Navigation Menu
Search code, repositories, users, issues, pull requests..., provide feedback.
We read every piece of feedback, and take your input very seriously.

Saved searches
Use saved searches to filter your results more quickly.
To see all available qualifiers, see our documentation .
- Notifications
Insulin pump case study retrieved from Ian Sommerville's Software Engineering, Tenth Edition https://software-engineering-book.com/case-studies/
opendesigncasestudies/InsulinPump-IanSommerville
Folders and files, repository files navigation, insulin pump case study - cited from ian sommerville software engineering, tenth edition.
https://software-engineering-book.com/case-studies/ This case study is part of the Open Design Case Study project. This work is licensed under the Creative Commons Attribution-NonCommercial 3.0 ( CC BY-NC 3.0 ) United States License.
Sommerville, I. (2016) Software Engineering. 10th Edition, Pearson Education Limited, Boston.
A personal software controlled insulin pump
This case study discusses the control software for a personal insulin pump, which is used by diabetics to mimic the function of the pancreas and hence control the level of glucose (sugar) in their blood. The level of blood sugar depends on what the system user has eaten, the speed of their digestive processes and the effectiveness of their body in metabolising blood sugar. Therefore, their is not a simple relationship between a blood sugar measurement and the amount of insulin to be injected. Rather, the control system has to make several measurements and assess the rate of change of blood sugar. Based on the current level and the rate and direction of change, the incremental amount of insulin to be injected is computed and injected using the micro-pump in the system. This is a safety-critical system as failure to inject the correct amount of insulin can have serious health consequences.
Stakeholder
- User (diabetic patient)
- Doctors (indirect user)
- Caregiver (indirect user)
- Nurses (indirect user)
Requirements
Description.
- Using readings from an embedded sensor, the system automatically measures the level of glucose in the users' body
- Consecutive readings are compared and, if they indicate that the level of glucose is rising, then insulin is injected to conteract this rise
- The ideal situation is a consistent level of sugar that is within some "safe" band
- Sugar levels can be categorized as "unsafe", "safe", and "undeseriable" where different amount of insulin will be injected (or not injected) to the users.
Detailed requirements can be found here
Constraints
- The system shall be available to deliver insulin when required
- The system shall perform reliably and deliver the correct amount of insulin to counteract the current level of sugar.
- If error condition occurs, alarm will be shown to the users based on the constraints stated in the detailed requirement specification
Quality Attributes
Reliability - (a) can inject the correct amount of dosage all the time (safety-critical) (b) must monitor blood sugar level at fixed intervals (c) if error occurs, must notify the users with the designated alarm condition (d) intermittent demand for service are made on the system
Availability - the pump should have a high level of availability but the nature of the diabetes is such that continous availability is unnecessary
Safety - The key safety requirements are that the operation of the system should never result in a very low level of blood sugar.
Environment
Entities and assumptions, design solution.
Architecture Model (hardware)

Activity Diagram of the insulin pump

Teaching Materials
Suggested usage.
The author use this case study to discuss general issues of safety and safety-critical systems. It is used in discussing issues of dependability specification and the use of formal specification techniques in dependable system engineering. Formal specification is especially valuable for this system as the computation to calculate the amount of insulin required is detailed and complex and quite difficult to specify using natural language. The formal specification in the Z notation is provided.
In lectures on dependability assurance, the author use examples from the insulin pump system to illustrate safety arguments and safety cases. Users of this case study can use the example more generally in lectures on requirements engineering, system modeling and embedded systems.
Other notes and resources
- Overview of a software controller insulin pump
- System overview slides
- Insulin Pump Requirements Specification
- Insulin Pump Z Schema
Help Center Help Center
- Help Center
- Trial Software
- Product Updates
- Documentation
Design Insulin Infusion Pump Using Model-Based Systems Engineering
This example uses:
- System Composer System Composer
- Requirements Toolbox Requirements Toolbox
- Simulink Simulink
- Simulink Test Simulink Test
- Stateflow Stateflow
This example shows you how to use a model-based systems engineering (MBSE) workflow to investigate optimal insulin infusion pump design. Insulin pumps are medical devices used by people with diabetes that mimic the human pancreas by delivering insulin continuously and delivering variable amounts of insulin with food intake.
The purpose of an insulin pump wearable device is to keep the blood glucose level of the wearer near a healthy set point by infusing insulin as needed and in response to food intake. This example shows a proposed insulin infusion pump system with two sensor and three pump variants that represent alternate design choices.
Begin by determining system requirements, then create detailed design models with code generation and verification tests. Finally, simulate the system architecture model that meets the evolving requirements.
Insulin Pump System Architecture Model
This figure shows the System Composer™ architecture model for the insulin pump system. This example uses Stateflow® blocks. If you do not have a Stateflow license, you can open and simulate the model but can only make basic changes, such as modifying block parameters.
The BGSensor component measures the blood glucose level. The Controller component makes a decision about the insulin rate. The Pump component provides insulin to the body using the InfusionSet . The Patient receives the treatment. The BGMeter calibrates the BGSensor . Finally, the HID (human interface device) component may be a mobile app on the phone for the patient to communicate with the system. The HID provides information to the PatientDataServer component, which sends analyses to the Clinician , Regulator , and Reimburser components.

System Requirements and Links
Use Requirements Toolbox™ to analyze the system requirements, further break them down into subsystem requirements, and link derived requirements to architectural components that satisfy them. A Requirements Toolbox license is required to link, trace, and manage requirements in System Composer.
Manage requirements and architecture together in the Requirements Perspective from Requirements Toolbox. Select Apps > Requirements Manager . To edit requirements, select Requirements > Requirements Editor or enter these commands to open the Requirements Editor (Requirements Toolbox) .

The requirements decomposition and analysis at this point represent these concerns:
Accuracy of delivery
Mitigations against over-infusion, which leads to dangerously low blood glucose levels
Fault analysis to prevent negative outcomes, for example, when the battery is depleted or the device runs out of medication
On the architecture model, select the requirements icon to see the requirements that are associated with the component. For example, below are the requirements linked to the Pump component.

Conversely, select a requirement to see the highlighted component by which the requirement is implemented. For example, the BGSensor component implements the Sense blood glucose requirement.

Outcome Analysis for Optimal Design Choice
Outcome analysis consists of a trade study where the goal is to maximize the business value of the design options based on calculations that sum up different component properties with weighting factors. Many are directly entered properties, such as non-recurring engineering (NRE) costs to develop the component. Compliance score, however, is a derived property that is based on different data for each type of component. These properties model the burden to an end user of the system. The compliance score includes these considerations:
Energy consumption
Size and weight
Mean time between failures (MTBF)
Sound level produced during operation
Ease of use
Navigate to Modeling > Profiles > Profile Editor , or enter this command.
A System Composer profile, defined in the Profile Editor , is composed of stereotypes with properties defined. You can apply stereotypes to components in the model to assign specific property values to each component.

The pump and sensor trade study includes these steps:
Collect all variant combinations.
Activate variants one by one to represent all the combinations.
Iterate over the model to calculate compliance and compute the outcome using the stored and calculated parameters.
Collect outcomes and weight them using the same units.
Provide the optimized option.
A Variant Component block named BGSensor contains two different sensor variants representing example sensors from different manufacturers.

The Variant Component block named Pump contains three different pumps in this example called PeristalticPump , SyringePump , and PatchPump .

To programmatically cycle between the different variant choice combinations, calculate compliance, and monitor the outcome to determine the optimal design choice, run OutcomeAnalysis.m . For more information on variant analysis, see Analysis Function Constructs .

The normalized outcome score is at a maximum for the SensorA + SyringePump combination. This design choice is optimal for the insulin pump.
Controller Implementation Model
Implement the insulin infusion pump controller in Simulink®. To access the Controller model, navigate to the InsulinInfusionPumpSystem architecture model and double-click the Controller component. The input ports in this implementation include User input , with user metrics that the insulin pump reads, and Hardware status , with information about the insulin pump. The block named ModeControl deteremines in which mode the insulin pump must operate.

The block named ModeControl contains a Stateflow chart with details on how to select the mode.
The three modes include:
Alarm mode, where the system is be suspended, repaired, and restarted once clear
Bolus delivery mode to deliver insulin quickly with food intake
Basal delivery mode to deliver insulin over a longer period of time to keep glucose levels steady throughout the day

After the mode is selected, this component behavior determines the insulin rate for the outport.

Verification and Validation Using Test Manager
You can use model-based design to verify architectural designs and system requirements. The abstract architecture model and the detailed Simulink design model are connected with traceable requirement links. This section requires a Simulink® Test™ license.
The Controller implementation model in Simulink demonstrates requirements traceability for the Alarm handling requirement.

Load and view the Simulink Test Manager (Simulink Test) using these commands.
The Alarm_Detection functional test verifies the Alarm handling requirement.

Double-click the Test Sequence block to view the steps in the test sequence. The steps define a scenario to verify the functioning of the alarm system.

To run this test, go back into the Simulink Test Manager (Simulink Test) .
Right-click the test Alarm_Detection in the Test Browser and select Run . In the Results and Artifacts section, view your test results. A passing test indicates that the system requirement Alarm handling is verified by the conditions defined in the Test Assessment Block:
Whether the alarm disables insulin delivery when there is low battery, occlusion (line blockage), or low medication (insulin)
Whether the system restarts after the issue has passed
- Profile Editor | Simulink Test Manager (Simulink Test) | Requirements Editor (Requirements Toolbox)
Related Topics
- Allocate and Trace Requirements from Design to Verification
- Compose Architectures Visually
- Analysis Function Constructs
- Simulate Mobile Robot with System Composer Workflow
- Calculate Endurance Using Quadcopter Architectural Design
- Model-Based Systems Engineering for Space-Based Applications
MATLAB Command
You clicked a link that corresponds to this MATLAB command:
Run the command by entering it in the MATLAB Command Window. Web browsers do not support MATLAB commands.
Select a Web Site
Choose a web site to get translated content where available and see local events and offers. Based on your location, we recommend that you select: .
- Switzerland (English)
- Switzerland (Deutsch)
- Switzerland (Français)
- 中国 (English)
You can also select a web site from the following list:
How to Get Best Site Performance
Select the China site (in Chinese or English) for best site performance. Other MathWorks country sites are not optimized for visits from your location.
- América Latina (Español)
- Canada (English)
- United States (English)
- Belgium (English)
- Denmark (English)
- Deutschland (Deutsch)
- España (Español)
- Finland (English)
- France (Français)
- Ireland (English)
- Italia (Italiano)
- Luxembourg (English)
- Netherlands (English)
- Norway (English)
- Österreich (Deutsch)
- Portugal (English)
- Sweden (English)
- United Kingdom (English)
Asia Pacific
- Australia (English)
- India (English)
- New Zealand (English)
Contact your local office

- Medical Device Development
- Biotech Instrument Development
- Inkjet & 3D Printer Development
- Commercial Product Development
- Custom Automation
- Pre-Production Manufacturing
- Hardware Design Services
- Software Development
- Systems Engineering
- Design for Manufacturability
- Verification Testing
- Modeling and Simulation
- Quality and Regulatory
- User Experience Design
- Intellectual Property and Innovation
- CNC Machine Shop and Model Shop
- Onsite 3D Printing
- Onsite Product Testing
- Network Infrastructure
- News and Blog
INSULIN PUMP DEVELOPMENT
Insulin pump development experience for patch style and traditional pumps.
NOVO has contributed to the mechanical, electrical, and software design of several insulin pump product families including pumps, controllers, consumables, and accessories. The image above shows the Medtronic 670G (a component of the first FDA approved artificial pancreas system) , the Tandem Diabetes t:slim, and the Unilife Imperium pumps. NOVO contributed to each of these designs, and to several others still under development. Our experience extends to every subsystem in either a belt-worn or wearable pump (patch pump), including core systems like the pump drive, automatic cannula insertion/retraction, primary container, occlusion sensing, electronics, and embedded software.
In addition to product development, NOVO has developed automated test equipment and process control automation for insulin pump manufacturing and quality control purposes.
NOVO has integrated peripheral devices such as wireless continuous glucose monitors (CGM devices) and blood glucose meters, in addition to developing pump control electronics and Bluetooth low energy (BLE) communications. The breadth of our experience in diabetes care device development made NOVO an obvious choice as a development partner for an automated insulin delivery system development program currently underway. Our strong understanding of the design and manufacturing of diabetes care devices for both hospital and ambulatory applications makes us a unique resource to product companies bringing new insulin pumps, pen-injectors, and CGM devices to market.
INSULIN PUMPS: TECHNICAL CHALLENGES
Insulin pumps present a unique combination of technical challenges related to usability, performance, and reliability requirements. For example, usability requirements drive a goal of minimizing the size of the device, which in turn complicates the achievement of performance and reliability goals. Miniature, precision drive trains, occlusion sensing, environmental hardening for shock and water ingress, electrostatic discharge (ESD) hardening, wireless communications, system integration, manufacturability, and most importantly, patient safety combine to increase the technical challenges in these designs. Regulatory requirements and manufacturing considerations for both high-volume consumables and lower-volume durable assemblies add further challenges to insulin pump development.
THE ENGINEERING BEHIND GREAT PRODUCTS
NOVO has a history of helping our clients solve the most challenging aspects of the durable and consumable designs on several insulin pump projects. We have worked closely with our clients’ operations departments to create automated test equipment and assembly automation. NOVO continues to build our expertise in this important medical device product category, and in the related category of on-body drug delivery devices , as we provide product, process, and manufacturing development support to our clients developing the next generation of diabetes care products.
Related case studies include:
- Insulin Pump Embedded Design
- Insulin Pump Controller Development
More Medical Device Case Studies
Case study autoinjector design and development for prefilled syringes.

Case Study BIOFEEDBACK POSTURE TRAINER

Case Study CATHETER DELIVERY SYSTEM DESIGN FOR A VASCULAR FILTER
Case Study CONTINUOUS GLUCOSE MONITOR DEVELOPMENT
Case Study CORDLESS PROPHY DEVICE
Case Study DIAGNOSTIC ARTHROSCOPE DESIGN

Case Study IN-VITRO STENT DELIVERABILITY MODEL

Case Study Inflation System Design for Intragastric Balloon

Case Study Insulin Pump Controller Development

Case Study Insulin Pump Embedded Design
Case Study INTRAGASTRIC BALLOON IMPLANT

Case Study Intramuscular Electroporation Delivery Device Design

Case Study MEDICAL DEVICE FAILURE ANALYSIS
Case Study Medical Device Ingress Protection

Case Study Miniature Travel CPAP Device

Case Study ON-BODY INJECTOR DEVELOPMENT
Case Study Oral Biopharmaceutical Delivery

Case Study PHOTOTHERAPY DEVICE DESIGNED FOR HOME USE
Case Study Single-Use CGM Auto-Applicator Development

Case Study Smart Pen Cap for Common Insulin Pens

Case Study Smart Prescription Bottle Cap
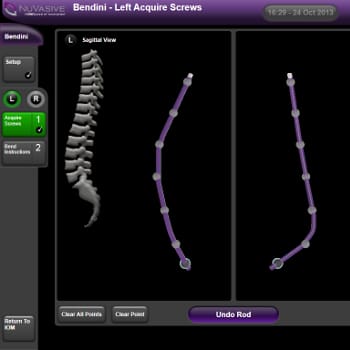
Case Study SPINAL ROD-BENDING SOFTWARE DEVELOPMENT
Case Study STENT CONFORMABILITY TEST METHOD

Case Study SURGICAL RFID DETECTION WAND
Case Study VACCINE REFRIGERATOR AND INVENTORY MANAGEMENT

Case Study Waist Measurement Device to Screen for Health Risks to Overweight Patients
Software Design Specification and Analysis of Insulin Dose to Adaptive Carbohydrate Algorithm for Type 1 Diabetic Patients
- First Online: 31 January 2021
Cite this chapter

- Ishaya Gambo 5 , 6 ,
- Rhodes Massenon 6 ,
- Terungwa Simon Yange 7 ,
- Rhoda Ikono 6 ,
- Theresa Omodunbi 6 &
- Kolawole Babatope 8
Part of the book series: Studies in Computational Intelligence ((SCI,volume 932))
1145 Accesses
1 Citations
Crucial in the development of a technological solution for managing insulin-dependent patients is an appropriate method of software engineering (SE) process. The SE process aims at providing supports for the design and implementation of a high-quality system by engaging patients and other healthcare professionals in knowing the exact specification for development. Primarily, insulin-dependent patients have a big challenge in determining the right dose of insulin to be injected according to measurements of glucose level and estimated carbohydrate from food intake. The result of this is often hypoglycaemia and hyperglycaemia, which may lead to coma and cause unfortunate death. The objective of this research work is to develop an adaptive model dedicated to the adjustment of insulin dose requirements in patients with type 1 diabetes. A mathematical model is formulated based on insulin sensitivity, basal insulin, bolus insulin, correction factor, carbohydrate counts, bolus on board equations and continuous blood glucose levels. We have evaluated the model by using root-mean-square error (RMSE) and mean absolute error (MAE) as parameters. The results showed the correct insulin dose values within the range of 4.76–0.18 IU for the fifty-two (52) patients with T1DM. Consequently, the developed model suggests a reduction in the incidence of hypoglycaemia, which can be used to predict the next insulin dose.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
World Health Organization (WHO). Global report on Diabetes 2016 Retrieve from http: www.who.intdiabetesglobal-report . Date consulted: 28th March 2019.
Soylu, S., Danisman, K., Sacu, I. E., & Alci, M. (2013). Closed-loop control of blood glucose level in type-1 diabetics: A simulation study. In Electrical and Electronics Engineering (ELECO), 2013 8th International Conference on (371–375). IEEE.
Google Scholar
American Diabetes Association. (2010). Standards of medical care in diabetes—2010. Diabetes Care, 33 (Supplement 1), S11–S61.
Article Google Scholar
Imran, S. A., Rabasa-Lhoret, R., Ross, S., & Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. (2013). Targets for glycemic control. The Canadian Journal of Diabetes, 37 (Suppl 1), S31–34.
Cryer, P. E. (2008). The barrier of hypoglycemia in diabetes. Diabetes, 57 (12), 3169–3176.
Miller, K. M., Xing, D., Tamborlane, W. V., Bergenstal, R. M., & Beck, R. W. (2013). Challenges and future directions of the T1D exchange clinic network and registry. Journal of Diabetes Science and Technology, 7 (4), 963–969.
The Diabetes Control and Complications Trial Research Group. (1993). Effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. New England Journal of Medicine, 329 (14), 977–986.
Gillespie, S. J., Kulkarni, K. A. R. M. E. E. N., & Daly, A. E. (1998). Using carbohydrate counting in diabetes clinical practice. Journal of the American Dietetic Association, 98 (8), 897–905.
Wylie-Rosett, J., Albright, A. A., Apovian, C., Clark, N. G., Delahanty, L., Franz, M. J., et al. (2007). 2006–2007 American Diabetes Association nutrition recommendations: Issues for practice translation. Journal of the American Dietetic Association, 107 (8), 1296–1304.
Rabasa-Lhoret, R., Garon, J., Langelier, H., Poisson, D., & Chiasson, J. L. (1999). Effects of meal carbohydrate content on insulin requirements in type 1 diabetic patients treated intensively with the basal-bolus (ultralente-regular) insulin regimen. Diabetes Care, 22 (5), 667–673.
Delahanty, L. M., & Halford, B. N. (1993). The role of diet behaviors in achieving improved glycemic control in intensively treated patients in the diabetes control and complications trial. Diabetes Care, 16 (11), 1453–1458.
Fu, S., Li, L., Deng, S., Zan, L., & Liu, Z. (2016). Effectiveness of advanced carbohydrate counting in type 1 diabetes mellitus: A systematic review and meta-analysis. Scientific Reports, 6, 37067.
Kawamura, T. (2007). The importance of carbohydrate counting in the treatment of children with diabetes. Pediatric Diabetes, 8, 57–62.
Sheard, N. F., Clark, N. G., Brand-Miller, J. C., Franz, M. J., Pi-Sunyer, F. X., Mayer-Davis, E., et al. (2004). Dietary Carbohydrate (amount and type) in the prevention and management of diabetes: a statement by the American Diabetes Association. Diabetes Care, 27 (9), 2266–2271.
American Diabetes Association. (2008). Clinical practice recommendations: Diagnosis and classification of diabetes mellitus. Diabetes Care, 31 (1), 55–60S.
Palerm, C. C., Zisser, H., Jovanovič, L., & Doyle, F. J., III. (2008). A run-to-run control strategy to adjust basal insulin infusion rates in type 1 diabetes. Journal of Process Control, 18 (3–4), 258–265.
Weijman, I., Ros, W. J., Rutten, G. E., Schaufeli, W. B., Schabracq, M. J., & Winnubst, J. A. (2005). The role of work-related and personal factors in diabetes self-management. Patient education and counselling, 59 (1), 87–96.
Morel, A., Lecoq, G., & Jourdain-Menninger, D. (2012). Evaluation de la prise en charge du diabète. Inspection Général des Affaires sociales, RM2012-033P .
Hovorka, R., Canonico, V., Chassin, L. J., Haueter, U., Massi-Benedetti, M., Federici, M. O., et al. (2004). Nonlinear model predictive control of glucose concentration in subjects with type 1 diabetes. Physiological Measurement, 25 (4), 905.
Steil, G. M., & Reifman, J. (2009). Mathematical modelling research to support the development of automated insulin delivery systems. Journal of Diabetes Science and Technology, 3 (2), 388–395.
Steil, G. M., Clark, B., Kanderian, S., & Rebrin, K. (2005). Modelling insulin action for development of a closed-loop artificial pancreas. Diabetes Technology & Therapeutics, 7 (1), 94–108.
Souto, D. L., & Rosado, E. L. (2010). Use of carb counting in the dietary treatment of diabetes mellitus. Nutricion Hospitalaria, 25 (1), 18–25.
Wilinska, M. E., Chassin, L. J., Schaller, H. C., Schaupp, L., Pieber, T. R., & Hovorka, R. (2004). Insulin kinetics in type-1 diabetes: continuous and bolus delivery of rapid acting insulin. IEEE Transactions on Biomedical Engineering, 52 (1), 3–12.
Ellingsen, C., Dassau, E., Zisser, H., Grosman, B., Percival, M. W., Jovanovič, L., et al. (2009). Safety constraints in an artificial pancreatic β cell: an implementation of model predictive control with insulin on board. Journal of Diabetes Science and Technology, 3 (3), 536–544.
Murphy, H. R., Elleri, D., Allen, J. M., Harris, J., Simmons, D., Rayman, G., et al. (2011). Closed-loop insulin delivery during pregnancy complicated by type 1 diabetes. Diabetes Care, 34 (2), 406–411.
Percival, M. W., Wang, Y., Grosman, B., Dassau, E., Zisser, H., Jovanovič, L., et al. (2011). Development of a multi-parametric model predictive control algorithm for insulin delivery in type 1 diabetes mellitus using clinical parameters. Journal of Process Control, 21 (3), 391–404.
Campos-Delgado, D. U., Femat, R., Hernández-Ordoñez, M., & Gordillo-Moscoso, A. (2005). Self-tuning insulin adjustment algorithm for type 1 diabetic patients based on multi-doses regime. Applied Bionics and Biomechanics, 2 (2), 61–71.
Bishop, F. K., Maahs, D. M., Spiegel, G., Owen, D., Klingensmith, G. J., Bortsov, A., et al. (2009). The carbohydrate counting in adolescents with type 1 diabetes (CCAT) study. Diabetes Spectrum, 22 (1), 56–62.
Lee, H., Buckingham, B. A., Wilson, D. M., & Bequette, B. W. (2009). A closed-loop artificial pancreas using model predictive control and a sliding meal size estimator.
Dias, V. M., Pandini, J. A., Nunes, R. R., Sperandei, S. L., Portella, E. S., Cobas, R. A., et al. (2010). Effect of the carbohydrate counting method on glycemic control in patients with type 1 diabetes. Diabetology & Metabolic Syndrome, 2 (1), 54.
Bell, K. J., Barclay, A. W., Petocz, P., Colagiuri, S., & Brand-Miller, J. C. (2014). Efficacy of carbohydrate counting in type 1 diabetes: A systematic review and meta-analysis. The Lancet Diabetes & Endocrinology, 2 (2), 133–140.
Finner, N., Quinn, A., Donovan, A., O’Leary, O., & O’Gorman, C. S. (2015). Knowledge of carbohydrate counting and insulin dose calculations in paediatric patients with type 1 diabetes mellitus. BBA Clinical, 4, 99–101.
Rhyner, D., Loher, H., Dehais, J., Anthimopoulos, M., Shevchik, S., Botwey, R. H., et al. (2016). Carbohydrate estimation by a mobile phone-based system versus self-estimations of individuals with type 1 diabetes mellitus: A comparative study. Journal of Medical Internet Research, 18 (5), e101.
Reiterer, F., Freckmann, G., & del Re, L. (2018). Impact of carbohydrate counting errors on glycemic control in type 1 diabetes. IFAC-PapersOnLine, 51 (27), 186–191.
Oviedo, S., Contreras, I., Bertachi, A., Quirós, C., Giménez, M., Conget, I., et al. (2019). Minimizing postprandial hypoglycemia in Type 1 diabetes patients using multiple insulin injections and capillary blood glucose self-monitoring with machine learning techniques. Computer Methods and Programs in Biomedicine, 178, 175–180.
Reiterer, F., & Freckmann, G. (2019). Advanced carbohydrate counting: An engineering perspective. Annual Reviews in Control, 48 (2019), 401–422.
Article MathSciNet Google Scholar
Ziegler, R., Waldenmaier, D., Kamecke, U., Mende, J., Haug, C., & Freckmann, G. (2020). Accuracy assessment of bolus and basal rate delivery of different insulin pump systems used in insulin pump therapy of children and adolescents. Pediatric Diabetes, 21 (4), 649–656.
Yin, R. K. (2017). Case study research and applications: Design and methods . Sage publications.
Runeson, P., Host, M., Rainer, A., & Regnell, B. (2012). Case study research in software engineering: Guidelines and examples . Wiley.
Willmott, C. J., & Matsuura, K. (2005). Advantages of the mean absolute error (MAE) over the root mean square error (RMSE) in assessing average model performance. Climate Research, 30 (1), 79–82.
Chai, T., & Draxler, R. R. (2014). Root mean square error (RMSE) or mean absolute error (MAE)?—Arguments against avoiding RMSE in the literature. Geoscientific model development, 7 (3), 1247–1250.
Li, J., & Fernando, C. (2016). Smartphone-based personalized blood glucose prediction. ICT Express, 2 (4), 150–154.
Pashkov, V. M., Gutorova, N. O., & Harkusha, A. (2016). Medical device software: defining key terms. Wiadomości lekarskie, 6 (2016), 813–817.
Download references
Author information
Authors and affiliations.
Institute of Computer Science, University of Tartu, Tartu, Estonia
Ishaya Gambo
Department of Computer Science and Engineering, Obafemi Awolowo University, Ile-Ife, Nigeria
Ishaya Gambo, Rhodes Massenon, Rhoda Ikono & Theresa Omodunbi
Department of Mathematics, Statistics and Computer Science, Federal University of Agriculture, Makurdi, Nigeria
Terungwa Simon Yange
Department of Medicine, Faculty of Clinical Sciences, Obafemi Awolowo University, Ile-Ife, Nigeria
Kolawole Babatope
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ishaya Gambo .

Editor information
Editors and affiliations.
Department of Computer Science and Engineering, National Institute of Technology Silchar, Silchar, Assam, India
Ripon Patgiri
Anupam Biswas
Rights and permissions
Reprints and permissions
Copyright information
© 2021 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Gambo, I., Massenon, R., Yange, T.S., Ikono, R., Omodunbi, T., Babatope, K. (2021). Software Design Specification and Analysis of Insulin Dose to Adaptive Carbohydrate Algorithm for Type 1 Diabetic Patients. In: Patgiri, R., Biswas, A., Roy, P. (eds) Health Informatics: A Computational Perspective in Healthcare. Studies in Computational Intelligence, vol 932. Springer, Singapore. https://doi.org/10.1007/978-981-15-9735-0_7
Download citation
DOI : https://doi.org/10.1007/978-981-15-9735-0_7
Published : 31 January 2021
Publisher Name : Springer, Singapore
Print ISBN : 978-981-15-9734-3
Online ISBN : 978-981-15-9735-0
eBook Packages : Intelligent Technologies and Robotics Intelligent Technologies and Robotics (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Llewellyn S, Procter R, Harvey G, et al. Facilitating technology adoption in the NHS: negotiating the organisational and policy context – a qualitative study. Southampton (UK): NIHR Journals Library; 2014 Jul. (Health Services and Delivery Research, No. 2.23.)

Facilitating technology adoption in the NHS: negotiating the organisational and policy context – a qualitative study.
Chapter 6 the insulin pump therapy case study.
- Introduction
This case study focuses on IPT, used in the treatment of type 1 diabetes. Type 1 diabetes is a long-term condition that affects around 250,000 people in the UK and requires lifelong treatment with insulin. 187 Rates of type 1 diabetes have been increasing over time, with the greatest increase in children younger than 5 years of age. People with type 1 diabetes are unable to produce the natural hormone insulin, which is needed to control and use glucose. Most people with the condition control their diabetes through multiple daily injections (MDIs) of insulin. IPT, also known as continuous subcutaneous insulin infusion (CSII), is an alternative method of treatment to insulin injections by syringes or insulin pens. The pump provides a CSII, thus replacing the need for MDIs and typically producing better control of blood glucose levels.
Insulin pump therapy was first introduced in the UK in the 1970s, initially within the context of research studies on type 1 diabetes. However, mainstream adoption of the technology was limited, linked to concerns about efficacy and safety and the potential financial burden on the NHS. 188 , 189 In the past decade, technological advances have resulted in a new generation of smaller, more portable, more efficient and user-friendly pumps with additional safety features. 189 – 191 Several studies in recent years have demonstrated the benefits of IPT in type 1 diabetes compared with MDIs. 190 , 192 – 199 In particular, it is suggested that the continuous infusion of insulin provides not only improvement in metabolic control, but also increased physiological and psychological well-being. 189 , 200 Other benefits include improved patient outcomes [e.g. lower glycated haemoglobin (HbA 1c ); HbA 1c is a measure of the average plasma glucose concentration over a period of time, in people with diabetes, a higher plasma glucose concentration indicates poorer control of blood glucose levels], reduction in all grades of hypoglycaemia (hypoglycaemia is a low blood glucose level, which is too low to provide sufficient energy for the normal body functions), a reduction in blood glucose concentrations, fewer blood glucose swings, and a lower daily insulin dosage compared with insulin injection therapy. 201 It is also suggested that quality of life for patients and their family and treatment satisfaction are likely to be better on pump treatment than on MDIs, 145 particularly for people who have experienced significant and continued control problems on MDIs. 201 However, comprehensive patient education, such as carbohydrate counting, and frequent self-monitoring of blood glucose or continuous glucose monitoring are necessary components of successful IPT. 190
The IPT case is the only one of the three technologies studied that had been the subject of a national technology appraisal process; however, this did not guarantee that the evidence base for the technology was universally accepted, as the findings illustrate. NICE issued technology appraisal guidance on IPT in 2003, which was further updated in 2008, and recommended it as a clinically effective and cost-effective treatment option for people with type 1 diabetes, whether adult or child, for whom MDIs have failed, and for children aged < 12 years if MDIs are not deemed practical or appropriate. 202 Alongside the technology appraisal, NICE produced a commissioning guide to help health service commissioners plan and deliver services in line with the guidance. This suggests that the standard benchmark rate for the uptake of IPT should be 12% of people with type 1 diabetes, and 33% for children younger than 12 years old.
A national working group on insulin pump services 203 used a variety of sources, including national registers, manufacturers’ records and published reports of pump practice in various countries, to estimate the uptake of IPT at an international level. These data suggested that some countries (USA, Israel and Germany) were using pumps for about 15–20% of people with type 1 diabetes. A typical figure for Europe (e.g. France, Sweden and the Netherlands) was around 10% of people with type 1 diabetes using insulin pumps for routine management. In contrast, overall UK pump usage was estimated at around 1% of people with type 1 diabetes using insulin pumps for routine management.
A subsequent review of IPT in England was undertaken by the Medical Technology Group in 2010. 204 They carried out a survey of all PCTs ( n = 152) in England to ascertain levels of insulin pump provision. Of these, 87.5% responded to the survey and the data indicated that the average rate of pump use was 3.7%, with rates across the country ranging from 0.25% to 13%. 204 Thus, although the rates were higher than those estimated by the Department of Health Working Group in 2007, they were still some way from the NICE recommended benchmark of 12% and considerably lower than in most other countries of comparable economic standing and level of health-care provision.
- The NHS Technology Adoption Centre project on insulin pump therapy
NHS Technology Adoption Centre selected IPT to go forward as a technology implementation project so that it could identify the challenges associated with implementing IPT and suggest ways to overcome them. Three NHS trusts were selected to work as implementation sites; these were organisations that responded to the NTAC call for implementation sites and were selected because they wanted to develop their IPT service in line with NICE guidance (see Chapter 4 for a more detailed description of the NTAC selection process). Three MSs were identified to work with the implementation sites, these were organisations that were already using insulin pumps with many of their patients and could provide clinical mentorship to the teams in the implementation sites. The experiences of the sites involved in the IPT project were collated into a HTWT guide. 205 This was intended as an online resource that could be used to help the adoption of IPT throughout the NHS.
- The case study
The main data collection took place in four NHS organisations: two NTAC implementation sites for IPT; one MS; and one organisation that had initially applied to be an implementation site, but was not selected by NTAC. This latter organisation was keen to increase the use of pump therapy and, as such, would be typical of the organisations that NTAC was aiming to target with the HTWT guide. In all sites, interviews were conducted with a range of individuals involved in implementing IPT, including commissioners, clinicians, diabetes nurse specialists and business/procurement managers. A total of 23 interviews were conducted across the four sites; details of the interview sample by site are provided in Table 3 .
Interviewees for the IPT case study
Alongside these qualitative interview data from the case study sites, supplementary sources of data included a survey of clinicians about IPT use in the NHS in England and documentary analysis of e-mail correspondence received by the patient support group INPUT between September and November 2011.
Before presenting the main findings relating to the IPT case, some brief background information on each of the four NHS organisations studies is outlined, along with a summary of the results of the survey we conducted on IPT uptake in England.
Implementation site 1
The first site that worked on IPT with NTAC was an NHS trust providing acute services that had been formed from the merger of two trusts some 8 years earlier. Diabetic services were provided at two separate sites and the acute trust had two main commissioners. The trust was in the process of applying for foundation trust status. One of the two main acute hospital sites had a history of interest in using IPT and was very receptive to the adoption of the technology; the second site was less enthusiastic about the technology. However, prior to their involvement with NTAC, there were no formalised processes or systems for managing the introduction of IPT at an individual patient or service level.
Implementation site 2
The second implementation site was an NHS foundation trust providing specialist children’s services, commissioned from a wide range of primary care organisations (around 17 in total). The pressure to provide IPT had particularly been driven from the patient population (children and parents); as a consequence, the diabetic team was keen to become more skilled and up to date in terms of providing pump services.
Mentor site
The MS was an NHS foundation trust, providing an integrated hospital, community and primary care diabetic service. The trust had originally applied to be part of the NTAC implementation project, but had been seen to be relatively well advanced in terms of the adoption of IPT and, therefore, did not meet the early adopter criteria. As a consequence, it was invited to act as a MS for IPT (although in reality they received limited requests for advice or information from the implementation sites). The trust had an internal manager with responsibility for commissioning, who acted as an interface with the PCT to develop and negotiate contracts, an arrangement that had worked particularly well in the introduction of IPT.
Non-implementation site
The fourth site was a specialist diabetes centre, hosted by an NHS foundation trust. The centre had only recently moved from a primary to secondary care setting, as a result of the changes to commissioning in the NHS. On account of its specialist status, the trust dealt with a large number of commissioners, across a wide geographical area. The introduction of IPT had been led by a clinical academic, who had submitted an application to become an NTAC implementation site for IPT. This application was unsuccessful; however, the consultant and some of his colleagues had continued to try to develop their pump service without NTAC's input.
- Uptake of insulin pump therapy: survey findings
As outlined in Chapter 3 , Research methods and Data analysis and Appendix 3 , we conducted an online survey of a network of UK clinicians actively engaged in trying to increase IPT uptake to assess the current level of uptake of IPT. Table 4 summarises the key findings from this survey, comparing estimated uptake of IPT in May 2012 with uptake levels 3 years previously.
Percentage of patients with diabetes estimated by respondents to be using IPT
These data suggest an increased use of IPT, in line with the findings of the Medical Technology Group survey of 2010. However, they also indicate that, in summer 2012, 65% of respondents reported that pump use was lower than 10% in their NHS trust (in other words, below the 12% target recommended in the NICE guidance). Moreover, of the 62 respondents working in trusts where the uptake rate of IPT was < 5% 3 years previously, only 29 (47%) had managed to raise this level to > 5%.
Our qualitative data collection attempted to explore in more depth the reasons for the lower than recommended uptake of IPT in the NHS in England, and the difficulties encountered in attempting to increase the uptake rate. Analysis of the findings reveals a number of key themes in terms of factors that appear to facilitate or hinder the adoption of IPT, ranging from those on the individual level, including patient- and clinician-focused factors, through to more organisational- and system-level issues relating to past history and experience of IPT including resourcing, financing and commissioning issues. Each of the key themes is discussed in more detail in the following sections.
- The patient pull for insulin pump therapy
A number of patient level factors appear to be important in terms of the adoption (or otherwise) of IPT. These include patient-driven demand for the service, acceptability of the technology and the importance of patient self-management of the technology. In relation to the first issue, views differed in terms of the extent to which patient demand was important in driving the introduction of the technology. In some cases, patient requests for IPT were seen to be a major driver:
We are very good at being ahead of our patients in what they know about either their condition or their treatment plan or their medications. But with insulin pumps, there was a feeling that we were only just one step ahead of our patients because they came in so suddenly really that the patients were asking for them and we were saying, ‘Oh, hang on a minute. We’re not so sure that we have the skills and knowledge to facilitate this for you.’ So there was a definite perceived, you know, we weren’t skilled enough to run these kind of programmes. Diabetic specialist nurse, IS2
By contrast, other interviewees did not perceive a significant patient demand for pumps; rather they felt that it tended to be the clinicians who raised the possibility of using a pump as an alternative to MDIs.
One of the things that I have noticed when I’ve been to meetings and so on elsewhere that some of the people say patients ask for pump therapy. It doesn’t happen in my experience very much . . . Yes it tends to be us that says, well actually I think you might benefit, would you consider a pump? There is also a significant turning down of going on pump therapy. Diabetic consultant, IS1
Linked to the above point, there was a fairly consistent view among many of the clinical staff interviewed that there were issues relating to patient acceptability of pumps that accounted for some of the lack of uptake of IPT. For some patients, this was about how the pump would fit in with their lifestyle or how they felt about having to be attached to a pump at all times; for others it was the requirements that went hand in hand with using a pump, for example the need for active self-management and regular blood glucose monitoring.
There are a significant number who think about it and then just don’t want even to take anything any further. They’re clear in their own minds that they don’t want a pump. And I think the minority are the ones who would be prepared to consider a pump and they then go on to have an assessment, a formal assessment. And of those who go forward, not everyone in the end decides that they want a pump. Having seen one, talked about it in more detail, quite a number of them have come to the conclusion that it’s not for them . . . Diabetic consultant 3, NIS
However, for others the pump was seen to provide them with a greater freedom to manage their diabetes, despite some initial concerns about the pump itself:
Well there’s the body image, which probably is more of a concern for females, understandably . . . There’s a classic quote where the partner calls them the Bionic Man or Bionic Woman because they are linked up to the machine. Certainly in my study . . . I think the patient experience is honestly amazingly positive. Even people who have apprehensions about going on the pump, but still probably want to do it because their control is not where they want to be or they’ve got severe hypos, generally speaking a few months into pump they would not go back to injections. Specialist medical trainee, NIS
The comments above represent a rather divergent set of opinions on patients’ views of IPT from the perspective of professionals delivering the service. It also appears that professionals’ views do not always correspond with the views of patients themselves. This is illustrated by some of the e-mail correspondence received by the patient support group, INPUT.
[My] consultant said: ‘If you use a pump you will be a failure to yourself.’ ‘It’s not suitable for you because you work.’ ‘If you have to miss a meal you’ll be in trouble because the insulin is going in all the time.’ Patient A with type 1 diabetes
My daughter has had diabetes for 4 years. The clinic team are saying it’s not the right time for a pump. She is aged 9, will be 10 in April. [Her] control is very poor. The diabetes clinic said ‘how would we work out her pump ratios when she’s so up and down?’ Parent of child B with diabetes
Achieving effective self-management of pumps
Despite some differences of opinion on the level of acceptability of pumps to patients, there was more consensus on the need for effective self-management by patients using pumps, including educational preparation and support to use the pump. This included regular monitoring of blood glucose and a level of understanding to interpret the results and adjust the pump settings accordingly.
When a patient goes on a pump, initially, it is . . . very time-consuming, the patient needs lots of education . . . the thing about the pump it, whether it works or not, depends on patients’ self management. Now, for a patient to be able to self manage the pump, they need to be, learn a lot about the pump. They need to learn all the pump functions, they need to know what to do if they hypo, if their blood sugars are high . . . Diabetic specialist nurse, IS1
In the case of children, the second implementation site had identified a number of additional criteria to determine which children were eligible to be started on pump therapy.
As long as they’ve got at least one English speaking parent because, as you can imagine, it’s quite tricky to train somebody who doesn’t read and speak English to use that level of technology. If you’ve got one parent that speaks and reads English, we can go for it. And we’ve had ten children in the last two years go straight onto insulin pumps from diagnosis. So there is no [lower age limit] – and they actually work really, really well for tiny babies. Diabetic specialist nurse, IS2
- The influence of clinicians on the implementation of insulin pump therapy
Clinician-related factors appeared particularly influential in the adoption of IPT; this was particularly the case at a consultant level. Where consultants providing a diabetic service were motivated and enthusiastic about IPT, they played an important role in leading the introduction of the technology.
. . . we have always been a forward looking trust I think, although we are only a district general hospital I do think that we have a philosophy of, the philosophy that we have in this department is that our patients should not miss out on any treatment that could be of benefit to them and that is available in the UK and that’s how it has always been. Diabetic consultant, IS1
Equally, where consultants were sceptical or wary of the technology, they represented a significant barrier to its implementation. For some clinicians, this appeared to relate to bad experiences of using pump in the earlier stages of developing the technology, alongside some more recent negative incidents.
And you have to remember that I did actually use pumps in 1982, so I’ve been using pumps for a very long time . . . So I’m not sceptical because I’ve had nothing to do with them. I’m sceptical because I’ve seen the good things and I’ve also seen the bad things about pumps. Like the girl we admitted last weekend, in very severe diabetic ketoacidosis [ketoacidosis is a dangerous complication of diabetes caused by a lack of insulin in the body], with a pH of 6.8, who nearly died, and I’m not egging this up because she’d . . . we hadn’t put her on a pump, she was put on a pump elsewhere and she didn’t self-manage properly. When she felt sick, she took her pump off and she was that close to dying. Diabetic consultant 3, NIS
In other cases, resistance appeared to be tied up with local politics and personalities:
And that’s one of the sort of barriers . . . internal barriers to implementation and it just kind of reflects the bigger picture I think and problems within the sort of structure of the team that was highlighted by this project. So I think some of the barriers to this project aren’t really barriers to this project, they’re more to do with identifying barriers that are within . . . our Diabetes Team . . . one individual in particular who was asked to be involved but because of other work commitments he wasn’t, couldn’t get involved but if it isn’t his project he doesn’t want to know and he has done quite a few things to try and sabotage the . . . and he’s still trying to do things to sabotage it. It’s not just in this, it’s in other things as well. And those are the sort of kind of things that actually aren’t necessarily surmountable. Project manager, IS1
- The strength of evidence for insulin pump therapy
One particular issue that emerged, and may partly account for the differences of opinion at a clinical level, was the strength of the evidence supporting the introduction of IPT. Some clinicians perceived the evidence supporting the use of IPT as rather ‘shaky’.
I don’t think we have the evidence yet and that is a really important question. I think it was an assumption when we started our journey with pumps really so hopefully if you can get people good at this then their needs for support get less. I don’t know if that’s born true and I think this comes back to your patient selection that you need to be very careful about the reasons that the patient is selecting a pump. The one thing that it does not do is take away your diabetes it makes it even more in the front and you know whether they are able to manage it themselves and again I think we haven’t to my knowledge got very good data on long-term outcomes from our pump clinic. Diabetic consultant 4, NIS
I’m not even a hundred per cent sure about the proven clinical benefits. If you have . . . do the proper randomised controlled trial, pump versus intensive therapy with an equivalent amount of input from health care professionals, you don’t get a great difference in Hb 1c or in anything else. You might get a difference in patient satisfaction in favour of pump, but that’s in folk who want to go onto the pump. So I think sometimes the benefits of pumps are a bit overstated. Diabetic consultant 3, NIS
This second quote raised an issue that a number of interviewees at site NIS referred to: whether it was the additional educational input and support that pump patients received that made the most difference, as opposed to the technology itself. However, at other sites, the evidence for pumps was much less disputed, with IPT being described as a ‘proven technology’. With the endorsement from the NICE technology appraisal that IPT was a clinically effective and cost-effective treatment, this has enabled organisations to develop a case for their commissioners for increased funding of IPT.
I suppose, on the surface, it looks expensive but there’s then the evidence to show that . . . that’s why NTAC have taken it on . . . because the improvement of the blood result, HbA 1c , in preventing complications . . . is a proven technology for improving progress with [diabetic] complications. Diabetic specialist nurse 2, IS1
PCTs have to follow NICE, have to be seen to be following NICE guidance, so I know it’s a time of austerity and the PCTs have other priorities, but a trust should always play the NICE guidance card and say, look you know, that’s why we’ve got NICE guidance because it’s the best outcomes for this group of patients. Diabetic specialist nurse, MS
However, there were some who were critical of the wording of the NICE guidance, which indicates that IPT should be used where MDIs have not been effective in controlling blood glucose levels. This led some interviewees to suggest that IPT was perceived as a ‘treatment of failure’:
And at the moment, very much NICE guidance is geared to pump use being used as a treatment of failure. So if you fail with your multiple daily injections, i.e. you’ve still got poor control, or you’ve still got low blood glucose. It’s a negative process . . . it’s sort of like a downward staircase on diabetes. Start on maybe twice daily injections or your control is not very good – let’s put you onto the next treatment, which might be structured education and basal bolus regime [a basal-bolus regimen, which includes an injection at each meal, attempts to roughly emulate how a non-diabetic person’s body delivers insulin]. Oh you’ve not done well with that, you’ve failed with that. Right, let’s go onto something else. And eventually you get to pump therapy. By that time, the question is, is the patient demoralised, is the clinician demoralised? Why is pump therapy used as the treatment of failure, if it’s supposedly the best treatment? Compared to cancer treatment, you wouldn’t . . . We’ll start with something but if you don’t get better then we’ll use the next one. Whereas in diabetes, it is like that. Specialist medical trainee, NIS
This could lead to a dilemma as to how to proceed with patients who might benefit from a pump, but did not meet the NICE criteria.
But there are definitely people whose diabetes control is okay but actually they probably would do better if they were on a pump, but that’s not covered by NICE guidance, so they’re currently excluded and you can’t really encourage them to let their HbA 1c s go up, which you know some of the conversations that not just us have had but we’ve you know . . . well do you let them increase their [blood glucose] . . . so they meet the criteria? Project manager, IS1
- The initial investment costs of insulin pump therapy
A key issue relating to the introduction of IPT was the service investment required, including the availability of specialist pump trained nurses, educational preparation of patients to use a pump, and the cost of purchasing pumps and related consumables. The upfront investment costs in terms of staff and patient education were seen to be significant. Furthermore, several interviewees raised questions about the extent to which investing in service development for the introduction of IPT took services away from non-pump users and could result in inequity.
And I think the other barrier is the fact that, certainly in [this organisation], we expend a great deal of health care time with the small number of people who are on pumps and that is to the detriment of people who are not on pumps. We have a limited number of physicians, nurses, dieticians and if they’re working with pumps, it means that they’re not working with our other non-pump population. And I think we have a tension there; that the more resources we devote to pumps, the less we’ve got for somebody else. So it’s not just the cost of the pump and the pump consumables, it’s the health care professional time as well. Diabetic consultant 3, NIS
Others raised concerns about the costs of the technology itself, suggesting that the cost was kept unnecessarily high by the producers, which, in turn, acted to limit the wider scale uptake of IPT.
So there’s been a lot of input from the pump manufacturers, but then there ought to be because prices of the pumps is ridiculous. And if you want to ask about the constraints, I’ve said it to everybody so I may as well say it to you, if an iPad can be 600 quid, why is an insulin pump £3,500? . . . And if – it strikes me that it’s not any technical – I don’t know anything about technology, but I think they’re milking it. And if you could even – that’s your – that’s your biggest constraint. Diabetic consultant 2, NIS
- Funding and commissioning insulin pump therapy
Each of the four sites had different arrangements in terms of the commissioning of IPT services. In the first implementation site (IS1), the trust had a close working relationship with commissioners. The PCT had been instrumental in driving the adoption of IPT and agreed to provide funding for the service without a formal business case.
So that’s when I got involved, I suppose that’s why they’re saying it’s been driven primarily by the PCT because once I became involved, I did actually start trying to structure it and say, you know, let’s look at it in a more comprehensive way; rather than just clinicians thinking this is a great thing to do, without any thought about how we’re going to pay for it. Commissioner, IS1
. . . unlike other places we don’t actually have anything signed between the PCTs and us, we didn’t have to do a business case or anything. We sat around the table. . .. Diabetic consultant, IS1
The second implementation site worked with a large number of commissioners, which could cause difficulties as different commissioning bodies had different ways of working.
I think what’s difficult is they all have a different way of working. For example, the main PCT that we work with . . . are quite happy to be invoiced directly by the insulin pump companies. I order a pump and the consumables from the pump company; they invoice the PCT; the PCT pay the bill. The patient then starts ordering consumables from the company and the PCT get the bill. That’s brilliant. I don’t have to have a cupboard full of stock. I don’t have to sign off invoices. I don’t have to get involved. So that works really well. Then you have another PCT, who shall remain nameless – there are two or three of them – where they insist on recharging back to the children’s hospital, who then have to pay the company, and then the company will supply. And then the bill will come back to the children’s hospital, we pay the bill, then we recharge the PCT . . . So you add another step. An unnecessary step in some respects, but it’s in there . . . And the other problem that that then causes is the PCTs do not get any bulk buying discount and they are missing a trick . . . Diabetic specialist nurse 1, IS2
In the MS, the acute trust had a commissioning manager who acted as a point of liaison and negotiation with the PCTs, which was seen to have played an important supporting role in the introduction of IPT.
. . . not every trust has got like my post and my team that can be the conduit so sometimes the individual departments struggle with, who do they speak to within the PCT or we can help with that. We facilitate it, we package it in a way we know the PCTs would be amenable and so I think that’s quite important . . . We call it commissioning, which is a bit of a daft name, . . . so we agree the contracts, we agree the quality in the case of the PCT, we’re always mindful of whatever we agree is, I’ve got an inward looking role and an outward looking role and we’ve got to keep that, that communication going both ways from my department basically. Commissioning manager, MS
In the fourth (non-implementation site; NIS), the organisation had been used to working on a block contract arrangement and had discretion over the use of their resources, which meant they could decide the level of investment they made in pumps. This was initiated when a clinical academic consultant came to work in the service and was interested in establishing a pump service. However, the consequence was that the service had largely grown around the practice of this particular clinician without being more widely adopted by or embedded in the rest of the organisation.
[The consultant] . . . was told in no uncertain terms that he could not develop a service bigger than that because he was an academic . . . It was not clear what his long-term shelf life would be . . . so he could then leave me with a huge number of people on pumps . . . We have to be quite careful that if he did move we’re then not left with a service and nobody and no budget to cover it. Clinical manager, NIS
In summary, the implementation and MSs were able to get the PCTs to fund IPT because of the NICE guidance and, as a consequence, they were not particularly worried about the lack of a national tariff for IPT. Indeed, having the PCT pay directly for the costs was seen to be an advantage, as a tariff only provides the average national cost, which may be less than the actual cost.
I guess the danger with having a specific HRG [for the pump service] is making sure it reflects the cost and responds to changes in cost. Whereas the advantage to us at the moment is we don’t have to worry about it. If someone’s having a pump, the PCT pays for it direct. Diabetic consultant, IS2
However, a persistent concern raised related to the infrastructure required to establish an IPT service, in terms of nurse specialist provision and patient education (as discussed in the previous section); this was a cost that the organisation typically had to absorb as part of setting up the IPT service.
- The role of NHS Technology Adoption Centre in supporting implementation
NHS Technology Adoption Centre clearly played a key role in supporting the implementation of IPT for the two implementation sites. This included acting as an initial catalyst to ‘do something’ about IPT, introducing a formal project management structure, providing a map of how to get started and implement IPT and developing the systems that were needed to support the use of the technology (see Chapter 4 for a description of the NTAC process of working with implementation sites).
I mean I think it was if you like the catalyst, participating in this project was the catalyst that we needed to get us to focus on this. Diabetic consultant, IS1
They [NTAC] were phenomenally helpful. We knew where we wanted to be, but weren’t sure of the map to use to get us there . . . NTAC were really good in helping us to get the people in the room who needed to be in the room, to have the right conversations. Project management – I think that’s what we really lack and what they did really well. Diabetic specialist nurse, IS2
That’s the difference with NTAC. It’s a project. One of the things that’s come out of it, I would say, is that there’s been joint working with commissioners and PCTs and a recognised procurement process . . . The whole problem in the past is that you had patients waiting for pumps but it was either applying for funding individually, . . . or finding an ad hoc way of doing it. Diabetic specialist nurse, IS1
The NTAC method of supporting technology adoption seemed particularly suited to the sites attempting to introduce IPT, providing a co-ordinating structure and project management that had been lacking, despite prior enthusiasm or intent to support IPT. The lack of a co-ordinated approach to IPT provision is equally apparent in some of the patient feedback received by the support group INPUT:
My consultant supports my belief that an insulin pump should now be tried as soon as possible and referred me to a Dr X at the X Diabetes Centre. Dr X has agreed that a pump should be tried soon, however he has warned me that there may be a delay of possibly 5 months before the local PCT gets around to agreeing to this proposal. Patient C with type 1 diabetes
I would like some guidance if possible on how much choice I have on the insulin pump that is provided for my 5 year old. We have had funding approved for a pump, although the pump of our choice – we are told is too expensive by the PCT. Our hospital have said they will support us in whatever pump we choose if we can obtain funding, but have also said that if we don’t make up our minds soon the funding will be retracted. Parent of child D with type 1 diabetes
Although acknowledging the important input that NTAC provided in structuring and signposting the implementation of IPT, there was also a view from some interviewees that the hard work only started once the NTAC project was coming to an end and they had to actually implement all the things they had planned on paper. In one of the two implementation sites, this responsibility sat with an internal project manager for IPT.
Well, it’s like anything; the pump project is in some way a theory. It was setting a structure, having guidelines, having a structure to every stage of pump therapy, but then, whatever we’ve decided now as part of this pump project – you’ve got to put it into practice! . . . That’s when the real hard work starts, doesn’t it? Up to now it’s been on paper. Diabetic specialist nurse 2, IS1
How to Why to guides
Overall, the case study sites had limited involvement with the HTWT guides. Although the two implementation sites had been involved in the development of the guides, the first site had received almost no contact from other organisations asking for information or advice since the publication of the guide. This was the same situation in the MS studied and there was a view among the interviewees that the guide could not substitute for the hands-on project management support provided through working with NTAC.
I’d like to be able to show you some examples of where the How to Why to guide has been used and someone’s done what we have done, but my worry is that it’s quite hard without that push from NTAC centrally. Diabetic specialist nurse, IS2
The exception to this was IS2, where one of the diabetic nurse specialists reported using the HTWT guide to direct people who contacted the trust for advice and guidance on setting up a pump service.
I’ve [directed] an awful lot of people to the How To Why To guide . . . I used to get a lot of calls saying, you know, ‘How do I get my pump service up and running? How do I get more kids on pumps? What do I do?’ and you’d spend, you know, forty minutes on the phone. The How To Why To guide has saved us loads of time because you can say, ‘Go on and track it through,’ and all those questions you’re likely to ask are answered. And there are templates on there for doing a business case.’ Diabetic specialist nurse 2, IS2
- Discussion and summary
Overall, the NTAC process seemed relatively well matched to the needs of IPT implementation sites; these were sites that were generally receptive to IPT and were already working with the technology, but often in an ad hoc way. NTAC brought a structured project management approach that enabled them to formalise the implementation of IPT. From an ANT perspective, the network of human and non-human actors was less complex in the IPT case, compared with the other two technologies studied in the research. Certainly, in the implementation sites, work had already been undertaken to address issues related to problematisation and interessement. However, the sites were lacking the guidance and ‘know-how’ to move further along the translation path in a systematic and structured way; the input from NTAC provided the support that these organisations needed. Through applying a project management approach and enlisting the involvement of key stakeholders, the NTAC model enabled and supported implementation sites to move through the translation path from problematisation/interessement to enrolment and mobilisation. Introducing IPT requires changes to the patient pathway and the design and delivery of diabetes services, for example in relation to patient and staff education and dedicated specialist nursing support. In turn, this requires a different set of networks and relationships to be developed. However, the fact that the evidence base for the technology was relatively well established and accepted (at least in the implementation sites) meant that the NTAC model of working had a good fit with the organisation’s needs in terms of developing this new network of actors, agreeing and co-ordinating roles, and embedding new structures and processes required to sustain the innovation.
The data reveal a marked contrast between the experiences and views of the two implementation sites and the NIS, where the receptivity to the technology appeared more mixed, particularly in relation to the benefits of IPT, the evidence supporting its adoption and the feasibility of establishing a wider adoption programme. From an ANT perspective, it could be hypothesised that the NIS was still at the problematisation stage, with human actors (particularly the clinicians) not agreeing on the nature of the problem and whether or how it needed to be addressed. This site did not receive the type of external input and support that NTAC provided to address the translational stages from problematisation through to interessement and beyond. The existence of an online resource in the form of a HTWT guide did not appear to provide a comparable substitute to the more ‘hands-on’ role provided by NTAC to actively facilitate the translational process. However, the question remains as to whether or not the NTAC process would be sufficient to address the barriers in the NIS. The issues at this site appeared to be more to do with differences of professional opinion over the value and benefit of the technology, rather than more practical problems of how to ensure implementation.
Included under terms of UK Non-commercial Government License .
- Cite this Page Llewellyn S, Procter R, Harvey G, et al. Facilitating technology adoption in the NHS: negotiating the organisational and policy context – a qualitative study. Southampton (UK): NIHR Journals Library; 2014 Jul. (Health Services and Delivery Research, No. 2.23.) Chapter 6, The insulin pump therapy case study.
- PDF version of this title (1.1M)
In this Page
Other titles in this collection.
- Health Services and Delivery Research
Recent Activity
- The insulin pump therapy case study - Facilitating technology adoption in the NH... The insulin pump therapy case study - Facilitating technology adoption in the NHS: negotiating the organisational and policy context – a qualitative study
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 9, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
Recall notice after more than 200 insulin pump users injured due to app malfunction
by Ernie Mundell

The U.S. Food and Drug Administration has issued a Class 1 recall—its most urgent kind—for an IOS app linked to a specific kind of insulin pump used by people with diabetes.
The recall notice , which the FDA says is a "correction" rather than a product removal, involves version 2.7 of the Apple iOS t:connect mobile app. It's used in conjunction with t:slim X2 insulin pump with Control-IQ technology.
The problem: The app can repeatedly crash and then restart. Over time, this drains the battery of the insulin pump, which must function in order for patients to stay healthy. Already, nearly 86,000 of the apps have been recalled due to this issue.
"Pump shutdown will cause insulin delivery to suspend, which could lead to an under-delivery of insulin and may result in hyperglycemia or even diabetic ketoacidosis , which can be a life-threatening condition due to high blood sugars and lack of insulin," the FDA explained in a statement released Thursday.
So far, the agency knows of 224 reported injuries from this malfunction as of April 15. No deaths have been reported.
Anyone who uses the t:slim X2 Insulin Pump Mobile App version 2.7 on the Apple iOS platform could be affected.
According to the FDA notice, Tandem Diabetes Care, Inc. has recalled version 2.7 of the t:connect mobile app, which was first released on the Apple iOS platform on Feb. 12, 2024. It works in conjunction with the t:slim X2 insulin pump, which delivers insulin under the skin at specific but varying rates.
Tandem is urging users to update the software to version 2.7.1 or later. It is available in the Apple App Store.
After that:
- Identify the software version of the t:connect mobile app by opening the app, clicking the "Setting" icon on an iPhone's screen, and then clicking "About."
- Complete the online form External Link Disclaimer to acknowledge receipt of this notice.
- Keep using the pump as described in the user guide, paying heed to all system alerts and alarms.
- Monitor pump battery level closely to ensure the pump is at or near full charge before going to sleep to help prevent pump shutdown.
- Begin charging device after the first low battery alert.
- Always carry backup supplies for insulin delivery in the case of insulin pump failure.
2024 HealthDay. All rights reserved.
Explore further
Feedback to editors

Artificial intelligence tool detects sex-related differences in brain structure

Over 20,000 people join UK search for new dementia treatments

Clinical trial: New drug makes exercise, everyday tasks easier for people with common heart condition
9 hours ago

Semaglutide can yield weight loss, lower heart issues for at least four years in non-diabetic adults with overweight
12 hours ago

Study reveals key role of glutamate tRNA fragments in brain aging and Alzheimer's disease
13 hours ago

New molecule mimics the anti-clotting action of blood-sucking organisms

Axons in female mammal brains may be more prone to concussions

Herpes cure with gene editing makes progress in laboratory studies
14 hours ago

Breast cancer risk variants identified for women of African ancestry

Variations in telomere lengthening genes may predispose some people to papillary thyroid cancer
Related stories.

FDA clears first smartphone app to deliver insulin doses
Feb 21, 2022

FDA warns of cybersecurity risk with certain Medtronic insulin pumps
Sep 20, 2022
Insulin pumps associated with lower risk of serious complications among young patients with type 1 diabetes
Oct 10, 2017

Medtronic expands recall to include more than 463,000 insulin pumps
Oct 5, 2021

Study shows hybrid pumps work very well in type 1 diabetes
Apr 24, 2024

Eli Lilly warns that two insulin products will be in short supply
Mar 25, 2024
Recommended for you

Peptide-based hydrogel shows promise for a wide range of tissue and organ repair

New synthetic biomarker technology differentiates between prior Zika and dengue infections
15 hours ago
Research takes electroretinography to the next level with a soft multi-electrode system
17 hours ago
Improving the safety of iPS cell-derived pancreatic islets by eliminating unwanted cells
20 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Hundreds injured after insulin pump software issue, FDA says

(CNN) - The Food and Drug Administration reports hundreds of people were injured by a malfunctioning insulin pump.
The problem was with T-Connect, the software that connects the T-Slim insulin pump to iPhones.
A glitch in the code caused the app to crash and relaunch, which can also drain the pump’s battery sooner than expected.
On March 26, Tandem Diabetics Care told affected owners to update their mobile phone apps to an updated version that corrects the issue.
About 85,000 people were running the problematic software at that time.
The FDA is now reporting the technical issue left to 224 injuries.
No deaths have been attributed to the problem.
Copyright 2024 CNN Newsource. All rights reserved.

Zurik: New photo appears to show NOPD’s Vappie enjoying wine with Mayor Cantrell while on duty

New Orleans woman ordered to stay away from Mayor Cantrell over stalking allegations

Severe Geomagnetic Storm Watch issued for the first time in almost 20 years

Bond set at $100k for NBA YoungBoy in prescription drug fraud case

Stream news and weather 24/7
Latest news.

Man accused of fatally shooting attorney in McDonald’s order dispute

County employees working 32-hour weeks report better job satisfaction, mental health

Employees praise Washington county's new 32-hour workweek

Search for 17-year-old girl’s remains continues; suspect in custody

104-year-old celebrates Mother’s Day with six generations of family

IMAGES
VIDEO
COMMENTS
This case study discusses the control software for a personal insulin pump, which is used by diabetics to mimic the function of the pancreas and hence control the level of glucose (sugar) in their blood.
A personal insulin pump. This case study discusses the control software for a personal insulin pump, which is used by diabetics to mimic the function of the pancreas and hence control the level of glucose (sugar) in their blood. The system hardware is illustrated schematically in the following diagram:
The insulin pump delivers one unit of insulin in response to a single pulse from a controller. Therefore, to deliver 10 units of insulin, the controller sends 10 pulses to the pump. Figure 2 is a UML activity model that illustrates how the software transforms an input blood sugar level to a sequence of commands that drive the insulin pump. Needle
Reference : Ian Sommerville Software engineering 9th Edition No copyright infringement intendedImage Courtesy of insulin pump: https://diabeteson.com/technic...
Insulin Pump System Design Introduction The insulin pump system is a portable, automated insulin pump which is used by diabetics to administer insulin as and when they require it at regular, periodic intervals. This document illustrates the important design decisions that were made during the production of both the insulin pump software and the ...
engineering design using the design of an insulin pump as a case study. Keywords: Biomedical design, User centred design, Design process, User-in-the-loop, Insulin pump Contact: Karia, Deval Indian Institute of Science Bangalore Centre for Product Design and Manufacturing India [email protected] 847
An Affordable Insulin Pump for Type-1 Diabetic Patients: A Case Study of User-in-the-Loop Approach to Engineering Design - Volume 1 Issue 1 ... we've outlined a user-in-the-loop approach to engineering design using the design of an insulin pump as a case study. Keywords. Biomedical design User centred design Design process User-in-the-loop ...
In this paper, we study how to use the Structured-Object-Based-Formal Language, which is called SOFL to develop a software system controlling an insulin pump, called the Generic Insulin Infusion Pump (GIIP). This case study facilitates the understanding of how SOFL can be applied to software systems related to medical devices in terms of the ...
Moreover, the field of biomedical engineering has paved the way for the creation of low-cost solutions, such as cost-effective insulin pumps for type-1 diabetic patients [55], and wearable devices ...
Copy Command. This example shows you how to use a model-based systems engineering (MBSE) workflow to investigate optimal insulin infusion pump design. Insulin pumps are medical devices used by people with diabetes that mimic the human pancreas by delivering insulin continuously and delivering variable amounts of insulin with food intake.
An insulin pump and its environment form a typical hybrid system that the discrete software controls directly the continuous physical injection. Therefore, it is a very interesting case study for formal development. When tried to construct a family of insulin pump following the requirements in using Event-B , we met the following problems ...
The Engineering Behind Great Products. NOVO's insulin pump embedded design contributions to this medical device development program, including the incorporation of control algorithms developed by Bigfoot, integrated a set of discrete devices into a body area network that functions as a closed loop medication delivery system—a major step ...
The modeling approach is described by means of an insulin infusion pump case study, including formal models, assurance case models, and an Arduino prototype. It is argued that manufacturers may conduct the requirements engineering process based on goals (Goal-Oriented Requirements Engineering (GORE)) by creating assurance cases in GSN and ...
An embedded control system for a personal insulin pump. This case study discusses the control software for a personal insulin pump, which is used by diabetics to mimic the function of the pancreas and hence control the level of glucose (sugar) in their blood. The system hardware is illustrated schematically in the following diagram:
Collects data from a blood sugar sensor and calculates the amount of insulin required to be injected. Calculation based on the rate of change of blood sugar levels. Sends signals to a micro-pump to deliver the correct dose of insulin. Safety-critical system as low blood sugars can lead to brain malfunctioning, coma and death; high-blood sugar ...
Software Engineering
THIS VIDEO EXPLAINS THE 3 CASE STUDIES OF SOFTWARE ENGINEERING EXPLAINEDAn insulin pump control systemA patient information system for mental health careA wi...
NOVO has contributed to the mechanical, electrical, and software design of several insulin pump product families including pumps, controllers, consumables, and accessories. The image above shows the Medtronic 670G (a component of the first FDA approved artificial pancreas system) , the Tandem Diabetes t:slim, and the Unilife Imperium pumps.
Case studies. Insulin pump control system: This is a system that collects data from a blood sugar sensor and calculates the amount of insulin required to be injected. Calculation is based on the rate of change of blood sugar levels. It is programmed to send signals to a micro-pump to deliver the correct dose of. insulin.
This amount is injected to the patient through the insulin pump before the patients start eating food. Furthermore, patients with T1DM can view the insulin report chart to avoid hypoglycaemia and hyperglycaemia, even in a coma. ... Case study research in software engineering: Guidelines and examples. Wiley. Google Scholar Willmott, C. J ...
This case study focuses on IPT, used in the treatment of type 1 diabetes. Type 1 diabetes is a long-term condition that affects around 250,000 people in the UK and requires lifelong treatment with insulin.187 Rates of type 1 diabetes have been increasing over time, with the greatest increase in children younger than 5 years of age. People with type 1 diabetes are unable to produce the natural ...
Description. This case study is used throughout the book to illustrate various aspects of embedded critical systems including specification and safety analysis. The material here is based on classwork that I set around this system where students had to write a simulation of it. General overview of the system. Use in teaching.
The displacement micropump with passive check valves is an attractive solution for precise insulin infusion in patients with type I diabetes. Unlike most insulin pumps that push insulin from a cartridge using a piston, a displacement micropump will first pull insulin from the reservoir before infusing it into the patient. This dual sequence introduces new challenges in terms of insulin ...
To ensure, as much as possible, that their efforts will be used for good, software engineers must commit themselves to making software engineering a beneficial and respected profession. Ethical principles 1. PUBLIC - Software engineers shall act consistently with the public interest. 2. CLIENT AND EMPLOYER - Software engineers shall act in a ...
by Ernie Mundell. The U.S. Food and Drug Administration has issued a Class 1 recall—its most urgent kind—for an IOS app linked to a specific kind of insulin pump used by people with diabetes ...
The problem was with T-Connect, the software that connects the T-Slim insulin pump to iPhones. A glitch in the code caused the app to crash and relaunch, which can also drain the pump's battery ...
The FDA is alerting users of the t:slim X2 insulin pump that an app used to regulate the device could be malfunctioning, and needs to be checked and updated if necessary to avoid injury. News. Medical Technology. Diabetes. More than 200 people with diabetes were injured when their insulin pumps shut down unexpectedly due to a problem with a ...