Heat Transfer Quiz: Convection, Conduction, And Radiation

Welcome to our Heat Transfer Quiz, where you can test your knowledge of the principles and mechanisms governing the transfer of heat! Heat transfer is a fundamental concept in physics and engineering, encompassing various processes by which thermal energy is exchanged between different systems. In this quiz, you'll encounter questions that cover the three main modes of heat transfer: conduction, convection, and radiation. You'll explore how heat flows through solids, liquids, and gases, and learn about the factors that influence heat transfer rates. Additionally, this quiz will challenge your understanding of specific heat capacities, thermal conductivity, and the Read more laws of thermodynamics as they relate to heat transfer phenomena. By engaging with this quiz, you'll have the opportunity to deepen your understanding of thermal physics and its practical applications in everyday life and various industries.

Heat Transfer Questions and Answers
Rising warm air currents is an example of:.
Rate this question:
A thermometer works because the liquid in its contracts when heated. This is an example of:
How is air heated in our atmosphere, true or false the sun directly heats the air in our atmosphere., true or false higher temperature means faster moving molecules., true or false air is a great conductor of heat., the reason why water boils, causing the circular motion is due to, when you get into a car with hot black leather in the middle of the summer, and you feel the seats become extremely hot, this is an example of, when the warmth of the sun heats rocks, this is an example of, when a metal spoon with a temperature of 20 degrees celsius is placed in a cup of water with a temperature of 90 degrees celsius, the spoon will heat up. this is an example of:, during a house fire, the smoke and flames rise, but the air down near the floor is cooler and less smoky. this is an example of:, what type of heat transfer occurs when heat moves from one molecule to another, when heat is given off by light, this type of heat is known as:, the transfer of heat between substances that are in direct contact with each other is called what.
Heat Transfer
.webp)
The ray in this picture is an example of:
The air in the hot air balloon is heated and circulates. this is an example of:, you walk barefoot on the hot street, and it burns your toes. this is an example of:, a boy sits to the side of a campfire. he is 10 feet away but still feels warm. this is an example of:, the transfer of energy in fluids or gasses:, light waves traveling through space:.
Quiz Review Timeline +
Our quizzes are rigorously reviewed, monitored and continuously updated by our expert board to maintain accuracy, relevance, and timeliness.
- Current Version
- May 13, 2024 Quiz Edited by ProProfs Editorial Team Expert Reviewed by Matt Balanda
- Jan 22, 2013 Quiz Created by Mr Duncan
Related Topics
- Temperature
- Educational Psychology
Recent Quizzes
Featured Quizzes
Popular Topics
- Heating Quizzes

Related Quizzes
Wait! Here's an interesting quiz for you.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

1.5: Heat Transfer, Specific Heat, and Calorimetry
- Last updated
- Save as PDF
- Page ID 4347

\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Learning Objectives
By the end of this section, you will be able to:
- Explain phenomena involving heat as a form of energy transfer
- Solve problems involving heat transfer
We have seen in previous chapters that energy is one of the fundamental concepts of physics. Heat is a type of energy transfer that is caused by a temperature difference, and it can change the temperature of an object. As we learned earlier in this chapter, heat transfer is the movement of energy from one place or material to another as a result of a difference in temperature. Heat transfer is fundamental to such everyday activities as home heating and cooking, as well as many industrial processes. It also forms a basis for the topics in the remainder of this chapter.
We also introduce the concept of internal energy, which can be increased or decreased by heat transfer. We discuss another way to change the internal energy of a system, namely doing work on it. Thus, we are beginning the study of the relationship of heat and work, which is the basis of engines and refrigerators and the central topic (and origin of the name) of thermodynamics.
Internal Energy and Heat
A thermal system has internal energy (also called thermal energy ) , which is the sum of the mechanical energies of its molecules. A system’s internal energy is proportional to its temperature. As we saw earlier in this chapter, if two objects at different temperatures are brought into contact with each other, energy is transferred from the hotter to the colder object until the bodies reach thermal equilibrium (that is, they are at the same temperature). No work is done by either object because no force acts through a distance (as we discussed in Work and Kinetic Energy ). These observations reveal that heat is energy transferred spontaneously due to a temperature difference. Figure \(\PageIndex{1}\) shows an example of heat transfer.
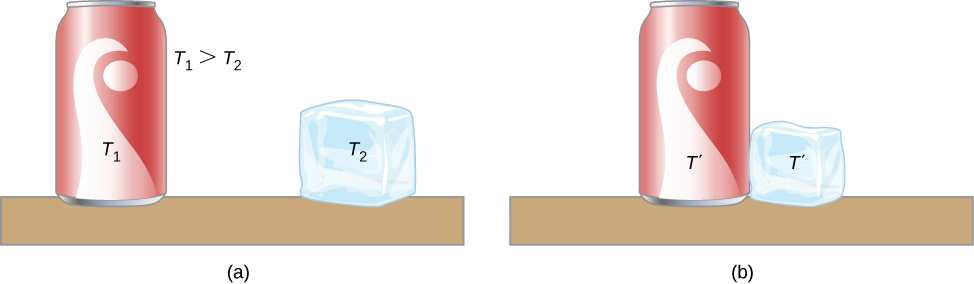
The meaning of “heat” in physics is different from its ordinary meaning. For example, in conversation, we may say “the heat was unbearable,” but in physics, we would say that the temperature was high. Heat is a form of energy flow, whereas temperature is not. Incidentally, humans are sensitive to heat flow rather than to temperature.
Since heat is a form of energy, its SI unit is the joule (J). Another common unit of energy often used for heat is the calorie (cal), defined as the energy needed to change the temperature of 1.00 g of water by \(1.00^oC\)—specifically, between \(14.5^oC\) and \(15.5^oC\) since there is a slight temperature dependence. Also commonly used is the kilocalorie (kcal), which is the energy needed to change the temperature of 1.00 kg of water by \(1.00^oC\). Since mass is most often specified in kilograms, the kilocalorie is convenient. Confusingly, food calories (sometimes called “big calories,” abbreviated Cal) are actually kilocalories, a fact not easily determined from package labeling.
Mechanical Equivalent of Heat
It is also possible to change the temperature of a substance by doing work, which transfers energy into or out of a system. This realization helped establish that heat is a form of energy. James Prescott Joule (1818–1889) performed many experiments to establish the mechanical equivalent of heat — the work needed to produce the same effects as heat transfer . In the units used for these two quantities, the value for this equivalence is
\[1.000 \, kcal = 4186 \, J.\] We consider this equation to represent the conversion between two units of energy. (Other numbers that you may see refer to calories defined for temperature ranges other than \(14.5^oC\) to \(15.5^oC\).)
Figure \(\PageIndex{2}\) shows one of Joule’s most famous experimental setups for demonstrating that work and heat can produce the same effects and measuring the mechanical equivalent of heat. It helped establish the principle of conservation of energy. Gravitational potential energy ( U ) was converted into kinetic energy ( K ), and then randomized by viscosity and turbulence into increased average kinetic energy of atoms and molecules in the system, producing a temperature increase. Joule’s contributions to thermodynamics were so significant that the SI unit of energy was named after him.
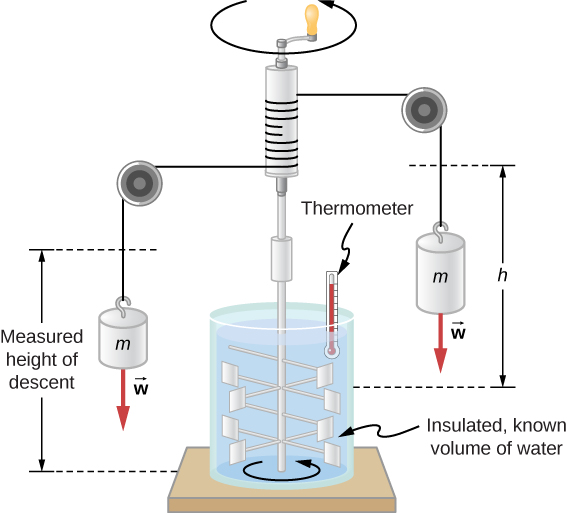
Increasing internal energy by heat transfer gives the same result as increasing it by doing work. Therefore, although a system has a well-defined internal energy, we cannot say that it has a certain “heat content” or “work content.” A well-defined quantity that depends only on the current state of the system, rather than on the history of that system, is known as a state variable . Temperature and internal energy are state variables. To sum up this paragraph, heat and work are not state variables .
Incidentally, increasing the internal energy of a system does not necessarily increase its temperature. As we’ll see in the next section, the temperature does not change when a substance changes from one phase to another. An example is the melting of ice, which can be accomplished by adding heat or by doing frictional work, as when an ice cube is rubbed against a rough surface.
Temperature Change and Heat Capacity
We have noted that heat transfer often causes temperature change. Experiments show that with no phase change and no work done on or by the system, the transferred heat is typically directly proportional to the change in temperature and to the mass of the system, to a good approximation. (Below we show how to handle situations where the approximation is not valid.) The constant of proportionality depends on the substance and its phase, which may be gas, liquid, or solid. We omit discussion of the fourth phase, plasma, because although it is the most common phase in the universe, it is rare and short-lived on Earth.
We can understand the experimental facts by noting that the transferred heat is the change in the internal energy, which is the total energy of the molecules. Under typical conditions, the total kinetic energy of the molecules \(K_{total}\) is a constant fraction of the internal energy (for reasons and with exceptions that we’ll see in the next chapter). The average kinetic energy of a molecule \(K_{ave}\) is proportional to the absolute temperature. Therefore, the change in internal energy of a system is typically proportional to the change in temperature and to the number of molecules, N . Mathematically, \(\Delta U \propto \Delta K_{total} = NK_{ave} \propto N\Delta T\). The dependence on the substance results in large part from the different masses of atoms and molecules. We are considering its heat capacity in terms of its mass, but as we will see in the next chapter, in some cases, heat capacities per molecule are similar for different substances. The dependence on substance and phase also results from differences in the potential energy associated with interactions between atoms and molecules.
Heat Transfer and Temperature Change
A practical approximation for the relationship between heat transfer and temperature change is:
\[Q = mc\Delta T,\]
where \(Q\) is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and \(\Delta T\) is the change in temperature. The symbol c stands for the specific heat (also called “ specific heat capacity ”) and depends on the material and phase. The specific heat is numerically equal to the amount of heat necessary to change the temperature of \(1.00 \, kg\) of mass by \(1.00^oC\). The SI unit for specific heat is \(J/(kg \times K)\) or \(J/(kg \times ^oC)\). (Recall that the temperature change \(\Delta T\) is the same in units of kelvin and degrees Celsius.)
Values of specific heat must generally be measured, because there is no simple way to calculate them precisely. Table \(\PageIndex{1}\) lists representative values of specific heat for various substances. We see from this table that the specific heat of water is five times that of glass and 10 times that of iron, which means that it takes five times as much heat to raise the temperature of water a given amount as for glass, and 10 times as much as for iron. In fact, water has one of the largest specific heats of any material, which is important for sustaining life on Earth.
The specific heats of gases depend on what is maintained constant during the heating—typically either the volume or the pressure. In the table, the first specific heat value for each gas is measured at constant volume, and the second (in parentheses) is measured at constant pressure. We will return to this topic in the chapter on the kinetic theory of gases.
In general, specific heat also depends on temperature. Thus, a precise definition of c for a substance must be given in terms of an infinitesimal change in temperature. To do this, we note that \(c = \frac{1}{m} \frac{\Delta Q}{\Delta T}\) and replace \(\Delta\) with d:
\[c = \dfrac{1}{m} \dfrac{dQ}{dT}.\]
Except for gases, the temperature and volume dependence of the specific heat of most substances is weak at normal temperatures. Therefore, we will generally take specific heats to be constant at the values given in the table.
Example \(\PageIndex{1}\): Calculating the Required Heat
A 0.500-kg aluminum pan on a stove and 0.250 L of water in it are heated from \(20.0^oC\) to \(80.0^oC\). (a) How much heat is required? What percentage of the heat is used to raise the temperature of (b) the pan and (c) the water?
We can assume that the pan and the water are always at the same temperature. When you put the pan on the stove, the temperature of the water and that of the pan are increased by the same amount. We use the equation for the heat transfer for the given temperature change and mass of water and aluminum. The specific heat values for water and aluminum are given in Table \(\PageIndex{1}\).
- Calculate the temperature difference: \[\Delta t = T_f - T_i = 60.0^oC.\]
- Calculate the mass of water. Because the density of water is \(1000 \, kg/m^3\), 1 L of water has a mass of 1 kg, and the mass of 0.250 L of water is \(m_w = 0.250 \, kg.\)
- Calculate the heat transferred to the water. Use the specific heat of water in Table \(\PageIndex{1}\): \[Q_w = m_wc_w\Delta T = (0.250 \, kg)(4186 \, J/kg ^oC)(60.0 ^oC) = 62.8 \, kJ.\]
- Calculate the heat transferred to the aluminum. Use the specific heat for aluminum in Table \(\PageIndex{1}\): \[Q_{A1} = m_{A1}c_{A1}\Delta T = (0.500 \, kg)(900 \, J/kg^oC)(60.0^oC) = 27.0 \, kJ.\]
- Find the total transferred heat: \[Q_{Total} = Q_W + Q_{A1} = 89.8 \, kJ.\]
Significance
In this example, the heat transferred to the container is a significant fraction of the total transferred heat. Although the mass of the pan is twice that of the water, the specific heat of water is over four times that of aluminum. Therefore, it takes a bit more than twice as much heat to achieve the given temperature change for the water as for the aluminum pan.
Example \(\PageIndex{2}\) illustrates a temperature rise caused by doing work. (The result is the same as if the same amount of energy had been added with a blowtorch instead of mechanically.)
Calculating the Temperature Increase from the Work Done on a Substance.
Truck brakes used to control speed on a downhill run do work, converting gravitational potential energy into increased internal energy (higher temperature) of the brake material (Figure \(\PageIndex{3}\)). This conversion prevents the gravitational potential energy from being converted into kinetic energy of the truck. Since the mass of the truck is much greater than that of the brake material absorbing the energy, the temperature increase may occur too fast for sufficient heat to transfer from the brakes to the environment; in other words, the brakes may overheat.

Calculate the temperature increase of 10 kg of brake material with an average specific heat of \(800 \, J/kg \cdot ^C\) if the material retains 10% of the energy from a 10,000-kg truck descending 75.0 m (in vertical displacement) at a constant speed.
We calculate the gravitational potential energy ( Mgh ) that the entire truck loses in its descent, equate it to the increase in the brakes’ internal energy, and then find the temperature increase produced in the brake material alone.
First we calculate the change in gravitational potential energy as the truck goes downhill:
\[Mgh = (10,000 \, kg)(9.80 \, m/s^2)(75.0 \, m) = 7.35 \times 10^6 \, J. \nonumber\]
Because the kinetic energy of the truck does not change, conservation of energy tells us the lost potential energy is dissipated, and we assume that 10% of it is transferred to internal energy of the brakes, so take \(Q = Mgh/10\). Then we calculate the temperature change from the heat transferred, using
\[\Delta T = \dfrac{7.35 \times 10^5 \, J}{(10 \, kg)(800 \, J/kg^oC)} = 92^oC. \nonumber\]
If the truck had been traveling for some time, then just before the descent, the brake temperature would probably be higher than the ambient temperature. The temperature increase in the descent would likely raise the temperature of the brake material very high, so this technique is not practical. Instead, the truck would use the technique of engine braking. A different idea underlies the recent technology of hybrid and electric cars, where mechanical energy (kinetic and gravitational potential energy) is converted by the brakes into electrical energy in the battery, a process called regenerative braking.
In a common kind of problem, objects at different temperatures are placed in contact with each other but isolated from everything else, and they are allowed to come into equilibrium. A container that prevents heat transfer in or out is called a calorimeter , and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is called calorimetry .
We will use the term “calorimetry problem” to refer to any problem in which the objects concerned are thermally isolated from their surroundings. An important idea in solving calorimetry problems is that during a heat transfer between objects isolated from their surroundings, the heat gained by the colder object must equal the heat lost by the hotter object, due to conservation of energy:
\[Q_{cold} + Q_{hot} = 0.\]
We express this idea by writing that the sum of the heats equals zero because the heat gained is usually considered positive; the heat lost, negative.
Calculating the Final Temperature in Calorimetry
Suppose you pour 0.250 kg of \(20.0^oC\) water (about a cup) into a 0.500-kg aluminum pan off the stove with a temperature of \(150^oC\). Assume no heat transfer takes place to anything else: The pan is placed on an insulated pad, and heat transfer to the air is neglected in the short time needed to reach equilibrium. Thus, this is a calorimetry problem, even though no isolating container is specified. Also assume that a negligible amount of water boils off. What is the temperature when the water and pan reach thermal equilibrium?
Originally, the pan and water are not in thermal equilibrium: The pan is at a higher temperature than the water. Heat transfer restores thermal equilibrium once the water and pan are in contact; it stops once thermal equilibrium between the pan and the water is achieved. The heat lost by the pan is equal to the heat gained by the water—that is the basic principle of calorimetry.
- Use the equation for heat transfer \(Q = mc\Delta T\) to express the heat lost by the aluminum pan in terms of the mass of the pan, the specific heat of aluminum, the initial temperature of the pan, and the final temperature: \[Q_{hot} = m_{A1}c_{A1}(T_f - 150^oC). \nonumber\]
- Express the heat gained by the water in terms of the mass of the water, the specific heat of water, the initial temperature of the water, and the final temperature: \[Q_{cold} = m_wc_w(T_f - 20.0^oC). \nonumber\]
- Note that \(Q_{hot} <0\) and \(Q_{cold} > 0 \) and that as stated above, they must sum to zero: \[Q_{cold} + Q_{hot} = 0\]\[Q_{cold} = -Q_{hot}\]\[m_wc_w(T_f - 20.0 ^C) = -m_{A1}c_{A1} (T_f - 150^oC). \nonumber\]
- This a linear equation for the unknown final temperature, \(T_f\). Solving for \(T_f\), \[T_f = \dfrac{m_{A1}c_{A1}(150^oC) + m_wc_w(20.0^oC)}{m_{A1}c_{A1} + m_wc_w}, \nonumber\] and insert the numerical values: \[T_f = \dfrac{(0.500 \, kg)(900 \, J/kg^oC)(150^oC) + (0.250 \, kg)(4186 \, J/kg^oC)(20.0^oC)}{(0.500 \, kg)(900 \, J/kg^oC) + (0.250 \, kg)(4186 \, J/kg^oC)} = 59.1 \, ^oC. \nonumber\]
Significance Why is the final temperature so much closer to \(20.0^oC\) than to \(150^oC\)? The reason is that water has a greater specific heat than most common substances and thus undergoes a smaller temperature change for a given heat transfer. A large body of water, such as a lake, requires a large amount of heat to increase its temperature appreciably. This explains why the temperature of a lake stays relatively constant during the day even when the temperature change of the air is large. However, the water temperature does change over longer times (e.g., summer to winter).
Exercise \(\PageIndex{3}\)
If 25 kJ is necessary to raise the temperature of a rock from \(25^oC\) to \(30^oC\), how much heat is necessary to heat the rock from \(45^oC\) to \(50^oC\)?
To a good approximation, the heat transfer depends only on the temperature difference. Since the temperature differences are the same in both cases, the same 25 kJ is necessary in the second case. (As we will see in the next section, the answer would have been different if the object had been made of some substance that changes phase anywhere between \(30^oC\) and \(50^oC\).)
Temperature-Dependent Heat Capacity
At low temperatures, the specific heats of solids are typically proportional to \(T^3\). The first understanding of this behavior was due to the Dutch physicist Peter Debye , who in 1912, treated atomic oscillations with the quantum theory that Max Planck had recently used for radiation. For instance, a good approximation for the specific heat of salt, NaCl, is \(c = 3.33 \times 10^4 \frac{J}{kg \cdot k}\left(\frac{T}{321 \, K}\right)^3\). The constant 321 K is called the Debye temperature of NaCl, \(\Theta_D\) and the formula works well when \(T < 0.04 \Theta_D\). Using this formula, how much heat is required to raise the temperature of 24.0 g of NaCl from 5 K to 15 K?
Because the heat capacity depends on the temperature, we need to use the equation \[c = \dfrac{1}{m} \dfrac{dQ}{dT}.\]
We solve this equation for Q by integrating both sides: \(Q = m \int_{T_1}^{T_2} cdT\).
Then we substitute the given values in and evaluate the integral:
\[Q = (0.024 \, kg) \int_{T1}^{T2} 333 \times 10^4 \dfrac{J}{kg \cdot K}\left(\dfrac{T}{321 \, K}\right)^3 dT = \left( 6.04 \times 10^{-4} \dfrac{J}{K^4}\right) T^4 |_{5 \, K}^{15 \, K} = 30.2 \, J.\]
Significance If we had used the equation \(Q = mc\Delta T\) and the room-temperature specific heat of salt, \(880 \, J/kg \cdot K\), we would have gotten a very different value.
Browse Course Material
Course info.
- Prof. Kripa K Varanasi
Departments
- Mechanical Engineering
As Taught In
- Thermodynamics
Learning Resource Types
Introduction to heat transfer, course description.

You are leaving MIT OpenCourseWare
12.1 Zeroth Law of Thermodynamics: Thermal Equilibrium
Section learning objectives.
By the end of this section, you will be able to do the following:
- Explain the zeroth law of thermodynamics
Teacher Support
The learning objectives in this section will help your students master the following standards:
- (G) analyze and explain everyday examples that illustrate the laws of thermodynamics, including the law of conservation of energy and the law of entropy.
Section Key Terms
[BL] [OL] Review the concept of heat as the transfer of energy due to a temperature difference.
[OL] Ask students what the direction of heat flow would be if an ice cube were melting in a glass of soda or if a glass of hot water were placed in a room. Give a few more examples. Ask students how long the heat transfer would take place. What causes the heat transfer to occur?
We learned in the previous chapter that when two objects (or systems ) are in contact with one another, heat will transfer thermal energy from the object at higher temperature to the one at lower temperature until they both reach the same temperature. The objects are then in thermal equilibrium , and no further temperature changes will occur if they are isolated from other systems. The systems interact and change because their temperatures are different, and the changes stop once their temperatures are the same. Thermal equilibrium is established when two bodies are in thermal contact with each other—meaning heat transfer (i.e., the transfer of energy by heat) can occur between them. If two systems cannot freely exchange energy, they will not reach thermal equilibrium. (It is fortunate that empty space stands between Earth and the sun, because a state of thermal equilibrium with the sun would be too toasty for life on this planet!)
If two systems, A and B, are in thermal equilibrium with each other, and B is in thermal equilibrium with a third system, C, then A is also in thermal equilibrium with C. This statement may seem obvious, because all three have the same temperature, but it is basic to thermodynamics. It is called the zeroth law of thermodynamics .
[AL] Ask students how we receive the energy of the sun, and yet the sun and Earth never reach thermal equilibrium.
Tips For Success
The zeroth law of thermodynamics is very similar to the transitive property of equality in mathematics: If a = b and b = c, then a = c.
You may be wondering at this point, why the wacky name? Shouldn’t this be called the first law of thermodynamics rather than the zeroth ? The explanation is that this law was discovered after the first and second laws of thermodynamics but is so fundamental that scientists decided it should logically come first.
As an example of the zeroth law in action, consider newborn babies in neonatal intensive-care units in hospitals. Prematurely born or sick newborns are placed in special incubators. These babies have very little covering while in the incubators, so to an observer, they look as though they may not be warm enough. However, inside the incubator, the temperature of the air, the cot, and the baby are all the same—that is, they are in thermal equilibrium. The ambient temperature is just high enough to keep the baby safe and comfortable.
[OL] [AL] Ask students to come up with more everyday examples of heat transfer that demonstrate the zeroth law of thermodynamics.
Work In Physics
Thermodynamics engineer.
Thermodynamics engineers apply the principles of thermodynamics to mechanical systems so as to create or test products that rely on the interactions between heat, work, pressure, temperature, and volume. This type of work typically takes place in the aerospace industry, chemical manufacturing companies, industrial manufacturing plants, power plants ( Figure 12.2 ), engine manufacturers, or electronics companies.
The need for energy creates quite a bit of demand for thermodynamics engineers, because both traditional energy companies and alternative ( green ) energy startups rely on interactions between heat and work and so require the expertise of thermodynamics engineers. Traditional energy companies use mainly nuclear energy and energy from burning fossil fuels, such as coal. Alternative energy is finding new ways to harness renewable and, often, more readily available energy sources, such as solar, water, wind, and bio-energy.
A thermodynamics engineer in the energy industry can find the most efficient way to turn the burning of a biofuel or fossil fuel into energy, store that energy for times when it’s needed most, or figure out how to best deliver that energy from where it’s produced to where it’s used: in homes, factories, and businesses. Additionally, he or she might also design pollution-control equipment to remove harmful pollutants from the smoke produced as a by-product of burning fuel. For example, a thermodynamics engineer may develop a way to remove mercury from burning coal in a coal-fired power plant.
Thermodynamics engineering is an expanding field, where employment opportunities are expected to grow by as much as 27 percent between 2012 and 2022, according to the U.S. Bureau of Labor Statistics. To become a thermodynamics engineer, you must have a college degree in chemical engineering, mechanical engineering, environmental engineering, aerospace engineering, civil engineering, or biological engineering (depending on which type of career you wish to pursue), with coursework in physics and physical chemistry that focuses on thermodynamics.
Grasp Check
What would be an example of something a thermodynamics engineer would do in the aeronautics industry?
- Test the fuel efficiency of a jet engine
- Test the functioning of landing gear
- Test the functioning of a lift control device
- Test the autopilot functions
Check Your Understanding
Use these questions to assess student achievement of the section’s Learning Objectives. If students are struggling with a specific objective, these questions will help identify which and direct students to the relevant content.
- When two objects in contact with each other are at the same pressure, they are said to be in thermal equilibrium.
- When two objects in contact with each other are at different temperatures, they are said to be in thermal equilibrium.
- When two objects in contact with each other are at the same temperature, they are said to be in thermal equilibrium.
- When two objects not in contact with each other are at the same pressure, they are said to be in thermal equilibrium.
What is the zeroth law of thermodynamics?
- Energy can neither be created nor destroyed in a chemical reaction.
- If two systems, A and B, are in thermal equilibrium with each other, and B is in thermal equilibrium with a third system, C, then A is also in thermal equilibrium with C.
- Entropy of any isolated system not in thermal equilibrium always increases.
- Entropy of a system approaches a constant value as temperature approaches absolute zero.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute Texas Education Agency (TEA). The original material is available at: https://www.texasgateway.org/book/tea-physics . Changes were made to the original material, including updates to art, structure, and other content updates.
Access for free at https://openstax.org/books/physics/pages/1-introduction
- Authors: Paul Peter Urone, Roger Hinrichs
- Publisher/website: OpenStax
- Book title: Physics
- Publication date: Mar 26, 2020
- Location: Houston, Texas
- Book URL: https://openstax.org/books/physics/pages/1-introduction
- Section URL: https://openstax.org/books/physics/pages/12-1-zeroth-law-of-thermodynamics-thermal-equilibrium
© Jan 19, 2024 Texas Education Agency (TEA). The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.
Have an account?

Heat Transfer
5th - 6th grade.
20 questions

Introducing new Paper mode
No student devices needed. Know more
- 1. Multiple Choice Edit 1.5 minutes 1 pt What is the definition of CONDUCTION? When heat is transferred from objects like rays of light or electromagnetically. a hot liquid or air that expands, becomes less dense, and rises or becomes more dense, and sinks. When heat transfers from objects that are touching. When heat transfers through the heater or AC in your house.
- 2. Multiple Choice Edit 1.5 minutes 1 pt What is the definition of CONVECTION When heat transfers through the heater or AC in your house. When heat transfers from objects that are touching. a hot liquid or air that expands, becomes less dense, and rises or becomes more dense, and sinks. hot liquid or air that expands, becomes less dense, and rises or becomes more dense, and sinks.
- 3. Multiple Choice Edit 1.5 minutes 1 pt What is the definition of RADIATION hot liquid or air that expands, becomes less dense, and rises or becomes more dense, and sinks. When heat is transferred from objects like rays of light or electromagnetically. When heat transfers from objects that are touching. When heat transfers through the heater or AC in your house.
- 4. Multiple Choice Edit 30 seconds 1 pt What kind of heat transfer happens when the sun is heating your body? Conduction Radiation Convection Density
- 5. Multiple Choice Edit 2 minutes 1 pt What heat transfer happens when you burn your hand by touching a fire? Radiation Conduction Convectio I don't know!!!!!!!!!!!
- 6. Multiple Choice Edit 2 minutes 1 pt When a pot is touching a stove that is on, what heat transfer is happening? I don't know?????? Radiation Conduction Convection
Explore all questions with a free account

Continue with email
Continue with phone
A Heat Transfer Textbook, 6th edition
Solutions manual
Solutions to more than 520 problems are on the following links.
Solutions for Chapter 1 (v1.01, 16 MB, February 2023)
Solutions for Chapter 2 (v1.0, 13 MB, August 2020)
Solutions for Chapter 3 (v1.0, 15 MB, August 2020)
Solutions for Chapters 4-10 (v1.07, 19 MB, 3 April 2024) Solutions for all problems in Chapters 4, 5, 6, 10, and most in Chapters 7, 8, 9.
Solutions for Chapter 11 (v1.07, 4 MB, 3 April 2024)
If additional solutions become available, they will be posted here.
The solutions that are handwritten were prepared decades ago, and some use property data that don’t precisely match today’s Appendix A. In most instances, the differences are small.
Between them, the authors have spent more than 100 years using various textbook solutions manuals. In our experience, none are without errors. If you happen to find one in our solutions manual, do let us know.
Lesson Plans
Energy transfer in earth's atmosphere.
Students will examine how radiation, conduction, and convection work together as a part of Earth’s Energy Budget to heat the atmosphere. They will further explore Earth’s Energy Budget through a set of animations and create their own energy budget that includes their school and surrounding area. As an extension, students will analyze a diagram of Earth’s Energy Budget designed by NASA and answer critical thinking questions to check for student understanding of the content.
Materials Required
- Internet Access
- Animations Earth's Response to Total Solar Irradiance and Earth’s Absorption of Different Wavelengths of Light
- Video Real World: Monitoring Earth's Energy Budget with CERES
- Google Slides Energy Transfer in Earth's Atmosphere
- Energy Transfer in Earth’s Atmosphere Cloze Notes
- Earth's Response to Total Solar Irradiance
- Absorption of Different Wavelengths of Light
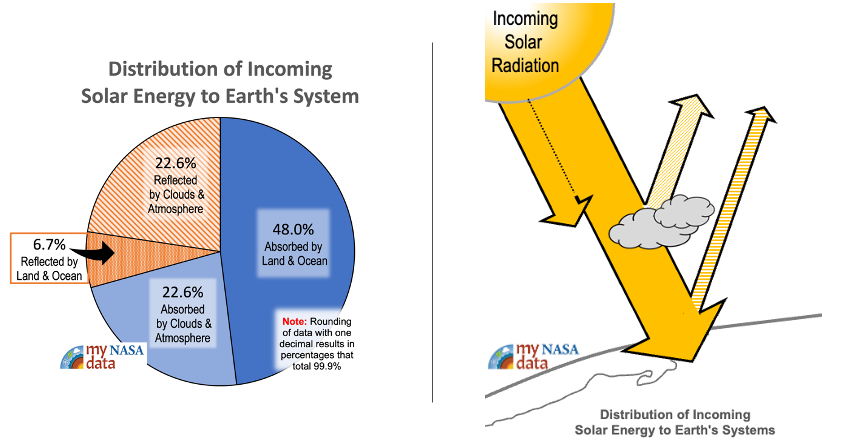
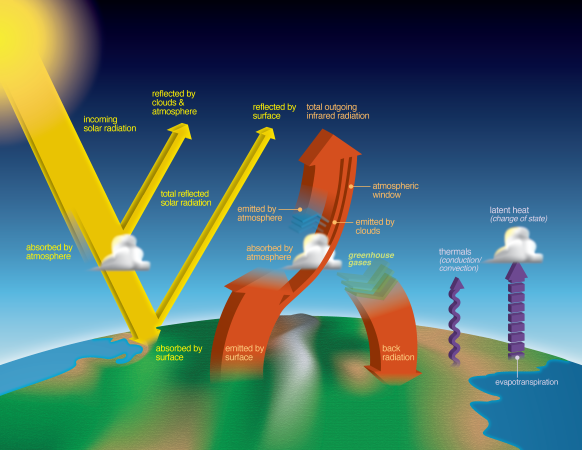
- Explain to students that these animations show how Earth’s solar radiation is reflected or absorbed in Earth’s atmosphere. Clarify that the Sun is Earth’s heat source and energy from the sun travels to Earth through electromagnetic waves to Earth’s surface. That energy gives off heat that is then transferred into other forms of heat energy, namely radiation, conduction, and convection.
- NASA uses CERES, a data collecting sensor on satellites, to measure what variable?
- How much of the Sun’s solar radiation is reflected back to space or absorbed by Earth’s atmosphere and clouds?
- What absorbs the remaining radiation?
- True or false: Darker surfaces tend to absorb more energy, whereas lighter surfaces tend to reflect more energy.
- Following the video, pass out the student handout Energy Transfer in Earth’s Atmosphere Cloze Notes .
- The teacher will go through the Google slides Energy Transfer in Earth's Atmosphere and elaborate on the content of each slide.
- Students will fill in the cloze notes as the teacher goes through each slide.
- Explain in your own words what is meant by the term “heat transfer”.
- Describe how radiation, conduction, and convection work together to heat Earth’s atmosphere.
- What is meant by a “balanced” Earth’s Energy Budget?
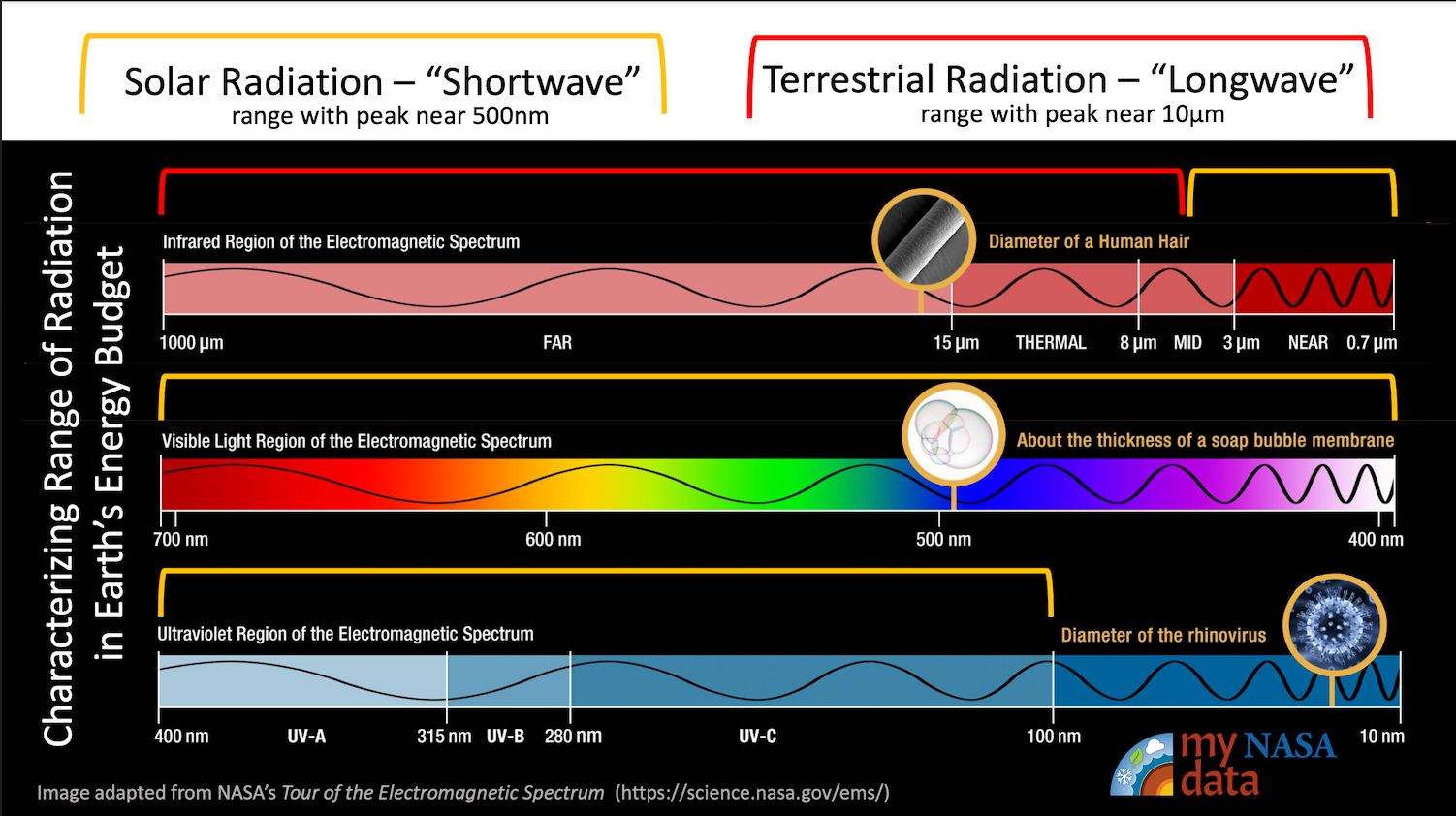
- Differentiate between shortwave and longwave radiation.
- Make sure to draw the Sun somewhere at the top of your paper.
- Draw any clouds that you see in the sky.
- Think about the geography of the land surrounding your school and sketch it. Is it hilly and mountainous or is the land flat?
- Draw your school on your paper. Make sure to label the parking lot and draw any vehicles found there.
- Next, think about any other surfaces found outside or near your school. Are there grassy areas, trees, or plant life? Is your school next to a body of water?
- After you have labeled the surfaces, draw any buildings, homes, or businesses that may be nearby.
- Draw the roads or highways that lead up to your school.
- Dark colors absorb a lot more heat than lighter ones because they absorb more light energy.
- Lighter regions are reflective and absorb less of the Sun’s energy.
- Using a yellow colored pencil, draw solid arrows from the sun to each structure or surface that will absorb the sun’s energy.
- Lastly, with the same colored pencil, draw dashed yellow arrows from any structure or surface on your diagram outward towards space that will reflect the sun’s energy.
Answer Key:
Teachers who are interested in receiving the answer key, please complete the Teacher Key Request and Verification Form . We verify that requestors are teachers prior to sending access to the answer keys as we’ve had many students try to pass as teachers to gain access.

Supported NGSS Performance Expectations
- MS-ESS2-1: Develop a model to describe the cycling of Earth's materials and the flow of energy that drives this process.
- MS-PS4-2: Develop and use a model to describe that waves are reflected, absorbed, or transmitted through various materials.
- As a homework assignment or extension activity, have students analyze NASA’s Earth’s Energy Budget diagram.
- Identify the objects in Earth’s atmosphere that are absorbing and reflecting the Sun’s energy.
- Latent heat in water vapor carries a percentage of what back into the atmosphere?
- Atmosphere:
- By looking at Earth’s Energy Budget and reviewing today’s class notes, explain how the energy budget can become imbalanced.
- Predict what will happen to Earth’s Energy Budget if the amount of greenhouse gas emissions continue to rise in the Earth’s atmosphere.
Technology Requirements
- Internet Required
- One-to-a-Group
Complementary Lesson Plans
Ins and outs of shortwave radiation.
Modeling Incoming Solar Radiation
Albedo Card Sort

Complementary Mini Lessons
Earth's heating imbalances.

Describing Radiation in Earth’s Energy Budget
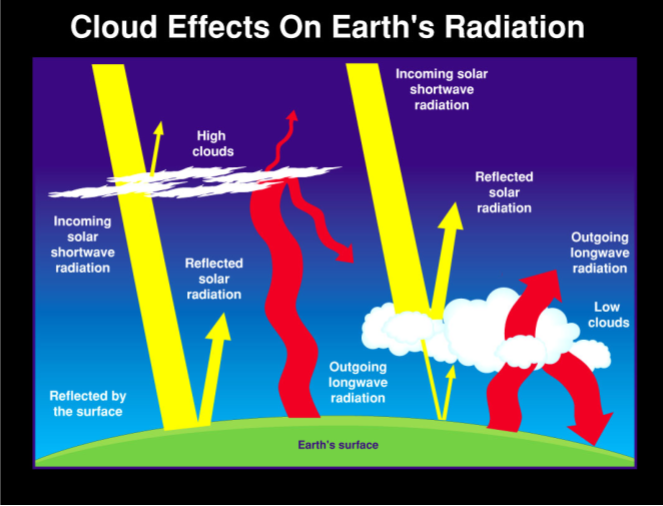
Examining a Simplified Model of Cloud Effects on Earth’s Energy Budget
Complementary Models
Earth's energy budget story map.
Student Resources
- Energy Transfer in Earth's Atmosphere Cloze Notes
Interactive Files
- > 90 minutes
My NASA Data Visualization Tool
- Earth System Data Explorer
Download this page
Not finding what you are looking for.

- Heat Introduction Classification
- Heat Transfer Conduction Convection And Radiation
Heat Transfer - Radiation, Convection And Conduction
Any matter which is made up of atoms and molecules has the ability to transfer heat. The atoms are in different types of motion at any time. The motion of molecules and atoms is responsible for heat or thermal energy and every matter has this thermal energy. The more the motion of molecules, more will be the heat energy. However, talking about heat transfer, it is nothing but the process of transfer of heat from a high-temperature body to a low temperature one.
What is Heat Transfer?
According to thermodynamic systems, heat transfer is defined as
“The movement of heat across the border of the system due to a difference in temperature between the system and its surroundings.”
Interestingly, the difference in temperature is said to be a ‘potential’ that causes the transfer of heat from one point to another.

How is Heat Transferred?
Heat can travel from one place to another in several ways . The different modes of heat transfer include :
Meanwhile, if the temperature difference exists between the two systems, heat will find a way to transfer from the higher to the lower system.

What is Conduction?
Conduction is defined as
The process of transmission of energy from one particle of the medium to another with the particles being in direct contact with each other.
An area of higher kinetic energy transfers thermal energy towards the lower kinetic energy area. High-speed particles clash with particles moving at a slow speed, as a result, slow speed particles increase their kinetic energy . This is a typical form of heat transfer and takes place through physical contact. Conduction is also known as thermal conduction or heat conduction.
Conduction Equation
The rate of conduction can be calculated by the following equation:
- Q is the transfer of heat per unit time
- K is the thermal conductivity of the body
- A is the area of heat transfer
- T hot is the temperature of the hot region
- T cold is the temperature of the cold region
- d is the thickness of the body
The coefficient of thermal conductivity shows that a metal body conducts heat better when it comes to conduction.
Conduction Examples
Following are the examples of conduction:
- Ironing of clothes is an example of conduction where the heat is conducted from the iron to the clothes.
- Heat is transferred from hands to ice cube resulting in the melting of an ice cube when held in hands.
- Heat conduction through the sand at the beaches. This can be experienced during summers. Sand is a good conductor of heat.
What is Convection?
Convection is defined as
The movement of fluid molecules from higher temperature regions to lower temperature regions.
Convection Equation
As the temperature of the liquid increases, the liquid’s volume also has to increase by the same factor and this effect is known as displacement. The equation to calculate the rate of convection is as follows:
- Q is the heat transferred per unit time
- h c is the coefficient of convective heat transfer
- T s is the surface temperature
- T f is the fluid temperature
Convection Examples
Examples of convection include:
- Boiling of water, that is molecules that are denser move at the bottom while the molecules which are less dense move upwards resulting in the circular motion of the molecules so that water gets heated.
- Warm water around the equator moves towards the poles while cooler water at the poles moves towards the equator.
- Blood circulation in warm-blooded animals takes place with the help of convection, thereby regulating the body temperature.
Learn more about Convection

What is Radiation?
Radiant heat is present in some or other form in our daily lives. Thermal radiations are referred to as radiant heat. Thermal radiation is generated by the emission of electromagnetic waves . These waves carry away the energy from the emitting body. Radiation takes place through a vacuum or transparent medium which can be either solid or liquid. Thermal radiation is the result of the random motion of molecules in matter. The movement of charged electrons and protons is responsible for the emission of electromagnetic radiation. Let us know more about radiation heat transfer.
Radiation heat transfer is measured by a device known as thermocouple. A thermocouple is used for measuring the temperature. In this device sometimes, error takes place while measuring the temperature through radiation heat transfer.
Radiation Equation
As temperature rises, the wavelength in the spectra of the radiation emitted decreases and shorter wavelengths radiations are emitted. Thermal radiation can be calculated by Stefan-Boltzmann law:
- P is the net power of radiation
- A is the area of radiation
- Tr is the radiator temperature
- Tc is the surrounding temperature
- e is emissivity and σ is Stefan’s constant (σ = 5.67 × 10 -8 Wm -2 K -4
Radiation Example
Following are the examples of radiation:
- Microwave radiation emitted in the oven is an example of radiation.
- UV rays coming from the sun is an example of radiation.
- The release of alpha particles during the decaying of Uranium-238 into Thorium-234 is an example of radiation.
Unit of Heat Transfer
To know more about heat transfer in detail, click on the video below.

Frequently Asked Questions – FAQs
What are the different modes of heat transfer.
The different modes of heat transfer are:
Give an example of radiation.
What is the si unit of heat.
SI unit of heat is Joules.
How is electromagnetic radiation emitted?
What is the movement of molecules in fluids from higher temperature regions to lower temperature regions known as.
It is known as convection.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Physics related queries and study materials
Your result is as below
Request OTP on Voice Call
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
This was so good, thank you very much!
Very good writing sir
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.

IMAGES
VIDEO
COMMENTS
Terms in this set (19) Heat. Flow of energy from one thing to another. Thermal Energy. Energy that speeds up molecules. Conduction. Energy moves from one object to another by touch. Conductor. Materials that transfer energy easily.
The movement of energy from a warmer object to a cooler object. Conduction. Heat transfer by direct contact particles of matter. Convection. The transfer of heat by the movement of a heated fluid in currents. Density. Measures how much mass there is in a volume of a substance.
Study with Quizlet and memorize flashcards containing terms like Explain how the temperature of a substance is related to the kinetic energy of its molecules., Describe the motion of molecules in an ice cube and in a radiator in winter. Describe the motion of molecules in these two substances when the ice cube is placed on the radiator., Analyze the reaction to determine whether the reaction ...
A. A whole metal spoon getting hot when one end is in hot soup. B. The inside of a car in the sun getting very hot. C. An asphalt road getting hotter in the sun than a concrete sidewalk. D. Fireplace fire heating a room on a cold day. 6.9A Learn with flashcards, games, and more — for free.
heat energy moves through a fluid (gas, liquid, solid if it shows plasticity) radiation. heat is transferred through electromagnetic rays matter is not needed at all to transfer energy; thermal energy travels through space. thermal energy. heat energy; when temperatures increase, molecules vibrate, expand, and rise. direct contact.
Study with Quizlet and memorize flashcards containing terms like 1. Throughout the reflection, make sure you have a copy of the 'Student Guide' and your data tables. Use the drop-down menus to complete the statements. - The independent variables in Part I, the ones intentionally manipulated, are the ________. - The independent variable in Part II, the one that is intentionally manipulated is ...
flat on her back. Vicente is going to spend the afternoon in his yard. He has the choice to get in the pool, lay on a cotton chaise longue, sit on the porcelain tile bordering the pool, or sit on a plastic chair. The specific heats of these items are: Water = 4.19 J/g•°C. Cotton = 1.30 J/g•°C. Porcelain = 1.07 J/g•°C.
This is called heat transfer. (Remember, we learned that energy transfer is when energy moves from one thing or place to another, but the energy type stays the same). Heat can transfer (or move) in 3 ways: conduction, convection, and radiation. As you read about the three types of heat transfer, pay attention to:
Convection is different from radiation and conduction, as radiation is the transfer of heat through electromagnetic waves, and conduction is the transfer of heat through direct contact between objects. Rate this question: 17 3. 2. A thermometer works because the liquid in its contracts when heated. This is an example of:
What is the main type of heat transfer that is making you feel cold? a. conduction b. convection c. solar d. induction 4. A material that slows down heat transfer is known as a/an: a. conductor b. thermometer c. insulator d. metal 5. When you heat up a material (such as water), a. Its molecules move more quickly b. Its molecules slow down c.
Experiments show that the heat transferred to or from a substance depends on three factors—the change in the substance's temperature, the mass of the substance, and certain physical properties related to the phase of the substance. The equation for heat transfer Q is. Q = m c Δ T, 11.7. where m is the mass of the substance and Δ T is the ...
Because the density of water is 1000kg / m3, 1 L of water has a mass of 1 kg, and the mass of 0.250 L of water is mw = 0.250kg. Calculate the heat transferred to the water. Use the specific heat of water in Table 1.5.1: Qw = mwcwΔT = (0.250kg)(4186J / kgoC)(60.0oC) = 62.8kJ. Calculate the heat transferred to the aluminum.
Level up on all the skills in this unit and collect up to 500 Mastery points! This unit examines the role of energy in physical and chemical processes. Learn about heat transfer, calorimetry, enthalpy of reaction, Hess's law, and more. Practice what you've learned and study for the AP Chemistry exam with more than 55 AP-aligned questions.
Course Description. This course is an introduction to the principal concepts and methods of heat transfer. The objectives of this integrated subject are to develop the fundamental principles and laws of heat transfer and to explore the implications of these principles for system behavior; to formulate the models necessary to study, …. Show more.
Teacher Support [BL] [OL] Review the concept of heat as the transfer of energy due to a temperature difference. [OL] Ask students what the direction of heat flow would be if an ice cube were melting in a glass of soda or if a glass of hot water were placed in a room. Give a few more examples. Ask students how long the heat transfer would take place. What causes the heat transfer to occur?
b) Increase turbulence in flow for enhancing heat transfer. c) Surface area is maximum to promote the rate of heat transfer. d) Increase temperature gradient so as to enhance heat transfer. View Answer. 17. A heating unit is made in the form of a vertical tube of 50 mm outside diameter and 1.2 m height.
Answer: d) Forced convection heat transfer. Explanation: It is the ratio of the heat transfer coefficient to the heat flow per unit temperature increase due to fluid velocity. It can only be used to transfer heat by forced convection. Q13: Define a Black Surface. Answer: Three characteristics describe a black surface:
1 pt. What is the definition of CONVECTION. When heat transfers through the heater or AC in your house. When heat transfers from objects that are touching. a hot liquid or air that expands, becomes less dense, and rises or becomes more dense, and sinks. hot liquid or air that expands, becomes less dense, and rises or becomes more dense, and ...
Heat Convection Convection is heat transfer by mass motion of a fluid such as air or water when the heated fluid is caused to move away from the source of heat, carrying energy with it. Convection above a hot surface occurs because hot air expands, becomes less dense, and rises (see Ideal Gas Law).Hot water is likewise less dense than cold water and rises, causing convection currents which ...
2. Multiple Choice. 30 seconds. 1 pt. What is the definition of CONDUCTION? When heat is transferred through waves of heat across a distance. When heat is transferred through circulation of liquid or gases. When heat transfers from objects that are touching. 3.
Solutions for Chapter 3 (v1.0, 15 MB, August 2020) Solutions for Chapters 4-10 (v1.07, 19 MB, 3 April 2024) Solutions for all problems in Chapters 4, 5, 6, 10, and most in Chapters 7, 8, 9. Solutions for Chapter 11 (v1.07, 4 MB, 3 April 2024) If additional solutions become available, they will be posted here. The solutions that are handwritten ...
Clarify that the Sun is Earth's heat source and energy from the sun travels to Earth through electromagnetic waves to Earth's surface. That energy gives off heat that is then transferred into other forms of heat energy, namely radiation, conduction, and convection. Next, show students the video Real World: Monitoring Earth's Energy Budget ...
According to thermodynamic systems, heat transfer is defined as. "The movement of heat across the border of the system due to a difference in temperature between the system and its surroundings.". Interestingly, the difference in temperature is said to be a 'potential' that causes the transfer of heat from one point to another. 2,48,152.