Loading metrics
Open Access

Origins of DNA replication
Affiliation Quantitative Biology, Friedrich Miescher Institute for Biomedical Research, Basel, Switzerland
* E-mail: [email protected]
- Babatunde Ekundayo,
- Franziska Bleichert

Published: September 12, 2019
- https://doi.org/10.1371/journal.pgen.1008320
- Reader Comments
19 Dec 2019: The PLOS Genetics Staff (2019) Correction: Origins of DNA replication. PLOS Genetics 15(12): e1008556. https://doi.org/10.1371/journal.pgen.1008556 View correction
In all kingdoms of life, DNA is used to encode hereditary information. Propagation of the genetic material between generations requires timely and accurate duplication of DNA by semiconservative replication prior to cell division to ensure each daughter cell receives the full complement of chromosomes . DNA synthesis of daughter strands starts at discrete sites, termed replication origins, and proceeds in a bidirectional manner until all genomic DNA is replicated. Despite the fundamental nature of these events, organisms have evolved surprisingly divergent strategies that control replication onset. Here, we discuss commonalities and differences in replication origin organization and recognition in the three domains of life.
Citation: Ekundayo B, Bleichert F (2019) Origins of DNA replication. PLoS Genet 15(9): e1008320. https://doi.org/10.1371/journal.pgen.1008320
Copyright: © 2019 Ekundayo, Bleichert. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding: This work was supported by the Novartis Research Foundation and the European Research Council under the European Union’s Horizon 2020 research and innovation program (ERC-STG-757909). The funders had no role in the preparation of the article.
Competing interests: The authors have declared that no competing interests exist.
Wikipedia Version : https://en.wikipedia.org/wiki/origins_of_dna_replication .
Introduction
In the second half of the 19th century, Gregor Mendel's pioneering work on the inheritance of traits in pea plants suggested that specific “factors” (today established as genes) are responsible for transferring organismal traits between generations [ 1 ]. Although proteins were initially assumed to serve as the hereditary material, Avery, MacLeod and McCarty established a century later DNA, which had been discovered by Friedrich Miescher , as the carrier of genetic information [ 2 ]. These findings paved the way for research uncovering the chemical nature of DNA and the rules for encoding genetic information, and ultimately led to the proposal of the double-helical structure of DNA by Watson and Crick [ 3 ]. This three-dimensional model of DNA illuminated potential mechanisms by which the genetic information could be copied in a semiconservative manner prior to cell division, a hypothesis that was later experimentally supported by Meselson and Stahl using isotope incorporation to distinguish parental from newly synthesized DNA [ 4 ][ 5 ]. The subsequent isolation of DNA polymerases, the enzymes that catalyze the synthesis of new DNA strands, by Kornberg and colleagues pioneered the identification of many different components of the biological DNA replication machinery, first in the bacterial model organism E . coli , but later also in eukaryotic life forms [ 6 ].
A key prerequisite for DNA replication is that it must occur with extremely high fidelity and efficiency exactly once per cell cycle to prevent the accumulation of genetic alterations with potentially deleterious consequences for cell survival and organismal viability [ 7 ]. Incomplete, erroneous, or untimely DNA replication events can give rise to mutations, chromosomal polyploidy or aneuploidy , and gene copy number variations, each of which in turn can lead to diseases, including cancer [ 8 ][ 9 ]. To ensure complete and accurate duplication of the entire genome and the correct flow of genetic information to progeny cells, all DNA replication events are not only tightly regulated with cell cycle cues but are also coordinated with other cellular events such as transcription and DNA repair [ 10 ][ 11 ][ 12 ].
DNA replication is divided into different stages ( Fig 1 ). During initiation, the replication machineries–termed replisomes–are assembled on DNA in a bidirectional fashion. These assembly loci constitute the start sites of DNA replication or replication origins. In the elongation phase, replisomes travel in opposite directions with the replication forks, unwinding the DNA helix and synthesizing complementary daughter DNA strands using both parental strands as templates. Once replication is complete, specific termination events lead to the disassembly of replisomes. As long as the entire genome is duplicated before cell division, one might assume that the location of replication start sites does not matter; yet, it has been shown that many organisms use preferred genomic regions as origins [ 13 ][ 14 ]. The necessity to regulate origin location likely arises from the need to coordinate DNA replication with other processes that act on the shared chromatin template to avoid DNA strand breaks and DNA damage [ 8 ][ 12 ][ 15 ][ 16 ][ 17 ][ 18 ][ 19 ].
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Models for bacterial ( A ) and eukaryotic ( B ) DNA replication initiation. A ) Circular bacterial chromosomes contain a cis -acting element, the replicator, that is located at or near replication origins. i ) The replicator recruits initiator proteins in a DNA sequence-specific manner, which results in melting of the DNA helix and loading of the replicative helicase onto each of the single DNA strands ( ii ). iii ) Assembled replisomes bidirectionally replicate DNA to yield two copies of the bacterial chromosome. B ) Linear eukaryotic chromosomes contain many replication origins. Initiator binding ( i ) facilitates replicative helicase loading ( ii ) onto duplex DNA to license origins. iii ) A subset of loaded helicases is activated for replisome assembly. Replication proceeds bidirectionally from origins and terminates when replication forks from adjacent active origins meet ( iv ).
https://doi.org/10.1371/journal.pgen.1008320.g001
The replicon model
More than five decades ago, Jacob , Brenner , and Cuzin proposed the replicon hypothesis to explain the regulation of chromosomal DNA synthesis in E . coli [ 20 ]. The model postulates that a diffusible, trans -acting factor, a so-called initiator, interacts with a cis -acting DNA element, the replicator, to promote replication onset at a nearby origin ( Fig 1A , i ). Once bound to replicators, initiators (often with the help of co-loader proteins) deposit replicative helicases onto DNA, which subsequently drive the recruitment of additional replisome components and the assembly of the entire replication machinery ( Fig 1A , ii ). The replicator thereby specifies the location of replication initiation events, and the chromosome region that is replicated from a single origin or initiation event is defined as the replicon.
A fundamental feature of the replicon hypothesis is that it relies on positive regulation to control DNA replication onset, which can explain many experimental observations in bacterial and phage systems [ 20 ]. For example, it accounts for the failure of extrachromosomal DNAs without origins to replicate when introduced into host cells. It further rationalizes plasmid incompatibilities in E . coli , where certain plasmids destabilize each other’s inheritance due to competition for the same molecular initiation machinery [ 21 ]. By contrast, a model of negative regulation (analogous to the replicon-operator model for transcription) fails to explain the above findings [ 20 ]. Nonetheless, research subsequent to Jacob’s, Brenner’s and Cuzin’s proposal of the replicon model has discovered many additional layers of replication control in bacteria and eukaryotes that comprise both positive and negative regulatory elements, highlighting both the complexity and the importance of restricting DNA replication temporally and spatially [ 22 ][ 23 ][ 24 ].
The concept of the replicator as a genetic entity has proven very useful in the quest to identify replicator DNA sequences and initiator proteins in prokaryotes , and to some extent also in eukaryotes , although the organization and complexity of replicators differ considerably between the domains of life (for reviews, see [ 25 ][ 26 ]). While bacterial genomes typically contain a single replicator that is specified by consensus DNA sequence elements and that controls replication of the entire chromosome ( Fig 1A ), most eukaryotic replicators–with the exception of budding yeast–are not defined at the level of DNA sequence; instead, they appear to be specified combinatorially by local DNA structural and chromatin cues [ 27 ][ 28 ][ 29 ][ 30 ] [ 31 ][ 32 ][ 33 ][ 34 ][ 35 ][ 36 ]. Eukaryotic chromosomes are also much larger than their bacterial counterparts, raising the need for initiating DNA synthesis from many origins simultaneously to ensure timely replication of the entire genome ( Fig 1B ). Additionally, many more replicative helicases are loaded than activated to initiate replication in a given cell cycle ( Fig 1B ). The context-driven definition of replicators and selection of origins suggests a relaxed replicon model in eukaryotic systems that allows for flexibility in the DNA replication program [ 25 ]. Although replicators and origins can be spaced physically apart on chromosomes, they often co-localize or are located in close proximity; for simplicity, we will thus refer to both elements as ‘origins’ throughout this review. Taken together, the discovery and isolation of origin sequences in various organisms represents a significant milestone towards gaining mechanistic understanding of replication initiation. In addition, these accomplishments had profound biotechnological implications for the development of shuttle vectors that can be propagated in bacterial, yeast, and mammalian cells [ 37 ][ 38 ][ 39 ].
Bacterial replication origins
Most bacterial chromosomes are circular and contain a single origin of chromosomal replication ( oriC ). Bacterial oriC regions are surprisingly diverse in size (ranging from 250 bp to 2 kbp), sequence, and organization [ 41 ][ 42 ]; nonetheless, their ability to drive replication onset typically depends on sequence-specific readout of consensus DNA elements by the bacterial initiator, a protein called DnaA [ 43 ][ 44 ][ 45 ][ 46 ]. Origins in bacteria are either continuous or bipartite and contain three functional elements that control origin activity: conserved DNA repeats that are specifically recognized by DnaA (called DnaA-boxes), an AT-rich DNA unwinding element (DUE), and binding sites for proteins that help regulate replication initiation (for reviews, see [ 13 ][ 47 ][ 48 ]; Fig 2A ). Interactions of DnaA both with the double-stranded (ds) DnaA-box regions and with single-stranded (ss) DNA in the DUE are important for origin activation and are mediated by different domains in the initiator protein: a helix-turn-helix (HTH) DNA binding element and an ATPase associated with various cellular activities ( AAA+ ) domain, respectively ( Fig 2B ) [ 49 ][ 50 ][ 51 ][ 52 ][ 53 ][ 54 ][ 55 ][ 56 ]. While the sequence, number, and arrangement of origin-associated DnaA-boxes vary throughout the bacterial kingdom, their specific positioning and spacing in a given species are critical for oriC function and for productive initiation complex formation [ 41 ][ 42 ][ 57 ][ 58 ][ 59 ][ 60 ][ 61 ].
A ) Schematic of the architecture of E . coli origin oriC , Thermotoga maritima oriC , and the bipartite origin in Helicobacter pylori . The DUE is flanked on one side by several high- and weak-affinity DnaA-boxes as indicated for E . coli oriC . B ) Domain organization of the E . coli initiator DnaA. The magenta circle indicates the single-strand DNA binding site. C ) Models for origin recognition and melting by DnaA. In the two-state model (left panel), the DnaA protomers transition from a dsDNA binding mode (mediated by the HTH-domains recognizing DnaA-boxes) to an ssDNA binding mode (mediated by the AAA+ domains). In the loop-back model, the DNA is sharply bent backwards onto the DnaA filament (facilitated by the regulatory protein IHF [ 40 ]) so that a single protomer binds both duplex and single-stranded regions. In either instance, the DnaA filament melts the DNA duplex and stabilizes the initiation bubble prior to loading of the replicative helicase (DnaB in E . coli ). HTH–helix-turn-helix domain, DUE–DNA unwinding element, IHF–integration host factor.
https://doi.org/10.1371/journal.pgen.1008320.g002
Among bacteria, E . coli is a particularly powerful model system to study the organization, recognition, and activation mechanism of replication origins. E . coli oriC comprises an approximately 260 bp region containing four types of initiator binding elements that differ in their affinities for DnaA and their dependencies on the co-factor ATP ( Fig 2A ). DnaA-boxes R1, R2, and R4 constitute high-affinity sites that are bound by the HTH domain of DnaA irrespective of the nucleotide-binding state of the initiator [ 43 ][ 62 ][ 63 ][ 64 ][ 65 ][ 66 ]. By contrast, the I, τ, and C-sites, which are interspersed between the R-sites, are low-affinity DnaA-boxes and associate preferentially with ATP-bound DnaA, although ADP-DnaA can substitute for ATP-DnaA under certain conditions [ 67 ][ 68 ][ 69 ][ 60 ]. Binding of the HTH domains to the high- and low-affinity DnaA recognition elements promotes ATP-dependent higher-order oligomerization of DnaA’s AAA+ modules into a right-handed filament that wraps duplex DNA around its outer surface, thereby generating superhelical torsion that facilitates melting of the adjacent AT-rich DUE ( Fig 2C ) [ 49 ][ 70 ][ 71 ][ 72 ]. DNA strand separation is additionally aided by direct interactions of DnaA’s AAA+ ATPase domain with triplet repeats, so-called DnaA-trios, in the proximal DUE region [ 73 ]. The engagement of single-stranded trinucleotide segments by the initiator filament stretches DNA and stabilizes the initiation bubble by preventing reannealing [ 53 ]. The DnaA-trio origin element is conserved in many bacterial species, indicating it is a key element for origin function [ 73 ]. After melting, the DUE provides an entry site for the E . coli replicative helicase DnaB, which is deposited onto each of the single DNA strands by its loader protein DnaC.
Although the different DNA binding activities of DnaA have been extensively studied biochemically and various apo , ssDNA-, or dsDNA-bound structures have been determined [ 52 ][ 53 ][ 54 ][ 71 ], the exact architecture of the higher-order DnaA- oriC initiation assembly remains unclear. Two models have been proposed to explain the organization of essential origin elements and DnaA-mediated oriC melting. The two-state model assumes a continuous DnaA filament that switches from a dsDNA binding mode (the organizing complex) to an ssDNA binding mode in the DUE (the melting complex) ( Fig 2C , left panel ) [ 71 ][ 74 ]. By contrast, in the loop-back model, the DNA is sharply bent in oriC and folds back onto the initiator filament so that DnaA protomers simultaneously engage double- and single-stranded DNA regions ( Fig 2C , right panel ) [ 75 ]. Elucidating how exactly oriC DNA is organized by DnaA remains thus an important task for future studies. Insights into initiation complex architecture will help explain not only how origin DNA is melted, but also how a replicative helicase is loaded directionally onto each of the exposed single DNA strands in the unwound DUE, and how these events are aided by interactions of the helicase with the initiator and specific loader proteins.
Archaeal replication origins
Archaeal replication origins share some but not all of the organizational features of bacterial oriC . Unlike bacteria, archaea often initiate replication from multiple origins per chromosome (one to four have been reported) [ 76 ][ 77 ][ 78 ][ 79 ][ 80 ][ 81 ][ 82 ] [ 83 ][ 42 ]; yet, archaeal origins also bear specialized sequence regions that control origin function (for recent reviews, see [ 84 ][ 85 ][ 86 ]). These elements include both DNA sequence-specific origin recognition boxes (ORBs or miniORBs) and an AT-rich DUE that is flanked by one or several ORB regions [ 82 ][ 87 ]. ORB elements display a considerable degree of diversity in terms of their number, arrangement, and sequence, both among different archaeal species and among different origins within in a single species [ 77 ][ 82 ][ 88 ]. An additional degree of complexity is introduced by the initiator, Orc1/Cdc6 in archaea, which binds to ORB regions. Archaeal genomes typically encode multiple paralogs of Orc1/Cdc6 that vary substantially in their affinities for distinct ORB elements and that differentially contribute to origin activities [ 82 ][ 89 ][ 90 ][ 91 ]. In Sulfolobus solfataricus , for example, three chromosomal origins have been mapped (oriC1, oriC2, and oriC3; Fig 3A ), and biochemical studies have revealed complex binding patterns of initiators at these sites ( Fig 3B ) [ 82 ][ 83 ][ 92 ][ 93 ]. The cognate initiator for oriC1 is Orc1-1, which associates with several ORBs at this origin [ 82 ][ 90 ]. OriC2 and oriC3 are bound by both Orc1-1 and Orc1-3 [ 82 ][ 90 ][ 93 ]. Conversely, a third paralog, Orc1-2, footprints at all three origins but has been postulated to negatively regulate replication initiation [ 82 ][ 93 ]. Additionally, the WhiP protein, an initiator unrelated to Orc1/Cdc6, has been shown to bind all origins as well and to drive origin activity of oriC3 in the closely related Sulfolobus islandicus [ 90 ][ 92 ]. Because archaeal origins often contain several adjacent ORB elements, multiple Orc1/Cdc6 paralogs can be simultaneously recruited to an origin and oligomerize in some instances [ 91 ][ 94 ]; however, in contrast to bacterial DnaA, formation of a higher-order initiator assembly does not appear to be a general prerequisite for origin function in the archaeal domain.
A ) The circular chromosome of Sulfolobus solfataricus contains three different origins. B ) Arrangement of initiator binding sites at two S . solfataricus origins, oriC1 and oriC2. Orc1-1 association with ORB elements is shown for oriC1. Recognition elements for additional Orc1/Cdc6 paralogs are also indicated, while WhiP binding sites have been omitted. C ) Domain architecture of archaeal Orc1/Cdc6 paralogs. The orientation of ORB elements at origins leads to directional binding of Orc1/Cdc6 and MCM loading in between opposing ORBs (in B ). (m)ORB–(mini-)origin recognition box, DUE–DNA unwinding element, WH–winged-helix domain.
https://doi.org/10.1371/journal.pgen.1008320.g003
Structural studies have provided insights into how archaeal Orc1/Cdc6 recognizes ORB elements and remodels origin DNA [ 94 ][ 95 ]. Orc1/Cdc6 paralogs are two-domain proteins and are composed of a AAA+ ATPase module fused to a C-terminal winged-helix fold ( Fig 3C ) [ 96 ][ 97 ][ 98 ]. DNA-complexed structures of Orc1/Cdc6 revealed that ORBs are bound by an Orc1/Cdc6 monomer despite the presence of inverted repeat sequences within ORB elements [ 94 ][ 95 ]. Both the ATPase and winged-helix regions interact with the DNA duplex but contact the palindromic ORB repeat sequence asymmetrically, which orients Orc1/Cdc6 in a specific direction on the repeat [ 94 ][ 95 ]. Interestingly, the DUE-flanking ORB or miniORB elements often have opposite polarities [ 77 ][ 82 ][ 91 ][ 99 ][ 100 ], which predicts that the AAA+ lid subdomains and the winged-helix domains of Orc1/Cdc6 are positioned on either side of the DUE in a manner where they face each other ( Fig 3B , bottom panel ) [ 94 ][ 95 ]. Since both regions of Orc1/Cdc6 associate with the minichromosome maintenance (MCM) replicative helicase [ 101 ][ 102 ], this specific arrangement of ORB elements and Orc1/Cdc6 is likely important for loading two MCM complexes symmetrically onto the DUE ( Fig 3B ) [ 82 ]. Surprisingly, while the ORB DNA sequence determines the directionality of Orc1/Cdc6 binding, the initiator makes relatively few sequence-specific contacts with DNA [ 94 ][ 95 ]. However, Orc1/Cdc6 underwinds and bends DNA, suggesting that it relies on a mix of both DNA sequence and context-dependent DNA structural features to recognize origins [ 94 ][ 95 ][ 103 ]. Notably, base pairing is maintained in the distorted DNA duplex upon Orc1/Cdc6 binding in the crystal structures [ 94 ][ 95 ], whereas biochemical studies have yielded contradictory findings as to whether archaeal initiators can melt DNA similarly to bacterial DnaA [ 90 ][ 91 ][ 104 ]. Although the evolutionary kinship of archaeal and eukaryotic initiators and replicative helicases indicates that archaeal MCM is likely loaded onto duplex DNA (see next section), the temporal order of origin melting and helicase loading, as well as the mechanism for origin DNA melting, in archaeal systems remains therefore to be clearly established. Likewise, how exactly the MCM helicase is loaded onto DNA needs to be addressed in future studies.
Eukaryotic replication origins
Origin organization, specification, and activation in eukaryotes are more complex than in bacterial or archaeal kingdoms and significantly deviate from the paradigm established for prokaryotic replication initiation. The large genome sizes of eukaryotic cells, which range from 12 Mbp in S . cerevisiae to 3 Gbp in humans, necessitates that DNA replication starts at several hundred (in budding yeast) to tens of thousands (in humans) origins to complete DNA replication of all chromosomes during each cell cycle (for recent reviews, see [ 32 ][ 23 ]). With the exception of S . cerevisiae and related Saccharomycotina species, eukaryotic origins do not contain consensus DNA sequence elements but their location is influenced by contextual cues such as local DNA topology, DNA structural features, and chromatin environment [ 25 ][ 31 ][ 33 ]. Nonetheless, eukaryotic origin function still relies on a conserved initiator protein complex to load replicative helicases onto DNA during the late M and G1 phases of the cell cycle, a step known as origin licensing ( Fig 1B ). [ 107 ] In contrast to their bacterial counterparts, replicative helicases in eukaryotes are loaded onto origin duplex DNA in an inactive, double-hexameric form and only a subset of them (10–20% in mammalian cells) is activated during any given S phase, events that are referred to as origin firing ( Fig 1B ) [ 108 ][ 109 ][ 110 ]. The location of active eukaryotic origins is therefore determined on at least two different levels, origin licensing to mark all potential origins, and origin firing to select a subset that permits assembly of the replication machinery and initiation of DNA synthesis. The extra licensed origins serve as backup and are activated only upon slowing or stalling of nearby replication forks, ensuring that DNA replication can be completed when cells encounter replication stress [ 111 ][ 112 ]. Together, the excess of licensed origins and the tight cell cycle control of origin licensing and firing embody two important strategies to prevent under- and overreplication and to maintain the integrity of eukaryotic genomes.
Early studies in S . cerevisiae indicated that replication origins in eukaryotes might be recognized in a DNA-sequence-specific manner analogously to those in prokaryotes. In budding yeast, the search for genetic replicators lead to the identification of autonomously replicating sequences (ARS) that support efficient DNA replication initiation of extrachromosomal DNA [ 113 ][ 114 ][ 115 ]. These ARS regions are approximately 100–200 bp long and exhibit a multipartite organization, containing A, B1, B2, and sometimes B3 elements that together are essential for origin function ( Fig 4 ) [ 116 ][ 117 ]. The A element encompasses the conserved 11 bp ARS consensus sequence (ACS) [ 118 ][ 119 ], which, in conjunction with the B1 element, constitutes the primary binding site for the heterohexameric origin recognition complex (ORC), the eukaryotic replication initiator [ 120 ][ 121 ][ 122 ][ 123 ]. Within ORC, five subunits are predicated on conserved AAA+ ATPase and winged-helix folds and co-assemble into a pentameric ring that encircles DNA ( Fig 4 ) [ 123 ][ 124 ][ 125 ]. In budding yeast ORC, DNA binding elements in the ATPase and winged-helix domains, as well as adjacent basic patch regions in some of the ORC subunits, are positioned in the central pore of the ORC ring such that they aid the DNA-sequence-specific recognition of the ACS in an ATP-dependent manner [ 123 ][ 126 ]. By contrast, the roles of the B2 and B3 elements are less clear. The B2 region is similar to the ACS in sequence and has been suggested to function as a second ORC binding site under certain conditions, or as a binding site for the replicative helicase core [ 127 ][ 128 ][ 129 ][ 130 ][ 131 ]. Conversely, the B3 element recruits the transcription factor Abf1, albeit B3 is not found at all budding yeast origins and Abf1 binding does not appear to be strictly essential for origin function [ 116 ][ 132 ][ 133 ].
Specific DNA elements and epigenetic features involved in ORC recruitment and origin function are summarized for S . cerevisiae , S . pombe , and metazoan origins. A schematic of the ORC architecture is also shown, highlighting the arrangement of the AAA+ and winged-helix domains into a pentameric ring that encircles origin DNA. Ancillary domains of several ORC subunits involved in targeting ORC to chromosomes are included. Other regions in ORC subunits may also be involved in initiator recruitment, either by directly or indirectly associating with partner proteins. A few examples are listed. Note that the BAH domain in S . cerevisiae Orc1 binds nucleosomes [ 105 ] but does not recognize H4K20me2 [ 106 ]. BAH–bromo-adjacent homology domain, WH–winged-helix domain, TFIIB–transcription factor II B-like domain in Orc6, G4 –G quadruplex, OGRE–origin G-rich repeated element.
https://doi.org/10.1371/journal.pgen.1008320.g004
Origin recognition in eukaryotes other than S . cerevisiae or its close relatives does not conform to the sequence-specific readout of conserved origin DNA elements. Pursuits to isolate specific chromosomal replicator sequences more generally in eukaryotic species, either genetically or by genome-wide mapping of initiator binding or replication start sites, have failed to identify clear consensus sequences at origins [ 134 ][ 135 ][ 136 ][ 137 ][ 138 ][ 139 ][ 140 ][ 141 ][ 142 ][ 143 ][ 144 ][ 145 ]. Thus, sequence-specific DNA-initiator interactions in budding yeast signify a specialized mode for origin recognition in this system rather than an archetypal mode for origin specification across the eukaryotic domain. Nonetheless, DNA replication does initiate at discrete sites that are not randomly distributed across eukaryotic genomes, arguing that alternative means determine the chromosomal location of origins in these systems. These mechanisms involve a complex interplay between DNA accessibility, nucleotide sequence skew (both AT-richness and CpG islands have been linked to origins), nucleosome positioning, epigenetic features, DNA topology and certain DNA structural features (e.g., G4 motifs), as well as regulatory proteins and transcriptional interference [ 13 ][ 14 ][ 30 ][ 31 ][ 33 ][ 146 ][ 147 ][ 139 ][ 148 ]. Importantly, origin properties vary not only between different origins in an organism and among species, but some can also change during development and cell differentiation. The chorion locus in Drosophila follicle cells constitutes a well-established example for spatial and developmental control of initiation events. This region undergoes DNA-replication-dependent gene amplification at a defined stage during oogenesis and relies on the timely and specific activation of chorion origins, which in turn is regulated by origin-specific cis-elements and several protein factors, including the Myb complex, E2F1, and E2F2 [ 149 ][ 150 ][ 151 ][ 152 ][ 153 ]. This combinatorial specification and multifactorial regulation of metazoan origins has complicated the identification of unifying features that determine the location of replication start sites across eukaryotes more generally.
To facilitate replication initiation, ORC assemblies from various species have evolved specialized auxiliary domains that are thought to aid initiator targeting to chromosomal origins or chromosomes in general ( Fig 4 ). For example, the Orc4 subunit in S . pombe ORC contains several AT-hooks that preferentially bind AT-rich DNA [ 154 ], while in metazoan ORC the TFIIB-like domain of Orc6 is thought to perform a similar function [ 155 ]. Metazoan Orc1 proteins also harbor a bromo-adjacent homology (BAH) domain that interacts with H4K20me2-nucleosomes [ 106 ]. Particularly in mammalian cells, H4K20 methylation has been reported to be required for efficient replication initiation, and the Orc1-BAH domain facilitates ORC association with chromosomes and Epstein-Barr virus origin-dependent replication [ 156 ][ 157 ][ 158 ][ 159 ][ 160 ]. Therefore, it is intriguing to speculate that both observations are mechanistically linked at least in a subset of metazoa, but this possibility needs to be further explored in future studies. In addition to the recognition of certain DNA or epigenetic features, ORC also associates directly or indirectly with several partner proteins that could aid initiator recruitment, including LRWD1, PHIP (or DCAF14), HMGA1a, among others ( Fig 4 ) [ 29 ][ 161 ][ 162 ][ 163 ][ 164 ][ 165 ][ 166 ][ 167 ]. Interestingly, Drosophila ORC, like its budding yeast counterpart, bends DNA and negative supercoiling has been reported to enhance DNA binding of this complex, suggesting that DNA topology and malleability might influence the location of ORC binding sites across metazoan genomes [ 27 ][ 123 ][ 168 ][ 169 ][ 170 ]. A molecular understanding for how ORC’s DNA binding regions might support the readout of structural properties of the DNA duplex in metazoans rather than of specific DNA sequences as in S . cerevisiae awaits high-resolution structural information of DNA-bound metazoan initiator assemblies. Likewise, how different epigenetic factors contribute to initiator recruitment in metazoan systems is poorly defined and is an important question that needs to be addressed in more detail.
Once recruited to origins, ORC and its co-factors Cdc6 and Cdt1 drive the deposition of the minichromosome maintenance 2–7 (Mcm2-7) complex onto DNA (for reviews see [ 107 ][ 171 ]). Like the archaeal replicative helicase core, Mcm2-7 is loaded as a head-to-head double hexamer onto DNA to license origins ( Fig 1B ) [ 108 ][ 109 ][ 110 ]. In S-phase, Dbf4-dependent kinase (DDK) and cyclin-dependent kinase (CDK) phosphorylate several Mcm2-7 subunits and additional initiation factors to promote the recruitment of the helicase co-activators Cdc45 and GINS, DNA melting, and ultimately bidirectional replisome assembly at a subset of the licensed origins ( Fig 1B ) [ 172 ][ 24 ]. In both yeast and metazoans, origins are free or depleted of nucleosomes, a property that is crucial for Mcm2-7 loading, indicating that chromatin state at origins regulates not only initiator recruitment but also helicase loading [ 140 ][ 173 ][ 174 ][ 175 ][ 176 ][ 177 ]. A permissive chromatin environment is further important for origin activation and has been implicated in regulating both origin efficiency and the timing of origin firing. Euchromatic origins typically contain active chromatin marks, replicate early, and are more efficient than late-replicating, heterochromatic origins, which conversely are characterized by repressive marks [ 23 ][ 175 ][ 178 ]. Not surprisingly, several chromatin remodelers and chromatin-modifying enzymes have been found to associate with origins and certain initiation factors [ 179 ][ 180 ], but how their activities impact different replication initiation events remains largely obscure. Remarkably, cis-acting “early replication control elements” (ERCEs) have recently also been identified to help regulate replication timing and to influence 3D genome architecture in mammalian cells [ 181 ]. Understanding the molecular and biochemical mechanisms that orchestrate this complex interplay between 3D genome organization, local and higher-order chromatin structure, and replication initiation is an exciting topic for further studies.
Why have metazoan replication origins diverged from the DNA sequence-specific recognition paradigm that determines replication start sites in prokaryotes and budding yeast? Observations that metazoan origins often co-localize with promoter regions in Drosophila and mammalian cells and that replication-transcription conflicts due to collisions of the underlying molecular machineries can lead to DNA damage suggest that proper coordination of transcription and replication is important for maintaining genome stability [ 135 ][ 137 ][ 139 ][ 142 ][ 182 ][ 16 ][ 17 ][ 19 ]. Recent findings also point to a more direct role of transcription in influencing the location of origins, either by inhibiting Mcm2-7 loading or by repositioning of loaded Mcm2-7 on chromosomes [ 183 ][ 148 ]. Sequence-independent (but not necessarily random) initiator binding to DNA additionally allows for flexibility in specifying helicase loading sites and, together with transcriptional interference and the variability in activation efficiencies of licensed origins, likely determines origin location and contributes to the co-regulation of DNA replication and transcriptional programs during development and cell fate transitions. Computational modeling of initiation events in S . pombe , as well as the identification of cell-type specific and developmentally-regulated origins in metazoans, are in agreement with this notion [ 136 ][ 144 ][ 184 ][ 185 ][ 186 ][ 187 ][ 188 ][ 148 ]. However, a large degree of flexibility in origin choice also exists among different cells within a single population [ 139 ][ 145 ][ 185 ], and the molecular mechanisms that lead to the heterogeneity in origin usage remain ill-defined. Mapping origins in single cells in metazoan systems and correlating these initiation events with single-cell gene expression and chromatin status will be important to elucidate whether origin choice is purely stochastic or controlled in a defined manner.
Concluding remarks
Although DNA replication is essential for genetic inheritance, defined, site-specific replication origins are technically not a requirement for genome duplication as long as all chromosomes are copied in their entirety to maintain gene copy numbers. Certain bacteriophages and viruses, for example, can initiate DNA replication by homologous recombination independent of dedicated origins [ 189 ]. Likewise, the archaeon Haloferax volcanii uses recombination-dependent initiation to duplicate its genome when its endogenous origins are deleted [ 78 ]. Similar non-canonical initiation events through break-induced or transcription-initiated replication have been reported in E . coli and S . cerevisiae [ 190 ][ 191 ][ 192 ][ 193 ][ 194 ]. Nonetheless, despite the ability of cells to sustain viability under these exceptional circumstances, origin-dependent initiation is a common strategy universally adopted across different domains of life. The controlled assembly of the replication machinery at origins likely confers long-term advantage to cells by allowing tight cell cycle regulation and by maintaining a specific replication dynamics. The divergent origin specification modes between prokaryotes and budding yeast on the one hand and metazoans on the other hand appear to reflect distinct needs to coordinate the spatiotemporal replication program with gene expression and cell differentiation programs to ensure not only genetic but also epigenetic inheritance and to preserve cell identity. Deciphering the underlying molecular mechanisms that modulate origin location, usage, and timing to define the replication program in metazoan systems represents an important major challenge in the field and will be essential to understand how dysregulation of these events are linked to human diseases. In addition, detailed studies of replication initiation have focused on a limited number of model systems. The extensively studied fungi and metazoa are both members of the opisthokont supergroup and exemplify only a small fraction of the evolutionary landscape in the eukaryotic domain [ 195 ]. Comparably few efforts have been directed at other eukaryotic model systems, such as kinetoplastids or tetrahymena [ 196 ][ 197 ][ 198 ][ 199 ][ 200 ] [ 201 ][ 202 ]. Surprisingly, these studies have revealed interesting differences both in origin properties and in initiator composition compared to yeast and metazoans. Further exploration of replication initiation mechanisms across different branches of the eukaryotic domain will likely yield unexpected insight into the diversity and evolution of this fundamental biological process.
Supporting information
S1 text. version history of the text file..
https://doi.org/10.1371/journal.pgen.1008320.s001
S2 Text. Peer reviews and response to reviews.
https://doi.org/10.1371/journal.pgen.1008320.s002
- View Article
- Google Scholar
- PubMed/NCBI
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

DNA Replication: the recombination connection
The failure to complete DNA replication is one of the major sources of genome instability that leads to aneuploidy, chromosome breakage, and chromosome rearrangements associated with human cancer. One of the surprising revelations of the past decade is that the completion of replication at so-called common fragile sites occurs very late in the cell cycle – at mitosis – through a process termed MiDAS (mitotic DNA synthesis). MiDAS has proven to be strongly related to another cancer-promoting phenomenon: the activation of alternative lengthening of telomeres (ALT) mechanisms. Our understanding of the mechanisms of ALT and MiDAS in mammalian cells has drawn heavily from recent advances in the study of break-induced replication (BIR) especially in budding yeast. Here we provide new insights on BIR, MiDAS and ALT pathways and their shared similarities.
DNA repair requires new DNA synthesis and the completion of DNA replication requires recombination.
It has been clear for more than 40 years that homologous recombination requires at least some new DNA synthesis to patch up the initiating lesion[ 1 – 3 ]; but only recently has it become evident that the completion of DNA replication often requires homologous recombination. The accurate transmission of genetic information demands that the entire genome be copied; even a small unreplicated region poses a challenge at mitosis, as it results in a bridge linking the sister chromatids. Such bridges may prevent mitosis entirely so that a diploid cell becomes tetraploid. But often these bridges will either create a lagging chromosome pair that will fail to be properly incorporated into the daughter nuclei (creating aneuploidy) or will be ruptured, resulting in unstable, broken-ended DNA that leads to a cascade of events leading to genome rearrangements that are the hallmark of many human cancers [ 4 , 5 ].
Cells have a number of strategies to re-start or complete replication [ 6 – 11 ]. One option is to process stalled replication forks so that they can use homologous recombination to re-initiate replication. Replication fork reversal forms a four-stranded Holliday junction that can be cleaved by structure-specific endonucleases such as Mus81 [ 12 – 14 ] ( Figure 1 ). Cleavage generates an intact chromatid and a single-ended partially replicated chromatid, which can then engage in homologous recombination (HR) through a process known as break-induced replication (BIR) ( Figure 2 ). Recombination-dependent DNA synthesis also becomes essential for the maintenance of eroded chromosome ends (telomeres) when cells lack the RNA-dependent DNA polymerase (telomerase) that maintains the ends that otherwise are shortened with every replication cycle. But beyond alternative lengthening of telomeres (ALT) , new studies have revealed that break-induced replication plays a central role in the completion of normal DNA replication. Surprisingly, specific regions of chromosomes are often only replicated when cells have entered mitosis. This process of mitotic DNA synthesis (MiDAS) uses a replication strategy different from normal semi-conservative DNA replication, through BIR. While we have been learning a great deal about ALT and MiDAS, our understanding of the mechanism(s) of BIR itself has grown appreciably, principally by studies in budding yeast, but also from mammalian cells. This review examines what we now know about BIR and how it accounts for both ALT and MiDAS.

Mus81-mediated resolution of a stalled replication fork. A. Replication can be stalled by DNA secondary structures, by DNA cross-links, or by R-loops in which RNA is paired with one of the DNA strands (green). B. Replication fork regression allows access of repair enzymes, such as the RNA-DNA hybrid ribonuclease (triangle) to remove the impediment. (C.) D. The structure-specific endonuclease Mus81 (with Eme1) cleaves a Holliday junction that is created by replication fork regression. Cleavage results in a broken chromatid end (E, F) that can be resected (G) so that the 3’ single-stranded end can engage in recombination to re-start replication (H).

Break-induced replication. BIR begins by stand invasion (A) to create a D-loop that can migrate as new DNA synthesis (red) is initiated at the invading 3’ end (B). Unlike normal DNA replication, BIR creates a long single-stranded intermediate (C) that becomes copied to form double-stranded DNA by a delayed lagging-strand synthesis (E), so that all the newly copied DNA is conservatively inherited (F).
Break-Induced Replication: what we have learned from budding yeast.
Recombination-dependent DNA replication in eukaryotes, which is generally called break-induced replication, first became evident in budding yeast in the maintenance of telomeres in cells lacking telomerase [ 15 ]. This process has also been studied by creating site-specific DSBs leading to ectopic recombination and the formation of nonreciproical translocations [ 16 , 17 ]. There are at least two distinct BIR pathways, both employing the Rad52 protein that is required for essentially all homologous recombination in yeast [ 15 , 18 ]. At telomeres, Type I events are dependent on the Rad51 strand exchange protein and all of its associated mediators [ 19 ] ( Figure 3 ). A second, Rad51-independent process (Type II) requires a different set of initiation factors including the Me11-Rad50 complex, the Rdh54 paralog of Rad54, and Rad52’s “cousin” Rad59 [ 20 , 21 ]; none of these proteins can promote canonical in vitro strand exchange that should be needed to start recombination, so there is much yet to be learned about Rad51-independent repair. Type II telomere maintenance also depends on the BLM helicase homolog, Sgs1, and likely on its associated Rmi1 and Top3 partners, all of which play an important role in mammalian ALT [ 22 ]. The SUMOylation of various telomere-associated proteins by Slx5/Slx8 STUbL proteins and the association of telomeres with nuclear pores is also critical to promote telomere recombination [ 23 , 24 ]. As discussed below the clustering of telomeres in mammalian cells is key in some ALT pathways.

Telomerase-independent maintenance of budding yeast telomeres. All chromosome ends have an irregular TG 1–3 sequence (hatched lines), but some ends have one or more copies of Y’ subtelomeric DNA sequences (blue rectangles), flanked by telomere sequences. In Rad51-dependent Type I events, recombination occurs between internal TG 1–3 telomere sequences and subtelomeric Y’ elements that proliferate these sequences to all chromosome ends. In Rad51-independent Type II events, recombination and replication occurs between the TG 1–3 sequences themselves.
Telomerase-independent telomere maintenance in yeast: a model for ALT
Rad51-independent repair is particularly effective with very short regions of homology; in fact, deleting Rad51 significantly elevates repair by this alternative pathway [ 25 ]. In yeast cells deleted for the RNA or protein components of telomerase, the Rad51-dependent pathway results in repair events that proliferate ~ 6 kb Y’ subtelomeric elements, whereas the Rad51-independent route results in large expansions of yeast telomere sequences ( Figure 3 ). The ability of the Rad51-independent pathway to deal with very short regions of homologies apparently allows it to deal efficiently with yeast telomeres, which are irregular TG 1–3 repeats instead of the regular TTAGGG repeats of mammalian cells. Although the different genetic dependencies of these processes identify two distinct routes, recent work in yeast telomere maintenance has shown that they sometimes intermingle into one more complex process [ 26 ].
Rad51-dependent BIR
Here we discuss primarily Rad51-dependent BIR, about which much more is known. Initially, it was thought that BIR was essentially a recombination-based mechanism to re-establish a replication fork, thus restoring coordinate leading- and lagging-strand synthesis performed by DNA polymerases ε and δ, respectively, resulting in semi-conservative DNA synthesis. But in fact, BIR leads to conservative replication in which all the newly-copied DNA is inherited at the repaired end [ 27 , 28 ] ( Figure 2 ). Moreover, the two strands of DNA are not simultaneously copied; rather there is a long first strand synthesis that remains single-stranded for a considerable time until the second (lagging strand-like) stand is copied [ 27 ]. First-strand synthesis, which can extend for > 100 kb, depends predominantly on DNA polymerase δ, which normally performs very short Okazaki fragment synthesis [ 29 ]. The ability of Pol δ to processively copy such extensive regions depends on several factors that are not required for normal replication, nor for short-patch DNA synthesis during DSB repair by gene conversion: the nonessential Pol32 subunit of the DNA polymerase δ complex and the 5’ to 3’ helicase Pif1 [ 30 , 31 ]. These factors are also needed when Pol δ takes over leading strand synthesis in a catalytically-dead Pol ε mutant [ 32 ]. BIR synthesis differs from normal replication in several other respects. First, it is nearly 1000-fold more error-prone and largely independent of mismatch repair [ 33 ], possibly because the normal Msh/Mlh mismatch repair machinery fails to track properly behind the replication bubble and because in BIR, the heteroduplex DNA created by strand invasion is rapidly displaced after new DNA synthesis is initiated [ 34 , 35 ]. The persistence of the single-stranded first new strand of DNA also makes it a target for cytidine deamination by enzymes such as the APOBEC family [ 36 ] ( Figure 4 ). Second, BIR exhibits frequent template jumps to create intrachromosomal deletions, translocations and truncations [ 16 , 37 ]. Such jumps readily occur between dispersed homologous sequences such as single LTR regions of transposons, but also between interstitial telomere-like sequences and normal telomeres [ 29 , 38 ] ( Figure 4 ). The increased frequency of dissociation of the BIR replication structure may also account for its being impaired by collisions with oncoming transcription [ 29 ].

Mutations arising during BIR. A. Frequent clustered mutations in persistent ssDNA intermediates caused by cytidine deaminases such as APOBEC. B. Structural variants resulting from dissociation of the newly copied BIR intermediate and invasion at ectopic sequences. C. Arrest of BIR and creation of chromosome truncations at interstitial telomere-like sequences.
BIR is error-prone
There is a lot of interest about DSB repair “pathway choice,” between nonhomologous end-joining, microhomology-mediated end-joining and various forms of homologous recombination [ 39 – 41 ]. There is an additional “indecision” when DSB repair can proceed by gene conversion, when both ends of the DSB share homology with a donor sequence. The principal mitotic gene conversion mechanism, synthesis-dependent strand annealing (SDSA) , is completed by second-end capture of the newly copied strand; but if second-end capture fails, the repair process can convert the outcome to BIR [ 42 , 43 ]. These outcomes are strongly influenced by the length of possible second-end homology and the ability of the Rad52 and Rad59 proteins to facilitate single-strand annealing, but they also reflect a contest between the BLM helicase, Sgs1, which opposes second-end capture, apparently dismantling the annealed ends (thus promoting BIR) and the FANCM helicase, Mph1, which promotes SDSA [ 42 , 43 ]. These results suggest that the initial steps in SDSA and BIR are similar and apparently interconvertible.
Our understanding of BIR is still at an early stage and new mutations affecting this process are still being uncovered. The recent finding that BIR depends on the ubiquitin ligase, Rad6, responsible for the monoubiquitylation of histone H2B and the subsequent methylation of histone H3, points toward a role for chromatin modifications in facilitating BIR [ 44 ], but no global screen for BIR-specific mutations has yet been done.
BIR in mammalian cells
Studies of BIR in mammalian systems confirm the conservation of core recombination/replication functions, including the POLD3 (yeast Pol32) subunit of DNA polymerase δ and PIF1 helicase [ 45 – 47 ]. Moreover, DNA repair by BIR in human cells is also accompanied by > 100-fold increased rate of mutagenesis [ 48 ]. As with yeast, there are both Rad51-dependent and -independent routes; but unlike yeast – where all HR depends on Rad52 – there are also both Rad52-dependent and -independent pathways [ 49 ]. This difference can be understood by the evolution of Rad52’s functions. Whereas yeast uses Rad52 both as a mediator, to load the Rad51 protein onto ssDNA at resected DSB ends, and as the principal strand-annealing protein, mammalian Rad52 has yielded most of its Rad51 loading functions to BRCA2. In vitro, human Rad52 still facilitates Rad51 loading, and this function may prove to be critical in some BIR pathways. As in yeast, Rad52’s annealing function is critical for Rad51-independent single-strand annealing [ 50 ].
Alternative lengthening of telomeres in mammals occurs by at least two different BIR processes.
A fraction of tumors and many cell lines are immortal without re-activating telomerase; they maintain their telomere length through ALT. ALT appears to be most similar to yeast Type II survivors, in that recombination/replication occurs within telomere sequences themselves; but since mammalian telomeres are long stretches of identical repeating sequence, Rad51-dependent events similar to Type I in yeast should also be possible. Indeed, in mammals there are several different pathways of telomere maintenance whose relative importance depends in part on how telomere replication and integrity is challenged. The most commonly described pathway strongly resembles yeast Type II in its Rad51-independence and its requirements for RAD52 and POLD3 [ 51 , 52 ]; but cells lacking RAD52 are able to carry out ALT. As in Type II yeast cells, ALT in mammalian cells depends on the BLM-TOP3A-RMI1 helicase (yeast Sgs1-Top3-Rmi1) [ 53 ].
There are several additional ALT pathways that are less well characterized; whether they are all variants of BIR is not known. At least one RAD52-independent ALT pathway also engages POLD3 and POLD4 [ 54 ], perhaps by both Rad51-dependent routes [ 54 ]. One complication in understanding these pathways is that “ALT” can be induced in many different ways, which may obscure some important differences. First there is naturally occurring ALT in telomerase-negative cells; but ALT-like events can also be generated by inhibiting telomere replication or by depleting a number of factors needed for telomere integrity including the shelterin complex that normally protects telomeres from degradation and prevents activating the ATR and ATM damage-alarm pathways that sense a true broken chromosome end [ 7 , 55 , 56 ]. Depleting histone chaperone ASF1 also triggers ALT [ 57 ]. An alternative approach has been to target DNA damage to telomeres, for example by creating a conditionally-expressed TRF1-Fok1 fusion endonuclease that will introduce breaks in telomeres ( Figure 5B ). Apparently, most telomeres are damaged within a cell, but how many cleavage events there are per telomere, or how often both sister-telomeres are cleaved, has not been assessed. Fok1-cleaved telomeres engage in several pathways of repair [ 58 , 59 ].

Induction of ALT through artificial telomere clustering. A. Induction of ALT by artificial telomere clustering. Multimers of SUMO and SUMO interacting motifs (SIM) are fused to a nuclear localization signal (NLS) and the RAP1 C-terminus (RCT) that binds to the RAP1-binding domain of the shelterin protein, TRF2. Multiple copies of this SUMO-SIM construct facilitate the formation of ABP-like liquid phase-separated condensates that recruit telomeres in the absence of the PML protein. Without a nuclear localization signal, the mCherry-tagged fusion forms condensates in the cytoplasm. When transported to the nucleus and when containing a RAP1 C-terminal domain that can interact with TRF2, telomeres are recruited to an APB-like body. Adapted from Min et al. [ 71 ]. Image courtesy of Jaewon Min. B. Induction of telomeric break-induced replication by creating DSBs in telomere regions by conditional expression of a TRF1-Fok1 fusion protein. Fok1-mediated telomere cleavage results in telomere clustering and recombination-induced replication of telomere ends [ 58 , 59 ].
Additional features of ALT
Beyond the simple maintenance of telomere length in telomerase-deficient mammalian cells, ALT is characterized by a number of additional features. A common phenotype is the presence of so-called C-circles, which are extrachromosomal circles with an intact CCCTAA (C-rich) strand but only a partial TTAGGG (G-rich) strand [ 60 ]. These circles are derived from lagging-strand telomere synthesis (that is, where CCCTAA is being synthesized toward the centromere using a presumably uninterrupted TTAGGG strand as template)[ 61 ]. The mechanism by which this occurs remains unknown, but internal telomere loops (i-loops) could be the intermediates of these circles [ 62 ]. The formation of C-circles and other features of ALT are also induced by treatment of cells with agents such as zeocin or camptothecin, which cause DNA breaks as well as replication fork stalling [ 63 ]. Inhibition of replication fork regression ( Figure 1 ) by depleting RAD51, SMARCAL1 or SLX4, also increased C-circle abundance [ 63 ]. These results suggest that replication fork breakage, but not fork stalling per se, leads to a Rad51-dependent ALT process. Under some circumstances there are also telomeres with 5’-end extensions, which may be derived from defective leading strand telomere synthesis. Normally, broken ends are found with 3’-ended single-stranded regions arising from 5’ to 3’ exonuclease digestion, so there must be a way in which the 5’ end is protected. Such 3’, C-rich ends may also be generated by rapid telomere deletion (deletions of t-loops) by a cleavage process involving the XRCC3 endonuclease [ 64 ].
ALT is often associated with – and apparently occurs within - ALT-associated promyelocytic leukemia (PML) Bodies (APBs) [ 65 ]. PML bodies are huge (up to 1 μm in diameter) phase-separated condensates including the many proteins besides the PML protein for which it is named. Telomere elongation in APBs is strongly dependent on the BLM helicase and its associated TOP3A and RMI1 proteins [ 66 ]. The accumulation of BLM in APBs depends on POLD3, suggesting a reinforcing mechanism that drives BIR [ 67 ]. Moreover, recruitment of BLM to telomeres, using an inducible fusion protein containing RMI1 and a domain of the Teb1 telomere binding protein, eliminated the need for PML [ 66 ]. Another 3’ to 5’ helicase, FANCM, also plays a critical role, apparently restraining BIR [ 53 ]. FANCM both remodels and promotes cleavage of stalled replication forks [ 6 ]; but it also disrupts R-loops formed by the non-coding TERRA transcript at telomeres [ 68 , 69 ].
ALT is also frequently associated with the loss of the ATRX protein that is important in establishing sister telomere cohesion, but removing ATRX does not necessarily lead to ALT [ 70 ]. The loss of ATRX might enable sister telomere ends to align out of register and thus engage in recombination or replication events that would result in elongated telomeres.
ALT often is associated with microscopically visible alterations of telomere structure (fragile telomeres) containing apparent breaks or gaps in the structure. In some instances, there is also an elevated level of telomeric sister-chromatid exchange, again revealed by microscopy through the use of co-orientation fluorescence in situ hybridization (CO-FISH). The prominence of these features varies depending on the variety of ways that telomeric BIR has been induced and studied. Lagging-strand telomeres are prone to form these structures when BLM is depleted or when the shelterin subunit, TRF1, which recruits BLM, is impaired [ 58 ]. Chromosome breaks under these circumstances are generated through the action of the SLX4/SLX1 structure-specific endonuclease; such breaks are repaired by a POLD3/POLD4-dependent process, presumably BIR [ 58 ]. When telomere damage is inflicted by the tethering of the Fok1 endonuclease to telomeres ( Figure 5B ), or in some naturally occurring ALT cell lines, BIR itself appears to create fragile telomeres [ 58 ]; but whether these fragile sites are similar to common fragile sites in other chromosome locations isn’t yet clear.
New approaches to the study of ALT
Two major advances have been made in the study of ALT, by inducing this process even in cells with normal telomerase activity. First, APB-mediated ALT can be greatly enhanced by creating condensates of telomeres by artificial means [ 51 , 71 ]. It was known that APB formation depends on the SUMOylation of the shelterin telomere binding proteins, notably PIAS4-mediated SUMOylation of TRF2 [ 67 ]. Recently it has been possible to assemble APBs by creating artificial proteins consisting of multimers of 6–10 copies of the SUMO protein and of the SUMO-interacting domains (SIM) [ 71 ]. Expression of these SUMO-SIM multimers drives the formation of aggregates that recruit telomeres and enhance ALT ( Figure 5A ). In this context ALT requires the BLM helicase and Rad52 to initiate late DNA synthesis that resembles MiDAS [ 71 ]. In yeast, although telomeres are normally organized in small clusters at the nuclear periphery, disruption of SUMO or the SUMO-targeted ubiquitin ligase, Slx5-Slx8, impairs type II telomere repair that is analogized to be similar to that occurring within ABPs [ 23 ] [ 24 ]. In keeping with the idea that telomeres must coalesce into APBs for efficient ALT, is that telomere clustering is inhibited by their association with the nuclear envelope via the SUN1 protein [ 72 ]. Another key target of SUMOylation is the Rad51-associated protein, Rad51AP1, whose precise role(s) in homologous recombination remain poorly defined, but which is critical for Rad52-dependent ALT [ 73 ]. Rad54, another Rad51-interacting protein, has been implicated in resolving recombination intermediates in ALT; its depletion leads to anaphase bridges [ 74 ].
A second remarkable advance was the demonstration that ALT can been induced in non-ALT cells by infecting them with the KSHV herpes virus [ 75 ]. KSHV itself apparently replicates its ~ 150 kb linear genome using break-induced replication; but infection also converts previously non-ALT cell lines into ALT. Infection results in recruitment of the Mre11-Rad50 complex, SLX4, SLX4IP and several nuclear receptors to telomeres as well as in the appearance of APBs and C-circles – that is, all the hallmarks of ALT. KSHV-infected cells became dependent on BIR factors (BLM, SLX4, POLD3, and RAD52) for their proliferation. Moreover, KSHV-infected cells can replicate its genome in G2/M-arrested cells, reminiscent of MiDAS. However, this viral induction of an ALT-like mechanism is not associated with the loss of ATRX, DAXX or ASF1. It is possible that the function of one of these proteins is impaired.

Completion of DNA replication is a surprisingly late event in the cell cycle (MiDAS).
The timing of DNA replication in different regions of the genome is critical for maintaining chromosome architecture and the landscape of chromatin modifications[ 76 ]. Some regions are programmed to replicate early, some late; but sometimes replication is not merely tardy, but decidedly late – occurring in cells that have already entered mitosis [ 77 – 79 ]. The discovery of MiDAS was accompanied by several other surprises. First, MiDAS depends on RAD52 and on POLD3 but not BRCA2 or presumably RAD51 [ 13 , 78 , 80 ]. Moreover, Edu labeling of cells arrested in G2/M and then released into mitosis showed that MIDAS most often appeared on only one of a pair of sister chromatids [ 78 ]; that is, like BIR in yeast, DNA synthesis was conservative ( Figure 6 ).

New DNA synthesis in MiDAS. DNA synthesis occurring in the prophase of mitosis was marked by the incorporation of EdU (red) and scored on spreads of condensed metaphase chromosomes (stained with DAPI; grey). Note several cases where the EdU lies only on one of the sister chromatids, and sometimes co-localizes with a visible gap/break in condensed chromosome arm (depicting the site of a common fragile site). To induce DNA replication perturbation, cells were exposed to aphidicolin (0.4 μM) during S-phase and G2, which slows DNA replication but doesn’t block it. Image courtesy of Anna Bizard and Ian Hickson.
The ability to label newly synthesized DNA with EdU has made it possible to map the locations of MiDAS. Two recent papers have shown that MIDAS occurs at so-called common fragile sites (CFS) , that are characterized by encoding long, transcribed genes in regions depleted for origins of DNA replication [ 81 , 82 ]. MiDAS at specific fragile sites, including microsatellites or FRAX exhibit similar patterns of mitotic DNA synthesis. MiDAS is greatly enhanced in cells treated with the DNA polymerase inhibitor aphidicolin or otherwise subjected to replication stress. These studies also revealed that the domains over which MiDAS occurs embrace regions as long as 0.5 Mb, but the length of individual MiDAS events is not known. From the intensity of Edu labeling compared to normal replication, MiDAS tract lengths are estimated to be about 10 kb (I. Hickson, personal communication). Other assays suggesting that BIR tracts are much shorter (a few kb) differ by when in the cell cycle the repair was induced or by constraints of the genetic assays employed [ 46 , 83 ]. Edu labeling in mitotic cells is also prominent at telomeres, especially in cells using ALT.
It is still early days in working out how MiDAS is regulated. In general, mitotic cells have highly condensed chromatin in which 3-dimensional contacts forming intrachromosomal domains are reduced and transcription is shut off [ 84 ]; but common fragile site regions in which replication has stalled and MiDAS can be triggered appear to maintain more open, accessible chromatin structure [ 85 ]. These regions have compaction-resistant properties marked by faulty condensin loading that are evident throughout the cell cycle and are exacerbated by replication stress. The less condensed chromatin should provide access of stalled forks to the MiDAS machinery. A key to understanding this aspect of repair is the finding that the ubiquitin ligase TRAIP facilitates the disassembly of the replisome to provide access to DNA repair factors [ 86 ].
Several studies have implicated the SLX4 scaffold protein and three of the proteins with which it is associated as key factors in promoting MiDAS under different conditions. Both the SLX1 and MUS81 structure-specific endonucleases have been identified in several studies [ 13 , 78 , 87 ], along with the helicase RTEL1 that can dismantle difficult-to-replicate DNA secondary structures [ 88 ]. Mus81 has also been implicated in budding yeast in limiting the length of BIR tracts when a replication fork encounters an endonuclease-nicked chromosome [ 89 ].
MiDAS can occur in budding yeast
Although BIR is obviously important in the life of budding yeast, it wasn’t clear if there would be a need for MiDAS in completing replication of such a simple genome with so little repetitive DNA. But indeed, MiDAS has been revealed in 20–40% of unperturbed budding yeast cells, displaying very similar properties to that in mammalian cells [ 90 ]. Here, the infamous “awesome power of yeast genetics” has made it possible to explore some aspects of MiDAS that will be important to explore in mammalian cells. The use of auxin-inducible degrons allows rapid inactivation and re-activation of proteins required for mitotic exit; under these conditions the resolution of anaphase bridges proved to be independent of Rad51 but dependent on the POLD3 homolog, Pol32. One striking finding is that there is a very high level of mutation in late-replicating subtelomeric regions that is dependent on MiDAS, consistent with the high mutation rate associated with BIR; however, some of this elevated mutation may be the consequence of repair events that occur after breakage of anaphase bridges.
Concluding Remarks
We have learned much about BIR, ALT and MiDAS in the past decade but many questions remain (see Outstanding Questions ). In many respects BIR, ALT and MiDAS appear very similar, but already it is clear that each has its own special features. How many distinct pathways are there in mammalian cells and how similar are they to the two major mechanisms defined in budding yeast? But even in yeast there are still many mysteries associated with the Rad51-independent pathway, as it is not clear what role several of the proteins so-far identified actually play and specifically how the initial strand invasion is accomplished. To what degree are the recombination/replication events triggered by inflicting DSBs within telomere sequences similar to those events that arise from the processing of stalled replication forks, both naturally and when various essential components are depleted? What are the structures of fragile sites at common fragile sites in chromosomes and at telomeres?
Outstanding Questions
What are the structures of the stalled intermediates in DNA replication that are found at common fragile sites and how are they processed to initiate MiDAS?
Although most chromosome regions are highly condensed in mitosis, even precluding transcription, how is the chromatin of stalled replication forks in CFS maintained in an accessible conformation?
What DNA damage or replication checkpoint signals are required to initiate MiDAS?
Do MiDAS or ALT lead to template switches similar to what is seen in budding yeast?
What is the chromosomal structure of a cytologically visible fragile telomere?
Are Rad51-dependent ALT events analogous to Type I events in yeast and do they rely on long homologies provided by the regular TTAGGG repeats?
In cells employing ALT for telomere maintenance how efficient is a model system? fix
How much homology is required for BIR template jumps and how are such jumps effected? Does the partially replicated strand remain associated with the DNA polymerase? Is Rad51 required to initiate a new search for homology?
How are chromosome breaks created by a site-specific cleavage of telomeres (e.g. with TRF1-Fok1) different from eroded telomeres?
A strategy that has worked so successfully in budding yeast, studying in detail the repair of a site-specific DSBs [ 91 ], has only begun to be exploited in mammalian cells. Placing defined homologous sequences very close to specific telomeres and then creating synchronously induced DSBs should provide a way to monitor BIR events that are able to extend to the end of the chromosome in mammals. One frequent and naturally occurring chromosome rearrangement, the recombination between related D4Z4 repeats at the ends of human chromosomes 4 and 10, leading to the asymmetric transfer of the chromosome 10 repeats to the ends of chromosome 4, might be a model for BIR [ 92 ].
Break-induced replication (BIR) results in conservative DNA replication, where both strands of newly copied DNA are inherited together. Synthesis of the second strand is delayed, resulting in single-stranded intermediates subject to frequent mutation.
New approaches to study alternative lengthening of telomeres (ALT) provide insights into the mechanisms of HR-dependent telomere maintenance. Infection of mammalian cells by Kaposi Herpes Simplex Virus (KHSV) imposes BIR-like telomere maintenance even in telomerase-positive cells.
The function of ALT-associated PML-bodies in telomere maintenance can now be studied by artificially tethering chromosome ends in a phase-separated condensates lacking PML. These studies have emphasized the role of SUMOylation of telomere-associated proteins.
Completing DNA replication surprisingly takes place after S phase, when cells have entered mitosis. Selective labeling of mitotic DNA synthesis (MiDAS) reveals that MiDAS occurs principally at cytologically-identified common fragile sites.
Acknowledgements
We are grateful for the comments of Ian Hickson, Zhe Yang, Anna Malkova, Liping Liu, and Xiaohua Wu. Research in the Haber lab has been supported by NIH grants GM20056, GM61722 and R35 GM127029.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests.
The authors declare no competing interests.
- Search Menu
- Advance Articles
- Collections
- Focus Collections
- Browse by cover
- High-Impact Research
- Author Guidelines
- Quick and Simple Author Support
- Focus Issues Call for Papers
- Submission Site
- Open Access Options
- Self-Archiving Policy
- Why Publish with Us?
- About Plant Physiology
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Results and discussion.
- MATERIALS AND METHODS
- ACKNOWLEDGMENTS
- LITERATURE CITED
- < Previous
Genome-Wide Analysis of the Core DNA Replication Machinery in the Higher Plants Arabidopsis and Rice
This work was supported by the National Science Foundation Plant Genome Research Initiative (grant no. 0421651) and an Integrative Graduate Education and Research Traineeship from the National Science Foundation (to R.W.S.).
Present address: Department of Molecular and Structural Biochemistry, North Carolina State University, Raleigh, NC 27695.
Corresponding author; e-mail [email protected] ; fax 919–513–1209.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors ( www.plantphysiol.org ) is: Linda Hanley-Bowdoin ( [email protected] ).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
www.plantphysiol.org/cgi/doi/10.1104/pp.107.101105
- Article contents
- Figures & tables
- Supplementary Data
Randall W. Shultz, Vinaya M. Tatineni, Linda Hanley-Bowdoin, William F. Thompson, Genome-Wide Analysis of the Core DNA Replication Machinery in the Higher Plants Arabidopsis and Rice, Plant Physiology , Volume 144, Issue 4, August 2007, Pages 1697–1714, https://doi.org/10.1104/pp.107.101105
- Permissions Icon Permissions
Core DNA replication proteins mediate the initiation, elongation, and Okazaki fragment maturation functions of DNA replication. Although this process is generally conserved in eukaryotes, important differences in the molecular architecture of the DNA replication machine and the function of individual subunits have been reported in various model systems. We have combined genome-wide bioinformatic analyses of Arabidopsis ( Arabidopsis thaliana ) and rice ( Oryza sativa ) with published experimental data to provide a comprehensive view of the core DNA replication machinery in plants. Many components identified in this analysis have not been studied previously in plant systems, including the GINS (go ichi ni san) complex (PSF1, PSF2, PSF3, and SLD5), MCM8, MCM9, MCM10, NOC3, POLA2, POLA3, POLA4, POLD3, POLD4, and RNASEH2. Our results indicate that the core DNA replication machinery from plants is more similar to vertebrates than single-celled yeasts ( Saccharomyces cerevisiae ), suggesting that animal models may be more relevant to plant systems. However, we also uncovered some important differences between plants and vertebrate machinery. For example, we did not identify geminin or RNASEH1 genes in plants. Our analyses also indicate that plants may be unique among eukaryotes in that they have multiple copies of numerous core DNA replication genes. This finding raises the question of whether specialized functions have evolved in some cases. This analysis establishes that the core DNA replication machinery is highly conserved across plant species and displays many features in common with other eukaryotes and some characteristics that are unique to plants.
DNA replication depends on the coordinated action of numerous multiprotein complexes. At the simplest level, it requires an initiator to establish the site of replication initiation, a helicase to unwind DNA, a polymerase to synthesize new DNA, and machinery to process the Okazaki fragments generated during discontinuous synthesis. Much is known about the DNA replication machinery in yeast ( Saccharomyces cerevisiae ) and animal model systems, but relatively little is known about the apparatus in plants. To gain insight into plant DNA replication components, we have combined published experimental information with our own bioinformatic analysis of genomic sequence data to examine the core DNA replication machinery in the model plants Arabidopsis ( Arabidopsis thaliana ) and rice ( Oryza sativa ).

Model depicting the core eukaryotic DNA replication machinery from initiation through Okazaki fragment maturation. A, Components of the preinitiation complex. DNA bound ORC recruits NOC3, CDC6, and CDT1 in early G1. Reiterative loading of 10 to 40 MCM complexes forms a licensed origin. After MCM loading is complete, CDC6 and CDT1 dissociate from the origin. B, At the G1/S transition a subset of licensed origins transition to an initiation complex. The precise order of events is not clear and may vary between systems. CDC45, TOPBP1, and MCM8-10 contribute to GINS complex loading, DNA unwinding, and recruitment of the polymerases. C, Components of the active DNA replication fork. MCM2-7, CDC45, and GINS unwind the duplex DNA. Leading strand synthesis is accomplished primarily by POLE. GINS increases the processivity of POLE. On the lagging strand, RPA stabilizes ssDNA, POLA lays down a short RNA/DNA primer and then is replaced by POLD, which completes the Okazaki fragment. RFC loads PCNA, which increases the processivity of POLD. The precise role of MCM8-10 in this process is not clear. D, The dominant mechanism of Okazaki fragment maturation requires FEN1 to cleave the RNA/DNA flap, resulting in a nick that is sealed by LIG1.
In recent years, there has been increased interest in plant DNA replication and in using plants as models for understanding DNA replication in eukaryotes. A detailed understanding of the core DNA replication machinery in plants will provide researchers with an important tool for understanding what makes plants unique with respect to replicative and developmental capacity and for investigating how plant strategies compare to the mechanisms employed by animals.
Core DNA replication genes in Arabidopsis and rice
At, Hs, Os, and Sc are Arabidopsis, human, rice, and yeast, respectively.
References: 1, Kimura et al. (2000a) ; 2, Witmer et al. (2003) ; 3, Collinge et al. (2004) ; 4, Masuda et al. (2004) ; 5, Diaz-Trivino et al. (2005) ; 6, Mori et al. (2005) ; 7, Gavin et al. (1995) ; 8, Li et al. (2005) ; 9, Sabelli et al. (1996) ; 10, Sabelli et al. (1999) ; 11, Stevens et al. (2002) ; 12, Dambrauskas et al. (2003) ; 13, Taliercio et al. (2005) ; 14, Dresselhaus et al. (2006) ; 15, Springer et al. (1995) ; 16, Springer et al. (2000) ; 17, Bastida and Puigdomenech (2002) ; 18, Holding and Springer (2002) ; 19, Castellano et al. (2001) ; 20, de Jager et al. (2001) ; 21, Ramos et al. (2001); 22, Castellano et al. (2004) ; 23, Raynaud et al. (2005) ; 24, Stevens et al. (2004) ; 25, Yokoi et al. (1997) ; 26, Burgers et al. (2001) ; 27, Uchiyama et al. (2002) ; 28, Garcia et al. (2006) ; 29, Kimura et al. (2001) ; 30, Egelkrout et al. (2002) ; 31, Kosugi and Ohashi (2002) ; 32, Toueille et al. (2002) ; 33, Castillo et al. (2003) ; 34, Raynaud et al. (2006) ; 35, Furukawa et al. (2003) ; 36, Furukawa et al. (2001) ; 37, Jenik et al. (2005) ; 38, Ronceret et al. (2005) ; 39, He and Mascarenhas (1998) ; 40, Yang and Sheila (2002) ; 41, Grelon et al. (2003) ; 42, Ishibashi et al. (2001) ; 43, Marwedel et al. (2003) ; 44, Ishibashi et al. (2005) ; 45, Ishibashi et al. (2006) ; 46, Xia et al. (2006) ; 47, Kimura et al. (2003) ; 48, Kimura et al. (2000b) ; 49, Taylor et al. (1998) ; 50, Sunderland et al. (2004) ; 51, Bonatto et al. (2005) ; 52, Sunderland et al. (2006) .
New gene models were predicted for these loci. NA, Gene was not identified; ns, no significant similarity was found.
We encountered numerous instances where the existing gene model resulted in a protein that either lacked highly conserved sequences or contained additional residues compared to other eukaryotic proteins. When available, plant-derived transcripts from GenBank and the TIGR Plant Transcript Assembly databases (TIGR-TA; Childs et al., 2007 ) were used to guide the prediction of a new gene model. In cases where full-length transcripts could not be identified, we used protein sequence alignments to create gene models that maximized sequence conservation with other eukaryotes ( Table I ). The predicted coding and resulting protein sequences are provided in Supplemental Text S1 .
Preinitiation
One of the first steps toward establishing a functional DNA replication fork is binding of the eukaryotic initiator complex termed the origin recognition complex (ORC) to DNA in late M and early G1 phases of the cell division cycle ( Dutta and Bell, 1997 ; Bryant et al., 2001 ; Bell, 2002 ; Bell and Dutta, 2002 ; DePamphilis, 2003 ). Next, CDC6 interacts with the origin-bound ORC, which together recruit a CDT1/MCM2-7 complex of proteins. Hydrolysis of ATP by ORC/CDC6 causes the release of CDT1 and structural alteration of the ring-shaped MCM complex, leading to its loading around DNA ( Randell et al., 2006 ; Ranjan and Gossen, 2006 ; Waga and Zembutsu, 2006 ). MCM loading is reiterated through the action of a single ORC/CDC6 complex resulting in the recruitment of 10 to 40 MCM complexes at each potential origin ( Blow and Dutta, 2005 ). The resulting protein/DNA assembly consisting of ORC1-6/CDC6/MCM2-7 is termed the prereplication complex (pre-RC), and sites containing this complex are considered licensed with the potential to serve as origins of replication ( Fig. 1A ).
All six ORC genes have been identified in Arabidopsis ( Gavin et al., 1995 ; Collinge et al., 2004 ; Masuda et al., 2004 ), and genes encoding ORC1 to 5 have been reported for rice ( Kimura et al., 2000a ; Li et al., 2005 ; Mori et al., 2005 ) and maize ( Zea mays ; Witmer et al., 2003 ). The Arabidopsis ORC proteins show 22% to 37% amino acid identity with human ORC subunits ( Table I ). Consistent with a role in DNA replication, Arabidopsis ORC transcripts have been shown to be abundant in proliferating tissues such as root tips, young leaves, and flower buds, and their expression induced upon cell cycle reentry following Suc starvation of cultured suspension cells ( Masuda et al., 2004 ; Diaz-Trivino et al., 2005 ). Interestingly, ZmORC3 ( Witmer et al., 2003 ) and AtORC5 - 6 ( Diaz-Trivino et al., 2005 ) transcripts are also abundant in postmitotic tissues, suggesting that plant ORC subunits may have additional functions in mature tissues.

Multiple sequence alignments of plant ORC6 and GINS complex proteins. A, ORC6. B, PSF1. C, PSF2. D, PSF3. E, SLD5. For all sections, protein sequences from the indicated plant species were aligned using the Clustal W algorithm. Black shading indicates identical residues in all aligned sequences. Gray shading denotes residues with similar chemical properties that are conserved in >50% of sequences aligned. Similar chemical properties of amino acid residues were defined as follows: DE, Acidic; AGILV, aliphatic; NQ, amide; FWY, aromatic; RHK, basic; ST, hydroxyl; CM, sulphur. Plant sequences were also aligned with proteins from other eukaryotes. Ascomycota includes sequences from yeast and Schizosaccharomyces pombe . Vertebrata includes Homo sapeins , Danio rerio , X. laevis , and Gallus gallus . An x indicates residues that are identical in all sequences from plants and the specified group. An o denotes residues with similar chemical properties that are conserved in >50% of plant sequences and 100% of sequences from the specified group. The plant species are designated as Ac, Ananas comosus ; Af, Aquilegia formosa × Aquilegia pubescens ; At, Arabidopsis; Bn, Brassica napus ; Br, B. rapa ; Cs, Citrus sinensis ; Ee, Euphorbia esula ; Ga, Gossypium arboreum ; Gm, G. max ; Gr, Gossypium raimondii ; Ha, Helianthus annus ; Ht, Helianthus petiolaris ; Hv, Hordeum vulgare ; In, Ipomoea nil ; Lc, Lotus corniculatus ; Ls, Lactuca serriola ; Lv, Lactuca virosa ; Mt, Medicago truncatula ; Nt, N. tabacum ; Os, O. sativa ; Pg, Picea glauca ; Ps, Picea sitchensis ; Pt, Populus trichocarpa ; Pta, Pinus taeda ; Sa, Senecio aethnensis ; Sb, Sorghum bicolor ; Sch, Solanum chacoense ; So, Saccharum officinarum ; St, Solanum tuberosum ; Ta, Triticum aestivum ; Zm, Z mays .
It has been reported that Arabidopsis has two CDC6 ( Ramos et al., 2001 ) and two CDT1 ( Castellano et al., 2004 ) genes. We identified one candidate CDC6 and two candidate CDT1 homologs in rice ( Table I ). OsCDC6 shares 51% and 59% amino acid identity with AtCDC6A (At2g29680) and AtCDC6B (At1g07270), respectively. The CDT1 proteins are more divergent. AtCDT1A (At2g31270) and AtCDT1B (At3g54710) are 37% identical while the two rice CDT1 proteins are 30% identical. Between Arabidopsis and rice, the amino acid identity ranges from 28% to 37% ( Table I ). Given that CDC6 and CDT1 have similar functions in all eukaryotes studied to date, it is likely that these proteins also act similarly in plants. However, the divergence between copies raises the possibility of additional activities.
The six-subunit MCM complex (MCM2-7) represents the putative eukaryotic replicative helicase ( Forsburg, 2004 ; Masai et al., 2005 ; Maiorano et al., 2006 ), and genes encoding one copy of each subunit have been identified in Arabidopsis ( Springer et al., 1995 ; Stevens et al., 2002 ; Masuda et al., 2004 ; Dresselhaus et al., 2006 ). MCM3 ( Sabelli et al., 1996 ; Sabelli et al., 1999 ) and MCM6 ( Dresselhaus et al., 2006 ) proteins have been identified in maize, and MCM3 has been reported in tobacco ( Nicotiana tabacum ; Dambrauskas et al., 2003 ). We identified strong candidates for each of the MCM2-7 proteins in rice ( Table I ). Importantly, these proteins contain the sequence features that define the MCM family, including Walker A and Walker B domains, a zinc finger region, and an Arg finger motif ( Forsburg, 2004 ; Maiorano et al., 2006 ).
Nucleolar complex-associated (NOC) proteins are conserved in eukaryotes and are involved in ribosome biogenesis ( Milkereit et al., 2001 ) and cell differentiation ( Tominaga et al., 2004 ). One member of this complex, NOC3, has been shown to interact with ORC and MCM proteins and is required for pre-RC formation in budding yeast ( Zhang et al., 2002 ). Our analysis indicates that Arabidopsis and rice both code for a NOC3 protein ( Table I ). The TAIR gene model for AtNOC3 (At1g79150.1) produces a protein of 496 amino acids that is missing conserved sequences in the C-terminal region. This model is based on a cDNA sequence in GenBank (accession NM_106566 ), but there is a second cDNA sequence in GenBank (accession AAC17047 ) with a different intron-exon structure that contains the conserved C-terminal portion of NOC3. It would be interesting to investigate whether these two AtNOC3 transcripts represent alternative splicing events or are simply artifacts. We assembled the available transcripts to predict a putative full-length AtNOC3 transcript ( Supplemental Text S1 ). Our results support the conclusion that a complete pre-RC is conserved in plants.
- Reference Manager
- Simple TEXT file
People also looked at
Editorial article, editorial: the dna replication machinery as therapeutic targets.

- 1 New England Biolabs, Inc., Ipswich, MA, United States
- 2 Biomolecular Labeling Laboratory, Institute for Bioscience and Biotechnology Research, Rockville, MD, United States
- 3 National Institute of Standards and Technology, Rockville, MD, United States
Editorial on the Research Topic The DNA Replication Machinery as Therapeutic Targets
Chromosomal DNA replication is a process conserved through all domains of life. For accurate and efficient duplication of the genetic information, DNA replication components must work together in a highly regulated and coordinated fashion. Due to a central role in cell proliferation, the DNA replication machinery is an attractive therapeutic target for treating bacterial and viral infections, autoimmune disorders, and cancer. This eBook, entitled “The DNA Replication Machinery as Therapeutic Targets” explores how DNA replication factors serve as targets for new generations of therapies.
In all organisms, the chromosomal replication process starts at a specific chromosomal region called an origin of replication, at which origin-binding proteins (OBP) bind and locally unwind the duplex DNA. Additional proteins interact with the OBP-DNA complex and are responsible for the assembly of the DNA helicase around the DNA. Once assembled, the helicase unwinds the duplex in an ATP-dependent manner and forms the initial replication bubble. The exposed short regions of single-stranded DNA (ssDNA) at the replication bubble are coated with ssDNA-binding protein (SSB). DNA primase, DNA polymerases, and the rest of the replication machinery are recruited to the SSB-ssDNA complex to initiate bidirectional DNA synthesis. Due to the antiparallel nature of duplex DNA, and the unidirectionality of DNA polymerases, one strand of the chromosome is synthesized continuously (leading strand) while the other is copied discontinuously (lagging strand) as a series of Okazaki fragments ( O'Donnell et al., 2013 ; Kelman and Kelman, 2014 ; Kunkel and Burgers, 2017 ). Although these processes are fundamentally conserved in the three domains: archaea, bacteria and eukarya; as well as viruses and bacteriophages, the proteins, and complexes involved differ ( Makarova and Koonin, 2013 ).
Because the replication machinery is composed of a variety of core proteins and regulatory factors, disruption of any of the proteins involved will inhibit the replication process and/or its efficiency, and lead to replication stress. Replication stress is induced by endogenous factors such as dNTP depletion, DNA secondary structures or crosslinks, or by exogenous chemotherapies that damage DNA, such as cisplatin ( Kitao et al., 2018 ). In human cells, replication stress occurs when DNA polymerases uncouples from the replisome and lags behind helicase unwinding ( Zeman and Cimprich, 2014 ). As a result, long stretches of ssDNA are exposed at the replication fork. Replication protein A (RPA, the eukaryotic SSB) binds to the extended stretches of ssDNA, depleting free RPA in the cell and causing replication fork collapse (which may lead to DNA breakage and cell death). Therefore, inducing replication stress by disrupting the replication machinery or its regulation are an attractive strategies for drug design ( O'Connor, 2015 ; Forment and O'Connor, 2018 ).
Each chapter in this eBook highlights a different therapeutic strategy to effectively target DNA replication. One approach to halt replication is to inhibit DNA polymerases via binding of small molecules to the active site. The three replicative DNA polymerases responsible for the duplication of chromosomal DNA in eukarya, DNA polymerases α, δ and ε (Pol α, Pol δ, and Pol ε), all belong to family B DNA polymerases. In order to develop nucleotide analogs as drugs, one needs to understand how DNA polymerases incorporate natural and modified nucleotides into DNA. The contribution by Daimon et al. adds to our understanding of the structure and function relationships of family B polymerases by solving the structure of two members of this family from Aeropyrum pernix ( Daimon et al. ). The incorporation of various classes of modified nucleotides and nucleotide terminators by another family B DNA polymerase from archaea is described by a review contributed by Gardner et al . These archaeal polymerases share sequence similarity with the eukaryotic enzymes and thus can serve as good model systems for the more complex eukaryotic replication ( Makarova et al., 2014 ).
In addition, the use of nucleotide analogs to treat human autoimmune disorders and cancer is summarized in a contribution by Berdis . Young explores mitochondrial replication and how incorporation of certain nucleoside chain terminator inhibitors by Pol γ (the mitochondrial-specific polymerase) can lead to unintended toxicity by shutting down mitochondrial genome replication ( Young ). In addition, certain classes of compounds target Pol γ in cancer cells to inhibit mitochondrial replication with the potential to induce tumor cell death ( Young ).
Despite a central role in copying the chromosome, the inherent processivity of DNA polymerases is low, and only a few nucleotides are incorporated at a time. However, in the replication complex, high processivity DNA synthesis is conferred by a ring-shaped protein, referred to as the DNA polymerase sliding clamp, that encircles DNA and tethers the polymerase catalytic unit to the DNA for processive DNA synthesis ( Indiani and O'Donnell, 2006 ). The sliding clamps cannot assemble themselves around the DNA and require an additional clamp loader complex that assembles the clamp around duplex DNA in an ATP-dependent manner. In addition to interacting with the polymerase, the sliding clamps of bacteria and eukarya also interact with dozens of other proteins involved in DNA replication, repair, and cell cycle progression ( Vivona and Kelman, 2003 ). Therefore, inhibitors of the bacterial and eukaryal sliding clamps are being developed as anti-cancer and anti-bacterial drugs ( Georgescu et al., 2008 ). The current knowledge on the development of sliding clamps inhibitors and their possible use as therapeutic agents is summarized in a review contribution by Altieri and Kelman .
Another key enzyme for cellular replication is the DNA helicase, the enzyme responsible for unwinding double-stranded DNA ahead of the replisome ( Sakakibara et al., 2009 ). The contribution by Datta and Brosh describes the current state of the art in designing helicase inhibitors as anti-cancer drugs, and the issues surrounding the use of helicase inhibitors ( Datta and Brosh ). In eukarya, the replicative helicase is a complex of three components, the heterohexameric minichromosome maintenance (MCM), the tetrameric GINS complex and the Cdc45 protein. These form the CMG (Cdc45, MCM, GINS) complex ( Onesti and MacNeill, 2013 ; O'Donnell and Li, 2018 ). Due to the essential role of CMG in chromosome replication, it is a prime target for anti-cancer drugs. The current efforts in the development of CMG inhibitors as anti-cancer drugs are summarized in a contribution by Seo and Kang . Instead of directly inhibiting an enzyme activity (such as DNA polymerase), another strategy is to deplete activity by downregulating gene expression or deregulating protein activity via the ubiquitination pathway ( Jang et al .).
In addition, while some drugs are effective on their own, in other cases multiple replication factors can be targeted simultaneously to disrupt multiple pathways and lead to more efficient and effective treatment strategies. Mycobacterium tuberculosis is a pathogenic bacterium that is the etiological agent of tuberculosis (TB), which kills more than a million people a year ( Bañuls et al., 2015 ). Reiche and coauthors summarize the current state of the development of drugs against the M. tuberculosis replication machinery, including drugs targeting the polymerase (Pol III), the sliding clamp, clamp loader, and other replication proteins ( Reiche et al. ). In addition, Reiche et al. demonstrate the increased effectiveness of a combination M. tuberculosis antibiotic strategy that depletes dNTP pools while inhibiting DNA polymerase activity ( Reiche et al. ). Another example of a combination strategy is the inhibition of DNA polymerase synthesis with nucleotide inhibitors in combination with DNA damaging agents to create DNA lesion that stall synthesis ( Berdis ).
Remaining Challenges and Future Opportunities
Despite effective inhibitors of DNA replication proteins, eventual resistance to these inhibitors leads to tumor recurrence and remains a challenge for long-term therapeutic efficacy. Therefore, it will be important to continue to study molecular mechanisms of tumor resistance to DNA replication inhibitors. For example, DNA polymerase mutants that effectively remove nucleotide chain terminators can lead to drug resistance, as can upregulation of lesion bypass DNA polymerases ( Berdis ).
We anticipate that knowledge of DNA replication protein expression, regulation, and biochemical properties will continue to address these challenges and accelerate the development of novel strategies for effective treatment. To reach potential as therapeutic targets, more high-resolution structural information is needed for all replisome proteins and complexes to understand important replisome active site architectures and protein interactions. High resolution replisome structures will enable models for docking small molecules to inhibit enzyme activities and disrupt essential replisome interactions.
Finally, new molecular tools will accelerate identification of new DNA replication drugs targets. CRISPR-Cas9 genome engineering tools have revolutionized many scientific disciplines and offer a powerful method to alter genes by either modifying gene sequence or introducing insertions or deletions to knock out gene function ( Doudna and Charpentier, 2014 ). Genome-wide CRISPR-Cas9 screens aim to disrupt all or a subset of genes in an organism to identify important genes in a pathway ( Sánchez-Rivera and Jacks, 2015 ; Peters et al., 2016 ). CRISPR-Cas9 genome-wide screens can be adapted to identify novel factors that confer either resistance or sensitivity to DNA replication inhibitors. Knowledge of these factors may inform future therapeutic strategies to design new drug classes or enhance the efficacy of current therapies.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
AG is employed and funded by New England Biolabs, Inc., a manufacturer and vendor of molecular biology reagents, including DNA replication and repair enzymes. This affiliation does not affect the author's impartiality, objectivity of data generation or its interpretation, adherence to journal standards and policies or availability of data.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Bañuls, A. L., Sanou, A., Anh, N. T., and Godreuil, S. (2015). Mycobacterium tuberculosis: ecology and evolution of a human bacterium. J. Med. Microbiol. 64, 1261–1269. doi: 10.1099/jmm.0.000171
PubMed Abstract | CrossRef Full Text | Google Scholar
Doudna, J. A., and Charpentier, E. (2014). Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346:1258096. doi: 10.1126/science.1258096
Forment, J. V., and O'Connor, M. J. (2018). Targeting the replication stress response in cancer. Pharmacol. Ther. 188, 155–167. doi: 10.1016/j.pharmthera.2018.03.005
Georgescu, R. E., Yurieva, O., Kim, S. S., Kuriyan, J., Kong, X. P., and O'Donnell, M. (2008). Structure of a small-molecule inhibitor of a DNA polymerase sliding clamp. Proc. Natl. Acad. Sci. U.S.A. 105, 11116–11121. doi: 10.1073/pnas.0804754105
Indiani, C., and O'Donnell, M. (2006). The replication clamp-loading machine at work in the three domains of life. Nat. Rev. Mol. Cell Biol. 7, 751–761. doi: 10.1038/nrm2022
Kelman, L. M., and Kelman, Z. (2014). Archaeal DNA replication. Annu. Rev. Genet. 48, 71–97. doi: 10.1146/annurev-genet-120213-092148
Kitao, H., Iimori, M., Kataoka, Y., Wakasa, T., Tokunaga, E., Saeki, H., et al. (2018). DNA replication stress and cancer chemotherapy. Cancer Sci. 109, 264–271. doi: 10.1111/cas.13455
Kunkel, T. A., and Burgers, P. M. J. (2017). Arranging eukaryotic nuclear DNA polymerases for replication: specific interactions with accessory proteins arrange Pols alpha, delta, and in the replisome for leading-strand and lagging-strand DNA replication. Bioessays 39:1700070. doi: 10.1002/bies.201700070
Makarova, K. S., and Koonin, E. V. (2013). Archaeology of eukaryotic DNA replication. Cold Spring Harb. Perspect. Biol. 5:a012963. doi: 10.1101/cshperspect.a012963
Makarova, K. S., Krupovic, M., and Koonin, E. V. (2014). Evolution of replicative DNA polymerases in archaea and their contributions to the eukaryotic replication machinery. Front. Microbiol. 5:354. doi: 10.3389/fmicb.2014.00354
O'Connor, M. J. (2015). Targeting the DNA damage response in cancer. Mol. Cell 60, 547–560. doi: 10.1016/j.molcel.2015.10.040
O'Donnell, M., Langston, L., and Stillman, B. (2013). Principles and concepts of DNA replication in bacteria, archaea, and eukarya. Cold Spring Harb. Perspect. Biol. 5:a010108. doi: 10.1101/cshperspect.a010108
O'Donnell, M. E., and Li, H. (2018). The ring-shaped hexameric helicases that function at DNA replication forks. Nat. Struct. Mol. Biol. 25, 122–130. doi: 10.1038/s41594-018-0024-x
Onesti, S., and MacNeill, S. A. (2013). Structure and evolutionary origins of the CMG complex. Chromosoma 122, 47–53. doi: 10.1007/s00412-013-0397-x
Peters, J. M., Colavin, A., Shi, H., Czarny, T. L., Larson, M. H., Wong, S., et al. (2016). A comprehensive, CRISPR-based functional analysis of essential genes in bacteria. Cell 165, 1493–1506. doi: 10.1016/j.cell.2016.05.003
Sakakibara, N., Kelman, L. M., and Kelman, Z. (2009). Unwinding the structure and function of the archaeal MCM helicase. Mol. Microbiol. 72, 286–296. doi: 10.1111/j.1365-2958.2009.06663.x
Sánchez-Rivera, F. J., and Jacks, T. (2015). Applications of the CRISPR-Cas9 system in cancer biology. Nat. Rev. Cancer 15, 387–395. doi: 10.1038/nrc3950
Vivona, J. B., and Kelman, Z. (2003). The diverse spectrum of sliding clamp interacting proteins. FEBS Lett. 546, 167–172. doi: 10.1016/S0014-5793(03)00622-7
Zeman, M. K., and Cimprich, K. A. (2014). Causes and consequences of replication stress. Nat. Cell Biol. 16, 2–9. doi: 10.1038/ncb2897
Keywords: DNA replication, replication stress, DNA polymerase, helicase, therapeutic targets
Citation: Gardner AF and Kelman Z (2019) Editorial: The DNA Replication Machinery as Therapeutic Targets. Front. Mol. Biosci. 6:35. doi: 10.3389/fmolb.2019.00035
Received: 27 March 2019; Accepted: 02 May 2019; Published: 21 May 2019.
Reviewed by:
Copyright © 2019 Gardner and Kelman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew F. Gardner, gardner@neb.com
This article is part of the Research Topic
The DNA Replication Machinery as Therapeutic Targets
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 13 February 2023
Revolutionizing DNA repair research and cancer therapy with CRISPR–Cas screens
- Samah W. Awwad 1 , 2 na1 ,
- Almudena Serrano-Benitez 1 , 2 na1 ,
- John C. Thomas 1 , 2 ,
- Vipul Gupta 2 &
- Stephen P. Jackson ORCID: orcid.org/0000-0001-9317-7937 1 , 2
Nature Reviews Molecular Cell Biology volume 24 , pages 477–494 ( 2023 ) Cite this article
12k Accesses
19 Citations
84 Altmetric
Metrics details
- DNA damage and repair
- High-throughput screening
All organisms possess molecular mechanisms that govern DNA repair and associated DNA damage response (DDR) processes. Owing to their relevance to human disease, most notably cancer, these mechanisms have been studied extensively, yet new DNA repair and/or DDR factors and functional interactions between them are still being uncovered. The emergence of CRISPR technologies and CRISPR-based genetic screens has enabled genome-scale analyses of gene–gene and gene–drug interactions, thereby providing new insights into cellular processes in distinct DDR-deficiency genetic backgrounds and conditions. In this Review, we discuss the mechanistic basis of CRISPR–Cas genetic screening approaches and describe how they have contributed to our understanding of DNA repair and DDR pathways. We discuss how DNA repair pathways are regulated, and identify and characterize crosstalk between them. We also highlight the impacts of CRISPR-based studies in identifying novel strategies for cancer therapy, and in understanding, overcoming and even exploiting cancer-drug resistance, for example in the contexts of PARP inhibition, homologous recombination deficiencies and/or replication stress. Lastly, we present the DDR CRISPR screen (DDRcs) portal , in which we have collected and reanalysed data from CRISPR screen studies and provide a tool for systematically exploring them.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
176,64 € per year
only 14,72 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
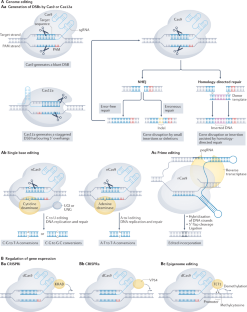
Similar content being viewed by others

A systematic CRISPR screen defines mutational mechanisms underpinning signatures caused by replication errors and endogenous DNA damage
Synthetic lethality prediction in DNA damage repair, chromatin remodeling and the cell cycle using multi-omics data from cell lines and patients.
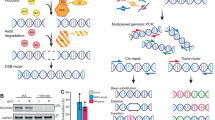
iMUT-seq: high-resolution DSB-induced mutation profiling reveals prevalent homologous-recombination dependent mutagenesis
Friedberg, E. C. A brief history of the DNA repair field. Cell Res. 18 , 3–7 (2008).
Article CAS PubMed Google Scholar
Lindahl, T. Instability and decay of the primary structure of DNA. Nature 362 , 709–715 (1993).
Lindahl, T. & Nyberg, B. Rate of depurination of native deoxyribonucleic acid. Biochemistry 11 , 3610–3618 (1972).
Lindahl, T. & Barnes, D. E. Repair of endogenous DNA damage. Cold Spring Harb. Symp. Quant. Biol. 65 , 127–134 (2000).
Jackson, S. P. & Bartek, J. The DNA-damage response in human biology and disease. Nature 461 , 1071–1078 (2009).
Article CAS PubMed PubMed Central Google Scholar
Doudna, J. A. & Charpentier, E. The new frontier of genome engineering with CRISPR–Cas9. Science 346 , 1258096 (2014).
Article PubMed Google Scholar
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315 , 1709–1712 (2007).
Makarova, K. S. et al. Evolution and classification of the CRISPR–Cas systems. Nat. Rev. Microbiol. 9 , 467–477 (2011).
Przybyla, L. & Gilbert, L. A. A new era in functional genomics screens. Nat. Rev. Genet. 23 , 89–103 (2022).
Baskar, R., Lee, K. A., Yeo, R. & Yeoh, K.-W. Cancer and radiation therapy: current advances and future directions. Int. J. Med. Sci. 9 , 193–199 (2012).
Article PubMed PubMed Central Google Scholar
Tchounwou, P. B., Dasari, S., Noubissi, F. K., Ray, P. & Kumar, S. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J. Exp. Pharmacol. 13 , 303–328 (2021).
Zagnoli-Vieira, G. & Caldecott, K. W. Untangling trapped topoisomerases with tyrosyl-DNA phosphodiesterases. DNA Repair. 94 , 102900 (2020).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12 , 31–46 (2022).
Pilié, P. G., Tang, C., Mills, G. B. & Yap, T. A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 16 , 81–104 (2019).
Dias, M. P., Moser, S. C., Ganesan, S. & Jonkers, J. Understanding and overcoming resistance to PARP inhibitors in cancer therapy. Nat. Rev. Clin. Oncol. 18 , 773–791 (2021).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434 , 917–921 (2005).
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434 , 913–917 (2005). Together with Farmer et al. (2005), this paper establishes that PARP inhibitors are strikingly and selectively toxic to cells bearing BRCA1 or BRCA2 deficiency, highlighting the potential for their use in HR-deficient cancers .
Lord, C. J. & Ashworth, A. PARP inhibitors: synthetic lethality in the clinic. Science 355 , 1152–1158 (2017).
Pilger, D., Seymour, L. W. & Jackson, S. P. Interfaces between cellular responses to DNA damage and cancer immunotherapy. Genes Dev. 35 , 602–618 (2021).
Gourley, C. et al. Moving from poly(ADP-ribose) polymerase inhibition to targeting DNA repair and DNA damage response in cancer therapy. J. Clin. Oncol. 37 , 2257–2269 (2019).
Lord, C. J., McDonald, S., Swift, S., Turner, N. C. & Ashworth, A. A high-throughput RNA interference screen for DNA repair determinants of PARP inhibitor sensitivity. DNA Repair. 7 , 2010–2019 (2008).
Bartz, S. R. et al. Small interfering RNA screens reveal enhanced cisplatin cytotoxicity in tumor cells having both BRCA network and TP53 disruptions. Mol. Cell. Biol. 26 , 9377–9386 (2006).
Smogorzewska, A. et al. A genetic screen identifies FAN1, a Fanconi anemia-associated nuclease necessary for DNA interstrand crosslink repair. Mol. Cell 39 , 36–47 (2010).
O’Connell, B. C. et al. A genome-wide camptothecin sensitivity screen identifies a mammalian MMS22L–NFKBIL2 complex required for genomic stability. Mol. Cell 40 , 645–657 (2010).
Doil, C. et al. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell 136 , 435–446 (2009).
Stewart, G. S. et al. The RIDDLE syndrome protein mediates a ubiquitin-dependent signaling cascade at sites of DNA damage. Cell 136 , 420–434 (2009).
Paulsen, R. D. et al. A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol. Cell 35 , 228–239 (2009).
López-Saavedra, A. et al. A genome-wide screening uncovers the role of CCAR2 as an antagonist of DNA end resection. Nat. Commun. 7 , 12364 (2016).
Adamson, B., Smogorzewska, A., Sigoillot, F. D., King, R. W. & Elledge, S. J. A genome-wide homologous recombination screen identifies the RNA-binding protein RBMX as a component of the DNA-damage response. Nat. Cell Biol. 14 , 318–328 (2012).
Acevedo-Arozena, A. et al. ENU mutagenesis, a way forward to understand gene function. Annu. Rev. Genom. Hum. 9 , 49–69 (2008).
Article CAS Google Scholar
Blomen, V. A. et al. Gene essentiality and synthetic lethality in haploid human cells. Science 350 , 1092–1096 (2015).
O’Loughlin, T. A. & Gilbert, L. A. Functional genomics for cancer research: applications in vivo and in vitro. Annu. Rev. Cancer Biol. 3 , 345–363 (2019).
Article Google Scholar
Forment, J. V. et al. Genome-wide genetic screening with chemically mutagenized haploid embryonic stem cells. Nat. Chem. Biol. 13 , 12–14 (2017).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR–Cas9 for genome engineering. Cell 157 , 1262–1278 (2014).
Shalem, O., Sanjana, N. E. & Zhang, F. High-throughput functional genomics using CRISPR–Cas9. Nat. Rev. Genet. 16 , 299–311 (2015).
Shalem, O. et al. Genome-scale CRISPR–Cas9 knockout screening in human cells. Science 343 , 84–87 (2014).
Wang, T. et al. Identification and characterization of essential genes in the human genome. Science 350 , 1096–1101 (2015).
Wang, T., Wei, J. J., Sabatini, D. M. & Lander, E. S. Genetic screens in human cells using the CRISPR–Cas9 system. Science 343 , 80–84 (2014).
Koike-Yusa, H., Li, Y., Tan, E.-P., del Castillo Velasco-Herrera, M. & Yusa, K. Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat. Biotechnol. 32 , 267–273 (2014).
Hart, T. et al. High-resolution CRISPR screens reveal fitness genes and genotype-specific cancer liabilities. Cell 163 , 1515–1526 (2015).
Gilbert, L. A. et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154 , 442–451 (2013).
Gilbert, L. A. et al. Genome-scale CRISPR-mediated control of gene repression and activation. Cell 159 , 647–661 (2014).
Perez-Pinera, P. et al. RNA-guided gene activation by CRISPR–Cas9-based transcription factors. Nat. Methods 10 , 973–976 (2013).
Kearns, N. A. et al. Functional annotation of native enhancers with a Cas9–histone demethylase fusion. Nat. Methods 12 , 401–403 (2015).
Thakore, P. I. et al. Highly specific epigenome editing by CRISPR–Cas9 repressors for silencing of distal regulatory elements. Nat. Methods 12 , 1143–1149 (2015).
Sanson, K. R. et al. Optimized libraries for CRISPR–Cas9 genetic screens with multiple modalities. Nat. Commun. 9 , 5416 (2018).
Iyer, V. S. et al. Designing custom CRISPR libraries for hypothesis-driven drug target discovery. Comput. Struct. Biotechnol. J. 18 , 2237–2246 (2020).
Wong, A. S. L. et al. Multiplexed barcoded CRISPR–Cas9 screening enabled by CombiGEM. Proc. Natl Acad. Sci. USA 113 , 2544–2549 (2016).
Shen, J. P. et al. Combinatorial CRISPR–Cas9 screens for de novo mapping of genetic interactions. Nat. Methods 14 , 573–576 (2017).
Najm, F. J. et al. Orthologous CRISPR–Cas9 enzymes for combinatorial genetic screens. Nat. Biotechnol. 36 , 179–189 (2018).
Han, K. et al. Synergistic drug combinations for cancer identified in a CRISPR screen for pairwise genetic interactions. Nat. Biotechnol. 35 , 463–474 (2017).
DeWeirdt, P. C. et al. Optimization of AsCas12a for combinatorial genetic screens in human cells. Nat. Biotechnol. 39 , 94–104 (2021).
Gier, R. A. et al. High-performance CRISPR–Cas12a genome editing for combinatorial genetic screening. Nat. Commun. 11 , 3455 (2020).
Dede, M., McLaughlin, M., Kim, E. & Hart, T. Multiplex enCas12a screens detect functional buffering among paralogs otherwise masked in monogenic Cas9 knockout screens. Genome Biol. 21 , 262 (2020).
Carleton, J. B., Berrett, K. C. & Gertz, J. Multiplex enhancer interference reveals collaborative control of gene regulation by estrogen receptor α-bound enhancers. Cell Syst. 5 , 333–344.e5 (2017).
Gasperini, M. et al. A genome-wide framework for mapping gene regulation via cellular genetic screens. Cell 176 , 1516 (2019).
Yeh, C. D., Richardson, C. D. & Corn, J. E. Advances in genome editing through control of DNA repair pathways. Nat. Cell Biol. 21 , 1468–1478 (2019).
DeWeirdt, P. C. et al. Genetic screens in isogenic mammalian cell lines without single cell cloning. Nat. Commun. 11 , 752 (2020). This study provides a strategy to create isogenic pairs of cells while avoiding single-cell cloning .
Bowden, A. R. et al. Parallel CRISPR–Cas9 screens clarify impacts of p53 on screen performance. eLife 9 , e55325 (2020).
Su, D. et al. CRISPR/CAS9-based DNA damage response screens reveal gene–drug interactions. DNA Repair. 87 , 102803 (2020).
Hussmann, J. A. et al. Mapping the genetic landscape of DNA double-strand break repair. Cell 184 , 5653–5669.e25 (2021). In this study, the authors develop a high-throughput screening approach called Repair-seq to map the genetic dependencies of DNA repair outcomes .
Zimmermann, M. et al. CRISPR screens identify genomic ribonucleotides as a source of PARP-trapping lesions. Nature 559 , 285–289 (2018).
Fugger, K. et al. Targeting the nucleotide salvage factor DNPH1 sensitizes BRCA -deficient cells to PARP inhibitors. Science 372 , 156–165 (2021).
Guo, J. U., Su, Y., Zhong, C., Ming, G. & Song, H. Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain. Cell 145 , 423–434 (2011).
Verma, P. et al. ALC1 links chromatin accessibility to PARP inhibitor response in homologous recombination-deficient cells. Nat. Cell Biol. 23 , 160–171 (2021).
Hewitt, G. et al. Defective ALC1 nucleosome remodeling confers PARPi sensitization and synthetic lethality with HRD. Mol. Cell 81 , 767–783.e11 (2021).
Juhász, S. et al. The chromatin remodeler ALC1 underlies resistance to PARP inhibitor treatment. Sci. Adv. 6 , eabb8626 (2020).
Blessing, C. et al. The oncogenic helicase ALC1 regulates PARP inhibitor potency by trapping PARP2 at DNA breaks. Mol. Cell 80 , 862–875.e6 (2020).
Ahel, D. et al. Poly(ADP-ribose)-dependent regulation of DNA repair by the chromatin remodeling enzyme ALC1. Science 325 , 1240–1243 (2009).
He, Y. J. et al. DYNLL1 binds to MRE11 to limit DNA end resection in BRCA1-deficient cells. Nature 563 , 522–526 (2018).
Barazas, M. et al. The CST complex mediates end protection at double-strand breaks and promotes PARP inhibitor sensitivity in BRCA1-deficient cells. Cell Rep. 23 , 2107–2118 (2018).
Dev, H. et al. Shieldin complex promotes DNA end-joining and counters homologous recombination in BRCA1-null cells. Nat. Cell Biol. 20 , 954–965 (2018).
Noordermeer, S. M. et al. The shieldin complex mediates 53BP1-dependent DNA repair. Nature 560 , 117–121 (2018). Together with Dev et al. (2018), this paper demonstrates that loss of the shieldin complex causes PARP1 resistance in BRCA1 -null cells .
Setiaputra, D. & Durocher, D. Shieldin — the protector of DNA ends. EMBO Rep. 20 , e47560 (2019).
Markiewicz-Potoczny, M. et al. TRF2-mediated telomere protection is dispensable in pluripotent stem cells. Nature 589 , 110–115 (2021).
Wang, C. et al. Genetic vulnerabilities upon inhibition of DNA damage response. Nucleic Acids Res. 49 , 8214–8231 (2021).
Pinzaru, A. M. et al. Replication stress conferred by POT1 dysfunction promotes telomere relocalization to the nuclear pore. Genes Dev. 34 , 1619–1636 (2020).
Adam, S. et al. The CIP2A–TOPBP1 axis safeguards chromosome stability and is a synthetic lethal target for BRCA-mutated cancer. Nat. Cancer 2 , 1357–1371 (2021).
Álvarez-Quilón, A. et al. Endogenous DNA 3′ blocks are vulnerabilities for BRCA1 and BRCA2 deficiency and are reversed by the APE2 nuclease. Mol. Cell 78 , 1152–1165.e8 (2020).
Mengwasser, K. E. et al. Genetic screens reveal FEN1 and APEX2 as BRCA2 synthetic lethal targets. Mol. Cell 73 , 885–899.e6 (2019).
Ceccaldi, R. et al. Homologous-recombination-deficient tumours are dependent on Pol-mediated repair. Nature 518 , 258–262 (2015).
Mateos-Gomez, P. A. et al. Mammalian polymerase promotes alternative NHEJ and suppresses recombination. Nature 518 , 254–257 (2015).
Feng, W. et al. Genetic determinants of cellular addiction to DNA polymerase θ. Nat. Commun. 10 , 4286 (2019).
Ramsden, D. A., Carvajal-Garcia, J. & Gupta, G. P. Mechanism, cellular functions and cancer roles of polymerase-θ-mediated DNA end joining. Nat. Rev. Mol. Cell Biol. 23 , 125–140 (2022).
Tang, M. et al. Genome-wide CRISPR screens reveal cyclin C as synthetic survival target of BRCA2. Nucleic Acids Res. 49 , 7476–7491 (2021).
Hustedt, N. et al. A consensus set of genetic vulnerabilities to ATR inhibition. Open. Biol. 9 , 190156 (2019).
Wang, C. et al. C17orf53 is identified as a novel gene involved in inter-strand crosslink repair. DNA Repair. 95 , 102946 (2020).
Huang, J.-W. et al. MCM8IP activates the MCM8-9 helicase to promote DNA synthesis and homologous recombination upon DNA damage. Nat. Commun. 11 , 2948 (2020).
Tucker, E. J. et al. Meiotic genes in premature ovarian insufficiency: variants in HROB and REC8 as likely genetic causes. Eur. J. Hum. Genet. 30 , 219–228 (2022).
Balmus, G. et al. ATM orchestrates the DNA-damage response to counter toxic non-homologous end-joining at broken replication forks. Nat. Commun. 10 , 87 (2019).
Mazouzi, A., Velimezi, G. & Loizou, J. I. DNA replication stress: causes, resolution and disease. Exp. Cell Res. 329 , 85–93 (2014).
Zeman, M. K. & Cimprich, K. A. Causes and consequences of replication stress. Nat. Cell Biol. 16 , 2–9 (2014).
Blackford, A. N. & Jackson, S. P. ATM, ATR, and DNA-PK: the trinity at the heart of the DNA damage response. Mol. Cell 66 , 801–817 (2017).
Cimprich, K. A. & Cortez, D. ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 9 , 616–627 (2008).
Lloyd, R. L. et al. Loss of cyclin C or CDK8 provides ATR inhibitor resistance by suppressing transcription-associated replication stress. Nucleic Acids Res. 49 , 8665–8683 (2021).
Wang, C. et al. Genome-wide CRISPR screens reveal synthetic lethality of RNASEH2 deficiency and ATR inhibition. Oncogene 38 , 2451–2463 (2019).
Ruiz, S. et al. A genome-wide CRISPR screen identifies CDC25A as a determinant of sensitivity to ATR inhibitors. Mol. Cell 62 , 307–313 (2016).
Schleicher, E. M. et al. Dual genome-wide CRISPR knockout and CRISPR activation screens identify mechanisms that regulate the resistance to multiple ATR inhibitors. PLoS Genet. 16 , e1009176 (2020).
Benslimane, Y. et al. Genome-wide screens reveal that resveratrol induces replicative stress in human cells. Mol. Cell 79 , 846–856.e8 (2020).
Wang, R. et al. DNA polymerase ι compensates for Fanconi anemia pathway deficiency by countering DNA replication stress. Proc. Natl Acad. Sci. USA 117 , 33436–33445 (2020).
Adeyemi, R. O. et al. The Protexin complex counters resection on stalled forks to promote homologous recombination and crosslink repair. Mol. Cell 81 , 4440–4456.e7 (2021).
Zhao, Y. et al. Applying genome-wide CRISPR to identify known and novel genes and pathways that modulate formaldehyde toxicity. Chemosphere 269 , 128701 (2021).
Cai, M.-Y. et al. Cooperation of the ATM and Fanconi anemia/BRCA pathways in double-strand break end resection. Cell Rep. 30 , 2402–2415.e5 (2020).
Schubert, L. et al. SCAI promotes error-free repair of DNA interstrand crosslinks via the Fanconi anemia pathway. EMBO Rep. 23 , e53639 (2022).
Maiani, E. et al. AMBRA1 regulates cyclin D to guard S-phase entry and genomic integrity. Nature 592 , 799–803 (2021).
Chaikovsky, A. C. et al. The AMBRA1 E3 ligase adaptor regulates the stability of cyclin D. Nature 592 , 794–798 (2021).
Olivieri, M. et al. A genetic map of the response to DNA damage in human cells. Cell 182 , 481–496.e21 (2020). This study identifies 890 genes whose loss causes either resistance or sensitivity to various DNA-damaging agents, and builds a genetic map of responses to DNA damage .
van der Weegen, Y. et al. ELOF1 is a transcription-coupled DNA repair factor that directs RNA polymerase II ubiquitylation. Nat. Cell Biol. 23 , 595–607 (2021).
Geijer, M. E. et al. Elongation factor ELOF1 drives transcription-coupled repair and prevents genome instability. Nat. Cell Biol. 23 , 608–619 (2021).
Carnie, C. J. & Jackson, S. P. The ELOF(1)ant in the room of TCR. Nat. Cell Biol. 23 , 584–586 (2021).
Liu, X. et al. ERCC6L2 promotes DNA orientation-specific recombination in mammalian cells. Cell Res. 30 , 732–744 (2020).
Feng, Y. et al. FAM72A antagonizes UNG2 to promote mutagenic repair during antibody maturation. Nature 600 , 324–328 (2021).
Hundley, F. V. et al. A comprehensive phenotypic CRISPR–Cas9 screen of the ubiquitin pathway uncovers roles of ubiquitin ligases in mitosis. Mol. Cell 81 , 1319–1336.e9 (2021).
Sanchez-Burgos, L. et al. Activation of the integrated stress response overcomes multidrug resistance in FBXW7-deficient cells. EMBO Mol. Med. 14 , e15855 (2022).
Liao, S., Maertens, O., Cichowski, K. & Elledge, S. J. Genetic modifiers of the BRD4-NUT dependency of NUT midline carcinoma uncovers a synergism between BETis and CDK4/6is. Genes Dev. 32 , 1188–1200 (2018).
Zhang, Q. et al. The WD40 domain of FBXW7 is a poly(ADP-ribose)-binding domain that mediates the early DNA damage response. Nucleic Acids Res. 47 , 4039–4053 (2019).
Hanna, R. E. et al. Massively parallel assessment of human variants with base editor screens. Cell 184 , 1064–1080.e20 (2021).
Cuella-Martin, R. et al. Functional interrogation of DNA damage response variants with base editing screens. Cell 184 , 1081–1097.e19 (2021).
Kweon, J. et al. A CRISPR-based base-editing screen for the functional assessment of BRCA1 variants. Oncogene 39 , 30–35 (2020).
Huang, C., Li, G., Wu, J., Liang, J. & Wang, X. Identification of pathogenic variants in cancer genes using base editing screens with editing efficiency correction. Genome Biol. 22 , 80 (2021).
Sangree, A. K. et al. Benchmarking of SpCas9 variants enables deeper base editor screens of BRCA1 and BCL2. Nat. Commun. 13 , 1318 (2022).
Koblan, L. W. et al. Efficient C•G-to-G•C base editors developed using CRISPRi screens, target-library analysis, and machine learning. Nat. Biotechnol. 39 , 1414–1425 (2021).
Chen, P. J. et al. Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 184 , 5635–5652.e29 (2021).
Fang, P., de Souza, C., Minn, K. & Chien, J. Genome-scale CRISPR knockout screen identifies TIGAR as a modifier of PARP inhibitor sensitivity. Commun. Biol. 2 , 335 (2019).
Kim, Y. et al. High-throughput functional evaluation of human cancer-associated mutations using base editors. Nat. Biotechnol. 40 , 874–884 (2022).
Pettitt, S. J. et al. Genome-wide and high-density CRISPR–Cas9 screens identify point mutations in PARP1 causing PARP inhibitor resistance. Nat. Commun. 9 , 1849 (2018).
Thompson, N. A. et al. Combinatorial CRISPR screen identifies fitness effects of gene paralogues. Nat. Commun. 12 , 1302 (2021).
Behan, F. M. et al. Prioritization of cancer therapeutic targets using CRISPR–Cas9 screens. Nature 568 , 511–516 (2019). This comprehensive study provides a systematic analysis of core-fitness genes and cancer type-specific fitness genes, and the Cancer Dependency Map (DepMap) .
Chan, E. M. et al. WRN helicase is a synthetic lethal target in microsatellite unstable cancers. Nature 568 , 551–556 (2019).
Cui, Y. et al. CRISP-view: a database of functional genetic screens spanning multiple phenotypes. Nucleic Acids Res. 49 , D848–D854 (2021).
Feng, X. et al. Genome-wide CRISPR screens using isogenic cells reveal vulnerabilities conferred by loss of tumor suppressors. Sci. Adv. 8 , eabm6638 (2022).
Tzelepis, K. et al. A CRISPR dropout screen identifies genetic vulnerabilities and therapeutic targets in acute myeloid leukemia. Cell Rep. 17 , 1193–1205 (2016).
Gallo, D. et al. CCNE1 amplification is synthetic lethal with PKMYT1 kinase inhibition. Nature 604 , 749–756 (2022).
Fong, P. C. et al. Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum-free interval. J. Clin. Oncol. 28 , 2512–2519 (2010).
Audeh, M. W. et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet 376 , 245–251 (2010).
Edwards, S. L. et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature 451 , 1111–1115 (2008).

COMMENTS
Introduction. DNA synthesis occurs during the S phase of the cell cycle and is ensured by the replisome, a molecular machine made of a large number of proteins acting in a coordinated manner to synthesize DNA at many genomic locations, the replication origins 1.Replication origin activation in space and time (or replication program) is set by a sequence of events, starting already at the end ...
DNA replication is the biological process by which an exact copy of a deoxyribonucleic acid (DNA) molecule is created and it is the basis for biological inheritance. ... Research Highlights 12 Jul ...
Accurate DNA replication is modulated by multiple replication‐associated proteins, which is fundamental to preserve genome stability. Abundant replication proteins are involved in tumorigenesis and development, implying these proteins act as therapeutic targets in clinical. Replication‐target cancer therapy emerges as the times require.
Unwinding of DNA at the origin and synthesis of new strands. results in replication forks growing bidir ectional from the origin. number of proteins are associated with the replication fork w hich ...
In all kingdoms of life, DNA is used to encode hereditary information. Propagation of the genetic material between generations requires timely and accurate duplication of DNA by semiconservative replication prior to cell division to ensure each daughter cell receives the full complement of chromosomes. DNA synthesis of daughter strands starts at discrete sites, termed replication origins, and ...
The bacterial DnaA-trio replication origin element specifies single-stranded DNA initiator binding. The bacterial chromosome replication origin contains an indispensable element composed of a ...
The failure to complete DNA replication is one of the major sources of genome instability that leads to aneuploidy, chromosome breakage, and chromosome rearrangements associated with human cancer. One of the surprising revelations of the past decade is that the completion of replication at so-called common fragile sites occurs very late in the ...
A paper in Molecular Cell reports the characterization of a second functional light strand promoter (LSP2) in the mitochondrial genome, challenging the view that mitochondrial DNA replication and ...
Our current view of DNA replication in eukaryotes, which comes mainly from in vitro studies using purified components of the DNA synthesome, is that both the initiation of leading strand DNA replication and discontinuous lagging strand synthesis require a switch from the use of the Pol‐α to either the Pol‐δ or Pol‐ϵ enzyme, as well as continuous recycling of the processivity factor ...
Genomic DNA replicates during the S phase of the cell cycle in a consistent order known as DNA replication timing. Replication timing is associated with gene regulation and influences mutation rates; however, the impacts of replication timing on human evolution are largely unknown. Here, we generated replication timing for humans, chimpanzees ...
Abstract. DNA replication is a fundamental process of the cell that ensures accurate duplication of the genetic information and subsequent transfer to daughter cells. Various pertubations, originating from endogenous or exogenous sources, can interfere with proper progression and completion of the replication process, thus threatening genome ...
DNA replica tion. and recombina tion. Bruce Alberts. National A cademy of Sciences, 2101 Constitut ion A venue, W ashington D C 20418, USA. Knowledge of the structure of DNA enabled scientists to ...
DNA synthesis in the in vitro system starts by covalent addition onto the 3'OH end at a random nick on a double-stranded DNA template and proceeds to generate a replication fork that moves at ...
Abstract. Core DNA replication proteins mediate the initiation, elongation, and Okazaki fragment maturation functions of DNA replication. Although this process is generally conserved in eukaryotes, important differences in the molecular architecture of the DNA replication machine and the function of individual subunits have been reported in various model systems.
As noted in their 1953 paper, Watson and Crick strongly suspected that the specific base pairings within the DNA double helix existed in order to ensure a controlled system of DNA replication ...
Chromosomal DNA replication is a process conserved through all domains of life. For accurate and efficient duplication of the genetic information, DNA replication components must work together in a highly regulated and coordinated fashion. Due to a central role in cell proliferation, the DNA replication machinery is an attractive therapeutic target for treating bacterial and viral infections ...
In eukaryotic cells, the response to replication stress is regulated by the kinase ATR, which stabilizes stalled replication forks and regulates firing of origins of DNA replication, cell cycle ...