Management of Vernal Keratoconjunctivitis
- Open access
- Published: 07 September 2013
- Volume 2 , pages 73–88, ( 2013 )

Cite this article
You have full access to this open access article

- Andrea Leonardi 1
6363 Accesses
64 Citations
90 Altmetric
12 Mentions
Explore all metrics
Vernal keratoconjunctivitis (VKC) is a relatively rare, chronic form of ocular allergy that can potentially cause severe visual complications. Affecting mainly children and young adults, it is an IgE- and T cell-mediated disease, leading to a chronic inflammation in which eosinophil, lymphocyte and structural cell activation are involved. Treatment of VKC requires a multiple approach that includes conservative measures and pharmacologic treatment. Patients and parents should be made aware of the long duration of disease, its chronic evolution and possible complications. Treatment should be based on the duration and frequency of symptoms and the severity of corneal involvement. Mast cell stabilizers and antihistamines have been proven to be effective for the treatment of mild to moderate forms of VKC. In the most severe cases, topical steroids can be used as rescue medication to reduce conjunctival and corneal inflammation. Immunomodulators that have been investigated for VKC treatment include topical ocular preparations of cyclosporine A and tacrolimus. Topical cyclosporine A has been proven to be effective in the long-term treatment of VKC, significantly improving signs and symptoms without significant side effects.
Similar content being viewed by others

An Update on the Therapeutic Approach to Vernal Keratoconjunctivitis

Omalizumab in Severe Refractory Vernal Keratoconjunctivitis in Children: Case Series and Review of the Literature
A patient-centered approach to vernal keratoconjunctivitis (vkc): a podcast.
Avoid common mistakes on your manuscript.
Introduction
Allergic conjunctivitis is a localized allergic condition frequently associated with rhinitis, but is often observed as the only or prevalent allergic phenomenon. Ocular allergy can involve all of the components of the ocular surface, including the lid and lid margin, conjunctiva and the lacrimal system. The term allergic conjunctivitis refers to a collection of hypersensitivity disorders that affect the lid and conjunctiva. Various clinical forms are included in the classification of ocular allergy: seasonal/intermittent allergic conjunctivitis (SAC), perennial/persistent allergic conjunctivitis (PAC), vernal keratoconjunctivitis (VKC), atopic keratoconjunctivitis (AKC) and drug-induced dermato-conjunctivitis [ 1 ]. Corneal involvement is typically restricted to the two most severe forms of ocular allergy, vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC), which requires particular care in their management.
The current review focuses on the practical management of VKC, a recurrent, bilateral, chronic allergic inflammatory disease of the ocular surface affecting mainly children and young adults and is characterized by incompletely identified pathogenic mechanisms. Several therapeutic measures are required to control signs and symptoms of the disease and avoid potential long-standing or permanent inflammatory sequelae. Although topical anti-allergic and anti-inflammatory eye drops are the mainstay of treatment for VKC, a gold standard treatment has not yet been established for this disease.
Vernal Keratoconjunctivitis
VKC is a severe inflammatory disease that appears in children and adolescents with seasonal recurrence. It is most often seen in boys and tends to resolve at puberty. It is a relatively rare, chronic form of ocular allergy that can cause severe visual complications [ 2 – 4 ]. VKC is more frequent in warmer, arid, windy climates, in the Mediterranean area, central Africa, Japan, India, and South America but is also reported in North America, China, Australia, Great Britain, and Sweden. VKC appears mainly seasonally but can be perennial, chronic or with acute exacerbations. It is an IgE- and T cell-mediated allergic reaction with additional, ill-defined, perhaps nonspecific, hypersensitivity responses. The etiology involves a variety of factors, including environmental allergens, climate, and genetic predisposition. Cytologic, biohumoral, immunohistologic, and molecular biologic studies indicate that VKC is a Th2 lymphocyte-mediated disease. Mast cells and eosinophils and their mediators play major roles in the clinical manifestations. In addition to typical Th2-derived cytokines, Th1-type cytokines, chemokines, growth factors, and enzymes are over-expressed [ 5 ]. Increased serum levels of IL-17 and antinuclear antibodies, together with a high association with familial history of autoimmune disorders suggest additional mechanisms involved in the development of VKC [ 6 , 7 ]. Tissue remodeling reactions, papillae formation of different sizes and shapes, stem cell deficiency, and various degrees of superficial corneal opacification, are further consequences of chronic inflammation [ 8 ]. Many elements contribute to this dramatic response, including epithelial changes, connective tissue deposition, edema, inflammatory cell infiltration, and glandular hypertrophy.
Clinical Forms
It is well known that two VKC populations can be defined: (1) those with positive test results, who generally also present with some other allergic manifestation, such as asthma, rhinitis, or eczema and (2) those with negative test results, and a negative personal and familial history of atopy.
The disease may present in three clinical forms: tarsal, limbal and the mixed form. Large papillae of different shape and size, usually greater than 1 mm in diameter, on the upper tarsal conjunctiva characterize the tarsal form, while Trantas’ dots and infiltrates on the limbus are typical of the limbal form. The mixed form is characterized by the presence of both forms in the same eye. VKC sufferers have a characteristic ropey, stringy mucous and/or serous discharge, and corneal complications, such as superficial punctate keratopathy (SPK), and shield ulcers are common. Moderate to intense conjunctival hyperemia, intense itching, photophobia, mild to moderate chemosis, foreign-body sensation, and pain are typical signs and symptoms which may be very intense upon awakening, causing frequently what is called the morning misery. While it is considered a long-term disease with an average duration of 4–8 years, VKC generally subsides before or just after puberty [ 1 , 2 , 4 ]. It can persist or reactivate after puberty, however, a VKC-like disease has been found in young adults without any history of allergic disease in childhood [ 9 ]. This new clinical entity is characterized by signs and symptoms similar to the typical VKC. Allergy test results, cytokine production, and quality of life are similar to those in pediatric VKC. It is still unclear if this is a sub-type of VKC with a later onset or a different clinical entity.
VKC is not difficult to diagnose by clinical examination. Trantas’ dots and large cobblestone papillae are indicative of the condition [ 1 , 2 ]. VKC is differentiated from other ocular allergic conditions, such as SAC, PAC, AKC, ocular rosacea in children, and infectious conjunctivitis, through a comprehensive clinical history and ophthalmic examination. It is important to note that while skin test results may be positive, VKC is not always closely related to allergen exposure, and climate is an equally important factor. Conjunctival scrapings or tear cytology can be useful, revealing increased leukocytes in the conjunctiva, particularly eosinophils [ 1 , 2 ].
Management of VKC
Understanding and treating VKC has been a challenge for ophthalmologists, since the pathogenesis is unclear and anti-allergic therapy often unsuccessful. VKC is an IgE- and T cell-mediated disease, leading to a chronic inflammation in which eosinophil, lymphocyte and structural cell activation characterize the conjunctival allergic reaction. Therefore, measures aimed at stabilization of mast cells or histamine receptor antagonists alone are frequently insufficient for controlling conjunctival inflammation and the frequent corneal involvement [ 1 , 2 ]. Currently available drugs may be merely palliative and do not extinguish the complex immune process that initiates and perpetuates the allergic ocular surface inflammation.
In a meta-analysis of randomized clinical trials evaluating topical treatments for VKC, only 10 of 21 studies were suitable for statistical analysis, and the authors concluded that the currently available topical drugs are effective in treating acute phases of VKC [ 10 ]. However, controlling VKC signs and symptoms may be a challenge even for expert ophthalmologists. Because of the chronicity and severity of the disease, avoidance of triggers and life-style planning must be accompanied by pharmacological treatments: topical ocular and non-ocular pharmacologic treatment, systemic pharmacologic treatments and immunotherapy.
Non-Pharmacologic Management
Patients and parents should be instructed regarding the nature and duration of the disease, clinical characteristics and possible complications. Psychological support may be necessary in severe cases. The first line of VKC management, when possible, is the identification of allergens and avoidance of those environmental factors that may exacerbate the disease. Avoiding exposure to nonspecific triggering factors, such as sun, wind, and salt water, with the use of sunglasses, hats with visors, and swimming goggles should be recommended. Frequent hand, face, and ear washing should also be suggested. Cold compresses may help as natural decongestant. Tear substitutes aid in stabilization of the tear film, act as an eyewash, and dilute the concentration of the allergens and mediators in tears. Eye drops containing herbal extracts, such as chamomile-containing preparations, should be avoided because they may cross-react with sensitizing allergens [ 11 ] (Table 1 ).
Topical Ocular Pharmacologic Treatment
Pharmacological treatment should be planned ahead in patients with a history of VKC and started in the early spring or continued all year, depending on the allergen exposure and duration of the symptoms. Currently available topical drugs for allergic conjunctivitis belong to several pharmacologic classes (Table 2 ): vasoconstrictors, antihistamines, mast cell stabilizers, ‘dual-acting’ agents (with antihistaminic and mast cell stabilizing properties), non-steroidal anti-inflammatory agents, corticosteroids and immunosuppressive drugs.
Mast Cell Stabilizers
Mast cell stabilizers are the first-line drugs for VKC. Topical mast cell stabilizers are generally safe and have minimal ocular side effects, although there may be some tolerability concerns, since transient burning or stinging may occur upon application. Several studies have demonstrated the efficacy of 2% and 4% sodium cromoglicate (DSCG, cromolyn), nedocromil sodium 2%, lodoxamide tromethamine 0.1%, and spaglumic acid 4% [ 12 – 14 ]. The recommended dosing schedule is 4–6 times daily, with a loading period of at least 7 days and an onset of activity after as much as 2 weeks. DSCG alone has limited effects in the treatment of VKC and is less well tolerated than newer anti-allergic compounds. Nedocromil appears to be more potent than DSCG [ 15 ], acting on multiple cells involved in allergic inflammation, including eosinophils, neutrophils, macrophages, mast cells, monocytes, and platelets.
Lodoxamide has long been available for the treatment of VKC. Its mechanism of action is thought to be similar to that of DSCG, since it was shown to prevent tryptase release [ 16 ]. Lodoxamide was shown to be more effective than DSCG for the inhibition of eosinophil activation, evaluated by measuring tear eosinophil cationic protein ECP before and after therapy [ 17 ], suggesting that lodoxamide has an effect on eosinophil activation. Inhibition of eosinophil activation and degranulation is the proposed mechanism for its efficacy against corneal signs such as keratitis and shield ulcers in severe allergic disease [ 17 ]. Lodoxamide was shown to be superior to placebo [ 18 ] and N -acetyl aspartyl glutamic acid (NAAGA) for treatment of VKC [ 19 ]. The recommended dosing schedule is four times daily. Lodoxamide may be used continuously for 3 months in children older than 2 years of age.
NAAGA 6% has been widely used in Europe as topical eye drops in the treatment of VKC [ 20 ]. NAAGA is known to inhibit leukotriene synthesis, histamine release by mast cells, and complement-derived anaphylatoxin production. This anti-allergic compound was also shown to directly inhibit leukocyte adhesion to endothelial cells induced by proinflammatory stimuli, and abrogates tumor necrosis factor α-induced expression of adhesion molecules on granulocytes and endothelial cells [ 21 ]. These pharmacological properties confer a potential anti-inflammatory activity.
Antihistamines
Antihistamines act via histamine receptor (HR) antagonism to block the inflammatory effects of endogenous histamine and prevent or relieve the associated signs and symptoms. Most antihistamines used in the treatment of allergy are H 1 receptor antagonists, although some agents may have affinity for other receptor subtypes [ 22 ]. H 2 antagonists have been shown to modulate both cell growth and migration [ 22 ]. Ocular drugs with antihistaminic activity may offer therapeutic advantages to patients with allergic conjunctivitis, including VKC, by inhibiting proinflammatory cytokine secretion from conjunctival epithelial cells [ 23 ]. The first-generation antihistamines pheniramine and antazoline have a long safety record, but are known for their burn upon instillation, the rapid onset and disappearance of their effects, and their limited potency [ 24 ]. These are still available in over-the-counter products, particularly in combination with vasoconstrictors.
The newer antihistamines are still H 1 antagonists, but have a longer duration of action (4–6 h), and are better tolerated than their predecessors. These include levocabastine hydrochloride 0.5% and emedastine difumarate 0.05%.
Levocabastine 0.05% eye drops alone, instilled four times daily for 3 months, was effective, safe, and well tolerated by patients with VKC; however, it was less effective than lodoxamide [ 25 ] or NAAGA [ 20 ]. Interestingly, in an animal model, levocabastine reduced the clinical aspects of the late-phase reaction and the conjunctival expression of alpha(4)beta(1) integrin by reducing infiltration of eosinophils [ 26 ].
Emedastine 0.05% appears to be more potent and selective than levocabastine [ 27 , 28 ]. Indeed, in direct comparison with levocabastine, emedastine proved significantly more effective in alleviating signs of seasonal allergic conjunctivitis [ 27 , 28 ]. No specific studies in VKC have been performed.
A meta-analysis of randomized clinical trials in VKC showed a large number of studies (20) evaluated the efficacy of common anti-allergic eye drops (levocabastine, lodoxamide, mipragoside, NAAGA, nedocromil sodium, DCG). Among these, lodoxamide appeared to be the most effective [ 10 ].
Topical Antihistamines with Multiple Anti-Inflammatory Activities
New antihistamines that combine mast cell stabilizing properties and histamine receptor antagonism, such as alcaftadine, azelastine, bepotastine, epinastine, ketotifen, and olopatadine, are presently available and show evident benefits in treating all forms of ocular allergy. The advantage offered by these agents is the rapidity of symptomatic relief given by immediate histamine receptor antagonism, which alleviates itching and redness, coupled with the long-term disease-modifying benefit of mast cell stabilization. All of these medications are well tolerated and none are associated with significant ocular drying effects. Although widely used in the treatment of VKC, specific studies in VKC are very few. Olopatadine and ketotifen have shown efficacy in relieving signs and symptoms in patients with VKC. These two drugs may have also anti-inflammatory properties, reducing eosinophil activation and cytokine release. A significant difference in favor of ketotifen-treated patients has being shown in a single-center, simple-masked study comparing these two drugs [ 29 ]. However, both drugs were efficient and safe relieving the main symptoms and signs of VKC, including itching, tearing, conjunctival hyperemia, mucous discharge and photophobia. Olopatadine hydrochloride 0.1% was effective for relieving the signs and symptoms of VKC and reducing the number of goblet cells during treatment [ 30 ].
Decongestants are a useful adjunctive therapy to mast cell stabilizers and/or antihistamines in mild and moderate forms of VKC, or in adult patients who have demonstrated a notable improvement in symptomatology after adolescence. However, topical decongestants do not reduce the allergic response because they do not antagonize any of the mediators of allergic inflammation. Burning or stinging on instillation is a common side effect. Prolonged use of topical decongestants, as well as the discontinuation of these agents following prolonged use, can lead to rebound hyperemia and conjunctivitis medicamentosa [ 31 ]. These events are usually associated with topical first-generation antihistamines such as pheniramine and antazoline that are available as over-the-counter products.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Generally NSAIDS employed in ocular allergy treatment inhibit both cyclooxygenase (COX)-1 and COX-2 enzymes. Ketorolac, diclofenac, and pranoprofen, may also be valid alternatives to steroids, since they have a proven effect on itching, intercellular adhesion molecule-1 expression, and tear tryptase levels [ 32 ]. Indomethacin 1% [ 33 ], ketorolac 0.5% [ 34 ], and diclofenac 0.1% have shown effectiveness in the treatment of VKC [ 35 ].
Topical Corticosteroids
Moderate to severe VKC needs repeated topical steroid treatment to downregulate conjunctival inflammation. Persistent severe symptoms, thick mucous discharge with moderate to severe corneal involvement, numerous and inflamed limbal infiltrates and/or giant papillae, indicate a need for corticosteroids. However, corticosteroids should be avoided as the first line of defense in the treatment of VKC. If steroids are used, those with low intraocular absorption, such as hydrocortisone, clobetasone, desonide, fluorometholone, loteprednol, difluprednate and rimexolone, should be used first. Dosages are chosen based on the inflammatory state of the eye, with therapy prescribed in pulses of 3–5 days. Loteprednol etabonate is usually indicated for 7–8 days in the treatment of the acute phase. Prednisolone, dexamethasone, or betamethasone should be used only when the above-mentioned first-choice steroids have proven ineffective. Steroid–antibiotic combination eye drops should be avoided, as VKC is an allergic inflammation, rather than an infection. Corticosteroids should not be recommended for long-term use because of possible ocular adverse effects, including increases in intraocular pressure (IOP), induction or exacerbation of glaucoma, formation of cataracts, delayed wound healing, and increased susceptibility to infection or superinfection. These adverse effects depend, in part, on the structure, dose, duration of treatment and gender disposition [ 36 ]. In fact, following daily administration of corticosteroids for 4–6 weeks, approximately one-third of the normal population will be “high or moderate responders” with an increase in IOP of between 6 and 15 mm Hg [ 36 ]. Forty-one of 145 (28.3%) patients with severe VKC in a Singapore case series developed a corticosteroid response, of which eight (5.5%) progressed to glaucoma [ 37 ]. Six of these patients ( n = 8 eyes) required trabeculectomy/mitomycin-C. The main risk factor for trabeculectomy was a greater increase in IOP from baseline, which was independent of potential confounders such as type and duration of corticosteroid use [ 38 ].
Calcineurin Inhibitors and Other Immunomodulators
Cyclosporine A (CsA) is effective in controlling VKC-associated ocular inflammation by blocking Th2 lymphocyte proliferation and interleukin-2 production. It inhibits histamine release from mast cells and basophils through a reduction in IL-5 production, and may reduce eosinophil recruitment and effects on the conjunctiva and cornea [ 39 ]. CsA is lipophilic and thus must be dissolved in an alcohol-oil base [ 39 ]. Unavailability of a commercial preparation of topical CsA, technical difficulties in dispensing eye drops and legal restrictions on its topical use in many countries, preclude its widespread use for VKC treatment. The 2% formulation has the longest track record, but lower concentrations (1%, 0.5%, and 0.05%) have been used and shown to be effective. So far, there is no general consensus regarding the minimum effective concentration of CsA. Only the 0.05% formulation is commercially available for the treatment of dry eye.
After long cycles of treatment, CsA has a marked steroid-sparing effect, potentially allowing control of symptomatology without steroids [ 40 ]. When necessary, additional topical steroids can be used in short cycles. Systemic absorption of CsA was not detectable by clinical laboratory methods. Burning and irritation are frequent side effects. Treatment can be prescribed seasonally or perennially, reducing doses in the non-active phases of the disease. Adverse events, such as bacterial or viral infections are rare, while IOP changes have not been reported.
CsA 1% or 2% emulsion in castor or olive oil instilled four times daily can be considered for treatment of moderate to severe VKC and can serve as a good alternative to steroids [ 39 , 41 ]. After 2 weeks, CsA 1% four times daily significantly reduced signs and symptoms and tear levels of ECP in a group of VKC patients [ 42 ].
CsA 1% was reported to be the minimum effective concentration in the treatment of shield ulcers, with recurrence observed at lower concentrations [ 43 ]. In a randomized, controlled trial, the effects of CsA 0.05% were similar to placebo [ 44 ]. Conversely, in another study, CsA 0.05% decreased the severity of symptoms and clinical signs significantly after 6 months and the need for steroids was reduced, suggesting that CsA at low doses is an effective steroid-sparing agent in VKC [ 45 ]. In a clinical prospective and observational study in 594 patients, CsA 0.1% was shown to be effective and safe for the treatment of VKC [ 46 ]. A recent systematic review and meta-analysis study suggests that topical CsA is effective and safe for the treatment of VKC, since signs and symptoms significantly improve after treatment, regardless of the CsA dosage [ 47 ].
A randomized, controlled two-year crossover study demonstrated the safety and efficacy of CsA 0.05% for long-term prevention of VKC relapses [ 40 ]. Patients treated with ketotifen had a risk of recurrences 2.4 times higher than patients treated with CsA. In addition, CsA significantly improved itching, photophobia and conjunctival hyperemia scores in comparison with ketotifen. These data are of great importance for the long-term management of pediatric patients at risk of visual impairment, whether due to steroid abuse or to continued recurrences of acute inflammation [ 40 ].
Tacrolimus is a potent drug, similar to CsA in its mode of action, but chemically distinct. A tacrolimus skin ointment is licensed for the treatment of moderate to severe atopic eyelid diseases and may have secondary benefits for AKC [ 48 – 50 ]. Conjunctival application of tacrolimus ointment 0.03% and 0.1% were effective, well tolerated, and safe in the treatment of severe allergic conjunctivitis [ 49 , 51 ]. In a multicenter, randomized, double-masked, placebo-controlled clinical trial, tacrolimus ophthalmic suspension 0.1% was shown to be effective in treating severe allergic conjunctivitis. Patients were treated twice daily for 4 weeks. Objective signs, subjective symptoms, giant papillae and corneal involvement were significantly improved. The most frequent tacrolimus-related adverse event was ocular irritation [ 52 ]. In the same study it is also reported that the dose of tacrolimus was based on the results from a previous dose-ranging study in which tacrolimus ophthalmic suspension 0.01%, 0.03%, and 0.1% were tested. Since 0.1% showed stronger improvement and similar safety profile compared with 0.01% and 0.03%, the 0.1% was considered an optimal dose.
Documentation on the quality, safety and efficacy of the different preparations used in the different clinical reports will be necessary before tacrolimus is granted orphan medication status for VKC.
A prospective double-masked randomized comparative trial comparing the efficacy of 0.1% tacrolimus ophthalmic ointment with CsA 2% showed that both were equally effective in the treatment of VKC [ 53 ].
Short-term, low-dose, topical mitomycin-C 0.01% has been considered for treating acute exacerbations in patients with severe VKC refractory to conventional treatment [ 54 ]. A significant decrease in signs and symptoms compared with the placebo group was shown at the end of the 2-week treatment period. Unavailability of commercial topical preparations, the short duration of studies, and the lack of data on the safety profile and long-term outcomes are major limitations in recommending mitomycin for the treatment of VKC.
Topical Non-Ocular Pharmacologic Treatment
The efficacy of intranasal corticosteroids in treating allergic nasal symptoms is well established. Recent data show a promising effect of intranasal corticosteroids on ocular symptoms of allergic rhinoconjunctivitis [ 55 ]. At the moment, no studies in VKC have been performed or reported in the literature. The mechanism by which an intranasal corticosteroid reduces ocular allergic symptoms has been under investigation; some effects on both the reflex neural activity and the local inflammation, facilitating nasolacrimal drainage, have been proposed [ 56 ]. Meta-analysis studies showed that there is no significant difference in improvement of eye symptoms between intranasal corticosteroids and oral antihistamines [ 57 ] including non-sedating antihistamines. Thus, in VKC patients with associated nasal symptoms, nasal corticosteroids may be beneficial.
Systemic Pharmacologic Treatment
Systemic treatment with oral antihistamines or antileukotrienes can reduce the severity of flare-ups and generalized hyper-reactivity. First-generation H 1 receptor antagonists may provide some relief of ocular itching, but are sedating and have anticholinergic effects such as dry mouth, dry eye, blurred vision and urinary retention. Second-generation antihistamines offer the same efficacy as their predecessors, but with a low-sedating profile and lack of anticholinergic activity. These drugs include acrivastine, cetirizine, ebastine, fexofenadine, loratadine and mizolastine. However, even their use has been associated with drying effects, particularly of the ocular surface [ 58 ]. Desloratadine and levocetirizine are considered a subsequent evolution of these second-generation agents and are preferred over first-generation antihistamines for the treatment of allergic conjunctivitis [ 59 ].
Aspirin 0.5–1 g per day has been shown as a steroid-sparing factor in the treatment of VKC [ 60 ]; however, it should be used with caution because of the well-known possible side effects.
In severe cases, systemic treatment with T-lymphocyte signal transduction inhibitors such as CsA or tacrolimus may ameliorate both the dermatologic and ocular manifestations in severe patients who are refractory to conventional treatment [ 61 ].
Omalizumab, an anti-IgE recombinant, humanized, non-anaphylactogenic antibody, directed against the receptor-binding domain of IgE, may be used in VKC patients with high levels of total serum IgE [ 62 ].
Specific Immunotherapy
Allergen-specific immunotherapy (SIT) is indicated only when a clearly defined systemic hypersensitivity to identified allergens exists. The choice of the allergen to be employed for SIT should be made in accordance with the combination of clinical history and results of skin prick test and specific serum IgE. Since the development of non-invasive formulations with better safety profiles, there is an increasing tendency to prescribe sublingual immunotherapy (SLIT) in young patients. A systematic review and meta-analysis of double-blind, placebo-controlled randomized controlled trials confirmed that SLIT reduces significantly ocular symptom scores in patients with allergic conjunctivitis with or without rhinitis [ 63 ]. The SIT treatment in IgE-positive patients with VKC was more effective than topical treatment in improving clinical symptoms and reducing total serum IgE [ 64 ].
Surgical Treatment
Supratarsal injection of either a short- or intermediate-acting corticosteroid has been proposed as a therapeutic approach to treating patients with refractory VKC [ 65 ]. Although significant symptomatic and clinical improvements have been reported, persistent increase in IOP occurred in one of 12 VKC patients [ 65 ].
Surgical removal of corneal plaques is recommended to alleviate severe symptoms and to allow for corneal re-epithelization. Giant papillae excision with intraoperative 0.02% mitomycin-C followed by CsA topical treatment may be indicated in cases of mechanical pseudoptosis or the presence of coarse giant papillae and continuous active disease [ 66 , 67 ].
Cryotherapy and/or excision of giant papillae should otherwise be avoided because these measures treat only the complications and not the underlying disease, and may induce unnecessary scarring. Amniotic membrane transplantation (AMT) following keratectomy has been described as a successful treatment for deep ulcers, in cases with slight stromal thinning [ 68 ]. The presence of residual membrane under the epithelium may affect postoperative corneal transparency. The treatment algorithm for corneal complication can be based on the Cameron clinical grading of shield ulcers [ 69 ]: Grade 1, ulcers received medical therapy alone; Grade 2 and Grade 3 ulcers received either medical therapy alone or medical therapy combined with debridement, AMT, or both. Using this approach, it was shown that Grade 1 ulcers respond well to medical therapy alone; Grade 2 ulcers occasionally may require additional debridement or AMT; Grade 3 ulcers are frequently refractory to medical therapy and require debridement and AMT for rapid re-epithelialization [ 70 ] .
Significant limbal stem cell deficiency as a complication of severe and persistent limbal inflammation has been treated with stem cell transplantation [ 71 , 72 ].
These and other more invasive procedures such as oral mucosal grafting should be avoided or considered only by ophthalmology experts in VKC management.
Practical Management of VKC
Treatment of VKC requires a multi-pronged approach that includes conservative measures and the use of drugs, as summarized in Table 3 . Close collaboration between ophthalmologists, allergists and pediatricians is recommended. Patients and parents should be made aware of the long duration of disease, its chronic evolution and possible complications.
It is recommended to use a clinical grading system to identify the more severe forms of VKC that are at higher risk of recurrences, corneal ulceration, and worsened final visual outcome [ 73 ]. A simple grading system has been proposed to formulate global guidelines for treating VKC (Table 4 ) [ 73 – 75 ].
The selection of a drug from the many available options is also based on geographical area, personal experience and preference of the treating physician, since there is no standard treatment and a lack of evidence to support choice of drug in the management of VKC.
Topical administration of mast cell stabilizers, with preference for those which have anti-eosinophil effects such as NAAGA and lodoxamide, should be started at the onset of the allergic symptoms and used continuously throughout the season. If monotherapy with mast cell stabilizers is not enough to prevent the symptoms, antihistamines or multiple-acting drugs such as olopatadine and ketotifen, 2–4 times a day should be added and continued for the entire season. Frequent instillations may be inconvenient, however, no significant side effects of these drugs have been reported with short- or long-term use. Preservative-free formulations should be recommended.
NSAIDs such as ketorolac, diclofenac and pranoprofen may be considered as steroid-sparing options. However, in clinical practice they have limited use in VKC management. Systemic treatment with oral antihistamines or antileukotrienes can reduce the severity of ocular flare-ups and the nonspecific hyper-reactivity typical of these patients. They should be started at the onset of symptoms and used continuously throughout the allergic season.
Moderate to severe VKC may require repeated topical steroid treatment to downregulate conjunctival inflammation and reduce cellular infiltrate. “Soft corticosteroids” may be considered preferentially as the first corticosteroid preparations to be used. Dosages are chosen based on the inflammatory state. An instillation frequency of 4 times per day for 5–10 days is recommended. Prednisolone, dexamethasone or betamethasone should be used as a second-line choice, or as first-line treatment in the most severe cases. A “pulsed” treatment of 3–5 days, in addition to the continuous use of mast cell stabilizers and topical antihistamines, is recommended. The use of ointment at night-time may be helpful in children when opening the eyes in the morning is difficult because of photophobia due to the epitheliotoxicity of released mediators while the eyes are closed.
CsA 1% or 2% can be considered for treatment of moderate to severe VKC. It decreases the severity of signs and symptoms and the need for steroids. No significant side effects, except for a burning sensation during administration, have been reported [ 39 ]. No randomized studies on dose–effect differences have been published. In clinical practice, one drop of CsA 1% from 2 to 4 times a day, depending on the severity of signs and symptoms, is effective for controlling the disease during seasonal exacerbations. Treatment can be suspended during winter until the first exacerbation of the new season. Adult patients respond better to CsA compared with any other therapeutic regimen [ 9 ].
If a patient does not respond to CsA, topical tacrolimus can be considered. Several experiences with tacrolimus have been reported using different preparations and concentrations. It seems to be more effective than CsA, and also effective in patients refractory to CsA. Randomized trials of topical tacrolimus are needed.
Corneal complications should be carefully monitored and anti-inflammatory therapy adjusted; in these cases, steroids should be used, since the pathogenesis of the ulcer is strictly immune-mediated. Corticosteroids are preferred over CsA, since they are more effective in inhibiting the inflammatory component of corneal damage (i.e., eosinophil- and neutrophil-liberated epithelial toxic mediators) [ 2 ].
Severe cases that do not respond to any of these topical therapies may require treatment with systemic corticosteroids (prednisone 1 mg/kg per day) for a short period of time.
If a systemic hypersensitivity to identified allergens exists, specific immunotherapy may be considered.
Leonardi A, Bogacka E, Fauquert JL, et al. Ocular allergy: recognizing and diagnosing hypersensitivity disorders of the ocular surface. Allergy. 2012;67(11):1327–37.
Article CAS PubMed Google Scholar
Leonardi A. Vernal keratoconjunctivitis: pathogenesis and treatment. Prog Retin Eye Res. 2002;21(3):319–39.
Tabbara KF. Ocular complications of vernal keratoconjunctivitis. Can J Ophthalmol. 1999;34(2):88–92.
CAS PubMed Google Scholar
Bonini S, Lambiase A, Marchi S, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term followup. Ophthalmology. 2000;107(6):1157–63.
Leonardi A, Sathe S, Bortolotti M, et al. Cytokines, matrix metalloproteases, angiogenic and growth factors in tears of normal subjects and vernal keratoconjunctivitis patients. Allergy. 2009;64(5):710–7.
Zicari AM, Nebbioso M, Lollobrigida V, et al. Vernal keratoconjunctivitis: atopy and autoimmunity. Eur Rev Med Pharmacol Sci. 2013;17(10):1419–23.
Zicari AM, Nebbioso M, Zicari A, et al. Serum levels of IL-17 in patients with vernal keratoconjunctivitis: a preliminary report. Eur Rev Med Pharmacol Sci. 2013;17(9):1242–4.
Kumagai N, Fukuda K, Fujitsu Y, et al. Role of structural cells of the cornea and conjunctiva in the pathogenesis of vernal keratoconjunctivitis. Prog Retin Eye Res. 2006;25(2):165–87.
Leonardi A, Lazzarini D, Motterle L, et al. Vernal keratoconjunctivitis-like disease in adults. Am J Ophthalmol. 2013;155(5):796–803.
Article PubMed Google Scholar
Mantelli F, Santos MS, Petitti T, et al. Systematic review and meta-analysis of randomised clinical trials on topical treatments for vernal keratoconjunctivitis. Br J Ophthalmol. 2007;91(12):1656–61.
Article CAS PubMed Central PubMed Google Scholar
Kumar S, Gupta N, Vivian AJ. Modern approach to managing vernal keratoconjunctivitis. Curr Allergy Asthma Rep. 2010;10(3):155–62.
Tabbara KF, Arafat NT. Cromolyn effects on vernal keratoconjunctivitis in children. Arch Ophthalmol. 1977;95(12):2184–6.
Bonini S, Barney NP, Schiavone M, et al. Effectiveness of nedocromil sodium 2% eyedrops on clinical symptoms and tear fluid cytology of patients with vernal conjunctivitis. Eye. 1992;6(Pt 6):648–52.
Caldwell DR, Verin P, Hartwich-Young R, et al. Efficacy and safety of lodoxamide 0.1% vs cromolyn sodium 4% in patients with vernal keratoconjunctivitis. Am J Ophthalmol. 1992;113(6):632–7.
Kjellman NI, Stevens MT. Clinical experience with Tilavist: an overview of efficacy and safety. Allergy. 1995;50(21 Suppl):14–22 (discussion 34–8).
Bonini S, Schiavone M, Magrini L, et al. Efficacy of lodoxamide eye drops on mast cells and eosinophils after allergen challenge in allergic conjunctivitis. Ophthalmology. 1997;104(5):849–53.
Leonardi A, Borghesan F, Avarello A, et al. Effect of lodoxamide and disodium cromoglycate on tear eosinophil cationic protein in vernal keratoconjunctivitis. Br J Ophthalmol. 1997;81(1):23–6.
Cerqueti PM, Ricca V, Tosca MA, et al. Lodoxamide treatment of allergic conjunctivitis. Int Arch Allergy Immunol. 1994;105(2):185–9.
Gunduz K, Ucakhan O, Budak K, et al. Efficacy of lodoxamide 0.1% versus N -acetyl aspartyl glutamic acid 6% ophthalmic solutions in patients with vernal keratoconjunctivitis. Ophthalmic Res. 1996;28(2):80–7.
Leonardi A, Bremond-Gignac D, Bortolotti M, et al. Clinical and biological efficacy of preservative-free NAAGA eye-drops versus levocabastine eye-drops in vernal keratoconjunctivitis patients. Br J Ophthalmol. 2007;91(12):1662–6.
Lapalus P, Moulin G, Bayer V, et al. Effects of a new anti-allergic agent: the magnesium salt of N -acetyl-aspartyl-glutamic acid on experimental allergic inflammation of the rabbit eye. Curr Eye Res. 1986;5(7):517–22.
Bielory L, Ghafoor S. Histamine receptors and the conjunctiva. Curr Opin Allergy Clin Immunol. 2005;5(5):437–40.
Yanni JM, Weimer LK, Sharif NA, et al. Inhibition of histamine-induced human conjunctival epithelial cell responses by ocular allergy drugs. Arch Ophthalmol. 1999;117(5):643–7.
Yanni JM, Sharif NA, Gamache DA, et al. A current appreciation of sites for pharmacological intervention in allergic conjunctivitis: effects of new topical ocular drugs. Acta Ophthalmol Scand Suppl. 1999;228:33–7.
PubMed Google Scholar
Verin P, Allewaert R, Joyaux JC, et al. Comparison of lodoxamide 0.1% ophthalmic solution and levocabastine 0.05% ophthalmic suspension in vernal keratoconjunctivitis. Eur J Ophthalmol. 2001;11(2):120–5.
Qasem AR, Bucolo C, Baiula M, et al. Contribution of alpha4beta1 integrin to the antiallergic effect of levocabastine. Biochem Pharmacol. 2008;76(6):751–62.
Secchi A, Leonardi A, Discepola M, et al. An efficacy and tolerance comparison of emedastine difumarate 0.05% and levocabastine hydrochloride 0.05%: reducing chemosis and eyelid swelling in subjects with seasonal allergic conjunctivitis. Emadine Study Group. Acta Ophthalmol Scand Suppl. 2000;230:48–51.
Secchi A, Ciprandi G, Leonardi A, et al. Safety and efficacy comparison of emedastine 0.05% ophthalmic solution compared to levocabastine 0.05% ophthalmic suspension in pediatric subjects with allergic conjunctivitis. Emadine Study Group. Acta Ophthalmol Scand Suppl. 2000;230:42–7.
Hida WT, Nogueira DC, Schaefer A, et al. Comparative study between 0.025% ketotifen fumarate and 0.1% olopatadine hydrochloride in the treatment of vernal keratoconjunctivitis. Arq Bras Oftalmol. 2006;69(6):851–6.
Corum I, Yeniad B, Bilgin LK, Ilhan R. Efficiency of olopatadine hydrochloride 0.1% in the treatment of vernal keratoconjunctivitis and goblet cell density. J Ocul Pharmacol Ther. 2005;21(5):400–5.
Spector SL, Raizman MB. Conjunctivitis medicamentosa. J Allergy Clin Immunol. 1994;94(1):134–6.
Leonardi A, Busato F, Fregona I, et al. Anti-inflammatory and antiallergic effects of ketorolac tromethamine in the conjunctival provocation model. Br J Ophthalmol. 2000;84(11):1228–32.
Gupta S, Khurana AK, Ahluwalia BK, Gupta NC. Topical indomethacin for vernal keratoconjunctivitis. Acta Ophthalmol (Copenh). 1991;69(1):95–8.
Article CAS Google Scholar
Sharma A, Gupta R, Ram J, Gupta A. Topical ketorolac 0.5% solution for the treatment of vernal keratoconjunctivitis. Indian J Ophthalmol. 1997;45(3):177–80.
D’Angelo G, Lambiase A, Cortes M, et al. Preservative-free diclofenac sodium 0.1% for vernal keratoconjunctivitis. Graefes Arch Clin Exp Ophthalmol. 2003;241(3):192–5.
McGhee CN, Dean S, Danesh-Meyer H. Locally administered ocular corticosteroids: benefits and risks. Drug Saf. 2002;25(1):33–55.
Ang M, Ti SE, Loh R, et al. Steroid-induced ocular hypertension in Asian children with severe vernal keratoconjunctivitis. Clin Ophthalmol. 2012;6:1253–8.
Ang M, Ho CL, Tan D, Chan C. Severe vernal keratoconjunctivitis requiring trabeculectomy with mitomycin C for corticosteroid-induced glaucoma. Clin Exp Ophthalmol. 2012;40(4):e149–55.
Article Google Scholar
Utine CA, Stern M, Akpek EK. Clinical review: topical ophthalmic use of cyclosporin A. Ocul Immunol Inflamm. 2010;18(5):352–61.
Lambiase A, Leonardi A, Sacchetti M, et al. Topical cyclosporine prevents seasonal recurrences of vernal keratoconjunctivitis in a randomized, double-masked, controlled 2-year study. J Allergy Clin Immunol. 2011;128(4):896–7 e9.
Google Scholar
Pucci N, Novembre E, Cianferoni A, et al. Efficacy and safety of cyclosporine eyedrops in vernal keratoconjunctivitis. Ann Allergy Asthma Immunol. 2002;89(3):298–303.
Leonardi A, Borghesan F, Faggian D, et al. Eosinophil cationic protein in tears of normal subjects and patients affected by vernal keratoconjunctivitis. Allergy. 1995;50(7):610–3.
Cetinkaya A, Akova YA, Dursun D, Pelit A. Topical cyclosporine in the management of shield ulcers. Cornea. 2004;23(2):194–200.
Daniell M, Constantinou M, Vu HT, Taylor HR. Randomised controlled trial of topical ciclosporin A in steroid dependent allergic conjunctivitis. Br J Ophthalmol. 2006;90(4):461–4.
Ozcan AA, Ersoz TR, Dulger E. Management of severe allergic conjunctivitis with topical cyclosporin a 0.05% eyedrops. Cornea. 2007;26(9):1035–8.
Ebihara N, Ohashi Y, Uchio E, et al. A large prospective observational study of novel cyclosporine 0.1% aqueous ophthalmic solution in the treatment of severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2009;25(4):365–72.
Wan KH, Chen LJ, Rong SS, et al. Topical Cyclosporine in the Treatment of Allergic Conjunctivitis: A Meta-analysis. Ophthalmology. 2013.
Rikkers SM, Holland GN, Drayton GE, et al. Topical tacrolimus treatment of atopic eyelid disease. Am J Ophthalmol. 2003;135(3):297–302.
Vichyanond P, Tantimongkolsuk C, Dumrongkigchaiporn P, et al. Vernal keratoconjunctivitis: result of a novel therapy with 0.1% topical ophthalmic FK-506 ointment. J Allergy Clin Immunol. 2004;113(2):355–8.
Virtanen HM, Reitamo S, Kari M, Kari O. Effect of 0.03% tacrolimus ointment on conjunctival cytology in patients with severe atopic blepharoconjunctivitis: a retrospective study. Acta Ophthalmol Scand. 2006;84(5):693–5.
Attas-Fox L, Barkana Y, Iskhakov V, et al. Topical tacrolimus 0.03% ointment for intractable allergic conjunctivitis: an open-label pilot study. Curr Eye Res. 2008;33(7):545–9.
Ohashi Y, Ebihara N, Fujishima H, et al. A randomized, placebo-controlled clinical trial of tacrolimus ophthalmic suspension 0.1% in severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2010;26(2):165–74.
Labcharoenwongs P, Jirapongsananuruk O, Visitsunthorn N, et al. A double-masked comparison of 0.1% tacrolimus ointment and 2% cyclosporine eye drops in the treatment of vernal keratoconjunctivitis in children. Asian Pac J Allergy Immunol. 2012;30(3):177–84.
Akpek EK, Hasiripi H, Christen WG, Kalayci D. A randomized trial of low-dose, topical mitomycin-C in the treatment of severe vernal keratoconjunctivitis. Ophthalmology. 2000;107(2):263–9.
Bielory L. Ocular symptom reduction in patients with seasonal allergic rhinitis treated with the intranasal corticosteroid mometasone furoate. Ann Allergy Asthma Immunol. 2008;100(3):272–9.
Baroody FM, Shenaq D, DeTineo M, et al. Fluticasone furoate nasal spray reduces the nasal-ocular reflex: a mechanism for the efficacy of topical steroids in controlling allergic eye symptoms. J Allergy Clin Immunol. 2009;123(6):1342–8.
Weiner JM, Abramson MJ, Puy RM. Intranasal corticosteroids versus oral H1 receptor antagonists in allergic rhinitis: systematic review of randomised controlled trials. BMJ. 1998;317(7173):1624–9.
Bielory L, Lien KW, Bigelsen S. Efficacy and tolerability of newer antihistamines in the treatment of allergic conjunctivitis. Drugs. 2005;65(2):215–28.
Hingorani M, Lightman S. Therapeutic options in ocular allergic disease. Drugs. 1995;50(2):208–21.
Abelson MB, Butrus SI, Weston JH. Aspirin therapy in vernal conjunctivitis. Am J Ophthalmol. 1983;95(4):502–5.
Anzaar F, Gallagher MJ, Bhat P, et al. Use of systemic T-lymphocyte signal transduction inhibitors in the treatment of atopic keratoconjunctivitis. Cornea. 2008;27(8):884–8.
de Klerk TA, Sharma V, Arkwright PD. Biswas S. J AAPOS: Severe vernal keratoconjunctivitis successfully treated with subcutaneous omalizumab; 2013.
Calderon MA, Penagos M, Sheikh A, et al. Sublingual immunotherapy for allergic conjunctivitis: Cochrane systematic review and meta-analysis. Clin Exp Allergy. 2011;41(9):1263–72.
Mahdy RA, Nada WM, Marei AA. Subcutaneous allergen-specific immunotherapy versus topical treatment in vernal keratoconjunctivitis. Cornea. 2012;31(5):525–8.
Holsclaw DS, Whitcher JP, Wong IG, Margolis TP. Supratarsal injection of corticosteroid in the treatment of refractory vernal keratoconjunctivitis. Am J Ophthalmol. 1996;121(3):243–9.
Tanaka M, Takano Y, Dogru M, et al. A comparative evaluation of the efficacy of intraoperative mitomycin C use after the excision of cobblestone-like papillae in severe atopic and vernal keratoconjunctivitis. Cornea. 2004;23(4):326–9.
Fujishima H, Fukagawa K, Satake Y, et al. Combined medical and surgical treatment of severe vernal keratoconjunctivitis. Jpn J Ophthalmol. 2000;44(5):511–5.
Tanaka M, Dogru M, Takano Y, et al. Quantitative evaluation of the early changes in ocular surface inflammation following MMC-aided papillary resection in severe allergic patients with corneal complications. Cornea. 2006;25(3):281–5.
Cameron JA. Shield ulcers and plaques of the cornea in vernal keratoconjunctivitis. Ophthalmology. 1995;102(6):985–93.
Reddy JC, Basu S, Saboo US, et al. Management, clinical outcomes, and complications of shield ulcers in vernal keratoconjunctivitis. Am J Ophthalmol. 2013;155(3):550–9 e1.
Sangwan VS, Jain V, Vemuganti GK, Murthy SI. Vernal keratoconjunctivitis with limbal stem cell deficiency. Cornea. 2011;30(5):491–6.
Sangwan VS, Murthy SI, Vemuganti GK, et al. Cultivated corneal epithelial transplantation for severe ocular surface disease in vernal keratoconjunctivitis. Cornea. 2005;24(4):426–30.
Sacchetti M, Lambiase A, Mantelli F, et al. Tailored approach to the treatment of vernal keratoconjunctivitis. Ophthalmology. 2010;117(7):1294–9.
Leonardi A, Lazzarini D, Bortolotti M, et al. Corneal confocal microscopy in patients with vernal keratoconjunctivitis. Ophthalmology. 2011.
Leonardi A, Lazzarini D, Bortolotti M, et al. Corneal confocal microscopy in patients with vernal keratoconjunctivitis. Ophthalmology. 2012;119(3):509–15.
Download references
Acknowledgments
Dr. Leonardi is the guarantor for this article, and takes responsibility for the integrity of the work as a whole. No funding or sponsorship was received for the publication of this article.
Conflict of interest
Dr. Leonardi declares no conflicts of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and affiliations.
Ophthalmology Unit, Department of Neuroscience, University of Padua, Padua, Italy
Andrea Leonardi
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Andrea Leonardi .
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Leonardi, A. Management of Vernal Keratoconjunctivitis. Ophthalmol Ther 2 , 73–88 (2013). https://doi.org/10.1007/s40123-013-0019-y
Download citation
Received : 16 May 2013
Published : 07 September 2013
Issue Date : December 2013
DOI : https://doi.org/10.1007/s40123-013-0019-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Anti-allergic treatment
- Cyclosporine A
- Grading system
- Immunomodulation
- Vernal keratoconjunctivitis
- Find a journal
- Publish with us
- Track your research
- Case report
- Open access
- Published: 12 June 2021
Vernal keratoconjunctivitis in twins: case report and literature review
- Maria Cristina Artesani 1 ,
- Mariacristina Esposito 2 ,
- Maurizio Mennini 1 ,
- Marco Andreani 3 ,
- Franco Locatelli 4 ,
- Luca Buzzonetti 2 &
- Alessandro Fiocchi 1
Italian Journal of Pediatrics volume 47 , Article number: 136 ( 2021 ) Cite this article
1673 Accesses
4 Citations
Metrics details
Vernal keratoconjunctivitis (VKC) is a chronic bilateral seasonal allergic inflammatory disease with a prevalence of < 1 case out of 10,000 in Europe [ 1 ], which occurs mainly in pediatric age. The diagnosis is generally confirmed by the finding at the ocular examination of conjunctival hyperemia, papillary hypertrophy in the tarsal conjunctiva, giant papillae and Trantas dots in the limbus region.
Few studies evaluated the association of specific HLA genes with VKC. In an Italian pediatric study, HLA class I A32 was found more frequent in familiar than sporadic forms of VKC [ 2 ]. In another pediatric population, patients with VKC presented more frequently HLA-DRB1*01 and DRB1*16, while the DRB1*13 was negatively associated with VKC. The DRB1*01 and DRB1*16 families of alleles are in strong linkage disequilibrium (LD) with the DQB1*05 allele, that was found significantly more frequent in VKC patients than in controls [ 3 ]. In this context, HLA analysis of monozygotic twin patients with VKC may provide useful information to clarify the haplotypes potentially implicated in the pathogenesis of the disease. Furthermore, differently from previous reported data, in our investigation we applied a next generation sequencing (NGS) typing approach to determine the different HLA alleles of class I and II present in the studied patients.
After obtaining the informed consent from patients and their parents and the approval of our local Ethics Committee, we describe here the assessment of HLA in a couple of monozygotic twins and in their father, all with VKC. Two 10-years-old Caucasian male monozygotic twins with history of mild intermittent allergic rhinoconjuctivitis to dust mites, as determined by positive skin prick testing, came to our observation due to the appearance of bilateral conjunctivitis in spring-summer time which responded only to steroid topic therapy. The patients complained of ocular itching, burning, watering and mucoid stringy discharge and intense photophobia. On slit-lamp examination, the children showed conjunctival hyperemia, papillary hypertrophy, giant papillae and Tranta’s nodules (Fig. 1 a, b). Vernal keratoconjunctivitis was diagnosed and the disease activity was graded, according to the Bonini VKC severity score [ 4 ], as severe (grade 3) for both twins. A successful topical immunosuppressant therapy with cyclosporin 1% was initiated. Their father was diagnosed with VKC, while their mother had no ocular symptoms or signs. We performed the HLA typing at high resolution of the DNA of the two patients and of all the family members available, by NGS.
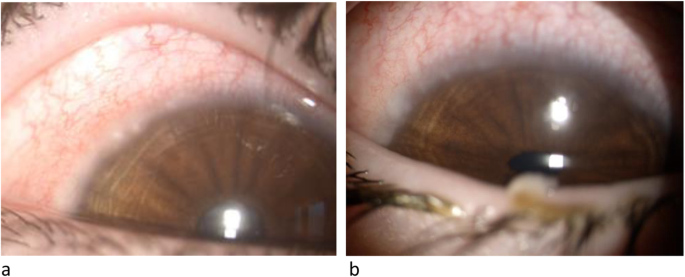
Slit-lamp examination, twin n.1 (a) and twin n.2 (b)
DNA samples were extracted using an EZ1 DSP DNA Blood kit (Qiagen - Thermo Fisher Scientific Walthman, Massacchussets, USA) on an automatic EZ1 Advanced XL instrument (Qiagen- Thermo Fisher Scientific Walthman, Massacchussets, USA) from peripheral blood samples. HLA genotyping was obtained after a library preparation, using the AllType kit (One Lambda, Canoga Park, California) and run on the Ion Torrent S5 XL platform (Thermo Fisher Scientific Walthman, Massacchussets, USA). These kits use a single multiplexed polymerase chain reaction (PCR) to amplify the full HLA-A/B/C/DQA1/DPA1 gene sequences and from exon 2 to the 30UTR of the HLADRB1/3/4/5/DQB1/DPB1 genes. Reads were analyzed using the HLA TypeStream Visual Software (TSV) (One Lambda), ver. 1.1.0.27232.
The twins’ haplotype is presented in detail in Table 1 , reporting the different whole HLA haplotypes of all the different family members investigated. The patients presented three different HLA alleles reported to be strongly associated with the developing of VKC: DQB1*05:01:01 and HLA-DRB1*01:01:01 alleles, inherited from the father, also affected by VKC, and the HLA-A*32:01:01 allele, present in the mother haplotype (Fig. 2 ). The mother presents also the HLA-DRB1*13:02:01 allele, considered protective for VKC [2].
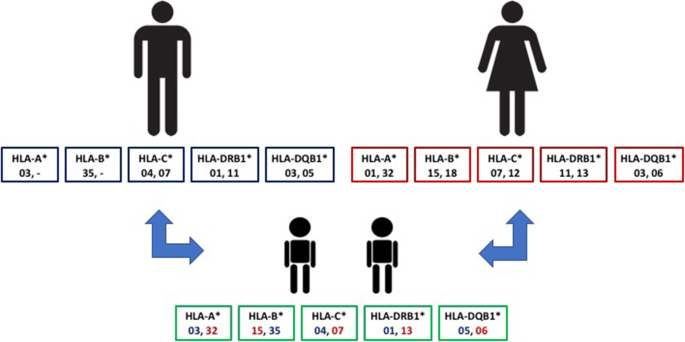
Familiar HLA typing
As reported by Zicari et al., DQB1*05 has been already associated to VKC, both in presence of DRB1*01 and DRB1*16 families of alleles typed at low resolution [ 3 ]. It is well known from the literature that these two families of alleles are in strong LD with DQB1*05:01 and DQB1*05:02, respectively, specified as DRB1*01:01-DQB1*05:01 and DRB1*16:01-DQB1*05:02.
VKC is an immune-based disorder, most likely with a genetic predisposition [ 5 ], but many questions about its pathogenesis remain still unanswered [ 6 ]. Further studies need to be carried out to elucidate the full spectrum of immune-genetics of VKC in order to obtain a real stratification of risk. At the moment, our case report suggests that the presence of VKC cases in families should be valorized and approached as a potentially genetically determined condition.
To identify other similar cases, a search of Medline via PubMed and Google Scholar was conducted using the following search strings: “keratoconjunctivis” or “vernal” or “vernal keratoconjunctivis” AND “twins”. The search was restricted to scientific literature published up to January 2021. We identified four reports of VKC in twins, but three of them did not evaluated the relevance of HLA assessment [ 7 , 8 , 9 ].
So only one case report described the assessment of HLA haplotypes A2, A11, B27, B61, DR1, and DR4 in a couple of twins affected by atopic dermatitis, allergic rhino-conjunctivitis and food allergy, but it is not possible to deduce from the text whether the described conjunctivitis could be a VKC or just an allergic conjunctivitis [ 10 ].
So far, at our knowledge, this is the first description of HLA haplotypes through NGS approach in twins with VKC.
Availability of data and materials
The datasets generated during and/or analysed during the current study available from the corresponding author on reasonable request.
Vichyanond P, Pacharn P, Pleyer U, Leonardi A. Vernal keratoconjunctivitis: a severe allergic eye disease with remodeling changes. Pediatr Allergy Immunol. 2014;25(4):314–22. https://doi.org/10.1111/pai.12197 .
Article PubMed Google Scholar
Pucci N, Azari C, Vierucci A. La cheratocongiuntivite vernal. Riv Immunol Allergol Pediatr. 2007;2:37–44.
Google Scholar
Zicari AM, Mora B, Lollobrigida V, Occasi F, Cesoni Marcelli A, Megiorni F, et al. Immunogenetic investigation in vernal keratoconjunctivitis. Pediatr Allergy Immunol. 2014;25(5):508–10. https://doi.org/10.1111/pai.12231 .
Bonini S, Sacchetti M, Mantelli F, Lambiase A. Clinical grading of vernal keratoconjunctivitis. Curr Opin Allergy Clin Immunol. 2007;7(5):436–41. https://doi.org/10.1097/ACI.0b013e3282efb726 .
Bonini S, Bonini S, Lambiase A, Magrini L, Rumi C, del Prete G, et al. Vernal keratoconjunctivitis: a model of 5q cytokine gene cluster disease. Int Arch Allergy Immunol. 1995;107(1–3):95–8. https://doi.org/10.1159/000236942 .
Article CAS PubMed Google Scholar
Zicari AM, Brindisi G, De Castro G, Lollobrigida V, Nebbioso M, Duse M. Is oxidative stress involved in vernal keratoconjunctivitis? Results from a pilot study in children. Pediatr Allergy Immunol. 2020;31(Suppl 26):52–6. https://doi.org/10.1111/pai.13382 .
Rosenthal WN, Insler MS. Vernal keratoconjunctivitis: new corneal findings in fraternal twins. Cornea. 1984-1985;3(4):288–90.
Article Google Scholar
Al-Okour KR, Odat TA. Vernal keratoconjunctivitis clinical features and complications in 123 patients in Gaza strip. JRMS. 2014;21(1):55–62. https://doi.org/10.12816/0002580 .
Darrell RW. Superior limbic keratoconjunctivitis in identical twins. Cornea. 1992;11(3):262–3. https://doi.org/10.1097/00003226-199205000-00013 .
Murakami Y, Matsui S, Kijima A, Kitaba S, Murota H, Katayama I. Cedar pollen aggravates atopic dermatitis in childhood monozygotic twin patients with allergic rhino conjunctivitis. Allergol Int. 2011;60(3):397–400. https://doi.org/10.2332/allergolint.10-CR-0268 .
Download references
Acknowledgments
Not applicable.
Authors disclose any financial support for the manuscript.
Author information
Authors and affiliations.
Translational Specialized Pediatrics Research Area, Allergic Diseases Research Unit, Bambino Gesù Children’s Hospital, IRCCS, Piazza San’Onofrio, 4, 00165, Rome, Italy
Maria Cristina Artesani, Maurizio Mennini & Alessandro Fiocchi
Ophthalmology Department, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
Mariacristina Esposito & Luca Buzzonetti
Laboratory of Immunogenetics of Transplant, Department of Pediatric Hematology/Oncology and of Cell and Gene Therapy, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
Marco Andreani
Department of Pediatric Hematology/Oncology and of Cell and Gene Therapy, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy
Franco Locatelli
You can also search for this author in PubMed Google Scholar
Contributions
MCA, the corresponding author, analyzed and interpreted the patient data and is the main contributor in writing the manuscript, ME performed the ocular examination, MM was a major contributor in writing the manuscript, MA performed the HLA examination and was a major contributor in writing the manuscript; FL, LB, AF have drafted the work. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Maria Cristina Artesani .
Ethics declarations
Competing interests.
Authors disclose any Conflict of interests related to the manuscript content.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Artesani, M.C., Esposito, M., Mennini, M. et al. Vernal keratoconjunctivitis in twins: case report and literature review. Ital J Pediatr 47 , 136 (2021). https://doi.org/10.1186/s13052-021-01073-w
Download citation
Received : 17 March 2021
Accepted : 17 May 2021
Published : 12 June 2021
DOI : https://doi.org/10.1186/s13052-021-01073-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Italian Journal of Pediatrics
ISSN: 1824-7288
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
RELATED TOPICS
INTRODUCTION
GPC is an inflammatory disorder that represents a reaction to lid movement over a foreign substance, such as hard contact lenses. Toxic conjunctivitis is not allergic in nature, but it is frequently confused with allergic ocular disease. It develops with protracted use of topical medications, mostly due to preservatives. GPC and toxic conjunctivitis are discussed in detail separately. (See "Giant papillary conjunctivitis" and "Toxic conjunctivitis" .)
Seasonal and perennial allergic conjunctivitis, the most common forms of ocular allergy, are also discussed separately. (See "Allergic conjunctivitis: Clinical manifestations and diagnosis" .)
EPIDEMIOLOGY
Males are more commonly affected than females. In one series, the male-to-female ratio was 3.2:1 in patients <20 years of age but was nearly equal in older patients [ 4 ]. Age at onset is generally before 10 years, with the earliest reported onset at five months of age [ 5 ], although VKC can infrequently occur in adults. Patients usually "outgrow" the disease with the onset of puberty.
Help | Advanced Search
Computer Science > Computation and Language
Title: text generation: a systematic literature review of tasks, evaluation, and challenges.
Abstract: Text generation has become more accessible than ever, and the increasing interest in these systems, especially those using large language models, has spurred an increasing number of related publications. We provide a systematic literature review comprising 244 selected papers between 2017 and 2024. This review categorizes works in text generation into five main tasks: open-ended text generation, summarization, translation, paraphrasing, and question answering. For each task, we review their relevant characteristics, sub-tasks, and specific challenges (e.g., missing datasets for multi-document summarization, coherence in story generation, and complex reasoning for question answering). Additionally, we assess current approaches for evaluating text generation systems and ascertain problems with current metrics. Our investigation shows nine prominent challenges common to all tasks and sub-tasks in recent text generation publications: bias, reasoning, hallucinations, misuse, privacy, interpretability, transparency, datasets, and computing. We provide a detailed analysis of these challenges, their potential solutions, and which gaps still require further engagement from the community. This systematic literature review targets two main audiences: early career researchers in natural language processing looking for an overview of the field and promising research directions, as well as experienced researchers seeking a detailed view of tasks, evaluation methodologies, open challenges, and recent mitigation strategies.
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .

Review of Related Literature (RRL)
Ai generator.
The Review of Related Literature (RRL) is a crucial section in research that examines existing studies and publications related to a specific topic. It summarizes and synthesizes previous findings, identifies gaps, and provides context for the current research. RRL ensures the research is grounded in established knowledge, guiding the direction and focus of new studies.
What Is Review of Related Literature (RRL)?
The Review of Related Literature (RRL) is a detailed analysis of existing research relevant to a specific topic. It evaluates, synthesizes, and summarizes previous studies to identify trends, gaps, and conflicts in the literature. RRL provides a foundation for new research, ensuring it builds on established knowledge and addresses existing gaps.
Format of Review of Related Literature (RRL)
The Review of Related Literature (RRL) is a critical part of any research paper or thesis . It provides an overview of existing research on your topic and helps to establish the context for your study. Here is a typical format for an RRL:
1. Introduction
- Purpose : Explain the purpose of the review and its importance to your research.
- Scope : Define the scope of the literature reviewed, including the time frame, types of sources, and key themes.
2. Theoretical Framework
- Concepts and Theories : Present the main theories and concepts that underpin your research.
- Relevance : Explain how these theories relate to your study.
3. Review of Empirical Studies
- Sub-theme 1 : Summarize key studies, including methodologies, findings, and conclusions.
- Sub-theme 2 : Continue summarizing studies, focusing on different aspects or variables.
- Sub-theme 3 : Include any additional relevant studies.
4. Methodological Review
- Approaches : Discuss the various methodologies used in the reviewed studies.
- Strengths and Weaknesses : Highlight the strengths and weaknesses of these methodologies.
- Gaps : Identify gaps in the existing research that your study aims to address.
5. Synthesis and Critique
- Integration : Integrate findings from the reviewed studies to show the current state of knowledge.
- Critique : Critically evaluate the literature, discussing inconsistencies, limitations, and areas for further research.
6. Conclusion
- Summary : Summarize the main findings from the literature review.
- Research Gap : Clearly state the research gap your study will address.
- Contribution : Explain how your study will contribute to the existing body of knowledge.
7. References
- Citation Style : List all the sources cited in your literature review in the appropriate citation style (e.g., APA, MLA, Chicago).
Review of Related Literature (RRL) 1. Introduction This review examines research on social media’s impact on mental health, focusing on anxiety and depression across various demographics over the past ten years. 2. Theoretical Framework Anchored in Social Comparison Theory and Uses and Gratifications Theory, this review explores how individuals’ social media interactions affect their mental health. 3. Review of Empirical Studies Adolescents’ Mental Health Instagram & Body Image : Smith & Johnson (2017) found Instagram use linked to body image issues and lower self-esteem among 500 high school students. Facebook & Anxiety : Brown & Green (2016) showed Facebook use correlated with higher anxiety and depressive symptoms in a longitudinal study of 300 students. Young Adults’ Mental Health Twitter & Stress : Davis & Lee (2018) reported higher stress levels among heavy Twitter users in a survey of 400 university students. LinkedIn & Self-Esteem : Miller & White (2019) found LinkedIn use positively influenced professional self-esteem in 200 young professionals. Adult Mental Health General Social Media Use : Thompson & Evans (2020) found moderate social media use associated with better mental health outcomes, while excessive use correlated with higher anxiety and depression in 1,000 adults. 4. Methodological Review Studies used cross-sectional surveys, longitudinal designs, and mixed methods. Cross-sectional surveys provided large data sets but couldn’t infer causation. Longitudinal studies offered insights into long-term effects but were resource-intensive. Mixed methods enriched data through qualitative insights but required careful integration. 5. Synthesis and Critique The literature shows a complex relationship between social media and mental health, with platform-specific and demographic-specific effects. However, reliance on self-reported data introduces bias, and many cross-sectional studies limit causal inference. More longitudinal and experimental research is needed. 6. Conclusion Current research offers insights into social media’s mental health impact but leaves gaps, particularly regarding long-term effects and causation. This study aims to address these gaps through comprehensive longitudinal analysis. 7. References Brown, A., & Green, K. (2016). Facebook Use and Anxiety Among High School Students . Psychology in the Schools, 53(3), 257-264. Davis, R., & Lee, S. (2018). Twitter and Psychological Stress: A Study of University Students . Journal of College Student Development, 59(2), 120-135. Miller, P., & White, H. (2019). LinkedIn and Its Effect on Professional Self-Esteem . Journal of Applied Psychology, 104(1), 78-90. Smith, J., & Johnson, L. (2017). The Impact of Instagram on Teen Body Image . Journal of Adolescent Health, 60(5), 555-560. Thompson, M., & Evans, D. (2020). The Relationship Between Social Media Use and Mental Health in Adults . Cyberpsychology, Behavior, and Social Networking, 23(4), 201-208.
Review of Related Literature (RRL) Examples
Review of related literature in research, review of related literature in research paper, review of related literature qualitative research.

Review of Related Literature Quantitative Research

More Review of Related Literature (RRL) Examples
- Impact of E-learning on Student Performance
- Effectiveness of Mindfulness in Workplace
- Green Building and Energy Efficiency
- Impact of Technology on Healthcare Delivery
- Effects of Nutrition on Cognitive Development in Children
- Impact of Employee Training Programs on Productivity
- Effects of Climate Change on Biodiversity
- Impact of Parental Involvement on Student Achievement
- Effects of Mobile Learning on Student Engagement
- Effects of Urban Green Spaces on Mental Health
Purpose of the Review of Related Literature (RRL)
The Review of Related Literature (RRL) serves several critical purposes in research:
- Establishing Context : It situates your research within the broader field, showing how your study relates to existing work.
- Identifying Gaps : It highlights gaps, inconsistencies, and areas needing further exploration in current knowledge, providing a clear rationale for your study.
- Avoiding Duplication : By reviewing what has already been done, it helps ensure your research is original and not a repetition of existing studies.
- Building on Existing Knowledge : It allows you to build on the findings of previous research, using established theories and methodologies to inform your work.
- Theoretical Foundation : It provides a theoretical basis for your research, grounding it in existing concepts and theories.
- Methodological Insights : It offers insights into the methods and approaches used in similar studies, helping you choose the most appropriate methods for your research.
- Establishing Credibility : It demonstrates your familiarity with the field, showing that you are well-informed and have a solid foundation for your research.
- Supporting Arguments : It provides evidence and support for your research questions, hypotheses, and objectives, strengthening the overall argument of your study.
How to Write Review of Related Literature (RRL)
Writing a Review of Related Literature (RRL) involves several key steps. Here’s a step-by-step guide:
1. Define the Scope and Objectives
- Determine the Scope : Decide on the breadth of the literature you will review, including specific themes, time frame, and types of sources.
- Set Objectives : Clearly define the purpose of the review. What do you aim to achieve? Identify gaps, establish context, or build on existing knowledge.
2. Search for Relevant Literature
- Identify Keywords : Use keywords and phrases related to your research topic.
- Use Databases : Search academic databases like Google Scholar, PubMed, JSTOR, etc., for relevant articles, books, and papers.
- Select Sources : Choose sources that are credible, recent, and relevant to your research.

3. Evaluate and Select the Literature
- Read Abstracts and Summaries : Quickly determine the relevance of each source.
- Assess Quality : Consider the methodology, credibility of the authors, and publication source.
- Select Key Studies : Choose studies that are most relevant to your research questions and objectives.
4. Organize the Literature
- Thematic Organization : Group studies by themes or topics.
- Chronological Organization : Arrange studies in the order they were published to show the development of ideas over time.
- Methodological Organization : Categorize studies by the methods they used.
5. Write the Review
- State the purpose and scope of the review.
- Explain the importance of the topic.
- Theoretical Framework : Present and discuss the main theories and concepts.
- Summarize key studies, including their methodologies, findings, and conclusions.
- Organize by themes or other chosen organizational methods.
- Methodological Review : Discuss the various methodologies used, highlighting their strengths and weaknesses.
- Synthesis and Critique : Integrate findings, critically evaluate the literature, and identify gaps or inconsistencies.
- Summarize the main findings from the literature review.
- Highlight the research gaps your study will address.
- State how your research will contribute to the existing knowledge.
6. Cite the Sources
- Use Appropriate Citation Style : Follow the required citation style (e.g., APA, MLA, Chicago).
- List References : Provide a complete list of all sources cited in your review.
What is an RRL?
An RRL summarizes and synthesizes existing research on a specific topic to identify gaps and guide future studies.
Why is RRL important?
It provides context, highlights gaps, and ensures new research builds on existing knowledge.
How do you write an RRL?
Organize by themes, summarize studies, evaluate methodologies, identify gaps, and conclude with relevance to current research.
What sources are used in RRL?
Peer-reviewed journals, books, conference papers, and credible online resources.
How long should an RRL be?
Length varies; typically 10-20% of the total research paper.
What are common RRL mistakes?
Lack of organization, insufficient synthesis, over-reliance on outdated sources, and failure to identify gaps.
Can an RRL include non-scholarly sources?
Primarily scholarly, but reputable non-scholarly sources can be included for context.
What is the difference between RRL and bibliography?
RRL synthesizes and analyzes the literature, while a bibliography lists sources.
How often should an RRL be updated?
Regularly, especially when new relevant research is published.
Can an RRL influence research direction?
Yes, it identifies gaps and trends that shape the focus and methodology of new research.
Text prompt
- Instructive
- Professional
10 Examples of Public speaking
20 Examples of Gas lighting
Omalizumab in Severe Refractory Vernal Keratoconjunctivitis in Children: Case Series and Review of the Literature
Affiliations.
- 1 Ophthalmology Department of Fondation A de Rothschild and Hôpital Bichat, Paris, France. [email protected].
- 2 Université Paris Diderot, Sorbonne Paris Cité, Paris, France. [email protected].
- 3 APHP-Hôpital Armand Trousseau, Centre de l'asthme et des allergies, Paris, France.
- 4 Université Pierre et Marie Curie, Paris, France.
- 5 Equipe EPAR, Institut Pierre Louis d'Epidémiologie et de Santé Publique, UMR_S1136, INSERM, Paris, France.
- 6 Ophthalmology Department of Fondation A de Rothschild and Hôpital Bichat, Paris, France.
- 7 Université Paris Diderot, Sorbonne Paris Cité, Paris, France.
- PMID: 27909980
- PMCID: PMC5449293
- DOI: 10.1007/s40123-016-0074-2
Introduction: Vernal keratoconjunctivis (VKC) is a severe form of pediatric ocular allergy, characterized by acute and chronic corneoconjunctival inflammation that may lead to visual sequelae. Although topical immunosuppressive drugs such as cyclosporine are usually effective, some severe forms may be refractory and require prolonged steroid therapy. Very few papers report the use of omalizumab in VKC in the literature. In the present study, we describe our clinical experience with omalizumab in severe VKC children.
Methods: We retrospectively reviewed the files of four boys treated with omalizumab because of severe VKC, defined as persistent corneal inflammation despite continuous topical 2% cyclosporine and steroid eye drops. We also performed a literature review.
Results: Four boys, aged 7-13 years old, were treated. All children had asthma and one had severe lid eczema. Two patients had required intrapalpebral depot-steroid injections. Omalizumab was administered every 2 weeks by subcutaneous injections, at doses varying from 450 to 600 mg per injection. Three patients out of four responded to the treatment, with a decrease in global symptoms (median symptom rating decreasing from 89 to 29 on a 100-mm visual analog scale), frequency and in duration of the inflammatory flares, and also a decreased need for topical steroid. Their median clinical grade decreased from 4 to 3 (Bonini grading). However, the response was incomplete and they still had inflammatory corneoconjunctival flares despite continuous topical cyclosporine. On the other hand, asthma and lid eczema were completely controlled in these three patients. The fourth child did not respond to omalizumab and needed oral steroids for his VKC and his asthma. Noticeably, this latter patient did not have detectable sensitization to any allergen, contrary to the other cases. The treatment was stopped in this refractory case, but is still ongoing in all other cases, with a median duration of 33 months (range 16-42 months). In the literature (four cases), omalizumab may have a more complete efficacy in some cases, but the results are still variable.
Conclusion: Omalizumab is an interesting treatment in severe refractory forms of VKC, but its efficacy is incomplete in these very severe cases.
Keywords: Children; Cyclosporine; IgE; Immunomodulation; Severe allergy; Steroids; VKC.
- Systematic Review
- Open access
- Published: 23 May 2024
Systematic literature review of real-world evidence for treatments in HR+/HER2- second-line LABC/mBC after first-line treatment with CDK4/6i
- Veronique Lambert ORCID: orcid.org/0000-0002-6984-0038 1 ,
- Sarah Kane ORCID: orcid.org/0009-0006-9341-4836 2 na1 ,
- Belal Howidi ORCID: orcid.org/0000-0002-1166-7631 2 na1 ,
- Bao-Ngoc Nguyen ORCID: orcid.org/0000-0001-6026-2270 2 na1 ,
- David Chandiwana ORCID: orcid.org/0009-0002-3499-2565 3 ,
- Yan Wu ORCID: orcid.org/0009-0008-3348-9232 1 ,
- Michelle Edwards ORCID: orcid.org/0009-0001-4292-3140 3 &
- Imtiaz A. Samjoo ORCID: orcid.org/0000-0003-1415-8055 2 na1
BMC Cancer volume 24 , Article number: 631 ( 2024 ) Cite this article
177 Accesses
1 Altmetric
Metrics details
Cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) combined with endocrine therapy (ET) are currently recommended by the National Comprehensive Cancer Network (NCCN) guidelines and the European Society for Medical Oncology (ESMO) guidelines as the first-line (1 L) treatment for patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative, locally advanced/metastatic breast cancer (HR+/HER2- LABC/mBC). Although there are many treatment options, there is no clear standard of care for patients following 1 L CDK4/6i. Understanding the real-world effectiveness of subsequent therapies may help to identify an unmet need in this patient population. This systematic literature review qualitatively synthesized effectiveness and safety outcomes for treatments received in the real-world setting after 1 L CDK4/6i therapy in patients with HR+/ HER2- LABC/mBC.
MEDLINE®, Embase, and Cochrane were searched using the Ovid® platform for real-world evidence studies published between 2015 and 2022. Grey literature was searched to identify relevant conference abstracts published from 2019 to 2022. The review was conducted in accordance with PRISMA guidelines (PROSPERO registration: CRD42023383914). Data were qualitatively synthesized and weighted average median real-world progression-free survival (rwPFS) was calculated for NCCN/ESMO-recommended post-1 L CDK4/6i treatment regimens.
Twenty records (9 full-text articles and 11 conference abstracts) encompassing 18 unique studies met the eligibility criteria and reported outcomes for second-line (2 L) treatments after 1 L CDK4/6i; no studies reported disaggregated outcomes in the third-line setting or beyond. Sixteen studies included NCCN/ESMO guideline-recommended treatments with the majority evaluating endocrine-based therapy; five studies on single-agent ET, six studies on mammalian target of rapamycin inhibitors (mTORi) ± ET, and three studies with a mix of ET and/or mTORi. Chemotherapy outcomes were reported in 11 studies. The most assessed outcome was median rwPFS; the weighted average median rwPFS was calculated as 3.9 months (3.3-6.0 months) for single-agent ET, 3.6 months (2.5–4.9 months) for mTORi ± ET, 3.7 months for a mix of ET and/or mTORi (3.0–4.0 months), and 6.1 months (3.7–9.7 months) for chemotherapy. Very few studies reported other effectiveness outcomes and only two studies reported safety outcomes. Most studies had heterogeneity in patient- and disease-related characteristics.
Conclusions
The real-world effectiveness of current 2 L treatments post-1 L CDK4/6i are suboptimal, highlighting an unmet need for this patient population.
Peer Review reports
Introduction
Breast cancer (BC) is the most diagnosed form of cancer in women with an estimated 2.3 million new cases diagnosed worldwide each year [ 1 ]. BC is the second leading cause of cancer death, accounting for 685,000 deaths worldwide per year [ 2 ]. By 2040, the global burden associated with BC is expected to surpass three million new cases and one million deaths annually (due to population growth and aging) [ 3 ]. Numerous factors contribute to global disparities in BC-related mortality rates, including delayed diagnosis, resulting in a high number of BC cases that have progressed to locally advanced BC (LABC) or metastatic BC (mBC) [ 4 , 5 , 6 ]. In the United States (US), the five-year survival rate for patients who progress to mBC is three times lower (31%) than the overall five-year survival rate for all stages (91%) [ 6 , 7 ].
Hormone receptor (HR) positive (i.e., estrogen receptor and/or progesterone receptor positive) coupled with negative human epidermal growth factor 2 (HER2) expression is the most common subtype of BC, accounting for ∼ 60–70% of all BC cases [ 8 , 9 ]. Historically, endocrine therapy (ET) through estrogen receptor modulation and/or estrogen deprivation has been the standard of care for first-line (1 L) treatment of HR-positive/HER2-negative (HR+/HER2-) mBC [ 10 ]. However, with the approval of the cyclin-dependent kinase 4/6 inhibitor (CDK4/6i) palbociclib in combination with the aromatase inhibitor (AI) letrozole in 2015 by the US Food and Drug Administration (FDA), 1 L treatment practice patterns have evolved such that CDK4/6i (either in combination with AIs or with fulvestrant) are currently considered the standard of care [ 11 , 12 , 13 , 14 , 15 , 16 , 17 ]. Other CDK4/6i (ribociclib and abemaciclib) in combination with ET are approved for the treatment of HR+/HER2- LABC/mBC; 1 L use of ribociclib in combination with an AI was granted FDA approval in March 2017 for postmenopausal women (with expanded approval in July 2018 for pre/perimenopausal women and for use in 1 L with fulvestrant for patients with disease progression on ET as well as for postmenopausal women), and abemaciclib in combination with fulvestrant was granted FDA approval in September 2017 for patients with disease progression following ET and as monotherapy in cases where disease progression occurs following ET and prior chemotherapy in mBC (with expanded approval in February 2018 for use in 1 L in combination with an AI for postmenopausal women) [ 18 , 19 , 20 , 21 ].
Clinical trials investigating the addition of CDK4/6i to ET have demonstrated significant improvement in progression-free survival (PFS) and significant (ribociclib) or numerical (palbociclib and abemaciclib) improvement in overall survival (OS) compared to ET alone in patients with HR+/HER2- advanced or mBC, making this combination treatment the recommended option in the 1 L setting [ 22 , 23 , 24 , 25 , 26 , 27 ]. However, disease progression occurs in a significant portion of patients after 1 L CDK4/6i treatment [ 28 ] and the optimal treatment sequence after progression on CDK4/6i remains unclear [ 29 ]. At the time of this review (literature search conducted December 14, 2022), guidelines by the National Comprehensive Cancer Network (NCCN) and the European Society for Medical Oncology (ESMO) recommend various options for the treatment of HR+/HER2- advanced BC in the second-line (2 L) setting, including fulvestrant monotherapy, mammalian target of rapamycin inhibitors (mTORi; e.g., everolimus) ± ET, alpelisib + fulvestrant (if phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha mutation positive [PIK3CA-m+]), poly-ADP ribose polymerase inhibitors (PARPi) including olaparib or talazoparib (if breast cancer gene/partner and localizer of BRCA2 positive [BRCA/PALB2m+]), and chemotherapy (in cases when a visceral crisis is present) [ 15 , 16 ]. CDK4/6i can also be used in 2 L [ 16 , 30 ]; however, limited data are available to support CDK4/6i rechallenge after its use in the 1 L setting [ 15 ]. Depending on treatments used in the 1 L and 2 L settings, treatment in the third-line setting is individualized based on the patient’s response to prior treatments, tumor load, duration of response, and patient preference [ 9 , 15 ]. Understanding subsequent treatments after 1 L CDK4/6i, and their associated effectiveness, is an important focus in BC research.
Treatment options for HR+/HER2- LABC/mBC continue to evolve, with ongoing research in both clinical trials and in the real-world setting. Real-world evidence (RWE) offers important insights into novel therapeutic regimens and the effectiveness of treatments for HR+/HER2- LABC/mBC. The effectiveness of the current treatment options following 1 L CDK4/6i therapy in the real-world setting highlights the unmet need in this patient population and may help to drive further research and drug development. In this study, we conducted a systematic literature review (SLR) to qualitatively summarize the effectiveness and safety of treatment regimens in the real-world setting after 1 L treatment with CDK4/6i in patients with HR+/HER2- LABC/mBC.
Literature search
An SLR was performed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions [ 31 ] and reported in alignment with the Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses (PRISMA) statement [ 32 ] to identify all RWE studies assessing the effectiveness and safety of treatments used for patients with HR+/HER2- LABC/mBC following 1 L CDK4/6i therapy and received subsequent treatment in 2 L and beyond (2 L+). The Ovid® platform was used to search MEDLINE® (including Epub Ahead of Print and In-Process, In-Data-Review & Other Non-Indexed Citations), Ovid MEDLINE® Daily, Embase, Cochrane Central Register of Controlled Trials, and Cochrane Database of Systematic Reviews by an experienced medical information specialist. The MEDLINE® search strategy was peer-reviewed independently by a senior medical information specialist before execution using the Peer Review of Electronic Search Strategies (PRESS) checklist [ 33 ]. Searches were conducted on December 14, 2022. The review protocol was developed a priori and registered with the International Prospective Register of Systematic Review (PROSPERO; CRD42023383914) which outlined the population, intervention, comparator, outcome, and study design (PICOS) criteria and methodology used to conduct the review (Table 1 ).
Search strategies utilized a combination of controlled vocabulary (e.g., “HER2 Breast Cancer” or “HR Breast Cancer”) and keywords (e.g., “Retrospective studies”). Vocabulary and syntax were adjusted across databases. Published and validated filters were used to select for study design and were supplemented using additional medical subject headings (MeSH) terms and keywords to select for RWE and nonrandomized studies [ 34 ]. No language restrictions were included in the search strategy. Animal-only and opinion pieces were removed from the results. The search was limited to studies published between January 2015 and December 2022 to reflect the time at which FDA approval was granted for the first CDK4/6i agent (palbociclib) in combination with AI for the treatment of LABC/mBC [ 35 ]. Further search details are presented in Supplementary Material 1 .
Grey literature sources were also searched to identify relevant abstracts and posters published from January 2019 to December 2022 for prespecified relevant conferences including ESMO, San Antonio Breast Cancer Symposium (SABCS), American Society of Clinical Oncology (ASCO), the International Society for Pharmacoeconomics and Outcomes Research (ISPOR US), and the American Association for Cancer Research (AACR). A search of ClinicalTrials.gov was conducted to validate the findings from the database and grey literature searches.
Study selection, data extraction & weighted average calculation
Studies were screened for inclusion using DistillerSR Version 2.35 and 2.41 (DistillerSR Inc. 2021, Ottawa, Canada) by two independent reviewers based on the prespecified PICOS criteria (Table 1 ). A third reviewer was consulted to resolve any discrepancies during the screening process. Studies were included if they reported RWE on patients aged ≥ 18 years with HR+/HER2- LABC/mBC who received 1 L CDK4/6i treatment and received subsequent treatment in 2 L+. Studies were excluded if they reported the results of clinical trials (i.e., non-RWE), were published in any language other than English, and/or were published prior to 2015 (or prior to 2019 for conference abstracts and posters). For studies that met the eligibility criteria, data relating to study design and methodology, details of interventions, patient eligibility criteria and baseline characteristics, and outcome measures such as efficacy, safety, tolerability, and patient-reported outcomes (PROs), were extracted (as available) using a Microsoft Excel®-based data extraction form (Microsoft Corporation, WA, USA). Data extraction was performed by a single reviewer and was confirmed by a second reviewer. Multiple publications identified for the same RWE study, patient population, and setting that reported data for the same intervention were linked and extracted as a single publication. Weighted average median real-world progression-free survival (rwPFS) values were calculated by considering the contribution to the median rwPFS of each study proportional to its respective sample size. These weighted values were then used to compute the overall median rwPFS estimate.
Quality assessment
The Newcastle-Ottawa scale (NOS) for nonrandomized (cohort) studies was used to assess the risk of bias for published, full-text studies [ 36 ]. The NOS allocates a maximum of nine points for the least risk of bias across three domains: (1) Formation of study groups (four points), (2) Comparability between study groups (two points), (3) Outcome ascertainment (three points). NOS scores can be categorized in three groups: very high risk of bias (0 to 3 points), high risk of bias (4 to 6), and low risk of bias (7 to 9) [ 37 ]. Risk of bias assessment was performed by one reviewer and validated by a second independent reviewer to verify accuracy. Due to limited methodological data by which to assess study quality, risk of bias assessment was not performed on conference abstracts or posters. An amendment to the PROSPERO record (CRD42023383914) for this study was submitted in relation to the quality assessment method (specifying usage of the NOS).
The database search identified 3,377 records; after removal of duplicates, 2,759 were screened at the title and abstract stage of which 2,553 were excluded. Out of the 206 reports retrieved and assessed for eligibility, an additional 187 records were excluded after full-text review; most of these studies were excluded for having patients with mixed lines of CDK4/6i treatment (i.e., did not receive CDK4/6i exclusively in 1 L) (Fig. 1 and Table S1 ). The grey literature search identified 753 records which were assessed for eligibility; of which 752 were excluded mainly due to the population not meeting the eligibility criteria (Fig. 1 ). In total, the literature searches identified 20 records (9 published full-text articles and 11 conference abstracts/posters) representing 18 unique RWE studies that met the inclusion criteria. The NOS quality scores for the included full-text articles are provided in Table S2 . The scores ranged from four to six points (out of a total score of nine) and the median score was five, indicating that all the studies suffered from a high risk of bias [ 37 ].
Most studies were retrospective analyses of chart reviews or medical registries, and all studies were published between 2017 and 2022 (Table S3 ). Nearly half of the RWE studies (8 out of 18 studies) were conducted in the US [ 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 ], while the remaining studies included sites in Canada, China, Germany, Italy, Japan, and the United Kingdom [ 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 ]. Sample sizes ranged from as few as 4 to as many as 839 patients across included studies, with patient age ranging from 26 to 86 years old.
Although treatment characteristics in the 1 L setting were not the focus of the present review, these details are captured in Table S3 . Briefly, several RWE studies reported 1 L CDK4/6i use in combination with ET (8 out of 18 studies) or as monotherapy (2 out of 18 studies) (Table S3 ). Treatments used in combination with 1 L CDK4/6i included letrozole, fulvestrant, exemestane, and anastrozole. Where reported (4 out of 18 studies), palbociclib was the most common 1 L CDK4/6i treatment. Many studies (8 out of 18 studies) did not report which specific CDK4/6i treatment(s) were used in 1 L or if its administration was in combination or monotherapy.
Characteristics of treatments after 1 L CDK4/6i therapy
Across all studies included in this review, effectiveness and safety data were only available for treatments administered in the 2 L setting after 1 L CDK4/6i treatment. No studies were identified that reported outcomes for patients treated in the third-line setting or beyond after 1 L CDK4/6i treatment. All 18 studies reported effectiveness outcomes in 2 L, with only two of these studies also describing 2 L safety outcomes. The distribution of outcomes reported in these studies is provided in Table S4 . Studies varied in their reporting of outcomes for 2 L treatments; some studies reported outcomes for a group of 2 L treatments while others described independent outcomes for specific 2 L treatments (i.e., everolimus, fulvestrant, or chemotherapy agents such as eribulin mesylate) [ 42 , 45 , 50 , 54 , 55 ]. Due to the heterogeneity in treatment classes reported in these studies, this data was categorized (as described below) to align with the guidelines provided by NCCN and ESMO [ 15 , 16 ]. The treatment class categorizations for the purpose of this review are: single-agent ET (patients who exclusively received a single-agent ET after 1 L CDK4/6i treatment), mTORi ± ET (patients who exclusively received an mTORi with or without ET after 1 L CDK4/6i treatment), mix of ET and/or mTORi (patients who may have received only ET, only mTORi, and/or both treatments but the studies in this group lacked sufficient information to categorize these patients in the “single-agent ET” or “mTOR ± ET” categories), and chemotherapy (patients who exclusively received chemotherapy after 1 L CDK4/6i treatment). Despite ESMO and NCCN guidelines indicating that limited evidence exists to support rechallenge with CDK4/6i after 1 L CDK4/6i treatment [ 15 , 16 ], two studies reported outcomes for this treatment approach. Data for such patients were categorized as “ CDK4/6i ± ET ” as it was unclear how many patients receiving CDK4/6i rechallenge received concurrent ET. All other patient groups that lacked sufficient information or did not report outcome/safety data independently (i.e., grouped patients with mixed treatments) to categorize as one of the treatment classes described above were grouped as “ other ”.
The majority of studies reported effectiveness outcomes for endocrine-based therapy after 1 L CDK4/6i treatment; five studies for single-agent ET, six studies for mTORi ± ET, and three studies for a mix of ET and/or mTORi (Fig. 2 ). Eleven studies reported effectiveness outcomes for chemotherapy after 1 L CDK4/6i treatment, and only two studies reported effectiveness outcomes for CDK4/6i rechallenge ± ET. Eight studies that described effectiveness outcomes were grouped into the “other” category. Safety data was only reported in two studies: one study evaluating the chemotherapy agent eribulin mesylate and one evaluating the mTORi everolimus.
Effectiveness outcomes
Real-world progression-free survival
Median rwPFS was described in 13 studies (Tables 2 and Table S5 ). Across the 13 studies, the median rwPFS ranged from 2.5 months [ 49 ] to 17.3 months [ 39 ]. Out of the 13 studies reporting median rwPFS, 10 studies reported median rwPFS for a 2 L treatment recommended by ESMO and NCCN guidelines, which ranged from 2.5 months [ 49 ] to 9.7 months [ 45 ].
Weighted average median rwPFS was calculated for 2 L treatments recommended by both ESMO and NCCN guidelines (Fig. 3 ). The weighted average median rwPFS for single-agent ET was 3.9 months ( n = 92 total patients) and was derived using data from two studies reporting median rwPFS values of 3.3 months ( n = 70) [ 38 ] and 6.0 months ( n = 22) [ 40 ]. For one study ( n = 7) that reported outcomes for single agent ET, median rwPFS was not reached during the follow-up period; as such, this study was excluded from the weighted average median rwPFS calculation [ 49 ].
The weighted average median rwPFS for mTORi ± ET was 3.6 months ( n = 128 total patients) and was derived based on data from 3 studies with median rwPFS ranging from 2.5 months ( n = 4) [ 49 ] to 4.9 months ( n = 25) [ 54 ] (Fig. 3 ). For patients who received a mix of ET and/or mTORi but could not be classified into the single-agent ET or mTORi ± ET treatment classes, the weighted average median rwPFS was calculated to be 3.7 months ( n = 17 total patients). This was calculated based on data from two studies reporting median rwPFS values of 3.0 months ( n = 5) [ 46 ] and 4.0 months ( n = 12) [ 49 ]. Notably, one study of patients receiving ET and/or everolimus reported a median rwPFS duration of 3.0 months; however, this study was excluded from the weighted average median rwPFS calculation for the ET and/or mTORi class as the sample size was not reported [ 53 ].
The weighted average median rwPFS for chemotherapy was 6.1 months ( n = 499 total patients), calculated using data from 7 studies reporting median rwPFS values ranging from 3.7 months ( n = 249) [ 38 ] to 9.7 months ( n = 121) [ 45 ] (Fig. 3 ). One study with a median rwPFS duration of 5.6 months was not included in the weighted average median rwPFS calculation as the study did not report the sample size [ 53 ]. A second study was excluded from the calculation since the reported median rwPFS was not reached during the study period ( n = 7) [ 41 ].
Although 2 L CDK4/6i ± ET rechallenge lacks sufficient information to support recommendation by ESMO and NCCN guidelines, the limited data currently available for this treatment have shown promising results. Briefly, two studies reported median rwPFS for CDK4/6i ± ET with values of 8.3 months ( n = 302) [ 38 ] and 17.3 months ( n = 165) (Table 2 ) [ 39 ]. The remaining median rwPFS studies reported data for patients classified as “Other” (Table S5 ). The “Other” category included median rwPFS outcomes from seven studies, and included a myriad of treatments (e.g., ET, mTOR + ET, chemotherapy, CDK4/6i + ET, alpelisib + fulvestrant, chidamide + ET) for which disaggregated median rwPFS values were not reported.
Overall survival
Median OS for 2 L treatment was reported in only three studies (Table 2 ) [ 38 , 42 , 43 ]. Across the three studies, the 2 L median OS ranged from 5.2 months ( n = 3) [ 43 ] to 35.7 months ( n = 302) [ 38 ]. Due to the lack of OS data in most of the studies, weighted averages could not be calculated. No median OS data was reported for the single-agent ET treatment class whereas two studies reported median OS for the mTORi ± ET treatment class, ranging from 5.2 months ( n = 3) [ 43 ] to 21.8 months ( n = 54) [ 42 ]. One study reported 2 L median OS of 24.8 months for a single patient treated with chemotherapy [ 43 ]. The median OS data in the CDK4/6i ± ET rechallenge group was 35.7 months ( n = 302) [ 38 ].
Patient mortality was reported in three studies [ 43 , 44 , 45 ]. No studies reported mortality for the single-agent ET treatment class and only one study reported this outcome for the mTORi ± ET treatment class, where 100% of patients died ( n = 3) as a result of rapid disease progression [ 43 ]. For the chemotherapy class, one study reported mortality for one patient receiving 2 L capecitabine [ 43 ]. An additional study reported eight deaths (21.7%) following 1 L CDK4/6i treatment; however, this study did not disclose the 2 L treatments administered to these patients [ 44 ].
Other clinical endpoints
The studies included limited information on additional clinical endpoints; two studies reported on time-to-discontinuation (TTD), two reported on duration of response (DOR), and one each on time-to-next-treatment (TTNT), time-to-progression (TTP), objective response rate (ORR), clinical benefit rate (CBR), and stable disease (Tables 2 and Table S5 ).
Safety, tolerability, and patient-reported outcomes
Safety and tolerability data were reported in two studies [ 40 , 45 ]. One study investigating 2 L administration of the chemotherapy agent eribulin mesylate reported 27 patients (22.3%) with neutropenia, 3 patients (2.5%) with febrile neutropenia, 10 patients (8.3%) with peripheral neuropathy, and 14 patients (11.6%) with diarrhea [ 45 ]. Of these, neutropenia of grade 3–4 severity occurred in 9 patients (33.3%) [ 45 ]. A total of 55 patients (45.5%) discontinued eribulin mesylate treatment; 1 patient (0.83%) discontinued treatment due to adverse events [ 45 ]. Another study reported that 5 out of the 22 patients receiving the mTORi everolimus combined with ET in 2 L (22.7%) discontinued treatment due to toxicity [ 40 ]. PROs were not reported in any of the studies included in the SLR.
The objective of this study was to summarize the existing RWE on the effectiveness and safety of therapies for patients with HR+/HER2- LABC/mBC after 1 L CDK4/6i treatment. We identified 18 unique studies reporting specifically on 2 L treatment regimens after 1 L CDK4/6i treatment. The weighted average median rwPFS for NCCN- and ESMO- guideline recommended 2 L treatments ranged from 3.6 to 3.9 months for ET-based treatments and was 6.1 months when including chemotherapy-based regimens. Treatment selection following 1 L CDK4/6i therapy remains challenging primarily due to the suboptimal effectiveness or significant toxicities (e.g., chemotherapy) associated with currently available options [ 56 ]. These results highlight that currently available 2 L treatments for patients with HR+/HER2- LABC/mBC who have received 1 L CDK4/6i are suboptimal, as evidenced by the brief median rwPFS duration associated with ET-based treatments, or notable side effects and toxicity linked to chemotherapy. This conclusion is aligned with a recent review highlighting the limited effectiveness of treatment options for HR+/HER2- LABC/mBC patients post-CDK4/6i treatment [ 56 , 57 ]. Registrational trials which have also shed light on the short median PFS of 2–3 months achieved by ET (i.e., fulvestrant) after 1 L CDK4/6i therapy emphasize the need to develop improved treatment strategies aimed at prolonging the duration of effective ET-based treatment [ 56 ].
The results of this review reveal a paucity of additional real-world effectiveness and safety evidence after 1 L CDK4/6i treatment in HR+/HER2- LABC/mBC. OS and DOR were only reported in two studies while other clinical endpoints (i.e., TTD, TTNT, TTP, ORR, CBR, and stable disease) were only reported in one study each. Similarly, safety and tolerability data were only reported in two studies each, and PROs were not reported in any study. This hindered our ability to provide a comprehensive assessment of real-world treatment effectiveness and safety following 1 L CDK4/6i treatment. The limited evidence may be due to the relatively short period of time that has elapsed since CDK4/6i first received US FDA approval for 1 L treatment of HR+/HER2- LABC/mBC (2015) [ 35 ]. As such, almost half of our evidence was informed by conference abstracts. Similarly, no real-world studies were identified in our review that reported outcomes for treatments in the third- or later-lines of therapy after 1 L CDK4/6i treatment. The lack of data in this patient population highlights a significant gap which limits our understanding of the effectiveness and safety for patients receiving later lines of therapy. As more patients receive CDK4/6i therapy in the 1 L setting, the number of patients requiring subsequent lines of therapy will continue to grow. Addressing this data gap over time will be critical to improve outcomes for patients with HR+/HER2- LABC/mBC following 1 L CDK4/6i therapy.
There are several strengths of this study, including adherence to the guidelines outlined in the Cochrane Handbook to ensure a standardized and reliable approach to the SLR [ 58 ] and reporting of the SLR following PRISMA guidelines to ensure transparency and reproducibility [ 59 ]. Furthermore, the inclusion of only RWE studies allowed us to assess the effectiveness of current standard of care treatments outside of a controlled environment and enabled us to identify an unmet need in this patient population.
This study had some notable limitations, including the lack of safety and additional effectiveness outcomes reported. In addition, the dearth of studies reporting PROs is a limitation, as PROs provide valuable insight into the patient experience and are an important aspect of assessing the impact of 2 L treatments on patients’ quality of life. The studies included in this review also lacked consistent reporting of clinical characteristics (e.g., menopausal status, sites of metastasis, prior surgery) making it challenging to draw comprehensive conclusions or comparisons based on these factors across the studies. Taken together, there exists an important gap in our understanding of the long-term management of patients with HR+/HER2- LABC/mBC. Additionally, the effectiveness results reported in our evidence base were informed by small sample sizes; many of the included studies reported median rwPFS based on less than 30 patients [ 39 , 40 , 41 , 46 , 49 , 51 , 60 ], with two studies not reporting the sample size at all [ 47 , 53 ]. This may impact the generalizability and robustness of the results. Relatedly, the SLR database search was conducted in December 2022; as such, novel agents (e.g., elacestrant and capivasertib + fulvestrant) that have since received FDA approval for the treatment of HR+/HER2- LABC/mBC may impact current 2 L rwPFS outcomes [ 61 , 62 ]. Finally, relative to the number of peer-reviewed full-text articles, this SLR identified eight abstracts and one poster presentation, comprising half (50%) of the included unique studies. As conference abstracts are inherently limited by how much content that can be described due to word limit constraints, this likely had implications on the present synthesis whereby we identified a dearth of real-world effectiveness outcomes in patients with HR+/HER2- LABC/mBC treated with 1 L CDK4/6i therapy.
Future research in this area should aim to address the limitations of the current literature and provide a more comprehensive understanding of optimal sequencing of effective and safe treatment for patients following 1 L CDK4/6i therapy. Specifically, future studies should strive to report robust data related to effectiveness, safety, and PROs for patients receiving 2 L treatment after 1 L CDK4/6i therapy. Future studies should also aim to understand the mechanism underlying CDK4/6i resistance. Addressing these gaps in knowledge may improve the long-term real-world management of patients with HR+/HER2- LABC/mBC. A future update of this synthesis may serve to capture a wider breadth of full-text, peer-reviewed articles to gain a more robust understanding of the safety, effectiveness, and real-world treatment patterns for patients with HR+/HER2- LABC/mBC. This SLR underscores the necessity for ongoing investigation and the development of innovative therapeutic approaches to address these gaps and improve patient outcomes.
This SLR qualitatively summarized the existing real-world effectiveness data for patients with HR+/HER2- LABC/mBC after 1 L CDK4/6i treatment. Results of this study highlight the limited available data and the suboptimal effectiveness of treatments employed in the 2 L setting and underscore the unmet need in this patient population. Additional studies reporting effectiveness and safety outcomes, in addition to PROs, for this patient population are necessary and should be the focus of future research.
PRISMA flow diagram. *Two included conference abstracts reported the same information as already included full-text reports, hence both conference abstracts were not identified as unique. Abbreviations: 1 L = first-line; AACR = American Association of Cancer Research; ASCO = American Society of Clinical Oncology; CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ESMO = European Society for Medical Oncology; ISPOR = Professional Society for Health Economics and Outcomes Research; n = number of studies; NMA = network meta-analysis; pts = participants; SABCS = San Antonio Breast Cancer Symposium; SLR = systematic literature review.
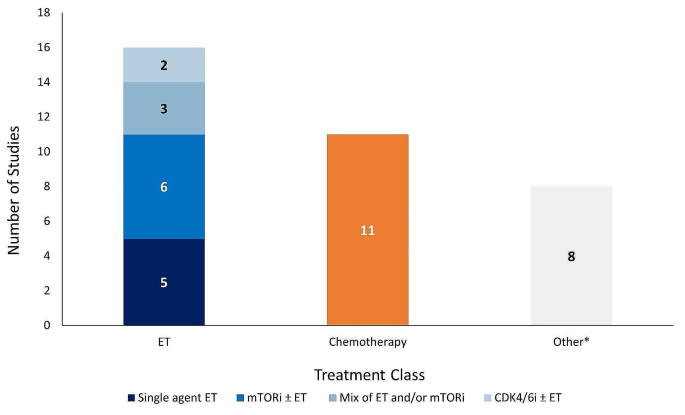
Number of studies reporting effectiveness outcomes exclusively for each treatment class. *Studies that lack sufficient information on effectiveness outcomes to classify based on the treatment classes outlined in the legend above. Abbreviations: CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ET = endocrine therapy; mTORi = mammalian target of rapamycin inhibitor.
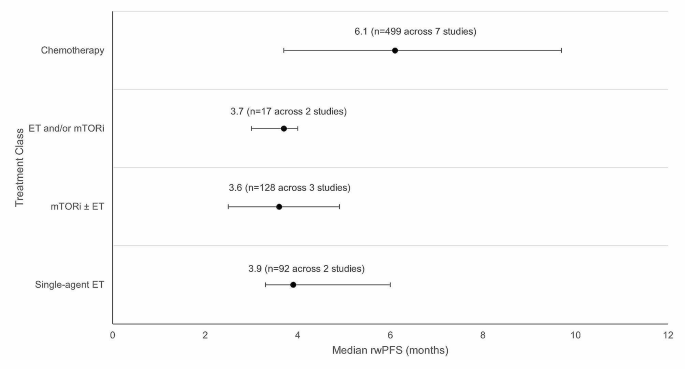
Weighted average median rwPFS for 2 L treatments (recommended in ESMO/NCCN guidelines) after 1 L CDK4/6i treatment. Circular dot represents weighted average median across studies. Horizontal bars represent the range of values reported in these studies. Abbreviations: CDK4/6i = cyclin-dependent kinase 4/6 inhibitor; ESMO = European Society for Medical Oncology; ET = endocrine therapy, mTORi = mammalian target of rapamycin inhibitor; n = number of patients; NCCN = National Comprehensive Cancer Network; rwPFS = real-world progression-free survival.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files]. This study is registered with PROSPERO (CRD42023383914).
Abbreviations
Second-line
Second-line treatment setting and beyond
American Association of Cancer Research
Aromatase inhibitor
American Society of Clinical Oncology
- Breast cancer
breast cancer gene/partner and localizer of BRCA2 positive
Clinical benefit rate
Cyclin-dependent kinase 4/6 inhibitor
Complete response
Duration of response
European Society for Medical Oncology
Food and Drug Administration
Human epidermal growth factor receptor 2
Human epidermal growth factor receptor 2 negative
Hormone receptor
Hormone receptor positive
Professional Society for Health Economics and Outcomes Research
Locally advanced breast cancer
Metastatic breast cancer
Medical Literature Analysis and Retrieval System Online
Medical subject headings
Mammalian target of rapamycin inhibitor
National Comprehensive Cancer Network
Newcastle Ottawa Scale
Objective response rate
Poly-ADP ribose polymerase inhibitor
Progression-free survival
Population, Intervention, Comparator, Outcome, Study Design
Partial response
Preferred Reporting Items for Systematic Literature Reviews and Meta-Analyses
Patient-reported outcomes
- Real-world evidence
San Antonio Breast Cancer Symposium
- Systematic literature review
Time-to-discontinuation
Time-to-next-treatment
Time-to-progression
United States
Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A, Breast, Cancer—Epidemiology. Risk factors, classification, prognostic markers, and current treatment Strategies—An. Updated Rev Cancers. 2021;13(17):4287.
Google Scholar
World Health Organization (WHO). Breast Cancer Facts Sheet [updated July 12 2023. https://www.who.int/news-room/fact-sheets/detail/breast-cancer .
Arnold M, Morgan E, Rumgay H, Mafra A, Singh D, Laversanne M, et al. Current and future burden of breast cancer: global statistics for 2020 and 2040. Breast. 2022;66:15–23.
Article PubMed PubMed Central Google Scholar
Wilkinson L, Gathani T. Understanding breast cancer as a global health concern. Br J Radiol. 2022;95(1130):20211033.
Article PubMed Google Scholar
Giaquinto AN, Sung H, Miller KD, Kramer JL, Newman LA, Minihan A et al. Breast Cancer Statistics, 2022. CA: A Cancer Journal for Clinicians. 2022;72(6):524– 41.
National Cancer Institute (NIH). Cancer Stat Facts: Female Breast Cancer [updated 2020. https://seer.cancer.gov/statfacts/html/breast.html .
American Cancer Society. Key Statistics for Breast Cancer [ https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html .
Zagami P, Carey LA. Triple negative breast cancer: pitfalls and progress. npj Breast Cancer. 2022;8(1):95.
Article CAS PubMed PubMed Central Google Scholar
Matutino A, Joy AA, Brezden-Masley C, Chia S, Verma S. Hormone receptor-positive, HER2-negative metastatic breast cancer: redrawing the lines. Curr Oncol. 2018;25(Suppl 1):S131–41.
Lloyd MR, Wander SA, Hamilton E, Razavi P, Bardia A. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: current and emerging role. Ther Adv Med Oncol. 2022;14:17588359221113694.
Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO International Consensus guidelines for advanced breast Cancer (ABC 4)†. Ann Oncol. 2018;29(8):1634–57.
Article CAS PubMed Google Scholar
US Food Drug Administration. Palbociclib (Ibrance) 2017 [updated March 31, 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/palbociclib-ibrance .
US Food Drug Administration. FDA expands ribociclib indication in HR-positive, HER2-negative advanced or metastatic breast cancer 2018 [updated July 18. 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-ribociclib-indication-hr-positive-her2-negative-advanced-or-metastatic-breast-cancer .
US Food Drug Administration. FDA approves abemaciclib for HR positive, HER2-negative breast cancer 2017 [updated Sept 28. 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-hr-positive-her2-negative-breast-cancer .
NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Breast Cancer 2022 [ https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf .
Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–95.
Beaver JA, Amiri-Kordestani L, Charlab R, Chen W, Palmby T, Tilley A, et al. FDA approval: Palbociclib for the Treatment of Postmenopausal Patients with estrogen Receptor-Positive, HER2-Negative metastatic breast Cancer. Clin Cancer Res. 2015;21(21):4760–6.
US Food Drug Administration. Ribociclib (Kisqali) [ https://www.fda.gov/drugs/resources-information-approved-drugs/ribociclib-kisqali#:~:text=On%20March%2013%2C%202017%2C%20the,hormone%20receptor%20(HR)%2Dpositive%2C .
US Food Drug Administration. FDA approves new treatment for certain advanced or metastatic breast cancers [ https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-certain-advanced-or-metastatic-breast-cancers .
US Food Drug Administration. FDA expands ribociclib indication in HR-positive, HER2-negative advanced or metastatic breast cancer. 2018 [ https://www.fda.gov/drugs/resources-information-approved-drugs/fda-expands-ribociclib-indication-hr-positive-her2-negative-advanced-or-metastatic-breast-cancer .
US Food Drug Administration. FDA approves abemaciclib as initial therapy for HR-positive, HER2-negative metastatic breast cancer [ https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-abemaciclib-initial-therapy-hr-positive-her2-negative-metastatic-breast-cancer .
Turner NC, Slamon DJ, Ro J, Bondarenko I, Im S-A, Masuda N, et al. Overall survival with Palbociclib and fulvestrant in advanced breast Cancer. N Engl J Med. 2018;379(20):1926–36.
Slamon DJ, Neven P, Chia S, Fasching PA, De Laurentiis M, Im SA, et al. Phase III randomized study of Ribociclib and Fulvestrant in hormone Receptor-Positive, human epidermal growth factor receptor 2-Negative advanced breast Cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–72.
Goetz MP, Toi M, Campone M, Sohn J, Paluch-Shimon S, Huober J, et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast Cancer. J Clin Oncol. 2017;35(32):3638–46.
Gopalan PK, Villegas AG, Cao C, Pinder-Schenck M, Chiappori A, Hou W, et al. CDK4/6 inhibition stabilizes disease in patients with p16-null non-small cell lung cancer and is synergistic with mTOR inhibition. Oncotarget. 2018;9(100):37352–66.
Watt AC, Goel S. Cellular mechanisms underlying response and resistance to CDK4/6 inhibitors in the treatment of hormone receptor-positive breast cancer. Breast Cancer Res. 2022;24(1):17.
Goetz M. MONARCH 3: final overall survival results of abemaciclib plus a nonsteroidal aromatase inhibitor as first-line therapy for HR+, HER2- advanced breast cancer. SABCS; 2023.
Munzone E, Pagan E, Bagnardi V, Montagna E, Cancello G, Dellapasqua S, et al. Systematic review and meta-analysis of post-progression outcomes in ER+/HER2– metastatic breast cancer after CDK4/6 inhibitors within randomized clinical trials. ESMO Open. 2021;6(6):100332.
Gennari A, André F, Barrios CH, Cortés J, de Azambuja E, DeMichele A, et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Annals of Oncology. 2021;32(12):1475-95.
European Society for Medical Oncology (ESMO). ESMO Metastatic Breast Cancer Living Guideline: ER-positive HER2-negative Breast Cancer [updated May 2023. https://www.esmo.org/living-guidelines/esmo-metastatic-breast-cancer-living-guideline/er-positive-her2-negative-breast-cancer .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Welch PM VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). www.training.cochrane.org/handbook : Cochrane; 2021.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18(3):e1003583.
McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 Guideline Statement. J Clin Epidemiol. 2016;75:40–6.
Fraser C, Murray A, Burr J. Identifying observational studies of surgical interventions in MEDLINE and EMBASE. BMC Med Res Methodol. 2006;6(1):41.
US Food Drug Administration. Palbociclib (Ibrance). Silver Spring, MD: US Food and Drug Administration; 2017.
Book Google Scholar
GA Wells BS, D O’Connell J, Peterson V, Welch M, Losos PT. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [ https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
Lo CK-L, Mertz D, Loeb M. Newcastle-Ottawa Scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14(1):45.
Martin JM, Handorf EA, Montero AJ, Goldstein LJ. Systemic therapies following progression on first-line CDK4/6-inhibitor treatment: analysis of real-world data. Oncologist. 2022;27(6):441–6.
Kalinsky KM, Kruse M, Smyth EN, Guimaraes CM, Gautam S, Nisbett AR et al. Abstract P1-18-37: Treatment patterns and outcomes associated with sequential and non-sequential use of CDK4 and 6i for HR+, HER2- MBC in the real world. Cancer Research. 2022;82(4_Supplement):P1-18-37-P1-18-37.
Choong GM, Liddell S, Ferre RAL, O’Sullivan CC, Ruddy KJ, Haddad TC, et al. Clinical management of metastatic hormone receptor-positive, HER2-negative breast cancer (MBC) after CDK 4/6 inhibitors: a retrospective single-institution study. Breast Cancer Res Treat. 2022;196(1):229–37.
Xi J, Oza A, Thomas S, Ademuyiwa F, Weilbaecher K, Suresh R, et al. Retrospective Analysis of Treatment Patterns and effectiveness of Palbociclib and subsequent regimens in metastatic breast Cancer. J Natl Compr Canc Netw. 2019;17(2):141–7.
Rozenblit M, Mun S, Soulos P, Adelson K, Pusztai L, Mougalian S. Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy. Breast Cancer Res. 2021;23(1):14.
Bashour SI, Doostan I, Keyomarsi K, Valero V, Ueno NT, Brown PH, et al. Rapid breast Cancer Disease Progression following cyclin dependent kinase 4 and 6 inhibitor discontinuation. J Cancer. 2017;8(11):2004–9.
Giridhar KV, Choong GM, Leon-Ferre R, O’Sullivan CC, Ruddy K, Haddad T, et al. Abstract P6-18-09: clinical management of metastatic breast cancer (MBC) after CDK 4/6 inhibitors: a retrospective single-institution study. Cancer Res. 2019;79:P6–18.
Article Google Scholar
Mougalian SS, Feinberg BA, Wang E, Alexis K, Chatterjee D, Knoth RL, et al. Observational study of clinical outcomes of eribulin mesylate in metastatic breast cancer after cyclin-dependent kinase 4/6 inhibitor therapy. Future Oncol. 2019;15(34):3935–44.
Moscetti LML, Riggi L, Sperduti I, Piacentini FOC, Toss A, Barbieri E, Cortesi L, Canino FMA, Zoppoli G, Frassoldati A, Schirone A, Dominici MECF. SEQUENCE OF TREATMENTS AFTER CDK4/6 THERAPY IN ADVANCED BREAST CANCER (ABC), A GOIRC MULTICENTER RETRO/ PROSPECTIVE STUDY. PRELIMINARY RESULTS IN THE RETROSPECTIVE SERIES OF 116 PATIENTS. Tumori. 2022;108(4S):80.
Menichetti AZE, Giorgi CA, Bottosso M, Leporati R, Giarratano T, Barbieri C, Ligorio F, Mioranza E, Miglietta F, Lobefaro R, Faggioni G, Falci C, Vernaci G, Di Liso E, Girardi F, Griguolo G, Vernieri C, Guarneri V, Dieci MV. CDK 4/6 INHIBITORS FOR METASTATIC BREAST CANCER: A MULTICENTER REALWORLD STUDY. Tumori. 2022;108(4S):70.
Marschner NW, Harbeck N, Thill M, Stickeler E, Zaiss M, Nusch A, et al. 232P Second-line therapies of patients with early progression under CDK4/6-inhibitor in first-line– data from the registry platform OPAL. Annals of Oncology. 2022;33:S643-S4
Gousis C, Lowe KMH, Kapiris M. V. Angelis. Beyond First Line CDK4/6 Inhibitors (CDK4/6i) and Aromatase Inhibitors (AI) in Patients with Oestrogen Receptor Positive Metastatic Breast Cancer (ERD MBC): The Guy’s Cancer Centre Experience. Clinical Oncology2022. p. e178.
Endo Y, Yoshimura A, Sawaki M, Hattori M, Kotani H, Kataoka A, et al. Time to chemotherapy for patients with estrogen receptor-positive breast Cancer and cyclin-dependent kinase 4 and 6 inhibitor use. J Breast Cancer. 2022;25(4):296–306.
Li Y, Li W, Gong C, Zheng Y, Ouyang Q, Xie N, et al. A multicenter analysis of treatment patterns and clinical outcomes of subsequent therapies after progression on palbociclib in HR+/HER2- metastatic breast cancer. Ther Adv Med Oncol. 2021;13:17588359211022890.
Amaro CP, Batra A, Lupichuk S. First-line treatment with a cyclin-dependent kinase 4/6 inhibitor plus an aromatase inhibitor for metastatic breast Cancer in Alberta. Curr Oncol. 2021;28(3):2270–80.
Crocetti SPM, Tassone L, Marcantognini G, Bastianelli L, Della Mora A, Merloni F, Cantini L, Scortichini L, Agostinelli V, Ballatore Z, Savini A, Maccaroni E. Berardi R. What is the best therapeutic sequence for ER-Positive/HER2- Negative metastatic breast cancer in the era of CDK4/6 inhibitors? A single center experience. Tumori. 2020;106(2S).
Nichetti F, Marra A, Giorgi CA, Randon G, Scagnoli S, De Angelis C, et al. 337P Efficacy of everolimus plus exemestane in CDK 4/6 inhibitors-pretreated or naïve HR-positive/HER2-negative breast cancer patients: A secondary analysis of the EVERMET study. Annals of Oncology. 2020;31:S382
Luhn P, O’Hear C, Ton T, Sanglier T, Hsieh A, Oliveri D, et al. Abstract P4-13-08: time to treatment discontinuation of second-line fulvestrant monotherapy for HR+/HER2– metastatic breast cancer in the real-world setting. Cancer Res. 2019;79(4Supplement):P4–13.
Mittal A, Molto Valiente C, Tamimi F, Schlam I, Sammons S, Tolaney SM et al. Filling the gap after CDK4/6 inhibitors: Novel Endocrine and Biologic Treatment options for metastatic hormone receptor positive breast Cancer. Cancers (Basel). 2023;15(7).
Ashai N, Swain SM. Post-CDK 4/6 inhibitor therapy: current agents and novel targets. Cancers (Basel). 2023;15(6).
Higgins JPTTJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). www.training.cochrane.org/handbook : Cochrane; 2022.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Serdar CC, Cihan M, Yücel D, Serdar MA. Sample size, power and effect size revisited: simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem Med (Zagreb). 2021;31(1):010502.
US Food Drug Administration. FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer [updated January 27 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer .
US Food Drug Administration. FDA approves capivasertib with fulvestrant for breast cancer [updated November 16 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-capivasertib-fulvestrant-breast-cancer .
Download references
Acknowledgements
The authors would like to acknowledge Joanna Bielecki who developed, conducted, and documented the database searches.
This study was funded by Pfizer Inc. (New York, NY, USA) and Arvinas (New Haven, CT, USA).
Author information
Sarah Kane, Belal Howidi, Bao-Ngoc Nguyen and Imtiaz A. Samjoo contributed equally to this work.
Authors and Affiliations
Pfizer, 10017, New York, NY, USA
Veronique Lambert & Yan Wu
EVERSANA, Burlington, ON, Canada
Sarah Kane, Belal Howidi, Bao-Ngoc Nguyen & Imtiaz A. Samjoo
Arvinas, 06511, New Haven, CT, USA
David Chandiwana & Michelle Edwards
You can also search for this author in PubMed Google Scholar
Contributions
VL, IAS, SK, BH, BN, DC, YW, and ME participated in the conception and design of the study. IAS, SK, BH and BN contributed to the literature review, data collection, analysis, and interpretation of the data. VL, IAS, SK, BH, BN, DC, YW, and ME contributed to the interpretation of the data and critically reviewed for the importance of intellectual content for the work. VL, IAS, SK, BH, BN, DC, YW, and ME were responsible for drafting or reviewing the manuscript and for providing final approval. VL, IAS, SK, BH, BN, DC, YW, and ME meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Corresponding author
Correspondence to Imtiaz A. Samjoo .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors of this manuscript declare that the research presented was funded by Pfizer Inc. and Arvinas. While the support from Pfizer Inc. and Arvinas was instrumental in facilitating this research, the authors affirm that their interpretation of the data and the content of this manuscript were conducted independently and without bias to maintain the transparency and integrity of the research. IAS, SK, BH, and BN are employees of EVERSANA, Canada, which was a paid consultant to Pfizer in connection with the development of this manuscript.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Lambert, V., Kane, S., Howidi, B. et al. Systematic literature review of real-world evidence for treatments in HR+/HER2- second-line LABC/mBC after first-line treatment with CDK4/6i. BMC Cancer 24 , 631 (2024). https://doi.org/10.1186/s12885-024-12269-8
Download citation
Received : 26 January 2024
Accepted : 16 April 2024
Published : 23 May 2024
DOI : https://doi.org/10.1186/s12885-024-12269-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- First-line CDK4/6i
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ophthalmol Ther
- v.12(2); 2023 Apr
- PMC10011216

Novel Insights in the Management of Vernal Keratoconjunctivitis (VKC): European Expert Consensus Using a Modified Nominal Group Technique
Annegret dahlmann-noor.
1 National Institute for Health Research Moorfields Biomedical Research Centre, Moorfields Eye Hospital National Health Service Foundation Trust, University College London Institute of Ophthalmology, London, UK
2 UCL Institute of Ophthalmology, London, UK
Stefano Bonini
3 Research Unit of Ophthalmology, University of Rome Campus Bio-Medico and Fondazione Policlinico Universitario Campus Bio-Medico, Rome, Italy
Dominique Bremond-Gignac
4 Department of Ophthalmology, University Hospital Necker-Enfants Malades, AP-HP, OPHTARA, Paris, France
5 INSERM Unit, UMRS 1138, Team 17, Paris University, Paris, France
Steffen Heegaard
6 Department of Ophthalmology and Pathology, Rigshospitalet, University of Copenhagen, Copenhagen, Denmark
Andrea Leonardi
7 Ophthalmology Unit, Department of Neuroscience, University of Padua, Padua, Italy
Jesús Montero
8 Clinica CARTUJAVISION, Ophthalmological Center, Seville, Spain
Eduardo D. Silva
9 CRI-OftaPed, Hospital Dona Estefânia, CHULC, Lisbon, Portugal
Associated Data
Data sharing is not applicable to this article as no datasets were generated or analysed during the consensus programme.
Introduction
Vernal keratoconjunctivitis (VKC) is a rare, severe allergic ocular disease, typically occurring in children and adolescents, that can have a significant impact on quality of life and lead to visual impairment. Long-term treatment may be necessary to tackle chronic inflammation and topical corticosteroid dependency must be minimised due to the risk of complications. There is a need for unified clinical guidance to aid the assessment, diagnosis and management of VKC across Europe. The aim of this expert panel (the EUR-VKC Group) was to provide clear guidance for primary care physicians and general ophthalmologists involved in the diagnosis and management of VKC.
An expert group of seven European ophthalmologists was convened and a modified nominal group technique used to develop key recommendations on VKC management. The recommendations were subject to up to two rounds of voting using a 5-point Likert scale to ascertain consensus and the strength of each recommendation. Consensus was set at a predetermined threshold of ≥ 75.0% of experts selecting ‘Strongly agree’ or ‘Agree’.
A total of 47 recommendations were developed relating to the assessment of key of VKC, guidance on who and when to refer, as well as treatment-escalation pathways, long-term follow-up, and supportive care and education. All recommendations reached consensus after two rounds. The group emphasise how timely diagnosis and treatment initiation that is appropriate to disease severity are crucial to benefit patients with VKC. Patients with signs (‘red flags’) indicating severe VKC, or persistent mild-to-moderate VKC that is non-responsive following 2–4 weeks of treatment, should be referred to a sub-specialist.
The EUR-VKC Group provides recommendations on the assessment, diagnosis, management, referral and follow-up of patients with VKC. It also provides a framework to facilitate collaboration between primary care physicians, general ophthalmologists and sub-specialists to improve the outcomes for patients with VKC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40123-023-00665-5.
Plain Language Summary
Vernal keratoconjunctivitis (VKC) is a rare, underdiagnosed, chronic allergic eye disease that typically occurs in children and adolescents. If left untreated, VKC can significantly damage the eye, potentially leading to long-term complications, visual impairment and a reduced quality of life for the child and their family and/or caregivers. In the absence of established guidelines, this consensus programme set out to gather expert insights on best practices for assessing and managing VKC across Europe. A group of seven European ophthalmologists engaged in the consensus programme. A total of 47 recommendations were developed relating to the assessment, diagnosis, management, referral and follow-up of patients with VKC. These 47 recommendations underwent two rounds of review and were revised, if necessary, following expert input. Recommendations where ≥ 75.0% of experts agreed were considered as having reached consensus and were included as final recommendations. The experts agreed that VKC can be classified as mild, moderate or severe, and should be managed according to severity in a stepwise manner, with treatment intensity escalating as the disease severity increases. Timely diagnosis and treatment initiation appropriate to the severity of VKC are crucial to prevent sight loss and improve the quality of life of children with VKC. Ongoing treatment may be necessary to tackle the chronic inflammation associated with the disease and, therefore, reliance on steroid eye drops should be reduced to avoid an increased risk of well-known complications. The experts concluded that mild VKC can be assessed and managed in primary care, but patients with severe VKC, or with moderate-to-severe VKC that does not respond to treatment within 2–4 weeks, should be referred to a VKC specialist.
Key Summary Points
Vernal keratoconjunctivitis (VKC) is a rare, recurrent, bilateral, chronic inflammatory eye disease with an important allergic component affecting the ocular surface that can cause severe visual complications. VKC mainly affects children before the age of 10 years, and is more common among males than females, although this difference may become less at older ages of onset [ 1 – 3 ]. VKC has a typical seasonal trend (but can be perennial), often worsening with acute exacerbations in spring and summer [ 1 , 2 , 4 ].
VKC is classified into limbal, tarsal and mixed forms based on the presence and location of the papillary reaction and inflammation on the conjunctiva [ 5 , 6 ]. Limbal VKC most commonly presents with Horner–Trantas dots, which are focal white spots, consisting of degenerated eosinophils and epithelial cell debris, found on top of limbal papillae. The tarsal form is characterised by the presence of giant, cobblestone-like papillae on the tarsal conjunctiva [ 5 ]. These papillae can differ in shape and size, but are usually defined as > 1.0 mm in diameter [ 1 ].
VKC has an estimated prevalence of 0.7–3.3 cases per 10,000 population, of which 0.3–1.4 cases per 10,000 are estimated to be severe [ 7 , 8 ]. The pathogenesis of VKC is not fully understood but results from a complex interplay between immune cells, including mast cells and eosinophils, and an immunoglobulin E (IgE)- and T cell-mediated allergic reaction, along with familial history and environmental factors [ 2 , 4 , 9 – 12 ].
Classical symptoms of VKC include photophobia, itching (with redness), increased tearing, stringy mucus discharge (or secretion), eye discomfort, pain or burning, and even blurred vision. Notable clinical signs include giant, cobblestone-like papillae on the upper tarsal conjunctiva, conjunctival hyperaemia, limbal papillae with or without Horner–Trantas dots, ptosis or pseudoptosis. Other notable signs include shield ulcers, plaques and superficial keratitis (or other corneal involvement), all of which are indicative of more severe disease [ 1 , 2 , 13 ].
Due to limited awareness, diagnosis of VKC can take several months, and during this time treatment may be suboptimal [ 14 , 15 ]. The inflammatory changes and associated tissue remodelling can lead to long-term complications and severe visual impairment [ 16 ], which if left untreated, can impact a patient’s vision and quality of life, interfering with everyday activities of childhood [ 17 , 18 ]. VKC resolves during puberty in most individuals, but some experience disease progression and permanent corneal damage [ 16 , 17 ].
Approximately 50% of cases of VKC show allergic sensitisation and, typically, two VKC populations exist: patients with positive allergy test results and a history of allergic manifestations, such as asthma, rhinitis or eczema; and those with negative allergy test results and no personal or familial history of atopy [ 16 , 19 ]. Various severity grading scales for VKC are used in research and specialist clinical practice, including the Bonini scale, but there is an unmet need for a grading scale that is universally accepted for everyday clinical practice [ 20 – 23 ]. Classifying mild, moderate and severe VKC based on the clinical presentation provides a distinction that can guide management [ 13 ]. Mild disease is typically treatment naive, with no sight-threatening signs. Disease is considered to be moderate if symptoms recur despite previous treatment with conventional medications, or if patients are treatment naive with limbitis, larger cobblestone-like papillae on the tarsal conjunctiva, punctate keratopathy or mucus discharge [ 13 ]. Severe disease exhibits a lack of response to prior treatments, with repeated flare-ups despite compliance with treatment, the presence of shield ulcers, significant corneal vascularisation and/or disabling symptoms such as frequent photophobia [ 13 ].
Treatment should follow a stepwise approach based on disease severity, progression and response to prior lines of treatment [ 13 ]. Non-pharmacological and pharmacological therapies are part of the treatment armamentarium for VKC and include topical ocular and non-ocular medications. Conventional topical therapies include antihistamines, mast cell stabilisers and dual-acting agents (i.e. antihistamine + mast cell stabiliser). Other therapies include topical anti-inflammatory agents such as corticosteroids and immunomodulators [e.g. ciclosporin A (CsA)] as well as other allergen-specific or systemic therapies [ 2 , 13 , 24 ]. Surgical interventions to remove corneal plaques and alleviate symptoms may also be considered in patients with severe VKC or recalcitrant disease [ 2 , 24 ].
Both the presentation and management of VKC may vary across countries, and with few clinical studies and no treatment guidelines, clinical practice is based on experience [ 13 ]. Regional recommendations have been previously published [ 1 , 13 , 25 ], but none has focused on the management of VKC across Europe. The aim of this expert panel was to evolve existing recommendations and close the gap between the latest evidence and expert insights to provide clear guidance for primary care physicians and general ophthalmologists to ensure accurate assessment and diagnosis, timely referral and optimal treatment for patients with VKC, and thus ultimately improve outcomes for patients.
The information and recommendations provided herein are based on the best available evidence, as interpreted by an expert panel of seven ophthalmologists from six countries in Europe (Denmark, France, Italy, Portugal, Spain, UK), all with specialism and interest in the ocular surface, VKC and paediatric ophthalmology (the EUR-VKC Group). The experts were identified from a variety of clinical and research roles, including authors of high-quality literature pertaining to the subject area, to promote heterogeneity and inclusion of a wide range of knowledge and experience.
The consensus programme was based on a multistep modified nominal group technique (also known as the Expert Panel method) [ 26 , 27 ], comprising an anonymous topic-generation stage and an iterative refinement process, before formal consensus and validation of key recommendations. This is a recognised approach used to gain consensus between specialists in a particular field where expert opinion is important in shaping judgements, and where the literature may be limited.
The process, which took place between June 2021 and June 2022, was led by Annegret Dahlmann-Noor, with regular input and approval from the rest of the EUR-VKC Group (see Fig. S1 in the electronic supplementary material). During the topic-generation stage, the lead asked EUR-VKC Group members to independently submit areas of clinical focus and define the scope of the issues or questions that needed to be addressed. These were then ranked, prioritised and categorised into groups, and ratified by the panel of experts. The topics included both contextual issues, relating to the definition and assessment of VKC, and practical issues, relating to different treatments and management strategies in different clinical scenarios.
A partial literature search was performed for each of the proposed topics. The level of evidence was assessed and agreed by the EUR-VKC Group, and only high-quality evidence was considered. The search included non-pharmacological interventions such as surgery, psychological support, lid hygiene and lubricants, as well as the following treatments: antihistamines, mast cell stabilisers, dual-acting agents, non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, calcineurin inhibitors/immunomodulators (CsA and tacrolimus), allergen-specific immunotherapy and biologics. Randomised controlled trials, systematic reviews of observational and interventional studies, high-quality case reports/case series, opinion articles and consensus papers on best practice in VKC were considered, as well as expert insights from the EUR-VKC Group.
Based on the available literature and expert insights, key recommendations were developed by the facilitator (Synergy Vision medical communications agency) and the lead, and presented in a series of EUR-VKC Group meetings. Discussions were led by the facilitator and lead, with the goal of clarifying any issues or recommendations. Secondary review followed an iterative process (offline), where recommendations were reassessed and revised according to group input, in no more than two rounds. While providing unbiased judgement during the initial topic-generation stage and offline review stages, this approach also allowed members the opportunity to listen to their peers’ opinions, thus increasing the likelihood of reaching consensus.
Following a final EUR-VKC Group meeting in February 2022, validation of the key recommendations took place with a formal survey – which was completed via an online platform, organised by the facilitator. EUR-VKC Group members were asked to anonymously vote on a series of recommendations about VKC using a 5-point Likert scale based on level of agreement. In each case, the members could select only one option: ‘Strongly agree’ (associated with a score of 2), ‘Agree’ (1), ‘Neutral’ (0), ‘Disagree’ (–1) or ‘Strongly disagree’ (–2). Only recommendations on which ≥ 75.0% of members agreed (consistent with other consensus programmes and nominal group techniques [ 26 , 27 ]), as predetermined by the EUR-VKC Group, are included as reaching consensus.
If consensus on a particular topic was not initially reached, members provided input to identify reasons for the lack of agreement. When warranted, the facilitator and lead revised recommendations according to feedback from the group and re-voting then occurred (for a maximum of two votes only).
For each recommendation, the value of the votes was averaged and the strength of the recommendation determined. Recommendations herein are presented with certainty and strength. Recommendations with average scores ≥ 1.6 were categorised as ‘Very strong’ (+ + +); ≥ 1.1 to < 1.6 as ‘Strong’ (+ +); and ≥ 0.7 to < 1.1 as ‘Moderate’ ( +).
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. All members of the EUR-VKC Group (expert panel) are authors of the paper.
Expert Panel Rounds
A total of 47 recommendations were developed relating to the assessment, diagnosis, management, referral and follow-up of patients with VKC. These 47 recommendations underwent two rounds of review and were revised, if necessary, following expert input. In the validation stage, all seven EUR-VKC Group members completed both rounds of voting in June 2022. Of the 47 recommendations included in the round 1 voting, consensus was reached for 41 (87.2%) and not reached for six (12.8%). These six recommendations were revised and included in round 2 voting, where consensus was subsequently reached. No recommendation did not reach consensus after two rounds of voting.
Staged Assessment of VKC
The EUR-VKC Group agreed on the importance of both primary care physicians and general ophthalmologists assessing the clinical and family history of allergy or atopic conditions (e.g. eczema, asthma, rhinitis), as presence of ocular allergy may require further assessment for differential diagnosis; possible external and environmental triggers/exacerbators of symptoms in patients suspected to have VKC; the impact of the condition on the patient’s quality of life; and the duration and seasonality of disease.
The group distinguished common signs and symptoms of VKC of which different specialties should be aware (Fig. 1 ). Both primary care physicians and ophthalmologists should understand the potential impact of these symptoms on patient quality of life.

Clinical signs and hallmark symptoms for the assessment and diagnosis of VKC. a If redness or ‘watery’ eyes are observed in isolation, referral may only be appropriate if there is no improvement in signs and symptoms following 1–2 weeks of conventional treatment (defined as typical first-line pharmacological therapy, including antihistamines, mast cell stabilisers or dual−acting agents). b Unless the general ophthalmologist is experienced and competent in the management of VKC. SPK superficial punctate keratitis, VKC vernal keratoconjunctivitis
If mild symptoms do not abate after 2 weeks of treatment, patients should be referred to an ophthalmologist. Patients with two or more clinical signs and symptoms of moderate VKC should be referred to a specialist ophthalmologist (indicated throughout this manuscript as an ophthalmologist who specialises in cornea, ocular surface or paediatric ophthalmology), and those with corneal involvement or corneal opacity with redness should be referred urgently.
General ophthalmologists should use slit-lamp examination to identify some of the hallmark signs of VKC: giant, cobblestone-like papillae on the upper tarsal conjunctiva, Horner–Trantas dots, stringy mucus discharge (or secretion), conjunctival hyperaemia, ptosis or pseudoptosis, as well as corneal complications (including shield ulcers, plaques, superficial punctate keratitis or other corneal involvement). In all patients suspected of having VKC, general ophthalmologists should be consulted to make the diagnosis and assess severity. They should evert the eyelids (if proficient and confident to do so, to avoid distress to the patient) to confirm the diagnosis by assessing the presence and size of cobblestone-like papillae on the upper tarsal conjunctiva.
General ophthalmologists should be aware of the differential diagnosis for VKC, which includes atopic keratoconjunctivitis (AKC), seasonal allergic conjunctivitis (SAC), perennial allergic conjunctivitis (PAC), giant papillary conjunctivitis and blepharokeratoconjunctivitis in some severe cases of ocular surface inflammation. If a diagnosis cannot be confirmed, then the patient should be referred to a specialist ophthalmologist (see Fig. 1 for ‘red flags’ indicating when urgent specialist referral is required). If available, general ophthalmologists should use fluorescein staining to detect corneal involvement, and any patient with severe corneal involvement (e.g. diffuse punctuate epitheliopathy, macroerosion/large epithelial defects) should be referred to an ophthalmologist specialised in cornea, ocular surface or paediatric ophthalmology, if the general ophthalmology is not experienced in treating VKC.
All patients with clinical signs or symptoms of moderate-to-severe VKC should be referred to a specialist ophthalmologist for further assessment, unless the general ophthalmologist is experienced and competent in the management of VKC.
A full list of recommendations relating to the staged assessment of patients with suspected signs and symptoms of VKC can be found in Table S1 in the electronic supplementary material.
‘Red Flags’ and Who and When to Refer
VKC can be classified as mild, moderate or severe, and this classification is important to determine next steps for the management of VKC. Mild disease may be managed in primary care with support from an ophthalmologist (if appropriate and depending on the healthcare system) to provide adequate follow-up. Moderate VKC may be managed by a general ophthalmologist until non-urgent referral to a specialist clinic, ideally within 1–2 months. Severe VKC should be managed by a specialist ophthalmologist.
The group agreed that any referral to a specialist should be based on disease severity and/or progression. Figure 1 provides a summary of the ‘red flags’ indicating urgent referral to a specialist ophthalmologist. If any of the ‘red flags’ are severe or sight threatening, or are worsening with treatment, then urgent referral (within 24 h) to a corneal specialist, paediatric ophthalmologist or emergency eye clinic is recommended.
A full list of recommendations regarding ‘red flags’ for urgent referral to a specialist ophthalmologist can be found in Table S2 in the electronic supplementary material.
Stepwise Management Approach Based on Severity and Progression of VKC
The EUR-VKC Group agreed that the patient and family/caregiver should always be provided with supportive care and education, with an emphasis on avoiding triggers or exacerbators and allergens, lid hygiene, and use of cold compresses and ocular lubricants/artificial tears (without preservatives, since these can cause allergies or may damage the corneal surface [ 28 , 29 ]). As first-line pharmacological therapy, dual-acting agents (e.g. olopatadine, azelastine hydrochloride, epinastine, ketotifen) may be considered rather than monotherapy with antihistamines or mast cell stabilisers (e.g. sodium cromoglycate, nedocromil, lodoxamide), depending on formulary, local recommendations and availability.
Short-pulse topical corticosteroids are effective to tackle inflammation and manage acute exacerbations or when the cornea is involved, and should be considered for patients with moderate-to-severe disease either alone, as an add-on to topical CsA, or as rescue therapy. However, the long-term use of corticosteroids is associated with an increased risk of adverse events including elevated intraocular pressure (IOP) and glaucoma, formation of cataracts, delayed wound healing and increased susceptibility to infection [ 2 , 30 ]. Notably, corticosteroid-induced glaucoma is a debilitating disease that may cause irreversible loss of vision and potentially blindness [ 31 , 32 ]. Because of these risks, topical corticosteroid eye drops should only be used in ‘short pulses’ (alone or in combination with topical CsA) under the supervision of an ophthalmologist, and repeat cycles avoided, where possible, to prevent dependency. The use of high-frequency or oral corticosteroids (in short pulses) may be appropriate for patients with persistent corneal complications or non-response to prior treatments, but should only be prescribed by a clinician experienced in the use of these medications.
Topical immunomodulators (e.g. CsA) should be considered for patients with moderate-to-severe or persistent VKC, as well as those with corticosteroid dependency, to provide long-term control. If short-pulse corticosteroids are used frequently, for a period of > 3 months, then topical CsA should be considered for long-term control. Topical CsA has shown a marked corticosteroid-sparing effect, potentially allowing control of symptomatology without corticosteroids [ 33 – 35 ]. CsA may not be appropriate for patients with moderate VKC without other signs of progression or risk of recurrences [ 2 , 25 , 36 ].
Oral antihistamines may be used as adjunctive therapy for mild flare-ups or in the case of allergic rhinitis, if required. Advanced systemic treatments (e.g. immunomodulators, biologics) should only be prescribed in appropriate settings (e.g. patients with recalcitrant disease or involving other allergic manifestations) and by clinicians experienced in their use. Allergen-specific immunotherapy is only recommended where there is clearly defined systemic hypersensitivity to an identified allergen. Access to allergen-specific immunotherapy may vary between countries and settings, and referrals should be made accordingly.
The EUR-VKC Group agreed that treatment of VKC should be escalated if there is no improvement in symptoms or if changes in conjunctival papillary or ocular surface clinical signs are observed within 2–4 weeks. In general, if there is no improvement within 2–4 weeks of treatment and symptoms remain persistent, then the patient should be referred to a specialist ophthalmologist. Patients with VKC who may benefit from surgical intervention (e.g. debridement for shield ulcers) should be referred to a corneal specialist or paediatric ophthalmologist, or an emergency eye clinic.
The full list of recommendations regarding the stepwise management of VKC can be found in Fig. 2 and in Table S3 in the electronic supplementary material.

Stepwise management of mild, moderate and severe VKC. a If available and depending on formulary and local recommendations. b CsA may not be appropriate for moderate VKC without other signs. c Recommendation revised from round 1 (where two out of seven group members did not agree, and the recommendation was ‘Weak’): the use of high-frequency corticosteroids or oral corticosteroids (‘short pulses’) may be appropriate for patients with persistent corneal complications or non-response to prior treatments but should only be prescribed by a corneal specialist or paediatric ophthalmologist. d Recommendation revised from round 1 (where two out of seven group members did not agree, and the recommendation was ‘Weak’): systemic treatment (e.g. with immunomodulators, such as CsA, biologics or antihistamines) should only be considered in patients recalcitrant or with non-response to prior therapy, or who have other allergic manifestations. e Recommendation revised from round 1 (where two out of seven group members did not agree, and the recommendation was ‘Weak’): allergen-specific immunotherapy is only recommended when clearly defined systemic hypersensitivity to an identified allergen exists. Patients requiring allergen-specific immunotherapy should be referred to an allergologist or specialist ophthalmologist. CsA ciclosporin A, VKC vernal keratoconjunctivitis
Other Treatments for VKC
The group agreed that before starting treatment with topical corticosteroids, general ophthalmologists should assess patients’ IOP for monitoring purposes to avoid potential issues associated with the development of glaucoma. Vasoconstrictors and NSAIDs are not recommended for the treatment of VKC as they do not target the specific inflammatory mechanisms associated with VKC. Vasoconstrictors used to address hyperaemia should be used with caution and only for short periods of time due to adverse events. In addition, products with herbal extracts, such as chamomile-containing eye drops, should be avoided as they may cross-react with allergens (e.g. Artemisia vulgaris ). Second- and third-generation systemic antihistamines may be preferred over older first-generation antihistamines due to their favourable efficacy/safety profile, pharmacokinetics, and lack of anticholinergic and sedative side effects [ 1 – 3 , 37 ].
The complete results for recommendations regarding other treatments for VKC can be found in Table S4 in the electronic supplementary material.
Long-Term Management and Flare-Ups of VKC
The EUR-VKC Group agreed that for patients with seasonal symptoms, follow-up appointments may be scheduled based on the pattern of previously observed exacerbations or arranged via patient-initiated follow-up with primary care. The duration and frequency of follow-up should depend on disease severity and progression, as well as treatment choice. Quality-of-life assessment is an important part of patient follow-up and should be included in the first consultation and then monitored every 6 months, or as most appropriate. Families and caregivers should be made aware that exacerbations can occur even with effective treatment. They should be aware of the need to access an eye clinic immediately if a flare occurs, and of how to access that clinic. If more than one flare-up occurs within 3 months, despite good treatment adherence, therapy should be stepped up and the patient should be referred to a specialist ophthalmologist. The use of topical corticosteroids over an extended period should be avoided but, if prescribed, should be monitored by an ophthalmologist to avoid complications (e.g. elevated IOP). Oral antihistamines can be used as adjunctive treatment for mild flare-ups or in the case of allergic rhinitis. Results from the VEKTIS study suggest that topical CsA drops used year-round may reduce the risk of exacerbations during the peak allergy season [ 35 ], and this should be discussed with the patient and their family and prescribed at the clinician’s discretion.
If a patient has been asymptomatic for approximately 12 months, then treatment de-escalation may be considered. Discharge back to primary care may be considered if there is no need for ongoing corticosteroids or topical CsA. If patients with VKC are discharged to primary care, the primary care physician should be made aware of the risk of recurrence and/or flare-ups as well as the steps to take in this situation, including triggers for referral and treatment-escalation pathways.
The full list of recommendations regarding long-term management and flare-ups of VKC can be found in Table S5 in the electronic supplementary material.
Key Information to Communicate with Patients and Caregivers on VKC
The group agreed that caregivers (and patients, if appropriate) should be informed that VKC is a chronic, recurrent condition that usually improves with age, but that excessive rubbing of itchy eyes can make the condition worse (with advice on a ‘no-touch zone’). Sunlight, wind, salty water, dust and heat can exacerbate VKC, so the use of sunglasses, hats, visors and swimming goggles may be considered. Furthermore, an air-filtration system in the home may provide relief. Common allergens can exacerbate VKC, and frequently washing the hands, face and hair can reduce exposure to these allergens. Cold compresses and preservative-free artificial tears can provide symptomatic relief.
It is important to reaffirm with patients and caregivers that adherence to treatment is important to ensure treatment success. Caregivers (and patients, if appropriate) should be made aware of the risks associated with the long-term use of corticosteroids and be advised to report any ocular adverse events. It may also be appropriate to educate on how best to manage treatment with topical CsA (e.g. it could be helpful to use preservative-free artificial tears prior to and after instillation or ‘cool’ the eye drops in the fridge to make administration easier) and advise that the effects will manifest over time and any issues with instillation likely improve with sustained use.
A multidisciplinary team consisting of an ophthalmologist, primary care physician, immunologist, paediatrician, allergologist and/or psychological support may be considered as part of collaborative management of patients with VKC (when appropriate).
Figure 3 summarises key information on VKC for patients and caregivers, while a full list of recommendations can be found in Table S6 in the electronic supplementary material.

Summary of the information that physicians should share with patients diagnosed with VKC and their caregivers. CsA ciclosporin A, VKC vernal keratoconjunctivitis
VKC is a rare, chronic disease requiring prompt management of symptoms to prevent exacerbations, flare-ups and complications [ 2 , 13 , 17 ]. Recent consensus initiatives have provided best-practice guidance on the principles for diagnosis, referral, initial and long-term management, and supportive care, but none has specifically addressed the unmet need for unified clinical guidance for primary care physicians and general ophthalmologists in the European region [ 1 , 4 , 13 ]. At present, clinical judgement and experience guide daily practice in managing patients with VKC [ 13 , 38 ]. Due to the lack of standardised recommendations and guidelines for diagnosis and management, treatment for VKC varies greatly across countries and regions [ 1 , 4 , 13 ]. This could result in suboptimal management of patients, increasing the risk of prolonged or permanent damage to the cornea and conjunctiva, which may lead to visual impairment [ 1 , 15 , 17 ]. In fact, inadequate counselling and unrealistic expectations, often resulting in the overuse or misuse of corticosteroids can be associated with complications. Over-medication with corticosteroids can cause vision loss, as can under-medication (although uncommon), and persistent inflammation resulting in corneal scarring and stem-cell damage.
Using the modified nominal group technique, the EUR-VKC Group of seven European ophthalmologists achieved consensus on best-practice recommendations for the assessment, diagnosis, management and follow-up of VKC in Europe.
Patients with VKC often initially present to primary care or emergency departments [ 13 ]. In both scenarios, the decision for referral should depend on disease severity and progression [ 2 ]. If any corneal involvement is present, the patient should be referred to a specialist. As VKC is a rare disease, it is anticipated that referral to an ophthalmologist from primary care should not significantly affect waiting lists.
Many studies have found an association between VKC and keratoconus [ 39 – 41 ], with prevalence reported as high as 26.8% among patients with VKC [ 39 ]. It may be hypothesised that an increase in protease activity or inflammatory cytokines may be exacerbated during forceful eye rubbing (without clear observation that it is a cause), as seen in patients with allergic conjunctivitis, potentially contributing to the development and progression of keratoconus [ 40 , 41 ]. However, this remains an unsettled issue because of contradictory results – such as that of a cross-sectional, single-centre study in Italy which found that only approximately 2% of a large series of patients with VKC had keratoconus [ 42 ]. The EUR-VKC Group agreed, as other researchers have suggested before, that there still lacks clarity on whether any possible link between ocular allergy (and particularly VKC) and keratoconus is only due to eye rubbing.
No single clinical feature viewed in isolation can accurately differentiate VKC from other ocular allergies such as AKC [ 1 ]. For very young children who are unable to communicate their symptoms, photophobia may be the only sign indicating the need for a complete ocular examination. However, VKC is not typically suspected based on one symptom or sign alone [ 1 , 43 ]. Therefore, a complete ocular examination is required by an ophthalmologist for an accurate diagnosis.
Eversion of the eyelids (to check for giant, cobblestone-like papillae of the upper tarsal conjunctiva) is not mandatory for primary care as it is fairly invasive and may cause distress if not done correctly, but it will likely be needed to confirm a diagnosis of VKC in most patients [ 43 ]. Eversion of the eyelids should be carried out by a competent and confident physician. Ophthalmologists should be able to recognise limbal inflammation and Horner–Trantas dots with or without slit-lamp examination.
Timely initiation and application of a stepwise treatment strategy, that is appropriate for the severity of the disease and inflammatory activity present, is crucial to prevent sight loss and improve the quality of life of children with VKC. Long-term treatment for chronic inflammation may be necessary, and the use of topical corticosteroids should be minimised to avoid complications. There is no consensus on the best approach for the use of anti-inflammatory agents to treat VKC – that is, whether to use topical CsA alone, initiate CsA at the same time as corticosteroids or use corticosteroids then CsA (e.g. such as a bridging approach in dry eye disease). A key question remains as to whether the repeated short-term use of corticosteroids (which are commonly prescribed by both non-specialists and specialists) may impact earlier intervention with more targeted treatments, such as topical CsA, and thus possibly prolong symptoms in the long term.
The EUR-VKC Group suggested that short-pulse corticosteroids are typically positioned before topical CsA in the treatment sequence. However, if corticosteroid pulses are prescribed frequently (e.g. more than three times), or considered for > 3 months, then topical CsA is indicated [ 35 , 43 , 44 ]. VKC may require long-term treatment, and corticosteroids are often used to provide early relief of symptoms [ 17 ] but are accompanied with long-term risks, such as increased IOP or steroid-induced glaucoma [ 31 ]. Measuring IOP before starting treatment allows the physician to monitor changes over time and identify any potential steroid-induced complications. Some ‘soft’ corticosteroids (e.g. loteprednol, hydrocortisone) may not completely resolve VKC exacerbations, and long-term treatment can lead to corticosteroid dependency. ‘High-potency’ topical corticosteroids (e.g. dexamethasone), used as a pulse therapy for 3–5 days without tapering, could be more efficacious in resolving exacerbations and less likely to increase IOP than the longer-term use of ‘soft’ corticosteroids [ 2 ].
The EUR-VKC Group agreed that topical CsA should be considered for long-term control, as CsA is the only immunomodulator indicated and approved in Europe to treat severe VKC (based on the time of the patient’s visit and the history of disease) [ 33 – 35 , 44 , 45 ]. Tacrolimus is an immunomodulator that is approved for the treatment of atopic dermatitis. It has shown some therapeutic effectiveness in VKC, improving ocular objective signs and reducing itching, congestion, tearing and foreign-body sensation [ 46 , 47 ]. However, tacrolimus is currently only indicated and approved for the treatment of VKC in Japan, and additional data from randomised clinical studies are needed to better understand its potential role in treating VKC in Europe [ 1 , 46 ].
It may be appropriate to provide a personalised management plan for each child, addressing each medication, the rationale for its use, and the frequency and method of administration. The EUR-VKC Group suggest discharging asymptomatic patients, patients with mild VKC and patients without a need for the ongoing use of corticosteroids or topical CsA to a primary care physician, who can provide fast access to follow-up appointments and, depending on the healthcare setting, discuss whether ongoing treatment can be managed in primary care or general ophthalmology.
All of the currently available treatments for VKC are palliative and do not extinguish the complex immune process that initiates and propagates the ocular inflammation associated with VKC [ 2 ]. Further investigation is required into the feasibility of steroid-sparing regimens in the treatment of VKC, as well as other potential options including combinations with mast cell stabilisers, antihistamines, calcineurin inhibitors [ 4 ] and/or oral montelukast [ 48 , 49 ]. Other areas of potential research include individualised treatment to improve outcomes and patient satisfaction [ 4 ], understanding the long-term impact on patient outcomes and the cost-effectiveness of treatments. In the future, a national patient association/patient representative(s) or non-VKC specialist physicians, may be engaged to validate or expand upon the findings of the EUR-VKC Group.
Strengths and Limitations
The modified nominal group technique was chosen rather than the Delphi consensus method due to the small number of contributors in the EUR-VKC Group and the rarity of the disease. The modified nominal group technique facilitates small-group discussions, giving all group members the opportunity to provide input in a timely manner. In the absence of research-based evidence, the technique provided a structured process to support the expert panel in evaluating key aspects of VKC assessment, diagnosis, treatment and follow-up, and in reaching consensus on recommendations for the benefit of primary care physicians and general ophthalmologists.
The EUR-VKC Group members represent a range of nationalities and each brought their unique background and experiences to the panel, thereby strengthening the consensus programme. One potential methodological issue with consensus programmes is the tendency for participants to feel pressure to conform to the group view. This was mitigated by using an anonymous online voting platform while allowing for rapid collection, analysis and dissemination of each round of results.
Conclusions
VKC is a rare, underdiagnosed, chronic allergic ocular disease that, if left untreated, can cause significant damage to the cornea and conjunctiva, which may lead to long-term complications, visual impairment and a detrimental impact on the quality of life of both the child and their family/caregivers. There is an unmet need for unified clinical guidance on the assessment, diagnosis and management of VKC in Europe. The EUR-VKC Group has provided recommendations on the assessment, diagnosis, management, referral and follow-up of patients with VKC, and should be used as a framework to facilitate further collaboration between primary care physicians, general ophthalmologists and specialists to improve the outcomes for patients with VKC.
Below is the link to the electronic supplementary material.
Acknowledgements
The authors of this paper are the members of the EUR-VKC Group.
The authors acknowledge Synergy Vision (London, UK), as the facilitator of the consensus programme. The authors thank Santen SA (Geneva, Switzerland), which provided funding and organised the group. Santen SA had no input into the outcomes from this programme.
Publication support for third-party writing assistance for this article, provided by Synergy Vision, UK, and all associated publication costs (including the journal’s Rapid Service fee) were funded by Santen SA.
Medical Writing and Editorial Assistance
The authors acknowledge Richard Maver (Synergy Vision, UK) for medical writing and editorial assistance based on the authors’ input and direction. This was funded by Santen SA in accordance with Good Publication Practice (GPP3) guidelines ( http://www.ismpp.org/gpp3 ).
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Author Contributions
Annegret Dahlmann-Noor facilitated the consensus programme and is the corresponding author. All other authors are listed alphabetically as they had equal input. All authors were involved in the conception and design of the consensus programme, collection, analysis and interpretation of the results, drafting and revising the article, and the decision to submit the manuscript for publication.
Disclosures
Annegret Dahlmann-Noor: Consultant/Advisor: Santen, SightGlass Vision, Théa. Honorarium recipient: CooperVision, Novartis, Santen, SightGlass Vision, Théa. Speaker: Santen. Stefano Bonini: Nothing to disclose. Dominique Bremond-Gignac: Consultant/Advisor: Alcon, Bausch, Novartis, Santen, Théa. Steffen Heegaard: Consultant/Advisor: Alcon, LEO Pharma, Sanofi, Santen, Théa. Andrea Leonardi: Consultant/Advisor/Speaker: Alcon, AstraZeneca, FAES FARMA, Santen, Seqirus, SIFI, Théa, URSAPHARM. Jesús Montero: Nothing to disclose. Eduardo D. Silva: Consultant/Advisor: Santen, Théa.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability

IMAGES
VIDEO
COMMENTS
To systematically review the literature on the treatment of VKC: Topical corticosteroids are the most effective therapy for VKC: High: Open in a separate window. Table 9. Therapy narrative reviews. Author Year Country No. of VKC studies N VKC patients Median age Outcome Results SANRA scale; Esposito et al.
Vernal keratoconjunctivitis (VKC) is a chronic, bilateral corneal and conjunctival problem which typically presents in young individuals. VKC is characterized by itching, photophobia, white mucous discharge, lacrimation, foreign body sensation, and pain due to corneal involvement of shield ulcers. Vernal keratoconjunctivitis is categorized within ocular diseases. The diagnosis is clinical, as ...
Vernal keratoconjunctivitis (VKC) is a recurrent, bilateral allergic inflammation of the conjunctiva.[1] The episodes are often periodic and have seasonal recurrences. Seasonal exacerbations characterize the condition in the initial stages with a peak incidence during spring and summer. Over time, the condition tends to become perennial. VKC is a subtype of allergic conjunctivitis.
Background. Vernal keratoconjunctivitis (VKC) is a severe ocular allergic disease that occurs primarily in children and is characterized by bilateral, recurrent, and chronic inflammation of the cornea and conjunctiva that may lead to loss of visual acuity and blindness.1, 2, 3 The incidence and prevalence rates of VKC vary with geography and season. In colder regions of Europe and the United ...
systematic literature review to provide a comprehensive overview of currently available diagnostic methods, management of VKC, and its treatments. Keywords Vernal keratoconjunctivitis · VKC · Ocular allergy Introduction Vernal keratoconjunctivitis (VKC) is a chronic bilateral keratoconjunctivitis typical of children. It usually mani-
Literature review current through: Jan 2024. ... VKC and AKC are chronic, bilateral, and severe forms of allergic inflammation affecting the ocular surface. These two relatively uncommon types of allergic eye disease can cause severe damage to the ocular surface, leading to corneal scarring and vision loss if not treated properly (this occurs ...
Vernal keratoconjunctivitis (VKC) is a chronic, progressive, and potentially sight-threatening form of ocular inflammatory disease that primarily affects children and young adults. ... Omalizumab in severe refractory vernal keratoconjunctivitis in children: case series and review of the literature. Ophthalmol Ther, 6 (2017), pp. 195-206, 10. ...
Publication types. Review. Vernal keratoconjunctivitis (VKC) is a chronic, bilateral corneal and conjunctival problem which typically presents in young individuals. VKC is characterized by itching, photophobia, white mucous discharge, lacrimation, foreign body sensation, and pain due to corneal involvement of shield ulcers. V ….
In this study, we performed a systematic literature review to provide a comprehensive overview of currently available diagnostic methods, management of VKC, and its treatments. Flow chart of the ...
To conduct a systematic review and meta-analysis to evaluate the efficacy of medical treatments on signs and symptoms of vernal keratoconjunctivitis (VKC) and to compare conventional drugs with new therapies.Data from 1749 patients with VKC were included in the meta-analysis. The mean age of patients was 11.2 years (95% confidence interval, 10.2-12.1 years) with a range of 3-38 years ...
Vernal keratoconjunctivitis (VKC) is a form of ocular allergy primarily affecting children. Considered a rare disease in Europe, its prevalence varies by geographic region and is poorly studied in the United Kingdom. ... A Review of the Literature and Current Best Practice Across Six Large United Kingdom Centers J Pediatr Ophthalmol Strabismus ...
VKC usually presents as one of 3 clinical phenotypes based on anatomical location. Limbal VKC is the most frequent form (54%) and more common in warmer climates; it presents with limbal swelling and the characteristic Trantas dots at the pericorneal limbus (Fig. 1 A).Tarsal VKC is more common in temperate regions and presents with the disease's hallmark giant papillae at the upper tarsal lids ...
VKC is an underdiagnosed and underrecognized ocular surface disease and epidemiological data are limited. VKC is most frequent in Asia, ... two case reports and a review of the literature. J Med Case Rep. (2020) 14: 70. 10.1186/s13256-020-02407-8 [PMC free article] [Google Scholar] 67. AlHaran DH. ...
Abstract. Vernal keratoconjunctivitis (VKC) is a chronic, progressive, and potentially sight-threatening form of ocular inflammatory disease that primarily affects children and young adults. Prevalence varies by region, ranging from <2 per 10,000 in in the United States to as high as 1,100 per 10,000 in parts of Africa.
There are no national guidelines for the management of VKC, and with little published data on effective treatments, there is a risk of misdiagnosis and delays in treatment.14 The aim of this review is to formulate best practice principles for diagnosis and management of VKC, based on clinical expertise and experience in the United Kingdom ...
Vernal keratoconjunctivitis (VKC) is a relatively rare, chronic form of ocular allergy that can potentially cause severe visual complications. Affecting mainly children and young adults, it is an IgE- and T cell-mediated disease, leading to a chronic inflammation in which eosinophil, lymphocyte and structural cell activation are involved. Treatment of VKC requires a multiple approach that ...
A systematic review of all randomised clinical trials on topical therapy for VKC published up to December 2005 underlined the lack of standardised outcome measures in VKC . Obvious efforts have been undertaken by several researchers to propose a solution for a composite index that integrates signs and symptoms in a fair and balance way [ 20 ].
Vernal keratoconjunctivitis (VKC) is a chronic bilateral seasonal allergic inflammatory disease with a prevalence of < 1 case out of 10,000 in Europe [], which occurs mainly in pediatric age.The diagnosis is generally confirmed by the finding at the ocular examination of conjunctival hyperemia, papillary hypertrophy in the tarsal conjunctiva, giant papillae and Trantas dots in the limbus region.
Literature review current through: Apr 2024. ... (PAC), vernal keratoconjunctivitis (VKC), atopic keratoconjunctivitis (AKC), and giant papillary conjunctivitis (GPC). VKC and AKC are chronic, bilateral, and severe forms of allergic inflammation affecting the ocular surface. These two relatively uncommon types of allergic eye disease can cause ...
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
Vernal keratoconjunctivitis (VKC) is a relatively rare, chronic form of ocular allergy that can potentially cause severe visual complications. Affecting mainly children and young adults, it is an IgE- and T cell-mediated disease, leading to a chronic inflammation in which eosinophil, lymphocyte and structural cell activation are involved.
Text generation has become more accessible than ever, and the increasing interest in these systems, especially those using large language models, has spurred an increasing number of related publications. We provide a systematic literature review comprising 244 selected papers between 2017 and 2024. This review categorizes works in text generation into five main tasks: open-ended text ...
The Review of Related Literature (RRL) is a crucial section in research that examines existing studies and publications related to a specific topic. It summarizes and synthesizes previous findings, identifies gaps, and provides context for the current research. RRL ensures the research is grounded in established knowledge, guiding the direction and focus of new studies.
Methods: We retrospectively reviewed the files of four boys treated with omalizumab because of severe VKC, defined as persistent corneal inflammation despite continuous topical 2% cyclosporine and steroid eye drops. We also performed a literature review. Results: Four boys, aged 7-13 years old, were treated. All children had asthma and one had ...
This systematic literature review qualitatively synthesized effectiveness and safety outcomes for treatments received in the real-world setting after 1 L CDK4/6i therapy in patients with HR+/ HER2- LABC/mBC. MEDLINE®, Embase, and Cochrane were searched using the Ovid® platform for real-world evidence studies published between 2015 and 2022. ...
Few studies in the literature integrate rainwater harvesting and firefighting. Thus, the general objective of this paper was to evaluate the potential use of rainwater as a source of water for firefighting. To do so, two approaches were proposed for the assessment. The first approach was the analysis of the existing literature. Two databases were evaluated as references in engineering fields ...
Ghauri A, Biswas S, Manzouri B. Management of vernal keratoconjunctivitis in children in the United Kingdom: a review of the literature and current best practice across six large United Kingdom centers. J Pediatr Ophthalmol Strabismus. 2022 doi: 10.3928/01913913-20220328-01. [Google Scholar]
Understanding the relationship between the intake of sugars and diet quality can inform public health recommendations. This systematic review synthesized recent literature on associations between sugar intake and diet quality in generally healthy populations aged 2 years or older. We searched databases from 2010 to 2022 for studies of any design examining associations between quantified sugar ...