Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State

Patient Case Presentation
Patient Mrs. B.C. is a 56 year old female who is presenting to her WHNP for her annual exam. She had to cancel her appointment two months ago and didn’t reschedule until now. Her last pap smear and mammogram were normal. Today, while performing her breast exam, her nurse practitioner notices dimpling in the left breast as the patient raises her arms over her head. When the NP mentions it to Mrs. B.C. she is surprised and denies noticing it before today. A firm, non-tender, immobile nodule is palpated in the upper quadrant of her breast . The NP then asks Mrs. B.C. how frequently she is performing breast self-exams, she admits to only doing them randomly when she remembers, which is about every few months. She reports no recent or abnormal drainage from her breast. Further examination reveals palpable axillary lymph nodes.
Mrs. B.C. is about 30 pounds overweight and walks her dog around her neighborhood every morning before work and every evening when she gets home. She reports drinking a glass of white wine before bed each night. She denies any history of tobacco use. She reports use of a combination birth control pill on and off for 25 years until she reached menopause. She is not currently taking any prescription medications.
Past Medical History
- Menarche (Age 10)
- Post-menopausal (Age 53)
- No other pertinent medical history
Family History:
- Father George- deceased from stroke (75 years old), history of hypertension, CAD, HLD
- Mother Maryanne alive- 76 years old, history of dementia, osteoporosis
- Brother Michael- alive, 57 years old, history of hypertension, CAD and cardiac stent placement (54 years old)
- Sister, Michelle- alive 53 years old, history of GERD, Asthma
- Brother- Jimmy- alive 50 years old, no past medical history
Social History:
Mrs. B.C. works Monday-Friday 8am-5pm at the local dentist’s office at the front desk as a schedule coordinator. She is planning to retire in a few years. In her spare time, she is involved in various community efforts to feed the homeless and helps to prepare dinners at her local church one night a week. She also enjoys cooking and baking at home, gardening, and nature photography.
Mrs. B.C. has two children. Her oldest son, Patrick, is 21 years old and is in his final year of pre-med. He is attending a public university about 2 hours away from home where he lives year-round. As an infant, Patrick was breastfed until 18 months when he self-weaned. Her daughter, Veronica, is 19 years old and lives at home while attending the local branch campus of a state university. She is in her second year of a business degree and then plans to transfer to the main campus next year. When Veronica was an infant she had difficulty latching onto the breast due to an undiagnosed tongue and lip ties resulting in Mrs. BC exclusively pumping and bottle feeding for six months. After six months, Mrs. B.C. was having a hard time keeping up while working and her found her supply diminished. Veronica had begun eating solid foods so Mrs. B.C. switched to supplemental formula, which was a big relief.
Mrs. B.C. was married to her now ex-husband Kent for 26 years. They divorced two years ago when Veronica was a senior in high school. They have remained friends and Kent lives 25 minutes away in a condo with his girlfriend. She also has two brothers who live nearby and a sister who lives out of state. Her 7 nieces and nephews range in age from 9 years old to 26 years old. Her father, George, passed away from a sudden stroke 4 years ago. Her mother, Maryanne, has dementia and is living in a nearby memory care facility. She also has many close friends.
Lessons learned from COVID-19: improving breast cancer care post-pandemic from the patient perspective
- Open access
- Published: 10 May 2024
- Volume 32 , article number 338 , ( 2024 )
Cite this article
You have full access to this open access article

- Charlotte Myers 1 ,
- Kathleen Bennett 2 &
- Caitriona Cahir 2
162 Accesses
Explore all metrics
Since the onset of the pandemic, breast cancer (BC) services have been disrupted in most countries. The purpose of this qualitative study is to explore the unmet needs, patient-priorities, and recommendations for improving BC healthcare post-pandemic for women with BC and to understand how they may vary based on social determinants of health (SDH), in particular socio-economic status (SES).
Thirty-seven women, who were purposively sampled based on SDH and previously interviewed about the impact of COVID-19 on BC, were invited to take part in follow-up semi-structured qualitative interviews in early 2023. The interviews explored their perspectives of BC care since the easing of COVID-19 government restrictions, including unmet needs, patient-priorities, and recommendations specific to BC care. Thematic analysis was conducted to synthesize each topic narratively with corresponding sub-themes. Additionally, variation by SDH was analyzed within each sub-theme.
Twenty-eight women (mean age = 61.7 years, standard deviation (SD) = 12.3) participated in interviews (response rate = 76%). Thirty-nine percent ( n = 11) of women were categorized as high-SES, while 61% ( n = 17) of women were categorized as low-SES. Women expressed unmet needs in their BC care including routine care and mental and physical well-being care, as well as a lack of financial support to access BC care. Patient priorities included the following: developing cohesion between different aspects of BC care; communication with and between healthcare professionals; and patient empowerment within BC care. Recommendations moving forward post-pandemic included improving the transition from active to post-treatment, enhancing support resources, and implementing telemedicine where appropriate. Overall, women of low-SES experienced more severe unmet needs, which in turn resulted in varied patient priorities and recommendations.
As health systems are recovering from the COVID-19 pandemic, the emphasis should be on restoring access to BC care and improving the quality of BC care, with a particular consideration given to those women from low-SES, to reduce health inequalities post-pandemic.
Similar content being viewed by others
Access to health services among culturally and linguistically diverse populations in the Australian universal health care system: issues and challenges

Connecting communities to primary care: a qualitative study on the roles, motivations and lived experiences of community health workers in the Philippines
COVID-19 impact on urban low-income individuals in Bangladesh: a qualitative content analysis
Avoid common mistakes on your manuscript.
Globally, health services for breast cancer (BC) across the cancer continuum were significantly disrupted and compromised due to the coronavirus disease (COVID-19) pandemic [ 1 ]. Breast screening services were curtailed or paused during periods of COVID-19 restrictions [ 2 ]. Active cancer treatment, such as surgery, radiotherapy, and chemotherapy, was disrupted and/ or modified to account for the level of restrictions in place [ 3 , 4 ]. Post-treatment care (i.e., routine care), which includes mammograms, follow-up appointments, blood tests, and other scans, was significantly disrupted during the pandemic [ 5 ]. Support services, which address the multi-disciplinary needs of those living with a diagnosis of cancer, including physiotherapy and psycho-oncology, were paused or modified during the pandemic [ 6 , 7 ].
Breast screening services generally resumed after government restrictions were lifted [ 8 ]; however, the impact of pausing breast screening services on BC diagnoses is only now becoming apparent with later stage and more symptomatic BC diagnoses [ 9 ] which may have a negative impact on survival rates in the future [ 10 ]. Furthermore, the issue of backlogs and waiting lists across the cancer continuum is continuing [ 11 ]. There are likely to be considerable unmet needs in healthcare for women with BC due to barriers such as availability, affordability, accessibility, and acceptability of BC services [ 12 ] which were apparent during the pandemic. Unmet needs, which can be measured by the difference between required healthcare services and received healthcare services, can assess the effectiveness in healthcare delivery [ 13 ]. A failure to address unmet needs can have a negative impact on an individual’s quality of life and other health outcomes [ 14 ]. Previous research conducted during the pandemic identified unmet needs for individuals living with a diagnosis of BC such as psychological and emotional support, management of side effects, complementary therapy, communication among healthcare providers, local health care services, and transportation [ 15 ]. However, similar research has not been conducted qualitatively post-pandemic to identify lasting unmet needs.
Considering the long-lasting consequences of the pandemic, the acquired knowledge and experience from COVID-19 can be used by healthcare providers and policy makers to improve BC care and to prepare health systems for future unexpected events [ 16 ]. Historically, pandemics and other crises have provided opportunities to strengthen health systems by exploiting faults in the pre-existing health system and exacerbating pre-existing health inequalities [ 17 ]. For example, low socio-economic status (SES) has been associated with higher disease burden [ 18 ] and decreased access to healthcare [ 19 ]. Within the context of non-communicable diseases, including BC, the social determinants of health (SDH) framework have been applied to the COVID-19 pandemic to explain health disparities [ 20 ]. Specific to BC care, SDH identified during the pandemic include age, race, insurance status, and region (1); however, it is unknown whether these SDH persist post-pandemic. Therefore, health systems should respond to the COVID-19 pandemic by addressing the multidisciplinary and personalized needs of all individuals to improve health equality.
The reorganization of BC services during the pandemic provides an opportunity to improve overall BC care and the experience and priorities of women with BC. To effectively address the needs of individuals, the patient experience and voice is integral for healthcare improvement. The aims of this qualitative study are (i) to explore and identify the unmet needs, patient-priorities, and recommendations for improvements in BC healthcare post-pandemic and (ii) to understand how they may vary for women with BC according to SDH, in particular SES.
Study design
A qualitative exploratory study was conducted using the consolidated criteria for reporting qualitative research (COREQ) guidelines [ 21 ].
Participants
Women with BC were initially enrolled into a prospective cohort study measuring the impact of the COVID-19 pandemic on BC healthcare services and women’s well-being using a questionnaire ( N = 387) [ 22 ]. In total, 37 women from this cohort, purposively sampled based on SDH, were interviewed about their experience of the COVID-19 pandemic on health care and well-being during the pandemic [ 23 ]. These 37 women were invited to take part in a follow-up qualitative study to explore the impact of COVID-19 post-pandemic on BC care. The initial SDH sampling strata in the baseline interviews were further refined to include age, education level, annual income, work status, health insurance, and region. SES was established by considering annual income, education level, and health insurance status, respectively. Regarding health insurance status in Ireland, eligibility for entire public coverage through a medical card is based primarily on income, while health status and age are also considered. A medical card entitles the individual to primary care and hospital services free at the point of access; however, only 32% of the population are eligible for such coverage [ 24 ]. This eligibility structure causes inequalities for health services [ 25 ]. Thus, nearly 50% of Irish citizens seek out additional private, or voluntary, health insurance for quicker access to care [ 26 ]. Additional clinical information was obtained from the survey components, including year of diagnosis and cancer stage. Further information on the overall study design, participant recruitment, and sampling strata can be found in Supplementary 1 .
Data collection
Semi-structured qualitative interviews were conducted between January and March 2023 via Microsoft Teams (General Data Protection Regulation compliant) using a topic guide developed from the analysis of the baseline qualitative study [ 23 ] and baseline and follow-up survey study [ 22 ] by two qualitatively trained researchers (CM, CC). The topic guide included questions to explore women’s experiences and perspectives of BC care since the easing of COVID-19 government restrictions, including unmet needs, patient priorities, and recommendations specific to BC care. The topic guide was adjusted by removing, adding, or rewording questions during the interview process, a process known as reflexivity in qualitative research, which reduces researcher bias in data collection [ 27 ]. The final topic guide can be found in Supplementary 2 . The interviews were anonymised and transcribed verbatim.
Data analysis
Interviews were analyzed using a codebook approach to thematic analysis within NVivo software, which identifies themes early in the analysis process and subsequently maps concepts around central patterns or relationships within the data [ 28 ]. The codebook approach utilizes both deductive reasoning (e.g., the creation of the topic guide as a preliminary codebook, aligning with the research objectives) and inductive reasoning (e.g., the addition of topics and codes as the interviews was conducted). The codebook approach to thematic analysis was conducted using the following steps: identifying existing code sources/code development; familiarization with new data, generating additional codes, identifying patterns around codes for themes, reviewing themes, defining and naming themes, and producing the report [ 28 , 29 ]. The data was organized by themes, summarized by sub-themes, and illustrated with quotations. Furthermore, cross-tabulation within NVivo was used to explore any variation in themes and sub-themes by SDH to associate patterns in variation. Evident variations were identified with a difference in proportion greater than 20%. SDH were then interpreted within the corresponding themes and sub-themes.
Ethical approval
Ethical approval was obtained from the Office for National Research Ethics Committee in Ireland (20-NREC-COV-078). Participation was voluntary, and participants were able to withdraw their consent at any point throughout the research study.
Participant characteristics
The follow-up interview study included 28 of the original participants which was a 76% response rate from the baseline interview study. There were nine women who were either lost to follow-up, uninterested, or deceased. Table 1 presents the clinical and demographic data of participants interviewed at follow-up. The average age for women in the study was 61.7 years (standard deviation (SD) = 12.3). Over half of women were diagnosed prior to 2020 (57%) and the majority of women reported an early stage (e.g. stage I–II) diagnosis (68%). Fifty-four percent of women were highly educated, and 68% of women reported not working due to unemployment, retirement, or as a result of BC. Half of women (50%) reported an annual household income of less than €40,000. Furthermore, 39% ( n = 11) of women were categorized as high SES, while 61% ( n = 17) of women were categorized as low SES. Supplementary 3 presents the refined SDH sampling strata by participant.
The three main themes, along with their sub-themes and corresponding codes, are described narratively below, and also summarized in Table 2 . SES was the only SDH that was associated with varied themes and sub-themes; therefore, SES is integrated narratively when evident.
Unmet needs in BC health care
Most women ( n = 26, 98%) mentioned at least one on-going unmet need specific to their BC health care, which negatively impacts their overall well-being since the onset of the COVID-19 pandemic.
Unmet needs in routine care for BC
Post-treatment care (i.e., routine care) includes follow-up appointments, exams, scans, and other tests that occur after active treatment, and most women reported an unmet need regarding their routine care ( n = 21). Many women experienced persisting delays and/or cancellations ( n = 16) since the pandemic. A higher proportion of women with low SES reported disruptions ( n = 12, 71%) compared to women with high SES ( n = 4, 36%) for routine appointments. Overall, these reported delays and/or cancellations were typically rescheduled and/or modified; however, women were dissatisfied without in-person annual mammograms: “Now, it was a drawback not being able to have my mammogram for two years and, well, I was keeping up with, you know, with my examinations myself, so I was kind of half OK, you know, that if anything was there, I would have felt it.” (P6, low SES).
Regarding routine care, many women expressed difficulty contacting their BC team ( n = 17). Additional concerns included delays with breast reconstruction procedures ( n = 6) and BC medication ( n = 11). A higher proportion of women from low SES backgrounds reported medication difficulties ( n = 9, 53%) compared to women from higher SES background ( n = 2, 18%), including disruptions with supply of BC medications and a lack of support for the side effects and consequences from tamoxifen: “When I was given tamoxifen, I wasn’t aware that other medications could actually reduce its’ effectiveness. That was something I discovered during the year, myself, on my own. So I had to bring that to the attention of my doctors and request that they be changed.” (P8, low SES).
Unmet needs in mental and physical healthcare
The combination of experiencing COVID-19 and having BC exacerbated both physical health and mental health for women, regardless of SES: “But the last six or eight months, again, I have felt very un-well and it just can’t be gotten to the bottom of, medically, you know… I don’t know whether it’s right or wrong or appropriate to attribute it to COVID or post-pandemic or is it just a fact of life post cancer?” (P28, high SES).
For physical health ( n = 13), women noted developing pain, co-morbidities, and fatigue which was attributed to the disruption and lack of access to healthcare services during the pandemic: “That side of my arm and breast is very sore, so maybe if I was going to physio, it mightn’t be so bad. It’s hard to lift up that arm. It’s very, very sore.” (P16, low SES).
For mental health ( n = 18), women expressed persisting depression, anxiety, and fear of cancer recurrence, which they believed would have improved post-pandemic, if they were able to access treatment: “Now I find in the last sort of six months, I’m struggling mentally. I’m a bit overwhelmed at times. I think that would be the best way to put it. And you know, the impact now of it, I suppose, is really taking effect now because you are dealing with the treatments and stuff like that. (P2, low SES).
However, these mental health needs were infrequently addressed through their BC care: “But they need to be more aware of the mental… health side of it as well. I don’t think there’s anything, well look, there is nothing done, there’s nothing there for it. That’s not part of the treatment plan and I think it should be.” (P7, low SES).
Unmet financial support to access BC healthcare
Throughout the pandemic, some women experienced financial difficulties ( n = 10), including disruption to work and income due to BC and/or COVID-19, resulting in an inability to pay for BC services and medications, and transportation issues. A higher proportion of women with low SES reported financial difficulties ( n = 9, 53%) compared to women with high SES ( n = 1, 9%): “And I wasn’t working, so I had no money at all. And so, I got sorted out. Well, it took a while and I think that’s hard on people because I had to like, actually beg for a medical card because I wasn’t even going to get the operation because I had no money to pay for an operation.” (P1, low SES).
The lack of financial support is still evident and on-going post-pandemic: “I have the hormone medication, the breast cancer one. So it is, that’s a bit of a pain every three months, having to pay that.” (P13, low SES).
Patient priorities for BC healthcare
Women proposed priorities for their BC care ( n = 28); which were personalized and included improving their health-related anxiety and overall well-being.
Cohesion in BC healthcare
Cohesion among multidisciplinary BC care (e.g., oncology, surgery, radiotherapy, GP, physiotherapy, psychology, and pharmacy), spanning from diagnosis through post-treatment care, was a top priority for all women ( n = 28). However, women’s experience with cohesive BC care varied; the majority of women described adequate BC cohesion ( n = 18); however, of the women who described poor BC cohesion ( n = 10), a higher proportion was of low SES ( n = 8, 80%): “It didn’t feel linked… it almost felt a bit like a conveyor belt system. And you know…you could be with radiology and you might say something or have a concern and they’d be like, well, that’s not really our department. So you need to phone oncology.” (P7, low SES).
Women with poor BC cohesion expressed that the different elements for BC care (e.g. radiology, oncology) were not linked and those who attended appointments in varying BC clinics addressed concern regarding clarity in patient information: “When you’re coming in as a patient, they should know your history… Like I’ve been asked, ‘So you had cancer on the left?’ And then I said, ‘Yeah but I had double mastectomy.’ ‘Oh, I didn’t know that’, you know? They’re the type of things you should know as a doctor or a nurse…you should read the notes. They should have a preliminary page that says this patient has had this, this, and this.” (P3, low SES).
On the other hand, women expressed better cohesion with continuity and on-going monitoring with BC health professionals post-treatment: “Now I’m really lucky because we know they’re there. So, for instance, next month I’m having… a breast CT…so I’m kind of back into six-monthly checks now again. So that’s where I am at the moment, [I’m] being watched quite carefully now.” (P23, high SES).
Many women discussed the importance of efficiency with appointments ( n = 13), including timely results and less delays: “I think the fact that the hospital…quite quickly geared up to being very, very efficient and they dealt with people when they arrived and soon as you were kind of finished, you were let go. There wasn’t any of the delays that would have been before.” (P24, high SES).
Communication with and between BC healthcare providers
Proper communication, including consistency and understandability, with health professionals was another top priority for women interviewed in the study ( n = 28). All women who expressed adequate cohesion in their BC care attributed it to good communication ( n = 18): “Since [the pandemic], it has been the same, consistent. If they give me an appointment, it goes ahead and there’s no changes. If you ring them… they answer the phone and you get on to them and you know the details are there and so everything is fine from that point of view.” (P21, high SES).
However, poor communication was a common concern for some women ( n = 10), and women’s experience with poor communication was attributed to the unmet need of fall-out from routine care: “It’s communication. It always comes back to communication, doesn’t it? And if you had somebody that you could just have a 5-min conversation with an’ it’s kind of like, just give me your opinion. Hear me. Hear me. First of all.” (P18, high SES).
To enhance communication, many women mentioned the use of telemedicine ( n = 21), especially during the pandemic when in-person appointments were limited: “To know that there was somebody picking up your file, looking at it, picking up the phone and checking in with you. It was very reassuring.” (P6, low SES).
Indeed, there were differences in women’s experiences with communication. Similar to cohesion, 80% ( n = 8) of women who expressed poor communication were of low SES, even with the use to telemedicine: “The phone call, like I said, you were just talking… there were no personal details. You know, you couldn’t show [them anything] over the phone.” (P9, low SES).
Additionally, women described the role of a BC nurse, junior doctor, or GP as an integral component to their BC care to enhance both communication and cohesion: “That continuity of care was very important… I found my oncology link nurse was extremely supportive… And I got that. But I imagine not everyone probably is that lucky.” (P20, high SES).
Patient empowerment in BC healthcare
Empowerment was another top priority, and most women ( n = 20) described the ability to be an active participant in their BC care. The personalization of BC care enhanced patient empowerment: “Everybody has different needs and different wants. And what I would find satisfactory, somebody else wouldn’t, you know?” (P10, low SES).
Furthermore, proper education and knowledge on BC treatment and results also improved mental well-being: “They really kind of involved me in a sense, showed me the evidence. And that really made a difference. I mean, that melted away any lingering anxiety I had. Now I’m just a new person. That could make a huge difference to somebody.” (P8, low SES).
However, several women did not experience empowerment and involvement within their BC care ( n = 5), which was more common among women of low SES ( n = 4, 80%). This lack of control caused health-related anxiety and worry: “It’s the effects of all the other things, you know? I find that a bit problematic… It’s a struggle. I don’t know what anyone even could do about it because I don’t know even myself.” (P1, low SES).
Women also discussed the importance of managing their BC care by understanding their specific pathway for treatment and care, and being aware of next steps: “I had my plan set out for me from the beginning of where we were going. Chemo, surgery, radiation. So you knew all that, which was great. You know, you weren’t second guessing it all the time. You knew you had a plan and the plan was going to plan.” (P2, low SES).
Recommendations to improve BC healthcare
Considering their unmet needs and priorities for proper and personalized BC care, all women ( n = 28) proposed improvements and recommendations to enhance BC care moving forward post-pandemic. Most of the recommendations addressed an unmet need and/or a patient priority.
Transition from active treatment to post-treatment
To address the unmet need of routine care fall-out, many women ( n = 17) suggested ways to improve the transition from active to post-treatment, a period of time when women feel abandoned from their habitual BC care. Women proposed continuity in care through continued contact with a designated BC nurse: “I think that could help a lot of people out, if you could ring the nurse and they could tell you what’s going on. It might be something very simple or, you know…it’s something that’s part of [the BC] because, as you know, the side effects are massive from medications.” (P17, low SES).
Women expressed the desire for clear communication on their BC care plan post-treatment: “I didn’t feel that they gave you…a little pamphlet or booklet or something that could give you directions if you have anything, anxieties… How do you get back into your normal life? And how do you deal with maybe upcoming events, something like that? It was just like dead stop.” (P2, low SES).
The promotion of local cancer support centers from the BC care team was a common suggestion to enhance the transition from active treatment to post-treatment: “There should be something, a follow-up from your treatment, as in a nurse even saying to you, ‘look, I think you should contact your local [support centre]’ or something but like I, I literally finished my treatment and that was it.” (P4, low SES).
Likewise, the use of local cancer support centers offered women supportive care to address physical and mental health unmet needs: “That psych-oncologist was brilliant and she arranged… she gave me the name and number of somebody in [support centre] to ring, which I did. And she said, ‘I think these services, these things, the thrive and survive… this would be really beneficial for you.’” (P18, high SES).
Support resources for BC care
In addition to support centers, many women ( n = 18) expressed the importance of support resources for their BC care to improve the transition from active treatment. To address the unmet need of financial support within BC care, women ( n = 9) suggested ways to improve the barrier of healthcare costs specific to BC: “Look, there’s definitely…grants and stuff like that. I never chase them because they made it too difficult for you to access. When you’re in the midst of a diagnosis and you’re trying to process everything, the last thing you want to do is go through your emails, try find pay slips and try to find, you know, bank statements…a letter from your oncologist should be enough. You know?” (P7, low SES).
Women also recommended ways to improve the patient experience with general issues such as transportation ( n = 8): “And I do think the volunteer drivers with the [support centre] that’s a massive plus. That’s how I used to get in because I would be very dopey when I finished treatment, so I wasn’t, it wasn’t safe for me to drive, so they were huge resource … and I wasn’t made aware of that in the hospital.” (P19, high SES).
Specific to BC, women discussed BC specific resources such as bras and wigs ( n = 9); however, there was limited knowledge on the accessibility and availability of such resources: “I’m only talking about what’s available locally… I don’t think there are those things here. Even down to…getting a proper fitting bra or where to go for it… I didn’t seem to realize that the mastectomy bra… you get those cheap or free for your first one.” (P27, high SES).
Telemedicine
The use of telemedicine was common throughout the pandemic ( n = 21), and women recommended the adaptation of telemedicine moving forward post-pandemic when feasible ( n = 7): “The use of technology has been…a positive. If I was to turn around and say, ‘can I have a video, you know, a phone consultation?’ I think most of the time, it wouldn’t be a problem if you were to ask for that rather than just having an actual face-to-face.” (P23, high SES).
More so, the adaptation of telemedicine can eliminate transportation barriers and reduce time spent waiting for appointments: “They were useful in the pandemic in that you couldn’t physically be in the same spot. You know, we were in lockdown. And I actually think sometimes it’s better to be able to do that rather than going up to spend 3 or 4 h in the hospital waiting to speak to somebody.” (P3, low SES).
The current study found that women with a diagnosis of BC are experiencing many unmet needs associated with their BC care post-pandemic. Unmet needs included disruption and discontinuation of routine BC care, a lack of treatments and support services to address women’s mental and physical well-being, and a lack of financial support for those women of low SES to help them access and obtain BC care. Considering such unmet needs, women identified their priorities for receiving adequate BC care and further proposed recommendations for improving BC care in the future. Cohesion within BC health care delivery and improved communication among BC healthcare providers were considered top priorities, both of which were perceived to empower women in managing their BC care. The following three recommendations addressed unmet needs and patient priorities: (1) improving the transition from active to post-treatment care, (2) enhancing and promoting support resources, and (3) appropriate adaptation of telemedicine.
Unmet supportive care needs were common for all BC patients throughout the pandemic, including physical and psychological needs, communication with clinicians, health system information needs, and other financial and social needs [ 30 ]. A previous quantitative study conducted during the pandemic found that unmet needs for BC survivors can be addressed with either comprehensive care or psychological and emotional support and women who reported more unmet needs also reported a significantly lower quality of life [ 15 ]. The results of our study found that women of low SES experienced greater disruption to routine care and increased financial difficulties specific to BC, which is consistent with research conducted prior to the pandemic [ 31 ]. It is likely the pandemic exacerbated pre-existing socio-economic inequalities in BC care; therefore, women who experienced greater unmet needs should be reintegrated into routine BC care along the entire cancer continuum [ 22 ].
The identification of patient priorities for personalized BC care ensures equality in BC care [ 32 ]. The women in our study identified cohesion and communication as top priorities; however, poorer cohesion and communication were both common for women from lower SES backgrounds. Personalized BC care should address comprehensive continuity for all women, regardless of SES, to improve equity in healthcare services. Women in this study proposed improving the transition from active to post-treatment by having a designated, or liaison, healthcare professional to support them with the transition. Research has shown the multidisciplinary benefits of a liaison nurse for cancer care, including physical, psychosocial, and communicative outcomes [ 33 ]. Cancer support centers also improve the transition from active treatment by providing cancer survivors a social and community network to address multidisciplinary needs [ 34 ]. However, the pandemic created barriers towards accessing such resources. Women should be made more aware of the availability of these centers, and other supportive care, directly from their BC care team.
Providing financial aid and transportation means to women in need, especially women from low SES backgrounds, can address health inequalities specific to accessing and obtaining BC care [ 35 ]. Strategies from a health systems level for reducing cancer-related inequalities include enhancing patient navigation along the cancer continuum and integrating telemedicine for routine care [ 36 ]. Furthermore, transportation barriers and auxiliary costs can be addressed with telemedicine, which was a widely utilized practice during the COVID-19 pandemic [ 37 ]. In addition, telemedicine can improve communication with continued contact with BC health professionals. As BC services recover from the COVID-19 pandemic, consideration should be given to the use of telemedicine in BC care and how it could be used more effectively to support women.
Strengths and limitations
The study has a number of strengths including the large number of women interviewed and the selection via stratified purposive sampling to ensure diverse representation. This study is one of few studies to associate SDH, in particular SES, with BC care experience. The interviews were conducted immediately following COVID-19 government restrictions; therefore, they were timely and represent experiences of the transition from pandemic restrictions. There is limited research post-pandemic from the patient perspective; therefore, it addresses an evidence gap. However, there are several limitations to the study regarding generalizability. The participants do not represent all women living with a diagnosis of BC. The study was conducted in Ireland, a country which experienced severe and longer periods of restrictions compared with other countries [ 38 ]. Ireland remains the only country within the European Union without universal healthcare, and health inequalities have been associated with health insurance status and SES [ 39 ]. To enhance health equality in BC care, the findings from this research, in tandem with previous related research conducted during the pandemic [ 22 ], suggest that all women with a diagnosis of BC should be entitled to a medical card to assist with healthcare costs, if needed. Despite differences in health care systems, women with BC may be experiencing similar unmet needs across different countries and further research across countries with varying health care systems is needed to fully understand unmet needs for women with BC post-pandemic and inequities in these unmet needs. Additional future research may include comparing individual SDH characteristics to determine what SDH characteristics have a greater influence on women’s experience with BC care.
The pandemic has impacted BC services considerably for women in Ireland with BC, and this study has identified a range of unmet needs in BC care, patient-centered priorities, and recommendations for addressing these unmet needs. These priorities and recommendations align with the goals of the national cancer strategy, which aims to put structures in place to allow for increased patient involvement in the delivery of BC care going forward [ 40 ]. As health systems are recovering from the COVID-19 pandemic, the emphasis should be on both restoring access to BC care and improving the quality of BC care to achieve the best possible health outcomes for women living with and beyond a diagnosis of BC. Particular consideration needs to be given to those women from lower socioeconomic groups, in order to reduce health inequalities, which have been further exacerbated by the pandemic. As health systems are recovering from the COVID-19 pandemic, an emphasis to restore and enhance better BC care should be essential, with consideration and emphasis from the patient perspective.
Myers C, Bennett K, Cahir C (2023) Breast cancer care amidst a pandemic: a scoping review to understand the impact of coronavirus disease 2019 on health services and health outcomes. Int J Qual Health Care 35(3)
Vanni G, Pellicciaro M, Materazzo M, Bruno V, Oldani C, Pistolese CA et al (2020) Lockdown of breast cancer screening for COVID-19: possible scenario. In Vivo 34(5):3047–53
Article CAS PubMed PubMed Central Google Scholar
Gasparri ML, Gentilini OD, Lueftner D, Kuehn T, Kaidar-Person O, Poortmans P (2020) Changes in breast cancer management during the Corona Virus Disease 19 pandemic: an international survey of the European Breast Cancer Research Association of Surgical Trialists (EUBREAST). Breast 52:110–115
Article PubMed PubMed Central Google Scholar
Lee J, Jung JH, Kim WW, Park CS, Park HY (2020) Patterns of delaying surgery for breast cancer during the COVID-19 outbreak in Daegu. South Korea Front Surg 7:576196
Article PubMed Google Scholar
Mo A, Chung J, Eichler J, Yukelis S, Feldman S, Fox JL et al (2021) Breast cancer survivorship care during the COVID-19 pandemic within an urban new york hospital system. Int J Radiat Oncol Biol Phys 111(3):e169–e170
Article PubMed Central Google Scholar
Helm EE, Kempski KA, Galantino MLA (2020) Effect of disrupted rehabilitation services on distress and quality of life in breast cancer survivors during the COVID-19 pandemic. Rehabil Oncol 38(4)
Archer S, Holch P, Armes J, Calman L, Foster C, Gelcich S et al (2020) “No turning back” psycho-oncology in the time of COVID-19: insights from a survey of UK professionals. Psychooncology 29(9):1430–1435
Pairawan SS, Olmedo Temich L, de Armas S, Folkerts A, Solomon N, Cora C et al (2021) Recovery of screening mammogram cancellations during COVID-19. Am Surg 87(10)
Toss A, Isca C, Venturelli M, Nasso C, Ficarra G, Bellelli V et al (2021) Two-month stop in mammographic screening significantly impacts on breast cancer stage at diagnosis and upfront treatment in the COVID era. ESMO Open 6(2)
Sprague BL, Lowry KP, Miglioretti DL, Alsheik N, Bowles EJA, Tosteson ANA et al (2021) Changes in mammography use by women’s characteristics during the first 5 months of the COVID-19 pandemic. J Natl Cancer Inst 113(9):1161–1167
Haribhai S, Bhatia K, Shahmanesh M (2023) Global elective breast- and colorectal cancer surgery performance backlogs, attributable mortality and implemented health system responses during the COVID-19 pandemic: a scoping review. PLOS Glob Public Health 3(4):e0001413
Rahman MM, Rosenberg M, Flores G, Parsell N, Akter S, Alam MA et al (2022) A systematic review and meta-analysis of unmet needs for healthcare and long-term care among older people. Heal Econ Rev 12(1):60
Article Google Scholar
Carr W, Wolfe S (1976) Unmet needs as sociomedical indicators. Int J Health Serv 6(3):417–430
Article CAS PubMed Google Scholar
Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ (2009) What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer 17(8):1117–1128
Miroševič Š, Prins J, Borštnar S, Besić N, Homar V, Selič-Zupančič P et al (2022) Factors associated with a high level of unmet needs and their prevalence in the breast cancer survivors 1–5 years after post local treatment and (neo)adjuvant chemotherapy during the COVID-19: a cross-sectional study. Front Psychol 13:969918
Kenis I, Theys S, Hermie E, Foulon V, Van Hecke A (2022) Impact of COVID-19 on the Organization of Cancer Care in Belgium: lessons learned for the (post-)pandemic future. Int J Environ Res Public Health 19(19):12456
Ahmed F, Ne Ahmed, Pissarides C, Stiglitz J (2020) Why inequality could spread COVID-19. Lancet Public Health 5(5):e240
Mamelund S-E, Shelley-Egan C, Rogeberg O (2021) The association between socioeconomic status and pandemic influenza: systematic review and meta-analysis. PLoS ONE 16(9):e0244346
McCarthy G, Shore S, Ozdenerol E, Stewart A, Shaban-Nejad A, Schwartz DL (2023) History repeating-how pandemics collide with health disparities in the United States. J Racial Ethn Health Disparities 10(3):1455–1465
Bambra C, Riordan R, Ford J, Matthews F (2020) The COVID-19 pandemic and health inequalities. J Epidemiol Community Health 74(11):964–968
PubMed Google Scholar
Tong A, Sainsbury P, Craig J (2007) Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 19(6):349–357
Myers C, Bennett K, Kelly C, Walshe J, O’Sullivan N, Quinn M et al (2023) Impact of COVID-19 on health care and quality of life in women with breast cancer. JNCI Cancer Spectr 7(3)
Myers CL, Waldron C, Bennett K, Cahir C (2022) COVID-19 and breast cancer care in Ireland: a qualitative study to explore the perspective of breast cancer patients on their health and health care. Cancer Res 82(12)
OECD/European Observatory on Health Systems and Policies (2019) Ireland: country health profile 2019. State of health in the EU, OECD Publishing, Paris/European observatory on health systems and policies. Brussels
McDaid D, Wiley M, Maresso A, Mossialos E (2009) Ireland: health system review. Health Syst Trans (European Observatory on Health Systems and Policies) 11(4)
The Health Insurance Authority (2022) Health insurance in Ireland: market report 2022
Dodgson JE (2019) Reflexivity in qualitative research. J Hum Lact 35(2):220–222
Braun V, Clarke V (2022) Conceptual and design thinking for thematic analysis. Qual Psychol 9(1):3
Fereday J, Muir-Cochrane E (2006) Demonstrating rigor using thematic analysis: a hybrid approach of inductive and deductive coding and theme development. Int J Qual Methods 5(1):80–92
Legge H, Toohey K, Kavanagh PS, Paterson C (2022) The unmet supportive care needs of people affected by cancer during the COVID-19 pandemic: an integrative review. J Cancer Survivorship 17(4):1036–1056
DeGuzman P, Colliton K, Nail CJ, Keim-Malpass J (2017) Survivorship care plans: rural, low-income breast cancer survivor perspectives. Clin J Oncol Nurs 21(6):692–698
Roux A, Cholerton R, Sicsic J, Moumjid N, French DP, Giorgi Rossi P et al (2022) Study protocol comparing the ethical, psychological and socio-economic impact of personalised breast cancer screening to that of standard screening in the “My Personal Breast Screening” (MyPeBS) randomised clinical trial. BMC Cancer 22(1):507
Kerr H, Donovan M, McSorley O (2021) Evaluation of the role of the clinical nurse specialist in cancer care: an integrative literature review. Eur J Cancer Care 30(3):e13415
McIllmurray MB, Thomas C, Francis B, Morris S, Soothill K, Al-Hamad A (2001) The psychosocial needs of cancer patients: findings from an observational study. Eur J Cancer Care 10(4):261–269
Article CAS Google Scholar
Kong YC, Wong LP, Ng CW, Taib NA, Bhoo-Pathy NT, Yusof MM et al (2020) Understanding the financial needs following diagnosis of breast cancer in a setting with universal health coverage. Oncologist 25(6):497–504
Dickerson JC, Ragavan MV, Parikh DA, Patel MI (2020) Healthcare delivery interventions to reduce cancer disparities worldwide. World J Clin Oncol 11(9):705–722
McGrowder DA, Miller FG, Vaz K, Anderson Cross M, Anderson-Jackson L, Bryan S et al (2021) The utilization and benefits of telehealth services by health care professionals managing breast cancer patients during the COVID-19 pandemic. Healthcare 9(10):1401
Hale T, Angrist N, Kira B, Petherick A, Phillips T, Webster S (2020) Variation in government responses to COVID-19. University of Oxford
Burke S, Pentony S (2011) Eliminating health inequalities: a matter of life and death. TASC: think-tank for action on social change
National Cancer Strategy 2017–2026 (2017) Department of Health, and National Patient Safety (NPS) Office: Ireland
Download references
Open Access funding provided by the IReL Consortium This work is independent research funded by the Royal College of Surgeons in Ireland (Strategic Academic Recruitment funding).
Author information
Authors and affiliations.
School of Population Health, RCSI University of Medicine and Health Sciences, 123 St Stephen’s, Green, Dublin, D02 YN77, Ireland
Charlotte Myers
Data Science Centre, School of Population Health, RCSI University of Medicine and Health Sciences, Dublin, Ireland
Kathleen Bennett & Caitriona Cahir
You can also search for this author in PubMed Google Scholar
Contributions
C.M., K.B., and C.C. contributed to the study conception and design. Material preparation, data collection and analysis were performed by C.M. The first draft of the manuscript was written by C.M. and K.B. and C.C. commented and edited versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Charlotte Myers .
Ethics declarations
Ethics approval.
The study was conducted in accordance with the Declaration of Helsinki of Good Clinical Practice guidelines. Ethical approval was obtained from the Office for National Research Ethics Committee in Ireland (20-NREC-COV-078). Participation was voluntary, and participants were able to withdraw their consent at any point throughout the research study.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 26 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Myers, C., Bennett, K. & Cahir, C. Lessons learned from COVID-19: improving breast cancer care post-pandemic from the patient perspective. Support Care Cancer 32 , 338 (2024). https://doi.org/10.1007/s00520-024-08540-0
Download citation
Received : 27 October 2023
Accepted : 01 May 2024
Published : 10 May 2024
DOI : https://doi.org/10.1007/s00520-024-08540-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Social determinants of health
- Quality of life
- Patient voice
- Health policy
Advertisement
- Find a journal
- Publish with us
- Track your research
- Around the Practice
- Between the Lines
- Contemporary Concepts
- Readout 360
- Insights from Experts at Mayo Clinic on Translating Evidence to Clinical Practice
- Optimizing Outcomes in Patients with HER2+ Metastatic Breast Cancer

- Conferences
- Publications
- Career Center
Patient Case: A 37-Year-Old Woman With HER2+/HR- Breast Cancer
- Vijayakrishna K. Gadi, MD, PhD
- Sara A. Hurvitz, MD
Adam Brufsky, MD, PhD, presents the case of a 37-year-old woman with HER2+/HR- metastatic breast cancer and polls the audience about screening for brain metastases.
EP: 1 . Patient Case: A 37-Year-Old Woman With HER2+/HR- Breast Cancer
EP: 2 . Management Approaches for Patients with HER2+ BC at Risk for Brain Metastases
EP: 3 . The Role of Prophylactic Therapy in HER2+ BC
Ep: 4 . decision making in selecting treatment in her2+ bc, ep: 5 . her2+ bc: considerations for sequencing treatment, ep: 6 . screening and recognition of ild in her2+ bc, ep: 7 . monitoring symptoms of ild in her2+ breast cancer, ep: 8 . management of ild in her2+ bc, ep: 9 . the evolving space of her2+ breast cancer.

EP: 10 . HER2-Positive Breast Cancer: Special Challenges and Expert Insight
Adam Brufsky, MD, PhD: Welcome to this CancerNetwork ® Around the Practice presentation, “HER2+ Breast Cancer Special Challenges and Expert Insight.” I am your host, Dr Adam Brufsky, from UPMC [University of Pittsburgh Medical Center] Hillman Cancer Center in Pittsburgh, Pennsylvania. Tonight we have a great panel of very enthusiastic investigators, all of whom are very experienced in this field and have lots to add to this discussion. We have Dr VK Gadi from University of Illinois Cancer Center in Chicago; Dr Sara Hurvitz from UCLA [University of California, Los Angeles] Jonsson Comprehensive Cancer Center in Los Angeles, California; and finally, Dr Neil Iyengar from Memorial Sloan Kettering Cancer Center in New York City.
Tonight, we’re going to talk about 3 challenges we face in our practices. The first is how to optimally treat patients with HER2-positive breast cancer and brain metastases. We’ll also discuss considerations for sequencing therapies and how we make sequencing decisions in second-, third-, and fourth-line HER2+ metastatic breast cancer. I’ll add parenthetically that it’s a comment on how far this field has come that we’re now debating third-, fourth-, and fifth-line therapy in HER2+ metastatic disease. Finally, we’ll talk about the identification and management of interstitial lung disease and how it impacts the next steps for therapy. During this time, we’ll review a single patient case. Instead of 3 cases, we’re going to do 1 case and add to it as we go along during this hour. We’re going to ask the audience several polling questions using an interactive polling platform.
Let’s start. We’d appreciate if people answer this question. How often do you screen asymptomatic patients with metastatic HER2+ breast cancer for brain metastases? Always, sometimes, rarely, or never?
The results show 20% are always screening, 60% sometimes, 20% rarely, and 0% never.
How often do you treat prophylactically for brain metastases in asymptomatic patients with HER2+ breast cancer? Always, sometimes, rarely, or never? Please fill out the poll.
This is interesting stuff. No one chose always, 25% do sometimes, 25% do rarely, and 2 votes for never. Interesting. Let’s move on and talk about a case. I’ll fill in the details of this case. It is hard to put it all in 1 or 2 slides. This is a 37-year-old woman who 3 or 4 years ago presented with a 5-cm lump in her right breast. She has the typical HER2+, IHC [immunohistochemistry] score of 3+, hormone receptor-negative breast cancer, and no other metastatic disease or LVF [left ventricular failure], and she feels well otherwise. We gave her neoadjuvant TCHP [docetaxel, carboplatin, trastuzumab, pertuzumab] for 6 cycles, and she did pretty well with it. Then she had a lumpectomy after being on radiation, had a pCR [pathologic complete response], and then was given trastuzumab and pertuzumab for the remainder of her therapy, which we can debate back and forth.
She did well for about 2.5 years and then came to the clinic complaining of increased fatigue and persistent cough. She had a chest CT scan that showed a 1.5-cm nodule in the left upper—not superior—lobe. She got a lung biopsy, which showed she has adenocarcinoma persistent with the breast primary that is IHC 3+ for HER2 and negative for ER [estrogen receptor] and PR [progesterone receptor]. She had a PET [positron emission tomography]/CT scan that showed no other bone or liver metastases, but did show several lung metastases, not just 1. Had she just had 1, we would have probably taken it out and called it a day. That’s a whole other question. She had 3 or 4 lung metastases, each of which is about 2 to 3 cm.
At this point, she was given a typical first-line regimen. She was given THP [docetaxel, trastuzumab, pertuzumab], and started on bisphosphonates every 3 months. She was given the docetaxel for probably 8 cycles and then complained of neuropathy. At that point, we discontinued that and just continued her on HP [trastuzumab, pertuzumab]. But it’s now 2 years later and a routine CT showed 3 liver metastases, each of which is about 1 or 2 cm in diameter. There is 1 that’s about 3 cm. She has 4 liver metastases, none of which are incredibly damaging to her organs, but she does clearly have visceral disease progression in her liver after THP [docetaxel, trastuzumab, pertuzumab] in the first line.
Here is the first polling question for the audience. You have a woman who has been on THP [docetaxel, trastuzumab, pertuzumab] for 2 years for metastatic disease. Would you screen her for brain metastases at that point? Obviously, she has had PET/CT, but would you actually screen an asymptomatic patient for brain metastases?
Let’s see what we’ve got. Fifty-fifty, right down the middle. This is going to be an interesting discussion with our esteemed audience and our esteemed group of investigators to see what they think.
If you did not know whether she had brain metastases, and you decide not to screen her, what would you give this patient in the absence of an MRI? She has progressive disease in her liver. She has been on HP [trastuzumab, pertuzumab] alone for about 18 months. Would you rechallenge her with THP [docetaxel, trastuzumab, pertuzumab], give her T-DM1 [trastuzumab emtansine], give her trastuzumab deruxtecan, or something else? The results show that 14% would rechallenge with THP [docetaxel, trastuzumab, pertuzumab], which is not a bad idea, 57% would give T-DM1 [trastuzumab emtansine], 29% would give trastuzumab deruxtecan, and 0% would give other.
Transcript edited for clarity.

52 UK Experience of Non-Radioisotope, Non-Magnetic Guided Breast Wide Local Excision and Sentinel Node Biopsy

Managing CDK4/6 Inhibitor, ADC Toxicity in Metastatic Breast Cancer
Sarah Donahue, MPH, NP, speaks to the importance of communicating potential adverse effects associated with treatments such as CDK4/6 inhibitors to patients with breast cancer.
Dato-DXd Shows Improved Tolerability Vs Chemo in HR+/HER2– Breast Cancer
Safety data further support datopotamab deruxtecan as a new treatment option in metastatic hormone receptor–positive, HER2-negative breast cancer.

HER2CLIMB-02 Trial Shows ‘Interesting Data’ in HER2+ Breast Cancer
Tucatinib plus trastuzumab emtansine shows a progression-free survival improvement in HER2-positive breast cancer in the phase 3 HER2CLIMB-02 trial, says Sara A. Hurvitz, MD, FACP.

55 Language as a Barrier to Deep Inspiration Breath Hold (DIBH) Radiation Therapy for Left Breast Cancer

56 Predictive Factors Correlating With Pathologic Complete Response Rates in Racially Diverse, Minority Populations Receiving Neoadjuvant Therapy for HR+/HER2– Breast Cancer
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Personalized circulating tumor DNA response to local radiotherapy in a patient with an early lobular breast cancer: A case report
Affiliations.
- 1 Department of Medicine and Oncology, Lady Davis Institute and Segal Cancer Centre, Jewish General Hospital, McGill University Montreal, Montreal, QC H3T 1E2, Canada.
- 2 Division of Oncology, Lady Davis Institute and Segal Cancer Centre, Jewish General Hospital, McGill University Montreal, Montreal, QC H3T 1E2, Canada.
- PMID: 38736743
- PMCID: PMC11082640
- DOI: 10.3892/ol.2024.14415
The detection of circulating tumor DNA (ctDNA) in the plasma of cancer patients is emerging as a very sensitive and specific prognostic biomarker. Previous studies with ctDNA have focused on the ability of ctDNA detection to predict micrometastatic and eventual clinical metastatic relapse. There are few data on the role of ctDNA in monitoring response to local therapy. The present study reports the case of a patient with early-stage lobular breast cancer, with a detectable ctDNA test which resolved with local radiotherapy to the breast. This case suggests that ctDNA is sensitive enough to detect the response of minimal residual disease, localized in the breast, to radiation therapy, and thus may assist in providing indications for local breast cancer treatment.
Keywords: biomarker; ctDNA; lobular breast cancer; radiotherapy prognostic.
Copyright © 2024, Spandidos Publications.
Publication types
- Case Reports
Grants and funding
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Breast cancer patient experiences through a journey map: A qualitative study
Roles Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Clinical Psychology and Psychobiology Department, Faculty of Psychology, University of Barcelona, Barcelona, Spain
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing
Affiliation Medical Oncology Department Hospital Universitario Central of Asturias, Oviedo, Spain
Roles Resources, Validation, Writing – review & editing
Affiliation Social Psychology and Quantitative Psychology Department, Faculty of Psychology, University of Barcelona, Barcelona, Spain
Affiliation Medical Oncology Department, Hospital Universitario Clínico San Carlos, Madrid, Spain
Affiliation Medical Oncology Department, Complexo Hospitalario Universitario de Ourense, Ourense, Spain
Affiliation Medical Oncology Department, Hospital Universitario La Paz, Madrid, Spain
Affiliation Medical Oncology Department, Hospital General Universitario de Elche, Elche, Spain
Affiliation Medical Oncology Department, Hospital Universitario Fundación Alcorcón, Madrid, Spain
Roles Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing
- Laura Ciria-Suarez,
- Paula Jiménez-Fonseca,
- María Palacín-Lois,
- Mónica Antoñanzas-Basa,
- Ana Fernández-Montes,
- Aranzazu Manzano-Fernández,
- Beatriz Castelo,
- Elena Asensio-Martínez,
- Susana Hernando-Polo,
- Caterina Calderon

- Published: September 22, 2021
- https://doi.org/10.1371/journal.pone.0257680
- Reader Comments
Registered Report Protocol
21 Dec 2020: Ciria-Suarez L, Jiménez-Fonseca P, Palacín-Lois M, Antoñanzas-Basa M, Férnández-Montes A, et al. (2020) Ascertaining breast cancer patient experiences through a journey map: A qualitative study protocol. PLOS ONE 15(12): e0244355. https://doi.org/10.1371/journal.pone.0244355 View registered report protocol
Breast cancer is one of the most prevalent diseases in women. Prevention and treatments have lowered mortality; nevertheless, the impact of the diagnosis and treatment continue to impact all aspects of patients’ lives (physical, emotional, cognitive, social, and spiritual).
This study seeks to explore the experiences of the different stages women with breast cancer go through by means of a patient journey.
This is a qualitative study in which 21 women with breast cancer or survivors were interviewed. Participants were recruited at 9 large hospitals in Spain and intentional sampling methods were applied. Data were collected using a semi-structured interview that was elaborated with the help of medical oncologists, nurses, and psycho-oncologists. Data were processed by adopting a thematic analysis approach.
The diagnosis and treatment of breast cancer entails a radical change in patients’ day-to-day that linger in the mid-term. Seven stages have been defined that correspond to the different medical processes: diagnosis/unmasking stage, surgery/cleaning out, chemotherapy/loss of identity, radiotherapy/transition to normality, follow-up care/the “new” day-to-day, relapse/starting over, and metastatic/time-limited chronic breast cancer. The most relevant aspects of each are highlighted, as are the various cross-sectional aspects that manifest throughout the entire patient journey.
Conclusions
Comprehending patients’ experiences in depth facilitates the detection of situations of risk and helps to identify key moments when more precise information should be offered. Similarly, preparing the women for the process they must confront and for the sequelae of medical treatments would contribute to decreasing their uncertainty and concern, and to improving their quality-of-life.
Citation: Ciria-Suarez L, Jiménez-Fonseca P, Palacín-Lois M, Antoñanzas-Basa M, Fernández-Montes A, Manzano-Fernández A, et al. (2021) Breast cancer patient experiences through a journey map: A qualitative study. PLoS ONE 16(9): e0257680. https://doi.org/10.1371/journal.pone.0257680
Editor: Erin J. A. Bowles, Kaiser Permanente Washington, UNITED STATES
Received: February 17, 2021; Accepted: September 3, 2021; Published: September 22, 2021
Copyright: © 2021 Ciria-Suarez et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: Relevant anonymized data excerpts from the transcripts are in the main body of the manuscript. They are supported by the supplementary documentation at 10.1371/journal.pone.0244355 .
Funding: This work was funded by the Spanish Society of Medical Oncology (SEOM) in 2018. The sponsor of this research has not participated in the design of research, in writing the report, or in the decision to submit the article for publication.
Competing interests: The authors have declared that no competing interests exist.
Introduction
Breast cancer is the most common cancer and the one that associates the highest mortality rates among Spanish women, with 32,953 new cases estimated to be diagnosed in Spain in 2020 [ 1 ]. Thanks to early diagnosis and therapeutic advances, survival has increased in recent years [ 2 ]. The 5-year survival rate is currently around 85% [ 3 , 4 ].
Though high, this survival rate is achieved at the expense of multiple treatment modalities, such as surgery, chemotherapy, radiotherapy, and hormone therapy, the side effects and sequelae of which can interfere with quality-of-life [ 5 ]. Added to this is the uncertainty surrounding prognosis; likewise, life or existential crises are not uncommon, requiring great effort to adjust and adapt [ 6 ]. This will not only affect the patient psychologically, but will also impact their ability to tolerate treatment and their socio-affective relations [ 7 ].
Several medical tests are performed (ultrasound, mammography, biopsy, CT, etc.) to determine tumor characteristics and extension, and establish prognosis [ 8 ]. Once diagnosed, numerous treatment options exist. Surgery is the treatment of choice for non-advanced breast cancer; chemotherapy, radiotherapy, and hormone therapy are adjuvant treatments with consolidated benefit in diminishing the risk of relapse and improving long-term survival [ 9 ]. Breast cancer treatments prompt changes in a person’s physical appearance, sexuality, and fertility that interfere with their identity, attractiveness, self-esteem, social relationships, and sexual functioning [ 10 ]. Patients also report more fatigue and sleep disturbances [ 11 ]. Treatment side effects, together with prognostic uncertainty cause the woman to suffer negative experiences, such as stress in significant relationships, and emotions, like anxiety, sadness, guilt, and/or fear of death with negative consequences on breast cancer patients’ quality-of-life [ 10 , 12 ]. Once treatment is completed, patients need time to recover their activity, as they report decreased bodily and mental function [ 13 ], fear of relapse [ 14 ], and changes in employment status [ 15 ]. After a time, there is a risk of recurrence influenced by prognostic factors, such as nodal involvement, size, histological grade, hormone receptor status, and treatment of the primary tumor [ 16 ]. Thirty percent (30%) of patients with early breast cancer eventually go on to develop metastases [ 17 ]. There is currently no curative treatment for patients with metastatic breast cancer; consequently, the main objectives are to prolong survival, enhance or maintain quality-of-life, and control symptoms [ 17 , 18 ]. In metastatic stages, women and their families are not only living with uncertainty about the future, the threat of death, and burden of treatment, but also dealing with the existential, social, emotional, and psychological difficulties their situation entails [ 18 , 19 ].
Supporting and accompanying breast cancer patients throughout this process requires a deep understanding of their experiences. To describe the patient’s experiences, including thoughts, emotions, feelings, worries, and concerns, the phrase “patient voice” has been used, which is becoming increasingly common in healthcare [ 20 ]. Insight into this “voice” allows us to delve deeper into the physical, emotional, cognitive, social, and spiritual effects of the patient’s life. This narrative can be portrayed as a “cancer journey", an experiential map of patients’ passage through the different stages of the disease [ 21 ] that captures the path from prevention to early diagnosis, acute care, remission, rehabilitation, possible recurrence, and terminal stages when the disease is incurable and progresses [ 22 ]. The term ‘patient journey’ has been used extensively in the literature [ 23 – 25 ] and is often synonymous with ‘patient pathway’ [ 26 ]. Richter et al. [ 26 ] state that there is no common definition, albeit in some instances the ‘patient journey’ comprises the core concept of the care pathway with greater focus on the individual and their perspective (needs and preferences) and including mechanisms of engagement and empowerment.
While the patient’s role in the course of the disease and in medical decision making is gaining interest, little research has focused on patient experiences [ 27 , 28 ]. Patient-centered care is an essential component of quality care that seeks to improve responsiveness to patients’ needs, values, and predilections and to enhance psychosocial outcomes, such as anxiety, depression, unmet support needs, and quality of life [ 29 ]. Qualitative studies are becoming more and more germane to grasp specific aspects of breast cancer, such as communication [ 27 , 30 ], body image and sexuality [ 31 , 32 ], motherhood [ 33 ], social support [ 34 ], survivors’ reintegration into daily life [ 13 , 15 ], or care for women with incurable, progressive cancer [ 17 ]. Nevertheless, few published studies address the experience of women with breast cancer from diagnosis to follow-up. These include a clinical pathway approach in the United Kingdom in the early 21st century [ 35 ], a breast cancer patient journey in Singapore [ 25 ], a netnography of breast cancer patients in a French specialized forum [ 28 ], a meta-synthesis of Australian women living with breast cancer [ 36 ], and a systematic review blending qualitative studies of the narratives of breast cancer patients from 30 countries [ 37 ]. Sanson-Fisher et al. [ 29 ] concluded that previously published studies had examined limited segments of patients’ experiences of cancer care and emphasized the importance of focusing more on their experiences across multiple components and throughout the continuum of care. Therefore, the aim of this study is to depict the experiences of Spanish breast cancer patients in their journey through all stages of the disease. To the best of our knowledge, there are no studies that examine the experience of women with breast cancer in Spain from diagnosis through treatment to follow-up of survivors and those who suffer a relapse or incurable disease presented as a journey map.
A map of the breast cancer patient’s journey will enable healthcare professionals to learn first-hand about their patients’ personal experiences and needs at each stage of the disease, improve communication and doctor-patient rapport, thereby creating a better, more person-centered environment. Importantly, understanding the transitional phases and having a holistic perspective will allow for a more holistic view of the person. Furthermore, information about the journey can aid in shifting the focus of health care toward those activities most valued by the patient [ 38 ]. This is a valuable and efficient contribution to the relationship between the system, medical team, and patients, as well as to providing resources dedicated to the patient’s needs at any given time, thus improving their quality of life and involving them in all decisions.
Study design and data collection
We conducted a qualitative study to explore the pathway of standard care for women with breast cancer and to develop a schematic map of their journey based on their experiences. A detailed description of the methodology is reported in the published protocol “Ascertaining breast cancer patient experiences through a journey map: A qualitative study protocol” [ 39 ].
An interview guide was created based on breast cancer literature and adapted with the collaboration of two medical oncologists, three nurses (an oncology nurse from the day hospital, a case manager nurse who liaises with the different services and is the ‘named’ point of contact for breast cancer patients for their journey throughout their treatment, and a nurse in charge of explaining postoperative care and treatment), and two psycho-oncologists. The interview covered four main areas. First, sociodemographic and medical information. Second, daily activities, family, and support network. Third, participants were asked about their overall perception of breast cancer and their coping mechanisms. Finally, physical, emotional, cognitive, spiritual, and medical aspects related to diagnosis, treatment, and side effects were probed. Additionally, patients were encouraged to express their thoughts should they want to expand on the subject.
The study was carried out at nine large hospitals located in six geographical areas of Spain. To evaluate the interview process, a pilot test was performed. Interviews were conducted using the interview guide by the principal investigator who had previous experience in qualitative research. Due to the Covid-19 pandemic, all interviews were completed online and video recorded with the consent of the study participants for subsequent transcription. Relevant notes were taken during the interview to document key issues and observations.
Participant selection and recruitment
Inclusion criteria were being female, over 18 years of age, having a diagnosis of histologically-confirmed adenocarcinoma of the breast, and good mental status. To ascertain the reality of women with breast cancer, most of the patients recruited (80%) had been diagnosed in the past 5 years. Patients (20%) were added who had been diagnosed more than 5 years earlier, with the aim of improving the perspective and ascertaining their experience after 5 years.
Medical oncologists and nurses working at the centers helped identify patients who met the inclusion criteria. Participants went to the sites for follow-up between December 2019 and January 2021. Eligible women were informed of the study and invited to participate during an in-person visit by these healthcare professionals. Those who showed interest gave permission to share their contact information (e-mail or telephone number) with the principal investigator, who was the person who conducted all interviews. The principal investigator contacted these women, giving them a more detailed explanation of the study and clarifying any doubts they may have. If the woman agreed to participate, an appointment was made for a videoconference.
A total of 21 women agreed to participate voluntarily in this research. With the objective of accessing several experiences and bolstering the transferability of the findings, selection was controlled with respect to subjects’ stage of cancer, guaranteeing that there would be a proportional number of women with cancer in all stages, as well as with relapses.
Data analysis
The data underwent qualitative content analysis. To assure trustworthiness, analyses were based on the system put forth by Graneheim, and Lundman [ 40 ]. Interviews were transcribed and divided into different content areas; units of meaning were obtained and introduced into each content area; meaning codes were extracted and added; codes were categorized in terms of differences and similarities, and themes were created to link underlying meanings in the categories. All members of the research team (core team, two medical oncologists, three nurses and two psycho-oncologists) reviewed the data and triangulated the outcomes between two sources of data: qualitative data from the interview and non-modifiable information, such as sociodemographic (i.e., age, marital status, having children) and clinical (i.e., cancer stage and surgery type) data. Following this process, we reached saturation of the interview data by the time we had completed 21 interviews.
Ethical considerations
This study was performed in accordance with the ethical standards of the Declaration of Helsinki, and its subsequent amendments. The study was approved by the Research Ethics Committee of University of Barcelona (Institutional Review Board: IRB00003099) and supported by the Bioethics Group of the Spanish Society of Medical Oncology (SEOM) 2018 grant. All participants received a written informed consent form that they signed prior to commencing with the interviews and after receiving information about the study.
Patient baseline characteristics
In total, 21 women with a mean age of 47 years (range, 34 to 61) were interviewed. Most of the study population was married (66.7%), had a college education (66.7%), and had 2 or more children (42.9%). All cancer stages were represented, up to 23.8% tumor recurrence, and most of the primary cancers had been resected (95.2%) (see Table 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0257680.t001
Description of the breast cancer patient journey
The women diagnosed with breast cancer describe the journey as a process tremendously affected by the different medical stages. Each stage has its own characteristics that condition the experiences, unleashing specific physical, emotional, cognitive, and social processes. Additionally, the patients perceive this entire process as pre-established journey they must undertake to save their life, with its protocols based on the type and stage of cancer.
“ People said to me , ‘What do you think ? ’ and I answered that there was nothing for me to think about because everything is done , I have to go on the journey and follow it and wait to see how it goes” (Patient 6)
Fig 1 displays the various phases of the journey that patients with breast cancer go through; nevertheless, each woman will go through some or others, depending on their type of cancer.
https://doi.org/10.1371/journal.pone.0257680.g001
Throughout the entire patient journey.
Processes of loss and reinterpretation of the new circumstance . What stands out the most in the process these women go through during the diagnosis and treatment of breast cancer is loss; specifically, the loss of health and a reinterpretation of the new circumstance and the new bodily reality. In the most extreme cases, the loss of health emerges with the fear of death that many women report at the time of diagnosis or during treatment, due to the distress generated. The loss of identity seems to be related to the evolutionary (existential) moment in which the woman is; there are patients who report feelings of disability or loss of attractiveness, or fear of not being able to get pregnant in the future, especially the youngest.
I felt a terrifying fear and thought , “You have cancer you tell yourself , you’re going to die tomorrow .” (Patient 6) I feel like after the hysterectomy , as a woman , I no longer have anything , only the physical . Sure , I look great , but I tell myself that it’s just a shell , the shell I inhabit , because as a woman , I only have one breast left . (Patient 6) At that moment , I had to make the decision that I was no longer going to be a mother . (Patient 14)
Personal change . Most of the women report that with the diagnosis of breast cancer, their life stands still and from that point forward, a different journey begins. The sole focus on this journey is the disease and its implications. During all those months, the patients stop working; they focus on their medical treatments, and reflect a lot on their current situation and on life. Most of the participants state, especially those who have already been discharged, that they know themselves better now; they take better care of themselves, and they enjoy their day-to-day and the small moments more, making the most of their time, with more initiatives and fewer trivial complaints.
Clearly , you’re not the same person you were before; I don’t think she’ll ever come back; your mindset changes completely and I have sequelae from all the treatments . (Patient 1) I re-think wasting energy on lost causes; what’s more , I’ve also learnt to say no . If I’m not in the mood to go somewhere , I just say no . (Patient 7) I take much more advantage of the present now , because you realize that things can change on any given day . (Patient 3)
Trust and appreciation for their physician . Most of the interviewees stated that they fully trusted the doctors who care for them, without question or objection to the treatments proposed. They reported that, as they go forward, they discuss the tests and treatments that are going to be performed, as well as possible side effects. Several stated that they are unaware of the stage of their cancer; similarly, most also do not know the benefits expressed in X% of the treatments. A few of the participants claimed that they did talk in detail about the different types of treatments with their oncologists, that they had sought another opinion, and one of them even reported having decided to stop chemotherapy, which was very hard for her, given her physician’s insistence that she continue.
The truth is that the oncologist didn’t say much about percentages; what she told me were the steps that I had to take; I thoroughly trusted her and she gave me a lot of peace of mind . (Patient 5) I told him , “I’m going to do whatever you tell me to . ” It never occurred to me to dispute whatever the oncologist might tell me . I was willing to do whatever was needed . (Patient 8)
Most of the women, at some point during the interview, state that they are grateful for the care they received and that, within the seriousness of their situation, there is a treatment for their condition.
I am super grateful for the treatment I’ve received and with the doctors assigned to me . (Patient 2) I’m very lucky; I’m only on my second line of treatment for metastasis and I’ve got a lot more ahead of me , but I consider myself lucky and I believe things are going very well . (Patient 20)
Role of the woman . We can see that the women adopt a role of care-givers and managers of their surroundings. They worry about the disease negatively affecting the people around them, which is why they make an effort to manage the family’s activity for when they can’t do it and they try to avoid being a physical burden or cause emotional distress to the people around them.
I was very strong ; I made everything easy for people , but making it very easy , doesn’t mean that it was easy for me , but that I made it easy for everyone . (Patient 8) I didn’t want to worry anyone because that’s just the way I am , I push forward and that’s that . (Patient 5)
Support network . In all cases, the family appears to be one of the elements that is most involved in the disease process. Within the family, the partner deserves special mention. The testimonies in this regard reveal a wide spectrum of possibilities that range from the feeling of having had great support to a lack of attention and understanding that, in many situations, causes the relationship to be strained or to end. Friends tend to appear more occasionally.
I can’t complain about my husband; he was up to the challenge , very attentive toward me and he fully understood how I was feeling ; I felt very supported . (Patient 14) We’ve had a period of a lot of arguing; I’ve had to sit down with him and tell him that life had changed for me . (Patient 18) I had a partner I had lived with for five and a half years and he told me , literally , that he looked at me like a little sister , no longer as a woman , and he left me , and that hurt me tremendously . (Patient 6)
On the other hand, many patients commented on the importance of social media, where they have met people in the same situation as them. They report feeling understood and in good company; likewise, they commented on the importance of being able to share their doubts and get to know about other experiences.
It’s a situation that only someone who has gone through can understand; you can have all the good intentions in the world , but if you haven’t gone through it , you can’t even begin to understand . (Patient 8)
Use of complementary treatments . Most patients follow conventional medical treatment. However, many resort to other disciplines that help them improve their quality-of-life, like dietary changes, getting more exercise than usual, visits to a psychologist or physical therapist, or using other integrative therapies, such as acupuncture, yoga, reiki, flowers of Bach, homeopathy, cannabis, or meditation.
I started to read a whole bunch of books to see what I could do to take care of myself in terms of nutrition and exercise ; you consider everything you can do . (Patient 5)
Diagnosis/unmasking.
This phase encompasses the time from when the woman detects some symptom or goes to a check-up until the medical diagnosis is made. For the woman, this is a time of a series of tests and results. We have observed that the procedures, especially the healthcare professionals that deal with the patients, and the timing vary, depending on the medical center where they are being cared for. Emotionally, this is one of the most complicated stages.
Emotional whirlwind . The wait to obtain test results has a huge emotional impact for the women, given that it is a time of great uncertainty and fear.
An entire month with all the anguish of finding out if you have something . (Patient 3) The worst part is waiting 15 days to find out the magnitude of the tragedy , if it’s throughout your entire body or only in your breast; you go through a brutal emotional whirlwind; the wait is horrible because there’s nothing else you can do , so that anguish that you carry inside is dreadful; it was hell for me . (Patient 10)
Additionally, the interviewees described many other emotions that included fear of death, fear of having no time, feeling of unreality, rage, anger, sadness, avoidance, denial…
The first thing I thought was that I was going to die and that I wouldn’t finish watching my children [grow up]; my father had died of lung cancer 25 years ago . (Patient 9) My only aim was to get back to normal , as if there were nothing wrong . (Patient 4) You have a lot of conflicting feelings; you wish this weren’t happening; you want to run away , but you say , “Where am I going to run to ? ” . (Patient 14)
Impact of medical communication . Several women comment that, when given the diagnosis, they dissociate because of the emotional impact and that they don’t listen to all the information that the medical professional is giving them.
I remember that she talked and talked , but I didn’t know what she was saying until she said , “Isabel , you’re going to be cured , okay ?”. (Patient 9)
During the diagnostic testing, the women are highly sensitive to the healthcare professionals’ words and gestures.
I looked at the face of the person who was doing the mammogram and that’s when I started to imagine the worst . (Patient 20) I say to them , “ But , is there a solution to this ? ” , and they say to me , “Don’t worry , I’m sure there is a solution . ” That “sure” is etched in my mind . (Patient 10)
Communication and managing their surroundings . After the diagnosis, the patients feel that they have to tell the people around them about their situation, especially those closest to them, the family. They all agree on how hard it is to share. Normally, the people it’s hardest to tell are their mother and their children. When they do, they try to put the most positive spin on it possible, in an attempt to keep them from worrying.
You no longer think only about yourself , you think , “Good grief , now I’ve got to tell my mother .” It’s hard . (Patient 16) I wanted to tell my kids the way I say things , always trying to look for the upside , and positive , although it was hard , but , anyway , in the end , it went well . When I finished , my husband told me , “You’ve convinced me that it’s no big deal .” (Patient 9) I told my son , “Son , don’t cry , your mom’s going to get over this , this is nothing .” (Patient 1)
During this period, the women contemplate how their situation will affect their surroundings and they try to organize it as much as possible.
I devoted myself to planning everything , to organizing what to do with my daughter , and to thinking about work , too , how I had left things at work . (Patient 4)
Surgery/cleaning out the cancer.
Uncertainty and fear . The participants express that before going into surgery, they are told about the kind of procedure that will be done, but that, depending on what they find and the analysis, it may change. In light of this, they exhibit an enormous feeling of uncertainty and fear. In addition, many voice concern about how the surgery will go.
They tell you conservative surgery , but if we open up and see something we didn’t see on the tests , then everything could change . (Patient 10) Aside from the anesthesia , that I’m terrified of , you spend several hours in surgery and you don’t really know how things will go; when they clean it out , they analyze it , and you go into the operating room and you don’t know what can happen . (Patient 9)
Feeling of loss . Considering that the breast is associated with an intimate, feminine part [of their body], many women experience the operation as a loss. This loss is more acute if the operation is a mastectomy and there is no reconstruction at the same time. The loss also involves a loss of identity, compounded by the side effects of chemotherapy, such as hair loss. The interviewees who had undergone mastectomy say that following surgery, when the bandaging is removed and the scar is revealed, is one of the most critical moments, which is why they express difficulty in managing it and appreciate the caring assistance from the professionals.
It is identification with yourself , you know , it’s what you’ve seen in the mirror , what you think you’re like and , suddenly , you’re no longer like that; there’s an incredible personal crisis because you no longer recognize what you’re seeing . (Patient 11) I closed my eyes and I removed the bandaging and I didn’t dare look … with my eyes , I imagined the worst . (Patient 12)
Acceptance or demand for more aggressive intervention . The patients perceive the surgery as essential to recovering their health, which is why the process is widely accepted. Some patients who demand a more invasive intervention, normally a bilateral mastectomy, do so because that way, they feel safer with respect to a possible relapse, as well as more comfortable esthetically.
If they have to remove my breast , let them take it; what I want is to get better . (Patient 16) They say that I am in full remission , so they only removed the lump , but at first , I said that I wanted my whole [breast] removed ; then they assessed how to do it . (Patient 13) They told me that I had a genetic mutation and more possibilities of developing breast cancer and , since I felt such rejection toward my remaining breast , I decided to get rid of that one , too . (Patient 20)
Chemotherapy/loss of identity.
The chemotherapy phase is one of the phases that affects the women’s lives the most, because of its physical impact and how long it lasts. No differences have been found in how they experience chemotherapy depending on whether it was neoadjuvant or adjuvant.
Negative impact of side effects . Chemotherapy is associated with many side effects that vary from one woman to another. Many indicate that they have suffered physical discomfort, such as fatigue, dysgeusia, pain, nausea and vomiting, mucositis, diarrhea, etc.
One day when I didn’t want to go to bed , I went to bed crying because I had the feeling that I wasn’t going to wake up . That day it was because I felt awful . (Patient 1)
Furthermore, all of the women suffer hair loss, which is one of the most-feared effects. Likewise, their body hair also falls out, especially on their face, and their weight fluctuates. All of these changes lead to a loss of identity that is experienced as taking away from their femininity. It must be remembered that oftentimes, chemotherapy is administered after surgery, further exacerbating this physical change. On top of all that, several women comment having to decide at the beginning of treatment whether to freeze their eggs or not; at that moment, many of them forfeit the possibility of becoming a mother or of becoming a mother again, which also adds to this loss of femininity.
Losing my hair was hard , but when it grew out again , I had an identity crisis . I didn’t recognize myself; people said I was really pretty like that , with my hair so short . I looked at myself in the mirror and I said that I’m not that woman , I can see that that woman is pretty , but it’s just that I don’t recognize myself . That’s not me or , it was like , I looked at myself and I didn’t recognize myself . That’s when I suffered a serious identity crisis , psychologically serious , but also serious because I sobbed because I looked at myself , but it wasn’t me . (Patient 6) Where’s that sexy lady , where is she ?, because you don’t feel good . I didn’t like myself at all . I was several sizes larger and I looked at myself and said , “What a monster . ” I didn’t feel good about myself . (Patient 1)
Many patients say that chemotherapy decreases their libido and dries up their mucous membranes, which is why they prefer not to have sex. For those who live as a couple, this situation can strain the relationship.
Sexually , I just didn’t feel like it , I wasn’t in the mood; not only did I not feel like it , my mucous membranes were dry and , what’s smore , I just couldn’t , I couldn’t , I felt bad for my husband , but he said , “Don’t worry .” (Patient 16)
Finally, some interviewees expressed a feeling of being poisoned by the treatment. These women tend to be highly focused on taking care of their body and have a very natural attitude toward life.
I had to really work my awareness that I was poisoning myself; at night I was at home and I thought that all that red liquid was circulating through my veins … I think I even had nightmares . (Patient 4)
Balance between caring for oneself and caring for others . The patients feel that it is time to take care of themselves, so they prioritize resting when they need it. Moreover, they worry about getting a haircut and, most of the times, they look for turbans and wigs. Some also learn how to put on make-up, which they rate as being very positive. On the other hand, those who have children or another person in their care, try to take care of them as much as they are able.
Around 11 : 00 , I no longer felt good , so I’d go to the armchair to rest and it’s like I had an angel , because I’d wake up a minute before I had to set the table and get lunch for my son who would be coming home from school . (Patient 1) While I was getting chemo , I went with the gadget and I told myself , “I’m going to teach you to apply make-up; for instance , your eyelashes are going to fall out . Make a line like this ” and at that moment when you look in the mirror , and we look like Fester in the Addams family . (Patient 13)
Vulnerability . The women experience great uncertainty and feelings of vulnerability the first times they receive chemotherapy, since they don’t know what side effects they will suffer.
With chemo , I started with a lot of fear and , later on , I became familiar with it little by little until the time comes when you go to the hospital like someone who’s going to pick up a bit of paper . (Patient 9)
In addition, those participants who join a social network or who are more closely tied to the hospital setting, know about the relapses and deaths of people around them diagnosed with breast cancer, which makes them feel highly vulnerable.
There are some people who leave the group because … it’s not like there are a lot of relapses and , geez , I think that it messes with your head . (Patient 13) We were almost always the same people at chemotherapy ; there was one guy who was really yellow who looked terrible and , there was one time when we stopped seeing him and another lady asked and the nurse said that he had died . (Patient 15)
At the same time, given the physical changes, especially those that have to do with body hair, many women feel observed when they leave home.
If I have to go out and take off my scarf because I’m hot or go straight out without any scarf on my head and whoever wants to look… let them ; I think that it’s up to us , the patients , to normalize the situation; unfortunately , there are more and more cases . (Patient 9)
Telling the kids . Since when the chemotherapy stage is going to entail many physical changes, the women look for ways to talk to their children about the treatment. Most of them comment that it is a complicated situation and all of them try to talk to their children in such a way as to protect them as much as possible.
I asked the nurse for help before I started chemotherapy to see if she had any pointers about how to talk about this with the kids and she recommended a story , but when I saw it , I didn’t like it … so , in the end , I decided to do it off the cuff . (Patient 10)
Radiotherapy/transition to normality.
The “last” treatment . When the patient reaches radiotherapy, normally, they have already spent several months undergoing physically aggressive medical procedures, which is why they feel exhausted. There is a physical exhaustion resulting from the previous treatments and made worse by the radiation therapy. Furthermore, many women also report feeling emotionally drained by the entire process. However, this is generally accompanied by joy and relief because they feel that they are in the final stage of treatment.
Emotionally , it’s a marathon that has to end up at some point . (Patient 10) For me , radiotherapy was like a lull in the battle , with a winning mind-set . (Patient 4)
Comparison with chemotherapy . There is a widespread perception that radiotherapy has fewer side effects than chemotherapy, although later, when they receive it, several patients suffer discomfort, above all fatigue and dizziness. Several report that at this point, they are mentally worn out and just want to be done with the process, which is why they have less information than about chemotherapy.
I feel like radiotherapy is unknown , that you think it’s more “light ” and it turns out not to be so light . (Patient 13)
Follow-up care/the “new” day-to-day.
Difficulty in getting back to normal . Once the patients are discharged, many feel that they need some time to recover, that it will be slow, in order to restore a more normalized pace of life. They are still working on their emotional and personal process.
When they tell you that you have cancer , they make it very clear : you have a goal; you have some months of chemo , some months of radio , and when you finish , you say , “And now , what do I do ?”. I say that because now I have to get back to my normal life , but I don’t feel normal . I still don’t feel cured , I’m not 100% . And you’re glad you’ve that you’ve finished it all and you’re alive , but at the same time , you say , “Gosh… this is very odd . ” It was a very strange feeling . (Patient 8)
Most patients report that their quality-of-life has diminished, due to the sequelae from the treatments. Lymphedema is one of the sequelae they name most often, although they also mention other symptoms, like digestive upset, weight issues, eye problems, scar pain, etc. The women who are on hormone therapy also suffer side effects, such as joint and muscle pain.
I have lymphedema and , although I have good mobility , I’m a little bit weak; when I go out for dinner , I generally order fish , because I can’t always cut meat well . (Patient 6)
Several interviewees also express difficulty in their affective-sexual relations. Many of them feel insecure because of all the physical changes; others have sequelae that hinder their relations, and still others are suffering symptoms of early menopause. This can cause problems in the couple and for those who don’t have a partner, suffer many complications when it comes to meeting other people.
I haven’t had sex with my husband for 2 years because , it’s also really complicated to get over; I’ve gone for pelvic physical therapy; I’ve used gels , but nothing works . (Patient 8) It’s taken me many months for me to have a relationship again; it’s been really hard because , even though everyone told me that I looked fine , I didn’t feel fine . My breast cancer had taken away all my attributes as a woman . (Patient 6)
Some women also experience difficulties when it comes to returning to work. Several state that they had been fired when they went back. They also report that when interviewing for a job, it’s complicated for them because they have to explain what happened and they mention the schedule of doctor’s visits that they have. Other women comment that they’ve been given early retirement or disability.
You go to the interview and if you tell them that you’ve had the disease , they look at you like you’re a weirdo . (Patient 13)
Breast reconstruction . How reconstruction is experienced, as well as its timing, are highly contingent upon they type of reconstruction. Each one has its pros and cons, but the opinions collected with respect to the type of reconstruction have been positive.
Although it took 18 months for the entire process to be over , I’m delighted with reconstruction with the expander . (Patient 16)
Some patients state that after the whole process, which has been long and complicated, they prefer not to undergo reconstruction immediately. In these cases, they report having felt a subtle pressure from the outside to undergo reconstruction.
Every time I went for my check-ups , they said , “You’re the only one left [who hasn’t undergone reconstruction]” and in the end , the truth is that I’m really happy because I think I look pretty . (Patient 12)
Check-ups and fear of relapse . Check-ups are one of the times that generate most worry and insecurity. The women remark that, starting a few days before and until they receive the results of the follow-up studies, they are more anxious about the possibility of relapse.
At every check-up my legs start shaking again and my stomach is in knots, although at my last one, everything turned out okay and I’m thrilled. (Patient 6) During the first stage , I did everything I had to do and I got over it , but it’s a lottery . You can do whatever you want , but it’s the luck of the draw and when you start going for check-ups , it’s like going to play Russian roulette . (Patient 8)
Maintenance hormone therapy . Hormone therapy is understood differently depending on age and on the major decision of whether or not to be a mother or to have another child. If the woman does not want to have more children, the treatment is accepted better. The patients who take it also report effects derived from menopause, for instance, joint pain or dry mucous membranes.
I did notice joint pain , but since I exercised , [I felt it] much less than my fellow women , although , for instance , when it comes to getting up from a chair , you get up like an old lady . (Patient 10)
Position of support . Several patients mention that, after discharge, they stay active on social media, they volunteer when they find out about someone or to participate in activities related to breast cancer, with the aim of being able to help other people who are in this situation.
It’s really good to meet other people who are going through the same thing , so , now that I’ve finished , I like it and I always help whenever I can , because I can share what was good for me . (Patient 13)
Relapse/starting over.
Emotional impact . The diagnosis of a relapse is experienced much the same as the initial diagnosis. All of the women report fear, although they also state that they are more familiar with the processes. Other emotions emerge, such as why me, blame, disbelief, etc.
Since they had told me that it wasn’t going to happen again , I believed it , of course , I wanted to believe it and it totally surprised me; I couldn’t stop crying and crying . (Patient 17)
Telling the family again . Patients repeat that telling the family about it again, especially the children and parents, is tough and they try to minimize it in an attempt to protect them emotionally.
On the very same day that I had my mammogram , my mother says that she wants to come a see the kids . We’re in the park , when she arrives , I have to tell her that everything’s fine and when we get home , I tell her everything . My mother’s devastated again and I tell her not to worry , that everything is going to be fine . (Patient 16)
Thinking about whether something could have been done differently . Several women comment that, after their relapse, they think about whether the treatment was enough or there must have been something they could have done to avoid the relapse.
You get furious , because you say , “I wasn’t supposed to get sick , because if , 2 years ago when the first microcalcifications appeared I had had them removed , then I wouldn’t have metastasis , or maybe I would . (Patient 19)
Metastatic breast cancer/time-limited chronic.
Re-interpreting the concept of metastasis . Most of the participants in this stage state that they have had to give new meaning to the word, “metastasis,” since their first perception was directly related to death. In this way, they come to understand that cancer can become chronic, although they now have to take medication and go to the hospital on a regular basis. Nevertheless, they know that their life expectancy may be a few years. The women who are involved in a group point out how hard it is to see their fellow member pass away.
What I now call my “ new normal” consists of lots of visits to the hospital and never going back to work . (Patient18)
They also state that at this stage, they do not identify with the disease generally known socially as “breast cancer”, where there is great emphasis placed on early detection and on their chances of being cured. This causes them to feel more isolated.
These pink ribbon campaigns hurt us because they tend to underscore that everything is going to turn out fine because breast cancer has a very high cure rate; there is huge lack of awareness . (Patient 20)
Physical and emotional discomfort . Most of the women in this stage report side effects from the treatments, although some comment that good quality-of-life can be preserved. On an emotional level, they say that they sometimes feel a certain agony due to not knowing how much longer the treatment will be effective. They live in a state of uncertainty that they try to cope with by focusing on their day-to-day and experience the good times deeply.
When I’m not in pain , I try not to even remember what I have and go out and have fun with my family and live . (Patient 20)
Several women who have children express with regret that they worry about their children enjoying them and remembering them when they were well. They are sad that they won’t be able to grow up in a normal family. Some also comment the impact this diagnosis is having on their partner.
What I don’t want is for them to carry this baggage of having a sick mother . (Patient 18)
A conflict with disability also appears, as many women report their desire to continue working, but feel that they can’t keep up with the pace of work. Additionally, several state that going through the medical board is a strenuous process, given that they look good physically.
It’s hard to deal with , I’m a non-practicing lawyer and I have degrees galore , but I worked the first year and I couldn’t continue . (Patient 21) Every year they call me again for the disability monitoring and they always threaten me . To be honest , the treatment doesn’t make me sick , but I don’t know how long it’s going to be like this . (Patient 19)
Social invisibility . The participants say that they do not have any physical signs of being ill, that they look fine, although they know and feel that inside, they are not well. They say that it is sometimes hard to manage socially, since on occasion, they feel misunderstood and disparaged.
I’m much sicker now , but people think or want to think that I’m fine . When I was doing chemo , it was like wearing a sign that said “cancer . ” (Patient 17)
This study describes the patient journey of women with breast cancer, specifying the different phases with the most relevant aspects of each, as well as the different cross-sectional features they report throughout the entire treatment process.
The results portray breast cancer as a process in which there is a striking feeling of loss of health and self-identity, changes in routines, personal and employment transformation, as well as emotional hardship during and after breast cancer treatment, aspects that are also reported in the literature [ 41 , 42 ]. Earlier studies state that experiencing cancer is highly stressful. It involves a major threat to life or physical integrity, in addition to mental health, interfering with the path, projects, and plans patients have for their life over the short, medium, and, on occasion, long term as well [ 6 ]. Along with reporting adverse physical and psychological impacts, patients also report positive ways in which they have grown psychologically or emotionally from the experience [ 7 , 42 ]. The diagnosis of breast cancer not only impacts the women individually, but also affects their surroundings. As reported in the literature, despite going through a very challenging time, the women struggle to put on a positive face and attempt to conserve the family’s well-being, specifically that of their children [ 7 ]. At the same time, the family is a fundamental source of support and usually provide indispensable support; however, it is not always effective, because family members do not fully understand the stresses involved in living with cancer [ 43 ]. Previous studies also reveal that for some women, their partners are one of their most significant supports; nonetheless, research also suggests that a cancer diagnosis predicts marital breakup more strongly for female survivors than males [ 44 ]. Our results reflect that the women frequently resort to other women in the same situation, possibly because they face significant unmet supportive care needs [ 30 ]. The need for social support may lead patients to seek social support groups consisting of people who are experiencing similar health crises, because such groups allow them to interact with those who best understand their suffering [ 43 ]. Another aspect that appears across the board is the relationship the participants have with the medical team. In this study, we have noted their trust in the medical team and acceptance of the treatments proposed without going into the clinical data of the disease and without needing to know the benefit provided by the treatment. Cancer patients are confronted with a potentially life-threatening [condition], feeling vulnerable, and need to rely heavily on their care providers, expecting the physician to act in their best interests [ 5 ]. Therefore, they need to have a close relationship, as well as comprehensive care [ 30 ]. Patients’ trust in a physician has been associated with a reduction of their fears and anxiety and [increased] satisfaction and adherence to treatment [ 5 , 30 ]. We believe that it would be important to provide patients with accurate information, so as to avoid misunderstandings (such as cancer being synonymous with death, regardless of stage) as several participants in this study have reported, which can lead them to believe that the risk of relapse with and without chemotherapy is much greater than the oncologists estimate [ 45 ]. We believe that in future studies that it would be worthwhile to examine the peculiarities of each kind of patient information with the aim of determining how to break it up and make it both comprehensible and tolerable to promote patients’ well-being.
A breast cancer diagnosis is generally unexpected and practically all patients suffer psychological distress, such as feelings of uncertainty, disbelief, hopelessness, vulnerability, anger, fear, anxiety, and sadness [ 46 , 47 ]. The literature has reported that many women experience peritraumatic distress or dissociation during the medical conversation in which they are given their diagnosis of cancer [ 48 ], which might account for the reactions of the respondents. Given that, when they receive their diagnosis, additional information is generally given to them, such as clinical aspects and preferred treatments. Repeating this information at subsequent appointments could contribute to improving communication with patient, since several participants stated that they found it hard to pay attention to the physician, given the emotional impact. Additionally, breast cancer patients tend to be diagnosed when they are relatively young, and often when they are in the middle furthering their career or raising children [ 12 ]. In spite of everything, the women try to put on as brave a face as they can and focus on maintaining their children’s well-being [ 7 ]. Telling children about their diagnosis is reportedly one of the biggest challenges; parents are usually unsure of how to tell them, because at the same time that they want it to be open and honest and cover their children’s developmental needs, they also want to protect them children [ 49 ].
Once diagnosed, breast cancer patients go through different treatments. The most salient experiences of these phases pertain to the impact of side effects on physical quality-of-life and psychological well-being, which is consistent with the literature [ 11 ]. Moreover, cancer therapy entails physical changes that affect their feminine identity, fertility, self-esteem, sexual functioning, and makes them more vulnerable [ 10 , 50 ]. Women described their inner self as being on an emotional rollercoaster with highs and lows throughout the various phases of treatment [ 7 ]. Given treatment side effects and sequelae, these women are more likely to experience physical symptoms and psychological disorders than patients with other kinds of tumors [ 51 ]. The side effects involve an acute sense of loss of health and quality-of-life, as well as identity and femininity. It would be interesting for future research to explore the therapies used in grief counseling with cancer patients, as understanding and exploring this perspective could comprise an additional clinical aid.
Once the women have completed their treatments, they gradually get back to normal and many contemplate returning to work. However, in line with our results, the literature reveals that even though they want to normalize their lives, female breast cancer survivors feel that they will never return to their baseline status [ 7 ]. A significant number of patients experience difficulties in physical, cognitive, and emotional functioning after their treatment, such as symptoms like lymphedema, fatigue, pain, sleep disorders, cancer-related cognitive impairment, emotional stress, symptoms of depression and anxiety, problems with relationships, reduced sexual identity, fertility problems, and fear of cancer relapse [ 13 , 14 ]. Furthermore, patients with hormone therapy suffer hot flashes, sweats, joint pain, weight gain, decreased libido, and low energy [ 52 ]. A sizeable number of these women also experience changes in employment status which can happen even 5–10 years following diagnosis [ 15 ]. Given that all these changes alter the structure of the woman’s everyday life, personalized care and treatment plans in cancer survivors are highlighted in the literature with extended specialized support being proposed that enables them to make a better psychosocially adjusted transition from treatment to follow-up [ 53 ] and advocating for the patient’s participation in all decisions that affect her during this period [ 54 ]. Further research is needed concerning how to structure the follow-up and support offered to these women during this stage so as to meet their needs and help them adjust to their new reality with the chronic sequelae caused by cancer and its treatment. On the other hand, the personal transformation of the initial stages of the journey are best seen during this phase. The literature shows that women who have had breast cancer report changes in their philosophy of life, such as embarking on a new life path, changing their priorities in life, as well as valuing life in general [ 42 ]. Most of the participants in our study place special emphasis on appreciating life, enjoying it more, and living each day to the fullest. Cancer survivors report being aware of how precarious life is, while also feeling the joy of being alive [ 55 ]. Similarly, they have been found to be more resilient and better able to repair their mood than healthy women [ 56 ].
About 5% of all patients with breast cancer are diagnosed when the disease is metastatic, whereas some 30% have suffered a relapse of an early breast cancer [ 17 ]. We saw that some women suffering a relapse after initial treatment with curative intent tend to wonder if the treatment was sufficient or if they should have done something more to prevent the relapse. Metastatic breast cancer is uncurable, which is why these women’s main psychosocial challenges are not the same as those who are diagnosed in early stages [ 18 ]. Faced with incurability, the women react with shock and fear of imminent death, but this anxiety diminishes once they begin treatment and learn that there are more treatment options [ 17 ]. During this phase, the interviewees reported impaired physical QoL and functioning, being hindered by pain, fatigue, or menopausal symptoms. Emotionally, they report suffering bouts of depression and anxiety, as well as fear because of the spread of their cancer. As for their relational QoL, their children’s welfare is their number one concern, especially for mothers of young children [ 17 , 57 ]. What’s more, these women felt isolated from society in general and, more specifically, from the non-advanced breast cancer community, inasmuch as they feel that nobody understands what they are going through [ 18 ]. A psychosocial approach is especially important in this phase to help these women to continuously adapt to the changes of their individual clinical situation and to the progression of the disease, thereby improving their coping.
Clinical implications
Having first-person information enables us to comprehend in detail the experiences of breast cancer patients, their situation, and emotional state, which favors holistic cancer care for health professionals.
Healthcare professionals should prepare women for a changed life situation, as well as to face prolonged, multimodal treatment (surgery, chemotherapy, hormone therapy, radiotherapy), and to confront physical and psychological sequelae, as well as the fear surrounding an uncertain prognosis. It is important to help them manage their expectations and fears and, to identify and address the issues and concerns that arise at different time points during treatment. The information and support offered should be adjusted to each woman’s individual needs, her life situation, her coping style, and the time and stage of their cancer. This more empathic, understanding outlook can also contribute to improving the physician-patient rapport, promoting communication, understanding, and shared decision-making.
Finally, a comprehensive understanding of the women’s psychosocial support endorses their belonging to groups of women with breast cancer, in which there is a relationship among equals. Further research is needed to specify the type needed so as to decrease both the impact of the death of women in the group, as well as the vast amount of information that they may end up obtaining, without needing it or requesting it.
Limitations
This study was performed with Spanish participants, which is why certain aspects cannot reflect the experiences of breast cancer patients from other countries, given the particularities of both the Spanish healthcare system and Spanish culture. Likewise, the data attained were specific to women with breast cancer, which can scarcely be extrapolated to individuals with other cancers. Moreover, the findings do not reflect men’s experiences with breast cancer and research with this group would enrich the field further. In addition, the age of our participants ranged from 34 to 61 years; hence the results should be interpreted for a middle-aged population and do not reflect the experiences of women diagnosed at very early or very old ages. Finally, we believe that there may be a bias regarding the women who agree to participate, as this group has probably accepted their condition more, as well as having worked on it more.
Despite these limitations, we hope that our findings can contribute to better understanding the experiences of women with breast cancer.
Acknowledgments
The authors are grateful to the investigators of the Neoetic study and the Bioetic Group of the Spanish Society of Medical Oncology (SEOM) for their contribution to this study. We would like to thank all the women who generously shared their experiences with us, the support of HealthyOnco ( www.healthyonco.com ), and Priscilla Chase Duran for editing and translating the manuscript.
- View Article
- Google Scholar
- PubMed/NCBI
Nursing Case Study for Breast Cancer
Watch More! Unlock the full videos with a FREE trial
Included In This Lesson
Study tools.
Access More! View the full outline and transcript with a FREE trial
Natasha is a 32-year-old female African American patient arriving at the surgery oncology unit status post left breast mastectomy and lymph node excision. She arrives from the post-anesthesia unit (PACU) via hospital bed with her spouse, Angelica, at the bedside. They explain that a self-exam revealed a lump, and, after mammography and biopsy, this surgery was the next step in cancer treatment, and they have an oncologist they trust. Natasha says, “I wonder how I will look later since I want reconstruction.”
What assessments and initial check-in activities should the nurse perform for this post-operative patient?
- Airway patency, respiratory rate (RR), peripheral oxygen saturation (SpO2), heart rate (HR), blood pressure (BP), mental status, temperature, and the presence of pain, nausea, or vomiting are assessed upon arrival. Medication allergies, social questioning (i.e. living situation, religious affiliation), as well as education preference are also vital. An admission assessment MUST include an examination of the post-op dressing and any drains in place. This should be documented accordingly.
- The hand-off should be thorough and may be standardized. Some institutions have implemented a formal checklist to provide a structure for the intrahospital transfer of surgical patients. Such instruments help to standardize processes thereby ensuring that clinicians have critical information when patient care is transferred to a new team. The nurse should also prepare to provide education based on surgeon AND oncologist guidance
What orders does the nurse expect to see in the chart?
- Post-op medications, dressing change and/or drain management, strict I&O, no BP/stick on the operative side (rationale is to help prevent lymphedema – Blood pressure (BP) measurement with a cuff on the ipsilateral arm has been posed as a risk factor for the development of LE after-breast cancer therapy for years, regardless of the amount of lymph node excision.)
- Parameters for calling the surgeon are also important. The nurse should also check for an oncology service consult.
After screening and assessing the patient, the nurse finds she is AAOx4 (awake, alert and oriented to date, place, person and situation). The PACU staff gave her ice due to dry mouth which she self-administers and tolerates well. She has a 20G IV in her right hand. She states her pain is 2 on a scale of 1-10 with 10 being the highest. Her wife asks when the patient can eat and about visiting hours. Natasha also asks about a bedside commode for urination and why she does not have a “pain medicine button”. Another call light goes off and the nurse’s clinical communicator (unit issued cell phone) rings.
The nurse heard in report about a Jackson-Pratt drain but there are no dressing change instructions, so she does not further assess the post-op dressing situation in order deal with everything going on at the moment. She then sits down to document this patient.
Medications ordered in electronic health record but not yet administered by PACU: Tramadol 50 mg q 6 hrs. Prn for mild to moderate pain. Oxycodone 5 mg PO q 4 hrs. Prn for moderate to severe pain (5-7 on 1-10 scale) Fentanyl 25 mcg IV q3hrs. Prn For breakthrough pain (no relieve from PO meds or greater than 8 on 1-10 scale) Lactated Ringers 125 mL/hr IV infusion, continuous x 2 liters Naloxone 0.4-2 mg IV/IM/SC; may repeat q2-3min PRN respiratory rate less than 6 bpm; not to exceed 10 mg
BP 110/70 SpO2 98% on Room Air HR 68bpm and regular Ht 157 cm RR 14 bpm Wt 53 kg Temp 36.°5C EBL 130mL CBC -WNL BMP Potassium – 5.4 mEq/L
What education should be conducted regarding post-op medications?
- New post-op pain guidelines rely less on patient-controlled analgesia (aka “pain medicine button”) than in previous years. Most facilities will have an approved standing protocol (i.e., “Multimodal analgesia and Opioid Prescribing recommendation” guideline) or standing orders. The patient must be instructed on how to rate pain using facility-approved tools (aka “pain scales”). She should also report any medication-related side effects and reinforce there is a reversal medication in case of an opioid overdose.
What are some medical and/or non-medical concerns the nurse may have at this point? If there are any, should they be brought up to the surgeon?
- The nurse may request an anti-emetic such as ondansetron 4 mg IV q 6 hrs prn nausea vomiting (N&V) since it is not uncommon post-op for the patient to have N&V. The rate of LR is a little high for such a small patient and could cause electrolyte imbalances. The nurse may also inquire about the oncologist being on the case and ask if the surgeon has discussed reconstruction with the patient yet. She may also want to ask about dressing change orders.
Natasha sleeps through the night with no complaints of pain. Lab comes to draw the ordered labs and the CNA takes vital signs. See below.
CBC HGB 7.2 g/dl HCT 21.6%
BMP Sodium 130 mEq/L Potassium 6.0 mEq/L BUN 5 mg/dL
BP 84/46 SpO2 91% on Room Air HR 109 RR 22 bpm
What should the nurse do FIRST? Is the nurse concerned about the AM labs? AM vital signs? Why or why not?
- Check the dressing and drain for bleeding (assess the patient). The patient should also sit up and allow staff to check the bed for signs of bleeding. Reinforce the dressing as needed. Record output from the drain (or review documentation of all the night’s drain output). Labs and vital signs indicate she may be losing blood.
Check the dressing and drain for BLEEDING (assess the patient). The patient should also sit up and allow staff to check the bed for signs of bleeding. Reinforce the dressing as needed. Record output from the drain (or review documentation of all the night’s drain output). Labs and vital signs indicate she may be losing blood.
What orders does the nurse anticipate from the surgeon?
- The nurse should expect an order to transfuse blood for this patient. Also, dressing reinforcement or change instructions are needed in the case of saturation)

How should the nurse address Natasha’s declaration? What alerts the nurse to a possible complication?
- First, the complication is that “Kingdom Hall” is the site of worship for Jehovah’s Witnesses. They do not accept ANY blood product, not even in emergencies. It is vital the nurse determines the patient’s affiliation and religious exceptions for medical care before moving forward. Next, employ therapeutic communication to elicit more details about Natasha’s concerns. Say things like, “tell me why you think you’re not attractive?” She may discuss reconstruction options or ask the patient to write down specific questions about this option to ask the provider later. Ask about getting family in to provide support. Seek information to give the patient about support groups and other resources available (as appropriate, ie. prosthetics, special undergarments/accessories, etc)
The surgeon orders 1 unit packed red blood cells to be infused. The nurse then goes to the patient to ask about religious affiliation and to discuss the doctor’s order. After verifying that Natasha is not a practicing Jehovah’s Witness, the nurse proceeds to prepare the transfusion.
What is required to administer blood or blood products?
- First, the patient’s CONSENT is required to give blood products. The nurse must also prepare to stay with the patient for at least the first 15 minutes of the transfusion taking a baseline set of V/S prior to infusion. Then, V/S per protocol (frequent). Education is also required. The patient should report feeling flushed, back or flank pain, shortness of breath, chest pain, chills, itching, hives. Normal saline ONLY for infusion setup and flushing: size IV 20g or higher. Always defer infusion time limits to “per policy” because this can differ vastly
How should the nurse respond to this question?
- Planning for post-op cancer treatment should have begun prior to the surgery. Ask the patient if she has discussed plans with her oncologist. Refer to any specialist documentation to see if this is mentioned. Remind the patient of the specialist’s assessment and planning information. Reinforce that testing of the tissue may change the course of treatment as well. Provide education AS PER THE PATIENT’S STATED PREFERENCE and/or resources based on what the plan includes (ie. chemotherapy, radiation, further surgery. Continually assess and reassess patient understanding. Include family and/or support with the patient’s approval.
View the FULL Outline
When you start a FREE trial you gain access to the full outline as well as:
- SIMCLEX (NCLEX Simulator)
- 6,500+ Practice NCLEX Questions
- 2,000+ HD Videos
- 300+ Nursing Cheatsheets
“Would suggest to all nursing students . . . Guaranteed to ease the stress!”
References:
View the full transcript, nursing case studies.

This nursing case study course is designed to help nursing students build critical thinking. Each case study was written by experienced nurses with first hand knowledge of the “real-world” disease process. To help you increase your nursing clinical judgement (critical thinking), each unfolding nursing case study includes answers laid out by Blooms Taxonomy to help you see that you are progressing to clinical analysis.We encourage you to read the case study and really through the “critical thinking checks” as this is where the real learning occurs. If you get tripped up by a specific question, no worries, just dig into an associated lesson on the topic and reinforce your understanding. In the end, that is what nursing case studies are all about – growing in your clinical judgement.
Nursing Case Studies Introduction
Cardiac nursing case studies.
- 6 Questions
- 7 Questions
- 5 Questions
- 4 Questions
GI/GU Nursing Case Studies
- 2 Questions
- 8 Questions
Obstetrics Nursing Case Studies
Respiratory nursing case studies.
- 10 Questions
Pediatrics Nursing Case Studies
- 3 Questions
- 12 Questions
Neuro Nursing Case Studies
Mental health nursing case studies.
- 9 Questions
Metabolic/Endocrine Nursing Case Studies
Other nursing case studies.
- Case Report
- Open access
- Published: 06 January 2021
Spontaneous regression of breast cancer with immune response: a case report
- Masahiro Ohara 1 ,
- Yumiko Koi 1 , 4 ,
- Tatsunari Sasada 1 ,
- Keiko Kajitani 1 ,
- Seishi Mizuno 2 ,
- Ai Takata 2 ,
- Atsuko Okamoto 2 ,
- Ikuko Nagata 2 ,
- Mie Sumita 2 ,
- Kaita Imachi 2 ,
- Mayumi Watanabe 2 ,
- Yutaka Daimaru 2 &
- Shingo Kawamura 3
Surgical Case Reports volume 7 , Article number: 10 ( 2021 ) Cite this article
6562 Accesses
3 Citations
1 Altmetric
Metrics details
Spontaneous regression (SR) is a rare phenomenon in which a cancer disappears or remits without treatment. We report a case of breast cancer that showed spontaneous tumor regression in the surgical specimen after core needle biopsy.
Case presentation
A 59-year-old woman came to our hospital complaining of a painful lump in the right breast. In the upper-outer quadrant of the right breast, a tumor with an unclear boundary, 30 mm in diameter, was palpable. In pathological findings from needle biopsy, the tumor was diagnosed as solid-type invasive ductal breast carcinoma. Partial coagulation necrosis was generated in estrogen receptor-negative, HER2-negative, and AE1/AE3-positive ductal carcinoma without infiltration of lymphocytes. Surgery for right breast cancer was then performed. Histological examination of the surgical specimen revealed the tumor was invasive ductal carcinoma with lymphocyte infiltration, coagulation necrosis, and fibrous tissue with hemosiderin. The tumor formed a solid nest, 3 mm in diameter, suggesting the possibility of SR.
Conclusions
Immune responses, infection, hormones, surgical stress, and ischemia have been reported as mechanisms of SR. The findings in this case strongly suggest that SR of breast cancer is associated with anti-tumor immune responses.
Spontaneous regression (SR) of cancer is a rare but well-documented biological phenomenon. SR is defined as “the partial or complete disappearance of a tumor in the absence of any treatment capable of regression” [ 1 , 2 ]. Breast cancer regression was reported in 43/741 cases of spontaneously regressing cancers compiled and summarized by Challis and Stam in a review of the period from 1900 to 1987 [ 3 ], and few additional reports have been published since then [ 4 , 5 , 6 , 7 , 8 , 9 ]. Various mechanisms are considered to be associated with this phenomenon, including immune mediation, tumor inhibition by growth factors and/or cytokines, induction of differentiation, hormonal mediation, and tumor necrosis.
Spontaneously induced T-cell-mediated immunological responses have recently gained attention in multidisciplinary cancer treatment, since more than 30% of durable clinical responses including complete response are observed just with administration of antibody to block the PD-1/PD-L1 inhibitory immunological checkpoint signal in various cancer patients [ 10 , 11 ]. Spontaneously induced immunological responses could thus also be an important mechanism in the SR of cancer.
We report herein a case of SR of breast cancer with induced immune responses. Immunohistochemically, we confirmed that partial coagulation necrosis was generated in estrogen receptor-negative, HER2-negative, and AE1/AE3-positive ductal carcinoma without infiltration of lymphocytes on preoperative pathological findings. Postoperative histopathological findings consequently showed that most tumor cells had been replaced by granulation tissue and residual ductal carcinoma had been driven into a smaller area by the infiltration of lymphocytes, suggesting that the SR of this breast cancer could be due to anti-tumor immune responses induced by unexplained inflammation.
A 59-year-old woman came to our hospital with a chief complaint of a painful lump in the right breast. She regularly visited her primary doctor for type 2 diabetes, hypertension, and hyperlipidemia. She was treated with metformin, olmesartan medoxomil/ azelnidipine, and pravastatin for less than 5 years. She had no family history associated with breast cancer. Reviewing her past history, she had received total hysterectomy at age of 47 for a uterine leiomyoma. A tumor with an unclear boundary was palpable in the upper-outer region of the right breast, about 30 mm in diameter along the major axis. Mammography revealed a mass with a clear boundary, 19 × 18 mm in size, in the middle outer portion of the right breast (Fig. 1 a, b). Ultrasonography revealed a smooth, round mass measuring 20 × 18 × 18 mm in size, in the upper-outer quadrant of the right breast. Subcutaneous fat tissue around the tumor appeared as a highly echogenic, edematous region (Fig. 1 c). In pathological findings from needle biopsy, the tumor was diagnosed as solid-type invasive ductal breast carcinoma. Partial coagulation necrosis was generated in estrogen receptor-negative, HER2-negative, and AE1/AE3-positive ductal carcinoma without infiltration of lymphocytes (Fig. 2 a–j, 3 a–e). Thirteen days after core needle biopsy, magnetic resonance imaging (MRI) was performed. MRI showed a smooth, round mass measuring 20 × 16 × 15 mm in size with slight hyperintensity on T1-weighted MRI and with high intensity on T2-weighted MRI. Only the marginal region of the tumor was enhanced (Fig. 1 e–g). Ring-type dedicated breast positron emission tomography showed a ring-shaped fluorodeoxyglucose accumulation in the right breast (Fig. 1 h). We performed ultrasonography just before surgery, showing that the tumor remained the same shape as before, although the size had decreased to 12 × 10 × 11 mm and the edema around the tumor had disappeared (Fig. 1 d). Right partial breast resection and sentinel lymph node biopsy was performed 53 days after core needle biopsy. On histological examination of the surgical specimen, the tumor was showed lymphocyte infiltration, coagulation necrosis, and fibrous tissue spread with hemosiderin deposition. The tumor formed a solid nest, 3 mm in diameter, suggesting the possibility of SR (Fig. 4 a–d). A significant aggregation of lymphocytes was observed around tumor cells. These lymphocytes comprised CD3-positive, CD4-positive, or CD8-positive T cells (Fig. 5 a–c) accompanied by aggregations of CD20-positive B-cells (Fig. 5 d), but few CD56-positive natural killer (NK) cells (Fig. 5 e). In addition, residual tumor cells in the surgical specimen did not express PD-L1 (Fig. 5 f).
Preoperative imaging findings. a Mediolateral oblique-view mammogram. b Craniocaudal-view mammogram. A mass with a clear boundary is recognized in the right breast. c Ultrasonogram at first visit. A smooth, round mass is apparent in the upper-outer quadrant of the right breast. Subcutaneous fat tissue around the tumor appears as a highly echogenic, edematous layer. d Ultrasonogram just before surgery. Edema around the tumor has disappeared. e T1-weighted magnetic resonance imaging (MRI). f T2-weighted MRI. g MRI with early gadolinium enhancement A smooth, round mass with slight hyperintensity on T1-weighted MRI and hyperintensity on T2-weighted MRI. Only the marginal region of the tumor appears enhanced. h Ring-type dedicated breast positron emission tomography. A ring-shaped accumulation of 18 F-fluorodeoxyglucose is evident in the right breast
Preoperative pathorological findings. The histopathological findings of the right breast from core needle biopsy ( a , f : hematoxylin and eosin (HE) × 40; b , g : HE × 200). Immunohistochemistry study for AE1/AE3, estrogen receptor (ER), and HER2 ( c , h : AE1/AE3 × 40; d , i : ER × 40; e , j : HER2 × 40). Small foci of atypical ductal cells are recognized (yellow arrow). Partial coagulation necrosis is generated in estrogen receptor-negative, HER2-negative, and AE1/AE3-positive ductal carcinoma without infiltration of lymphocytes
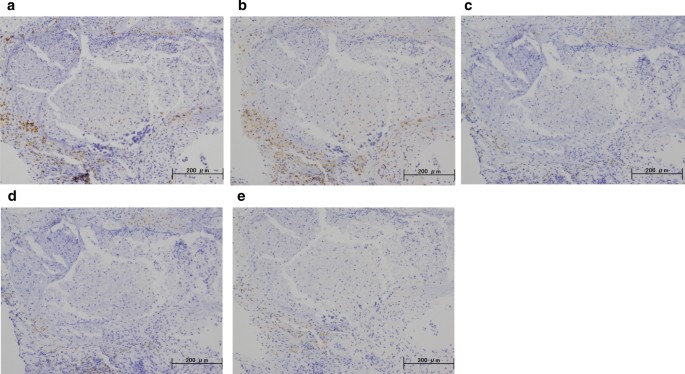
Immunohistochemical staining for core needle biopsy specimens. Immunohistochemistry study for immunological surface markers ( a CD3; b CD4; c CD8; d CD20; e CD56. All original magnifications are × 200). The necrotic area in needle biopsy did not contain immune cells
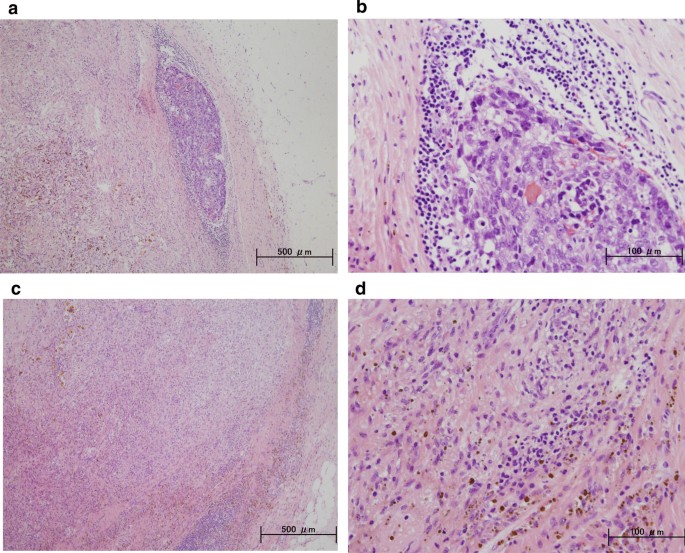
Postoperative histopathological findings. Histopathological findings of the resected breast tissue ( a , c : hematoxylin and eosin (HE) × 40; b , d : HE × 200). The tumor shows lymphocyte infiltration, coagulation necrosis, and fibrous tissue with hemosiderin deposition
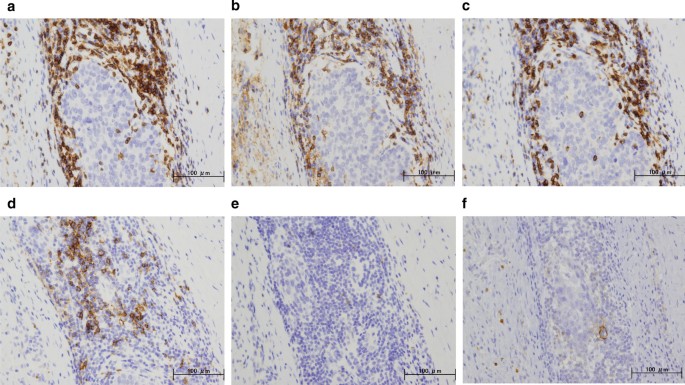
Immunohistochemical staining for the primary breast cancer. Immunohistochemistry study for immunological surface markers ( a CD3; b CD4; c CD8; d CD20; e CD56; f PD-L1. All original magnifications are × 200). Immunohistochemical staining for the primary breast cancer. CD3-positive ( a ), CD4-positive ( b ), and CD8-positive T-cells ( c ), aggregation of CD20-positive B-cells ( d ), and occasional CD56-positive NK cells are detected ( e ). Ductal carcinoma cells are immunonegative for PD-L1 ( f )
Adjuvant radiation therapy (50 Gy in 25 fractions) to the whole breast was performed. The patient is being followed-up closely, with examinations every 3–4 months, and is undergoing regular breast examinations, breast ultrasonography, mammography and tumor marker evaluations (carcinoma antigen 15–3 and carcinoembryonic antigen). After 16 months of follow-up, we have not observed any signs of cancer relapse, and the patient has remained free of the disease.
SR of breast cancer is a rare event that is recognized in the medical literature, but is still an unexpected phenomenon. Due to the rarity of SR, case reports and studies of the reported single cases remain restricted by the lack of sufficient data on a number of biological behaviors and their clinical significance.
Possible mechanisms underlying spontaneous cancer regression include immune system or hormonal mediation, tumor inhibition by growth factors/cytokines, induction of differentiation, elimination of a carcinogen, tumor necrosis, angiogenesis inhibition, psychological factors, apoptosis, and epigenetic mechanisms [ 1 , 2 ]. This phenomenon has been speculated to be possibly related to trauma or infection [ 1 , 4 ]. In the current case, the patient could not remember any traumatic or infectious events involving the site. Furthermore, she did not change her pattern of living and her medication regimen was not changed before surgery. Although the patient took metformin and pravastatin for 5 years, the possibility could not be denied that metformin and pravastatin has played an important role in this regression. Retrospective studies have demonstrated that metformin and statin decreased incidence and recurrence rate of breast cancer potentially [ 12 , 13 , 14 , 15 ]. Pain symptoms at the tumor site and the edema around the tumor on ultrasonography showed the existence of inflammation, irrespective of cause. Preoperative histopathological findings revealed tumor generation and necrosis without infiltration of inflammatory cells at that time.
Immunogenic cell death (ICD), a newly defined form of cell death, may involve recruitment of the host immune system, thereby resulting in immune memory and advantageous systemic effects. ICD of cancer cells can induce effective antitumor immune responses through activation of dendritic cells (DCs) and consequent activation of specific T-cell responses [ 16 , 17 ]. ICD is defined as several steps resulting in the translocation of calreticulin to the cell surface (an “eat-me” signal for DCs) and the release of danger signals such as HMGB1 and ATP, which are essential for the promotion of CD8 T-cell anticancer responses [ 18 ]. ICD can be induced by chemotherapeutic agents such as anthracyclines and oxaliplatin, or radiotherapy and photodynamic therapy, or some physical therapies [ 19 ]. In the present case, surgical specimens showed tumor cells surrounded by abundant lymphocytes, while core needle biopsy specimens had not contained tumor-infiltrating lymphocytes. Unknown ICD-derived anti-tumor immunity was speculated to have caused residual tumor regression.
The roles and subsets of tumor-infiltrating lymphocytes have been discussed in cases involving SR of breast cancer [ 5 , 6 ]. Both CD4- and CD8-positive subsets of CD3-positive T-cells have been implicated in the genesis of SR [ 6 ], although NK cells were suggested in another case [ 5 ]. In the present case, the aggregated cells were mainly CD3-, CD8-, or CD4-positive T-cells, while CD56-positive NK cells were not observed, consistent with a previous report [ 6 ]. A few reports have discussed the triggers generating antitumor immunity during spontaneous tumor regression. The initiating event might be related to the trauma from biopsy, as suggested by Maillet et al. [ 5 ]. To the best of our knowledge, this is the first case to suggest that ICD of tumor cells could induce anti-tumor immunity resulting in SR of breast cancer based on pre- and postoperative pathological findings. Furthermore, the immune response could potentially have continued and the patient might have achieved complete remission without surgery, as the final tumor cells did not express PD-L1 despite the infiltration of abundant lymphocytes.
Our case distinctly indicates that SR of breast cancer is associated with ICD. One limitation of our study was that we could not show concrete reasons and molecular markers for ICD. Recognizing the presence of SR and ICD of breast cancer is important, and a more detailed understanding of the mechanisms underlying SR and ICD would provide significant implications for cancer prevention and therapeutics.
Availability of data and materials
All data analyzed during this study are included within the manuscript. The datasets used and/or analyzed during this study are available from the first author on reasonable request.
Abbreviations
- Spontaneous regression
- Immunogenic cell death
Dendritic cells
Magnetic resonance imaging
Hematoxylin and eosin
Cole WH. Spontaneous regression of cancer and the importance of finding its cause. Natl Cancer Inst Monogr. 1976;44:5–9.
CAS PubMed Google Scholar
Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201–9.
Article CAS Google Scholar
Challis GB, Stam HJ. The spontaneous regression of cancer: a review of cases from 1900 to 1987. Acta Oncol. 1990;29:545–50.
Dussan C, Zubor P, Fernandez M, Yabar A, Szunyogh N, Visnovsky J. Spontaneous regression of a breast carcinoma: a case report. Gynecol Obstet Invest. 2008;65:206–11.
Article Google Scholar
Maiche AG, Jekunen A, Rissanen P, Virkkunen P, Halavaara J, Turunen JP. Sudden tumour regression with enhanced natural killer cell accumulation in a patient with stage IV breast cancer. Eur J Cancer. 1994;30A:1642–6.
Tokunaga E, Okano S, Nakashima Y, Yamashita N, Tanaka K, Akiyoshi S, et al. Spontaneous regression of breast cancer with axillary lymph node metastasis: a case report and review of literature. Int J Clin Exp Pathol. 2014;7:4371–80.
PubMed PubMed Central Google Scholar
Maillet L, Chopin N, Treilleux I, Bachelot T, Tredan O, Faure C, et al. Spontaneous regression of breast cancer after biopsy. About two cases Gynecol Obstet Fertil. 2014;42:269–72.
Ito E, Nakano S, Otsuka M, Mibu A, Karikomi M, Oinuma T, et al. Spontaneous breast cancer remission: a case report. Int J Surg Case Rep. 2016;25:132–6.
Cserni G, Serfozo O, Ambrózay É, Markó L, Krenács L. Spontaneous pathological complete regression of high-grade triple-negative breast cancer with axillary metastasis. Pol J Pathol. 2019;70:139–43.
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Col NF, Ochs L, Springmann V, Aragaki AK, Chlebowski RT. Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat. 2012;135:639–46.
Lega IC, Austin PC, Gruneir A, Goodwin PJ, Rochon PA, Lipscombe LL. Association between metformin therapy and mortality after breast cancer: a population-based study. Diabetes Care. 2013;36:3018–26.
Lv H, Shi D, Fei M, Chen Y, Xie F, Wang Z, et al. Association between statin use and prognosis of breast cancer: a meta-analysis of cohort studies. Front Oncol. 2020. https://doi.org/10.3389/fonc.2020.5562430 ( eCollection 2020 ).
Article PubMed PubMed Central Google Scholar
Van Wyhe RD, Rahal OM, Woodward WA. Effect of statins on breast cancer recurrence and mortality: a review. Breast Cancer (Dove Med Press). 2017;9:559–65.
Google Scholar
Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–75.
Spisek R, Dhodapkar MV. Towards a better way to die with chemotherapy: role of heat shock protein exposure on dying tumor cells. Cell Cycle. 2007;6:1962–5.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72.
Zhou J, Wang G, Chen Y, Wang H, Hua Y, Cai Z. Immunogenic cell death in cancer therapy: present and emerging inducers. J Cell Mol Med. 2019;23:4854–65.
Download references
Acknowledgements
We would like to thank Forte ( https://www.forte-science.co.jp/ ) for English language editing.
No commercial, public, or nonprofit organizations financially supported this research.
Author information
Authors and affiliations.
Department of Breast Surgery, Hiroshima General Hospital, 1-3-3 Jigozen, Hatsukaichi, Hiroshima, 738-8503, Japan
Masahiro Ohara, Yumiko Koi, Tatsunari Sasada & Keiko Kajitani
Section of Pathological Research and Laboratory, Hiroshima General Hospital, 1-3-3 Jigozen, Hatsukaichi, Hiroshima, 738-8503, Japan
Seishi Mizuno, Ai Takata, Atsuko Okamoto, Ikuko Nagata, Mie Sumita, Kaita Imachi, Mayumi Watanabe & Yutaka Daimaru
Suzumine Imanaka Clinic, 4-2-31, Inokuchi, Nishi-ku, Hatsukaichi, Hiroshima, 733-0842, Japan
Shingo Kawamura
Department of Breast Oncology, National Hospital Organization Kyushu Cancer Center, 3-1-1 Notame, Minami-ku, Fukuoka, 811-1395, Japan
You can also search for this author in PubMed Google Scholar
Contributions
SK provided the clinical data included in the text. MO wrote the manuscript draft and critically revised the manuscript for important intellectual content. YK, TS, and KK contributed to the conception of the work and interpreted and revised the results of PET-CT, MRI, mammograms, and ultrasonograms included in this report. SM, AT, AO, IN, MS, KI, and MW collected and analyzed the histopathological data. YD diagnosed the disease pathologically. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Masahiro Ohara .
Ethics declarations
Ethics approval and consent to participate.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent for publication
Written consent to publish this information was obtained from the patient. Proof of consent to publish from the patient can be requested at any time.
Competing interests
All the authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Ohara, M., Koi, Y., Sasada, T. et al. Spontaneous regression of breast cancer with immune response: a case report. surg case rep 7 , 10 (2021). https://doi.org/10.1186/s40792-020-01103-5
Download citation
Received : 18 September 2020
Accepted : 26 December 2020
Published : 06 January 2021
DOI : https://doi.org/10.1186/s40792-020-01103-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Breast cancer
- Open access
- Published: 15 May 2024
Dietary supplement intake in women with breast cancer before and after diagnosis: results from the SUCCESS C trial
- Dagmar Hauner 1 ,
- Anna Mang 1 ,
- Lara Donik 1 ,
- Florian Schederecker 1 , 2 ,
- Dorothy Meyer 1 ,
- Brigitte Rack 3 ,
- Wolfgang Janni 3 &
- Hans Hauner 1
BMC Cancer volume 24 , Article number: 591 ( 2024 ) Cite this article
134 Accesses
Metrics details
There is little evidence that dietary supplements are beneficial for patients with breast cancer; therefore, they are usually not recommended by treatment guidelines. The aim of the present analysis was to assess the prevalence of dietary supplement (DS) intake among women before and after a breast cancer diagnosis.
Participants in the SUCCESS C lifestyle intervention study, a randomized controlled trial in women with newly diagnosed intermediate- to high-risk breast cancer, completed two questionnaires on dietary supplement intake 24 months (QS1) and 48 months (QS2) after beginning the lifestyle intervention. The study was registered on 12.17.2008 under the EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ , trial registration number: 2008-005453-38. The questionnaires collected data on DS intake during the 5-year period prediagnosis (QS1) and in the period postdiagnosis (QS2). Multivariate logistic regression models were fitted to examine differences in DS intake between the two intervention groups. The groups were then pooled to examine differences in DS use between the prediagnostic and postdiagnostic period.
A total of 320 questionnaires from 58.5% of intervention group completers and 416 questionnaires from 46.6% of low-level intervention group completers were included in the analysis. Overall, 20.2% of all respondents reported taking DS prior to their diagnosis. After a cancer diagnosis, the percentage of women taking DS significantly increased to 56.4% (p for time effect < 0.0001). No differences in DS intake between the intervention groups were observed. Single or combined preparations of vitamins and minerals/trace elements were the most frequently reported supplements. Notably, a 9-fold increase in vitamin D intake was reported postdiagnosis, where the proportion of women increased from 3.8 to 34.5%.
A 3-fold increase in the reported intake of dietary supplements was seen in women after a breast cancer diagnosis. These observations underscore the need to incorporate patient education surrounding the use of dietary supplements in a treatment care plan, particularly addressing the negligible benefits as well as the potential risks and treatment interactions.
Peer Review reports
Dietary supplement (DS) intake has gained popularity among patients with a wide range of diseases and can be obtained without a prescription in most countries. DS are subject to European law in the European Union [ 1 ] and complemented by national regulations. In Germany, DS are defined as food products that supplement general nutrition and consist of a “concentrate of nutrients or other substances with a nutrition-specific or physiological effect, alone or in composition” [ 2 ]. Importantly, DS do not require approval before they are marketed, and in Germany, in contrast to other countries such as Denmark and France, there is no defined upper intake level [ 3 ]. Only the amounts of the individual ingredients, per serving, must be indicated with reference to the recommended daily intake and an accompanying warning not to exceed the recommended daily amount [ 2 ]. National consumer surveys show that over one-quarter (27.6%) of adults in Germany report taking DS [ 4 , 5 ].
The use of DS is also rather common among cancer patients [ 6 , 7 ], and is particularly high in women with breast cancer, with reported rates ranging between 45 and 87% of patients [ 6 , 8 , 9 , 10 , 11 , 12 , 13 , 14 ]. This behavior is in clear contrast to recommendations from expert panels, which universally discourage the use of supplements for both cancer survivors in general and breast cancer patients [ 15 , 16 ]. Because of this discrepancy, we were interested in examining the prevalence of DS intake among participants in the lifestyle modification part of the German SUCCESS C trial before and after a breast cancer diagnosis.
The SUCCESS C trial
Data was obtained from the SUCCESS C trial, an open-label, multicenter, randomized controlled study that examined the effect of two different chemotherapy regimens ( n = 3642) as well as the effect of a comprehensive lifestyle intervention program on disease-free survival in women with newly diagnosed HER2/neu-negative intermediate-risk to high-risk breast cancer [ 17 , 18 , 19 ]. The study and all experimental protocols were approved by the Heinrich Heine University Düsseldorf Ethics Committee and was registered on 12.17,2008 under the EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ , identifier: 2008-005453-38. All participants provided written informed consent.
The focus of the 2-year lifestyle intervention was on moderate weight loss in breast cancer patients with a BMI between 24 and 40 kg/m² ( n = 2292). Not later than six weeks after surgery, 2292 women were randomized to either the lifestyle intervention group (IG) or the low-level intervention group (LLIG). The lifestyle intervention started either 3 or 6 months after the completion of chemotherapy. Women in the IG received an individualized, telephone-based program promoting an energy-reduced, healthy diet and regular physical activity, whereas the LLIG received general recommendations for a healthy lifestyle [ 18 ]. Figure 1 shows a participation flowchart. From the 776 women (IG) and 1009 women (LLIG) who began the lifestyle program, at total of 547 in the IG (70.5%) and 893 in the LLIG (88.5%) completed the 2-year intervention. Specific information or advice on dietary supplements was not delivered to either group.
Assessment of dietary supplement intake
Participants in both groups were requested to record their food intake, using 7-day dietary records, as well as their physical activity. These data were collected at five time points (i.e., baseline, 6, 12, 24 and 48 months after starting the lifestyle intervention). All participants were also requested to fill in two additional questionnaires on DS intake, developed by our research team and added to the dietary record forms, at 24 months (i.e. the end of the lifestyle intervention, QS1) and 48 months (i.e. 24 months later, QS2). The questionnaires collected data on DS intake for the following periods: (1) within 5 years prior to a breast cancer diagnosis (T0), and (2) postdiagnosis at 24 months (T1) and 48 months (T2) after starting the lifestyle intervention (Table S1 ).
Participants were asked whether they used DS, and if so, to report the dosage, frequency (i.e. how often in a day), and product name of any vitamins, minerals/trace elements, and/or any combinations of vitamins and minerals and/or any other dietary supplements.
Statistical methods
This secondary analysis used data from 736 women who completed questionnaires on DS intake. Descriptive data were derived from QS1 and reported as mean values ± standard deviation (SD).
The components and dosages of DS were calculated from questionnaires, when available. Any drugs that were misclassified as DS by participants were excluded from analysis.
Analyses were performed with IBM SPSS Statistics version 26 (IBM, Armonk, NJ, USA) and R software version 4.0.3 (RStudio Inc, Boston MA, USA). The proportion of women taking DS were compared between the two intervention groups using binary logistic regression models. The primary aim of this analysis was to investigate any differences in DS prevalence before (T0) and after a breast cancer diagnosis (T1) for the entire cohort by fitting binary logistic regression models. We further investigated the change in DS intake in the period postdiagnosis (i.e. the change between T1 and T2). Only women who filled out questionnaires at both time points were included in the descriptive data presentation.
Subgroups were determined a priori and analyses were performed to compare the following groups: participants with a BMI < 30 kg/m² vs. BMI ≥ 30 kg/m², and participants with positive vs. negative hormone receptor status. Participants were also subcategorized into three age groups: < 51 years, 51–64 years and ≥ 65 years to observe any differences in DS use. The SUCCESS C study was not powered to detect differences between subgroups; hence mainly descriptive reporting of between-group differences was considered the most appropriate choice rather than using interaction tests to assess subgroup effects. This approach was chosen to reduce the risk of Type 1 errors [ 20 ]. All models were adjusted for age at baseline, weight at baseline and chemotherapy arm. A two-tailed p value of ≤ 0.05 was considered statistically significant.
In total, 342 women in the IG and 434 women in the LLIG filled out the QS1, of which 320 (from 58.5% of the IG completers) and 416 (46.6% of the LLIG completers) could be included in the analyses. For QS2, we obtained 276 (IG) and 368 (LLIG) questionnaires, of which 216 (IG) and 288 (LLIG) provided complete questionnaires at both time points to assess the change in DS use over time (Fig. 1 ). The discrepancy in the number of collected questionnaires versus those included in the analyses was due to several questionnaires containing either insufficient or implausible responses. Comparing women whose QS1 were missing or incomplete ( n = 712) to those who were included in this study ( n = 736), we found that the non-included women were younger (55.5 vs. 57.4 years), had a higher BMI (29.4 vs. 28.6 kg/m 2 ) and an increased waist circumference (94.6 vs. 92.9 cm). Moreover, a larger proportion of these women were premenopausal (37.4% vs. 27.6%), smokers (17.1% vs. 11.0%) and underwent an anthracyclin-based chemotherapy regimen (52.5% vs. 47.0%) (all p-values < 0.05).
The baseline participant characteristics on dietary supplement intake are presented in Table 1 and stratified by intervention group. Findings were generally comparable in both groups. The mean age was 57.4 years (both groups) and mean BMI was 28.8 kg/m² (IG) and 28.5 kg/m² (LLIG). Baseline weight was comparable in both groups (IG 78.1 kg and LLIG 77.3 kg). Most participants had tumor staging T1 or T2, N0 or N1 status, and G2 or G3 grading. The majority of women were hormone receptor positive (IG 83.7% and LLIG 79.3%). Most women, 77.2% (IG) and 79.6% (LLIG), underwent breast-conserving surgery, whereas 18.8% (IG) and 17.5% (LLIG) had mastectomies. All participants received adjuvant chemotherapy and most underwent radiation therapy (IG 90.9% and LLIG 89.9%). Hypertension was noted in 40.0% (IG) and 39.2% (LLIG) of the participants, diabetes mellitus in 4.7% (IG) and 4.8% (LLIG), and coronary heart disease in 0% (IG) and 1.0% (LLIG) (Table 1 ).
Total intake and intake of specific groups of dietary supplements
Prediagnostic DS use was reported by 20.2% and postdiagnostic use by 56.4% of all women. Results were similar when groups were split into IG and LLIG, with no significant between-group differences at either time point (Table 2 ). After a breast cancer diagnosis, a significant 2.8-fold increase (p-value for time effect < 0.0001) in DS use was found in all participants, which was also similar when observing the behavior of each group separately (2.6-fold in IG and 2.9-fold in LLIG, with no significant group difference) (Table 2 ).
We found that 13.9% of women reported taking any mineral/trace mineral, 11.1% any vitamin, and 8.3% any combination of vitamins and/or minerals before diagnosis. Postdiagnosis, the use of DS increased significantly by 4.1-fold for any vitamin (45.9% of women), 3.2-fold for any mineral/trace element (44.7% of women), and 3.9-fold for any combination of vitamins and/or minerals (31.9% of women) (p for time effect < 0.0001 for all three). Similar results were found for the IG and the LLIG separately, with no significant between-group differences for either time point. The consumption of herbal supplements (T0 4.5% vs. T1 11.8%), omega-3 fatty acid supplements (T0 1.5% vs. T1 2.9%) and other supplements (T0 1.6% vs. T1 8.0%) was less frequently reported (Table 2 ).
Intake of specific micronutrients
We found that prediagnosis, women most frequently took magnesium (9.9%), followed by zinc (5.4%), vitamin C (5.2%), B vitamins (4.6%) and vitamin D (3.8%). Fewer women reported taking vitamin E (2.2%), calcium (2.3%), selenium (1.6%), beta-carotene (1.2%) and vitamin A (1.1%). After a breast cancer diagnosis, 1 out of 3 women (34.5%) reported taking vitamin D. A considerable increase in intake was also reported for B vitamins (21.6%), magnesium (21.2%), zinc (19.6%), selenium (17.4%), vitamin C (13.6%) and calcium (12.8%). Fewer women reported taking vitamin E (8.8%), vitamin A (6.5%), and beta-carotene (5.2%). The increase in DS use postdiagnosis was particularly notable for selenium, vitamin D and calcium (p for time effect < 0.0001). No significant between-group differences were found when comparing DS use in the IG vs. LLIG (Table 3 ).
Dosage of specific micronutrients
The dosage of selected micronutrients that were taken postdiagnosis is shown in Table S2 . The median daily dosage values were 310.0 mg/d for vitamin C, 22.0 µg/d for vitamin D, 38.0 mg/d for vitamin E, 600.0 mg/d for calcium, 300.0 mg/d for magnesium, 100.0 mg/d for selenium, and 10.0 mg/d for zinc. A high percentage of women who reported using selected micronutrients took dosages that exceeded the reference values for daily intake (Table S3 ), such as vitamin C (85.5%), vitamin E (80.0%), zinc (78.6%), selenium (66.4%), and vitamin D (50.7%), followed by magnesium (31.3%), and calcium (14.6%) (Table S2 ). Women taking dosages exceeding the tolerable upper intake level (UL) (Table S3 ) were reported for magnesium (53.1%), zinc (20.0%) vitamin E (13.3%), selenium (8.4%), vitamin C (4.4%), vitamin D (3.0%), and calcium (2.3%) (Table S2 ).
Subgroup analyses
A similar significant increase in the intake of total DS was found for women with a BMI < 30 kg/m² and women with a BMI ≥ 30 kg/m² (p for time effect < 0.0001) as well as for women with and without anti-hormonal therapy (data not shown). Similar increases in postdiagnosis DS use were seen when participants were classified into three age-range categories (T0 vs. T1 in women < 51 years old: from 22.1 to 58.4%; 51–64 years old: from 19.2 to 56.4% and in women ≥ 65 years old: from 20.3 to 54.2%).
Temporal changes of postdiagnosis DS intake
No significant differences were observed when analysis was restricted to participants who filled out both QS1 and QS2 at the end of active lifestyle intervention (T1) and at a follow-up two years later (T2). Overall, DS intake was reported by 58.3% ( n = 294) of women at T1 and 57.9% ( n = 292) at T2; 48.0% reported taking any vitamin supplement at T1 and 46.6% at T2, 46.6% took any mineral/trace mineral at T1 and 43.1% at T2 and 42.3% took any combination of vitamins/minerals and/or other dietary supplements at T1 and 41.5% at T2 (Table S4 ).
The main finding of this secondary analysis was that women increased their use of DS almost 3-fold, from 20.2 to 56.4%, after receiving a breast cancer diagnosis. The proportion of participants taking DS was similar when the cohort was split into their respective study intervention groups (i.e., IG and LLIG). Confounders, such as age, BMI or type of cancer treatment did not substantively change the outcomes.
Data from a nationally representative sample showed that 28.0% of women in Germany report taking DS, with even higher rates observed among older women (e.g. 43.2% of women aged 65–80 years) [ 4 ]. Our findings show that the percentage of SUCCESS C participants who took DS was clearly higher than those who were part of the national sample, irrespective of age. The prevalence we report is comparable to findings from a recent German survey that found that 59.8% of women with breast cancer took DS after their diagnosis [ 14 ]. Notably, another German study in women with breast cancer observed that almost twice as many women compared to our cohort (36%) took DS prediagnosis and observed only a moderate increase in prevalence after diagnosis (45%) [ 13 ].
Our observations of a postdiagnostic increase in DS intake is in line with other cross-sectional and prospective studies. European studies have reported postdiagnostic DS intake rates of 62.8% in women with cancer [ 10 ] and 68.3% in individuals with breast cancer [ 21 ]. It is noteworthy that US-American studies show higher prevalence rates in both the prediagnostic (i.e. 54 − 84%) [ 9 , 10 , 12 ] and postdiagnostic periods (i.e. 60.6% and 87.0%) [ 6 , 7 , 9 , 11 , 12 , 22 ], reflecting a generally higher DS intake compared to European studies. Of interest, some of these studies showed that adult cancer survivors with a healthier lifestyle, lower BMI, higher diet quality, and higher physical activity were more likely to use DS [ 7 , 21 , 23 ].
Single or combined preparations of vitamins and minerals/trace minerals were the most frequently reported DS in the SUCCESS-C trial, with a significant 3- to 4-fold increase postdiagnosis, a finding that is in line with data from another European study [ 10 ]. The widespread use of DS, reported in these studies, is in striking contrast to current recommendations for breast cancer survivors. The Continuous Update Expert Report from 2018 [ 15 ] as well as national expert statements [ 24 ] explicitly do not recommend the use of DS in this population. In addition, the US Preventive Services Task Force recently released an updated report on the evidence for the efficacy and safety of DS for the prevention of cancer and cardiovascular diseases. In this report, a clear recommendation against beta carotene and vitamin E supplements for cancer patients was given [ 16 ]. The authors also noted that only insufficient evidence was found to evaluate the benefits and potential harms of multivitamins, vitamins B3, B6, C and D, calcium, selenium, and folic acid (both with and without vitamin B12) on cancer outcomes [ 16 ]. In accordance with this report, literature confirms that there is not only no proven benefit for DS, but also the potential for negative effects which must be considered when taking any DS [ 16 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 ].
Given compelling evidence indicating the absence of benefits from dietary supplements, their widespread use by a majority of breast cancer patients is concerning. One reason for this discrepancy is the inadequate translation of established scientific knowledge and research findings into clinical practice to inform patient care and improve health literacy. In the SUCCESS C trial, no specific information on the use of DS was delivered to either group. Therefore, the marked increase in DS intake following a cancer diagnosis, found in this analysis, likely did not result from medical recommendations provided by their oncologists. A large breast cancer trial observed that one-third of participants were advised to take DS by their clinicians and 10% were asked to discontinue DS intake, while 51% of the patients did not receive any advice [ 32 ]. These findings suggest that the widespread use of DS is not medically supervised. Other research indicates that the majority of physicians are unaware of DS use among their cancer patients [ 6 ]. A web-based survey conducted as part of the NutriNet Sante Cohort study noted potentially adverse DS intake patterns among a significant number of cancer patients. Examples include concurrently taking vitamin E and anticoagulants or smokers taking beta-carotene supplements [ 21 ].
The general lack of awareness among clinicians on their cancer patients’ use of DS aligns with a growing trend toward self-medication and a heightened interest in complementary and alternative medicine [ 33 ]. At the same time, predatory marketing campaigns targeting cancer patients and offering deceptive or unsupported health claims are common. Patients are vulnerable to such messages and frequently believe that DS can prevent nutritional deficiencies and thereby support their immune system. Furthermore, many patients hope or expect that DS can mitigate the side effects of conventional cancer therapies and improve their quality of life [ 34 ].
Vitamin D supplementation showed the most dramatic increase postdiagnosis in the present study: from 3.8 to 34.5% of participants. Similarly, in a French study, vitamin D was clearly preferred among all single-ingredient DS, where 47.7% of women with breast cancer reported regular intake [ 21 ]. The reasons for these high intake rates are unclear. According to current recommendations by the World Cancer Research Fund there is insufficient evidence for general vitamin D use for this population [ 15 , 24 ], although some guidelines recommend routine measurement of serum 25(OH)D in oncology patients and supplementation if a deficiency is detected [ 35 ]. For osteoporosis prevention in postmenopausal women, consuming the recommended daily amounts of calcium and vitamin D should be ensured through a balanced diet. If the recommended levels cannot be achieved through food then supplements should be given to fill nutrient gaps [ 36 ]. Vitamin D supplements are principally recommended when clinical osteoporosis is diagnosed [ 36 ]. In breast cancer patients, prevention of therapy-associated bone loss is advised. However, vitamin D and calcium supplementation should be medically indicated and tailored to the individual patient [ 24 ]. Therefore, the increase in vitamin D supplementation we observed in this cohort is plausible and at least partially justified.
A high-quality diet is associated with a better breast cancer prognosis [ 37 ] and routine monitoring for nutritional imbalances and weight change is important for all cancer patients [ 38 , 39 ]. During chemotherapy and radiotherapy, an adequate intake of micronutrients should be ensured according to physiological requirements [ 24 ]. However, possible drug interactions must be considered. While some antioxidants might reduce side effects, antagonistic effects of antioxidants and other nutrients may compromise the therapeutic efficiency of chemotherapy and radiotherapy, thereby affecting prognostic outcomes [ 24 , 25 , 27 , 30 , 40 , 41 ]. Hence, unnecessary and unmonitored consumption of dietary supplements should be avoided, particularly in excessive doses.
Finally, there is also a financial burden on patients arising from out-of-pocket costs for DS that cannot be ignored. Many cancer patients suffer from financial hardship as a consequence of their illness due to diverse additional costs [ 42 ], a reduction in working hours [ 43 ], a loss of revenue and an increased likelihood of unemployment [ 43 ]. Around 20–30% of cancer survivors do not return to the workplace [ 42 ]. Financial challenges could potentially exacerbate the disease burden and hinder the adoption of a healthy lifestyle. For these reasons, clinicians bear the responsibility of addressing costs associated with DS use within the patient treatment plan to prevent avoidable additional expenses [ 15 , 39 ].
Our manuscript presents several strengths that contribute to the robustness of our findings. Firstly, our study was able to analyze data on DS intake from a sizable cohort of 736 participants. Additionally, we collected data from DS use in both the pre- and postdiagnostic periods, providing a nuanced understanding of changes in DS intake behavior. Furthermore, detailed baseline characteristics of participating women were documented, enhancing the reliability and applicability of our results. The nationwide recruitment of women from over 200 gynecological practices across Germany underscores the potential generalizability of our findings to adult German women with overweight or obesity and Her2/neu-negative breast cancer.
However, several weaknesses also merit consideration. Notably, data on DS use was available from only around one-third of the initially randomized participants and around 40% of those who commenced the lifestyle intervention. This may have resulted in an overall discrepancy between the DS use we report in this study and behavior from the entire cohort, although other studies have reported similar findings. Additionally, we lacked information regarding the reasons for DS intake among participants and relied solely on retrospective self-reporting. Lastly, the study was limited to women with a BMI between 24 and 40 kg/m² and Her2/neu-negative breast cancer who received chemotherapy, potentially restricting the generalizability of our findings to a broader population. These weaknesses highlight areas for further investigation and consideration in future research endeavors.
In conclusion, results from this study show that the proportion of women taking DS markedly increased after a diagnosis of breast cancer. Given the inadequate scientific evidence supporting the general benefit and safety of additional DS intake, patients with breast cancer should receive fundamental guidance from their treating physicians regarding DS. Consideration of DS use should be a recurring and consistently addressed aspect of comprehensive cancer care.
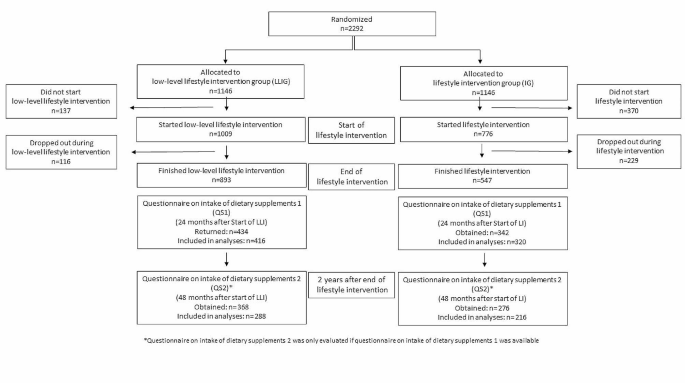
Flow diagram of the lifestyle intervention part of SUCCESS C Trial and the analysis of the intake of dietary supplements
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Dietary supplements
Intervention group
Low-level intervention group
Dietary supplement questionnaire
GUIDELINE 2002/46/EG OF THE EUROPEAN PARLIAMENT AND COUNCIL of June 10. 2002 on the adaptation of laws of the legal regulation of member states relating to supplements. Official Journal of European Communities: The European Parliament and the Council of the European Union 2021 [ https://eur-lex.europa.eu/legal-content/DE/TXT/PDF/?uri=CELEX:02002L0046-20170726&qid=1545903950901&from=DE
Verordnung über Nahrungsergänzungsmittel. (Nahrungsergänzungsmittelverordnung - NemV): Bundesministerium der Justiz und für Verbraucherschutz; [updated 2017; cited October 26, 2022. https://www.gesetze-im-internet.de/nemv/NemV.pdf .
Weißenborn A, Bakhiya N, Demuth I, Ehlers A, Ewald M, Niemann B, et al. Höchstmengen für Vitamine Und Mineralstoffe in Nahrungsergänzungsmitteln. J Consumer Prot Food Saf. 2018;13(1):25–39.
Article Google Scholar
Bundesministerium für Ernährung LuV. Ergebnisbericht Teil 1: Nationale Verzehrsstudie II, die bundesweite Befragung Zur Ernährung Von Jugendlichen Und Erwachsenen. Volume 5. Contract No.: Max Rubner-Institut; 2008.
Google Scholar
Bundesministerium für Ernährung LuV. Ergebnisbericht Teil 2: Nationale Verzehrsstudie II, Die bundesweite Befragung zur Ernährung von Jugendlichen und Erwachsenen: Max Rubner-Institut. 2008.
Velicer CM, Ulrich CM. Vitamin and mineral supplement use among US adults after cancer diagnosis: a systematic review. J Clin Oncol. 2008;26(4):665–73.
Article CAS PubMed Google Scholar
Miller PE, Vasey JJ, Short PF, Hartman TJ. Dietary supplement use in adult cancer survivors. Oncol Nurs Forum. 2009;36(1):61–8.
Article PubMed PubMed Central Google Scholar
Newman V, Rock CL, Faerber S, Flatt SW, Wright FA, Pierce JP. Dietary supplement use by women at risk for breast cancer recurrence. The women’s healthy eating and living Study Group. J Am Diet Assoc. 1998;98(3):285–92.
Kwan ML, Greenlee H, Lee VS, Castillo A, Gunderson EP, Habel LA, et al. Multivitamin use and breast cancer outcomes in women with early-stage breast cancer: the Life after Cancer Epidemiology study. Breast Cancer Res Treat. 2011;130(1):195–205.
Velentzis LS, Keshtgar MR, Woodside JV, Leathem AJ, Titcomb A, Perkins KA, et al. Significant changes in dietary intake and supplement use after breast cancer diagnosis in a UK multicentre study. Breast Cancer Res Treat. 2011;128(2):473–82.
Greenlee H, Kwan ML, Kushi LH, Song J, Castillo A, Weltzien E, et al. Antioxidant supplement use after breast cancer diagnosis and mortality in the Life after Cancer Epidemiology (LACE) cohort. Cancer. 2012;118(8):2048–58.
Greenlee H, Kwan ML, Ergas IJ, Strizich G, Roh JM, Wilson AT, et al. Changes in vitamin and mineral supplement use after breast cancer diagnosis in the pathways Study: a prospective cohort study. BMC Cancer. 2014;14:382.
Jung AY, Cai X, Thoene K, Obi N, Jaskulski S, Behrens S, et al. Antioxidant supplementation and breast cancer prognosis in postmenopausal women undergoing chemotherapy and radiation therapy. Am J Clin Nutr. 2019;109(1):69–78.
Article PubMed Google Scholar
Holzapfel C, Kocsis A, Jaeckel B, Martignoni M, Hauner D, Hauner H. Dietary habits and intake of nutritional supplements in patients of outpatient cancer clinics. Ernährungs Umschau. 2020;67(3):60–8.
Research WCRFIAIfC. Continuous Update Project Expert Report. 2018. Survivors of breast and other cancers [ https://www.wcrf.org/wp-content/uploads/2021/02/Cancer-Survivors.pdf .
Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, Coker TR, et al. Vitamin, Mineral, and Multivitamin supplementation to prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2022;327(23):2326–33.
Rack B, Andergassen U, Neugebauer J, Salmen J, Hepp P, Sommer H, et al. The German SUCCESS C study - the first European lifestyle study on breast Cancer. Breast Care (Basel). 2010;5(6):395–400.
Hauner D, Rack B, Friedl T, Hepp P, Janni W, Hauner H. Rationale and description of a lifestyle intervention programme to achieve moderate weight loss in women with non-metastatic breast cancer: the lifestyle intervention part of the SUCCESS C study. BMJ Nutr Prev Health. 2020;3(2):213–9.
de Gregorio A, Janni W, Friedl TWP, Nitz U, Rack B, Schneeweiss A, et al. The impact of anthracyclines in intermediate and high-risk HER2-negative early breast cancer-a pooled analysis of the randomised clinical trials PlanB and SUCCESS C. Br J Cancer. 2022;126(12):1715–24.
Wang X, Piantadosi S, Le-Rademacher J, Mandrekar SJ. Statistical considerations for subgroup analyses. J Thorac Oncol. 2021;16(3):375–80.
Pouchieu C, Fassier P, Druesne-Pecollo N, Zelek L, Bachmann P, Touillaud M, et al. Dietary supplement use among cancer survivors of the NutriNet-Santé cohort study. Br J Nutr. 2015;113(8):1319–29.
Poole EM, Shu X, Caan BJ, Flatt SW, Holmes MD, Lu W, et al. Postdiagnosis supplement use and breast cancer prognosis in the after breast Cancer Pooling Project. Breast Cancer Res Treat. 2013;139(2):529–37.
Article CAS PubMed PubMed Central Google Scholar
Li K, Kaaks R, Linseisen J, Rohrmann S. Consistency of vitamin and/or mineral supplement use and demographic, lifestyle and health-status predictors: findings from the European prospective investigation into Cancer and Nutrition (EPIC)-Heidelberg cohort. Br J Nutr. 2010;104(7):1058–64.
S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms, Langversion 4.4 [Internet]. AWMF Registernummer: 032-045OL. 2021a. https://register.awmf.org/assets/guidelines/032-045OLl_S3_Mammakarzinom_2021-07.pdf .
D’Andrea GM. Use of antioxidants during chemotherapy and radiotherapy should be avoided. CA Cancer J Clin. 2005;55(5):319–21.
Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;2012(3):Cd007176.
PubMed PubMed Central Google Scholar
Harvie M. Nutritional supplements and cancer: potential benefits and proven harms. Am Soc Clin Oncol Educ Book. 2014:e478–86.
Navarro VJ, Barnhart H, Bonkovsky HL, Davern T, Fontana RJ, Grant L, et al. Liver injury from herbals and dietary supplements in the U.S. Drug-Induced Liver Injury Network. Hepatology. 2014;60(4):1399–408.
Rayman MP, Winther KH, Pastor-Barriuso R, Cold F, Thvilum M, Stranges S, et al. Effect of long-term selenium supplementation on mortality: results from a multiple-dose, randomised controlled trial. Free Radic Biol Med. 2018;127:46–54.
Vernieri C, Nichetti F, Raimondi A, Pusceddu S, Platania M, Berrino F, et al. Diet and supplements in cancer prevention and treatment: clinical evidences and future perspectives. Crit Rev Oncol Hematol. 2018;123:57–73.
Chen F, Du M, Blumberg JB, Ho Chui KK, Ruan M, Rogers G, et al. Association among Dietary Supplement Use, Nutrient Intake, and Mortality among U.S. adults: a Cohort Study. Ann Intern Med. 2019;170(9):604–13.
Zirpoli GR, Brennan PM, Hong CC, McCann SE, Ciupak G, Davis W, et al. Supplement use during an intergroup clinical trial for breast cancer (S0221). Breast Cancer Res Treat. 2013;137(3):903–13.
Micke O, Bruns F, Glatzel M, Schönekaes K, Micke P, Mücke R, et al. Predictive factors for the use of complementary and alternative medicine (CAM) in radiation oncology. Eur J Integr Med. 2009;1(1):19–25.
Tank M, Franz K, Cereda E, Norman K. Dietary supplement use in ambulatory cancer patients: a survey on prevalence, motivation and attitudes. J Cancer Res Clin Oncol. 2021;147(7):1917–25.
S3-Leitlinie Komplementärmedizin in der Behandlung von onkologischen PatientInnen, Kurzversion 1.1 [Internet]. Deutsche Krebshilfe, AWMF. 2021b. https://register.awmf.org/assets/guidelines/032-055OLk_Komplementaermedizin-in-der-Behandlung-von-onkologischen-PatientInnen-2021-11.pdf .
Prophylaxe eVDO. Diagnostik und Therapie der Osteoporose bei postmenopausalen Frauen und bei Männern, Langfassung. 2017. Contract No.: AWMF-Register-Nr.: 183/001.
Weigl J, Hauner H, Hauner D. Can Nutrition Lower the risk of recurrence in breast Cancer? Breast Care (Basel). 2018;13(2):86–91.
Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11–48.
Muscaritoli M, Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin Nutr. 2021;40(5):2898–913.
Lawenda BD, Kelly KM, Ladas EJ, Sagar SM, Vickers A, Blumberg JB. Should supplemental antioxidant administration be avoided during chemotherapy and radiation therapy? J Natl Cancer Inst. 2008;100(11):773–83.
Ambrosone CB, Zirpoli GR, Hutson AD, McCann WE, McCann SE, Barlow WE, et al. Dietary supplement use during chemotherapy and survival outcomes of patients with breast Cancer enrolled in a Cooperative Group Clinical Trial (SWOG S0221). J Clin Oncol. 2020;38(8):804–14.
Arbeitsgemeinschaft Soziale Arbeit in der Onkologie (ASO). [ https://www.krebsgesellschaft.de/onko-internetportal/basis-informationen-krebs/leben-mit-krebs/beratung-und-hilfe/armutsrisiko-krebs.html .
Hernandez D, Schlander M. Income loss after a cancer diagnosis in Germany: an analysis based on the socio-economic panel survey. Cancer Med. 2021;10(11):3726–40.
Download references
Acknowledgements
The study was initially funded by unrestricted grants given to WJ from Pfizer, Sanofi-Aventis, Chugai and Veridex. We wish to thank Dr. Lynne Stecher for initial statistical advice. We also appreciate the excellent contribution by the almeda GmbH Munich lifestyle coaches, led by Mrs. Rose, for performing the telephone-based lifestyle intervention. We thank Mrs. Heidrun-Lorsbach, CEO of the CRO Alcedis, Giessen, for support with data collection. Finally, we would like to thank all the study centers, their contributing staff members and all participating women.
HH received funding for the analysis of dietary supplement intake by the Else Kröner Fresenius Foundation, Bad Homburg, Germany (grant no. 2016_A31), the Bavarian State Ministry of Health (project no. 89397) and the Technical University of Munich, Germany. The study design, data collection and analysis, decision to publish, and preparation of the manuscript were independent from the funder.
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and affiliations.
Institute of Nutritional Medicine, Else Kröner Fresenius Center for Nutritional Medicine, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany
Dagmar Hauner, Anna Mang, Lara Donik, Florian Schederecker, Dorothy Meyer & Hans Hauner
Chair of Epidemiology, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany
Florian Schederecker
Department of Gynecology and Obstetrics, University Hospital Ulm, Ulm, Germany
Brigitte Rack & Wolfgang Janni
You can also search for this author in PubMed Google Scholar
Contributions
HH, DH and BR designed the lifestyle intervention arm of the SUCCESS C trial. WJ was responsible for the design of the complete SUCCESS C trial. BR and WJ were responsible for supervision and support of the study centers. DH supervised the lifestyle program conducted by lifestyle coaches of almeda, a health care provider in Munich. DH and the almeda team were responsible for data collection and management. AM supported the analysis of DS intake, LD and FS performed the final statistical analyses. DH, DM and HH wrote the manuscript. All authors reviewed and commented on the manuscript.
Corresponding author
Correspondence to Hans Hauner .
Ethics declarations
Ethics approval and consent to participate.
The study was approved by the Heinrich Heine University Düsseldorf Ethics Committee. The protocol was registered under the EU Clinical Trials Register https://www.clinicaltrialsregister.eu/ , identifier: 2008-005453-38. All women gave written informed consent to participate in the trial.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hauner, D., Mang, A., Donik, L. et al. Dietary supplement intake in women with breast cancer before and after diagnosis: results from the SUCCESS C trial. BMC Cancer 24 , 591 (2024). https://doi.org/10.1186/s12885-024-12341-3
Download citation
Received : 01 February 2024
Accepted : 06 May 2024
Published : 15 May 2024
DOI : https://doi.org/10.1186/s12885-024-12341-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Dietary supplement use
- Inadequate intake
- Excess intake
- Breast cancer
- Micronutrients
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- ACS Foundation
- Diversity, Equity, and Inclusion
- ACS Archives
- Careers at ACS
- Federal Legislation
- State Legislation
- Regulatory Issues
- Get Involved
- SurgeonsPAC
- About ACS Quality Programs
- Accreditation & Verification Programs
- Data & Registries
- Standards & Staging
- Membership & Community
- Practice Management
- Professional Growth
- News & Publications
- Information for Patients and Family
- Preparing for Your Surgery
- Recovering from Your Surgery
- Jobs for Surgeons
- Become a Member
- Media Center
Our top priority is providing value to members. Your Member Services team is here to ensure you maximize your ACS member benefits, participate in College activities, and engage with your ACS colleagues. It's all here.
- Membership Benefits
- Find a Surgeon
- Find a Hospital or Facility
- Quality Programs
- Education Programs
- Member Benefits
- Cancer Programs News
- Cancer Programs News: May 16
- Update on the PROMPT Study...
Update on the PROMPT Study for Breast Patients
May 16, 2024
In the May issue of the Bulletin of the American College of Surgeons , authors from the NAPBC and ACS Cancer Research Program discuss the Patient-Reported Observations on Medical Procedure Timeliness for Breast Patients (PROMPT) study . PROMPT was a two-year quality collaborative that examined the time interval from screening to diagnosis to first treatment for patients with breast disease.
In This Issue
New: ajcc protocol version 9 webinar recordings now available.
The authors of the American Joint Committee on Cancer (AJCC) Version 9 Appendix and Cervix Uteri protocols have recorded brief webinars discussing the protocols’ contents.
Call for Cancer Research Program Implementation Research Committee Members
The ACS Cancer Research Program (CRP) is launching the Implementation Research Committee and is seeking new members.
Save the date: CSSP Webinar Technical Standards for Breast Cancer Surgery
The Cancer Surgery Standards Program (CSSP), in collaboration with the National Accreditation Program for Breast Centers (NAPBC), will host an educational webinar for surgeons on the technical standards for breast cancer surgery.
Cancer Programs Accreditation Fees Update
ACS Cancer Programs annual accreditation fees have been adjusted for 2023. \
New Prostate Cancer Awareness Month Marketing Tools Available
September is Prostate Cancer Awareness Month. According to the American Cancer Society Cancer Facts & Figures 2023, there will be an estimated 288,300 new cases of prostate cancer diagnosed in the US and 34,700 men will die from the disease this year.
Upcoming CAnswer Forum Maintenance
The CAnswer Forum will be undergoing routine maintenance on Monday, July 31 at 8 pm CT.
American Cancer Society Updates and Resources
“I Love You Get Screened” campaign, Second Annual National Lung Cancer Screening Day, and more.
CoC Recognizes Accredited Sites
The ACS CoC recognizes these cancer sites for demonstrating their commitment to providing high-quality, patient-centered cancer care to patients and the community by recently earning CoC reaccreditation.
NAPBC Recognizes Accredited Sites
The ACS NAPBC recognizes these sites for demonstrating their commitment to providing high-quality, patient-centered cancer care to patients and the community by recently earning NAPBC reaccreditation.
The Latest from Cancer Programs
Save the date for upcoming Cancer Program events.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

A case-case analysis of women with breast cancer: predictors of interval vs screen-detected cancer
Nickolas dreher.
1 University of California San Francisco, San Francisco, CA, USA
2 The Icahn School of Medicine at Mount Sinai, New York, NY, USA
Madeline Matthys
Edward hadeler.
3 University of Miami Miller School of Medicine, Miami, FL, USA
Yiwey Shieh
Irene acerbi, fiona m. mcauley, michelle melisko, martin eklund.
4 Karolinska Institutet, Stockholm, Sweden
Jeffrey A. Tice
Laura j. esserman, laura j. van ‘t veer.
Authors’ contributions: All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Nickolas Dreher, Madeline Matthys, and Edward Hadeler. The first draft of the manuscript was written by Nickolas Dreher and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Associated Data
The Breast Cancer Surveillance Consortium (BCSC) model is a widely-used risk model that predicts five- and ten-year risk of developing invasive breast cancer for healthy women aged 35–74 years. Women with high BCSC risk may also be at elevated risk to develop interval cancers, which present symptomatically in the year following a normal screening mammogram. We examined the association between high BCSC risk (defined as the top 2.5% by age) and breast cancers presenting as interval cancers.
We compared the mode of detection and tumor characteristics of patients in the top 2.5% BCSC risk by age with age-matched (1:2) patients in the lower 97.5% risk. We constructed logistic regression models to estimate the odds ratio (OR) of presenting with interval cancers, and poor-prognosis tumor features, between women from the top 2.5% and bottom 97.5% of BCSC risk.
Our analysis included 113 breast cancer patients in the top 2.5% of risk for their age and 226 breast cancer patients in the lower 97.5% of risk. High-risk patients were more likely to have presented with an interval cancer within one year of a normal screening, OR 6.62 (95% CI 3.28–13.4, p<0.001). These interval cancers were also more likely to be larger, node positive, and higher stage.
Conclusion:
Breast cancer patients in the top 2.5% of BCSC risk for their age were more likely to present with interval cancers. The BCSC model could be used to identify healthy women who may benefit from intensified screening.
Introduction
Interval cancers are invasive breast cancers that present symptomatically within 12 months of a normal screening mammogram. These cancers include both those that develop after a mammogram and those that were not detected (but did exist) at the previous screening mammograms. Interval cancers tend to be more aggressive and faster-growing than screen-detected cancers.[ 1 – 4 ] Identifying women who are at increased risk for interval breast cancers could inform screening strategies, as these women may benefit from supplemental or more frequent screening and risk reduction. However, no consensus regarding how to risk-stratify women for interval breast cancer risk exists. The Breast Cancer Surveillance Consortium (BCSC) model is a validated and widely used risk prediction tool that predicts five- and ten-year risk of developing invasive breast cancer for women age 35–74.[ 5 ] It bases risk prediction on age, race/ethnicity, presence of first degree relative with breast cancer, prior biopsies/benign breast disease, and Breast Imaging-Reporting and Data System (BI-RADS) breast density.[ 5 , 6 ] Past work by Kerlikowske et al. has suggested that the combination of BCSC risk and BI-RADS breast density is one method upon which risk-stratification for interval cancer could be based.[ 7 ]
However, both the BCSC model and breast density itself are correlated with age: as age increases, BCSC score increases and breast density decreases. Providers may be wary of basing recommendations for screening frequency and modality on risk models (such as BCSC) that may enrich for increased screening as age increases. Further, tumor characteristics and morbidity vary by age, with younger women being at increased risk of developing poor prognosis tumors and interval cancers.[ 7 – 9 ] In contrast to using an absolute risk cutoff to identify high risk women, an alternative method is to use age-specific cutoffs. Age-specific BCSC risk distributions are generated directly by the BCSC, and aggregate 5-year age groups have been described in the literature.[ 5 ] The WISDOM study, run by the Athena Breast Health Network, uses these distributions to establish a threshold of the top 2.5% of risk for each age group to initiate counseling on prevention interventions and annual screening. Prior thresholds were not sufficiently high to motivate interest in embarking on risk reduction strategies.[ 10 ] The top 2.5% by age threshold consistently identifies women with lifetime risk of 23–28%, and 20% of these women elect to pursue prevention interventions. This is why it was chosen for the high risk threshold to trigger for annual screening and prevention counseling in WISDOM.[ 10 ]
This study’s primary aim is to validate this top 2.5% by age threshold by determining if these women are more likely to present with interval cancers rather than screen-detected cancers. We also evaluated whether these interval cancers have more aggressive features to confirm the clinical relevance of detecting interval cancers.
Patients and Methods
We conducted a case-case analysis of women treated for invasive breast cancer at the University of California San Francisco Breast Care Center (UCSF BCC). This study included only women with a confirmed diagnosis of invasive breast cancer previously undergoing standard mammography screening. Between 2013 and 2017, 896 patients completed the Athena intake questionnaire (described in Measures ) in the BCC and had available BI-RADS breast density and pathology data. We identified the 180 women in the top 2.5% of BCSC risk for their age. Women were excluded from the study if they deviated from standard screening intervals by having an increased screening frequency (more than 1 mammogram per year) or if they had an “interval” cancer detected more than one year after their prior clear mammogram. Ultimately, information on mode of detection (screen detected versus interval breast cancer) was available for 113 women in the top 2.5% of risk for their age. We then used a random number generator to select age-matched (±1 year) women from the lower 97.5% who also had method of detection available. ( Figure 1 ).

Selection of the study group from women seen at the UCSF Breast Care Center from 2013–2017. Top 2.5% threshold determined from distributions of BCSC 5-year risk estimates.
The UCSF BCC is part of the Athena Breast Health Network, a breast cancer clinical care and research collaborative that includes breast care clinics from five University of California hospitals and Sanford Health in South Dakota.[ 11 ] Athena collects patient characteristics and outcome data across the entire care spectrum from screening and prevention to treatment and survivorship. At the UCSF BCC, questionnaires are distributed to all patients presenting with a new breast problem.
The Athena intake questionnaire at the BCC collects race/ethnicity, family history, personal cancer history, history of prior biopsies, presence of comorbidities, and psychosocial and physical quality of life metrics.[ 12 ] These questionnaires contain all the variables included in the BCSC model ( http://tools.bcsc-scc.org/BC5yearRisk/ ) except for BI-RADS density. Using the electronic medical record, we exported BI-RADS density based on the last negative screening mammogram prior to diagnosis and used it for the BCSC risk assessment. While the BCSC model is intended for women without a history of breast cancer, this allowed for a retrospective estimate of each patient’s 5-year risk of developing cancer at the approximate time of their diagnosis with the assumption that breast density stayed relatively stable between the last negative mammogram and density.[ 13 ] The 97.5 th BCSC risk percentile for each age ( Supplementary Table A ) was estimated by applying the BCSC risk calculator to data collected from more than six million mammograms from eight breast imaging registries across the country.[ 14 ]
The BCSC risk score was calculated for eligible women (those between the ages of 40–74 without a diagnosis of breast cancer prior to the current diagnosis) who completed the online intake questionnaire and whose BI-RADS density was available ( Figure 1 ). These BCSC scores were based on the patient’s age at time of intake. Medical records for all patients in the top 2.5% of risk for their age and two age-matched (±1 year) cases from the bottom 97.5% of risk were reviewed to determine method of cancer detection.
The UCSF Cancer Center registry contains pathology and outcome data linked to state and national registries, and has been described previously.[ 15 , 16 ] We collected information on each patient’s histology, grade, stage, nodal involvement, hormone receptor status, and tumor size from the Registry. If data were not available for a patient, we imported these fields from the UCSF surgical registry. The UCSF BCC maintains an internal surgical registry that is updated weekly with pathology reports from recent surgeries. This dataset, updated in near real-time, was included to capture data that were not yet reported in other registries.
Our primary outcome focused on interval cancers, defined as invasive breast cancers that presented within one year of a normal mammogram, BI-RADS score 1 or 2. Tumor characteristics including hormone receptor status, grade, size, nodal involvement and stage were imported from the registries based on patient medical record number and approximate diagnosis date.
Statistical Analysis
We compared the proportion of interval cancers between the two age-matched groups using conditional logistic regressions in R. We also used logistic regressions to compare tumor characteristics between interval cancers and screen-detected cancers. All tests were two-sided with alpha of 0.05.
In addition to comparing patients in the top 2.5% of risk for their age to patients from the lower 97.5%, we examined two additional risk stratification criteria from the literature: patients with extremely dense breasts (BI-RADS d) or a very high BCSC score irrespective of age (>4.00% 5-year risk of developing breast cancer).[ 7 ] This was an adjunct analysis included to address potential questions from the reader. However, it is important to note that the sample used in this study is not matched based on these two criteria.
Patient Characteristics
Of the 339 patients included in the final analysis, 113 fell in the top 2.5% of risk for their age, and they were compared to 226 from the lower 97.5% of risk ( Figure 1 ). Table 1 summarizes demographic information from the patients included in the analysis. Women in the top 2.5% of risk for their age tended to have higher breast density and more frequently reported a first degree relative with breast cancer and a personal history of breast biopsy (p<0.001 for all comparisons).
Baseline characteristics and demographic data for women in the top 2.5% of risk for their age (n=113) and age-matched women from the lower 97.5% (n=226).
Interval cancer risk by BCSC risk group
Patients from the top 2.5% of risk for their age were more likely to present with an interval cancer within one year of a normal screening mammogram compared to patients in the lower 97.5% of risk, OR 6.62 (95% CI 3.28–13.4, p<0.001) ( Table 2 ). Similar results were seen when we expanded the analysis to include “late-interval” cancers, those discovered within two years of a normal screening mammogram.
Association between three risk stratification criteria and interval cancers. The three risk stratification criteria included the BCSC top 2.5%, BI-RADS d (extremely dense), or BCSC 5-year cancer risk >4.00% (very high).
We also compared the top 2.5% by age threshold to two other common risk stratification criteria: extremely dense breasts (BI-RADS d) or a very high BCSC score irrespective of age (>4.00% 5-year risk of developing breast cancer) ( Table 2 ). The BCSC top 2.5% by age threshold was most strongly associated with interval cancer risk. The mean age for the BCSC top 2.5% threshold was between that of extremely dense breasts and 4% 5-year BCSC risk. Furthermore, a substantial number of women in the top 2.5% of risk for their age would not have been identified by these other risk cutoffs. Specifically, 49 of 113 (43%) women would only be flagged for increased risk using the top 2.5% by age threshold – and these women show a similarly high percentage of interval cancers (32.7%).
Tumor characteristics of interval cancers
Interval cancers had more aggressive features than cancers detected via screening mammogram. Interval cancers were more likely to be lymph node positive (odds ratio, OR 3.24, 95% CI 1.76 – 5.96, p<0.001) and larger than two centimeters (OR 3.49, 95% CI 1.82 – 6.70, p<0.001). Thus, they were more likely to be stage II or higher (OR 4.88, 95% CI 2.34 – 10.2, p<0.001). Likewise, interval cancers tended to be grade 3 and hormone receptor negative, although these trends were not statistically significant ( Table 3 ).
Tumor characteristics of interval cancers compared to screen-detected cancers from 339 breast cancer patients seen at the UCSF Breast Care Center. Certain components of pathology were not available for all patients, most notably tumor size. The ratios represent number of patients with the characteristic per those with data available.
In this study, we compared breast cancer patients in the BCSC top 2.5% of risk for their age to patients from the remaining 97.5%. We found that women in the top 2.5% of risk for their age, who have double the risk of getting breast cancer relative to the average women, had more than six-fold higher odds of presenting with interval cancers. Furthermore, the interval cancers detected in this study were of clinical relevance as they followed trends outlined in the literature and tended to have more aggressive features.
Our study extends the literature by validating an alternative approach to risk stratification, which considers the distribution of risk among similarly aged women, as a predictor of interval cancer risk.[ 17 ] This allows providers to identify women at high risk without selecting certain age groups, as would BCSC score or density alone. A numeric threshold, identical for all ages, also fails to recognize the range of risk in each age group and does not account for lifetime risk. A 1.5% 5-year risk in a 40-year-old, for example, is associated with a much higher lifetime risk than a 1.5% 5-year risk in a 75-year-old. Many patients in the top 2.5% of risk for their age have extremely dense or heterogeneously dense breasts, which may mask tumors and contribute to interval cancer prevalence. However, if density alone drove this effect, we would expect to see the highest interval cancer prevalence in patients with BI-RADS d density. To the contrary, the data presented in this manuscript demonstrate that the top 2.5% by age threshold had the highest proportion of interval cancers when compared to other previously reported risk stratification criteria such as extremely dense breasts (BIRADS d) or a 4% absolute 5-year risk. However, it is important to recognize that this study was not designed to compare these criteria, and in creating the BIRADS d or 4% absolute risk groups age-matching was broken. Further research is necessary to effectively compare risk-stratification criteria; this analysis was included to address common questions from readers but is largely beyond the scope of this work.
We also replicated previous work showing interval cancers to be enriched for aggressive features and linked to poor prognosis.[ 7 , 18 ] In a large case-case study of 431,480 women, Kirsh et al. found interval cancers were more likely to be higher stage, higher grade, estrogen receptor negative, and progesterone receptor negative when compared to screen-detected tumors. We replicated these findings for stage, and while our study may not have been sufficiently powered to detect significant differences in grade and hormone receptor status, it should be noted that trends in our results were aligned with previous findings in the literature.[ 1 , 2 ]
Our work should be interpreted in light of several limitations. First, this was a case-case analysis and our sample size may have limited the precision of our estimates and ability to detect small differences between groups. Larger cohort studies in multiracial/multiethnic populations are needed to validate our main findings. Such studies would also make our work more generalizable, given our study predominately included white patients. Second, we did not review the most recent mammogram to confirm that the tumor represented a “true” interval cancer – rather than merely a missed tumor due to human error in the initial reading. However, missed interval cancers have also been shown to have more aggressive features compared to screen-detected cancers, although to a lesser extent.[ 1 ] Furthermore, these data reflect the limits of what is understood in clinical practice. Ultimately, if this sampling includes tumors that should have been screen-detected, it should only underestimate the unique characteristics of interval cancers. Third, women with higher risk are often offered more intensive screening due to the presence of risk factors such as dense breasts or positive family history. This may also bias these results, but we expect the bias to be toward the null, given that we expect increased screening to decrease interval cancer prevalence in high-risk groups.
Our results have several important clinical implications. Since interval cancers tend to present at later stages and lead to worse prognosis, it follows that a goal of breast cancer screening should be to detect interval cancers at an earlier, more treatable stage. However, increasing screening frequency for all women would likely lead to unsustainable resource usage and unintended effects such as false positives. As such, there is a clear need for risk stratification criteria that can identify women at elevated risk of interval cancers so that they can receive targeted screening and prevention. However, providers may be wary of using existing criteria that tend to select specific age groups for a variety of reasons – such as the prevalence of indolent tumors in older women.[ 19 , 20 ] Our results suggest that a simple top 2.5% by age threshold, based on a widely used risk-assessment tool, may effectively identify women with higher odds of developing interval cancers. This threshold is already being used to target preventative efforts (such as chemoprevention and lifestyle changes) by providers in the Athena Breast Health Network and in the WISDOM (Women Informed to Screen Depending on Measures of risk) Study, a randomized trial of personalized versus annual breast cancer screening that uses the BCSC model as well as genetic predisposition (mutations and polygenic risk).[ 21 , 22 ] Women in the personalized arm who are in the top 2.5% of risk for their age are assigned to annual screening and active outreach for risk reduction counseling; those whose 5-year risk is over 6% get screening every 6 months, alternating annual mammography with annual MRI.
Future work should aim to validate whether the top 2.5% by age threshold is associated with a similar increase in the likelihood of interval cancers in large cohort studies. These studies may also determine that a different sensitivity is optimal, such as top 1% or 5% by age. Cohort studies should ideally be powered to compare alternative risk-stratification criteria and examine the link between BCSC score and other features of aggressiveness, such as HER2 positivity, triple-negative/basal subtype, or high grade or proliferation.
Implications
Breast cancer patients whose BCSC risk, at the time they were diagnosed with breast cancer, was in the top 2.5% of predicted breast cancer risk for their age are significantly more likely to have their cancers detected in the interval between screening mammograms. These interval cancers were more likely to be higher grade and later stage, and thus may be linked to poor prognosis. Women in this elevated-risk category may benefit from tailored screening strategies or preventative interventions such as chemoprevention. A prospective validation is underway in the WISDOM study.
Supplementary Material
Acknowledgments.
We are extremely grateful to Karla Kerlikowske and her team at the San Francisco Mammography Registry (SFMR) for their guidance contextualizing this research and their willingness to collaborate. The SFMR provided access to data that was not ultimately used in this study. We would also like to thank Ann Griffin from the UCSF Cancer Registry and Patrick Wang from the UCSF Breast Care Center Internship Program. Data collection and sharing was supported by the National Cancer Institute-funded Breast Cancer Surveillance Consortium (HHSN261201100031C). You can learn more about the BCSC at: http://www.bcsc-research.org/ . Yiwey Shieh was supported by funding from the National Cancer Institute (1K08CA237829) and the MCL consortium. Dr. Esserman is supported by funding from the NCI MCL consortium (U01CA196406). We would also like to thank the dedicated Athena investigators and advocates for their continued work and support.
Yiwey Shieh was supported by funding from the National Cancer Institute (1K08CA237829) and the MCL consortium. Laura Esserman is supported by funding from the NCI MCL consortium (U01CA196406).
Conflicts of Interest: The authors declare no potential conflicts of interest.
Ethics approval: This work was approved by the UCSF Institutional Review Board and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate: All participants consented to have their data used for research that may result in publication.
Consent for publication: All participants consented to have their data used for research that may result in publication.
Availability of data and material: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability: Code used in this analysis will be made available from the corresponding author on reasonable request.

COMMENTS
A widely accepted evidence-based treatment approach used in patients with early HER2-positive breast cancer is surgery, followed by adjuvant therapy, for patients with clinical stage T1N0 disease ...
The most common psychiatric co-morbidities in breast cancer patients are anxiety and depression [36,37]. Patients with breast cancer may experience anxiety and/or depression at any stage of the disease. Dastan and Buzlu reported that 35% of female patients with breast cancer experienced anxiety. In this case, the support that the patient ...
Patient Case Presentation. Patient Mrs. B.C. is a 56 year old female who is presenting to her WHNP for her annual exam. She had to cancel her appointment two months ago and didn't reschedule until now. Her last pap smear and mammogram were normal. Today, while performing her breast exam, her nurse practitioner notices dimpling in the left ...
This study presents a case of a female patient with initial triple-negative breast cancer who transformed to ER, PR, and HER2-positive status after recurrence and metastasis. Consequently, she underwent systematic treatment, including chemotherapy, targeted therapy, cranial radiotherapy, and maintenance treatment.
This is a qualitative study in which 21 women with breast cancer or survivors were interviewed. Participants were recruited at 9 large hospitals in Spain and intentional sampling methods were applied. Data were collected using a semi-structured interview that was elaborated with the help of medical oncologists, nurses, and psycho-oncologists.
Methods: This is a qualitative study in which 21 women with breast cancer or survivors were interviewed. Participants were recruited at 9 large hospitals in Spain and intentional sampling methods were applied. Data were collected using a semi-structured interview that was elaborated with the help of medical oncologists, nurses, and psycho ...
Case Report Open Access 26 May 2021 Potential of ultra-high-resolution photon-counting CT of bone metastases: initial experiences in breast cancer patients E. Wehrse
Materials and methods. This prospective, cross-sectional, case-control study was conducted at a single center. The inclusion criteria were operable breast cancer patients treated at Hangzhou ...
study was conducted on 392 women who underwent breast cancer surgery at Hangzhou Cancer Hospital from January 1, 2013, to December 31, 2022. Operable breast cancer patients who had
Ruta Rao, MD, presents the case of a 36-year-old woman with metastatic HER2+ breast cancer and brain metastases. News. ... Findings suggest that patients with breast cancer and consistently negative circulating tumor DNA tests tend to have improved clinical outcomes.
example, in a series of 8422 patients enrolled on International Breast Cancer Study Group trials between 1978 and 1999, the rate of node-negativity for medial compared to lateral/central tumors was 44 versus 33 percent, respectively. The most likely explanation for this difference is preferential drainage of some medial tumors to the IM nodes.
Adam M. Brufsky, MD, PhD: Let's talk about this case. This is a 48-year-old woman who presented to her primary care physician a number of years ago with a lump in her breast. She had a 4.4-cm left breast mass and 3 palpable axillary lymph nodes. Her ultrasound and mammogram confirmed these physical findings.
Hepin* had been diagnosed with triple negative breast cancer late in 2014, before going on to have surgery. Her treatment was initially successful, and for a number of years she led an active lifestyle. But in May 2018 she started to notice a change. 'I was feeling more tired than usual - yawning and flagging easily,' she explains.
Background. Since the onset of the pandemic, breast cancer (BC) services have been disrupted in most countries. The purpose of this qualitative study is to explore the unmet needs, patient-priorities, and recommendations for improving BC healthcare post-pandemic for women with BC and to understand how they may vary based on social determinants of health (SDH), in particular socio-economic ...
Abstract. Breast cancer is Malignment cell growth in the breast. If the cancer isn't treated, it will spread to other parts of the body. Except for skin cancer, the most frequent type of cancer among women is breast cancer. Mammograms can detect breast cancer early, possibly before it has spread. Main symptoms and/or important clinical findings: A 58-year-old female patient registered to the ...
1 Introduction. Breast cancer remains the most common cancer diagnosed among women in the United States and is the second leading cause of cancer-related deaths, with approximately 41,000 patients projected to die from this disease in 2018 alone. The prognosis for patients with metastatic breast cancer varies based on many factors including estrogen receptor (ER), progesterone receptor (PR ...
Video. Adam Brufsky, MD, PhD, presents the case of a 37-year-old woman with HER2+/HR- metastatic breast cancer and polls the audience about screening for brain metastases. Now Viewing. EP: 1. Patient Case: A 37-Year-Old Woman With HER2+/HR- Breast Cancer. EP: 2. Management Approaches for Patients with HER2+ BC at Risk for Brain Metastases. EP: 3.
Some studies have small sample sizes, limited quality, and lack research support from evidence-based medicine, so there is a need to assess the impact of CM on breast cancer patients through systematic evaluation and meta-analysis. 2 Objectives. To explore the impact of CM on breast cancer patients and provide evidence-based clinical care support.
This patient's breast cancer is negative for ER and PR. Immunohistochemistry staining results for HER-2 are shown in Figure 3. HER-2 IHC is scored as 2+ (equivocal) for HER-2, demonstrating weak to moderate complete membrane staining in >10% of tumor cells. Due to this result, the sample is tested reflexively by FISH.
The present study reports the case of a patient with early-stage lobular breast cancer, with a detectable ctDNA test which resolved with local radiotherapy to the breast. This case suggests that ctDNA is sensitive enough to detect the response of minimal residual disease, localized in the breast, to radiation therapy, and thus may assist in ...
Background Breast cancer is one of the most prevalent diseases in women. Prevention and treatments have lowered mortality; nevertheless, the impact of the diagnosis and treatment continue to impact all aspects of patients' lives (physical, emotional, cognitive, social, and spiritual). Objective This study seeks to explore the experiences of the different stages women with breast cancer go ...
Outline. Natasha is a 32-year-old female African American patient arriving at the surgery oncology unit status post left breast mastectomy and lymph node excision. She arrives from the post-anesthesia unit (PACU) via hospital bed with her spouse, Angelica, at the bedside. They explain that a self-exam revealed a lump, and, after mammography and ...
Spontaneous regression (SR) of cancer is a rare but well-documented biological phenomenon. SR is defined as "the partial or complete disappearance of a tumor in the absence of any treatment capable of regression" [1, 2].Breast cancer regression was reported in 43/741 cases of spontaneously regressing cancers compiled and summarized by Challis and Stam in a review of the period from 1900 to ...
Abstract and Figures. In this case study, a women aged 40 was diagnosed with Metastatic breast cancer. Metastatic breast cancer is a complex multi-stage disease involving the expansion of ...
There is little evidence that dietary supplements are beneficial for patients with breast cancer; therefore, they are usually not recommended by treatment guidelines. The aim of the present analysis was to assess the prevalence of dietary supplement (DS) intake among women before and after a breast cancer diagnosis. Participants in the SUCCESS C lifestyle intervention study, a randomized ...
In the May issue of the Bulletin of the American College of Surgeons, authors from the NAPBC and ACS Cancer Research Program discuss the Patient-Reported Observations on Medical Procedure Timeliness for Breast Patients (PROMPT) study.PROMPT was a two-year quality collaborative that examined the time interval from screening to diagnosis to first treatment for patients with breast disease.
The clinical survival data were obtained from TCGA pan-cancer atlas. Additional expression profile data were obtained from the Molecular Taxonomy of Breast Cancer International Consortium (METABRIC) and Gene Expression Omnibus (GEO) database . Our study excluded patients who had a survival time of less than 30 days or missing data.
In this study, we compared breast cancer patients in the BCSC top 2.5% of risk for their age to patients from the remaining 97.5%. We found that women in the top 2.5% of risk for their age, who have double the risk of getting breast cancer relative to the average women, had more than six-fold higher odds of presenting with interval cancers ...
Objectives: Several studies have indicated that dietary interventions may offer protection against the development of cardiac damage in the case of anthracycline-induced cardiomyopathy (AIC). The goal of this study was to assess whether an evidence-based cardioprotective diet can be effective in preventing AIC in patients with breast cancer. Design: Randomized, open-label, controlled trial ...
There is an indication for a well-designed case-control study using the duplex ultrasound neck vein examination, comparing patients treated for breast cancer with healthy women. METHODS The venous drainage of the breasts is described and the cerebrospinal venous systems (CSVS) of six patients with treated breast cancer were assessed using duplex ultrasound to measure venous blood volume flows ...