

Systematic Review
- Library Help
- What is a Systematic Review (SR)?
Steps of a Systematic Review
- Framing a Research Question
- Developing a Search Strategy
- Searching the Literature
- Managing the Process
- Meta-analysis
- Publishing your Systematic Review
Forms and templates

Image: David Parmenter's Shop
- PICO Template
- Inclusion/Exclusion Criteria
- Database Search Log
- Review Matrix
- Cochrane Tool for Assessing Risk of Bias in Included Studies
• PRISMA Flow Diagram - Record the numbers of retrieved references and included/excluded studies. You can use the Create Flow Diagram tool to automate the process.
• PRISMA Checklist - Checklist of items to include when reporting a systematic review or meta-analysis
PRISMA 2020 and PRISMA-S: Common Questions on Tracking Records and the Flow Diagram
- PROSPERO Template
- Manuscript Template
- Steps of SR (text)
- Steps of SR (visual)
- Steps of SR (PIECES)
Adapted from A Guide to Conducting Systematic Reviews: Steps in a Systematic Review by Cornell University Library
Source: Cochrane Consumers and Communications (infographics are free to use and licensed under Creative Commons )
Check the following visual resources titled " What Are Systematic Reviews?"
- Video with closed captions available
- Animated Storyboard
- << Previous: What is a Systematic Review (SR)?
- Next: Framing a Research Question >>
- Last Updated: May 8, 2024 1:44 PM
- URL: https://lib.guides.umd.edu/SR
Reference management. Clean and simple.
How to write a systematic literature review [9 steps]

What is a systematic literature review?
Where are systematic literature reviews used, what types of systematic literature reviews are there, how to write a systematic literature review, 1. decide on your team, 2. formulate your question, 3. plan your research protocol, 4. search for the literature, 5. screen the literature, 6. assess the quality of the studies, 7. extract the data, 8. analyze the results, 9. interpret and present the results, registering your systematic literature review, frequently asked questions about writing a systematic literature review, related articles.
A systematic literature review is a summary, analysis, and evaluation of all the existing research on a well-formulated and specific question.
Put simply, a systematic review is a study of studies that is popular in medical and healthcare research. In this guide, we will cover:
- the definition of a systematic literature review
- the purpose of a systematic literature review
- the different types of systematic reviews
- how to write a systematic literature review
➡️ Visit our guide to the best research databases for medicine and health to find resources for your systematic review.
Systematic literature reviews can be utilized in various contexts, but they’re often relied on in clinical or healthcare settings.
Medical professionals read systematic literature reviews to stay up-to-date in their field, and granting agencies sometimes need them to make sure there’s justification for further research in an area. They can even be used as the starting point for developing clinical practice guidelines.
A classic systematic literature review can take different approaches:
- Effectiveness reviews assess the extent to which a medical intervention or therapy achieves its intended effect. They’re the most common type of systematic literature review.
- Diagnostic test accuracy reviews produce a summary of diagnostic test performance so that their accuracy can be determined before use by healthcare professionals.
- Experiential (qualitative) reviews analyze human experiences in a cultural or social context. They can be used to assess the effectiveness of an intervention from a person-centric perspective.
- Costs/economics evaluation reviews look at the cost implications of an intervention or procedure, to assess the resources needed to implement it.
- Etiology/risk reviews usually try to determine to what degree a relationship exists between an exposure and a health outcome. This can be used to better inform healthcare planning and resource allocation.
- Psychometric reviews assess the quality of health measurement tools so that the best instrument can be selected for use.
- Prevalence/incidence reviews measure both the proportion of a population who have a disease, and how often the disease occurs.
- Prognostic reviews examine the course of a disease and its potential outcomes.
- Expert opinion/policy reviews are based around expert narrative or policy. They’re often used to complement, or in the absence of, quantitative data.
- Methodology systematic reviews can be carried out to analyze any methodological issues in the design, conduct, or review of research studies.
Writing a systematic literature review can feel like an overwhelming undertaking. After all, they can often take 6 to 18 months to complete. Below we’ve prepared a step-by-step guide on how to write a systematic literature review.
- Decide on your team.
- Formulate your question.
- Plan your research protocol.
- Search for the literature.
- Screen the literature.
- Assess the quality of the studies.
- Extract the data.
- Analyze the results.
- Interpret and present the results.
When carrying out a systematic literature review, you should employ multiple reviewers in order to minimize bias and strengthen analysis. A minimum of two is a good rule of thumb, with a third to serve as a tiebreaker if needed.
You may also need to team up with a librarian to help with the search, literature screeners, a statistician to analyze the data, and the relevant subject experts.
Define your answerable question. Then ask yourself, “has someone written a systematic literature review on my question already?” If so, yours may not be needed. A librarian can help you answer this.
You should formulate a “well-built clinical question.” This is the process of generating a good search question. To do this, run through PICO:
- Patient or Population or Problem/Disease : who or what is the question about? Are there factors about them (e.g. age, race) that could be relevant to the question you’re trying to answer?
- Intervention : which main intervention or treatment are you considering for assessment?
- Comparison(s) or Control : is there an alternative intervention or treatment you’re considering? Your systematic literature review doesn’t have to contain a comparison, but you’ll want to stipulate at this stage, either way.
- Outcome(s) : what are you trying to measure or achieve? What’s the wider goal for the work you’ll be doing?
Now you need a detailed strategy for how you’re going to search for and evaluate the studies relating to your question.
The protocol for your systematic literature review should include:
- the objectives of your project
- the specific methods and processes that you’ll use
- the eligibility criteria of the individual studies
- how you plan to extract data from individual studies
- which analyses you’re going to carry out
For a full guide on how to systematically develop your protocol, take a look at the PRISMA checklist . PRISMA has been designed primarily to improve the reporting of systematic literature reviews and meta-analyses.
When writing a systematic literature review, your goal is to find all of the relevant studies relating to your question, so you need to search thoroughly .
This is where your librarian will come in handy again. They should be able to help you formulate a detailed search strategy, and point you to all of the best databases for your topic.
➡️ Read more on on how to efficiently search research databases .
The places to consider in your search are electronic scientific databases (the most popular are PubMed , MEDLINE , and Embase ), controlled clinical trial registers, non-English literature, raw data from published trials, references listed in primary sources, and unpublished sources known to experts in the field.
➡️ Take a look at our list of the top academic research databases .
Tip: Don’t miss out on “gray literature.” You’ll improve the reliability of your findings by including it.
Don’t miss out on “gray literature” sources: those sources outside of the usual academic publishing environment. They include:
- non-peer-reviewed journals
- pharmaceutical industry files
- conference proceedings
- pharmaceutical company websites
- internal reports
Gray literature sources are more likely to contain negative conclusions, so you’ll improve the reliability of your findings by including it. You should document details such as:
- The databases you search and which years they cover
- The dates you first run the searches, and when they’re updated
- Which strategies you use, including search terms
- The numbers of results obtained
➡️ Read more about gray literature .
This should be performed by your two reviewers, using the criteria documented in your research protocol. The screening is done in two phases:
- Pre-screening of all titles and abstracts, and selecting those appropriate
- Screening of the full-text articles of the selected studies
Make sure reviewers keep a log of which studies they exclude, with reasons why.
➡️ Visit our guide on what is an abstract?
Your reviewers should evaluate the methodological quality of your chosen full-text articles. Make an assessment checklist that closely aligns with your research protocol, including a consistent scoring system, calculations of the quality of each study, and sensitivity analysis.
The kinds of questions you'll come up with are:
- Were the participants really randomly allocated to their groups?
- Were the groups similar in terms of prognostic factors?
- Could the conclusions of the study have been influenced by bias?
Every step of the data extraction must be documented for transparency and replicability. Create a data extraction form and set your reviewers to work extracting data from the qualified studies.
Here’s a free detailed template for recording data extraction, from Dalhousie University. It should be adapted to your specific question.
Establish a standard measure of outcome which can be applied to each study on the basis of its effect size.
Measures of outcome for studies with:
- Binary outcomes (e.g. cured/not cured) are odds ratio and risk ratio
- Continuous outcomes (e.g. blood pressure) are means, difference in means, and standardized difference in means
- Survival or time-to-event data are hazard ratios
Design a table and populate it with your data results. Draw this out into a forest plot , which provides a simple visual representation of variation between the studies.
Then analyze the data for issues. These can include heterogeneity, which is when studies’ lines within the forest plot don’t overlap with any other studies. Again, record any excluded studies here for reference.
Consider different factors when interpreting your results. These include limitations, strength of evidence, biases, applicability, economic effects, and implications for future practice or research.
Apply appropriate grading of your evidence and consider the strength of your recommendations.
It’s best to formulate a detailed plan for how you’ll present your systematic review results. Take a look at these guidelines for interpreting results from the Cochrane Institute.
Before writing your systematic literature review, you can register it with OSF for additional guidance along the way. You could also register your completed work with PROSPERO .
Systematic literature reviews are often found in clinical or healthcare settings. Medical professionals read systematic literature reviews to stay up-to-date in their field and granting agencies sometimes need them to make sure there’s justification for further research in an area.
The first stage in carrying out a systematic literature review is to put together your team. You should employ multiple reviewers in order to minimize bias and strengthen analysis. A minimum of two is a good rule of thumb, with a third to serve as a tiebreaker if needed.
Your systematic review should include the following details:
A literature review simply provides a summary of the literature available on a topic. A systematic review, on the other hand, is more than just a summary. It also includes an analysis and evaluation of existing research. Put simply, it's a study of studies.
The final stage of conducting a systematic literature review is interpreting and presenting the results. It’s best to formulate a detailed plan for how you’ll present your systematic review results, guidelines can be found for example from the Cochrane institute .

Jump to navigation

Cochrane Training
Chapter 1: starting a review.
Toby J Lasserson, James Thomas, Julian PT Higgins
Key Points:
- Systematic reviews address a need for health decision makers to be able to access high quality, relevant, accessible and up-to-date information.
- Systematic reviews aim to minimize bias through the use of pre-specified research questions and methods that are documented in protocols, and by basing their findings on reliable research.
- Systematic reviews should be conducted by a team that includes domain expertise and methodological expertise, who are free of potential conflicts of interest.
- People who might make – or be affected by – decisions around the use of interventions should be involved in important decisions about the review.
- Good data management, project management and quality assurance mechanisms are essential for the completion of a successful systematic review.
Cite this chapter as: Lasserson TJ, Thomas J, Higgins JPT. Chapter 1: Starting a review. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook .
1.1 Why do a systematic review?
Systematic reviews were developed out of a need to ensure that decisions affecting people’s lives can be informed by an up-to-date and complete understanding of the relevant research evidence. With the volume of research literature growing at an ever-increasing rate, it is impossible for individual decision makers to assess this vast quantity of primary research to enable them to make the most appropriate healthcare decisions that do more good than harm. By systematically assessing this primary research, systematic reviews aim to provide an up-to-date summary of the state of research knowledge on an intervention, diagnostic test, prognostic factor or other health or healthcare topic. Systematic reviews address the main problem with ad hoc searching and selection of research, namely that of bias. Just as primary research studies use methods to avoid bias, so should summaries and syntheses of that research.
A systematic review attempts to collate all the empirical evidence that fits pre-specified eligibility criteria in order to answer a specific research question. It uses explicit, systematic methods that are selected with a view to minimizing bias, thus providing more reliable findings from which conclusions can be drawn and decisions made (Antman et al 1992, Oxman and Guyatt 1993). Systematic review methodology, pioneered and developed by Cochrane, sets out a highly structured, transparent and reproducible methodology (Chandler and Hopewell 2013). This involves: the a priori specification of a research question; clarity on the scope of the review and which studies are eligible for inclusion; making every effort to find all relevant research and to ensure that issues of bias in included studies are accounted for; and analysing the included studies in order to draw conclusions based on all the identified research in an impartial and objective way.
This Handbook is about systematic reviews on the effects of interventions, and specifically about methods used by Cochrane to undertake them. Cochrane Reviews use primary research to generate new knowledge about the effects of an intervention (or interventions) used in clinical, public health or policy settings. They aim to provide users with a balanced summary of the potential benefits and harms of interventions and give an indication of how certain they can be of the findings. They can also compare the effectiveness of different interventions with one another and so help users to choose the most appropriate intervention in particular situations. The primary purpose of Cochrane Reviews is therefore to inform people making decisions about health or health care.
Systematic reviews are important for other reasons. New research should be designed or commissioned only if it does not unnecessarily duplicate existing research (Chalmers et al 2014). Therefore, a systematic review should typically be undertaken before embarking on new primary research. Such a review will identify current and ongoing studies, as well as indicate where specific gaps in knowledge exist, or evidence is lacking; for example, where existing studies have not used outcomes that are important to users of research (Macleod et al 2014). A systematic review may also reveal limitations in the conduct of previous studies that might be addressed in the new study or studies.
Systematic reviews are important, often rewarding and, at times, exciting research projects. They offer the opportunity for authors to make authoritative statements about the extent of human knowledge in important areas and to identify priorities for further research. They sometimes cover issues high on the political agenda and receive attention from the media. Conducting research with these impacts is not without its challenges, however, and completing a high-quality systematic review is often demanding and time-consuming. In this chapter we introduce some of the key considerations for potential review authors who are about to start a systematic review.
1.2 What is the review question?
Getting the research question right is critical for the success of a systematic review. Review authors should ensure that the review addresses an important question to those who are expected to use and act upon its conclusions.
We discuss the formulation of questions in detail in Chapter 2 . For a question about the effects of an intervention, the PICO approach is usually used, which is an acronym for Population, Intervention, Comparison(s) and Outcome. Reviews may have additional questions, for example about how interventions were implemented, economic issues, equity issues or patient experience.
To ensure that the review addresses a relevant question in a way that benefits users, it is important to ensure wide input. In most cases, question formulation should therefore be informed by people with various relevant – but potentially different – perspectives (see Chapter 2, Section 2.4 ).
1.3 Who should do a systematic review?
Systematic reviews should be undertaken by a team. Indeed, Cochrane will not publish a review that is proposed to be undertaken by a single person. Working as a team not only spreads the effort, but ensures that tasks such as the selection of studies for eligibility, data extraction and rating the certainty of the evidence will be performed by at least two people independently, minimizing the likelihood of errors. First-time review authors are encouraged to work with others who are experienced in the process of systematic reviews and to attend relevant training.
Review teams must include expertise in the topic area under review. Topic expertise should not be overly narrow, to ensure that all relevant perspectives are considered. Perspectives from different disciplines can help to avoid assumptions or terminology stemming from an over-reliance on a single discipline. Review teams should also include expertise in systematic review methodology, including statistical expertise.
Arguments have been made that methodological expertise is sufficient to perform a review, and that content expertise should be avoided because of the risk of preconceptions about the effects of interventions (Gøtzsche and Ioannidis 2012). However, it is important that both topic and methodological expertise is present to ensure a good mix of skills, knowledge and objectivity, because topic expertise provides important insight into the implementation of the intervention(s), the nature of the condition being treated or prevented, the relationships between outcomes measured, and other factors that may have an impact on decision making.
A Cochrane Review should represent an independent assessment of the evidence and avoiding financial and non-financial conflicts of interest often requires careful management. It will be important to consider if there are any relevant interests that may constitute a conflict of interest. There are situations where employment, holding of patents and other financial support should prevent people joining an author team. Funding of Cochrane Reviews by commercial organizations with an interest in the outcome of the review is not permitted. To ensure that any issues are identified early in the process, authors planning Cochrane Reviews should consult the Conflict of Interest Policy . Authors should make complete declarations of interest before registration of the review, and refresh these annually thereafter until publication and just prior to publication of the protocol and the review. For authors of review updates, this must be done at the time of the decision to update the review, annually thereafter until publication, and just prior to publication. Authors should also update declarations of interest at any point when their circumstances change.
1.3.1 Involving consumers and other stakeholders
Because the priorities of decision makers and consumers may be different from those of researchers, it is important that review authors consider carefully what questions are important to these different stakeholders. Systematic reviews are more likely to be relevant to a broad range of end users if they are informed by the involvement of people with a range of experiences, in terms of both the topic and the methodology (Thomas et al 2004, Rees and Oliver 2017). Engaging consumers and other stakeholders, such as policy makers, research funders and healthcare professionals, increases relevance, promotes mutual learning, improved uptake and decreases research waste.
Mapping out all potential stakeholders specific to the review question is a helpful first step to considering who might be invited to be involved in a review. Stakeholders typically include: patients and consumers; consumer advocates; policy makers and other public officials; guideline developers; professional organizations; researchers; funders of health services and research; healthcare practitioners, and, on occasion, journalists and other media professionals. Balancing seniority, credibility within the given field, and diversity should be considered. Review authors should also take account of the needs of resource-poor countries and regions in the review process (see Chapter 16 ) and invite appropriate input on the scope of the review and the questions it will address.
It is established good practice to ensure that consumers are involved and engaged in health research, including systematic reviews. Cochrane uses the term ‘consumers’ to refer to a wide range of people, including patients or people with personal experience of a healthcare condition, carers and family members, representatives of patients and carers, service users and members of the public. In 2017, a Statement of Principles for consumer involvement in Cochrane was agreed. This seeks to change the culture of research practice to one where both consumers and other stakeholders are joint partners in research from planning, conduct, and reporting to dissemination. Systematic reviews that have had consumer involvement should be more directly applicable to decision makers than those that have not (see online Chapter II ).
1.3.2 Working with consumers and other stakeholders
Methods for working with consumers and other stakeholders include surveys, workshops, focus groups and involvement in advisory groups. Decisions about what methods to use will typically be based on resource availability, but review teams should be aware of the merits and limitations of such methods. Authors will need to decide who to involve and how to provide adequate support for their involvement. This can include financial reimbursement, the provision of training, and stating clearly expectations of involvement, possibly in the form of terms of reference.
While a small number of consumers or other stakeholders may be part of the review team and become co-authors of the subsequent review, it is sometimes important to bring in a wider range of perspectives and to recognize that not everyone has the capacity or interest in becoming an author. Advisory groups offer a convenient approach to involving consumers and other relevant stakeholders, especially for topics in which opinions differ. Important points to ensure successful involvement include the following.
- The review team should co-ordinate the input of the advisory group to inform key review decisions.
- The advisory group’s input should continue throughout the systematic review process to ensure relevance of the review to end users is maintained.
- Advisory group membership should reflect the breadth of the review question, and consideration should be given to involving vulnerable and marginalized people (Steel 2004) to ensure that conclusions on the value of the interventions are well-informed and applicable to all groups in society (see Chapter 16 ).
Templates such as terms of reference, job descriptions, or person specifications for an advisory group help to ensure clarity about the task(s) required and are available from INVOLVE . The website also gives further information on setting and organizing advisory groups. See also the Cochrane training website for further resources to support consumer involvement.
1.4 The importance of reliability
Systematic reviews aim to be an accurate representation of the current state of knowledge about a given issue. As understanding improves, the review can be updated. Nevertheless, it is important that the review itself is accurate at the time of publication. There are two main reasons for this imperative for accuracy. First, health decisions that affect people’s lives are increasingly taken based on systematic review findings. Current knowledge may be imperfect, but decisions will be better informed when taken in the light of the best of current knowledge. Second, systematic reviews form a critical component of legal and regulatory frameworks; for example, drug licensing or insurance coverage. Here, systematic reviews also need to hold up as auditable processes for legal examination. As systematic reviews need to be both correct, and be seen to be correct, detailed evidence-based methods have been developed to guide review authors as to the most appropriate procedures to follow, and what information to include in their reports to aid auditability.
1.4.1 Expectations for the conduct and reporting of Cochrane Reviews
Cochrane has developed methodological expectations for the conduct, reporting and updating of systematic reviews of interventions (MECIR) and their plain language summaries ( Plain Language Expectations for Authors of Cochrane Summaries ; PLEACS). Developed collaboratively by methodologists and Cochrane editors, they are intended to describe the desirable attributes of a Cochrane Review. The expectations are not all relevant at the same stage of review conduct, so care should be taken to identify those that are relevant at specific points during the review. Different methods should be used at different stages of the review in terms of the planning, conduct, reporting and updating of the review.
Each expectation has a title, a rationale and an elaboration. For the purposes of publication of a review with Cochrane, each has the status of either ‘mandatory’ or ‘highly desirable’. Items described as mandatory are expected to be applied, and if they are not then an appropriate justification should be provided; failure to implement such items may be used as a basis for deciding not to publish a review in the Cochrane Database of Systematic Reviews (CDSR). Items described as highly desirable should generally be implemented, but there are reasonable exceptions and justifications are not required.
All MECIR expectations for the conduct of a review are presented in the relevant chapters of this Handbook . Expectations for reporting of completed reviews (including PLEACS) are described in online Chapter III . The recommendations provided in the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement have been incorporated into the Cochrane reporting expectations, ensuring compliance with the PRISMA recommendations and summarizing attributes of reporting that should allow a full assessment of the methods and findings of the review (Moher et al 2009).
1.5 Protocol development
Preparing a systematic review is complex and involves many judgements. To minimize the potential for bias in the review process, these judgements should be made as far as possible in ways that do not depend on the findings of the studies included in the review. Review authors’ prior knowledge of the evidence may, for example, influence the definition of a systematic review question, the choice of criteria for study eligibility, or the pre-specification of intervention comparisons and outcomes to analyse. It is important that the methods to be used should be established and documented in advance (see MECIR Box 1.5.a , MECIR Box 1.5.b and MECIR Box 1.5.c ).
Publication of a protocol for a review that is written without knowledge of the available studies reduces the impact of review authors’ biases, promotes transparency of methods and processes, reduces the potential for duplication, allows peer review of the planned methods before they have been completed, and offers an opportunity for the review team to plan resources and logistics for undertaking the review itself. All chapters in the Handbook should be consulted when drafting the protocol. Since systematic reviews are by their nature retrospective, an element of knowledge of the evidence is often inevitable. This is one reason why non-content experts such as methodologists should be part of the review team (see Section 1.3 ). Two exceptions to the retrospective nature of a systematic review are a meta-analysis of a prospectively planned series of trials and some living systematic reviews, as described in Chapter 22 .
The review question should determine the methods used in the review, and not vice versa. The question may concern a relatively straightforward comparison of one treatment with another; or it may necessitate plans to compare different treatments as part of a network meta-analysis, or assess differential effects of an intervention in different populations or delivered in different ways.
The protocol sets out the context in which the review is being conducted. It presents an opportunity to develop ideas that are foundational for the review. This concerns, most explicitly, definition of the eligibility criteria such as the study participants and the choice of comparators and outcomes. The eligibility criteria may also be defined following the development of a logic model (or an articulation of the aspects of an extent logic model that the review is addressing) to explain how the intervention might work (see Chapter 2, Section 2.5.1 ).
MECIR Box 1.5.a Relevant expectations for conduct of intervention reviews
A key purpose of the protocol is to make plans to minimize bias in the eventual findings of the review. Reliable synthesis of available evidence requires a planned, systematic approach. Threats to the validity of systematic reviews can come from the studies they include or the process by which reviews are conducted. Biases within the studies can arise from the method by which participants are allocated to the intervention groups, awareness of intervention group assignment, and the collection, analysis and reporting of data. Methods for examining these issues should be specified in the protocol. Review processes can generate bias through a failure to identify an unbiased (and preferably complete) set of studies, and poor quality assurance throughout the review. The availability of research may be influenced by the nature of the results (i.e. reporting bias). To reduce the impact of this form of bias, searching may need to include unpublished sources of evidence (Dwan et al 2013) ( MECIR Box 1.5.b ).
MECIR Box 1.5.b Relevant expectations for the conduct of intervention reviews
Developing a protocol for a systematic review has benefits beyond reducing bias. Investing effort in designing a systematic review will make the process more manageable and help to inform key priorities for the review. Defining the question, referring to it throughout, and using appropriate methods to address the question focuses the analysis and reporting, ensuring the review is most likely to inform treatment decisions for funders, policy makers, healthcare professionals and consumers. Details of the planned analyses, including investigations of variability across studies, should be specified in the protocol, along with methods for interpreting the results through the systematic consideration of factors that affect confidence in estimates of intervention effect ( MECIR Box 1.5.c ).
MECIR Box 1.5.c Relevant expectations for conduct of intervention reviews
While the intention should be that a review will adhere to the published protocol, changes in a review protocol are sometimes necessary. This is also the case for a protocol for a randomized trial, which must sometimes be changed to adapt to unanticipated circumstances such as problems with participant recruitment, data collection or event rates. While every effort should be made to adhere to a predetermined protocol, this is not always possible or appropriate. It is important, however, that changes in the protocol should not be made based on how they affect the outcome of the research study, whether it is a randomized trial or a systematic review. Post hoc decisions made when the impact on the results of the research is known, such as excluding selected studies from a systematic review, or changing the statistical analysis, are highly susceptible to bias and should therefore be avoided unless there are reasonable grounds for doing this.
Enabling access to a protocol through publication (all Cochrane Protocols are published in the CDSR ) and registration on the PROSPERO register of systematic reviews reduces duplication of effort, research waste, and promotes accountability. Changes to the methods outlined in the protocol should be transparently declared.
This Handbook provides details of the systematic review methods developed or selected by Cochrane. They are intended to address the need for rigour, comprehensiveness and transparency in preparing a Cochrane systematic review. All relevant chapters – including those describing procedures to be followed in the later stages of the review – should be consulted during the preparation of the protocol. A more specific description of the structure of Cochrane Protocols is provide in online Chapter II .
1.6 Data management and quality assurance
Systematic reviews should be replicable, and retaining a record of the inclusion decisions, data collection, transformations or adjustment of data will help to establish a secure and retrievable audit trail. They can be operationally complex projects, often involving large research teams operating in different sites across the world. Good data management processes are essential to ensure that data are not inadvertently lost, facilitating the identification and correction of errors and supporting future efforts to update and maintain the review. Transparent reporting of review decisions enables readers to assess the reliability of the review for themselves.
Review management software, such as Covidence and EPPI-Reviewer , can be used to assist data management and maintain consistent and standardized records of decisions made throughout the review. These tools offer a central repository for review data that can be accessed remotely throughout the world by members of the review team. They record independent assessment of studies for inclusion, risk of bias and extraction of data, enabling checks to be made later in the process if needed. Research has shown that even experienced reviewers make mistakes and disagree with one another on risk-of-bias assessments, so it is particularly important to maintain quality assurance here, despite its cost in terms of author time. As more sophisticated information technology tools begin to be deployed in reviews (see Chapter 4, Section 4.6.6.2 and Chapter 22, Section 22.2.4 ), it is increasingly apparent that all review data – including the initial decisions about study eligibility – have value beyond the scope of the individual review. For example, review updates can be made more efficient through (semi-) automation when data from the original review are available for machine learning.
1.7 Chapter information
Authors: Toby J Lasserson, James Thomas, Julian PT Higgins
Acknowledgements: This chapter builds on earlier versions of the Handbook . We would like to thank Ruth Foxlee, Richard Morley, Soumyadeep Bhaumik, Mona Nasser, Dan Fox and Sally Crowe for their contributions to Section 1.3 .
Funding: JT is supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care North Thames at Barts Health NHS Trust. JPTH is a member of the NIHR Biomedical Research Centre at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. JPTH received funding from National Institute for Health Research Senior Investigator award NF-SI-0617-10145. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
1.8 References
Antman E, Lau J, Kupelnick B, Mosteller F, Chalmers T. A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts: treatment for myocardial infarction. JAMA 1992; 268 : 240–248.
Chalmers I, Bracken MB, Djulbegovic B, Garattini S, Grant J, Gulmezoglu AM, Howells DW, Ioannidis JP, Oliver S. How to increase value and reduce waste when research priorities are set. Lancet 2014; 383 : 156–165.
Chandler J, Hopewell S. Cochrane methods – twenty years experience in developing systematic review methods. Systematic Reviews 2013; 2 : 76.
Dwan K, Gamble C, Williamson PR, Kirkham JJ, Reporting Bias Group. Systematic review of the empirical evidence of study publication bias and outcome reporting bias: an updated review. PloS One 2013; 8 : e66844.
Gøtzsche PC, Ioannidis JPA. Content area experts as authors: helpful or harmful for systematic reviews and meta-analyses? BMJ 2012; 345 .
Macleod MR, Michie S, Roberts I, Dirnagl U, Chalmers I, Ioannidis JP, Al-Shahi Salman R, Chan AW, Glasziou P. Biomedical research: increasing value, reducing waste. Lancet 2014; 383 : 101–104.
Moher D, Liberati A, Tetzlaff J, Altman D, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009; 6 : e1000097.
Oxman A, Guyatt G. The science of reviewing research. Annals of the New York Academy of Sciences 1993; 703 : 125–133.
Rees R, Oliver S. Stakeholder perspectives and participation in reviews. In: Gough D, Oliver S, Thomas J, editors. An Introduction to Systematic Reviews . 2nd ed. London: Sage; 2017. p. 17–34.
Steel R. Involving marginalised and vulnerable people in research: a consultation document (2nd revision). INVOLVE; 2004.
Thomas J, Harden A, Oakley A, Oliver S, Sutcliffe K, Rees R, Brunton G, Kavanagh J. Integrating qualitative research with trials in systematic reviews. BMJ 2004; 328 : 1010–1012.
For permission to re-use material from the Handbook (either academic or commercial), please see here for full details.
Easy guide to conducting a systematic review
Affiliations.
- 1 Discipline of Child and Adolescent Health, University of Sydney, Sydney, New South Wales, Australia.
- 2 Department of Nephrology, The Children's Hospital at Westmead, Sydney, New South Wales, Australia.
- 3 Education Department, The Children's Hospital at Westmead, Sydney, New South Wales, Australia.
- PMID: 32364273
- DOI: 10.1111/jpc.14853
A systematic review is a type of study that synthesises research that has been conducted on a particular topic. Systematic reviews are considered to provide the highest level of evidence on the hierarchy of evidence pyramid. Systematic reviews are conducted following rigorous research methodology. To minimise bias, systematic reviews utilise a predefined search strategy to identify and appraise all available published literature on a specific topic. The meticulous nature of the systematic review research methodology differentiates a systematic review from a narrative review (literature review or authoritative review). This paper provides a brief step by step summary of how to conduct a systematic review, which may be of interest for clinicians and researchers.
Keywords: research; research design; systematic review.
© 2020 Paediatrics and Child Health Division (The Royal Australasian College of Physicians).
Publication types
- Systematic Review
- Research Design*
Systematic Reviews and Meta Analysis
- Getting Started
- Guides and Standards
- Review Protocols
- Databases and Sources
- Randomized Controlled Trials
- Controlled Clinical Trials
- Observational Designs
- Tests of Diagnostic Accuracy
- Software and Tools
- Where do I get all those articles?
- Collaborations
- EPI 233/528
- Countway Mediated Search
- Risk of Bias (RoB)
Systematic review Q & A
What is a systematic review.
A systematic review is guided filtering and synthesis of all available evidence addressing a specific, focused research question, generally about a specific intervention or exposure. The use of standardized, systematic methods and pre-selected eligibility criteria reduce the risk of bias in identifying, selecting and analyzing relevant studies. A well-designed systematic review includes clear objectives, pre-selected criteria for identifying eligible studies, an explicit methodology, a thorough and reproducible search of the literature, an assessment of the validity or risk of bias of each included study, and a systematic synthesis, analysis and presentation of the findings of the included studies. A systematic review may include a meta-analysis.
For details about carrying out systematic reviews, see the Guides and Standards section of this guide.
Is my research topic appropriate for systematic review methods?
A systematic review is best deployed to test a specific hypothesis about a healthcare or public health intervention or exposure. By focusing on a single intervention or a few specific interventions for a particular condition, the investigator can ensure a manageable results set. Moreover, examining a single or small set of related interventions, exposures, or outcomes, will simplify the assessment of studies and the synthesis of the findings.
Systematic reviews are poor tools for hypothesis generation: for instance, to determine what interventions have been used to increase the awareness and acceptability of a vaccine or to investigate the ways that predictive analytics have been used in health care management. In the first case, we don't know what interventions to search for and so have to screen all the articles about awareness and acceptability. In the second, there is no agreed on set of methods that make up predictive analytics, and health care management is far too broad. The search will necessarily be incomplete, vague and very large all at the same time. In most cases, reviews without clearly and exactly specified populations, interventions, exposures, and outcomes will produce results sets that quickly outstrip the resources of a small team and offer no consistent way to assess and synthesize findings from the studies that are identified.
If not a systematic review, then what?
You might consider performing a scoping review . This framework allows iterative searching over a reduced number of data sources and no requirement to assess individual studies for risk of bias. The framework includes built-in mechanisms to adjust the analysis as the work progresses and more is learned about the topic. A scoping review won't help you limit the number of records you'll need to screen (broad questions lead to large results sets) but may give you means of dealing with a large set of results.
This tool can help you decide what kind of review is right for your question.
Can my student complete a systematic review during her summer project?
Probably not. Systematic reviews are a lot of work. Including creating the protocol, building and running a quality search, collecting all the papers, evaluating the studies that meet the inclusion criteria and extracting and analyzing the summary data, a well done review can require dozens to hundreds of hours of work that can span several months. Moreover, a systematic review requires subject expertise, statistical support and a librarian to help design and run the search. Be aware that librarians sometimes have queues for their search time. It may take several weeks to complete and run a search. Moreover, all guidelines for carrying out systematic reviews recommend that at least two subject experts screen the studies identified in the search. The first round of screening can consume 1 hour per screener for every 100-200 records. A systematic review is a labor-intensive team effort.
How can I know if my topic has been been reviewed already?
Before starting out on a systematic review, check to see if someone has done it already. In PubMed you can use the systematic review subset to limit to a broad group of papers that is enriched for systematic reviews. You can invoke the subset by selecting if from the Article Types filters to the left of your PubMed results, or you can append AND systematic[sb] to your search. For example:
"neoadjuvant chemotherapy" AND systematic[sb]
The systematic review subset is very noisy, however. To quickly focus on systematic reviews (knowing that you may be missing some), simply search for the word systematic in the title:
"neoadjuvant chemotherapy" AND systematic[ti]
Any PRISMA-compliant systematic review will be captured by this method since including the words "systematic review" in the title is a requirement of the PRISMA checklist. Cochrane systematic reviews do not include 'systematic' in the title, however. It's worth checking the Cochrane Database of Systematic Reviews independently.
You can also search for protocols that will indicate that another group has set out on a similar project. Many investigators will register their protocols in PROSPERO , a registry of review protocols. Other published protocols as well as Cochrane Review protocols appear in the Cochrane Methodology Register, a part of the Cochrane Library .
- Next: Guides and Standards >>
- Last Updated: Feb 26, 2024 3:17 PM
- URL: https://guides.library.harvard.edu/meta-analysis
The Graduate Health & Life Sciences Research Library at Georgetown University Medical Center
Systematic reviews.
- Should I do a systematic review?
- Writing the Protocol
- Building a Systematic Search
- Where to Search
- Managing Project Data
- How can a DML librarian help?
Guides and Standards
- The Cochrane Handbook The Cochrane Handbook has become the de facto standard for planning and carrying out a systematic review. Chapter 6, Searching for Studies, is most helpful in planning your review.
- Finding What Works in Health Care: Standards for Systematic Reviews The IOM standards promote objective, transparent, and scientifically valid systematic reviews. They address the entire systematic review process, from locating, screening, and selecting studies for the review, to synthesizing the findings (including meta-analysis) and assessing the overall quality of the body of evidence, to producing the final review report.
- PRISMA Standards The Preferred Reporting Items for Systematic Reviews and Meta-Analyses is an evidence-based minimum set of items for reporting in systematic reviews and meta-analyses. A 27-item checklist, PRISMA focuses on randomized trials but can also be used as a basis for reporting systematic reviews of other types of research, particularly evaluations of interventions.
What is a systematic review?
A systematic literature review is a research methodology designed to answer a focused research question. Authors conduct a methodical and comprehensive literature synthesis focused on a well-formulated research question. Its aim is to identify and synthesize all of the scholarly research on a particular topic, including both published and unpublished studies. Systematic reviews are conducted in an unbiased, reproducible way to provide evidence for practice and policy-making and identify gaps in research. Every step of the review, including the search, must be documented for reproducibility.
Researchers in medicine may be most familiar with Cochrane Reviews, which synthesize randomized controlled trials to evaluate specific medical interventions. Systematic reviews are conducted in many other fields, though the type of evidence analyzed varies with the research question.
When to use systematic review methodology
Systematic reviews require more time and manpower than traditional literature reviews. Before beginning a systematic review, researchers should address these questions:
Is there is enough literature published on the topic to warrant a review?
Systematic reviews are designed to distill the evidence from many studies into actionable insights. Is there a body of evidence available to analyze, or does more primary research need to be done?
Can your research question be answered by a systematic review?
Systematic review questions should be specific and clearly defined. Questions that fit the PICO (problem/patient, intervention, comparison, outcome) format are usually well-suited for the systematic review methodology. The research question determines the search strategy, inclusion criteria, and data that you extract from the selected studies, so it should be clearly defined at the start of the review process.
Do you have a protocol outlining the review plan?
The protocol is the roadmap for the review project. A good protocol outlines study methodology, includes the rationale for the systematic review, and describes the key question broken into PICO components. It is also a good place to plan out inclusion/exclusion criteria, databases that will be searched, data abstraction and management methods, and how the studies will be assessed for methodological quality.
Do you have a team of experts?
A systematic review is team effort. Having multiple reviewers minimizes bias and strengthens analysis. Teams are often composed of subject experts, two or more literature screeners, a librarian to conduct the search, and a statistician to analyze the data.
Do you have the time that it takes to properly conduct a systematic review?
Systematic reviews typically take 12-18 months.
Do you have a method for discerning bias?
There are many types of bias, including selection, performance, & reporting bias, and assessing the risk of bias of individual studies is an important part of your study design.
Can you afford to have articles in languages other than English translated?
You should include all relevant studies in your systematic review, regardless of the language they were published in, so as to avoid language bias.
Which review is right for you?
If your project does not meet the above criteria, there are many more options for conducting a synthesis of the literature. The chart below highlights several review methodologies. Reproduced from: Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009 Jun;26(2):91-108. doi: 10.1111/j.1471-1842.2009.00848.x . Review. PubMed PMID: 19490148
- Next: Writing the Protocol >>
- Last Updated: May 1, 2024 1:01 PM
- URL: https://guides.dml.georgetown.edu/systematicreviews
The Responsible Use of Electronic Resources policy governs the use of resources provided on these guides. © Dahlgren Memorial Library, Georgetown University Medical Center. Unless otherwise stated, these guides may be used for educational or academic purposes as long as proper attribution is given. Please seek permission for any modifications, adaptations, or for commercial purposes. Email [email protected] to request permission. Proper attribution includes: Written by or adapted from, Dahlgren Memorial Library, URL.

Literature Reviews
- Getting started
What is a literature review?
Why conduct a literature review, stages of a literature review, lit reviews: an overview (video), check out these books.
- Types of reviews
- 1. Define your research question
- 2. Plan your search
- 3. Search the literature
- 4. Organize your results
- 5. Synthesize your findings
- 6. Write the review
- Artificial intelligence (AI) tools
- Thompson Writing Studio This link opens in a new window
- Need to write a systematic review? This link opens in a new window

Contact a Librarian
Ask a Librarian
Definition: A literature review is a systematic examination and synthesis of existing scholarly research on a specific topic or subject.
Purpose: It serves to provide a comprehensive overview of the current state of knowledge within a particular field.
Analysis: Involves critically evaluating and summarizing key findings, methodologies, and debates found in academic literature.
Identifying Gaps: Aims to pinpoint areas where there is a lack of research or unresolved questions, highlighting opportunities for further investigation.
Contextualization: Enables researchers to understand how their work fits into the broader academic conversation and contributes to the existing body of knowledge.

tl;dr A literature review critically examines and synthesizes existing scholarly research and publications on a specific topic to provide a comprehensive understanding of the current state of knowledge in the field.
What is a literature review NOT?
❌ An annotated bibliography
❌ Original research
❌ A summary
❌ Something to be conducted at the end of your research
❌ An opinion piece
❌ A chronological compilation of studies
The reason for conducting a literature review is to:

Literature Reviews: An Overview for Graduate Students
While this 9-minute video from NCSU is geared toward graduate students, it is useful for anyone conducting a literature review.

Writing the literature review: A practical guide
Available 3rd floor of Perkins

Writing literature reviews: A guide for students of the social and behavioral sciences
Available online!

So, you have to write a literature review: A guided workbook for engineers

Telling a research story: Writing a literature review

The literature review: Six steps to success

Systematic approaches to a successful literature review
Request from Duke Medical Center Library

Doing a systematic review: A student's guide
- Next: Types of reviews >>
- Last Updated: May 17, 2024 8:42 AM
- URL: https://guides.library.duke.edu/litreviews

Services for...
- Faculty & Instructors
- Graduate Students
- Undergraduate Students
- International Students
- Patrons with Disabilities
- Harmful Language Statement
- Re-use & Attribution / Privacy
- Support the Libraries

- Library Hours
- Strategic Plan
- Giving to the Libraries
- Jobs at the Libraries
- Find Your Librarian
- View All →
- Google Scholar
- Research Guides
- Textbook/Reserves
- Government Documents
- Get It For Me
- Print/Copy/Scan
- Renew Materials
- Study Rooms
- Use a Computer
- Borrow Tech Gear
- Student Services
- Faculty Services
- Users with Disabilities
- Visitors & Alumni
- Special Collections
- Find Information
Basics of Systematic Reviews
- About Systematic Review
Types of Reviews
Literature review.
Collects key sources on a topic and discusses those sources in conversation with each other
- Standard for research articles in most disciplines
- Tells the reader what is known, or not known, about a particular issue, topic, or subject
- Demonstrates knowledge and understanding of a topic
- Establishes context or background for a case or argument
- Helps develop the author’s ideas and perspective
Rapid Review
Thorough methodology but with process limitations in place to expeditethe completion of a review.
- For questions that require timely answers
- 3-4 months vs. 12-24 months
- Limitations - scope, comprehensiveness bias, and quality of appraisal
- Discusses potential effects that the limited methods may have had on results
Scoping Review
Determine the scope or coverage of a body of literature on a given topic and give clear indication of the volume of literature and studies available as well as an overview of its focus.
- Identify types of available evidence in a given field
- Clarify key concepts/definitions in the literature
- Examine how research is conducted on a certain topic or field
- Identify key factors related to a concept
- Key difference is focus
- Identify and analyze knowledge gaps
Systematic Review
Attempts to identify, appraise, and summarize all empirical evidence that fits pre-specified eligibility criteria to answer a specific research question.
- clearly defined question with inclusion/exclusion criteria
- rigorous and systematic search of the literature
- thorough screening of results
- data extraction and management
- analysis and interpretation of results
- risk of bias assessment of included studies
Meta-Analysis
Used to systematically synthesize or merge the findings of single, independent studies, using statistical methods to calculate an overall or ‘absolute’ effect.
- Combines results from multiple empirical studies
- Requires systematic review first
- Use well recognized, systematic methods to account for differences in sample size, variability (heterogeneity) in study approach and findings (treatment effects)
- Test how sensitive their results are to their own systematic review protocol
For additional types of reviews please see these articles:
- Sutton, A., Clowes, M., Preston, L. and Booth, A. (2019), Meeting the review family: exploring review types and associated information retrieval requirements. Health Info Libr J, 36: 202-222. https://doi.org/10.1111/hir.12276
- Grant, M.J. and Booth, A. (2009), A typology of reviews: an analysis of 14 review types and associated methodologies. Health Information & Libraries Journal, 26: 91-108. https://doi.org/10.1111/j.1471-1842.2009.00848.x
- << Previous: About Systematic Review
- Next: Sources >>
- Last Updated: May 17, 2024 10:04 AM
- URL: https://libguides.utsa.edu/systematicreview
- Library Locations
- Staff Directory
- 508 Compliance
- Site Search
- © The University of Texas at San Antonio
- Information: 210-458-4011
- Campus Alerts
- Required Links
- UTSA Policies
- Report Fraud
- Open access
- Published: 14 May 2024
Protocol for a scoping review study on learning plan use in undergraduate medical education
- Anna Romanova ORCID: orcid.org/0000-0003-1118-1604 1 ,
- Claire Touchie 1 ,
- Sydney Ruller 2 ,
- Victoria Cole 3 &
- Susan Humphrey-Murto 4
Systematic Reviews volume 13 , Article number: 131 ( 2024 ) Cite this article
93 Accesses
Metrics details
The current paradigm of competency-based medical education and learner-centredness requires learners to take an active role in their training. However, deliberate and planned continual assessment and performance improvement is hindered by the fragmented nature of many medical training programs. Attempts to bridge this continuity gap between supervision and feedback through learner handover have been controversial. Learning plans are an alternate educational tool that helps trainees identify their learning needs and facilitate longitudinal assessment by providing supervisors with a roadmap of their goals. Informed by self-regulated learning theory, learning plans may be the answer to track trainees’ progress along their learning trajectory. The purpose of this study is to summarise the literature regarding learning plan use specifically in undergraduate medical education and explore the student’s role in all stages of learning plan development and implementation.
Following Arksey and O’Malley’s framework, a scoping review will be conducted to explore the use of learning plans in undergraduate medical education. Literature searches will be conducted using multiple databases by a librarian with expertise in scoping reviews. Through an iterative process, inclusion and exclusion criteria will be developed and a data extraction form refined. Data will be analysed using quantitative and qualitative content analyses.
By summarising the literature on learning plan use in undergraduate medical education, this study aims to better understand how to support self-regulated learning in undergraduate medical education. The results from this project will inform future scholarly work in competency-based medical education at the undergraduate level and have implications for improving feedback and supporting learners at all levels of competence.
Scoping review registration:
Open Science Framework osf.io/wvzbx.
Peer Review reports
Competency-based medical education (CBME) has transformed the approach to medical education to focus on demonstration of acquired competencies rather than time-based completion of rotations [ 1 ]. As a result, undergraduate and graduate medical training programs worldwide have adopted outcomes-based assessments in the form of entrustable professional activities (EPAs) comprised of competencies to be met [ 2 ]. These assessments are completed longitudinally by multiple different evaluators to generate an overall impression of a learner’s competency.
In CBME, trainees will progress along their learning trajectory at individual speeds and some may excel while others struggle to achieve the required knowledge, skills or attitudes. Therefore, deliberate and planned continual assessment and performance improvement is required. However, due to the fragmented nature of many medical training programs where learners rotate through different rotations and work with many supervisors, longitudinal observation is similarly fragmented. This makes it difficult to determine where trainees are on their learning trajectories and can affect the quality of feedback provided to them, which is a known major influencer of academic achievement [ 3 ]. As a result, struggling learners may not be identified until late in their training and the growth of high-performing learners may be stifled [ 4 , 5 , 6 ].
Bridging this continuity gap between supervision and feedback through some form of learner handover or forward feeding has been debated since the 1970s and continues to this day [ 5 , 7 , 8 , 9 , 10 , 11 ]. The goal of learner handover is to improve trainee assessment and feedback by sharing their performance and learning needs between supervisors or across rotations. However, several concerns have been raised about this approach including that it could inappropriately bias subsequent assessments of the learner’s abilities [ 9 , 11 , 12 ]. A different approach to keeping track of trainees’ learning goals and progress along their learning trajectories is required. Learning plans (LPs) informed by self-regulated learning (SRL) theory may be the answer.
SRL has been defined as a cyclical process where learners actively control their thoughts, actions and motivation to achieve their goals [ 13 ]. Several models of SRL exist but all entail that the trainee is responsible for setting, planning, executing, monitoring and reflecting on their learning goals [ 13 ]. According to Zimmerman’s SRL model, this process occurs in three stages: forethought phase before an activity, performance phase during an activity and self-reflection phase after an activity [ 13 ]. Since each trainee leads their own learning process and has an individual trajectory towards competence, this theory relates well to the CBME paradigm which is grounded in learner-centredness [ 1 ]. However, we know that medical students and residents have difficulty identifying their own learning goals and therefore need guidance to effectively partake in SRL [ 14 , 15 , 16 , 17 ]. Motivation has also emerged as a key component of SRL, and numerous studies have explored factors that influence student engagement in learning [ 18 , 19 ]. In addition to meeting their basic psychological needs of autonomy, relatedness and competence, perceived learning relevance through meaningful learning activities has been shown to increase trainee engagement in their learning [ 19 ].
LPs are a well-known tool across many educational fields including CBME that can provide trainees with meaningful learning activities since they help them direct their own learning goals in a guided fashion [ 20 ]. Also known as personal learning plans, learning contracts, personal action plans, personal development plans, and learning goals, LPs are documents that outline the learner’s roadmap to achieve their learning goals. They require the learner to self-identify what they need to learn and why, how they are going to do it, how they will know when they are finished, define the timeframe for goal achievement and assess the impact of their learning [ 20 ]. In so doing, LPs give more autonomy to the learner and facilitate objective and targeted feedback from supervisors. This approach has been described as “most congruent with the assumptions we make about adults as learners” [ 21 ].
LP use has been explored across various clinical settings and at all levels of medical education; however, most of the experience lies in postgraduate medical education [ 22 ]. Medical students are a unique learner population with learning needs that appear to be very well suited for using LPs for two main reasons. First, their education is often divided between classroom and clinical settings. During clinical training, students need to be more independent in setting learning goals to meet desired competencies as their education is no longer outlined for them in a detailed fashion by the medical school curriculum [ 23 ]. SRL in the workplace is also different than in the classroom due to additional complexities of clinical care that can impact students’ ability to self-regulate their learning [ 24 ]. Second, although most medical trainees have difficulty with goal setting, medical students in particular need more guidance compared to residents due to their relative lack of experience upon which they can build within the SRL framework [ 25 ]. LPs can therefore provide much-needed structure to their learning but should be guided by an experienced tutor to be effective [ 15 , 24 ].
LPs fit well within the learner-centred educational framework of CBME by helping trainees identify their learning needs and facilitating longitudinal assessment by providing supervisors with a roadmap of their goals. In so doing, they can address current issues with learner handover and identification as well as remediation of struggling learners. Moreover, they have the potential to help trainees develop lifelong skills with respect to continuing professional development after graduation which is required by many medical licensing bodies.
An initial search of the JBI Database, Cochrane Database, MEDLINE (PubMed) and Google Scholar conducted in July–August 2022 revealed a paucity of research on LP use in undergraduate medical education (UGME). A related systematic review by van Houten–Schat et al. [ 24 ] on SRL in the clinical setting identified three interventions used by medical students and residents in SRL—coaching, LPs and supportive tools. However, only a couple of the included studies looked specifically at medical students’ use of LPs, so this remains an area in need of more exploration. A scoping review would provide an excellent starting point to map the body of literature on this topic.
The objective of this scoping review will therefore be to explore LP use in UGME. In doing so, it will address a gap in knowledge and help determine additional areas for research.
This study will follow Arksey and O’Malley’s [ 26 ] five-step framework for scoping review methodology. It will not include the optional sixth step which entails stakeholder consultation as relevant stakeholders will be intentionally included in the research team (a member of UGME leadership, a medical student and a first-year resident).
Step 1—Identifying the research question
The overarching purpose of this study is to “explore the use of LPs in UGME”. More specifically we seek to achieve the following:
Summarise the literature regarding the use of LPs in UGME (including context, students targeted, frameworks used)
Explore the role of the student in all stages of the LP development and implementation
Determine existing research gaps
Step 2—Identifying relevant studies
An experienced health sciences librarian (VC) will conduct all searches and develop the initial search strategy. The preliminary search strategy is shown in Appendix A (see Additional file 2). Articles will be included if they meet the following criteria [ 27 ]:
Participants
Medical students enrolled at a medical school at the undergraduate level.
Any use of LPs by medical students. LPs are defined as a document, usually presented in a table format, that outlines the learner’s roadmap to achieve their learning goals [ 20 ].
Any stage of UGME in any geographic setting.
Types of evidence sources
We will search existing published and unpublished (grey) literature. This may include research studies, reviews, or expert opinion pieces.
Search strategy
With the assistance of an experienced librarian (VC), a pilot search will be conducted to inform the final search strategy. A search will be conducted in the following electronic databases: MEDLINE, Embase, Education Source, APA PsycInfo and Web of Science. The search terms will be developed in consultation with the research team and librarian. The search strategy will proceed according to the JBI Manual for Evidence Synthesis three-step search strategy for reviews [ 27 ]. First, we will conduct a limited search in two appropriate online databases and analyse text words from the title, abstracts and index terms of relevant papers. Next, we will conduct a second search using all identified key words in all databases. Third, we will review reference lists of all included studies to identify further relevant studies to include in the review. We will also contact the authors of relevant papers for further information if required. This will be an iterative process as the research team becomes more familiar with the literature and will be guided by the librarian. Any modifications to the search strategy as it evolves will be described in the scoping review report. As a measure of rigour, the search strategy will be peer-reviewed by another librarian using the PRESS checklist [ 28 ]. No language or date limits will be applied.
Step 3—Study selection
The screening process will consist of a two-step approach: screening titles/abstracts and, if they meet inclusion criteria, this will be followed by a full-text review. All screening will be done by two members of the research team and any disagreements will be resolved by an independent third member of the team. Based on preliminary inclusion criteria, the whole research team will first pilot the screening process by reviewing a random sample of 25 titles/abstracts. The search strategy, eligibility criteria and study objectives will be refined in an iterative process. We anticipate several meetings as the topic is not well described in the literature. A flowchart of the review process will be generated. Any modifications to the study selection process will be described in the scoping review report. The papers will be excluded if a full text is not available. The search results will be managed using Covidence software.
Step 4—Charting the data
A preliminary data extraction tool is shown in Appendix B (see Additional file 3 ). Data will be extracted into Excel and will include demographic information and specific details about the population, concept, context, study methods and outcomes as they relate to the scoping review objectives. The whole research team will pilot the data extraction tool on ten articles selected for full-text review. Through an iterative process, the final data extraction form will be refined. Subsequently, two members of the team will independently extract data from all articles included for full-text review using this tool. Charting disagreements will be resolved by the principal and senior investigators. Google Translate will be used for any included articles that are not in the English language.
Step 5—Collating, summarising and reporting the results
Quantitative and qualitative analyses will be used to summarise the results. Quantitative analysis will capture descriptive statistics with details about the population, concept, context, study methods and outcomes being examined in this scoping review. Qualitative content analysis will enable interpretation of text data through the systematic classification process of coding and identifying themes and patterns [ 29 ]. Several team meetings will be held to review potential themes to ensure an accurate representation of the data. The PRISMA Extension for Scoping Reviews (PRISMA-ScR) will be used to guide the reporting of review findings [ 30 ]. Data will be presented in tables and/or diagrams as applicable. A descriptive summary will explain the presented results and how they relate to the scoping review objectives.
By summarising the literature on LP use in UGME, this study will contribute to a better understanding of how to support SRL amongst medical students. The results from this project will also inform future scholarly work in CBME at the undergraduate level and have implications for improving feedback as well as supporting learners at all levels of competence. In doing so, this study may have practical applications by informing learning plan incorporation into CBME-based curricula.
We do not anticipate any practical or operational issues at this time. We assembled a team with the necessary expertise and tools to complete this project.

Availability of data and materials
All data generated or analysed during this study will be included in the published scoping review article.
Abbreviations
- Competency-based medical education
Entrustable professional activity
- Learning plan
- Self-regulated learning
- Undergraduate medical education
Frank JR, Snell LS, Cate OT, et al. Competency-based medical education: theory to practice. Med Teach. 2010;32(8):638–45.
Article PubMed Google Scholar
Shorey S, Lau TC, Lau ST, Ang E. Entrustable professional activities in health care education: a scoping review. Med Educ. 2019;53(8):766–77.
Hattie J, Timperley H. The power of feedback. Rev Educ Res. 2007;77(1):81–112.
Article Google Scholar
Dudek NL, Marks MB, Regehr G. Failure to fail: the perspectives of clinical supervisors. Acad Med. 2005;80(10 Suppl):S84–7.
Warm EJ, Englander R, Pereira A, Barach P. Improving learner handovers in medical education. Acad Med. 2017;92(7):927–31.
Spooner M, Duane C, Uygur J, et al. Self-regulatory learning theory as a lens on how undergraduate and postgraduate learners respond to feedback: a BEME scoping review : BEME Guide No. 66. Med Teach. 2022;44(1):3–18.
Frellsen SL, Baker EA, Papp KK, Durning SJ. Medical school policies regarding struggling medical students during the internal medicine clerkships: results of a National Survey. Acad Med. 2008;83(9):876–81.
Humphrey-Murto S, LeBlanc A, Touchie C, et al. The influence of prior performance information on ratings of current performance and implications for learner handover: a scoping review. Acad Med. 2019;94(7):1050–7.
Morgan HK, Mejicano GC, Skochelak S, et al. A responsible educational handover: improving communication to improve learning. Acad Med. 2020;95(2):194–9.
Dory V, Danoff D, Plotnick LH, et al. Does educational handover influence subsequent assessment? Acad Med. 2021;96(1):118–25.
Humphrey-Murto S, Lingard L, Varpio L, et al. Learner handover: who is it really for? Acad Med. 2021;96(4):592–8.
Shaw T, Wood TJ, Touchie T, Pugh D, Humphrey-Murto S. How biased are you? The effect of prior performance information on attending physician ratings and implications for learner handover. Adv Health Sci Educ Theory Pract. 2021;26(1):199–214.
Artino AR, Brydges R, Gruppen LD. Chapter 14: Self-regulated learning in health professional education: theoretical perspectives and research methods. In: Cleland J, Duning SJ, editors. Researching Medical Education. 1st ed. John Wiley & Sons; 2015. p. 155–66.
Chapter Google Scholar
Cleland J, Arnold R, Chesser A. Failing finals is often a surprise for the student but not the teacher: identifying difficulties and supporting students with academic difficulties. Med Teach. 2005;27(6):504–8.
Reed S, Lockspeiser TM, Burke A, et al. Practical suggestions for the creation and use of meaningful learning goals in graduate medical education. Acad Pediatr. 2016;16(1):20–4.
Wolff M, Stojan J, Cranford J, et al. The impact of informed self-assessment on the development of medical students’ learning goals. Med Teach. 2018;40(3):296–301.
Sawatsky AP, Halvorsen AJ, Daniels PR, et al. Characteristics and quality of rotation-specific resident learning goals: a prospective study. Med Educ Online. 2020;25(1):1714198.
Article PubMed PubMed Central Google Scholar
Pintrich PR. Chapter 14: The role of goal orientation in self-regulated learning. In: Boekaerts M, Pintrich PR, Zeidner M, editors. Handbook of self-regulation. 1st ed. Academic Press; 2000. p. 451–502.
Kassab SE, El-Sayed W, Hamdy H. Student engagement in undergraduate medical education: a scoping review. Med Educ. 2022;56(7):703–15.
Challis M. AMEE medical education guide No. 19: Personal learning plans. Med Teach. 2000;22(3):225–36.
Knowles MS. Using learning contracts. 1 st ed. San Francisco: Jossey Bass; 1986.
Parsell G, Bligh J. Contract learning, clinical learning and clinicians. Postgrad Med J. 1996;72(847):284–9.
Article CAS PubMed PubMed Central Google Scholar
Teunissen PW, Scheele F, Scherpbier AJJA, et al. How residents learn: qualitative evidence for the pivotal role of clinical activities. Med Educ. 2007;41(8):763–70.
Article CAS PubMed Google Scholar
van Houten-Schat MA, Berkhout JJ, van Dijk N, Endedijk MD, Jaarsma ADC, Diemers AD. Self-regulated learning in the clinical context: a systematic review. Med Educ. 2018;52(10):1008–15.
Taylor DCM, Hamdy H. Adult learning theories: Implications for learning and teaching in medical education: AMEE Guide No. 83. Med Teach. 2013;35(11):e1561–72.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Peters MDJ, Godfrey C, McInerney P, Munn Z, Tricco AC, Khalol H. Chapter 11: Scoping reviews. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis. JBI; 2020. https://synthesismanual.jbi.global. . Accessed 30 Aug 2022.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–6.
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–88.
Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–73.
Venables M, Larocque A, Sikora L, Archibald D, Grudniewicz A. Understanding indigenous health education and exploring indigenous anti-racism approaches in undergraduate medical education: a scoping review protocol. OSF; 2022. https://osf.io/umwgr/ . Accessed 26 Oct 2022.
Download references
Acknowledgements
Not applicable.
This study will be supported through grants from the Department of Medicine at the Ottawa Hospital and the University of Ottawa. The funding bodies had no role in the study design and will not have any role in the collection, analysis and interpretation of data or writing of the manuscript.
Author information
Authors and affiliations.
The Ottawa Hospital – General Campus, 501 Smyth Rd, PO Box 209, Ottawa, ON, K1H 8L6, Canada
Anna Romanova & Claire Touchie
The Ottawa Hospital Research Institute, Ottawa, Canada
Sydney Ruller
The University of Ottawa, Ottawa, Canada
Victoria Cole
The Ottawa Hospital – Riverside Campus, Ottawa, Canada
Susan Humphrey-Murto
You can also search for this author in PubMed Google Scholar
Contributions
AR designed and drafted the protocol. CT and SH contributed to the refinement of the research question, study methods and editing of the manuscript. VC designed the initial search strategy. All authors reviewed the manuscript for final approval. The review guarantors are CT and SH. The corresponding author is AR.
Authors’ information
AR is a clinician teacher and Assistant Professor with the Division of General Internal Medicine at the University of Ottawa. She is also the Associate Director for the internal medicine clerkship rotation at the General campus of the Ottawa Hospital.
CT is a Professor of Medicine with the Divisions of General Internal Medicine and Infectious Diseases at the University of Ottawa. She is also a member of the UGME Competence Committee at the University of Ottawa and an advisor for the development of a new school of medicine at Toronto Metropolitan University.
SH is an Associate Professor with the Department of Medicine at the University of Ottawa and holds a Tier 2 Research Chair in Medical Education. She is also the Interim Director for the Research Support Unit within the Department of Innovation in Medical Education at the University of Ottawa.
CT and SH have extensive experience with medical education research and have numerous publications in this field.
SR is a Research Assistant with the Division of General Internal Medicine at the Ottawa Hospital Research Institute.
VC is a Health Sciences Research Librarian at the University of Ottawa.
SR and VC have extensive experience in systematic and scoping reviews.
Corresponding author
Correspondence to Anna Romanova .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. prisma-p 2015 checklist., 13643_2024_2553_moesm2_esm.docx.
Additional file 2: Appendix A. Preliminary search strategy [ 31 ].
Additional file 3: Appendix B. Preliminary data extraction tool.
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Romanova, A., Touchie, C., Ruller, S. et al. Protocol for a scoping review study on learning plan use in undergraduate medical education. Syst Rev 13 , 131 (2024). https://doi.org/10.1186/s13643-024-02553-w
Download citation
Received : 29 November 2022
Accepted : 03 May 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s13643-024-02553-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Downloadable Content
Depression and Anxiety Among US Born Children of Unauthorized Immigrants: A Systematic Literature Review
- Masters Thesis
- Jimenez, Ana
- Park, Hyun-Sun
- Jacobsen, Jeanna
- Sorrell-Medina, Zayda
- Social Work
- California State University, Northridge
- Dissertations, Academic -- CSUN -- Social Work.
- discrimination
- deportation
- mental health
- unauthorized immigrants
- http://hdl.handle.net/20.500.12680/qj72pf83q
- by Ana Jimenez

Items in ScholarWorks are protected by copyright, with all rights reserved, unless otherwise indicated.
Do alpha blockers reduce the risk of urinary retention post-transperineal prostate biopsy? A systematic narrative review
- Original Article
- Open access
- Published: 17 May 2024
- Volume 42 , article number 332 , ( 2024 )
Cite this article
You have full access to this open access article

- Zein Alhamdani ORCID: orcid.org/0000-0002-9222-6781 1 ,
- Samuel Poppenbeek 2 ,
- Damien Bolton 1 ,
- Lih-Ming Wong 1 , 2 &
- Kapil Sethi 1 , 2
102 Accesses
Explore all metrics
Transperineal Prostate Biopsy (TPB) is a commonly used technique for the diagnosis of prostate cancer due to growing concerns related to infectious complications associated with transrectal ultrasound-guided prostate biopsy (TRUSB). TPB is associated with an infective complication rate of near zero, however, acute urinary retention (AUR) remains the leading complication causing morbidity. Previously in TRUSB, there was weak evidence that alpha-blockers reduce AUR rates, and their usage has been extrapolated to clinical practice with TPB. This review aims to explore if there is an evidence base for using alpha-blockers to prevent AUR following TPB.
A systematic approach was used to search Ovid Medline and Embase using keywords related to “Transperineal” and “Retention”. Articles were then screened by applying inclusion and exclusion criteria to find studies that compared alpha-blocker recipients to no alpha-blocker use in the perioperative period and the subsequent effect on AUR in TPB.
361 records were identified in the initial search to produce 5 studies included in the final review. No randomised controlled trials (RCTs) were identified. One observational study showed a reduction in AUR rate from 12.5% to 5.3% with a single dose of tamsulosin. A previous systematic review of complications associated with prostate biopsy concluded there may be a potential benefit to alpha-blockers given in the TPB perioperative period. Three observational studies demonstrated a harmful effect related to alpha-blocker use; however, this was well explained by their clear limitations.
Based on this review and the extrapolation from TRUSB data, perioperative alpha-blockers may offer some weak benefits in preventing AUR following TPB. However, there is significant scope and need for an RCT to further develop the evidence base further given the significant gap in the literature and lack of a standard alpha blocker protocol in TPB.
Similar content being viewed by others

Update on the current evidence for Tm:YAG vapoenucleation of the prostate 2014
Transurethral enucleation of the prostate versus transvesical open prostatectomy for large benign prostatic hyperplasia: a systematic review and meta-analysis of randomized controlled trials, prostatic artery embolization in treating benign prostatic hyperplasia: a systematic review.
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer is the most commonly diagnosed cancer in men across the world [ 1 ]. To diagnose prostate cancer, a biopsy of the suspected lesion is first required to histologically confirm the type of cancer and stage of disease. Previously, the most common method of biopsy was trans-rectal ultrasound guided biopsy (TRUSB), which involves the use of an ultrasound probe to visualise the prostate and facilitate a needle through the rectum to acquire the tissue samples. There is growing concern related to the infective complications associated with this technique, however, as the needle passes through the rectum into the prostatic tissues leading to sepsis in up to 6.3% of cases [ 2 ].
This trend has shifted the biopsy technique to favour the transperineal biopsy (TPB). This method allows the sterilisation of the perineum before needle access is obtained and has been shown to have a sepsis complication rate of near zero [ 3 , 4 , 5 ]. Other benefits of a transperineal approach compared to a transrectal include the ability to access the anterior prostate, resulting in a greater cancer detection rate [ 6 ]. The main complication associated with TPB however is now acute urinary retention (AUR). Previously, in TRUSB, there have been two clinical trials that have demonstrated a benefit to the use of perioperative Tamsulosin, a uroselective alpha-blocking medication, to prevent AUR complications [ 7 , 8 ]. It is hypothesised that the needle sample taken during the biopsy leads to local tissue damage in the prostate. This causes oedema, swelling and bleeding or possible trauma to the urethra itself that then results in an outlet obstruction and AUR [ 2 ]. Alpha-blockers like Tamsulosin are hypothesised to relieve this [ 9 ]. This benefit has then been extrapolated to the TPB by some clinicians hoping to see the same advantage seen in the two RCTs on TRUSB [ 7 , 8 ]. There is no clinical standard or established protocol for their usage, and not every clinician prescribes them as part of their practice when taking a TPB. This review sets out to examine if there is an evidence base for their use in TPB with a systematic approach, and examines the question, do perioperative alpha-blockers reduce the risk of AUR following TPB?
Selection criteria
Types of studies.
Initially, the search criteria were restricted to only include randomised controlled trials (RCTs) comparing the benefit of perioperative alpha-blockers to a control on the reported urinary retention rates, however to the best of our knowledge there has been no RCT examining this in the setting of TPB. Given this gap in the literature, the search was widened to include retrospective studies, prospective cohort studies, national database series, and systematic reviews reporting alpha-blocker usage during TPB.
Types of participants
Papers were included if they examined men undergoing prostate biopsy via a transperineal approach. No limitations were placed on the specific technique used within the transperineal approach given the wide variety used in clinical practice.
Types of interventions and comparators
Studies were included if they commented on using of perioperative alpha-blockers and allowed a direct comparison to participants not taking alpha-blockers in the same period. The exact prophylactic regime of alpha-blocker use was not limited.
Types of outcome measures
Studies were included if they reported the AUR rates following TPB and could give a breakdown of the impact of an alpha-blocker in the perioperative period. In this review, AUR was defined by the need for temporary catheterisation between biopsy and follow-up review in the days/weeks after the biopsy. Additional information regarding further analysis of the risk factors for AUR following TPB was also sought; however, this was not part of the strict inclusion criteria concerning the aim of this study.
A summary of the inclusion/exclusion criteria can be found in Appendix 2.
Search methods for identification of studies
Electronic searches.
On 15th November 2023, two independent investigators (Z.A and S.P) utilised the electronic databases Embase and Ovid Medline® were searched around the key terms transperineal AND urinary retention along with their relevant MeSH headings combined with the OR Boolean operator. The entire search strategies can be found in Appendix 1 .
Data collection and extraction
Selection of studies.
Using the online tool Covidence [ 10 ] which is a web-based collaboration software platform that streamlines the production of systematic reviews, the titles and abstracts of all studies identified as part of the search underwent initial screening. According to the inclusion and exclusion criteria, each study is marked as either yes, no, or unclear, with being marked as no resulting in exclusion from further evaluation. The papers marked yes or unclear then underwent full-text screening using the same inclusion and exclusion criteria to yield the final selection of studies. Any disputes in articles included or excluded were resolved by the use of a third author (K.S).
All studies included were assessed for their risk of bias utilising the Study Quality Assessment Tools provided by National Heart, Lung and Blood Institute [ 11 ] which assesses the rate of participation, justification for patient population and recruitment, time, exposure measures, outcome assessment, follow-up and adjustment for confounding factors. Each study was rated ‘good’, ‘fair’, or ‘poor’ according to the estimated risk of bias by individual assessment (Z.A and S.P), and any rating disagreements were resolved using a third author (K.S) to reach consensus.
Extraction of data
Using an excel template, the selected studies underwent data extraction. The type of study, aims, biopsy technique, anaesthetic type, number of biopsy cores, data collection methods, centre type, number of participants, breakdown of alpha-blocker use and AUR rates were collected.
Search results
The literature searches yielded 361 articles (315 from Embase and 46 from Medline). The online tool Covidence matched 37 records as duplicates and removed them from the pool resulting in 324 records entering the initial screening. Of these 324, 86 texts were identified as potentially fulfilling the inclusion and exclusion criteria and underwent full-text screening. Of these 86, 50 were excluded due to no mention of alpha-blocker usage in their methods, and 20 were excluded as there was either a missing comparator or lack of data that prevented the effect of alpha-blocker usage from being established. Six were removed due to the original article being in a non-English language with no available translation, and five were excluded due to their editorial nature. This left five studies to be evaluated as fulfilling the inclusion and exclusion criteria. A flow diagram using the PRISMA template is presented in Fig. 1 .
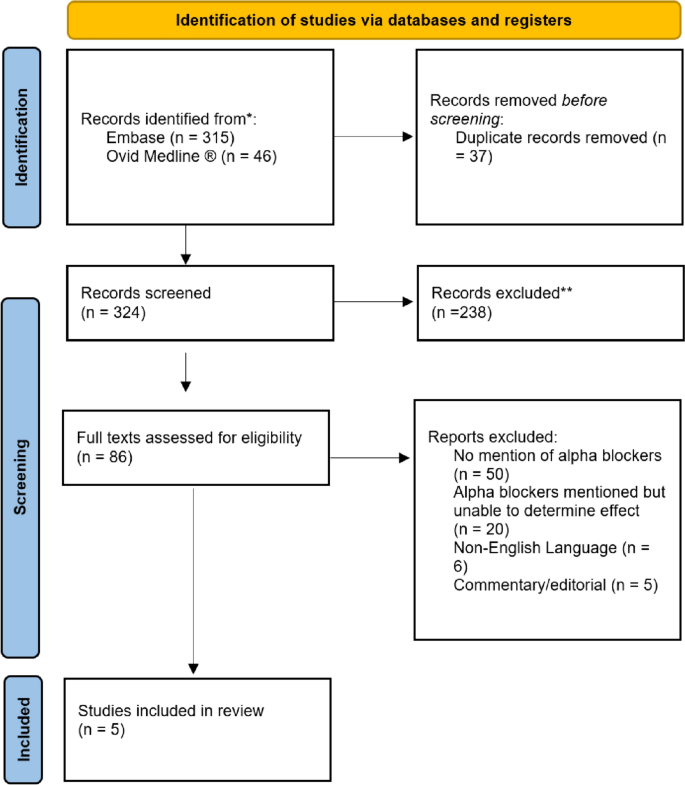
PRISMA diagram outlining the search strategy. 324 records were identified from Embase and Ovid Medline after accounting for duplicates. 238 records were removed at the initial title and abstract screening with a further 86 at full-text screening, leaving 5 records included in the review
Characteristics of included studies
Five studies met the literature search criteria and were eligible for inclusion. Table 1 , Table 2 , Table 3 is an overview of the type of each study, its aims and the alpha-blocker protocol utilised.
Design and participants
Three of the five studies were of a prospective cohort study design at a single tertiary centre [ 12 , 13 , 14 ]. Kum et al. [ 15 ] was a retrospective data collection at a single tertiary centre, and Loeb et al. [ 16 ] was a systematic review examining the complications of prostate biopsy.
Of the prospective and retrospective studies, participants were all males suspected of prostate cancer undergoing either surveillance or diagnostic biopsies.
Biopsy technique and alpha-blocker regimens
The biopsy technique varied across the different studies. Muthuveloe et al. [ 12 ] used a transperineal template-guided approach, taking systematic biopsies with a 5 mm brachytherapy grid and a minimum of 24 cores. Kum et al. [ 15 ] used a similar technique while collecting additional targeted biopsy cores based on MP-MRI results when required. Namekawa et al. [ 14 ] used a predetermined 16-core systematic biopsy technique. Ekwueme et al. [ 13 ] explored a modified template-guided technique, using 22 mm long cores to avoid sampling the base of the peri-urethral area. They achieved this due to the length of the cephalo-caudal prostate increasing as the sampling moves centrally, hypothesising that reducing trauma to this region would reduce the AUR complication rates. There were no studies that met the criteria examining a freehand technique.
In terms of peri-operative alpha blocker regimes, two studies prescribed tamsulosin. Kum et al. [ 15 ] used 400 μg on the day of the procedure (given to 238/243 patients) followed by a five to seven-day course post-operatively given to all patients who were not initially on alpha-blocker therapy, whilst Muthuveloe et al. [ 12 ] only gave a single 400 μg dose of tamsulosin on the day of the procedure to 59 out of their 200 patients.
Two studies did not prescribe a prophylactic alpha-blocker on the day of or following the procedure. Ekwueme et al. [ 13 ] however, did report that 43 of their patients were on a long-term dose of 400 μg daily tamsulosin, including on the day of the procedure. Namekawa et al. [ 14 ] reported that 566/1663 had a history of alpha-blocker use but did not give a further breakdown of the exact medications and their usage during the perioperative period.
Data collection methods
The three prospective cohort studies all used similar methods to collect their data. Muthuveloe et al. [ 12 ] recruited their initial 200 participants and then retrospectively reviewed their case notes and databases. Namekawa et al. [ 14 ] delivered purpose-built daily questionnaires for 7 days following biopsy to their 2086 initially identified patients. The questionnaire focused on adverse events experienced after the biopsy. They received valid responses from 1663 patients which then underwent further analysis. Ekwueme et al. [ 13 ] did not describe how they collected their data on their prospective cohort other than to say their local audit committee approved it.
The systematic review by Loeb et al. [ 16 ] used the search terms Prostate Biopsy AND Complications to search PubMed and Embase retrieving 4402 records to be screened that were then narrowed down to 213 studies included in the final synthesis. 25 of these studies examined the morbidity following TPB.
Urinary retention rates
Two studies reported a potential benefit of using of an alpha-blocker in the perioperative period. Muthuveloe et al. [ 12 ] gave 59 of their 200 patients prophylactic tamsulosin and reported that the AUR rate dropped from 14.9% to 5.3% with no significant difference between the baseline characteristics of the two groups.
In their systematic review, Loeb et al. [ 16 ] found that the range of AUR varied across 24 studies examining TPB to be between 1.6 and 8.8%, with a single 25th outlier study at 20.6%. They concluded that this increase could be due to the lack of routine perioperative alpha-blockers, which was standard in the other 24 studies.
The two studies that only reported a history of, or current alpha-blocker usage demonstrated potential harm for perioperative tamsulosin on AUR rates. In the study by Ekwueme et al. [ 13 ], the patients on long-term 400 μg tamsulosin experienced urinary retention at a rate of 9.3% compared to 4.4% of those not taking an alpha-blocker. Similarly, Namekawa et al. [ 14 ] found a urinary retention rate of 19.6% compared to 12.2% in those taking alpha-blockers versus those that were not respectively.
The last study by Kum et al. [ 15 ] similarly reported that the rates of urinary retention were higher in the perioperative tamsulosin group at 13.0% compared to 0%; however, only 5 out of the 243 patients did not receive perioperative tamsulosin.
The shift away from TRUSB in recent years has largely been driven by the desire to reduce the risk of infective complications and sepsis. A national database review of 486,467 prostate biopsies in the United Kingdom National Health Service (NHS) found that the rates of sepsis had more than doubled in the two years to 2019 compared to the entire decade at 1.12% [ 17 ]. This has primarily been thought to be due to emerging fluoroquinolone resistance that has been confirmed in multiple studies and a systematic review prompting a shift towards TPB in the hope of reducing the burden of infective complications [ 2 , 16 , 18 , 19 , 20 ]. This shift has significantly dropped the morbidity related to sepsis with the procedure, however, AUR is now the leading complication after biopsy [ 21 ]. Perioperative tamsulosin is thought to reduce the rates of urinary retention through its uroselective alpha blockade mechanism.
Five studies examined the effect of the perioperative use of alpha blockers and the impact on AUR rates.
Evidence for alpha blockers preventing AUR in TPB
Muthuveloe et al. [ 12 ] in their small prospective cohort series of 200 participants, reported AUR rates dropped from 12.5% to 5.3% when perioperative tamsulosin was used as part of their biopsy procedure in 59 of 200 participants, concluding there is a benefit for their usage in TPB. Measuring the effect of alpha blockers was not a direct aim of their observational study however, and there were no formal treatment groups or randomisation to determine the prescription of alpha blockers leading to a risk of selection bias. The baseline demographics for the study were given which included age, median PSA and pre-procedural PIRADS score on MRI, however, the authors did not provide a breakdown for their alpha blocker and non-alpha blocker groups, only stating that they were not different at baseline. There were no mentions of prostate volume or long-term alpha-blocker use in the study design, which other authors have suggested may increase the likelihood of urinary retention [ 13 , 14 , 16 ]. This leads to a risk of confounding bias as there was no mention of controlling for these factors.
In their systematic review, Loeb et al. [ 16 ] also concluded that alpha blockers may be beneficial in reducing AUR in TPB. They examined 25 studies that reported on the complications following TPB as part of their study, noting that AUR rates were between 1.6% and 8.8% for 24 of the studies where alpha blockers were given in the perioperative period, with a single outlier study at 20.6% not using alpha-blockers. The limitations of this conclusion however are significant, given the studies were all performed with differing biopsy techniques, surgeons, and study populations, making a single outlier study not an unexpected finding, but still worth commenting on as part of this review given the paucity of evidence.
Evidence against alpha blockers preventing AUR in TPB
Three studies identified in this review reported a potential harmful effect for alpha blockers in reducing AUR.
In their small retrospective data analysis, Kum et al. [ 15 ] reported that their AUR was 12.8% for those taking alpha-blockers, compared to 0% for those that were not. This study was severely limited by its participant numbers and statistical power, with only 5/243 patients observed to be not taking alpha blockers in the perioperative period. AUR rates have been reported to be as low as 1–2% following biopsy, and a larger population than five would be required to power the study significantly enough to make a comment on the potential impact of alpha blockers, cautioning any conclusions drawn regarding their use here due to a potential selection bias.
Likewise, the remaining two studies in this review demonstrating the harmful effect for alpha blockers are significantly limited. Ekwueme et al. [ 13 ] and Namekawa et al. [ 14 ] reported their AUR rates increased by 111% and 61%, respectively, following the addition of alpha blockers. However, their study had patients already prescribed long-term alpha blockers before the biopsy continuing them in the perioperative period. As a result, there is a serious risk for selection and confounding bias, as these patients are more likely to enter retention at baseline given their indication for long-term alpha blocker prescription. This selection bias severely limits the conclusions that can be drawn from these two observational studies regarding prophylactic use in the perioperative period only, and the harm effect can be well explained in this setting. It is unlikely that the alpha-blockers were causing the observed increase in AUR rates.
Limitations and gaps in the literature
There remains a significant gap in the literature concerning the prophylactic use of alpha blockers to prevent AUR following TPB. According to the literature search, there have been no RCTs examining this subject. Neither have there been any observational studies directly assessing this question. The data extracted in this review is drawn from the minor findings of studies outside of their aims and thereby is susceptible to selection and information bias. Our conclusions are based on data that has been collected without methods designed to specifically limit the number of potential confounding factors and improve the validity of the results regarding the effects of alpha blockers. Randomised controlled trial comparisons are needed in this field to accurately represent this risk and reduce the risk of selection and information bias.
In addition to this, much of the observational literature around TPB already has the entire population taking perioperative alpha-blockers based on the evidence from TRUSB, meaning the potential benefit or harm cannot be assessed in that population without a control comparator. There is significant scope for an RCT to be developed to fill this gap in the literature, and to provide an evidence base to continue this practice that it appears has solely been extrapolated from TRUSB.
Predicting AUR retention risk
One study included in this review focused on assessing the risk factors for AUR following TPB. Kum et al. [ 15 ] concluded that the factors associated with an increased risk of urinary retention were patients of advanced age (> 68.7 years); those with a larger prostate volume (> 75 cc; a higher number of biopsy cores taken (> 35) and a higher international prostate symptom score (IPSS) before biopsy. These conclusions are reflected in the larger literature, with several studies also finding that a greater number of core biopsies, larger prostatic volume and older age are independent risk factors for entering AUR [ 4 , 22 , 23 , 24 ]. Patients also on long term alpha blockers are more likely to enter retention. [ 13 , 14 ]. As such, this population of patients are the most likely to benefit from alpha-blockers in terms of risk stratification and should, at the very least, be considered for a prophylactic perioperative regime.
Another early identified risk factor associated with developing AUR following TPB was the use of muscle relaxants as part of the general anaesthetic due to their anti-muscarinic effects [ 25 ]. Subsequently, it was recommended that paralytics be avoided in the procedure [ 25 ], and they have largely been phased out of practice [ 26 ].
Alpha-blocker protocol
There is no standard clinical protocol or guideline for prophylactic alpha-blockers. Muthuveloe et al. [ 12 ] used a single dose of 400 μg tamsulosin the day of the procedure to achieve their reduction in AUR rates and the published literature reports similar varying practices from single doses of 400 μg to 2-week courses of 800 μg starting two prior to biopsy [ 15 ]. Further research is required to determine the optimum protocol.
AUR remains the leading complication after TPB. This review found a significant gap in the literature regarding the evidence base for perioperative alpha-blockers to prevent AUR following TPB, however there is some weak evidence from observational studies that there may be benefit. The scope and need for an RCT remains. There is no current evidence-based alpha-blocker protocol to prevent retention and clinical practice varies significantly in terms of their usage. In terms of triaging risk, older patients with larger prostatic volumes undergoing a high number of core biopsies appear to be most at risk of entering retention and would therefore benefit the most from the use of perioperative alpha-blockers.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Abbreviations
Transperineal Prostate Biopsy
Transrectal Ultrasound Guided Prostate Biopsy
Acute Urinary Retention
Randomised Controlled Trial
National Health Service
Leslie, S.W., et al., Prostate Cancer , in StatPearls . 2024, StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.: Treasure Island (FL).
Borghesi M et al (2017) Complications after systematic, random, and image-guided prostate biopsy. Eur Urol 71(3):353–365
Article PubMed Google Scholar
Rafiq M et al (2022) Sepsis rates after template prostate biopsy with single-dose prophylactic antibiotic. Cent European J Urol 75(2):205–208
CAS PubMed PubMed Central Google Scholar
Kohl T et al (2022) Comprehensive analysis of complications after transperineal prostate biopsy without antibiotic prophylaxis: results of a multicenter trial with 30 days’ follow-up. Prostate Cancer Prostatic Dis 25(2):264–268
Article CAS PubMed Google Scholar
Stefanova V et al (2019) Transperineal prostate biopsies using local anesthesia: experience with 1,287 prostate cancer detection rate, complications and patient tolerability. J Urol 201(6):1121–1126
Thomson A et al (2020) Transperineal prostate biopsy: a review of technique. Transl Androl Urol 9(6):3009–3017
Article PubMed PubMed Central Google Scholar
Bozlu M et al (2003) Voiding impairment after prostate biopsy: does tamsulosin treatment before biopsy decrease this morbidity? Urology 62(6):1050–1053
Chung SJ et al (2015) The preventive effect of tamsulosin on voiding dysfunction after prostate biopsy: a prospective, open-label, observational study. Int Urol Nephrol 47(5):711–715
Dunn CJ, Matheson A, Faulds DM (2002) Tamsulosin: a review of its pharmacology and therapeutic efficacy in the management of lower urinary tract symptoms. Drugs Aging 19(2):135–161
Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org .
National Heart, Lung, and Blood Institute. "Study Quality Assessment Tools [ https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools ]." (2019).
Muthuveloe D et al (2016) The detection and upgrade rates of prostate adenocarcinoma following transperineal template-guided prostate biopsy - a tertiary referral centre experience. Cent European J Urol 69(1):42–47
PubMed PubMed Central Google Scholar
Ekwueme K et al (2013) Transperineal template-guided saturation biopsy using a modified technique: outcome of 270 cases requiring repeat prostate biopsy. BJU Int 111(8):E365–E373
Namekawa T et al (2015) Prospective evaluation of the safety of transrectal ultrasound-guided transperineal prostate biopsy based on adverse events. Int J Clin Oncol 20(6):1185–1191
Kum F, Jones A, Nigam R (2019) Factors influencing urinary retention after transperineal template biopsy of the prostate: outcomes from a regional cancer centre. World J Urol 37(2):337–342
Loeb S et al (2013) Systematic review of complications of prostate biopsy. Eur Urol 64(6):876–892
Tamhankar AS et al (2020) The clinical and financial implications of a decade of prostate biopsies in the NHS: analysis of Hospital Episode Statistics data 2008–2019. BJU Int 126(1):133–141
Zaytoun OM et al (2011) Emergence of fluoroquinolone-resistant Escherichia coli as cause of postprostate biopsy infection: implications for prophylaxis and treatment. Urology 77(5):1035–1041
Carignan A et al (2012) Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: time to reassess antimicrobial prophylaxis? Eur Urol 62(3):453–459
Wagenlehner FM et al (2013) Infective complications after prostate biopsy: outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur Urol 63(3):521–527
Devetzis K, Kum F, Popert R (2021) Recent advances in systematic and targeted prostate biopsies. Res Rep Urol 13:799–809
Skouteris V, Stone N (2022) MP09-05 comprehensive analysis of morbidity following transperineal prostate biopsy. J Urol 207(Supplement 5):e136
Article Google Scholar
Pepe P, Aragona F (2014) Prostate biopsy: results and advantages of the transperineal approach–twenty-year experience of a single center. World J Urol 32(2):373–377
Pepe P, Aragona F (2013) Morbidity after transperineal prostate biopsy in 3000 patients undergoing 12 vs 18 vs more than 24 needle cores. Urology 81(6):1142–1146
Willis S, Bott S, Montgomery B (2013) Urinary retention following transperineal template prostate biopsy – study of risk factors. J Clic Urology 6(1):55–58
Berry B et al (2020) Comparison of complications after transrectal and transperineal prostate biopsy: a national population-based study. BJU Int 126(1):97–103
Download references
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and affiliations.
Department of Urology, University of Melbourne, Austin Health, 145 Studley Rd, Heidelberg VIC, Melbourne, 3084, Australia
Zein Alhamdani, Damien Bolton, Lih-Ming Wong & Kapil Sethi
Department of Surgery, University of Melbourne, St Vincent’s Health, Melbourne, Australia
Samuel Poppenbeek, Lih-Ming Wong & Kapil Sethi
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the writeup and edits of the final draft.
Corresponding author
Correspondence to Zein Alhamdani .
Ethics declarations
Conflict of interest.
There are no conflict of interests in any of the authors in regards to this study.
Ethical statement
This study was conducted in line with the values of the Committee on Publication Ethics.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix 1 Search strategy
Appendix 2 inclusion and exclusion criteria, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Alhamdani, Z., Poppenbeek, S., Bolton, D. et al. Do alpha blockers reduce the risk of urinary retention post-transperineal prostate biopsy? A systematic narrative review. World J Urol 42 , 332 (2024). https://doi.org/10.1007/s00345-024-05001-5
Download citation
Received : 08 February 2024
Accepted : 16 April 2024
Published : 17 May 2024
DOI : https://doi.org/10.1007/s00345-024-05001-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Transperineal Prostate biopsy
- Urinary retention
- Alpha blockers
- Systematic review
- Uro-oncology
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.112(1); Jan-Feb 2015

Systematically Reviewing the Literature: Building the Evidence for Health Care Quality
There are important research and non-research reasons to systematically review the literature. This article describes a step-by-step process to systematically review the literature along with links to key resources. An example of a graduate program using systematic literature reviews to link research and quality improvement practices is also provided.
Introduction
Systematic reviews that summarize the available information on a topic are an important part of evidence-based health care. There are both research and non-research reasons for undertaking a literature review. It is important to systematically review the literature when one would like to justify the need for a study, to update personal knowledge and practice, to evaluate current practices, to develop and update guidelines for practice, and to develop work related policies. 1 A systematic review draws upon the best health services research principles and methods to address: What is the state of the evidence on the selected topic? The systematic process enables others to reproduce the methods and to make a rational determination of whether to accept the results of the review. An abundance of articles on systematic reviews exist focusing on different aspects of systematic reviews. 2 – 9 The purpose of this article is to describe a step by step process of systematically reviewing the health care literature and provide links to key resources.
Systematic Review Process: Six Key Steps
Six key steps to systematically review the literature are outlined in Table 1 and discussed here.
Systematic Review Steps
1. Formulate the Question and Refine the Topic
When preparing a topic to conduct a systematic review, it is important to ask at the outset, “What exactly am I looking for?” Hopefully it seems like an obvious step, but explicitly writing a one or two sentence statement of the topic before you begin to search is often overlooked. It is important for several reasons; in particular because, although we usually think we know what we are searching for, in truth our mental image of a topic is often quite fuzzy. The act of writing something concise and intelligible to a reader, even if you are the only one who will read it, clarifies your thoughts and can inspire you to ask key questions. In addition, in subsequent steps of the review process, when you begin to develop a strategy for searching the literature, your topic statement is the ready raw material from which you can extract the key concepts and terminology for your strategies. The medical and related health literature is massive, so the more precise and specific your understanding of your information need, the better your results will be when you search.
2. Search, Retrieve, and Select Relevant Articles
The retrieval tools chosen to search the literature should be determined by the purpose of the search. Questions to ask include: For what and by whom will the information be used? A topical expert or a novice? Am I looking for a simple fact? A comprehensive overview on the topic? Exploration of a new topic? A systematic review? For the purpose of a systematic review of journal research in the area of health care, PubMed or Medline is the most appropriate retrieval tool to start with, however other databases may be useful ( Table 2 ). In particular, Google Scholar allows one to search the same set of articles as PubMed/MEDLINE, in addition to some from other disciplines, but it lacks a number of key advanced search features that a skilled searcher can exploit in PubMed/MEDLINE.
Examples of Electronic Bibliographic Databases Specific to Health Care
Note: These databases may be available through university or hospital library systems.
An effective way to search the literature is to break the topic into different “building blocks.” The building blocks approach is the most systematic and works the best in periodical databases such as PubMed/MEDLINE. The “blocks” in a “building blocks” strategy consist of the key concepts in the search topic. For example, let’s say we are interested in researching about mobile phone-based interventions for monitoring of patient status or disease management. We could break the topic into the following concepts or blocks: 1. Mobile phones, 2. patient monitoring, and 3. Disease management. Gather synonyms and related terms to represent each concept and match to available subject headings in databases that offer them. Organize the resulting concepts into individual queries. Run the queries and examine your results to find relevant items and suggest query modifications to improve your results. Revise and re-run your strategy based on your observations. Repeat this process until you are satisfied or further modifications produce no improvements. For example in Medline, these terms would be used in this search and combined as follows: cellular phone AND (ambulatory monitoring OR disease management), where each of the key word phrases is an official subject heading in the MEDLINE vocabulary. Keep detailed notes on the literature search, as it will need to be reported in the methods section of the systematic review paper. Careful noting of search strategies also allows you to revisit a topic in the future and confidently replicate the same results, with the addition of those subsequently published on your topic.
3. Assess Quality
There is no consensus on the best way to assess study quality. Many quality assessment tools include issues such as: appropriateness of study design to the research objective, risk of bias, generalizability, statistical issues, quality of the intervention, and quality of reporting. Reporting guidelines for most literature types are available at the EQUATOR Network website ( http://www.equator-network.org/ ). These guidelines are a useful starting point; however they should not be used for assessing study quality.
4. Extract Data and Information
Extract information from each eligible article into a standardized format to permit the findings to be summarized. This will involve building one or more tables. When making tables each row should represent an article and each column a variable. Not all of the information that is extracted into the tables will end up in the paper. All of the information that is extracted from the eligible articles will help you obtain an overview of the topic, however you will want to reserve the use of tables in the literature review paper for the more complex information. All tables should be introduced and discussed in the narrative of the literature review. An example of an evidence summary table is presented in Table 3 .
Example of an evidence summary table
Notes: BP = blood pressure, HbA1c = Hemoglobin A1c, Hypo = hypoglycemic, I = Internet, NS = not significant, PDA = personal digital assistant, QOL = quality of life, SMBG = self-monitored blood glucose, SMS = short message service, V = voice
5. Analyze and Synthesize Data and information
The findings from individual studies are analyzed and synthesized so that the overall effectiveness of the intervention can be determined. It should also be observed at this time if the effect of an intervention is comparable in different studies, participants, and settings.
6. Write the Systematic Review
The PRISMA 12 and ENTREQ 13 checklists can be useful resources when writing a systematic review. These uniform reporting tools focus on how to write coherent and comprehensive reviews that facilitate readers and reviewers in evaluating the relative strengths and weaknesses. A systematic literature review has the same structure as an original research article:
TITLE : The systematic review title should indicate the content. The title should reflect the research question, however it should be a statement and not a question. The research question and the title should have similar key words.
STRUCTURED ABSTRACT: The structured abstract recaps the background, methods, results and conclusion in usually 250 words or less.
INTRODUCTION: The introduction summarizes the topic or problem and specifies the practical significance for the systematic review. The first paragraph or two of the paper should capture the attention of the reader. It might be dramatic, statistical, or descriptive, but above all, it should be interesting and very relevant to the research question. The topic or problem is linked with earlier research through previous attempts to solve the problem. Gaps in the literature regarding research and practice should also be noted. The final sentence of the introduction should clearly state the purpose of the systematic review.
METHODS: The methods provide a specification of the study protocol with enough information so that others can reproduce the results. It is important to include information on the:
- Eligibility criteria for studies: Who are the patients or subjects? What are the study characteristics, interventions, and outcomes? Were there language restrictions?
- Literature search: What databases were searched? Which key search terms were used? Which years were searched?
- Study selection: What was the study selection method? Was the title screened first, followed by the abstract, and finally the full text of the article?
- Data extraction: What data and information will be extracted from the articles?
- Data analysis: What are the statistical methods for handling any quantitative data?
RESULTS: The results should also be well-organized. One way to approach the results is to include information on the:
- Search results: What are the numbers of articles identified, excluded, and ultimately eligible?
- Study characteristics: What are the type and number of subjects? What are the methodological features of the studies?
- Study quality score: What is the overall quality of included studies? Does the quality of the included studies affect the outcome of the results?
- Results of the study: What are the overall results and outcomes? Could the literature be divided into themes or categories?
DISCUSSION: The discussion begins with a nonnumeric summary of the results. Next, gaps in the literature as well as limitations of the included articles are discussed with respect to the impact that they have on the reliability of the results. The final paragraph provides conclusions as well as implications for future research and current practice. For example, questions for future research on this topic are revealed, as well as whether or not practice should change as a result of the review.
REFERENCES: A complete bibliographical list of all journal articles, reports, books, and other media referred to in the systematic review should be included at the end of the paper. Referencing software can facilitate the compilation of citations and is useful in terms of ensuring the reference list is accurate and complete.
The following resources may be helpful when writing a systematic review:
CEBM: Centre for Evidence-based Medicine. Dedicated to the practice, teaching and dissemination of high quality evidence based medicine to improve health care Available at: http://www.cebm.net/ .
CITING MEDICINE: The National Library of Medicine Style Guide for Authors, Editors, and Publishers. This resource provides guidance in compiling, revising, formatting, and setting reference standards. Available at http://www.ncbi.nlm.nih.gov/books/NBK7265/ .
EQUATOR NETWORK: Enhancing the QUAlity and Transparency Of health Research. The EQUATOR Network promotes the transparent and accurate reporting of research studies. Available at: http://www.equator-network.org/ .
ICMJE RECOMMENDATIONS: International Committee of Medical Journal Editors Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals. The ICJME recommendations are followed by a large number of journals. Available at: http://www.icmje.org/about-icmje/faqs/icmje-recommendations/ .
PRISMA STATEMENT: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. Authors can utilize the PRISMA Statement checklist to improve the reporting of systematic reviews and meta-analyses. Available at: http://prisma-statement.org .
THE COCHRANE COLLABORATION: A reliable source for making evidence generated through research useful for informing decisions about health. Available at: http://www.cochrane.org/ .
Examples of Systematic Reviews To Link Research and Quality Improvement
Over the past 17 years more than 300 learners, including physicians, nurses, and health administrators have completed a course as part of a Master of Health Administration or a Master of Science in Health Informatics degree at the University of Missouri. An objective of the course is to educate health informatics and health administration professionals about how to utilize a systematic, scientific, and evidence-based approach to literature searching, appraisal, and synthesis. Learners in the course conduct a systematic review of the literature on a health care topic of their choosing that could suggest quality improvement in their organization. Students select topics that make sense in terms of their core educational competencies and are related to their work. The categories of topics include public health, leadership, information management, health information technology, electronic medical records, telehealth, patient/clinician safety, treatment/screening evaluation cost/finance, human resources, planning and marketing, supply chain, education/training, policies and regulations, access, and satisfaction. Some learners have published their systematic literature reviews 14 – 15 . Qualitative comments from the students indicate that the course is well received and the skills learned in the course are applicable to a variety of health care settings.
Undertaking a literature review includes identification of a topic of interest, searching and retrieving the appropriate literature, assessing quality, extracting data and information, analyzing and synthesizing the findings, and writing a report. A structured step-by-step approach facilitates the development of a complete and informed literature review.
Suzanne Austin Boren, PhD, MHA, (above) is Associate Professor and Director of Academic Programs, and David Moxley, MLIS, is Clinical Instructor and Associate Director of Executive Programs. Both are in the Department of Health Management and Informatics at the University of Missouri School of Medicine.
Contact: ude.iruossim.htlaeh@snerob

None reported.

IMAGES
VIDEO
COMMENTS
Method details Overview. A Systematic Literature Review (SLR) is a research methodology to collect, identify, and critically analyze the available research studies (e.g., articles, conference proceedings, books, dissertations) through a systematic procedure [12].An SLR updates the reader with current literature about a subject [6].The goal is to review critical points of current knowledge on a ...
Literature reviews establish the foundation of academic inquires. However, in the planning field, we lack rigorous systematic reviews. In this article, through a systematic search on the methodology of literature review, we categorize a typology of literature reviews, discuss steps in conducting a systematic literature review, and provide suggestions on how to enhance rigor in literature ...
The best reviews synthesize studies to draw broad theoretical conclusions about what a literature means, linking theory to evidence and evidence to theory. This guide describes how to plan, conduct, organize, and present a systematic review of quantitative (meta-analysis) or qualitative (narrative review, meta-synthesis) information.
A systematic review is a type of review that uses repeatable methods to find, select, and synthesize all available evidence. ... Systematic review vs. literature review. A literature review is a type of review that uses a less systematic and formal approach than a systematic review. Typically, an expert in a topic will qualitatively summarize ...
Image by TraceyChandler. Steps to conducting a systematic review. Quick overview of the process: Steps and resources from the UMB HSHSL Guide. YouTube video (26 min); Another detailed guide on how to conduct and write a systematic review from RMIT University; A roadmap for searching literature in PubMed from the VU Amsterdam; Alexander, P. A. (2020).
Screen the literature. Assess the quality of the studies. Extract the data. Analyze the results. Interpret and present the results. 1. Decide on your team. When carrying out a systematic literature review, you should employ multiple reviewers in order to minimize bias and strengthen analysis.
1.1 Why do a systematic review? Systematic reviews were developed out of a need to ensure that decisions affecting people's lives can be informed by an up-to-date and complete understanding of the relevant research evidence. With the volume of research literature growing at an ever-increasing rate, it is impossible for individual decision ...
Systematic reviews involve the application of scientific methods to reduce bias in review of literature. The key components of a systematic review are a well-defined research question, comprehensive literature search to identify all studies that potentially address the question, systematic assembly of the studies that answer the question, critical appraisal of the methodological quality of the ...
How to Perform a Systematic Literature Review A Guide for Healthcare Researchers, Practitioners and Students. ... The systematic review is a rigorous method of collating and synthesizing evidence from multiple studies, producing a whole greater than the sum of parts. This textbook is an authoritative and accessible guide to an activity that is ...
systematic'' reviews which do not have these features, and which are sometimes also described as a ''conven-tional literature review,''53 ''scoping review,''54 or ''nar-rative review.''55 In the past, there has been considerable confusion and inconsistency in the termin-ology used around systematic reviews,56 in part ...
A systematic review is a type of study that synthesises research that has been conducted on a particular topic. Systematic reviews are considered to provide the highest level of evidence on the hierarchy of evidence pyramid. Systematic reviews are conducted following rigorous research methodology. To minimise bias, systematic reviews utilise a ...
Systematic reviews are characterized by a methodical and replicable methodology and presentation. They involve a comprehensive search to locate all relevant published and unpublished work on a subject; a systematic integration of search results; and a critique of the extent, nature, and quality of evidence in relation to a particular research question. The best reviews synthesize studies to ...
A review earns the adjective systematic if it is based on a clearly formulated question, identifies relevant studies, appraises their quality and summarizes the evidence by use of explicit methodology. It is the explicit and systematic approach that distinguishes systematic reviews from traditional reviews and commentaries.
A well-designed systematic review includes clear objectives, pre-selected criteria for identifying eligible studies, an explicit methodology, a thorough and reproducible search of the literature, an assessment of the validity or risk of bias of each included study, and a systematic synthesis, analysis and presentation of the findings of the ...
1. Decide on your team. When carrying out a systematic literature review, you should employ multiple reviewers in order to minimize bias and strengthen analysis. A minimum of two is a good rule of thumb, with a third to serve as a tiebreaker if needed. 2.
Systematic literature reviews (SRs) are a way of synthesising scientific evidence to answer a particular research question in a way that is transparent and reproducible, while seeking to include all published evidence on the topic and appraising the quality of th is evidence. SRs have become a major methodology
Abstract. Performing a literature review is a critical first step in research to understanding the state-of-the-art and identifying gaps and challenges in the field. A systematic literature review is a method which sets out a series of steps to methodically organize the review. In this paper, we present a guide designed for researchers and in ...
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
A systematic literature review is a research methodology designed to answer a focused research question. Authors conduct a methodical and comprehensive literature synthesis focused on a well-formulated research question. Its aim is to identify and synthesize all of the scholarly research on a particular topic, including both published and ...
Literature reviews can be narrative or systematic, with narrative reviews aiming to provide a descriptive overview of selected literature, without undertaking a systematic literature search. By contrast, systematic reviews use explicit and replicable methods in order to retrieve all available literature pertaining to a specific topic to answer ...
What is a literature review? Definition: A literature review is a systematic examination and synthesis of existing scholarly research on a specific topic or subject. Purpose: It serves to provide a comprehensive overview of the current state of knowledge within a particular field. Analysis: Involves critically evaluating and summarizing key findings, methodologies, and debates found in ...
A systematic review collects all possible studies related to a given topic and design, and reviews and analyzes their results [ 1 ]. During the systematic review process, the quality of studies is evaluated, and a statistical meta-analysis of the study results is conducted on the basis of their quality. A meta-analysis is a valid, objective ...
Systematic literature reviews (SRs) are a way of synt hesising scientific evidence to answer a particular. research question in a way that is transparent and reproducible, while seeking to include ...
Systematic Review. Attempts to identify, appraise, and summarize all empirical evidence that fits pre-specified eligibility criteria to answer a specific research question. clearly defined question with inclusion/exclusion criteria. rigorous and systematic search of the literature. thorough screening of results. data extraction and management.
Literature searches will be conducted using multiple databases by a librarian with expertise in scoping reviews. Through an iterative process, inclusion and exclusion criteria will be developed and a data extraction form refined. ... (UGME). A related systematic review by van Houten-Schat et al. on SRL in the clinical setting identified three ...
You do not have access to any existing collections. You may create a new collection. ... A Systematic Literature Review. Study Purpose: The purpose of this systemic literature review was to explore how stress from having parents who are unauthorized immigrants impacts the likelihood of US born children (ages 5 to 19) of developing depression or ...
A systematic review is a summary of the medical literature that uses explicit and reproducible methods to systematically search, critically appraise, and synthesize on a specific issue. It synthesizes the results of multiple primary studies related to each other by using strategies that reduce biases and random errors.[ 7 ]
In order to understand the body of works that emerged from the global south region and their area of focus in computing education, we conducted a systematic review of the literature. This research investigates the prominent CER journals and conferences to discern the kind of research that has been published and how much contribution they have ...
A previous systematic review of complications associated with prostate biopsy concluded there may be a potential benefit to alpha-blockers given in the TPB perioperative period. ... is significant scope and need for an RCT to further develop the evidence base further given the significant gap in the literature and lack of a standard alpha ...
Systematic reviews that summarize the available information on a topic are an important part of evidence-based health care. There are both research and non-research reasons for undertaking a literature review. It is important to systematically review the literature when one would like to justify the need for a study, to update personal ...