Your session is about to expire
Clinical research project management: back to basics.
Clinical trials require care and precision regarding planning, coordination, and collaboration. The stakes are high, with participants’ health and well-being and significant investments of both time and money on the line. That’s why clinical research project managers are necessary – to ensure coordinated and collaborative efforts between numerous departments, teams, and vendors that adhere to the study protocol as well as regulatory and ethical standards. In this article, we will take an in-depth look at the basics of clinical research project management.

What is project management in clinical research?
Clinical trials involve several complex, dynamic parts with different boundaries/areas of responsibility, and personnel with specific skill sets and qualifications. As a consequence, successful clinical trials require organizers who are adept at project management.
Clinical trials can be thought of as large-scale, complex projects with multiple milestones and parallel workstreams, including:
- Study and protocol design
- Study startup
- Site activation
- Recruitment
- Documentation (promotional material, informed consent forms, case report forms, protocol documents, surveys, etc.)
- IRB and IEC approval
- Site management
- Investigational product distribution and management of study materials
- Laboratories (analyses, lab tests, imaging, etc.)
- Reporting and regulatory affairs
Why is project management in clinical research important?
Clinical research involves multiple stakeholders. Project management can essentially be thought of as monitoring progress and keeping everyone involved on the same page. Project management is critical for the success of a clinical trial; it helps the different teams stay on schedule, adhere to protocol, and communicate amongst one another, as well as meet the trial objectives/targets, maintain quality standards, stay within budget, and follow regulatory requirements.
The following are three major reasons why project management in clinical research is important:
Timeline management
Project management ensures the delivery of clinical trial objectives and sub-objectives within the allocated time and budget. This is important because nearly 85% of all clinical trials experience delays. [1] Project management techniques can allow investigators to plan ahead if it looks like a deadline is not going to be met, so they can reallocate resources and priorities to accelerate the process, or otherwise notify teams responsible for tasks that would be affected by the resultant delay and adjust the timelines.
Streamline and facilitate communications
It is important for the various stakeholders involved in a clinical trial to be on the same page. Keeping all of these actors updated and facilitating communication amongst them is another task of the project management team. Lack of communication in clinical trials can have negative consequences on the quality of research. [2] An efficient project manager or management team can streamline communication and collaboration between multiple teams and departments, further increasing the transparency of the individual but interdependent operations.
Quality control (QC)
Quality control is another important aspect of clinical research project management. Quality standards are often stringent, but this is designed to protect the safety of participants and the general population. Clinical trials that fail to adhere to or meet quality standards will not be considered to have provided sufficient evidence on the effectiveness and/or safety of an investigational drug. Researchers and investigators tend to be under a lot of pressure as trials are often on tight budgets and timelines, but it is important that quality not be sacrificed in order to meet other objectives.
Project management helps sponsors/investigators manage all objectives and sub-tasks in a clinical trial while still prioritizing adherence to quality and regulatory standards. Part of the task of the project management team may even be to define internal quality standards for specific tasks, objectives, and/or teams.
What does a clinical research project manager do?
A clinical research project manager coordinates with other departments, teams, and personnel involved in the clinical trial to ensure the organized completion of clearly defined tasks. They also manage external vendors such as central laboratories or technology providers. Project managers will monitor the progress of all tasks and objectives to keep the study on track according to its protocol, including timeline and budget, and also communicate with stakeholders such as the sponsor to keep them up to date.
What is the clinical research project manager responsible for?
The responsibilities of a clinical research project manager depend on the specifics of the trial and its complexity, but they generally include the following:
- Planning : This includes general planning of the trial, including the internal organization between departments/teams, how tasks should be executed in order to comply with regulations, how many and which sites to involve, whether external tools/solutions need to be contracted, etc.
- Budgeting : Making sure that the study’s resources are allocated appropriately to the different teams/tasks, within the overall study budget, also providing room for unexpected costs or delays.
- Vendor identification/selection : Negotiation with vendors, technology providers, and suppliers.
- Scheduling : Scheduling the objectives/sub-tasks of the clinical trial and monitoring activities to make sure they are completed on time.
- Liaising : Acting as a central point of contact for members of the project team and sponsors.
- Task delegation : Assigning tasks to team members and updating them about their responsibilities, as well as deadlines and expectations.
What are the key topics included in a clinical research project plan?
The project manager may organize all of the above-mentioned tasks and responsibilities into a document or repository referred to as a clinical research project plan. This plan would formally outline standard protocols for aspects of the clinical research project management, such as:
- Timeline : The timeline should clearly outline specific tasks for each team/department, including their expected initiation and completion dates, and the project manager will ensure tasks and teams are on track. Clinical trials often get delayed, so it is useful to have protocols in place regarding how to deal with potential delays. [3]
- Budget : Often related to unexpected delays, it is not uncommon for projects to end up over budget. The project plan should clearly define budgets, both for teams and for individual tasks, and should outline how deviations from budgets should be dealt with.
- Stakeholder management : The project management plan should outline the content and dates of formal reports for keeping stakeholders updated about the trial’s progress.
- Documentation : The project plan should outline how documentation should be collected, organized, stored, and verified in order to ensure compliance with laws as well as ethical and clinical standards as established by the WHO and ICH guidelines for Clinical Good Practice.
- Site management : Although site monitoring is usually a separate responsibility in clinical trials, the project plan may include instructions and guidelines for individual study sites regarding adherence to protocol, tasks, and timelines. In addition, sites should have clear guidelines on who to contact in the case of any problems, questions, or adverse events that may arise during the trial.
- Data management : The project plan should specify protocol for the collection, secure storage, management, validation, and cleaning of subject information and trial data, in accordance with quality standards and applicable regulations. Proper data management ties in closely with quality assurance, and sound results require healthy data.
Tips for successful clinical research project planning and management
Here are 4 specific tips and ideas for maximizing the efficacy of project management functions in clinical research.
1. Plan with flexibility
Delays can be hard to avoid, especially in the recruitment stage, and they are costly to sponsors. Nonetheless, proper consideration of these potential delays in the timeline (i.e., allowing for some degree of flexibility) can make the difference between the delays simply setting the trial back a few weeks, or ending in the entire trial being canceled.
While delays aren’t ideal, proper planning can allow the sponsors to absorb these delays without them leading to completely missed deadlines and/or cancellation of the trial; in the end, cancellation likely represents a much more significant waste of resources than delays. The same logic can be applied to flexibility in budgeting, as delays may imply additional costs; if these are less unexpected, they can be better absorbed within the trial budget without setting it entirely off track.
2. Identify possible risks and establish mitigation strategies
Perform a thorough analysis of the protocol and utilize specialist knowledge in the fields of medicine and clinical research management to identify and create a list of risks that could arise throughout the clinical trial. Planning ahead of time will allow sponsors to respond rapidly to these risk factors and mitigate them, without having to perform lengthy analyses and coming up with mitigation strategies when it may be too late.
Some potential risks to consider include recruitment delays or low accrual, adverse events, patient dropouts, protocol breaches, problems with study drug supply or distribution, technical failures (of software systems, medical equipment, etc.), and data integrity issues, to name a few. Start with the risks that pose the greatest threat to the integrity of the study, i.e., those which would result in it being canceled, stopped, or rendering the results unusable.
3. Use project management tools
Constantly reviewing all aspects of the clinical trial is a daunting task, so the use of specialized and customizable software solutions can be helpful. There are many such solutions available, from general project management tools to dedicated clinical trial management systems ( CTMS ). These tools can be of significant help in managing, organizing, and overviewing all of the aspects of project management discussed previously, acting as a sort of central dashboard as well as a “safeguard” for the project management plan and tasks.
4. Leverage data automation tools and functions
Similarly to the previous point, data management is another aspect of clinical trials (and clinical trial project management) that can benefit greatly from the assistance of technological tools. Lots of data management functions, including organization, cleanup, and validation, can be streamlined or even completely automated through data processing tools, which are sometimes integrated directly into CTMS or other clinical trial monitoring solutions. The benefits of healthy data include enhanced regulatory compliance and faster progression to data analysis and results once the study data has been collected. Data can also be improved at the source through the use of electronic reporting/collection/recording methods such as:
- Electronic patient-reported outcomes ( ePRO )
- Electronic trial master files ( eTMF )
- Electronic clinical outcome assessments ( eCOA )
- Electronic case report forms ( eCRF )
Conclusions
Clinical research project management is a vital function for keeping the numerous separate yet highly interconnected parts involved in a clinical trial operating in coordination and on track with protocol, budget, timelines, and regulations. There are numerous strategies and tools that can facilitate clinical trial project management tasks and help improve clinical trial quality and speed while still ensuring patient safety and regulatory compliance.
Other Trials to Consider
Atezolizumab and Bevacizumab in combination with TACE
Low-level laser therapy, telehealth advance care planning, project think, medical follow-up appointment, esym app usage, test, subjects categorised as "low risk" or "not low risk", popular categories.
Asthma Clinical Trials 2024
Stroke Clinical Trials 2024
Ulcerative Colitis Clinical Trials 2024
Cardiology Clinical Trials 2024
Medulloblastoma Clinical Trials 2024
MRSA Clinical Trials 2024
Paid Clinical Trials in San Diego, CA
Familial Hypercholesterolemia Clinical Trials 2024
Keloid Clinical Trials 2023
Acute Myeloid Leukemia Clinical Trials 2023
Popular guides.

Clinical Project Manager
Clinical trial management certification.

Demo Clinical Project Manager Training
Research Project Manager Certification
CCRPS Research Manager Graduates obtained job roles including:
Clinical Trial Project Manager, Research Nurse Manager, Clinical Research Coordinator-Data Manager, Clinical Research Associate, Transdisciplinary Research Project Manager, IT Project Manager in Clinical Research, Publicly Funded Research Project Manager (2024 CCRPS Graduate LinkedIn Survey).
Clinical Research Project Manager Training
Advanced Clinical Research Associate Certification (ACRAC)
Introduction
CME Handout
Common Terminology Used In Clinical Research - Reference Glossary
Commonly Used Abbreviations and Terms in Clinical Research
An Overview of ICH GCP
CFR 21 Part 11
Ethics of Research Involving Children
Ethics of Research Involving Mentally Incapacitated
Ethics of Research Involving Pregnant Women and Fetuses
Fundamentals of Project Management
Project Management Fundamentals
PMBOK Summary - Mandatory Project Management Review
Clinical Trial Project Management
Importance of Project Management
Roles and Relationships in Clinical Trials
Role of a Project Sponsor
ICH GCP E6 Section 5 - Sponsor/CRO Responsibilities
Institutional Review Board/Ethics Committee (IRB/EC) (Requirements, sIRB, Application, Exemptions, Expedited Review, Continuation, and Reporting)
Data Safety Monitoring board- DSMB
Stakeholders in Clinical Trials (Sponsor, Project Manager, IRB, PI, CRA, CRC, Site Staff, Data Team/DSMB, Patients)
Contract Research Organizations (Delegation, Responsibilities, Management )
ICH GCP E6 Sections 2-4 Principles, IRB, & Investigator Roles
ICH GCP E6 Section 4 - Reporting Responsibilities of the Investigators
Skills of a Project Manager
Essential skills of a Project Manager
Technical skills for Project Management
Project Team
Managing a Project Team
Project Management Documents
Regulatory Documents
Regulatory Documents in Clinical Trials
Delegation of Authority Log – DOAL
Investigators Brochure (IB)
Trial Master File
Essential Regulatory Documents Binder Tab Organization (Trial Master File)
Trial Master File Reference Guide
New Drug Application
The Investigational New Drug (IND) & New Drug Application (NDA) Process
Investigator Initiated Multi-Center Trials
IND and IDE AE Reporting
Safety Reporting Requirements for Sponsor Investigators of An IND
Problem Solving in Project Management
Problem Solving as a Project Manager
Project Failures and Statistics
Project Reporting Styles
Avoiding Project Failure
Budgeting for Clinical Trials and Projects
Project Budgeting
Payments and Budgeting for Investigators and Site
Advertisement Aid in Subject Recruitment and Retention
Clinical Trial Design
Advanced Designs of Clinical Trials
Advanced Review of Phases of Clinical Trials (Preclinical & Phase 0-4)
Randomized Controlled Trials (Randomization, Allocation Concealment, Validity, Blinding, Controls, Outcomes, Fidelity)
Blinding and Unblinding in Clinical Trials
The Clinical Trial Protocol - Advanced Mastery Review
Inclusion and Exclusion Criteria in Clinical Research (Writing, Assessing for Broad vs. Narrow, Organ Dysfunction, Older Adults, Pediatrics, Pregnant Women)
Protocol Deviations and Violations (Major, Minor, Exceptions, Resolution)
Project Management Scheduling and Tracking
Basics of Project Scheduling
Project Progress Tracking
Project Management Planning Process
Project Management Plan
Closing a Project
Project Delays
Process Mapping
Metric Tracking
Duties of a Successful Project Manager
Roles and Responsibilties of a Project Manager
Project Management Success Factors
Adverse Events
Advanced Review of Adverse Events
Site Selection and Visits
Types of Monitoring Visits (Selection, Initiation, Routine, Close-Out)
Site and Investigator Selection Criteria (Process, Criteria, Investigator Selection, Agreements, Decision-Making)
Site Selection/Qualification Pre-Study Visit (SSV/SQV) (Before, During, After, Letters, Checklists, and Report)
Audit and Inspections
Audits and Inspections in Clinical Trials
Clinical Trial Data Audits
FDA Warning Letter
Quality Control and Safety
Quality Control in Clinical Trials ( QC/QA, KQI, QMS, Checklist)
ICH GCP - Safety of Human Subjects in Clinical Research
Technology in Trials (IVRS, CTMS, EDC)
Clinical Trial Management System-CTMS
ICH GCP - Trial Management, Data Handling, and Record Keeping
An Overview of Remote Monitoring - COVID-19 Update
Centralized Monitoring
Interactive Response Technologies in Clinical Trials (IVRS, IWRS, IRT, RTSM)
Pharmacovigilance and Regulatory Affairs
Advanced Practice of Pharmacovigilance
Regulatory Affairs for Clinical Trials
Investigational Product and Labs
Investigational Product Storage and Dispensing
Investigational Product Accountability in Clinical Trials
Local and Central Labs in Clinical Trials (Local, Regional, Central, GLCP, CLIA Cert, Lab Audit Checklist)
Patient Recruitment, Retention, and Compliance
Patient Recruitment in Clinical Trials
Patient Engagement and Retention in Clinical Trials
Patient Adherence and Compliance in Clinical Trials
Project Manager Job Readiness
Project Manager Skills Interview Questions
Interview Questions
Competency Examination
Competency Exam

About this course
- Required: Prior clinical research or project management experience.
- Length: 100 hours. Online, self paced, start anytime.
- ACCRE, Joint Accreditation with AMA, ANCC, ACPE for 17.5 CME. Online certificate. Exam score 70% or higher on 2 attempts.
Enroll Schedule Advising
CCRPS Reviews
Clinical Research Project Manager Certification
Navigating the Course
Natalie johnson.
I was originally intimidated by the materials and all that I had to learn. The syllabus, the instructors and the course material were easy to follow and lear...
I was originally intimidated by the materials and all that I had to learn. The syllabus, the instructors and the course material were easy to follow and learn from. I am very happy I completed the course.
Advanced Clinical Research Project Manager Certification ...
Roger andersen.
There is extensive material in this course. It is highly relevant to managing clinical trials.
Advanced Clinical Research Project Manager
Ellen lyrtzis.
The clinical trial project manager is responsible for different aspects of the clinical trial process, such as setting timelines, developing budgets, and overseeing data analysis. To become a clinical trial manager, you can gain experience in project management or clinical research roles. Clinical trial manager certification will increase your chances of getting hired.
To become a clinical project manager, one must first obtain a Bachelor's degree in a health-related field. Additionally, developing skills for project management and participating in relevant courses are necessary.
Experience can be gained by volunteering or interning in clinical trials with pharmaceutical companies or medical research centers.
Pursuing an advanced degree or getting certified as a Clinical Project Manager with open up more opportunities.
Clinical Trial Manager
Clinical Trial Managers are responsible for planning and overseeing all aspects of clinical research projects. This includes making sure the project is conducted according to regulations and best practices. They also manage budgets, timelines, and resources to ensure the project is completed successfully.
Clinical Project Managers plan and execute clinical research projects by coordinating with internal and external stakeholders, regulatory authorities, and by develop key documents like protocols, consent forms, investigator brochures, budget sheets, study reports, and final reports.
Clinical Research Manager
Clinical Research Managers make sure that data is being collected and analyzed correctly, and that everyone is compliant. Clinical Project Managers design research studies and monitoring projects in terms of cost, budgeting, quality assurance, and risk assessment.
The Clinical Trials Management Certificate program is designed to provide students with the knowledge and skills necessary to design, implement, and manage clinical trial protocols.
This Clinical Trials Design & Management Certificate Program introduces learners to the fundamentals of clinical trials design and management.
The program covers principles and regulations of clinical trial design, analysis techniques for statistical analysis, quality control and assurance, data management and reporting.
Students will also gain an understanding of risk assessment strategies, study site selection, protocol implementation and monitoring, resource management as well as safety requirements for conducting clinical trials.
Clinical Trial Manager Salary
Clinical manager salary.
Clinical trial manager salaries vary based on experience, location, and company size. The average hourly rate for a clinical trial manager is $30-$60 per hour.
Clinical trial manager salary
The monthly salary of a clinical trial manager typically ranges from $5,000 to $10,000. Those with more experience or who work at well-known organizations can earn up to $20,000 per month.
Clinical project manager salary
Annual clinical project manager salary ranges between $60,000 and $120,000 dollars per year. The median salary for a clinical trial manager is approximately $82,500 across all industries and geographies
What does a Research Project Manager do?
Clinical Project Managers are responsible for creating project plans, timelines, budgets, and communication with vendors and stakeholders. They also provide training to personnel involved in the project, establish systems to track project progress, and identify risks associated with the project.
Clinical Project Managers make sure that clinical research projects go well by working groups like contract research organizations (CROs), internal departments, and external vendors. Clinical Project Managers make sure that projects follow the research protocols, good clinical practices (GCPs), applicable regulations, and standards.
Research Project Managers also develop protocols for data collection and analysis, prepare reports for regulatory submissions, coordinate activities related to safety monitoring, and provide support to staff during project-related training sessions or workshops.
Trial Project Managers make sure that data is collected accurately according to guidelines from the FDA or EMA. This includes finding risks associated with the project; assessing their impacts; planning ways to reduce the risks; and also planning how to use resources so that everything runs smoothly.
The most advanced clinical trial project management training available
Take the fast track.
Take the fast track to a lucrative career as a Clinical Research Project Manager to start earning salaries of $100k+
Get advanced training
Get the most advanced training - ACRPM is recognized as a gold standard by many CROs in the industry thanks to its comprehensive training
Work at your own pace
Work at your own pace from wherever you are with flexible online training. The 100+ modules included can be completed in as little as 2 weeks
Requirements
Designed for those holding a minimum of a BA in Science, ACRPM is internationally accredited to ACCRE, ACCME, ACPE, ANCC, and Transcelerate Biopharma. In other words, upon completion of the course and the final exam, you will have a level of knowledge equivalent to (and beyond!) that of a senior CRA.
ACRPM features 100+ modules, or 250 hours, of on-demand online training (worth 17.5 CME credits). The course has been put together by clinical trial project managers, enabling students to build a deep knowledge of the industry.
Certification
This course can be completed in as little as two weeks, with certification and a letter of recommendation awarded after completing a final exam. ACRPM also provides you with tools to help you find a job, including resume and interview guides, giving you a further edge over other applicants for the same position.
Clinical Project Manager Guide
Clinical Project Managers are responsible for clinical research activities and initiatives in the healthcare, pharmaceutical, and biotechnology sectors. This includes creating and implementing project plans, developing timelines, overseeing budgets, managing resources, and ensuring compliance with regulatory requirements. CPMs typically work with cross-functional teams that may include clinicians, scientists, regulatory affairs specialists, data management personnel, software developers/engineers, project sponsors/coordinators.
The career path of a Clinical Research Project Manager is very rewarding. You get to use your clinical research expertise to develop new treatments for existing medical conditions and to create new treatments for future medical needs. Additionally, you get to work with leading doctors, scientists, and other professionals who are working towards improving healthcare outcomes. You also have the opportunity to build relationships with key stakeholders including pharmaceutical companies, governmental agencies, and funding bodies which leads to greater satisfaction in your career.
Research project managers are responsible for planning and executing research studies throughout their entire life cycle. This includes activities such as designing experiments or surveys, selecting appropriate experimental methods or sampling techniques, recruiting participants for studies or surveys, collecting data from multiple sources, analyzing results using statistical methods or software programs, interpreting results and preparing recommendations for further action.
The clinical project manager definition refers to a professional who is in charge of developing clinical research activities from the beginning to the end. This includes making a plan for the scope and timeline of the research project; making sure that the project meets all regulatory requirements; organizing the technical aspects of data collection; coordinating meetings between stakeholders; monitoring progress and deadlines; utilizing risk management processes; providing guidance to staff members; ensuring quality control of data collection processes; providing reports to stakeholders and company executives regarding project status updates as needed.
Working as a clinical project manager can be challenging due to the complexity of the tasks involved. Clinical projects require significant planning, coordination and supervision between multiple stakeholders. Additionally, time management can be difficult due to deadlines that must be met and ever-changing regulatory requirements. Other challenges include managing competing priorities and ensuring proper communication between team members. It is also important for clinical project managers to stay current on trends, technologies and best practices within their field so that they can remain competitive in their work.
The core duties of a clinical project manager vary depending on the industry but typically include planning and organizing activities related to assigned projects; developing budgets; coordinating resources; monitoring progress; overseeing quality control standards; ensuring adherence to safety procedures; providing leadership for teams; communicating with stakeholders throughout the duration of the project; developing plans for corrective action if needed; preparing reports for upper management; analyzing data related to performance metrics; maintaining records of all activities related to assigned projects; and staying informed of changes in regulations relating to their area of expertise.
To be a good clinical project manager, it is important to have experience working in healthcare, like in a hospital. It is also helpful to be good at organizing and communicating, as well as understanding the medical environment and the rules that govern it. Additionally, clinical project managers should know about principles related to project management, like budgeting, scheduling, and risk management. Those who have earned a healthcare-related degree or certificate (like in nursing or health information management) may have a better chance of being successful in the role.
A clinical project manager oversees clinical research studies from design through implementation. This includes working with teams of physicians, pharmacists, nurses, statisticians or other health professionals. Responsibilities include making sure the project runs smoothly while staying within the budget and timeline. As part of their role they must understand FDA regulations when conducting trials in the US or ICH guidelines when conducting international studies. Additionally they must be able to identify potential risks associated with each study and develop strategies for mitigating them throughout all stages of the study process.
The average salary for a Clinical Project Manager is approximately $85,000 per year. Hourly pay for Clinical Project Managers usually ranges from around $41 to $58 per hour, with an average rate of approximately $49.50 per hour. Monthly salaries typically range from around $7,083 to $9,833 per month or more.
•Clinical Project Manager – Research and Development: This person is responsible for making sure clinical research studies are organized and run smoothly. • Senior Clinical Project Manager: This person provides guidance to project teams and management when they are developing clinical research projects. • Global Clinical Trial Program Manager: This person creates global clinical trial plans that meet operational requirements while following regulatory guidelines. • Clinical Data Management Project Manager: This person oversees all aspects of data collection, management, analysis and reporting associated with a clinical research project from start to finish. • Regulatory Affairs/Clinical Project Manager: monitors and reports on the regulatory submissions for clinical trials taking place in different countries. • Clinical Operations Project Manager:: oversees and coordinates the daily operations of clinical research projects to ensure that they meet quality assurance standards set out by IRBs or FDA .
Achieving certification as a Clinical Project Manager is a way to show that you are an expert in project management within the healthcare and medical research industries. This certification allows individuals to demonstrate their understanding of clinical project management concepts and skills, which are necessary for ensuring successful outcomes for initiatives within complex clinical research environments. To obtain a Clinical Project Manager Certification, you need extensive knowledge and experience in clinical project management, which can be obtained through formal education, training courses, and hands-on experience.
Certification as a clinical project manager provides healthcare professionals with the skills and knowledge necessary to effectively manage complex clinical trials, research projects, and quality improvement initiatives. There are several key reasons to get certified, including professional credibility, a competitive edge in the job market, increased knowledge base, improved efficiency in completing tasks related to clinical project management, and networking opportunities.
- Professional Scrum Product Owner (PSPO)
- SAFe for Government
- Professional Scrum Master (PSM)
- Certified ScrumMaster
- PMI-ACP Exam Prep
- Leading SAFe® 6.0 Certification
- SAFe Scrum Master
- Certified Scrum Product Owner (CSPO)
- SAFe for Teams
- Agile Scrum Foundation
- AgilePM Foundation and Practitioner Certification
- Agile Scrum Master (ASM)
- Kanban Training
- Scrum Fundamentals
PMP Certification
Project Management Fundamentals
CAPM Exam Prep
- Change Management Foundation and Practitioner Certification
- PRINCE2 Foundation & Practitioner Certification (7th Edition)
- PRINCE2 Agile Foundation & Practitioner Certification
- Business Analysis Foundation and Practitioner Certification
- Microsoft Project Training
- JIRA Certification Training
- Lean Project Management
- ITIL 4 Foundation
- VeriSM™ Foundation
- SIAM Foundation
- SIAM Professional
- 7 QC Tools Training
- Minitab Essentials
- Lean Six Sigma Yellow Belt
- Six Sigma Awareness
- Lean Six Sigma Green Belt
- Design for Six Sigma
- Lean Six Sigma Black Belt
- Lean Fundamentals
- Value Stream Mapping
- Quality by Design
- Quality Function Deployment
- BPM and Six Sigma
- RCA through Six Sigma
- DevOps Foundation
- DevOps Master
- DevOps Professional
- Continuous Delivery Architecture
- COBIT 5 Certification
- Corporate Group Training
- 1-to-1 Training
- Join as a Trainer

- Best Project Management Blogs
What Does a Clinical Project Manger Do? Roles & Responsibilities

Clinical Project Managers (CPM) play a crucial role in advancing the progress of a clinical trial. The significance of CPMs lies in their ability to navigate complex clinical trials, ensuring precision, compliance, and efficiency.
As stewards of the entire research lifecycle, from planning to execution, Clinical Project Managers wield strategic thinking, leadership insight, and a deep understanding of regulatory landscapes.
In a world where the pursuit of groundbreaking therapies intensifies, the demand for skilled managers is reaching new heights, with organizations recognizing their pivotal role in trial success.
This blog explores the pivotal responsibilities of clinical project managers and sheds light on why their expertise is becoming increasingly coveted, underscoring the crucial role they play in shaping the future of healthcare.
Table of Contents:
What is a Clinical Project Manager?
What does a clinical project manager do, skills required to become a clinical project manager.
- Essential Certifications or Degrees Required to become a Clinical Project Manager
How to Become a Clinical Project Manager?
Salary and job outlook for a clinical project manager.
A Clinical Project Manager (CPM) is an experienced expert in clinical research and healthcare management who is responsible for managing and coordinating the different aspects of clinical trials.
This multifaceted role encompasses strategic planning, execution, and monitoring of clinical research projects to ensure they adhere to regulatory standards, timelines, and budgets.
Clinical Project Manager acts as a connecting point between research teams, sponsors, regulatory authorities, and other stakeholders, facilitating effective communication and collaboration.
Clinical Project Manager responsibilities include protocol development, risk management , team leadership, and navigating the complexities of regulatory compliance. By leveraging their expertise in project management, scientific understanding, and regulatory knowledge, they contribute significantly to successful clinical trials, ultimately advancing medical knowledge and bringing novel treatments to needy patients.
A Clinical Project Manager (CPM) is a pivotal figure in clinical trials, overseeing the intricate processes that lead to the successful execution of healthcare research. Their role encompasses many responsibilities, blending scientific expertise with project management skills to ensure the seamless progression of clinical trials.
Other key roles and responsibilities of a Clinical Project Manager:
- Strategic Planning: Develop comprehensive plans for the initiation, execution, and completion of clinical trials, aligning them with project goals and timelines
- Protocol Development: Contribute to the creation and refinement of study protocols, outlining the methodology, objectives, and criteria for participant selection
- Site Selection: Identify and evaluate suitable clinical trial sites, considering factors such as patient demographics, facilities, and regulatory compliance
- Regulatory Compliance: Navigate and ensure adherence to the complex web of regulatory requirements, obtaining necessary approvals and permissions for the clinical trial
- Budget Oversight: Manage the financial aspects of the clinical trial, ensuring adherence to the allocated budget and making informed decisions to optimize resource utilization
- Data Integrity: Oversee data collection and management processes, emphasizing the importance of data accuracy, completeness, and compliance with regulatory standards
- Problem Resolution: Address challenges and obstacles that may arise during the trial, making decisions that safeguard patient safety and ensure the integrity of the study
- Quality Assurance: Maintain a focus on the overall quality of the clinical trial, implementing measures to uphold ethical standards, patient welfare, and the reliability of research outcomes
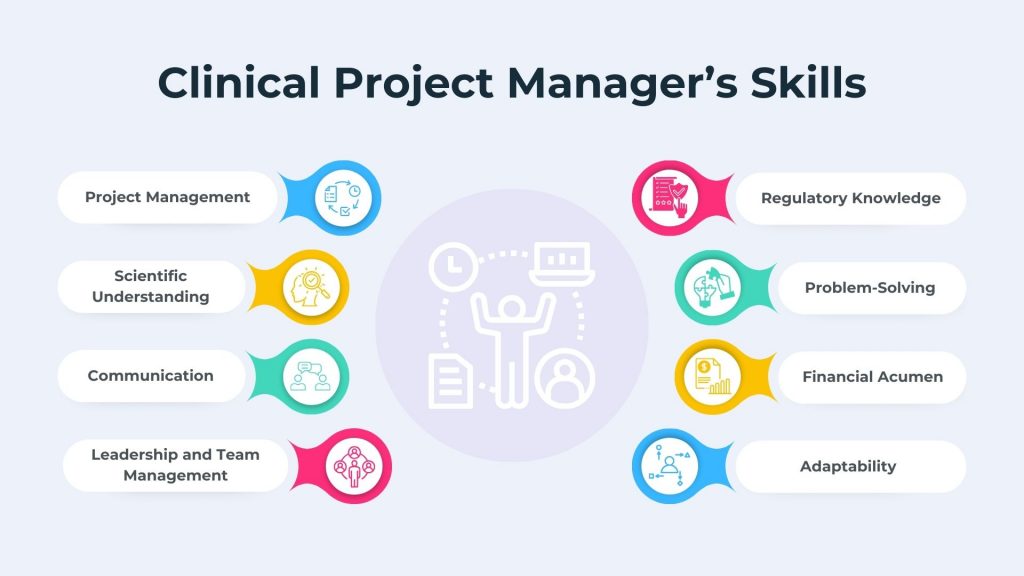
Becoming a successful clinical project manager requires a diverse set of skills that combines scientific knowledge, project management proficiency, and effective communication. Below are some of the key skills that a clinical project manager is required to excel in the role:
1. Project Management Skills
- Planning and Organization: Ability to develop and execute comprehensive project plans, ensuring all aspects of the clinical trial are well-coordinated
- Time Management: Efficiently allocate resources, manage timelines, and prioritize tasks to meet project milestones
- Risk Management: Identify potential risks and proactively implement strategies to mitigate them, ensuring smooth project progression
2. Scientific Understanding Skills
- Clinical Research Knowledge: Familiarity with the principles and processes of clinical research, including study design, protocols, and ethical considerations
- Medical Terminology: Ability to understand and interpret medical and scientific terminology crucial for effective communication with research teams and stakeholders
3. Communication Skills
- Interpersonal Communication: Build strong professional relationships with diverse stakeholders , including research teams, sponsors, regulatory authorities, and site personnel
- Presentation Skills: Effectively convey complex information clearly and concisely, verbally and in written form
4. Leadership and Team Management Skills
- Team Building: Foster collaboration and cohesion within cross-functional teams, inspiring motivation and commitment to project goals
- Decision-Making: Make informed decisions promptly, especially in high-pressure situations, to address challenges and keep the project on track
5. Regulatory Knowledge and Skills
- Regulatory Compliance: Stay updated on and ensure adherence to relevant regulations and guidelines governing clinical trials in different regions
- Ethical Considerations: Understand and navigate the ethical considerations in clinical research, prioritizing patient safety and welfare
6. Problem-Solving Skills
- Critical Thinking: Analyze complex situations, identify root causes of issues, and develop effective solutions to keep the project moving forward
7. Financial Acumen
- Budget Management: Proficiency in managing project budgets, optimizing resource allocation, and ensuring financial accountability throughout the trial
8. Adaptability Skills
- Flexibility: Navigate unforeseen challenges and changes in project scope with adaptability, adjusting strategies and plans as needed
- Learning Agility: Stay abreast of advancements in clinical research, project management methodologies, and regulatory requirements
Essential Certifications or Degrees Required to Become a Clinical Project Manager
Becoming a Clinical Project Manager requires a combination of education, relevant degrees, and professional certifications. The specific requirements may vary based on the employer, industry sector, and the clinical trials complexity.
Here are some essential certifications and degrees that can enhance the qualifications of individuals aspiring to become Clinical Project Managers:

1. Educational Background
- Bachelor’s Degree: A bachelor’s degree in an appropriate field such as life sciences, healthcare, nursing, pharmacy, or a related discipline is frequently the minimum educational requirement
- Advanced Degrees: While not always mandatory, having a master’s degree (e.g., Master of Public Health, Master of Science in Clinical Research) or a Ph.D. can be advantageous, especially for more senior or specialized roles
2. Project Management Professional (PMP) Certification
The PMP certification is offered by the Project Management Institute (PMI), is widely recognized, and demonstrates proficiency in project management principles. It is valuable for Clinical Project Managers as they oversee complex clinical trials.
Achieve global recognition with the PMP certification from Invensis Learning. Benefit from expert trainers, flexible learning options, and success guarantees to propel your career to new heights. Enroll now to access exclusive discounts and become a certified leader in project management.
3. Certified Clinical Research Professional (CCRP) Certification
The Certified Clinical Research Professional (CCRP) certification is a professional designation offered by the Society of Clinical Research Associates (SoCRA). It is a worldwide recognized credential that demonstrates an individual’s skills and understanding of the principles and practices of clinical research.
4. Project Management Fundamentals (PMF) Certification
The Project Management Fundamentals (PMF) Certification is an entry-level credential offered by the Association for Project Management (APM) that validates an individual’s understanding of the fundamental principles and practices of project management. It is designed for those new to the field or wanting to formalize their project management knowledge.
5. Certified Clinical Project Manager (CCPM) Certification
The Certified Clinical Project Manager (CCPM) certification is a professional designation offered by various organizations that demonstrates an individual’s expertise in managing clinical trials and research projects. It validates their ability to effectively plan, execute, monitor, and evaluate clinical research studies, ensuring adherence to regulatory and ethical guidelines.
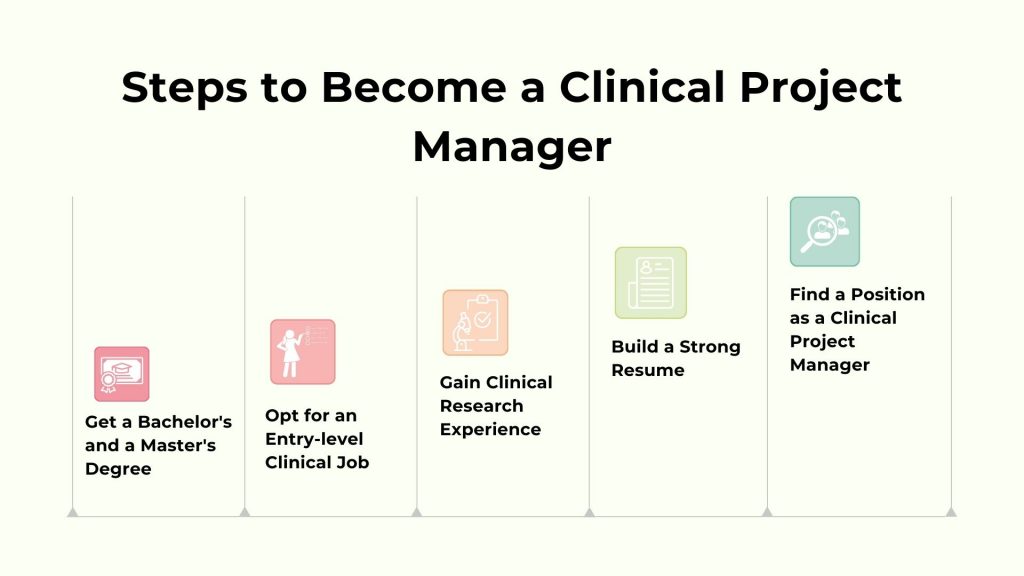
Becoming a Clinical Project Manager involves a strategic combination of education, experience, and professional development. Here’s a step-by-step guide on how to embark on a career as a Clinical Project Manager:
1. Get a Bachelor’s and a Master’s Degree
Embark on your journey by earning a bachelor’s degree in a relevant field, such as life sciences or healthcare. This foundational step equips you with essential knowledge for a career in clinical research.
To enhance your qualifications further, pursue a master’s degree, opting for specialized programs like a Master’s in Public Health (MPH) or a Master’s in Clinical Research.
2. Opt for an Entry-level Clinical Job
Kickstart your career with an entry-level position in clinical research, such as a Clinical Research Assistant or Coordinator. These roles expose you to the day-to-day operations of clinical trials, providing valuable insights into research protocols, data management, and regulatory compliance.
3. Gain Clinical Research Experience
Actively seek hands-on experience in clinical research, engaging in tasks like patient recruitment and study coordination. Develop a strong understanding of Good Clinical Practice (GCP) guidelines and ethical considerations. This practical experience lays the groundwork for a well-rounded skill set and prepares you for more advanced roles.
4. Build a Strong Resume
Create an effective resume that highlights your educational background, relevant coursework, and practical experience. Emphasize key skills such as attention to detail, data management, and knowledge of regulatory standards. Include certifications, like GCP, to underscore your commitment to maintaining high-quality standards in clinical research.
5. Find a Position as a Clinical Project Manager
Progress in your career by applying for roles with increasing responsibilities, focusing on project management within clinical trials. Leverage your educational background, practical experience, and certifications to showcase readiness for a Clinical Project Manager role. Highlight your ability to lead teams, manage timelines, and strictly adhere to regulatory standards.
Before switching any career, individuals should know two main things: one is salary growth and the other one is job opportunities. The salary and job outlook for a Clinical Project Manager (CPM) can vary based on factors such as experience, education, location, and the specific industry within healthcare or clinical research. It’s essential to note that salary trends and job outlook may evolve over time.
Salary of a Clinical Project Manager
The salary prospects for a clinical project manager are generally quite positive. They play a crucial role in the healthcare industry, overseeing the planning, execution, and monitoring of clinical trials and research projects.
Their expertise in project management, clinical research methodology, and regulatory compliance ensures the successful completion of these studies, leading to the development of new drugs, treatments, and medical devices.
Experience is a significant factor in determining salary. The salary ranges for clinical project managers are as follows:
Clinical project managers have the potential to experience significant salary growth throughout their careers. With increasing experience, specialized skills, and advanced certifications, clinical project managers can advance into senior-level positions with higher earning potential.
Additionally, the demand for clinical project managers is expected to grow faster than average in the coming years, further contributing to positive salary prospects.
Job Outlook of a Clinical Project Manager
The job outlook for clinical project managers is exceptionally promising, driven by the increasing demand for clinical trials, the growing complexity of research projects, and the expanding healthcare needs of an aging population.
As per the US Bureau of Labor Statistics (BLS) , employment of medical and health services managers, which includes clinical project managers, will expand by 32% from 2020 to 2030, much faster than the average for all professions. This growth is related to the aging population and the increasing demand for healthcare services.
Here are some specific factors that contribute to the positive job outlook for clinical project managers:
- Increasing demand for clinical trials
- Growing complexity of clinical trials
- The aging population and rising healthcare needs
- Expansion of medical group practices
Clinical project managers can pursue diverse career paths and advance into senior-level positions with increasing responsibilities and higher compensation.
Some potential career trajectories include:
- Clinical Research Associate (CRA)
- Clinical Research Coordinator
- Clinical Research Manager
- Director of Clinical Research
- Clinical Research Program Manager
- Clinical Research Portfolio Manager
- Clinical Project Manager Specialist
- Clinical Project Manager Lead
- Global Clinical Project Manager
- Senior Clinical Project Manager
- Executive Clinical Project Manager
A clinical project manager plays a pivotal role in the healthcare industry, ensuring the successful execution of clinical trials and research projects. Their expertise in project management, clinical research methodology, and regulatory compliance is crucial for bringing new drugs, devices, and therapies to patients, improving healthcare outcomes, and advancing medical knowledge.
If you are passionate about healthcare, have strong organizational skills, and possess a keen eye for detail, a career as a clinical project manager could be a rewarding and fulfilling path. With the right education, experience, and certifications, you can significantly impact the future of healthcare by overseeing the development of life-saving treatments and technologies.
Change Management Foundation and Practiitioner Certification Training
EXIN Business Analysis Foundation and Practitioner Training
PRINCE2 Foundation and Practitioner Certification Training
RELATED ARTICLES MORE FROM AUTHOR

What is Quality Assurance (QA) in Project Management?

What is Sensitivity Analysis in Project Management?

Mind Mapping in Project Management: How to Use It?
Leave a reply.
Save my name, email, and website in this browser for the next time I comment.
- 14,520 Likes
- 444 Followers
- 96,500 Subscribers
- 2,170 Followers
Related Articles

How to Convince your Employer to Pay for Lean Six Sigma...

The Impact of Artificial Intelligence on Project Management

Why PMOs are More Popular than Ever
Top 9 DevOps Collaboration Tools

Kanban vs. Scrum – Which Works Best for Enterprises in 2024
Popular posts.

The Project Management Life Cycle Explained

Roles and Responsibilities of a Quality Control Inspector

7 Cs of Effective Communication with Example

Top 5 Factors for Project Success

Quality Analyst Job Role and Responsibilities- Explained!
Suggested posts.
- 7 Cs of Effective Communication with Examples
- Project Management Lifecycle
- Project Success Factors
- Quality Control Inspector Job Description
- Risk Management Examples
- QA Manager Job Description
- Quality Management Team Roles and Responsibilities
- Risk Management Tools & Techniques
- Quality Analyst Job Description
- What is Business Value
- Who are Project Stakeholders
- Importance of Project Management
- What is Project Management
- Project Management Skills
- Project Manager Job Description
- Agile Project Manager Interview Questions
- Risk and Compliance Manager Job Description
- Risk Management Process
- Project Scope Management
- Healthcare Project Manager Job Description
- Six Sigma Project Examples
- Risk Analysis Methods
- ITIL Service Lifecycle
- Risk Manager Job Description
POPULAR CATEGORIES
- Best Project Management Blogs 264
- Top Agile Blog Posts 158
- Top Blogs on Quality Management 126
- Latest IT Service Management Blogs 108
- Trending Articles on DevOps 65
- Popular Blogs on IT Security and Governance 55
- Top Blogs on Professional Development 33
- Top Infographics Collection 8
Download E-book Blog
Thank You for submitting your enquiry. One of our training consultants will get in touch with you shortly.
50+ Training and Certification Programs - Upskill Today Learn more about our training programs.

Connect with us:
What to know about project management for clinical trials.

Completing any multi-part task requires organization, coordination, and discipline — and, of course, clinical trials are no exeption. Planning a research study, launching a trial, and keeping things running smoothly requires knowledge and expertise, which is why clinical trial project managers are so vital to the process.
From creating a plan, communicating updates, calculating risks, and addressing any mistakes that arise , solid project management is a necessity to ensure medical research is allowed to move forward. Here, we’ll discuss the role of the clinical trial project manager, as well as tips for developing project plans, stakeholder involvement, communication, IRB submission, and summarizing lessons learned.
What is project management in clinical trials?
The Project Management Institute (PMI) defines project management as the "application of knowledge, skills, tools, and techniques to project activities to meet the project requirements." Project management combines expertise in scope, time, cost, quality, risk management, communication, and stakeholder management in order to move through the five basic phases of any project :
- Project initiation. This phase involves developing an idea, understanding the necessity of the project, and identifying the key decision-makers
- Project planning. This is the phase for making a plan and outlining the work required, including prioritization, budget, schedule, and resources
- Project execution. This is where tasks are distributed by informing all teams of their responsibilities and deadlines
- Project monitoring. This entails implementing project tracking to compare the current project status and progress with the original plan, adjusting as needed
- Project closure. The final phase, where project managers reflect on project success and key learnings for next time
When concerning clinical trials, project management brings all of these phases together to ensure set up, enrollment, operations, and reporting are all done smoothly and effectively.
Roles of a clinical trial project manager
A clinical trial project manager may have different responsibilities specific to each trial, but in general, they will be tasked with vendor selection, budget oversight, IRB submissions, report creation, and meeting planning, all of which are detailed below.
Vendor selection: Because conducting a clinical trial requires many different elements, outside vendors will often be brought in to provide expertise in certain aspects of a study. This is most common for specialized elements such as Interactive Web Response Systems (IWRS) , electronic patient-reported outcome (ePRO) technology, and clinical trial patient recruitment . Often, it will be the project manager's job to vet these vendors and assist with comparing options.
Timeline and budget oversight: Though every study begins with a specific timeline and budget, nearly 80% of all clinical trials are delayed due to difficulties in patient recruitment, which causes many to exceed their budget. In these situations, a project manager can leverage their expertise to hold the trial team accountable for the time and money spent on the study, in addition to managing expectations should these elements begin to change.
IRB submissions: Any patient-facing materials involved in research studies must be reviewed and submitted for Institutional Review Board (IRB) approval, and gathering these materials is often the responsibility of the clinical trial project manager. Because every IRB is different, the project manager will need to look at previous submissions and any templates that are available to ensure the study's particular IRB requirements are met.
Report generation: As part of tracking the progress of a trial, clinical trial project managers should regularly generate and distribute reports on various aspects of a study’s progress. These reports can often be automated so they are not a time-consuming task, but they do play an essential role in keeping key stakeholders looped in on the progress of the research.
Meeting coordination: Occasionally, the trial’s key stakeholders may need to meet to review a trial’s progress and address any roadblocks. The project manager will likely be charged with planning and leading these meetings to ensure that all details are covered and relevant updates are provided.
Tips for effective project management in clinical trials
Create a detailed project plan
One of the best ways to circumvent delays and issues in a clinical trial is to create a detailed project plan before the study launches. This plan should include a timeline of milestones, key dates, a task schedule, and any other relevant pieces that can keep the project on track. Some people may prefer pen and paper for this, but there are also many online resources available to help project managers keep track of details and stay on schedule.
Anticipate risk management demands
Every project will have some level of risk, so it’s wise to acknowledge what points of contention may arise and plan accordingly. A few examples of risks associated with clinical trials include:
- Long wait times for IRB approval
- Delays in patient recruitment
- Turnover among site staff
- Changes to the trial protocol
Before the project starts, it can be helpful to come together as a team to discuss potential risks that may arise, share past experiences, and determine how they can be prevented or handled if they do arise.
Understand IRB requirements
In most studies, the project manager will also be tasked with gathering materials and submitting them for approval from the Institutional Review Board (IRB). Every IRB will vary on its guidelines, but IRB administrators should be able to answer any questions to ensure a smooth and efficient process.
Even if the responsibility of IRB submission is a task for the project manager, it can be helpful to enlist additional team members to provide a second set of eyes before the materials are submitted. It is also wise to create a checklist of elements that should be included in the IRB packet to ensure nothing gets left out.
Foster open communication between sites and sponsors
Another important communication piece for project managers is to share feedback from sites back with the sponsor of the trial. Communication is particularly important in relation to sharing updates with sponsors about recruitment or screening challenges the site may be facing.
Communication tips for project management
One of the most important jobs of a clinical trial project manager is to ensure clear and effective communication with multiple stakeholders. To manage this communication from the onset, it can be useful to create a list of stakeholders, the updates they'll need, and how often they should be informed.
To manage this communication, the RACI project management method can provide a helpful framework to organize stakeholders into four categories based on their involvement in the project and communication needs.
- Responsible: The responsible party is the main point person for communication – this is the stakeholder who does the actual work of this part of the project. For example, when submitting outreach material to the IRB, the person responsible for creating the material may be the lead on the marketing team or the contact at a clinical trial recruitment company.
- Accountable: The accountable person is generally the manager of the responsible party and may wish to be involved in only some of the updates related to the project. Generally, if the responsible party needs approval from their manager, they should do so before sharing updates more broadly.
- Consulted: The consulted party would be any additional stakeholders who should weigh in on a project. The responsible or accountable party can generally help project managers determine who should be involved in the consultation.
- Informed: These are people who are simply kept up-to-date on the progress of the project at appropriate intervals.
A paper on managing clinical trials published by the National Institutes of Health also mentions the importance of keeping the investigators themselves in the loop about a trial, stating, "Investigators need to feel valued and part of an inclusive team answering an important clinical question, so providing regular feedback that ensures they feel involved must be central to a trial's communication strategy." Because the investigators may also have busy clinical practices in addition to being part of the trial, it’s important to respect their time while making them feel involved and informed.
Project managers’ roles after a clinical trial
Project management is an ever-evolving skill and there are lessons to be learned from even the most successful project execution. It’s important that project managers evaluate each trial after it's complete in order to analyze trends and plan for the next one while key learnings are still top of mind. As Hubspot puts it, "a productive project post-mortem is a chance to fully unpack a project's trajectory and dig deeper into why things unfolded the way they did.”
Hosting a project post-mortem meeting involving key stakeholders is advisable. Sending a pre-meeting questionnaire can streamline the process of gathering thoughts on what went well, what didn't, and what could be done better next time. The agenda should include a recap of the project's goals and a review of the results so that any discrepancies can be addressed and everyone is able to come up with actionable takeaways for the future.
If you're interested in learning more about how Antidote begins the clinical trial recruitment process and manages the project throughout, download our recruitment template below.
Download now

Get our latest blog posts in your inbox

The Clinical Research Project Management Association (CRPM) was established to bring together clinical research professionals who utilize project management tools and methodologies to assist them in achieving project deliverables on time and within budget.
CRPM aims to connect members of the Clinical Research Project Management (CRPM) community through online groups, in-person meet-ups, monthly webinars, and retreats for project managers that target expanding the CRPM network throughout the clinical research industry.
CRPM strives to educate the CRPM community through professional and career development opportunities including articles, webinars, and in-person events to empower CRPMs with the skills and education needed to perform their roles efficiently and effectively.
CRPM is dedicated to improving the health, both mental and physical, of CRPMs in our community. CRPM is dedicated to seeing CRPMs as a whole person, not a productivity tool, which includes supporting the physical and mental wellness of CRPMs in a demanding career field. Health and wellness topics will be featured throughout the various platforms used in the CRPM community to support CRPMs
CRPM aspires to provide a space that helps CRPMs grow personally and professionally in their careers, recognizing that their success depends on a balanced approach to both their work and personal lives.
Vision of CRPM
CRPM envisions a future where Clinical Research Project Managers (CRPMs) have access to a supportive community that provides the resources, education, and tools needed to succeed in their careers. We are committed to promoting the health and wellness of our members, recognizing that their success depends on a balanced approach to both their work and personal lives. CRPM aims to foster a vibrant community of CRPMs that prioritize personal and professional growth while achieving a healthy and sustainable lifestyle.
The CRPM is a community of clinical research professionals dedicated to expanding our knowledge in project management and clinical research. We strive to connect CRPMs through a range of platforms, including online groups, in-person meet-ups, monthly webinars, and retreats, to expand the CRPM network throughout the clinical research industry. We are committed to promoting the health and wellness of our community, recognizing that the success of CRPMs depends on taking care of the whole individual.
CRPM Advisory Council
The Advisory Council for CRPM is a group of experienced and respected individuals in the clinical research industry who provide guidance and support to the organization’s leadership team. Members of the Advisory Council are selected based on their expertise in clinical research project management, healthcare, academia, and business.
The role of the Advisory Council is to provide strategic advice to the CRPM leadership team on matters related to the organization’s goals, mission, and vision. Council members also serve as ambassadors for CRPM, promoting the organization’s mission and objectives within their respective networks and industries.
Through regular communication and collaboration with the CRPM leadership team, the Advisory Council helps to ensure that the organization is well-positioned to meet the needs of its members and to advance the field of clinical research project management.
CRPM Members
CRPM Membership is open to clinical research professionals who utilize project management tools and methodologies to assist them in achieving project deliverables on time and within budget. Members gain the skills, knowledge, and connections needed to excel in their roles and advance their careers through exclusive educational and career development opportunities, networking events, industry news, and resources. CRPM members also have access to a variety of online resources, including webinars, forums, and other tools designed to help them stay informed and up-to-date on the latest trends and best practices in clinical research project management.
Learn more about CRPM Membership .
CRPM Volunteers
Volunteering with CRPM provides clinical research project managers with the opportunity to give back to the profession, connect with like-minded professionals, and gain valuable experience and skills that can help them advance their careers. Volunteers have the chance to make a meaningful contribution to the field of clinical research project management, helping to shape the industry’s future.
Learn more about Volunteering with CRPM .
CRPM Ambassadors
CRPM Ambassadors are members of the CRPM community who are passionate about the organization’s mission and goals and want to help spread the word about CRPM within their networks and beyond.
As a CRPM Ambassador, members act as advocates for the organization, sharing information about CRPM with their colleagues, friends, and other contacts. CRPM Ambassadors play an important role in helping the organization grow and expand its reach within the clinical research project management community.
Learn more about CRPM Ambassadors .
Project Management: Introduction to Tools and Templates
By: melissa harris, mpa, ccrp director of interventional resources & clinical trials unit pennington biomedical research center at lsu.
Abstract: Project management involves many complex components and moving parts. Prior to initiating a clinical trial, various types of project tools and templates can be used to successfully plan and execute a clinical trial. This article highlights tools that are readily available for project management, including Microsoft Excel, Access, Visio, Outlook, and SharePoint, as well as Web-based applications. Monitoring progress through various tracking mechanisms ensures successful clinical trial execution from recruitment through retention and follow-up.
Project Management
The project management life cycle for clinical trials is comprised of:
- Study start-up
- Team management
- Clinical assessment
- Intervention.
The project management tools covered in this article are described in relation to the project management life cycle. Examples are from clinical trials in academia; however, the tools can be used in any research setting.
At any given time, the project manager is shuffling plates and trying not to drop one. Project management can be considered similar to riding a bike. The project manager should be able to get on the bike or project and do the same thing on each ride, or in this case, from research project to research project. Unfortunately, in clinical research, the bike is “on fire,” the project manager is “on fire,” and everybody working on the clinical trial is “on fire.” This article provides tools to help douse the fire and continue to move forward on the research project.
There are many components of a study, from the research idea through analyzing the data and publishing results (Table 1). The most difficult parts of a project are study start-up and keeping the study going when recruitment is not going well. The project manager and study team try to complete the first four components of a study (research idea, protocol, grant, and institutional review board review) in as compacted an amount of time as possible. Sometimes this requires having a very strong foundation. Tools and processes can be rotated from study to study, enabling the project team to move through the cycle fairly quickly.
The clinical trial lifespan includes:
- Trial initiation and timeline management
- Creating and managing the budget
- Protocol/consent preparations, institutional review board (IRB) submissions, and revisions
- Development of processes and the manual of procedures (MOP)
- Liaison for contracts, subawards, and community partnerships
- Identifying and managing resources (staffing)
- Training/certification plans and tracking
- Ongoing communication and clinical trial oversight.
Tools help project managers calm the chaos of clinical trials. There are many project management tools, including:
- Microsoft Office Excel, Word, Access, Outlook, and SharePoint
Office 365 is not covered in this article, outside of Outlook, however, Office 365 offers a multitude of online apps.
Tools for Study Start-up
Tools for study start-up include organizational charts, timelines, and process flows (Table 2). A research program organizational chart documents a clear chain of command. It can create camaraderie and outlines responsibilities so that staff know to whom to report. The organizational chart also identifies people who have specialized positions, such as blinded staff. Visio is an easy tool to use in developing organizational charts.
Timelines provide milestone time points for various stages of start-up to be completed. They are crucial and should be revised constantly to align with the pace of the study. When developing a timeline, the author starts with the expected date of the first randomization or site activation and works backwards. The timeline maps the amount of time necessary for each step involved in study start-up. The timeline establishes clear goals for study staff so that each staff member knows the due date for assigned tasks.
Process flows, also known as flowcharts, allow a process and the steps in the process to be viewed at a glance. The author uses flowcharts for all extensive processes within a protocol, investigator brochure, or manual of procedures. It is much more efficient to refer to a flowchart when working with a study participant or working on other study tasks than to have to pull out a large document and search for the necessary information.
Cross-functional flowcharts, also known as swim lanes, demonstrate the process steps in sequential order and show who does each task. Decision channels (yes/no) can also be included in a cross-functional flowchart.
The cycle of implementing the project and maintaining it is extremely important. Implementation must constantly be re-evaluated to determine whether it is working. A project may be working; however, it could be more efficient. Without monitoring, the project manager will never know if project efficiency could be improved. Monitoring includes identifying inefficiencies, assessing the cost-benefit ratios, and monitoring expenses against the budget.
Tools for Team Management
Team management is a major component of running a clinical trial. The author spends much of her day reading emails from people who are updating her on the work that they are doing on a study. This makes it difficult for her to accomplish her tasks for that day. Regular team meetings are an important form of communication. Team meetings can minimize the need for many emails. They can be done electronically, through teleconferences, Web conferences, online reporting systems, etc.
Effective team management also requires ongoing communication with internal affiliates (other departments) and external affiliates (community partners). A 10-minute telephone call twice a month may be sufficient to communicate with internal and external affiliates. Ongoing communication on the study’s outcomes/progress is also necessary with regulators, funders, and other external affiliates.
Document libraries, calendars, and action items are good tools for team management (Table 3). Document libraries provide a central location for all departmental or project-specific files. They may be housed on shared drives such as Google drives or Dropbox. Since these shared drives do not comply with the Health Insurance Portability and Accountability Act, some universities do not allow their use. Universities often use tools such as SharePoint, OneDrive, and Basecamp, which staff can access from anywhere. A document library automatically backs up the documents every night.
Basecamp allows the project manager to set up study teams and provide different levels of access to documents for different team members. Assignments, schedules, and bookmarking of certain materials can also be done using Basecamp. In SharePoint, the project manager can create folders and list documents. SharePoint and Basecamp both track edits to documents.
Project managers and study staff use calendars, such as Outlook, extensively for scheduling appointments or responsibilities, participant scheduling, and study-specific calendars. Appointment reminder alerts are a key benefit of calendars. The author maintains a personal calendar and a department calendar to oversee staff activities via a central destination to book participant visits and other study related meetings.
The department calendar is color-coded so that people can easily see the type of visit: green for assessment visits, purple for remote data monitoring, and yellow for phone call visits to name a few examples. Red indicates something important, such as not scheduling participants for visits requiring online RedCap surveys on a day that Internet access will be shut off or when the center will be closed. Calendars also show when staff will be out of the office. Patient identifiers and notes can also be put into calendars so that staff can reference the invite for patient information.
Action items are a key component of team management. Pennington Biomedical Research Center does action items with the Interventional Resources Unit for administrative activities and study specific tasks for every study. Action items clarify tasks to be completed by members of the study team. Each action item is associated with a responsible person and the deadline. If study team members cannot meet their deadlines, they need to notify the author because her deadlines are contingent on team members meeting their deadlines. Action items also increase accountability by providing clear expectations.
Using SharePoint, the responsible staff member can update action items as she/he completes them so that the author does not to have to receive emails documenting this. SharePoint can also send notifications, emailing a staff member when she/he is assigned to a task. SharePoint can be used to prioritize tasks. This author has experienced a major challenge with Generation X and Z team members is who may have difficulty prioritizing. In the author’s experience, these generations may more often work on the last task assigned to them instead of the most important task.
Tools for Recruiting
Recruitment is the costliest part of clinical trials. Table 4 highlights tools for recruitment:
- Advertising timeline
- Recruitment goal tracking
- Recruitment budget tracking
- Participant flow diagram
- Enrollment predictions.
The author develops an advertising timeline that is separate from the overall study timeline. The advertising timeline has recruiting and advertising tasks, with color coding for tasks that have been completed, and yield rates of completed events. Pennington Biomedical Research Center does a great deal of community-based recruitment. The yield rates (number of participants randomized) of completed events show the most effective recruitment methods for each quarter or year. This enables staff to repeat the most effective recruitment methods.
Tracking recruitment goals is very helpful. Many of the clinical trials conducted at Pennington Biomedical Research Center are funded by the National Institutes of Health (NIH) or another government agency or department. These trials have quarterly recruitment goals. The author usually uses more aggressive goals than the NIH’s goals, since it is easier to recruit participants earlier in the grant when the project is novel and exciting to potential study participants that may be reached during the recruitment process.
Achieving recruitment goals requires providing the study team with clear expectations. Tracking enables project managers to assess monthly/quarterly randomization goals to see when the clinical research site was most successful and to identify effective recruitment methods that can be used again.
Tracking spending on recruitment is also helpful. The most expensive recruitment methods, such as television and radio advertising, may not be providing the most participants. Tracking spending and sources of participants enables the project manager to assess the cost effectiveness of advertising campaigns and adjust them as needed. Pennington Biomedical Research Center has different departments for recruitment and advertising. The departments have designated budgets over the study year yet coordinate marketing and outreach activities to maximize recruitment reach.
The participant flow diagram is one of the author’s most important tools. A participant flow diagram tracks what is happening in real time, allowing project managers to see where participants are in any part of the study flow. It also lets study team members see when potential research participants and enrolled participants are lost due to exclusionary criteria or dropouts. A participant flow diagram documents the ratio of phone screens to randomization and the number of participants in the pipeline.
Screening yields can also be reviewed through the participant flow diagram. Pennington Biomedical Research Center always assesses why the clinical research site is losing potential subjects. This sometimes enables the project manager to make changes. For example, by tracking screening yields, Pennington Biomedical Research Center has found that people were being excluded from a study in the phone screen because they did not understand a question. In response to this problem, the question was clarified. Enrollment predictions can also be done with a participant flow diagram, and the pending pipeline can be assessed. Finally, retention rates can be assessed with the same diagram by looking at the number of completed, anticipated, and pending visits at each follow-up time point. This could allow for the project manager to identify whether a particular follow-up visit is problematic in return rates for study participants. This could lead to more intensive staff contact for said visits to work to improve these rates for future visit windows.
Tools for Clinical Assessment
Electronic case report forms (CRFs), visit scheduling, and visit windows are tools for clinical assessment (Table 5). The world is moving toward electronic CRFs. Some sites and PIs may be reluctant to eliminate paper especially with specific clinical trial populations such as the elderly. However, technology is moving clinical and research practices towards paperless data entry. In this author’s experience, many industry and pharmaceutical clinical trials are paperless or at least using electronic data capture options in many of their trials.
RedCap, built at Vanderbilt University, is a secure Web application for electronic data capture. Various levels of access can be set up for different staff members. Participant self-reported forms captured via surveys are part of RedCap. These surveys can be sent by email. Rather than call participants to collect information such as adverse events and weight, RedCap can send out automated emails on a timer to collect this information. Pennington Biomedical Research Center sets these up in advance and only has to contact participants when they do not complete the surveys.
Visit scheduling windows can be set in various electronic platforms. Pennington Biomedical Research Center uses Outlook or Sharepoint for visit schedules for some trials. The visit schedules show the start and stop time, preventing double booking of staff. If the visit includes laboratory testing, the system can send an alert to the laboratory with an appointment reminder. RedCap also does visit scheduling. The visit schedule can be printed for study participants or for the study folder.
Tools for Intervention
Real-time data capture, adherence and compliance reporting, and retention tracking are tools for intervention (Table 6). Pennington Biomedical Research Center does many large multi-site exercise clinical trials or trials with many participants. In one study, 300 participants came to the center three times a week. Instead of writing all of the exercise prescriptions and data capture on paper, staff created the Exercise Database for Intervention (EDIN) to capture the exercise data in real-time using laptops on rolling carts. iPads can also be used to collect data in real time.
With real-time data capture, the data are automatically entered into a website or clinical trial management system. Real-time data capture also allows compliance reports to be generated instantly.
Other data capture tools include Fitabase, heart rate monitors, and body trace scales. When Fitbits are used in a study, its Fitabase can be used to look at data for all participants together. Fitabase provides more data than the data that are available on the app. Participants do need to sync their Fitbits in order for researchers to use Fitabase.
PolarÔ Heart Rates Monitor and Zephyr can be used to monitor heart rates, including monitoring the heart rates of a group of people at once. Body trace scales are sent home with the participants, where they transmit weight wirelessly to Pennington Biomedical Research Center. Study staff can review trends and share individual data with each participant.
Adherence and compliance reporting is necessary because it is important for participants to stay in the study and to comply with the intervention. Project managers and study teams need to monitor compliance. Pennington Biomedical Research Center extracts adherence and compliance information and puts it in a format that will resonate with investigators. Staff create monthly or weekly participant compliance reports depending on the speed of the study. These reports can show what is happening between groups or within a group.
Since all of the information is in the system, staff can generate reports for participants such as score cards or report cards. Participants often enjoy receiving these reports. If a participant is not doing something well, this is an opportunity for study staff to discuss any challenges and ways to overcome those challenges.
In order to facilitate intervention retention, Pennington Biomedical Research Center does case assessment to identify thresholds for adherence or compliance. Any participant who reaches the threshold for poor compliance is assigned to a study staff member who acts as a case manager and troubleshoots problems.
Staff also assess reasons for poor compliance to identify trends. They adjust screening and/or retention methods based on assessment results.
The author is often asked how Pennington Biomedical Research Center tracks contacts with research participants. It is important to know why participants miss visits and the number of times that study staff call them. Clinical research sites must have a retention/participant contact system in place such as a SharePoint list. Pennington Biomedical Research Center’s retention/participant contact system lets the author see the visit window and when to call. She assigns a study staff member to call participants on the specified dates.
Take-Home Messages
Clinical research sites should not rely solely on the successes or failures of past programs or models. It is often necessary to tailor tools to a specific study. In order to be successful over time, clinical research sites must establish:
- A strong infrastructure
- Clear operational procedures
- A variety of tools to monitor research programs, study teams, and research participants.
Continual evaluation and revision of the research program is necessary. If something works very well, keep doing it. If something does not work, reevaluate it and shift to a more effective strategy. Project managers and study staff must be willing to adapt and change. Some study staff members may require more micro-management than others. The author uses electronic platforms to manage study staff, which is less confronting than managing them face to face.
Project managers should create a versatile study team that matches the needs of the research program and has a great deal of information. Team members will be different. Some may be very technologically savvy while others may not be technologically savvy.
Increasing efficiencies by saving minutes a day does matter. This can reduce staff burden, burnout, and turnover. Project managers and study staff should work smarter, not harder.
Study Components
- Choose a topic
- Create a hypothesis
- Develop a plan
- Submit a grant for funding
- Submit the protocol for approval
- Market the study to the target population
- Screen potential participants by telephone
- Orient participants
- Obtain informed consent
- Inclusion/exclusion criteria
- Compliance assessment
- Perform initial assessments
- Conduct study group
- Monitor progress
- Test outcomes for changes
- Prove/disprove hypothesis
- Assess outcomes
- Publish findings
- Outlines chain of command
- Outlines responsibilities
- Helpful for all study staff to appreciate where they fall and where other’s fall
- Provides pre-identified time points for completion of various stages of start-up
- Establishes clear goals for study staff
- Ever-changing with the pace of the study
- Also known as flowcharts
- Allows process and steps to be viewed at a glance
- Identifies actions within a process in a sequential order
- Provides specifics of process steps with relevant “if, then” scenarios
- Central location for all departmental or project-specific files
- Increases accessibility
- Increases dissemination of information
- Archived history of all documents, processes, data, etc.
- Document security with automatic nightly backups
- Staff scheduling for designated appointments or responsibilities
- Participant scheduling for appointments or procedures
- Study-specific calendar for meetings, visits, etc.
- Clear assignment of staff to participant visits
- Appointment reminder alerts
- Ease of identifying other staff members’ availability
- Reduces double booking staff and appointments
- Color coding to easily identify appointments
- Clarify tasks to be completed by the study team
- Provide study team pending action items with associated deadlines
- Assign actions items to designated staff
- Increase accountability through clear expectations
Tools for Recruitment
- Timeline of recruitment and advertisement events
- Yield rates of completed events
- Identify and track study recruitment requirements
- Assess monthly/quarterly randomization goals
- Monitor progress
- Adjust advertising campaigns as needed
- Identify and track study recruitment budget
- Assess cost effectiveness of advertising campaigns
- Determines screening yields
- Assess where participants are lost due to dropouts or exclusionary criteria
- Determine ratio of phone screens to randomization
- Stage of the process for pending participants
- Management of N in each arm of the trial
- Follow-up visit completion rates
- Assess pending pipeline
- Use throughput rates to predict enrollment
- Predict quantity needed to reach goals
- Paperless electronic data capture systems
- Participant self-reported forms captured via surveys
- Auto-generated timed survey requests for completion
- Calculated visit windows for follow-up testing
- Tracking scheduled and actual visit dates
- Pending visit reports
Tools for Intervention
- Real-time data
- Easily accessible compliance reports
- Assess participant compliance monthly
- Identify discrepancies and any areas of concern
- Generating reports for participants
- Tracking participants’ attendance and reasons by the individual, group, cohort, month, etc.
- Tracking retention procedures
- Tracking contact attempts during dropout recovery
2 thoughts on “Project Management: Introduction to Tools and Templates”
where is the tools template?
Hey, fellow readers! I just finished reading the article on project management tools and templates, and I couldn’t resist leaving a comment here. First off, I want to thank the author for putting together such a comprehensive and informative piece. As someone who’s relatively new to the project management world, this article was like a goldmine of practical tips and resources.
The way the author explained various project management tools, from Gantt charts to PERT diagrams, was incredibly helpful. I always found these concepts a bit overwhelming, but the article managed to break them down into digestible chunks, making it easier for me to understand their applications. The best part is that they provided links to free templates and software, which is a lifesaver for anyone on a budget.
Moreover, the insights on how to choose the right tools for different types of projects were enlightening. Understanding that not all projects are the same and tailoring our approach accordingly is crucial for success. I’ve already bookmarked this article for future reference and plan to explore the recommended tools further.
Lastly, I want to express my gratitude for the tips on how to collaborate effectively with teams using these tools. As a project manager, fostering good communication and collaboration is essential, and the author’s suggestions will undoubtedly prove invaluable in my journey.
Great job on this article! I can’t wait to dive deeper into project management armed with these newfound knowledge and resources. Keep up the fantastic work, and I’ll be eagerly awaiting more insightful pieces from this blog. Cheers!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
This site uses Akismet to reduce spam. Learn how your comment data is processed .
- Search Menu
- Browse content in Arts and Humanities
- Browse content in Archaeology
- Anglo-Saxon and Medieval Archaeology
- Archaeological Methodology and Techniques
- Archaeology by Region
- Archaeology of Religion
- Archaeology of Trade and Exchange
- Biblical Archaeology
- Contemporary and Public Archaeology
- Environmental Archaeology
- Historical Archaeology
- History and Theory of Archaeology
- Industrial Archaeology
- Landscape Archaeology
- Mortuary Archaeology
- Prehistoric Archaeology
- Underwater Archaeology
- Urban Archaeology
- Zooarchaeology
- Browse content in Architecture
- Architectural Structure and Design
- History of Architecture
- Residential and Domestic Buildings
- Theory of Architecture
- Browse content in Art
- Art Subjects and Themes
- History of Art
- Industrial and Commercial Art
- Theory of Art
- Biographical Studies
- Byzantine Studies
- Browse content in Classical Studies
- Classical History
- Classical Philosophy
- Classical Mythology
- Classical Literature
- Classical Reception
- Classical Art and Architecture
- Classical Oratory and Rhetoric
- Greek and Roman Epigraphy
- Greek and Roman Law
- Greek and Roman Papyrology
- Greek and Roman Archaeology
- Late Antiquity
- Religion in the Ancient World
- Digital Humanities
- Browse content in History
- Colonialism and Imperialism
- Diplomatic History
- Environmental History
- Genealogy, Heraldry, Names, and Honours
- Genocide and Ethnic Cleansing
- Historical Geography
- History by Period
- History of Emotions
- History of Agriculture
- History of Education
- History of Gender and Sexuality
- Industrial History
- Intellectual History
- International History
- Labour History
- Legal and Constitutional History
- Local and Family History
- Maritime History
- Military History
- National Liberation and Post-Colonialism
- Oral History
- Political History
- Public History
- Regional and National History
- Revolutions and Rebellions
- Slavery and Abolition of Slavery
- Social and Cultural History
- Theory, Methods, and Historiography
- Urban History
- World History
- Browse content in Language Teaching and Learning
- Language Learning (Specific Skills)
- Language Teaching Theory and Methods
- Browse content in Linguistics
- Applied Linguistics
- Cognitive Linguistics
- Computational Linguistics
- Forensic Linguistics
- Grammar, Syntax and Morphology
- Historical and Diachronic Linguistics
- History of English
- Language Acquisition
- Language Evolution
- Language Reference
- Language Variation
- Language Families
- Lexicography
- Linguistic Anthropology
- Linguistic Theories
- Linguistic Typology
- Phonetics and Phonology
- Psycholinguistics
- Sociolinguistics
- Translation and Interpretation
- Writing Systems
- Browse content in Literature
- Bibliography
- Children's Literature Studies
- Literary Studies (Asian)
- Literary Studies (European)
- Literary Studies (Eco-criticism)
- Literary Studies (Romanticism)
- Literary Studies (American)
- Literary Studies (Modernism)
- Literary Studies - World
- Literary Studies (1500 to 1800)
- Literary Studies (19th Century)
- Literary Studies (20th Century onwards)
- Literary Studies (African American Literature)
- Literary Studies (British and Irish)
- Literary Studies (Early and Medieval)
- Literary Studies (Fiction, Novelists, and Prose Writers)
- Literary Studies (Gender Studies)
- Literary Studies (Graphic Novels)
- Literary Studies (History of the Book)
- Literary Studies (Plays and Playwrights)
- Literary Studies (Poetry and Poets)
- Literary Studies (Postcolonial Literature)
- Literary Studies (Queer Studies)
- Literary Studies (Science Fiction)
- Literary Studies (Travel Literature)
- Literary Studies (War Literature)
- Literary Studies (Women's Writing)
- Literary Theory and Cultural Studies
- Mythology and Folklore
- Shakespeare Studies and Criticism
- Browse content in Media Studies
- Browse content in Music
- Applied Music
- Dance and Music
- Ethics in Music
- Ethnomusicology
- Gender and Sexuality in Music
- Medicine and Music
- Music Cultures
- Music and Religion
- Music and Media
- Music and Culture
- Music Education and Pedagogy
- Music Theory and Analysis
- Musical Scores, Lyrics, and Libretti
- Musical Structures, Styles, and Techniques
- Musicology and Music History
- Performance Practice and Studies
- Race and Ethnicity in Music
- Sound Studies
- Browse content in Performing Arts
- Browse content in Philosophy
- Aesthetics and Philosophy of Art
- Epistemology
- Feminist Philosophy
- History of Western Philosophy
- Metaphysics
- Moral Philosophy
- Non-Western Philosophy
- Philosophy of Science
- Philosophy of Language
- Philosophy of Mind
- Philosophy of Perception
- Philosophy of Action
- Philosophy of Law
- Philosophy of Religion
- Philosophy of Mathematics and Logic
- Practical Ethics
- Social and Political Philosophy
- Browse content in Religion
- Biblical Studies
- Christianity
- East Asian Religions
- History of Religion
- Judaism and Jewish Studies
- Qumran Studies
- Religion and Education
- Religion and Health
- Religion and Politics
- Religion and Science
- Religion and Law
- Religion and Art, Literature, and Music
- Religious Studies
- Browse content in Society and Culture
- Cookery, Food, and Drink
- Cultural Studies
- Customs and Traditions
- Ethical Issues and Debates
- Hobbies, Games, Arts and Crafts
- Lifestyle, Home, and Garden
- Natural world, Country Life, and Pets
- Popular Beliefs and Controversial Knowledge
- Sports and Outdoor Recreation
- Technology and Society
- Travel and Holiday
- Visual Culture
- Browse content in Law
- Arbitration
- Browse content in Company and Commercial Law
- Commercial Law
- Company Law
- Browse content in Comparative Law
- Systems of Law
- Competition Law
- Browse content in Constitutional and Administrative Law
- Government Powers
- Judicial Review
- Local Government Law
- Military and Defence Law
- Parliamentary and Legislative Practice
- Construction Law
- Contract Law
- Browse content in Criminal Law
- Criminal Procedure
- Criminal Evidence Law
- Sentencing and Punishment
- Employment and Labour Law
- Environment and Energy Law
- Browse content in Financial Law
- Banking Law
- Insolvency Law
- History of Law
- Human Rights and Immigration
- Intellectual Property Law
- Browse content in International Law
- Private International Law and Conflict of Laws
- Public International Law
- IT and Communications Law
- Jurisprudence and Philosophy of Law
- Law and Politics
- Law and Society
- Browse content in Legal System and Practice
- Courts and Procedure
- Legal Skills and Practice
- Primary Sources of Law
- Regulation of Legal Profession
- Medical and Healthcare Law
- Browse content in Policing
- Criminal Investigation and Detection
- Police and Security Services
- Police Procedure and Law
- Police Regional Planning
- Browse content in Property Law
- Personal Property Law
- Study and Revision
- Terrorism and National Security Law
- Browse content in Trusts Law
- Wills and Probate or Succession
- Browse content in Medicine and Health
- Browse content in Allied Health Professions
- Arts Therapies
- Clinical Science
- Dietetics and Nutrition
- Occupational Therapy
- Operating Department Practice
- Physiotherapy
- Radiography
- Speech and Language Therapy
- Browse content in Anaesthetics
- General Anaesthesia
- Neuroanaesthesia
- Browse content in Clinical Medicine
- Acute Medicine
- Cardiovascular Medicine
- Clinical Genetics
- Clinical Pharmacology and Therapeutics
- Dermatology
- Endocrinology and Diabetes
- Gastroenterology
- Genito-urinary Medicine
- Geriatric Medicine
- Infectious Diseases
- Medical Toxicology
- Medical Oncology
- Pain Medicine
- Palliative Medicine
- Rehabilitation Medicine
- Respiratory Medicine and Pulmonology
- Rheumatology
- Sleep Medicine
- Sports and Exercise Medicine
- Clinical Neuroscience
- Community Medical Services
- Critical Care
- Emergency Medicine
- Forensic Medicine
- Haematology
- History of Medicine
- Browse content in Medical Dentistry
- Oral and Maxillofacial Surgery
- Paediatric Dentistry
- Restorative Dentistry and Orthodontics
- Surgical Dentistry
- Browse content in Medical Skills
- Clinical Skills
- Communication Skills
- Nursing Skills
- Surgical Skills
- Medical Ethics
- Medical Statistics and Methodology
- Browse content in Neurology
- Clinical Neurophysiology
- Neuropathology
- Nursing Studies
- Browse content in Obstetrics and Gynaecology
- Gynaecology
- Occupational Medicine
- Ophthalmology
- Otolaryngology (ENT)
- Browse content in Paediatrics
- Neonatology
- Browse content in Pathology
- Chemical Pathology
- Clinical Cytogenetics and Molecular Genetics
- Histopathology
- Medical Microbiology and Virology
- Patient Education and Information
- Browse content in Pharmacology
- Psychopharmacology
- Browse content in Popular Health
- Caring for Others
- Complementary and Alternative Medicine
- Self-help and Personal Development
- Browse content in Preclinical Medicine
- Cell Biology
- Molecular Biology and Genetics
- Reproduction, Growth and Development
- Primary Care
- Professional Development in Medicine
- Browse content in Psychiatry
- Addiction Medicine
- Child and Adolescent Psychiatry
- Forensic Psychiatry
- Learning Disabilities
- Old Age Psychiatry
- Psychotherapy
- Browse content in Public Health and Epidemiology
- Epidemiology
- Public Health
- Browse content in Radiology
- Clinical Radiology
- Interventional Radiology
- Nuclear Medicine
- Radiation Oncology
- Reproductive Medicine
- Browse content in Surgery
- Cardiothoracic Surgery
- Gastro-intestinal and Colorectal Surgery
- General Surgery
- Neurosurgery
- Paediatric Surgery
- Peri-operative Care
- Plastic and Reconstructive Surgery
- Surgical Oncology
- Transplant Surgery
- Trauma and Orthopaedic Surgery
- Vascular Surgery
- Browse content in Science and Mathematics
- Browse content in Biological Sciences
- Aquatic Biology
- Biochemistry
- Bioinformatics and Computational Biology
- Developmental Biology
- Ecology and Conservation
- Evolutionary Biology
- Genetics and Genomics
- Microbiology
- Molecular and Cell Biology
- Natural History
- Plant Sciences and Forestry
- Research Methods in Life Sciences
- Structural Biology
- Systems Biology
- Zoology and Animal Sciences
- Browse content in Chemistry
- Analytical Chemistry
- Computational Chemistry
- Crystallography
- Environmental Chemistry
- Industrial Chemistry
- Inorganic Chemistry
- Materials Chemistry
- Medicinal Chemistry
- Mineralogy and Gems
- Organic Chemistry
- Physical Chemistry
- Polymer Chemistry
- Study and Communication Skills in Chemistry
- Theoretical Chemistry
- Browse content in Computer Science
- Artificial Intelligence
- Computer Architecture and Logic Design
- Game Studies
- Human-Computer Interaction
- Mathematical Theory of Computation
- Programming Languages
- Software Engineering
- Systems Analysis and Design
- Virtual Reality
- Browse content in Computing
- Business Applications
- Computer Security
- Computer Games
- Computer Networking and Communications
- Digital Lifestyle
- Graphical and Digital Media Applications
- Operating Systems
- Browse content in Earth Sciences and Geography
- Atmospheric Sciences
- Environmental Geography
- Geology and the Lithosphere
- Maps and Map-making
- Meteorology and Climatology
- Oceanography and Hydrology
- Palaeontology
- Physical Geography and Topography
- Regional Geography
- Soil Science
- Urban Geography
- Browse content in Engineering and Technology
- Agriculture and Farming
- Biological Engineering
- Civil Engineering, Surveying, and Building
- Electronics and Communications Engineering
- Energy Technology
- Engineering (General)
- Environmental Science, Engineering, and Technology
- History of Engineering and Technology
- Mechanical Engineering and Materials
- Technology of Industrial Chemistry
- Transport Technology and Trades
- Browse content in Environmental Science
- Applied Ecology (Environmental Science)
- Conservation of the Environment (Environmental Science)
- Environmental Sustainability
- Environmentalist Thought and Ideology (Environmental Science)
- Management of Land and Natural Resources (Environmental Science)
- Natural Disasters (Environmental Science)
- Nuclear Issues (Environmental Science)
- Pollution and Threats to the Environment (Environmental Science)
- Social Impact of Environmental Issues (Environmental Science)
- History of Science and Technology
- Browse content in Materials Science
- Ceramics and Glasses
- Composite Materials
- Metals, Alloying, and Corrosion
- Nanotechnology
- Browse content in Mathematics
- Applied Mathematics
- Biomathematics and Statistics
- History of Mathematics
- Mathematical Education
- Mathematical Finance
- Mathematical Analysis
- Numerical and Computational Mathematics
- Probability and Statistics
- Pure Mathematics
- Browse content in Neuroscience
- Cognition and Behavioural Neuroscience
- Development of the Nervous System
- Disorders of the Nervous System
- History of Neuroscience
- Invertebrate Neurobiology
- Molecular and Cellular Systems
- Neuroendocrinology and Autonomic Nervous System
- Neuroscientific Techniques
- Sensory and Motor Systems
- Browse content in Physics
- Astronomy and Astrophysics
- Atomic, Molecular, and Optical Physics
- Biological and Medical Physics
- Classical Mechanics
- Computational Physics
- Condensed Matter Physics
- Electromagnetism, Optics, and Acoustics
- History of Physics
- Mathematical and Statistical Physics
- Measurement Science
- Nuclear Physics
- Particles and Fields
- Plasma Physics
- Quantum Physics
- Relativity and Gravitation
- Semiconductor and Mesoscopic Physics
- Browse content in Psychology
- Affective Sciences
- Clinical Psychology
- Cognitive Psychology
- Cognitive Neuroscience
- Criminal and Forensic Psychology
- Developmental Psychology
- Educational Psychology
- Evolutionary Psychology
- Health Psychology
- History and Systems in Psychology
- Music Psychology
- Neuropsychology
- Organizational Psychology
- Psychological Assessment and Testing
- Psychology of Human-Technology Interaction
- Psychology Professional Development and Training
- Research Methods in Psychology
- Social Psychology
- Browse content in Social Sciences
- Browse content in Anthropology
- Anthropology of Religion
- Human Evolution
- Medical Anthropology
- Physical Anthropology
- Regional Anthropology
- Social and Cultural Anthropology
- Theory and Practice of Anthropology
- Browse content in Business and Management
- Business Strategy
- Business Ethics
- Business History
- Business and Government
- Business and Technology
- Business and the Environment
- Comparative Management
- Corporate Governance
- Corporate Social Responsibility
- Entrepreneurship
- Health Management
- Human Resource Management
- Industrial and Employment Relations
- Industry Studies
- Information and Communication Technologies
- International Business
- Knowledge Management
- Management and Management Techniques
- Operations Management
- Organizational Theory and Behaviour
- Pensions and Pension Management
- Public and Nonprofit Management
- Strategic Management
- Supply Chain Management
- Browse content in Criminology and Criminal Justice
- Criminal Justice
- Criminology
- Forms of Crime
- International and Comparative Criminology
- Youth Violence and Juvenile Justice
- Development Studies
- Browse content in Economics
- Agricultural, Environmental, and Natural Resource Economics
- Asian Economics
- Behavioural Finance
- Behavioural Economics and Neuroeconomics
- Econometrics and Mathematical Economics
- Economic Systems
- Economic History
- Economic Methodology
- Economic Development and Growth
- Financial Markets
- Financial Institutions and Services
- General Economics and Teaching
- Health, Education, and Welfare
- History of Economic Thought
- International Economics
- Labour and Demographic Economics
- Law and Economics
- Macroeconomics and Monetary Economics
- Microeconomics
- Public Economics
- Urban, Rural, and Regional Economics
- Welfare Economics
- Browse content in Education
- Adult Education and Continuous Learning
- Care and Counselling of Students
- Early Childhood and Elementary Education
- Educational Equipment and Technology
- Educational Strategies and Policy
- Higher and Further Education
- Organization and Management of Education
- Philosophy and Theory of Education
- Schools Studies
- Secondary Education
- Teaching of a Specific Subject
- Teaching of Specific Groups and Special Educational Needs
- Teaching Skills and Techniques
- Browse content in Environment
- Applied Ecology (Social Science)
- Climate Change
- Conservation of the Environment (Social Science)
- Environmentalist Thought and Ideology (Social Science)
- Natural Disasters (Environment)
- Social Impact of Environmental Issues (Social Science)
- Browse content in Human Geography
- Cultural Geography
- Economic Geography
- Political Geography
- Browse content in Interdisciplinary Studies
- Communication Studies
- Museums, Libraries, and Information Sciences
- Browse content in Politics
- African Politics
- Asian Politics
- Chinese Politics
- Comparative Politics
- Conflict Politics
- Elections and Electoral Studies
- Environmental Politics
- European Union
- Foreign Policy
- Gender and Politics
- Human Rights and Politics
- Indian Politics
- International Relations
- International Organization (Politics)
- International Political Economy
- Irish Politics
- Latin American Politics
- Middle Eastern Politics
- Political Methodology
- Political Communication
- Political Philosophy
- Political Sociology
- Political Behaviour
- Political Economy
- Political Institutions
- Political Theory
- Politics and Law
- Public Administration
- Public Policy
- Quantitative Political Methodology
- Regional Political Studies
- Russian Politics
- Security Studies
- State and Local Government
- UK Politics
- US Politics
- Browse content in Regional and Area Studies
- African Studies
- Asian Studies
- East Asian Studies
- Japanese Studies
- Latin American Studies
- Middle Eastern Studies
- Native American Studies
- Scottish Studies
- Browse content in Research and Information
- Research Methods
- Browse content in Social Work
- Addictions and Substance Misuse
- Adoption and Fostering
- Care of the Elderly
- Child and Adolescent Social Work
- Couple and Family Social Work
- Developmental and Physical Disabilities Social Work
- Direct Practice and Clinical Social Work
- Emergency Services
- Human Behaviour and the Social Environment
- International and Global Issues in Social Work
- Mental and Behavioural Health
- Social Justice and Human Rights
- Social Policy and Advocacy
- Social Work and Crime and Justice
- Social Work Macro Practice
- Social Work Practice Settings
- Social Work Research and Evidence-based Practice
- Welfare and Benefit Systems
- Browse content in Sociology
- Childhood Studies
- Community Development
- Comparative and Historical Sociology
- Economic Sociology
- Gender and Sexuality
- Gerontology and Ageing
- Health, Illness, and Medicine
- Marriage and the Family
- Migration Studies
- Occupations, Professions, and Work
- Organizations
- Population and Demography
- Race and Ethnicity
- Social Theory
- Social Movements and Social Change
- Social Research and Statistics
- Social Stratification, Inequality, and Mobility
- Sociology of Religion
- Sociology of Education
- Sport and Leisure
- Urban and Rural Studies
- Browse content in Warfare and Defence
- Defence Strategy, Planning, and Research
- Land Forces and Warfare
- Military Administration
- Military Life and Institutions
- Naval Forces and Warfare
- Other Warfare and Defence Issues
- Peace Studies and Conflict Resolution
- Weapons and Equipment

- < Previous chapter
- Next chapter >

21 Research project management
- Published: February 2016
- Cite Icon Cite
- Permissions Icon Permissions
What is a project? - Stage 1: Definition: defining and agreeing what the project is about - Stage 2: Planning: planning how the project will be conducted - Stage 3: Implementation and control: running the project - Stage 4: Close out: delivery and the end of the project
Signed in as
Institutional accounts.
- Google Scholar Indexing
- GoogleCrawler [DO NOT DELETE]
Personal account
- Sign in with email/username & password
- Get email alerts
- Save searches
- Purchase content
- Activate your purchase/trial code
Institutional access
- Sign in with a library card Sign in with username/password Recommend to your librarian
- Institutional account management
- Get help with access
Access to content on Oxford Academic is often provided through institutional subscriptions and purchases. If you are a member of an institution with an active account, you may be able to access content in one of the following ways:
IP based access
Typically, access is provided across an institutional network to a range of IP addresses. This authentication occurs automatically, and it is not possible to sign out of an IP authenticated account.
Sign in through your institution
Choose this option to get remote access when outside your institution. Shibboleth/Open Athens technology is used to provide single sign-on between your institution’s website and Oxford Academic.
- Click Sign in through your institution.
- Select your institution from the list provided, which will take you to your institution's website to sign in.
- When on the institution site, please use the credentials provided by your institution. Do not use an Oxford Academic personal account.
- Following successful sign in, you will be returned to Oxford Academic.
If your institution is not listed or you cannot sign in to your institution’s website, please contact your librarian or administrator.
Sign in with a library card
Enter your library card number to sign in. If you cannot sign in, please contact your librarian.
Society Members
Society member access to a journal is achieved in one of the following ways:
Sign in through society site
Many societies offer single sign-on between the society website and Oxford Academic. If you see ‘Sign in through society site’ in the sign in pane within a journal:
- Click Sign in through society site.
- When on the society site, please use the credentials provided by that society. Do not use an Oxford Academic personal account.
If you do not have a society account or have forgotten your username or password, please contact your society.
Sign in using a personal account
Some societies use Oxford Academic personal accounts to provide access to their members. See below.
A personal account can be used to get email alerts, save searches, purchase content, and activate subscriptions.
Some societies use Oxford Academic personal accounts to provide access to their members.
Viewing your signed in accounts
Click the account icon in the top right to:
- View your signed in personal account and access account management features.
- View the institutional accounts that are providing access.
Signed in but can't access content
Oxford Academic is home to a wide variety of products. The institutional subscription may not cover the content that you are trying to access. If you believe you should have access to that content, please contact your librarian.
For librarians and administrators, your personal account also provides access to institutional account management. Here you will find options to view and activate subscriptions, manage institutional settings and access options, access usage statistics, and more.
Our books are available by subscription or purchase to libraries and institutions.
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Rights and permissions
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
ACRP Certification
With a 30-year legacy, acrp certification is the most reputable credentialing program in clinical research. since 1992, more than 40,000 professionals and their employers have come to trust acrp certification as the mark of excellence in clinical research., “joining acrp and becoming certified was the best thing i ever did to jumpstart my career in research and has opened many doors for me.”, jeri burr, ms, rn, ped-bc, ccra, facrp acrp certified since 1999, benefits of certification >, start your next career journey by exploring acrp’s flagship certification and subspecialty credential programs below. each program provides specific information and resources on exam eligibility, exam content, how to apply, scheduling & testing, how to prepare, and after your test. if you have questions, explore the faq page or the acrp certification handbook ., acrp certified professional, acrp-cp ® is a credential formally recognizing clinical research professionals of all types, regardless of their roles or functional activities on the clinical study team., learn more >, certified clinical research associate, ccra ® is a credential formally recognizing clinical research professionals with experience monitoring and supervising the conduct and progress of clinical trials on behalf of a sponsor., certified clinical research coordinator, ccrc ® is a credential formally recognizing clinical research professionals with experience coordinating and facilitating clinical trial activities in adherence to gcp, under the direction of a principal investigator., certified principal investigator, cpi ® is a credential formally recognizing clinical research professionals with experience as a principal investigator or sub investigator on multiple studies., acrp medical device professional, acrp-mdp ® is a credential formally recognizing clinical research professionals with specialized knowledge in medical device clinical trials. candidates must be acrp certified to sit for this exam., acrp project manager, acrp-pm ® is a credential formally recognizing clinical research professionals with specialized knowledge in project management. candidates must be acrp certified to sit for this exam., the academy’s acrp-cp, ccrc, ccra, and cpi programs are accredited by the national commission for certifying agencies (ncca), which sets internationally recognized standards for the development and operation of certification programs. the standards assure that a program is valid, reflects current practice, and treats candidates fairly and are based on the established processes for developing certification exams., the most respected certification thought leaders in the country agree that ncca accreditation is the gold standard when it comes to accreditation of programs that certify professionals working in healthcare—including the clinical research industry. when you’re searching for certification programs, make sure you always look for the ncca-accredited program seal., bring certification to your team —acrp certification is an ideal solution for managers looking to enhance team knowledge, grow leaders, and signify to others that their study teams are among the best of the best. learn more >.

Review of Myopia Management
Myopia research highlights from the arvo 2024 meeting.

May 10, 2024
By Dwight Akerman, OD, MBA, FAAO, FBCLA, FIACLE
Once again, myopia research took center stage at the Association for Research in Vision and Ophthalmology (ARVO) Meeting , held May 5–9, 2024, in Seattle, WA. The meeting featured more than 150 posters and papers on various aspects of myopia and myopia management. Here are 20 noteworthy myopia-related posters and papers:
- 3-year myopia control efficacy can be predicted from 1-year data https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4036500&external=true
- A Century of Myopia in the Netherlands: Unveiling the Emerging Epidemic https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4048429&external=true
- Efficacy of a next-generation design of ophthalmic lenses for myopia control: Six-month results of the CEME Study https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4045244&external=true
- Predictive factors associated with incident myopia in childhood. https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4047734&external=true
- Dietary Omega-3 Polyunsaturated Fatty Acids as a Protective Factor of Myopia: Hong Kong Children Eye Study. https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4045255&external=true
- A Randomized Controlled Trial for Myopia Progression Control Using Catenary Power Profile Contact Lenses: 12-month Effectiveness and Safety. https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4049857&external=true
- Assessing the 2-year Efficacy of Atropine, Orthokeratology, and Combined Therapies: Myopia Control and Choroidal change – Insights from an Age-stratified Randomized Controlled Trial https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4043459&external=true
- Biometrically defining myopia with the Refractive Mechanism Map: Relationship with myopia progression and treatment efficacy. https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4048987&external=true
- Control of Myopia Using Diffusion Optics Technology (DOT) Spectacle Lenses in a Chinese population. https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4048229&external=true
- Defocus Incorporated Multiple Segment lenses and 0.025% atropine for myopia control in a European population: 12-month results of a randomized clinical trial https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4045828&external=true
- Do past axial elongation and myopia progression predict the future? Results from the BLINK Study https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4049687&external=true
- Five-Year Clinical Trial of Low-concentration Atropine for Myopia Progression (LAMP) Study: Phase 4 Report. https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4048205&external=true
- Highly Aspherical Lenslet Target (HALT) technology in combination with low-dose atropine to control myopia progression https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4049211&external=true
- Incidence and progression of myopia in young adults. https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4050576&external=true
- Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004—A reappraisal and model https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4027817&external=true
- Influence of first-time correction on myopia progression and axial elongation in myopic children wearing spectacle lenses with and without aspherical lenslets https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4049944&external=true
- The myopia control effect in children wearing orthokeratology lenses with different back optical zone diameters https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4027873&external=true
- Myopia control efficacy through Emmetropic Progression Ratio:1-year of spectacle wear with cylindrical annular refractive elements (CARE). https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4035816&external=true
- The short-term effects of spectacle-based myopia management interventions on dynamic vision. https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4045535&external=true
- Projection of Glaucoma Burden Worldwide in 2060 with Myopia Surge https://eppro02.ativ.me/web/page.php?nav=false&page=IntHtml&project=ARVO24&id=4050544&external=true

Recommended for you

How Is Canada Addressing Myopia? Find Out From CORE’s Dr. Debbie Jones
sponsored content April 15, 2024 Following her recent studies on “Myopia in Practice” and...

Chinese Conference Covers ‘Frontier in Vision Science Summit – Myopia Research’
April 15, 2024 SHANGHAI, China – The Asia Optometric Management Academy (AOMA) in partnership...
Why Different FDA Indications Matter
April 15, 2024 Andrew Neukirch, OD The relationships my team and I cultivate with...

IMAGES
VIDEO
COMMENTS
ACRPM is a network of clinical research project managers who use project management tools and methodologies to deliver projects on time and within budget. Join ACRPM to access resources, events, articles, and opportunities to connect, learn, and grow in the field.
Learn how to plan, execute and close out a clinical trial with guidance on documentation, human subjects protection, project management plan, personnel and communication management, and problem diagnosis and correction. This module provides templates, examples and tips for each phase of the trial process.
Learn the basics of clinical research project management, including its importance, responsibilities, and key topics. Find out how to plan, coordinate, and monitor complex clinical trials with multiple stakeholders and vendors.
Learn what clinical project managers do, what skills they need and how much they earn in this online program. Explore the curriculum, tracks and career outlook for clinical research management.
Learn what clinical research project management is and why it is essential for the successful execution of clinical trials. Explore the roles and benefits of project managers in ensuring study quality, participant safety, timely execution, resource optimization, risk management, and more.
Clinical trial management certification prepares students by teaching them how to effectively oversee clinical studies, ensuring adherence to protocols, budget, and timelines. Accredited and trusted by over 1,100 students. Salary range 65k-143k+. Complete in 2-4 weeks in 80-100 hours. Free clinical project manager job coaching after completion.
Learn what a clinical project manager does, what skills and qualifications are required, and how to become one. Explore the roles and responsibilities of a clinical project manager in clinical trials, from planning to execution, and the essential certifications and degrees to pursue.
Learn how to plan, execute, monitor, and close a clinical trial with project management skills. Find out the roles of a clinical trial project manager, how to create a detailed plan, anticipate risks, understand IRB requirements, and foster open communication with stakeholders.
The key areas of expertise for clinical trial project managers are communication, relationships, influence, planning, attention to detail, and following instructions. Strong teamwork skills are also essential when working on projects as well as people management skills. You will need to be adaptable to a rapidly changing environment with ...
The CRPM is a community of clinical research professionals dedicated to expanding our knowledge in project management and clinical research. We strive to connect CRPMs through a range of platforms, including online groups, in-person meet-ups, monthly webinars, and retreats, to expand the CRPM network throughout the clinical research industry.
The clinical project manager is a professional who applies the definition of project management to the field of clinical research to ensure that all stages of a clinical trial are properly managed, that the objectives of the trial are achieved on time, on budget, and according to the GCP, and that the safety of the subjects participating in the ...
By: Melissa Harris, MPA, CCRPDirector of Interventional Resources & Clinical Trials UnitPennington Biomedical Research Center at LSU Abstract: Project management involves many complex components and moving parts. Prior to initiating a clinical trial, various types of project tools and templates can be used to successfully plan and execute a clinical trial. This article highlights tools that
Here's a list of six steps that you can follow when trying to pursue a career as a clinical project manager: 1. Earn a bachelor's degree. You can start pursuing a career in clinical project management by earning an undergraduate degree in an applicable field. Many clinical project managers have bachelor's degrees in biology, health, life ...
Pearl #1: Project management of clinical trials is a science with valuable tools at your disposal. We tend to think the day-to-day business of managing a clinical trial is not the "scientific part." However, project management is a science and has well-defined evidence-based methods. Recognizing this is the first step in successfully managing ...
ACRP-PM Specialty. ACRP-PM® (ACRP Project Manager) is a credential formally recognizing clinical research professionals with specialized knowledge in project management. Candidates must be ACRP Certified to sit for this exam. This trusted mark of excellence in clinical research is awarded to clinical researchers who have demonstrated ...
Abstract. This review introduces a conceptual framework for understanding stakeholder management (ShM) in the clinical and community-based research environment. In recent years, an evolution in practice has occurred in many applicants for public and non-governmental funding of public health research in hospital settings.
Clinical research is an alternative terminology used to describe medical research. Clinical research involves people, and it is generally carried out to evaluate the efficacy of a therapeutic drug, a medical/surgical procedure, or a device as a part of treatment and patient management. ... and project management, clinical trial operations at ...
There are 5 modules in this course. In this course, you'll learn about the more advanced elements of managing clinical trials. From anticipating and planning for protocol events to conducting systematic reviews to synthesize evidence, you and your study team need the skills to implement best practices throughout the trial process.
This is an intensive two-day course which provides detailed and practical guidance on the different project management skills used by study sponsors and Contract Research Organizations (CROs), so helps you decide how to apply these skills in your situation. The course is specifically designed for clinical research project management, with ...
What is a project? - Stage 1: Definition: defining and agreeing what the project is about - Stage 2: Planning: planning how the project will be conducted - Stage 3: Implementation and control: running the project - Stage 4: Close out: delivery and the end of the project.
The history of clinical research dates to 650 BCE, and the ethical rules underwent evolution between CE 1135 and 1204. In the modern scientific era (19th to 20th century) clinical trial were the requisite part of drug development. ... Clinical Trial Project Management provides a detailed overview of how to conduct clinical trials in an ...
According to 'payscale.com', remuneration for this profile ranges from $84,168 for early-career to $120,501 for experienced Clinical Research Project Managers, with a mid-career median of $107,649 [2]. A Clinical Research Project Manager (henceforth CRPM) is also known as a Clinical Research Manager or a Clinical Trial Manager.
ACRP Project Manager ACRP-PM ® is a credential formally recognizing clinical research professionals with specialized knowledge in project management. Candidates must be ACRP Certified to sit for this exam. Learn More > ... With more than 16,500 members, the Association of Clinical Research Professionals (ACRP) is the only non-profit ...
Apply for Project Management Intern, Clinical Research (Remote,) - Vaccines & GPHS job with Thermo Fisher Scientific in Remote, North Carolina, United States of America. Students & Internships jobs at Thermo Fisher Scientific
May 10, 2024 By Dwight Akerman, OD, MBA, FAAO, FBCLA, FIACLE Once again, myopia research took center stage at the Association for Research in Vision and Ophthalmology (ARVO) Meeting, held May 5-9, 2024, in Seattle, WA. The meeting featured more than 150 posters and papers on various aspects of myopia and myopia management. Here are 20 noteworthy myopia-related posters and papers: 3-year ...
The Department of Medicine's newly formed Division of Hospital Medicine is pleased to announce our search for a Unit Administrator to oversee clinical and administrative business operations. This position will report to the Division Administrator and will be responsible for managing and coordinating various business-related functions.The successful candidate will: Oversee management of the ...