Advances in Breast Cancer Research
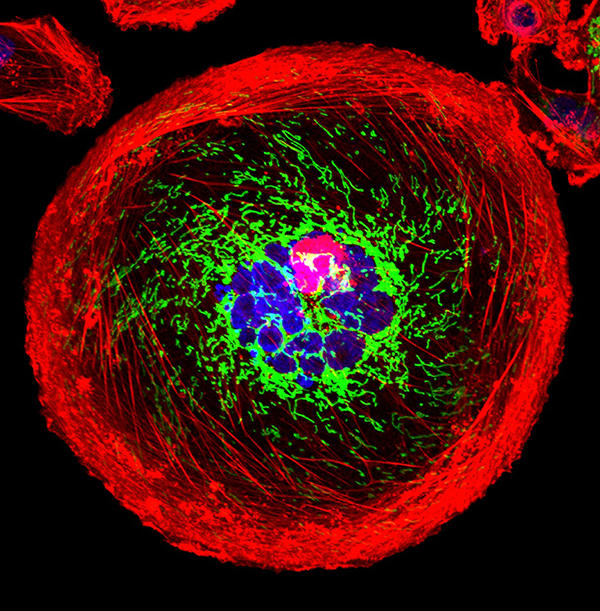
A polyploid giant cancer cell (PGCC) from triple-negative breast cancer.
NCI-funded researchers are working to advance our understanding of how to prevent, detect, and treat breast cancer. They are also looking at how to address disparities and improve quality of life for survivors of the disease.
This page highlights some of what's new in the latest research for breast cancer, including new clinical advances that may soon translate into improved care, NCI-supported programs that are fueling progress, and research findings from recent studies.

Early Detection of Breast Cancer
Breast cancer is one of a few cancers for which an effective screening test, mammography , is available. MRI ( magnetic resonance imaging ) and ultrasound are also used to detect breast cancer, but not as routine screening tools for people with average risk.
Ongoing studies are looking at ways to enhance current breast cancer screening options. Technological advances in imaging are creating new opportunities for improvements in both screening and early detection.
One technology advance is 3-D mammography , also called breast tomosynthesis . This procedure takes images from different angles around the breast and builds them into a 3-D-like image. Although this technology is increasingly available in the clinic, it isn’t known whether it is better than standard 2-D mammography , for detecting cancer at a less advanced stage.
NCI is funding a large-scale randomized breast screening trial, the Tomosynthesis Mammographic Imaging Screening Trial (TMIST) , to compare the number of advanced cancers detected in women screened for 5 years with 3-D mammography with the number detected in women screened with 2-D mammography.
Two concerns in breast cancer screening, as in all cancer screening, are:
- the potential for diagnosing tumors that would not have become life-threatening ( overdiagnosis )
- the possibility of receiving false-positive test results, and the anxiety that comes with follow-up tests or procedures
As cancer treatment is becoming more individualized, researchers are looking at ways to personalize breast cancer screening. They are studying screening methods that are appropriate for each woman’s level of risk and limit the possibility of overdiagnosis.
For example, the Women Informed to Screen Depending on Measures of Risk (WISDOM) study aims to determine if risk-based screening—that is, screening at intervals that are based on each woman’s risk as determined by her genetic makeup, family history , and other risk factors—is as safe, effective, and accepted as standard annual screening mammography.
WISDOM is also making a focused effort to enroll Black women in the trial. Past studies tended to contain a majority of White women and therefore, there is less data on how screening can benefit Black women. Researchers are taking a number of steps to include as many Black women as possible in the study while also increasing the diversity of all women enrolled.
Breast Cancer Treatment
The mainstays of breast cancer treatment are surgery , radiation , chemotherapy , hormone therapy , and targeted therapy . But scientists continue to study novel treatments and drugs, along with new combinations of existing treatments.
It is now known that breast cancer can be divided into subtypes based on whether they:
- are hormone receptor (HR) positive which means they express estrogen and/or progesterone receptors ( ER , PR )
Shortening Radiation Therapy for Some with Early Breast Cancer
A condensed course was as effective and safe as the standard course for women with higher-risk early-stage breast cancer who had a lumpectomy.
As we learn more about the subtypes of breast cancer and their behavior, we can use this information to guide treatment decisions. For example:
- The NCI-sponsored TAILORx clinical trial. The study, which included patients with ER-positive, lymph node-negative breast cancer, found that a test that looks at the expression of certain genes can predict which women can safely avoid chemotherapy.
- The RxPONDER trial found that the same gene expression test can also be used to determine treatment options in women with more advanced breast cancer. The study found that some postmenopausal women with HR positive, HER-2 negative breast cancer that has spread to several lymph nodes and has a low risk of recurrence do not benefit from chemotherapy when added to their hormone therapy.
- The OFSET trial is comparing the addition of chemotherapy to usual treatment ( ovarian function suppression plus hormone therapy) to usual treatment alone in treating premenopausal estrogen receptor (ER)-positive/HER2-negative breast cancer patients who are at high risk of their cancer returning. This will help determine whether or not adding chemotherapy helps prevent the cancer from returning.
Genomic analyses, such as those carried out through The Cancer Genome Atlas (TCGA) , have provided more insights into the molecular diversity of breast cancer and eventually could help identify even more breast cancer subtypes. That knowledge, in turn, may lead to the development of therapies that target the genetic alterations that drive those cancer subtypes.
HR-Positive Breast Cancer Treatment
Hormone therapies have been a mainstay of treatment for HR-positive cancer. However, there is a new focus on adding targeted therapies to hormone therapy for advanced or metastatic HR-positive cancers. These treatments could prolong the time until chemotherapy is needed and ideally, extend survival. Approved drugs include:

Drug Combo Effective for Metastatic Breast Cancer in Younger Women
Ribociclib plus hormone therapy were superior to standard chemotherapy combos in a recent trial.
- Palbociclib (Ibrance) , ribociclib (Kisqali) , and everolimus (Afinitor) have all been approved by the FDA for use with hormone therapy for treatment of advanced or metastatic breast cancer. Ribociclib has been shown to increase the survival of patients with metastatic breast cancer . It has also shown to slow the growth of metastatic cancer in younger women when combined with hormone therapy.
- Elacestrant (Orserdu) is approved for HR-positive and HER2-negative breast cancer that has a mutation in the ESR1 gene, and has spread. It is used in postmenopausal women and in men whose cancer has gotten worse after at least one type of hormone therapy.
- Abemaciclib (Verzenio) can be used with or after hormone therapy to treat advanced or metastatic HR-positive, HER2-negative breast cancer. In October 2021, the Food and Drug Administration ( FDA ) approved abemaciclib in combination with hormone therapy to treat some people who have had surgery for early-stage HR-positive, HER2-negative breast cancer.
- Alpelisib (Piqray) is approved to be used in combination with hormone therapy to treat advanced or metastatic HR-positive, HER2-negative breast cancers that have a mutation in the PIK3CA gene .
- Sacituzumab govitecan-hziy (Trodelvy) is used for HR-positive and HER2-negative breast cancer that has spread or can't be removed with surgery. It is used in those who have received hormone therapy and at least two previous treatments. It has shown to extend the amount of time that the disease doesn't get worse ( progression-free survival ) and also shown to improve overall survival .
HER2-Positive Breast Cancer Treatment
The FDA has approved a number of targeted therapies to treat HER2-positive breast cancer , including:
- Trastuzumab (Herceptin) has been approved to be used to prevent a relapse in patients with early-stage HER2-positive breast cancer.
- Pertuzumab (Perjeta) is used to treat metastatic HER2-positive breast cancer, and also both before surgery ( neoadjuvant ) and after surgery ( adjuvant therapy ).
- Trastuzumab and pertuzumab together can be used in combination with chemotherapy to prevent relapse in people with early-stage HER2-positive breast cancer. Both are also used together in metastatic disease, where they delay progression and improve overall survival.
- Trastuzumab deruxtecan (Enhertu) is approved for patients with advanced or metastatic HER2-positive breast cancer who have previously received a HER2-targeted treatment. A 2021 clinical trial showed that the drug lengthened the time that people with metastatic HER2-positive breast cancer lived without their cancer progressing. The trial also showed that it was better at shrinking tumors than another targeted drug, trastuzumab emtansine (Kadcyla).
- Tucatinib (Tukysa) is approved to be used in combination with trastuzumab and capecitabine (Xeloda) for HER2-positive breast cancer that cannot be removed with surgery or is metastatic. Tucatinib is able to cross the blood–brain barrier, which makes it especially useful for HER2-positive metastatic breast cancer, which tends to spread to the brain.
- Lapatinib (Tykerb) has been approved for treatment of some patients with HER2-positive advanced or metastatic breast cancer, together with capecitabine or letrozole.
- Neratinib Maleate (Nerlynx) can be used in patients with early-stage HER2-positive breast cancer and can also be used together with capecitabine (Xeloda) in some patients with advanced or metastatic disease.
- Ado-trastuzumab emtansine (Kadcyla) is approved to treat patients with metastatic HER2-positive breast cancer who have previously received trastuzumab and a taxane . It's also used in some patients with early-stage HER2-positive breast cancer who have completed therapy before surgery ( neoadjuvant ) and have residual disease at the time of surgery.
HER2-Low Breast Cancer
A newly defined subtype, HER2-low, accounts for more than half of all metastatic breast cancers. HER2-low tumors are defined as those whose cells contain lower levels of the HER2 protein on their surface. Such tumors have traditionally been classified as HER2-negative because they did not respond to drugs that target HER2.
However, in a clinical trial, trastuzumab deruxtecan (Enhertu) improved the survival of patients with HER2-low breast cancer compared with chemotherapy , and the drug is approved for use in such patients.

Immunotherapy Improves Survival in Triple-Negative Breast Cancer
For patients whose tumors had high PD-L1 levels, pembrolizumab with chemo helped them live longer.
Triple-Negative Breast Cancer Treatment
Triple-negative breast cancers (TNBC) are the hardest to treat because they lack both hormone receptors and HER2 overexpression , so they do not respond to therapies directed at these targets. Therefore, chemotherapy is the mainstay for treatment of TNBC. However, new treatments are starting to become available. These include:
- Sacituzumab govitecan-hziy (Trodelvy) is approved to treat patients with TNBC that has spread to other parts of the body . Patients must have received at least two prior therapies before receiving the drug.
- Pembrolizumab (Keytruda) is an immunotherapy drug that is approved to be used in combination with chemotherapy for patients with locally advanced or metastatic TNBC that has the PD-L1 protein. It may also be used before surgery (called neoadjuvant ) for patients with early-stage TNBC, regardless of their PD-L1 status.
- PARP inhibitors, which include olaparib (Lynparza) and talazoparib (Talzenna) , are approved to treat metastatic HER2-negative or triple-negative breast cancers in patients who have inherited a harmful BRCA gene mutation. Olaparib is also approved for use in certain patients with early-stage HER2-negative or triple-negative breast cancer.
- Drugs that block the androgen receptors or prevent androgen production are being tested in a subset of TNBC that express the androgen receptor.
For a complete list of drugs for breast cancer, see Drugs Approved for Breast Cancer .
NCI-Supported Breast Cancer Research Programs
Many NCI-funded researchers working at the NIH campus, as well as across the United States and world, are seeking ways to address breast cancer more effectively. Some research is basic, exploring questions as diverse as the biological underpinnings of cancer and the social factors that affect cancer risk. And some are more clinical, seeking to translate this basic information into improving patient outcomes. The programs listed below are a small sampling of NCI’s research efforts in breast cancer.
TMIST is a randomized breast screening trial that compares two Food and Drug Administration (FDA)-approved types of digital mammography, standard digital mammography (2-D) with a newer technology called tomosynthesis mammography (3-D).
The Breast Specialized Programs of Research Excellence (Breast SPOREs) are designed to quickly move basic scientific findings into clinical settings. The Breast SPOREs support the development of new therapies and technologies, and studies to better understand tumor resistance, diagnosis, prognosis, screening, prevention, and treatment of breast cancer.
The NCI Cancer Intervention and Surveillance Modeling Network (CISNET) focuses on using modeling to improve our understanding of how prevention, early detection, screening, and treatment affect breast cancer outcomes.
The Confluence Project , from NCI's Division of Cancer Epidemiology and Genetics (DCEG) , is developing a research resource that includes data from thousands of breast cancer patients and controls of different races and ethnicities. This resource will be used to identify genes that are associated with breast cancer risk, prognosis, subtypes, response to treatment, and second breast cancers. (DCEG conducts other breast cancer research as well.)
The Black Women’s Health Study (BWHS) Breast Cancer Risk Calculator allows health professionals to estimate a woman’s risk of developing invasive breast cancer over the next 5 years. With the NCI-funded effort, researchers developed a tool to estimate the risk of breast cancer in US Black women. The team that developed the tool hopes it will help guide more personalized decisions on when Black women—especially younger women—should begin breast cancer screening.
The goal of the Breast Cancer Surveillance Consortium (BCSC) , an NCI-funded program launched in 1994, is to enhance the understanding of breast cancer screening practices in the United States and their impact on the breast cancer's stage at diagnosis, survival rates, and mortality.
There are ongoing programs at NCI that support prevention and early detection research in different cancers, including breast cancer. Examples include:
- The Cancer Biomarkers Research Group , which promotes research in cancer biomarkers and manages the Early Detection Research Network (EDRN) . EDRN is a network of NCI-funded institutions that are collaborating to discover and validate early detection biomarkers. Within the EDRN, the Breast and Gynecologic Cancers Collaborative Group conducts research on breast and ovarian cancers.
- NCI's Division of Cancer Prevention houses the Breast and Gynecologic Cancer Research Group which conducts and fosters the development of research on the prevention and early detection of breast and gynecologic cancers.
Breast Cancer Survivorship Research
NCI’s Office of Cancer Survivorship, part of the Division of Cancer Control and Population Sciences (DCCPS), supports research projects throughout the country that study many issues related to breast cancer survivorship. Examples of studies funded include the impact of cancer and its treatment on physical functioning, emotional well-being, cognitive impairment , sleep disturbances, and cardiovascular health. Other studies focus on financial impacts, the effects on caregivers, models of care for survivors, and issues such as racial disparities and communication.
Breast Cancer Clinical Trials
NCI funds and oversees both early- and late-phase clinical trials to develop new treatments and improve patient care. Trials are available for breast cancer prevention , screening , and treatment .
Breast Cancer Research Results
The following are some of our latest news articles on breast cancer research and study updates:
- Can Some People with Breast Cancer Safely Skip Lymph Node Radiation?
- Study Adds to Debate about Mammography in Older Women
- Pausing Long-Term Breast Cancer Therapy to Become Pregnant Appears to Be Safe
- A Safer, Better Treatment Option for Some Younger Women with Breast Cancer
- Shorter Course of Radiation Is Effective, Safe for Some with Early-Stage Breast Cancer
- Pembrolizumab Improves Survival in Advanced Triple-Negative Breast Cancer
View the full list of Breast Cancer Research Results and Study Updates .
Your Account
Manage your account, subscriptions and profile.
MyKomen Health
ShareForCures
In Your Community
In Your Community
View resources and events in your local community.
Change your location:

Susan G. Komen®
One moment can change everything.
What’s New in Breast Cancer
This section gives an overview of new breast cancer treatment breakthroughs and recent developments in research that are fueling new ways to assess risk, and prevent, detect, diagnose and treat breast cancer. Advances in breast cancer care are evaluated through a rigorous process that includes clinical trials and regulatory approvals before being considered standards of care and included in breast cancer care guidelines. Komen’s research team monitors the rapidly evolving breast cancer landscape, and here we will highlight new breast cancer treatment breakthroughs, innovations in technology or key advances that may be added or are new to guidelines. We will share these research advancements to empower patients with knowledge to help them make informed decisions with their doctors.
Use these links to jump to the topics below.
- Emerging Areas in Metastatic Breast Cancer Treatment
- Clinical Trials
Treatments and Drugs
For patients, new treatments can mean more options and more hope. Researchers are working to develop new breast cancer treatment breakthroughs, such as more effective drugs that will specifically target breast cancer cells, minimize side effects and prevent breast cancer cells from coming back. While some treatments increase the effectiveness of existing drugs, others may offer new, innovative strategies for attacking tumor cells.
As of August 2023, the following new treatments and drugs are currently in clinical trials and have not yet received FDA approval:
- A new antibody-drug conjugate called datopotamab deruxtecan (Dato-DXd) is currently being evaluated in three Phase 3 clinical trials for advanced estrogen receptor-positive (ER+) [2] breast cancer, metastatic triple negative [ 3 ] breast cancer and early triple negative [ 4 ] breast cancer (TNBC). Dato-DXd specifically targets a protein called TROP2, a biomarker that can be used to target cancer cells instead of healthy cells. Another TROP2-targeting therapy called sacituzumab govitecan has already been approved for TNBC and estrogen-receptor-positive breast cancer. Dato-DXd uses a different chemotherapy drug and delivery system compared to sacituzumab govitecan.
- HER2 is a common treatment target for breast cancer. This new drug targets HER3, a biomarker related to HER2, which is associated with poor breast cancer outcomes. About 10% to 20% of newly diagnosed breast cancers are HER2-positive. At the 2023 American Society for Clinical Oncology (ASCO) Annual Meeting, researchers announced positive results for a Phase 2 clinical trial studying HER3-DXd, a new HER3-targeting antibody-drug conjugate for people with metastatic breast cancer . [ 1 ]. While the study found that 35% of patients responded positively to HER3-DXd, researchers will continue to evaluate which patients could benefit most from this drug through future Phase 3 clinical trials.
- CDK4/6 inhibitors are commonly used to treat estrogen receptor-positive breast cancer, but a new CDK4/6 inhibitor called trilaciclib is being tested to treat TNBC. Results from a Phase 2 clinical trial showed that trilaciclib improved outcomes for people with advanced TNBC, and the drug is currently being evaluated in the Phase 3 PRESERVE 2 clinical trial [ 5 ]. Researchers believe that unlike currently available CDK4/6 inhibitors, trilaciclib may improve response to immunotherapy and mitigate some of the side effects of chemotherapy . If this clinical trial is successful, this would be the first CDK4/6 inhibitor approved for people with TNBC.
New and improved technologies may be able to increase the speed and accuracy of detecting, diagnosing or monitoring breast cancer for progression and response to treatment.
- Doctors may use PET scans, or positron emission tomography, to scan for evidence that breast cancer has spread or metastasized. Once breast cancer has spread, the metastases may have evolved to a different type of breast cancer than the original tumor. These differences mean the metastases and the original tumor may not respond to the same treatments. A diagnostic imaging agent called Cerianna (fluoroestradiol F-18 or FES PET) allows doctors to use PET scans to learn if estrogen receptors are present in metastatic lesions. If a person has metastatic lesions that are estrogen receptor-positive, they may respond well to hormone therapy. This agent was recently incorporated in the National Comprehensive Cancer Network (NCCN) guidelines [ 6 ] as an option for some people with metastatic or recurrent estrogen receptor-positive breast cancer to consider [ 7 ].
- Ovarian suppression increases the effectiveness of hormone therapy in some premenopausal women but comes with additional side effects that can affect quality of life. A study presented at the 2022 San Antonio Breast Cancer Symposium [ 8 ] suggests that the Breast Cancer Index , a tumor profiling test that looks at genes to predict how likely a cancer is to metastasize, may be able to identify premenopausal women that would benefit most from ovarian suppression. This test would give doctors a new tool to personalize treatment for premenopausal women with estrogen receptor-positive breast cancer. More data are needed to confirm these results.
- Doctors are getting closer to identifying which patients with early HER2-positive breast cancer can safely avoid chemotherapy by using the HER2DX genomic test. HER2DX is the first test specifically designed to identify HER2-positive patients at high and low risk for recurrence . For some people, being able to avoid chemotherapy without comprising long-term outcomes will lead to a better quality of life.

Research can take decades to reach the bedside, but what discoveries are just around the corner for patients? Susan G. Komen shares all of this and more through Breast Cancer Breakthroughs, a virtual education series focusing on the new science and technology advancements that are poised to make a difference for patients in the near future. Sign up for Breast Cancer Breakthroughs to never miss an episode.

Kimberly’s Story: Finding Joy in the Midst of a Metastatic Breast Cancer Diagnosis
After Kimberly Reinika’s mother passed away in 2019 from ovarian cancer, she worried that it would ultimately take her life, too. “That was the cancer I was checking for,” she said.
Approaches to Care
With knowledge gained from clinical trials, researchers are seeking new ways to improve patient outcomes while using existing drugs. Some new breast cancer treatment breakthroughs are the result of combining certain drugs, finding which patients can skip certain elements of treatment or changing the order of their treatments to maximize effectiveness or minimize side effects.
- Patients with early estrogen receptor-positive breast cancer generally have a good prognosis, but some people have a higher risk of recurrence for as long as 20 years. Researchers are seeking new strategies to reduce this risk of recurrence. CDK4/6 inhibitors are used to treat advanced breast cancer, but the Phase 3 NATALEE clinical trial, presented at the 2023 American Society of Clinical Oncology Annual Meeting [ 9 ], found that using the CDK4/6 inhibitor ribociclib for two years in the adjuvant setting reduced the risk of recurrence for people with estrogen receptor-positive breast cancer.
- Inflammatory breast cancer is difficult to diagnose because its symptoms often mimic infections. Additionally, because some medical professionals don’t see it often, they may lack experience in recognizing and treating inflammatory breast cancer. In partnership with the Inflammatory Breast Cancer Research Foundation and the Milburn Foundation, Susan G. Komen launched a first-of-its kind diagnostic tool for inflammatory breast cancer. Through this scoring system, the tool considers the defining features of inflammatory breast cancer and provides data that can help providers accurately determine whether a person has inflammatory breast cancer. The goal of this tool is to increase the accuracy of diagnosing inflammatory breast cancer so that people will receive the appropriate care they need to treat this aggressive disease.
- Immunotherapy targets the immune system to help the body fight off tumors. Immunotherapy is currently only available for some patients with triple negative breast cancer, but researchers are aiming to bring this cutting edge therapy to more people. In a recent announcement [ 10 ], positive results were announced for a clinical trial that evaluated the immunotherapy drug pembrolizumab in patients with early estrogen receptor-positive breast cancer. Komen will be closely monitoring the results of this study at upcoming scientific conferences and hopes to see more promising data suggesting that a new treatment option may soon be available for patients with early estrogen receptor-positive breast cancer.
- Clinical trials are often designed using the maximum tolerated dose of a drug. However, many drugs may give the same effect with a smaller dose that results in fewer side effects for the patient. The X-7/7 clinical trial, which was presented at the 2023 ASCO Annual Meeting, tested the impact of a new treatment schedule for the chemotherapy drug capecitabine to treat metastatic breast cancer. Researchers found that people who took a higher dose of capecitabine over fewer days had fewer side effects and were able to remain on their treatment longer compared to the standard regimen. This new approach can improve the quality of life for those living with metastatic breast cancer without compromising the effectiveness of their treatments.
Komen will be closely monitoring the results of these studies and more at upcoming scientific conferences and hopes to see more promising data regarding new ways to prevent, detect, diagnose and treat breast cancer.

It Looks Promising: Uncovering New Possibilities in Breast Cancer Prevention
Is breast cancer prevention possible? Komen Scientific Advisory Board Member Dr. Kornelia Polyak is exploring a new strategy to identify and eliminate cell precursors from which tumors can grow.

Help discover cures to breast cancer, faster. New treatment breakthroughs for breast cancer come from researchers learning from people who have breast cancer, but our current data sources only represent a small portion of the breast cancer community. Help us discover the cures to breast cancer, faster, by joining ShareForCures.
What’s New in Breast Cancer References
- Hamilton, E. P., et al. (2023). “A phase 2 study of HER3-DXd in patients (pts) with metastatic breast cancer (MBC).” Journal of Clinical Oncology 41(16_suppl): 1004-1004. https://meetings.asco.org/abstracts-presentations/219699
- https://classic.clinicaltrials.gov/ct2/show/NCT05104866
- https://clinicaltrials.gov/study/NCT05374512
- https://classic.clinicaltrials.gov/ct2/show/NCT05629585
- https://classic.clinicaltrials.gov/ct2/show/NCT04799249
- https://www.gehealthcare.com/about/newsroom/press-releases/ge-healthcare-announces-fes-pet-imaging-recommendation-in-nccn-clinical-practice-guidelines-in-oncology-nccn-guidelines
- https://www.nccn.org/patients/guidelines/content/PDF/breast-invasive-patient.pdf (page 16)
- https://www.sabcs.org/Portals/SABCS2016/2022%20SABCS/SABCS%202022%20Abstract%20Report.pdf?ver=2022-12-08-111637-860
- Stroyakovskiy, D., et al. (2023). “Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer: Primary results from the phase III NATALEE trial.” Journal of Clinical Oncology 41(17_suppl): LBA500-LBA500.
- https://www.merck.com/news/merck-announces-phase-3-keynote-756-trial-met-primary-endpoint-of-pathological-complete-response-pcr-rate-in-patients-with-high-risk-early-stage-er-her2-breast-cancer/
TOOLS & RESOURCES
NEED HELP OR MORE INFORMATION?
1-877 GO KOMEN (1-877-465-6636)
Educational Resources
Komen Financial Assistance Program

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

New study finds triple-negative breast cancer tumors with an increase in immune cells have lower risk of recurrence after surgery
Share this:.
By Kelley Luckstein
A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
TNBC is a breast cancer subtype that does not respond to drugs that target the estrogen receptor or the HER2 protein. It grows rapidly, is more likely to spread beyond the breast before diagnosis and is more likely to recur than other breast cancers. TNBC represents about 15% of all breast cancers and is more common in younger people and in women of African American, Hispanic and Indian descent. Immune cells, also known as tumor-infiltrating lymphocytes, or TILs, are naturally existing immune system cells that can move from the bloodstream into a tumor and can recognize and destroy cancer cells.

"This is an important finding because it highlights that the abundance of TILs in breast tissue is a prognostic biomarker in people with early-stage triple-negative breast cancer, even when chemotherapy is not administered," says Roberto Leon-Ferre, M.D. , a breast medical oncologist at Mayo Clinic Comprehensive Cancer Center and first author of the study. "The study's findings may inspire future clinical trials to explore whether patients with a favorable prognosis (high TILs) can avoid intensive chemotherapy regimens."
"This meta-analysis confirms robustly the prognostic value of TILs that we have previously reported in TNBC patients treated with chemotherapy and expands it to patients treated without chemotherapy," says Sarah Flora Jonas, Ph.D., a statistician at Gustave Roussy and co-first author of the study. "Future studies may allow the use of this biomarker along with standard clinicopathological factors to inform treatment decisions in TNBC patients."
"Of interest, the first report suggesting that an increased number of immune cells being associated with better prognosis in breast cancer patients was described by doctors at Mayo Clinic more than 100 years ago," says Roberto Salgado, M.D., co-chair of the International Immuno-Oncology Biomarker Working Group; co-lead of the study; and pathologist from the Peter MacCallum Cancer Centre, Melbourne, Australia, and ZAS Hospitals, Antwerp, Belgium. "It took a global effort and a century later to reexamine this biomarker and bring it closer to application in patient care."

"TILs are not currently measured or reported in the routine examination of tissue samples of breast cancer," says co-senior author, Matthew Goetz, M.D. , a medical oncologist at Mayo Clinic Comprehensive Cancer Center and the Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, M.D. "While prior studies have focused on measuring TILs in people treated with chemotherapy, this is the largest study to comprehensively demonstrate that the presence of TILs influences the natural behavior of breast cancer in people who have surgery and/or radiation with no additional medical treatment."
For this study, Mayo Clinic and Gustave Roussy researchers, in collaboration with the International Immuno-Oncology Biomarker Working Group, led 11 additional groups to collect data on 1,966 participants with early-stage TNBC who only underwent surgery with or without radiation therapy but did not receive chemotherapy. The participants had been followed for a median of 18 years. The results showed that higher levels of TILs in breast cancer tissue were associated with lower recurrence rates among participants with early-stage TNBC.
"Five years after surgery, 95% of participants with small tumors, stage 1 TNBC, and whose tumors had high TILs were alive, compared to 82% of patients whose tumors had low TILs. Importantly, the breast cancer recurrence rate was significantly lower among patients whose tumors had high TILs," says co-senior author, Stefan Michiels, Ph.D. , head of Oncostat team, Gustave Roussy, Inserm U1018, University Paris-Saclay. "With nearly 2,000 participants involved in the study, we have now assembled the largest international cohort across three continents of people with TNBC in which the primary treatment was surgery without chemotherapy."
"The results of this study could lead to a recommendation to include TILs in the pathology reports of early-stage TNBC worldwide, as it has the potential to inform clinicians and patients when they discuss treatment options," says Dr. Salgado.
Furthermore, this biomarker would only require a visual evaluation by a pathologist looking through a microscope, meaning there are no additional costs associated with identifying the presence of immune cells. This could be particularly beneficial to regions with limited resources, adds Dr. Leon-Ferre.
Most people with early-stage TNBC undergo chemotherapy either before or after surgery, including people with stage 1 breast cancer. Most people receive multiple chemotherapy drugs in combination, which can cause significant side effects. Currently, the main factors considered to determine the course of chemotherapy treatment for each person are the tumor size and whether the cancer has spread to the lymph nodes. However, the authors identified that the number of TILs further influences the risk of future recurrence.
The researchers plan to evaluate TILs as biomarkers in prospective clinical trials evaluating chemotherapy selection based on TIL levels. Ongoing efforts to conduct additional research with other potential biomarkers are underway.
For a complete list of authors, disclosures and funding, see the full paper here .
Learn more about breast cancer and find a clinical trial at Mayo Clinic.
Join the Breast Cancer Support Group on Mayo Clinic Connect , an online community moderated by Mayo Clinic for patients and caregivers.
Also, read these articles:
- Understanding triple-negative breast cancer and its treatment
- 17-gene signature linked to remission after triple-negative breast cancer treatment
A version of this article was originally published as a press release on the Mayo Clinic News Network .
Related Posts

Dr. Maria Linnaus discusses the link between obesity and cancer risk and how bariatric surgery may reduce that risk.

Dr. Dawn Mussallem, a Mayo Clinic lifestyle medicine expert, says consuming soy products in moderation can be beneficial.

Dr. Jesse Bracamonte discusses the importance of cancer screenings as well as preventive screenings for diabetes and cardiovascular disease.
Scientists create tailored drug for aggressive breast cancer
Scientists have used breast cancer cells' weakness against themselves by linking a tumour-selective antibody with a cell-killing drug to destroy hard-to-treat tumours.
The research, published today in Clinical Cancer Research by a team from King's College London and funded by Breast Cancer Now, marks a new method in cancer treatment.
The discovery is particular to triple negative breast cancer, which makes up 15% of all diagnosed breast cancer. This type of breast cancer is typically aggressive, resistant to chemotherapy, has a lower survival rate and is more common in women under 40.
Usual treatment involves surgery, chemotherapy and radiotherapy, however this type of cancer can evade the drugs and return to spread again.
The scientists conducted data analysis using over 6000 breast cancer samples to investigate the properties of breast cancer cells that are associated with aggressive and chemotherapy-resistant cancers.
They studied the cancer's biology, what is expressed in the tumour and the cell surface, and the cell's insides to understand how the cancer cells escape from cancer drugs. They established the presence of the cancer cell surface marker EGFR along with oncogenic molecules cyclin-dependent kinases (CDK), which are responsible for cell division and proliferation.
They used this knowledge against the cancer cells to link cetuximab, a tumour-selective antibody that targets the EGFR protein expressed in this type of cancer, with a CDK-blocking drug to create a tailored drug for breast cancer. Because the antibody drug conjugate specifically targets the cancer cell, it may be possible to administer a lower inhibitor dose than usual which means it's less toxic for the patient.
Lead author Professor Sophia Karagiannis, from King's College London, said: "We were on the hunt for cancer's vulnerabilities and now we've found out how we can guide our therapies to one of these. We combined these two drugs to create a tailored antibody drug conjugate for patients with this aggressive cancer. The antibody guides the toxic drug directly to the cancer cell which offers the possibility for a lower dose and less adverse side effects to be experienced.
"More work needs to be done before this therapy can reach the clinic, but we expect that this can offer new treatment options for cancers with unfavourable prognosis. Beyond this antibody drug conjugate, we hope that our concept will lead the way for new antibody drug conjugates of this type to be tailored to patient groups likely to benefit."
Lead research scientist Dr Anthony Cheung from King's College London said: ''Triple negative breast cancer represents a molecularly and clinically diverse disease. By exploiting EGFR overexpression and dysregulated cell cycle molecules in selected patient groups, the antibody drug conjugate, but not the antibody alone, could stop the cancer cell from dividing and engender cytotoxic functions specifically against the cancer cells.''
Dr Simon Vincent, director of services, support and influencing at Breast Cancer Now, which funded this research, said: "Each year, around 8,000 women in the UK are diagnosed with triple negative breast cancer, which is typically more aggressive than other breast cancers and more likely to return or spread following treatment.
"This exciting research has not only improved our understanding of the properties of aggressive breast cancer cells that are resistant to chemotherapy but has also brought us closer to developing a targeted therapy that destroys these cancer cells while minimising side effects for patients.
"While further research is needed before this treatment can be used in people, this is an exciting step forward in developing targeted therapies for triple negative breast cancer, and we look forward to seeing how these findings could lead to new and effective ways of tackling this devastating disease."
- Breast Cancer
- Lung Cancer
- Colon Cancer
- Brain Tumor
- Ovarian Cancer
- Monoclonal antibody therapy
- Chemotherapy
- Mammography
- Breast cancer
- Esophageal cancer
Story Source:
Materials provided by King's College London . Note: Content may be edited for style and length.
Journal Reference :
- Anthony Cheung, Alicia M. Chenoweth, Annelie Johansson, Roman Laddach, Naomi Guppy, Jennifer Trendell, Benjamina Esapa, Antranik Mavousian, Blanca Navarro-Llinas, Syed Haider, Pablo Romero-Clavijo, Ricarda M. Hoffmann, Paolo Andriollo, Khondaker Miraz Rahman, Paul Jackson, Sophia Tsoka, Sheeba Irshad, Ioannis Roxanis, Anita Grigoriadis, David E. Thurston, Christopher J. Lord, Andrew N.J. Tutt, Sophia N. Karagiannis. Anti-EGFR antibody-drug conjugate carrying an inhibitor targeting CDK restricts triple-negative breast cancer growth . Clinical Cancer Research , 2024; DOI: 10.1158/1078-0432.CCR-23-3110
Cite This Page :
Explore More
- Future Climate Impacts Put Whale Diet at Risk
- Charge Your Laptop in a Minute?
- Caterpillars Detect Predators by Electricity
- 'Electronic Spider Silk' Printed On Human Skin
- Engineered Surfaces Made to Shed Heat
- Innovative Material for Sustainable Building
- Human Brain: New Gene Transcripts
- Epstein-Barr Virus and Resulting Diseases
- Origins of the Proton's Spin
- Symbiotic Bacteria Communicate With Plants
Trending Topics
Strange & offbeat.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Breast Cancer Treatments: Updates and New Challenges
Anna burguin.
1 Department of Molecular Medicine, Faculty of Medicine, Université Laval, Quebec City, QC G1T 1C2, Canada; [email protected]
2 Cancer Research Center, CHU de Québec-Université Laval, Quebec City, QC G1V 4G2, Canada; [email protected]
Caroline Diorio
3 Department of Preventive and Social Medicine, Faculty of Medicine, Université Laval, Quebec City, QC G1T 1C2, Canada
Francine Durocher
Associated data.
The study did not report any data.
Breast cancer (BC) is the most frequent cancer diagnosed in women worldwide. This heterogeneous disease can be classified into four molecular subtypes (luminal A, luminal B, HER2 and triple-negative breast cancer (TNBC)) according to the expression of the estrogen receptor (ER) and the progesterone receptor (PR), and the overexpression of the human epidermal growth factor receptor 2 (HER2). Current BC treatments target these receptors (endocrine and anti-HER2 therapies) as a personalized treatment. Along with chemotherapy and radiotherapy, these therapies can have severe adverse effects and patients can develop resistance to these agents. Moreover, TNBC do not have standardized treatments. Hence, a deeper understanding of the development of new treatments that are more specific and effective in treating each BC subgroup is key. New approaches have recently emerged such as immunotherapy, conjugated antibodies, and targeting other metabolic pathways. This review summarizes current BC treatments and explores the new treatment strategies from a personalized therapy perspective and the resulting challenges.
1. Introduction
Breast cancer (BC) is the most frequent cancer and the second cause of death by cancer in women worldwide. According to Cancer Statistics 2020, BC represents 30% of female cancers with 276,480 estimated new cases and more than 42,000 estimated deaths in 2020 [ 1 ].
Invasive BC can be divided into four principal molecular subtypes by immunohistological technique based on the expression of the estrogen receptor (ER), the progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) [ 2 ]. Luminal A BC (ER+ and/or PR+, and HER2-) represents around 60% of BC and is associated with a good prognosis [ 3 ]. Luminal B BC (ER+ and/or PR+, and HER2+) represents 30% of BC and is associated with high ki67 (>14%), a proliferation marker, and a poor prognosis [ 4 ]. HER2 BC (ER-, PR-, and HER2+) represents 10% of BC and is also associated with a poor prognosis [ 5 ]. Lastly, triple-negative BC (TNBC) (ER-, PR-, and HER2-) represents 15–20% of BC and is associated with more aggressivity and worse prognosis compared to other BC molecular subtypes and often occurs in younger women [ 6 ]. Characteristics of BC by molecular subtypes are described in Figure 1 .

Characteristics of breast cancer molecular subtypes. ER: estrogen receptor; PR: progesterone receptor; HER2: human epidermal growth factor receptor 2; TNBC: triple-negative breast cancer. a . Frequency derived from Al-thoubaity et al. [ 12 ] and Hergueta-Redondo et al. [ 13 ]. b . Grade derived from Engstrom et al. [ 14 ]. c . Prognosis derived from Hennigs et al. [ 15 ] and Fragomeni et al. [ 16 ]. d . The 5–year survival rate derived from the latest survival statistics of SEER [ 7 ].
The 5-year relative BC-specific survival rate of BC is encouraging with 90.3% for all subtypes and stages. However, for metastatic BC the 5-year relative cancer-specific survival rate is still low: 29% regardless of subtype and can drop to 12% for metastatic TNBC [ 7 ]. This clearly indicates that strategies of treatment for metastatic BC patients are not effective enough to ensure a good survival rate. Thus, it is crucial to find new solutions for the treatment of metastatic BC and especially TNBC.
Treatment choice is based on the grade, stage, and BC molecular subtype to have the most personalized, safe, and efficient therapy. The grade describes the appearance of tumor cells compared to normal cells. It includes tubule differentiation, nuclear pleomorphism, and the mitotic count [ 8 ]. The stage is used to classify the extent of cancer in the body and is defined using the TNM system comprising tumor size, lymph node status, and the presence of metastases [ 9 ]. For non-metastatic BC, the strategic therapy involves removing the tumor by complete or breast-conserving surgery with preoperative (neoadjuvant) or postoperative (adjuvant) radiotherapy and systemic therapy including chemotherapy, and targeted therapy. Targeted therapy comprises endocrine therapy for hormone receptor-positive (HR+) BC and anti-HER2 therapy for HER2+ BC. Unfortunately, there is no available targeted therapy for the TNBC subtype. For metastatic BC the priority is to contain tumor spread as this type of BC remains incurable. The same systemic therapies are used to treat metastatic BC [ 10 ].
Challenges in the treatment of BC including dealing with treatment resistance and recurrence. Indeed, 30% of early-stage BC have recurrent disease, mostly metastases [ 11 ]. Thus, it is crucial to develop new strategic therapies to treat each BC subgroup effectively.
This review will summarize current treatments for invasive BC, the underlying resistance mechanisms and explore new treatment strategies focusing on personalized therapy and the resulting challenges.
2. Common Treatments for All Breast Cancer Subtypes
In addition to surgery, radiotherapy and chemotherapy are used routinely to treat all BC subtypes [ 17 ].
2.1. Surgery
The most standard breast surgery approaches are either total excision of the breast (mastectomy), usually followed by breast reconstruction, or breast-conserving surgery (lumpectomy). Lumpectomy entails the excision of the breast tumor with a margin of surrounding normal tissue. The recommended margins status is defined as “no ink on tumor”, meaning no remaining tumor cells at the tissue edge [ 18 ]. Studies show that total mastectomy and lumpectomy plus irradiation are equivalent regarding relapse-free and overall survival (OS) [ 19 ]. Contraindications for breast-conserving surgery include the presence of diffuse microcalcifications (suspicious or malignant-appearing), disease that cannot be incorporated by local excision with satisfactory cosmetic result, and ATM (ataxia-telangiesctasia mutated) mutation (biallelic inactivation) [ 18 ].
The surgery to remove axillary lymph nodes is useful to determine cancerous cell spread and for therapeutic purposes. For instance, axillary lymph node dissection (ALND) can improve survival rated by removing remaining tumor cells. ALND used to be the goal standard for removing positive lymph nodes. However, clinical trials showed that sentinel lymph node biopsy (SLNB) had the same effect as ALND regarding disease-free survival (DFS) and OS [ 20 ]. Other clinical trials demonstrated that ALND was not necessary for all patients with positive lymph nodes. Moreover, most patients who receive radiation and systemic treatment after SLNB have negative lymph nodes as these treatments are sufficient in eliminating residual tumor cells [ 21 ].
2.2. Radiotherapy
Radiation therapy has been used to treat cancer since Röngten discovered the X-ray in 1895 [ 22 ]. High-energy radiations are applied to the whole breast or a portion of the breast (after breast-conservative surgery), chest wall (after mastectomy), and regional lymph nodes [ 23 ]. A meta-analysis showed that radiation following conservative surgery offered more benefits to patients with higher-risk BC while patients with small, low-grade tumors could forego radiation therapy [ 24 ]. Postmastectomy radiation to the chest wall in patients with positive lymph nodes is associated with decreased recurrence risk and BC mortality compared to patients with negative lymph nodes [ 25 ]. A radiation boost to the regional node radiation treatment can be incorporated after mastectomy for patients at higher risk for recurrence [ 26 ]. This additional radiation boost to regional nodes following mastectomy is associated with improved (DFS) but is also associated with an increase in radiation toxicities such as pneumonitis and lymphedema [ 27 ]. Radiotherapy can be administered concurrently with personalized therapy (anti-HER2 therapy or endocrine therapy).
As one of the major side effects of radiotherapy is cardiotoxicity, it is critical to minimize exposure to the heart and lungs [ 28 ]. Additional techniques can be used to reduce the radiation exposure to the heart, lungs, and normal tissue such as prone positioning, respiratory control, or intensity-modulated radiotherapy [ 29 ].
Advanced invasive BC can exhibit radiation therapy resistance [ 30 ]. The hypoxic tumor microenvironment, which lacks oxygen, leads to increased cell proliferation, apoptosis resistance, and radiotherapy resistance [ 31 ]. The major player of this resistance is the HIF-1α (hypoxia-inducible factor 1 alpha) protein [ 32 ]. Indeed, HIF-1α overexpression is caused by low oxygen levels within the microenvironment and promotes the maintenance of hypoxia by allowing tumoral cells to survive in a hypoxic microenvironment [ 33 , 34 , 35 ]. Cancer stem cells (CSC) could also have a role in radiation therapy resistance [ 36 ]. CSC can self-renew and initiate subpopulations of differential progeny, and a hypoxic microenvironment is ideal for CSC survival and proliferation [ 37 , 38 ].
Radiation therapy is used to treat all BC subtypes, but its implication is more important for TNBC, as there is no personalized therapy for this subtype. It has been shown that radiotherapy benefits TNBC patients both after conserving surgery and mastectomy [ 39 ].
2.3. Chemotherapy
BC chemotherapy comprises several families of cytotoxic drugs, including alkylating agents, antimetabolites and tubulin inhibitors [ 40 ]. Cyclophosphamide is a nitrogen mustard alkylating agent causing breakage of the DNA strands [ 41 ]. The mechanism of action for anthracyclines (doxorubicin, daunorubicin, epirubicin, and idarubicin) includes DNA intercalation, thereby inhibiting macromolecular biosynthesis [ 42 ]. Taxanes, including docetaxel and paclitaxel, bind to microtubules and prevent their disassembly, leading to cell cycle arrest and apoptosis [ 43 ].
Chemotherapy can be administered in the neoadjuvant or adjuvant setting and for metastatic BC treatment.
2.3.1. Neoadjuvant Chemotherapy (NAC)
Neoadjuvant chemotherapy was initially administered for non-metastatic but inoperable BC, defined as unreachable tumors [ 44 ]. Then, chemotherapy was used before the surgery for operable tumors to facilitate breast conservation [ 45 ].
Studies demonstrated that chemotherapy administered before surgery is as effective as administered after surgery [ 46 , 47 , 48 ]. The NSABP-B-18 trial compared the effects of doxorubicin and cyclophosphamide administered either postoperatively or preoperatively. This trial showed that NAC reduces the rate of axillary metastases in node-negative BC patients [ 48 ].
Some patients fail to achieve pathologic complete response after a full course of NAC. Unfortunately, there is no consensus regarding the treatment strategy to follow for patients with residual disease after surgery [ 49 , 50 ]. The BC subtype plays an important role in the response to NAC. Indeed, TNBC and HER2+ BC are more likely to be sensitive to chemotherapy. Hence, NAC is a good strategy to maximize pathologic complete response in these BC subtypes [ 45 ].
2.3.2. Adjuvant Chemotherapy
Adjuvant chemotherapy is administered to BC patients with lymph nodes metastases or a high risk of recurrence [ 51 ]. The standard chemotherapy treatment comprises an anthracycline and a taxane. The two most common regimens are cyclophosphamide and doxorubicin for four cycles followed by paclitaxel for four cycles. Then patients are given the previous combination of therapies followed by either weekly paclitaxel for 12 weeks, or docetaxel every 3 weeks for four cycles [ 52 , 53 ].
Like neoadjuvant therapy, patients with HR-negative BC receive more benefits from adjuvant therapy (i.e., reduction of BC recurrence and mortality) than HR+ BC patients [ 54 ]. However, for patients with HR+, node-negative BC associated with a high Oncotype recurrence score (≥31), calculated from the expression of 16 BC-related genes and 5 reference genes, adjuvant chemotherapy reduces the risk of recurrence [ 55 ]. The TAILORx clinical trial showed that HR+ BC patients with a low Oncotype recurrence score do not benefit from chemotherapy alone [ 56 ].
According to the molecular BC subtype, chemotherapy can be administered with targeted therapies. Patients with HR+ BC should receive endocrine therapy after chemotherapy is completed, and HER2+ BC patients should receive trastuzumab combined with chemotherapy [ 57 ]. For TNBC patients, front-line therapy includes a combination of taxane and anthracycline [ 58 ].
One of the major drawbacks of chemotherapy is its side effects. The early side effects (0–6 months of treatment) involve fatigue, alopecia, cytopenia (reduction in the number of normal blood cells), muscle pain, neurocognitive dysfunction, and chemo-induced peripheral neuropathy. The chronic or late side effects (after 6 months of treatment) include cardiomyopathy, second cancers, early menopause, sterility, and psychosocial impacts [ 59 ].
As mentioned previously in this review, chemotherapy is composed of taxanes, anthracyclines and cyclophosphamide. Each of these molecules can lead to resistance in BC patients [ 60 ].
One mechanism of resistance is by overexpressing p-glycoprotein, an ATP-binding cassette (ABC) family member, which confers resistance to anthracycline and taxanes [ 61 ]. Breast cancer resistance protein (BCRP), another ABC family member, induces resistance to anthracycline but not taxanes when overexpressed [ 62 ]. Microtubule alterations can also lead to taxane resistance. The overexpression of β-tubulin III induces paclitaxel resistance [ 63 ]. Moreover, mutations in microtubule-associated proteins (MAPs) affect microtubule dynamics and improve taxane resistance [ 64 ]. Multiple enzymes are known to be involved in the cyclophosphamide detoxification, leading to its resistance. For example, aldehyde dehydrogenase upregulation detoxifies aldophosphamide a type of cyclophosphamide, and mutations in glutathione S-transferases, enzymes involved in drug-metabolizing conjugation reactions, can also affect cyclophosphamide detoxification [ 65 , 66 ].
Surgery, radiotherapy, and chemotherapy are complementary strategies in the treatment of BC patients. However, they are not sufficient to effectively treat all BC molecular subtypes, as they do not have the same response to radiotherapy or chemotherapy. Thus, personalized therapies are essential in the process for BC treatment.
3. Current Personalized Treatments for Breast Cancer: Strengths and Weaknesses
The current strategies of treatment are principally based on the tumor progression and BC molecular subtypes in order to offer the most personalized treatment for BC patients. The algorithm of BC treatment is represented in Figure 2 .

Breast cancer treatment flow diagram. ( A ). Early-stage breast cancer. ( B ). Metastatic/advanced breast cancer. a Neoadjuvant chemotherapy for HR+ BC patients is not systematic. It is mainly administered to luminal B BC patients and/or elder BC patients. HR+: hormone receptors positive; HER2+: human epidermal growth factor receptor 2 positive; TNBC: triple-negative breast cancer; AIs: aromatase inhibitors; T-DM1: trastuzumab-emtansine.
3.1. Endocrine Therapy
Endocrine therapy is the main strategy to treat HR positive invasive BC. The purpose of this therapy is to target the ER directly (selective estrogen receptors modulators and degraders) or the estrogen synthesis (aromatase inhibitors) [ 67 ]. The most common types of endocrine therapy are selective estrogen receptor modulators (SERMs), selective modulators estrogen receptor degraders (SERDs), and aromatase inhibitors (AIs) [ 68 ]. Endocrine therapy mechanism of action and resistance are described in Figure 3 .

Endocrine therapy mechanisms of action and resistance. The left part of the figure shows the mechanism of endocrine therapy through aromatase inhibitors, tamoxifen, and fulvestrant. The right part of the figure describes the mechanisms of resistance to endocrine therapy through the epigenetic modifications, the increase of coactivators and cell cycle actors, and the activation of other signaling pathways. Estrogens can go through the plasma membrane by a. diffusion as they are small non-polar lipid soluble molecules; b. binding to membrane ER initiating the activation of Ras/Raf/MAPK and PI3K/Akt signaling pathways which are blocked by tamoxifen. 1: inhibition of ER dimerization; 2: blockage of nucleus access; 3: ER degradation. ER: estrogen receptor; AIB1: amplified in breast cancer 1; IGF-1R: insulin growth factor receptor 1; IGF: insulin growth factor; HER: human epidermal receptors; EGF: epidermal growth factor; HB-EGF: heparin-binding EGF-like growth factor; TGF-α: transforming growth factor alpha; MEK/MAPK: mitogen activated protein kinase; PI3K: phosphoinositide 3-kinase; mTOR: mammalian target of rapamycin; Me: methylation; Ac: acetylation.
3.1.1. Selective Estrogen Receptor Modulators (SERMs)
SERMs, such as tamoxifen, toremifene, bazedoxifene, and raloxifene, are antiestrogens that compete with estrogen by binding to the ER. This binding changes the conformation of the ER ligand-binding domain, and once ER is translocated to the nucleus, it blocks co-factor recruitment and subsequent genes transcription involved in cell cycle progression (cyclin D1), cell proliferation (like IGF-1), or cell migration (collagenase) [ 69 , 70 ].
The most used SERMs is tamoxifen, approved by the US Food and Drugs Administration (FDA) in 1977. It is an adjuvant therapy orally administered for 5 to 10 years according to tumor aggressivity. Tamoxifen adjuvant treatment reduces recurrence risk by 50% for the first 5 years and 30% for the next 5 years [ 71 ]. Tamoxifen is given to either premenopausal or postmenopausal patients. However, for high-risk premenopausal patients, adding ovarian suppression is more effective than tamoxifen alone [ 72 ]. Tamoxifen can also be administered as neoadjuvant treatment, especially for elderly BC patients [ 73 ]. However, studies have demonstrated no difference in OS for ER+ BC patients when neoadjuvant tamoxifen is compared to surgery [ 74 , 75 ].
Other SERMs have since been developed, such as toremifene approved by the FDA in 1997 [ 76 ]. Studies comparing the effect of toremifene and tamoxifen in premenopausal patients with ER+ advanced BC have shown that toremifene efficacy and safety are similar to tamoxifen [ 77 , 78 ]. Bazedoxifene and raloxifene are administered as prevention treatment to postmenopausal patients at high risk of developing invasive BC and for preventing osteoporosis [ 79 , 80 , 81 ].
The most frequent adverse events of SERMs are hot flushes, nausea, vomiting, vaginal bleeding/discharges, and increased risk of thromboembolic events [ 82 ]. Of note, about 40% of HR+ BC patients will develop resistance to SERMs [ 83 ]. SERMs resistance can occur by the loss of ER expression or functions. Epigenetic modifications such as hypermethylation of CpG islands or histone deacetylation can lead to transcriptional repression of ER [ 84 ]. Another potential mechanism for ER expression loss is the overpopulation of ER-negative cells in heterogenous ER+ tumors [ 85 ]. Mutations in the ligand-binding domain of ER gene ( ESR1 ) inhibit the binding of estrogen to the ER leading to the abolition of downstream signaling. Moreover, abnormal splicing can lead to truncated, nonfunctional ER protein [ 86 , 87 ]. Another explanation for SERMs resistance is the abnormal expression of ER coregulators [ 88 ]. Coregulators are very important in the ER pathway as they can increase or decrease ER activity depending on incoming signals [ 89 ]. The most studied coregulator involved in SERMs resistance is the AIB1 (Amplified in breast cancer 1) coactivator protein, often overexpressed in resistant breast tumors [ 90 ]. In particular, in ER+ cells that overexpress HER2, there is a crosstalk between HER2 and AIB1. HER2 induces phosphorylation of AIB1 leading to evasion and subsequent activation of the ER signaling pathway even though it is inhibited by SERMs [ 91 ]
3.1.2. Selective Estrogen Receptor Degraders (SERDs)
To counteract the large proportion of tamoxifen-resistant tumors, a new type of therapeutic agents with a different mechanism of action has been developed: SERDs. In contrast to SERMs, SERDs completely block the ER signaling pathway.
Fulvestrant is the main SERD administered. It was discovered by Wakeling and collaborators in 1987 and demonstrated pure anti-estrogen activity [ 92 ]. Fulvestrant binds to ER with a higher affinity than tamoxifen. Once it binds to the ER, it inhibits receptor dimerization and then blocks ER translocation to the nucleus leading to its degradation [ 93 , 94 , 95 ].
Fulvestrant is administered by intramuscular injections, and common adverse effects are nausea, pain, and headaches [ 96 ]. Fulvestrant is approved to treat postmenopausal and premenopausal patients with ovarian function suppression, with ER+ advanced or metastatic BC on prior endocrine therapy [ 97 ]. More recently (in 2017), fulvestrant was approved as first-line monotherapy for advanced ER+ breast cancer [ 98 ]. According to the 2021 NCCN guidelines, fulvestrant combined with endocrine therapy or CDK4/6 inhibitors is one of the preferred regimens for second-line therapy in ER+ advanced or metastatic BC [ 99 ]. The combination of fulvestrant with other endocrine therapies has not shown any advantages over fulvestrant used in monotherapy [ 100 , 101 ]. Clinical studies have shown benefits from fulvestrant when administered in higher doses to patients with ESR1 -mutated advanced BC [ 102 , 103 ]. Indeed, ESR1 mutations occur in nearly 20% of cases of ER+ BC [ 86 ].
However, fulvestrant can lead to resistance by different mechanisms. For example, by upregulating the PI3K (phosphatidylinositol 3-kinase), mTOR (mammalian target of rapamycin) and Ras-ERK (extracellular signal-regulated kinase) signaling pathways. PI3K/Akt/mTOR is a downstream signaling pathway of ER activation and plays an important role in antiestrogen therapy resistance [ 104 ]. PI3K pathway activation can occur independently of ER by binding to the epidermal growth factor (EGF) [ 105 ]. Moreover, it has been shown that Akt overexpression leads to fulvestrant resistance [ 106 ]. IGF-1R activation (insulin-like growth factor 1 receptor) may be another mechanism of resistance to fulvestrant. IGF-1R expression is involved in cell survival and promotes metastatic cell proliferation. The interaction between IGF-1R and ER initiates the activation of IGF-1R/MAPK (mitogen-activated protein kinase) and IGF-1R/PI3K signaling leading to antiestrogen resistance [ 107 ].
3.1.3. Aromatase Inhibitors (AIs)
Aromatase is a cytochrome P50 enzyme involved in the synthesis of androgens and estrogens [ 108 ]. Aromatase is found in the breast, uterus, and other estrogen-sensitive tissues in specific levels depending on menopausal status [ 109 , 110 ]. Aromatase expression is increased in breast tumors and associated with high estrogen levels. Therefore, high expression of aromatase promotes ER+ tumor proliferation [ 111 ].
Aromatase inhibitors (AIs) block aromatase enzyme activity, leading to the inhibition of estrogen synthesis. Current AIs can be classified into two categories: steroidal AIs and non-steroidal AIs [ 112 ]. Exemestane, a steroidal AI, has a steroid-like structure similar to androstenedione, which is the aromatase substrate. Exemestane irreversibly binds to the aromatase substrate-binding site leading to its inactivation [ 113 ]. Non-steroidal AIs include letrozole and anastrozole. They both bind non-covalently and competitively to the aromatase substrate-binding site and prevent the binding of androgens by saturating the binding site [ 112 ].
AIs are an oral treatment administered only to postmenopausal women (including patients that become postmenopausal following ovarian suppression). It is administered alone or in combination with tamoxifen as adjuvant therapy for HR+ BC patients [ 114 , 115 , 116 , 117 ]. AIs can be administered for 5 years or 2–3 years if followed by tamoxifen and up to 5 years after previous tamoxifen or AI treatment. For advanced or metastatic HR+ BC, AIs can be delivered as first-line and second-line therapy. Patients who become postmenopausal after or during the 5 years of tamoxifen treatment can receive AIs, such as letrozole, as an extended treatment strategy [ 118 , 119 ].
Estrogens have protective effects on the cardiovascular system by regulating serum lipids concentrations and increasing vasodilatation [ 120 ]. Hence, AIs might increase the risk of developing cardiovascular diseases by reducing estrogen levels in the blood [ 121 ]. Other adverse effects of AIs include hot flushes, vaginal dryness, fatigue, and osteoporosis [ 122 ]. ER+ tumors can acquire AI resistance. Some mechanisms of AI resistance are similar to those conferring SERM or SERD resistance, such as ESR1 mutations, epigenetic modifications, and PI3K pathway upregulation [ 123 ]. However, other mechanisms of action are involved in AI resistance. For example, the upregulation of cyclin-dependent kinase 4 (CDK4) or cyclin-dependent kinase 6-retinoblastoma (CDK6-RB) pathways can lead to an estrogen-dependent cell progression [ 124 ]. Clinical studies have shown better benefits from CDK4-CDK6 inhibitors in combination with AIs compared to AIs alone [ 125 , 126 ].
Endocrine therapy is a well-established treatment strategy for HR+ tumors. Over the last decades, SERMs, SERDs and AIs have been proven as safe and effective personalized therapy for HR+ BC patients, and these therapeutic strategies have shown continued improvements. However, the main drawback of endocrine therapy is acquired or de novo resistance [ 127 ]. Hence, it is essential to develop new therapeutic agents that use different modes of action to treat HR+ BC more efficiently.
3.2. Anti-HER2 Therapy
The overexpression of HER2 is associated with worse survival outcome compared to HR-positive/HER2-negative BC [ 128 , 129 ]. Hence, therapies targeting HER2 are essential to treat HER2-positive BC. The current anti-HER2 therapies comprise antibodies that target specific HER2 epitopes, tyrosine kinase inhibitors (TKIs) and, more recently, antibody-drug conjugates (ADCs) [ 130 ]. Anti-HER2 mechanisms of action and resistance are described in Figure 4 .

Anti-HER2 therapy mechanisms of action and resistance. The left part of the figure describes the mechanism of action of anti-HER2 therapy through anti-HER2 antibody (trastuzumab and pertuzumab), tyrosine kinase inhibitors (lapatinib and nerotinib), and trastuzumab-emtansine (T-DM1). The right part of the figure describes the mechanism of resistance to anti-HER2 therapy through constitutive active p95 HER2 fragment, activation of other signaling pathways, and rapid recycling of HER2-T-DM1. ADCC: antibody-dependent cellular cytotoxicity; HER2: human epidermal growth factor receptor 2; EGF: epidermal growth factor, HB-EGF: heparin-binding EGF-like growth factor; TGF-α: transforming growth factor alpha; T-DM1: trastuzumab-emtansine; IGF-1R: insulin growth factor receptor 1; IGF: insulin growth factor; HGF: hepatocyte growth factor; MEK/MAPK: mitogen activated protein kinase; PI3K: phosphoinositide 3-kinase; mTOR: mammalian target of rapamycin; PTEN: phosphatase and tensin homolog.
3.2.1. Antibodies Targeting HER2
The first developed HER2-targeted antibody, trastuzumab (Herceptin), was approved by the FDA in 1998 [ 131 , 132 ]. Trastuzumab targets subdomain IV of the HER2 extracellular domain. However, the mechanism underlying trastuzumab’s therapeutic effect is not well understood. Multiple studies have reported hypotheses to explain trastuzumab’s mechanism of action. For instance, trastuzumab may inhibit the formation of the HER2-HER3 heterodimer, known to be the most oncogenic pair in the HER family [ 133 ]. It could also inhibit the formation of the active p95 HER2 fragment by preventing cleavage of the HER2 extracellular domain [ 134 ]. An indirect antitumor effect could be activating antibody-dependent cellular cytotoxicity (ADCC) by engaging with Fc receptors on immune effector cells [ 135 ].
Initially, trastuzumab was approved for administration in metastatic HER2+ BC, increasing the clinical benefits of first-line chemotherapy [ 132 ]. Trastuzumab has also demonstrated its efficacy and safety in early-stage HER2+ BC. It is given as neoadjuvant or adjuvant therapy in combination with other anti-HER2 treatments and/or with chemotherapy [ 136 , 137 , 138 ]. The recommended dose for intravenous trastuzumab is 4 mg/kg followed by 2 mg/kg weekly for 1 year in the adjuvant setting for early-stage HER2+ BC and until disease-free progression for metastatic HER2+ BC [ 139 ].
Pertuzumab (Perjeta) is another antibody that targets the HER2 extracellular domain but binds to subdomain II. Once it binds to HER2, pertuzumab prevents HER2 heterodimerization with other HER family members, leading to inhibition of downstream signaling pathways [ 140 ]. Like trastuzumab, one of pertuzumab’s indirect antitumor effects is activating the ADCC pathway [ 141 ]. Multiple clinical trials have shown that pertuzumab, combined with trastuzumab and chemotherapy, improved OS in metastatic HER2+ BC patients compared to trastuzumab and chemotherapy alone [ 142 , 143 , 144 , 145 ]. The benefits of pertuzumab have also been shown in early-stage HER2+ BC, as pertuzumab can be used in the neoadjuvant or adjuvant setting combined with trastuzumab and chemotherapy [ 146 , 147 , 148 , 149 ]. Pertuzumab is administered in fixed doses of 840 mg followed by 420 mg every three weeks [ 150 ].
Despite the major positive impacts of trastuzumab and pertuzumab in HER2+ BC treatment, only one-third of BC patients with HER2+ tumors benefit from anti-HER2 antibodies [ 151 ]. One of the hypotheses explaining this resistance concerns structural modifications of HER2, which hinder antibody binding. Alternative splicing can lead to a truncated isoform lacking the extracellular domain, thus forming a constitutive active p95 HER2 fragment [ 152 ]. The overexpression of other tyrosine kinases can bypass the signaling pathways mediated by HER2. It has been shown that cells overexpressing IGF-1R overcome cell cycle arrest by increasing CDK2 kinase activity [ 153 ]. Moreover, the overexpression of c-Met (a hepatic growth factor receptor) synergizes with HER2 signaling to confer resistance to anti-HER2 antibodies. Indeed, c-Met physically interacts with HER2, and c-Met depletion renders cells more sensitive to trastuzumab [ 154 , 155 ]. Another hypothesis for anti-HER2 antibody resistance is intracellular alterations in HER2 downstream signaling pathways. HER2 activates PI3K/Akt signaling, and PTEN (phosphatase and tensin homolog) is a well-known inhibitor of this pathway [ 156 ]. Tumors with a loss of PTEN function and/or constitutive activation of PI3K due to alteration mutations achieve worse therapeutic outcomes with trastuzumab [ 157 , 158 ].
3.2.2. Tyrosine Kinase Inhibitors (TKIs)
Since tumors may be resistant to anti-HER2 antibodies, new approaches have been developed. TKIs such as lapatinib, neratinib, or pyrotinib are small molecules that compete with ATP at the catalytic domain of the receptor to prevent tyrosine phosphorylation and HER2 downstream signaling [ 159 ].
Lapatinib is a dual EGFR/HER2 TKI blocking both HER1 and HER2 activation [ 160 ]. In metastatic BC, clinical trials have shown that lapatinib offers more benefits than chemotherapy alone [ 161 , 162 , 163 ]. The effects of lapatinib in the neoadjuvant/adjuvant setting have also been evaluated. As a neoadjuvant treatment, lapatinib plus trastuzumab combined with chemotherapy were more efficient than chemotherapy combined with lapatinib or trastuzumab alone [ 164 ]. Lapatinib as adjuvant treatment showed modest antitumor efficacy compared to placebo in a randomized, controlled, and multicenter phase III trial (TEACH) [ 165 ]. For luminal B (ER/PR+; HER2+) advanced or metastatic BC, lapatinib can be administered in combination with AIs.
Neratinib is an irreversible TKI targeting HER1, HER2, and HER4 [ 166 ]. The FDA approved Neratinib in 2017 as an extended adjuvant treatment for patients with HER2+ early-stage BC and combination with trastuzumab in the adjuvant setting [ 167 , 168 ]. Neratinib can be delivered in combination with capecitabine as a third-line and beyond therapy for HER2+ advanced or metastatic BC.
More recently, pyrotinib, a new generation TKI targeting HER1, HER2 and HER4, has been developed [ 169 ]. Pyrotinib is still under clinical trials to prove its efficacy and safety [ 170 ]. However, in 2018, the Chinese State Drug Administration approved pyrotinib in combination with or after chemotherapy treatment for patients with HER2+ advanced or metastatic BC [ 171 ].
Despite the recent development of TKI treatments, patients can still exhibit intrinsic or acquired resistance to these agents. Three mechanisms of action have been hypothesized: (1) activation of compensatory pathways, (2) HER2 tyrosine kinase domain mutation, and (3) other gene amplification [ 172 ]. For instance, activation of the PI3K/Akt pathway and FOXO3A (Forkhead transcription factor) by the upregulation of HER3 can lead to lapatinib resistance [ 173 ]. Other tyrosine kinases can be involved, such as c-Met, also known to be implicated in trastuzumab resistance. C-Met induces the activation of PI3K/Akt signaling in lapatinib-resistant BC [ 174 ]. Mutations in the HER2 tyrosine kinase domain lead to the constitutive activation of HER2 by substituting individual amino acids [ 175 ]. Lastly, it has been shown that the amplification of the NIBP (TRAPPC9, Trafficking Protein Particle Complex 9) gene occurs in HER2+ lapatinib-resistant tumors. The inhibition of NIBP makes resistant cells sensitive to lapatinib [ 176 ].
3.2.3. Trastuzumab-Emtansine (T-DM1)
Trastuzumab-emtansine (T-DM1) is an antibody-drug conjugate (ADC), which is a conjugate of trastuzumab and a cytotoxic molecule, DM1, a derivative of maytansine [ 177 ]. T-DM1 binds to HER2 with the trastuzumab part. The formed complex is then internalized for degradation, releasing DM1 metabolites into the cytoplasm. DM1 then inhibits microtubule assembly causing cell death [ 178 , 179 ]. Thus, T-DM1 consists of the antitumor effects of trastuzumab and those associated with DM1 metabolites [ 180 ].
Three phase III clinical trials have evaluated the safety and efficacy of T-DM1 for HER2+ metastatic BC [ 181 , 182 , 183 ]. They have shown that T-DM1 improves OS and DFS of HER2+ metastatic BC patients compared to lapatinib in combination with trastuzumab or chemotherapy [ 181 , 182 , 183 ]. T-DM1 as neoadjuvant treatment has less efficacy compared with trastuzumab or pertuzumab with chemotherapy [ 146 ]. This suggests that T-DM1 should not be administered as a neoadjuvant treatment but as a first-line or second-line therapy for HER2+ metastatic BC. The 2021 NCCN guidelines recommend using T-DM1 as second-line therapy for HER2+ advanced or metastatic BC [ 99 ].
The mechanism of action of T-DM1 involves those related to trastuzumab and DM1, so the observed resistance to T-DM1 could come from interference in one or both constituents [ 184 ]. The mechanism of T-DM1 resistance has been hypothesized to involve (1) the loss of trastuzumab mediated activity, (2) the dysfunctional intracellular trafficking of T-DM1, and (3) the impairment of DM1 mediated cytotoxicity [ 185 ].
As previously described in this review, the reduction of trastuzumab effects can occur by reduced HER2 expression, dysregulation of PI3K signaling, or the activation of alternative tyrosine kinase receptors [ 153 , 154 , 156 , 186 ]. The alteration of HER2-T-DM1 complex internalization can go through a rapid recycling of HER2 to the plasma membrane leading to the inhibition of DM1 metabolism released into the cytoplasm [ 187 ]. The internalization of the HER2-T-DM1 complex occurs through the formation of lysosomes. These vesicles enclose lysosomal enzymes involved in HER2-T-DM1 complex degradation. In T-DM1-resistant tumors, the level of lysosomal enzymes is inhibited [ 188 , 189 ]. T-DM1 also disrupts microtubule assembly causing incomplete spindle formation resulting in mitotic catastrophe and apoptosis [ 190 ]. Cells resistant to T-DM1 can avoid this process by reducing the induction of Cyclin-B1, an enzyme essential for cell cycle progression [ 191 ].
HER2+ BC are aggressive and associated with poor prognosis and metastasis, and recurrences. Anti-HER2 therapy has greatly improved the management of HER2+ BC. However, 25% of early-stage HER2+ BC patients will have a recurrence after the initial anti-HER2 treatment [ 192 ]. The emergence of new therapeutic agents specific for HER2+ BC provides new hope to treat this particularly aggressive BC subtype.
3.3. PARP Inhibitors
The prevalence of BRCA (Breast Cancer genes) mutations in TNBC patients is approximately 20% [ 193 ]. BRCA1 and BRCA2 are proteins involved in the DNA damage response to repair DNA lesions [ 194 ]. Mutations in BRCA 1/2 genes are associated with an increased risk of breast and ovarian cancers [ 195 ].
PARP (poly-(ADP-ribose) polymerase protein) proteins are also involved in the DNA damage response as they recruit DNA repair proteins, such as BRCA1 and BRCA2, to the damage site [ 196 ]. PARP inhibitors (PARPi) were developed to inhibit DNA repair in BRCA-mutated BC since cells defective in BRCA functions cannot repair DNA damage when PARP is inhibited [ 197 ]. The principal PARPis currently in clinical development are olaparib, talazoparib, veliparib, and rucaparib [ 198 ]. PARP inhibitors mechanisms of action and resistance are described in Figure 5 .

PARP inhibitors mechanisms of action and resistance. The left part of the figure describes the mechanism of PARP inhibitors in the context of BRCA mutated breast cancer. The right part of the figure describes the mechanism of resistance to PARP inhibitors through secondary intragenic mutations restoring BRCA proteins functions and the decrease of the recruitment of nucleases (MUS81 or MRE11) to protect the replication fork. PARP: poly-(ADP-ribose) polymerase protein; PARPi: PARP inhibitors; BRCA: breast cancer protein; MUS81: methyl methanesulfonate ultraviolet sensitive gene clone 81; MRE11: meiotic recombination 11.
3.3.1. Olaparib
Olaparib is the first FDA-approved PARPi for the treatment of BRCA -mutated BC [ 199 ]. Phase I and phase II trials evaluating the effects of olaparib monotherapy in germline BRCA-mutated (gBRCAm) BC proved its clinical benefits by improving progression-free survival (PFS) [ 200 , 201 , 202 , 203 ]. The phase III, randomized, open-label, OlympiAD trial compared olaparib monotherapy vs. standard chemotherapy in patients with BRCA mutated HER2-negative BC. This trial showed that olaparib has better efficacy and tolerability than standard chemotherapy for this group of patients [ 204 ]. Olaparib has also been tested in combination with chemotherapy. A phase I study evaluated the effects of olaparib in combination with paclitaxel in unselected TNBC patients [ 205 ]. The overall response rate (ORR) for these patients was 37%. Two phase I studies evaluating the combination of olaparib with cisplatin or carboplatin in gBRCAm BC patients showed improved ORR [ 206 , 207 ].
3.3.2. Talazoparib
Talazoparib has the highest PARP-DNA trapping efficiency among the PARPis [ 208 ]. A phase II trial testing the effects of talazoparib on gBRCAm early-stage BC showed decreased tumor size in all patients included [ 209 ]. Other phase I and II trials with gBRCAm BC patients receiving talazoparib confirmed the efficiency of this PARPi [ 210 , 211 ]. The EMBRACA study, an open-label phase III trial, compared talazoparib monotherapy to chemotherapy in gBRCAm, HER2-negative BC patients [ 212 ]. PFS and ORR were improved with talazoparib compared to chemotherapy alone.
3.3.3. Veliparib
Veliparib has been mostly evaluated in combination with chemotherapy. For example, the phase II multicenter I-SPY2 trial tested the combination of veliparib and neoadjuvant chemotherapy in unselected TNBC patients [ 213 ]. The predicted complete response rate (pCR) was 51% with veliparib and chemotherapy vs. 26% in the control arm (chemotherapy alone). The phase II BROCADE study evaluated the combination of veliparib with carboplatin and paclitaxel in gBRCAm BC patients [ 214 ]. The ORR was improved with the combination of veliparib and chemotherapy compared to chemotherapy alone. Lastly, the phase III BRIGHTNESS study evaluated the addition of veliparib to carboplatin in the standard neoadjuvant chemotherapy setting [ 211 ]. The addition of veliparib showed no further benefit to chemotherapy.
3.3.4. Rucaparib
Rucaparib is the second PARPi that has been FDA approved for gBRCAm BC patients [ 215 ]. Intravenous rucaparib was tested in a phase II trial of gBRCAm BC patients [ 216 ]. Stable disease, meaning no tumor development, was reported in 44% of patients. Rucaparib was also tested in combination with chemotherapy in unselected TNBC patients [ 217 ]. This phase I study showed that rucaparib could be safely used in combination with chemotherapy. The phase II, a randomized BRE09-146 trial, evaluated rucaparib in combination with cisplatin vs. cisplatin alone in gBRCAm patients with residual disease following neoadjuvant therapy [ 218 ]. DFS was similar in the two arms, as low-dose rucaparib did not affect cisplatin toxicity. However, the rucaparib dose may not have been sufficient to inhibit PARP activity.
Tumor cells can become resistant to PARPi by different mechanisms [ 219 ].
First, secondary intragenic mutations that restore BRCA proteins functions can lead to PARPi resistance [ 220 ]. These genetic events can lead to the expression of nearly full-length proteins or full-length wild-type proteins with complete restored functions [ 221 ]. This has been reported mostly in ovarian cancer patients, and it has also been demonstrated in BC cell line models [ 222 ]. Tumor cells with missense mutations conserving the N-terminal and C-terminal domains of BRCA proteins also lead to poor PARPi response [ 223 ]. Another mechanism of action leading to PARPi resistance is decreased expression of PARP enzymes. Indeed, tumor cells with low PARP1 expression acquire resistance to veliparib [ 224 ].
In addition, tumor cells can find alternative mechanisms to protect the replication fork. It has been shown that PARPi-resistant cells can reduce the recruitment of the MRE11 (meiotic recombination 11) nuclease to the damage site, leading to the protection of the fork by blocking its access [ 225 ]. Another study has shown that BRCA2 -mutated tumors acquired PARPi resistance by reducing the recruitment of the MUS81 (methyl methanesulfonate ultraviolet sensitive gene clone 81) nuclease to protect the replication fork [ 226 ].
Chemotherapy has been the pioneer treatment strategy for TNBC for decades. The development of PARPis has been a major improvement in the treatment of TNBC and, more specifically, gBRCAm TNBC, as they have shown more benefits over chemotherapy [ 227 ]. However, TNBC is a heterogenous BC subtype, and PARPis cannot treat all TNBCs as it is administered only for gBRCAm TNBC [ 228 ]. Therefore, it is necessary to develop specific targeted therapies to treat each TNBC subtype.
4. New Strategies and Challenges for Breast Cancer Treatment
4.1. emerging therapies for hr-positive breast cancer.
As mentioned in Section 3.1 , the major mechanisms of action of current endocrine therapy resistance occur via (1) the mTOR/PI3K/Akt signaling pathway and (2) the actors of the cell cycle progression CDK4/6. Therefore, emerging therapies for HR+ BC mainly target these pathways to bypass estrogen-independent cell survival [ 229 ]. The most recent completed clinical trials on emerging therapies for HR+ BC are presented in Table 1 .
Most recent completed clinical trial on emerging therapies for HR-positive breast cancer.
HR+: hormone receptors positive; HER2-: human epidermal growth factor receptor 2 negative; MBC: metastatic breast cancer; BC: breast cancer; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; pCR: pathologic complete response; HR: hazard ratio.
4.1.1. mTOR/PI3K/AKT Pathway Inhibitors
The mTOR/PI3K/Akt pathway inhibitors can be divided into different categories according to the target in the pathway. Specific inhibitors have been developed to target all or specific isoforms of PI3K, mTORC1 and Akt [ 251 ].
Pan-Pi3K Inhibitors
Pan-PI3K inhibitors target all PI3K isoforms resulting in significant off-target effects. The main pan-PI3K inhibitors are buparlisib and pictilisib [ 252 ]. Multiple clinical trials have tested the effects of pan-PI3K inhibitors in luminal BC.
The phase III randomized double-blinded BELLE-2 trial compared buparlisib combined with fulvestrant, to fulvestrant monotherapy in luminal A advanced or metastatic BC patients [ 230 ]. The results of this trial showed a modest improvement in PFS when buparlisib was added to fulvestrant. Another phase III clinical trial (BELLE-3) studied the effects of buparlisib plus fulvestrant in luminal A advanced or metastatic BC patients with no benefits from endocrine therapy [ 231 ]. Though PFS was significantly improved with buparlisib, there were severe adverse effects such as hyperglycemia, dyspnea, or pleural effusion. Lastly, the phase II/III BELLE-4 clinical trial evaluated buparlisib plus paclitaxel in HER2-negative locally advanced or metastatic BC patients [ 232 ]. The addition of buparlisib to paclitaxel did not improve PFS in these patients. Thus, further studies on buparlisib in HR+ BC were not conducted. The phase II randomized, double-blinded FERGI clinical trial analyzed the effects of pictilisib plus fulvestrant in luminal A BC patients resistant to AI [ 233 ]. The addition of pictilisib to fulvestrant did not improve PFS. Moreover, severe adverse effects occurred when the dose of pictilisib was increased. These results were confirmed for pictilisib plus paclitaxel, as the phase II PEGGY study showed no benefit from pictilisib in PI3K-mutated HER2-negative BC patients [ 234 ].
Hence, pan-PI3K inhibitors are not optimal to treat HR+ BC due to their toxicity and lack of efficacy.
Isoform-Specific PI3K Inhibitors
To sort out issues related to off-target effects and toxicities with pan-PI3K inhibitors, isoform-specific PI3K inhibitors have been developed. These isoform-specific PI3K inhibitors can specifically target the PI3K p110α, p110β, p110δ, and p110γ isoforms [ 252 ]. Multiple clinical trials have tested the effects of isoform-specific PI3K inhibitors.
PI3K p110α is the most commonly mutated isoform in BC [ 253 ]. Alpelisib is the first FDA-approved PI3K p110α isoform inhibitor. A phase Ib clinical trial tested the effects of alpelisib and letrozole in patients with ER+ metastatic BC refractory to endocrine therapy [ 235 ]. The clinical benefit of the alpselisib and letrozole combination was higher for patients with PI3K-mutated BC, but clinical activity was still observed in patients with non-mutated tumors. The phase III randomized SOLAR-1 clinical trial compared the effects of alpelisib plus fulvestrant to fulvestrant alone in luminal A advanced BC patients who received no benefits from prior endocrine therapy [ 236 ]. The addition of alpelisib improved PFS for patients with PI3K-mutated BC.
Taselisib targets the PI3K p110α, p110γ and p110δ isoforms [ 254 ]. Taselisib was tested in the SANDPIPER study, a phase III randomized clinical trial, in combination with fulvestrant in patients with ER+ metastatic BC resistant to AIs [ 238 ]. Although the addition of taselisib slightly improved PFS, further clinical trials with taselisib were interrupted since high rates of severe adverse events were detected.
mTORC1 Inhibitors
mTORC1 inhibitors, such as everolimus, block the mTORC1 dependent phosphorylation of s6k1 [ 255 ]. The BOLERO-2 phase III randomized clinical trial investigated the effects of exemestane with or without everolimus in AI-resistant ER+ metastatic BC patients [ 240 ]. The combination of everolimus and exemestane improved PFS. The TAMRAD phase II randomized open-label study compared the effects of tamoxifen with or without everolimus in AI-resistant luminal A BC patients [ 241 ]. This study showed an improvement in overall survival (OS) when everolimus was given in combination with tamoxifen. The findings of these two clinical trials led to FDA approval of everolimus. More recently, the PrE0102 phase II randomized clinical trial showed that the addition of everolimus to fulvestrant improved PFS of patients with AI-resistant ER+ BC compared to fulvestrant alone [ 242 ].
Akt Inhibitors
Akt inhibitors target all Akt isoforms as Akt 1, 2, and 3 isoforms share very similar structures [ 256 ]. Capivasertib is the principal Akt inhibitor under investigation in different clinical trials. The FAKTION phase II multi-centered randomized clinical trial compared the effects of capivasertib plus fulvestrant to fulvestrant plus placebo in AI-resistant luminal A advanced BC patients [ 243 ]. PFS was significantly improved with the combination of capivasertib and fulvestrant in comparison with the placebo arm.
The AKT1 E17K activating mutation is the most common in Akt and occurs in approximately 7% of ER+ metastatic BC. This mutation in the Akt lipid-binding pocket leads to constitutive Akt activation by modifying its localization to the membrane [ 257 ]. A phase I study analyzed the effects of capivasertib alone or in combination with fulvestrant in a cohort of patients with AKT1 E17K mutation ER+ metastatic BC [ 244 ]. Capivasertib, in combination with fulvestrant, demonstrated clinically meaningful activity and better tolerability compared to capivasertib alone.
4.1.2. CDK4/6 Inhibitors
There are currently three CDK4/6 inhibitors approved to treat HR+/HER2- metastatic BC: palbociclib, ribociclib, and abemaciclib. They can be administered as first-line treatment combined with AIs or as second-line treatment combined with fulvestrant [ 258 ].
First-Line Treatment
Palbociclib, a highly selective CDK4/6 inhibitor, is the first FDA-approved CDK4/6 inhibitor as first-line treatment combined with AIs for metastatic or advanced HR+ BC patients [ 259 ].
PALOMA-1 is an open-label, randomized phase II study that evaluated the effects of palbociclib in combination with letrozole vs. letrozole alone as first-line treatment for HR+ advanced BC patients [ 126 ]. The addition of palbociclib to letrozole significantly improved PFS in HR+ BC patients. A phase III study was performed (PALOMA-2) to confirm these findings and expand the efficacy and safety of palbociclib, [ 245 ]. This double-blinded clinical trial tested the combination of palbociclib and letrozole in postmenopausal BC patients without prior systemic therapy for advanced BC. The addition of palbociclib to letrozole significantly improved PFS and ORR.
Ribociclib is the second FDA-approved CDK4/6 inhibitor for first-line treatment in postmenopausal advanced BC patients in combination with AIs [ 260 ]. The phase III MONALEESA-2 clinical trial results showed improved PFS and ORR with the combination of ribociclib and letrozole in HR+ metastatic BC patients. The clinical benefits and manageable tolerability observed with ribociclib and letrozole are maintained with longer follow-up compared to letrozole alone [ 247 ].
Abemaciclib has been tested in the phase III randomized double-blinded MONARCH-3 study [ 250 ]. HR+ advanced BC patients with no prior systemic therapy received abemaciclib plus anastrozole or letrozole or AIs plus placebo in the control arm. PFS and ORR were significantly improved with the combination of abemaciclib and AIs.
Second-Line Treatment
As second-line treatment, palbociclib can be given in combination with fulvestrant in advanced or metastatic BC patients with disease progression after endocrine therapy [ 261 ]. This was confirmed in the phase III multi-centered randomized double-blinded PALOMA-3 trial [ 246 ]. BC patients who received palbociclib plus fulvestrant had significantly longer PFS compared to fulvestrant plus placebo.
The phase III MONALEESA-3 study tested the effects of ribociclib plus fulvestrant in patients with HR+ advanced BC who received prior endocrine therapy in the advanced setting [ 248 ]. The PFS and ORR were significantly improved when ribociclib was added to fulvestrant. Thus, ribociclib plus fulvestrant can be considered as second-line treatment for these BC patients.
Abemaciclib has been recently approved in combination with fulvestrant for HR+ advanced or metastatic BC patients with disease progression after endocrine therapy. This was based on the results of the phase III, double-blinded MONARCH 2 study [ 249 ]. The combination of abemaciclib and fulvestrant demonstrated a significant improvement of PFS and ORR compared to fulvestrant plus placebo in HR+ metastatic BC patients who experienced relapse or progression after prior endocrine therapy.
mTOR/PI3K/Akt inhibitors and CDK4/6 inhibitors show great promise for advanced HR+ BC resistant to endocrine therapy. To leverage the potential of these two types of therapies, some preclinical studies have evaluated a triple therapy combination including PI3K inhibitors, CDK4/6 inhibitors, and endocrine therapy (see the summarized table at the end of the manuscript) [ 262 ].
4.2. New Strategic Therapies for HER2-Positive Breast Cancer
As mentioned in Section 3.2 , HER2+ BC is currently treated with specific HER2 targeting antibodies or tyrosine kinase inhibitors (TKIs), and more recently, with TDM-1, an antibody-drug conjugate. These treatments have greatly improved HER2+ BC survival. However, 25% of HER2+ BC patients will still develop resistance to anti-HER2 treatment. Hence, new therapeutic strategies are emerging, such as new antibodies targeting HER2, new TKIs, vaccines, and PI3K/mTOR and CDK4/6 inhibitors [ 263 ]. The most recent completed clinical trials on new strategies for HER2+ BC treatment are gathered in Table 2 .
Most recent completed clinical trials on emerging therapies for HER2+ breast cancer.
HER2+: human epidermal growth factor receptor 2 positive; ER+: estrogen receptor positive; HLA2/3: human leucocyte antigen 2/3; MBC: metastatic breast cancer; BC: breast cancer; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; DFS: disease-free survival OS: overall survival GM-CSF: granulocyte macrophage colony-stimulated factor; HR: hazard ratio.
4.2.1. New Antibodies
Novel types of antibodies have been developed to target HER2+ BC more efficiently. They can be divided into three categories: antibody-drug conjugates (ADC), modified antibodies, and bispecific antibodies.
Antibody-Drug Conjugates (ADC)
ADCs are the combination of a specific monoclonal antibody and a cytotoxic drug that is released in the antigen-expressing cells [ 280 ]. The most common ADC is TDM-1, and the promising results with TDM-1 have led to the development of new ADCs.
Trastuzumab-deruxtecan (DS-8201a) is a HER2-targeting antibody (trastuzumab) linked to a DNA topoisomerase I inhibitor (deruxtecan) [ 281 ]. A phase I study demonstrated that DS-8201a had antitumor activity even with HER2 low-expressing tumors [ 282 ]. These results led to phase II and phase III clinical trials. The DESTINY-Breast01 clinical trial is an open-labeled, single-group, multicentered phase II study [ 264 ] was evaluated in HER2+ metastatic BC patients who received prior TDM-1 treatment. DS-8201a showed durable antitumor activity for these patients. Two phase III clinical trials are currently evaluating DS-8201a. DESTINY-Breast02 (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03523585","term_id":"NCT03523585"}} NCT03523585 ) is comparing DS-8201a to standard treatment (lapatinib or trastuzumab) in HER2+ metastatic BC patients previously treated with TDM-1. The DESTINY-Breast03 (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03529110","term_id":"NCT03529110"}} NCT03529110 ) trial is evaluating the effects of DS-8201a vs. TDM-1 in HER2+ metastatic BC patients with prior trastuzumab and taxane treatment.
Trastuzumab-duocarmycin (SYD985) is a HER-2 targeting antibody (trastuzumab) conjugate with a cleavable linker-duocarmycin payload that causes irreversible alkylation of the DNA in tumor cells leading to cell death [ 283 ]. A dose-escalation phase I study evaluated the effects of SYD85 in BC patients with variable HER2 status and refractory to standard cancer treatment [ 284 ]. Trastuzumab-duocarmycin showed clinical activity in heavily pretreated HER2+ metastatic BC patients, including TDM-1 resistant and HER2-low BC patients. After these promising results, a phase I expansion cohort study was performed on the same type of patients (heavily pretreated HER2+ or HER2-low BC patients) [ 265 ]. This study confirmed previous results on the efficacy of STD985. A phase III clinical trial (TULIP-ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03262935","term_id":"NCT03262935"}} NCT03262935 ) is ongoing to compare SYD985 to the treatment chosen by the physician in HER2+ metastatic BC patients. Other ADCs are under clinical trials to test their safety and activity for HER2+ advanced BC patients. RC48 is an anti-HER2 antibody conjugated with monomethyl auristatin E that demonstrated promising efficacy and a manageable safety profile in an open-labeled, multicentered phase II study (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT02881138","term_id":"NCT02881138"}} NCT02881138 ) [ 248 ]. PF06804103 conjugates an anti-HER2 monoclonal antibody and the cytotoxic agent, Aur0101. In a phase I study (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03284723","term_id":"NCT03284723"}} NCT03284723 ), PF06804103 showed manageable toxicity and promising antitumor activity [ 249 ]. XMT1522 showed encouraging results in a dose-escalation phase I study (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT02952729","term_id":"NCT02952729"}} NCT02952729 ) [ 250 ]. MEDI4276, which targets two different HER2 epitopes and is linked to a microtubule inhibitor, showed promising clinical activity in a phase I study (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT02576548","term_id":"NCT02576548"}} NCT02576548 ) [ 254 ] (see the summarized table at the end of the manuscript).
Chimeric Antibody
Margetuxumab (MGAH22) is a human/mouse chimeric IgG1 targeting HER2 monoclonal antibody. It is based on trastuzumab as it binds to the same epitope (subdomain IV or HER2 extracellular domain) but with an enhanced Fcγ domain. The substitution of five amino acids into the IgG1 Fc domain increases CD16A affinity, a receptor found on macrophages and natural-killer cells, and decreases CD32B affinity, leading to increased antibody-dependent cell-mediated cytotoxicity (ADCC) [ 285 ]. A phase I study evaluated margetuximab toxicity and tumor activity on HER2+ BC patients for whom no standard treatment was available [ 266 ]. This study showed promising single-agent activity of margetuximab as well as good tolerability. The phase III randomized open-labeled SOPHIA clinical trial (ClinicalTrials.gov Identifier: {"type":"clinical-trial","attrs":{"text":"NCT02492711","term_id":"NCT02492711"}} NCT02492711 ) compared margetuximab plus chemotherapy vs. trastuzumab plus chemotherapy in pretreated HER2+ advanced BC patients [ 286 ]. The combination of margetuximab and chemotherapy significantly improved the PFS of patients compared to trastuzumab plus chemotherapy. This study is still under investigation to collect data on OS (see the summarized table at the end of the manuscript).
Bispecific Antibodies
Bispecific antibodies (BsAbs) can target two different epitopes in the same or different receptors by combining the functionality of two monoclonal antibodies [ 287 ]. MCLA-128 targets both HER2 and HER3 and have an enhanced ADCC activity [ 288 ]. A phase I/II study evaluated the safety, tolerability, and antitumor activity of MCLA-128 in patients with pretreated HER2+ metastatic BC.
Preliminary results showed encouraging clinical benefits of MCLA-128. An open-labeled, multicentered phase II study (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03321981","term_id":"NCT03321981"}} NCT03321981 ) is ongoing to evaluate the effects of MCLA-128 in combination with trastuzumab and chemotherapy in HER2+ metastatic BC patients.
ZW25 is a BsAb biparatopic that binds the IV and II subdomains of the HER2 extracellular domain, the binding epitopes of trastuzumab and pertuzumab, respectively [ 289 ]. The efficacy of ZW25 was evaluated in a phase I study given alone or in combination with chemotherapy in patients with advanced or metastatic HER2+ BC. The results of this study showed promising antitumor activity, and no-dose limiting was observed.
T-cell bispecific antibodies (TCBs) are another type of BsAbs recently developed. TCBs target the CD3-chain of the T-cell receptor and tumor-specific antigens, resulting in lymphocyte activation and tumor cell death [ 290 ].
GBR1302 targets both HER2 and CD3 receptors and directs T-cells to HER2+ tumor cells. A phase II study (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03983395","term_id":"NCT03983395"}} NCT03983395 ) is ongoing to determine the safety profile of the GBR1302 single agent in previously treated HER2+ metastatic BC. PRS-343 targets HER2 and the immune receptor CD137, a member of the tumor necrosis factor receptor family. Two clinical trials are investigating the effects of PRS-343 monotherapy (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03330561","term_id":"NCT03330561"}} NCT03330561 ) or in combination with other treatments (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03650348","term_id":"NCT03650348"}} NCT03650348 ) (see the summarized table at the end of the manuscript).
4.2.2. HER2-Derived Peptide Vaccines
One of the strategies of immunotherapy is activating the patient’s immune system to kill cancer cells. Vaccination is an emerging approach to induce a tumor-specific immune response by targeting tumor-associated antigens, such as HER2 [ 291 ]. HER2-derived peptide vaccines comprise different parts of the HER2 molecule, such as E75 (extracellular domain), GP2 (transmembrane domain), and AE37 (intracellular domain) [ 292 ].
E75 (HER2/neu 369–377: KIFGSLAFL) has high affinity for HLA2 and HLA3 (human leucocyte antigen) that can stimulate T-cells against HER2 overexpressing tumor cells [ 293 ]. The efficacy of the E75 vaccine to prevent BC recurrence has been evaluated in a phase I/II study, in which high-risk HER2+ HLA2/3+ BC patients received the E75 vaccine [ 269 ]. The results demonstrated the safety and clinical efficacy of the vaccine as PFS was improved in the E75-vaccinated group compared to the unvaccinated group. Other clinical trials are currently investigating the efficacy of the E75 vaccine on HER2+ BC (see he summarized table at the end of the manuscript).
GP2 (654-662: IISAVVGIL) is a subdominant epitope with poor affinity for HLA2 [ 294 ]. A phase I trial evaluating the effects of a GP2 vaccine in disease-free BC patients showed that it was safe and tolerable with HER2-specific immune response [ 295 ]. The GP2 vaccine has been tested in a randomized, open-labeled phase II study to prevent BC recurrence. The patients that received the GP2 vaccine had HER2+ and HLA2+ BC and were disease-free with a high risk of recurrence at the time of the study [ 270 ]. The results demonstrated that the GP2 vaccine was safe and clinically beneficial for patients with HER2+ BC who received the full vaccine series.
AE37 (Ii-key hybrid of MHC II peptide AE36 (HER2/neu 776–790)) can stimulate CD8+ and CD4+ cells. A randomized, single-blinded phase II study evaluated the effects of an AE37 vaccine to prevent BC recurrence. Patients with a high risk of recurrence and HER2+ BC received the AE37 vaccine [ 271 ]. The vaccination demonstrated no benefit in the overall intention-to-treat analysis, a method that considers the randomized treatment to avoid bias happening after the randomization [ 296 ]. However, the study showed that the AE37 vaccine was safe, and results suggested that it could be effective for HER2-low BC, such as TNBC.
4.2.3. New Tyrosine Kinase Inhibitors (TKIs)
As previously described in this review (see Section 3.2.2 Tyrosine kinase inhibitors (TKIs)), TKIs are small molecules targeting the HER2 intracellular catalytic domain [ 159 ]. New TKIs have been developed with better efficacy and less toxicity in the treatment of HER2+ metastatic BC, such as tucatinib and poziotinib.
Tucatinib is a TKI with high selectivity for HER2, leading to less EGFR-related toxicities, common with other HER TKIs [ 297 ]. A phase I dose-escalation trial evaluated the combination of tucatinib and trastuzumab in BC patients with progressive HER2+ brain metastases [ 298 ]. This study showed preliminary evidence of tucatinib efficacy and tolerability in these patients. Tucatinib was also tested in combination with TDM-1 in a phase Ib trial in HER2+ metastatic BC patients with heavy pre-treatment [ 299 ]. The combination of tucatinib and TDM-1 showed acceptable toxicity and antitumor activity in these patients. Tucatinib was FDA approved in combination with trastuzumab and capecitabine for patients with advanced or metastatic HER2+ BC who received prior anti-HER2 in the metastatic setting [ 300 ]. This was based on the results of the phase II HER2CLIMB clinical trial, where HER2+ metastatic BC patients received tucatinib or placebo in combination with trastuzumab and capecitabine [ 267 ]. The addition of tucatinib to trastuzumab and capecitabine improved PFS and OS of heavily pretreated HER2+ metastatic BC patients.
Poziotinib is a pan-HER kinase inhibitor that irreversibly inhibits the HER family members’ kinase activity [ 301 ]. A phase I study evaluated the efficacy and tolerability of poziotinib in advanced solid tumors. The results showed encouraging antitumor activity against different types of HER2+ cancers as poziotinib was safe and well-tolerated by the patients [ 302 ]. The phase II NOV120101-203 study evaluated the safety and efficacy of poziotinib monotherapy in heavily pretreated HER2+ metastatic BC patients [ 268 ]. Poziotinib showed meaningful activity in these patients with no severe toxicities.
4.2.4. mTOR/PI3K Inhibitors and CDK4/6 Inhibitors
As mentioned in the previous Section 4.1 , mTOR/PI3K inhibitors and CDK4/6 inhibitors have been evaluated as potential new strategic therapies for HR+ BC resistant to endocrine therapy. The mTOR/PI3K signaling pathway and CDK4/6 also play a role in the mechanisms leading to treatment resistance in HER2+ BC [ 303 ]. Thus, targeting them with mTOR/PI3K and CDK4/6 inhibitors is also being investigated in HER2+ BC subtype.
mTOR/PI3K Inhibitors
Alpelisib and taselisib are PI3K isoform-specific inhibitors that were also evaluated in HR+ BC [ 235 , 236 , 238 , 253 , 254 ]. A phase I study evaluated alpelisib in combination with trastuzumab and LJM716 (a HER3-targeted antibody) in patients with PI3KCA mutant HER2+ metastatic BC [ 272 ]. Unfortunately, the results of this study were limited by high gastrointestinal toxicity. Another phase I study tested alpelisib in combination with TDM-1 in HER2+ metastatic BC patients pretreated with trastuzumab [ 273 ]. The combination of alpelisib and TDM-1 demonstrated tolerability and antitumor activity in trastuzumab-resistant HER2+ metastatic BC patients. Taselisib is being tested in an ongoing phase Ib dose-escalation trial in combination with anti-HER2 therapies (trastuzumab, pertuzumab and TDM-1) in HER2+ advanced BC patients (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT02390427","term_id":"NCT02390427"}} NCT02390427 ).
Copanlisib is a highly selective and potent pan-class I PI3K inhibitor [ 304 ]. A phase Ib (PantHER) study evaluated the tolerability and activity of copanlisib in combination with trastuzumab in heavily pretreated HER2+ metastatic BC patients [ 274 ]. The combination of copanlisib and trastuzumab was safe and tolerable. Preliminary evidence of tumor stability was observed in these patients.
Everolimus is a mTORC1 inhibitor also tested in HR+ BC [ 240 , 241 , 242 ]. Everolimus was tested in phase III clinical trials, in combination with trastuzumab and docetaxel (BOLERO-1), or in combination with trastuzumab and vinorelbine (BOLERO-3) in trastuzumab-resistant advanced HER2+ BC [ 275 , 276 ]. Unfortunately, results showed an increase of adverse effects with everolimus. Moreover, the BOLERO-1 clinical trial showed no improvement in PFS with the combination of trastuzumab and everolimus. By contrast, PFS was significantly longer when everolimus was added to vinorelbine in BOLERO-3. A study analyzing the molecular alterations found in patients in the BOLERO-1 and BOLERO-3 clinical trials demonstrated that HER2+ BC patients could derive more benefit from everolimus if the tumors had PI3KCA mutations, PTEN loss or a hyperactive PI3K pathway [ 305 ].
CDK4/6 Inhibitors
Palbociclib, ribociclib and abemaciclib are CDK4/6 inhibitors that have been FDA approved to treat HR+ BC as first-line treatments [ 247 , 250 , 259 ]. They have also been evaluated in multiple clinical trials for advanced HER2+ BC. Palbociclib has been tested in combination with trastuzumab in the phase II SOLTI-1303 PATRICIA clinical trial in heavily pretreated advanced HER2+ BC patients [ 277 ]. Palbociclib combined with trastuzumab demonstrated safety and encouraging survival outcomes in these patients. Palbociclib has also been evaluated in combination with TDM-1 in HER2+ advanced BC patients pretreated with trastuzumab and taxane therapy [ 306 ]. The results of this phase I/Ib study showed safety, tolerability, and antitumor activity in these patients.
Ribociclib was evaluated in a phase Ib/II trial in combination with trastuzumab to treat advanced HER2+ BC patients previously treated with multiple anti-HER2 therapies [ 278 ]. The combination of ribociclib and trastuzumab was safe, but there was limited activity in heavily pretreated patients. The conclusions of this study suggest that CDK4/6 inhibitor/anti-HER2 combination should be administered in patients with few previous therapies.
Abemaciclib has been tested in the phase II randomized open-labeled MonarcHER trial in combination with trastuzumab with or without fulvestrant vs. trastuzumab with standard chemotherapy in HR+/HER2+ BC patients [ 279 ]. The combination of abemaciclib, trastuzumab, and fulvestrant significantly improved PFS in these patients, with a tolerable safety profile.
There are multiple ongoing clinical trials for advanced HER2+ BC testing the combination of palbociclib, trastuzumab, pertuzumab, and anastrozole (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03304080","term_id":"NCT03304080"}} NCT03304080 ); or palbociclib and trastuzumab plus letrozole (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT03054363","term_id":"NCT03054363"}} NCT03054363 ). Preliminary results are expected around July 2021 and March 2022, respectively (see he summarized table at the end of the manuscript).
A great proportion of HER2+ BC patients develop resistance to traditional anti-HER2 therapies, and 40–50% of patients with advanced HER2+ BC develop brain metastases [ 307 ]. Thus, developing new therapies to overcome resistance is essential. The therapeutic strategies that have been described in this section provide new hope for HER2+ BC patients, especially for advanced or metastatic HER2+ BC patients.
4.3. Emerging Therapies for Triple Negative Breast Cancer (TNBC)
TNBC is the most aggressive BC subtype. The fact that TNBC lacks ER and PR expression and does not overexpress HER2, combined with its high heterogeneity, has contributed to the difficulties in developing efficient therapies [ 308 ]. Thus, multiple strategic therapies have been developed to treat all TNBC subtypes. These include conjugated antibodies, targeted therapy, and immunotherapy. An overview of the most recent and completed clinical trials on emerging therapies for TNBC is presented in Table 3 .
Most recent completed clinical trials on emerging therapies for TNBC.
TNBC: triple negative breast cancer; HER2: human epidermal growth factor receptor; HR: hormonal receptor; MBC: metastatic breast cancer; BC: breast cancer; AR: androgen receptor; PPV: personalized peptide vaccine; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; IDFS: invasive disease-free survival; OS: overall survival; TTP: time to progression; pCR: pathologic complete response; HR: hazard ratio.
4.3.1. Antibodies-Drug Conjugates (ADC)
Antibody drug conjugates (ADCs) deliver a cytotoxic drug into the tumor cell by the specific binding of an antibody to a surface molecule [ 280 ]. Multiple ADCs have been investigated in TNBC such as sacituzumab govitecan, ladiratuzumab vedotin, or trastuzumab deruxtecan.
Sacituzumab govitecan combines an antibody targeting trophoblast antigen 2 (Trop-2) and a topoisomerase I inhibitor SN-38 [ 334 ]. Trop-2, a CA 2+ signal transducer, is expressed in 90% of TNBCs and is associated with poor prognosis [ 335 , 336 ]. A single-arm, multicentered phase I/II study evaluated sacituzumab govitecan in heavily pretreated metastatic TNBC patients [ 336 , 337 ]. The efficacy and safety of scituzumab govitecan was shown in these patients, as it was associated with durable objective response. Based on these results, a randomized phase III trial (ASCENT) tested sacituzumab govitecan compared to single-agent chemotherapy chosen by the physician in patients with relapsed or refractory metastatic TNBC [ 309 ]. Sacituzumab govitecan significantly improved PFS and OS of metastatic TNBC patients compared to chemotherapy.
Ladiratuzumab vedotin is composed of a monoclonal antibody targeting the zinc transporter LIV-1 and a potent microtubule disrupting agent, monoethyl auristatin E (MMAE) [ 338 ]. LIV-1 is a transmembrane protein with potent zinc transporter and metalloproteinase activity, expressed in more than 70% of metastatic TNBC tumors [ 339 ]. All clinical trials investigating ladiratuzumab vedotin are still ongoing. A dose-escalation phase I study is evaluating the safety and efficacy of ladiratuzumab vedotin in heavily pretreated metastatic TNBC patients (ClinicalTrials.gov identifier: {"type":"clinical-trial","attrs":{"text":"NCT01969643","term_id":"NCT01969643"}} NCT01969643 ). Preliminary results showed encouraging antitumor activity and tolerability of ladiratuzumab vedotin with an objective response rate of 32% [ 340 ]. The estimated study completion date is June 2023. Two phase Ib/II trials are testing ladiratuzumab vedotin in combination with immunotherapy agents in metastatic TNBC patients, such as pembrolizumab (ClinicalTrials.gov Identifier: {"type":"clinical-trial","attrs":{"text":"NCT03310957","term_id":"NCT03310957"}} NCT03310957 ) with expected preliminary results in February 2022, or in combination with multiple immunotherapy-based treatments (ClinicalTrials.gov Identifier: {"type":"clinical-trial","attrs":{"text":"NCT03424005","term_id":"NCT03424005"}} NCT03424005 ) with expected preliminary results in January 2023.
Trastuzumab deruxtecan is an ADC developed as a treatment for metastatic HER2+ BC patients. Its mechanism of action is described in Section 3.2 . Even though trastuzumab deruxtecan was developed to treat HER2+ BC, it showed antitumor activity in HER2-low tumors in a phase I study [ 282 ]. Based on these results, an ongoing open-labeled, multicentered phase III study (ClinicalTrials.gov Identifier: {"type":"clinical-trial","attrs":{"text":"NCT03734029","term_id":"NCT03734029"}} NCT03734029 ) is recruiting patients with HER2-low metastatic BC to test trastuzumab deruxtecan vs. standard treatment chosen by the physician. Preliminary results are expected in January 2023 (see Table 4 ).
Ongoing clinical trials on emerging therapies for BC treatment for all BC molecular subtypes.
TNBC: triple negative breast cancer; HER2: human epidermal growth factor receptor 2; ER: estrogen receptor; MBC: metastatic breast cancer; BC: breast cancer; HR: hormonal receptor; PFS: progression free survival; CBR: clinical benefit rate; ORR: objective response rate; DFS: disease-free survival; OS: overall survival; TTP: time to progression. pCR: pathologic complete response; GM-CSF: granulocyte macrophage colony-stimulated factor; DLT: dose-limiting toxicities; MTD: maximum tolerated dose; TTF: time to treatment failure; TTR: time to treatment response; iDFS: invasive disease-free survival; RFS: recurrence free survival; DDFS: distant disease-free survival; iEFS: invasive events-free survival; CR: clinical response; DoCB: duration of clinical benefit; SD: stable disease; DoR: duration of response; IAEs: incidence of adverse events; TDR: treatment discontinuation rate; PR: partial response; DCR: disease control rate; HR: hazard ratio.
4.3.2. Targeted Therapies
Targeted therapy is the current standard of care to treat HR+ and HER2+ BC, but it cannot be administered to patients with TNBC as these tumors lack the expression of these biomarkers. Hence, the next logical step is to identify biomarkers associated with TNBC to develop specific targeted therapies. Several emerging targeted therapies are being clinically trialed with limited or mixed results.
VEGF and EGFR Inhibitors
Vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR) are overexpressed in most TNBC patients [ 341 , 342 ]. Bevacizumab and cetuximab are antibodies developed to specifically target VEGF and EGFR, respectively. Unfortunately, clinical trials studying the effects of these antibodies in TNBC patients demonstrated limited results. The phase III, randomized BEATRICE study evaluating adjuvant bevacizumab-continuing therapy in TNBC demonstrated no significant benefit in OS [ 310 ]. A phase II trial evaluating the impact of adding bevacizumab or cisplatin to neoadjuvant chemotherapy to stage II to III TNBC concluded that further investigation of bevacizumab in this setting was unlikely [ 311 ].
The phase II randomized TBCRC 001 trial testing the combination of cetuximab and carboplatin in stage IV TNBC showed a response in fewer than 20% of patients [ 312 ]. Another randomized phase II study compared the effects of cetuximab plus cisplatin to cisplatin alone in metastatic TNBC patients. Adding cetuximab to cisplatin prolonged PFS and OS, warranting further investigation of cetuximab in TNBC [ 313 ]. Based on these results, bevacizumab is not recommended for the treatment of TNBC.
mTOR/PI3K/AKT Inhibitors
mTOR/PI3K/Akt signaling pathway is an important target involving all BC subtypes. Inhibitors of mTOR, PI3K, and Akt have been tested in HR+ and HER2+ BC patients and have also been tested in TNBC patients. The mTOR inhibitor everolimus has been tested in a randomized phase II trial in combination with chemotherapy vs. chemotherapy alone in stage II/III TNBC patients [ 314 ]. Unfortunately, the addition of everolimus was associated with more adverse effects, without improving pCR or clinical response. A phase I study testing the combination of everolimus and eribulin in metastatic TNBC patients showed that this combination was safe, but the efficacy was modest [ 343 ].
The Akt inhibitor ipatasertib has been tested in combination with paclitaxel (vs. placebo) for metastatic TNBC patients in the phase II multicentered double-blinded randomized LOTUS trial [ 315 ]. The results showed improved PFS when patients received ipatasertib. Another phase II double-blinded randomized trial, FAIRLANE, testing neoadjuvant ipatasertib plus paclitaxel for early TNBC, showed no clinically or statistically significant improvement in the pCR rate, but ipatasertib’s antitumor effect was more pronounced in patients with PI3K/AKT1/PTEN-altered tumors [ 316 ]. Capivasertib, another Akt inhibitor, has been tested in combination with paclitaxel (vs. placebo), first-line therapy for metastatic TNBC patients in the phase II double-blinded randomized PAKT trial [ 317 ]. The addition of capivasertib to paclitaxel significantly improved PFS and OS, with better benefits for patients with PI3K/AKT1/PTEN-altered tumors.
Androgen Receptor Inhibitors
The androgen receptor (AR) is a steroidal hormonal receptor that belongs to the nuclear receptor family and is expressed in 10% to 50% of TNBC tumors [ 344 ]. Tumors expressing AR have better prognosis but are less responsive to chemotherapy [ 345 ]. Multiple clinical trials have tested AR inhibitors in TNBC [ 318 , 319 , 320 ].
Bicalutamide, an AR agonist, was tested in a phase II study in patients with AR+, HR- metastatic BC [ 318 ]. The results showed promising efficacy and safety for these patients.
Enzalutamide, a nonsteroidal antiandrogen, has been tested in a phase II study in patients with locally advanced or metastatic AR+ TNBC [ 319 ]. Enzalutamide demonstrated significant clinical activity and tolerability, warranting further investigation.
Abiraterone, a selective inhibitor of CYP17, has been evaluated in combination with prednisone in AR+ locally advanced or metastatic TNBC patients [ 320 ]. This combination was beneficial for 20% of the patients.
Several clinical trials are currently testing AR inhibitors alone or combined with other treatments for TNBC patients; expecting results between 2022 and 2027 (see Table 4 ).
4.3.3. Immunotherapy
Targeted antibodies.
The immune system plays a crucial role in BC development and progression. Tumor cells can escape the immune system by regulating T-cell activity leading to the inhibition of immune response [ 346 , 347 ]. Two principal biomarkers found in TNBC are associated with this bypass: the programmed cell death protein receptor (PD-1) and its ligand PDL-1, and the cytotoxic T lymphocyte-associated protein 4 (CTLA-4) [ 348 ].
PD-1 is an immune checkpoint receptor expressed on the surface of activated T-cells. PDL-1, its ligand, is expressed on the surface of dendritic cells or macrophages. The interaction of PD-1 and PDL-1 inhibits T-cell response [ 349 ]. CTLA-4 is expressed on T-cells and inhibits T-cell activation by binding to CD80/CD86, leading to decreased immune response [ 350 ].
Atezolizumab, an anti-PDL-1 antibody, has demonstrated safety and efficacy in a phase I study for metastatic TNBC patients [ 351 ]. Based on these results, atezolizumab was tested in combination with nab-paclitaxel for unresectable locally advanced or metastatic TNBC in the phase III double-blinded placebo-controlled randomized Impassion130 study [ 321 ]. Atezolizumab plus nab-paclitaxel prolonged PFS and OS in both the intention-to-treat population and PDL1+ subgroup. Another double-blinded, randomized phase III study (Impassion031) compared atezolizumab in combination with nab-paclitaxel and anthracycline-based chemotherapy vs. placebo for early-stage TNBC [ 322 ]. This combination significantly improved pCR with an acceptable safety profile.
Durvalumab, another anti-PDL-1 antibody, has been tested in combination with an anthracycline taxane-based neoadjuvant therapy for early TNBC in the randomized phase II GeparNuevo study [ 323 ]. This combination increased pCR rate, particularly in patients pretreated with durvalumab monotherapy before chemotherapy. Another randomized phase II study, SAFIRO BREAST-IMMUNO, compared durvalumab to maintenance chemotherapy in a cohort including TNBC patients [ 324 ]. Results showed that durvalumab, as a single agent therapy, could improve outcomes in TNBC patients. A phase I study tested durvalumab in combination with multiple TNBC therapies: PARP inhibitor olaparib and VEGFR1-3 inhibitor cediranib for patients with recurrent cancers including TNBC [ 325 ]. This combination was well tolerated and showed preliminary antitumor activity in all of these patients.
The safety and efficacy of avelumab, another anti-PDL-1 antibody, was evaluated in the phase Ib JAVELIN study in patients with locally advanced or metastatic BC, including TNBC [ 326 ]. Avelumab showed an acceptable safety profile and clinical activity, particularly in tumors expressing PDL-1.
Pembrolizumab is an anti-PD-1 antibody that has been tested in multiple clinical trials. The phase Ib KEYNOTE-012 study demonstrated the safety and efficacy of pembrolizumab on advanced TNBC patients [ 352 ]. Based on these results, the phase II KEYNOTE-086 study evaluated pembrolizumab monotherapy for pretreated or non-pretreated metastatic TNBC patients [ 327 , 353 ]. Pembrolizumab monotherapy showed a manageable safety profile and durable antitumor activity for both pretreated and non-pretreated subgroups. The randomized open-labeled phase III KEYNOTE-119 trial compared pembrolizumab monotherapy to standard chemotherapy in metastatic TNBC [ 354 ]. Pembrolizumab monotherapy did not significantly improve OS compared to chemotherapy in these patients. These findings suggest that pembrolizumab should be investigated in a combinational approach rather than in monotherapy. Based on these results, pembrolizumab was tested in combination with chemotherapy (vs. placebo) for pretreated locally recurrent or metastatic TNBC patients in the phase III double-blinded randomized KEYNOTE-355 trial [ 328 ]. The combination of pembrolizumab plus chemotherapy significantly and clinically improved PFS compared to chemotherapy plus placebo. Pembrolizumab has also been evaluated for early TNBC as neoadjuvant therapy in combination with chemotherapy (vs. placebo) in the phase III KEYNOTE-522 trial [ 329 ]. The combination of pembrolizumab plus chemotherapy significantly improved pCR rate in these patients compared to placebo plus chemotherapy.
Tremelimumab is an anti-CTLA-4 antibody. A dose-escalation phase I study evaluating the safety and efficacy of tremelimumab in patients with metastatic BC showed good tolerability [ 330 ].
Vaccination is an emerging approach to prevent recurrence in high-risk BC patients. As mentioned earlier, TNBC is the most aggressive BC subtype with a higher risk of distant recurrence [ 331 ]. Thus, developing vaccines to prevent recurrence in TNBC patients is of great interest.
Takahashi et al. have developed a novel regimen of personalized peptide vaccination (PPV) based on the patient’s immune system to select vaccine antigens from a pool of peptide candidates [ 332 ]. They performed a phase II study where metastatic recurrent BC patients with prior chemotherapy and/or hormonal therapies received a series of personalized vaccines. This vaccination demonstrated safety, possible clinical benefit, and immune response, especially for TNBC patients [ 332 ]. A multicentered, randomized, double-blinded phase III study analyzed the effects of sialyl-TN keyhole limpet hemocyanin (STn-KLH) on metastatic BC patients [ 333 ]. STn-KLH consists of a synthetic STn, an epitope expressed in BC and associated with aggressive and metastatic tumors, and a high molecular weight protein carrier KLH [ 355 ]. Stn-KLH demonstrated good tolerability, but no benefits in time to progression (TTP) or survival were found. Thus, this vaccination is not recommended for metastatic BC patients [ 333 ].
PVX-410 is a multiple peptide vaccine that activates T-cell to target tumor cells and was developed to treat myeloma. A phase Ib/II study demonstrated the safety and immunogenicity in myeloma patients [ 356 ]. Based on these results, a PVX-410 vaccine is currently being tested to treat TNBC in multiple clinical trials (see Table 4 ).
Finding new treatments for TNBC is an ongoing challenge. The therapeutic strategies that have been described in this section offer great hope to treat TNBC patients. However, because TNBC is highly heterogeneous, it is difficult to find a single treatment efficient for all TNBC subtypes [ 228 ].
5. Conclusions
This review clearly demonstrates that the treatment of BC is complex and is constantly evolving with a large number of ongoing clinical trials on emerging therapies. Indeed, the BC molecular subtype will determine the personalized therapeutic approach, such as targeted treatments like endocrine therapy for HR+ BC or anti-HER2 therapy for HER2+ BC. These therapies have demonstrated their safety and efficacy in treating BC over the years. However, it is essential to go beyond these conventional treatments as BC is a complex disease and not all patients can benefit from personalized treatment. One of the major challenges in BC treatment is finding effective therapies to treat TNBC patients since conventional targeted therapies cannot be administered for this specific BC subtype, which has the worst survival outcomes.
Another important issue in BC treatment is the acquisition of treatment resistance. This is a common phenomenon for either endocrine therapy, anti-HER2 therapy, and chemotherapy.
Hence, understanding the mechanisms underlying drug resistance is a good strategy to develop novel treatments for BC. For example, the mTOR/PI3K/Akt pathway is involved in the mechanism of resistance in all BC molecular subtypes, and thus developing specific inhibitors targeting this pathway is a promising BC treatment approach.
Acknowledgments
The authors would also like to thank team members from the C.D. and F.D. research groups for their valuable assistance.
Abbreviation
Author contributions.
A.B. conceptualized and drafted the manuscript. F.D. and C.D. supervised the project. All authors did critical revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
This work was supported by the “Fond de recherche du Québec–Santé (FRQS)” associated with the Canadian Tumor Repository Network (CTRNet). Caroline Diorio is a senior Research Scholar from the FRSQ. Anna Burguin holds a Bourse d’excellence en recherche sur le cancer du sein—Faculté de médecine-Université Laval.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Data availability statement, conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Breast Cancer Research

Breast Cancer Risk Factors
Breast Cancer Research is presenting our Retrospective Collection on "Breast Cancer Risk Factors." Celebrating 'Breast Cancer Awareness Month (1 October- 31 October)', with this Collection, we aim to gain valuable insights into the multifaceted aspects of breast cancer risk to promote awareness, prevention, and early detection.
NEW CROSS-JOURNAL COLLECTIONS Find out more by clicking the links below:
Artif icial Intelligence in Breast Imaging PDGFB in Br east Cancer Initiation,Progression, and Metastasis

Aims and scope
- Most accessed
Deep learning of mammogram images to reduce unnecessary breast biopsies: a preliminary study
Authors: Chang Liu, Min Sun, Dooman Arefan, Margarita Zuley, Jules Sumkin and Shandong Wu

Reporting on patient’s body mass index (BMI) in recent clinical trials for patients with breast cancer: a systematic review
Authors: Josephine Van Cauwenberge, Karen Van Baelen, Marion Maetens, Tatjana Geukens, Ha Linh Nguyen, Ines Nevelsteen, Ann Smeets, Anne Deblander, Patrick Neven, Stijn Koolen, Hans Wildiers, Kevin Punie and Christine Desmedt
Infrared laser moxibustion for cancer-related fatigue in breast cancer survivors: a randomized controlled trial
Authors: Huijuan Mao, Ming Jin, Lulu Xie, Ni Mao, Xubo Shen, Junchao Chen, Xuefen Chen, Jun J. Mao and Xueyong Shen
Association of area- and volumetric-mammographic density and breast cancer risk in women of Asian descent: a case control study
Authors: Shivaani Mariapun, Weang-Kee Ho, Mikael Eriksson, Nur Aishah Mohd Taib, Cheng-Har Yip, Kartini Rahmat, Per Hall and Soo-Hwang Teo
Fusogenic vesicular stomatitis virus combined with natural killer T cell immunotherapy controls metastatic breast cancer
Authors: Adam Nelson, Nichole McMullen, Simon Gebremeskel, Roberto De Antueno, Duncan Mackenzie, Roy Duncan and Brent Johnston
Most recent articles RSS
View all articles
Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib
Authors: Nusayba Bagegni, Shana Thomas, Ning Liu, Jingqin Luo, Jeremy Hoog, Donald W. Northfelt, Matthew P. Goetz, Andres Forero, Mattias Bergqvist, Jakob Karen, Magnus Neumüller, Edward M. Suh, Zhanfang Guo, Kiran Vij, Souzan Sanati, Matthew Ellis…
Choosing the right cell line for breast cancer research
Authors: Deborah L Holliday and Valerie Speirs
Breast asymmetry and predisposition to breast cancer
Authors: Diane Scutt, Gillian A Lancaster and John T Manning
Triple-negative breast cancer molecular subtyping and treatment progress
Authors: Li Yin, Jiang-Jie Duan, Xiu-Wu Bian and Shi-cang Yu
Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer
Authors: Suzanne A Eccles, Eric O Aboagye, Simak Ali, Annie S Anderson, Jo Armes, Fedor Berditchevski, Jeremy P Blaydes, Keith Brennan, Nicola J Brown, Helen E Bryant, Nigel J Bundred, Joy M Burchell, Anna M Campbell, Jason S Carroll, Robert B Clarke, Charlotte E Coles…
Most accessed articles RSS

Editor-in-Chief
Lewis Chodosh , University of Pennsylvania, USA

Trending in the Media
Click here to see the most popular articles published in Breast Cancer Research in the past three months.

BCR's 20th Anniversary
20 years ago Breast Cancer Research published its first articles with BMC. Well-respected in the field, the journal has continually placed in the first quartile of the ‘Oncology’ category of Journal Citation Reports. Over the past decade, Breast Cancer Research (BCR) has also become the highest ranked breast cancer focused title in the field.
Look back at the journal’s milestone achievements and article highlights .

Featured Review - Artificial intelligence in mammographic phenotyping of breast cancer risk: a narrative review
In this review, we provide a useful reference for AI researchers investigating image-based breast cancer risk assessment while indicating key priorities and challenges that, if properly addressed, could accelerate the implementation of AI-assisted risk stratification to future refine and individualize breast cancer screening strategies.
Springer Nature Oncology Portfolio
Discover the range of academic oncology titles at Springer Nature here .

FRIDAY, May 24, 2024 (HealthDay News) -- New AI can help detect breast cancer that is spreading to other parts of the body, without the need for biopsies, a new study finds.
The AI analyzes MRI scans to detect the presence of cancer cells in the lymph nodes under the arms, researchers said.
In clinical practice, the AI could help avoid 51% of unnecessary surgical biopsies to test lymph nodes for cancer, while correctly identifying 95% of patients whose breast cancer had spread, results showed.
Most breast cancer deaths are due to cancer that’s spread elsewhere, and the cancer typically first spreads to an armpit lymph node, explained lead researcher Dr. Basak Dogan , director of breast imaging research at UT Southwestern Medical Center.
U.S. Cities With the Most Homelessness

Finding cancer that’s spread to a lymph node “is critical in guiding treatment decisions, but traditional imaging techniques alone do not have enough sensitivity” to effectively detect it, Dogan said in a medical center news release.
Patients with benign findings from MRI exams or needle biopsies often must undergo surgical lymph node biopsy anyway, because those tests can miss a good number of cancer cells that have spread past the breast, Dogan said.
Researchers trained the AI by feeding the program MRI scans from 350 newly diagnosed breast cancer patients known to have cancer in their lymph nodes.
Testing showed that the newly developed AI was significantly better at identifying these patients than human doctors using MRI or ultrasound, researchers reported recently in the journal Radiology: Imaging Cancer .
“That’s an important advancement because surgical biopsies have side effects and risks, despite having a low probability of a positive result confirming the presence of cancer cells,” Dogan explained. “Improving our ability to rule out [cancer cells in lymph nodes] during a routine MRI -- using this model -- can reduce that risk while enhancing clinical outcomes.”
More information
The American Cancer Society has more about breast cancer .
SOURCE: UT Southwestern, news release, May 21, 2024
Copyright © 2024 HealthDay . All rights reserved.
Join the Conversation
Tags: breast cancer
America 2024

Health News Bulletin
Stay informed on the latest news on health and COVID-19 from the editors at U.S. News & World Report.
Sign in to manage your newsletters »
Sign up to receive the latest updates from U.S News & World Report and our trusted partners and sponsors. By clicking submit, you are agreeing to our Terms and Conditions & Privacy Policy .
You May Also Like
The 10 worst presidents.
U.S. News Staff Feb. 23, 2024

Cartoons on President Donald Trump
Feb. 1, 2017, at 1:24 p.m.

Photos: Obama Behind the Scenes
April 8, 2022

Photos: Who Supports Joe Biden?
March 11, 2020

U.S. Military Deaths, by War
Elliott Davis Jr. May 25, 2024

Takeaways From the NCAA’s Settlement
Laura Mannweiler May 24, 2024

Noncitizen Voting: the Fiction and Facts
Aneeta Mathur-Ashton May 24, 2024

Quiz: Who Said What in Trump’s Trial?
U.S. News Staff May 24, 2024

CDC: COVID-19 Strains Are on the Rise
Cecelia Smith-Schoenwalder May 24, 2024

Consumers See Worsening Job Market
Tim Smart May 24, 2024

- IT’S ADVANCING OUR UNDERSTANDING OF BREAST CANCER
- IT’S SAVING LIVES, IMPROVING OUTCOMES
- IT’S LEADING TO PREVENTION & A CURE
- RESEARCH IS THE REASON STORIES
- Our Approach
- The Ground We’ve Gained
- Areas of Focus
- Meet Our Researchers
- Collaborative Initiatives
- Start Your Fundraiser
- Make a planned gift
- Game for BCRF
- Other Ways to Give
- Become a Partner
- Find an event
- Our History
- Board of Directors
- Scientific Advisors
- Corporate Partners
- Affiliate Organizations
- Major Donors
- Blog: The Progress Report
- Podcasts: Investigating Breast Cancer
- Video Series: Behind the Breakthroughs
- Stories: Research is the reason
- BCRF Publications
- Research is the reason
Research Moves Us Forward
Your gift fuels the research that gives people who have breast cancer longer, healthier lives.
The Breast Cancer Research Foundation is dedicated to ending breast cancer by advancing the world’s most promising research.
This year, BCRF is the largest private funder of breast cancer research—and metastatic breast cancer research—worldwide and is the highest-rated breast cancer research organization in the country.

THIS YEAR'S INVESTMENT
One-third of our research program is dedicated to metastasis.
METASTATIC BREAST CANCER
BCRF explains this form and highlights our MBC investment.
BREAST CANCER RACIAL DISPARTIES
Explore how BCRF researchers are working to end disparities.

Research Is the Reason
Nasreen is grateful to research for the fact that her metastatic breast cancer has responded well to treatment.
From Our Blog

Research Is the Reason I’m Here for My Daughters

What to Know About the USPSTF's New Breast Cancer Screening Recommendations

5 Facts About Breast Cancer in Younger Women

Signs of Breast Cancer: What to Know

The Alcohol and Breast Cancer Connection

BCRF Honors William P. Lauder with Evelyn H. Lauder Spirit of Philanthropy Award
Meet our researchers.
Learn more about the over 250 investigators BCRF funds.
FUNDRAISE FOR BCRF
We make it easy to make a difference. Get started today.
SHOP PINK, SAVE LIVES
Support brands and products that fuel BCRF’s research.
MAKE A PLANNED GIFT
Use our FreeWill tool to build your legacy: a world without breast cancer.
Get The Latest
Connect with us.
Please remember BCRF in your will planning. Learn More
Breast Cancer Research Foundation 28 West 44th Street, Suite 609, New York, NY 10036
General Office: 646-497-2600 | Toll Free: 1-866-346-3228 [email protected] | BCRF is a 501 (c)(3) | EIN: 13-3727250
- Privacy Policy
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 24, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
AI might help spot breast cancer's spread without biopsy
by Dennis Thompson
New AI can help detect breast cancer that is spreading to other parts of the body, without the need for biopsies, a new study finds.
The AI analyzes MRI scans to detect the presence of cancer cells in the lymph nodes under the arms, researchers said.
In clinical practice , the AI could help avoid 51% of unnecessary surgical biopsies to test lymph nodes for cancer, while correctly identifying 95% of patients whose breast cancer had spread, results showed.
Most breast cancer deaths are due to cancer that's spread elsewhere, and the cancer typically first spreads to an armpit lymph node, explained lead researcher Dr. Basak Dogan, director of breast imaging research at UT Southwestern Medical Center.
Finding cancer that's spread to a lymph node "is critical in guiding treatment decisions, but traditional imaging techniques alone do not have enough sensitivity" to effectively detect it, Dogan said in a medical center news release.
Patients with benign findings from MRI exams or needle biopsies often must undergo surgical lymph node biopsy anyway, because those tests can miss a good number of cancer cells that have spread past the breast, Dogan said.
Researchers trained the AI by feeding the program MRI scans from 350 newly diagnosed breast cancer patients known to have cancer in their lymph nodes.
Testing showed that the newly developed AI was significantly better at identifying these patients than human doctors using MRI or ultrasound, researchers reported in the journal Radiology: Imaging Cancer .
"That's an important advancement because surgical biopsies have side effects and risks, despite having a low probability of a positive result confirming the presence of cancer cells," Dogan explained. "Improving our ability to rule out [cancer cells in lymph nodes ] during a routine MRI—using this model—can reduce that risk while enhancing clinical outcomes."
Copyright © 2024 HealthDay . All rights reserved.
Explore further
Feedback to editors

New technique detects novel biomarkers for kidney diseases with nephrotic syndrome
May 25, 2024

In experiments, mice get ill from raw milk carrying bird flu virus
Understanding a broken heart—study finds link between stress and recurrent heart failure

Genetic cause of rare childhood immune disorders discovered

New surgical tool moves tiny bioparticles with robotics and acoustic energy
The link between defective autophagy and pancreatitis could point to new treatments

Possible association between tattoos and lymphoma revealed

Walkability in neighborhoods linked to health, study of siblings shows

Scientists uncover new treatment pathway for rare 'spider web' childhood brain tumors
How COVID-19 'breakthrough' infections alter your immune cells
Related stories.

Some breast cancer patients can retain lymph nodes, avoiding lymphedema
Apr 13, 2024

Study suggests it may be safe to de-escalate surgery in middle-aged breast cancer patients
May 22, 2024
Clinical trial: Less extensive breast cancer surgery results in fewer swollen arms
Apr 4, 2024
Breast cancer patients can safely avoid extensive removal of lymph nodes if they respond well to systemic treatment
Mar 21, 2024

Neoadjuvant chemotherapy may help some breast cancer patients skip regional nodal irradiation
Jan 15, 2024
Axillary surgery may not be necessary for all women with invasive breast cancer
Oct 22, 2020
Recommended for you

Colon cancers are rising among the young: New study outlines the warning signs

New mechanism of immune evasion in squamous cell carcinoma offers potential for improved treatment
Harnessing the power of viruses to kill cancers

New biomarker predicts success of immunotherapy in kidney cancer
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

Conquering Breast Cancer Using Supercomputers, Data, and Mathematical Modeling
Stampede2, lonestar6, corral systems help advance tumor models, treatment options.
- by Jorge Salazar
- May 20, 2024
- Feature Story

Breast cancer leads worldwide among cancers in women, claiming nearly 670,000 lives in 2022 according to the World Health Organization. TACC supercomputers give scientists the computational resources and innovative data analysis tools they need to make new discoveries in understanding and treating breast cancer. The following examples illustrate different strategies where advanced computing is making strides in conquering breast cancer.
Digital Twins in Oncology
Mathematical modeling has helped improve predictions of how triple-negative breast cancer (TNBC) tumors will respond to treatment, according to research led by Tom Yankeelov of the Oden Institute for Computational Engineering and Sciences at UT Austin.
TNBC cells lack three commonly overexpressed biomarkers in breast cancer — receptors of estrogen, progesterone, and the human epidermal growth factor receptor 2 (HER2). TNBC is an aggressive form of breast cancer with fewer treatment options and is more common in Black women and all women under 40.
Yankeelov co-authored a 2022 study published in the journal Cancer Research that used MRI data from 56 patients with TNBC to develop calibrated models to achieve early, patient-specific resolved predictions of the tumor response of patients with TNBC.

“Using patient specific imaging data, we calibrated our biology-based, mathematical model to make predictions of how tumors grow in space and time,” Yankeelov said. “These predictions have shown to be highly accurate when predicting the response of triple negative breast cancer patients to standard neoadjuvant chemotherapy.” This type of chemotherapy is widely accepted as the standard-of-care for early TNBC, but it comes with concerns of clinical benefits versus harm from the treatment.
Improved predictions provide physicians with guidance on whether a particular treatment is likely to work. “If our model predicts that the treatment is going to be beneficial, then they have more confidence staying the course with chemotherapy. Conversely, if our model predicts that the treatment is not going to be beneficial, then they have more confidence finding an alternative intervention,” Yankeelov said.
Over the last eight years, TACC has provided extensive computational support for our research efforts via Lonestar5, Lonestar6, and Frontera. TACC has been there every step of the way as we develop methods for improving the treatment of, and outcomes for, patients battling cancer. Thomas Yankeelov, Oden Institute
Yankeelov’s mathematical models describe how tumor cells change in space and time due to factors such as how the cells migrate, how they proliferate, and how they respond to therapy.
“What we do is make MRI measurements that let us calibrate those model parameters based on an individual patient's MRI measurements," Yankeelov said. "Once the model is calibrated, we run it forward to predict how that patient's tumor will grow in space and time —this prediction can then be compared to actual measurements in the patient at a future time. It is these predictions that we are getting surprisingly good at.”
Going forward, his lab’s goal is to go beyond making a prediction of whether a patient will respond to therapies or not. Instead, it is about using mathematical modeling to identify an optimal intervention strategy.
“If you have a model that can accurately predict the spatial and temporal development of a tumor, then we use a supercomputer to try an array of treatment schedules to identify the one that works best. That is, we use the mathematical model to build a 'digital twin' to try a myriad of treatment schedules to identify the one with the highest probability of success. That is where the research and field is going,” Yankeelov added.
Yankeelov’s lab used TACC’s Stampede2 and supercomputer and Corral high performance storage in developing digital twins. It's a fast turnaround — the goal is to get the digital twins to work within 24 hours of getting a patient's data to help a physician with treatment decisions within 24 hours, according to Yankeelov. To reach that goal requires access to a supercomputer.
"Over the last eight years, TACC has provided extensive computational support for our research efforts via Lonestar5, Lonestar6, and Frontera," Yankeelov said. "Indeed, it started within the first weeks of our arrival in Austin where TACC staff visited our lab to provide a rapid tutorial on how to start using the systems. TACC has been there every step of the way as we develop methods for improving the treatment of — and outcomes for — patients battling cancer."
HER2+ and Combined Therapies
HER2+ breast cancer overexpresses the gene that makes the HER2 protein —it is characterized as an aggressive breast cancer that can respond well to treatments such as Trastuzumab (a monoclonal antibody), which typically is administered in combination with Doxorubicin (a chemotherapy drug). The challenge for researchers and physicians lies in optimizing the combination of these two drugs to maximize treatment efficacy.

“I developed several mathematical models to assess their ability to replicate experimental data with mice receiving various drug combinations obtained by our collaborator Anna Sorace ,” said Ernesto Lima of the Oden Institute.
Lima co-authored along with Yankeelov a 2022 study published in Computational Methods in Applied Mechanics and Engineering. It developed a family of models to capture the effects of combination Trastuzumab and Doxorubicin on tumor growth to optimize the outcome of the combination therapy while minimizing the dosage and thereby the toxic side-effects necessary to achieve tumor control.
“We created 10 models and calibrated them using the experimental data," Lima said. "Calibration involves adjusting parameters, such as the proliferation rate, which dictates how fast the tumor volume increases over time to align the model's output with the experimental data."

Lima was awarded supercomputer allocations through The University of Texas Research Cyberinfrastructure project on TACC’s Lonestar6 system to calibrate the models, computations that when parallelized ran 13 times faster than in serial. Parallelization takes large calculations and divides them into smaller ones that run simultaneously, versus running the calculations one-at-a-time.
After identifying the best model to replicate the data, Lima’s team optimized the treatment protocol.
“Using our model, we determined the optimal order and timing of drug delivery to maximize treatment efficacy. One treatment protocol, with the same drug amount as in the experiments, achieved a 45 percent reduction in tumor size compared to the experimental controls,” he said.
The team sought ways to maintain treatment efficacy while reducing the drug concentration because of potential toxicity. “We successfully reduced the concentration of Doxorubicin by almost 43 percent, while maintaining the same treatment outcome as in the experiments,” Lima added.
"Without TACC, our ability to explore diverse treatment options and solve complex mathematical models, driving forward our understanding of tumor biology, would be significantly hindered," he continued.
To validate their theoretical results, Sorace and her team are evaluating the identified protocols in a new set of experiments with mice. Preliminary results are hopeful — they suggest that the experimental protocol is more effective than the original protocols. However, there is a long road ahead before they can enter clinical trials.
“The experiments were done with a limited number of doses per drug and treatment protocols," Lima concluded. "However, the framework itself could be applied to different types of treatments where you have multiple drugs being delivered.”
Biopsy Data Gold Mine
UT Austin has gained a veritable goldmine of de-identified breast cancer data and preserved frozen tissue samples of other carcinomas, thanks to a generous donation in the spring of 2024 from James L. (Jim) Wittliff and his wife and collaborator, Mitzie, of the University of Louisville School of Medicine.

“This Database and Tissue Biorepository contains among the most highly quantified datasets of breast cancer biomarkers in the world, with several of the assays such as those for estrogen and progestin receptor proteins representing gold standard breast cancer tests," said Wittliff.
In the 1980s, Wittliff was co-developer with NEN/DuPont of these latter two biomarker tests which were approved by the FDA. More than 5,000 frozen pristine breast, endometrial, ovarian, and colon cancer biopsies and nuclear pellets containing DNA that were collected from patients that Wittliff’s Clinical Laboratory served and curated through a lifetime of research have been transferred and are now stored at the Dell Medical School. In addition, a treasure trove of de-identified comprehensive biomarker and clinical data will be stored and managed at TACC.
“Our immediate goal is to analyze these data, probably in the context of the NIH’s The Cancer Genome Atlas Program and other data," said Ari Kahn of the Life Sciences Computing Group at TACC.
Wittliff is energized to expedite the use of the comprehensive data and unique samples to advance cancer diagnosis, treatment approaches, and ways to assess risk of recurrence of carcinomas, and is excited to support UT Austin, his alma mater, with this amazing gift. TACC will steward the data on TACC's Corral system and is planning on making it available in the future to other scientists online through tools such as a web portal. Ari Kahn, Life Sciences Computing Group, TACC
The irreplaceable biopsies are now preserved for other scientists to use for clinical trials in silico and to develop future companion diagnostic tests. Many of the tissue specimens have data associated with them such as protein tumor markers; genomic data on gene expression; patient characteristics such as age, sex, and smoking history; disease properties such as tumor size and pathology; and clinical follow-up such as surgeries and chemotherapy treatments.
“Wittliff is energized to expedite the use of the comprehensive data and unique samples to advance cancer diagnosis, treatment approaches, and ways to assess risk of recurrence of carcinomas, and is excited to support UT Austin, his alma mater, with this amazing gift,” Kahn added. “TACC will steward the data on TACC's Corral system and is planning on making it available in the future to other scientists online through tools such as a web portal."
Cancer and AI
Artificial intelligence has emerged as a tool for the sciences helping researchers make progress on biological problems such as high throughput virtual drug screening and planning chemical synthesis pathways. According to Yankeelov, it is important to point out the fundamental limitations of AI in what it can inform scientists about the most important problems in oncology.

"In studying cancer, the problem with the AI approach is that cancer is a notoriously heterogeneous disease. In fact, it is not just one disease — it is more than 100 diseases. The issue is with needing a training set to calibrate an AI algorithm," Yankeelov said.
For example, consider a patient with TNBC cancer who contracts one of the five different subtypes of triple-negative breast cancer that are labeled.
“To use an AI-based approach to predict how this patient needs to be treated, one needs to have a training data set that consists of that subtype of triple-negative breast cancer in addition to all of the possible therapeutic regimens that could be received," Yankeelov said.
"That training set does not exist, and it will never exist because the diseases are getting more specifically labelled and the treatments are getting more targeted. Furthermore, even if it did exist, it does not account for the unique characteristics of this patient's cancer because the patient is different than everyone else in that training set."
Challenging Road Ahead
Cancer remains one of the biggest health challenges facing society. According to Yankeelov and Lima, the computational resources provided by TACC are essential in advancing tumor models and treatment options by facilitating rigorous testing and refinement of various mathematical models.
TACC offers scientists the computational resources they need to make discoveries that are effective for breast cancer patients. Rising survival rates over the past decade for breast cancer offer a glimmer of hope, thanks to awareness campaigns and increased funding for research.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- News & Views
- Published: 22 May 2024
Breast cancer
Gaining ground in personalized breast cancer therapy: lesson learned from PHERGain
- Maria Vittoria Dieci ORCID: orcid.org/0000-0002-3967-9861 1 , 2 &
- Valentina Guarneri 1 , 2
Nature Reviews Clinical Oncology ( 2024 ) Cite this article
70 Accesses
1 Altmetric
Metrics details
- Therapeutics
De-escalation of treatment for HER2 + breast cancer is a priority, given the increase in cure rates owing in part to improved HER2-targeted therapies. In this regard, the neoadjuvant approach provides the ideal platform to test less-intensive treatment regimens. Here we highlight a study that demonstrated the role of the metabolic response after dual HER2 blockade as a method of selecting patients who are most likely to benefit from chemotherapy-free neoadjuvant therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Pérez-García, J. M. et al. 3-year invasive disease-free survival with chemotherapy de-escalation using an 18 F-FDG-PET-based, pathological complete response-adapted strategy in HER2-positive early breast cancer (PHERGain): a randomised, open-label, phase 2 trial. Lancet 403 , 1649–1659 (2024).
Article PubMed Google Scholar
Miglietta, F., Dieci, M. V., Griguolo, G. & Guarneri, V. Neoadjuvant approach as a platform for treatment personalization: focus on HER2-positive and triple-negative breast cancer. Cancer Treat. Rev. 98 , 102222 (2021).
Article CAS PubMed Google Scholar
Llombart-Cussac, A. et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol. 18 , 545–554 (2017).
Guarneri, V. et al. De-escalated therapy for HR+/HER2+ breast cancer patients with Ki67 response after 2-week letrozole: results of the PerELISA neoadjuvant study. Ann. Oncol. 30 , 921–926 (2019).
Article CAS PubMed PubMed Central Google Scholar
Tolaney, S. M. et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 24 , 273–285 (2023).
Pérez-García, J. M. et al. Chemotherapy de-escalation using an 18 F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): a multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 22 , 858–871 (2021).
van Mackelenbergh, M. T. et al. Pathologic complete response and individual patient prognosis after neoadjuvant chemotherapy plus anti-human epidermal growth factor receptor 2 therapy of human epidermal growth factor receptor 2-positive early breast cancer. J. Clin. Oncol. 41 , 2998–3008 (2023).
Squifflet, P. et al. Re-evaluation of pathologic complete response as a surrogate for event-free and overall survival in human epidermal growth factor receptor 2-positive, early breast cancer treated with neoadjuvant therapy including anti-human epidermal growth factor receptor 2 therapy. J. Clin. Oncol. 41 , 2988–2997 (2023).
Dieci, M. V. et al. Type of adjuvant endocrine therapy and disease-free survival in patients with early HR-positive/HER2-positive BC: analysis from the phase III randomized ShortHER trial. NPJ Breast Cancer 9 , 6 (2023).
Prat, A. et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. J. Natl. Cancer Inst. 112 , 46–54 (2020).
Download references
Author information
Authors and affiliations.
Department of Surgery, Oncology and Gastroenterology, University of Padova, Padova, Italy
Maria Vittoria Dieci & Valentina Guarneri
Oncology 2, Veneto Institute of Oncology IOV-IRCCS, Padova, Italy
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Valentina Guarneri .
Ethics declarations
Competing interests.
M.V.D. has acted as a consultant and/or advisor of AstraZeneca, Daiichi Sankyo, Eli Lilly, Exact Sciences, Gilead, MSD, Novartis, Pfizer, Roche and Seagen. V.G. has acted as a consultant and/or advisor board membership for AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, Exact Sciences, Gilead, Menarini Stemline, Merck Serono, MSD, Novartis, Olema Oncology, Pfizer, and Pierre Fabre, has acted as a speaker for AstraZeneca, Daiichi Sankyo, Eli Lilly, Exact Sciences, Gilead, GSK, Menarini Stemline, Novartis, Roche and Zentiva and has received personal fees for expert testimony from Eli Lilly.
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Dieci, M.V., Guarneri, V. Gaining ground in personalized breast cancer therapy: lesson learned from PHERGain. Nat Rev Clin Oncol (2024). https://doi.org/10.1038/s41571-024-00907-w
Download citation
Published : 22 May 2024
DOI : https://doi.org/10.1038/s41571-024-00907-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.

IMAGES
VIDEO
COMMENTS
This page highlights what's new and recent research in the detection and treatment of breast cancer. New methods to detect breast cancer, such as 3-D mammography, are described. Treatments tailored to breast cancer subtypes are discussed, including targeted therapies.
Ribociclib plus Endocrine Therapy in Early Breast Cancer. D. Slamon and OthersN Engl J Med 2024;390:1080-1091. In patients with stage II or III early breast cancer, the addition of ribociclib to ...
Patients with new metastatic triple-negative breast cancer at initial diagnosis were also eligible for the trial. Patients who received taxane, gemcitabine, or platinum agents as neoadjuvant or ...
The long-sought discovery of HER2 as an actionable and highly sensitive therapeutic target was a major breakthrough for the treatment of highly aggressive HER2-positive breast cancer, leading to ...
In this international, open-label, randomized, phase 3 trial, we randomly assigned patients with HR-positive, HER2-negative early breast cancer in a 1:1 ratio to receive ribociclib (at a dose of ...
Breast cancer is caused by the development of malignant cells in the breast. The malignant cells originate in the lining of the milk glands or ducts of the breast (ductal epithelium). Breast ...
HER2 is a common treatment target for breast cancer. This new drug targets HER3, a biomarker related to HER2, which is associated with poor breast cancer outcomes. About 10% to 20% of newly diagnosed breast cancers are HER2-positive. At the 2023 American Society for Clinical Oncology (ASCO) Annual Meeting, researchers announced positive results ...
By Kelley Luckstein. A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
Possible environmental causes of breast cancer have also received more attention in recent years. While much of the science on this topic is still in its earliest stages, this is an area of active research. Breast cancer prevention. Researchers are looking for ways to help reduce breast cancer risk, especially for women who are at high risk.
Panels A-C show the number of clinical trials in breast cancer since early 2000, by immunotherapeutic approach (A), by trial setting (B), and by trial phase (C).Panel D shows the major immune ...
The research, published today in Clinical Cancer Research by a team from King's College London and funded by Breast Cancer Now, marks a new method in cancer treatment.. The discovery is particular ...
To compare the compartmentalized diffusion-weighted models, intravoxel incoherent motion (IVIM) and restriction spectrum imaging (RSI), in characterizing breast lesions and normal fibroglandular tissue. Litong He, Yanjin Qin, Qilan Hu, Zhiqiang Liu, Yunfei Zhang and Tao Ai. Breast Cancer Research 2024 26 :71.
Address reprint requests to Dr. Tutt at the Breast Cancer Now Toby Robins Research Centre, the Institute of Cancer Research, 237 Fulham Rd., London SW3 6JB, United Kingdom, or at [email protected].
Breast cancer (BC) is the most frequent cancer and the second cause of death by cancer in women worldwide. According to Cancer Statistics 2020, BC represents 30% of female cancers with 276,480 estimated new cases and more than 42,000 estimated deaths in 2020 [ 1 ]. Invasive BC can be divided into four principal molecular subtypes by ...
Breast Cancer Research is an international, peer-reviewed online journal, publishing original research, reviews, editorials and reports. Open access research articles of exceptional interest are published in all areas of biology and medicine relevant to breast cancer, including normal mammary gland biology, with special emphasis on the genetic, biochemical, and cellular basis of breast cancer.
Despite tremendous advances in breast cancer research and treatment over the past three decades—leading to a reduction in breast cancer mortality of over 40% in some high-income countries—gross inequities remain, with many groups being systematically left behind, ignored, and even forgotten.
Nature Cancer (2022) New research shows that comprehensively characterized patient-derived xenografts (PDXs) of breast cancer can be adapted to high-throughput drug screening and can be used as ...
FRIDAY, May 24, 2024 (HealthDay News) -- New AI can help detect breast cancer that is spreading to other parts of the body, without the need for biopsies, a new study finds. The AI analyzes MRI ...
The mission of the Breast Cancer Research Foundation is to prevent and cure breast cancer by advancing the world's most promising breast cancer research. Why Research . ... 28 West 44th Street, Suite 609, New York, NY 10036. General Office: 646-497-2600 | Toll Free: 1-866-346-3228
Increased blood draws for ultrasensitive ctDNA and CTCs detection in early breast cancer patients. Alfonso Alba-Bernal. Ana Godoy-Ortiz. Emilio Alba. Article Open Access 15 May 2024.
by Dennis Thompson. New AI can help detect breast cancer that is spreading to other parts of the body, without the need for biopsies, a new study finds. The AI analyzes MRI scans to detect the ...
Breast cancer researchers are enlisting supercomputers in making new discoveries to improve treatment and understanding of the deadly disease. TACC systems and expertise are giving scientists much-needed data and computational resources for modeling tumor growth, optimizing treatment combinations, analyzing biopsy collections, and more.
Breast cancer cases in patients under 40 are on the rise. New research sheds light on the comparatively rare cancer, for both oncologists and patients. In 2016, 21-year-old Jessica Florence found ...
De-escalation of treatment for HER2+ breast cancer is a priority, given the increase in cure rates owing in part to improved HER2-targeted therapies. In this regard, the neoadjuvant approach ...