Browse Course Material
Course info.
- Prof. Donald Sadoway

Departments
- Materials Science and Engineering
As Taught In
- Chemical Engineering
Learning Resource Types
Introduction to solid state chemistry, 26. acids & bases.
« Previous | Next »
Session Overview
Prerequisites.
Before starting this session, you should be familiar with:
- the Molecules and Bonding module ( Session 7 through Session 12 )
- Solutions ( Session 25 )
Learning Objectives
After completing this session, you should be able to:
- Compare the acid-base models of Arrhenius, Brønsted-Lowry, and Lewis, and know the salient features of each.
- Write the general acid-base reaction.
- Explain the chemical basis of acid strength and the pH measurement.
- Describe the behaviors of conjugate acid-base pairs.
- Explain how dissociation affects ionic compounds.
- Solve specific acid-base reaction problems.
Lecture Video
- Download video
- Download transcript
Lecture Slides (PDF - 2.2MB)
Lecture Summary
This lecture introduces the chemical models and behaviors of acids and bases. Starting from the historical origins (“acid” derives from the Latin acidus , meaning “sour”), Prof. Sadoway discusses the evolving acid-base models of Lavoisier (1776), Arrhenius (1887), Brønsted and Lowry (1923), and Lewis (1923-1938).
The lecture proceeds to cover:
- The general acid-base reaction
- Conjugate acid-base pairs
- The dissociation process of ionic compounds
- Solving acid-base reaction problems
Problems (PDF)
Solutions (PDF)
Textbook Problems
For further study, supplemental readings.
Djerassi, C., and R. Hoffmann. Oxygen: A Play in Two Acts . New York, NY: Wiley-VCH, 2001. ISBN: 9783527304134. See also the study guide and other publisher resources .
Brandis, Kerry. Acid-Base Physiology . See Chapter 1 of this online tutorial/textbook, which applies acid-base chemistry to physiology.
Antoine Lavoisier
Svante Arrhenius — 1903 Nobel Prize in Chemistry
Johannes Nicolaus Brønsted
Martin Lowry
Gilbert N. Lewis
Other OCW and OER Content

You are leaving MIT OpenCourseWare
Find what you need to study
8.1 Introduction to Acids and Bases
3 min read • january 8, 2023
Jillian Holbrook
Attend a live cram event
Review all units live with expert teachers & students

Types of Acids and Bases
Arrhenius definition.
There are two main schools of thought for what should be the definitions of acids and bases. The Arrhenius definition categorizes an acid as any compound that increases the concentration of hydrogen ions ([H+]) in a solution. Conversely, the Arrhenius definition of a base is any compound that increases the concentration of hydroxide ions ([OH-]) in solution. Essentially, the Arrhenius definition of an acid/base is anything that yields H+ or OH- respectively in water.
For example, HCl ( hydrochloric acid ) can be described as an Arrhenius acid because, in water, the following reaction occurs:
HCl → H+ + Cl-
Thus, HCl yields an H+ ion in water, making it an Arrhenius acid.
The concentrations of hydronium ions and hydroxide ions are often reported as pH and pOH , respectively:
pH = −log[H3O+]
pOH = −log[OH−]
pH of Water
Water autoionizes , meaning a proton is transferred from one water molecule to another to produce a hydronium ion (H₃O⁺) and a hydroxide ion (OH⁻), with the equilibrium constant Kw :
Kw = [H3O+][OH−] = 1.0 × 10−14 at 25°C In pure water, pH = pOH , making it a neutral solution because pH = pOH = 7.0. However, the value of Kw is temperature dependent, so the pH of pure, neutral water deviates from 7.0 at temperatures other than 25ºC.
Brønsted-Lowry Definition
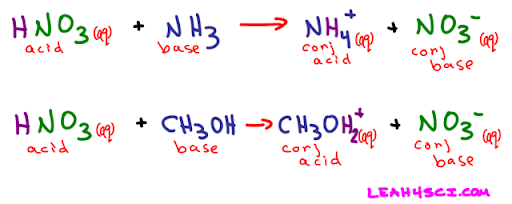
Meanwhile, the Brønsted-Lowry definition of acids and bases defines acids and bases in the form of a donation reaction . Basically, an acid/base is seen as an H+ donator or accepter (the acid donates, the base accepts):
HA + B- → HB + A-
In this example, HA is the acid, which donates an H+ ion to the B- ion (the base) to form HB and A-.
Get used to seeing HA and B- as sample acids and bases; it is a common notation! A and B could be stand-ins for any number of ions.
Quick thinkers may be wondering about the reverse reaction in the case that HA + B- is an equilibrium reaction :
HA + B- ⇄ HB + A-

The Hydronium Ion
A consequence of Bronsted Acids and Bases is that when an acid dissolves in water, it needs something to donate its H+ to! In this case, it donates it to water, creating H3O+ or hydronium . Thus, we can see the dissolution of an acid both in an Arrhenius sense as HA <--> H+ + A- and a Bronsted sense as HA + H2O <--> H3O+ + A-. Both of these essentially mean the same thing (and when we learn pH , we'll find that [H+] = [H3O+]), but they display the difference between Arrhenius and Bronsted Acids.
Conjugate Acids and Bases
How do we classify HB and A- then? Would A- be a base and HB be an acid if the reaction were reversed? The answer is short: yes. However, they are given a special name: HB is called the conjugate acid of B-, and A- is called the conjugate base of HA.
In the case that HA is a weak acid and B- is a weak base , HB and A- would be considered to have significant acidity/basicity. The conjugate acid/base of a strong base/acid does not have acidity/basicity . The weaker the acid/base, the stronger the conjugate base/acid.
Key Terms to Review ( 15 )
Bronsted Acids and Bases
Donation Reaction
Equilibrium Constant Kw
Hydrochloric Acid
Hydronium Ion
Neutral Solution
Proton Transfer
Strong Acid/Base
Water Autoionizes

Stay Connected
© 2024 Fiveable Inc. All rights reserved.
AP® and SAT® are trademarks registered by the College Board, which is not affiliated with, and does not endorse this website.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
High school chemistry
Course: high school chemistry > unit 7.
- Quiz 1 Solutions, acids, and bases
- TPC and eLearning
- What's NEW at TPC?
- Read Watch Interact
- Practice Review Test
- Teacher-Tools
- Subscription Selection
- Seat Calculator
- Ad Free Account
- Edit Profile Settings
- Classes (Version 2)
- Student Progress Edit
- Task Properties
- Export Student Progress
- Task, Activities, and Scores
- Metric Conversions Questions
- Metric System Questions
- Metric Estimation Questions
- Significant Digits Questions
- Proportional Reasoning
- Acceleration
- Distance-Displacement
- Dots and Graphs
- Graph That Motion
- Match That Graph
- Name That Motion
- Motion Diagrams
- Pos'n Time Graphs Numerical
- Pos'n Time Graphs Conceptual
- Up And Down - Questions
- Balanced vs. Unbalanced Forces
- Change of State
- Force and Motion
- Mass and Weight
- Match That Free-Body Diagram
- Net Force (and Acceleration) Ranking Tasks
- Newton's Second Law
- Normal Force Card Sort
- Recognizing Forces
- Air Resistance and Skydiving
- Solve It! with Newton's Second Law
- Which One Doesn't Belong?
- Component Addition Questions
- Head-to-Tail Vector Addition
- Projectile Mathematics
- Trajectory - Angle Launched Projectiles
- Trajectory - Horizontally Launched Projectiles
- Vector Addition
- Vector Direction
- Which One Doesn't Belong? Projectile Motion
- Forces in 2-Dimensions
- Being Impulsive About Momentum
- Explosions - Law Breakers
- Hit and Stick Collisions - Law Breakers
- Case Studies: Impulse and Force
- Impulse-Momentum Change Table
- Keeping Track of Momentum - Hit and Stick
- Keeping Track of Momentum - Hit and Bounce
- What's Up (and Down) with KE and PE?
- Energy Conservation Questions
- Energy Dissipation Questions
- Energy Ranking Tasks
- LOL Charts (a.k.a., Energy Bar Charts)
- Match That Bar Chart
- Words and Charts Questions
- Name That Energy
- Stepping Up with PE and KE Questions
- Case Studies - Circular Motion
- Circular Logic
- Forces and Free-Body Diagrams in Circular Motion
- Gravitational Field Strength
- Universal Gravitation
- Angular Position and Displacement
- Linear and Angular Velocity
- Angular Acceleration
- Rotational Inertia
- Balanced vs. Unbalanced Torques
- Getting a Handle on Torque
- Torque-ing About Rotation
- Properties of Matter
- Fluid Pressure
- Buoyant Force
- Sinking, Floating, and Hanging
- Pascal's Principle
- Flow Velocity
- Bernoulli's Principle
- Balloon Interactions
- Charge and Charging
- Charge Interactions
- Charging by Induction
- Conductors and Insulators
- Coulombs Law
- Electric Field
- Electric Field Intensity
- Polarization
- Case Studies: Electric Power
- Know Your Potential
- Light Bulb Anatomy
- I = ∆V/R Equations as a Guide to Thinking
- Parallel Circuits - ∆V = I•R Calculations
- Resistance Ranking Tasks
- Series Circuits - ∆V = I•R Calculations
- Series vs. Parallel Circuits
- Equivalent Resistance
- Period and Frequency of a Pendulum
- Pendulum Motion: Velocity and Force
- Energy of a Pendulum
- Period and Frequency of a Mass on a Spring
- Horizontal Springs: Velocity and Force
- Vertical Springs: Velocity and Force
- Energy of a Mass on a Spring
- Decibel Scale
- Frequency and Period
- Closed-End Air Columns
- Name That Harmonic: Strings
- Rocking the Boat
- Wave Basics
- Matching Pairs: Wave Characteristics
- Wave Interference
- Waves - Case Studies
- Color Addition and Subtraction
- Color Filters
- If This, Then That: Color Subtraction
- Light Intensity
- Color Pigments
- Converging Lenses
- Curved Mirror Images
- Law of Reflection
- Refraction and Lenses
- Total Internal Reflection
- Who Can See Who?
- Formulas and Atom Counting
- Atomic Models
- Bond Polarity
- Entropy Questions
- Cell Voltage Questions
- Heat of Formation Questions
- Reduction Potential Questions
- Oxidation States Questions
- Measuring the Quantity of Heat
- Hess's Law
- Oxidation-Reduction Questions
- Galvanic Cells Questions
- Thermal Stoichiometry
- Molecular Polarity
- Quantum Mechanics
- Balancing Chemical Equations
- Bronsted-Lowry Model of Acids and Bases
- Classification of Matter
- Collision Model of Reaction Rates
- Density Ranking Tasks
- Dissociation Reactions
- Complete Electron Configurations
- Elemental Measures
- Enthalpy Change Questions
- Equilibrium Concept
- Equilibrium Constant Expression
- Equilibrium Calculations - Questions
- Equilibrium ICE Table
- Intermolecular Forces Questions
- Ionic Bonding
- Lewis Electron Dot Structures
- Limiting Reactants
- Line Spectra Questions
- Mass Stoichiometry
- Measurement and Numbers
- Metals, Nonmetals, and Metalloids
- Metric Estimations
- Metric System
- Molarity Ranking Tasks
- Mole Conversions
- Name That Element
- Names to Formulas
- Names to Formulas 2
- Nuclear Decay
- Particles, Words, and Formulas
- Periodic Trends
- Precipitation Reactions and Net Ionic Equations
- Pressure Concepts
- Pressure-Temperature Gas Law
- Pressure-Volume Gas Law
- Chemical Reaction Types
- Significant Digits and Measurement
- States Of Matter Exercise
- Stoichiometry Law Breakers
- Stoichiometry - Math Relationships
- Subatomic Particles
- Spontaneity and Driving Forces
- Gibbs Free Energy
- Volume-Temperature Gas Law
- Acid-Base Properties
- Energy and Chemical Reactions
- Chemical and Physical Properties
- Valence Shell Electron Pair Repulsion Theory
- Writing Balanced Chemical Equations
- Mission CG1
- Mission CG10
- Mission CG2
- Mission CG3
- Mission CG4
- Mission CG5
- Mission CG6
- Mission CG7
- Mission CG8
- Mission CG9
- Mission EC1
- Mission EC10
- Mission EC11
- Mission EC12
- Mission EC2
- Mission EC3
- Mission EC4
- Mission EC5
- Mission EC6
- Mission EC7
- Mission EC8
- Mission EC9
- Mission RL1
- Mission RL2
- Mission RL3
- Mission RL4
- Mission RL5
- Mission RL6
- Mission KG7
- Mission RL8
- Mission KG9
- Mission RL10
- Mission RL11
- Mission RM1
- Mission RM2
- Mission RM3
- Mission RM4
- Mission RM5
- Mission RM6
- Mission RM8
- Mission RM10
- Mission LC1
- Mission RM11
- Mission LC2
- Mission LC3
- Mission LC4
- Mission LC5
- Mission LC6
- Mission LC8
- Mission SM1
- Mission SM2
- Mission SM3
- Mission SM4
- Mission SM5
- Mission SM6
- Mission SM8
- Mission SM10
- Mission KG10
- Mission SM11
- Mission KG2
- Mission KG3
- Mission KG4
- Mission KG5
- Mission KG6
- Mission KG8
- Mission KG11
- Mission F2D1
- Mission F2D2
- Mission F2D3
- Mission F2D4
- Mission F2D5
- Mission F2D6
- Mission KC1
- Mission KC2
- Mission KC3
- Mission KC4
- Mission KC5
- Mission KC6
- Mission KC7
- Mission KC8
- Mission AAA
- Mission SM9
- Mission LC7
- Mission LC9
- Mission NL1
- Mission NL2
- Mission NL3
- Mission NL4
- Mission NL5
- Mission NL6
- Mission NL7
- Mission NL8
- Mission NL9
- Mission NL10
- Mission NL11
- Mission NL12
- Mission MC1
- Mission MC10
- Mission MC2
- Mission MC3
- Mission MC4
- Mission MC5
- Mission MC6
- Mission MC7
- Mission MC8
- Mission MC9
- Mission RM7
- Mission RM9
- Mission RL7
- Mission RL9
- Mission SM7
- Mission SE1
- Mission SE10
- Mission SE11
- Mission SE12
- Mission SE2
- Mission SE3
- Mission SE4
- Mission SE5
- Mission SE6
- Mission SE7
- Mission SE8
- Mission SE9
- Mission VP1
- Mission VP10
- Mission VP2
- Mission VP3
- Mission VP4
- Mission VP5
- Mission VP6
- Mission VP7
- Mission VP8
- Mission VP9
- Mission WM1
- Mission WM2
- Mission WM3
- Mission WM4
- Mission WM5
- Mission WM6
- Mission WM7
- Mission WM8
- Mission WE1
- Mission WE10
- Mission WE2
- Mission WE3
- Mission WE4
- Mission WE5
- Mission WE6
- Mission WE7
- Mission WE8
- Mission WE9
- Vector Walk Interactive
- Name That Motion Interactive
- Kinematic Graphing 1 Concept Checker
- Kinematic Graphing 2 Concept Checker
- Graph That Motion Interactive
- Two Stage Rocket Interactive
- Rocket Sled Concept Checker
- Force Concept Checker
- Free-Body Diagrams Concept Checker
- Free-Body Diagrams The Sequel Concept Checker
- Skydiving Concept Checker
- Elevator Ride Concept Checker
- Vector Addition Concept Checker
- Vector Walk in Two Dimensions Interactive
- Name That Vector Interactive
- River Boat Simulator Concept Checker
- Projectile Simulator 2 Concept Checker
- Projectile Simulator 3 Concept Checker
- Hit the Target Interactive
- Turd the Target 1 Interactive
- Turd the Target 2 Interactive
- Balance It Interactive
- Go For The Gold Interactive
- Egg Drop Concept Checker
- Fish Catch Concept Checker
- Exploding Carts Concept Checker
- Collision Carts - Inelastic Collisions Concept Checker
- Its All Uphill Concept Checker
- Stopping Distance Concept Checker
- Chart That Motion Interactive
- Roller Coaster Model Concept Checker
- Uniform Circular Motion Concept Checker
- Horizontal Circle Simulation Concept Checker
- Vertical Circle Simulation Concept Checker
- Race Track Concept Checker
- Gravitational Fields Concept Checker
- Orbital Motion Concept Checker
- Angular Acceleration Concept Checker
- Balance Beam Concept Checker
- Torque Balancer Concept Checker
- Aluminum Can Polarization Concept Checker
- Charging Concept Checker
- Name That Charge Simulation
- Coulomb's Law Concept Checker
- Electric Field Lines Concept Checker
- Put the Charge in the Goal Concept Checker
- Circuit Builder Concept Checker (Series Circuits)
- Circuit Builder Concept Checker (Parallel Circuits)
- Circuit Builder Concept Checker (∆V-I-R)
- Circuit Builder Concept Checker (Voltage Drop)
- Equivalent Resistance Interactive
- Pendulum Motion Simulation Concept Checker
- Mass on a Spring Simulation Concept Checker
- Particle Wave Simulation Concept Checker
- Boundary Behavior Simulation Concept Checker
- Slinky Wave Simulator Concept Checker
- Simple Wave Simulator Concept Checker
- Wave Addition Simulation Concept Checker
- Standing Wave Maker Simulation Concept Checker
- Color Addition Concept Checker
- Painting With CMY Concept Checker
- Stage Lighting Concept Checker
- Filtering Away Concept Checker
- InterferencePatterns Concept Checker
- Young's Experiment Interactive
- Plane Mirror Images Interactive
- Who Can See Who Concept Checker
- Optics Bench (Mirrors) Concept Checker
- Name That Image (Mirrors) Interactive
- Refraction Concept Checker
- Total Internal Reflection Concept Checker
- Optics Bench (Lenses) Concept Checker
- Kinematics Preview
- Velocity Time Graphs Preview
- Moving Cart on an Inclined Plane Preview
- Stopping Distance Preview
- Cart, Bricks, and Bands Preview
- Fan Cart Study Preview
- Friction Preview
- Coffee Filter Lab Preview
- Friction, Speed, and Stopping Distance Preview
- Up and Down Preview
- Projectile Range Preview
- Ballistics Preview
- Juggling Preview
- Marshmallow Launcher Preview
- Air Bag Safety Preview
- Colliding Carts Preview
- Collisions Preview
- Engineering Safer Helmets Preview
- Push the Plow Preview
- Its All Uphill Preview
- Energy on an Incline Preview
- Modeling Roller Coasters Preview
- Hot Wheels Stopping Distance Preview
- Ball Bat Collision Preview
- Energy in Fields Preview
- Weightlessness Training Preview
- Roller Coaster Loops Preview
- Universal Gravitation Preview
- Keplers Laws Preview
- Kepler's Third Law Preview
- Charge Interactions Preview
- Sticky Tape Experiments Preview
- Wire Gauge Preview
- Voltage, Current, and Resistance Preview
- Light Bulb Resistance Preview
- Series and Parallel Circuits Preview
- Thermal Equilibrium Preview
- Linear Expansion Preview
- Heating Curves Preview
- Electricity and Magnetism - Part 1 Preview
- Electricity and Magnetism - Part 2 Preview
- Vibrating Mass on a Spring Preview
- Period of a Pendulum Preview
- Wave Speed Preview
- Slinky-Experiments Preview
- Standing Waves in a Rope Preview
- Sound as a Pressure Wave Preview
- DeciBel Scale Preview
- DeciBels, Phons, and Sones Preview
- Sound of Music Preview
- Shedding Light on Light Bulbs Preview
- Models of Light Preview
- Electromagnetic Radiation Preview
- Electromagnetic Spectrum Preview
- EM Wave Communication Preview
- Digitized Data Preview
- Light Intensity Preview
- Concave Mirrors Preview
- Object Image Relations Preview
- Snells Law Preview
- Reflection vs. Transmission Preview
- Magnification Lab Preview
- Reactivity Preview
- Ions and the Periodic Table Preview
- Periodic Trends Preview
- Intermolecular Forces Preview
- Melting Points and Boiling Points Preview
- Reaction Rates Preview
- Ammonia Factory Preview
- Stoichiometry Preview
- Nuclear Chemistry Preview
- Gaining Teacher Access
- Tasks and Classes
- Tasks - Classic
- Subscription
- Subscription Locator
- 1-D Kinematics
- Newton's Laws
- Vectors - Motion and Forces in Two Dimensions
- Momentum and Its Conservation
- Work and Energy
- Circular Motion and Satellite Motion
- Thermal Physics
- Static Electricity
- Electric Circuits
- Vibrations and Waves
- Sound Waves and Music
- Light and Color
- Reflection and Mirrors
- About the Physics Interactives
- Task Tracker
- Usage Policy
- Newtons Laws
- Vectors and Projectiles
- Forces in 2D
- Momentum and Collisions
- Circular and Satellite Motion
- Balance and Rotation
- Electromagnetism
- Waves and Sound
- Atomic Physics
- Forces in Two Dimensions
- Work, Energy, and Power
- Circular Motion and Gravitation
- Sound Waves
- 1-Dimensional Kinematics
- Circular, Satellite, and Rotational Motion
- Einstein's Theory of Special Relativity
- Waves, Sound and Light
- QuickTime Movies
- About the Concept Builders
- Pricing For Schools
- Directions for Version 2
- Measurement and Units
- Relationships and Graphs
- Rotation and Balance
- Vibrational Motion
- Reflection and Refraction
- Teacher Accounts
- Task Tracker Directions
- Kinematic Concepts
- Kinematic Graphing
- Wave Motion
- Sound and Music
- About CalcPad
- 1D Kinematics
- Vectors and Forces in 2D
- Simple Harmonic Motion
- Rotational Kinematics
- Rotation and Torque
- Rotational Dynamics
- Electric Fields, Potential, and Capacitance
- Transient RC Circuits
- Light Waves
- Units and Measurement
- Stoichiometry
- Molarity and Solutions
- Thermal Chemistry
- Acids and Bases
- Kinetics and Equilibrium
- Solution Equilibria
- Oxidation-Reduction
- Nuclear Chemistry
- NGSS Alignments
- 1D-Kinematics
- Projectiles
- Circular Motion
- Magnetism and Electromagnetism
- Graphing Practice
- About the ACT
- ACT Preparation
- For Teachers
- Other Resources
- Newton's Laws of Motion
- Work and Energy Packet
- Static Electricity Review
- Solutions Guide
- Solutions Guide Digital Download
- Motion in One Dimension
- Work, Energy and Power
- TaskTracker
- Other Tools
- Algebra Based Physics
- Frequently Asked Questions
- Purchasing the Download
- Purchasing the CD
- Purchasing the Digital Download
- About the NGSS Corner
- NGSS Search
- Force and Motion DCIs - High School
- Energy DCIs - High School
- Wave Applications DCIs - High School
- Force and Motion PEs - High School
- Energy PEs - High School
- Wave Applications PEs - High School
- Crosscutting Concepts
- The Practices
- Physics Topics
- NGSS Corner: Activity List
- NGSS Corner: Infographics
- About the Toolkits
- Position-Velocity-Acceleration
- Position-Time Graphs
- Velocity-Time Graphs
- Newton's First Law
- Newton's Second Law
- Newton's Third Law
- Terminal Velocity
- Projectile Motion
- Forces in 2 Dimensions
- Impulse and Momentum Change
- Momentum Conservation
- Work-Energy Fundamentals
- Work-Energy Relationship
- Roller Coaster Physics
- Satellite Motion
- Electric Fields
- Circuit Concepts
- Series Circuits
- Parallel Circuits
- Describing-Waves
- Wave Behavior Toolkit
- Standing Wave Patterns
- Resonating Air Columns
- Wave Model of Light
- Plane Mirrors
- Curved Mirrors
- Teacher Guide
- Using Lab Notebooks
- Current Electricity
- Light Waves and Color
- Reflection and Ray Model of Light
- Refraction and Ray Model of Light
- Classes (Legacy Version)
- Teacher Resources
- Subscriptions

- Newton's Laws
- Einstein's Theory of Special Relativity
- About Concept Checkers
- School Pricing
- Newton's Laws of Motion
- Newton's First Law
- Newton's Third Law
Chemistry: Acids and Bases
Acids and Bases
Assignment 1: acids and bases @ home.

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

7.1: Acid-Base Buffers
- Last updated
- Save as PDF
- Page ID 164771

Skills to Develop
- Describe the composition and function of acid–base buffers
- Calculate the pH of a buffer before and after the addition of added acid or base using the Henderson-Hasselbalch approximation
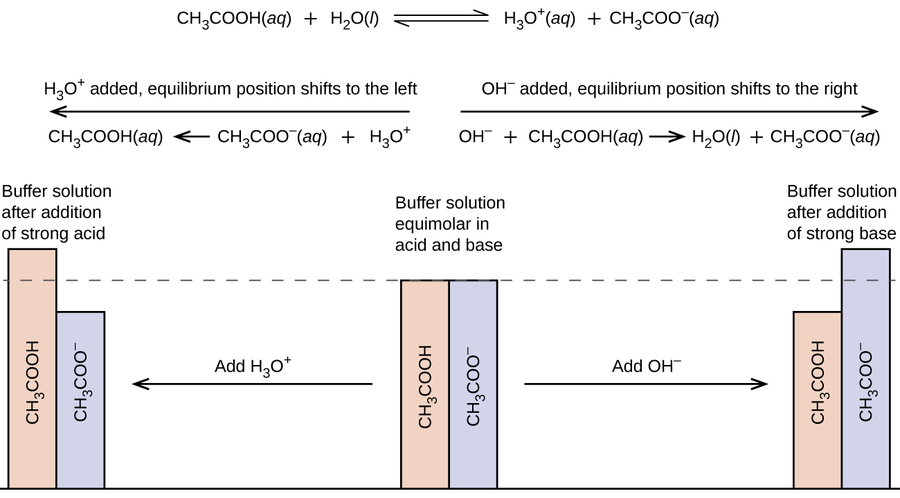
A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer . Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure \(\PageIndex{1}\)). A solution of acetic acid (\(\ce{CH3COOH}\) and sodium acetate \(\ce{CH3COONa}\)) is an example of a buffer that consists of a weak acid and its salt. An example of a buffer that consists of a weak base and its salt is a solution of ammonia (\(\ce{NH3(aq)}\)) and ammonium chloride (\(\ce{NH4Cl(aq)}\)).

Figure \(\PageIndex{1}\): (a) The unbuffered solution on the left and the buffered solution on the right have the same pH (pH 8); they are basic, showing the yellow color of the indicator methyl orange at this pH. (b) After the addition of 1 mL of a 0.01-M HCl solution, the buffered solution has not detectably changed its pH but the unbuffered solution has become acidic, as indicated by the change in color of the methyl orange, which turns red at a pH of about 4. (credit: modification of work by Mark Ott)
How Buffers Work
In order for a buffer to "resist" the effect of adding strong acid or strong base, it must have both an acidic and a basic component. However, you cannot mix any two acid/base combination together and get a buffer. If you mix HCl and NaOH, for example, you will simply neutralize the acid with the base and obtain a neutral salt, not a buffer. For a buffer to work, both the acid and the base component must be part of the same equilibrium system - that way, neutralizing one or the other component (by adding strong acid or base) will transform it into the other component, and maintain the buffer mixture.
Therefore, a buffer must consist of a mixture of a weak conjugate acid-base pair.
The pH a buffer maintains is determined by the nature of the conjugate pair and the concentrations of both components. A mixture of acetic acid and sodium acetate is acidic because the K a of acetic acid is greater than the K b of its conjugate base acetate. It is a buffer because it contains both the weak acid and its salt. Hence, it acts to keep the hydronium ion concentration (and the pH) almost constant by the addition of either a small amount of a strong acid or a strong base. If we add a base such as sodium hydroxide, the hydroxide ions react with the few hydronium ions present. Then more of the acetic acid reacts with water, restoring the hydronium ion concentration almost to its original value:
The pH changes very little. If we add an acid such as hydrochloric acid, most of the hydronium ions from the hydrochloric acid combine with acetate ions, forming acetic acid molecules:
Thus, there is very little increase in the concentration of the hydronium ion, and the pH remains practically unchanged (Figure \(\PageIndex{2}\)).
A mixture of ammonia and ammonium chloride is basic because the K b for ammonia is greater than the K a for the ammonium ion. It is a buffer because it also contains the salt of the weak base. If we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion concentration almost to its original value:
If we add an acid (hydronium ions), ammonia molecules in the buffer mixture react with the hydronium ions to form ammonium ions and reduce the hydronium ion concentration almost to its original value:
The three parts of the following example illustrate the change in pH that accompanies the addition of base to a buffered solution of a weak acid and to an unbuffered solution of a strong acid.
Example \(\PageIndex{1}\): pH Changes in Buffered and Unbuffered Solutions
Acetate buffers are used in biochemical studies of enzymes and other chemical components of cells to prevent pH changes that might change the biochemical activity of these compounds.
- Calculate the pH of an acetate buffer that is a mixture with 0.10 M acetic acid and 0.10 M sodium acetate.
- Calculate the pH after 1.0 mL of 0.10 M NaOH is added to 100 mL of this buffer, giving a solution with a volume of 101 mL.
For comparison, calculate the pH after 1.0 mL of 0.10 M NaOH is added to 100 mL of a solution of an unbuffered solution with a pH of 4.74 (e.g. a 1.8 × 10 −5 - M solution of HCl). The volume of the final solution is 101 mL.
To determine the pH of the buffer solution we use a typical equilibrium calculation (as illustrated in earlier Examples):
\[\ce{CH3CO2H}(aq)+\ce{H2O}(l)⇌\ce{H3O+}(aq)+\ce{CH3CO2-}(aq) \]
The equilibrium constant for CH 3 CO 2 H is not given, so we look it up in Table E1: K a = 1.8 × 10 −5 . With [CH 3 CO 2 H] = \(\ce{[CH3CO2- ]}\) = 0.10 M and [H 3 O + ] = ~0 M , the reaction shifts to the right to form H 3 O + .
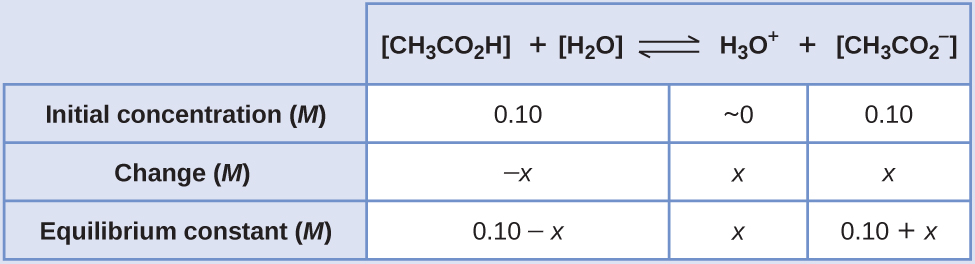
\[x=1.8×10^{−5}\:M\]
\[\ce{[H3O+]}=0+x=1.8×10^{−5}\:M\]
4. Check the work . If we calculate all calculated equilibrium concentrations, we find that the equilibrium value of the reaction coefficient, Q = K a .
(b) Calculate the pH after 1.0 mL of 0.10 M NaOH is added to 100 mL of this buffer, giving a solution with a volume of 101 mL.
First, we calculate the concentrations of an intermediate mixture resulting from the complete reaction between the acid in the buffer and the added base. Then we determine the concentrations of the mixture at the new equilibrium:
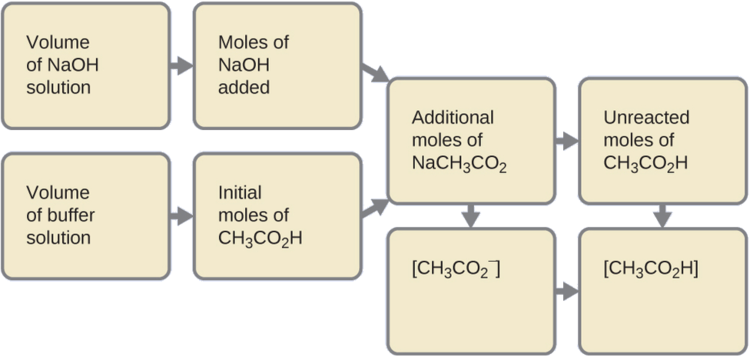
\[\mathrm{0.0010\cancel{L}×\left(\dfrac{0.10\:mol\: NaOH}{1\cancel{L}}\right)=1.0×10^{−4}\:mol\: NaOH} \]
\[\mathrm{0.100\cancel{L}×\left(\dfrac{0.100\:mol\:CH_3CO_2H}{1\cancel{L}}\right)=1.00×10^{−2}\:mol\:CH_3CO_2H} \]
\[\mathrm{(1.0×10^{−2})−(0.01×10^{−2})=0.99×10^{−2}\:mol\:CH_3CO_2H} \]
[\mathrm{(1.0×10^{−2})+(0.01×10^{−2})=1.01×10^{−2}\:mol\:NaCH_3CO_2} \]
4. Find the molarity of the products. After reaction, CH 3 CO 2 H and NaCH 3 CO 2 are contained in 101 mL of the intermediate solution, so:
\[\ce{[NaCH3CO2]}=\mathrm{\dfrac{1.01×10^{−2}\:mol}{0.101\:L}}=0.100\:M \]
Now we calculate the pH after the intermediate solution, which is 0.098 M in CH 3 CO 2 H and 0.100 M in NaCH 3 CO 2 , comes to equilibrium. The calculation is very similar to that in part (a) of this example:

This series of calculations gives a pH = 4.75. Thus the addition of the base barely changes the pH of the solution.
(c) This 1.8 × 10 −5 - M solution of HCl has the same hydronium ion concentration as the 0.10- M solution of acetic acid-sodium acetate buffer described in part (a) of this example. The solution contains:
As shown in part (b), 1 mL of 0.10 M NaOH contains 1.0 × 10 −4 mol of NaOH. When the NaOH and HCl solutions are mixed, the HCl is the limiting reagent in the reaction. All of the HCl reacts, and the amount of NaOH that remains is:
The concentration of NaOH is:
The pOH of this solution is:
The pH changes from 4.74 to 10.99 in this unbuffered solution. This compares to the change of 4.74 to 4.75 that occurred when the same amount of NaOH was added to the buffered solution described in part (b).
Exercise \(\PageIndex{1}\)
Show that adding 1.0 mL of 0.10 M HCl changes the pH of 100 mL of a 1.8 × 10 −5 M HCl solution from 4.74 to 3.00.
Initial pH of 1.8 × 10 −5 M HCl; pH = −log[H 3 O + ] = −log[1.8 × 10 −5 ] = 4.74
Moles of H 3 O + added by addition of 1.0 mL of 0.10 M HCl: 0.10 moles/L × 0.0010 L = 1.0 × 10 −4 moles; final pH after addition of 1.0 mL of 0.10 M HCl:
\[\mathrm{pH=−log[H_3O^+]=−log\left(\dfrac{total\: moles\:H_3O^+}{total\: volume}\right)=−log\left(\dfrac{1.0×10^{−4}\:mol+1.8×10^{−6}\:mol}{101\:mL\left(\dfrac{1\:L}{1000\:mL}\right)}\right)=3.00} \]
The Henderson-Hasselbalch Approximation
We have seen in Example \(\PageIndex{1}\) how the pH of a buffer may be calculated using the ICE table method. The method requires knowing the concentrations of the conjugate acid-base pair and the \(K_a\) or \(K_b\) of the weak acid or weak base. However, there is a simpler method using the same information in a convenient formula, based on a rearrangement of the equilibrium equation for the dissociation of a weak acid.
The simplified ionization reaction of any weak acid is \(HA \leftrightharpoons H^+ + A^−\), for which the equilibrium constant expression is as follows:
This equation can be rearranged as follows:
\[[H^+]=K_a\dfrac{[HA]}{[A^−]} \label{Eq6}\]
Taking the logarithm of both sides and multiplying both sides by −1,
\[ \begin{align} −\log[H^+] &=−\log K_a−\log\left(\dfrac{[HA]}{[A^−]}\right) \\[4pt] &=−\log{K_a}+\log\left(\dfrac{[A^−]}{[HA]}\right) \label{Eq7} \end{align}\]
Replacing the negative logarithms in Equation \(\ref{Eq7}\) to obtain pH, we get,
\[pH=pK_a+\log \left( \dfrac{[A^−]}{[HA]} \right) \label{Eq8}\]
or, more generally,
\[pH=pK_a+\log\left(\dfrac{[base]}{[acid]}\right) \label{Eq9}\]
Equation \(\ref{Eq8}\) and Equation \(\ref{Eq9}\) are both forms of the Henderson-Hasselbalch approximation , named after the two early 20th-century chemists who first noticed that this rearranged version of the equilibrium constant expression provides an easy way to calculate the pH of a buffer solution. In general, the validity of the Henderson-Hasselbalch approximation may be limited to solutions whose concentrations are at least 100 times greater than their \(K_a\) values (the "x is small" assumption).
There are three special cases where the Henderson-Hasselbalch approximation is easily interpreted without the need for calculations:
- \([base] = [acid]\): Under these conditions, \[\dfrac{[base]}{[acid]} = 1\] in Equation \(\ref{Eq9}\). Because \(\log 1 = 0\), \[pH = pK_a\] regardless of the actual concentrations of the acid and base.
- \([base]/[acid] = 10\): In Equation \(\ref{Eq9}\), because \(\log 10 = 1\), \[pH = pK_a + 1.\]
- \([base]/[acid] = 100\): In Equation \(\ref{Eq9}\), because \(\log 100 = 2\), \[pH = pK_a + 2.\]
Each time we increase the [base]/[acid] ratio by 10, the pH of the solution increases by 1 pH unit. Conversely, if the [base]/[acid] ratio is 0.1, then pH = \(pK_a\) − 1. Each additional factor-of-10 decrease in the [base]/[acid] ratio causes the pH to decrease by 1 pH unit.
If [base] = [acid] for a buffer, then pH = \(pK_a\). Changing the ratio by a factor of 10 changes the pH by ±1 unit.
Example \(\PageIndex{2}\)
What is the pH of a solution that contains
- 0.135 M \(HCO_2H\) and 0.215 M \(HCO_2Na\)? (The \(pK_a\) of formic acid is 3.75.)
- 0.0135 M \(HCO_2H\) and 0.0215 M \(HCO_2Na\)?
- 0.119 M pyridine and 0.234 M pyridine hydrochloride? (The \(pK_b\) of pyridine is 8.77.)
Given : concentration of acid, conjugate base, and \(pK_a\); concentration of base, conjugate acid, and \(pK_b\)
Asked for : pH
Substitute values into either form of the Henderson-Hasselbalch approximation (Equation \(\ref{Eq8}\) or Equation \(\ref{Eq9}\)) to calculate the pH.
According to the Henderson-Hasselbalch approximation (Equation \(\ref{Eq8}\)), the pH of a solution that contains both a weak acid and its conjugate base is
\[pH = pK_a + \log([A−]/[HA]).\]
Inserting the given values into the equation,
\[\begin{align*} pH &=3.75+\log\left(\dfrac{0.215}{0.135}\right) \\[4pt] &=3.75+\log 1.593 \\[4pt] &=3.95 \end{align*}\]
This result makes sense because the \([A^−]/[HA]\) ratio is between 1 and 10, so the pH of the buffer must be between the \(pK_a\) (3.75) and \(pK_a + 1\), or 4.75.
This is identical to part (a), except for the concentrations of the acid and the conjugate base, which are 10 times lower. Inserting the concentrations into the Henderson-Hasselbalch approximation,
\[\begin{align*} pH &=3.75+\log\left(\dfrac{0.0215}{0.0135}\right) \\[4pt] &=3.75+\log 1.593 \\[4pt] &=3.95 \end{align*}\]
This result is identical to the result in part (a), which emphasizes the point that the pH of a buffer depends only on the ratio of the concentrations of the conjugate base and the acid, not on the magnitude of the concentrations. Because the [A − ]/[HA] ratio is the same as in part (a), the pH of the buffer must also be the same (3.95).
In this case, we have a weak base, pyridine (Py), and its conjugate acid, the pyridinium ion (\(HPy^+\)). We will therefore use Equation \(\ref{Eq9}\), the more general form of the Henderson-Hasselbalch approximation, in which “base” and “acid” refer to the appropriate species of the conjugate acid–base pair. We are given [base] = [Py] = 0.119 M and \([acid] = [HPy^{+}] = 0.234\, M\). We also are given \(pK_b = 8.77\) for pyridine, but we need \(pK_a\) for the pyridinium ion. Recall that the \(pK_b\) of a weak base and the \(pK_a\) of its conjugate acid are related:
\[pK_a + pK_b = pK_w.\]
Thus \(pK_a\) for the pyridinium ion is \(pK_w − pK_b = 14.00 − 8.77 = 5.23\). Substituting this \(pK_a\) value into the Henderson-Hasselbalch approximation,
\[\begin{align*} pH=pK_a+\log \left(\dfrac{[base]}{[acid]}\right) \\[4pt] &=5.23+\log\left(\dfrac{0.119}{0.234}\right) \\[4pt] & =5.23 −0.294 \\[4pt] &=4.94 \end{align*}\]
Once again, this result makes sense: the \([B]/[BH^+]\) ratio is about 1/2, which is between 1 and 0.1, so the final pH must be between the \(pK_a\) (5.23) and \(pK_a − 1\), or 4.23.
Exercise \(\PageIndex{2}\)
- 0.333 M benzoic acid and 0.252 M sodium benzoate?
- 0.050 M trimethylamine and 0.066 M trimethylamine hydrochloride?
The \(pK_a\) of benzoic acid is 4.20, and the \(pK_b\) of trimethylamine is also 4.20.
The Henderson-Hasselbalch approximation ((Equation \(\ref{Eq8}\)) can also be used to calculate the pH of a buffer solution after adding a given amount of strong acid or strong base, as demonstrated in Example \(\PageIndex{3}\).
Example \(\PageIndex{3}\)
The buffer solution in Example \(\PageIndex{2}\) contained 0.135 M \(HCO_2H\) and 0.215 M \(HCO_2Na\) and had a pH of 3.95.
- What is the final pH if 5.00 mL of 1.00 M \(HCl\) are added to 100 mL of this solution?
- What is the final pH if 5.00 mL of 1.00 M \(NaOH\) are added?
Given : composition and pH of buffer; concentration and volume of added acid or base
Asked for : final pH
- Calculate the amounts of formic acid and formate present in the buffer solution. Then calculate the amount of acid or base added.
- Construct a table showing the amounts of all species after the neutralization reaction. Use the final volume of the solution to calculate the concentrations of all species. Finally, substitute the appropriate values into the Henderson-Hasselbalch approximation (Equation \(\ref{Eq9}\)) to obtain the pH.
The added \(HCl\) (a strong acid) or \(NaOH\) (a strong base) will react completely with formate (a weak base) or formic acid (a weak acid), respectively, to give formic acid or formate and water. We must therefore calculate the amounts of formic acid and formate present after the neutralization reaction.
A We begin by calculating the millimoles of formic acid and formate present in 100 mL of the initial pH 3.95 buffer:
The millimoles of \(H^+\) in 5.00 mL of 1.00 M HCl is as follows:
B Next, we construct an ICE table:
\[HCO^{2−} (aq) + H^+ (aq) \rightarrow HCO_2H (aq) \]
The final amount of \(H^+\) in solution is given as “∼0 mmol.” For the purposes of the stoichiometry calculation, this is essentially true, but remember that the point of the problem is to calculate the final \([H^+]\) and thus the pH. We now have all the information we need to calculate the pH. We can use either the lengthy procedure of Example \(\PageIndex{1}\) or the Henderson–Hasselbach approximation. The latter approach is much simpler. The Henderson-Hasselbalch approximation requires the concentrations of \(HCO_2^−\) and \(HCO_2H\), which can be calculated using the number of millimoles (\(n\)) of each and the total volume (\(VT\)). Substituting these values into the Henderson-Hasselbalch approximation,
\[pH=pK_a+\log \left( \dfrac{[HCO_2^−]}{[HCO_2H]} \right)=pK_a+\log\left(\dfrac{n_{HCO_2^−}/V_f}{n_{HCO_2H}/V_f}\right)=pK_a+\log \left(\dfrac{n_{HCO_2^−}}{n_{HCO_2H}}\right)\]
Because the total volume appears in both the numerator and denominator, it cancels. We therefore need to use only the ratio of the number of millimoles of the conjugate base to the number of millimoles of the weak acid. So
\[pH=pK_a+\log\left(\dfrac{n_{HCO_2^−}}{n_{HCO_2H}}\right)=3.75+\log\left(\dfrac{16.5\; mmol}{18.5\; mmol}\right)=3.75 −0.050=3.70\]
Once again, this result makes sense on two levels. First, the addition of \(HCl \)has decreased the pH from 3.95, as expected. Second, the ratio of \(HCO_2^−\) to \(HCO_2H\) is slightly less than 1, so the pH should be between the \(pK_a\) and \(pK_a\) − 1.
A The procedure for solving this part of the problem is exactly the same as that used in part (a). We have already calculated the numbers of millimoles of formic acid and formate in 100 mL of the initial pH 3.95 buffer: 13.5 mmol of \(HCO_2H\) and 21.5 mmol of \(HCO_2^−\). The number of millimoles of \(OH^-\) in 5.00 mL of 1.00 M \(NaOH\) is as follows:
B With this information, we can construct an ICE table.
\[HCO_2H (aq) + OH^− (aq) \rightarrow HCO^−_2 (aq) + H_2O (l) \]
The final amount of \(OH^-\) in solution is not actually zero; this is only approximately true based on the stoichiometric calculation. We can calculate the final pH by inserting the numbers of millimoles of both \(HCO_2^−\) and \(HCO_2H\) into the simplified Henderson-Hasselbalch expression used in part (a) because the volume cancels:
\[pH=pK_a+\log \left(\dfrac{n_{HCO_2^−}}{n_{HCO_2H}}\right)=3.75+\log \left(\dfrac{26.5\; mmol}{8.5\; mmol} \right)=3.75+0.494=4.24\]
Once again, this result makes chemical sense: the pH has increased, as would be expected after adding a strong base, and the final pH is between the \(pK_a\) and \(pK_a\) + 1, as expected for a solution with a \(HCO_2^−/HCO_2H\) ratio between 1 and 10.
Exercise \(\PageIndex{3}\)
The buffer solution from Example \(\PageIndex{2}\) contained 0.119 M pyridine and 0.234 M pyridine hydrochloride and had a pH of 4.94.
- What is the final pH if 12.0 mL of 1.5 M \(NaOH\) are added to 250 mL of this solution?
- What is the final pH if 12.0 mL of 1.5 M \(HCl\) are added?
Either concentrations OR amounts (in moles or millimoles) of the acidic and basic components of a buffer may be used in the Henderson-Hasselbalch approximation, because the volume cancels out in the ratio of [base]/[acid].
The results obtained in Example \(\PageIndex{3}\) and its corresponding exercise demonstrate how little the pH of a well-chosen buffer solution changes despite the addition of a significant quantity of strong acid or strong base. Suppose we had added the same amount of \(HCl\) or \(NaOH\) solution to 100 mL of an unbuffered solution at pH 3.95 (corresponding to \(1.1 \times 10^{−4}\) M HCl). In this case, adding 5.00 mL of 1.00 M \(HCl\) would lower the final pH to 1.32 instead of 3.70, whereas adding 5.00 mL of 1.00 M \(NaOH\) would raise the final pH to 12.68 rather than 4.24. (Try verifying these values by doing the calculations yourself.) Thus the presence of a buffer significantly increases the ability of a solution to maintain an almost constant pH.
Medicine: The Buffer System in Blood
The normal pH of human blood is about 7.4. The carbonate buffer system in the blood uses the following equilibrium reaction:
\[\ce{CO2}(g)+\ce{2H2O}(l)⇌\ce{H2CO3}(aq)⇌\ce{HCO3-}(aq)+\ce{H3O+}(aq)\]
The concentration of carbonic acid, H 2 CO 3 is approximately 0.0012 M , and the concentration of the hydrogen carbonate ion, \(\ce{HCO3-}\), is around 0.024 M . Using the Henderson-Hasselbalch equation and the p K a of carbonic acid at body temperature, we can calculate the pH of blood:
\[\mathrm{pH=p\mathit{K}_a+\log\dfrac{[base]}{[acid]}=6.1+\log\dfrac{0.024}{0.0012}=7.4}\]
The fact that the H 2 CO 3 concentration is significantly lower than that of the \(\ce{HCO3-}\) ion may seem unusual, but this imbalance is due to the fact that most of the by-products of our metabolism that enter our bloodstream are acidic. Therefore, there must be a larger proportion of base than acid, so that the capacity of the buffer will not be exceeded.
Lactic acid is produced in our muscles when we exercise. As the lactic acid enters the bloodstream, it is neutralized by the \(\ce{HCO3-}\) ion, producing H 2 CO 3 . An enzyme then accelerates the breakdown of the excess carbonic acid to carbon dioxide and water, which can be eliminated by breathing. In fact, in addition to the regulating effects of the carbonate buffering system on the pH of blood, the body uses breathing to regulate blood pH. If the pH of the blood decreases too far, an increase in breathing removes CO 2 from the blood through the lungs driving the equilibrium reaction such that [H 3 O + ] is lowered. If the blood is too alkaline, a lower breath rate increases CO 2 concentration in the blood, driving the equilibrium reaction the other way, increasing [H + ] and restoring an appropriate pH.
A solution containing a mixture of an acid and its conjugate base, or of a base and its conjugate acid, is called a buffer solution. Unlike in the case of an acid, base, or salt solution, the hydronium ion concentration of a buffer solution does not change greatly when a small amount of acid or base is added to the buffer solution. The base (or acid) in the buffer reacts with the added acid (or base).
Key Equations
- p K a = −log K a
- p K b = −log K b
- \(\mathrm{pH=p\mathit{K}_a+\log\dfrac{[A^- ]}{[HA]}}\)
Contributors
Paul Flowers (University of North Carolina - Pembroke), Klaus Theopold (University of Delaware) and Richard Langley (Stephen F. Austin State University) with contributing authors. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Download for free at http://cnx.org/contents/[email protected] ).

IMAGES
VIDEO
COMMENTS
A weak acid is a hydrogen-bearing molecular compound that ionizes only slightly in water solution. Using HX (aq) as the formula of a weak acid dissolved in water, the ionization equilibrium is. HX (aq) H + (aq) + X- (aq) (1.7) H 2 O (l) + HX (aq) H 3 O + (aq) + X - (aq) and the associated equilibrium expression is.
Hydrogen gas is highly flammable. Identify two acids and two bases that you use or come into contact with in an average week. Identify uses for each substance. I use citric acid everyday when I eat foods with preservatives and citrus fruits. I use magnesium hydroxide everyday when I put on my deodorant.
Numerical Answers. 1. Acids in order of increasing strength: \(acid\,B<acid\,C<acid\,A\). Given the same initial concentration of each acid, the highest percent of ionization is acid A because it is the strongest acid.
A molecule or ion that loses or "donates" a proton is acting as an acid; a species that receives or "accepts" that proton plays the role of a base. Defining an acid as a substance that simply "dissociates" into its component parts. HA → H+ + AB- (13.1.1) (13.1.1) H A → H + + A B -.
Hydrogen gas is highly flammable. Identify two acids and two bases that you use or come into contact with in an average week. Identify uses for each substance. Examples of common acids include vinegar, lemon juice, and soda. Examples of common bases include ammonia, bleach, and antacids. Common uses of acids include flavoring and preserving ...
This page contains materials for the session on acid-base chemistry. It features a 1-hour lecture video, and also presents the prerequisites, learning objectives, reading assignment, lecture slides, homework with solutions, and resources for further study.
Basically, an acid/base is seen as an H+ donator or accepter (the acid donates, the base accepts): HA + B- → HB + A-. In this example, HA is the acid, which donates an H+ ion to the B- ion (the base) to form HB and A-. Get used to seeing HA and B- as sample acids and bases; it is a common notation! A and B could be stand-ins for any number of ...
Quiz 1. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.
Unit 5: Acids & Bases Assignment 1: An Introduction to Acids & Bases 1. What is the difference between a strong electrolyte and a weak electrolyte? For your answer you should define these terms and explain what makes an electrolytic solution strong or weak. 2. Classify each of the following as either an acid or a base: a.
Problem Set AB1: Strong Acids, Strong Bases, [H+], and [OH-] Relate the [H +] or [OH -] concentration to the concentration of a strong acid or a strong base. Includes 8 problems. Problem Set AB2: pH and pOH 1. Calculate the pH or pOH of an acidic or basic solution if given the hydronium or hydroxide ion concentration. Includes 7 problems.
Study with Quizlet and memorize flashcards containing terms like the independent variable, the one that is intentionally manipulated, is ____ the dependent variable, the one that you measure the response in, is _____, does taking 1 mL of solution Y (pure bleach) out of the first beaker change the solution's concentration?, what would the concentration of the second beaker be? and more.
The O atom is donating an electron pair to the H + ion, making the base an electron pair donor and the acid an electron pair acceptor. The N atom is donating a lone pair to B in BF 3, Hence NH 3 is the Lewis base and BF 3 is the Lewis acid. 11.E: Acids and Bases (Exercises) Problems and select solutions to the chapter.
Acid-Base Solutions 1.2.4 - PhET Interactive Simulations
Assignment 1: Acids and Bases @ Home. Click Assignment_1.pdf link to view the file.. Titration Simulation Site
Submit electronically to the Loud Cloud assignment box. The instructor may also require paper submissions. ... if overfull empty until it reaches 0 into a waste beaker. -using a pipette add the desired amount of acid/base into a beaker and place below the pipette. -slowly after measuring the intial volume add titrant and record new volume ...
Acid base assignment. Subject. Chemistry. 999+ Documents. Students shared 3828 documents in this course. Level Standard. School Bluffton High - Bluffton-SC. Academic year: 2015/2016. Uploaded by: Lizeth Francia. Virginia Commonwealth University. 0 followers. 4 Uploads. 0 upvotes. Follow. Recommended for you. 4.
An acid-base reaction is an equilibrium reaction between conjugate acid-base pairs. What is a strong acid and give the 3 examples you need to know. A strong acid is one which is almost COMPLETELY IONISED in (dilute) aqueous solution. HCl (hydrochloric acid), HNO3 (nitric acid) and H2SO4 (sulfuric acid).
There are three primary theories of acid-base chemistry that are often taught together: Arrhenius theory, Brønsted-Lowry theory, and Lewis acid-base theory. Each theory is introduced below. Figure 7.1A. 1 7.1 A. 1: Hierarchal definitions of acids and bases via the three primary theories. These theories are designed to be "superset" of the ...
Unit 11.1 Nucleic Acids; UNIT 8, ASSIGNMENT 1; Family Summary Sheets Completed; Related documents. Jan 2022 Mock Exam Print Paper; ... Preview text. Acid/Base. Investigate acid-base equilibria in order and to understand buffer action to optimise acid-base titration procedures. Unit 13: Applications of Inorganic Chemistry. P2/P3 - Demonstrate ...
A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer.Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure \(\PageIndex{1}\)).
a solution consisting of a base and its conjugate acid or an acid and its conjugate base. Buffers help maintain the pH of a solution by absorbing the effects of small volumes of acids and bases. In this case, the mixture of the base Na2HPO4 and its conjugate acid NaH2PO4 is the buffer. Identify the method you will use to measure the pH of ...
1 Unit 5: Acids & Bases Assignment 2 Page 3 of 4 . Name: ANSWER KEY 5 11. A 2.67 g sample of hydrogen fluoride gas (HF) is dissolved in sufficient water to make 1.05 L of solution at 25°C to form an acidic solution. Hydrogen fluoride is a weak acid with K a = 6.6 × 10-4.