- Research article
- Open access
- Published: 04 June 2021

Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews
- Israel Júnior Borges do Nascimento 1 , 2 ,
- Dónal P. O’Mathúna 3 , 4 ,
- Thilo Caspar von Groote 5 ,
- Hebatullah Mohamed Abdulazeem 6 ,
- Ishanka Weerasekara 7 , 8 ,
- Ana Marusic 9 ,
- Livia Puljak ORCID: orcid.org/0000-0002-8467-6061 10 ,
- Vinicius Tassoni Civile 11 ,
- Irena Zakarija-Grkovic 9 ,
- Tina Poklepovic Pericic 9 ,
- Alvaro Nagib Atallah 11 ,
- Santino Filoso 12 ,
- Nicola Luigi Bragazzi 13 &
- Milena Soriano Marcolino 1
On behalf of the International Network of Coronavirus Disease 2019 (InterNetCOVID-19)
BMC Infectious Diseases volume 21 , Article number: 525 ( 2021 ) Cite this article
16k Accesses
29 Citations
13 Altmetric
Metrics details
Navigating the rapidly growing body of scientific literature on the SARS-CoV-2 pandemic is challenging, and ongoing critical appraisal of this output is essential. We aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Nine databases (Medline, EMBASE, Cochrane Library, CINAHL, Web of Sciences, PDQ-Evidence, WHO’s Global Research, LILACS, and Epistemonikos) were searched from December 1, 2019, to March 24, 2020. Systematic reviews analyzing primary studies of COVID-19 were included. Two authors independently undertook screening, selection, extraction (data on clinical symptoms, prevalence, pharmacological and non-pharmacological interventions, diagnostic test assessment, laboratory, and radiological findings), and quality assessment (AMSTAR 2). A meta-analysis was performed of the prevalence of clinical outcomes.
Eighteen systematic reviews were included; one was empty (did not identify any relevant study). Using AMSTAR 2, confidence in the results of all 18 reviews was rated as “critically low”. Identified symptoms of COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%) and gastrointestinal complaints (5–9%). Severe symptoms were more common in men. Elevated C-reactive protein and lactate dehydrogenase, and slightly elevated aspartate and alanine aminotransferase, were commonly described. Thrombocytopenia and elevated levels of procalcitonin and cardiac troponin I were associated with severe disease. A frequent finding on chest imaging was uni- or bilateral multilobar ground-glass opacity. A single review investigated the impact of medication (chloroquine) but found no verifiable clinical data. All-cause mortality ranged from 0.3 to 13.9%.
Conclusions
In this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic were of questionable usefulness. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards.
Peer Review reports
The spread of the “Severe Acute Respiratory Coronavirus 2” (SARS-CoV-2), the causal agent of COVID-19, was characterized as a pandemic by the World Health Organization (WHO) in March 2020 and has triggered an international public health emergency [ 1 ]. The numbers of confirmed cases and deaths due to COVID-19 are rapidly escalating, counting in millions [ 2 ], causing massive economic strain, and escalating healthcare and public health expenses [ 3 , 4 ].
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ]. The living map of COVID-19 evidence, curated by the Evidence for Policy and Practice Information and Co-ordinating Centre (EPPI-Centre), contained more than 40,000 records by February 2021 [ 6 ]. More than 100,000 records on PubMed were labeled as “SARS-CoV-2 literature, sequence, and clinical content” by February 2021 [ 7 ].
Due to publication speed, the research community has voiced concerns regarding the quality and reproducibility of evidence produced during the COVID-19 pandemic, warning of the potential damaging approach of “publish first, retract later” [ 8 ]. It appears that these concerns are not unfounded, as it has been reported that COVID-19 articles were overrepresented in the pool of retracted articles in 2020 [ 9 ]. These concerns about inadequate evidence are of major importance because they can lead to poor clinical practice and inappropriate policies [ 10 ].
Systematic reviews are a cornerstone of today’s evidence-informed decision-making. By synthesizing all relevant evidence regarding a particular topic, systematic reviews reflect the current scientific knowledge. Systematic reviews are considered to be at the highest level in the hierarchy of evidence and should be used to make informed decisions. However, with high numbers of systematic reviews of different scope and methodological quality being published, overviews of multiple systematic reviews that assess their methodological quality are essential [ 11 , 12 , 13 ]. An overview of systematic reviews helps identify and organize the literature and highlights areas of priority in decision-making.
In this overview of systematic reviews, we aimed to summarize and critically appraise systematic reviews of coronavirus disease (COVID-19) in humans that were available at the beginning of the pandemic.
Methodology
Research question.
This overview’s primary objective was to summarize and critically appraise systematic reviews that assessed any type of primary clinical data from patients infected with SARS-CoV-2. Our research question was purposefully broad because we wanted to analyze as many systematic reviews as possible that were available early following the COVID-19 outbreak.
Study design
We conducted an overview of systematic reviews. The idea for this overview originated in a protocol for a systematic review submitted to PROSPERO (CRD42020170623), which indicated a plan to conduct an overview.
Overviews of systematic reviews use explicit and systematic methods for searching and identifying multiple systematic reviews addressing related research questions in the same field to extract and analyze evidence across important outcomes. Overviews of systematic reviews are in principle similar to systematic reviews of interventions, but the unit of analysis is a systematic review [ 14 , 15 , 16 ].
We used the overview methodology instead of other evidence synthesis methods to allow us to collate and appraise multiple systematic reviews on this topic, and to extract and analyze their results across relevant topics [ 17 ]. The overview and meta-analysis of systematic reviews allowed us to investigate the methodological quality of included studies, summarize results, and identify specific areas of available or limited evidence, thereby strengthening the current understanding of this novel disease and guiding future research [ 13 ].
A reporting guideline for overviews of reviews is currently under development, i.e., Preferred Reporting Items for Overviews of Reviews (PRIOR) [ 18 ]. As the PRIOR checklist is still not published, this study was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 statement [ 19 ]. The methodology used in this review was adapted from the Cochrane Handbook for Systematic Reviews of Interventions and also followed established methodological considerations for analyzing existing systematic reviews [ 14 ].
Approval of a research ethics committee was not necessary as the study analyzed only publicly available articles.
Eligibility criteria
Systematic reviews were included if they analyzed primary data from patients infected with SARS-CoV-2 as confirmed by RT-PCR or another pre-specified diagnostic technique. Eligible reviews covered all topics related to COVID-19 including, but not limited to, those that reported clinical symptoms, diagnostic methods, therapeutic interventions, laboratory findings, or radiological results. Both full manuscripts and abbreviated versions, such as letters, were eligible.
No restrictions were imposed on the design of the primary studies included within the systematic reviews, the last search date, whether the review included meta-analyses or language. Reviews related to SARS-CoV-2 and other coronaviruses were eligible, but from those reviews, we analyzed only data related to SARS-CoV-2.
No consensus definition exists for a systematic review [ 20 ], and debates continue about the defining characteristics of a systematic review [ 21 ]. Cochrane’s guidance for overviews of reviews recommends setting pre-established criteria for making decisions around inclusion [ 14 ]. That is supported by a recent scoping review about guidance for overviews of systematic reviews [ 22 ].
Thus, for this study, we defined a systematic review as a research report which searched for primary research studies on a specific topic using an explicit search strategy, had a detailed description of the methods with explicit inclusion criteria provided, and provided a summary of the included studies either in narrative or quantitative format (such as a meta-analysis). Cochrane and non-Cochrane systematic reviews were considered eligible for inclusion, with or without meta-analysis, and regardless of the study design, language restriction and methodology of the included primary studies. To be eligible for inclusion, reviews had to be clearly analyzing data related to SARS-CoV-2 (associated or not with other viruses). We excluded narrative reviews without those characteristics as these are less likely to be replicable and are more prone to bias.
Scoping reviews and rapid reviews were eligible for inclusion in this overview if they met our pre-defined inclusion criteria noted above. We included reviews that addressed SARS-CoV-2 and other coronaviruses if they reported separate data regarding SARS-CoV-2.
Information sources
Nine databases were searched for eligible records published between December 1, 2019, and March 24, 2020: Cochrane Database of Systematic Reviews via Cochrane Library, PubMed, EMBASE, CINAHL (Cumulative Index to Nursing and Allied Health Literature), Web of Sciences, LILACS (Latin American and Caribbean Health Sciences Literature), PDQ-Evidence, WHO’s Global Research on Coronavirus Disease (COVID-19), and Epistemonikos.
The comprehensive search strategy for each database is provided in Additional file 1 and was designed and conducted in collaboration with an information specialist. All retrieved records were primarily processed in EndNote, where duplicates were removed, and records were then imported into the Covidence platform [ 23 ]. In addition to database searches, we screened reference lists of reviews included after screening records retrieved via databases.
Study selection
All searches, screening of titles and abstracts, and record selection, were performed independently by two investigators using the Covidence platform [ 23 ]. Articles deemed potentially eligible were retrieved for full-text screening carried out independently by two investigators. Discrepancies at all stages were resolved by consensus. During the screening, records published in languages other than English were translated by a native/fluent speaker.
Data collection process
We custom designed a data extraction table for this study, which was piloted by two authors independently. Data extraction was performed independently by two authors. Conflicts were resolved by consensus or by consulting a third researcher.
We extracted the following data: article identification data (authors’ name and journal of publication), search period, number of databases searched, population or settings considered, main results and outcomes observed, and number of participants. From Web of Science (Clarivate Analytics, Philadelphia, PA, USA), we extracted journal rank (quartile) and Journal Impact Factor (JIF).
We categorized the following as primary outcomes: all-cause mortality, need for and length of mechanical ventilation, length of hospitalization (in days), admission to intensive care unit (yes/no), and length of stay in the intensive care unit.
The following outcomes were categorized as exploratory: diagnostic methods used for detection of the virus, male to female ratio, clinical symptoms, pharmacological and non-pharmacological interventions, laboratory findings (full blood count, liver enzymes, C-reactive protein, d-dimer, albumin, lipid profile, serum electrolytes, blood vitamin levels, glucose levels, and any other important biomarkers), and radiological findings (using radiography, computed tomography, magnetic resonance imaging or ultrasound).
We also collected data on reporting guidelines and requirements for the publication of systematic reviews and meta-analyses from journal websites where included reviews were published.
Quality assessment in individual reviews
Two researchers independently assessed the reviews’ quality using the “A MeaSurement Tool to Assess Systematic Reviews 2 (AMSTAR 2)”. We acknowledge that the AMSTAR 2 was created as “a critical appraisal tool for systematic reviews that include randomized or non-randomized studies of healthcare interventions, or both” [ 24 ]. However, since AMSTAR 2 was designed for systematic reviews of intervention trials, and we included additional types of systematic reviews, we adjusted some AMSTAR 2 ratings and reported these in Additional file 2 .
Adherence to each item was rated as follows: yes, partial yes, no, or not applicable (such as when a meta-analysis was not conducted). The overall confidence in the results of the review is rated as “critically low”, “low”, “moderate” or “high”, according to the AMSTAR 2 guidance based on seven critical domains, which are items 2, 4, 7, 9, 11, 13, 15 as defined by AMSTAR 2 authors [ 24 ]. We reported our adherence ratings for transparency of our decision with accompanying explanations, for each item, in each included review.
One of the included systematic reviews was conducted by some members of this author team [ 25 ]. This review was initially assessed independently by two authors who were not co-authors of that review to prevent the risk of bias in assessing this study.
Synthesis of results
For data synthesis, we prepared a table summarizing each systematic review. Graphs illustrating the mortality rate and clinical symptoms were created. We then prepared a narrative summary of the methods, findings, study strengths, and limitations.
For analysis of the prevalence of clinical outcomes, we extracted data on the number of events and the total number of patients to perform proportional meta-analysis using RStudio© software, with the “meta” package (version 4.9–6), using the “metaprop” function for reviews that did not perform a meta-analysis, excluding case studies because of the absence of variance. For reviews that did not perform a meta-analysis, we presented pooled results of proportions with their respective confidence intervals (95%) by the inverse variance method with a random-effects model, using the DerSimonian-Laird estimator for τ 2 . We adjusted data using Freeman-Tukey double arcosen transformation. Confidence intervals were calculated using the Clopper-Pearson method for individual studies. We created forest plots using the RStudio© software, with the “metafor” package (version 2.1–0) and “forest” function.
Managing overlapping systematic reviews
Some of the included systematic reviews that address the same or similar research questions may include the same primary studies in overviews. Including such overlapping reviews may introduce bias when outcome data from the same primary study are included in the analyses of an overview multiple times. Thus, in summaries of evidence, multiple-counting of the same outcome data will give data from some primary studies too much influence [ 14 ]. In this overview, we did not exclude overlapping systematic reviews because, according to Cochrane’s guidance, it may be appropriate to include all relevant reviews’ results if the purpose of the overview is to present and describe the current body of evidence on a topic [ 14 ]. To avoid any bias in summary estimates associated with overlapping reviews, we generated forest plots showing data from individual systematic reviews, but the results were not pooled because some primary studies were included in multiple reviews.
Our search retrieved 1063 publications, of which 175 were duplicates. Most publications were excluded after the title and abstract analysis ( n = 860). Among the 28 studies selected for full-text screening, 10 were excluded for the reasons described in Additional file 3 , and 18 were included in the final analysis (Fig. 1 ) [ 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 ]. Reference list screening did not retrieve any additional systematic reviews.
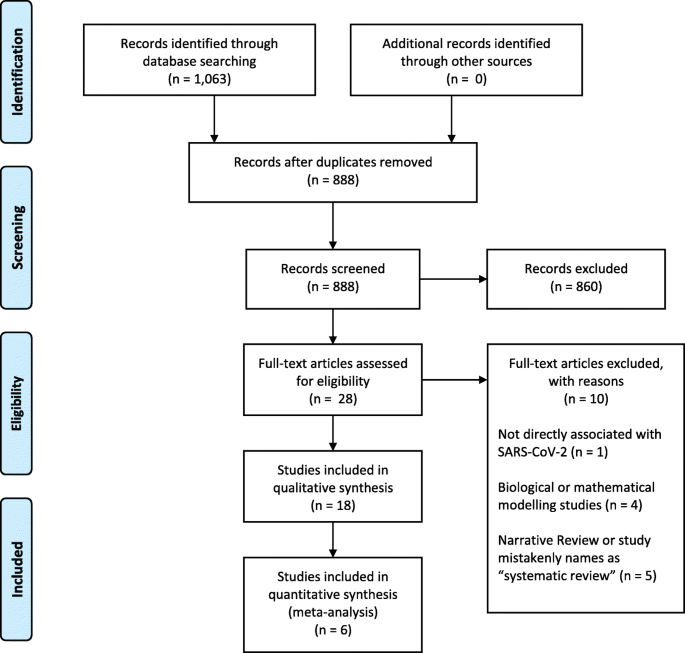
PRISMA flow diagram
Characteristics of included reviews
Summary features of 18 systematic reviews are presented in Table 1 . They were published in 14 different journals. Only four of these journals had specific requirements for systematic reviews (with or without meta-analysis): European Journal of Internal Medicine, Journal of Clinical Medicine, Ultrasound in Obstetrics and Gynecology, and Clinical Research in Cardiology . Two journals reported that they published only invited reviews ( Journal of Medical Virology and Clinica Chimica Acta ). Three systematic reviews in our study were published as letters; one was labeled as a scoping review and another as a rapid review (Table 2 ).
All reviews were published in English, in first quartile (Q1) journals, with JIF ranging from 1.692 to 6.062. One review was empty, meaning that its search did not identify any relevant studies; i.e., no primary studies were included [ 36 ]. The remaining 17 reviews included 269 unique studies; the majority ( N = 211; 78%) were included in only a single review included in our study (range: 1 to 12). Primary studies included in the reviews were published between December 2019 and March 18, 2020, and comprised case reports, case series, cohorts, and other observational studies. We found only one review that included randomized clinical trials [ 38 ]. In the included reviews, systematic literature searches were performed from 2019 (entire year) up to March 9, 2020. Ten systematic reviews included meta-analyses. The list of primary studies found in the included systematic reviews is shown in Additional file 4 , as well as the number of reviews in which each primary study was included.
Population and study designs
Most of the reviews analyzed data from patients with COVID-19 who developed pneumonia, acute respiratory distress syndrome (ARDS), or any other correlated complication. One review aimed to evaluate the effectiveness of using surgical masks on preventing transmission of the virus [ 36 ], one review was focused on pediatric patients [ 34 ], and one review investigated COVID-19 in pregnant women [ 37 ]. Most reviews assessed clinical symptoms, laboratory findings, or radiological results.
Systematic review findings
The summary of findings from individual reviews is shown in Table 2 . Overall, all-cause mortality ranged from 0.3 to 13.9% (Fig. 2 ).
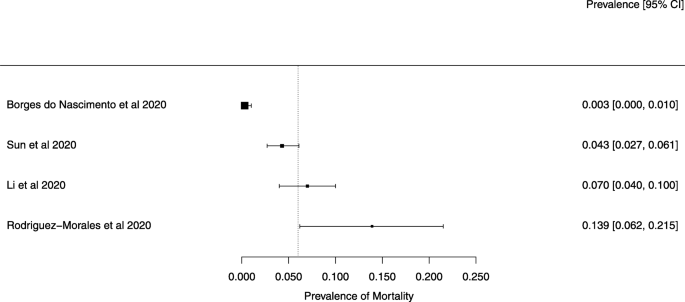
A meta-analysis of the prevalence of mortality
Clinical symptoms
Seven reviews described the main clinical manifestations of COVID-19 [ 26 , 28 , 29 , 34 , 35 , 39 , 41 ]. Three of them provided only a narrative discussion of symptoms [ 26 , 34 , 35 ]. In the reviews that performed a statistical analysis of the incidence of different clinical symptoms, symptoms in patients with COVID-19 were (range values of point estimates): fever (82–95%), cough with or without sputum (58–72%), dyspnea (26–59%), myalgia or muscle fatigue (29–51%), sore throat (10–13%), headache (8–12%), gastrointestinal disorders, such as diarrhea, nausea or vomiting (5.0–9.0%), and others (including, in one study only: dizziness 12.1%) (Figs. 3 , 4 , 5 , 6 , 7 , 8 and 9 ). Three reviews assessed cough with and without sputum together; only one review assessed sputum production itself (28.5%).
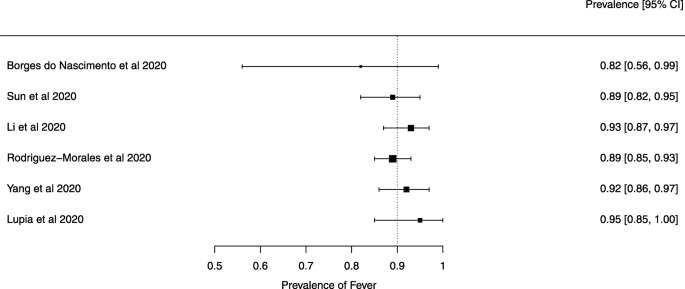
A meta-analysis of the prevalence of fever
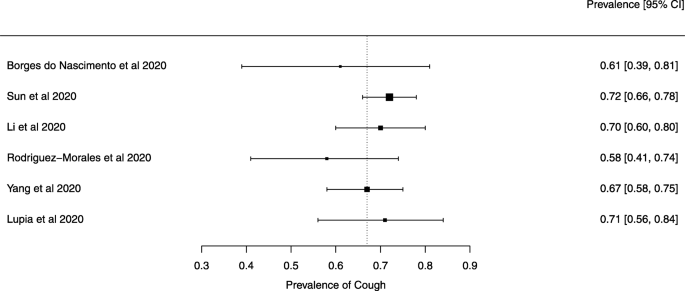
A meta-analysis of the prevalence of cough
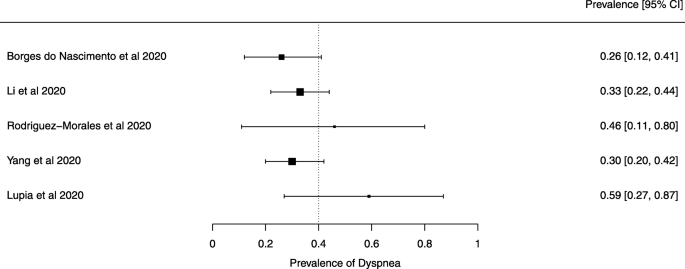
A meta-analysis of the prevalence of dyspnea
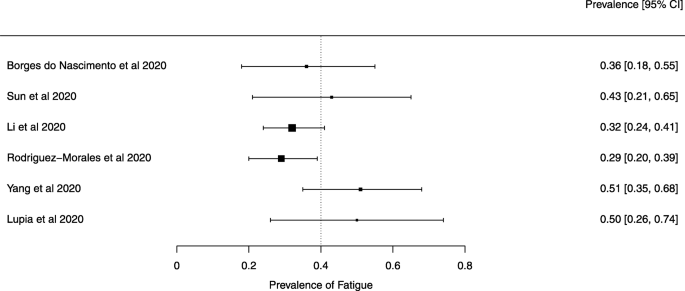
A meta-analysis of the prevalence of fatigue or myalgia
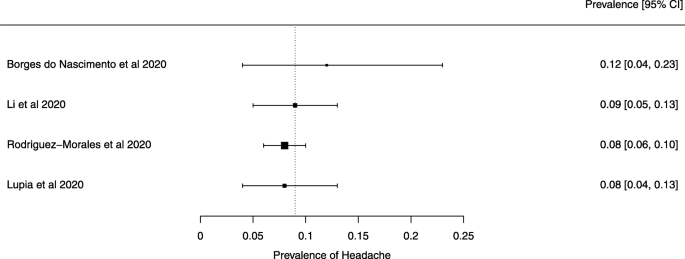
A meta-analysis of the prevalence of headache
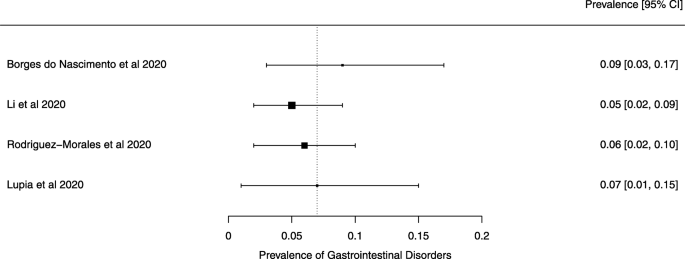
A meta-analysis of the prevalence of gastrointestinal disorders
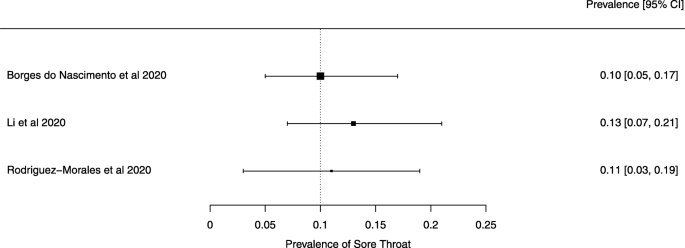
A meta-analysis of the prevalence of sore throat
Diagnostic aspects
Three reviews described methodologies, protocols, and tools used for establishing the diagnosis of COVID-19 [ 26 , 34 , 38 ]. The use of respiratory swabs (nasal or pharyngeal) or blood specimens to assess the presence of SARS-CoV-2 nucleic acid using RT-PCR assays was the most commonly used diagnostic method mentioned in the included studies. These diagnostic tests have been widely used, but their precise sensitivity and specificity remain unknown. One review included a Chinese study with clinical diagnosis with no confirmation of SARS-CoV-2 infection (patients were diagnosed with COVID-19 if they presented with at least two symptoms suggestive of COVID-19, together with laboratory and chest radiography abnormalities) [ 34 ].
Therapeutic possibilities
Pharmacological and non-pharmacological interventions (supportive therapies) used in treating patients with COVID-19 were reported in five reviews [ 25 , 27 , 34 , 35 , 38 ]. Antivirals used empirically for COVID-19 treatment were reported in seven reviews [ 25 , 27 , 34 , 35 , 37 , 38 , 41 ]; most commonly used were protease inhibitors (lopinavir, ritonavir, darunavir), nucleoside reverse transcriptase inhibitor (tenofovir), nucleotide analogs (remdesivir, galidesivir, ganciclovir), and neuraminidase inhibitors (oseltamivir). Umifenovir, a membrane fusion inhibitor, was investigated in two studies [ 25 , 35 ]. Possible supportive interventions analyzed were different types of oxygen supplementation and breathing support (invasive or non-invasive ventilation) [ 25 ]. The use of antibiotics, both empirically and to treat secondary pneumonia, was reported in six studies [ 25 , 26 , 27 , 34 , 35 , 38 ]. One review specifically assessed evidence on the efficacy and safety of the anti-malaria drug chloroquine [ 27 ]. It identified 23 ongoing trials investigating the potential of chloroquine as a therapeutic option for COVID-19, but no verifiable clinical outcomes data. The use of mesenchymal stem cells, antifungals, and glucocorticoids were described in four reviews [ 25 , 34 , 35 , 38 ].
Laboratory and radiological findings
Of the 18 reviews included in this overview, eight analyzed laboratory parameters in patients with COVID-19 [ 25 , 29 , 30 , 32 , 33 , 34 , 35 , 39 ]; elevated C-reactive protein levels, associated with lymphocytopenia, elevated lactate dehydrogenase, as well as slightly elevated aspartate and alanine aminotransferase (AST, ALT) were commonly described in those eight reviews. Lippi et al. assessed cardiac troponin I (cTnI) [ 25 ], procalcitonin [ 32 ], and platelet count [ 33 ] in COVID-19 patients. Elevated levels of procalcitonin [ 32 ] and cTnI [ 30 ] were more likely to be associated with a severe disease course (requiring intensive care unit admission and intubation). Furthermore, thrombocytopenia was frequently observed in patients with complicated COVID-19 infections [ 33 ].
Chest imaging (chest radiography and/or computed tomography) features were assessed in six reviews, all of which described a frequent pattern of local or bilateral multilobar ground-glass opacity [ 25 , 34 , 35 , 39 , 40 , 41 ]. Those six reviews showed that septal thickening, bronchiectasis, pleural and cardiac effusions, halo signs, and pneumothorax were observed in patients suffering from COVID-19.
Quality of evidence in individual systematic reviews
Table 3 shows the detailed results of the quality assessment of 18 systematic reviews, including the assessment of individual items and summary assessment. A detailed explanation for each decision in each review is available in Additional file 5 .
Using AMSTAR 2 criteria, confidence in the results of all 18 reviews was rated as “critically low” (Table 3 ). Common methodological drawbacks were: omission of prospective protocol submission or publication; use of inappropriate search strategy: lack of independent and dual literature screening and data-extraction (or methodology unclear); absence of an explanation for heterogeneity among the studies included; lack of reasons for study exclusion (or rationale unclear).
Risk of bias assessment, based on a reported methodological tool, and quality of evidence appraisal, in line with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method, were reported only in one review [ 25 ]. Five reviews presented a table summarizing bias, using various risk of bias tools [ 25 , 29 , 39 , 40 , 41 ]. One review analyzed “study quality” [ 37 ]. One review mentioned the risk of bias assessment in the methodology but did not provide any related analysis [ 28 ].
This overview of systematic reviews analyzed the first 18 systematic reviews published after the onset of the COVID-19 pandemic, up to March 24, 2020, with primary studies involving more than 60,000 patients. Using AMSTAR-2, we judged that our confidence in all those reviews was “critically low”. Ten reviews included meta-analyses. The reviews presented data on clinical manifestations, laboratory and radiological findings, and interventions. We found no systematic reviews on the utility of diagnostic tests.
Symptoms were reported in seven reviews; most of the patients had a fever, cough, dyspnea, myalgia or muscle fatigue, and gastrointestinal disorders such as diarrhea, nausea, or vomiting. Olfactory dysfunction (anosmia or dysosmia) has been described in patients infected with COVID-19 [ 43 ]; however, this was not reported in any of the reviews included in this overview. During the SARS outbreak in 2002, there were reports of impairment of the sense of smell associated with the disease [ 44 , 45 ].
The reported mortality rates ranged from 0.3 to 14% in the included reviews. Mortality estimates are influenced by the transmissibility rate (basic reproduction number), availability of diagnostic tools, notification policies, asymptomatic presentations of the disease, resources for disease prevention and control, and treatment facilities; variability in the mortality rate fits the pattern of emerging infectious diseases [ 46 ]. Furthermore, the reported cases did not consider asymptomatic cases, mild cases where individuals have not sought medical treatment, and the fact that many countries had limited access to diagnostic tests or have implemented testing policies later than the others. Considering the lack of reviews assessing diagnostic testing (sensitivity, specificity, and predictive values of RT-PCT or immunoglobulin tests), and the preponderance of studies that assessed only symptomatic individuals, considerable imprecision around the calculated mortality rates existed in the early stage of the COVID-19 pandemic.
Few reviews included treatment data. Those reviews described studies considered to be at a very low level of evidence: usually small, retrospective studies with very heterogeneous populations. Seven reviews analyzed laboratory parameters; those reviews could have been useful for clinicians who attend patients suspected of COVID-19 in emergency services worldwide, such as assessing which patients need to be reassessed more frequently.
All systematic reviews scored poorly on the AMSTAR 2 critical appraisal tool for systematic reviews. Most of the original studies included in the reviews were case series and case reports, impacting the quality of evidence. Such evidence has major implications for clinical practice and the use of these reviews in evidence-based practice and policy. Clinicians, patients, and policymakers can only have the highest confidence in systematic review findings if high-quality systematic review methodologies are employed. The urgent need for information during a pandemic does not justify poor quality reporting.
We acknowledge that there are numerous challenges associated with analyzing COVID-19 data during a pandemic [ 47 ]. High-quality evidence syntheses are needed for decision-making, but each type of evidence syntheses is associated with its inherent challenges.
The creation of classic systematic reviews requires considerable time and effort; with massive research output, they quickly become outdated, and preparing updated versions also requires considerable time. A recent study showed that updates of non-Cochrane systematic reviews are published a median of 5 years after the publication of the previous version [ 48 ].
Authors may register a review and then abandon it [ 49 ], but the existence of a public record that is not updated may lead other authors to believe that the review is still ongoing. A quarter of Cochrane review protocols remains unpublished as completed systematic reviews 8 years after protocol publication [ 50 ].
Rapid reviews can be used to summarize the evidence, but they involve methodological sacrifices and simplifications to produce information promptly, with inconsistent methodological approaches [ 51 ]. However, rapid reviews are justified in times of public health emergencies, and even Cochrane has resorted to publishing rapid reviews in response to the COVID-19 crisis [ 52 ]. Rapid reviews were eligible for inclusion in this overview, but only one of the 18 reviews included in this study was labeled as a rapid review.
Ideally, COVID-19 evidence would be continually summarized in a series of high-quality living systematic reviews, types of evidence synthesis defined as “ a systematic review which is continually updated, incorporating relevant new evidence as it becomes available ” [ 53 ]. However, conducting living systematic reviews requires considerable resources, calling into question the sustainability of such evidence synthesis over long periods [ 54 ].
Research reports about COVID-19 will contribute to research waste if they are poorly designed, poorly reported, or simply not necessary. In principle, systematic reviews should help reduce research waste as they usually provide recommendations for further research that is needed or may advise that sufficient evidence exists on a particular topic [ 55 ]. However, systematic reviews can also contribute to growing research waste when they are not needed, or poorly conducted and reported. Our present study clearly shows that most of the systematic reviews that were published early on in the COVID-19 pandemic could be categorized as research waste, as our confidence in their results is critically low.
Our study has some limitations. One is that for AMSTAR 2 assessment we relied on information available in publications; we did not attempt to contact study authors for clarifications or additional data. In three reviews, the methodological quality appraisal was challenging because they were published as letters, or labeled as rapid communications. As a result, various details about their review process were not included, leading to AMSTAR 2 questions being answered as “not reported”, resulting in low confidence scores. Full manuscripts might have provided additional information that could have led to higher confidence in the results. In other words, low scores could reflect incomplete reporting, not necessarily low-quality review methods. To make their review available more rapidly and more concisely, the authors may have omitted methodological details. A general issue during a crisis is that speed and completeness must be balanced. However, maintaining high standards requires proper resourcing and commitment to ensure that the users of systematic reviews can have high confidence in the results.
Furthermore, we used adjusted AMSTAR 2 scoring, as the tool was designed for critical appraisal of reviews of interventions. Some reviews may have received lower scores than actually warranted in spite of these adjustments.
Another limitation of our study may be the inclusion of multiple overlapping reviews, as some included reviews included the same primary studies. According to the Cochrane Handbook, including overlapping reviews may be appropriate when the review’s aim is “ to present and describe the current body of systematic review evidence on a topic ” [ 12 ], which was our aim. To avoid bias with summarizing evidence from overlapping reviews, we presented the forest plots without summary estimates. The forest plots serve to inform readers about the effect sizes for outcomes that were reported in each review.
Several authors from this study have contributed to one of the reviews identified [ 25 ]. To reduce the risk of any bias, two authors who did not co-author the review in question initially assessed its quality and limitations.
Finally, we note that the systematic reviews included in our overview may have had issues that our analysis did not identify because we did not analyze their primary studies to verify the accuracy of the data and information they presented. We give two examples to substantiate this possibility. Lovato et al. wrote a commentary on the review of Sun et al. [ 41 ], in which they criticized the authors’ conclusion that sore throat is rare in COVID-19 patients [ 56 ]. Lovato et al. highlighted that multiple studies included in Sun et al. did not accurately describe participants’ clinical presentations, warning that only three studies clearly reported data on sore throat [ 56 ].
In another example, Leung [ 57 ] warned about the review of Li, L.Q. et al. [ 29 ]: “ it is possible that this statistic was computed using overlapped samples, therefore some patients were double counted ”. Li et al. responded to Leung that it is uncertain whether the data overlapped, as they used data from published articles and did not have access to the original data; they also reported that they requested original data and that they plan to re-do their analyses once they receive them; they also urged readers to treat the data with caution [ 58 ]. This points to the evolving nature of evidence during a crisis.
Our study’s strength is that this overview adds to the current knowledge by providing a comprehensive summary of all the evidence synthesis about COVID-19 available early after the onset of the pandemic. This overview followed strict methodological criteria, including a comprehensive and sensitive search strategy and a standard tool for methodological appraisal of systematic reviews.
In conclusion, in this overview of systematic reviews, we analyzed evidence from the first 18 systematic reviews that were published after the emergence of COVID-19. However, confidence in the results of all the reviews was “critically low”. Thus, systematic reviews that were published early on in the pandemic could be categorized as research waste. Even during public health emergencies, studies and systematic reviews should adhere to established methodological standards to provide patients, clinicians, and decision-makers trustworthy evidence.
Availability of data and materials
All data collected and analyzed within this study are available from the corresponding author on reasonable request.
World Health Organization. Timeline - COVID-19: Available at: https://www.who.int/news/item/29-06-2020-covidtimeline . Accessed 1 June 2021.
COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available at: https://coronavirus.jhu.edu/map.html . Accessed 1 June 2021.
Anzai A, Kobayashi T, Linton NM, Kinoshita R, Hayashi K, Suzuki A, et al. Assessing the Impact of Reduced Travel on Exportation Dynamics of Novel Coronavirus Infection (COVID-19). J Clin Med. 2020;9(2):601.
Chinazzi M, Davis JT, Ajelli M, Gioannini C, Litvinova M, Merler S, et al. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368(6489):395–400. https://doi.org/10.1126/science.aba9757 .
Article CAS PubMed PubMed Central Google Scholar
Fidahic M, Nujic D, Runjic R, Civljak M, Markotic F, Lovric Makaric Z, et al. Research methodology and characteristics of journal articles with original data, preprint articles and registered clinical trial protocols about COVID-19. BMC Med Res Methodol. 2020;20(1):161. https://doi.org/10.1186/s12874-020-01047-2 .
EPPI Centre . COVID-19: a living systematic map of the evidence. Available at: http://eppi.ioe.ac.uk/cms/Projects/DepartmentofHealthandSocialCare/Publishedreviews/COVID-19Livingsystematicmapoftheevidence/tabid/3765/Default.aspx . Accessed 1 June 2021.
NCBI SARS-CoV-2 Resources. Available at: https://www.ncbi.nlm.nih.gov/sars-cov-2/ . Accessed 1 June 2021.
Gustot T. Quality and reproducibility during the COVID-19 pandemic. JHEP Rep. 2020;2(4):100141. https://doi.org/10.1016/j.jhepr.2020.100141 .
Article PubMed PubMed Central Google Scholar
Kodvanj, I., et al., Publishing of COVID-19 Preprints in Peer-reviewed Journals, Preprinting Trends, Public Discussion and Quality Issues. Preprint article. bioRxiv 2020.11.23.394577; doi: https://doi.org/10.1101/2020.11.23.394577 .
Dobler CC. Poor quality research and clinical practice during COVID-19. Breathe (Sheff). 2020;16(2):200112. https://doi.org/10.1183/20734735.0112-2020 .
Article Google Scholar
Bastian H, Glasziou P, Chalmers I. Seventy-five trials and eleven systematic reviews a day: how will we ever keep up? PLoS Med. 2010;7(9):e1000326. https://doi.org/10.1371/journal.pmed.1000326 .
Lunny C, Brennan SE, McDonald S, McKenzie JE. Toward a comprehensive evidence map of overview of systematic review methods: paper 1-purpose, eligibility, search and data extraction. Syst Rev. 2017;6(1):231. https://doi.org/10.1186/s13643-017-0617-1 .
Pollock M, Fernandes RM, Becker LA, Pieper D, Hartling L. Chapter V: Overviews of Reviews. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane. 2020. Available from www.training.cochrane.org/handbook .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020). Cochrane. 2020; Available from www.training.cochrane.org/handbook .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. The impact of different inclusion decisions on the comprehensiveness and complexity of overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):18. https://doi.org/10.1186/s13643-018-0914-3 .
Pollock M, Fernandes RM, Newton AS, Scott SD, Hartling L. A decision tool to help researchers make decisions about including systematic reviews in overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):29. https://doi.org/10.1186/s13643-018-0768-8 .
Hunt H, Pollock A, Campbell P, Estcourt L, Brunton G. An introduction to overviews of reviews: planning a relevant research question and objective for an overview. Syst Rev. 2018;7(1):39. https://doi.org/10.1186/s13643-018-0695-8 .
Pollock M, Fernandes RM, Pieper D, Tricco AC, Gates M, Gates A, et al. Preferred reporting items for overviews of reviews (PRIOR): a protocol for development of a reporting guideline for overviews of reviews of healthcare interventions. Syst Rev. 2019;8(1):335. https://doi.org/10.1186/s13643-019-1252-9 .
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Open Med. 2009;3(3):e123–30.
Krnic Martinic M, Pieper D, Glatt A, Puljak L. Definition of a systematic review used in overviews of systematic reviews, meta-epidemiological studies and textbooks. BMC Med Res Methodol. 2019;19(1):203. https://doi.org/10.1186/s12874-019-0855-0 .
Puljak L. If there is only one author or only one database was searched, a study should not be called a systematic review. J Clin Epidemiol. 2017;91:4–5. https://doi.org/10.1016/j.jclinepi.2017.08.002 .
Article PubMed Google Scholar
Gates M, Gates A, Guitard S, Pollock M, Hartling L. Guidance for overviews of reviews continues to accumulate, but important challenges remain: a scoping review. Syst Rev. 2020;9(1):254. https://doi.org/10.1186/s13643-020-01509-0 .
Covidence - systematic review software. Available at: https://www.covidence.org/ . Accessed 1 June 2021.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Borges do Nascimento IJ, et al. Novel Coronavirus Infection (COVID-19) in Humans: A Scoping Review and Meta-Analysis. J Clin Med. 2020;9(4):941.
Article PubMed Central Google Scholar
Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. https://doi.org/10.1186/s40249-020-00646-x .
Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–83. https://doi.org/10.1016/j.jcrc.2020.03.005 .
Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–8. https://doi.org/10.1007/s00392-020-01626-9 .
Article CAS PubMed Google Scholar
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–83. https://doi.org/10.1002/jmv.25757 .
Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63(3):390–1. https://doi.org/10.1016/j.pcad.2020.03.001 .
Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014 .
Lippi G, Plebani M. Procalcitonin in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chim Acta. 2020;505:190–1. https://doi.org/10.1016/j.cca.2020.03.004 .
Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–8. https://doi.org/10.1016/j.cca.2020.03.022 .
Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–95. https://doi.org/10.1111/apa.15270 .
Lupia T, Scabini S, Mornese Pinna S, di Perri G, de Rosa FG, Corcione S. 2019 novel coronavirus (2019-nCoV) outbreak: a new challenge. J Glob Antimicrob Resist. 2020;21:22–7. https://doi.org/10.1016/j.jgar.2020.02.021 .
Marasinghe, K.M., A systematic review investigating the effectiveness of face mask use in limiting the spread of COVID-19 among medically not diagnosed individuals: shedding light on current recommendations provided to individuals not medically diagnosed with COVID-19. Research Square. Preprint article. doi : https://doi.org/10.21203/rs.3.rs-16701/v1 . 2020 .
Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol. 2020;55(5):586–92. https://doi.org/10.1002/uog.22014 .
Pang J, Wang MX, Ang IYH, Tan SHX, Lewis RF, Chen JIP, et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel coronavirus (2019-nCoV): a systematic review. J Clin Med. 2020;9(3):623.
Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. https://doi.org/10.1016/j.tmaid.2020.101623 .
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. https://doi.org/10.2214/AJR.20.23034 .
Sun P, Qie S, Liu Z, Ren J, Li K, Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J Med Virol. 2020;92(6):612–7. https://doi.org/10.1002/jmv.25735 .
Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–5. https://doi.org/10.1016/j.ijid.2020.03.017 .
Bassetti M, Vena A, Giacobbe DR. The novel Chinese coronavirus (2019-nCoV) infections: challenges for fighting the storm. Eur J Clin Investig. 2020;50(3):e13209. https://doi.org/10.1111/eci.13209 .
Article CAS Google Scholar
Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwanica. 2006;15(1):26–8.
Google Scholar
Suzuki M, Saito K, Min WP, Vladau C, Toida K, Itoh H, et al. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117(2):272–7. https://doi.org/10.1097/01.mlg.0000249922.37381.1e .
Rajgor DD, Lee MH, Archuleta S, Bagdasarian N, Quek SC. The many estimates of the COVID-19 case fatality rate. Lancet Infect Dis. 2020;20(7):776–7. https://doi.org/10.1016/S1473-3099(20)30244-9 .
Wolkewitz M, Puljak L. Methodological challenges of analysing COVID-19 data during the pandemic. BMC Med Res Methodol. 2020;20(1):81. https://doi.org/10.1186/s12874-020-00972-6 .
Rombey T, Lochner V, Puljak L, Könsgen N, Mathes T, Pieper D. Epidemiology and reporting characteristics of non-Cochrane updates of systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(3):471–83. https://doi.org/10.1002/jrsm.1409 .
Runjic E, Rombey T, Pieper D, Puljak L. Half of systematic reviews about pain registered in PROSPERO were not published and the majority had inaccurate status. J Clin Epidemiol. 2019;116:114–21. https://doi.org/10.1016/j.jclinepi.2019.08.010 .
Runjic E, Behmen D, Pieper D, Mathes T, Tricco AC, Moher D, et al. Following Cochrane review protocols to completion 10 years later: a retrospective cohort study and author survey. J Clin Epidemiol. 2019;111:41–8. https://doi.org/10.1016/j.jclinepi.2019.03.006 .
Tricco AC, Antony J, Zarin W, Strifler L, Ghassemi M, Ivory J, et al. A scoping review of rapid review methods. BMC Med. 2015;13(1):224. https://doi.org/10.1186/s12916-015-0465-6 .
COVID-19 Rapid Reviews: Cochrane’s response so far. Available at: https://training.cochrane.org/resource/covid-19-rapid-reviews-cochrane-response-so-far . Accessed 1 June 2021.
Cochrane. Living systematic reviews. Available at: https://community.cochrane.org/review-production/production-resources/living-systematic-reviews . Accessed 1 June 2021.
Millard T, Synnot A, Elliott J, Green S, McDonald S, Turner T. Feasibility and acceptability of living systematic reviews: results from a mixed-methods evaluation. Syst Rev. 2019;8(1):325. https://doi.org/10.1186/s13643-019-1248-5 .
Babic A, Poklepovic Pericic T, Pieper D, Puljak L. How to decide whether a systematic review is stable and not in need of updating: analysis of Cochrane reviews. Res Synth Methods. 2020;11(6):884–90. https://doi.org/10.1002/jrsm.1451 .
Lovato A, Rossettini G, de Filippis C. Sore throat in COVID-19: comment on “clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis”. J Med Virol. 2020;92(7):714–5. https://doi.org/10.1002/jmv.25815 .
Leung C. Comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1431–2. https://doi.org/10.1002/jmv.25912 .
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. Response to Char’s comment: comment on Li et al: COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(9):1433. https://doi.org/10.1002/jmv.25924 .
Download references
Acknowledgments
We thank Catherine Henderson DPhil from Swanscoe Communications for pro bono medical writing and editing support. We acknowledge support from the Covidence Team, specifically Anneliese Arno. We thank the whole International Network of Coronavirus Disease 2019 (InterNetCOVID-19) for their commitment and involvement. Members of the InterNetCOVID-19 are listed in Additional file 6 . We thank Pavel Cerny and Roger Crosthwaite for guiding the team supervisor (IJBN) on human resources management.
This research received no external funding.
Author information
Authors and affiliations.
University Hospital and School of Medicine, Universidade Federal de Minas Gerais, Belo Horizonte, Minas Gerais, Brazil
Israel Júnior Borges do Nascimento & Milena Soriano Marcolino
Medical College of Wisconsin, Milwaukee, WI, USA
Israel Júnior Borges do Nascimento
Helene Fuld Health Trust National Institute for Evidence-based Practice in Nursing and Healthcare, College of Nursing, The Ohio State University, Columbus, OH, USA
Dónal P. O’Mathúna
School of Nursing, Psychotherapy and Community Health, Dublin City University, Dublin, Ireland
Department of Anesthesiology, Intensive Care and Pain Medicine, University of Münster, Münster, Germany
Thilo Caspar von Groote
Department of Sport and Health Science, Technische Universität München, Munich, Germany
Hebatullah Mohamed Abdulazeem
School of Health Sciences, Faculty of Health and Medicine, The University of Newcastle, Callaghan, Australia
Ishanka Weerasekara
Department of Physiotherapy, Faculty of Allied Health Sciences, University of Peradeniya, Peradeniya, Sri Lanka
Cochrane Croatia, University of Split, School of Medicine, Split, Croatia
Ana Marusic, Irena Zakarija-Grkovic & Tina Poklepovic Pericic
Center for Evidence-Based Medicine and Health Care, Catholic University of Croatia, Ilica 242, 10000, Zagreb, Croatia
Livia Puljak
Cochrane Brazil, Evidence-Based Health Program, Universidade Federal de São Paulo, São Paulo, Brazil
Vinicius Tassoni Civile & Alvaro Nagib Atallah
Yorkville University, Fredericton, New Brunswick, Canada
Santino Filoso
Laboratory for Industrial and Applied Mathematics (LIAM), Department of Mathematics and Statistics, York University, Toronto, Ontario, Canada
Nicola Luigi Bragazzi
You can also search for this author in PubMed Google Scholar
Contributions
IJBN conceived the research idea and worked as a project coordinator. DPOM, TCVG, HMA, IW, AM, LP, VTC, IZG, TPP, ANA, SF, NLB and MSM were involved in data curation, formal analysis, investigation, methodology, and initial draft writing. All authors revised the manuscript critically for the content. The author(s) read and approved the final manuscript.
Corresponding author
Correspondence to Livia Puljak .
Ethics declarations
Ethics approval and consent to participate.
Not required as data was based on published studies.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Search strategies used in the study.
Additional file 2: Appendix 2.
Adjusted scoring of AMSTAR 2 used in this study for systematic reviews of studies that did not analyze interventions.
Additional file 3: Appendix 3.
List of excluded studies, with reasons.
Additional file 4: Appendix 4.
Table of overlapping studies, containing the list of primary studies included, their visual overlap in individual systematic reviews, and the number in how many reviews each primary study was included.
Additional file 5: Appendix 5.
A detailed explanation of AMSTAR scoring for each item in each review.
Additional file 6: Appendix 6.
List of members and affiliates of International Network of Coronavirus Disease 2019 (InterNetCOVID-19).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Borges do Nascimento, I.J., O’Mathúna, D.P., von Groote, T.C. et al. Coronavirus disease (COVID-19) pandemic: an overview of systematic reviews. BMC Infect Dis 21 , 525 (2021). https://doi.org/10.1186/s12879-021-06214-4
Download citation
Received : 12 April 2020
Accepted : 19 May 2021
Published : 04 June 2021
DOI : https://doi.org/10.1186/s12879-021-06214-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Coronavirus
- Evidence-based medicine
- Infectious diseases
BMC Infectious Diseases
ISSN: 1471-2334
- General enquiries: [email protected]
- Contact Tracing
- Pandemic Data Initiative
- Events & News
JHU has stopped collecting data as of
After three years of around-the-clock tracking of COVID-19 data from...

What is the JHU CRC Now?
The Johns Hopkins Coronavirus Resource Center established a new standard for infectious disease tracking by publicly providing pandemic data in near real time. It began Jan. 22, 2020 as the COVID-19 Dashboard , operated by the Center for Systems Science and Engineering and the Applied Physics Laboratory . But the map of red dots quickly evolved into the global go-to hub for monitoring a public health catastrophe. By March 3, 2020, Johns Hopkins expanded the site into a comprehensive collection of raw data and independent expert analysis known as the Coronavirus Resource Center (CRC) – an enterprise that harnessed the world-renowned expertise from across Johns Hopkins University & Medicine.
Why did we shut down?
After three years of 24-7 operations, the CRC is ceasing its data collection efforts due to an increasing number of U.S. states slowing their reporting cadences. In addition, the federal government has improved its pandemic data tracking enough to warrant the CRC’s exit. From the start, this effort should have been provided by the U.S. government. This does not mean Johns Hopkins believes the pandemic is over. It is not. The institution remains committed to maintaining a leadership role in providing the public and policymakers with cutting edge insights into COVID-19. See details below.
Ongoing Johns Hopkins COVID-19 Resources
The Hub — the news and information website for Johns Hopkins — publishes the latest updates on COVID-19 research about vaccines, treatments, and public health measures.
The Johns Hopkins Bloomberg School of Public Health maintains the COVID-19 Projects and Initiatives page to share the latest research and practice efforts by Bloomberg faculty.
The Johns Hopkins Center for Health Security has been at the forefront of providing policymakers and the public with vital information on how to mitigate disease spread.
The Johns Hopkins International Vaccine Access Center offers an online, interactive map-based platform for easy navigation of hundreds of research reports into vaccine use and impact.
Johns Hopkins Medicine provides various online portals that provide information about COVID-19 patient care, vaccinations, testing and more.
Accessing past data
Johns Hopkins maintains two data repositories for the information collected by the Coronavirus Resource Center between Jan. 22, 2020 and March 10, 2023. The first features global cases and deaths data as plotted by the Center for Systems Science and Engineering. The second features U.S. and global vaccination data , testing information and demographics that were maintained by Johns Hopkins University’s Bloomberg Center for Government Excellence .
How to use the CSSE GitHub
- Click on csse_covid_19_data to access chronological 'time series' data.
- Click on csse_covid_19_time_series to access daily data for cases and deaths.
- Click on 'confirmed_US' for U.S. cases and “deaths_US” for U.S. fatalities; and “confirmed_global” for international cases and 'deaths_global' for international fatalities.
- Click “View Raw.”
- Left click to “Save As” as spreadsheet.
How to use the GovEx GitHub
- Visit the U.S. 'data dictionary' and the global 'data dictionary' to understand the vaccine, testing and demographic data available for your use.
- Example: Click on either 'us_data' or 'global_data' for vaccine information.
- Click on 'time_series' to access daily reports for either the U.S. or the world.
- Select either 'vaccines' or “doses administered” for the U.S. or the world.
- For either database, click 'view raw' to view the data or to save it to a spreadsheet.
Government Resources
Us cases & deaths.
The U.S. Centers for Disease Control and Prevention maintains a national data tracker.
GLOBAL TRENDS
The World Health Organization provides information about global spread.
The CDC also provides a vaccine data tracker for the U.S., while Our World In Data from Oxford University provides global vaccine information.
HOSPITAL ADMISSIONS
The CDC and the U.S. Department of Health and Human Services have provided hospital admission data in the United States.
THANK YOU TO ALL CONTRIBUTORS TO THE JHU CRC TEAM
Thank you to our partners, thank you to our funders, special thanks to.
Aaron Katz, Adam Lee, Alan Ravitz, Alex Roberts, Alexander Evelo, Amanda Galante, Amina Mahmood, Angel Aliseda Alonso, Anna Yaroslaski, Arman Kalikian, Beatrice Garcia, Breanna Johnson, Cathy Hurley, Christina Pikas, Christopher Watenpool, Cody Meiners, Cory McCarty, Dane Galloway, Daniel Raimi Zlatic, David Zhang, Doug Donovan, Elaine Gehr, Emily Camacho, Emily Pond, Ensheng Dong, Eric Forte, Ethel Wong, Evan Bolt, Fardin Ganjkhanloo, Farzin Ahmadi, Fernando Ortiz-Sacarello, George Cancro, Grant Zhao, Greta Kinsley, Gus Sentementes, Heather Bree, Hongru Du, Ian Price, Jan LaBarge, Jason Williams, Jeff Gara, Jennifer Nuzzo, Jeremy Ratcliff, Jill Rosen, Jim Maguire, John Olson, John Piorkowski, Jordan Wesley, Joseph Duva, Joseph Peterson, Josh Porterfield, Joshua Poplawski, Kailande Cassamajor, Kevin Medina Santiago, Khalil Hijazi, Krushi Shah, Lana Milman, Laura Asher, Laura Murphy, Lauren Kennell, Louis Pang, Mara Blake, Marianne von Nordeck, Marissa Collins, Marlene Caceres, Mary Conway Vaughan, Meg Burke, Melissa Leeds, Michael Moore, Miles Stewart, Miriam McKinney Gray, Mitch Smallwood, Molly Mantus, Nick Brunner, Nishant Gupta, Oren Tirschwell, Paul Nicholas, Phil Graff, Phillip Hilliard, Promise Maswanganye, Raghav Ramachandran, Reina Chano Murray, Roman Wang, Ryan Lau, Samantha Cooley, Sana Talwar, Sara Bertran de Lis, Sarah Prata, Sarthak Bhatnagar, Sayeed Choudury, Shelby Wilson, Sheri Lewis, Steven Borisko, Tamara Goyea, Taylor Martin, Teresa Colella, Tim Gion, Tim Ng, William La Cholter, Xiaoxue Zhou, Yael Weiss
CRC in the Media
Time names crc go-to data source.
TIME Magazine named the Johns Hopkins Coronavirus Resource Center one of its Top 100 Inventions for 2020.
Research!America Praises CRC Work
Research!America awarded the CRC a public health honor for providing reliable real time data about COVID-19.
CRC Earns Award From Fast Company
The Johns Hopkins Coronavirus Resource Center wins Fast Company’s 2021 Innovative Team of the Year award.
Public Service

Lauren Gardner Wins Lasker Award for Service
Lauren Gardner, co-creator of the COVID-19 Dashboard, won the 2022 Lasker-Bloomberg Public Service Award.
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
Coronavirus (COVID-19)
How americans view the coronavirus, covid-19 vaccines amid declining levels of concern.
Just 20% of the public views the coronavirus as a major threat to the health of the U.S. population and only 10% are very concerned about getting a serious case themselves. In addition, a relatively small share of U.S. adults (28%) say they’ve received an updated COVID-19 vaccine since last fall.
How the Pandemic Has Affected Attendance at U.S. Religious Services
During the pandemic, a stable share of U.S. adults have been participating in religious services in some way – either virtually or in person – but in-person attendance is slightly lower than it was before COVID-19. Among Americans surveyed across several years, the vast majority described their attendance habits in roughly the same way in both 2019 and 2022.
Mental health and the pandemic: What U.S. surveys have found
Here’s a look at what surveys by Pew Research Center and other organizations have found about Americans’ mental health during the pandemic.
Sign up for our weekly newsletter
Fresh data delivery Saturday mornings
Just 20% of the public views the coronavirus as a major threat to the health of the U.S. population and only 10% are very concerned about getting a serious case themselves. In addition, a relatively small share of U.S. adults (28%) say they’ve received an updated COVID-19 vaccine since last fall.
Online Religious Services Appeal to Many Americans, but Going in Person Remains More Popular
About a quarter of U.S. adults regularly watch religious services online or on TV, and most of them are highly satisfied with the experience. About two-in-ten Americans (21%) use apps or websites to help with reading scripture.
About a third of U.S. workers who can work from home now do so all the time
About a third of workers with jobs that can be done remotely are working from home all the time, according to a new Pew Research Center survey.
Economy Remains the Public’s Top Policy Priority; COVID-19 Concerns Decline Again
Americans now see reducing the budget deficit as a higher priority for the president and Congress to address than in recent years. But strengthening the economy continues to be the public’s top policy priority.
At least four-in-ten U.S. adults have faced high levels of psychological distress during COVID-19 pandemic
58% of those ages 18 to 29 have experienced high levels of psychological distress at least once between March 2020 and September 2022.
Key findings about COVID-19 restrictions that affected religious groups around the world in 2020
Our study analyzes 198 countries and territories and is based on policies and events in 2020, the most recent year for which data is available.
How COVID-19 Restrictions Affected Religious Groups Around the World in 2020
Nearly a quarter of countries used force to prevent religious gatherings during the pandemic; other government restrictions and social hostilities related to religion remained fairly stable.
What Makes Someone a Good Member of Society?
Most in advanced economies say voting, taking steps to reduce climate change and getting a COVID-19 vaccine are ways to be a good member of society; fewer say this about attending religious services.
REFINE YOUR SELECTION
Research teams.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
© 2024 Pew Research Center
New Report Reviews Evidence on Long COVID Diagnosis, Risk, Symptoms, and Functional Impact for Patients
- A formal COVID-19 diagnosis or positive test is not necessary to consider Long COVID diagnosis.
- Long COVID can cause more than 200 symptoms and affects each person differently. Long COVID is associated with a wide range of new or worsening health conditions impacting multiple organ systems. These can include cardiovascular, respiratory, mental health, gastrointestinal, nervous system, and metabolic symptoms. The report includes a full listing of all symptoms and conditions that have been associated with Long COVID.
- The risk of Long COVID increases with the severity of COVID-19 illness. The report estimates that people whose infection was sufficiently severe to necessitate hospitalization are 2 to 3 times more likely to experience Long COVID than are those who were not hospitalized, and among individuals who were hospitalized, those requiring life support in an intensive care unit may be twice as likely to experience Long COVID. However, people with mild disease can also develop Long COVID, and given the much higher number of people with mild versus severe disease, they make up the majority of people with Long COVID.
- There is no curative treatment for Long COVID. Management of the condition is based on current knowledge about treating its symptoms and health effects. As with other complex chronic health conditions, medical treatment for Long COVID is focused on managing symptoms and optimizing quality of life and function.
- Recovery from Long COVID varies among individuals, and the data on recovery trajectories are rapidly evolving. While there is evidence that many people with Long COVID symptoms have improved by 12 months, data beyond that time frame is limited but suggestive that recovery might plateau or progress at a slower rate.
- Socioeconomic status, geographic location, health literacy, and race and ethnicity all affect access to health care — and have contributed to disparities in access to COVID-19 testing, vaccination, and therapeutics, including treatments for acute infection and specialized rehabilitation clinics for Long COVID.
- Complex, infection-associated chronic conditions are not new, and Long COVID shares many features with conditions like myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and fibromyalgia.
- Although the majority of children, including adolescents, recover fully from COVID-19, some develop Long COVID and experience persistent or intermittent symptoms that can reduce their quality of life. This can result in increased school absences and decreased participation and performance in school, sports, and other social activities. The trajectory for recovery in children and adolescents is better than in adults. More research is needed to understand Long COVID in children, as information from adult studies may not be directly applicable.
Featured Publication
Long-Term Health Effects of COVID-19: Disability and Function Following SARS-CoV-2 Infection
Since the onset of the coronavirus disease 2019 (COVID-19) pandemic in early 2020, many individuals infected with the virus that causes COVID-19, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), have continued to experience lingering symptoms for months or even years following infection. Some symptoms can affect a person ability to work or attend school for an extended period of time. Consequently, in 2022, the Social Security Administration requested that the National Academies convene a committee of relevant experts to investigate and provide an overview of the current status of diagnosis, treatment, and prognosis of long-term health effects related to Long COVID. This report presents the committee conclusions.
- Press Release
Recent News
New Commission on Investment Imperatives for a Healthy Nation
Supporting Caregivers in Academic STEMM
Scientific Forum on Science in the Age of AI
Fostering Future Leaders and a More Inclusive Vision for Science
- Load More...
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 05 May 2021
COVID research: a year of scientific milestones
For just over a year of the COVID-19 pandemic, Nature highlighted key papers and preprints to help readers keep up with the flood of coronavirus research. Those highlights are below. For continued coverage of important COVID-19 developments, go to Nature’s news section .
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-020-00502-w
Reprints and permissions
- Medical research
- Epidemiology
Brain fluid probed by ultrasound using squishy cubes
News & Views 05 JUN 24
This injectable gel can help to diagnose brain injury — then it disappears
News 05 JUN 24

MDMA therapy for PTSD rejected by FDA panel
Father’s diet influences son’s metabolic health through sperm RNA
A virally encoded high-resolution screen of cytomegalovirus dependencies
Article 05 JUN 24

Huge amounts of bird-flu virus found in raw milk of infected cows

‘Preposterous’: Anthony Fauci denies cover-up of COVID origins during tense hearing
News 03 JUN 24
Faculty Positions, Aging and Neurodegeneration, Westlake Laboratory of Life Sciences and Biomedicine
Applicants with expertise in aging and neurodegeneration and related areas are particularly encouraged to apply.
Hangzhou, Zhejiang, China
Westlake Laboratory of Life Sciences and Biomedicine (WLLSB)
Faculty Positions in Chemical Biology, Westlake University
We are seeking outstanding scientists to lead vigorous independent research programs focusing on all aspects of chemical biology including...
School of Life Sciences, Westlake University
Postdoctoral Associate- Cancer Epidemiology
Houston, Texas (US)
Baylor College of Medicine (BCM)
Head of Climate Science and Impacts Team (f/m/d)
You will play a pivotal role in shaping the scientific outputs and supporting the organisation's mission and culture. Your department has 20+ staff.
Ritterstraße 3, 10969 Berlin
Climate Analytics gGmbH
Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, Warmly Welcomes Talents Abroad
Qiushi Chair Professor; Qiushi Distinguished Scholar; ZJU 100 Young Researcher; Distinguished researcher
No. 3, Qingchun East Road, Hangzhou, Zhejiang (CN)
Sir Run Run Shaw Hospital Affiliated with Zhejiang University School of Medicine
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Along with Stanford news and stories, show me:
- Student information
- Faculty/Staff information
We want to provide announcements, events, leadership messages and resources that are relevant to you. Your selection is stored in a browser cookie which you can remove at any time using “Clear all personalization” below.
A new, nano-scale look at how the SARS-CoV-2 virus replicates in cells may offer greater precision in drug development, a Stanford University team reports in Nature Communications . Using advanced microscopy techniques, the researchers produced what might be some of the most crisp images available of the virus’s RNA and replication structures, which they witnessed form spherical shapes around the nucleus of the infected cell.
“We have not seen COVID infecting cells at this high resolution and known what we are looking at before,” said Stanley Qi , Stanford associate professor of bioengineering in the Schools of Engineering and of Medicine and co-senior author of the paper. “Being able to know what you are looking at with this high resolution over time is fundamentally helpful to virology and future virus research, including antiviral drug development.”
Blinking RNA
The work illuminates molecular-scale details of the virus’ activity inside host cells. In order to spread, viruses essentially take over cells and transform them into virus-producing factories, complete with special replication organelles. Within this factory, the viral RNA needs to duplicate itself over and over until enough genetic material is gathered up to move out and infect new cells and start the process over again.
The Stanford scientists sought to reveal this replication step in the sharpest detail to date. To do so, they first labeled the viral RNA and replication-associated proteins with fluorescent molecules of different colors. But imaging glowing RNA alone would result in fuzzy blobs in a conventional microscope. So they added a chemical that temporarily suppresses the fluorescence. The molecules would then blink back on at random times, and only a few lit up at a time. That made it easier to pinpoint the flashes, revealing the locations of the individual molecules.
Using a setup that included lasers, powerful microscopes, and a camera snapping photos every 10 milliseconds, the researchers gathered snapshots of the blinking molecules. When they combined sets of these images, they were able to create finely detailed photos showing the viral RNA and replication structures in the cells. “We have highly sensitive and specific methods and also high resolution,” said Leonid Andronov, co-lead author and Stanford chemistry postdoctoral scholar. “You can see one viral molecule inside the cell.”
The resulting images, with a resolution of 10 nanometers, reveal what might be the most detailed view yet of how the virus replicates itself inside of a cell. The images show magenta RNA forming clumps around the nucleus of the cell, which accumulate into a large repeating pattern. “We are the first to find that viral genomic RNA forms distinct globular structures at high resolution,” said Mengting Han, co-lead author and Stanford bioengineering postdoctoral scholar.
Video showing the different colored fluorescent labels blinking on and off, revealing more precise locations for individual molecules. | Leonid Andronov, Moerner Laboratory
The clusters help show how the virus evades the cell’s defenses, said W. E. Moerner , the paper’s co-senior author and Harry S. Mosher Professor of Chemistry in the School of Humanities and Sciences. “They’re collected together inside a membrane that sequesters them from the rest of the cell, so that they’re not attacked by the rest of the cell.”
Nanoscale drug testing
Compared to using an electron microscope, the new imaging technique can allow researchers to know with greater certainty where virus components are in a cell thanks to the blinking fluorescent labels. It also can provide nanoscale details of cell processes that are invisible in medical research conducted through biochemical assays. The conventional techniques “are completely different from these spatial recordings of where the objects actually are in the cell, down to this much higher resolution,” said Moerner. “We have an advantage based on the fluorescent labeling because we know where our light is coming from.”
Seeing exactly how the virus stages its infection holds promise for medicine. Observing how different viruses take over cells may help answer questions such as why some pathogens produce mild symptoms while others are life-threatening. The super-resolution microscopy can also benefit drug development. “This nanoscale structure of the replication organelles can provide some new therapeutic targets for us,” said Han. “We can use this method to screen different drugs and see its influence on the nanoscale structure.”
Indeed, that’s what the team plans to do. They will repeat the experiment and see how the viral structures shift in the presence of drugs like Paxlovid or remdesivir. If a candidate drug can suppress the viral replication step, that suggests the drug is effective at inhibiting the pathogen and making it easier for the host to fight the infection.
The researchers also plan to map all 29 proteins that make up SARS-CoV-2 and see what those proteins do across the span of an infection. “We hope that we will be prepared to really use these methods for the next challenge to quickly see what’s going on inside and better understand it,” said Qi.
For more information
Acknowledgements: Additional Stanford co-authors include postdoctoral scholar Yanyu Zhu, PhD student Ashwin Balaji, former PhD student Anish Roy, postdoctoral scholar Andrew Barentine, research specialist Puja Patel, and Jaishree Garhyan, director of the In Vitro Biosafety Level-3 Service Center . Moerner is also a member of Stanford Bio-X and the Wu Tsai Neurosciences Institute, and a faculty fellow of Sarafan ChEM-H . Qi is also a member of Bio-X, the Cardiovascular Institute , the Maternal & Child Health Research Institute (MCHRI), the Stanford Cancer Institute, and the Wu Tsai Neurosciences Institute, an institute scholar at Sarafan ChEM-H , and a Chan Zuckerberg Biohub – San Francisco Investigator.
This research was funded by the National Institute of General Medical Sciences of the National Institutes of Health. We also acknowledge use of the Stanford University Cell Sciences Imaging Core Facility.
Taylor Kubota, Stanford University: [email protected]
Announcements
Updates on campus events, policies, construction and more.
- Dean’s 2023 State of the School address available online
- Notice of data security incident
- COVID-19: Medical Campus updates
close
Information for Our Community
Whether you are part of our community or are interested in joining us, we welcome you to Washington University School of Medicine.
- Prospective Students
- Current Students
- Alumni & Friends
- Administrators
- Researchers
- Job Seekers
Risk of death from COVID-19 lessens, but infection still can cause issues 3 years later
Study also shows that patients hospitalized within 30 days after infection face 29% higher death risk in 3rd year compared with those not infected
by Kristina Sauerwein • May 30, 2024
New findings on long COVID by Washington University School of Medicine in St. Louis and the Veterans Affairs St. Louis Health Care system reveal that COVID-19 patients who were hospitalized within the first 30 days after infection face a 29% higher risk of death in the third year post-infection compared with people who have not had the virus. However, the three-year death risk marks a significant decline compared with such risk at previous time points post-infection. The study also shows that even people with mild COVID-19 still experienced new health problems related to the infection three years later.
New findings on long COVID — long-term effects on health experienced by many who have had COVID-19 — present a good-news, bad-news situation, according to a study at Washington University School of Medicine in St. Louis and the Veterans Affairs St. Louis Health Care system.
The bad news: COVID-19 patients who were hospitalized within the first 30 days after infection face a 29% higher risk of death in the third year compared with people who have not had the virus. However, the three-year death risk still marks a significant decline compared with such risk at the one- and two-year marks post-infection. The findings also show that even people with mild COVID-19 were still experiencing new health problems related to the infection three years later.
The good news: The increased risk of death diminishes significantly one year after a SARS-CoV-2 infection among people who were not hospitalized for the virus. This demographic accounts for most people who have had COVID-19.
The new research, published May 30 in Nature Medicine, tracked the virus’s health effects in people three years after being infected with the original strain of COVID-19 in 2020. That year, about 20 million people tested positive for the virus in the U.S. The new study assessed the risk of death and 80 adverse health conditions in people three years after being diagnosed with COVID-19.
“We aren’t sure why the virus’s effects linger for so long,” said senior author Ziyad Al-Aly, MD, a Washington University clinical epidemiologist and a global leader in long COVID research. “Possibly it has to do with viral persistence, chronic inflammation, immune dysfunction or all the above. We tend to think of infections as mostly short-term illnesses with health effects that manifest around the time of infection. Our data challenges this notion. I feel COVID-19 continues to teach us — and this is an important new lesson — that a brief, seemingly innocuous or benign encounter with the virus can still lead to health problems years later.”
Up to 10% of people infected with the virus experience long COVID, according to federal data.
Al-Aly’s prior research has documented COVID-19’s damage to nearly every human organ, contributing to diseases and conditions affecting the lungs, heart, brain, and the body’s blood, musculoskeletal and gastrointestinal (GI) systems.
Such studies with longer follow-up are limited, said Al-Aly, a nephrologist who treats patients at the Washington University-affiliated John J. Cochran Veterans Hospital in midtown St. Louis. “Addressing this knowledge gap is critical to enhance our understanding of long COVID and will help inform care for people suffering from long COVID.”
Al-Aly and his team analyzed millions of de-identified medical records in a database maintained by the U.S. Department of Veterans Affairs, the nation’s largest integrated health-care system. The study included more than 114,000 veterans with mild COVID-19 who did not require hospitalization; more than 20,000 hospitalized COVID-19 patients; and 5.2 million veterans with no COVID-19 diagnosis. Patients were enrolled in the study from March 1, 2020, to Dec. 31, 2020, and followed for at least three years, until Dec. 31, 2023. Patients included people of diverse ages, races and sexes; statistical modeling ensured parity in representation.
In the third year after infection, COVID-19 patients who had been hospitalized experienced a 34% elevated health risk across all organ systems compared with people who did not have COVID. That number is down from a 182% increased risk one year after a COVID infection and a 57% risk two years after.
Among nonhospitalized patients, researchers found a 5% increased risk in suffering from long COVID in the third year after infection. This translates into 41 more health problems per 1,000 persons – a small but not trivial burden. The long-term health effects in the third year primarily affected the GI, pulmonary and neurological systems. By comparison, the risk was increased by 23% one year after infection and increased by 16% two years after.
In the analysis, researchers also measured and compared the number of healthy life-years lost due to COVID-19. They found that among the nonhospitalized, at three years after infection, COVID-19 had contributed to 10 lost years of healthy life per 1,000 persons. By comparison, three years post-infection, those hospitalized for COVID-19 had experienced 90 lost years of healthy life per 1,000 persons.
For context, in the U.S., heart disease and cancer each cause about 50 lost years of healthy life per 1,000 persons, while stroke contributes to 10 lost years of healthy life per 1,000 persons.
“That a mild SARS-CoV-2 infection can lead to new health problems three years down the road is a sobering finding,” said Al-Aly, who is also director of the Clinical Epidemiology Center at the VA St. Louis Health Care System, and head of the research and development service. “The problem is even worse for people with severe SARS-CoV-2 infection. It is very concerning that the burden of disease among hospitalized individuals is astronomically higher.”
“COVID-19 is a serious threat to the long-term health and well-being of people and it should not be trivialized,” he said.
The extended trajectory for long COVID may change as researchers incorporate data from years beyond 2020. At that time, vaccines and antivirals had not been developed. Similarly, Al-Aly’s analysis does not consider subsequent variants such as omicron or delta.
“Even three years out, you might have forgotten about COVID-19, but COVID hasn’t forgotten about you,” Al-Aly said. “People might think they’re out of the woods, because they had the virus and did not experience health problems. But three years after infection, the virus could still be wreaking havoc and causing disease or illness in the gut, lungs or brain.”
- Click to share on Facebook (Opens in new window)
- Click to share on Twitter (Opens in new window)
- Click to share on Pinterest (Opens in new window)
- Click to share on LinkedIn (Opens in new window)
Editors' Picks

June 6, 2024
MD/PhD student creates crosswords for major newspapers.
Medical Campus & Community, Profiles
May 17, 2024
Response to updated vaccine is shaped by earlier vaccines yet generates broadly neutralizing antibodies.
News Release

April 19, 2024
Gift from Andy Newman supports world-changing research on the gut microbiome.
Medical Campus & Community, News Release

The link between poor housing conditions and COVID-19 infection
Robb, Katharine, Rowana Ahmed, John Wong, Elissa Ladd, and Jorrit de Jong. “ Substandard housing and the risk of COVID-19 infection and disease severity: A retrospective cohort study. ” SSM - Population Health 25 (March 2024): 101629.
Faculty Authors

Jorrit de Jong
What’s the issue.
Following the peak of the COVID-19 pandemic, researchers continue to investigate the factors related to the spread of disease—and how city leaders can better respond before, during, and after such crises. Housing conditions, for example, can play a role in infectious disease risk. Substandard housing—which might feature poor ventilation, overcrowding, and dampness—can create an environment favorable to respiratory disease.
So, did poor housing conditions lead to more—and more severe—cases of COVID-19 infection during the pandemic?
What does the research say?
Researchers from the Bloomberg Center for Cities at Harvard University and the MGH Institute of Health Professions studied the connections between poor housing conditions and COVID-19 infection and severity during the first year of the pandemic. They combined city housing data with healthcare data for residents of Chelsea, Massachusetts—a densely populated city with high levels of substandard housing.
The researchers found that:
- Living in substandard housing was linked to higher COVID-19 infection risk, even after adjusting for factors like age, income, and race.
- This increased risk was only observed during lockdown and early reopening; after stay-at-home restrictions lifted there was no difference in COVID-19 risk between residents of substandard versus adequate housing.
- Substandard housing was not linked to greater risk of severe COVID-19 disease.
- Leaders can leverage city housing data for pandemic response and longer-term solutions.
They conclude, “The results demonstrate the value of combining cross-sector datasets to yield new insights and solutions. Existing city data can be leveraged to identify and prioritize 1) high-risk areas for future pandemic response activities, and 2) for longer-term solutions that address social determinants of health through safe and affordable housing.”
More from HKS
Media narratives have generally blamed democrats for housing crises in cities. research suggests that is not the full story., advancing progress in cities around the globe, doctors’ political beliefs influence covid-19 treatment recommendations, study suggests.
Get smart & reliable public policy insights right in your inbox.
Advertisement
Supported by
New Report Underscores the Seriousness of Long Covid
The National Academies said the condition could involve up to 200 symptoms, make it difficult for people to work and last for months or years.
- Share full article

By Pam Belluck
One of the nation’s premier medical advisory organizations has weighed in on long Covid with a 265-page report that recognizes the seriousness and persistence of the condition for millions of Americans.
More than four years since the start of the coronavirus pandemic, long Covid continues to damage many people’s ability to function, according to the National Academies of Sciences, Engineering and Medicine, a nongovernmental institution that advises federal agencies on science and medicine.
“Long Covid can impact people across the life span, from children to older adults, as well as across sex, gender, racial, ethnic and other demographic groups,” it said, concluding that “long Covid is associated with a wide range of new or worsening health conditions and encompasses more than 200 symptoms involving nearly every organ system.”
Here are some of the National Academies’ findings, drafted by a committee of 14 doctors and researchers:
How many people have long Covid?
The report cited data from 2022 suggesting that nearly 18 million adults and nearly a million children in the United States have had long Covid at some point. At the time of that survey, about 8.9 million adults and 362,000 children had the condition.
Surveys showed that the prevalence of long Covid decreased in 2023 but, for unclear reasons, has risen this year. As of January, data showed nearly 7 percent of adults in the United States had long Covid.
Diagnosis and consequences
There is still no standardized way to diagnose the condition and no definitive treatments to cure it. “There is no one-size-fits-all approach to rehabilitation, and each individual will need a program tailored to their complex needs,” the National Academies said, advising that doctors should not require patients to have a positive coronavirus test to be diagnosed with long Covid.
The report said that some of the most troublesome symptoms — like brain fog and chronic fatigue — can prevent people from returning to work and should make them eligible for disability payments, though their symptoms may not fit the Social Security Administration’s current disability categories.
“Long Covid can result in the inability to return to work (or school for children and adolescents), poor quality of life, diminished ability to perform activities of daily living, and decreased physical and cognitive function for six months to two years or longer,” the report said.
People most at risk
People who become more seriously ill from their initial coronavirus infection are more likely to have long-term symptoms. Those who were sick enough to be hospitalized were two to three times as likely to develop long Covid.
But, the report said, “even individuals with a mild initial course of illness can develop long Covid with severe health effects.” And “given the much higher number of people with mild versus severe disease, they make up the great majority of people with long Covid.”
Women are about twice as likely to develop long Covid. Other risk factors include not being adequately vaccinated against the coronavirus, having preexisting medical conditions or disabilities and smoking.
Long Covid in children
Children are less likely than adults to develop long Covid and are more likely to recover from it, but some children “experience persistent or intermittent symptoms that can reduce their quality of life” and “result in increased school absences and decreased participation and performance in school, sports and other social activities,” the report said.
Recovery from long Covid
Some people recover with time, and there’s some evidence that after a year, many people’s symptoms have diminished. But some research suggests that recovery slows down or plateaus after that first year, the report said.
Because long Covid varies so widely from person to person and affects so many body systems, each case must be approached individually.
For some people, “returning to work too early may result in health deterioration, and a gradual return to work plan may be advised,” the report said, especially for people with post-exertional malaise, a symptom that involves depleted energy or setbacks after doing activities that involve physical or mental exertion.
Employers may need to offer accommodations to returning employees, like allowing them to take frequent breaks or work remotely.
Some similarities to other chronic conditions
“Long Covid appears to be a chronic illness, with few patients achieving full remission,” the report said.
Some symptoms are like those of other conditions that emerge following infections, including myalgic encephalomyelitis/chronic fatigue syndrome, fibromyalgia and postural orthostatic tachycardia syndrome.
The biological cause of the symptoms is unclear. Theories include inflammation, fragments of remaining virus and immune system dysregulation.
Equity issues make long Covid worse
Long Covid presents more obstacles for people who face economic challenges or discrimination because of their race or ethnicity, where they live or how much education they have.
Such patients may encounter more skepticism about their symptoms, may be less able to take time off from work and may live farther from long Covid clinics or treatment programs.
Pam Belluck is a health and science reporter, covering a range of subjects, including reproductive health, long Covid, brain science, neurological disorders, mental health and genetics. More about Pam Belluck
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Elsevier - PMC COVID-19 Collection

The impact of COVID-19 on research
a Department of Pediatric Urology and Pediatric Surgery, Hopital Pellegrin-Enfants, CHU Bordeaux, France
b Service de chirurgie et urologie pédiatrique, hôpital Lapeyronie, CHU de Montpellier et Université de Montpellier, France
G.M.A. Beckers
c Department of Urology, Section of Pediatric Urology, AmsterdamUMC, Location VUmc, Amsterdam, the Netherlands
d Indiana University, 702 Barnhill Drive, Suite 4230, Indianapolis, IN, USA
A.J. Nieuwhof-Leppink
e Department of Medical Psychology and Social Work, Urology, Wilhelmina Children’s Hospital, University Medical Center Utrecht, PO box 85090, 3508 AB, Utrecht, the Netherlands
Magdalena Fossum
f Department of Pediatric Surgery, Copenhagen University Hospital Rigshospitalet, DK-2100, Denmark
g Department of Women's and Children's Health, Bioclinicum, Floor 10, Karolinska Institutet, SE-171 76, Stockholm, Sweden
K.W. Herbst
h Division of Urology, Department of Research, Connecticut Children's Medical Center, Hartford, CT, USA
i Hospital for Sick Chidlren, Univeristy of Toronto, Canada
Coronavirus disease 2019 (COVID-19) has swept across the globe causing hundreds of thousands of deaths, shutting down economies, closing borders and wreaking havoc on an unprecedented scale. It has strained healthcare services and personnel to the brink in many regions and will certainly deeply mark medical research both in the short and long-term.
Prior to the COVID pandemic, virology research (including influenza) represented less than 2% of all biomedical research. However, the number of laboratories and investigators that have pivoted to address COVID related research questions is astonishing, likely comprising 10–20% of current biomedical investigation, showing the incredible adaptability of the research community [ 1 ]. The multinational support rapidly infused for COVID-19 research is in the billions of euros [ 2 ]. The sharing of research findings and research data has never been as rapid and efficient [ 3 ]. The crisis has also brought disease, health, and healthcare back to the forefront of societal issues, and will have a lasting impact on public spending. However, with all this optimism and focus, there is a downside.
To begin, the COVID-19 crisis has led to a massive influx of publications. Not only are specialty journals being flooded with submissions by authors being unwittingly granted much needed writing time, but publications on COVID have literally inundated us. More than 20,000 papers have been published since December 2019, many in prestigious journals. There are also an increasing number of studies being uploaded to preprint servers, such as BioRxiv, for rapid dissemination prior to any peer review. However, we cannot assume that the time and quality available for peer review is able to keep pace with the explosion of publication. There is need for increased caution in the wake of this massive influx of submissions, especially since we are increasingly seeing these results being picked up by the media and diffused to a less attuned audience. In recent weeks, several prestigious journals, including the Lancet and the New England Journal of Medicine, have published retractions of earlier and potentially major COVID-related findings [ 4 , 5 ]. On June 15, 2020, The New York Times highlighted potential lapses in the peer review process affecting major scientific journals [ 6 ].
We must strive to improve scientific quality always. The current debate over the use of hydroxychloroquine further illustrates the undermining of the scientific process when faced with global desperation for ready-made truths and solutions [ 4 , 7 , 8 ]. Science needs time, and good science needs a lot of it for data to grow and knowledge to evolve, but this process is ill-prepared to handle the rush for solutions to the COVID crises.
Moreover, just as COVID-19 has shown social, racial, and economic health disparities, the pandemic seems also to have accentuated existing gender inequalities within the field of research [ 9 ]. Indeed, early analyses suggest that female academics are publishing less and starting fewer research projects than their male peers. This might be an effect of the lockdown and the fact that more women than are men are juggling caring for families and children despite both “working” from home [ 10 , 11 ].
Travel, social, and funding restrictions will also take a serious toll on scientific research worldwide. Research staff and resources have been purposely and purposefully prioritized to COVID-19 activities above all else. Distancing and transmission issues have caused most non-COVID clinical research to be suspended, causing a reduction in recruitment of research subjects and a delay in data entry into clinical trial databases [ 12 ]. Research-related hiring has been suspended because of travel restrictions and young researchers might soon find themselves out of a job if their subject is not the pandemic. Indeed, though government-funded medical research bodies worldwide say they are committed to maintaining the continuity and breadth of biomedical research, how the economic downfall will influence government spending remains to be seen. Furthermore, research funding that relies on public fundraising is expected to drop substantially and many researchers will see a significant decrease in funding opportunities [ 13 ]. The global impact the crisis will have on the economy makes it hard to imagine that future research funding will not be substantially affected.
During this crisis, many resources were understandably redirected toward preparing for and caring for COVID-19 patients, but the collateral damage to so many patients with non-COVID-19 medical conditions that did not receive, or failed to seek, treatment will surely emerge [ 14 ]. Finally, children have also paid a high price for the redirecting of medical resources, with delays in their medical and surgical management, as well as vaccinations [ 15 , 16 ]. This may be especially problematic when many aspects of pediatric care is based on their developmental clock, which even the pandemic cannot stop. Whether this was the best option will certainly be analyzed in retrospect. Congenital anomalies alone account for over 400,000 deaths worldwide every year, and inflict a considerable burden both on children, families, and healthcare systems [ 17 ]. Thus, it is essential that funding for medical research does not follow the same pattern with a disproportionate decrease in funding for non-COVID research including pediatric and developmental urology.
COVID-19 has already changed the world, not only because of the disease itself, but because of the long-term effects of the world's reaction to the pandemic. While the pandemic may have brought with it some silver linings, it is crucial that the scientific community conduct current and future research broadly and openly, lest future pandemic preparedness in research repeat the hard-fought lessons of today.
COVID Select Subcommittee Releases Dr. Fauci’s Transcript, Highlights Key Takeaways in New Memo
WASHINGTON — Today, Select Subcommittee on the Coronavirus Pandemic Chairman Brad Wenstrup (R-Ohio) released the transcript from Dr. Anthony Fauci’s transcribed interview. Dr. Fauci served as the Director of the National Institute of Allergy and Infectious Diseases (NIAID) and was the face of America’s public health response during the COVID-19 pandemic. His closed door, 14-hour, two-day testimony in January 2024 has served as a critical component of the Select Subcommittee’s investigations into the origins of COVID-19, pandemic-era domestic policy failures, and improvements to the United States’ public health system. In conjunction with the transcript, the Select Subcommittee also released a new staff memo that highlights the key takeaways from Dr. Fauci’s transcribed interview. The memo can be found here .
The Select Subcommittee also released four additional transcripts from senior public health officials. These transcripts, as well as Dr. Fauci’s transcript, can be found below:
- Dr. Anthony Fauci Part 1
- Dr. Anthony Fauci Part 2
- Dr. Hugh Auchincloss
- Dr. Cliff Lane
- Greg Folkers
- Gray Handley
Below are important exchanges from Dr. Fauci’s transcribed interview:
SOCIAL DISTANCING : The “6 feet apart” social distancing recommendation forced on Americans by federal health officials was arbitrary and not based on science. Dr. Fauci testified that this guidance — which shut down schools and small businesses nationwide — “sort of just appeared” and was not based on any scientific studies.
Majority Counsel: “ Do you recall when discussions regarding, kind of, the at least a 6 foot threshold began? ”
Dr. Fauci: “The 6 foot in the school?”
Majority Counsel: “Six foot overall. I mean, 6-foot was applied at businesse s—”
Dr. Fauci: “Yeah.”
Majority Counsel: “ —it was applied in schools, it was applied here. At least how the messaging was applied was that 6-foot distancing was the distance that needed to be— “
Dr. Fauci: “ You know, I don’t recall. It sort of just appeared. I don’t recall, like, a discussion of whether it should be 5 or 6 or whatever. It was just that 6 foot is— ”
Majority Counsel: “ Did you see any studies that supported 6 feet? ”
Dr. Fauci: “ I was not aware of studies that in fact, that would be a very difficult study to do. ”
MASKING : Dr. Fauci testified that he did not recall any supporting evidence for masking children. Concerningly, mask-wearing has been associated with learning loss and severe speech development issues in America’s children.
Majority Counsel: “ Do you recall reviewing any studies or data supporting masking for children? ”
Dr. Fauci: “ You know, I might have, Mitch, but I don’t recall specifically that I did. I might have. ”
Majority Counsel: “ Since the — there’s been a lot of studies that have come out since the pandemic started, but specifically on this there have been significant on kind of like the learning loss and speech and development issues that have been associated with particularly young children wearing masks while they’re growing up. They can’t see their teacher talk and can’t learn how to form words. Have you followed any of those studies? ”
Dr. Fauci: “ No. But I believe that there are a lot of conflicting studies too, that there are those that say, yes, there is an impact, and there are those that say there’s not. I still think that’s up in the air. ”
TRAVEL RESTRICTIONS : Dr. Fauci unequivocally agreed with EVERY travel restriction issued by the Trump Administration at the height of the COVID-19 pandemic. This testimony runs counter to the public narrative that the Trump Administration’s travel restrictions were xenophobic. During his transcribed interview, the Biden Administration’s counsel curiously prohibited Dr. Fauci from answering questions on whether he recommended the travel restrictions.
Majority Counsel: “ Did you agree with President Trump’s decision to restrict travel from China? ”
Dr. Fauci: “ I did , and I said there were caveats to restrictions. I agreed with it, but I said we have to be careful because sometimes when you do restrictions they have negative consequences in that you don’t have open access to help or even information. But fundamentally, I agreed at that time, since we had almost no infections that we knew of in our country, that at least a temporary restriction would be important. ”
Majority Counsel: “ Did you also agree with the EU travel restriction? ”
Dr. Fauci: “ I agreed with the suggestion that that be done, yes. ”
Majority Counsel: “ Did you agree with the U.K. travel restriction? ”
Dr. Fauci: “ Yes, I did. ”
Majority Counsel: “ Did you recommend instituting travel restrictions in response to the pandemic? ”
Biden Administration Official: “ I’m going to step in here .”
VACCINE MANDATES: Dr. Fauci admitted that vaccine mandates during the COVID-19 pandemic could increase vaccine hesitancy in the future. He also claimed that these mandates were not sufficiently studied ahead of the pandemic. Previously, Dr. Fauci advocated “that when you make it difficult for people in their lives, they lose their ideological bullshit, and they get vaccinated.”
Majority Counsel: “ Do you think mandating vaccines can result in some hesitancy? ”
Dr. Fauci: “ I think one of the things that we really need to do after the fact, now, to — you know, after-the-game, after-the-event evaluation of things that need to be done, we really need to take a look at the psyche of the country, have maybe some social-type studies to figure out, does the mandating of vaccines in the way the country’s mental framework is right now, does that actually cause more people to not want to get vaccinated, or not? I don’t know. But I think that’s something we need to know. ”
LAB LEAK THEORY : Dr. Fauci acknowledged that the lab leak hypothesis is not a conspiracy theory. This comes nearly four years after prompting the publication of the now infamous “Proximal Origin” paper that attempted to vilify and disprove the lab leak hypothesis.
Majority Counsel: “ Just you sitting here today, do you think the possibility or the hypothesis that the coronavirus emerged from a laboratory accident is a conspiracy theory? ”
Dr. Fauci: “ Well, it’s a possibility. I think people have made conspiracy aspects from it. And I think you have to separate the two when you keep an open mind, that it could be a lab leak or it could be a natural occurrence. I’ve mentioned in this committee that I believe the evidence that I’ve seen weighs my opinion towards one, which is a natural occurrence, but I still leave an open mind. So I think that in and of itself isn’t inherently a conspiracy theory, but some people spin off things from that that are kind of crazy .”
GAIN-OF-FUNCTION RESEARCH : Dr. Fauci repeatedly played semantics with the definition of “gain-of-function” research in an effort to avoid conceding that the NIH’s funded this dangerous research in China . As the head of NIAID and the face of America’s response to the pandemic, Dr. Fauci certainly understood the common definition of “gain-of-function.” Yet, he repeatedly refused — both behind closed doors and to Sen. Rand Paul during a 2021 hearing — to clarify a general understanding of the term and instead only referred to his own “operative definition.
Dr. Fauci: “ So, when I, to repeat, when I’m asked is something gain of function, I’m referring to the operative definition of gain of function according to the framework of the 3PCO…That’s my definition. That is the regulatory operational definition. And as we were talking about before, other people use the word “gain of function” this, “gain of function” that, and everybody’s got their own interpretation of it. But when you’re deciding whether a grant should be funded, this is the operational definition. And when I was asked anywhere by the Congress, by the Senate, by Senator Paul this is what I was referring to .”
CONFLICTS OF INTEREST : Dr. Fauci claimed that his staff had no conflicts of interest regarding the origins of COVID-19, yet his Senior Advisor — Dr. David Morens — was “best-friends” with disgraced and soon-to-be debarred EcoHealth Alliance President Dr. Peter Daszak . Considering Dr. Morens worked under Dr. Fauci’s leadership for more than 20 years, it seems highly unlikely that Dr. Fauci was genuinely unaware of this relationship.
Majority Counsel: “ I was wondering if you had thoughts on whether Dr. Daszak should have filed competing interest statements when he was weighing in on these issues, whether through the National Academies or other venues. ”
Dr. Fauci: “ You know, I hesitate to speculate about what someone else should do. The only people that I am involved with is my own staff, who we’ve mentioned many times in this discussion, who don’t have a conflict of interest. ”
GRANT APPROVAL : Dr. Fauci testified that he signed off on every foreign and domestic NIAID grant without reviewing the proposals. He was also unable to confirm if NIAID has ANY mechanisms to conduct oversight of the foreign laboratories they fund . NIAID’s flawed grant process — which relies heavily on trusting its grantees without verifying — leaves opportunities for adversaries to exploit.
Majority Counsel: “ Who gives the final approval? ”
Dr. Fauci: “ You know, technically, I sign off on each council, but I don’t see the grants and what they are. I never look at what grants are there. It’s just somebody at the end of the council where they’re all finished and they go, ‘Here,’ and you sign it .”
Majority Counsel: “ Okay. So to your knowledge, NIAID wouldn’t kind of independently verify the biosafety of a foreign lab? ”
Dr. Fauci: “ Again, I’d have to say I’m not sure. To my knowledge, I wouldn’t be able to make a statement that I would be confident it would be. ”
Majority Counsel: “ Do you know if NIAID grants go through any type of national security review as part of the process? ”
Dr. Fauci: “ National security review? ”
Majority Counsel: “ So, like, through the National Security Council or— “
Dr. Fauci: “ No. ”
Majority Counsel: “ —or anyone in the [intelligence community]— “
Dr. Fauci: “ Not to my knowledge .”
Majority Counsel: “ I guess what we’re trying to learn going forward is, obviously, U.S. labs are vetted, certified, and there’s a standard of how U.S. labs operate. Are foreign labs held to the same standard as U.S. labs when they receive U.S. money, or are they the standards of the country in which they operate? ”
Dr. Fauci: “ I am not certain. I have heard again, I think it was subsequent to of course, that was never brought up. ”
Majority Counsel: “ Uh huh. ”
Dr. Fauci: “ When I was the director, no one ever asked me, you know, who determines, you know, what the standards of a foreign lab are. But so the answer to your question is I don’t know, okay? ”
FEIGNED IGNORANCE : Dr. Fauci claimed he “did not recall” numerous issues and events surrounding the pandemic more than 100 times . Specifically, Dr. Fauci testified that despite the fact EcoHealth Alliance was conducting risky gain-of-function research in China, he did not know any details about the grant, nor did he maintain a relationship with its President, Dr. Peter Daszak.
Majority Counsel: “ Do you recall when you first found out that the year 5 progress report was missing from the EcoHealth grant? ”
Dr. Fauci: “ I don’t recall precisely. It was somewhere on a briefing that the staff gave to me. I don’t know exactly when that was. It could have been later. I don’t know. ”
Majority Counsel: “ Okay. Do you think, just to the best of your recollection, whether it was before you were aware that the year 5 progress report was late before May 2021 or it would have been after? ”
Dr. Fauci: “ I don’t recall. ”

IMAGES
COMMENTS
The WHO COVID-19 Research Database was a resource created in response to the Public Health Emergency of International Concern (PHEIC). It contained citations with abstracts to scientific articles, reports, books, preprints, and clinical trials on COVID-19 and related literature. The WHO Covid-19 Research Database was maintained by the WHO ...
Key Events in the Effort to Determine the Origins of the Covid-19 Pandemic. The joint WHO-China technical report published in March 2021 rated a zoonotic spillover as a "likely to very likely ...
The impact on research in progress prior to COVID-19 was rapid, dramatic, and no doubt will be long term. The pandemic curtailed most academic, industry, and government basic science and clinical ...
The research community has responded by publishing an impressive number of scientific reports related to COVID-19. The world was alerted to the new disease at the beginning of 2020 [ 1 ], and by mid-March 2020, more than 2000 articles had been published on COVID-19 in scholarly journals, with 25% of them containing original data [ 5 ].
Research Open Access 29 May 2024 Scientific Reports Volume: 14, P: 12365 PMMA dialyzers modulate both humoral and cell-mediate immune response to anti-COVID-19 vaccine (BNT162b2) in a cohort of ...
Explore a collection of articles and other resources on the Coronavirus (Covid-19) outbreak, including clinical reports, management guidelines, and commentary.
COVID-19 can involve persistence, sequelae, and other medical complications that last weeks to months after initial recovery. This systematic review and meta-analysis aims to identify studies ...
COVID-19 is moderately infectious with a relatively high mortality rate, but the information available in public reports and published literature is rapidly increasing. The aim of this review is to summarize the current understanding of COVID-19 including causative agent, pathogenesis of the disease, diagnosis and treatment of the cases, as ...
Here, we report safety and efficacy findings from the phase 2/3 part of a global phase 1/2/3 trial evaluating the safety, immunogenicity, and efficacy of 30 μg of BNT162b2 in preventing Covid-19 ...
In this rapid living systematic evidence synthesis and meta-analysis, we searched EMBASE and the US National Institutes of Health's iSearch COVID-19 Portfolio, supplemented by manual searches of COVID-19-specific sources, until Dec 1, 2022, for studies that reported vaccine effectiveness immediately and at least 112 days after a primary vaccine series or at least 84 days after a booster dose.
Find COVID-19 datasets, data tools, and publications to use in research. EXPLORE COVID-19 DATA. Learn how NIH is supporting research in COVID-19 testing, treatments, and vaccines.
Coronavirus (COVID-19) research. Medical, social, and behavioral science articles from Sage Sage believes in the power of the social and behavioral sciences to convert the best medical research into policies, practices, and procedures to improve - and even save - lives. This collection includes the latest medical research from Sage related ...
Community‐based studies in five countries show consistent strong benefits from early rollouts of COVID‐19 vaccines. By the beginning of June 2021, almost 11% of the world's population had received at least one dose of a coronavirus disease 2019 (COVID‐19) vaccine. 1 This represents an extraordinary scientific and logistic achievement — in 18 months, researchers, manufacturers and ...
The Johns Hopkins International Vaccine Access Center offers an online, interactive map-based platform for easy navigation of hundreds of research reports into vaccine use and impact. Treatment Johns Hopkins Medicine provides various online portals that provide information about COVID-19 patient care, vaccinations, testing and more.
At least four-in-ten U.S. adults have faced high levels of psychological distress during COVID-19 pandemic. 58% of those ages 18 to 29 have experienced high levels of psychological distress at least once between March 2020 and September 2022. short readsNov 29, 2022.
There have been around 96,000 reported cases of coronavirus disease 2019 (COVID-2019) and 3300 reported deaths to date (05/03/2020). The disease is transmitted by inhalation or contact with infected droplets and the incubation period ranges from 2 to 14 d. The symptoms are usually fever, cough, sore throat, breathlessness, fatigue, malaise ...
COVID-19 Resource Centre. As of January 2024, changes have been made to our COVID-19 Resource Centre. A COVID-19 Collection is available where you can continue to explore and access The Lancet Group's COVID-19 research, reviews, commentary, news, and analysis as it is published.
A new National Academies report for the Social Security Administration presents findings about Long COVID, including that it can cause more than 200 symptoms and that some effects can impact an individual's ability to function for six months to two years or longer after COVID-19 infection.
If COVID-19 was a defining health event, the global responses to COVID-19 were a defining health policy experience (4, 5).The swiftness of global responses, their extensiveness, and direct implications for billions of people's lives were historically unique: The responses to the 1918 influenza pandemic, in comparison, were largely localized, while the global response to the HIV pandemic was ...
Andrew McGuire at the Fred Hutchinson Cancer Research Center in Seattle, Washington, and his colleagues collected blood from ten people who had recovered from COVID-19; they collected additional ...
more likely to delay graduation due to COVID-19 and are 41% more likely to report that COVID-19 impacted their major choice. Further, COVID-19 nearly doubled the gap between higher- and lower-income students' expected GPA.4 There also is substantial variation in the pandemic's e ect on preference for online learning,
The clusters help show how the virus evades the cell's defenses, said W. E. Moerner, the paper's co-senior author and Harry S. Mosher Professor of Chemistry in the School of Humanities and ...
This demographic accounts for most people who have had COVID-19. The new research, published May 30 in Nature Medicine, tracked the virus's health effects in people three years after being infected with the original strain of COVID-19 in 2020. That year, about 20 million people tested positive for the virus in the U.S.
The protective effects of vaccination and prior infection against severe Covid-19 are reviewed, with proposed directions for future research, including mucosal immunity and intermittent vaccine boo...
The COVID-19 pandemic led to an increase in racial and ethnic disparities among children in attendance to recommended well-child visits, according to an AHRQ study. ... New Research and Evidence From AHRQ. White Paper: Making Healthcare Safer IV: Summary of Findings on Year 1 Topics.
What does the research say? Researchers from the Bloomberg Center for Cities at Harvard University and the MGH Institute of Health Professions studied the connections between poor housing conditions and COVID-19 infection and severity during the first year of the pandemic. They combined city housing data with healthcare data for residents of ...
Covid-19 Guidance. Symptoms and Treatment ... The report said that some of the most troublesome symptoms — like brain fog and chronic fatigue — can prevent people from returning to work and ...
The impact of COVID-19 on research. Coronavirus disease 2019 (COVID-19) has swept across the globe causing hundreds of thousands of deaths, shutting down economies, closing borders and wreaking havoc on an unprecedented scale. It has strained healthcare services and personnel to the brink in many regions and will certainly deeply mark medical ...
His closed door, 14-hour, two-day testimony in January 2024 has served as a critical component of the Select Subcommittee's investigations into the origins of COVID-19, pandemic-era domestic policy failures, and improvements to the United States' public health system.
To do this, we used major sources of global data, including the University of Oxford's Covid-19 Government Response Tracker and the Johns Hopkins Covid-19 dashboard, on the use of any of 19 ...