- Case report
- Open access
- Published: 13 September 2021

Critical iron deficiency anemia with record low hemoglobin: a case report
- Audrey L. Chai ORCID: orcid.org/0000-0002-5009-0468 1 ,
- Owen Y. Huang 1 ,
- Rastko Rakočević 2 &
- Peter Chung 2
Journal of Medical Case Reports volume 15 , Article number: 472 ( 2021 ) Cite this article
30k Accesses
6 Citations
2 Altmetric
Metrics details
Anemia is a serious global health problem that affects individuals of all ages but particularly women of reproductive age. Iron deficiency anemia is one of the most common causes of anemia seen in women, with menstruation being one of the leading causes. Excessive, prolonged, and irregular uterine bleeding, also known as menometrorrhagia, can lead to severe anemia. In this case report, we present a case of a premenopausal woman with menometrorrhagia leading to severe iron deficiency anemia with record low hemoglobin.
Case presentation
A 42-year-old Hispanic woman with no known past medical history presented with a chief complaint of increasing fatigue and dizziness for 2 weeks. Initial vitals revealed temperature of 36.1 °C, blood pressure 107/47 mmHg, heart rate 87 beats/minute, respiratory rate 17 breaths/minute, and oxygen saturation 100% on room air. She was fully alert and oriented without any neurological deficits. Physical examination was otherwise notable for findings typical of anemia, including: marked pallor with pale mucous membranes and conjunctiva, a systolic flow murmur, and koilonychia of her fingernails. Her initial laboratory results showed a critically low hemoglobin of 1.4 g/dL and severe iron deficiency. After further diagnostic workup, her profound anemia was likely attributed to a long history of menometrorrhagia, and her remarkably stable presentation was due to impressive, years-long compensation. Over the course of her hospital stay, she received blood transfusions and intravenous iron repletion. Her symptoms of fatigue and dizziness resolved by the end of her hospital course, and she returned to her baseline ambulatory and activity level upon discharge.
Conclusions
Critically low hemoglobin levels are typically associated with significant symptoms, physical examination findings, and hemodynamic instability. To our knowledge, this is the lowest recorded hemoglobin in a hemodynamically stable patient not requiring cardiac or supplemental oxygen support.
Peer Review reports
Anemia and menometrorrhagia are common and co-occurring conditions in women of premenopausal age [ 1 , 2 ]. Analysis of the global anemia burden from 1990 to 2010 revealed that the prevalence of iron deficiency anemia, although declining every year, remained significantly high, affecting almost one in every five women [ 1 ]. Menstruation is considered largely responsible for the depletion of body iron stores in premenopausal women, and it has been estimated that the proportion of menstruating women in the USA who have minimal-to-absent iron reserves ranges from 20% to 65% [ 3 ]. Studies have quantified that a premenopausal woman’s iron storage levels could be approximately two to three times lower than those in a woman 10 years post-menopause [ 4 ]. Excessive and prolonged uterine bleeding that occurs at irregular and frequent intervals (menometrorrhagia) can be seen in almost a quarter of women who are 40–50 years old [ 2 ]. Women with menometrorrhagia usually bleed more than 80 mL, or 3 ounces, during a menstrual cycle and are therefore at greater risk for developing iron deficiency and iron deficiency anemia. Here, we report an unusual case of a 42-year-old woman with a long history of menometrorrhagia who presented with severe anemia and was found to have a record low hemoglobin level.
A 42-year-old Hispanic woman with no known past medical history presented to our emergency department with the chief complaint of increasing fatigue and dizziness for 2 weeks and mechanical fall at home on day of presentation.
On physical examination, she was afebrile (36.1 °C), blood pressure was 107/47 mmHg with a mean arterial pressure of 69 mmHg, heart rate was 87 beats per minute (bpm), respiratory rate was 17 breaths per minute, and oxygen saturation was 100% on room air. Her height was 143 cm and weight was 45 kg (body mass index 22). She was fully alert and oriented to person, place, time, and situation without any neurological deficits and was speaking in clear, full sentences. She had marked pallor with pale mucous membranes and conjunctiva. She had no palpable lymphadenopathy. She was breathing comfortably on room air and displayed no signs of shortness of breath. Her cardiac examination was notable for a grade 2 systolic flow murmur. Her abdominal examination was unremarkable without palpable masses. On musculoskeletal examination, her extremities were thin, and her fingernails demonstrated koilonychia (Fig. 1 ). She had full strength in lower and upper extremities bilaterally, even though she required assistance with ambulation secondary to weakness and used a wheelchair for mobility for 2 weeks prior to admission. She declined a pelvic examination. No bleeding was noted in any part of her physical examination.
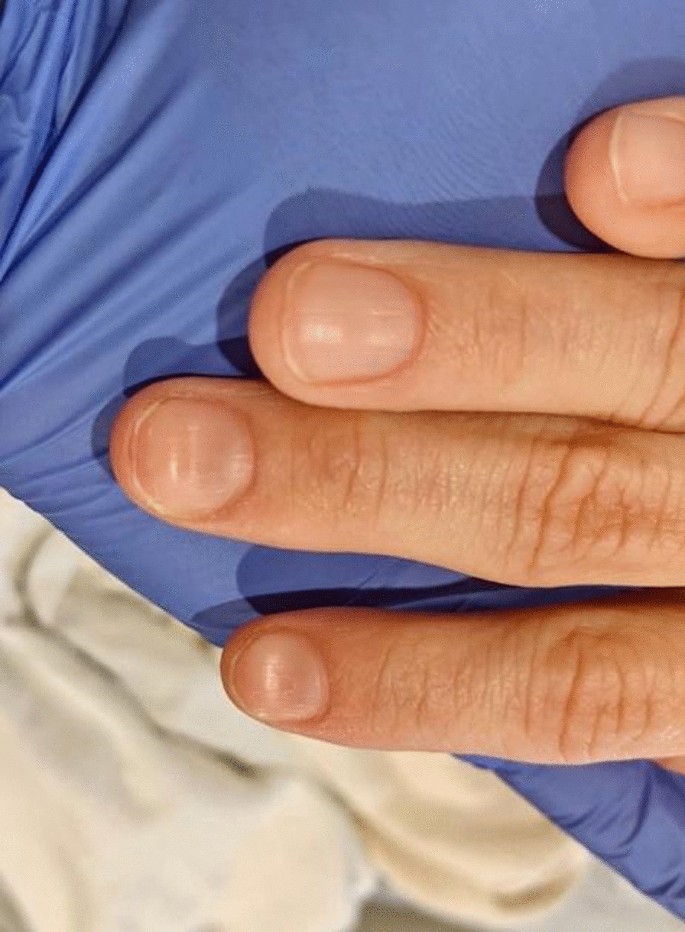
Koilonychia, as seen in our patient above, is a nail disease commonly seen in hypochromic anemia, especially iron deficiency anemia, and refers to abnormally thin nails that have lost their convexity, becoming flat and sometimes concave in shape
She was admitted directly to the intensive care unit after her hemoglobin was found to be critically low at 1.4 g/dL on two consecutive measurements with an unclear etiology of blood loss at the time of presentation. Note that no intravenous fluids were administered prior to obtaining the hemoglobin levels. Upon collecting further history from the patient, she revealed that she has had a lifetime history of extremely heavy menstrual periods: Since menarche at the age of 10 years when her periods started, she has been having irregular menstruation, with periods occurring every 2–3 weeks, sometimes more often. She bled heavily for the entire 5–7 day duration of her periods; she quantified soaking at least seven heavy flow pads each day with bright red blood as well as large-sized blood clots. Since the age of 30 years, her periods had also become increasingly heavier, with intermittent bleeding in between cycles, stating that lately she bled for “half of the month.” She denied any other sources of bleeding.
Initial laboratory data are summarized in Table 1 . Her hemoglobin (Hgb) level was critically low at 1.4 g/dL on arrival, with a low mean corpuscular volume (MCV) of < 50.0 fL. Hematocrit was also critically low at 5.8%. Red blood cell distribution width (RDW) was elevated to 34.5%, and absolute reticulocyte count was elevated to 31 × 10 9 /L. Iron panel results were consistent with iron deficiency anemia, showing a low serum iron level of 9 μg/dL, elevated total iron-binding capacity (TIBC) of 441 μg/dL, low Fe Sat of 2%, and low ferritin of 4 ng/mL. Vitamin B12, folate, hemolysis labs [lactate dehydrogenase (LDH), haptoglobin, bilirubin], and disseminated intravascular coagulation (DIC) labs [prothrombin time (PT), partial thromboplastin time (PTT), fibrinogen, d -dimer] were all unremarkable. Platelet count was 232,000/mm 3 . Peripheral smear showed erythrocytes with marked microcytosis, anisocytosis, and hypochromia (Fig. 2 ). Of note, the patient did have a positive indirect antiglobulin test (IAT); however, she denied any history of pregnancy, prior transfusions, intravenous drug use, or intravenous immunoglobulin (IVIG). Her direct antiglobulin test (DAT) was negative.
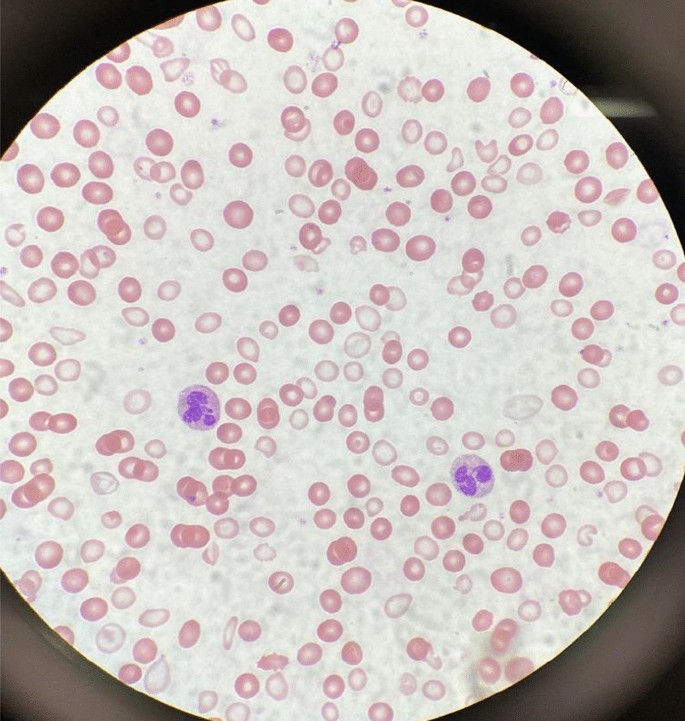
A peripheral smear from the patient after receiving one packed red blood cell transfusion is shown. Small microcytic red blood cells are seen, many of which are hypochromic and have a large zone of pallor with a thin pink peripheral rim. A few characteristic poikilocytes (small elongated red cells also known as pencil cells) are also seen in addition to normal red blood cells (RBCs) likely from transfusion
A transvaginal ultrasound and endometrial biopsy were offered, but the patient declined. Instead, a computed tomography (CT) abdomen and pelvis with contrast was performed, which showed a 3.5-cm mass protruding into the endometrium, favored to represent an intracavitary submucosal leiomyoma (Fig. 3 ). Aside from her abnormal uterine bleeding (AUB), the patient was without any other significant personal history, family history, or lab abnormalities to explain her severe anemia.
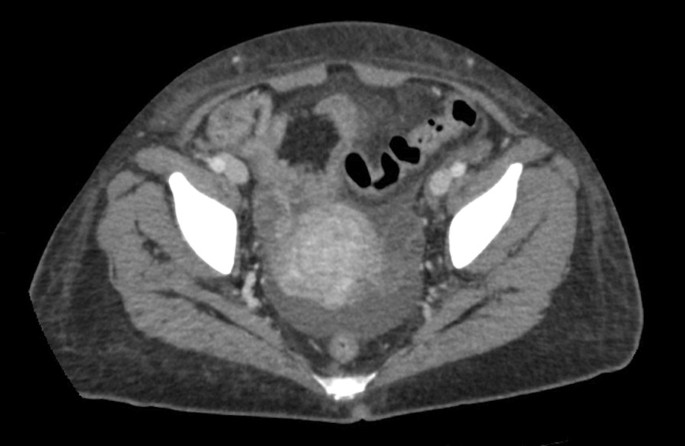
Computed tomography (CT) of the abdomen and pelvis with contrast was obtained revealing an approximately 3.5 × 3.0 cm heterogeneously enhancing mass protruding into the endometrial canal favored to represent an intracavitary submucosal leiomyoma
The patient’s presenting symptoms of fatigue and dizziness are common and nonspecific symptoms with a wide range of etiologies. Based on her physical presentation—overall well-appearing nature with normal vital signs—as well as the duration of her symptoms, we focused our investigation on chronic subacute causes of fatigue and dizziness rather than acute medical causes. We initially considered a range of chronic medical conditions from cardiopulmonary to endocrinologic, metabolic, malignancy, rheumatologic, and neurological conditions, especially given her reported history of fall. However, once the patient’s lab work revealed a significantly abnormal complete blood count and iron panel, the direction of our workup shifted towards evaluating hematologic causes.
With such a critically low Hgb on presentation (1.4 g/dL), we evaluated for potential sources of blood loss and wanted to first rule out emergent, dangerous causes: the patient’s physical examination and reported history did not elicit any concern for traumatic hemorrhage or common gastrointestinal bleeding. She denied recent or current pregnancy. Her CT scan of abdomen and pelvis was unremarkable for any pathology other than a uterine fibroid. The microcytic nature of her anemia pointed away from nutritional deficiencies, and she lacked any other medical comorbidities such as alcohol use disorder, liver disease, or history of substance use. There was also no personal or family history of autoimmune disorders, and the patient denied any history of gastrointestinal or extraintestinal signs and/or symptoms concerning for absorptive disorders such as celiac disease. We also eliminated hemolytic causes of anemia as hemolysis labs were all normal. We considered the possibility of inherited or acquired bleeding disorders, but the patient denied any prior signs or symptoms of bleeding diatheses in her or her family. The patient’s reported history of menometrorrhagia led to the likely cause of her significant microcytic anemia as chronic blood loss from menstruation leading to iron deficiency.
Over the course of her 4-day hospital stay, she was transfused 5 units of packed red blood cells and received 2 g of intravenous iron dextran. Hematology and Gynecology were consulted, and the patient was administered a medroxyprogesterone (150 mg) intramuscular injection on hospital day 2. On hospital day 4, she was discharged home with follow-up plans. Her hemoglobin and hematocrit on discharge were 8.1 g/dL and 24.3%, respectively. Her symptoms of fatigue and dizziness had resolved, and she was back to her normal baseline ambulatory and activity level.
Discussion and conclusions
This patient presented with all the classic signs and symptoms of iron deficiency: anemia, fatigue, pallor, koilonychia, and labs revealing marked iron deficiency, microcytosis, elevated RDW, and low hemoglobin. To the best of our knowledge, this is the lowest recorded hemoglobin in an awake and alert patient breathing ambient air. There have been previous reports describing patients with critically low Hgb levels of < 2 g/dL: A case of a 21-year old woman with a history of long-lasting menorrhagia who presented with a Hgb of 1.7 g/dL was reported in 2013 [ 5 ]. This woman, although younger than our patient, was more hemodynamically unstable with a heart rate (HR) of 125 beats per minute. Her menorrhagia was also shorter lasting and presumably of larger volume, leading to this hemoglobin level. It is likely that her physiological regulatory mechanisms did not have a chance to fully compensate. A 29-year-old woman with celiac disease and bulimia nervosa was found to have a Hgb of 1.7 g/dL: she presented more dramatically with severe fatigue, abdominal pain and inability to stand or ambulate. She had a body mass index (BMI) of 15 along with other vitamin and micronutrient deficiencies, leading to a mixed picture of iron deficiency and non-iron deficiency anemia [ 6 ]. Both of these cases were of reproductive-age females; however, our patient was notably older (age difference of > 20 years) and had a longer period for physiologic adjustment and compensation.
Lower hemoglobin, though in the intraoperative setting, has also been reported in two cases—a patient undergoing cadaveric liver transplantation who suffered massive bleeding with associated hemodilution leading to a Hgb of 0.6 g/dL [ 7 ] and a patient with hemorrhagic shock and extreme hemodilution secondary to multiple stab wounds leading to a Hgb of 0.7 g/dL [ 8 ]. Both patients were hemodynamically unstable requiring inotropic and vasopressor support, had higher preoperative hemoglobin, and were resuscitated with large volumes of colloids and crystalloids leading to significant hemodilution. Both were intubated and received 100% supplemental oxygen, increasing both hemoglobin-bound and dissolved oxygen. Furthermore, it should be emphasized that the deep anesthesia and decreased body temperature in both these patients minimized oxygen consumption and increased the available oxygen in arterial blood [ 9 ].
Our case is remarkably unique with the lowest recorded hemoglobin not requiring cardiac or supplemental oxygen support. The patient was hemodynamically stable with a critically low hemoglobin likely due to chronic, decades-long iron deficiency anemia of blood loss. Confirmatory workup in the outpatient setting is ongoing. The degree of compensation our patient had undergone is impressive as she reported living a very active lifestyle prior to the onset of her symptoms (2 weeks prior to presentation), she routinely biked to work every day, and maintained a high level of daily physical activity without issue.
In addition, while the first priority during our patient’s hospital stay was treating her severe anemia, her education became an equally important component of her treatment plan. Our institution is the county hospital for the most populous county in the USA and serves as a safety-net hospital for many vulnerable populations, most of whom have low health literacy and a lack of awareness of when to seek care. This patient had been experiencing irregular menstrual periods for more than three decades and never sought care for her heavy bleeding. She, in fact, had not seen a primary care doctor for many years nor visited a gynecologist before. We emphasized the importance of close follow-up, self-monitoring of her symptoms, and risks with continued heavy bleeding. It is important to note that, despite the compensatory mechanisms, complications of chronic anemia left untreated are not minor and can negatively impact cardiovascular function, cause worsening of chronic conditions, and eventually lead to the development of multiorgan failure and even death [ 10 , 11 ].
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Kassebaum NJ. The global burden of anemia. Hematol Oncol Clin. 2016;30(2):247–308.
Article Google Scholar
Donnez J. Menometrorrhagia during the premenopause: an overview. Gynecol Endocrinol. 2011;27(sup1):1114–9.
Cook JD, Skikne BS, Lynch SR, Reusser ME. Estimates of iron sufficiency in the US population. Blood. 1986;68(3):726–31.
Article CAS Google Scholar
Palacios S. The management of iron deficiency in menometrorrhagia. Gynecol Endocrinol. 2011;27(sup1):1126–30.
Can Ç, Gulactı U, Kurtoglu E. An extremely low hemoglobin level due to menorrhagia and iron deficiency anemia in a patient with mental retardation. Int Med J. 2013;20(6):735–6.
Google Scholar
Jost PJ, Stengel SM, Huber W, Sarbia M, Peschel C, Duyster J. Very severe iron-deficiency anemia in a patient with celiac disease and bulimia nervosa: a case report. Int J Hematol. 2005;82(4):310–1.
Kariya T, Ito N, Kitamura T, Yamada Y. Recovery from extreme hemodilution (hemoglobin level of 0.6 g/dL) in cadaveric liver transplantation. A A Case Rep. 2015;4(10):132.
Dai J, Tu W, Yang Z, Lin R. Intraoperative management of extreme hemodilution in a patient with a severed axillary artery. Anesth Analg. 2010;111(5):1204–6.
Koehntop DE, Belani KG. Acute severe hemodilution to a hemoglobin of 1.3 g/dl tolerated in the presence of mild hypothermia. J Am Soc Anesthesiol. 1999;90(6):1798–9.
Georgieva Z, Georgieva M. Compensatory and adaptive changes in microcirculation and left ventricular function of patients with chronic iron-deficiency anaemia. Clin Hemorheol Microcirc. 1997;17(1):21–30.
CAS PubMed Google Scholar
Lanier JB, Park JJ, Callahan RC. Anemia in older adults. Am Fam Phys. 2018;98(7):437–42.
Download references
Acknowledgements
Not applicable.
No funding to be declared.
Author information
Authors and affiliations.
Department of Medicine, University of Southern California, Los Angeles, CA, USA
Audrey L. Chai & Owen Y. Huang
Division of Pulmonary, Critical Care and Sleep Medicine, Department of Medicine, University of Southern California, Los Angeles, CA, USA
Rastko Rakočević & Peter Chung
You can also search for this author in PubMed Google Scholar
Contributions
AC, OH, RR, and PC managed the presented case. AC performed the literature search. AC, OH, and RR collected all data and images. AC and OH drafted the article. RR and PC provided critical revision of the article. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Audrey L. Chai .
Ethics declarations
Ethics approval and consent to participate, consent for publication.
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Chai, A.L., Huang, O.Y., Rakočević, R. et al. Critical iron deficiency anemia with record low hemoglobin: a case report. J Med Case Reports 15 , 472 (2021). https://doi.org/10.1186/s13256-021-03024-9
Download citation
Received : 25 March 2021
Accepted : 21 July 2021
Published : 13 September 2021
DOI : https://doi.org/10.1186/s13256-021-03024-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Menometrorrhagia
- Iron deficiency
- Critical care
- Transfusion
Journal of Medical Case Reports
ISSN: 1752-1947
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Advertisement
Introduction
Epidemiology, pathophysiology of iron deficiency, etiology of iron deficiency, diagnosing iron deficiency, advances and controversies in the treatment of iron deficiency, future perspectives, acknowledgments, iron deficiency.
- Split-Screen
- Request Permissions
- Cite Icon Cite
- Search Site
- Open the PDF for in another window
Clara Camaschella; Iron deficiency. Blood 2019; 133 (1): 30–39. doi: https://doi.org/10.1182/blood-2018-05-815944
Download citation file:
- Ris (Zotero)
- Reference Manager
Iron deficiency anemia affects >1.2 billions individuals worldwide, and iron deficiency in the absence of anemia is even more frequent. Total-body (absolute) iron deficiency is caused by physiologically increased iron requirements in children, adolescents, young and pregnant women, by reduced iron intake, or by pathological defective absorption or chronic blood loss. Adaptation to iron deficiency at the tissue level is controlled by iron regulatory proteins to increase iron uptake and retention; at the systemic level, suppression of the iron hormone hepcidin increases iron release to plasma by absorptive enterocytes and recycling macrophages. The diagnosis of absolute iron deficiency is easy unless the condition is masked by inflammatory conditions. All cases of iron deficiency should be assessed for treatment and underlying cause. Special attention is needed in areas endemic for malaria and other infections to avoid worsening of infection by iron treatment. Ongoing efforts aim at optimizing iron salts–based therapy by protocols of administration based on the physiology of hepcidin control and reducing the common adverse effects of oral iron. IV iron, especially last-generation compounds administered at high doses in single infusions, is becoming an effective alternative in an increasing number of conditions because of a more rapid and persistent hematological response and acceptable safety profile. Risks/benefits of the different treatments should be weighed in a personalized therapeutic approach to iron deficiency.
Iron balance is essential for all cell life. Iron homeostatic mechanisms evolved to avoid iron excess and the generation of harmful reactive oxygen species by reutilizing body iron and limiting its uptake from the environment. The inevitable other side of the coin is the easy development of iron deficiency.
Iron deficiency is the depletion of total-body iron, especially of macrophage and hepatocyte iron stores. Because the largest amount of iron is consumed for hemoglobin (Hb) synthesis to produce 200 billion erythrocytes daily, anemia is the more evident sign of iron deficiency, and iron deficiency anemia is often considered synonymous with iron deficiency. However, iron deficiency is a broader condition that often precedes the onset of anemia or indicates deficiency in organs/tissues other than those involved in erythropoiesis, such as skeletal muscles and the heart, the latter highly iron dependent for myoglobin and energy production to sustain mechanical contraction.
This article reviews the mechanisms of adaptation to iron deficiency and related anemia, examines how improved knowledge is influencing treatment, and discusses areas that remain uncertain from the biological and clinical perspectives.
According to the Global Burden of Disease Study 2016, iron deficiency anemia is 1 of the 5 leading causes of years lived with disability burden and is the first cause in women. 1 Adopting the World Health Organization–recommended cutoff for anemia (Hb <13 g/dL in males, <12 g/dL in females, <11g/dL during pregnancy), a worldwide survey showed that in 2010, anemia still affected one third of the population, with approximately half of the cases resulting from iron deficiency. The estimate is that ∼1.24 billion individuals experience iron deficiency anemia, although with huge variations from low- to high-income countries. 2 The global prevalence of iron deficiency without anemia remains elusive, although the suggested figure is at least double that of iron deficiency anemia. The problem becomes even more relevant if we take into account functional iron deficiency, which occurs when iron is hardly mobilized from stores, as in chronic inflammations/infections or when the vigorous erythropoietic expansion by exogenous or endogenous erythropoietin (EPO) causes an acute disproportion between iron demand and supply.
Globally, iron deficiency anemia has relevant medical and social impacts, accounting for impairment of cognitive performance in young children, 3 adverse outcomes of pregnancy for both mothers and newborns, 4 decreased physical and working capacities in adults, and cognitive decline in the elderly. 5 , 6 From available data, the relative contribution of iron deficiency to these negative outcomes is difficult to disassociate from that of anemia.
Iron deficiency deeply affects iron homeostasis, inducing adaptive mechanisms on the hepcidin-ferroportin (FPN) axis, the iron regulatory protein (IRP)/iron responsive element (IRE) machinery, and other regulators. The aim is to optimize iron usage by erythropoiesis and to counteract the physiological inhibition of iron absorption.
Mechanisms of adaptation
Systemic regulation.
Liver hepcidin is the master hormone that physiologically limits iron entry into plasma. Binding to its receptor FPN, hepcidin blocks iron export both by occluding the exporter central cavity 7 and by inducing its degradation. 8 Because of the high FPN expression on professional iron exporter cells, such as enterocytes and macrophages, hepcidin suppression in iron deficiency enhances both iron absorption and its release from macrophages to plasma. Multiple factors downregulate hepcidin transcription ( Figure 1 ). The BMP-SMAD signaling pathway is repressed, because in iron deficiency, expression of BMP6 ligand is low, 9 the BMP coreceptor HJV is cleaved by TMPRSS6, 10 and TFR2 is removed from the cell surface. 11 In addition, the histone deacetylase HDAC3 erases activation markers from the hepcidin locus, 12 providing an epigenetic contribution to hormone suppression. The function of ERFE, released by erythroid cells stimulated by erythropoietin, 13 is less relevant in iron deficiency without anemia, because hepcidin is downregulated when iron deficiency is induced in Erfe −/− mice. 12 However, ERFE plays a role in the presence of anemia and hypoxia. 13

Mechanisms of hepcidin inhibition in iron deficiency anemia. Main cells/organs involved in hepcidin (HAMP) inhibition in iron deficiency are illustrated. In the hepatocytes, bone morphogenic protein (BMP)-SMAD signaling, the main activator of hepcidin, is low because low levels of BMP6 are produced by liver sinusoidal endothelial cells (L-SEC), the BMP coreceptor hemojuvelin (HJV) is cleaved from the hepatocyte surface by the transmembrane serine protease 6 (TMPRSS6), and the second transferrin receptor (TFR2) is not stabilized on the cell surface in the absence of the ligand diferric transferrin (TF). Low hepcidin levels increase iron absorption by enterocytes and recycling by macrophages through increased activity of the iron exporter FPN. In mild iron deficiency in the absence of hypoxia, increased EPO sensitivity is due to the loss of TFR2 on erythroblast surfaces. Histone deacetylase 3 (HIDAC3) participates in hepcidin suppression by erasing markers of activation at the hepcidin locus. In iron deficiency anemia, hypoxia increases EPO. Increased ERFE fully blocks the hepcidin pathway, although the molecular mechanism of hepcidin inhibition by ERFE remains unknown (?). BMPR, BMP receptor; CP, ceruloplasmin; DCYTB, duodenal cytochrome B; DMT1, divalent metal transporter 1; EPOR, EPO receptor; HEPH, hephestin.
Local mechanisms increase intestinal iron absorption. Hypoxia-inducible factor 2α (HIF2α) upregulates the expression of both the brush border machinery (DMT1 and DCYTB) that uptakes iron from the lumen and the iron exporter FPN at the basolateral membrane by binding hypoxia-responsive elements of these gene promoters. 14
Macrophages rapidly recycle iron derived from the phagocytosis of senescent red cells ( Figure 1 ). However, the absolute amount of iron recycled from hypochromic erythrocytes by heme-oxygenase 1 decreases in parallel with the severity of iron deficiency, because Hb content per cell (mean corpuscular Hb [MCH]) is reduced. A novel mechanism related to erythrocyte FPN, which is highly expressed in iron deficiency, may contribute to maintaining circulating iron levels. 15 Low serum hepcidin levels ultimately determine the amount of iron entering the circulation ( Figure 1 ).
Cellular regulation
Cellular iron content is controlled by IRPs that in iron deficiency bind stem-loop sequences (IREs) in the untranslated regions (UTRs) of iron genes to posttranscriptionally coordinate proteins of iron absorption, export, use, and storage. 16 Binding to 3′UTR IREs, IRPs stabilize the messenger RNA of TFRC and DMT1 ; binding to 5′UTR IREs, they repress translation of ferritin, FPN, 5′-aminolevulinate synthase 2 (ALAS2), and HIF2α. To avoid deleterious iron retention in iron-deficient enterocytes and maturing erythroblasts, an alternative isoform of FPN lacking 5′UTR IRE escapes IRP control 15 while remaining sensitive to the hepcidin effect.
Other IRP-independent mechanisms optimize iron use in low-iron states. mTOR inhibition activates tristetraprolin, which reduces both TFR1 and FPN expression to save iron for tissue metabolic needs. 17 Cells may recover their own iron stored in ferritin. In iron deficiency, ferritin is delivered to autophagosomes for degradation (ferritinophagy) by nuclear receptor coactivator 4, which in contrast is proteasome degraded in iron-replete cells. 18 Reduced ferritinophagy makes NcoA4 knockout mice susceptible to hypoferremia and iron deficiency. 19 Ferritinophagy has been shown to provide iron for erythroid differentiation in vitro 20 and in zebrafish. 21 It has not been assessed whether ferritin is reduced in plasma when it undergoes ferritinophagy. Although serum ferritin is the best biomarker of iron deficiency, the mechanisms of its release as well as its function in the circulation remain mysterious. 22
Iron-restricted erythropoiesis
Iron restriction limits the expansion of early erythropoiesis and optimizes iron use by terminal erythropoiesis. In vitro iron deprivation blunts the EPO responsiveness of early progenitors through inactivation of iron-dependent aconitase, which suppresses isocitrate production. 23 Accordingly, iron or isocitrate treatment restores erythroid lineage differentiation. 24 EPO is not elevated in mice with iron deficiency without anemia. 25 , 26 However, in the same condition, terminal erythropoiesis is modified, with decreased apoptosis and increased number of late erythroblasts. The same phenotype, expression of increased EPO sensitivity, is recapitulated by the genetic loss of the EPOR partner TFR2 in mice; this condition mimics iron deficiency, 27 because TFR2 is lost from the membrane when diferric TF is reduced. 11 , 28
With the development of anemia and hypoxia, EPO levels increase exponentially, and multiple mediators, such as erythroferrone, 13 GDF15, 29 and PDGF-BB, 30 suppress hepcidin to enhance iron supply. In this process, a role of soluble TFR (sTFR), an accepted biomarker of iron deficiency, 31 although reasonable, remains unproven.
Because of the increased number of erythroblasts and limited iron supply, heme content per cell is reduced. Globin translation is also impaired by low heme; the stress sensor heme-regulated inhibitor (HRI) phosphorylates the elongation initiation factor 2a (eIF2A) to block translation, concomitantly increasing ATF4, which inhibits the translation regulator mTOR. 32 The heme/globin coordination improves erythropoiesis, producing microcytic (low mean corpuscular volume)/hypochromic (low MCH) erythrocytes. The optimization of erythropoiesis might preserve iron for vital functions within a global body economy. However, the mechanism is not fully effective, because even in the absence of anemia, other organs may become iron deficient.
Individuals at risk
Reflecting high iron requirements, infants, preschool children (age <5 years), young menstruating women, and women in the second/third trimester of pregnancy and postpartum are the most affected groups. 33 , 34 Adolescents also are susceptible to iron deficiency because of rapid growth.
In Western countries, other healthy individuals may be at risk. These include vegetarians, especially vegans, because of diet restriction and blood donors. 35 The RISE study, which evaluated the iron status of >2000 frequent blood donors in the United States, showed that two thirds of women and half of men were iron deficient. 36 Elite endurance athletes are at risk because of inflammation-induced increased hepcidin and blood losses. Females are more affected in all the groups listed here.
Iron deficiency with or without anemia may be isolated or secondary to a causative disorder or occur in the context of multiple pathological conditions (eg, in the elderly). Iron deficiency is usually acquired and exceptionally inherited.
Acquired iron deficiency
In developing countries, iron deficiency anemia is nutritional, resulting from reduced intake of bioavailable iron ( Table 1 ), and often associated with infections causing hemorrhages, such as hookworm infestation or schistosomiasis. In Western societies, other than in individuals at risk, iron depletion results from chronic bleeding and/or reduced iron absorption, disorders that may be more relevant than anemia itself ( Table 1 ). For this reason, considering age, sex, clinical history, and symptoms, identification of the underlying cause is an essential part of the patient’s workup. 33 , 34
Main causes of absolute iron deficiency/iron deficiency anemia
ESA, erythropoiesis-stimulating agent; H 2 antagonists, histamine receptor blockers; IRIDA, iron-refractory iron deficiency anemia; PNH, paroxysmal nocturnal hemoglobinuria.
More common in developing countries.
Rarely resulting from gene mutations other than TMPRSS6 . 100
Absolute iron deficiency may be masked by comorbidities (eg, in the elderly, and in the setting of renal failure). Anemia in the elderly has multiple causes. 37 Iron deficiency accounts for ∼30% of cases, resulting from low intake, reduced absorption (atrophic gastritis, use of proton pump inhibitors), gastrointestinal blood losses (antithrombotic drugs, angiodysplasia, peptic ulcer, hemorrhoids, and even colorectal cancer). Unfortunately, being obscured by comorbidities, it often remains undiagnosed, 38 while even mild anemia worsens the outcome of associated disorders and influences mortality. 39 Patients with chronic kidney disease (CKD) are prone to absolute iron deficiency because of reduced absorption 40 and blood loss at dialysis, at an estimated rate of up to 2 to 3 g per year. 41 However, high hepcidin levels and inflammation, which reduce iron mobilization from stores, may mask absolute deficiency. A recognized cause of dysregulation of iron metabolism is obesity, which may lead to iron deficiency, especially after bariatric surgery because of global absorption impairment ( Table 1 ). 42 In Western countries, as a result of increased life expectancy, these types of iron deficiency are expected to increase in coming years.
Considering the need for balancing iron demand and supply, specific clinical settings are characterized by acute restriction of iron for erythropoiesis. The best-known example is treatment with erythropoiesis-stimulating agents. Another example is postoperative anemia that follows major surgery. Recovery from anemia may be limited or delayed because of preexisting unrecognized iron deficiency that becomes evident after surgery and/or cytokine-induced defective iron mobilization.
Genetics of iron deficiency
IRIDA 43 is a rare recessive condition resulting from mutations of TMPRSS6 , 44 , 45 leading to an inability to cleave the BMP coreceptor HJV and inhibit hepcidin. 10 High hepcidin in IRIDA patients impairs iron absorption, counteracting an essential compensatory mechanism to sustain erythropoiesis. IRIDA patients are refractory to oral iron supplementation. 46 , 47 IV iron is indicated when anemia is severe, but it may be only partially effective. In adults, especially men, anemia may be less evident than in children, while iron deficiency and microcytosis persist. 48
Populations studies suggest that susceptibility to iron deficiency is in part influenced by genetics. Studies of blood donors have strengthened the hypothesis that genetic variants of iron genes, especially TMPRSS6 and HFE , reported to influence iron parameters 49 , 50 and hepcidin, 51 may predispose to or protect individuals from iron deficiency. 52
In a novel murine model, genetic iron deficiency anemia was caused by loss of the enzyme of the sulfur assimilation pathway bisphosphate-3′-nucleotidase (Bpnt1). Iron deficiency anemia characterizes both germinal and intestinal conditional Bpnt 1 knockout mice, establishing a novel link between sulfur and iron homeostasis. 53
Clinical signs and symptoms of iron deficiency anemia are limited and often neglected. The most important, fatigue, is unspecific. Alterations of epithelial cells such as dry mouth, cheilitis, atrophic glossitis, Plummer-Vinson pharyngeal webs, and hair loss are observed in longstanding deficiency. Restless leg syndrome reveals iron deficiency in a proportion of cases. 54 , 55 In the elderly, iron deficiency anemia may cause heart failure or angina. For a detailed discussion of symptoms in iron deficiency anemia, readers are referred elsewhere. 34 , 56
A correct diagnosis requires laboratory tests. Low serum ferritin levels are the hallmark of absolute iron deficiency, reflecting exhausted stores. Levels <30 mg/L are the accepted threshold that identifies mild cases; in the presence of anemia, ferritin levels are usually lower (<10-12 mg/L). In the absence of inflammations/infections, serum ferritin shows the best correlation with bone marrow stainable iron, once the gold standard in assessing depletion of iron stores. 33 , 34
Measuring TF saturation (<16%) is unnecessary for diagnosis, although it has diagnostic value in functional deficiency when serum ferritin is unreliable. Hepcidin levels, which are low/undetectable in absolute iron deficiency, are also unnecessary. Exceptions are the rare IRIDA patients who show low TF saturation and normal/high hepcidin and serum ferritin levels, reflecting increased macrophage iron. Measuring serum hepcidin may be diagnostic of this atypical iron deficiency, provided that inflammation is excluded. 57
sTFR and its relationship to ferritin (sTFR/logFt index) are good indicators of iron-deficient erythropoiesis, 58 , 59 but tests to measure these indicators are scarcely available in clinics. Reduction of MCH and mean corpuscular volume and increased (>6% in CKD) hypochromic red cells (with MCH <28 pg) occur relatively late because of the erythrocyte lifespan. Reticulocyte Hb content may reveal rapid changes in erythropoietic activity. Early reduction (<26 pg) may occur after erythropoiesis-stimulating agent treatment and early increase after iron supplementation. 60 When heme is low, zinc is incorporated into protoporphyrin-IX, levels of which become elevated and measurable in mature erythrocytes. 60
All tissues are assumed to be iron deficient when ferritin is low. No specific test assesses tissue (eg, cardiac or muscle) iron deficiency when ferritin is unreliable, such as in inflammation. Perception of this deficiency by patients is highly variable. Clinical diagnosis relies on deterioration of the specific organ (eg, heart) function or on unspecific symptoms, the most popular being fatigue. Alternatively, the diagnosis is based on a positive outcome after iron supplementation, such as in heart failure. 61
To correctly diagnose iron deficiency in the context of multiple comorbidities, such as in inflammation, ferritin threshold <100 mg/L or even higher values are suggested, in combination with low (<20%) TF saturation. 62 Although these arbitrary cutoffs likely overestimate iron deficiency, they are largely used for therapeutic decisions. The diagnosis of absolute iron deficiency is also challenging in the elderly; proposed cutoffs between >30 and <100 mg/L are based on small studies. 37 , 38 This supports the need for well-designed prospective clinical trials and development of biomarkers for tissue iron deficiency.
The etiological cause of iron deficiency should be addressed in all cases and, whenever possible, eliminated. Iron treatment should be started immediately, even in the absence of anemia, especially in symptomatic patients. 63 , 64 A systematic review of the efficacy of iron supplementation in iron-deficient nonanemic individuals concluded that treatment (any type) increased Hb and ferritin and reduced self-reported fatigue but did not improve physical performance or maximal oxygen consumption. 65
The choice of iron compound and the route of administration are largely dependent on the presence and degree of anemia, reversibility of the underlying cause, clinical status (age, sex, longstanding vs recent onset), and in some instances patient preference.
Oral iron supplementation
Iron salts such as iron sulfate, fumarate, and gluconate remain a mainstay of therapy in absolute iron deficiency. Mounting evidence indicates that low doses are more effective and better tolerated than the traditionally recommended 100 to 200 mg of elementary iron per day. Because absorption of nonheme iron is modest (5% to 28% at the fastest), 66 high doses may result in ROS-mediated toxicity of nonabsorbed iron on intestinal mucosa. Common adverse effects, such as nausea, vomiting, constipation, or diarrhea, may lead to noncompliance with therapy in 30% to 70% of cases 67 and jeopardize the prolonged (several months) treatment planned. Importantly, even a mild increase in serum iron activates hepcidin to limit iron absorption. This physiological response was exploited to design the most appropriate dose and schedule of oral iron administration in iron-deficient nonanemic women. In short-term studies that used stable iron isotopes, supplementation with iron sulfate (60-240 mg) induced hepcidin increase for up to 48 hours, limiting the absorption of the subsequent doses. 68 In another trial in which participants were randomly assigned to receive 60 mg of iron per day for 14 days or on alternate day for 28 days, fractional iron absorption was significantly greater in the latter group (21.8% vs 16.3%). In a study comparing 2 groups of women who were receiving 120 mg of iron sulfate per day either as a single or 2 divided doses, the first group showed smaller serum hepcidin increases. 69 Altogether these elegant studies indicate that changing the administration from daily to alternate-day schedules and from divided to single doses increases the efficacy of treatment in iron-deficient nonanemic individuals and has the potential to improve tolerability. An ongoing study in women with iron deficiency anemia 70 is assessing whether the alternate-day protocol should also be recommended in the presence of anemia, 71 when hypoxia further increases intestinal iron absorption and fully suppresses hepcidin. 14
Other adverse effects of unabsorbed iron include alterations in the composition of the gut microbiome, with reduction of beneficial Lactobacillus and Bifidobacterium bacteria, enhancement of potential pathogens ( Enterobacteriaceae ), and increased inflammation and diarrhea, as shown in African children. 72 , 73
The minimal dose used for iron supplementation is 60 mg per day. Lower doses (37.5 mg per day) of oral iron have proven useful in blood donors to limit deferrals from donations. 36
A prophylactic treatment with iron sulfate (60 mg in adults and 30 mg in children) has been recommended in world areas characterized by high prevalence of iron deficiency anemia. 74 However, the validity of universal supplementation in countries with high prevalence of malaria and/or other infections is controversial. Epidemiological 75 and in vitro studies have shown that iron deficiency is an adaptation process protecting from Plasmodium virulence and that its correction may increase infection severity. 76 , 77 Recent evidence shows that FPN expressed in erythrocytes is functional and reduced by the high hepcidin levels induced by iron supplementation. This would increase erythrocyte iron content, favoring the parasite growth. 15 In these cases, iron supplementation should occur in association with antimalarial treatment. 78 Another problem is related supplemented iron causing gut dysbiosis and diarrhea. To avoid the latter effects, a future solution is the development of iron compounds bioavailable only to humans and not to pathogens.
There is great interest in the development of compounds better tolerated than iron salts; numerous compounds have been proposed (eg, sucrosomial iron, heme iron polypeptide, iron containing nanoparticles), but studies are limited. 79 Sucrosomial iron has been tested in patients with CKD, 80 but the mechanism of absorption and the real benefits are uncertain. In the same condition, the phosphate binder iron ferric citrate simultaneously corrects both hyperphosphatemia and iron deficiency; its double effect is being tested in a clinical trial in CKD. 81 A phase 3 trial of ferric maltol provided positive results on iron deficiency anemia in inflammatory bowel diseases. 82 Rigorously designed clinical trials are needed to confirm the efficacy of these iron preparations.
The natural compound extracted from the bark of the Taiwanese tree hinokitiol restores iron transport in cells lacking transporters, such as DMT1 or FPN. 83 Exploiting the iron gradient that, in the absence of the transporter, is formed across membranes, hinokitiol restores transport direction both in vitro and in zebrafish, but no data are available on its chronic use in mice.
The alternative for patients intolerant or unresponsive to oral compounds is IV iron. 47 Once limited by the risk of severe hypersensitivity reactions, this route of administration is currently more widely used as a result of the improved safety profile of last-generation compounds. Established indications to IV iron are reduced absorption capacity in the presence of gastrointestinal disorders or bariatric surgery, severe anemia (Hb <7-8 g/dL), high hepcidin resulting from concomitant inflammation, and rarely IRIDA and when a fast recovery is desirable ( Table 2 ). Advantages are the more rapid effect and the negligible gastrointestinal toxicity. 67 IV iron is more effective than oral iron in CKD patients treated with erythropoiesis-stimulating agents 41 , 84 and avoids oxidative damage to the intestinal mucosa in active inflammatory bowel diseases. 85 In the latter disorders, IV iron preserves the normal microbiome, which would be disrupted by oral iron. 40 The European Crohn’s and Colitis Organization recommends IV iron as a first-line therapy for patients with active disease and Hb <10 g/dL and oral iron in inactive disease/mild anemia patients, 86 the latter being more likely to have absolute iron deficiency.
Indication for IV iron therapy
IBD, inflammatory bowel disease.
IV iron is available in different forms; iron gluconate and iron sucrose require repeated infusions, whereas ferric carboxymaltose, ferumoxytol, low molecular weight iron dextran, and iron isomaltoside may be administered in high doses to rapidly replace the total iron deficit (usually 1-1.5 g) in 1 or 2 infusions. 56 The stable carbohydrate shell of the latter compounds prevents free iron release, a feature that increases their safety. 56 The high-dose schedule avoids repeated hospital visits (eg, for patients with reduced mobility, such as the elderly, for whom oral therapy can be particularly disturbing) 38 and is convenient when a fast recovery is needed (such as in the second and third trimesters of pregnancy or in postpartum anemia 87 and the prevention of repeated cycles of therapy [eg, in heavy uterine bleeding]). 88 In addition to the prompt Hb increase, this protocol rapidly reconstitutes stores, 89 making the advantages (single access, accelerated recovery, limited need for blood tests) outweigh the disadvantages (cost, invasiveness, risk of reactions). However, this decision should be carefully made on an individual basis.
High-dose IV iron may increase Hb or iron stores before surgery predicted to induce heavy bleeding. This is a kind of prevention of acute postoperative anemia and an alternative to blood transfusions, which are associated with several postoperative complications, including infections. Patient blood management programs that limit blood transfusions by perioperative iron use reduce morbidity and negative prognoses in high-risk interventions. 90 A randomized trial of IV iron administration at postoperative day 1 vs standard care showed less anemia and reduction of transfusions and infections in the iron arm in major orthopedic and abdominal surgeries. 91 The iron infusion approach might be especially valuable in surgery candidates prone to iron deficiency, such as young women or patients with colorectal cancer. 92
An important issue concerning IV iron is safety. Because iron is a growth factor for several pathogens, iron therapy is contraindicated in infections. The risk of infection after IV iron is still a matter of controversy. Increased risk was found in a meta-analysis evaluating trials of IV iron to spare transfusions, 93 and caution was suggested in dialysis patients. 94 Another meta-analysis of >10 000 patients receiving different IV compounds or oral iron or placebo did not find different risks of infection. 95 Long-term studies are needed in patients with different disorders.
Hypophosphatemia after ferricarboxymaltose is usually transient and reversible, although rarely, severe cases have been reported after repeated infusions. 96 Minor/moderate infusion reactions (nausea, pruritus, urticaria, flushing, back or thoracic pain), often self-limited, may be observed in 1:200 infusions and more serious reactions (hypotension, dyspnea) in 1:200 000. 95 Although globally IV treatment seems safe, the number of reported patients is usually too limited to detect extremely rare anaphylactic reactions, such as those once caused by high molecular weight iron dextran. Their unclear pathogenesis is ascribed to the release of iron particles in the circulation and has been interpreted as a “complement activation-related pseudo-allergy.” 97 (p5029) Personnel who administer IV iron must be prepared to manage any type of reaction, including exceptionally severe ones. 98
The superior efficacy of IV vs oral iron is undisputable and expected; the long-term adverse effects of ROS generation in cases of therapy-induced positive iron balance have been scarcely explored, although overtreatment might occur in functional rather than in absolute iron deficiency. A recent analysis in CKD concluded that patients seemed to tolerate positive iron balance, because iron that was not used was safely stored in reticule-endothelial cells. 99 However, in the absence of data and iron toxicity tests, it is advisable to regularly assess iron status when high doses are repeatedly administered.
Although advances in understanding iron metabolism and regulation are systematically providing novel insights, additional studies are needed before iron therapy becomes a personalized approach in all cases. These studies should aim at discovering markers of tissue iron deficiency, investigate novel schedules of iron administration based on iron physiology, provide clearer indications to high-dose IV iron, and contribute long-term evaluations of treatment outcomes.
The author thanks Domenico Girelli for his valuable advice and criticism and Alessia Pagani for help with the figure.
Contribution: C.C. conceived, wrote, and reviewed the paper.
Conflict-of-interest disclosure: C.C. is an advisor for Vifor Iron Core and has received honoraria from Vifor Pharma.
Correspondence: Clara Camaschella, Division of Genetics and Cell Biology, San Raffaele Scientific Institute, Via Olgettina, 58, 20132 Milan, Italy; e-mail: [email protected] .
This feature is available to Subscribers Only
- Previous Article
- Next Article
Email alerts
Affiliations.
- Current Issue
- First edition
- Collections
- Submit to Blood
- About Blood
- Subscriptions
- Public Access
- Permissions
- Blood Classifieds
- Advertising in Blood
- Terms and Conditions
American Society of Hematology
- 2021 L Street NW, Suite 900
- Washington, DC 20036
- TEL +1 202-776-0544
- FAX +1 202-776-0545
ASH Publications
- Blood Advances
- Blood Neoplasia
- Blood Vessels, Thrombosis & Hemostasis
- Hematology, ASH Education Program
- ASH Clinical News
- The Hematologist
- Publications
- Privacy Policy
- Cookie Policy
- Terms of Use
This Feature Is Available To Subscribers Only
Sign In or Create an Account

MATTHEW W. SHORT, LTC, MC, USA, AND JASON E. DOMAGALSKI, MAJ, MC, USA
Am Fam Physician. 2013;87(2):98-104
Patient information : See related handout on iron deficiency anemia , written by the authors of this article.
Author disclosure: No relevant financial affiliations to disclose.
Iron deficiency is the most common nutritional disorder worldwide and accounts for approximately one-half of anemia cases. The diagnosis of iron deficiency anemia is confirmed by the findings of low iron stores and a hemoglobin level two standard deviations below normal. Women should be screened during pregnancy, and children screened at one year of age. Supplemental iron may be given initially, followed by further workup if the patient is not responsive to therapy. Men and postmenopausal women should not be screened, but should be evaluated with gastrointestinal endoscopy if diagnosed with iron deficiency anemia. The underlying cause should be treated, and oral iron therapy can be initiated to replenish iron stores. Parenteral therapy may be used in patients who cannot tolerate or absorb oral preparations.
Iron deficiency anemia is diminished red blood cell production due to low iron stores in the body. It is the most common nutritional disorder worldwide and accounts for approximately one-half of anemia cases. 1 , 2 Iron deficiency anemia can result from inadequate iron intake, decreased iron absorption, increased iron demand, and increased iron loss. 3 Identifying the underlying etiology and administering the appropriate therapy are keys to the evaluation and management of this condition.
Diagnosis of iron deficiency anemia requires laboratory-confirmed evidence of anemia, as well as evidence of low iron stores. 4 Anemia is defined as a hemoglobin level two standard deviations below normal for age and sex ( Table 1 ) . 5
A complete blood count can be helpful to determine the mean corpuscular volume or red blood cell size. Although iron deficiency is the most common cause of microcytic anemia, up to 40 percent of patients with iron deficiency anemia will have normocytic erythrocytes. 2 As such, iron deficiency should still be considered in all cases of anemia unless the mean corpuscular volume is greater than 95 μm 3 (95 fL), because this cutoff has a sensitivity of 97.6 percent. 6 Other causes of microcytosis include chronic inflammatory states, lead poisoning, thalassemia, and sideroblastic anemia. 1
The following diagnostic approach is recommended in patients with anemia and is outlined in Figure 1 . 2 , 6 – 11 A serum ferritin level should be obtained in patients with anemia and a mean corpuscular volume less than 95 μm 3 . Ferritin reflects iron stores and is the most accurate test to diagnose iron deficiency anemia. 7 Although levels below 15 ng per mL (33.70 pmol per L) are consistent with a diagnosis of iron deficiency anemia, using a cutoff of 30 ng per mL (67.41 pmol per L) improves sensitivity from 25 to 92 percent, and specificity remains high at 98 percent. 8 , 12 Ferritin is also an acute phase reactant and can be elevated in patients with chronic inflammation or infection. In patients with chronic inflammation, iron deficiency anemia is likely when the ferritin level is less than 50 ng per mL (112.35 pmol per L). 7 Ferritin values greater than or equal to 100 ng per mL (224.70 pmol per L) generally exclude iron deficiency anemia. 9 , 10
In patients with no inflammatory states and in whom the ferritin level is indeterminate (31 to 99 ng per mL [69.66 to 222.45 pmol per L]), further tests can be performed to ascertain iron status. Values consistent with iron deficiency include a low serum iron level, low transferrin saturation, and a high total iron-binding capacity. 2
Soluble transferrin receptor and erythrocyte protoporphyrin testing, or bone marrow biopsy can be considered if the diagnosis remains unclear. 2 The soluble transferrin receptor level is an indirect measure of erythropoiesis and is increased in patients with iron deficiency anemia. 8 Another benefit of this test is that the soluble transferrin receptor level is unaffected by inflammatory states and can help identify concomitant iron deficiency anemia in patients with anemia of chronic disease. 12 Erythrocyte protoporphyrin is a heme precursor and accumulates in the absence of adequate iron stores. 11 If other tests are indeterminate and suspicion for iron deficiency anemia persists, the absence of stainable iron in a bone marrow biopsy is considered the diagnostic standard. 2
MEN AND POSTMENOPAUSAL WOMEN
Asymptomatic men and postmenopausal women should not be screened for iron deficiency anemia. Testing should be performed in patients with signs and symptoms of anemia, and a complete evaluation should be performed if iron deficiency is confirmed. 13
PREGNANT WOMEN
The American Academy of Family Physicians, U.S. Preventive Services Task Force, and Centers for Disease Control and Prevention recommend routine screening of asymptomatic pregnant women for iron deficiency anemia. 4 , 11 , 14 The American College of Obstetricians and Gynecologists recommends screening for anemia and implementing iron therapy if iron deficiency anemia is confirmed. 15 The defined values consistent with anemia in pregnancy are hemoglobin levels less than 11 g per dL (110 g per L) in the first or third trimester, or less than 10.5 g per dL (105 g per L) in the second trimester. 16 A maternal hemoglobin level of less than 6 g per dL (60 g per L) has been associated with poor fetal outcomes, including death. 15
The American Academy of Pediatrics recommends universal hemoglobin screening and evaluation of risk factors for iron deficiency anemia in all children at one year of age. 16 Risk factors include low birth weight, history of prematurity, exposure to lead, exclusive breastfeeding beyond four months of life, and weaning to whole milk and complementary foods without iron-fortified foods. 16 The Centers for Disease Control and Prevention recommends screening children from low-income or newly immigrated families at nine to 12 months of age, and consideration of screening for preterm and low-birth-weight infants before six months of age if they are not given iron-fortified formula. 14 The U.S. Preventive Services Task Force found insufficient evidence for screening in asymptomatic children six to 12 months of age and does not make recommendations for other ages. 4 A meta-analysis showed that infants in whom cord clamping was delayed for up to two minutes after birth had a reduced risk of low iron stores for up to six months. 17 Larger randomized studies that include maternal outcomes are needed before delayed cord clamping can be recommended for general practice.
Once iron deficiency anemia is identified, the goal is to determine the underlying etiology. Causes include inadequate iron intake, decreased iron absorption, increased iron demand, and increased iron loss ( Table 2 ) . 5 , 7 , 18 , 19
Iron Therapy
Premenopausal women with a negative evaluation for abnormal uterine bleeding can be given a trial of iron therapy. In children and pregnant women, iron therapy should be tried initially. Current guidelines recommend empiric treatment in children up to two years of age and in pregnant women with iron deficiency anemia; however, if the hemoglobin level does not increase by 1 g per dL (10 g per L) after one month of therapy in children or does not improve in pregnant women, further evaluation may be indicated. 4 , 15 , 16 In pregnant patients, poor compliance or intolerance should be considered, and parenteral iron may produce a better response. 15
The evaluation should begin with a thorough history and physical examination to help identify the cause of iron deficiency. The history should focus on potential etiologies and may include questions about diet, gastrointestinal (GI) symptoms, history of pica or pagophagia (i.e., compulsive consumption of ice), signs of blood loss (e.g., epistaxis, menorrhagia, melena, hematuria, hematemesis), surgical history (e.g., gastric bypass), and family history of GI malignancy. Patients with iron deficiency anemia are often asymptomatic and have limited findings on examination. Further evaluation should be based on risk factors ( Figure 2 ) . 10 , 15 , 17 – 21
PREMENOPAUSAL WOMEN
Excessive menstruation is a common cause of iron deficiency anemia in premenopausal women in developed countries; however, a GI source (particularly erosive lesions in the stomach or esophagus) is present in 6 to 30 percent of cases. 20 , 22 , 23 If the gynecologic workup is negative and the patient does not respond to iron therapy, endoscopy should be performed to exclude an occult GI source. 20 , 22 , 23
Excessive or irregular menstrual bleeding affects 9 to 14 percent of all women and can lead to varying degrees of iron deficiency anemia. 24 Etiologies include thyroid disease, uncontrolled diabetes mellitus, polycystic ovary syndrome, coagulopathies, uterine fibroids, endometrial hyperplasia, hyperprolactinemia, and use of antipsychotics or antiepileptics. Initial evaluation includes a history, physical examination, and pregnancy and thyroid-stimulating hormone tests. An endometrial biopsy should be considered in women 35 years and younger who have conditions that could lead to unopposed estrogen exposure, in women older than 35 years who have suspected anovulatory bleeding, and in women with abnormal uterine bleeding that does not respond to medical therapy. 25
In men and postmenopausal women, GI sources of bleeding should be excluded. Current recommendations support upper and lower endoscopy; however, there are no clear guidelines about which procedure should be performed first or if the second procedure is necessary if a source is found on the first study. 18 Lesions that occur simultaneously in the upper and lower tracts are rare, occurring in only 1 to 9 percent of patients. 18 However, one study showed that 12.2 percent of patients diagnosed with celiac disease and iron deficiency anemia had a secondary source of anemia, including three cases of colon cancer. 26 A study of patients with iron deficiency anemia of unknown etiology in the primary care setting found that 11 percent had newly diagnosed GI cancer. 27 Additionally, a cohort study found that 6 percent of patients older than 50 years and 9 percent of those older than 65 years will be diagnosed with a GI malignancy within two years of a diagnosis of iron deficiency anemia. 28 Celiac serology should also be considered for all adults presenting with iron deficiency anemia. 18 Upper endoscopy with duodenal biopsies should be performed to confirm the diagnosis after positive serologic testing and to evaluate for additional etiologies. 29
In patients in whom endoscopy may be contraindicated because of procedural risk, radiographic imaging may offer sufficient screening. The sensitivity of computed tomographic colonography for lesions larger than 1 cm is greater than 90 percent. 7 The use of barium enema is less reliable, but may be of use if colonoscopy or computed tomographic colonography is not available.
If initial endoscopy findings are negative and patients with iron deficiency anemia do not respond to iron therapy, repeat upper and lower endoscopy may be justified. In some instances, lesions may not be detected on initial examination (e.g., missed mucosal erosions in a large hiatal hernia, suboptimal preparation for colonoscopy, inadequate biopsy of a suspected lesion). 13 Colonoscopy can fail to diagnose up to 5 percent of colorectal tumors. 13
Additional evaluation of the small intestine is not necessary unless there is inadequate response to iron therapy, the patient is transfusion dependent, or fecal occult blood testing suggests that the patient has had obscure GI bleeding with the source undiscovered on initial or repeat endoscopy. 30 In these cases, further evaluation with capsule endoscopy should be considered. 30 Enteroscopy is an upper endoscopy procedure using a longer scope to visualize the proximal jejunum; it should be reserved to treat or biopsy lesions identified by capsule endoscopy. This test is a second-line technique for evaluating the small bowel because it is complicated by the level of sedation and duration of procedure. 13 Magnetic resonance imaging enteroclysis, computed tomographic enterography, or barium studies may also be considered, but have a limited ability to identify most small bowel lesions, which are mucosal and flat. 7
UNDERLYING CAUSE
Patients with an underlying condition that causes iron deficiency anemia should be treated or referred to a subspecialist (e.g., gynecologist, gastroenterologist) for definitive treatment.
ORAL IRON THERAPY
The dosage of elemental iron required to treat iron deficiency anemia in adults is 120 mg per day for three months; the dosage for children is 3 mg per kg per day, up to 60 mg per day. 1 An increase in hemoglobin of 1 g per dL after one month of treatment shows an adequate response to treatment and confirms the diagnosis. 16 In adults, therapy should be continued for three months after the anemia is corrected to allow iron stores to become replenished 7 ( Figure 3 6 , 28 , 31 ) .
Adherence to oral iron therapy can be a barrier to treatment because of GI adverse effects such as epigastric discomfort, nausea, diarrhea, and constipation. These effects may be reduced when iron is taken with meals, but absorption may decrease by 40 percent. 1 Medications such as proton pump inhibitors and factors that induce gastric acid hyposecretion (e.g., chronic atrophic gastritis, recent gastrectomy or vagotomy) are associated with reduced absorption of dietary iron and iron tablets. 31
PARENTERAL IRON THERAPY
Parenteral therapy may be used in patients who cannot tolerate or absorb oral preparations, such as those who have undergone gastrectomy, gastrojejunostomy, bariatric surgery, or other small bowel surgeries. The most common indications for intravenous therapy include GI effects, worsening symptoms of inflammatory bowel disease, unresolved bleeding, renal failure–induced anemia treated with erythropoietin, and insufficient absorption in patients with celiac disease. 32
Parenteral treatment options are outlined in Table 3 . 2 , 16 Serious adverse effects have occurred in up to 0.7 percent of patients receiving iron dextran, with 31 recorded fatalities reported between 1976 and 1996. 32 , 33 Iron sucrose and sodium ferric gluconate (Ferrlecit) have greater bio-availability and a lower incidence of life-threatening anaphylaxis compared with iron dextran. 2 Approximately 35 percent of patients receiving iron sucrose have mild adverse effects (e.g., headache, nausea, diarrhea). 7 One small study cited similar adverse effect profiles between intravenous iron dextran and sodium ferric gluconate, with only one serious adverse effect reported in the iron dextran group. 34 If this finding is duplicated in larger studies, it could support the use of iron dextran over sodium ferric gluconate, because the total dose can be given in one sitting. A newer formulation, ferumoxytol, can be given over five minutes and supplies 510 mg of elemental iron per infusion, allowing for greater amounts of iron in fewer infusions compared with iron sucrose. 2
There are no standard recommendations for follow-up after initiating therapy for iron deficiency anemia; however, one suggested course is to recheck complete blood counts every three months for one year. If hemoglobin and red blood cell indices remain normal, one additional complete blood count should be obtained 12 months later. A more practical approach is to recheck the patient periodically; no further follow-up is necessary if the patient is asymptomatic and the hematocrit level remains normal. 7
BLOOD TRANSFUSION
There is no universally accepted threshold for transfusing packed red blood cells in patients with iron deficiency anemia. Guidelines often specify certain hemoglobin values as indications to transfuse, but the patient's clinical condition and symptoms are an essential part of deciding whether to transfuse. 35 Transfusion is recommended in pregnant women with hemoglobin levels of less than 6 g per dL because of potentially abnormal fetal oxygenation resulting in non-reassuring fetal heart tracings, low amniotic fluid volumes, fetal cerebral vasodilation, and fetal death. 15 If transfusion is performed, two units of packed red blood cells should be given, then the clinical situation should be reassessed to guide further treatment. 35
Data Sources: A PubMed search was completed in Clinical Queries using the key terms iron deficiency and anemia. The search included meta-analyses, randomized controlled trials, controlled trials, and reviews. Searches were also performed using Essential Evidence Plus, the Cochrane database, the National Guideline Clearinghouse database, the Trip Database, DynaMed, and the Agency for Healthcare Research and Quality evidence reports. Search date: January 10, 2012.
World Health Organization. Iron Deficiency Anaemia: Assessment, Prevention, and Control: A Guide for Programme Managers . Geneva, Switzerland: World Health Organization; 2001.
Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Therap Adv Gastroenterol. 2011;4(3):177-184.
WHO Global Database on Anaemia. Worldwide Prevalence of Anaemia 1993–2005 . Geneva, Switzerland: World Health Organization; 2008.
U. S. Preventive Services Task Force. Screening for iron deficiency anemia, including iron supplementations for children and pregnant women: recommendation statement. Am Fam Physician. 2006;74(3):461-464.
Van Vranken M. Evaluation of microcytosis. Am Fam Physician. 2010;82(9):1117-1122.
Ioannou GN, Spector J, Scott K, Rockey DC. Prospective evaluation of a clinical guideline for the diagnosis and management of iron deficiency anemia. Am J Med. 2002;113(4):281-287.
Goddard AF, James MW, McIntyre AS, Scott BB British Society of Gastroenterology. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60(10):1309-1316.
Mast AE, Blinder MA, Gronowski AM, Chumley C, Scott MG. Clinical utility of the soluble transferrin receptor and comparison with serum ferritin in several populations. Clin Chem. 1998;44(1):45-51.
Knovich MA, Storey JA, Coffman LG, Torti SV, Torti FM. Ferritin for the clinician. Blood Rev. 2009;23(3):95-104.
Galloway MJ, Smellie WS. Investigating iron status in microcytic anaemia. BMJ. 2006;333(7572):791-793.
Assessing the iron status of populations: report of a joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level, Geneva, Switzerland, 6–8 April 2004. Geneva: World Health Organization, Centers for Disease Control and Prevention; 2005.
Skikne BS, Punnonen K, Caldron PH, et al. Improved differential diagnosis of anemia of chronic disease and iron deficiency anemia: a prospective multicenter evaluation of soluble transferrin receptor and the sTfR/log ferritin index. Am J Hematol. 2011;86(11):923-927.
Bermejo F, García-López S. A guide to diagnosis of iron deficiency and iron deficiency anemia in digestive diseases. World J Gastroenterol. 2009;15(37):4638-4643.
Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47(RR-3):1-29.
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 95: anemia in pregnancy. Obstet Gynecol. 2008;112(1):201-207.
Baker RD, Greer FR Committee on Nutrition, American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age). Pediatrics. 2010;126(5):1040-1050.
Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full-term neonates: systematic review and meta-analysis of controlled trials. JAMA. 2007;297(11):1241-1252.
Liu K, Kaffes AJ. Iron deficiency anaemia: a review of diagnosis, investigation and management. Eur J Gastroenterol Hepatol. 2012;24(2):109-116.
British Columbia Ministry of Health. Iron deficiency—investigation and management. http://www.bcguidelines.ca/guideline_iron_deficiency.html . Accessed November 13, 2012.
Carter D, Maor Y, Bar-Meir S, Avidan B. Prevalence and predictive signs for gastrointestinal lesions in premenopausal women with iron deficiency anemia. Dig Dis Sci. 2008;53(12):3138-3144.
American College of Obstetricians and Gynecologists Committee on Adolescent Health Care; American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114(6):1439-1443.
Green BT, Rockey DC. Gastrointestinal endoscopic evaluation of pre-menopausal women with iron deficiency anemia. J Clin Gastroenterol. 2004;38(2):104-109.
Park DI, Ryu SH, Oh SJ, et al. Significance of endoscopy in asymptomatic premenopausal women with iron deficiency anemia. Dig Dis Sci. 2006;51(12):2372-2376.
Fraser IS, Langham S, Uhl-Hochgraeber K. Health-related quality of life and economic burden of abnormal uterine bleeding. Expert Rev Obstet Gynecol. 2009;4(2):179-189.
ACOG Committee on Practice Bulletins—Gynecology, American College of Obstetricians and Gynecologists. ACOG practice bulletin: management of anovulatory bleeding. Int J Gynaecol Obstet. 2001;72(3):263-271.
Hopper AD, Leeds JS, Hurlstone DP, Hadjivassiliou M, Drew K, Sanders DS. Are lower gastrointestinal investigations necessary in patients with coeliac disease?. Eur J Gastroenterol Hepatol. 2005;17(6):617-621.
Yates JM, Logan EC, Stewart RM. Iron deficiency anaemia in general practice: clinical outcomes over three years and factors influencing diagnostic investigations. Postgrad Med J. 2004;80(945):405-410.
Ioannou GN, Rockey DC, Bryson CL, Weiss NS. Iron deficiency and gastrointestinal malignancy: a population-based cohort study. Am J Med. 2002;113(4):276-280.
Lewis NR, Scott BB. Systematic review: the use of serology to exclude or diagnose coeliac disease (a comparison of the endomysial and tissue transglutaminase antibody tests). Aliment Pharmacol Ther. 2006;24(1):47-54.
Sidhu R, Sanders DS, Morris AJ, McAlindon ME. Guidelines on small bowel enteroscopy and capsule endoscopy in adults. Gut. 2008;57(1):125-136.
Ajmera AV, Shastri GS, Gajera MJ, Judge TA. Suboptimal response to ferrous sulfate in iron-deficient patients taking omeprazole. Am J Ther. 2012;19(3):185-189.
Maslovsky I. Intravenous iron in a primary-care clinic. Am J Hematol. 2005;78(4):261-264.
Silverstein SB, Rodgers GM. Parenteral iron therapy options. Am J Hematol. 2004;76(1):74-78.
Eichbaum Q, Foran S, Dzik S. Is iron gluconate really safer than iron dextran?. Blood. 2003;101(9):3756-3757.
Murphy MF, Wallington TB, Kelsey P, et al.; British Committee for Standards in Haematology, Blood Transfusion Task Force. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113(1):24-31.
Continue Reading
More in AFP
More in pubmed.
Copyright © 2013 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Learn how UpToDate can help you.
Select the option that best describes you
- Medical Professional
- Resident, Fellow, or Student
- Hospital or Institution
- Group Practice
- Patient or Caregiver
- Find in topic
CALCULATORS
Related topics.
INTRODUCTION
The evaluation and management of iron deficiency in other populations is presented in separate topic reviews:
● Children – (See "Iron deficiency in infants and children <12 years: Screening, prevention, clinical manifestations, and diagnosis" and "Iron deficiency in infants and children <12 years: Treatment" .)
● Adolescents – (See "Iron requirements and iron deficiency in adolescents" .)
● Pregnancy – (See "Anemia in pregnancy" and "Nutrition in pregnancy: Dietary requirements and supplements", section on 'Iron' .)
Iron Deficiency Anemia
(anemia of chronic blood loss; chlorosis).
- Pathophysiology |
- Symptoms and Signs |
- Diagnosis |
- Treatment |
- Key Points |
Iron deficiency is the most common cause of anemia and usually results from blood loss; malabsorption, such as occurs in celiac disease, is a much less common cause. Symptoms are usually nonspecific. Red blood cells tend to be microcytic and hypochromic, and iron stores are low, as shown by low serum ferritin and low serum iron levels with high serum total iron-binding capacity. Once the diagnosis is made, occult blood loss should be suspected until proven otherwise. Treatment involves iron replacement and treatment of the cause of blood loss.
(See also Overview of Decreased Erythropoiesis .)
Pathophysiology of Iron Deficiency Anemia
Iron is distributed in active metabolic and storage pools. Total body iron is about 3.5 g in healthy men and 2.5 g in women; the difference relates to women's smaller body size and dearth of stored iron because of iron loss due to menses. The distribution of body iron is
Hemoglobin: 2 g (men), 1.5 g (women)
Ferritin: 1 g (men), 0.6 g (women)
Hemosiderin: 300 mg
Myoglobin: 200 mg
Tissue enzymes (heme and nonheme): 150 mg
Transport-iron compartment: 3 mg
Iron absorption
Ascorbic acid is the only common food element known to increase nonheme iron absorption.
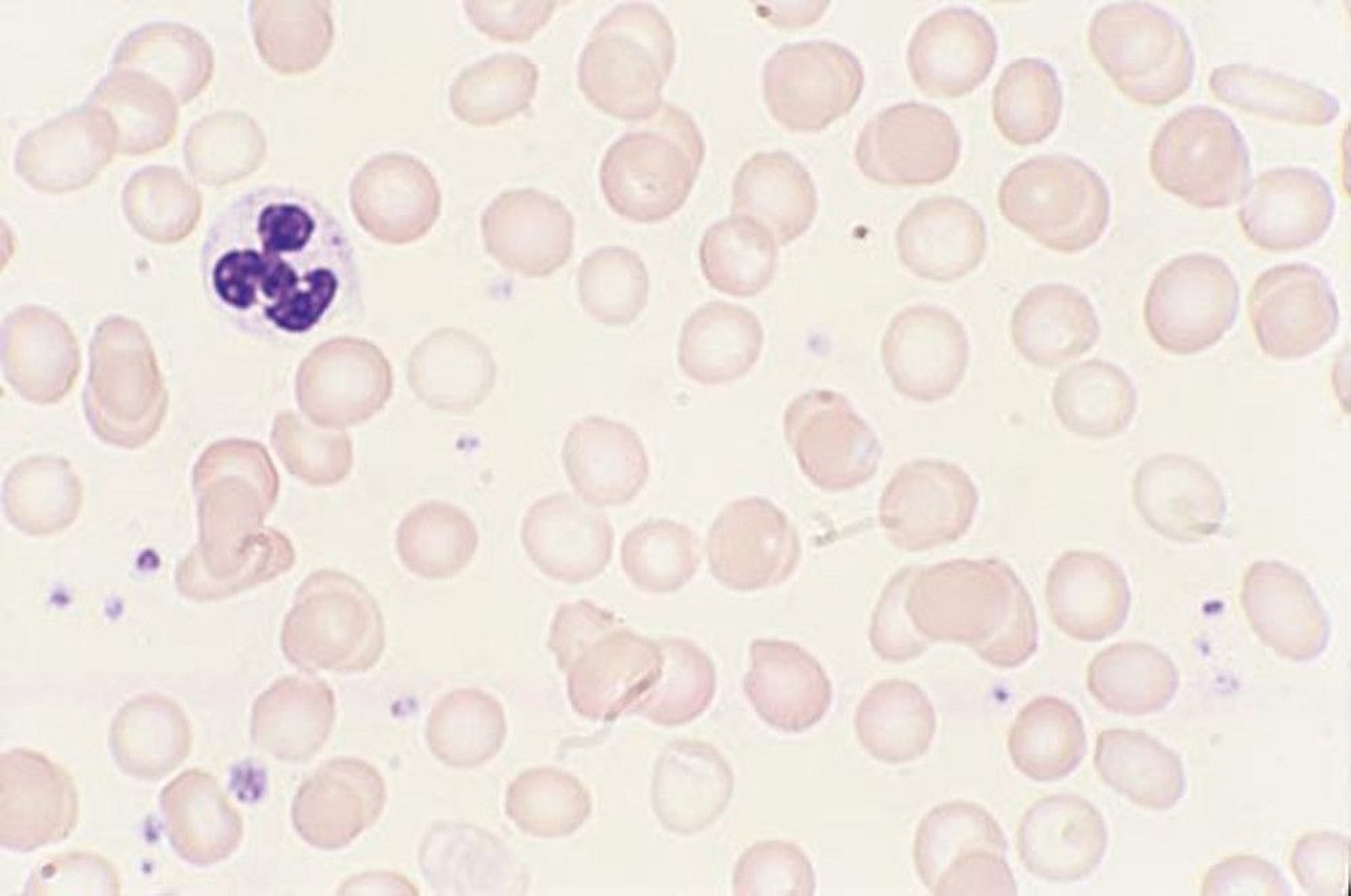
By permission of the publisher. From Tefferi A, Li C. In Atlas of Clinical Hematology . Edited by JO Armitage. Philadelphia, Current Medicine, 2004.
The average American diet, which contains 6 mg of elemental iron/1000 kcal of food, is adequate for iron homeostasis. Of about 15 mg/day of dietary iron, adults absorb only 1 mg, which is the approximate amount lost daily by cell desquamation from the skin and intestine. In iron depletion, absorption increases due to the suppression of hepcidin, a key regulator of iron metabolism; however, absorption rarely increases to > 6 mg/day unless supplemental iron is added ( 1 ). Children have a greater need for iron and appear to absorb more to meet this need.
Iron transport and usage
Iron from intestinal mucosal cells is transferred to transferrin, an iron-transport protein synthesized in the liver; transferrin can transport iron from cells (intestinal, macrophages) to specific receptors on erythroblasts, placental cells, and liver cells. For heme synthesis, transferrin transports iron to the erythroblast mitochondria, which insert the iron into protoporphyrin IX for it to become heme. Transferrin (plasma half-life, 8 days) is extruded for reutilization. Synthesis of transferrin increases with iron deficiency but decreases with any type of chronic disease.
Iron storage and recycling
Iron not used for erythropoiesis is transferred by transferrin to the storage pool; iron is stored in 2 forms:
Hemosiderin
The most important storage form is ferritin (a heterogeneous group of proteins surrounding an iron core), which is a soluble and active storage fraction located in the liver (in hepatocytes), bone marrow, and spleen (in macrophages); in red blood cells (RBCs); and in serum. Iron stored in ferritin is readily available for any body requirement. Circulating (serum) ferritin level parallels the size of the body stores (1 ng/mL = 8 mg of iron in the storage pool).
The second storage pool of iron is in hemosiderin, which is relatively insoluble and is stored primarily in the liver (in Kupffer cells) and in the bone marrow (in macrophages).
Because iron absorption is so limited, the body recycles and conserves iron. Transferrin binds and recycles available iron from aging RBCs undergoing phagocytosis by mononuclear phagocytes. This mechanism provides approximately 90 to 95% of the daily iron needed .
Iron deficiency
Iron deficiency develops in stages. In the first stage, iron requirement exceeds intake, causing progressive depletion of bone marrow iron stores. As stores decrease, absorption of dietary iron increases in compensation. During later stages, deficiency impairs RBC synthesis, ultimately causing anemia.
Severe and prolonged iron deficiency also may cause dysfunction of iron-containing cellular enzymes.
Pathophysiology reference
1. Nemeth E, Tuttle MS, Powelson J, et al: Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306(5704):2090–2093, 2004.
Etiology of Iron Deficiency Anemia
Because nonheme iron is poorly absorbed, dietary iron barely meets the daily requirement for most people. Even so, men who eat a typical Western diet are unlikely to become iron deficient solely as a result of dietary deficiency. However, even modest losses, increased requirements, iatrogenic phlebotomy, or decreased caloric intake can contribute to iron deficiency.
Blood loss is the major cause of iron deficiency. In men and postmenopausal women, the most frequent cause of blood loss is chronic occult bleeding, usually from the gastrointestinal tract (eg, due to peptic ulcer disease , malignancy, hemorrhoids, or vascular ectasias ). Intestinal bleeding due to hookworm infection is a common cause in low-resource countries. In premenopausal women, cumulative menstrual blood loss (mean, 0.5 mg iron/day) is a common cause. Less common causes include urinary blood loss, recurrent pulmonary hemorrhage (see Diffuse Alveolar Hemorrhage ) and chronic intravascular or traumatic (exercise-induced) hemolysis when the amount of iron released during hemolysis exceeds the plasma haptoglobin-binding capacity.
Increased iron requirements may contribute to iron deficiency. From birth to age 2 and during adolescence, when rapid growth requires a large iron intake, dietary iron often is inadequate. During pregnancy, the fetal iron requirement increases the maternal iron requirement (see Anemia in Pregnancy) despite the absence of menses. Lactation also increases the iron requirement.
Decreased iron absorption can result from gastrectomy or malabsorption syndromes such as celiac disease , atrophic gastritis , Helicobacter pylori infection , achlorhydria, short bowel syndrome , and rarely IRIDA (iron-refractory iron deficiency anemia). Rarely, absorption is decreased by dietary deprivation due to undernutrition.
Symptoms and Signs of Iron Deficiency Anemia
Most symptoms of iron deficiency are due to anemia. Such symptoms include fatigue, loss of stamina, shortness of breath, weakness, dizziness, and pallor. Another common symptom is restless leg syndrome (RLS), which is an unpleasant urge to move the legs during periods of inactivity.
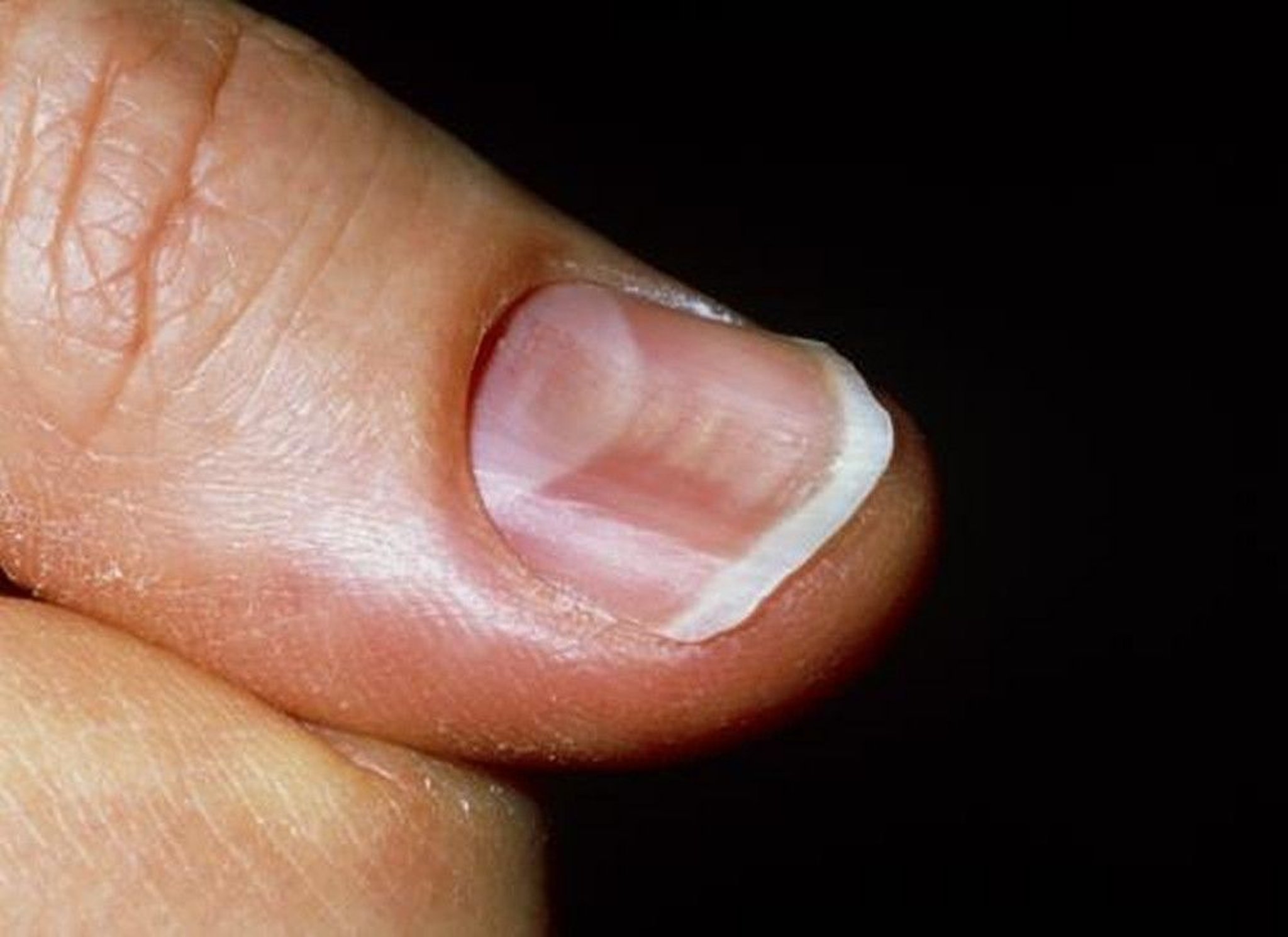
DR P. MARAZZI/SCIENCE PHOTO LIBRARY
In addition to the usual manifestations of anemia, some uncommon symptoms occur in severe iron deficiency. Patients may have pica , an abnormal craving to eat nonfood substances (eg, ice, dirt, paint, starch, ashes). Other symptoms of severe deficiency include glossitis, cheilosis, and concave nails (koilonychia).
Diagnosis of Iron Deficiency Anemia
Complete blood count (CBC), serum iron, iron-binding capacity, serum ferritin, transferrin saturation, reticulocyte count, red cell distribution width (RDW), and a peripheral blood smear
Iron deficiency anemia is suspected in patients with chronic blood loss or microcytic anemia, particularly if pica is present. In such patients, a CBC, serum iron and iron-binding capacity, and serum ferritin and reticulocyte count are obtained (see table Typical Serum Values for Iron, Iron-Binding Capacity, Ferritin, and Transferrin Saturation ).
Iron and iron-binding capacity (and transferrin saturation) are measured because their relationship is important. Various tests exist; the range of normal values relates to the test used and varies from laboratory to laboratory. Serum iron level is low in iron deficiency and in many chronic diseases and is elevated in hemolytic disorders and in iron-overload syndromes . The iron-binding capacity increases in iron deficiency, while the transferrin saturation decreases.
Serum ferritin levels closely correlate with total body iron stores. The range of normal in most laboratories is 30 to 300 ng/mL (67.4 to 674.1 pmol/L), and the mean is 88 ng/mL (197.7 pmol/L) in men and 49 ng/mL (110.1 pmol/L) in women. Low levels ( < 30 ng/mL [67.4 pmol/L]) are specific for iron deficiency. However, ferritin is an acute-phase reactant, and levels increase in inflammatory and infectious disorders (eg, hepatitis ), and neoplastic disorders (especially acute leukemia , Hodgkin lymphoma , and gastrointestinal tract tumors). In these disorders, a serum ferritin level up to 100 ng/mL remains compatible with iron deficiency.
The reticulocyte count is low in iron deficiency. The peripheral smear generally reveals hypochromic red cells with significant anisopoikilocytosis, which is reflected in a high red cell distribution width (RDW).
The most sensitive and specific criterion for iron-deficient erythropoiesis is absent bone marrow stores of iron, although a bone marrow examination is rarely needed.
Stages of iron deficiency
Laboratory test results help stage iron deficiency anemia.
Stage 1 is characterized by decreased bone marrow iron stores; hemoglobin (Hb) and serum iron remain normal, but the serum ferritin level falls to < 30 ng/mL ( < 67.4 pmol/L). The compensatory increase in iron absorption causes an increase in iron-binding capacity (transferrin level).
During stage 2, erythropoiesis is impaired. Although the transferrin level is increased, the serum iron level decreases; transferrin saturation decreases. Erythropoiesis is impaired when serum iron falls to < 50 mcg/dL ( < 9 micromol/L) and transferrin saturation to > 8.5 mg/L).
During stage 3, anemia with normal-appearing RBCs and indices develops.
During stage 4, microcytosis and then hypochromia develop.
During stage 5, iron deficiency affects tissues, resulting in symptoms and signs.
Diagnosis of iron deficiency anemia prompts consideration of its cause, usually bleeding. Patients with obvious blood loss (eg, women with menorrhagia) may require no further testing. Men and postmenopausal women without obvious blood loss should undergo evaluation of the gastrointestinal (GI) tract, because anemia may be the only indication of an occult GI cancer. Rarely, chronic epistaxis or genitourinary bleeding is underestimated by the patient and requires evaluation in patients with normal GI study results.
Differentiation from other microcytic anemias
Iron deficiency anemia must be differentiated from other microcytic anemias (see table Differential Diagnosis of Microcytic Anemia Due to Decreased RBC Production ). If tests exclude iron deficiency in patients with microcytic anemia, then the anemia of chronic disease and structural Hb abnormalities (eg, hemoglobinopathies ) are considered. Clinical features, Hb studies (eg, Hb electrophoresis and Hb A2), and genetic testing (eg, for alpha-thalassemia ) may help distinguish these entities.
Treatment of Iron Deficiency Anemia
Oral supplemental iron
Parenteral iron
Iron therapy without pursuit of the cause is poor practice; a bleeding site should be sought even in cases of mild anemia.
Oral iron 1
Parenteral iron causes a more rapid therapeutic response than oral iron does but can cause adverse effects, most commonly allergic reactions or infusion reactions (eg, fever, arthralgias, myalgias). Severe anaphylactoid reactions
Patients who do not tolerate oral iron
Patients for whom oral iron is ineffective
Patients who steadily lose large amounts of blood because of capillary or vascular disorders (eg, hereditary hemorrhagic telangiectasia )
Patients with a need for expedient iron repletion due to severe anemia, elective surgery, or third trimester of pregnancy
The dose of parenteral iron is calculated based on weight and current hemoglobin level but generally an initial cumulative dose of 1000 mg is sufficient.
Oral iron therapy should continue for ≥ 6 months after correction of hemoglobin levels to replenish tissue stores, and iron studies should be rechecked at least 4 weeks after parenteral iron to ensure adequate repletion. The response to treatment is assessed by serial Hb measurements until normal RBC values are achieved. Hb rises little for 2 weeks but then rises 0.7 to 1 g/week until near normal, at which time the rate of increase tapers. Anemia should be corrected within 2 months. A subnormal response suggests continued hemorrhage, underlying infection or cancer, insufficient iron intake, or malabsorption of oral iron. If the symptoms of anemia, such as fatigue, weakness, and shortness of breath, do not abate following resolution of the anemia, an alternative cause should be sought.

Treatment reference
1. Moretti D, Goede JS, Zeder C, et al : Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood 126(17):1981-1989, 2015. doi: 10.1182/blood-2015-05-642223
Iron deficiency anemia is usually caused by blood loss (eg, gastrointestinal, menstrual) but may be due to hemolysis, malabsorption, or increased demand for iron (eg, in pregnancy, lactation, periods of rapid growth in children).
Differentiate iron deficiency anemia from other microcytic anemias (eg, anemia of chronic disease, hemoglobinopathies).
Measure serum iron, iron-binding capacity, and serum ferritin levels.
Iron deficiency typically causes low serum iron, high iron-binding capacity, and low serum ferritin levels.
Always seek a cause of iron deficiency, even when anemia is mild.
Oral iron supplements are usually adequate; use of parenteral iron is reserved for select patients.

- Cookie Preferences

Copyright © 2024 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Disclaimer » Advertising
- HealthyChildren.org

- Previous Article
- Next Article
INTRODUCTION
Definitions, iron requirements for infants (up to 12 completed months of age), preterm infants, term infants (birth through 12 completed months of age), iron requirements for toddlers (1–3 years of age), prevalence of id and ida, id and neurodevelopment, prevention of id and ida, term, breastfed infants, term, formula-fed infants, toddlers (1–3 years of age), screening for id and ida, development of this report, lead authors, committee on nutrition, 2009–2010, diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age).
This document is copyrighted and is property of the American Academy of Pediatrics and its Board of Directors. All authors have filed conflict of interest statements with the American Academy of Pediatrics. Any conflicts have been resolved through a process approved by the Board of Directors. The American Academy of Pediatrics has neither solicited nor accepted any commercial involvement in the development of the content of this publication.
- Split-Screen
- Article contents
- Figures & tables
- Supplementary Data
- Peer Review
- CME Quiz Close Quiz
- Open the PDF for in another window
- Get Permissions
- Cite Icon Cite
- Search Site
Robert D. Baker , Frank R. Greer , The Committee on Nutrition; Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0–3 Years of Age). Pediatrics November 2010; 126 (5): 1040–1050. 10.1542/peds.2010-2576
Download citation file:
- Ris (Zotero)
- Reference Manager
This clinical report covers diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants (both breastfed and formula fed) and toddlers from birth through 3 years of age. Results of recent basic research support the concerns that iron-deficiency anemia and iron deficiency without anemia during infancy and childhood can have long-lasting detrimental effects on neurodevelopment. Therefore, pediatricians and other health care providers should strive to eliminate iron deficiency and iron-deficiency anemia. Appropriate iron intakes for infants and toddlers as well as methods for screening for iron deficiency and iron-deficiency anemia are presented.
Iron deficiency (ID) and iron-deficiency anemia (IDA) continue to be of worldwide concern. Among children in the developing world, iron is the most common single-nutrient deficiency. 1 In industrialized nations, despite a demonstrable decline in prevalence, 2 IDA remains a common cause of anemia in young children. However, even more important than anemia itself is the indication that the more common ID without anemia may also adversely affect long-term neurodevelopment and behavior and that some of these effects may be irreversible. 3 , 4 Because of the implications for pediatric health care providers and their patients, this report reviews and summarizes this information.
This clinical report is a revision and extension of a previous policy statement published in 1999, 5 which addressed iron fortification of formulas. This report covers diagnosis and prevention of ID and IDA in infants (both breastfed and formula fed) and toddlers aged 1 through 3 years.
Anemia: A hemoglobin (Hb) concentration 2 SDs below the mean Hb concentration for a normal population of the same gender and age range, as defined by the World Health Organization, the United Nations Children's Fund, and United Nations University. 6 On the basis of the 1999–2002 US National Health and Nutrition Examination Survey, anemia is defined as a Hb concentration of less than 11.0 g/dL for both male and female children aged 12 through 35 months. 7 , 8 For certain populations (ie, people living at high altitudes), adjustment of these values may be necessary.
Iron sufficiency: A state in which there is sufficient iron to maintain normal physiologic functions.
Iron deficiency: A state in which there is insufficient iron to maintain normal physiologic functions. ID results from inadequate iron absorption to accommodate an increase in requirements attributable to growth or resulting from a long-term negative iron balance. Either of these situations leads to a decrease in iron stores as measured by serum ferritin (SF) concentrations or bone marrow iron content. ID may or may not be accompanied by IDA.
Iron-deficiency anemia: An anemia (as defined above) that results from ID.
Iron overload: The accumulation of excess iron in body tissues. Iron overload usually occurs as a result of a genetic predisposition to absorb and store iron in excess amounts, the most common form of which is hereditary hemochromatosis. Iron overload can also occur as a complication of other hematologic disorders that result in chronic transfusion therapy, repeated injections of parenteral iron, or excessive iron ingestion.
Recommended dietary allowance for iron: The average daily dietary intake that is sufficient to meet the nutrient requirements of nearly all individuals (97%–98%) of a given age and gender.
Adequate intake for iron: This term is used when there is not enough information to establish a recommended dietary allowance for a population (eg, term infants, 0–6 months of age). The adequate intake is based on the estimated average nutrient intake by a group (or groups) of healthy individuals.
Eighty percent of the iron present in a newborn term infant is accreted during the third trimester of pregnancy. Infants born prematurely miss this rapid accretion and are deficient in total body iron. A number of maternal conditions, such as anemia, maternal hypertension with intrauterine growth restriction, or diabetes during pregnancy, can also result in low fetal iron stores in both term and preterm infants.
The deficit of total body iron in preterm infants increases with decreasing gestational age. It is worsened by the rapid postnatal growth that many infants experience and by frequent phlebotomies without adequate blood replacement. On the other hand, sick preterm infants who receive multiple transfusions are at risk of iron overload. The use of recombinant human erythropoietin to prevent transfusion therapy in preterm infants will further deplete iron stores if additional supplemental iron is not provided. The highly variable iron status of preterm infants, along with their risks for ID as well as toxicity, precludes determining the exact requirement, but it can be estimated to be between 2 and 4 mg/kg per day when given orally. 9
The Institute of Medicine (IOM) 10 used the average iron content of human milk to determine the adequate intake of 0.27 mg/day for term infants from birth through 6 months' completed age. The average iron content of human milk was determined to be 0.35 mg/L, and the average milk intake of an exclusively breastfed infant was determined to be 0.78 L/day. Multiplying these 2 numbers determined the adequate intake of 0.27 mg/day for term infants from birth through 6 months of age in the IOM report. The IOM further reasoned that there should be a direct correlation between infant size and human milk ingestion; therefore, no correction need be made for infant weight. It should be pointed out, however, that although bigger infants may ingest more milk, there is a large variation in iron concentration of human milk, and there is no guarantee that the iron content of the maternal milk matches the needs of the infant for iron.
For infants from 7 to 12 months' completed age, the recommended dietary allowance for iron, according to the IOM, is 11 mg/day, which was determined by using a factorial approach. The amount of iron lost, primarily from sloughed epithelial cells from skin and the intestinal and urinary tracts, was added to the amounts of iron required for increased blood volume, increased tissue mass, and storage iron during this period of life. It was noted that the iron needs of infants do not suddenly jump from 0.27 to 11 mg/day at 6 months of age; this disjuncture is the result of the use of very different methods of determining these values. However, it is clear that healthy, term newborn infants require very little iron early in life compared with the significant amounts of iron required after 6 months of age.
Using a similar factorial approach as described for infants 7 to 12 months' completed age, the IOM determined that the recommended dietary allowance for iron for children from 1 through 3 years of age is 7 mg/day. 9
There are currently no national statistics for the prevalence of ID and IDA in infants before 12 months' completed age. Hay et al 11 reported on a cohort of 284 term Norwegian infants. Using the definitions provided by Dallman 12 in an IOM report, the prevalence of ID at 6 months of age was 4% and increased to 12% at 12 months of age.
The prevalence of ID and IDA among toddlers (1–3 years of age) in the United States is listed in Table 1 and was derived from National Health and Nutrition Examination Survey data collected between 1999 and 2002. 7 , 8 The overall prevalence of anemia and possibly ID and IDA in infants and toddlers has declined since the 1970s. 2 Although there is no direct proof, this decline has been attributed to use of iron-fortified formulas and iron-fortified infant foods provided by the Special Supplemental Program for Women, Infants, and Children (WIC) in the early 1970s and the decrease in use of whole cow milk for infants. 8 Still, ID remains relatively common and occurs in 6.6% to 15.2% of toddlers, depending on ethnicity and socioeconomic status. The prevalence of IDA is 0.9% to 4.4%, again depending on race/ethnicity and socioeconomic status, 7 , 8 but only accounts for approximately 40% of the anemia in toddlers ( Table 1 ). These numbers are comparable to data collected in other industrialized countries. 13 , 14
ID, IDA, and Anemia in the 1999–2002 National Health and Nutrition Examination Survey, 7 Children 12 to 35 Months of Age
Shown are the unweighted number and weighted percentage and SEs for all children with complete data for Hb, SF, transferrin saturation, and zinc protoporphyrin. Anemia was defined as a Hb concentration of <11.0 g/dL; ID 7 was defined as an abnormal value for at least 2 of 3 indicators: SF (abnormal cutoff: <10 μg/dL), zinc protoporphyrin (>1.42 μmol/L red blood cells), and transferrin saturation (<10%); and IDA was defined as anemia plus ID.
Proportion of row descriptor of all children in analytic sample ( N = 672).
Children with income data ( N = 623).
Estimate is statistically unreliable. Relative SE (SE of estimate/estimate × 100) ≥ 30%.
Any member of household who received benefits from WIC in the previous 12 months: children with food-security data ( N = 668).
Related to the problem of ID/IDA is the interaction of iron and lead. Results of both animal and human studies have confirmed that IDA increases intestinal lead absorption. 15 , – , 17 A reasonably well-established epidemiologic association has been made between IDA and increased lead concentrations. 18 Thus, primary prevention of IDA could also serve as primary prevention of lead poisoning. This possibility is all the more attractive, because lead has been reported to induce neurotoxicity at even very low blood concentrations. 19 , 20 In addition, preexisting IDA decreases the efficiency of lead chelation therapy, and iron supplementation corrects this effect. In contrast, iron supplementation in a child with IDA who also has lead poisoning without chelation therapy seems to increase blood lead concentrations and decrease basal lead excretion. 21 , 22 The effect of iron supplementation on blood lead concentrations in iron-replete children with or without lead poisoning is not known. Thus, in theory, selective rather than universal iron supplementation would be more likely to reduce lead poisoning and its potential harmful effects on these children.
The possible relationship between ID/IDA and later neurobehavioral development in children is the subject of many reports. 3 , 23 , – , 31 Results of a preponderance of studies have demonstrated an association between IDA in infancy and later cognitive deficits. Lozoff et al 3 , 25 have reported detecting cognitive deficits 1 to 2 decades after the iron-deficient insult during infancy. However, it has been difficult to establish a causal relationship because of the many confounding variables and the difficulty in designing and executing the large, randomized controlled trials necessary to distinguish small potential differences. The authors of a Cochrane Database systematic review, in which the question of whether treatment of IDA improved psychomotor development was examined, stated that there was inconclusive but plausible evidence (only 2 randomized controlled trials) demonstrating improvement if the treatment extended for more than 30 days. 27 McCann and Ames 28 recently reviewed the evidence of a causal relationship between ID/IDA and deficits in cognitive and behavioral function. They concluded that for IDA, there is at least some support for causality, but because specificity for both cause and effect have not been established unequivocally, it is premature to conclude the existence of a causal relationship between IDA and cognitive and behavioral performance. For ID, some evidence of causality exists, but it is less than that for IDA. 28
It is known that iron is essential for normal neurodevelopment in a number of animal models. ID affects neuronal energy metabolism, the metabolism of neurotransmitters, myelination, and memory function. These observations would explain the behavioral findings in human infants that have been associated with ID. 29 , – , 31 Therefore, taking into account that iron is the world's most common single-nutrient deficiency, it is important to minimize IDA and ID among infants and toddlers, even if an unequivocal relationship between IDA and ID and neurodevelopmental outcomes has yet to be established.
Iron status is a continuum. At one end of the spectrum is IDA, and at the other end is iron overload. ID and IDA are attributable to an imbalance between iron needs and available iron that results in a deficiency of mobilizable iron stores and is accompanied by changes in laboratory measurements that include Hb concentration, mean corpuscular Hb concentration, mean corpuscular volume, reticulocyte Hb concentration (abbreviated in the literature as CHr) content, total iron-binding capacity, transferrin saturation, zinc protoporphyrin, SF concentration, and serum transferrin receptor 1 (TfR1) concentration. Measurements that are used to describe iron status are listed in Table 2 .
Spectrum of Iron Status
Confounded by the presence of inflammation. If SF is normal or increased and the CRP level is normal, then there is no ID. If SF is decreased, then ID is present regardless of the measure of CRP. If SF is normal or increased and the CRP level is increased, then the presence of ID cannot be determined.
Modified from American Academy of Pediatrics, Committee on Nutrition. Iron deficiency. In: Kleinman RE, ed. Pediatric Nutrition Handbook . 5th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2004:304.
In a child with ID, as the Hb concentration falls 2 SDs below the mean for age and gender, IDA is present, by definition; for infants at 12 months of age, this is 11.0 mg/dL. 7 , 8 When IDA accounted for most cases of anemia in children, “anemia” and “IDA” were roughly synonymous, and a simple measurement of Hb concentration was sufficient to make a presumptive diagnosis of anemia attributable to ID. Particularly in industrialized nations, the prevalence of ID and IDA has decreased, and other causes of anemia, such as hemolytic anemias, anemia of chronic disease, and anemia attributable to other nutrient deficiencies, have become proportionately more common. 32
No single measurement is currently available that will characterize the iron status of a child. The limitations of using Hb concentration as a measure of iron status are its lack of specificity and sensitivity. Factors that limit erythropoiesis or result in chronic hemolysis, such as genetic disorders and chronic infections, may result in low Hb concentrations. Vitamin B 12 or folate deficiency, although uncommon in the pediatric population, also can result in a low Hb concentration. The lack of sensitivity is largely attributable to the marked overlap in Hb concentrations between populations with iron sufficiency and those with ID. 33 Thus, to identify ID or IDA, Hb concentration must be combined with other measurements of iron status. Once the diagnosis of IDA has been established, however, following Hb concentration is a good measure of response to treatment.
In establishing the definitive iron status of an individual, it is desirable to use the fewest tests that will accurately reflect iron status. Any battery of tests must include Hb concentration, because it determines the adequacy of the circulating red cell mass and whether anemia is present. One or more tests must be added to the determination of Hb concentration if ID or IDA is to be diagnosed. The 3 parameters that provide discriminatory information about iron status are SF, CHr, and TfR1 concentrations.
SF is a sensitive parameter for the assessment of iron stores in healthy subjects 34 , – , 36 ; 1 μg/L of SF corresponds to 8 to 10 mg of available storage iron. 34 , 37 , 38 Measurement of SF concentration is widely used in clinical practice and readily available. Cook et al 36 selected an SF concentration below 12 μg/L as diagnostic for ID after a comprehensive population survey in the United States. Thus, a cutoff value of 12 μg/L has been widely used for adults and denotes depletion of iron stores. In children, a cutoff value of 10 μg/L has been suggested. 39 Because SF is an acute-phase reactant, concentrations of SF may be elevated in the presence of chronic inflammation, infection, malignancy, or liver disease, and a simultaneous measurement of C-reactive protein (CRP) is required to rule out inflammation. Although Brugnara et al 40 found SF concentration to be less accurate than either the CHr or TfR1 concentration in establishing iron status of children, combining SF concentration with a determination of CRP is currently more readily available to assess iron stores and is a reliable screening test as long as the CRP level is not elevated 41 ( Table 2 ).
CHr and TfR1 concentrations are not affected by inflammation (infection), malignancy, or anemia of chronic disease and, thus, would be preferable as biomarkers for iron status. Only the CHr assay is currently available for use in children. The CHr content assay has been validated in children, and standard values have been determined. 40 , 42 The CHr assay provides a measure of iron available to cells recently released from the bone marrow. CHr content can be measured by flow cytometry, and 2 of the 4 automated hematology analyzers commonly used in the United States have the capability to measure CHr. 43 A low CHr concentration has been shown to be the strongest predictor of ID in children 40 , 42 , 43 and shows much promise for the diagnosis of ID when the assay becomes more widely available.
TfR1 is a measure of iron status, detecting ID at the cellular level. TfR1 is found on cell membranes and facilitates transfer of iron into the cell. When the iron supply is inadequate, there is an upregulation of TfR1 to enable the cell to compete more effectively for iron, and subsequently, more circulating TfR1 is found in serum. An increase in serum TfR1 concentrations is seen in patients with ID or IDA, although it does not increase in serum until iron stores are completely exhausted in adults. 44 , – , 46 However, the TfR1 assay is not widely available, and standard values for infants and children have yet to be established.
Thus, to establish a diagnosis of IDA, the following sets of tests can be used at the present time (when coupled with determination of a Hb concentration of <11 g/dL): (1) SF and CRP measurements or (2) CHr measurement. For diagnosing ID without anemia, measure either (1) SF and CRP or (2) CHr.
Another approach to making the diagnosis of IDA in a clinically stable child with mild anemia (Hb concentration between 10 and 11 g/dL) is to monitor the response to iron supplementation, especially if a dietary history indicates that the diet is likely to be iron deficient. An increase in Hb concentration of 1 g/dL after 1 month of therapeutic supplementation has been used to signify the presence of IDA. This approach requires that iron supplementation be adequate, iron be adequately absorbed, and patient compliance with adequate follow-up can be ensured. However, because only 40% of the cases of anemia identified at 12 months of age will be secondary to IDA ( Table 1 ), strong consideration should be given to establishing a diagnosis of IDA by using the screening tests described previously.
The preterm infant (<37 weeks' gestation) who is fed human milk should receive a supplement of elemental iron at 2 mg/kg per day starting by 1 month of age and extending through 12 months of age. 47 This can be provided as medicinal iron or in iron-fortified complementary foods. Preterm infants fed a standard preterm infant formula (14.6 mg of iron per L) or a standard term infant formula (12.0 mg of iron per L) will receive approximately 1.8 to 2.2 mg/kg per day of iron, assuming a formula intake of 150 mL/kg per day. Despite the use of iron-containing formulas, 14% of preterm infants develop ID between 4 and 8 months of age. 48 Thus, some formula-fed preterm infants may need an additional iron supplement, 47 although there is not enough evidence to make this a general recommendation at this time. Exceptions to this iron-supplementation practice in preterm infants would be infants who received multiple transfusions during hospitalization, who might not need any iron supplementation.
Infants who are born at term usually have sufficient iron stores until 4 to 6 months of age. 49 Infants born at term have high Hb concentration and high blood volume in proportion to body weight. They experience a physiologic decline in both blood volume and Hb concentration during the first several months of life. These facts have led to the supposition that breastfed infants need very little iron. It is assumed that the small amount of iron in human milk is sufficient for the exclusively breastfed infant. The World Health Organization recommends exclusive breastfeeding for 6 months, and the American Academy of Pediatrics (AAP) has recommended exclusive breastfeeding for a minimum of 4 months but preferably for 6 months. Exclusive breastfeeding for more than 6 months has been associated with increased risk of IDA at 9 months of age. 49 , 50 Recommendations for exclusive breastfeeding for 6 months do not take into account infants who are born with lower-than-usual iron stores (low birth weight infants, infants of diabetic mothers), a condition that also has been linked to lower SF concentrations at 9 months of age. 51 In a double-blind study, Friel et al 52 demonstrated that exclusively breastfed infants supplemented with iron between 1 and 6 months of age had higher Hb concentration and higher mean corpuscular volume at 6 months of age than did their unsupplemented peers. Supplementation also resulted in better visual acuity and higher Bayley Psychomotor Developmental Indices at 13 months. Thus, it is recommended that exclusively breastfed term infants receive an iron supplementation of 1 mg/kg per day, starting at 4 months of age and continued until appropriate iron-containing complementary foods have been introduced ( Tables 3 and 4 ). For partially breastfed infants, the proportion of human milk versus formula is uncertain; therefore, beginning at 4 months of age, infants who receive more than one-half of their daily feedings as human milk and who are not receiving iron-containing complementary foods should also receive 1 mg/kg per day of supplemental iron.
Foods to Increase Iron Intake and Iron Absorption
Note that all figures are rounded.
Baby food values are generally based on generic jar, not branded jar; 3 oz of table-food meat = 85 g; a 2.5-oz jar of baby food = 71 g (an infant would not be expected to eat 3 oz [approximately the size of a deck of cards] of pureed table meat at a meal).
Source of iron value was obtained from a manufacturer of this type of molasses.
Source of iron values in foods: US Department of Agriculture, Agricultural Research Service. USDA National Nutrient Database for Standard Reference, Release 20: Nutrient Data Laboratory home page. Available at: www.ars.usda.gov/ba/bhnrc/ndl .
Selected Good Vitamin C Sources to Increase Iron Absorption
For the term, formula-fed infant, the level of iron fortification of formula to prevent ID remains controversial. 53 , 54 For more than 25 years, 12 mg of iron per L has been the level of fortification in standard term infant formulas in the United States, consistent with guidelines of WIC for iron-fortified formula (at least 10 mg/L), thus creating a natural experiment. The level of 12 mg/L was determined by calculating the total iron needs of the child from 0 to 12 months of age, assuming average birth weight and average weight gain during the first year. The calculation also assumed that formula was the only source of iron during this period. Others have recommended lower amounts of iron in infant formula, 55 and there have been studies to examine iron-fortification levels of less than 12 mg/L. 56 , – , 61 However, it is the conclusion of the AAP that infant formula that contains 12 mg of elemental iron per L is safe for its intended use. Although there has been some concern about linear growth in iron-replete infants given medicinal iron, 62 no published studies have convincingly documented decreased linear growth in iron-replete infants receiving formulas containing high amounts of iron. Evidence is also insufficient to associate formulas that contain 12 mg of iron per L with gastrointestinal symptoms. At least 4 studies have shown no adverse effects. 63 , – , 66 Reports have conflicted on whether iron fortification is associated with increased risk of infection. Decreased incidence, increased incidence, and no change in number of infections have all been reported. 67 , 68 The authors of a recent systematic review concluded that “iron supplementation has no apparent harmful effect on the overall incidence of infectious illnesses in children, though it slightly increases the risk of developing diarrhoea.” 69 Finally, when examining specifically infants given formula with 12 mg of iron per L, Singhal et al 70 were “unable to identify adverse health effects in older infants and toddlers consuming a high iron-containing formula.” They found no difference between controls and the treatment group in incidence of infection, gastrointestinal problems, or general morbidity.
The iron requirement for toddlers is 7 mg/day. Ideally, the iron requirements of toddlers would be met and ID/IDA would be prevented with naturally iron-rich foods rather than iron supplementation. These foods include those with heme sources of iron (ie, red meat) and nonheme sources of iron (ie, legumes, iron-fortified cereals) ( Table 3 ). Foods that contain vitamin C (ascorbic acid), such as orange juice, aid in iron absorption and are listed in Table 4 . Foods that contain phytates (found in soy) reduce iron absorption. Through public education and altering feeding practices, the amount of iron available to older infants and toddlers via a normal diet could be maximized ( Table 3 ).
In developing countries, iron requirements of older infants and toddlers have been met by iron fortification of various foods, including corn flour, 71 soy sauce, 72 fish sauce, 73 and rice. 74 However, there are many technical and practical barriers to a successful fortification program for toddlers. Not the least of these barriers is the determination of which foods to fortify with iron. In the United States, fortification of infant formula and infant cereal has been credited with the decline in IDA. However, toddlers in the United States typically do not eat enough of any other food to serve as a vehicle for iron fortification. Universal food fortification for all ages is problematic, given the possible adverse effects of iron in certain subsets of older children and adults.
As an alternative for toddlers who do not eat adequate amounts of iron-containing food ( Table 3 ), iron supplements are available in the form of iron sulfate drops and chewable iron tablets or as a component of either liquid or chewable multivitamins. Iron sprinkles with or without additional zinc are available in Canada. Barriers to adequate iron supplementation are (1) lack of education for care providers and patients, (2) poor compliance made worse by the perception of adverse effects, including nausea, vomiting, constipation, stomach upset, and teeth staining, (3) cost, (4) current federal supplemental nutrition programs not providing iron supplements, and (5) risk of iron overload.
The AAP has concluded that universal screening for anemia should be performed with determination of Hb concentration at approximately 1 year of age. Universal screening would also include an assessment of risk factors associated with ID/IDA: history of prematurity or low birth weight; exposure to lead; exclusive breastfeeding beyond 4 months of age without supplemental iron; and weaning to whole milk or complementary foods that do not include iron-fortified cereals or foods naturally rich in iron ( Table 3 ). Additional risk factors include the feeding problems, poor growth, and inadequate nutrition typically seen in infants with special health care needs as well as low socioeconomic status, especially children of Mexican American descent, as identified in the recent National Health and Nutrition Examination Survey 8 , 75 ( Table 1 ). Selective screening can be performed at any age when these risk factors for ID and IDA have been identified, including risk of inadequate iron intake according to dietary history.
It has been acknowledged that screening for anemia with a Hb determination neither identifies children with ID nor specifically identifies those with IDA. 76 In the United States, 60% of anemia is not attributable to ID, and most toddlers with ID do not have anemia ( Table 2 ). It is also known that there is poor follow-up testing and poor documentation of improved Hb concentrations. In 1 study, 14% of the children had a positive screening result for anemia. However, only 18.3% of these children with a positive screening result had follow-up testing performed, and of that group, only 11.6% had documented correction of low Hb levels. 77 Therefore, for infants identified with a Hb concentration of less than 11.0 mg/dL or identified with significant risk of ID or IDA as described previously, SF and CRP or CHr levels in addition to Hb concentration should be measured to increase the sensitivity and specificity of the diagnosis. In addition, the AAP, the World Health Organization, and the European Society for Pediatric Gastroenterology, Hepatology and Nutrition also support the use of the measurement of TfR1 as a screening test once the method has been validated and normal values for infants and toddlers have been established.
Another step to improve the current screening system is to use technology-based reminders for screening and follow-up of infants and toddlers with a diagnosis of ID/IDA. Reminders could be incorporated into electronic health records, and there should be documentation that Hb concentrations have returned to the normal range. The efficacy of any program for minimizing ID and IDA should be tracked scientifically and evaluated through well-planned surveillance programs.
Given that iron is the world's most common single-nutrient deficiency and there is some evidence of adverse effects of both ID and IDA on cognitive and behavioral development, it is important to minimize ID and IDA in infants and toddlers without waiting for unequivocal evidence. Controversies remain regarding the timing and methods used for screening for ID/IDA as well as regarding the use of iron supplements to prevent ID/IDA. Although further study is required to generate higher levels of evidence to settle these controversies, the currently available evidence supports the following recommendations.
Term, healthy infants have sufficient iron for at least the first 4 months of life. Human milk contains very little iron. Exclusively breastfed infants are at increasing risk of ID after 4 completed months of age. Therefore, at 4 months of age, breastfed infants should be supplemented with 1 mg/kg per day of oral iron beginning at 4 months of age until appropriate iron-containing complementary foods (including iron-fortified cereals) are introduced in the diet (see Table 3 ). For partially breastfed infants, the proportion of human milk versus formula is uncertain; therefore, beginning at 4 months of age, partially breastfed infants (more than half of their daily feedings as human milk) who are not receiving iron-containing complementary foods should also receive 1 mg/kg per day of supplemental iron.
For formula-fed infants, the iron needs for the first 12 months of life can be met by a standard infant formula (iron content: 10–12 mg/L) and the introduction of iron-containing complementary foods after 4 to 6 months of age, including iron-fortified cereals ( Table 3 ). Whole milk should not be used before 12 completed months of age.
The iron intake between 6 and 12 months of age should be 11 mg/day. When infants are given complementary foods, red meat and vegetables with higher iron content should be introduced early ( Table 3 ). To augment the iron supply, liquid iron supplements are appropriate if iron needs are not being met by the intake of formula and complementary foods.
Toddlers 1 through 3 years of age should have an iron intake of 7 mg/day. This would be best delivered by eating red meats, cereals fortified with iron, vegetables that contain iron, and fruits with vitamin C, which augments the absorption of iron ( T3 , Tables 3 and 4 ). For toddlers not receiving this iron intake, liquid supplements are suitable for children 12 through 36 months of age, and chewable multivitamins can be used for children 3 years and older.
All preterm infants should have an iron intake of at least 2 mg/kg per day through 12 months of age, which is the amount of iron supplied by iron-fortified formulas. Preterm infants fed human milk should receive an iron supplement of 2 mg/kg per day by 1 month of age, and this should be continued until the infant is weaned to iron-fortified formula or begins eating complementary foods that supply the 2 mg/kg of iron. An exception to this practice would include infants who have received an iron load from multiple transfusions of packed red blood cells.
Universal screening for anemia should be performed at approximately 12 months of age with determination of Hb concentration and an assessment of risk factors associated with ID/IDA. These risk factors would include low socioeconomic status (especially children of Mexican American descent [ Table 1 ]), a history of prematurity or low birth weight, exposure to lead, exclusive breastfeeding beyond 4 months of age without supplemental iron, and weaning to whole milk or complementary foods that do not include iron-fortified cereals or foods naturally rich in iron ( Table 3 ). Additional risk factors are the feeding problems, poor growth, and inadequate nutrition typically seen in infants with special health care needs. For infants and toddlers (1–3 years of age), additional screening can be performed at any time if there is a risk of ID/IDA, including inadequate dietary iron intake.
If the Hb level is less than 11.0 mg/dL at 12 months of age, then further evaluation for IDA is required to establish it as a cause of anemia. If there is a high risk of dietary ID as described in point 6 above, then further testing for ID should be performed, given the potential adverse effects on neurodevelopmental outcomes. Additional screening tests for ID or IDA should include measurement of:
SF and CRP levels; or
CHr concentration.
If a child has mild anemia (Hb level of 10–11 mg/d) and can be closely monitored, an alternative method of diagnosis would be to document a 1 g/dL increase in plasma Hb concentration after 1 month of appropriate iron-replacement therapy, especially if the history indicates that the diet is likely to be iron deficient.
Use of the TfR1 assay as screening for ID is promising, and the AAP supports the development of TfR1 standards for use of this assay in infants and children.
If IDA (or any anemia) or ID has been confirmed by history and laboratory evidence, a means of carefully tracking and following infants and toddlers with a diagnosis of ID/IDA should be implemented. Electronic health records could be used not only to generate reminder messages to screen for IDA and ID at 12 months of age but also to document that IDA and ID have been adequately treated once diagnosed.
This report was written by the primary authors after extensive review of the literature using PubMed, previous AAP reports, Cochrane reviews, and reports from other groups. 1 , 6 , 7 , 48 , 77
The report was also submitted to the following sections and committees of the AAP that were asked to comment on the manuscript: Committee on Fetus and Newborn (COFN); Committee on Psychosocial Aspects of Child and Family Health (COPACFH); Section on Administration and Practice Management (SOAPM); Section on Developmental and Behavioral Pediatrics (SODBP); Section on Gastroenterology, Hepatology, and Nutrition (SOGHN); Section on Hematology and Oncology (SOHO); and Section on Breast Feeding (SOBr).
Additional comments were sought from the Centers for Disease Control and Prevention (CDC), the Department of Agriculture (WIC), the National Institutes of Health (NIH), and the Food and Drug Administration (FDA), because these governmental agencies were involved in the development of the statement and will necessarily deal with its impact. As it was developed it was extensively reviewed and revised by members of the AAP Committee on Nutrition, who unanimously approved this clinical report. It is openly acknowledged that where the highest levels of evidence are absent, the opinions and suggestions of members of the Committee on Nutrition as well as other groups consulted for this statement were taken into consideration in developing this clinical report.
Robert D. Baker, MD, PhD, Former Committee Member
Frank R. Greer, MD, Immediate Past Chairperson
Jatinder J. S. Bhatia, MD, Chairperson
Steven A. Abrams, MD
Stephen R. Daniels, MD, PhD
Marcie Beth Schneider, MD
Janet Silverstein, MD
Nicolas Stettler, MD, MSCE
Dan W. Thomas, MD
Laurence Grummer-Strawn, PhD
Centers for Disease Control and Prevention
Rear Admiral Van S. Hubbard, MD, PhD
National Institutes of Health
Valérie Marchand, MD
Canadian Paediatric Society
Benson M. Silverman, MD
Food and Drug Administration
Valery Soto, MS, RD, LD
US Department of Agriculture
Debra L. Burrowes, MHA
The guidance in this report does not indicate an exclusive course of treatment or serve as a standard of medical care. Variations, taking into account individual circumstances, may be appropriate.
All clinical reports from the American Academy of Pediatrics automatically expire 5 years after publication unless reaffirmed,revised, or retired at or before that time.
iron deficiency
iron-deficiency anemia
serum ferritin
Institute of Medicine
Special Supplemental Program for Women, Infants, and Children
reticulocyte hemoglobin
transferrin receptor 1
C-reactive protein
American Academy of Pediatrics
Competing Interests
Re: screening for iron deficiency.
To the Editors:
We are gratified that Drs Meyers, Lozoff and Georgieff agree with our stance of the importance of preventing irons deficiency (ID) and iron deficiency anemia (IDA) especially in infants and young children because of possible detrimental impact on neurodevelopment ( 1,2,3,4 ). Their letter highlights one of the many perplexing issues in deciding how to deal with the problem of ID and IDA, the lack of a single, straight- forward test that accurately reflects iron status across its spectrum. In our clinical report (5)we suggest three options for screening tests: 1) Hemoglobin (Hb), serum ferritin (SF) and C-reactive protein (CRP); 2) Hb and reticulocyte hemoglobin concentration (CHr); and 3) Hb and tranferrin receptor 1 (TfR1). The first set of tests is simple to interpret and readily available. The second set is straight-forward, has been validated in the pediatric age group, but is not available to all. The third is also straight-forward, but is the least widely available and has not been completely validated for the age groups considered in this report. Yet, this is the set of tests recommended by the World Health Organization (6). Drs Meyers, Lozoff and Georgieff mention erythrocyte protoporphyrin , zinc protoporphyrin, mean corpuscular volume, red cell distribution width and transferrin saturation as possible additions or alternatives to the tests suggested in our report. These, among other screening options were considered, but if anything, adding them to the screening process makes the interpretation of the results even more complicated for the practitioner. The bottom line is that there is no ideal screening test at the present time. For the reasons pointed out in our report we are pushing for further development of either CHr or TfR1. Either of these tests with the addition of an Hb determination would offer an accurate assessment of iron status.
Robert D. Baker, MD, PhD Frank R. Greer, MD
1. McCann JC, Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr. 2007;85(4):931-945 2. Logan S, Martins S, Gilbert R. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia. Cochrane Database Syst Rev. 2001;(2):CD001444 3. Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron-deficiency anemia in healthy full-term infants. Pediatrics. 2003;112(4):846-854 4. Grantham-McGregor S. Does iron-deficiency anemia affect child development? Pediatrics. 2003;112(4):978 5. Baker RD, Greer FR. Clinical Report – Diagnosis and prevention of iron deficiency and iron deficiency anemia in infants and young children (0-3 years of age).Pediatrics. 2010;104: 119-123 6. World Health Organization. Assessing the Iron Status of Populations: Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level. Geneva, Switzerland; April 6-8, 2004. Available at: http://whqlibdoc.who.int/publications/2004/9241593156_eng.pdf. Accessed September 29, 2008
Conflict of Interest:
None declared
screening for iron deficiency
We applaud the AAP Committee on Nutrition’s new recommendations (1) for screening young children for iron deficiency (ID) and iron deficiency anemia (IDA) and the important emphasis placed on preventing, identifying, and treating ID without anemia, as evidence now suggests this condition may have adverse neurodevelopmental consequences. However, as noted by the report and numerous investigators in the field, the detection of pre- anemic ID is difficult since widely accessible sensitive and specific tests are not available. The report recommends the use of serum ferritin (SF) with C-reactive protein (CRP), reticulocyte hemoglobin (CHr), or soluble transferrin receptor 1 (TfR1) to screen children at risk for ID or those with low hemoglobin (Hb). But as noted in the report, CHr is measured only by a limited number of cell counters currently in use, while TfR1 is not yet commercially available. The report does not mention the use of erythrocyte protoporphyrin (EP)/zinc protoporphyrin (ZPP), mean corpuscular volume (MCV), red cell distribution width (RDW) or transferrin saturation (TS), which have been in wide use for ID screening for decades, and which are more readily available at relatively low cost. ZPP, in particular, has been demonstrated to be highly suited for ID screening in young children. (2-7) Furthermore, the Committee’s statement that “SF is a sensitive parameter for the assessment of iron stores in healthy subjects” cites 3 references, all of which are studies of adults. (8-10) Evidence suggests that SF is not sensitive for screening for ID in infants. For example, in a study of one-year-old infants with screening hemoglobin < 11.5 g/dl, Dallman et al reported that only 29% of those who responded to an iron challenge had had a low SF at the time of screening. (11) Until the problems with accessibility of CHr and Tfr1 are solved, we feel that, at a minimum, the new recommendations should include readily available tests such TS (with CRP), red cell measures included in a complete blood count, and/or ZPP as suggested screening tests for ID. For the child whose MCV and/or RDW is abnormal but other screening parameters are normal, the clinician might consider obtaining additional tests of iron sufficiency (SF, TS, ZPP) or a trial of iron therapy.
REFERENCES 1) Baker RD, Greer FR, the Committee on Nutrition. Clinical report - diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics 2010;126:1040- 1050
2) Yip R, Schwartz S, Deinard A. Screening for iron deficiency with The erythrocyte protoporphyrin test. Pediatrics 1983;72:214-219
3) Siegel JM, LaGrone DH. The use of zinc protoporphyrin in screening young children for iron deficiency. Clin Pediatr 1994;33:473-479
4) Rettmer RL,m Carlson TH, Origenes ML Jr, et al. Zinc protoporphyrin/heme ratio for diagnosis of preanemic iron deficiency Pediatrics 1999;104:e37
5) Mei Z, Parvanta I, Cogswell ME et al. Erythrocyte protoporphyrin or hemoglobin: which is a better screening test for iron deficiency in children and women? Am J Clin Nutr 2003;77:1229-1233
6) Labbé RF, Dewanji A. Iron assessment tests: transferrin receptor vis-à-vis zinc protoporphyrin. Clin Biochem 2003;37:165-174
7) Crowell R, Ferris AM, Wood RJ, et al. Comparative effectiveness of zinc protoporphyrin and hemoglobin concentrations in identifying iron deficiency in a group of low-income, preschool-aged children: practical implications of recent illness. Pediatrics 2006;118:224-232
8) Jacobs A, Miller F, Worwood M, Beamish MR, Wardrop CA. Ferritin in the serum of normal subjects and patients with iron deficiency and iron overload. Br Med J. 1972;4(5834):206 –208
9) Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973;26(10):770 –772
10) Cook JD, Lipschitz DA, Miles LE, Finch CA. Serum ferritin as a measure of iron stores in normal subjects. Am J Clin Nutr. 1974;27(7):681– 687
11) Dallman PR, Reeves JD, Driggers DA, Lo YET. Diagnosis of iron deficiency: the limitations of laboratory tests in predicting response to iron treatment in 1-year-old infants. J Pediatr 1981;99:376-381
Response to comments on iron recommendations
Re: CR120445, Diagnosis and prevention of iron deficiency and iron deficiency anemia in infants and young children (0-3 years)(1)
Iron nutriture has always been a difficult, controversial, but important topic in pediatrics. It is not surprising that the AAP’s clinical report on iron has generated a number letters. We thank Dr Schanler, Dr Furman and Drs Hernell and Lönnerdahl for their comments on our report on iron.
Their comments focus on the recommendation that full-term exclusively breastfed babies receive iron supplementation starting at four months of age and continuing until a complementary dietary source of iron is established. In making this recommendation, we weighed the potential harm of not supplementing these infants with the potential harm of providing supplemental iron. We readily admit that the evidence on either side of this equation is not yet certain; however, we concluded that there was substantial and growing evidence of behavioral and developmental harm from iron deficiency (ID) and scant and yet to be established evidence of deleterious effects from iron supplementation. We also concluded that exclusively breastfed infants are at risk of becoming iron deficient. One objection voiced by each of these letters is that our report cites only one study that reports on exclusively breastfed, term babies who received iron or placebo in a blinded manner (Friel, et al)(2). While the authors of our report felt that Friel’s study was the best available, there are other studies that show that breastfed babies are at risk of ID and iron deficiency anemia (IDA) and/or that iron supplementation improves iron status of these infants (Pizzaro et al.(3) Calvo et al.(4) Arvas et al.(5) Innis et al.(6) Zeigler et al.(7) Hokama et al.(8)). The paper by Calvos et al. includes a calculation that concludes that the iron required by an infant in the first year exceeds, by several fold, the total amount of iron available in breastmilk. Despite the fact that the calculation is theoretical and open to question, it is provocative. Others have pointed out that by the time an exclusively breastfed infant doubles his birthweight, supplemental iron is necessary since breast milk alone will not supply the iron to support the infant’s needs.
In questioning the recommendation, Dr Furman challenges the validity of the study by Friel. In particular she points out the small sample size, the high drop out rate, and the questionable power of the study. Of course a larger study would be more reassuring that the correct conclusions were made. Large, powerful studies protect against type II errors. Since statistical differences in visual acuity psychomotor development were documented, the validity of the conclusion is not in question. It would, for instance, not be correct to use Friel’s data to try to demonstrate that iron supplementation caused no harm. For this a much larger study would be necessary. The drop out rate reflects the real life problems inherent in studies of this sort. We believe that one can draw conclusions regarding neurodevelopment from Dr. Friel’s data and that those conclusion are scientifically justified. It is important to note that we based our overall assessment of the relationship between iron status and neurodevelopment on many studies(9,10,11), including meta- analyses(12,13), not just on Dr. Friel’s work.
Dr Furman cites a recent study by Ziegler et al.7 We assert that this study actually supports our recommendations. The study examined iron status and not neurodevelopment. It demonstrated a statistically significant “moderate” improvement in iron status in supplemented infants. The difference did not last beyond the time of supplementation. Combining the conclusions of this study with those of Dr Lozoff (9,10,11) that tracked neurodevelopmental changes two decades after iron deficiency has been corrected, we would contend that it is important to prevent any period of iron deficiency in infants and young children.
Each of the critiques from Schanler, Furman and from Hernell and Lönnerdahl mention “harm” from iron supplementation. To substantiate harm they point to a study, co-authored by Hernell and Lönnerdahl,(14) which reported that a small number of exclusively breastfed Swedish babies (n =31) supplemented with iron beginning at 4 months had decreased length (one centimeter) despite responding with a rise in serum hemoglobin. The impact on length did not occur in the Honduran comparison group. We await further studies confirming this finding, but we could find no previous reports of such an effect. Several subsequent studies including Friel’s(2) and Zeigler’s (7) looked for an effect on linear growth, but found none, though these studies were not powered to exclude harm. The Swedish infants, supplemented or not, had a positive Z-score for length at all time points measured. An alternative explanation for the small effect on growth could be “catch down growth”. There is considerable “adjustment” in growth during the first year. In developed countries babies are larger and tend to catch down while smaller babies catch up. In Lönnerdahl’s study the supplemented Swedish infants were significantly larger than the unsupplemented babies at the outset. Thus the larger Swedish babies may have been exhibiting normal “catch down” growth rather than an adverse effect of iron supplementation. While proving no harm is statistically difficult, we would like to point out that for approximately the past 30 years standard formula in the United States has contained 12 mg/L iron which by most calculation is more than double the concentration needed to supply the iron requirements. No harmful effects have been documented. We realize that both human milk and formula are complex matrices, and iron absorption from human milk is different from formula and absorption from both is different from supplemental iron; however, once absorption has taken place, iron from all sources is treated similarly. By any criteria, the less than a centimeter difference in length while maintaining a positive z score for length could not be classified as harm.
Lönnerdahl et al. have used their finding of increased Hb in response to supplements in Swedish breastfed infants in their study to argue that the infants have disregulation of iron absorption (15). Dr. Furman also brings up the idea of disregulation in her criticism. We found this explanation intriguing, but unsubstantiated. At six months the iron absorption from human milk was reported as 11.9+7.4% in iron supplemented infants and 17.8+12.2% in unsupplemented infants. There were just 6 infants in the supplemented group and 19 in the unsupplemented group. While there was no statistical difference, these numbers are far too few to prove “no difference”. We await further studies to either confirm or refute the conclusion of Lönnerdahl et al. Since “no difference” was the basis for the disregulation hypothesis, we question disregulation of iron absorption during the first six months of life. Iron is a highly regulated metal. Regulation occurs at the level of intestinal lumen, the apical and basal lateral surfaces of the enterocyte as well as within the enterocyte. There is regulation by transport proteins as well as entrance and exit for storage sites. The utilization of iron at the level of the erythron is regulated as well as the salvage of iron via macrophages. For Lönnerdahl’s explanation to be valid there would necessarily be a breakdown at least two levels of regulation, not merely at the level of absorption but also at the erythron. We offer an alternative explanation for Lönnerdahl’s findings, that the increase in Hb in apparent response to iron supplementation suggests that the additional iron is needed by these breastfed infants to increase erythropoesis.
Hernell and Lönnerdahl argue that the prevalence of iron deficiency is very low. However, we would point out that the prevalence data is based solely on hematologic criteria which are set somewhat arbitrarily and may not be relevant to consideration of neurobehavioral development. In our deliberations we were persuaded that the potential negative and long-lasting influence of iron deficiency (iron deficiency taken to mean body iron content low enough to hinder optimal function) overpowered any possible negative effects. Dr Schanler suggests that instead of universal supplementation of exclusively breastfed infants, that we perform screening of “at risk” infants. We address, in the report, the difficulty in identifying “at risk” infants. We also highlight the problems inherent to a targeted screening program. In Dr. Betsy Lozoff’s editorial (16) that accompanied Dr Friel’s paper, she states “No simple accurate way of identifying breastfed infants at 1-2 months who will later become iron deficient is available now or in the foreseeable future.” The recommendation for universal supplementation avoids the issue of screening. It would be difficult to decide whom to screen, because risk factors in addition to exclusive breastfeeding may include infants of diabetic mothers, maternal iron deficiency, twins, late preterm infants, children of minority mothers, children not in daycare, lower SES, such that the population to be screened encompasses a large percentage of most physician practices in the United States..What tests to use for screening and what levels would be considered abnormal are also problematic.. Also, from a practical point of view, it would be difficult to persuade parents and pediatricians to agree to screening studies that would necessitate a venipuncture.
Dr. Schanler suggests that rather than recommending iron supplements other means of improving the iron deficit of breastfeeding infants be exploited. In particular Dr Schanler mentions early cord clamping. This clinical report is specifically directed toward the pediatrician and the authors of our report felt that recommendations for early versus late cord clamping is not under the direct control of pediatricians, but the American College of Obstetrics and Gynecology; thus while the AAP and/or the Section on Breastfeeding may want to advocate for late cord clamping in a joint statement with our obstetrical colleagues, this is beyond the purview of this report.
We stand by the conclusions of our report, specifically with regards to iron supplementation of breastfed infants. While the definitive studies have not been done, a mounting body of evidence supports that iron deficiency is associated with developmental and behavioral changes. Data from the present pediatrics population indicates that iron deficiency is common at 12 months of age. Iron deficiency occurs long before there is overt anemia and may have long lasting consequences. Because of the very low iron content of human milk, exclusively breastfed infants are a risk of iron deficiency. Supplementing breastfed infants would protect them. Why put these infants at any risk, when no appreciable harm of iron supplementation has been convincingly demonstrated?
Robert D. Baker, MD, PhD, FAAP Frank R. Greer, MD, FAAP
1. Baker RD, Greer FR and the American Academy of Pediatrics Committee on Nutrition. Clinical report diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatr, published on line Oct 5, 2010. DOI:10.1542/peds.2010-2576.
2. Friel JK, Aziz K, Andrews WL, Harding SV, Courage ML and Adams RJ. A double-masked randomized control trial of iron supplementation in early infancy in healthy term breast-fed infants. J Pediatr 2003; 143: 582-586.
3. Pizarro F, Yip R, Dallman PR, Olivares M, Hertrampf E, Walter T. Iron status with different feeding regimens: relevance to screening and prevention of iron deficiency. J Pediatr 1991; 118:687-692.
4. Calvo EB, Galindo AC, Aspres NB. Iron status of exclusively breast-fed infants. Pediatr 1992; 90:375-379.
5. Arvas A, Elgörmüs Y, Gür E, Alikaþifoðlu M, Çelebri A. Iron status in breast-fed full-term infants. Turkish J Pediatr 2000; 42:22-26.
6. Innes SM, Nelson CM, Wadsworth LD, MacLaren IA, Lwanga D. Incidence of iron deficiency anemia and depleted iron stores among nine- month old infants in Vancouver , Canada. Canada J Public Health 1997; 88:80-84.
7. Ziegler EE, Nelson SE, Jeter JM. Iron supplementation of breastfed infants from an early age. Am J Clin Nutr 2009; 89:525-532.
8. Hokama T. Levels of serum ferritin and total body iron among infants with different feeding regimens. Acta Pediatr Japn 1993; 35:298- 301.
9. Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. New Engl J Med. 1991; 325:687-694.
10. Lozoff B, Jimenez E, Hagan J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatr 2000; 105(4). Available at: www.pediatrics.org/cgi/content/full/105/4/e51
11. Lozoff B, De Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and delvelopmental effects of preventing iron-deficiency anemia in healthy full-term infants [Published correction appears in Pediatr 2004; 113(6):1853]. Pediatr 2003; 112(4): 846-854.
12. Logan S, Martins S, Gilbert R. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anemia. Cochrane Database Syst Rev. 2001;(2):CD001444.
13. McCann JC, Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr. 2007;85(4):931-945
14. Dewey KG, Domellöf M, Cohen RJ, Landa Rivera R, Hernell O, Lönnerdal B. Iron supplementation effects growth and morbidity of breast- fed infants: results of a randomized trial in Sweden and Honduras. J Nutr. 2002;132(11):3249-3255.
15. Domellöf M, Lönnerdahl B, Abrams SA, Hernell O. Iron absoption in breast-fed infants: effect of age, iron status, iron supplements, and complementary foods. Am J Clin Nutr 2002; 76: 198-204.
16. Lozoff B. Do breast-fed benefit from iron before 6 months? J Pediatr 2003; 143:554-556.
Recommendations on iron questioned
We read with interest the recently published Clinical Report – Diagnosis and Prevention of Iron Deficiency and Iron-Deficiency Anemia in Infants and Young Children (0-3 Years of Age) by RD Baker, FR Greer and the Committee on Nutrition of the American Academy of Pediatrics (1), but were astonished to find that the authors recommend changing the recommendation on provision of iron, now to include all breastfed infants, based on one (1) clinical study and at the same time ignoring clinical studies suggesting adverse effects of this practice. This is especially surprising as they in their introductory part emphasize the need for larger studies and systematic reviews for evaluating the potential correlation between iron deficiency anemia (IDA) and iron deficiency (ID) and neurodevelopment, and conclude that “an unequivocal relationship between IDA and ID and neurodevelopmental outcomes has yet to be established”. In the study by Friel et al (2), which is the basis for the new recommendations, term breastfed infants were randomly selected to receive either 7.5 mg/day of elemental iron as ferrous sulfate or placebo from one month (study entry) to 6 months of age and anthropometry and hematological indexes were evaluated at entry and at 3.5, 6 and 12 months of age. In addition, mental and psychomotor developmental indexes (MDI and PDI) were assessed by the Bayley scales and visual acuity assessed by Teller cards at 12 to 18 months of age. One problem, which the authors acknowledge, is that the study was underpowered, partially due to the low initial breastfeeding rate in the population studied, and partially due to the high dropout rate. In fact, the authors´ power calculation arrived to the conclusion that 100 infants would be needed in each group to detect a 5 percent difference in MDI and PDI, but at 12 months of age only 26 and 20 infants, respectively, were available for intention-to-treat analyses, and only 24 and 17 had received iron for more than 30 days of the intended 150 days. There was a trend toward improved visual acuity with iron supplementation, which became significant only when excluding non- compliers. It is questionable whether an effect on visual acuity as measured by Teller cards can be based on 17 and 23 infants at a mean age of 13 months (12-18 months) (3,4). Power calculations for anthropometry and hematological indexes were not reported, but it is generally agreed that for anthropometry considerably larger sample sizes are needed. From what we have learned during the last decades on the association of the intakes of docosahexaenoic acid (DHA) and neurodevelopment interpretations of the effect of single nutrients based on underpowered studies warrant caution (3-5). Friel et al found a difference of 7 points in PDI and mean values for both groups were within the normal range. The new recommendations is to give iron supplements, 1 mg/kg/day, to all breastfed infants (if breast milk constitute more than half of their daily feedings) from 4 months of age until appropriate iron-containing complementary foods are introduced into the diet. This is based on the observation by Friel et al that infants in the intervention group had significantly higher hemoglobin (Hb) concentration and mean corpuscular volume (MCV) value than the infants in the placebo group at 6 months of age, based on 28 and 21 infants, respectively (2). Iron supplements prevented the decrease in Hb concentration seen in breastfed infants not given supplementary iron and reduced the decrease in serum ferritin level. In our studies comparing various levels of iron content in infant formulas we found no difference in Hb concentration reflecting the difference in iron intake, and more iron in the formula did not prevent the decrease in Hb concentration between 1 and 6 months of age (6-7). In our study on the effects of giving exclusively breastfed infants iron supplements as iron drops we found that giving supplements between 4 and 6 months increased Hb (8). Thus, it seems that giving iron as supplements affects Hb differently from giving more iron as fortification in formulas (9). Our interpretation is that increased Hb does not necessarily reflect previous ID or IDA but the effect may rather reflect immature metabolism of surplus iron. When we compared Honduran infants (with initially lower iron status) with Swedish infants (with satisfactory iron status) the change in Hb between 4 and 6 months was very similar (+ 5g/L in both groups), emphasizing that Hb increases with iron supplementation regardless of initial iron status. Thus, the suggestion by the authors to use Hb response to iron supplementation as a diagnostic tool for IDA (1) is in our view highly questionable in that age group. It should be noted that in the study by Friel et al (2) there was a slower decrease in serum ferritin in the intervention group, but the values continued to decrease until 12 months of age, with no difference between the groups at that age, also reflecting that supplemental iron between 1 and 6 months did not affect iron stores at 12 months, supporting our observation that supplemental iron in contrast to fortification iron is not incorporated into ferritin during the first half of infancy (9). These shifts in associations between dietary iron intake, and Hb and serum ferritin, respectively may be due to developmental changes in the channeling of dietary iron to erythropoiesis relative to storage, in the absence of IDA (10). We find it notable that the authors do not clearly discriminate between iron fortification, i.e. iron content in infant formulas, and iron supplementation, i.e. medicinal iron given as iron drops, when discussing potential adverse effects of high iron intakes. We have shown that iron supplements to iron-replete Swedish breastfed infants at the same level as now recommended in the clinical report had significant negative effect on linear growth. In contrast, there was no obvious effect on growth in the Honduran cohort of the same study. However, when the latter infants were divided into iron deficient and iron-replete infants, a negative effect was seen in the iron-replete subgroup (11). This most likely explains why this adverse effect has not been noted in more studies as most populations studied include a significant proportion of iron deficient infants and/or children, thus obscuring a negative effect on iron- replete infants. In fact, when initial infant iron status has been measured and groups have been studied separately with regard to outcome an adverse effect has been noticed in several studies (12-14). While these studies were performed in developing countries, it is interesting that a recent study by Ziegler et al in which the effect of medicinal iron and iron fortified cereals between four and nine months of age was evaluated (i.e. a design similar to ours), a significant reduction in length gain and a trend towards reduced weight gain was noted (15). Our suggestion that iron in fortified foods is handled differently from medicinal iron and that this needs to be taken into account when recommendations are given (9) is thus in agreement with the study by Ziegler et al (15) who observed the adverse effect in the infants given medicinal iron, but not in the group given fortified cereals. While we agree with the authors that few adverse effects have been noted for “high” iron fortified infant formulas (12 mg/L), we still believe that this level is unnecessarily high and some caution is warranted. This is also the position taken by the ESPGHAN coordinated international expert group on a global standard for the composition of infant formulas (16). Although iron may be better absorbed from breast milk than from infant formula it seems unreasonable that infant formula should contain approximately 4,000 % more iron than the average concentration in breast milk! Several studies have shown similar iron status in infants receiving infant formulas containing 4 or 7-8 mg iron/L, and in fact, we have shown similar iron status in infants fed formula containing 1.8 mg iron/L up till 6 months of age (7). Iron is a known pro-oxidant and having a high luminal concentration of iron may not be beneficial, although the adverse consequences may not be immediately apparent. Infant formula containing high level of iron has been shown to be less protective against oxidative stress than breast milk in vitro (17), but clinical studies on this are scarce. Although we believe that the risk of adverse effects is lower with iron fortification than with medicinal iron, a recent study by Lozoff et al. suggests a long term negative effect of high iron formula on neurodevelopment (18). We also find it surprising that Baker and Greer do not discuss the problem of diagnosing ID and IDA during infancy when iron metabolism obviously is in dynamic change, i.e. if the same cut-offs for Hb and serum ferritin should be used to define ID and IDA throughout infancy. We have suggested that this may not be the case (19). Nor do they discuss particular risk groups for ID among the population of term breastfed infants, e.g. those with birth weight between 2,500 and 3,000 g (20). Friel and collaborators concluded from their study: “A larger study that focuses on the long-term developmental outcomes is needed before recommendations can be considered regarding the whole population of breast -fed infants”. We are surprised that Baker and Greer on behalf of the Committee on Nutrition of the American Academy of Pediatrics (1) reached a different conclusion. Neither do we believe that recommending iron supplements to the population of breastfed infants at large is appropriate, nor that an iron fortification level of infant formulas as high as 12 mg/L is necessary. In both cases we find the lack of an evidenced-based approach remarkable, particularly as these recommendations will be used for US infants in general.
Olle Hernell, MD, PhD Bo Lönnerdal PhD Department of Clinical Sciences/Pediatrics Department of Nutrition Umeå University University of California Sweden Davis, CA, USA
References 1. Baker RD, Greer FR, and the Committee of Nutrition of the American Academy of Pediatrics. Clinical Report – Diagnosis and prevention of iron deficiency and iron deficiency anemia in infants and young children (0-3 years of age). Pediatrics 2010;126(5):1-11 2. Friel JK, Aziz K, Andrews WL, Harding SV, Courage ML, Adams RJ. A double-masked, randomized controlled trial of iron supplementation in early infancy in healthy term breast-fed infants. J Pediatr 2003;143:582-6 3. SanGiovannia JP, Catherine S. Berkey CS, Dwyer JT, Colditz GA. Dietary essential fatty acids, long-chain polyunsaturated fatty acids, and visual resolution acuity in healthy fullterm infants: a systematic review. Early Hum Dev. 2000;57(3):165-88 4. Simmer K, Patole SK, Rao SC. Longchain polyunsaturated fatty acid supplementation in infants born at term. Cochrane Database Syst Rev. 2008 Jan 23;(1):CD000376 5. Beyerlein A, Hadders-Algra M, Kennedy K, Fewtrell M, Singhal A, Rosenfeld E, Lucas A, Bouwstra H, Koletzko B, von Kries R. Infant formula supplementation with long-chain polyunsaturated fatty acids has no effect on bayley developmental scores at 18 months of age—IPD meta-analysis of 4 large clinical trials. J Pediatr Gastroenterol Nutr 2010;50(1):79-84 6. Lönnerdal B, Hernell O. Iron, zinc, copper and selenium status of breast-fed infants and infants fed trace element fortified milk-based infant formula. Acta Paediatr 1994;83(4):367-73 7. Hernell O, Lönnerdal B. Iron status of infants fed low-iron formula: no effect of added bovine lactoferrin or nucleotides. Am J Clin Nutr 2002;76(4):858-64 8. Domellöf M, Cohen RJ, Dewey KG, Hernell O, Rivera LL, Lönnerdal B. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J Pediatr 2001;138(5):679-87 9. Domellöf M, Lind T, Lönnerdal B, Persson LA, Dewey KG, Hernell O. Effects of mode of oral iron administration on serum ferritin and haemoglobin in infants. Acta Paediatr 2008;97(8):1055-60 10. Lind T, Hernell O, Lönnerdal B, Stenlund H, Domellöf M, Persson LA. Dietary iron intake is positively associated with hemoglobin concentration during infancy but not during the second year of life. J Nutr 2004;134(5):1064-70 11. Dewey KG, Domellöf M, Cohen RJ, Landa Rivera L, Hernell O, Lönnerdal B. Iron supplementation affects growth and morbidity of breast-fed infants: results of a randomized trial in Sweden and Honduras. J Nutr 2002;132(11):3249-55 12. Idjradinata P, Watkins WE, Pollitt E. Adverse effect of iron supplementation on weight gain of iron-replete young children. Lancet. 1994;343:1252-4 13. Majumdar I, Paul P, Talib VH, Ranga S. The effect of iron therapy on the growth of iron-replete and iron- deplete children. J Trop Pediatr. 2003;49:84-8 14. Lind T, Seswandhana R, Persson LA, Lönnerdal B. Iron supplementation of iron-replete Indonesian infants is associated with reduced weight-for- age. Acta Paediatr 2008;97:770-5 15. Ziegler EE, Nelson SE, Jeter JM. Iron status of breastfed infants is improved equally by medicinal iron and iron-fortified cereal. Am J Clin Nutr 2009;90(1):76-87 16. Koletzko B, Baker S, Cleghorn G, Neto UF, Gopalan S, Hernell O, Hock QS, Jirapinyo P, Lonnerdal B, Pencharz P, Pzyrembel H, Ramirez-Mayans J, Shamir R, Turck D, Yamashiro Y, Zong-Yi D. Global standard for the composition of infant formula: recommendations of an ESPGHAN coordinated international expert group. J Pediatr Gastroenterol Nutr 2005;41(5):584-99 17. Friel JK, Martin SM, Langdon M, Herzberg GR, Buettner GR. Milk from mothers of both premature and full-term infants provides better antioxidant protection than does infant formula. Pediatr Res 2002;51(5):612-8 18. Lozoff B, Castillo M, Smith JB. Poorer developmental outcome with 12 mg/L iron-fortified formula in infancy. Abstract 2225, Pediatric Academic Societies Annual meeting, Honolulu, HI, 2008, EPASS2008: 635340.2 19. Domellöf M, Dewey KG, Lönnerdal B, Cohen RJ, Hernell O. The diagnostic criteria for iron deficiency in infants should be reevaluated. J Nutr. 2002;132:3680-6 20. Yang Z, Lönnerdal B, Adu-Afarwuah S, Brown KH, Chaparro CM, Cohen RJ, Domellöf, M, Hernell O, Lartey A Dewey KG. Prevalence and predictors of iron deficiency in fully breastfed infants at 6 mo of age: comparison of data from 6 studies. Am J Clin Nutr 2009;89:1433–40
Red meats for dietary iron: a concerning AAP recommendation
As a family physician, nutrition enthusiast, public-health researcher, and parent of a toddler, I read with great interest the AAP clinical report on the diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children.1 The report addresses two important deficiency states and, in general, gives sound clinical advice based on the best available evidence.
Where the report raises concern is in its statements about red meat. For instance the authors talk about “heme sources of iron (ie, red meat) …”. Red meat is certainly a source of heme iron, and if one allows that “ie” is a typo for “eg”, than the reader has no cause for concern. However, later in the article, summary recommendations suggest reader concern is actually warranted (the authors seem unduly biased in favor of red meat over other heme—and over non-heme—sources of iron). For instance, recommendations state that for 1 to 3 year olds, sufficient iron would “best be delivered by eating red meats, cereals fortified with iron, vegetables that contain iron, and fruits with vitamin C”.
“Best be delivered by eating red meats”? (And red meats as the first and most prominently featured source of iron?) Data from the report’s own Table 3 (Foods to Increase Iron Intake and Iron Absorption) suggests why red meats should not be listed first, or considered best. In fact, the elemental iron available in red meats pales in comparison to that from shellfish. While there may be arguments to avoid excessive shellfish consumption in toddlers,2 the elemental iron in red meats also pales in comparison to that from legumes (eg, soybeans/tofu and lentils). It is, of course, possible that the authors lump “legumes” with other “vegetables that contain iron” in their recommendation, and further consider non-heme (ie, plant-derived) iron sources inferior to heme (ie, animal-derived) sources due to absorbability. Yet while non-heme iron itself may be less absorbable, heme sources of iron may be far less preferable for other reasons.
Particularly less-preferable is red meat. One need only consider direct implications for health (eg, food safety3, chronic-disease risk4, 5, and potential early mortality6) to understand why—although other implications for overall community and world wellness are also important (eg, human rights considerations7, environmental justice issues8, and the impact on climate change9). Given that dietary behaviors develop throughout childhood,10 why would AAP recommend starting children down a carnivorous path towards potentially poor personal, public, and planetary health?
It would appear that recommendations for red meat in the AAP iron- deficiency statement represent either a misreading of the facts, unsupported personal biases, or insidious industry influence. The report’s conflict-of-interest disclosure reassures against the third possibility, but neither of the two remaining options is comforting. When the health of children is at stake, only the soundest dietary guidance will do. In the case of iron, and diet in general, I would submit that AAP consider modifying its recommendations to be consistent with general advice for healthful eating: e.g. “For adequate iron and good nutrition, focus mostly on eating legumes and other vegetables, whole grains, and fruit. If you choose to consume animal products, choose fish and shellfish over other meats, particularly red meat”.
No conflicts of interest to disclose
Sean C. Lucan, MD, MPH, MS Department of Family and Social Medicine Montefiore Medical Center Albert Einstein College of Medicine 1300 Morris Park Ave Mazer Building, Room 410 Bronx, NY 10461 Tel: (718) 430-3667 Fax: (718) 430-8645
References:
1. Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics. 2010;126(5):1040-1050.
2. United States Food and Drug Administration. What You Need to Know About Mercury in Fish and Shellfish: 2004 EPA and FDA Advice For Women Who Might Become Pregnant, Women Who are Pregnant, Nursing Mothers, Young Children. http://www.fda.gov/food/foodsafety/product- specificinformation/seafood/foodbornepathogenscontaminants/methylmercury/ucm115662.htm. Published 2004. Accessed November 15, 2010.
3. Price L. Prevalence of high-priority antibiotic resistant bacteria in the US food supply. American Public Health Association 138th Annual Meeting and Expo. http://apha.confex.com/apha/138am/webprogram/Paper232107.html. Published 2010.
4. Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major Dietary Protein Sources and Risk of Coronary Heart Disease in Women. Circulation. 2010.
5. Key TJ, Schatzkin A, Willett WC, Allen NE, Spencer EA, Travis RC. Diet, nutrition and the prevention of cancer. Public Health Nutr. 2004;7(1A):187-200.
6. Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010;153(5):289-298.
7. Human Rights Watch. Blood, Sweat, and Fear: Workers’ Rights in U.S. Meat and Poultry Plants. http://www.hrw.org/en/reports/2005/01/24/blood-sweat-and-fear-0. Published 2004.
8. Wenz PS. Environmental justice. Albany: State University of New York Press; 1988.
9. Walsh B. Meat: Making Global Warming Worse. Time magazine Wednesday, September 10, 2008.
10. Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101(3 Pt 2):539-549.
Exclusively breastfed infants: iron recommendations are premature
Dear Editor,
The new AAP recommendations for prevention of iron deficiency and iron deficiency anemia conclude that “exclusively breastfed term infants [should] receive an iron supplement of 1 mg/kg/day starting at 4 months of age.” (1) Prevention of iron deficiency and iron deficiency anemia are important public health goals. But while late preterm and low birth weight infants and other infants with risk for low iron stores may benefit from iron supplementation, this recommendation is controversial with respect to healthy full term infants with birth weight over 2500 grams, who represent the largest proportion of exclusively breastfeeding infants in the US.
The study referenced in the AAP recommendation included 77 full term infants randomized to 7.5 mg of iron daily or placebo from one to six months of age. (2) By 6 months of age, 26 infants (34%) had dropped out of the study, 15 (19%) were noncompliant with iron/placebo treatment, and most in both groups were receiving formula (i.e. not exclusively breastfeeding), with all but 3 infants also receiving cereal. Although the study initially was powered to evaluate development, enrollment stopped prior to the full number needed, and so the results regarding neurodevelopmental outcomes are interesting but not conclusive or scientifically defensible. This particular data set is not ideal for support of a significant AAP policy recommendation.
A more recent study enrolled 75 full term infants and supplemented 37 with 7 mg/day of iron and 38 with placebo from age 1 to 5.5 months; 63 infants completed the intervention (16% drop out rate).(3) Two infants (6%) in the placebo group were iron deficient by 5.5 months. The study was not powered to test the hypothesis that iron supplementation prevents iron deficiency, and the effect of treatment on iron stores was “modest” and did not extend beyond the period of supplementation.
Finally, among exclusively breastfed Swedish infants at 6 months of age, there was no statistically significant difference between those who had received iron from 4 months of age and those who had received placebo in measures of iron sufficiency including MCV, serum ferritin, zinc protoporphyrin or transferrin receptors.(4) Although iron supplementation increased hemoglobin levels, it did not decrease the already low prevalence of iron deficiency or iron deficiency anemia (2.9%) observed in these infants. In this study, infants over 6 months of age with lower body iron stores absorbed more iron than iron-replete peers, while iron absorption among infants 4-6 months of age was not apparently related to the sufficiency of iron stores, suggesting that self-regulatory mechanisms develop in later infancy. (4) Thus it appears that 4-6 month olds will absorb the additional iron they receive whether they need it or not.
There is very limited information about the effect of giving iron to iron-replete infants. A negative effect of iron supplementation on linear growth among iron-replete infants has been suggested though not confirmed. (5) Whether saturation of lactoferrin due to iron supplementation could diminish its immunomodulatory functions and increase the risk of infection among exclusively breastfed infants has not been studied. Finally, although biological plausibility certainly exists with regards to a hypothesized relationship between iron deficiency and neurodevelopmental outcome, evidence among infants below the age of 2 years is extremely limited, and studies are handicapped by small sample size and lack of randomization.(6 )
The great majority of healthy full term exclusively breastfed infants with birth weights over 2500 grams in the US do not have iron deficiency by 6 months of age. These infants do not need iron, and it is not known whether iron supplementation negatively affects growth (or risk of infection). There is also not yet evidence that iron supplementation as recommended improves developmental outcome among the minority of infants who are iron deficient and could theoretically benefit from iron.
The new recommendation will generate a great deal of prescribing. It may generate additional testing of iron status and hemoglobin as parents will appropriately ask if their infant is “OK”. And while infants in studies are treated to cherry flavored and palatable iron suspensions, insurance-covered iron drops are not as tasty, and parents and infants may object to the drops. Although none of these latter factors is a “deal breaker”, each should be considered.
The AAP has PROS (Pediatric Research in Office Settings), a large network of practices that collaborate in performing office-based research. Why not harness this resource to study the question of whether iron supplementation, as compared to non-supplementation, from 4-6 months of age among exclusively breastfed infants with birth weight >2500 grams (1) prevents iron deficiency, and (2) has a beneficial effect on developmental outcome, and (3) is at least neutral with respect to growth and risk of infection. This seems to be a golden opportunity to make sure that recommendations are based on evidence gathered in studies with an optimal sample size, powered to answer the question(s) at hand.
I recognize that a great deal of hard work and thought has gone into these current recommendations, and that they utilize the best available information well considered by experts. However, I believe it would be preferable to do the best study possible, and then make a recommendation based on that evidence.
Thank you for your consideration,
Lydia Furman, M.D.
2. Freil JK, Aziz K, Andrews WL, Harding SV, Courage ML and Adams RJ. A double-masked randomized control trial of iron supplementation in early infancy in healthy term breast-fed infants. J Pediatr 2003; 143: 582-586.
3. Ziegler EE, Nelson SE, and Jeter JM. Iron supplementation of breastfed infants from an early age. Am J Clin Nutr 2009; 89: 525-532.
4. Domellöf M, Cohen RJ, Dewey KG, Hernell O, Rivera LL and Lönnerdal B. Iron supplementation of breast-fed Honduran and Swedish infants from 4 to 9 months of age. J Pediatr 2001;138: 679–687. 5. Dewey KG, Magnus Domellő M, Cohen RJ, Rivera LL, Hernell H and Lőnnerdal B. Iron Supplementation Affects Growth and Morbidity of Breast-Fed Infants: Results of a Randomized Trial in Sweden and Honduras J. Nutr. 132: 3249–3255, 2002.
6. McCann JC and Ames BN. An overview of evidence for a causal relation between iron deficiency during development and deficits in cognitive or behavioral function. Am J Clin Nutr 2007; 85: 931–945.
Concerns with early universal iron supplementation of breastfeeding infants
October 27, 2010
RE: Concerns with early universal iron supplementation of breastfeeding infants
We have major concerns about universal iron supplementation at 4 months in breastfeeding infants, reported by Drs Baker and Greer, “Clinical Report-Diagnosis and Prevention of Iron Deficiency and Iron- Deficiency Anemia in Infants and Young Children (0-3 Years of Age).”
We point out that as a clinical recommendation for millions of infants, supplementary iron drops beginning at 4 months of age is inconsistent with previous recommendations of the American Academy of Pediatrics.1-3 The only supportive data for this recommendation comes from 1 study where 77 breastfed term newborns were supplemented with iron at some time between 1 and 6 months of age.4 Follow-up studies found ‘improved’ psychomotor but not cognitive development at 13 months. It has been pointed out that this outcome is unusual and the 13 month exam is not necessarily predictive of overall developmental outcome.5
We would like the authors to acknowledge other ways to ensure that breastfeeding infants have adequate iron status. We suggest that delayed cord clamping at birth be included in their recommendations and that screening of ‘at risk’ infants be used as a guide to determine iron supplementation before 6 months.1, 6
The Clinical Report does not address potential harms of supplementation nor does it discuss the difference in bioavailability of iron contained in human milk vs. iron-fortified fluids and foods. Given that research has shown potential harm in infant growth and morbidity when iron supplementation is provided to iron-sufficient infants one wonders if universal iron supplementation will be deleterious to the population of developing infants who are breastfeeding exclusively.7
Furthermore, in a relatively recent US study the prevalence of iron deficiency anemia is low (3%) among unsupplemented breastfed infants in the first 6 mo.8
Lastly, the authors acknowledge that this report was submitted for review to the Section on Breastfeeding of the American Academy of Pediatrics. It did not mention that we disagreed and provided our additional recommendations, 2 years ago. The manuscript infers that the Section, along with many other groups, endorsed this report. This is wrong and will mislead the medical community.
We would welcome a discussion of science and changes in recommendations that are evidence-based. We do not have issues with screening at risk populations. We further request that the section “Development of this Report,” be retracted and removed from publication.
Richard J. Schanler, MD, FAAP Chairperson, AAP Section on Breastfeeding
Section Executive Committee:
Lori Feldman-Winter, MD, FAAP Camden, NJ Susan Landers, MD, FAAP Austin, TX Lawrence Noble, MD, FAAP Elmhurst, NY Kinga Szucs, MD, FAAP Carmel, IN Laura Viehmann, MD, FAAP Cumberland, RI
1. American Academy of Pediatrics, Section on Breastfeeding. Breastfeeding and the Use of Human Milk. Pediatrics 2005; 115:496-506.
2. American Academy of Pediatrics, American College of Obstetricians and Gynecologists. Breastfeeding Handbook for Physicians. Elk Grove Village, IL: American Academy of Pediatrics; 2006.
3. American Academy of Pediatrics. Pediatric Nutrition Handbook. Pediatric Nutrition Handbook 2009.
4. Friel JK, Aziz A, Andrews WL, Harding SV, Courage ML, Adams RJ. A double-masked, randomized control trial of iron supplementation in early infancy in healthy term breast-fed infants. J Pediatr 2003; 143:582-6.
5. Lozoff B. Do breast-fed babies benefit from iron before 6 months? J Pediatr 2003; 143:554-6.
6. Hutton EK, Hassan ES. Late vs early clamping of the umbilical cord in full term neonates: systemic review and meta-analysis. JAMA 2007; 297:1241-52.
7. Dewey KG, Domellöf M, Cohen RJ, Landa Rivera L, Hernell O, Lonnerdal B. Iron supplementation affects growth and morbidity of breast- fed infants: results of a randomized trial in Sweden and Honduras. J Nutr 2002; 132:3249-55.
8. Ziegler EE, Nelson SE, Jeter JM. Iron supplementation of breastfed infants from an early age. Am J Clin Nutr 2009; 89:525-32.
Advertising Disclaimer »
Citing articles via
Email alerts.

Affiliations
- Editorial Board
- Editorial Policies
- Journal Blogs
- Pediatrics On Call
- Online ISSN 1098-4275
- Print ISSN 0031-4005
- Pediatrics Open Science
- Hospital Pediatrics
- Pediatrics in Review
- AAP Grand Rounds
- Latest News
- Pediatric Care Online
- Red Book Online
- Pediatric Patient Education
- AAP Toolkits
- AAP Pediatric Coding Newsletter
First 1,000 Days Knowledge Center
Institutions/librarians, group practices, licensing/permissions, integrations, advertising.
- Privacy Statement | Accessibility Statement | Terms of Use | Support Center | Contact Us
- © Copyright American Academy of Pediatrics
This Feature Is Available To Subscribers Only
Sign In or Create an Account
- Publications
- Conferences & Events
- Professional Learning
- Science Standards
- Awards & Competitions
- Instructional Materials
- Free Resources
- American Rescue Plan
- For Preservice Teachers
- NCCSTS Case Collection
- Partner Jobs in Education
- Interactive eBooks+
- Digital Catalog
- Regional Product Representatives
- e-Newsletters
- Bestselling Books
- Latest Books
- Popular Book Series
- Prospective Authors
- Web Seminars
- Exhibits & Sponsorship
- Conference Reviewers
- National Conference • Denver 24
- Leaders Institute 2024
- National Conference • New Orleans 24
- Submit a Proposal
- Latest Resources
- Professional Learning Units & Courses
- For Districts
- Online Course Providers
- Schools & Districts
- College Professors & Students
- The Standards
- Teachers and Admin
- eCYBERMISSION
- Toshiba/NSTA ExploraVision
- Junior Science & Humanities Symposium
- Teaching Awards
- Climate Change
- Earth & Space Science
- New Science Teachers
- Early Childhood
- Middle School
- High School
- Postsecondary
- Informal Education
- Journal Articles
- Lesson Plans
- e-newsletters
- Science & Children
- Science Scope
- The Science Teacher
- Journal of College Sci. Teaching
- Connected Science Learning
- NSTA Reports
- Next-Gen Navigator
- Science Update
- Teacher Tip Tuesday
- Trans. Sci. Learning
MyNSTA Community
- My Collections
A Case of Iron Deficiency Anemia
By David F. Dean (rr)
Share Start a Discussion

“Dolores Welborn,” a 28-year-old attorney, is pregnant with her first child. Lately she has been tiring easily and is often short of breath. She has also had periods of light-headedness, cramping in her legs, and a sore tongue. Students read a brief clinical history and a description of signs and symptoms, then answer a set of directed questions designed to probe the underlying anatomy, physiology, and pathology of the Dolores's condition. In the process, they learn about the human hemolymphatic system. The case has been used in a sophomore-level course in human anatomy and physiology taught to pre-med and nursing students as well as in senior-level elective course in general physiology taken primarily by pre-med students.
Download Case
Date Posted
- The structure of hemoglobin and the role played by iron in the transport of oxygen.
- The means by which iron is transported and stored in the body.
- The incidence and causes of IDA.
- The red blood cell indices and how they are used to characterize anemia.
- How IDA is prevented and treated.
Iron deficiency anemia; hemoglobin; red blood cell indices; transport of oxygen; blood oxygen; apotransferin; transferring; ferritin
Subject Headings
EDUCATIONAL LEVEL
Undergraduate lower division, Undergraduate upper division
TOPICAL AREAS
TYPE/METHODS
Teaching Notes & Answer Key
Teaching notes.
Case teaching notes are protected and access to them is limited to paid subscribed instructors. To become a paid subscriber, purchase a subscription here .
Teaching notes are intended to help teachers select and adopt a case. They typically include a summary of the case, teaching objectives, information about the intended audience, details about how the case may be taught, and a list of references and resources.
Download Notes
Answer Keys are protected and access to them is limited to paid subscribed instructors. To become a paid subscriber, purchase a subscription here .
Download Answer Key
Materials & Media
Supplemental materials, you may also like.
Web Seminar
Join us on Tuesday, June 4, 2024, from 7:00 PM to 8:30 PM ET, to learn about the free lesson plans and storyline units designed for high school studen...
Join us on Thursday, October 24, 2024, from 7:00 PM to 8:00 PM ET, to learn about all NSTA Teacher Awards available and how to apply.Did you come up w...
Join us on Thursday, September 19, 2024, from 7:00 PM to 8:00 PM ET, to learn about NSTA election process for new and interested members of the Board ...
Sorry. You need a frames capable broswer to view this page.
Iron absorption in adults with sickle cell anemia: a stable-isotope approach
- Original Contribution
- Published: 09 May 2024
Cite this article

- Juliana Omena¹ ORCID: orcid.org/0000-0003-4639-1898 1 na1 ,
- Flávia Fioruci Bezerra¹ ORCID: orcid.org/0000-0002-6594-4323 1 na1 ,
- Vanessa Monteiro Voll¹ ORCID: orcid.org/0000-0002-0939-0071 1 ,
- Bernardo Ferreira Braz 2 , 3 ,
- Ricardo Erthal Santelli ORCID: orcid.org/0000-0001-9812-6098 2 , 3 ,
- Carmen Marino Donangelo ORCID: orcid.org/0000-0002-4243-4179 4 ,
- Gustavo Federico Jauregui 5 ,
- Andrea Soares Ribeiro 6 ,
- Cláudia dos Santos Cople Rodrigues ORCID: orcid.org/0000-0001-9497-556X 1 &
- Marta Citelli ORCID: orcid.org/0000-0003-1380-3729 1
41 Accesses
Explore all metrics
Iron absorption in sickle cell anemia (SCA) remains unclear and studies in adults with SCA are scarce. The aim of this study was to evaluate the iron absorption SCA adults and its association with iron status and hepcidin concentration.
SCA patients ( n = 13; SCA total ) and control participants ( n = 10) ingested an oral stable iron isotope ( 57 Fe). Iron absorption was measured by inductively coupled plasma mass spectrometry (ICP-MS) 14 days after isotope administration. Patients with ≥ 1000 ng/mL serum ferritin were considered to present iron overload (IO) (SCAio+; n = 3) and others classified without IO (SCAio-; n = 10).
Iron absorption in the control group ranged from 0.3 to 26.5% (median = 0.9%), while it varied from 0.3 to 5.4% in SCAio+ (median = 0.5%) and from 0.3 to 64.2% in the SCAio- (median = 6.9%). Hepcidin median values were 14.1 ng/mL (3.0–31.9 ng/mL) in SCAio-, 6.2 ng/mL (3.3–7.8 ng/mL) in SCAio + and 6.2 ng/mL (0.6–9.3 ng/mL) in control. Iron absorption was associated with ferritin level ( r = − 0.641; p = 0.018) and liver iron concentration (LIC; r = − 0.786; p = 0.036) in the SCA total group.
Our data suggest that SCAio- individuals may be at risk of developing primary IO. Simultaneously, secondary IO may induce physiological adaptation, resulting in reduced iron absorption. Further studies evaluating intestinal iron absorption using larger sample sizes should be conducted to help establish a safe nutrition approach to be adopted and to ensure the security of food-fortifying public policies for these patients.
Trial registration
This trial was registered at www.ensaiosclinicos.gov.br (Identifier RBR-4b7v8pt).
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others
Iron deficiency markers in patients undergoing iron replacement therapy: a 9-year retrospective real-world evidence study using healthcare databases

Evaluation of iron stores in hemodialysis patients on maintenance ferric Carboxymaltose dosing

Monitoring oral iron therapy in children with iron deficiency anemia: an observational, prospective, multicenter study of AIEOP patients (Associazione Italiana Emato-Oncologia Pediatrica)
Data availability.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conran N, Franco-Penteado CF, Costa FF (2009) Newer aspects of the pathophysiology of sickle cell disease vaso-occlusion. Hemoglobin 33(1):1–16. https://doi.org/10.1080/03630260802625709
Article CAS PubMed Google Scholar
Rees DC, Williams TN, Gladwin MT (2010) Sickle-cell disease. Lancet 376(9757):2018–2031. https://doi.org/10.1016/S0140-6736(10)61029-X
Modell B, Darlinson M (2008) Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ 86(6):480–487. https://doi.org/10.2471/blt.06.036673
Article PubMed PubMed Central Google Scholar
Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, Krishnamurti L et al (2018) Sickle cell disease. Nat Rev Dis Primers 4:18010. https://doi.org/10.1038/nrdp.2018.10
Article PubMed Google Scholar
Harmatz P, Butensky E, Quirolo K, Williams R, Ferrell L, Moyer T et al (2000) Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood 96(1):76–79. https://doi.org/10.1182/blood.V96.1.76
Josephson CD, Su LL, Hillyer KL, Hillyer CD (2007) Transfusion in the patient with sickle cell disease: a critical review of the literature and transfusion guidelines. Transfus Med Rev 21(2):118–133. https://doi.org/10.1016/j.tmrv.2006.11.003
Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C et al (1998) Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med 339(1):5–11. https://doi.org/10.1056/NEJM199807023390102
Adams RJ, Brambilla D, Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators (2005) Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med 353(26):2769–2778. https://doi.org/10.1056/NEJMoa050460
Inati A, Khoriaty E, Mussalam KM (2011) Iron sickle-cell disease: what have we learned over the years? Pediatr Blood Cancer 56(2):182–190. https://doi.org/10.1002/pbc.22721
Thuret I (2013) Post-transfusional iron overload in the haemoglobinopathies. C R Biol 336(3):164–172. https://doi.org/10.1016/j.crvi.2012.09.010
Cohen A, Masera G, Zoumbos N, Uysal Z, Boulet D, Watman N et al (2005) Effect of iron intake on control of body iron in patients with Thalassemia major treated with Deferasirox (Exjade®, ICL670). Blood 106(11):822. https://doi.org/10.1182/blood.V106.11.822.822
Article Google Scholar
Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Capellini MD et al (2008) Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol 80(2):168–176. https://doi.org/10.1111/j.1600-0609.2007.00985.x
Article CAS PubMed PubMed Central Google Scholar
Hoffbrand AV, Taher A, Cappellini MD (2012) How I treat transfusional iron overload. Blood 120(18):3657–3669. https://doi.org/10.1182/blood-2012-05-370098
Ward R (2010) An update on disordered iron metabolism and iron overload. Hematology 15(5):311–317. https://doi.org/10.1179/102453310X12647083621164
Ganz T, Nemeth E (2012) Hepcidin and iron homeostasis. Biochim Biophys Acta Mol Cell Res 1823(9):1434–1443. https://doi.org/10.1016/j.bbamcr.2012.01.014
Article CAS Google Scholar
Muckenthaler MU, Rivella S, Hentze MW, Galy B (2017) A Red Carpet for Iron Metabolism. Cell 168(3):344–361. https://doi.org/10.1016/j.cell.2016.12.034
Ganz T, Nemeth E (2006) Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol 290(2):G199–203. https://doi.org/10.1152/ajpgi.00412.2005
Lynch S, Pfeiffer CM, Georgieff MK, Brittenham G, Fairweather-Tait S, Hurrell RF, McArdle HJ, Raiten DJ (2018) Biomarkers of Nutrition for Development (BOND)-Iron review. J Nutr 148(suppl1):1001S–1067S. https://doi.org/10.1093/jn/nxx036
Nemeth E (2008) Iron regulation and erythropoiesis. Curr Opin Hematol 15(3):169–175. https://doi.org/10.1097/MOH.0b013e3282f73335
Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T (2003) Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101(7):2461–2463. https://doi.org/10.1182/blood-2002-10-3235
Camaschella C, Nai A, Silvestri L (2020) Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 105(2):260–272. https://doi.org/10.3324/haematol.2019.232124
Omena J, Cople-Rodrigues CS, Cardoso JDA, Soares AR, Fleury MK, Brito FSB et al (2018) Serum hepcidin concentration in individuals with sickle cell anemia: basis for the dietary recommendation of iron. Nutrients 10(4):498. https://doi.org/10.3390/nu10040498
Mangaonkar AA, Thawer F, Son J, Ajebo G, Xu H, Barrett NJ et al (2020) Regulation of iron homeostasis through the erythroferrone-hepcidin axis in sickle cell disease. Br J Haematol 189(6):1204–1209. https://doi.org/10.1111/bjh.16498
Ringelhann B, Konotey-Ahulu F, Dodu SR (1970) Studies on iron metabolism in sickle cell anaemia, sickle cell haemoglobin C disease, and haemoglobin C disease using a large volume liquid scintillation counter. J Clin Pathol 23(2):127–134. https://doi.org/10.1136/jcp.23.2.127
Borges R, Mancuso A, Camey S, Leotti V, Hirakata V, Azambuja G, Castro S (2021) Power and Sample Size for Health Researchers a tool for calculating sample size and statistical power designed for health researchers. Clin Biomed Res 40(4). https://doi.org/10.22491/2357-9730.109542
Porter J, Garbowski M (2013) Consequences and management of iron overload in sickle cell disease. Hematol Am Soc Hematol Educ Program 2013:447–456. https://doi.org/10.1182/asheducation-2013.1.447
Walczyk T, von Blanckenburg F (2002) Natural iron isotope variations in human blood. Science 295(5562):2065–2066. https://doi.org/10.1126/science.1069389
Abrams SA (1999) Using stable isotopes to assess mineral absorption and utilization by children. Am J Clin Nutr 70(6):955–964. https://doi.org/10.1093/ajcn/70.6.955
van den Heuvel EG, Muys T, Pellegrom H, Bruyntjes JP, van Dokkum W, Spanhaak S, Schaafsma G (1998) A new method to measure iron absorption from the enrichment of 57Fe and 58Fe in young erythroid cells. Clin Chem 44(3):649–654
Fidler MC, Davidsson L, Zeder C, Walczyk T, Hurrell RF (2003) Iron absorption from ferrous fumarate in adult women is influenced by ascorbic acid but not by Na2EDTA. Br J Nutr 90(6):1081–1085. https://doi.org/10.1079/bjn2003995
Junqueira-Franco MVM, Dutra de Oliveira JE, Nutti MR, Pereira HS, Carvalho JLV, Abrams SA et al (2018) Iron absorption from beans with different contents of iron, evaluated by stable isotopes. Clin Nutr ESPEN 25:121–125. https://doi.org/10.1016/j.clnesp.2018.03.120
International Atomic Energy Agency (IAEA) (2012) Assessment of iron bioavailability in humans using stable iron isotope techniques. Vienna: International Atomic Energy Agency 77 p. No.: 21
Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF (1994) A double stable isotope technique for measuring iron absorption in infants. Br J Nutr 7(3):411–424. https://doi.org/10.1079/bjn19940148
Chen Z, Griffin IJ, Plumlee LM, Abrams SA (2005) High resolution inductively coupled plasma mass spectrometry allows rapid assessment of iron absorption in infants and children. J Nutr 135(7):1790–1795. https://doi.org/10.1093/jn/135.7.1790
Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C et al (2000) Hepatic iron concentration and total body iron stores in Thalassemia major. N Engl J Med 343(5):327–331. https://doi.org/10.1056/NEJM200008033430503
St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK et al (2005) Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105(2):855–861. https://doi.org/10.1182/blood-2004-01-0177
Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA et al (2009) R2* magnetic resonance imaging of the liver in patients with iron overload. Blood 113(20):4853–4855. https://doi.org/10.1182/blood-2008-12-191643
Labranche R, Gilbert G, Cerny M, Vu KN, Soulières D, Olivié D et al (2018) Liver iron quantification with MR imaging: a primer for radiologists. Radiographics 38(2):392–412. https://doi.org/10.1148/rg.2018170079
Institute of Medicine (US) Panel on Micronutrients (2001) Dietary reference intakes for. In: Vitamin A, Vitamin K (eds) Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press (US)
Teixeira TV, Da Silva ACF, Rodrigues CDSC, Brito FDSB, Canella DS, Citelli M (2023) Food Consumption of people with Sickle Cell Anemia in a Middle-Income Country. Nutrients15(6):1478. https://doi.org/10.3390/nu15061478
Li H, Kazmi JS, Lee S, Zhang D, Gao X, Maryanovich M et al (2023) Dietary iron restriction protects against vaso-occlusion and organ damage in murine sickle cell disease. Blood 141(2):194–199. https://doi.org/10.1182/blood.2022016218
Sarria B, Dainty JR (2010) Comparison of faecal monitoring and area under the curve techniques to determine iron absorption in humans using stable isotope labelling. J Trace Elem Med Biol 24(3):157–160. https://doi.org/10.1016/j.jtemb.2010.01.010
Sudarev VV, Dolotova SM, Bukhalovich SM, Bazhenov SV, Ryzhykau YL, Uversky VN et al (2023) Ferritin self-assembly, structure, function, and biotechnological applications. Int J Biol Macromol 224:319–343. https://doi.org/10.1016/j.ijbiomac.2022.10.126
Cook JD, Lipschitz DA, Miles LEM, Finch CA (1974) Serum ferritin as a measure of iron stores in normal subjects. Am J Clin Nutr 27(7):681–687. https://doi.org/10.1093/ajcn/27.7.681
Lynch SR, Skikne BS, Cook JD (1989) Food iron absorption in idiopathic hemochromatosis. Blood 74(6):2187–2193
Roe MA, Collings R, Dainty JR, Swinkels DW, Fairweather-Tait SJ (2009) Plasma hepcidin concentrations significantly predict interindividual variation in iron absorption in healthy men. Am J Clin Nutr 89(4):1088–1091. https://doi.org/10.3945/ajcn.2008.27297
Zimmermann MB, Troesch B, Biebinger R, Egli I, Zeder C, Hurrell RF (2009) Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am J Clin Nutr 90(5):1280–1287. https://doi.org/10.3945/ajcn.2009.28129
Erlandson ME, Walden B, Stern G, Hilgartner MW, Wehman J, Smith CH (1962) Studies on congenital hemolytic syndromes, IV. Gastrointestinal absorption of iron. Blood 19:359–378
Download references
Acknowledgements
The authors express their gratitude to the volunteers who participated in this study. They would also like to acknowledge the excellent technical assistance provided by Isis Rodrigues, Viviane F.Meneses, Clarice M. Carvalho, Elizabeth Pereira, Verônica Barbosa, and Valdilene L. Souza.
This study was supported by the Ministry of Health (process # 777022/2012); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process # 408401/2017-6); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) (Finance Code 001); Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (process # E-26-010.100930/2018 and E-26/200.963/2022).
Author information
Flávia Fioruci Bezerra and Vanessa Monteiro Voll have contributed equally to this work.
Authors and Affiliations
Nutrition Institute, Rio de Janeiro State University, São Francisco Xavier Street, 524, 12144F, Maracanã, Rio de Janeiro, 20550-900, Brazil
Juliana Omena¹, Flávia Fioruci Bezerra¹, Vanessa Monteiro Voll¹, Cláudia dos Santos Cople Rodrigues & Marta Citelli
Institute of Chemistry, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil
Bernardo Ferreira Braz & Ricardo Erthal Santelli
National Institute of Science and Technology of Bioanalytics (INCTBio), Campinas, Brazil
School of Nutrition, University of the Republic, Montevideo, Uruguay
Carmen Marino Donangelo
Radiology Department, Pedro Ernesto University Hospital, Rio de Janeiro, Brazil
Gustavo Federico Jauregui
Hematology Department, Pedro Ernesto University Hospital, Rio de Janeiro, Brazil
Andrea Soares Ribeiro
You can also search for this author in PubMed Google Scholar
Contributions
MC, JO, FFB, and CSCR designed the research; JO, MC, and VMV conducted the research; JO, VMV, MC, BFB and RES conducted the laboratory analysis; FFB and CMD helped to interpret the data and provided critical suggestions and comments; GFJ and ASR conducted the MRI procedures; JO, FFB and MC performed the statistical analysis, wrote the manuscript, and had primary responsibility for the final content. All authors read, contributed and approved the final manuscript. None of the authors declared any personal or financial conflict of interest.
Corresponding authors
Correspondence to Juliana Omena¹ or Marta Citelli .
Ethics declarations
Ethical approval.
The study was conducted in accordance with the Declaration of Helsinki principles. The Ethical Committee of Hemorio (419/17; 2.788.659) and Pedro Ernesto University Hospital (2.695.418) approved the study protocol. Written informed consent was obtained from each participant.
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Omena¹, J., Bezerra¹, F.F., Voll¹, V.M. et al. Iron absorption in adults with sickle cell anemia: a stable-isotope approach. Eur J Nutr (2024). https://doi.org/10.1007/s00394-024-03417-8
Download citation
Received : 01 November 2023
Accepted : 22 April 2024
Published : 09 May 2024
DOI : https://doi.org/10.1007/s00394-024-03417-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Iron overload
- Liver iron concentration
- Bioavailability
- Find a journal
- Publish with us
- Track your research
Should I take an iron supplement? Here’s what the science says.
If you have iron deficiency anemia, you should take an iron supplement

I’ve heard that iron supplements may ease fatigue. Should I start taking one?
If you have iron-deficiency anemia, you should take an iron supplement. But here’s an interesting pro-tip I share with my patients: Don’t take it every day. A study found that taking an iron supplement every other day can optimize iron absorption — and may mitigate side effects such as nausea and constipation.
Iron is an essential micronutrient that helps produce healthy red blood cells. People with an iron deficiency often feel exhausted. Some may find it hard to catch their breath or notice paler skin (though this may be less obvious among those with black or brown skin tones). Iron deficiency can also lead to a racing heart, headaches and odd cravings, such as for ice, chalk or clay.
Get Well+Being tips straight to your inbox

It’s normal to lose a small amount of the body’s iron stores through the skin or during menses. But you can develop a health problem called iron-deficiency anemia (one of several forms of anemia) if you lose more than you’re absorbing through your diet. Iron-deficiency anemia is a condition in which red blood cells aren’t able to carry oxygen efficiently to the rest of the body. It is characterized by both low levels of iron and red blood cells and diagnosed with blood tests, including a complete blood count and an iron panel.
Young children, people who have heavy menses or are pregnant, and older people should be particularly vigilant. A study published in the Annals of Internal Medicine last year found that about 1 in 4 healthy adults, age 70 and older, developed anemia within five years of enrolling in the study and that taking a low-dose daily aspirin increased that risk by 20 percent.
If you are feeling tired all the time, but have not been diagnosed with iron-deficiency anemia, work with your physician to investigate other possible causes.
What causes low iron levels?
Pregnancy: During pregnancy, iron requirements multiply to support the mother and growing fetus (as if pregnant people needed another reason to feel exhausted), and so we screen for it routinely. Taking an iron supplement is safe during pregnancy and while breastfeeding.
Blood loss: Heavy bleeding during menses or from the gastrointestinal tract (which may not always be noticeable in your stool) both lead to iron depletion. Unexplained iron-deficiency anemia is a common reason to get a colonoscopy (and often also an upper endoscopy) because we don’t want to miss an important cause such as cancer.
Issues with iron absorption: Certain medical conditions make it hard to absorb iron from our gut — for instance, post-bariatric surgery, celiac disease, inflammatory bowel disease or gastritis (which becomes more common as we age). Depending on the condition, people may need intravenous iron instead of an oral supplement.
Iron-poor diet: Certain plants such as spinach or legumes are high in iron. However, our bodies absorb the iron contained in meat and fish much more efficiently. People on plant-based diets may be at a higher risk of iron deficiency, but that’s usually mitigated in countries like the United States, where foods such as cereals and flour are generally fortified with iron.
The right way to take an iron supplement
Here are some ways to make iron supplements more effective:
Take it on an empty stomach: Ingesting the supplement an hour before eating or two hours after eating will help maximize absorption.
Swallow it with a glass of orange juice — and skip the milk and coffee: Vitamin C has been shown to promote iron absorption by creating a more acidic environment that helps iron dissolve. A 2020 randomized-controlled trial found that vitamin C supplementation in pill form did not improve iron absorption, but getting a healthy amount of vitamin C in your diet, such as through orange juice, could boost iron absorption fourfold. On the other hand, calcium-containing foods and beverages, including milk and yogurt, and polyphenol-containing drinks such as tea and coffee — can all decrease iron absorption. It’s okay to consume these at other times in the day — but avoid them around the same time as you take your iron supplement.
Help yourself to a stool softener: Constipation is a big reason people stop taking iron, so get on top of it before things back up! I usually tell my patients to preemptively take a stool softener (I typically start with psyllium) for the duration that they’re on iron supplements.
Rethink your antacid: Antacids such as omeprazole can interfere with iron absorption because they decrease acidity. Many people are on medications such as omeprazole for longer than they really need to be, so ask your physician if your daily antacid still makes sense and whether you can try coming down on the dose — or perhaps off it entirely.
Avoid “enteric-coated” capsules: These formulations — which apply a special coating meant to avoid degradation early in the digestive tract — sound good in theory, but because iron is absorbed by the first parts of the small intestine, “enteric-coated” tablets can bypass that key area of your gut so you won’t absorb as much as you should.
What I want my patients to know
Iron-deficiency anemia can occur slowly — often over several months — so it can be hard to pinpoint exactly when you started to feel differently. And some of my female patients with heavy menses have normalized feeling fatigued, telling themselves “they’re just out of shape.”
Iron-deficiency anemia can be resolved (usually fairly easily), but we need to investigate its cause and treat it, if we can. Otherwise, people with long-term iron deficiency are at risk of heart problems and infections.
A prescription for better living
- Taking care of your skin doesn’t have to be expensive. Here’s a simple, science-backed routine for day and night.
- Our nails are a unique window into our overall health. Here’s what to know about the diseases associated with distinctive nail changes.
- Do you get sleepy in the afternoon after eating lunch? The most likely culprit is your circadian rhythm, but what you eat may also play a role.
- Does acupuncture work for chronic pain? The evidence for using it to treat headaches and back pain is convincing.
- Next time you get a leg cramp, try taking a sip of pickle juice. Athletes have long used it to ward off cramps, and studies show it may actually work.
- Are salads actually good for you ? It depends on what you add to it, especially your choice of dressing.

J.D.’s Hematopoietic Issues and Iron Deficiency Anemia
Hematopoietic: J.D. is a 37 years old white woman who presents to her gynecologist complaining of a 2-month history of intermenstrual bleeding, menorrhagia, increased urinary frequency, mild incontinence, extreme fatigue, and weakness. Her menstrual period occurs every 28 days and lately there have been 6 days of heavy flow and cramping. She denies abdominal distension, back-ache, and constipation. She has not had her usual energy levels since before her last pregnancy.
Past Medical History (PMH): Upon reviewing her past medical history, the gynecologist notes that her patient is a G5P5with four pregnancies within four years, the last infant having been delivered vaginally four months ago. All five pregnancies were unremarkable and without delivery complications. All infants were born healthy. Patient history also reveals a 3-year history of osteoarthritis in the left knee, probably the result of sustaining significant trauma to her knee in an MVA when she was 9 years old. When asked what OTC medications she is currently taking for her pain and for how long she has been taking them, she reveals that she started taking ibuprofen, three tablets each day, about 2.5 years ago for her left knee. Due to a slowly progressive increase in pain and a loss of adequate relief with three tablets, she doubled the daily dose of ibuprofen. Upon the recommendation from her nurse practitioner and because long-term ibuprofen use can cause peptic ulcers, she began taking OTC omeprazole on a regular basis to prevent gastrointestinal bleeding. Patient history also reveals a 3-year history of HTN for which she is now being treated with a diuretic and a centrally acting antihypertensive drug. She has had no previous surgeries.
Case Study Questions
- Name the contributing factors on J.D that might put her at risk to develop iron deficiency anemia.
- Within the case study, describe the reasons why J.D. might be presenting constipation and or dehydration.
- Why Vitamin B12 and folic acid are important on the erythropoiesis? What abnormalities their deficiency might cause on the red blood cells?
- The gynecologist is suspecting that J.D. might be experiencing iron deficiency anemia. In order to support the diagnosis, list and describe the clinical symptoms that J.D. might have positive for Iron deficiency anemia.
- If the patient is diagnosed with iron deficiency anemia, what do you expect to find as signs of this type of anemia? List and describe.
- Labs results came back for the patient. Hb 10.2 g/dL; Hct 30.8%; Ferritin 9 ng/dL; red blood cells are smaller and paler in color than normal. Research list and describe for appropriate recommendations and treatments for J.D.
Cardiovascular Mr. W.G. is a 53-year-old white man who began to experience chest discomfort while playing tennis with a friend. At first, he attributed his discomfort to the heat and having had a large breakfast. Gradually, however, discomfort intensified to a crushing sensation in the sternal area and the pain seemed to spread upward into his neck and lower jaw. The nature of the pain did not seem to change with deep breathing. When Mr. G. complained of feeling nauseated and began rubbing his chest, his tennis partner was concerned that his friend was having a heart attack and called 911 on his cell phone. The patient was transported to the ED of the nearest hospital and arrived within 30 minutes of the onset of chest pain. In route to the hospital, the patient was placed on nasal cannula and an IV D5W was started. Mr. G. received aspirin (325 mg po) and 2 mg/IV morphine. He is allergic to meperidine (rash). His pain has eased slightly in the last 15 minutes but is still significant; was 9/10 in severity; now7/10. In the ED, chest pain was not relieved by 3 SL NTG tablets. He denies chills.
- For patients at risk of developing coronary artery disease and patients diagnosed with acute myocardial infarct, describe the modifiable and non-modifiable risk factors.
- What would you expect to see on Mr. W.G. EKG and which findings described on the case are compatible with the acute coronary event?
- Having only the opportunity to choose one laboratory test to confirm the acute myocardial infarct, which would be the most specific laboratory test you would choose and why?
- How do you explain that Mr. W.G temperature has increased after his Myocardial Infarct, when that can be observed and for how long? Base your answer on the pathophysiology of the event.
- Explain to Mr. W.G. why he was experiencing pain during his Myocardial Infarct. Elaborate and support your answer.
Submission Instructions:
- Include both case studies in your post.
- Your initial post should be at least 500 words, formatted and cited in current APA style with support from at least 2 academic sources.

WhatsApp us
Ferric carboxymaltose infusion versus oral iron supplementation for preoperative iron deficiency anaemia in patients with colorectal cancer (FIT): a multicentre, open-label, randomised, controlled trial
Collaborators.
- FIT collaborative group : Annette A van Zweeden , Daniel Hess , Hilko A Swank , Lisette Scholten , Jarmila D W van der Bilt , Marilou A Jansen , Peter van Duijvendijk , Donna Bezuur , Michele Carvello , Caterina Foppa , Wouter H de Vos Tot Nederveen Cappel , Ritch T J Geitenbeek , Lara van Woensel , Steve M M De Castro , Caroline Wientjes , Stefan van Oostendorp
Affiliations
- 1 Department of Surgery, Amsterdam UMC, Location AMC, Amsterdam, Netherlands.
- 2 Department of Gastroenterology, Amsterdam UMC, Location AMC, Amsterdam, Netherlands.
- 3 Department of Surgery, Amsterdam UMC, Location VUmc, Amsterdam, Netherlands.
- 4 Department of Surgery, Meander Medical Centre, Amersfoort, Netherlands; Department of Surgery, University Medical Centre Groningen, Groningen, Netherlands.
- 5 Department of Surgery, Spaarne Gasthuis, Hoofddorp, Netherlands.
- 6 Department of Gastroenterology, Amstelland Hospital, Amstelveen, Netherlands.
- 7 Department of Surgery, TergooiMC, Hilversum, Netherlands.
- 8 Department of Internal Medicine, Antonius Hospital, Sneek, Netherlands.
- 9 Department of Surgery, Albert Schweitzer Hospital, Dordrecht, Netherlands.
- 10 Department of Surgery, Onze Lieve Vrouwe Gasthuis, Amsterdam, Netherlands.
- 11 Department of Surgery, Hospital Gelderse Vallei, Ede, Netherlands.
- 12 Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; Division of Colon and Rectal Surgery, IRCCS Humanitas Research Hospital, Rozzano, Milan, Italy.
- 13 Department of Surgery, Flevo Hospital, Almere, Netherlands.
- 14 Department of Surgery, Gelre Hospital, Apeldoorn, Netherlands.
- 15 Department of Surgery, Haaglanden Medical Centre, Den Haag, Netherlands.
- 16 Department of Surgery, Isala Hospital, Zwolle, Netherlands.
- 17 Epidemiology and Data Science, Amsterdam UMC, location University of Amsterdam, Amsterdam, Netherlands; Amsterdam Public Health Methodology, Amsterdam, Netherlands.
- 18 Department of Internal Medicine, Amsterdam UMC, Location AMC, Amsterdam, Netherlands.
- 19 Department of Surgery, Amsterdam UMC, Location AMC, Amsterdam, Netherlands; IBD Unit, Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita Salute San Raffaele, Milan, Italy. Electronic address: [email protected].
- PMID: 36863386
- DOI: 10.1016/S2352-3026(22)00402-1
Background: A third of patients with colorectal cancer who are eligible for surgery in high-income countries have concomitant anaemia associated with adverse outcomes. We aimed to compare the efficacy of preoperative intravenous and oral iron supplementation in patients with colorectal cancer and iron deficiency anaemia.
Methods: In the FIT multicentre, open-label, randomised, controlled trial, adult patients (aged 18 years or older) with M0 stage colorectal cancer scheduled for elective curative resection and iron deficiency anaemia (defined as haemoglobin level of less than 7·5 mmol/L (12 g/dL) for women and less than 8 mmol/L (13 g/dL) for men, and a transferrin saturation of less than 20%) were randomly assigned to either 1-2 g of ferric carboxymaltose intravenously or three tablets of 200 mg of oral ferrous fumarate daily. The primary endpoint was the proportion of patients with normalised haemoglobin levels before surgery (≥12 g/dL for women and ≥13 g/dL for men). An intention-to-treat analysis was done for the primary analysis. Safety was analysed in all patients who received treatment. The trial was registered at ClincalTrials.gov, NCT02243735 , and has completed recruitment.
Findings: Between Oct 31, 2014, and Feb 23, 2021, 202 patients were included and assigned to intravenous (n=96) or oral (n=106) iron treatment. Treatment began a median of 14 days (IQR 11-22) before surgery for intravenous iron and 19 days (IQR 13-27) for oral iron. Normalisation of haemoglobin at day of admission was reached in 14 (17%) of 84 patients treated intravenously and 15 (16%) of 97 patients treated orally (relative risk [RR] 1·08 [95% CI 0·55-2·10]; p=0·83), but the proportion of patients with normalised haemoglobin significantly increased for the intravenous treatment group at later timepoints (49 [60%] of 82 vs 18 [21%] of 88 at 30 days; RR 2·92 [95% CI 1·87-4·58]; p<0·0001). The most prevalent treatment-related adverse event was discoloured faeces (grade 1) after oral iron treatment (14 [13%] of 105), and no treatment-related serious adverse events or deaths were observed in either group. No differences in other safety outcomes were seen, and the most common serious adverse events were anastomotic leakage (11 [5%] of 202), aspiration pneumonia (5 [2%] of 202), and intra-abdominal abscess (5 [2%] 202).
Interpretation: Normalisation of haemoglobin before surgery was infrequent with both treatment regimens, but significantly improved at all other timepoints following intravenous iron treatment. Restoration of iron stores was feasible only with intravenous iron. In selected patients, surgery might be delayed to augment the effect of intravenous iron on haemoglobin normalisation.
Funding: Vifor Pharma.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Publication types
- Randomized Controlled Trial
- Multicenter Study
- Anemia, Iron-Deficiency* / complications
- Anemia, Iron-Deficiency* / etiology
- Colorectal Neoplasms* / complications
- Colorectal Neoplasms* / drug therapy
- Colorectal Neoplasms* / surgery
- Dietary Supplements
- Hemoglobins
- ferric carboxymaltose
Associated data
- ClinicalTrials.gov/NCT02243735
Investing in Women's Health: Let's start by addressing our iron levels
Global researcher and advocate for the improvement of child and maternal nutrition, angeline jeyakumar, discusses iron deficiency, a common issue in women's health, for national women's health month.
May is designated as National Women’s Health Month, a time to raise awareness about the various health concerns that impact women across all age groups. This commemoration reminds women to prioritize their overall wellbeing and seek proper medical care whenever necessary.
This year, the Office on Women’s Health, a part of the U.S. Department of Health and Human Services and the agency overseeing the National Women’s Health Week from May 12 to 18, aims to “empower women to take charge of their health journeys and shine a light on health issues unique to women.” The agency also indicated on its website that it hopes that this year’s commemoration will set the stage for women to freely voice their health needs and concerns.
While contemplating this year’s theme, an issue came to mind – iron deficiency – often stigmatized and seldom openly discussed by women due to its association with menstrual bleeding and socioeconomic ranking. Yet, it is an issue that warrants the attention of public health stakeholders.
Iron deficiency anemia, a condition in which blood lacks adequate healthy red blood cells, stands out as an endemic nutritional, lifestyle and biological issue among women from all backgrounds. Women of reproductive ages and those approaching menopause are particularly at risk due to factors such as heavy menstrual bleeding and the increased demand for iron as gestational age increases. A 2022 Centers for Disease Control study found that anemia prevalence among pregnant women enrolled in the Women, Infants and Children Program increased 13% from 2008 to 2018 while a survey by the same agency found that a total of about 250,000 women between ages 25 and 64 visited a physician’s office for anemia-related issues in 2016 and in 2021, about 38,000 women in the same age range visited an emergency room for similar issues. Young women are not spared. Studies show that 40% of young women between ages 12 and 21 suffered from anemia resulting from insufficient iron levels. Yet with these concerning statistics, in a country with advanced medical care, the health agency revealed that surveillance of anemia during pregnancy in the U.S. is limited, highlighting a gap in monitoring and addressing this crucial issue.
Although anemia is categorized as a nutritional deficiency, it is a chronic condition that frequently presents hidden symptoms. In the absence of coordinated national efforts to effectively manage iron loss, women must take proactive measures to increase nutritional and health awareness.
Here are a few practical steps to increase iron intake and eat healthily:
- Pack up on iron-rich foods – incorporating green leafy vegetables, sprouted and fermented foods into your diet can enhance the absorption of iron into the body.
- Have a nourishing beginning to your day – opting for a breakfast rich protein and micronutrient-packed vegetables offers a healthier alternative to processed cereals. Beware of cereals that claim high iron content; their hidden sugars may contribute to health issues such as obesity and diabetes. Legumes such as lentils are a better breakfast option as they are rich in fiber, protein, vitamins and minerals while low in fat. They also contain complex carbohydrates, offering sustained energy.
- Making healthier food choices – monitoring grocery purchases and opting for cooked meals using fresh produce improves nutrition and reduces consumption of unhealthy additives and preservatives in processed foods. Fresh fruits, vegetables, lean proteins and whole grains provide a variety of vitamins, minerals and antioxidants that promote overall health.
- While fad diets promise quick weight loss, their effects are short-lived. It is important to adopt a sustainable dietary pattern for long-term health. This includes eating balanced food choices, portion control and nutrient-dense foods.
- Regular exercise stimulates the cardiovascular system, leading to better circulation and oxygen delivery throughout the body. This enhances endurance, stamina and energy levels. A combination of aerobics, strength training and flexibility exercises maximizes these benefits. Obesity, on the other hand, hinders the absorption and usage of iron, making it a risk factor for anemia.
- Excessive alcohol intake and smoking cigarettes can hinder the body from absorbing important nutrients, vitamins and minerals such as vitamin B12, folate and calcium, which are necessary for overall well-being. Limiting alcohol consumption and practicing moderation can preserve nutrient absorption and promote good health.
In line with this year’s theme, I urge women to actively educate themselves about their bodies. It is important for them to openly communicate their health concerns with medical experts without any fear of judgment or negative consequences that may impact their professional or social lives. Women, let us take a proactive approach and regularly monitor our iron levels if we experience any concerning symptoms such as extreme fatigue, numbness or tingling sensations in the hands and feet, dizziness, changes in the color of the whites of our eyes, pale skin and other related issues.
About the author
Angeline Jeyakumar is a global researcher and advocate for the improvement of child and maternal nutrition. She currently serves as a public health nutrition specialist for the University of Nevada, Reno Extension and is an assistant professor in the College of Agriculture, Biotechnology & Natural Resources’ Department of Nutrition . Read more about her background.

By: Angeline Jeyakumar
Optimism Series The Past is the Key to our Future
Associate Professor Andrew Zuza in the Nevada Bureau of Mines and Geology discusses how an old-fashion summer field course helps train earth science students to tackle future global issues

University Libraries key map publications
Recent collection award ensures access to critical resources for Nevadans

The Outdoors A Natural Path to Wellness
Carly Boren discusses the restorative power of nature and its positive impacts on well-being for Mental Health Awareness Month

bodymind Aiyana Graham
University Undergraduate Researcher and NURA awardee learns through and creates art highlighting the marginalized

Editor's Picks

Father and son set to receive doctoral degrees May 17

Strong advisory board supports new Supply Chain and Transportation Management program in College of Business

Sagebrushers season 3 ep. 4: Associate Professor Thomas White

Geoffrey Blewitt elected to the National Academy of Sciences
Nevada Today
‘The Jewish heart of campus’
Rabbi Dani Libersohn and his wife Rochel are dedicated to creating a safe, welcoming environment for Jewish students at the University of Nevada, Reno through Chabad

College of Engineering will graduate Jay and Nathan Thom with Ph.Ds in Computer Science & Engineering
Industry leaders set clear direction to serve fast-growing economic sector in Nevada
University of Nevada, Reno once again nationally recognized as a Voter Friendly Campus
The acknowledgment marks the University’s 5th year in a row to receive this title

Spring 2024 Senior Scholars
The University of Nevada, Reno honors twelve graduating students who have achieved the highest grade-point average for their respective college or school

University of Nevada, Reno to confer more than 3,000 degrees during May 2024 commencement
Five in-person ceremonies held Thursday through Saturday, May 16-18, on the University Quad

University launches program to increase number of Nevada organic producers
Grow Organic Nevada aims to help meet increasing demand for organic products

Big data, advancements in GPS and a search for dark matter earn the Nevada Bureau of Mines and Geology Professor of Geodesy the prestigious nomination

IMAGES
VIDEO
COMMENTS
Mother alive at 50 years old. Diagnosis of iron deficiency anemia at 24 years old during pregnancy with patient - on daily supplement. Otherwise healthy. Father alive at 52 years old. Diagnosis of hypertension - controlled with diet and exercise. Otherwise healthy.
Iron deficiency anemia is one of the most common causes of anemia seen in women, with menstruation being one of the leading causes. Excessive, prolonged, and irregular uterine bleeding, also known as menometrorrhagia, can lead to severe anemia. ... Studies have quantified that a premenopausal woman's iron storage levels could be approximately ...
The World Health Organization estimated worldwide prevalence of anemia to be 42% in children, 29% in non-pregnant women, and 38% in pregnant women in 2011. 1 In 2013, iron deficiency (ID) was identified as the predominant cause of anemia among the 1.93 billion anemic people (27% of the world's population) globally, making iron deficiency anemia (IDA) a major global health issue. 2,3 The ...
Recent studies have demonstrated sucrosomial iron to be non-inferior to parenteral iron in patients with anaemia secondary to coeliac disease, ... Efficacy of sodium feredetate versus ferrous sulfate in iron deficiency anemia in preganant women. Int J Reprod Contracept Obstet Gynecol 2017; 6:1978. 10.18203/2320-1770.ijrcog20171961 ...
Case Studies /. Anemia in a 42-year-old woman. Brought to you by Merck & Co, Inc., Rahway, NJ, USA (known as MSD outside the US and Canada) — dedicated to using leading-edge science to save and improve lives around the world. Learn more about the MSD Manuals and our commitment to Global Medical Knowledge.
According to the Global Burden of Disease Study 2016, iron deficiency anemia is 1 of the 5 leading causes of years lived with disability burden and is the first cause in women. 1 Adopting the World Health Organization-recommended cutoff for anemia (Hb <13 g/dL in males, <12 g/dL in females, <11g/dL during pregnancy), a worldwide survey showed that in 2010, anemia still affected one third of ...
The dosage of elemental iron required to treat iron deficiency anemia in adults is 120 mg per day for three months; the dosage for children is 3 mg per kg per day, up to 60 mg per day. 1 An ...
The diagnosis of iron deficiency (low iron stores, as measured by iron studies or other testing) is a major public health goal and an important aspect of the care of many adults. This topic will review the causes of iron deficiency in adults and an approach to the diagnostic evaluation. Treatment of iron deficiency in adults is discussed ...
Iron deficiency anemia is one of the most common causes of anemia seen in women, with menstruation being one of the leading causes. Excessive, prolonged, and irregular uterine bleeding, also known as menometrorrhagia, can lead to severe anemia. In this case report, we present a case of a premenopausal woman with menometrorrhagia leading to ...
Anemia is defined as hemoglobin below two standard deviations of the mean for the age and gender of the patient. Iron is an essential component of the hemoglobin molecule. The most common cause of anemia worldwide is iron deficiency, which results in microcytic and hypochromic red cells on the peripheral smear. Several causes of iron deficiency vary based on age, gender, and socioeconomic status.
In iron-deficiency anemia, iron studies usually show decreased ferritin and serum iron levels, an elevated serum transferrin level and a high total iron binding capacity. Unless there is clear history of low dietary intake of iron, the clinician should initiate evaluation for a source of bleeding or a malabsorptive process. Beyond infancy ...
Iron deficiency affects more than 2 billion people worldwide, 1 and iron-deficiency anemia remains the top cause of anemia, as confirmed by the analysis of a large number of reports on the burden ...
Nurse Michael works on an inpatient Medical-Surgical unit and is caring for Hannah, a 26-year-old female with a history of Crohn disease who was admitted for iron deficiency anemia. After settling Hannah in her room, Nurse Michael goes through the steps of the Clinical Judgment Measurement Model to make clinical decisions about Hannah's care ...
Most symptoms of iron deficiency are due to anemia. Such symptoms include fatigue, loss of stamina, shortness of breath, weakness, dizziness, and pallor. Another common symptom is restless leg syndrome (RLS), which is an unpleasant urge to move the legs during periods of inactivity. Koilonychia. Image.
Iron deficiency (ID) and iron-deficiency anemia (IDA) continue to be of worldwide concern. Among children in the developing world, iron is the most common single-nutrient deficiency. 1 In industrialized nations, despite a demonstrable decline in prevalence, 2 IDA remains a common cause of anemia in young children. However, even more important than anemia itself is the indication that the more ...
Objectives. The structure of hemoglobin and the role played by iron in the transport of oxygen. The means by which iron is transported and stored in the body. The incidence and causes of IDA. The red blood cell indices and how they are used to characterize anemia. How IDA is prevented and treated.
Blood pressure. 110/75 mm Hg. Respiratory rate. 12 bpm. Temperature. 98.6°F. On follow-up, MR's laboratory results are indicative of celiac disease (Table 2). The provider deferred a ...
Physical examination is positive for pale conjunctiva, mild spooning of nails, and a II/VI systolic murmur at left lower sternal border. Stools are negative for occult blood. Labs: Complete blood count (CBC) - Hg 7.1 gm/dl, Hct 23%, WBC 5,400/mm3 (differential is normal), platelets 450,000/mm3; Mean Corpuscular volume (MCV) is 74 fl (normal 85 ...
Left untreated, however, iron-deficiency anemia can make you feel tired and weak. You may notice pale skin and cold hands and feet. Iron-deficiency anemia can also cause you to feel dizzy or lightheaded. Occasionally, it can cause chest pain, a fast heartbeat and shortness of breath.
Studies have shown that HbA1c levels are a strong predictor of maternal and foetal complications during pregnancy. ... Api O, Breyman C, Çetiner M, Demir C, Ecder T. Diagnosis and treatment of iron deficiency anemia during pregnancy and the postpartum period: iron deficiency anemia working group consensus report. Turk J Obstet Gynecol. 2015;12 ...
Semantic Scholar extracted view of "Evaluating the Association Between Iron Deficiency Anemia and Febrile Convulsion Among Children Aged 6-60 Months Admitted to a Tertiary Care Hospital in Eastern India: A Case-Control Study" by Sandip K Mandal et al.
The diagnosis of iron deficiency anemia can be made either by the laboratory demonstration an iron-deficient state or evaluating the response to a therapeutic trial of iron replacement. Since the anemia itself is rarely life-threatening, the most important part of treatment is the identification of the cause—especially a source of occult ...
A 39-year-old woman was referred to our institution for evaluation of anemia. She was known to have multiple comorbidities and had a baseline hemoglobin concentration of approximately 10.5 g/dL. About 6 months before her referral, the patient began having recurrent episodes of severe anemia, with hemoglobin values as low as 3.5 g/dL.
Purpose Iron absorption in sickle cell anemia (SCA) remains unclear and studies in adults with SCA are scarce. The aim of this study was to evaluate the iron absorption SCA adults and its association with iron status and hepcidin concentration. Methods SCA patients (n = 13; SCAtotal) and control participants (n = 10) ingested an oral stable iron isotope (57Fe). Iron absorption was measured by ...
If you have iron-deficiency anemia, you should take an iron supplement. But here's an interesting pro-tip I share with my patients: Don't take it every day. A study found that taking an iron ...
Case Study Questions. Name the contributing factors on J.D that might put her at risk to develop iron deficiency anemia. Within the case study, describe the reasons why J.D. might be presenting constipation and or dehydration. Why Vitamin B12 and folic acid are important on the erythropoiesis?
Anemia is the most common dose-limiting toxicity of olaparib. However, few studies have analyzed the clinical features of olaparib-induced anemia. This study investigated the clinical features of olaparib-induced anemia. Additionally, the role of folate or vitamin B12 in olaparib-induced anemia was examined.
Methods: In the FIT multicentre, open-label, randomised, controlled trial, adult patients (aged 18 years or older) with M0 stage colorectal cancer scheduled for elective curative resection and iron deficiency anaemia (defined as haemoglobin level of less than 7·5 mmol/L (12 g/dL) for women and less than 8 mmol/L (13 g/dL) for men, and a ...
In the absence of coordinated national efforts to effectively manage iron loss, women must take proactive measures to increase nutritional and health awareness. Here are a few practical steps to increase iron intake and eat healthily: Pack up on iron-rich foods - incorporating green leafy vegetables, sprouted and fermented foods into your ...