With a herpes vaccine on the horizon, will the stigma persist?

When I was 9 years old, I came down with a terrifying bout of pneumonia and ended up in the hospital for a week. I remember having a panic attack when I couldn’t catch my breath and my mother, scared, called a doctor for help. He arrived quickly and calmed me down with his gentle bedside manner. He helped me take deep, slow breaths through an oxygen mask, to the tune of his voice as he counted down from 10. I egged myself on, knowing I had to relax or I’d get transferred to the local children’s hospital.
A few days later, I arrived back at school. I was in fourth grade. I had recovered but still had trouble breathing. Whenever my immune system runs low, I’m at greater risk for a herpes outbreak , and that’s exactly what happened. My face had erupted in giant, oozing cold sores. During recess, I sat on a bench alone listening to Coldplay’s “Yellow” on my Walkman. A group of older, prettier and more popular girls approached me. I pulled one of my earbuds out to hear what they were saying, only to find they were taunting me. “AIDS Face.” “Pimple Mouth.” “Zit Lips.”
I’ve had herpes for as long as I can remember, likely contracting the virus as a grabbing toddler reaching for my mother’s face.
As these cruel names were hurled at me, I trembled, cried and hugged my legs to my chest. When treating cold sores, time is of the essence. The second you feel a tingle, you need to treat the afflicted area. This helps mitigate the severity of the breakout . However, for a period of my childhood, I chose inaction, too traumatized by the stigma to do anything about it anymore. Instead, I leaned into being the weird kid and a social pariah, allowing my face to be riddled with herpes. While being infected with the virus is common and technically not a big deal, I was astronomically ashamed and isolated. In pop culture, the word herpes is near synonymous with dirty and that’s how I felt — dirty.
I’ve had herpes for as long as I can remember, likely contracting the virus as a grabbing toddler reaching for my mother’s face. Over the decades, I have spent a considerable amount of time agonizing over how to skip work, school and social events. When hiding from the world, I have tried every home remedy, topical cream and ointment and antiviral drug available. Sadly, there is no cure for herpes, only options to limit or prevent outbreaks . But a new vaccine on the horizon could prove to be a game changer.
Moderna is developing a vaccine using mRNA technology to treat the herpes simplex virus (HSV). There are two HSV virus types — HSV-1, the one I have, that affects the mouth, face and genitals, and HSV-2, which predominantly affects the genitals. However, both viruses can spread to other parts of the body. In the United States, of people aged 14 to 49, 47.8 percent have HSV-1 and 11.9 percent have HSV-2 , according to the Centers for Disease Control and Prevention. Many people living with herpes don’t know they have it, which means these figures may be far greater. HSV remains latent in the body , staying alive through the lifelong infection of a given person. When reactivated, HSV results in visible outbreaks. The vaccine will protect against HSV-2 and provide cross-protection for HSV-1 as a suppressive antiviral treatment.
The CDC recommends against widespread testing for herpes as, alongside the risk of false positives, “the risk of shaming and stigmatizing people outweighs the potential benefits.” Throughout my life, the social stigma surrounding herpes has proven more disastrous for my mental health than the virus itself. For so long, I assumed I wasn’t likable, let alone loveable. I believed I would be consigned to a life without sex and intimacy, having internalized harmful myths about a generally harmless infection. When I’ve had an outbreak, I’ve often chosen abstinence over disclosure, too fearful of rejection to open up. Interestingly, many people don’t even realize that having had chickenpox or shingles means they’ve been infected by a member of the herpes family . (Moderna is also developing a vaccine that would reduce the rate of the varicella-zoster virus that causes shingles.) But it’s the sexual component of HSV-1 and 2 that remains socially lethal.
The CDC recommends against widespread testing for herpes as, alongside the risk of false positives, “the risk of shaming and stigmatizing people outweighs the potential benefits.”
Much of the hysteria affecting the social status of herpes has been generated by the media and pharmaceutical companies. A 1982 TIME magazine cover labeled genital herpes “Today’s Scarlet Letter.” Authors of the cover story , John Leo and Maureen Dowd, posited that it could cause the sexual revolution to grind to a shrieking halt. Even more dramatic, the story argued that herpes was “altering sexual rites in America, changing courtship patterns, sending thousands of sufferers spinning into months of depression and self-exile and delivering a numbing blow to the one-night stand.”
Given the stigma around HIV at the time, perhaps the increased awareness about herpes did make some people change their sexual behaviors, but we also know that any activity that was deemed sexually deviant was used as a scapegoat to make sex seem shameful. A 2016 Vice exposé found that, starting in the 1970s, there is evidence that “big pharma” likely conjured up and perpetuated stigma to increase sales of a new drug, one that couldn’t be used to treat all members of the herpes family. To advertise the drug, herpes had to be pushed as a disease worthy of attention, the answer to which was a sex panic.
In the age of medical misinformation, vaccines themselves are misunderstood. For example, in general, they have been said to cause autism, despite no scientific evidence. The misinformation seems to increase when it comes to newly available ones; look no further than conspiracy theories swirling around Covid-19 vaccines , which were rumored to contain infertility agents or spread HIV — another notoriously stigmatized STI. The mRNA technology used to create these life-saving Covid-19 vaccines opened up the door for those Moderna is currently developing to treat herpes. In the near future, it’s possible that people will be prevented from ever getting herpes and that those with it won’t have to suffer through outbreaks anymore. I’ve wondered if the social stigma will persist and if kids like myself will be spared the pain I have experienced since childhood.
Deidre Olsen is an award-nominated writer based in Berlin. She is writing a memoir about self-destruction, healing and resilience.
Early Hints at a Gene Therapy Cure for Herpes
By Dennis Thompson HealthDay Reporter

WEDNESDAY, May 15, 2024 (HealthDay News) -- An experimental gene therapy could one day provide a first-ever cure for genital and oral herpes, researchers report.
The gene therapy removed 90% or more of oral herpes infection in lab mice, and it also suppressed how much virus an infected animal shed, according to results published May 13 in the journal Nature Communications .
The experimental therapy involves an injection of gene-editing molecules that seek out herpes virus hiding in the body, researchers said.
“Herpes is very sneaky. It hides out among nerve cells and then reawakens and causes painful skin blisters,” explained researcher Dr. Keith Jerome , a professor in the Vaccine and Infectious Disease Division at Fred Hutch Cancer Center in Seattle.

U.S. Cities With the Most Homelessness

The gene-editing cocktail includes laboratory-modified delivery viruses that contain an enzyme that works like “molecular scissors,” the researchers said.
Once the delivery virus reaches a cluster of nerves where herpes is hiding, it releases the enzyme. The enzyme then snips away at the herpes virus's genes, to either damage or destroy it.
The enzyme “cuts in two different places in the herpes virus’s DNA,” said lead researcher Martine Albert , a principal staff scientist at Fred Hutch. “These cuts damage the virus so much that it can’t repair itself. Then the body’s own repair systems recognize the damaged DNA as foreign and get rid of it.”
An estimated 3.7 billion people younger than 50 have herpes simplex virus 1, which causes oral herpes, according to the World Health Organization.
Another 491 million people 15 to 49 have herpes simplex 2, which causes genital herpes.
This experimental therapy eliminated 90% of facial infection and 97% of genital infection in lab mice who were infected with herpes simplex 1, researchers say.
It took about a month for the treated mice to reach these reductions, and the reduction of virus appeared to become more complete over time.
“If you talk to people living with herpes, many are worried about whether their infection will transmit to others,” Jerome said in a cancer center news release. “Our new study shows that we can reduce both the amount of virus within the body and how much virus is shed.”
This new therapy represents a streamlined approach to attacking herpes.
In a 2020 study, the research team used three different delivery viruses armed with two different enzymes to attack herpes. This study used just one delivery virus and one enzyme capable of cutting the virus DNA in two places.
“Our streamlined gene-editing approach is effective at eliminating the herpes virus and has less side effects to the liver and nerves,” Jerome said. “This suggests that the therapy will be safer for people and easier to make, since it has fewer ingredients.”
Researchers are preparing to translate the findings into treatments for humans that can be tested in clinical trials.
“We’re collaborating with numerous partners as we approach clinical trials so we align with federal regulators to ensure safety and effectiveness of the gene therapy,” Jerome said.
The team also is adapting the gene-editing technology to target herpes simplex 2 viruses.
More information
The Cleveland Clinic has more about herpes simplex .
SOURCE: Fred Hutch Cancer Center, news release, May 13, 2024
Copyright © 2024 HealthDay . All rights reserved.
Join the Conversation
Tags: genetics , sexually transmitted diseases
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

Key Moments From Cohen Cross-Examination
Laura Mannweiler May 16, 2024

Brown v. Board in Pictures
Lauren Camera and Avi Gupta May 16, 2024

Privilege Claim Signals Fed-Up Biden
Aneeta Mathur-Ashton May 16, 2024

Kim Tees Up Shift in New Jersey Politics
Louis Jacobson May 16, 2024

New Home Construction Holds Steady
Tim Smart May 16, 2024

Who Is Prime Minister Robert Fico?
Laura Mannweiler May 15, 2024

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Vaccines (Basel)

Insights into the Novel Therapeutics and Vaccines against Herpes Simplex Virus
Shiza malik.
1 Bridging Health Foundation, Rawalpindi 46000, Pakistan
2 Department of Microbiology, Institute of Medicine, Tribhuvan University Teaching Hospital, Kathmandu 44600, Nepal
3 Department of Microbiology, Dr. D. Y. Patil Medical College, Hospital and Research Center, Dr. D. Y. Patil Vidyapeeth, Pune 411018, Maharashtra, India
4 Department of Medicine, School of Health Sciences, Foundation University Islamabad, DHA Phase I, Islamabad 44000, Pakistan
Khalid Muhammad
5 Department of Biology, College of Science, UAE University, Al Ain 15551, United Arab Emirates
Yasir Waheed
6 Office of Research, Innovation, and Commercialization (ORIC), Shaheed Zulfiqar Ali Bhutto Medical University, Islamabad 44000, Pakistan
7 Gilbert and Rose-Marie Chagoury School of Medicine, Lebanese American University, Byblos 1401, Lebanon
Associated Data
Not applicable.
Herpes simplex virus (HSV) is a great concern of the global health community due to its linked infection of inconspicuous nature and resultant serious medical consequences. Seropositive patients may develop ocular disease or genital herpes as characteristic infectious outcomes. Moreover, the infectious nature of HSV is so complex that the available therapeutic options have been modified in certain ways to cure it. However, no permanent and highly effective cure has been discovered. This review generates insights into the available prophylactic and therapeutic interventions against HSV. A methodological research approach is used for study design and data complication. Only the latest data from publications are acquired to shed light on updated therapeutic approaches. These studies indicate that the current antiviral therapeutics can suppress the symptoms and control viral transmission up to a certain level, but cannot eradicate the natural HSV infection and latency outcomes. Most trials that have entered the clinical phase are made part of this review to understand what is new within the field. Some vaccination approaches are also discussed. Moreover, some novel therapeutic options that are currently in research annals are given due consideration for future development. The data can enable the scientific community to direct their efforts to fill the gaps that remain unfilled in terms of therapies for HSV. The need is to integrate scientific efforts to produce a proper cure against HSV to control the virus spread, resistance, and mutation in future disease management.
1. Introduction
Herpes simplex virus (HSV) belongs to the family of Alphaherpesvirinae with a characteristic double-stranded DNA structural composition [ 1 ]. Its two main serotypes, HSV-1 and HSV-2, are mainly known for their links with infectious diseases [ 2 ]. According to estimates by WHO, approximately 70–90% of the population worldwide is seropositive for HSV-2, which makes it a great concern for the healthcare community regarding the possibility of developing infections (James & Kimberlin, 2015b) [ 3 ]. HSV-1 is considered the main causative agent of ocular infection that may occur in patients already having diseases such as genital lesions, keratitis or retinal necrosis, encephalitis, iridocyclitis, or conjunctivitis [ 4 , 5 , 6 ]. Some studies have also established links between longstanding HSV-1 serology with psychological complications, including Alzheimer’s disease [ 7 ]. On the other hand, HSV-2 is predominantly linked with characteristic genital herpes disease of the herpes virus. These infections are worldwide, irrespective of the developing or industrialized national standing [ 8 ].
HSV-2 virus is a sexually transmitted infectious agent that is prevalent in approximately 536 million people worldwide, standing at an annual incidence rate of about 23.6 million cases, as per the updates by the CDC [ 9 , 10 ]. The genital area is the prime target of viral infection; however, it may also cause infections similar to HSV-1, including necrotizing stromal keratitis in the eyes, meningitis, encephalitis, and neurological complications [ 11 ]. However, not all HSV-2-positive cases develop genital herpes or ulcerative and vesicular lesions because the virus mostly remains in a latency phase that makes it possible to transmit to other members of the populace without getting noticed and without exhibiting any infectious outcomes [ 12 ]. In simple words, the sexual transmission may not be coupled with a clinical history or symptomatology of genital herpes. The symptomology makes the viral presence rarely fatal, but the same is not the case for babies from infected mothers and pregnant ladies, due to their immunocompromised system and susceptibility to easily acquire infection [ 12 , 13 , 14 ].
The most prevalent feature that makes HSV infection complications is its ability to enter a nonreplicating latency period, which enables it to survive long periods of inactivation within the host and gives it the ability to reactivate and infect the host under different external and internal stimuli [ 12 , 13 ]. The latency period is mostly asymptomatic; thus, viral transmission during this period remains unrecognized. This aspect is considered the major reason for the large-scale seroprevalence of HSV [ 13 ]. Additionally, it can infect almost every type of cell for the characteristic receptor recognition strategies, making it pertinent to large-scale population spread [ 10 ]. This property is associated with the presence of hundreds of diverse glycoproteins in HSV lipid bilayers that alter its receptor recognition and viral entry into host cells [ 8 , 15 , 16 , 17 , 18 , 19 ]. Furthermore, it uses multiple strategies for viral fusion, endocytosis, and transmission among cells, all of which cause further complications its treatment procedures [ 20 , 21 ]. Thus, most of the therapeutic studies are limited in terms of their effectiveness and inability to eradicate infections.
The complex nature of the virus, in terms of symptomatic infections and asymptomatic presence and recurrence, makes its cure difficult; for this reason, no vaccination or therapeutic cure has been devised that could completely eradicate HSV infection [ 21 , 22 ]. Moreover, the lifelong presence of HSV-2 infection further complicates the treatment procedures because it may require prolonged administration of standard proposed treatments, which is in line with its resistive nature [ 12 , 23 ]. In this review, we discuss the primary nature of HSV that makes it complicated for the design of therapeutics. This understanding can set the pace for further insight into the therapeutic interventions designed to date [ 24 ]. We discuss how the antiviral strategies are designed knowing the complexities associated with viral entry, infection, and persistence. The focus is on therapeutic developments that may hold hope for future control over HSV infections.
2. Materials and Methods
A systematic approach was used to gather the latest data regarding the different dimensions of H. simplex virus infection and the accumulated therapeutic and vaccination strategies. We searched electronic sources such as Google Scholar, Pub Med, NIH (National Library of Medicine), Scopus (Elsevier), and Web of Science. Moreover, the official websites of WHO, CDC, UNAID, and FDA were also used to obtain the statistical results and latest updates regarding HSV epidemiology, as well as ongoing treatment efforts. As the studies mainly incorporated the data regarding therapeutics against HSV viruses, the major research terms were “Herpes simplex virus”, “HSV infection”, “therapeutics against HSV”, “antiviral agents”, “vaccines against HSV”, “therapies against HSV”, “novel therapeutic approaches”, and some other linked search terms. After a thorough analysis of the dates, abstracts, titles, and journals of research publications, they were made part of this review. The process of information gathering was not limited to a few studies but rather collected from research compilations in the form of original research articles, reviews, short commentaries, case reports, and letters to the editors. Lastly, the search strategy was limited to incorporating data from 2010 to 2022 to add only the most recent advances related to HSV disease management.
3. Results and Discussion
3.1. the pathological biology of hsv and associated infection.
As explained earlier, HSV belongs to the family of neurotropic alpha herpesviruses, which are well known for latency features [ 4 , 12 ]. The virus particle consists of an internal dense electron core that contains the reproductive material in the form of double-stranded DNA [ 25 ]. All age groups of people are prone to developing HSV infection. Some other pathogenic species of viruses belonging to the same DNA herpes family of viruses include varicella-zoster virus (VZV), Epstein–Barr virus (EBV), cytomegalovirus, and human herpes types 6, 7, and 8 [ 26 ]. The two subtypes of HSV-1 and HSV-2 share close genomic relevance with >80% of the amino-acid identity profile. Moreover, both share the common infectious nature of causing oral and genital ulcerations [ 26 , 27 ].
HSV DNA has been well studied and formulated to encode 70+ genes. The genomic portion is enveloped by a viral capsid of icosahedral shape that in turn displays >162 known protein units (capsomers) [ 28 , 29 ]. The virus capsid is further surrounded by a lipid bilayer envelope consisting of tegument proteins and several dozen glycoproteins on the surface. Of this GP, five glycoproteins with known functions are gB, gC, gD, gH, and gl [ 30 ]. These proteins facilitate host viral coordination in terms of attachment, binding, and host cell penetration and entry [ 31 , 32 ]. The complex mechanism of HSV host interaction and its latency period present scientists with a great challenge [ 33 ]. The host virus entry mechanisms, cellular interaction, and infectious cycles are, thus, the prime focus of understanding for scientists developing therapeutics. This is discussed in relevance to HSV therapeutics in a later section of this article to describe to the readers the procedures, limitations, and progress achieved in this domain [ 27 , 34 ].
The virus Infection mainly begins with the epithelial cells of the skin or mucosal surfaces. The virus particle then trickles down to nerve endings and nerve axons, where the virus undergoes persistent infection within the trigeminal or lumbosacral ganglia region [ 12 , 35 ]. After establishing virus progeny in this area, it returns to the mucosal and skin surfaces to produce oral or genital ulcers. At other times, it remains asymptomatic, associated with viral shedding and silent transmission to other hosts [ 36 , 37 ]. This asymptomatic and silent transmission nature makes HSV spread quite extensively unnoticed in the population, as most cases remain subclinical and, thus, hidden from diagnosis [ 1 , 21 ]. The clinical manifestations of HSV-1 and HSV-2 vary depending on the age group, entry route, host immune response, and initial or recurrent nature of the infection [ 1 ].
HSV-1 is linked with episodes of genital and neonatal herpes, in addition to mainly causing oral and facial infections [ 38 ]. The disease incidences are higher in HICs, though the disease occurrence rates in neonates are lower compared to the incidence in children in LICs, where ≥90% people acquire HSV-1 infection by adolescence, which makes it a great healthcare burden for the world [ 10 ]. The major disease outcomes of HSV-1 infection include herpes labialis, gingivostomatitis, HSV-linked infectious encephalitis, keratitis, and pharyngitis [ 39 ]
HSV-2, on the other hand, is mainly dependent on sexual transmission and is associated with genital ulceration (genital ulcer disease (GUD)). Apart from the risk of neonatal herpes and possibly being associated with the development of neurological disorders such as Alzheimer’s at a later age, an increased risk of developing HIV infection is linked to HSV-2 infection [ 1 , 12 , 14 ]. The disease incidence is continuously rising on an annual basis due to the silent nature among sexual partners with high HSV-2 seroprevalence [ 3 , 13 ]. Additionally, the incidence rates are mostly outlined for high-income countries where R&D is progressively outlined in databases, while, in the case of low-income countries, the incidence rates are still unknown [ 10 ]. Neonatal herpes infections are often the major causes of increased morbidity and mortality rates. Most importantly, HSV-2 infection and transmission are linked with up to threefold increased incidence of HIV epidemics, with incidence rates up to 23–50% being the major concern regarding healthcare management. Moreover, the clinical complications in terms of life-threatening incidences may be accounted for in immunocompromised individuals [ 40 ].
The major diagnostic tests performed for HSV detection mainly include polymerase chain reaction, type-specific serological essays, viral culture, and antigen detection assays, which differentiate between the two subtypes of HSV [ 21 , 41 ]. The main treatment strategies against genital herpes mainly include antiviral treatments, such as acyclovir, valacyclovir, or famciclovir among other antiviral agents mentioned by WHO guidelines [ 3 , 9 , 22 ]. Most of these treatment regimens require continuous application to reduce the symptoms without permanently dealing with the virus prevalence. In the upcoming sections, we discuss the various therapeutic strategies and vaccination efforts against HSV carried out recently, as well as outline the futuristic perspective for HSV treatment.
3.2. Current Vaccination Efforts against HSV
Currently, no vaccine has been specified against HSV infection; however, several vaccination candidates are in research annals for vaccine development. Most of the clinical efforts are directed toward HSV-2 for its greater infectious outcomes, but HSV-2 vaccines will have benefits against both subtypes of viruses because of their sequence homology [ 42 , 43 , 44 ]. Several lines of research show that HSV vaccination is feasible. Successful vaccination strategies against varicella-zoster virus by using replated herpes virus biology, bovine herpesvirus-1, and herpesvirus-1 (pseudorabies virus) indicate that effective vaccine efforts against HSV can be successful [ 44 ]. Similarly, work on vaccines along with antiviral adjuvants has also presented preventive abilities against herpes zoster infection, with proven 97% efficacy in phase III trials [ 43 , 44 , 45 ].
Studies on human papillomavirus vaccination protocols are also helping with the understanding of immunomodulatory regulation which can be effectively induced with vaccination against HSV [ 43 , 46 ]. Some other research groups tested herpes vaccine trials with truncated glycoprotein D2 (Gd2T) vaccines tested on thousands of HSV-positive cases and demonstrated vaccine efficacy of up to 58% against HSV-1 with little or no effect against HSV-2. These studies indicated that immunomodulation against HSV-1 can be achieved with antibody titers, but the same cannot yet be shown for HSV-2 [ 21 , 30 ]. Additionally, the viral sequencing, widescale data availability, and genetic profiling for both HSV-1 and HSV-2 predict that better vaccination protocols could be implemented with the identification of potential targets for therapeutic development. With proper and coordinated efforts among bioinformaticians and clinicians, these efforts could be successful [ 46 , 47 ].
The rigorous efforts put forward for vaccination development come in two major forms, preventive and therapeutic vaccination protocols [ 48 ]. The former provides pre-exposure immune-protective responses against HSV-2 infection development tested over the long term up to phase III trials [ 43 ]. These trials have mostly been limited to HIC. It is hypothesized that the same preventive vaccination practices in LMIC could produce better results in terms of disease mitigation and adaptive management. For this cause, the geographic strain diversity must be accounted for during vaccination development to ensure the nonrestrictive geographic nature of HSV vaccination. Scientists propose that, if any candidate vaccine was found to be effective for both HSV subtypes, it could be shifted from adolescents to infants for the possible prevention option [ 14 , 49 ].
Therapeutic vaccines, in contrast, are being tested to reduce the disease symptoms and viral transmission across the hosts, for the larger benefit of public health management. For this route, the targeted populace is mostly HSV-2-infected persons. Some therapeutic candidates have been checked up to phase I and II trials with proven antiviral effects, as well as decreased viral shedding and lesion formation in infected subjects [ 30 , 48 ]. Similar to the first line of preventive vaccinations, this route has also been tested mostly in HIC without solid testing in LMICs [ 20 ]; a limitation to these preclinical trials on animals is that they cannot be assumed to be effective in humans since the host viral interaction could be different in people. Thus, certain limitations are attached to phase I and II trials. Therefore, some effort should be put into clinical experimentation for rapid disease management in the future [ 47 ].
3.3. Outlining the Current Vaccines against HSV
Various vaccine candidates are in research annals for preclinical and clinical evaluation. Currently, there is no specific FDA-approved vaccine against HSV infection [ 9 ]. The ongoing clinical and preclinical trials are based on the rational understanding of HSV biology and immunopathogenesis in host cells. Some of the latest vaccine designs, their working mechanism, and ongoing trials are outlined briefly in the Table 1 .
An update on current vaccine candidates against HSV.
3.3.1. Subunit Vaccines against HSV
Subunit vaccines are composed of viral components, such as glycoproteins and protein subunits, which undergo protective immune responses to the host [ 50 ]. They have proven safer, stable, and effective for HPV vaccination design and immunization design, but still lack clinical experimental success against HSV [ 51 ]. They mostly use viral glycoproteins and antigenic mediators such as Gb/Gd/gE in their antiviral design. This type of vaccine varies in function and procures the inhibition of viral entry, viral shedding, transmission across cells, and immune-evasive responses [ 42 , 50 , 51 ]. Novel experiments are ongoing that link multiple herpes antigens and peptide epitopes in one vaccination protocol. Approximately 80–300 open readings frames (ORFs) identified by multi-omics technologies are under consideration for antigenic breadth generation and efficiency in subunit vaccines against HSV [ 42 , 52 , 53 ].
This method of vaccination provides a gateway to present complex antigenic composition to the immune system that may include T- and B-cell epitopes [ 22 , 72 ]. For testing the efficacy of these vaccines, several recombinant protein formats have been tested that are conceptually similar and undergo the introduction of HSV ORFs (complete or near complete), into bacterial or other vector systems [ 54 , 73 ]. Moreover, these vaccine combinations with certain adjuvants and vaccine formats have opened a new route to explore options for HSV vaccination in the future.
3.3.2. Vectored/DNA/RNA Vaccines against HSV
DNA- and mRNA-based vaccines have been in research annals for a long time now. The same approach has been successfully used in COVID vaccination design and is now being successfully utilized against HSV [ 74 , 75 ]. Experiments have been conducted on animal models to check the efficacy of nucleoside-modified mRNA-based vaccines against HSV-2 infection [ 75 ]. The results indicated a therapeutic reduction in symptoms within animal models in a dose-dependent manner. Moreover, these vaccines stimulate immune responses in the form of neutralizing antibodies [ 76 ]. Studies have shown that DNA is a better candidate for its stability, synthesis characteristics, and purification protocol and can be better managed compared to mRNA [ 29 , 62 ].
DNA-based vector vaccines have shown efficacy even better than subunit vaccines but not as effective as live-attenuated vaccines [ 29 , 62 ]. Moreover, some clinical concerns in the form of side-effects remain linked to the application of vehicle vector carriers [ 77 ]. Thus, adenovirus vector-based vaccines exhibit a better stability profile than mRNA vaccines. Recent studies showed the use of Vaccinia and MVA vectors for the deployment of transgenetic expression and virulence in tested subjects against different viral diseases caused by HIV, influenza, measles, flavivirus, and malaria vectors [ 11 , 45 ]. Thus, these insights into vaccination approaches compel scientists to drain effective vaccination efforts against HSV [ 78 ].
3.3.3. Live-Attenuated Vaccines against HSV
The live-attenuated vaccination method has been the most used and effective method against viral infections through history, such as smallpox vaccination, poliomyelitis, measles, mumps, rubella virus, rotavirus, and many other infections [ 63 ]. The mechanism of inactivation often includes chemical or radiation-based inactivation of virus particles. One of the antiviral vaccine candidates derived for chicken pox virus/HSV-3 (varicella-zoster virus (VZV)) is also based on a live-attenuated virus vaccination protocol [ 62 ]. It is safe and well tolerated with a highly effective profile that controls viral reactivation. This and several other examples guide a more effective vaccination protocol to be designed on the basis of this mechanism [ 43 , 62 , 63 , 64 ].
Live-attenuated vaccination has also contributed to the development of FDA-approved oncolytic virotherapy against herpes simplex virus known as (TVEC or Imlygic), which limits virus replication and regulates human immunity, and which is used for treating human melanoma [ 63 ]. Following this approach, novel vaccination drives are being tailored in medical science to reduce the side-effects and induce long-term immunity against HSV infection, with the aim of achieving prophylactic and therapeutic goals to reduce viral infection and reduce the disease symptomology [ 43 , 62 ]. Moreover, efforts are being directed to reducing the neurotropism and latency associated with HSV while designing the live-attenuated vaccination regimens. Thus, by introducing certain insertions and/or or deletions in the viral progeny, the vaccination attempts show disrupting neuronal retrograde transport and the respective inability of HSV to affect neuronal cells [ 32 , 38 , 65 ]. Some important clinical ongoing trials in this regard are provided in Table 1 .
3.3.4. Peptide Vaccines against HSV
Peptide vaccines are developed on the principle that a single molecular entity or peptide epitope could generate massive immune responses to protect against a particular disease. In this regard, immunization with immuno-dominant T-cell epitopes or neutralizing epitopes has been tested and found to be protective [ 58 ]. This system of vaccination has shown better outcomes upon the combined application of certain adjuvants such as heat-shock proteins that may be expressed in recombinant viruses or bacterial expression systems [ 37 ]. However, the complications and limitations associated with the widespread human population and differential immune responses that may entail immunodominant responses by a certain peptide hinder the development of peptide-based vaccines [ 25 , 31 ]. However, efforts are still in research annals to develop better vaccination options for both serotypes of HSV.
3.3.5. Killed-Virus Vaccines against HSV
Similar to the live-attenuated mechanism, this mechanism involves variations in terms of killed virus vaccination to avoid the risk of reactivation of viruses in subjects. Traditionally, phenol chemicals and UV light treatments have been used for this purpose, but other methods of viral inactivation have also been used more recently [ 79 , 80 ]. This approach is used as immunotherapy but remains underrated as it only provides little help to regress the viral infection, which is a property of natural infection. Recent advances have been made, and some newer studies are in progress that use sonication, chemicals, radiation, UV light, formaldehyde treatment, or their combination to cause viral death [ 80 , 81 , 82 ]. Moreover, experiments are performed by regulating the dosage amount, time, route, and number of administrations and combination with adjuvants to check the efficacy. However, further work is necessary to deduce the efficiency of this vaccination method [ 80 , 83 ].
3.3.6. Fractionated-Virus Vaccines against HSV
In these protocols, HSV vaccines are prepared by subjecting the infected cultured cells to various procedures, which inactivate the virus particles while partially purifying some viral protein subsets [ 82 , 84 ]. Trials are ongoing on such vaccination methods. In simple terms, viral characteristic proteins such as those used in peptide vaccines (e.g., gPs) are mixed with inactive virus particles and with some adjuvants to produce a binding effect of the vaccine. Previous studies have shown little or no effect on immune responses; thus, this approach requires further work to induce productive clinical outcomes [ 30 , 31 ].
3.3.7. Discontinuously Replicating Virus Vaccines against HSVs
In this method, some important genes required for viral replication or transmission are either deleted or replaced with other genes. The method is mainly used to study the functionalities of different proteins; however, the same approach is often used for designing vaccines [ 22 , 73 ]. These viruses may undergo replication but are unable to further infect the cell because they are transmission noncompetent. Because of this effect, they are termed as discontinuously replicating viruses. They exhibit the property of inability to restimulate periodically to have a recurrent, peripheral lytic replication cycle [ 47 , 64 ]. They have been checked in animal subjects for creating strong immune responses, with some candidates entering clinical trials, as indicated in Table 1 . However, further work is required for effective clinical improvement in vaccination implications.
3.3.8. Replication-Competent Live-Virus Vaccines against HSV
As the name indicates, these vaccines exhibit the replication property of viruses but undergo certain insertions and or deletion of encoded genes for application. They generate broad-scale immunostimulatory effects, including reactions from T and B cells and neutralizing antibodies. [ 85 ] They undergo the presentation of a complex mixture of epitopes with only a few missing genes. In the case of latency and reactivation from virus progeny, an endogenous re-boosting effect is created [ 12 ]. However, the limitation is that the possibility of mutation and reactivity with the wildtype strain of HSV in an immunocompromised individual may alter the vaccine mechanism. Moreover, complications may also be faced in terms of viral strain production and serological testing of HSV infection [ 12 , 13 ]. Several genetic studies have been conducted to understand which genes can be deleted for the preparation of replication-competent vaccines. This method is similar or identical to the method used in live-attenuated vaccination [ 61 , 63 ].
3.4. Therapeutics and Antiviral Strategies against HSV
When HSV infection was initially identified as a health concern, several therapeutic trials were put into research trials for evaluating different drugs against it. So much research was conducted around the time of the discovery of acyclovir back in the 1980s [ 21 ]. The search has not stopped even now, and new therapeutics are being developed that focus on different mechanisms of antiviral action [ 86 , 87 , 88 ]. This may include various approaches such as virus entry inhibitors, fusion, or virus-release inhibitors. Among these trials, N-docosanol (an entry inhibitor) is the only FDA-approved drug that is used to counter herpes labialis but not recurrent genital herpes or ocular infection [ 48 ]. More effective therapies are required to contain the global burden associated with HSV infections. Some of the major drugs with varying mechanisms of action are briefly described in the next section and a summary has been presented in form of Table 2 at the end of this section.
3.4.1. Receptor Targeting Therapeutics against HSV
These therapeutics work by preventing the receptor virus binding phenomena by targeting HSV entry molecules/receptors or glycoproteins on the host cell surface. They demonstrate both prophylactic and therapeutic efficiencies against HSV [ 38 , 47 , 89 ].
Anti-Heparan Sulfate Peptides
Two important receptor peptides, G1 and G2, have a role in binding to the cell surface receptors of HS (present in almost all cell types) and targeting them to block HSV-1 infection [ 55 ]. This phenomenon has been dose-dependently checked in cell line-based experiments. These results indicate the potential benefit of the inhibition of viral replication and cell-to-cell viral spread [ 56 ]. Similarly, experiments on animal models exhibited their prophylactic properties against ocular and genital infections. The overall number of genital lesions was reduced in tested subjects. However, a limitation of these drugs is the presence of HS receptors on all cells; thus, the drugs may produce side-effects in healthy cells, while there is a need to prevent the associated cytotoxicity [ 11 , 55 , 56 ].
Apolipoprotein E
Apolipoprotein E (apoE) is a glycoprotein that helps in viral attachment and entry by binding directly to heparin sulfate proteoglycans in the extracellular matrix of the host cell membrane [ 90 ]. Specifically, the tandem repeat dimer peptide, apoEdp, exhibits antiviral activity against both HSV 1 and HSV 2, as well as HIV. The effective results of these drugs have been shown to induce corneal infection along with immunomodulation in terms of downregulated proinflammatory and angiogenic cytokines [ 40 , 90 ]. Moreover, the drugs exhibited low or no systematic toxicity in mouse models. Their effect has been comparatively evaluated to be the same as that of the currently in-use drug trifluoro thymidine (TFT) against HSV-1. The therapeutic effects have largely been shown to reduce infection symptomology in animal models [ 40 , 90 ].
AC-8-Potential Cationic Peptide
AC-8 is an igG FAB fragment that exhibits antiviral properties by binding to the glycoprotein D receptor [ 57 ]. This drug has shown efficacy in terms of reducing corneal vascularization and keratitis in mouse models. This property is produced due to the essential role of Gd in the herpes virus entry mechanism, which AC-8 successfully targets to prevent a subsequent infection. It also reduces cytotoxicity and inflammation even after repeated usage [ 25 , 37 , 56 ].
3.4.2. Nucleic Acid-Based Molecules
Aptamers are compounds that can bind with targeted molecules with a high affinity. They have characteristic features similar to antibodies; they fold in a different sequence-specific conformation determined by the target agents [ 91 ]. Several aptamer compounds have been proposed as antiviral agents in different infectious diseases, including HIV, cytomegalovirus, and recently against glycoproteins of HSV viruses [ 61 ]. RNA aptamers are major candidates under study that exhibit the antiviral potential to neutralize HSV species. Their highly specific nature allows scientists to define and manufacture specifically targeted aptamers that do not show a reaction against other viruses [ 61 , 91 , 92 ].
Dermaseptins
These form a family of associated poly cationic peptides derived from frog species. They exhibit antiviral properties against HSV species [ 93 ]. They interfere with the virus–host interaction owing to the positively charged amino acids that bind with the opposing negative charged heparin sulfate molecules of host cells [ 36 , 55 , 56 , 93 ]. Experiments showed they were effective against acyclovir-resistant HSV-1 species and had a reduced cytotoxic profile. They work well at low pH levels, which may allow them to remain active in the genital tract [ 55 , 56 ]. Some important cation ion peptides belonging to dermaseptins are indicated in Table 2 .
3.4.3. Viral Glycoprotein Targeting Therapeutics
As explained earlier, virus surface glycoproteins play an important function in fusion and viral entry into the host cell. They are positively charged molecules; hence, polyanionic compounds with negative charges could be designed and used to inhibit HSV fusion and replication in vitro by targeting the glycoprotein/sulfate compound complex [ 27 , 30 , 31 , 32 ]. Some important polyanionic compounds that have been used in research experiments are described briefly below.
Nanoparticles with Affinity to Bind GPs
Recent advances in nanotechnology-based therapeutics have presented newer methods for tackling viral infections. Hence, various experiments have been designed that may inculcate the properties of metallic nanostructure-based compounds with high affinity to bind viral glycoproteins [ 94 , 95 ]. As the virus binds to the HS with its surface gPs, a strategy could be devised simply by targeting the gPs. Some important nanoparticle species such as gold nanoparticles (AuNPs), tin oxide (SnO), zinc oxide (ZnO), mercaptoethane sulfonate (Au-MES), and some other important species are under research [ 94 , 95 , 96 , 97 ]. Moreover, the latest studies have demonstrated dual effectivity in terms of viral fusion inhibition and immune stimulation to protect against viral diseases. The overall effect is reduced virus entry, replication, transmission, mutation, and highly induced immune response against these virus infections. Moreover, the conjugation with other drugs and adjuvants may also provide added value to antiviral therapeutics [ 94 , 96 ].
K-5 and SP-510-50 Compounds
Since the presence of HSV-2 infection increases the likelihood of catching HIV-1 infection, therapeutics are being designed for a combined and simultaneous effect against both. In this regard, polyanionic K-5 compounds present a major therapeutic option to address this issue [ 30 , 31 ]. They work by inhibiting free virion infection by interfering with GPs and subsequently preventing cellular cross-transmission in vitro. With more advanced clinical experimentation, these compounds could be used against the sexual transmission of HSV and HIV diseases [ 48 ]. Similarly, SP-510-50 works as an antibody toward the gD of virus particles and, thus, provides antiviral infectivity in HSV patients. Their effect is bound to their dosage applicability for infection prevention [ 85 , 89 ]. They exhibited twofold better results compared to the commercial trifluoridine (TFT) using a lower dose. Moreover, the overall disease symptomology was reduced by their application [ 38 ].
Dendrimers are composed of an amino-acid or carbohydrate conformation that is arranged in macromolecular compositions. Like nanoparticles, they exhibit good antiviral activities for their size [ 58 ]. Moreover, their characteristics, such as ease of preparation, ability to display a wide variety of surface molecules, easier functionalization, and targeted effect against viral gPs and the host cell surface make them an important therapeutic candidate for HSV treatment [ 31 ]. The surface characteristics make them eligible to bind multiple drug regimens, with a high and multidrug payload. Their successful application against HSV is in research annals. The purpose of these trials is to properly establish the safety, tolerability, toxicity, and systematic pharmacokinetic properties of these agents [ 31 , 58 ]. Some important ongoing trials are shown in Table 2 .
3.4.4. Targeting the Downstream Signaling Cascades
Targeting various downstream molecules that conduct cell signaling to induce viral infection is an important strategy that has been the focus of cell biology and bioinformatics recently. These studies allow the exploration of wide-spectrum molecular entities that could be used to design targeted therapies [ 98 ]. For example, studies have demonstrated the mechanism of different viruses that use actin and myosin-dependent pathways for the internalization of viruses in the cell [ 99 ]. The same property is exhibited by HSV which is involved in phagocytic uptake by corneal fibroblasts and retinal epithelial cells [ 98 , 100 ]. The underlying mechanisms are controlled by various kinases such as cyclic AMP-dependent protein kinase A, Akt/PKB, and ribosomal kinases p70 and p85, which play important roles in establishing cellular fusion [ 98 , 99 , 101 ]. Thus, inhibitor therapies are being designed against PI3K kinases to regulate the cellular surfing, entry, and viral infection in targeted cells. Successful results have been acquired in vitro, while next-level studies are still ongoing.
3.5. Antimicrobial Peptides against HSV
Antimicrobial peptides (AMPs) are positively charged short oligopeptides found in virtually all organisms which exhibit diversity in structure and function. They are synthesized and processed to play a vital role in initial immune responses against injury and infections. Some examples of such AMPs in humans include defensins, transferrins, hepcidin, cathelicidins, human antimicrobial proteins, histones, AMP-derived chemokines, and antimicrobial neuropeptides. AMPs have widely been studied for their potential antiviral properties. Defensins have been shown to play a protective role against HSV by blocking virus entry and other stages of the virus life cycle [ 102 , 103 ]. Several studies have shown a vital role of AMPs against various viral infections; therefore, AMPs can be effectively used as excellent therapeutic agents against HSV [ 104 ].
3.6. Some of the Latest Therapeutic Options
3.6.1. compounds derived from marine resources (algal species).
The widespread HSV positivity in the human population has compelled the scientific community to continuously remain engaged in proposing different therapeutic regimens against HSV infections [ 21 , 46 , 48 ]. The traditionally used drugs such as acyclovir, ganciclovir, valaciclovir, and foscarnet are good options for HSV treatment; however, the development of drug resistance in patients and the ability for viruses to develop a mutation in strains has compelled scientists to look for other options [ 105 ]. Marine-based products, such as those derived from algal populations, bacterial species, fungal biomass, sponges, tunicates, echinoderms, and mollusk seaweeds, are important organisms from which these drug candidates are being derived [ 105 ]. Caulerpin is one of such candidate drugs that has its origin in marine algae and works well as an antioxidant, antifungal, antibacterial agent, and acetylcholinesterase (AChE) inhibitor [ 106 ]. It functions to inhibit the stages of the replication cycle [ 107 ]. Moreover, its application as an alternative to traditional acyclovir is under consideration. In addition to caulerpin, various other algal species (~40) are in research and development for exhibiting anti-HSV properties in resistant infections. They exhibit antiviral activity ranging from 50% to 80% for both species of HSV [ 21 , 46 , 48 , 105 , 106 ]. Different algae with antiviral properties are shown in Table 2 . These studies allow the scientific community to delve deeper into marine-based and plant-based products to find a cure for HSV.
3.6.2. Mucus Penetrating Particles
Since mucus formation is an unfortunate characteristic of the common summer cold, concurrent HSV and common cold infections could present a hurdle in drug delivery and penetration of the targeted cells [ 95 ]. Owing to the mucoadhesive characteristics exhibited by common drugs, some studies have been conducted to design mucous penetrating particles mainly based on nanoparticles. These neoformations easily penetrate the tissues of the sinuses and vagina and, thus, establish successful delivery of drugs to tissues of interest [ 95 , 96 ]. Moreover, they provide the opportunity to surface coat the particles with multiple antiviral drugs and enable better absorption of the nanosized particles for a more profound effect. Overall, MPPs improved drug binding, distribution, retention, and dosages, as well as reduced toxicity, in HSV model experiments [ 95 , 96 , 108 ].
3.6.3. Plant-Derived Therapeutic Options
Similar to algal-derived drug candidates, some recent studies have indicated the therapeutic potential of some plant-based products ( Table 2 ). Like other drug regimens, they inhibit the virus entry and replication cycle by acting as potent inhibitors of various glycoproteins specific to different antiviral plant agents [ 109 ]. Antiviral agents such as neem bark extract (NBE) derived from Azardirachta indica and cyanovirin-n (CV-N) derived from Nostoc ellipsosporum, as well as peri-acylated gossylic nitriles derived from gossypol, are some of the important drug candidates exhibiting efficient anti-HSV profiles [ 42 , 47 , 48 , 109 , 110 ]. However, the potent anti-HSV profiling, toxicity studies, pharmacokinetic profiling, and antidrug comparative studies remain to be conducted in detail to provide the benefits associated with plant-based herbal therapies [ 109 ].
3.6.4. Combined Therapies
Knowing the scope of HSV disease implications, scientists are now gathering their research focus toward combined therapies since a certain specific drug or vaccine has not yet been shown to eradicate HSV infection [ 21 , 30 , 48 , 61 ]. Therefore, more integrated and coordinated efforts are being put forth in the form of combined therapies, where several drug combinations are checked for their effect against HSV. Most of the individual drug regimens have already gone through scientific examination to establish their antiviral character. Hence, the purpose of combined therapies is to only evaluate multiplex combined antiviral effects against HSV infection [ 46 , 48 ]. Various experiments in research annals have been carried out in vitro, in animal models, and in clinical trials. Similarly, more specific studies are in the research phase against proven anti-HSV drugs such as acyclovir and acycloguanosine in terms of evaluating their cytotoxic and pharmacokinetic profiles and upgrading them by nano-scaling or conjugating with nanoparticle formulations for effective low dosage implications [ 59 , 94 , 95 , 96 , 97 ]. These latest studies have provided a doorway to the resistance that develops over time in patients. The new formulation offers lower dosage, more targeted delivery, and enhanced efficacy in tested subjects. Therefore, the field of combined therapy against HSV is a major player in the future drug and vaccination designs against HSV. A brief overview of these therapeutic strategies against HSV have been covered in a summarized version in Table 2 below.
Ongoing trials for HSV drugs.
4. Conclusions
Several vaccines and drug trials are in progress against HSV. They provide a promising therapeutic potential in individual studies. However, no profound and specific therapy has been established until now that could tackle the problem of HSV infection worldwide. The need is to establish more coordinated and integrated studies with the cooperation of scientists, doctors, and pharmacies to take drug testing one step ahead in clinical practice. This is important because the expected viral mutations present the threat of the development of another mutant HSV that could then become another complication for HSV treatment and prevention. Therefore, the most effective approach for future therapeutic development will be to develop modern drug-design approaches such as those based on plant products and nanotechnology, and to carry out more combined therapies for large-scale and broad-spectrum antiviral and immunostimulatory effects so that HSV complications can be successfully addressed in the coming years.
Abbreviations
Funding statement.
This research received no external funding.
Author Contributions
Conceptualization, S.M. and Y.W.; methodology, S.M., R.S., K.M. and Y.W.; validation, S.M., O.A., R.S. and K.M.; formal analysis, S.M., O.A., R.S., K.M. and Y.W.; resources K.M. and Y.W.; data curation, S.M., O.A., R.S., K.M. and Y.W.; writing—original draft preparation, S.M.; writing—review and editing, S.M., K.M. and Y.W.; supervision, Y.W.; project administration, Y.W. funding acquisition, Y.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Informed consent statement, data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
Herpes virus articles from across Nature Portfolio
Herpesvirus is an infectious agent belonging to the virus family Herpesviridae that causes latent and lytic infections in a wide range of animals and humans. There are 8 herpesvirus types currently known to infect humans, including Herpes simplex viruses, varicella-zoster virus, Epstein-Barr virus, Kaposi's sarcoma-associated herpesvirus and human cytomegalovirus.
Latest Research and Reviews
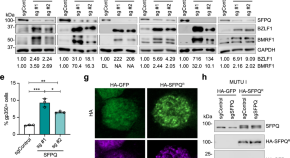
The nucleic acid binding protein SFPQ represses EBV lytic reactivation by promoting histone H1 expression
Here, Murray-Nerger et al use a genome-wide CRISPR/Cas9 screen to show that the nuclear protein SFPQ suppresses lytic reactivation of Epstein-Barr virus by promoting the expression and accumulation of linker histone H1 on the viral genome.
- Laura A. Murray-Nerger
- Clarisel Lozano
- Benjamin E. Gewurz
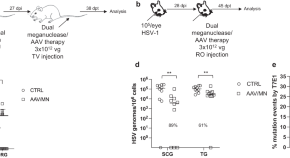
Gene editing for latent herpes simplex virus infection reduces viral load and shedding in vivo
The main challenge for anti-HSV therapy is to target latent virus in ganglionic neurons. Here, the authors report a well-tolerated anti-HSV gene editing approach against HSV which targets latent HSV genomes and leads to reductions of ganglionic viral loads, and viral shedding upon reactivation in mouse models.
- Martine Aubert
- Anoria K. Haick
- Keith R. Jerome
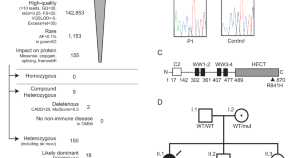
Herpes simplex encephalitis due to a mutation in an E3 ubiquitin ligase
Encephalitis is a rare and severe complication of Herpes Simplex type 1 infection. Here, Bibert et al describe a genetic variant in a 2-year-old affected child that impairs interferon production in neuronal cells and enhances viral replication.
- Stéphanie Bibert
- Mathieu Quinodoz
- Pierre-Yves Bochud
Highly stable and immunogenic CMV T cell vaccine candidate developed using a synthetic MVA platform
- Marcal Yll-Pico
- Yoonsuh Park
- Don J. Diamond
Neuronal miR-9 promotes HSV-1 epigenetic silencing and latency by repressing Oct-1 and Onecut family genes
Here, the authors identify neuron-specific miR-9 that potentially blocks HSV-1 neuronal replication by targeting host OCT-1 and ONECUT transcription factors involved in epigenetic activation of HSV-1 productive-cycle genes. Thus miR-9 promotes viral epigenetic silencing and latent infection in neurons.
Human cytomegalovirus exploits STING signaling and counteracts IFN/ISG induction to facilitate infection of dendritic cells
Human cytomegalovirus (HCMV) is a ubiquitous pathogen associated with morbidity and mortality in the immunocompromised or immunonaive context. Here the authors show that HCMV exploits STING signalling and subverts the interferon response to support infection of monocyte derive dendritic cells.
- Bibiana Costa
- Jennifer Becker
- Ulrich Kalinke
News and Comment
EBV linked to multiple sclerosis
Two recent papers implicate Epstein–Barr virus (EBV) as a trigger for the development of multiple sclerosis and provide mechanistic insights into EBV-mediated development of the disease.
- Andrea Du Toit
Motor-powered trafficking
This study provides insights into the neuroinvasive mechanism of neurotropic alphaherpesviruses, which involves viral assimilation and repurposing of a cellular motor protein.
Making the cut
This study reports the development of a CRISPR –Cas9 therapeutic to target herpes simplex virus 1 and treat herpetic stromal keratitis in mice.
Controlling the nucleus from a distance
This paper found that cytoplasmic control of intranuclear polarity by human cytomegalovirus leads to segregation of viral DNA from heterochromatin, thus promoting virus replication.
- Ashley York
Exploiting peroxisomes
New activities for old antibiotics
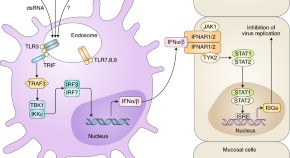
Topical administration of aminoglycoside antibiotics has been shown to induce expression of interferon-stimulated genes in dendritic cells, inducing an antiviral state in the vaginal and lung mucosa that increases resistance to infection with herpes simplex virus 1, influenza and Zika viruses.
- Jeffrey I. Cohen
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Interest Areas
- Systems Immunology and Genomics
Harvey M. Friedman

- Cell and Molecular Biology
Description of Research Expertise
Selected publications.
© The Trustees of the University of Pennsylvania | Site best viewed in a supported browser . | Report Accessibility Issues and Get Help | Privacy Policy | Site Design: DART Web Team. | Sitemap
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
June 17, 2021 report
Tweak to pritelivir allows treatment of latent herpes infections
by Bob Yirka , Medical Xpress
A team of researchers at Innovative Molecules GmbH, working with several other institutions in Germany, has developed a small-molecule therapy for the treatment of latent herpes simplex virus infections. In their paper published in the journal Science Translational Medicine , the group describes tweaking a drug used for treating active herpes infections for treatments of both active and latent herpes infections in rodents.
Herpes is a viral disease that can affect the skin and sometimes the nervous system . The oral version of the disease is called HSV-1 and the genital version HSV-2. People with the disease typically exhibit cold sores and can experience itching or pain—sometimes, such as with infants or people with compromised immune systems, the disease can become life threatening if it impacts the nervous system. The disease has two states: active and dormant. As the names suggest, symptoms generally appear during active periods and cease during dormant times. Treatment for the disease works only for active infections—therapies typically target viral DNA polymerase. Such treatments do not work for latent infections, because the viruses hide in the nervous system where the drugs cannot reach them. In this new effort, the researchers have tweaked a drug previously developed by Innovative Molecules called pritelivir—it has been used to treat active herpes infections, but does so in a different way: by targeting a different enzyme—it unwinds the viral DNA strand, rather than reducing its ability to build its DNA. It was chosen for tweaking due to its small size.
The tweaking by the team involved changing out a sulfonamide for a sulfoximine to remove undesired off-target effects. They also changed one of the aromatic groups to make the molecule even smaller, allowing it to enter the central nervous system. The team has named the new therapy IM-250.
Testing of the new therapy showed it prevented death in mice that were given lethal loads of HSV-1 viruses. They also found that it reduced symptoms in guinea pigs infected with HSV-2 and prevented the reemergence of symptoms even after the therapy was stopped.
© 2021 Science X Network
Explore further
Feedback to editors

Likelihood of kids and young people smoking and vaping linked to social media use
8 hours ago

Primary health coverage found to have prevented more than 300,000 child deaths in four Latin American countries

Global life expectancy projected to increase by nearly 5 years by 2050 despite various threats

Number of people experiencing poor health, early death from metabolism-related risk factors has increased since 2000

Men at greater risk of major health effects of diabetes than women, study suggests


Discovery of a master neuron that controls movement in worms has implications for human disease
10 hours ago

First US trial of varenicline for e-cigarette cessation shows positive results
11 hours ago

Examining the mechanisms and clinical potential of a promising non-opioid pain therapy candidate
Machine learning method for predicting glioma mutations shows promise for personalized treatment

Study finds brain wiring predicted adolescents' emotional health during COVID-19 pandemic
12 hours ago
Related Stories

Discovery reveals mechanism that turns herpes virus on and off
Nov 14, 2019

Drug for rare disorder shows promise for treating herpes viruses
Dec 7, 2020

Scientists describe new herpes treatment strategy
Dec 3, 2014
Are we getting closer to a herpes vaccine?
Nov 16, 2020

New gene therapy approach eliminates at least 90% latent herpes simplex virus 1
Aug 18, 2020

Commonalities found between viral infections and ovarian cancer
Feb 18, 2021
Recommended for you

Researchers profile clinical, gene and protein changes in 'brain fog' from long COVID
15 hours ago

'Trojan Horse' weight loss drug found to be more effective than available therapies

Despite its 'nothingburger' reputation, COVID-19 remains deadlier than the flu
20 hours ago

Link between COVID-19 vaccine complication and rare 'common cold' blood disease
16 hours ago
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
A Guide To Herpes
Home / Clinical Trials / A Guide to Herpes – Cures & Clinical Trials
Herpes Clinical Trial Recruiting Now
What is herpes.
Herpes is a sexually transmitted disease (STD) caused by the herpes simplex virus (HSV). It is one of the most common STDs in the world, and it is estimated that approximately one in eight people have been infected by the virus. Around 80% of those infected don’t even know they have the virus. It is impossible to know exactly how many people have the virus, since many cases are asymptomatic or never diagnosed.
What Causes Herpes?
Herpes is caused by two different viruses: HSV1 and HSV2. HSV1 usually causes sores around the mouth, while HSV2 causes genital herpes.
The infection is spread by skin-to-skin contact, and it can be transmitted through oral, vaginal, and anal sex, and kissing. It can also be spread through contact with lesions from other areas of the body. The virus goes into the body through small lesions in the skin, or through the mucosae in the mouth, penis, vagina, cervix, or anus.
After the infection, the patient might develop unspecific symptoms such as fever, fatigue, nausea, myalgia, adenopathy, along with the characteristic sores. Sores are small blisters which can sting or burn. They are usually grouped in clusters and they become crusted before healing. They don’t leave scars and resolve spontaneously. In some cases, patients won’t develop sores, instead displaying only irritated skin. Women can also present with vaginal discharge. Some people can be infected and remain asymptomatic; however, they can still spread the virus to other people.
After the first episode, the virus can become latent and remain in sensory nerves. This is known as the latent stage; afterwards, the virus can affect the skin again, usually along the pathway of the nerve where it has remained. These are the recurring episodes of the virus.
Fortunately, outbreaks tend to become less frequent and painful over time.
Risk factors for acquiring the infection include:
- Multiple sex partners
- Previous STDs
- Female gender
- Having unprotected sex
- Early age during first sexual intercourse
Match to Herpes Clinical Trials
How is herpes diagnosed.
A sample will be taken from a sore and tested to determine whether HSV is present in the lesion, thus confirming the diagnosis. However, a negative result does not rule out herpes. Samples should be taken from new ulcers, where it is more likely to find the virus.
Blood tests are also carried out to determine the presence of antibodies against HSV. This test can determine if the infection is new or a repeat outbreak. It is usually very difficult, if not impossible, to point to the exact moment a person was infected with the virus. If herpes is diagnosed, tests should be carried out to discard other STDs, since they can exist as comorbidities.
What Are Some Herpes Clinical Trial Recruiting Now?
Treating herpes.
Herpes Cure Information
>> Match With A Herpes Trial Here
There isn’t a cure for herpes, and although the sores heal in days or weeks, the virus never leaves the body. However, some medications can help make the outbreak pass faster.
NSAIDs such as paracetamol can be taken to reduce the discomfort during an outbreak. Ice packs, salt baths, and local anesthetic creams can also be applied.
Over-the-counter medications:
- Abreva Docosanol
Antiviral medications:
- Valacyclovir
- Famciclovir
- Penciclovir: only available for topical application
The dosage and length of treatment with these medications will depend on the location and chronicity of the lesions. Immunocompromised patients can develop life-threatening infections due to HSV (such as encephalitis or pneumonitis), and in these cases, acyclovir is often used in high doses. If acyclovir-resistant HSV is encountered, it is usually treated with cidofovir and foscarnet; however, these drugs can cause kidney toxicity. Prophylactic treatment can also be administered with antiviral drugs to prevent or shorten future outbreaks.
Lifestyle Changes
If you have been infected with the herpes simplex virus, make sure to adopt habits that minimize the risk of infecting other people, such as using condoms to prevent infection during intercourse, avoiding having multiple sex partners, avoiding intercourse during an active outbreak. Be honest with your partners about having the virus, and consider contacting previous partners if you aren’t sure where you became infected.
Make sure to discuss all your therapeutic options with your doctor, since the proper treatment can make outbreaks shorter and less painful. Seek professional help if you are having difficulty coping with the diagnosis. It is normal to feel shock, anger and embarrassment after diagnosis; however, it is important to know that although there is a stigma associated with herpes, it is a manageable condition that hardly ever leads to serious complications and you can lead a normal life after being infected.
Tips for Friends and Family
- Be kind to your partner if they have HSV and don’t make them feel ashamed or stigmatize the disease. Practice safe sex, and ask your doctor about prophylactic treatment to minimize the risk of infection.
- Herpes can’t be spread through contact with personal objects, so there is no danger in sharing objects like towels, sheets, toilets, or going swimming together.
- Avoid intimate contact with your loved one when they have active sores.
- Consider couples counseling to deal with the diagnosis.
- If you are interested, look for clinical trials near me
- Knott, L. (Oct 16th, 2017) Genital Herpes. Recovered from https://patient.info/health/sexually-transmitted-infections-leaflet/genital-herpes
- Genital herpes. 2015. Recovered from https://www.betterhealth.vic.gov.au/health/conditionsandtreatments/genital-herpes
Other Conditions
- Acute Aortic Dissection
- Ankylosing Spondylitis
- Alzheimer’s
- Auditory Loss and Deafness
- Autoimmune Hepatitis
- Bronchiectasis
- Breast Cancer
- Esophageal Cancer
- Lung Cancer
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer
- Compensation
- COPD Treatments
- Coronavirus
- Crohn’s Disease
- Diet And Nutrition
- Endometriosis
- Fibromyalgia
- Hepatitis B
- Hepatitis C
- Mindfulness
- Multiple Sclerosis
- Myasthenia Gravis
- Ophthalmology
- Osteoarthritis
- Parkinson’s Disease
- PBC and PSC
- Plant Based Diet
- Plastic Surgery
- Pulmonary Fibrosis
- Ulcerative Colitis
- Urinary Tract Infections
- Weight Loss
Paid Vaccine Trial For Women 16-17 Years Old
CMVictory: a vaccine trial for women between 16-17 years old.
Paid Clinical Trials Nationwide
Nationwide clinical trials offering up to $3,000 for participation and treatment.
Paid Vaccine Study For Teens
Vaccine study that aims to protect teens ages 12–17 against Epstein-Barr virus (EBV) and infectious mononucleosis (mono).
Paid Clinical Studies Nationwide
Sign up to receive email notifications on clinical trials for individual conditions in your local area.
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Sexually Transmitted Infections (STIs)
Genital Herpes
- Health Topics A-Z
About Genital Herpes
Genital herpes is a common sexually transmitted infection (STI) that can be treated.
- People who are sexually active can get genital herpes.
- This fact sheet answers basic questions about genital herpes.

What is genital herpes?
Genital herpes is an STI caused by two types of viruses – herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2).
Oral herpes
HSV-1 often causes oral herpes, which can result in cold sores or fever blisters on or around the mouth. However, most people with oral herpes do not have any symptoms. Most people with oral herpes get it during childhood or young adulthood from non-sexual contact with saliva.
Genital herpes is common in the United States (U.S.). In 2018, CDC estimates show there were 572,000 new genital herpes infections in the U.S. among people aged 14 to 49. 1
Signs and symptoms
Genital herpes often has no symptoms, but it can cause serious health problems, even without symptoms., how do i know i have genital herpes.
Most people with genital herpes have no symptoms or have very mild symptoms. Mild symptoms may go unnoticed or be mistaken for other skin conditions like a pimple or ingrown hair. Because of this, most people do not know they have a herpes infection.
Herpes Outbreak
Herpes sores usually appear as one or more blisters on or around the genitals, rectum or mouth. This is known as having an "outbreak". The blisters break and leave painful sores that may take a week or more to heal. Flu-like symptoms (e.g., fever, body aches, or swollen glands) also may occur during the first outbreak.
People who experience an initial outbreak of herpes can have repeated outbreaks, especially if they have HSV-2. However, repeat outbreaks are usually shorter and less severe than the first outbreak. Although genital herpes is a lifelong infection, the number of outbreaks may decrease over time.
See your healthcare provider if you notice any of these symptoms. You should also see a provider if your partner has an STI or symptoms of one. Symptoms can include an unusual sore, a smelly genital discharge, burning when peeing, or bleeding between periods.
Risk factors
What is the link between genital herpes and hiv.
Herpes infection can cause sores or breaks in the skin or lining of the mouth, vagina, and rectum. This provides a way for HIV to enter the body. Even without visible sores, herpes increases the number of immune cells in the lining of the genitals. HIV targets immune cells for entry into the body. Having both HIV and genital herpes increases the chance of spreading HIV to a HIV-negative partner during oral, vagina, or anal sex.
How it spreads
How is genital herpes spread.
You can get genital herpes by having vaginal, anal, or oral sex with someone who has the infection. You can get herpes if you have contact with:
- A herpes sore
- Saliva from a partner with an oral herpes infection
- Genital fluids from a partner with a genital herpes infection
- Skin in the oral area of a partner with oral herpes
- Skin in the genital area of a partner with genital herpes
You also can get genital herpes from a sex partner who does not have a visible sore or is unaware of their infection. It is also possible to get genital herpes if you receive oral sex from a partner with oral herpes.
You will not get herpes from toilet seats, bedding, or swimming pools. You also will not get it from touching objects, such as silverware, soap, or towels.
If you have more questions about herpes, consider discussing your concerns with a healthcare provider.
Is there a link between genital herpes and oral herpes?
Yes. Oral herpes caused by HSV-1 can spread from the mouth to the genitals through oral sex . This is why some cases of genital herpes are due to HSV-1.
How can I prevent genital herpes?
The only way to completely avoid STIs is to not have vaginal, anal, or oral sex.
If you are sexually active, you can do the following things to lower your chances of getting genital herpes:
- Being in a long-term mutually monogamous relationship with a partner who does not have herpes.
- Using condoms the right way every time you have sex.
Be aware that not all herpes sores occur in areas that a condom can cover. Also, the skin can release the virus (shed) from areas that do not have a visible herpes sore. For these reasons, condoms may not fully protect you from getting herpes.
If your sex partner(s) has/have genital herpes, you can lower your risk of getting it if:
- Your partner takes an anti-herpes medicine every day. This is something your partner should discuss with his or her healthcare provider.
- You avoid having vaginal, anal, or oral sex when your partner has herpes symptoms (i.e., during an "outbreak").
I'm pregnant. If I have genital herpes, how can I protect my baby from getting it?
If you are pregnant and have genital herpes, prenatal care visits are very important. Some research suggest that a genital herpes infection may lead to miscarriage or make it more likely to deliver your baby too early. You can pass herpes to your unborn child before birth, but it more commonly passes during delivery. This can lead to a deadly infection in your baby (called neonatal herpes). It is important that you avoid getting genital herpes during pregnancy. Tell your healthcare provider if you have ever had a genital herpes diagnosis or symptoms. Also tell them about any possible exposure to genital herpes.
If you have genital herpes, you may need to take anti-herpes medicine towards the end of your pregnancy. This medicine may reduce your risk of having signs or symptoms of genital herpes when you deliver. At the time of delivery, your healthcare provider should carefully examine you for herpes sores. If you have signs or symptoms of genital herpes at delivery, a 'C-section' is likely to occur.
Testing and diagnosis
How will my healthcare provider know if i have genital herpes.
Your healthcare provider may diagnose genital herpes by simply looking at any sores that are present. Providers can also take a sample from the sore(s) and test it. If sores are not present, a blood test may be used to look for HSV antibodies.
Have an honest and open talk with your healthcare provider about herpes testing and other STDs.
Please note: A herpes blood test can help determine if you have herpes infection. It cannot tell you who gave you the infection or when you got the infection.
Treatment and recovery
Is there a cure for genital herpes.
There is no cure for genital herpes. However, there are medicines that can prevent or shorten outbreaks. A daily anti-herpes medicine can make it less likely to pass the infection on to your sex partner(s).
Can I still have sex if I have herpes?
If you have herpes, you should talk to your sex partner(s) about their risk. Using condoms may help lower this risk but it will not get rid of the risk completely. Having sores or other symptoms of herpes can increase your risk of spreading the disease. Even if you do not have any symptoms, you can still infect your sex partners.
You may have concerns about how genital herpes will impact your health, sex life, and relationships. While herpes is not curable, it is important to know that it is manageable with medicine. Daily suppressive therapy (i.e., daily use of antiviral medication) can lower your risk of spreading the virus to others. Talk to a healthcare provider about your concerns and treatment options.
A genital herpes diagnosis may affect how you will feel about current or future sexual relationships. Knowing how to talk to sexual partners about STIs is important.
What happens if I don't get treated?
Genital herpes can cause painful genital sores and can be severe in people with suppressed immune systems.
If you touch your sores or fluids from the sores, you may transfer herpes to another body part like your eyes. Do not touch the sores or fluids to avoid spreading herpes to another part of your body. If you do touch the sores or fluids, quickly wash your hands thoroughly to help avoid spreading the infection.
If you are pregnant, there can be problems for you and your unborn fetus, or newborn baby. See "I'm pregnant. How could genital herpes affect my baby?" for information about this.
- Mertz GJ. Asymptomatic shedding of herpes simplex virus 1 and 2: implications for prevention of transmission. J Infect Dis , 2008. 198(8): 1098–1100.
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Health and social care
- Public health
- Health protection
- Immunisation
- Herpes zoster (shingles) immunisation programme 2022 to 2023: evaluation reports
- UK Health Security Agency
Shingles vaccine coverage (England): annual report of the financial year 2022 to 2023
Updated 14 May 2024
Applies to England

© Crown copyright 2024
This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3 or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: [email protected] .
Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned.
This publication is available at https://www.gov.uk/government/publications/herpes-zoster-shingles-immunisation-programme-2022-to-2023-evaluation-reports/shingles-vaccine-coverage-england-annual-report-of-the-financial-year-2022-to-2023
Adults eligible from April 2022 to March 2023 and vaccinated to the end of June 2023
The shingles vaccination programme was introduced in September 2013. The routine programme offers the vaccine to those turning 70 and patients remain eligible for the vaccine until their 80th birthday.
This annual shingles report presents vaccine coverage in single year cohorts turning 70 to 80 years old between 1 April 2022 and 31 March 2023 measured at the end of June 2023.
Cumulative shingles vaccine coverage for all adults aged between 70 and 80 years old continues to increase year on year through opportunistic vaccination.
Coverage in the cohort that turned 70 years old was 5.6 percentage points higher in June 2023 than in June 2022. This increase is likely due to recovery of the immunisation programme following disruption due to the restrictions put in place in response to the coronavirus (COVID-19) pandemic in the 2020 to 2021 period.
When estimates were taken at the end of June, coverage was highest among those turning 79 years old in the 2022 to 2023 financial year (83%) and lowest among those turning 70 years old (36.8%) and 71 years old (50.8%).
Coverage in the cohort that turned 70 years old was lowest in London (27.4%) and highest in South West (38.4%). Coverage in the cohort that turned 79 years old was lowest in London (77.5%) and highest in South West (84.3%).
Introduction
The aim of the routine shingles (herpes zoster) vaccination programme is to prevent severe morbidity from shingles in groups at the highest risk. The shingles vaccination programme began on 1 September 2013, offering the Zostavax shingles vaccine to all 70 years olds, together with a catch-up programme for older cohorts. Since the programme began, the eligibility criteria have changed several times (see the Appendix ). In the 2022 to 2023 financial year, adults became eligible at 70 years old as catch-up cohorts ended in the 2020 to 2021 financial year ( Table 1 ). Those who have previously been offered the shingles vaccine remain eligible until their 80th birthday ( 1 , 2 , 3 , 4 ).
For the first 5 years of the vaccination programme, vaccine coverage was monitored in England by Public Health England ( PHE ) through monthly collections via automatic uploads of GP practice-level data using the ImmForm website. To continue to accurately evaluate cumulative vaccine coverage following a change in eligibility criteria on 1 April 2017, PHE changed the monthly collections to quarterly extractions, and vaccine coverage was also calculated each quarter to reflect the new delivery model ( Appendix ). It is important to note that vaccine coverage for adults who have become eligible under the revised criteria is not directly comparable to previous cumulative vaccine coverage estimates using the former eligibility criteria ( 5 ).
As a live viral vaccine, the Zostavax shingles vaccine is contraindicated for immunosuppressed individuals. From 1 September 2021, these individuals have been offered the recombinant Shingrix vaccine.
This annual report presents vaccine coverage in cohorts turning 70 to 80 years old between 1 April 2022 and 31 March 2023 who have been offered the Zostavax vaccine.
The coverage data presented in this report was collected at the GP practice level and was automatically uploaded via participating GP IT suppliers to the ImmForm website each quarter. The data was then validated and analysed by UKHSA to check data completeness, identify, and query any anomalous results and describe epidemiological trends.
Vaccine coverage was defined as the number of patients in each birth cohort, who received the shingles vaccine as a proportion of the total number of registered patients in that birth cohort (denominator).
Cumulative vaccine coverage was calculated for all adults born between 1 April 1942 and 31 March 1953 (that is, adults turning 70 to 80 years old between 1 April 2022 and 31 March 2023) and who were vaccinated by 23 June 2023, for each financial year birth cohort. A more detailed breakdown of the different cohorts can be found in Table 1 .
Data quality
GP practices reporting were as follows:
- for data collected in June, 6,033 out of a total of 6,443 (93.6%) GP practices reported shingles vaccine coverage data
- 2 GP IT suppliers (EMIS and TPP) provided this coverage data
Birth cohorts
Table 1. birth and shingles vaccine eligibility dates for cohorts included in the annual 2022 to 2023 coverage report, vaccine coverage for birth cohorts turning 70 to 80 from quarter 1 to quarter 4 (april 2022 to march 2023).
Coverage in those aged 70 has increased by 5.6% to 36.8% compared with 2021 to 2022 (31.2%) and is higher than pre-pandemic levels reported for the period of 2018 to 2019 (31.9%). This suggests a positive recovery of the immunisation programme compared to the last annual data for 2020 to 2021 (see Figure 1 ).
Cumulative vaccine coverage for each birth cohort continues to increase each year through opportunistic vaccination (see Figure 2 and Table 1 ). The highest cumulative coverage when assessed in quarter 4 was observed among those turning 79 years old (83%). These patients were first offered the shingles vaccine as the earliest routine birth cohort from 2013 to 2014.
The lowest cumulative coverage when assessed in quarter 4 was observed among cohorts aged 70 (36.8%). This cohort became eligible in the 2022 to 2023, and therefore had the shortest period of eligibility for vaccination.
Coverage in the cohort that turned 70 years old was lowest in London (27.4%) and highest in South West (38.4%). Coverage in the cohort that turned 79 years old was lowest in London (77.5%) and highest in South West (84.3%) (Table 2).
Table 2. National cumulative shingles vaccine coverage for adults turning 70 to 80 years old between 1 April 2022 and 31 March 2023 and vaccinated by 23 June 2023, by NHS England commissioning region
¥ Those aged 80 born between 2 September 1942 and 31 March 1943 were eligible for vaccination since 1 September 2013. Those aged 79 and born between 1 April 1942 and 1 September 1942 were eligible at age 78 as a catch-up cohort in 2020 to 2021.
Table 3. Change in national cumulative shingles vaccine coverage from June 2022 to June 2023 by birth cohort
Cumulative vaccine coverage for each of the earlier routine cohorts (now aged 71 to 78) continues to increase each year. In the 2022 to 2023 financial year, coverage in those aged 70 was higher by 5.6 percentage points when compared to 2021 to 2022. This increase is likely due to recovery of the immunisation programme following disruption due to the restrictions put in place in response to the coronavirus (COVID-19) pandemic in the 2020 to 2021 period.
Cumulative vaccine coverage for each birth cohort continues to increase each year through opportunistic vaccination. Coverage was highest in June among those turning 79 years old in the 2022 to 2023 financial year (83%) who had been eligible for the longest and lowest among those turning 70 years old (36.8%) and 71 years old (50.8%).
Previous routine and catch-up cohorts remain eligible for vaccination until their 80th birthday and longer-term vaccine coverage data has shown increases in coverage in these cohorts in subsequent years (Table 3) ( 5 ).
Results assessing the impact of shingles vaccination in the 5 years after the introduction of the programme in England showed large reductions in both GP consultations and hospitalisations for herpes zoster and post-herpetic neuralgia ( 7 ). Therefore, GPs should continue to offer the shingles vaccine to all eligible patients, particularly in regions with lower coverage.
Appendix. Date and eligibility criteria for the shingles vaccination programme since 1 September 2013
1 september 2013.
Eligibility criteria: in the first year of the programme (2013 to 2014), the vaccine was routinely offered to adults aged 70 years on 1 September 2013 (born between 2 September 1942 and 1 September 1943 and to adults aged 79 on 1 September 2013 (born between 2 September 1933 and 1 September 1934) as part of the catch-up campaign.
1 September 2014
Eligibility criteria: in the second year of the programme (1 September 2014 to 31 August 2015), the vaccine was routinely offered to adults aged 70 years on 1 September 2014 (born between 2 September 1943 and 1 September 1944). The second year of the programme also included 2 catch-up cohorts comprised of adults aged 78 on 1 September 2014 (born between 2 September 1935 and 1 September 1936), and adults aged 79 on 1 September 2014 (born between 2 September 1934 and 1 September 1935). In addition, those who became eligible as 70 year olds from 1 September 2013 but had not yet been immunised were also eligible.
1 September 2015
Eligibility criteria: in the third year of the programme (1 September 2015 to 31 August 2016), the vaccine was routinely offered to adults aged 70 years on 1 September 2015 (born between 2 September 1944 and 1 September 1945). The third year of the programme also included a catch-up cohort of adults aged 78 on 1 September 2015 (born between 2 September 1936 and 1 September 1937). In addition, patients who became eligible in the first 2 years of the programme but have not been vaccinated against shingles remain eligible until their 80th birthday (patients aged 71, 72 and 79 on 1 September 2015).
1 September 2016
Eligibility criteria: in the fourth year of the programme (1 September 2016 to 31 August 2017), the vaccine was routinely offered to adults aged 70 years on 1 September 2016 (born between 2 September 1945 and 1 September 1946). The fourth year of the programme also included a catch-up cohort of adults aged 78 on 1 September 2016 (born between 2 September 1937 and 1 September 1938). In addition, patients who became eligible in the first 3 years of the programme but have not been vaccinated against shingles remain eligible until their 80th birthday (patients aged 71, 72, 73 and 79 on 1 September 2016).
1 April 2017
Eligibility criteria: the eligibility criteria for receiving the shingles vaccine was simplified so that individuals become eligible on their 70th birthday (routine cohort) or their 78th birthday (catch-up cohort).
1 September 2020
Eligibility criteria: as of 1 September 2020, all individuals in the catch-up cohorts (born 2 September 1933 to 1 September 1942) have been offered the shingles vaccine as they became 78 years old, thus completing the catch-up programme which started on 1 September 2013.
Figure 1. Comparison of national cumulative shingles vaccine coverage of those turning 70 to 76 from April 2020 to March 2021 measured in June 2021, those turning 70 to 76 from April 2021 to March 2022 measured in June 2022 and those turning 70 to 76 from April 2022 to March 2023 measured in June 2023
Figure 2. comparison of shingles coverage on 23 june 2022 to coverage on 23 june 2023 by birth cohort.
1. NHS Choices website. ‘ Who can have the shingles vaccine? ’ (2018)
2. UKHSA . ( Shingles vaccination: eligibility poster - GOV.UK (www.gov.uk)
3. UKHSA . ‘ Vaccination against shingles: information for healthcare professionals ’
4. UKHSA . ‘ Shingles: guidance and vaccination programme ’
5. UKHSA website. ‘ Shingles vaccine uptake ’
6. UKHSA . Shingles (herpes zoster): the Green Book, chapter 28a
7. Andrews N, Stowe J, Kuyumdzhieva G, Sile B, Yonova I, Lusignan S, Ramsay M and Amirthalingam G (2020). ‘ Impact of the herpes zoster vaccination programme on hospitalised and general practice consulted herpes zoster in the 5 years after its introduction in England: a population-based study ’ British Medical Journal Open.
Is this page useful?
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. Please fill in this survey .
Wisp Begins Offering Diagnostics Through Partnership With TBD Health
Wisp members can now order TBD Health’s STI/STD testing kits on Wisp’s website for free delivery.
- Copy Link Copy Link

Sexual health company Wisp expanded its partnership with TBD Health, the company shared with MedCity News. On Wednesday, it began offering access to TBD Health’s sexually transmitted infection/disease (STI/STD) testing kits on its site.
San Francisco-based Wisp is a virtual health provider for vaginal health, reproductive health and sexual health. Its direct-to-consumer platform offers birth control, as well as treatments for urinary tract infections, genital herpes, bacterial vaginosis and yeast infections. TBD Health , based in New York City, is an online and in-person sexual healthcare platform. It offers at-home STD testing and emergency contraception, as well as telemedicine consults. Its in-person clinics are located in Las Vegas and Denver.
In January, the companies announced that Wisp telehealth patients could be referred to TBD Health’s in-person clinics to receive STD testing, PrEP prescriptions, UTI treatments and other services. Through the expanded partnership, Wisp members can now order TBD Health’s STI/STD testing kits on Wisp’s website for free delivery. They take the test by providing a urine sample and return the kit to one of TBD Health’s partner labs. If the test is positive, then they will consult with a TBD clinician to receive treatment and understand the next steps.

A Deep-dive Into Specialty Pharma
A specialty drug is a class of prescription medications used to treat complex, chronic or rare medical conditions. Although this classification was originally intended to define the treatment of rare, also termed “orphan” diseases, affecting fewer than 200,000 people in the US, more recently, specialty drugs have emerged as the cornerstone of treatment for chronic and complex diseases such as cancer, autoimmune conditions, diabetes, hepatitis C, and HIV/AIDS.
“We are on a continued mission to offer patients the treatments and care they need when they need it with no hassle, judgment, and no insurance required. … This partnership is helping us to further democratize access to sexual health care and marks the first time we are experimenting with at-home diagnostics on our platform,” said Monica Cepak, CEO of Wisp, in an email.
Wisp is paying TBD Health for its inventory, Cepak added.
The company’s announcement comes at a time when STI cases are on the rise. More than 2.5 million cases of syphilis, gonorrhea and chlamydia were reported in the U.S. in 2022, according to the Centers for Disease Control and Prevention . Syphilis cases rose by 80% in the U.S. between 2018 and 2022.
“We recognize that there has never been a greater need for STI/STD treatments and testing options,” Cepak stated. “Through this partnership, we are meeting patient demand while simultaneously working to educate consumers on what symptoms look like and what preventative and post-diagnosis options are.”

With the Rise of AI, What IP Disputes in Healthcare Are Likely to Emerge?
Munck Wilson Mandala Partner Greg Howison shared his perspective on some of the legal ramifications around AI, IP, connected devices and the data they generate, in response to emailed questions.
The company also recently added two new medications to its site: M. Hominis Treatment and Ureaplasma Treatment . These bacteria don’t typically lead to issues, but can cause pain, discharge, vaginal odor and challenges with fertility if an imbalance occurs. The medications were created to “effectively combat the imbalance of different bacterias and quickly aid uncomfortable genital symptoms,” Cepak said.
Other companies that offer sexual health support for consumers include Ro and Hims and Hers.
More From MedCity News

Innovation Doesn’t Always Have To Involve The Latest Tech, MD Anderson Exec Says

Virginia Medicaid Taps Aeroflow Health for Lactation Services

It’s Past Time to Prevent Polypharmacy in U.S. Healthcare

J&J Joins Chase for Hot Immunology Target With $850M Proteologix Acquisition

IMAGES
VIDEO
COMMENTS
Photo by Robert Hood / Fred Hutch News Service. Two years after scientists showed that an experimental gene therapy for herpes can knock out most latent infection in mice, new tests reveal that it also suppresses the amount of transmissible virus shed by the treated animals. It is encouraging news from researchers at Fred Hutchinson Cancer ...
June 14, 2022 - Eurocine Vaccines AB announced that it entered into a research and collaboration agreement with Redbiotec AG that transfers the exclusive global rights to develop, manufacture, and commercialize vaccine candidates against Herpes Simplex Virus Type 2, HSV-2, based on the technologies developed by Redbiotec.
A new vaccine on the horizon could prove to be a game changer for the many people living with herpes. Aneta Pucia / EyeEm/Getty Images April 12, 2022, 10:23 PM UTC
This multi-country research study is recruiting 332 healthy participants aged 18-40 years or those aged 18-60 years with recurrent genital herpes. Known as GSK3943104A , GSK Study #215336 is forecasted to conclude in May 2026. This HSVTI study will explore different formulations and be conducted in two parts:
This new therapy represents a streamlined approach to attacking herpes. In a 2020 study, the research team used three different delivery viruses armed with two different enzymes to attack herpes.
BioNtech has dosed the first patient with its BNT163 herpes vaccine candidate designed to prevent genital lesions as part of a first-in-human Phase 1 clinical research study, the German vaccine ...
mRNA-1608 Herpes Vaccine Candidate Clinical Trials, Dosage, Indication, News, Side Effects. Moderna Inc.'s herpes simplex virus vaccine candidate is a Messenger RNA () vaccine targeted against HSV-2 disease.On February 18, 2022, Moderna Inc. stated that it expects an HSV-2 vaccine could provide cross-protection against HSV-1. With mRNA-1608, Moderna aims to induce a strong antibody response ...
So much research was conducted around the time of the discovery of acyclovir back in the 1980s . The search has not stopped even now, and new therapeutics are being developed that focus on different mechanisms of antiviral action [86,87,88]. This may include various approaches such as virus entry inhibitors, fusion, or virus-release inhibitors.
Herpes virus articles from across Nature Portfolio. Herpesvirus is an infectious agent belonging to the virus family Herpesviridae that causes latent and lytic infections in a wide range of ...
Pritelivir is a new class of drugs that targets the DNA of the virus and stops it from replicating. It has received FDA approval and is taken orally each day. Scientists also are working on other ...
Read the latest research on the herpes virus, including new treatment options. ... Jan. 25, 2022 — A new study has opened the door to a new approach to attacking herpesviruses. The study ...
A novel herpes vaccine has achieved a nearly 100-percent success rate in animal testing, with researchers hoping to soon move into human safety and efficacy trials.
We expect our vaccine candidate for prevention of genital herpes to proceed to human trials by summer 2022. We are now working on a vaccine as treatment for genital herpes that will reduce the frequency of outbreaks for people already infected. ... a Senior Research Scientist evaluating efficacy of HSV vaccines as therapy for genital herpes in ...
In response to the persistent health challenges of herpes simplex virus 1 (HSV-1) and HSV-2, today the National Institutes of Health released the Strategic Plan for Herpes Simplex Virus Research. An NIH-wide HSV Working Group developed the plan, informed by feedback from more than 100 representatives of the research and advocacy communities and interested public stakeholders.
Oct. 26, 2022 — Two new studies support development of a broadly applicable treatment for neurodegenerative diseases that targets a molecule that serves as the central executioner in the death ...
Reprints. Early efforts to produce a protein-based vaccine for herpes failed. But a new mRNA approach has outperformed the efficacy of the past vaccines in preclinical trials and is expected to be introduced in clinical trials in the second half of 2022, investigators say. This new approach for a prophylactic genital herpes vaccine showed great ...
Your source for the latest research news. Follow: ... the anti-influenza agent baloxavir, 3 natural products previously shown to exhibit anti-herpes simplex virus (HSV) activity, and two 8 ...
Current research has identified other candidates that may provide protective benefits alone or combined with gD. Monoclonal antibodies for therapy or prevention of transmission. As an example, a multi-country research study is recruiting 332 healthy adult participants with recurrent genital herpes.
Credit: CC0 Public Domain. A team of researchers at Korea Advanced Institute of Science and Technology, working with a colleague from Myunggok Medical Research Center, has found that test mice ...
Research team introduces new tool to boost battle against childhood undernutrition. May 10, 2024. How herpes hijacks a ride into cells. ... Scientists describe new herpes treatment strategy. Dec 3 ...
Herpes can hide in the nerve cells for a long time before activating, which makes finding a cure challenging. There is currently no cure, but research on vaccines is ongoing. Most people with ...
HSV1 usually causes sores around the mouth, while HSV2 causes genital herpes. The infection is spread by skin-to-skin contact, and it can be transmitted through oral, vaginal, and anal sex, and kissing. It can also be spread through contact with lesions from other areas of the body.
Herpes sores usually appear as one or more blisters on or around the genitals, rectum or mouth. This is known as having an "outbreak". The blisters break and leave painful sores that may take a week or more to heal. Flu-like symptoms (e.g., fever, body aches, or swollen glands) also may occur during the first outbreak.
Vaccine coverage for birth cohorts turning 70 to 80 from quarter 1 to quarter 4 (April 2022 to March 2023) Coverage in those aged 70 has increased by 5.6% to 36.8% compared with 2021 to 2022 (31.2 ...
Medical research in Haiti has long had to contend with the country's instability. Before February, gangs already controlled an estimated 80% of Port-au-Prince, the capital and largest city. The violence has forced 60% of GHESKIO's staff to leave since 2021; the son of its director, Jean William "Bill" Pape, was kidnapped in November ...
More than 2.5 million cases of syphilis, gonorrhea and chlamydia were reported in the U.S. in 2022, according to the Centers for Disease Control and Prevention. Syphilis cases rose by 80% in the U ...
From a sick focus to health focus, From paying for visits and hospitalizations to paying for population health. From ignoring the conditions of people's lives to focusing on where people live, work, play, pray, shop and age. As this shift continues, nurses will be in the forefront of addressing what matters to people for their health, in the ...