

Paid Clinical Studies
Make a difference, serving the knoxville area for over 12 years.
New Phase Research & Development is a clinical research company located in Knoxville, Tennessee. With our dedication to research and patient care, New Phase has become a premier research site in the region. Our research dedicated facility offers subjects the security of a highly educated staff while providing the luxury of a well maintained and comfortable facility to visit throughout the study process. Interested in Paid Clinical Trials?

Enrolled Subjects
Visits completed.
* In 2023 alone*
Volunteers in Database
Want to join a paid clinical study.
Choose a study that fits you. Volunteers are able to review the program and it will be carefully explained by our professional medical team. If you’re interested in Paid Clinical Research check out our current Enrolling Studies.

We do not focus on trial volume but instead, we carefully select each trial for our facility and focus on enrollment and performance. Our full-time research team consists of dedicated professionals who work together to achieve one common goal; successful trials.
“Awesome place to go to do research trials! Staff is very friendly. I like that it is a small business created in Knoxville.”
“ I felt so welcomed when I entered this research center from the friendly front office to the medical staff. Building is spotless! Give this place a try if you’re looking to get into a clinical trial. “
“Great screening tools to get the perfect fit between test subject and investigative team. Warm, personable staff.”

- Patient Login
- Partner Login
- Become A Member
- Search Clinical Trials
- About Clinical Trials
- Virtual Clinical Trials
Latest Blog Posts
Services for research sites.
- Study Listings
- Clinical Ad Network™
- Patient Direct Mail Alerts
- Create Partner Account
National Recruitment Services
- Multicenter Study Listings
- Digital Marketing Strategies
- Site Identification Services
- Direct to Patient Advertising
News And Information
- Case Studies
- Patient Recruitment Insider Blog
- For Research Sites
- Study Screening Websites
Research Site Profile
- Clinic Profile List
New Phase Research and Development
6914 Office Park Cir Knoxville, TN 37909
Research Site Introduction:
New Phase Research & Development is a clinical research company located in Knoxville, Tennessee. With our dedication to research and patient care, New Phase has become a premier research site in the region. Our research dedicated facility offers subjects the security of a highly educated staff while providing the luxury of a well maintained and comfortable facility to visit throughout the study process.
Research Site Description:
We currently operate as a fully dedicated clinical research facility in West Knoxville.
We have a secured double locked drug closet equipped with pin code access and deadbolt. We also maintain a drug storage refrigerator that is independently locked. Both the storage area and refrigerator are equipped with a digital glycol controlled min/max thermometer. These monitors are set to alarm staff of any deviations from the set range. Designated site staff maintain daily drug logs. We maintain an active emergency action plan and are located approximately 6 miles from the nearest hospital. We are equipped with a fully stocked crash cart that includes oxygen and an AED.
Patient Demographics:
Over 20,000 volunteers in our database and growing! In the last year alone, we have enrolled 670 patients for 20 studies, with a total of over 2,700 visits scheduled.
Additional Information:
With a focus on quality data, outstanding subject care, and active recruitment, New Phase has become a leader in successful clinical trials at a site level.
Contact Information:
Give us a call at (865) 200-8364 or visit our website at NEWPHASEONLINE.com to see more information on our current studies and qualifications.
Active Studies:
There are no active studies for this clinic.
Nearby listings:
- Asthma - Knoxville TN
- Asthma (Adults and Adolescents) - Knoxville TN
- Post-Surgical Neuropathic Pain - Knoxville TN
- Migraines - Knoxville TN
- High Blood Pressure (Hypertension) - Knoxville TN
- Vitiligo At Home - Gatlinburg TN
- Lymphoma At Home - Gatlinburg TN
- Diffuse Large B-Cell Lymphoma (DLBCL) At Home - Gatlinburg TN
- Duchenne Muscular Dystrophy (DMD) At Home - Gatlinburg TN

© 1998-2024 | All trademarks are property of their legal owners. | All Rights Reserved
ClinicalConnection.com is a resource that provides information on clinical trials and other research opportunities worldwide.
ClinicalConnection.com does not conduct or endorse this research. Please consult your physician before participating.
- Artificial Intelligence in Medicine: Transformative Medical Breakthroughs
- Exploring the Microbiome: Gut Health, Probiotics and Clinical Trials
- Nutrition and Clinical Trials: Latest Clinical Research on Diet's Role in Disease
- Personalized Medicine: The Power of Genetics in Clinical Trials
Useful Links
1998-2024 © All Rights Reserved. Privacy Policy | Terms of Service
New Phase Research and Development
- Is this your listing? Link it to your account so you can update it at any time.
- Don't have an account? Register Now
Contact Center or Provider

Contact Information
Center information, currently enrolling trials.
New Phase Research and Development is a full-time dedicated research facility in Knoxville, Tenn. Our facility offers subjects the security of a highly educated staff while providing the luxury of a well-maintained and comfortable facility to visit throughout the study process.
Research Experience
New Phase specializes in many therapeutic areas. As a multispecialty organization for more than 10 years, we have acquired experience in a wide range of clinical trials. To date we have conducted trials in the following indications:
- Type I diabetes
- Type II diabetes
- Overactive bladder
- Lactose intolerance
- Osteoarthritis
- Rheumatoid arthritis
- GERD/acid reflux
- Neuropathy (traumatic and diabetic)
- Fibromyalgia
- Allergy/rhinitis
- Hypertension
- Hyperlipidemia
- High triglycerides
- Low testosterone
- Weight loss
Facility Description
Due to our dedication to research and patient care, New Phase has become a premier research site in our region. We perform on-site ECGs and PFTs. Some of our specialized equipment includes laboratory with centrifuge, a 24-hour climate-controlled secured drug storage closet, temperature-monitored freezer/refrigerators, -70-degree Celsius freezer and a fully stocked crash cart. New Phase maintains relationships with local facilities that perform MRI/imaging, colonoscopies and other specialized procedures.
Investigator Experience
Dr. Evelyne Davidson, MD, Principal Investigator Dr. Davidson is a board-eligible internist with more than 37 years of experience in patient care and management. Dr. Davidson has been a principal investigator at New Phase Research since 2012 and has conducted more than 90 clinical trials. Dr. Davidson has extensive experience in most primary care indications and is actively involved in the care of every clinical research subject. Unlike most principal investigators, Dr. Davidson not only provides oversight, but also has an active role in the day-to-day coordination of each trial.
Dr. Natalie Clarke, MD, Principal Investigator Dr. Clarke began working at New Phase in March 2016. She graduated from Penn State University with a Bachelor of Science degree in Biology in 1996. After college, she participated in two years of research investigating the effects of various vasodilators on atherosclerotic arteries. From 1998-2002, she attended The Medical College of Georgia, earning her MD and completed her anesthesiology residency in 2006. Dr. Clarke moved from Georgia to Knoxville in 2010. While working at New Phase, Dr. Clarke has been involved in several studies including ones pertaining to diabetic neuropathy, IBS, COPD, asthma, rheumatoid arthritis, lupus and chronic low back pain.
Staff Experience
In addition to two hands-on principal investigators, we maintain a full-time staff of research professionals. Our dedicated team is committed to ethical excellence and adheres to strict training and quality improvement standards.
Patient Demographics
New Phase Research and Development has a dedicated recruitment team with an extensive volunteer database readily available. Our marketing team utilizes web-based and social media outlets, along with traditional advertising methods.
Upcoming Events
Demystifying ibcs: a guide to compliance and success, cutting through the noise: use cases for imaging core labs and endpoint adjudication, magi@home clinical research conference 2024, featured products.

Surviving an FDA GCP Inspection: Resources for Investigators, Sponsors, CROs and IRBs

Best Practices for Clinical Trial Site Management
Featured stories.

Thought Leadership: Remote Patient Monitoring Gives New View of Safety in Cardiac Clinical Trials

Ask the Experts: Applying Quality by Design to Protocols

Clinical Trials Need Greater Representation of Obese Patients, Experts Say

FDA IT Modernization Plan Prioritizes Data-Sharing, AI, Collaboration and More
Standard operating procedures for risk-based monitoring of clinical trials, the information you need to adapt your monitoring plan to changing times..
New Phase Clinical Research and Development
Photos & videos.
See all 1 photos

You Might Also Consider

The Allergy, Asthma & Sinus Center
1.1 miles away from this business
Richard S. said "Welcome to East Tennessee, this week with frigid cold temperatures and dry air inside your home; especially if you have gas heat. The doctors at the Allergy, Asthma, and Sinus Center can diagnose your symptoms from local dust,…" read more
in Allergists
Clinch River Home Health Care
15.8 miles away from this business
Little Company with a Big Heart Clinch River Home Health's mission is to provide affordable in-home skilled and personal care to all residents regardless of age, sex, race, or religious affiliation; especially those with limited… read more
in Physical Therapy

New Hope Healthcare Institute
7.3 miles away from this business
An important facet of treatment at New Hope Healthcare includes during- and aftercare support services. It is our experience that those who participate in alumni groups after treatment have a much higher chance of remaining sober. read more
in Counseling & Mental Health, Rehabilitation Center
About the Business
New Phase Research & Development is a clinical research site located in Knoxville, TN. With our dedication to research and patient care, New Phase has become a premier research site in the region. Our research dedicated facility offers subjects the security of a highly educated staff while providing the luxury of a well maintained and comfortable facility to visit throughout the study process. …
Location & Hours
Suggest an edit
6914 Office Park Cir
Knoxville, TN 37909
Amenities and More
Ask the community.
Ask a question
Yelp users haven’t asked any questions yet about New Phase Clinical Research and Development .
Recommended Reviews
- 1 star rating Not good
- 2 star rating Could’ve been better
- 3 star rating OK
- 4 star rating Good
- 5 star rating Great
Select your rating
1 review that is not currently recommended

Knoxville Family Chiropractic
3.6 miles away from this business
We love our Patients and have been passionate about providing the very best Chiropractic care for over 25 years! This location is a new location for us and the same great care Dr. Lundgren and his Team have provided to thousands of… read more
in Chiropractors

The Eye Group - Kingston Pike
7.8 miles away from this business
Chris B. said "My family has been going to the Eye Group for many years, and they are the best in the business. I couldn't be more pleased with Dr. Lain and the staff here. You can tell that they really care and they go the extra mile for their…" read more
in Optometrists, Laser Eye Surgery/lasik
Browse Nearby
Restaurants
Things to Do
Family Practice
Other Medical Centers Nearby
Find more Medical Centers near New Phase Clinical Research and Development
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- For Patients
- Learn About Drug and Device Approvals
- The Drug Development Process
Step 3: Clinical Research
While preclinical research answers basic questions about a drug’s safety, it is not a substitute for studies of ways the drug will interact with the human body. “Clinical research” refers to studies, or trials, that are done in people. As the developers design the clinical study, they will consider what they want to accomplish for each of the different Clinical Research Phases and begin the Investigational New Drug Process (IND), a process they must go through before clinical research begins.
On this page you will find information on:
Designing Clinical Trials
Clinical Research Phase Studies
The Investigational New Drug Process
Asking for FDA Assistance
FDA IND Review Team
Researchers design clinical trials to answer specific research questions related to a medical product. These trials follow a specific study plan, called a protocol , that is developed by the researcher or manufacturer. Before a clinical trial begins, researchers review prior information about the drug to develop research questions and objectives. Then, they decide:
Who qualifies to participate (selection criteria)
How many people will be part of the study
How long the study will last
Whether there will be a control group and other ways to limit research bias
How the drug will be given to patients and at what dosage
What assessments will be conducted, when, and what data will be collected
How the data will be reviewed and analyzed
Clinical trials follow a typical series from early, small-scale, Phase 1 studies to late-stage, large scale, Phase 3 studies.
What are the Clinical Trial Phases?
Watch this video to learn about the three phases of clinical trials.
Study Participants: 20 to 100 healthy volunteers or people with the disease/condition.
Length of Study: Several months
Purpose: Safety and dosage
During Phase 1 studies, researchers test a new drug in normal volunteers (healthy people). In most cases, 20 to 80 healthy volunteers or people with the disease/condition participate in Phase 1. However, if a new drug is intended for use in cancer patients, researchers conduct Phase 1 studies in patients with that type of cancer.
Phase 1 studies are closely monitored and gather information about how a drug interacts with the human body. Researchers adjust dosing schemes based on animal data to find out how much of a drug the body can tolerate and what its acute side effects are.
As a Phase 1 trial continues, researchers answer research questions related to how it works in the body, the side effects associated with increased dosage, and early information about how effective it is to determine how best to administer the drug to limit risks and maximize possible benefits. This is important to the design of Phase 2 studies.
Approximately 70% of drugs move to the next phase
Study Participants: Up to several hundred people with the disease/condition.
Length of Study: Several months to 2 years
Purpose: Efficacy and side effects
In Phase 2 studies, researchers administer the drug to a group of patients with the disease or condition for which the drug is being developed. Typically involving a few hundred patients, these studies aren't large enough to show whether the drug will be beneficial.
Instead, Phase 2 studies provide researchers with additional safety data. Researchers use these data to refine research questions, develop research methods, and design new Phase 3 research protocols.
Approximately 33% of drugs move to the next phase
Study Participants: 300 to 3,000 volunteers who have the disease or condition
Length of Study: 1 to 4 years
Purpose: Efficacy and monitoring of adverse reactions
Researchers design Phase 3 studies to demonstrate whether or not a product offers a treatment benefit to a specific population. Sometimes known as pivotal studies, these studies involve 300 to 3,000 participants.
Phase 3 studies provide most of the safety data. In previous studies, it is possible that less common side effects might have gone undetected. Because these studies are larger and longer in duration, the results are more likely to show long-term or rare side effects
Approximately 25-30% of drugs move to the next phase
Study Participants: Several thousand volunteers who have the disease/condition
Purpose: Safety and efficacy
Phase 4 trials are carried out once the drug or device has been approved by FDA during the Post-Market Safety Monitoring
Learn more about Clinical Trials .
Drug developers, or sponsors , must submit an Investigational New Drug (IND) application to FDA before beginning clinical research.
In the IND application, developers must include:
Animal study data and toxicity (side effects that cause great harm) data
Manufacturing information
Clinical protocols (study plans) for studies to be conducted
Data from any prior human research
Information about the investigator
Drug developers are free to ask for help from FDA at any point in the drug development process, including:
Pre-IND application, to review FDA guidance documents and get answers to questions that may help enhance their research
After Phase 2, to obtain guidance on the design of large Phase 3 studies
Any time during the process, to obtain an assessment of the IND application
Even though FDA offers extensive technical assistance, drug developers are not required to take FDA’s suggestions. As long as clinical trials are thoughtfully designed, reflect what developers know about a product, safeguard participants, and otherwise meet Federal standards, FDA allows wide latitude in clinical trial design.
The review team consists of a group of specialists in different scientific fields. Each member has different responsibilities.
Project Manager: Coordinates the team’s activities throughout the review process, and is the primary contact for the sponsor.
Medical Officer: Reviews all clinical study information and data before, during, and after the trial is complete.
Statistician: Interprets clinical trial designs and data, and works closely with the medical officer to evaluate protocols and safety and efficacy data.
Pharmacologist: Reviews preclinical studies.
Pharmakineticist: Focuses on the drug’s absorption, distribution, metabolism, and excretion processes.Interprets blood-level data at different time intervals from clinical trials, as a way to assess drug dosages and administration schedules.
Chemist: Evaluates a drug’s chemical compounds. Analyzes how a drug was made and its stability, quality control, continuity, the presence of impurities, etc.
Microbiologist: Reviews the data submitted, if the product is an antimicrobial product, to assess response across different classes of microbes.
The FDA review team has 30 days to review the original IND submission. The process protects volunteers who participate in clinical trials from unreasonable and significant risk in clinical trials. FDA responds to IND applications in one of two ways:
Approval to begin clinical trials.
Clinical hold to delay or stop the investigation. FDA can place a clinical hold for specific reasons, including:
Participants are exposed to unreasonable or significant risk.
Investigators are not qualified.
Materials for the volunteer participants are misleading.
The IND application does not include enough information about the trial’s risks.
A clinical hold is rare; instead, FDA often provides comments intended to improve the quality of a clinical trial. In most cases, if FDA is satisfied that the trial meets Federal standards, the applicant is allowed to proceed with the proposed study.
The developer is responsible for informing the review team about new protocols, as well as serious side effects seen during the trial. This information ensures that the team can monitor the trials carefully for signs of any problems. After the trial ends, researchers must submit study reports.
This process continues until the developer decides to end clinical trials or files a marketing application. Before filing a marketing application, a developer must have adequate data from two large, controlled clinical trials.
AFWERX Selects Othram for SBIR Phase II Contract to Advance Forensic Intelligence Through Forensic DNA Sequencing
New research and development effort will aim to leverage advanced forensic dna testing to enhance the capabilities of the department of the air force..
The Woodlands, TX — June 4, 2024
Othram, the leading forensic sequencing laboratory for law enforcement, has been selected by AFWERX for a $1.25 million SBIR Phase II contract focused on advancing forensic DNA sequencing technology to address pressing challenges in the Department of the Air Force (DAF). The Air Force Research Laboratory and AFWERX have partnered to streamline the Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR) process by accelerating the small business experience through faster proposal to award timelines, changing the pool of potential applicants by expanding opportunities to small business, and eliminating bureaucratic overhead by continually implementing process improvement changes in contract execution. The DAF began offering the Open Topic SBIR/STTR program in 2018 which expanded the range of innovations the DAF funded.
As of May 24, 2024, Othram’s research and development team is now working to refine innovative capabilities that will strengthen the national defense of the United States of America. Othram’s Forensic-Grade Genome Sequencing® technology will be leveraged to accelerate DNA-assisted identifications of missing U.S. service members and other unidentified persons. This includes identifying human remains by the Defense Prisoner of War/Missing in Action (POW/MIA) Accounting Agency (DPAA) and the Armed Forces Medical Examiner System (AFMES) and assisting AFMES with AFOSI cold cases.
Forensic-Grade Genome Sequencing® has been instrumental in solving numerous active and cold cases for local, state, and federal agencies across the United States and internationally. More forensic genetic genealogy cases have been solved with Othram FGGS® than any other method.
Note: The views expressed are those of the author and do not necessarily reflect the official policy or position of the Department of the Air Force, the Department of Defense, or the U.S. government.
About Othram
Othram is the world’s first private DNA laboratory built specifically to apply the power of modern parallel sequencing to forensic evidence. Othram’s scientists are experts at recovery, enrichment, and analysis of human DNA from trace quantities of degraded or contaminated materials. Founded in 2018, and located in The Woodlands, Texas, our team works with academic researchers, forensic scientists, medical examiners, and law enforcement agencies to achieve results when other approaches fail. Follow Othram on Twitter @OthramTech or visit Othram.com to learn how we can help you with your case. Visit dnasolves.com to learn how anyone can make a difference in helping solve the next cold case.
The Air Force Research Laboratory is the primary scientific research and development center for the Department of the Air Force. AFRL plays an integral role in leading the discovery, development, and integration of affordable warfighting technologies for our air, space and cyberspace force. With a workforce of more than 12,500 across nine technology areas and 40 other operations across the globe, AFRL provides a diverse portfolio of science and technology ranging from fundamental to advanced research and technology development. For more information, visit afresearchlab.com .
About AFWERX
As the innovation arm of the DAF and a directorate within the Air Force Research Laboratory, AFWERX brings cutting-edge American ingenuity from small businesses and start-ups to address the most pressing challenges of the DAF. AFWERX employs approximately 370 military, civilian and contractor personnel at five hubs and sites executing an annual $1.4 billion budget. Since 2019, AFWERX has executed over 6,100 new contracts worth more than $4 billion to strengthen the U.S. defense industrial base and drive faster technology transition to operational capability. For more information, visit afwerx.com .
The best case scenario.
Forensic evidence will degrade over time. Don't lose your evidence or allow it to be destroyed by inadequate testing or inexperienced consultants. We work with forensic professionals, medical examiners and law enforcement globally to achieve results the first time, even when other approaches have failed. Learn how Othram can help you solve your case and support you from crime scene to courtroom.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Science and Technology Directorate
- IRD Strategic Plan Prepares DHS for Future Homeland Security Challenges
News Release: Innovation, Research and Development Strategic Plan Prepares DHS for Future Homeland Security Challenges
For immediate release s&t public affairs , 202-286-9047.
Plan will prepare DHS to meet emerging technological needs and maximize strategic impact.
WASHINGTON - The Department of Homeland Security (DHS) released the first-ever department-wide Innovation, Research and Development (IRD) Strategic Plan , articulating key investment goals over the next seven fiscal years. Developed at the direction of Secretary Alejandro N. Mayorkas, the Strategic Plan serves as a blueprint for DHS to keep pace with technology by leveraging research and development to address homeland security challenges.
“This visionary roadmap, informed by scientific efforts, will empower DHS and its components to reduce risks to the homeland through optimized innovation, research and development investments,” said Dr. Dimitri Kusnezov, DHS Under Secretary for Science and Technology. “The technologies resulting from our IRD investments play a critical role in equipping the Department’s front-line operators with necessary tools to outpace our adversaries and enhance our preparedness and response capabilities.”
In 2022, Secretary Mayorkas tasked the DHS Science and Technology Directorate (S&T) with examining DHS’s execution of research and development (R&D), including through developing a coordinated strategy focused on areas for long-term Departmental research. The resulting IRD Strategic Plan will help the Department and its partners make coordinated, integrated investments. In addition to capturing current IRD efforts already underway – compiling data from every DHS component and office – it provides an overview of complementary efforts led by federal, state, local, tribal, territorial, nongovernmental and private sector entities.
From this analysis of common research fields, the plan highlights eight Strategic Priority Research Areas (SPRAs) and future capabilities that DHS needs across its missions. The SPRAs will enhance the coordination of R&D across DHS while giving a demand signal for industry, interagency, academic, and international communities about future partnership opportunities. The Strategic Priority Research Areas for Fiscal Years 2024-2030 are:
- Advanced Sensing – next-generation sensor capabilities to provide enhanced detection performance against a broad spectrum of threats.
- Artificial Intelligence (AI) and Autonomous Systems – automated technologies to provide predictions, recommendations, or decisions across a wide variety of operating environments, including means to deal with adversarial AI.
- Biotechnology – augmented capabilities to predict, detect, and defend against current and emerging bioagents and biotechnologies of concern.
- Climate Change – technologies to strengthen climate adaptation/resilience, improve equity, protect critical infrastructure, and reduce carbon emissions.
- Communications and Networking – enhanced communications and networking capabilities, while maintaining security and resiliency.
- Cybersecurity – enhanced resiliency, protection, and operational assurance across data, software, hardware, and communications networks.
- Data Integration, Analytics, Modeling and Simulation – enhanced, integrated data ecosystems, analytics, and modeling to enable better and more accurate data-driven insights, predictions, and decisions.
- Digital Identity and Trust – enhanced capabilities to establish and verify both individuals’ identities and the validity, integrity, and privacy of associated data.
In collaboration with stakeholders from across DHS, S&T is advancing implementation of the Strategic Plan by developing IRD investment roadmaps for each SPRA. These roadmaps will inform the Department’s budget process for FY 2027 and beyond.
More information about the IRD Strategic Plan and its priorities can be found at DHS IRD Strategic Plan FY24-30 | Homeland Security .
- Science and Technology
- Strategic Plan
NCBE Publishes Results of NextGen Pilot Test Research
Media contact: NCBE: [email protected]
MADISON, WISCONSIN, June 3, 2024—The National Conference of Bar Examiners (NCBE) has published its first analysis of NextGen bar exam performance data , focused on the pilot testing phase, one of three stages of implementation research being conducted in preparation for the launch of the NextGen bar exam. Insights gained through pilot testing have already been used to inform the development of the new exam, which will be administered for the first time in July 2026.
Over 2,500 law students and recently licensed lawyers participated in the pilot test, which was conducted in four separate administrations between August 2022 and April 2023. Pilot test participants answered drafts of new types of questions being developed for the NextGen exam and then provided feedback on the questions and on their overall experience taking the pilot test.
The results of the pilot test have helped solidify several key details about the new exam. For example, one of the questions NCBE researchers sought to answer was how much time examinees need to answer the new exam questions—important information because the bar exam is intended to test knowledge and skills, not speed. Exam developers will use this data and additional information gathered during subsequent research phases to ensure that sufficiently prepared examinees can complete the exam within the time given.
Researchers were also interested in learning whether examinees would benefit from access to legal resources such as the Federal Rules of Evidence (FRE) during the exam. During the pilot test, some participants were given access to the FRE and others were not. Researchers compared the performance of these groups and determined that participants who used the FRE took longer to answer the relevant questions compared to those who did not, without increasing their scores. As a result of these findings, it was determined that extensive legal reference material such as the FRE will not be provided on the NextGen exam. Relevant, targeted resources will still be provided for some question types, such as performance tasks, and questions on certain topics within the foundational concepts and principles (see the NextGen content scope for more information).
One important area of NextGen research is whether the new question types being developed for the exam might help reduce performance differences between groups of examinees (for example, men and women). The pilot test yielded some promising results on this front, which NCBE psychometric experts will continue to study during subsequent research phases.
The next stages of research for the NextGen exam have already begun. Field testing was conducted in January 2024 at law schools across the US, and a full-length prototype exam will be administered nationwide in October of this year. In the months ahead, NCBE will issue additional research briefs to share the results of this testing.
To date, 19 jurisdictions have announced plans to adopt the NextGen bar exam. The new exam is being developed by the National Conference of Bar Examiners (NCBE), which currently develops bar exam content for 54 of 56 US jurisdictions. In the US, the highest court in each jurisdiction has authority over the admission of attorneys to practice in its courts, aided by its own bar admissions agency. The NextGen bar exam will replace the current Uniform Bar Examination (UBE) and, like the UBE, will serve as the basis for score portability between participating jurisdictions.
Designed to reflect the work performed by newly licensed attorneys, the NextGen bar exam will test nine areas of legal doctrine (civil procedure, contract law, evidence, torts, business associations, constitutional law, criminal law, real property, family law) and seven foundational lawyering skills (legal research, legal writing, issue spotting and analysis, investigation and evaluation, client counseling and advising, negotiation and dispute resolution, client relationship and management). Tenets of attorney ethics will also be tested in conjunction with other topics and skills.
The new exam will balance the skills and knowledge needed in litigation and transactional legal practice and will reflect many of the key changes that law schools are making to their own curricula, building on the successes of clinical legal education programs, alternative dispute resolution programs, legal research, and legal writing and analysis programs. See https://www.ncbex.org/exams/nextgen/content-scope for detailed outlines of the legal doctrine and skills that will be tested on the exam.
The subjects and skills to be tested were developed through a multi-year, nationwide legal practice analysis focused on the most important knowledge and skills for newly licensed lawyers (defined as lawyers within their first three years in practice).
Like the current bar exam, the NextGen bar exam will be administered, and the written portions graded, by the individual US jurisdictions. The exam will be administered over one and a half days, with six hours of testing time on day one and three hours on day two. The current bar exam is typically administered in 12 hours over two full days.
# # #
About the National Conference of Bar Examiners
The National Conference of Bar Examiners (NCBE), headquartered in Madison, Wisconsin, is a not-for-profit corporation founded in 1931. NCBE promotes fairness, integrity, and best practices in bar admissions for the benefit and protection of the public, in pursuit of its vision of a competent, ethical, and diverse legal profession. Best known for developing bar exam content used by 54 US jurisdictions, NCBE serves admission authorities, courts, the legal education community, and candidates by providing high-quality assessment products, services, and research; character investigations; and informational and educational resources and programs. In 2026, NCBE will launch the next generation of the bar examination, ensuring that the exam continues to test the knowledge, skills, and abilities required for competent entry-level legal practice in a changing profession. For more information, visit the NCBE website at https://www.ncbex.org .
About the Next Generation of the Bar Exam
Set to debut in July 2026, the NextGen bar exam will test a broad range of foundational lawyering skills, utilizing a focused set of clearly identified fundamental legal concepts and principles needed in today’s practice of law. The skills and concepts to be tested were developed through a multi-year, nationwide legal practice analysis, focused on the most important knowledge and skills for newly licensed lawyers. Designed to balance the skills and knowledge needed in litigation and transactional legal practice, the exam will reflect many of the key changes that law schools are making today. NCBE is committed to ensuring a systematic, transparent, and collaborative implementation process, informed by input from and participation by stakeholders, and guided by best practices and the professional standards for high-stakes testing. For more information, visit https://nextgenbarexam.ncbex.org/ or https://www.ncbex.org/exams/nextgen .
- Jurisdictions
- Registration
- ADHD Medical Documentation Guidelines
- Accommodation Decisions
- Accommodations FAQs
- Apply For Test Accommodations
- Extension Requests
- How To Prepare Your Request
- Important Dates for MPRE Test Accomodations
- Learning Disabilities Medical Documentation Guidelines
- MPRE Stop-The-Clock Breaks
- MPRE Test Accommodations Privacy Policy
- Medical Documentation Guidelines For MPRE Test Accommodations
- Neurocognitive Disorders
- Physical and Chronic Health-Related Disabilities
- Psychological Disabilities
- Test Conditions
- Visual Disabilities
- Test Day Policies
- Score Portability
- Minimum Scores
- Maximum Score Age
- Local Components
- UBE Jurisdictions
- Implementation Timeline
- Integrated Question Sets
- Multiple-Choice
- Performance Task
- Content Scope
- Character & Fitness
- MBE Score Services
- MPRE Score Services
- UBE Score Services
- Bar Exam Results by Jurisdiction
- Technical Advisory Panel
- Covington Award
- Validity and Fairness Research Award
- Publications
- Media Resources
- Job Announcements
- Next Generation of The Bar Exam
- Diversity and Inclusion
- News/Resources
- NextGen Bar Exam
- Help & Support

40 Facts About Elektrostal
Written by Lanette Mayes
Modified & Updated: 01 Jun 2024
Reviewed by Jessica Corbett

Elektrostal is a vibrant city located in the Moscow Oblast region of Russia. With a rich history, stunning architecture, and a thriving community, Elektrostal is a city that has much to offer. Whether you are a history buff, nature enthusiast, or simply curious about different cultures, Elektrostal is sure to captivate you.
This article will provide you with 40 fascinating facts about Elektrostal, giving you a better understanding of why this city is worth exploring. From its origins as an industrial hub to its modern-day charm, we will delve into the various aspects that make Elektrostal a unique and must-visit destination.
So, join us as we uncover the hidden treasures of Elektrostal and discover what makes this city a true gem in the heart of Russia.
Key Takeaways:
- Elektrostal, known as the “Motor City of Russia,” is a vibrant and growing city with a rich industrial history, offering diverse cultural experiences and a strong commitment to environmental sustainability.
- With its convenient location near Moscow, Elektrostal provides a picturesque landscape, vibrant nightlife, and a range of recreational activities, making it an ideal destination for residents and visitors alike.
Known as the “Motor City of Russia.”
Elektrostal, a city located in the Moscow Oblast region of Russia, earned the nickname “Motor City” due to its significant involvement in the automotive industry.
Home to the Elektrostal Metallurgical Plant.
Elektrostal is renowned for its metallurgical plant, which has been producing high-quality steel and alloys since its establishment in 1916.
Boasts a rich industrial heritage.
Elektrostal has a long history of industrial development, contributing to the growth and progress of the region.
Founded in 1916.
The city of Elektrostal was founded in 1916 as a result of the construction of the Elektrostal Metallurgical Plant.
Located approximately 50 kilometers east of Moscow.
Elektrostal is situated in close proximity to the Russian capital, making it easily accessible for both residents and visitors.
Known for its vibrant cultural scene.
Elektrostal is home to several cultural institutions, including museums, theaters, and art galleries that showcase the city’s rich artistic heritage.
A popular destination for nature lovers.
Surrounded by picturesque landscapes and forests, Elektrostal offers ample opportunities for outdoor activities such as hiking, camping, and birdwatching.
Hosts the annual Elektrostal City Day celebrations.
Every year, Elektrostal organizes festive events and activities to celebrate its founding, bringing together residents and visitors in a spirit of unity and joy.

Has a population of approximately 160,000 people.
Elektrostal is home to a diverse and vibrant community of around 160,000 residents, contributing to its dynamic atmosphere.
Boasts excellent education facilities.
The city is known for its well-established educational institutions, providing quality education to students of all ages.
A center for scientific research and innovation.
Elektrostal serves as an important hub for scientific research, particularly in the fields of metallurgy , materials science, and engineering.
Surrounded by picturesque lakes.
The city is blessed with numerous beautiful lakes , offering scenic views and recreational opportunities for locals and visitors alike.
Well-connected transportation system.
Elektrostal benefits from an efficient transportation network, including highways, railways, and public transportation options, ensuring convenient travel within and beyond the city.
Famous for its traditional Russian cuisine.
Food enthusiasts can indulge in authentic Russian dishes at numerous restaurants and cafes scattered throughout Elektrostal.
Home to notable architectural landmarks.
Elektrostal boasts impressive architecture, including the Church of the Transfiguration of the Lord and the Elektrostal Palace of Culture.
Offers a wide range of recreational facilities.
Residents and visitors can enjoy various recreational activities, such as sports complexes, swimming pools, and fitness centers, enhancing the overall quality of life.
Provides a high standard of healthcare.
Elektrostal is equipped with modern medical facilities, ensuring residents have access to quality healthcare services.
Home to the Elektrostal History Museum.
The Elektrostal History Museum showcases the city’s fascinating past through exhibitions and displays.
A hub for sports enthusiasts.
Elektrostal is passionate about sports, with numerous stadiums, arenas, and sports clubs offering opportunities for athletes and spectators.
Celebrates diverse cultural festivals.
Throughout the year, Elektrostal hosts a variety of cultural festivals, celebrating different ethnicities, traditions, and art forms.
Electric power played a significant role in its early development.
Elektrostal owes its name and initial growth to the establishment of electric power stations and the utilization of electricity in the industrial sector.
Boasts a thriving economy.
The city’s strong industrial base, coupled with its strategic location near Moscow, has contributed to Elektrostal’s prosperous economic status.
Houses the Elektrostal Drama Theater.
The Elektrostal Drama Theater is a cultural centerpiece, attracting theater enthusiasts from far and wide.
Popular destination for winter sports.
Elektrostal’s proximity to ski resorts and winter sport facilities makes it a favorite destination for skiing, snowboarding, and other winter activities.
Promotes environmental sustainability.
Elektrostal prioritizes environmental protection and sustainability, implementing initiatives to reduce pollution and preserve natural resources.
Home to renowned educational institutions.
Elektrostal is known for its prestigious schools and universities, offering a wide range of academic programs to students.
Committed to cultural preservation.
The city values its cultural heritage and takes active steps to preserve and promote traditional customs, crafts, and arts.
Hosts an annual International Film Festival.
The Elektrostal International Film Festival attracts filmmakers and cinema enthusiasts from around the world, showcasing a diverse range of films.
Encourages entrepreneurship and innovation.
Elektrostal supports aspiring entrepreneurs and fosters a culture of innovation, providing opportunities for startups and business development .
Offers a range of housing options.
Elektrostal provides diverse housing options, including apartments, houses, and residential complexes, catering to different lifestyles and budgets.
Home to notable sports teams.
Elektrostal is proud of its sports legacy , with several successful sports teams competing at regional and national levels.
Boasts a vibrant nightlife scene.
Residents and visitors can enjoy a lively nightlife in Elektrostal, with numerous bars, clubs, and entertainment venues.
Promotes cultural exchange and international relations.
Elektrostal actively engages in international partnerships, cultural exchanges, and diplomatic collaborations to foster global connections.
Surrounded by beautiful nature reserves.
Nearby nature reserves, such as the Barybino Forest and Luchinskoye Lake, offer opportunities for nature enthusiasts to explore and appreciate the region’s biodiversity.
Commemorates historical events.
The city pays tribute to significant historical events through memorials, monuments, and exhibitions, ensuring the preservation of collective memory.
Promotes sports and youth development.
Elektrostal invests in sports infrastructure and programs to encourage youth participation, health, and physical fitness.
Hosts annual cultural and artistic festivals.
Throughout the year, Elektrostal celebrates its cultural diversity through festivals dedicated to music, dance, art, and theater.
Provides a picturesque landscape for photography enthusiasts.
The city’s scenic beauty, architectural landmarks, and natural surroundings make it a paradise for photographers.
Connects to Moscow via a direct train line.
The convenient train connection between Elektrostal and Moscow makes commuting between the two cities effortless.
A city with a bright future.
Elektrostal continues to grow and develop, aiming to become a model city in terms of infrastructure, sustainability, and quality of life for its residents.
In conclusion, Elektrostal is a fascinating city with a rich history and a vibrant present. From its origins as a center of steel production to its modern-day status as a hub for education and industry, Elektrostal has plenty to offer both residents and visitors. With its beautiful parks, cultural attractions, and proximity to Moscow, there is no shortage of things to see and do in this dynamic city. Whether you’re interested in exploring its historical landmarks, enjoying outdoor activities, or immersing yourself in the local culture, Elektrostal has something for everyone. So, next time you find yourself in the Moscow region, don’t miss the opportunity to discover the hidden gems of Elektrostal.
Q: What is the population of Elektrostal?
A: As of the latest data, the population of Elektrostal is approximately XXXX.
Q: How far is Elektrostal from Moscow?
A: Elektrostal is located approximately XX kilometers away from Moscow.
Q: Are there any famous landmarks in Elektrostal?
A: Yes, Elektrostal is home to several notable landmarks, including XXXX and XXXX.
Q: What industries are prominent in Elektrostal?
A: Elektrostal is known for its steel production industry and is also a center for engineering and manufacturing.
Q: Are there any universities or educational institutions in Elektrostal?
A: Yes, Elektrostal is home to XXXX University and several other educational institutions.
Q: What are some popular outdoor activities in Elektrostal?
A: Elektrostal offers several outdoor activities, such as hiking, cycling, and picnicking in its beautiful parks.
Q: Is Elektrostal well-connected in terms of transportation?
A: Yes, Elektrostal has good transportation links, including trains and buses, making it easily accessible from nearby cities.
Q: Are there any annual events or festivals in Elektrostal?
A: Yes, Elektrostal hosts various events and festivals throughout the year, including XXXX and XXXX.
Elektrostal's fascinating history, vibrant culture, and promising future make it a city worth exploring. For more captivating facts about cities around the world, discover the unique characteristics that define each city . Uncover the hidden gems of Moscow Oblast through our in-depth look at Kolomna. Lastly, dive into the rich industrial heritage of Teesside, a thriving industrial center with its own story to tell.
Was this page helpful?
Our commitment to delivering trustworthy and engaging content is at the heart of what we do. Each fact on our site is contributed by real users like you, bringing a wealth of diverse insights and information. To ensure the highest standards of accuracy and reliability, our dedicated editors meticulously review each submission. This process guarantees that the facts we share are not only fascinating but also credible. Trust in our commitment to quality and authenticity as you explore and learn with us.
Share this Fact:
Novotech Launches Dedicated Early Phase Unit for Biotechs Leveraging the Australian and New Zealand Clinical Environment
SAN DIEGO, June 03, 2024 (GLOBE NEWSWIRE) — Novotech, the global full-service Contract Research Organization (CRO) that partners with biotech companies to accelerate the development of advanced and novel therapeutics at every phase, today announced the launch of a dedicated early phase unit focussed on leveraging the unique advantage of Australian and New Zealand (ANZ) for early phase clinical development.
This Early Phase Strategic Delivery Unit (EP SDU) is the first in a series of new Strategic Delivery Units (SDUs) to be launched by Novotech over the course of 2024, each one being a dedicated delivery unit with specialist teams and tailored processes, for different types of complex clinical trials.
Novotech has long been a partner of choice for biotechs wanting to conduct early phase trials in ANZ and benefit from the clinical and scientific excellence, attractive financial rebates, and rapid start-up with streamlined regulatory processes.
Novotech CEO Dr John Moller said: “Our Early Phase Strategic Delivery Unit ensures that, as our global operations continue to grow, our commitment to excellence in Phase I delivery across ANZ remains a core and integral part of Novotech's service offering. This continues to serve as a platform for our biotech clients to expedite their early phase work before expanding into later phase global development with Novotech.”
Lynda Bluck, Director of Early Phase Project Management, who brings over 20 years of clinical trial experience said: “The EP SDU consists of a multidisciplinary team of experts in early phase clinical trials who provide support, expertise, guidance, and oversight to all our early phase projects, ensuring consistent high quality operational delivery.”
“ANZ is an attractive destination for early phase biotechs as it offers rapid, cost-effective, and high-quality clinical development solutions,” said Bluck.
“Novotech has more than 30 years of experience in conducting early phase clinical trials in ANZ with a reputation for consistently delivering high quality data that is accepted by international regulatory agencies.”
“Our team offers proven optimized processes for rapid and efficient execution of Phase I clinical trials and are committed to helping our sponsors navigate both the preclinical and clinical stages of drug development.”
Bluck said central to the EP SDU’s offering is integrated engagement with Novotech's world-class Drug Development Consulting (DDC) team.
“The DDC is a full-service global product development and strategic regulatory group that supports sponsors through every phase of clinical development. The expert team is particularly integral in providing guidance and support prior to entering Phase I and ensuring our sponsors are HREC (IRB equivalent) ready,” said Scott Schliebner, Novotech’s Vice President of Drug Development Consulting.
Novotech's DDC team provides comprehensive "lab to launch" program development services, including regulatory strategy and operations, CMC and product/analytical consulting, clinical oversight, toxicology and other nonclinical consulting, quality/GMP consulting, and electronic submissions. We have a proven track record of successful FDA meetings and approvals.
The teams have the expertise to support projects over their entire lifecycle, all the way from preclinical development through to approval and post-marketing.
About Novotech Novotech-CRO.com
Founded in 1997, Novotech is a global full-service clinical Contract Research Organization (CRO) focused on partnering with biotech companies to accelerate the development of advanced and novel therapeutics at every phase.
Recognized for its industry-leading contributions, Novotech has received numerous prestigious awards, including the CRO Leadership Award 2023, the Asia Pacific Cell & Gene Therapy Clinical Trials Excellence 2023, the Asia-Pacific Contract Research Organization Company of the Year Award since 2006.
The Company offers a comprehensive suite of services including laboratories, Phase I facilities, drug development consulting, regulatory expertise, and has experience with over 5,000 clinical projects, including Phase I to Phase IV clinical trials and bioequivalence studies. With a presence in 34 office locations and a dedicated team of 3,000+ professionals worldwide, Novotech is a trusted end-to-end strategic partner of choice.
For more information or to speak to an expert team member visit www.Novotech-CRO.com
Media Contact
David James
AU: +61 2 8218 2144
USA: +1 415 951 3228
Asia: +65 3159 3427

- Policy & Compliance
- Changes Coming To NIH Applications and Peer Review In 2025
Changes Coming to NIH Applications and Peer Review in 2025
This page serves as a central location where you can learn more about multiple changes coming in 2025 that will affect the submission and review of NIH grant applications.
These changes include updates to the peer review and submission of most research project grants, fellowships, and training grants; Common Forms for NIH biographical sketch and Current and Pending (Other) Support; updated instructions for reference letters; and the transition to FORMS-I application instructions. Although each of these initiatives has specific goals, they are all meant to simplify, clarify, and/or promote greater fairness towards a level playing field for applicants throughout the application and review processes.
Upcoming Webinars
Learn more and have the opportunity to ask questions at the following upcoming webinars:
- June 5, 2024 : Webinar on Updates to NIH Training Grant Applications (registration open)
- September 19, 2024 : Webinar on Revisions to the Fellowship Application and Review Process (registration open)
Simplified Review Framework for Most Research Project Grants (RPGs)
NIH is implementing a simplified framework for the peer review of the majority of competing research project grant (RPG) applications, beginning with submissions with due dates on or after January 25, 2025.

Revisions to the NIH Fellowship Application and Review Process
NIH is revising the fellowship review criteria used to evaluate fellowship applications and modifying the PHS Fellowship Supplemental Form to align with the restructured review criteria beginning with submissions with due dates on or after January 25, 2025.

Updates to Training Grant Applications
The NIH Training Program applications are undergoing changes that take effect for submissions with due dates on or after January 25, 2025.

Updates to Reference Letter Instructions for Referees
NIH is updating the instructions for reference letters submitted for due dates on or after January 25, 2025 to provide more structure so letters will better assist reviewers in understanding the candidate’s strengths, weaknesses, and potential to pursue a productive career in biomedical science. Updated instructions will be posted on the NIH Grants page for Reference Letters as soon as they are available (later in 2024).

Updated Application Forms and Instructions (FORMS-I)
NIH is updating application forms to support many of the changes coming in 2025. These new forms will provide the needed form fields to efficiently implement policy updates and align form instructions and field labels with current terminology. Updated application forms will be posted with active funding opportunities in the Fall of 2024, and updated instructions will be available on the How to Apply - Application Guide at that time.

Common Forms for Biographical Sketch and Current and Pending (Other) Support
NIH is adopting the Biographical Sketch Common Form and the Current and Pending (Other) Support Common Form in 2025. Information on the timing and details of implementation are expected in the coming months.

NIH will provide applicants with plenty of training and resources throughout 2024. The below resources discuss the collective changes coming in January 2025. Additional resources for each initiative can be found on their respective pages.
- Overview of Grant Application and Review Changes for Due Dates on or after January 25, 2025: NOT-OD-24-084
- Drop-in slides on changes coming in 2025 (PowerPoint)
This page last updated on: May 17, 2024
- Bookmark & Share
- E-mail Updates
- Help Downloading Files
- Privacy Notice
- Accessibility
- National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892
- NIH... Turning Discovery Into Health
IOWA Research Park

Perspective Therapeutics to Present at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) Annual Meeting 2024
SEATTLE, May 20, 2024 (GLOBE NEWSWIRE) -- Perspective Therapeutics, Inc. ("Perspective" or "the Company") (NYSE AMERICAN: CATX), a radiopharmaceutical company that is pioneering advanced treatment applications for cancers throughout the body, today announced the Company will present information pertaining to the Company's sponsored studies of its assets at the Society of Nuclear Medicine & Molecular Imaging ("SNMMI") Annual Meeting 2024, which is being held in Toronto, Canada, from June 8-11, 2024. The Company notes that results based on investigator-initiated use of its assets are also being presented.
"We are greatly encouraged by the progress of our clinical and preclinical programs," said Thijs Spoor, CEO of Perspective. "The upcoming presentations at the SNMMI meeting will showcase our activities with VMT-α-NET and other promising therapies, underlining our commitment to advancing care for patients battling challenging tumor types."
[ 212 Pb]VMT-α-NET for the Treatment of Neuroendocrine Tumors The abstract describing Perspective's trial in progress of the Phase 1/2a dose escalation study (NCT05636618) in patients with unresectable or metastatic somatostatin receptor type 2 ("SSTR2")-positive neuroendocrine tumors ("NETs") who have not received prior peptide receptor radionuclide therapies ("PRRT") stated the trial's status as of January 15, 2024, consistent with the abstract submission deadline for the SNMMI conference. Updates on this trial as of March 7, 2024 were provided during the Company's investor update on March 18, 2024, accessible on the events page of the Company's website. This study, in conjunction with the Phase 0 imaging study (NCT05111509), would inform the recommended Phase 2 dose of [ 212 Pb]VMT-α-NET.
Perspective, in collaborating independent investigators, is evaluating dosimetry and the applicability of patient-specific dosing as determined by a target renal absorbed dose for [ 212 Pb]VMT-α-NET. An abstract reported pooled data from ten patients recruited in the Company's sponsored Phase 0 imaging study and an investigator sponsored absorbed dose escalation study. The investigator reported [ 212 Pb]VMT-α-NET was prescribed to three patients at individualized doses of 5.3, 7.9, and 13.3 mCi (cumulatively, delivered over two cycles) while targeting renal absorbed dose of 3.5Gy. Higher levels of targeted renal absorbed doses are in the protocol for subsequent cohorts of the investigator sponsored study.
The Company has been informed that updated results from the investigator initiated trial of [ 212 Pb]VMT-α-NET in India have been accepted for presentation by the SNMMI conference. The investigator enrolled adult patients with histologically confirmed NETs and metastatic medullary thyroid carcinomas. The investigator informed the Company that the update includes results from 12 patients at a later data cutoff date than previous presentations. The most recent prior public update by the investigator was during the 36 th Annual Congress of the European Association of Nuclear Medicine (EANM) in September 2023.
Preclinical Progress on Targeted Therapies for Melanoma and Tumor Environments
The Company will present preclinical data for [ 212 Pb]VMT01, a targeted α-particle radionuclide therapy (α-TRT) targeting the melanocortin 1 receptor ("MC1R"), used in combination with immune checkpoint inhibitors ("ICIs") in diverse murine melanoma models with high (B16-F10), mid (YUMM-D3) and low (YUMMwt) expressions of MC1R. These data provide a rationale for advancing the combination of [ 212 Pb]VMT01 and ICIs to clinical investigations. The ongoing Phase 1/2a study of [ 212 Pb]VMT01 is being amended to evaluate the safety and tolerability of Perspective's [ 212 Pb]VMT01 in combination with the ICI nivolumab in patients with histologically confirmed melanoma and MC1R-positive imaging scans.
The final presentation will introduce [ 203/212 Pb]-PSV-359, a novel cyclic peptide developed to target fibroblast activation protein alpha ("FAP"), a protein abundantly expressed by cancer-associated fibroblasts in tumor lesions and involved in promoting disease progression. [ 203/212 Pb]-PSV-359 has a proprietary targeting moiety designed by Perspective to optimize "theranostic" applications, representing a promising avenue for addressing FAP expressing cancers regardless of disease site.
About Perspective Therapeutics, Inc. Perspective Therapeutics, Inc., is a radiopharmaceutical development company that is pioneering advanced treatment applications for cancers throughout the body. The Company has proprietary technology that utilizes the alpha emitting isotope 212 Pb to deliver powerful radiation specifically to cancer cells via specialized targeting peptides. The Company is also developing complementary imaging diagnostics that incorporate the same targeting peptides, which provide the opportunity to personalize treatment and optimize patient outcomes. This "theranostic" approach enables the ability to see the specific tumor and then treat it to potentially improve efficacy and minimize toxicity.
The Company's melanoma (VMT01) and neuroendocrine tumor (VMT-α-NET) programs have entered Phase 1/2a imaging and therapy trials for the treatment of metastatic melanoma and neuroendocrine tumors at several leading academic institutions. The Company has also developed a proprietary 212 Pb generator to secure key isotopes for clinical trial and commercial operations.
For more information, please visit the Company's website at www.perspectivetherapeutics.com .
Safe Harbor Statement This press release contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995. Statements in this press release that are not statements of historical fact are forward-looking statements. Words such as "may," "will," "should," "expect," "plan," "anticipate," "could," "intend," "target," "project," "estimate," "believe," "predict," "potential" or "continue" or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements in this press release include statements concerning, among other things, the Company's ability to pioneer advanced treatment applications for cancers throughout the body; the Company's expectation that its clinical and preclinical programs will continue to progress; the Company's belief that it will showcase its activities with VMT-α-Net and other promising therapies at the Society of Nuclear Medicine and Molecular Imaging meeting, underlining the Company's commitment to advancing care for patients battling challenging tumor types; the Company's belief that [ 203/212 Pb]-PSV-359, a novel cyclic peptide, has the ability to target fibroblast activation protein alpha, a protein abundantly expressed by cancer-associated fibroblasts in tumor lesions and involved in promoting disease progression; the Company's belief that it has designed a proprietary targeting moiety for [ 203/212 Pb]PSV359 to optimize "theranostic" applications, representing a promising avenue for addressing FAP expressing cancers regardless of disease site; the Company's prediction that complementary imaging diagnostics that incorporate certain targeting peptides provide the opportunity to personalize treatment and optimize patient outcomes; the Company's expectation that its "theranostic" approach enables the ability to see specific tumors and then treat it to potentially improve efficacy and minimize toxicity; the Company's ability to develop a proprietary 212 Pb generator to secure key isotopes for clinical trial and commercial operations; the Company's clinical development plans and the expected timing thereof; the expected timing for availability and release of data; expectations regarding the potential market opportunities for the Company's product candidates; the potential functionality, capabilities, and benefits of the Company's product candidates and the potential application of these product candidates for other disease indications; the Company's expectations, beliefs, intentions, and strategies regarding the future; the Company's intentions to improve important aspects of care in cancer treatment; and other statements that are not historical fact.
The Company may not actually achieve the plans, intentions or expectations disclosed in the forward-looking statements and you should not place undue reliance on the forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause the Company's actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation, the potential that regulatory authorities may not grant or may delay approval for the Company's product candidates; uncertainties and delays relating to the design, enrollment, completion, and results of clinical trials; unanticipated costs and expenses; pre-clinical and early clinical trials may not be indicative of the results in later clinical trials; clinical trial results may not support regulatory approval or further development in a specified indication or at all; actions or advice of regulatory authorities may affect the design, initiation, timing, continuation and/or progress of clinical trials or result in the need for additional clinical trials; the Company's ability to obtain and maintain regulatory approval for the Company's product candidates; delays, interruptions or failures in the manufacture and supply of the Company's product candidates; the size and growth potential of the markets for the Company's product candidates, and the Company's ability to service those markets; the Company's cash and cash equivalents may not be sufficient to support its operating plan for as long as anticipated; the Company's expectations, projections and estimates regarding expenses, future revenue, capital requirements, and the availability of and the need for additional financing; the Company's ability to obtain additional funding to support its clinical development programs; the availability or potential availability of alternative products or treatments for conditions targeted by the Company that could affect the availability or commercial potential of its product candidates; the ability of the Company to manage growth and successfully integrate its businesses; the Company's ability to maintain its key employees; sufficient training and use of the Company's products and product candidates; the market acceptance and recognition of the Company's products and product candidates; the Company's ability to maintain and enforce its intellectual property rights; the Company's ability to maintain its therapeutic isotope supply agreement with the Department of Energy; the Company's ability to continue to comply with the procedures and regulatory requirements mandated by the FDA for additional trials, Phase 1 and 2 approvals, Fast Track approvals, and 510(k) approval and reimbursement codes; and any changes in applicable laws and regulations. Other factors that may cause the Company's actual results to differ materially from those expressed or implied in the forward-looking statements in this press release are described under the heading "Risk Factors" in the Company's most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (the "SEC"), in the Company's other filings with the SEC, and in the Company's future reports to be filed with the SEC and available at www.sec.gov . Forward-looking statements contained in this news release are made as of this date. Unless required to do so by law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
Perspective Therapeutics IR: Annie Cheng [email protected]
Russo Partners, LLC Nic Johnson / Adanna G. Alexander, Ph.D.
- Find a Program
- Find A Contractor

- 2024 Announcements
- 2023 Announcements
- 2022 Announcements
- 2021 Announcements
- Social Media
- Press Release Email List
$39.6 Million Is Now Available To Low Income New Yorkers For Home Energy Efficiency and Electrification Upgrades
New york becomes first state to offer u.s. department of energy home energy rebate funding; funds now available through state’s empower+ program.
May 30, 2024
Governor Kathy Hochul today joined U.S. Department of Energy (DOE) Secretary Granholm and White House Senior Advisor John Podesta at the Andromeda Community Initiative in Queens to celebrate New York State becoming the first state in the nation to offer the first phase of Inflation Reduction Act (IRA) Home Electrification and Appliance Rebates (HEAR) Program funding to consumers. The initial $39.6 million formula grant will expand the reach of New York’s EmPower+ program by allowing more low-income families to improve their homes with energy efficiency and electrification upgrades that will reduce energy costs and transition away from burning fossil fuels in their homes. Today's announcement supports the State’s nation-leading Climate Leadership and Community Protection Act (Climate Act) goal to reduce greenhouse gas emissions by 85 percent by 2050 and requirement for at least 35 percent, with a goal of 40 percent of the benefits of clean energy investments supporting disadvantaged communities.
“As the first state in the nation to offer these Inflation Reduction Act rebates, we are expanding access to home improvements that will save New Yorkers money on their energy bills and reduce our reliance on fossil fuels,” Governor Hochul said . “Thanks to our strong partnership between New York State, the Biden Administration, and U.S. Department of Energy, we are making important progress to make the clean energy transition affordable for all New Yorkers.”
U.S. Secretary of Energy Jennifer M. Granholm said , “From tax credits to rebates, the Biden-Harris Administration is determined to lower costs for American families and change the economics of home energy bill. New York is leading the charge as states across the country gear up to launch their Home Energy Rebates program— delivering jobs, savings, and healthier homes.”
Today’s announcement represents the first step in New York State deploying these IRA rebates to residents. Funding for the full portfolio of IRA Home Energy Rebate programs and deployment of additional rebates is expected later this year. The State is eligible to receive a total of $317.7 million through the DOE’s IRA Home Energy Rebate programs: $159.3 million for the Home Efficiency Rebates program and $158.4 million for HEAR program.
In April 2024, the U.S DOE approved the New York State Energy Research and Development Authority’s (NYSERDA) partial-scope application for this initial funding, which allows NYSERDA’s low income EmPower+ program to expand its current reach by providing funding for additional energy efficiency and beneficial electrification improvements, such as insulation and air sealing, heat pumps for space and water heating, and any necessary electrical upgrades to support those improvements. Beginning today, eligible owners of one- to four-family households that have a household income below 80 percent of the Area Median Income or participate in a utility payment assistance program will automatically be enrolled to benefit from IRA HEAR incentives when they apply to the EmPower+ program. Incentives are provided to residents through EmPower+ contractors to reduce the cost of installing energy efficiency and electrification upgrades in a home or apartment, making them more affordable for New Yorkers. For more information including the application process and eligibility requirements, please visit NYSERDA’s website .
NYSERDA is currently working towards developing and submitting the full HEAR application to enable rebate offers to moderate-income residents, up to 150 percent of area median income, and to owners of larger low- and moderate-income multifamily buildings, including high-efficiency electric appliance rebates.
NYSERDA President and CEO Doreen M. Harris said , “The Inflation Reduction Act will be critical in helping New Yorkers make affordable home efficiency upgrades while also leveraging the state’s strong commitment to reduce emissions and confront the global challenge of climate change. With this funding, the State’s expanded EmPower+ program will focus on those who need assistance the most by providing the opportunity to reap the benefits of high-performance, energy-saving measures throughout their homes.”
Senate Majority Leader Charles Schumer said , “The Home Electrification and Appliance Rebates/HEAR program is the secret sauce of our groundbreaking Inflation Reduction Act: it saves consumers money on energy costs while reducing the polluting greenhouse gasses driving global warming. With this $40 million federal boost, the HEAR program will reduce the cost of installing energy efficiency and electrification upgrades in a home or apartment, making them more affordable for New Yorkers while radically reducing carbon pollution. I am proud to stand with Secretary Granholm and the Biden administration to announce this major investment in clean energy and environmental justice and thank Governor Hochul for helping New York achieve a healthier and brighter future.”
House Minority Leader Hakeem Jeffries said , “This $39.6 million in Inflation Reduction Act rebate funding will allow New Yorkers to breathe cleaner, safer air, put money back in the pockets of families and help bring this nation-leading climate plan to life. In the last Congress, House Democrats, in partnership with President Biden and Senate Democrats, passed the transformational Inflation Reduction Act to create the largest investment in combating the climate crisis in the history of the world. I am grateful to President Biden, the U.S. Department of Energy and Governor Hochul for their partnership in securing this important funding for New York.”
Representative Grace Meng said , “Thank you to Governor Hochul, Secretary Granholm and Senior Advisor Podesta for coming to Queens to commemorate these millions of dollars for home energy efficiency and electrification upgrades. As New York’s representative on the House of Representatives’ Regional Leadership Council – which works to promote and implement legislation signed by President Biden – I’m thrilled that our state will be the first in the nation to offer this rebate funding. It will go a long way toward reducing energy costs and ensuring a cleaner, healthier future for New Yorkers. I’m so proud that I helped to pass the Bipartisan Infrastructure Law which continues to deliver needed resources across our state.”
Representative Adriano Espaillat said , “As we enter the summer months, I commend Governor Hochul on today's announcement expanding New York's EmPower+program, making it more accessible for low-income families who rely on this funding to ensure energy efficiency for their homes. New York is once again leading the way in innovation and implementation of the IRA Home Electrification and Appliance Rebates (HEAR) Program to put families first and make energy efficiency more affordable for all.”
Representative Joe Morelle said , “Too many families continue to feel the pressure of rising costs. From healthcare to prescription drugs to heating and cooling their homes and other basic necessities, I’ve heard horrific stories of people cutting corners to make ends meet, something we cannot allow to continue. Thanks to federal funds I secured as a part of the Inflation Reduction Act, this Department of Energy program will help ensure people’s homes are updated with energy-saving and cost-saving technologies, all at a reduced cost. I’m grateful to Governor Hochul for her leadership, and I look forward to continuing our work together to uplift families in Monroe County and across New York State.”
Representative Jamaal Bowman said , “We need to make bold investments in renewable energy everywhere—from our schools to our homes. Heating our houses in the winter, keeping them cool in the summer, and using our stoves and other appliances should not contribute to the climate catastrophe we are facing. I am so excited to join Governor Hochul and Secretary Granholm in announcing nearly $40 million in funding now available to New Yorkers for home energy efficiency and electrification upgrades and rebates—with even more to come. I am especially proud that these investments focus on low-income New Yorkers and marginalized groups who are most impacted by the harms of the climate crisis and the burden of high electricity bills. The Home Energy Rebate Programs are a direct result of our transformational Inflation Reduction Act, which is consistently putting money back in people’s pockets. This new funding will lower costs for hardworking New Yorkers and support New York in reaching its climate goals.”
Representative Ritchie Torres said , “I am proud to join Governor Hochul in announcing a massive $39.6 million investment in energy rebate funding for low-income New Yorkers. This money is the direct result of the unprecedented green energy investments in President Joe Biden and Congressional Democrats’ landmark Infrastructure Reduction Act (IRA). The increased energy efficiency brought by the HEAR Program’s retrofits will save low-income Bronxites money. It will also reduce carbon emissions – especially important in a borough with the highest rates of asthma and other respiratory illnesses in the city. This program is an excellent example of how Democratic policy is working to impact the daily lives of those in our district and beyond. It is often a privilege to think about energy efficiency when considering housing, due to the limited access of resources, but with the IRA it is feasible. For a community that already struggles with accessibility, this is a step in the right direction.”
Representative Dan Goldman said , “As we transition into a cleaner, greener economy, it is vital that no one – regardless of their circumstance – is left behind. Through President Biden and House Democrats' Inflation Reduction Act, we can finally ensure that everyone has access to the energy and efficiency upgrades that have revolutionized our nation's green infrastructure. I'm proud that under Governor Kathy Hochul's leadership, New York is the first in the nation to roll out these rebates, allowing low-income families across the state to participate in our nation's green transition.”
Building Performance Contractors Association President Hal Smith said , “On behalf of New York’s Building Performance contractors, hats off to everyone who has helped to lead the way for the whole country. It is a great time to be a New Yorker!”
Rewiring America CEO Ari Matusiak said , “Today marks a landmark moment in the implementation of the largest climate bill in history. We congratulate New York for becoming the first state in the nation to roll out the Home Electrification and Appliance Rebates and applaud the Department of Energy for prioritizing getting these dollars out the door and into communities. By crafting a program that meets the needs of its communities and directing this first tranche of funding exclusively to low-income households that stand to benefit the most from lower energy bills and healthier homes, New York is taking an important step in ensuring that the transition to an all-electric future is an equitable one. We’re eager to see the next batch of rebate programs roll out in the coming months and to see states follow New York’s lead in thoughtfully designing effective programs that make the most of this historic opportunity.”
Climate & Energy at Natural Resources Defense Council New York Policy Director Samantha Wilt said , “New York is the first state to leverage the IRA's Home Electrification and Appliance Rebates Program to invest in the homes of the state's most vulnerable families while cutting climate pollution. These funds are being invested in a way that drives benefits to the New Yorkers who need them most and ensures that the harms from burning fossil fuels are reduced in disadvantaged communities. With this investment, New York will be delivering more all-electric appliances, insulation, and wiring upgrades for seriously improved, zero-emission homes.”
Buildings are one of the most significant sources of greenhouse gas emissions in New York State and through NYSERDA and utility programs over $6.8 billion is being invested to decarbonize buildings. By improving energy efficiency in buildings and advancing statewide installations of onsite storage, renewables and electric vehicle charging equipment, the State will reduce its carbon pollution and advance toward the ambitious target of reducing on-site energy consumption by 185 TBtu by 2025, the equivalent of powering 1.8 million homes.
New York State's Nation-Leading Climate Plan
New York State's climate agenda calls for an orderly and just transition that creates family-sustaining jobs, continues to foster a green economy across all sectors and ensures that at least 35 percent, with a goal of 40 percent, of the benefits of clean energy investments are directed to disadvantaged communities. Guided by some of the nation’s most aggressive climate and clean energy initiatives, New York is advancing a suite of efforts – including the New York Cap-and-Invest program (NYCI) and other complementary policies – to reduce greenhouse gas emissions 40 percent by 2030 and 85 percent by 2050 from 1990 levels. New York is also on a path to achieving a zero-emission electricity sector by 2040, including 70 percent renewable energy generation by 2030, and economywide carbon neutrality by mid-century. A cornerstone of this transition is New York's unprecedented clean energy investments, including more than $28 billion in 61 large-scale renewable and transmission projects across the State, $6.8 billion to reduce building emissions, $3.3 billion to scale up solar, nearly $3 billion for clean transportation initiatives and over $2 billion in NY Green Bank commitments. These and other investments are supporting more than 170,000 jobs in New York’s clean energy sector as of 2022 and over 3,000 percent growth in the distributed solar sector since 2011. To reduce greenhouse gas emissions and improve air quality, New York also adopted zero-emission vehicle regulations, including requiring all new passenger cars and light-duty trucks sold in the State be zero emission by 2035. Partnerships are continuing to advance New York’s climate action with more than 400 registered and more than 130 certified Climate Smart Communities, nearly 500 Clean Energy Communities, and the State’s largest community air monitoring initiative in 10 disadvantaged communities across the State to help target air pollution and combat climate change.
Media Inquiries
- Executive Chamber, Phone: 518-474-8418, Ext. n/a Email: [email protected]
Thermodynamics of titanium oxides in metallurgical slags
- Published: 02 September 2015
- Volume 2015 , pages 346–353, ( 2015 )
Cite this article

- A. V. Alpatov 1 &
- S. N. Paderin 2
72 Accesses
Explore all metrics
The energy parameters of the model of a pseudoregular ionic solution are estimated for binary oxide phase diagrams in seven systems containing titanium oxide. The obtained parameters are compared to the available theoretical and experimental data on the thermodynamic properties of TiO 2 in liquid binary systems. The model of a pseudoregular ionic solution is extended to the liquid eight-component FeO-MnO-CaO-MgO-SiO 2 -CrO 1.5 -AlO 1.5 -TiO 2 system, as applied to metallurgical slags containing titanium oxides.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
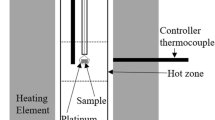
Phase Equilibrium Study of the CaO-SiO2-MgO-Al2O3-TiO2 System at 1300°C and 1400°C in Air
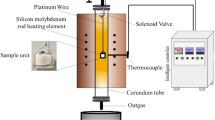
Phase Equilibria of Ti-bearing Electric Furnace Slags in the CaO-MgO-SiO2-13%Al2O3-50%TiO2 System
Thermodynamic analysis of the deoxidizing ability of strontium in liquid iron in the presence of aluminum.
N. A. Smirnova, Molecular Theory of Solutions (Khimiya, Leningrad, 1987).
Google Scholar
A. V. Senin, O. V. Kuznetsova, A. A. Lykasov, et al., “Calculation of the activities of the components of oxide melts in terms of the model of an ideal solution of interaction proiducts,” in Structure and Properties of Metallic and Slag Melts. Vol. 3. Structure and Properties of Slag Melts (YuUrGU, Chelyabinsk, 2004).
V. A. Kozheurov, Thermodynamics of Metallurgical Slags (Metallurgiya, Sverdlovsk, 1955).
Ban-Ya Shiro and Jae-Dong Shim, “Application of regular solution model for the equilibrium of distribution of oxygen between liquid iron and steelmaking slags,” Can. Met. Quart. 21 (4), 319–328 (1982).
Article Google Scholar
Shiro Ban-Ya, “Mathematical expression of slag–metal reaction in steelmaking process by quadratic formalism based on the regular solution model,” ISIJ Intern. 33 (1), 2–11 (1993).
E. M. Vil’gel’m and G. G. Mikhailov, “On the application of the thermodynamics of ionic melts,” in Physicochemical Studies of Metallurgical Processes (UPI, Sverdlovsk, 1978), Vol. 6.
A. D. Pelton and M. Blander, “Thermodynamic analysis of ordered liquid solutions by modified quasichemical approach—application to silicate slags,” Met. Trans. B 17 , 805–815 (1986).
V. I. Antonenko and D. Ya. Povolotskii, “Electrostatic version of the theory of ionic solutions and its application to calculating the metal–slag interaction,” Izv. Chelyab. Nauchn. Tsentra UrO RAN, No. 2 , 71–80 (1999).
D. R. Gaskell, “Thermodynamic models of liquid silicates,” Can. Met. Quart. 20 (1), 3–19 (1981).
P. Sastri and A.K. Lahiri, “Applicability of central atom models to binary silicate and aluminate melts,” Met. Trans. B 16 , 325–331 (1985).
A. G. Ponomarenko, “Problems of the thermodynamics of phases of variable compositions that have a collective electron system: I. The free energy of the phase,” Zh. Fiz. Khim. XLVIII (7), 1668–1671 (1974).
A. G. Ponomarenko and E. P. Mavrenova, “Problems of the thermodynamics of phases of variable compositions that have a collective electron system: I I. Estimation of the energy parameters,” Zh. Fiz. Khim. XLVIII (7), 1672–1674 (1974).
S. N. Paderin and A. V. Alpatov, “Energy parameters in the model of regular ionic solutions as applied to metallurgical slags,” Elektrometallurgiya, No. 9 , 34–41 (2008).
Atlas of Slags , (Metallurgiya, Moscow, 1985).
E. T. Turkdogan, Physical Chemistry of High Temperature Technology (Academic, New York, 1980).
G. Eriksson and A.D. Pelton, “Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the MnO–TiO 2 , MgO–TiO 2 , FeO–TiO 2 , Ti 2 O 3 –TiO 2 , Na 2 O–TiO 2 and K 2 O–TiO 2 systems,” Met. Trans. B 24 , 795–805 (1993).
Y. Q. Hou, G. Xie, D. P. Tao, and X. H. Yu, “Application of modified quasi-regular solution model to binary metallurgical molten slag systems,” J. Iron Steel Res. Intern. 17 (10), 13–17 (2010).
Shiro Ban-Ya, A. Chiba, and A. Hikosaka, “Thermodynamics of Fe t O–M x O y (M x O y = CaO, SiO 2 , TiO 2 and Al 2 O 3 ) binary melts in equilibrium with solid iron,” Tetsu-to-hagane 66 , 1484–1493 (1980).
Y. B. Kang and H. G. Lee, “Experimental study of phase equilibria in the MnO–“TiO 2 ”–“Ti 2 O 3 ” system,” ISIJ Intern. 45 (11), 1543–1551 (2005).
B. K. D. P. Rao and D. R. Gaskel, “The thermodynamic activity of MnO in melts containing SiO 2 , B 2 O 3 and TiO 2 ,” Met. Trans. B 12 , 469–477 (1981).
T. G. Kim, W. K. Lee, J. H. Park, D. J. Min, and H. S. Song, “Sulfide capacity and phase equilibria of MnO–TiO 2 –MnS system at1723K,” ISIJ Intern. 41 (12), 1460–1464 (2001).
M. Ito, K. Morita, and N. Sano, “Thermodynamics of the MnO–SiO 2 –TiO 2 system at1673K,” ISIJ Intern. 37 (9), 839–843 (1997).
M. Ohta and K. Morita, “Thermodynamics of the MnO–Al 2 O 3 –TiO 2 system,” ISIJ Intern. 39 (12), 1231–1238 (1999).
A. S. Berezhnoi, Multicomponent Oxide Systems (Naukova Dumka, Kiev, 1970).
Download references
Author information
Authors and affiliations.
Baikov Institute of Metallurgy and Materials Science, Russian Academy of Sciences, Leninskii pr. 49, Moscow, 119991, Russia
A. V. Alpatov
OAO Elektrostal’ Metallurgical Works, Elektrostal’, Moscow oblast, Russia
S. N. Paderin
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to A. V. Alpatov .
Additional information
Original Russian Text © A.V. Alpatov, S.N. Paderin, 2015, published in Metally, 2015, No. 3, pp. 11–18.
Rights and permissions
Reprints and permissions
About this article
Alpatov, A.V., Paderin, S.N. Thermodynamics of titanium oxides in metallurgical slags. Russ. Metall. 2015 , 346–353 (2015). https://doi.org/10.1134/S003602951505002X
Download citation
Received : 21 July 2014
Published : 02 September 2015
Issue Date : May 2015
DOI : https://doi.org/10.1134/S003602951505002X
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Phase Diagram
- RUSSIAN Metallurgy
- Energy Parameter
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
New Phase Research & Development is a clinical research company located in Knoxville, Tennessee. With our dedication to research and patient care, New Phase has become a premier research site in the region. Our research dedicated facility offers subjects the security of a highly educated staff while providing the luxury of a well maintained and ...
New Phase Research & Development, Knoxville, Tennessee. 14,540 likes · 3 talking about this · 79 were here. New Phase Research & Development is a Knoxville based Clinical Research Site Management...
3. New Phase Research & Development | 363 followers on LinkedIn. A New Phase in Clinical Trials. | A dedicated clinical research facility located in the heart of West Knoxville focused on the ...
New Phase Research & Development is a clinical research company located in Knoxville, Tennessee. With our dedication to research and patient care, New Phase has become a premier research site in the region. Our research dedicated facility offers subjects the security of a highly educated staff while providing the luxury of a well maintained and ...
New Phase Research and Development is a full-time dedicated research facility in Knoxville, Tenn. Our facility offers subjects the security of a highly educated staff while providing the luxury of a well-maintained and comfortable facility to visit throughout the study process. Research Experience. New Phase specializes in many therapeutic areas.
NEW PHASE RESEARCH & DEVELOPMENT LLC Hospitals and Health Care Knoxville, Tennessee 6 followers
184 Followers, 264 Following, 341 Posts - New Phase Research (@newphaseresearch) on Instagram: "New Phase Research & Development is a clinical research site located in Knoxville, TN. New Phase has become a premier research site in the region."
New Phase Research & Development is a clinical research company located in Knoxville Tennessee. With our dedication to research and patient care, New Phase has become a premier research site in the region. Our research dedicated facility offers subjects the security of a highly educated staff while providing the luxury of a well maintained and ...
New Phase Research & Development is a clinical research company. It enrolls patients with osteoarthritis of the knee, asthma, COPD, gastroparesis, diabetic neuropathy, and other conditions.
New Phase Research & Development is a clinical research site located in Knoxville, TN. With our dedication to research and patient care, New Phase has become a premier research site in the region. Our research dedicated facility offers subjects the security of a highly educated staff while providing the luxury of a well maintained and ...
About New Phase Research & Development. New Phase Research & Development is located at 6914 Office Pk Cir NW in Knoxville, Tennessee 37909. New Phase Research & Development can be contacted via phone at 865-200-8364 for pricing, hours and directions.
Specialties: New Phase Research & Development is a clinical research site located in Knoxville, TN. With our dedication to research and patient care, New Phase has become a premier research site in the region. Our research dedicated facility offers subjects the security of a highly educated staff while providing the luxury of a well maintained and comfortable facility to visit throughout the ...
New Phase Research & Development locations. 5.0. Knoxville, TN. Show all locations. Companies. New Phase Research & Development. Find out what works well at New Phase Research & Development from the people who know best. Get the inside scoop on jobs, salaries, top office locations, and CEO insights. Compare pay for popular roles and read about ...
This year, the company plans to open multiple new Phase Ib and II clinical studies for tuvusertib and M9466, key assets from its broad portfolio of DNA damage response (DDR) inhibitors; has advanced its lead antibody-drug conjugate (ADC), M9140, to Phase Ib based on positive signs of clinical benefit, and plans to expand to additional tumors; and progress M3554, its next ADC based on the ...
Watch this video to learn about the three phases of clinical trials. Clinical Research Phase Studies. Phase 1. Study Participants: 20 to 100 healthy volunteers or people with the disease/condition ...
New research and development effort will aim to leverage advanced forensic DNA testing to enhance the capabilities of the Department of the Air ... has been selected by AFWERX for a $1.25 million SBIR Phase II contract focused on advancing forensic DNA sequencing technology to address pressing challenges in the Department of the Air Force (DAF
The Company offers a comprehensive suite of services including laboratories, Phase I facilities, drug development consulting, regulatory expertise, and has experience with over 5,000 clinical ...
FOR IMMEDIATE RELEASE S&T Public Affairs, 202-286-9047. Plan will prepare DHS to meet emerging technological needs and maximize strategic impact. WASHINGTON - The Department of Homeland Security (DHS) released the first-ever department-wide Innovation, Research and Development (IRD) Strategic Plan, articulating key investment goals over the next seven fiscal years.
The National Conference of Bar Examiners (NCBE) has published its first analysis of NextGen bar exam performance data, focused on the pilot testing phase, one of three stages of implementation research being conducted in preparation for the launch of the NextGen bar exam. Insights gained through pilot testing have already been used to inform the development of the new exam, which will be ...
40 Facts About Elektrostal. Elektrostal is a vibrant city located in the Moscow Oblast region of Russia. With a rich history, stunning architecture, and a thriving community, Elektrostal is a city that has much to offer. Whether you are a history buff, nature enthusiast, or simply curious about different cultures, Elektrostal is sure to ...
SAN DIEGO, June 03, 2024 (GLOBE NEWSWIRE) — Novotech, the global full-service Contract Research Organization (CRO) that partners with biotech companies to accelerate the development of advanced and novel therapeutics at every phase, today announced the launch of a dedicated early phase unit focussed on leveraging the unique advantage of Australian and New Zealand (ANZ) for early phase ...
These new forms will provide the needed form fields to efficiently implement policy updates and align form instructions and field labels with current terminology. Updated application forms will be posted with active funding opportunities in the Fall of 2024, and updated instructions will be available on the How to Apply - Application Guide at ...
This study, in conjunction with the Phase 0 imaging study (NCT05111509), would inform the recommended Phase 2 dose of [212Pb]VMT-α-NET.Perspective, in collaborating independent investigators, is evaluating dosimetry and the applicability of patient-specific dosing as determined by a target renal absorbed dose for [212Pb]VMT-α-NET.
Governor Kathy Hochul today joined U.S. Department of Energy (DOE) Secretary Granholm and White House Senior Advisor John Podesta at the Andromeda Community Initiative in Queens to celebrate New York State becoming the first state in the nation to offer the first phase of Inflation Reduction Act (IRA) Home Electrification and Appliance Rebates (HEAR) Program funding to consumers.
The energy parameters of the model of a pseudoregular ionic solution are estimated for binary oxide phase diagrams in seven systems containing titanium oxide. The obtained parameters are compared to the available theoretical and experimental data on the thermodynamic properties of TiO2 in liquid binary systems. The model of a pseudoregular ionic solution is extended to the liquid eight ...