Physical Review B
Covering condensed matter and materials physics.
- Collections
- Editorial Team

RKKY signals characterizing the topological phase transitions in Floquet Dirac semimetals
Hou-jian duan, shi-ming cai, xing wei, yong-chi chen, yong-jia wu, ming-xun deng, ruiqiang wang, and mou yang, phys. rev. b 109 , 205149 – published 23 may 2024.
- No Citing Articles
Recently, the Floquet Na 3 Bi -type material has been proposed as an ideal platform for realizing various phases, i.e., the spin-degenerate Dirac semimetal (DSM) can be turned into the Weyl semimetal (WSM), and even to the Weyl half-metal (WHM). Instead of the conventional electrical methods, we use the RKKY interaction to characterize the topological phase transitions in this paper. It is found that detecting the Ising term J I is feasible for distinguishing the phase transition of DSM/WSM, since the emergence of J I is induced by the broken spin degeneracy. For the case with impurities deposited on z axis (the line connecting the Weyl points), the Heisenberg term J H coexists with J I in the WSM, while J H is filtered out and only J I survives in the WHM. This magnetic filtering effect is a reflection of the fully spin-polarized property (one spin band is in the WSM phase while the other is gapped) of the WHM, and it can act a signal to capture the phase transition of WSM/WHM. This signal can not be disturbed unless the direction of the impurities greatly deviates from z axis. Interestingly, as the impurities are moved into the x − y plane, there arises another signal (a dip structure for J H at the phase boundary), which can also identify the phase transition of WSM/WHM. Furthermore, we have verified that all magnetic signals are robust to the term that breaks the electron-hole symmetry. Besides characterizing the phase transitions, our results also suggest that the Floquet DSMs are power platforms for controlling the magnetic interaction.
- Received 3 January 2024
- Revised 1 April 2024
- Accepted 8 May 2024
DOI: https://doi.org/10.1103/PhysRevB.109.205149
©2024 American Physical Society
Physics Subject Headings (PhySH)
- Research Areas
- Physical Systems
Authors & Affiliations
- Guangdong Basic Research Center of Excellence for Structure and Fundamental Interactions of Matter, Guangdong Provincial Key Laboratory of Quantum Engineering and Quantum Materials, School of Physics, South China Normal University , Guangzhou 510006, China and Guangdong-Hong Kong Joint Laboratory of Quantum Matter, Frontier Research Institute for Physics, South China Normal University , Guangzhou 510006, China
- * [email protected]
- † [email protected]
Article Text (Subscription Required)
References (subscription required).
Vol. 109, Iss. 20 — 15 May 2024
Access Options
- Buy Article »
- Log in with individual APS Journal Account »
- Log in with a username/password provided by your institution »
- Get access through a U.S. public or high school library »

Authorization Required
Other options.
- Buy Article »
- Find an Institution with the Article »
Download & Share
Schematic of Na 3 Bi -type DSMs with two Dirac points located on k z axis with positions (0, 0, ± k 0 ) ( k 0 = M 0 / M 1 ), each of which contains two spin-resolved Weyl points with opposite chiralities. Along z axis, a beam of off-resonant CPL is assumed to be irradiated.
Evolution of the k z -axis dispersion with different values of k A , which change the material from (a) DSM to (b) WSM, and then to (d) WHM. The solid (dashed) lines denote the spin-up (spin-down) bands. (c) The k z -axis dispersion for the phase boundary ( k A = k c ) between the WSM and the WHM. The related low-energy dispersion is shown in (e), where the spin-up band around the Weyl point k 0 , + is linear in all directions while the spin-down band exhibits a semi-Dirac shape around the Γ point (i.e., linear in k x axis but disperses quadratically in k z axis). Here, k 0 , + = ( M 0 − M 2 k A 2 − λ ) / M 1 , k c = M 0 / ( M 2 − v 0 2 / ℏ Ω ) with ℏ Ω = 2 eV and ε 0 is temporarily dropped (i.e., ε 0 = 0 ). Parameters M 0 = − 0.08686 eV , M 1 = − 10.6424 eV Å 2 , M 2 = − 10.3610 eV Å 2 , v 0 = 2.4598 eV Å are extracted from Na 3 Bi [ 27 ] material.
The RKKY components (a) J H and (b) J I versus the light intensity k A with different impurity distances. Impurities are deposited on the z axis, u F = 0 and C = J 2 / ( 2 π ) 3 . The vertical dotted lines denote the phase boundary ( k A = k c ) between the WSM and the WHM.
Spatial dependence of J H with (a) k A = 0.37 k c in the WSM ( 0 < k A < k c ), (b) k A = k c , and (c) k A = 1.18 k c in the WHM. The hollow circles in (b) denote the analytical result of the Eq. ( B10 ) in the Appendix pp2 , and the solid lines in (a)–(c) refer to the numerical results calculated from Eqs. ( 9, 10 ), ( 12 ), ( 14 ).
(a)–(c) The Heisenberg term J H versus the light intensity k A with u F = 0 and φ R = π / 4 . Here, the vertical dotted lines denote the phase boundary ( k A = k c ) between the WSM and the WHM. (a), (b) Different polarization angles θ R are considered with R k 0 = 14 . (c) Impurities are placed in x − y plane (i.e., θ R = π / 2 ) with different impurity distances R .
(a) θ R -dependent J H in the WHM phase ( k A = 0.95 k 0 ) with φ R = 0.25 π and R k 0 = 14 . (b) The relationship between κ z and κ ∥ . Here, the coordinate system of real space is consistent with that of k space. The asterisk denotes the value of κ = κ z 2 + κ ∥ 2 1 taken in the direction of θ R .
(a)–(d) Spatial dependence of the Heisenberg term J H with (a) k A = 0.37 k c in the WSM ( 0 < k A < k c ), (b) k A = k c , and (c) k A = 1.24 k c in the WHM. The dashed lines denote the long-range asymptotic results for J H . Here, c 0 = − 0.0165 R − 3.5 , κ ∥ 0 = E g / 2 v − , and φ R = 0.25 π .
(a)–(d) Energy dispersion along the k z axis for different phases in the presence of ε 0 ( k ) . Other parameters are the same as that in Figs. 2 – 2 . u c in (c) refers to the specific Fermi energy at which the spin-down conduction band touches the valence band. (e) The energy of the Weyl points of different spins versus k A . Parameters C 0 = − 0.06382 eV , C 1 = 8.7536 eV Å 2 , C 2 = − 8.4008 eV Å 2 are extracted from Na 3 Bi [ 27 ] material.
(a) The Heisenberg term J H as a function of k A and u F . (b) k A -dependent RKKY components J H and J I with u F = u c . u c is the specific Fermi energy as depicted in Fig. 8 , and the red solid line (enlarged in the illustration) refers to the Ising term J I for a small interval of k A in the WHM. All results in (a)–(b) are calculated by considering the effect of ε 0 , and impurities are deposited on z axis with R k 0 = 14 .
The Heisenberg term J H as a function of k A with u F = u c and R k 0 = 14 . Here, impurities are placed in x − y plane with φ R = 0.25 π . The vertical dotted lines denote the phase boundary ( k A = k c ) between the WSM and the WHM.
Sign up to receive regular email alerts from Physical Review B
- Forgot your username/password?
- Create an account
Article Lookup
Paste a citation or doi, enter a citation.
Physical Review Research
- Collections
- Editorial Team
- Open Access
Dynamical phase transitions of information flow in random quantum circuits
J.-z. zhuang, y.-k. wu, and l.-m. duan, phys. rev. research 5 , l042043 – published 26 december 2023.
- No Citing Articles
Supplemental Material
We study how the information flows in many-body dynamics governed by random quantum circuits and discover a rich set of dynamical phase transitions in this information flow. The phase-transition points and their critical exponents are established across Clifford and Haar random circuits through finite-size scaling. The flow of both classical and quantum information, measured respectively by Holevo and coherent information, shows similar dynamical phase transition behaviors. We investigate how the phase transitions depend on the initial location of the information and the final probe region, and find ubiquitous behaviors in these transitions, revealing interesting properties about the information propagation and scrambling in this quantum many-body model. Our paper underscores rich behaviors of the information flow in large systems with numerous phase transitions, thereby sheds light on the understanding of quantum many-body dynamics.
- Received 15 April 2023
- Revised 7 August 2023
- Accepted 4 December 2023
DOI: https://doi.org/10.1103/PhysRevResearch.5.L042043
Published by the American Physical Society under the terms of the Creative Commons Attribution 4.0 International license. Further distribution of this work must maintain attribution to the author(s) and the published article's title, journal citation, and DOI.
Published by the American Physical Society
Physics Subject Headings (PhySH)
- Research Areas
Authors & Affiliations
- 1 Center for Quantum Information, Institute for Interdisciplinary Information Sciences, Tsinghua University, Beijing 100084, People's Republic of China
- 2 Hefei National Laboratory, Hefei 230088, People's Republic of China
- * [email protected]
- † [email protected]
Article Text
Vol. 5, Iss. 4 — December - December 2023
Subject Areas
- Quantum Physics

Authorization Required
Other options.
- Buy Article »
- Find an Institution with the Article »
Download & Share
Model for probing information dynamics. (a) Information is encoded into an S -qubit source in an N -qubit system with periodic boundary condition. Then after t layers of brick-wall-structured random circuits, we trace out the environment and retrieve the information from the remaining M -qubit measurement subsystem. Each “brick” (green rectangle) represents a random operation between the two nearby qubits. Here N = 4 for illustration. (b) In each two-qubit random operation, we first apply a CNOT gate. Then independently for each qubit, we randomly apply a Hadamard or phase gate diag ( 1 , e i π / 2 ) with equal probability. (c) An example of source and measurement subsystem. For ease of expression, they are consecutively selected according to the 16 equal segments of the system.
Time evolution of average normalized Holevo information h ( τ ) under eight system sizes from N = 240 (blue) to N = 800 (red). We fix s ≡ S N = 2 16 and the measurement subsystem m ≡ M N = 6 16 . At the three DPT points, the curve becomes sharp as N grows. We denote them from left to right as the τ e , τ a , and τ s point. For the τ a point, we show an additional curve to illustrate its position. The inset further demonstrates the transition by finite-size scaling of ∂ τ h . We find the critical exponent ν 0 = 1.25 and scale the τ axis near each of the three DPT points in the same way τ i ′ ( τ ) = ( τ − τ i ) N 1 ν 0 where i ∈ { e , a , s } . All of the eight curves collapse. Each data point in the inset is obtained from over 6 × 10 4 samples.
Dynamics of h ( τ ) under different selections of the source S and the measurement subsystem M . (a) We change the relative position between S and M . We keep S inside M and fix s = 2 16 , m = 6 16 . Each group of curves is from various system sizes and labeled by the corresponding relative position 16 l , where l is the normalized distance between the right boundaries of S and M . The escape point's position τ e is proportional to l . The scrambled point τ s stays invariant. (b) We change 2 16 ≤ m ≤ 1 2 and fix S in the middle of M . Each group of curves is labeled by 16 m . τ s is proportional to m . For clarity, only part of the calculated h ( τ ) curves are shown.
Universality of DPT points in quantum circuit ansatz. (a) Dynamics of average normalized coherent information c ( τ ) . We again specify s = 2 16 and m = 6 16 . S is inside (outside) of M with the boundary distance l = 1 (denoted as l = − 1 ). For l = 1 , up to three DPT points τ e , τ a , and τ r can be observed. Additionally, c crosses from positive to negative at τ s . For l = − 1 , the τ a point does not exist and a DPT at τ s appears. (b) Information dynamics in Haar random circuits (HRC, solid line) and uniform sampling Clifford random circuits (uCRC, dashed line). Here, we set the system size from N = 12 (blue) to N = 28 (red) and fix s = 1 , m = 1 2 . h ( τ ) in both systems exhibit similar behavior. The inset shows the scaling behavior of ∂ τ h | τ s − δ τ s + δ ∝ N 1 ν 0 under both HRC and uCRC where the same critical exponent ν 0 = 1.25 is applied. τ s is from the thermodynamic limit of uCRC and δ = 0.012 is a constant.
Sign up to receive regular email alerts from Physical Review Research
Reuse & Permissions
It is not necessary to obtain permission to reuse this article or its components as it is available under the terms of the Creative Commons Attribution 4.0 International license. This license permits unrestricted use, distribution, and reproduction in any medium, provided attribution to the author(s) and the published article's title, journal citation, and DOI are maintained. Please note that some figures may have been included with permission from other third parties. It is your responsibility to obtain the proper permission from the rights holder directly for these figures.
- Forgot your username/password?
- Create an account
Article Lookup
Paste a citation or doi, enter a citation.
Suggestions or feedback?
MIT News | Massachusetts Institute of Technology
- Machine learning
- Social justice
- Black holes
- Classes and programs
Departments
- Aeronautics and Astronautics
- Brain and Cognitive Sciences
- Architecture
- Political Science
- Mechanical Engineering
Centers, Labs, & Programs
- Abdul Latif Jameel Poverty Action Lab (J-PAL)
- Picower Institute for Learning and Memory
- Lincoln Laboratory
- School of Architecture + Planning
- School of Engineering
- School of Humanities, Arts, and Social Sciences
- Sloan School of Management
- School of Science
- MIT Schwarzman College of Computing
Scientists use generative AI to answer complex questions in physics
Press contact :, media download.
*Terms of Use:
Images for download on the MIT News office website are made available to non-commercial entities, press and the general public under a Creative Commons Attribution Non-Commercial No Derivatives license . You may not alter the images provided, other than to crop them to size. A credit line must be used when reproducing images; if one is not provided below, credit the images to "MIT."
Previous image Next image
When water freezes, it transitions from a liquid phase to a solid phase, resulting in a drastic change in properties like density and volume. Phase transitions in water are so common most of us probably don’t even think about them, but phase transitions in novel materials or complex physical systems are an important area of study.
To fully understand these systems, scientists must be able to recognize phases and detect the transitions between. But how to quantify phase changes in an unknown system is often unclear, especially when data are scarce.
Researchers from MIT and the University of Basel in Switzerland applied generative artificial intelligence models to this problem, developing a new machine-learning framework that can automatically map out phase diagrams for novel physical systems.
Their physics-informed machine-learning approach is more efficient than laborious, manual techniques which rely on theoretical expertise. Importantly, because their approach leverages generative models, it does not require huge, labeled training datasets used in other machine-learning techniques.
Such a framework could help scientists investigate the thermodynamic properties of novel materials or detect entanglement in quantum systems, for instance. Ultimately, this technique could make it possible for scientists to discover unknown phases of matter autonomously.
“If you have a new system with fully unknown properties, how would you choose which observable quantity to study? The hope, at least with data-driven tools, is that you could scan large new systems in an automated way, and it will point you to important changes in the system. This might be a tool in the pipeline of automated scientific discovery of new, exotic properties of phases,” says Frank Schäfer, a postdoc in the Julia Lab in the Computer Science and Artificial Intelligence Laboratory (CSAIL) and co-author of a paper on this approach.
Joining Schäfer on the paper are first author Julian Arnold, a graduate student at the University of Basel; Alan Edelman, applied mathematics professor in the Department of Mathematics and leader of the Julia Lab; and senior author Christoph Bruder, professor in the Department of Physics at the University of Basel. The research is published today in Physical Review Letters.
Detecting phase transitions using AI
While water transitioning to ice might be among the most obvious examples of a phase change, more exotic phase changes, like when a material transitions from being a normal conductor to a superconductor, are of keen interest to scientists.
These transitions can be detected by identifying an “order parameter,” a quantity that is important and expected to change. For instance, water freezes and transitions to a solid phase (ice) when its temperature drops below 0 degrees Celsius. In this case, an appropriate order parameter could be defined in terms of the proportion of water molecules that are part of the crystalline lattice versus those that remain in a disordered state.
In the past, researchers have relied on physics expertise to build phase diagrams manually, drawing on theoretical understanding to know which order parameters are important. Not only is this tedious for complex systems, and perhaps impossible for unknown systems with new behaviors, but it also introduces human bias into the solution.
More recently, researchers have begun using machine learning to build discriminative classifiers that can solve this task by learning to classify a measurement statistic as coming from a particular phase of the physical system, the same way such models classify an image as a cat or dog.
The MIT researchers demonstrated how generative models can be used to solve this classification task much more efficiently, and in a physics-informed manner.
The Julia Programming Language , a popular language for scientific computing that is also used in MIT’s introductory linear algebra classes, offers many tools that make it invaluable for constructing such generative models, Schäfer adds.
Generative models, like those that underlie ChatGPT and Dall-E, typically work by estimating the probability distribution of some data, which they use to generate new data points that fit the distribution (such as new cat images that are similar to existing cat images).
However, when simulations of a physical system using tried-and-true scientific techniques are available, researchers get a model of its probability distribution for free. This distribution describes the measurement statistics of the physical system.
A more knowledgeable model
The MIT team’s insight is that this probability distribution also defines a generative model upon which a classifier can be constructed. They plug the generative model into standard statistical formulas to directly construct a classifier instead of learning it from samples, as was done with discriminative approaches.
“This is a really nice way of incorporating something you know about your physical system deep inside your machine-learning scheme. It goes far beyond just performing feature engineering on your data samples or simple inductive biases,” Schäfer says.
This generative classifier can determine what phase the system is in given some parameter, like temperature or pressure. And because the researchers directly approximate the probability distributions underlying measurements from the physical system, the classifier has system knowledge.
This enables their method to perform better than other machine-learning techniques. And because it can work automatically without the need for extensive training, their approach significantly enhances the computational efficiency of identifying phase transitions.
At the end of the day, similar to how one might ask ChatGPT to solve a math problem, the researchers can ask the generative classifier questions like “does this sample belong to phase I or phase II?” or “was this sample generated at high temperature or low temperature?”
Scientists could also use this approach to solve different binary classification tasks in physical systems, possibly to detect entanglement in quantum systems (Is the state entangled or not?) or determine whether theory A or B is best suited to solve a particular problem. They could also use this approach to better understand and improve large language models like ChatGPT by identifying how certain parameters should be tuned so the chatbot gives the best outputs.
In the future, the researchers also want to study theoretical guarantees regarding how many measurements they would need to effectively detect phase transitions and estimate the amount of computation that would require.
This work was funded, in part, by the Swiss National Science Foundation, the MIT-Switzerland Lockheed Martin Seed Fund, and MIT International Science and Technology Initiatives.
Share this news article on:
Press mentions.
Researchers at MIT and elsewhere have developed a new machine-learning model capable of “predicting a physical system’s phase or state,” report Kyle Wiggers and Devin Coldewey for TechCrunch .
Previous item Next item
Related Links
- Frank Schäfer
- Alan Edelman
- Computer Science and Artificial Intelligence Laboratory
- Department of Mathematics
- Department of Electrical Engineering and Computer Science
Related Topics
- Mathematics
- Computer science and technology
- Artificial intelligence
- Computer modeling
- Computer Science and Artificial Intelligence Laboratory (CSAIL)
- Electrical Engineering & Computer Science (eecs)
Related Articles

Technique could efficiently solve partial differential equations for numerous applications
Computational model captures the elusive transition states of chemical reactions

Explained: Generative AI

From physics to generative AI: An AI model for advanced pattern generation
More mit news.

Using art and science to depict the MIT family from 1861 to the present
Read full story →

Convening for cultural change

Q&A: The power of tiny gardens and their role in addressing climate change

In international relations, it’s the message, not the medium

A modest intervention that helps low-income families beat the poverty trap

Understanding why autism symptoms sometimes improve amid fever
- More news on MIT News homepage →
Massachusetts Institute of Technology 77 Massachusetts Avenue, Cambridge, MA, USA
- Map (opens in new window)
- Events (opens in new window)
- People (opens in new window)
- Careers (opens in new window)
- Accessibility
- Social Media Hub
- MIT on Facebook
- MIT on YouTube
- MIT on Instagram
Help | Advanced Search
Computer Science > Machine Learning
Title: phase transitions in the output distribution of large language models.
Abstract: In a physical system, changing parameters such as temperature can induce a phase transition: an abrupt change from one state of matter to another. Analogous phenomena have recently been observed in large language models. Typically, the task of identifying phase transitions requires human analysis and some prior understanding of the system to narrow down which low-dimensional properties to monitor and analyze. Statistical methods for the automated detection of phase transitions from data have recently been proposed within the physics community. These methods are largely system agnostic and, as shown here, can be adapted to study the behavior of large language models. In particular, we quantify distributional changes in the generated output via statistical distances, which can be efficiently estimated with access to the probability distribution over next-tokens. This versatile approach is capable of discovering new phases of behavior and unexplored transitions -- an ability that is particularly exciting in light of the rapid development of language models and their emergent capabilities.
Submission history
Access paper:.
- HTML (experimental)
- Other Formats
References & Citations
- Google Scholar
- Semantic Scholar
BibTeX formatted citation
Bibliographic and Citation Tools
Code, data and media associated with this article, recommenders and search tools.
- Institution
arXivLabs: experimental projects with community collaborators
arXivLabs is a framework that allows collaborators to develop and share new arXiv features directly on our website.
Both individuals and organizations that work with arXivLabs have embraced and accepted our values of openness, community, excellence, and user data privacy. arXiv is committed to these values and only works with partners that adhere to them.
Have an idea for a project that will add value for arXiv's community? Learn more about arXivLabs .
- Affiliate Program

- UNITED STATES
- 台灣 (TAIWAN)
- TÜRKIYE (TURKEY)
- Academic Editing Services
- - Research Paper
- - Journal Manuscript
- - Dissertation
- - College & University Assignments
- Admissions Editing Services
- - Application Essay
- - Personal Statement
- - Recommendation Letter
- - Cover Letter
- - CV/Resume
- Business Editing Services
- - Business Documents
- - Report & Brochure
- - Website & Blog
- Writer Editing Services
- - Script & Screenplay
- Our Editors
- Client Reviews
- Editing & Proofreading Prices
- Wordvice Points
- Partner Discount
- Plagiarism Checker
- APA Citation Generator
- MLA Citation Generator
- Chicago Citation Generator
- Vancouver Citation Generator
- - APA Style
- - MLA Style
- - Chicago Style
- - Vancouver Style
- Writing & Editing Guide
- Academic Resources
- Admissions Resources
Effective Transition Words for Research Papers
What are transition words in academic writing?
A transition is a change from one idea to another idea in writing or speaking and can be achieved using transition terms or phrases. These transitions are usually placed at the beginning of sentences, independent clauses, and paragraphs and thus establish a specific relationship between ideas or groups of ideas. Transitions are used to enhance cohesion in your paper and make its logical development clearer to readers.
Types of Transition Words
Transitions accomplish many different objectives. We can divide all transitions into four basic categories:
- Additive transitions signal to the reader that you are adding or referencing information
- Adversative transitions indicate conflict or disagreement between pieces of information
- Causal transitions point to consequences and show cause-and-effect relationships
- Sequential transitions clarify the order and sequence of information and the overall structure of the paper
Additive Transitions
These terms signal that new information is being added (between both sentences and paragraphs), introduce or highlight information, refer to something that was just mentioned, add a similar situation, or identify certain information as important.
Adversative Transitions
These terms and phrases distinguish facts, arguments, and other information, whether by contrasting and showing differences; by conceding points or making counterarguments; by dismissing the importance of a fact or argument; or replacing and suggesting alternatives.
Causal Transitions
These terms and phrases signal the reasons, conditions, purposes, circumstances, and cause-and-effect relationships. These transitions often come after an important point in the research paper has been established or to explore hypothetical relationships or circumstances.
Sequential Transitions
These transition terms and phrases organize your paper by numerical sequence; by showing continuation in thought or action; by referring to previously-mentioned information; by indicating digressions; and, finally, by concluding and summing up your paper. Sequential transitions are essential to creating structure and helping the reader understand the logical development through your paper’s methods, results, and analysis.
How to Choose Transitions in Academic Writing
Transitions are commonplace elements in writing, but they are also powerful tools that can be abused or misapplied if one isn’t careful. Here are some ways to ensure you are using transitions effectively.
- Check for overused, awkward, or absent transitions during the paper editing process. Don’t spend too much time trying to find the “perfect” transition while writing the paper.
- When you find a suitable place where a transition could connect ideas, establish relationships, and make it easier for the reader to understand your point, use the list to find a suitable transition term or phrase.
- Similarly, if you have repeated some terms again and again, find a substitute transition from the list and use that instead. This will help vary your writing and enhance the communication of ideas.
- Read the beginning of each paragraph. Did you include a transition? If not, look at the information in that paragraph and the preceding paragraph and ask yourself: “How does this information connect?” Then locate the best transition from the list.
- Check the structure of your paper—are your ideas clearly laid out in order? You should be able to locate sequence terms such as “first,” “second,” “following this,” “another,” “in addition,” “finally,” “in conclusion,” etc. These terms will help outline your paper for the reader.
For more helpful information on academic writing and the journal publication process, visit Wordvice’s Academic Resources Page. And be sure to check out Wordvice’s professional English editing services if you are looking for paper editing and proofreading after composing your academic document.
Wordvice Tools
- Wordvice APA Citation Generator
- Wordvice MLA Citation Generator
- Wordvice Chicago Citation Generator
- Wordvice Vancouver Citation Generator
- Wordvice Plagiarism Checker
- Editing & Proofreading Guide
Wordvice Resources
- How to Write the Best Journal Submissions Cover Letter
- 100+ Strong Verbs That Will Make Your Research Writing Amazing
- How to Write an Abstract
- Which Tense to Use in Your Abstract
- Active and Passive Voice in Research Papers
- Common Phrases Used in Academic Writing
Other Resources Around the Web
- MSU Writing Center. Transition Words.
- UW-Madison Writing Center. Transition Words and Phrases.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Phase Transition of Gels—A Review of Toyoich Tanaka’s Research
In 70’s, the extensive studies about the gel science has begun with the discovery of the volume phase transition of gel at the physics department of Massachusetts Institute of Technology. After the discovery of the volume phase transition of gel, the phenomenon was extensively studied and advanced by the discoverer, the late Professor Toyoichi Tanaka, who deceased on 20 May 2000 in the halfway of his research. In this paper, we would like to review his research to clarify his deep insight into the science of gels.
1. Introduction
Gel is one of polymer system that consists of the three-dimensional cross-linked network of polymers and a huge amount of solvent. It has been well established that the polymer gels swell and/or shrink in response to the changes of environmental conditions that the gel is surrounded. The equilibrium swelling behaviors of the hydrogel under various external conditions have been studied extensively and the results are summarized in Flory’s “Bible” [ 1 ]. It is shown by Flory that the equilibrium swelling ratio of the gel is determined by the combination of several interaction parameters that contribute to the osmotic pressure of the gel; the rubber elasticity, the interaction between polymer and solvent, and the degree of ionization of the polymer network of gel. It is found that the osmotic pressure of the gel that calculated theoretically well explains the experimental results of the swelling behavior of ionized gels that consist of methacrylic acid and divinylbenzene [ 2 , 3 ]. Besides, it is also reported that the polyelectrolyte gel shows a discontinuous transition behavior in its length-force curve under constrained conditions [ 4 ]. Therefore, the theoretical framework of the swelling behavior of gel are already constructed in early 50’s with many experimental results. The concept of the volume phase transition of the polymer gel are suggested theoretically about twenty years after Flory [ 5 ]. However, it took further ten years for the observation of the volume phase transition in actual system of polymer gels by the late Professor Toyoichi Tanaka of Massachusetts Institute of Technology. Professor Tanaka was an experimental scientist but he was also passionate about the theoretical study. As seen in the later, his research papers are the beautiful collaboration of the experimental study and the theoretical analysis of the experimental results. This feeling of excitement is always noticeable in his professional talks, and it is clearly seen in many of his research papers.
In the end of the 70’s, he discovered the volume phase transition phenomenon of polymer gel. This discovery became one of the most frequently cited works of Tanaka in the period. After the discovery, numerous followers all over the world studied various phase transitions in gels. It was, however, Tanaka whose comprehensive vision made the difference. He examined phase transitions in many different types of gels systematically. The results are summarized in four types of weak interactions that operate in water. He has achieved a systematic understanding, namely, when gel shows the discontinuous volume change it is a first order phase transition. On the other hand, if the volume change of the gel is continuous, it is a second order transition. This gave rise to industrial applications of responsive gels, such as the heat sensitive gel, the gel that sense the electric and magnetic fields, the light sensitive gel, responsive gels to pH and chemicals. In this review, we would like to looking back the discovery of the phenomenon and the advancements of the phase transition of gel through Tanaka’s scientific research. We believe that such a review is still worth publishing for the young scientists who wish to start studying the gel sciences.
2. Equilibrium Property of Gel
2.1. discovery of the volume phase transition of gel.
When a gel is soaked into a solvent, the gel attains a thermodynamic equilibrium state. We recognize that the gel is in the equilibrium state when the gel reaches at an equilibrium volume. The volume of the gel, namely, the equilibrium state of the gel is uniquely determined by a set of environmental conditions. We, thus, define the volume ratio of gel, V / V 0 , as the measure of the volume of the gel at an equilibrium state where V and V 0 are the volume of the gel at an equilibrium state and that at the reference state. The choice of the reference state is rather arbitrary. Hereafter, we choose the diameter, d 0 , of the cylindrical capillary as the reference state, which we soaked into the monomer solution and then the gelation reaction takes place. The diameter d of the rod shaped gel thus obtained are measured under various experimental conditions. Then the volume swelling ratio of the gel is calculated by ( d / d 0 ) 3 = V / V 0 . The ratio of the diameters of the gel d / d 0 is occasionally employed as the swelling ratio of the gel. It should be noted here that the reference state itself is a function of experimental conditions such as the composition of monomer and cross-linker, the gelation temperature, and so forth. The swelling behaviors of gels are measured and discussed thus far. The studies that have been made before the discovery of the volume phase transition of gel are described in the Flory’s book [ 1 ].
In 1978, however, Tanaka discovered a new phenomenon that the volume of poly-(acrylamide) gel changes discontinuously at a certain conditions [ 6 ]. In this study, he realized the important roles played by the ionic group on the polymer chain. He, then, studied the swelling behaviors of gel by changing the ionization of the polymer network of the gel systematically [ 7 ]. The results are shown in Figure 1 . The hydrolyzed poly(acrylamide) gels in alkaline solution are employed in this study. The degree of ionization of polymer chain is changed by changing the duration of hydrolyze reaction. The results obtained in these earlier studies are, therefore, not quantitative enough. The quantitative studies on the effects of the ionic group in the polymer chain is made later. However, the characteristic features of the volume phase transition of gel are well shown in these experimental results: for instance, the presence of the critical point, the temperature dependence of the transition point, the degree of volume change at the transition point, and so forth. The behaviors of the characteristic features can be intuitively explained by the effects of the ionic group in the polymer network. This is the discovery of the volume phase transition phenomenon in polymer gels. He, then, establish the theory of the volume phase transition of gel to describe the experimental results.

The volume phase transition of ionic poly(acrylamide) gels. The equilibrium swelling ratio of the gels in the mixed solvent system of water and acetone. The time of hydrolyzed reaction are given in the figure as, for instance, 6 Days. Reproduced with permission from Ref. [ 8 ]. © 1986, The Physical Society of Japan.

2.2. How the Volume Phase Transition of the Gel Occurs
2.2.1. analogy with the gas-liquid phase transition.
It may be worth glancing over the similarity between the gas-liquid phase transition and the volume phase transition of gel. The phase behavior of the gas-liquid system, for instance water, is well understood and the phase diagram in ( V − p ) space has been established. It is well known that water is in the liquid state below 100 °C under the atmospheric pressure. It, however, becomes vapor above 100 °C at a pressure of 1 atm. The volume of water reversibly changes about 1700 hold when water transforms into vapor and vice versa . The spatial distribution of the density of water fluctuates in time and space. The fluctuations of the density diverges at the phase transition point. Microscopically, the transition point is determined by the balance of the thermal motion and the attractive interaction, which is called as the van der Waals interaction for the sake of simplicity. The thermal motion of the water molecules becomes dominant at higher temperatures, say above 100 °C, that promote the change of water molecule from the liquid state to the vapor state. On the other hand, the attractive force by the van der Waals interaction becomes dominant at lower temperatures, and hence, the liquid state of water is preferable. This is a rough sketch of the gas-liquid phase transition.
Tanaka thought that similar phenomenon occur in polymer gels if the segment of polymer chain in the polymer network of gel is regarded as the “gas” molecule. The appearance of the phase transition in the gel is, however, considerably modified from that of the gas-liquid phase transition because the “gas” molecule of the polymer gel are connected each other through the chemical bonds including the cross-links to make an infinite polymer network of the gel. The segments of polymer network are, therefore, forbidden to expand infinitely and hence the equilibrium volume is limited. Besides, the elastic property of the gel also deforms the appearance of the phase transition since the elastic property is a characteristic feature of solid and both the gas and liquid does not show the elasticity except for the bulk modulus. For instance, the pattern that appears on the surface of the swelling gel is a typical example of the effects of the elasticity of the polymer network. The patterns that appear in the shrinking process are also induced by the complicated interactions between the elasticity, the destruction of the polymer network, and the over cooling effects of the system. These points are shortly discussed later. However, we get a benefit from the presence of the polymer network since we can study the phase transition of the gel only by the naked eye observation of the gel; a so-called “five cents experiment”.The fact that the volume phase transition of gel occurs in liquid solvent under atmospheric conditions is another advantage of the studying the volume phase transition of gels. In addition to these benefits of the polymer gel, the elastic properties of the gel due to the polymer network gives rise the unique behaviors to the phase transition of the gel such as the formation of patterns in both the swelling and the shrinking processes.
2.2.2. Analogy with the Gas-Liquid Phase Transition
Tanaka established the equation of state of the gel taking into account following four forces that create the osmotic pressure of the gel [ 1 , 7 ].
- Osmotic pressure due to the rubber elasticity; π re .
- Osmotic pressure due to the interaction between polymer and solvent; π ps .
- Osmotic pressure of counter ions; π ion .
- Osmotic pressure due to the mixing entropy; π mix .
According to the similarity of the configuration between the gel and the rubber, the osmotic pressure, π , due to the rubber elasticity is calculated on the basis of the Gaussian statistics.
Here, ν represents the number of elastically active chain in the unit volume of the gel at the reference state and V / V 0 is the swelling ratio of gel, respectively.
The volume of gel is also changed by the interactions between the segments of the polymer and the solvent molecules. When the affinity between polymer segments is preferable than that between the polymer segment and the solvent molecule, the gel tends to shrink. In contrast, the gel tends to swell if the affinity between the polymer segment and the solvent molecule is preferable than that between polymer segments. The osmotic pressure due to the interactions between polymer segments and solvent molecules are reasonably expressed by Flory-Huggins theory of solutions as
where Δ F is given by
Here, ϕ and υ 0 represent the volume fraction of the polymer network in the gel and the volume of solvent molecule. The free energies Δ F PP , Δ F SS , and Δ F PS are the free energy of contacts between two polymer segments, between two solvent molecules, and between polymer segment and solvent, respectively. The volume fraction of the polymer network of gel is related with the volume of the gel as ϕ = ϕ 0 ( V 0 / V ) where ϕ 0 is the volume fraction of the polymer network at the reference state. The factor ϕ 2 represents the probability of contact between two polymer segments and Δ F changes with the composition of the solvent. Here, Tanaka focus his attention to two body interaction.
The osmotic pressure due to the ionizable group in the polymer chains of the polymer network of gel plays crucial roles in the discontinuous volume change of the gel. When the gel, in which the polymer chain of the gel contains ionic groups, is soaked in pure water, the ionic groups tend to dissociate into positively and negatively charged groups. The dissociation of ionizable groups is governed by the dissociation equilibrium. Both the positive charge and the negative charge emerges in the gel but the numbers of positive charge, n + , and negative charge, n − , are the same by the conservation law of charges. Accordingly, the number of excess charge in the gel, n gel , is always zero, n gel = ( + e ) n + + ( − e ) n − = 0 where e is the elementary electric charge. The gel is, therefore, always electrically neutral as a whole even it contains the ionic group in the polymer network of gel. In the earlier studies, the poly(acrylamide) gel was hydrolyzed to introduce the ionic group in the gel by which acrylamide is transformed into acrylic acid. A part of the polymer chain of the hydrolyzed poly(acrylamide) gel is negatively charged. The positive counter ion of the proton emerges in the gel as a result of the dissociation. The protons, thus, freely diffuse within the polymer network of the gel. The counter ion, however, may not diffuses out of the gel by the conservation law of charges. The counter ions, thus, confined within the polymer network of gel create a pressure to the wall of the gel. The osmotic pressure due to the confined counter ion in the gel is given as follows.
Here, f represents the number of counter ion that emerges from an elastically active polymer chain of the polymer network. and hence, f ν corresponds to the number of counter ion in a unit volume of the gel in the reference state.
The thermodynamic relationships for the polymer solutions are discussed by Flory using the lattice model. According to Flory, the entropy of mixing, Δ S , is approximately given as follows [ 1 ].
Here, n is the number of the solvent molecule. Then, the osmotic pressure due to the entropy of mixing is obtained.
The equation above can be rewritten as follows using a relationship n = ( V / υ 0 ) ( 1 − ϕ ) .
We, thus, obtained all relationship that contribute to the osmotic pressure of gel. The equation of state of the gel is, then, obtained by summing up the Equations ( 1 ), ( 2 ), ( 4 ) and ( 6 ).
The total osmotic pressure, π = π re + π ps + π ion + π mix , is the sum of each contribution. The properties of gel at an equilibrium state can be described by the Equation ( 7 ). The Equation ( 7 ) is constructed on the assumption of homogeneous gel. This limits the application of the theory because the volume phase transition proceeds through biphasic heterogeneous states although the initial and the final states of the gel are homogeneous. The patterns that appear in the swelling and the shrinking processes of the gel are typical examples.
2.2.3. Theoretical Swelling Curve of Gel
The swelling curve of the gel can be deduced from Equation ( 7 ) by setting that the osmotic pressure is zero, π = 0 , at an equilibrium state of gel.
The left hand member of this equation corresponds to the reduced temperature, τ , that depends only on the temperature, T , and the free energy of interaction between polymer and solvent, Δ F . It is clear from above Equation ( 8 ) that the change of temperature and the change of quality of the solvent causes the same result against the change of the volume of gel if proper solvent is chosen, namely, the variation in temperature and the variation in the quality of the solvent are equivalent. By using the Equation ( 8 ), the equilibrium swelling ratio of the gel, V / V 0 , can be calculated as a function of the reduced temperature, τ = 1 − Δ F / k T , and results are given in Figure 2 . The theoretical swelling curves of the gel, that given in Figure 2 , correspond to the gels of various ionization, f . Some important results are deduced from the series of swelling curves of the gel shown in Figure 2 . First of all, the swelling curve of the non-ionic gel, f = 0 , is a continuous function of the reduced temperature. Then, it becomes discontinuous as increasing the degree of ionization, f . It is enough to ionize one segment in the polymer chain, f = 1 , to induce the discontinuous volume change to the gel. It is also clear that the discrete volume change at the transition point becomes larger as increasing the degree of ionization of the polymer chain. Finally, the reduced temperature at the volume phase transition point becomes lower with the degree of ionization of the polymer chain. These results well explain the experimental results of the swelling behavior of the ionized poly(acrylamide) gel that shown in Figure 1 .

The theoretical swelling curve of gel calculated by Equation ( 8 ). The swelling curves are calculated for various values of ionic component on the active chain of the gel, f . Reproduced with permission from Ref. [ 8 ]. © 1986, The Physical Society of Japan.
2.2.4. Critical Conditions for Discontinuous Volume Phase Transition
The logarithmic term in the theoretical swelling curve of gel, Equation ( 8 ), is expanded for further discussion.
Scaling of above equation yields as
represent the scaled reduced temperature and scaled density of the polymer network, respectively.
The parameter, S , in Equation ( 10 ) is given as follows.
In this approximation, the shape of the reduced equation of state is determined only by a single parameter, S . S solely determines whether the volume change is continuous or discontinuous. Theoretical calculation suggests a critical value of S = S 0 = 243 above which the phase transition is discontinuous and below which it is continuous.
The value of S , which determines the swelling curve of the gel uniquely, can be rewritten further by using the parameters that describe the segment of polymer chain. Let us consider an effective polymer chain consisting of n freely jointed segments of radius a and persistent length b . The volume of solvent is assumed to be a 3 . When the interaction is neglected among the segments constituting the polymer, the average end-to-end distance of the chain is given as R ∼ b n 1 / 2 . Since number of network chain in a unit volume of gel is ν ∼ 1 / R , then, the parameter S is written as follows.
This equation shows that the shape of the swelling curve of gel is uniquely determined by two physical factors. The one is b / a and the other is f . The value b / a represents the stiffness of the polymer chain and f is the number of the ionized group in the polymer chain. In summary, the volume change of the gel at the phase transition becomes larger and the transition temperature becomes lower when the polymer chain becomes stiff and/or the number of ionized group on the chain becomes lager. These two parameters are related through Equation ( 14 ).
2.2.5. Volume Phase Transition in Various Gels
After the discovery of the volume phase transition in poly(acrylamide) gel, extensive experimental studies were made to confirm the theoretical description of the volume phase transition phenomena of gel. Tanaka classified the volume phase transition of gel into following four classes according to the driving forces of the phase transition.
- Van der Waals interaction.
- Hydrophobic interaction.
- Hydrogen bond.
- Electrical interaction between charges.
Here, we address some important experimental studies corresponding to the classifications briefly.
The phase transition behaviors of poly(acrylamide) gel are given in Figure 1 in detail. Tanaka classified the interaction that controlled the phase transition of poly(acrylamide) gel as van der Waals type because it plays important role in establishing the mean field theory of the volume phase transition of gel just like a van der Waals gas was.
According to the theory, Equation ( 14 ), the discontinuous volume phase transition of gel is observable whenever the polymer chain is stiff enough even though the gel is not ionized, f = 0 . A typical example of such system is poly(N-isopropylacrylamide) gel that is shown in Figure 3 [ 9 ]. It was found that this gel collapses at higher temperature and swells at lower temperature. The volume of poly(N-isopropylacrylamide) gel decreases discontinuously when the temperature is raised to 33.2 °C in pure water. The phase transition of poly(N-isopropylacrylamide) gel occurs due to the hydrophobic interactions between bulky side group of the polymer chain; N-isopropyl group. The fact that poly(N-isopropylacrylamide) gel shows the volume phase transition under pure water aids the further systematic studies of the volume phase transition of gel. For instance, the effects of the ionization on the volume phase transition of gel are clearly shown in the co-polymer gels of N-isopropylacrylamide and acrylic acid as shown in Figure 4 [ 10 ]. The characteristic behaviors of the swelling curves can be clearly observed in this figure. According to the theory, the phase transition behavior of the gel is independent of the sign of the charge. The expected results were obtained and shown in Figure 5 [ 11 ].

The swelling curves of non-ionic poly(N-isopropylacrylamide) gels. Reproduced with permission from Ref. [ 8 ]. © 1986, The Physical Society of Japan.

The swelling curves of ionic poly(N-isopropylacrylamide) gels. The gels are ionized by co-polymerization of a desired amount of sodium acrylate. Reproduced with permission from Ref. [ 8 ]. © 1986, The Physical Society of Japan.

The swelling curves of positively charged gels and negatively charged gels. The desired amounts of (methacrylamidopropyl)trimethylammonium chloride is co-polymerized in the case of positively charged acrylamide gel. On the other hand, sodium acrylate is introduced into the polymer network of acrylamide by co-polymerization. Reproduced with permission from Ref. [ 8 ]. © 1986, The Physical Society of Japan.
3. Dynamic Property of Gel
The polymer chain that constructs the polymer network of gel is flexible and it fluctuates in time and space even in the equilibrium state. The fluctuations of the network causes the fluctuations of the refractive index that scatters the light. It is shown both theoretically and experimentally that the time-correlation function of the fluctuation of scattered light intensity is expressed by the diffusion of the polymer network of the gel. Besides, the elasticity of the the polymer network and the diffusion coefficient of the polymer network are determined from the intensity of the scattered light and the decay rate of the fluctuation, respectively. On the other hand, the macroscopic swelling and shrinking processes of the gel are analyzed by the equation of motion of the gel. We will see that the results obtained from the light scattering experiments and that from the macroscopic swelling experiments yields the consistent picture for the dynamics of the gel.
3.1. Collective Diffusion of Polymer Network
Let us consider a unit cube of the polymer network of a gel alone with the density ρ . The polymer network of gel is regarded as a uniform elastic material. Then, the displacement of the unit cube from the average position r is expressed by the displacement vector u = u ( r , t ) . The displacement vector is governed by the wave equation.
Here, represents the longitudinal modulus of the network. The polymer network, however, E l moves in the sea of solvent in the gel. Therefore, the frictional force due to the solvent, which we assume to be proportional to the velocity of polymer chain ∂ u / ∂ t , affects the motion of the chain. The wave Equation ( 15 ) above is, then, modified to be follows.
The inertia term in Equation ( 16 ) is much smaller than the two terms in the right hand member in usual case of gels. Neglecting the inertia term, we obtain following diffusion equation.
The Equation ( 17 ) is the collective diffusion equation of gel and D coop = E l / f the collective diffusion coefficient of gel. Equation ( 17 ) indicates that the polymer chain of the network collectively diffuses with a diffusion coefficient D coop .
3.2. Swelling Behavior
The collective diffusion equation of the gel, Equation ( 17 ), was solved under the initial and the boundary conditions for a spherical gel and compared with the experimental results of the kinetics of the swelling in spherical gels [ 12 ]. It is found that the collective diffusion coefficient of gel becomes the order of D coop ∼ 10 − 7 cm 2 /s. The results indicate that the collective diffusion coefficient of the polymer network is about 1/100 of the smaller molecules such as the monomer, which is the order of D ∼ 10 − 5 cm 2 /s. This is a direct experimental demonstration of the collective motion of the polymer network. It is clear from the dimension analysis of Equation ( 17 ) that the characteristic time that governs the swelling process, [ Time ] , is proportional to the square of the characteristic length scale, L .
The results indicate that the response time of the swelling and collapse against stimuli becomes smaller if the size of the gel becomes smaller as shown in Figure 6 . For instance, the swelling time of the gel about 1cm size is of the order of one day while the swelling time of the gel of 1 μ m size becomes of the order of 10 − 3 s. The results are in good agreement with the Equation ( 18 ) [ 13 ]. The result obtained from these swelling experiments of the gel provide the important information not only for designing the experimental study of the gel but also for the practical use of the gel in industry.

Swelling time and shrinking time of gels. The measurements are made in poly(N-isopropylacrylamide) gel particles [ 13 ]. Reproduced with permission from Ref. [ 8 ]. © 1986, The Physical Society of Japan.
3.3. Light Scattering from Collective Mode of Gel
The collective diffusion mode of the gel gives rise the light scattering. The space–time correlation function of the scattered light electric field is proportional to the spatial Fourier transform of the time-correlation function of the density fluctuations. It is given by an exponential decay function with the amplitude that is inversely proportional to the longitudinal modulus of the gel, E l = K + 4 μ 3 . Here, K and μ are the bulk modulus and the shear modulus of the polymer network, respectively. On the other hand, the decay rate is proportional to the collective diffusion coefficient.
where f represents the friction coefficient between the polymer network of the gel and the solvent. Here, q represents the scattering vector. The result, above Equation ( 19 ), was confirmed by measuring the correlation function of light scattered from poly(acrylamide) gel in water [ 14 ]. The temperature dependence of the light scattering revealed the critical behavior in poly(acrylamide) gel as shown in Figure 7 [ 15 ]. Tanaka made this study before the discovery of the volume phase transition of gel. The intensity of the scattered light diverges and the relaxation time slows down to zero as the temperature approaches the critical point. The results indicate that the fluctuations of polymer network increases to infinity and the relaxation rate slows down infinitely. The gel becomes opaque as a results of non-uniform spatial density distribution of the polymer network in the vicinity of the critical point. The opacity of the gel, however, disappears and the gel becomes transparent reversibly when the distance from the critical point is increased. It is found that the divergence of the fluctuation of the polymer network is well explained by the mode-mode coupling theory [ 16 ].

The critical slowing down in poly (acrylamide) gel observed by the light scattering measurements. Reproduced with permission from Ref. [ 8 ]. © (1986) The Physical Society of Japan.
3.4. Critical Phenomena
It is well known that the critical phenomena can be seen in a wide variety of material systems expanding from gas to solid. In most case of gel, we recognize it by the appearance of the strong opalescence. The gel, thus, gets opaque near the critical point. The light scattering results indicate that the relaxation rate decreases toward zero in the vicinity of the critical point as shown in Figure 7 . Thus, we expect
The longitudinal modulus of the gel, which is independently determined from the intensity of the scattered light, also becomes zero near the critical point, Equation ( 20 ).
These results, Equations ( 21 ) and ( 22 ), are consistent because D coop = E l / f . It is, however, extremely desirable to measure the friction coefficient of gel in the vicinity of the critical point of the gel, though it can be estimated from the collective diffusion coefficient and the longitudinal elastic modulus of the gel by Equation ( 20 ). The experimental study of the critical behavior of the friction coefficient of the gel was made much later than the finding of the critical phenomena of the gel. A new apparatus for the friction measurement should be constructed, and then, the frictional properties of gels were studied [ 17 , 18 ]. The results are given in Figure 8 . In this figure, the friction coefficient of the gel is normalized by the viscosity of water, f / η , because the flow rate of water depends on the viscosity of flowing fluid. The friction of the poly (acrylamide) gel is almost constant in the temperature region studied. In contrast, the friction of poly(N-isopropylacrylamide) gel decreases about three orders of magnitude in the vicinity of the volume phase transition temperature of this gel, T ∼ 33 °C. We finally find the critical behavior of friction

Reversible decrease of gel-solvent friction observed by the mechanical measurements. The inset shows the linear plot of the friction.
The friction of the gel, therefore, decreases to zero in the vicinity of the volume phase transition point of the gel. The fluid of the gel easily flows through the gel when the critical point is approached. Here, we obtained the complete set of the critical behaviors of the gel. Namely, the longitudinal modulus of the gel becomes smaller in the vicinity of the critical point, E l → 0 . The gel gets opaque because the intensity of the scattered light diverges as I ∝ E l − 1 . At the same time, the fluctuations of the density of the polymer network becomes slower, D coop → 0 . The density fluctuation of the polymer network creates both the dense regions and the dilute regions of polymer network within the gel. Since the collective diffusion coefficient of the gel becomes zero in the vicinity of the critical point, the distribution of the dense regions and the dilute regions becomes spatially pinned. The solvent of the gel, therefore, flows through the gel easily because the dilute regions serve as the open pore for the solvent flow, f → 0 . This is a rough picture of the critical phenomena in the gel.
Finally, we present the experimental results on the critical kinetics of swelling and shrinking of the gel. The light scattering from the gel indicates that the collective diffusion coefficient of the gel becomes zero when the gel approaches the critical point, Equation ( 21 ). It may be natural to ask how the swelling and shrinking of the gel are affected near the critical point of the gel. The experiments are made on the spherical poly(N-isoropylacrylamide) gel of sub-millimeter in size [ 13 ]. The results are given in Figure 9 . It is clear from the results that the transition rate strongly depends on the temperature. Thus, the total rate of the volume change depends both on the initial position and final position of the gel in the phase diagram. The swelling and the shrinking of the gel become infinitely slow at the critical point where the volume of the gel shows the discontinuous transition.

Critical kinetics of the gel. The swelling curve of the gel ( a ); the temperature dependence of the transition rate of the volume change ( b ); and the temperature dependence of the thermal expansion coefficient of the gel ( c ). Reproduced with permission from Ref. [ 8 ]. © 1986, The Physical Society of Japan.
4. Concluding Remarks
We quickly reviewed the research work of Professor Toyoichi Tanaka here. We mainly focused our attention to the early works of the volume phase transition of the gel. Although the theory that he had constructed is of mean field type, it explains the experimental results rather well. Therefore, we believe that it will be still a good guideline for young scientist who entering into the gel science. Because of the limited space, many exotic results were not addressed here. We hope readers to cite other works published by Tanaka, which could not cited here, for deepen their knowledge about the science of gels.
In the end of this review, we would like to address about some future works of gels. First one is related to the critical phenomena. In the section of critical phenomena, we found that physical parameters tend to disappear at the critical point, namely,
The results intuitively depicts the state of the gel at the critical point very well. The results are, however, still qualitative because these results were obtained in different gels of acrylamide and N-isopropylacrylamide. Therefore, systematic measurements of D coop , E l , and f in the same gel are required for further quantitative understanding of the critical phenomena of the gel. It may be possible to discuss the relationship between these parameters theoretically through the critical exponents for these parameters [ 19 ].
Second subject is related to the pattern formation in the gel. It has been reported that beautiful patterns are formed both in swelling and shrinking processes of the gel. The swelling pattern of the gel were analyzed and results suggest that the mechanical instability at the surface of the swelling gel plays important roles for the formation of the swelling pattern [ 20 ]. In contrast, the shrinking patterns of the gel are yet to be analyzed in detail. The gel forms various patterns in the shrinking process. It is only suggested that the relationship between the final patterns and the shrinking conditions in a form of the “ phase diagram of shrinking patterns ” [ 21 ]. Few experimental studies were reported in which the confocal laser scanning microscopy is employed. Such studies suggest that the destruction of the polymer network occurs during the formation of the shrinking patterns [ 22 ]. Besides, in the case of the bubble formation process, the observation results strongly suggest that the constant volume conditions in the initial state of the shrinking process plays essential roles for the pattern formation of the gel. It further suggest that the shrinking pattern formation process may be related to the non-equilibrium steady state of the shrinking gel [ 23 ].
The third example is related to the last project of Tanaka. When we discuss in the section of the volume phase transition of various gels, we left the experimental results about the volume phase transition of gel due to the electrical charges. It may be natural to design the gel that contains both positively charged segments and negatively charged segments to clarify the effects of the interaction between electrical charges of polymer chain on the volume phase transition of the gel. The concentration of proton, i.e., pH, may also be the natural choice of the external variable to observe the volume phase transition in such gels. It is reasonably assumed that the gel that contains both the positively charged segments and the negatively charged segments swell both at lower and higher pH regions and it collapses into compact state in the intermediate pH region because we know that either positively and negatively charged gels swell lower pH region and higher pH region, as shown in Figure 10 schematically. He, however, discovered entirely new volume phase transition phenomena in the gels that contains both the positively charged segments and the negatively charged segments, namely, multiple volume phase transition of the gel [ 24 ]. The gel shows many stable swollen state against pH change. The phenomenon is believed to occur by the cooperative interaction between the hydrogen bonding, the repulsive force between the same charges, and the attractive force between opposite charges. The similar behaviors were observed in the chemically cross-linked biopolymer gels. A totally new idea was born in Tanaka’s mind through the studies of the multiple phases of gel. He found the similarity of the origin between the multiple volume phase transition of gel and the structure transition in the heteropolymer system. He, then, moved to study the phase transition of the heteropolymer systems theoretically [ 25 , 26 ]. After these studies, his idea was expanded to establish the molecular recognition system by the heteropolymer gel with the idea of imprinting; such system are known only in biological molecules as proteins. He wanted to prepare a heteropolymer gel in which some information is imprinted within its structure in such a way that some degree of molecular self-assembly would be achieved in the shrunken state of the gel. He actually make significant progress in this direction [ 27 , 28 ]. It was demonstrated experimentally in some heteropolymer gels that imprinted molecular information leads to minimize the frustrations in the gel. Nevertheless, this program remains incomplete. Some researchers pointed out that the imprinting of information has very little chance to succeed and some were skeptical about his idea. However, I believe that Tanaka was seeing the answer for the problem of “ what is life ” behind the research of heteropolymer gels. Tanaka sometime expressed this problem as “ the origin of life ”. Apart from such a big problem, we believe that above ambitious studies will open a new insight into the gel science as well as the life science. Although the path to success may be narrow and steep, young scientists should try it. The big scientific achievements are not found on convenient paved roads as suggested by Feynman in the title of his book, “Perfectly reasonable deviations from the beaten track”. Tanaka left many seeds of science to be solved. I believe that solving of these problems will contribute to the deeper understanding of the gel science.

A simple estimation of the swelling curve of gel that contains both the positive charges and the negative charges.
Acknowledgments
The author thanks the Physical Society of Japan for the permission to copy the figures and to use in this review. Figure 1 , Figure 2 , Figure 3 , Figure 4 , Figure 5 , Figure 6 , Figure 7 and Figure 9 are copied from the review paper of Toyoichi Tanaka entitled as “Phase transition in gels” that is written in Japanese. The review was published in the bulletin of the Physical Society of Japan (Japanese title ’Butsuri’) 542-552, 41, (1986). The figures are copied and properly transferred into english for use in this review.
Funding Statement
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Conflicts of interest.
The author declares no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Phase Transitions

Subject Area and Category
- Materials Science (miscellaneous)
- Instrumentation
Taylor and Francis Ltd.
Publication type
01411594, 10290338
Information
How to publish in this journal
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a journal, it expresses how central to the global scientific discussion an average article of the journal is.
Evolution of the number of published documents. All types of documents are considered, including citable and non citable documents.
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric.
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years. Journal Self-citation is defined as the number of citation from a journal citing article to articles published by the same journal.
Evolution of the number of total citation per document and external citation per document (i.e. journal self-citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
Evolution of the percentage of female authors.
Evolution of the number of documents cited by public policy documents according to Overton database.
Evoution of the number of documents related to Sustainable Development Goals defined by United Nations. Available from 2018 onwards.
Leave a comment
Name * Required
Email (will not be published) * Required
* Required Cancel
The users of Scimago Journal & Country Rank have the possibility to dialogue through comments linked to a specific journal. The purpose is to have a forum in which general doubts about the processes of publication in the journal, experiences and other issues derived from the publication of papers are resolved. For topics on particular articles, maintain the dialogue through the usual channels with your editor.

Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2024. Data Source: Scopus®

Cookie settings
Cookie Policy
Legal Notice
Privacy Policy
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 25 May 2024
Enhancement of phase transition temperature through hydrogen bond modification in molecular ferroelectrics
- Yu-An Xiong 1 na1 ,
- Sheng-Shun Duan 2 na1 ,
- Hui-Hui Hu 1 na1 ,
- Jie Yao 1 ,
- Qiang Pan 1 ,
- Tai-Ting Sha 1 ,
- Xiao Wei 2 ,
- Hao-Ran Ji 1 ,
- Jun Wu ORCID: orcid.org/0000-0002-9912-5238 2 &
- Yu-Meng You ORCID: orcid.org/0000-0002-4258-8733 1
Nature Communications volume 15 , Article number: 4470 ( 2024 ) Cite this article
2 Altmetric
Metrics details
- Crystal engineering
- Ferroelectrics and multiferroics
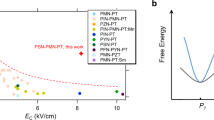
Molecular ferroelectrics are attracting great interest due to their light weight, mechanical flexibility, low cost, ease of processing and environmental friendliness. These advantages make molecular ferroelectrics viable alternatives or supplements to inorganic ceramics and polymer ferroelectrics. It is expected that molecular ferroelectrics with good performance can be fabricated, which in turns calls for effective chemical design strategies in crystal engineering. To achieve so, we propose a hydrogen bond modification method by introducing the hydroxyl group, and successfully boost the phase transition temperature ( T c ) by at least 336 K. As a result, the molecular ferroelectric 1-hydroxy-3-adamantanammonium tetrafluoroborate [(HaaOH)BF 4 ] can maintain ferroelectricity until 528 K, a T c value much larger than that of BTO (390 K). Meanwhile, micro-domain patterns, in stable state for 2 years, can be directly written on the film of (HaaOH)BF 4 . In this respect, hydrogen bond modification is a feasible and effective strategy for designing molecular ferroelectrics with high T c and stable ferroelectric domains. Such an organic molecule with varied modification sites and the precise crystal engineering can provide an efficient route to enrich high- T c ferroelectrics with various physical properties.
Similar content being viewed by others
Tailoring the coercive field in ferroelectric metal-free perovskites by hydrogen bonding

The fundamentals and applications of ferroelectric HfO2
Simultaneously achieving giant piezoelectricity and record coercive field enhancement in relaxor-based ferroelectric crystals
Introduction.
Due to the unique feature of switchable spontaneous polarization, ferroelectric are widely used in industrial and commercial applications, such as ferroelectric random access memories, piezoelectric sonar, sensors, and electromechanical transformers 1 , 2 , 3 , 4 . A great majority of excellent and advanced ferroelectrics are based on inorganic ceramics and polymers, like BaTiO 3 (BTO), Pb(Zr,Ti)O 3 (PZT), polyvinylidene fluoride, etc. 5 , 6 , 7 , 8 , 9 . In recent years, molecular ferroelectrics emerge to be a focus of research, with the advantages of mechanical flexibility, lightweight, environmental friendliness, low cost, and ease of processing into films. Over a century has passed since the discovery of the molecular ferroelectric Rochelle salt. Recently, abundant progress has been made in molecular ferroelectrics due to the convenient modification and design of crystal engineering 10 , 11 , 12 , 13 , 14 . The current research on molecular ferroelectrics is focused on new materials, methodological advancements, improving performance, etc. The practical applications of molecular ferroelectrics still need further exploration. Meanwhile, molecular ferroelectrics can serve as a complement to inorganic and polymeric materials in certain specialized applications. With continuous performance upgrade, molecular ferroelectrics can outperform inorganic and polymer ferroelectrics. For instance, diisopropylammonium bromid has the spontaneous polarization of 23 μC cm −2 (close to that of BTO) 15 ; 2-(hydroxymethyl)−2-nitro-1,3-propanediol obtains 48 crystallographically equivalent polarization directions, the largest amount among molecular ferroelectrics 16 ; trimethylchloromethyl ammonium trichloromanganese (II) has a high piezoelectric coefficient ( d 33 = 383 pC N −1 ) 17 , 18 ; and trimethylchloromethyl ammonium tetrachlorogallium(III) has a large piezoelectric voltage coefficient ( g 33 = 1318 × 10 −3 Vm N −1 ) 19 . In addition, molecular engineering, such as morphotropic phase boundaries 20 , bandgap regulation 21 , 22 , and ferroelectric domain engineering, such as vortex domain structures 23 , 24 , strain-induced periodic domain structures 25 and design of charged domain walls 26 , have been successfully carried out in molecular ferroelectrics. Based on these, researchers have introduced the quasi-spherical theory, introducing homochirality, and H/F substitution to summarize the molecular design principles of ferroelectrochemistry. In this sense, organic cations with more modification sites can be modified in a more diversified way. This has boosted the development of molecular ferroelectrics 12 . Consequently, it is promising that organic cations with more modification sites can facilitate the crystal engineering and performance optimization of molecular ferroelectrics.
As a significant intermolecular force, the hydrogen bond plays a key role in inducing ferroelectric polarization and promoting the phase transition temperature ( T c ). As we all know, molecules based on the hydroxyl group, a fundamental contributor to inducing the hydrogen bond, have been synthesized in molecular ferroelectrics. Typical representatives include 2-(hydroxymethyl)-2-nitro-1,3-propanediol 16 , R / S -3-quinuclidinol 27 , 28 and N-fluormethyltropine 29 . However, these molecules cannot maintain ferroelectricity at a high temperature, as the hydrogen bond network is not tight enough. Therefore, it is urgent to clarify and explore the feasibility of using hydrogen bonds to design molecular ferroelectrics effectively. The appropriate host and guest need to be selected to construct a hydrogen bond network of intermolecular interactions. This is a critical step to introduce ferroelectric polarization and improve T c values.
As a large spherical molecule, adamantane has modification sites of 10 C atoms, which has more C atoms and higher mass than its counterparts in molecular ferroelectrics, such as 1,4-diazabicyclo[2.2.2]octane, quinuclidine, and tropine. Here, based on the hydrogen bond modification strategy, 1-hydroxy-3-adamantanammonium (HaaOH + ) and BF 4 − were chosen as the organic guest and host respectively to form a molecular ferroelectric (Supplementary Fig. 1 ). Also, by modifying the hydrogen bond of non-ferroelectric 1-adamantanammonium tetrafluoroborate [(Haa)BF 4 ] which has large-scale adamantane molecules, we successfully introduced polarization and enhanced T c by at least 336 K. To our knowledge, the temperature enhancement has reached a high level in molecular ferroelectrics (Supplementary Table 1 ). And the ferroelectricity was retained until the decomposition temperature of (HaaOH)BF 4 ( T d = 528 K), which was closed to T c of [(2-aminoethyl)trimethylphosphanium]PbBr 4 , a high T c in reported molecular ferroelectrics 30 . This further proved that hydrogen bond modification was an effective strategy in designing molecular ferroelectrics. The regular micron ferroelectric domain pattern was written on the thin film of (HaaOH)BF 4 , a pattern in stable state for more than 2 years. Based on the stable polarization, the piezoelectric energy-harvesting device of (HaaOH)BF 4 has good efficient piezoelectric performance, which can light up 9 blue light-emitting diodes (LEDs) and has sensitive self-powered sensing. This will promote the application of molecular ferroelectrics in micro-nano electronic devices. Undoubtedly, the design strategy of hydrogen bond modification has great significance in optimizing the performance of molecular ferroelectrics, and the adamantane organic molecule with such a large number of modification sites will also provide more possibilities to develop molecular ferroelectrics.
The colorless and transparent bulk crystals of (HaaOH)BF 4 and (Haa)BF 4 were obtained by slowly evaporating the deionized aqueous solutions of 1-hydroxy-3-adamantanamine and 1-adamantanamine in HBF 4 at room temperature, respectively. The bulk phase purity was verified by powder X-ray diffraction (PXRD) (Supplementary Fig. 2 ). Single-crystal X-ray diffraction analyses indicate that (Haa)BF 4 was crystallized in the orthorhombic central symmetric space group Pnma (point group mmm) at 293 K (Supplementary Table 2 ). It contains a Haa + organic cation and a BF 4 ‒ anion in a unit (Fig. 1b ), and both the organic cation and anion are in partial disorder at room temperature. Hydroxyl modification was used to induce hydrogen bonds on the Haa + organic cations of the compound (Haa)BF 4 . As shown in Fig. 1a , the designed organic cation HaaOH + , as the template guest, was assembled with BF 4 ‒ into the hydrogen-bonded host-guest compound (HaaOH)BF 4 . Single-crystal X-ray diffraction analyses indicate that (HaaOH)BF 4 was crystallized in the orthorhombic non-centrosymmetrical polar space group Pna 2 1 (point group mm 2) at 293 K through hydrogen bond modification (Supplementary Table 2 ). This suggests that (HaaOH)BF 4 may be a ferroelectric. The crystal morphologies of (Haa)BF 4 and (HaaOH)BF 4 (Supplementary Fig. 2 ) are the same as that predicted by the Bravais, Friedel, Donnay, and Harker (BFDH) method (Supplementary Figs. 3 and 4 ). As denoted by the symmetry of the crystal point group, the polarization of (HaaOH)BF 4 is along the c -axis. Using the Berry phase method in VASP and based on the crystal structure, the simulated polarization was calculated to be 4.21 μC cm −2 along the [001] direction at 293 K 31 , 32 .

Asymmetric units of a (HaaOH)BF 4 and b (Haa)BF 4 . c Packing view along the c -axis of (HaaOH)BF 4 .The anions and cations are ordered. The dotted lines represent hydrogen bonding interactions. d Packing view along the c -axis of (Haa)BF 4 . The anions and cations are orientationally disordered. Parts of hydrogen atoms are omitted for clarity.
From the perspective of crystallographic stacking, only three N–H ⋯ F hydrogen bonds, which can be divided into two types, are formed on each Haa + organic cation. As depicted in Fig. 1d , guest-guest hydrogen bonds are absent between organic cations, and molecules are not fixed in an orderly state. However, in addition to the electrostatic effect, the hydrogen bond of (HaaOH)BF 4 has a significant effect in inducing the polarization. There are four various types of hydrogen bonds between anions and cations in (HaaOH)BF 4 (Supplementary Table 3 ). And five hydrogen bonds are formed on a HaaOH + organic cation. In addition to the two N–H ⋯ F hydrogen bonds, there are also two N–H ⋯ O and one O–H ⋯ F hydrogen bonds. Molecules are orderly arranged under a stable hydrogen bond network (Fig. 1c ). The crystal structure diagram shows that the hydroxy-modified organic cations are connected by N1–H1B ⋯ O1 to form guest-guest interactions (Supplementary Fig. 5 ). Besides, each organic cation connects to the same BF 4 ‒ anion through N1–H1C ⋯ F1B and O1–H1 ⋯ F1A. Meanwhile, the three F atoms on each BF 4 ‒ anion connect with two HaaOH + organic cations through N1–H1A ⋯ F1C, N1–H1C ⋯ F1B, and O1–H1 ⋯ F1A. The hydrogen bond network is constructed in (HaaOH)BF 4 through hydroxyl modification, resulting in the stable arrangement of ordered organic and inorganic ions.
We simulated and analyzed the organic cations of Haa + and HaaOH + through Hirshfeld surface analysis 33 . As for the two compounds, their anions are the same, while the organic cations are different. This difference is the main inducing factor of the varying crystal structures and phase transition behaviors. Then the electron density distributions around the HaaOH + (Fig. 2a ) and Haa + (Fig. 2b ) were analyzed respectively. Specifically, the hydrogen bond formed between the internal and external molecules of the Hirshfeld surface has strengthened the intermolecular force and reduced the distance between the donor and the receptor. Meanwhile, the calculated standard distance ( d norm ) decreases and the d norm surface is displayed quite distinctly on the Hirshfeld surface in red (Fig. 2a, b ). Similarly, hydrogen bonding has also shortened the atomic distance inside and outside the Hirshfeld surface ( d e and d i are also of small values). In addition, d e and d i increases as the distance between the hydrogen bond and the point on the Hirshfeld surface increases. Thus, peaks are formed on the 2D fingerprint plots of (HaaOH)BF 4 (Fig. 2c ) and (Haa)BF 4 (Fig. 2d ). As shown in the decomposed fingerprint plots, the contribution proportions of the H ⋯ F contacts of (HaaOH)BF 4 and (Haa)BF 4 in the Hirshfeld surface area were 37.1 % and 46.3 %, respectively. This is related to the host-guest interaction between organic cations and inorganic anions. Among all host-guest interactions in (HaaOH)BF 4 , the strongest is the O–H ⋯ F hydrogen bond with d O ⋯ F of 2.812 Å. Two N–H ⋯ F hydrogen bonds with d N ⋯ F of 2.931 Å and 2.961 Å also play a major part (Fig. 2a ). However, due to the disorder of H and F atoms, (Haa)BF 4 possesses more object-object interactions. As a result, the proportion of H ⋯ F in (HaaOH)BF 4 is slightly lower than that of (Haa)BF 4 . But significant differences still exist in the host-guest interaction between (HaaOH)BF 4 and (Haa)BF 4 .

Hirshfeld surfaces of the guest cations in (HaaOH)BF 4 a and (Haa)BF 4 b whose d norm values are in the range of −0.6215 (red) to 1.4037 (blue) and −0.5902 (red) to 1.5298 (blue), respectively. The red spots represent the strong short-term contacts between neighboring atoms. Decomposed fingerprint plots and proportion for the H ⋯ O, H ⋯ F, and H ⋯ H contacts of the guest cations in (HaaOH)BF 4 c and (Haa)BF 4 d on the Hirshfeld surfaces are displayed respectively.
The adjacent organic cations in (HaaOH)BF 4 and (Haa)BF 4 have completely different guest-guest interactions. In the decomposed fingerprint plots shown in Fig. 2c , (HaaOH)BF 4 has a pair of symmetrical peaks, which is contributed to the N–H ⋯ O hydrogen bond in HaaOH + . Because the atoms forming the N–H ⋯ O hydrogen bond are symmetrically organized on the Hirshfeld surface based on internal molecules, the 2D fingerprint plot shows symmetric peaks between d e and d i that have the same value. The guest-guest H ⋯ O interaction accounts for 8.1 % and 0 % in (HaaOH)BF 4 , and (Haa)BF 4 , respectively (Fig. 2c, d ). And the d N ⋯ O of the N–H ⋯ O hydrogen bond is 2. 801 Å, the shortest among all hydrogen bonds in (HaaOH)BF 4 . This implies that the attractive guest-guest interaction between adjacent organic cations is due to hydrogen bond modification. Furthermore, the intermolecular interactions were visualized quantitatively based on the energy framework analysis (Supplementary Fig. 6 ) 34 , 35 . The calculated energies between ions were represented by cylinders and are proportionate to the cylinders’ thickness (Supplementary Fig. 7 ). As shown in the 3D topologies of the energy framework, ions were strongly connected through hydrogen bonds. These strong interactions form several stable columns, which would be difficult to destroy.
The chemical design method of hydrogen bond modification has formed stronger intermolecular interactions. This increases the energy barrier to the free rotation of HaaOH + cations and calls for a higher T c for the order-disorder transition of (HaaOH)BF 4 . The differential scanning calorimetry (DSC) measurement shows that the T c of (Haa)BF 4 is 192 K (Supplementary Fig. 8 ). The temperature-dependent single-crystal X-ray diffraction shows that the low-temperature phase of (Haa)BF 4 crystallizes in the space group P 2 1 / c (point group 2/ m ). And the distance between atoms and the intermolecular force have changed a little from the high-temperature phase due to the ordering of organic cations and anions and structural phase transition (Supplementary Fig. 9 ). To our amazement, the introduction of the hydroxyl group has changed the guest-guest and host-guest interactions of (HaaOH)BF 4 and formed a network of intermolecular forces (Supplementary Fig. 7 ). This makes (HaaOH)BF 4 should have a higher T c . However, under the combined affection of hydrogen bonds and large HaaOH + cations, the potential T c is higher than the low decomposition temperature ( T d = 528 K) (Supplementary Fig. 10 ). Meanwhile, the dielectric constant and loss in (HaaOH)BF 4 were probed by the temperature- and frequency-dependent dielectric permittivity measurements across various frequencies ranging from 500 Hz to 1 MHz (Supplementary Fig. 11 ). The real part ( ε ′) (Supplementary Fig. 11a ) and the imaginary part ( ε ″) (Supplementary Fig. 11b ) of the dielectric constant were found to gradually increase upon increasing the temperature and absent of structural phase transition. Thus, the crystallographic phase transition cannot be obtained before decomposition. (HaaOH)BF 4 experiences no crystal phase transitions and still maintains its ferroelectricity until T d , a temperature close to the high T c = 534 K of reported molecular ferroelectrics 30 . More importantly, the T c enhancement (at least 336 K), as compared to T c of the parent compound (Haa)BF 4 , is high among reported enhancements for molecular ferroelectrics. The enhancement is larger than the previous record of 288 K from [(4-methoxyanilinium)(18-crown-6)][BF 4 ] ( T c = 127 K) to [(4-methoxyanilinium)(1-crown-6)][bis(trifluoromethanesulfonyl)ammonium] ( T c = 415 K) 36 . This verifies that the hydrogen bond modification is effective in designing molecular ferroelectrics with a high T c . Therefore, further exploration on the correlation between molecular structure and ferroelectricity is made possible.
Ferroelectric materials, possessing spontaneous polarization ( P s ) with hysteresis effects, can be used for information storage, and the polarization of ferroelectric thin films can be switched at low voltages. Thin films of molecular ferroelectrics, represented by (HaaOH)BF 4 , can be prepared at low temperature in a cost-effective and easy way. This makes these molecular ferroelectrics suitable for preparing ferroelectric electronic devices. By dropping the homogeneous deionized water solution of (HaaOH)BF 4 onto a fresh ozone-treated indium tin oxide (ITO)-coated conductive glass, the continuous block crystal film with high coverage was grown at the controlled temperature of 333 K. Then based on the measurements of the typical polarization−voltage ( P – V ) hysteresis loop, the ferroelectricity of (HaaOH)BF 4 has been verified. The typical ferroelectric current density−voltage ( J – V ) curve and P – V hysteresis loop were measured on the film of (HaaOH)BF 4 by the double-wave method at room temperature. This indicates that (HaaOH)BF 4 has obtained the reversible spontaneous polarization (Fig. 3 ). The typical ferroelectric J – V curve shows two opposite current peaks. According to the J – V curve, we obtained the perfect P – V hysteresis loop by integrating the current. With an applied voltage, the P s value rapidly increases and reaches the maximum value of about 4.1 μC cm −2 for thin film, which is close to the estimated value of 4.21 μC cm −2 depending on the Berry phase method. The hysteresis loop is a significant feature of ferroelectric molecules, which proves (HaaOH)BF 4 as a ferroelectric. In this respect, the modification of hydrogen bond is an effective strategy to successfully introduce ferroelectric polarization.

J – V curve and P – V hysteresis loop of the (HaaOH)BF 4 film at room temperature.
Ferroelectricity characterization was carried out on the film of (HaaOH)BF 4 via the piezoresponse force microscopy (PFM) technology, realizing the polarization switching at micro and nano scales. We used the probe to scan across the surface of the thin film under the contact mode while applying an ac voltage to the ferroelectric samples simultaneously. Then polarization-dependent deformation occurred to the samples under the ac voltage due to the inverse piezoelectric effect. This can produce a nondestructive visualization of ferroelectric domains with ultra-high spatial resolution and help obtain the polarization information of these ferroelectric samples. PFM contains both lateral (LPFM) and vertical (VPFM) modes, corresponding to the in-plane and out-of-plane polarization components, respectively. According to the intensity and orientation of polarization components, the PFM amplitude and phase data can be obtained, respectively. Using the BFDH method, the crystal plane with dominant growth directions is predicted to be (110), and the polarization of (HaaOH)BF 4 is distributed in an in-plane way along the c -axis. Therefore, the domain structure of the (HaaOH)BF 4 film was detected by LPFM. Figure 4 a, b show the as-grown domain structure of the (HaaOH)BF 4 film. The PFM phase imaging (Fig. 4b ) shows a clear domain structure with a 180° contrast, due to the different polarization directions on both sides of the PFM probe. As depicted in Fig. 4a , the PFM amplitude imaging shows clear domain walls, that is, the boundary between two domains, which conform to the domain structure of PFM phase imaging. The domain wall displays the lowest amplitude signals. Since the amplitude signals of different domains have no obvious differences, the domains show a 180° polarization distribution. This also indicates that (HaaOH)BF 4 may be a uniaxial molecular ferroelectric. Besides, the clear distribution of as-grown domains has no correlation with the surface morphology of the thin film (Supplementary Fig. 12 ), demonstrating that (HaaOH)BF 4 possesses spontaneous polarization in different directions.

The lateral phase ( a ) and lateral amplitude ( b ) of the pristine domain on the as-grown thin film of (HaaOH)BF 4 are displayed. The final state of LPFM amplitude ( c ), phase ( d ), and topography ( e ) images for the 90 × 90 μm 2 region were observed after V dc was applied in the order of +110 V and −110 V twice to switch fourfold box-in-box domains. f Phase and amplitude signals as functions of the tip voltage for a selected point in the off-field period, showing local PFM hysteresis loops.
In addition to the as-grown domains and domain walls, ferroelectrics are also characteristic of reversible polarization under an applied electric field. Thanks to the switchable polarization, arbitrary micron patterns of domains can be written on ferroelectrics. Based on this, we characterized the local polarization switching behavior on the (HaaOH)BF 4 thin film through switching spectroscopy PFM (SSPFM) measurement. The reversal of polarization was recorded off-field by PFM phase and amplitude signals when the dc voltage ( V dc ), a triangular pulse square wave, and superimposed ac voltage were applied to the film through the conductive tip (Fig. 4f ). Evidently, the PFM phase curve shows a square-shaped hysteresis loop with a 180° contrast under the electric field, and the PFM amplitude curve shows a typical butterfly shape. These curves indicate the switching and hysteresis behaviors of ferroelectric polarization in (HaaOH)BF 4 , which are obtained in the off-field period. Meanwhile, temperature-dependent SSPFM measurements indicate that the required voltages for polarization switching decrease as the temperature increases and the polarization switching still occurs at 473 K (Supplementary Fig. 13 ). Furthermore, we selected a 90×90 μm 2 region and applied the dc bias to the (HaaOH)BF 4 crystal film through the PFM probe to achieve clear domain switching. The selected region on the film is in initial single-domain state (Supplementary Fig. 14 ). With the PFM probe, V dc was applied to a selected rectangular region that became smaller and smaller, in the order of +110 V and −110 V twice. The domain in the same region of the film was switched four times under the applied bias at room temperature. Supplementary Fig. 14 vividly shows the meticulous PFM imaging of each polarization reversal process. Evidently, a significant 180° PFM phase contrast exists between different domains, and adjacent domains are separated by clear domain walls with weak amplitude signals. This proves that the polarization in the (HaaOH)BF 4 film can be reversed back and forth, conforming to the principle of ferroelectric polarization. Figure 4c, d displays the fourfold box-in-box domain structures in the final state, which are almost the same as the preset ones. Because the new domain nucleation energy of (HaaOH)BF 4 is lower than the domain growth energy, the domain is easier to nucleate and difficult to diffuse. No obvious change is observed in the morphology of the scanning region before and after domain switching, as shown in Supplementary Fig. 14 . And the domain structure written by the electric field can remain stable until 443 K (Supplementary Fig. 15 ) and for over 2 years at room temperature (Supplementary Figs. 15 and 16 ). Meanwhile, the (HaaOH)BF 4 film can still be switched under the external electric field at the temperature of 413 K (Supplementary Fig. 17 ). This rebuts the assumption that domain switching of (HaaOH)BF 4 is caused by charge injection, and further corroborates its intrinsic ferroelectric switching. Furthermore, the written box-in-box domain pattern proves that (HaaOH)BF 4 has switchable spontaneous polarization and can conduct the stable domain configuration on the micron scale.
The resonant PFM mode is widely used for the electromechanical coupling of ferroelectric materials, which detects the surface deformation excited by the electric field 17 , 20 . With PFM, the local piezoresponse of (HaaOH)BF 4 and (Haa)BF 4 thin films can be obtained. The two samples were both driven at the cantilever-sample resonance frequency of 10 V. The amplitude signal obtained is matched with the Damped Simple Harmonic Oscillator model (Supplementary Fig. 18 ), and the inherent piezoelectric response can be obtained through quality factor correction and resonant amplification. Supplementary Fig. 18 shows the obvious piezoelectric response of (HaaOH)BF 4 and a positive linear relationship between the inherent piezoelectric and the driving voltage. On the contrary, (Haa)BF 4 has no piezoelectric response along different crystal axis directions as expected (Supplementary Fig. 19 ). The piezoelectric coefficient d 33 along the corresponding polarization direction of (HaaOH)BF 4 is 22 pC N −1 , according to the quasi-static method (Berlincourt method) (Fig. 5a ). And the value of g 33 can be evaluated through the formula of g 33 = d 33 / ɛ 33 , in which the dielectric permittivity ɛ 33 can be derived from ɛ ’ = ɛ 33 / ɛ 0 ( ɛ ’ = 21). Based on the results of d 33 and ɛ ’ (Supplementary Fig. 11 , 19 ), the g 33 of (HaaOH)BF 4 is about 165.7 × 10 −3 Vm N −1 , which is higher than that of PZT-based piezoelectric ceramics (about 20 to 40 × 10 −3 Vm N −1 ).

a Diagram of the piezoelectric coefficient d 33 of the (HaaOH)BF 4 crystal along the [001] direction using the quasi-static method. b Schematic illustration of piezoelectric energy-harvesting devices. c Generated V OC of energy-harvesting devices based on (HaaOH)BF 4 and (Haa)BF 4 polycrystalline samples under a periodical vertical force of 17 N at a frequency of 10 Hz, and the V OC of the device without samples under the same conditions. d V OC of the (HaaOH)BF 4 energy-harvesting device under forward (blue line) and reverse (red line) electrical connections. e V OC of the energy-harvesting device containing (HaaOH)BF 4 with different periodical vertical forces applied (3, 13, 22, and 31 N) at a frequency of 10 Hz. f Linear fitting of the V OC of the (HaaOH)BF 4 energy-harvesting device as a function of the applied force.
To exploit the piezoelectric response with the energy-harvesting capability of (HaaOH)BF 4 , we fabricated a device with the structure of electrode-(HaaOH)BF 4 -electrode through the package of polydimethylsiloxane (PDMS) (Fig. 5b ). The device exhibited an open-circuit voltage ( V OC ) of about 3.5 V under a 10 Hz periodic vertical pressure of 17 N. Under the same condition, the blank PDMS device and (Haa)BF 4 device were tested, whose V OC were close to 0 and were lower than (HaaOH)BF 4 device (Fig. 5c ). This indicates that the V OC of the (HaaOH)BF 4 device is induced by piezoelectricity. Similarly, switching-polarity tests were conducted to verify that the generated output signals indeed originate from the piezoelectric phenomenon. The reversal polarization test realized by electronic reverse connection also produces corresponding reverse transformation of V OC (Fig. 5d ). The reversible electrical signals indicated that the detected outputs were generated by the compression-induced strain of the (HaaOH)BF 4 devices 37 . And this rules out the possibility that V OC comes from the change of system capacitance 38 . Additionally, voltage peak values between compressing and releasing conditions were different and asymmetric. This can be explained by variations in the strain rate during the application and removal of stress on the devices 39 . It is obvious that the V OC of the (HaaOH)BF 4 device increases gradually with the pressure rising from 3 N to 31 N at a stable frequency (Fig. 5e ). Figure 5f displays a good linear relationship between the pressure and V OC . Meanwhile, the electrical V OC of the device were measured under various force frequencies (Supplementary Fig. 20 ). The generated electrical output performance is noticeable and capable of responding to different external force frequencies. The output current of the (HaaOH)BF 4 device was measured under a pressure of 20 N, exhibiting a maximum of ~0.31 μA (Supplementary Fig. 21 ). And the piezoelectric device of (HaaOH)BF 4 can realize long-term sensing with the voltage maintained for ~5 V after at least 7000 cycles (Supplementary Fig. 22 ). Furthermore, the output signals of the (HaaOH)BF 4 device at high temperatures have also been verified, which maintained the output voltages of around 2 V at 503 K (Supplementary Fig. 23 ). This is consistent with the temperature-dependent piezoresponse measurements, which indicate that the sample can maintain piezoelectricity until T d (Supplementary Fig. 24 ). This indicates the potential of (HaaOH)BF 4 device for monitoring the working status and vibrations of mechanical components in high-temperature environments. Evidently, the design strategy of hydrogen bond modification can successfully build intermolecular force networks in molecular materials to achieve an orderly molecule arrangement and introduce polarity. And the molecular ferroelectric (HaaOH)BF 4 , with a high T d and stable domains, will not only promote the conversion of mental-free materials into electromechanical converters but also be a promising source for flexible electronic devices.
Due to insufficient development in power and sensing technologies, operating long-duration missions in unstructured environments remain a difficult task for robots 40 , 41 . Non-metallic (HaaOH)BF 4 , possessing good mechanical-to-current conversion capabilities, can be potentially applied to robots to achieve mechanical energy harvesting and self-powered tactile sensing under external mechanical stimuli. Additionally, the output performance of the device was measured by connecting various external load resistors. The output voltage gradually rose as the external load resistance was increased, while the output current decreased (Supplementary Fig. 25a ). The obtained maximum output power density was approximately 1.2 μW cm −2 at a load resistance of 4 × 10 7 Ω (Supplementary Fig. 25b ). In our proof-of-concept study, the (HaaOH)BF 4 device lit 9 blue LEDs (3.0-3.2 V, 60–64 mW) under the periodic mechanical tapping without using any external power unit (such as a capacitor) (Fig. 6a and Supplementary Movie 1 ). In addition, by housing the (HaaOH)BF 4 device directly on the robot’s surface, it is readily available to detect external mechanical stimuli or collisions. The energy harvester consists of the top conductive adhesive tape, (HaaOH)BF 4 , and the bottom conductive adhesive tape, encapsulated by PDMS. The top conductive adhesive tape is electrically grounded (Supplementary Fig. 26 ). Through this configuration, charges originating from the human body and those induced by the triboelectric effect between the human body and the device can dissipate into the ground. Consequently, the impact of additional charges, apart from the piezoelectric effect, is eliminated. As shown in Fig. 6b , the output piezoelectric response signals vary a lot according to different tapping forces (Supplementary Movie 2 ), although light tapping can still produce distinct signal responses. Meanwhile, the piezoelectric sensor responded to the pressure quickly, with a fast response time of 10.24 ms under a pressure upon finger tapping (Supplementary Fig. 27 ). The two applications further prove non-metallic (HaaOH)BF 4 -based devices as reliable applicants for robots equipped with energy harvesting and mechanical stimuli sensing.

a Illuminate LEDs through mechanical tapping driving. b Signals of the output voltage in the process of light, normal, and heavy tapping on a dummy. Inset that describes the robot is from Pixabay Web site and undergoes a free license.
In summary, we successfully designed the non-ferroelectric (Haa)BF 4 as the molecular ferroelectric (HaaOH)BF 4 through hydrogen bond modification. The modification of the hydroxyl group has not only introduced ferroelectric polarization but also promoted T c of (HaaOH)BF 4 by at least 336 K. By analyzing the intermolecular force of two compounds’ structure, we found that (HaaOH)BF 4 obtained more H ⋯ O hydrogen bonds and guest-guest interactions. Due to the lattice intermolecular force formed in the crystal, (HaaOH)BF 4 has a higher T c , which is even higher than its T d (528 K). And the ferroelectricity of (HaaOH)BF 4 can be maintained until T d , which is close to the high T c (534 K) as reported among molecular ferroelectrics. This proves hydrogen bond modification as an effective strategy for designing molecular ferroelectrics, stabling ferroelectric domain structure, and optimizing the phase transition temperature. Meanwhile, the fabrication of adamantane with so many modification sites can hopefully optimize the properties of molecular ferroelectrics. Furthermore, stable micron ferroelectric domain structures have been constructed on the film of molecular ferroelectric (HaaOH)BF 4 . These structures can retain stable until 443 K and for over 2 years at room temperature. The piezoelectric properties of the flexible sample were detected by the energy-harvesting device capable of mechanical energy harvesting and self-powered sensing. The precise molecular design strategy and crystal engineering are crucial to further optimize and promote the development of molecular ferroelectrics. Such an organic molecule with a variety of modification sites also provides opportunities and platforms to enhance modern energies and develop micro-nano electronic devices.
Synthesis of single crystals
All reagents and solvents in the syntheses were of reagent grade and used without further purification. Slight excess of tetrafluoroboric acid (48 wt. % in H 2 O, 20,12 g, 0.11 mol) and proper amount of deionized water (100 mL) was added to the beaker, and then adamantan-1-amine (15.13 g, 0.1 mol) or 3-amino-1-adamantanol (16.73 g, 0.1 mol) was added to the solution and stirred for 20 min at room temperature. Transparent and colorless crystal of 1-adamantanammonium tetrafluoroborate [(Haa)BF 4 ] or 1-hydroxy-3-adamantanammonium tetrafluoroborate [(HaaOH)BF 4 ] can be obtained by slowly evaporating at room temperature.
Thin film preparation
The thin films of [(Haa)BF 4 ] and [(HaaOH)BF 4 ] were prepared by drop-coating approach on ITO/glass substrate. The deionized aqueous solutions of [(Haa)BF 4 ] and [(HaaOH)BF 4 ] with 0.08 g/ml concentration were prepared. And then 15 μL of the solution was dropped on 1 × 1 μm 2 ITO/glass at 353 K. The transparent films can be obtained after the solution has evaporated.
Measurements
Methods of single-crystal X-ray crystallography, powder X-ray diffraction, differential scanning calorimetry, thermogravimetric analysis, dielectric measurements, ferroelectric hysteresis loop measurements, piezoresponse force microscopy characterization, piezoelectric coefficient measurements, preparation and measurements of piezoelectric energy-harvesting devices, calculate condition, Hershefield surface analysis and energy framework analysis were described in the Supplementary Information.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data generated and analyzed in this study are included in the Article and its Supplementary Information. And all relevant data that support the findings of this study are available from the corresponding authors upon request. The crystal structures generated in this study have been deposited in the Cambridge Crystallographic Data Centre under accession code CCDC: 2192545, 2192429, and 2314039. The data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif , or by emailing [email protected], or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Lines M. E., Glass A. M. Principles and Applications of Ferroelectrics and Related Materials .(Oxford Univ. Press, 2001).
Bain A. K., Chand P. Ferroelectrics: Principles and Applications (John Wiley & Sons, 2017).
Scott, J. F. Applications of modern ferroelectrics. Science 315 , 954–959 (2007).
Article ADS CAS PubMed Google Scholar
Dragan, D. Ferroelectric, dielectric and piezoelectric properties of ferroelectric thin films and ceramics. Rep. Prog. Phys. 61 , 1267 (1998).
Article ADS Google Scholar
Liu, Y. et al. Ferroelectric polymers exhibiting behaviour reminiscent of a morphotropic phase boundary. Nature 562 , 96–100 (2018).
Haertling, G. H. Ferroelectric ceramics: history and technology. J. Am. Ceram. Soc. 82 , 797–818 (1999).
Article CAS Google Scholar
Li, F. et al. Ultrahigh piezoelectricity in ferroelectric ceramics by design. Nat. Mater. 17 , 349–354 (2018).
Lovinger, A. J. Ferroelectric polymers. Science 220 , 1115–1121 (1983).
Qiu, C. et al. Transparent ferroelectric crystals with ultrahigh piezoelectricity. Nature 577 , 350–354 (2020).
Whatmore, R. W., You, Y.-M., Xiong, R.-G. & Eom, C.-B. 100 years of ferroelectricity—a celebration. APL Mater. 9 , 070401 (2021).
Article ADS CAS Google Scholar
Shi, P.-P. et al. Symmetry breaking in molecular ferroelectrics. Chem. Soc. Rev. 45 , 3811–3827 (2016).
Article CAS PubMed Google Scholar
Liu, H.-Y., Zhang, H.-Y., Chen, X.-G. & Xiong, R.-G. Molecular design principles for ferroelectrics: ferroelectrochemistry. J. Am. Chem. Soc. 142 , 15205–15218 (2020).
Pan, Q., Xiong, Y.-A., Sha, T.-T. & You, Y.-M. Recent progress in the piezoelectricity of molecular ferroelectrics. Mater. Chem. Front. 5 , 44–59 (2021).
Xiong, Y.-A. et al. Recent progress in molecular ferroelectrics with perovskite structure. Chin. Sci. Bull. 65 , 916–930 (2020).
Article Google Scholar
Fu, D.-W. et al. Diisopropylammonium bromide is a high-temperature molecular ferroelectric crystal. Science 339 , 425–428 (2013).
Ai, Y., Zeng, Y.-L., He, W.-H., Huang, X.-Q. & Tang, Y.-Y. Six-fold vertices in a single-component organic ferroelectric with most equivalent polarization directions. J. Am. Chem. Soc. 142 , 13989–13995 (2020).
You, Y.-M. et al. An organic-inorganic perovskite ferroelectric with large piezoelectric response. Science 357 , 306–309 (2017).
Lv, H.-P., Liao, W.-Q., You, Y.-M. & Xiong, R.-G. Inch-size molecular ferroelectric crystal with a large electromechanical coupling factor on par with barium titanate. J. Am. Chem. Soc. 144 , 22325–22331 (2022).
Wang, B. et al. Achievement of a giant piezoelectric coefficient and piezoelectric voltage coefficient through plastic molecular-based ferroelectric materials. Matter 5 , 1296–1304 (2022).
Liao, W.-Q. et al. A molecular perovskite solid solution with piezoelectricity stronger than lead zirconate titanate. Science 363 , 1206–1210 (2019).
Yao, J. et al. Hybrid organic–inorganic perovskite ferroelectrics bring light to semiconducting applications: Bandgap engineering as a starting point. APL Mater. 9 , 040901 (2021).
Ye, H.-Y. et al. Bandgap engineering of lead-halide perovskite-type ferroelectrics. Adv. Mater. 28 , 2579–2586 (2016).
Zhang, H.-Y. et al. Observation of vortex domains in a two-dimensional lead iodide perovskite ferroelectric. J. Am. Chem. Soc. 142 , 4925–4931 (2020).
Tang, Y.-Y. et al. Organic ferroelectric vortex–antivortex domain structure. J. Am. Chem. Soc. 142 , 21932–21937 (2020).
Song X.-J. et al. The first demonstration of strain-controlled periodic ferroelectric domains with superior piezoelectric response in molecular materials. Adv. Mater. 35 , 2211584 (2023).
Xiong, Y.-A. et al. Rational design of molecular ferroelectrics with negatively charged domain walls. J. Am. Chem. Soc. 144 , 13806–13814 (2022).
Li, P.-F. et al. Organic enantiomeric high-Tc ferroelectrics. Proc. Natl Acad. Sci. USA 116 , 5878–5885 (2019).
Article ADS CAS PubMed PubMed Central Google Scholar
Li, P.-F. et al. Anomalously rotary polarization discovered in homochiral organic ferroelectrics. Nat. Commun. 7 , 13635 (2016).
Xiong, Y.-A. et al. A nickel(II) nitrite based molecular perovskite ferroelectric. Angew. Chem. Int. Ed. 58 , 8857–8861 (2019).
Zhang, H.-Y. et al. Large electrostrictive coefficient in a two-dimensional hybrid perovskite ferroelectric. J. Am. Chem. Soc. 143 , 1664–1672 (2021).
King-Smith, R. D. & Vanderbilt, D. Theory of polarization of crystalline solids. Phys. Rev. B 47 , 1651–1654 (1993).
Vanderbilt, D. & King-Smith, R. D. Electric polarization as a bulk quantity and its relation to surface charge. Phys. Rev. B 48 , 4442–4455 (1993).
Spackman, M. A. & Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 11 , 19–32 (2009).
Turner, M. J., Grabowsky, S., Jayatilaka, D. & Spackman, M. A. Accurate and efficient model energies for exploring intermolecular interactions in molecular crystals. J. Phys. Chem. Lett. 5 , 4249–4255 (2014).
Turner, M. J., Thomas, S. P., Shi, M. W., Jayatilaka, D. & Spackman, M. A. Energy frameworks: insights into interaction anisotropy and the mechanical properties of molecular crystals. Chem. Commun. 51 , 3735–3738 (2015).
Song, X.-J. et al. Record enhancement of curie temperature in host–guest inclusion ferroelectrics. J. Am. Chem. Soc. 143 , 5091–5098 (2021).
Ding, R. et al. High-performance piezoelectric nanogenerators composed of formamidinium lead halide perovskite nanoparticles and poly(vinylidene fluoride). Nano Energy 37 , 126–135 (2017).
Yang, R., Qin, Y., Li, C., Dai, L. & Wang, Z. L. Characteristics of output voltage and current of integrated nanogenerators. Appl. Phys. Lett. 94 , 022905 (2009).
Chen, H. et al. Piezoelectric nanogenerator based on in situ growth all-inorganic CsPbBr3 perovskite nanocrystals in PVDF fibers with long-term stability. Adv. Funct. Mater. 31 , 2011073 (2021).
Aubin, C. A. et al. Towards enduring autonomous robots via embodied energy. Nature 602 , 393–402 (2022).
Duan, S. et al. Water-modulated biomimetic hyper-attribute-gel electronic skin for robotics and skin-attachable wearables. ACS Nano 17 , 1355–1371 (2023).
Download references
Acknowledgements
This work was supported by the National Key R&D Program of China (Grant No. 2021YFA1200700 (Y.-M.Y.)), the National Natural Science Foundation of China (Grant No. 21925502 (Y.-M.Y.), and 223B2502 (Y.-A.X.)), Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX23_0224 (Y.-A.X.)), the China Scholarship Council program (Project ID: 202306090116 (Y.-A.X.)) and “the Fundamental Research Funds for the Central Universities, China”.
Author information
These authors contributed equally: Yu-An Xiong, Sheng-Shun Duan, Hui-Hui Hu.
Authors and Affiliations
Jiangsu Key Laboratory for Science and Applications of Molecular Ferroelectrics, Southeast University, Nanjing, 211189, People’s Republic of China
Yu-An Xiong, Hui-Hui Hu, Jie Yao, Qiang Pan, Tai-Ting Sha, Hao-Ran Ji & Yu-Meng You
Joint International Research Laboratory of Information Display and Visualization, School of Electronic Science and Engineering, Southeast University, Nanjing, 210096, People’s Republic of China
Sheng-Shun Duan, Xiao Wei & Jun Wu
You can also search for this author in PubMed Google Scholar
Contributions
Y.-M.Y. and J.W. conceived and supervised the project. Y.-A.X. prepared the samples and performed the PFM measurements, piezoelectric energy-harvesting devices, and analysis. S.-S.D. prepared and test the power supply and stimuli sensing of piezoelectric devices. H.-H.H. measured the thermodynamic properties and sorted out the article. J.Y. performed the single-crystal measurement and analysis. Q.P. contributed to P–V loop measurements. T.-T.S., X.W., and H.-R.J. participated in the production and performance testing of piezoelectric devices. Y.-M.Y., J.W., Y.-A.X., S.-S.D., and H.-H.H. analyzed the data and results. Y.-A.X., S.-S.D., and H.-H.H. wrote the manuscript with input from all the other authors. All authors discussed the results and contributed to the manuscript preparation.
Corresponding authors
Correspondence to Jun Wu or Yu-Meng You .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Xiangyu Gao, Kanghua Li, and Guifu Zou for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, description of additional supplementary files, supplementary movie 1, supplementary movie 2, reporting summary, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Xiong, YA., Duan, SS., Hu, HH. et al. Enhancement of phase transition temperature through hydrogen bond modification in molecular ferroelectrics. Nat Commun 15 , 4470 (2024). https://doi.org/10.1038/s41467-024-48948-0
Download citation
Received : 28 November 2023
Accepted : 20 May 2024
Published : 25 May 2024
DOI : https://doi.org/10.1038/s41467-024-48948-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Ultrasonication-assisted synthesis of transition metal carbide of MXene: an efficient and promising material for photocatalytic organic dyes degradation of rhodamine B and methylene blue in wastewater
- Research Article
- Published: 27 May 2024
Cite this article

- Gautam Kumar 1 ,
- Amit Ahlawat 1 , 2 ,
- Hema Bhardwaj 1 ,
- Gaurav Kumar Sahu 1 ,
- Pawan S. Rana 2 &
- Partima R. Solanki ORCID: orcid.org/0000-0003-1679-2180 1
Water pollutants of non-biodegradable toxic aromatic dye including Methylene blue (MB) and Rhodamine (RhB) are extremely carcinogenic thiazines used in various industries such as leather industry, paper industry, and the dyeing industry. The presence of dyes in wastewater causes severe threats to human health that are responsible for various harmful chronic or acute diseases and also shows an adverse impact on the environment as it reduces transparency and is harmful to water microorganisms. To overcome severe issues, many traditional techniques have been used to remove toxic pollutants, but these methods are insufficient to remove chemically stable dyes that remain in the treated wastewater. However, the photocatalytic degradation process is an efficient approach to degrade the dye up to the maximum extent with improved efficiency. Therefore, in this work, a new class of two-dimensional (2D) transition metal carbide of Titanium Carbide (Ti 3 C 2 Tx) MXene material was used for the organic dyes degradation such as MB and RhB using a photocatalytic process. A layered structure of hexagonal lattice symmetry of Ti 3 C 2 Tx MXene was successfully synthesized from the Titanium Aluminum Carbide of Ti 3 AlC 2 bulk phase using an exfoliation process. Further, the XRD spectrum confirms the transformation of bulk MAX phase having (002) plane at 9.2° to Ti 3 C 2 Tx MXene of (002) plane at 8.88° confirms the successful removal of Al layer from MAX phase. A smooth, transparent, thin sheet-like morphology of Ti 3 C 2 Tx nanosheet size were found to be in the range of 70 to 150 nm evaluated from TEM images. Also, no holes or damages in the thin sheets were found after the treatment with strong hydrofluoric acid confirms the formation Ti 3 C 2 Tx layered sheets. The synthesized Ti 3 C 2 Tx MXene possesses excellent photocatalytic activity for the degradation of dyes MB, RhB, and mixtures of MB and RhB dyes. MB dye degraded with a degradation percentage efficiency of 99.32% in 30 min, while RhB dye was degraded upto 98.9% in 30 min. Also, experiments were conducted for degradation of mixture of MB and RhB dyes by UV light, and the degradation percentage efficiency were found to be 98.9% and 99.75% for mixture of MB and RhB dye in 45 min, respectively. Moreover, reaction rate constant ( k ) was determined for each dye of MB, RhB, and mixtures of MB and RhB and was found to be 0.0215 min −1 and 0.0058 min −1 , and for mixtures, it was 0.0020 min −1 and 0.009 min −1 , respectively.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Data Availability
Data will be made available on request.
Ahlawat Pawan S and Solanki, Pratima R A and R (2024) A simple and highly efficient approach towards the degradation of methylene blue and study the impact of degraded water on seed germination of cicer arietinum. Nano express
Ahlawat A, Dhiman TK, Solanki PR, Rana PS (2023) Facile synthesis of carbon dots via pyrolysis and their application in photocatalytic degradation of rhodamine B (RhB). Environ Sci Pollut Res 1–8
Ahlawat A, Rana PS, Solanki PR (2021) Studies of photocatalytic and optoelectronic properties of microwave synthesized and polyethyleneimine stabilized carbon quantum dots. Mater Lett 305:130830
Article CAS Google Scholar
Akhavan O, Ghaderi E (2009) Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J Phys Chem C 113:20214–20220
Alhabeb M, Maleski K, Anasori B et al (2017) Guidelines for synthesis and processing of two-dimensional titanium carbide (Ti3C2T x MXene). Chem Mater 29:7633–7644
Ali Khan A, Tahir M (2021) Constructing S-scheme heterojunction of CoAlLa-LDH/g-C3N4 through monolayer Ti3C2-MXene to promote photocatalytic CO2 re-forming of methane to solar fuels. ACS Appl Energy Mater 5:784–806
Article Google Scholar
Alsoruji G, Moustafa EB, Alzahrani MA, Taha MA (2022) Preparation of silicon bronze-based hybrid nanocomposites with excellent mechanical, electrical, and wear properties by adding the Ti3AlC2 MAX phase and granite via powder metallurgy. Silicon 1–11
Assad H, Fatma I, Kumar A et al (2022) An overview of MXene-based nanomaterials and their potential applications towards hazardous pollutant adsorption. Chemosphere 298:134221
Atout H, Álvarez MG, Chebli D et al (2017) Enhanced photocatalytic degradation of methylene blue: preparation of TiO2/reduced graphene oxide nanocomposites by direct sol-gel and hydrothermal methods. Mater Res Bull 95:578–587
Bashir I, Lone FA, Bhat RA, et al (2020) Concerns and threats of contamination on aquatic ecosystems. Bioremediation biotechnol sustain approaches to pollut degrad 1–26
Chen C, Boota M, Urbankowski P et al (2018) Effect of glycine functionalization of 2D titanium carbide (MXene) on charge storage. J Mater Chem A 6:4617–4622
Chen F, Ma T, Zhang T et al (2021) Atomic-level charge separation strategies in semiconductor-based photocatalysts. Adv Mater 33:2005256
Chen I-WP, Kashale AA, Pan Y-H (2023) Hydrofluoric acid-free synthesis of Ti3C2T x MXene nanostructures for energy applications. ACS Appl Nano Mater 6:1985–1995
Chen J, Chen K, Tong D et al (2015) CO 2 and temperature dual responsive “smart” MXene phases. Chem Commun 51:314–317
Chen W, Lei J, Wang Y et al (2019) Direct generation of Mn-doped ZnS quantum dots/alginate nanocomposite beads based on gelation and in situ synthesis of quantum dots. Macromol Mater Eng 304:1–8. https://doi.org/10.1002/mame.201800681
Chiu WS, Khiew PS, Cloke M et al (2010) Photocatalytic study of two-dimensional ZnO nanopellets in the decomposition of methylene blue. Chem Eng J 158:345–352. https://doi.org/10.1016/j.cej.2010.01.052
Coleman JN, Lotya M, O’Neill A, et al (2011) Two-dimensional nanosheets produced by liquid exfoliation of layered materials. Science (80- ) 331:568–571
Driscoll N, Maleski K, Richardson AG, et al (2020) Fabrication of Ti3C2 MXene microelectrode arrays for in vivo neural recording. JoVE (Journal Vis Exp e60741
Fakhraee M, Akhavan O (2019) Ultrahigh permeable C2N-inspired graphene nanomesh membranes versus highly strained C2N for reverse osmosis desalination. J Phys Chem B 123:8740–8752
Fard AK, Mckay G, Chamoun R et al (2017) Barium removal from synthetic natural and produced water using MXene as two dimensional (2-D) nanosheet adsorbent. Chem Eng J 317:331–342
Feng W, Luo H, Wang Y et al (2018) Ultrasonic assisted etching and delaminating of Ti3C2 Mxene. Ceram Int 44:7084–7087
Guo K, Wu Z, Chen C, Fang J (2022) UV/chlorine process: an efficient advanced oxidation process with multiple radicals and functions in water treatment. Acc Chem Res 55:286–297
Guo Z, Zhou J, Zhu L, Sun Z (2016) MXene: a promising photocatalyst for water splitting. J Mater Chem A 4:11446–11452
Han R, Ma X, Xie Y et al (2017) Preparation of a new 2D MXene/PES composite membrane with excellent hydrophilicity and high flux. Rsc Adv 7:56204–56210
Hojjati-Najafabadi A, Mansoorianfar M, Liang T et al (2022) Magnetic-MXene-based nanocomposites for water and wastewater treatment: a review. J Water Process Eng 47:102696
Hong S, Ming F, Shi Y et al (2019) Two-dimensional Ti3C2T x MXene membranes as nanofluidic osmotic power generators. ACS Nano 13:8917–8925
Ibrahim Y, Meslam M, Eid K et al (2022) A review of MXenes as emergent materials for dye removal from wastewater. Sep Purif Technol 282:120083. https://doi.org/10.1016/j.seppur.2021.120083
Ikram M, Raza A, Imran M et al (2020) Hydrothermal synthesis of silver decorated reduced graphene oxide (rGO) nanoflakes with effective photocatalytic activity for wastewater treatment. Nanoscale Res Lett 15:1–11
Im JK, Sohn EJ, Kim S et al (2021) Review of MXene-based nanocomposites for photocatalysis. Chemosphere 270:129478
Iqbal MA, Ali SI, Amin F et al (2019a) La-and Mn-codoped bismuth ferrite/Ti3C2 MXene composites for efficient photocatalytic degradation of Congo red dye. ACS Omega 4:8661–8668
Iqbal MA, Tariq A, Zaheer A et al (2019b) Ti3C2-MXene/bismuth ferrite nanohybrids for efficient degradation of organic dyes and colorless pollutants. ACS Omega 4:20530–20539
Jamdar M, Monsef R, Ganduh SH et al (2024) Unraveling the potential of sonochemically achieved DyMnO3/Dy2O3 nanocomposites as highly efficient visible-light-driven photocatalysts in decolorization of organic contamination. Ecotoxicol Environ Saf 269:115801. https://doi.org/10.1016/j.ecoenv.2023.115801
Jia J, Hou Z, He N et al (2023) Fabrication, microstructure and properties of Ti3C2Tx MXene nanosheets reinforced Cu composites. J Mater Res Technol 23:503–514
Jiang X, Kuklin AV, Baev A et al (2020) Two-dimensional MXenes: from morphological to optical, electric, and magnetic properties and applications. Phys Rep 848:1–58
Joseph L, Jun B-M, Jang M et al (2019) Removal of contaminants of emerging concern by metal-organic framework nanoadsorbents: a review. Chem Eng J 369:928–946
Khazaei M, Arai M, Sasaki T et al (2013) Novel electronic and magnetic properties of two-dimensional transition metal carbides and nitrides. Adv Funct Mater 23:2185–2192
Krzanowski JE, Leuchtner RE (1997) Chemical, mechanical, and tribological properties of pulsed-laser-deposited titanium carbide and vanadium carbide. J Am Ceram Soc 80:1277–1280
Kumar S, Lei Y, Alshareef NH et al (2018) Biofunctionalized two-dimensional Ti3C2 MXenes for ultrasensitive detection of cancer biomarker. Biosens Bioelectron 121:243–249
Li Z, Guo Y, Yue H et al (2021) Electrochemical determination of epinephrine based on Ti3C2Tx MXene-reduced graphene oxide/ITO electrode. J Electroanal Chem 895:115425
Liu G, Zou J, Tang Q et al (2017) Surface modified Ti3C2 MXene nanosheets for tumor targeting photothermal/photodynamic/chemo synergistic therapy. ACS Appl Mater Interfaces 9:40077–40086
Lukatskaya MR, Mashtalir O, Ren CE, et al (2013) Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science (80- ) 341:1502–1505
Ma D, Wang W, Wang Q, et al (2024) A novel visible-light-driven Z-scheme C3N5/BiVO4 heterostructure with enhanced photocatalytic degradation performance. Environ Sci Pollut Res 1–12
Maleski K, Alhabeb M (2019) Top-down MXene synthesis (selective etching). 2D met carbides nitrides struct prop appl 69–87
Mashtalir O, Naguib M, Mochalin VN et al (2013) Intercalation and delamination of layered carbides and carbonitrides. Nat Commun 4:1716
Meidanchi A, Akhavan O (2014) Superparamagnetic zinc ferrite spinel–graphene nanostructures for fast wastewater purification. Carbon N Y 69:230–238
Naguib M, Kurtoglu M, Presser V et al (2011) Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv Mater 23:4248–4253
Naguib M, Unocic RR, Armstrong BL, Nanda J (2015) Large-scale delamination of multi-layers transition metal carbides and carbonitrides “MXenes.” Dalt Trans 44:9353–9358
Näslund L-Å, Persson I (2022) XPS spectra curve fittings of Ti3C2Tx based on first principles thinking. Appl Surf Sci 593:153442
Natu V, Benchakar M, Canaff C et al (2021) A critical analysis of the X-ray photoelectron spectra of Ti3C2Tz MXenes. Matter 4:1224–1251
Nazim M, Khan AAP, Asiri AM, Kim JH (2021) Exploring rapid photocatalytic degradation of organic pollutants with porous CuO nanosheets: synthesis, dye removal, and kinetic studies at room temperature. ACS Omega 6:2601–2612
Nehra P, Rana PS, Singh S (2023) Remediation of recalcitrant pollutants in water solution using visible light responsive cerium-doped tungsten trioxide nanoparticles. Environ Sci Pollut Res 30:70094–70108
Nicolosi V, Chhowalla M, Kanatzidis MG, et al (2013) Liquid exfoliation of layered materials. Science (80- ) 340:1226419
Othman Z, Sinopoli A, Mackey HR, Mahmoud KA (2021) Efficient photocatalytic degradation of organic dyes by AgNPs/TiO2/Ti3C2T x MXene composites under UV and solar light. ACS Omega 6:33325–33338
Pal H, Chatterjee KN, Sharma D (2017) Water footprint of denim industry. In: Sustainability in denim. Elsevier, pp 111–123
Pereloma E, Edmonds DV (2012) Phase transformations in steels: diffusionless transformations, high strength steels, modelling and advanced analytical techniques. Elsevier
Book Google Scholar
Pouramini Z, Mousavi SM, Babapoor A et al (2023) Recent advances in MXene-based nanocomposites for wastewater purification and water treatment: a review. Water 15:1267
Qian R, Zong H, Schneider J et al (2019) Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: an overview. Catal Today 335:78–90
Qu J, Teng D, Zhang X et al (2022) Preparation and regulation of two-dimensional Ti3C2Tx MXene for enhanced adsorption–photocatalytic degradation of organic dyes in wastewater. Ceram Int 48:14451–14459
Rahighi R, Hosseini-Hosseinabad SM, Zeraati AS et al (2022) Two-dimensional materials in enhancement of membrane-based lithium recovery from metallic-ions-rich wastewaters: a review. Desalination 543:116096. https://doi.org/10.1016/j.desal.2022.116096
Sachidhanandham A, Periyasamy AP (2021) Environmentally friendly wastewater treatment methods for the textile industry. In: Handbook of nanomaterials and nanocomposites for energy and environmental applications. Springer, pp 2269–2307
Salavati-Niasari M (2006) Ship-in-a-bottle synthesis, characterization and catalytic oxidation of styrene by host (nanopores of zeolite-Y)/guest ([bis(2-hydroxyanil)acetylacetonato manganese(III)]) nanocomposite materials (HGNM). Microporous Mesoporous Mater 95:248–256. https://doi.org/10.1016/j.micromeso.2006.05.025
Saxena S, Raja ASM, Arputharaj A (2017) Challenges in sustainable wet processing of textiles. Text cloth sustain sustain text chem process 43–79
Sayem A, Suvo AH, Syed IM, Bhuiyan MA (2024) Effective adsorption and visible light driven enhanced photocatalytic degradation of rhodamine B using ZnO nanoparticles immobilized on graphene oxide nanosheets. Results Phys 107471
Seling TR, Katzbaer RR, Thompson KL et al (2024) Transition metal-doped CuO nanosheets for enhanced visible-light photocatalysis. J Photochem Photobiol A Chem 448:115356
Sengupta A, Rao BVB, Sharma N et al (2020) Comparative evaluation of MAX, MXene, NanoMAX, and NanoMAX-derived-MXene for microwave absorption and Li ion battery anode applications. Nanoscale 12:8466–8476
Shindhal T, Rakholiya P, Varjani S et al (2021) A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 12:70–87
Solangi NH, Karri RR, Mazari SA et al (2023) MXene as emerging material for photocatalytic degradation of environmental pollutants. Coord Chem Rev 477:214965
Song X, Wang Y, Wang C et al (2019) Solar-intensified ultrafiltration system based on porous photothermal membrane for efficient water treatment. ACS Sustain Chem Eng 7:4889–4896
Szuplewska A, Rozmysłowska-Wojciechowska A, Poźniak S et al (2019) Multilayered stable 2D nano-sheets of Ti2 NT x MXene: synthesis, characterization, and anticancer activity. J Nanobiotechnology 17:1–14
Teymourinia H, Salavati-Niasari M, Amiri O, Safardoust-Hojaghan H (2017) Synthesis of graphene quantum dots from corn powder and their application in reduce charge recombination and increase free charge carriers. J Mol Liq 242:447–455
Thirumal V, Yuvakkumar R, Kumar PS et al (2021) Efficient photocatalytic degradation of hazardous pollutants by homemade kitchen blender novel technique via 2D-material of few-layer MXene nanosheets. Chemosphere 281:130984
Thirunavukkarasu A, Nithya R, Sivashankar R (2020) A review on the role of nanomaterials in the removal of organic pollutants from wastewater. Rev Environ Sci Bio/technol 19:751–778
Tripta RPS (2023) Structural, optical, electrical, and photocatalytic application of NiFe2O4@NiO nanocomposites for methylene blue dye. Ceram Int 49:13520–13530. https://doi.org/10.1016/j.ceramint.2022.12.227
Tunesi MM, Soomro RA, Han X et al (2021) Application of MXenes in environmental remediation technologies. Nano Converg 8:1–19
Vasseghian Y, Dragoi E-N, Almomani F (2022) A comprehensive review on MXenes as new nanomaterials for degradation of hazardous pollutants: deployment as heterogeneous sonocatalysis. Chemosphere 287:132387
Warsi A-Z, Aziz F, Zulfiqar S et al (2022) Synthesis, characterization, photocatalysis, and antibacterial study of WO3, MXene and WO3/MXene nanocomposite. Nanomaterials 12:713
Xu N, Wang W, Zhu Z, et al (2022) Recent developments in photocatalytic water treatment technology with MXene material: a review. Chem Eng J Adv 100418
Xu T, Zhang J, Guo H et al (2021) Antifouling fibrous membrane enables high efficiency and high-flux microfiltration for water treatment. ACS Appl Mater Interfaces 13:49254–49265
Yan Y, Wei Z, Duan X et al (2023) Merits and limitations of radical vs. nonradical pathways in persulfate-based advanced oxidation processes. Environ Sci Technol 57:12153–12179
Zamhuri A, Lim GP, Ma NL et al (2021) MXene in the lens of biomedical engineering: synthesis, applications and future outlook. Biomed Eng Online 20:1–24
Zhao L, Dong B, Li S et al (2017) Interdiffusion reaction-assisted hybridization of two-dimensional metal–organic frameworks and Ti3C2T x nanosheets for electrocatalytic oxygen evolution. ACS Nano 11:5800–5807
Zhao W-Y, Zhou M, Yan B et al (2018) Waste conversion and resource recovery from wastewater by ion exchange membranes: state-of-the-art and perspective. Ind Eng Chem Res 57:6025–6039
Zhou W, Zhu J, Wang F et al (2017) One-step synthesis of Ceria/Ti3C2 nanocomposites with enhanced photocatalytic activity. Mater Lett 206:237–240
Zhu M, Huang Y, Deng Q et al (2016) Highly flexible, freestanding supercapacitor electrode with enhanced performance obtained by hybridizing polypyrrole chains with MXene. Adv Energy Mater 6:1600969
Zhu Q, Liu N, Zhang N et al (2018) Efficient photocatalytic removal of RhB, MO and MB dyes by optimized Ni/NiO/TiO2 composite thin films under solar light irradiation. J Environ Chem Eng 6:2724–2732
Zinatloo-Ajabshir S, Salehi Z, Amiri O, Salavati-Niasari M (2019) Simple fabrication of Pr2Ce2O7 nanostructures via a new and eco-friendly route; a potential electrochemical hydrogen storage material. J Alloys Compd 791:792–799
Download references
Acknowledgements
The authors of this paper acknowledge the AIRF and JNU for providing the TEM facility. One of the authors, Amit Ahlawat, is thankful to UGC, India, for giving financial support through fellowship (NTA Ref No. 201610066606). Hema Bhardwaj, is thankful to DBT, for giving financial support through the RA fellowship (DBT-RA/2022/July/N/2383). PRS thanks to Department of Health Research, Ministry of Health and Family Welfare, Government of India through (Grant No: R.11013/46/2021-GIA/HR) for financial support for this research work. PSR also acknowledges DST, India, for the DST-FIST project (ref. no: SR/ FST/PS-I/2012/32), sanctioned to the Department of Physics, DCRUST, Murthal, Haryana.
Author information
Authors and affiliations.
Nano-Bio Laboratory, Special Centre for Nanoscience, Jawaharlal Nehru University, New Delhi, 110067, India
Gautam Kumar, Amit Ahlawat, Hema Bhardwaj, Gaurav Kumar Sahu & Partima R. Solanki
Hydrogen Energy Lab, Department of Physics, DCRUST, Murthal, Sonepat, Haryana, 131001, India
Amit Ahlawat & Pawan S. Rana
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the study’s conception and design. Material preparation and data collection and analysis were performed by Gautam Kumar, Amit Ahlawat, Hema Bhardwaj, and Gaurav Kumar Sahu. The first draft of the manuscript was written by Gautam Kumar, Amit Ahlawat, and Hema Bhardwaj, and all authors commented on previous versions of the manuscript. Pratima R. Solanki has formulated the concept as well as the preparation of the manuscript. All the authors have read and approved the draft. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Partima R. Solanki .
Ethics declarations
Ethical approval.
This manuscript does not contain any studies with human or animal subjects.
Consent to participate
Not applicable.
Consent to publish
Competing interests.
The authors declare no competing interests.
Additional information
Gautam Kumar, Amit Ahlawat, and Hema Bhardwaj contributed equally to this study.
Responsible Editor: George Z. Kyzas
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Kumar, G., Ahlawat, A., Bhardwaj, H. et al. Ultrasonication-assisted synthesis of transition metal carbide of MXene: an efficient and promising material for photocatalytic organic dyes degradation of rhodamine B and methylene blue in wastewater. Environ Sci Pollut Res (2024). https://doi.org/10.1007/s11356-024-33505-5
Download citation
Received : 29 December 2023
Accepted : 26 April 2024
Published : 27 May 2024
DOI : https://doi.org/10.1007/s11356-024-33505-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Two-dimensional nanosheet
- Ti 3 C 2 Tx MXene
- Photocatalysis
- Organic pollutants
- Dyes removal
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Phase Transitions will not consider papers in the field of metallurgy.. Phase Transitions publishes both research papers and invited articles devoted to special topics. Major review papers are particularly welcome. A further emphasis of the journal is the publication of a selected number of small workshops, which are at the forefront of their field.
Abstract. The discovery and control of new phases of matter is a central endeavour in materials research. The emergence of atomically thin 2D materials, such as transition-metal dichalcogenides ...
Based on the research of the phase-transition mechanism, modulation methods, such as element doping, electric field (current and gating), and tensile/compression strain, as well as employing ...
The majority of the studies have been in 2D systems that require complicated external fields. 3D phase transitions (e.g. from FCC phase to AuCu phase) have been observed using X-ray scattering 32 ...
Explore the latest full-text research PDFs, articles, conference papers, preprints and more on PHASE TRANSITION. Find methods information, sources, references or conduct a literature review on ...
sizezero-temperature phase transitions we studyinthis paper. We formally define the order parameter and the effective ... ZEROTH, FIRST, AND SECOND-ORDER PHASE … PHYSICAL REVIEW RESEARCH 5, 043243 (2023) where x ∈ Rd is the input data, y = y(x) the label, W(i) the model parameters, D the number of hidden layers, the ran-
the research on the application of neural networks to analyze phases and phase transitions are: 1) To what extent the ... data reveal information about the phase transition? In this paper we investigate these two questions. To analyze the influence of the network architecture, we employ a neural architecture search (NAS) [10], [11] strategy,
Lattice models exhibit significant potential in investigating phase transitions, yet they encounter numerous computational challenges. To address these issues, this study introduces a Monte Carlo-based approach that transforms lattice models into a network model with intricate inter-node correlations. This framework enables a profound analysis of Ising, JQ, and XY models. By decomposing the ...
Recently, the Floquet Na 3 Bi-type material has been proposed as an ideal platform for realizing various phases, i.e., the spin-degenerate Dirac semimetal (DSM) can be turned into the Weyl semimetal (WSM), and even to the Weyl half-metal (WHM).Instead of the conventional electrical methods, we use the RKKY interaction to characterize the topological phase transitions in this paper.
The phase transition can keep the heat flux from decreasing as it can help to keep the temperature difference. ... In this paper, a new multiple PCMs arrangement is proposed in the circumferential direction of a concentric tube. ... Natural Science Foundation of Hunan Province, China (Grant No: 2020JJ5734) and the Fundamental Research Funds for ...
2. Published online: 17 Feb 2024. Explore the current issue of Phase Transitions, Volume 97, Issue 3, 2024.
The phase-transition points and their critical exponents are established across Clifford and Haar random circuits through finite-size scaling. The flow of both classical and quantum information, measured respectively by Holevo and coherent information, shows similar dynamical phase transition behaviors. ... Our paper underscores rich behaviors ...
The phase transition process of ternary cathode materials corresponds to the release of oxygen and the variation in heat. Observing Figs. 6 a and c, it can be noticed that the phase transition temperatures demonstrated in the XRD graph, varying with energy density, align perfectly with the peak temperature variation with energy density in the ...
The research is published today in Physical Review Letters. Detecting phase transitions using AI. While water transitioning to ice might be among the most obvious examples of a phase change, more exotic phase changes, like when a material transitions from being a normal conductor to a superconductor, are of keen interest to scientists.
View a PDF of the paper titled Phase Transitions in the Output Distribution of Large Language Models, by Julian Arnold and 3 other authors. View PDF HTML (experimental) Abstract: In a physical system, changing parameters such as temperature can induce a phase transition: an abrupt change from one state of matter to another. Analogous phenomena ...
Phase change materials (PCMs) are preferred in thermal energy storage applications due to their excellent storage and discharge capacity through melting and solidifications. PCMs store energy as a Latent heat-base which can be used back whenever required. The liquefying rate (melting rate) is a significant parameter that decides the suitability of.
Phase Transitions will not consider papers in the field of metallurgy.. Phase Transitions publishes both research papers and invited articles devoted to special topics. Major review papers are particularly welcome. A further emphasis of the journal is the publication of a selected number of small workshops, which are at the forefront of their field.
Here are some of the most useful transition words for research papers. 1-888-627-6631; [email protected]; Jobs; FAQ; About Us About Us; ... These transitions often come after an important point in the research paper has been established or to explore hypothetical relationships or circumstances. Purpose: Common Terms: Common Phrases:
After the discovery of the volume phase transition of gel, the phenomenon was extensively studied and advanced by the discoverer, the late Professor Toyoichi Tanaka, who deceased on 20 May 2000 in the halfway of his research. In this paper, we would like to review his research to clarify his deep insight into the science of gels.
Scope. Phase Transitions is the only journal devoted exclusively to this important subject. It provides a focus for papers on most aspects of phase transitions in condensed matter. Although emphasis is placed primarily on experimental work, theoretical papers are welcome if they have some bearing on experimental results.
Impact of Co-doped TiO2 nanoparticles on the physical properties of a nematic liquid crystal. Soumaya Alaya et al. Article | Published online: 17 Feb 2024. View all latest articles. All journal articles featured in Phase Transitions vol 23 issue 1.
These proceedings collect papers on phase transitions. Topics include: nucleation theory, neutron-induced vaporization of superheated liquids, kinetics of nucleation, glass formation, Co-Zr glasses, uranium-based metallic glasses, x-ray scattering technique, phase formation in thin-film lateral-diffusion couples, and solute trapping.
Meanwhile, the total width of the three filterable bandgaps accounts for 65.5% at 6200 Hz and below. In this paper, the band structure is obtained by using the method of finite element simulation. The generation of topological phase transition induced by the rotation angle of the scatterer is analyzed.
A paper demonstrating that the scattering from the thermal bath is key for the photoinduced phase transition in VO 2. Article Google Scholar Download references
Here the authors report evidence of magnetic phase transitions in a polycrystalline non-collinear antiferromagnet, which are explained by a phenomenological model with topological orbital momenta ...
Liquid metals, in the form of micro/nanodroplets, have emerged as unique filler materials owing to their unrivaled coupled fluidic and metallic properties (24-28).Liquid metal-embedded soft materials with high electrical and thermal conductivity (29, 30), tunable conductivity (31-33), and self-healing capability have been reported.In most cases, the tuning of thermal, electrical, or ...
—This paper presents a new control methodology for achieving smooth gait transitions for a hexapod robot using central pattern generators (CPGs). The approach involves modifying the Phase Oscillator within the CPG network to enable smooth transitions between different gaits in order to improve the adaptability to changing environmental conditions. The foot trajectory generator is designed ...
Fig. 1: Comparison of crystal structures between (HaaOH)BF 4 and (Haa)BF 4 at 293 K. Fig. 2: Hirshfeld surface analyses of (HaaOH)BF 4 and (Haa)BF 4. Fig. 4: Domain structure and domain switching ...
Volume 7 1986. Volume 6 1985-1986. Volume 5 1985. Volume 4 1983-1984. Volume 3 1982-1983. Volume 2 1981-1982. Volume 1 1979-1980. Browse the list of issues and latest articles from Phase Transitions.
The present work was focused on the synthesis of Ti 3 C 2 Tx MXene material from the Ti 3 AlC 2 bulk phase using an exfoliation process and ultrasonic-assisted delamination process. The synthesized Ti 3 C 2 Tx MXene was characterized using various spectroscopy and microscopy techniques that confirmed the successful removal of the aluminum (Al) layer from the bulk phase and transformed it into ...