Breast cancer nursing interventions and clinical effectiveness: a systematic review
Affiliations.
- 1 Division of Cancer Services, Princess Alexandra Hospital, Metro South Health, Woolloongabba, Queensland, Australia [email protected].
- 2 School of Nursing and Cancer and Palliative Care Outcomes Centre, Queensland University of Technology, Brisbane, Queensland, Australia.
- 3 School of Medicine, Griffith University, Brisbane, Queensland, Australia.
- 4 McGrath Foundation, North Sydney, New South Wales, Australia.
- PMID: 32499405
- DOI: 10.1136/bmjspcare-2019-002120
Objectives: To examine the effects of nurse-led interventions on the health-related quality of life, symptom burden and self-management/behavioural outcomes in women with breast cancer.
Methods: Cochrane Controlled Register of Trials (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Medline and Embase databases were searched (January 1999 to May 2019) to identify randomised controlled trials (RCTs) and controlled before-and-after studies of interventions delivered by nurses with oncology experience for women with breast cancer. Risk of bias was evaluated using the revised Cochrane risk-of-bias tool for randomised trials . Intervention effects were synthesised by cancer trajectory using The Omaha System Intervention Classification Scheme .
Results: Thirty-one RCTs (4651 participants) were included. All studies were at risk of bias mainly due to inherent limitations such as lack of blinding and self-report data. Most studies (71%; n=22) reported at least one superior intervention effect. There were no differences in all outcomes between those who receive nurse-led surveillance care versus those who received physical led or usual discharge care. Compared with control interventions, there were superior teaching, guidance and counselling (63%) and case management (100%) intervention effects on symptom burden during treatment and survivorship. Effects of these interventions on health-related quality of life and symptom self-management/behavioural outcomes were inconsistent.
Discussion: There is consistent evidence from RCTs that nurse-led surveillance interventions are as safe and effective as physician-led care and strong evidence that nurse-led teaching, guidance and counselling and case management interventions are effective for symptom management. Future studies should ensure the incorporation of health-related quality of life and self-management/behavioural outcomes and consider well-designed attentional placebo controls to blind participants for self-report outcomes.
Protocol registration: The International Prospective Register of Systematic Reviews (PROSPERO): CRD42020134914).
Keywords: breast; quality of life; survivorship; symptoms and symptom management.
© Author(s) (or their employer(s)) 2020. No commercial re-use. See rights and permissions. Published by BMJ.

Publication types
- Systematic Review
- Breast Neoplasms / nursing*
- Disease Management
- Hospice and Palliative Care Nursing / methods*
- Palliative Care / methods*
- Practice Patterns, Nurses' / statistics & numerical data*
- Quality of Life*
- Randomized Controlled Trials as Topic
- Treatment Outcome
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Current issue
- Instructions for Authors
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 10, Issue 3
- Breast cancer nursing interventions and clinical effectiveness: a systematic review
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0003-0248-7046 Raymond Javan Chan 1 , 2 ,
- Laisa Teleni 2 ,
- Suzanne McDonald 2 ,
- Jaimon Kelly 3 ,
- Jane Mahony 4 ,
- Kerryn Ernst 4 ,
- Kerry Patford 4 ,
- James Townsend 4 ,
- Manisha Singh 4 and
- Patsy Yates 2
- 1 Division of Cancer Services , Princess Alexandra Hospital, Metro South Health , Woolloongabba , Queensland , Australia
- 2 School of Nursing and Cancer and Palliative Care Outcomes Centre , Queensland University of Technology , Brisbane , Queensland , Australia
- 3 School of Medicine , Griffith University , Brisbane , Queensland , Australia
- 4 McGrath Foundation , North Sydney , New South Wales , Australia
- Correspondence to Professor Raymond Javan Chan, Division of cancer Services, Princess Alexandra Hospital, Woolloongabba, QLD 4029, Australia; Raymond.Chan{at}qut.edu.au
Objectives To examine the effects of nurse-led interventions on the health-related quality of life, symptom burden and self-management/behavioural outcomes in women with breast cancer.
Methods Cochrane Controlled Register of Trials (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Medline and Embase databases were searched (January 1999 to May 2019) to identify randomised controlled trials (RCTs) and controlled before-and-after studies of interventions delivered by nurses with oncology experience for women with breast cancer. Risk of bias was evaluated using the revised Cochrane risk-of-bias tool for randomised trials . Intervention effects were synthesised by cancer trajectory using The Omaha System Intervention Classification Scheme .
Results Thirty-one RCTs (4651 participants) were included. All studies were at risk of bias mainly due to inherent limitations such as lack of blinding and self-report data. Most studies (71%; n=22) reported at least one superior intervention effect. There were no differences in all outcomes between those who receive nurse-led surveillance care versus those who received physical led or usual discharge care. Compared with control interventions, there were superior teaching, guidance and counselling (63%) and case management (100%) intervention effects on symptom burden during treatment and survivorship. Effects of these interventions on health-related quality of life and symptom self-management/behavioural outcomes were inconsistent.
Discussion There is consistent evidence from RCTs that nurse-led surveillance interventions are as safe and effective as physician-led care and strong evidence that nurse-led teaching, guidance and counselling and case management interventions are effective for symptom management. Future studies should ensure the incorporation of health-related quality of life and self-management/behavioural outcomes and consider well-designed attentional placebo controls to blind participants for self-report outcomes.
Protocol registration The International Prospective Register of Systematic Reviews (PROSPERO): CRD42020134914).
- quality of life
- survivorship
- symptoms and symptom management
https://doi.org/10.1136/bmjspcare-2019-002120
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Breast care nurses (BCNs) are a specialist workforce to support people with breast cancer through their disease experience, predominantly in well-resourced healthcare systems. 1 BCNs were initially introduced into the health systems in the UK, Australia, USA and parts of Europe over the last three decades to facilitate continuity of care and psychosocial support. 2 3 Yates et al defined the BCN as ‘a registered nurse who applies advanced knowledge of the health needs, preferences and circumstances of women with breast cancer to optimise the individual’s health and well-being at various phases across the continuum of care, including diagnosis, treatment, rehabilitation, follow-up and palliative care’. 4
As early as the introduction of BCNs, there was a recognition that the model of care for BCNs should be informed by the best available evidence. 2 5 At the early stage of implementation, there were a few randomised controlled trials (RCTs) 6–10 providing direct evidence to inform the model of care. 5 In addition to the evidence from these RCTs, the model was also informed by evidence-based recommendations, relevant management guidelines and training curricula available at the time. 2 3
Over the past 20 years, there have been significant changes in the landscape of breast cancer treatment. People with a breast cancer diagnosis are now living longer, especially among those with early stage disease. In developed countries, the overall 5-year relative survival rate is approximately 90% in the USA, UK and Australia. 11 A 2008 Cochrane review of five randomised studies examined the effects of supportive care provided by specialist BCNs, three of which tested psychosocial interventions during diagnosis and early treatment and one study each that tested supportive care during radiotherapy and post-treatment follow-up interventions. 1 The review concluded that there was limited evidence to inform BCNs’ model of care. 1 Over the past 10 years, the literature has grown enormously. A recent scoping review conducted by Charalambous et al 12 identified 214 RCT, quasi-RCT and controlled before-and-after (CBA) studies assessing the impacts of nurse-led interventions offered to patients with any cancer diagnosis. 12 However, this review did not use systematic review methods and was not breast cancer specific. Therefore, the findings were not directly relevant for understanding the BCN’s role in supporting patients with breast cancer. A systematic review of the current literature examining the effects of nurse-led interventions for people with breast cancer is warranted, to inform the evolution of the BCN model within Australia and internationally.
This was a systematic review of RCTs and high-quality CBA studies (study designs as defined by the Cochrane Effective Practice Organisation of Care) 13 that examined the effects of interventions provided by specialist cancer nurses to patients with breast cancer. This review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement 14 (protocol registration: The International Prospective Register of Systematic Reviews (PROSPERO): CRD42020134914).
Search strategy
A complete list of searches is listed in online supplementary A . The search terms were devised by the study authors. Cochrane CENTRAL, CINAHL, Medline and Embase databases were searched up to May 2019. The search strategy was adapted for use with each bibliographic database and included Medical Subject Headings (MeSH) terms, where available, and keywords relating to the population (breast care/cancer nurses) and any structured interventions (eg, nurse-led services, psychoeducational programmes, cognitive–behavioural therapies and symptom management programmes). The search was limited to English articles published in the past 20 years (1999–2019) to account for significant changes in treatment and patient experiences that have occurred over the past 20 years due to the advances in treatment options and increased expectation of the specialist cancer nurse role. Reference lists of relevant articles were hand searched, and authors of any included articles were searched for additional articles.
Supplemental material
Selection criteria.
Studies were included where: (1) interventionists were nurses with experience in oncology; (2) the effects of interventions delivered by these nurses could be isolated and evaluated against a comparison group (ie, usual care or other control); (3) the population was patients with breast cancer (and their family members) or the healthcare system. Studies of patients with mixed cancer diagnoses were only included if ≥80% of the study sample comprised patients with breast cancer or where the results for patients with breast cancer were reported separately. Studies that focused on family members and excluded patients, or studies where the effects of the nurse interventionist could not be separated from other interventionists, were excluded. Outcomes of interest were guided by a systematic review examining the effects of nurse-led services in chronic disease management. 15 These included any valid measures of patient-reported outcomes (ie, health-related quality of life (HRQoL), symptom burden, self-management and behaviour), survival and measures of patient and/or family member satisfaction or perception of the quality of care, service delivery and healthcare professional satisfaction.
Data collection
Records identified by the search strategy were imported into Endnote and duplicates were removed. Titles and abstracts of records were independently screened for relevance by two reviewers (SM and LT). Full reports of all potentially relevant trials were then retrieved independently assessed for eligibility based on the inclusion criteria by two reviewers (LT and SM). Disagreements were resolved in consultation between the two reviewers, or with a third reviewer (RC) where consensus could not be reached. Reference lists of articles were screened for additional potentially relevant references.
A standardised data extraction template was piloted for use with three studies and refined before extracting data from the remaining included studies. Data extracted included study design, aim of the study, setting or nature of healthcare service, location, characteristics of the nurse interventionists, key components of the intervention (dose, administration and content), length of the study, outcome measures, data collection methods, reported results (specific to the primary and secondary outcomes), reported conclusions, strengths and limitations. Data were extracted by one reviewer (LT) and cross checked for accuracy by a second reviewer (SM).
Data synthesis
Outcomes were not pooled as the outcomes were not considered comparable across trials. Given the clinical heterogeneity across the studies with a number of different study populations (pre, during and post various treatment types, and survivorship), a narrative approach to synthesis was used. Mean difference for continuous data or ORs for categorical data and 95% CIs were calculated between intervention and control groups at the study endpoint where authors reported significant findings ( online supplementary table 2 ). All studies were categorised by stage of cancer trajectory in which the intervention was delivered (ie, diagnosis, treatment, survivorship, end of life) and intervention and problem classification schemes using the Omaha System 16 with additional descriptors provided by Charalambous et al 12 to guide decisions. The Omaha System 16 provides a taxonomy for classifying interventions and categorising problems addressed by these interventions. In addition to the guidance provided by the scheme, we categorised interventions into the four categories ( treatments and procedures, case management, surveillance, and teaching, guidance and counselling ). The Omaha System 16 categorises problems into four domains: environmental (ie, material resource, physical surroundings), psychosocial (ie, behaviour, emotion, communication and relationships), physiological (ie, functions and processes that maintain life) and health-related behaviours (ie, patterns of activity that maintain wellness, recovery or decrease risk of disease).
Risk of bias
Risk of bias was assessed using the revised Cochrane risk-of-bias tool for randomised trials (RoB 2, Version 15 March 2019), 17 which evaluates risk of bias arising from the following five domains: (1) randomisation process; (2) deviations from the intended interventions; (3) missing outcome data; (4) measurement of the outcome and (5) selection of the reported result. Each domain was assigned a risk of bias (low risk, some concerns or high risk) based on the domain algorithm and an overall judgement (low risk, some concerns or high risk) was made using the described criteria. Two reviewers independently assessed the risk of bias for each included study (SM and JK). Disagreements were resolved by a third reviewer (LT).
Overview of included studies
A total of 39 articles reporting 31 RCTs (4651 participants, range 18–408) and no CBA studies were included in this review ( figure 1 ) from the initial search, with no new articles identified from the snowball search. Characteristics of included studies and interventions are summarised in online supplementary table 1 and the results of included studies are summarised in online supplementary table 2 . Studies were predominantly conducted in the USA (n=10), UK (n=4), Australia (n=3) and Sweden (n=3). The aims of studies were to establish the effectiveness, 18–44 pilot test the feasibility or acceptability, 45–49 and/or to conduct an economic evaluation 50–53 of the intervention(s).
- Download figure
- Open in new tab
- Download powerpoint
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram showing the selection of studies. BC, breast cancer; RCT, randomised controlled trial.
Interventionist experience, qualifications and training
All included studies indicated that the nurse interventionist(s) had experience in cancer care with 10 studies reporting specific experience in breast cancer care ( online supplementary table 1 ). Five studies reported the actual or minimum required years of experience in cancer care and/or breast cancer care (range 3–20 years). 34 38 43 44 46 Nurse interventionist qualifications were described in four studies, two included masters-prepared nurses, 36 46 one described the interventionist requirements as high diploma degree in nursing or higher degree 44 and one interventionist was the primary author whose qualifications were listed as RN and PhD. 41 Sixteen studies described interventionist training. 19 23–25 27–31 33 34 36 40 43 47 Training content included administering and adhering to the intervention and/or research protocols, human research ethics and evidence-based practice updates. Trainer expertise was described in two studies 23 25 (ie, specialist nurses, oncologists clinical psychologist) and five studies described training duration (range 8 hours to 12 months). 19 25 33 34 43
The risk of bias in each study is outlined in table 1 . All studies were judged to be at high overall risk of bias for at least one outcome. Across all studies, the lowest risk of bias was in the randomisation process. The domains with the highest risk of bias across all studies were measurement of the outcome, missing outcome data and deviations from interventions. The largest contributors to risk of bias was lack of information about missing data and its potential impact on the true value of the outcome and the impact of the lack of blinding on self-report outcomes.
- View inline
Risk-of-bias judgements of included studies
Intervention effects
A heat map of intervention effects by Omaha intervention category and cancer trajectory is summarised in table 2 . Twenty-two studies (71%) reported at least one superior nurse-led intervention effect ( table 2 ). Of the studies that reported superior nurse-led intervention effects on HRQoL (32%) and that measured secondary outcomes, all had at least 1 and 83% had at least 2 superior effects on secondary outcomes ( online supplementary tables 3–9 ). Eighteen RCTs were conducted during treatment, 12 during survivorship and 1 conducted from diagnosis to survivorship. Problem domains were psychosocial (n=10), health-related behaviours (n=8) or a combination of both (n=12) with one study also including physiological . Most of the study interventions were categorised as teaching, guidance and counselling (n=19; 2569 participants). All teaching, guidance and counselling interventions were structured and delivered by at least one (range: 1–7) oncology nurse. These interventions were mostly delivered individually with the exception of two group education interventions (254 total participants). 31 39 Five study interventions were categorised as case management (606 participants) and compared with usual care. Three interventions were unstructured, requiring patient-initiated contact 20 40 48 and two were structured 44 46 interventions delivered face-to-face, by telephone and/or online by an experienced oncology nurse. In addition to case management, two interventions included counselling and education. Seven study interventions were categorised as surveillance (1476 participants). These interventions replaced usual follow-up care with nurse-led follow-up care with the exception of Wells et al 50 who replaced usual hospital postoperative discharge with nurse-led early discharge. Surveillance interventions were predominantly delivered by telephone (n=6) and unstructured (n=4) requiring patient-initiated contact with a BCN or other experienced oncology nurse. There were no treatment and procedure interventions.
The distribution of intervention effects by Omaha system intervention category on all health-related outcomes across the cancer trajectory
During treatment
The most tested interventions during treatment were teaching, guidance and counselling (n=15; 1987 participants). Of these, three tested interventions in addition to usual care and compared with a usual care 18 42 or other non-descript control 38 and 12 lacked sufficient information to judge whether usual care was part of the intervention arm(s). Of these 12 RCTs, 6 were compared with usual care, 19 23 25 27 39 47 5 compared with attentional controls or other interventions 24 28–30 43 and 1 did not describe the control condition. 21 No intervention was inferior to control for any outcome and 67% (n=10) were superior in at least one outcome. Superior intervention effects were most common for symptom resolution or overall burden (n=8, 67%). Superior effects were also reported in 60% (n=4) of the studies that measured self-management/behavioural outcomes and 33% (n=4) of the studies that measured HRQoL. One RCT that compared cognitive–behavioural therapy delivered by a nurse versus cognitive–behavioural therapy delivered by a psychologist versus usual care reported greater patient satisfaction in the intervention groups, with greater perceived benefits from the nurse-led intervention. 26 Moreover, this study reported that usual care incurred higher hospitalisation and total healthcare costs. 54
Case management intervention studies evaluated in 2 studies of 300 participants collectively were compared with usual care. In one study, the intervention was delivered in addition to usual care 44 while the other study was unclear. 40 Both studies reported superior effects on symptom burden outcomes, equivocal effects on self-management/behavioural outcomes and one study reported equivocal effects on HRQoL. One RCT evaluated health service/resource use and costs throughout four or six cycles of chemotherapy. 44 53 In those undergoing four cycles of chemotherapy, intervention participants incurred a higher per patient cost with an equivocal quality-adjusted life years (QALYs) compared with usual care. In comparison, those undergoing six cycles, intervention participants incurred lower per patient costs and fewer emergency department visits, but fewer QALYs compared with usual care.
One surveillance intervention study (108 participants) 50 compared early nurse-led postoperative discharge with usual hospital discharge. Wells et al 50 reported equivocal effects of the early nurse-led discharge for HRQoL, symptom burden and self-management/behavioural outcomes. However, nurse-led postoperative early discharge saved 2.26 bed days per patient and was associated with a reduction in cancellations of 2.9 per month and subsequent cost savings. Moreover, Wells et al 50 reported that significantly more interventions participants would opt for the same care again.
During survivorship
The most tested interventions during survivorship were surveillance (n=6, 1368 participants), all of which compared nurse-led follow-up care (including annual mammography) with usual physician-led follow-up care. Four surveillance interventions relied on patient-initiated contacts and were delivered via telephone 22 34 49 or combined telephone/face to face 51 and two mimicked usual physician follow-up care as per study site. Irrespective of the mode or structure of follow-up care, nurse-led intervention effects were equivocal to physician-led care for HRQoL (n=4), symptom burden (n=6), self-management/behavioural outcomes (n=1) and 5-year time to all-cause death (n=1; table 1 ). Moreover, of the three studies that measured patient satisfaction with care, only Beaver et al 33 found significantly greater patient satisfaction in the nurse-led intervention arm compared with usual care. In terms of health service/resource use and costs, Koinberg et al 51 reported that fewer visits to the physician resulted in lower per patient cost. Similarly, Kimman et al 36 compared nurse led with usual follow-up care with and without a group education programme and reported that nurse-led follow-up care combined with the exercise education was the most cost effective in terms of QALYs.
There were four teaching, guidance and counselling interventions (582 participants). Although most studies reported significant improvements in symptom burden (three of four studies) and self-management/behaviour (two of three studies), only two of four studies reported improvements in HRQoL, one of which was not sustained at final follow-up of 16 weeks ( table 1 ). Three studies aimed to improve symptoms using counselling and exercise, 31 cognitive–behavioural therapy 41 or psychoeducation 45 and one tested the effect of a nurse-developed survivorship care plan in conjunction with general practitioner (GP)-led care. 37 Only one study 37 measured patient satisfaction with care, reporting that satisfaction with coordination and continuity of care was equivocal between those under GP-led follow-up care with and without a nurse-developed supportive care plan.
There were two case management interventions (95 participants). Mertz et al 48 replaced usual rehabilitation care with nurse-led follow-up and counselling and reported significant improvements in symptom burden (ie, distress, anxiety, depression) and satisfaction with treatment and rehabilitation, but no difference in HRQoL. Kim et al 46 conducted a telephone-based diet and exercise interventions matched to the patient’s stage of change. Compared with usual care, there were significantly greater improvements in symptom burden, stages of change and emotional functioning. However, the intervention had equivocal effects on all other HRQoL measures, anxiety and physical activity compared with usual care.
Diagnosis to survivorship
One study was conducted from diagnosis through to survivorship, testing a 2-year nurse-led case management intervention (211 participants) compared with usual care. 20 The nurse-led case management had superior effects on disease-specific HRQoL, but only for unmarried women at 1 month; otherwise, the effects were equivocal to usual care over 12 months for the unmarried subgroup and entire participant. Improvements in mood disturbance were greater in the intervention arm at 1 and 3 months, but this difference was only sustained to 6 months in women with no family history of breast cancer. There was no difference in costs between nurse-led intervention and usual care, and most (75%) of the intervention nurse-related costs and time was attributable to the first 6 months of the intervention. Patient satisfaction was not investigated in this study.
To the best of our knowledge, this is the largest systematic review examining high-level evidence of the effectiveness of interventions delivered by nurses with experience in oncology for patients across the postdiagnosis breast cancer trajectory, including 31 original research studies of 4651 participants. While several reviews of nurse-led interventions in cancer exist in the literature, 1 55–58 this review focusses on interventions delivered to patients with breast cancer by nurses with oncology experience or expertise for relevant outcomes guided by taxonomy developed by Chan et al . 15 Using the Omaha intervention categorisation system, 16 we identified three intervention categories implemented during breast cancer treatment and survivorship or over the entire breast cancer trajectory. Compared to the 2008 Cochrane review, 1 our review not only provides inclusion of new studies published over the past 12 years, it also has a wider inclusion of studies that are delivered by any nurses with oncology experience, as opposed to only those with a specialist title of BCNs.
The most tested intervention was teaching, guidance and counselling in this review. This finding is consistent with Charalambous et al 12 scoping review that reported teaching, guidance and counselling interventions are most tested in both breast cancer and during treatment in any cancer. However, Charalambous’s review had a wide focus including all cancer types and did not specifically report effectiveness outcomes for these interventions for patients with breast cancer. 12 The current review suggests that during treatment, most teaching, guidance and counselling intervention studies reported at least one superior outcome (67%) including HRQoL (n=4), symptom burden (n=8), self-management/behavioural outcomes (n=4), patient satisfaction (n=4) and health service/resource use and costs outcomes (n=1).
Similar to teaching, guidance and counselling interventions, there is consistent evidence from RCTs that case management is effective for managing symptom burden in patients with breast cancer whether during treatment, 40 44 survivorship 46 48 or across the entire cancer trajectory. 20 The effectiveness of case management interventions on HRQoL and self-management/behaviour is less clear with superior intervention effects reported in 50% (n=2) and 25% (n=1), respectively. One study measured and reported greater patient satisfaction with treatment and rehabilitation in the nurse-led intervention group compared with usual care and two studies reported no overall difference in health service/resource use or costs outcomes. Thus far, evidence of effectiveness from the wider care coordination literature is conflicting. In a systematic review including 59 studies of patients with mixed-cancer types, it was reported that care coordination had no effect on HRQoL and symptom burden, but that patients with coordinated care felt more satisfied. 59 A more recent systematic review of observational studies and RCTs (n=52) in patients with cancer reported that coordination improved 81% of outcomes in patients and resulted in significantly higher odds of appropriate healthcare utilisation. 60
Surveillance interventions were the second most tested intervention in breast cancer and the most tested during survivorship and exclusively involved nurse-led follow-up care or early postoperative discharge instead of usual follow-up care or hospital discharge, respectively. The included RCTs consistently reported that nurse-led surveillance interventions in the survivorship phase were comparable to physician-led clinics for HRQoL, 22 34 36 49 50 symptom burden, 22 32–34 36 49 50 survival 32 and self-management/behavioural outcomes. 36 50 These studies also indicated that nurse-led follow-up care had greater health service/resource use and cost benefits 32 36 50 and patient satisfaction with care. 33 50 It is important to ensure that any nurse-led or shared-care model capitalises on the full potential of a nurse interventionist by preparing nurses to incorporate concurrent teaching, guidance and counselling and surveillance interventions. However, future studies should consider the additional preparation of nurses when evaluating cost effectiveness.
We did not identify any study with an overall inferior intervention effect for any outcome; however, one study did have conflicting health utility/costs outcomes. Lai et al 53 reported lower per person costs, but significantly fewer QALY health gains in the nurse-led case management intervention group that included counselling for self-care strategies and psychosocial support compared with usual care in a subgroup of patients receiving six cycles of chemotherapy, but not four cycles. However, this was a feasibility study and the authors recognise the limitations of a relatively small sample size (n=124) and the potential underestimation of QALY benefit from using a transformed quality of life score.
Similar to a previous review that had a broader focus of nurse-led services in all chronic conditions, 15 we identified insufficient reporting of interventionist qualifications and their levels of experience in oncology. Of the 31 included studies, only three studies listed the educational requirements for the interventionist. Further, although study-specific training was described by 16 studies, the depth of information about the content, duration and trainer expertise was highly variable and generally poorly reported. As such, we are unable to make recommendations about the skilling or requirements of nurses for implementing these interventions. Future studies should include such information as it is important for implementation of evidence into practice.
All studies were assessed as being at high risk of bias for at least one outcome. The Cochrane Revised Risk of Bias tool deems an overall high risk of bias for any study outcome with a high risk in at least one domain or some concerns in multiple domains in a way that substantially lowers confidence in the result. 17 The main attributor to overall high risk judgements was the lack blinding. Previous Cochrane reviews of psychosocial interventions in breast cancer highlighted blinding as a limitation of these complex interventions, but recommend that as a minimum the blinding of outcome assessors should be considered to improve the rigour of these studies. 61 62 However, blinding of outcome assessors for participant-reported outcomes (eg, HRQoL) where the participant is the assessor is not feasible in an unblinded complex intervention. In the current review, the lack of blinding, contributed to the missing outcome data domain where missing data in the outcome likely depended on its true value (particularly for self-reported outcomes), placing studies at risk of social desirability bias. Several studies attempted an attentional control which is highly recommended for these types of RCTs 63 ; however, reporting of blinding in these studies was often absent. In addition, it was often unclear as to whether participants were blinded or whether the control activities, such as providing information and resources on breast cancer and providing support, were associated with the study outcomes. One exception was Matthews et al 41 who implemented a previously tested attentional placebo control. Future studies should consider adopting attentional placebo control or a well-designed attentional control and provide information on blinding.
The majority of RCTs with a large sample size reported a positive effect of nurse-led teaching guidance and counselling interventions on symptom burden during treatment and survivorship. The benefits of these nurse-led interventions are less clear for HRQoL and self-management/behavioural outcomes and there is insufficient evidence of effects on survival outcomes, health service/resource use and cost, and perceived intervention benefits and patient satisfaction. Findings from this review suggest that teaching, guidance and counselling interventions are effective for alleviating symptom burden, but more research is needed to evaluate the effects on HRQoL, self-management/behaviour, survival, health service/resource use and cost, perceived intervention benefits and patient satisfaction.
During treatment, there is consistent evidence from two studies with large sample sizes that nurse-led case management interventions delivered online or via telephone have superior effects on symptom burden and equivocal effects on self-management/behavioural outcomes compared with usual care. 40 44 There is, however, insufficient evidence of effect on HRQoL. During survivorship, the evidence for effectiveness of nurse-led case management is less clear. Two small pilot studies report superior intervention effects on symptom burden compared with usual care or control; however, there was no information provided on the control condition in one study. Moreover, the effects on HRQoL self-management/behavioural outcomes are conflicting and no study reported survival, satisfaction or health service/resource use and costs. This suggests that case management interventions are effective for alleviating symptom burden during treatment, but its effects during survivorship are less clear. Therefore, there is a need for well-powered, well-controlled trials evaluating all outcomes, particularly during survivorship.
There is consistent evidence from randomised trials that nurse-led surveillance interventions, whether with structured or patient-initiated contact, are equivocal to usual care for HRQoL, symptom burden and self-management/behavioural outcomes during survivorship and treatment. There is insufficient evidence of effect on survival outcomes, health service/resource use and cost, and perceived intervention benefits and patient satisfaction. This suggests that nurse-led follow-up care is as safe and effective as physician-led follow-up care, but future research should evaluate the effects of these interventions on survival and impacts of the interventions on health service or resource use and costs.
This was a comprehensive systematic review following a predefined, registered review protocol. We included high-level evidence from RCTs to evaluate the effectiveness of nursing interventions. We implemented a taxonomy of outcomes recommended by a systematic review of nurse-led interventions 15 and synthesised the interventions using well-established categorisation systems (The Omaha System) for interventions and problem domains. 16
There were some limitations to this review. We conducted a comprehensive search of the literature and while all efforts were made to identify relevant studies, it is possible that some were excluded due to the lack of adequate information about the interventionist. All included studies were at high risk of bias for at least one outcome. The heterogeneous nature of the included studies did not allow statistical pooling of data. Collating studies using the Omaha System categories may have oversimplified the intervention effects, particularly the case management and teaching, guidance and counselling interventions which were often complex. However, we included detailed information about the designs and interventions of each included study in our report. We only included publications in English and most included study interventions were conducted in the USA, thereby limiting the generalisability of our findings.
There is consistent evidence from randomised trials that nurse-led surveillance interventions are as safe and effective as physician-led care and the majority of RCTs indicate that nurse-led teaching, guidance and counselling and case management interventions are effective for alleviating symptom burden. Overall, there are a lack of studies evaluating intervention effects on survival outcomes, patient satisfaction and perceived intervention benefits and health service/resource use and costs. Future studies should evaluate the HRQoL and self-management/behavioural outcomes and consider using a well-designed attentional placebo control to blind participants for self-report outcomes. Such studies should consider the Medical Research Council (MRC) complex intervention framework to gain better insights into which mechanisms are effective, thus providing deeper insights into these interventions which would then facilitate replication.
- Cruickshank S ,
- Kennedy C ,
- Lockhart K , et al
- Liebert B ,
- White K , et al
- Claassen S , et al
- Moore A , et al
- National Breast Cancer Centre
- Maguire P ,
- Tait A , et al
- McArdle JM ,
- George WD ,
- McArdle CS , et al
- Baum M , et al
- Wilkinson S ,
- International Agency for Research on Cancer
- Charalambous A ,
- Campbell P , et al
- Cochrane Effective Practice and Organisation of Care (EPOC)
- Shamseer L ,
- Clarke M , et al
- Bradford N , et al
- Sterne JAC ,
- Savović J ,
- Page MJ , et al
- Wengström Y ,
- Häggmark C ,
- Strander H , et al
- Nissen MJ ,
- Swenson KK , et al
- Nezu AM , et al
- Sandgren AK ,
- Hargraves M , et al
- Schofield P ,
- Weih L , et al
- Sjödén P-O ,
- Bergh J , et al
- Dorros SM , et al
- Meneses KD ,
- Loerzel VW , et al
- Sikorskii A ,
- Tamkus D , et al
- Fillion L ,
- Leblond F , et al
- Koinberg I-L ,
- Fridlund B ,
- Engholm G-B , et al
- Tysver-Robinson D ,
- Campbell M , et al
- Sheppard C ,
- Higgins B ,
- Wise M , et al
- Kimman ML ,
- Bloebaum MM ,
- Dirksen CD , et al
- Dirksen CD ,
- Voogd AC , et al
- Grunfeld E ,
- Julian JA ,
- Pond G , et al
- Debess J , et al
- Schjolberg TK ,
- Henriksen N , et al
- Børøsund E ,
- Cvancarova M ,
- Moore SM , et al
- Matthews EE ,
- Berger AM ,
- Schmiege SJ , et al
- Han K , et al
- Ching SSY ,
- Wong FKY , et al
- Heidrich SM ,
- Egan JJ , et al
- Lee HS , et al
- Freysteinson WM ,
- Deutsch AS ,
- Davin K , et al
- Dunn-Henriksen AK ,
- Kroman N , et al
- Kirshbaum MN ,
- Stephenson J , et al
- Donnan P , et al
- Koinberg I ,
- Engholm G-B ,
- Genell A , et al
- Brandberg Y ,
- Feldman I , et al
- Browall M ,
- Forsberg C ,
- Wengström Y
- Tan J-Y , et al
- de Graaf E ,
- Teunissen SCCM
- Langbecker DH ,
- Haggstrom D ,
- Han PKJ , et al
- Jassim GA ,
- Whitford DL ,
- Hickey A , et al
- Mustafa M ,
- Carson-Stevens A ,
- Gillespie D , et al
- Aycock DM ,
- Helvig A , et al
Contributors All authors planned the research. RC, JM, KE, KP, JT, MS and PY conceptualised the review and research questions. RC, LT and SM designed the protocol and conducted the research. SM conducted searches. SM, JK and LT screened and evaluated studies. LT extracted study data. All authors contributed to reporting the research. RC and LT synthesised study results and took lead on writing the manuscript and all authors discussed the results and provided critical feedback on the manuscript. RC is responsible for the overall content as guarantor.
Funding The McGrath Foundation provided funding for this project.
Competing interests This research was a collaboration between McGrath Foundation and Queensland University of Technology. RC received grant funding from McGrath Foundation to conduct this research. JM, KE, KP, MS and JT are employees of the McGrath Foundation but were not involved in the systematic review beyond scoping the research questions, interpreting the data synthesis and reviewing the manuscript.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- ADVERTISEMENT FEATURE Advertiser retains sole responsibility for the content of this article
The new normal for breast cancer research
Produced by
One lost year of breast cancer research funding can amount to ten years of lost research advances. Credit: Steve Gschmeissner/Science Photo Library/Getty Images
Published November 2020
The COVID-19 pandemic has impacted just about every aspect of breast cancer care. Patients and doctors have been forced to adapt to the new normal, and the Breast Cancer Research Foundation (BCRF) is no different. Dorraya El-Ashry, the Chief Scientific Officer of BCRF, explains how the foundation has navigated the crisis and is committed to data sharing.
How has the pandemic aff ected the BCRF research community?
Breast cancer research faces a steep cut in funding. BCRF’s researchers depend on our support to make discoveries, mount clinical trials, and keep labs in full operation. If we fall short, the impact on breast cancer research will be profound.
When social distancing measures were at their height, many labs closed and clinical trials were placed on hold. The biggest danger is not what happened over the last few months — many researchers adapted and found new ways to continue. The real danger is if research funding is diminished, it will put a stop to the progress we’ve made in the last decades, essentially an increase in preventable deaths.
With science, you can’t just turn the tap off and then turn it back on. There’s loss of lab personnel, loss of research models, labs that may need to close and data that is lost because the experiments couldn’t be finished. The consequence of decreased funding is much more long term — one year of lost funding could result in 10 years of lost advances.

Dorraya El-Ashry, Chief Scientific Officer of the Breast Cancer Research Foundation. Credit: Breast Cancer Research Foundation
After 30 years spent as a breast cancer research scientist, I had a lot of ideas for how to help steer this global, scientific endeavor to eradicate breast cancer. One area I had high hopes for was to increase investments in breast cancer disparities research. Prior to the pandemic, we were working towards that goal to secure expanded funding for this specific area of research. Now the field faces a future of diminished funding, and yet, it’s clear that this area of research is needed now more than ever as disparities in health outcomes has become a central issue in the social justice movement. My hope is that, as we come out of COVID-19 downturn, this becomes an area we can pursue with deeper investments.
What are some of your other research priorities?
There are two ends of the spectrum that we need to target: Finding curative treatments for patients living with breast cancer, and preventing breast cancer from happening in the first place. Certainly novel therapeutics and precision medicine are some of the most urgent areas—we need treatments that can prolong lives and effect cures for women with metastatic breast cancer today. And we have to be thinking about the ultimate end goal, which is to prevent breast cancer. In order to best target these two goals, we need to support an entire spectrum of research—from basic science studying the roots of the disease to clinical trials moving treatments from the lab to patients.
In 2019, BCRF partnered with Springer Nature to help make research datasets publicly accessible for papers published by npj Breast Cancer . Why undertake this pilot programme?
A key tenet of BCRF’s mission is to connect investigators around the world, and this partnership with Springer Nature is a critical tool in making that happen. We all know that submitting research results in scientific journals and presenting them at major conferences is what allows investigators to review and comment on the work of others, but what’s missing are the raw data. So, we wanted to facilitate data-sharing among researchers who publish in npj Breast Cancer by helping to make all their datasets available in an accessible way. This will help researchers validate published results and allow scientists to ask new questions, generate hypotheses, and query their data from and with these datasets.
How is BCRF diff erent from other funders of breast cancer science?
BCRF was founded by Evelyn Lauder and Larry Norton with a vision to provide the best minds in breast cancer research with the security and freedom to pursue novel ideas. Rather than funding research projects with proven outcomes, we invite investigators with demonstrated track records in the field to submit proposals —proposals that are innovative and hold the potential to advance the field in some way, but don’t require an established result at the outset. We are in the 27th year of funding breast cancer research in this way — now supporting the scientists that have been involved in every major breast cancer research breakthrough to date.
How can you tell?
In this past year, we initiated a process of impact reviews, starting with a group of 40 investigators who have had BCRF awards for many years. And by any number of measures — publication rates, citation rates, other grant funding generated from BCRF-supported work— the impact of their work has been extremely significant. The most significant measure: the impact on patient lives over the last three decades. Breast cancer mortality rates have declined by 40 percent in that time in the U.S. Early-stage breast cancers have a five-year survival rate of over 90 percent. Nonetheless we remain committed to the women and men that continue to be diagnosed with metastatic breast cancer. And with estimates of a rise in advanced breast cancer cases as a result of delays in diagnosis and treatment during the pandemic, the research we fund today has the potential to save even more lives tomorrow.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Open access
- Published: 14 May 2024
Enhancing pathological complete response prediction in breast cancer: the role of dynamic characterization of DCE-MRI and its association with tumor heterogeneity
- Xinyu Zhang 1 ,
- Xinzhi Teng 1 ,
- Jiang Zhang 1 ,
- Qingpei Lai 1 &
- Jing Cai 1 , 2
Breast Cancer Research volume 26 , Article number: 77 ( 2024 ) Cite this article
330 Accesses
Metrics details
Early prediction of pathological complete response (pCR) is important for deciding appropriate treatment strategies for patients. In this study, we aimed to quantify the dynamic characteristics of dynamic contrast-enhanced magnetic resonance images (DCE-MRI) and investigate its value to improve pCR prediction as well as its association with tumor heterogeneity in breast cancer patients.
The DCE-MRI, clinicopathologic record, and full transcriptomic data of 785 breast cancer patients receiving neoadjuvant chemotherapy were retrospectively included from a public dataset. Dynamic features of DCE-MRI were computed from extracted phase-varying radiomic feature series using 22 CAnonical Time-sereis CHaracteristics. Dynamic model and radiomic model were developed by logistic regression using dynamic features and traditional radiomic features respectively. Various combined models with clinical factors were also developed to find the optimal combination and the significance of each components was evaluated. All the models were evaluated in independent test set in terms of area under receiver operating characteristic curve (AUC). To explore the potential underlying biological mechanisms, radiogenomic analysis was implemented on patient subgroups stratified by dynamic model to identify differentially expressed genes (DEGs) and enriched pathways.
A 10-feature dynamic model and a 4-feature radiomic model were developed (AUC = 0.688, 95%CI: 0.635–0.741 and AUC = 0.650, 95%CI: 0.595–0.705) and tested (AUC = 0.686, 95%CI: 0.594–0.778 and AUC = 0.626, 95%CI: 0.529–0.722), with the dynamic model showing slightly higher AUC (train p = 0.181, test p = 0.222). The combined model of clinical, radiomic, and dynamic achieved the highest AUC in pCR prediction (train: 0.769, 95%CI: 0.722–0.816 and test: 0.762, 95%CI: 0.679–0.845). Compared with clinical-radiomic combined model (train AUC = 0.716, 95%CI: 0.665–0.767 and test AUC = 0.695, 95%CI: 0.656–0.714), adding the dynamic component brought significant improvement in model performance (train p < 0.001 and test p = 0.005). Radiogenomic analysis identified 297 DEGs, including CXCL9, CCL18, and HLA-DPB1 which are known to be associated with breast cancer prognosis or angiogenesis. Gene set enrichment analysis further revealed enrichment of gene ontology terms and pathways related to immune system.
Dynamic characteristics of DCE-MRI were quantified and used to develop dynamic model for improving pCR prediction in breast cancer patients. The dynamic model was associated with tumor heterogeniety in prognostic-related gene expression and immune-related pathways.
Introduction
Breast cancer is one of the most common malignant in women. In 2020, there were around 2.3 million women newly diagnosed with and over 600,000 women died of breast cancer worldwide [ 1 ]. Recently, neoadjuvant chemotherapy (NAC) has become increasingly used in breast cancer systemic treatment. NAC was initially used in inoperable breast cancer to enable surgical resection, and expanded to other types of breast cancer for increasing the chance of breast conservation owing to its remarkable efficacy [ 2 ]. Current NAC treatment schemes are determined by hormone receptor (HR) status and human epidermal growth factor receptor 2 (HER2) status as recommended by American Society of Clinical Oncology (ASCO) [ 3 ]. Pathological complete response (pCR), defined as no residual disease in breast and axillary region after NAC, is a validated prognostic factor to assess treatment response and associated with long-term outcome [ 4 ]. However, only 10-50% patients achieved pCR, varying according to their receptor subtypes [ 5 ]. Moreover, the assessment of pCR status is performed at surgery after completion of NAC, prior to which non-responders have suffered from the toxicity and side effects caused by NAC. Therefore, it is essential to identify patients who are likely to achieve pCR before NAC to avoid unnecessary complications and maximize potential benefits.
Dynamic contrast-enhanced MRI (DCE-MRI) is the clinical routine for breast cancer assessment. It has high sensitivity in diagnosis and treatment monitoring [ 6 , 7 ]. Through acquisition of sequential images before, during, and after the administration of contrast agent, DCE-MRI provides valuable information about tissue perfusion and contrast agent enhancement dynamics associated with tumor angiogenesis [ 8 ]. Radiomics extracts high-dimentional image features that are imperceptible to human eyes to non-invasively quantify tumor characteristics [ 9 ]. Radiomic analysis of breast DCE-MRI has been used for pCR prediction in many previous studies, most of which only used radiomic features from one or several phases while ignoring the dynamic information [ 10 , 11 ]. Recently, attempts have been made to leverage the dynamic information embedded in DCE-MRI for pCR prediction by combining radiomic features extracted from different DCE-MRI phases. For instance, Peng et al. calculated delta-features between two different phases for pCR prediction [ 12 ]; Li et al. employed simple statistical patterns of radiomic features extracted from different phases for pCR prediction and achieved better performance compared to single-phase features, demonstrating the value of multi-phase information [ 13 ]. In BMMR2 challenge, radiomic features from kinetic maps, such as peak enhancement maps and signal enhancement ratio maps, were used to predict pCR [ 14 ]. However, the entire time series of radiomic features has not been fully explored and may contain additional information for tumor characterization. On the other hand, feature-based representation of time series data like 22 CAnonical Time-series CHaracteristics (Catch22) can capture the dynamic properties of time series data and was used in various tasks [ 15 , 16 ]. Accordingly, there developed an assumption that the dynamics of radiomic feature series extracted by Catch22 can characterize the dynamic information in DCE-MRI and improve pCR prediction of breast cancer patients.
In this study, we aimed to systematically extract dynamic properties of radiomic feature series from DCE-MRI to improve treatment response prediction of breast cancer patients. To achieve this, a large number of dynamic features were extracted by Catch22 from DCE-MRI feature series, and a dynamic model was then built for pCR prediction. Various combinations of dynamic models and existing radiomic and clinical models were developed to find the optimal one as the final model. In addition, radiogenomic analysis of binarized dynamic model predictions was conducted to explore its association with tumor heterogeneity and biological process. Figure 1 shows the overall workflow of this study.
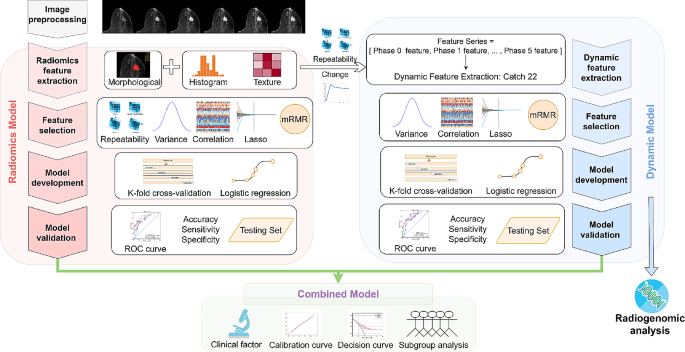
Workflow of the study. Firstly, the collected DCE-MR images were preprocessed by normalization and discretization. Radiomic features were extracted from multiple phases of DCE-MRI, while dynamic features were extracted from radiomic feature series. Feature selection, model development, and model validation were then conducted separately for radiomic model and dynamic model. Subsequently, combined models were developed by integrating radiomic, dynamic, and clinical information and their performance were evaluated. In addition, radiogenomic analysis was performed on dynamic model to investigate potential biological mechanisms
Materials and methods
Patient data.
A total of 985 stage II/III locally advanced breast cancer patients enrolled in the multi-center I-SPY2 trial (clinical trial number: NCT01042379) during 2010 to 2016 were collected from the publicly available dataset on The Cancer Image Archive [ 17 , 18 , 19 ]. Institutional review board approval was waived due to the use of public data. The detailed descriptions of I-SPY2 trial have been reported by previous paper [ 20 ]. All the patients underwent MR examination and percutaneous biopsy before receiving NAC. After the completion of NAC, patients underwent surgical resection to assess residual disease. The exclusion criteria included: (1) incomplete image or clinicopathologic data; (2) deviations from the prescribed scanning protocol; (3) insufficient image quality.
Clinicopathologic data
Clinicopathologic data including HR, HER2, MammaPrint status (MP), pCR, and other patient characteristics was provided by the dataset. HR and HER2 were deternmined by immunohistochemical (IHC) staining or fluorescence in-situ hybridization (FISH) of tissues obtained during pre-treatment biopsy. HR was determined as positive when ≥ 5% tumor staining for ER and/or PgR was seen. HER2 was determined as positive by IHC 3 + or FISH overexpression [ 21 ]. The surrogate of treatment response pCR was defined as no residual disease in breast and axillary lymph nodes after NAC and obtained by post-treatment surgery [ 22 ].
Imaging data and tumor segmentation
The scanning process of DCE-MRI can be found on TCIA website [ 17 , 18 ]. DCE-MRI scanning protocol details are provided in Supplementary Material Table S1 . The pre-contrast phase and five post-contrast phases were used for radiomic feature extraction and subsequent analysis.
The region of interest was segmented by functional tumor volume (FTV) included in the dataset. The calculation of FTV involved background filtering, estimating signal enhancement ratio, and applying a peak enhancement threshold in a manual-defined 3D bounding box [ 23 ].
Image preprocessing and feature extraction
Image normalization was performed across different DCE-MRI phases of the same patient to preserve the dynamic information. All the images were isotropically resampled to 1*1*1mm 3 , and discretized by a fixed bin width of 5. More details of image preprocessing can be found in Supplementary Material Figure S1 . Radiomic features were extracted from each phase of DCE-MRI using PyRadiomics package version 3.0.1 following the standardization and definitions in the image biomarker standadization initiative [ 24 , 25 ]. The extracted features included morphological features ( n = 14), first-order features ( n = 17), and texture features ( n = 79). The repeatability of radiomic features was evaluated by perturbation which involved random translation, rotation, and contour randomization of original masks [ 26 , 27 , 28 ]. Features with high-repeatabliity (intraclass correlation coefficient, ICC ≥ 0.9 [ 29 ]) were retained for better model repeatability. The fluctuation of radiomic features was measured by performing a single-sample t test on the variations between features from different phases. Radiomic feature series were constructed by concatenating the high-repeatable and phase-varying first-order and texture features. Dynamic features were extracted from radiomic feature series using the 22 CAnonical Time-series Characteristics (catch22) feature set, specifically designed for capturing the dynamic properties of time series data, such as distributions and outliers, linear and non-linear autocorrelation, and so on [ 30 ].
Model development and evaluation
The development of radiomic model and dynamic model followed the same process including feature selection and model building. The features with low variances were removed first to retain those providing more information. Then, features with significant correlations with pCR were identified by MannWhitney U test, where a p value smaller than 0.05 was defined as significant. LASSO was subsequently used to select the independently discriminative features. Finally, features were ranked by minimum redundancy and maximum relevance (mRMR) algorithm considering the relevance to pCR and redundancy at the same time [ 31 ]. Clinical factors that are commonly used in clinical decision making and have significant associations with pCR were identified and used in developing clinical model. Combined models were constructed using logistic regression with clinical factors, prediction score of the dynamic model, and prediction score of radiomic model as variables. Different combination strategies were adopted, including combining two of the three variables respectively, as well as the combination of all three together. The independence of the components in the combined models were examined by their coefficients and p values. All the models were developed using Logistic Regression with 10-fold cross-validation in training set and tested in independent testing set.
The pCR prediction performance of the candidate models was assessed by various metrics, including area under receiver operating characteristic curve (AUC), accuracy, sensitivity, and specificity. AUCs were calculated by continuous prediction (the probability) and the other metrics were calculated by binary prediction (pCR or non-pCR) dichotomized by Youden index. An AUC typically ranges from 0 to 1 while AUC equal to one means a perfect descrimination ability. The optimal model was determined by the highest internal validation AUC in the training set. Heatmap was employed to visualize the relationships between different models and their association with clinical factors. SHapley Additive Explanations (SHAP), a method to interpret and explain the output of machine learning models, was employed to evaluate the importance of each component in the model with the highest AUC [ 32 ]. In our case, where the model output is the probability of achieving pCR, the SHAP values for each parameter ranges from − 1 to 1 and a larger absolute value means a higher importance for model output. Calibration curves and Brier scores were used to further evaluate the alignment between model-predicted probabilities and actual probabilities. Brier score measures the accuracy of probabilistic predictions and takes the value from 0 to 1, for which 0 means a perfect prediction. Decision curve analysis was performed to evaluate the clinical benefit obtained by the optimal model [ 33 ]. Besides, to further demonstrate the generalizability of the optimal model, its association with pCR in various pre-defined molecular subtypes, namely HR + HER2-, HR + HER2+, HR-HER2-, and HR-HER2+, and patients receiving different treatments were evaluated.
Radiogenomic analysis
To examine whether the dynamic model can reflect tumor heterogeneity and its association with gene expression, we collected paired total mRNA expression data from National Center for Biotechnology Information (NCBI) [ 34 ]. Patients were divided into DYN + and DYN- groups according to the binary prediction of dynamic model. Student t test was performed to identify differentially expressed genes (DEGs) between the two groups. An absolute log-2 fold change larger than 0.25 and a p value smaller than 0.05 were used as cutoff. Enriched Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways were identified by gene set enrichment analysis (GSEA) of DEGs [ 35 , 36 , 37 , 38 , 39 ]. A p value smaller than 0.05 and false discovery rate (FDR) smaller than 0.25 were considered statistically significant.
Statistical analysis and software
For statistical analysis, Chi-Square test or Fisher’s exact test was used for categorical variables and MannWhitney U test was used for continuous variables. A two-tailed p-value smaller than 0.05 was considered statistically significant. The 95% CIs for AUCs were calculated according to DeLong’s methods [ 40 ]. DeLong test was used to compare the AUCs of two independent models and likelihood ratio test was used to compare the model fit of nested models to demonstrate the improvements conferred by the additional factors in complex models. The statistical analysis was carried out on R4.2.2 [ 41 ] and Python3.7 [ 42 ]. Logistic regression was carried out by package scikit-learn 1.0.2 [ 43 ]. Radiogenomic analysis was conducted using packages scanpy 1.9.3 [ 44 ] and gseapy 1.1.0 [ 45 ].
Patient characteristics
A total of 785 patients with complete imaging data and clinicopathological record constituted the entire patient cohort in this study and were divided into training set and testing set with a ratio of 3:1 (Fig. 2 ). As shown in Table 1 , there is no significant difference in all of the patient characteristics between training set and testing set. The characteristics of pCR and non-pCR patients were tabulated in Supplementary Material Table S2 . Significant association with pCR was observed in treatment, HR, HER2, and MP, while the other characteristics were independent of pCR.
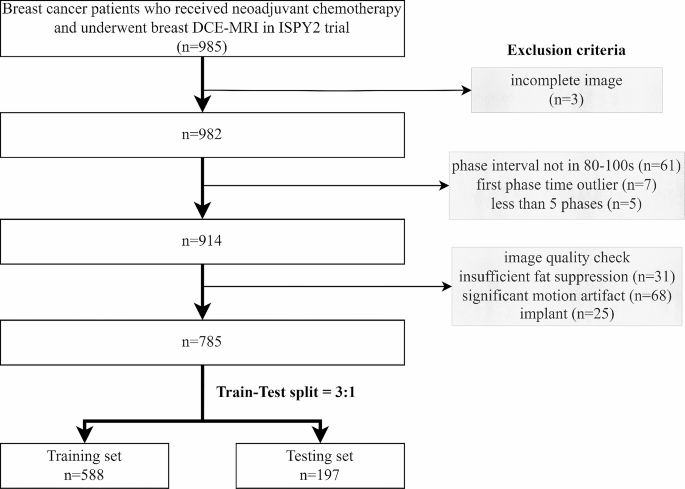
Patient cohort and train-test split
Feature repeatability and feature change
The summarized results of feature repeatability and feature variation are shown in Supplementary Material Figure S2 and Figure S3 . After removing the duplicate shape features and features with low repeatability, there were 480 radiomic features retained for each patient. For dynamic feature extraction, features repeatable in all the DCE-MRI phases and changing across different phases were retained. A total of 1232 dynamic features were extracted from 56 selected radiomic feature series for each patient and used in further analysis. An example of the dynamic feature is shown in Supplementary Material Table S3 .
Different models in pCR prediction
A 10-feature dynamic model and a 4-feature radiomic model were developed separately (Supplementary Material Table S4 ). Dynamic model achieved higher AUC than radiomic model in both training set (0.688 vs. 0.650) and testing set (0.686 vs. 0.626) (Fig. 3 (a)(b)), but the difference was not statistically significant (p value = 0.181 and 0.222). Dynamic model also had better performance in terms of accuracy and specificity, while the sensitivity was the same as radiomic model (Table 2 ). The significance of each feature in dynamic model and radiomic model was evaluated by the odds ratio and tabulated in Supplementary Material Table S5 and S6 .
Among the clinicopathological variables provided in the dataset, treatment, HR, HER2, and MP were significantly associated with pCR (Supplementary Material Table S2 ). Since we intended to study biomarkers and MP requires expensive genomic test, only HR and HER2 were retained for further analysis. Table 3 summarized the metrics for pCR prediction performance of clinical model and combined models. Clinical-radiomic-dynamic (CRD) model achieved the highest training and testing AUC (Fig. 3 (c)(d)), accuracy, and specificity, while clinical model had the highest sensitivity among all the models. The clinical factors, the dynamic model, and the radiomic model demonstrated independent value in CRD model as indicated by their coefficients and p values (Supplementary Material Table S7 ). Compared with clinical-radiomic (CR) model, CRD model shown significant improvement in both training and testing performance, indicating the additive value of dynamic model. Figure 3 (e) shows the heatmap of the predicted probability by different models. Models containing dynamic features were clustered as similar models, showing the distinctive characteristic of dynamic features. The dynamic model was not associated with HR and HER2, demonstrating the independent value of dynamic model.
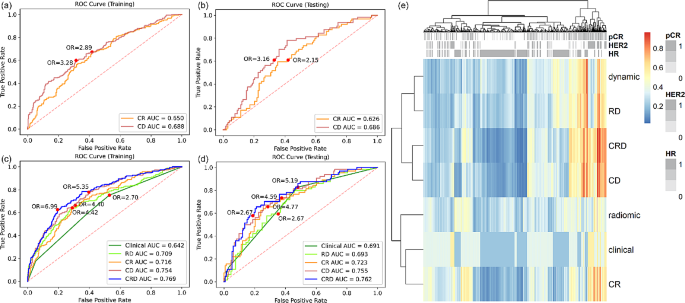
Reciever operating characteristic (ROC) curve analysis of dynamic model and radiomic model in ( a ) training set and ( b ) testing set. ROC analysis of clinical model, radiomic-dynamic (RD) model, clinical-radiomic (CR) model, clinical-dynamic (CD) model, and clinical-radiomic-dynamic (CRD) model in ( c ) training set and ( d ) testing set. ( e ) Heatmap of predicted probability by different models
Evaluation of the optimal model
Overall, CRD model has the best performance in pCR prediction. The calibration curves of CRD model had Brier score of 0.174 and 0.180 in training and testing set respectively (Fig. 4 (a)), indicating well-alignment between predicted probablities and actual probabilities. The decision curve analysis of CRD model demonstrated its clinical usefulness by higher net benefit gain compared to clinical model and the other combined models (Fig. 4 (b)).
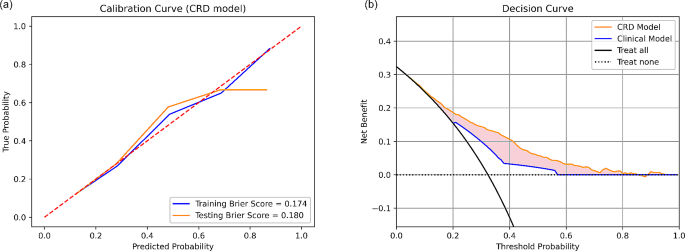
Calibration curve and decision curve analysis
The SHAP value analysis of CRD model shown high importance of dynamic model, which was comparable to HR. The importance of radiomic model and HER2 was a little bit lower, but still had significant effect on the model output (Fig. 5 (a)). The CRD model was also used in stratifying pCR and non-pCR patients under different pre-defined molecular subtypes. It shown a significant stratification ability in all the four molecular subtypes with odds ratio (OR) of 2.88–8.42. (Fig. 5 (b)). In the analysis of patients receiving different drugs, except for the marginally significant performance in Pertuzumab arm, CRD model shown significant association with pCR with OR of 2.88–10.93 in the other treatment arms (Fig. 5 (c)).
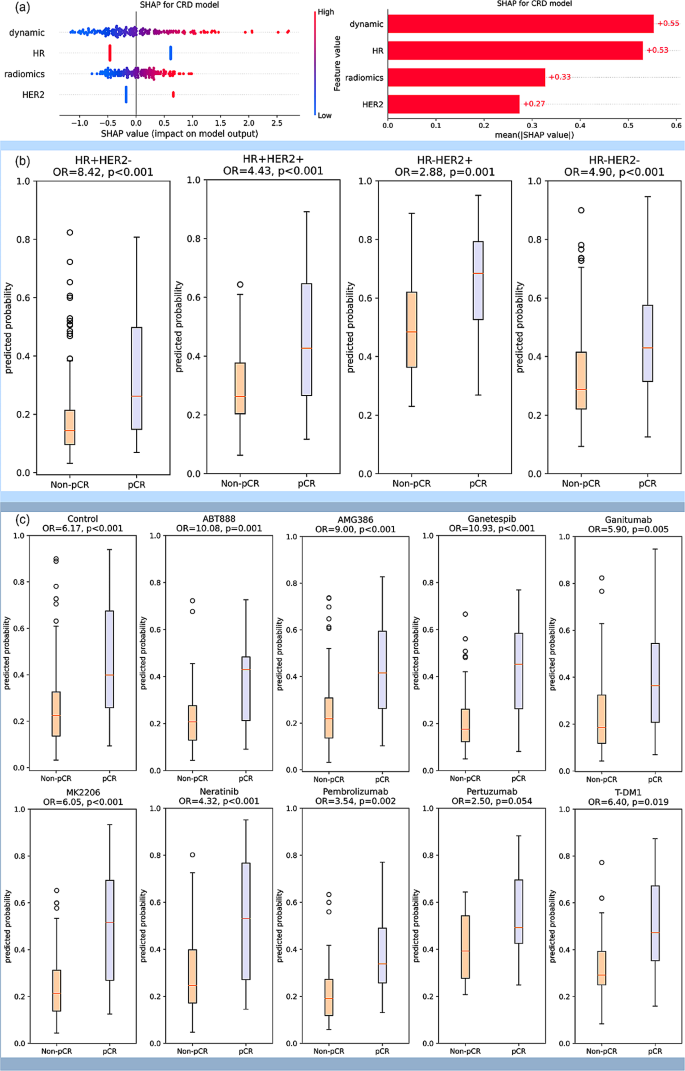
( a ) SHAP analysis for interpretable component importance of CRD model. The beaswarm plot shows how each variable influence model output on single data where one dot represents one patient (left). The mean absolute SHAP value reflects the global effect of each variable on model output (right). ( b ) Box plots showing the predictive ability of CRD model in patient subgroups of various molecular subtypes. The molecular subtypes were defined by the status of HR and HER2, namely HR + HER2-, HR + HER2+, HR-HER2+, and HR-HER2-. The box plots indicate that the CRD model yields significantly distinct prediction probabilities for patients with pCR and non-pCR in all the four molecular subtypes. ( c ) Box plots showing the predictive ability of CRD model in patients receiving various treatments. Patients received standard care (control) or standard care plus one trial agent (ABT888, AMG386, Ganetespib, Ganitumab, MK2206, Neratinib, Pembrolizumab, Pertuzumab, T-DM1) in the trial. The box plots suggest that CRD model demonstrates the capability to differentiate pCR and non-pCR patients across various treatment drugs in this trial, with the exception of a marginal significance observed in Pertuzumab. All the p values were obtained by student t test. OR: odds ratio
DEGs and enriched pathways
In DEG analysis, a total of 196 up-regulated genes and 101 down-regulated genes in DYN + group were identified. As compared with HR- group and HER2 + group, which also associate with better pCR outcome in ISPY2 trial, there are 7 common up-regulated genes and 22 common down-regulated genes (Fig. 6 (a)). In GSEA by GO terms, there are 36 biological processes, 3 cellular components, 2 molecular functions enriched in DYN+ (Fig. 6 (b)), many of which are associated with immune system. There are 4 enriched pathways in GSEA by KEGG, in which 3 pathways are related to viral disease and 1 pathway is related to immune disease (Fig. 6 (c)).
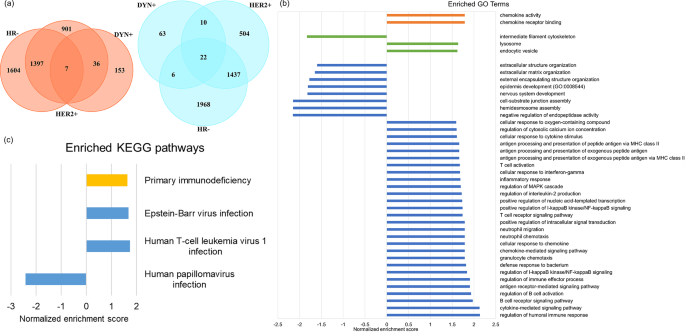
( a ) Up-regulated (left) and down-regulated (right) DEGs in DYN+, HR-, and HER2 + group. ( b ) Enriched GO terms in DYN + group. ( c ) Enriched KEGG pathways in DYN + group
While the dynamic information in DCE-MRI has shown potential in various clinical applications, the exploration of DCE-MRI-derived radiomic feature series has remained limited. This study systematically extracted dynamic features from DCE-MRI-derived radiomic feature series using feature-based time series analysis method and built dynamic model for pCR prediction. Adding dynamic model to exisitng clinical and radiomic model can improve pCR prediction. Radiogenomic analysis revealed correlations of dynamic model with some breast cancer prognosis-related genes and pathways, providing the potential biological explanations for the additive value.
The change in DCE-MR image appearances caused by the flow of contrast agent may contain valuable information for pCR prediction. Previous studies have employed delta features and statistical distributions to characterize the relevant dynamic information [ 12 , 13 ]. However, the former method may provide limited information by utilizing only two of multiple DCE-MR phases, while the latter method disregards the temporal information that is crucial for reflecting the directional flow of contrast agent. A recently published paper implemented several classical time series analysis algorithms in DCE-MRI-derived radiomic feature series and achieved an accuracy of 0.852 in breast cancer diagnosis, demonstrating the significance of serial information as well as the feasibility and efficacy of time series analysis [ 46 ]. In our study, we used radiomic features to comprehensively describe DCE-MR image appearance and adopted Catch22 to systematically analyze the dynamics of radiomic feature series. The Catch22 feature set takes into account both the temporal order and relative magnitude of series values. It has been successfully implemented in many time series analysis applications, such as breath signal and heart rate. To the best of our knowledge, it is the first study to apply a systematic feature-based time series analysis method to DCE-MRI for pCR prediction. Our results demonstrated the utility of the extracted dynamic features by showing a modestly higher AUC of dynamic model in comparison to the conventional radiomic model. Furthermore, the dynamic features provided additive value to the existing methods, as evidenced by a significantly improved model performance compared with both clinical model and CR model. Overall, we have demonstrated the feasibility and efficacy of extracting dynamic information through feature-based time series analysis and the potential of dynamic features in facilitating pCR prediction. Besides, our method offers the advantage of interpretability as Catch22 provides clear definition for each dynamic feature. And it is adaptable to different time series length which is frequently encountered in real-world clinical practice due to the variations of machines and scan settings. Our method demonstrates the potential to be implemented in real clinical practice, although further validation is required to confirm its performance in diverse settings.
Both single-modal and multi-modal models were developed in this study. While the imaging-based model and clinical model appeared to have similar performance, the combined models shown better performance than individual models. The CRD model achieved the highest AUC, which is significantly better than RD model and clinical model alone, indicating that imaging features and clinical factors may provide distinct and complementary information for pCR prediction. Subgroup analysis of the CRD model was conducted to further explore the effectiveness of CRD model under various conditions. Breast cancer is a highly heterogeneous disease characterized by various HR and HER2 status, resulting in four molecular subtypes. Our results on molecular subtype analysis resulted in varying effect size by OR ranging from 2.88 to 8.42, where a larger OR indicates a stronger predictive ability. While CRD model is significantly associated with pCR in all the molecular subtypes, our results suggested that CRD model has stronger predictive ability for patients of HR + HER2-. The CRD model was also evaluated by its effect for patients receiving different drugs, resulting in the largest OR in Ganetespib and marginally significant OR in Pertuzumab. This indicates the various predictive value of CRD model for various treatment drugs and assists the clinicians to decide applicable scenarios. In general, CRD model shown generalizability acorss various molecular subtypes and various treatment drugs. However, due to the nature of trial data, the patient numbers are small in each subgroups and further validation on larger cohort is required to confirm the results.
It is believed that radiomics is able to detect the underlying biological processes in the human body by analyzing image textures that are imperceptible to human eyes. Moreover, pre-treatment radiomics mostly reflect the baseline tumor characteristics, which is the result of various biological processes and associated with treatment response. Previous studies indicated the representativeness of image phenotypes for the biological characteristics by demonstrating their similar predictive ability to pCR [ 47 ]. However, few radiomics study has linked the image phenotype to biological processes through radiogenomic analysis [ 48 ]. In this study, we conducted a radiogenomic analysis to associate our dynamic model to the genomic profiles of breast tumors, providing insight into the underlying biological mechanisms of radiomics. Some DEGs in our DYN + subgroup is associated with better prognosis of breast cancer patients. For example, the DYN + subgroup has higher expression of CXCL9 which was demonstrated to associate with higher pCR rate in breast cancer patients receiving NAC in previous study [ 49 ]; HLA-DPB1 was up-regulated in DYN + subgroup and was also assocaited with more tumor infiltrating lymphcytes and thereby better prognosis [ 50 ]. On the other hand, DEGs such as CCL18 is associated with angiogenesis in breast cancer, demonstrating the potential of DYN to represent the dynamics in DCE-MRI [ 51 ].
Our study has several limitations. Due to the retrospective nature of the study, it is possible that our results suffer from spectrum bias and information bias, which may compromise the overall strength of evidence of our study. Besides, our study included a medium sample size ( n = 785) without external validation, which may not be representative enough for the large population of breast cancer patients. Therefore, further study is needed to externally validate our methods and conclusions in a prospective manner. Also, our study only employed DCE-MRI, while multi-parametric MR images could be available in clinics. Further exploration on incorporating other MR images is warranted.
Conclusions
In conclusion, this study quantified the dynamic characteristics of DCE-MRI by calculating dynamic properties of radiomic feature series and developed a dynamic model. The dynamic model can aid in improving pCR prediction of breast cancer patients receiving NAC. The potential biological underpinnings of the dynamic model was explored by demonstrating its association with tumor heterogeneity in gene expression. Further investigations on more biological associations and assisting treatment selection are warranted.
Data availability
The dataset used in this study is a public dataset available at The Cancer Image Archive with accession code 70230072. Dynamic and radiomics features that support the findings of this study can be found here: https://github.com/Xinyu-Z000/DCE-MRI-dynamics.
Breast cancer. Accessed December 6. 2023. https://www.who.int/news-room/fact-sheets/detail/breast-cancer .
Bonadonna G. Evolving concepts in the systemic adjuvant treatment of breast Cancer. Cancer Res. 1992;52(8):2127–37.
CAS PubMed Google Scholar
Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast Cancer: ASCO Guideline. J Clin Oncol. 2021;39(13):1485–505. https://doi.org/10.1200/JCO.20.03399 .
Article CAS PubMed PubMed Central Google Scholar
Wang-Lopez Q, Chalabi N, Abrial C, et al. Can pathologic complete response (pCR) be used as a surrogate marker of survival after neoadjuvant therapy for breast cancer? Crit Rev Oncol Hematol. 2015;95(1):88–104. https://doi.org/10.1016/j.critrevonc.2015.02.011 .
Article PubMed Google Scholar
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72. https://doi.org/10.1016/S0140-6736(13)62422-8 .
Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol. 2008;18(7):1307–18. https://doi.org/10.1007/s00330-008-0863-7 .
Fowler AM, Mankoff DA, Joe BN. Imaging neoadjuvant therapy response in breast Cancer. Radiology. 2017;285(2):358–75. https://doi.org/10.1148/radiol.2017170180 .
Xiao J, Rahbar H, Hippe DS, et al. Dynamic contrast-enhanced breast MRI features correlate with invasive breast cancer angiogenesis. Npj Breast Cancer. 2021;7(1):1–9. https://doi.org/10.1038/s41523-021-00247-3 .
Article CAS Google Scholar
Saha A, Harowicz MR, Grimm LJ, et al. A machine learning approach to radiogenomics of breast cancer: a study of 922 subjects and 529 DCE-MRI features. Br J Cancer. 2018;119(4):508–16. https://doi.org/10.1038/s41416-018-0185-8 .
Granzier RWY, Ibrahim A, Primakov SP, et al. MRI-Based Radiomics Analysis for the Pretreatment Prediction of Pathologic Complete Tumor Response to Neoadjuvant systemic therapy in breast Cancer patients: a Multicenter Study. Cancers. 2021;13(10):2447. https://doi.org/10.3390/cancers13102447 .
Article PubMed PubMed Central Google Scholar
Fan M, Wu G, Cheng H, Zhang J, Shao G, Li L. Radiomic analysis of DCE-MRI for prediction of response to neoadjuvant chemotherapy in breast cancer patients. Eur J Radiol. 2017;94:140–7. https://doi.org/10.1016/j.ejrad.2017.06.019 .
Article CAS PubMed Google Scholar
Peng S, Chen L, Tao J, et al. Radiomics Analysis of Multi-phase DCE-MRI in Predicting Tumor response to neoadjuvant therapy in breast Cancer. Diagnostics. 2021;11(11):2086. https://doi.org/10.3390/diagnostics11112086 .
Li Q, Xiao Q, Li J, Wang Z, Wang H, Gu Y. Value of Machine Learning with multiphases CE-MRI radiomics for early prediction of Pathological Complete Response to Neoadjuvant Therapy in HER2-Positive invasive breast Cancer. Cancer Manag Res. 2021;13:5053–62. https://doi.org/10.2147/CMAR.S304547 .
Li W, Partridge SC, Newitt DC, et al. Breast multiparametric MRI for prediction of Neoadjuvant Chemotherapy response in breast Cancer: the BMMR2 challenge. Radiol Imaging Cancer. 2024;6(1):e230033. https://doi.org/10.1148/rycan.230033 .
Quicke P, Sun Y, Arias-Garcia M, et al. Voltage imaging reveals the dynamic electrical signatures of human breast cancer cells. Commun Biol. 2022;5(1):1–14. https://doi.org/10.1038/s42003-022-04077-2 .
Orlova Y, Gorobtsov A, Sychev O, Rozaliev V, Zubkov A, Donsckaia A. Method for determining the Dominant type of human breathing using motion capture and Machine Learning. Algorithms. 2023;16(5):249. https://doi.org/10.3390/a16050249 .
Article Google Scholar
ISPY2 trial data 2. https://doi.org/10.7937/TCIA.KK02-6D95 .
ISPY2 trial data1. https://doi.org/10.7937/TCIA.D8Z0-9T85 .
Clark K, Vendt B, Smith K, et al. The Cancer Imaging Archive (TCIA): maintaining and operating a Public Information Repository. J Digit Imaging. 2013;26(6):1045–57. https://doi.org/10.1007/s10278-013-9622-7 .
Wang H, Yee D. I-SPY 2: a neoadjuvant adaptive clinical trial designed to improve outcomes in high-risk breast Cancer. Curr Breast Cancer Rep. 2019;11(4):303–10. https://doi.org/10.1007/s12609-019-00334-2 .
Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol off J Am Soc Clin Oncol. 2007;25(28):4414–22. https://doi.org/10.1200/JCO.2007.10.6823 .
Spring LM, Fell G, Arfe A, et al. Pathologic Complete Response after neoadjuvant chemotherapy and impact on breast Cancer recurrence and survival: a Comprehensive Meta-analysis. Clin Cancer Res off J Am Assoc Cancer Res. 2020;26(12):2838–48. https://doi.org/10.1158/1078-0432.CCR-19-3492 .
Hylton NM, Gatsonis CA, Rosen MA, et al. Neoadjuvant chemotherapy for breast Cancer: functional tumor volume by MR Imaging predicts recurrence-free survival—results from the ACRIN 6657/CALGB 150007 I-SPY 1 TRIAL. Radiology. 2016;279(1):44–55. https://doi.org/10.1148/radiol.2015150013 .
Zwanenburg A, Vallières M, Abdalah MA, et al. The image Biomarker Standardization Initiative: standardized quantitative Radiomics for High-Throughput Image-based phenotyping. Radiology. 2020;295(2):328–38. https://doi.org/10.1148/radiol.2020191145 .
van Griethuysen JJM, Fedorov A, Parmar C, et al. Computational Radiomics System to Decode the Radiographic phenotype. Cancer Res. 2017;77(21):e104–7. https://doi.org/10.1158/0008-5472.CAN-17-0339 .
Zwanenburg A, Leger S, Agolli L, et al. Assessing robustness of radiomic features by image perturbation. Sci Rep. 2019;9(1):614. https://doi.org/10.1038/s41598-018-36938-4 .
Teng X, Zhang J, Ma Z, et al. Improving radiomic model reliability using robust features from perturbations for head-and-neck carcinoma. Front Oncol. 2022;12:974467. https://doi.org/10.3389/fonc.2022.974467 .
Zhang J, Teng X, Zhang X, et al. Comparing effectiveness of image perturbation and test retest imaging in improving radiomic model reliability. Sci Rep. 2023;13(1):18263. https://doi.org/10.1038/s41598-023-45477-6 .
Koo TK, Li MY. A Guideline of selecting and reporting Intraclass correlation coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155–63. https://doi.org/10.1016/j.jcm.2016.02.012 .
Lubba CH, Sethi SS, Knaute P, Schultz SR, Fulcher BD, Jones NS. catch22: CAnonical time-series CHaracteristics. Data Min Knowl Discov. 2019;33(6):1821–52. https://doi.org/10.1007/s10618-019-00647-x .
Ding C, Peng H. Minimum redundancy feature selection from microarray gene expression data. J Bioinform Comput Biol. 2005;03(02):185–205. https://doi.org/10.1142/S0219720005001004 .
Lundberg SM, Lee SI. A Unified Approach to Interpreting Model Predictions. In: Advances in Neural Information Processing Systems . Vol 30. Curran Associates, Inc.; 2017. Accessed December 6, 2023. https://proceedings.neurips.cc/paper_files/paper/2017/hash/8a20a8621978632d76c43dfd28b67767-Abstract.html .
Fitzgerald M, Saville BR, Lewis RJ, Decision Curve Analysis. JAMA. 2015;313(4):409–10. https://doi.org/10.1001/jama.2015.37 .
NCBI. site.
Ashburner M, Ball CA, Blake JA, et al. Gene Ontology: tool for the unification of biology. Nat Genet. 2000;25(1):25–9. https://doi.org/10.1038/75556 .
The Gene Ontology Consortium, Aleksander SA, Balhoff J, et al. The Gene Ontology knowledgebase in 2023. Genetics. 2023;224(1):iyad031. https://doi.org/10.1093/genetics/iyad031 .
Kanehisa M, Furumichi M, Sato Y, Kawashima M, Ishiguro-Watanabe M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023;51(D1):D587–92. https://doi.org/10.1093/nar/gkac963 .
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. https://doi.org/10.1093/nar/28.1.27 .
Kanehisa M. Toward understanding the origin and evolution of cellular organisms. Protein Sci Publ Protein Soc. 2019;28(11):1947–51. https://doi.org/10.1002/pro.3715 .
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a Nonparametric Approach. Biometrics. 1988;44(3):837–45. https://doi.org/10.2307/2531595 .
Ihaka R, Gentleman R. R: a Language for Data Analysis and Graphics. J Comput Graph Stat. 1996;5(3):299–314. https://doi.org/10.1080/10618600.1996.10474713 .
Van Rossum G, Drake FL. Python 3 reference Manual. CreateSpace; 2009.
Pedregosa F, Varoquaux G, Gramfort A et al. Scikit-learn: machine learning in Python. Mach Learn PYTHON .
Wolf FA, Angerer P, Theis FJ. SCANPY: large-scale single-cell gene expression data analysis. Genome Biol. 2018;19(1):15. https://doi.org/10.1186/s13059-017-1382-0 .
Fang Z, Liu X, Peltz G. GSEApy: a comprehensive package for performing gene set enrichment analysis in Python. Bioinformatics. 2023;39(1):btac757. https://doi.org/10.1093/bioinformatics/btac757 .
Prinzi F, Orlando A, Gaglio S, Vitabile S. Breast cancer classification through multivariate radiomic time series analysis in DCE-MRI sequences. Expert Syst Appl. 2024;249:123557. https://doi.org/10.1016/j.eswa.2024.123557 .
Teng X, Zhang J, Zhang X, et al. Noninvasive imaging signatures of HER2 and HR using ADC in invasive breast cancer: repeatability, reproducibility, and association with pathological complete response to neoadjuvant chemotherapy. Breast Cancer Res. 2023;25(1):77. https://doi.org/10.1186/s13058-023-01674-9 .
Bismeijer T, van der Velden BHM, Canisius S, et al. Radiogenomic analysis of breast Cancer by linking MRI phenotypes with Tumor Gene expression. Radiology. 2020;296(2):277–87. https://doi.org/10.1148/radiol.2020191453 .
Liu H, Yang Z, Lu W, et al. Chemokines and chemokine receptors: a new strategy for breast cancer therapy. Cancer Med. 2020;9(11):3786–99. https://doi.org/10.1002/cam4.3014 .
Lyu L, Yao J, Wang M et al. Overexpressed Pseudogene HLA-DPB2 Promotes Tumor Immune Infiltrates by Regulating HLA-DPB1 and Indicates a Better Prognosis in Breast Cancer. Front Oncol . 2020;10. Accessed December 19, 2023. https://www.frontiersin.org/articles/ https://doi.org/10.3389/fonc.2020.01245 .
Lin L, Chen YS, Yao YD, et al. CCL18 from tumor-associated macrophages promotes angiogenesis in breast cancer. Oncotarget. 2015;6(33):34758–73.
Download references
This research was partly supported by research grants of Mainland-Hong Kong Joint Funding Scheme (MHKJFS) (MHP/005/20), Shenzhen Basic Research Program (JCYJ20210324130209023), Project of Strategic Importance Fund (P0035421), Projects of RISA (P0043001) and Projects of RI-IWEAR (P0038684) from The Hong Kong Polytechnic University, Innovation and Technology Fund (ITS/047/22), Health and Medical Research Fund (HMRF 09200576), the Health Bureau, The Government of the Hong Kong Special Administrative Region.
Author information
Authors and affiliations.
Department of Health Technology and Informatics, The Hong Kong Polytechnic University, Hong Kong, China
Xinyu Zhang, Xinzhi Teng, Jiang Zhang, Qingpei Lai & Jing Cai
The Hong Kong Polytechnic University Shenzhen Research Institute, Shenzhen, China
You can also search for this author in PubMed Google Scholar
Contributions
XY.Z. and J.C. contributed to the conceptualization and all the authors were involved in study design. Data collection and preprocessing were performed by XZ.T. and J.Z. Data analysis and model development were performed by XY.Z. The draft of the manuscript was written by XY.Z. XZ.T., J.Z., QP.L., and J.C. commented and revised on previous versions of the manuscript. All the authors read and approve the final version of the manuscript.
Corresponding author
Correspondence to Jing Cai .
Ethics declarations
Ethics approval and consent to participate.
Not applicable. A public dataset was used.
Consent for publication
Competing interests.
The authors declare no relevant financial or non-financial interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Zhang, X., Teng, X., Zhang, J. et al. Enhancing pathological complete response prediction in breast cancer: the role of dynamic characterization of DCE-MRI and its association with tumor heterogeneity. Breast Cancer Res 26 , 77 (2024). https://doi.org/10.1186/s13058-024-01836-3
Download citation
Received : 05 February 2024
Accepted : 07 May 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s13058-024-01836-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Treatment response prediction
Breast Cancer Research
ISSN: 1465-542X
- Submission enquiries: [email protected]
Measuring serum oestrogen levels in breast cancer survivors using vaginal oestrogens: a systematic review
- Open access
- Published: 23 May 2024
Cite this article
You have full access to this open access article

- Antonia Pearson ORCID: orcid.org/0000-0001-6966-5586 1 ,
- Jill Chen 2 ,
- Haryana M. Dhillon ORCID: orcid.org/0000-0003-4039-5169 2 &
- Belinda E. Kiely 1 , 3
33 Accesses
2 Altmetric
Explore all metrics
Vaginal oestrogens can be used to treat genitourinary symptoms in women with early breast cancer. Studies evaluating vaginal oestrogens have commonly measured serum oestrogen levels as a surrogate marker of safety, but methods vary. We sought to summarise the data on serum oestrogen measurement in women with breast cancer using vaginal oestrogens to better understand the methods, levels and reliability.
We searched Medline, Embase, CENTRAL, SCOPUS and CINAHL from inception to October 2023 for clinical studies where serum oestrogen was measured in women with a history of early breast cancer using vaginal oestrogens. Studies with a reported testing methodology were included.
Nine studies met the inclusion criteria for this systematic review. Methods used to measure oestradiol and oestriol in selected studies included mass spectrometry and immunoassays; several studies used more than one with variable concordance. Mass spectrometry detected oestradiol levels down to a lower limit between 1.0 pg/mL and 3.0 pg/mL. Immunoassays such as ELISA (enzyme-linked immunosorbent assay), ECLIA (enhanced chemiluminiscence immunoassay) and RIA (radioimmunoassay) had lower detection limits ranging between 0.8 pg/mL and 10 pg/mL. Studies were heterogeneous in testing techniques used, timing of testing, and the population including with subsequent varying results in the effect on oestrogens reported.
Conclusions
Adopting consistent and standardised methods of measuring oestrogens in clinical trials involving women with early breast cancer on vaginal oestrogens is critical. Serum oestrogens are used as a surrogate marker of safety in this population, and good-quality data are necessary to enable clinicians and patients to feel confident in prescribing and taking vaginal oestrogens. Mass spectrometry, although more expensive, gives more reliable results when dealing with very low levels of oestrogens often found in women on aromatase inhibitors, compared to immunoassays.
Avoid common mistakes on your manuscript.
Introduction
Genitourinary Syndrome of Menopause (GSM) or vulvovaginal atrophy is reported in up to 75% of women after breast cancer (BC) [ 1 ]. Symptoms are more prevalent in women with BC compared to peers without [ 2 , 3 ]. Chemotherapy, treatment-induced early and rapid onset of menopause and endocrine therapy can all contribute to GSM. Changes in genital tissues, with decreasing blood flow and secretions, increased pH, epithelial thinning and loss of elasticity occur from chronic oestrogen depletion [ 4 ]. GSM can negatively impact sexual function and health-related quality of life [ 5 ], presenting as follows: vaginal dryness; vaginal itch or burning; dysuria; urinary urgency; urinary incontinence; dyspareunia; loss of libido; dysfunction of arousal and orgasm; and increased urinary tract infections [ 4 , 6 ].
Treatments for GSM include vaginal moisturisers and lubricants, oral or transdermal hormone replacement therapy, vaginal oestrogen therapies, and vaginal laser [ 7 ]. For women with a history of BC, particularly hormone receptor-positive (HR+) early breast cancer (EBC), systemic hormone-based therapies are not recommended due to concerns they increase BC recurrences by stimulating tumour growth [ 4 ]. However, vaginal oestrogens are considered safe for women with BC based on several studies showing no increased risk of de novo BC, [ 8 , 9 , 10 ] or recurrences [ 11 , 12 , 13 ]. International guidelines support use of vaginal oestrogens in women with BC, when non-hormonal interventions are unsuccessful in treating symptoms [ 14 , 15 ]. Cold et al.’s cohort study [ 16 ] has renewed debate regarding vaginal oestrogen safety. Overall, there was no increased BC recurrences or mortality in women with EBC using vaginal oestrogens, but a subgroup analysis showed increased recurrences in women on aromatase inhibitors (AI) but not in women on tamoxifen [ 16 ], which may lead to some preferences for tamoxifen as a result.
To definitively establish safety, a large, prospective, randomised trial of vaginal oestrogens in women with BC assessing recurrences and mortality is required. Such a trial would require thousands of women and many years of follow-up, making it is unlikely to be conducted. Instead, measurement of serum oestrogen levels in women using vaginal oestrogens has been a key method of assessing safety.
The primary oestrogens are oestrone (E1), oestradiol (E2) and oestriol (E3). Available vaginal oestrogen preparations contain oestradiol and oestriol, so measurement of these oestrogens in the serum is of greatest interest. Oestradiol is the most frequently tested oestrogen. Oestriol is less potent and shorter acting than oestradiol and cannot be converted back into oestradiol [ 17 , 18 ]. The problem with measuring oestrogen levels is the lack of clearly defined safe levels of serum oestrogen for women with BC. The normal range for median oestradiol in postmenopausal women ranges from 5 to 27 pg/ml [ 19 , 20 ]. For women on AIs, which suppress oestradiol by up to 99% [ 20 ], serum oestradiol levels are typically lower with mean levels reported between 2 and 10 pg/ml [ 19 , 20 ], and with ultrasensitive testing median oestradiol levels as low as < 0.1 pg/ml [ 20 ]. Previous studies used multiple methodologies for measuring oestrogens with varying sensitivity and reliability, making interpretation challenging. For example, results from pooled individual patient data from nine prospective case–control studies revealed an increased relative risk of BC recurrence of 1.29 (95% CI, 1.15–1.44, p < 0.001) for every doubling of oestradiol (E2) concentration [ 21 ]. One study defined a prolonged rise of oestradiol as > 10 pg/mL and > 10 pg/mL above baseline on 2 consecutive blood tests > 2 weeks apart [ 22 ].
Any method used to measure serum oestrogens in clinical trials and practice must meet these criteria: ability to detect very low levels; high specificity; and reproducibility [ 23 ]. Commercially available oestradiol testing is often unable to detect very low levels of oestradiol (E2) induced by AI. Mass spectrometry (liquid chromatography mass spectrometry [LC-MS] or gas chromatography mass spectrometry [GC-MS]) and immunoassays have been used in clinical trials, but do not always show concordance [ 24 ]. Mass spectrometry appears more accurate at detecting low levels of oestrogens [ 20 , 23 , 25 ]. However, access and cost prohibit use outside clinical trials.
Our systematic review aimed to (i) identify methods used to test oestradiol, oestriol and other hormones; (ii) summarise E2 and E3 levels reported; (iii) assess the quality of reported assays, in women with HR+ EBC using vaginal oestrogens.
Our systematic review was conducted according to PRISMA guidelines [ 26 ]. Searches were conducted in October 2023 using five databases: Medline, Embase, CENTRAL (Cochrane Register of Controlled Trials), SCOPUS, and CINAHL (Cumulative Index to Nursing and Allied Health Literature). Table 3 shows search terms used. Reference lists of relevant studies were hand searched and a Google Scholar search performed to identify additional studies.
Eligible studies included randomised controlled trials (RCTs) and other clinical intervention studies. Study inclusion criteria were postmenopausal women with a history of HR+ EBC receiving any form of vaginal oestrogen treatment. To be included, oestradiol and/or oestriol had to be measured and techniques and testing parameters reported. Studies involving systemic (oral or transdermal) oestrogen treatments were excluded. Conference abstracts were permitted where required information was accessible.
Following database searches, studies were imported to Covidence [ 27 ], and duplicates identified. Two authors (AP; JC) independently screened titles and abstracts to determine eligibility. Full manuscripts were independently reviewed by both authors, with disagreements reviewed by BK.
Data extracted included design, duration, inclusion criteria, menopausal status and age, use and type of endocrine therapy, type of vaginal oestrogen (including dose, frequency, duration), control group, sample size, oestrogen testing timepoints, oestrogens measured (for each: level, testing method, unit of measurement, detection limits, normal range), other sex hormones measured. Study quality and risk of bias were to be assessed if possible.
The PRISMA flow diagram (Fig. 1 ) depicts 789 citations identified, of which 201 were duplicates and 547 excluded on title and abstract screening. After full-text review, nine studies [ 22 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 ] met the inclusion criteria (See Table 1 ). Of these, three were RCTs, two case–control studies, and four single-arm studies. Oestrogen testing methodology is outlined in Table 2 .
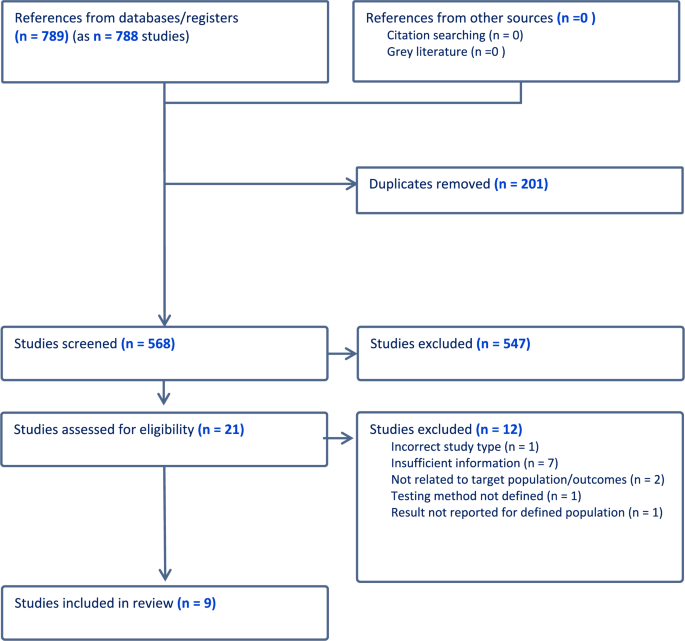
PRISMA flow diagram
Methods used to measure oestradiol and oestriol in selected studies included mass spectrometry and immunoassays; several studies used more than one with variable concordance [ 22 , 33 ]. Mass spectrometry detected oestradiol levels down to a lower limit between 1.0 pg/mL and 3.0 pg/mL [ 22 , 29 , 33 , 34 , 35 , 36 ]. Immunoassays such as ELISA (enzyme-linked immunosorbent assay), ECLIA (enhanced chemiluminiscence immunoassay) and RIA (radioimmunoassay) had lower detection limits ranging between 0.8 pg/mL and 10 pg/mL [ 22 , 28 , 30 , 32 , 33 , 34 , 36 ].
The highest quality and largest study was Sanchez-Rovira et al.’s trial of vaginal oestriol [ 35 ]. Sixty-one women with HR+ EBC taking an AI for > 6 months were randomised to a 0.005% oestriol gel (50mcg oestriol per application) or placebo moisturising gel daily for 3 weeks then twice weekly for 9 weeks. Serum oestrogen levels were measured at baseline, 3, 8, and 12 weeks via ultrasensitive LC-MS (detection limit of 3 pg/mL for oestradiol and 1 pg/mL for oestriol) [ 37 ]. There was no significant rise in E2 detected. A small rise in E3 was detected in the treatment group in the first 3 weeks returning to same level as the placebo group by 12 weeks (median, [interquartile range IQR], E3 = 0.5 pg/mL [0.5 to 7.3] in the treatment group and 0.5 pg/mL [0.5 to 0.5] placebo group, p = 0.140). Oestrone, follicle stimulating hormone (FSH) and luteinising hormone (LH) had no significant changes detected at any timepoint.
The REVIVE study [ 33 ], an ongoing open-label RCT, reported serum hormone levels for the first eight participants. This study compares a vaginal oestradiol ring (Estring®) which releases approximately 7.5mcg oestradiol daily for 90 days to a polycarbophil-based vaginal moisturiser (Replens ™) in women with HR+ EBC taking an AI. Two different ELISA kits were used to quantify oestradiol (E2) with a detection limit of 3 pg/mL. There was concordance between two ELISA kit measurements for 5/6 patients, but one had different results: E2 < 3 pg/mL versus E2 67 pg/mL. Samples from three patients were analysed using LC-MS/MS with a detection limit of 1 pg/mL. The values of E2 between different testing methods (ELISA and LC-MS/MS) varied markedly. One patient had higher readings of E2 on both ELISA kits compared to LC-MS/MS, and the degree of elevation varied between the two kits. Another patient had elevation in E2 at 2 weeks on LC-MS/MS, not detected on ELISA. The third patient was taking exemestane, an AI known to cause aberrant results via immunoassays due to cross-reactivity [ 20 , 38 ]. Her results showed the anticipated rise in E2 on ELISA, but corresponding LC-MS/MS testing showed an elevated level of E2 at baseline but levels < 2 pg/mL at all other timepoints, attributed to a laboratory error. Although the RCT design is sound, this sub-study is fraught with issues: small sample size ( N = 8), heterogeneity of testing methodologies, and discordant results.
Melisko et al. [ 22 ] conducted a randomised open-label phase 2 study comparing vaginal testosterone cream to a vaginal oestradiol ring (Estring®) in 76 women with HR + EBC on AI for > 30 days. The vaginal testosterone cream was used daily for 2 weeks then three times per week for 10 weeks for the first 24 participants then changed to three times per week for the 12-week study duration for the subsequent 12 participants. Both RIA and ultrasensitive LC-MS methods were used to measure oestradiol (E2) with a detection limit of 3 pmol/L (0.82 pg/mL) for RIA and no reported detection limit for LC-MS. The authors predefined < 10 pg/mL as the expected postmenopausal level of oestradiol, and the primary safety endpoint was defined as < 25% of participants having a persistent elevation of E2 (> 10 pg/mL and at least > 10 pg/mL above baseline on two consecutive blood tests more than two weeks apart) and was met for both arms in this study. 63 participants had E2 measured with both methods and surprisingly of these, 25 (40%) had baseline E2 above the 10 pg/mL threshold with LC-MS and 9 (14%) with RIA. In the oestradiol ring arm, 4/35 participants had a transient rise in E2 (range 11–29 pg/mL) but none had persistent elevation. No differences were detected in mean E2 levels between LC-MS and RIA at baseline (17.7 [SD 28.5] pg/mL and 17.9 [SD 44.1] pg/mL, respectively) or at week 4 (7.8 [SD 15.0] pg/mL and 2.9 [SD 13.4] pg/mL, respectively). There was some variability of reported E2 levels between RIA and LC-MS on review of matched samples, but discordant E2 elevation was rare (2/63 participants with matched samples). Similar to REVIVE [ 33 ], Melisko et al. found that LC-MS was more likely than RIA to detect an early rise in E2, 19% of participants had elevated E2 at 4 weeks with LC–MS vs. 1.5% with RIA. This study had a reasonable sample size, with most patients (63/76) having matched samples, allowing greater reliability of concordance assessment for testing methods. Although very discordant results were rare, there was still variability and RIA failed to detect early rises in E2 which were detected with LC-MS.
A prospective, open-label, single-arm study by Kendall et al. which showed a persistent rise in oestradiol (E2) levels, [ 32 ] is often used as a basis for concern regarding safety of vaginal oestrogens. In this study, six women with HR+ EBC on AI for > 6 months used a 25mcg vaginal oestradiol pessary (Vagifem®) daily for two weeks, then twice weekly for 10 weeks. Oestradiol (E2) was measured using RIA with a detection limit of 3 pmol/L (0.82 pg/mL). One participant on exemestane had high baseline levels of E2 attributed to cross-reactivity with immunoassays [ 38 ]. E2 levels increased at two weeks in 5/6 participants (from median 0.82 pg/mL at baseline to 19.61 pg/mL) and decreasing to median 4.36 pg/mL at 4 weeks and stayed at this level for most participants. However, two participants had persistently raised E2 when tested between 7 and 10 weeks (37.32 pg/mL and 59.65 pg/mL), one of whom was on exemestane. This study has several limitations including a very small sample size, no randomisation, heterogeneity in testing schedules, and variable reported oestrogen levels. The study also used a 25mcg oestradiol pessary, which has been subsequently replaced by a lower dose 10mcg preparation (Vagifem Low®).
Donders et al. [ 29 ] enrolled 16 women with HR+ EBC on AI for > 6 months to take a vaginal tablet containing 100 million acidophilus KS400 and 0.03 mg oestriol (Gynoflor®) daily for 4 weeks, then three times per week for 8 weeks. The authors used a highly sensitive GC-MS to quantify oestradiol (E2) and oestriol (E3) with a detection limit of 1 pg/mL and 10 pg/mL, respectively. As expected, there was no rise in E2 (as oestriol does not back convert to oestradiol [ 17 , 18 ]), but there was a small transient rise in E3, most pronounced after the first dose. Oestrone, LH, FSH and sex hormone-binding globulin (SBHG) were tested, with no change detected except in FSH which had a small but significant decrease at 4 weeks. Again, this study is limited by a very small sample size and its single-arm design.
A short study by Pfeiler et al. [ 34 ] assessed use of oestriol 0.5 mg pessaries (Ovestin®) daily for two weeks in 10 women with HR + EBC on anastrozole. Oestradiol (E2) was quantified at baseline and 2 weeks using both ECLIA (detection limit of 10 pg/mL) and GC-MS (unreported detection limit). No significant change was reported for E2 or E3, but mean FSH and LH levels decreased significantly from baseline to 2 weeks (LH − 10.8%, p = 0.02; FSH − 12.8%, p = 0.01). This is a very small, single-arm study of two-week duration; thus, the duration of effect on FSH and LH remains unknown. Both ECLIA and GC-MS were performed, but detection limits were much higher for ECLIA than in other studies, so small rises in E2 could have been missed.
Biglia et al.’s [ 28 ] non-randomised three-arm study compared 0.25 mg oestriol cream, 12.5mcg oestradiol pessaries (Vagifem®), and a vaginal moisturiser (Replens™) in 26 women with HR + EBC on endocrine treatment. Unlike other studies reviewed, women on AI were not permitted in the vaginal oestrogen groups (but permitted in moisturiser group). RIA was used to quantify oestradiol (E2) with a detection limit of 5 pg/mL. No significant difference was found in E2 levels from baseline to week 12 or additionally, in E3, E1, LH, FSH, testosterone and SHBG. Although there were no reported issues with E2 RIA testing, the lower detection limit of 5 pg/mL is not as sensitive as methods used in other studies. Given most patients were not on an AI, the impact of small rises in oestradiol is less concerning.
Wills et al.’s prospective case–control study enrolled 48 women with HR+ EBC or an increased risk of developing BC (all on endocrine therapy) and compared cases of those on vaginal oestrogens for > 3 months (25mcg oestradiol pessary twice weekly or vaginal oestradiol ring inserted every 90 days), with a no vaginal oestrogen control group [ 30 ]. Oestradiol (E2) levels were measured using RIA with a detection limit of 3 pmol/L (0.82 pg/mL). For women using oestrogen pessaries for > 3 months, pre-insertion levels of E2 were not elevated compared to controls, although 12 h post-insertion E2 levels were raised. For those using the oestrogen ring for > 3 months, pre-insertion mean E2 levels were already elevated compared to controls suggesting a persistent elevation in E2 in this group. This study implemented a novel approach testing oestrogen levels pre- and post-insertion in women who had used vaginal oestrogens for > 3 months, with the intent of capturing whether persistent elevation in oestrogens occurred before insertion and after.
Streff et al. conducted a prospective study of vaginal oestradiol rings (Estring®) in women with HR+ EBC on AI [ 36 ]. This study included 8 prospective participants and 6 retrospective participants who had oestradiol (E2) quantification via tandem mass spectrometry or ECLIA with a variety of laboratories and reference ranges. Baseline E2 levels were in the expected range, but after commencing the oestradiol ring, 6/8 prospective participants had a transient rise in E2 (at week 4) which returned to baseline levels by week 16. The study quality was low due to small sample size, heterogeneous testing methods and no clear definition of the detection limits used.
Due to the heterogeneity of studies included in this systematic review, a formal bias assessment was not possible.
We identified a variety of testing methodologies used to quantify serum oestradiol (E2) and oestriol (E3) from included studies. Some used multiple techniques with mixed concordance across testing methods. Overall, the quality of studies was poor with considerable heterogeneity of testing techniques, timing and populations. There was also a mixture of concordant and discordant results when more than one methodology was used. This creates a confusing landscape to determine the impact vaginal oestrogens may have on oestrogen levels in women with BC. In addition, the impact of small, and often transient elevations in serum oestrogen levels on outcomes of women with BC remains unknown.
Folkerd et al. [ 39 ] cautioned against false interpretations from studies testing serum oestrogens with potentially erroneous quantification methods. The REVIVE study highlights challenges of using different testing methodologies for measurement of low levels of oestradiol (E2), with discordant oestrogen levels between methods [ 33 ].
Immunoassay methodologies (RIA, ECLIA, ELISA) can detect low levels of oestrogens, but are prone to inaccuracies [ 39 ], and cannot detect levels as low as mass spectrometry. Caution is required for patients taking exemestane, an AI known to falsely elevate oestradiol levels when immunoassays are used [ 38 ]. Immunoassays appear less likely to detect early rises in oestradiol levels in women using vaginal oestrogens compared to mass spectrometry [ 22 , 33 ]. A recent study of women with EBC on letrozole showed no concordance between oestrogen levels measured with immunoassays compared to mass spectrometry [ 40 ]. Given multiple confounding issues with results from sensitive immunoassays, mass spectrometry appears the most reliable testing methodology in women with BC on vaginal oestrogens, particularly those on AI.
Vaginal oestrogen formulations varied considerably between included studies. Kendall et al. [ 32 ] and Wills et al. [ 30 ] used 25mcg oestradiol tablets (Vagifem®), replaced by lower dosage 10mcg formulation (Vagifem® Low), which remains effective for symptom relief with less systemic absorption [ 41 , 42 ]. Oestradiol is also the oestrogen used in the ring (Estring®), whereas the other formulations were oestriol based, a less potent oestrogen which cannot be converted to oestradiol [ 17 , 18 ].
Oestrogen levels increase after initiation of vaginal oestrogens due to a denuded vaginal epithelium, tapering over time as the epithelium recovers and absorption decreases [ 43 , 44 ]. This transient rise was demonstrated in several studies [ 22 , 29 , 30 , 32 , 35 , 36 ]. When considering the impact of vaginal oestrogens on BC recurrence risk, transient raised oestrogen levels are less concerning than a persistent rise. Oestrogen levels should be measured after vaginal oestrogens have been used for a few months. Of studies showing a persistent rise in oestrogen [ 30 , 32 ], both assessed women using higher dose vaginal oestradiol products than typically recommended in women with BC. Wills et al. [ 30 ] showed persistent elevations of oestradiol (E2) at 60 days post-insertion in women using an oestradiol ring; however, in the oestradiol 25mcg tablet group, there were no elevations in oestradiol after 3 months of treatment, suggesting that any rise in E2 post-oestradiol tablet insertion is not persistent. Contrastingly, Kendall et al. [ 32 ] found persistently elevated E2 in two patients using 25mcg vaginal oestradiol tablets for 7–10 weeks, but no week 12 test was performed. This may reflect a true significant rise in E2 but may be due to inaccuracies of RIA.
Several studies in our systematic review are of low quality, and reasons include small sample size; retrospective design; mixed methodologies for testing oestrogens; inconsistencies in timing of oestrogen testing; lack of randomisation; and frequent lack of a control group. Despite significant limitations, these studies are often used to justify withholding vaginal oestrogens from women experiencing GSM because concerns use could increase BC recurrence risk.
It is essential that larger, more robust, and reliable studies are performed for this population. This includes a more uniform approach to oestrogen testing in clinical trials and practice. A better understanding of oestrogen levels and fluctuations over time in postmenopausal women with EBC not using vaginal oestrogens is essential; future studies should include a control group. Larger RCTs are needed to demonstrate the impact of longer-term use of vaginal oestrogens on oestrogen levels, given factors potentially influencing them including adherence, testing methodology, sample storage, timing of testing, and cross-reactivity with immunoassays. Relying on small studies to suggest a safety signal is problematic.
Limitations of our systematic review include the small number of included studies and heterogeneity of their design, size, intervention, and testing methods. Consequently, we were unable to perform a formal quality and bias assessment.
Inconsistent measurement and reporting of serum E2 and E3 levels in women with EBC on endocrine therapy creates uncertainty in safety of vaginal oestrogens, generating reluctance in clinicians to prescribe and patients to use vaginal oestrogens, even when symptoms are severe. More robust and standardised methods of measuring E2/E3 are critical to improve our understanding of the impact of vaginal oestrogens on serum oestrogen levels. In the absence of prospective randomised trials assessing BC outcomes, measurement of serum oestrogens remains the main method of assessing safety. Good quality data are required to increase confidence of women and clinicians worldwide.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
Aromatase inhibitor
Breast Cancer
Early breast cancer
Enhanced chemiluminiscence immunoassay
Enzyme-linked immunosorbent assay
Follicle stimulating hormone
Gas chromatography mass spectrometry
- Genitourinary syndrome of menopause
Hormone receptor positive
Liquid chromatography mass spectrometry
Lutenising hormone
Randomised controlled trial
Radioimmunoassay
Sex hormone-binding globulin
Vaginal oestrogen
Mazzarello S et al (2015) Management of urogenital atrophy in breast cancer patients: a systematic review of available evidence from randomized trials. Breast Cancer Res Treat 152(1):1–8
Article CAS PubMed Google Scholar
Lester J, Bernhard L, Ryan-Wenger N (2012) A self-report instrument that describes urogenital atrophy symptoms in breast cancer survivors. West J Nurs Res 34(1):72–96
Article PubMed Google Scholar
Goodwin PJ et al (1999) Risk of menopause during the first year after breast cancer diagnosis. J Clin Oncol 17(8):2365–2370
Lester J et al (2015) Atrophic vaginitis in breast cancer survivors: a difficult survivorship issue. J Pers Med 5(2):50–66
Article PubMed PubMed Central Google Scholar
Salvatore S et al (2015) Sexual function after fractional microablative CO(2) laser in women with vulvovaginal atrophy. Climacteric 18(2):219–225
Portman DJ, Gass ML (2014) Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Menopause 21(10):1063–1068
Biglia N et al (2015) Genitourinary syndrome of menopause in breast cancer survivors: are we facing new and safe hopes? Clin Breast Cancer 15(6):413–420
Collaborative Group on Hormonal Factors in Breast Cancer (2019) Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. The Lancet
Bhupathiraju SN, Bhupathiraju SN, Grodstein F, Stampfer MJ, Willett WC, Crandall CJ, Shifren JL, Manson JE et al (2019) Vaginal estrogen use and chronic disease risk in the Nurses’ Health Study. Menopause 26(6):603–61
Article Google Scholar
Crandall CJ et al (2018) Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause 25(1):11–20
Dew JE, Wren BG, Eden JA (2003) A cohort study of topical vaginal estrogen therapy in women previously treated for breast cancer. Climacteric 6(1):45–52
O’Meara ES et al (2001) Hormone replacement therapy after a diagnosis of breast cancer in relation to recurrence and mortality. J Natl Cancer Inst 93(10):754–762
Le Ray I et al (2012) Local estrogen therapy and risk of breast cancer recurrence among hormone-treated patients: a nested case-control study. Breast Cancer Res Treat 135(2):603–609
Faubion SS et al (2022) The 2022 hormone therapy position statement of The North American Menopause Society. Menopause 29(7):767–794
Stuenkel CA et al (2015) Treatment of symptoms of the menopause: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 100(11):3975–4011
Cold S et al (2022) Systemic or vaginal hormone therapy after early breast cancer: a Danish Observational Cohort Study. J Natl Cancer Inst 114(10):1347–1354
Head KA (1998) Estriol: safety and efficacy. Altern Med Rev 3(2):101–113
CAS PubMed Google Scholar
van der Vies J (1982) The pharmacology of oestriol. Maturitas 4(4):291–299
Wang S et al (2005) Recombinant cell ultrasensitive bioassay for measurement of estrogens in postmenopausal women. J Clin Endocrinol Metab 90(3):1407–1413
Santen RJ et al (2007) Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids 72(8):666–671
Key T et al (2002) Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst 94(8):606–616
Melisko ME et al (2017) Vaginal testosterone cream vs estradiol vaginal ring for vaginal dryness or decreased libido in women receiving aromatase inhibitors for early-stage breast cancer a randomized clinical trial. JAMA Oncol 3(3):313–319
Rosner W et al (2013) Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab 98(4):1376–1387
Article CAS PubMed PubMed Central Google Scholar
Dowsett M, Folkerd E (2004) Deficits in plasma oestradiol measurement in studies and management of breast cancer. Breast Cancer Res 7(1):1
Jaque J et al (2013) Deficiencies in immunoassay methods used to monitor serum Estradiol levels during aromatase inhibitor treatment in postmenopausal breast cancer patients. Springerplus 2(1):5
Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. www.covidence.org . Accessed 2023
Biglia N et al (2010) Low-dose vaginal estrogens or vaginal moisturizer in breast cancer survivors with urogenital atrophy: a preliminary study. Gynecol Endocrinol 26(6):404–412
Donders G et al (2014) Ultra-low-dose estriol and Lactobacillus acidophilus vaginal tablets (Gynoflor()) for vaginal atrophy in postmenopausal breast cancer patients on aromatase inhibitors: pharmacokinetic, safety, and efficacy phase I clinical study. Breast Cancer Res Treat 145(2):371–379
Wills S et al (2012) Effects of vaginal estrogens on serum estradiol levels in postmenopausal breast cancer survivors and women at risk of breast cancer taking an aromatase inhibitor or a selective estrogen receptor modulator. J Oncol Pract 8(3):144–149
Hirschberg AL et al (2020) Efficacy and safety of ultra-low dose 0.005% estriol vaginal gel for the treatment of vulvovaginal atrophy in postmenopausal women with early breast cancer treated with nonsteroidal aromatase inhibitors: a phase II, randomized, double-blind, placebo-controlled trial. Menopause 27(5):526–534
Kendall A et al (2006) Caution: vaginal estradiol appears to be contraindicated in postmenopausal women on adjuvant aromatase inhibitors. Ann Oncol 17(4):584–587
Niravath P et al (2017) Challenges of measuring accurate estradiol levels in aromatase inhibitor-treated postmenopausal breast cancer patients on vaginal estrogen therapy. Pharmacol Res Perspect 5(4):7
Pfeiler G et al (2011) Vaginal estriol to overcome side-effects of aromatase inhibitors in breast cancer patients. Climacteric 14(3):339–344
Sanchez-Rovira P et al (2020) A phase II prospective, randomized, double-blind, placebo-controlled and multicenter clinical trial to assess the safety of 0.005% estriol vaginal gel in hormone receptor-positive postmenopausal women with early stage breast cancer in treatment with aromatase inhibitor in the adjuvant setting. Oncologist 25(12):e1846-1854
Streff A, Streff A, Chu-Pilli M, Stopeck A, Chalasani P et al (2021) Changes in serum estradiol levels with Estring in postmenopausal women with breast cancer treated with aromatase inhibitors. Support Care Cancer 29:187
Sanchez Rovira P et al (2016) A phase II prospective, randomized, double-blind, placebo-controlled multi-centre clinical trial to assess the safety of 0.005% estriol vaginal gel in hormone receptor-positive postmenopausal women with early stage breast cancer in treatment with aromatase inhibitors (AIs) in the adjuvant setting-Initial safety results. Eur J Cancer 2:S44
Google Scholar
Mandic S et al (2017) Falsely elevated serum oestradiol due to exemestane therapy. Ann Clin Biochem 54(3):402–405
Folkerd EJ, Lonning PE, Dowsett M (2014) Interpreting plasma estrogen levels in breast cancer: caution needed. J Clin Oncol 32(14):1396–1400
Faltinová M et al (2023) Effects of letrozole on serum estradiol and estrone in postmenopausal breast cancer patients and tolerability of treatment: a prospective trial using a highly sensitive LC-MS/MS (liquid chromatography-tandem mass spectrometry) method for estrogen measurement. Breast Cancer Res Treat 201(3):425–435
Goldfarb SB et al (2012) Limited absorption of low dose 10 μg intravaginal 17-β estradiol (vagifem) in postmenopausal women with breast cancer on aromatase inhibitors. Cancer Res. https://doi.org/10.1158/0008-5472.SABCS12-P2-12-05
Goldfarb SB et al (2013) Use of intravaginal 17-β estradiol to improve sexual function and menopausal symptoms in postmenopausal women with breast cancer on aromatase inhibitors. J Clin Oncol. https://doi.org/10.1200/jco.2013.31.15_suppl.9610
Heimer G, Englund D (1984) Estriol: absorption after long-term vaginal treatment and gastrointestinal absorption as influenced by a meal. Acta Obstet Gynecol Scand 63(6):563–567
Buhling KJ et al (2012) Systemic bioavailability of estriol following single and repeated vaginal administration of 0.03 mg estriol containing pessaries. Arzneimittelforschung 62(8):378–83
Download references
Open Access funding enabled and organized by CAUL and its Member Institutions. The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and affiliations.
Sydney Medical School, University of Sydney, Camperdown, NSW, Australia
Antonia Pearson & Belinda E. Kiely
Psycho-Oncology Cooperative Research Group, Faculty of Science, School of Psychology, The University of Sydney, Camperdown, NSW, Australia
Jill Chen & Haryana M. Dhillon
NHMRC Clinical Trials Centre, University of Sydney, Camperdown, NSW, Australia
Belinda E. Kiely
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Dr Antonia Pearson and Jill Chen. The first draft of the manuscript was written by Antonia Pearson, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Antonia Pearson .
Ethics declarations
Conflict of interest.
Author TP has received conference support from GSK and Novartis Author JC declares they have no financial interests. Author HD has received speaker and consultant honoraria from MDS, BMS, Janssen. Author BEK reports: honoraria for speaking from Novartis, Eisai, MSD Oncology, Gilead; payment for advisory board participation from Gilead and Novartis; support for conferences from Novartis, Pfizer and MSD Oncology.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Pearson, A., Chen, J., Dhillon, H.M. et al. Measuring serum oestrogen levels in breast cancer survivors using vaginal oestrogens: a systematic review. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07364-0
Download citation
Received : 07 February 2024
Accepted : 24 April 2024
Published : 23 May 2024
DOI : https://doi.org/10.1007/s10549-024-07364-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Vaginal atrophy
- Vaginal estrogen
- Estrogen testing
- Find a journal
- Publish with us
- Track your research
Breast Cancer Research Results and Study Updates
See Advances in Breast Cancer Research for an overview of recent findings and progress, plus ongoing projects supported by NCI.
Some people with no evidence of cancer in nearby lymph nodes after presurgical chemotherapy can skip radiation to that area without increasing the risk of the cancer returning, a clinical trial found. But some experts caution that more details are needed.
For women in their 70s and older, the risk of overdiagnosis with routine screening mammography is substantial, a new study suggests. The findings highlight the need for conversations between older women and their health care providers about the potential benefits and harms of continuing screening mammography.
Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
For younger women with advanced breast cancer, the combination of ribociclib (Kisqali) and hormone therapy was much better at shrinking metastatic tumors than standard chemotherapy treatments, results from an NCI-funded clinical trial show.
In a large clinical trial, a condensed course of radiation therapy was as effective and safe as a longer standard course for those with higher-risk early-stage breast cancer who had a lumpectomy. This shorter radiation course makes treatment less of a burden for patients.
Adding the immunotherapy drug pembrolizumab (Keytruda) to chemotherapy can help some patients with advanced triple-negative breast cancer live longer. In the KEYNOTE-355 trial, overall survival improved among patients whose tumors had high levels of the PD-L1 protein.
People with metastatic breast cancer whose tumors had low levels of HER2 protein lived longer after treatment with trastuzumab deruxtecan (Enhertu) than those treated with standard chemotherapy, results of the DESTINY-Breast04 clinical trial show.
NCI researchers have shown that an experimental form of immunotherapy that uses an individual’s own tumor-fighting immune cells could potentially be used to treat people with metastatic breast cancer who have exhausted all other treatment options.
Most breast cancer risk tools were developed with data mainly from White women and don’t work as well for Black women. A new tool that estimates risk for Black women may help identify those who might benefit from earlier screening, enabling earlier diagnosis and treatment.
In people with metastatic HER2-positive breast cancer, the targeted drug trastuzumab deruxtecan (Enhertu) markedly lengthened progression-free survival compared with trastuzumab emtansine (Kadcycla), new study results show.
In a large clinical trial, women with HR-positive, HER2-negative metastatic breast cancer treated with ribociclib (Kisqali) and letrozole (Femara) as their initial treatment lived approximately 1 year longer than women treated with letrozole only.
Women with early-stage breast cancer who had one or both breasts surgically removed (a unilateral or bilateral mastectomy) had lower scores on a quality-of-life survey than women who had breast-conserving surgery, a new study has found.
For women undergoing chemotherapy for breast cancer, meeting the national physical activity guidelines may help alleviate cognitive issues, a new study suggests. The benefits may be even greater for patients who were physically active before treatment.
Sacituzumab govitecan (Trodelvy) now has regular FDA approval for people with locally advanced or metastatic triple-negative breast cancer (TNBC). The update follows last year’s accelerated approval of the drug for people with TNBC.
For some people with ER-positive breast cancer, a new imaging test may help guide decisions about receiving hormone therapy, according to a new study. The test can show whether estrogen receptors in tumors are active and responsive to estrogen.
The test, which helps guide treatment decisions, was not as good at predicting the risk of death from breast cancer for Black patients as for White patients, a new study has found. The findings highlight the need for greater racial diversity in research studies.
The drug abemaciclib (Verzenio) may be a new treatment option for people with the most common type of breast cancer, with new study findings suggesting that it can reduce the risk of the cancer returning.
Fertility preservation for young women with breast cancer doesn’t increase their risk of dying in the ensuing decades, a new study affirmed. Experts said the findings support routinely offering fertility preservation to patients who want it.
Some postmenopausal women with HR-positive, HER2-negative breast cancer may not benefit from chemotherapy and can safely forgo the treatment, according to clinical trial results presented at the San Antonio Breast Cancer Symposium.
A heart-related event, like a heart attack, may make breast cancer grow faster, a new study suggests. In mice, heart attacks accelerated breast tumor growth and human studies linked cardiac events with breast cancer recurrence, researchers reported.
FDA has approved sacituzumab govitecan (Trodelvy) for the treatment of triple-negative breast cancer that has spread to other parts of the body. Under the approval, patients must have already undergone at least two prior treatment regimens.
Women with high-risk breast cancer who engaged in regular exercise before their cancer diagnosis and after treatment were less likely to have their cancer return or to die compared with women who were inactive, a recent study found.
Researchers have developed a “microscaled” approach to analyze the proteins and genetic changes (proteogenomics) of a tumor that uses tissue from a core needle biopsy. The analyses can provide important information that may help guide treatment.
Tucatinib improved survival for women in the HER2CLIMB trial, including some whose cancer had spread to the brain. Trastuzumab deruxtecan improved survival and shrank many tumors in the DESTINY-Breast01 trial, which led to its accelerated approval.
A TAILORx analysis shows women with early-stage breast cancer and high recurrence scores on the Oncotype DX who received chemotherapy with hormone therapy had better long-term outcomes than what would be expected from hormone therapy alone.
Men with breast cancer may be more likely to die of the disease than women, particularly during the first 5 years after diagnosis, a new study suggests. The higher likelihood of death was linked in part to undertreatment and later diagnosis.
In a survey of nearly 600 breast cancer survivors, researchers found that the cost of care factored into the decisions the women made about what type of surgery to get. Many women also reported never discussing costs with their physicians.
FDA has expanded the approved use of the drug ado-trastuzumab emtansine (Kadcyla), also called T-DM1, to include adjuvant treatment in some women with early-stage HER2-positive breast cancer.
Many women diagnosed with ovarian and breast cancer are not undergoing tests for inherited genetic mutations that can provide important information to help guide decisions about treatment and longer-term cancer screening, a new study has found.
FDA has approved atezolizumab (Tecentriq) in combination with chemotherapy for the treatment of some women with advanced triple-negative breast cancer. This is the first FDA-approved regimen for breast cancer to include immunotherapy.
The build-up of connective tissue around some types of cancer can act as a barrier to immunotherapy. A new study uses a bone marrow transplant drug, plerixafor, to break down this barrier and improve the efficacy of immune checkpoint inhibitors in animal models of breast cancer.
A new study in mice shows that disrupting the relationship between breast cancer cells that spread to bone and normal cells surrounding them makes the cancer cells sensitive to treatment.
In women with early-stage breast cancer, two clinical trials have shown that both whole- and partial-breast radiation therapy are effective at preventing the cancer from returning after breast-conserving surgery.
Researchers are testing a topical-gel form of the drug tamoxifen to see if it can help prevent breast cancer as effectively as the oral form of the drug but with fewer side effects.
Findings from a clinical study and a mouse study may shed light on genetic risk factors for developing cancer-related cognitive problems in older breast cancer survivors. The results suggest a gene associated with Alzheimer’s disease may play a role.
Arsenic trioxide and retinoic acid work together to target the master regulator protein Pin1, a new study shows. In cancer cell lines and mice, the drug combination slowed the growth of triple-negative breast cancer tumors.
FDA has expanded the approved uses of ribociclib (Kisqali) for women with advanced breast cancer, including new uses in pre- and postmenopausal women. It’s the first approval under a new FDA program to speed the review of cancer drugs.
Using a liquid biopsy to test for tumor cells circulating in blood, researchers found that, in women with breast cancer, the presence of these cells could identify women at risk of their cancer returning years later.
Findings from the TAILORx clinical trial show chemotherapy does not benefit most women with early breast cancer. The new data, released at the 2018 ASCO annual meeting, will help inform treatment decisions for many women with early-stage breast cancer.
Do cancer study participants want to receive their genetic test results? A recent study involving women with a history of breast cancer tested an approach for returning genetic research results and evaluated the impact those results had on the women.
Researchers compared the risk of death for women with breast cancer who had low skeletal muscle mass, or sarcopenia, at the time of their cancer diagnosis and women who had adequate muscle mass.
Some people who have been treated for breast cancer or lymphoma have a higher risk of developing congestive heart failure than people who haven’t had cancer, results from a new study show.
FDA has approved the CDK4/6 inhibitor abemaciclib (Verzenio) as a first-line treatment in some women with advanced or metastatic breast cancer. Under the approval, the drug must be used in combination with an aromatase inhibitor.
A new study in mice raises the possibility that using microscopic, oxygen-carrying bubbles may improve the effectiveness of radiation therapy in the treatment of breast cancer.
The drug olaparib (Lynparza®) is the first treatment approved by the Food and Drug Administration for patients with metastatic breast cancer who have inherited mutations in the BRCA1 or BRCA2 genes.
Joint pain caused by aromatase inhibitors in postmenopausal women with breast cancer can cause some women to stop taking the drugs. Reducing their symptoms may translate into better adherence to therapy.
A new study suggests that the cells in treatment-resistant tumors in women with metastatic breast cancer share important characteristics that could potentially make tumors vulnerable to therapies that otherwise might not have been considered.
A large nationwide clinical trial called TMIST has been launched to compare two techniques used for mammograms: tomosynthesis, often called 3D mammography, and standard 2D digital mammography.
FDA approved abemaciclib (Verzenio™) for the treatment of some people with advanced or metastatic HR-positive, HER2-negative breast cancer whose disease has progressed after treatment with hormone therapy.
Long-term results from a large clinical trial confirm that, for some women with early-stage breast cancer who have lumpectomy as their surgical treatment, a less extensive lymph node biopsy approach is sufficient.
When given at the same time, two immune checkpoint inhibitors were ineffective against breast cancer growth in mice, a new study found. The combination was more effective and safer if the two inhibitors were given in a specific sequence.
FDA has expanded its approval of fulvestrant (Faslodex®) as a standalone treatment for postmenopausal women with advanced HR-positive, HER2-negative breast cancer who have not previously undergone endocrine therapy.
Many women who receive taxane-based chemotherapy to treat breast cancer experience long-term nerve damage, or peripheral neuropathy, data from a large clinical trial show.
Researchers recognized the potential of endoxifen as a treatment for breast cancer and, with NCI support, developed the compound into a drug now being tested in clinical trials.
Researchers have used modified stem cells to deliver a cancer drug selectively to metastatic breast cancer tumors in mice. The stem cells target metastatic tumors by homing in on the stiff environment that typically surrounds them.
FDA has approved neratinib for patients with early-stage HER2-positive breast cancer who have finished at least 1 year of adjuvant therapy with trastuzumab.
Many survivors of early-stage breast cancer prefer that their oncologist handle aspects of routine medical care usually overseen by primary care practitioners, leading to concerns about gaps in care.
Results from the first large prospective study of breast and ovarian cancer risk in women with inherited mutations in the BRCA 1 or BRCA2 genes confirm the high risks estimated from earlier, retrospective studies.
Two clinical trials show that trastuzumab emtansine (T-DM1) improves survival compared with other standard treatments for patients with HER2-positive metastatic breast cancer that has progressed after treatment with other HER2-targeted drugs.
Using one of the largest collections of tumor samples from African Americans with breast cancer, researchers tried to assess the extent to which the molecular characteristics on these tumors might help to explain breast cancer disparities.
A new study shows that the number of women in the United States living with distant metastatic breast cancer (MBC), the most severe form of the disease, is growing. This is likely due to the aging of the U.S. population and improvements in treatment.
In a randomized trial, low-income women who role-played talking with their doctor about their survivorship care plan in a counseling session reported receiving more of their recommended care than women who did not get counseling.
The FDA has approved a new targeted therapy, ribociclib, and expanded its earlier approval of another targeted therapy, palbociclib, for some women with metastatic breast cancer.
Researchers have found that duloxetine (Cymbalta®), a drug most commonly used to treat depression, may also reduce joint pain caused by aromatase inhibitors in some women being treated for early-stage breast cancer.
- Member Benefits
- Communities
- Grants and Scholarships
- Student Nurse Resources
- Member Directory
- Course Login
- Professional Development
- Organizations Hub
- ONS Course Catalog
- ONS Book Catalog
- ONS Oncology Nurse Orientation Program™
- Account Settings
- Help Center
- Print Membership Card
- Print NCPD Certificate
- Verify Cardholder or Certificate Status

- Trouble finding what you need?
- Check our search tips.

Clinical Journal of Oncology Nursing
Current issue: number 3 / june 2024.
Explore topics including:
- Building resilience in new graduate RNs
- Improving vaccination documentation rates among patients with breast cancer
- Incorporating music therapy with acupuncture in cancer pain management

Number 3 / June 2024 Articles


- Get Press Releases

- Media Contacts
- News Releases
- Photos & B-Roll Downloads
- VUMC Facts and Figures
- Credo Award
- DAISY Award
- Elevate Team Award
- Health, Yes
- Employee Spotlight
- Five Pillar Leader Award
Patient Spotlight
- Pets of VUMC
- Tales of VUMC Past
- All Voice Stories
Explore by Highlight
- Education & Training
- Growth & Finance
- Leadership Perspectives
- VUMC People
Explore by Topic
- Emergency & Trauma
- Genetics & Genomics
- Health Equity
- Health Policy
- Tech & Health
- Women's Health
Explore by Location
- Monroe Carell Jr. Children’s Hospital at Vanderbilt
- Vanderbilt Bedford County Hospital
- Vanderbilt Health One Hundred Oaks
- Vanderbilt Health Affiliated Network
- Vanderbilt-Ingram Cancer Center
- Vanderbilt Kennedy Center
- Vanderbilt Psychiatric Hospital
- Vanderbilt Wilson County Hospital
- Vanderbilt Stallworth Rehabilitation Hospital
- Vanderbilt Tullahoma Harton Hospital
- Vanderbilt University Hospital

- Photos & B-Roll Downloads
Featured Story


A gunshot to the head. A long recovery. Then a wedding.
May 14, 2024, breast cancer risk variants identified for women of african ancestry.
A study led by researchers from Vanderbilt-Ingram Cancer Center sheds light on some of the genetic variants that make breast cancer more deadly for women of African ancestry and significantly reduces the disparity in knowledge for assessing their genomic risk factors.
The study, which was published May 13 in Nature Genetics , is the largest genome-wide association study ever conducted among women of African ancestry for breast cancer, the researchers said. Analyzing 18,034 cases and 22,104 controls of African ancestry, they identified genetic variants at 12 loci associated with breast cancer risk at the genome-wide significant level. Of them, variants in three loci were associated with risk of triple-negative breast cancer (TNBC). Approximately 8% of African-ancestry women carry all six risk variants in these loci, and these women are 4.2 times more likely to be diagnosed with TNBC than those who carry none or only one of the variants.
The new data put women of African ancestry on a more equitable status with women of European and Asian ancestry for deriving breast cancer polygenic risk scores (PRS) — a calculation based on genetic markers and variants for assessing cancer susceptibility. Including the newly identified genomic markers into PRS analyses significantly improved the performance of the prediction of breast cancer risk for women of African ancestry.

“We have worked with researchers from more than 15 institutions in the U.S. and Africa to establish this large genetic consortium. Data put together in this consortium have been and will continue to be used by researchers around the world to address significant questions related to breast cancer etiology and genetics,” said the study’s corresponding author Wei Zheng , MD, PhD, MPH, the Anne Potter Wilson Professor of Medicine and director of the Vanderbilt Epidemiology Center.
Breast cancer is the most commonly diagnosed cancer among women in the United States, and TNBC is a more aggressive subtype that women of West African ancestry are more likely to develop than women of other ethnic backgrounds.
Guochong Jia, PhD, MPH, a postdoctoral fellow at Vanderbilt University Medical Center, is the study’s lead author.
Other Vanderbilt authors on the study are Jie Ping, PhD, Xingyi Guo, PhD, Ran Tao, PhD, Bingshan Li, PhD, Xiao-Ou Shu, MD, PhD, MPH, Tuya Pal, MD, Sonya Reid, MD, MPH, Oiuyin Cai, MD, PhD, and Jirong Long, PhD.
The Vanderbilt researchers received support from the National Institutes of Health (R01CA202981, R01CA235553).
Related Articles
May 1, 2024
Study finds 500 new blood pressure genes.
An analysis of the genomes of more than 1 million people of European ancestry, conducted by several of the world’s leading genomic centers, including Vanderbilt University Medical Center, has identified more than 2,000 independent genetic signals for blood pressure.
By Bill Snyder

May 23, 2024
When is it safe to leave a child home alone; can cicadas hurt your hearing; building healthy habits; and other news stories with vumc sources.

December 20, 2023
Achievements, accolades highlight past year at vumc.
2023 was a year full of achievements and accolades for Vanderbilt University Medical Center.
By VUMC News and Communications
Most younger women who want kids after breast cancer are successful, research data shows
About two-thirds of the women in the study had a baby after diagnosis.
Among younger women with breast cancer, it may be possible for many to have a baby after their diagnosis thanks to advances in breast cancer care, new research suggests.
In a study of about 200 women ages 40 and younger with non-metastatic breast cancer who wanted children, roughly three-quarters were able to become pregnant after diagnosis, and about two-thirds had a baby.
The research will be presented on Monday, June 3, at the 2024 ASCO Annual Conference, a major medical conference of the American Society of Clinical Oncology. However, it has not yet been peer-reviewed or published as a full manuscript in a journal.
MORE: Major disparities exist in women of color's access to breast cancer care, report finds
Doctors say this may give hope to the growing number of younger people being diagnosed with breast cancer who want to preserve their fertility.
"This is indeed great news for young breast cancer survivors," Dr. Julie R. Gralow, Chief Medical Officer of ASCO and oncologist who specializes in breast cancer, told ABC News. "Achieving a pregnancy after breast cancer diagnosis is both possible and safe."
For women diagnosed at earlier ages, fertility may be of great concern and importance, but experts point out that in this study, only 16% of women said they desired a baby after their diagnosis.
"I think this may represent the overall reluctance of young women with this diagnosis interrupting their lives at such a young age to pursue pregnancy," Dr. Julia Foldi, assistant professor of medicine in hematology/oncology, University of Pittsburgh School of Medicine, told ABC News.
In the study, women with more financial security were more likely to become pregnant, and fertility preservation, such as egg freezing, nearly tripled the odds of having a baby. The older the patient, the less likelihood of having a baby or getting pregnant.
"While we can't impact the age at diagnosis, we can make sure that all young women diagnosed with breast cancer receive information prior to beginning treatment about options to increase the chance of a future pregnancy, and also have access to those options," Gralow said.
Doctors hope this research helps counsel women who desire pregnancy after their breast cancer diagnosis and highlights the importance of having access to fertility preservation services, which can be costly.
"Timely access to fertility preservation can be very challenging due to lack of available resources and infrastructure, financial barriers, and much more," Dr. Kimia Sorouri, research fellow, Dana-Farber Cancer Institute, Boston, Massachusetts, and one of the study authors told ABC News.
Trending Reader Picks

Valedictorian gives speech after father’s funeral
- May 23, 4:27 PM

This viral creator is making sustainable fashion
- May 23, 3:05 PM

UN court order demanding Israel to halt Gaza offensive further isolates US position
- May 25, 12:04 AM
"Ensuring that women have the resources necessary to enable them to benefit from this technology, including insurance coverage for fertility preservation, will go a long way towards ensuring access to care for those women who have yet to complete their reproductive plan," Dr. Sigal Klipstein, InVia Fertility Specialists in Chicago and former chair of the ACOG Committee on Ethics, told ABC News.
MORE: Finalized guidance drops breast cancer screening age to 40 for women with average risk
Researchers also found several factors that were not associated with fertility outcomes in this study, including having a history of infertility, never giving birth before diagnosis, tumor characteristics, cancer treatment, race, and ethnicity.
"It is particularly impressive data because almost 70% of the women in this study had received chemotherapy, which can reduce fertility," Gralow said.
In this study, the typical age at the time of breast cancer diagnosis was 32 years old, and the average time to pregnancy was 4 years after diagnosis. Most of the women in this study were non-Hispanic white and had their breast cancer diagnosed in earlier stages of the disease.
"This should encourage folks to get their screening done and know that if they are diagnosed earlier on, that's less likely to impact their future fertility goals," Dr. Elizabeth Langen, associate clinical professor of obstetrics and gynecology, University of Michigan Health System, told ABC News.
In guidance by the U.S. Preventive Services Task Force, women with average risk are now recommended to start breast cancer screening at age 40.
This finalized guidance, in part, reflects a worrying trend of more women being diagnosed with breast cancer at a younger age.
"While this study provides great hope for women with a diagnosis of breast cancer, it is important to be cognizant of the fact that not all women will have success," Klipstein said. "Expeditious counseling, availability of and access to fertility preservation options are the elements that often make the difference between having or not having the family that women desire."
Dr. Jade A Cobern, MD, MPH, a licensed and practicing physician board certified in pediatrics and preventive medicine, is a member of the ABC News Medical Unit.
Related Topics
- Breast Cancer

If you’ve tried meditating but can’t sit still, here’s how — and why — to try again
- May 15, 8:37 AM

Newborn’s photo shoot goes viral for grumpy faces
- May 23, 8:57 PM
ABC News Live
24/7 coverage of breaking news and live events
Scientists create tailored drug for aggressive breast cancer
Scientists have used breast cancer cells' weakness against themselves by linking a tumour-selective antibody with a cell-killing drug to destroy hard-to-treat tumours.
The research, published today in Clinical Cancer Research by a team from King's College London and funded by Breast Cancer Now, marks a new method in cancer treatment.
The discovery is particular to triple negative breast cancer, which makes up 15% of all diagnosed breast cancer. This type of breast cancer is typically aggressive, resistant to chemotherapy, has a lower survival rate and is more common in women under 40.
Usual treatment involves surgery, chemotherapy and radiotherapy, however this type of cancer can evade the drugs and return to spread again.
The scientists conducted data analysis using over 6000 breast cancer samples to investigate the properties of breast cancer cells that are associated with aggressive and chemotherapy-resistant cancers.
They studied the cancer's biology, what is expressed in the tumour and the cell surface, and the cell's insides to understand how the cancer cells escape from cancer drugs. They established the presence of the cancer cell surface marker EGFR along with oncogenic molecules cyclin-dependent kinases (CDK), which are responsible for cell division and proliferation.
They used this knowledge against the cancer cells to link cetuximab, a tumour-selective antibody that targets the EGFR protein expressed in this type of cancer, with a CDK-blocking drug to create a tailored drug for breast cancer. Because the antibody drug conjugate specifically targets the cancer cell, it may be possible to administer a lower inhibitor dose than usual which means it's less toxic for the patient.
Lead author Professor Sophia Karagiannis, from King's College London, said: "We were on the hunt for cancer's vulnerabilities and now we've found out how we can guide our therapies to one of these. We combined these two drugs to create a tailored antibody drug conjugate for patients with this aggressive cancer. The antibody guides the toxic drug directly to the cancer cell which offers the possibility for a lower dose and less adverse side effects to be experienced.
"More work needs to be done before this therapy can reach the clinic, but we expect that this can offer new treatment options for cancers with unfavourable prognosis. Beyond this antibody drug conjugate, we hope that our concept will lead the way for new antibody drug conjugates of this type to be tailored to patient groups likely to benefit."
Lead research scientist Dr Anthony Cheung from King's College London said: ''Triple negative breast cancer represents a molecularly and clinically diverse disease. By exploiting EGFR overexpression and dysregulated cell cycle molecules in selected patient groups, the antibody drug conjugate, but not the antibody alone, could stop the cancer cell from dividing and engender cytotoxic functions specifically against the cancer cells.''
Dr Simon Vincent, director of services, support and influencing at Breast Cancer Now, which funded this research, said: "Each year, around 8,000 women in the UK are diagnosed with triple negative breast cancer, which is typically more aggressive than other breast cancers and more likely to return or spread following treatment.
"This exciting research has not only improved our understanding of the properties of aggressive breast cancer cells that are resistant to chemotherapy but has also brought us closer to developing a targeted therapy that destroys these cancer cells while minimising side effects for patients.
"While further research is needed before this treatment can be used in people, this is an exciting step forward in developing targeted therapies for triple negative breast cancer, and we look forward to seeing how these findings could lead to new and effective ways of tackling this devastating disease."
- Breast Cancer
- Lung Cancer
- Colon Cancer
- Brain Tumor
- Ovarian Cancer
- Monoclonal antibody therapy
- Chemotherapy
- Mammography
- Breast cancer
- Esophageal cancer
Story Source:
Materials provided by King's College London . Note: Content may be edited for style and length.
Journal Reference :
- Anthony Cheung, Alicia M. Chenoweth, Annelie Johansson, Roman Laddach, Naomi Guppy, Jennifer Trendell, Benjamina Esapa, Antranik Mavousian, Blanca Navarro-Llinas, Syed Haider, Pablo Romero-Clavijo, Ricarda M. Hoffmann, Paolo Andriollo, Khondaker Miraz Rahman, Paul Jackson, Sophia Tsoka, Sheeba Irshad, Ioannis Roxanis, Anita Grigoriadis, David E. Thurston, Christopher J. Lord, Andrew N.J. Tutt, Sophia N. Karagiannis. Anti-EGFR antibody-drug conjugate carrying an inhibitor targeting CDK restricts triple-negative breast cancer growth . Clinical Cancer Research , 2024; DOI: 10.1158/1078-0432.CCR-23-3110
Cite This Page :
Explore More
- Caterpillars Detect Predators by Electricity
- 'Electronic Spider Silk' Printed On Human Skin
- Engineered Surfaces Made to Shed Heat
- Innovative Material for Sustainable Building
- Human Brain: New Gene Transcripts
- Epstein-Barr Virus and Resulting Diseases
- Origins of the Proton's Spin
- Symbiotic Bacteria Communicate With Plants
- Birdsong and Human Voice: Same Genetic Blueprint
- Molecular Dysregulations in PTSD and Depression
Trending Topics
Strange & offbeat.
Sarah Harding breast cancer research project is successfully identifying at-risk young women
Sarah Harding died aged 39 after being diagnosed with breast cancer. One of the Girls Aloud singer's final hopes was to find ways of spotting the disease early when it's easier to treat.
By Charlotte Leeming, north of England correspondent
Sunday 19 May 2024 02:43, UK

A groundbreaking breast cancer research project launched in memory of the late Girls Aloud singer Sarah Harding is already successfully identifying young women at increased risk of getting the disease.
The BCAN-RAY (Breast Cancer Risk Assessment in Young Women) was launched a year ago in the singer's name after she died from the disease in 2021 at the age of 39.
While she was having treatment, the star said she was "really keen" for more research into why young women are being diagnosed without a family history of the disease.
One of the singer's final hopes was to find ways of spotting the disease early when it's easier to treat.
The BCAN-RAY is one of the only projects in the world trying to identify which women in their 30s are most at risk.
About 2,300 women under 40 are diagnosed with the disease each year in the UK, according to Breast Cancer Now.
The two-year study is using money from Cancer Research UK, the Christie Charity, and the Sarah Harding Breast Cancer Appeal - backed by her family and former bandmates.
More on Cancer

Seinfeld star Michael Richards: I probably would've died in eight months without surgery for prostate cancer

Girl, 10, cuts off 13 inches of hair to make wig for cancer patient

Proteins in blood could warn of cancer seven years before diagnosis, scientists find
Related Topics:
- Sarah Harding
It looks at risk factors most commonly found in young women with the disease and will form a model to identify them in future.
Read more: Proteins in blood could warn of cancer seven years before diagnosis New cancer treatment gives hope as diagnoses rise Girls Aloud kick off reunion tour dedicated to Sarah Harding
Anna Housley, 39, from Hale, Greater Manchester, is one of the women taking part in the trial. After being tested last year the mother of two was surprised to find she's at increased risk.
With no history of the disease in her family, she told Sky News: "I'm really grateful that I have been found because now I know that I'm going to be looked after and I can be screened."
Keep up with all the latest news from the UK and around the world by following Sky News
Speaking about the work of Harding, she said: "All I can say really is thanks to her for being such a brave advocate to young women."
The new information means she's now eligible for annual mammograms and medication should she want it.
It's hoped all women will eventually be able to have a risk assessment when they reach 30.

A thousand women in the Greater Manchester area will take part, including 250 with breast cancer who don't have a family history of the disease.
Saliva samples will hopefully help experts identify certain types and patterns of genes that could raise a woman's risk.
Be the first to get Breaking News
Install the Sky News app for free

These will be considered with factors such as period timing, breast tissue density, alcohol consumption and use of the pill.
Harding's consultant Dr Sacha Howell from Manchester University NHS Foundation Trust, who is leading the study, said of the singer: "I think she'd be absolutely thrilled that she was part of this and her legacy is that we will be helping more and more young women like her.
"But what we're all hoping is that by detecting those cancers earlier, they won't unfortunately have that end result that Sarah did, which was to pass away with the disease."
Harding's legacy won't just be her successful music career, it will also be her work in raising awareness around breast cancer and potentially giving many more women in their 30s a future.
Related Topics
Fertility Treatments Safe for Breast Cancer Survivors With Cancer-Linked Genes
By Ernie Mundell HealthDay Reporter

MONDAY, May 20, 2024 (HealthDay News) -- Fertility treatments such as in vitro fertilization (IVF) and other methods don't boost the odds for tumor recurrence in young women who've survived breast cancer and carry the BRCA cancer genes, a reassuring, new report finds.
The issue had been in question because breast tissue can be sensitive to hormones and many assisted reproductive techniques (ARTs) involve a temporary boost in estrogen.
However, the new Italian study "provides the first evidence" that fertility procedures are safe in women with those variants of the BRCA1 and BRCA 2 genes that are known to raise risks for breast and ovarian cancers, said study lead author Matteo Lambertini .
He spoke in a news release from the European Society for Medical Oncology (ESMO). Lambertini's team presented its findings last week at ESMO's annual meeting in Lugano, Switzerland.
U.S. Cities With the Most Homelessness

The new findings "provide reassuring evidence for these women and their doctors to consider when discussing the risks and benefits of using ART to preserve their chances of having a baby following completion of anti-cancer therapies,” said Lambertini, an associate professor of medical oncology at the University of Genova.
As the researchers explained, women in their reproductive years faced with a breast cancer diagnosis often opt to freeze their eggs, since some treatments can send them into early menopause. Doing so often involves boosting circulating levels of estrogen, however.
“We have previously been concerned that increasing hormone levels for fertility preservation techniques before starting breast cancer treatment may increase the risk of cancer recurrence in the future," Lambertini explained.
Investigating the issue, his team tracked 2000 to 2020 data on nearly 5,000 women with BRCA1/2 variants linked to cancer, all of who were diagnosed with breast cancer at the age of 40 or younger.
They compared the risk for a recurrence of breast cancer in 107 of these women who had a pregnancy using ART with 436 who conceived naturally.
The result: Five years after successful conception, there was no difference in rates of recurrent breast cancer between the two groups.
There were also no differences in terms of complications from pregnancy.
The only difference found was that women conceiving with fertility treatments had more miscarriages and fewer induced abortions, compared to those conceiving naturally, the team found.
Lambertini noted that the study population was small, but that was a necessary evil: Breast cancer is rare in women under 40, and only about one in every six of those cases involve BRCA genes.
"We put together centers from all over the world to collect data on this unique group of patients,” he explained.
Study co-author Ann Partridge is professor of medicine at Harvard Medical School and vice chair of medical oncology at the Dana-Farber Cancer Center in Boston.
She said the new findings “provide reassuring evidence that pursuing fertility preservation before undergoing breast cancer treatment or using the products of fertility preservation [eggs or embryos] or undergoing fertility preservation after surviving breast cancer, all appear to be safe from a cancer standpoint and in terms of the baby’s outcome."
Because these findings were presented at a medical meeting, they should be considered preliminary until published in a peer-reviewed journal.
More information
Find out more about the BRCA genes at the U.S. National Cancer Institute .
SOURCE: European Society for Medical Oncology (ESMO), news release, May 16, 2024
Copyright © 2024 HealthDay . All rights reserved.
Join the Conversation
Tags: pregnancy , cancer , breast cancer
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

Takeaways From the NCAA’s Settlement
Laura Mannweiler May 24, 2024

Noncitizen Voting: the Fiction and Facts
Aneeta Mathur-Ashton May 24, 2024

Quiz: Who Said What in Trump’s Trial?
U.S. News Staff May 24, 2024

CDC: COVID-19 Strains Are on the Rise
Cecelia Smith-Schoenwalder May 24, 2024

Consumers See Worsening Job Market
Tim Smart May 24, 2024

Biden vs. the Border
Elliott Davis Jr. May 23, 2024

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Cancers (Basel)

Breast Cancer Risk Assessment and Primary Prevention Advice in Primary Care: A Systematic Review of Provider Attitudes and Routine Behaviours
Sarah bellhouse.
1 Manchester Centre for Health Psychology, Division of Psychology and Mental Health, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester M13 9PL, UK; [email protected] (R.E.H.); [email protected] (D.P.F.)
Rhiannon E. Hawkes
Sacha j. howell.
2 Division of Cancer Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester Academic Health Science Centre, Manchester M13 9PL, UK; [email protected]
Louise Gorman
3 NIHR Greater Manchester Patient Safety Translational Research Centre, University of Manchester, Manchester Academic Health Science Centre, Manchester M13 9PL, UK; [email protected]
David P. French
Associated data.
The data presented in this review are available in Supplementary Material S2 .
Simple Summary
There is growing international interest in adopting a risk-based approach to breast cancer screening, where an individual’s risk would inform screening practices. It has been suggested that primary care will contribute to the delivery of this service by conducting risk assessment and providing primary prevention advice. The aim of our review was to understand what primary care providers think and feel about performing these tasks by examining their attitudes and typical activity in clinical practice (routine behaviours). The results suggest that primary care providers mainly assess breast cancer risk by collecting family history information but feel less comfortable advising on risk-reducing medications. Primary care will need to proactively assess breast cancer risk for women to get the most benefit from risk-based screening and prevention. To promote risk assessment and prevention activities, improved education/training and changes to resources (integrated risk assessment tools, better patient materials etc.) will be necessary.
Implementing risk-stratified breast cancer screening is being considered internationally. It has been suggested that primary care will need to take a role in delivering this service, including risk assessment and provision of primary prevention advice. This systematic review aimed to assess the acceptability of these tasks to primary care providers. Five databases were searched up to July–August 2020, yielding 29 eligible studies, of which 27 were narratively synthesised. The review was pre-registered (PROSPERO: CRD42020197676). Primary care providers report frequently collecting breast cancer family history information, but rarely using quantitative tools integrating additional risk factors. Primary care providers reported high levels of discomfort and low confidence with respect to risk-reducing medications although very few reported doubts about the evidence base underpinning their use. Insufficient education/training and perceived discomfort conducting both tasks were notable barriers. Primary care providers are more likely to accept an increased role in breast cancer risk assessment than advising on risk-reducing medications. To realise the benefits of risk-based screening and prevention at a population level, primary care will need to proactively assess breast cancer risk and advise on risk-reducing medications. To facilitate this, adaptations to infrastructure such as integrated tools are necessary in addition to provision of education.
1. Introduction
Population based screening programmes aim to detect asymptomatic cancers at an earlier stage to reduce mortality rates and the need for aggressive treatments associated with long term morbidities [ 1 ]. A substantial reduction in breast cancer related mortality has been observed since the introduction of mammographic screening programmes [ 2 , 3 ]. However, harms of breast cancer screening include overdiagnosis and false positive test results. Overdiagnosis refers to the diagnosis of breast cancers via screening that would never have caused any clinically apparent symptoms over the course of a person’s lifetime [ 4 ]. A false positive result is an abnormality on a screening test that necessitates further investigations, ultimately ruling out the presence of cancer. Whether the benefits of screening outweigh the known harms has been much debated [ 2 , 5 ].
Adopting an alternative risk-based approach to breast cancer screening has the potential to improve the benefit to harm ratio [ 6 ]. The development of risk algorithms, such as the Gail and Tyrer–Cuzick models, has made estimation of an individual’s breast cancer risk possible [ 7 , 8 ]. The provision of personalised breast cancer risk estimates would allow screening and prevention services to be offered that are commensurate with the degree of risk, thus improving benefit to harm ratios [ 9 , 10 ]. In the UK, the Predicting Risk of Cancer at Screening (PROCAS) study demonstrated that breast cancer risk information can be collected and communicated to women participating in a population-based mammographic screening programme [ 11 ]. International trials are currently ongoing to establish the effectiveness of a risk-based screening regimen in comparison to standard screening practices [ 12 , 13 ].
A key benefit of risk estimation is the ability to identify women at increased risk, affording them the opportunity to benefit from preventative strategies. There are two strategies that have proven benefit in reducing breast cancer risk. The use of selective oestrogen receptor modulators and aromatase inhibitors, commonly referred to as chemoprevention or risk-reducing medication, have been shown to reduce breast cancer incidence [ 14 , 15 ]. Furthermore, evidence suggests 15–40% of breast cancers may be preventable by engaging in health-related behaviours such as increased physical activity and reduced alcohol intake [ 16 ]. Clinical guidance acknowledges the need to discuss lifestyle related risk factors in relation to breast cancer risk but the care setting where this discussion should take place is not specified [ 17 ].
As the first point of healthcare contact for the general population, primary care has been repeatedly identified as the most opportune setting to conduct breast cancer risk assessment [ 18 , 19 ]. Secondly, primary care providers have a critical role in delivering preventive health care services to the general population as evidenced by their current role in assessment and management of cardiovascular and diabetes risk [ 20 , 21 ].
As the likely roles of primary care in delivering risk-based screening and prevention will be risk assessment and provision of primary prevention advice including prescription of risk-reducing medication, it is important to assess acceptability of these activities. Acceptability is increasingly being recognised as an important component of the feasibility of complex interventions in guidance documents such as the Medical Research Council (MRC) framework [ 22 ].
A previous review identified a considerable evidence base related to the acceptability of primary care involvement in risk-based screening and prevention [ 19 ]. This review identified numerous barriers reported by primary care providers in relation to their proposed roles which suggests concerns about the acceptability of this approach. However, the scope of the review was limited as it did not examine key participant-reported evaluations of acceptability, such as confidence, as recommended by an evidence-based framework of acceptability [ 23 ]. Furthermore, the review did not quantify the strength of individual barriers and facilitators nor examine potential sources of variation such as country and healthcare specialty. The latter is important to investigate as countries vary substantially in how primary healthcare is delivered, including differences in training requirements and what types of providers are considered part of the primary care workforce [ 18 ]. Consequently, implementation of risk-based screening and prevention will likely differ across countries [ 19 ].
The present systematic review aimed to provide a robust and in-depth examination of acceptability beyond identification of barriers and facilitators. It achieves this by employing the theoretical framework of acceptability which recognises the value of participant-reported evaluations of acceptability in addition to behavioural assessments [ 23 ]. However, as primary care providers’ significant knowledge deficits in this area have been described extensively in previous systematic reviews [ 19 , 24 , 25 , 26 ], the present review did not assess the extent to which primary care providers understand breast cancer risk assessment and management.
Specific objectives were to summarise the evidence base on:
- ratings of acceptability (including, attitudes, opinions, beliefs, feelings, barriers or facilitators) by primary care providers with respect to (1) breast cancer risk assessment and (2) primary prevention advice
- the performance of routine behaviours by primary care providers regarding (1) breast cancer risk assessment and (2) primary prevention advice
- sources of variation in acceptability and behaviours
The protocol of this systematic review was pre-registered in PROSPERO (CRD42020197676) and follows the reporting guidelines detailed in the PRISMA statement [ 27 ]. The protocol covered both quantitative and qualitative literature but for reasons of space only the quantitative findings are reported here.
2.1. Search Strategy
The following electronic databases were searched: MEDLINE, EMBASE, CINAHL Plus, PsycINFO (each up to 10 July 2020) and ProQuest Dissertations & Theses Global (up to 26 August 2020). Databases were searched from 1989 as the first breast cancer risk model incorporating multiple breast cancer risk factors was published in this year [ 7 ]. Search terms were produced using medical subject headings (MeSH), other index terms, keywords and appropriate synonyms (see Supplementary Material S1 ) and refined with the input of a librarian with expertise in systematic review searching. The strategy was tailored in accordance with the technical language of each database. The searches were limited to articles for which the full text was available in English. Forward and backward citation searches and a lead author search were performed for all included papers. Relevant reviews were hand-searched and researchers with expertise in the area were contacted to identify any additional articles not retrieved by the searches.
2.2. Eligibility Criteria
Studies were included in the review if they met the following criteria:
Studies conducted with both primary and secondary care providers were only included if it was possible to separately identify those findings relevant to primary care providers.
- Data had to be reported about risk assessment and/or providing primary prevention advice in the context of breast cancer. Studies focusing on cancer risk or primary prevention whereby data specific to breast cancer could not be extracted were excluded.
- (a) Acceptability defined as anticipated or experiential cognitive and emotional responses. Studies had to report one or more of the following outcomes using quantitative methodologies: attitudes, opinions (e.g., perceptions of responsibility), beliefs, feelings (e.g., confidence), barriers or facilitators.
- (b) Routine behaviours defined as typical or regular activity in clinical practice. Frequency of behaviours reported in a specific timeframe were not eligible for inclusion. Hypothetical clinical scenarios/vignettes or reflections on previous clinical cases were ineligible as these methods ascertain the action taken in a specific situation which may not be indicative of routine behaviours.
- Studies: Full empirical articles of any quantitative design published in the English language. Grey literature including PhD theses, dissertations and unpublished research were eligible for inclusion. Additionally, baseline surveys of intervention studies designed to improve breast cancer risk assessment behaviours or provision of primary prevention advice were included.
2.3. Selection and Coding of Studies
The search results were downloaded into Endnote and duplicates were removed. The library was then uploaded to Rayyan [ 29 ] to complete screening. The first author screened all titles and abstracts and a second reviewer (RH) independently screened 30% (k = 945) of these (97% agreement). Full text articles were obtained for all records that appeared to be eligible or could not be confidently excluded (k = 124). The first author read all full text articles and assessed these against the eligibility criteria. A second reviewer (RH) read 50% of the full text articles (k = 62) and disagreements regarding the eligibility of an article were resolved by discussion. In ambiguous cases, additional reviewers were consulted (DF, SH) and consensus was reached.
2.4. Data Extraction
Following full text review, detailed information on study characteristics (authors, country, study design and outcome measures), sample characteristics (sample size, age and sex) and outcome data relevant to the objectives were extracted by the first author for all eligible articles. A second reviewer (RH) verified the data extraction by independently extracting primary outcome data for 50% (15/29) of eligible articles.
2.5. Quality Assessment
The Mixed Methods Appraisal Tool (MMAT) was deemed most suitable for quality assessment due to its demonstrated reliability and inclusion of quality criteria specifically designed to appraise quantitative descriptive study designs such as surveys [ 30 ]. Criteria were categorised as ‘yes’, ‘somewhat’, ‘no’ or ‘can’t tell’. The response option of ‘somewhat’ was added to reflect when a criterion had been partially fulfilled but lacked some key indicators of quality. This enabled a more nuanced approach to quality appraisal. The authors of the tool discourage the use of a scoring metric therefore a narrative description of quality is provided.
In line with MMAT recommendation, two authors (SB and DF) discussed which quality indicators were most important to consider for each criterion listed and following this a coding scheme was devised and agreed upon. All studies were appraised using the criteria for quantitative descriptive designs to assess the quality of the survey design and outcome measures which were of most interest to the review. A 50% rate of response was a priori regarded as satisfactory for avoidance of nonresponse bias, in line with response rates observed in previously published provider surveys [ 31 ]. Two authors (SB and RH) independently appraised the quality of the remaining studies. Reviewers met on three separate occasions to check the reliability of decisions and any disagreements were discussed and resolved. During these meetings, the coding scheme was also reviewed and refined in line with discussions to ensure consistency and fairness in coding.
2.6. Synthesis of the Evidence
A meta-analysis was deemed inappropriate as studies varied widely in outcomes, measurement scales and study populations. Instead, a narrative synthesis was conducted with findings tabulated [ 32 ]. The outcomes from each study were organised into categories initially based on what the authors of each individual study stated the data was measuring (i.e., barrier, facilitator, confidence etc.). Additional outcomes that had not been explicitly measured as barriers or facilitators (e.g., beliefs, feelings, etc.) were reviewed and categorised as such depending on whether they could reasonably be considered to promote or impede performance of the behaviour. For example, a negative affective attitude such as discomfort was categorised as a barrier. Consensus was reached on these decisions through discussion with additional reviewers (DF and SH). Supplementary Material S2 provides full details of the outcomes included per study, the raw data extracted from each study, and how each outcome was categorised.
To aid interpretation and allow meaningful patterns to be identified, outcomes were categorised into broader themes depending on content (see Supplementary Material S2 ). Initial themes were identified by the first author. These themes were then refined and agreed upon following several rounds of consultation with additional reviewers (DF and SH). The findings were synthesised across the included studies.
3.1. Study Characteristics
The searches identified 6750 articles, of which 3164 remained after duplicates were removed ( Figure 1 ). A total of 29 studies were eligible for inclusion (see Supplementary Table S1 for list of excluded studies and reasons). Years of publication ranged from 1997 to 2020. Twenty-seven studies were included in the synthesis. Two were excluded due to using measurement scales that could not be meaningfully compared to other studies [ 33 , 34 ]. More than half of the included studies were conducted in the USA (k = 14) ( Table 1 ). Sample sizes ranged from 28 [ 35 ] to 1311 [ 36 ] individuals. The most commonly studied population were physicians. The majority (24/27, 89%) of studies assessed at least one outcome relevant to breast cancer risk assessment. In comparison, fewer studies assessed outcomes pertinent to primary prevention (9/27, 33%). No studies investigating health-related behaviours within the context of breast cancer risk were identified so primary prevention findings are limited to risk-reducing medications only.

PRISMA flow diagram of study selection.
Characteristics of studies included in the synthesis ( n = 27).
Notes. * Nippert et al. (2014)—France, the Netherlands, UK and Germany; Mainous et al. (2013)—USA and Canada. 1 These studies recruited other professional groups in addition to physicians and nursing staff, namely physician assistants, midwives and residents.
3.2. Perceived Practice Responsibilities with Respect to Both Risk Assessment and Primary Prevention
Primary care providers’ perceptions of responsibility with respect to tasks implicated in breast cancer risk assessment and primary prevention were examined in several studies. Taking a family history was overwhelmingly perceived as a primary care responsibility (88.8–98.1%; Table 2 ). Additionally, primary care providers readily identified counselling about risk and providing follow up support post genetic testing as practice responsibilities. In comparison, discussion of genetic testing and disclosure of results were less likely to be perceived as primary care responsibilities. Inter-country differences were apparent in a study that recruited participants from four European countries [ 37 ]. GPs from France ascribed most practice responsibilities to themselves whereas GPs from the UK considered genetic risk and genetic testing to be the responsibility of genetic specialists. The most commonly assumed responsibilities for primary prevention were writing ongoing prescriptions for risk-reducing medications and initiating discussions about preventative measures.
Primary care providers’ perceived responsibilities in breast cancer risk assessment and primary prevention.
4. Risk Assessment
4.1. barriers and facilitators.
For conducting breast cancer risk assessment, the most commonly endorsed barriers were insufficient education/training followed by discomfort discussing breast density and performing the assessment ( Table 3 ). The least frequently endorsed barriers included lack of primary care responsibility and concern about the implications of risk assessment for women with respect to causing unnecessary anxiety or impacting screening behaviour. None of the included studies investigated factors that could help facilitate breast cancer risk assessment behaviour.
Primary care providers’ perceptions of barriers associated with conducting breast cancer risk assessment.
4.2. Perceived Confidence
Primary care providers reported highest levels of confidence in taking a family history (60.7–65.5%) and reassuring low-risk patients (46.0–67.7%) ( Table 4 ). Very low levels of confidence were observed for using the Gail model to calculate breast cancer risk (8.6%).
Primary care providers’ perceived confidence in performing breast cancer risk assessment behaviours.
4.3. Routine Behaviours
The discussion and collection of breast cancer family history was reported to be a common task ( Table 5 ). Rates were particularly high when the situational context increased the saliency of the topic matter; for example, during a discussion about a woman’s health history or when a woman presented with concerns about breast cancer risk (90.4–92.6%). In comparison, routine collection of family history during a new patient appointment was found to be lower (48.4–69.3%) in two studies conducted in the UK [ 42 , 43 ]. Reported use of multi-factorial risk assessment tools was low with estimates ranging from 3 to 50.9%.
Primary care providers’ reported behaviours with respect to breast cancer risk assessment.
Professional specialty and training level were found to be associated with reported behaviours. A higher proportion of providers specialising in obstetrics and gynaecology reported using risk assessment tools in comparison to family and internal medicine providers [ 44 , 45 ]. Additionally, qualified physicians were significantly more likely to report routinely assessing family history and using the Gail model compared to residents in training [ 31 , 44 ].
5. Primary Prevention Advice
5.1. barriers and facilitators.
Overall, there was higher endorsement of barriers for primary prevention than risk assessment. The most prevalent barriers for providing primary prevention advice were concern and discomfort prescribing risk-reducing medication and insufficient education/training, in line with barriers to risk assessment ( Table 6 ). Furthermore, a greater proportion of primary care providers were more likely to report they see fewer patients for whom risk-reducing medications are indicated in comparison to patients suitable for risk assessment (39.6% vs. 12.5%). The majority of primary care providers did not report beliefs indicating scepticism about the evidence base underpinning risk-reducing medications. More specifically, few indicated that they believed the risks of prescribing risk-reducing medications outweighed the benefits (6.5–20.5%) or expressed doubts about effectiveness of risk-reducing medications (1.0–31.5%). Lack of scepticism was a consistent finding reported across all five studies that assessed this outcome.
Primary care providers’ perceptions of barriers associated with providing primary prevention advice.
Primary care providers endorsed all facilitators to a relatively high degree (32.0–61.6%; Table 7 ). Availability of provisions to discuss risk-reducing options more effectively was endorsed as the strongest facilitator for providing primary prevention advice.
Primary care providers’ perceptions of facilitators associated with providing primary prevention advice.
Providers specialising in women’s health reported feeling more comfortable using a breast cancer risk assessment tool and prescribing risk-reducing medication [ 46 ]. These providers were also less likely to agree that the risks of prescribing risk-reducing medications outweighed the benefits in comparison to other primary care providers [ 45 ].
5.2. Perceived Confidence and Routine Behaviours
Primary care providers reported low levels of confidence in providing advice/information to patients about risk-reducing medications (24%) [ 41 ]. Only one study reported a behavioural outcome relevant to primary prevention wherein 13.5% reported discussing chemoprevention ‘usually’ or ‘always’ [ 31 ].
5.3. Quality Assessment
Overall, study quality was poor ( Table 8 ). A detailed breakdown of quality assessment by question for each study is available in Supplementary Material S3 . For the majority of studies, external validity was likely to be low due to reliance on recruitment through single institutions and sampling via medical association membership lists with limited coverage of the target population. For example, membership of the American Medical Association has been declining with the most recent estimate suggesting only 15% of practising US doctors are members [ 61 ]. Inadequate reporting of how outcome measures were developed was common across studies. Furthermore, none of the outcomes of interest were assessed using standardised measures with demonstrated reliability and validity; response rates lower than 50% were reported in k = 14 (48%) studies [ 34 , 35 , 36 , 37 , 39 , 41 , 45 , 47 , 48 , 50 , 51 , 55 , 58 , 60 ].
Quality assessment results for studies included in the review ( n = 29).
6. Discussion
6.1. summary of main findings.
The results from this systematic review indicate that primary care providers typically take a reactive role in breast cancer risk assessment that is predominantly focused on collection of family history and provision of support following identification of increased risk. Reported use of multi-factorial risk assessment tools was low. Primary care providers reported higher discomfort and lower confidence with respect to prescribing risk-reducing medications when compared to risk assessment. However, few providers reported beliefs suggestive of doubts about the evidence base underpinning risk-reducing medications. Insufficient education/training and perceived discomfort were amongst the most commonly endorsed barriers reported for both activities. The strongest facilitators for offering risk-reducing medication related to availability of provisions such as clear guidelines and tools to facilitate identification of suitable patients. Professional background, training and country were identified as sources of variation in acceptability and behaviours. The methodological quality of included studies was generally poor and common limitations were high nonresponse rates and use of non-standardised outcome measures.
6.2. Relevance to Existing Literature
Previous systematic reviews have consistently identified primary care providers’ lack of knowledge about breast cancer risk assessment and management as a significant barrier to engagement [ 19 , 24 , 25 , 62 ]. In line with this, the present review found that insufficient education/training was a prevalent barrier reported for both risk assessment and primary prevention.
Prior to this review, widespread reticence by primary care providers to discuss and prescribe risk-reducing medications has been recognised [ 60 , 63 , 64 , 65 ]. In line with previous findings, this review found that primary care providers report high levels of discomfort and low levels of confidence associated with risk-reducing medications. However, the present review also offers novel insight: few primary care providers reported scepticism about the evidence base underpinning risk-reducing medication. This suggests that the perceived discomfort towards risk-reducing medication is not solely attributable to a lack of knowledge. The present findings on facilitators instead highlight that there is a need for more structural approaches, such as the use of guidelines or prompts, to facilitate primary care involvement in breast cancer risk assessment and management practices.
This review has been the first to investigate sources of variation in acceptability and behaviours. Examination of routine behaviours illustrated that primary care providers infrequently report using multi-factorial risk assessments such as the Gail or Tyrer–Cuzick models [ 7 , 8 ]. Nonetheless, family history collection was reported as a common behaviour and perceived as a core task for the majority of primary care providers. Lower levels of routine family history taking were reported by two UK studies [ 42 , 43 ]. However, given the age of these studies (1997 and 2001), these findings may not be reflective of current clinical practice. Nevertheless, present guidelines in the UK and Europe discourage primary care providers from proactively identifying women with a family history of breast cancer [ 17 , 37 ]. A survey of GPs and breast surgeons from four different European countries revealed strong disapproval of the current purely reactive approach to family history assessment [ 66 ]. Therefore, the present guidelines are likely to hinder optimal promotion of risk assessment and primary prevention activities in UK and European primary care settings.
Professional background was also associated with outcomes. Providers specialising in women’s health issues reported feeling more comfortable with respect to both risk assessment and primary prevention as evidenced by greater reported use of quantitative risk assessments and fewer negative views about the risks of risk-reducing medications. These perceptions are likely to be the result of specialised knowledge acquired through additional training. For instance, an understanding of the diagnosis and clinical management of hereditary breast and ovarian cancer syndrome is considered essential for obstetrician/gynaecologists [ 67 ]. Therefore, in countries such as the USA, primary care providers specialising in women’s health may be more prepared to assume greater responsibility for assessment and management of breast cancer risk because of their knowledge and experience acquired through training and routine practice.
6.3. Limitations
The present review has identified methodological biases present in the primary studies. Firstly, high rates of nonresponse were observed in a significant proportion of the studies. Primary care providers who respond to surveys might have more positive views of breast cancer risk assessment and primary prevention than non-responders which may lead to overestimations of acceptability. Furthermore, there was a reliance on convenience sampling procedures in many studies. Outcomes were not assessed using standardised measures with demonstrated reliability and validity. In addition, older studies included in the review may be a poor reflection of current clinical practice given the significant advances made in breast cancer risk assessment and management in recent years. Primary prevention outcomes tended to be assessed in more recent studies which is in line with risk-reducing medications being a relatively new option in comparison to risk assessment. Nonetheless, findings were largely consistent across studies suggesting that methodological limitations with sampling and publication date did not unduly affect the overall conclusions.
Substantial heterogeneity across included studies was evident and therefore a meta-analysis was not possible. To allow meaningful patterns to be identified via narrative synthesis, the research team decided how outcomes were categorised and to some extent this process was subjective. Nevertheless, analytical processes were reviewed in reflective team meetings to achieve consensus and ensure a rigorous and robust synthesis.
Additionally, a wide range of values were observed for some outcomes indicating uncertainty about the average values presented. This is likely to be the result of heterogeneous outcome measurements, as well as differences in samples included. Consequently, caution is warranted when drawing conclusions about the precision of estimating strength of outcomes.
Finally, and perhaps surprisingly, the review did not identify any studies investigating primary care providers’ perceptions of discussing health-related behaviours within the context of breast cancer risk reduction. There is, however, an evidence base focusing on cancer risk more generally. Inclusion of this literature may have provided a more comprehensive understanding of primary care’s perceived role in primary prevention than was possible in this review.
6.4. Implications and Future Research Directions
The present review suggests that provision of education/training will be necessary but not sufficient to facilitate primary care involvement in breast cancer risk assessment and primary prevention. The findings on facilitators and routine behaviour indicate that adapting infrastructure and providing prompts to utilise available resources are essential to increase the likelihood of primary care providers routinely conducting both activities. For instance, the integration of risk assessment and management tools into practice software or access to web-based applications would facilitate the desired behaviours, as has been demonstrated for cardiovascular risk assessment and management [ 20 ]. Several prototype tools for breast cancer risk assessment have been subject to usability and acceptability testing [ 68 , 69 ]. Primary care providers have expressed concerns about the amount of time needed to complete such tools and highlighted the lack of guidance on clinical management as a significant barrier to use. Therefore, future tool development should focus on streamlining the process and incorporating risk reduction recommendations to increase uptake in routine practice. Additionally, future research should focus on developing and evaluating the impact of educational interventions on knowledge assimilation. This will identify what implementation support primary care will require to fulfil their proposed roles in risk assessment and primary prevention. However, it is worth noting evidence which suggests not all women may be in favour of primary care performing these roles [ 70 ]. Therefore, further research assessing the acceptability of this approach to women is needed.
Nursing staff were underrepresented in the included studies. Decision makers have suggested that nurses could assume increased duties in risk assessment and management to support implementation of risk-based screening and prevention [ 71 ]. In relation to primary prevention, general practitioners have been found to perceive intervening on obesity as an inappropriate use of their time in comparison to nurses who report feeling responsible for raising the topic [ 72 ]. Given the important role of health-related behaviours in reducing breast cancer risk, it would be timely to compare and contrast the views of primary care nurses and physicians to determine their respective roles in implementing prevention recommendations for breast cancer.
Additionally, there is a clear need for more research using populations outside the US to understand the feasibility of primary care assessing and managing breast cancer risk in different healthcare contexts. Future primary studies would benefit from assessing similar outcomes across studies using measures with demonstrated reliability and validity. Wider and more representative sampling frames should be used to obtain better coverage of the target population. Furthermore, recruitment strategies that build personal connections with potential participants such as using physician recruiters ought to be considered to reduce nonresponse rates [ 73 ].
7. Conclusions
Within the context of implementing risk-based breast cancer screening and prevention, the findings of this review suggest that primary care providers are more likely to accept an increased role in breast cancer risk assessment compared to advising on risk-reducing medications. Adaptations to infrastructure will be necessary to promote enactment of breast cancer risk assessment and management behaviours in addition to provision of education. To fully realise the benefits of risk-based breast cancer screening and prevention, guidelines will need to be reviewed to ensure promotion of a proactive approach to breast cancer risk assessment in primary care.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13164150/s1 , Material S1: Search terms for each database. Material S2: Data extracted from all primary studies, showing how each eligible outcome mapped onto broader themes reported in the main analyses. Material S3: Summary and detailed results of the quality appraisal using the Mixed Methods Appraisal Tool. Table S1: A list of 95 excluded studies and reasons for exclusion.
Author Contributions
Conceptualization, S.B., D.P.F., S.J.H. and L.G.; methodology, S.B., D.P.F., S.J.H. and L.G.; formal analysis, S.B., D.P.F. and S.J.H.; investigation, S.B. and R.E.H.; data curation, S.B.; writing—original draft preparation, S.B.; writing—review and editing, D.P.F., S.J.H., R.E.H. and L.G.; visualization, S.B.; supervision, D.P.F., S.J.H. and L.G.; project administration, S.B.; funding acquisition, D.P.F. and S.J.H. All authors have read and agreed to the published version of the manuscript.
S.B. is funded by a Manchester Cancer Research Centre PhD studentship. D.P.F and S.J.H were supported by the NIHR Manchester Biomedical Research Centre (IS-BRC-1215-20007). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Institutional Review Board Statement
This systematic review synthesises previously published data and does not include new data that require ethical approval and consent.
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.

IMAGES
VIDEO
COMMENTS
Prior learning: Prior learning and experience assessed to identify learning needs. Breast care nurse must have a minimum of 3 years experience within the role and have completed a level 3 module in breast care nursing or cancer nursing. Outcome measure: Certificate of level 3 relevant learning; 3 years within the role.
1 Division of Cancer Services, Princess Alexandra Hospital, Metro South Health, Woolloongabba, Queensland, Australia [email protected]. 2 School of Nursing and Cancer and Palliative Care Outcomes Centre, Queensland University of Technology, Brisbane, Queensland, Australia. 3 School of Medicine, Griffith University, Brisbane, Queensland ...
Methods Cochrane Controlled Register of Trials (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Medline and Embase databases were searched (January 1999 to May 2019) to identify randomised controlled trials (RCTs) and controlled before-and-after studies of interventions delivered by nurses with oncology experience for women with breast cancer.
In an analysis of over 4000 women who had been diagnosed with breast cancer, aspirin use was associated with a lower risk of distant recurrence and breast cancer death. 22 For example, compared with nonusers, women who used aspirin 7 days per week had a 64% lower risk of breast cancer death (95% CI = 0.24, 0.54; Figure 3 ).
The Association of Women's Health, Obstetric and Neonatal Nurses (AWHONN) strongly encourages nurses to work with women to develop individualized plans for breast cancer screening based on their age, health status, breast cancer risk assessment, and personal values. AWHONN supports access to screening mammography and other breast health related services with no out of pocket costs.
Most women who develop breast cancer in a high-income country will survive; the opposite is true for women in most low-income and many middle-income countries . In 2020 breast cancer mortality-to-incidence ratio (MIR) as a representative indicator of 5-year survival rates was 0.30 globally . Taking into consideration the clinical extent of ...
The current BC early detection strategies mainly rely on BC education and awareness (patient, community, and health professional education), breast health awareness, breast self-detection, and clinical breast exams (CBE). 10,11 Treatment of BC often involves a combination of surgical resection, radiotherapy, and medications (hormonal therapy ...
Advanced breast cancer focus: Advanced breast cancer was a secondary topic; chemotherapy, breast cancer, palliative care, and introduction to cancer nursing were the primary topics of this programme. PPI involvement: Not reported. Curriculum model: Not reported. Programme development methods: Not reported.
Kimman et al, 2011 36 (Netherlands) Different interventions in 4 groups 1. Regular follow-up visits at 3, 6, 9, 12, and 18 mo. 2. Nurse-led telephone follow-up, control by mammography at 12 mo and telephone interviews by a breast cancer nurse during the routine follow-up months. 3.
No Time to Lose. Gürsoy, Ayla PhD. Author Information. Cancer Nursing: November/December 2015 - Volume 38 - Issue 6 - p 493-494. doi: 10.1097/NCC.0000000000000305. Free. Metrics. Breast cancer continues to threaten women's health all over the world despite the many advances in treatments in recent years. Nurses need to actively use their ...
Ribociclib plus Endocrine Therapy in Early Breast Cancer. D. Slamon and OthersN Engl J Med 2024;390:1080-1091. In patients with stage II or III early breast cancer, the addition of ribociclib to ...
Introduction. The number of survivors of breast cancer (BrCa) has increased since 1990 because of advancements in biomedical technology and medical care that have increased early diagnosis and treatment (World Cancer Research Fund International, 2020).Worldwide, it has been reported that 2,261,419 new cases of BrCa were diagnosed among women, making BrCa the most common cancer worldwide ...
To determine the impact of breast conservation on quality of life and identify treatment-related and other demographic factors associated with post-breast cancer treatment quality of life. A ...
Cancer is an international interdisciplinary journal publishing articles on the latest clinical cancer research findings, spanning ... Because breast cancer is a priority health condition and important ... integrated into the Romanian national health care system. 52 Undergraduate training in palliative care for medical and nursing students is ...
The COVID-19 pandemic has impacted just about every aspect of breast cancer care. Patients and doctors have been forced to adapt to the new normal, and the Breast Cancer Research Foundation (BCRF ...
Breast cancer is the second most common cancer in women, surpassed only by skin cancer (National Institute of Health, 2019). In 2019, approximately 268,000 women were diagnosed with breast cancer (National Cancer Institute, 2020). Approximately seven out of a hundred women will develop breast cancer before the age of seventy (Centers for Disease
Patient data. A total of 985 stage II/III locally advanced breast cancer patients enrolled in the multi-center I-SPY2 trial (clinical trial number: NCT01042379) during 2010 to 2016 were collected from the publicly available dataset on The Cancer Image Archive [17,18,19].Institutional review board approval was waived due to the use of public data.
Purpose Vaginal oestrogens can be used to treat genitourinary symptoms in women with early breast cancer. Studies evaluating vaginal oestrogens have commonly measured serum oestrogen levels as a surrogate marker of safety, but methods vary. We sought to summarise the data on serum oestrogen measurement in women with breast cancer using vaginal oestrogens to better understand the methods ...
Posted: January 20, 2023. Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
Results. Two main themes emerged: Challenges in caring for breast cancer patients and Nurses' psychological distress.The late diagnosis was very challenging for the nurses. Low health literacy regarding breast cancer disease and treatment, patients' financial difficulties, minimal oncology nursing education, and technology in healthcare systems were also major challenges.
The Oncology Nursing Society (ONS) is a professional association that represents 100,000 nurses and is the professional home to more than 35,000 members. ONS is committed to promoting excellence in oncology nursing and the transformation of cancer care. Since 1975, ONS has provided a professional community for oncology nurses, developed evidence-based education programs and treatment ...
Breast cancer is the most common malignancy in the female sex; although recent therapies have significantly changed the natural history of this cancer, it remains a significant challenge. In the past decade, evidence has been put forward that some oncogenic viruses may play a role in the development of sporadic breast cancer; however, data are scattered and mostly reported as sparse case ...
Each bimonthly issue of Cancer Nursing™ addresses the whole spectrum of problems arising in the care and support of cancer patients--prevention and early detection, geriatric and pediatric cancer nursing, medical and surgical oncology, ambulatory care, nutritional support, psychosocial aspects of cancer, patient responses to all treatment modalities, and specific nursing interventions.
A study led by researchers from Vanderbilt-Ingram Cancer Center sheds light on some of the genetic variants that make breast cancer more deadly for women of African ancestry and significantly reduces the disparity in knowledge for assessing their genomic risk factors.. The study, which was published May 13 in Nature Genetics, is the largest genome-wide association study ever conducted among ...
Among younger women with breast cancer, it may be possible for many to have a baby after their diagnosis thanks to advances in breast cancer care, new research suggests.. In a study of about 200 ...
Scientists have used breast cancer cells' weakness against themselves by linking a tumour-selective antibody with a cell-killing drug to destroy hard-to-treat tumours. The research, published ...
May 10, 2024. By UCLA School of Nursing. 1 min read. Erin Rosales, a first-year MSN-MECN program student, was selected to present her research at the annual UCLA Jonsson Comprehensive Cancer Symposium on May 8, 2024. Rosales, who joined UCLA from the Bay Area, previously worked as a Clinical Research Coordinator at Stanford and UCSF. She earned ...
Sarah Harding died aged 39 after being diagnosed with breast cancer. One of the Girls Aloud singer's final hopes was to find ways of spotting the disease early when it's easier to treat.
Investigating the issue, his team tracked 2000 to 2020 data on nearly 5,000 women with BRCA1/2 variants linked to cancer, all of who were diagnosed with breast cancer at the age of 40 or younger.
Disclosing breast cancer genetic test results [37,39] 37.2 [27.4-47.0] Country: GPs from Germany were significantly more likely to assume responsibility for disclosing breast cancer genetic test results in comparison to GPs from France, the Netherlands and the UK (43.7% vs. 23.5%, 11.6% and 16.9%) Primary prevention