- Lupe_Grau Cancel

Laboratory Products for “Research Use Only” (RUO) – Often a Dangerous Claim
Manufactures use the “Research Use Only” (RUO) label to declare that their products should not be used in diagnostic procedures. This enables them to avoid the time-consuming and costly documentation required for conformity-assessed in vitro diagnostic medical devices (CE-IVDs). Nevertheless, some medical laboratories, for example, still use RUO products in diagnostic procedures, sometimes even with the knowledge of the manufacturers. This can have consequences – not just for manufacturers and operators, but for patients as well.
In this article, you will learn:
- What the “Research Use Only” label (RUO) means
- What the requirements for RUO products are
- How to avoid legal problems
- What alternatives there are to RUO products
1. “Research Use Only” – what does it mean?
Labeling products for “research use only” has far-reaching consequences. It means the products are barely subject to any regulatory controls under the IVDR. As a result, for a lot of manufacturers and operators, they are desirable alternatives to more costly and time-intensive conformity-assessed in-vitro diagnostic medical devices (CE-IVDs) that must comply with the applicable legal requirements.
a) Institutions affected
The following institutions, in particular, use RUO products:
- Medical laboratories can use RUO products, but this makes them the manufacturer with all the consequences this entails. You can find more information on “lab developed tests” in our article “ The E U Regulates Medical Laboratories. Are Laboratory Developed Tests Still Allowed? ”
- If medical laboratories use RUO products for purposes other than research then, in the worst case, this makes them liable for damages as well as criminally liable.
- Therefore, medical laboratories should inform themselves about the parameters for RUO products and possible alternatives .
- Manufacturers Manufacturers use RUO products as components for their IVDs. They should, therefore, make sure that they know all the requirements in detail before labeling a product as “RUO”.
b) Definition
There is no uniform definition of “research use only” products. In general, they can be understood to be what the name implies, i.e., products to be used for analysis that are intended to be used for scientific research purposes only.
They primarily differ from medical devices in that they cannot be used for medical purposes.
However, the understanding of “research use only” is different in Europe and the USA.
Definition in Europe
In Europe, the MEDDEV 2.14/2 guidance document (IVD Guidance: Research Use Only products – A guide for manufacturers and notified bodies) provides clues as to the definition of RUOs. This guidance was written within the framework of the now obsolete Directive 98/79/EC on in vitro diagnostic medical devices (IVDD) and, in the absence of an up-to-date replacement, it can still be considered the state of the art.
MEDDEV 2.14/2 states:
“for a product to be categorized as an RUO product it must have no intended medical purpose or objective."
Source: MEDDEV 2.14/2 rev.1
This means that an RUO product must not have even a rudimentary medical purpose.
However, in the case of tests developed in-house by a laboratory (LDTs), this restriction does not apply provided that the products are not sold to other companies. The guidance gives the following specific examples of LDTs that may be designated “research use only” under this requirement:
- PCR enzymes
- Gel component agars
The IVDR also addresses RUO products.
“device for performance study’ means a device intended by the manufacturer to be used in a performance study.
A device intended to be used for research purposes, without any medical objective, shall not be deemed to be a device for performance study; ”
Source: IVDR Art. 2(45)
Thus, the IVDR, like MEDDEV 2.14/1 (IVD Medical Device Borderline and Classification issues), draws a distinction between RUO products and “devices for performance studies.”
Again, the key aspect of the definition is the RUO product’s lack of medical purpose.
To be classed an RUO product, it is vital that the product does not serve a medical purpose. Even a suspected medical purpose is enough for a device to be no longer considered an RUO product.
(See MEDDEV 2.14/1 section 1.1 4.)
Definition in the USA
In 2013, the FDA published a guidance document on RUOs entitled “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only.”
This guidance defines RUO products as follows:
“ An RUO product is an IVD product that is in the laboratory research phase of development and is being shipped or delivered for an investigation that is not subject to part 812” [NB: Part 812 concerns the provision of devices for performance evaluation purposes as a preliminary step to IVDs]
Source: FDA guidance “Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only”
Some examples of products that the FDA believes fall into this research phase of development are:
- Tests that are in development to identify test kit methodology, necessary components, and analytes to be measured
- Instrumentation, software, or other electrical/mechanical components under development to determine correct settings, subcomponents, subassemblies, basic operational characteristics, and possible use methods
- Reagents under development to determine production methods, purification levels, packaging needs, shelf life, storage conditions, etc.
Therefore, according to the FDA, a clearly visible RUO label must be affixed specifically to products that are in a research phase.
c) What are the consequences of using the “Research Use Only” label?
Normally, IVDs are subject to regulatory requirements (for example, according to the IVDR or FDA) based on their risk class.
However, RUO products do not fall within the definition of “in vitro diagnostic medical devices” given by the IVDR or the relevant FDA regulations . This means that these regulations do not apply to RUO products.
Definition: In vitro diagnostic medical devices (IVDs) in the EU
“‘In vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:
(a) concerning a physiological or pathological process or state;
(b) concerning congenital physical or mental impairments;
(c) concerning the predisposition to a medical condition or a disease;
(d) to determine the safety and compatibility with potential recipients;
(e) to predict treatment response or reactions;
(f) to define or monitoring therapeutic measures.
Specimen receptacles shall also be deemed to be in vitro diagnostic medical devices;”
Source: Article 2 IVDR
Definition: In vitro diagnostic medical devices (IVDs) in the USA
“In vitro diagnostic products are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body.”
Source: 21 CFR 809.3
Therefore, the requirements of the IVDR do not apply to RUO products. In the USA, they are exempt from cGMP and the FDA's quality regulations.
Depending on the product, they may still have to comply with requirements that are not specifically intended for IVDs (such as the REACH regulation for chemicals or the Machinery Directive ).
Since RUO products are thus subject to considerably fewer controls than IVDs, it is necessary to severely restrict their use.
Therefore, in particular they may not be used to:
- Make diagnoses
- Conduct performance studies
2. Use and misuse of “Research Use Only” labels
A) what should ruo products be used for.
As the name “research use only” indicates, products with RUO labeling are intended for research purposes only. RUO products are particularly attractive for the research sector due to the simplified process and lower hurdles for placing them on the market.
MEDDEV. 2.14/2 rev.1 provides a precise list of areas where RUO products may potentially be used:
- Basic research
- Pharmaceutical research
- Better identification and quantification of individual chemical substances or ligands in biological specimens
- In house manufacturing of so called “home brew kits” for research purposes
And of areas where the use of RUOs is expressly not permitted:
- Use of raw materials which are labeled “for “research use only” but which are incorporated into a finished product
- So called “research use” products being tested against a comparator IVD product that bears the CE mark
- Products for market studies/ feasibility studies
b) What RUO products are often used for
However, the low hurdles are also the reason why RUO products are often used for purposes they are not intended for. This poses significant dangers for manufacturers, operators and patients.
Sale of RUO products to medical laboratories
RUO products are sold by manufacturers to medical laboratories. Although doctors sometimes also conduct research, this is not really the main purpose of a medical laboratory. Therefore, when discussing sales with doctors, it should always be assumed that there is a medical reason behind the use of the product.
This means that anyone who knowingly sells RUO products to medical laboratories is potentially under suspicion of using the pretext “for research use only” to ignore an intended medical purpose and thus avoid responsibility for a medical device.
There are certainly laboratory products that clearly have no specific medical purpose, e.g.:
- Nutrient media
- Reaction vessels
- Washing solutions
These products are best labeled as “general laboratory supplies” rather than “RUO”.
Avoid reference to any specific diagnostic procedures in your advertising materials for products that clearly do not have a medical purpose. You should always stay on the technical or purely analytical level.
The issue with analyte specific reagents
Whether an RUO product contains analyte specific reagents, e.g., primary antibodies, FISH probes, PCR primers and probes, and sequencing panels, can be critical. In some cases, a medical purpose can be inferred just from the description of the product's performance.
This would be the case if a manufacturer of a RUO-labeled kit for the detection of viral genes specifies a number of copies per ml of blood that the kit can detect.
ASR in the USA
The FDA abbreviates the term “analyte specific reagents” to “ASR” and defines it as follows:
“Analyte specific reagents (ASR's) are antibodies, both polyclonal and monoclonal, specific receptor proteins, ligands, nucleic acid sequences, and similar reagents which, through specific binding or chemical reaction with substances in a specimen, are intended for use in a diagnostic application for identification and quantification of an individual chemical substance or ligand in biological specimens.”
Source: 21CFR864.4020 a)
In other words, US law says that, by definition, ASRs have a diagnostic purpose.
Exception: The sale of ASRs to IVD manufacturers as components for manufacturing kits or to non-clinical laboratories for research and development without compliance with regulatory requirements is permitted.
ASR in the EU EU law does not contain this exception. Nor does the term “analyte specific reagent” does appear in any of the applicable EU regulations. Therefore, such products may have a general laboratory purpose in the EU, depending on the justification. This means they do not fall under the IVDR if the manufacturer defines the intended purpose accordingly. However, if the manufacturer assigns a medical or diagnostic purpose to these products, the regulatory hurdles will very high once the IVDR comes into full effect (currently scheduled for May 26, 2022).
This means that the crucial factor is whether manufacturers have clearly defined the intended purpose and whether communication with customers (e.g., in advertising materials) is in line with this purpose.
Further information
You can find out more about the intended purpose of medical devices here: Intended purpose and intended use
Use of RUO products in medical laboratories
It is not just manufacturers for whom the sale of RUOs to medical laboratories represents a problem. The laboratories themselves may also not be acting in line with their status as operators and may, as a result, be liable under certain circumstances.
- Medical laboratories are free to develop in-house tests themselves. In such cases, RUO products are often used in diagnostic procedures. Even under the IVDD, MEDDEV 2.14/2 was critical of this. However, with the new In Vitro Diagnostic Medical Device Regulation (IVDR) , the EU is explicitly placing more restrictions on the routine use of such lab developed tests . Read more in our article The EU Is Regulating Medical Laboratories. Are Laboratory Developed Tests Still Allowed? .
- Due to the low regulatory hurdles, purchasing RUO products is very affordable. As a result, medical laboratories prefer them over expensive CE-IVD devices if they can achieve the same level of performance. Nevertheless, the use of RUO products for purposes other than research, even in cases where they provide similar results, is not permitted.

3. Consequences of incorrect classification
Lack of controls can have a negative effect on quality. As a result, the relevant bodies (e.g., authorities during inspections) take a closer look at whether a product is actually intended for “research use only”.
Manufacturers should also be aware that simply sticking an RUO label on a product does not on its own mean that the product no longer has to comply with requirements for IVDs that would otherwise apply.
In its guidance document on RUO , the FDA writes that only the actual intended use qualifies a product as RUO – or doesn’t. The FDA also uses marketing materials or other general factors as evidence of the intended purpose.
"Because these products are exempt from most regulatory controls, it is important that they are not distributed for clinical diagnostic uses. Mere placement of an RUO or IUO label on an IVD product does not render the device exempt from otherwise applicable clearance, approval, or other requirements. FDA may determine that the device is intended for use in clinical diagnosis based on other evidence, including how the device is marketed. ”
Manufacturers and operators who misuse the RUO label could face severe penalties, as such behavior can cause serious harm to patients or even the general public.
a) Consequences for manufacturers and operators
Improperly selling IVDs with an RUO label or using RUO products for purposes other than research is not a trivial offense.
Manufacturers who demonstrably hide or aim to hide a diagnostic purpose behind the RUO label should expect legal consequences in Germany. The same applies for operators who misuse RUO products. There is the possibility of a fine or even prison sentences. In addition, there is potential liability for harm suffered by patients.
b) Consequences in the USA
There are also severe penalties in the USA. If an RUO label is deemed to have been incorrectly used for a product, the product would be considered misbranded under sections 502(a) and 502(o) of 21 US Code, 352(a), 352(o) [A1] and would be considered adulterated under section 501(f) of 21 US Code 351(f).
c) Consequences for patients
However, the consequences can be even worse for patients. After all, the regulatory requirements for IVDs aren’t just plucked out of thin air to annoy manufacturers and operators. The regulations are intended to protect patients against incorrect results and subsequent wrong decisions. False-negative results can lull patients into a false sense of security and an existing disease may worsen undetected. One example would be the metastasis of an undetected cancer due to a test not performing as intended.
Some incorrect diagnoses could even be so severe that they can cause the death of a lot of people: an undetected viral infection can cost many lives in the early stages of an epidemic or pandemic, as the coronavirus pandemic sadly demonstrated.
4. Alternatives to “research use only” products
To avoid legal problems and risks for third parties, manufacturers and users should use alternatives to RUO products in borderline cases.
These alternatives don’t always have to be CE-IVDs. Depending on the specific situation, the following alternatives can be considered based on the intended purpose:
a) Products for general laboratory use
According to the MEDDEV 2.14/1 (IVD Medical Device Borderline and Classification Issues) guidance, it is a product's characteristics that determine whether it can be classified as a product for general laboratory use or not.
- If, based on its characteristics, a product is not specifically intended to be used for in vitro diagnostic examinations, it is not an IVD.
- Manufacturers cannot label products for general laboratory use as IVDs.
RUO products used for a better identification and quantification of individual chemical substances or ligands in biological specimens
Source: MEDDEV 2.14/2
Such products must have a general use. However, use as an IVD does not have to be ruled out, provided the product is not made specifically for a particular test. According to MEDDEV 2.14/2, even the aforementioned analyte specific reagents (ASRs) without a medical purpose fall into this category.
There are several advantages to using products for general laboratory use instead of RUO products:
- The product does not fall under the IVD Directive or the IVDR, which saves you a lot of time and money.
- Laboratories that use these products for in-house procedures are not in danger of being accused of using RUO products in routine diagnostic procedures.
However, the disadvantage is that the medical laboratory is responsible for ensuring that the examination conforms with the IVDR. This can make the product less interesting because the regulatory requirements entail a lot of work.
b) Lab developed tests with class A CE-IVDs Manufacturers may sell general laboratory reagents, which can be authorized as IVDs under the IVDR, to medical laboratories.
In combination with the ASRs developed in-house, laboratories can validate and use these products as lab developed tests (LDTs).
Read our article on lab developed tests to find out what laboratories should be aware of.
c) “For performance evaluation only” as a preliminary stage for certified IVDs
The IVDR defines " device for performance studies ” as follows:
“‘Device for performance study’ means a device intended by the manufacturer to be used in a performance study.”
Source: IVDR 2017/746/EU
These devices must already be safe, as far as possible, and meet the relevant general safety and performance requirements.
5. Ways to protect yourself
Manufacturers, operators and patients can take the following steps to avoid legal and other negative consequences when using RUO products:
a) Manufacturers
In the case of manufacturers, it is particularly important that they narrowly define the intended purpose of their product.
Analyte specific reagents should only be labeled as RUO products for specific non-medical purposes.
Example: SARS-CoV-2 and its mutations: a test kit that uses specific primers and probes to distinguish the variants B.1.1.7 (alpha variant) and B.1.351 (beta variant) from the initial variant following a positive result may be an RUO product if it is only intended to be used to determine the prevalence of the variant in the population. A specific intended purpose in this case would be: “Intended solely for epidemiological research for the purpose of surveying the prevalence of SARS-CoV-2 variants in the general population.” If a medical laboratory subsequently, based on new findings, used this test to provide the best possible treatment for infection by a specific variant, this would be an off-label use. The laboratory would then be responsible for the test's conformity.
Provided the manufacturer did not advertise the product with this clinical benefit, it would be adequately protected.
b) Operators
Operators should record exactly what they use IVDs and RUO products for.
Medical laboratories are operators of medical devices and IVDs and, therefore, are responsible for only using medical devices according to their intended purpose and in accordance with the generally accepted rules of the technology. This is stipulated in Section 4 of the German Medizinprodukte-Betreiberverordnung (MPBetreibV (German)). To be on the safe side, laboratories should keep a record of which medical devices and IVDs are in operation and routine use. This record should include a reference to the applicable test procedure and the intended purpose of the IVD.
This record can also be used to identify investigational procedures for which there are no adequate CE-IVDs available on the market. The lack of alternatives would justify the use of RUOs (as lab developed tests) in validated processes it has developed in-house, provided that the laboratory checks and can demonstrate that the general safety and performance requirements and the additional requirements of Article 5(5) of the IVDR are met.
Read more about the requirements for LDTs in our article on the topic .
c) Patients
Patients lack the knowledge to recognize what is and isn’t an RUO on their own. They are often given little to no information about the test they are undergoing. So, patients should follow this basic rule: ask your doctor or pharmacist!
- Patients can ask for the complete test report from the laboratory so that they can get a second opinion in case of doubt. The report should also indicate which specific test was performed.
- Patients should inform themselves about how “well” or “poorly” a test works, as well as the benefit-risk ratio.
- In the future, patients and doctors will also be able to get information about medical devices from EUDAMED and use this information to decide whether or not the test was performed with certified and thus legally compliant IVDs.
6. Conclusion
In the opinion of the EU Commission and the FDA, products “for research use only" have no place in diagnostics. To be used for diagnostic purposes, products have to go through the necessary controls. But these controls do not apply to RUO products.
Anyone who ignores this prohibition and uses or sells RUO products for purposes other than pure research is playing with fire. Manufacturers and operators run the risk of legal trouble and could even endanger patients’ health. Therefore, RUO products should only be used for research purposes. For other uses, manufacturers and operators should use the alternatives mentioned.
Our tip is: if you, as a manufacturer or medical laboratory, find that an RUO product is particularly well-suited for in vitro diagnostics, consider whether further development and conformity assessment to make it an IVD is worthwhile. We will be happy to help you work out which of the three alternatives to RUOs mentioned above is the best alternative to your product as part of our IVD authorization strategy consultation. If necessary, we can also help you ensure your product development conforms with the regulations.

Dr. Diana Gabriel

A quick overview: Our
Starter-Kit
Always up to date: Our
Back To Top
Privacy settings
We use cookies on our website. Some of them are essential, while others help us improve this website and your experience.
Individual Cookie Settings
Only accept required cookies.
Privacy Notes Imprint
Here is an overview of all cookies use
Required Cookies
These cookies are needed to let the basic page functionallity work correctly.
Show Cookie Informationen
Hide Cookie Information
Provide load balancing functionality.
Provides functions across pages.
Hubspot Forms
Used for the google recaptcha verification for online forms.
Cookies for Statistics
Statistic cookies anonymize your data and use it. These information will help us to learn, how the users are using our website.
Google Analytics
Tracking and analys of traffic on our websites.
Cookies for Marketing
Marketing cookies from thrid parties will be used to show personal advertisment. They use them to track users outside of their own web page.
Keeping track of a visitor's identity. It is passed to HubSpot on form submission and used when deduplicating contacts. It contains an opaque GUID to represent the current visitor. It also introduces cookies from linked in for marketing reasons.
LinkedIn conversion tracking.
Cookies for external Content
Content for Videoplatforms und Social Media Platforms will be disabled automaticly. To see content from external sources, you need to enable it in the cookie settings.
Google Maps
Used to display google maps on our Websites. Google uses cookies to identify and track users.
An Introduction to Research Use Only (RUO)
In this blog, we recap our eBook, “An Introduction to Research Use Only (RUO)” – Click HERE to download the entire publication.
Learn how it differs from adjacent labels, the FDA and EU guidance, its appropriate use, and the consequences of mislabeling products RUO.
Introduction
In the complex world of medical device development, regulation, and distribution, finding the appropriate label to put on a device may not be simple. When is one label appropriate over another? Does a device need to go through additional testing, verification, or validation? And what are the consequences of using the wrong label? In this eBook, we’ll cover the differences between Research Use Only (RUO) and a medical device – although, it’s generally a very clear distinction.
Using the right language and label is critical to complying with best practices. This is why Regulatory Affairs works with the regulatory bodies to ensure that the limitations of the product are properly documented. In a rush to get products to market, it may be tempting to use a Research Use Only (RUO) label to avoid additional regulatory processes while still empowering other researchers and developers. However, there are risks to using the RUO label inappropriately that can have serious consequences for developers, users, and patients. In fact, mislabeling a product is illegal, and punishable. You can see an example warning letter the FDA sent to Carolina Liquid Chemistries Corp after finding intentional mislabeling in 2019 here.
This introduction will provide an overview of the Research Use Only label, how it differs from similar, adjacent labels, its appropriate use, and the consequences of mislabeling products RUO.
What is Research Use Only (RUO)?
The label Research Use Only (RUO) is generally used to indicate products that are intended for scientific research only. They cannot be used for diagnostic or medical purposes. However, there is no standard definition of “research use only,” and the label has slightly different meanings in the European Union and the United States. With the IVDR regulations, RUO products that are being used in the LDT space are going to be revisited and potentially reclassified as a medical device. With this new classification, teams will likely need to follow design controls, best practices, and industry standards.
What is the FDA guidance on Research Use Only products?
Under the FDA’s guidance issued in 2013 , a product labeled Research Use Only is an In Vitro Diagnostic (IVD) product “that is in the laboratory research phase of development and is being shipped or delivered for an investigation that is not subject to part 812.” The agency includes in this category:
- “Tests that are in development to identify test kit methodology, necessary components, and analytes to be measured.
- “Instrumentation, software, or other electrical/mechanical components under development to determine correct settings, subcomponents, subassemblies, basic operational characteristics, and possible use methods.
- “Reagents under development to determine production methods, purification levels, packaging needs, shelf life, storage conditions, etc.”
The European guidance document MEDDEV 2.14/2 states that a product categorized as an RUO product “must have no intended medical purpose or objective.” The guidance does exempt some tests developed for in-house use as long as the products are not sold to other companies. Some examples of items that can be classified as “research use only” under this exemption include PCR enzymes, gel component agars, and primers.
RELATED: FDA released new draft guidance of premarket submissions for medical devices – are you ready?
What is the difference between ruo and ivd.
An IVD is an “In Vitro Diagnostic Medical Device,” and the general term applies to any device or product that either alone or with other products is intended to be used for diagnostic, monitoring, or compatibility purposes. There are four different regulatory levels for IVDs:
- Research Use Only (RUO)
- General Laboratory Use (GLU)
- For Performance Studies Only (PSO)
- In Vitro Diagnostic Medical Device (IVD)
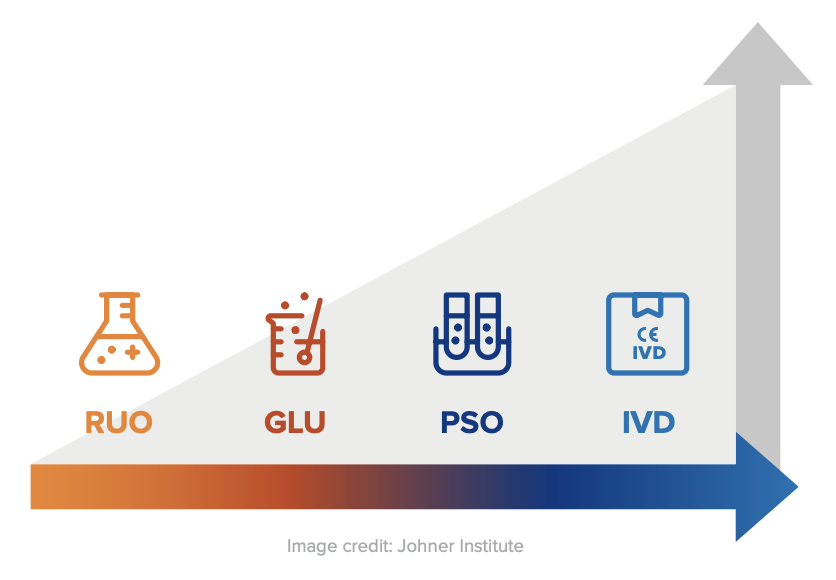
The simplest explanation for these different levels is that each increasing level requires more testing and oversight. Research Use Only products are at the lowest level of regulation, and In Vitro Diagnostic Medical Devices are at the highest level. Occasionally in the US, products will be labeled as “RUO IVD,” which means an in vitro device that is intended for research use only.
Products labeled with the “CE-IVD” label indicate that they have progressed through the applicable regulatory process and standards (such as IVDD or IVDR). These products are approved for diagnostic use and must include the IVD symbol to be used for medical purposes.
In the EU, as of May 2022, IVDs must comply with Regulation (EU) 2017/746 (IVDR) . The IVDR defines IVDs as follows:
“‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:
(a) concerning a physiological or pathological process or state; (b) concerning congenital physical or mental impairments; (c) concerning the predisposition to a medical condition or a disease; (d) to determine the safety and compatibility with potential recipients; (e) to predict treatment response or reactions; (f) to define or monitoring therapeutic measures.”
All IVDs that comply with the IVDR must carry the CE Mark if marketed in the EU.
Research Use Only products are not subject to regulatory requirements in either the US or the EU, but because they don’t meet the same compliance standards as IVDs, they must be clearly labeled as RUO products and cannot be used for medical purposes.
A known exception is the lab developed test (LDT) pathway for clinical purposes.
What are the requirements for an RUO product?
In the US, RUO products are basically unregulated and do not need to meet any specific requirements to carry the RUO label. The FDA does not specify any restrictions or limitations on RUO products, provided they are clearly labeled “For Research Use Only. Not for use in diagnostic procedures.” For this reason, RUO products can be an excellent solution for laboratories that need research materials for testing and research purposes. Because products with the RUO label do not require extensive testing, verification, and validation, they tend to be more cost-effective for research purposes.
The EU rules are similar. Because RUO products do not have clinical applications, they are not considered medical devices, and there are no requirements for RUO products defined by either the IVDD or the IVDR. These products should not be marked with the IVD mark, and they should be clearly labeled as “Research Use Only.”
RELATED: See how Jama Software ® helped Össur improve the mobility of millions by replacing process rigidity with speed and agility.
Are there alternatives to ruo labels.
Given the significant differences between labeling a product as RUO and labeling a product as IVD, manufacturers and users can’t be too careful when it comes to assigning labels or using products for specific purposes. If there is a risk to using products labeled as RUO, manufacturers and users should opt for products that have attained a higher compliance level. For example, for a doctor’s office or home use, IVD is the right path. For clinical purposes or hospital labs, RUO could be used as LDT as long as they are CAP/CLIA certified, such was the case with COVID-19 testing kits when the pandemic first hit.
For products that meet a higher degree of compliance, it is possible to assign General Laboratory Use (GLU), Performance Studies Only (PSO), or even In Vitro Diagnostic Medical Device (IVD) labels. However, depending on the intended use for the Research Use Only products, pursuing these additional levels of compliance may or may not make sense.
What is CLIA certification?
CLIA stands for Clinical Laboratory Improvement Amendments. The Centers for Medicare & Medicaid Services (CMS) regulates all clinical laboratory testing performed on humans in the United States through CLIA.
What is a CAP accreditation?
CAP stands for The College of American Pathologists (CAP) . The purpose of CAP laboratory accreditation is to ensure laboratories provide precise test results for accurate patient diagnoses, meet CLIA and CAP requirements, and demonstrate compliance with professionally and scientifically sound and approved laboratory operating standards.
What are RUO products used for?
As the name implies, RUO projects should be used for research purposes only. They may be used for basic research, pharmaceutical research, or in-house manufacturing of “home brew kits” for research purposes and potentially for clinical applications via the LDT pathway. RUO products are specifically not to be used to make diagnoses, conduct performance studies, or as a substitute or comparator for a CE-IVD device. They may also not be used for market or feasibility studies. Raw ingredients labeled as RUO products may not be incorporated into a finished IVD product.
Learn more about the advantages and disadvantages of the RUO label (and more) by downloading the entire eBook HERE .
- Recent Posts
- [Webinar Recap] Key Systems Engineering Skills: Critical Thinking and Problem Framing - March 5, 2024
- Jama Connect® Features in Five: Medical Device & Life Sciences Solution 2.0 – Part 2 - July 28, 2023
- Jama Connect® Features in Five: Medical Device & Life Sciences Solution 2.0 – Part 1 - July 21, 2023
USA 135 SW Taylor Suite 200 Portland, Oregon, 97204
EUROPE Amsterdam Queens Tower Delflandlaan 1, 1062EA Amsterdam The Netherlands
© 2024 Jama Software
- JAMA CONNECT
- Product Overview
- Pricing and Licensing
- Success Programs
- Resource Library
- User Community
- Privacy Policy
- Privacy and Security
- Preferences
Welcome to the IDT family!
Your product is now available from Integrated DNA Technologies.
Many of the Swift products you have grown to love are now part of our new complete portfolio, xGen™ NGS. Through this new partnership we are pleased to offer you comprehensive next generation sequencing solutions.
xGen NGS—made for you.
Unsure of what products are available? Or, perhaps you’d like guidance on which products are compatible? If so, try our xGen NGS Solutions Builder Tool today.
Find Archer now at IDT!
All Archer information is now available on IDT’s website. You can view Archer assays alongside IDT’s xGen™ NGS portfolio to find the best next generation sequencing solution for your lab.
Confidently detect more with Archer NGS assay solutions for your solid tumor, blood cancer, immune profiling, and genetic disease research.
Explore how our NEW cGMP gRNA manufacturing service can accelerate your CRISPR therapeutics project

- Order history

- 1-800-328-2661
- How to order and request a quote
- Videos & webinars
Choose your region, country/territory, and preferred language
Sars-cov-2 research use only primer and probe sets.
IDT offers a wide array of primer and probe sets, as well as plasmid controls, for the identification of SARS-CoV-2 (2019-nCoV). Primers and probes are manufactured in a template-free environment and certified template-free to cycle 45 by NTC testing.
SARS-CoV-2 Research Use Only qPCR Primer & Probe Kit: N1, N2 & RP*
- Manufactured in a certified, template-free environment under ISO 13485:2016
- Confirmed template-free to cycle 45 by NTC testing
- Next-day shipping
*These products are identical in sequence to the component(s) previous supplied with the 2019-nCov CDC EUA Kit, 1000 rxn, PN 10006770, which has been discontinued; none of these products have been qualified by the CDC.
SARS-CoV-2 Research Use Only qPCR Primers & Probes for N1, N2 & RP*
Sars-cov-2 & influenza research use only qpcr primers & probes for infa, infb, sc2 & rnase p, sars-cov-2 research use only primers & probes for envelope & rdrp genes, sars-cov-2 research use only plasmid controls.
- NGS sequence-verified
nCoV-N control: Severe acute respiratory syndrome coronavirus 2 isolate Wuhan-Hu-1, complete genome (GenBank: NC_045512.2)
MERS control: Middle East respiratory syndrome-related coronavirus isolate KNIH/002_05_2015, complete genome (GenBank: MK796425.1)
SARS control: Bat SARS-like coronavirus isolate bat-SL-CoVZC45, complete genome (GenBank: MG772933.1)
2019-nCoV_RdRp (ORF1ab) control: Severe acute respiratory syndrome coronavirus 2 isolate Wuhan-Hu-1, complete genome (GenBank: NC_045512.2)
2019-nCoV_E control: Severe acute respiratory syndrome coronavirus 2 isolate Wuhan-Hu-1, complete genome (GenBank: NC_045512.2)
Don’t see what you need for your nCoV experiments?
We can accommodate custom requests.
Safety data sheets (SDSs)
Product information, certificates of analysis (coas).
Find COAs by batch or lot number
- The ARTIC platform for genomic surveillance of SARS-CoV-2
For research use only. Not for use in diagnostic procedures. Unless otherwise agreed to in writing, IDT does not intend these products to be used in clinical applications and does not warrant their fitness or suitability for any clinical diagnostic use. Purchaser is solely responsible for all decisions regarding the use of these products and any associated regulatory or legal obligations.
NOTE: None of the primer, probe or kit products on this page have been qualified by the CDC.
ATTO is a trademark of ATTO-TEC GmbH.
Cy is a registered trademark of Cytiva.
We use cookies to offer you a better browsing experience and analyse our website performance. We also use third-party cookies to further customise your experience showing relevant content while you are navigating on third-party platforms.

Our Priorities MedTech Europe strives to support our dynamic sector in meeting the needs of patients and health systems. To achieve this, we focus on engaging with healthcare stakeholders on key issues from regulations and market access to digital health and Brexit, among others.
- COVID-19 Information Hub
- Interactions with the Medical Community
- Access to Medical Technology
- Medical Technology Regulations
- Digital Health
- International
- Environmental and Social Sustainability
- Market Data
- Research and Innovation
- Innovative Health Initiative (IHI)
Sector Groups MedTech Europe sector groups bring together company experts to drive forward key healthcare domains, helping to address issues facing these sectors and shaping their future. We have dedicated groups focused on cardiovascular health, ophthalmology, diabetes, orthopaedics, and AMR/HAI.
- Antimicrobial Resistance (AMR) and Healthcare Associated Infections (HAIS)
- Cardiovascular
- Homecare & Community Care
- Orthopaedic

Real stories of people’s lives transformed by medtech.

Your platform for dialogue about medical technologies.
Search on this website
Research Use Only Products

What are Research Use Only (RUO) products? Research Use Only (RUO) products are a distinct category of in vitro diagnostics (IVDs) exclusively tailored for laboratory research. RUOs encompass specialised reagents, equipment, and materials crucial for scientific investigations, contributing significantly to the development of cutting-edge tools and solutions for research applications.
Research Use Only (RUO) products play a crucial role in medical research and innovative management of many patients. These specialised products, which include laboratory reagents and equipment, are exclusively designed for research in controlled laboratory environments. As essential tools for medical and scientific investigations, experimentation, and analysis, RUOs contribute to developing innovative solutions and advancements in medical research.
For example: RUO products can be used for Fundamental Research, in Pharmaceutical Research to find new drug compounds, and for a better identification and quantification of individual chemical substances. In diagnostics research, RUO products are essential to the development of new diagnostic assays and tools.
Unlike in vitro diagnostic medical devices (IVDs), RUOs are dedicated to facilitating research initiatives and are not intended for direct medical procedures with human patients. RUOs are not defined in the EU’s In Vitro Diagnostic Medical Devices Regulation 2017/746 (IVDR); they are regulated by the EU General Product Safety Regulation and other applicable EU legislations. Manufacturers of RUO products clearly label them as “Research Use Only” and use the RUO label.
From a production and specifications general perspective, the knowledge and processes needed to manufacture RUOs are very similar to those needed to manufacture CE marked IVDs. Many companies which operate in the IVD space will have RUO products in their portfolio. RUOs will generally have a similar chemical and physical composition compared to IVDs, but their intended purpose will be different. While RUO or IVDs might seem similar in their appearance and specifications, unambiguous and documented evidence associating the use of devices with in vitro diagnostic examination procedures is required to qualify a device as an IVD.
RUOs provide researchers and scientists – including those operating in medical laboratories – with valuable resources to advance in the understanding of disease, in drug discovery, in the development of new therapies and diagnostic tools. Laboratories or research consortia often collaborate with RUO manufacturers to tailor products to meet specific research needs and requirements, fostering a collaborative environment and contributing to the continuous evolution of research tools and solutions.
One critical application of RUO is to enable medical laboratories to develop in-house assays to e.g. diagnose rare and emerging conditions or to improve the current knowledge and management of specific diseases for which no adequate CE marked IVDs exist. This not only fulfils a critical and imminent healthcare need but is also a key stepping stone in the eventual development of IVDs. A poignant example of this was the development of COVID-19 assays during the early phase of the pandemic – initially, reference laboratories developed in house assays test for the SARS-CoV-2 virus, and shortly afterwards, commercial IVDs began to reach the market in order to fulfil a critical need during the global health crisis. However, it is worth noting that the use of in-house assays is regulated in IVDR and is subject to certain conditions.
In essence, RUO products provide researchers and physicians with the necessary tools to conduct experiments and studies, contributing to the overall progress in medical research. Their intended use in laboratory settings supports the development of new technologies and innovative solutions for various research applications.
Share this page
MedTech Europe Manifesto for 2024 – 2029
Empowering Patients, Inspiring Innovation
Sign up for your monthly newsletter
By clicking the Subscribe button, you give consent to MedTech Europe AISBL to use of the information you provided and send you content on the services you selected. We will ensure that the information is processed confidentially, and will only share it with third party providers that assist in providing these services. These providers may be located outside the EU; in this case, we will ensure that they are subject to a legal framework adequate in safeguarding your data, in compliance with European data protection law. You can unsubscribe, change your preferences or update your information at any time by clicking on the unsubscribe button available on all messages. For more information on how MedTech Europe will handle your personal data, please refer to our Privacy Policy . You can contact us at [email protected] for further questions related to your privacy and your rights.
Thermo Fisher Scientific
Research Use Only (RUO) Recombinant Antibodies for Biotech and Pharmaceutical Research
Authors: Haripriya Sridharan and Aparna Chandrasekaran
Research use only (RUO) antibodies are used in basic and applied research. Differing fundamentally in their end application from therapeutic or diagnostic antibodies, RUO antibodies are not meant for patient or clinical use; however, they are an essential component in a biotech or pharmaceutical scientist’s toolkit and are used in a myriad of ways during drug or diagnostic development. For example, they are extensively used as research tools to study biological processes and therapeutic targets, such as measuring protein levels after a drug treatment.
Advantages of recombinant monoclonal antibodies
One of the critical considerations for biotech or pharmaceutical research is antibody reproducibility. The traditional choice for monoclonal antibodies were hybridomas derived from the fusion of an antibody producing B cell with a myeloma cell line. However, hybridomas are plagued with challenges such as genetic drift, which can cause a drop in antibody titer over time.
Recombinant antibodies are a new generation of monoclonal antibodies developed in vitro by cloning immunogen-specific antibody genes into expression vectors. Since recombinant antibodies are defined by their sequence, they offer several advantages over traditional hybridomas, such as lot-to-lot reproducibility and the option to use an animal-free production system, in addition to specificity and sensitivity. Furthermore, recombinant antibody expression can be conducted at any scale in a high-throughput manufacturing environment along with a guaranteed long-standing supply of antibody, thus making them an excellent tool for testing multiple samples or for long-term studies.
Due to these advantages, many hybridomas are being converted to recombinant antibodies. During these conversions, the antibody encoding genes from the hybridoma cell lines are cloned into expression vectors. The recombinant antibody has the same antigen binding sequences as the parental hybridoma thereby, retaining the same antigen specificity as the hybridoma. For example, as shown in Figure 1, a rabbit hybridoma encoding an antibody recognizing somatostatin receptor 2 (SSTR2) protein was converted to a rabbit recombinant monoclonal antibody with specificity for SSTR2.
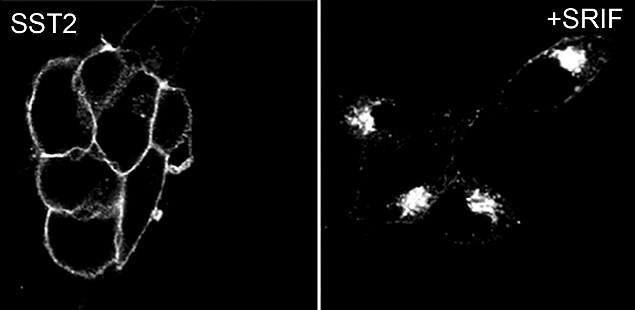
Figure 1: Recombinant antibody testing data for SSTR2 Recombinant Rabbit Monoclonal Antibody (Cat. No. 704011).
A) HEK-293 cells stably transfected with SSTR2, after 24 hours, cells were treated with 1 µM somatostatin-14 (SRIF) and then fixed and permeabilized. The specimens were incubated with SSTR2 Recombinant Rabbit Monoclonal Antibody (Cat. No. 704011, 1:1000 dilution). Cells were then incubated with Alexa 488-conjugated secondary antibody for 2 hours at room temperature, mounted and examined. Immunofluorescence analysis shows internalization of SST2 receptor from the plasma membrane to perinuclear cluster of the vesicles upon treatment with somatostatin-14 (SRIF – somatotropin release inhibiting factor). Altered expression of the protein upon cell treatment demonstrates antibody specificity.
B) Sections of human neuroendocrine tumor (NET) were dewaxed, microwaved in citric acid and incubated with SSTR2 Recombinant Rabbit Monoclonal Antibody (Cat. No. 704011, 1:1000 dilution). Sections were then sequentially treated with biotinylated anti-rabbit IgG and AB solution. Sections were then developed in DAB and lightly counterstained with hematoxylin.
Recombinant antibodies can be offered in a multitude of formats, including full-length antibodies from a single species or chimeric antibodies, as well as, antibody fragments such as single chain fragment variable (scFv) or antigen-binding fragment (Fab). Each of these formats offers unique benefits that are determined by their end usage and application. In the following sections, we highlight two such examples that were developed to serve distinct research needs.
A GPCR/G-protein complex stabilizing scFv recombinant monoclonal antibody
The scFv16 antibody fragment stabilizes active trimeric G protein complexes by recognizing the interface between G alpha and G beta/gamma subunits and is a valuable tool for CRYO-EM studies of GPCR protein complexes. This scFv is offered as a Recombinant Mouse Monoclonal Antibody (Cat. No. 703976) to serve customers focused on CRYO-EM applications of GPCR protein complexes (Figure 2). The product formulation was specifically developed to ensure compatibility for CRYO-EM applications.
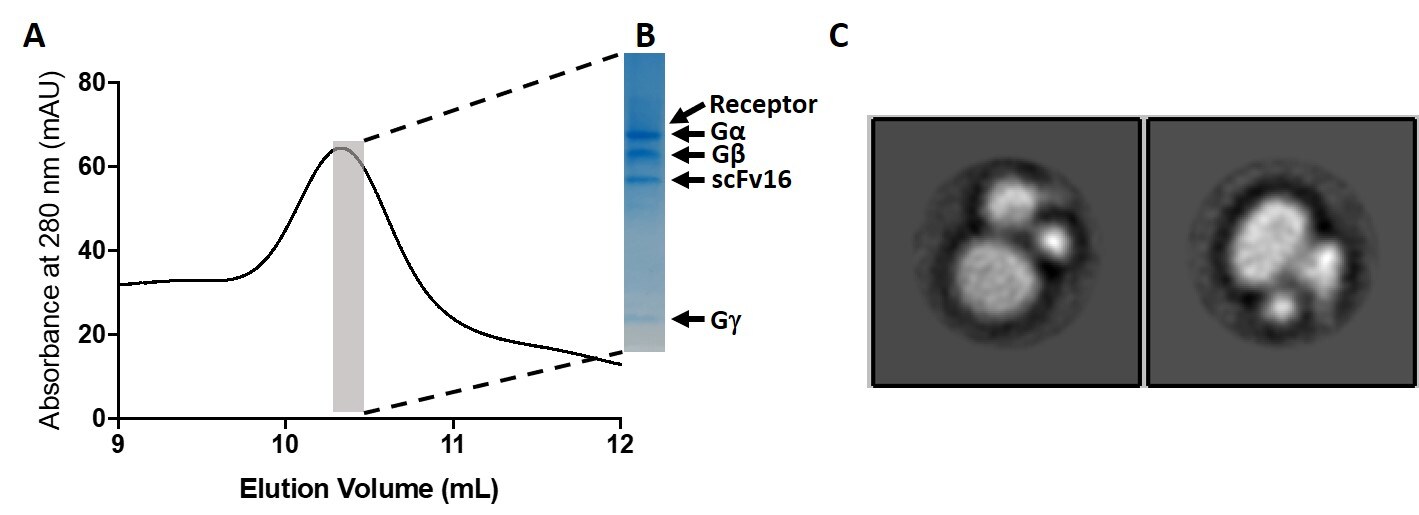
Figure 2: Recombinant antibody testing data for GPCR/G-protein complex-stabilizing scFv Recombinant Mouse Monoclonal Antibody (scFv16) (Cat. No. 703976).
A tagged GPCR was used to pull down a GPCR-heterotrimeric G protein complex that is stabilized by scFv16. A) Size exclusion chromatography (SEC) chromatogram of the purified complex. B) Highlighted sample from SEC was run on SDS-PAGE gel and Coomassie stained. All components of the active complex are present. C) Direct visualization of GPCR-G protein complex by negative stain TEM showing features characteristic for active GPCR complexes that are intact. Data courtesy Dr. David M. Thal, Monash Institute of Pharmaceutical Sciences, Monash University.
Recombinant antibodies for SARS-CoV-2 Spike protein
Sometimes researchers may have specific species requirements for an antibody to ensure compatibility with the rest of the assay or product development process. In these cases, the antigen-binding region (variable regions or the Fab fragment) of the antibody can be fused to the backbone of the required species. Having the same antigen-binding region gives the researcher complete confidence that the antigen-binding specificity is retained. Recombinant antibodies that recognize the spike protein of SARS-CoV-2 were developed as either fully human or human-rabbit chimeric antibodies to serve different research or product development requirements (Figure 3). For example, antibodies with a human constant region are useful as controls for developing kits to detect and characterize immune responses to SARS-CoV-2. At the same time, many laboratories routinely use rabbit antibodies to understand viral biology using immunoassays. Since a key focus area of SARS-CoV-2 research was to study the neutralization of the virus, some of these antibodies were tested and found to neutralize the ACE2-Spike protein interaction (Figure 4), making them useful controls for these experiments. The development of SARS-CoV-2 specific recombinant monoclonal antibodies, including the application and specificity testing, is discussed in further detail in a separate blog ‘Specific and neutralizing recombinant antibodies to SARS-CoV-2’ .
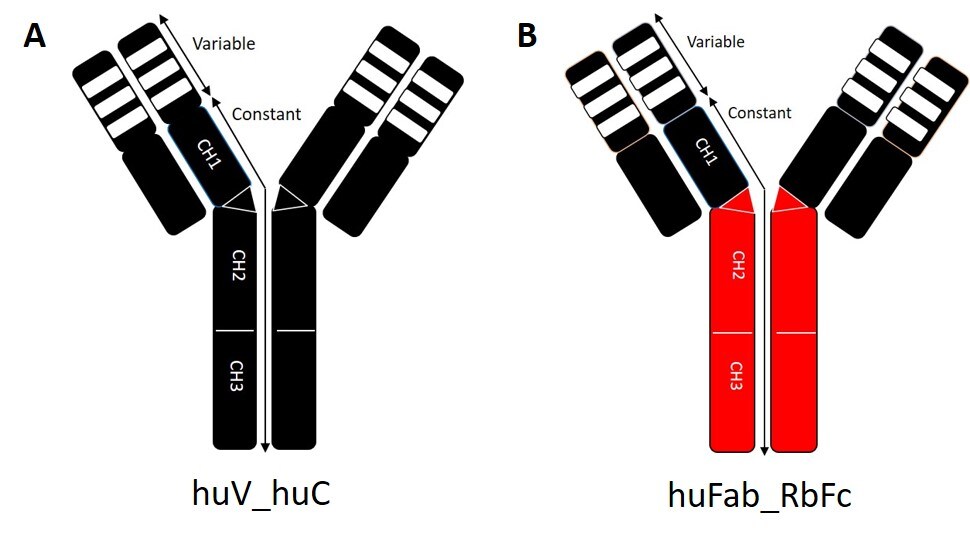
Figure 3: Schematic representation of the SARS-CoV-2 antibody backbones
A) Fully human backbone by grafting onto a human IgG1
B) Human Fab and rabbit Fc chimeric backbone by grafting onto a rabbit IgG.
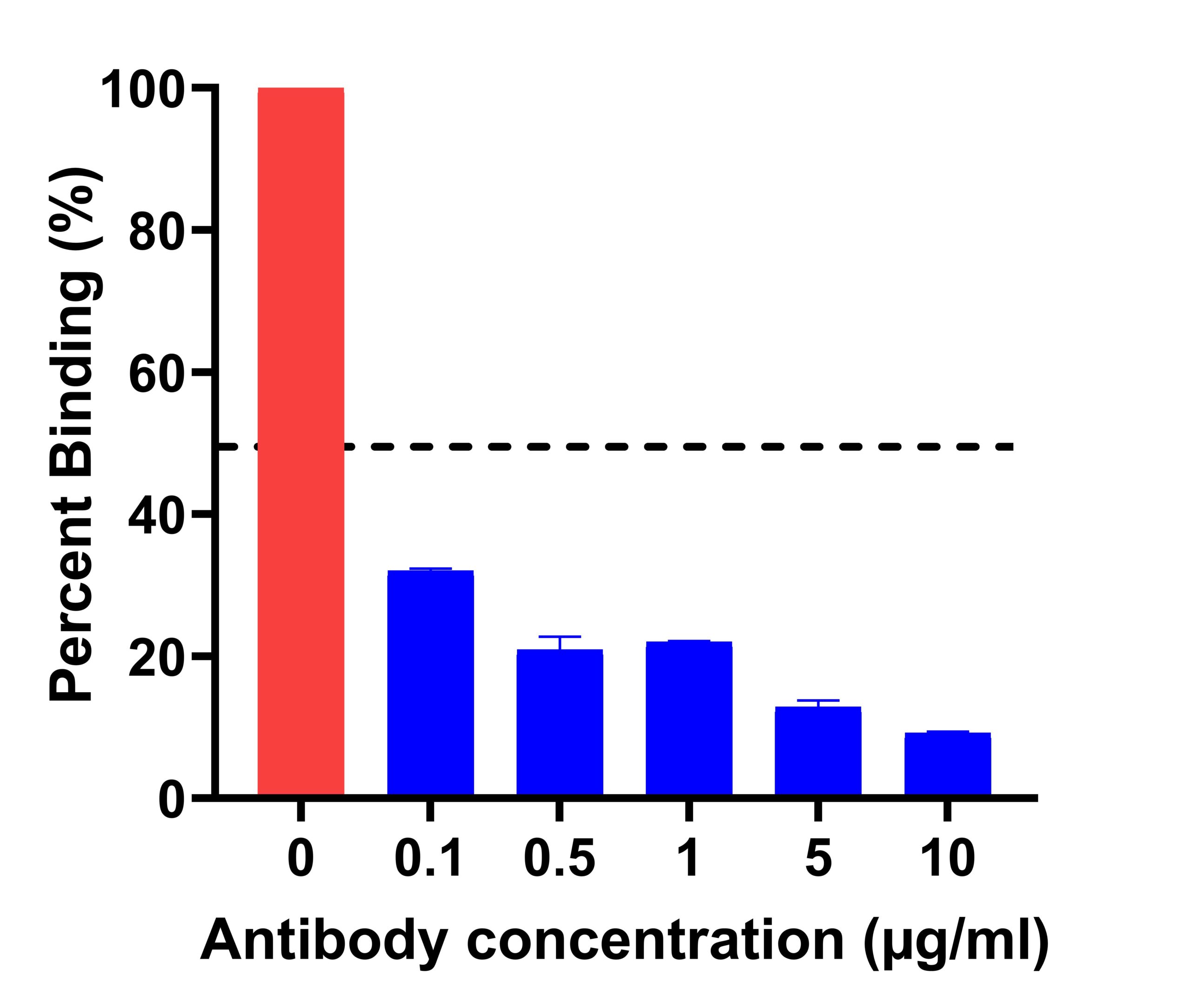
Figure 4: Spike protein RBD-ACE2 interaction blocking by SARS-CoV-2 Recombinant Monoclonal Antibody (Cat. No. 703973)
ELISA based SARS-CoV-2 inhibitor screening assay shows that the binding of SARS-CoV-2 Spike Protein RBD to human ACE2 was inhibited in the presence of the SARS-CoV-2 Recombinant Monoclonal Antibody (Cat. No. 703973). X-axis represents antibody concentrations and Y-axis represents percent binding signal of human ACE2 to SARS-CoV-2 Spike Protein RBD. The dotted line represents 50% inhibition.
RUO antibodies used in biotech and pharmaceutical research must often fit very specific criteria to maximize their utility as controls or reagents in various workflows. The examples described in this blog highlight how these requirements are being anticipated and met in order to provide optimal antibody performance and reproducibility to support the biotech and pharmaceutical research communities.
Additional Antibody Blogs:
Let’s get ‘specific’ about the TNFR pathway!
DIY Neurons for antibody validation
Translate to Invitrogen antibodies for your ribosomal protein research!
Drivers of the Chromosomal Passenger Complex
PRMTs: Role in epigenetic regulation
Using Blockers to Unlock Secretory Proteins
Specific and neutralizing recombinant antibodies to SARS-CoV-2
Staining Your Way into Cells: Exploring Cell and Organelle Markers
Is it a T-Cell or B-Cell? Antibodies for Immunophenotyping
December 1, 2021 at 12:50 am
I didn’t know that recombinant monoclonal antibodies have advantages. I will be sure to try them. Maybe they could make a difference.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *

Get news and research reviews on the topic of your choice, right in your inbox.
Subscribe Now
- Select your country/region * Select your country/region United States Canada Afghanistan Albania Algeria American Samoa Andorra Angola Anguilla Antarctica Antigua and Barbuda Argentina Armenia Aruba Australia Austria Azerbaijan Bahamas Bahrain Bangladesh Barbados Belarus Belgium Belize Benin Bermuda Bhutan Bolivia Bosnia and Herzegovina Botswana Bouvet Island Brazil British Indian Ocean Territory Brunei Darussalam Bulgaria Burkina Faso Burundi Cambodia Cameroon Cape Verde Cayman Islands Central African Republic Chad Chile China Christmas Island Cocos (Keeling) Islands Colombia Comoros Congo Congo, The Democratic Republic of Cook Islands Costa Rica Cote D'Ivoire Croatia Cuba Cyprus Czech Republic Denmark Djibouti Dominica Dominican Republic East Timor Ecuador Egypt El Salvador Equatorial Guinea Eritrea Estonia Ethiopia Falkland Islands (Malvinas) Faroe Islands Fiji Finland Fmr Yugoslav Rep of Macedonia France French Guiana French Polynesia French Southern Territories Gabon Gambia Georgia Germany Ghana Gibraltar Greece Greenland Grenada Guadeloupe Guam Guatemala Guinea Guinea-Bissau Guyana Haiti Heard and McDonald Islands Holy See (Vatican City State) Honduras Hong Kong Hungary Iceland India Indonesia Iran (Islamic Republic Of) Iraq Ireland Israel Italy Jamaica Japan Jordan Kazakstan Kenya Kiribati Korea, Democratic People's Rep Korea, Republic of Kuwait Kyrgyzstan Lao People's Democratic Rep Latvia Lebanon Lesotho Liberia Libyan Arab Jamahiriya Liechtenstein Lithuania Luxembourg Macau Madagascar Malawi Malaysia Maldives Mali Malta Marshall Islands Martinique Mauritania Mauritius Mayotte Mexico Micronesia, Federated States Moldova, Republic of Monaco Mongolia Montserrat Morocco Mozambique Myanmar Namibia Nauru Nepal Netherlands Netherlands Antilles New Caledonia New Zealand Nicaragua Niger Nigeria Niue Norfolk Island Northern Mariana Islands Norway Oman Pakistan Palau Panama Papua New Guinea Paraguay Peru Philippines Pitcairn Poland Portugal Puerto Rico Qatar Reunion Romania Russian Federation Rwanda Saint Helena Saint Kitts and Nevis Saint Lucia Saint Pierre and Miquelon Samoa San Marino Sao Tome and Principe Saudi Arabia Senegal Seychelles Sierra Leone Singapore Slovakia Slovenia Solomon Islands Somalia South Africa Spain Sri Lanka Sth Georgia & Sth Sandwich Is St Vincent and the Grenadines Sudan Suriname Svalbard and Jan Mayen Swaziland Sweden Switzerland Syrian Arab Republic Taiwan, Province of China Tajikistan Tanzania, United Republic of Thailand Togo Tokelau Tonga Trinidad and Tobago Tunisia Turkey Turkmenistan Turks and Caicos Islands Tuvalu Uganda Ukraine United Arab Emirates United Kingdom Uruguay US Minor Outlying Islands Uzbekistan Vanuatu Venezuela Vietnam Virgin Islands (British) Virgin Islands (U.S.) Wallis and Futuna Islands Western Sahara Yemen Yugoslavia Zambia Zimbabwe
- Pick a category * Pick a category Accelerating Microscopy Advancing Materials Advancing Mining Analyzing Metals Behind the Bench Examining Food Identifying Threats Life in the Lab
- I would like to receive information about content, events, products, services and promotions from Thermo Fisher Scientific and its affiliates. I agree for the Thermo Fisher Scientific group (thermofisher.com/legalentities) to contact me by email and/or telephone to inform me of events, products, services and/or promotions offered in connection with the brands Thermo Scientific™, Applied Biosystems™, Invitrogen™, Gibco™, Ion Torrent ™, Unity Lab Services™, Fisher Scientifc™. I can withdraw my consent and unsubscribe at any time by emailing [email protected] By submitting my data, I give consent to the collection, processing and use of my personal data in accordance with the Thermo Fisher Scientific Policy (thermofisher.com/privacypolicy).
- Name This field is for validation purposes and should be left unchanged.
- Featured News
- Artificial Intelligence
- Bioprocessing
- Drug Discovery
- Genome Editing
- Infectious Diseases
- Translational Medicine
- Browse Issues
- Learning Labs
- eBooks/Perspectives
- GEN Biotechnology
- Re:Gen Open
- New Products
- Conference Calendar
- Get GEN Magazine
- Get GEN eNewsletters

Oversight of Research Use Only Products
By Jeffrey N. Gibbs
March 1, 2010 (Vol. 30, No. 5)
RUO Assays and Instruments Face Greater Scrutiny
The FDA actively regulates medical devices intended for diagnostic use. Diagnostic kits intended for diagnostic use face the full panoply of FDA regulation. In sharp contrast, research use only (RUO) products are essentially unregulated. In fact, although RUO products are often discussed as though they are a kind of medical device, RUOs are not devices at all.
A commercially important class of products, RUOs are defined very briefly by FDA regulations. RUO products are described as products “in the laboratory research phase of development and not represented as an effective in vitro diagnostic product.” This definition has created some uncertainty as to what products fall into the RUO category.
The same regulation establishing the RUO category requires that RUO products bear the following labeling statement: “For Research Use Only—Not for use in diagnostic procedures.” Although not authorized by the regulation, many companies have shortened the statement to just the first clause. FDA regulations do not prescribe any other restrictions or limitations on RUO products beyond this labeling statement. Thus, FDA regulations define the category and prescribe labeling, and nothing more.
Given that RUO products are not intended to diagnose “a disease or other condition,” it is not clear that they are even subject to FDA’s jurisdiction. The intended use of an RUO product—research, not diagnosis—presumptively removes it from the definition of a device and FDA’s authority.
In any event, aside from bearing the mandated statement, RUO products are not regulated by the agency. For example, they do not need to be listed with FDA or comply with the Quality System Regulation (QSR). They can be sold without any FDA clearance or approval. As a practical matter an RUO is essentially unregulated by FDA.
Over the years, the paramount regulatory issue for products bearing the RUO label has been whether or not they actually do belong within the RUO category. There have been multiple instances in which RUO products have become widely used by laboratories for clinical applications. There have also been a number of occasions where companies have labeled products as RUO but then promoted them for diagnostic use. In some instances, companies have made specific diagnostic claims for their assay or instrument but still labeled the product as RUO.
Biomarker kits are often labeled as RUO because it is not known whether the product has any clinical use or, if so, what that use might be. The assay’s developer may expect that a particular biological substance will be of some clinical value, but not be sure what that value is. Labeling a product RUO, allows it to get into the hands of researchers who can then evaluate whether the product may be potentially valuable for some specific diagnostic purpose.
Often, no clinical use is ever identified. Some assays maintain their true RUO status indefinitely. While the product may be helpful to researchers in understanding basic biological mechanisms, a diagnostic use may never be discovered.

Guidance Documents
FDA has initiated several attempts to try to regulate RUO products more tightly. In the early 1990s, FDA issued a draft Compliance Policy Guide (CPG) document that sought to significantly restrict the availability of RUO products. This guidance document went through several iterations but was never finalized. There is still no guidance document setting out FDA’s policy regarding RUO products, however, reports have recently surfaced that a new RUO policy may finally be released.
One of the elements set forth in the draft CPG was that the distributor of the RUO product should receive a certification from the laboratory customer that the product will be used for research purposes only. Although the CPG was not adopted, some vendors have asked laboratories to sign some type of acknowledgement form. While this will help support a vendor’s position that its product is intended only for research use, it is not currently required. FDA has, however, “encouraged” some instrument suppliers to adopt certification programs.
Concerned by the proliferation of RUO products, in 1997 FDA tried a different tack. That year, FDA promulgated the Analyte Specific Reagent (ASR) regulation. ASRs were broadly defined as the building blocks of diagnostic assays. Unlike RUOs, ASRs were subject to FDA requirements, including QSRs and Medical Device Reporting. This regulation was prompted, in part, by the belief that it would result in the availability of higher quality materials for laboratory tests and displace some of the lower quality RUOs.
To some degree, that plan succeeded. Many different products were offered to laboratories as ASRs. However, while many of these were basic chemical components, more complex products were also sold as ASRs. Ultimately, FDA concluded that the ASR regulation was being used as a vehicle for products that didn’t fit the intent of the regulation.
FDA therefore released a guidance document in 2007 that substantially curbed the availability of ASRs by prohibiting companies from combining more than one active component. With the advent of molecular diagnostics, selling a single component was often impracticable, e.g., a primer and probe pair need to be offered together. This narrow interpretation of ASRs has essentially precluded the sale of ASRs for use in molecular diagnostics. Somewhat predictability, a number of companies responded by relabeling their ASRs as RUOs. This has helped lead to a renewed focus on RUOs by FDA.
For years, the principal regulatory question for products labeled as RUOs has been whether they qualify for this classification and hence are not subject to regulation as devices. While FDA has not issued either a regulation or guidance delineating how companies can promote RUOs, the agency has taken enforcement action against a number of RUO companies.
Even absent regulations or guidance, it is apparent that in FDA’s view a product forfeits its RUO status if certain types of claims are made—claims that the product can diagnose a disease or condition, provide clinical sensitivity or specificity data, or offers a clinical benefit. Correspondingly, the instructions for use (IFU) accompanying the product need to be brief.
While the bulk of RUO products have been assays, the RUO category also encompasses instruments and equipment. This can present its own set of regulatory challenges, particularly when an IVD applicant has used an RUO instrument in conjunction with developing its assay, a situation that is now occurring with greater frequency.
The utilization of RUO instruments in assay development has led to the submission of applications that reference RUO instruments. This may result in naming the RUO instrument in the draft IFU, i.e., the applicant states that the assay is to be performed on an RUO instrument, or the data for the IVD were generated on an RUO instrument.
While FDA had accepted these practices, that has seemingly changed. Therefore, an IVD company that has tested and validated its assay on an RUO instrument or is using RUO assays in its test system should discuss with FDA at an early stage how to address the regulatory implications that may arise from this situation. Simultaneously, companies that are selling RUO-labeled instruments that are being widely used in diagnostics may find that they will be receiving more regulatory scrutiny from FDA.
Over the past few years, RUO products have received relatively little attention from FDA. That regulatory lull seems to be ending.
Jeffrey N. Gibbs ( [email protected] ) is a director at Hyman, Phelps & McNamara. Web: www.hpm.com.
Single-Cell Cloning Remains a Challenge
New approach to nanopore sequencing that is sure to catch your....
Enzyme-Linked Immunosorbent Assay (ELISA) Kits

An ELISA (Enzyme-Linked Immunosorbent Assay) is a multi-well plate-based immunoassay within which one of the assay components, typically an antibody or sample, is adsorbed onto a solid surface, in this case, a plate. Offering rapid, quantitative, and sensitive analyte detection at relatively low cost, ELISAs represent one of the simplest assay formats to perform. Furthermore, the ease of adapting an ELISA to a higher throughput screening method empowers researchers to test large sample numbers in a single run.
Read more about
- Sandwich, Direct, and Competitive ELISAs
- Conferma ® ELISAs and Belysa ® Immunoassay Curve Fitting Software
Subscribe to Our Newsletter
Choose up to 4 products to compare, sandwich, direct, and competitive elisa s.
Our ELISAs are a convenient and user-friendly method for studying soluble protein biomarkers in a variety of matrices including serum, plasma, cell culture supernatant, or lysate. Our ELISA offering consists of >1,000 Research Use Only assays, against a wide variety of species including human, mouse, rat, and various other animal models (Agricultural and Companion). Each assay comes with a specific protocol and the required reagents to run a 96-well plate. ELISAs primarily exist in a 96-well format with the vendor providing the reagents necessary to detect and quantitate the target. There are a variety of ELISA methods including Sandwich, Direct, and Competitive.
The sandwich assay uses a pair of monoclonal antibodies (mAbs) against different epitopes against the same target. The primary mAb, bound to the plate, pulls the protein out of the solution while the second mAb is used to complete the “sandwich” and provide the signal which will indicate the presence of the target. A known amount of the recombinant version of the protein is provided with the kit, allowing the user to create a standard curve against which the signal from one of the samples will be interpreted. The majority of our portfolio is in this sandwich ELISA format.
The direct ELISA and competitive assays are rarer. The direct assay uses a single mAb to detect the sample which is bound to the plate. The mAb is then bound by a reporter secondary antibody to provide the signal.
The competitive assay has a known amount of the biomarker pre-bound to the plate. A labeled antibody is co-incubated with the sample which “mops up” the antibody depending on the concentration of the target in the sample. The free antibody is then able to bind to the antigen on the plate and provide a signal after the sample is washed away. In this case, the signal is inversely proportional to the concentration of the target biomarker.
Conferma ® ELISA s and Belysa ® Immunoassay Curve Fitting Software
The Conferma ® ELISA brand consists of sandwich assays designed to minimize lot-to-lot variability at the critical reagent level, a key concern of assay users. Evaluating the individual lots of critical reagents with a range of analytical techniques aligned with a detailed quality control (QC) protocol allows us to control and limit variability over time.
In order to allow scientists to evaluate the reproducibility of their method we further designed the Belysa ® Immunoassay Curve Fitting Software . With this tool, a user can examine their sandwich immunoassay to ensure parameters such as %CV (coefficient of variation) and %Recovery were as expected. Then they can move on to compare the standard curves of multiple plates to confirm that their method was consistent.
Related Product Resources
This article offers 4 popular ELISA protocols: Sandwich ELISA protocol, Phosphorylation Assay Procedure, EIA Assay Procedure, & Cell-based Assay Procedure.
Troubleshoot and optimize ELISAs using this guide that includes solutions to some of the most common sources of problems for assay development.
There are several ELISA configurations, with the most common formats being sandwich, competitive and signaling assays.
Learn about the Conferma ® ELISA development and manufacturing methods that provide strong sample detection and long-term assay and lot consistency, giving you confidence in your research.
Explore how to effortlessly monitor immunoassay method reproducibility using Belysa ® analysis software.
Stay up to date on the latest immunoassay research trends and our newest product offerings by subscribing to our Analyte Update newsletter or explore our current immunoassay offerings in the Analyte Quarterly .
To continue reading please sign in or create an account.
Research. Development. Production.
We are a leading supplier to the global Life Science industry with solutions and services for research, biotechnology development and production, and pharmaceutical drug therapy development and production.
© 2024 Merck KGaA, Darmstadt, Germany and/or its affiliates. All Rights Reserved.
Reproduction of any materials from the site is strictly forbidden without permission.
- English - EN
- Español - ES

Purpose (+) Scope
This post is about the fundamental distinctions between an IVD product and a RUO product. The emphasis is placed on the regulatory requirements of the European Union (EU) and the United States (US).
in vitro Diagnostic (IVD) Medical Device
An “in vitro diagnostic (IVD) medical device” is defined generically as a device that, whether used alone or in combination, is intended to the manufacturer for the in vitro examination of specimens derived from the human body solely and principally to provide information for diagnostic, monitoring or compatibility purposes. IVDs can be reagents, calibrators, control materials, specimen receptacles, software, instruments, apparatus or other articles.
Usually, IVDs are classified based on their risk levels. Each risk class is linked to a given conformity assessment type who is itself linked to specific regulatory requirements.
Depending of the jurisdiction, the IVD classes may be covered by separate national regulations.
IVDs can be sterile, not sterile, intended to be used by lay people (self-testing devices) or not by health professionals within or outside a laboratory environment (near-patient testing devices).
Regulations, norms and standards (such as ISO 13485) apply for IVDs and they are, in most of the countries, subject to product registration. Extensive validations (such as scientific validity, analytical and clinical performances, stability studies, etc.) are required to be performed for IVDs and expected to be approved by certification body or regulatory agency. After approval, IVDs they must bear the IVD symbol (Fig. 1).

IVDs in the EU
In the EU, the IVDs are regulated by the Directive 98/79/EC (IVDD) . In May 2022, a new law governing the IVDs will be fully applicable: The Regulation (EU) 2017/746 (IVDR) . Passed the IVDR different transition periods, all IVDs in the European Economic Area (EEA) would need to comply with the requirements of the IVDR.
As a reminder, please note that all IVDs compliant with the EU requirements must carry the CE Mark (Fig. 2).
There no major differences between the definition of an IVD under the IVDD (Art.1.2.b) and the IVDR (Art.2(2)). Both texts define an IVD as the following:
“‘in vitro diagnostic medical device’ means any medical device which is a reagent, reagent product, calibrator, control material, kit, instrument, apparatus, piece of equipment, software or system, whether used alone or in combination, intended by the manufacturer to be used in vitro for the examination of specimens, including blood and tissue donations, derived from the human body, solely or principally for the purpose of providing information on one or more of the following:
(a) concerning a physiological or pathological process or state;
(b) concerning congenital physical or mental impairments;
(c) concerning the predisposition to a medical condition or a disease;
(d) to determine the safety and compatibility with potential recipients;
(e) to predict treatment response or reactions;
(f) to define or monitoring therapeutic measures.
Note that the IVDR specifies the following: “Specimen receptacles shall also be deemed to be in vitro diagnostic medical devices”.

IVDs in the U.S.
The US Food and Drug Administration (FDA) defines IVDs in Title 21 of the Code of Federal Regulation (CFR) Part 809.3 as “products that are those reagents, instruments, and systems intended for use in diagnosis of disease or other conditions, including a determination of the state of health, in order to cure, mitigate, treat, or prevent disease or its sequelae. Such products are intended for use in the collection, preparation, and examination of specimens taken from the human body.”
IVDs are devices as defined in Section 210(h) of the Federal Food, Drug and Cosmetic Act (FD&C Act) and can be also biological devices subject to Section 351 of the Public Health Service Act (PHS Act).
It is important to know that IVD products must be labelled “For In Vitro Diagnostic Use” (as per 21 CFR 809.10(a)(4)) or carry the IVD symbol.
The FDA proposes on its website a whole overview of how it regulates IVD products. See section Recommended Reading .
Research Use Only (RUO) Products
RUO means Research Use Only. It means that a given product is basically for that, for research such as basic laboratory research, performance investigation, design investigation, etc.
Norms and standards don’t apply here. Depending of the countries, the RUO products can be considered or not as ineffective IVDs. Therefore, depending of the countries and jurisdictions, the RUO products can be more or less regulated.
RUOs in the EU
RUO products are not subject to the IVDD not the IVDR. As they lack a clinical application, there are not qualified as medical devices by law.
The IVDD and the IVDR don’t define the term “Research Use Only” explicitly and no requirements are defined.
Manufacturers of RUO products should not apply the CE Mark of these products and clearly label them as “Research Use Only” and use the RUO label (Fig.3).
In the EU, a distinction is made between RUO products and IVDs for Performance Testing. IVDs introduced in European laboratories to establish their performance characteristics also are subject to the IVDD and IVDR. Such products cannot carry the CE Mark as their performance have not been established yet. IVDD and IVDR have specific performance documentation and notification requirements for these products.
For more information: The EU Commission has published a MEDDEV guidance concerning Research Use Only products. See section Recommended Reading. Note this guidance is not aligned with the IVDR and that a MDCG Guidance for RUO products is still under development at this moment.

RUO in the U.S.
The 21 CFR 809.10 and 21 CFR 864 define four types of IVDs: General Purpose Reagent (GPR), Investigational Use Only (IUO), Analyte Specific Reagent (ASR) and Research Use Only (RUO). This is why in the U.S., RUOs are also called RUO IVDs – In contrast, in EU, only the term RUO prevails.
As per the 21 CFR, RUO products are IVD products in the laboratory research phase of development and not represented as effective IVDs (21 CFR 809.10(c)(2)(i)). In essence, RUO products are reagents, instruments, or systems under development and evaluated for their potential use as IVDs (Evaluation of design, performance, usability, etc.).
RUO product are essentially unregulated in the U.S but must be labelled with the following statement: “For Research Use Only. Not for use in diagnostic procedures”. Labelling a product as such permits it to be used by researchers, who can evaluate usefulness for a specific diagnostic purpose. Beyond the labelling statement, FDA regulations do not mandate any other restrictions or limitations on RUO products, and RUO manufacturers do not have to register or list their RUO products with FDA or comply with manufacturing standards. RUO products can be offered for sale without any FDA clearance or approval.
RUO products can be also used in conducting nonclinical laboratory research with goals other than commercial IVD product development and are used in basic life science research and not intended for further clinical diagnostic use development. In this case, these RUOs are used to carry out research and are not, themselves, the object of the research.
As good marketing practices, RUO products must never be represented as effective IVD products. And no specific disease, condition, or diagnostic performance claims can be made for RUO products. In the other side, an IVD product that is inappropriately labelled as RUO may be also considered misbranded or adulterated due to the lack of premarket notification (510(k)) or premarket approval (PMA) if distributed/or labelled for clinical diagnostic purpose.
For more information, the FDA has published a guidance regarding IVD products labelled for Research Use Only. See section Recommended Reading .
Recommended Reading
Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only, FDA Guidance, 2013
IVD Guidance : Research Use Only products, MEDDEV. 2.14/2 rev.1, 2004
Overview of IVD Regulation, FDA website
Manufacturer IVD, EC website
Medical Devices – Sector, EC website
CFR Title 21
Directive 98/79/EC (IVDD)
Regulation (EU) 2017/746 IVDR: 02017R0746 — EN — 05.05.2017 — 000.003 (consolidated version)

Template – Product Qualification and Classification under the EU IVDR 2017/746 (v.1.0)

Checklist – ISO 13485 2016 Internal Audit (v.1.0)

Template – SOP Master Validation Test Plan (v.1.0)

Template – Technical Documentation Table of Content according to the IVDR (v1.0)

Template – SOP Technical Documentation according the IVDR (v1.0)

How to Create a Project Timeline in Simple Steps

How to create a Project Timeline with VisualMaker?

The IVD Product Types: RUO, IUO, GPR, ASR

Claims under the MDR and the IVDR (Art. 7/7)

The Investigational Device Exemption (IDE)

Conformity Assessment Options for Products Failing under the MDR
#regulatoryandmore
- Search Menu
- Sign in through your institution
- Advance Articles
- Clinical Case Studies
- Journal Club
- Clinical Chemistry Podcasts
- Clinical Trainee Council
- Special Issues
- Clinical Chemistry Guide to Scientific Writing
- Clinical Chemistry Guide to Manuscript Review
- Author Guidelines
- Submission Site
- Self-Archiving Policy
- Call for Papers
- Why Publish?
- About Clinical Chemistry
- Editorial Board
- Advertising & Corporate Services
- Journals on Oxford Academic
- Books on Oxford Academic
Article Contents
2 nonstandard abbreviations:.
- < Previous
“Research Use Only” Reagents: Is There an Imperative for Increased FDA Oversight?
- Article contents
- Figures & tables
- Supplementary Data
Timothy J O'Leary, “Research Use Only” Reagents: Is There an Imperative for Increased FDA Oversight?, Clinical Chemistry , Volume 57, Issue 12, 1 December 2011, Pages 1681–1683, https://doi.org/10.1373/clinchem.2011.174268
- Permissions Icon Permissions
On June 1, 2011, the US Food and Drug Administration (FDA) 2 Office of In Vitro Diagnostic Device Evaluation and Safety issued draft guidance for industry and FDA staff intended to provide guidance regarding the FDA's thinking about in vitro diagnostic device (IVD) products labeled for “Research Use Only” (RUO) or “Investigational Use Only” (IUO) ( 1 ). Reagents may be marketed under either of these labels without FDA premarket review and are partially or totally exempt from compliance with the Quality Systems Regulation (21 CFR 820). Therefore, an IVD manufacturer might it very tempting to avoid the trouble and expense of a 510(k) or premarket-approval submission by labeling a product as RUO or IUO, despite knowing full well that the product is likely being used as an IVD under conditions in which there is no research protocol and no oversight by an institutional review board.
There is no doubt that the FDA's intent is to improve patient safety. Compliance with the Quality Systems Regulation provides assurance that an IVD meets manufacturing specifications and that lot-to-lot variations are minimized and well understood. FDA clearance provides further assurance that the performance characteristics of laboratory tests are sufficiently well understood as to enable their intelligent use. Furthermore, the use by clinical laboratories of FDA-cleared IVD products likely reduces interlaboratory variation in testing and increases the ease with which results can be “ported” from one healthcare facility to another, potentially reducing healthcare costs that arise from duplicate testing. Nevertheless, the laboratory community is right to be concerned about the guidance document statement that manufacturers should not sell RUO or IUO reagents “to laboratories that they know use the product for clinical diagnostics use.” This statement could have unintended consequences that adversely affect patient care. In particular, the molecular diagnostics community has expressed concern that such reagents as PCR primers and sequencing reagents and equipment could become unavailable and that this outcome could affect many aspects of medical care, including newborn screening, HLA testing, and human papilloma virus genotyping, among others.
When considering approaches by which the FDA and the laboratory community can improve patient care and safety, it is important to consider the overall medical and regulatory environment in which laboratory tests that currently use RUO and IOU IVDs are conducted. In the remainder of this Opinion, I consider potential deficits in laboratory testing that currently do not rely on FDA-cleared IVDs and discuss principles that the FDA and other agencies may wish to consider when determining whether a regulatory solution is the most appropriate way to address the perceived deficits in laboratory testing.
The design and implementation of regulations entail substantial costs on the part of both regulator and regulated, both of which must ultimately be borne by the public. Therefore, regulators should take a measured approach when deciding to implement a regulatory solution to a perceived problem. In my opinion, such decisions should be based on a careful and narrow definition of the public health problem to be addressed, a scientifically valid and quantitative assessment of the magnitude of this problem, and strong evidence that the overall cost of implementing the regulatory approach (including both the cost to the regulator and the cost to the regulated entities) is cost-effective. The regulatory reasoning and the cost–benefit analysis should be published together to facilitate public scrutiny and comment. Implementation of this approach would unquestionably be associated, at least initially, with an adverse financial impact on regulatory agencies, because the assessment of regulatory impact would undoubtedly be more costly than is currently the case. The overall cost to the public seems likely to be offset, at least in part, by avoidance and/or rescission of ineffective and costly regulatory interventions. The recent FDA guidance document does not implement such an approach, so I consider some of the issues that the agency may have attempted to address.
Several potential problems are associated with the use of RUO and IUO reagents in clinical laboratory testing: ( a ) the creation of an uneven playing field for manufacturers; ( b ) the perception that manufacturers or laboratories are defying FDA regulatory authority; and ( c ) the reporting of inaccurate, misleading, or inconsistent results, either within a laboratory or among several laboratories. There is no question that the cost avoidance produced by ignoring regulatory requirements creates an economic environment that gives unfair advantage to commercial manufacturers, compared with institutions that play by the FDA interpretation of the law. Both the clinical investigations and the paperwork requirements associated with FDA submissions are costly. These costs may come at the expense of profit or at the expense of healthcare organizations and insurers (including the federal government).
Inaccurate or misleading results can occur when a clinical laboratory result does not mean what a clinician believes that it means. That situation could arise, for example, if an RUO or IUO reagent is not what the vendor says it is or performs in a manner that both is inconsistent with what the vendor states and is unexpected by the clinical laboratory. It is thus incumbent on laboratories to conduct their quality-assurance activities in a manner that ensures that reported results mean exactly what they purport to mean, whether or not a laboratory test has been cleared by the FDA. Under the framework proposed above, increased regulatory effort by the FDA might be appropriate if evidence of harm exists, although CLIA also provides a framework for achieving this objective.
Although the FDA has not officially elucidated the reason for issuing the guidance document, there have been a number of reports of inaccurate testing with laboratory-developed tests. Perhaps the most prominent is prescribing Herceptin® based on the results of immunohistochemical tests for HER2 (human epidermal growth factor receptor 2) overexpression. 3 Some of these results have depended on the use of uncalibrated laboratory-developed tests; indeed, some authors have postulated that as much as 20% of immunohistochemical HER2 testing used for selecting patients for Herceptin therapy has not been correlated, either directly or indirectly, with response to the drug ( 2 ). If true, that finding demonstrates a clear failure of the CLIA framework alone to adequately protect public health, and it seems likely that the burden of regulatory compliance, if narrowly directed to this issue, is proportionate to the problem. Inaccurate or misleading results have also been associated with direct-to-consumer genetic testing ( 3 ), which is widespread. The magnitude of the harm associated with such testing is uncertain but may be considerable, and oversight under the CLIA mechanism has failed to address the issue.
The potential reach of the FDA guidance document is broader than necessary to deal with HER2 assessment and direct-to-consumer testing. There are substantial numbers of important laboratory-developed tests for which only RUO and IUO reagents are currently available. The FDA guidance document (which, I should note, is nonbinding) could lead to withdrawal of these tests from clinical practice. In my opinion, FDA officials should work to minimize the likelihood of such an occurrence. In addition, there is a risk that innovative new tests for which only RUO and IUO reagents are available will not be deployed in a timely way, further compromising patient well-being. There is clearly a role in medical practice for laboratory-developed and -validated tests that will, of necessity, use reagents that have not passed FDA muster. The failure of CLIA oversight mechanisms is not a reason for the FDA to act unilaterally. Enforcement of both the Food Drug and Cosmetics Act and the Public Health Service Act [which created the CLIA 88 (CLIA amendments of 1988) framework] falls to the Department of Health and Human Services. It is thus appropriate for the FDA and the Centers for Medicare and Medicaid Services to work cooperatively, not only with each other but also with industry and laboratory communities, to develop a robust framework for reducing inaccurate and unreliable laboratory testing while maintaining access to high-quality laboratory testing and minimizing its economic burden.
US Food and Drug Administration
in vitro diagnostic device
Research Use Only
Investigational Use Only
human epidermal growth factor receptor 2
CLIA amendments of 1988.
HER2 is used in this Opinion to refer to the protein encoded by the gene with HUGO-approved gene symbol ERBB2 [v-erb-b2 erythroblastic leukemia viral oncogene homolog 2, neuro/glioblastoma derived oncogene homolog (avian)], as HER2 is the name commonly used in practice.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article .
Authors' Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the Disclosures of Potential Conflict of Interest form. Potential conflicts of interest:
Employment or Leadership: T.J. O'Leary, Association for Molecular Pathology and Journal of Molecular Diagnostics .
Consultant or Advisory Role: None declared.
Stock Ownership: None declared.
Honoraria: None declared.
Research Funding: None declared.
Expert Testimony: None declared.
Disclaimer: The views or opinions expressed in this paper are those of the author and are not to be construed as official or as representing the views of the Department of Veterans Affairs or any other entity of the United States government.
U.S. Food and Drug Administration . Draft guidance for industry and FDA staff-commercially distributed in vitro diagnostic products labeled for research use only or investigational use only: frequently asked questions . http://www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm253307.htm (Accessed October 2011) .
Carlson B . HER2 tests: How do we choose? Biotechnol Healthc 2008 ; 5 : 23 – 7 .
Google Scholar
U.S. Government Accountability Office . Direct-to-consumer genetic tests: misleading test results are further complicated by deceptive marketing and other questionable practices . http://www.gao.gov/new.items/d10847t.pdf (Accessed October 2011) .
Email alerts
Citing articles via.
- Recommend to Your Librarian
- Advertising and Corporate Services
- Journals Career Network
Affiliations
- Online ISSN 1530-8561
- Print ISSN 0009-9147
- Copyright © 2024 Association for Diagnostics & Laboratory Medicine
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.

Research Use Only or IVD: What’s Right for Your Lab?
by Tina Sobania | Clinical , Molecular

Publish Date: September 13, 2018
There are many misconceptions in the clinical industry regarding laboratory quality control materials. With numerous products available and manufacturers using various labeling practices, how do you know what’s best for your laboratory?
To help clear up the confusion, we’re answering two important questions clinical laboratorians have about quality control products.
Are diagnostic system controls IVDs?
One common misconception is that materials used for quality control of diagnostic systems are not themselves in vitro diagnostics (IVDs). However, the U.S. Food & Drug Administration (FDA) has written regulations citing quality control material as medical devices. For example, 21 CFR 862.1660 , Mulit-Analyte Controls Unassayed under Clinical Chemistry, and more recently 21 CFR 866.3920 , classify Class II controls requiring FDA 510(k) review under microbiology.
It’s important to understand that if a manufacturer for controls of nucleic acid amplification states its product works with a specific instrument or assay in its labeling or marketing literature, the FDA considers the material to be a Class II IVD and requires a 510(k) review . The FDA has established special controls for this type of material to ensure the product is properly labeled, performs according to claims and remains stable. In addition, IVD material must be manufactured under the FDA’s current Good Manufacturing Practices (cGMP).
Should “Research Use Only” products be used for quality control?
The second misconception clinical laboratories should be aware of involves material labeled as Research Use Only (RUO). RUO labeling is intended for products that are still under development and are not commercially distributed. A developer would use this labeling to ship product for “investigation relating to product development” as explained by the FDA in guidance document, Distribution of In Vitro Diagnostic Products Labeled for Research Use Only or Investigation Use Only .
Another factor one must consider is products labeled RUO are not required to be manufactured in accordance with cGMP and FDA Quality System Regulation. Lack of manufacturing controls may be detrimental to the quality of the control material. As such, clinical laboratories using RUO quality control materials to ensure the quality of testing may be placing patients at unnecessary risk.
Key Takeaway
To maintain the highest possible quality of your diagnostic testing, it’s best to choose materials that have been manufactured by a cGMP compliant facility under the FDA QSR, and when necessary reviewed by the FDA. Materials clearly labeled as IVDs provide that assurance and lower your laboratory’s risk.
Follow the links below to find all the FDA regulations cited in this post.
- 21 CFR 862.1660 CFR – Code of Federal Regulations Title 21, Subchapter H – Medical Devices
- 21 CFR 866.3920 CFR – Code of Federal Regulations Title 21, Subchapter H – Medical Devices
- Distribution of In Vitro Diagnostic Products Labeled for Research Use Only of Investigation Use Only
Written by Tina Sobania
You may also like.

MLS and MLT Career Opportunities
As a manufacturer of quality controls used in clinical diagnostics, we see firsthand the difficulties that laboratory...

The Challenges of Diagnosing and Treating Secondary Infections
In the past two decades, there have been six major global outbreaks of infectious diseases. While these infections may...
Trackbacks/Pingbacks
- Put Your Best Plate Forward – #CreepyCultures is Back! – Microbiologics Blog - […] Read Next – Research Use Only or IVD: What’s Right for Your Lab? […]
- Clinical Case File: Group A Streptococcus – Microbiologics Blog - […] Read Next – Research Use Only or IVD: What’s Right for Your Lab? […]
- Dear Stanley: Molecular QC Best Practices – Microbiologics Blog - […] Read Next – Research Use Only or IVD: What’s Right for Your Lab? […]
- Our Top Posts from 2018 – Microbiologics Blog - […] 1. Research Use Only or IVD: What’s Right for Your Lab? […]
- LDT Oversight Deliberation Continues – Microbiologics Blog - […] Read Next – Research Use Only or IVD: What’s Right for Your Lab? […]

- Pharmaceutical
- Events and Webinars
- Uncategorized

1-800-599-2847 microbiologics.com [email protected]
QUICK LINKS
CATEGORIES RESOURCES ABOUT US CONTACT US SITE MAP PRIVACY POLICY
- Visit GenomeWeb on Twitter
- Visit GenomeWeb on LinkedIn
Request an Annual Quote
FDA Releases Guidance on Research Use, Investigational Use Only IVDs
NEW YORK (GenomeWeb News) – The Food and Drug Administration has released a guidance document that lays out and clarifies the rules for how in vitro diagnostic products for research use only (RUO) and investigational use only (IUO) may be used, labeled, or marketed.
FDA created the guidance on RUOs and IUOs, which has been in development for several years , because it is concerned that unapproved or uncleared IVDs are being used for clinical diagnostic use, even though their performance characteristics and manufacturing controls have not met the agency's clinical standards.
The agency allows an investigational device exemption (IDE) for medical devices that enables them to be used in research without receiving premarket approval or 510(k) clearance, but a lack of clarity in the exemption has created a loophole that makes it possible for RUOs and IOUs to seep into clinical use.
The worry is that healthcare providers could be misled about the approved applications for RUO and IUO tests, and patients could be harmed. To avoid such harms, FDA wants this guidance to inform IVD makers on how to comply and ensure that devices are being used as they were intended.
The new FDA document covers the appropriate and inappropriate uses for IVDs, as well as requirements for their labeling, manufacturing, and marketing.
To qualify as an IDE, devices must be non-invasive, must not be used as a diagnostic procedure without confirmation of the diagnosis by another medically established diagnostic, and must not require an invasive sampling procedure that presents a risk.
The guidance states that RUOs must be labeled, "For Research Use Only. Not for use in Diagnostic Procedures." IUOs must be labeled, "For Investigational Use Only. The performance characteristics of this product have not been established.''
Product labeling alone may not keep IVDs from being marketed for clinical uses, so the guidance provides details regarding how the RUOs and IUOs may be marketed, and the kinds of promotional statements that would conflict with the devices' RUO and IUO status.
Filed under
Genialis, pancan partner to validate biomarker for kras-inhibitor response, benefit, study describes brain phenotypes, transcriptomic features linked to math ability, learning outcomes, people in the news at quantum-si, johns hopkins university, new products posted to genomeweb: tempus, personalis, dovetail genomics, 10x genomics, more, qiagen, myriad genetics to develop sequencing-based homologous recombination deficiency kit, momentum builds for amplification-free molecular diagnostics, but significant hurdles remain premium, uk nhs declines to accelerate grail galleri implementation following early data review, labs, doctors still grapple with patient recontact despite streamlined variant reclassification premium, acla sues fda over laboratory-developed test final rule.
- News and Events
- Find Your Sales Rep

- Anticoagulation Measurement Kits
- Buffers & Diluents
- Calibrators & Controls
- Chromogenic Substrates
- Coagulation Kits
- Enzymes & Other Proteins
- Factor Deficient Plasmas
- Fibrinolysis Kits
- Hemostasis Autoimmunity
- Inhibitor Plasmas
- Misc. Reagents
- Phospholipids
- alpha2-Antiplasmin (a2AP)
- Fibronectin
- Fletcher Trait
- Plasminogen Activator Inhibitor (PAI-1)
- Plasminogen
- Tissue Plasminogen Activator (tPA)
- Urokinase Plasminogen Activator (uPA)
- Vitronectin
- Anti-Xa Assays
- Direct Thrombin Inhibitors
- Factor X (FX)
- Heparin Kits
- Thrombin Generation
- Anticoagulation Measurement
- Factor XI (FXI)
- Plasmin-alpha2-Antiplasmin complex (PAP)
- Prekallikrein
- Reference Plasmas
- APC Resistance
- Calibration Plasma (Hemostasis)
- Controls, Hemostasis
- Factor IX (FIX)
- von Willebrand Factor (vWF)
- Activated Protein C (APC)
- Antiplasmin (Plasmin Inhibitor)
- Antithrombin
- Enzyme Inhibitors
- Factor XIa (FXIa)
- Glandular Kallikrein
- Plasminogen-SK (Plg)
- Platelet Factor 3 (PF3)
- Prothrombin (FII)
- Chymotrypsin & Anti-chymotrypsin
- Factor VIII (FVIII)
- Factor Xa inhibitor
- Factor Xa (FXa)
- Factor XIIa (FXIIa)
- Prekallikrein Activator (PKA)
- Proteolytic Activity
- PSA Enzyme Activity
- Tissue Factor Pathway Inhibitor (TFPI)
- Urokinase (uPA)
- APC Resistance (Factor V Leiden)
- Direct Thrombin Inhibitors (DTI)
- C1-Esterase Inhibitor (C1-INH)
- Factor IXa (FIXa)
- Factor XIII (FXIII)
- Microparticles
- Prothrombin
- Corn Trypsin Inhibitor
- Streptokinase
- Thrombomodulin
- von Willebrand factor (vWF)
- Factor II (FII)
- Factor V (FV)
- Factor VII (FVII)
- Factor XII (FXII)
- High Molecular Weight Kininogen
- Prekallikrein (Fletcher)
- Urokinase-Type Plasminogen Activator (uPA)
- Anti-Beta2 Glycoprotein I
- Anti-Cardiolipin
- Anti-Phosphatidylserine
- Anti-Prothrombin
- Lupus Anticoagulant (LA)
- Calcium Chloride (CaCl2)
- Sodium Chloride (NaCl)
- Multiplate® 5.0
- Ceveron® 100 Series
- Ceveron® alpha
- Consumables, Buffers, and System Solution
- Plasma Calibrators and Controls
- Anticoagulant Measurement
- Replacement Parts
- Ceveron® m-Series Instruments
- Ceveron® m-Series Consumables
- Ceveron® m Accesorsories
- Consumables
- Ceveron® MFU 500
- Cytokeratin 18/Keratin 18 (CK18/K18)
- Mac-2 Binding Protein (M2BP)/Galectin-3 Binding Protein
- Osteopontin (OPN)
- Adiponectin
- Lipocalin-2/Neutrophil Gelatinase Associated-Lipocalin (NGAL)
- Fibroblast Growth Factor (FGF)
- Proteinase 3 (PR3)
- Soluble CD163 (sCD163)
- Alpha1-Antitrypsin (AAT, A1AT)
- Anti-ASGPR IgG
- Hyaluronic Acid (HA)
- C Reactive Protein (CRP)
- Alpha GST (aGST)
- Fatty Acid Binding Protein (FABP)
- Insulin/Proinsulin
- Neutrophil Elastase (NE)
- Plasminogen Activator Inhibitor 1 (PAI-1)
- Retinol Binding Protein 4 (RBP-4)
- All Metabolic
- Metabolic Syndrome
- Type I Diabetes
- Animal Assays
- All Cardiovascular
- All Oncology
- All Veterinary Biomarkers
- Transplantation
- All Other Biomarkers
- Bone Metabolism
- Other Kidney Diseases
- Cytokeratin 18
- All Pulmonary
- Other Liver Diseases
- DILI/Hepatotoxicity
- NETosis Research
- COVID-19 Serology
- Vitellogenin
Volition Nu Q® Discover H3.1 ELISA Research Use Only Kit
- Description
- Specifications
Volition’s Nu.Q ® Discover H3.1 ELISA Research Use Only kit detects quantitative levels of circulating intact nucleosomes containing Histone H3 in plasma. Nucleosomes are repeating subunits of DNA and histone proteins that constitute human chromatin. During cellular damage, such as apoptosis or necrosis, chromatin is fragmented into oligo- or mono- nucleosomes which can be released into the blood stream.
A specific cell death process involved with immune function and results in the release of nucleosomes into biological fluids is known as NETosis. In this process, neutrophils produce neutrophil extracellular traps (NETs), which are long web-like strands of chromatin structures containing nucleosomes and antimicrobial proteins. NETs function to trap pathogens (viral, bacterial, cancer, etc) and are thought to be protective when properly controlled but harmful if not. Systemically elevated levels of NETs are associated with immune-mediated pathologies and poor outcomes.
Given their relation to disease, measuring NETs and the process of NETosis in human samples is important. Currently, there are several common methodologies that can directly measure NETs, including NET microscopy and NET flow cytometry. Both methods require NET formation to be captured in process and laborious sample handling. NETosis ELISA methodologies measure NET components and include dsDNA, histones, nucleosomes, citrullinated histone, granular proteins in complex with DNA, and granular proteins alone. These can be measured via a variety of NETosis ELISA kits (MPO ELISA, MPO+DNA Complex ELISA, citH3 ELISA, total nucleosome ELISA). Some of these NETosis ELISA kits are available commercially, but most are in-house developed assays. Volition’s Nu.Q® Discover H3.1 ELISA Research Use Only kit offers a set of standards composed of recombinant nucleosomes, controls and a standardized output of ng/mL and is able to be used as a surrogate measure of NETs or NETosis via ELISA.
The Nu.Q ® Discover H3.1 ELISA Research Use Only kit is a powerful research tool for a wide range of research areas that study nucleosomes and neutrophil extracellular traps (NETs) such as:
- Coagulation
- Auto-immune Diseases
- Neurodegenerative Diseases
- Inflammation
- NETopathies (neutrophil extracellular trap associated diseases)
Assay Principle:
The Nu.Q ® Discover H3.1 ELISA Research Use Only kit is a sandwich format colorimetric assay for research use only (RUO). The sample is added with the assay buffer to the well coated with anti-H3.1 antibody. After a first incubation followed by a washing step, the detection antibody coupled to HRP is added. After a second incubation and washing step, the TMB substrate is added. The absorbance is read after addition of stop solution. The signal obtained is proportional to the concentration of H3.1 nucleosome present in the sample. This colorimetric assay is provided in a 96-stripwell format, enabling high and low throughput analysis.
For Research Use Only. Not for use in diagnostic procedures.
All reagents are exclusively for Research Use Only – Not for Use in Diagnostic Procedures
Material required but not provided
- Deionized water.
- Adjustable or preset single-channel pipettes able to dispense 20μL.
- Multi-channel pipettes able to dispense 50μL to 200μL.
- Disposable pipette tips.
- Disposable reagent reservoirs.
- Vortex mixer.
- Volumetric cylinders.
- Container for biohazardous waste.
- Microplate incubator with orbital shaking facility (shaker at 700 rpm).
- Microplate reader equipped with 450nm filter
- Absorbent paper.
- Light-tight plate cover.
REMARK: For best assay efficiency, we recommend the use of a qualified pipettes, shaker, and reader.
Standardized
Analytically validated quantitative assays calibrated in ng/ml using recombinant nucleosome standards for use in EDTA plasma. The Nu.Q ® H3.1 ELISA Assay was developed under the CLSI guidelines.
Quantification
Recombinant nucleosomes enable absolute quantification of levels of circulating H3.1 nucleosomes, ideal to measure apoptosis or release of histone complexes in research.
Detection Range
The reportable range of the Nu.Q ® Discover H3.1 ELISA Assay is 22.7 ng/mL to 650 ng/mL.
Analytical Performance
Sensitivity: The Lower Limit of Detection is 9.1ng/mL.
Specificity: Nucleosome epitope specific antibody enables detection of only intact nucleosomes
Interferences
The following potential interfering substances were tested and have shown not to interfere on the assay when present below the concentrations defined in the following table:
Species Cross-Reactivity
Nucleosome structure is conserved across species. H3.1 has been evaluated in samples taken from human and multiple animal models including mouse, dogs, cats, horses, rats, and pigs.
Please use in conjunction with the instructions for use.
FAQ Volition Nu Q® Discover H3.1 ELISA RUO Kit
Does this kit measure NETs?
Nucleosomes are DNA-histone complexes that are released during the process of NETosis. Measuring nucleosomes in plasma serves as a surrogate marker of NET formation. This allows researchers to assess the extent of NETosis in various disease states, such as thrombosis, autoimmune disorders, and infectious diseases with a robust and standardized assay. It is worth noting that nucleosomes alone may not provide a comprehensive understanding of NETosis. Additional surragate markers, such as specific NET-associated proteins (MPO or NE), citrullinated histone H3 (H3Cit), or other functional assays, are often used in conjunction with nucleosome measurements to obtain a more comprehensive view of NETosis dynamics.
My lab already measures NETs with our own assay, how is this kit adding to what I do?
Because there are not many commercial assays available for NET measurement, many labs have generated their own in-house assays. While these in-house assays provide insightful information, they leave a gap in standardization and the data generated is difficult to compare within and between areas of study. In addition, these processes are often VERY labor intensive and may be limited to labs that have access to cell culture methodologies for in-house production of standards. The Volition Nu Q® Discover H3.1 ELISA RUO Kit provides continuity for high throughput analysis by including standards, controls, and data outputs in ng/mL. The kit was developed under CLSI guidelines and has been well characterized for many analytical parameters (intra and inter-assay variability, interferences, limits of detection, etc.).
Is there a method to measure free H3?
The Nu Q® Discover H3.1 ELISA Research Use Only Kit is specific for detection of intact nucleosomes and does not detect free histones or free DNA. There are ELISA assays by other manufacturers that claim to measure H3 alone, but Volition has not done any work with them. They are typically made up of 2 H3 antibodies.
Can this kit also recognize EETs (eosinophil extracellular traps)? If so, how can we estimate the levels of NETs?
The Nu Q® Discover H3.1 ELISA Research Use Only Kit is specific for detection of intact nucleosomes. Nucleosomes are released during cell death and can include events related to NETosis. Volition has not tested out H3.1 Nu.Q assay on EETs, but if nucleosomes are intact in EETs then we would expect that we would be able to detect them.
Is it possible to stain tissue samples with this biomarker?
The Nu Q® Discover H3.1 ELISA Research Use Only is an ELISA kit. The antibodies are not available for purchase outside of the kit format. One would think that there are antibodies that could be used to stain H3.1, but it is unknown if there are antibodies that can specifically detect intact nucleosomes in tissue samples.
How popular and in use is flow cytometry for NETosis?
Flow cytometry for measuring NETosis requires specific instrumentation and needs to capture NET formation while it is occurring. These hurdles limit the widespread use of the technique, but there are certainly reports using the technique.
Can you measure NETs in the whole blood, plasma, serum?
Publications indicate that NETs have been found in a variety of body fluids and cell culture conditions including blood, sputum, synovial fluid, urine, saliva, wounds, tears, and CSF. While the Volition Nu Q® Discover H3.1 RUO ELISA kit has been characterized with human EDTA plasma samples, other sample types have been tested within Volition’s R&D. While the kit will work with serum samples, they should be avoided because the process of preparing serum can activate immune cells and produce NETs, resulting in false positives.
Does the sample have to be fresh?
No, the samples can be fresh or frozen.
Can you measure NETs from other species or is it only human nucleosomes?
The Volition Nu Q® Discover H3.1 ELISA RUO Kit has been evaluated in samples taken from human and multiple animal models including mice, dogs, cats, horses, rats, and pigs. The sample volume required is 20uL per well, making it an effective kit for small sample collections.
Nucleosome structure is conserved across species. While not every species has been tested, the conservation of H3 increases the likelihood that the Volition Nu Q® Discover H3.1 ELISA RUO Kit will work with most model systems.
Do anticoagulants affect the assay?
Preanalytical variables and sample collection conditions for clinical samples are under review by KOLs in the field. Time from collection to analysis, blood temperature, anticoagulation, etc. have all been shown to impact the measurement of NETs in human blood samples, but the extent is to be determined. Volition does not recommend the assay to be used with heparinized plasma and has some anti-coagulant data. Please reach out to have a conversation about a specific anticoagulant.
Have you tested this kit with airway samples, human sputum supernatants or bronchoalveolar lavage?
While BAL or sputum have not been tested with this kit specifically, the chances of success are high because of successes with other sample types and other neutrophil focused assays. Also, the ProAxsis ProteaseTag® Active Neutrophil Elastase Immunoassay might be an additional fit for these needs.
Has this assay been tested with in vitro generated NETs?
Volition has tested in vitro stimulated neutrophils in limited situations. If this is of interest to you, we recommend reaching out to DiaPharma to discuss the project in more detail.
H3.1 Nu.Q is not binding to H3Cit but H3, so how this is a marker for NETosis?
Measuring nucleosomes in plasma serves as a surrogate marker of NET formation as nucleosomes are severely elevated when NETs are produced. This surrogate allows researchers to correlate the extent of NETosis in various disease states with a robust and standardized assay. Nucleosomes alone may not provide a comprehensive understanding of NETosis and additional markers, such as MPO or NE, citrullinated histone H3 (H3Cit), are often used in conjunction with nucleosome measurements to obtain a more comprehensive view of NETosis dynamics.
Can H3Cit also be released from cells other than neutrophils?
Publications suggest that PAD4 is expressed in cells of hematopoietic origin and include neutrophils, eosinophils, and monocytes. H3Cit is mostly reported in the context of NETs but there are some reports that it is found in cancer as well (Zhu et al. Molecular Cancer (2021) 20:90; https://doi.org/10.1186/s12943-021-01373-z).
Nucleosomes can be from any apoptotic cells including NETosis, so do you have kit for CitH3- specific as (PAD) NETosis-specific?
A CitH3-specific kit (H3R8 Cit kit) is in development by Volition and DiaPharma will announce when the RUO version is officially available in the US and Canadian markets. Currently, there is a prototype version available for evaluation. Please inquire with DiaPharma if you have an interest in being a test site for the kit now.
Can you detect NETs in healthy subjects with your assay and if so, what are the values compared to sepsis patients for example?
Turnover rate for nucleosomes in healthy subjects is fast. Therefore, nucleosome levels seen in frozen plasma from healthy control groups are detectable, but less than 60 ng/mL. Studies with sepsis or COVID subjects are in the several hundred ng/ml and greater.
It was not clear to me how you determine nucleosomes are neutrophil specific?
This cannot be determined with the existing assay; however, Volition has performed NGS on sister samples and confirmed that the DNA is derived from neutrophils using methylation-based cell of origin analysis.
Product Information
- Package Inserts

Research Use Only
Mycare pyschiatry antipsychotic assays †.
- Total Risperidone / Paliperidone
- Total Aripiprazole
MyCare Oncology Assays †
- 5-Fluorouracil (My5-FU)
The MyCare Assay Kits are intended for the in vitro quantitative measurement of analytes in human serum using automated clinical chemistry analysers. The assays are for research use only (RUO), not for use in diagnostic procedures.
† Registration Status: – Research Use Only. Not for use in diagnostic procedures.
Fast. Simple. Accurate.
Rapid accurate easy to perform tests
Multi-analyte calibrators and controls, request information.
Please fill out the below contact form. You can also email [email protected] or call us at +1 (610) 419-6731 .

116 Research Drive, Bethlehem, PA 18015, USA
Email: [email protected] Fax: +1 (484) 547-0590 Phone: +1 (610) 419-6731
Online Privacy Notice Legal Statement
© 2024 MyCare Tests - USA. All Rights Reserved, Saladax Biomedical Inc.
- Clozapine Assay Kit
- Download Product Catalog
- Package Inserts
- Safety Data Sheets
- Analyser Applications
- Product Catalog
- Contract Research & Manufacturing
- Knowledge Center
- Change Region: EU / ME
- Change Region: CA
Search our Collections & Repository
- Advanced Search
- Custom Query
All these words:
For very narrow results
This exact word or phrase:
When looking for a specific result
Any of these words:
Best used for discovery & interchangable words
None of these words:
Recommended to be used in conjunction with other fields
Publication Date Range:
Document Type:
Collection:
Query Builder
For additional assistance using the Custom Query please check out our Help Page
Research use only 2019-novel coronavirus (2019-nCoV) real-time RT-PCR primers and probes
May 29, 2020
- By Centers for Disease Control and Prevention (U.S.)
- Corporate Authors: Centers for Disease Control and Prevention (U.S.)
- Description: Disclaimer: These sequences are intended to be used for the purposes of respiratory virus surveillance and research. The recipient agrees to use them in compliance with all applicable laws and regulations. Every effort has been made to assure the accuracy of the sequences, but CDC cannot provide any warranty regarding their accuracy. The recipient can acknowledge the source of sequences in any oral presentations or written publications concerning the research project by referring to the Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention, Atlanta, GA, USA. Last Updated: May 29, 2020 rt-pcr-panel-primer-probes.pdf More ▼ -->
- Subjects: [+] Clinical Laboratory Techniques Coronavirus Infections/diagnosis COVID-19 Nucleic Acid Testing DNA Primers Real-Time Polymerase Chain Reaction
- Document Type: Pamphlet (or booklet)
- Collection(s): Stephen B. Thacker CDC Library collection
- Main Document Checksum: [+] urn:sha256:b212c6778216d0a52f9c27151f204e2dd8af974ad75d5d3e7cb3b99ef3bb25cf
- Download URL: https://stacks.cdc.gov/view/cdc/88834/cdc_88834_DS1.pdf
Supporting Files
You may also like.
Checkout today's featured content at stacks.cdc.gov
Exit Notification/Disclaimer Policy
- The Centers for Disease Control and Prevention (CDC) cannot attest to the accuracy of a non-federal website.
- Linking to a non-federal Website does not constitute an endorsement by CDC or any of its employees of the sponsors or the information and products presented on the website.
- You will be subject to the destination website's privacy policy when you follow the link.
- CDC is not responsible for Section 508 compliance (accessibility) on other federal or private websites.

Novaplex™ MPXV Assay*

Single-tube assay designed to detect Monkeypox Virus
Key features :.
- Designed to detect Western African and Congo Basin clades
- Compatible with Seegene's automation solutions and other automation solutions

ORDERING INFORMATION

COMMENTS
Please use the document number 1723 to identify the guidance you are requesting. Or, contact: Office of Communication, Outreach and Development, HFM-40 Center for Biologics Evaluation and Research ...
Definition in Europe. In Europe, the MEDDEV 2.14/2 guidance document (IVD Guidance: Research Use Only products - A guide for manufacturers and notified bodies) provides clues as to the definition of RUOs. This guidance was written within the framework of the now obsolete Directive 98/79/EC on in vitro diagnostic medical devices (IVDD) and, in the absence of an up-to-date replacement, it can ...
Clinical laboratory professionals may not pause to remember that these labels stand for In Vitro Diagnostics (IVD) and Research Use Only (RUO). Even clinical laboratory professionals who are familiar with these regulatory designations for assays or instruments sometimes do not realize the full significance that these labels have for certain ...
There are four different regulatory levels for IVDs: Research Use Only (RUO) General Laboratory Use (GLU) For Performance Studies Only (PSO) In Vitro Diagnostic Medical Device (IVD) The simplest explanation for these different levels is that each increasing level requires more testing and oversight. Research Use Only products are at the lowest ...
Thermo Fisher Scientific GMP products can support your efforts to produce products that function consistently as intended. We follow quality standards in manufacturing, testing, documentation, and proven use. Our - CTS products, intended for use in GMP production, are manufactured at sites that are FDA registered, ISO 13485 certified, and ...
SARS-CoV-2 Research Use Only Primer and Probe Sets. IDT offers a wide array of primer and probe sets, as well as plasmid controls, for the identification of SARS-CoV-2 (2019-nCoV). Primers and probes are manufactured in a template-free environment and certified template-free to cycle 45 by NTC testing. Ordering.
Research Use Only (RUO) products play a crucial role in medical research and innovative management of many patients. These specialised products, which include laboratory reagents and equipment, are exclusively designed for research in controlled laboratory environments. As essential tools for medical and scientific investigations ...
Research product (Research Use Only/RUO) is medical device and in-vitro medical device product that is in research development stage and has not been approved to be used for clinical purposes; or which is declared RUO by the authorized body in country of origin of the manufacturer. Category 3. 1.
Research use only (RUO) antibodies are used in basic and applied research; differing fundamentally in the end application from therapeutic or diagnostic antibodies. ... For example, antibodies with a human constant region are useful as controls for developing kits to detect and characterize immune responses to SARS-CoV-2. At the same time, many ...
Diagnostic kits intended for diagnostic use face the full panoply of FDA regulation. In sharp contrast, research use only (RUO) products are essentially unregulated.
Our ELISA offering consists of >1,000 Research Use Only assays, against a wide variety of species including human, mouse, rat, and various other animal models (Agricultural and Companion). Each assay comes with a specific protocol and the required reagents to run a 96-well plate.
The 21 CFR 809.10 and 21 CFR 864 define four types of IVDs: General Purpose Reagent (GPR), Investigational Use Only (IUO), Analyte Specific Reagent (ASR) and Research Use Only (RUO). This is why in the U.S., RUOs are also called RUO IVDs - In contrast, in EU, only the term RUO prevails. As per the 21 CFR, RUO products are IVD products in the ...
On June 1, 2011, the US Food and Drug Administration (FDA) 2 Office of In Vitro Diagnostic Device Evaluation and Safety issued draft guidance for industry and FDA staff intended to provide guidance regarding the FDA's thinking about in vitro diagnostic device (IVD) products labeled for "Research Use Only" (RUO) or "Investigational Use Only" (IUO) ().
August 29, 2011. College of American Pathologists 1350 I Street, NW, Suite 590 Washington, DC 20005 (202) 354-7100 (202) 354-7155 - fax (800) 392-9994 www.cap.org. The Food and Drug Administration (FDA) released a draft guidance entitled, "Commercially Distributed in Vitro Diagnostic Products Labeled for Research Use Only or Investigational ...
01. Introduction. This document has been developed as a result of the outcome of initial discussions on "research only products" at the Medical Devices Expert Group (MDEG) meeting of July 2003. It aims to clarify a number of issues raised by Competent Authorities with regard to products labeled as "For Research Use Only" (RUO) and their ...
The second misconception clinical laboratories should be aware of involves material labeled as Research Use Only (RUO). RUO labeling is intended for products that are still under development and are not commercially distributed. A developer would use this labeling to ship product for "investigation relating to product development" as ...
Save for later. NEW YORK (GenomeWeb News) - The Food and Drug Administration has released a guidance document that lays out and clarifies the rules for how in vitro diagnostic products for research use only (RUO) and investigational use only (IUO) may be used, labeled, or marketed. FDA created the guidance on RUOs and IUOs, which has been ...
The Nu.Q ® Discover H3.1 ELISA Research Use Only kit is a sandwich format colorimetric assay for research use only (RUO). The sample is added with the assay buffer to the well coated with anti-H3.1 antibody. After a first incubation followed by a washing step, the detection antibody coupled to HRP is added. After a second incubation and ...
The MyCare Assay Kits are intended for the in vitro quantitative measurement of analytes in human serum using automated clinical chemistry analysers. The assays are for research use only (RUO), not for use in diagnostic procedures. Download RUO Product Catalog † Registration Status: - Research Use Only. Not for use in diagnostic procedures.
Research use only 2019-novel coronavirus (2019-nCoV) real-time RT-PCR primers and probes May 29, 2020. By Centers for ... Disclaimer: These sequences are intended to be used for the purposes of respiratory virus surveillance and research. The recipient agrees to use them in compliance with all applicable laws and regulations. Every effort has ...
Intended use. cobas® HBV RNA for use on the cobas® 5800/6800/8800 Systems ( cobas® HBV RNA) is an automated real-time RT-PCR assay for the in vitro quantitative detection of circulating HBV RNA in EDTA plasma and serum. cobas® HBV RNA is intended for research use only and is not for use in diagnostic procedures.
Novaplex™ MPXV Assay*. Monkeypox is an acute zoonotic infection disease caused by the monkeypox virus (MPXV). The disease is mainly transmitted by animals, but it can also be. transmitted from person to person through physical contact, body fluids, or even contaminated objects, showing symptoms such as fever, severe headache, and. muscle pain.
Researchers can also use the QIAseq Multimodal DNA/RNA Lib Kit for generating DNA-only or RNA-only libraries. It is the first NGS multimodal kit on the market that is compatible with a wide range of input samples, including blood, Formalin-Fixed Paraffin-Embedded (FFPE) samples, and cell-free DNA (cfDNA).