Writing an Introduction for a Scientific Paper
Dr. michelle harris, dr. janet batzli, biocore.
This section provides guidelines on how to construct a solid introduction to a scientific paper including background information, study question , biological rationale, hypothesis , and general approach . If the Introduction is done well, there should be no question in the reader’s mind why and on what basis you have posed a specific hypothesis.
Broad Question : based on an initial observation (e.g., “I see a lot of guppies close to the shore. Do guppies like living in shallow water?”). This observation of the natural world may inspire you to investigate background literature or your observation could be based on previous research by others or your own pilot study. Broad questions are not always included in your written text, but are essential for establishing the direction of your research.
Background Information : key issues, concepts, terminology, and definitions needed to understand the biological rationale for the experiment. It often includes a summary of findings from previous, relevant studies. Remember to cite references, be concise, and only include relevant information given your audience and your experimental design. Concisely summarized background information leads to the identification of specific scientific knowledge gaps that still exist. (e.g., “No studies to date have examined whether guppies do indeed spend more time in shallow water.”)
Testable Question : these questions are much more focused than the initial broad question, are specific to the knowledge gap identified, and can be addressed with data. (e.g., “Do guppies spend different amounts of time in water <1 meter deep as compared to their time in water that is >1 meter deep?”)
Biological Rationale : describes the purpose of your experiment distilling what is known and what is not known that defines the knowledge gap that you are addressing. The “BR” provides the logic for your hypothesis and experimental approach, describing the biological mechanism and assumptions that explain why your hypothesis should be true.
The biological rationale is based on your interpretation of the scientific literature, your personal observations, and the underlying assumptions you are making about how you think the system works. If you have written your biological rationale, your reader should see your hypothesis in your introduction section and say to themselves, “Of course, this hypothesis seems very logical based on the rationale presented.”
- A thorough rationale defines your assumptions about the system that have not been revealed in scientific literature or from previous systematic observation. These assumptions drive the direction of your specific hypothesis or general predictions.
- Defining the rationale is probably the most critical task for a writer, as it tells your reader why your research is biologically meaningful. It may help to think about the rationale as an answer to the questions— how is this investigation related to what we know, what assumptions am I making about what we don’t yet know, AND how will this experiment add to our knowledge? *There may or may not be broader implications for your study; be careful not to overstate these (see note on social justifications below).
- Expect to spend time and mental effort on this. You may have to do considerable digging into the scientific literature to define how your experiment fits into what is already known and why it is relevant to pursue.
- Be open to the possibility that as you work with and think about your data, you may develop a deeper, more accurate understanding of the experimental system. You may find the original rationale needs to be revised to reflect your new, more sophisticated understanding.
- As you progress through Biocore and upper level biology courses, your rationale should become more focused and matched with the level of study e ., cellular, biochemical, or physiological mechanisms that underlie the rationale. Achieving this type of understanding takes effort, but it will lead to better communication of your science.
***Special note on avoiding social justifications: You should not overemphasize the relevance of your experiment and the possible connections to large-scale processes. Be realistic and logical —do not overgeneralize or state grand implications that are not sensible given the structure of your experimental system. Not all science is easily applied to improving the human condition. Performing an investigation just for the sake of adding to our scientific knowledge (“pure or basic science”) is just as important as applied science. In fact, basic science often provides the foundation for applied studies.
Hypothesis / Predictions : specific prediction(s) that you will test during your experiment. For manipulative experiments, the hypothesis should include the independent variable (what you manipulate), the dependent variable(s) (what you measure), the organism or system , the direction of your results, and comparison to be made.
If you are doing a systematic observation , your hypothesis presents a variable or set of variables that you predict are important for helping you characterize the system as a whole, or predict differences between components/areas of the system that help you explain how the system functions or changes over time.
Experimental Approach : Briefly gives the reader a general sense of the experiment, the type of data it will yield, and the kind of conclusions you expect to obtain from the data. Do not confuse the experimental approach with the experimental protocol . The experimental protocol consists of the detailed step-by-step procedures and techniques used during the experiment that are to be reported in the Methods and Materials section.
Some Final Tips on Writing an Introduction
- As you progress through the Biocore sequence, for instance, from organismal level of Biocore 301/302 to the cellular level in Biocore 303/304, we expect the contents of your “Introduction” paragraphs to reflect the level of your coursework and previous writing experience. For example, in Biocore 304 (Cell Biology Lab) biological rationale should draw upon assumptions we are making about cellular and biochemical processes.
- Be Concise yet Specific: Remember to be concise and only include relevant information given your audience and your experimental design. As you write, keep asking, “Is this necessary information or is this irrelevant detail?” For example, if you are writing a paper claiming that a certain compound is a competitive inhibitor to the enzyme alkaline phosphatase and acts by binding to the active site, you need to explain (briefly) Michaelis-Menton kinetics and the meaning and significance of Km and Vmax. This explanation is not necessary if you are reporting the dependence of enzyme activity on pH because you do not need to measure Km and Vmax to get an estimate of enzyme activity.
- Another example: if you are writing a paper reporting an increase in Daphnia magna heart rate upon exposure to caffeine you need not describe the reproductive cycle of magna unless it is germane to your results and discussion. Be specific and concrete, especially when making introductory or summary statements.
Where Do You Discuss Pilot Studies? Many times it is important to do pilot studies to help you get familiar with your experimental system or to improve your experimental design. If your pilot study influences your biological rationale or hypothesis, you need to describe it in your Introduction. If your pilot study simply informs the logistics or techniques, but does not influence your rationale, then the description of your pilot study belongs in the Materials and Methods section.
How will introductions be evaluated? The following is part of the rubric we will be using to evaluate your papers.
Get science-backed answers as you write with Paperpal's Research feature

How to Write a Research Paper Introduction (with Examples)

The research paper introduction section, along with the Title and Abstract, can be considered the face of any research paper. The following article is intended to guide you in organizing and writing the research paper introduction for a quality academic article or dissertation.
The research paper introduction aims to present the topic to the reader. A study will only be accepted for publishing if you can ascertain that the available literature cannot answer your research question. So it is important to ensure that you have read important studies on that particular topic, especially those within the last five to ten years, and that they are properly referenced in this section. 1 What should be included in the research paper introduction is decided by what you want to tell readers about the reason behind the research and how you plan to fill the knowledge gap. The best research paper introduction provides a systemic review of existing work and demonstrates additional work that needs to be done. It needs to be brief, captivating, and well-referenced; a well-drafted research paper introduction will help the researcher win half the battle.
The introduction for a research paper is where you set up your topic and approach for the reader. It has several key goals:
- Present your research topic
- Capture reader interest
- Summarize existing research
- Position your own approach
- Define your specific research problem and problem statement
- Highlight the novelty and contributions of the study
- Give an overview of the paper’s structure
The research paper introduction can vary in size and structure depending on whether your paper presents the results of original empirical research or is a review paper. Some research paper introduction examples are only half a page while others are a few pages long. In many cases, the introduction will be shorter than all of the other sections of your paper; its length depends on the size of your paper as a whole.
- Break through writer’s block. Write your research paper introduction with Paperpal Copilot
Table of Contents
What is the introduction for a research paper, why is the introduction important in a research paper, craft a compelling introduction section with paperpal. try now, 1. introduce the research topic:, 2. determine a research niche:, 3. place your research within the research niche:, craft accurate research paper introductions with paperpal. start writing now, frequently asked questions on research paper introduction, key points to remember.
The introduction in a research paper is placed at the beginning to guide the reader from a broad subject area to the specific topic that your research addresses. They present the following information to the reader
- Scope: The topic covered in the research paper
- Context: Background of your topic
- Importance: Why your research matters in that particular area of research and the industry problem that can be targeted
The research paper introduction conveys a lot of information and can be considered an essential roadmap for the rest of your paper. A good introduction for a research paper is important for the following reasons:
- It stimulates your reader’s interest: A good introduction section can make your readers want to read your paper by capturing their interest. It informs the reader what they are going to learn and helps determine if the topic is of interest to them.
- It helps the reader understand the research background: Without a clear introduction, your readers may feel confused and even struggle when reading your paper. A good research paper introduction will prepare them for the in-depth research to come. It provides you the opportunity to engage with the readers and demonstrate your knowledge and authority on the specific topic.
- It explains why your research paper is worth reading: Your introduction can convey a lot of information to your readers. It introduces the topic, why the topic is important, and how you plan to proceed with your research.
- It helps guide the reader through the rest of the paper: The research paper introduction gives the reader a sense of the nature of the information that will support your arguments and the general organization of the paragraphs that will follow. It offers an overview of what to expect when reading the main body of your paper.
What are the parts of introduction in the research?
A good research paper introduction section should comprise three main elements: 2
- What is known: This sets the stage for your research. It informs the readers of what is known on the subject.
- What is lacking: This is aimed at justifying the reason for carrying out your research. This could involve investigating a new concept or method or building upon previous research.
- What you aim to do: This part briefly states the objectives of your research and its major contributions. Your detailed hypothesis will also form a part of this section.
How to write a research paper introduction?
The first step in writing the research paper introduction is to inform the reader what your topic is and why it’s interesting or important. This is generally accomplished with a strong opening statement. The second step involves establishing the kinds of research that have been done and ending with limitations or gaps in the research that you intend to address. Finally, the research paper introduction clarifies how your own research fits in and what problem it addresses. If your research involved testing hypotheses, these should be stated along with your research question. The hypothesis should be presented in the past tense since it will have been tested by the time you are writing the research paper introduction.
The following key points, with examples, can guide you when writing the research paper introduction section:
- Highlight the importance of the research field or topic
- Describe the background of the topic
- Present an overview of current research on the topic
Example: The inclusion of experiential and competency-based learning has benefitted electronics engineering education. Industry partnerships provide an excellent alternative for students wanting to engage in solving real-world challenges. Industry-academia participation has grown in recent years due to the need for skilled engineers with practical training and specialized expertise. However, from the educational perspective, many activities are needed to incorporate sustainable development goals into the university curricula and consolidate learning innovation in universities.
- Reveal a gap in existing research or oppose an existing assumption
- Formulate the research question
Example: There have been plausible efforts to integrate educational activities in higher education electronics engineering programs. However, very few studies have considered using educational research methods for performance evaluation of competency-based higher engineering education, with a focus on technical and or transversal skills. To remedy the current need for evaluating competencies in STEM fields and providing sustainable development goals in engineering education, in this study, a comparison was drawn between study groups without and with industry partners.
- State the purpose of your study
- Highlight the key characteristics of your study
- Describe important results
- Highlight the novelty of the study.
- Offer a brief overview of the structure of the paper.
Example: The study evaluates the main competency needed in the applied electronics course, which is a fundamental core subject for many electronics engineering undergraduate programs. We compared two groups, without and with an industrial partner, that offered real-world projects to solve during the semester. This comparison can help determine significant differences in both groups in terms of developing subject competency and achieving sustainable development goals.
Write a Research Paper Introduction in Minutes with Paperpal
Paperpal Copilot is a generative AI-powered academic writing assistant. It’s trained on millions of published scholarly articles and over 20 years of STM experience. Paperpal Copilot helps authors write better and faster with:
- Real-time writing suggestions
- In-depth checks for language and grammar correction
- Paraphrasing to add variety, ensure academic tone, and trim text to meet journal limits
With Paperpal Copilot, create a research paper introduction effortlessly. In this step-by-step guide, we’ll walk you through how Paperpal transforms your initial ideas into a polished and publication-ready introduction.
How to use Paperpal to write the Introduction section
Step 1: Sign up on Paperpal and click on the Copilot feature, under this choose Outlines > Research Article > Introduction
Step 2: Add your unstructured notes or initial draft, whether in English or another language, to Paperpal, which is to be used as the base for your content.
Step 3: Fill in the specifics, such as your field of study, brief description or details you want to include, which will help the AI generate the outline for your Introduction.
Step 4: Use this outline and sentence suggestions to develop your content, adding citations where needed and modifying it to align with your specific research focus.
Step 5: Turn to Paperpal’s granular language checks to refine your content, tailor it to reflect your personal writing style, and ensure it effectively conveys your message.
You can use the same process to develop each section of your article, and finally your research paper in half the time and without any of the stress.
The purpose of the research paper introduction is to introduce the reader to the problem definition, justify the need for the study, and describe the main theme of the study. The aim is to gain the reader’s attention by providing them with necessary background information and establishing the main purpose and direction of the research.
The length of the research paper introduction can vary across journals and disciplines. While there are no strict word limits for writing the research paper introduction, an ideal length would be one page, with a maximum of 400 words over 1-4 paragraphs. Generally, it is one of the shorter sections of the paper as the reader is assumed to have at least a reasonable knowledge about the topic. 2 For example, for a study evaluating the role of building design in ensuring fire safety, there is no need to discuss definitions and nature of fire in the introduction; you could start by commenting upon the existing practices for fire safety and how your study will add to the existing knowledge and practice.
When deciding what to include in the research paper introduction, the rest of the paper should also be considered. The aim is to introduce the reader smoothly to the topic and facilitate an easy read without much dependency on external sources. 3 Below is a list of elements you can include to prepare a research paper introduction outline and follow it when you are writing the research paper introduction. Topic introduction: This can include key definitions and a brief history of the topic. Research context and background: Offer the readers some general information and then narrow it down to specific aspects. Details of the research you conducted: A brief literature review can be included to support your arguments or line of thought. Rationale for the study: This establishes the relevance of your study and establishes its importance. Importance of your research: The main contributions are highlighted to help establish the novelty of your study Research hypothesis: Introduce your research question and propose an expected outcome. Organization of the paper: Include a short paragraph of 3-4 sentences that highlights your plan for the entire paper
Cite only works that are most relevant to your topic; as a general rule, you can include one to three. Note that readers want to see evidence of original thinking. So it is better to avoid using too many references as it does not leave much room for your personal standpoint to shine through. Citations in your research paper introduction support the key points, and the number of citations depend on the subject matter and the point discussed. If the research paper introduction is too long or overflowing with citations, it is better to cite a few review articles rather than the individual articles summarized in the review. A good point to remember when citing research papers in the introduction section is to include at least one-third of the references in the introduction.
The literature review plays a significant role in the research paper introduction section. A good literature review accomplishes the following: Introduces the topic – Establishes the study’s significance – Provides an overview of the relevant literature – Provides context for the study using literature – Identifies knowledge gaps However, remember to avoid making the following mistakes when writing a research paper introduction: Do not use studies from the literature review to aggressively support your research Avoid direct quoting Do not allow literature review to be the focus of this section. Instead, the literature review should only aid in setting a foundation for the manuscript.
Remember the following key points for writing a good research paper introduction: 4
- Avoid stuffing too much general information: Avoid including what an average reader would know and include only that information related to the problem being addressed in the research paper introduction. For example, when describing a comparative study of non-traditional methods for mechanical design optimization, information related to the traditional methods and differences between traditional and non-traditional methods would not be relevant. In this case, the introduction for the research paper should begin with the state-of-the-art non-traditional methods and methods to evaluate the efficiency of newly developed algorithms.
- Avoid packing too many references: Cite only the required works in your research paper introduction. The other works can be included in the discussion section to strengthen your findings.
- Avoid extensive criticism of previous studies: Avoid being overly critical of earlier studies while setting the rationale for your study. A better place for this would be the Discussion section, where you can highlight the advantages of your method.
- Avoid describing conclusions of the study: When writing a research paper introduction remember not to include the findings of your study. The aim is to let the readers know what question is being answered. The actual answer should only be given in the Results and Discussion section.
To summarize, the research paper introduction section should be brief yet informative. It should convince the reader the need to conduct the study and motivate him to read further. If you’re feeling stuck or unsure, choose trusted AI academic writing assistants like Paperpal to effortlessly craft your research paper introduction and other sections of your research article.
1. Jawaid, S. A., & Jawaid, M. (2019). How to write introduction and discussion. Saudi Journal of Anaesthesia, 13(Suppl 1), S18.
2. Dewan, P., & Gupta, P. (2016). Writing the title, abstract and introduction: Looks matter!. Indian pediatrics, 53, 235-241.
3. Cetin, S., & Hackam, D. J. (2005). An approach to the writing of a scientific Manuscript1. Journal of Surgical Research, 128(2), 165-167.
4. Bavdekar, S. B. (2015). Writing introduction: Laying the foundations of a research paper. Journal of the Association of Physicians of India, 63(7), 44-6.
Paperpal is a comprehensive AI writing toolkit that helps students and researchers achieve 2x the writing in half the time. It leverages 21+ years of STM experience and insights from millions of research articles to provide in-depth academic writing, language editing, and submission readiness support to help you write better, faster.
Get accurate academic translations, rewriting support, grammar checks, vocabulary suggestions, and generative AI assistance that delivers human precision at machine speed. Try for free or upgrade to Paperpal Prime starting at US$19 a month to access premium features, including consistency, plagiarism, and 30+ submission readiness checks to help you succeed.
Experience the future of academic writing – Sign up to Paperpal and start writing for free!
Related Reads:
- Scientific Writing Style Guides Explained
- 5 Reasons for Rejection After Peer Review
- Ethical Research Practices For Research with Human Subjects
- 8 Most Effective Ways to Increase Motivation for Thesis Writing
Practice vs. Practise: Learn the Difference
Academic paraphrasing: why paperpal’s rewrite should be your first choice , you may also like, how to write a high-quality conference paper, academic editing: how to self-edit academic text with..., measuring academic success: definition & strategies for excellence, phd qualifying exam: tips for success , ai in education: it’s time to change the..., is it ethical to use ai-generated abstracts without..., what are journal guidelines on using generative ai..., quillbot review: features, pricing, and free alternatives, what is an academic paper types and elements , should you use ai tools like chatgpt for....

- Langson Library
- Science Library
- Grunigen Medical Library
- Law Library
- Connect From Off-Campus
- Accessibility
- Gateway Study Center

Email this link
Writing a scientific paper.
- Writing a lab report
What is a "good" introduction?
Citing sources in the introduction, "introduction checklist" from: how to write a good scientific paper. chris a. mack. spie. 2018..
- LITERATURE CITED
- Bibliography of guides to scientific writing and presenting
- Peer Review
- Presentations
- Lab Report Writing Guides on the Web
This is where you describe briefly and clearly why you are writing the paper. The introduction supplies sufficient background information for the reader to understand and evaluate the experiment you did. It also supplies a rationale for the study.
- Present the problem and the proposed solution
- Presents nature and scope of the problem investigated
- Reviews the pertinent literature to orient the reader
- States the method of the experiment
- State the principle results of the experiment
It is important to cite sources in the introduction section of your paper as evidence of the claims you are making. There are ways of citing sources in the text so that the reader can find the full reference in the literature cited section at the end of the paper, yet the flow of the reading is not badly interrupted. Below are some example of how this can be done: "Smith (1983) found that N-fixing plants could be infected by several different species of Rhizobium." "Walnut trees are known to be allelopathic (Smith 1949, Bond et al. 1955, Jones and Green 1963)." "Although the presence of Rhizobium normally increases the growth of legumes (Nguyen 1987), the opposite effect has been observed (Washington 1999)." Note that articles by one or two authors are always cited in the text using their last names. However, if there are more than two authors, the last name of the 1st author is given followed by the abbreviation et al. which is Latin for "and others".
From: https://writingcenter.gmu.edu/guides/imrad-reports-introductions
- Indicate the field of the work, why this field is important, and what has already been done (with proper citations).
- Indicate a gap, raise a research question, or challenge prior work in this territory.
- Outline the purpose and announce the present research, clearly indicating what is novel and why it is significant.
- Avoid: repeating the abstract; providing unnecessary background information; exaggerating the importance of the work; claiming novelty without a proper literature search.
- << Previous: ABSTRACT
- Next: METHODS >>
- Last Updated: Aug 4, 2023 9:33 AM
- URL: https://guides.lib.uci.edu/scientificwriting
Off-campus? Please use the Software VPN and choose the group UCIFull to access licensed content. For more information, please Click here
Software VPN is not available for guests, so they may not have access to some content when connecting from off-campus.

Scientific Reports
What this handout is about.
This handout provides a general guide to writing reports about scientific research you’ve performed. In addition to describing the conventional rules about the format and content of a lab report, we’ll also attempt to convey why these rules exist, so you’ll get a clearer, more dependable idea of how to approach this writing situation. Readers of this handout may also find our handout on writing in the sciences useful.
Background and pre-writing
Why do we write research reports.
You did an experiment or study for your science class, and now you have to write it up for your teacher to review. You feel that you understood the background sufficiently, designed and completed the study effectively, obtained useful data, and can use those data to draw conclusions about a scientific process or principle. But how exactly do you write all that? What is your teacher expecting to see?
To take some of the guesswork out of answering these questions, try to think beyond the classroom setting. In fact, you and your teacher are both part of a scientific community, and the people who participate in this community tend to share the same values. As long as you understand and respect these values, your writing will likely meet the expectations of your audience—including your teacher.
So why are you writing this research report? The practical answer is “Because the teacher assigned it,” but that’s classroom thinking. Generally speaking, people investigating some scientific hypothesis have a responsibility to the rest of the scientific world to report their findings, particularly if these findings add to or contradict previous ideas. The people reading such reports have two primary goals:
- They want to gather the information presented.
- They want to know that the findings are legitimate.
Your job as a writer, then, is to fulfill these two goals.
How do I do that?
Good question. Here is the basic format scientists have designed for research reports:
- Introduction
Methods and Materials
This format, sometimes called “IMRAD,” may take slightly different shapes depending on the discipline or audience; some ask you to include an abstract or separate section for the hypothesis, or call the Discussion section “Conclusions,” or change the order of the sections (some professional and academic journals require the Methods section to appear last). Overall, however, the IMRAD format was devised to represent a textual version of the scientific method.
The scientific method, you’ll probably recall, involves developing a hypothesis, testing it, and deciding whether your findings support the hypothesis. In essence, the format for a research report in the sciences mirrors the scientific method but fleshes out the process a little. Below, you’ll find a table that shows how each written section fits into the scientific method and what additional information it offers the reader.
Thinking of your research report as based on the scientific method, but elaborated in the ways described above, may help you to meet your audience’s expectations successfully. We’re going to proceed by explicitly connecting each section of the lab report to the scientific method, then explaining why and how you need to elaborate that section.
Although this handout takes each section in the order in which it should be presented in the final report, you may for practical reasons decide to compose sections in another order. For example, many writers find that composing their Methods and Results before the other sections helps to clarify their idea of the experiment or study as a whole. You might consider using each assignment to practice different approaches to drafting the report, to find the order that works best for you.
What should I do before drafting the lab report?
The best way to prepare to write the lab report is to make sure that you fully understand everything you need to about the experiment. Obviously, if you don’t quite know what went on during the lab, you’re going to find it difficult to explain the lab satisfactorily to someone else. To make sure you know enough to write the report, complete the following steps:
- What are we going to do in this lab? (That is, what’s the procedure?)
- Why are we going to do it that way?
- What are we hoping to learn from this experiment?
- Why would we benefit from this knowledge?
- Consult your lab supervisor as you perform the lab. If you don’t know how to answer one of the questions above, for example, your lab supervisor will probably be able to explain it to you (or, at least, help you figure it out).
- Plan the steps of the experiment carefully with your lab partners. The less you rush, the more likely it is that you’ll perform the experiment correctly and record your findings accurately. Also, take some time to think about the best way to organize the data before you have to start putting numbers down. If you can design a table to account for the data, that will tend to work much better than jotting results down hurriedly on a scrap piece of paper.
- Record the data carefully so you get them right. You won’t be able to trust your conclusions if you have the wrong data, and your readers will know you messed up if the other three people in your group have “97 degrees” and you have “87.”
- Consult with your lab partners about everything you do. Lab groups often make one of two mistakes: two people do all the work while two have a nice chat, or everybody works together until the group finishes gathering the raw data, then scrams outta there. Collaborate with your partners, even when the experiment is “over.” What trends did you observe? Was the hypothesis supported? Did you all get the same results? What kind of figure should you use to represent your findings? The whole group can work together to answer these questions.
- Consider your audience. You may believe that audience is a non-issue: it’s your lab TA, right? Well, yes—but again, think beyond the classroom. If you write with only your lab instructor in mind, you may omit material that is crucial to a complete understanding of your experiment, because you assume the instructor knows all that stuff already. As a result, you may receive a lower grade, since your TA won’t be sure that you understand all the principles at work. Try to write towards a student in the same course but a different lab section. That student will have a fair degree of scientific expertise but won’t know much about your experiment particularly. Alternatively, you could envision yourself five years from now, after the reading and lectures for this course have faded a bit. What would you remember, and what would you need explained more clearly (as a refresher)?
Once you’ve completed these steps as you perform the experiment, you’ll be in a good position to draft an effective lab report.
Introductions
How do i write a strong introduction.
For the purposes of this handout, we’ll consider the Introduction to contain four basic elements: the purpose, the scientific literature relevant to the subject, the hypothesis, and the reasons you believed your hypothesis viable. Let’s start by going through each element of the Introduction to clarify what it covers and why it’s important. Then we can formulate a logical organizational strategy for the section.
The inclusion of the purpose (sometimes called the objective) of the experiment often confuses writers. The biggest misconception is that the purpose is the same as the hypothesis. Not quite. We’ll get to hypotheses in a minute, but basically they provide some indication of what you expect the experiment to show. The purpose is broader, and deals more with what you expect to gain through the experiment. In a professional setting, the hypothesis might have something to do with how cells react to a certain kind of genetic manipulation, but the purpose of the experiment is to learn more about potential cancer treatments. Undergraduate reports don’t often have this wide-ranging a goal, but you should still try to maintain the distinction between your hypothesis and your purpose. In a solubility experiment, for example, your hypothesis might talk about the relationship between temperature and the rate of solubility, but the purpose is probably to learn more about some specific scientific principle underlying the process of solubility.
For starters, most people say that you should write out your working hypothesis before you perform the experiment or study. Many beginning science students neglect to do so and find themselves struggling to remember precisely which variables were involved in the process or in what way the researchers felt that they were related. Write your hypothesis down as you develop it—you’ll be glad you did.
As for the form a hypothesis should take, it’s best not to be too fancy or complicated; an inventive style isn’t nearly so important as clarity here. There’s nothing wrong with beginning your hypothesis with the phrase, “It was hypothesized that . . .” Be as specific as you can about the relationship between the different objects of your study. In other words, explain that when term A changes, term B changes in this particular way. Readers of scientific writing are rarely content with the idea that a relationship between two terms exists—they want to know what that relationship entails.
Not a hypothesis:
“It was hypothesized that there is a significant relationship between the temperature of a solvent and the rate at which a solute dissolves.”
Hypothesis:
“It was hypothesized that as the temperature of a solvent increases, the rate at which a solute will dissolve in that solvent increases.”
Put more technically, most hypotheses contain both an independent and a dependent variable. The independent variable is what you manipulate to test the reaction; the dependent variable is what changes as a result of your manipulation. In the example above, the independent variable is the temperature of the solvent, and the dependent variable is the rate of solubility. Be sure that your hypothesis includes both variables.
Justify your hypothesis
You need to do more than tell your readers what your hypothesis is; you also need to assure them that this hypothesis was reasonable, given the circumstances. In other words, use the Introduction to explain that you didn’t just pluck your hypothesis out of thin air. (If you did pluck it out of thin air, your problems with your report will probably extend beyond using the appropriate format.) If you posit that a particular relationship exists between the independent and the dependent variable, what led you to believe your “guess” might be supported by evidence?
Scientists often refer to this type of justification as “motivating” the hypothesis, in the sense that something propelled them to make that prediction. Often, motivation includes what we already know—or rather, what scientists generally accept as true (see “Background/previous research” below). But you can also motivate your hypothesis by relying on logic or on your own observations. If you’re trying to decide which solutes will dissolve more rapidly in a solvent at increased temperatures, you might remember that some solids are meant to dissolve in hot water (e.g., bouillon cubes) and some are used for a function precisely because they withstand higher temperatures (they make saucepans out of something). Or you can think about whether you’ve noticed sugar dissolving more rapidly in your glass of iced tea or in your cup of coffee. Even such basic, outside-the-lab observations can help you justify your hypothesis as reasonable.
Background/previous research
This part of the Introduction demonstrates to the reader your awareness of how you’re building on other scientists’ work. If you think of the scientific community as engaging in a series of conversations about various topics, then you’ll recognize that the relevant background material will alert the reader to which conversation you want to enter.
Generally speaking, authors writing journal articles use the background for slightly different purposes than do students completing assignments. Because readers of academic journals tend to be professionals in the field, authors explain the background in order to permit readers to evaluate the study’s pertinence for their own work. You, on the other hand, write toward a much narrower audience—your peers in the course or your lab instructor—and so you must demonstrate that you understand the context for the (presumably assigned) experiment or study you’ve completed. For example, if your professor has been talking about polarity during lectures, and you’re doing a solubility experiment, you might try to connect the polarity of a solid to its relative solubility in certain solvents. In any event, both professional researchers and undergraduates need to connect the background material overtly to their own work.
Organization of this section
Most of the time, writers begin by stating the purpose or objectives of their own work, which establishes for the reader’s benefit the “nature and scope of the problem investigated” (Day 1994). Once you have expressed your purpose, you should then find it easier to move from the general purpose, to relevant material on the subject, to your hypothesis. In abbreviated form, an Introduction section might look like this:
“The purpose of the experiment was to test conventional ideas about solubility in the laboratory [purpose] . . . According to Whitecoat and Labrat (1999), at higher temperatures the molecules of solvents move more quickly . . . We know from the class lecture that molecules moving at higher rates of speed collide with one another more often and thus break down more easily [background material/motivation] . . . Thus, it was hypothesized that as the temperature of a solvent increases, the rate at which a solute will dissolve in that solvent increases [hypothesis].”
Again—these are guidelines, not commandments. Some writers and readers prefer different structures for the Introduction. The one above merely illustrates a common approach to organizing material.
How do I write a strong Materials and Methods section?
As with any piece of writing, your Methods section will succeed only if it fulfills its readers’ expectations, so you need to be clear in your own mind about the purpose of this section. Let’s review the purpose as we described it above: in this section, you want to describe in detail how you tested the hypothesis you developed and also to clarify the rationale for your procedure. In science, it’s not sufficient merely to design and carry out an experiment. Ultimately, others must be able to verify your findings, so your experiment must be reproducible, to the extent that other researchers can follow the same procedure and obtain the same (or similar) results.
Here’s a real-world example of the importance of reproducibility. In 1989, physicists Stanley Pons and Martin Fleischman announced that they had discovered “cold fusion,” a way of producing excess heat and power without the nuclear radiation that accompanies “hot fusion.” Such a discovery could have great ramifications for the industrial production of energy, so these findings created a great deal of interest. When other scientists tried to duplicate the experiment, however, they didn’t achieve the same results, and as a result many wrote off the conclusions as unjustified (or worse, a hoax). To this day, the viability of cold fusion is debated within the scientific community, even though an increasing number of researchers believe it possible. So when you write your Methods section, keep in mind that you need to describe your experiment well enough to allow others to replicate it exactly.
With these goals in mind, let’s consider how to write an effective Methods section in terms of content, structure, and style.
Sometimes the hardest thing about writing this section isn’t what you should talk about, but what you shouldn’t talk about. Writers often want to include the results of their experiment, because they measured and recorded the results during the course of the experiment. But such data should be reserved for the Results section. In the Methods section, you can write that you recorded the results, or how you recorded the results (e.g., in a table), but you shouldn’t write what the results were—not yet. Here, you’re merely stating exactly how you went about testing your hypothesis. As you draft your Methods section, ask yourself the following questions:
- How much detail? Be precise in providing details, but stay relevant. Ask yourself, “Would it make any difference if this piece were a different size or made from a different material?” If not, you probably don’t need to get too specific. If so, you should give as many details as necessary to prevent this experiment from going awry if someone else tries to carry it out. Probably the most crucial detail is measurement; you should always quantify anything you can, such as time elapsed, temperature, mass, volume, etc.
- Rationale: Be sure that as you’re relating your actions during the experiment, you explain your rationale for the protocol you developed. If you capped a test tube immediately after adding a solute to a solvent, why did you do that? (That’s really two questions: why did you cap it, and why did you cap it immediately?) In a professional setting, writers provide their rationale as a way to explain their thinking to potential critics. On one hand, of course, that’s your motivation for talking about protocol, too. On the other hand, since in practical terms you’re also writing to your teacher (who’s seeking to evaluate how well you comprehend the principles of the experiment), explaining the rationale indicates that you understand the reasons for conducting the experiment in that way, and that you’re not just following orders. Critical thinking is crucial—robots don’t make good scientists.
- Control: Most experiments will include a control, which is a means of comparing experimental results. (Sometimes you’ll need to have more than one control, depending on the number of hypotheses you want to test.) The control is exactly the same as the other items you’re testing, except that you don’t manipulate the independent variable-the condition you’re altering to check the effect on the dependent variable. For example, if you’re testing solubility rates at increased temperatures, your control would be a solution that you didn’t heat at all; that way, you’ll see how quickly the solute dissolves “naturally” (i.e., without manipulation), and you’ll have a point of reference against which to compare the solutions you did heat.
Describe the control in the Methods section. Two things are especially important in writing about the control: identify the control as a control, and explain what you’re controlling for. Here is an example:
“As a control for the temperature change, we placed the same amount of solute in the same amount of solvent, and let the solution stand for five minutes without heating it.”
Structure and style
Organization is especially important in the Methods section of a lab report because readers must understand your experimental procedure completely. Many writers are surprised by the difficulty of conveying what they did during the experiment, since after all they’re only reporting an event, but it’s often tricky to present this information in a coherent way. There’s a fairly standard structure you can use to guide you, and following the conventions for style can help clarify your points.
- Subsections: Occasionally, researchers use subsections to report their procedure when the following circumstances apply: 1) if they’ve used a great many materials; 2) if the procedure is unusually complicated; 3) if they’ve developed a procedure that won’t be familiar to many of their readers. Because these conditions rarely apply to the experiments you’ll perform in class, most undergraduate lab reports won’t require you to use subsections. In fact, many guides to writing lab reports suggest that you try to limit your Methods section to a single paragraph.
- Narrative structure: Think of this section as telling a story about a group of people and the experiment they performed. Describe what you did in the order in which you did it. You may have heard the old joke centered on the line, “Disconnect the red wire, but only after disconnecting the green wire,” where the person reading the directions blows everything to kingdom come because the directions weren’t in order. We’re used to reading about events chronologically, and so your readers will generally understand what you did if you present that information in the same way. Also, since the Methods section does generally appear as a narrative (story), you want to avoid the “recipe” approach: “First, take a clean, dry 100 ml test tube from the rack. Next, add 50 ml of distilled water.” You should be reporting what did happen, not telling the reader how to perform the experiment: “50 ml of distilled water was poured into a clean, dry 100 ml test tube.” Hint: most of the time, the recipe approach comes from copying down the steps of the procedure from your lab manual, so you may want to draft the Methods section initially without consulting your manual. Later, of course, you can go back and fill in any part of the procedure you inadvertently overlooked.
- Past tense: Remember that you’re describing what happened, so you should use past tense to refer to everything you did during the experiment. Writers are often tempted to use the imperative (“Add 5 g of the solid to the solution”) because that’s how their lab manuals are worded; less frequently, they use present tense (“5 g of the solid are added to the solution”). Instead, remember that you’re talking about an event which happened at a particular time in the past, and which has already ended by the time you start writing, so simple past tense will be appropriate in this section (“5 g of the solid were added to the solution” or “We added 5 g of the solid to the solution”).
- Active: We heated the solution to 80°C. (The subject, “we,” performs the action, heating.)
- Passive: The solution was heated to 80°C. (The subject, “solution,” doesn’t do the heating–it is acted upon, not acting.)
Increasingly, especially in the social sciences, using first person and active voice is acceptable in scientific reports. Most readers find that this style of writing conveys information more clearly and concisely. This rhetorical choice thus brings two scientific values into conflict: objectivity versus clarity. Since the scientific community hasn’t reached a consensus about which style it prefers, you may want to ask your lab instructor.
How do I write a strong Results section?
Here’s a paradox for you. The Results section is often both the shortest (yay!) and most important (uh-oh!) part of your report. Your Materials and Methods section shows how you obtained the results, and your Discussion section explores the significance of the results, so clearly the Results section forms the backbone of the lab report. This section provides the most critical information about your experiment: the data that allow you to discuss how your hypothesis was or wasn’t supported. But it doesn’t provide anything else, which explains why this section is generally shorter than the others.
Before you write this section, look at all the data you collected to figure out what relates significantly to your hypothesis. You’ll want to highlight this material in your Results section. Resist the urge to include every bit of data you collected, since perhaps not all are relevant. Also, don’t try to draw conclusions about the results—save them for the Discussion section. In this section, you’re reporting facts. Nothing your readers can dispute should appear in the Results section.
Most Results sections feature three distinct parts: text, tables, and figures. Let’s consider each part one at a time.
This should be a short paragraph, generally just a few lines, that describes the results you obtained from your experiment. In a relatively simple experiment, one that doesn’t produce a lot of data for you to repeat, the text can represent the entire Results section. Don’t feel that you need to include lots of extraneous detail to compensate for a short (but effective) text; your readers appreciate discrimination more than your ability to recite facts. In a more complex experiment, you may want to use tables and/or figures to help guide your readers toward the most important information you gathered. In that event, you’ll need to refer to each table or figure directly, where appropriate:
“Table 1 lists the rates of solubility for each substance”
“Solubility increased as the temperature of the solution increased (see Figure 1).”
If you do use tables or figures, make sure that you don’t present the same material in both the text and the tables/figures, since in essence you’ll just repeat yourself, probably annoying your readers with the redundancy of your statements.
Feel free to describe trends that emerge as you examine the data. Although identifying trends requires some judgment on your part and so may not feel like factual reporting, no one can deny that these trends do exist, and so they properly belong in the Results section. Example:
“Heating the solution increased the rate of solubility of polar solids by 45% but had no effect on the rate of solubility in solutions containing non-polar solids.”
This point isn’t debatable—you’re just pointing out what the data show.
As in the Materials and Methods section, you want to refer to your data in the past tense, because the events you recorded have already occurred and have finished occurring. In the example above, note the use of “increased” and “had,” rather than “increases” and “has.” (You don’t know from your experiment that heating always increases the solubility of polar solids, but it did that time.)
You shouldn’t put information in the table that also appears in the text. You also shouldn’t use a table to present irrelevant data, just to show you did collect these data during the experiment. Tables are good for some purposes and situations, but not others, so whether and how you’ll use tables depends upon what you need them to accomplish.
Tables are useful ways to show variation in data, but not to present a great deal of unchanging measurements. If you’re dealing with a scientific phenomenon that occurs only within a certain range of temperatures, for example, you don’t need to use a table to show that the phenomenon didn’t occur at any of the other temperatures. How useful is this table?
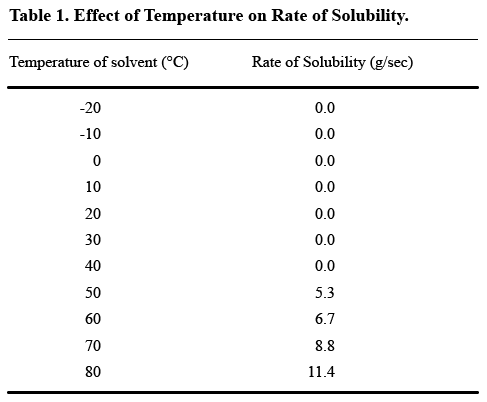
As you can probably see, no solubility was observed until the trial temperature reached 50°C, a fact that the text part of the Results section could easily convey. The table could then be limited to what happened at 50°C and higher, thus better illustrating the differences in solubility rates when solubility did occur.
As a rule, try not to use a table to describe any experimental event you can cover in one sentence of text. Here’s an example of an unnecessary table from How to Write and Publish a Scientific Paper , by Robert A. Day:
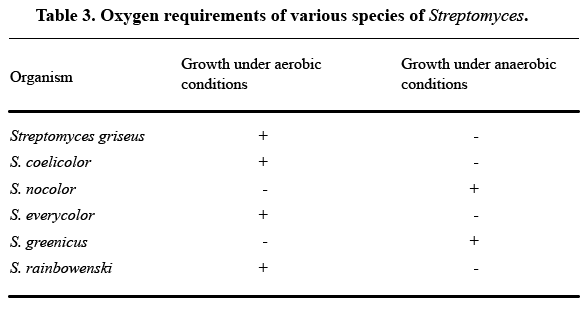
As Day notes, all the information in this table can be summarized in one sentence: “S. griseus, S. coelicolor, S. everycolor, and S. rainbowenski grew under aerobic conditions, whereas S. nocolor and S. greenicus required anaerobic conditions.” Most readers won’t find the table clearer than that one sentence.
When you do have reason to tabulate material, pay attention to the clarity and readability of the format you use. Here are a few tips:
- Number your table. Then, when you refer to the table in the text, use that number to tell your readers which table they can review to clarify the material.
- Give your table a title. This title should be descriptive enough to communicate the contents of the table, but not so long that it becomes difficult to follow. The titles in the sample tables above are acceptable.
- Arrange your table so that readers read vertically, not horizontally. For the most part, this rule means that you should construct your table so that like elements read down, not across. Think about what you want your readers to compare, and put that information in the column (up and down) rather than in the row (across). Usually, the point of comparison will be the numerical data you collect, so especially make sure you have columns of numbers, not rows.Here’s an example of how drastically this decision affects the readability of your table (from A Short Guide to Writing about Chemistry , by Herbert Beall and John Trimbur). Look at this table, which presents the relevant data in horizontal rows:
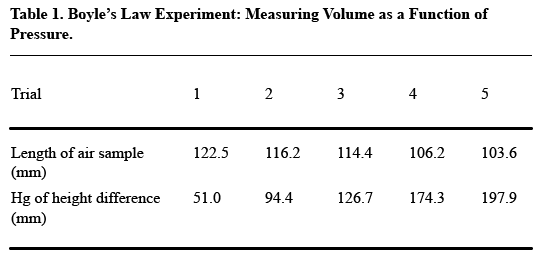
It’s a little tough to see the trends that the author presumably wants to present in this table. Compare this table, in which the data appear vertically:
The second table shows how putting like elements in a vertical column makes for easier reading. In this case, the like elements are the measurements of length and height, over five trials–not, as in the first table, the length and height measurements for each trial.
- Make sure to include units of measurement in the tables. Readers might be able to guess that you measured something in millimeters, but don’t make them try.
- Don’t use vertical lines as part of the format for your table. This convention exists because journals prefer not to have to reproduce these lines because the tables then become more expensive to print. Even though it’s fairly unlikely that you’ll be sending your Biology 11 lab report to Science for publication, your readers still have this expectation. Consequently, if you use the table-drawing option in your word-processing software, choose the option that doesn’t rely on a “grid” format (which includes vertical lines).
How do I include figures in my report?
Although tables can be useful ways of showing trends in the results you obtained, figures (i.e., illustrations) can do an even better job of emphasizing such trends. Lab report writers often use graphic representations of the data they collected to provide their readers with a literal picture of how the experiment went.
When should you use a figure?
Remember the circumstances under which you don’t need a table: when you don’t have a great deal of data or when the data you have don’t vary a lot. Under the same conditions, you would probably forgo the figure as well, since the figure would be unlikely to provide your readers with an additional perspective. Scientists really don’t like their time wasted, so they tend not to respond favorably to redundancy.
If you’re trying to decide between using a table and creating a figure to present your material, consider the following a rule of thumb. The strength of a table lies in its ability to supply large amounts of exact data, whereas the strength of a figure is its dramatic illustration of important trends within the experiment. If you feel that your readers won’t get the full impact of the results you obtained just by looking at the numbers, then a figure might be appropriate.
Of course, an undergraduate class may expect you to create a figure for your lab experiment, if only to make sure that you can do so effectively. If this is the case, then don’t worry about whether to use figures or not—concentrate instead on how best to accomplish your task.
Figures can include maps, photographs, pen-and-ink drawings, flow charts, bar graphs, and section graphs (“pie charts”). But the most common figure by far, especially for undergraduates, is the line graph, so we’ll focus on that type in this handout.
At the undergraduate level, you can often draw and label your graphs by hand, provided that the result is clear, legible, and drawn to scale. Computer technology has, however, made creating line graphs a lot easier. Most word-processing software has a number of functions for transferring data into graph form; many scientists have found Microsoft Excel, for example, a helpful tool in graphing results. If you plan on pursuing a career in the sciences, it may be well worth your while to learn to use a similar program.
Computers can’t, however, decide for you how your graph really works; you have to know how to design your graph to meet your readers’ expectations. Here are some of these expectations:
- Keep it as simple as possible. You may be tempted to signal the complexity of the information you gathered by trying to design a graph that accounts for that complexity. But remember the purpose of your graph: to dramatize your results in a manner that’s easy to see and grasp. Try not to make the reader stare at the graph for a half hour to find the important line among the mass of other lines. For maximum effectiveness, limit yourself to three to five lines per graph; if you have more data to demonstrate, use a set of graphs to account for it, rather than trying to cram it all into a single figure.
- Plot the independent variable on the horizontal (x) axis and the dependent variable on the vertical (y) axis. Remember that the independent variable is the condition that you manipulated during the experiment and the dependent variable is the condition that you measured to see if it changed along with the independent variable. Placing the variables along their respective axes is mostly just a convention, but since your readers are accustomed to viewing graphs in this way, you’re better off not challenging the convention in your report.
- Label each axis carefully, and be especially careful to include units of measure. You need to make sure that your readers understand perfectly well what your graph indicates.
- Number and title your graphs. As with tables, the title of the graph should be informative but concise, and you should refer to your graph by number in the text (e.g., “Figure 1 shows the increase in the solubility rate as a function of temperature”).
- Many editors of professional scientific journals prefer that writers distinguish the lines in their graphs by attaching a symbol to them, usually a geometric shape (triangle, square, etc.), and using that symbol throughout the curve of the line. Generally, readers have a hard time distinguishing dotted lines from dot-dash lines from straight lines, so you should consider staying away from this system. Editors don’t usually like different-colored lines within a graph because colors are difficult and expensive to reproduce; colors may, however, be great for your purposes, as long as you’re not planning to submit your paper to Nature. Use your discretion—try to employ whichever technique dramatizes the results most effectively.
- Try to gather data at regular intervals, so the plot points on your graph aren’t too far apart. You can’t be sure of the arc you should draw between the plot points if the points are located at the far corners of the graph; over a fifteen-minute interval, perhaps the change occurred in the first or last thirty seconds of that period (in which case your straight-line connection between the points is misleading).
- If you’re worried that you didn’t collect data at sufficiently regular intervals during your experiment, go ahead and connect the points with a straight line, but you may want to examine this problem as part of your Discussion section.
- Make your graph large enough so that everything is legible and clearly demarcated, but not so large that it either overwhelms the rest of the Results section or provides a far greater range than you need to illustrate your point. If, for example, the seedlings of your plant grew only 15 mm during the trial, you don’t need to construct a graph that accounts for 100 mm of growth. The lines in your graph should more or less fill the space created by the axes; if you see that your data is confined to the lower left portion of the graph, you should probably re-adjust your scale.
- If you create a set of graphs, make them the same size and format, including all the verbal and visual codes (captions, symbols, scale, etc.). You want to be as consistent as possible in your illustrations, so that your readers can easily make the comparisons you’re trying to get them to see.
How do I write a strong Discussion section?
The discussion section is probably the least formalized part of the report, in that you can’t really apply the same structure to every type of experiment. In simple terms, here you tell your readers what to make of the Results you obtained. If you have done the Results part well, your readers should already recognize the trends in the data and have a fairly clear idea of whether your hypothesis was supported. Because the Results can seem so self-explanatory, many students find it difficult to know what material to add in this last section.
Basically, the Discussion contains several parts, in no particular order, but roughly moving from specific (i.e., related to your experiment only) to general (how your findings fit in the larger scientific community). In this section, you will, as a rule, need to:
Explain whether the data support your hypothesis
- Acknowledge any anomalous data or deviations from what you expected
Derive conclusions, based on your findings, about the process you’re studying
- Relate your findings to earlier work in the same area (if you can)
Explore the theoretical and/or practical implications of your findings
Let’s look at some dos and don’ts for each of these objectives.
This statement is usually a good way to begin the Discussion, since you can’t effectively speak about the larger scientific value of your study until you’ve figured out the particulars of this experiment. You might begin this part of the Discussion by explicitly stating the relationships or correlations your data indicate between the independent and dependent variables. Then you can show more clearly why you believe your hypothesis was or was not supported. For example, if you tested solubility at various temperatures, you could start this section by noting that the rates of solubility increased as the temperature increased. If your initial hypothesis surmised that temperature change would not affect solubility, you would then say something like,
“The hypothesis that temperature change would not affect solubility was not supported by the data.”
Note: Students tend to view labs as practical tests of undeniable scientific truths. As a result, you may want to say that the hypothesis was “proved” or “disproved” or that it was “correct” or “incorrect.” These terms, however, reflect a degree of certainty that you as a scientist aren’t supposed to have. Remember, you’re testing a theory with a procedure that lasts only a few hours and relies on only a few trials, which severely compromises your ability to be sure about the “truth” you see. Words like “supported,” “indicated,” and “suggested” are more acceptable ways to evaluate your hypothesis.
Also, recognize that saying whether the data supported your hypothesis or not involves making a claim to be defended. As such, you need to show the readers that this claim is warranted by the evidence. Make sure that you’re very explicit about the relationship between the evidence and the conclusions you draw from it. This process is difficult for many writers because we don’t often justify conclusions in our regular lives. For example, you might nudge your friend at a party and whisper, “That guy’s drunk,” and once your friend lays eyes on the person in question, she might readily agree. In a scientific paper, by contrast, you would need to defend your claim more thoroughly by pointing to data such as slurred words, unsteady gait, and the lampshade-as-hat. In addition to pointing out these details, you would also need to show how (according to previous studies) these signs are consistent with inebriation, especially if they occur in conjunction with one another. To put it another way, tell your readers exactly how you got from point A (was the hypothesis supported?) to point B (yes/no).
Acknowledge any anomalous data, or deviations from what you expected
You need to take these exceptions and divergences into account, so that you qualify your conclusions sufficiently. For obvious reasons, your readers will doubt your authority if you (deliberately or inadvertently) overlook a key piece of data that doesn’t square with your perspective on what occurred. In a more philosophical sense, once you’ve ignored evidence that contradicts your claims, you’ve departed from the scientific method. The urge to “tidy up” the experiment is often strong, but if you give in to it you’re no longer performing good science.
Sometimes after you’ve performed a study or experiment, you realize that some part of the methods you used to test your hypothesis was flawed. In that case, it’s OK to suggest that if you had the chance to conduct your test again, you might change the design in this or that specific way in order to avoid such and such a problem. The key to making this approach work, though, is to be very precise about the weakness in your experiment, why and how you think that weakness might have affected your data, and how you would alter your protocol to eliminate—or limit the effects of—that weakness. Often, inexperienced researchers and writers feel the need to account for “wrong” data (remember, there’s no such animal), and so they speculate wildly about what might have screwed things up. These speculations include such factors as the unusually hot temperature in the room, or the possibility that their lab partners read the meters wrong, or the potentially defective equipment. These explanations are what scientists call “cop-outs,” or “lame”; don’t indicate that the experiment had a weakness unless you’re fairly certain that a) it really occurred and b) you can explain reasonably well how that weakness affected your results.
If, for example, your hypothesis dealt with the changes in solubility at different temperatures, then try to figure out what you can rationally say about the process of solubility more generally. If you’re doing an undergraduate lab, chances are that the lab will connect in some way to the material you’ve been covering either in lecture or in your reading, so you might choose to return to these resources as a way to help you think clearly about the process as a whole.
This part of the Discussion section is another place where you need to make sure that you’re not overreaching. Again, nothing you’ve found in one study would remotely allow you to claim that you now “know” something, or that something isn’t “true,” or that your experiment “confirmed” some principle or other. Hesitate before you go out on a limb—it’s dangerous! Use less absolutely conclusive language, including such words as “suggest,” “indicate,” “correspond,” “possibly,” “challenge,” etc.
Relate your findings to previous work in the field (if possible)
We’ve been talking about how to show that you belong in a particular community (such as biologists or anthropologists) by writing within conventions that they recognize and accept. Another is to try to identify a conversation going on among members of that community, and use your work to contribute to that conversation. In a larger philosophical sense, scientists can’t fully understand the value of their research unless they have some sense of the context that provoked and nourished it. That is, you have to recognize what’s new about your project (potentially, anyway) and how it benefits the wider body of scientific knowledge. On a more pragmatic level, especially for undergraduates, connecting your lab work to previous research will demonstrate to the TA that you see the big picture. You have an opportunity, in the Discussion section, to distinguish yourself from the students in your class who aren’t thinking beyond the barest facts of the study. Capitalize on this opportunity by putting your own work in context.
If you’re just beginning to work in the natural sciences (as a first-year biology or chemistry student, say), most likely the work you’ll be doing has already been performed and re-performed to a satisfactory degree. Hence, you could probably point to a similar experiment or study and compare/contrast your results and conclusions. More advanced work may deal with an issue that is somewhat less “resolved,” and so previous research may take the form of an ongoing debate, and you can use your own work to weigh in on that debate. If, for example, researchers are hotly disputing the value of herbal remedies for the common cold, and the results of your study suggest that Echinacea diminishes the symptoms but not the actual presence of the cold, then you might want to take some time in the Discussion section to recapitulate the specifics of the dispute as it relates to Echinacea as an herbal remedy. (Consider that you have probably already written in the Introduction about this debate as background research.)
This information is often the best way to end your Discussion (and, for all intents and purposes, the report). In argumentative writing generally, you want to use your closing words to convey the main point of your writing. This main point can be primarily theoretical (“Now that you understand this information, you’re in a better position to understand this larger issue”) or primarily practical (“You can use this information to take such and such an action”). In either case, the concluding statements help the reader to comprehend the significance of your project and your decision to write about it.
Since a lab report is argumentative—after all, you’re investigating a claim, and judging the legitimacy of that claim by generating and collecting evidence—it’s often a good idea to end your report with the same technique for establishing your main point. If you want to go the theoretical route, you might talk about the consequences your study has for the field or phenomenon you’re investigating. To return to the examples regarding solubility, you could end by reflecting on what your work on solubility as a function of temperature tells us (potentially) about solubility in general. (Some folks consider this type of exploration “pure” as opposed to “applied” science, although these labels can be problematic.) If you want to go the practical route, you could end by speculating about the medical, institutional, or commercial implications of your findings—in other words, answer the question, “What can this study help people to do?” In either case, you’re going to make your readers’ experience more satisfying, by helping them see why they spent their time learning what you had to teach them.
Works consulted
We consulted these works while writing this handout. This is not a comprehensive list of resources on the handout’s topic, and we encourage you to do your own research to find additional publications. Please do not use this list as a model for the format of your own reference list, as it may not match the citation style you are using. For guidance on formatting citations, please see the UNC Libraries citation tutorial . We revise these tips periodically and welcome feedback.
American Psychological Association. 2010. Publication Manual of the American Psychological Association . 6th ed. Washington, DC: American Psychological Association.
Beall, Herbert, and John Trimbur. 2001. A Short Guide to Writing About Chemistry , 2nd ed. New York: Longman.
Blum, Deborah, and Mary Knudson. 1997. A Field Guide for Science Writers: The Official Guide of the National Association of Science Writers . New York: Oxford University Press.
Booth, Wayne C., Gregory G. Colomb, Joseph M. Williams, Joseph Bizup, and William T. FitzGerald. 2016. The Craft of Research , 4th ed. Chicago: University of Chicago Press.
Briscoe, Mary Helen. 1996. Preparing Scientific Illustrations: A Guide to Better Posters, Presentations, and Publications , 2nd ed. New York: Springer-Verlag.
Council of Science Editors. 2014. Scientific Style and Format: The CSE Manual for Authors, Editors, and Publishers , 8th ed. Chicago & London: University of Chicago Press.
Davis, Martha. 2012. Scientific Papers and Presentations , 3rd ed. London: Academic Press.
Day, Robert A. 1994. How to Write and Publish a Scientific Paper , 4th ed. Phoenix: Oryx Press.
Porush, David. 1995. A Short Guide to Writing About Science . New York: Longman.
Williams, Joseph, and Joseph Bizup. 2017. Style: Lessons in Clarity and Grace , 12th ed. Boston: Pearson.
You may reproduce it for non-commercial use if you use the entire handout and attribute the source: The Writing Center, University of North Carolina at Chapel Hill
Make a Gift
Writing up a Research Report
- First Online: 04 January 2024
Cite this chapter

- Stefan Hunziker 3 &
- Michael Blankenagel 3
386 Accesses
A research report is one big argument about how and why you came up with your conclusions. To make it a convincing argument, a typical guiding structure has developed. In the different chapters, there are distinct issues that need to be addressed to explain to the reader why your conclusions are valid. The governing principle for writing the report is full disclosure: to explain everything and ensure replicability by another researcher.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Barros, L. O. (2016). The only academic phrasebook you’ll ever need . Createspace Independent Publishing Platform.
Google Scholar
Field, A. (2016). An adventure in statistics. The reality enigma . SAGE.
Field, A. (2020). Discovering statistics using IBM SPSS statistics (5th ed.). SAGE.
Früh, M., Keimer, I., & Blankenagel, M. (2019). The impact of Balanced Scorecard excellence on shareholder returns. IFZ Working Paper No. 0003/2019. https://zenodo.org/record/2571603#.YMDUafkzZaQ . Accessed: 9 June 2021.
Pearl, J., & Mackenzie, D. (2018). The book of why: The new science of cause and effect. Basic Books.
Yin, R. K. (2013). Case study research: Design and methods (5th ed.). SAGE.
Download references
Author information
Authors and affiliations.
Wirtschaft/IFZ, Campus Zug-Rotkreuz, Hochschule Luzern, Zug-Rotkreuz, Zug, Switzerland
Stefan Hunziker & Michael Blankenagel
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Stefan Hunziker .
Rights and permissions
Reprints and permissions
Copyright information
© 2024 Springer Fachmedien Wiesbaden GmbH, part of Springer Nature
About this chapter
Hunziker, S., Blankenagel, M. (2024). Writing up a Research Report. In: Research Design in Business and Management. Springer Gabler, Wiesbaden. https://doi.org/10.1007/978-3-658-42739-9_4
Download citation
DOI : https://doi.org/10.1007/978-3-658-42739-9_4
Published : 04 January 2024
Publisher Name : Springer Gabler, Wiesbaden
Print ISBN : 978-3-658-42738-2
Online ISBN : 978-3-658-42739-9
eBook Packages : Business and Management Business and Management (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Privacy Policy

Home » Research Report – Example, Writing Guide and Types
Research Report – Example, Writing Guide and Types
Table of Contents

Research Report
Definition:
Research Report is a written document that presents the results of a research project or study, including the research question, methodology, results, and conclusions, in a clear and objective manner.
The purpose of a research report is to communicate the findings of the research to the intended audience, which could be other researchers, stakeholders, or the general public.
Components of Research Report
Components of Research Report are as follows:
Introduction
The introduction sets the stage for the research report and provides a brief overview of the research question or problem being investigated. It should include a clear statement of the purpose of the study and its significance or relevance to the field of research. It may also provide background information or a literature review to help contextualize the research.
Literature Review
The literature review provides a critical analysis and synthesis of the existing research and scholarship relevant to the research question or problem. It should identify the gaps, inconsistencies, and contradictions in the literature and show how the current study addresses these issues. The literature review also establishes the theoretical framework or conceptual model that guides the research.
Methodology
The methodology section describes the research design, methods, and procedures used to collect and analyze data. It should include information on the sample or participants, data collection instruments, data collection procedures, and data analysis techniques. The methodology should be clear and detailed enough to allow other researchers to replicate the study.
The results section presents the findings of the study in a clear and objective manner. It should provide a detailed description of the data and statistics used to answer the research question or test the hypothesis. Tables, graphs, and figures may be included to help visualize the data and illustrate the key findings.
The discussion section interprets the results of the study and explains their significance or relevance to the research question or problem. It should also compare the current findings with those of previous studies and identify the implications for future research or practice. The discussion should be based on the results presented in the previous section and should avoid speculation or unfounded conclusions.
The conclusion summarizes the key findings of the study and restates the main argument or thesis presented in the introduction. It should also provide a brief overview of the contributions of the study to the field of research and the implications for practice or policy.
The references section lists all the sources cited in the research report, following a specific citation style, such as APA or MLA.
The appendices section includes any additional material, such as data tables, figures, or instruments used in the study, that could not be included in the main text due to space limitations.
Types of Research Report
Types of Research Report are as follows:
Thesis is a type of research report. A thesis is a long-form research document that presents the findings and conclusions of an original research study conducted by a student as part of a graduate or postgraduate program. It is typically written by a student pursuing a higher degree, such as a Master’s or Doctoral degree, although it can also be written by researchers or scholars in other fields.
Research Paper
Research paper is a type of research report. A research paper is a document that presents the results of a research study or investigation. Research papers can be written in a variety of fields, including science, social science, humanities, and business. They typically follow a standard format that includes an introduction, literature review, methodology, results, discussion, and conclusion sections.
Technical Report
A technical report is a detailed report that provides information about a specific technical or scientific problem or project. Technical reports are often used in engineering, science, and other technical fields to document research and development work.
Progress Report
A progress report provides an update on the progress of a research project or program over a specific period of time. Progress reports are typically used to communicate the status of a project to stakeholders, funders, or project managers.
Feasibility Report
A feasibility report assesses the feasibility of a proposed project or plan, providing an analysis of the potential risks, benefits, and costs associated with the project. Feasibility reports are often used in business, engineering, and other fields to determine the viability of a project before it is undertaken.
Field Report
A field report documents observations and findings from fieldwork, which is research conducted in the natural environment or setting. Field reports are often used in anthropology, ecology, and other social and natural sciences.
Experimental Report
An experimental report documents the results of a scientific experiment, including the hypothesis, methods, results, and conclusions. Experimental reports are often used in biology, chemistry, and other sciences to communicate the results of laboratory experiments.
Case Study Report
A case study report provides an in-depth analysis of a specific case or situation, often used in psychology, social work, and other fields to document and understand complex cases or phenomena.
Literature Review Report
A literature review report synthesizes and summarizes existing research on a specific topic, providing an overview of the current state of knowledge on the subject. Literature review reports are often used in social sciences, education, and other fields to identify gaps in the literature and guide future research.
Research Report Example
Following is a Research Report Example sample for Students:
Title: The Impact of Social Media on Academic Performance among High School Students
This study aims to investigate the relationship between social media use and academic performance among high school students. The study utilized a quantitative research design, which involved a survey questionnaire administered to a sample of 200 high school students. The findings indicate that there is a negative correlation between social media use and academic performance, suggesting that excessive social media use can lead to poor academic performance among high school students. The results of this study have important implications for educators, parents, and policymakers, as they highlight the need for strategies that can help students balance their social media use and academic responsibilities.
Introduction:
Social media has become an integral part of the lives of high school students. With the widespread use of social media platforms such as Facebook, Twitter, Instagram, and Snapchat, students can connect with friends, share photos and videos, and engage in discussions on a range of topics. While social media offers many benefits, concerns have been raised about its impact on academic performance. Many studies have found a negative correlation between social media use and academic performance among high school students (Kirschner & Karpinski, 2010; Paul, Baker, & Cochran, 2012).
Given the growing importance of social media in the lives of high school students, it is important to investigate its impact on academic performance. This study aims to address this gap by examining the relationship between social media use and academic performance among high school students.
Methodology:
The study utilized a quantitative research design, which involved a survey questionnaire administered to a sample of 200 high school students. The questionnaire was developed based on previous studies and was designed to measure the frequency and duration of social media use, as well as academic performance.
The participants were selected using a convenience sampling technique, and the survey questionnaire was distributed in the classroom during regular school hours. The data collected were analyzed using descriptive statistics and correlation analysis.
The findings indicate that the majority of high school students use social media platforms on a daily basis, with Facebook being the most popular platform. The results also show a negative correlation between social media use and academic performance, suggesting that excessive social media use can lead to poor academic performance among high school students.
Discussion:
The results of this study have important implications for educators, parents, and policymakers. The negative correlation between social media use and academic performance suggests that strategies should be put in place to help students balance their social media use and academic responsibilities. For example, educators could incorporate social media into their teaching strategies to engage students and enhance learning. Parents could limit their children’s social media use and encourage them to prioritize their academic responsibilities. Policymakers could develop guidelines and policies to regulate social media use among high school students.
Conclusion:
In conclusion, this study provides evidence of the negative impact of social media on academic performance among high school students. The findings highlight the need for strategies that can help students balance their social media use and academic responsibilities. Further research is needed to explore the specific mechanisms by which social media use affects academic performance and to develop effective strategies for addressing this issue.
Limitations:
One limitation of this study is the use of convenience sampling, which limits the generalizability of the findings to other populations. Future studies should use random sampling techniques to increase the representativeness of the sample. Another limitation is the use of self-reported measures, which may be subject to social desirability bias. Future studies could use objective measures of social media use and academic performance, such as tracking software and school records.
Implications:
The findings of this study have important implications for educators, parents, and policymakers. Educators could incorporate social media into their teaching strategies to engage students and enhance learning. For example, teachers could use social media platforms to share relevant educational resources and facilitate online discussions. Parents could limit their children’s social media use and encourage them to prioritize their academic responsibilities. They could also engage in open communication with their children to understand their social media use and its impact on their academic performance. Policymakers could develop guidelines and policies to regulate social media use among high school students. For example, schools could implement social media policies that restrict access during class time and encourage responsible use.
References:
- Kirschner, P. A., & Karpinski, A. C. (2010). Facebook® and academic performance. Computers in Human Behavior, 26(6), 1237-1245.
- Paul, J. A., Baker, H. M., & Cochran, J. D. (2012). Effect of online social networking on student academic performance. Journal of the Research Center for Educational Technology, 8(1), 1-19.
- Pantic, I. (2014). Online social networking and mental health. Cyberpsychology, Behavior, and Social Networking, 17(10), 652-657.
- Rosen, L. D., Carrier, L. M., & Cheever, N. A. (2013). Facebook and texting made me do it: Media-induced task-switching while studying. Computers in Human Behavior, 29(3), 948-958.
Note*: Above mention, Example is just a sample for the students’ guide. Do not directly copy and paste as your College or University assignment. Kindly do some research and Write your own.

Applications of Research Report
Research reports have many applications, including:
- Communicating research findings: The primary application of a research report is to communicate the results of a study to other researchers, stakeholders, or the general public. The report serves as a way to share new knowledge, insights, and discoveries with others in the field.
- Informing policy and practice : Research reports can inform policy and practice by providing evidence-based recommendations for decision-makers. For example, a research report on the effectiveness of a new drug could inform regulatory agencies in their decision-making process.
- Supporting further research: Research reports can provide a foundation for further research in a particular area. Other researchers may use the findings and methodology of a report to develop new research questions or to build on existing research.
- Evaluating programs and interventions : Research reports can be used to evaluate the effectiveness of programs and interventions in achieving their intended outcomes. For example, a research report on a new educational program could provide evidence of its impact on student performance.
- Demonstrating impact : Research reports can be used to demonstrate the impact of research funding or to evaluate the success of research projects. By presenting the findings and outcomes of a study, research reports can show the value of research to funders and stakeholders.
- Enhancing professional development : Research reports can be used to enhance professional development by providing a source of information and learning for researchers and practitioners in a particular field. For example, a research report on a new teaching methodology could provide insights and ideas for educators to incorporate into their own practice.
How to write Research Report
Here are some steps you can follow to write a research report:
- Identify the research question: The first step in writing a research report is to identify your research question. This will help you focus your research and organize your findings.
- Conduct research : Once you have identified your research question, you will need to conduct research to gather relevant data and information. This can involve conducting experiments, reviewing literature, or analyzing data.
- Organize your findings: Once you have gathered all of your data, you will need to organize your findings in a way that is clear and understandable. This can involve creating tables, graphs, or charts to illustrate your results.
- Write the report: Once you have organized your findings, you can begin writing the report. Start with an introduction that provides background information and explains the purpose of your research. Next, provide a detailed description of your research methods and findings. Finally, summarize your results and draw conclusions based on your findings.
- Proofread and edit: After you have written your report, be sure to proofread and edit it carefully. Check for grammar and spelling errors, and make sure that your report is well-organized and easy to read.
- Include a reference list: Be sure to include a list of references that you used in your research. This will give credit to your sources and allow readers to further explore the topic if they choose.
- Format your report: Finally, format your report according to the guidelines provided by your instructor or organization. This may include formatting requirements for headings, margins, fonts, and spacing.
Purpose of Research Report
The purpose of a research report is to communicate the results of a research study to a specific audience, such as peers in the same field, stakeholders, or the general public. The report provides a detailed description of the research methods, findings, and conclusions.
Some common purposes of a research report include:
- Sharing knowledge: A research report allows researchers to share their findings and knowledge with others in their field. This helps to advance the field and improve the understanding of a particular topic.
- Identifying trends: A research report can identify trends and patterns in data, which can help guide future research and inform decision-making.
- Addressing problems: A research report can provide insights into problems or issues and suggest solutions or recommendations for addressing them.
- Evaluating programs or interventions : A research report can evaluate the effectiveness of programs or interventions, which can inform decision-making about whether to continue, modify, or discontinue them.
- Meeting regulatory requirements: In some fields, research reports are required to meet regulatory requirements, such as in the case of drug trials or environmental impact studies.
When to Write Research Report
A research report should be written after completing the research study. This includes collecting data, analyzing the results, and drawing conclusions based on the findings. Once the research is complete, the report should be written in a timely manner while the information is still fresh in the researcher’s mind.
In academic settings, research reports are often required as part of coursework or as part of a thesis or dissertation. In this case, the report should be written according to the guidelines provided by the instructor or institution.
In other settings, such as in industry or government, research reports may be required to inform decision-making or to comply with regulatory requirements. In these cases, the report should be written as soon as possible after the research is completed in order to inform decision-making in a timely manner.
Overall, the timing of when to write a research report depends on the purpose of the research, the expectations of the audience, and any regulatory requirements that need to be met. However, it is important to complete the report in a timely manner while the information is still fresh in the researcher’s mind.
Characteristics of Research Report
There are several characteristics of a research report that distinguish it from other types of writing. These characteristics include:
- Objective: A research report should be written in an objective and unbiased manner. It should present the facts and findings of the research study without any personal opinions or biases.
- Systematic: A research report should be written in a systematic manner. It should follow a clear and logical structure, and the information should be presented in a way that is easy to understand and follow.
- Detailed: A research report should be detailed and comprehensive. It should provide a thorough description of the research methods, results, and conclusions.
- Accurate : A research report should be accurate and based on sound research methods. The findings and conclusions should be supported by data and evidence.
- Organized: A research report should be well-organized. It should include headings and subheadings to help the reader navigate the report and understand the main points.
- Clear and concise: A research report should be written in clear and concise language. The information should be presented in a way that is easy to understand, and unnecessary jargon should be avoided.
- Citations and references: A research report should include citations and references to support the findings and conclusions. This helps to give credit to other researchers and to provide readers with the opportunity to further explore the topic.
Advantages of Research Report
Research reports have several advantages, including:
- Communicating research findings: Research reports allow researchers to communicate their findings to a wider audience, including other researchers, stakeholders, and the general public. This helps to disseminate knowledge and advance the understanding of a particular topic.
- Providing evidence for decision-making : Research reports can provide evidence to inform decision-making, such as in the case of policy-making, program planning, or product development. The findings and conclusions can help guide decisions and improve outcomes.
- Supporting further research: Research reports can provide a foundation for further research on a particular topic. Other researchers can build on the findings and conclusions of the report, which can lead to further discoveries and advancements in the field.
- Demonstrating expertise: Research reports can demonstrate the expertise of the researchers and their ability to conduct rigorous and high-quality research. This can be important for securing funding, promotions, and other professional opportunities.
- Meeting regulatory requirements: In some fields, research reports are required to meet regulatory requirements, such as in the case of drug trials or environmental impact studies. Producing a high-quality research report can help ensure compliance with these requirements.
Limitations of Research Report
Despite their advantages, research reports also have some limitations, including:
- Time-consuming: Conducting research and writing a report can be a time-consuming process, particularly for large-scale studies. This can limit the frequency and speed of producing research reports.
- Expensive: Conducting research and producing a report can be expensive, particularly for studies that require specialized equipment, personnel, or data. This can limit the scope and feasibility of some research studies.
- Limited generalizability: Research studies often focus on a specific population or context, which can limit the generalizability of the findings to other populations or contexts.
- Potential bias : Researchers may have biases or conflicts of interest that can influence the findings and conclusions of the research study. Additionally, participants may also have biases or may not be representative of the larger population, which can limit the validity and reliability of the findings.
- Accessibility: Research reports may be written in technical or academic language, which can limit their accessibility to a wider audience. Additionally, some research may be behind paywalls or require specialized access, which can limit the ability of others to read and use the findings.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Data Collection – Methods Types and Examples

Delimitations in Research – Types, Examples and...

Research Process – Steps, Examples and Tips

Research Design – Types, Methods and Examples

Institutional Review Board – Application Sample...

Evaluating Research – Process, Examples and...

Principles of Sound Scientific Writing: “The Introduction, Setting the State for your Scientific Paper”
The School of Medicine ’s Office of Faculty will host the next session in the Principles of Sound Scientific Writing series Wednesday, May 22, from 6 to 8 p.m. in Samson Pavilion, Room 271 (TEAL Classroom).
The topic of this session will be “The Introduction, Setting the State for your Scientific Paper.” Led by Goutham Rao, participants will discuss principles of writing an effective introduction to a scientific manuscript.
The session’s objectives are to:
- Explain the importance of writing a clear, persuasive introduction to a scientific paper;
- Explain the application of the 5Cs paradigm to writing an introduction;
- Adopt a rational, step-wise strategy to create a sound introduction.
Refreshments will be served at 5:30 p.m.
The fee to participate in this workshop is $25.
Complete the registration form.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 13 May 2024
Vaccine hesitancy and trust in sub-Saharan Africa
- Kerstin Unfried 1 &
- Jan Priebe 1 , 2
Scientific Reports volume 14 , Article number: 10860 ( 2024 ) Cite this article
325 Accesses
1 Altmetric
Metrics details
- Applied mathematics
- Information technology
- Scientific data
Lack of trust is a primary reason behind the global rise in vaccine hesitancy. Existing research on the trust—vaccine hesitancy nexus has almost exclusively focused on COVID-19 with the vast majority of studies examining industrialized countries. In this study, we investigated the influence of trust in different policy-relevant actors (government, science, media, pharmaceutical companies, society) on vaccine hesitancy for recently available vaccines related to polio and HPV which we benchmark against a COVID-19 vaccine. Leveraging unique primary data on 5203 individuals from six countries (Ghana, Kenya, Nigeria, South Africa, Tanzania, and Uganda), we showed that individuals’ trust in the government and society are key predictors of vaccine hesitancy. Furthermore, we demonstrated that these relationships are remarkably stable across vaccine, disease, and country contexts.
Similar content being viewed by others

Conspiratorial thinking as a precursor to opposition to COVID-19 vaccination in the US: a multi-year study from 2018 to 2021
Despite misinformation, low trust, and conflict in Somalia, high demand for vaccines and a negative endorsement effect of non-state authorities

Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021
Introduction.
Immunization is one of the most cost-effective public health interventions and has helped save millions of lives globally. Despite many success stories, the progress made in tackling vaccine-preventable diseases has in many settings come under threat by increases in vaccine hesitancy, i.e. the reluctance or refusal to vaccinate despite the availability of vaccines 1 . As such vaccine hesitancy can lead to the prolongation and resurgence of vaccine-preventable diseases and cause substantial negative social and economic consequences. It is estimated that annually around 1.5 million deaths could be avoided globally if vaccination rates were higher 2 , 3 .
Vaccine hesitancy is primarily a trust issue 4 , 5 . Especially in contexts of high uncertainty, trust in pivotal institutions is a common heuristic used to shortcut decision-making with incomplete information. Systematic and literature reviews have documented a negative relationship between trust in institutions that are involved in the production, supply, distribution, and monitoring of vaccines and vaccine hesitancy 6 , 7 . Specifically, vaccine hesitancy has been related to levels of trust in the national and local government 5 , 8 , 9 , manufacturers/pharmaceutical firms 9 , 10 , healthcare systems including physicians 10 , 11 , 12 , 13 , and science 4 , 12 , 14 , 15 .
Understanding the nexus between trust and vaccine hesitancy matters for policy making as it helps to design optimal information campaigns and distribution of vaccines, among others. Despite its importance, there is a notable knowledge gap with respect to low- and middle-income countries (LMICs) in which most vaccine-preventable deaths occur. Existing research has almost exclusively focused on (i) richer countries or (ii) COVID-19 vaccines. This gap is worrisome given that existing evidence suggests that vaccine hesitancy is highly context-specific, varying across place, time, and vaccine type 14 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 .
The objective of this study is to fill this gap by analyzing the relationship between trust and vaccine hesitancy in the context of six sub-Saharan African countries with respect to vaccines for polio, HPV, and COVID-19. In early 2023 we gathered primary data via online surveys in Ghana, Kenya, Nigeria, South Africa, Tanzania, Uganda. In total, we collected information from 5203 adults on vaccine hesitancy concerning three types of vaccines (polio (nOPV2); HPV (GARDASIL4, CERVARIX); COVID-19 (COMIRANTY)) and trust in five distinct types of institutions (government, media, science, pharmaceutical companies, and society). The selection of vaccines was based on the countries’ national strategies to integrate these vaccines into their (standard) immunization programs. Employing a multivariate regression framework, we estimated in this paper to what extend institution-specific trust influences vaccine-specific vaccination intentions. Importantly, our study design allows to explicitly compare trust- and vaccine-specific factors, enabling us to gauge to what extent results obtained from the abundant research on COVID-19 vaccine hesitancy can be transferred to other disease and vaccine contexts.
The focus of our study on sub-Saharan Africa (SSA) was motivated by three considerations. First, vaccination and vaccine acceptance rates in SSA are particularly low in international comparisons 14 , 24 , 27 , 28 , and have witnessed a substantial decline in recent years 29 , 30 , 31 . Second, SSA represents a cultural and historical context that differs from other world regions in important ways as various controversies concerning vaccinations and trust are SSA-specific. For instance, widespread rumours exist that SSA is a testing ground for new vaccines and that Africans are used as guinea pigs in vaccine trials 32 . These rumours have contributed to vaccine hesitancy 27 , 33 , 34 . In this context, colonial medical experimentation in SSA has been found to still diminish trust into modern medicine, today 35 . Third, despite most of the world’s vaccine-preventable deaths occurring in SSA, issues of trust and vaccine hesitancy appear to be understudied for SSA 36 with existing quantitative studies being confined to COVID-19 and a limited set of trust indicators 37 , 38 , 39 . Beyond COVID-19 vaccines, evidence on vaccine hesitancy in SSA primarily stems from correlational evidence related to vaccination rates but not to measures of vaccine hesitancy 8 or from small-scale qualitative studies 40 , 41 .
Our study also speaks to the literature on the role of societal trust for vaccine hesitancy. With the existing literature focusing on individual trust factors, societal aspects of trust in vaccination are often neglected or disregarded 14 . Apart from individual health benefits, vaccinations create beneficial externalities that help achieve herd immunity; health benefits to other non-vaccinated members of society. The economic & psychology literature has highlighted that decisions to contribute to a collective good (vaccinations) depends on an individual’s expectations about the cooperation of other society members and an individual’s trust in others 42 , 43 . Hence, individual trust and beliefs about vaccination decisions of others can play a relevant role in overcoming vaccine hesitancy. By examining individual trust towards vaccination decisions of other society members, we add to the literature investigating the role of prosocial motivations in health preventive behavior 44 , 45 , 46 .
Sample description, vaccination status and vaccine hesitancy
The study sample comprised 5203 individuals living in six SSA countries (Ghana, Kenya, Nigeria, South Africa, Tanzania, Uganda). The number of respondents per country is presented in Supplementary Table B1 in Appendix B . The age of respondents ranged from 18 to 75 years, with a mean of 29.15 and a median age of 27 years. About 65% of respondents were male and 35% were female. As common in online samples, respondents tended to be well-educated (about 73% possess tertiary education). Supplementary Table B2 in Appendix B provides additional summary statistics describing the socio-demographic characteristics of our total sample and Supplementary Table B3 in Appendix B reports the descriptive statistics by country.
We start with discussing the vaccination status and vaccine hesitancy rates among our survey respondents. Panel A of Fig. 1 depicts the percentage of study participants that were vaccinated against COVID-19, polio, and HPV by country. The numbers are based on individuals’ self-reports and capture whether respondents received at least one vaccination per disease. A majority of respondents stated that they were vaccinated against polio (89.74%) and COVID-19 (71.42%), while a minority said that they received any vaccination against HPV (about 10%). The national vaccination rates based on statistics from WHO, UNICEF, and national reports are reported in Supplementary Table B4 in Appendix B . Comparing these official statistics to the self-reported vaccination status in our sample, we see that generally vaccination figures match quite well. The vaccination status for COVID-19 was higher compared to the published official vaccination rates in the respective countries. We believe that this is due to the circumstance that respondents in our sample were better educated and predominantly resided in urban areas. Additionally, the rate of vaccinated persons against HPV are lower in our study sample compared to the national statistics that might be explained by the difference in the underlying population sample.
Vaccination status and vaccine hesitancy. The Figure presents the percentage of participants that reported to have received at least one vaccine against the respective disease (vaccine status) and vaccine hesitancy rates by country. Panel ( A ) presents the average vaccination status for COVID-19, polio, and HPV based on the self-reported answers in the study sample. Panel ( B ) depicts vaccine hesitancy rates measured by participants’ intention to get vaccinated with a new vaccine (COVID-19, polio, HPV).
Furthermore, Fig. 1 shows considerable variations across countries and disease. Vaccination status were highest in Kenya and Uganda, and lowest in Nigeria, South Africa and Tanzania.
Panel B of Fig. 1 depicts the share of vaccine-hesitant respondents by country. Vaccine hesitancy was defined as (i) having no intention to get vaccinated (if the person is not yet vaccinated) or (ii) having no intention to recommend the vaccination to other family members (if respondent was already vaccinated against the disease). [Likewise, the second part of the question allowed us to measure vaccination attitudes also in cases in which vaccinations are mainly recommended for females (HPV) or children (polio).] About 9% of our sample were classified as vaccine hesitant. Vaccine hesitancy differed substantially across countries and ranged between 8.3% in Kenya and 17.15% in South Africa.
Trust and vaccine hesitancy
Employing linear probability models—as described in section ’ Empirical strategy ”—we estimated by ordinary least squares (OLS) how individuals’ level of trust affects vaccine hesitancy. The adopted regression framework controlled for a number of individual and country-specific characteristics as well as vaccine type. As explanatory variables we considered trust in science, pharmaceutical firms, the government, media, and society. These variables captured respondents’ level of trust into the respective institutions with regard to health matters. We proxied trust in society from a behavioral perspective; respondents’ beliefs about the extent to which other members of society intend to get vaccinated with the specific vaccine. A detailed description of each measure is presented in section Variable construction . All trust variables were standardized so that effect sizes can be compared across trust measures and with respect to other studies. Figure 2 depicts the estimated effect sizes and the related confidence intervals (at 90% and 95% levels).
Trust and vaccine hesitancy. The Figure reports coefficient estimates and confidence intervals at the 95 and 90 percent level. Results are obtained by OLS. Controls included are age, gender, marital and employment status, wealth, education, religion, personality traits, and vaccination history as well as country and vaccine-type fixed effects. Robust standard errors are used.
Our results suggested that individuals’ level of trust in the government, in pharmaceutical firms, and society are strong predictors of vaccine hesitancy. These coefficients were negative and statistically significant at the 95% confidence level. This means that persons with lower levels of trust in the national government, pharmaceutical companies, and society were more likely to be vaccine-hesitant. The estimated coefficient on governmental trust was about twice as large as the one for societal trust and trust in pharmaceutical firms. A one standard deviation higher level of trust in the government reduced the likelihood to be vaccine-hesitant by around five percentage points. Levels of trust in the national media and science appeared unrelated to vaccine hesitancy.
Regarding other variables included in the regression framework, we found that being female and vaccination status were negatively correlated with vaccine hesitancy (statistically significant at the 90% level). Prior literature on gender differences in vaccine hesitancy is rare for the context of Africa. Mainly focusing on COVID-19 it shows mixed findings 47 , 48 , 49 . Though gender differences are important to study, this topic is beyond the scope of this paper. Other individual-level controls seemed to rather be unrelated to vaccine hesitancy. The full set of regression results is shown in Supplementary Table B5 in Appendix B .
We performed several robustness checks to assess the sensitivity of our results. First, we re-estimated our main specification by using non-linear probit models (see Supplementary Fig. B1 in Appendix A ). In addition to trust in government, society, and pharmaceutical companies, the coefficient of trust in science was negative and became statistically significant (90% level). However, this effect could not be confirmed in the sub-samples, though admittedly these samples had less statistical power (see Supplementary Figs. A2 and A3 in Appendix A ). Second, we used an alternative dependent variable. More specifically, we replaced the previous measure of vaccine hesitancy with a variable that captured the confidence in vaccinations. The respective results (Supplementary Fig. A4 in Appendix A ) were similar to our previous findings, demonstrating that trust in the government, society, and pharmaceutical firms were positively associated with vaccination confidence. Third, we used alternative definitions of our principal independent variables. Using regression specifications with binary trust measures that distinguished between persons with higher and lower trust we obtained similar results as before (see Supplementary Table B6 in Appendix B ). Lastly, Supplementary Table B7 in Appendix B contains results from a range of further robustness checks related to (i) the clustering of standard errors and (ii) alternative covariate specifications. Columns (1) to (3) report estimates with standard errors being clustered at the country, vaccine-type, or country-vaccine-type level. Column (4) includes additional controls that measure respondents’ tendency to answer survey questions strategically (social desirability bias), while column (5) controls for treatment indicators of an experiment that was part of the survey for another study and unrelated to our topic. By and large, we found that the results related to trust in the government and society were robust to these model modifications.
Context-specific effects
We now turn to the analysis of context-specific effects. To investigate variations across study contexts, we re-estimated our main OLS specifications for different sub-samples. First, we focused on vaccine-type specific effects. As respondents were asked to state vaccination intentions on one of three vaccines only (randomized), each sub-sample consisted of about one third of the total number of observations. Figure 3 displays our results.
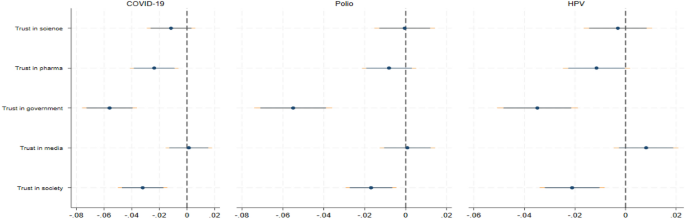
Trust and vaccine hesitancy by disease. The Figure reports coefficient estimates and confidence intervals at the 90 and 95 percent level. Results are obtained by OLS (see section “ Empirical strategy ”) for sub-samples. Controls included are age, gender, marital and employment status, wealth, education, religion, personality traits, and vaccination history as well as country and vaccine-type fixed effects. Robust standard errors are used.
Overall, we found very consistent patterns. Across all three samples, trust in the government, pharmaceutical industry, and society was negatively correlated (conditionally) with vaccine hesitancy.
Second, we analysed country differences. Figure 4 presents the regression estimations for each of the six countries: Ghana, Kenya, Nigeria, South Africa, Tanzania, and Uganda.
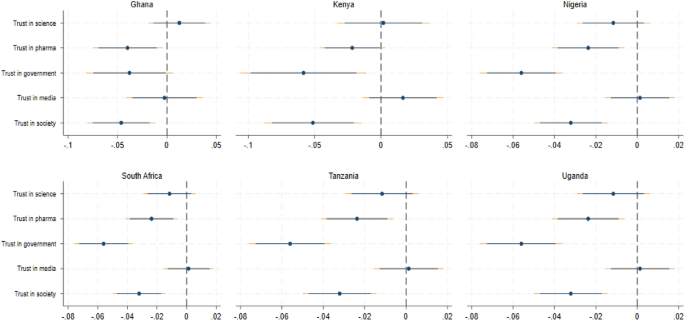
Trust and vaccine hesitancy by country. The Figure reports coefficient estimates and confidence intervals at the 90 and 95 percent level. Results are obtained by OLS (see section “ Empirical strategy ”) for sub-samples. Controls included are age, gender, marital and employment status, wealth, education, religion, personality traits, and vaccination history as well as country and vaccine-type fixed effects. Robust standard errors are used.
For all countries, we found a statistically significant negative correlation between trust in the society with vaccine hesitancy. Trust in the government and in pharmaceutical firms was statistically significant and negatively correlated with vaccine hesitancy in the majority of countries. Exceptions were South Africa for trust in pharmaceutical firms and Tanzania for trust in the government. Trust in science seemed to be uncorrelated to vaccine hesitancy in most countries except for South Africa.
While COVID-19 and HPV are viruses that were circulating in all countries, not all countries in our study sample had recently experienced a polio outbreak. Moreover, the polio vaccine nOPV2 is distinct from the other two types of vaccines as it is only used in emergencies and not part of the routine immunization programs. This might cause a bias of the estimates related to polio if survey participants found it hard to imagine the hypothetical case of a polio outbreak in their country. To see in how far our results are sensitive to such a situation, we distinguished between countries that experienced a polio outbreak in the last few years and others and present the results of the sub-samples in Supplementary Fig. A5 in Appendix A . In both samples, we found a negative correlation between trust in the government and vaccine hesitancy. Additionally, we found a negative correlation between trust in the society and vaccine hesitancy but only for the sample of countries that have experienced a polio outbreak.
Immunization is one of the most cost-effective health interventions to tackle vaccine-preventable diseases. Across the world vaccine hesitancy is widely acknowledged to be one of the largest threats to achieve the widespread adoption and roll-out of vaccinations. As such the observed increasing rates of vaccine hesitancy pose a tremendous challenge for global health and the targets set under the Sustainable Development Goal #3 on good health and well-being. A better understanding of the determinants of vaccine hesitancy is pivotal to design effective policies to boost immunization rates.
In this study, we examined the relationship between trust and vaccine hesitancy. Leveraging primary data from six SSA countries, our analysis showed that low levels of trust in the government go along with a higher likelihood of vaccine hesitancy. Our results also emphasized the role of societal trust and social norms in the decision to get vaccinated. Specifically, we found that individuals are more likely to get vaccinated if (they believed that) others do so. Lastly, we found empirical evidence for a lack of trust in pharmaceutical companies to influence vaccine hesitancy.
The second part of our analysis relates to vaccine and country-specific effects in the relationship between trust and vaccine hesitancy. We showed that the relationship between trust and vaccine hesitancy was much less context-specific as expected. Most importantly, we demonstrated that the same trust factors that were responsible for vaccine hesitancy against COVID-19 helped explain vaccine hesitancy against other vaccines and diseases (HPV, polio). Moreover, the trust-related drivers of vaccine hesitancy were highly comparable across all six study countries.
Our results corroborate previous literature documenting that low levels of trust in the government is a pivotal predictor of vaccine hesitancy. We add to the existing literature by expanding the analysis of the trust-vaccine hesitancy nexus to other disease and vaccine contexts. Hence, a more comprehensive understanding of vaccine hesitancy in SSA has become possible. In contrast to several other studies, we found no robust evidence for a relationship between trust in media and science on vaccine hesitancy. Moreover, we provided new evidence on how trust in society in regards to social norms influences vaccine hesitancy. While our measure of societal trust is adjusted for the specific disease and vaccine type, previous research had to rely on very aggregated measures of societal trust (e.g. overall trust in strangers 12 ). The inclusion of trust in society specified as social norm could be an explanation for the null findings of trust in media and science, if those factors are mainly operating through the societal channel.
Our study focused on six English-speaking African countries. While these countries comprise a diverse geographical, cultural, and health setting, it remains unclear in how far the relation between trust in crucial institutions and vaccine hesitancy applies to other African countries. Studies that were conducted in the context of COVID-19 in other African countries showed similar results, supporting the context-unspecific relation between trust in pivotal institutions and vaccine hesitancy. For instance, a negative relation between trust in pharmaceutical firms and vaccine hesitancy was found in Cameroon 50 . Several studies found a negative relation between vaccine hesitancy and trust in public institutions in the context of various African countries 8 , 51 .
A final thought relates to gender aspects. Preventive health behavior and preferences often tend to differ between men and women 52 , 53 . Therefore and considering that HPV vaccines are targeted towards young girls and women, trust-related effects on vaccine hesitancy might differ by gender. Split sample estimates that are illustrated in Supplementary Fig. A6 in Appendix A , however, revealed high similarities in the relationship between trust and vaccine hesitancy across gender and vaccine types.
Several policy conclusions can be derived on basis of our findings. First, our results imply a rather universal relationship between trust in the government and vaccine hesitancy, enabling the use of more targeted policies to address this specific trust issue. Second, to increase vaccination acceptance, policymakers should focus on emphasizing the social acceptance of vaccination. Third, when new vaccines enter the market, raised mistrust in pharmaceutical firms can decrease vaccination up-take.
Finally, we would like to point out some limitations of our study. First, the cross-sectional nature of data did not allow us to identify causal effects. By controlling for a large set of individual characteristics in combination with country and vaccine-type fixed effects, we, however, were able to control for several potentially confounding factors. Second, our measure of vaccine hesitancy captured respondents’ intention to get vaccinated. Bussink-Coorend et al. 54 discussed the challenge to conceptualize vaccine hesitancy and the lack of a precise definition. While we show the robustness of our results to an alternative measure (vaccination confidence), we did not consider cognitive and affective aspects of indecisiveness. Third, using social media to recruit respondents, our sample was rather representative of a social media user population, but however, not representative of the national population in the respective countries. Lastly, participants in our survey were adults, yet polio and HPV vaccinations are mainly given to children and teenagers. While the prior literature showed that parents often decide for their children 55 , 56 , 57 , adult responses might be biased and not fully capture vaccine hesitancy of the target group.
Data collection and sample description
Our analyses were based on primary data that we collected between February to March 2023 via online surveys in six SSA countries (Ghana, Kenya, Nigeria, South Africa, Tanzania, and Uganda). The surveys were implemented on the UniPark platform. We recruited participants through paid advertisements on Facebook. Advertisements targeted all Facebook users in the target countries. The Facebook ads stated that survey participants had the chance to win phone credit upon successful completion of the survey. 12,975,764 persons saw the advertisements. Facebook users that were interested in participating could click on a link and were directed to our surveys on UniPark. Respondents had to be adults (18 years or older). Informed consent was obtained from all participants at the beginning of each survey. Respondents did not receive any compensation for survey participation. However, in order to motivate participation, we distributed twelve 5G mobile phone credits among all survey participants. The twelve persons were randomly selected after the finalization of the surveys. We obtained ethical approval from the ethical commission of the medical association in Hamburg, called ‘Ethik-Kommission der Ar̈ztekammer Hamburg’. All methods were performed in accordance with relevant guidelines and regulations.
The surveys gathered information on a number of individual characteristics, attitudes towards health topics, health knowledge, and health behavior. The surveys were completed by 5203 participants. During the data cleaning process, we dropped observations that did not pass quality controls (attention checks; participants that typically live outside of the targeted countries). The majority of participants were from Kenya. The average age of respondents was 29.15 years. 35% of respondents were female. Summary statistics on the main variables are presented in Supplementary Table B2 in Appendix B . In general, the sample appeared to be representative of the social media user population. It was, however, not representative of the general population.
Experimental set-up
The survey comprised three parts. Part 1 collected information on the socio-demographic characteristics of the respondents, including age, gender, religion, wealth, among others. In Part 2 respondents were randomly assigned to one of the following three vaccine-type groups: COVID-19, HPV, polio. Respondents in the COVID-19 group received a short text about the COMIRNATY BA.4/BA.5 vaccine, whereas respondents in the HPV group received a short text describing the GARDASIL4 and CERVARIX vaccine. Respondents in the polio group read a text about the nOPV2 vaccine. The texts are presented in Supplementary Appendix C together with the survey questionnaire. In Part 3 all respondents received identical survey questions measuring trust into the different institutions and vaccine hesitancy. We performed balance tests that are presented in Supplementary Table B9 in Appendix B . We showed that overall the samples are highly comparable with respect to almost all socio-demographic characteristics. An exception was gender that we included as control in all regressions.
The three vaccines
We considered information regarding three vaccines in the survey: the Comirnaty Ba.4/Ba.5 vaccine against COVID-19, the Gardasil-4 against HPV, and the nOPV2 vaccine against polio. We selected these three vaccines because of their current relevance. For most countries these vaccines have been recently integrated in the national immunization program or are planned to do so. LMICs started HPV vaccination for young girls in 2012. Yet, the use of the GARDASIL4 and CERVARIX vaccine is currently small. Nigeria and Ghana planned to include the vaccine in their vaccination program this year. Kenya, Tanzania, Uganda, and South Africa have included it in their national campaigns already. Referring to COVID-19, the COMIRNATY BA.4/BA.5 vaccine is an adapted version of the mRNA COVID-19 vaccine Comirnaty (Pfizer/BioNTech) and recommended for use in people age 12 and older. It is approved in several countries (EU, US, Canada), but still in the process to be approved in most African countries. Regarding polio, the nOPV2 vaccine is a novel oral poliomyelitis (polio) vaccine that was developed to address the increasing risk of vaccine-derived polio-virus type 2 (cVDPV2). It received a recommendation for use in November 2020. The vaccine is used only for polio outbreak response. Hence, it is distributed according to outbreaks. It has been used in several African countries. The highest share of nOPV2 doses went to Nigeria 58 . All three vaccines have been approved by recognized authorities. For instance, the COVID-19 and HPV vaccine received EMA approval in 2006 and 2022 59 , 60 , and the polio vaccine has been authorized for the WHO Emergency Use Listing 61 .
Variable construction
Our outcome of interest was vaccine hesitancy. In the survey, respondents were presented a short text about a new vaccine and asked about their agreement with the following statement “I intend to get vaccinated against [vaccine type] with the new vaccine or will encourage one of my family members to do so”. The question varies across randomized sub-groups (COVID-19, polio, HPV). Respondents could answer on a 7-point Likert scale that ranged from 0 “strongly disagree” to 6 “strongly agree”. Based on this survey item, we constructed a binary indicator of vaccine hesitancy that takes the value one if responses to the survey item were below 3. For robustness checks, we used vaccination confidence as an alternative outcome of interest. Vaccination confidence was an index (average value) constructed on basis of three survey items: (i) “I worry about the side effects of vaccines.”, (ii) “After getting vaccinated, I feel protected.” and (iii) “I believe that vaccines often cause more harm than good.” Respondents gave their agreement to the three statements on a 7 point Likert scale. The first and third item entered the score in reversed form.
The main explanatory variables in our study were measures of trust in various institutions. We standardized all trust variables to be able to compare magnitudes. Trust in science and media was measured with the respondents’ evaluation of the trustworthiness of health-related information from the named institution on a 4 point Likert scale ranging from 0 “not at all trustworthy” to 3 “a lot trustworthy”. Trust in pharmaceutical firms was measured with the respondents agreement to the following question item “I believe that Western countries use pharmaceutical companies to exploit African people for their own purposes.” in a reverse order. Participants could agree or disagree on a 7 point Likert scale. We constructed an index for trust in government using the average score of the following three question items: (i) “In your opinion, how trustworthy are health-related information from the government?”, answered on a 4 point Likert scale, (ii) “How much do you trust in the ministry of health in your country?” answered with a 7 point Likert scale, and (iii) the respondent’s agreement to the statement “I believe that governmental regulations in my country ensure quality vaccines and drugs.” on a 7 point Likert scale. Lastly, trust in society was assessed with the respondent’s belief about the share of persons in the country that would like to get vaccinated with the described vaccine. Supplementary Table B8 in Appendix B presents the correlation matrix of our trust measures.
Lastly, we considered a wide range of individual characteristics. First, the following socio-demographic characteristics were considered: gender (0 = male, 1 = female), age in number of years, highest level of education (ranging from no education to university/tertiary education), married (indicator whether person is married), work status ((self-)employed = 1, 0 otherwise), wealth (very poor, poor, average, rich, very rich relative to others), and religion (Islam, Christianity, traditional beliefs, no religion, other). The socio-economic factors were based on self-reported statements. Moreover, we assessed two personality traits (agreeableness and openness to new experiences) using TIPI personality test items. Moreover, we controlled for past vaccinations including indicator variables that identify persons that have been vaccinated against polio, HPV, and COVID-19 based on self-reported responses.
Empirical strategy
In order to estimate the relationship between trust in different institutions and vaccine hesitancy, we run a multivariate linear probability model. We regressed an indicator variable that captures whether an individual intends to get vaccinated on trust measures using the following OLS model:
where \(Y_{ivc}\) was a binary indicator that captures whether respondent i in country c had no intention to get vaccinated with vaccine v . The principal explanatory variables of institutional trust were included in the matrix \(\textbf{Trust}_{ic}\) . We considered trust in science, the national government, national media, pharmaceutical firms, and society. \(\textbf{X}_{ic}\) was a matrix of individual-level characteristics including socio-demographics (age, gender, religion, education, wealth, employment and marital status, and personality traits) and a person’s own vaccination history regarding polio, HPV, and COVID-19. \(\eta _{v}\) related to vaccine-type fixed effects, \(\phi _{c}\) represented country fixed effects, and \(\varepsilon _{iv}\) was the error term. Standard errors were robust. As common with cross-sectional regressions, the specification estimated conditional correlations between trust and vaccine hesitancy. We do not claim any causality as we cannot completely rule out omitted variable bias or reverse causality. All statistical analyses were conducted with Stata 18.
Data availability
Data and code of the analysis is available upon request from the corresponding author.
Larson, H. J., Gakidou, E. & Murray, C. J. The vaccine-hesitant moment. N. Engl. J. Med. 387 , 58–65 (2022).
Article CAS PubMed Google Scholar
WHO. Ten threats to global health in 2019. Website. The World Health Organization. (2019). https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 .
Jansen, V. A. et al. Measles outbreaks in a population with declining vaccine uptake. Science 301 , 804–804 (2003).
Carrieri, V., Guthmuller, S. & Wübker, A. Trust and COVID-19 vaccine hesitancy. Sci. Rep. 13 , 9245 (2023).
Article ADS CAS PubMed PubMed Central Google Scholar
Pertwee, E., Simas, C. & Larson, H. J. An epidemic of uncertainty: Rumors, conspiracy theories and vaccine hesitancy. Nat. Med. 28 , 456–459 (2022).
Sapienza, A. & Falcone, R. The role of trust in COVID-19 vaccine acceptance: Considerations from a systematic review. Int. J. Environ. Res. Public Health 20 , 665 (2022).
Article PubMed PubMed Central Google Scholar
Adhikari, B., Cheah, P. Y. & von Seidlein, L. Trust is the common denominator for COVID-19 vaccine acceptance: A literature review. Vaccine: X 100213 (2022).
Stoop, N., Hirvonen, K. & Maystadt, J.-F. Institutional mistrust and child vaccination coverage in Africa. BMJ Glob. Health 6 , e004595 (2021).
Galdikiene, L., Jaraite, J. & Kajackaite, A. Trust and vaccination intentions: Evidence from Lithuania during the COVID-19 pandemic. PLoS One 17 , e0278060 (2022).
Article CAS PubMed PubMed Central Google Scholar
Riad, A. et al. Prevalence and drivers of COVID-19 vaccine hesitancy among Czech university students: National cross-sectional study. Vaccines 9 , 948 (2021).
Solís Arce, J. et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 27 , 1385–1394 (2021).
Jennings, W. et al. Lack of trust, conspiracy beliefs, and social media use predict COVID-19 vaccine hesitancy. Vaccines 9 , 593 (2021).
Rozek, L. S. et al. Understanding vaccine hesitancy in the context of COVID-19: The role of trust and confidence in a seventeen-country survey. Int. J. Public Health 66 , 636255 (2021).
Sturgis, P., Brunton-Smith, I. & Jackson, J. Trust in science, social consensus and vaccine confidence. Nat. Hum. Behav. 5 , 1528–1534 (2021).
Article PubMed Google Scholar
Cadeddu, C. et al. Vaccine hesitancy and trust in the scientific community in Italy: Comparative analysis from two recent surveys. Vaccines 9 , 1206 (2021).
Caserotti, M. et al. Associations of COVID-19 risk perception with vaccine hesitancy over time for Italian residents. Soc. Sci. Med. 272 , 113688 (2021).
Collis, A. et al. Global survey on COVID-19 beliefs, behaviours and norms. Nat. Hum. Behav. 6 , 1310–1317 (2022).
Kanyanda, S., Markhof, Y., Wollburg, P. & Zezza, A. Acceptance of COVID-19 vaccines in sub-Saharan Africa: Evidence from six national phone surveys. BMJ Open 11 , e055159 (2021).
Kutasi, K. et al. Understanding hesitancy with revealed preferences across COVID-19 vaccine types. Sci. Rep. 12 , 13293 (2022).
Larson, H. J. et al. The state of vaccine confidence 2016: Global insights through a 67-country survey. EBioMedicine 12 , 295–301 (2016).
Lazarus, J. V. et al. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat. Commun. 13 , 3801 (2022).
Lazarus, J. V. et al. A survey of COVID-19 vaccine acceptance across 23 countries in 2022. Nat. Med. 29 , 366–375 (2023).
Patwary, M. M. et al. COVID-19 vaccine acceptance among low-and lower-middle-income countries: A rapid systematic review and meta-analysis. Vaccines 10 , 427 (2022).
Sallam, M. COVID-19 vaccine hesitancy worldwide: A concise systematic review of vaccine acceptance rates. Vaccines 9 , 160 (2021).
Solís Arce, J. S. et al. COVID-19 vaccine acceptance and hesitancy in low-and middle-income countries. Nat. Med. 27 , 1385–1394 (2021).
Sprengholz, P., Eitze, S., Korn, L., Siegers, R. & Betsch, C. The power of choice: Experimental evidence that freedom to choose a vaccine against COVID-19 improves willingness to be vaccinated. Eur. J. Intern. Med. 87 , 106–108 (2021).
Cooper, S., Betsch, C., Sambala, E. Z., Mchiza, N. & Wiysonge, C. S. Vaccine hesitancy-a potential threat to the achievements of vaccination programmes in Africa. Hum. Vaccines Immunother. 14 , 2355–2357 (2018).
Article Google Scholar
Dinga, J. N. et al. Quantitative synthesis of factors associated with COVID-19 vaccine acceptance and vaccine hesitancy in 185 countries. Vaccines 12 , 34 (2023).
de Figueiredo, A., Temfack, E., Tajudeen, R. & Larson, H. Declining trends in vaccine confidence across sub-Saharan Africa: A large-scale cross-sectional modeling study. Hum. Vaccines Immunother. 2213117 (2023).
Wiegand, M. et al. Global declines in vaccine confidence from 2015 to 2022: A large-scale retrospective analysis. Available at SSRN 4438003 (2023).
Shen, A. K., Grundmeier, R. W. & Michel, J. J. Trends in vaccine refusal and acceptance using electronic health records from a large pediatric hospital network, 2013–2020: Strategies for change. Vaccines 10 , 1688 (2022).
Archibong, B. & Annan, F. ‘We are not guinea pigs’: The effects of disclosure of medical misconduct on vaccine compliance. Tech. Rep. https://doi.org/10.2139/ssrn.3765793 (2021).
Dubé, E., Vivion, M. & MacDonald, N. E. Vaccine hesitancy, vaccine refusal and the anti-vaccine movement: Influence, impact and implications. Expert Rev. Vaccines 14 , 99–117 (2015).
Mutombo, P. N. et al. COVID-19 vaccine hesitancy in Africa: A call to action. Lancet Glob. Health 10 , e320–e321 (2022).
Lowes, S. & Montero, E. The legacy of colonial medicine in Central Africa. Am. Econ. Rev. 111 , 1284–1314 (2021).
Larson, H. J. et al. Measuring trust in vaccination: A systematic review. Hum. Vaccines Immunother. 14 , 1599–1609 (2018).
Miner, C. et al. Acceptance of COVID-19 vaccine among sub-Saharan Africans (SSA): A comparative study of residents and diasporan dwellers. BMC Public Health. 23 (2023).
Strupat, C., Shigute, Z., Bedi, A. & Rieger, M. Willingness to take COVID-19 vaccination in low-income countries: Evidence from Ethiopia. PLoS One 17 , e0264633 (2022).
Wollburg, P., Markhof, Y., Kanyanda, S. & Zezza, A. The evolution of COVID-19 vaccine hesitancy in Sub-Saharan Africa: Evidence from panel survey data. BMC Public Health. 17 (2023).
Démolis, R. et al. A rapid qualitative assessment of oral cholera vaccine anticipated acceptability in a context of resistance towards cholera intervention in Nampula, Mozambique. Vaccine 36 , 6497–6505 (2018).
Vinck, P., Pham, P. N., Bindu, K. K., Bedford, J. & Nilles, E. J. Institutional trust and misinformation in the response to the 2018–19 Ebola outbreak in North Kivu, DR Congo: A population-based survey. Lancet Infect. Dis. 19 , 529–536 (2019).
Ostrom, E. Collective action and the evolution of social norms. J. Econ. Perspect. 14 , 137–158 (2000).
Kocher, M. G., Martinsson, P., Matzat, D. & Wollbrant, C. The role of beliefs, trust, and risk in contributions to a public good. J. Econ. Psychol. 51 , 236–244 (2015).
Jordan, J. J., Yoeli, E. & Rand, D. G. Don’t get it or don’t spread it: Comparing self-interested versus prosocial motivations for COVID-19 prevention behaviors. Sci. Rep. 11 , 20222 (2021).
Heiman, S. L. et al. Descriptive norms caused increases in mask wearing during the COVID-19 pandemic. Sci. Rep. 13 , 11856 (2023).
Campos-Mercade, P., Meier, A. N., Schneider, F. H. & Wengström, E. Prosociality predicts health behaviors during the COVID-19 pandemic. J. Public Econ. 195 , 104367 (2021).
Acheampong, T. et al. Examining vaccine hesitancy in Sub-Saharan Africa: A survey of the knowledge and attitudes among adults to receive COVID-19 vaccines in Ghana. Vaccines 9 , 814 (2021).
Kahn, K. et al. COVID-19 vaccine hesitancy in rural South Africa: Deepening understanding to increase uptake and access. J. Glob. Health. 12 (2022).
Orangi, S. et al. Assessing the level and determinants of COVID-19 vaccine confidence in Kenya. Vaccines 9 , 936 (2021).
Dinga, J. N., Sinda, L. K. & Titanji, V. P. Assessment of vaccine hesitancy to a COVID-19 vaccine in Cameroonian adults and its global implication. Vaccines 9 , 175 (2021).
Seydou, A. Who wants COVID-19 vaccination? In 5 West African countries, hesitancy is high, trust low. Tech. Rep., Afrobarometer . Afrobarometer Dispatch No. 432 (2021).
Schünemann, J., Strulik, H. & Trimborn, T. The gender gap in mortality: How much is explained by behavior?. J. Health Econ. 54 , 79–90 (2017).
Galasso, V. et al. Gender differences in COVID-19 attitudes and behavior: Panel evidence from eight countries. Proc. Natl. Acad. Sci. 117 , 27285–27291 (2020).
Bussink-Voorend, D., Hautvast, J. L., Vandeberg, L., Visser, O. & Hulscher, M. E. A systematic literature review to clarify the concept of vaccine hesitancy. Nat. Hum. Behav. 6 , 1634–1648 (2022).
Damnjanović, K. et al. Parental decision-making on childhood vaccination. Front. Psychol. 9 , 735 (2018).
Callaghan, T., Motta, M., Sylvester, S., Trujillo, K. L. & Blackburn, C. C. Parent psychology and the decision to delay childhood vaccination. Soc. Sci. Med. 238 , 112407 (2019).
Burdette, A. M., Gordon-Jokinen, H. & Hill, T. D. Social determinants of HPV vaccination delay rationales: Evidence from the 2011 National Immunization Survey-Teen. Prev. Med. Rep. 1 , 21–26 (2014).
WHO. Safety profile of nOPV2 vaccine. Website (2023). The World Health Organization. https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/poliovirus-vaccines .
EMA. Adapted vaccine targeting BA.4 and BA.5 Omicron variants and original SARS-CoV-2 recommended for approval. Tech. Rep., European Medicines Agency (2022). https://www.ema.europa.eu/en/news/adapted-vaccine-targeting-ba4-ba5-omicron-variants-original-sars-cov-2-recommended-approval (accessed 15 Nov 2023).
EMA. Gardasil. Tech. Rep., European Medicines Agency (2022). https://www.ema.europa.eu/en/medicines/human/EPAR/gardasil#authorisation-details-section (accessed 15 Nov 2023).
WHO. Safety profile of nOPV2 vaccine. Tech. Rep., World Health Organization (2023). https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/poliovirus-vaccines (accessed 15 Nov 2023).
Download references
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Health Economics Research Group, Bernhard Nocht Institute for Tropical Medicine (BNITM), Hamburg, Germany
Kerstin Unfried & Jan Priebe
Hamburg Center for Health Economics (HCHE), Hamburg, Germany
You can also search for this author in PubMed Google Scholar
Contributions
Both authors contributed to the design of the survey and its implementation. K.U. analysed the results. Both authors wrote and reviewed the manuscript.
Corresponding authors
Correspondence to Kerstin Unfried or Jan Priebe .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary information., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Unfried, K., Priebe, J. Vaccine hesitancy and trust in sub-Saharan Africa. Sci Rep 14 , 10860 (2024). https://doi.org/10.1038/s41598-024-61205-0
Download citation
Received : 29 August 2023
Accepted : 02 May 2024
Published : 13 May 2024
DOI : https://doi.org/10.1038/s41598-024-61205-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: AI and Robotics newsletter — what matters in AI and robotics research, free to your inbox weekly.
AlphaFold 3 predicts the structure and interactions of all of life’s molecules
May 08, 2024
[[read-time]] min read
Introducing AlphaFold 3, a new AI model developed by Google DeepMind and Isomorphic Labs. By accurately predicting the structure of proteins, DNA, RNA, ligands and more, and how they interact, we hope it will transform our understanding of the biological world and drug discovery.

Inside every plant, animal and human cell are billions of molecular machines. They’re made up of proteins, DNA and other molecules, but no single piece works on its own. Only by seeing how they interact together, across millions of types of combinations, can we start to truly understand life’s processes.
In a paper published in Nature , we introduce AlphaFold 3, a revolutionary model that can predict the structure and interactions of all life’s molecules with unprecedented accuracy. For the interactions of proteins with other molecule types we see at least a 50% improvement compared with existing prediction methods, and for some important categories of interaction we have doubled prediction accuracy.
We hope AlphaFold 3 will help transform our understanding of the biological world and drug discovery. Scientists can access the majority of its capabilities, for free, through our newly launched AlphaFold Server , an easy-to-use research tool. To build on AlphaFold 3’s potential for drug design, Isomorphic Labs is already collaborating with pharmaceutical companies to apply it to real-world drug design challenges and, ultimately, develop new life-changing treatments for patients.
Our new model builds on the foundations of AlphaFold 2, which in 2020 made a fundamental breakthrough in protein structure prediction . So far, millions of researchers globally have used AlphaFold 2 to make discoveries in areas including malaria vaccines, cancer treatments and enzyme design. AlphaFold has been cited more than 20,000 times and its scientific impact recognized through many prizes, most recently the Breakthrough Prize in Life Sciences . AlphaFold 3 takes us beyond proteins to a broad spectrum of biomolecules. This leap could unlock more transformative science, from developing biorenewable materials and more resilient crops, to accelerating drug design and genomics research.
7PNM - Spike protein of a common cold virus (Coronavirus OC43): AlphaFold 3’s structural prediction for a spike protein (blue) of a cold virus as it interacts with antibodies (turquoise) and simple sugars (yellow), accurately matches the true structure (gray). The animation shows the protein interacting with an antibody, then a sugar. Advancing our knowledge of such immune-system processes helps better understand coronaviruses, including COVID-19, raising possibilities for improved treatments.
How AlphaFold 3 reveals life’s molecules
Given an input list of molecules, AlphaFold 3 generates their joint 3D structure, revealing how they all fit together. It models large biomolecules such as proteins, DNA and RNA, as well as small molecules, also known as ligands — a category encompassing many drugs. Furthermore, AlphaFold 3 can model chemical modifications to these molecules which control the healthy functioning of cells, that when disrupted can lead to disease.
AlphaFold 3’s capabilities come from its next-generation architecture and training that now covers all of life’s molecules. At the core of the model is an improved version of our Evoformer module — a deep learning architecture that underpinned AlphaFold 2’s incredible performance. After processing the inputs, AlphaFold 3 assembles its predictions using a diffusion network, akin to those found in AI image generators. The diffusion process starts with a cloud of atoms, and over many steps converges on its final, most accurate molecular structure.
AlphaFold 3’s predictions of molecular interactions surpass the accuracy of all existing systems. As a single model that computes entire molecular complexes in a holistic way, it’s uniquely able to unify scientific insights.
7R6R - DNA binding protein: AlphaFold 3’s prediction for a molecular complex featuring a protein (blue) bound to a double helix of DNA (pink) is a near-perfect match to the true molecular structure discovered through painstaking experiments (gray).
Leading drug discovery at Isomorphic Labs
AlphaFold 3 creates capabilities for drug design with predictions for molecules commonly used in drugs, such as ligands and antibodies, that bind to proteins to change how they interact in human health and disease.
AlphaFold 3 achieves unprecedented accuracy in predicting drug-like interactions, including the binding of proteins with ligands and antibodies with their target proteins. AlphaFold 3 is 50% more accurate than the best traditional methods on the PoseBusters benchmark without needing the input of any structural information, making AlphaFold 3 the first AI system to surpass physics-based tools for biomolecular structure prediction. The ability to predict antibody-protein binding is critical to understanding aspects of the human immune response and the design of new antibodies — a growing class of therapeutics.
Using AlphaFold 3 in combination with a complementary suite of in-house AI models, Isomorphic Labs is working on drug design for internal projects as well as with pharmaceutical partners. Isomorphic Labs is using AlphaFold 3 to accelerate and improve the success of drug design — by helping understand how to approach new disease targets, and developing novel ways to pursue existing ones that were previously out of reach.
AlphaFold Server: A free and easy-to-use research tool
8AW3 - RNA modifying protein: AlphaFold 3’s prediction for a molecular complex featuring a protein (blue), a strand of RNA (purple), and two ions (yellow) closely matches the true structure (gray). This complex is involved with the creation of other proteins — a cellular process fundamental to life and health.
Google DeepMind’s newly launched AlphaFold Server is the most accurate tool in the world for predicting how proteins interact with other molecules throughout the cell. It is a free platform that scientists around the world can use for non-commercial research. With just a few clicks, biologists can harness the power of AlphaFold 3 to model structures composed of proteins, DNA, RNA and a selection of ligands, ions and chemical modifications.
AlphaFold Server helps scientists make novel hypotheses to test in the lab, speeding up workflows and enabling further innovation. Our platform gives researchers an accessible way to generate predictions, regardless of their access to computational resources or their expertise in machine learning.
Experimental protein-structure prediction can take about the length of a PhD and cost hundreds of thousands of dollars. Our previous model, AlphaFold 2, has been used to predict hundreds of millions of structures, which would have taken hundreds of millions of researcher-years at the current rate of experimental structural biology.
Sharing the power of AlphaFold 3 responsibly
With each AlphaFold release, we’ve sought to understand the broad impact of the technology , working together with the research and safety community. We take a science-led approach and have conducted extensive assessments to mitigate potential risks and share the widespread benefits to biology and humanity.
Building on the external consultations we carried out for AlphaFold 2, we’ve now engaged with more than 50 domain experts, in addition to specialist third parties, across biosecurity, research and industry, to understand the capabilities of successive AlphaFold models and any potential risks. We also participated in community-wide forums and discussions ahead of AlphaFold 3’s launch.
AlphaFold Server reflects our ongoing commitment to share the benefits of AlphaFold, including our free database of 200 million protein structures. We’ll also be expanding our free AlphaFold education online course with EMBL-EBI and partnerships with organizations in the Global South to equip scientists with the tools they need to accelerate adoption and research, including on underfunded areas such as neglected diseases and food security. We’ll continue to work with the scientific community and policy makers to develop and deploy AI technologies responsibly.
Opening up the future of AI-powered cell biology
7BBV - Enzyme: AlphaFold 3’s prediction for a molecular complex featuring an enzyme protein (blue), an ion (yellow sphere) and simple sugars (yellow), along with the true structure (gray). This enzyme is found in a soil-borne fungus (Verticillium dahliae) that damages a wide range of plants. Insights into how this enzyme interacts with plant cells could help researchers develop healthier, more resilient crops.
AlphaFold 3 brings the biological world into high definition. It allows scientists to see cellular systems in all their complexity, across structures, interactions and modifications. This new window on the molecules of life reveals how they’re all connected and helps understand how those connections affect biological functions — such as the actions of drugs, the production of hormones and the health-preserving process of DNA repair.
The impacts of AlphaFold 3 and our free AlphaFold Server will be realized through how they empower scientists to accelerate discovery across open questions in biology and new lines of research. We’re just beginning to tap into AlphaFold 3’s potential and can’t wait to see what the future holds.
Related stories

New support for AI advancement in Central and Eastern Europe

Bringing Gemini to Google Workspace for Education

8 new accessibility updates across Lookout, Google Maps and more

100 things we announced at I/O 2024

How Google’s AI model Gemini got its name

How The FA uses Google Cloud AI to identify future England football stars
Let’s stay in touch. Get the latest news from Google in your inbox.

Cultural Relativity and Acceptance of Embryonic Stem Cell Research
Article sidebar.
Main Article Content
There is a debate about the ethical implications of using human embryos in stem cell research, which can be influenced by cultural, moral, and social values. This paper argues for an adaptable framework to accommodate diverse cultural and religious perspectives. By using an adaptive ethics model, research protections can reflect various populations and foster growth in stem cell research possibilities.
INTRODUCTION
Stem cell research combines biology, medicine, and technology, promising to alter health care and the understanding of human development. Yet, ethical contention exists because of individuals’ perceptions of using human embryos based on their various cultural, moral, and social values. While these disagreements concerning policy, use, and general acceptance have prompted the development of an international ethics policy, such a uniform approach can overlook the nuanced ethical landscapes between cultures. With diverse viewpoints in public health, a single global policy, especially one reflecting Western ethics or the ethics prevalent in high-income countries, is impractical. This paper argues for a culturally sensitive, adaptable framework for the use of embryonic stem cells. Stem cell policy should accommodate varying ethical viewpoints and promote an effective global dialogue. With an extension of an ethics model that can adapt to various cultures, we recommend localized guidelines that reflect the moral views of the people those guidelines serve.
Stem cells, characterized by their unique ability to differentiate into various cell types, enable the repair or replacement of damaged tissues. Two primary types of stem cells are somatic stem cells (adult stem cells) and embryonic stem cells. Adult stem cells exist in developed tissues and maintain the body’s repair processes. [1] Embryonic stem cells (ESC) are remarkably pluripotent or versatile, making them valuable in research. [2] However, the use of ESCs has sparked ethics debates. Considering the potential of embryonic stem cells, research guidelines are essential. The International Society for Stem Cell Research (ISSCR) provides international stem cell research guidelines. They call for “public conversations touching on the scientific significance as well as the societal and ethical issues raised by ESC research.” [3] The ISSCR also publishes updates about culturing human embryos 14 days post fertilization, suggesting local policies and regulations should continue to evolve as ESC research develops. [4] Like the ISSCR, which calls for local law and policy to adapt to developing stem cell research given cultural acceptance, this paper highlights the importance of local social factors such as religion and culture.
I. Global Cultural Perspective of Embryonic Stem Cells
Views on ESCs vary throughout the world. Some countries readily embrace stem cell research and therapies, while others have stricter regulations due to ethical concerns surrounding embryonic stem cells and when an embryo becomes entitled to moral consideration. The philosophical issue of when the “someone” begins to be a human after fertilization, in the morally relevant sense, [5] impacts when an embryo becomes not just worthy of protection but morally entitled to it. The process of creating embryonic stem cell lines involves the destruction of the embryos for research. [6] Consequently, global engagement in ESC research depends on social-cultural acceptability.
a. US and Rights-Based Cultures
In the United States, attitudes toward stem cell therapies are diverse. The ethics and social approaches, which value individualism, [7] trigger debates regarding the destruction of human embryos, creating a complex regulatory environment. For example, the 1996 Dickey-Wicker Amendment prohibited federal funding for the creation of embryos for research and the destruction of embryos for “more than allowed for research on fetuses in utero.” [8] Following suit, in 2001, the Bush Administration heavily restricted stem cell lines for research. However, the Stem Cell Research Enhancement Act of 2005 was proposed to help develop ESC research but was ultimately vetoed. [9] Under the Obama administration, in 2009, an executive order lifted restrictions allowing for more development in this field. [10] The flux of research capacity and funding parallels the different cultural perceptions of human dignity of the embryo and how it is socially presented within the country’s research culture. [11]
b. Ubuntu and Collective Cultures
African bioethics differs from Western individualism because of the different traditions and values. African traditions, as described by individuals from South Africa and supported by some studies in other African countries, including Ghana and Kenya, follow the African moral philosophies of Ubuntu or Botho and Ukama , which “advocates for a form of wholeness that comes through one’s relationship and connectedness with other people in the society,” [12] making autonomy a socially collective concept. In this context, for the community to act autonomously, individuals would come together to decide what is best for the collective. Thus, stem cell research would require examining the value of the research to society as a whole and the use of the embryos as a collective societal resource. If society views the source as part of the collective whole, and opposes using stem cells, compromising the cultural values to pursue research may cause social detachment and stunt research growth. [13] Based on local culture and moral philosophy, the permissibility of stem cell research depends on how embryo, stem cell, and cell line therapies relate to the community as a whole. Ubuntu is the expression of humanness, with the person’s identity drawn from the “’I am because we are’” value. [14] The decision in a collectivistic culture becomes one born of cultural context, and individual decisions give deference to others in the society.
Consent differs in cultures where thought and moral philosophy are based on a collective paradigm. So, applying Western bioethical concepts is unrealistic. For one, Africa is a diverse continent with many countries with different belief systems, access to health care, and reliance on traditional or Western medicines. Where traditional medicine is the primary treatment, the “’restrictive focus on biomedically-related bioethics’” [is] problematic in African contexts because it neglects bioethical issues raised by traditional systems.” [15] No single approach applies in all areas or contexts. Rather than evaluating the permissibility of ESC research according to Western concepts such as the four principles approach, different ethics approaches should prevail.
Another consideration is the socio-economic standing of countries. In parts of South Africa, researchers have not focused heavily on contributing to the stem cell discourse, either because it is not considered health care or a health science priority or because resources are unavailable. [16] Each country’s priorities differ given different social, political, and economic factors. In South Africa, for instance, areas such as maternal mortality, non-communicable diseases, telemedicine, and the strength of health systems need improvement and require more focus [17] Stem cell research could benefit the population, but it also could divert resources from basic medical care. Researchers in South Africa adhere to the National Health Act and Medicines Control Act in South Africa and international guidelines; however, the Act is not strictly enforced, and there is no clear legislation for research conduct or ethical guidelines. [18]
Some parts of Africa condemn stem cell research. For example, 98.2 percent of the Tunisian population is Muslim. [19] Tunisia does not permit stem cell research because of moral conflict with a Fatwa. Religion heavily saturates the regulation and direction of research. [20] Stem cell use became permissible for reproductive purposes only recently, with tight restrictions preventing cells from being used in any research other than procedures concerning ART/IVF. Their use is conditioned on consent, and available only to married couples. [21] The community's receptiveness to stem cell research depends on including communitarian African ethics.
c. Asia
Some Asian countries also have a collective model of ethics and decision making. [22] In China, the ethics model promotes a sincere respect for life or human dignity, [23] based on protective medicine. This model, influenced by Traditional Chinese Medicine (TCM), [24] recognizes Qi as the vital energy delivered via the meridians of the body; it connects illness to body systems, the body’s entire constitution, and the universe for a holistic bond of nature, health, and quality of life. [25] Following a protective ethics model, and traditional customs of wholeness, investment in stem cell research is heavily desired for its applications in regenerative therapies, disease modeling, and protective medicines. In a survey of medical students and healthcare practitioners, 30.8 percent considered stem cell research morally unacceptable while 63.5 percent accepted medical research using human embryonic stem cells. Of these individuals, 89.9 percent supported increased funding for stem cell research. [26] The scientific community might not reflect the overall population. From 1997 to 2019, China spent a total of $576 million (USD) on stem cell research at 8,050 stem cell programs, increased published presence from 0.6 percent to 14.01 percent of total global stem cell publications as of 2014, and made significant strides in cell-based therapies for various medical conditions. [27] However, while China has made substantial investments in stem cell research and achieved notable progress in clinical applications, concerns linger regarding ethical oversight and transparency. [28] For example, the China Biosecurity Law, promoted by the National Health Commission and China Hospital Association, attempted to mitigate risks by introducing an institutional review board (IRB) in the regulatory bodies. 5800 IRBs registered with the Chinese Clinical Trial Registry since 2021. [29] However, issues still need to be addressed in implementing effective IRB review and approval procedures.
The substantial government funding and focus on scientific advancement have sometimes overshadowed considerations of regional cultures, ethnic minorities, and individual perspectives, particularly evident during the one-child policy era. As government policy adapts to promote public stability, such as the change from the one-child to the two-child policy, [30] research ethics should also adapt to ensure respect for the values of its represented peoples.
Japan is also relatively supportive of stem cell research and therapies. Japan has a more transparent regulatory framework, allowing for faster approval of regenerative medicine products, which has led to several advanced clinical trials and therapies. [31] South Korea is also actively engaged in stem cell research and has a history of breakthroughs in cloning and embryonic stem cells. [32] However, the field is controversial, and there are issues of scientific integrity. For example, the Korean FDA fast-tracked products for approval, [33] and in another instance, the oocyte source was unclear and possibly violated ethical standards. [34] Trust is important in research, as it builds collaborative foundations between colleagues, trial participant comfort, open-mindedness for complicated and sensitive discussions, and supports regulatory procedures for stakeholders. There is a need to respect the culture’s interest, engagement, and for research and clinical trials to be transparent and have ethical oversight to promote global research discourse and trust.
d. Middle East
Countries in the Middle East have varying degrees of acceptance of or restrictions to policies related to using embryonic stem cells due to cultural and religious influences. Saudi Arabia has made significant contributions to stem cell research, and conducts research based on international guidelines for ethical conduct and under strict adherence to guidelines in accordance with Islamic principles. Specifically, the Saudi government and people require ESC research to adhere to Sharia law. In addition to umbilical and placental stem cells, [35] Saudi Arabia permits the use of embryonic stem cells as long as they come from miscarriages, therapeutic abortions permissible by Sharia law, or are left over from in vitro fertilization and donated to research. [36] Laws and ethical guidelines for stem cell research allow the development of research institutions such as the King Abdullah International Medical Research Center, which has a cord blood bank and a stem cell registry with nearly 10,000 donors. [37] Such volume and acceptance are due to the ethical ‘permissibility’ of the donor sources, which do not conflict with religious pillars. However, some researchers err on the side of caution, choosing not to use embryos or fetal tissue as they feel it is unethical to do so. [38]
Jordan has a positive research ethics culture. [39] However, there is a significant issue of lack of trust in researchers, with 45.23 percent (38.66 percent agreeing and 6.57 percent strongly agreeing) of Jordanians holding a low level of trust in researchers, compared to 81.34 percent of Jordanians agreeing that they feel safe to participate in a research trial. [40] Safety testifies to the feeling of confidence that adequate measures are in place to protect participants from harm, whereas trust in researchers could represent the confidence in researchers to act in the participants’ best interests, adhere to ethical guidelines, provide accurate information, and respect participants’ rights and dignity. One method to improve trust would be to address communication issues relevant to ESC. Legislation surrounding stem cell research has adopted specific language, especially concerning clarification “between ‘stem cells’ and ‘embryonic stem cells’” in translation. [41] Furthermore, legislation “mandates the creation of a national committee… laying out specific regulations for stem-cell banking in accordance with international standards.” [42] This broad regulation opens the door for future global engagement and maintains transparency. However, these regulations may also constrain the influence of research direction, pace, and accessibility of research outcomes.
e. Europe
In the European Union (EU), ethics is also principle-based, but the principles of autonomy, dignity, integrity, and vulnerability are interconnected. [43] As such, the opportunity for cohesion and concessions between individuals’ thoughts and ideals allows for a more adaptable ethics model due to the flexible principles that relate to the human experience The EU has put forth a framework in its Convention for the Protection of Human Rights and Dignity of the Human Being allowing member states to take different approaches. Each European state applies these principles to its specific conventions, leading to or reflecting different acceptance levels of stem cell research. [44]
For example, in Germany, Lebenzusammenhang , or the coherence of life, references integrity in the unity of human culture. Namely, the personal sphere “should not be subject to external intervention.” [45] Stem cell interventions could affect this concept of bodily completeness, leading to heavy restrictions. Under the Grundgesetz, human dignity and the right to life with physical integrity are paramount. [46] The Embryo Protection Act of 1991 made producing cell lines illegal. Cell lines can be imported if approved by the Central Ethics Commission for Stem Cell Research only if they were derived before May 2007. [47] Stem cell research respects the integrity of life for the embryo with heavy specifications and intense oversight. This is vastly different in Finland, where the regulatory bodies find research more permissible in IVF excess, but only up to 14 days after fertilization. [48] Spain’s approach differs still, with a comprehensive regulatory framework. [49] Thus, research regulation can be culture-specific due to variations in applied principles. Diverse cultures call for various approaches to ethical permissibility. [50] Only an adaptive-deliberative model can address the cultural constructions of self and achieve positive, culturally sensitive stem cell research practices. [51]
II. Religious Perspectives on ESC
Embryonic stem cell sources are the main consideration within religious contexts. While individuals may not regard their own religious texts as authoritative or factual, religion can shape their foundations or perspectives.
The Qur'an states:
“And indeed We created man from a quintessence of clay. Then We placed within him a small quantity of nutfa (sperm to fertilize) in a safe place. Then We have fashioned the nutfa into an ‘alaqa (clinging clot or cell cluster), then We developed the ‘alaqa into mudgha (a lump of flesh), and We made mudgha into bones, and clothed the bones with flesh, then We brought it into being as a new creation. So Blessed is Allah, the Best of Creators.” [52]
Many scholars of Islam estimate the time of soul installment, marked by the angel breathing in the soul to bring the individual into creation, as 120 days from conception. [53] Personhood begins at this point, and the value of life would prohibit research or experimentation that could harm the individual. If the fetus is more than 120 days old, the time ensoulment is interpreted to occur according to Islamic law, abortion is no longer permissible. [54] There are a few opposing opinions about early embryos in Islamic traditions. According to some Islamic theologians, there is no ensoulment of the early embryo, which is the source of stem cells for ESC research. [55]
In Buddhism, the stance on stem cell research is not settled. The main tenets, the prohibition against harming or destroying others (ahimsa) and the pursuit of knowledge (prajña) and compassion (karuna), leave Buddhist scholars and communities divided. [56] Some scholars argue stem cell research is in accordance with the Buddhist tenet of seeking knowledge and ending human suffering. Others feel it violates the principle of not harming others. Finding the balance between these two points relies on the karmic burden of Buddhist morality. In trying to prevent ahimsa towards the embryo, Buddhist scholars suggest that to comply with Buddhist tenets, research cannot be done as the embryo has personhood at the moment of conception and would reincarnate immediately, harming the individual's ability to build their karmic burden. [57] On the other hand, the Bodhisattvas, those considered to be on the path to enlightenment or Nirvana, have given organs and flesh to others to help alleviate grieving and to benefit all. [58] Acceptance varies on applied beliefs and interpretations.
Catholicism does not support embryonic stem cell research, as it entails creation or destruction of human embryos. This destruction conflicts with the belief in the sanctity of life. For example, in the Old Testament, Genesis describes humanity as being created in God’s image and multiplying on the Earth, referencing the sacred rights to human conception and the purpose of development and life. In the Ten Commandments, the tenet that one should not kill has numerous interpretations where killing could mean murder or shedding of the sanctity of life, demonstrating the high value of human personhood. In other books, the theological conception of when life begins is interpreted as in utero, [59] highlighting the inviolability of life and its formation in vivo to make a religious point for accepting such research as relatively limited, if at all. [60] The Vatican has released ethical directives to help apply a theological basis to modern-day conflicts. The Magisterium of the Church states that “unless there is a moral certainty of not causing harm,” experimentation on fetuses, fertilized cells, stem cells, or embryos constitutes a crime. [61] Such procedures would not respect the human person who exists at these stages, according to Catholicism. Damages to the embryo are considered gravely immoral and illicit. [62] Although the Catholic Church officially opposes abortion, surveys demonstrate that many Catholic people hold pro-choice views, whether due to the context of conception, stage of pregnancy, threat to the mother’s life, or for other reasons, demonstrating that practicing members can also accept some but not all tenets. [63]
Some major Jewish denominations, such as the Reform, Conservative, and Reconstructionist movements, are open to supporting ESC use or research as long as it is for saving a life. [64] Within Judaism, the Talmud, or study, gives personhood to the child at birth and emphasizes that life does not begin at conception: [65]
“If she is found pregnant, until the fortieth day it is mere fluid,” [66]
Whereas most religions prioritize the status of human embryos, the Halakah (Jewish religious law) states that to save one life, most other religious laws can be ignored because it is in pursuit of preservation. [67] Stem cell research is accepted due to application of these religious laws.
We recognize that all religions contain subsets and sects. The variety of environmental and cultural differences within religious groups requires further analysis to respect the flexibility of religious thoughts and practices. We make no presumptions that all cultures require notions of autonomy or morality as under the common morality theory , which asserts a set of universal moral norms that all individuals share provides moral reasoning and guides ethical decisions. [68] We only wish to show that the interaction with morality varies between cultures and countries.
III. A Flexible Ethical Approach
The plurality of different moral approaches described above demonstrates that there can be no universally acceptable uniform law for ESC on a global scale. Instead of developing one standard, flexible ethical applications must be continued. We recommend local guidelines that incorporate important cultural and ethical priorities.
While the Declaration of Helsinki is more relevant to people in clinical trials receiving ESC products, in keeping with the tradition of protections for research subjects, consent of the donor is an ethical requirement for ESC donation in many jurisdictions including the US, Canada, and Europe. [69] The Declaration of Helsinki provides a reference point for regulatory standards and could potentially be used as a universal baseline for obtaining consent prior to gamete or embryo donation.
For instance, in Columbia University’s egg donor program for stem cell research, donors followed standard screening protocols and “underwent counseling sessions that included information as to the purpose of oocyte donation for research, what the oocytes would be used for, the risks and benefits of donation, and process of oocyte stimulation” to ensure transparency for consent. [70] The program helped advance stem cell research and provided clear and safe research methods with paid participants. Though paid participation or covering costs of incidental expenses may not be socially acceptable in every culture or context, [71] and creating embryos for ESC research is illegal in many jurisdictions, Columbia’s program was effective because of the clear and honest communications with donors, IRBs, and related stakeholders. This example demonstrates that cultural acceptance of scientific research and of the idea that an egg or embryo does not have personhood is likely behind societal acceptance of donating eggs for ESC research. As noted, many countries do not permit the creation of embryos for research.
Proper communication and education regarding the process and purpose of stem cell research may bolster comprehension and garner more acceptance. “Given the sensitive subject material, a complete consent process can support voluntary participation through trust, understanding, and ethical norms from the cultures and morals participants value. This can be hard for researchers entering countries of different socioeconomic stability, with different languages and different societal values. [72]
An adequate moral foundation in medical ethics is derived from the cultural and religious basis that informs knowledge and actions. [73] Understanding local cultural and religious values and their impact on research could help researchers develop humility and promote inclusion.
IV. Concerns
Some may argue that if researchers all adhere to one ethics standard, protection will be satisfied across all borders, and the global public will trust researchers. However, defining what needs to be protected and how to define such research standards is very specific to the people to which standards are applied. We suggest that applying one uniform guide cannot accurately protect each individual because we all possess our own perceptions and interpretations of social values. [74] Therefore, the issue of not adjusting to the moral pluralism between peoples in applying one standard of ethics can be resolved by building out ethics models that can be adapted to different cultures and religions.
Other concerns include medical tourism, which may promote health inequities. [75] Some countries may develop and approve products derived from ESC research before others, compromising research ethics or drug approval processes. There are also concerns about the sale of unauthorized stem cell treatments, for example, those without FDA approval in the United States. Countries with robust research infrastructures may be tempted to attract medical tourists, and some customers will have false hopes based on aggressive publicity of unproven treatments. [76]
For example, in China, stem cell clinics can market to foreign clients who are not protected under the regulatory regimes. Companies employ a marketing strategy of “ethically friendly” therapies. Specifically, in the case of Beike, China’s leading stem cell tourism company and sprouting network, ethical oversight of administrators or health bureaus at one site has “the unintended consequence of shifting questionable activities to another node in Beike's diffuse network.” [77] In contrast, Jordan is aware of stem cell research’s potential abuse and its own status as a “health-care hub.” Jordan’s expanded regulations include preserving the interests of individuals in clinical trials and banning private companies from ESC research to preserve transparency and the integrity of research practices. [78]
The social priorities of the community are also a concern. The ISSCR explicitly states that guidelines “should be periodically revised to accommodate scientific advances, new challenges, and evolving social priorities.” [79] The adaptable ethics model extends this consideration further by addressing whether research is warranted given the varying degrees of socioeconomic conditions, political stability, and healthcare accessibilities and limitations. An ethical approach would require discussion about resource allocation and appropriate distribution of funds. [80]
While some religions emphasize the sanctity of life from conception, which may lead to public opposition to ESC research, others encourage ESC research due to its potential for healing and alleviating human pain. Many countries have special regulations that balance local views on embryonic personhood, the benefits of research as individual or societal goods, and the protection of human research subjects. To foster understanding and constructive dialogue, global policy frameworks should prioritize the protection of universal human rights, transparency, and informed consent. In addition to these foundational global policies, we recommend tailoring local guidelines to reflect the diverse cultural and religious perspectives of the populations they govern. Ethics models should be adapted to local populations to effectively establish research protections, growth, and possibilities of stem cell research.
For example, in countries with strong beliefs in the moral sanctity of embryos or heavy religious restrictions, an adaptive model can allow for discussion instead of immediate rejection. In countries with limited individual rights and voice in science policy, an adaptive model ensures cultural, moral, and religious views are taken into consideration, thereby building social inclusion. While this ethical consideration by the government may not give a complete voice to every individual, it will help balance policies and maintain the diverse perspectives of those it affects. Embracing an adaptive ethics model of ESC research promotes open-minded dialogue and respect for the importance of human belief and tradition. By actively engaging with cultural and religious values, researchers can better handle disagreements and promote ethical research practices that benefit each society.
This brief exploration of the religious and cultural differences that impact ESC research reveals the nuances of relative ethics and highlights a need for local policymakers to apply a more intense adaptive model.
[1] Poliwoda, S., Noor, N., Downs, E., Schaaf, A., Cantwell, A., Ganti, L., Kaye, A. D., Mosel, L. I., Carroll, C. B., Viswanath, O., & Urits, I. (2022). Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthopedic reviews , 14 (3), 37498. https://doi.org/10.52965/001c.37498
[2] Poliwoda, S., Noor, N., Downs, E., Schaaf, A., Cantwell, A., Ganti, L., Kaye, A. D., Mosel, L. I., Carroll, C. B., Viswanath, O., & Urits, I. (2022). Stem cells: a comprehensive review of origins and emerging clinical roles in medical practice. Orthopedic reviews , 14 (3), 37498. https://doi.org/10.52965/001c.37498
[3] International Society for Stem Cell Research. (2023). Laboratory-based human embryonic stem cell research, embryo research, and related research activities . International Society for Stem Cell Research. https://www.isscr.org/guidelines/blog-post-title-one-ed2td-6fcdk ; Kimmelman, J., Hyun, I., Benvenisty, N. et al. Policy: Global standards for stem-cell research. Nature 533 , 311–313 (2016). https://doi.org/10.1038/533311a
[4] International Society for Stem Cell Research. (2023). Laboratory-based human embryonic stem cell research, embryo research, and related research activities . International Society for Stem Cell Research. https://www.isscr.org/guidelines/blog-post-title-one-ed2td-6fcdk
[5] Concerning the moral philosophies of stem cell research, our paper does not posit a personal moral stance nor delve into the “when” of human life begins. To read further about the philosophical debate, consider the following sources:
Sandel M. J. (2004). Embryo ethics--the moral logic of stem-cell research. The New England journal of medicine , 351 (3), 207–209. https://doi.org/10.1056/NEJMp048145 ; George, R. P., & Lee, P. (2020, September 26). Acorns and Embryos . The New Atlantis. https://www.thenewatlantis.com/publications/acorns-and-embryos ; Sagan, A., & Singer, P. (2007). The moral status of stem cells. Metaphilosophy , 38 (2/3), 264–284. http://www.jstor.org/stable/24439776 ; McHugh P. R. (2004). Zygote and "clonote"--the ethical use of embryonic stem cells. The New England journal of medicine , 351 (3), 209–211. https://doi.org/10.1056/NEJMp048147 ; Kurjak, A., & Tripalo, A. (2004). The facts and doubts about beginning of the human life and personality. Bosnian journal of basic medical sciences , 4 (1), 5–14. https://doi.org/10.17305/bjbms.2004.3453
[6] Vazin, T., & Freed, W. J. (2010). Human embryonic stem cells: derivation, culture, and differentiation: a review. Restorative neurology and neuroscience , 28 (4), 589–603. https://doi.org/10.3233/RNN-2010-0543
[7] Socially, at its core, the Western approach to ethics is widely principle-based, autonomy being one of the key factors to ensure a fundamental respect for persons within research. For information regarding autonomy in research, see: Department of Health, Education, and Welfare, & National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (1978). The Belmont Report. Ethical principles and guidelines for the protection of human subjects of research.; For a more in-depth review of autonomy within the US, see: Beauchamp, T. L., & Childress, J. F. (1994). Principles of Biomedical Ethics . Oxford University Press.
[8] Sherley v. Sebelius , 644 F.3d 388 (D.C. Cir. 2011), citing 45 C.F.R. 46.204(b) and [42 U.S.C. § 289g(b)]. https://www.cadc.uscourts.gov/internet/opinions.nsf/6c690438a9b43dd685257a64004ebf99/$file/11-5241-1391178.pdf
[9] Stem Cell Research Enhancement Act of 2005, H. R. 810, 109 th Cong. (2001). https://www.govtrack.us/congress/bills/109/hr810/text ; Bush, G. W. (2006, July 19). Message to the House of Representatives . National Archives and Records Administration. https://georgewbush-whitehouse.archives.gov/news/releases/2006/07/20060719-5.html
[10] National Archives and Records Administration. (2009, March 9). Executive order 13505 -- removing barriers to responsible scientific research involving human stem cells . National Archives and Records Administration. https://obamawhitehouse.archives.gov/the-press-office/removing-barriers-responsible-scientific-research-involving-human-stem-cells
[11] Hurlbut, W. B. (2006). Science, Religion, and the Politics of Stem Cells. Social Research , 73 (3), 819–834. http://www.jstor.org/stable/40971854
[12] Akpa-Inyang, Francis & Chima, Sylvester. (2021). South African traditional values and beliefs regarding informed consent and limitations of the principle of respect for autonomy in African communities: a cross-cultural qualitative study. BMC Medical Ethics . 22. 10.1186/s12910-021-00678-4.
[13] Source for further reading: Tangwa G. B. (2007). Moral status of embryonic stem cells: perspective of an African villager. Bioethics , 21(8), 449–457. https://doi.org/10.1111/j.1467-8519.2007.00582.x , see also Mnisi, F. M. (2020). An African analysis based on ethics of Ubuntu - are human embryonic stem cell patents morally justifiable? African Insight , 49 (4).
[14] Jecker, N. S., & Atuire, C. (2021). Bioethics in Africa: A contextually enlightened analysis of three cases. Developing World Bioethics , 22 (2), 112–122. https://doi.org/10.1111/dewb.12324
[15] Jecker, N. S., & Atuire, C. (2021). Bioethics in Africa: A contextually enlightened analysis of three cases. Developing World Bioethics, 22(2), 112–122. https://doi.org/10.1111/dewb.12324
[16] Jackson, C.S., Pepper, M.S. Opportunities and barriers to establishing a cell therapy programme in South Africa. Stem Cell Res Ther 4 , 54 (2013). https://doi.org/10.1186/scrt204 ; Pew Research Center. (2014, May 1). Public health a major priority in African nations . Pew Research Center’s Global Attitudes Project. https://www.pewresearch.org/global/2014/05/01/public-health-a-major-priority-in-african-nations/
[17] Department of Health Republic of South Africa. (2021). Health Research Priorities (revised) for South Africa 2021-2024 . National Health Research Strategy. https://www.health.gov.za/wp-content/uploads/2022/05/National-Health-Research-Priorities-2021-2024.pdf
[18] Oosthuizen, H. (2013). Legal and Ethical Issues in Stem Cell Research in South Africa. In: Beran, R. (eds) Legal and Forensic Medicine. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-32338-6_80 , see also: Gaobotse G (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[19] United States Bureau of Citizenship and Immigration Services. (1998). Tunisia: Information on the status of Christian conversions in Tunisia . UNHCR Web Archive. https://webarchive.archive.unhcr.org/20230522142618/https://www.refworld.org/docid/3df0be9a2.html
[20] Gaobotse, G. (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[21] Kooli, C. Review of assisted reproduction techniques, laws, and regulations in Muslim countries. Middle East Fertil Soc J 24 , 8 (2020). https://doi.org/10.1186/s43043-019-0011-0 ; Gaobotse, G. (2018) Stem Cell Research in Africa: Legislation and Challenges. J Regen Med 7:1. doi: 10.4172/2325-9620.1000142
[22] Pang M. C. (1999). Protective truthfulness: the Chinese way of safeguarding patients in informed treatment decisions. Journal of medical ethics , 25(3), 247–253. https://doi.org/10.1136/jme.25.3.247
[23] Wang, L., Wang, F., & Zhang, W. (2021). Bioethics in China’s biosecurity law: Forms, effects, and unsettled issues. Journal of law and the biosciences , 8(1). https://doi.org/10.1093/jlb/lsab019 https://academic.oup.com/jlb/article/8/1/lsab019/6299199
[24] Wang, Y., Xue, Y., & Guo, H. D. (2022). Intervention effects of traditional Chinese medicine on stem cell therapy of myocardial infarction. Frontiers in pharmacology , 13 , 1013740. https://doi.org/10.3389/fphar.2022.1013740
[25] Li, X.-T., & Zhao, J. (2012). Chapter 4: An Approach to the Nature of Qi in TCM- Qi and Bioenergy. In Recent Advances in Theories and Practice of Chinese Medicine (p. 79). InTech.
[26] Luo, D., Xu, Z., Wang, Z., & Ran, W. (2021). China's Stem Cell Research and Knowledge Levels of Medical Practitioners and Students. Stem cells international , 2021 , 6667743. https://doi.org/10.1155/2021/6667743
[27] Luo, D., Xu, Z., Wang, Z., & Ran, W. (2021). China's Stem Cell Research and Knowledge Levels of Medical Practitioners and Students. Stem cells international , 2021 , 6667743. https://doi.org/10.1155/2021/6667743
[28] Zhang, J. Y. (2017). Lost in translation? accountability and governance of Clinical Stem Cell Research in China. Regenerative Medicine , 12 (6), 647–656. https://doi.org/10.2217/rme-2017-0035
[29] Wang, L., Wang, F., & Zhang, W. (2021). Bioethics in China’s biosecurity law: Forms, effects, and unsettled issues. Journal of law and the biosciences , 8(1). https://doi.org/10.1093/jlb/lsab019 https://academic.oup.com/jlb/article/8/1/lsab019/6299199
[30] Chen, H., Wei, T., Wang, H. et al. Association of China’s two-child policy with changes in number of births and birth defects rate, 2008–2017. BMC Public Health 22 , 434 (2022). https://doi.org/10.1186/s12889-022-12839-0
[31] Azuma, K. Regulatory Landscape of Regenerative Medicine in Japan. Curr Stem Cell Rep 1 , 118–128 (2015). https://doi.org/10.1007/s40778-015-0012-6
[32] Harris, R. (2005, May 19). Researchers Report Advance in Stem Cell Production . NPR. https://www.npr.org/2005/05/19/4658967/researchers-report-advance-in-stem-cell-production
[33] Park, S. (2012). South Korea steps up stem-cell work. Nature . https://doi.org/10.1038/nature.2012.10565
[34] Resnik, D. B., Shamoo, A. E., & Krimsky, S. (2006). Fraudulent human embryonic stem cell research in South Korea: lessons learned. Accountability in research , 13 (1), 101–109. https://doi.org/10.1080/08989620600634193 .
[35] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC medical ethics, 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6
[36] Association for the Advancement of Blood and Biotherapies. https://www.aabb.org/regulatory-and-advocacy/regulatory-affairs/regulatory-for-cellular-therapies/international-competent-authorities/saudi-arabia
[37] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: Interviews with researchers from Saudi Arabia. BMC medical ethics , 21 (1), 35. https://doi.org/10.1186/s12910-020-00482-6
[38] Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: Interviews with researchers from Saudi Arabia. BMC medical ethics , 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6
Culturally, autonomy practices follow a relational autonomy approach based on a paternalistic deontological health care model. The adherence to strict international research policies and religious pillars within the regulatory environment is a great foundation for research ethics. However, there is a need to develop locally targeted ethics approaches for research (as called for in Alahmad, G., Aljohani, S., & Najjar, M. F. (2020). Ethical challenges regarding the use of stem cells: interviews with researchers from Saudi Arabia. BMC medical ethics, 21(1), 35. https://doi.org/10.1186/s12910-020-00482-6), this decision-making approach may help advise a research decision model. For more on the clinical cultural autonomy approaches, see: Alabdullah, Y. Y., Alzaid, E., Alsaad, S., Alamri, T., Alolayan, S. W., Bah, S., & Aljoudi, A. S. (2022). Autonomy and paternalism in Shared decision‐making in a Saudi Arabian tertiary hospital: A cross‐sectional study. Developing World Bioethics , 23 (3), 260–268. https://doi.org/10.1111/dewb.12355 ; Bukhari, A. A. (2017). Universal Principles of Bioethics and Patient Rights in Saudi Arabia (Doctoral dissertation, Duquesne University). https://dsc.duq.edu/etd/124; Ladha, S., Nakshawani, S. A., Alzaidy, A., & Tarab, B. (2023, October 26). Islam and Bioethics: What We All Need to Know . Columbia University School of Professional Studies. https://sps.columbia.edu/events/islam-and-bioethics-what-we-all-need-know
[39] Ababneh, M. A., Al-Azzam, S. I., Alzoubi, K., Rababa’h, A., & Al Demour, S. (2021). Understanding and attitudes of the Jordanian public about clinical research ethics. Research Ethics , 17 (2), 228-241. https://doi.org/10.1177/1747016120966779
[40] Ababneh, M. A., Al-Azzam, S. I., Alzoubi, K., Rababa’h, A., & Al Demour, S. (2021). Understanding and attitudes of the Jordanian public about clinical research ethics. Research Ethics , 17 (2), 228-241. https://doi.org/10.1177/1747016120966779
[41] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[42] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[43] The EU’s definition of autonomy relates to the capacity for creating ideas, moral insight, decisions, and actions without constraint, personal responsibility, and informed consent. However, the EU views autonomy as not completely able to protect individuals and depends on other principles, such as dignity, which “expresses the intrinsic worth and fundamental equality of all human beings.” Rendtorff, J.D., Kemp, P. (2019). Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. In: Valdés, E., Lecaros, J. (eds) Biolaw and Policy in the Twenty-First Century. International Library of Ethics, Law, and the New Medicine, vol 78. Springer, Cham. https://doi.org/10.1007/978-3-030-05903-3_3
[44] Council of Europe. Convention for the protection of Human Rights and Dignity of the Human Being with regard to the Application of Biology and Medicine: Convention on Human Rights and Biomedicine (ETS No. 164) https://www.coe.int/en/web/conventions/full-list?module=treaty-detail&treatynum=164 (forbidding the creation of embryos for research purposes only, and suggests embryos in vitro have protections.); Also see Drabiak-Syed B. K. (2013). New President, New Human Embryonic Stem Cell Research Policy: Comparative International Perspectives and Embryonic Stem Cell Research Laws in France. Biotechnology Law Report , 32 (6), 349–356. https://doi.org/10.1089/blr.2013.9865
[45] Rendtorff, J.D., Kemp, P. (2019). Four Ethical Principles in European Bioethics and Biolaw: Autonomy, Dignity, Integrity and Vulnerability. In: Valdés, E., Lecaros, J. (eds) Biolaw and Policy in the Twenty-First Century. International Library of Ethics, Law, and the New Medicine, vol 78. Springer, Cham. https://doi.org/10.1007/978-3-030-05903-3_3
[46] Tomuschat, C., Currie, D. P., Kommers, D. P., & Kerr, R. (Trans.). (1949, May 23). Basic law for the Federal Republic of Germany. https://www.btg-bestellservice.de/pdf/80201000.pdf
[47] Regulation of Stem Cell Research in Germany . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-germany
[48] Regulation of Stem Cell Research in Finland . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-finland
[49] Regulation of Stem Cell Research in Spain . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-spain
[50] Some sources to consider regarding ethics models or regulatory oversights of other cultures not covered:
Kara MA. Applicability of the principle of respect for autonomy: the perspective of Turkey. J Med Ethics. 2007 Nov;33(11):627-30. doi: 10.1136/jme.2006.017400. PMID: 17971462; PMCID: PMC2598110.
Ugarte, O. N., & Acioly, M. A. (2014). The principle of autonomy in Brazil: one needs to discuss it ... Revista do Colegio Brasileiro de Cirurgioes , 41 (5), 374–377. https://doi.org/10.1590/0100-69912014005013
Bharadwaj, A., & Glasner, P. E. (2012). Local cells, global science: The rise of embryonic stem cell research in India . Routledge.
For further research on specific European countries regarding ethical and regulatory framework, we recommend this database: Regulation of Stem Cell Research in Europe . Eurostemcell. (2017, April 26). https://www.eurostemcell.org/regulation-stem-cell-research-europe
[51] Klitzman, R. (2006). Complications of culture in obtaining informed consent. The American Journal of Bioethics, 6(1), 20–21. https://doi.org/10.1080/15265160500394671 see also: Ekmekci, P. E., & Arda, B. (2017). Interculturalism and Informed Consent: Respecting Cultural Differences without Breaching Human Rights. Cultura (Iasi, Romania) , 14 (2), 159–172.; For why trust is important in research, see also: Gray, B., Hilder, J., Macdonald, L., Tester, R., Dowell, A., & Stubbe, M. (2017). Are research ethics guidelines culturally competent? Research Ethics , 13 (1), 23-41. https://doi.org/10.1177/1747016116650235
[52] The Qur'an (M. Khattab, Trans.). (1965). Al-Mu’minun, 23: 12-14. https://quran.com/23
[53] Lenfest, Y. (2017, December 8). Islam and the beginning of human life . Bill of Health. https://blog.petrieflom.law.harvard.edu/2017/12/08/islam-and-the-beginning-of-human-life/
[54] Aksoy, S. (2005). Making regulations and drawing up legislation in Islamic countries under conditions of uncertainty, with special reference to embryonic stem cell research. Journal of Medical Ethics , 31: 399-403.; see also: Mahmoud, Azza. "Islamic Bioethics: National Regulations and Guidelines of Human Stem Cell Research in the Muslim World." Master's thesis, Chapman University, 2022. https://doi.org/10.36837/ chapman.000386
[55] Rashid, R. (2022). When does Ensoulment occur in the Human Foetus. Journal of the British Islamic Medical Association , 12 (4). ISSN 2634 8071. https://www.jbima.com/wp-content/uploads/2023/01/2-Ethics-3_-Ensoulment_Rafaqat.pdf.
[56] Sivaraman, M. & Noor, S. (2017). Ethics of embryonic stem cell research according to Buddhist, Hindu, Catholic, and Islamic religions: perspective from Malaysia. Asian Biomedicine,8(1) 43-52. https://doi.org/10.5372/1905-7415.0801.260
[57] Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[58] Lecso, P. A. (1991). The Bodhisattva Ideal and Organ Transplantation. Journal of Religion and Health , 30 (1), 35–41. http://www.jstor.org/stable/27510629 ; Bodhisattva, S. (n.d.). The Key of Becoming a Bodhisattva . A Guide to the Bodhisattva Way of Life. http://www.buddhism.org/Sutras/2/BodhisattvaWay.htm
[59] There is no explicit religious reference to when life begins or how to conduct research that interacts with the concept of life. However, these are relevant verses pertaining to how the fetus is viewed. (( King James Bible . (1999). Oxford University Press. (original work published 1769))
Jerimiah 1: 5 “Before I formed thee in the belly I knew thee; and before thou camest forth out of the womb I sanctified thee…”
In prophet Jerimiah’s insight, God set him apart as a person known before childbirth, a theme carried within the Psalm of David.
Psalm 139: 13-14 “…Thou hast covered me in my mother's womb. I will praise thee; for I am fearfully and wonderfully made…”
These verses demonstrate David’s respect for God as an entity that would know of all man’s thoughts and doings even before birth.
[60] It should be noted that abortion is not supported as well.
[61] The Vatican. (1987, February 22). Instruction on Respect for Human Life in Its Origin and on the Dignity of Procreation Replies to Certain Questions of the Day . Congregation For the Doctrine of the Faith. https://www.vatican.va/roman_curia/congregations/cfaith/documents/rc_con_cfaith_doc_19870222_respect-for-human-life_en.html
[62] The Vatican. (2000, August 25). Declaration On the Production and the Scientific and Therapeutic Use of Human Embryonic Stem Cells . Pontifical Academy for Life. https://www.vatican.va/roman_curia/pontifical_academies/acdlife/documents/rc_pa_acdlife_doc_20000824_cellule-staminali_en.html ; Ohara, N. (2003). Ethical Consideration of Experimentation Using Living Human Embryos: The Catholic Church’s Position on Human Embryonic Stem Cell Research and Human Cloning. Department of Obstetrics and Gynecology . Retrieved from https://article.imrpress.com/journal/CEOG/30/2-3/pii/2003018/77-81.pdf.
[63] Smith, G. A. (2022, May 23). Like Americans overall, Catholics vary in their abortion views, with regular mass attenders most opposed . Pew Research Center. https://www.pewresearch.org/short-reads/2022/05/23/like-americans-overall-catholics-vary-in-their-abortion-views-with-regular-mass-attenders-most-opposed/
[64] Rosner, F., & Reichman, E. (2002). Embryonic stem cell research in Jewish law. Journal of halacha and contemporary society , (43), 49–68.; Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[65] Schenker J. G. (2008). The beginning of human life: status of embryo. Perspectives in Halakha (Jewish Religious Law). Journal of assisted reproduction and genetics , 25 (6), 271–276. https://doi.org/10.1007/s10815-008-9221-6
[66] Ruttenberg, D. (2020, May 5). The Torah of Abortion Justice (annotated source sheet) . Sefaria. https://www.sefaria.org/sheets/234926.7?lang=bi&with=all&lang2=en
[67] Jafari, M., Elahi, F., Ozyurt, S. & Wrigley, T. (2007). 4. Religious Perspectives on Embryonic Stem Cell Research. In K. Monroe, R. Miller & J. Tobis (Ed.), Fundamentals of the Stem Cell Debate: The Scientific, Religious, Ethical, and Political Issues (pp. 79-94). Berkeley: University of California Press. https://escholarship.org/content/qt9rj0k7s3/qt9rj0k7s3_noSplash_f9aca2e02c3777c7fb76ea768ba458f0.pdf https://doi.org/10.1525/9780520940994-005
[68] Gert, B. (2007). Common morality: Deciding what to do . Oxford Univ. Press.
[69] World Medical Association (2013). World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA , 310(20), 2191–2194. https://doi.org/10.1001/jama.2013.281053 Declaration of Helsinki – WMA – The World Medical Association .; see also: National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. (1979). The Belmont report: Ethical principles and guidelines for the protection of human subjects of research . U.S. Department of Health and Human Services. https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html
[70] Zakarin Safier, L., Gumer, A., Kline, M., Egli, D., & Sauer, M. V. (2018). Compensating human subjects providing oocytes for stem cell research: 9-year experience and outcomes. Journal of assisted reproduction and genetics , 35 (7), 1219–1225. https://doi.org/10.1007/s10815-018-1171-z https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6063839/ see also: Riordan, N. H., & Paz Rodríguez, J. (2021). Addressing concerns regarding associated costs, transparency, and integrity of research in recent stem cell trial. Stem Cells Translational Medicine , 10 (12), 1715–1716. https://doi.org/10.1002/sctm.21-0234
[71] Klitzman, R., & Sauer, M. V. (2009). Payment of egg donors in stem cell research in the USA. Reproductive biomedicine online , 18 (5), 603–608. https://doi.org/10.1016/s1472-6483(10)60002-8
[72] Krosin, M. T., Klitzman, R., Levin, B., Cheng, J., & Ranney, M. L. (2006). Problems in comprehension of informed consent in rural and peri-urban Mali, West Africa. Clinical trials (London, England) , 3 (3), 306–313. https://doi.org/10.1191/1740774506cn150oa
[73] Veatch, Robert M. Hippocratic, Religious, and Secular Medical Ethics: The Points of Conflict . Georgetown University Press, 2012.
[74] Msoroka, M. S., & Amundsen, D. (2018). One size fits not quite all: Universal research ethics with diversity. Research Ethics , 14 (3), 1-17. https://doi.org/10.1177/1747016117739939
[75] Pirzada, N. (2022). The Expansion of Turkey’s Medical Tourism Industry. Voices in Bioethics , 8 . https://doi.org/10.52214/vib.v8i.9894
[76] Stem Cell Tourism: False Hope for Real Money . Harvard Stem Cell Institute (HSCI). (2023). https://hsci.harvard.edu/stem-cell-tourism , See also: Bissassar, M. (2017). Transnational Stem Cell Tourism: An ethical analysis. Voices in Bioethics , 3 . https://doi.org/10.7916/vib.v3i.6027
[77] Song, P. (2011) The proliferation of stem cell therapies in post-Mao China: problematizing ethical regulation, New Genetics and Society , 30:2, 141-153, DOI: 10.1080/14636778.2011.574375
[78] Dajani, R. (2014). Jordan’s stem-cell law can guide the Middle East. Nature 510, 189. https://doi.org/10.1038/510189a
[79] International Society for Stem Cell Research. (2024). Standards in stem cell research . International Society for Stem Cell Research. https://www.isscr.org/guidelines/5-standards-in-stem-cell-research
[80] Benjamin, R. (2013). People’s science bodies and rights on the Stem Cell Frontier . Stanford University Press.
Mifrah Hayath
SM Candidate Harvard Medical School, MS Biotechnology Johns Hopkins University
Olivia Bowers
MS Bioethics Columbia University (Disclosure: affiliated with Voices in Bioethics)
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License .

IMAGES
VIDEO
COMMENTS
Table of contents. Step 1: Introduce your topic. Step 2: Describe the background. Step 3: Establish your research problem. Step 4: Specify your objective (s) Step 5: Map out your paper. Research paper introduction examples. Frequently asked questions about the research paper introduction.
Dr. Michelle Harris, Dr. Janet Batzli,Biocore. This section provides guidelines on how to construct a solid introduction to a scientific paper including background information, study question, biological rationale, hypothesis, and general approach. If the Introduction is done well, there should be no question in the reader's mind why and on ...
Define your specific research problem and problem statement. Highlight the novelty and contributions of the study. Give an overview of the paper's structure. The research paper introduction can vary in size and structure depending on whether your paper presents the results of original empirical research or is a review paper.
The introduction supplies sufficient background information for the reader to understand and evaluate the experiment you did. It also supplies a rationale for the study. Goals: Present the problem and the proposed solution. Presents nature and scope of the problem investigated. Reviews the pertinent literature to orient the reader.
Here is the basic format scientists have designed for research reports: Introduction; ... Thinking of your research report as based on the scientific method, but elaborated in the ways described above, may help you to meet your audience's expectations successfully. We're going to proceed by explicitly connecting each section of the lab ...
Emphasizing the importance of the proposed research and how the gaps will be addressed. Stating the research problem/ questions. Stating the hypotheses briefly. Figure 17.1 depicts how the introduction needs to be written. A scientific paper should have an introduction in the form of an inverted pyramid.
INTRODUCTION. A scientific paper is the formal lasting record of a research process. It is meant to document research protocols, methods, results and conclusions derived from an initial working hypothesis. ... Experimental reports deal with research that tests a research hypothesis through an established protocol, and, in the case of health ...
This format is often used for lab reports as well as for reporting any planned, systematic research in the social sciences, natural sciences, or engineering and computer sciences. Introduction - Make a case for your research. The introduction explains why this research is important or necessary or important. Begin by describing the problem or ...
When writing your research paper introduction, there are several key elements you should include to ensure it is comprehensive and informative. A hook or attention-grabbing statement to capture the reader's interest. It can be a thought-provoking question, a surprising statistic, or a compelling anecdote that relates to your research topic.
Standard Research Report Components. Scientific and technical research reports generally follow a conventional format that includes a title, an abstract, a reference (or Literature Cited) section and the components of the IMRAD structure: ... Introduction. focuses on the overall issue, problem, or question that your research addresses.
Scientific papers based on experimentation typically include five predominant sections: Abstract, Introduction, Methods, Results, and Discussion.This structure is a widely accepted approach to writing a research paper, and has specific sections that parallel the scientific method.
Introduction Sections in Scientific Research Reports (IMRaD) The goal of the introduction in an IMRaD* report is to give the reader an overview of the literature in the field, show the motivation for your study, and share what unique perspective your research adds. To introduce readers to your material and convince them of the research value ...
4.1 Introduction to Writing a Research Report. ... This does not change with the length of your research paper. An article in a scientific journal has the same basic structure (and issues to address) as a bachelor's thesis, master's thesis, and doctoral dissertation. In the following, we describe each section's structure and challenges.
AUTHORS. 1. The person who did the work and wrote the paper is generally listed as the first author of a research paper. 2. For published articles, other people who made substantial contributions to the work are also listed as authors. Ask your mentor's permission before including his/her name as co-author.
1. Introduction. Writing scientific papers is currently the most accepted outlet of research dissemination and scientific contribution. A scientific paper is structured by four main sections according to IMRAD (Introduction, Methods, Results, and Discussion) style ().To quote Plato, the Greek philosopher, "the beginning is half of the whole", and the introduction is probably one of the ...
Scientific Papers. Scientific papers are for sharing your own original research work with other scientists or for reviewing the research conducted by others. As such, they are critical to the ...
To learn in more detail the guidelines to write a great Introduction section, check out this course: How to write a strong introduction for your research paper References: 1. Araújo C G. 2014. Detailing the writing of scientific manuscripts: 25-30 paragraphs. Arquivos Brasileiros de Cardiologia 102 (2): e21-e23 2. Boxman R and Boxman E. 2017.
The introduction of a scientific article is where the author needs to articula... Learn how to write an introduction to a research paper using a simple formula. The introduction of a scientific ...
Components of Research Report are as follows: Introduction. ... An experimental report documents the results of a scientific experiment, including the hypothesis, methods, results, and conclusions. Experimental reports are often used in biology, chemistry, and other sciences to communicate the results of laboratory experiments. ...
This section describes an organizational structure commonly used to report experimental research in many scientific disciplines, the IMRAD format: Introduction, Methods, Results, And Discussion. Although the main headings are standard for many scientific fields, details may vary; check with your instructor, or, if submitting an article to a journal, refer to the instructions to authors.…
3. Aim. The aim identifies what is going to be tested in the experiment. This should be short, concise and clear. Example. The aim of the experiment is to test whether light is required for photosynthesis to occur.
Explain the importance of writing clear, persuasive introduction to a scientific paper; Explain the application of the 5Cs paradigm to writing an introduction; Adopt a rational, step-wise strategy to create a sound introduction. Refreshments will be served at 5:30 p.m. The fee to participate in this workshop is $25. Complete the registration form.
This study was supported in part by the German Research Foundation (CRC 1525 grant no. 453989101, projects C3 and PS1) and the German Federal Ministry of Education and Research (BMBF, grants ...
Lack of trust is a primary reason behind the global rise in vaccine hesitancy. Existing research on the trust—vaccine hesitancy nexus has almost exclusively focused on COVID-19 with the vast ...
Google DeepMind's newly launched AlphaFold Server is the most accurate tool in the world for predicting how proteins interact with other molecules throughout the cell. It is a free platform that scientists around the world can use for non-commercial research. With just a few clicks, biologists can harness the power of AlphaFold 3 to model structures composed of proteins, DNA, RNA and a ...
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Hi-net seismic data required in the paper are available from the National Research Institute for Earth Science and Disaster Prevention (NIED; www.hinet.bosai.go.jp).
Voices in Bioethics is currently seeking submissions on philosophical and practical topics, both current and timeless. Papers addressing access to healthcare, the bioethical implications of recent Supreme Court rulings, environmental ethics, data privacy, cybersecurity, law and bioethics, economics and bioethics, reproductive ethics, research ethics, and pediatric bioethics are sought.
Abstract. An article primarily includes the following sections: introduction, materials and methods, results, discussion, and conclusion. Before writing the introduction, the main steps, the heading and the familiarity level of the readers should be considered. Writing should begin when the experimental system and the equipment are available.
A complete understanding of the human brain begins with elucidation of its structural properties at a subcellular level. To provide a valuable resource for the scientific community and to better understand the structure of the human temporal cortex, Shapson-Coe et al. performed an electron microscopy reconstruction of a cubic millimeter of human temporal cortex.
In the winter of 2020, the main authors of this paper discovered a large dinosaur track assemblage in Fujian Longyan, southeastern China., Three main track layers were identified, and the site has been dubbed the Longxiang tracksite. One of the track layers contained three occurrences of didactyl tracks.