An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.192(31); 2020 Aug 4


Obesity in adults: a clinical practice guideline
- Obesity is a prevalent, complex, progressive and relapsing chronic disease, characterized by abnormal or excessive body fat (adiposity), that impairs health.
- People living with obesity face substantial bias and stigma, which contribute to increased morbidity and mortality independent of weight or body mass index.
- This guideline update reflects substantial advances in the epidemiology, determinants, pathophysiology, assessment, prevention and treatment of obesity, and shifts the focus of obesity management toward improving patient-centred health outcomes, rather than weight loss alone.
- Obesity care should be based on evidence-based principles of chronic disease management, must validate patients’ lived experiences, move beyond simplistic approaches of “eat less, move more,” and address the root drivers of obesity.
- People living with obesity should have access to evidence-informed interventions, including medical nutrition therapy, physical activity, psychological interventions, pharmacotherapy and surgery.
Obesity is a complex chronic disease in which abnormal or excess body fat (adiposity) impairs health, increases the risk of long-term medical complications and reduces lifespan. 1 Epidemiologic studies define obesity using the body mass index (BMI; weight/height 2 ), which can stratify obesity-related health risks at the population level. Obesity is operationally defined as a BMI exceeding 30 kg/m 2 and is subclassified into class 1 (30–34.9), class 2 (35–39.9) and class 3 (≥ 40). At the population level, health complications from excess body fat increase as BMI increases. 2 At the individual level, complications occur because of excess adiposity, location and distribution of adiposity and many other factors, including environmental, genetic, biologic and socioeconomic factors ( Box 1 ). 11
Complications of obesity
Adipose tissue not only influences the central regulation of energy homeostasis, but excessive adiposity can also become dysfunctional and predispose the individual to the development of many medical complications, such as:
- Type 2 diabetes 3
- Gallbladder disease 4
- Nonalcoholic fatty liver disease 5
Excess and ectopic body fat are important sources of adipocytokines and inflammatory mediators that can alter glucose and fat metabolism, leading to increased cardiometabolic and cancer risks, and thereby reducing disease-free duration and life expectancy by 6 to 14 years. 1 , 7 , 8 It is estimated that 20% of all cancers can be attributed to obesity, independent of diet. 9 Obesity increases the risk of the following cancers: 10
- Colon (both sexes)
- Kidney (both sexes)
- Esophagus (both sexes)
- Endometrium (women)
- Postmenopausal breast (women)
Over the past 3 decades, the prevalence of obesity has steadily increased throughout the world, 12 and in Canada, it has increased threefold since 1985. 13 Importantly, severe obesity has increased more than fourfold and, in 2016, affected an estimated 1.9 million Canadian adults. 13
Obesity has become a major public health issue that increases health care costs 14 , 15 and negatively affects physical and psychological health. 16 People with obesity experience pervasive weight bias and stigma, which contributes (independent of weight or BMI) to increased morbidity and mortality. 17
Obesity is caused by the complex interplay of multiple genetic, metabolic, behavioural and environmental factors, with the latter thought to be the proximate cause of the substantial rise in the prevalence of obesity. 18 , 19 A better understanding of the biological underpinnings of this disease has emerged in recent years. 19 The brain plays a central role in energy homeostasis by regulating food intake and energy expenditure ( Box 2 ). 24
Appetite regulation 20 – 23
- The control of appetite is complex and involves the integration of the central neural circuits including the hypothalamus (homeostatic control), the mesolimbic system (hedonic control) and the frontal lobe (executive control).
- The crosstalk between homeostatic and hedonic eating is influenced by mediators from adipose tissue, the pancreas, gut and other organs.
- Cognitive functions in the prefrontal cortex exert executive control on food choices and the decision to eat. The interconnectivity of these neural networks drives eating behaviour and has been shown to be altered in obesity.
Decreased food intake and increased physical activity lead to a negative energy balance and trigger a cascade of metabolic and neurohormonal adaptive mechanisms. 25 , 26 Therapies that target these alterations in neurohormonal mechanisms can become effective tools in the long-term management of obesity. 27
Novel approaches to diagnose and assess obesity in clinical practice have been proposed. 11 , 18 , 19 , 28 Although BMI is widely used to assess and classify obesity (adiposity), it is not an accurate tool for identifying adiposity-related complications. 19 Waist circumference has been independently associated with an increase in cardiovascular risk, but it is not a good predictor of visceral adipose tissue on an individual basis. 29 Integration of both BMI and waist circumference in clinical assessment may identify the higher-risk phenotype of obesity better than either BMI or waist circumference alone, particularly in those individuals with lower BMI. 30 , 31 In addition to BMI and waist circumference measurements, a comprehensive history to identify the root causes of obesity, appropriate physical examination and relevant laboratory investigations will help to identify those who will benefit from treatment. 32
The Edmonton obesity staging system has been proposed to guide clinical decisions from the obesity assessment and at each BMI category (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.191707/-/DC2 ). 28 This 5-stage system of obesity classification considers metabolic, physical and psychological parameters to determine the optimal obesity treatment. In population studies, it has been shown to be a better predictor of all-cause mortality when compared with BMI or waist circumference measurements alone. 33 , 34
There is a recognition that obesity management should be about improved health and well-being, and not just weight loss. 34 – 36 Because the existing literature is based mainly on weight-loss outcomes, several recommendations in this guideline are weight-loss centred. However, more research is needed to shift the focus of obesity management toward improving patient-centred health outcomes, rather than weight loss alone.
Despite growing evidence that obesity is a serious chronic disease, it is not effectively managed within our current health system. 37 , 38 Canadian health professionals feel ill equipped to support people living with obesity. 39 – 41 Biased beliefs about obesity also affect the level and quality of health care that patients with obesity receive. 42 The dominant cultural narrative regarding obesity fuels assumptions about personal irresponsibility and lack of willpower and casts blame and shame upon people living with obesity. 41 Importantly, obesity stigma negatively influences the level and quality of care for people living with obesity. 42
With increased knowledge of the disease state and better approaches to assess and manage obesity, it is timely to update the 2006 Canadian clinical practice guideline. 43 The goal of this update is to disseminate to primary care practitioners evidence-informed options for assessing and treating people living with obesity. Importantly, this guideline incorporates the perspectives of people with lived experience and of interprofessional primary care providers with those of experts on obesity management, and researchers. This article is a summary of the full guideline, which is available online ( http://obesitycanada.ca/guidelines/ ).
The target users for this guideline are primary health care professionals. The guideline may also be used by policy-makers and people affected by obesity and their families. The guideline is focused on obesity in adults. The recommendations are intended to serve as a guide for health care providers; clinical discretion should be used by all who adopt these recommendations. Resource limitations and individual patient preferences may make it difficult to put every recommendation into practice, but the guideline is intended to improve the standard of, and access to, care for individuals with obesity in all regions of Canada.

Recommendations
This clinical practice guideline informs the arc of the patient journey and clinical management approach in the primary care setting. The guideline recommendations are shown in Table 1 .
Recommendations on management of obesity in adults *
Note: ALT = alanine aminotransferase, BMI = body mass index.
A complete description of the recommendations and supporting evidence are available in the 19 chapters of the full guideline ( http://obesitycanada.ca/guidelines/ ). This synopsis outlines a discussion of the guiding principles that the executive committee determined as important for advancing clinical practice in Canada.
There are 5 steps in the patient arc to guide a health care provider in the care of people living with obesity. Each step is outlined below with highlights of the relevant recommendations and a discussion of supporting evidence.
- Recognition of obesity as a chronic disease by health care providers, who should ask the patient permission to offer advice and help treat this disease in an unbiased manner.
- Assessment of an individual living with obesity, using appropriate measurements, and identifying the root causes, complications and barriers to obesity treatment.
- Discussion of the core treatment options (medical nutrition therapy and physical activity) and adjunctive therapies that may be required, including psychological, pharmacologic and surgical interventions.
- Agreement with the person living with obesity regarding goals of therapy, focusing mainly on the value that the person derives from health-based interventions.
- Engagement by health care providers with the person with obesity in continued follow-up and reassessments, and encouragement of advocacy to improve care for this chronic disease.
Step 1: Recognition of obesity as a chronic disease and obtaining patient permission
Primary care providers should recognize and treat obesity as a chronic disease, caused by abnormal or excess body fat accumulation (adiposity), which impairs health, with increased risk of premature morbidity and mortality. 1 , 2 , 18 , 44 – 47
Obesity is a complex and heterogeneous chronic disease that does not present in the same way in all patients and that requires individualized treatment and long-term support like any other complex chronic disease.
Weight bias in health care settings can reduce the quality of care for patients living with obesity. 42 A key to reducing weight bias, stigma and discrimination in health care settings is for health care providers to be aware of their own attitudes and behaviours toward individuals living with obesity. 48 This can be achieved by completing a self-assessment tool, like the Implicit Association Test, for weight bias. 49 A full description and supporting evidence for weight bias recommendations are available online ( http://obesitycanada.ca/guidelines/ ) in the chapter titled “Reducing weight bias in obesity management, practice and policy.”
Health care providers should not assume that all patients living with obesity are prepared to initiate obesity management. Health care providers should ask the patient permission to discuss obesity, and if the patient permits, then a discussion on treatment can begin. 50 , 51
Step 2: Assessment
Primary care clinicians should promote a holistic approach to health with a focus on health behaviours in all patients and address the root causes of weight gain with care to avoid stigmatizing and overly simplistic narratives.
Direct measurement of height, weight and waist circumference and calculation of BMI should be included in routine physical examination for all adults. Although BMI has its limitations, it remains a valuable tool for screening purposes and for population health indices. 52 For persons with increased BMI (between 25 mg/m 2 and 34.9 mg/m 2 ), waist circumference should be regularly measured to identify individuals with increased visceral adiposity and adiposity-related health risks. 53
Root causes of obesity include biological factors such as genetics, epigenetics, neurohormonal mechanisms, associated chronic diseases and obesogenic medications, sociocultural practices and beliefs, social determinants of health, built environment, individual life experiences like adverse childhood experiences, and psychological factors such as mood, anxiety, binge-eating disorder, attention-deficit/hyperactivity disorder, self-worth and identity. 50 Working with people to understand their context and culture, and integrate their root causes, allows for the development of personalized plans. These plans can be integrated into long-term therapeutic relationships with chronic disease follow-up of obesity and related comorbidities, including addressing the root causes of obesity such as existing conditions and obesogenic medications.
We recommend obtaining a comprehensive history to identify these root causes of weight gain, as well as physical, mental and psychosocial barriers. Physical examination, laboratory, diagnostic imaging and other investigations should be carried out based on clinical judgment. We also recommend measuring blood pressure in both arms and obtaining fasting glucose or glycated hemoglobin values and a lipid panel to determine cardiometabolic risk, and when indicated, alanine aminotransferase to screen for nonalcoholic fatty liver disease.
Step 3: Discussion of treatment options
Adults living with obesity should receive individualized care plans that address their root causes of obesity and that provide support for behavioural change (e.g., nutrition, physical activity) and adjunctive therapies, which may include psychological, pharmacologic and surgical interventions.
Nutrition and exercise
All individuals, regardless of body size or composition, would benefit from adopting a healthy, well-balanced eating pattern and engaging in regular physical activity. Aerobic activity (30–60 min) on most days of the week can lead to a small amount of weight and fat loss, improvement in cardiometabolic parameters, and weight maintenance after weight loss. 54
Weight loss and weight-loss maintenance require a long-term reduction in caloric intake. Long-term adherence to a healthy eating pattern that is personalized to meet individual values and preferences, while fulfilling nutritional needs and treatment goals, is an important element of managing health and weight.
Medical nutrition therapy is a foundation for chronic disease management, including obesity management. 55 , 56 However, medical nutrition therapy should not be used in isolation in obesity management, as sustaining weight loss may be difficult long term because of compensatory mechanisms in the brain that promote positive caloric intake by increasing hunger and ultimately causing weight gain. 57 , 58 Instead, medical nutrition therapy, in combination with other interventions (psychological, pharmacologic, surgical), should be tailored to meet an individual’s health-related or weight-related outcomes. 56 , 59
The weight loss achieved with health behavioural changes is usually 3%–5% of body weight, which can result in meaningful improvement in obesity-related comorbidities. 60 The amount of weight loss varies substantially among individuals, depending on biological and psychosocial factors and not simply on individual effort.
The weight at which the body stabilizes when engaging in healthy behaviours can be referred to as the “best weight”; this may not be an “ideal” weight on the BMI scale. Achieving an “ideal” BMI may be very difficult. If further weight loss is needed to improve health and well-being beyond what can be achieved with behavioural modification, then more intensive pharmacologic and surgical therapeutic options can be considered.
Psychological and behavioural interventions
All health interventions such as healthy eating and physical activity strategies, medication adherence or surgery preparation and adjustment approaches rest on behaviour change. 61 Psychological and behavioural interventions are the “how to” of change. They empower the clinician to guide the patient toward recommended behaviours that can be sustained over time. 60 A full description of psychological and behavioural interventions and supporting evidence are available online ( http://obesitycanada.ca/guidelines/ ) in the chapter titled “Effective psychological and behavioural interventions in obesity management.”
Pharmacotherapy
We recommend adjunctive pharmacotherapy for weight loss and weight-loss maintenance for individuals with BMI ≥ 30 kg/m 2 or BMI ≥ 27 kg/m 2 with adiposity-related complications, to support medical nutrition therapy, physical activity and psychological interventions. Options include liraglutide 3.0 mg, naltrexone-bupropion combination and orlistat. Pharmacotherapy augments the magnitude of weight loss beyond that which health behaviour changes can achieve alone and is important in the prevention of weight regain. 62 – 66 A full description and supporting evidence are available online ( http://obesitycanada.ca/guidelines/ ) in the chapter titled “Pharmacotherapy in obesity management.”
Bariatric surgery
Bariatric surgery may be considered for people with BMI ≥ 40 kg/m 2 or BMI ≥ 35 kg/m 2 with at least 1 obesity-related disease. The decision regarding the type of surgery should be made in collaboration with a multidisciplinary team, balancing the patient’s expectations, medical condition, and expected benefits and risks of the surgery. A full description and supporting evidence are available online ( http://obesitycanada.ca/guidelines/ ) in the chapters titled “Bariatric surgery: selection and preoperative workup,” “Bariatric surgery: options and outcomes” and “Bariatric surgery: postoperative management.”
Step 4: Agreement regarding goals of therapy
Because obesity is a chronic disease, managing it in the long term involves patient–provider collaboration. 67 Health care providers should talk with their patients and agree on realistic expectations, person-centred treatments and sustainable goals for behaviour change and health outcomes. 68
Helpful actions in primary care consultations to mitigate antifat stigma include explicitly acknowledging the multiple determinants of weight-disrupting stereotypes of personal failure or success attached to body composition; focusing on behavioural interventions to improve overall health; and redefining success as healthy behaviour change regardless of body size or weight. 69
As this disease is chronic in nature, the treatment plan must be long term. Health care providers and patients should design and agree on a personalized action plan that is practical and sustainable and addresses the drivers of weight gain. 70
Step 5: Follow-up and advocacy
There is a need to advocate for more effective care for people living with obesity. This includes improving the education and lifelong learning of health care providers to be able to deliver effective, evidence-based obesity care. We also need to support allocation of health care resources to improve access to effective behavioural, pharmacologic and surgical therapeutic options.
There are substantial barriers affecting access to obesity care in Canada, including a profound lack of interdisciplinary obesity management programs, a lack of adequate access to health care providers with expertise in obesity, long wait times for referrals and surgery, and the high costs of some treatments., 37 , 71 – 73 In general, health care professionals are poorly prepared to treat obesity. 74 None of the anti-obesity medications available in Canada is listed as a benefit on any provincial or territorial formulary and none is covered under any provincial public drug benefit or pharmacare program. 71 Wait times for bariatric surgery in Canada are the longest of any surgically treatable condition. 37 , 71 Although access to bariatric surgery has increased in some parts of Canada, it is still limited in most provinces and nonexistent in the 3 territories. 37 , 71 , 75 Patients referred to bariatric surgery can wait as long as 8 years before meeting a specialist or receiving the surgery.
The lack of access to obesity treatments is contributing to rising levels of severe obesity in Canada. 46 Canadians affected by obesity are left to navigate a complex landscape of weight-loss products and services, many of which lack a scientific rationale and openly promote unrealistic and unsustainable weight-loss goals. 76
Composition of participating groups
Obesity Canada and the Canadian Association of Bariatric Physicians and Surgeons assembled an executive committee and steering committee with broad expertise and geographic representation. The executive committee (comprising 2 co-chairs [S.W., D.C.W.L.], a primary care physician [D.C.-S.], a psychologist [M.V.], a bariatric surgeon [L.B.] and a nephrologist [A.M.S.]) provided overall vision and oversight for the guideline process.
The steering committee ( n = 16) consisted of some lead authors of each chapter and a person living with obesity; this committee identified additional researchers (chapter leads and authors) to write each chapter. The executive committee and steering committee met in person in April 2017 and December 2017 and at least monthly by phone.
Chapter leads and chapter authors ( n = 60) were selected based on their expertise in clinical practice and research in the field of obesity medicine. The number of chapter authors per chapter ranged from 2 to 4. Some chapter leads identified additional authors to participate in writing each chapter.
We engaged people living with obesity ( n = 7) through participation of the Public Engagement Committee of Obesity Canada. One member of the Public Engagement Committee (I.P.) was assigned to the steering committee for this guideline. The Public Engagement Committee met by phone once per month. We obtained contributions from committee members through online surveys, focus groups and individual conversations.
We engaged Indigenous community members through a focus group ( n = 14). Additionally, we obtained the insights of health care providers working with Indigenous communities via a consensus-building process between these clinicians and chapter authors, carried out over the spring of 2019, which further grounded evidence in clinical practice. Details are available online ( http://obesitycanada.ca/guidelines/ ) in the chapter titled “Obesity management with Indigenous Peoples.”
Obesity Canada staff, consultants and volunteers ( n = 15) provided administrative support and project coordination for the guideline development process. Table 2 outlines the guideline development process and the responsibilities of each group of participants.
Summary of guideline development process
Note: AGREE = Appraisal of Guidelines for Research and Evaluation, MERST = McMaster Evidence Review and Synthesis Team; PICO(T) = Population, Intervention, Comparison, Outcome, Time.
Selection of priority topics
The executive committee conducted a mind-mapping exercise to identify the scope of the guideline and the broad sections and chapters (April–June 2017). 79 A total of 19 different sections and chapters were prioritized. The steering committee developed PI/PECOT (Population, Intervention or Exposure, Comparison, Outcome, Time) questions for each chapter at an in-person meeting on Dec. 15–16, 2017, resulting in 179 questions to guide the literature search. All clinical questions were developed with the assistance of the McMaster Evidence Review and Synthesis Team (MERST; previously the McMaster Evidence-Based Practice Centre) in the appropriate format (e.g., PICO [T] for therapeutics and treatments, PEO for qualitative questions).
Literature review and quality assessment
The McMaster Evidence Review and Synthesis Team supported the guideline development through literature searches based on the PI/PECOT questions for each chapter. A health sciences librarian, based at McMaster Health Sciences Library (Hamilton, Ont.), used this information to create search strategies for the MEDLINE and Embase databases. The searches were for peer-reviewed and published literature in the English language; the search dates were January 2006 to June 2018. There were 14 searches that mapped directly to the chapters and another 7 searches that helped provide context for various chapters. Search strategies are available on the obesity guideline webpage ( http://obesitycanada.ca/guidelines/ ). Once a search was conducted, the results were uploaded to EndNote, where the duplicates were removed and the final set of citations was uploaded to DistillerSR software for selection and review. 80 In addition to the electronic searches, the chapter authors identified additional citations and added them to the main search results.
Two reviewers completed screening of article titles and abstracts and independently selected studies for possible inclusion. Any citation that was selected for inclusion by either reviewer was moved to full-text review. One or more authors of the relevant chapter conducted reviews of full-text articles for relevancy. Selected citations were then assessed for their methodological quality using the Shekelle approach. 77 , 81 Each citation was categorized into prevention, treatment, evaluation of diagnostic properties or prognosis. Once that selection was made, the appropriate methods worksheet was displayed in the DistillerSR platform, from which the methodological questions were answered and a level of evidence generated based on the type and quality of the study. The levels of evidence informed the strength of the recommendations and were generated from the methods worksheets ( Box 3 ). 77
Classification schemes 77
Category of evidence
- Level 1a: Evidence from meta-analysis of randomized controlled trials (RCTs)
- Level 1b: Evidence from at least 1 RCT
- Level 2a: Evidence from at least 1 controlled study without randomization
- Level 3: Evidence from nonexperimental descriptive studies, such as comparative studies, correlation studies and case–control studies
- Level 4: Evidence from expert committee reports or opinions or clinical experience of respected authorities, or both
Strength of recommendation
- Grade A: Directly based on level 1 evidence
- Grade B: Directly based on level 2 evidence or extrapolated recommendation from category 1 evidence
- Grade C: Directly based on level 3 evidence or extrapolated recommendation from level 1 or 2 evidence
- Grade D: Directly based on level 4 evidence or extrapolated recommendation from level 1, 2 or 3 evidence
Adapted with permission from BMJ Publishing Group Limited. Shekelle PG, Woolf SH, Eccles M, et al. Developing clinical guidelines. West J Med 1999;170:348-51.
Development of recommendations
Recommendations were formulated by the steering committee, chapter leads and chapter authors based on the highest level of evidence available (Box 3). 77 Chapter leads and authors reviewed the type and strength of the available evidence (level) and added the study reference that provided the highest level of evidence for the specific recommendation.
Recognizing the importance of qualitative research in addressing questions pertinent to the care of people living with obesity, content experts in qualitative research (S.K., X.R.S., D.C.S., L.C., S.R.M.) were involved in the review of all materials informing these recommendations. Consensus appraisal of evidence quality by reviewers with expertise in qualitative methods informed the level of evidence in these recommendations.
Some grade D recommendations were formulated based on expert committee reports, opinions or clinical experience of respected authorities, and referenced accordingly; other grade D recommendations formulated by chapter authors were noted with “Consensus ” after the grade D.
Chapter authors used a standardized terminology to make the recommendation more specific. The actionable verbs used for each of the recommendations were informed by the literature ( Table 3 ). 82 – 84
Definitions of actionable verbs used in the recommendations 82 – 84
We used an iterative process to finalize the recommendations. Methodologists from MERST provided an independent review of recommendations that had a grade between A and C, for which they examined the clarity of wording and the fidelity of the recommendations with the evidence. Two methodologists (a primary and secondary reviewer) reviewed each recommendation, using checklists as a guide for assigning levels of evidence to each citation. The methodologists met, discussed and reached consensus on grading the recommendations, and reported their suggestions regarding revisions to the wording or grading to the executive committee. Chapter leads edited the recommendations based on the MERST review process.
The executive committee voted on each recommendation, to ensure consensus. If a recommendation did not reach 100% agreement, the executive committee discussed the recommendation in depth until consensus was achieved. The chapter leads subsequently modified the wording of this recommendation, as required, and the executive committee approved the newly worded recommendation. The executive committee provided final approval of all the recommendations. All the recommendations included in this guideline achieved 100% agreement.
External review
External reviewers (primary care health care professionals and people living with obesity [ n = 7]) reviewed the recommendations for relevance and feasibility. We made some modifications to reflect language and the context of the primary care setting. A separate external peer review was conducted for each chapter.
Management of competing interests
Funding came from the Canadian Institutes of Health Research Strategic Patient-Oriented Research initiative, Obesity Canada’s Fund for Obesity Collaboration and Unified Strategies (FOCUS) initiative, the Canadian Association of Bariatric Physicians and Surgeons, and in-kind support from the scientific and professional volunteers engaged in the process. The views of the funding body have not influenced the content of the guideline. All committee members (executive and steering committees), chapter leads and chapter authors were volunteers and not remunerated for their services.
The executive committee developed and managed the competing interest policy and procedures for mitigating bias. The policy and disclosures of competing interest are available on the guideline website. All participants were required to disclose potential competing interests. We maintained detailed competing interest declarations throughout the process for all members of the steering and executive committees, as well as the participating methodologists from MERST. We used the International Committee of Medical Journal Editors’ disclosure form, with the addition of government funding sources.
Individuals with relevant disclosures were not excluded from conducting the critical appraisals or voting on recommendations. However, the executive committee asked individuals with direct competing interests to abstain from voting in the areas in which they had the conflict. Any discussion regarding off-label use of drugs included the caveat that the use was off label.
As mentioned earlier, methodologists from MERST who had no competing interests reviewed and graded 78 each included study to ensure the evidence had been appropriately assessed. They also reviewed the recommendations (graded between A and C) to ensure that recommendations were aligned with the evidence. Finally, we conducted an external review process to assess the feasibility of the recommendations and evaluate for the presence of bias.
Implementation
Obesity Canada and the Canadian Association of Bariatric Surgeons and Physicians have created a joint guideline website ( http://obesitycanada.ca/guidelines ) that hosts the full guideline; interim updates; a quick reference guide; key messages; health care provider tools, slide kits, videos and webinars; and resources for people living with obesity and their support systems, in English and French. The guideline will be hosted on the website as a living document. Each chapter lead will monitor evidence related to this guideline and will collaborate with the executive committee to update the recommendations if new evidence becomes available that could influence the recommendations. A framework for implementation (5As Framework) is available in Appendix 2.
More than 10 years after the release of the first Canadian obesity guideline in 2006, access to obesity care remains an issue in Canada. 37 , 71 Obesity is not officially recognized as a chronic disease by the federal, provincial and territorial, and municipal governments, despite declarations by the Canadian Medical Association 85 and the World Health Organization. 86 The lack of recognition of obesity as a chronic disease by public and private payers, health systems, the public and media has a trickle-down effect on access to treatment. 72 Obesity continues to be treated as a self-inflicted condition, which affects the type of interventions and approaches that are implemented by governments or covered by health benefit plans. 87
Implementation of this guideline will require targeted policy action, as well as advocacy efforts and engagement from people living with obesity, their families and health care providers. Canadian organizations have come together to change the narrative regarding obesity in Canada, to eliminate weight bias and obesity stigma, and to change the way health care systems and policies approach obesity. 88 This guideline will be used to assist in advocacy efforts to federal and provincial governments to improve the care of individuals with obesity.
Other guidelines
In 2006, the first evidence-based Canadian clinical practice guideline on the prevention and management of obesity in adults and children was released. 43 In 2015, the Canadian Task Force on Preventive Health Care, in collaboration with scientific staff of the Public Health Agency of Canada and the McMaster Evidence Review and Synthesis Centre, released a set of recommendations for prevention of weight gain and use of behavioural and pharmacologic interventions to manage overweight and obesity in adults in primary care. 89 This guideline was not designed to “apply to people with BMI of 40 or greater, who may benefit from specialized bariatric programs” and reviewed only intervention trials conducted in settings generalizable to Canadian primary care. The guideline also did not include surgical treatments.
Gaps in knowledge
The recommendations in this guideline are informed by the best level of evidence available in 2020. We acknowledge that ongoing research will continue to inform and advance obesity management. 90 , 91
Current treatment options, apart from surgical intervention, rarely yield sustained weight loss beyond 20%, and for some people living with obesity, this level of weight loss may be inadequate for the resolution or improvement of many adiposity-related medical complications. There is a need for more treatment options to meet the needs of people with obesity. Weight regain continues to be a challenge for many patients who have received treatment. 92
Obesity is a prevalent, complex chronic disease that affects a large number of adults in Canada and globally, and yet only a small fraction of people living with obesity who could benefit from treatment have access to care. This updated evidence-informed guideline is an attempt to enhance access and care by people living with obesity through recognition among health care providers that obesity requires long-term treatment. The newer insights into appetite regulation and the pathophysiology of obesity have opened new avenues for treating this chronic disease. Reducing weight bias and stigma, understanding the root causes of obesity, and promoting and supporting patient-centred behavioural interventions and appropriate treatment by health care providers — preferably with the support of interdisciplinary care teams — will raise the standards of care and improve the well-being of people living with obesity. Dissemination and implementation of this guideline are integral components of our goals to address this prevalent chronic disease. Much more effort is needed to close the gaps in knowledge through obesity research, education, prevention and treatment.
Acknowledgements
The authors thank Obesity Canada staff members Dawn Hatanaka, Nicole Pearce, Brad Hussey, Robert Fullerton and Patti Whitefoot-Bobier for their coordinating support as well as their contributions for the development of the Obesity Guidelines website, online resources, tables and figures. The authors also thank members of the Obesity Canada Public Engagement Committee (Lisa Schaffer, Candace Vilhan, Kelly Moen, Doug Earle, Brenndon Goodman), who contributed to the creation of the research questions and reviewed key messages for individuals living with obesity and recommendations for health care providers. The authors also thank McMaster Evidence Review and Synthesis Team (MERST) member Donna Fitzpatrick, who played a critical role in developing the methods needed for the guideline; and thank the reviewers whose comments helped to improve the chapters and this manuscript. The authors thank Barbara Kermode-Scott and Brad Hussey for editing the guidelines, Elham Kamran and Rubin Pooni for research assistance, and Jordan Tate from the Physician Learning Program at the University of Alberta for designing the 5As framework for the guideline.
This article is available in French at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.191707/-/DC1
CMAJ Podcasts: author interview at https://www.cmaj.ca/lookup/doi/10.1503/cmaj.191707/tab-related-content
Competing interests: Sean Wharton reports receiving honoraria and travel expenses and has participated in academic advisory boards for Novo Nordisk, Bausch Health, Eli Lilly and Janssen. Sean Wharton is also the medical director of a medical clinic specializing in weight management and diabetes. David Lau reports receiving grants and research support from AstraZeneca, Novo Nordisk and the Canadian Institutes of Health Research (CIHR); speaker bureau fees from AstraZeneca, Bausch Health, Boehringer Ingelheim, Diabetes Canada, Eli Lilly, Merck and Novo Nordisk; and consulting fees from Amgen, AstraZeneca, Bausch Health, Boehringer Ingelheim, Gilead, HLS Therapeutics, Janssen, Eli Lilly and Novo Nordisk. Michael Vallis is a member of advisory boards for Novo Nordisk, Bausch Health and LifeScan. Michael Vallis has also received consulting fees from Bausch Health, LifeScan, Novo Nordisk and Sanofi, and speaking fees from Novo Nordisk, Sanofi, Bausch Health, Abbott and AbbVie. Arya Sharma reports receiving speaker’s bureau and consulting fees from Novo Nordisk, Bausch Pharmaceuticals and Astra-Zeneca. Laurent Biertho reports receiving grants from Johnson and Johnson and Medtronic, and is a member of advisory boards for Novo Nordisk and Bausch Health, outside the submitted work. Denise Campbell-Scherer has no personal financial relationships, but reports receiving research funding from the following sources in the past 3 years: Novo Nordisk Alberta Diabetes Fund (NOVAD), a peer-reviewed grant that is a partnership between the University Hospital Foundation, Novo Nordisk and Alberta Innovates joint funders; Alberta Innovates Health Solutions (Cancer Prevention Research Opportunity and Collaborative Research and Innovation Opportunities competitions), CIHR (Strategy for Patient-Oriented Research and Knowledge-to-Action competitions); Northern Alberta Family Medicine Fund; and the Alberta Cancer Prevention and Legacy Fund. She also reports receiving knowledge transfer funding from the following sources in the past 3 years: an unrestricted education grant from Obesity Canada, funded by Novo Nordisk Global; a Worldwide University Network Meeting Grant; an Agency for Healthcare Research and Quality R13 grant for a Healthcare Effectiveness and Outcomes Research; and a Physician Learning Program grant from Alberta Health and the Alberta Medical Association. Angela Alberga reports receiving the following grants: the Santé Award from Fonds de Recherche du Quebec, the Mitacs Accelerate Grant, and the Concordia University Start-up Team Grant, outside the submitted work. Jennifer Brown reports receiving nonfinancial support from Novo Nordisk, and personal fees from Bausch Health, Dietitians of Canada, Obesity Canada and the Canadian Association of Bariatric Physicians & Surgeons. Yoni Freedhoff is the co-owner of the Bariatric Medical Institute and Constant Health, which provide weight management services; Constant Health has received a grant from Novo Nordisk. Yoni Freedhoff is also the author of The Diet Fix: Why Diets Fail and How to Make Yours Work published by Crown Publishing Group, and receives royalties for the book. In addition, he is the sole author of the Weighty Matters blog and a column for Medscape and many other op-eds and articles in which he has publicly expressed opinions about the treatment, management and prevention of obesity. Yoni Freedhoff also regularly speaks on topics related to obesity and receives honoraria and travel costs and expenses for same. Michel Gagner reports receiving speaker honoraria from Ethicon, WL Gore and Medtronic; consulting fees from Novo Nordisk, Bausch Health and Lexington Medical; and holds stock options with Lexington Medical. Margaret Hahn reports receiving consulting fees from Alkermes. Marie-France Langlois reports receiving personal fees from Novo Nordisk, Valeant, Merck Canada, Sanofi, Eli Lilly and Boehringer Ingelheim; a grant from Merck Canada; and other fees from AstraZeneca and from TIMI (Thrombolysis in Myocardial Infarction) Study Group for diabetes clinical research as a principal investigator, all outside the submitted work. David Macklin reports receiving personal fees from Novo Nordisk and Bausch Health, outside the submitted work. Priya Manjoo reports receiving personal fees from Novo Nordisk, Bausch Health and Sanofi; and grants from Boehringer Ingelheim, Sanofi and AstraZeneca, outside the submitted work. Marie-Philippe Morin reports receiving speaker honoraria from Novo Nordisk, Bausch Health, Eli Lilly, Boehringer Ingelheim, Nestlé Health Science, Janssen and AstraZeneca; research subvention from Novo Nordisk and Sanofi; and consultation honoraria from Novo Nordisk, Bausch Health, Eli Lilly, Boehringer Ingelheim, Janssen and AstraZeneca. Sue Pedersen reports receiving personal fees from Novo Nordisk, Bausch Health, Janssen, Eli Lilly, Merck, AstraZeneca, Boehringer Ingelheim, Sanofi, Pfizer; grants from Eli Lilly, AstraZeneca, Boehringer Ingelheim and Sanofi; and nonfinancial support from Novo Nordisk, Bausch Health, Janssen, Eli Lilly, AstraZeneca, Boehringer Ingelheim and Sanofi, outside the submitted work. Megha Poddar reports receiving honoraria for continuing medical education (CME) from Novo Nordisk, Bausch Health, Boehringer Ingelheim, Eli Lilly, Jenssen, Merck, the Canadian Collaborative Research Network and the Antibody Network; education grants from Novo Nordisk and Bausch Health; fees for mentorship from Novo Nordisk; fees for membership of advisory boards from Novo Nordisk and Bausch Health; and a quality improvement project grant from Boehringer Ingelheim. Paul Poirier reports receiving fees for consulting and continuing medical education from AstraZeneca, Boehringer Ingelheim, Janssen, Eli Lilly, Novo Nordisk, Valeant and Bausch Health, outside the submitted work. Judy Shiau reports receiving personal fees from Novo Nordisk and Bausch Health, outside the submitted work. Diana Sherifali reports receiving consulting fees for advice regarding chronic disease and diabetes management from Merck, and a grant from Obesity Canada to support the literature review process, during the conduct of the study. John Sievenpiper reports receiving grants from CIHR, the Nutrition Trialists Fund at the University of Toronto, the International Nut and Dried Fruit Council Foundation, the Tate & Lyle Nutritional Research Fund at the University of Toronto, the American Society for Nutrition, the Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto, the National Dried Fruit Trade Association, PSI Graham Farquharson Knowledge Translation Fellowship, the Diabetes Canada Clinician Scientist award, the Banting & Best Diabetes Centre Sun Life Financial New Investigator Award, the Canada Foundation for Innovation, and the Ministry of Research and Innovation’s Ontario Research Fund. Dr. Sievenpiper has received personal fees from Perkins Coie LLP, Tate & Lyle, Dairy Farmers of Canada, PepsiCo, Food-Minds LLC, European Fruit Juice Association, International Sweeteners Association, Nestlé Health Science, Canadian Society for Endocrinology and Metabolism, GI Foundation, Pulse Canada, Wirtschaftliche Vereinigung Zucker e.V., Abbott, Biofortis, the European Food Safety Authority, the Physicians Committee for Responsible Medicine, the Soy Nutrition Institute and the Comité Européen des Fabricants de Sucre. Dr. Sievenpiper has received nonfinancial support from Tate & Lyle, PepsiCo, FoodMinds LLC, European Fruit Juice Association, International Sweeteners Association, Nestlé Health Science, Wirtschaftliche Vereinigung Zucker e.V., Abbott, Biofortis, the European Food Safety Authority and the Physicians Committee for Responsible Medicine, Kellogg Canada, American Peanut Council, Barilla, Unilever, Unico Primo, Loblaw Companies, WhiteWave Foods, Quaker, California Walnut Commission, Almond Board of California, outside the submitted work. Dr. Sievenpiper is a member of the International Carbohydrate Quality Consortium and the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the Study of Diabetes, Canadian Cardiovascular Society, and Obesity Canada, and holds appointments as an Executive Board Member of the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes, and as Director of the Toronto 3D Knowledge Synthesis and Clinical Trials Foundation. He is also an unpaid scientific adviser for the Program in Food Safety, Nutrition and Regulatory Affairs and the Carbohydrates Committee of the International Life Science Institute North America. He has a spousal relationship with an employee of Anheuser-Busch InBev. Sanjeev Sockalingam reports receiving honoraria from Bausch Health Canada within the last 36 months. Valerie Taylor reports receiving speaker fees from Sunovion. Shahebina Walji reports receiving consulting or advisory board fees from Novo Nordisk, Bausch Health and Takeda and speaker’s bureau fees from Novo Nordisk and Bausch Health. Shahebina Walji also reports selling Optifast Meal replacements through a weight management centre Optifast is a product produced and sold by Nestlé. No other competing interests were declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the work and the acquisition, analysis, and interpretation of data. All of the authors drafted the manuscript, revised it critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: Funding for this initiative was provided by Obesity Canada, the Canadian Association of Bariatric Physicians and Surgeons, and the Canadian Institutes of Health Research through a Strategy for Patient-Oriented Research grant, with no participants or authors receiving any personal funding for their creation.
The Science of Obesity Management: An Endocrine Society Scientific Statement
Affiliations.
- 1 Department of Clinical Obesity, Pennington Biomedical Research Center, Louisiana State University, Baton Rouge, Louisiana.
- 2 Institute of Health Metrics and Evaluation University of Washington, Seattle, Washington.
- 3 Department of Medicine, Mayo Clinic, Rochester, Minnesota.
- 4 Redstone Global Center for Prevention and Wellness, Milken Institute School of Public Health, George Washington University, Washington, District of Columbia.
- 5 Northwestern Feinberg School of Medicine, Chicago, Illinois.
- 6 Department of Pediatrics, University of Colorado Children Hospital, Denver, Colorado.
- 7 Department of Psychiatry, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania.
- 8 Kaiser Permanente Colorado, Denver, Colorado.
- 9 Department of Nutrition and Epidemiology, Harvard T.H. Chan School of Public Health, Boston, Massachusetts.
- 10 University of Pittsburgh, Pittsburgh, Pennsylvania.
- 11 Oregon Health and Science University, Portland, Oregon.
- 12 Department of Surgery, University of Colorado Denver, Aurora, Colorado.
- 13 Children's Hospital Colorado, Aurora, Colorado.
- PMID: 29518206
- PMCID: PMC5888222
- DOI: 10.1210/er.2017-00253
The prevalence of obesity, measured by body mass index, has risen to unacceptable levels in both men and women in the United States and worldwide with resultant hazardous health implications. Genetic, environmental, and behavioral factors influence the development of obesity, and both the general public and health professionals stigmatize those who suffer from the disease. Obesity is associated with and contributes to a shortened life span, type 2 diabetes mellitus, cardiovascular disease, some cancers, kidney disease, obstructive sleep apnea, gout, osteoarthritis, and hepatobiliary disease, among others. Weight loss reduces all of these diseases in a dose-related manner-the more weight lost, the better the outcome. The phenotype of "medically healthy obesity" appears to be a transient state that progresses over time to an unhealthy phenotype, especially in children and adolescents. Weight loss is best achieved by reducing energy intake and increasing energy expenditure. Programs that are effective for weight loss include peer-reviewed and approved lifestyle modification programs, diets, commercial weight-loss programs, exercise programs, medications, and surgery. Over-the-counter herbal preparations that some patients use to treat obesity have limited, if any, data documenting their efficacy or safety, and there are few regulatory requirements. Weight regain is expected in all patients, especially when treatment is discontinued. When making treatment decisions, clinicians should consider body fat distribution and individual health risks in addition to body mass index.
Publication types
- Research Support, N.I.H., Extramural
- Research Support, Non-U.S. Gov't
- Obesity / diagnosis
- Obesity / drug therapy
- Obesity / surgery
- Obesity / therapy*
- Practice Guidelines as Topic*
- Societies, Medical*
Grants and funding
- P30 DK046200/DK/NIDDK NIH HHS/United States
- R01 DK040484/DK/NIDDK NIH HHS/United States
- R01 DK045343/DK/NIDDK NIH HHS/United States
- R37 DK040484/DK/NIDDK NIH HHS/United States
- Open access
- Published: 03 November 2021
The association between obesity and quality of life: a retrospective analysis of a large-scale population-based cohort study
- J. Stephenson 1 ,
- C. M. Smith 1 ,
- B. Kearns 2 ,
- A. Haywood 2 &
- P. Bissell 1
BMC Public Health volume 21 , Article number: 1990 ( 2021 ) Cite this article
14k Accesses
60 Citations
63 Altmetric
Metrics details
The relationship between obesity and health-related quality of life (HRQoL) may be confounded by factors such as multimorbidity. The aim of the study was to explore this relationship, controlling for long-term conditions and other health, lifestyle and demographic factors in a general adult population. There was specific interest in the impact of high weight status, measured by body mass index (BMI) levels (obesity, morbid obesity) compared with individuals of normal weight.
Health, lifestyle and demographic data were collected from 64,631 individuals aged 16 years and over registered in the Yorkshire Health Study; a long-term cohort study. Data were collected in 2 waves: from patients attending GP surgeries in the South Yorkshire region; and using online recruitment across the entire Yorkshire and Humber area. Univariable and multivariable regression methods were utilised to identify factors associated with HRQoL as measured by the EQ-5D summary score. Long-term conditions were tested as both covariates and mediating factors on the causal pathway between obesity and HRQoL.
Increasing levels of obesity are associated with reduced HRQoL, although this difference is negligible between those of normal weight and those who are overweight. Individuals with obesity and morbid obesity score 4.9 and 11.3 percentage points less on the EQ-5D summary scale respectively than those of normal weight. Concurrent physical, and particularly mental health-related long-term conditions are substantively related to HRQoL: those with 3 or more reported mental or physical health conditions score 29.8 and 14.6 percentage points less on the EQ-5D summary scale respectively than those with fewer conditions. Long-term conditions can be conceptualised as lying on the causal path between obesity and HRQoL, but there is weak evidence for a partial mediating relationship only.
Conclusions
To conclude, in agreement with the established literature we have found a clear inverse relationship between increasing weight status and decreasing HRQoL and confirmed the mediating role of long-term conditions in the reduction of HRQoL in people with obesity. Nevertheless, a high BMI remains independently related to HRQoL, suggesting that ‘healthy people with obesity’ may be in transition to an unhealthy future.
Peer Review reports
Health-related quality of life (HRQoL) is a broad subjective concept that encompasses both physical and mental health, which are themselves in complex relationships with other external factors such as health, socio-economic status, the environment and other factors [ 1 ]. Obesity is a condition of ‘abnormal or excessive fat accumulation that may impair health’, defined by the WHO [ 2 ] as a body mass index (BMI) greater than 30 kg/m 2 , with a BMI of more than 40 kg/m 2 defined as morbid obesity. The aetiology of obesity is complex and multifaceted, stemming from biological, behavioural and environmental causes [ 3 ].
Worldwide obesity has tripled since 1975, and in 2016, 1.9 billion adults (39% of the worldwide adult population) were considered to be overweight: i.e. have a BMI in the range 25 kg/m 2 ≤ BMI < 30 kg/m 2 ; and 650 million (13% of the worldwide population) were considered to have obesity: i.e. have a BMI in the range BMI ≤ 30 kg/m 2 [ 2 ]. In England in 2018, 63% of adults were classified as being overweight or having obesity, with 2 and 4% of men and women respectively being defined as having morbid obesity: i.e. have a BMI in the range BMI ≤ 40 kg/m 2 [ 4 ]. It has been predicted that by 2050 Britain could be a mainly obese society [ 3 ]. Connelly reported a noticeable increase in the proportion of the United Kingdom population at very high risk of chronic disease due to their weight [ 4 ]. Physical associations include long-term health conditions such as Type 2 diabetes, hypertension, dyslipidaemia, coronary artery disease, stroke, various cancers, reduced reproductive function, osteoarthritis, liver and gall bladder disease, chronic pain and adverse respiratory effects [ 3 , 5 , 6 ]. The proportion of individuals reporting long-term conditions (LTCs) has been shown to increase linearly with increasing BMI, and to be independently related to BMI, after adjusting for age and gender [ 7 ]. Similarly, the number of reported LTCs increases with BMI, with 25 and 42% of individuals with moderate and morbid obesity respectively reporting 3 or more LTCs, compared with 12% of normal weight individuals. In addition to physical disease, obesity is also associated with mental health conditions: sleep disorders, anxiety, depression low self-esteem, motivational disorders, eating disorders, impaired body image [ 1 , 8 , 9 , 10 ] and serious psychiatric disorders [ 10 , 11 ].
Obesity is associated with physical, mental and economic consequences. The economic consequences of obesity are substantial and increasing [ 12 ]. In the UK alone it is estimated that by 2050 the societal and business costs of obesity will reach £49.9billion per year [ 3 ]. These costs have been categorised by Seidall [ 13 ] as direct costs from treating obesity and its related diseases; societal costs arising from loss of work due to increased absence, physical limitations, lower life expectancy and unemployment benefits; and personal costs stemming e.g. from stigmatisation and discrimination leading to lower incomes and higher healthcare costs. Physical and mental long-term conditions can impact both on each other and Health Related Quality of Life [ 6 , 14 , 15 , 16 ], and the relationship between obesity and HRQoL can be both mediated and confounded by the presence of comorbidities [ 17 , 18 ] and other effects such as medication [ 11 ] and polypharmacy [ 19 ].
The Yorkshire Health Study (YHS) is an observational cohort study of health and lifestyle in Yorkshire and the Humber [ 20 , 21 ] supported by NIHR CLAHRC (Collaboration for Leadership in Applied Health Research and Care). Adults (aged 16 and over) residing in the in the Yorkshire and Humber region of England are eligible to enter.
The data, from 70,836 adults, was collected in two waves: the first 27,813 were recruited via GP surgeries in South Yorkshire between 2010 and 2012; the second wave of data collection, from 2013 to 2015 utilised online recruitment and the National Clinical Research Network to recruit 43,023 participants. The majority of participants, whether recruited in Waves 1 or 2, completed one survey only. It is well established that there is an inverse relationship between QoL and obesity [ 12 , 17 , 22 , 23 , 24 ]. There are many research studies that demonstrate improved quality of life following both dietary and surgical weight loss [ 25 , 26 , 27 ].
The aim of this study was to utilise a large, contemporary cohort from the UK to explore the relationships between obesity and HRQoL, controlling for LTCs and other health, lifestyle and demographic factors in a general adult population; considering specifically the impact of high levels of BMI (obesity and morbid obesity) in comparison to BMI levels corresponding to individuals of normal weight.
Personal (age, gender, academic history, employment status, socio-economic status, quality of life), health (history of diabetes, physical and mental long-term conditions, frequency of visits to health care professionals, frequency of visits to hospital, days off work due to sickness) and lifestyle (smoking status, weekly levels of walking and exercise) data were collected from participants who responded to either Wave 1 and/or the full version of the questionnaire administered in Wave 2 of the YHS.
HRQoL, as measured by the EQ-5D summary index (measured on a scale from 0 to 1, with higher values representing higher QoL, and derived from scores on individual EQ-5D domains of mobility, self-care, activities, pain and anxiety), was considered to be the outcome measure in the current investigation. The key predictor variable was weight status, measured using BMI, categorised for the purposes of the current investigation as Normal weight (18 kg/m 2 ≤ BMI < 25 kg/m 2 ); Overweight (25 kg/m 2 ≤ BMI < 30 kg/m 2 ), Obese (30 kg/m 2 ≤ BMI < 40 kg/m 2 ), and Morbidly obese (BMI ≥ 40 kg/m 2 ). This variable was collected in both waves of the survey. Individuals with BMI less than 18 kg/m 2 were not included in the analysis, as BMIs in this range may be indicative of illness or eating disorder. An investigation into the relationship between QoL and BMI using the first wave only of the YHS [ 17 ] revealed the relationship to be monotonic and approximately linear in individuals with BMI values of 18 kg/m 2 or more: inclusion of underweight individuals’ results in a curvilinear effect.
Additionally, a number of variables, also collected in one or both waves of the survey, were collected and examined for potential inclusion as covariates in the analysis (Table 1 ). The first mentioned category of the categorical variables above was considered to be the reference category in all cases.
In addition to modelling the LTC variables as covariates in a multiple regression model, these variables were assessed for their effect as mediating variables on the causal pathway between BMI and QoL; in the light of findings by Doll et al. [ 7 ] that the proportion of individuals reporting LTCs, and the number of reported LTCs are significantly predicted by BMI in controlled models.
Physical exercise (including activities such as swimming, playing football, cycling and aerobics) and walking time (including walking to work, to shops and leisure walking) in the week preceding data collection were estimated using the mid-point of options presented as ranges of times (none; 0–1 h per week; 2–3 h per week etc.) offered to respondents as response categories.
The data set was checked before analysis for errors. Any values outside of theoretical or plausible ranges were deleted or replaced with a limiting value as appropriate, with limits for inclusion of BMI values obtained using guidelines. The extent and nature of data missingness was investigated. Missing values were assessed for nature of missingness using Little’s test for data missing completely at random (MCAR) and separate variance t -tests and cross-tabulations. Data missing at random (MAR) was inferred if the MCAR test was statistically significant but missingness could be predicted from variables other than the outcome variable from separate variance t -tests and cross-tabulations. Following verification of missing data on key variables to be MCAR or MAR, complete case analysis was used with respect to both the key predictor variable (weight status as measured by BMI category) and the outcome measure (EQ-5D score) with no imputation conducted on these variables. Controlling variables with more than 5% missing values on remaining cases were dropped from further analysis. Controlling variables with less than 5% missing values that could be shown or inferred to be MCAR or MAR were imputed using expectation maximisation.
The data were summarised descriptively, by weight status (BMI category) and as a full cohort. A series of simple (univariable) regression models were conducted on valid cases, with imputation where necessary and appropriate, considering both the key variable of weight status and each controlling variable in turn as predictors. Controlling variables showing some substantive relationship with the outcome measure were carried forward for inclusion in a subsequent main effects multiple linear regression analysis alongside weight status. Included variables were assessed for collinearity and regression assumptions for the final multiple model were checked post-estimation using residual plots.
Model transferability was assessed by cross-validation. A regression equation was constructed based on a random 80% of cases with model coefficients used to obtain predicted values on the remaining validation sample. The correlation between predicted and actual values in the validation sample was then compared with the corresponding statistic for the main sample; with low or no reductions representing good model transferability.
Ethical approval for the YHS was granted by the NHS Research Ethics Committee (09/H1306/97).
Valid data were collected on 64,631 individuals. Data checking revealed a small proportion of certain variables with implausible or impossible data values. These were investigated on an individual basis and deleted or amended where necessary.
Calculated BMI values of the cleaned data set ranged from 8.32 to 85.9 kg/m 2 ; with a mean value of 26.7 kg/m 2 (SD 5.50 kg/m 2 ). The BMI ranges and corresponding frequencies associated with each original and merged category are summarised in Table 2 .
A summary of participant characteristics (by weight status) before imputation and variable deletion is summarised in Table 3 ; with data based on respondents from whom a valid weight status could be deduced.
While most differences across groups were statistically significant at the 5% significance level, reflecting the large sample size, few substantive differences across groups were observed. Uni-variable tests of significance revealed low effect sizes (measured by the ϕ and partial-η 2 statistics) of less than 5% for most reported variables in the table above. However, some cross-group differences of non-negligible magnitude were observed with respect to gender, diabetes status and academic qualifications. A higher proportion of women than men were in the group with morbid obesity; however, overall mean male BMI (26.9 kg/m 2 ; SD 4.83 kg/m 2 ) was higher than the mean female BMI (26.6 kg/m 2 ; SD 5.84 kg/m 2 ). The proportion of those in the Normal weight group who were qualified to degree level or above was, at 12.1%, more than double that of those in the group with obesity (5.5%) and over 3 times that of those in the group with morbid obesity (3.5%). The proportion of those in the Normal weight group who suffered from 3 or more long-term mental health-related conditions was, at 6.7%, less than half that of those in the group with obesity (15.7%) and less than a third that of those in the group with morbid obesity (24.5%).
Little’s test for MCAR using all quantitative variables with complete or near-complete cases revealed no evidence that missing EQ-5D scores were not MCAR ( p = 0.408). Separate variance t-tests revealed no evidence that missing weight statuses were not MAR. The variables corresponding to diabetes status, employment status, IMD, exercise levels, alcohol consumption and days off work due to sickness were not carried forward for consideration due to excessive proportions of missing values on these variables.
P -values, parameter estimates, associated confidence intervals, and effect sizes (using the partial-η 2 statistic) from a series of univariable regression analyses conducted the outcome measure of EQ-5D score on an imputed data set including the key predictor variable and all controlling variables with complete or near-complete set of cases as identified in Table 3 above, are summarised in Table 4 .
A mediation analysis revealed that both of the variables modelling mental or physical health-related LTCs exhibited some mediating effect on the relationship between weight status and HRQoL. All paths in the mediation models considering weight status as a predictor, and the mental or physical health-related LTCs in turn as mediators were significant. Path coefficients for weight status were revealed to be − 0.010 in a univariable regression of QoL on weight status; − 0.007 in a model including the variable modelling mental health LTCs and − 0.007 in a model including the variable modelling physical health LTCs. Hence while conditions for partial mediation were met, the conditions were full mediation were not met. The substantive mediating effect was low and weight status continued to significantly predict the outcome in the presence of the mediating variable. Hence analysis proceeded with LTCs being modelled as a controlling covariate.
The simple regression models suggested that age, presence/absence of long-term conditions, level of contact with health professions in last 3 months, number of hours per week spent walking, and number of hospital outpatient visits in previous 3 months should be included alongside a weight status category in a multiple model. As strong evidence for statistical significance was expected in most cases due to the size of the data set, assessments for inclusion were made primarily on the basis of effect sizes, with an associated partial-η 2 statistic of about 0.025 or more considered to indicate grounds for inclusion of a particular variable. As the predictor variable of key contextual interest, this did not apply to any of the weight status categories. Model parameters from this multiple model are summarised in Table 5 .
The R 2 and adjusted-R 2 statistics for this model were both 0.390; representing a moderately good fit to the data. No evidence for collinearity was revealed, with variance inflation factors all within tolerable limits. Analysis of residuals revealed no clear evidence for violations of regression assumptions, with normally distributed standardised residuals which exhibited no clear pattern when plotted against standardised predicted values. The model showed very good cross-validation properties, with negligible loss in correlation computed from the validation sample fitted values against predictions from the training sample model coefficients.
Hence controlling for other categorical factors and covariates, compared to individuals in the Normal weight category; HRQoL was essentially the same in individuals in the Overweight category; slightly lower (4.9 percentage points less on the EQ-5D summary index) in individuals in the Obese category and lower (11.3 percentage points less on the EQ-5D summary index) in individuals in the Morbidly obese category. Hence the effect of morbid obesity, compared to normal weight, has approximately the same impact as 3 or more physical long-term conditions or an increase in age of about 55 years. Amongst the controlling variables, those with the greatest substantive effect on QoL were mental and physical health-related LTCs: those with 3 or more mental health conditions scored 29.8 percentage points less on the EQ-5D summary index than those with 2 or fewer conditions; and those with 3 or more physical health conditions scored 14.6 percentage points less on the EQ-5D summary index than those with 2 or fewer conditions. Higher quality of life was also reported by younger people, by those who saw health professionals more infrequently and spent less time visiting hospital as an outpatient, and by those who spent more time walking.
Key findings
The analysis has revealed a clear relationship indicating lower levels of QoL with weight status defined by categories of increasing BMI in individuals with BMIs in the range of 18 kg/m 2 and above. This monotonic decrease in QoL, recorded in groups categorised by increasing BMI, is consistent with both the findings relating to the individual EQ-5D items in the analysis by Kearns et al. [ 17 ] of the first wave of the YHS data, and the wider literature [ 12 , 23 ]. The effect on QoL of weight status category is substantial, particularly for those in the highest BMI category. This reduction in QoL as a result of increasing BMI is greater than that found linked to cancer, myocardial infarction and diabetes, and similar to having schizophrenia, heart failure or kidney failure (Sullivan 2001). However, the EQ-5D summary index is a highly negatively skewed measure, with about one third of our respondents scoring the maximum value of 1.00 and over half of respondents scoring 0.84 or more.
Comparing the estimates and magnitudes thereof of the weight status variables in the simple and multiple models reveals that the effect of weight status is smaller in the multiple (controlled) model. The variables corresponding to mental and physical health-related LTCs in the multiple model appear to be of greater effect on QoL than weight status itself. This may be due to a proportion of the residual variance ascribed to weight status in the simple model being ascribed to other variables in the multiple model; specifically, LTCs, which are already known to be related to weight status from the descriptive analysis and is reflected in the 2007 Sach analysis of BMI and quality of life. It may also reflect the status of obesity as a risk factor for many LTCs [ 3 , 5 , 6 , 7 , 8 ]. However, there are no changes of direction of association of parameter coefficients or substantial changes in parameter estimates or inferences of significance between the models. Further work considering the impact of specific individual conditions may be beneficial.
The mediation analysis reveals that the presence of mental or physical health-related LTCs has a limited partial mediating effect on the underlying relationship between weight status and QoL. In the current analysis, LTCs are analysed as controlling factors. Nonetheless, LTCs can alternatively be conceptualised as lying on the causal path between BMI and QoL [ 1 , 10 , 17 ]; although the direct link between BMI and QoL is stronger and more intuitive. Further model-testing work is needed to establish the existence of, and direction of associations between other constructs represented in the YHS.
The unique contribution of BMI to QoL is consistent with Scottish data [ 18 ] which found an independent relationship between obesity and Quality of Life. This is in contrast to the ‘Healthy Obesity’ hypothesis and may represent a subset of the population ‘in transition’ to unhealthy obesity [ 28 ] via metabolic syndrome, not measured in our study.
The largest unique effect in the multiple model was the presence of 3 or more mental health LTCs. This may be an artefact of the data, explained by a presumed higher likelihood of MH LTCs being related in our sample, compared to the ‘independence’ of the physical domains of LTC. The second biggest effect is degree of contact with a health professional, which we presume is acting as a proxy measure for general health.
Strengths and limitations
The strengths of the YHS are its large sample size which allows for an exploration of detailed obesity categories, comprehensive examination of a wide range of variables, and the use of EQ-5D which measures HRQoL using public preferences.
Most measures captured by the YHS are self-reported and may not be completely reliable; particularly those requiring accurate recall, such as activity levels or levels of contact with healthcare professionals over an extended period of time; or the ability of respondents to distinguish between, for example, hospital visits as an out-patient or day case. The key predictor of BMI requires accurate self-reporting of both height and body weight in appropriate units. In addition, self-reported height and weight are respectively over and underestimated in both men and women (Niedhammer 2000, Spencer 2002, Taylor 2006). In the current study, analysis was restricted to variables which were derived from items elicited in both waves of the questionnaire.
The fit of the multiple regression model to the data, though of moderately high magnitude, may have been constrained in magnitude by uncertainties in the integrity of certain measures and the limited availability of variables for which an acceptable proportion of valid cases were available. Nonetheless, a moderately good fit was obtained and cross-validation procedures revealed that model portability is good; it should be expected that the model will perform equally well on samples other than that from which parameter coefficients were derived.
Implications for future work
This study has demonstrated that further work is needed to establish the existence of, and direction of associations; for example, it seems plausible that not only can factors such as BMI and exercise impact on quality of life (as was assumed in this analysis), but also that variables such as exercise level and BMI are correlated with a plausible association in either direction. A number of models are required to be tested for model fit using, for example, a confirmatory factor analysis approach in order to ensure that an optimal series of relationships are tested.
To conclude, in agreement with the established literature we have found a clear inverse relationship between increasing weight status and decreasing QoL, using a large regional cohort study. We have investigated the influence of other demographic, lifestyle and health related domains on this relationship and confirmed the mediating role of LTCs in the reduction of QoL in people with obesity. Nevertheless, a high weight status remains independently related to QoL, suggesting that the ‘healthy obese’ may be in transition to an unhealthy future.
Availability of data and materials
Anonymised data and details regarding using the resource for recruiting participants to studies can be gathered by contacting Professor Elizabeth Goyder ( [email protected] ). Multi-disciplinary collaboration is strongly encouraged.
Abbreviations
Body mass index
Health-related quality of life
- Quality of life
- Long-term conditions
Taylor VH, Forhan M, Vigod SN, McIntyre RS, Morrison KM. The impact of obesity on quality of life. Best Pract Res Clin Endocrinol Metab. 2013;27(2):139–46. https://doi.org/10.1016/j.beem.2013.04.004 .
Article PubMed Google Scholar
World Health Organization. Obesity and overweight. 2018. Accessed 10 June 2020 http://www.who.int/mediacentre/factsheets/fs311/en/ .
Butland B, Jebb S, Kopelman P, McPherson K, Thomas S, Mardell J, et al. Foresight. Tackling obesities: future choices—project report. London: Government Office for Science; 2007. http://www.foresight.gov.uk .
Google Scholar
Conolly A, Craig S. Health survey for England 2018 - overweight and obesity in adults and children. Leeds: NHS Digital, NHS; 2019.
Chu DT, Nguyet NT, Dinh TC, Lien NV, Nguyen KH, Ngoc VT, et al. An update on physical health and economic consequences of overweight and obesity. Diab Metab Syndr. 2018;12(6):1095–100. https://doi.org/10.1016/j.dsx.2018.05.004 .
Article Google Scholar
Okifuji A, Hare BD. The association between chronic pain and obesity. J Pain Res. 2015;8:399. https://doi.org/10.2147/JPR.S55598 .
Article PubMed PubMed Central Google Scholar
Doll HA, Petersen SE, Stewart-Brown SL. Obesity and physical and emotional well-being: associations between body mass index, chronic illness, and the physical and mental components of the SF-36 questionnaire. Obes Res. 2000;8(2):160–70. https://doi.org/10.1038/oby.2000.17 .
Article CAS PubMed Google Scholar
Chu DT, Nguyet NT, Nga VT, Lien NV, Vo DD, Lien N, et al. An update on obesity: mental consequences and psychological interventions. Diab Metab Syndr. 2019;13(1):155–60. https://doi.org/10.1016/j.dsx.2018.07.015 .
Scott KM, Bruffaerts R, Simon GE, Alonso J, Angermeyer M, De Girolamo G, et al. Obesity and mental disorders in the general population: results from the world mental health surveys. Int J Obes. 2008;32(1):192–200. https://doi.org/10.1038/sj.ijo.0803701 .
Article CAS Google Scholar
Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, Van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006;63(7):824–30. https://doi.org/10.1001/archpsyc.63.7.824 .
Holt RI, Peveler RC. Obesity, serious mental illness and antipsychotic drugs. Diabetes Obes Metab. 2009;11(7):665–79. https://doi.org/10.1111/j.1463-1326.2009.01038.x .
Dixon JB. The effect of obesity on health outcomes. Mol Cell Endocrinol. 2010;316(2):104–8. https://doi.org/10.1016/j.mce.2009.07.008 .
Seidell JC. Societal and personal costs of obesity. Exper Clin Endocrinol Diab. 1998;106(S 02):7–10.
Kearns B, Rafia R, Leaviss J, Preston L, Brazier JE, Palmer S, et al. The cost-effectiveness of changes to the care pathway used to identify depression and provide treatment amongst people with diabetes in England: a model-based economic evaluation. BMC Health Serv Res. 2017;17(1):78. https://doi.org/10.1186/s12913-017-2003-z .
Naylor C, Parsonage M, McDaid D, Knapp M, Fossey M, Galea A: Long-term conditions and mental health. The cost of co-morbidities. The King’s Fund and Centre for Mental Health; 2012. http://www.kingsfund.org.uk/sites/files/kf/field/field_publication_file/long-term-conditions-mental-health-costcomorbidities-naylor-feb12.pdf .
Wu M, Brazier JE, Kearns B, Relton C, Smith C, Cooper CL. Examining the impact of 11 long-standing health conditions on health-related quality of life using the EQ-5D in a general population sample. Eur J Health Econ. 2015;16(2):141–51. https://doi.org/10.1007/s10198-013-0559-z .
Kearns B, Ara R, Young T, Relton C. Association between body mass index and health-related quality of life, and the impact of self-reported long-term conditions–cross-sectional study from the South Yorkshire cohort dataset. BMC Public Health. 2013;13(1):1009. https://doi.org/10.1186/1471-2458-13-1009 .
Ul-Haq Z, Mackay DF, Fenwick E, Pell JP. Impact of metabolic comorbidity on the association between body mass index and health-related quality of life: a Scotland-wide cross-sectional study of 5,608 participants. BMC Public Health. 2012;12(1):143. https://doi.org/10.1186/1471-2458-12-143 .
Silveira EA, Dalastra L, Pagotto V. Polypharmacy, chronic diseases and nutritional markers in community-dwelling older. Revista Brasileira de Epidemiologia. 2014;17(4):818–29. https://doi.org/10.1590/1809-4503201400040002 .
Green MA, Little E, Cooper R, Relton C, Strong M. Investigation of social, demographic and health variations in the usage of prescribed and over-the-counter medicines within a large cohort (South Yorkshire, UK). BMJ Open. 2016;6(9):e012038. https://doi.org/10.1136/bmjopen-2016-012038 .
Stephenson J, Smith CM, Goyder EC, et al. Cohort profile update: the Yorkshire health study. Int J Epidemiology. (in press).
Jia H, Lubetkin EI. The impact of obesity on health-related quality-of-life in the general adult US population. J Public Health. 2005;27(2):156–64. https://doi.org/10.1093/pubmed/fdi025 .
Kolotkin RL, Andersen JR. A systematic review of reviews: exploring the relationship between obesity, weight loss and health-related quality of life. Clin Obes. 2017;7(5):273–89. https://doi.org/10.1111/cob.12203 .
Article CAS PubMed PubMed Central Google Scholar
Sach TH, Barton GR, Doherty M, Muir KR, Jenkinson C, Avery AJ. The relationship between body mass index and health-related quality of life: comparing the EQ-5D, EuroQol VAS and SF-6D. Int J Obes. 2007;31(1):189–96. https://doi.org/10.1038/sj.ijo.0803365 .
Carson TL, Hidalgo B, Ard JD, Affuso O. Dietary interventions and quality of life: a systematic review of the literature. J Nutr Educ Behav. 2014;46(2):90–101. https://doi.org/10.1016/j.jneb.2013.09.005 .
Dixon JB, Dixon ME, O’Brien PE. Quality of life after lap-band placement: influence of time, weight loss, and comorbidities. Obes Res. 2001;9(11):713–21. https://doi.org/10.1038/oby.2001.96 .
Hachem A, Brennan L. Quality of life outcomes of bariatric surgery: a systematic review. Obes Surg. 2016;26(2):395–409. https://doi.org/10.1007/s11695-015-1940-z .
Smith GI, Mittendorfer B, Klein S. Metabolically healthy obesity: facts and fantasies. J Clin Investig. 2019;129(10):3978–89. https://doi.org/10.1172/JCI129186 .
Relton C, Bissell P, Smith C, Blackbrun J, Cooper CL, Nicholl J, et al. South Yorkshire cohort: a ‘cohort trials facility’ study of health and weight - protocol for the recruitment phase. BMC Public Health. 2011;11(1):640. https://doi.org/10.1186/1471-2458-11-640 .
Download references
Acknowledgements
This report is independent research funded by the National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care (CLAHRC) Yorkshire and Humber. The views expressed in this publication are those of the authors and not necessarily those of the Yorkshire Health Study Management Team or Steering Committee, National Institute for Health Research or the Department of Health and Social Care.
This work was supported by the National Institute for Health Research (NIHR) Collaborations for Leadership in Applied Health Research and Care (CLAHRC) Yorkshire and Humber (NIHR200166). Analysis was supported by the Universities of Huddersfield and Sheffield.
Author information
Authors and affiliations.
School of Human & Health Sciences, University of Huddersfield, Queensgate, Huddersfield, HD1 3DH, UK
J. Stephenson, C. M. Smith & P. Bissell
School of Health and Related Research, University of Sheffield, Sheffield, UK
B. Kearns & A. Haywood
You can also search for this author in PubMed Google Scholar
Contributions
PB, AH, CS and BK had the original concept. JS and CS designed the work. All authors agreed the methodology. JS performed the statistical analyses. All authors interpreted the results. CS and JS drafted the manuscript. All authors fed back comments. All authors read and approved the final manuscript.
Corresponding author
Correspondence to J. Stephenson .
Ethics declarations
Ethics approval and consent to participate.
The protocol for the South Yorkshire Cohort (the early name for the Yorkshire Health Study) was approved by the NHS Research Ethics Committee on 27 April 2010 (09/H1306/97). All methods were carried out in accordance with relevant guidelines and regulations. Informed consent was obtained from all participants based on the principle of ‘patient-centred informed consent’ i.e. where patient information and consent aim to replicate that of real world routine healthcare rather than conform to the needs of standard trial designs. Therefore all cohort patients consented to provide observational data at the outset, be contacted again, and for their information to be used to look at the benefit of healthcare treatments; however, consent to “try” a particular intervention in the future was sought only from those offered that intervention. This method of obtaining consent replicates the ‘patient-centred’ information and consent procedures that exist in routine health care, where clinicians provide patients with the information they need, at the time they need it. The consent procedure is described fully in the South Yorkshire Cohort Protocol [ 29 ]. Research on human data was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.

Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Stephenson, J., Smith, C.M., Kearns, B. et al. The association between obesity and quality of life: a retrospective analysis of a large-scale population-based cohort study. BMC Public Health 21 , 1990 (2021). https://doi.org/10.1186/s12889-021-12009-8
Download citation
Received : 08 March 2021
Accepted : 21 September 2021
Published : 03 November 2021
DOI : https://doi.org/10.1186/s12889-021-12009-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Download PDF
- CME & MOC
- Share X Facebook Email LinkedIn
- Permissions
Obesity Management in Adults : A Review
- 1 Department of Medicine, Division of General Internal Medicine and Clinical Innovation, New York University Grossman School of Medicine, New York, New York
- 2 Department of Population Health, New York University Grossman School of Medicine, New York, New York
- 3 Family Health Centers at NYU Langone, New York, New York
- 4 Division of General Internal Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland
- 5 Division of General Internal Medicine, University of Minnesota Medical School, Minneapolis
- 6 Division of General Internal Medicine, University of Colorado School of Medicine, Aurora
- 7 New York Harbor Veteran Affairs, New York, New York
- Medical News & Perspectives What to Know About Zepbound, the Newest Antiobesity Drug Jennifer Abbasi JAMA
- Medical News & Perspectives What to Know About Wegovy’s Rare but Serious Adverse Effects Kate Ruder, MSJ JAMA
- Medical News & Perspectives More Data Emerge for New Antiobesity Drugs Kate Ruder, MSJ JAMA
Importance Obesity affects approximately 42% of US adults and is associated with increased rates of type 2 diabetes, hypertension, cardiovascular disease, sleep disorders, osteoarthritis, and premature death.
Observations A body mass index (BMI) of 25 or greater is commonly used to define overweight, and a BMI of 30 or greater to define obesity, with lower thresholds for Asian populations (BMI ≥25-27.5), although use of BMI alone is not recommended to determine individual risk. Individuals with obesity have higher rates of incident cardiovascular disease. In men with a BMI of 30 to 39, cardiovascular event rates are 20.21 per 1000 person-years compared with 13.72 per 1000 person-years in men with a normal BMI. In women with a BMI of 30 to 39.9, cardiovascular event rates are 9.97 per 1000 person-years compared with 6.37 per 1000 person-years in women with a normal BMI. Among people with obesity, 5% to 10% weight loss improves systolic blood pressure by about 3 mm Hg for those with hypertension, and may decrease hemoglobin A 1c by 0.6% to 1% for those with type 2 diabetes. Evidence-based obesity treatment includes interventions addressing 5 major categories: behavioral interventions, nutrition, physical activity, pharmacotherapy, and metabolic/bariatric procedures. Comprehensive obesity care plans combine appropriate interventions for individual patients. Multicomponent behavioral interventions, ideally consisting of at least 14 sessions in 6 months to promote lifestyle changes, including components such as weight self-monitoring, dietary and physical activity counseling, and problem solving, often produce 5% to 10% weight loss, although weight regain occurs in 25% or more of participants at 2-year follow-up. Effective nutritional approaches focus on reducing total caloric intake and dietary strategies based on patient preferences. Physical activity without calorie reduction typically causes less weight loss (2-3 kg) but is important for weight-loss maintenance. Commonly prescribed medications such as antidepressants (eg, mirtazapine, amitriptyline) and antihyperglycemics such as glyburide or insulin cause weight gain, and clinicians should review and consider alternatives. Antiobesity medications are recommended for nonpregnant patients with obesity or overweight and weight-related comorbidities in conjunction with lifestyle modifications. Six medications are currently approved by the US Food and Drug Administration for long-term use: glucagon-like peptide receptor 1 (GLP-1) agonists (semaglutide and liraglutide only), tirzepatide (a glucose-dependent insulinotropic polypeptide/GLP-1 agonist), phentermine-topiramate, naltrexone-bupropion, and orlistat. Of these, tirzepatide has the greatest effect, with mean weight loss of 21% at 72 weeks. Endoscopic procedures (ie, intragastric balloon and endoscopic sleeve gastroplasty) can attain 10% to 13% weight loss at 6 months. Weight loss from metabolic and bariatric surgeries (ie, laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass) ranges from 25% to 30% at 12 months. Maintaining long-term weight loss is difficult, and clinical guidelines support the use of long-term antiobesity medications when weight maintenance is inadequate with lifestyle interventions alone.
Conclusion and Relevance Obesity affects approximately 42% of adults in the US. Behavioral interventions can attain approximately 5% to 10% weight loss, GLP-1 agonists and glucose-dependent insulinotropic polypeptide/GLP-1 receptor agonists can attain approximately 8% to 21% weight loss, and bariatric surgery can attain approximately 25% to 30% weight loss. Comprehensive, evidence-based obesity treatment combines behavioral interventions, nutrition, physical activity, pharmacotherapy, and metabolic/bariatric procedures as appropriate for individual patients.
Read More About
Elmaleh-Sachs A , Schwartz JL , Bramante CT , Nicklas JM , Gudzune KA , Jay M. Obesity Management in Adults : A Review . JAMA. 2023;330(20):2000–2015. doi:10.1001/jama.2023.19897
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

Overweight, obesity and weight management
- Sport, Exercise and Health Sciences
Publisher statement
Publication date, administrator link.
- https://repository.lboro.ac.uk/account/articles/9609809
Usage metrics

- Other health sciences not elsewhere classified

- Systematic Review
- Open access
- Published: 13 May 2024
Sarcopenia and sarcopenic obesity among older adults in the nordic countries: a scoping review
- Fereshteh Baygi 1 na1 ,
- Sussi Friis Buhl 1 na1 ,
- Trine Thilsing 1 ,
- Jens Søndergaard 1 &
- Jesper Bo Nielsen 1
BMC Geriatrics volume 24 , Article number: 421 ( 2024 ) Cite this article
351 Accesses
Metrics details
Sarcopenia and sarcopenic obesity (SO) are age-related syndromes that may compromise physical and mental health among older adults. The Nordic countries differ from other regions on prevalence of disease, life-style behavior, and life expectancy, which may impact prevalence of sarcopenia and SO. Therefore, the aim of this study is to review the available evidence and gaps within this field in the Nordic countries.
PubMed, Embase, and Web of science (WOS) were searched up to February 2023. In addition, grey literature and reference lists of included studies were searched. Two independent researcher assessed papers and extracted data.
Thirty-three studies out of 6,363 searched studies were included in this scoping review. Overall prevalence of sarcopenia varied from 0.9 to 58.5%. A wide prevalence range was still present for community-dwelling older adults when definition criteria and setting were considered. The prevalence of SO ranged from 4 to 11%, according to the only study on this field. Based on the included studies, potential risk factors for sarcopenia include malnutrition, low physical activity, specific diseases (e.g., diabetes), inflammation, polypharmacy, and aging, whereas increased levels of physical activity and improved dietary intake may reduce the risk of sarcopenia. The few available interventions for sarcopenia were mainly focused on resistance training with/without nutritional supplements (e.g., protein, vitamin D).
The findings of our study revealed inadequate research on SO but an increasing trend in the number of studies on sarcopenia. However, most of the included studies had descriptive cross-sectional design, small sample size, and applied different diagnostic criteria. Therefore, larger well-designed cohort studies that adhere to uniform recent guidelines are required to capture a full picture of these two age-related medical conditions in Nordic countries, and plan for prevention/treatment accordingly.
Peer Review reports
The number of older adults with age-related disorders is expected to increase worldwide [ 1 , 2 ]. Sarcopenia and sarcopenic obesity (SO) are both age-related syndromes that may compromise the physical and mental health of older adults and increase their need for health care services in old age [ 3 , 4 ], and this may challenge the sustainability of health care systems economically and by shortage of health care personnel [ 5 ].
Sarcopenia is characterized by low muscle mass in combination with low muscle strength [ 4 ]. SO is characterized by the co-existence of obesity (excessive adipose tissue) and sarcopenia [ 3 ]. Sarcopenia and SO are both associated with physical disability, risk of falls, morbidity, reduced quality of life and early mortality [ 4 , 6 , 7 , 8 , 9 ]. In SO the consequences of sarcopenia and obesity are combined and maximized [ 4 , 6 , 7 , 8 ].
Etiology of sarcopenia and SO is multifactorial and closely linked to multimorbidity [ 3 , 7 , 8 , 9 , 10 ]. Nevertheless, lifestyle and behavioral components particularly diet and physical activity, are important interrelated factors that potentially can be modified. Physical inactivity and sedentary behavior may accelerate age-related loss of muscle mass, reduce energy expenditure, and increase risk of obesity [ 3 , 11 ]. In addition, weight cycling (the fluctuations in weight following dieting and regain) and an unbalanced diet (particularly inadequate protein intake) may accelerate loss of muscle mass and increase severity of sarcopenia and SO in older adults [ 3 , 12 ]. International guideline for the treatment of sarcopenia emphasizes the importance of resistance training potentially in combination with nutritional supplementation to improve muscle mass and physical function [ 13 ]. Similar therapeutic approach is suggested for treatment of SO [ 14 ]. However, more research is needed to confirm optimal treatment of SO [ 14 ].
According to a recently published meta-analysis the global prevalence of sarcopenia ranged from 10 to 27% in populations of older adults ≥ 60 years [ 15 ]. Further the global prevalence of SO among older adults was 11% [ 8 ]. So, sarcopenia and SO are prevalent conditions, with multiple negative health outcomes and should be given special attention [ 16 ]. Despite the large burden on patients and health care systems, the awareness of the importance of skeletal muscle maintenance in obesity is low among clinicians and scientists [ 3 , 16 ].
A recent meta-analysis on publication trends revealed that despite an increase in global research on sarcopenia, the Nordic countries were only limitedly represented [ 6 ]. Nordic countries may differ from other regions on aspects associated with the prevalence and trajectory of sarcopenia and SO and challenge the representativeness of research findings from other parts of the world. These include a different prevalence pattern of noncommunicable diseases [ 17 ], different life-style behavior and life-style associated risk factors [ 15 , 18 ], and higher life expectancy [ 18 ].
The Nordic countries including Sweden, Finland, Iceland, Norway, Denmark, and three autonomous areas (Åland Islands, Greenland and Faroe Islands) share common elements of social and economic policies such as a comprehensive publicly financed health care system [ 18 , 19 ]. Additionally, these countries have a strong tradition of collaboration including a common vision of a socially sustainable region by promoting equal health and inclusive participation in society for older adults [ 20 ]. Therefore, more insight into the etiology, prevalence, and risk factors for sarcopenia and SO among older adults is a prerequisite for the development and implementation of effective strategies to prevent and treat these complex geriatric conditions in this geographic region. So, the aim of this study is to conduct a scoping review to systematically identify and map the available evidence while also addressing knowledge gaps and exploring the following research questions: (1) What are the prevalence of sarcopenia and SO in older adults living in the Nordic countries? (2) Which risk factors or contributing conditions are involved in the development of sarcopenia and SO in the Nordic Countries? (3) Which interventions to prevent or counteract negative health outcomes of sarcopenia and SO have been tested or implemented among older adults living in the Nordic countries?
Identification of relevant studies
The development and reporting of this review were done by following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [ 21 ].
The literature search was developed to target three main areas: Sarcopenia, sarcopenic obesity, and aging (See Appendix 1 for full search strategy). All studies published before the end of February 2023 were included in this scoping review. The optimal sensitivity of search was obtained by simultaneous search of the following databases: PubMed, Embase, and Web of science (WOS). Additionally, a detailed search for grey literature was performed in relevant databases (e.g., Research Portal Denmark, Libris, Oria, Research.fi). Besides, reference lists of the included studies were reviewed to identify eligible studies. Duplicates and non-peer reviewed evidence (e.g., PhD thesis) were excluded but if the latter contained published articles of relevance, these were included. If more than one publication on similar outcomes (e.g., prevalence) were based on a single study, just one publication was included. Data were extracted from large studies with combined data from several countries only when findings were presented separately for the Nordic countries.
Inclusion and exclusion criteria
The inclusion criteria were as follow : Broad selection criteria were used to be comprehensive: (1) studies with any outcome (e.g., prevalence, risk factors, etc.) to address our research questions on sarcopenia and SO, (2) studies on subjects with age ≥ 60 years in any type of settings (e.g., community, nursing homes, general practice, hospital, outpatients, homecare, etc.), (3) studies using any definition of sarcopenia and SO without restriction for criteria and cutoff values, (4) all type of study designs (e.g., randomized control trials, cohort studies, cross-sectional, etc.), (5) studies should be conducted in the Nordic countries The exclusion criteria are as follow : (1) studies without relevant outcome to sarcopenia or SO, (2) studies without sufficient information to determine eligibility.
Study selection and data extraction
Two independent researchers screened literature and conducted data extraction. Any discrepancies between them were resolved through discussion.
First, duplicates were removed by using EndNote 20.6 software, then titles and abstracts were screened to narrow down the list of potentially eligible studies. Finally, the full text review was done to examine in detail the studies that were not excluded in first step. For more clarification, the reasons for the exclusion were recorded (Fig. 1 ).
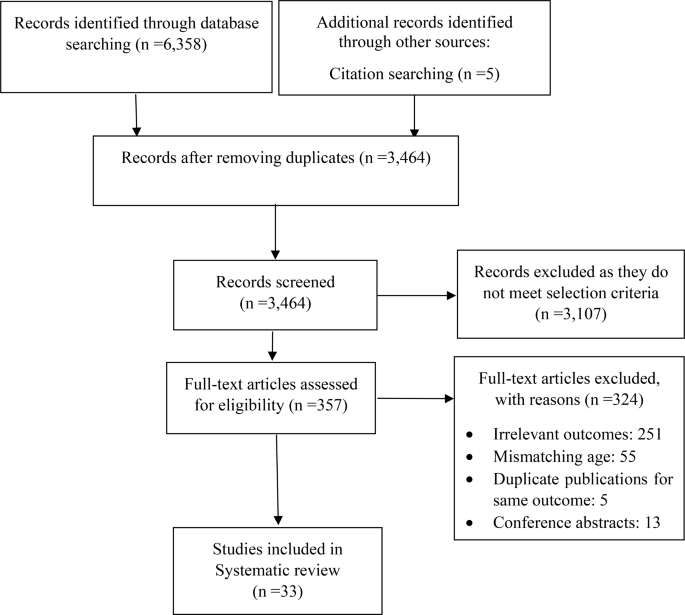
PRISMA diagram for searching resources
The following information was extracted: (1) study characteristics (e.g., first author’s name, country, year of publication), (2) characteristics of the target population (e.g., age, sex), (3) study design, setting, intervention duration and follow-up time (if applicable), measurements, tools, criteria, and results.
Study selection
A combined total of 6,358 studies were identified through the initial electronic database and grey literature searches. An additional five articles were identified through other sources (citation searching). After removing duplication, 3,464 articles remained. A total of 3107 articles were excluded based on screening titles and abstracts. Out of the remaining 357 studies, 324 were excluded after the full-text review. Finally, 33 studies met our inclusion criteria and were included in this current scoping review [ 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 ] (Fig. 1 ).
Study characteristics
Table 1 summarized characteristics of the included studies.
The number of documents showed an increasing trend between 2020 and 2021. A peak in the number of publications was observed in 2021 (24.2% of all documents). All the studies were conducted across four (Denmark, Norway, Sweden, and Finland) out of the five Nordic countries and three autonomous areas. The highest contribution in this field was made by Sweden ( n = 12).
Most studies were conducted in community-dwelling settings [ 22 , 23 , 24 , 28 , 30 , 31 , 35 , 36 , 38 , 39 , 40 , 42 , 45 , 46 , 47 , 48 , 49 , 54 ]. Seven studies included patients with acute diseases (hospital-setting) [ 26 , 27 , 33 , 37 , 50 , 51 , 52 ], while four studies included patients with chronic conditions (out-patient setting) [ 25 , 32 , 41 , 44 ], and one study including nursing-home residents [ 34 ]. In terms of study design, most of the studies were observation studies with a cross-sectional or longitudinal design ( 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 33 , 34 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 ), while three studies [ 32 , 35 , 46 ] applied interventions. It appears, however, that one study [ 32 ] out of the above three interventions is sub-project conducted within the framework of larger intervention program. Sample size ranged from 49 in a cross-sectional case control study [ 52 ] to 3334 in a cohort study [ 30 ].
Five studies were among males only [ 22 , 24 , 36 , 45 , 53 ] and three studies included females only [ 38 , 47 , 54 ]. The rest of the studies had a mixed sample. Top subject area was sarcopenia (31 out of the 33 included studies), and on this subject, publications were categorized into the following research areas (with some studies addressing more areas): prevalence [ 22 , 23 , 24 , 25 , 26 , 27 , 29 , 30 , 33 , 35 , 36 , 37 , 40 , 42 , 44 , 45 , 47 , 49 , 50 , 51 , 52 , 53 , 54 ], risk factors [ 24 , 27 , 28 , 30 , 31 , 34 , 38 , 40 , 42 , 44 , 47 , 49 , 50 , 51 ], and effectiveness of interventions on sarcopenia or indicator of sarcopenia [ 32 , 35 , 46 ].
In most studies sarcopenia was defined according to the criteria set by the European Working Group on Sarcopenia in Older People in the updated version from 2019 (EWGSOP2) ( n = 15) or the original version from 2010 (EWGSOP) ( n = 14). However, in some studies multiple criteria such as EWGSOP, EWGSOP2, and National Institutes of Health Sarcopenia Project definition (FNIH) were applied [ 27 , 39 , 43 ], and in other studies alternative criteria were used [ 26 , 33 , 35 , 45 , 57 ].
Different assessment methods of muscle mass including Dual energy X-ray absorptiometry (DXA) [ 22 , 24 , 25 , 27 , 29 , 30 , 32 , 33 , 38 , 39 , 40 , 41 , 45 , 46 , 47 , 52 , 53 , 54 ], Bioelectrical Impedance Analysis (BIA) [ 28 , 31 , 34 , 44 , 48 , 49 ], Bioimpedance Spectroscopy (BIS) [ 35 , 42 , 43 ], Computed Tomography (CT) [ 33 ], and Computed Tomography Angiogram (CTA) [ 26 ] were used in the included studies.
SO were defined by the co-existence of sarcopenia with obesity. Studies on SO used the EWGSOP2 criteria [ 39 ], or the EWGSOP2 criteria for hand grip strength only (probable sarcopenia) [ 23 ] in combination with obesity estimated from BMI cut points [ 23 , 39 ], waist circumference [ 23 , 39 ], and fat mass percentage [ 39 ]. Lastly, one study used measures of body composition measures that reflect adiposity as estimates of SO [ 48 ].
Four studies reported the prevalence of “probable sarcopenia” [ 23 , 30 , 36 , 45 ], while two studies reported the prevalence of sarcopenia and comorbidities (e.g., osteopenia, pre-frailty, malnutrition) [ 33 , 40 ].
Narrative synthesis
Due to the heterogeneity of the studies in definition of sarcopenia, settings, and sample size, the overall reported prevalence was variable and ranged from 0.9% [ 54 ] to 58.5% [ 26 ]. However, according to the most commonly used criteria (EWGSOP2) the highest (46%) and lowest (1%) prevalence of sarcopenia was reported in Sweden among inpatients in geriatric care [ 27 ], and community-dwelling older adults [ 30 ], respectively.
Prevalence of sarcopenia according to population and definition criteria is illustrated in Table 2 . Higher prevalence rates of sarcopenia were found in females compared to males among community-dwelling older adults [ 49 ] and in older adults acutely admitted to hospital [ 51 ]. Further, acutely admitted female patients also presented with more severe sarcopenia compared to male patients [ 51 ].
Frequency of sarcopenia was higher (9.1–40.0%) in patients with diabetes (with and without complications of charcot osteoarthropathy), compared to age-matched healthy adults [ 52 ].
The prevalence of “probable sarcopenia” ranged between 20.4% (reduced muscle strength only) and 38.1% (fulfilling one of the following criteria: reduced muscle strength, reduced muscle mass, or low physical function) in Finnish community-dwelling adults [ 23 , 36 ], while longitudinal studies on Swedish community-dwelling old (70 years) and very old adults (≥ 85 years) the prevalence of “probable sarcopenia” (reduced muscle strength only) ranged from 1.8 to 73%, respectively [ 30 , 45 ]. Lastly, in a Swedish study among nursing home residents the prevalence of probable sarcopenia was 44% (evaluated by an impaired chair stand test) [ 34 ].
Prevalence of Osteosarcopenia (sarcopenia and osteoporosis) was 1.5% [ 36 ], and the prevalence of co-occurrence of all three following conditions: pre-frail, malnutrition, and sarcopenia was 7% [ 34 ].
We only identified two studies with prevalence of SO [ 39 ] and probable SO [ 23 ]. The prevalence of SO in a Swedish population was 4% and 11% in females and males, respectively, while the prevalence of probable SO among Finnish community-dwelling ranged between 5.8% and 12.6%, depending on the criteria to define the obesity (e.g., BMI, waist circumference, etc.) [ 23 ].
Several studies investigated aspects of etiology and risk factors for sarcopenia [ 24 , 27 , 28 , 30 , 31 , 34 , 36 , 38 , 40 , 42 , 43 , 44 , 47 , 49 , 50 , 51 ] and one study focused on SO [ 49 ]. Higher physical activity was associated with a decreased likelihood of sarcopenia [ 30 ]. In addition, adhering to world health organization (WHO) guidlines for physical activity and the Nordic nutritional recommendations for protein intake was positively associated with greater physical function and lower fat mass in older female community-dwellers [ 38 ]. In older adults who are physically active, eating a healthy diet (based on the frequency of intake of favorable food like fish, fruits, vegetables, and whole grains versus unfavorable foods like red/processed meats, desserts/sweets/sugar-sweetened beverages, and fried potatoes) was associated with lower risk of sarcopenia [ 28 ]. Further, among older adults who already meet the physical activity guidelines, additional engagement in muscle-strengthening activities was associated with a lower sarcopenia risk score and improved muscle mass and chair rise time [ 31 ].
Associations between sarcopenia, risk of sarcopenia and malnutrition or nutritional status was identified in geriatric patients [ 27 , 51 ], older patients with hip fracture [ 50 ], nursing home residents [ 34 ] and in community-dwelling older adults [ 49 ]. Moreover, the importance of nutritional intake was investigated in the following studies [ 24 , 36 , 47 ]. A study among community-dwelling men revealed an inverse association between total energy intake, protein intake (total, plant, and fish protein), intake of dietary fibers, fat (total and unsaturated), and vitamin D with sarcopenia status [ 36 ]. In a cohort of 71-year-old men a dietary pattern characterized by high consumption of fruit, vegetables, poultry, rice and pasta was associated with lower prevalence of sarcopenia after 16 years [ 24 ]. A longitudinal Finnish study on sarcopenia indices among postmenopausal older women, showed that lower adherence to the Mediterranean (focuses on high consumption of olive oil) or Baltic Sea (focuses on the dietary fat quality and low-fat milk intake) diets resulted in higher loss of lean mass over a 3-year period [ 47 ]. Further, a higher adherence to the Baltic Sea diet was associated with greater lean mass and better physical function, and higher adherence to the Mediterranean diet was associated with greater muscle quality [ 47 ].
In a study of patients with hip fracture age, polypharmacy, and low albumin levels was associated with sarcopenia [ 50 ]. Exocrine pancreatic insufficiency was an independent risk factor for sarcopenia [ 44 ]. This study also revealed that sarcopenia was associated with reduced quality of life, physical function, and increased risk of hospitalization [ 44 ]. In a longitudinal study of community-dwelling adults (+ 75 years) at risk of sarcopenia, high physical function, muscle strength, muscle mass and low BMI predicted better physical function and reduced need for care after four years [ 42 ]. Furthermore, in community-dwelling adults with sarcopenia, muscle mass, muscle strength and physical function are independent predictors of all-cause mortality. As a result, they have been proposed by researchers as targets for the prevention of sarcopenia-related over-mortality [ 43 ]. Lastly, community-dwelling older adults with sarcopenia had lower bone mineral density compared to those without sarcopenia and they were more likely to develop osteoporosis (Osteosarcopenia) [ 40 ].
Regarding SO risk factors, a longitudinal study among community-dwelling older adults in Finland found that SO (operationalized by measures of adiposity) were associated with poorer physical function after ten years [ 48 ].
Our literature search identified three randomized controlled trials investigating the effectiveness of interventions to prevent or counteract sarcopenia in older adults of Norway, Finland, and Sweden, respectively [ 32 , 35 , 46 ]. The Norwegian study [ 32 ] was a double-blinded randomized controlled trial (RCT). The study included those who were at risk of developing sarcopenia, including patients with chronic obstructive pulmonary disease (COPD) or individuals who showed diagnostic indications of sarcopenia. Participants received either vitamin D 3 or placebo supplementation for 28 weeks. Additionally, resistance training sessions were provided to all participants from weeks 14 to 27. Vitamin D supplementation did not significantly affect response to resistance training in older adults at risk of sarcopenia with or without COPD [ 32 ].
Furthermore, a RCT among pre-sarcopenic Swedish older adults investigated the effectiveness of three weekly sessions of instructor-led progressive resistance training in combination with a non-mandatory daily nutritional supplement (175 kcal, 19 g protein) compared to control group. The 10 weeks intervention resulted in significant between group improvements of physical function and a significant improvement in body composition in the intervention group [ 46 ].
Another intervention study revealed that a 12-month intervention with two daily nutritional supplements (each containing 20 g whey protein) did not attenuate the deterioration of physical function and muscle mass in sarcopenic older community-dwelling adults compared to isocaloric placebo supplements or no supplementation. All participants were given instructions on home-based exercises, importance of dietary protein and vitamin D supplementation [ 35 ].
Based on our broad literature search 33 studies were identified that concerned sarcopenia and SO and met the inclusion criteria. However, research on SO was very limited with only three studies identified. Narrative synthesis of the included studies revealed that the most reported classification tool for sarcopenia in Nordic countries was the EWGSOP2. Moreover, some studies estimated sarcopenia using EWGSOP. The overall prevalence of sarcopenia in Nordic countries according to EWGSOP2 ranged between 1% and 46% [ 25 , 28 ]. The prevalence of SO, however, was reported only in one study in Sweden (4–11%) [ 39 ]. Even though the previous systematic reviews and meta-analysis have reported the prevalence of sarcopenia and SO in different regions and settings (e.g., community-dwelling, nursing home, etc.) [ 8 , 15 , 55 , 56 ], this current scoping review is to the best of our knowledge the first study that provides an overview of research on sarcopenia and SO in the Nordic countries.
Based on our findings from 24 studies, there were large variability in prevalence of sarcopenia in studies conducted in the Nordic countries. We think that the wide variation in estimated prevalence of sarcopenia in our scoping review might be due to a different definition/diagnostic criterion (e.g., EWGSOP, EWGSOP2, FNIH), methodology to measure muscle mass (DXA, BIA, CT), and heterogeneity in characteristics of the study population (e.g., setting, age, medical conditions, co-occurrence of multiple risk factors). A previous study on prevalence of sarcopenia in Swedish older people showed significant differences between prevalence of sarcopenia based on EWGSOP2 and EWGSOP1 [ 29 ]. Therefore, researchers stressed that prevalence is more dependent on cut-offs than on the operational definition [ 29 , 57 ]. Further, we know that various international sarcopenia working groups have issued expert consensus and such diagnostic criteria are being updated [ 4 , 58 ]. Since the revision of criteria focuses primarily on the adjustment of cut-off values, the main reason for differences in prevalence even when using an updated version of one diagnosis criteria is modification in cut-off values. For instance, if the cut-off value for gait speed was increased by 0.2 m/s, the prevalence of sarcopenia may increase by 8.5% [ 57 ]. Meaning that even a small change in cut-off value can have a big impact on how sarcopenia is diagnosed. Besides when we take definition criteria into account (Table 2 ), the prevalence of sarcopenia is still variable in the population of community-dwelling adults for instance. We believe it is basically because studies have applied different assessment tools and tests to identify older adults with low muscle mass and muscle strength, although using the same definition criteria (Table 1 ). Previous studies have illustrated that choice of methodology to assess muscle strength (e.g., hand grip strength, chair rise) [ 59 ] and muscle mass (e.g., DXA, BIA, anthropometry) [ 60 , 61 , 62 ] in older adults may impact findings and this variability may explain some of the variability in our findings. So, adherence to the latest uniform diagnostic criteria for future studies is recommended to simplify the comparison of findings within the same country, across countries, and regions. Moreover, we suggest that medical community particularly GPs to come to an agreement on assessment methods for muscle mass and muscle strength and the use of one set of definition criteria for sarcopenia.
In previous meta-analyses [ 15 ], sub-group analyses based on region and classification tool, revealed that the prevalence of sarcopenia was higher in European studies using EWGSOP (12%) compared to rest of the studies using Asian Working Group for Sarcopenia (AWGS), FNIH, and EWGSOP (3%) [ 15 ]. In our scoping review, we also found a high prevalence of sarcopenia in Nordic countries. Longevity and life expectancy is higher in the Nordic countries compared to estimates for rest of the world [ 18 ], which means that in this region many people reach old age, and consequently they are more likely to be diagnosed with sarcopenia as an age-related disorder. Therefore, the authors of this current scoping review emphasis the importance of preventive strategies targeted major risk factors and effective interventions to limit the consequences of sarcopenia in the Nordic populations. Besides, we think that the health care system in the Nordic countries should be better equipped with the necessary healthcare resources for both a timely diagnosis and dealing with this major age-related issue in the years to come. However, due to the limitations regarding the timely diagnosis, we highly recommend a comprehensive approach including establishment of support services, implement educational programs, offer training for health care professionals, and engage the community.
Many countries have conducted research on SO [ 7 , 39 , 63 , 64 , 65 ]. Based on our findings, however, among the Nordic countries only Sweden and Finland have investigated the prevalence of probable SO and SO [ 23 , 29 ]. Besides, we only found one study investigating the association between body adiposity and physical function over time [ 54 ]. We did not find any literature on risk factors or interventions among older adults with SO in this region. Therefore, we call on medical and research community in Nordic countries to attach importance to screening of SO in elderly people to capture a full picture of this public health risk to aging society and allocate healthcare resources accordingly.
In terms of risk factors for sarcopenia, our study revealed that malnutrition, low levels of physical activity, specific diseases (e.g., diabetes, osteoporosis), inflammation, polypharmacy (multiple medicines), BMI, and ageing are potential risk factor for sarcopenia in populations of the Nordic region. However, evidence on risk factors derived mainly from cross-sectional associations [ 27 , 28 , 30 , 31 , 34 , 40 , 44 , 49 , 50 , 51 ], and only to a limited extend from longitudinal studies [ 24 , 38 , 43 , 47 ]. Therefore, the associations between risk factors and sarcopenia should be interpreted with caution due to the possibility of reverse causality and confounding affecting the results. Moreover, our findings on risk factors mainly came from community-dwelling older adults, and only to a limited extend hospital and nursing home settings. We think that risk factors may vary depending on population characteristics (e.g., age, sex, health condition) and setting (e.g., hospital, nursing home, community). Therefore, we encourage researchers of the Nordic countries to perform well-designed prospective cohort studies in different settings to enhance the possibility to establish causal inference as well as understanding degree and direction of changes over time.
A recently published meta-analyses revealed a higher risk of having polypharmacy in Europe among individuals with sarcopenia compared to people without this condition [ 66 ]. A nationwide register-based study in Swedish population also showed that the prevalence of polypharmacy has increased in Sweden over the last decade [ 67 ]. Sarcopenia itself is associated with morbidity (identified by specific disease or inflammatory markers) and different health-related outcomes (e.g., disability) [ 7 ]; therefore, future research should investigate whether polypharmacy is a major factor to sarcopenia development [ 66 ]. Although we lack information on polypharmacy in Nordic countries other than Sweden, we encourage researchers in this region to examine the above research gap in their future studies.
According to previous studies physiological changes in ageing include systemic low-grade inflammation which results in insulin resistance, affect protein metabolism and leads to increased muscle wasting [ 68 ]. Acute and chronic disease may increase the inflammatory response and accelerate age-related loss of muscle mass and increase risk of sarcopenia [ 68 , 69 ]. Hence, we think that special attention should be made by health care professionals particularly GPs to older adults with acute or chronic conditions to limit the risk of sarcopenia.
Literature from the Nordic countries also indicated that higher levels of physical activity and different dietary patterns (e.g., higher protein intake, fruit, vegetables, fibers) were associated with reduced risk of sarcopenia or improvement in indicators of sarcopenia. There was a large heterogeneity in the studied aspect which makes direct comparison of studies difficult. Nevertheless, according to findings from a recent systematic review of meta-analyses on sarcopenia the identified risk factors are in alignment with previously identified risk factors globally [ 70 ]. Other potential lifestyle-related risk factors suggested from the above meta-analysis included smoking and extreme sleep duration. However, we did not identify studies investigating these health behaviors in the Nordic populations. Therefore, high-quality cohort studies are needed to deeply understand such associations with the risk of sarcopenia.
In this current review, we only found three intervention studies in Nordic countries. However, two of them were sub-projects of big intervention programs, meaning that such studies were not designed explicitly for the prevention/treatment of sarcopenia. Therefore, explicit intervention studies on sarcopenia in this region is recommended.
We believe that on a global level, research on sarcopenia will carry on with nutrition, exercise, and understanding of molecular mechanisms. Furthermore, examining the link between sarcopenia and other medical conditions/diseases would be the next step [ 6 ]. In the Nordic countries, however, already performed studies have a basic and descriptive design, so that, well-designed research and advanced analyses are lacking. Hence, we recommend conducting large well-designed and adequately powered studies to (a) explore the scale of this age-related health issue on country and regional level, (b) investigate the patterns of physical activity and sedentary behavior to understand if this should be a target in older adults with SO and sarcopenia, (c) determine whether elderly populations are suffering from nutritional deficiency or are at risk of malnutrition. The latest can support further studies to assess the impact of combined physical activity and dietary intake, which are still lacking globally [ 6 ].
A previous systematic review on therapeutic strategies for SO revealed that exercise-based interventions (e.g., resistance training) reduced total adiposity and consequently improved body composition. However, evidence of other therapeutic strategies (e.g., nutritional supplementation) was limited due to scarcity of data and lack of unique definition for SO [ 69 ]. Therefore, authors suggested that more research should be done to clarify optimal treatment options for various age-groups and not only for older adults [ 14 ].
In our scoping review, the included studies, did not provide a status of either SO or the prevention/treatment methods in this region. We believe that SO is practically neglected in clinical practice and research as well, and this is mainly because it is difficult to separate it from general obesity. The consequence of lacking knowledge in this research area is that when older adults with SO are recommended weight loss- a frequently used strategy for management of general obesity- this may accelerate the loss of muscle mass and increase the severity of the sarcopenia [ 3 ]. Consequently, we think that this issue may have adverse effects both on patients (e.g., decreasing quality of their life) and on the health care system (e.g., increasing the health care demands) of this region. Therefore, we encourage researchers to perform cohort studies to understand the epidemiology and etiological basis of SO, which are poorly understood even on a global scale [ 8 ]. We think that the consensus definition on SO from the European Society for Clinical Nutrition and Metabolism (ESPEN) and European Association for the Study of Obesity (EASO) which was published in 2022 [ 3 ], can positively affect the ability to define studies on prevalence and prevention of SO. Besides, we recommend conducting further research to find the optimal treatment for SO and reduce its adverse consequences both at individual and society levels. Additionally, we think that the concepts of sarcopenia and SO might be somehow unfamiliar to health care personnel. Therefore, it is highly recommended that more information be provided to bring their attention to the significance of prevention, timely diagnosis, and treatment of these two aging disorders.
Strengths and limitations of the study
This is the first study providing an overview of available evidence on sarcopenia and SO among older adults in the Nordic countries. These countries have important similarities in welfare sectors and on a population level and we believe that our findings will be a significant benefit for researchers and health care providers to understand the knowledge gaps and plan for future studies in this geographical region. However, the current scoping review has limitations. This review was limited to studies among individuals more than 60 years old which may limit the overview of available research in this field, as well as understanding risk factors, confounders for prevention, and the potential for early detection of these two diseases in younger age population. The included cross-sectional studies in our review cannot provide information on causality of the associations.
Sarcopenia and SO are generally prevalent syndromes among older adults in Nordic countries, even though the prevalence of them varies according to the criteria for definition, population, and setting. Research among older adults with SO was very limited in this region. Besides, studies on risk factors were primarily cross-sectional and only few intervention studies were identified. Therefore, we encourage researchers performing well-designed studies (e.g., prospective cohorts) to understand the epidemiology and etiological basis of these two age-related disorders. For the next step, implementation of interventions targeting risk factors (e.g., combined physical activity and dietary intake) and evaluating of their impact on prevention or treatment of sarcopenia and SO is recommended. Furthermore, for the comprehensive advancement of muscle health in older adults, we recommend implementing interventions directed at health care personnel and encouraging more collaboration among clinicians, professional societies, researchers, and policy makers to ensure comprehensive and effective approach to health care initiatives.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
sarcopenic obesity
Web of science
Preferred Reporting Items for Systematic Reviews and Meta-analyses
European Working Group on Sarcopenia in Older People in the updated version from 2019
National Institutes of Health Sarcopenia Project definition
Dual energy X-ray absorptiometry
Bioelectrical Impedance Analysis
Bioimpedance Spectroscopy
Computed Tomography
Computed Tomography Angiogram
World Health Organization
General Practitioner
Randomized Controlled Trial
Chronic Obstructive Pulmonary Disease
European Association for the Study of Obesity
United, Nations, Department of Economic and Social Affairs., Population Division (2019). World Population Prospects 2019: Highlights (ST/ESA/SER.A/423).
United, Nations, Department of Economic and Social Affairs., Population Division (2019). World Population Ageing 2019: Highlights (ST/ESA/SER.A/430).
Donini LM, Busetto L, Bischoff SC, Cederholm T, Ballesteros-Pomar MD, Batsis JA, Bauer JM, Boirie Y, Cruz-Jentoft AJ, Dicker D, Frara S, Frühbeck G, Genton L, Gepner Y, Giustina A, Gonzalez MC, Han HS, Heymsfield SB, Higashiguchi T, Laviano A, Lenzi A, Nyulasi I, Parrinello E, Poggiogalle E, Prado CM, Salvador J, Rolland Y, Santini F, Serlie MJ, Shi H, Sieber CC, Siervo M, Vettor R, Villareal DT, Volkert D, Yu J, Zamboni M, Barazzoni R. Definition and diagnostic criteria for sarcopenic obesity: ESPEN and EASO Consensus Statement. Obes Facts. 2022;15(3):321–35. doi: 10.1159/000521241. Epub 2022 Feb 23. PMID: 35196654; PMCID: PMC9210010.
Article PubMed PubMed Central Google Scholar
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. https://doi.org/10.1093/ageing/afy169 . Erratum in: Age Ageing. 2019;48(4):601. PMID: 30312372; PMCID: PMC6322506.
Article PubMed Google Scholar
Cylus J, Figueras J, Normand C. Will population ageing spell the end of the welfare state? A review of evidence and policy options [Internet]. Sagan A, Richardson E, North J, White C, editors. Copenhagen (Denmark): European Observatory on Health Systems and Policies; 2019. PMID: 31820887.
Yuan D, Jin H, Liu Q, Zhang J, Ma B, Xiao W, Li Y. Publication trends for Sarcopenia in the World: a 20-Year bibliometric analysis. Front Med (Lausanne). 2022;9:802651. https://doi.org/10.3389/fmed.2022.802651 . PMID: 35223902; PMCID: PMC8873525.
Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, Meinow B, Fratiglioni L. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10(4):430–9. https://doi.org/10.1016/j.arr.2011.03.003 . Epub 2011 Mar 23. PMID: 21402176.
Gao Q, Mei F, Shang Y, Hu K, Chen F, Zhao L, Ma B. Global prevalence of sarcopenic obesity in older adults: a systematic review and meta-analysis. Clin Nutr. 2021;40(7):4633–41. https://doi.org/10.1016/j.clnu.2021.06.009 . Epub 2021 Jun 21. PMID: 34229269.
Molino S, Dossena M, Buonocore D, Verri M. Sarcopenic obesity: an appraisal of the current status of knowledge and management in elderly people. J Nutr Health Aging. 2016;20(7):780-8. https://doi.org/10.1007/s12603-015-0631-8 . PMID: 27499312.
Khadra D, Itani L, Tannir H, Kreidieh D, El Masri D, El Ghoch M. Association between sarcopenic obesity and higher risk of type 2 diabetes in adults: a systematic review and meta-analysis. World J Diabetes. 2019;10(5):311–23. https://doi.org/10.4239/wjd.v10.i5.311 . PMID: 31139318; PMCID: PMC6522758.
Aggio DA, Sartini C, Papacosta O, Lennon LT, Ash S, Whincup PH, Wannamethee SG, Jefferis BJ. Cross-sectional associations of objectively measured physical activity and sedentary time with Sarcopenia and sarcopenic obesity in older men. Prev Med. 2016;91:264–72. Epub 2016 Aug 26. PMID: 27575317; PMCID: PMC5061552.
Rossi AP, Rubele S, Calugi S, Caliari C, Pedelini F, Soave F, Chignola E, Vittoria Bazzani P, Mazzali G, Dalle Grave R, Zamboni M. Weight cycling as a risk factor for low muscle mass and strength in a population of males and females with obesity. Obesity (Silver Spring). 2019;27(7):1068–1075. https://doi.org/10.1002/oby.22493 . PMID: 31231958.
Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J, Bauer JM, Pahor M, Clark BC, Cesari M, Ruiz J, Sieber CC, Aubertin-Leheudre M, Waters DL, Visvanathan R, Landi F, Villareal DT, Fielding R, Won CW, Theou O, Martin FC, Dong B, Woo J, Flicker L, Ferrucci L, Merchant RA, Cao L, Cederholm T, Ribeiro SML, Rodríguez-Mañas L, Anker SD, Lundy J, Gutiérrez Robledo LM, Bautmans I, Aprahamian I, Schols JMGA, Izquierdo M, Vellas B. International clinical practice guidelines for sarcopenia (ICFSR): screening, diagnosis and management. J Nutr Health Aging. 2018;22(10):1148–1161. https://doi.org/10.1007/s12603-018-1139-9 . PMID: 30498820.
Poggiogalle E, Parrinello E, Barazzoni R, Busetto L, Donini LM. Therapeutic strategies for sarcopenic obesity: a systematic review. Curr Opin Clin Nutr Metab Care. 2021;24(1):33–41. https://doi.org/10.1097/MCO.0000000000000714 . PMID: 33323715.
Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP, Celis-Morales C. Global prevalence of Sarcopenia and severe Sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13(1):86–99. https://doi.org/10.1002/jcsm.12783 . Epub 2021 Nov 23. PMID: 34816624; PMCID: PMC8818604.
Prado CM, Wells JC, Smith SR, Stephan BC, Siervo M. Sarcopenic obesity: a critical appraisal of the current evidence. Clin Nutr. 2012;31(5):583–601. https://doi.org/10.1016/j.clnu.2012.06.010 . Epub 2012 Jul 17. PMID: 22809635.
Article CAS PubMed Google Scholar
Balaj M, Huijts T, McNamara CL, Stornes P, Bambra C, Eikemo TA. Non-communicable diseases and the social determinants of health in the nordic countries: findings from the European Social Survey (2014) special module on the social determinants of health. Scand J Public Health. 2017;45(2):90–102. Epub 2017 Jan 27. PMID: 28128015.
Nordic Burden of Disease Collaborators. Life expectancy and disease burden in the Nordic countries: results from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet Public Health. 2019;4(12): e658-e669. doi: 10.1016/S2468-2667(19)30224-5. Epub 2019 Nov 20. PMID: 31759894; PMCID: PMC7098475.
Stockmarr A, Hejgaard T, Matthiessen J. Obesity prevention in the Nordic Countries. Curr Obes Rep. 2016;5(2):156 – 65. https://doi.org/10.1007/s13679-016-0206-y . PMID: 27033877.
Cuadrado A, Stjernberg M, Huynh D. Active and healthy ageing: heterogenous perspectives and nordic indicators. Nordens välfärdscenter/Nordic Welfare Centre; 2022.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336 – 41. https://doi.org/10.1016/j.ijsu.2010.02.007 . Epub 2010 Feb 18. Erratum in: Int J Surg. 2010;8(8):658. PMID: 20171303.
Sallfeldt ES, Mallmin H, Karlsson MK, Mellström D, Hailer NP, Ribom EL. Sarcopenia prevalence and incidence in older men - a MrOs Sweden study. Geriatr Nurs. 2023 Mar-Apr;50:102–8. https://doi.org/10.1016/j.gerinurse.2023.01.003 . Epub 2023 Feb 10. PMID: 36774676.
Sääksjärvi K, Härkänen T, Stenholm S, Schaap L, Lundqvist A, Koskinen S, Borodulin K, Visser M. Probable Sarcopenia, obesity, and risk of all-cause mortality: a pooled analysis of 4,612 participants. Gerontology. 2023;69(6):706–15. Epub 2023 Jan 30. PMID: 36716714.
Karlsson M, Becker W, Cederholm TE, Byberg L. A posteriori dietary patterns in 71-year-old Swedish men and the prevalence of Sarcopenia 16 years later. Br J Nutr Camb Univ Press. 2022;128(5):909–20. https://doi.org/10.1017/S0007114521003901 .
Article CAS Google Scholar
Dolin TG, Mikkelsen MK, Jakobsen HL, Vinther A, Zerahn B, Nielsen DL, Johansen JS, Lund CM, Suetta C. The prevalence of Sarcopenia and cachexia in older patients with localized colorectal cancer. J Geriatr Oncol. 2023;14(1):101402. Epub 2022 Nov 21. PMID: 36424269.
Paajanen P, Lindström I, Oksala N, Väärämäki S, Saari P, Mäkinen K, Kärkkäinen JM. Radiographically quantified Sarcopenia and traditional cardiovascular risk assessment in predicting long-term mortality after endovascular aortic repair. J Vasc Surg. 2022;76(4):908–e9152. Epub 2022 Mar 31. PMID: 35367563.
Sobestiansky S, Åberg AC, Cederholm T. Sarcopenia and malnutrition in relation to mortality in hospitalised patients in geriatric care - predictive validity of updated diagnoses. Clin Nutr ESPEN. 2021;45:442–8. Epub 2021 Jul 16. PMID: 34620352.
Papaioannou KG, Nilsson A, Nilsson LM, Kadi F. Healthy eating is Associated with Sarcopenia Risk in physically active older adults. Nutrients. 2021;13(8):2813. https://doi.org/10.3390/nu13082813 . PMID: 34444973; PMCID: PMC8401667.
Article CAS PubMed PubMed Central Google Scholar
Wallengren O, Bosaeus I, Frändin K, Lissner L, Falk Erhag H, Wetterberg H, Rydberg Sterner T, Rydén L, Rothenberg E, Skoog I. Comparison of the 2010 and 2019 diagnostic criteria for Sarcopenia by the European Working Group on Sarcopenia in Older people (EWGSOP) in two cohorts of Swedish older adults. BMC Geriatr. 2021;21(1):600. https://doi.org/10.1186/s12877-021-02533-y . PMID: 34702174; PMCID: PMC8547086.
Scott D, Johansson J, Gandham A, Ebeling PR, Nordstrom P, Nordstrom A. Associations of accelerometer-determined physical activity and sedentary behavior with Sarcopenia and incident falls over 12 months in community-dwelling Swedish older adults. J Sport Health Sci. 2021;10(5):577–84. Epub 2020 Feb 5. PMID: 34088651; PMCID: PMC8500807.
Veen J, Montiel-Rojas D, Nilsson A, Kadi F. Engagement in muscle-strengthening activities lowers Sarcopenia Risk in older adults already adhering to the Aerobic Physical Activity guidelines. Int J Environ Res Public Health. 2021;18(3):989. https://doi.org/10.3390/ijerph18030989 . PMID: 33499423; PMCID: PMC7908493.
Mølmen KS, Hammarström D, Pedersen K, Lian Lie AC, Steile RB, Nygaard H, Khan Y, Hamarsland H, Koll L, Hanestadhaugen M, Eriksen AL, Grindaker E, Whist JE, Buck D, Ahmad R, Strand TA, Rønnestad BR, Ellefsen S. Vitamin D3 supplementation does not enhance the effects of resistance training in older adults. J Cachexia Sarcopenia Muscle. 2021;12(3):599–628. https://doi.org/10.1002/jcsm.12688 . Epub 2021 Mar 31. PMID: 33788419; PMCID: PMC8200443.
Simonsen C, Kristensen TS, Sundberg A, Wielsøe S, Christensen J, Hansen CP, Burgdorf SK, Suetta C, de Heer P, Svendsen LB, Achiam MP, Christensen JF. Assessment of Sarcopenia in patients with upper gastrointestinal tumors: prevalence and agreement between computed tomography and dual-energy x-ray absorptiometry. Clin Nutr. 2021;40(5):2809–16. Epub 2021 Mar 26. PMID: 33933747.
Faxén-Irving G, Luiking Y, Grönstedt H, Franzén E, Seiger Å, Vikström S, Wimo A, Boström AM, Cederholm T. Do malnutrition, sarcopenia and frailty overlap in nursing-home residents? J Frailty Aging. 2021;10(1):17–21. https://doi.org/10.14283/jfa.2020.45 . PMID: 33331617.
Björkman MP, Suominen MH, Kautiainen H, Jyväkorpi SK, Finne-Soveri HU, Strandberg TE, Pitkälä KH, Tilvis RS. Effect of protein supplementation on physical performance in older people with sarcopenia-a randomized controlled trial. J Am Med Dir Assoc. 2020;21(2):226–e2321. Epub 2019 Nov 14. PMID: 31734121.
Jyväkorpi SK, Urtamo A, Kivimäki M, Strandberg TE. Macronutrient composition and sarcopenia in the oldest-old men: the Helsinki businessmen study (HBS). Clin Nutr. 2020;39(12):3839–41. https://doi.org/10.1016/j.clnu.2020.04.024 . Epub 2020 Apr 24. PMID: 32376097.
Probert N, Lööw A, Akner G, Wretenberg P, Andersson ÅG. A comparison of patients with hip fracture, ten years apart: morbidity, malnutrition and sarcopenia. J Nutr Health Aging. 2020;24(8):870–877. https://doi.org/10.1007/s12603-020-1408-2 . PMID: 33009538.
Sjöblom S, Sirola J, Rikkonen T, Erkkilä AT, Kröger H, Qazi SL, Isanejad M. Interaction of recommended levels of physical activity and protein intake is associated with greater physical function and lower fat mass in older women: Kuopio osteoporosis risk Factor- (OSTPRE) and fracture-Prevention Study. Br J Nutr. 2020;123(7):826–39. Epub 2020 Jan 8. PMID: 31910914; PMCID: PMC7054249.
von Berens Å, Obling SR, Nydahl M, Koochek A, Lissner L, Skoog I, Frändin K, Skoglund E, Rothenberg E, Cederholm T. Sarcopenic obesity and associations with mortality in older women and men - a prospective observational study. BMC Geriatr. 2020;20(1):199. https://doi.org/10.1186/s12877-020-01578-9 . PMID: 32517653; PMCID: PMC7285448.
Nielsen BR, Andersen HE, Haddock B, Hovind P, Schwarz P, Suetta C. Prevalence of muscle dysfunction concomitant with osteoporosis in a home-dwelling Danish population aged 65–93 years -the Copenhagen Sarcopenia Study. Exp Gerontol. 2020;138:110974. https://doi.org/10.1016/j.exger.2020.110974 . Epub 2020 May 25. PMID: 32464171.
Van Ancum JM, Alcazar J, Meskers CGM, Nielsen BR, Suetta C, Maier AB. Impact of using the updated EWGSOP2 definition in diagnosing Sarcopenia: a clinical perspective. Arch Gerontol Geriatr 2020 Sep-Oct;90:104125. https://doi.org/10.1016/j.archger.2020.104125 . Epub 2020 May 23. PMID: 32534364.
Björkman M, Jyväkorpi SK, Strandberg TE, Pitkälä KH, Tilvis RS. Sarcopenia indicators as predictors of functional decline and need for care among older people. J Nutr Health Aging. 2019;23(10):916–922. https://doi.org/10.1007/s12603-019-1280-0 . PMID: 31781719.
Björkman MP, Pitkala KH, Jyväkorpi S, Strandberg TE, Tilvis RS. Bioimpedance analysis and physical functioning as mortality indicators among older sarcopenic people. Exp Gerontol. 2019;122:42–6. https://doi.org/10.1016/j.exger.2019.04.012 . Epub 2019 Apr 24. PMID: 31026498.
Olesen SS, Büyükuslu A, Køhler M, Rasmussen HH, Drewes AM. Sarcopenia associates with increased hospitalization rates and reduced survival in patients with chronic pancreatitis. Pancreatology. 2019;19(2):245–51. https://doi.org/10.1016/j.pan.2019.01.006 . Epub 2019 Jan 14. PMID: 30665702.
Sobestiansky S, Michaelsson K, Cederholm T. Sarcopenia prevalence and associations with mortality and hospitalisation by various sarcopenia definitions in 85–89 year old community-dwelling men: a report from the ULSAM study. BMC Geriatr. 2019;19(1):318. https://doi.org/10.1186/s12877-019-1338-1 . PMID: 31747923; PMCID: PMC6864927.
Vikberg S, Sörlén N, Brandén L, Johansson J, Nordström A, Hult A, Nordström P. Effects of resistance training on functional strength and muscle mass in 70-Year-old individuals with pre-sarcopenia: a randomized controlled trial. J Am Med Dir Assoc. 2019;20(1):28–34. Epub 2018 Nov 7. PMID: 30414822.
Isanejad M, Sirola J, Mursu J, Rikkonen T, Kröger H, Tuppurainen M, Erkkilä AT. Association of the baltic sea and mediterranean diets with indices of sarcopenia in elderly women, OSPTRE-FPS study. Eur J Nutr. 2018;57(4):1435–48. https://doi.org/10.1007/s00394-017-1422-2 . Epub 2017 Mar 16. PMID: 28303397.
Mikkola TM, von Bonsdorff MB, Salonen MK, Simonen M, Pohjolainen P, Osmond C, Perälä MM, Rantanen T, Kajantie E, Eriksson JG. Body composition as a predictor of physical performance in older age: a ten-year follow-up of the Helsinki Birth Cohort Study. Arch Gerontol Geriatr. 2018 Jul-Aug;77:163–8. doi: 10.1016/j.archger.2018.05.009. Epub 2018 May 14. PMID: 29783137; PMCID: PMC5994345.
Ottestad I, Ulven SM, Øyri LKL, Sandvei KS, Gjevestad GO, Bye A, Sheikh NA, Biong AS, Andersen LF, Holven KB. Reduced plasma concentration of branched-chain amino acids in sarcopenic older subjects: a cross-sectional study. Br J Nutr. 2018;120(4):445–53. Epub 2018 Jun 18. PMID: 29909813.
Steihaug OM, Gjesdal CG, Bogen B, Kristoffersen MH, Lien G, Ranhoff AH. Sarcopenia in patients with hip fracture: a multicenter cross-sectional study. PLoS ONE. 2017;12(9):e0184780. https://doi.org/10.1371/journal.pone.0184780 . PMID: 28902873; PMCID: PMC5597226.
Jacobsen EL, Brovold T, Bergland A, Bye A. Prevalence of factors associated with malnutrition among acute geriatric patients in Norway: a cross-sectional study. BMJ Open. 2016;6(9):e011512. https://doi.org/10.1136/bmjopen-2016-011512 . PMID: 27601491; PMCID: PMC5020767.
Jansen RB, Christensen TM, Bülow J, Rørdam L, Holstein PE, Svendsen OL. Sarcopenia and body composition in diabetic Charcot osteoarthropathy. J Diabetes Complications. 2015 Sep-Oct;29(7):937–42. https://doi.org/10.1016/j.jdiacomp.2015.05.020 . Epub 2015 Jun 5. PMID: 26139557.
Frost M, Nielsen TL, Brixen K, Andersen M. Peak muscle mass in young men and Sarcopenia in the ageing male. Osteoporos Int. 2015;26(2):749–56. https://doi.org/10.1007/s00198-014-2960-6 . Epub 2014 Nov 22. PMID: 25416073.
Patil R, Uusi-Rasi K, Pasanen M, Kannus P, Karinkanta S, Sievänen H. Sarcopenia and osteopenia among 70-80-year-old home-dwelling finnish women: prevalence and association with functional performance. Osteoporos Int. 2013;24(3):787–96. https://doi.org/10.1007/s00198-012-2046-2 . Epub 2012 Jun 12. PMID: 22688541.
Papadopoulou SK, Tsintavis P, Potsaki P, Papandreou D. Differences in the prevalence of sarcopenia in community-dwelling, nursing home and hospitalized individuals. a systematic review and meta-analysis. J Nutr Health Aging. 2020;24(1):83–90. https://doi.org/10.1007/s12603-019-1267-x . PMID: 31886813.
Mayhew AJ, Amog K, Phillips S, Parise G, McNicholas PD, de Souza RJ, Thabane L, Raina P. The prevalence of sarcopenia in community-dwelling older adults, an exploration of differences between studies and within definitions: a systematic review and meta-analyses. Age Ageing. 2019;48(1):48–56. https://doi.org/10.1093/ageing/afy106 . PMID: 30052707.
Cao M, Lian J, Lin X, Liu J, Chen C, Xu S, Ma S, Wang F, Zhang N, Qi X, Xu G, Peng N. Prevalence of Sarcopenia under different diagnostic criteria and the changes in muscle mass, muscle strength, and physical function with age in Chinese old adults. BMC Geriatr. 2022;22(1):889. https://doi.org/10.1186/s12877-022-03601-7 . PMID: 36418979; PMCID: PMC9682713.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European working group on sarcopenia in older people. sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39(4):412–23. https://doi.org/10.1093/ageing/afq034 . Epub 2010 Apr 13. PMID: 20392703; PMCID: PMC2886201.
Verstraeten LMG, de Haan NJ, Verbeet E, van Wijngaarden JP, Meskers CGM, Maier AB. Handgrip strength rather than chair stand test should be used to diagnose s in geriatric rehabilitation inpatients: restoring health of acutely unwell adulTs (RESORT). Age Ageing. 2022;51(11):afac242. https://doi.org/10.1093/ageing/afac242 . PMID: 36413590; PMCID: PMC9681126.
Cheng KY, Chow SK, Hung VW, Wong CH, Wong RM, Tsang CS, Kwok T, Cheung WH. Diagnosis of sarcopenia by evaluating skeletal muscle mass by adjusted bioimpedance analysis validated with dual-energy X-ray absorptiometry. J Cachexia Sarcopenia Muscle. 2021;12(6):2163–73. Epub 2021 Oct 4. PMID: 34609065; PMCID: PMC8718029.
Sousa-Santos AR, Barros D, Montanha TL, Carvalho J, Amaral TF. Which is the best alternative to estimate muscle mass for sarcopenia diagnosis when DXA is unavailable? Arch Gerontol Geriatr. 2021 Nov-Dec;97:104517. https://doi.org/10.1016/j.archger.2021.104517 . Epub 2021 Sep 3. PMID: 34547538.
González Correa CH, Marulanda Mejía F, Castaño González PA, Vidarte Claros JA, Castiblanco Arroyabe HD. Bioelectrical impedance analysis and dual x-ray absorptiometry agreement for skeletal muscle mass index evaluation in sarcopenia diagnosis. Physiol Meas. 2020;41(6):064005. https://doi.org/10.1088/1361-6579/ab8e5f . PMID: 32348971.
Hwang B, Lim JY, Lee J, Choi NK, Ahn YO, Park BJ. Prevalence rate and associated factors of sarcopenic obesity in Korean elderly population. J Korean Med Sci. 2012;27(7):748–55. https://doi.org/10.3346/jkms.2012.27.7.748 . Epub 2012 Jun 29. PMID: 22787369; PMCID: PMC3390722.
Kera T, Kawai H, Hirano H, Kojima M, Fujiwara Y, Ihara K, Obuchi S. Differences in body composition and physical function related to pure Sarcopenia and sarcopenic obesity: a study of community-dwelling older adults in Japan. Geriatr Gerontol Int. 2017;17(12):2602–9. https://doi.org/10.1111/ggi.13119 . Epub 2017 Jun 28. PMID: 28657168.
Aibar-Almazán A, Martínez-Amat A, Cruz-Díaz D, Jiménez-García JD, Achalandabaso A, Sánchez-Montesinos I, de la Torre-Cruz M, Hita-Contreras F. Sarcopenia and sarcopenic obesity in Spanish community-dwelling middle-aged and older women: Association with balance confidence, fear of falling and fall risk. Maturitas. 2018;107:26–32. Epub 2017 Oct 7. PMID: 29169576.
Prokopidis K, Giannos P, Reginster JY, Bruyere O, Petrovic M, Cherubini A, Triantafyllidis KK, Kechagias KS, Dionyssiotis Y, Cesari M, Ibrahim K, Scott D, Barbagallo M, Veronese N, the Task Force on Pharmaceutical Strategy of the European Geriatric Medicine Society (EuGMS). Special interest group in Systematic Reviews and Meta-analyses and sarcopenia is associated with a greater risk of polypharmacy and number of medications: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14(2):671–683. https://doi.org/10.1002/jcsm.13190 . Epub 2023 Feb 13. PMID: 36781175; PMCID: PMC10067503.
Zhang N, Sundquist J, Sundquist K, Ji J. An increasing Trend in the prevalence of polypharmacy in Sweden: a nationwide register-based study. Front Pharmacol. 2020;11:326. https://doi.org/10.3389/fphar.2020.00326 . PMID: 32265705; PMCID: PMC7103636.
Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. 2017;8:1045. https://doi.org/10.3389/fphys.2017.01045 . PMID: 29311975; PMCID: PMC5733049.
Riuzzi F, Sorci G, Arcuri C, Giambanco I, Bellezza I, Minelli A, Donato R. Cellular and molecular mechanisms of sarcopenia: the S100B perspective. J Cachexia Sarcopenia Muscle. 2018;9(7):1255–68. https://doi.org/10.1002/jcsm.12363 . Epub 2018 Nov 30. PMID: 30499235; PMCID: PMC6351675.
Yuan S, Larsson SC. Epidemiology of sarcopenia: prevalence, risk factors, and consequences. Metabolism. 2023;144:155533. https://doi.org/10.1016/j.metabol.2023.155533 . Epub 2023 Mar 11. PMID: 36907247.
Download references
Acknowledgements
Not applicable.
Open access funding provided by University of Southern Denmark
This work was done without any fund.
Author information
Fereshteh Baygi, Sussi Friis Buhl contributed equally to this work.
Authors and Affiliations
Research Unit of General Practice, Department of Public Health, University of Southern Denmark, Odense, Denmark
Fereshteh Baygi, Sussi Friis Buhl, Trine Thilsing, Jens Søndergaard & Jesper Bo Nielsen
You can also search for this author in PubMed Google Scholar
Contributions
FB conceived and designed the review, participated in literature review, data extraction, interpretation of the results and wrote the manuscript. SFB designed the review, participated in literature review, data extraction, and revised the manuscript. TT, JBN and JS contributed to the conception of the study and revised the manuscript critically. All the authors approved the final manuscript.
Corresponding authors
Correspondence to Fereshteh Baygi or Sussi Friis Buhl .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Baygi, F., Buhl, S.F., Thilsing, T. et al. Sarcopenia and sarcopenic obesity among older adults in the nordic countries: a scoping review. BMC Geriatr 24 , 421 (2024). https://doi.org/10.1186/s12877-024-04970-x
Download citation
Received : 12 November 2023
Accepted : 12 April 2024
Published : 13 May 2024
DOI : https://doi.org/10.1186/s12877-024-04970-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Sarcopenic obesity
- Nordic countries
BMC Geriatrics
ISSN: 1471-2318
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Share full article
Advertisement
Supported by
Obesity, Weight-Loss Drugs and Ultraprocessed Foods
More from our inbox:, donald trump, husband, the g.o.p. and latino voters, better alternatives to animal-to-human transplants.

To the Editor:
Re “ What a Year on Ozempic Taught Me ,” by Johann Hari (Opinion guest essay, May 12):
As an internal medicine physician for over 40 years, I am in agreement with Mr. Hari that current obesity patterns are driven by diets laden with processed foods and short on fruits, vegetables and whole grains. I also like the idea of treating obesity as a medical condition rather than a moral failure or sign of weakness.
Individuals can decide about Ozempic and similar drugs taking into consideration the known benefits and risks as well as the unknowns. These are not easy decisions, and I’m sure more will be learned over time.
Not discussed in Mr. Hari’s excellent article is the need for the entire population, including those who are not overweight, to eat a well-balanced diet that largely omits ultraprocessed foods and is generous with fruits and vegetables. There are many proven benefits, including reducing the risks of heart disease, cancer and Type 2 diabetes. Even if one does not lose an ounce, moving a diet in this direction will be beneficial.
The same goes for increasing exercise, a proven wellness strategy.
Jonathan Freudman San Rafael, Calif.
Ultraprocessed foods, it’s all their fault! So why do you go to the supermarket and buy them? The food industry is not forcing you to do that.
I grew up in the 1960s and ’70s in a family of six children, and my mother, who worked outside the home, made us lunches and dinners every day from scratch. I raised my four children in the 1980s and ’90s doing the same, with my husband’s help, since we both had careers.
No one is obese in our family. If it helps you to take this drug, fine. But stop complaining that the food industry is to blame. Go to the kitchen and cook!
Mary Ann Morigault Marseille, France
I read Johann Hari’s guest essay on Ozempic, and as someone who lost about 90 pounds without the help of any weight-loss drug, I wholeheartedly agree with his sentiments about weight not being a competition with other people, but rather with the food industry.
I struggled with diet and exercise my entire life until 2019, when I really got serious about trying to lose the weight I had put on. I did most of the fads — keto, intermittent fasting, making sure to hit 10,000 steps every day — but really the biggest hurdle was just being hungry and having to wait it out.
Ozempic or Wegovy would have absolutely helped me lose weight, but I certainly don’t begrudge people for being able to take it now when I couldn’t in my weight-loss journey. I eventually became a much more exercise-focused person, and I believe that lifestyle change is what allowed me to keep the weight off these last few years.
I think these drugs are excellent avenues to weight loss for those that can afford them, but I worry that without changing lifestyle or diet, these drugs treat symptoms rather than the root cause.
I don’t think weight-loss drugs are cheating, and I don’t judge people for using a tool that was not available for me. But I do worry that the use of these drugs will not lead to a positive long-term outcome without continued use of the drugs.
Evan Bennett Atlanta
Re “ What Kind of Husband Behaves Like Trump? ,” by Jessica Bennett (Opinion, May 10):
The answer to that question is that Donald Trump was not the only president accused of straying from his marriage vows. Historians have identified several other presidents who allegedly had extramarital affairs.
The list includes Warren G. Harding, Franklin D. Roosevelt, Dwight D. Eisenhower, John F. Kennedy, Lyndon B. Johnson, George H.W. Bush and Bill Clinton. There is also the case of Grover Cleveland, who admitted he fathered an out-of-wedlock child before being elected.
Your newspaper and most of the readers whose letters are published find enough to criticize about Mr. Trump. But please leave him alone on this matter.
Diana Klebanow Queens The writer is a former adjunct professor of political science at Long Island University, Brooklyn campus.
Jessica Bennett asks about former President Donald Trump, “What’s more honorable than a husband going to great lengths to conceal his affair?”
For the benefit of those who may miss the note of irony in Ms. Bennett’s question, I’ll posit this answer: Being faithful to your spouse, for starters.
Eric Orner Tarrytown, N.Y.
Re “ As Trump Fan, Pastor Signals a Latino Shift ” (front page, May 6):
MAGA Republicans are cynically manipulating Latino voters. Their strategy is to emphasize common ground on issues like law and order, religious and family values, patriotism and escape from oppression. Democrats urgently need to confront this false narrative.
A Republican Party that brands many immigrants as criminals is no friend of Latinos. Republicans who attack civil liberties, such as voting rights and reproductive freedom, represent the same oppression that many Latinos fought so hard to escape in their homelands. Republicans who vote against affordable health care weaken Latino families.
The MAGA insurrectionists who savagely attacked police officers on Jan. 6 are the enemies of law and order. Republicans are not patriots. Donald Trump has zero religious values.
Latino voters could determine the fate of our democracy. Democrats need to spend a lot more time in Latino churches clearly explaining that a vote for MAGA Republicans is a direct betrayal of God, country and family.
Ken Hargis Los Altos, Calif.
Re “ Patient With Kidney Transplanted From a Pig Has Died ” (news article, May 13):
Richard Slayman’s death highlights the tragic need for better solutions to end-stage kidney disease. More than 100,000 patients are waiting for organ transplants each year in the U.S. But animal-to-human transplantation, or xenotransplantation, causes more problems than it solves.
The transmission of viruses and other pathogens from pigs can be attenuated through genetic engineering, special animal confinement procedures and screening measures, but total prevention cannot be guaranteed, and potential harms could be devastating.
We are just beginning to grapple with the risks that animal agriculture poses to workers, consumers and the environment; we should not be creating an entirely new mode of large-scale animal production.
Clinical practice of xenotransplantation has been “just around the corner” for decades, yet we still have little to show for it. Organ preservation, expanded criteria donations, living donations, opt-out policies, automated referrals, regenerative therapies, and improved disease prevention and management could all together put a significant dent in — if not eliminate — the organ wait list.
These safer, more tangible ways to reduce demand for and increase supply of organs could use the same amount of attention and investment given to xenotransplantation.
Catharine E. Krebs Harpers Ferry, W.Va. The writer is a medical research program manager at the Physicians Committee for Responsible Medicine.
- Arnold School of Public Health
- Location Location
- Contact Contact
- Colleges and Schools
- 2024 News Archive
May graduate preps for career in psychiatric epidemiology
May 21, 2024 | Erin Bluvas, [email protected]
Born in Oklahoma, Paige Jones moved around before finding her home in Columbia – by far the largest of the seven places she had lived previously. She chose it to be closer to family, who had relocated here while she was an undergrad at the University of Mississippi, but she soon found it was where she wanted to stay for graduate school.

“I have really enjoyed living in Columbia,” she says. “There’s lots to do without being overwhelmingly large, and the student culture has been really great. It has been easy to make friends and connections within the Arnold School, which was something I was a bit worried about moving to a new city, particularly with the effects of COVID.”
Jones originally thought she’d become a therapist but found herself more interested in the research side of her bachelor’s program in psychology. After moving to Columbia, she worked in a USC research lab where an Arnold School alum introduced her to the field of public health as a career.
She enrolled in the M.S. in Epidemiology program, combining her two areas of interest into psychiatric epidemiology as her focus. Jones built on her research experience as a graduate assistant for epidemiology assistant professor Matthew Lohman and clinical associate professor of neuropsychiatry and behavioral science Eve Fields. She gained additional experience as a statistics and research analyst for the South Carolina Department of Health and Environmental Control – exploring the intersection of mental health with maternal and child health.
Jones believes that her coursework and work experiences have prepared her for positions in a research lab, health department or hospital settings. She is also open to pursuing a doctoral degree.
“I think my degree prepared me well for any of these roles,” she says. “I feel very confident in my SAS and overall data analysis skills, which has greatly helped me in my current job with DHEC. I think the opportunity to complete a thesis has further benefitted me in leading a research project and really applying all the data and scientific writing skills from my coursework.”
Every professor I have had or worked with has been great, and they truly want you to do well and provide you the knowledge to do that.
Her Arnold School mentors played a big role in her program, and she found Lohman as well as biostatistics clinical assistant professor Andrew Ortaglia to be particularly impactful.
“Every professor I have had or worked with has been great, and they truly want you to do well and provide you the knowledge to do that,” Jones says. “I am really grateful for all of Dr. Lohman’s guidance and patience during my thesis, as well being a very effective and kind teacher of complicated epidemiologic methods in class. I am also very grateful for Dr. Ortaglia’s biostatistics courses, and his commitment to teaching good statistics while challenging us to really consider the why behind what methods we use. Both have influenced the way I think about things in work and my commitment to doing meaningful research in any area of epidemiology.”
As she prepares to graduate next month, Jones has advice for future students.
“It’s important to get involved as much as possible – taking opportunities to learn new skills and make connections.”

Find Out More
Epidemiologists design and conduct investigations aimed at improving the health of groups of people by combining knowledge from the social sciences, medicine, biology, the environment, and statistics.

Meet Our Class of 2024
The Arnold School is proud of our 2024 graduates, who will go on to change the world locally and globally. Learn about some of the other outstanding individuals who completed one of our 34 programs this year.
Challenge the conventional. Create the exceptional. No Limits.

IMAGES
VIDEO
COMMENTS
Obesity is a complex chronic disease in which abnormal or excess body fat (adiposity) impairs health, increases the risk of long-term medical complications and reduces lifespan. 1 Epidemiologic studies define obesity using the body mass index (BMI; weight/height 2), which can stratify obesity-related health risks at the population level.Obesity is operationally defined as a BMI exceeding 30 kg ...
Introduction. Obesity, defined as a body mass index (BMI) greater or equal to 30 kg/m 2, is a chronic disease increasing in prevalence worldwide. 1 There are multiple treatment modalities for managing obesity, including surgery, pharmacological treatments, medical management, as well as behavioral modifications. Each treatment modality varies in efficacy, complications, side effects, long-term ...
The estimated obesity-related health care costs are as high as $147. billion per year. Despite the alarming health risks and increasing health care costs, the. rates of screening and counseling for obesity in the primary care setting are merely 30%. This project outlines weight-management counseling strategies.
Obesity is a prevalent, complex, progressive and relapsing chronic disease, characterized by abnormal or excessive body fat (adiposity), that impairs health. People living with obesity face ...
1 INTRODUCTION. Globally, overweight and obesity is an increasing public health problem, 1, 2 with over 100 million children and over 600 million adults estimated to be living with obesity. 2 Obesity is associated with cardiovascular disease, hypertension, type 2 diabetes mellitus, certain cancers, hyperlipidemia, sleep apnea, osteoarthritis, liver and gall bladder disease, and gynecological ...
Abstract and Figures. Abstract Obesity is a chronic metabolic disease characterized by an increase of body fat stores. It is a gateway to ill health, and has become one of the leading causes of ...
Some genetic and lifestyle factors affect an individual's likelihood of adult obesity; thus, the significant clusters of obesity observed in specific geographical regions and contexts also signal the impact of socioeconomic and environmental factors in "obesogenic" environments [13].Understanding the causes and determinants of obesity is a critical step toward creating effective policy and ...
Obesity is associated with and contributes to a shortened life span, type 2 diabetes mellitus, cardiovascular disease, some cancers, kidney disease, obstructive sleep apnea, gout, osteoarthritis, and hepatobiliary disease, among others. Weight loss reduces all of these diseases in a dose-related manner-the more weight lost, the better the outcome.
The relationship between obesity and health-related quality of life (HRQoL) may be confounded by factors such as multimorbidity. The aim of the study was to explore this relationship, controlling for long-term conditions and other health, lifestyle and demographic factors in a general adult population. There was specific interest in the impact of high weight status, measured by body mass index ...
Abstract Obesity is a chronic, multifactorial, and morbid disease. In the United States, 69% of adults are overweight or have obesity, and the global prevalence of obesity is increasing. Obesity is influenced by genetic, neurologic, metabolic, enteric, and behavioral processes. It remains a key modifiable risk factor for many comorbid ...
A health sciences librarian developed and implemented the search strategy. The search strategy included 3 main concepts: obesity, Canada, and education; the full search strategy for Medline is presented in Table 1.The following databases were searched: Medline (via PubMed), Embase (Ovid interface), Eric (Proquest interface), Education Database (Proquest interface), Canadian Business & Current ...
Adult Obesity Management: Knowledge, Attitudes, and Practices of Alberta Family Physicians by ... No part of this thesis has been previously published. iv Acknowledgements There are many people I would like to acknowledge for their critical help in making this thesis possible. First, I would like to express deep gratitude to my supervisor, Dr ...
Obesity affects approximately 42% of adults in the US. Behavioral interventions can attain approximately 5% to 10% weight loss, GLP-1 and glucose-dependent insulinotropic polypeptide/GLP-1 receptor agonists attain approximately 8% to 21% weight loss, and bariatric surgery attains approximately 25% to 30% weight loss.
Thesis Advisor: Andrew Wise, PhD. ABSTRACT. This study evaluated the effectiveness of the Coordinated School Health Program model. in reducing childhood obesity rates using state-level data collected by the Centers for Disease. Control and Prevention (CDC) for the years 1994, 2000, and 2006.
There is significant research evidence to demonstrate that physical activity can produce weight loss, weight maintenance and positive health effects in the overweight and obese. However, it can be difficult to get this population sufficiently active to achieve these benefits. This thesis reports on a series of studies that explore physical activity used alone and in conjunction with other ...
carbohydrate is a crucial factor in the obesity epidemic. 18 Soft drinks, alcoholic beverages and fast food tend to be calorie rich. In Britain, there has been a signi cant rise in the amount of ...
The undersigned certify that they have read and hereby recommend for acceptance by the University of Dar es Salaam a dissertation entitled: Prevalence and Implications of Overweight and Obesity in Children's health and Learning Behaviour, in fulfillment of the requirements for the degree of Masters of Arts (Education) of the
Obesity in children is defined as a BMI-z score above the 95th percentile (Ogden C. L., Carroll, Curtin, Lamb, & Flegal, 2010). The increasing prevalence of childhood obesity worldwide is considered a major global public health problem. While many factors contribute to the development of obesity, an imbalance between
Sarcopenia and sarcopenic obesity (SO) are age-related syndromes that may compromise physical and mental health among older adults. The Nordic countries differ from other regions on prevalence of disease, life-style behavior, and life expectancy, which may impact prevalence of sarcopenia and SO. Therefore, the aim of this study is to review the available evidence and gaps within this field in ...
In 2019, the estimated annual medical cost of obesity in the U.S. was $173 billion. Medical costs for adults with obesity were $1,861 higher than those not living with the disease. For millions of ...
Not discussed in Mr. Hari's excellent article is the need for the entire population, including those who are not overweight, to eat a well-balanced diet that largely omits ultraprocessed foods ...
Her Arnold School mentors played a big role in her program, and she found Lohman as well as biostatistics clinical assistant professor Andrew Ortaglia to be particularly impactful. "Every professor I have had or worked with has been great, and they truly want you to do well and provide you the knowledge to do that," Jones says.
0.63%. 1. Eli Lilly. Thanks to its smash-hit drugs for type 2 diabetes and obesity, Mounjaro and Zepbound, Eli Lilly ( LLY -2.02%) is on a tear. In the first quarter, its revenue jumped by 26% to ...