- Case Report
- Open access
- Published: 07 August 2009

Primary abdominal ectopic pregnancy: a case report
- Recep Yildizhan 1 ,
- Ali Kolusari 1 ,
- Fulya Adali 2 ,
- Ertan Adali 1 ,
- Mertihan Kurdoglu 1 ,
- Cagdas Ozgokce 1 &
- Numan Cim 1
Cases Journal volume 2 , Article number: 8485 ( 2009 ) Cite this article
21k Accesses
17 Citations
Metrics details
Introduction
We present a case of a 13-week abdominal pregnancy evaluated with ultrasound and magnetic resonance imaging.
Case presentation
A 34-year-old woman, (gravida 2, para 1) suffering from lower abdominal pain and slight vaginal bleeding was transferred to our hospital. A transabdominal ultrasound and magnetic resonance imaging were performed. The diagnosis of primary abdominal pregnancy was confirmed according to Studdiford's criteria. A laparatomy was carried out. The placenta was attached to the mesentery of sigmoid colon and to the left abdominal sidewall. The placenta was dissected away completely and safely. No postoperative complications were observed.
Ultrasound examination is the usual diagnostic procedure of choice. In addition magnetic resonance imaging can be useful to show the localization of the placenta preoperatively.
Abdominal pregnancy, with a diagnosis of one per 10000 births, is an extremely rare and serious form of extrauterine gestation [ 1 ]. Abdominal pregnancies account for almost 1% of ectopic pregnancies [ 2 ]. It has reported incidence of one in 2200 to one in 10,200 of all pregnancies [ 3 ]. The gestational sac is implanted outside the uterus, ovaries, and fallopian tubes. The maternal mortality rate can be as high as 20% [ 3 ]. This is primarily because of the risk of massive hemorrhage from partial or total placental separation. The placenta can be attached to the uterine wall, bowel, mesentery, liver, spleen, bladder and ligaments. It can be detach at any time during pregnancy leading to torrential blood loss [ 4 ]. Accurate localization of the placenta pre-operatively could minimize blood loss during surgery by avoiding incision into the placenta [ 5 ]. It is thought that abdominal pregnancy is more common in developing countries, probably because of the high frequency of pelvic inflammatory disease in these areas [ 6 ]. Abdominal pregnancy is classified as primary or secondary. The diagnosis of primary abdominal pregnancy was confirmed according to Studdiford's criteria [ 7 ]. In these criteria, the diagnosis of primary abdominal pregnancy is based on the following anatomic conditions: 1) normal tubes and ovaries, 2) absence of an uteroplacental fistula, and 3) attachment exclusively to a peritoneal surface early enough in gestation to eliminate the likelihood of secondary implantation. The placenta sits on the intra-abdominal organs generally the bowel or mesentery, or the peritoneum, and has sufficient blood supply. Sonography is considered the front-line diagnostic imaging method, with magnetic resonance imaging (MRI) serving as an adjunct in cases when sonography is equivocal and in cases when the delineation of anatomic relationships may alter the surgical approach [ 8 ]. We report the management of a primary abdominal pregnancy at 13 weeks.
The patient was a 34-year-old Turkish woman, gravida 2 para 1 with a normal vaginal delivery 15 years previously. Although she had not used any contraceptive method afterwards, she had not become pregnant. She was transferred to our hospital from her local clinic at the gestation stage of 13 weeks because of pain in the lower abdomen and slight vaginal bleeding. She did not know when her last menstrual period had been, due to irregular periods. At admission, she presented with a history of abdominal distention together with steadily increasing abdominal and back pain, weakness, lack of appetite, and restlessness with minimal vaginal bleeding. She denied a history of pelvic inflammatory disease, sexually transmitted disease, surgical operations, or allergies. Blood pressure and pulse rate were normal. Laboratory parameters were normal, with a hemoglobin concentration of 10.0 g/dl and hematocrit of 29.1%. Transvaginal ultrasonographic scanning revealed an empty uterus with an endometrium 15 mm thick. A transabdominal ultrasound (Figure 1 ) examination demonstrated an amount of free peritoneal fluid and the nonviable fetus at 13 weeks without a sac; the placenta measured 58 × 65 × 67 mm. Abdominal-Pelvic MRI (Philips Intera 1.5T, Philips Medical Systems, Andover, MA) in coronal, axial, and sagittal planes was performed especially for localization of the placenta before she underwent surgery. A non-contrast SPAIR sagittal T2-weighted MRI strongly suggested placental invasion of the sigmoid colon (Figure 2 ).
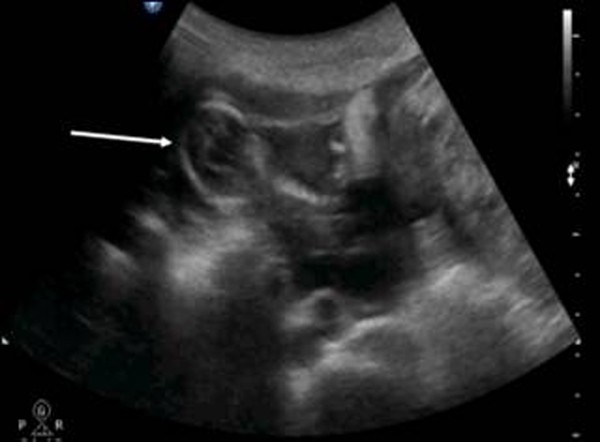
Pelvic ultrasound scanning . Diffuse free intraperitoneal fluid was seen around the fetus and small bowel loops.
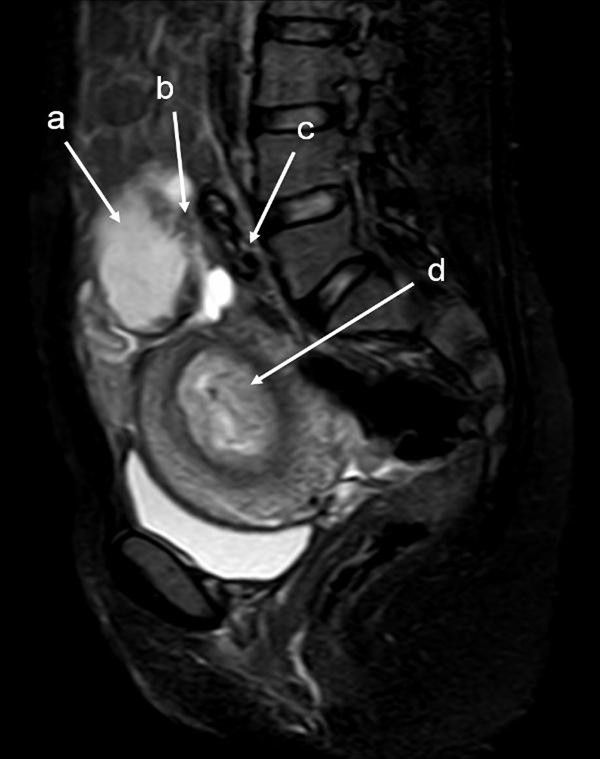
T2W SPAIR sagittal MRI of lower abdomen demonstrating the placental invasion . Placenta (a) , invasion area (b) , sigmoid colon (c) , uterine cavity (d) .
Under general anesthesia, a median laparotomy was performed and a moderate amount of intra-abdominal serohemorrhagic fluid was evident. The placenta was attached tightly to the mesentery of sigmoid colon and was loosely adhered to the left abdominal sidewall (Figure 3 ). The fetus was localized at the right of the abdomen and was related to the placenta by a chord. The placenta was dissected away completely and safely from the mesentery of sigmoid colon and the left abdominal sidewall. Left salpingectomy for unilateral hydrosalpinx was conducted. Both ovaries were conserved. After closure of the abdominal wall, dilatation and curettage were also performed but no trophoblastic tissue was found in the uterine cavity. As a management protocol in our department, we perform uterine curettage in all patients with ectopic pregnancy gently at the end of the operation, not only for the differential diagnosis of ectopic pregnancy, but also to help in reducing present or possible postoperative vaginal bleeding.
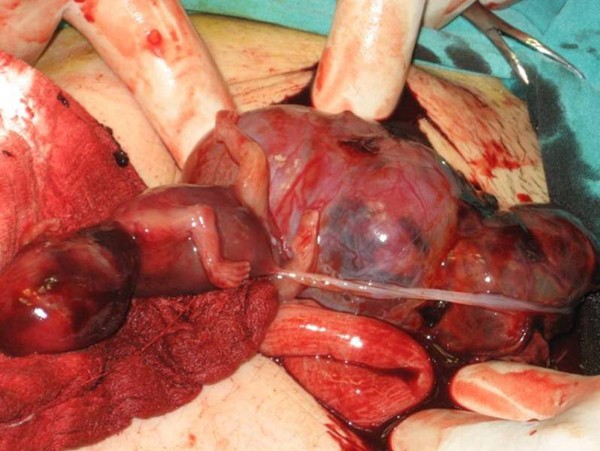
Fetus, placenta and bowels .
The patient was awakened, extubated, and sent to the room. The patient was discharged on post-operative day five with the standard of care at our hospital.
In the present case, we were able to demonstrate primary abdominal pregnancy according to Studdiford's criteria with the use of transvaginal and transabdominal ultrasound examination and MRI. In our case, both fallopian tubes and ovaries were intact. With regard to the second criterion, we did not observe any uteroplacental fistulae in our case. Since abdominal pregnancy at less than 20 weeks of gestation is considered early [ 9 ], our case can be regarded as early, and so we dismissed the possibility of secondary implantation.
The recent use of progesterone-only pills and intrauterine devices with a history of surgery, pelvic inflammatory disease, sexually transmitted disease, and allergy increases the risk of ectopic pregnancy. Our patient had not been using any contraception, and did not report a history of the other risk factors.
The clinical presentation of an abdominal pregnancy can differ from that of a tubal pregnancy. Although there may be great variability in symptoms, severe lower abdominal pain is one of the most consistent findings [ 10 ]. In a study of 12 patients reported by Hallatt and Grove [ 11 ], vaginal bleeding occurred in six patients.
Ultrasound examination is the usual diagnostic procedure of choice, but the findings are sometimes questionable. They are dependent on the examiner's experience and the quality of the ultrasound. Transvaginal ultrasound is superior to transabdominal ultrasound in the evaluation of ectopic pregnancy since it allows a better view of the adnexa and uterine cavity. MRI provided additional information for patients who needed precise diagnosing. After the diagnosis of abdominal pregnancy became definitive, it was essential to determine the localization of the placenta. Meanwhile, MRI may help in surgical planning by evaluating the extent of mesenteric and uterine involvement [ 12 ]. Non-contrast MRI using T 2 -weighted imaging is a sensitive, specific, and accurate method for evaluating ectopic pregnancy [ 13 ], and we used it in our case.
Removal of the placental tissue is less difficult in early pregnancy as it is likely to be smaller and less vascular. Laparoscopic removal of more advanced abdominal ectopic pregnancies, where the placenta is larger and more invasive, is different [ 14 ]. Laparoscopic treatment must be considered for early abdominal pregnancy [ 15 ].
Complete removal of the placenta should be done only when the blood supply can be identified and careful ligation performed [ 11 ]. If the placenta is not removed completely, it has been estimated that the remnant can remain functional for approximately 50 days after the operation, and total regression of placental function is usually complete within 4 months [ 16 ].
In conclusion, ultrasound scanning plus MRI can be useful to demonstrate the anatomic relationship between the placenta and invasion area in order to be prepared preoperatively for the possible massive blood loss.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-chief of this journal.
Abbreviations
Magnetic Resonance Imaging
Spectral Presaturation Attenuated by Inversion Recovery.
Yildizhan R, Kurdoglu M, Kolusari A, Erten R: Primary omental pregnancy. Saudi Med J. 2008, 29: 606-609.
PubMed Google Scholar
Ludwig M, Kaisi M, Bauer O, Diedrich K: The forgotten child-a case of heterotopic, intra-abdominal and intrauterine pregnancy carried to term. Hum Reprod. 1999, 14: 1372-1374. 10.1093/humrep/14.5.1372.
Article CAS PubMed Google Scholar
Alto WA: Abdominal pregnancy. Am Fam Physician. 1990, 41: 209-214.
CAS PubMed Google Scholar
Ang LP, Tan AC, Yeo SH: Abdominal pregnancy: a case report and literature review. Singapore Med J. 2000, 41: 454-457.
Martin JN, Sessums JK, Martin RW, Pryor JA, Morrison JC: Abdominal pregnancy: current concepts of management. Obstet Gynecol. 1988, 71: 549-557.
Maas DA, Slabber CF: Diagnosis and treatment of advanced extra-uterine pregnancy. S Afr Med J. 1975, 49: 2007-2010.
Studdiford WE: Primary peritoneal pregnancy. Am J Obstet Gynecol. 1942, 44: 487-491.
Google Scholar
Wagner A, Burchardt A: MR imaging in advanced abdominal pregnancy. Acta Radiol. 1995, 36: 193-195. 10.3109/02841859509173377.
Gaither K: Abdominal pregnancy-an obstetrical enigma. South Med J. 2007, 100: 347-348.
Article PubMed Google Scholar
Onan MA, Turp AB, Saltik A, Akyurek N, Taskiran C, Himmetoglu O: Primary omental pregnancy: case report. Hum Reprod. 2005, 20: 807-809. 10.1093/humrep/deh683.
Hallatt JG, Grove JA: Abdominal pregnancy: a study of twenty-one consecutive cases. Am J Obstet Gynecol. 1985, 152: 444-449.
Malian V, Lee JH: MR imaging and MR angiography of an abdominal pregnancy with placental infarction. AJR Am J Roentgenol. 2001, 177: 1305-1306.
Yoshigi J, Yashiro N, Kinoshito T, O'uchi T, Kitagaki H: Diagnosis of ectopic pregnancy with MRI: efficacy of T2-weighted imaging. Magn Reson Med Sci. 2006, 5: 25-32. 10.2463/mrms.5.25.
Kwok A, Chia KKM, Ford R, Lam A: Laparoscopic management of a case of abdominal ectopic pregnancy. Aust N Z J Obstet Gynaecol. 2002, 42: 300-302. 10.1111/j.0004-8666.2002.300_1.x.
Pisarska MD, Casson PR, Moise KJ, Di Maio DJ, Buster JE, Carson SA: Heterotopic abdominal pregnancy treated at laparoscopy. Fertil Steril. 1998, 70: 159-160. 10.1016/S0015-0282(98)00104-6.
France JT, Jackson P: Maternal plasma and urinary hormone levels during and after a successful abdominal pregnancy. Br J Obstet Gynaecol. 1980, 87: 356-362.
Download references
Author information
Authors and affiliations.
Department of Obstetrics and Gynecology, School of Medicine, Yuzuncu Yil University, Van, Turkey
Recep Yildizhan, Ali Kolusari, Ertan Adali, Mertihan Kurdoglu, Cagdas Ozgokce & Numan Cim
Department of Radiology, Women and Child Hospital, Van, Turkey
Fulya Adali
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Recep Yildizhan .
Additional information
Competing interests.
The authors declare that they have no competing interests.
Authors' contributions
All authors were involved in patient's care. RY, AK and FA analyzed and interpreted the patient data regarding the clinical and radiological findings of the patient and prepared the manuscript. EA, MK and CO edit and coordinated the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Yildizhan, R., Kolusari, A., Adali, F. et al. Primary abdominal ectopic pregnancy: a case report. Cases Journal 2 , 8485 (2009). https://doi.org/10.4076/1757-1626-2-8485
Download citation
Received : 12 January 2009
Accepted : 19 June 2009
Published : 07 August 2009
DOI : https://doi.org/10.4076/1757-1626-2-8485
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sigmoid Colon
- Ectopic Pregnancy
- Pelvic Inflammatory Disease
- Lower Abdominal Pain
- Transabdominal Ultrasound
Cases Journal
ISSN: 1757-1626
A Live 13 Weeks Ruptured Ectopic Pregnancy: A Case Report
Affiliations.
- 1 Obstetrics and Gynecology, Faculty of Medicine, King Abdulaziz University Hospital, Jeddah, SAU.
- 2 Obstetrics and Gynecology, King Abdulaziz University Hospital, Jeddah, SAU.
- PMID: 33209549
- PMCID: PMC7667717
- DOI: 10.7759/cureus.10993
Ectopic pregnancy is a pregnancy that occurs outside the uterus, most commonly in the fallopian tube. It is usually suspected if a pregnant woman experiences any of these symptoms during the first trimester: vaginal bleeding, lower abdominal pain, and amenorrhea. An elevated BhCG level above the discriminatory zone (2000 mIU/ml) with an empty uterus on a transvaginal ultrasound is essential for confirming ectopic pregnancy diagnosis. Such pregnancy can be managed medically with methotrexate or surgically via laparoscopy or laparotomy depending on the hemodynamic stability of the patient and the size of the ectopic mass. In this case study, we report on a 38-year-old woman, G3P2+0 who presented to King Abdulaziz University Hospital's emergency department with a history of amenorrhea for three months. She was unsure of her last menstrual period and her main complaint was generalized abdominal pain. Upon examination, she was clinically unstable and her abdomen was tender on palpation and diffusely distended. Her BhCG level measured 113000 IU/ml and a bedside pelvic ultrasound showed an empty uterine cavity, as well as a live 13 weeks fetus (measured by CRL). The fetus was seen floating in the abdominal cavity and surrounded by a moderate amount of free fluid, suggestive of ruptured tubal ectopic pregnancy. The patient's final diagnosis was live ruptured 13 weeks tubal ectopic pregnancy which was managed successfully through an emergency laparotomy with a salpingectomy.
Keywords: ectopic pregnancy; ruptured ectopic pregnancy; tubal pregnancy.
Copyright © 2020, Gari et al.
Publication types
- Case Reports
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- Global health
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 16, Issue 9
- Abdominal ectopic pregnancy
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- http://orcid.org/0000-0001-5499-415X Louise Dunphy ,
- Stephanie Boyle ,
- Nadia Cassim and
- Ajay Swaminathan
- Department of Obstetrics and Gynaecology , Leighton Hospital , Crewe , UK
- Correspondence to Dr Louise Dunphy; Louise.Dunphy{at}doctors.org.uk
An ectopic pregnancy (EP) accounts for 1–2% of all pregnancies, of which 90% implant in the fallopian tube. An abdominal ectopic pregnancy (AEP) is defined as an ectopic pregnancy occurring when the gestational sac is implanted in the peritoneal cavity outside the uterine cavity or the fallopian tube. Implantation sites may include the omentum, peritoneum of the pelvic and abdominal cavity, the uterine surface and abdominal organs such as the spleen, intestine, liver and blood vessels. Primary abdominal pregnancy results from fertilisation of the ovum in the abdominal cavity and secondary occurs from an aborted or ruptured tubal pregnancy. It represents a very rare form of an EP, occurring in <1% of cases. At early gestations, it can be challenging to render the diagnosis, and it can be misdiagnosed as a tubal ectopic pregnancy. An AEP diagnosed >20 weeks’ gestation, caused by the implantation of an abnormal placenta, is an important cause of maternal–fetal mortality due to the high risk of a major obstetric haemorrhage and coagulopathy following partial or total placental separation. Management options include surgical therapy (laparoscopy±laparotomy), medical therapy with intramuscular or intralesional methotrexate and/or intracardiac potassium chloride or a combination of medical and surgical management. The authors present the case of a multiparous woman in her early 30s presenting with heavy vaginal bleeding and abdominal pain at 8 weeks’ gestation. Her beta-human chorionic gonadotropin (bHCG) was 5760 IU/L (range: 0–5), consistent with a viable pregnancy. Her transvaginal ultrasound scan suggested an ectopic pregnancy. Laparoscopy confirmed an AEP involving the pelvic lateral sidewall. Her postoperative 48-hour bHCG was 374 IU/L. Due to the rarity of this presentation, a high index of clinical suspicion correlated with the woman’s symptoms; bHCG and ultrasound scan is required to establish the diagnosis to prevent morbidity and mortality.
- Obstetrics and gynaecology
- Emergency medicine
https://doi.org/10.1136/bcr-2022-252960
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
Abdominal pregnancies have been defined as serosal pregnancies occurring within the peritoneal cavity but excluding those pregnancies that are tubal, ovarian, intraligamentous or the result of a secondary implantation of primary tubal implantation. 1 The majority of abdominal ectopic pregnancies (AEPs) implant in the pelvis. An AEP is the rarest and the most serious type of extrauterine pregnancy. The pouch of Douglas (POD) is the most common location of an AEP followed by the mesosalpinx and omentum. In contrast to tubal ectopic pregnancies (EPs), AEPs may go undetected until an advanced gestational age. The absence of consistent clinical features makes the diagnosis difficult to establish. In 1944, Studdiford 1 defined the criteria used to diagnose a primary abdominal pregnancy. There are multiple risk factors that predispose patients to an EP, including a history of pelvic inflammatory disease, smoking, fallopian tube surgery, previous EP, endometriosis and assisted reproduction techniques. However, only 50% of women with an AEP have any associated risk factors. 2 An AEP can reach full-term gestation, with a viable fetus and subsequent perinatal survival, however, these are rare cases. 3 Surgery is the preferred procedure for an AEP, and the best option is to remove the entire sac including the fetus, membranes and the placenta.
Case presentation
A multiparous woman in her early 30s presented to the Emergency Gynaecology Clinic at 8 weeks’ gestation based on her last menstrual period with heavy vaginal bleeding and severe abdominal pain. She reported a 2-week history of mild vaginal bleeding and a 48-hour history of heavy bleeding with clots. It was a spontaneous conception. She had stopping taking her contraceptive pill 3 months previously. She described early pregnancy symptoms such as nausea and vomiting. Her medical history included asthma and ulcerative colitis. Her obstetrics history included two normal vaginal deliveries at term and a miscarriage at 6 weeks’ gestation. Her gynaecological history included a diagnosis of endometriosis. Her medications included salbutamol inhaler 100 mcg four times per day and sertraline 50 mg at night. She had a regular 28-day menstrual cycle. Her cervical smear was up to date. She did not have a Mirena intrauterine device in situ. Her observations were stable (Sp0 2 98% on air, respiratory rate of 14, BP 118/75 mm Hg, heart rate 79 beats per minute and temperature of 36.2°C). Physical examination confirmed generalised abdominal tenderness with guarding in the right iliac fossa. Speculum and bimanual palpation confirmed heavy vaginal bleeding. The cervical os was closed. Adnexal fullness was palpable on the right.
Investigations
Her haemoglobin on admission was 144 g/L (range: 115–165 g/L). Her beta-human chorionic gonadotropin (bHCG) was 5760 IU/L (range: 0–5), consistent with a viable pregnancy. A transvaginal ultrasound scan (TV USS) showed an endometrial thickness of 5.6 mm. Both ovaries appeared normal. Towards the midline of the pelvis, a mass was seen measuring 42×38 mm. The mass contained a cyst that appeared to contain an echogenic focus within, measuring 16 mm in diameter ( figure 1 ). The ultrasound findings raised the suspicion of a fetal pole. No fetal cardiac pulsations were seen. The mass appeared to be separate from the ovaries. The appearance raised the suspicion of an EP. There was no obvious free fluid seen in the pelvis. Her repeat bHCG at 48 hours was 4427 IU/L and her repeat TV USS showed a crown-rump length of 16.5 mm (suggesting a gestation of 8+1 weeks) ( figure 2 ). There was no free fluid. Both ovaries were normal. An EP was observed on the right side, measuring 38×27 mm. Her 96-hour bHCG was 3481 IU/L ( table 1 ).
- View inline
- Download figure
- Open in new tab
- Download powerpoint
Transvaginal ultrasound scan showing a lesion measuring 42×38 mm towards the midline of the pelvis. The lesion contained a cyst that appeared to contain an echogenic focus within, measuring 16 mm in diameter.
Repeat transvaginal ultrasound scan showing a crown-rump length (CRL) of 16.5 mm, suggesting a gestation of 8+1 weeks.
Differential diagnosis
The clinical symptoms correlated with her sonographic findings and her bHCG suggested an EP. Due to her abdominal pain and raised bHCG she was consented for a diagnostic laparoscopy.
A diagnostic laparoscopy was performed. An indwelling urinary catheter was inserted. The cervix appeared normal. An infra-umbilical incision was performed and the Veress needle inserted. Intraperitoneal entry was confirmed with Palmers, hanging drop and pressure tests. The uterus appeared of normal size and was mobile. The fallopian tubes were normal. A mass extending from the POD and right pelvic sidewall in close proximity to the ovary was observed. It measured 4×3 cm in diameter ( figures 3 and 4 ). The upper abdomen and the liver appeared normal. Superficial peritoneal endometriosis was observed. The ectopic pregnancy was separated from the POD and the pelvic lateral sidewall. The ureter was noted. Bipolar diathermy achieved haemostasis. A pelvic drain was inserted. The estimated blood loss was 1.5 L.
A mass extending from the pouch of Douglas and right pelvic sidewall in close proximity to the ovary was observed. It measured 4×3 cm in diameter.
A mass was observed extending from the right pelvic sidewall.
Outcome and follow-up
She remained haemodynamically stable. Her haemoglobin 24 hours postoperatively was 134 g/L. Her drain was removed as it contained <50 mL of serosanguineous fluid. Her bHCG 48 hours later was 374 IU/L. Serial monitoring of her bHCG was scheduled until it was negative. Follow-up was scheduled in the Gynaecology Clinic and histology confirmed an EP.
An AEP was first reported in 1708 as an autopsy finding. 4 Due to its rarity, only case reports or small case series exist in the literature. It has an estimated incidence of 1:10 000–25 000 live births. 1 An abdominal pregnancy is the only type of EP that can advance beyond 20 weeks of gestational age. In 1942, Studdiford 1 established three criteria for the diagnosis of a primary abdominal pregnancy ( Box 1 ). Watrowski et al recently expanded the classic Studdiford criteria, following a case of an omental pregnancy invading the peritoneum of the POD. 5 6 Since 1952, <50 cases of hepatic pregnancy have been described in the literature, with the most frequent site of implantation being the inferior surface of the right lobe. 7 An AEP can be classified as early or late. An early AEP is one that presents at or before 20 weeks’ gestation and a late AEP presents after 20 weeks’ gestation. An AEP can also be further classified as primary or secondary. Primary abdominal pregnancy occurs when the fertilised ovum implants directly into the peritoneal cavity; it is the less common type. In 1968, Friedrich and Rankin 8 proposed that to be a true primary AEP, the pregnancy should be <12 weeks’ gestation and the trophoblastic attachments should be related solely to the peritoneal surface. Secondary abdominal pregnancy occurs when the fertilised ovum first implants in the fallopian tube or uterus and then due to fimbrial abortion or rupture of the fallopian tube or uterus, the fetus subsequently develops in the mother’s abdominal cavity. Classification of an AEP although academically interesting is of limited clinical value. Risk factors include fallopian tube injury, pelvic inflammatory disease, endometriosis, multiparity and assisted reproductive techniques. Thirty-seven per cent of cases have a history of a tubal EP and 61% have a fallopian tube factor. 3 It has even been reported after bilateral salpingectomy in a patient who underwent in vitro fertilisation (IVF). 9 Studies have also suggested that the use of cocaine may be a risk factor for an AEP. 10
The Studdiford criteria for the diagnosis of a primary peritoneal pregnancy (L Dunphy)
The presence of normal tubes and ovaries.
No evidence of a uteroperitoneal fistula.
A pregnancy exclusively related to the peritoneal surface.
No evidence of secondary implantation following initial primary tubal nidation.
Several theories on the pathophysiology of an AEP have been postulated but the aetiology remains to be fully elucidated. Berghella and Wolf 11 refuted the diagnosis of primary implantation. Paternoster and Santarossa 12 suggested that delayed ovulation occurring close to menses may reverse the fertilised ovum in its tubal course by retrograde menstrual flow. Cavanagh 13 postulated that fertilisation may occur in the posterior cul-de-sac where sperm is known to accumulate and that an ovum could lay there due to dependent flow of peritoneal fluid. Dmowski et al 9 and Iwama et al 14 hypothesised that a retroperitoneal AEP may occur due to migration of the embryo along lymphatic channels. Reports have also described an AEP after a hysterectomy. It has been suggested that IVF may predispose to an AEP via uterine perforation at the time of embryo transfer, migration of an oocyte into the abdominal cavity with subsequent abdominal fertilisation by spermatozoa or migration through a micro-fistulous tract through the uterine isthmus.
Early diagnosis is difficult to establish as women may present with a plethora of symptoms or be asymptomatic. There are no pathognomonic symptoms of an AEP that distinguish it from a tubal pregnancy. It may present as diffuse pain accompanied by signs of incipient pregnancy. At an advanced gestation, the woman may present with non-specific symptoms, such as abdominal pain, vaginal bleeding or decreased, absent and painful fetal movements. 15 Nausea, vomiting and an intense persistent desire to defecate may be prominent symptoms, when the pregnancy implants on bowel. A history of irregular bleeding is less frequently reported than in tubal EPs. Fetal movements may also be reported high in the upper abdomen or there may be absent fetal movements. The fetus may be easily palpable and the presentation may be transverse. Speculum and bimanual examination may show a closed and effaced cervix. Oxytocin may also fail to stimulate the gestational mass. The patient may also present with haemodynamic compromise and shock due to rupture of the AEP.
A suboptimal rise in serum bHCG is a useful marker, but it is not sufficient to establish the diagnosis. However, laboratory investigations may reveal excessively elevated alpha-fetoprotein levels. TV USS is the main imaging modality. However, it only has a sensitivity of 50%. Sonographic features include an empty uterus. The classic ultrasound finding is the absence of myometrial tissue between the maternal bladder and the pregnancy. 16 Other sonographic features suggestive of the diagnosis may include poor definition of the placenta, oligohydramnios and an unusual fetal lie ( box 2 ). However, it can be challenging to differentiate it from a tubal EP at an early gestation, especially when it implants in the vicinity of the adnexa. CT and MRI can also be used for further evaluation. MRI is the gold standard for evaluating placental implantation and preoperative planning. However, it is not uncommon to diagnose an AEP for the first time at laparotomy or laparoscopy performed for a tubal EP. Chen’s review of 17 cases showed that the rate of preoperative diagnosis remained low with only 29.41% of cases diagnosed preoperatively. 17 The accuracy of diagnosis increased with an increase in gestational age and the appearance of the fetal heartbeat. The differential diagnosis includes an EP at other locations, an intrauterine pregnancy in a rudimentary horn, placental abruption or uterine rupture. A retroflexed uterus and the presence of a uterine leiomyoma (fibroid) can also make the diagnosis difficult to render. A false-negative diagnosis as an intrauterine pregnancy can occur. A false-positive diagnosis with cervical, intramural, isolated uterine horn and a bicornuate pregnancy can also occur.
. Sonographic features of an abdominal pregnancy
An absence of myometrial tissue between the maternal bladder and the pregnancy, abdominal wall and pregnancy.
An empty uterus.
Poor definition of the placenta.
Oligohydramnios.
Unusual fetal lie.
There is no established guidance available for the diagnosis and management of an AEP. An AEP can reach full-term gestation, with a viable fetus and subsequent perinatal survival; however, these are exceptional cases. 18 Between the years 2008 and 2013, 38 cases of advanced abdominal pregnancy resulting in a live birth were reported. 19 Twenty-one per cent of neonates had a birth defect such as limb defects, craniofacial and joint abnormalities, for example, talipes equinovarus, as well as central nervous system malformations. 20 Due to abnormal placental implantation, cases of advanced AEP carry multiple risks such as haemorrhage, infection, disseminated intravascular coagulation, fistula formation and pre-eclampsia. 21 Expectant management to gain fetal maturity has been attempted and has been successful in a few cases. 3 The conservative approach by Marcellin et al 22 carried a pregnancy until 32 weeks uneventfully. Similarly, Beddock et al 23 described a viable fetus at 37 weeks’ gestation without maternal or fetal complications. There are no strong clinical predictors for successful medical therapy and the decision for medical therapy must be individualised based on the distinctive characteristics of each case. Medical management includes methotrexate (local or systemic), local instillation of potassium chloride, hyperosmolar glucose, prostaglandins, danazol, etoposide and mifepristone. 24 Medical management is commonly used where potentially life-threatening bleeding is anticipated, such as an AEP involving the liver or the spleen. Primary methotrexate therapy has a high failure rate due to the advanced gestational age at which an AEP is diagnosed. Postoperatively, histology showing evidence of trophoblast proliferation with neovascularisation involving the organ or structure the pregnancy was attached to confirms the diagnosis of an EAP. 23
Angiographic arterial embolisation can be used as first-line treatment of an AEP with the aim of avoiding surgery or by reducing the vascularity of the placenta making surgery safer. 25 Selective embolisation of vessels supplying the placenta should be considered to control the haemorrhage postoperatively from the retained placenta. Historically, an AEP was managed surgically. However, in 1993, Balmaceda et al 26 reported laparoscopic management of an AEP at 7 weeks’ gestation. Abossolo et al 27 also described successful management of a first trimester AEP with significant intraoperative haemorrhage laparoscopically. 27 Laparoscopy can be performed at early gestations as removal of the small and less vascular placental tissue is easier. At late gestations, surgery is the main treatment. The entire sac including the fetus, membranes and the placenta should be removed. 28 However, termination of pregnancy should also be offered due to the adverse maternal and fetal sequelae. The fetus can be delivered easily; the key issue is how to manage the placenta. Bleeding from the placental site can be a life-threatening complication during laparotomy as an AEP lacks the haemostatic mechanisms exerted by myometrial contractions, therefore, it fails to constrict the placental vasculature. Indeed, a complicated AEP with the placenta feeding off the sacral plexus has also been described. 29 There are two options. The placenta can be removed after ligating the placental blood supply. A good separation technique and adequate vascular management should be performed. The second option is to leave the placenta in situ after ligating the umbilical cord. 30 However, this option is associated with sepsis, abscess formation, delayed haemorrhage due to retained placental remnants, adhesions, coagulopathy, ongoing pre-eclampsia and failure of lactogenesis. When the placenta remains in situ, two methods of follow-up are available. The first is the use of methotrexate to accelerate tissue absorption. The role of methotrexate to hasten placental resorption is discouraged by most authors due to the risk of infection. The second method is expectant management, which consists of monitoring the bHCG levels and performing an ultrasound scan. Chen et al 17 waited for self-absorption in cases where the placenta was left in situ and followed up the patients for a maximum period of 26 months. It has been suggested that there is a similarity with the intrauterine placenta accreta spectrum cases and an AEP, especially in the management of the placenta. 17 In Honduras in 1956, a case of an AEP with a dead fetus that developed into a lithopedion, derived from the Greek words lithos (stone) and paedion (child), a term used to describe an AEP in which the fetus dies but cannot be reabsorbed by the mother’s body was described. 31 Ultrasonographic-guided fetocide of a 14.5 weeks’ gestation to prevent further development and initiate the process of natural resorption has been reported. 32
Abdominal pregnancy has a maternal mortality rate between 0.5% and 18%, which is >7 times higher compared with that of other EPs. 33 34 This is due to the risk of massive haemorrhage from a partially or totally separated placenta at any stage of pregnancy. Its perinatal mortality rate is between 40% and 95%. 35 A high index of clinical suspicion and a multi-disciplinary approach is required to prevent adverse maternal and fetal outcomes.
Learning points
Abdominal ectopic pregnancies (AEPs) present with a plethora of non-specific symptoms and may go undetected until an advanced gestational age when the patient presents with a history of recurrent abdominal pain, the presence of fetal movements high in the upper abdomen or absence of fetal movements. Clinical manifestations may also include soreness during fetal movements, easily palpable parts of the fetal body and a transverse presentation.
There are no specific criteria to diagnose an AEP and it may be missed on ultrasound. However, a classic sign on ultrasound scan is the absence of echo signs of myometrium between the mother’s bladder and the fetus. Additional signs may include poor visualisation of the placenta, oligohydramnios and a transverse presentation. MRI is the gold standard for evaluating placental attachment and vascular connections.
The mainstay of treatment of advanced AEP is surgery, but the optimal approach has not been determined. Management of the placenta remains controversial. Maternal morbidity and mortality is high as AEPs typically implant on highly vascularised surfaces. Separation of the placenta can occur at any gestation and may lead to life-threatening maternal haemorrhage. Maternal and fetal outcomes are determined by haemodynamic status and gestational age at presentation.
Ethics statements
Patient consent for publication.
Consent obtained directly from patient(s).
- Studdiford WE
- Kopelman ZA ,
- Keyser EA ,
- Watrowski R ,
- Cao Y , et al
- Friedrich EG ,
- Dmowski WP ,
- Ding J , et al
- Smith DM , et al
- Berghella V ,
- Paternoster DM ,
- Santarossa C
- Tsutsumi S ,
- Igarashi H , et al
- Mansuria S ,
- Guido RS , et al
- Li C , et al
- Mascarenhas L ,
- Aragon-Charry J ,
- Santos-Bolívar J ,
- Torres-Cepeda D , et al
- Masukume G ,
- Sengurayi E ,
- Muchara A , et al
- Marcellin L ,
- Lamau M-C , et al
- Beddock R ,
- Naepels P ,
- Gondry C , et al
- Raughley MJ ,
- Frishman GN
- Balmaceda JP ,
- Bernardini L ,
- Asch RH , et al
- Abossolo T ,
- Sommer JC ,
- Dancoisne P , et al
- Chueh H-Y , et al
- Feldman J ,
- Madan M , et al
Contributors The following authors were responsible for drafting of the text, sourcing and editing of clinical images, investigation results, drawing original diagrams and algorithms, and critical revision for important intellectual content: LD: wrote the case report; SB: literature review; NC: literature review; AS: literature review. LD, SB, NC and AS gave final approval of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Read the full text or download the PDF:
- Research article
- Open access
- Published: 12 February 2021
Determinants of ectopic pregnancy among pregnant women attending referral hospitals in southwestern part of Oromia regional state, Southwest Ethiopia: a multi-center case control study
- Urge Gerema ORCID: orcid.org/0000-0001-5286-5100 1 ,
- Tilahun Alemayehu 1 ,
- Getachew Chane 1 ,
- Diliab Desta 1 &
- Amenu Diriba 2
BMC Pregnancy and Childbirth volume 21 , Article number: 130 ( 2021 ) Cite this article
8930 Accesses
4 Citations
Metrics details
Ectopic pregnancy is an abnormal condition in which implantation of the blastocyst occurs outside the endometrium of the uterus. It is gynecological important, particularly in the developing world, because of associated with enormous rate of high morbidity, during the first trimester of pregnancy. A better understanding of its risk factors can help to prevent its prevalence. However, the determinants of ectopic pregnancy are not well understood and few researches conducted in our country were based on secondary data covering small scale area. This study aimed to identify determinants of ectopic pregnancy among pregnant women attending referral hospitals in Southwestern part of Oromia regional state, Southwest Ethiopia.
Hospital-based case control study was employed from June 1 to September 30, 2019. The study was conducted in five referral hospitals in Southwestern part of Oromia regional state. Final sample size includes 59 cases and 118 controls. Data were entered by using Epidata version 3.1 and analyzed using SPSS version 23. Descriptive statistics were used to explore the data. All explanatory variables with p -value of < 0.25 in bi-variable analysis, then entered into multivariable logistic regression. Associated factors were identified at 95% confidence interval ( p < 0.05).
Out of 177 (59 cases and 118 controls) participants, 174 (58 cases and 116 controls) were participating in the study. Prior two or more induced abortions [AOR = 3.95:95% CI: 1.22–13.05], previous history of caesarean section [AOR = 3.4:95% CI: 1.11–10.94], marital status (being single) [AOR = 4.04:95%CI: 1.23–13.21], reporting prior recurrent sexual transmitted infection [AOR = 2.25:95%CI: 1.00–5.51], prior history of tubal surgery [AOR = 3.32:95%CI: 1.09–10.13], were more likely to have an ectopic pregnancy with their respective AOR with 95%CI.
It was found that having a history of more than two induced abortions during previous pregnancies, marital status (single), recurrent sexual transmitted infection, prior history of tubal surgery and experiencing prior caesarean section were found to be determinants of ectopic pregnancy. Hospitals should give emphasis on prevention and early detection of risks of ectopic pregnancy and create awareness in order to reduce the burden of ectopic pregnancy.
Peer Review reports
Ectopic pregnancy (EP) is an abnormal condition in which implantation of the blastocyst occurs outside the endometrium of the uterus. These abnormal sites of implantation in decreasing order of frequency include uterine tube (tubal pregnancy), abdominal cavity or on the mesentery (abdominal pregnancy), and in the ovaries (ovarian pregnancy) [ 1 , 2 ]. Blastocysts that do not implant in the uterine wall are generally unable to develop normally because; the space is incapable for developing blastocyst. Ectopic pregnancy can cause ruptures of fallopian tube, cervix and abdomen on which they are implanted. Rupture of ectopic pregnancy result in severe bleeding, many organ damage, and maternal mortality [ 3 , 4 , 5 ]. It is obstetrical and gynecological important, especially in the developing world, because of the high maternal morbidity and mortality associated with it and the enormous threat to life particularly in the first trimester pregnancy [ 2 ]. It occurs in approximately 1–2% of pregnancies [ 4 , 5 , 6 , 7 ]. It is one of the top leading causes of maternal mortality in the first trimester and accounts for 10–15% of all maternal deaths [ 7 ]. Ectopic pregnancy is the leading cause of maternal morbidity and mortality worldwide [ 8 ].
In western world, the prevalence of ectopic pregnancy is approximately 2% in the general population, but as high as 20% in patients who have undergo tubal surgery, previous ectopic pregnancy [ 8 ]. The prevalence of ectopic pregnancy has an increasing trend during the last three decades throughout the world especially in developing countries where early diagnosis is low [ 3 ].
The causes of ectopic pregnancy are not well understood. However, multiple risk factors have been associated with ectopic pregnancy, although some patients may not have any risk factor to developed ectopic pregnancy. The main function of the oviduct is to provide the optimal environment for the transport and maturation of ovum and sperm for the establishment of pregnancy. Most data suggest that ectopic pregnancy causes from both abnormal zygote transport and change in the tubal environment, which enables abnormal implantation to occur [ 9 , 10 , 11 ].
In spite of different research done on the prevalence of ectopic pregnancy, however, the determinants of ectopic pregnancy are not well understood and few researches published in our country were based on secondary data covering small scale area and the study area has different characteristics cultural, religious, socio-demographic characteristics, sexual behavior, beliefs, contraception usage and practice from other area. The study was aimed to identify risk factors of ectopic pregnancy among pregnant women attending referral hospitals in Southwestern part of Oromia Regional state, Southwest Ethiopia.
This study result would worth to detect the potential risk factors of ectopic pregnancy in the study setup which would have further advantages to minimize morbidity and mortality of patients due to ectopic pregnancy. With regard to the preventable factors associated with ectopic pregnancy in the current population, this study is an important piece of work that could serve as an important source of information to design prevention strategies or to conduct further investigations.
Study setting, design and population
A multi-centered hospital-based case control study was conducted among pregnant women attending referral hospitals in Southwestern parts of Oromia regional state, Southwest Ethiopia from June 1 to September 30, 2019. All hospitals are teaching and referral hospital that gave general and specialized clinical services including ANC, family planning, delivery service & treatment obstetric complications are some of the services provided in gynecologic and obstetric ward. These services have been delivered by senior midwives, gynecologists/obstetricians. All pregnant women attending gynecology and obstetrics department of JMC (Jimma Medical Center), WURH (Wollega University Referral Hospital), NRH (Nekemte Referral Hospital), AURH (Ambo Referral Hospital) and MKRH (Mettu Karl Referral Hospital) during the four-month study period were source population.
Study population: For cases all pregnant women who had been confirmed by ultrasound and HCG to have EP in the inpatient department of gynecology and obstetrics of each hospital were recruited. For controls: Controls were sampled pregnant women confirmed by ultrasound and HCG to have intra uterine pregnancy at the prenatal clinic in department of gynecology and obstetrics of each hospital.
Eligibility criteria
Inclusion criteria for cases.
admitted women who had been confirmed by ultrasound and HCG to have EP in the inpatient department of gynecology and obstetrics of each hospital. For controls: Controls were sampled pregnant women confirmed by ultrasound and HCG to have intra uterine pregnancy at the prenatal clinic in department of gynecology and obstetrics of each hospital.
Exclusion criteria for both cases and controls
Women with serious medical conditions and couldn’t give consent were excluded from the study.
Case definition
Case pregnant women diagnosed by hCG and ultrasound to have ectopic pregnancy confirmed by Obstetrician/gynecologist [ 12 ].
Pregnant women diagnosed by hCG and ultrasound to have intrauterine pregnancy confirmed by Obstetrician/gynecologist [ 12 ].
Sample size and sampling procedure
The required sample size was determined by using Epi-info version 7 statistical software for unmatched case-control study design. Results from similar studies were used to approximate the sample size in different potential risk factors of ectopic pregnancy. In a study report from India prior tubal surgery was a significant risk factor for ectopic pregnancy [ 13 ]. A case control study in western Ethiopia at Nekemte hospital marital status was a significant risk factor for ectopic pregnancy [ 14 ]. Similarly, case control study done in Turkey Ankara previous history of ectopic pregnancy was a significant risk factor for ectopic pregnancy [ 15 ]. Using these reports as starting point, similar assumptions P1: proportion among cases and p 2: proportion of among controls AOR: Adjusted odds ratio at 95% ( Zα/2 = 1.96) level of confidence, Power of study = 80% Ratio of cases to controls = 1:2 (Table 1 ).
From the above three significant risk factors of ectopic pregnancy, previous history of ectopic pregnancy gives the large sample size which gives total of 177 study participants (59 cases and 118 controls). In the selected five referral hospitals the number of pregnant. Women registered during the 2018 G. C HMIS report over 4 months at JMC, WURH, NRH, AURH and MKRH were 1707, 1085, 679, 1489 and 1219 respectively.
The calculated sample size was proportionally allocated based on the estimated number of pregnant women in selected referral hospitals. Therefore (16 cases and 32 controls) from JMC, (10 cases and 20 controls) from WURH, (7 cases and 14 controls) from NRH, (14 cases and 28 controls) from AURH and (12 cases and 28 controls) from MKRH. Then, the study participant was selected using consecutive sampling technique.
Data collection tools and procedures
The data were collected by face to face interview using semi structured questionnaire addressing socio-demographic and obstetric, gynecologic, behavioral, surgical history and contraceptive characteristics of study participants which was developed after reviewing different literatures. Fifteen trained data collectors and five supervisors were involved in the process.
Data quality control
The urine sample collection was done through standardized, and sterile technique by professional laboratory technologists, ultrasound was calibrated before the procedure. The diagnosis of pregnancy was confirmed by Trans abdominal ultrasonography combined to the hCG.
Data quality was ensured during data collection, coding, entry and analysis. During data collection adequate training and follow up was provided to data collectors and supervisors. Incomplete checklists were returned back to the data collector for completion. Codes were given to the questionnaires and during the data collection so that any identified errors was traced back using the codes.
Data processing and analysis
Collected data were rechecked for completeness, consistency and coded before data entry. Data were entered using Epi data version 3.1 and data from five hospitals were merged together, and then exported to the Statistical Package for Social Science (SPSS) version 23 for analysis. Descriptive analysis was conducted to explore the data and present some variables. Bi-variable binary logistic regression analysis was executed to select candidate variable for multivariable binary logistic regression to identify the predictors. Variables with p -value of less than 0.25 were selected for multivariable logistic regression. Odds ratio (OR) and 95% confidence intervals (CI) were used to describe the association between ectopic pregnancy and potential risk factors. Variables with a p -value < 0.05 in multi-variable analysis was considered as a significant risk factor for ectopic pregnancy.
Socio-demographic characteristics
In this prospective case control study conducted over four-months from June 1 to September 30, 2019 at five government referral hospitals found in Southwestern part Oromia, Ethiopia. A total of 174 pregnant women; 58 Cases (EP) and 116 Controls (IUP) were participated. The mean age was 26 (± 5.54), 26 (± 4.87 years for cases and controls respectively.
Almost two-third (63.8%) of cases and 79 (68.1%) of controls were aged between 21 and 30 years. Eighteen (31%) cases and 39(33%) of controls were orthodox in religion and 37(63.8%) of cases and 79 (68.1%) of controls were Oromo by their ethnicity and 37 (63.8%) cases and 95(81.9%) of controls were married. About 18 (31%) cases and 46 (39.7%) controls were house wives in occupation (Table 2 ).
Behavioral characteristics
Only one case (1.7%) and two controls (1.7%) had occasional history of cigarette smoking and only 18(31.1%) cases and 34(29.3%) controls history of occasionally alcohol consumption before current pregnancy (Table 3 ).
Obstetrics and surgical history of participants
As indicated in the Table 4 , three of the cases (5.1%) and another three women in the control group (2.6%) had prior history of ectopic pregnancy. Seven women in each of the study groups (12.0% of the cases and 6.0% of the controls) had more than two prior history of spontaneous abortion. Similarly, 8 (13.8%) cases and 6 (5.1%) controls reported two or more prior history of induced abortions. This study shows that 10(17.2%) of cases and 6(5.1%) controls had caesarean section before current pregnancy. Eleven (18.1%) of cases and 6(5.2%) controls had at least one tubal pregnancy before current pregnancy for any reason (Table 4 ).
Gynecologic and contraceptive history of participants
About 36.2% (21/58) of the cases and 16 (13.7%) controls had prior history of a recurrent.
STD/STI. Majority 42 (72.4%) of the cases and 92 (79.3%) controls had prior history of oral contraceptive use. Only 6 (10.3%) cases and 15(12.9%) controls had history of IUCD use.
Twenty (34.4%) cases and 18 (10.8%) controls reported practice of emergency contraceptives pills use before the current conception (Table 5 ).
Factors associated with ectopic pregnancy
Findings from bi-variable logistic regression analysis showed that marital status, prior history of induced abortions, prior history of spontaneous abortions, prior history of tubal surgery, prior history of caesarean section, prior history of tubal ligation and history of recurrent STD/STI had associated with ectopic pregnancy with p-value of < 0.25, However, in.
multivariable regression analysis, history of two or more induced abortions [AOR = 3.42:95%CI: 1.06–11.05], prior history of caesarean section [AOR = 3.48:95% CI: 1.14–10.13], prior history of tubal surgery [AOR = 3.32:95%CI: 1.09–10.13], marital status (being single) [AOR = 3.23:95%CI:1.02–10.22], prior recurrent STD/STI [AOR = 3.08:95%CI: 1.38–6.88} remained statistically significant risk factor for ectopic pregnancy (Table 6 ).
This was a multi-centered hospital based case control study which, was aimed to identify determinants of ectopic pregnancy among pregnant women attending referral hospitals in Southwestern parts of Oromia regional state, Southwest Ethiopia. Being single were independent predictors of ectopic pregnancy. A similar association was reported in studies done in west Ethiopia Nekemte and Uganda [ 14 , 16 ]. The association between being single and ectopic pregnancy infection could be explained by the fact that single women engaged in multiple sexual partners following successive infection, ascending infection result in adhesions, impede the morula retention of movement causing implantation in the tube and other site.
Having more than two times history of induced abortion found was statistically significant relation with ectopic pregnancy. This finding was supported by a study done in; India, Tigray, Ethiopia and Nigeria [ 17 , 18 , 19 ]. The association might be explained by most abortions are illegal different countries and usually performed in poor aseptic conditions. Thus, increasing post-abortion sepsis risk and subsequent PID.
Women who had a prior history of recurrent STI were significantly associated with ectopic pregnancy. This finding was similar to studies done in Ethiopia, Ghana [ 20 ]. The association between STD/STI and ectopic pregnancy might be successive infection, ascending infection result in salphingitis leads to tubal dysfunction, decrease cilia density; ciliary beat this result in retention of morula in the fallopian tube and implantation of blastocyst in the fallopian tube and other site.
Women having at least one caesarean section for previous pregnancy were independently associated with ectopic pregnancy. This study supported by a study done in Turkey [ 15 ]. The underlying mechanism of association between previous caesarean section and occurrence of ectopic pregnancy is might be due to increased pelvic infection and adhesion after caesarean section which disturbs the micro environment of the tube and implantation of blastocyst in the tube.
In the present study, women who had a prior history of tubal surgery were statistically significant with ectopic pregnancy. This study is supported by study done in Egypt and Uganda [ 16 ]. The association might be explained by the scar on the Fallopian tube may interfere with the ovum transport and implantation of blastocyst in the Fallopian tube.
I did not find any association between appendectomy, prior use of IUCD, cigarette smoking, alcohol drinking and previous history of ectopic pregnancy, previous tubal surgery with present study, probably the number of the studied participants was too small.
Limitations and strengths of the study
Due to a small number of cases obtained from each hospital, this study did not compare among the five hospitals with regard to risk factors of EP and study assesses history of exposure retrospectively, it may be prone to recall and selection bias by nature during the data collection time. The study has some strengths this study used the primary data from the participants. Further, the study was multi-centered hospital based case control.
Conclusions
It was found that having a history of more than two induced abortions during previous pregnancy, marital status (single), experiencing at least one caesarean section for previous pregnancies, prior history of STD/STI and using emergency contraceptive pills during the cycle of conception were found important determinants of ectopic pregnancy in the study population. Women with history of previous induced abortion and previous caesarean section STD/STI should be followed up carefully, even in the absence of symptoms should always be counseled about the possibility of ectopic pregnancy and the associated risks.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Adjusted odd ratio
Ambo University Referral Hospital
Crude odd ratio
- Ectopic pregnancy
chorionic gonadotropin
Health Management Information system
Intra uterine pregnancy
Jimma Medical Center
Mettu Karl Referral Hospital
Nekemte Referral Hospital
Sexual transmitted diseases
Sexual transmitted infection
Bhuria V, Nanda S, Chauhan M, Malhotra V. A retrospective analysis of ectopic pregnancy at a tertiary care Centre: one year study. Int J Reproduct Contracept Obstetr Gynecol. 2016;5:2224–7.
Gaym A. Maternal mortality studies in Ethiopia--magnitude, causes and trends. Ethiop Med J. 2009;47(2):95–108.
PubMed Google Scholar
Yeasmin MS, Uddin MJ, Hasan E. A clinical study of ectopic pregnancies in a tertiary care hospital of Chittagong, Bangladesh. Chattagram Maa-O-Shishu Hosp Med Coll J. 2014;13(3):1–4.
Article Google Scholar
Gharoro EP, Igbafe AA. Ectopic pregnancy revisited in Benin City, Nigeria: analysis of 152 cases. Acta Obstet Gynecol Scand. 2002;81(12):1139–43.
Article CAS Google Scholar
Basnet R, Pradhan N, Bharati L, Bhattarai N, Bb B, Sharma B. To determine the risk factors associated with ectopic pregnancy. Asian J Pharm Clin Res. 2015;8(2):93–7.
Google Scholar
Porricelli JD, Boyd JH. Analytical Techniques for Predicting Grounded Ship Response. Engineering computer opt economics inc Annapolis md; 1983.
Dabota BY. Management and outcome of ectopic pregnancy in developing countries. Ectopic Pregnancy. 2011;109:10–8.
Panchal D, Vaishnav G, Solanki K. Study of management in patient with ectopic pregnancy. Infection. 2011;33:55.
Shaw JL, Dey SK, Critchley HO, Horne AW. Current knowledge of the etiology of human tubal ectopic pregnancy. Hum Reprod Update. 2010;16(4):432–44.
Jacob L, Kalder M, Kostev K. Risk factors for ectopic pregnancy in Germany: a retrospective study of 100,197 patients. GMS German Med Sci. 2017;15:33–4.
John CO, Alegbleye JO. Ectopic pregnancy experience in a tertiary health facility in South-South Nigeria. Nigerian Health J. 2016;16(1):2–5.
Schoenwolf, G.C., Bleyl, S.B., Brauer, P.R., Francis-West, P.H. & Philippa H. (2015). Larsen's human embryology (5th ed.). New York; Edinburgh: Churchill Livingstone.
Bhandari G, Yadav KK, Shah R. Ectopic pregnancy and its risk factors: a case control study in Nepalese women. J BP Koirala Inst Health Sci. 2018;1(2):30–4.
Kebede Y, Dessie G. Determinants of ectopic pregnancy among pregnant women who were managed in Nekemte referral hospital, Oromia Region, Ethiopia. J Preg Child Health. 2018;5(370):2.
Karaer A, Avsar FA, Batioglu S. Risk factors for ectopic pregnancy: a case-control study. Aust N Z J Obstet Gynaecol. 2006;46(6):521–7.
Dp M, Lugobe H, Ssemujju A. Factors associated with ectopic pregnancy at Mbarara University teaching Hospital in South Western Uganda. Reprod Med. 2018;2(4):2–7.
Kassebaum NJ, Barber RM, Bhutta ZA, Dandona L, Gething PW, Hay SI, Kinfu Y, Larson HJ, Liang X, Lim SS, Lopez AD. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1775–812.
Abebe D, Tukue D, Aregay A, Gebremariam L. Magnitude and associated factors with ectopic pregnancy treated in Adigrt hospital, Tigray region, Northern Ethiopia. Int J Res Pharm Sci. 2017;7(1):30–39.
Lawani OL, Anozie OB, Ezeonu PO. Ectopic pregnancy: a life-threatening gynecological emergency. Int J Women's Health. 2013;5:515.
Chow JM, Yonekura ML, Richwald GA, Greenland S, Sweet RL, Schachter J. The association between chlamydia trachomatis and ectopic pregnancy: a matched-pair, case-control study. JAMA. 1990;263(23):3164–7.
Download references

Acknowledgments
We would like to thank Jimma University for allowing me to conduct this study. Also we would like to thanks the study participants, data collectors and supervisors.
There is no funding for this study.
Author information
Authors and affiliations.
Department of Biomedical sciences (Anatomy course unit), Jimma University, Jimma, Ethiopia
Urge Gerema, Tilahun Alemayehu, Getachew Chane & Diliab Desta
Department of gynecology and obstetrics, Wellega University, Wellega, Ethiopia
Amenu Diriba
You can also search for this author in PubMed Google Scholar
Contributions
UG involved in conceiving the idea, study design, data analysis and interpretation, writing the manuscript and managing the overall progress of the study. TA, G CH, DD and AD involved in study design, data analysis and in revising the manuscript. The final manuscript was read and approved by all the authors.
Corresponding author
Correspondence to Urge Gerema .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval or clearance letter RPSCMF/0132/19 was obtained from institutional review board (IRB) of Institute of Health, Jimma University. Permission letter was written to respective hospitals administration office, and the study was commencing after receiving formal permission from them. The Institutional review board approved the verbal consent. Due to low literacy level informed verbal consent was obtained from each respondent after they had been taken through the respondent information sheet. Data collectors maintained confidentiality through excluding names or any other personal identifiers from data collection sheets and reports.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Gerema, U., Alemayehu, T., Chane, G. et al. Determinants of ectopic pregnancy among pregnant women attending referral hospitals in southwestern part of Oromia regional state, Southwest Ethiopia: a multi-center case control study. BMC Pregnancy Childbirth 21 , 130 (2021). https://doi.org/10.1186/s12884-021-03618-7
Download citation
Received : 03 January 2020
Accepted : 03 February 2021
Published : 12 February 2021
DOI : https://doi.org/10.1186/s12884-021-03618-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Intrauterine pregnancy
- Determinants
- Southwest Ethiopia
BMC Pregnancy and Childbirth
ISSN: 1471-2393
- Submission enquiries: [email protected]
- General enquiries: [email protected]

ERIN HENDRIKS, MD, RACHEL ROSENBERG, MD, AND LINDA PRINE, MD
Am Fam Physician. 2020;101(10):599-606
Author disclosure: No relevant financial affiliations.
Ectopic pregnancy occurs when a fertilized ovum implants outside of the uterine cavity. In the United States, the estimated prevalence of ectopic pregnancy is 1% to 2%, and ruptured ectopic pregnancy accounts for 2.7% of pregnancy-related deaths. Risk factors include a history of pelvic inflammatory disease, cigarette smoking, fallopian tube surgery, previous ectopic pregnancy, and infertility. Ectopic pregnancy should be considered in any patient presenting early in pregnancy with vaginal bleeding or lower abdominal pain in whom intrauterine pregnancy has not yet been established. The definitive diagnosis of ectopic pregnancy can be made with ultrasound visualization of a yolk sac and/or embryo in the adnexa. However, most ectopic pregnancies do not reach this stage. More often, patient symptoms combined with serial ultrasonography and trends in beta human chorionic gonadotropin levels are used to make the diagnosis. Pregnancy of unknown location refers to a transient state in which a pregnancy test is positive but ultrasonography shows neither intrauterine nor ectopic pregnancy. Serial beta human chorionic gonadotropin levels, serial ultrasonography, and, at times, uterine aspiration can be used to arrive at a definitive diagnosis. Treatment of diagnosed ectopic pregnancy includes medical management with intramuscular methotrexate, surgical management via salpingostomy or salpingectomy, and, in rare cases, expectant management. A patient with diagnosed ectopic pregnancy should be immediately transferred for surgery if she has peritoneal signs or hemodynamic instability, if the initial beta human chorionic gonadotropin level is high, if fetal cardiac activity is detected outside of the uterus on ultrasonography, or if there is a contraindication to medical management.
Ectopic pregnancy occurs when a fertilized ovum implants outside of the uterine cavity. The prevalence of ectopic pregnancy in the United States is estimated to be 1% to 2%, but this may be an underestimate because this condition is often treated in the office setting where it is not tracked. 1 , 2 The mortality rate for ruptured ectopic pregnancy has steadily declined over the past three decades, and from 2011 to 2013 accounted for 2.7% of pregnancy-related deaths. 1 , 3 Risk factors for ectopic pregnancy are listed in Table 1 4 , 5 ; however, one-half of women with diagnosed ectopic pregnancy have no identified risk factors. 4 – 6 The overall rate of pregnancy (including ectopic) is less than 1% when a patient has an intrauterine device (IUD). However, in the rare case that a woman does become pregnant while she has an IUD, the prevalence of ectopic pregnancy is as high as 53%. 7 , 8 There is no difference in ectopic pregnancy rates between copper or progestin-releasing IUDs. 9
Making the Diagnosis
Signs and symptoms.
Ectopic pregnancy should be considered in any pregnant patient with vaginal bleeding or lower abdominal pain when intrauterine pregnancy has not yet been established ( Table 2 ) . 10 Vaginal bleeding in women with ectopic pregnancy is due to the sloughing of decidual endometrium and can range from spotting to menstruation-equivalent levels. 10 This endometrial decidual reaction occurs even with ectopic implantation, and the passage of a decidual cast may mimic the passage of pregnancy tissue. Thus, a history of bleeding and passage of tissue cannot be relied on to differentiate ectopic pregnancy from early intrauterine pregnancy failure.
The nature, location, and severity of pain in ectopic pregnancy vary. It often begins as a colicky abdominal or pelvic pain that is localized to one side as the pregnancy distends the fallopian tube. The pain may become more generalized once the tube ruptures and hemoperitoneum develops. Other potential symptoms include presyncope, syncope, vomiting, diarrhea, shoulder pain, lower urinary tract symptoms, rectal pressure, or pain with defecation. 11
The physical examination can reveal signs of hemodynamic instability (e.g., hypotension, tachycardia) in women with ruptured ectopic pregnancy and hemoperitoneum. 12 Patients with unruptured ectopic pregnancy often have cervical motion or adnexal tenderness. 13 Sometimes the ectopic pregnancy itself can be palpated as a painful mass lateral to the uterus. There is no evidence that palpation during the pelvic examination leads to an increased risk of rupture. 10
BETA HUMAN CHORIONIC GONADOTROPIN
Beta human chorionic gonadotropin (β-hCG) can be detected in pregnancy as early as eight days after ovulation. 14 The rate of increase in β-hCG levels, typically measured every 48 hours, can aid in distinguishing normal from abnormal early pregnancy. In a viable intrauterine pregnancy with an initial β-hCG level less than 1,500 mIU per mL (1,500 IU per L), there is a 99% chance that the β-hCG level will increase by at least 49% over 48 hours. 15 As the initial β-hCG level increases, the rate of increase over 48 hours slows, with an increase of at least 40% expected for an initial β-hCG level of 1,500 to 3,000 mIU per mL (1,500 to 3,000 IU per L) and 33% for an initial β-hCG level greater than 3,000 mIU per mL. 15 A slower-than-expected rate of increase or a decrease in β-hCG levels suggests early pregnancy loss or ectopic pregnancy. The rate of increase slows as pregnancy progresses and typically plateaus around 100,000 mIU per mL (100,000 IU per L) at 10 weeks' gestation. 16 A decrease in β-hCG of at least 21% over 48 hours suggests a likely failed intrauterine pregnancy, whereas a smaller decrease should raise concern for ectopic pregnancy. 17
The discriminatory level is the β-hCG level above which an intrauterine pregnancy is expected to be seen on transvaginal ultrasonography; it varies with the type of ultrasound machine used, the sonographer, and the number of gestations. A combination of β-hCG level greater than the discriminatory level and ultrasonography that does not show an intrauterine pregnancy should raise concern for early pregnancy loss or an ectopic pregnancy. 5 The discriminatory zone was previously defined as a β-hCG level of 1,000 to 2,000 mIU per mL (1,000 to 2,000 IU per L); however, this cutoff can miss some intrauterine pregnancies that do not become apparent until a slightly higher β-hCG level is achieved. Therefore, in a desired pregnancy, it is recommended that a discriminatory level as high as 3,500 mIU per mL (3,500 IU per L) be used to avoid misdiagnosis and interruption of a viable pregnancy, although most pregnancies will be visualized by the time the β-hCG level reaches 1,500 mIU per mL. 18 , 19
TRANSVAGINAL ULTRASONOGRAPHY
Intrauterine pregnancy visualized on transvaginal ultrasonography essentially rules out ectopic pregnancy except in the exceedingly rare case of heterotopic pregnancy. 5 The definitive diagnosis of ectopic pregnancy can be made with ultrasonography when a yolk sac and/or embryo is seen in the adnexa; however, ultrasonography alone is rarely used to diagnose ectopic pregnancy because most do not progress to this stage. 5 More often, the patient history is combined with serial quantitative β-hCG levels, sequential ultrasonography, and, at times, uterine aspiration to arrive at a final diagnosis of ectopic pregnancy.
PREGNANCY OF UNKNOWN LOCATION
Ultrasonography showing neither intrauterine nor ectopic pregnancy in a patient with a positive pregnancy test is referred to as a pregnancy of unknown location. In a desired pregnancy, β-hCG levels and serial ultrasonography combined with patient reports of pain or bleeding guide management. 20 In an undesired pregnancy or when the possibility of a viable intrauterine pregnancy has been excluded, manual vacuum aspiration of the uterus can evaluate for chorionic villi that differentiate intrauterine pregnancy loss from ectopic pregnancy. If chorionic villi are seen, further workup is unnecessary, and exposure to methotrexate can be avoided ( Figure 1 ) . 5 , 15 – 17 , 21 If chorionic villi are not seen after uterine aspiration, it is imperative to initiate treatment for ectopic pregnancy or repeat β-hCG measurement in 24 hours to ensure at least a 50% decrease. Ectopic precautions and serial β-hCG levels should be continued until the level is undetectable.
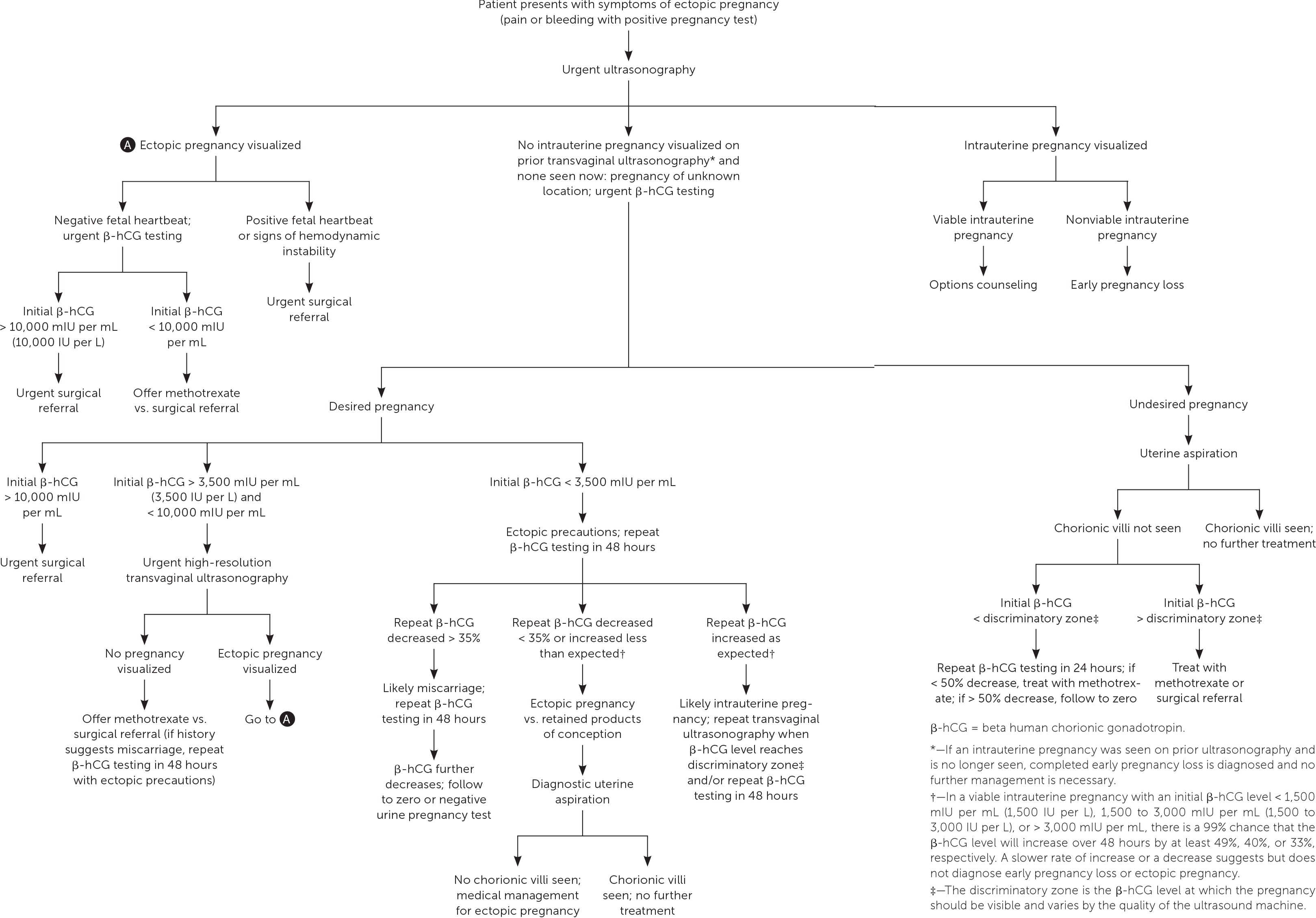
Management of Ectopic Pregnancy
It is appropriate for family physicians to treat hemodynamically stable patients in conjunction with their primary obstetrician. Patients with suspected or confirmed ectopic pregnancy who exhibit signs and symptoms of ruptured ectopic pregnancy should be emergently transferred for surgical intervention. If ectopic pregnancy has been diagnosed, the patient is deemed clinically stable, and the affected fallopian tube has not ruptured, treatment options include medical management with intramuscular methotrexate or surgical management with salpingostomy (removal of the ectopic pregnancy while leaving the fallopian tube in place) or salpingectomy (removal of part or all of the affected fallopian tube). The decision to manage the ectopic pregnancy medically or surgically should be informed by individual patient factors and preferences, clinical findings, ultrasound findings, and β-hCG levels. 12 Expectant management is rare but can be considered with close follow-up for patients with suspected ectopic pregnancy who are asymptomatic and have β-hCG levels that are very low and continue to decrease. 5
MEDICAL MANAGEMENT
Intramuscular methotrexate is the only medication appropriate for the management of ectopic pregnancy. A folate antagonist, it interrupts the rapidly dividing cells of the ectopic pregnancy, which are then resorbed by the body. 22 Its success rate decreases with higher initial β-hCG levels ( Table 3 ) . 23 Contraindications to methotrexate include renal insufficiency; moderate to severe anemia, leukopenia, or thrombocytopenia; liver disease or alcoholism; active peptic ulcer disease; and breastfeeding. 5 Therefore, a complete blood count and comprehensive metabolic panel should be obtained before it is administered.
Several methotrexate regimens have been studied, including a single-dose protocol, a two-dose protocol, and a multi-dose protocol ( Table 4 ) . 5 The single-dose protocol carries the lowest risk of adverse effects, whereas the two-dose protocol is more effective than the single-dose protocol in patients with higher initial β-hCG levels. 24 There is no consistent evidence or consensus regarding the cutoff above which a two-dose protocol should be used, so clinicians should choose a regimen based on the initial β-hCG level and ultrasound findings, as well as patient preference regarding effectiveness vs. the risk of adverse effects. In general, the single-dose protocol should be used in patients with β-hCG levels less than 3,600 mIU per mL (3,600 IU per L), and the two-dose protocol should be considered for patients with higher initial β-hCG levels, especially those with levels greater than 5,000 mIU per mL. Multidose protocols carry a higher risk of adverse effects and are not preferred. 25
Before administering methotrexate, β-hCG levels should be measured on days 1, 4, and 7 of treatment. The first measurement helps the clinician decide between the one- and two-dose protocols. Levels commonly increase between days 1 and 4, but should decrease by at least 15% between days 4 and 7. If this decrease does not occur, the clinician should discuss with the patient whether she prefers to repeat the course of methotrexate or pursue surgical treatment. If the β-hCG level does decrease by at least 15% between days 4 and 7, the patient should return for weekly β-hCG measurements until levels become undetectable, which can take up to eight weeks. 26
Close follow-up is critical for the safe use of methotrexate in women with ectopic pregnancies. Patients should be counseled that the risk of rupture persists until β-hCG levels are undetectable, and that they should seek emergency care if signs of ectopic pregnancy occur. It is common for patients to experience some abdominal pain two to three days after administration of methotrexate. This pain can be managed expectantly as long as there are no signs of rupture. 5 Gastrointestinal adverse effects (e.g., abdominal pain, vomiting, nausea) and vaginal spotting are common. Patients should be counseled to avoid taking folic acid supplements and nonsteroidal anti-inflammatory drugs, which can decrease the effectiveness of methotrexate, and to avoid anything that may mask the symptoms of ruptured ectopic pregnancy (e.g., narcotic analgesics, alcohol) and activities that increase the risk of rupture (e.g., vaginal intercourse, vigorous exercise). Sunlight exposure during treatment can cause methotrexate dermatitis and should be avoided. 5 Other adverse effects of methotrexate include alopecia and elevation of liver enzymes. Patients should be counseled to avoid repeat pregnancy until at least one ovulatory cycle after the serum β-hCG level becomes undetectable, although some experts recommend waiting three months so that the methotrexate can be cleared completely. 27 There is no evidence that methotrexate therapy affects future fertility. 28
SURGICAL MANAGEMENT
Overall, surgical management has a higher success rate for ectopic pregnancy than methotrexate. 5 The initial β-hCG level at which to transfer a patient for possible surgical treatment depends on local standards, although a level of 5,000 mIU per mL (5,000 IU per L) is commonly used. 5 , 11 Ultrasound visualization of an embryo with fetal cardiac activity outside of the uterus is an indication for urgent transfer for surgical management. 5 , 25 Additionally, social factors that preclude frequent laboratory testing (e.g., poor telephone access, work and family obligations, lack of transportation) can make surgical management the safer option 5 ( Table 5 5 , 11 ) . In cases where methotrexate is contraindicated or not preferred by the patient, surgical management can usually be performed laparoscopically if the patient is hemodynamically stable. Surgical options include salpingostomy or salpingectomy. Randomized trials have shown no difference in sequelae between methotrexate administration and fallopian tube–sparing laparoscopic surgery, including rates of future intrauterine pregnancy and risk of future ectopic pregnancy. 29 The decision whether to remove the fallopian tube or leave it in place depends on the extent of damage to the tube (evaluated intraoperatively) and the patient's desire for future fertility.
EXPECTANT MANAGEMENT
Expectant management can be considered for patients whose peak β-hCG level is below the discriminatory zone and is decreasing, but has plateaued or is decreasing more slowly than expected for a failed intrauterine pregnancy. 30 In cases where the initial β-hCG level is 200 mIU per mL (200 IU per L) or less, 88% of patients will have successful spontaneous resolution of the pregnancy; however, rates of spontaneous resolution decrease with higher β-hCG levels. 31 Patient counseling must include the risks of spontaneous rupture, hemorrhage, and need for emergency surgery. Patients who choose expectant management should have β-hCG levels monitored every 48 hours, and medical or surgical management should be recommended if β-hCG levels do not decrease sufficiently. 5
This article updates a previous article on this topic by Barash, et al. 12
Data Sources: An evidence summary from Essential Evidence Plus was reviewed and relevant studies referenced. Additionally, a PubMed search was completed in Clinical Queries using the key terms ectopic pregnancy, first trimester bleeding, and pregnancy of unknown location. The search included meta-analyses, guidelines, and reviews. Also searched were the Cochrane database, DynaMed, and the National Guideline Clearinghouse. Search dates: October 26, 2018, through January 14, 2020.
Creanga AA, Shapiro-Mendoza CK, Bish CL, et al. Trends in ectopic pregnancy mortality in the United States: 1980–2007. Obstet Gynecol. 2011;117(4):837-843.
Marion LL, Meeks GR. Ectopic pregnancy: history, incidence, epidemiology, and risk factors. Clin Obstet Gynecol. 2012;55(2):376-386.
Creanga AA, Syverson C, Seed K, et al. Pregnancy-related mortality in the United States, 2011–2013. Obstet Gynecol. 2017;130(2):366-373.
Ankum WM, Mol BW, Van der Veen F, et al. Risk factors for ectopic pregnancy: a meta-analysis. Fertil Steril. 1996;65(6):1093-1099.
ACOG practice bulletin no. 193: tubal ectopic pregnancy [published correction appears in Obstet Gynecol . 2019;133(5):1059]. Obstet Gynecol. 2018;131(3):e91-e103.
Barnhart KT, Sammel MD, Gracia CR, et al. Risk factors for ectopic pregnancy in women with symptomatic first-trimester pregnancies. Fertil Steril. 2006;86(1):36-43.
Backman T, Rauramo I, Huhtala S, et al. Pregnancy during the use of levonorgestrel intrauterine system. Am J Obstet Gynecol. 2004;190(1):50-54.
Hardeman J, Weiss BD. HardemanJWeissBDIntrauterine devices: an update. Am Fam Physician2014;89(6):445–450. Accessed November 9, 2019. https://www.ncbi.nlm.nih.gov/pubmed/24695563?dopt=Abstract
Bosco-Lévy P, Gouverneur A, Langlade C, et al. Safety of levonorgestrel 52 mg intrauterine system compared to copper intrauterine device: a population-based cohort study. Contraception. 2019;99(6):345-349.
Crochet JR, Bastian LA, Chireau MV. Does this woman have an ectopic pregnancy?: the rational clinical examination systematic review. JAMA. 2013;309(16):1722-1729.
Newbatt E, Beckles Z, Ullman R, et al.; Guideline Development Group. Ectopic pregnancy and miscarriage: summary of NICE guidance. BMJ. 2012;345:e8136.
Barash JH, Buchanan EM, Hillson C. BarashJHBuchananEMHillsonCDiagnosis and management of ectopic pregnancy. Am Fam Physician2014;90(1):34–40. Accessed November 9, 2019. https://www.aafp.org/afp/2014/0701/p34.html
Ramakrishnan K, Scheid DC. Ectopic pregnancy: forget the “classic presentation” if you want to catch it sooner. J Fam Pract. 2006;55(5):388-395.
Stewart BK, Nazar-Stewart V, Toivola B. Biochemical discrimination of pathologic pregnancy from early, normal intrauterine gestation in symptomatic patients. Am J Clin Pathol. 1995;103(4):386-390.
Barnhart KT, Guo W, Cary MS, et al. Differences in serum human chorionic gonadotropin rise in early pregnancy by race and value at presentation. Obstet Gynecol. 2016;128(3):504-511.
Barnhart KT, Sammel MD, Rinaudo PF, et al. Symptomatic patients with an early viable intrauterine pregnancy: HCG curves redefined. Obstet Gynecol. 2004;104(1):50-55.
Barnhart K, Sammel MD, Chung K, et al. Decline of serum human chorionic gonadotropin and spontaneous complete abortion: defining the normal curve. Obstet Gynecol. 2004;104(5 pt 1):975-981.
Doubilet PM, Benson CB, Bourne T, et al.; Society of Radiologists in Ultrasound Multispecialty Panel on Early First Trimester Diagnosis of Miscarriage and Exclusion of a Viable Intrauterine Pregnancy. Diagnostic criteria for nonviable pregnancy early in the first trimester. N Engl J Med. 2013;369(15):1443-1451.
Connolly A, Ryan DH, Stuebe AM, et al. Reevaluation of discriminatory and threshold levels for serum β-hCG in early pregnancy. Obstet Gynecol. 2013;121(1):65-70.
Rodgers SK, Chang C, DeBardeleben JT, et al. Normal and abnormal US findings in early first-trimester pregnancy: review of the Society of Radiologists in Ultrasound 2012 consensus panel recommendations. Radiographics. 2015;35(7):2135-2148.
Reproductive Health Access Project. Diagnosis and treatment of ectopic pregnancy algorithm. June 2019. Accessed June 29, 2019. https://www.reproductiveaccess.org/resource/ectopic-algorithm
Stika CS. Methotrexate: the pharmacology behind medical treatment for ectopic pregnancy. Clin Obstet Gynecol. 2012;55(2):433-439.
Menon S, Colins J, Barnhart KT. Establishing a human chorionic gonadotropin cutoff to guide methotrexate treatment of ectopic pregnancy: a systematic review. Fertil Steril. 2007;87(3):481-484.
Yang C, Cai J, Geng Y, et al. Multiple-dose and double-dose versus single-dose administration of methotrexate for the treatment of ectopic pregnancy: a systematic review and meta-analysis. Reprod Biomed Online. 2017;34(4):383-391.
Practice Committee of American Society for Reproductive Medicine. Medical treatment of ectopic pregnancy: a committee opinion. Fertil Steril. 2013;100(3):638-644.
Barnhart KT, Gosman G, Ashby R, et al. The medical management of ectopic pregnancy: a meta-analysis comparing “single dose” and “multidose” regimens. Obstet Gynecol. 2003;101(4):778-784.
Hospira. Methotrexate injection, USP [package insert]. October 2011. Accessed November 9, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/011719s117lbl.pdf
Ohannessian A, Loundou A, Courbière B, et al. Ovarian responsiveness in women receiving fertility treatment after methotrexate for ectopic pregnancy: a systematic review and meta-analysis. Hum Reprod. 2014;29(9):1949-1956.
Hajenius PJ, Mol F, Mol BW, et al. Interventions for tubal ectopic pregnancy. Cochrane Database Syst Rev. 2007(1):CD000324.
van Mello NM, Mol F, Verhoeve HR, et al. Methotrexate or expectant management in women with an ectopic pregnancy or pregnancy of unknown location and low serum hCG concentrations? A randomized comparison. Hum Reprod. 2013;28(1):60-67.
Korhonen J, Stenman UH, Ylöstalo P. Serum human chorionic gonadotropin dynamics during spontaneous resolution of ectopic pregnancy. Fertil Steril. 1994;61(4):632-636.
Continue Reading

More in AFP
More in pubmed.
Copyright © 2020 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PMC10602312

Global burden and trends of ectopic pregnancy: An observational trend study from 1990 to 2019
Shufei Zhang
Department of Obstetrics and Gynecology, Renmin Hospital of Wuhan University, Wuhan, Hubei Province, P.R.C
Jianfeng Liu
Jianming tang, associated data.
All relevant data are within the manuscript and its Supporting Information files.
Ectopic pregnancy (EP) is one of the leading causes of death in women in early pregnancy, and the mortality of EP have gradually decreased over time in developed countries such as the United Kingdom and the United States. However, epidemiological information on EP has been lacking in recent years, so we analyzed EP data over a thirty-year period from 1990–2019 with the help of Global Burden of Disease study (GBD) data to fill this gap.
According to the EP data in GBD for the three decades from 1990 to 2019, we used estimated annual percentage changes (EAPC) to assess the trend of age-standardized incidence rate (ASIR), age-standardized death rate (ASDR) and age-standardized disability adjusted life years (AS-DALYs) trends in EP and to explore the correlation between socio-demographic index (SDI) stratification, age stratification and EP.
Global ASIR, ASDR, AS-DALYs for EP in 2019 are 170.33/100,000 persons (95% UI: 133.18 to 218.49), 0.16/100,000 persons (95% UI, 0.14 to 0.19) and 9.69/100,000 persons (95% UI, 8.27 to 11.31), respectively. At the overall level, ASDR is significantly negatively correlated with SDI values (R = -0.699, p < 0.001). Besides that, ASDR and AS-DALYs have basically the same pattern. In addition, iron deficiency is one of the risk factors for EP.
Conclusions
In the past three decades, the morbidity, mortality and disease burden of EP have gradually decreased. It is noteworthy that some economically disadvantaged areas are still experiencing an increase in all indicators, therefore, it is more important to strengthen the protection of women from ethnic minorities and low-income groups.
1. Introduction
Ectopic pregnancy (EP) is the implantation of the gestational sac outside the uterine cavity and its typical clinical manifestations are menopause, abdominal pain, and vaginal bleeding. And EP is a significant cause of maternal morbidity and unexpected death worldwide [ 1 , 2 ]. Tubal infection resulting from upper genital tract infection is a major cause of EP, and infectious agents like Mycoplasma genitalium and Chlamydia trachomatis are important risk factors.
Developed countries with well-established healthcare systems have relatively reliable epidemiological data. Previous studies have shown an increase in the incidence of EP in countries like the United States, with the rate rising from 4.5 to 9.4 cases per 1000 reported pregnancies between 1970 and 1978 [ 3 ]. By 1989, there was a fourfold increase compared to 1970 [ 4 ]. Not only this, but other developed countries such as Canada, New Zealand, and the United Kingdom have shown similar increasing trends in the incidence of EP [ 5 – 7 ]. On the contrary, developing countries, particularly in Africa, have limited epidemiological data on EP, and only a few early studies indicate an increasing trend in EP incidence [ 8 ]. A study conducted in China showed that the prevalence of EP was around 2.5% in 2004 and exhibited an overall decreasing trend from 2011 to 2020. However, a change in fertility policy in 2015 resulted in an increase in the proportion of EP among individuals aged 35 years and older [ 9 ]. It is worrisome that underdeveloped countries lack comprehensive epidemiological data on EP due to poor medical and economic conditions. Consequently, assessing the prevalence of risk factors such as Chlamydia trachomatis infection and pelvic inflammatory disease is the only way to speculate, making the EP situation in underdeveloped countries less optimistic [ 10 ].
Despite a significant decrease in EP mortality in countries like the United Kingdom and the United States at the end of the last century, it remains high in developing countries [ 1 ]. The incidence of EP continues to rise, resulting in a substantial disease burden. However, global epidemiological studies of EP are still lacking.
Explore results from the 2019 Global Burden of Disease study (GBD 2019), which published epidemiological data related to 369 diseases/injuries and 286 causes of death, covered EP in 204 countries and territories from 1990 to 2019 [ 11 ]. In this context, we analyzed the GBD data from 1990 to 2019, examining various aspects such as incidence, mortality, and risk factors associated with EP, aiming to support the management of EP patients worldwide and inform public health policy development.
2. Materials and methods
2.1 data acquisition.
In GBD, EP is defined as pregnancy occurring outside of the uterus. ( https://www.healthdata.org/results/gbd_summaries/2019/ectopic-pregnancy-level-4-cause ). Study data was obtained from GBD 2019 was modeled by the Institute for Health Metrics and Evaluation (IHME) [ 12 ].
The Socio-demographic Index (SDI) is a composite indicator of development status strongly correlated with health outcomes. It is the geometric mean of 0 to 1 indices of total fertility rate under the age of 25, mean education for those ages 15 and older and lag distributed income per capita. As a composite, a location with an SDI of 0 would have a theoretical minimum level of development relevant to health, while a location with an SDI of 1 would have a theoretical maximum level [ 13 ] ( https://ghdx.healthdata.org/gbd-2019 ). SDI data was obtained from Global Health Data Exchange (GHDx).
2.2 Statistical analysis
To evaluate trends in incidence rate, death rate, and burden of EP, we calculated relevant assessment indicators, namely annual age-standardized incidence rate (ASIR), age-standardized mortality rate (ASDR), and age-standardized DALYs rate (AS-DALYs). Not only that, we used annual percentage change (EAPC) to accurately evaluate the trend of ASR [ 14 ].
The EAPC is calculated by fitting the linear regression line: Y = α + βx + ε, where y represents ln(ASR) and x refers to the calendar year. The value of EAPC equals 100 × (exp(β) − 1) and its 95% CI is attainable in the regression model [ 15 , 16 ]. And the ASR was obtained as follows:
In the i th age subgroup, ai is represented as age class. wi denotes the number of persons (or weight), where i is equal to the selected reference standard population [ 17 ]. when both the EAPC value and its 95% CI >0, we consider its ASR to be on an upward trend; when both the EAPC value and 95% CI <0, we consider its ASR to be on an downward trend; In other cases, we consider the ASR to be stable [ 18 ]. We use Pearson’s correlation coefficient (R) to represent the strength of the correlation, and all analyses and data visualization are done in R software (version 4.2.1, http://www.r-project.org/ , Auckland, New Zealand).
3.1 Distribution and trends in the incidence rate of EP
At the global level, there were 6.7 million (95% UI: 5.2 to 8.6) incident cases of EP in 2019, with an ASIR of 170.33/100,000 persons (95% UI: 133.18 to 218.49). The number of cases in 2019 was 0.1% lower than in 1990 (95% UI: -0.16 to -0.04). It is worth drawing our attention to the fact that only the number of incidence cases in the low SDI region increased by 0.53% (95% UI: 0.48 to 0.58) during these three decades ( Table 1 ). On observation from the GBD regions and countries level, the three countries with the highest ASIR are Niger, Papua New Guinea, and Chad; the three countries with the lowest ASIR are Australia, South Africa, and Poland ( Fig 1A and S1 Table ).

Distribution of (A) ASIR, (B) ASDR, (C) AS-DALYs and (D) EAPC-ASIR in various countries and regions in 2019.
In the global overall level analysis, incidence rates were lower in 2019 than in 1990 for all age stages, with peak incidence rates occurring in the 25–29 age group. incidence rates were significantly higher in women aged 25–34 years, which also appears to be consistent with a sexually active period for women. incidence rates in 2019 were generally lower than 1990 incidence rates, and interestingly, incidence rates in high SDI regions in the 30+ stage exceeded those in 1990, while incidence rates in high-moderate SDI regions exceeded those in 1990 in the 35+ stage. Not only that, the peak prevalence in each region was largely consistent with the global level, with the prevalence peaking at 25–29 years of age in both 1990 and 2019, while the high SDI region showed a pushed-back peak in 2019 ( Fig 2 ).

On observation from the GBD regions and countries level, the three countries with the highest Case changes are Qatar, Afghanistan, and Somalia; the three countries with the lowest Case changes are the Northern Mariana Islands, Albania, and Puerto Rico (S1 Fig). And the three countries with the highest EAPC are Russian Federation, Italy, and Belarus; the three countries with the lowest EAPC are Oman, Nepal, and Kuwait ( Fig 1D ).
3.2 Distribution and trends in the DALYs rate of EP
At the global level, there were 0.34 million (95% UI: 0.30–0.38) DALYs in 1990 and 0.38 million (95% UI: 0.32–0.44) DALYs in 2019. In the past 30 years, the AS-DALYs rate decreased with an EAPC of -0.84% (95%CI: from -0.98 to -0.7), dropping from 12.46/100,000 persons (95% UI, 11.09 to 13.91) in 1990 to 9.69/100,000 persons (95% UI, 8.27 to 11.31) in 2019. Over the past three decades, DALYs in middle SDI to high SDI regions have become lower and maintained the highest EAPC of -4.53 (-4.75 to -4.3) with a clear downward trend, while DALYs in low and low-middle SDI regions have not only not decreased but even slightly increased, not only that, the downward trend of EAPC values is not obvious. Looking at the global regions, DALYs generally decreased, but the four regions in Sub-Saharan Africa had the highest AS-DALYs at 60.76/100,000 (44.5 to 77.37). While the Caribbean had the most pronounced increase at 4.45% (3.07 to 6.12), EAPC also had the most pronounced upward trend at 6.11% (5.22 to 7.02) ( Table 2 ). On observation from the GBD regions and countries level, the three countries with the highest AS-DALYs are Chad, Mauritania, and Senegal; the three countries with the lowest AS-DALYs are Poland, Singapore, Cyprus ( Fig 1B and S2 Table ). The age distribution pattern of DALYs rate in most regions was largely consistent with that of death rate ( S2 and S3 Figs).
3.3 Distribution and trends in the death rate of EP
As we mentioned earlier, EP does not cause serious consequences if detected in time, but rupture of EP is a gynecological emergency that can lead to death in women [ 18 ]. Globally, there were 5749 (95% UI: 5107 to 6435) deaths from EP in 1990 and 6452 (95% UI: 5496 to 7513) deaths from EP in 2019. Over the last 30 years, ASDR has decreased and continues to show a downward trend from 0.22/100,000 (95% UI, 0.19 to 0.24) in 1990 decreasing to 0.16/100,000 (95% UI, 0.14 to 0.19) in 2019, with an EAPC of -0.91% (95% CI: -1.04 to -0.78).
In terms of SDI, lower SDI does seem to lead to higher ASDR, and this difference can even be tens of times higher. 2019 ASDR in low SDI areas was 0.68 (0.56 to 0.82), while ASDR in high SDI areas was only 0.01 (0.01 to 0.01), and EAPC was higher at -2.95% (-3.13 to– 2.77), suggesting a more pronounced downward trend in high SDI regions along with a decrease in mortality ( Table 3 ).
Looking at the GBD region and country level, the three countries with the highest ASDR were Mauritania, Chad, and Senegal; earlier detection of EP did not result in death, so the lowest countries were not listed ( Fig 1C and S3 Table ). Unlike acute diseases such as acute myocardial infarction, EP has a relatively low lethality rate. Globally, mortality from EP has declined after three decades compared with 1990, but the pattern of its age distribution is essentially the same as before ( S2 Fig ). By combining morbidity and mortality data, we performed a cluster analysis at the national and regional levels to find countries with similar annual increases. Based on the results of the cluster analysis, 59 countries (or regions) were classified in the "Significant increase" group, including the Netherlands, China, the United Kingdom, and Germany. 90 countries (or regions) were classified in the "Minor increase" group, including Thailand, Mexico, Egypt, and the Republic of Korea. 24 countries (or regions) were classified in the "Stable or minor decrease" group, including Canada, Cuba, Niger, and Chad. The remaining 39 countries (or regions) are classified in the "Significant decrease" group, including Oman, Sudan, Libya, and Yemen ( Fig 3 ).

3.4 Correlation analysis of EP related ASIR, ASDR, AS-DALYs and different SDI
In 2019, a significant association was detected between EAPC and ASIR, (ρ = 0.34, p < 0.001), and similarly, a significant association was observed between EAPC and ASDR, (ρ = 0.29, p < 0.001) ( Fig 4A ). We can observe the ASIR and its expected levels for different SDI regions and countries. High-income North America, High-income Asia Pacific, and Australasia closely followed expected trends over the study period. However, in many other regions, the actual situation is far from the forecast curve, for example, the level in regions such as Oceania and Southern Latin America is much higher than the expected level, however, the level in regions such as Tropical Latin America and Southern Sub-Saharan Africa is much lower than the expected level. The ASIR in most regions gradually declines smoothly along with the rise in SDI, but there are some regions where the ASIR fluctuates sharply, such as Central Asia and Eastern Europe ( Fig 4B ).

Correlation analysis. (A) The correlation between EAPC and ASIR/ASDR in 2019. (B) ASIR for EP for different regions and (C) countries and territories by SDI, 1990–2019.
At the overall level, ASDR is significantly negatively correlated with SDI values (R = -0.699, p < 0.001). Most of the high SDI regions have limited their ASDR to a very low level, close to zero, but almost all sub-Saharan Africa regions have higher than expected ASDR values. The case of AS-DALYs is basically a replica of the above ( S4A and S4C Fig ).
In 2019, there was an inverse relationship between the ASIR of EP and SDI at the national level, with some exceptions ( Fig 4C ). A similar pattern was observed in the relationship between ASDR and AS-DALYs in relationship to SDI. We can find that some countries are much higher than expected levels, such as Russian Federation, Austria, and Papua New Guinea, while countries at lower SDI, such as Somalia, Chad, and Niger, generally have higher incidence rate, death rate and AS-DALYs, which is consistent with our findings mentioned above ( S4B and S4D Fig ).
3.5 EP-related risk factors
According to the GBD 2019, we only found one risk factor associated with EP, iron deficiency. Iron deficiency is classified as " Child and maternal malnutrition " and " Behavioral risks ". Globally, 21.9% of deaths and DALYs in patients with EP can be attributed to iron deficiency. Among the five SDI tiers, only the low SDI regions exceeded the global average for the proportion attributed to iron deficiency, reaching 22.8% and 22.9% for the proportion attributed to deaths and DALYs. We can also observe a gradual decrease in the contribution of iron deficiency to deaths and DALYs from low SDI regions to high SDI regions until the proportions reach a minimum of 11.1% and 10.8%, respectively. Regionally, the highest proportion of deaths and DALYs due to iron deficiency was found in sub-Saharan Africa and the lowest in Western Europe, which is consistent with our results above. It is worth noting that all Sub-Saharan Africa regions had more than 20% of EP deaths and DALYs attributable to iron deficiency. Only South Asia and Western Saharan Africa exceeded the global average for both indicators ( Fig 5 ).

4. Discussion
EP is a leading cause of maternal morbidity and unexpected death worldwide. The incidence of EP is increasing in developed countries like the United States, while the mortality rate of EP in developing countries continues to rise, leading to a significant disease burden. Previous epidemiological studies have primarily focused on specific countries or regions, lacking a comprehensive global analysis to guide the prevention and treatment of EP [ 19 ]. Therefore, this study utilizes GBD 2019 data to provide a comprehensive overview of global epidemiological trends and patterns of EP over the past three decades. This analysis can contribute to the formulation of health policies and the effective allocation of healthcare resources.
EP is highly preventable and treatable, and with early detection, the chances of successful treatment are high, leading to a low risk of mortality. From a global perspective, there has been a decrease in the ASIR, ASDR, and AS-DALYs of EP in 2019 compared to 1990. This decreasing trend indicates improvements in the prevention and treatment of EP, which can be attributed to advancements in various aspects, including social living conditions. The age structure of EP incidence remains stable, with the highest incidence observed in the 25–29 years age group. Furthermore, the trends of mortality and DALYs rates with age are remarkably similar. This similarity may be due to the fact that DALYs in EP are primarily associated with fatal cases. However, it is surprising to note that both mortality and DALYs peak at an earlier age compared to 1990, suggesting a younger age profile of EP deaths. A survey conducted in Washington State, USA, between 1987 and 2014 revealed a decrease in EP hospitalization rates. However, there was an increase in EP-related mortality/severe morbidity among females aged 25–34 years, which aligns with the findings of our study. This outcome may be influenced by increasing low-risk EP patients receiving outpatient methotrexate therapy [ 20 ].
SDI represents a country or region’s combined educational, economic, and medical levels. In our analysis of EP incidence rates, we observed a significant decline in EP incidence rates in regions with low SDI, low-middle SDI, and middle SDI. The rate of decline in these regions was higher than the global average. However, in regions with higher SDI, the rate of decline was relatively low. We hypothesize that this discrepancy may be because the ASIR in high SDI regions was already lower than the ASIR in low SDI regions in 2019, as early as 1990. Consequently, the potential for further decline was originally smaller, resulting in a less pronounced decreasing trend. Further research and investigation are necessary to better understand the underlying factors contributing to these trends.
Not only did the ASDR and AS-DALYs show varying degrees of decline in different SDI regions, but the correlation analysis also revealed a negative correlation between all three ASR indicators and SDI. Notably, Sub-Saharan African regions had higher than expected values of ASDR and AS-DALYs, indicating a concerning health situation for women in these low SDI regions. Iceland is a highly developed country, belonging to the High SDI countries, and a study showed that there were no maternal deaths from EP in Iceland from 1985–2009, which is inseparable from good living conditions, universal education levels, and a comprehensive national health care system. These factors play crucial roles in preventing and managing EP incidences [ 21 ]. In contrast, low-income groups may face challenges such as lower levels of medical literacy and poorer living and medical conditions. Consequently, these factors could contribute to higher morbidity and mortality rates from EP in low SDI areas compared to high SDI areas [ 22 ].
Interestingly, when we analyze the data by age groups, we observe a delayed peak age of incidence in both High SDI areas and High-Middle SDI areas in 2019. In fact, in some age groups, the incidence rates have even exceeded the rates observed thirty years earlier. This delayed peak age of incidence and the increased incidence may be due to a variety of reasons. Firstly, women in higher SDI areas may have higher levels of education and contraceptive use. This may result in fewer early pregnancies and cases of younger EP, thus delaying the peak incidence of EP [ 23 , 24 ]. Furthermore, it has been suggested that there is a negative correlation between the human development index and fertility rates. Therefore, we can hypothesize that the increased years of education in women may have led to a delay in the age of childbearing, consequently causing a delay in the onset of EP and an increased incidence at certain ages [ 25 ].
The EP situation in underdeveloped countries is concerning. The incidence of EP in underdeveloped countries such as Niger and Chad is still among the highest in the world due to a series of implication effects caused by economic backwardness. In addition, the low level of treatment leads to a high mortality rate of EP, whose DALYs can be hundreds or even thousands of times higher than those in developed countries. Previous research has highlighted significant disparities in the management of EP based on factors such as race, region, and access to reliable health insurance. For instance, some women may delay seeking medical care due to a lack of dependable health insurance, consequently increasing their risk of complications and death related to EP [ 26 ]. Furthermore, it has been observed that minority populations exhibit higher rates of reproductive tract infections, which can contribute to more severe cases of EP [ 22 ]. Addressing these disparities and closing the gap in EP management is challenging. It is influenced by long-standing economic and medical inequalities and limitations associated with factors like educational access and religious beliefs.
Previous studies have identified several common risk factors associated with EP, including reproductive mycoplasma infections, Chlamydia trachomatis infections, pelvic inflammatory disease, and the use of assisted reproductive technology and IUDs [ 27 – 29 ]. However, according to the GBD 2019, iron deficiency has emerged as a significant risk factor for EP. The study revealed that more than 20% of EP deaths and DALYs in sub-Saharan Africa were attributed to iron deficiency. Even in regions classified as having a High SDI, the proportion of EP-related deaths and DALYs associated with iron deficiency exceeded 10%. This finding offers a new direction for EP-related research. Furthermore, research has indicated that serum zinc levels are significantly higher in EP patients, while serum copper levels are lower. The copper/zinc ratio has shown potential as a novel diagnostic tool for EP. However, further basic research is needed to verify whether iron deficiency or other micronutrient deficiencies contribute to the development of EP.
The present study has several notable strengths. Firstly, it provides a comprehensive analysis of EP morbidity, mortality, and DALYs over a period of 30 years (1990–2019), offering a valuable overview of the global burden of EP. Additionally, the study examines the influencing factors associated with EP by comparing its conditions in different SDI and age groups, thereby providing new insights and directions for further research on EP. This study is the first-ever global epidemiological study on EP, making it an important reference for EP management. However, despite these strengths, there are some limitations that should be acknowledged. Firstly, the study relies on data from the GBD estimation, which may introduce uncertainty due to variations in data availability across countries and regions. Moreover, the lack of a comprehensive EP surveillance system in certain low and Middle SDI countries/regions further contributes to potential data limitations. Secondly, the study does not differentiate between subtypes or pathological types of EP in the GBD analysis, hindering a more detailed disease analysis. Furthermore, it is worth noting that only one EP-related risk factor, iron deficiency, was included in the study, while common factors such as Chlamydia trachomatis infections and pelvic inflammatory disease were not identified as significant risk factors. This could be considered an incomplete and potentially biased representation of EP risk factors. However, modifying the availability of such data can be challenging.
5. Conclusion
Over the past 30 years, there has been a gradual decrease in the global morbidity, mortality, and DALYs associated with EP. However, it is crucial to note that these indicators are still increasing in economically disadvantaged regions. This trend may be attributed to various factors such as ethnicity, economic status, and educational levels. It is therefore essential to develop effective public health policies that address these disparities and provide enhanced protection for women belonging to ethnic minorities and low-income groups. Additionally, promoting early diagnosis and treatment of EP should be prioritized to mitigate its impact on these vulnerable populations.
Supporting information
(A) ASDR for EP for different regions and (B) countries and territories by SDI, 1990–2019; (C)AS-DALYs for EP for different regions and (D) countries and territories by SDI, 1990–2019.
Acknowledgments
The authors thank all staff for their contributions to the GBD database.
Funding Statement
This project was funded by The National Key Research and Development Program of China (2021YFC2701300), Hubei Key Research and Development Program (2022BCA045) and The National Natural Science Foundation of China (81971364 & 82001527). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability
- PLoS One. 2023; 18(10): e0291316.
Decision Letter 0
20 Apr 2023
PONE-D-22-31092Global burden and trends of ectopic pregnancy: An observational trend study from 1990 to 2019PLOS ONE
Dear Dr. Hong,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
==============================
ACADEMIC EDITOR: Please insert comments here and delete this placeholder text when finished. Be sure to:
I agree with the reviewer's comment.
Please submit your revised manuscript by Jun 04 2023 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
- A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
- A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
- An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: https://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols . Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols .
We look forward to receiving your revised manuscript.
Kind regards,
Gang Qin, PhD, MD
Academic Editor
Journal Requirements:
When submitting your revision, we need you to address these additional requirements.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
https://journals.plos.org/plosone/s/file?id=wjVg/PLOSOne_formatting_sample_main_body.pdf and
https://journals.plos.org/plosone/s/file?id=ba62/PLOSOne_formatting_sample_title_authors_affiliations.pdf
2. Thank you for stating the following financial disclosure:
"The National Key Research and Development Program of China (2018YFC2002204), Hubei Key Research and Development Program (2022BCA045) and The National Natural Science Foundation of China (81971364 & 82001527)."
Please state what role the funders took in the study. If the funders had no role, please state: "The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript."
If this statement is not correct you must amend it as needed.
Please include this amended Role of Funder statement in your cover letter; we will change the online submission form on your behalf.
3. PLOS requires an ORCID iD for the corresponding author in Editorial Manager on papers submitted after December 6th, 2016. Please ensure that you have an ORCID iD and that it is validated in Editorial Manager. To do this, go to ‘Update my Information’ (in the upper left-hand corner of the main menu), and click on the Fetch/Validate link next to the ORCID field. This will take you to the ORCID site and allow you to create a new iD or authenticate a pre-existing iD in Editorial Manager. Please see the following video for instructions on linking an ORCID iD to your Editorial Manager account: https://www.youtube.com/watch?v=_xcclfuvtxQ
4. Please include captions for your Supporting Information files at the end of your manuscript, and update any in-text citations to match accordingly. Please see our Supporting Information guidelines for more information: http://journals.plos.org/plosone/s/supporting-information .
5. We note that Figure 1 in your submission contain [map/satellite] images which may be copyrighted. All PLOS content is published under the Creative Commons Attribution License (CC BY 4.0), which means that the manuscript, images, and Supporting Information files will be freely available online, and any third party is permitted to access, download, copy, distribute, and use these materials in any way, even commercially, with proper attribution. For these reasons, we cannot publish previously copyrighted maps or satellite images created using proprietary data, such as Google software (Google Maps, Street View, and Earth). For more information, see our copyright guidelines: http://journals.plos.org/plosone/s/licenses-and-copyright .
We require you to either (1) present written permission from the copyright holder to publish these figures specifically under the CC BY 4.0 license, or (2) remove the figures from your submission:
a. You may seek permission from the original copyright holder of Figure 1 to publish the content specifically under the CC BY 4.0 license.
We recommend that you contact the original copyright holder with the Content Permission Form ( http://journals.plos.org/plosone/s/file?id=7c09/content-permission-form.pdf ) and the following text:
“I request permission for the open-access journal PLOS ONE to publish XXX under the Creative Commons Attribution License (CCAL) CC BY 4.0 ( http://creativecommons.org/licenses/by/4.0/ ). Please be aware that this license allows unrestricted use and distribution, even commercially, by third parties. Please reply and provide explicit written permission to publish XXX under a CC BY license and complete the attached form.”
Please upload the completed Content Permission Form or other proof of granted permissions as an "Other" file with your submission.
In the figure caption of the copyrighted figure, please include the following text: “Reprinted from [ref] under a CC BY license, with permission from [name of publisher], original copyright [original copyright year].”
b. If you are unable to obtain permission from the original copyright holder to publish these figures under the CC BY 4.0 license or if the copyright holder’s requirements are incompatible with the CC BY 4.0 license, please either i) remove the figure or ii) supply a replacement figure that complies with the CC BY 4.0 license. Please check copyright information on all replacement figures and update the figure caption with source information. If applicable, please specify in the figure caption text when a figure is similar but not identical to the original image and is therefore for illustrative purposes only.
The following resources for replacing copyrighted map figures may be helpful:
USGS National Map Viewer (public domain): http://viewer.nationalmap.gov/viewer/
The Gateway to Astronaut Photography of Earth (public domain): http://eol.jsc.nasa.gov/sseop/clickmap/
Maps at the CIA (public domain): https://www.cia.gov/library/publications/the-world-factbook/index.html and https://www.cia.gov/library/publications/cia-maps-publications/index.html
NASA Earth Observatory (public domain): http://earthobservatory.nasa.gov/
Landsat: http://landsat.visibleearth.nasa.gov/
USGS EROS (Earth Resources Observatory and Science (EROS) Center) (public domain): http://eros.usgs.gov/#
Natural Earth (public domain): http://www.naturalearthdata.com/
5. Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
Additional Editor Comments:
I agree with the reviewer's comment.
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: Yes
2. Has the statistical analysis been performed appropriately and rigorously?
3. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
4. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
5. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: I appreciate the authors for this intensive research work on the topic “Global burden and trends of ectopic pregnancy: An observational trend study from 1990 to 2019”. The presentation of the data seems strong, and the paper is generally well-written, with an appropriate focus. However, this study may be accepted for publication with the following suggestions and comments for further improvements.
Comment 1: The abstract is written with an appropriate focus.
Introduction
Comment 2: The introduction section is poorly written.
Comment 3: In addition to the current contents of the introduction section, add at least two paragraphs in the social context like.
1. What is the situation of EP in developed, developing, and underdeveloped countries? elaborate.
2. How is it changing over time?
3. Where it is still high from the previous literature.
4. Elaborate if it is declining, then why is your study important?
Material and Methods
Comment 4: Written Properly.
Results and Discussion
Comment 5: Figure resolution is very poor. Please provide high-resolution figures.
Comment 6: Add some discussion for underdeveloped and developing countries’ situations.
Comment 7: The conclusion is written very well.
6. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy .
Reviewer #1: Yes: Mayank Singh
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/ . PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at gro.solp@serugif . Please note that Supporting Information files do not need this step.
Submitted filename: Comments and Suggestions.docx
Author response to Decision Letter 0
22 Apr 2023
Dear Editor and reviewer,
Thank you for your letter and for the reviewers' comments concerning our manuscript entitled " Global burden and trends of ectopic pregnancy: An observational trend study from 1990 to 2019" (PONE-D-22-31092). Those comments are all valuable and very helpful for revising and improving our paper, as well as the important guiding significance to our researches. Images cannot be uploaded in this section of the submission system, so we have also uploaded a word version including the illustrations, which you can see at the end of the PDF.
First, here is our response to the Journal Requirements.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming.
First of all, we would like to thank the journal again for their guidance, and we have reworked the manuscript style as requested by the journal.
2. Thank you for stating the following financial disclosure: "The National Key Research and Development Program of China (2018YFC2002204), Hubei Key Research and Development Program (2022BCA045) and The National Natural Science Foundation of China (81971364 & 82001527)."
Please state what role the funders took in the study. If the funders had no role, please state: "The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript."
The above funds are our funders, but "The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. " And we will explain this again in the cover letter.
We double-checked our account and found that the corresponding author's ORCID iD exists in the system, as shown below.
We have added the title of the supporting information at the end of the manuscript and updated the references in the manuscript accordingly.
Thank you for your reminder that we take copyright issues very seriously, so we immediately went to investigate the source of the map. Since our map was generated by R, we investigated the source of the map in the R package (as shown below) and found that it came from Natural Earth ( http://www.naturalearthdata.com/ ), which is in the public domain and therefore does not require special attribution, but we will make the source of the Figure clear in the manuscript.
6. Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
We have checked the reference list, and we can now be sure that it is correct.
Here is a point-by-point response to the comments and concerns of the reviewers.
Thank you very much for your recognition of our work, and I believe that with your suggestions, our article will be more rigorous and more valuable.
We are very sorry for the poorly written introduction, we have revised the manuscript according to your comments and added the key missing parts you suggested, thank you very much for your pointers.
Your suggestions were meticulous and thoughtful, and we have added to the content of the manuscript.
We are very sorry that it affects the readability of the article. The images we uploaded in the system are very clear, but the image quality is compressed when the system automatically generates PDF files, so you can download the original images we uploaded by clicking the download button on the top right corner of the image (as shown in the picture), thank you very much for your patience.
As you suggested we have added a discussion of underdeveloped and developing countries to the manuscript.
We are very grateful to the editor and reviewers for giving us this opportunity to revise the manuscript. We have tried our best to improve the manuscript, made some changes to the manuscript, and responded to the reviewers' questions one by one. We have uploaded the Manuscript and Revised Manuscript with Track Changes. We appreciate for editor and reviewers' warm work earnestly, and hope that the correction will meet with approval. Once again, thank you very much for your comments and suggestions.
Thank you and best regards.
Yours sincerely,
Corresponding author:
Li Hong, Ph.D.
E-mail: nc.ude.uhw@ilgnoh_rd
Submitted filename: Response to Reviewers.docx
Decision Letter 1
PONE-D-22-31092R1Global burden and trends of ectopic pregnancy: An observational trend study from 1990 to 2019PLOS ONE
ACADEMIC EDITOR: Pls revise according to the reviewer's suggestions.
Please submit your revised manuscript by Aug 17 2023 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at gro.solp@enosolp . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
1. If the authors have adequately addressed your comments raised in a previous round of review and you feel that this manuscript is now acceptable for publication, you may indicate that here to bypass the “Comments to the Author” section, enter your conflict of interest statement in the “Confidential to Editor” section, and submit your "Accept" recommendation.
Reviewer #2: (No Response)
2. Is the manuscript technically sound, and do the data support the conclusions?
Reviewer #2: Partly
3. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #2: No
4. Have the authors made all data underlying the findings in their manuscript fully available?
Reviewer #2: Yes
5. Is the manuscript presented in an intelligible fashion and written in standard English?
6. Review Comments to the Author
Reviewer #2: Introduction:
1. The introduction section is poorly written
2. There is no need to discuss diagnosis and treatment in the introduction
1. Study data was obtained from The Global Burden of Disease Study 2019 was modeled by by the Institute for Health Metrics and Evaluation (IHME). SDI data obtained from Global Health Data Exchange (GHDx)
2. Global Burden of Disease Study 2019 should be introduced in details
3. SDI classes should be explained
4. How is EP defined in GED protocols?
5. Age-standardized incidence rate, mortality rate and DALY rate were defined in GBD, why haven't researchers used them and recalculated?
6. “EAPCs were calculated using a linear regression model as follows: ln (ASR) = α + β x + ε,” is incorrect
7. R language version 4.2.1 is incorrect, R software is correct
1. 3.1 Distribution and trends in the incidence rate of EP by age or year???
2. Figures resolution is very poor. Please provide high-resolution figures
3. Figure 2 is unclear
4. the 0-14 and e 55+ years age groups should be excluded
5. the results of EP-related risk factors not well presented
1. discussion section is weak
2. advantage and limitation were missed
7. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
Reviewer #2: Yes: Fatemeh khosravi Shadmani
Author response to Decision Letter 1
17 Jul 2023
Dear Editors and Reviewers,
Thank you for your letter and for the reviewers' comments concerning our manuscript entitled " Global burden and trends of ectopic pregnancy: An observational trend study from 1990 to 2019" (PONE-D-22-31092R1). Those comments are all valuable and very helpful for revising and improving our paper, as well as the important guiding significance to our research.
1. Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
Thank you for your patience in reviewing the manuscript, we have checked all the references to ensure that none of them have been withdrawn, and although we have changed some references, we can be sure that the reference list is correct.
Here are our responses to the reviewers' general comments
We truly appreciate your suggestions on our manuscript and are very sorry that the article's readability has been reduced due to our writing, so we have tried our best to polish the language in the revised manuscript. As for the statistical issues, we have also made changes and deletions based on your suggestions. We hope the revised manuscript could be acceptable to you.
Comments on the introduction:
We sincerely apologize for our poor writing, and we have carefully and completely rewritten the Introduction section of the manuscript.
Thank you very much for your suggestions, we couldn't agree with you more, so we have removed the relevant content and have carefully rewritten this section, and we hope that these changes will fulfill your requirements for the manuscript.
Comments on the Methods:
1. Study data was obtained from The Global Burden of Disease Study 2019 was modeled by the Institute for Health Metrics and Evaluation (IHME). SDI data obtained from Global Health Data Exchange (GHDx)
First of all, thank you very much for your suggestions, we have added this important information to the revised manuscript.
Line 62-64, “Study data was obtained from GBD 2019 was modeled by the Institute for Health Metrics and Evaluation (IHME)” was added.
Line 70-71, “SDI data was obtained from Global Health Data Exchange (GHDx)” was added.
We are very sorry for our omission of this important section, so we have added an introduction to the Global Burden of Disease Study 2019 in the revised manuscript.
Line 53-55, “Explore results from the 2019 Global Burden of Disease study (GBD 2019), which published epidemiological data related to 369 diseases/injuries and 286 causes of death, covered EP in 204 countries and territories from 1990 to 2019” was added.
References:
http://doi.org/10.1016/j.ajp.2023.103677
We are very sorry that our description of SDI was not clear, so we have made it explicit in the revised manuscript.
Line 65-69, “The Socio-demographic Index (SDI) is a composite indicator of development status strongly correlated with health outcomes. It is the geometric mean of 0 to 1 indices of total fertility rate under the age of 25, mean education for those ages 15 and older and lag distributed income per capita. As a composite, a location with an SDI of 0 would have a theoretical minimum level of development relevant to health, while a location with an SDI of 1 would have a theoretical maximum level” was added.
https://ghdx.healthdata.org/sites/default/files/record-attached-files/IHME_GBD_2019_SDI_1950_2019_INFO_SHEET_Y2021M08D16.PDF
4. How is EP defined in GBD protocols?
Thank you very much for your suggestion regarding the definition of ectopic pregnancy, which is a very important issue. We have managed to find the GBD definition of ectopic pregnancy after a careful search of the sources and have added it to the revised manuscript.
Line 61, “In GBD, EP is defined as pregnancy occurring outside of the uterus.” was added.
https://www.healthdata.org/results/gbd_summaries/2019/ectopic-pregnancy-level-4-cause
Thank you very much for your suggestion, we have calculated the Age-standardized incidence rate, mortality rate, and DALY rate in the table and abbreviated them as ASIR, ASDR, and AS-DALYs in the Method section and have used them for calculation and visualization in the results.
Thank you for your correction, we have corrected it in the revised manuscript.
Line 77-78, “EAPCs were calculated using a linear regression model as follows: ln (ASR) = α + β x + ε” was corrected as “The EAPC is calculated by fitting the linear regression line: Y = α + βx + ε, where y represents ln(ASR) and x refers to the calendar year.”.
Thanks again for the correction; we have also corrected it in the revised manuscript.
Line 87, “R language” was corrected as “R software”.
Comments on the Results:
Thank you very much for your patience, we have corrected several titles that did not make sense in the revised manuscript.
Line 91, “3.1 Distribution and trends in the incidence rate of EP by age or year” was corrected as “3.1 Distribution and trends in the incidence rate of EP”.
Line 115, “3.2 Distribution and trends in the DALYs rate of EP by age or year” was corrected as “3.2 Distribution and trends in the DALYs rate of EP”.
Line 133, “3.3 Distribution and trends in the Death rate of EP by age or year” was corrected as “3.3 Distribution and trends in the Death rate of EP”.
We are very sorry that the unclear figures have caused problems for your review, we have remade and uploaded all the figures and ensured their clarity. However, the journal requires that figures should not exceed 10M in size, so there may be some figures with crowded and unclear details.
Thanks for your suggestion, we have recreated and uploaded a clear figure 2.
We acknowledge that it may be unreasonable to discuss ectopic pregnancies in women under 14 years old or over 55 years old, therefore, we excluded cases in these two age groups and re-visualized them, as shown in Figure 2, S4 Figure, and S5 Figure.
Thank you for your suggestion and we recognize the limitations of this study in terms of the risk factors associated with ectopic pregnancy. This is because GBD 2019 contains only one risk factor for ectopic pregnancy, iron deficiency, and therefore we have only explored it in the manuscript. We have also rewritten this section in the revised manuscript, and we hope that these changes will help the reader better understand the contents of the manuscript.
Comments on the discussion:
Thank you very much for your patient suggestions, we have carefully rewritten the discussion section of the manuscript. The revised manuscript provides a comprehensive analysis of the condition of ectopic pregnancy on several levels.
We recognize that an exploration of the advantages and limitations of this study was missing from our manuscript, so in the revised manuscript, we have added this section. Thanks to your suggestions, this makes our manuscript better.
Line 271-286, “The present study has several notable strengths. Firstly, it provides a comprehensive analysis of EP morbidity, mortality, and DALYs over a period of 30 years (1990-2019), offering a valuable overview of the global burden of EP. Additionally, the study examines the influencing factors associated with EP by comparing its conditions in different SDI and age groups, thereby providing new insights and directions for further research on EP. This study is the first-ever global epidemiological study on EP, making it an important reference for EP management. However, despite these strengths, there are some limitations that should be acknowledged. Firstly, the study relies on data from the GBD estimation, which may introduce uncertainty due to variations in data availability across countries and regions. Moreover, the lack of a comprehensive EP surveillance system in certain low and Middle SDI countries/regions further contributes to potential data limitations. Secondly, the study does not differentiate between subtypes or pathological types of EP in the GBD analysis, hindering a more detailed disease analysis. Furthermore, it is worth noting that only one EP-related risk factor, iron deficiency, was included in the study, while common factors such as Chlamydia trachomatis infections and pelvic inflammatory disease were not identified as significant risk factors. This could be considered an incomplete and potentially biased representation of EP risk factors. However, modifying the availability of such data can be challenging.” was added.
We are very grateful to the editor and reviewers for giving us this opportunity to revise the manuscript. We have tried our best to improve the manuscript, made some changes to the manuscript, and responded to the reviewers' questions one by one. We have uploaded the Manuscript and Revised Manuscript with Track Changes. We appreciate for editor and reviewers' warm work earnestly and hope that the correction will meet with approval. Once again, thank you very much for your comments and suggestions.
Decision Letter 2
29 Aug 2023
PONE-D-22-31092R2
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/ , click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at gro.solp@gnillibrohtua .
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact gro.solp@sserpeno .
Additional Editor Comments (optional):
Acceptance letter
31 Aug 2023
Dear Dr. Hong:
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now with our production department.
If your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information please contact gro.solp@sserpeno .
If we can help with anything else, please email us at gro.solp@enosolp .
Thank you for submitting your work to PLOS ONE and supporting open access.
PLOS ONE Editorial Office Staff
on behalf of
Dr. Gang Qin

IMAGES
VIDEO
COMMENTS
Ectopic pregnancy is a pregnancy that occurs outside the uterus, most commonly in the fallopian tube. It is usually suspected if a pregnant woman experiences any of these symptoms during the first trimester: vaginal bleeding, lower abdominal pain, and amenorrhea. An elevated BhCG level above the discriminatory zone (2000 mIU/ml) with an empty ...
In a case-control study by Li at al, the risk of ectopic pregnancy was approximately four-fold higher for current oral contraceptive users and more than 20-fold higher in current IUD users compared to women currently not using contraception.7 Additionally, IUD use increases the risk that an ectopic pregnancy will implant at a more distal site.8 ...
Objective FDP1.1: Ectopic Pregnancy. Describe risk factors, characteristic morphologic findings, potential outcomes, and the medical/surgical options for management of ectopic pregnancy in relation to the pathogenesis and likelihood of adverse consequences. Competency 2: Organ System Pathology; Topic: Female Reproductive—Disorders of ...
Abdominal pregnancy, with a diagnosis of one per 10000 births, is an extremely rare and serious form of extrauterine gestation [ 1 ]. Abdominal pregnancies account for almost 1% of ectopic pregnancies [ 2 ]. It has reported incidence of one in 2200 to one in 10,200 of all pregnancies [ 3 ]. The gestational sac is implanted outside the uterus ...
An ectopic pregnancy is one in which the fertilised ovum is implanted and develops outside the normal endome-trial cavity, i.e. in the fallopian tubes, cervix, ovary or in the abdominal cavity. Commonest site is the fallopian tube. About 1 in 100 to 150 pregnancies are ectopic. The risk factors are age, history of infertility, smoking, history ...
Pregnancy occurring outside the normal uterine cavity is termed ectopic pregnancy. Its estimated incidence in the general population is 1%- 2%.1 A tubal stump pregnancy, a rare event with only a handful of cases described in the lit-erature, occurs when a blastocyst implants within the rem-nant of fallopian tube following salpingectomy.2 ...
One patient had a history of ectopic pregnancy. Serum levels of β-hCG in women with suspected ectopic pregnancy were less than 700 mIU/ml in four patients (25%), 700-1500 mIU/ml in five patients (31.25%) and more than 1500 mIU/ml in seven patients (43.75%). Out of 16 cases, 10 (62.5%) cases were suspected clinically as ectopic pregnancy.
Such pregnancy can be managed medically with methotrexate or surgically via laparoscopy or laparotomy depending on the hemodynamic stability of the patient and the size of the ectopic mass. In this case study, we report on a 38-year-old woman, G3P2+0 who presented to King Abdulaziz University Hospital's emergency department with a history of ...
Objective FDP1.1: Ectopic Pregnancy. Describe risk factors, characteristic morphologic findings, potential outcomes, and the medical/surgical options for management of ectopic preg-nancy in relation to the pathogenesis and likelihood of adverse consequences. Competency 2: Organ System Pathology; Topic: Female Reproductive—Disorders of ...
An ectopic pregnancy (EP) accounts for 1-2% of all pregnancies, of which 90% implant in the fallopian tube. An abdominal ectopic pregnancy (AEP) is defined as an ectopic pregnancy occurring when the gestational sac is implanted in the peritoneal cavity outside the uterine cavity or the fallopian tube. Implantation sites may include the omentum, peritoneum of the pelvic and abdominal cavity ...
nancy: previous ectopic pregnancy, previous tubal sur-gery, evidence of tubal pathology, and in utero exposure to diethystilbestrol.4 A history of genital infections, infer-tility, and more than one lifetime sexual partner carried only a moderately increased risk of ectopic pregnancy.4 A more recent case-control study from France identified
Similarly, case control study done in Turkey Ankara previous history of ectopic pregnancy was a significant risk factor for ectopic pregnancy . Using these reports as starting point, similar assumptions P1: proportion among cases and p 2: proportion of among controls AOR: Adjusted odds ratio at 95% ( Zα/2 = 1.96) level of confidence, Power of ...
PDF | On May 1, 2020, Ufaque Muzaffar and others published Risk factors for ectopic pregnancy in women: A case control study | Find, read and cite all the research you need on ResearchGate
In a case-control study comparing 243 women using an IUD and suffering from EP to 140 IUD users with an intrauterine pregnancy, Bouyer . [] have described that progesterone IUD, duration of IUD use, and pelvic pain after the insertion of the IUD are the factors increasing the risk of EP in IUD users. In addition, other influencing factors ...
Ectopic pregnancy occurs when a fertilized ovum implants outside of the uterine cavity. The prevalence of ectopic pregnancy in the United States is estimated to be 1% to 2%, but this may be an ...
Background: Ectopic pregnancy (EP) is a condition presenting as a major health problem for women of childbearing age. This study aimed to identify potential risk factors for EP and to evaluate the contribution of the risk factors associated to EP. Materials and Methods: This retrospective nested case-control study was conducted from 2006 to 2011.
In a clinical setting, sonography can diagnose an ectopic pregnancy in conjunction with beta-human chorionic gonadotropin (B-hCG) levels above 1500 mIU. A case is presented in which the diagnosis of ectopic pregnancy was supported through sonographic features. Medication was given to terminate the ectopic pregnancy; however, surgery was later ...
TEACHING CASE. CASE: A 36-year-old G1P0010 woman presents to the office with onset of light vaginal bleeding, which she feels is not her menstrual period, and mild right lower quadrant pain, which she rates as 2/10. The pain is intermittent and crampy, and is not associated with urination. There is no nausea or vomiting.
Results. There were 119 ectopic pregnancies during the study period. The incidence of ectopic pregnancy is 2.81/100 deliveries. Ectopic pregnancy was common in 26-30 years (54.6%), the minimum age at diagnosis was 18 years and maximum age was 40 years with a mean age of 28.79 years and SD of 4.256. Most of the patients were primigravida—47 ...
Ectopic pregnancy (EP) is the most common cause of pregnancy-related death in the first trimester, and sonography is the imaging method of choice when it comes to evaluating a woman with a suspected ectopic pregnancy. 1 The vast majority of ectopic pregnancies occur within the fallopian tube, comprising 95% of all ectopic pregnancies. 2 However, the remaining 5% of ectopics are nontubal in origin.
Ectopic pregnancy happens approximately 1.5-2.0% among all pregnancies, but accounts for 5-10% of all pregnancy-related deaths. 1, 2 Ninety-five percent of ectopic pregnancies occur in the fallopian tube, and approximately 2.5% of cases occur in the interstitial position. 3, 4 Approximately 10% of patients with ectopic pregnancies will ...
PDF. Save. Management of Non-Tubal Ectopic Pregnancies: A Single Center Experience ... A rare case of intramural ectopic pregnancy diagnosed by sonography and treated surgically shows that early detection of intamural ectopy pregnancy is needed to prevent complications like uterine rupture and helps preserve fertility. ... tool for case series ...
Methods. According to the EP data in GBD for the three decades from 1990 to 2019, we used estimated annual percentage changes (EAPC) to assess the trend of age-standardized incidence rate (ASIR), age-standardized death rate (ASDR) and age-standardized disability adjusted life years (AS-DALYs) trends in EP and to explore the correlation between socio-demographic index (SDI) stratification, age ...