An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Exp Ther Med
- v.22(2); 2021 Aug

Updates and new medical treatments for vitiligo (Review)
David emmanuel kubelis-lópez.
1 Department of Dermatology, Faculty of Medicine and University Hospital ‘Dr. José Eleuterio González’, Universidad Autónoma de Nuevo León, Monterrey, Nuevo León 64460, México
Natalia Aranza Zapata-Salazar
Salvador luis said-fernández.
2 Department of Biochemistry and Molecular Medicine, Faculty of Medicine and University Hospital ‘Dr. José Eleuterio González’, Universidad Autónoma de Nuevo León, Monterrey, Nuevo León 64460, México
Celia Nohemí Sánchez-Domínguez
Mauricio andrés salinas-santander.
3 Department of Research, Faculty of Medicine Saltillo Unit, Universidad Autónoma de Coahuila, Saltillo 25000, México
Herminia Guadalupe Martínez-Rodríguez
Osvaldo tomás vázquez-martínez, uwe wollina.
4 Department of Dermatology and Allergology and Skin Cancer Center, Städtisches Klinikum Dresden, D-01067 Dresden, Germany
Torello Lotti
5 Department of Dermatology and Venereology, University of Rome G. Marconi, I-00193 Rome, Italy
6 Department of Dermatology and Communicable Diseases, First Medical State University of Moscow I. M. Sechenev Ministry of Health, Moscow 119991, Russia
Jorge Ocampo-Candiani
Associated data.
Not applicable.
Vitiligo is a multifactorial disease characterized by the loss of skin pigment, which results in achromic macules and patches. There are currently several medical treatments available, which aim to arrest progression and induce skin repigmentation. These treatments alone or combined have exhibited varying degrees of pigmentation, and the majority are safe and effective. All therapies for vitiligo are limited, and no known treatment can consistently produce repigmentation in all patients. Individualized treatment is appropriate according to the location, clinical presentation and the presence of disease activity. The present review summarizes the medical treatments available for vitiligo: Systemic and topic pharmacological therapies, physical and depigmentation treatments. Several treatments are still underway and have not yet been approved. However, due to the promising preliminary results, these are also mentioned in the present review.
1. Introduction
Vitiligo is the most common disorder of depigmentation, and in 2012 its worldwide prevalence ranged from 0.06-2.28% ( 1 , 2 ). It is characterized by the absence of pigment in the skin, secondary to the loss of melanocytes ( 1 , 3 ). Melanocytes are found in several tissues in the skin, hair follicles, eyes, inner ear, bones, heart and brain ( 4 ). Melanocytes are found in the basal layer of the epidermis and together with the surrounding keratinocytes form the epidermal unit, whose main function is to produce and distribute melanin by a complex process called melanogenesis ( 4-7 ). Melanin is a pigment with two forms, eumelanin (brown/black or black) and pheomelanin (red/yellow) ( 6 , 8 ). It has light-absorbing properties that confer photoprotection ( 4 , 6-8 ). Melanogenesis is determined genetically but is influenced by several intrinsic and extrinsic factors ( 5 ). The intrinsic factors are released by surrounding cells, including keratinocytes, fibroblasts, inflammatory, neural and endocrine cells ( 4 , 5 , 9 , 10 ). The extrinsic factors include ultraviolet radiation and drugs ( 5 ). Among the inducers and positive regulators of melanogenesis are L-tyrosine and L-DOPA (pigment precursors), ultraviolet radiation and the melanocortin 1 receptor ( 5 , 9 , 10 ). The latter is considered the most important positive regulator ( 5 , 9 , 10 ).
The pathogenesis of vitiligo is unknown, but an autoimmune hypothesis prevails and is supported by several factors: Its association with other autoimmune diseases, the high level of antibodies against melanocytes found in 10% of patients with vitiligo, susceptibility loci associated with vitiligo found in genome-wide association studies that encode immunomodulatory proteins, and lastly, an inflammatory infiltrate that is observed at the margin of active lesions ( 11 , 12 ). In the biochemical theory, the damage to melanocytes is due to an imbalance in oxidative stress; a higher level of hydrogen peroxide in patients with vitiligo and increased superoxide dismutase activity reinforce this theory ( 11 ). Another hypothesis is the melanocytorrhagy theory, which proposes that defective cell adhesion leads to detachment and transepidermal loss of melanocytes with exposure of autoantigens and activation of the immune system leading to melanocyte injury ( 3 ). Finally, the convergence theory states that a combination of several pathways is necessary for the development of vitiligo, such as genetic background, susceptibility to environmental changes, altered epidermal microenvironment, an intrinsic melanocyte defect and an autoimmune response ( 13 , 14 ). Clinically, vitiligo manifests as achromic macules and patches that increase in number and size over time ( 15 ). Treatment strategies aim to arrest the disease, achieve repigmentation and prevent relapse ( 16 ). The present review discusses the pharmacological, physical and depigmentation treatment options for vitiligo, used either as monotherapy or in combination. In general, combining therapies results in superior outcomes ( 17 ).
2. Pharmacological treatment
Topical treatment corticosteroids.
Corticosteroids' main therapeutic effect in vitiligo is modulation and inhibition of inflammation ( 18 ). Topical corticosteroids (TCS), either potent (betamethasone valerate) or very potent (clobetasol propionate), are considered first-line therapy for vitiligo ( 19 , 20 ). The sun-exposed areas have a better response to treatment, while acral regions generally exhibit a poor response ( 18 ). High potency TCS are recommended to treat small areas of the body; in the areas more sensitive to TCS, namely the face, neck, genitals or intertriginous regions where absorption may be higher and more side effects may present, topical calcineurin inhibitors (TCI) or lower potency steroids are preferred ( 21 , 22 ). The application of daily TCS for up to 3 months is recommended ( 18 ). After that, an intermittent regimen can be used for up to 6 months, and if no response is seen after 3-4 months, the application should be discontinued ( 18 , 21 , 23 ). In a meta-analysis, Njoo et al ( 20 ) reported the effectiveness of TCS in localized vitiligo, measured as the percentage achieving ≥75% repigmentation, which was comparable with potent (56%) and very potent (55%) TCS. To increase the probability of a therapeutic response when TCS is used as monotherapy, very potent TCS may be preferred ( 17 ).
The side effects of TCS include atrophy, striae, telangiectasias, hypertrichosis and acneiform reactions ( 18 ). The most frequent local side effect is atrophy, which depends on diverse factors, including age, site of application, the potency of the TCS and the presence of occlusion. In vitiligo, sometimes long treatments are required ( 24 ). ‘Corticosteroid holidays’ which are weeks without TCS, along with tapering from high to mild potency can be used to minimize side effects ( 24 ). In addition to local side effects, systemic absorption may cause adrenal suppression ( 25 ). Kwinter et al ( 25 ) performed a retrospective study in pediatric patients with vitiligo treated with moderate to high potency TCS. The results demonstrated that cortisol levels were abnormal in 29% of patients, and the potential risks associated were lesions located in the head and neck ( 25 ). These undesirable effects can be minimized in the pediatric population by using soft steroids, which are esterified corticosteroids that retain their anti-inflammatory effects and have fewer systemic side effects ( 26 ), including mometasone furoate and methylprednisolone aceponate ( 26 ).
Calcineurin inhibitors
Calcineurin inhibitors are immunomodulators and an off-label treatment for vitiligo ( 24 ). They function by inhibiting calcineurin, a pro-inflammatory protein in lymphocytes and dendritic cells that induces the transcription of interleukin (IL)-2 and tumor necrosis factor-α (TNF-α) ( 27 ). Its inhibition decreases cytokine formation, and induces melanocyte and melanoblast proliferation ( 27 ). TCIs, such as tacrolimus (0.03 or 0.1%) and pimecrolimus (1%) are recommended for the head and neck areas as they have less side effects, mainly the lack of atrophy risk ( 18 , 21 ). TCI can be applied twice daily for a minimum of 6 months. When beneficial effects are observed, treatment can be prolonged according to results ( 18 ). Moderate daily sun exposure is recommended during treatment ( 18 ). Another usage of TCI is during the intermittent treatment schemes of TCS, whereby on days TCS was not applied, TCI can be used to ensure a continuous treatment ( 22 ).
The efficacy of TCI as monotherapy in a systemic review and meta-analysis by Lee et al ( 28 ), demonstrated ≥25% repigmentation in 55%, ≥50% repigmentation in 38.5%, and ≥75% repigmentation in 18.1% of patients. The results in children were ≥25% repigmentation in 66.4% and ≥75% repigmentation in 31.7% patients. A better response was achieved on the face and neck followed by the trunk and extremities and the least response was observed in the hands and feet ( 28 ). In a meta-analysis by Chang et al ( 29 ), comparing the efficacy of TCI to TCS, TCI was less effective than TCS in achieving ≥50% repigmentation but was comparable to TCS in achieving ≥75% repigmentation ( 29 ).
TCI can be used as monotherapy or in combination. Ebrahim et al ( 30 ) performed a study comparing the application of tacrolimus 0.1% alone or in combination with microneedling (fine needles to create micro-holes in the skin) in patients with localized stable vitiligo. Both groups applied tacrolimus daily, but the combination group also received microneedling and tacrolimus application every 2 weeks for up to 12 sessions. The results exhibited earlier pigmentation and ≥75% pigmentation in 50.00% of patients in the combination group compared with 29.92% in the monotherapy group ( 30 ).
Another combination was studied by Abd-Elazim et al ( 31 ) in a randomized placebo-controlled study in patients with stable generalized vitiligo. In each patient, three lesions of similar size were chosen. One lesion was treated with tacrolimus 0.03% daily, another with a combination of monthly microdermabrasion (light abrasion of the skin) and daily tacrolimus 0.03%, and the last was treated with placebo. The combination group achieved moderate to excellent response (≥50% repigmentation) in 65.7% of lesions compared with monotherapy with tacrolimus in 25.8% of lesions ( 31 ).
The side effects of TCI include burning sensation, pruritus and increased susceptibility to infection (herpes simplex and molluscum contagiosum) ( 24 ).
Vitamin D3 analogs
Vitamin D sources are the diet or synthesis by the skin with UVB light from 7-dehydrocholesterol ( 32 , 33 ). The classical pathway to obtain the hormonally active form of vitamin D is by hydroxylation to 25-hydroxyvitamin D3; mainly in the liver, and it is subsequently converted to 1,25-hydroxyvitamin D3 in the kidney, which is the active form of the vitamin ( 32 , 33 ). An alternative pathway of vitamin D3 activation is the biologically active metabolites produced by the action of cytochrome P450 family 11 subfamily A member 1 (CYP11A1) ( 34-37 ). Another source of active vitamin D3 is through the synthesis by antigen-presenting cells, T cells and B cells ( 38 ). These cell types can also respond to the stimulation of vitamin D, which may be associated with the ability to maintain self-tolerance and to promote protective immunity against infections ( 38 ).
Topical vitamin D3 analogs (D3A) are not effective as monotherapy for vitiligo but are useful as adjuvants to other therapies due to their immunomodulatory effects inhibiting T-cell activity, enhancement of melanocyte development and induction of melanogenesis ( 27 , 39 , 40 ). The maximum recommended dose is 100 g weekly on 30% of the body surface with the combination of calcipotriol 0.005% and betamethasone 0.05% for 4 weeks using the ointment and 8 weeks for the cream ( 24 ).
Efficacy of the combination therapy calcipotriol 0.005% and betamethasone dipropionate 0.05% was studied by Kumaran et al ( 41 ) in a randomized controlled trial, where each drug was given alone or in combination to patients with localized vitiligo. Marked repigmentation (50-75%) was achieved in 6.7% of patients in the calcipotriol, 13.3% in the betamethasone and 26.7% in the combination groups, respectively. Moderate repigmentation (25-50%) was observed in 33.3% of patients in the calcipotriol, 46.7% in the betamethasone and 46.7% in the combination groups, respectively. No patient achieved >75% repigmentation, but combined therapy resulted in faster repigmentation ( 41 ).
The transdermal delivery of drugs may be increased using microneedling. This was studied by Ibrahim et al ( 42 ) with the combination of microneedling with calcipotriol 0.05 mg/g and betamethasone 0.5 mg, compared with microneedling with tacrolimus 0.03%. The patients received both therapies in two different lesions. The creams were applied daily and microneedling was performed every 2 weeks for a maximum of 12 sessions. The combination with calcipotriol and betamethasone exhibited 76-100% repigmentation in 60% of patients compared with 32% in the combination with tacrolimus, concluding that the combination of calcipotriol and betamethasone with microneedling was superior and also effective in sites resistant to therapy (elbow, knees, extremities and acral area) ( 42 ). D3A is safe in both children and adults, only mild irritation has been reported ( 24 ).
Pseudocatalase/superoxide dismutase
Oxidative stress and accumulation of hydrogen peroxide (H 2 O 2 ) are believed to play roles in vitiligo. High levels of H 2 O 2 accumulate in the epidermis of the lesions ( 43 ). These are toxic to melanocytes, inhibit tyrosinase, and cause the deactivation of catalase (a peroxisomal enzyme catalyzing the reduction of H 2 O 2 to water and oxygen) ( 43 ). The efficacy of topical pseudocatalase is variable. In a pilot, randomized, placebo-controlled trial performed by Naini et al ( 43 ) using topical pseudocatalase/superoxide dismutase gel, no significant changes in the lesion area and perifollicular pigmentation were observed. In a study performed in a pediatric population by Schallreuter et al ( 44 ), patients were treated with twice daily application of pseudocatalase PC-KUS activated with low-dose narrow-band UVB (nb-UVB). The results demonstrated a halt of disease progression in 70/71 patients; >75% repigmentation was achieved in 92.9% of children with lesions located on the face/neck, 78.6% on the trunk, 72.7% on the extremities and 9.4% on the hands/feet ( 44 ). Bakis-Petsoglou et al ( 45 ) evaluated topical pseudocatalase and nb-UV in a double-blind, placebo-controlled, randomized, single-center trial in patients with active vitiligo. No added benefit was observed with the combination therapy ( 45 ). A study performed by Alshiyab et al ( 46 ) , comparing the efficacy of tacrolimus 0.1% ointment to tacrolimus 0.1% ointment plus topical pseudocatalase/superoxide dismutase gel in the treatment of children with localized vitiligo, demonstrated that there was no significant difference in repigmentation percentages between the two groups. However, information on side effects and safety of pseudocatalase is lacking ( 47 ). Current data are not in favor of an additional effect of topical catalase compared with UVB alone ( 45 ).
5-gluorouracil (5-FU)
Topical 5-FU is mainly used for the treatment of premalignant and malignant skin lesions ( 48 ). The observation of hyperpigmentation following therapy with this drug led to its use in vitiligo ( 48 ). The mechanisms of repigmentation of 5-FU may include stimulation of follicular melanocytes with migration during epithelization and by increasing the number of melanosomes in keratinocytes ( 49 , 50 ). The efficacy of monotherapy with 5-FU has been reported by Tsuji-Takuo and Hamada ( 48 ). A 5-FU cream was applied following epidermal abrasion, once daily for 7-10 days and >75% repigmentation was observed in 64% of patients ( 48 ).
Several studies have combined laser therapy with topical 5-FU ( 49 , 51 , 52 ). Abdelwahab et al ( 49 ) performed a study to assess the effect of 5-FU in monotherapy compared with its combination with ablative erbium: YAG (2,940 nm) laser in non-segmental vitiligo. Erbium: YAG laser was applied using the surgical handpiece with a spot size of 4 mm and a fluence of 60 J/cm 2 . A total of two to three passes were given with an endpoint of pinpoint bleeding, receiving three treatment sessions every 4-6 weeks. 5-FU cream was used daily for 2 weeks after each session. The range of repigmentation in the combined treatment was 0-70%, with <25% repigmentation in 73.3 and 50-75% repigmentation in 10% of patients; the range of repigmentation in the monotherapy group was 0-5% ( 49 ). Anbar et al ( 51 ) also used erbium-YAG laser in combination with topical 5-FU but in periungual vitiligo. The laser was used with a spot size of 5 mm and a fluence of 2.1 J/cm 2 . The endpoint was pinpoint bleeding with usually three passes required. Topical 5-FU cream was applied daily until inflammation with erythema, moderate oozing and crustation occurred. The sessions with erbium-YAG laser were repeated until 100% repigmentation was achieved or for a maximum of three successive sessions performed with no further improvement observed. The results were ≥75% repigmentation in 33.3% of patients, 26-74% repigmentation in 33.3 and ≤25% repigmentation or none in 33.3% ( 51 ).
CO 2 laser with topical 5-FU was studied in acral vitiligo by Mohamed et al ( 52 ) . The laser was employed at a rate of 1-2 Hz in level 2 pulse control and a power of 0.9 W to deliver single pulses using the single-spot handpiece. In the abraded area, 5-FU was applied daily for 7 days, and CO 2 laser sessions were repeated monthly until healing or a maximum of 5 sessions. The results demonstrated >75% repigmentation in 49.8% of the lesions and 50-75% repigmentation in 6.1% of the lesions ( 52 ).
Mina et al ( 53 ) performed a study comparing microneedling with either topical tacrolimus or topical 5-FU. In each patient, two patches of vitiligo were treated. First, microneedling with a Dermapen at the lowest speed and a depth of 0.25-0.50 mm according to the area was performed, then one patch was treated with a solution of 5-FU (50 mg/ml) and the other with tacrolimus 0.03% ointment. Patients were advised to continue the treatment with daily application of either 5-FU or tacrolimus for 2 weeks, accordingly. Microneedling in combination with topical treatment was repeated every 2 weeks for a maximum of 12 sessions. The reported efficacy with the combination of 5-FU was >75% repigmentation in 48% of patients, 51-75% repigmentation in 4 and 26-50% repigmentation in 20% of patients compared with 16, 24 and 36%, respectively, in the tacrolimus group. The 5-FU group also presented a faster response to repigmentation ( 53 ). The side effects of 5-FU are hyperpigmentation, scarring, infection, ulceration and delayed wound healing ( 47 , 53 ).
Methotrexate (MTX)
MTX is a folate antagonist that appears to decrease the number of T cells producing TNF-α, consequently having anti-inflammatory, immunomodulatory and antiproliferative effects ( 54 ). In a recent case report ( 54 ) in a patient with stable vitiligo, significant repigmentation was observed following treatment with topical MTX 1% gel applied twice daily for 12 weeks, along with folic acid supplementation. No side effects were reported. However, further studies are required to determine the efficacy and safety of MTX ( 54 ).
Prostaglandin F2 alpha analogs
Treatment of ocular hypertension with prostaglandin F2 alpha analogs (PF2A) is common ( 55 ). The observation of iris and periocular skin hyperpigmentation in patients with glaucoma led to its use in vitiligo ( 55 ). This hyperpigmentation seems to be due to an increase in melanogenesis ( 56 ).
In a preliminary study performed by Kanokrungsee et al ( 57 ), the efficacy of bimatoprost 0.01% solution was assessed in patients with non-segmental facial vitiligo compared with tacrolimus 0.1% ointment. Both topical drugs were applied twice daily for 12 weeks. Repigmentation was observed in 60 and 50% of the patients in the bimatoprost and tacrolimus groups, respectively. In addition, >50% repigmentation was achieved in 20% of patients in the bimatoprost group compared with 10% in the tacrolimus group, although no statistically significant differences were observed between the two groups ( 57 ). Latanoprost efficacy was evaluated in a double-blind clinical control trial by Nowroozpoor Dailami et al ( 58 ). Patients enrolled had generalized or focal vitiligo involving the eyelids. Latanoprost 0.005% gel was applied twice daily for 12 weeks and was compared with placebo. Improvement in pigmentation was observed in 45.66±14.87 and 2.32±0.85% in the case and control groups, respectively ( 58 ). The side effects of PF2A are minimal and periorbital hyperpigmentation is infrequent ( 24 ).
Basic fibroblast growth factor (bFGF)-derived peptide
bFGF effect in vitiligo is through melanocyte migration ( 59 ). The efficacy of bFGF as monotherapy was assessed by Kamala Subhashini et al ( 59 ) in a comparative study in patients receiving monotherapy with either bFGF 0.1% solution or betamethasone valerate 0.1% ointment. Both groups applied their respective drug daily for 16 weeks. The bFGF group reported >75% repigmentation in 45% of patients, 50-75% repigmentation in 35 and <50% repigmentation in 20%, compared with 0, 7 and 13%, respectively, in the betamethasone group. Also, 80% of patients exhibited no response in the betamethasone group ( 59 ). Shah et al ( 60 ) performed an open-label, randomized, prospective study using bFGF related decapeptide solution in combination with tacrolimus 0.1% ointment compared with monotherapy with tacrolimus 0.1% in patients with stable vitiligo. Both treatments were applied daily. The interim analysis at 6 months was >50% repigmentation in 22.5% of patients in the combination group compared with 6.8% of patients in the monotherapy group ( 60 ). The side effects include dry skin, burning sensation and skin irritation ( 24 ).
Janus kinase (JAK) inhibitors
JAK inhibitors used in vitiligo are tofacitinib (a JAK1/3 inhibitor) and ruxolitinib (a JAK 1/2 inhibitor) ( 61 ). Their mechanism of action is through downregulation of the JAK-STAT pathway, which decreases interferon-gamma (IFN-γ), which is also associated with the cell-mediated immunity in vitiligo ( 61 ). Hamzavi et al ( 62 ) performed a phase 2 open-label trial study with 11 patients with vitiligo. Ruxolitinib 1.5% cream was applied twice daily for 20 weeks in up to 10% of the body surface area or 3.75 g per application. Results were evaluated using the vitiligo area scoring index (VASI) ( 62 ), with a statistically significant overall mean improvement of 27% in patients who completed the trial, with a better response in lesions located in the face than in other sites ( 63 ). A recent phase 2 study by Rosmarin et al ( 64 ) evaluated the efficacy and safety of ruxolitinib cream at three different concentrations (0.15, 0.5 and 1.5%) compared with placebo, for up to 52 weeks. Patients were classified into four different groups of ruxolitinib: 1.5% twice daily, 1.5% once daily, 0.5% once daily and 0.15% once daily. Efficacy was evaluated using the percentage of patients achieving ≥50% improvement in the baseline facial VASI (F-VASI50). The ruxolitinib 1.5% once and twice daily groups achieved a F-VASI50 in 50 and 45% of patients, respectively, at 24 weeks compared with 3% in the placebo group ( 64 ).
Mobasher et al ( 65 ) performed an open-label study with tofacitinib 2% cream twice daily in 16 patients with vitiligo. Notably, patients were allowed concomitant use of TCS, TCI, supplements, or phototherapy during the study. Repigmentation was observed in 81.2% of patients. In addition, >90% repigmentation was observed in four patients, 25-75% repigmentation in five patients, 5-15% repigmentation in four patients, no change in two patients, and slow progression in one patient, with more improvement in facial lesions compared with other sites ( 65 ). The side effects of JAK inhibitors include erythema, pruritus, hyperpigmentation and transient acne ( 63 , 64 ).
Systemic treatment Corticosteroids
The main objective of systemic corticosteroids (SCS) use is to suppress the immune response, and with that stabilize the disease, favoring repigmentation ( 66 ). SCS is administrated to treat rapidly progressive active vitiligo ( 18 ). Pulse therapy with SCS is preferred to decrease the potential side-effects ( 67 ). Patients undergoing therapy with SCS should be monitored for blood pressure, glucose levels, weight, waist circumference and infections, as well as an ophthalmic examination every 6-12 months ( 24 ).
Several schemes for SCS have been reported. Imamura and Tagami ( 68 ) performed a study on 17 patients with generalized vitiligo and five patients with localized vitiligo. They used several oral corticosteroids (prednisolone, betamethasone, paramethasone acetate and methylprednisolone) at different doses, which were gradually decreased to a maintenance dose; effectiveness was assessed at 6 months. The results demonstrated >75% pigmentation in at least one patch in 35% of patients with generalized vitiligo, and repigmentation became evident at 4 weeks in most cases ( 68 ). Kim et al ( 66 ) also performed a study with continuous use of SCS in patients with active vitiligo. Oral prednisolone was given the first 2 months at 0.3 mg/kg of body weight, the third month at half of the initial dose, and the fourth month at half of the previous dose. The results exhibited arrest of vitiligo progression in 87.7% of patients and repigmentation in 70.4% of patients ( 66 ). Using the same scheme, Banerjee et al ( 69 ) observed arrest in 90% of patients and repigmentation in 76% of patients with active vitiligo.
Pasricha and Khaitan ( 70 ) used a therapy based on pulses of either betamethasone or dexamethasone at 5 mg orally for 2 consecutive days every week; treatments were continued until complete repigmentation or 4 months of continuous treatment with no further improvement. The results were halt in active disease in 89% of patients with 5 mg dose after 1-3 months and repigmentation was observed in 80% of patients after 2-4 months of treatment. The extent of repigmentation was 76-99% in 15.0% of patients, 51-75% in 7.5% of patients, 26-50% in 25.0% of patients, 10-25% in 17.5% of patients and <10% in 35.0% of patients ( 70 ). Kanwar et al ( 71 ) reported a retrospective study with a cohort of 444 patients with active vitiligo, using a low-dose oral mini-pulse therapy with a dose of 2.5 mg/day on 2 consecutive days every week. Arrest in disease activity was achieved in 91.8% of patients, during follow up 12.25% of these patients experienced one or two relapses in activity ( 71 ). Another scheme of oral pulse therapy was used by Radakovic-Fijan et al ( 72 ) administering 10 mg of dexamethasone 2 consecutive days for 24 weeks. The arrest of vitiligo activity was achieved in 88% of patients with active vitiligo and most of the patients (72.4%) had no response to repigmentation ( 72 ).
Seiter et al ( 67 ) also implemented a therapy based on pulses but with an intravenous route. Methylprednisolone was intravenously administered for 3 consecutive days at a dose of 8 mg/kg of body weight. The treatment was repeated at 4 and 8 weeks if tolerated. Active vitiligo progression was halted in 85% of patients and repigmentation in 71% of patients. Patients with stable vitiligo had no change in pigmentation ( 67 ).
The side effects of SCS are weight gain, transient weakness, fatigue, insomnia, acne, agitation, menstrual disturbances, hypertension, metallic taste, pruritus, headache, flush symptoms and hypertrichosis ( 67 , 70 , 72 ).
Apremilast is a phosphodiesterase 4 inhibitor that acts by increasing intracellular cyclic adenosine monophosphate (cAMP) ( 73 ). Apremilast application in vitiligo is due to its immunomodulation properties, increasing cAMP concentration results in the decreased production of pro-inflammatory mediators (IL-23, IL-17, TNF-α and IFN-γ) and an increase in anti-inflammatory mediators, such as IL-10( 73 ). Apremilast is approved for the treatment of moderate to severe plaque psoriasis ( 73 ). The first case report of its use in vitiligo was by Huff and Gottwald ( 74 ) in a patient that failed other therapies. Apremilast (30 mg twice daily) was administered for 13 months, and two intramuscular injections of 60 mg of triamcinolone acetonide were simultaneously applied. The results were repigmentation in 60-70% of the chest and extremities ( 74 ). More recently, a pilot study performed by Majid et al ( 73 ) reported a case series of 13 patients with rapidly progressing non-segmental vitiligo treated with apremilast 30 mg twice daily for 3 months after initial titration. The patients could use topical tacrolimus on the exposed parts of the body. The results were stabilization in all patients and partial repigmentation in 61.5% of patients ( 73 ). The side effects of apremilast include headache, nausea, vomiting, weight loss, depression and abdominal pain ( 73 , 74 ).
JAK inhibitors
JAK inhibitors are not only topically used. Craiglow and King ( 75 ) reported the case of a 50-year-old female with widespread and progressive vitiligo treated with oral tofacitinib citrate 5 mg daily for 5 months, with nearly complete repigmentation of the forehead and hands, while other areas exhibited partial repigmentation ( 75 ). Improvement of vitiligo was also observed in two case reports of female patients treated with tofacitinib 5 mg twice daily for rheumatoid arthritis ( 76 , 77 ). Liu et al ( 61 ) reported a case series of 10 patients treated with tofacitinib 5-10 mg, once or twice daily for at least 3 months. During the study, suction blister sampling was performed on responding and nonresponding areas, revealing an inhibition of the autoimmune response in both. Repigmentation was observed in 50% of patients at sites of low-dose nb-UVB phototherapy or sun-exposed areas; with these findings, the authors suggest that low-level light may be required for melanocyte regeneration and repigmentation during treatment with JAK inhibitors ( 61 ). The side effects were upper respiratory infections, weight gain, arthralgia and mild elevation of lipid levels ( 61 ).
Minocycline
This oral antibiotic was studied as a therapeutic option for vitiligo after in vitro analysis suggested that minocycline protects melanocytes from oxidative stress and prevents their loss in the early stages of the disease ( 78 ). To evaluate this, Parsad and Kanwar ( 79 ) performed a study on 32 patients with gradually progressive vitiligo. Patients were treated with 100 mg of minocycline daily for 3 months, the arrest of activity was achieved in 90.6% of patients, and moderate to marked repigmentation was observed in 21.8% of patients ( 79 ). Singh et al ( 80 ) performed a randomized controlled study to evaluate the efficacy and tolerability of oral minocycline compared with oral mini-pulse corticosteroids in patients with active vitiligo. The minocycline group received 100 mg daily, while the corticosteroid group received dexamethasone 2.5 mg on 2 consecutive days every week. Efficacy was evaluated using the vitiligo disease activity score (VIDA) ( 81 ) and VASI. Although not statistically significant at the end of treatment, VIDA and VASI scores decreased in both groups with comparable results, suggesting both drugs are effective to halt vitiligo activity ( 80 ). Another prospective comparative trial by Siadat et al ( 82 ) compared minocycline 100 mg daily to nb-UVB phototherapy in patients with unstable vitiligo during 3 months of treatment. Vitiligo was active in 100% of patients at the beginning of the trial; however, this decreased to 66.1 and 23.8% in the minocycline and nb-UVB groups, respectively, following treatment ( 82 ). The side effects of minocycline are nausea, gastrointestinal complaint, headache, and hyperpigmentation of the nails, oral mucosa or skin ( 80 , 82 ).
Statins are lipid-lowering drugs. Their role in vitiligo is due to anti-inflammatory and immunomodulatory effects that cause inhibition of CD8 T-cell proliferation, chemokines, proinflammatory mediators, and the expression of proinflammatory adhesion molecules ( 83 , 84 ). In addition, inhibition of IFN-γ production decreases the expression of major histocompatibility complex II and inhibition of activated T cell ( 83-86 ). Statins also exert antioxidant properties by upregulating the transcription factor nuclear erythroid 2-related factor, resulting in a reduction of reactive oxygen species and activation of the antioxidant response in melanocytes ( 83 ). Statins increment the production of tyrosinase mRNA and increase the effect of α-melanocyte-stimulating hormone on melanocytes resulting in improved melanogenesis ( 83 ). There is only one case report of unexpected improvement of vitiligo in a patient treated with a high dose of simvastatin ( 87 ). However, studies using statins have reported no benefit in vitiligo ( 85 , 86 , 88 ).
MTX is commonly used for multiple inflammatory and autoimmune diseases ( 89 ). Most of the initial reports of improvement in vitiligo treated with MTX were in patients using it for concomitant rheumatoid arthritis or psoriatic arthritis. The dosage of MTX varied from 7.5-25.0 mg weekly, along with folic acid supplementation. The results ranged from arrest in vitiligo activity to significant repigmentation ( 89 , 90 ). In a prospective study performed by Nageswaramma et al ( 91 ), 20 patients with unstable vitiligo were treated with MTX 15 mg weekly and folic acid supplementation. The results were moderate repigmentation in 70% of patients and arrest in progression in 90% of patients. However, the efficacy of MTX in vitiligo is variable. In an uncontrolled pilot study by Alghamdi and Khurrum ( 89 ), no clinical improvement was observed with MTX 25 mg weekly for 6 months. A randomized comparative study performed by Singh et al ( 92 ) compared MTX 10 mg weekly to oral corticosteroid mini pulses with 2.5 mg of dexamethasone on 2 consecutive days for 24 weeks. Both groups had a similar reduction in the VIDA score at the end of the study. New lesions developed during treatment in 23% of patients in the MTX group and 28% of patients in the corticosteroid group ( 92 ). ElGhareeb et al ( 93 ) performed a study with 42 patients to assess the efficacy and safety of oral MTX and oral mini pulse of dexamethasone, used either alone or in combination. Patients were randomly divided into three groups. Group A received 15 mg of MTX divided into three doses, with a 12 h weekly interval. Group B received 5 mg of dexamethasone daily on 2 successive days every week. Group C received a combination of both protocols. All groups received the treatment for 3 months. The results demonstrated a significant decrease in disease extension in group C compared with the other groups ( 93 ). The side effects of MTX are hepatotoxicity, idiosyncratic pulmonary toxicity, pancytopenia, nausea, vomiting and diarrhea ( 24 ).
Azathioprine
Azathioprine is an immunosuppressant that inhibits DNA synthesis in immune effector cells ( 47 ). There is a study of its use in vitiligo performed by Madarkar et al ( 94 ), comparing azathioprine 50 mg twice daily to betamethasone 5 mg on 2 consecutive days every week for 6 months. Remarkable improvements were observed in both groups, and the authors suggest that both therapies are equally effective in vitiligo ( 94 ). Radmanesh and Saedi ( 95 ) performed a study on 60 patients randomized into two groups. The first group received azathioprine calculated at 0.60-0.75 mg/kg per day (maximum dosage 50 mg) combined with twice-weekly oral psoralen (methoxypsoralen 0.3-0.4 mg/kg) plus UVA. The second group only received oral psoralen plus UVA (PUVA). Both groups were followed for 4 months. The results exhibited earlier repigmentation at 5 oral PUVA sessions and greater repigmentation (58.4%) in the combination group compared with the oral PUVA monotherapy group at 8 sessions with 24.8% repigmentation ( 95 ). The side effects of azathioprine include myelosuppression, hepatotoxicity, gastric irritation, increased susceptibility to infections (herpes simplex and human papillomavirus) and hypersensitivity syndrome ( 24 ).
Cyclosporine
Cyclosporine is a calcineurin inhibitor with immunomodulatory action. Taneja et al ( 96 ) performed an open-label, single-arm study in 18 patients with progressive vitiligo using cyclosporine at a dose of 3 mg/kg/day, divided into two doses for 12 weeks. Progression of vitiligo was arrested in 61% of the patients and repigmentation was observed in 81% of the patients ( 96 ). A pilot study was performed by Mutalik et al ( 97 ) in patients with localized stable vitiligo treated with autologous nonculture melanocyte-keratinocyte cell transplant (NCMKT). The objective was to assess the efficacy of cyclosporine to prevent the perilesional depigmentation halo seen after NCMKT surgery. The treatment group received cyclosporine postoperatively for 3 weeks at 3 mg/kg/day followed by cyclosporine for 6 weeks at 1.5 mg/kg/day. The results were >75% repigmentation in 100% of patients in the cyclosporine group compared with 28% of patients in the group without treatment. In the latter group, most patients (52%) achieved 25-50% repigmentation. The authors concluded that postoperative cyclosporine allowed a uniform and complete repigmentation following NCMKT ( 97 ).
The side effects of cyclosporine are renal dysfunction, hypertension, gingival hyperplasia, hypercalcemia, hyperuricemia, nausea, abdominal discomfort, tremor, headache, arthralgias and hypertrichosis ( 24 , 96 ).
Mycophenolate mofetil (MM)
MM inhibits de novo purine synthesis in T and B lymphocytes through inhibition of the enzyme inosine-5' monophosphate dehydrogenase ( 98 ). Bishnoi et al ( 98 ) evaluated MM efficacy in stabilizing non-segmental vitiligo. Mofetil mycophenolate up to 1 g twice daily was compared with dexamethasone 2.5 mg on 2 successive days weekly for 180 days. The arrest of disease activity was achieved in 80% of patients in the corticosteroid group compared with 72% of patients in the MM group. The most common side effects in the MM group were nausea and diarrhea. Treatment was discontinued in two patients in the MM group due to leucopenia and transaminitis, respectively ( 98 ).
3. Physical therapy
Phototherapy narrow-band uvb.
Ultraviolet radiation, more markedly UVB (wavelength of 280-320 nm) than UVA (wavelength of 320-400 nm), has several systemic effects, such as activation of the central hypothalamic-pituitary-adrenal axis, activation of the proopiomelanocortin pathway in the arcuate nucleus of the hypothalamus, immunosuppressor and opioidogenic effects ( 99-101 ). These effects are through upregulation of the local neuroendocrine axes ( 99-101 ). The mechanism of action of nb-UVB (wavelength of 311 nm) phototherapy in vitiligo is through immunosuppression, induction of melanocyte differentiation, melanin production and migration of melanocytes from perilesional skin ( 22 , 102 ). Total body nb-UVB is recommended for widespread vitiligo >15-20% of the body surface area and for rapidly progressive vitiligo ( 18 ).
Regarding phototherapy with nb-UVB, The Vitiligo Working Group recommends three sessions every week as an optimal frequency of administration ( 103 ). Regardless of the patients phototype, the initial dose is 200 mJ/cm 2 ( 103 ). Following a phototherapy session, pink erythema lasting less than 24 h is desired; if this does not occur, the dose can be increased by 10-20% each session until pink erythema is achieved ( 103 ). The same dose is held until erythema disappears, then once again the dosage is incremented ( 103 ). The response to treatment should be assessed after 18-36 sessions and because of the existence of slow responders, at least 72 sessions are recommended before discontinuing therapy ( 103 ). There is no maximum number of sessions in patients with phototypes IV-VI, and no recommendation was made for other phototypes ( 103 ). The maximum acceptable dose is 1,500 mJ/cm 2 for the face and 3,000 mJ/cm 2 for the body ( 103 ).
Treatment response to monotherapy with nb-UVB phototherapy was evaluated in a systematic review and meta-analysis by Bae et al ( 104 ), where ≥25% repigmentation was achieved in 62.1% of patients at 3 months, 74.2% of patients at 6 months and 75% of patients at 12 months. In addition, >75% repigmentation was observed in 13, 19.2 and 35.7% of patients at 3, 6 and 12 months, respectively. According to the site, the best response was observed on the face and neck, followed by the trunk, extremities, and lastly the hands and feet ( 104 ).
In a meta-analysis performed by Lee et al ( 28 ), a combination of nb-UVB or excimer laser (EL) with TCI exhibited a mild response (≥25% repigmentation) in 89.5% of patients, a moderate response (≥50% repigmentation) in 72.9% of patients and a marked response (≥75% repigmentation) in 47.5% of patients. The authors suggest that this combination has a synergistic effect ( 28 ). In another meta-analysis by Li et al ( 105 ), the benefit of nb-UVB combined with topical D3A, or nb-UVB combined with TCI compared to nb-UVB alone was investigated, where no significant superior effect was observed in the combined therapy group ( 105 ). However, this meta-analysis revealed that combining TCI and nb-UVB may improve the clinical response in the face and neck ( 105 ). The combination of nb-UVB with systemic therapies includes the study by Tovar-Garza et al ( 106 ), evaluating the efficacy of oral mini-pulse of dexamethasone 4 mg on 2 consecutive days weekly with nb-UVB and topical clobetasol compared with nb-UVB and topical clobetasol. A total of 92% of patients achieved disease arrest with dexamethasone, nb-UVB and clobetasol, compared with 53% of patients with nb-UVB and clobetasol ( 106 ). In a meta-analysis, Phan et al ( 107 ) studied the effectiveness of JAK inhibitors used with UVB phototherapy; improved efficacy was reported with the combination of both treatments. A good response was observed in 11.1% of patients receiving JAK inhibitor alone, compared with 88.9% of patients with concurrent phototherapy ( 107 ). Khemis et al ( 108 ) evaluated efficacy using apremilast 30 mg twice daily in combination with nb-UVB compared with placebo and nb-UVB in 80 patients. Apremilast combined with nb-UVB did not exhibit an additional benefit in repigmentation compared with nb-UVB alone ( 108 ). Lim et al ( 109 ) performed a randomized control trial of nb-UVB alone compared to afamelanotide 16 mg subcutaneously applied monthly for 4 months along with nb-UVB. The results demonstrated repigmentation response of 48.6% in the combination therapy group compared with 33.26% in the nb-UVB monotherapy group at day 168( 109 ). In a meta-analysis, Chang et al ( 110 ) evaluated the efficacy of combining nb-UVB with fractional CO 2 laser, but no additional benefit in repigmentation was observed. The adverse reactions of nb-UVB include burning, erythema, pruritus, xerosis, photoaging and photodamage ( 47 ).
PUVA radiation (wavelength of 320-340 nm) induces melanogenesis by immunosuppression and promoting a favorable milieu for the growth of melanocytes ( 18 , 111 ). This treatment, considered a second-line therapy, requires topical application or ingestion of psoralen and exposure to UVA ( 18 ). The oral psoralens are given 1-3 h before the UVA radiation, some examples are 8-methoxypsoralen (0.6-0.8 mg/kg), 5-methoxypsoralen (1.2-1.8 mg/kg) and trimethylpsoralen (0.6 mg/kg) ( 18 ). Topical PUVA uses the psoralen as a cream or ointment (8-methoxypsoralen 0.001%) and is applied 30 min before UVA radiation. The advantages of topical PUVA include fewer treatments, smaller cumulative UVA doses, less systemic and ocular phototoxicity ( 18 ). PUVA therapy should be given for at least 6 months before considering the patient non-responsive and for a maximal response, continuous therapy is required for up to 1-2 years ( 18 ). Efficacy of PUVA phototherapy reported by Bae et al ( 104 ) in a systematic review and meta-analysis was ≥25% repigmentation in 51.4% of patients at 6 months and 61.6% of patients at 12 months; ≥75% repigmentation in 8.5% of patients at 6 months and 13.6% of patients at 1 year ( 104 ). Parsad et al ( 112 ) compared treatment with nb-UVB to PUVA and marked to complete repigmentation was observed in 41.9% of patients with nb-UVB compared with 23.6% of patients with PUVA. Bhatnagar et al ( 113 ) compared treatment for induction of stability with nb-UVB or PUVA; vitiligo was arrested in 80% of patients using nb-UVB and only 40% of patients with PUVA. The side effects of PUVA are phototoxicity, headache, dizziness, depression, insomnia, hyperactivity, bronchoconstriction, tachycardia, ankle edema, nausea, vomiting, pruritus, xerosis, photoaging, hyperpigmentation, hypertrichosis, increased risk of non-melanoma skin cancer, and liver and eye toxicity ( 114 , 115 ).
Laser therapy EL
Excimer light (wavelength of 308 nm) in excimer lamps and EL are useful for targeted phototherapy ( 116 ). The mechanism of action is a direct cytotoxic effect on T cells, and stimulation of melanocyte migration and proliferation in hair follicles ( 117 ). In a systematic review and meta-analysis by Lopes et al ( 118 ) no significant difference in efficacy was observed between excimer lamps, EL and nb-UVB in achieving ≥50 and ≥75% repigmentation. In a systematic review and meta-analysis, Bae et al ( 119 ) reported that the combination of excimer laser/EL and TCI were more effective than monotherapy with EL, also the treatment failure rate was reduced with the combination therapy ( 119 ). The side effects of EL are pruritus, burning sensation and dryness ( 118 ).
Combined Fraxel Erbium and UVA1 laser
Lotti et al ( 120 ) investigated a combined laser and topical latanoprost approach in 30 adults with vitiligo, with active or stable localized disease. Initially, the vitiliginous lesions were treated with a single passage of Fraxel Herbium laser, with a wavelength of 1,540 nm and an energy level of 1,800 mJ/P. Immediately after obtaining columnar areas of epidermal ablation, they applied latanoprost 0.005% solution onto each skin lesion. After 24 h, the skin lesions were irradiated with a UVA1 laser (355 nm) for 20 min. The treatment was repeated every 21 days, for 9 months. A total of 27 patients (90%) obtained >75% repigmentation, while three patients (10%) achieved 50-75% repigmentation ( 120 ).
4. Depigmentation therapies
These therapies are generally recommended for extensive and refractory vitiligo, when >50% of the body surface is affected or if cosmetically sensitive areas are the major component involved ( 18 , 19 ). Monobenzyl ether of hydroquinone (MBEH) 10% is applied topically daily the first month, then MBEH 20% is applied daily for 1 month, and after that twice daily. The concentration can be increased to 30-40% if the areas are unresponsive, if tolerated. In general, patients present depigmentation after 3-6 months in areas distal to the application ( 19 , 121 ). Other treatment options are 4-methoxyphenol, 88% phenol solution, laser and cryotherapy ( 121 ).
5. Conclusions
Vitiligo treatment can sometimes be frustrating due to the inconsistency in clinical improvement and its relapsing feature. Therapy should be individualized according to the type of vitiligo, presence of activity and the side-effect profile of the drug used. All therapies for vitiligo are limited, no known treatment can consistently produce repigmentation in all patients. Further basic and clinical investigation is required to better understand the pathogenesis of vitiligo and provide new targets for therapy. There are multiple upcoming therapies, and most information of these new treatments are case reports or series. However, more randomized controlled trials are required to better evaluate their efficacy.
Acknowledgements
Funding statement.
Funding: No funding was received.
Availability of data and materials
Authors' contributions.
JOC, DEKL, NAZS, SLSF, CNSD, MASS, HGMR and OTVM performed the literature review and collected the data. JOC, DEKL, NAZS, SLSF, CNSD, MASS, HGMR and OTVM drafted the initial manuscript. JOC, DEKL, NAZS, SLSF, CNSD, MASS, HGMR, OTVM, UW and TL improved the manuscript. JOC, DEKL, NAZS, SLSF, CNSD, MASS, HGMR, OTVM, UW and TL critically revised the manuscript for important intellectual content. Data sharing is not applicable. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Patient consent for publication, competing interests.
The authors declare that they have no competing interests.

FDA Approves New Vitiligo Treatment, Ruxolitinib (Opzelura)
The JAK inhibitor cream is the first medication that can restore pigment in people with this autoimmune disease.
On July 18, the U.S. Food and Drug Administration (FDA) approved ruxolitinib ( Opzelura ) cream 1.5 percent as a treatment for the most common form of vitiligo, according to a statement by Incyte, the manufacturer of the drug.
Vitiligo is a chronic autoimmune condition that causes patches of skin to lose pigment and turn milky white. The most prevalent form is nonsegmental (also known as generalized) vitiligo, in which white patches appear symmetrically on both sides of the body, such as on both hands or both knees, often covering large areas.
Ruxolitinib is the first medication that can restore pigment in patients with nonsegmental vitiligo. The FDA approved Incyte’s ruxolitinib cream for adults and children ages 12 and up.
“This approval is monumental,” says Daniel Gutierrez, MD , assistant professor of dermatology at NYU Grossman School of Medicine and dermatologist at NYU Langone Health in New York City, who was not involved in the drug development. “With Opzelura, we will have an FDA-approved pharmaceutical treatment option that can actually bring back color in patients who have vitiligo,” says Dr. Gutierrez.
He adds that prior to ruxolitinib, the only FDA-approved medication for vitiligo was monobenzyl ether of hydroquinone, a topical drug that removes pigment from skin to even out tones.
What Is Vitiligo?
Researchers estimate that between 1.9 and 2.8 million adults in the United States have vitiligo, with perhaps 40 percent of adults with vitiligo going undiagnosed.
Vitiligo causes immune cells to destroy melanocytes, the skin cells that produce pigment, according to the National Institute of Arthritis and Musculoskeletal and Skin Diseases . “This makes vitiligo much more noticeable in patients of color — people whose skin is much more richly pigmented — because there is going to be much more of a contrast between the unaffected skin and the skin affected by the vitiligo,” says Gutierrez.
Vitiligo can occur at any age, but most people experience the initial symptoms before age 30.
About 50 Percent of People Using Ruxolitinib Had Significant Repigmentation After One Year
Ruxolitinib belongs to a class of drugs called Janus kinase (JAK) inhibitors. While doctors prescribe oral JAK inhibitors for diseases such as rheumatoid arthritis, ruxolitinib is the only topical JAK inhibitor approved in the United States.
The FDA previously approved ruxolitinib for mild to moderate atopic dermatitis (eczema) , in the fall of 2021.
JAK inhibitors work by decreasing the activity of the immune system, blocking certain enzymes that cause inflammation.
Patients using ruxolitinib apply the cream twice daily to the affected areas, covering up to 10 percent of their body’s surface area. It may take 24 weeks or more for people with vitiligo to see satisfactory results, according to Incyte.
The FDA based its approval on data from a clinical trial program that compared ruxolitinib to a placebo cream in more than 600 people (age 12 and older) with nonsegmental vitiligo. Investigators used the Vitiligo Area Scoring Index (VASI), a tool used to gauge disease severity and to measure improvements in face and body repigmentation.
In the two trials, by week 24 approximately 30 percent of people treated with ruxolitinib experienced significant improvements (at least 75 percent) as measured by VASI, which was the goal of the study. At one year, about 50 percent of those using the medication achieved that level of repigmentation.
“People using Opzelura had much more improvement in their vitiligo — very meaningful — compared to the placebo,” says Gutierrez.
The most common side effects seen in the trials were application-site acne, redness and itchiness, pharynx and nasal cavity inflammation, headache, urinary tract infection, and fever.
Ruxolitinib Comes With a Black Box Warning
The FDA added a black box warning to ruxolitinib, based on data showing that people taking oral JAK inhibitors faced a small increased risk of serious infections, major heart issues, clotting (thrombosis), cancer, and even death.
“However, in the clinical trials for people using ruxolitinib as a topical cream, the concentrations of the drug found in the blood were observed to be much lower compared to people who take ruxolitinib orally,” says Gutierrez. The same risks were not observed in the ruxolitinib trials, but the FDA is taking a “better safe than sorry” approach by including a warning on the box, he adds.
A conversation with your healthcare provider is the best way to determine whether the benefits of ruxolitinib outweigh the potential risks, as well as the need for any baseline and/or ongoing monitoring.
Patients Can Use Ruxolitinib on Their Face
Although dermatologists sometimes prescribe topical steroids off-label for vitiligo, there are risks when applying these medications to the face — the area where loss of pigment can impact appearance the most, says Gutierrez.
When used on the face, topical steroids can cause an acne-like rash that can persist for many months, called perioral dermatitis . Plus, “they can cause atrophy or dispigmentation, meaning you can have skin color changes. They can also thin the skin, cause stretch marks, and cause the growth of small blood vessels in the area,” Gutierrez says.
Ruxolitinib does not pose these risks, notes Gutierrez.
FDA Approval Means Better Access to Vitiligo Treatment
The FDA’s approval of ruxolitinib will definitely improve access to the drug by validating it as medically necessary. “Because vitiligo just creates a color change in the skin — there’s no itching or dermatitis under normal circumstances — sometimes it’s considered a cosmetic condition, meaning it’s not medically necessary to treat,” Gutierrez says. As a result, some insurers have declined to cover vitiligo treatments , according to the Vitiligo Research Foundation .
“However, this condition can dramatically impact how a patient sees themselves and how they present to the world. Vitiligo can cause significant psychological distress and negatively impact quality of life,” says Gutierrez.
“Vitiligo disproportionately impacts patients of color,” he adds. “This approval is an important step in improving a health disparity that does exist, and hopefully there will be more treatment options for vitiligo in the pipeline.”
How Much Will Ruxolitinib Cost?
The current Wholesale Acquisition Cost pricing is $1,950 for a 60 gram tube of Opzelura, according to Gabriella Greig, a spokesperson for Incyte. The actual cost to the consumer will vary depending on insurance coverage and how much of the cream is required for treatment.
“Incyte is committed to working with insurance providers in the U.S. to ensure eligible patients who can benefit from Incyte’s products have access to them,” says Greig. The company offers a copay savings card on its website for people with commercial insurance.
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- News & Events for Human Drugs
FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older
FDA has approved Opzelura (ruxolitinib) cream for the treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older. Opzelura is a topical Janus kinase (JAK) inhibitor currently approved for the topical short-term and non-continuous chronic treatment of mild to moderate atopic dermatitis in non-immunocompromised patients 12 years of age and older, whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
Opzelura is the first FDA-approved pharmacologic treatment to address repigmentation in vitiligo patients. Opzelura is applied twice a day to affected areas of up to 10% of the body’s surface area. Satisfactory patient response may require treatment with Opzelura for more than 24 weeks.
Disease or Condition
Nonsegmental vitiligo is the most common form of vitiligo. The condition involves loss of pigment (depigmentation) in patches of skin. Depigmentation may occur on the face, neck, and scalp, and around body openings such as the mouth and genitals, as well as areas that tend to experience rubbing or impact, such as the hands and arms.
Vitiligo is considered to be an autoimmune disorder. In people with vitiligo, the immune system appears to attack the pigment cells (melanocytes) in the skin. Many people with vitiligo are also affected by at least one other autoimmune disorder.
Effectiveness
Safety and effectiveness of Opzelura were demonstrated in two clinical trials, NCT04052425 and NCT04057573 . In both trials, subjects with nonsegmental vitiligo were randomized to treatment with Opzelura or placebo cream twice daily for 24 weeks, followed by an additional 28 weeks of treatment with Opzelura for all subjects. At the end of the 24-week treatment period, 30% of Opzelura patients had at least 75% improvement in the facial Vitiligo Area Scoring Index, compared with 10% of placebo patients.
Safety Information
The most common adverse reactions associated with Opzelura are application site acne, application site itching, common cold, headache, urinary tract infection, application site redness, and fever. Use of Opzelura in combination with therapeutic biologics, other JAK inhibitors, or potent immunosuppressants such as azathioprine or cyclosporine is not recommended.
Serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis have been observed in patients treated with JAK inhibitors for inflammatory conditions.
See full prescribing information for additional information on risks associated with Opzelura.
Designation
Opzelura received priority review for this indication.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 30 June 2020
- Correction 06 July 2020
Temprian Therapeutics: developing a gene-based treatment for vitiligo
- Charles Schmidt 0
Charles Schmidt is a freelance writer in Portland, Maine.
You can also search for this author in PubMed Google Scholar
Temprian Therapeutics is a spin-off from Northwestern University in Chicago, Illinois.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
185,98 € per year
only 3,65 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41586-020-01808-5
This article is part of Nature Outlook: The Spinoff Prize 2020 , an editorially independent supplement produced with the financial support of third parties. About this content .
Updates & Corrections
Correction 06 July 2020 : An earlier version of this profile gave the wrong specialty for Caroline Le Poole and the wrong campus for Northwestern University.
Mosenson, J. A. et al. Sci. Transl. Med. 5 , 174ra28 (2013).
Article PubMed Google Scholar
Henning, S. W. et al. J. Invest. Dermatol. 138 , 2531–2539 (2018).
Download references
Related Articles

- Biotechnology
- Drug discovery
- Therapeutics
Vaccine-enhancing plant extract could be mass produced in yeast
News & Views 08 MAY 24

Computationally restoring the potency of a clinical antibody against Omicron
Article 08 MAY 24
Complete biosynthesis of QS-21 in engineered yeast
How to kill the ‘zombie’ cells that make you age
News Feature 15 MAY 24
Dual-action obesity drug rewires brain circuits for appetite
News & Views 15 MAY 24

Experimental obesity drug packs double punch to reduce weight
News 15 MAY 24
Lab-grown sperm and eggs: ‘epigenetic’ reset in human cells paves the way
News 21 MAY 24
In vitro reconstitution of epigenetic reprogramming in the human germ line
Article 20 MAY 24
Airway hillocks are injury-resistant reservoirs of unique plastic stem cells
Article 01 MAY 24
Postdoctoral Fellow
New Orleans, Louisiana
Tulane University School of Medicine
Postdoctoral Associate - Immunology
Houston, Texas (US)
Baylor College of Medicine (BCM)
Postdoctoral Associate
Vice president, nature communications portfolio.
This is an exciting opportunity to play a key leadership role in the market-leading journal Nature Portfolio and help drive its overall contribution.
New York City, New York (US), Berlin, or Heidelberg
Springer Nature Ltd
Senior Postdoctoral Research Fellow
Senior Postdoctoral Research Fellow required to lead exciting projects in Cancer Cell Cycle Biology and Cancer Epigenetics.
Melbourne University, Melbourne (AU)
University of Melbourne & Peter MacCallum Cancer Centre
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Updates and new medical treatments for vitiligo (Review)
Affiliations.
- 1 Department of Dermatology, Faculty of Medicine and University Hospital 'Dr. José Eleuterio González', Universidad Autónoma de Nuevo León, Monterrey, Nuevo León 64460, México.
- 2 Department of Biochemistry and Molecular Medicine, Faculty of Medicine and University Hospital 'Dr. José Eleuterio González', Universidad Autónoma de Nuevo León, Monterrey, Nuevo León 64460, México.
- 3 Department of Research, Faculty of Medicine Saltillo Unit, Universidad Autónoma de Coahuila, Saltillo 25000, México.
- 4 Department of Dermatology and Allergology and Skin Cancer Center, Städtisches Klinikum Dresden, D-01067 Dresden, Germany.
- 5 Department of Dermatology and Venereology, University of Rome G. Marconi, I-00193 Rome, Italy.
- 6 Department of Dermatology and Communicable Diseases, First Medical State University of Moscow I. M. Sechenev Ministry of Health, Moscow 119991, Russia.
- PMID: 34093753
- PMCID: PMC8170669
- DOI: 10.3892/etm.2021.10229
Vitiligo is a multifactorial disease characterized by the loss of skin pigment, which results in achromic macules and patches. There are currently several medical treatments available, which aim to arrest progression and induce skin repigmentation. These treatments alone or combined have exhibited varying degrees of pigmentation, and the majority are safe and effective. All therapies for vitiligo are limited, and no known treatment can consistently produce repigmentation in all patients. Individualized treatment is appropriate according to the location, clinical presentation and the presence of disease activity. The present review summarizes the medical treatments available for vitiligo: Systemic and topic pharmacological therapies, physical and depigmentation treatments. Several treatments are still underway and have not yet been approved. However, due to the promising preliminary results, these are also mentioned in the present review.
Keywords: combined modality therapy; immunosuppressive agents; phototherapy; skin lightening preparations; therapy; vitiligo.
Copyright: © Kubelis-López et al.
Publication types
Grants and funding.
Vitiligo Research

Vitiligo Advancements and Discoveries
There has been an increase in the amount of research being undertaken in vitiligo over recent years and dermatologists have an improved understanding of the natural history and different types of the condition. Here you will find a brief summary of research into several areas, with references to the original research articles, for those of you who wish to follow these up.
Researchers are looking at:
- The effectiveness of existing treatments;
- Possible causes of vitiligo;
- How the condition develops;
- Segmental vitiligo;
- The association of vitiligo with other conditions;
- The psychological effects of vitiligo.
It is hoped that the improvements in scientific understanding will in future lead to more effective treatments for vitiligo.
Are psychological interventions important for vitiligo patients?
Yes, a survey of vitiligo patients and healthcare professionals found that psychological interventions are important for managing the impact of vitiligo on patients’ lives.
A survey was conducted to identify psychological interventions for vitiligo. The survey was funded by the UK Dermatology Clinical Trials Network and involved patients and health professionals. The survey recorded personal data and focused on the effect of vitiligo on normal life, as well as the most difficult problems faced by patients and which approaches would be helpful.
- Patients with vitiligo reported key issues such as acceptance of their disease, the duration of the disease and managing embarrassment.
- Other concerns were participating in sporting activities and exposure to sunlight.
- Interventions considered useful by professionals to address these issues included cognitive behavioural therapy (CBT), acceptance and commitment therapy (ACT), and mindfulness therapy.
Psychological interventions for vitiligo are a research priority, but there is little published on appropriate therapy from both patient and clinician perspectives. The unique survey referenced here is therefore of value to the future treatment of vitiligo patients.
Will piperine treat vitiligo?
Although promising results have been seen in cell and animal studies, and early work toward clinical trials in humans is underway, the effectiveness and safety of piperine as a treatment for vitiligo in humans has yet to be fully established.
Ongoing research is being conducted, but funding is needed to support further studies. Therefore, it is unclear at this time whether piperine will ultimately prove to be an effective treatment for vitiligo.
Amala Soumyanath led the research that discovered piperine as a potential treatment for vitiligo. In her own words, she shares the story of her research journey and provides an update on the latest developments. Become a member today and access more resources and stories like this.
How was piperine discovered as a potential treatment for vitiligo?
Piperine was discovered as a potential treatment for vitiligo through research and testing of herbal extracts , where a water extract of black pepper was found to stimulate melanocyte growth and dendrite formation. The compound responsible for this effect was identified as piperine, which could be developed for use in treating vitiligo.
How was piperine validated as a “lead” molecule for the treatment of vitiligo?
Piperine was validated as a “lead” molecule for the treatment of vitiligo through studies conducted at King’s College London. They tested extracts from various herbs and found that piperine from black pepper was the most effective at stimulating the growth of pigment cells. Further studies were conducted to make chemical variations (analogs) of piperine and two of these analogs showed good activity.
All three compounds, piperine, THP, and RCHP, were found to stimulate the growth of pigment cells in mice, causing their skin to visibly darken. These studies allowed the researchers to secure international patents for the use of piperine and its analogs to treat vitiligo.
How was piperine’s effectiveness and safety in treating vitiligo validated?
Piperine’s effectiveness and safety in treating vitiligo were validated through a detailed plan for a clinical trial of piperine in patients with vitiligo. Prior to the clinical study, experiments were conducted to investigate the effects of piperine on human pigment cells, including melanocytes from the uninvolved skin of a vitiligo patient.
Piperine was found to stimulate the replication of human melanocytes in culture and when grown within a reconstructed skin model. Colleagues in OHSU’s Biomedical Engineering and Dermatology departments used innovative optical methods to image pigmentation and melanocytes in the skin models.
What were its effects on human pigment cells and melanoma?
Experiments funded by AdPharma, Inc. showed that piperine has an inhibitory effect on cultured melanoma cells and prevents melanoma cell growth in a reconstructed full-skin model. To further study this aspect, the HGF mouse model of melanoma was introduced to OHSU.
The effects of piperine in this model are currently being studied with pilot funding from the Department of Dermatology’s Jesse Ettelson Fund for the Advancement of Dermatology Research. These ongoing studies are essential to establish the safety of piperine.
What is the status of piperine for treating vitiligo in humans?
In 2013, the appointment of Professor Sancy Leachman, a dermatologist and expert in pigment cell biology, gave a significant boost to the project of developing piperine as a new treatment for vitiligo. Dr. Pamela Cassidy and Eric Smith also joined the team, and a core group is working to bring this discovery to the clinic. The current status of piperine as a treatment for vitiligo in humans remains unclear.
Amala Soumyanath’s Personal and Professional Journey to Develop a Treatment for Vitiligo
Amala Soumyanath’s journey began when she received a phone call from Maxine Whitton, an MBE-awarded vitiligo service provider, sparking an idea to develop piperine as a potential treatment for vitiligo. With dedication and persistence, Amala’s knowledge of drug development processes led her to develop piperine to the point of being tested in humans.
Her personal experience with vitiligo, developing noticeable patches in 2006, fueled her drive to find a treatment for this difficult condition. Alongside a team of talented researchers at OHSU, they continue to evaluate piperine’s efficacy and understand its effects on melanocytes, with Dr. Sancy Leachman leading the project and Amala as the ongoing champion.
Is piperine the new treatment for vitiligo?
Amala Soumyanath and her team at OHSU are developing piperine as a potential treatment for vitiligo. A “proof of concept” human study demonstrating piperine’s safety and efficacy could attract large pharmaceutical companies to move forward with the project, but funding is needed. Donations of any size can make a real difference to the project’s progress. While piperine shows promise as a treatment for vitiligo, further research is required before it can be established as a new treatment.
How can you help?
The team at OHSU is reaching out to the general community for funding to support their ongoing studies on piperine for vitiligo at both the clinical and basic science levels. Donations of any size from those affected by vitiligo or anyone interested in supporting the research can be made online to the Vitiligo Research Fund .
Read Amala Soumyanath’s full story here .
What impact does vitiligo have on a person’s quality of life?
Vitiligo can have a moderate to severe impact on a person’s quality of life, including depression, stigmatization, and impaired sex lives. The location of the lesions and cultural values related to appearance and status may also play a role. Research has found that:
- Quality of life is closely related to the patients’ apprehensions about their disease, psychosocial adjustment, and psychiatric morbidity.
- British Asian women with vitiligo often feel visibly different and have experienced stigmatization due to cultural values related to appearance, status, and myths linked to the cause of the condition.
- Quality of life impairment in women affected with vitiligo assessed using the DLQI was equal to the impairment caused by psoriasis.
- Vitiligo had a negative impact on the sex lives of women with vitiligo.
To learn more about the impact vitiligo has on an individual and their quality of life you can find the full articles below:
- Quality of life of patients with vitiligo attending the Regional Dermatology Training Center in Northern Tanzania
- Depression, anxiety and health‐related quality of life in children and adolescents with vitiligo
- Quality of life and psychological adaptation of Korean adolescents with vitiligo
- Vitiligo linked to stigmatization in British South Asian women: a qualitative study of the experiences of living with vitiligo
- Effect of vitiligo on self‐reported health‐related quality of life
- The Problems in Sexual Functions of Vitiligo and Chronic Urticaria Patients
Can thyroid issues cause vitiligo?
There is evidence to suggest that thyroid issues can be associated with vitiligo. The frequency of thyroid disease in vitiligo patients is higher compared to the general population, and it is recommended that all patients with vitiligo have their thyroid function checked.
In the course of their clinical work, dermatologists discovered:
- the frequency of thyroid disease in vitiligo patients was 15.1%,
- autoimmune thyroid disease was 14.3%
- and the presence of thyroid-specific autoantibodies was 20.8%.
To learn more about the association between thyroid issues and vitiligo you can find the full article here .
Does vitiligo increase your risk of skin cancer?
Although patients with vitiligo have a tendency to burn in the sun, a survey conducted by a team from The Netherlands found that patients with vitiligo have a threefold lower probability of developing malignant melanoma and non-melanoma skin cancer. The reasons for this are not yet fully understood.
Read the entire survey here and learn more about this on BBC iPlayer .
What is segmental vitiligo?
Segmental vitiligo is a form of vitiligo that presents with patches distributed unilaterally and locally . It has been compared with a possible mosaic or neurogenic background, but its distribution pattern is not entirely similar to any other skin condition. Cutaneous mosaicism may be involved in segmental vitiligo. However, the underlying mechanism of segmental vitiligo is still unknown.
Learn more about the distribution pattern of segmental vitiligo here .
How is vitiligo classified?
Segmental vitiligo is classified separately from all other forms of vitiligo, with the term ‘vitiligo’ being used as an umbrella term for all non-segmental forms, including mixed vitiligo in which segmental and non-segmental vitiligo are combined and which is considered a subgroup of vitiligo.
Experts recommend that disease stability is best assessed based on the stability of individual lesions rather than the overall stability of the condition.
Read the entire article about the classification of vitiligo here .
What is the Koebner phenomenon in relation to vitiligo and how can it be assessed?
The Koebner phenomenon (KP) refers to the development of vitiligo within an area of skin that has been damaged by localised, often mild trauma (e.g. an injury). Dr. N van Geel and colleagues of Ghent have looked at this phenomenon. They developed a new assessment method for KP, taking into account both the history and clinical examination of people with vitiligo; this seems to be a useful and valuable tool for assessing KP in daily practice.
The results support the hypothesis that KP may be used to assess and predict the course of vitiligo (access the entire article here ).
What is the relationship between Halo Nevi and vitiligo?
Halo nevi are common moles with a white ring around them, showing the sort of pigment loss that is seen in vitiligo. They may represent a distinct condition, but in some cases, they may be an initiating factor in the development of vitiligo, according to research by Dr. van Geel and researchers (access the entire article here ).
What are the mechanisms of pathogenesis of vitiligo?
The pathogenesis of vitiligo is believed to involve oxidative stress, which leads to an imbalance between reactive oxygen species (ROS) and the body’s ability to detoxify them. (Access the entire article here ).
According to research (access the entire article here ):
- Mitochondria within melanocytes and blood cells generate reactive oxygen species (ROS) that may be relevant in vitiligo development.
- Modification of membrane lipid components in vitiligo cells may cause mitochondrial impairment and the production of intracellular ROS after exposure to mild stress.
- Autoimmunity plays a role in the pathogenesis of vitiligo, with tyrosine hydroxylase identified as an autoantigen target.
- Tyrosine hydroxylase antibodies are more frequent in people with active non-segmental vitiligo (23%) but not in the segmental type.
How does vitiligo affect the layers of skin?
Genetic studies show that susceptibility to vitiligo is related to proteins or parts of the pigment cell involved in the immune system (access the entire article here ). Research from Dalian, China, reveals that alterations in skin biophysical properties, such as stratum corneum (SC) hydration, melanin and erythema index, are lower in vitiligo-affected skin (access the entire article here ).
However, no difference in skin surface acidity was observed, and the SC integrity was similar in involved and uninvolved areas. Barrier recovery in vitiligo-involved areas was significantly delayed compared to uninvolved areas.
What are the systemic treatment options for vitiligo?
It is difficult to find a systemic treatment for vitiligo at the moment (one that affects the whole body). Some of the commonly used systemic treatments for vitiligo include:
- Ginkgo biloba – taking 60 mg of Ginkgo biloba BID was associated with a significant improvement in total Vitiligo Area Scoring Index (VASI) and Vitiligo European Task Force (VETF) scores, but more clinical trials are needed (access the entire article here ).
- Piperine – has been suggested as a potential treatment for vitiligo, yet only a few studies have been conducted and most have been on animals (access the entire article here ).
- Cosmetic camouflage – not only conceals the depigmented patches but has been shown to improve the quality of life in patients (access the entire article here )
What are the surgical treatment options for vitiligo?
Surgical treatment options for vitiligo involve transplanting melanocytes from normally pigmented skin to the depigmented areas and are only suitable for patients with stable vitiligo. It has been proven that suspending melanocytes in the patient’s own serum (plasma in the blood) can improve the effectiveness of the transplant (access the entire article here ).
Another new procedure called ReCell involves taking a sample of normal skin, separating out the skin cells, and spraying them onto the vitiligo patches (access the entire article here). Studies comparing Recell with conventional transplantation have shown varying degrees of repigmentation, but it is not widely available in the UK (access the entire article here ).
What are effective topical treatments and light therapies for vitiligo?
Creams or ointments, known as topical immunomodulators, are usually the first line of treatment for vitiligo. Topical tacrolimus and pimecrolimus have been found to be effective for localised vitiligo. Targeted narrow-band ultraviolet B (UVB) light treatment using the Excimer laser is also known to be effective, but not widely available. Other lasers such as the Q-switched ruby laser have been shown to induce depigmentation more quickly, but with more discomfort.
To learn more about effective topical treatments and light therapies for vitiligo you can find the full articles below:
- Comparative Therapeutic Evaluation of Different Topicals and Narrow Band Ultraviolet B Therapy
- Pimecrolimus: a new choice in the treatment of vitiligo?
- Laser for treating vitiligo: a randomized study
- Treatment of vitiligo: advantages and disadvantages, indications for use and outcomes
Table of contents
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
July 21, 2022
FDA approves first topical treatment for vitiligo

The U.S. Food and Drug Administration has approved Opzelura (ruxolitinib) as the first topical treatment for vitiligo.
The 1.5 percent cream is approved for continuous topical use twice daily to affected areas of up to 10 percent of body surface area in patients aged 12 years and older. More than 24 weeks of treatment may be needed for satisfactory patient response.
The approval was based on results from the TRuE-V clinical trials , in which more than 600 patients were randomly assigned to Opzelura or placebo. At week 24, 30 percent of patients treated with Opzelura achieved ≥75 percent improvement from baseline in the facial Vitiligo Area Scoring Index (F-VASI75) versus 8 to 13 percent of patients treated with placebo. Approximately half of Opzelura-treated patients achieved F-VASI75 at week 52.
"There have been no FDA-approved therapies available to date and the approval of Opzelura therefore marks a significant milestone ," David Rosmarin, M.D., from Tufts Medical Center in Boston, said in a company press release. "I welcome a medical treatment that helps my patients with nonsegmental vitiligo who are interested in potentially reversing the depigmentation caused by their disease."
Approval was granted to Incyte.
Copyright © 2022 HealthDay . All rights reserved.
Explore further
Feedback to editors

Regular fish oil supplement use might increase first-time heart disease and stroke risk

Pedestrians may be twice as likely to be hit by electric/hybrid cars as petrol/diesel ones
Study finds jaboticaba peel reduces inflammation and controls blood sugar in people with metabolic syndrome
2 hours ago

Study reveals how extremely rare immune cells predict how well treatments work for recurrent hives
4 hours ago

Researchers find connection between PFAS exposure in men and the health of their offspring

Researchers develop new tool for better classification of inherited disease-causing variants

Specialized weight navigation program shows higher use of evidence-based treatments, more weight lost than usual care

Exercise bouts could improve efficacy of cancer drug
Drug-like inhibitor shows promise in preventing flu

Scientists discover a novel way of activating muscle cells' natural defenses against cancer using magnetic pulses
Related stories.

Topical cream shows promise in treatment of skin pigmentation disease, vitiligo
Jun 17, 2019
FDA approves first treatment for eosinophilic esophagitis
May 26, 2022

Atogepant prevents migraine in adults
Jun 15, 2022
FDA approves Skyrizi for moderately to severely active Crohn disease
Jun 20, 2022
FDA approves Rinvoq for treatment of atopic dermatitis
Jan 19, 2022
Skyrizi approved for psoriatic arthritis
Jan 26, 2022
Recommended for you
Cancer drug shows powerful anti-tumor activity in animal models of several different tumor types
6 hours ago

Hope for a cure for visceral leishmaniasis, an often fatal infectious disease
8 hours ago
Matcha mouthwash shown to inhibit bacteria that cause periodontitis
11 hours ago

New AI algorithm may improve autoimmune disease prediction and therapies
May 20, 2024
Let us know if there is a problem with our content
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
A new treatment is restoring skin coloration to some with vitiligo. It's giving patients hope.

- Vitiligo is an autoimmune disorder that leads to the loss of skin pigmentation.
- A recent study shows a medicated cream called ruxolitinib is extremely effective in about one-third of patients.
- The cream is giving patients hope that even if they don't benefit from the treatment there will soon be others.
Sarah Hayden owns a lot of turtlenecks. She also has chunky necklaces and tons of makeup – anything to cover up the blotchy white skin on her face, neck, hands and knees.
When she was about 23, Hayden was diagnosed with vitiligo, a noncontagious skin condition that leads to the loss of pigmentation. It struck first on the back of her neck and then, as with many people, slowly progressed under her eyes and across her face, before jumping down to her knees, elbows and fingertips.
Once, when she stepped into a hot tub, a woman made a comment about how people with skin conditions shouldn't be in hot tubs and paraded herself and her daughter out. Hayden pretended it didn't matter. That she still felt beautiful on the inside.
It was only when she joined a clinical trial for an experimental drug and her vitiligo began to recede that she "realized that having the skin condition affected me more than I thought it did," said Hayden, now 41, of Hood River, Oregon.
The medicated cream she used as part of that trial, ruxolitinib, was approved by the Food and Drug Administration this summer. Late Wednesday, The New England Journal of Medicine published a study showing ruxolitinib cream is extremely effective in about one-third of patients who use it for at least six months.
What is vitiligo and its treatment?
Somewhere between half a percent and 2% of people worldwide have vitiligo, which is now understood to be an autoimmune disorder, where the immune system attacks the cells in the skin that provide pigment.
Famous people with vitiligo include Michael Jackson, model Winnie Harlow, actor and director Jon Hamm, comedian Steve Martin, commentator and comedian Joe Rogan, and NFL player-turned coach Karl Dunbar.
The condition can be particularly distressing for people with naturally dark skin because the light blotches stand out even more.
Only one treatment has previously been approved by the FDA and it removes more coloration, to avoid blotchiness, rather than restoring the skin's natural color as ruxolitinib cream can sometimes do.
Right now, many patients are treated with steroids, which don't work well, or given controlled phototherapy sessions, which can be hard to access for people who live far from a center that offers it, said Dr. David Rosmarin, a lead author on the new study.
MORE: For patients with earliest stage of breast cancer, how much treatment is enough?

How ruxolitinib works
The use of ruxolitinib cream reflects a new understanding of vitiligo, Rosmarin said. It works by tamping down an overactive immune response. "We are now better at modulating, rebalancing" that arm of the immune system, Rosmarin said.
About 30% of the 450 people who received active treatment as part of two studies saw a dramatic improvement in facial pigmentation after six months. Up to half did after a year of treatment, indicating that the cream became more potent over time. More than 80% of people in both trials were white and only 3% to 5% were Black or Asian.
Formulated as a cream ruxolitinib does not affect the whole body, so side effects are relatively minor, usually just some acne where the cream is used, Rosmarin said.
Ruxolitinib cream seems to work best on the head and neck, with hands and feet the hardest to repigment, Rosmarin said. It's not yet known whether someone can take ruxolitinib for a period of time and then stop, or whether vitiligo will return without constant dosing.
Commercial insurers and Medicare have been covering ruxolitinib cream, Rosmarin said, now that there's "broad agreement in the medical community that it is a medical not cosmetic condition and treatment should be covered." One tube of the cream costs about $2,000 and can last anywhere from a few weeks to a few months, depending on how much skin needs to be covered.
The amount of time a patient had vitiligo didn't affect their likelihood of success.
People who had poliosis vitiligo, or a total loss of pigmentation leading to pure white hairs, did not improve on the drug, said Dr. Brett King, who was not directly involved in the study but consults for Incyte, the Delaware-based company that makes ruxolitinib cream and sells it under the brand name Opzelura.
Researchers are also developing other approaches to treating vitiligo for those who don't see much improvement with ruxolitinib cream, Rosmarin said.
The study and recent approval of ruxolitinib cream "sets a pathway for other treatments to hopefully move forward," he said. "This is just the start."
RESEARCH: These rats have human cells in their brains. They may help scientists understand autism and schizophrenia.
The origins of the new approach date to 2017 when King, an associate professor of dermatology at Yale University, decided to test a rheumatoid arthritis drug in a mouse that works in the same way as ruxolitinib. It seemed to help, so he gave it to a patient who had the condition "from nose to toes," he said. Her dramatic improvement "was our first clue" that his approach might work.
"When you have an observation, but in particular one anchored in science, not just in chance observation, it leads to paradigm shifts in how we think about disease and treat disease," King said.
The success with ruxolitinib will encourage other drug companies to develop vitiligo treatments, he said.
Because it takes so long to see a benefit from ruxolitinib cream, it would be useful to find a way of distinguishing patients who will respond well from those who won't, Dr. Liv Eidsmo wrote in an editorial accompanying the new study.
Eidsmo, a dermatologist and researcher at Karolinska University Hospital in Sweden, also pointed out that it's not clear what will happen if patients stop using the cream and raised concerns about the lack of diversity among trial participants, who were largely white.
Still, she wrote, thanks to ruxolitinib, "patients with vitiligo finally have the hope of efficient treatments."
WHAT DOCS WANT YOU TO KNOW: Study raises questions about colonoscopies
'It gave me back something I lost'
Hayden is one of those lucky patients.
The cream has returned about 90% of the pigment to her face. Even her hands, considered the hardest to reach for the drug, have mostly cleared up.
"It gave me back something I lost," said Hayden, who was wearing a V-neck shirt and a thin gold necklace on a recent video call. On weekends, she no longer insists on wearing makeup before leaving the house.
An instructional coach in her town's school district, Hayden had almost no side effects from the cream and didn't find it a burden to spread on her face, chest and hands twice a day during the two-year trial. "It was just part of my morning routine," she said, "and it was part of my bedtime routine after I brushed my teeth."
The treatment changed her relationship with the sun. Before, she would wear high SPF sunblock, a hat and long sleeves if she was in the sun for long periods. With vitiligo, she said "you are either normal or burnt," there was no such thing as a tan.
During the trial, she said, she was encouraged to get limited amounts of sun exposure to promote skin regeneration. "Now, I feel like when I'm in the sunlight, it's regenerative instead of harmful," she said. "I have to be careful, but it's not the same burden as it was."
Hayden said she's grateful to have been able to participate in the research to help others avoid some of the same challenges she's faced.
"That really kind of fueled my 'why' for engaging in the trial," she said. "Not just for my own hope and excitement – which it did 100% and I would do it again in a heartbeat – but also potentially having this available to other people living with vitiligo."
Contact Karen Weintraub at [email protected].
Health and patient safety coverage at USA TODAY is made possible in part by a grant from the Masimo Foundation for Ethics, Innovation and Competition in Healthcare. The Masimo Foundation does not provide editorial input.
Patient Burden of Nonsegmental Vitiligo: A US Real-World Survey of Dermatologists and Their Patients
- Original Research
- Open access
- Published: 16 May 2024
Cite this article
You have full access to this open access article

- David Rosmarin ORCID: orcid.org/0000-0003-2786-0708 1 ,
- Jennifer H. Lofland ORCID: orcid.org/0000-0002-1015-3823 2 ,
- Simran Marwaha ORCID: orcid.org/0000-0003-0380-0372 3 ,
- James Piercy ORCID: orcid.org/0000-0001-7015-1021 3 ,
- Peter Anderson ORCID: orcid.org/0000-0002-2171-8228 3 &
- Jinan Liu ORCID: orcid.org/0009-0002-6042-5476 2
176 Accesses
1 Altmetric
Explore all metrics
Introduction
Vitiligo is a chronic autoimmune disease characterized by destruction of melanocytes, leading to skin depigmentation. Vitiligo can have a high quality-of-life burden and profound impact on psychosocial well-being. The objectives of this study were to describe the self-reported patient burden among patients with nonsegmental vitiligo with ≤ 10% affected body surface area, summarize the physician-reported psychosocial and psychological impact of vitiligo on patient lives, and describe disease characteristics and treatment history, goals, and satisfaction.
Data were drawn from the Adelphi Vitiligo Disease Specific Programme™, a real-world, cross-sectional survey with retrospective data collection of physicians and patients with vitiligo, collected in the United States between October 2021 and April 2022. Separate surveys for dermatologists and patients contained questions on clinical and demographic characteristics of patients with vitiligo and burden of vitiligo. Treatment history, goals, and satisfaction were assessed together with the impact of vitiligo on quality of life.
Sixty-one dermatologists provided data for 326 patients with ≤ 10% affected body surface area (adults, n = 221; adolescents, n = 105); 90 of those patients also responded to the survey. The most common treatments were topical corticosteroids, topical calcineurin inhibitors, and narrow-band ultraviolet-B phototherapy, with the main treatment goal being repigmentation. Physician-reported treatment satisfaction was 56%; 25% of patients reported frustration with treatment options. Physicians reported impact of vitiligo on everyday life in 46% of patients. Patients reported 12.7% overall work impairment; mean scores for Hospital Anxiety and Depression Scale anxiety and depression domains were 3.5 and 2.2, respectively, and mean Vitiligo-specific Quality of Life index score was 26.9. Patients with facial involvement experienced higher burden than those without.
A high patient burden was reported by dermatologists and their patients with vitiligo who had ≤ 10% affected body surface area, including psychosocial and psychological consequences. These findings highlight an unmet need in the treatment of vitiligo.
Plain Language Summary
Vitiligo is a chronic disease in which cells that produce the skin pigment melanin are attacked, causing patches of skin to lose color and become pale. Vitiligo can have emotional impacts such as social or psychological distress that can affect the day-to-day well-being of individuals. However, there is a lack of studies that assess the ways that vitiligo affects the everyday lives of people with the condition in the United States. Dermatologists and people with vitiligo answered survey questions on treatment goals, any vitiligo treatments currently and previously used, and how satisfied they were with the results of treatment. The surveys also contained questions that assessed the impact of vitiligo on everyday life. Sixty-one dermatologists answered questions about 326 patients and 90 of those patients also provided their own answers to the survey questions. Both dermatologists and patients reported that restoring color to patches of pale skin was their goal in treating vitiligo. However, dermatologists and patients both reported that they were dissatisfied with the results of available treatments. Dermatologists and patients both reported that vitiligo impacted aspects of everyday life. Emotional and psychological impacts such as anxiety and depression were reported, as well as negative effects on patients’ work and social lives due to vitiligo. These results confirm that vitiligo impacts the day-to-day well-being of patients. Furthermore, this study highlights that there is a need for improvements in the treatment of vitiligo.
Similar content being viewed by others

Microneedling and Its Use in Hair Loss Disorders: A Systematic Review

Alopecia Areata: an Update on Etiopathogenesis, Diagnosis, and Management
Lebrikizumab Provides Rapid Clinical Responses Across All Eczema Area and Severity Index Body Regions and Clinical Signs in Adolescents and Adults with Moderate-to-Severe Atopic Dermatitis
Avoid common mistakes on your manuscript.
Vitiligo is a chronic autoimmune disease of the skin that targets melanocytes, causing patches of skin depigmentation [ 1 ]. The diagnosed prevalence of vitiligo is estimated to be approximately 0.1–1.5% of the US population (approximately 1.9 million diagnosed US patients), with estimates suggesting that up to 40% of US adults with vitiligo may be undiagnosed [ 2 , 3 ]. Most patients with vitiligo have lesions affecting ≤ 10% of their body surface area (BSA), with the face being a commonly affected region [ 4 ].
A high level of clinical and emotional burden has been previously associated with vitiligo. Studies have documented a high prevalence of low self-esteem [ 5 , 6 ], social isolation, stigma [ 7 , 8 ], disease-specific discrimination [ 9 , 10 ], and anxiety, hopelessness, depression, and depressive symptoms [ 11 ] among patients with vitiligo. Patients report feeling stigmatized and experience unnecessary negative and antagonistic attention in public, which results in them feeling ostracized. Furthermore, the emotional distress patients with vitiligo experience has been shown to negatively impact their employment, cause social withdrawal, and lead to substance use disorders [ 12 ]. In dermatologic diseases, including atopic dermatitis and psoriasis, the presence of lesions in visible areas (e.g., face) has been associated with particularly high disease burden [ 13 , 14 ].
There is currently no cure for vitiligo and a historical lack of efficacious treatment, leaving patients and physicians frustrated and dissatisfied with available treatment options [ 15 ]. In July 2022, ruxolitinib cream was approved by the US Food and Drug Administration (FDA) for the treatment of nonsegmental vitiligo (NSV) in patients aged 12 years and older, for application to lesions affecting ≤ 10% BSA [ 16 ]. This approval marks the first pharmacologic treatment approved for repigmentation of NSV and augments the current treatment landscape for the disease [ 17 ]. Understanding the disease burden and real-world implications across the spectrum of NSV, not solely among those with higher BSA involvement, is necessary to guide the future evaluation and further research of FDA-approved therapies and products in development for the treatment of NSV.
The objectives of this study were to describe the self-reported patient burden among patients with NSV affecting ≤ 10% BSA, who constitute the majority of real-world patients with vitiligo and would be candidates for ruxolitinib cream treatment. We summarize the physician-reported impact of vitiligo on their patients’ lives, focusing on psychosocial and psychological consequences among patients with and without facial involvement and describe patients’ disease characteristics and treatment history, goals, and satisfaction.
Study Design
This is a secondary analysis of data from the Adelphi Vitiligo Disease Specific Programme™ (DSP) collected in the United States between October 2021 and April 2022, before the FDA approval of ruxolitinib cream for the treatment of NSV. For these analyses, only patients who had NSV with ≤ 10% affected BSA were included.
Data Source
The Vitiligo DSP is a large, multinational, cross-sectional survey with retrospective data collection, including dermatologists and their patients presenting with NSV in a real-world clinical setting. The DSP describes current disease management, disease burden impact, and associated treatment effects, both clinical- and dermatologist-perceived. A complete description of the survey methodology has been previously published and validated [ 18 , 19 , 20 ].
A geographically diverse sample of dermatologists was recruited to participate in the DSP, which received ethics exemption from the Pearl Institutional Review Board (study protocol number #21-ADRW-122) based on the use of aggregated and de-identified patient data. Dermatologists were eligible to participate in the survey if they were personally responsible for treatment decisions and management of patients with vitiligo and had a minimum monthly workload of 6 patients diagnosed with NSV. Patients were eligible for inclusion in the Vitiligo DSP if they were aged ≥ 18 years (adult population) or between 12 and 17 years (adolescent population), had a physician-confirmed diagnosis of NSV, were not involved in ongoing clinical trials, and visited the dermatologist for consultation. Both physicians and patients consented to take part in the research.
Dermatologists were instructed to complete a patient record form (PRF) for their next 6 consecutively consulted patients who visited for routine care. This form contained detailed information including patient demographics, disease characteristics (including affected BSA and facial involvement), treatment history and satisfaction with current treatment regimen, and disease control achieved. Facial involvement was defined as vitiligo affecting the head and neck area including eyes, ears, scalp, and the rest of the face. Completion of the patient record form was undertaken through consultation of existing patient clinical records, as well as the judgment and diagnostic skills of the respondent physician, which is entirely consistent with how decisions are made in routine clinical practice. It should be noted that the survey was designed to facilitate understanding of real-world clinical practice, and thus dermatologists could only report on data they had at hand at the time of the consultation. Therefore, data collected represent the evidence that physicians hold when making any clinical treatment and other management decisions at the time of consultation.
All data were collected in such a way that patients and dermatologists could not be directly identified; all data were aggregated and de-identified before receipt. Each survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act of 1996 [ 21 ] and Health Information Technology for Economic and Clinical Health Act legislation [ 22 ]. Each patient for whom the dermatologist completed a form was then invited to voluntarily complete a patient self-completed questionnaire (PSC). PSCs were completed by patients independently from their dermatologist and were returned in a sealed envelope, ensuring the patient response was kept confidential from their dermatologist. The PSC contained detailed questions on current symptomatic burden, disease characteristics (including comorbid conditions), heath-related quality of life, level of satisfaction with their treatment regimen and disease control achieved, over-the-counter therapies, and other patient-reported outcomes. PSCs included the Work Productivity and Activity Impairment (WPAI) questionnaire (completed by adult patients only), the Hospital Anxiety and Depression Scale (HADS), and the Vitiligo-specific Quality of Life instrument (VitiQoL). The WPAI measures time missed from work (absenteeism), impairment at work (presenteeism), and impairment of regular activities. WPAI scores are reported as percentage impairment due to the disease [ 23 ]. The HADS score details symptoms of anxiety and depression, using a 0–3 scale across 7 questions [ 24 ]. For HADS, a total score ≥ 8 out of a possible 21 indicates symptoms of anxiety or depression [ 24 ]. The VitiQoL is a 15-question patient-reported outcome measure assessing vitiligo-specific aspects of life quality using a 7-point Likert scale (0–6), which provides a scoring scale of 0–90; higher scores indicate poorer quality of life [ 25 ].
Data Analysis
For the analysis of patient characteristics, mean, standard deviation, and range were calculated for continuous variables, and frequency counts and percentages for categorical variables. All analyses were conducted in Stata v16 (StataCorp, Texas, USA) [ 26 ]. Missing data were not imputed. Data were analyzed using descriptive statistics.
Study Population and Demographics
In total, 61 US physicians (all dermatologists) completed PRFs for 326 patients with NSV who had ≤ 10% affected BSA. Of these patients, 221 were adults (≥ 18 years) and 105 were adolescents (12–17 years; Table 1 ). Mean (SD) age of all patients was 30.7 (16.4) years, and 199 (61%) had Fitzpatrick skin types I–III. Mean (SD) time since diagnosis was 27.2 (40.8) months, with a mean (SD) affected BSA of 7.6% (5.7%) at diagnosis and 5.6% (3.1%) at time of analysis. Overall, 152 patients (47%) had facial involvement; characteristics for patients with facial involvement are detailed in Table 1 .
A total of 90 patients who had NSV with ≤ 10% affected BSA completed a PSC, of whom 42 (47%) patients had facial involvement. Mean (SD) age of patients completing a PSC was 33.8 (15.9) years, and 65 (72%) of these patients had Fitzpatrick skin types I–III. Mean time since diagnosis was 30.6 (60.0) months, with a mean (SD) affected BSA of 7.3% (4.4%) at diagnosis and 5.5% (2.7%) at time of analysis.
Physician-Reported Regions Affected
For 326 patients with ≤ 10% affected BSA (including those with facial involvement), the physician-reported body regions most affected were head and neck (57%), upper limbs (57%), trunk/torso (31%), and lower limbs (29%; Fig. 1 a). Physicians considered the most bothersome regions to be head and neck for 52% of patients, followed by upper limbs (37%), trunk/torso (15%), and lower limbs for 9% of patients. Among patients with facial involvement, physicians considered the most bothersome regions to be head and neck for 96%, trunk/torso for 4%, upper limbs for 11%, and lower limbs for 1% (Fig. 1 b).
Physician-reported a overall and b most bothersome regions currently affected a ; BSA body surface area. a Physicians could select more than one region
Physician- and Patient-Reported Treatment
At the time of the survey, physicians reported that their 326 patients were receiving an average of 1.4 prescribed treatments, with 61% receiving monotherapy, 35% receiving combination therapy, and 4% not currently receiving prescribed treatment (i.e., untreated). Overall, 38% of patients were receiving topical calcineurin inhibitors (TCI), 30% were receiving moderate-potency topical corticosteroids (TCS), and 12% were receiving narrow-band ultraviolet-B (UVB) phototherapy. Among 152 patients with facial involvement, the prescription of TCIs and narrow-band UVB increased to 43% and 17%, respectively (Fig. 2 a).
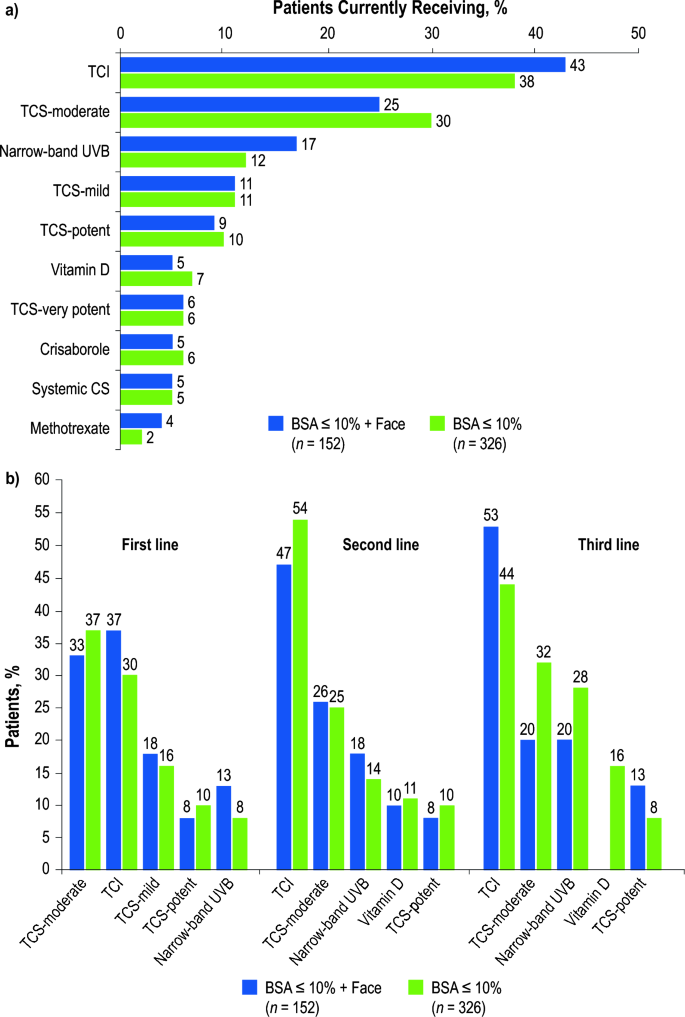
Physician-reported most commonly used treatment regimens a currently and b by treatment line a ; BSA body surface area, CS corticosteroid, TCI topical calcineurin inhibitor, TCS topical corticosteroid, UVB ultraviolet-B phototherapy. a Ruxolitinib cream was not approved in the United States at the time of the analysis
Overall, physicians reported that moderate-potency TCS were the most common first-line therapy (37%), and TCI were the most commonly prescribed second- (54%) and third-line therapies (44%; Fig. 2 b). Among patients with facial involvement, TCI were the most commonly prescribed first- (37%), second- (47%), and third-line (53%) therapies. Additionally, prescription of narrow-band UVB increased with each line of therapy. Physicians reported that cosmetics or skin camouflage creams were used by 19% of all patients (facial involvement, 33%).
Furthermore, 37/90 (41%) patients reported using over-the-counter medications. Among these 37 patients, the most commonly reported treatments used were vitamins (38%), creams or oils (35%), and makeup/camouflage cream (22%). Patients also reported spending a mean (SD) of US$17.19 ($36.92) over the last month on pharmacy/drug store nonprescription medicines for their vitiligo.
Physician- and Patient-Reported Treatment Goals
The most common physician-reported treatment goal was repigmentation of lesions for 77% (251/326) of patients, followed by shrinkage of lesions in 47% (154/326; Fig. 3 a). Physicians reported long-term safety of treatment as a goal in 35% (113/326) of patients. For patients with physician-reported data available, repigmentation of lesions was the main physician-reported reason for prescribing the current treatment [50% of patients (146/290); Fig. 3 b]. Reduction of steroid use (36%; 103/290) and overall safety (33%; 95/290) were also considered important reasons for treatment selection.
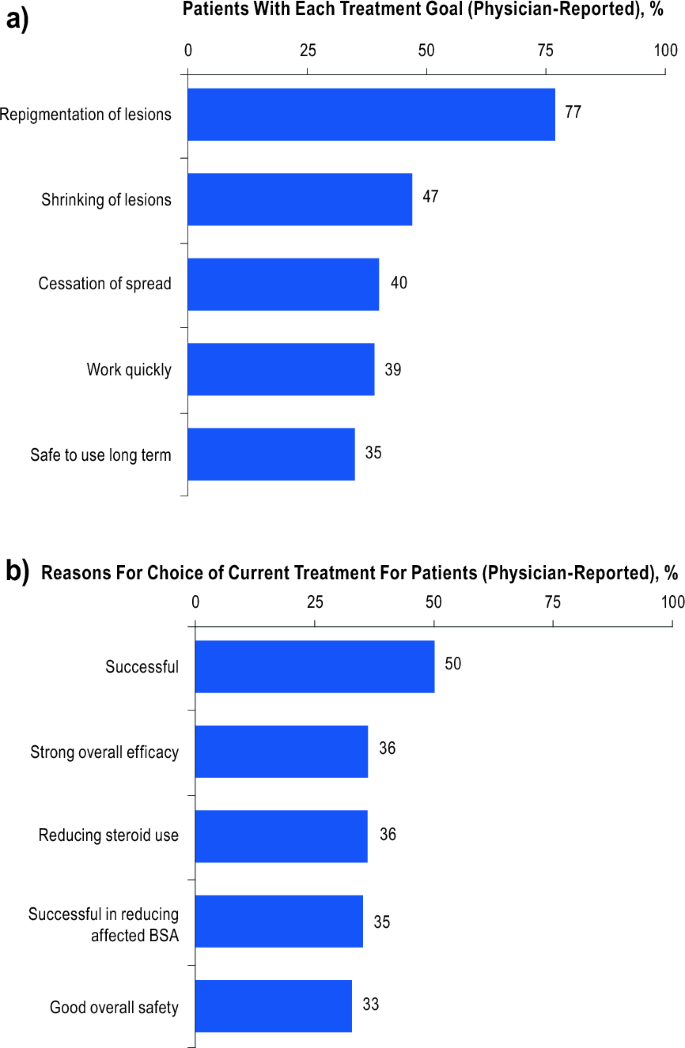
Physician-reported a treatment goals and b reasons for choice of current treatment for 326 patients with BSA ≤ 10%; BSA body surface area
Repigmentation of lesions was the main treatment goal for 67% (60/90) of patients, which rose to 74% (31/42) for patients with facial involvement (Figure S1 ). Shrinkage of lesions was reported as the main treatment goal by 61% (55/90). Among all patients, 64% (58/90) wanted a treatment that worked quickly, and 54% (49/90) wanted a treatment to stop the spread of their vitiligo; of those patients with facial involvement, 60% (25/42) wanted a treatment that worked well to specifically shrink more noticeable areas. Overall, 42% (38/90) of patients also reported wanting a treatment that was safe to use long term, as did 52% (22/42) of patients with facial involvement.
Physician- and Patient-Reported Satisfaction with Current Treatment
Physicians were satisfied with current treatment outcomes for only 56% of their 326 patients and believed best realistic control had been achieved for only 51% (facial involvement, 45%). Top reasons for treatment dissatisfaction were not initially inducing repigmentation (66%), lack of sustained efficacy (24%), and treatment ineffectiveness for obtaining complete depigmentation (14%).
Among the 90 patients, 14% were dissatisfied with current treatment results (facial involvement, 24%; Figure S2a). Main reasons for dissatisfaction included not liking the way the treatment was taken (23%) and not improving quality of life (21%). For the 42 patients with facial involvement, not improving vitiligo noticeability (27%) and lack of improved quality of life (23%) were most frequently reported as reasons for dissatisfaction. Patients reported feeling frustrated with available treatment options (25%; facial involvement, 37%). Patients reported being fairly (50%) and completely (39%) happy with the way their physician treated their vitiligo (Figure S2b), and were very confident (20%) or somewhat confident (58%) in managing their condition (Figure S2c).
Physician-Reported Psychosocial Impact
Physicians reported that 46% of their 326 patients were impacted by vitiligo in everyday life, with 47% experiencing emotional impact and 44% having low self-esteem (Fig. 4 ). Overall, physicians indicated that 30% avoided social situations, 19% avoided sports or other outdoor activities, and 16% avoided professional situations or intimacy. Physicians also reported that 21% felt socially isolated (facial involvement, 31%), and 14% experienced bullying or abuse (facial involvement, 22%). Physicians also reported that bullying, abuse, or social ostracization were experienced by 22% of all adolescent patients (facial involvement, 39%). Furthermore, physicians reported that 28% of their patients experienced depression and 37% experienced anxiety (facial involvement, 37% and 47%, respectively). Concerningly, 9% of all patients had suicidal ideation (facial involvement, 13%).
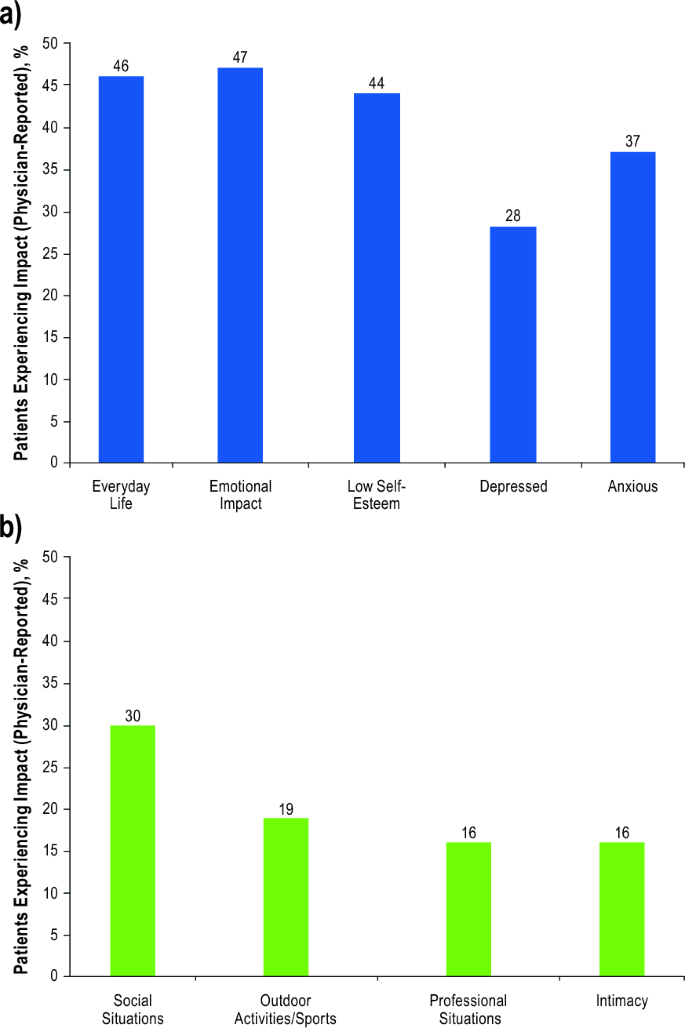
Physician-reported a impact of vitiligo on patients’ lives and b situations patients avoid for 326 patients with BSA ≤ 10%; BSA body surface area
Patient-Reported Vitiligo Impact
When patients reported how they felt at the time they received their vitiligo diagnosis, 33% of the 90 patients worried what other people would think (facial involvement, 38%; Figure S3a). Patients reported that their physician understood the impact vitiligo had on their life “quite well” (54%) or “completely” (39%). Overall, 20% reported feeling socially isolated (facial involvement, 29%); however, only 6% reported experiencing bullying or abuse (facial involvement, 10%). Additionally, 53% self-reported having to cover or hide their vitiligo; 31% used makeup to cover vitiligo on their face, and 18% used makeup to cover vitiligo elsewhere on their body (Figure S3b). Patients spent a mean (SD) time of 57 (100) minutes per day covering vitiligo on their face and 30 (48) minutes covering vitiligo on their body. Overall, 21% reported depression (facial involvement, 26%), and 32% reported anxiety (facial involvement, 38%).
Patient-Reported Impact of Vitiligo on Quality of Life and Work Productivity
Work productivity and activity impairment (wpai).
According to the WPAI questionnaire, 43 patients reported a mean (SD) of 12.7% (15.7%) overall work impairment [facial involvement ( n = 18), 13.5% (15.4%)], and 68 reported a mean (SD) of 14.1% (18.6%) activity impairment due to their vitiligo [facial involvement ( n = 32), 16.6% (21.0%); Figure S4a].
Hospital Anxiety and Depression Scale (HADS)
For the anxiety domain of the HADS, the mean (SD) score for the 90 patients was 3.5 (3.6), with 4.2 (4.1) in patients with facial involvement (Figure S4b). However, 14% of patients reported a score ≥ 8, suggesting either borderline abnormal or abnormal levels of anxiety (facial involvement, 22%).
For the depression domain of the HADS, the mean (SD) score for all patients was 2.2 (3.2), with 2.9 (3.8) in patients with facial involvement. However, 9% of patients reported a score ≥ 8, suggesting either borderline abnormal or abnormal levels of depression (facial involvement, 14%).
Vitiligo-Specific Quality of Life (VitiQoL) Instrument
The mean (SD) score on the VitiQoL instrument for the 89 patients with data available and the 42 patients with facial involvement was 26.9 (22.9) and 32.5 (25.5), respectively (Figure S4c), indicating moderate quality-of-life impairment [ 27 ].
This study highlights unmet needs in the treatment of vitiligo among patients with ≤ 10% affected BSA. Despite treatment, patients reported minimal or no changes in their vitiligo, exposing them to a high degree of emotional burden. Many patients experienced low self-esteem, avoided social situations, felt socially isolated or experienced bullying/abuse, and reported symptoms of depression and anxiety. Our study was conducted before FDA approval of ruxolitinib cream for topical treatment of NSV, and, in line with treatment guidelines [ 28 , 29 ], physicians prescribed mainly TCI, moderate-potency TCS, or narrow-band UVB phototherapy.
In our study, both physicians and patients reported similar treatment goals, namely repigmentation, shrinkage of lesions (especially noticeable areas), and prevention of the spread of vitiligo. Patients also wanted a treatment that was safe long term and had few or no side effects. However, physicians and patients were dissatisfied with disease control and treatment options available at the time of the study due to a lack of sustained efficacy, including not inducing initial repigmentation and ineffective complete depigmentation. The limited long-term success of current management strategies and the lack of effective treatment options are current barriers in the treatment of vitiligo [ 30 ]. Dissatisfaction rates are often high among physicians and their patients with vitiligo [ 15 , 31 , 32 ], commonly due to issues with sustained repigmentation [ 33 ], as depigmentation recurs in ~ 40% of cases after therapeutic intervention [ 34 ]. Patients, especially those with facial involvement, were also frustrated with treatment outcomes; a quarter of patients reported no improvement in quality of life after treatment.
Nearly half of physicians reported that vitiligo had a negative impact on their patients’ day-to-day life, including low self-esteem, avoidance of social situations, social isolation, bullying, abuse, social ostracization, depression, anxiety, and suicidal ideation. Patients’ reports were consistent with those of their physicians. Recently, the emphasis placed on addressing the emotional burden of vitiligo [ 35 ] and integrating patient-oriented measures in diagnosis and treatment decisions [ 36 ] has been shown to increase treatment adherence, patient satisfaction, and quality of life [ 35 ]. In our study, patients were satisfied with their physician’s awareness of the impact vitiligo had on their daily life, felt fairly or completely happy with the way their physician treated their vitiligo, were involved in treatment decisions, and felt supported by their dermatologists in managing their condition. Patients resorted to over-the-counter medications and spent a significant amount of time using camouflaging makeup to cover or hide their vitiligo. Over a third reported being worried what people would think, and patient-reported low self-esteem, social isolation, and avoidance were high, indicating significant emotional burden. Social avoidance behavior is reported to stem from the fear of negative social encounters including rude remarks and questions about their disease [ 7 , 8 ]. Patients with vitiligo also report feeling embarrassed and disfigured, leading to limited personal relationships [ 7 , 9 ] and sexual disorders [ 37 , 38 ].
Furthermore, our findings are consistent with previous studies indicating depression and anxiety to be the most common psychological comorbidities among patients with vitiligo. A meta-analysis of 29 studies, capturing data on 2530 patients with vitiligo with varying disease severity, found that a quarter of patients had depression and 1 in 7 had anxiety [ 10 ]. In our study, physicians reported depression in nearly one-third of patients and anxiety in two-fifths, with increased suicidal ideation among patients who had facial involvement. These percentages were higher than general population estimates from the United States, where depression and anxiety were reported to affect 19% and 15% of people, respectively [ 39 ]. It is also likely that the prevalence of depression and anxiety is underreported in vitiligo, consistent with other autoimmune conditions [ 40 , 41 , 42 ].
Most studies capturing data on the burden of vitiligo report on patients with a greater extent of affected BSA (often > 25%) [ 43 ], more noticeable lesions (Fitzpatrick skin types IV–VI or lesions on the face, neck, or hands), or those with genital involvement [ 44 ]. However, our study specifically evaluated patients with vitiligo who had ≤ 10% affected BSA and included those with and without facial involvement. As such, our data encompass an underreported cohort of patients with vitiligo and demonstrate that high levels of disease burden occur irrespective of disease extent. Our findings indicate that the disease burden associated with vitiligo is high even among those with a more limited disease extent and may be driven by a lack of efficacious treatment options. Physicians (all dermatologists) were aware of the high disease burden among their patients with vitiligo who had ≤ 10% affected BSA and were dissatisfied with disease control and treatments available at the time of the study. Although patients felt that their dermatologists understood the impact vitiligo had on their daily lives, they were also frustrated with current treatment outcomes and options.
Limitations of this study include that participating patients may not reflect the general population with vitiligo. Firstly, our study included only patients with ≤ 10% affected BSA, consistent with the labeling for ruxolitinib cream. Secondly, data collection was restricted to patients who visited their dermatologist for consultation during the study period. Thirdly, patients who visited their physician more frequently were more likely to be included in the survey than those not consulting their physician as frequently. Additionally, we acknowledge that the results may not be generalizable to patients who are treated by healthcare personnel other than dermatologists or to patients with > 10% affected BSA. To avoid selection bias, physicians were asked to provide data for a consecutive series of patients; however, there were no formal patient selection verification procedures. Recall bias is a common limitation of surveys; however, the data for these analyses were collected at the time of each patient’s appointment, an approach expected to reduce the likelihood of recall bias, particularly since reporting was about current clinical characteristics, treatment experience, and patient burden. Despite the limitations described above, the DSP sample is internally consistent and enables us to accurately describe any differences in clinical or health-related quality-of-life characteristics, management approach, treatment patterns, and burden for patients with vitiligo with or without facial involvement.
Further research would enable identification of additional factors that might be drivers of greater burden among patients with vitiligo. In the global VALIANT survey, patients with vitiligo reported substantial psychosocial burden, which was greatest among patients with > 5% affected BSA, Fitzpatrick skin types IV–VI, and facial or hand lesions versus their counterparts. However, patients with < 1% affected BSA, Fitzpatrick skin types I–III and no facial or hand lesions also reported considerable burden [ 45 ]. Investigation of these and other factors, including affected body regions, age groups, and sex, would help further our understanding of vitiligo burden.
Conclusions
Dermatologists and their patients reported a high patient burden associated with vitiligo limited to ≤ 10% affected BSA, including psychosocial and psychological consequences. Facial involvement was common, with head/neck lesions considered by physicians to be the most bothersome for patients. Repigmentation was the most common dermatologist- and patient-reported treatment goal; however, both dermatologists and patients reported being dissatisfied with disease control and available treatments at the time of the survey. Both dermatologists and patients reported bullying, abuse, and psychosocial comorbidities including depression. Patients also reported spending a considerable amount of time each day covering their vitiligo, experienced work productivity and activity impairment, and had reduced quality of life. Considered together, these findings suggest a significant unmet need concerning treatment and management of this chronic disease in the United States, warranting a focus on managing vitiligo using a full range of treatment options including those more recently approved by the FDA.
Data Availability
Simran Marwaha, James Piercy, Peter Anderson, and Jinan Liu had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All data, i.e., methodology, materials, data and data analysis, that support the findings of this survey are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Peter Anderson at [email protected].
Rodrigues M, Ezzedine K, Hamzavi I, Pandya AG, Harris JE, Vitiligo Working Group. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77(1):1–13.
Article CAS PubMed Google Scholar
Gandhi K, Ezzedine K, Anastassopoulos KP, et al. Prevalence of vitiligo among adults in the United States. JAMA Dermatol. 2022;158(1):43–50.
Article PubMed Google Scholar
Krüger C, Schallreuter KU. A review of the worldwide prevalence of vitiligo in children/adolescents and adults. Int J Dermatol. 2012;51(10):1206–12.
Hamzavi IH, Bibeau K, Grimes P, et al. Exploring the natural and treatment history of vitiligo: perceptions of patients and healthcare professionals from the global VALIANT study. Br J Dermatol. 2023;189(5):569–77.
Bergqvist C, Ezzedine K. Vitiligo: a review. Dermatology. 2020;236(6):571–92.
Porter JR, Beuf AH, Lerner A, Nordlund J. Psychosocial effect of vitiligo: a comparison of vitiligo patients with “normal” control subjects, with psoriasis patients, and with patients with other pigmentary disorders. J Am Acad Dermatol. 1986;15(2):220–4.
Krüger C, Schallreuter KU. Stigmatisation, avoidance behaviour and difficulties in coping are common among adult patients with vitiligo. Acta Derm Venereol. 2015;95(5):553–8.
Ongenae K, Beelaert L, van Geel N, Naeyaert JM. Psychosocial effects of vitiligo. J Eur Acad Dermatol Venereol. 2006;20(1):1–8.
Hamidizadeh N, Ranjbar S, Ghanizadeh A, Parvizi MM, Jafari P, Handjani F. Evaluating prevalence of depression, anxiety and hopelessness in patients with vitiligo on an Iranian population. Health Qual Life Outcomes. 2020;18(1):20.
Article PubMed PubMed Central Google Scholar
Osinubi O, Grainge MJ, Hong L, et al. The prevalence of psychological comorbidity in people with vitiligo: a systematic review and meta-analysis. Br J Dermatol. 2018;178(4):863–78.
Lai Y, Yew Y, Kennedy C, Schwartz R. Vitiligo and depression: a systematic review and meta-analysis of observational studies. Br J Dermatol. 2017;177(3):708–18.
Simons RE, Zevy DL, Jafferany M. Psychodermatology of vitiligo: psychological impact and consequences. Dermatol Ther. 2020;33(3): e13418.
Silverberg JI, Simpson B, Abuabara K, et al. Prevalence and burden of atopic dermatitis involving the head, neck, face, and hand: a cross sectional study from the TARGET-DERM AD cohort. J Am Acad Dermatol. 2023;89(3):519–28.
Lebwohl M, Langley RG, Paul C, et al. Evolution of patient perceptions of psoriatic disease: results from the understanding psoriatic disease leveraging insights for treatment (UPLIFT) survey. Dermatol Ther (Heidelb). 2022;12(1):61–78.
Narayan V, Uitentuis S, Luiten R, Bekkenk M, Wolkerstorfer A. Patients’ perspective on current treatments and demand for novel treatments in vitiligo. J Eur Acad Dermatol Venereol. 2021;35(3):744–8.
Opzelura™ (ruxolitinib cream). Full prescribing information. Incyte Corporation, Wilmington; 2023.
Sidbury R, Alikhan A, Bercovitch L, et al. Guidelines of care for the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023;89(1):e1–20.
Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: Disease-Specific Programmes–a means to understand. Curr Med Res Opin. 2008;24(11):3063–72.
Babineaux SM, Curtis B, Holbrook T, Milligan G, Piercy J. Evidence for validity of a national physician and patient-reported, cross-sectional survey in China and UK: the Disease Specific Programme. BMJ Open. 2016;6(8): e010352.
Article CAS PubMed PubMed Central Google Scholar
Higgins V, Piercy J, Roughley A, et al. Trends in medication use in patients with type 2 diabetes mellitus: a long-term view of real-world treatment between 2000 and 2015. Diabetes Metab Syndr Obes. 2016;9:371–80.
US Department of Health and Human Services. Summary of the HIPAA Privacy Rule. 2003 [cited 2023 June 19]; http://www.hhs.gov/sites/default/files/privacysummary.pdf
Health Information Technology (HITECH). Health Information Technology Act. 2009 [cited 2023 June 19]; https://www.healthit.gov/sites/default/files/hitech_act_excerpt_from_arra_with_index.pdf
Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–65.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Lilly E, Lu PD, Borovicka JH, et al. Development and validation of a vitiligo-specific quality-of-life instrument (VitiQoL). J Am Acad Dermatol. 2013;69(1):e11–8.
StataCorp. Stata Statistical Software: Release 16. College Station: StataCorp LLC 2019.
Picardo M, Huggins RH, Jones H, Marino R, Ogunsola M, Seneschal J. The humanistic burden of vitiligo: a systematic literature review of quality-of-life outcomes. J Eur Acad Dermatol Venereol. 2022;36(9):1507–23.
Eleftheriadou V, Atkar R, Batchelor J, et al. British Association of Dermatologists guidelines for the management of people with vitiligo 2021. Br J Dermatol. 2022;186(1):18–29.
Feng Y, Lu Y. Advances in vitiligo: update on therapeutic targets. Front Immunol. 2022. https://doi.org/10.3389/fimmu.2022.986918 .
Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396(10244):110–20.
Eleftheriadou V, Thomas K, Whitton M, Batchelor J, Ravenscroft J. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167(4):804–14.
Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet. 2015;386(9988):74–84.
Kubelis-López DE, Zapata-Salazar NA, Said-Fernández SL, et al. Updates and new medical treatments for vitiligo. Exp Ther Med. 2021;22(2):1–11.
Article Google Scholar
Abdel-Malek ZA, Jordan C, Ho T, Upadhyay PR, Fleischer A, Hamzavi I. The enigma and challenges of vitiligo pathophysiology and treatment. Pigment Cell Melanoma Res. 2020;33(6):778–87.
Huggins RH, Gardner J, Pandya AG. Patient support, education, and compliance. In: Gupta S, Olsson MJ, Parsad D, Lim HW, van Geel N, Pandya AG, eds. Vitiligo: medical and surgical management. Wiley; 2018. pp. 69–75.
van Geel N, Lommerts JE, Bekkenk MW, et al. Development and validation of a patient-reported outcome measure in vitiligo: the Self Assessment Vitiligo Extent Score (SA-VES). J Am Acad Dermatol. 2017;76(3):464–71.
Maamri A, Badri T. Sexual disorders in patients with vitiligo. Tunis Med. 2021;99(5):504.
PubMed PubMed Central Google Scholar
Sarhan D, Mohammed GF, Gomaa AH, Eyada MM. Female genital dialogues: female genital self-image, sexual dysfunction, and quality of life in patients with vitiligo with and without genital affection. J Sex Marital Ther. 2016;42(3):267–76.
Olfson M, Shea S, Feder A, et al. Prevalence of anxiety, depression, and substance use disorders in an urban general medicine practice. Arch Fam Med. 2000;9(9):876–83.
Li N, Chan E, Peterson S. The economic burden of depression among adults with rheumatoid arthritis in the United States. J Med Econ. 2019;22(4):372–8.
Peterson S, Piercy J, Blackburn S, Sullivan E, Karyekar CS, Li N. The multifaceted impact of anxiety and depression on patients with rheumatoid arthritis. BMC Rheumatol. 2019;3:43.
Sruamsiri R, Kaneko Y, Mahlich J. The underrated prevalence of depression in Japanese patients with rheumatoid arthritis—evidence from a Nationwide survey in Japan. BMC Rheumatol. 2017;1:5.
Bibeau K, Pandya A, Ezzedine K, et al. Vitiligo prevalence and quality of life among adults in Europe, Japan and the USA. J Eur Acad Dermatol Venereol. 2022;36(10):1831–44.
Grimes P, Miller M. Vitiligo: patient stories, self-esteem, and the psychological burden of disease. Int J Womens Dermatol. 2018;4(1):32–7.
Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159(10):1124–8.
Download references
Acknowledgements
The authors thank the physicians and their patients with vitiligo who participated in this study.
Medical Writing and Editorial Assistance
Medical writing support under the guidance of the authors was provided by Rebecca Charlton at Adelphi Real World (Bollington, UK) and was funded by Incyte Corporation (Wilmington, DE, USA), in accordance with Good Publication Practice (GPP) guidelines. Editorial assistance was provided by ICON (Blue Bell, PA, USA) and was funded by Incyte.
Data collection was undertaken by Adelphi Real World as part of an independent survey, entitled the Adelphi Vitiligo Disease Specific Programme™ (DSP), a wholly owned Adelphi program. Incyte Corporation (Wilmington, DE, USA) are subscribers to the Adelphi Vitiligo DSP on which this analysis is based. Incyte did not influence the original survey through either contribution to the design of questionnaires or data collection. Sponsorship of the study and the journal’s Rapid Service Fee were funded by Incyte Corporation. Permission to use the Hospital Anxiety and Depression Scale (HADS) was granted prior to its inclusion in this study.
Author information
Authors and affiliations.
Indiana University School of Medicine, Indianapolis, IN, USA
David Rosmarin
Incyte Corporation, 1801 Augustine Cut-Off, Wilmington, DE, 19803, USA
Jennifer H. Lofland & Jinan Liu
Adelphi Real World, Bollington, UK
Simran Marwaha, James Piercy & Peter Anderson
You can also search for this author in PubMed Google Scholar
Contributions
David Rosmarin, Jennifer H. Lofland, Simran Marwaha, James Piercy, Peter Anderson, and Jinan Liu were involved in 1) conception or design, or analysis and interpretation of data; 2) drafting and revising the article; 3) providing intellectual content of critical importance to the work described; and 4) final approval of the version to be published, and therefore meet the criteria for authorship in accordance with the International Committee of Medical Journal Editors (ICMJE) guidelines. In addition, all named authors take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Corresponding author
Correspondence to Jinan Liu .
Ethics declarations
Conflict of interest.
David Rosmarin has received honoraria as a consultant for AbbVie, Abcuro, AltruBio, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Concert, Dermavant Sciences, Dermira, Incyte Corporation, Janssen, Kyowa Kirin, Lilly, Novartis, Pfizer, Regeneron Pharmaceuticals, Revolo Biotherapeutics, Sanofi, Sun Pharmaceuticals, UCB, and Viela Bio; has received research support from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Dermira, Galderma, Incyte Corporation, Janssen, Lilly, Merck, Novartis, Pfizer, and Regeneron Pharmaceuticals; and has served as a paid speaker for AbbVie, Amgen, Celgene, Janssen, Lilly, Novartis, Pfizer, Regeneron Pharmaceuticals, and Sanofi. Jennifer H. Lofland and Jinan Liu are employees and stockholders of Incyte Corporation. Simran Marwaha, James Piercy, and Peter Anderson are employees of Adelphi Group, which was contracted by Incyte Corporation to perform this analysis.
Ethical Approval
The Adelphi Vitiligo Disease Specific Programme™ received ethics exemption from the Pearl Institutional Review Board (study protocol number #21-ADRW-122) based on the use of aggregated and de-identified patient data. All data were collected in such a way that patients and dermatologists could not be identified directly; all data were aggregated and de-identified before receipt. Each survey was performed in full accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act of 1996 and Health Information Technology for Economic and Clinical Health Act legislation. Both physicians and patients consented to take part in the research.
Additional information
Prior Presentation: This manuscript is based on work that has been previously presented at the following conferences: Rosmarin D, Liu J, Marwaha S, et al. Patient Burden of Nonsegmental Vitiligo: Perspectives From US Patients. Presented at Global Vitiligo Foundation Annual Scientific Symposium, March 16, 2023, New Orleans, LA, USA; Joish VN, Rosmarin D, Lofland JH, et al. Management and Impact of Vitiligo on Patient Lives: Survey of US Dermatologists. Presented at Maui Derm NP + PA Summer, June 22–25, 2022, Colorado Springs, CO, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 294 KB)
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
Rosmarin, D., Lofland, J.H., Marwaha, S. et al. Patient Burden of Nonsegmental Vitiligo: A US Real-World Survey of Dermatologists and Their Patients. Dermatol Ther (Heidelb) (2024). https://doi.org/10.1007/s13555-024-01165-5
Download citation
Received : 08 February 2024
Accepted : 10 April 2024
Published : 16 May 2024
DOI : https://doi.org/10.1007/s13555-024-01165-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Psychosocial comorbidities
- Disease burden
- Find a journal
- Publish with us
- Track your research
The Vitiligo Research Foundation
Firmly committed to curing vitiligo, the VR Foundation is a 501(c)3 non-profit, focused research organization. Our mission is to accelerate the end of suffering for millions of people who live with vitiligo through research, support and education.
VR Foundation is a consultative member of the United Nations Economic and Social Council (UN ECOSOC).
Discover the World's First AI-Guide on Vitiligo, available in 50+ languages! Get insights on the latest research, natural remedies, nutrition, and mental health, all in user-friendly language.
Discover the World's First AI-Guide on Vitiligo, available in 50+ languages!
News All News
- 21 May `24 Push for a Google Doodle on World Vitiligo Day 2024 Let’s raise the beat for WVD-2024 and ask Google for a special Doodle dedicated to World Vitiligo Day on June 25th! Here’s the idea: if we send enough requests to the Google Do... More
- 07 May `24 Update: Vitiligo Insurance Coverage Navigating the insurance maze for vitiligo treatments can be as unpredictable as the condition itself. Vitiligo, often misunderstood as merely a cosmetic issue, is in fact a ser... More
- 07 May `24 Understanding the Timeline for Vitiligo Treatment Treatment times can vary widely due to factors like health, genetic makeup, and where the spots appear. Here's what you need to know: Early Treatment is Key: Catching new spo... More
Quick Links
Learn ABC`s of Vitiligo
Join our community
Watch cool videos on vitiligo
Vote the World Vitiligo Day
World Vitiligo Map
Find and connect with vitiligo researcher, doctor or support group nearby.
Prof. Klaus Fritz, MD accepts vitiligo patients at the Dermatology and Laser Center, which offers systemic and topical therapies, excimer laser and all other kinds of phototherapy.
Reduitstrasse 13 D 76829 Landau Germany

What is Vitiligo?
Vitiligo is a long-term condition that causes pale, white patches to develop on the skin - in severe cases these cover the whole body. Vitiligo affects one in every hundred people and can strike anyone at any time, often leading to depression and isolation - especially amongst children.
Donate Today
Our work is entirely funded by private donations – we receive no money from government. Your money will help us continue funding research into vitiligo and supporting people affected by the condition.
Bookstore Other Books
This book is a practical, step-by-step guide for general practitioners. It contains a collection of protocols and tips regarding how to diagnose, treat and follow-up with vitiligo patients. Each of ten chapters features the experiences and opinions of vitiligo specialists from across the world – scientists, doctors and academics with vast experience and knowledge, who are all recognized as leaders in their field.

What does everyone need to know about vitiligo? Renowned author’s answer to this most difficult of questions uniquely combines the hard-won knowledge of traditional medicine with the stunning revelations of cutting-edge scientific research.
Subscribe to Vitiligo News
Events other events.
- Jun, 2024 24 WVD-2024 headquarters at Cali, Colombia 🇨🇴 World Vitiligo Day 2024 The World Vitiligo Day (WVD) Committee proudly announces the Cali, Colombia as the host for the global WVD 2024 celebrations. This momentous event will be held under the esteeme...
- Jun, 2024 19 Marriott Hotel Indianapolis, IN, USA 🇺🇸 World Vitiligo Day - USA 2024 The GVF and the Wonderfully Made Indianapolis Vitiligo Community are thrilled to announce a vibrant series of events celebrating World Vitiligo Day 2024 in Indianapolis, IN. Fi...
Answers To Frequently Asked Questions
I support the petition to designate June 25 as Vitiligo World Day and save millions of people worldwide from social isolation
FAQ Other Questions
Vitiligo's progression and response to treatment can vary significantly among individuals, making it a particularly unpredictable skin condition. Based on the VALIANT study from...
It's very unlikely. We have specifically looked into claims that gluten-free diet may ease symptoms of vitiligo, or completely reverse it, and found no firm scientific evidence ...
There are so many different ways that people try and spell or even pronounce Vitiligo. Here are some common mis-spellings: bitiligo, vitigo, vitaligo, vitilago, vitiglio, vita...
Though it is not always easy to treat vitiligo, there is much to be gained by clearly understanding the diagnosis, the future implications, treatment options and their outcomes.
Many people deal with vitiligo while remaining in the public eye, maintaining a positive outlook, and having a successful career.
By taking a little time to fill in the anonymous questionnaire, you can help researchers better understand and fight vitiligo.
Project Background
The study of multifactorial diseases, such as vitiligo, requires analysis of complex interplay of symptoms, treatments and outcomes across a large number of people. Population surveys and biobanks are indispensable research tools, required for downstream therapy development. Even small collections of biosamples may be extremely precious for researcher in academic institution or biopharma company.
Until recently, vitiligo researchers were generally limited to conducting studies on patient samples they could acquire themselves. When the Foundation started there were no centralized biological database along with the pre-existing body of the clinical management or the historical study data, which is required in order to proceed with the development of specific therapies. We have run a special investigation study to determine whether VRF shall establish its own biobank.
Then the project's leadership crafted a careful strategy for vitiligo biobank development, with special attention paid to the security and confidentiality of the donor's information. 'Future proofing' involves collecting and processing samples to permit the widest possible range of scientific uses, while avoiding approaches that would impede possible future uses.
We have started the first Vitiligo Biobank with a 100+ sample collection from the completed research project in genetics in late January 2013. Three months later, it held approximately 1,000 biosamples and detailed clinical profiles. Our target number is 10,000 samples and we encourage patients to donate samples . The primary biorepository is located in Moscow (Russia) with networked locations in 11 countries.
News Center
Children with skin diseases suffer stigma, bullying and depression, first large study to look at mental health problems in children with chronic skin conditions.

When someone walks briskly past David Artz, 16, on the sidewalk, he immediately thinks they are trying to get away from him. This is how his young mind works. He has a chronic skin disease that makes his skin red, scaly and rough. He has alopecia and is bald.
David has grown up feeling stigmatized and teased–someone to be avoided because he might be “contagious.”
“It makes me sad and tearful when I think people are avoiding me because of how I look,” Artz said. “It’s hard to be different.”
A new Northwestern Medicine study published in JAMA Dermatology shows Artz’s experience is common for children and teens with chronic skin diseases.
The majority of children and teens with chronic skin diseases such as acne, eczema, psoriasis, alopecia areata (hair loss) and vitiligo (pigment loss) feel stigmatized by peers for their condition and are sometimes bullied, the study reports. As a result, these children have a poor quality of life that includes suffering from depression, anxiety and impaired relationships with their peers.
“These chronic skin conditions can be tremendously life-altering, including shaping psychosocial development,” said corresponding study author Amy Paller, MD, MS , the chair and Walter J. Hamlin Professor of Dermatology and a pediatric dermatologist at the Ann & Robert H. Lurie Children’s Hospital of Chicago.
Having a chronic skin disease during childhood is not uncommon. Eczema affects more than 10 percent of school-aged children. Among teenagers, acne affects more than 90 percent and psoriasis 1 percent.
This is the first large, multi-site study of the psychosocial impact of skin diseases in children and teens.
The study showed that 73 percent of 1,671 children had experienced a measurable stigma, which was strongly associated with poor quality of life.
The disease severity and visibility as rated by the child (age eight and older) was quite different from that of the doctor’s ratings, suggesting the need to ask the child about the disease and its impacts.
The investigators used a newly developed scoring tool for stigma in school-aged children (PROMIS Pediatric Stigma) and collaborated with 31 sites in the Pediatric Dermatology Research Alliance to measure the extent of stigma, depression, anxiety, and poor peer relationships — and their association with an impaired quality of life.
“Stigma, which is when something false and negative is attached to an individual, can have a profound effect on children’s and teens’ mental health,” Paller said. “For example, a child with dark scales on the body can be called ‘dirty’ by other kids or a child with a hair loss issue can be shunned by other children who fear the hair loss is contagious.”
That can lead the child to internalize these thoughts, so these become their own perceptions. The false beliefs can convince other people around them that it’s true when it’s not. These kids often feel embarrassed or ashamed.”
The majority of the bullying and teasing occurs in school, Paller said.
“These painful experiences can shape a child’s personality into adulthood and erode self-confidence,” Paller said. “Children may underestimate their abilities and worry about taking social risks. They don’t feel good enough and this shame may affect them lifelong.”
Kids also may not be able to concentrate because they are worried in school, affecting their performance, Paller said.
“The study results should encourage clinicians to aggressively treat skin disorders in children and consider referral to evaluation and counseling of the child and potentially family if mental health issues occur,” Paller said.
Doctors need to ask children and parents about the impact of these diseases — stigma, mental health, how it impacts life — not just note the observable clinical manifestations.
It’s important to refer families to dermatologists for optimal treatment to decrease severity and visibility, which contribute to psychosocial impacts.”
Paller also suggested parents ask teachers to discuss the skin disease in the classroom, so other children understand it better. “Try to diminish the stigma through education and talk about and recognize bullying,” Paller said.
Meantime, things are looking up for David this year. He has made a new friend, a buddy he goes fishing with after school. “I found a friend who doesn’t care about me having a skin issue. He sees me on the inside for who I am. These are the friends you need to be around.”
Related Posts
Gene therapies could transform treatment of rare blood disorders, tech can’t replace human coaches in obesity treatment, new mechanisms underlying tumor variety in brain cancers discovered.
Comments are closed.
Type above and press Enter to search. Press Esc to cancel.
News : New treatment could reverse hair loss caused by an autoimmune skin disease
A microneedle patch that delivers immune-regulating molecules can teach T cells not to attack hair follicles, helping hair to regrow.
Anne Trafton | MIT News
Researchers at MIT, including at IMES, Brigham and Women’s Hospital (BWH), and Harvard Medical School (HMS), have developed a potential new treatment for alopecia areata, an autoimmune disorder that causes hair loss and affects people of all ages, including children.
For most patients with this type of hair loss, there is no effective treatment. The team developed a microneedle patch that can be painlessly applied to the scalp and releases drugs that help to rebalance the immune response at the site, halting the autoimmune attack.
In a study of mice, the researchers found that this treatment allowed hair to regrow and dramatically reduced inflammation at the treatment site, while avoiding systemic immune effects elsewhere in the body. This strategy could also be adapted to treat other autoimmune skin diseases such as vitiligo, atopic dermatitis, and psoriasis, the researchers say.
“This innovative approach marks a paradigm shift. Rather than suppressing the immune system, we’re now focusing on regulating it precisely at the site of antigen encounter to generate immune tolerance,” says Natalie Artzi , a principal research scientist in MIT’s Institute for Medical Engineering and Science (IMES), an associate professor of medicine at HMS and BWH, and an associate faculty member at the Wyss Institute of Harvard University.
Artzi and Jamil R. Azzi, an associate professor of medicine at HMS and BWH, are the senior authors of the new study , which appears in the journal Advanced Materials . Nour Younis, a BWH postdoc, and Nuria Puigmal, a BWH postdoc and former MIT research affiliate, are the lead authors of the paper.
The researchers are now working on launching a company to further develop the technology, led by Puigmal, who was recently awarded a Harvard Business School Blavatnik Fellowship.
Direct delivery
Alopecia areata, which affects more than 6 million Americans, occurs when the body’s own T cells attack hair follicles, leading the hair to fall out. The only treatment available to most patients — injections of immunosuppressant steroids into the scalp — is painful and patients often can’t tolerate it.
Some patients with alopecia areata and other autoimmune skin diseases can also be treated with immunosuppressant drugs that are given orally, but these drugs lead to widespread suppression of the immune system, which can have adverse side effects.
“This approach silences the entire immune system, offering relief from inflammation symptoms but leading to frequent recurrences. Moreover, it increases susceptibility to infections, cardiovascular diseases, and cancer,” Artzi says.
A few years ago, at a working group meeting in Washington, Artzi happened to be seated next to Azzi (the seating was alphabetical), an immunologist and transplant physican who was seeking new ways to deliver drugs directly to the skin to treat skin-related diseases.
Their conversation led to a new collaboration, and the two labs joined forces to work on a microneedle patch to deliver drugs to the skin. In 2021, they reported that such a patch can be used to prevent rejection following skin transplant. In the new study, they began applying this approach to autoimmune skin disorders.
“The skin is the only organ in our body that we can see and touch, and yet when it comes to drug delivery to the skin, we revert to systemic administration. We saw great potential in utilizing the microneedle patch to reprogram the immune system locally,” Azzi says.
The microneedle patches used in this study are made from hyaluronic acid crosslinked with polyethylene glycol (PEG), both of which are biocompatible and commonly used in medical applications. With this delivery method, drugs can pass through the tough outer layer of the epidermis, which can’t be penetrated by creams applied to the skin.
“This polymer formulation allows us to create highly durable needles capable of effectively penetrating the skin. Additionally, it gives us the flexibility to incorporate any desired drug,” Artzi says. For this study, the researchers loaded the patches with a combination of the cytokines IL-2 and CCL-22. Together, these immune molecules help to recruit regulatory T cells, which proliferate and help to tamp down inflammation. These cells also help the immune system learn to recognize that hair follicles are not foreign antigens, so that it will stop attacking them.
Hair regrowth
The researchers found that mice treated with this patch every other day for three weeks had many more regulatory T cells present at the site, along with a reduction in inflammation. Hair was able to regrow at those sites, and this growth was maintained for several weeks after the treatment ended. In these mice, there were no changes in the levels of regulatory T cells in the spleen or lymph nodes, suggesting that the treatment affected only the site where the patch was applied.
In another set of experiments, the researchers grafted human skin onto mice with a humanized immune system. In these mice, the microneedle treatment also induced proliferation of regulatory T cells and a reduction in inflammation.
The researchers designed the microneedle patches so that after releasing their drug payload, they can also collect samples that could be used to monitor the progress of the treatment. Hyaluronic acid causes the needles to swell about tenfold after entering the skin, which allows them to absorb interstitial fluid containing biomolecules and immune cells from the skin.
Following patch removal, researchers can analyze samples to measure levels of regulatory T cells and inflammation markers. This could prove valuable for monitoring future patients who may undergo this treatment.
The researchers now plan to further develop this approach for treating alopecia, and to expand into other autoimmune skin diseases.
The research was funded by the Ignite Fund and Shark Tank Fund awards from the Department of Medicine at BWH.
* Originally published in MIT News.
MINI REVIEW article
Advances in vitiligo: update on therapeutic targets.

- Department of Dermatology, Jiangsu Province People’s Hospital and Nanjing Medical University First Affiliated Hospital, Nanjing, China
Vitiligo, whose treatment remains a serious concern and challenge, is an autoimmune skin disease characterized by patches of depigmentation. The increasing application of molecular-targeted therapy in skin diseases, such as psoriasis and systemic lupus erythematosus, has dramatically improved their condition. Besides, there is a favorable effect of repigmentation in the treatment of the above diseases combined with vitiligo, implying that molecular-targeted therapy may also have utility in vitiligo treatment. Recently, the role of cytokine and signaling pathways in vitiligo pathogenesis are increasingly recognized. Thus, investigations are underway targeting the molecules described above. In this paper, we present a synopsis of current practices in vitiligo treatment and introduce the improvement in identifying new molecular targets and applying molecular-targeted therapies, including those under development in vitiligo treatment, providing valuable insight into establishing further precision medicine for vitiligo patients.
1 Introduction
Vitiligo is a primary, circumscribed, or generalized depigmentation of the skin and mucosa, related to genetic factors, self-destruction of melanocytes, cytokines, autoimmunity, and oxidative stress ( 1 ). While the detailed molecular mechanisms still require further investigation. In recent years, various studies have showed that the IFN-γ-CXCL9/10-CXCR3 axis appears to be important in vitiligo, via inhibiting melanogenesis, inducing apoptosis of melanocytes, and further recruiting T cells to the skin. These are all involved in the JAK/STAT pathway. In addition, cytokine, including HSP70i, IL-15, IL-17/23, TNF as well as wnt signaling pathway, Tregs, miRNAs have also been proved to be involved in the pathogenesis of vitiligo.
Vitiligo can be treated by different modalities of phototherapy, surgical procedures, and topical therapies, such as glucocorticosteroids, immunosuppressive agents, calcineurin inhibitors, and vitamin D. However, current treatments for vitiligo remain suboptimal, which may not be equally effective in all vitiligo patients, and it would be inconvenient for patients to visit clinics for phototherapy. Targeted therapies, such as biologics targeting cytokines and small-molecule inhibitors targeting intracellular signaling molecules, are recently emerging as promising therapeutics for autoimmune diseases. Their applications also promote our understanding of the detailed molecular mechanism of vitiligo and are essential for guiding a more precise vitiligo treatment. In this article, details of the roles that related cytokines and pathways play as well as the efficacy of targeted therapy have been described.
2 Current treatment
Topical, systemic treatment, and phototherapy are useful for stabilization and repigmentation of vitiligo. Treatment modalities are chosen in the individual patient, based on disease severity, disease activity (stable versus progressive disease), patient preference (including cost and accessibility), and response evaluation. For rapidly progressive disease, low-dose oral glucocorticoids and phototherapy are useful in stabilizing the disease. Therapeutic options for stable, segmental vitiligo include topical therapies (eg, topical corticosteroids, topical calcineurin inhibitors), targeted phototherapy, and surgical therapy (tissue grafts and cellular grafts) ( Table 1 ) ( 14 ). In recent years, attempts have been made to improve the repigmentation of vitiligo phototherapy by combination therapies, including NB-UVB with glucocorticoids ( 15 ), and topical calcineurin inhibitors ( 16 ). While their positive results were not confirmed in all studies. However, the method of treatment described, which were nonspecific, general, off-label, non-targeted with modest efficacy led to the problem of recurrence after stopping treatment. Therefore, efforts should be made to achieve a more comprehensive understanding of vitiligo pathogenesis to develop novel effective therapies ( Table 2 ).
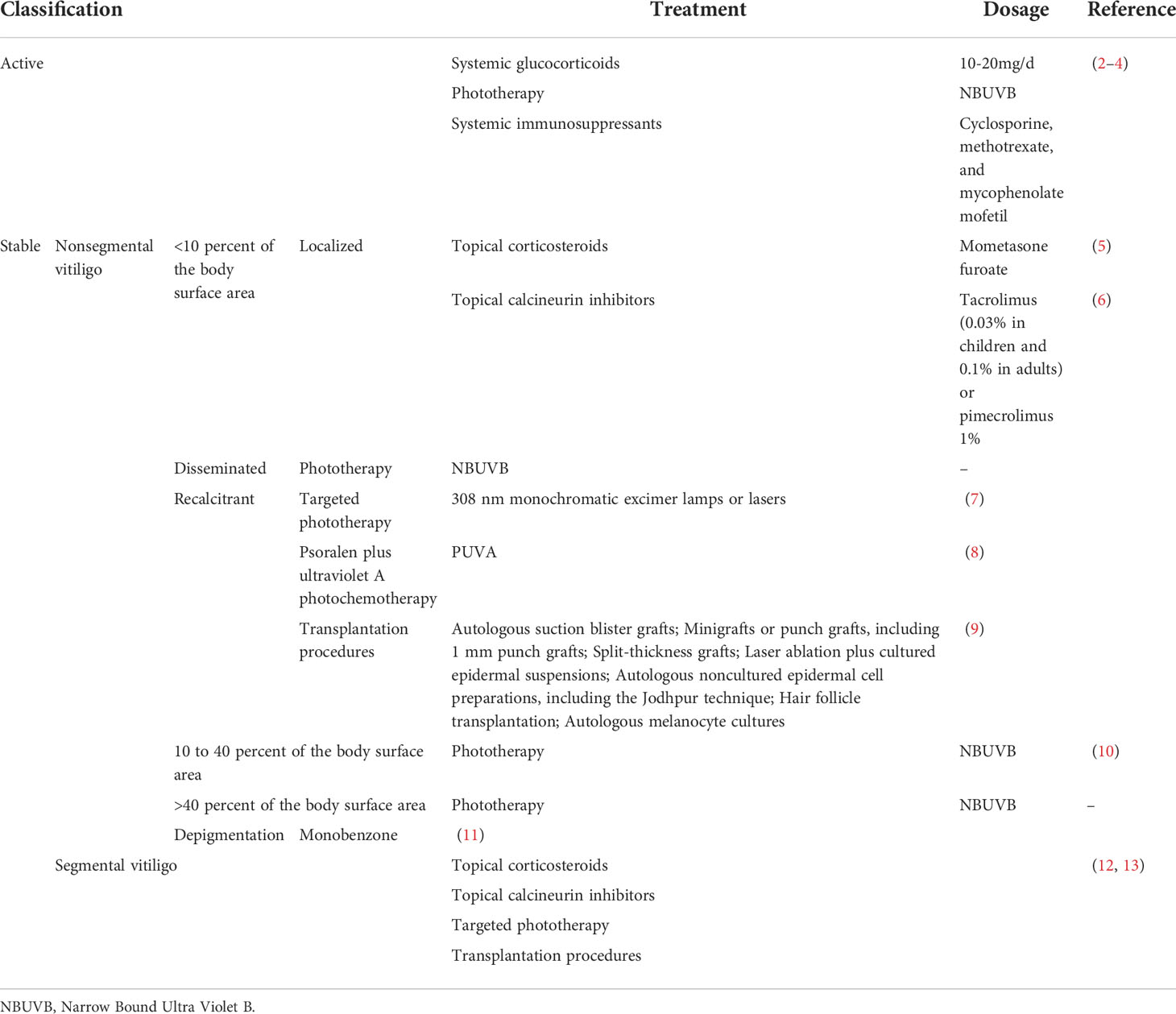
Table 1 Current treatment modalities for vitiligo.
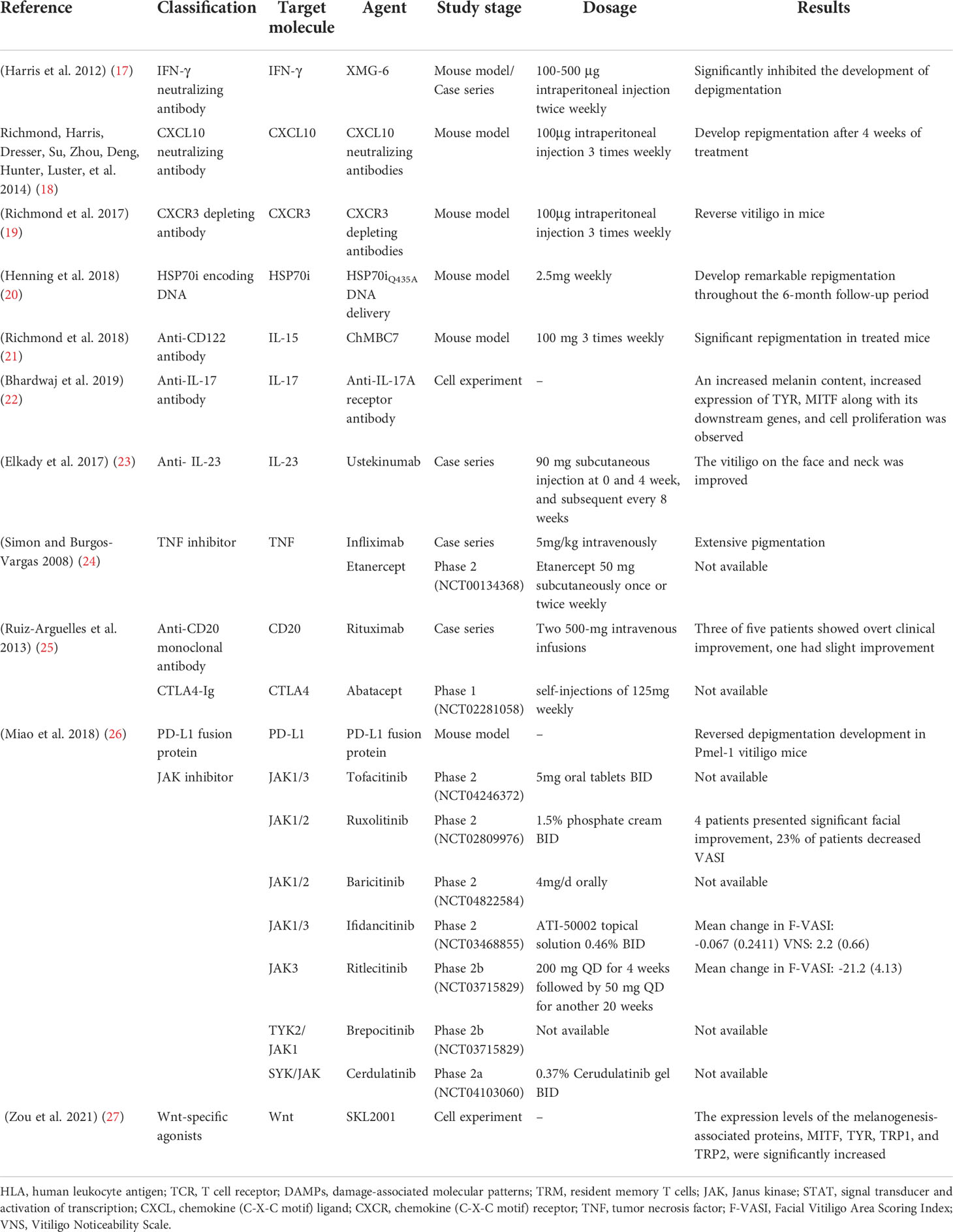
Table 2 Molecular-targeted therapies for the treatment of vitiligo.
3 Small molecules
3.1 emerging therapeutics targeting janus-activated kinase (jak) signaling.
The Janus kinases family consists of JAK1, JAK2, JAK3, and TYK2, which is engaged in the important JAK/STAT pathway, exhibiting pleiotropic effects on transducing multiple extracellular signals involved in regulating proliferative signaling, differentiation, migration, and apoptotic properties ( 28 ).
There are no licensed JAK/STAT inhibitors available against dermatological problems, however, some of them (ruxolitinib and tofacitinib) are used to treat other conditions such as myelofibrosis and RA. However, off-label usage of these medications in the treatment of vitiligo has shown promising outcomes.
JAK-STAT inhibitors promote Sonic Hedgehog and Wnt signaling in epidermal pigmentation, with the former inducing the migration, proliferation, and differentiation of melanocyte ( 29 ). Expanding our knowledge of these medications’ efficacy and safety profiles, as well as their use in dermatological conditions, is critical for establishing their risk-benefit ratio.
3.1.1 Tofacitinib
Tofacitinib is an FDA-cleared JAK1/3 inhibitor for treating RA, PsA, and active ulcerative colitis.
Tofacitinib 5-10 mg QD/BID has demonstrated superior efficacy against vitiligo, with improvement ratios of 5.4% in 5/10 patients with sun-exposed areas or areas treated only with phototherapy ( 30 ), and a reduced rate in vitiligo area scoring index (VASI) score of 4.68 at baseline to 3.95 at 5 months in another trial ( 31 ). In addition, a decline in the number of CD8 + T cells and chemokines, such as CXCL9 and CXCL10 has been observed after tofacitinib treatment, but no variations were observed for the percentage of melanocyte-specific T cells ( 30 ).
Unfortunately, this oral medication is associated with a host of systemic side effects, including infections, malignancies, and cytopenia. Thus, topical JAK inhibitors may be more preferred. 11 vitiligo patients treated with 2% tofacitinib cream twice a day in conjunction with NB-UVB therapy thrice-weekly demonstrated a mean improvement of 70% in facial VASI. There was also a significant difference between facial and non-facial lesions (P=0.022) ( 32 ).
3.1.2 Ruxolitinib
Ruxolitinib, the first Jakinib to get FDA approval, is a JAK1/2 inhibitor designed to deal with polycythemia vera and intermediate- and high-risk primary myelofibrosis ( 33 ).
Studies have shown that except for JAK inhibition, ruxolitinib also inhibited the differentiation and migration of DCs in vitiligo, increasing CD8 + cytotoxic T cell responses ( 34 ). In a double-blind phase 2 trial, 157 recruited vitiligo patients were randomized, in a 1:1:1:1:1 ratio, to receive topical ruxolitinib cream 1.5% BID, 1.5% QD, 0.5% QD, 0.15% QD, or a vehicle for 24 weeks, with the result showing considerably decreased CXCL9 and CXCL10 expression in 1.5% BID and 1.5% QD groups, and more individuals in groups receiving ruxolitinib cream 1.5% BID, 1.5% QD and 0.5% QD achieving F-VASI50, during which 1.5% BID group produced the highest responses in F-VASI50 (58%), F-VASI75 (52%), and F-VASI90 (33%). Besides, three positive responsive groups demonstrated significant repigmentation of vitiligo lesions and acceptable tolerability with a follow-up period of 52 weeks ( 35 ). Vitiligo on the face appears to respond more vigorously to therapy than non-facial lesions, reinforced by a 20-week, open-label trial in which patients with significant facial involvement experienced a 76% improvement in facial VASI scores ( 36 ). Furthermore, better repigmentation rates could be achieved both in oral and topical ruxolitinib treatment combined with phototherapy ( 37 ).
3.1.3 Baricitinib
Baricitinib is a selective JAK1/2 inhibitor that inhibits signal transduction of numerous proinflammatory cytokines ( 38 ), approved for the treatment of RA. To our knowledge, there was only one case report describing repigmentation in vitiligo patients with baricitinib 4 mg daily for the treatment of RA. Besides, an ongoing phase 2 trial (NCT04822584) in which patients received a combination therapy of baricitinib 4mg/d and phototherapy is being performed.
3.1.4 Ifidancitinib (ATI-50002)
Ifidancitinib is another dual JAK1/3 inhibitor for alopecia areata treatment, which is now undergoing phase II clinical trials for its application in vitiligo treatment. Patients with facial NSV(NCT03468855) receiving topical ATI-50002 BID for 24 weeks presented with an improved F-VASI and the Vitiligo Noticeability Scale (VNS) ( 39 ).
3.1.5 Ritlecitinib (PF-06651600) and Brepocitinib (PF-06700841)
Ritlecitinib, an irreversible inhibitor of JAK3 and tyrosine kinase applicable to the treatment of moderate-to-severe RA ( 40 ) and Brepocitinib, a TYK2/JAK1 inhibitor, are currently undergoing evaluation of their efficacy and safety profile in active NSV in combination with phototherapy (NCT03715829) ( 41 ).
3.1.6 Cerdulatinib (PRT062070)
Cerdulatinib, an SYK/JAK dual kinase inhibitor ( 42 ), has been assessed (NCT04103060) for its safety and tolerability for vitiligo treatment in topical formation (0.37% cerudulatinib gel BID).
However, additional studies are needed to determine the best-suited drug regimen and recommended dosage forms and doses to attain the optimum curative effect and minimal toxicity. As the occurrence of depigmentation after the withdrawal of JAK inhibitors, the mechanisms underlying need further exploration, and more work need to be done to corroborate the effectiveness in combination with other therapies.
3.2 Wnt signaling and its agonists
It has been shown that Wnt/β-catenin signaling plays a pivotal role in the proliferation, migration, and differentiation of melanocytes in vitiligo patients ( 29 ), which could be inhibited by oxidative stress ( 43 ). In addition, the Wnt/β-catenin pathway participates in the activation of MITF and its downstream enzymes ( 44 ). Intradermal injection of IWR-1 (inhibitor of Wnt response 1), a chemical inhibitor of β-catenin activation, and small interfering RNA (siRNA) against Wnt7α suppressed the number of epidermal melanocytes ( 45 ). This evidence suggested that stimulation of Wnt signaling may be an adjuvant therapy for vitiligo treatment. Micro-injury ( 46 ) as well as some phenanthridine-derived Wnt-specific agonists binding with the Axin protein have been proved to promote melanogenesis ( 47 ) and induce repigmentation.
3.3 Emerging therapeutics targeting microRNAs (miRNAs)
MiRNAs, which are a highly conservative small class of non-coding RNA molecules, participate in mRNA expression regulation via degradation or repression of mRNA translation ( 48 ). Previous studies have demonstrated that miRNAs were associated with genetic polymorphisms (e.g., miR-196a-2 rs11614913), immune response (e.g., miR-133b, miR-224-3p, miR-4712-3p, miR-3940-5p, miR-21−5p), oxidative stress (e.g., miR-135a, miR-9, miR-34a, miR-183, miR-184, miR-1, miR-25, miR-211, miR-383, miR-577, miR-421) and melanocyte functions (e.g., miR-434-5p, miR-330-5p, miR-137, miR-148, miR-145, miR-155, miR-203, miR-125, miR-377, miR-2909, miR-200c, hsa-miR-149-5p) ( 49 – 54 ), participating in pathological mechanism of vitiligo. These findings suggest that miRNAs may be involved in vitiligo pathogenesis via the modulation of vital genes expression in melanocytes and serve as novel therapeutic targets for vitiligo therapy.
There are two strategies for the therapeutic application of miRNAs: 1) anti-miRNAs, locked-nucleic acids (LNA), or antagomiRs ( 55 ) can be used to counteract the over-activation of miRNA. Short tandem target mimic (STTM)- miR-508-3p has been validated to upregulate SOX6 expression, leading to increased expression of key melanogenic genes CREB, MITF, TYR, and TYRP1/2 with increased melanogenesis ( 56 ). Besides, STTM-miR-143-5p also upregulates the expression of MYO5A, leading to an increase in the level of MITF, TYR, TYRP1, melanin, and Rab27a ( 57 ). 2) miRNA replacement, involving the reintroduction of a gene-suppressor miRNA mimic or AAV (adeno-associated virus)-mediated miRNA gain-of-function to modulate gene expression ( 55 ). A study demonstrated that the migratory capacity of melanocytes was altered by the application of miR-211 mimic through the p53-TRPM1/miR-211-MMP9 axis ( 58 ).
3.4 Emerging therapeutics targeting regulatory T-cells (Tregs)
Tregs are a suppressive CD4 + T cell subset that possesses a capacity to suppress self-reactive T cell activation and expansion ( 59 ). A clear decrease in Treg cells was observed in vitiligo skin within lesional, non-lesional, and perilesional sections ( 60 ), indicating that increasing the number of Tregs with normal function might be an important therapeutic intervention for vitiligo treatment.
Infusing purified populations of Tregs is the most direct way for the supply of Tregs. The current methods mainly include polyclonally-expanded Tregs, antigen-specific Tregs, and engineered Treg cells. In a mouse model of vitiligo, adoptive transfer of polyclonal Tregs may be effective in the short-term ( 61 ), which might however impart systemic immunosuppression ( 62 ). Besides, a TCR transgenic mouse with spontaneous vitiligo, receiving CAR Tregs treatment, developed a significant delay in depigmentation ( 63 ).
However, a limitation of infusing purified populations of Tregs might be the technical difficulty for therapeutic agent delivery to specific cells. A topical application of Tregs or the combination with CCR4 Treg homing receptor ligand CCL22 ( 64 ) by local needle-free jet injection of DNA ( 20 ) or CCL22-encoding plasmid DNA ( 64 ) may help resolve that issue. Besides, various strategies have been applied towards the modulation of Tregs function by targeting Treg-intrinsic pathways and functional modulators for Tregs. HO-1, a functional modulator of Tregs, was decreased in vitiligo Tregs. Treatment with Hemin, an agonist of HO-1, was found to enhance HO-1-induced restoration of Tregs function by up-regulating IL-10 expression ( 65 ). In addition, therapeutic method for microbiota modulation, such as neomycin treatment can significantly delay depigmentation in vitiligo mice and promote the infiltration of Tregs to the skin ( 66 ). Rapamycin, an inhibitor of PI3Kakt-mTORC1 signaling ( 67 ), efficiently halts the depigmentation process by increasing the abundance of Treg in h3TA2 mice, which effect lasted till 6 weeks after treatment ( 61 ). At present, a phase 2 clinical trial(NCT05342519) is underway for assessing the efficacy of the application of 0.1% topical rapamycin ( 68 ) (2022). In addition, nanoparticles containing rapamycin and autoantigen HEL46-61(NPHEL46-61/Rapa) were synthesized, the administration of which halted the disease progression ( 69 ). Also, the calcium-NFATc1-signaling pathway may be involved in defective Tregs function, indicating a potential therapeutic target for vitiligo treatment ( 70 ).
4 Cytokine-targeted therapies
Multiple monoclonal antibodies are available for vitiligo treatment, targeting IFN-γ, CXCL10, CXCR3, HSP70i, IL-15, IL-17/23, and TNF. In addition to full-size immunoglobulin, affibodies and nanobodies, composed of considerably smaller proteins, are currently being developed, which have higher bioavailability as well as affinity and specificity to the targeted molecules.
4.1 IFN-γ and the inhibitors
The IFN-γ-CXCL9/10-CXCR3 axis may be crucial for vitiligo pathogenesis, contributing to disease progression by inhibiting melanogenesis, inducing apoptosis of melanocytes, and further recruiting T cells to the skin ( Figure 1 ) ( 71 ). A study showed a higher expression of IFN-γ mRNA in non-lesional and perilesional skin, especially in active vitiligo ( 72 ), which is associated with disease activity ( 73 ).
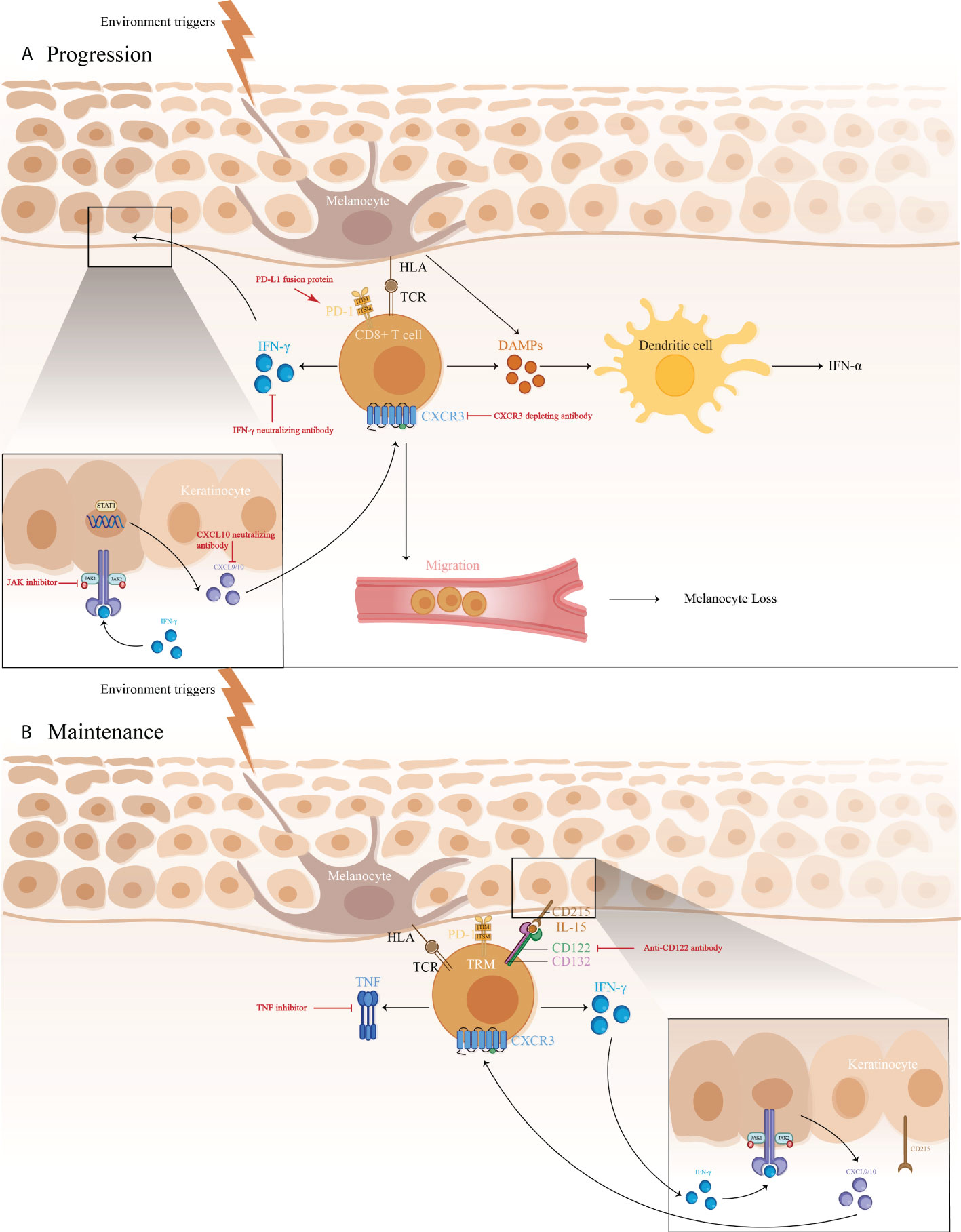
Figure 1 1) The immune pathogenesis of vitiligo: (A) CD8 + T cell expression of IFN-γ in vitiligo lesions activated the JAK/STAT pathway after binding to IFN-γ receptor, thus facilitating the release of CXCL9/10. The binding of CXCL9/10 to CXCR3 increased CXCR3+ T cells recruitment; (B) Maintenance of vitiligo lesions was influenced by the function of IL-15-dependent TRM cells, which produce IFN-γ and TNF-α. 2)Targeted therapeutic interventions in vitiligo mainly include therapies targeting IFN-γ-CXCL9/10-CXCR3 axis (IFN-γ neutralizing antibody, CXCL10 neutralizing antibody, and CXCR3 depleting antibody, as well as JAK inhibitors), anti-CD122 antibody (IL-15 receptor subunit) to decrease IFN-γ production and deplete autoreactive CD8 + TRM cells, TNF inhibitor to inhibit autoantibody production, and PD-L1 fusion protein to reduce the numbers of melanocyte-reactive T cells.
Anti-IFN-γ can have been proved to be effective in rheumatoid arthritis (RA), multiple sclerosis (MS), prevention of corneal rejection, autoimmune skin diseases, and others. In a recent study, vitiligo induction mice, treated with intraperitoneal injection with IFN-γ neutralizing antibody (XMG-6) at a dose of 100-500 μg twice a week, presented with significant improvement of depigmentation ( 17 ), with the same trend observed in vitiligo patients. Four patients who received intradermal perilesional injections presented with repigmentation of the treated area and boundary retreat ( 74 ). More research is warranted to be initiated for further definition of the role that IFN-γ plays in vitiligo and to examine whether IFN-γ neutralization would be more viable in reversing skin depigmentation.
4.2 CXCL10 and the inhibitors
Recent studies report a Th1/IFN-γ immune response in both human and a mouse model of vitiligo that involves elevated CXCL9, 10, and 11 productions, among which CXCL10 participated in the targeted migration of T cells ( 18 ), triggering an immune cell infiltration at the early stage ( 72 ), and involved in the downregulation of keratinocyte glycoprotein non-metastatic melanoma protein B (GPNMB) ( 75 ). A study showed that mice receiving CXCL10 neutralizing antibodies developed more repigmentation after 4 weeks’ treatment, which continued for an additional 4 weeks ( 18 ), thereby supporting CXCL10 suppression as a great therapeutic strategy.
4.3 CXCR3 antibodies
CXCR3 has been proved to be expressed in skin lesions, autoreactive T cells ( 18 ), and the vast majority of skin infiltrating CD8 + resident memory T cells (TRM), which stimulate the secretion of IFN-γ and TNF-α ( 76 ).
In a study, vitiligo mice with >75% depigmentation on their tails are treated with CXCR3 depleting antibodies for 7-8 weeks, which significantly reversed the clinical disease in a perifollicular pattern and a diminution of PMEL in the epidermis, with slightly reduced host CD8 + T cell numbers ( 19 ) compared to neutralizing antibody treatment ( 18 ). Although these results are preliminary, they may provide justification for further studies in targeting CXCR3 in vitiligo ( 19 ), which proposes the use of a depleting Ab to create a greater clinical efficacy by removing autoreactive cells rather than modulating their migration phenotype.
4.4 Inducible HSP70 (HSP70i) DNA
Indeed, HSP70i is the core participant in vitiligo predominantly through HSP70i-plasmacytoid dendritic cells (pDCs)-IFN-α-CXCL9 and CXCL10-cytotoxic T lymphocyte (CTL) axis. Pmel-1 mice vaccinated with HSP70i encoding DNA exhibited significant depigmentation, rarely seen in models knockout for HSP70i, indicating that elevated HSP70i expression alone would be enough to induce depigmentation in vitiligo prone animals ( 77 ). A study revealed that the expression of HSP-70 mRNA in skin lesions of active vitiligo patients was much higher ( 78 ), correlated with the disease activity.
Blocking HSP70i activity might have the potential to reverse vitiligo development. A recent study showed that a Sinclair swine, receiving HSP70iQ435A-encoding DNA treatment, showed remarkable repigmentation with an initial influx of T cells and increased CD4/CD8 ratios ( 20 ), which was also detected in mice with HSP70i Q435A -encoding DNA treatment, resulting in 76% restoration of skin pigmentation. Furthermore, the treatment halted T cells accumulation and transition to T cell phenotype in mice and human skin, engaging HSP70i Q435A DNA delivery as a potent effective therapeutic intervention for vitiligo ( 79 ).
4.5 IL-15 and the inhibitors
It has been established that IL-15 seems to participate in IL-17 regulation and maintenance of TRM signals ( 80 ), with the latter responsible for long-term maintenance and potential relapse of vitiligo ( 81 ). The study has demonstrated a higher serum level of IL-15 in vitiligo patients than in controls, highly associated with epidermal H 2 O 2 content and the disease activity ( 82 , 83 ).
In vitiligo mice, an anti-CD122 antibody that targets IL-15 signaling was reported to effectively reverse depigmentation. Anti-CD122 therapy, either systemically or locally, decreases TRM-induced IFN-γ production and results in long-term repigmentation. These findings consider CD122-targeted drugs as a valid therapy method, which results in effective and long-lasting responses in vitiligo and other tissue-specific autoimmune disorders involving TRM ( 21 ).
4.6 PD-1/PD-L1 pathway
Involvement of the PD-1/PD-L1 pathway has been shown in many autoimmune diseases, including RA, MS, and vitiligo. PD-L1 expression was found limited in normal skin, and only expressed on dermal T cells, and increased in primary melanocytes and fibroblasts after exposure to IFN-γ. No such effect was seen in vitiligo patients, indicating the absence of self-protection ability for melanocytes against T-cell attack during vitiligo pathogenesis. In agreement with this, treatment with PD-L1 fusion protein reduced the numbers of melanocyte-reactive T cells, inhibited the activation of Vβ12-expressing T cells, and increased Tregs numbers, reversing depigmentation in a Pmel-1 T-cell receptor transgenic vitiligo mouse model ( 26 ). However, PD-L1 treatment may still call for extended phototherapy treatment, especially NB-UVB therapy, which likely upregulates PD-L1 expression in an NF-κB-dependent manner ( 84 ), indicating a combination use of local PD-1/PD-L1 agonistic treatment and NB-UVB therapy as a promising option.
4.7 Other cytokine-targeted therapies under investigation
4.7.1 il-17/23 and the inhibitors.
Studies on the effect of IL-17/23 in vitiligo resulted in contradictory findings. On one hand, Th17 cells and IL-17 in vitiligo patients may inhibit function-related factors, repress melanogenesis, and dramatically induct other Th17 type cytokines as well as IL-1β production from dermal fibroblasts and keratinocytes ( 85 ). Elevated Th17 cells and IL-17/23 levels in skin lesions and serum of vitiligo patients, were positively correlated with disease activity ( 86 , 87 ), and decreased after narrowband ultraviolet B (NBUVB) treatment ( 88 ). Primary melanocyte culture showed an increased expression of MITF and its downstream genes, increased melanin pigment, and cell proliferation after blockade with anti-IL-17RA ( 22 ). Besides, incidences of repigmentation have been documented in ustekinumab treatment of vitiligo ( 23 ). However, secukinumab treatment in patients with active non‐segmental vitiligo (NSV) contributed to disease progression in 7/8 patients with no general reduction in CXCL9/10, sCD25/27, Th1 cells, or cytotoxic cells, resulting in early termination of study ( 89 ). There are also reports of ustekinumab-induced new-onset vitiligo and alopecia areata. The above studies showed IL-17/23 signal may not play a direct role in vitiligo pathogenesis, which needs further investigation to confirm this conjecture.
4.7.2 TNF and the inhibitors
As an anti-inflammatory mediator, TNF-α is considered to play a role in vitiligo, which may promote apoptosis in melanocytes, induce B-cell activation, increase autoantibody production, and inhibit melanogenesis ( 90 ). Recent data has shown a significantly higher expression of TNF-α in vitiligo skin. TNF inhibitors are beneficial in the treatment of plaque-type psoriasis, psoriatic arthritis (PsA), RA, and inflammatory bowel disease (IBD), arousing growing interest in their use in vitiligo.
Infliximab is a chimeric anti-TNF-α monoclonal antibody specifically binding to both soluble and membrane-bound TNF ( 91 , 92 ). Intravenous infliximab is widely licensed in the treatment of RA, psoriasis, ankylosing spondylitis (AS), IBD, uveitis, and Behcet’s disease. A 24-year-old patient with ankylosing spondylitis and refractory vitiligo improved significantly following six months of infliximab therapy at a dose of 5mg/kg intravenously in weeks 0, 2, and 6, and then every eight weeks for ten months ( 24 ). Besides, Etanercept is a monoclonal antibody targeted against TNF-α ( 93 ), which has been approved for the treatment of RA, juvenile RA, AS, psoriasis, and PsA. Treatment with etanercept 50 mg subcutaneously once or twice weekly for at least 2 months has shown a great curative effect on established vitiligo ( 94 ).
However, it has been shown that anti‐TNF‐α agents, especially adalimumab and infliximab ( 95 ), may exacerbate established vitiligo and induce new-onset vitiligo during treatment of other autoimmune diseases, including AS ( 96 ), Crohn’s disease ( 97 ), ulcerative colitis ( 98 ), psoriasis ( 99 ), and RA ( 100 ). The mechanism responsible for the TNF-α inhibitors-induced vitiligo is not fully understood. On the one hand, TNF-α inhibitors may increase the nucleosome-mediated autoantibody formation, interfere with the cytotoxic T-cell suppression of autoreactive B cells, and decrease Treg synthesis and activation. Additionally, infliximab increases pDC-produced IFN-γ, participating in further T cells recruiting. Although very rare, new-onset or exacerbations of vitiligo can occur in the anti‐TNF‐α treatment of other autoimmune diseases, the risk of which must not be ignored.
4.7.3 Rituximab
Rituximab has specific affinity for the B-lymphocyte transmembrane protein, CD20, which is expressed on B cells ( 101 ), participating in the activation of the CD8 + T cells and the ensuing autoreactive reaction ( 102 ). Rituximab is licensed for the treatment of lymphomas, leukemias, transplant rejection crisis, and a series of autoimmune diseases ( 103 , 104 ). An intravenous infusion of Rituximab was administered to five active disseminated vitiligo patients, the three of whom exhibited a considerable improvement in both the disease’s symptoms and histology ( 25 ).
4.7.4 Abatacept
Abatacept, a fusion protein consisting of IgG1 coupled to the extracellular domain of CTLA-4 via the immunoglobulin’s Fc region, was licensed for treating moderate to severe RA. Ten eligible patients with active vitiligo have been included to receive self-injections of 125mg abatacept weekly from week 0 to week 24. Secondary endpoints will be evaluated during a 32-week follow-up visit ( 105 ).
5 Future therapeutic prospects
As a future direction, new therapeutic approaches should be developed to reduce vitiligo progression. Among the new approaches being developed, the strategy of targeting the IFN-γ-CXCL9/10-CXCR3 axis has been clinically tested. OPZELURA has been indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older. MiRNA-based therapeutics are also in development. However, the absence of organ or tissue selectivity may also lead to off-target side effects, which must be considered and excluded in the process of miRNA-based therapeutics development. Besides, a suitable vector system, as well as the assurance of chemical and biological stability should also be taken into account. Adoptive Treg cell therapy has also been the research hotspot in recent years. However, it has always been a difficult point for reassurance for safety and the development of the delivery system.
Treating vitiligo remains a challenge. As is presented in this paper, a greater variety of precision treatments is currently being studied. With a better understanding and further validation of these therapeutic targets, patients can be stratified to achieve individualized treatment.
6 Conclusion
Current models of treatment for vitiligo are often nonspecific and general. Various therapy options are available for active vitiligo patients, including systemic glucocorticoids, phototherapy, and systemic immunosuppressants. While stable vitiligo patients may benefit from topical corticosteroids, topical calcineurin inhibitors, phototherapy, as well as transplantation procedures. Recently, a better understanding of the pathophysiological processes of vitiligo led to the advent of novel targeted therapies. To date, JAK inhibitors are the only category that has been proved to have a good tolerability profile and functional outcomes in vitiligo treatment, even though the risk of activation of latent infection and systemic side effects still existed, like other immunosuppressive agents. Research is in progress to investigate the important cytokines involved in the pathogenesis of vitiligo, including IFN-γ, CXCL10, CXCR3, HSP70i, IL-15, IL-17/23, and TNF, the blockade of which has undergone preliminary attempts in animal models and some patients. In addition, studies on miRNA-based therapeutics as well as adoptive Treg cell therapy are still primary, and more studies are necessary.
Author contributions
YFF and YL contributed to the conceptual design, writing, editing, and generation of figures for this manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ezzedine K, Eleftheriadou V, Whitton M, van Geel N. Vitiligo. Lancet (London England) (20159988) 386:74–84. doi: 10.1016/S0140-6736(14)60763-7
CrossRef Full Text | Google Scholar
2. Bishnoi A, Vinay K, Kumaran MS, Parsad D. “Oral mycophenolate mofetil as a stabilizing treatment for progressive non-segmental vitiligo: results from a prospective, randomized, investigator-blinded pilot study”. Arch Dermatol Res (2021) 313(5):357–65. doi: 10.1007/s00403-020-02108-8
PubMed Abstract | CrossRef Full Text | Google Scholar
3. Garza-Mayers AC, Kroshinsky D. “Low-dose methotrexate for vitiligo”. Drugs Dermatol (2017) 16(7):705–6.
Google Scholar
4. Singh H, Kumaran MS, Bains A, Parsad D. A randomized comparative study of oral corticosteroid minipulse and low-dose oral methotrexate in the treatment of unstable vitiligo. Dermatology (2015) 231(3):286–90. doi: 10.1159/000433424
5. Taieb A, Alomar A, Bohm M, Dell'anna ML, De Pase A, Eleftheriadou V, et al. Guidelines for the management of vitiligo: The European dermatology forum consensus. Br J Dermatol (2013) 168(1):5–19. doi: 10.1111/j.1365-2133.2012.11197.x
6. Lee JiH, Kwon HS, Jung HMi, Lee H, Kim GM, Yim HW, et al. Treatment outcomes of topical calcineurin inhibitor therapy for patients with vitiligo: A systematic review and meta-analysis. JAMA Dermatol (2019) 155(8):929–38. doi: 10.1001/jamadermatol.2019.0696
7. Lopes C, Trevisani VF, Melnik T. Efficacy and safety of 308-nm monochromatic excimer lamp versus other phototherapy devices for vitiligo: A systematic review with meta-analysis. Am J Clin Dermatol (2016) 17(1):23–32. doi: 10.1007/s40257-015-0164-2
8. Zubair R, Hamzavi IH. Phototherapy for vitiligo. Dermatol Clinics (2020) 38(1):55–62. doi: 10.1016/j.det.2019.08.005
9. Mohammad TF, Hamzavi IH. Surgical therapies for vitiligo. Dermatol Clinics (2017) 35(2):193–203. doi: 10.1016/j.det.2016.11.009
10. Mohammad TF, Al-Jamal M, Hamzavi IH, Harris JE, Leone G, Cabrera R, et al. The vitiligo working group recommendations for narrowband ultraviolet b light phototherapy treatment of vitiligo. J Am Acad Dermatol (2017) 76(5):879–88. doi: 10.1016/j.jaad.2016.12.041
11. Grimes PE, Nashawati R. Depigmentation therapies for vitiligo. Dermatol Clinics (2017) 35(2):219.
12. Mulekar SV, Al Eisa A, Delvi MB, Al Issa A, Al Saeed AH. Childhood vitiligo: A long-term study of localized vitiligo treated by noncultured cellular grafting. Pediatr Dermatol (2010) 27(2):132–6. doi: 10.1111/j.1525-1470.2009.00978.x
13. Mulekar SV. Long-term follow-up study of segmental and focal vitiligo treated by autologous, noncultured melanocyte-keratinocyte cell transplantation. Arch Dermatol (2004) 140(10):1211–5. doi: 10.1001/archderm.140.10.1211
14. Bohm M, Schunter JA, Fritz K, Salavastru C, Dargatz S, Augustin M, et al. S1 guideline: Diagnosis and therapy of vitiligo. J Dtsch Dermatol Ges (2022) 20(3):365–78. doi: 10.1111/ddg.14713
15. Batchelor JM, Thomas KS, Akram P, Azad J, Bewley A, Chalmers JR, et al. Home-based narrowband UVB, topical corticosteroid or combination for children and adults with vitiligo: HI-light vitiligo three-arm RCT. Health Technol Assess (2020) 24(64):1–128. doi: 10.3310/hta24640
16. Li R, Qiao M, Wang X, Zhao X, Sun Q. Effect of narrow band ultraviolet b phototherapy as monotherapy or combination therapy for vitiligo: A meta-analysis. Photodermatol Photoimmunol Photomed (2017) 33(1):22–31. doi: 10.1111/phpp.12277
17. Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol (2012) 132(7):1869–76. doi: 10.1038/jid.2011.463
18. Rashighi M, Agarwal P, Richmond JM, Harris TH, Dresser K, Su M-W, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Trans Med (2014) 6(223):223ra23. doi: 10.1126/scitranslmed.3007811
19. Richmond JM, Masterjohn E, Chu R, Tedstone J, Youd ME, Harris JE. CXCR3 depleting antibodies prevent and reverse vitiligo in mice. J Invest Dermatol (2017) 137(4):982–5. doi: 10.1016/j.jid.2016.10.048
20. Henning SW, Fernandez MF, Mahon JP, Duff R, Azarafrooz F, Guevara-Patino JA, et al. HSP70iQ435A-encoding DNA repigments vitiligo lesions in Sinclair swine. J Invest Dermatol (2018) 138(12):2531–9. doi: 10.1016/j.jid.2018.06.186
21. Richmond JM, Strassner JP, Zapata L, Garg M, Riding RL, Refat MA, et al. Antibody blockade of IL-15 signaling has the potential to durably reverse vitiligo. Sci Trans Med (2018) 10(450):eaam7710. doi: 10.1126/scitranslmed.aam7710
22. Bhardwaj S, Bhatia A, Kumaran MS, Parsad D. Role of IL-17A receptor blocking in melanocyte survival: A strategic intervention against vitiligo. Exp Dermatol (2019) 28(6):682–9. doi: 10.1111/exd.13773
23. Elkady A, Bonomo L, Amir Y, Vekaria AS, Guttman-Yassky E. Effective use of ustekinumab in a patient with concomitant psoriasis, vitiligo, and alopecia areata. JAAD Case Rep (2017) 3(6):477–9. doi: 10.1016/j.jdcr.2017.07.009
24. Simon JA, Burgos-Vargas R. Vitiligo improvement in a patient with ankylosing spondylitis treated with infliximab. Dermatology (2008) 216(3):234–5. doi: 10.1159/000112932
25. Ruiz-Arguelles A, Garcia-Carrasco M, Jimenez-Brito G, Sanchez-Sosa S, Perez-Romano B, Garces-Eisele J, et al. Treatment of vitiligo with a chimeric monoclonal antibody to CD20: A pilot study. Clin Exp Immunol (2013) 174(2):229–36. doi: 10.1111/cei.12168
26. Miao X, Xu R, Fan B, Chen J, Li X, Mao W, et al. PD-L1 reverses depigmentation in pmel-1 vitiligo mice by increasing the abundance of tregs in the skin. Sci Rep (2018) 8(1):1605. doi: 10.1038/s41598-018-19407-w
27. Zou DP, Chen YM, Zhang LZ, Yuan XH, Zhang YJ, Inggawati A, et al. SFRP5 inhibits melanin synthesis of melanocytes in vitiligo by suppressing the wnt/beta-catenin signaling. Genes Dis (2021) 8(5):677–88. doi: 10.1016/j.gendis.2020.06.003
28. Rawlings JS, Rosler KM, Harrison DA. The JAK/STAT signaling pathway. J Cell Sci (2004) 117(Pt 8):1281–3. doi: 10.1242/jcs.00963
29. Birlea SA, Costin GE, Roop DR, Norris DA. Trends in regenerative medicine: Repigmentation in vitiligo through melanocyte stem cell mobilization. Med Res Rev (2017) 37(4):907–35. doi: 10.1002/med.21426
30. Liu LY, Strassner JP, Refat MA, Harris JE, King BA. Repigmentation in vitiligo using the janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol (2017) 77(4):675–82.e1. doi: 10.1016/j.jaad.2017.05.043
31. Vu M, Heyes C, Robertson SJ, Varigos GA, Ross G. Oral tofacitinib: A promising treatment in atopic dermatitis, alopecia areata and vitiligo. Clin Exp Dermatol (2017) 42(8):942–4. doi: 10.1111/ced.13290
32. Mobasher P, Guerra R, Li SJ, Frangos J, Ganesan AK, Huang V. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Br J Dermatol (2020) 182(4):1047–9. doi: 10.1111/bjd.18606
33. Deisseroth A, Kaminskas E, Grillo J, Chen W, Saber H, Lu HL, et al. U.S. food and drug administration approval: ruxolitinib for the treatment of patients with intermediate and high-risk myelofibrosis. Clin Cancer Res (2012) 18(12):3212–7. doi: 10.1158/1078-0432.CCR-12-0653
34. Heine A, Held SA, Daecke SN, Wallner S, Yajnanarayana SP, Kurts C, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo . Blood (2013) 122(7):1192–202. doi: 10.1182/blood-2013-03-484642
35. Rosmarin D, Pandya AG, Lebwohl M, Grimes P, Hamzavi I, Gottlieb AB, et al. Ruxolitinib cream for treatment of vitiligo: A randomised, controlled, phase 2 trial. Lancet (2020) 396(10244):110–20. doi: 10.1016/S0140-6736(20)30609-7
36. Rothstein B, Joshipura D, Saraiya A, Abdat R, Ashkar H, Turkowski Y, et al. Treatment of vitiligo with the topical janus kinase inhibitor ruxolitinib. J Am Acad Dermatol (2017) 76(6):1054–60.e1. doi: 10.1016/j.jaad.2017.02.049
37. Gianfaldoni S, Tchernev G, Wollina U, Roccia MG, Fioranelli M, Lotti J, et al. Micro - focused phototherapy associated to janus kinase inhibitor: A promising valid therapeutic option for patients with localized vitiligo. Open Access Maced J Med Sci (2018) 6(1):46–8. doi: 10.3889/oamjms.2018.042
38. Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs (2014) 23(8):1067–77. doi: 10.1517/13543784.2014.918604
39. A study of ATI-50002 topical solution for the treatment of vitiligo (2018). Available at: https://clinicaltrials.gov/ct2/show/NCT03468855 .
40. Robinson MF, Damjanov N, Stamenkovic B, Radunovic G, Kivitz A, Cox L, et al. Efficacy and safety of PF-06651600 (Ritlecitinib), a novel JAK3/TEC inhibitor, in patients with moderate-to-Severe rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Rheumatol (2020) 72(10):1621–31. doi: 10.1002/art.41316
41. A phase 2b study to evaluate the efficacy and safety profile of PF-06651600 and PF-06700841 in active non-segmental vitiligo subjects (2018). Available at: https://clinicaltrials.gov/ct2/show/NCT03715829 .
42. Coffey G, Betz A, DeGuzman F, Pak Y, Inagaki M, Baker DC, et al. The novel kinase inhibitor PRT062070 (Cerdulatinib) demonstrates efficacy in models of autoimmunity and b-cell cancer. J Pharmacol Exp Ther (2014) 351(3):538–48. doi: 10.1124/jpet.114.218164
43. Regazzetti C, Joly F, Marty C, Rivier M, Mehul B, Reiniche P, et al. Transcriptional analysis of vitiligo skin reveals the alteration of WNT pathway: A promising target for repigmenting vitiligo patients. J Invest Dermatol (2015) 135(12):3105–14. doi: 10.1038/jid.2015.335
44. Harris JE. Melanocyte regeneration in vitiligo requires WNT beneath their wings. J Invest Dermatol (2015) 135(12):2921–3. doi: 10.1038/jid.2015.372
45. Yamada T, Hasegawa S, Inoue Y, Date Y, Yamamoto N, Mizutani H, et al. Wnt/beta-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol (2013) 133(12):2753–62. doi: 10.1038/jid.2013.235
46. Han X, Chang Li, Qiu Z, Lin M, Wang Y, Liu D, et al. Micro-injury induces hair regeneration and vitiligo repigmentation through wnt/β-catenin pathway. Stem Cells Dev (2022) 31(5-6):111–8. doi: 10.1089/scd.2021.0276
47. Yang BJ, Fan SR, Zhang XF, Cai JY, Ruan T, Xiang ZR, et al. Design, synthesis and structure-activity relationship optimization of phenanthridine derivatives as new anti-vitiligo compounds. Bioorg Chem (2022) 119:105582. doi: 10.1016/j.bioorg.2021.105582
48. Baek D, Villen J, Shin C, Camargo FD, Gygi SP, Bartel DP. The impact of microRNAs on protein output. Nature (2008) 455(7209):64–71. doi: 10.1038/nature07242
49. Li L. The role of MicroRNAs in vitiligo: Regulators and therapeutic targets. Ann Dermatol (2020) 32(6):441–51. doi: 10.5021/ad.2020.32.6.441
50. Huo J, Liu T, Li F, Song X, Hou X. MicroRNA215p protects melanocytes via targeting STAT3 and modulating Treg/Teff balance to alleviate vitiligo. Mol Med Rep (2021) 23(1):51. doi: 10.3892/mmr.2020.11689
51. Vaish U, Kumar AA, Varshney S, Ghosh S, Sengupta S, Sood C, et al. Micro RNAs upregulated in vitiligo skin play an important role in its aetiopathogenesis by altering TRP1 expression and keratinocyte-melanocytes cross-talk. Sci Rep (2019) 9(1):10079. doi: 10.1038/s41598-019-46529-6
52. Zhao C, Wang D, Wang X, Mao Y, Xu Z, Sun Y, et al. Down-regulation of exosomal miR-200c derived from keratinocytes in vitiligo lesions suppresses melanogenesis. J Cell Mol Med (2020) 24(20):12164–75. doi: 10.1111/jcmm.15864
53. Li L, Xie Z, Qian X, Wang T, Jiang M, Qin J, et al. Identification of a potentially functional circRNA-miRNA-mRNA regulatory network in melanocytes for investigating pathogenesis of vitiligo. Front Genet (2021) 12:663091. doi: 10.3389/fgene.2021.663091
54. Shang Z, Li H. Altered expression of four miRNA (miR-1238-3p, miR-202-3p, miR-630 and miR-766-3p) and their potential targets in peripheral blood from vitiligo patients. J Dermatol (2017) 44(10):1138–44. doi: 10.1111/1346-8138.13886
55. Kwekkeboom RF, Lei Z, Doevendans PA, Musters RJ, Sluijter JP. Targeted delivery of miRNA therapeutics for cardiovascular diseases: opportunities and challenges. Clin Sci (Lond) (2014) 127(6):351–65. doi: 10.1042/CS20140005
56. Liu B, Zhang J, Yang S, Ji K, Liu X, Du B, et al. Effect of silencing microRNA-508 by STTM on melanogenesis in alpaca (Vicugna pacos). Gene (2018) 678:343–8. doi: 10.1016/j.gene.2018.08.011
57. Qi S, Liu B, Zhang J, Liu X, Dong C, Fan R. Knockdown of microRNA1435p by STTM technology affects eumelanin and pheomelanin production in melanocytes. Mol Med Rep (2019) 20(3):2649–56. doi: 10.3892/mmr.2019.10492
58. Su M, Miao F, Jiang S, Shi Y, Luo L, He X, et al. Role of the p53−TRPM1/miR−211−MMP9 axis in UVB−induced human melanocyte migration and its potential in repigmentation. Int J Mol Med (2020) 45(4):1017–26. doi: 10.3892/ijmm.2020.4478
59. Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med (2001) 193(11):1295–302.
PubMed Abstract | Google Scholar
60. Klarquist J, Denman CJ, Hernandez C, Wainwright DA, Strickland FM, Overbeck A, et al. Reduced skin homing by functional treg in vitiligo. Pigment Cell Melanoma Res (2010) 23(2):276–86. doi: 10.1111/j.1755-148X.2010.00688.x
61. Chatterjee S, Eby JM, Al-Khami AA, Soloshchenko M, Kang HK, Kaur N, et al. A quantitative increase in regulatory T cells controls development of vitiligo. J Invest Dermatol (2014) 134(5):1285–94. doi: 10.1038/jid.2013.540
62. Alzhrani A, Bottomley M, Wood K, Hester J, Issa F. Identification, selection, and expansion of non-gene modified alloantigen-reactive tregs for clinical therapeutic use. Cell Immunol (2020) 357:104214. doi: 10.1016/j.cellimm.2020.104214
63. Mukhatayev Z, Dellacecca ER, Cosgrove C, Shivde R, Jaishankar D, Pontarolo-Maag K, et al. Antigen specificity enhances disease control by tregs in vitiligo. Front Immunol (2020) 11:581433. doi: 10.3389/fimmu.2020.581433
64. Eby JM, Kang HK, Tully ST, Bindeman WE, Peiffer DS, Chatterjee S, et al. CCL22 to activate treg migration and suppress depigmentation in vitiligo. J Invest Dermatol (2015) 135(6):1574–80. doi: 10.1038/jid.2015.26
65. Zhang Q, Cui T, Chang Y, Zhang W, Li S, He Y, et al. HO-1 regulates the function of treg: Association with the immune intolerance in vitiligo. J Cell Mol Med (2018) 22(9):4335–43. doi: 10.1111/jcmm.13723
66. Dellacecca ER, Cosgrove C, Mukhatayev Z, Akhtar S, Engelhard VH, Rademaker AW, et al. Antibiotics drive microbial imbalance and vitiligo development in mice. J Invest Dermatol (2020) 140(3):676–687 e6. doi: 10.1016/j.jid.2019.08.435
67. Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity (2010) 33(3):301–11. doi: 10.1016/j.immuni.2010.09.002
68. Daily topical rapamycin for vitiligo (2022). Available at: https://clinicaltrials.gov/ct2/show/NCT05342519 .
69. Zhang X, Liu D, He M, Lin M, Tu C, Zhang B. Polymeric nanoparticles containing rapamycin and autoantigen induce antigen-specific immunological tolerance for preventing vitiligo in mice. Hum Vaccin Immunother (2021) 17(7):1923–9. doi: 10.1080/21645515.2021.1872342
70. Giri PS, Bharti AH, Begum R, Dwivedi M. Calcium controlled NFATc1 activation enhances suppressive capacity of regulatory T cells isolated from generalized vitiligo patients. Immunology (2022). doi: 10.1111/imm.13538
71. Yang L, Wei Y, Sun Y, Shi W, Yang J, Zhu L, et al. Interferon-gamma inhibits melanogenesis and induces apoptosis in melanocytes: A pivotal role of CD8+ cytotoxic T lymphocytes in vitiligo. Acta Derm Venereol (2015) 95(6):664–70. doi: 10.2340/00015555-2080
72. Maouia A, Sormani L, Youssef M, Helal AN, Kassab A, Passeron T. Differential expression of CXCL9, CXCL10, and IFN-gamma in vitiligo and alopecia areata patients. Pigment Cell Melanoma Res (2017) 30(2):259–61. doi: 10.1111/pcmr.12559
73. Shi F, Erf GF. IFN-gamma, IL-21, and IL-10 co-expression in evolving autoimmune vitiligo lesions of smyth line chickens. J Invest Dermatol (2012) 132(3 Pt 1):642–9. doi: 10.1038/jid.2011.377
74. Skurkovich S, Skurkovich B, Kelly J. Anticytokine therapy, particularly anti-IFN-gamma, in Th1-mediated autoimmune diseases. Expert Rev Clin Immunol (2005) 1(1):11–25. doi: 10.1586/1744666X.1.1.11
75. Biswas KB, Takahashi A, Mizutani Y, Takayama S, Ishitsuka A, Yang L, et al. GPNMB is expressed in human epidermal keratinocytes but disappears in the vitiligo lesional skin. Sci Rep (2020) 10(1):4930. doi: 10.1038/s41598-020-61931-1
76. Boniface K, Jacquemin Clément, Darrigade A-S, Dessarthe Benoît, Martins C, Boukhedouni N, et al. Vitiligo skin is imprinted with resident memory CD8 T cells expressing CXCR3. J Invest Dermatol (2018) 138(2):355–64. doi: 10.1016/j.jid.2017.08.038
77. Mosenson JA, Zloza A, Klarquist J, Barfuss AJ, Guevara-Patino JA, Poole ICLe. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell melanoma Res (2012) 25(1):88–98. doi: 10.1111/j.1755-148X.2011.00916.x
78. Doss RW, El-Rifaie AA, Abdel-Wahab AM, Gohary YM, Rashed LA. Heat shock protein-70 expression in vitiligo and its relation to the disease activity. Indian J Dermatol (2016) 61(4):408–12. doi: 10.4103/0019-5154.185704
79. Mosenson JA, Zloza A, Nieland JD, Garrett-Mayer E, Eby JM, Huelsmann EJ, et al. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med (2013) 5(174):174ra28. doi: 10.1126/scitranslmed.3005127
80. Tokura Y, Phadungsaksawasdi P, Kurihara K, Fujiyama T, Honda T. Pathophysiology of skin resident memory T cells. Front Immunol (2020) 11:618897. doi: 10.3389/fimmu.2020.618897
81. Richmond JM, Strassner JP, Rashighi M, Agarwal P, Garg M, Essien KI, et al. Resident memory and recirculating memory T cells cooperate to maintain disease in a mouse model of vitiligo. J Invest Dermatol (2019) 139(4):769–78. doi: 10.1016/j.jid.2018.10.032
82. Chen X, Guo W, Chang Y, Chen J, Kang P, Yi X, et al. Oxidative stress-induced IL-15 trans-presentation in keratinocytes contributes to CD8(+) T cells activation via JAK-STAT pathway in vitiligo. Free Radic Biol Med (2019) 139:80–91. doi: 10.1016/j.freeradbiomed.2019.05.011
83. Atwa MA, Ali SMM, Youssef N, Mahmoud Marie RE. Elevated serum level of interleukin-15 in vitiligo patients and its correlation with disease severity but not activity. J Cosmet Dermatol (2021) 20(8):2640–4. doi: 10.1111/jocd.13908
84. Wang W, Chapman NM, Zhang B, Li M, Fan M, Laribee RN, et al. Upregulation of PD-L1 via HMGB1-activated IRF3 and NF-kappaB contributes to UV radiation-induced immune suppression. Cancer Res (2019) 79(11):2909–22. doi: 10.1158/0008-5472.CAN-18-3134
85. Kotobuki Y, Tanemura A, Yang L, Itoi S, Wataya-Kaneda M, Murota H, et al. Dysregulation of melanocyte function by Th17-related cytokines: significance of Th17 cell infiltration in autoimmune vitiligo vulgaris. Pigment Cell Melanoma Res (2012) 25(2):219–30. doi: 10.1111/j.1755-148X.2011.00945.x
86. Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol (2011) 36(3):292–7. doi: 10.1111/j.1365-2230.2010.03972.x
87. Vaccaro M, Cannavo SP, Imbesi S, Cristani M, Barbuzza O, Tigano V, et al. Increased serum levels of interleukin-23 circulating in patients with non-segmental generalized vitiligo. Int J Dermatol (2015) 54(6):672–4. doi: 10.1111/ijd.12392
88. Hegazy RA, Fawzy MM, Gawdat HI, Samir N, Rashed LA. T helper 17 and tregs: A novel proposed mechanism for NB-UVB in vitiligo. Exp Dermatol (2014) 23(4):283–6. doi: 10.1111/exd.12369
89. Speeckaert R, Mylle S, van Geel N. IL-17A is not a treatment target in progressive vitiligo. Pigment Cell melanoma Res (2019) 32(6):842–7. doi: 10.1111/pcmr.12789
90. Birol A, Kisa U, Kurtipek GS, Kara F, Kocak M, Erkek E, et al. Increased tumor necrosis factor alpha (TNF-alpha) and interleukin 1 alpha (IL1-alpha) levels in the lesional skin of patients with nonsegmental vitiligo. Int J Dermatol (2006) 45(8):992–3. doi: 10.1111/j.1365-4632.2006.02744.x
91. Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol (2010) 10(5):301–16.
92. Horiuchi T. Transmembrane TNF-α: structure, function and interaction with anti-TNF agents. Rheumatology (2010) 49(7):1215–28.
93. Spencer-Green and G. Etanercept (Enbrel): update on therapeutic use. Ann Rheum Dis (2000) 59 Suppl 1(90001):i46–9.
94. Webb KC, Tung R, Winterfield LS, Gottlieb AB, Eby JM, Henning SW, et al. Tumour necrosis factor-α inhibition can stabilize disease in progressive vitiligo. Br J Dermatol (2015) 173(3):641–50. doi: 10.1111/bjd.14016
95. Bae JM, Kim M, Lee HH, Kim KJ, Shin H, Ju HJ, et al. Increased risk of vitiligo following anti-tumor necrosis factor therapy: A 10-year population-based cohort study. J Invest Dermatol (2018) 138(4):768–74. doi: 10.1016/j.jid.2017.11.012
96. Toussirot E, Salard D, Algros MP, Aubin F. [Occurrence of vitiligo in a patient with ankylosing spondylitis receiving adalimumab]. Ann Dermatol Venereol (2013) 140(12):801–2. doi: 10.1016/j.annder.2013.09.158
97. Jung JM, Lee YJ, Won CH, Chang SE, Lee MW, Choi JH, et al. Development of vitiligo during treatment with adalimumab: A plausible or paradoxical response?". Ann Dermatol (2015) 27(5):620–1. doi: 10.5021/ad.2015.27.5.620
98. Ryu TH, Lee DW, Choi JE, Ahn HH, Kye YC, Seo SH. A type II segmental vitiligo developed under infliximab treatment for ulcerative colitis. Ann Dermatol (2017) 29(6):826–7. doi: 10.5021/ad.2017.29.6.826
99. Smith DI, Heffernan MP. Vitiligo after the resolution of psoriatic plaques during treatment with adalimumab. J Am Acad Dermatol (2008) 58(2 Suppl):S50–2. doi: 10.1016/j.jaad.2006.05.035
100. Carvalho CLDB, Ortigosa LCM. Segmental vitiligo after infliximab use for rheumatoid arthritis–a case report. Anais bras dermatol (2014) 89(1):154–6. doi: 10.1590/abd1806-4841.20142887
101. Banchereau J, Rousset F. Human b lymphocytes: phenotype, proliferation, and differentiation. Adv Immunol (1992) 52:125–262. doi: 10.1016/s0065-2776(08)60876-7
102. Lin X, Tian H, Xianmin M. Possible roles of b lymphocyte activating factor of the tumour necrosis factor family in vitiligo autoimmunity. Med Hypotheses (2011) 76(3):339–42. doi: 10.1016/j.mehy.2010.10.034
103. Eisenberg R, Albert D. B-cell targeted therapies in rheumatoid arthritis and systemic lupus erythematosus. Nat Clin Pract Rheumatol (2006) 2(1):20–7. doi: 10.1038/ncprheum0042
104. Ioannou Y, Isenberg DA. Current concepts for the management of systemic lupus erythematosus in adults: A therapeutic challenge. Postgrad Med J (2002) 78(924):599–606. doi: 10.1136/pmj.78.924.599
105. Open-label pilot study of abatacept for the treatment of vitiligo . Available at: https://clinicaltrials.gov/ct2/show/NCT02281058 .
Keywords: vitiligo, targeted therapy, JAK inhibitors, biological, treatment, miRNA - microRNA, Treg
Citation: Feng Y and Lu Y (2022) Advances in vitiligo: Update on therapeutic targets. Front. Immunol. 13:986918. doi: 10.3389/fimmu.2022.986918
Received: 05 July 2022; Accepted: 04 August 2022; Published: 31 August 2022.
Reviewed by:
Copyright © 2022 Feng and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Lu, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Dior Expands Partnership with Charlize Theron to High Jewelry and Skincare Ambassador
Boots online doctor introduces 24-hour skin condition diagnosis and new treatment services, anessa launches sunshine project to promote children’s well-being across asia, nivea launches “proud in your skin” campaign with pflag national, walmart inc. posts strong quarterly results and raises full-year outlook, superdrug celebrates 60th anniversary with major expansion plans, california bill targets ‘sephora kids’ with skincare ban for children under 13, &be launches limited edition pokémon color-coded cosmetic sunscreen collection, cerave expands sun safety day initiative to major league ballparks, street talk: hair care episode six | gen x ‘dry hair’.

ASA Announces 2024 Founders and Research Achievement Award Winners at SID Annual Meeting
Posted by Global Cosmetics News | May 21, 2024 | Marketing , North America |

THE WHAT? The American Skin Association (ASA) announced the recipients of the 2024 Inaugural Founders Award and the 2024 Research Achievement Awards at the Society for Investigative Dermatology (SID) Annual Meeting. These awards honor members of the dermatology community who have made significant contributions to the field.
THE DETAILS For over three decades, ASA has recognized dermatologists and researchers who embody the inspirational characteristics of the late Dr. David Martin Carter and Dr. George Hambrick, ASA’s co-founders. This year, ASA presented the first-ever Founders Award to Dr. Kim Yancey of UT Southwestern Medical Center. Dr. Yancey is celebrated for his four decades of teaching, leadership, and investigative research in dermatology.
Additionally, the 2024 Research Achievement Awards were given to several distinguished physicians for their contributions to dermatology. The recipients include:
- Psoriasis: Dr. Joseph F. Merola, UT Southwestern Medical Center
- Autoimmune & Inflammatory Skin Disorders and Vitiligo: Dr. David Fiorentino, Stanford Medicine
- Skin Cancer, Melanoma, and Pigment Cell Disorders: Dr. Madeleine Duvic, MD Anderson Cancer Center
- Discovery: Dr. Thomas S. Kupper, Dana-Farber Cancer Institute
- Translational Research: Dr. Laura Korb Ferris, University of Pittsburgh
- Community Education/Outreach: SPOTS – Sun Protection Outreach Teaching by Students led by Dr. Sofia Chaudhry and Dr. M. Laurin Council
THE WHY? ASA’s awards celebrate exceptional contributions to dermatology, recognizing the vital work of physicians who advance research and education in skin health. These honors highlight the importance of ongoing research and community outreach in combating skin diseases and fostering a deeper understanding of dermatological health. Through these awards, ASA continues to honor the legacy of its founders while inspiring the next generation of dermatologists.
LATEST NEWS

Boots Online Doctor Introduces 24-Hour S...

ANESSA Launches Sunshine Project to Prom...

ASA Announces 2024 Founders and Research...

NIVEA Launches “Proud In Your Skin...

Dior Expands Partnership with Charlize T...

Walmart Inc. Posts Strong Quarterly Resu...

Superdrug Celebrates 60th Anniversary wi...
California bill targets ‘sephora k....

&be Launches Limited Edition Pokémon...

CeraVe Expands Sun Safety Day Initiative...

COSMETICS NEWS WIRE

IN CONVERSATION WITH...

STREET TALK

60 SECOND PITCH

FORMULA FRIDAY

LIVE FROM THE LAB

PROFESSIONAL BEAUTY

TOP 100 BRANDS

JOBS & PEOPLE


How Does Vitiligo Affect Mental Health? Tips To Support Your Loved Ones
V itiligo is a skin condition that affects millions of people worldwide, causing loss of pigmentation in patches of skin. While it's not physically painful, it can have a significant impact on a person's self-esteem and mental well-being. While the condition itself is not life-threatening, the psychological toll it takes on those affected can be significant, often leading to feelings of embarrassment, low self-esteem, and even depression.
A study found that the majority of people with vitiligo deal with low confidence and reduced self-esteem. The impact of vitiligo on mental health may go way beyond interpersonal relationships and daily functioning. Many people with vitiligo report experiencing anxiety or distress when engaging in activities such as swimming or intimate relationships, fearing judgment or rejection from others.
Hence, it is essential to recognize the psychological impact of vitiligo and provide support and resources to those affected. But how to do it?
Here we have listed five tips through which you can extend support to your loved ones dealing with vitiligo.
Educate Yourself:
Understanding what vitiligo is and how it affects your loved one can help you provide better support. Take time to research about the skin condition, its causes, and treatment options. This will not only show your loved one that you care but also help you offer informed advice and encouragement.
Be Empathetic:
Vitiligo can be emotionally challenging for some people. Your loved one may struggle with feelings of self-consciousness, insecurity, or even depression. It's essential to be empathetic and validate their feelings. Let them know that you're there to listen and support them, no matter what they're going through.
Encourage Self-Care:
Self-care practices, such as maintaining a healthy lifestyle, wearing sunscreen, and practising stress-relief techniques, can help manage vitiligo symptoms and improve overall well-being. Encourage your loved one to prioritize self-care and offer to join them in activities that promote relaxation and self-confidence.
Promote Positive Body Image:
Society often sets unrealistic standards of beauty for people, which can make others feel uncomfortable, especially people with vitiligo. As a supportive friend or family member, it's essential to promote a positive body image and celebrate your loved one's unique beauty. Compliment them on their strengths, talents, and personality traits, rather than focusing solely on their appearance.
Offer Practical Support:
Sometimes, practical assistance can make a significant difference in managing vitiligo. Whether it's helping them find a dermatologist specializing in vitiligo treatment, accompanying them to appointments, or assisting with skincare routines, offering practical support shows that you're committed to their well-being.


- General Dermatology
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Psoriatic Arthritis
- Atopic Dermatitis
- Skin Cancer
- Hidradenitis Suppurativa
- Pigmentary Disorders
- Pediatric Dermatology
- Practice Management
Reviewing the Skin Cancer Pipeline: A Look at the Last 10 Years
In recognition of Skin Cancer Awareness Month, Dermatology Times is reviewing research and strides in skin cancer treatment over the last decade.

The Cutaneous Connection: A Reminder of Patient Impact

Journal Digest: May 20

The Cutaneous Connection: Make Data-Driven Decisions For Personalized AD Treatment

Pearls From the Diversity in Dermatology Conference

QUIZ: Dermoscopy and Melanoma Detection
2 Commerce Drive Cranbury, NJ 08512
609-716-7777


COMMENTS
1. Introduction. Vitiligo is the most common disorder of depigmentation, and in 2012 its worldwide prevalence ranged from 0.06-2.28% (1,2).It is characterized by the absence of pigment in the skin, secondary to the loss of melanocytes (1,3).Melanocytes are found in several tissues in the skin, hair follicles, eyes, inner ear, bones, heart and brain ().
On July 18, the U.S. Food and Drug Administration (FDA) approved ruxolitinib ( Opzelura) cream 1.5 percent as a treatment for the most common form of vitiligo, according to a statement by Incyte ...
Vitiligo is a chronic autoimmune disease that causes skin depigmentation. ... (nejmoa2118828_research-summary.pdf ... Harris JE. Interfering with the IFN-γ/CXCL10 pathway to develop new targeted ...
Latest Research and Reviews. ... Vitiligo is an autoimmune condition that results in skin depigmentation due to melanocyte loss, but the root causes are not well understood. Here they identify ...
Vitiligo affects 0.5 to 2% of the population worldwide. This chronic autoimmune disease manifests as pale skin patches, caused by local death of pigment-producing melanocytes. The extent and percei...
Action. FDA has approved Opzelura (ruxolitinib) cream for the treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older. Opzelura is a topical Janus kinase (JAK ...
Temprian Therapeutics is a spin-off from Northwestern University in Chicago, Illinois. Vitiligo is an autoimmune disease that affects an estimated 50 million people worldwide. It isn't life ...
Vitiligo is a multifactorial disease characterized by the loss of skin pigment, which results in achromic macules and patches. ... Updates and new medical treatments for vitiligo (Review) Exp Ther Med. 2021 Aug;22(2) :797. ... 3 Department of Research, Faculty of Medicine Saltillo Unit, Universidad Autónoma de Coahuila, ...
Vitiligo Advancements and Discoveries. There has been an increase in the amount of research being undertaken in vitiligo over recent years and dermatologists have an improved understanding of the natural history and different types of the condition. Here you will find a brief summary of research into several areas, with references to the ...
The U.S. Food and Drug Administration has approved Opzelura (ruxolitinib) as the first topical treatment for vitiligo. The 1.5 percent cream is approved for continuous topical use twice daily to ...
USA TODAY. 0:00. 3:02. Vitiligo is an autoimmune disorder that leads to the loss of skin pigmentation. A recent study shows a medicated cream called ruxolitinib is extremely effective in about one ...
Vitiligo is a common autoimmune-related depigmenting disease resulting from epidermal melanocyte loss. It is characterized by white patches in the skin or mucous, affecting around 0.1-2% of the ... Treatment update for vitiligo based on autoimmune inhibition and melanocyte protection: Expert Opinion on Therapeutic Targets: Vol 27 , No 3 - Get ...
Phase 3 vitiligo clinical trial results to test topical ruxolitinib as a new treatment. I've recently received many requests for an update on the new ruxolitinib cream to treat vitiligo. To remind everyone, we initially reported that oral ruxolitinib, a Janus Kinase inhibitor initially approved to treat a type of blood cancer, reversed ...
When Dr. Anand K. Ganesan started a vitiligo specialty practice in 2018 at the UCI Health Beckman Laser Institute & Medical Clinic, it was to find new therapies to reverse the disfiguring skin disorder.. Vitiligo — pronounced vit-ill-EYE-go — causes white patches, often on the face and hands, the result of the immune system destroying the skin's pigment-producing cells.
Introduction Vitiligo is a chronic autoimmune disease characterized by destruction of melanocytes, leading to skin depigmentation. Vitiligo can have a high quality-of-life burden and profound impact on psychosocial well-being. The objectives of this study were to describe the self-reported patient burden among patients with nonsegmental vitiligo with ≤ 10% affected body surface area ...
The latest consensus statement released in JAMA Dermatology on March 13, 2024, sets a new standard in the USA for vitiligo treatment among children, teenagers, and young adults. This pioneering guideline, led by Drs. Yael Renert-Yuval and Nanette ... 2024-03-13.
We address every phase in the vitiligo drug discovery process, from basic research all the way to the clinic, in order for new treatments to reach patients. At the Vitiligo Research Foundation, we have only one incentive: to bring a vitiligo treatment to market for nearly a 100 million people worldwide who are affected by this devastating ...
A new Northwestern Medicine study published in JAMA Dermatology shows Artz's experience is common for children and teens with chronic skin diseases. The majority of children and teens with chronic skin diseases such as acne, eczema, psoriasis, alopecia areata (hair loss) and vitiligo (pigment loss) feel stigmatized by peers for their ...
A microneedle patch that delivers immune-regulating molecules can teach T cells not to attack hair follicles, helping hair to regrow.Anne Trafton | MIT NewsResearchers at MIT, including at IMES, Brigham and Women's Hospital (BWH), and Harvard Medical School (HMS), have developed a potential new treatment for alopecia areata, an autoimmune disorder that causes hair loss and affects people of ...
Among the new approaches being developed, the strategy of targeting the IFN-γ-CXCL9/10-CXCR3 axis has been clinically tested. OPZELURA has been indicated for the topical treatment of nonsegmental vitiligo in adult and pediatric patients 12 years of age and older. MiRNA-based therapeutics are also in development.
Additionally, the 2024 Research Achievement Awards were given to several distinguished physicians for their contributions to dermatology. The recipients include: Psoriasis: Dr. Joseph F. Merola, UT Southwestern Medical Center; Autoimmune & Inflammatory Skin Disorders and Vitiligo: Dr. David Fiorentino, Stanford Medicine
Encourage Self-Care: Self-care practices, such as maintaining a healthy lifestyle, wearing sunscreen, and practising stress-relief techniques, can help manage vitiligo symptoms and improve overall ...
This Skin Cancer Awareness Month, Dermatology Times is recapping the last 10 years of news in the skin cancer drug pipeline. November 2015: Cobimetinib is approved by the FDA On November 10, 2015, the FDA approved Genentech's Cotellic (cobimetinib) for use with Zelboraf (vemurafenib) in treating advanced melanoma with BRAF V600E or V600K mutations.