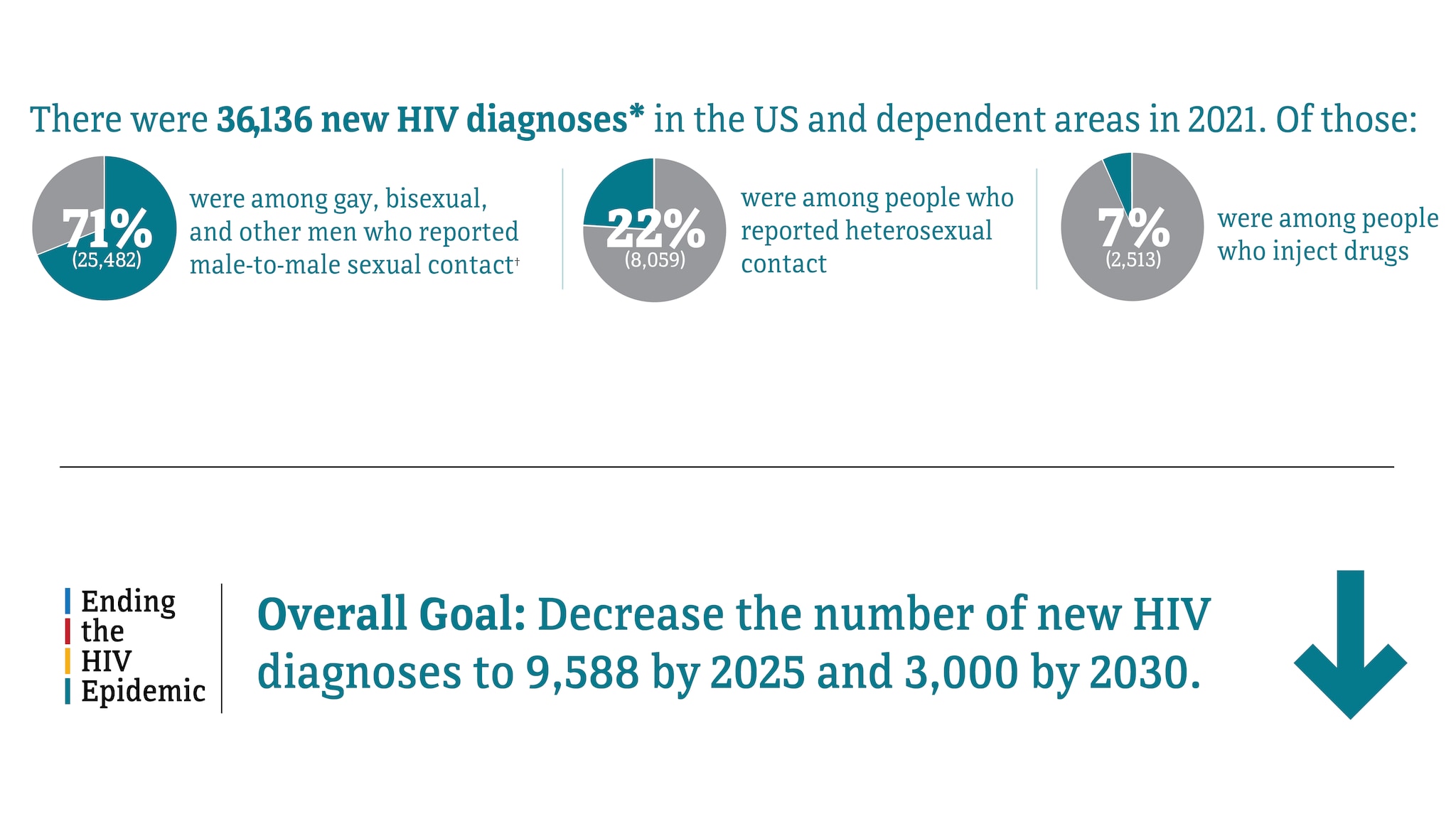
† Includes infections attributed to male-to-male sexual contact and injection drug use (men who reported both risk factors).
Source: CDC. Diagnoses of HIV infection in the United States and dependent areas, 2021. HIV Surveillance Report 2023;34.
HIV diagnoses in the US and Dependent areas for the most-affected subpopulations, 2021*†
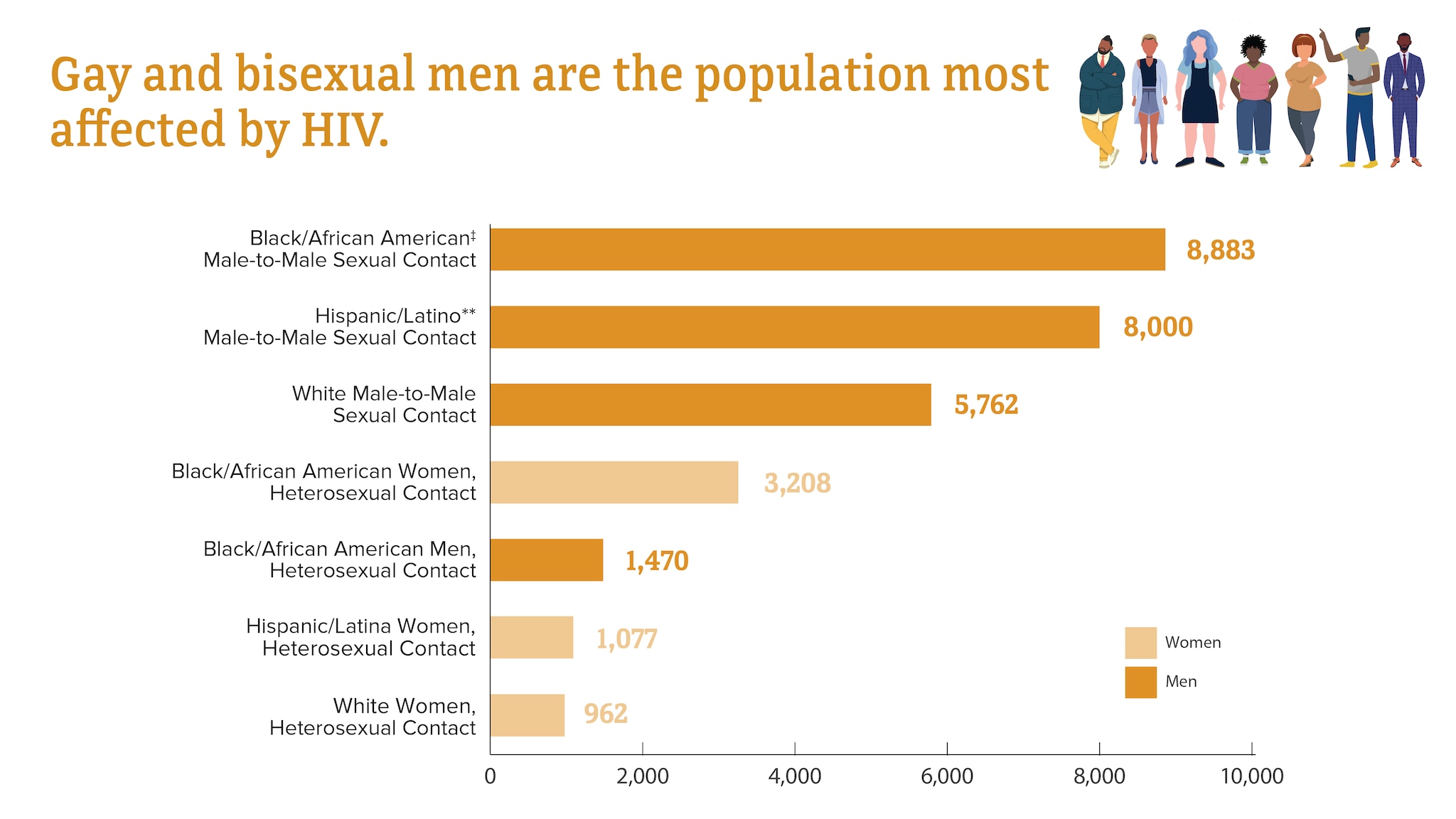
Subpopulations representing 2% or less of all people who received an HIV diagnosis in 2021 are not represented in this chart.
† Transmission category is classified based on a hierarchy of risk factors most likely responsible for HIV transmission. Classification is determined based on the person’s assigned sex at birth. Data have been statistically adjusted to account for missing transmission category.
‡ Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
** Hispanic/Latino people can be of any race.
Source: CDC. Diagnoses of HIV infection in the United States and dependent areas, 2021. HIV Surveillance Report 2023;34.
HIV diagnoses among transgender people
In 2021, transgender people accounted for 2% (868) of the 36,136 new HIV diagnoses.
- Transgender women accounted for 2% (812) of new HIV diagnoses.
- Transgender men accounted for less than 1% (56) of new HIV diagnoses.
HIV diagnoses among transgender people in the US and dependent areas by race and ethnicity, 2021*
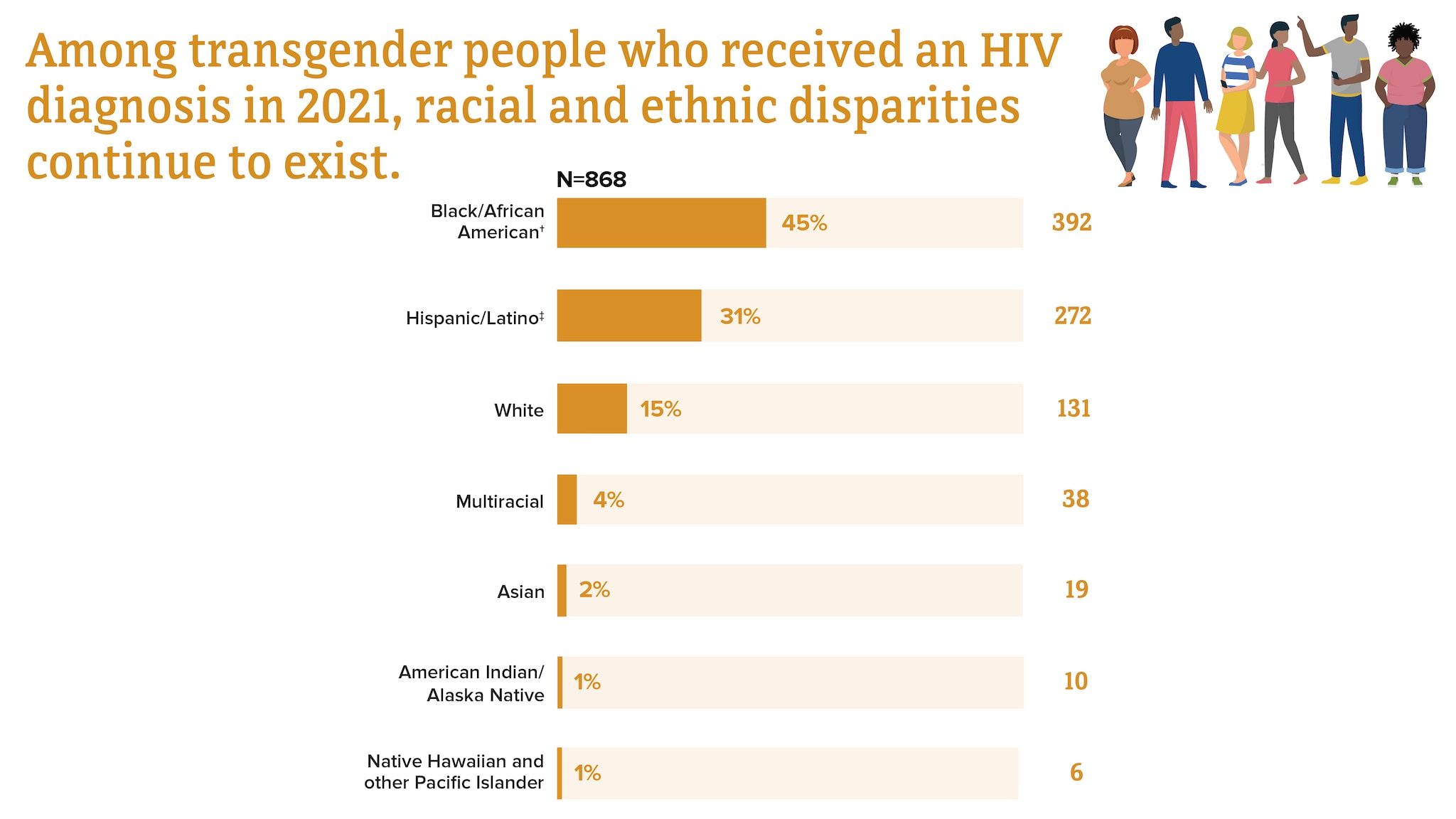
† Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
‡ Hispanic/Latino people can be of any race.
Trends in HIV diagnoses among transgender people in the US and dependent areas by race and ethnicity, 2017-2021*
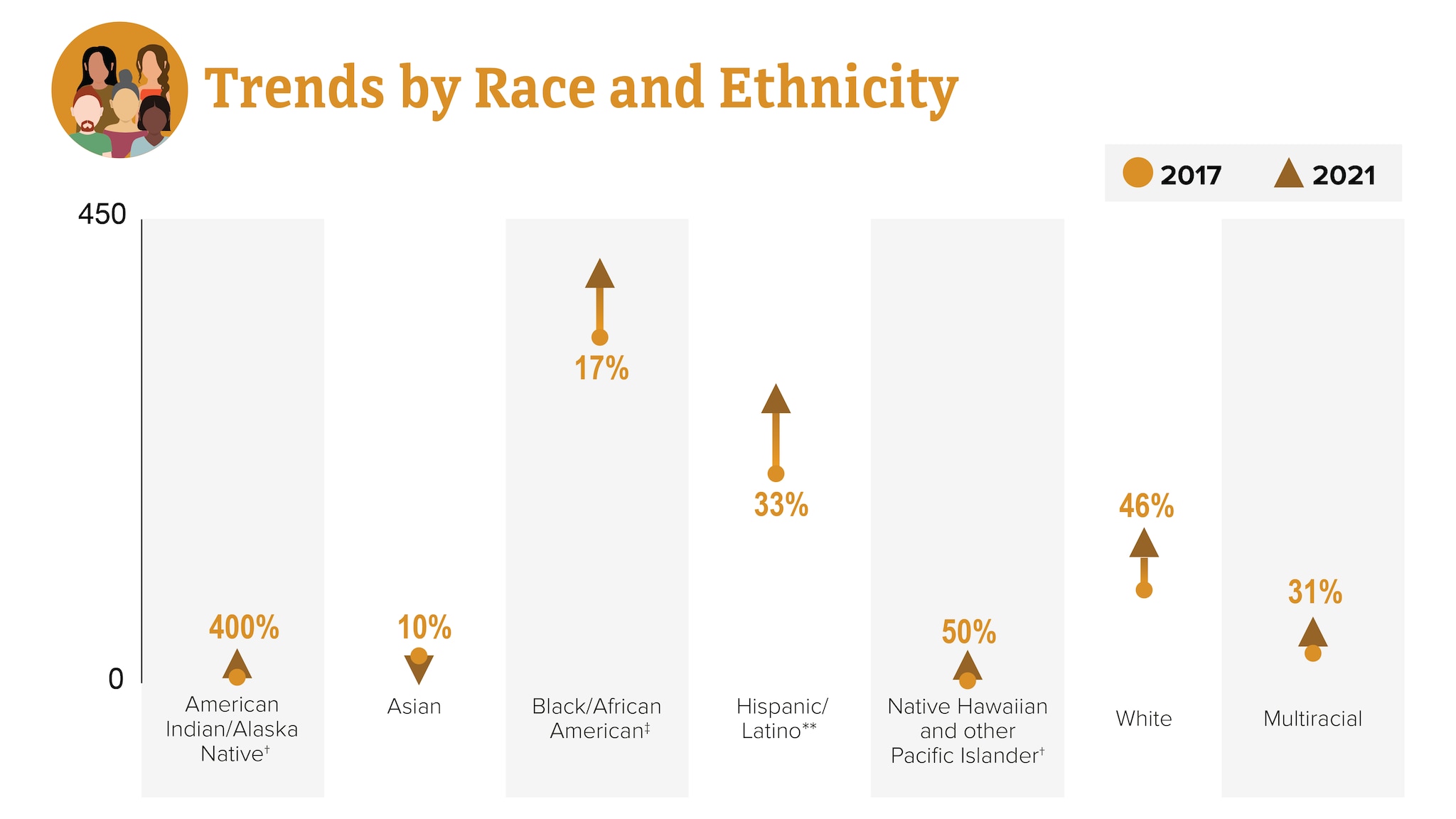
† Changes in subpopulations with fewer HIV diagnoses can lead to a large percentage increase or decrease.
‡ Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
** Hispanic/Latino people can be of any race.
HIV diagnoses among gay and bisexual men
Gay, bisexual, and other men who reported male-to-male sexual contact are the population most affected by HIV. In 2021, gay and bisexual men accounted for 71% (25,482) of the 36,136 new HIV diagnoses and 86% of diagnoses among all men.
HIV diagnoses among gay and bisexual men in the US and dependent areas by race and ethnicity, 2021*†
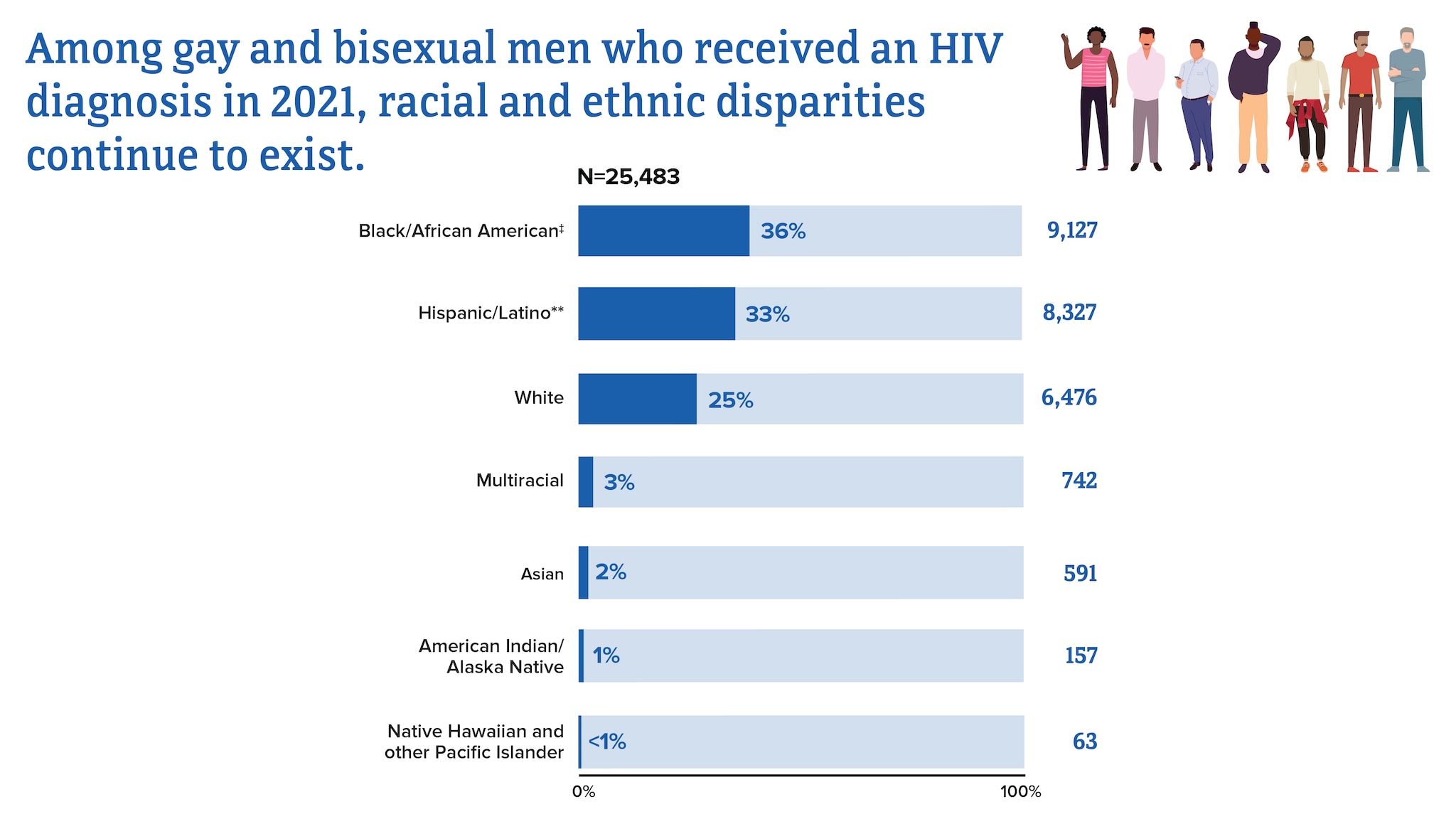
* Among people aged 13 and older
†Includes infections attributed to male-to-male sexual contact and injection drug use (men who reported both risk factors).
‡ Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
From 2017 to 2021, HIV diagnoses decreased 6% among gay and bisexual men overall. But trends varied for different groups of gay and bisexual men.
Trends in HIV diagnoses among gay and bisexual men in the US and dependent areas by race and ethnicity, 2017-2021*
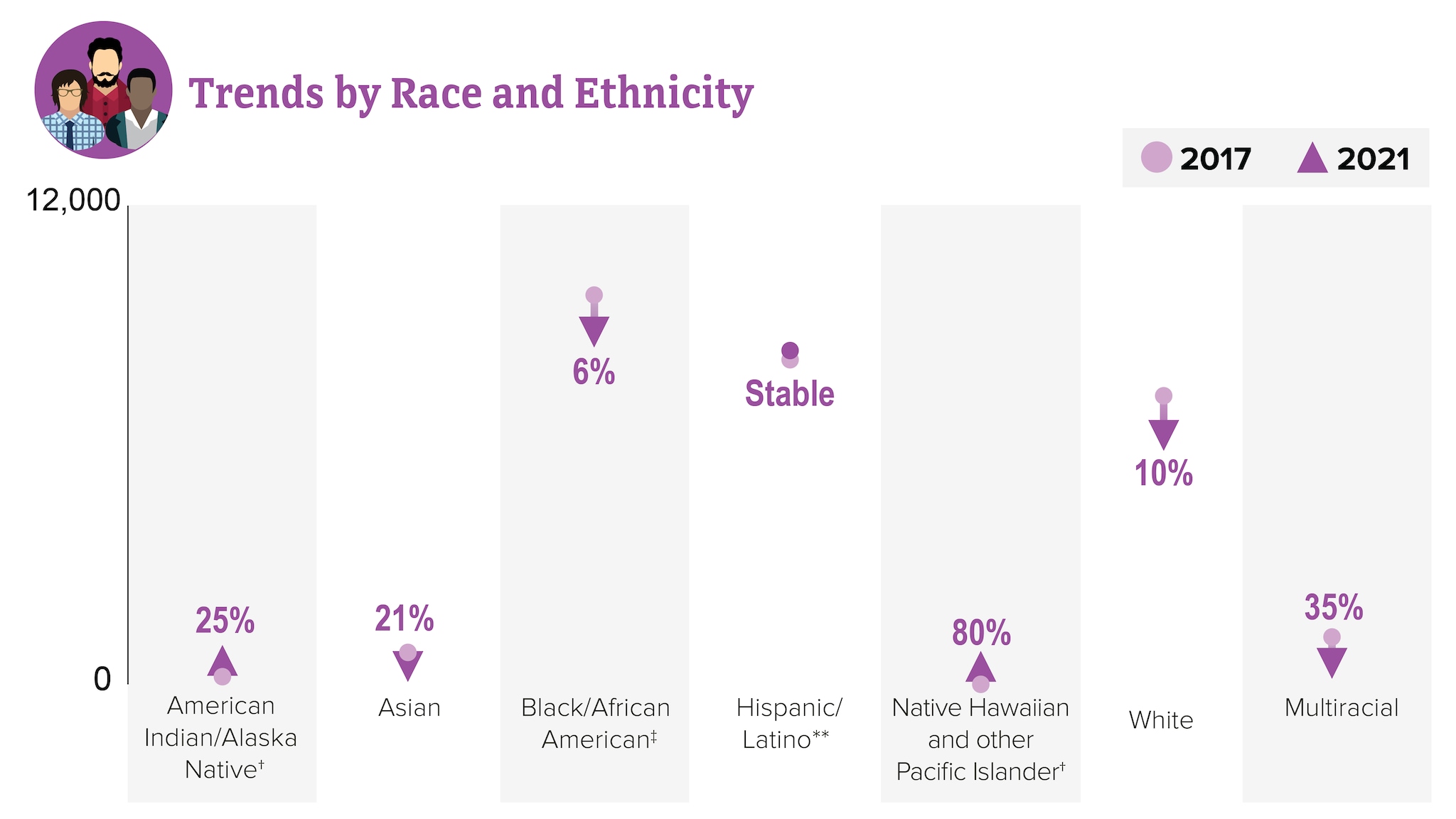
HIV diagnoses among people who reported heterosexual contact
Men and women who reported heterosexual contact continue to be affected by HIV. In 2021, people reporting heterosexual contact accounted for 22% (8,059) of the 36,136 new HIV diagnoses.
- Men reporting heterosexual contact accounted for 7% (2,523) of new HIV diagnoses.
- Women reporting heterosexual contact accounted for 15% (5,536) of new HIV diagnoses.
HIV diagnoses among people who reported heterosexual contact in the US and dependent areas by race and ethnicity, 2021*
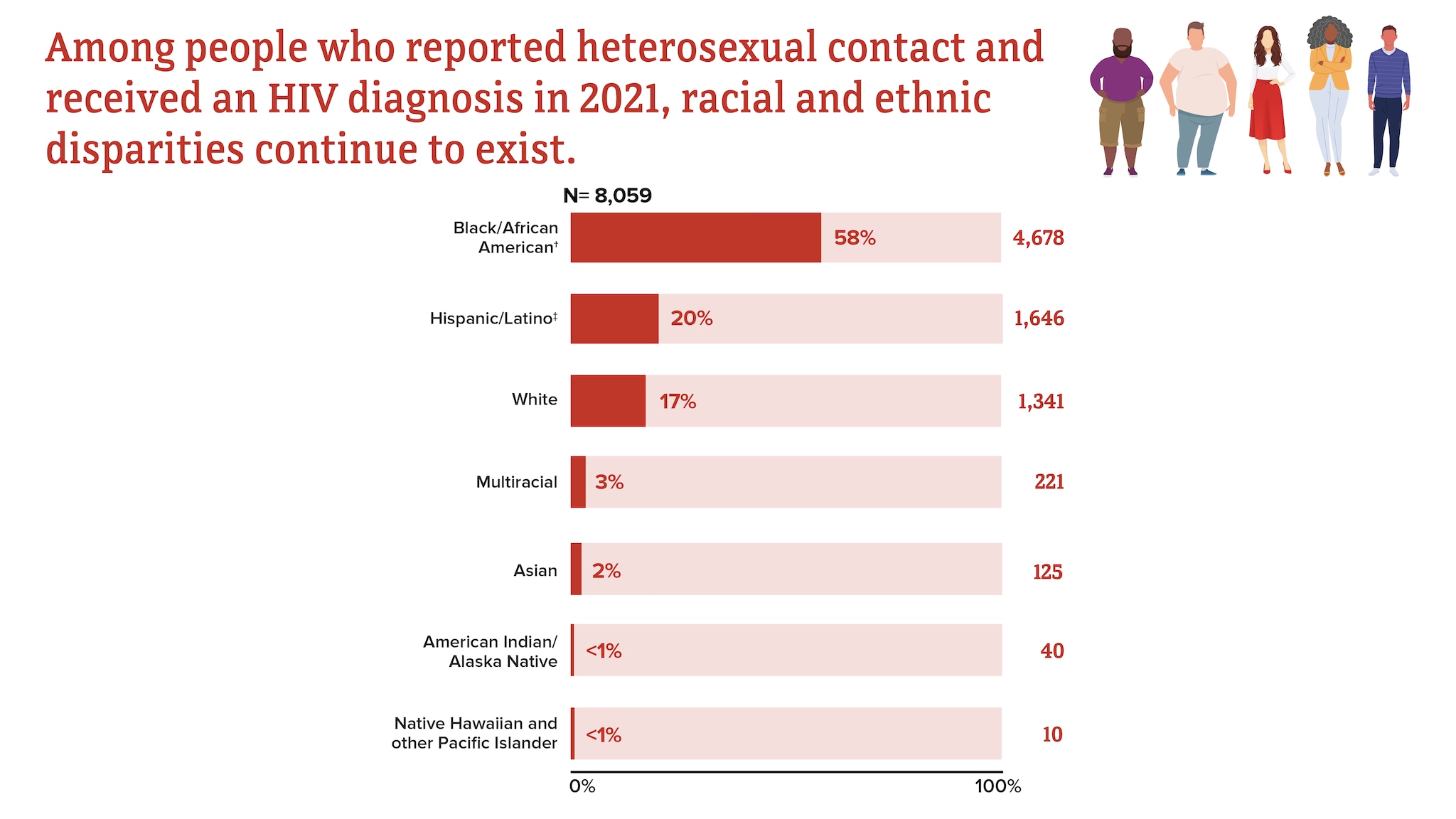
† Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
‡ Hispanic/Latino people can be of any race.
From 2017 to 2021, HIV diagnoses from heterosexual contact decreased 12% overall.
Trends in HIV diagnoses among people who reported heterosexual contact in the US and dependent areas, 2017-2021*†
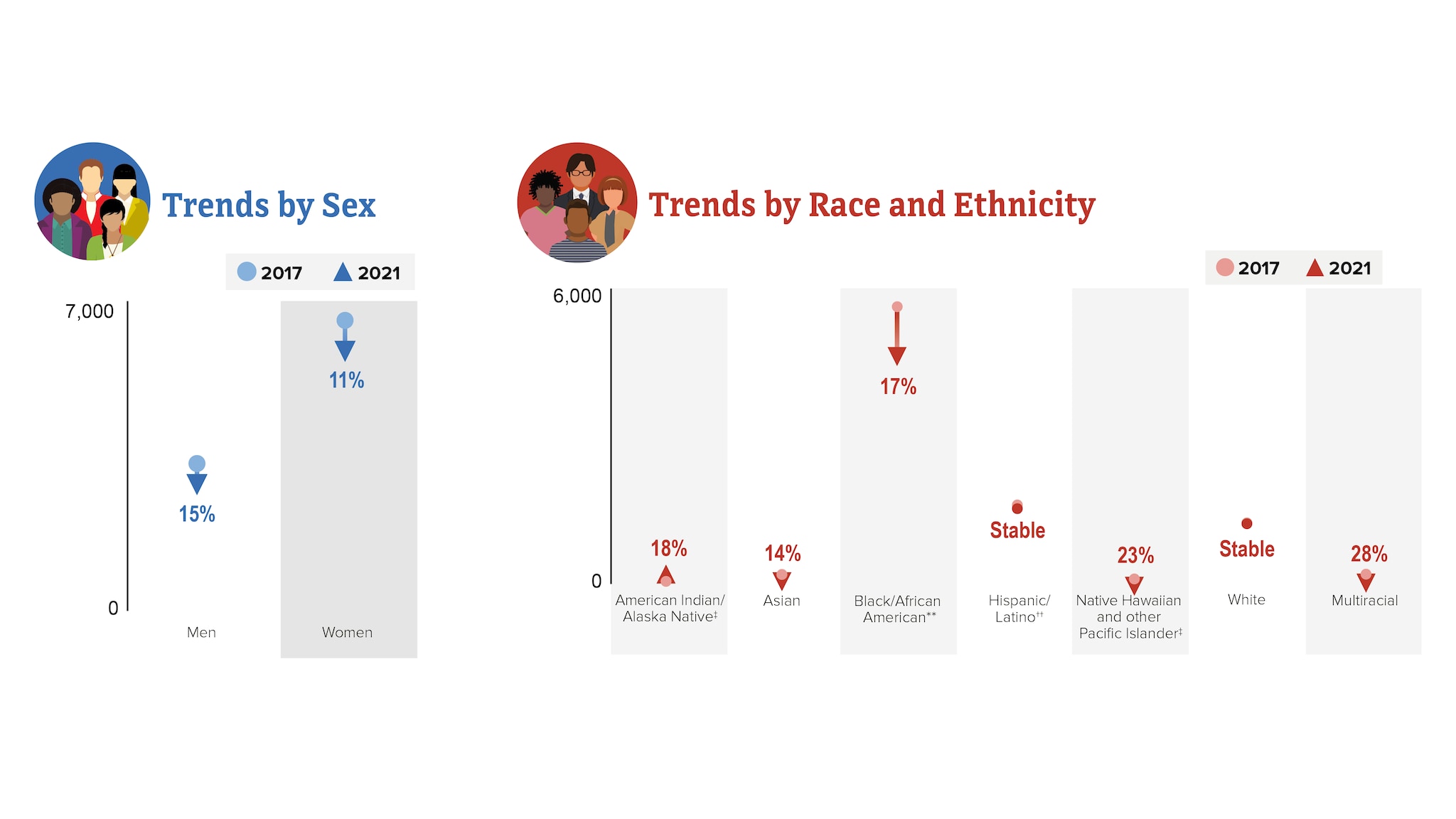
† Based on assigned sex at birth and includes transgender people.
‡ Changes in subpopulations with fewer HIV diagnoses can lead to a large percentage increase or decrease.
** Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
†† Hispanic/Latino people can be of any race.
HIV diagnoses among people who inject drugs (PWID)
In 2021, PWID accounted for 7% (2,512) of the 36,136 new HIV diagnoses.
- Men who inject drugs accounted for 4% (1,436) of new HIV diagnoses.
- Women who inject drugs accounted for 3% (1,076) of new HIV diagnoses.
HIV diagnoses among people who inject drugs in the US and dependent areas by race and ethnicity, 2021*
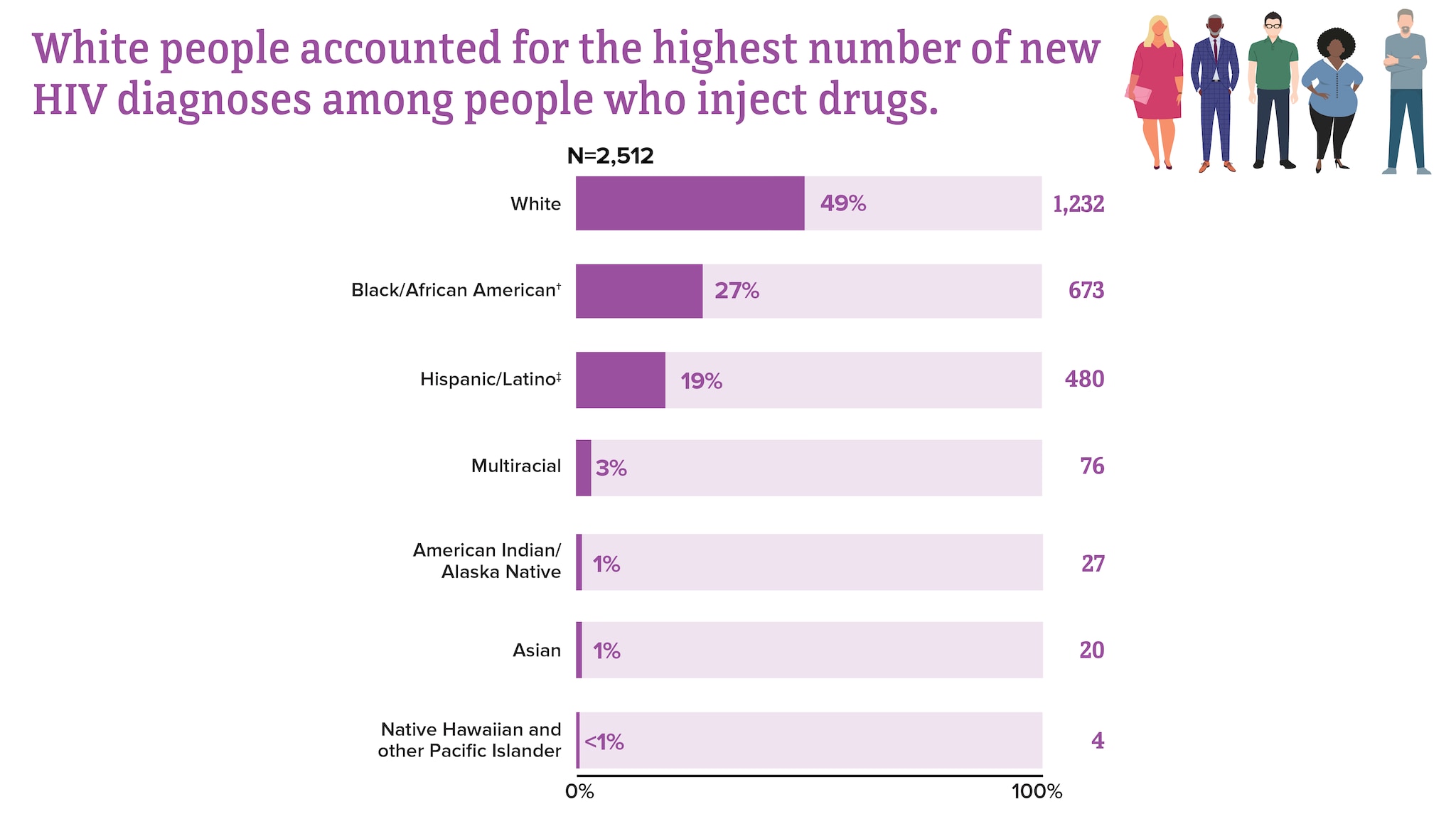
† Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
From 2017 to 2021, HIV diagnoses remained stable among PWID overall.
Trends in HIV diagnoses among people who inject drugs in the US and dependent areas, 2017-2021*†‡
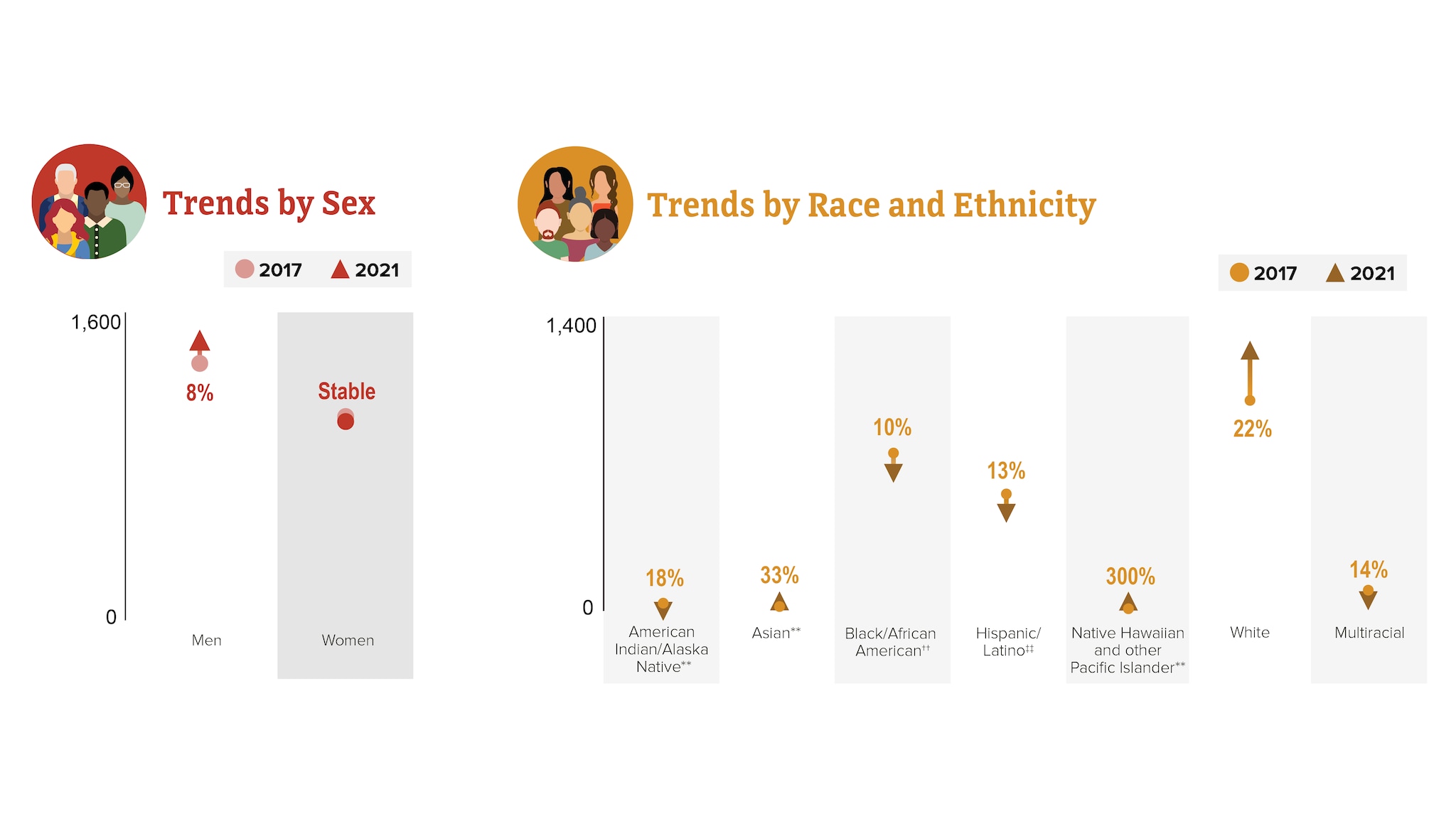
† Includes infections attributed to male-to-male sexual contact and injection drug use (men who reported both risk factors).
‡ Based on assigned sex at birth and includes transgender people.
** Changes in subpopulations with fewer HIV diagnoses can lead to a large percentage increase or decrease.
†† Black refers to people having origins in any of the Black racial groups of Africa. African American is a term often used for people of African descent with ancestry in North America.
‡‡ Hispanic/Latino people can be of any race.
HIV diagnoses by region
HIV diagnoses are not evenly distributed regionally in the US and dependent areas.
Rates of HIV diagnoses in the US and dependent areas by region, 2021*†
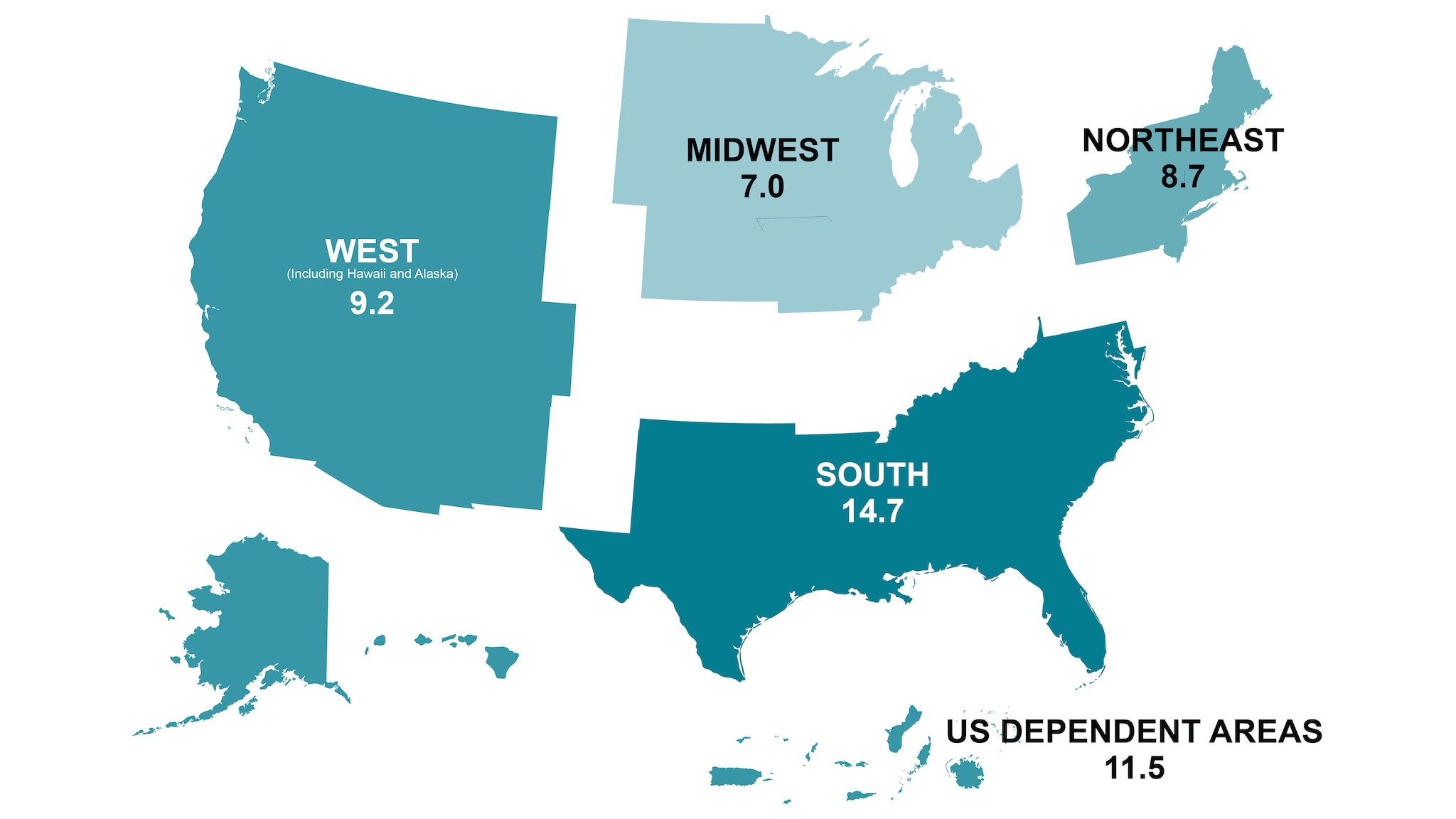
*Rates are per 100,000 people.
† Among adults, adolescents, and children under the age of 13.
Knowledge of status
Knowledge of status refers to the estimated percentage of people with HIV who have received an HIV diagnosis.
Knowledge of HIV status in the US, 2021*
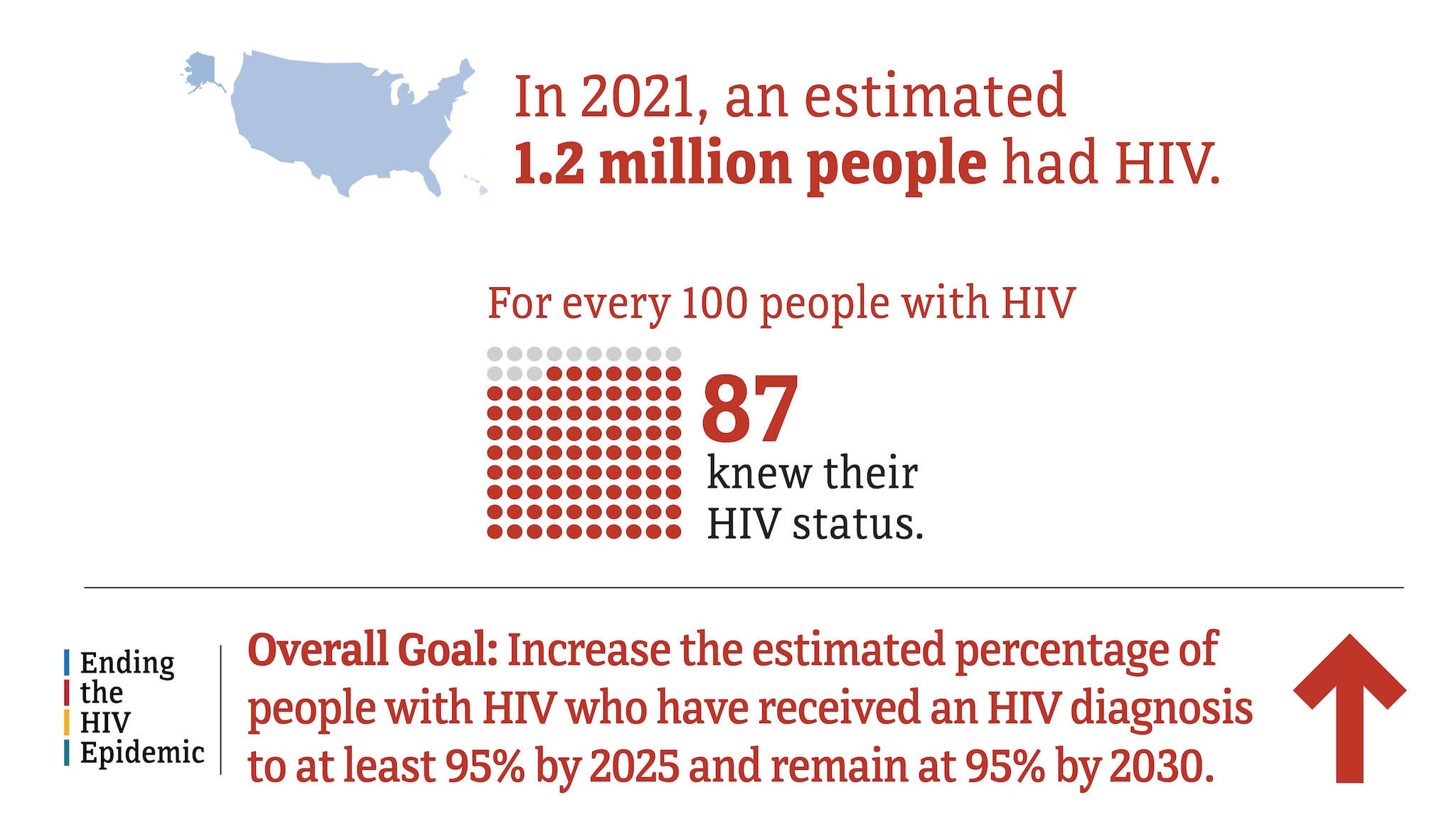
Knowledge of HIV status in the US by transmission category, 2021*
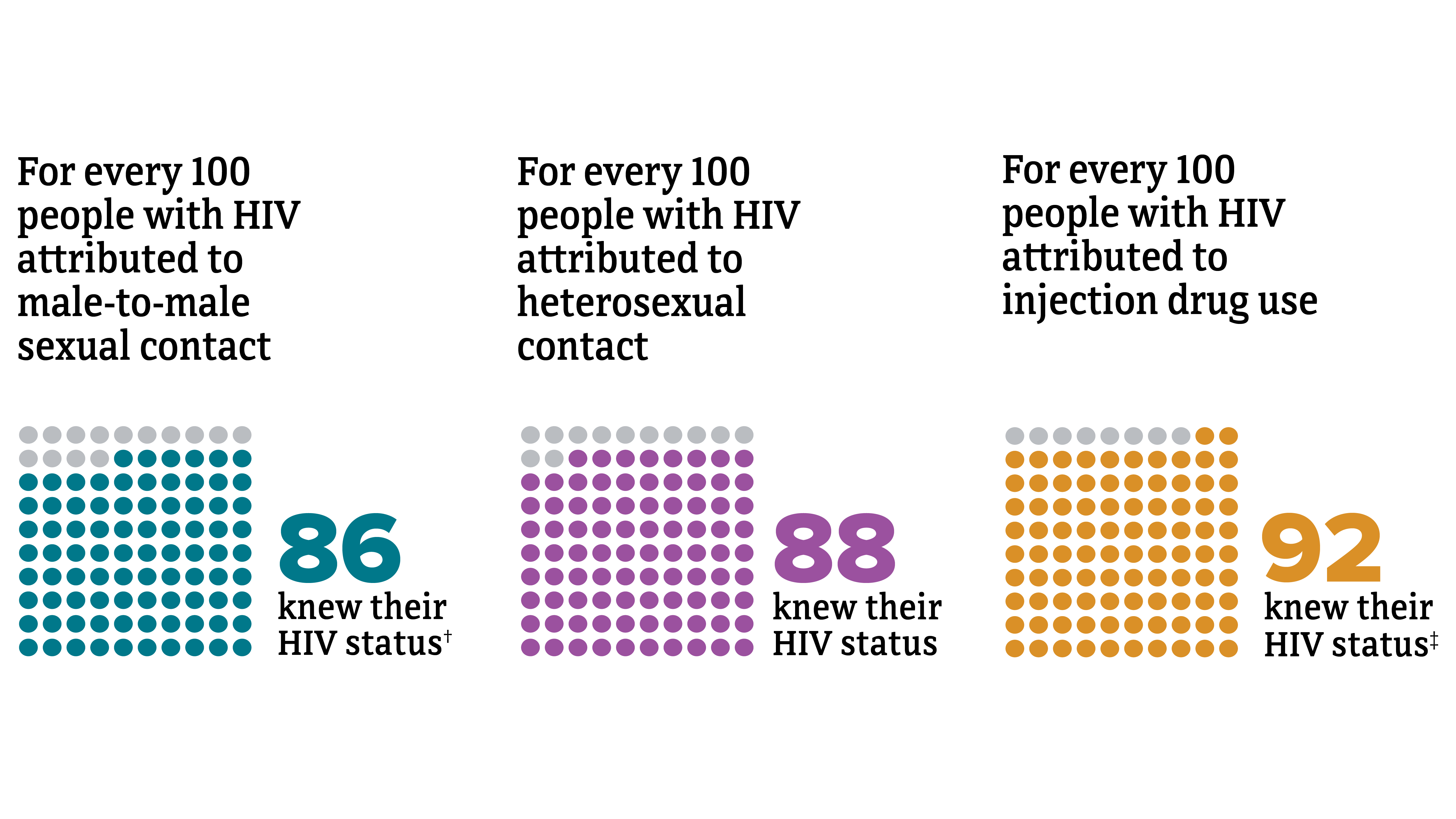
† Includes infections attributed to male-to-male sexual contact only .
‡ Includes infections attributed to injection drug use only. Among men with HIV attributed to male-to-male sexual contact and injection drug use, 92% knew they had HIV.
Knowledge of HIV status in the US by region, 2021*
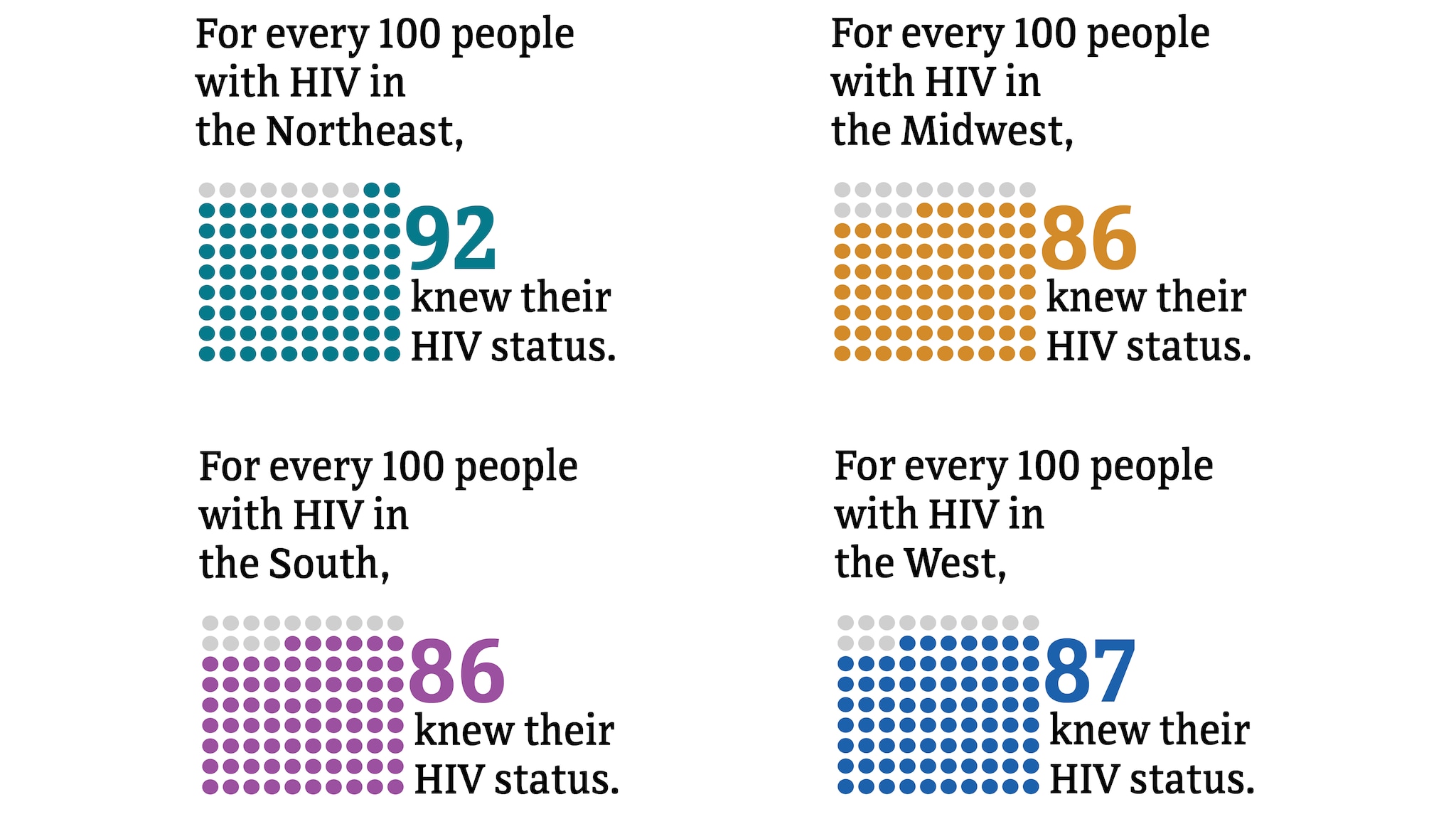
PrEP coverage
PrEP (pre-exposure prophylaxis) coverage refers to the estimated percentage of people with indications for PrEP classified as having been prescribed PrEP.
PrEP coverage in the US and Puerto Rico, 2021

Source: CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2021 . HIV Surveillance Supplemental Report 2023;28(4).
PrEP coverage in the US and Puerto Rico by area of residence, 2021*
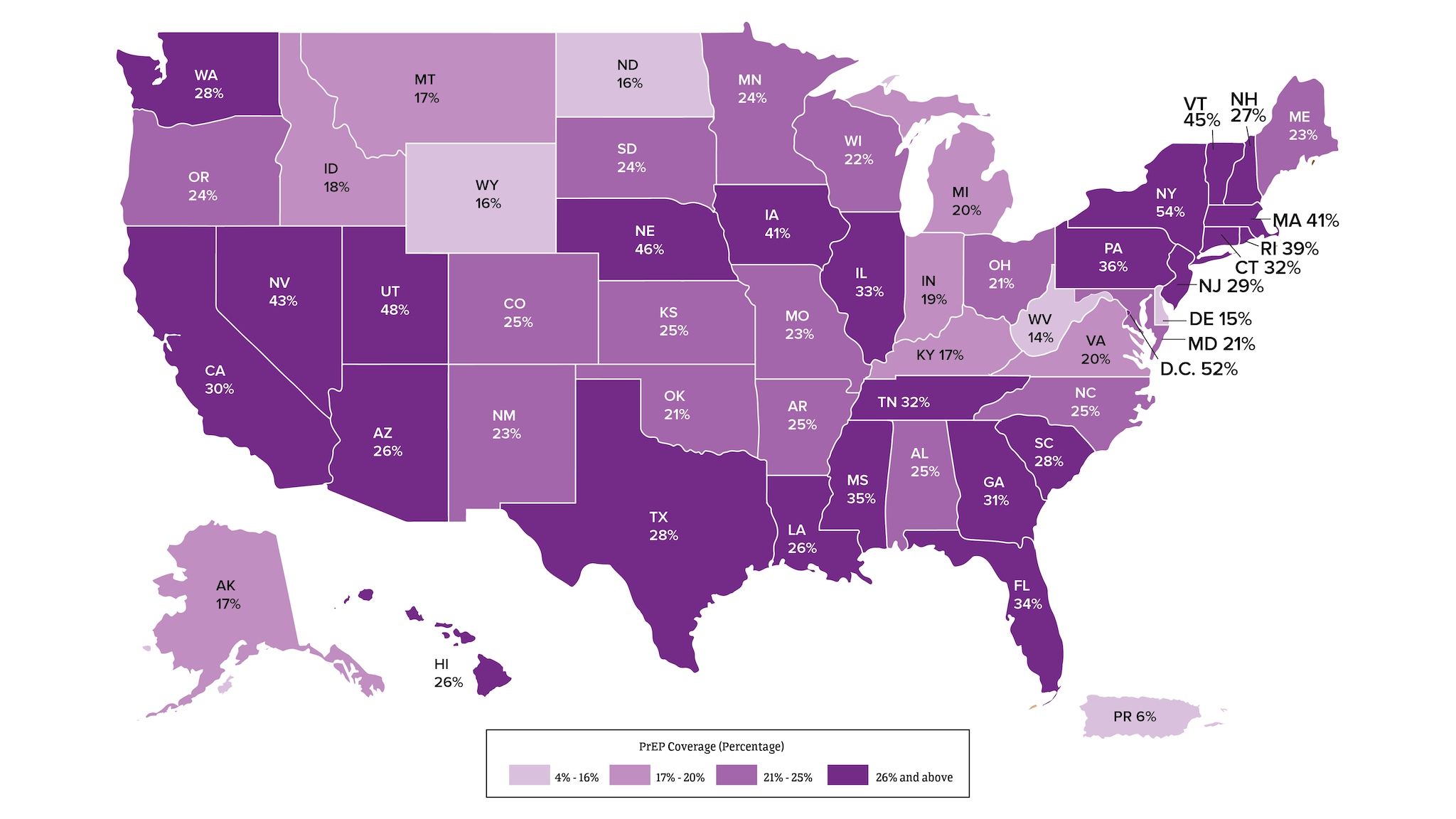
*Among people aged 16 and older.
Viral suppression and barriers to care
Viral suppression refers to the percentage of people with diagnosed HIV who have less than 200 copies of HIV per milliliter of blood.
HIV care continuum among people with diagnosed HIV in 47 states and the District of Columbia, 2021*
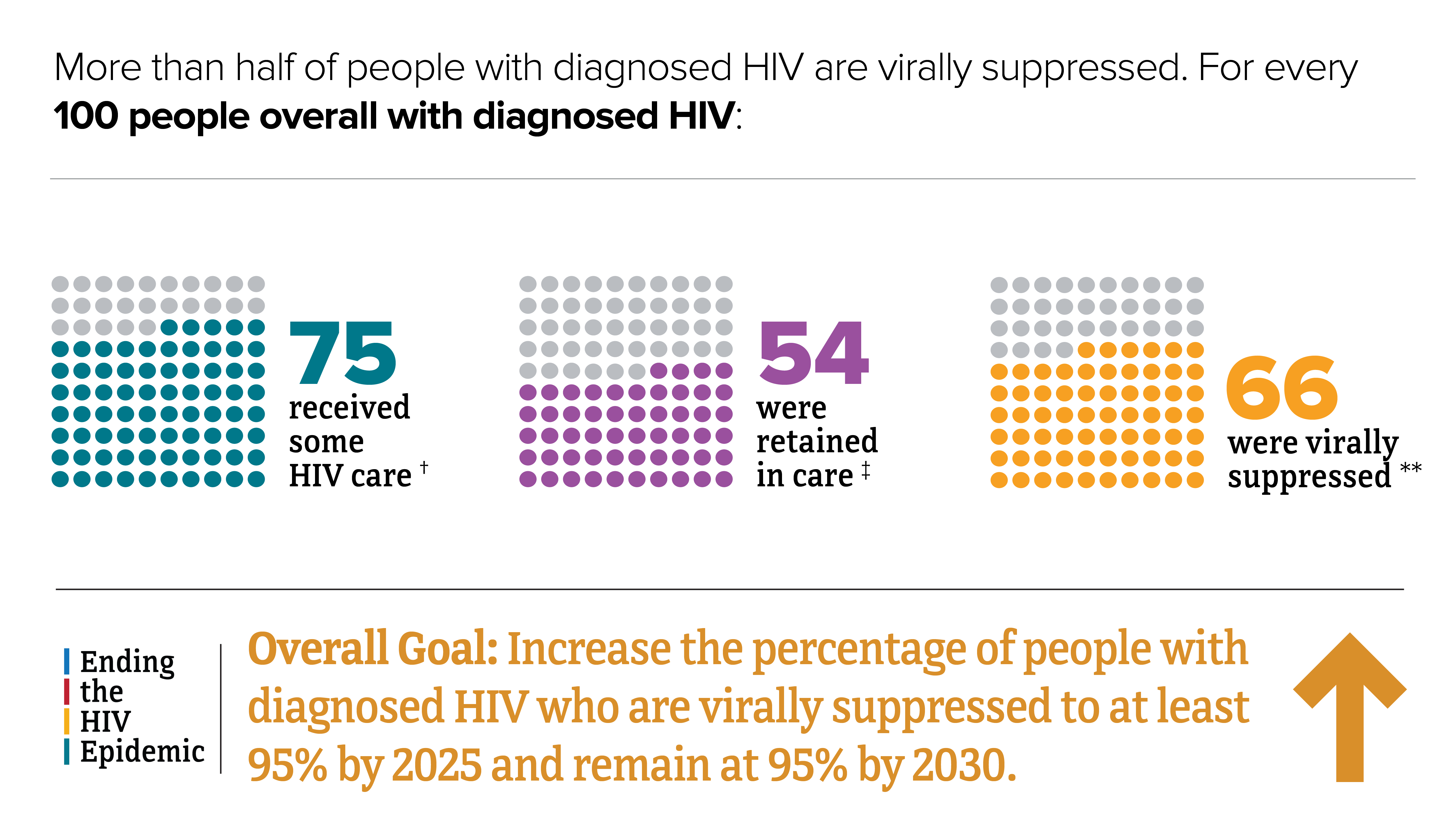
*Among people aged 13 and older.
†At least 1 viral load or CD4 test.
‡Had 2 viral load or CD4 tests at least 3 months apart in a year.
**Based on most recent viral load test.
HIV care continuum among transgender people with diagnosed HIV in 47 states and the District of Columbia, 2021*
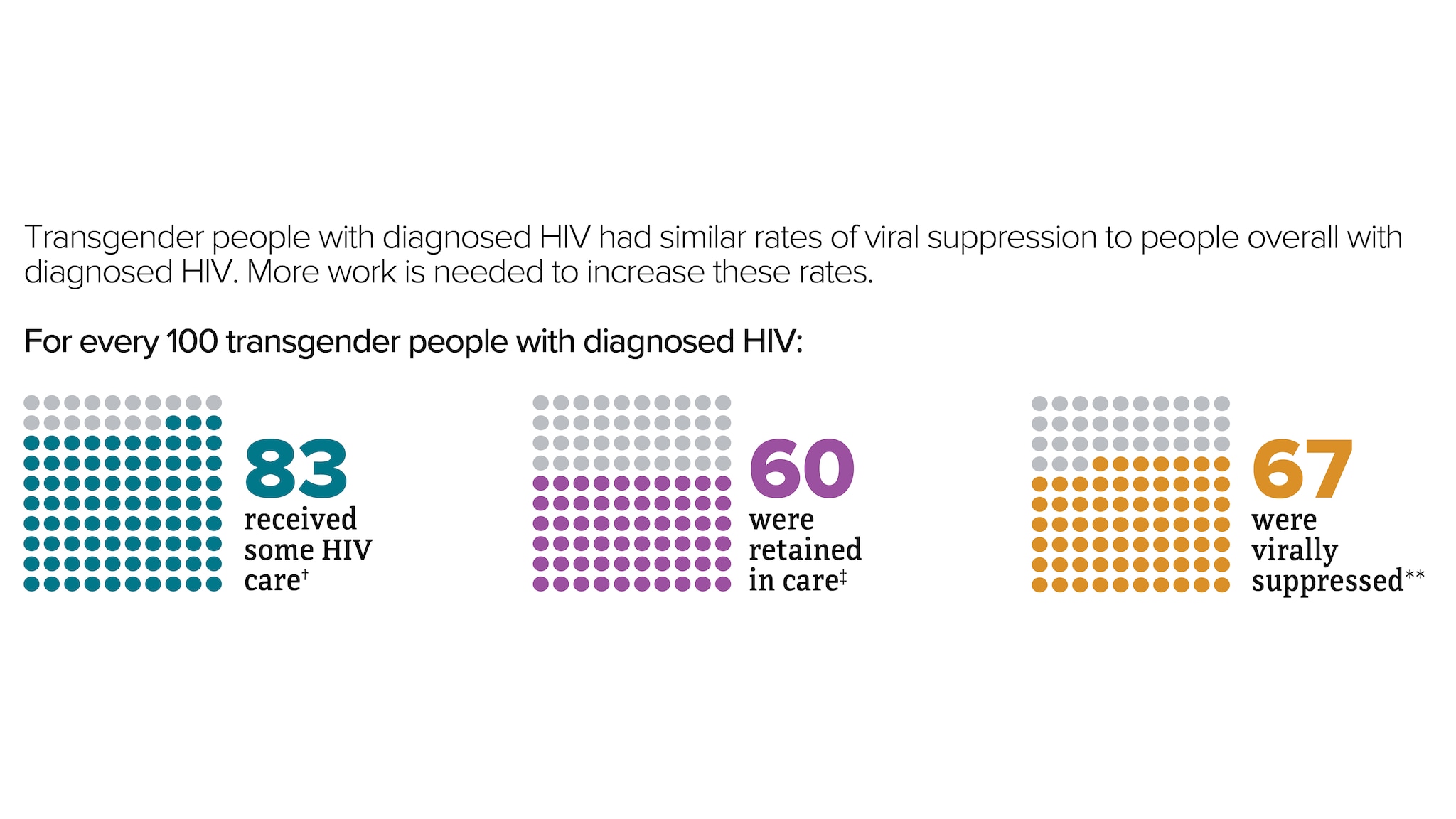
*Among people aged 13 and older.
‡Had 2 viral load or CD4 tests at least 3 months apart in a year.
**Based on most recent viral load test.
HIV care continuum among people with diagnosed HIV in 47 states and the District of Columbia by transmission category, 2021*
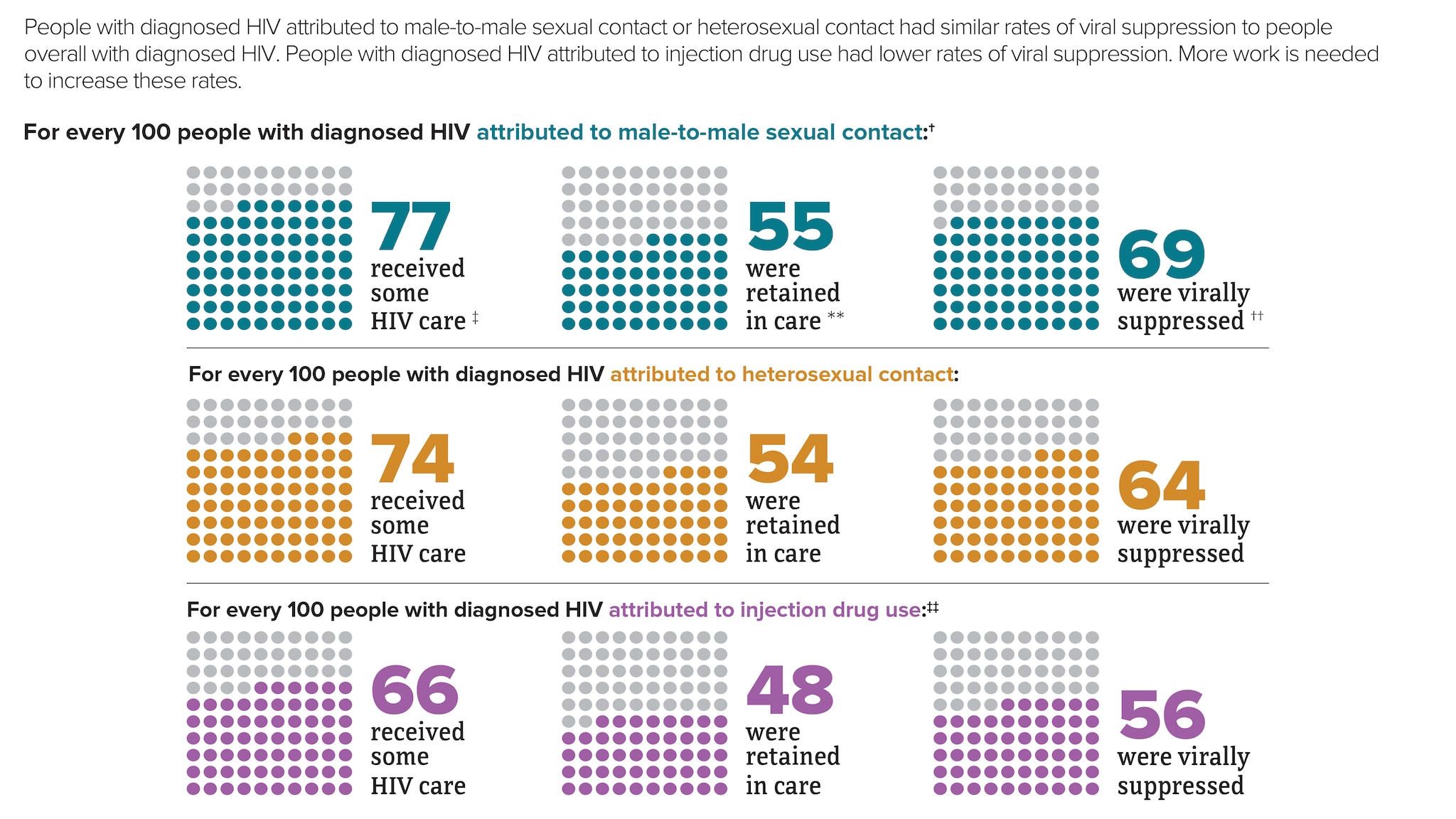
† Includes infections attributed to male-to-male sexual contact only.
‡ At least 1 viral load or CD4 test.
** Had 2 viral load or CD4 tests at least 3 months apart in a year.
†† Based on most recent viral load test.
‡‡ Includes infections attributed to injection drug use only. For every 100 men with HIV attributed to male-to-male sexual contact and injection drug use, 78 received some HIV care, 56 were retained in care, and 65 were virally suppressed.
HIV care continuum among people with diagnosed HIV in 47 states and the District of Columbia by region, 2021*
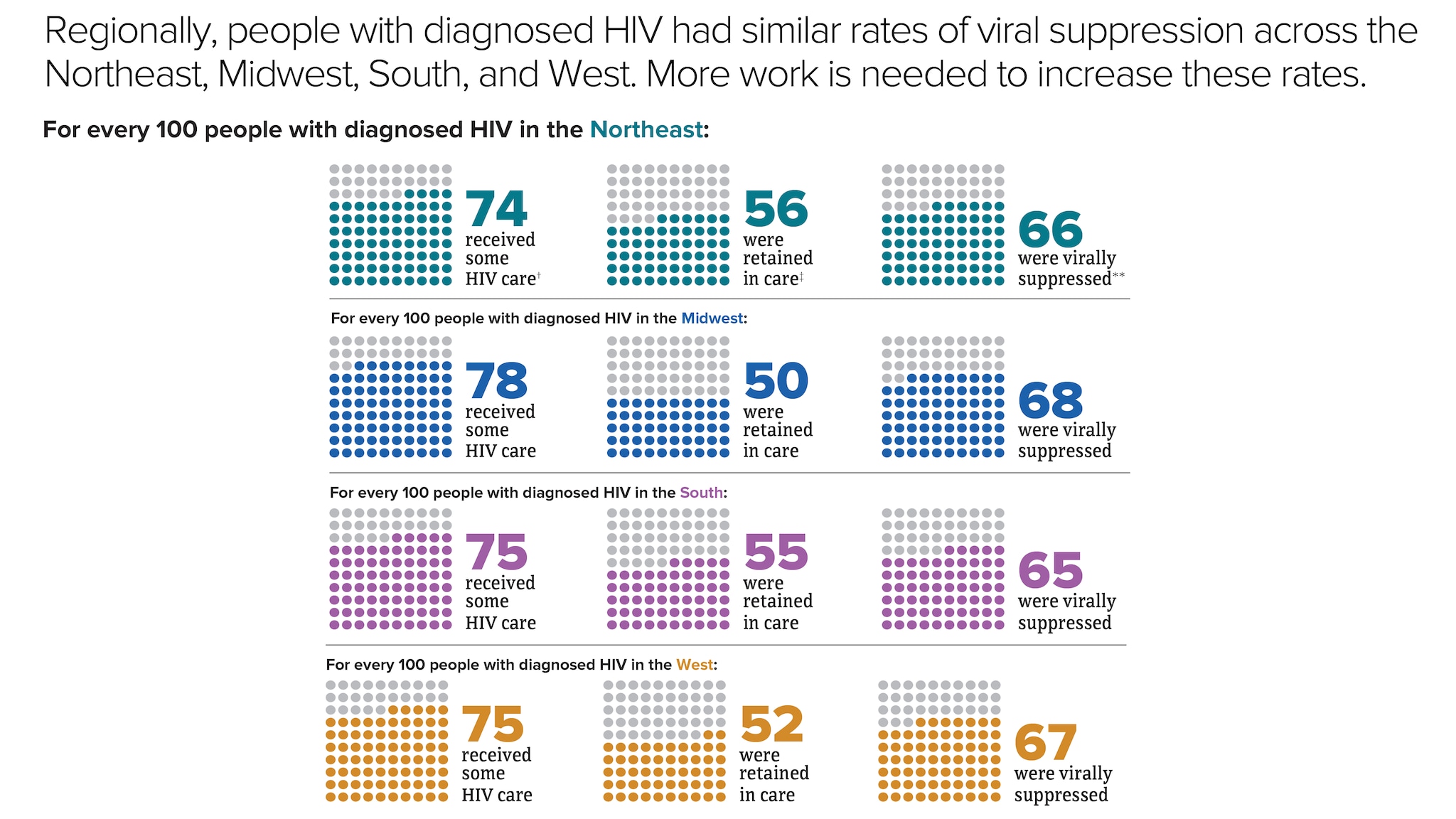
Source: CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2021 . HIV Surveillance Supplemental Report 2023;28(4).
People with diagnosed HIV in the US and dependent areas by age, 2021
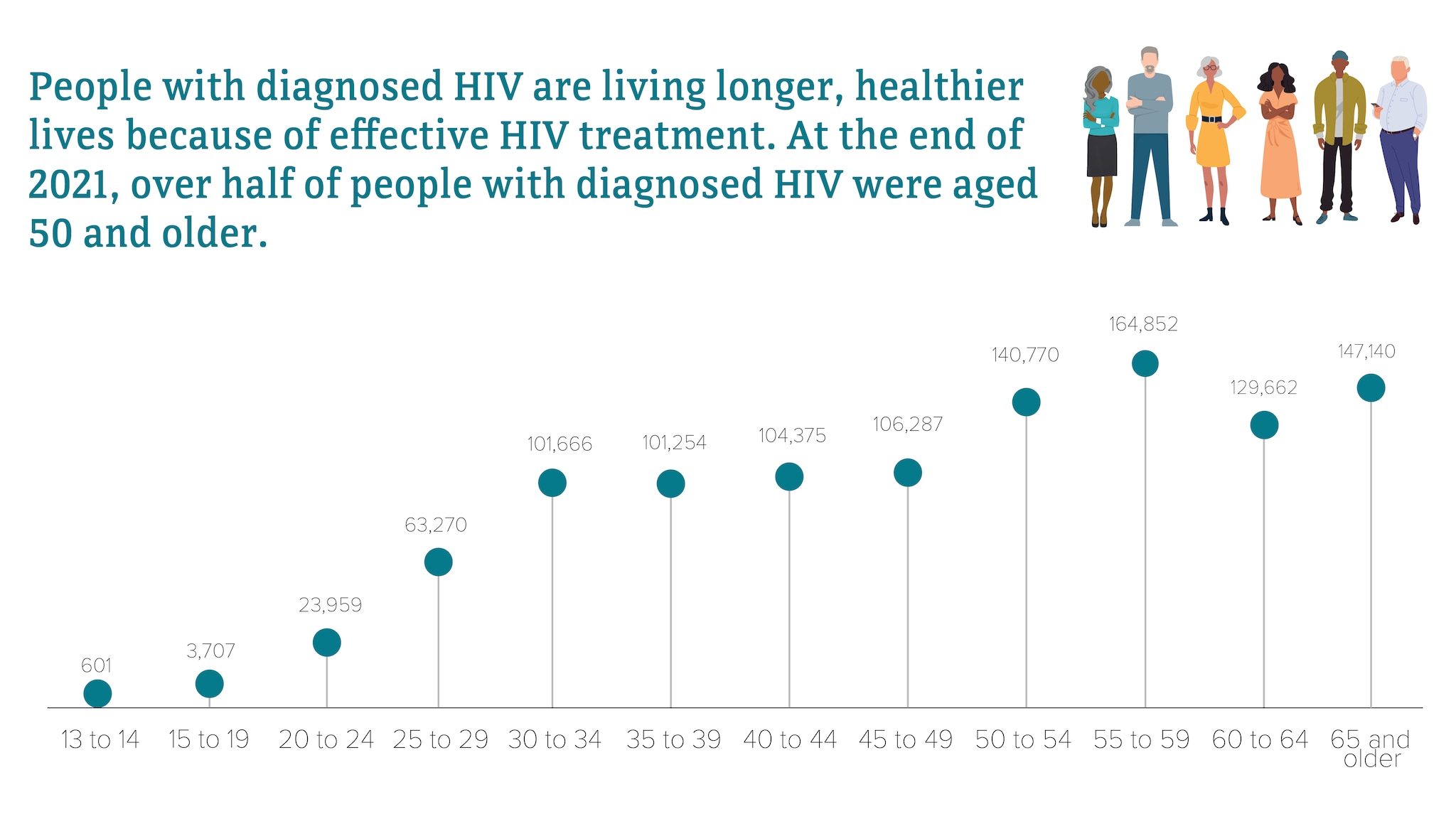
Most HIV cases occur in metropolitan areas with 500,000 or more people. The South has the highest number of people living with HIV, but if population size is taken into account, the Northeast has the highest rate of people living with HIV.
Rates of people with diagnosed HIV in the US and dependent areas by region of residence, 2021*†
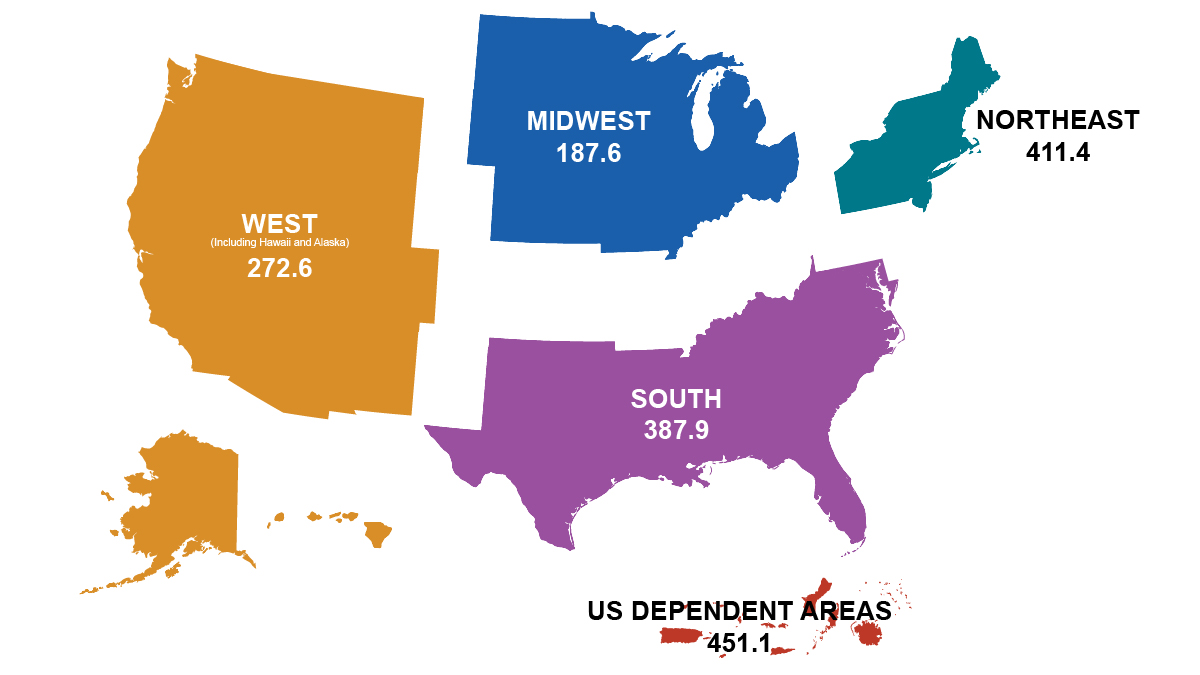
* Rates per 100,000 people.
† Includes adults, adolescents, and children under the age of 13.
Although many people taking HIV medicine are virally suppressed, some people with HIV are currently not virally suppressed or do not maintain viral suppression over time. Some challenges with achieving and maintaining viral suppression include HIV stigma, physical health, mental health, and structural issues—such as food insecurity, unemployment, and unstable housing or homelessness.
Median HIV stigma score among people with diagnosed HIV in the US, 2020*
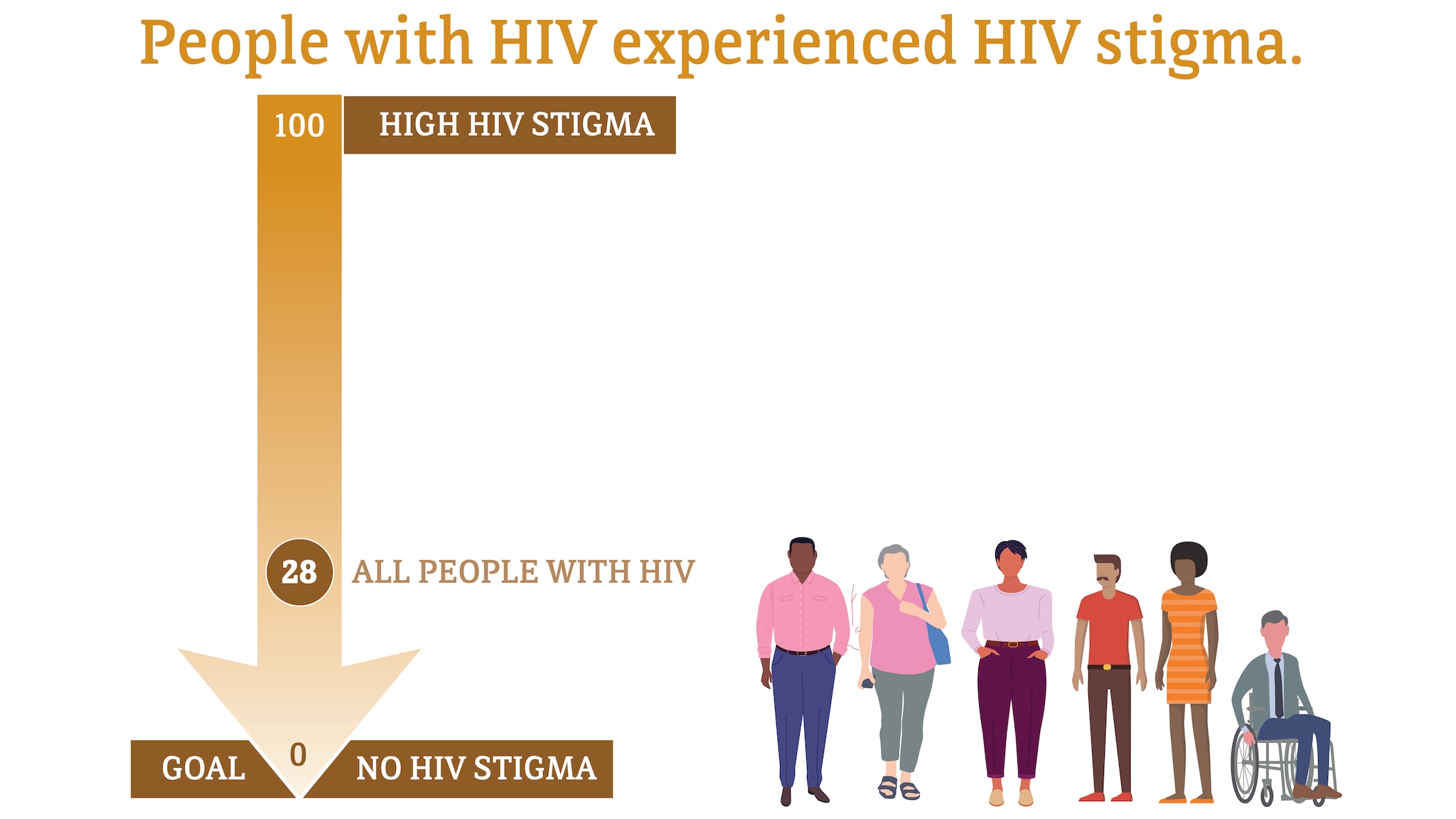
Median HIV stigma scores are presented based on a ten-item scale ranging from 0 (no stigma) to 100 (high stigma) that measures personalized stigma during the past 12 months, current disclosure concerns, current negative self-image, and current perceived public attitudes about people with HIV.
* Among people aged 18 and older.
Source: CDC. Behavioral and clinical characteristics of persons with diagnosed HIV infection—Medical Monitoring Project, United States 2020 cycle (June 2020–May 2021) . HIV Surveillance Special Report 2022;29.
Self-rated health among people with diagnosed HIV in the US, 2020*
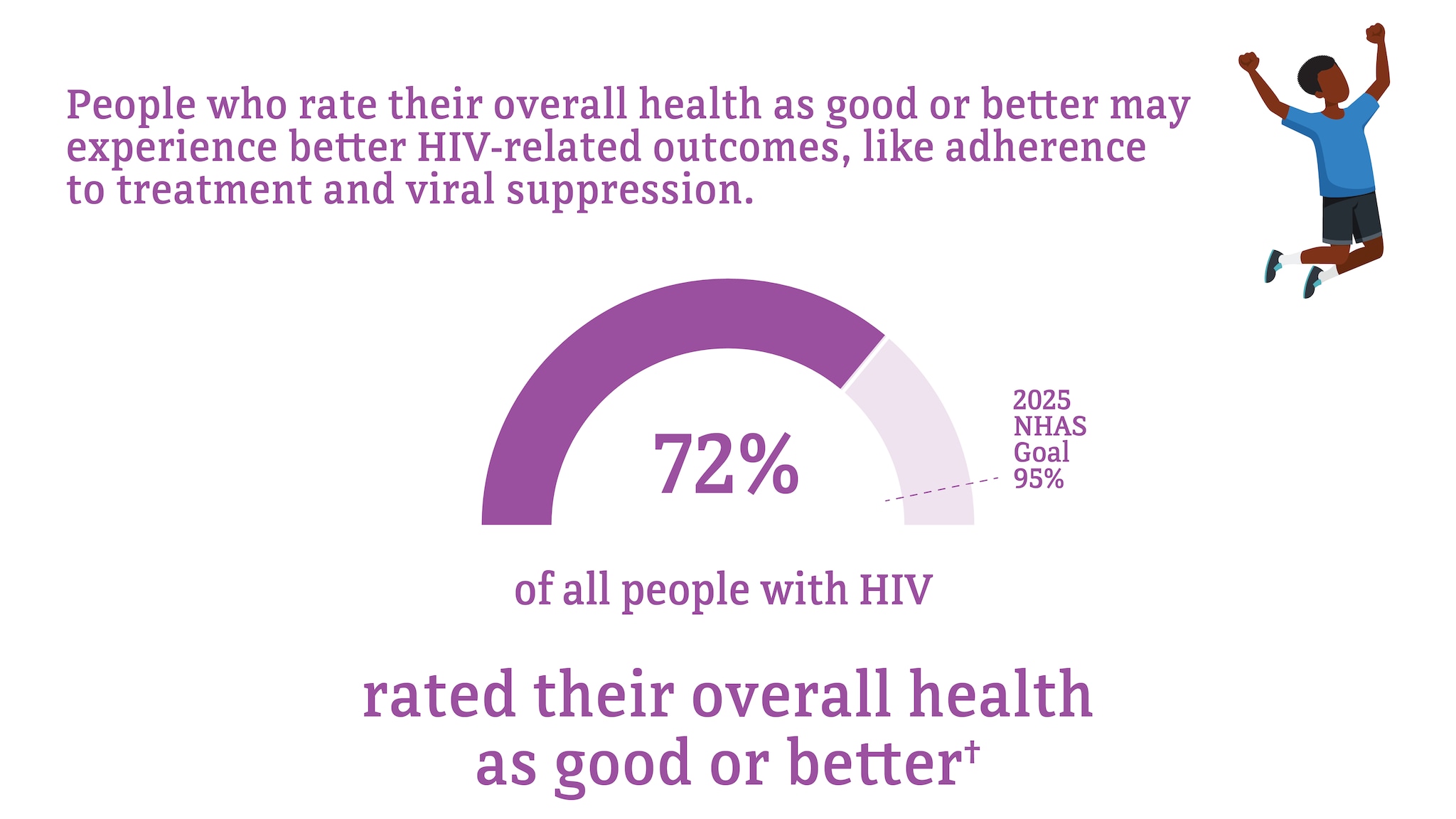
* Among people aged 18 and older.
† Good or better self-rated health is defined as rating one’s health as good, very good, or excellent (as opposed to poor or fair) at the time of interview.
Source: CDC. Quality of life and HIV stigma—Indicators for the National HIV/AIDS Strategy, 2022–2025, CDC Medical Monitoring Project, 2017–2020 cycles . HIV Surveillance Special Report 2022;30.
Unmet need for services from a mental health professional among people with diagnosed HIV in the US, 2020*†
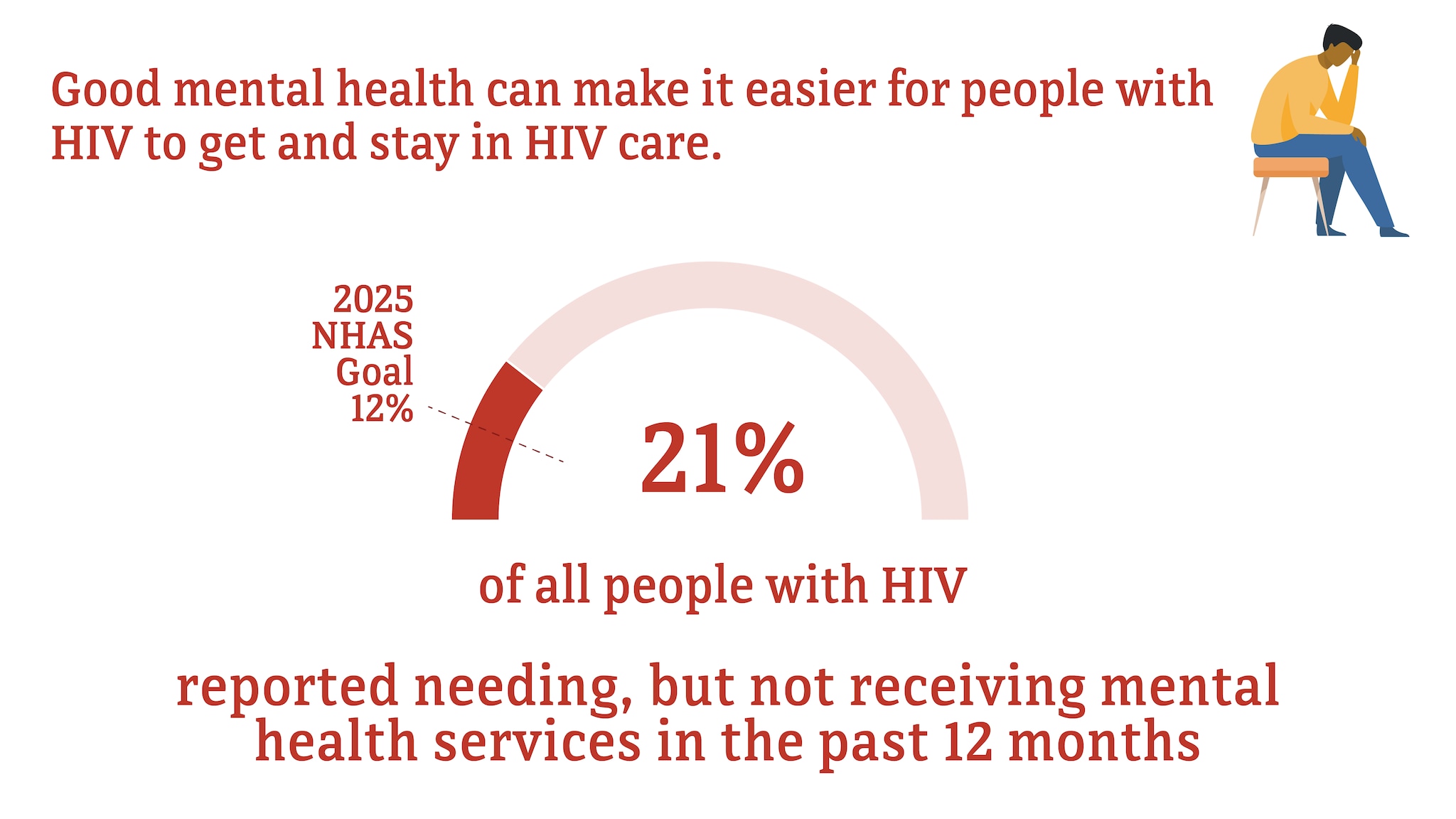
† Among people with diagnosed HIV who reported an unmet need for mental health services in the past 12 months.
Source: CDC. Quality of life and HIV stigma—Indicators for the National HIV/AIDS Strategy, 2022–2025, CDC Medical Monitoring Project, 2017–2020 cycles . HIV Surveillance Special Report 2022;30.
Food insecurity, unemployment, and unstable housing among people with diagnosed HIV in the US, 2020*
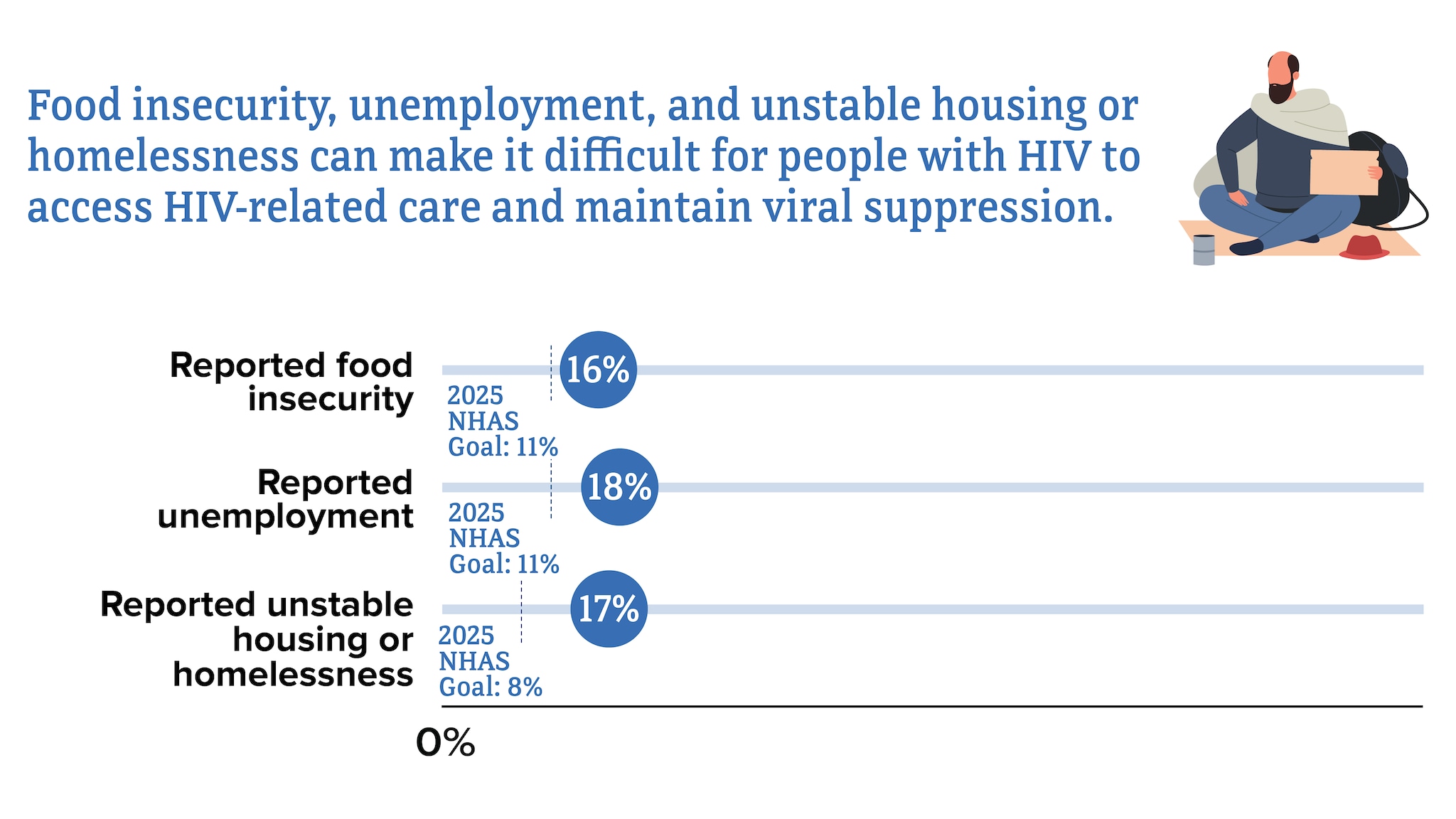
* Among people with HIV aged 18 and older.
Learn about HIV and how the virus is transmitted, how to protect yourself and others, and how to live well with HIV. Also learn how HIV impact the American people.
- Introduction
- Conclusions
- Article Information
Dark blue boxes indicate RRs, and horizontal bars indicate 95% CIs. Sizes of dark blue boxes are proportional to the inverse variance. The light blue diamond indicates the pooled RR estimate and 95% CI in the random-effects model meta-analysis. RRs are maximally adjusted estimates as reported by studies (see eTable 1 in Supplement 1 for adjustment variables). Badr et al 32 for RR of all-cause death and Postigo et al 28 for RR of MACE were crude estimates calculated by this study’s authors based on number of participants and number of events reported for patients living with HIV and control groups. The definition of MACE for Shitole et al 18 and Postigo et al 28 was death or cardiovascular admissions.
RRs are shown for recurrent acute coronary syndrome (ACS) (A), heart failure (HF) admission (B), cardiovascular (CV) death (C), and restenosis (D). Dark blue boxes indicate RRs, and horizontal bars indicate 95% CIs. Sizes of dark blue boxes are proportional to the inverse variance. The light blue diamond indicates the pooled RR estimate and 95% CI in the random-effects model meta-analysis.
eTable 1. Additional Patient Characteristics by Study for Patients Living With HIV and Patients in Control Groups
eTable 2. Comparison of Patient Characteristics Between Patients Living With HIV and Patients in Control Groups
eTable 3. Clinical Outcomes, Relative Risks, and Adjustment Variables by Study
eTable 4. Sensitivity Analysis of Pooled Relative Risks Calculated Using Knapp-Hartung Method for Random-Effects Model Meta-Analysis
eTable 5. Quality Assessment of Included Studies With Newcastle-Ottawa Scale
eFigure 1. Study Flow Sheet
eFigure 2. Pooled Relative Risks for Patients Living With HIV vs Patients in Control Groups for TLR and TVR
eFigure 3. Pooled Unadjusted Relative Risks for Patients Living With HIV vs Patients in Control Groups for All-Cause Mortality, MACE, and Recurrent ACS
eFigure 4. Funnel Plot of Relative Risks for All-Cause Mortality and MACE
eMethods. Detailed Description of Statistical Analysis
Data Sharing Statement
See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Haji M , Capilupi M , Kwok M, et al. Clinical Outcomes After Acute Coronary Syndromes or Revascularization Among People Living With HIV : A Systematic Review and Meta-Analysis . JAMA Netw Open. 2024;7(5):e2411159. doi:10.1001/jamanetworkopen.2024.11159
Manage citations:
© 2024
- Permissions
Clinical Outcomes After Acute Coronary Syndromes or Revascularization Among People Living With HIV : A Systematic Review and Meta-Analysis
- 1 Department of Medicine, Alpert Medical School of Brown University, Providence, Rhode Island
- 2 Department of Medicine, Washington University School of Medicine in St Louis, St Louis, Missouri
- 3 Department of Medicine, Duke Global Health Institute and Duke Clinical Research Institute, Duke University, Durham, North Carolina
- 4 Global Health Institute, University of Washington, Seattle
- 5 Infectious Disease Section, Michael E. DeBakey VA Medical Center, Houston, Texas
- 6 Department of Medicine, Baylor College of Medicine, Houston, Texas
- 7 Pharmacy Service, Michael E. DeBakey VA Medical Center, Houston, Texas
- 8 Center of Innovation, Providence VA Medical Center, Providence, Rhode Island
- 9 Evidence Synthesis Program Center, Providence VA Health Care System, Providence, Rhode Island
- 10 Department of Medicine, Providence VA Medical Center, Providence, Rhode Island
- 11 Department of Pharmacy, University of Rhode Island, Providence
- 12 Department of Health Services, Policy and Practice, Brown University, Providence, Rhode Island
Question What are the postdischarge outcomes for patients living with HIV after acute coronary syndromes or coronary revascularization?
Findings In this systematic review and meta-analysis of 15 studies involving 9499 patients living with HIV and 1 531 117 patients without HIV, patients living with HIV had a higher risk of all-cause mortality, major adverse cardiovascular events, recurrent acute coronary syndromes, and admission for heart failure after the index event, despite being approximately 11 years younger at the time of the event. Patients living with HIV were more likely to be current smokers and engage in illicit drug use and had higher triglyceride and lower high-density lipoprotein cholesterol levels than those without HIV.
Meaning This analysis highlights the need for attention toward secondary prevention strategies to address poor outcomes of cardiovascular disease among patients living with HIV.
Importance Clinical outcomes after acute coronary syndromes (ACS) or percutaneous coronary interventions (PCIs) in people living with HIV have not been characterized in sufficient detail, and extant data have not been synthesized adequately.
Objective To better characterize clinical outcomes and postdischarge treatment of patients living with HIV after ACS or PCIs compared with patients in an HIV-negative control group.
Data Sources Ovid MEDLINE, Embase, and Web of Science were searched for all available longitudinal studies of patients living with HIV after ACS or PCIs from inception until August 2023.
Study Selection Included studies met the following criteria: patients living with HIV and HIV-negative comparator group included, patients presenting with ACS or undergoing PCI included, and longitudinal follow-up data collected after the initial event.
Data Extraction and Synthesis Data extraction was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. Clinical outcome data were pooled using a random-effects model meta-analysis.
Main Outcome and Measures The following clinical outcomes were studied: all-cause mortality, major adverse cardiovascular events, cardiovascular death, recurrent ACS, stroke, new heart failure, total lesion revascularization, and total vessel revascularization. The maximally adjusted relative risk (RR) of clinical outcomes on follow-up comparing patients living with HIV with patients in control groups was taken as the main outcome measure.
Results A total of 15 studies including 9499 patients living with HIV (pooled proportion [range], 76.4% [64.3%-100%] male; pooled mean [range] age, 56.2 [47.0-63.0] years) and 1 531 117 patients without HIV in a control group (pooled proportion [range], 61.7% [59.7%-100%] male; pooled mean [range] age, 67.7 [42.0-69.4] years) were included; both populations were predominantly male, but patients living with HIV were younger by approximately 11 years. Patients living with HIV were also significantly more likely to be current smokers (pooled proportion [range], 59.1% [24.0%-75.0%] smokers vs 42.8% [26.0%-64.1%] smokers) and engage in illicit drug use (pooled proportion [range], 31.2% [2.0%-33.7%] drug use vs 6.8% [0%-11.5%] drug use) and had higher triglyceride (pooled mean [range], 233 [167-268] vs 171 [148-220] mg/dL) and lower high-density lipoprotein-cholesterol (pooled mean [range], 40 [26-43] vs 46 [29-46] mg/dL) levels. Populations with and without HIV were followed up for a pooled mean (range) of 16.2 (3.0-60.8) months and 11.9 (3.0-60.8) months, respectively. On postdischarge follow-up, patients living with HIV had lower prevalence of statin (pooled proportion [range], 53.3% [45.8%-96.1%] vs 59.9% [58.4%-99.0%]) and β-blocker (pooled proportion [range], 54.0% [51.3%-90.0%] vs 60.6% [59.6%-93.6%]) prescriptions compared with those in the control group, but these differences were not statistically significant. There was a significantly increased risk among patients living with HIV vs those without HIV for all-cause mortality (RR, 1.64; 95% CI, 1.32-2.04), major adverse cardiovascular events (RR, 1.11; 95% CI, 1.01-1.22), recurrent ACS (RR, 1.83; 95% CI, 1.12-2.97), and admissions for new heart failure (RR, 3.39; 95% CI, 1.73-6.62).
Conclusions and Relevance These findings suggest the need for attention toward secondary prevention strategies to address poor outcomes of cardiovascular disease among patients living with HIV.
The widespread use of effective antiretroviral therapies (ARTs) has led to increased survivorship among people living with HIV. Therefore, people living with HIV are experiencing an increased prevalence of age-related disease, such as cardiovascular disease (CVD). 1 , 2 The increase in CVD in this population has been attributed to multiple factors, including increasing age, the increase in burden of traditional CVD factors and psychosocial risk factors, the long-term metabolic effects of ART, and the low-grade immune activation of chronic HIV. 1 , 3 - 8
Epidemiological studies have shown that compared with populations without HIV, people living with HIV have a higher risk of coronary artery disease, acute coronary syndromes (ACS), and heart failure, with onset at younger ages. 4 , 9 - 12 Given this earlier emergence of CVD among people living with HIV, there has been significant attention and evidence generated for primary prevention strategies involving statins. 13 , 14 In conjunction with these studies, characterization of longitudinal CVD outcomes is important to identify strategies for secondary prevention and further improve survivorship among people living with HIV. Studies on clinical outcomes after ACS and percutaneous coronary interventions (PCIs) among patients living with HIV have shown higher rates of recurrent coronary disease and mortality compared with patients in HIV-negative control groups. 11 , 15 - 17 However, this association has not been characterized in sufficient detail in current literature, and extant data have not been adequately synthesized. We conducted a systematic review and meta-analysis of longitudinal studies of patients living with HIV after ACS or PCIs to better characterize clinical outcomes and postdischarge treatment compared with patients in HIV-negative control groups.
We report this systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses ( PRISMA ) reporting guideline. This study was not preregistered. Please see the eMethods in Supplement 1 for a detailed description of methods used in this meta-analysis, as recommended by the International Committee of Medical Journal Editors.
We searched Ovid MEDLINE, Embase, and Web of Science for all available articles from inception to August 2023 for the key terms coronary artery disease , myocardial infarction , non-fatal myocardial infarction , acute coronary syndrome , revascularization , percutaneous coronary intervention , and secondary prevention . We also reviewed references of relevant articles.
Articles were screened by 2 reviewers (M.H. and M.C.) by title and abstract and later by full text. We included studies if they fulfilled the following criteria: patients living with HIV and a comparator group of patients without HIV (control group) included, patients with obstructive coronary artery disease presenting with ACS or undergoing revascularization through PCI included, and longitudinal follow-up data on clinical outcomes after initial event collected. We initially also searched for studies that discussed outcomes after stroke and peripheral artery disease.
We extracted the following data where available using standardized forms: study characteristics, baseline demographics (ie, age, sex, and race and ethnicity) and other characteristics (ie, underlying comorbidities, revascularization strategies, and postdischarge medications) of HIV-positive and HIV-negative control populations, HIV-specific characteristics (use of ART, CD4 count, and viral load), number of events by group and hazard ratios (HRs) of clinical outcomes (ie, all-cause mortality, major adverse cardiovascular events [MACE], cardiovascular death, recurrent ACS, stroke, total lesion revascularization, total vessel revascularization, and admission for heart failure). We extracted maximally adjusted HRs where available, as well as unadjusted (crude) or minimally adjusted HRs for clinical outcomes. We captured data on race and ethnicity to help assess the full scope of diversity among patients living with HIV and how applicable our data may be within the global population of people living with HIV. Race and ethnicity were self-reported in the study by Shitole et al. 18 In the other studies reporting this information, data were obtained from review of medical records, including electronic health records. Reported race and ethnicity categories included African American, American Indian, Asian, Hispanic, Pacific Islander, White, and other. We primarily report aggregated data for Black, White, and Hispanic populations only given that there were limited data available on other races and ethnicities.
We combined summary study characteristics (eg, mean age, percentage male and female, percentage Black and White, and percentage Hispanic) across studies using study sizes as analytical weights to provide estimates of pooled means or percentages. The δ and P values comparing summary study-level characteristics (means or prevalences pooled across studies) between HIV-positive and HIV-negative groups were calculated from a linear regression model of each variable on HIV status weighted by the number of participants for each study (ie, a fixed-effects meta-regression). When HRs were not reported, we calculated crude risk ratios from the number of events in each group. In 2 studies, 15 , 19 data were reported as odds ratios. We pooled HRs of clinical outcomes across studies using a random-effects model meta-analysis, estimating between-study heterogeneity using the DerSimonian-Laird method. 20 As a sensitivity analysis, we also estimated between-study heterogeneity using the residual maximum likelihood method and calculated variances ( P values and CIs) of pooled relative risk (RR) estimates using modifications proposed by Knapp and Hartung. 21 For the purpose of the meta-analysis, we considered odds ratios, risk ratios, and HRs as equivalent measures of RR.
We assessed between-study heterogeneity using the Cochran Q statistic and I 2 statistic, which estimates the percentage of total variation across studies due to true between-study difference rather than chance. 22 , 23 We did not explore heterogeneity further owing to the limited numbers of studies available for most comparisons.
The quality of included studies was assessed using the Newcastle-Ottawa Scale for cohort studies. 24 We visually inspected funnel plots to assess the risk of publication bias. We also performed the Egger test for small study bias, although this was limited by the small number of studies that were generally available for investigated outcomes. Where there were P values trending toward small study bias, we performed trim and fill analyses to help assess the impact of the bias on pooled estimates (even if Egger test P values did not reach statistical significance). A 2-sided P value less than .05 was considered statistically significant. For the meta-analysis of RRs, we report point estimates and 95% CIs. All analyses were performed using Stata software statistical software version 15 (StataCorp).
An initial search yielded 3263 studies, which were screened using titles, abstracts, and full texts. Studies reviewing patient outcomes after diagnoses and interventions of peripheral artery disease and stroke were limited, reporting mainly in-hospital outcomes, short-term follow-up, or results without non-HIV comparator groups, and were not further considered in this meta-analysis. We identified 15 studies 11 , 15 , 16 , 18 , 25 - 35 of post-ACS or revascularization outcomes from 2003 to 2023 that met inclusion criteria (eFigure 1 in Supplement 1 ). Of identified studies, 2 were abstracts. 30 , 31 All were retrospective cohort studies except for 3 prospective studies ( Table 1 ). 11 , 26 , 30
Details of patient characteristics and outcomes by study are presented in Table 1 and eTable 1 in Supplement 1 . A total of 9499 patients living with HIV (pooled proportion [range], 76.4% [64.3%-100%] male; pooled mean [range] age, 56.2 [47.0-63.0] years; pooled proportion [range], 10.1% [95% CI, 7.0%-62.5%] Black; 8.1% [95% CI, 0.4%-54.6%] Hispanic, and 13.1% [95% CI, 7.2%-64.0%] White) and 1 531 117 patients in control groups without HIV (pooled proportion [range], 61.7% [59.7%-100%] male; pooled mean [range] age, 67.7 [42.0-69.4] years; pooled proportion [range], 3.3% [95% CI, 2.5%-21.4%] Black, 3.6% [95% CI, 0.7%-36.3%] Hispanic, and 21.1% [95% CI, 14.3%-68.0%] White) who experienced ACS or underwent coronary revascularization were included in the meta-analysis. Summary baseline characteristics of study participants and comparisons of patients living with HIV with patients in control groups are presented in Table 2 and eTable 2 in Supplement 1 . The mean age of patients living with HIV was 11.1 years (95% CI, 6.2-16.0 years) less than that of patients in HIV-negative control groups ( P < .001). HIV-positive and control populations were similarly male dominant. Patients living with HIV were statistically significantly more likely to be current smokers (pooled proportion [range], 59.1% [24.0%-75.0%] smokers vs 42.8% [26.0%-64.1%] smokers; P < .001) and engage in illicit drug use (pooled proportion [range], 31.2% [2.0%-33.7%] drug use vs 6.8% [0%-11.5%] drug use; P < .001) and had significantly higher pooled mean (range) triglyceride (233 [167-268] vs 171 [148-220] mg/dL; P = .01) and lower pooled mean (range) high-density lipoprotein cholesterol (40 [26-43] vs 46 [29-46] mg/dL; P = .03) levels. (To convert triglycerides and cholesterol to millimoles per liter, multiply by 0.0113 and 0.0259, respectively.) There were similar proportions of patients with diabetes, hypertension, and a family history of coronary artery disease in the 2 groups ( Table 2 ; eTable 2 in Supplement 1 ).
Patients with HIV had been diagnosed with HIV for a pooled mean (range) of 11.2 (8.5-12.0) years. From 9 studies 11 , 16 , 18 , 26 , 28 , 29 , 31 , 34 , 35 that provided these data, a pooled proportion (range) of 75.2% (50.0%-94.1%) of patients living with HIV were receiving ART and 47.6% (25.0%-85.6%) had previously received protease inhibitor therapy. The pooled mean (range) CD4 count was 377 (318-462) cells/mm 3 among patients living with HIV, and most of these patients (pooled proportion [range], 77.8% [63.3%-94.6%]) had a viral load less of than 200 copies per mL ( Table 2 ).
Among 13 studies 11 , 15 , 16 , 18 , 25 - 29 , 31 - 34 that reported data on ACS, patients living with HIV and those in control groups presented similarly with ST-segment elevation myocardial infarction, non–ST-segment elevation myocardial infarction, and unstable angina. Additionally, the groups received PCIs or coronary artery bypass graft surgery at similar proportions. After revascularization, pooled mean (range) left ventricular ejection fraction values were similar between groups (49.4% [44.0%-55.4%] vs 50.9% [48.0%-54.8%]). On postdischarge follow up, patients living with HIV had a lower proportion (range) of statin (53.3% [45.8%-96.1%] vs 59.9% [58.4%-99.0%]) and β-blocker (54.0% [51.3%-90.0%] vs 60.6% [59.6%-93.6%]) prescription compared with patients in control groups, but these differences were not statistically significant ( Table 2 ; eTable 2 in Supplement 1 ).
Over a pooled mean (range) follow-up of a mean of 16.2 (3.0-60.8) months after ACS or revascularization, patients living with HIV had a significantly higher adjusted risk of all-cause mortality (pooled adjusted RR, 1.64; 95% CI, 1.32-2.04), MACE (RR, 1.11; 95% CI, 1.01-1.22), recurrent ACS (RR, 1.83; 95% CI, 1.12-2.97), and heart failure readmission (RR, 3.39; 95% CI, 1.73-6.62) ( Figure 1 ), as well as restenosis (RR, 2.40; 95% CI, 1.13-5.09) ( Figure 2 ) compared with patients in HIV-negative control groups (pooled mean [range] follow-up, 11.9 [3.0-60.8] months). For CV death, total vessel revascularization, and total lesion revascularization, pooled HRs showed no significantly higher risk among patients living with HIV compared with patients in control groups (eFigure 2 in Supplement 1 ). RRs of clinical outcomes and adjustment variables included in multivariate models that were reported by each study are presented in eTable 3 in Supplement 1 . Sensitivity analyses specifying an alternative method for the random-effects model yielded comparable results (eTable 4 in Supplement 1 ). In a separate subsidiary analysis, there was no association between HIV status and risk of post–ACS or PCI mortality, recurrent ACS, or MACE outcomes in the unadjusted (minimally adjusted in some studies) model (eFigure 3 in Supplement 1 ).
There was generally low heterogeneity across studies for most outcomes ( Figure 1 and Figure 2 ). Visual inspection of the funnel plot for publication bias assessment and Egger tests did not suggest the presence of significant publication bias (eFigure 4 in Supplement 1 ). For the all-cause mortality outcome, the Egger test for bias was borderline, and so we performed trim and fill analysis; this yielded similar results (RR, 1.61; 95% CI, 1.30-2.00). Included studies were of moderate to high quality based on the Newcastle-Ottawa Scale, indicating a low to moderate risk of bias (eTable 5 in Supplement 1 ).
We performed a literature-based systematic review and meta-analysis of 15 studies of longitudinal clinical outcomes after ACS or revascularization from 2003 to 2023, comprising a total of 9499 patients living with HIV and 1 531 117 patients without HIV in control groups. We found that patients living with HIV were younger and had a higher risk of all-cause mortality, MACE, recurrent ACS, and heart failure after the index event. We also noted lower rates of statin and β-blocker prescription after discharge among patients living with HIV. Overall, these findings highlight the need to develop and implement strategies for secondary prevention of CVD among patients living with HIV.
The increased mortality, recurrence of ACS, and heart failure admissions among patients living with HIV may be attributed to increased traditional CVD risk factors, psychosocial factors, HIV-related chronic inflammation, and long-term effects of ART. 11 , 16 These factors are equally difficult to control after an initial coronary event. 19 , 35 , 36 The study by Boccara et al 11 from 2020 compared its findings with those of their first, 2011 study 37 and noted an increased rate of recurrence of ACS in patients living with HIV; the authors also noted persistent smoking and chronic inflammation as factors associated with some of the greatest increases in risk for recurrent disease. This further reinforces the need for a multifaceted approach to secondary prevention.
Of note, our study found suboptimal statin prescription in patients living with HIV after ACS or revascularization, which is consistent with results of other retrospective studies. 11 , 18 , 19 , 26 , 28 , 38 - 42 These findings and those of the Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults (REPRIEVE) trial, 14 which demonstrated the benefits of pitavastatin for primary prevention of atherosclerotic cardiovascular disease among patients living with HIV, highlight the need for a concerted effort to improve guideline-directed statin prescription and adherence among these patients. 43 Additionally, the higher prevalence of smoking and higher triglyceride levels we found among patients living with HIV highlight areas for optimization, with the goal of improving secondary prevention of atherosclerotic cardiovascular disease. Differences in statin and β-blocker prescriptions on follow-up were not statistically significant, although patients living with HIV had numerically lower percentages for both outcomes.
Our pooled estimates for postdischarge antiplatelet therapy are influenced by the study from Parks et al, 33 which defined antiplatelet use as a filled prescription for clopidogrel, ticagrelor, prasugrel, or ticlopidine and as a retrospective observational study, could not reliably exclude patients with type 2 myocardial infarctions who would not typically qualify for these therapies. In that study’s sensitivity analyses of patients who received coronary angiography, percentages of patients with postdischarge antiplatelet therapies were significantly higher. We performed an analysis of aggregate postdischarge antiplatelet therapy rates excluding data from Parks et al, 33 and aggregate data for postdischarge antiplatelet therapy was much higher.
Few studies reported race or ethnicity of participants, leading to overall low aggregate percentages of White and Black patients living with HIV in our analysis, which is not representative of the global population of these patients. Race and ethnicity in most studies were obtained from review of electronic health records, except in the study by Shitole et al, 18 in which race and ethnicity were self-reported. The analysis of race and ethnicity was skewed by 2 studies; in 1 study, 44 most of the population’s race and ethnicity was unknown, and in the other study, 19 the population was mainly Hispanic. Likewise, the percentage of patients who underwent PCIs was lower than expected for a typical population presenting with ACS. This was also contributed by the Parks et al study, 33 which included patients with type 2 myocardial infarctions, who were not candidates for PCIs in their analysis.
Most studies in our analysis included patients receiving ART with low viral loads and CD4 counts greater than 200 cells/mm 3 , indicating patients with good control of their HIV disease, who are representative of people living with HIV in the current era. 1 , 4 , 7 , 45 We found 8 studies 11 , 16 , 26 - 28 , 31 , 34 , 35 that reported use of protease inhibitors among approximately 50% of patients living with HIV (47.6%). Protease inhibitors are known to have metabolic effects associated with CVD, presenting a plausible explanation for the difference in hypertriglyceridemia between patients living with HIV and patients without HIV in our study. 46 Modern ART regimens have transitioned away from the use of protease inhibitors and now include integrase inhibitors. 7 Conflicting data have emerged around the possible association of integrase inhibitors with increased incidence of CVD. 47 , 48 Therefore, further research on long-term outcomes associated with ART will be essential to primary and secondary prevention of CVD among patients living with HIV.
The period after ACS or PCI provides additional opportunity to introduce aggressive interventions to improve CVD risk factors in patients living with HIV, and these interventions may involve multidisciplinary teams. Ensuring access to and engagement of cardiologists for patients living with HIV will be important to improve outcomes, especially among underrepresented racial and ethnic minorities. 49 Input from pharmacists can also help with optimal selection of statin types, other lipid-lowering agents, and dosages to avoid drug interactions and drug-related adverse effects and maximize adherence to these therapies. Additionally, input from addiction medicine specialists and psychologists can help address underlying mental health disorders (eg, depression and anxiety) and behavioral risk factors (eg, smoking, alcohol use, and cocaine use). In our study, patients living with HIV were more likely to be smokers and engage in illicit drug use, similar to contemporary studies that also show that these behaviors are associated with an overall increased mortality in patients living with HIV despite adequate control of their underlying infection. 50 Likewise, assistance from social workers can help to mitigate social determinants associated with diet and the ability to afford crucial medications. 36 , 51 - 53 Addressing this latter aspect is critically important to improve secondary outcomes of CVD in patients living with HIV because despite increased prescription rates for cardioprotective medications, patients living with HIV have been found to be less likely to fill these medications. 38 , 42 , 52 A multifaceted or multidisciplinary intervention to address psychosocial barriers to cardiovascular care may have the potential to limit mortality and morbidity after ACS or PCI for patients living with HIV.
The findings of this meta-analysis should be considered in context of several limitations. First, given that this was a literature based meta-analysis of aggregate published data, we were unable to compare the association between HIV status and CVD outcomes by clinically important subgroup, such as age, race and ethnicity, or sex. Second, the degree of adjustment for confounders in RR estimates is limited to what is reported in individual studies, is not consistent across studies, and may be inadequate overall. For instance, very few studies accounted for HIV-specific characteristics. However, the goal of the meta-analysis was to understand the difference in secondary CVD outcomes stratified by HIV status regardless of factors that may be contributing to them. We also performed a comparison between maximally adjusted and unadjusted or minimally adjusted RRs to provide further insight into the association. Our analysis showed that there was no association between HIV status and post-ACS or -PCI mortality, recurrent ACS, or MACE outcomes in the unadjusted model. This is likely due to the reverse confounding effect of age given that patients living with HIV were significantly younger than patients in control groups, with a difference of 11 years in pooled mean age across studies. Third, most studies included in this review evaluated patients living with HIV who lived in high-income countries, which may limit generalizability to the global population of patients living with HIV. Fourth, we were not able to perform subgroup analyses of patients who had ACS and were treated medically vs PCI, as well as those who received PCI for stable coronary disease, because these data were not reported separately. Future assessment of outcomes within these subgroups would be important for preventative efforts. Fifth, we were unable to identify timelines for prescription of or adherence to ART or cardioprotective medications based on these aggregate data. Understanding these trends will also be an important focus for secondary prevention in future studies.
In this literature based systematic review and meta-analysis of longitudinal studies from 2000 to 2023, we found that patients living with HIV were significantly younger than patients in control groups. Patients living with HIV had a significantly higher risk of all-cause mortality, MACE, recurrent ACS, and admission for heart failure after the index event compared with patients in control groups.
Patients living with HIV were also significantly more likely to be current smokers and engage in illicit drug use and had higher triglyceride levels at baseline. As more data emerge for primary prevention, this analysis highlights the need for optimization of secondary prevention strategies to address poor outcomes of CVD among patients living with HIV. Future studies can focus on assessing the role of aggressive interventions, including use of multidisciplinary teams to target important risk factors and improve prescription of and adherence to cardioprotective medications among patients living with HIV after ACS or PCI.
Accepted for Publication: March 7, 2024.
Published: May 14, 2024. doi:10.1001/jamanetworkopen.2024.11159
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2024 Haji M et al. JAMA Network Open .
Corresponding Author: Sebhat Erqou, MD, PhD, Department of Medicine, Providence VA Medical Center, 830 Chalkstone Ave, Providence, RI 02908 ( [email protected] ).
Author Contributions: Drs Haji and Erqou had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Haji, Ashong, Richard, Wu, Erqou.
Acquisition, analysis, or interpretation of data: Haji, Capilupi, Kwok, Ibrahim, Bloomfield, Longenecker, Rodriguez-Barradas, Jutkowitz, Taveira, Sullivan, Rudolph, Wu, Erqou.
Drafting of the manuscript: Haji, Capilupi, Kwok, Taveira, Erqou.
Critical review of the manuscript for important intellectual content: Capilupi, Kwok, Ibrahim, Bloomfield, Longenecker, Rodriguez-Barradas, Ashong, Jutkowitz, Taveira, Richard, Sullivan, Rudolph, Wu.
Statistical analysis: Kwok, Erqou.
Obtained funding: Erqou.
Administrative, technical, or material support: Haji, Capilupi, Kwok, Jutkowitz, Sullivan, Rudolph, Wu.
Supervision: Bloomfield, Taveira, Richard, Rudolph, Wu, Erqou.
Conflict of Interest Disclosures: Dr Longenecker reported receiving personal fees from Theratechnologies advisory board outside the submitted work. Dr Jutkowitz reported receiving grants from the Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development during the conduct of the study. Dr Rudolph reported receiving grants from the National Institute on Aging during the conduct of the study. No other disclosures were reported.
Funding/Support: This study was supported by a VISN 1 Career Development Award from the Department of Veterans Affairs, Veterans Health Administration, to Dr Erqou. Dr Erqou was also funded by the Center for Aids Research, Rhode Island Foundation, and Lifespan Cardiovascular Institute. Drs Sullivan, Rudolph, and Wu were funded by grants CIN 13-419 and C19-20-213 from the VA Health Services Research and Development Center of Innovation in Long Term Services and Supports.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the US government.
Data Sharing Statement: See Supplement 2 .
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Español (Spanish)
HIV Treatment
What is a drug interaction.
- A drug interaction is a reaction between two (or more) drugs or between a drug and a food, beverage, or supplement. Taking a drug while having certain medical conditions can also cause a drug interaction. For example, taking a nasal decongestant if you have high blood pressure may cause an unwanted reaction.
A drug interaction can affect how a drug works or cause unwanted side effects.
- Treatment with HIV medicines (called antiretroviral therapy or ART ) helps people with HIV live longer, healthier lives and reduces the risk of HIV transmission . But drug interactions can complicate HIV treatment.
- Health care providers carefully consider potential drug interactions before recommending an HIV treatment regimen . Before taking HIV medicines, tell your health care provider about all prescription and nonprescription medicines, vitamins, nutritional supplements, and herbal products you are taking or plan to take.
What is a drug interaction?
Medicines help us feel better and stay healthy. But sometimes drug interactions can cause problems. There are three types of drug interactions:
- Drug-drug interaction : A reaction between two (or more) drugs.
- Drug-food interaction : A reaction between a drug and a food or beverage.
- Drug-condition interaction: A reaction that occurs when taking a drug while having a certain medical condition. For example, taking a nasal decongestant if you have high blood pressure may cause an unwanted reaction.
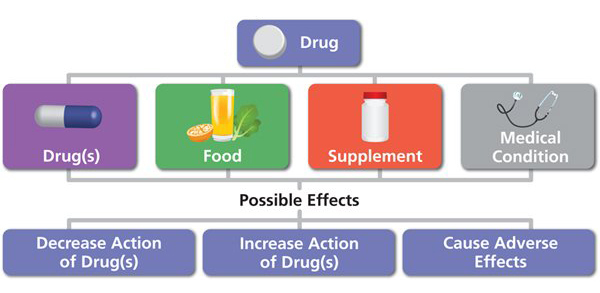
Do HIV medicines ever cause drug interactions?
Treatment with HIV medicines (called antiretroviral therapy or ART ) helps people with HIV live longer, healthier lives and reduces the risk of HIV transmission . But drug interactions, especially drug-drug interactions, can complicate HIV treatment.
Drug-drug interactions between different HIV medicines and between HIV medicines and other medicines are common. Before recommending an HIV treatment regimen , health care providers carefully consider potential drug-drug interactions between HIV medicines. They also ask about other medicines a person may be taking. For example, some HIV medicines may make hormonal birth control less effective, so women using hormonal contraceptives may need to use an additional or different method of birth control to prevent pregnancy. For more information about using birth control and HIV medicines at the same time, view the HIV and Birth Control infographic from HIVinfo.
Can drug-food interactions and drug-condition interactions affect people taking HIV medicines?
Yes, the use of HIV medicines can lead to both drug-food interactions and drug-condition interactions.
Food can affect the absorption of some HIV medicines and increase or reduce the concentration of the medicine in the blood. Depending on the HIV medicine, the change in concentration may be helpful or harmful. Directions on how to take HIV medicines specify whether to take the medicine with food or on an empty stomach. Some HIV medicines can be taken with or without food, because food does not affect their absorption.
Conditions, such as kidney disease, hepatitis, and pregnancy, can affect how the body processes HIV medicines. The dosing of some HIV medicines may need to be adjusted in people with certain medical conditions.
How can a person avoid drug interactions?
You can take the following steps to avoid drug interactions:
- Tell your health care provider about all prescription and nonprescription medicines you are taking or plan to take. Also tell your health care provider about any vitamins, nutritional supplements, and herbal products you take.
- Tell your health care provider about any other conditions you may have, such as high blood pressure or diabetes.
- What is the medicine used for?
- How should I take the medicine?
- While taking the medicine, should I avoid any other medicines or certain foods or beverages?
- Can I take this medicine safely with the other medicines that I am taking? Are there any possible drug interactions I should know about? What are the signs of those drug interactions?
- In the case of a drug interaction, what should I do?
- Take medicines according to your health care provider's instructions. Always read the information and directions that come with a medicine. Drug labels and package inserts include important information about possible drug interactions.
This fact sheet is based on information from the following sources:
From the Department of Health and Human Services:
- Drug-Drug Interactions
- Considerations for Antiretroviral Use in Special Patient Populations: HIV and the Older Person , Transgender People with HIV , and Women with HIV
From the U.S. Food and Drug Administration:
- Drug Interactions: What You Should Know
From the National Institute on Aging:
- Safe Use of Medicines for Older Adults
Also see the HIV Source collection of HIV links and resources.

IMAGES
VIDEO
COMMENTS
Researchers a step closer to a cure for HIV Date: March 26, 2024 Source: University of Western Ontario Summary: A new study shows virus-like particle can effectively 'shock and kill' latent HIV ...
More than 3,600 HIV and infectious disease researchers from 73 countries gathered in Denver and virtually from March 3-6 this year for CROI, an annual scientific meeting on the latest research that can help accelerate global progress in the response to HIV and other infectious diseases, including STIs and viral hepatitis.
Future focus. Investing in innovative strategies that meet the needs of individuals won't just be key to ending the HIV/AIDS pandemic by 2030. It will also help to ensure that global health ...
The search for an HIV cure. Currently, there are approximately 38 million people living with HIV around the world and over half of them receive antiretroviral therapy. Despite the progress made in treatment, in 2021 there were an estimated 1.5 million new cases and 650,000 people died from AIDS-related illnesses worldwide, according to UNAIDS.
If ART is discontinued, HIV will awaken from its dormant state, begin to replicate, and cause acquired immunodeficiency syndrome (AIDS). To create a cure, researchers have been attempting to drive HIV out of latency and target it for destruction. A new clinical trial led by Cynthia Gay, MD, MPH, associate professor of infectious diseases, David ...
An effective and scalable cure strategy is a top priority for the HIV research field; this Review discusses recent advances, knowledge gaps, and priority research areas for the next 5 years.
Emory's Gavegnano Group demonstrates ability to remove key barrier to an HIV cure. The results of a novel study presented by Emory researchers during the International AIDS Society (IAS) Conference in Brisbane, Australia, have revealed exciting findings in the pursuit of an HIV cure. The study, led by Monica Reece, a PhD candidate in Emory ...
Nature Medicine (2023) The development of antiretroviral therapy for the prevention and treatment of HIV infection has been marked by a series of remarkable successes. However, the efforts to ...
Investing in research to find a cure for HIV is focused on two broad aims: sustained viral remission and, in the longer term, viral eradication. Viral latency and sanctuaries. Latent HIV reservoirs—small amounts of HIV that persist in people taking ART—present a significant challenge to finding a cure for HIV. Latent reservoirs remain in ...
Nearly $17 billion was spent worldwide on HIV -vaccine research from 2000 to 2021. Nearly $1 billion more is spent annually, according to the Joint United Nations Program on HIV/AIDS and the ...
BEAUBIEN: Cabotegravir is the first injectable form of HIV pre-exposure prophylaxis, a treatment also known as PrEP. Up until now, PrEP was available only in an oral form in which pills had to be ...
Even with effective treatment, some go into a resting, or latent, state - so they still contain the DNA, or genetic material, of HIV, even if not actively producing new virus. Most people with HIV ...
NIAID. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has launched a Phase 1 clinical trial evaluating three experimental HIV vaccines based on a messenger RNA (mRNA) platform—a technology used in several approved COVID-19 vaccines. NIAID is sponsoring the study, called HVTN 302 ...
The NIH Record. The NIH Record, founded in 1949, is the biweekly newsletter for employees of the National Institutes of Health. Published 25 times each year, it comes out on payday Fridays. Scientists from NIAID's Vaccine Research Center and colleagues used cutting-edge technology to reveal new insights into cellular reservoirs of HIV.
These latest findings represent an ongoing multi-pronged approach in Gilead's HIV cure research program. The data were presented at the Conference on Retroviruses and Opportunistic Infections (CROI) 2023 in Seattle.
New research unveils initial insights into the mechanisms by which stem cell transplantation can eliminate the virus responsible for AIDS. New findings from Oregon Health & Science University help explain how at least five individuals have been cured of HIV following stem cell transplants. This research paves the way toward the potential development of a widespread remedy for the virus that ...
NIH's Dr. Carl Dieffenbach Discusses Highlights of HIV Cure, Treatment and Prevention Research from CROI 2022. 02-17-2022 In an HIV.gov video conversation yesterday, NIH's Dr. Carl Dieffenbach discussed some of the pivotal HIV research advances presented this week at the 2022….
Since it was first identified in 1983, HIV has infected more than 85 million people and caused some 40 million deaths worldwide. While medication known as pre-exposure prophylaxis, or PrEP, can ...
The new drugs are so effective in people who have fully suppressed virus that many only need to use two medications to maintain HIV treatment and control. New research is investigating ways to deliver the medications differently, such as a shot that lasts several months, or maybe someday even implantable medication delivery mechanisms so that ...
Aug. 8, 2023 — Some HIV-1 carriers who have received an early antiretroviral treatment during several years are able to control the virus for a long term after treatment interruption. However ...
While a regular vaccine targets about 1 in 1,000 B cells, a vaccine trying to activate a broad HIV response would have to hit cells as rare as 1 in 1 million or more, said Shane Crotty, a ...
People with HIV are about 14 times more likely to develop TB disease, have poorer TB treatment outcomes and have three-fold higher mortality during TB treatment compared to people without HIV. Despite advances in the screening, diagnosis, treatment and prevention of TB disease, TB remains the leading cause of death among people with HIV worldwide.
According to CDC's latest estimates, annual HIV infections dropped from 9,300 in 2017 to 6,100 in 2021 among 13- to 24-year-olds. Declines among young gay and bisexual males (who account for roughly 80% of new infections in this age group) drove the trend, falling from an estimated 7,400 infections to about 4,900 during the timeframe.
Advances in care over the last 30 years have helped transform HIV from a fatal disease into a chronic, manageable condition for many people. 1 Innovations in treatment and access are at the core ...
HIV infections among people who inject drugs (PWID) In 2021, PWID accounted for 8% (2,500) of the 32,100 estimated new HIV infections. Men who inject drugs accounted for 4% (1,400) of estimated new HIV infections. Women who inject drugs accounted for 3% (1,100) of estimated new HIV infections.
HIV/AIDS treatment and research information from the US federal government. Skip to main content Menu 1-800-448-0440 (1 p.m. to 4 p.m. ET) [email protected] ... HIV/AIDS and opportunistic infection drugs and investigational HIV/AIDS drugs. Drugs . Services Locator . GLOSSARY Medical dictionary of HIV-related terms. Glossary . Connect with us ...
About This Study "Likelihood of trying long-acting injectable antiretroviral therapy (LAI ART) among women with HIV in nine sites across the United States" was published online on April 12, 2024, in Journal of Acquired Immune Deficiency Syndromes.The lead author is Tara McCrimmon, M.P.H., M.I.A., of the Department of Sociomedical Sciences at Columbia University Mailman School of Public Health ...
Epidemiological studies have shown that compared with populations without HIV, people living with HIV have a higher risk of coronary artery disease, acute coronary syndromes (ACS), and heart failure, with onset at younger ages. 4,9-12 Given this earlier emergence of CVD among people living with HIV, there has been significant attention and ...
Treatment with HIV medicines (called antiretroviral therapy or ART) helps people with HIV live longer, healthier lives and reduces the risk of HIV transmission.But drug interactions, especially drug-drug interactions, can complicate HIV treatment. Drug-drug interactions between different HIV medicines and between HIV medicines and other medicines are common.