
Home » Cord Blood Banking » Stem cell case studies

Stem cell case studies
Read our stem cell case studies to discover how umbilical cord cells have been used to treat conditions such as leukaemia, stroke, brain injury and autism.
Since 1988, cord blood stem cells have been used to treat a growing number of diseases and disorders.
The first transplant was for a 5-year-old boy called Matthew Farrow, who received his sister’s cord blood to treat Fanconi anaemia. Children with the condition are only expected to live into their teenage years, but Matthew is now a healthy 30-year-old with a family of his own.
Fanconi anaemia is just one of more than 80 potential diseases cured by cord blood and in the past three decades, there have been more and more stem cell success stories from all around the world. You can find out more about these case studies below.

Jay’s journey
In 2016, little Jay Shetty took part in a pioneering clinical trial for cerebral palsy at Duke University in the USA. After he was diagnosed with cerebral palsy at a young age, his parents decided to store his brother’s stem cells with Cells4Life .
In 2017, Jay received those cells in a single injection that was overseen by Dr Joanne Kurtzberg from Duke’s medical centre.
“His muscle rigidity has reduced, and his vision has improved,” says Jay’s mother, Shilpa. “We definitely noticed a difference.”
The Promise of Stem Cells in Treating Fibromyalgia
Fibromyalgia is a complex condition that affects millions of people worldwide, causing chronic pain, fatigue, and various other symptoms. Despite its prevalence, the exact cause of fibromyalgia remains unknown, making it hard to treat effectively. However, recent...

Could Umbilical Cord Stem Cells Help Ovarian Ageing?
A new study has shown that mesenchymal stem cells derived from both the umbilical cord and from fat tissues may be able to provide a therapy for ovarian ageing. In a trial conducted using mice, researchers at the Centre for Reproductive Medicine in Beijing saw...
Stem Cells Shown to Aid Repair After Cardiac Arrest
In a study first published in April’s edition of Advanced Functional Materials, researchers found that stem cells boost natural repair following cardiac arrest. The most common consequence of cardiac arrest is brain injury. Decreased blood flow and oxygen to the brain...
FIND OUT MORE, REQUEST YOUR WELCOME PACK TODAY
Connect with us.

Pin It on Pinterest
- MEMBER DIRECTORY
- Member Login
- Publications
- Clinician Well-Being
- Culture of Health and Health Equity
- Fellowships and Leadership Programs
- Future of Nursing
- U.S. Health Policy and System Improvement
- Healthy Longevity
- Human Gene Editing
- U.S. Opioid Epidemic
- Staff Directory
- Opportunities
- Action Collaborative on Decarbonizing the U.S. Health Sector
- Climate Communities Network
- Communicating About Climate Change & Health
- Research and Innovation
- Culture of Health
- Fellowships
- Emerging Leaders in Health & Medicine
- Culture & Inclusiveness
- Digital Health
- Evidence Mobilization
- Value Incentives & Systems
- Substance Use & Opioid Crises
- Reproductive Health, Equity, & Society
- Credible Sources of Health Information
- Emerging Science, Technology, & Innovation
- Pandemic & Seasonal Influenza Vaccine Preparedness and Response
- Preventing Firearm-Related Injuries and Deaths
- Vital Directions for Health & Health Care
- NAM Perspectives
- All Publications
- Upcoming Events
- Past Events
- MEMBER HOME

Regenerative Medicine: Case Study for Understanding and Anticipating Emerging Science and Technology

Introduction
This case study was developed as one of a set of three studies, focusing on somewhat mature but rapidly evolving technologies. These case studies are intended to draw out lessons for the development of a cross-sectoral governance framework for emerging technologies in health and medicine. The focus of the case studies is the governance ecosystem in the United States, though where appropriate, the international landscape is included to provide context. Each of these case studies:
- describes how governance of the technology has developed within and across sectors and how it has succeeded, created challenges, or fallen down;
- outlines ethical, legal, and social issues that arise within and across sectors;
- considers a multitude of factors (market incentives, intellectual property, etc.) that shape the evolution of emerging technologies; and
- identifies key stakeholders.
Each case study begins with two short vignettes designed to highlight and make concrete a subset of the ethical issues raised by the case (see Box 1 and Box 2 ). These vignettes are not intended to be comprehensive but rather to provide a sense of the kinds of ethical issues being raised today by the technology in question.

The cases are structured by a set of guiding questions, outlined subsequently. These questions are followed by the historical context for the case to allow for clearer understanding of the trajectory and impact of the technology over time and the current status (status quo) of the technology. The bulk of the case consists of a cross-sectoral analysis organized according to the following sectors: academia, health care/nonprofit, government, private sector, and volunteer/consumer. Of note, no system of dividing up the world will be perfect—there will inevitably be overlap and imperfect fits. For example, “government” could be broken into many categories, including international, national, tribal, sovereign, regional, state, city, civilian, or military. The sectoral analysis is further organized into the following domains: science and technology, governance and enforcement, affordability and reimbursement, private companies, and social and ethical considerations. Following the cross-sectoral analysis is a broad, nonsectoral list of additional questions regarding the ethical and societal implications raised by the technology.
The next section of the case is designed to broaden the lens beyond the history and current status of the technology at the center of the case. The “Beyond” section highlights additional technologies in the broad area the focal technology occupies (e.g., neurotechnology), as well as facilitating technologies that can expand the capacity or reach of the focal technology. The “Visioning” section is designed to stretch the imagination to envision the future development of the technology (and society), highlighting potential hopes and fears for one possible evolutionary trajectory that a governance framework should take into account.
Finally, lessons learned from the case are identified—including both the core case and the visioning exercise. These lessons will be used, along with the cases themselves, to help inform the development of a cross-sectoral governance framework, intended to be shaped and guided by a set of overarching principles. This governance framework will be created by a committee of the National Academies of Sciences, Engineering, and Medicine (https://www.nationalacademies.org/our-work/creating-a-framework-for-emerging-science-technology-and-innovation-in-health-and-medicine).
Case Study: Regenerative Medicine
Regenerative medicine as a field is quite broad but is generally understood to focus on the regeneration, repair, and replacement of cells, tissues, and organs to restore function (Mason and Dunnill, 2008). The aspect of regenerative medicine on which this case study focuses relates to the ability to treat—or cure—genetic hematologic disease safely and effectively, and the significant trade-offs that come with these novel therapies.
The story of this therapy begins in the history of bone marrow transplants. The medicinal value of bone marrow has long been recognized and was first discussed in the 1890s as a potential treatment (administered orally) of “diseases believed to be characterized by defective hemogenesis” (Quine, 1896).
While allogeneic bone marrow transplant (in which stem cells from a donor are collected and transplanted into the recipient) may be the most broadly known form of hematopoietic stem and progenitor cell (HSPC) transplant, a range of other cell types are also used. HSPCs used in transplant can be either allogeneic (i.e., from a donor) or autologous (i.e., from the person who will also receive the transplant). The cells used in transplant research and clinical care can come from bone marrow, peripheral blood stem cells (PBSCs), umbilical cord blood, and pluripotent stem cell-derived cells.
A major challenge throughout the history of HSPC transplantation has been the dire risks associated with these transplants, including the morbidity and mortality caused by immunological reactions between the transplanted cells and the tissues of the recipient. In particular, graft-versus-host disease (GVHD) is a serious response in which the transplanted stem cells view the recipient’s tissues as foreign and mount an immune response, attacking the recipient’s body. If an autologous transplant is not possible given the nature of the disease to be treated, an immunologically well-matched healthy donor for allogeneic transplant is critical. For genetic hematologic disease, a new approach that would not only treat but cure the condition is now being tested: genetic modification of the patient’s own HSPCs to correct or compensate for the defect, followed by transplantation of the corrected autologous cells.
This challenge of matching transplantable cells to patients has driven evolution within the field of regenerative medicine, including logistical fixes in the form of HSPC registries and banks to technological approaches including the use of pluripotent stem cell-derived cell sources and genome editing (e.g., clustered regularly interspaced short palindromic repeats [CRISPR]).
This challenge of immunological matching has also driven significant ethical challenges, even beyond the substantial risks of HSPC transplantation itself. In contrast to many novel technologies, where finances are a primary barrier to access, in the case of regenerative medicine, there is the additional barrier of biology. People who are not of European descent have a lower probability of finding well-matched donors than do people of European descent. Furthermore, genetic hematologic diseases like sickle cell disease (SCD) and thalassemia, for which HSPC transplant is the only established cure (and a fraught one, at that), have struggled to garner the financial and grant support needed to move research forward. This challenge persists despite SCD being three times more prevalent in the United States than cystic fibrosis, which has historically benefited from generous public and private funding (Farooq et al., 2020; Wailoo and Pemberton, 2006). All of this stands on a background of long-understood barriers even to standard of care (e.g., adequate pain management) for individuals with SCD in particular (Haywood et al., 2009). Together, these facts raise concerns related to equity and access at multiple stages of research, development, and clinical care.
Finally, advances in this science have also attracted the attention of those who are willing to take advantage of patients under the guise of cutting-edge therapy, creating a robust market of direct-to-consumer (DTC) cell-based services and interventions that at best waste time and money and at worst cause serious harm or death (Bauer et al., 2018).
Guiding Questions
(derived from global neuroethics summit delegates, 2018; mathews, 2017).
The following guiding questions were used to frame and develop this case study.
- Historical context: What are the key scientific antecedents and ethics touchstones?
- Status quo: What are the key questions, research areas, and products/applications today?
- Cross-sectoral footprint: Which individuals, groups, and institutions have an interest or role in emerging biomedical technology?
- Ethical and societal implications: What is morally at stake? What are the sources of ethical controversy? Does this technology or application raise different and unique equity concerns?
Additional guiding questions to consider include the following:
- Key assumptions around technology: What are the key assumptions of both the scientists around the technology and the other stakeholders that may impede communication and understanding or illuminate attitudes?
- International context and relevant international comparisons: How are the technology and associated ethics and governance landscape evolving internationally?
- Legal and regulatory landscape: What are the laws and policies that currently apply, and what are the holes or challenges in current oversight?
- Social goals of the research: What are the goals that are oriented toward improving the human condition? Are there other goals?
Historical Context
What are the key scientific antecedents and ethics touchstones, hspc transplant.
HSPC transplant was initially only attempted in terminally ill patients (Thomas, 1999). The first recorded bone marrow transfusion was given to a 19-year-old woman with aplastic anemia in 1939 (Osgood et al., 1939). This was long before the Nuremburg Code, the Declaration of Helsinki, or the Belmont Report and anything like current understandings of informed consent (NCPHSBBR, 1979; Rickham, 1964; International Military Tribunal, 1949). There was also little understanding of the factors associated with graft failure—no attempts at bone marrow transfusions succeeded, and all patients died. Despite this early experience, the consequences of World War II, particularly the need to improve radiation and burn injury treatment, propelled this work forward (de la Morena and Gatti, 2010).
As human transplant work continued, experiments in mice and dogs in the 1950s and 1960s showed that after lethal radiation, these animals would recover if given autologous bone marrow. However, if given allogeneic marrow, the animal would reject the graft and die or accept the graft but then die from “wasting syndrome,” which later came to be understood as GVHD (Mannick et al., 1960; Billingham and Brent, 1959; Barnes et al., 1956; Rekers et al., 1950). It became clear that close immunologic matching between donor and recipient and management of GVHD in the recipient would be vital to the success of allogeneic bone marrow transplants (de la Morena and Gatti, 2010).
A 1970 accounting of the reported experience with HSPC transplants to date described approximately 200 allogeneic stem cell graft attempts (six involving fetal tissue) in subjects aged less than 1 to over 80 years, most of which had taken place between 1959 and 1962 (Bortin, 1970). (Of note, there were likely scores of unreported cases; in fact, the author ended the article with a call for reporting of all HSPC transplant attempts to the newly established American College of Surgeons-National Institutes of Health Organ Transplant Registry.) Of the reported cases (which often included the subjects’ initials), only 11 individuals were “unequivocal” allogeneic chimeras, and of those, only five were still alive at the time their case was reported. Many of the reported subjects died of opportunistic infections or GVHD, the noting of which often did not capture the true human toll of these deaths. For many years, even “success” (i.e., engraftment of the transplanted marrow) ended in death due to these other causes (Mathé et al., 1965; Thomas et al., 1959). As Donnall Thomas, a pioneer and leader in the field who won the Nobel Prize for “discoveries concerning organ and cell transplantation in the treatment of human disease” in 1990, reflected years later, “the experience with allogeneic transplants had been so dismal that questions were raised about whether or not such studies should be continued” (Thomas, 2005; Nobel Prize, 1990). In fact, the dismal experience with HSPC transplant eventually led most investigators to discontinue this work in humans, the focus returning for a time to animal studies (Little and Storb, 2002).
However, the discovery of human leukocyte antigen (HLA) in 1958 by Jean Dausset, which helps the immune system differentiate between what is “self” and what is foreign, and subsequent advances in the understanding of HLA matching and immunosuppression during the 1960s and 1970s led to a resumption of human clinical trials (Nobel Prize, 1980). In 1971, the first successful use of HSPC transplant to treat leukemia was reported (Granot and Storb, 2020). The following decades saw additional developments in HSPC transplant, improving the safety of the intervention, thus enabling its consideration for treatment of a broader array of blood diseases, including the hemoglobinopathies (Granot and Storb, 2020; Apperley, 1993).
The first use of HSPC transplant to cure thalassemia was in 1981, in a 16-month-old child, with an HLA-identical sibling donor—this patient was alive and thalassemia-free more than 20 years later (Bhatia and Walters, 2008; Thomas et al., 1982). Thalassemia major (the most serious form of the disease) requires chronic blood transfusion and chelation for life, a process which leads to gradual iron buildup and related organ damage, including heart failure, which is a common cause of death. Life expectancy for treated patients has increased substantially and varies by thalassemia type and treatment compliance, but patients can now live into their 40s and beyond (Pinto et al., 2019).
The first cure of SCD via HSPC transplant was incidental. An 8-year-old girl with acute myeloid leukemia (AML) was successfully treated for her leukemia with a bone marrow transplant, curing her SCD in the process (Johnson et al., 1984). By this time, life expectancy for an individual with SCD had improved substantially, reaching the mid-20s due to advances in understanding and treatment of the disease (particularly the use of antibiotics to manage the frequent infections that plagued those with the disease) (Wailoo, 2017; Prabhakar et al., 2010). The first five patients, all children, in whom HSPC transplants were used intentionally to treat SCD were reported in 1988 (Vermylen et al., 1988). As Vermylen and colleagues reported, “In all cases there was complete cessation of vaso-occlusive episodes and haemolysis” (Vermylen et al., 1988).
Around this same time, there were also advancements in the sources of transplantable hematopoietic cells, expanding beyond bone marrow to include peripheral blood stem cells and umbilical cord blood (Gluckman et al., 1989; Kessinger et al., 1988). Cord blood was particularly appealing for a number of reasons, including that it is less immunogenic than the other cell sources, reducing the risk of GVHD.
The development of cord blood transplant has a very different origin story to that of bone marrow, beginning with a hypothesis and the founding of a company (Ballen et al., 2013). The company, Biocyte Corporation (later PharmaStem Therapeutics), funded the early work and held two short-lived patents over the isolation, preservation, and culture of umbilical cord blood (Shyntum and Kalkreuter, 2009). The longevity of the science has thankfully surpassed that of the company that launched it. The first cord blood–based HSPC transplant was conducted with the approval of the relevant institutional review boards (IRBs) and the French National Ethics Committee, to treat a 5-year-old boy with Fanconi anemia using cells from the birth of an unaffected, HLA-matched sister (Ballen et al., 2013; Gluckman et al., 1989). The success of the early cases (the 5-year-old boy was still alive and well 25 years later) led to the use of unrelated cord blood transplant and expansion of use beyond malignant disease (Ballen et al., 2013; Kurtzberg et al., 1996). Benefits of cord blood include noninvasive collection, ability to cryopreserve characterized tissue for ready use, reduced likelihood of transmitting infections, and lower immunogenicity relative to bone marrow, enabling imperfect HLA matching and expanding access, in particular for people not of European descent (Barker et al., 2010; Gluckman et al., 1997). Cord blood HSPC transplant was first used primarily in children, because it was thought that the relatively low number of cells in a cord blood unit would limit its use in adults, but over time, as techniques and supportive care have improved, so has success of cord blood transplant in adults (Eapen et al., 2010; Ballen et al., 2007). Today, cord blood is widely used for HSPC transplants in both children and adults, with outcomes as good as or better than with bone marrow. Despite these advancements, however, allogeneic HSPC transplant continued to depend on the availability of HLA-matched donors.
Public HSPC Banks
Unfortunately, only about 35 percent of patients have HLA-matched siblings, so patients have needed to look beyond their immediate family for matched donors. This need led to the creation of HLA-typed donor registries, starting with the founding of the Europdonor registry in the Netherlands in 1970 and the International Blood and Marrow Transplant Registry at the Medical College of Wisconsin in 1972 (McCann and Gale, 2018). In 1986, the National Marrow Donor Program (NMDP), which operates the Be the Match registry, was founded by the U.S. Navy. Other registries in the United States and Europe followed, and by 1988, there were eight active registries around the world with more than 150,000 donors (van Rood and Oudshoorn, 2008). The Bone Marrow Donors Worldwide network, which connected these registries, was formed in 1988 to facilitate the identification of potential donors, and in 2017 its activities were taken over by the World Marrow Donor Association (WMDA) (Oudshoorn et al., 1994). Today, the combined registry includes more than 37,600,000 donors and more than 800,000 cord blood units from 54 different countries (see Figure 1 ) (WMDA, 2021; Petersdorf, 2010).

However, even with tremendous global collaboration to identify and make available donor information, access is not equal. The NMDP estimates suggest that while approximately 90 percent of people of European descent will identify a well-matched unrelated marrow donor, the same will be true for only about 70 percent of people of Asian or Hispanic descent and 60 percent of those of African descent (Pidala et al., 2013). Causes for this disparity include higher HLA diversity among these populations compared to those of European descent and smaller numbers of racial and ethnic minority volunteers in donor registries and ultimately available for transplant (Sacchi et al., 2008; Kollman et al., 2004).
Private HSPC Banks
Alongside the public registries, trading on the success of cord blood HSPC transplants and playing on the fears of new parents, a thriving market of private cord blood banks has developed (Murdoch et al., 2020). These for-profit private banks market their services—collecting and storing cord blood for potential future personal use—as insurance policies for the health of one’s newborn, without much data to support the claim. While donation of cord blood to a public bank is free to the donor, costs associated with private banking include a collection fee (US$1,350–$2,300) and annual storage fees ($100–$175/year), which are unlikely to be covered by health insurance (Shearer et al., 2017). At the same time, public banks are held to transparent, rigorous storage and quality standards that do not apply to private banks, leading to lower overall quality of cord blood in private banks (Shearer et al., 2017; Sun et al., 2010; Committee on Obstetric Practice, 2008). Finally, cord blood stored in public banks is 30 times more likely to be accessed for clinical use than samples stored in private banks, and there is broad professional consensus, and associated professional guidance, that public banking is preferable to private banking (Shearer et al., 2017; Ballen et al., 2015). Despite these differences, in 2017, there were about 800,000 cord blood units in public banks, compared with more than 5 million in private banks (Kurtzberg, 2017).
New HSPC Sources
While adult stem cell sources (bone marrow, peripheral blood, and cord blood) have dominated research and clinical care for many decades, in the late 1990s and mid-2000s, new tools were added in the form of several pluripotent stem cell types, including embryonic stem cells, embryonic germ cells, nuclear transfer (NT)-derived stem cells, and most recently, induced pluripotent stem cells (iPSCs) (Tachibana et al., 2013; Yu et al., 2007; Takahashi and Yamanaka, 2006; Shamblott et al., 1998; Thomson et al., 1998). In contrast to the previous cell sources, which are restricted to repopulating blood cell types, these new pluripotent stem cells can turn into any of the approximately 220 cell types in the human body and have a correspondingly diverse array of potential applications. For the purposes of this case, the authors focus on the use of these cells in hematologic disease, but understanding some of the history of the development and use of these cells is helpful for the broader goals of the case. Importantly, these new cell types emerged in a very different regulatory and societal environment than the environment in which bone marrow transplants were first being developed.
The first derivations of human embryonic stem cells (ESCs) and embryonic germ cells (EGCs) were published in 1998 (Shamblott et al., 1998; Thomson et al., 1998). Both of these seminal papers concluded with discussion of the potential for the use of these cells in transplantation-based treatments and cures and emphasized the need to address the challenge of immune rejection, either through the development of cell banks, akin to the registries described previously, or through the genetic modification of the cells to create universal donor cells or to match the particular cellular therapy to the particular patient.
Unlike bone marrow or cord blood, however, the source of these cells was human embryos and fetal tissue, and at the time of these publications, there was already a notable history of governance of these tissues (Matthews and Yang, 2019; Green, 1995; NIH, 1994). In addition, the Dickey-Wicker Amendment had been in place for 3 years, prohibiting the use of federal funds to create human embryos for research or to conduct research in which human embryos are “destroyed, discarded, or knowingly subjected to risk of injury or death” (104th Congress, 1995). Within weeks of the papers’ publication, a legal opinion was issued from the Department of Health and Human Services (HHS) interpreting Dickey-Wicker with regard to the new research (Rabb, 1995). Though federal dollars could not be used to create ESCs or EGCs, it was determined that federal dollars could be used to conduct research with pluripotent stem cells thus derived. This interpretation was supported later that year by a report of the National Bioethics Advisory Commission (NBCA, 1999). This did not, however, settle the issue.
A year later, President George W. Bush was elected following a campaign in which he made clear his opposition to this research (Cimons, 2001). In August 2001, in his first address to the nation, President Bush announced that federal funding would be permitted for research using the approximately 60 ESC lines already in existence at the time of his announcement, but not for research with newly derived lines (CNN, 2001). The president seemed to be attempting to walk a fine line between allowing promising research to move forward and not causing the federal government (and taxpayers) to be complicit in the destruction of human embryos. Ultimately, many of these 60 approved “Bush lines” proved impossible to access or difficult to work with. Furthermore, the accounting required in institutions and laboratories working with both “Bush lines” and newer lines was daunting (Murugan, 2009).
As ethical and policy debates raged, states began passing their own legislation governing human ESC research, beginning with California, and creating over time a patchwork of state-level policy that ranged from providing government funding for ESC research, as in California, to classifying the work as a felony, such as in Arizona (CIRM.ca.gov, n.d.; Justia US Law, 2020). In 2005, Congress passed its own bill that would permit federal funding of research with an expanded number of human ESC lines, but the bill was subsequently vetoed by President Bush (109th Congress, 2005). The same year, the National Research Council and the Institute of Medicine published its tremendously influential report titled Guidelines for Human Embryonic Stem Cell Research (IOM and NRC, 2005). These guidelines led to highly effective self-regulation in the field, as the Guidelines were adopted across the United States at institutions conducting human ESC research (Robertson, 2010). The Guidelines recommended the creation of a new institutional oversight committee to review ESC research, similar to IRBs, among other recommendations. The Guidelines remained the primary source of governance for ESC research through the end of the Bush administration.
An additional scientific innovation during this time was the announcement of the creation of iPSCs in 2006 (Nobel Prize, 2012; Takahashi and Yamanaka, 2006). iPSCs are derived from somatic tissue, not embryonic or fetal tissue, through the introduction of a small set of transcription factors that effectively reset the mature cell back to a pluripotent state. This concept had actually been introduced as an alternative to ESCs by President Bush’s bioethics commission, though it had been met with skepticism, and Shinya Yamanaka’s announcement at the 2006 International Society for Stem Cell Research (ISSCR) annual meeting stunned the assembled scientists (Scudellari, 2016; The President’s Council on Bioethics, 2005). This scientific end-run around the destruction of human embryos led to a flood of new researchers, as scientists now needed only somatic cells, rather than highly regulated embryonic or fetal tissue, to participate in this new wave of regenerative medicine research.
By the end of President Bush’s second term, in addition to the National Academies’ Guidelines, guidelines were also issued from the ISSCR and a number of other academic groups (ISSCR, n.d.; The Hinxton Group, 2006). Internationally, as in the United States, a patchwork of policy responses had emerged, ranging from very restrictive to permissive to supportive, leading both domestically and internationally to a degree of “brain drain” as some scientists relocated to jurisdictions that permitted this research (Verginer and Riccaboni, 2021; Levine, 2012).
When President Barack Obama took office in 2009, he issued an Executive Order reversing former president Bush’s prior actions (White House, 2009). Rather than establishing the final rules himself, he permitted funding of ESC research “to the extent permitted by law” (a nod to the Dickey-Wicker Amendment) and charged the National Institutes of Health (NIH) with developing guidelines for such funding. The NIH guidelines, which largely followed the Guidelines, were finalized in July 2009 and were promptly tied up in a years-long battle in the courts until the Supreme Court declined to hear the final appeal in 2013, leaving the NIH guidelines intact (NIH, 2013, 2009).
Genetic Modification
The final piece of the regenerative medicine puzzle is the need to overcome immune rejection of transplanted cells. As noted in the initial HPSC papers, potential ways to overcome immune rejection (in the absence of iPSCs) included both banking of a large number of diverse cell lines and genetic modification of the cells intended for transplant, although at the time the technology to do so did not exist (Faden et al., 2003). Gene therapy of this sort had been contemplated for years, and gene transfer trials had begun in the 1990s using the tools scientists had at the time (IOM, 2014). Governance structures grew up around these trials, including the transition of the Recombinant DNA Advisory Committee (RAC) from reviewing NIH-funded research involving recombinant DNA (rDNA) to reviewing gene transfer protocols (IOM, 2014). Of note, though the RAC served as a model internationally for the governance of rDNA research, its mandate was repeatedly questioned and its work critiqued, even as its role evolved (IOM, 2014). As the pace and volume of gene transfer research picked up, the pace of review slowed. Responding not only to the resulting critiques but also the accumulated experience and data, the RAC relaxed restrictions and expedited reviews where possible, ultimately pivoting again to a focus on novel protocols, and leaving more straightforward protocols to the U.S. Food and Drug Administration (FDA) to approve or deny (IOM, 2014). But the original vision of genetically tailored cellular therapy articulated in the 1998 papers did not become possible until almost 15 years later.
In 2012, the publication of the paper that introduced clustered regularly interspaced short palindromic repeats-CRISPR associated protein 9 (CRISPR-Cas9) launched a new era of genetic modification (Jinek et al., 2012). This new tool dramatically improved upon prior gene editing tools with respect to technical ease, speed, and cost, putting the kind of editing imagined in the 1998 papers within reach.
What are the key questions, research areas, and products or applications today?
Hspc transplant access.
Today, median health care costs for HSPC (including the procedure and 3 months of follow-up) in the United States are approximately $140,000–$290,000, depending on the type of procedure (Broder et al., 2017). While 200-day nonremission mortality has decreased substantially since 2000, it remains high (11%) (McDonald et al., 2020). The risks of transplant remain a significant barrier to access, in particular for those with nonmalignant disease, such as SCD. Beyond this, and as noted previously, there are significant ethnic and racial disparities in access to HSPC transplant, largely due to the relatively lower probability of identifying a well-matched HSPC donor (Barker et al., 2019). A recent study demonstrated that while White patients of European descent have a 75 percent chance of finding a well-matched (8/8 HLA-matched) donor, for White Americans of Middle Eastern or North African descent, the probability is 46 percent (Gragert et al., 2014). For Hispanic, Asian, Pacific Islander, and Native American individuals, the probability of such a match ranges from 27 to 52 percent, and for Black Americans, the probability is 16–19 percent (Gragert et al., 2014). Contributing to these disparities for racial and ethnic minority groups are higher HLA diversity, smaller numbers of racial and ethnic minority volunteers in donor registries, and the higher rates at which matched minority volunteers become unavailable for donation (e.g., due to inability to reach the volunteer or medical deferral due to diabetes, asthma, infectious disease, or other identified condition) (Sacchi et al., 2008; Kollman et al., 2004). Giving preference to 8/8 HLA-matched pairs therefore benefits White patients and disadvantages patients of color, but removing this preference might result in higher rates of graft failure. Attempts to balance these competing considerations raise ethical questions about justice and beneficence.
Another ethical question in HSPC transplantation revolves around compensation or incentives for donation. Increasing the number and availability of HSCP donors would improve the probability of identifying an appropriate unrelated match for patients in need of a transplant, but the 1984 National Organ Transplant Act (NOTA) banned the sale of bone marrow and organs, making the provision of financial incentives to donate illegal (98th Congress, 1983). Nonetheless, debates over the ethics of providing incentives to encourage the donation of bone marrow and HSCs persist among bioethicists and health economists. In an effort to reduce disincentives to donate, the federal government offers up to 1 work week of leave for federal employees who donate bone marrow, and most states have followed suit for state employees (Lacetera et al., 2014). Some states also offer tax deductions for nonmedical donation-related costs, and there is some evidence that these types of legislation do lead to modest increases in donation rates (Lacetera et al., 2014).
Although removing disincentives to donation is generally considered ethically acceptable, there is more debate about whether offering financial incentives for donation equates to a morally problematic commodification of the human body. In 2011, the 9th Circuit held in Flynn v. Holder that compensation for the collection of PBSCs does not violate NOTA’s ban on compensation (Cohen, 2012). In response, a coalition of cell therapy organizations published a statement arguing that this decision would mean that donors would no longer be motivated by altruism, and that people seeking to sell PBSCs might withhold important health information (Be the Match, 2012). After a regulatory back-and-forth over the status of PBSCs, HHS withdrew a proposed rule that would have effectively reversed Flynn v. Holder, so the current state of the law allows compensation for PBSCs (Todd, 2017).
Genetic Hematologic Disease: The Case of Sickle Cell Disease
Although Linus Pauling declared sickle cell disease (SCD) to be the first “molecular disease” (i.e., the first disease understood at the molecular level) in 1949, and it has long been considered an ideal target for gene therapy given that it is predominantly caused by a single mutation in the HBB gene and its phenotypic consequences are in a circulating cell type, developing a cure has not been as straightforward as hoped (Pauling et al., 1949). Though the presentation of SCD can vary significantly, clinical effects include anemia, painful vaso-occlusive crises, acute chest syndrome, splenic sequestration, stroke, chronic pulmonary and renal dysfunction, growth retardation, and premature death (OMIM, n.d.a.).
Standard treatment for SCD consists primarily of preventative and supportive care, including prophylactic penicillin, opioids for severe chronic pain, hydroxyurea, and transfusion therapy (Yawn et al., 2014). Such care has dramatically increased the life expectancy of those living with SCD (median survival in the United States is in the mid- to late 40s) (Wailoo, 2017; Ballas et al., 2016; Prabhakar et al., 2010). At the same time, this care costs more than $35,000 annually, and many patients have difficulty accessing such high-quality care, particularly adequate pain management (Bergman and Diamond, 2013; Haywood, 2013; Haywood et al., 2009; Kauf et al., 2009; Smith et al., 2006). Until recently, the only evidence-based cure for SCD and beta-thalassemia major was allogeneic hematopoietic cell transplantation (HCT), which comes with significant costs and risks (Bhatia and Walters, 2008).
Despite the fact that SCD is one of the most common genetic diseases worldwide and it was the first genetic disease to be molecularly defined, it has received relatively little research funding over the years, an observation that has been a frequent subject of critique (Farooq et al., 2020; Demirci et al., 2019; Benjamin, 2011; Smith et al., 2006; Scott, 1970). In contrast to better-funded diseases, such as cystic fibrosis and Duchenne muscular dystrophy, which are more common in White individuals of European descent, in the United States, SCD predominantly affects non-Hispanic Black and Hispanic populations, including 1 in 365 Black individuals and 1 in 16,300 Hispanic individuals (OMIM, n.d.b., n.d.c.; CDC, 2022). This disparity in research funding despite disease prevalence is part of the larger story of the impacts of structural racism in the United States and on its medical system (The New York Times, 2019; IOM, 2003; HHS and AHRQ, 2003).
Furthermore, as noted previously, those of African and Hispanic ancestry are less likely to be able to identify a suitable match in the existing registries. Due to this difficulty, the improvements in treatment not focused on an HSPC transplant, and the risks of such a transplant, relatively few patients with SCD are treated with HSPC transplant (Yawn et al., 2014; Benjamin, 2011). Gene therapy delivered in the context of an autologous HSPC transplant offers the possibility not only of a safer cure but also broader access by eliminating the need to identify a matched donor.
Recently, the promise of regenerative medicine and gene therapy for genetic hematologic disease appears to be coming to fruition (Ledford, 2020; Stein, 2020; Kolata, 2019). While a number of approaches are currently in various stages of preclinical and clinical research, two promising clinical trials involve the induction of fetal hemoglobin (rather than direct correction of the disease-causing mutation in the HBB gene) (Demirci et al., 2019). Fetal hemoglobin is the predominant globin type in the second and third trimester fetus and for the first few months of life, at which point production shifts from fetal to adult hemoglobin. It has long been recognized that SCD does not present until after this shift occurs (Watson et al., 1948). Furthermore, some patients with the causative SCD mutation are nonetheless asymptomatic, due to also having inherited hereditary persistence of fetal hemoglobin mutations (Stamatoyannopoulos et al., 1975). These findings and others suggested that inducing fetal hemoglobin, even in the presence of a faulty HBB gene, could mitigate the disease.
The first trial uses a viral vector to introduce into autologous bone marrow a short hairpin RNA (shRNA) that inhibits the action of the BCL11A gene. BCL11A is an inhibitor of fetal hemoglobin, so when BCL11A is inhibited, fetal hemoglobin can be produced (Esrick et al., 2021). The second trial—the first published study to use CRISPR to treat a genetic disease—includes both patients with SCD and with transfusion-dependent ß-thalassemia (Frangoul et al., 2021). In this trial, CRISPR-Cas9 is used to target the BCL11A gene to affect the same de-repression of fetal hemoglobin as in the first trial. Both trials, which have collectively enrolled more than 15 patients, have reduced or eliminated the clinical manifestation of disease in all patients thus far, though it remains to be seen how long-lasting this effect will be. However, the first trial was recently suspended after participants in the first trial and a related trial developed acute myeloid leukemia (AML) or myelodysplastic syndrome (MDS) (Liu, 2021); an investigation is under way regarding the cause of the AML and MDS. Marketing of a treatment for transfusion-dependent ß -thalassemia currently approved and available in the European Union (EU) was also suspended, as that treatment is manufactured using the same vector (BB305 lentiviral vector) used in the current trials, and it is possible that the vector is the source of the serious adverse events in the research participants.
Further challenges remain, including technical challenges, such as the possibility that gene editing tools, as they are derived from bacterial systems, will provoke an immune response; and concerns about financial access, given the anticipated cost of such curative therapies (ICER, 2021; Kim et al., 2018). In addition, despite the technical ease of the technology and designing new nucleic acid targets, intellectual property protecting CRISPR has, to date, narrowed the number of developers actively pursuing CRISPR-based clinical trials (Sherkow, 2017). At the same time, this new technology might also solve a number of ethical issues around HSPC transplants, including by expanding biological access to HSPC transplant and mitigating the concerns raised by the creation of “savior siblings” for HLA-matched cord blood transplantation for older siblings (Kahn and Mastroianni, 2004).
Unproven Cell-Based Interventions
A long-standing challenge in the field of regenerative medicine is the DTC marketing of unproven cell-based interventions. Since at least the 2000s, unscrupulous scientists and health professionals in the United States and internationally have been offering “stem cell therapy” at significant cost, often to vulnerable individuals, and without a legitimate scientific or medical basis (Knoepfler and Turner, 2018; Murdoch et al., 2018; Regenberg et al., 2009; Enserink, 2006). From 2009 to 2016, the number of such clinics in the United States doubled annually (Knoepfler and Turner, 2018). While the clinics look legitimate, their claims are fantastical, promising to treat or cure everything from knee pain to Parkinson’s disease. Such clinics are often vague about the cell sources involved in the interventions offered, but sometimes they claim to use bone marrow, cord blood, embryonic stem cells, and iPSCs, as well as other types of autologous adult stem cells (e.g., adipose, olfactory) and a range of other cell types, cell sources, and cell mixtures (Murdoch et al., 2018). While such interventions launch from legitimate science and scientific potential, the claims exceed and diverge from what is proven. The interventions are at best very expensive placebos and at worst could cause serious harm or death (Bauer et al., 2018).
Over time, attempts have been made to rein in these clinics by the FDA, the Federal Trade Commission (FTC), the ISSCR, individual customers and their lawyers, and others, but these attempts have faced a number of challenges (Pearce, 2020). The ISSCR, the primary professional society for those engaged in regenerative medicine, has struggled for years against such clinics. Early on, they attempted to establish a mechanism to publicly vet these clinics, though the effort was abandoned in part due to push back from the clinics’ lawyers (Taylor et al., 2010; personal communication from ISSCR Leadership, n.d.). In part because the majority of US-based clinics offer autologous interventions (removing and then reintroducing the patient’s own cells), the FDA struggled to clarify the line between medical practice and their regulatory authority. The FDA began issuing occasional warning letters to these clinics starting in 2011, though the letters were issued infrequently (Knoepfler and Turner, 2018). Under this relatively weak enforcement, the market expanded dramatically, and pressure increased on the FDA to take meaningful action (Knoepfler, 2018; Turner and Knoepfler, 2016).
In late 2017, the FDA took several significant steps to curtail these clinics, including using U.S. marshals to seize product from a California clinic, bringing a lawsuit against a Florida clinic, and publishing largely celebrated finalized guidance outlining a risk-based approach to the regulation of regenerative medicine products (FDA, 2019; Pew Research Center, 2019). The following year, the FTC took independent action against clinics making false claims about their interventions, and Google banned advertising for “unproven or experimental medical techniques such as most stem cell therapy, cellular (non-stem) therapy, and gene therapy” (Biddings, 2019; Fair, 2018). In 2019, the FDA won their case against US Stem Cells in Florida, significantly strengthening their ability to regulate these clinics (Wan and McGinley, 2019). Following the establishment of clear regulatory authority over at least a subset of clinics, FDA has begun to step up its enforcement (Knoepfler, 2020; Wan and McGinley, 2019; FDA, 2018). Increased action is anticipated following the end of the 3-year grace period established in the 2017 guidance, though there is some concern about the capacity of the agency to make significant headway against the more than 600 clinics now in operation—a worry bolstered by a 2019 study suggesting that despite increased enforcement, the unproven stem cell market seems to have shifted rather than contracted (Knoepfler, 2019; Pew Research Center, 2019). What seems clear is that it will take a collective and multipronged approach to ensure that the cell-based interventions to which patients have access are safe and effective (Lomax et al., 2020; Pew Research Center, 2019; Master et al., 2017; Zarzeczny et al., 2014).
Cross-Sectoral Footprint
The cross-sectoral analysis is structured according to sectors (see Figure 2 ) and domains (science and technology, governance and enforcement, end-user affordability and insurance reimbursement [affordability and reimbursement], private companies, and social and ethical considerations). The sectors described subsequently are intended to be sufficiently broad to encompass a number of individuals, groups, and institutions that have an interest or role in regenerative medicine. Health care is the primary nonprofit actor of interest, and so in this structure, “health care” has replaced “nonprofit,” though other nonprofit actors may have a role in this and other emerging technologies, and, of course, not all health care institutions are nonprofits.

Today, many regenerative medicine technologies are researched, developed, and promoted by a scientific-industrial complex largely driven by market-oriented goals. The development of various components of regenerative medicine may be altered by differing intellectual property regimes. This larger ecosystem is also embedded in a broad geopolitical context, in which the political and the economic are deeply intertwined, shaping national and regional investment and regulation. The political economy of emerging technologies involves and affects not only global markets and regulatory systems across different levels of government but also nonstate actors and international governance bodies. Individuals and societies subsequently adopt emerging technologies, adjusting their own values, attitudes, and norms as necessary, even as these technologies begin to shape the environments where they are deployed or adopted. Furthermore, individual and collective interests may change as the “hype cycle” of an emerging technology evolves (Gartner, 2022). Stakeholders in this process may include scientific and technological researchers, business firms and industry associations, government officials, civil society groups, worker safety groups, privacy advocates, and environmental protection groups, as well as economic and social justice–focused stakeholders (Marchant et al., 2014).
This intricate ecosystem of stakeholders and interests may be further complicated by the simultaneous introduction of other technologies and platforms with different constellations of ethical issues, modes of governance, and political economy contexts. In the following sections, this ecosystem is disaggregated and organized for ease of presentation. It is important to keep in mind that there are entanglements and feedback loops between and among the different sectors, such that pulling on a single thread in one sector often affects multiple areas and actors across the broader ecosystem.
Cross-Sectoral Analysis
For the purposes of this case study, the primary actors within the academic sector are academic and clinical researchers and the professional societies that represent them.
Science and technology: This case involves a tremendous amount of research and development that has taken place in and grown out of academia, including preclinical and clinical HSPC transplant research; human ESC, EGC, and iPSC research; and genome editing.
Governance and enforcement: Current work at research institutions is governed by IRBs and REBs, stem cell research oversight committees, and institutional animal care and use committees, among other bodies. In addition, research funding bodies, academic publication standards, and scientific and professional societies (i.e., self-regulation) also have a role to play—in particular, the ISSCR and its role in the governance of pluripotent stem cell research and in addressing clinics offering unproven cell-based therapies. The National Academies of Sciences, Engineering, and Medicine played a critical role in the governance of ESC research, particularly from 2005 until 2010.
Affordability and reimbursement: While not strictly a matter of patient affordability, it is important to reiterate, as noted previously, that funding available for academic research has disproportionately benefited those with diseases such as cystic fibrosis and Duchenne muscular dystrophy, which are more common in White individuals of European descent, compared to SCD, which in the United States is more prevalent among non-Hispanic Black and Hispanic populations (Farooq et al., 2020; Demirci et al., 2019; Benjamin, 2011; Smith et al., 2006; Scott, 1970).
Private companies: Academic–industry research partnerships, including industry-funded clinical trials, are involved in this space; for example, the CRISPR-based clinical trial was funded by two biotechnology companies (Frangoul et al., 2021). Such partnerships are often predicated on exclusive intellectual property licenses to “surrogate licensors” (Contreras and Sherkow, 2017).
Social and ethical considerations: Extensive bioethics literature exists on the ethical, legal, and societal issues raised by human subjects research, first-in-human clinical trials, stem cell research, clinics offering unproven cell-based interventions, genome editing, health disparities, and structural racism. Much has also been written on the role of intellectual property and data and materials sharing in the context of human tissue research and genome editing.
Health Care
Given the focus of CESTI on health and medicine, for the purpose of this case study, the primary actors within the nonprofit sector are those involved in health care, including hematopoietic stem and progenitor cell registries, health insurance companies, and medical profession associations.
Science and technology: HSPC transplants have been clinically available for decades, but research and improvement in this space continue.
Governance and enforcement: Today, the WMDA serves as the accrediting body for registries and promulgates regulations and standards to which the registries adhere on issues like the organization of a registry, the recruitment of volunteer donors, and the collection and transportation of HPCs (WMDA, 2022; Hurley et al., 2010). These standards represent the minimum guidelines for registries, which “demonstrate their commitment to comply with WMDA Standards through the WMDA accreditation process” (Hurley et al., 2010). Other groups involved in the governance of aspects of HSPC transplant are included in Table 1.

It is important to note that the “nonprofit” label in this context is somewhat fraught. Many (perhaps most) health care organizations are very much in the business of making money. One of these is the NMDP, which operates Be the Match, and which has diversified its portfolio over time, including the launch in 2016 of Be the Match BioTherapies, which partners with dozens of cell and gene therapy companies, supplying cells and services to “advance the development of life-saving cell and gene therapies” (Be the Match, 2021a,b).
The FDA generally has authority to regulate bone marrow transplantation through its oversight of bone marrow itself as a human cellular tissue product (HCT/P) and, therefore, a “biologic” (U.S. Code § 262, n.d.). Typically, biologic products are required to submit to the FDA’s premarket review process, including the filing of an investigative new drug application and clinical trials. With that said, the FDA has exempted certain types of bone marrow transplantation procedures from such review: namely, bone marrow products that are used in a same-day surgical procedure and those that are only “minimally manipulated” (FDA, 2020). Importantly, while the FDA’s minimally manipulated exception broadly applies to autologous therapy, including the sort of therapy private cord blood banks are intended to plan for, it only applies to allogenic therapy if derived from a “first-degree or second-degree blood relative”; allogenic therapy using cells from more distant relatives requires the FDA’s premarket review (FDA, 2020).
Cord blood matching and donor priority is controlled by the NMDP and regulated by the FDA (CFR, 2012). However, because cord blood therapy is almost always allogenic and usually from anonymized donors unrelated to the patient, cord blood HSPC transplant generally does not fulfill the FDA’s “minimal manipulation” exemptions for HCT/P (FDA, 2020). As such, a total of eight public cord blood banks have applied for, and received, approval from the FDA for their cord blood products (FDA, 2022). Generally, public banks are held to transparent, rigorous storage and quality standards that do not apply to private banks, leading to lower overall quality of cord blood in private banks (Shearer et al., 2017; Sun et al., 2010; Committee on Obstetric Practice, 2008).
The American Academy of Pediatrics has taken a position on private versus public cord blood banks and supports public banking, as do the American Medical Association and the American Congress of Obstetricians and Gynecologists (AMA, n.d.; ACOG, 2019; Shearer et al., 2017).
Affordability and reimbursement: Both public and private insurers in the United States tend to distinguish autologous from allogenic bone marrow therapies, covering autologous transplantation for some indications and allogenic transplantation for others (CMS, 2016).
Leaving aside the broader issues of health insurance and health care affordability in the United States, annual and lifelong care costs for genetic hematologic diseases like SCD and thalassemia are considerable—the yearly cost of standard of care for a patient with SCD is more than $35,000 (Kauf et al., 2009). Novel therapies—both pharmacologic and those based on HSPC transplants—are anticipated to be extraordinarily expensive, if proven safe and effective. For example, the drugs Oxbryta and Adakveo, approved in 2019 for treating SCD, are estimated to cost $84,000 and $88,000 per year, respectively (ICER, 2021; Sagonowsky, 2020). CART-T cell therapy, which as another novel, genetically modified cell-based therapy may be a reasonable bellwether for the cost of the SCD therapies described previously, costs at least $373,000 for a single infusion before hospital and other associated costs (Beasley, 2019). Many patients suffering from these diseases are from historically marginalized and underserved populations that tend to have lower levels of income. In addition, as therapies become more bespoke, scaling will increasingly become a challenge, from both a regulatory and delivery perspective. However, these delivery challenges may also open new business opportunities.
While donation of cord blood to a public bank is free to the donor, costs associated with private banking include a collection fee ($1,350–$2,300) and annual storage fees ($100–$175 a year), which are unlikely to be covered by health insurance (Shearer et al., 2017).
Private companies: Many private companies advertise private cord blood banking to new parents as a form of biological insurance; however, the costs of collection and storage are not generally covered by medical insurance (private companies offering unproven cell-based interventions are included under the private sector rather than health care).
Social and ethical considerations: Significant literature exists on health disparities and racism in medicine, including their impact on patients with SCD in particular. As noted previously, the likely high costs of these therapies raise serious concerns about access. There is also literature on ethical issues raised by the private cord blood market.
Private Sector
For the purposes of this case study, the primary actors within the private sector are companies involved in basic and translational regenerative medicine research and clinics offering unproven cell-based interventions.
Science and technology: Many private biotechnology companies are involved in regenerative medicine and genome editing research and development. A recent analysis predicted that the global CRISPR genome editing market (including CRISPR products, applications, and end-users) could grow from about $850 million in 2019 to $10 billion by 2030 (BIS Research, n.d.). In the United States, there are more than 600 clinics offering unproven cell-based interventions.
Governance and enforcement: The ISSCR attempted to establish a mechanism to publicly vet clinics selling unproven cell-based interventions, though the effort was ended in part due to push back from the clinics’ lawyers (personal communication from ISSCR leadership, n.d.; Taylor et al., 2010). In part because the majority of U.S. clinics offer autologous interventions (removing and then reintroducing the patient’s own cells), the FDA has struggled to clarify the line between medical practice and their regulatory authority in this space. Under relatively weak enforcement, the market expanded dramatically.
In late 2017, the FDA took several significant steps to curtail these clinics, including using U.S. marshals to seize product from a California clinic, bringing a lawsuit against a Florida clinic, and publishing largely celebrated finalized guidance that outlined a risk-based approach to the regulation of regenerative medicine products (FDA, 2019; Pew Research Center, 2019). The following year, the FTC took independent action against clinics making false claims about their interventions, and Google banned advertising for “unproven or experimental medical techniques such as most stem cell therapy, cellular (non-stem) therapy, and gene therapy” (Biddings, 2019; Fair, 2018). In 2019, the FDA won their case against U.S. stem cells in Florida, significantly strengthening their ability to regulate these clinics (Wan and McGinley, 2019). Following the establishment of clear regulatory authority over at least a subset of clinics, the FDA has begun to step up its enforcement (Knoepfler, 2020; Wan and McGinley, 2019; FDA, 2018).
Affordability and reimbursement: Unproven cell-based interventions can cost anywhere from several thousand dollars to tens of thousands of dollars (Regenberg et al., 2009). These costs are not covered by insurance. Patients have engaged in public fundraising campaigns, including on crowdfunding sites, to raise the money necessary to access the unproven intervention.
Private companies: There are far too many companies offering unproven cell-based interventions to list, though a recent accounting can be found in a supplemental table to Turner and Knoepfler, 2016.
Social and ethical considerations: Many have written about the ethical and policy issues raised by DTC unproven cell-based interventions and private cord blood banks, including issues related to truth-telling, taking advantage of historically marginalized and underserved individuals, and significant financial costs and physical risk in the absence of demonstrable benefit, among other issues.
For the purposes of this case study, the primary actors within the government sector are the FDA, the FTC, the NIH, and other regulatory bodies.
Science and technology: The federal government, and especially the NIH, has funded a tremendous amount of the research outlined in this case and is a critical part of the biotechnology research and development ecosystem.
Governance and enforcement: NOTA banned the sale of bone marrow and organs (98th Congress, 1983). Nonetheless, debates over the ethics of providing incentives to encourage the donation of bone marrow and HSCs persist among bioethicists and health economists. In an effort to reduce disincentives to donate, the federal government offers up to 1 week of leave for federal employees who donate bone marrow, and most states have followed suit for state employees. Some states also offer tax deductions for nonmedical donation-related costs, and there is some evidence that these types of legislation do lead to modest increases in donation rates (Lacetera et al., 2014).
Regarding pluripotent stem cell research, current governance of federally funded research includes the Dickey-Wicker Amendment and NIH’s 2009 guidelines, which remain in effect.
A notable approach to governance of cell-based interventions in Japan and elsewhere is the implementation of a sunset provision for therapy approvals (Maeda et al., 2015). Combined with post-market surveillance, this mechanism creates a default that a provisionally approved therapy comes off the market after a defined period of time unless proven safe and effective. While this model has faced challenges in Japan due to the pressure to keep approved interventions on the market, it has been more successful than similar provisions implemented for drug approvals in Europe (Maeda et al., 2015).
A significant challenge of HSPC transplants, combined with CRISPR and other technologies going forward, will be monitoring for late effects and the governance structures associated with that process.
Affordability and reimbursement: Proven HSPC transplants may be covered by public funding schemes; unproven cell-based interventions are not.
Private companies: N/A
Social and ethical considerations: Concerns in this sector include the disproportionate lack of research funding available for genetic hematologic disease, such as SCD and thalassemia; public funding of embryonic stem cell research; and the role of the public in decision-making about research that bears on questions of human meaning (Frangoul et al., 2021).
Volunteer/Consumer
For the purposes of this case study, the primary actors within the volunteer/consumer sector are patients and consumers seeking regenerative medicine–based solutions to their medical concerns. It is important to keep in mind that many members of “the public” nationally and internationally never have the opportunity to be patients or consumers of emerging technologies, and so do not show up in the following analysis. These members of the public may nonetheless be affected by the development, deployment, and use of such technologies, and those impacts should be taken into account.
Science and technology: There are few approved regenerative medicine–based therapies in the United States or internationally beyond those described previously, though there are many clinical trials under way.
Governance and enforcement: The ISSCR attempted to establish a mechanism to publicly vet clinics selling unproven cell-based interventions, though the effort was abandoned in part due to push back from the clinics’ lawyers (Personal communication from ISSCR leadership, n.d.; Taylor et al., 2010). The ISSCR does have educational materials available for the public on this topic (A Closer Look at Stem Cells, 2022).
In 2018, the FTC took independent action against clinics making false claims about their interventions, and Google banned advertising for “unproven or experimental medical techniques such as most stem cell therapy, cellular (non-stem) therapy, and gene therapy” (Biddings, 2019; Fair, 2018). Reducing access to information about these clinics could lead to decreased use by customers. Direct action against the clinics by the FDA is described in the “Private Sector” section.
Affordability and reimbursement: As noted previously, unproven cell-based interventions can cost anywhere from several thousand dollars to tens of thousands of dollars (Regenberg et al., 2009). These costs are not covered by insurance. Patients have engaged in public fundraising campaigns, including on crowdfunding sites, to raise the money necessary to access the unproven intervention.
Private companies: Clinics offering unproven cell-based interventions and private cord blood banks are covered in the “Health Care” and “Private Sector” sections.
Social and ethical considerations: There are significant concerns about safety, therapeutic misconception among consumers, and use in children and other historically marginalized and underserved groups whose members lack the capacity to consent.
Ethical and Societal Implications
What is morally at stake what are the sources of ethical controversy does this technology/application raise different and unique equity concerns.
In outlining the concerns of the authors in terms of the use of this technology, we considered the following ethical dimensions, as outlined in the recent National Academies of Sciences, Engineering, and Medicine report, A Framework for Addressing Ethical Dimensions of Emerging and Innovative Biomedical Technologies: A Synthesis of Relevant National Academies Reports (NASEM, 2019).
- Promote societal value
- Minimize negative societal impact
- Protect the interests of research participants
- Advance the interests of patients
- Maximize scientific rigor and data quality
- Engage relevant communities
- Ensure oversight and accountability
- Recognize appropriate government and policy roles
It is important to keep in mind that different uses of this technology in different populations and contexts will raise different constellations of issues. For example, HSPC transplants for malignancies raise different issues than the same therapy for SCD; both of these are of course quite different than the many uses of unproven cell-based interventions in patients outside standard clinical care. Some of the specific concerns might include the following:
- How should the risks and benefits of first-in-human clinical trials be weighed?
- How should the risks/benefits of (ideal) existing standards of care be balanced against the risks/benefits of novel attempts at cures?
- What should be the role of the public in the governance of research and applications that bear on questions of human meaning?
- How can regulators more effectively address clinics offering DTC unproven cell-based interventions, including issues related to truth-telling, taking advantage of historically marginalized and underserved people, and significant risks in the absence of demonstrable benefit?
- In the DTC marketplace, how can the safety of interventions offered be ensured, and how can therapeutic misconception among consumers, including parents of sick children, be avoided?
- How can and should historical and ongoing health disparities, structural racism, and racism in medicine be taken into account in the assessment of new technologies?
- What are the benefits and challenges of intellectual property and data and materials sharing in the context of human tissue research and genome editing?
- What is the role of science and data in the governance of the private cord blood market?
- What is the appropriate governance response when the relevant regulatory authority lacks sufficient funds to execute its authority?
Beyond Regenerative Medicine
As noted at the beginning of this case, regenerative medicine, its applications, and its implications are very broad. The same work that enabled the development of iPSCs, and therefore the matching of cellular therapies to particular individuals, has also led to improved understanding of the processes of cellular aging and senescence (Svendsen, 2013). Despite the significant increase in average human lifespan, there has yet to be an equivalent increase in the human health span (Christensen et al., 2009). Diseases and conditions associated with age contribute to this discrepancy, causing older adults to spend more time in physiological deficiency, and have encouraged the scientific community to develop therapies that slow or even reverse the effects of aging (Beyret et al., 2018). The discovery of the ability to reverse cellular fate has encouraged researchers to better understand the biological process of aging, which could provide insight into the development of therapies to extend healthy longevity (Takahashi and Yamanaka, 2006). Several rejuvenation methods involving blood factors, metabolic changes, senescent cell ablation, and differing levels of cellular reprogramming are currently under investigation (Mahmoudi et al., 2019). Specific areas of interest include further research into the role of telomere shortening in cellular senescence and the ability of telomerase to counteract such shortening and extend cellular lifespan as well as applications of reprogramming aged stem cells into iPSCs or directly into tissue-specific stem cells (Spehar et al., 2020; Bernadotte et al., 2016; Nobel Prize, 2009; Bodnar et al., 1998). Moreover, genetic modifications to rejuvenate or extend the therapeutic effects of aged stem cells could enhance treatment capabilities for a multitude of diseases, including metabolic and neurodegenerative disorders (Navarro Negredo et al., 2020; Zhou et al., 2020; Ahmed et al., 2017).
Despite recent advances in the field of regenerative medicine, many challenges remain. Although the ability to reprogram cells in vitro is well documented, more work is needed to establish best practices for in vivo manipulation and to assess long-term outcomes in nonhuman animals before such therapies can be translated to the clinic (Beyret et al., 2018; Mertens et al., 2018). In addition, tampering with the natural safeguards that exist to prevent cellular reprogramming can lead to unintended consequences such as tumor growth (Brumbaugh et al., 2019; Abad et al., 2013). However, recent work to counteract the negative effects of aging shows promise that such challenges can be overcome. For example, Ocampo et al. explored partial cellular reprogramming by inducing temporary expression of the Yamanaka factors, Oct4, Sox2, Klf4, and c-Myc (OSKM) in vivo in mice (Ocampo et al., 2016). The results of their experiments demonstrated decreased cellular and physiological signs of aging; increased lifespan of progeroid mice; and shortened recovery time for older mice with metabolic diseases and muscle injury, all without the side effect of tumor growth. Continued investigation into the potential of regenerative medicine gives scientists the opportunity to better understand the aging process and to perhaps translate innovative therapies into the clinic to counteract the maladies that accompany old age.
As alluded to previously, it is possible to foresee numerous future scenarios regarding the evolution of regenerative medicine. In an effort to probe the kinds of worries that the authors have about the trajectories of emerging technologies, to expand the range of lessons learned from each case, and ultimately to “pressure test” the governance framework, the authors have developed a brief “visioning” narrative that pushes the technology presented in the core case 10–15 years into the future, playing out one plausible (but imagined) trajectory. The narrative was developed iteratively in collaboration with a case-specific working group, with additional feedback from all members of CESTI. All reviewers are acknowledged in the back matter of this paper. Each narrative is told from a particular perspective and is designed to highlight the social shifts that shape and are shaped by the evolving technology.
Regenerative Medicine Case Visioning Narrative
Perspective: Potential but conflicted off-label user
It is 2035. After the COVID-19 pandemic, mRNA delivery technology has expanded significantly. Scientists are now readily able to temporarily (and with some genome editing techniques, permanently) express synthetic proteins in a wide variety of cell types using lipid nanoparticle (LNP)-encased synthetic mRNA molecules. The mRNA mixture is delivered via simple intramuscular injection or intravenous infusion. In addition, researchers have made significant advancements in directing mRNA-LNPs to specific tissues.
Meanwhile, research on cellular rejuvenation has yielded dramatic insights into mechanisms of “turning back the cellular clock” via partial reprogramming. Researchers can now rejuvenate cellular function and growth. This can be accomplished by the transient (and careful) expression of the four Yamanaka factors—Oct3/4, Sox2, Klf4, and c-Myc—in a wide variety of human cell types. Researchers can now also provide safeguards to avoid the risk of tumor development. Early research in animals using a combination of mRNA-LNP and rejuvenation technology has produced startling insights. The technology appears to not only reverse aging in animals but also seems to extend youthful life—in some instances, for example, youthful life in treated mice was up to twice as long as in nontreated controls. Upon publication of these results, testing on extending the breeding life of racehorses quickly began. The implications for the technology are vast.
This marriage of mRNA delivery technology and cellular rejuvenation research has yielded two therapeutics, developed by LioRNA Therapeutics, which can dramatically reverse age-related conditions. A variant of this technology was first approved for use in pets. The first, an intramuscular shot delivered once every 5 to 10 years, rejuvenates T cell production to combat age-related deterioration; it is, essentially, an immune booster for aging. The second is a therapy designed to speed up healing of certain injuries in a variety of tissue types otherwise similarly affected by age-related deterioration. The results are astounding. Injuries that would have taken months to heal in older populations now take weeks; infections that would have claimed the lives of elderly patients are now easily surmountable with standard treatment. In addition, both therapies—as animal models indicated—seem to reverse the effects of aging. Whether they extend patients’ lifespans is, as of 2035, unclear but expected by many. Notably, however, LioRNA’s therapies do not cross the blood–brain barrier. The therapies are approved in the United States and the European Union in 2031.
The popular press—with help from LioRNA’s marketing team—hails the therapy as a “miracle” and a fulfillment of decades of promises of regenerative medicine. Scientists advocate the science behind the treatment through popular scientific outlets including TEDx talks and conferences, as well as YouTube and other forms of social media. The therapy is also immediately co-opted by professional athletes seeking to recover more quickly from their injuries. Amateur athletes in a variety of injury-inducing sports follow suit and post their accomplishments online. Bodybuilders have also adopted the therapy off-label to “naturally and undetectably” increase the benefits of strenuous exercise without the risk of injury. This extension of the therapy beyond its relatively narrow intended use raises its prestige, and the general public takes an interest. A number of adventurous younger people and wellness seekers get the shot off-label for enhancement purposes and brag about their newfound vigor on social media. This includes a number of celebrities and social media influencers.
In addition, because of a substantial global excess of idle mRNA LNP manufacturing facilities, left over from their dramatic expansion in 2022 to end the COVID-19 pandemic, a number of “wildcat” wellness clinics begin to attempt to copy LioRNA’s therapies to offer them as an “anti-aging cure.” Safety concerns related to these clinics abound.
By 2035, off-label use of LioRNA’s therapies (and copycats from various clinics) begin to take hold in various segments of the population. The treatment is especially popular in high-income areas where anti-aging interventions are popular (e.g., Los Angeles and South Korea). Some of the interest in the technology may also be related to public financial austerity programs around the world with respect to health care for the elderly. Seeing a tremendous increase in the cost of gerontological care, especially as the populations of high-income countries age, well-off governments around the world have begun to restrict a variety of health care interventions for the elderly. Not knowing whether they will have adequate care when they are older, taking LioRNA’s therapy (or getting it from a wildcat clinic) is, to many, a sensible “hedging of their bets.”
While the technology has not yet transformed society, it is on the cusp of doing so. Patients (and practitioners, some of whom are ardent advocates of the technology) are faced with a number of issues as they navigate a series of choices about whether to use LioRNA’s anti-aging therapy for purposes beyond its narrow label.
Scott Oliveri, a 59-year-old, healthy, widowed, middle-class heating, ventilation, and air conditioning engineer in Ohio with two sons, is faced with many of these issues. Scott has seen the results of numerous friends—his peer age group—taking LioRNA’s therapy, some on-label, others off. The increased physical activity in Scott’s peer group—largely, greater participation in recreational sports—has induced a form of peer pressure to obtain the therapy or be left out of these popular activities. In addition, Scott desires—but is conflicted about—receiving the therapy so he can continue to work and delay his retirement. Scott is both concerned about the longevity of a social safety net for the elderly (e.g., Medicare), and philosophically uncomfortable with the safety net. He finds assessing his insurance coverage prior to treatments to be complicated.
Gerontological Disease Management
The existence of LioRNA’s therapy has begun to revolutionize gerontological disease management. The ethics of its use in patients among the medical community is hotly contested. After watching patients senesce or succumb to accidents, many practitioners have now begun advocating patients get the rejuvenation shot. In particular, the unintended effect of LioRNA’s therapy on muscle production seems to miraculously stave off aging-related sarcopenia. As a consequence, in the United States, many physicians prescribe the treatment off-label or, even where indicated, prescribe it for the primary purpose of achieving rejuvenation benefits in their patients. In other instances, when physicians attempt to discuss healthy living and healthy aging with their patients, they are often cut short by discussions surrounding getting the shot, even for aging-related diseases for which the shot has little effect.
Use of the therapy, on- or off-label, is complicated by the fact that the therapies do not cross the blood–brain barrier. As a consequence, the effect of the technology on age-related dementia and other mental impairments is, as of 2035, entirely unclear. Early data suggest a risk of differential aging: the number of older, healthy, and physically able patients with declining mental acuity appears to be high. Alarmingly, in a subset of patients, the therapy has differential effects across tissues (e.g., it is shown to successfully rejuvenate muscle, but does not have the same effect on an injured tendon), which results in severe chronic pain. This is discounted by some physicians but is a topic of significant concern for others. Scott is vaguely aware of these concerns, but, as informed by his peer group, he believes these “side effects” to be small. In addition, Scott’s primary care physician, who he has seen for 20 years, is not a gerontological specialist and is not as up to date on these nuances of LioRNA’s therapy as other colleagues.
Insurance Practice
Insurers are initially hesitant to widely cover the LioRNA therapies, and they only partially reimburse or cover the therapy (and only where the primary indication—age-related immune deficiencies and injury recovery—is present). Some insurers, however, seeing the enormous benefit of the therapy beyond its label (and its cost-effectiveness), and begin to mandate the treatment as top-line therapy before covering others, especially where “injury” is present, using an intentionally broad definition. Less desirable interventions, some of which are, by clinical estimations, inferior to mRNA anti-aging treatment, become second-line therapy, if used at all. In addition, some insurers have negotiated value-based agreements with LioRNA, which have proven remarkably successful for both LioRNA and some payers, especially those covering aging populations. As a consequence, many patients, presented with their insurers’ directives, are induced to choose the therapy for a wide variety of conditions even where they would not otherwise choose the treatment. Scott, however, is 59 years old and healthy and is below the age and indication cutoff for many of these incentive programs. He has had difficulty getting an answer from his insurer as to whether and what extent he would be reimbursed for treatment. Scott has heard that friends his age who have injured themselves playing recreational sports or otherwise by accident were entitled to full reimbursement from their insurers. Scott has joked that one “needs to get hurt to get insurance to pay for the shot.”
Equity, Access, and Medical Tourism
Some uninsured patients, the “worried middle aged,” and LioRNA enthusiasts begin to visit wildcat clinics in the United States for either cheaper versions of the shot or for certain modifications, including tissue targeting for reproductive issues. Given the safety profile of these compounded treatments, many are injured as a result; it is also unclear if the modified forms of the technology work as advertised, as the evidence is mixed. Other patients resort to anti-aging medical tourism where mRNA-LNP manufacturing capacity is most available (notably India, following the increase in manufacturing capacity post COVID-19 pandemic). This has the effect of raising the therapy’s price globally and diminishing access to the poorest among a number of low- and middle-income countries, despite the increase in mRNA-LNP production capacity. This is lamented by a number of public health researchers who point out, correctly, that the world’s poorest are the most negatively affected by aging relative to other groups. In this sense, differential access will likely have a significant and negative impact on health equity.
The popularity of LioRNA’s therapy, and excess mRNA-LNP manufacturing capacity on a contract basis worldwide, has spurred a major biohacking movement. Biohackers are developing their own versions of the LioRNA therapies and also creating their modifications, both for disease-treatment and for enhancement purposes. Online, biohackers share mRNA sequences for synthesis, manufacture, and injection, including modifications pertaining to various age-related concerns (e.g., age-related vision loss). Some of these experiments appear to be successful. Others, however, are less so, including complications pertaining to cancer risk so studiously avoided in LioRNA’s commercial products. Scott—otherwise unsure as to whether his insurer will cover the therapy—has been encouraged by one of his sons to visit a wildcat clinic to receive the therapy, on the premise that “it’s cheaper” and that he doesn’t “need to worry about insurance.” Scott has also heard from his son that a hobbyist could make a hacked version for him. Scott is concerned about safety issues, some of which have been present in the news and on social media.
Social Context of Aging
LioRNA’s technology, and its popularity, has made aging-related infirmities, once a normal facet of life, increasingly viewed as treatable maladies. Among the elderly and aging, aging-related health conditions are increasingly viewed with skepticism, in the same manner as contracting a communicable but preventable illness. There is peer social pressure to “get the shot,” exacerbated by social and other electronic media. In addition, declining standards in elderly home care facilities is leveraged to encourage those who are aging to use LioRNA’s treatments. Children of aging parents, worried about their care, are also pressuring their parents to use the therapy. Beyond all of this, there is a popular fear (yet to be appreciably realized) that the widespread use of the therapy will result in the diminishment of social safety nets for the elderly, including social security and Medicare. Scott is worried about these same issues and is cognizant of not wanting to be a burden on his children. As uptake of the therapy in his peer group increases, and he becomes more aware of memories of his parents aging, Scott is leaning toward accepting the therapy.
One of the therapy’s first, as well as most public and prominent, uses is among professional athletes. Again, because one of the treatment’s primary indications is rapidly healing from injury, physicians routinely prescribe the therapy to injured athletes. Some team physicians are selected, in part, on their willingness to prescribe the treatment to aging but valuable franchise athletes. All major professional sports see significant uptake of the therapy among their athletes, with significant pressure placed on the organizations’ collective bargaining efforts regarding whether the therapy is properly characterized as an “enhancement.” The therapy also becomes popular among amateur athletes who see it as a way to ward off injury. Scott, a sports enthusiast, is similarly moved by these efforts and their popularization online.
Regulation and Liability
The LioRNA treatments also challenge several precepts regarding regulation and consumer safety. On regulation, after years of developing guidelines regarding modular therapies (e.g., CAR-T and CRISPR-based therapies), the almost limitless indications and ease of modification of the therapies have challenged the FDA’s ability to police the line between biologic and medical practice, and between a commercial manufacturer and a compounding laboratory. This is largely complicated by reluctance, both in the White House and in Congress, to allow the FDA to take a more active enforcement role in shutting down the wildcat clinics and biohackers dedicated to producing variants of LioRNA’s treatments. Beyond this, the popularity of the therapy, and its introduction outside typical commercial channels in many cases, has complicated litigation concerning consumer safety.
Regenerative Medicine Case Study: Lessons Learned
Following are some of the lessons drawn from the preceding core case and visioning exercise that can inform the development of a cross-sectoral governance framework for emerging technologies focused on societal benefit.
- It is important to consider and articulate the role of the public in decision making regarding research and technology development bearing on questions of human meaning.
- Particularly in the absence of existing binding law or guidance, the National Academies (and other nongovernmental organizations) can play a critical role in governance, even when guidance is voluntary and nonbinding.
- Underfunded/understaffed agencies cannot effectively regulate every technology that falls within their mandate.
- The private sector can play a role in governance gaps (e.g., Google’s action regarding stem cell clinic ads).
- The governance ecosystem around a technology will evolve with the technology.
- A state-by-state regulatory patchwork can stifle innovation and reduce or reshape the workforce in a field.
- Public perception of a technology may shift in response to positive clinical developments.
- Early, public success or failure can have an outsized impact on the development of a technology.
- Politics throws a spanner in the works.
- There is a critical need for trustworthy institutions at all stages and levels of technology governance.
- Special attention must be paid to research and technologies to which not all patients have access due to limits on knowledge or availability of genetic variation in the research, product, or patient (biological access).
- Special attention must be paid to the impact of compounding inequities (e.g., biological access and structural racism).
- Sometimes scientific and technological solutions can be found to ethical concerns.
- Special attention must be paid to technologies based on human tissues and data (i.e., human tissue or data as product).
- Japan’s governance approach involving sunset provisions for therapy approvals, combined with post-market surveillance is an interesting model that has met with some success.
- Well-timed public pressure can prompt oversight.
- Society’s response to a technology’s off-label uses (including for enhancement) can shape its evolution as much as uptake of its intended use.
- Social structures (and future expectation of social structures) can influence uptake and vary across the globe.
- Technology can change social structures themselves (e.g., views on aging, injury).
- Access to and distribution of technology by nonlegacy players can affect use cases and uptake (e.g., the role of biohackers or the do-it-yourself community).
- Insurance coverage shapes uptake.
- For modular technologies (e.g., mRNA-LNPs), excess manufacturing capacity may act as a driver of secondary use and associated innovation.
Join the conversation!
Download the graphics below and share on social media!

- 98th Congress. 1983. S. 2048—National Organ Transplant Act. Available at: https://www.congress.gov/bill/98th-congress/senate-bill/2048 (accessed September 6, 2022).
- 104th Congress. 1995. H.R. 2880—Balanced Budget Downpayment Act. Available at: https://www.congress.gov/bill/104th-congress/house-bill/2880 (accessed September 6, 2022).
- 109th Congress. 2005. H.R. 810—Stem Cell Research Enhancement Act of 2005. Available at: https://www.congress.gov/bill/109th-congress/house-bill/810 (accessed September 6, 2022).
- A Closer Look at Stem Cells. 2022. The ISSCR patient handbook on stem cell therapies. Available at: https://www.closerlookatstemcells.org/patient-resources (accessed September 7, 2022).
- Abad, M., L. Mosteiro, C. Pantoja, M. Cañamero, T. Rayon, I. Ors, O. Graña, D. Megías, O. Domínguez, D. Martínez, M. Manzanares, S. Ortega, and M. Serrano. 2013. Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 502(7471):340–345. https://doi.org/10.1038/nature12586.
- Ahmed, A. S., M. H. Sheng, S. Wasnik, D. J. Baylink, and K. W. Lau. 2017. Effect of aging on stem cells. World Journal of Experimental Medicine 7(1):1–10. https://doi.org/10.5493/wjem.v7.i1.1.
- American College of Obstetricians and Gynecologists (ACOG). 2019. Umbilical cord blood banking, committee opinion number 771. Available at: https://www.acog.org/clinical/clinical-guidance/committee-opinion/articles/2019/03/umbilical-cord-blood-banking (accessed September 7, 2022).
- American Medical Association (AMA). n.d. Code of medical ethics opinion 6.1.5. Available at: https://www.ama-assn.org/delivering-care/ethics/umbilical-cord-blood-banking (accessed September 7, 2022).
- Apperley, J. F. 1993. Bone marrow transplant for the haemoglobinopathies: Past, present and future. Bailliere’s Clinical Haematology 6(1):299–325. https://doi.org/10.1016/s0950-3536(05)80074-5.
- Ballas, S. K., E. D. Pulte, C. Lobo, and G. Riddick-Burden. 2016. Case series of octogenarians with sickle cell disease. Blood 128(19):2367–2369. https://doi.org/10.1182/blood-2016-05-715946.
- Ballen, K. K., F. Verter, and J. Kurtzberg. 2015. Umbilical cord blood donation: Public or private? Bone Marrow Transplantation 50(10):1271–1278. https://doi.org/10.1038/bmt.2015.124.
- Ballen, K. K., E. Gluckman, and H. E. Broxmeyer. 2013. Umbilical cord blood transplantation: The first 25 years and beyond. Blood 122(4):491–498. https://doi.org/10.1182/blood-2013-02-453175.
- Ballen, K. K., T. R. Spitzer, B. Y. Yeap, S. McAfee, B. R. Dey, E. Attar, R. Haspel, G. Kao, D. Liney, E. Alyea, S. Lee, C. Cutler, V. Ho, R. Soiffer, and J. H. Antin. 2007. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biology of Blood and Marrow Transplantation 13(1):82–89. https://doi.org/10.1016/j.bbmt.2006.08.041.
- Barker, J. N., K. Boughan, P. B. Dahi, S. M. Devlin, M. A. Maloy, K. Naputo, C. M. Mazis, E. Davis, M. Nhaissi, D. Wells, C. Cooper, D. M. Ponce, N. Kernan, A. Scaradavou, S. A. Giralt, E. B. Papadopoulos, and I. Politikos. 2019. Racial disparities in access to HLA-matched unrelated donor transplants: A prospective 1312-patient analysis. Blood Advances 3(7):939–944. https://doi.org/10.1182/bloodadvances.2018028662.
- Barker, J. N., C. E. Byam, N. A. Kernan, S. S. Lee, R. M. Hawke, K. A. Doshi, D. S. Wells, G. Heller, E. B. Papadopoulos, A. Scaradavou, J. W. Young, and M. R. van den Brink. 2010. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biology of Blood and Marrow Transplantation 16(11):1541–1548. https://doi.org/10.1016/j.bbmt.2010.08.011.
- Barnes, D. W. H., M. J. Corp, J. F. Loutit, and F. E. Neal. 1956. Treatment of murine leukaemia with x rays and homologous bone marrow. British Medical Journal 2(4993):626–627. https://doi.org/10.1136/bmj.2.4993.626.
- Bauer, G., M. Elsallab, and M. Abou-El-Enein. 2018. Concise review: A comprehensive analysis of reported adverse events in patients receiving unproven stem cell-based interventions. Stem Cells Translational Medicine 7(9):676–685. https://doi.org/10.1002/sctm.17-0282.
- Be the Match. 2021a. Be the Match BioTherapies History. Available at: https://bethematchbiotherapies.com/about-us/history (accessed September 7, 2022).
- Be the Match. 2021b. Our Cell and Gene Therapy Partners. Available at: https://bethematchbiotherapies.com/about-us/our-partners (accessed September 7, 2022).
- Be the Match. 2012. Coalition says PBSC donor compensation poses health risks to patients and donors. Available at: https://bethematch.org/templates/displaynewsrelease/?id=707 (accessed September 6, 2022).
- Beasley, D. 2019. Expensive Gilead, Novartis cancer therapies losing patients to experimental treatments. Reuters, July 30. Available at: https://www.reuters.com/article/us-gilead-novartis-trials-focus/expensive-gilead-novartis-cancer-therapies-losing-patients-to-experimental-treatments-idUSKCN1UP10O (accessed September 7, 2022).
- Benjamin, R. 2011. Organized ambivalence: When sickle cell disease and stem cell research converge. Ethnicity & Health 16(4–5):447–463. https://doi.org/10.1080/13557858.2011.552710.
- Bergman, E. J., and N. J. Diamond. 2013. Sickle cell disease and the “difficult patient” conundrum. The American Journal of Bioethics 13(4):3–10. https://doi.org/10.1080/15265161.2013.767954.
- Bernadotte, A., V. M. Mikhelson, and I. M. Spivak. 2016. Markers of cellular senescense. Telomere shortening as a marker of cellular senescence. Aging 8(1):3–11. https://doi.org/10.18632/aging.100871.
- Beyret, E., P. Martinez Redondo, A. Platero Luengo, and J. C. Izpisua Belmonte. 2018. Elixir of life: Thwarting aging with regenerative reprogramming. Circulation Research 122(1):128–141. https://doi.org/10.1161/CIRCRESAHA.117.311866.
- Bhatia, M., and M. C. Walters. 2008. Hematopoietic cell transplantation for thalassemia and sickle cell disease: Past, present and future. Bone Marrow Transplantation 41(2):109–117. https://doi.org/10.1038/sj.bmt.1705943.
- Biddings, A. 2019. A new policy on advertising for speculative and experimental medical treatments. Google Ads Help. Available at: https://support.google.com/google-ads/answer/9475042 (accessed September 6, 2022).
- Billingham, R. E., and L. Brent. 1959. Quantitative studies on tissue transplantation immunity. IV. Induction of tolerance in newborn mice and studies on the phenomenon of runt disease. Philosophical Transactions of the Royal Society of London B 242:439–477. https://doi.org/10.1098/rstb.1959.0008.
- BIS Research. n.d. Global CRISPR gene editing market. Available at: https://bisresearch.com/industry-report/crispr-gene-editing-market.html (accessed September 7, 2022).
- Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279(5349):349–352. https://doi.org/10.1126/science.279.5349.349.
- Bortin, M. M. 1970. A compendium of reported human bone marrow transplants. Transplantation 9(6):571–587. https://doi.org/ 10.1097/00007890-197006000-00006.
- Broder, M. S., T. P. Quock, E. Chang, S. R. Reddy, R. Agarwal-Hashmi, S. Arai, and K. F. Villa. 2017. The cost of hematopoietic stem-cell transplantation in the United States. American Health & Drug Benefits 10(7):366–374. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5726064 (accessed September 6, 2022).
- Brumbaugh, J., B. Di Stefano, and K. Hochedlinger. 2019. Reprogramming: Identifying the mechanisms that safeguard cell identity. Development 146(23):dev182170. https://doi.org/10.1242/dev.182170.
- U.S. Centers for Disease Control and Prevention (CDC). 2022. Data & statistics on sickle cell disease. Available at: https://www.cdc.gov/ncbddd/sicklecell/data.html (accessed September 6, 2022).
- Centers for Medicare & Medicaid Services (CMS). 2016. Stem cell transplantation (formerly 110.8.1.). Available at: https://www.cms.gov/medicare-coverage-database/view/ncd.aspx?NCDId=366 (accessed September 7, 2022).
- Christensen, K., G. Doblhammer, R. Rau, and J. W. Vaupel. 2009. Ageing populations: The challenges ahead. Lancet 374(9696):1196–1208. https://doi.org/10.1016/S0140-6736(09)61460-4.
- Cimons, M. 2001. Bush is a threat to US stem-cell research. Nature Medicine 7(3):263–263. https://doi.org/10.1038/85369.
- CIRM.ca.gov. n.d. Text of proposed laws: Proposition 71. Available at: https://www.cirm.ca.gov/sites/default/files/files/about_cirm/prop71.pdf (accessed September 6, 2022).
- CNN. 2001. President George W. Bush’s address on stem cell research. Available at: https://edition.cnn.com/2001/ALLPOLITICS/08/09/bush.transcript (accessed September 6, 2022).
- Code of Federal Regulations (CFR). 2012. 21 CFR 1271—Human cells, tissues, and cellular and tissue-based products. Available at: https://www.govinfo.gov/app/details/CFR-2012-title21-vol8/CFR-2012-title21-vol8-part1271 (accessed September 7, 2022).
- Cohen, I. G. 2012. Selling bone marrow—Flynn v. Holder. New England Journal of Medicine 366:296–297. https://doi.org/10.1056/NEJMp1114288.
- Committee on Obstetric Practice and Committee on Genetics. 2008. ACOG committee opinion number 399, February 2008: Umbilical cord blood banking. Obstetrics and Gynecology 111(2 Pt 1):475–477. https://doi.org/10.1097/AOG.0b013e318166603c.
- Contreras, J. L., and J. S. Sherkow. 2017. CRISPR, surrogate licenses, and scientific discovery. Science 355(6326):698–700. https://doi.org/10.1126/science.aal4222.
- de la Morena, M. T., and R. A. Gatti. 2010. A history of bone marrow transplantation. Immunology and Allergy Clinics of North America 30(1):1–15. https://doi.org/10.1016/j.iac.2009.11.005.
- Demirci, S., A. Leonard, J. J. Haro-Mora, N. Uchida, and J. F. Tisdale. 2019. CRISPR/Cas9 for sickle cell disease: Applications, future possibilities, and challenges. Advances in Experimental Medicine and Biology 1144:37–52. https://doi.org/10.1007/5584_2018_331.
- Department of Health and Human Services and Agency for Healthcare Research and Quality (HHS and AHRQ). 2003. National Healthcare Disparities Report. Rockville, MD: Department of Health and Human Services and Agency for Healthcare Research and Quality. Available at: https://repository.library.georgetown.edu/bitstream/handle/10822/711771/NHDR2003.pdf?sequence=1&isAllowed=y (accessed September 6, 2022).
- Eapen, M., V. Rocha, G. Sanz, A. Scaradavou, M. J. Zhang, W. Arcese, A. Sirvent, R. E. Champlin, N. Chao, A. P. Gee, L. Isola, M. J. Laughlin, D. I. Marks, S. Nabhan, A. Ruggeri, R. Soiffer, M. M. Horowitz, E. Gluckman, J. E. Wagner, Center for International Blood and Marrow Transplant Research, Acute Leukemia Working Party and Eurocord, European Group for Blood and Marrow Transplantation, and National Cord Blood Program, New York Blood. 2010. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: A retrospective analysis. Lancet Oncology 11(7):653–660. https://doi.org/10.1016/S1470-2045(10)70127-3.
- Enserink, M. 2006. Biomedicine. Selling the stem cell dream. Science 313(5784):160–163. https://doi.org/10.1126/science.313.5784.160.
- Esrick, E. B., L. E. Lehmann, A. Biffi, M. Achebe, C. Brendel, M. F. Ciuculescu, H. Daley, B. MacKinnon, E. Morris, A. Federico, D. Abriss, K. Boardman, R. Khelladi, K. Shaw, H. Negre, O. Negre, S. Nikiforow, J. Ritz, S.-Y. Pai, W. B. London, C. Dansereau, M. M. Heeney, M. Armant, J. P. Manis, and D. A. Williams. 2021. Post-transcriptional genetic silencing of BCL11A to treat sickle cell disease. New England Journal of Medicine 384:205–215. https://doi.org/10.1056/NEJMoa2029392.
- Faden, R. R., L. Dawson, A. S. Bateman-House, D. M. Agnew, H. Bok, D. W. Brock, A. Chakravarti, X. J. Gao, M. Greene, J. A. Hansen, P. A. King, S. J. O’Brien, D. H. Sachs, K. E. Schill, A. Siegel, D. Solter, S. M. Suter, C. M. Verfaillie, L. B. Walters, and J. D. Gearhart. 2003. Public stem cell banks: Considerations of justice in stem cell research and therapy. The Hastings Center Report 33(6):13–27. https://doi.org/10.2307/3527822.
- Fair, L. 2018. Stemming unproven stem cell therapy claims. Federal Trade Commission Business Blog, October 18. Available at: https://www.ftc.gov/business-guidance/blog/2018/10/stemming-unproven-stem-cell-therapy-claims (accessed September 6, 2022).
- Farooq, F., P. J. Mogayzel, S. Lanzkron, C. Haywood, and J. J. Strouse. 2020. Comparison of US federal and foundation funding of research for sickle cell disease and cystic fibrosis and factors associated with research productivity. JAMA Network Open 3(3):e201737. https://doi.org/ 10.1001/jamanetworkopen.2020.1737.
- U.S. Food and Drug Administration (FDA). 2022. Approved cellular and gene therapy products. Available at: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (accessed September 7, 2022).
- FDA. 2020. Regulatory considerations for human cells, tissues, and cellular and tissue-based products: Minimal manipulation and homologous use. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal (accessed September 7, 2022).
- FDA. 2019. Framework for the regulation of regenerative medicine products. Available at: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/framework-regulation-regenerative-medicine-products (accessed September 6, 2022).
- FDA. 2018. Letter regarding FDA’s regenerative medicine framework and enforcement discretion period for HCT/Ps. Available at: https://www.fda.gov/media/119936/download (accessed September 6, 2022).
- Frangoul, H., D. Altshuler, M. D. Cappellini, Y.-S. Chen, J. Domm, B. K. Eustace, J. Foell, J. de la Fuente, S. Grupp, R. Handgretinger, T. W. Ho, A. Kattamis, A. Kernytsky, J. Lekstrom-Himes, A. M. Li, F. Locatelli, M. Y. Mapara, M. de Montalembert, D. Rondelli, A. Sharma, S. Sheth, S. Soni, M. H. Steinberg, D. Wall, A. Yen, and S. Corbacioglu. 2021. CRISPR-Cas9 gene editing for sickle cell disease and ß-thalassemia. New England Journal of Medicine 384(3):252–260. https://doi.org/10.1056/NEJMoa2031054.
- Gartner. 2022. Gartner hype cycle. Available at: https://www.gartner.com/en/research/methodologies/gartner-hype-cycle (accessed September 6, 2022).
- Global Neuroethics Summit Delegates, K. S. Rommelfanger, S.-J. Jeong, A. Ema, T. Fukushi, K. Kasai, K. M. Ramos, A. Salles, and I. Singh. 2018. Neuroethics questions to guide ethical research in the international brain initiatives. Neuron 100(1):19–36. https://doi.org/10.1016/j.neuron.2018.09.021.
- Gluckman, E., V. Rocha, A. Boyer-Chammard, F. Locatelli, W. Arcese, R. Pasquini, J. Ortega, G. Souillet, E. Ferreira, J. P. Laporte, M. Fernandez, and C. Chastang. 1997. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord transplant group and the European blood and marrow transplantation group. New England Journal of Medicine 337(6):373–381. https://doi.org/10.1056/NEJM199708073370602.
- Gluckman, E., H. A. Broxmeyer, A. D. Auerbach, H. S. Friedman, G. W. Douglas, A. Devergie, H. Esperou, D. Thierry, G. Socie, P. Lehn, S. Cooper, D. English, J. Kurtzberg, J. Bard, and E. A. Boyse. 1989. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. New England Journal of Medicine 321(17):1174–1178. https://doi.org/10.1056/NEJM198910263211707.
- Gragert, L., M. Eapen, E. Williams, J. Freeman, S. Spellman, R. Baitty, R. Hartzman, J. D. Rizzo, M. Horowitz, D. Confer, and M. Maiers. 2014. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. Registry. New England Journal of Medicine 371(4):339–348. https://doi.org/10.1056/NEJMsa1311707.
- Granot, N. and R. Storb. 2020. History of hematopoietic cell transplantation: Challenges and progress. Haematologica 105(12). https://doi.org/10.3324/haematol.2019.245688.
- Green, R. M. 1995. Report of the Human Embryo Research Panel. Kennedy Institute of Ethics Journal 5(1):83–84.
- Haywood Jr., C. 2013. Disrespectful care in the treatment of sickle cell disease requires more than ethics consultation. The American Journal of Bioethics 13(4):12–14. https://doi.org/10.1080/15265161.2013.768857.
- Haywood, Jr., C., M. C. Beach, S. Lanzkron, J. J. Strouse, R. Wilson, H, Park, C. Witkop, E. B. Bass, and J. B. Segal. 2009. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. Journal of the National Medical Association 101(10):1022–1033. https://doi.org/ 10.1016/s0027-9684(15)31069-5.
- The Hinxton Group. 2006. An international consortium on stem cells, ethics, and law. February 24. Available at: http://www.hinxtongroup.org/au_trans_cs.html (accessed September 6, 2022).
- Hurley, C. K., L. Foeken, M. Horowitz, B. Lindberg, M. McGregor, N. Sacchi, and the WMDA Accreditation and Regulatory Committees. 2010. Standards, regulations and accreditation for registries involved in the worldwide exchange of hematopoietic stem cell donors and products. Bone Marrow Transplant 45(5):819–824. https://doi.org/10.1038/bmt.2010.8.
- Institute for Clinical and Economic Review (ICER). 2021. Crizanlizumab, voxelotor, and l-glutamine for sickle cell disease: Effectiveness and value: Evidence report. Available at: https://icer.org/wp-content/uploads/2021/02/ICER_SCD_Evidence-Report_031220-FOR-PUBLICATION.pdf (accessed September 6, 2022).
- Institute of Medicine (IOM). 2014. Oversight and review of clinical gene transfer protocols: Assessing the role of the Recombinant DNA Advisory Committee. Washington, DC: The National Academies Press. https://doi.org/10.17226/18577.
- Institute of Medicine (IOM). 2003. Unequal treatment: Confronting racial and ethnic disparities in health care. Washington, DC: The National Academies Press. https://doi.org/10.17226/12875.
- Institute of Medicine and National Research Council (IOM and NRC). 2005. Guidelines for human embryonic stem cell research. Washington, DC: The National Academies Press. https://doi.org/10.17226/11278.
- International Military Tribunal. 1949. Trials of war criminals before the Nuremberg Military Tribunals under Control Council law no. 10 Nurenberg, October 1946–April 1949. Washington, DC: Government Printing Office, 1950. Available at: https://lccn.loc.gov/2011525364 (accessed September 5, 2022).
- International Society for Stem Cell Research (ISSCR). n.d. Guidelines for stem cell research and clinical translation. Available at: https://www.isscr.org/guidelines (accessed September 6, 2022).
- Jinek, M., K. Chylinski, I. Fonfara, M. Hauer, J. A. Doudna, and E. Charpentier. 2012. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337(6096):816–821. https://doi.org/10.1126/science.1225829.
- Johnson, F. L., A. T. Look, J. Gockerman, M. R. Ruggiero, L. Dalla-Pozza, and F. T. Billings, 3rd. 1984. Bone-marrow transplantation in a patient with sickle-cell anemia. New England Journal of Medicine 311(12):780–783. https://doi.org/10.1056/NEJM198409203111207.
- Justia US Law. 2020. 2020 Arizona revised statutes title 36—Public health and safety. § 36-2313 Destructive human embryonic stem cell research; violation; classification. Available at: https://law.justia.com/codes/arizona/2020/title-36/section-36-2313 (accessed September 6, 2022).
- Kahn, J. P., and A. C. Mastroianni. 2004. Creating a stem cell donor: A case study in reproductive genetics. Kennedy Institute of Ethics Journal 14(1):81–96. https://doi.org/10.1353/ken.2004.0017.
- Kauf, T. L., T. D. Coates, L. Huazhi, N. Mody-Patel, and A. G. Hartzema. 2009. The cost of health care for children and adults with sickle cell disease. American Journal of Hematology 84(6):323–327. https://doi.org/10.1002/ajh.21408.
- Kessinger, A., J. O. Armitage, J. D. Landmark, D. M. Smith, and D. D. Weisenburger. 1988. Autologous peripheral hematopoietic stem cell transplantation restores hematopoietic function following marrow ablative therapy. Blood 71(3):723–727. https://doi.org/10.1182/blood.V71.3.723.723.
- Kim, S., T. Koo, H.-G. Jee, H.-Y. Cho, G. Lee, D.-G. Lim, H. S. Shin, and J.-S. Kim. 2018. CRISPR RNAs trigger innate immune responses in human cells. Genome Research 28(3):367–373. https://doi.org/10.1101/gr.231936.117.
- Knoepfler, P. 2020. Historic spring storm of FDA letters to stem cell clinic industry. The Niche, May 17. Available at: https://ipscell.com/2020/05/historic-spring-storm-of-fda-letters-to-stem-cell-clinic-industry (accessed September 6, 2022).
- Knoepfler, P. S. 2019. Rapid change of a cohort of 570 unproven stem cell clinics in the USA over 3 years. Regenerative Medicine 14(8):735–740. https://doi.org/10.2217/rme-2019-0064.
- Knoepfler, P. S. 2018. Too much carrot and not enough stick in new stem cell oversight trends. Cell Stem Cell 23(1):18–20. https://doi.org/10.1016/j.stem.2018.06.004.
- Knoepfler, P. S., and L. G. Turner. 2018. The FDA and the U.S. direct-to-consumer marketplace for stem cell interventions: A temporal analysis. Regenerative Medicine 13(1):19–27. https://doi.org/10.2217/rme-2017-0115.
- Kolata, G. 2019. These patients had sickle-cell disease. Experimental therapies might have cured them. The New York Times, January 27. Available at: https://www.nytimes.com/2019/01/27/health/sickle-cell-gene-therapy.html (accessed September 6, 2022).
- Kollman, C., E. Abella, R. L. Baitty, P. G. Beatty, R. Chakraborty, C. L. Christiansen, R. J. Hartzman, C. K. Hurley, E. Milford, J. A. Nyman, T. J. Smith, G. E. Switzer, R. K. Wada, and M. Setterholm. 2004. Assessment of optimal size and composition of the U.S. National Registry of Hematopoietic Stem Cell Donors. Transplantation 78(1):89–95. https://doi.org/10.1097/01.TP.0000132327.40702.97.
- Kurtzberg, J. 2017. A history of cord blood banking and transplantation. Stem Cells Translational Medicine 6(5):1309–1311. https://doi.org/10.1002/sctm.17-0075.
- Kurtzberg, J., M. Laughlin, M. L. Graham, C. Smith, J. F. Olson, E. C. Halperin, G. Ciocci, C. Carrier, C. E. Stevens, and P. Rubinstein. 1996. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. New England Journal of Medicine 335(3):157–166. https://doi.org/10.1056/NEJM199607183350303.
- Lacetera, N., M. Macis, and S. S. Stith. 2014. Removing financial barriers to organ and bone marrow donation: The effect of leave and tax legislation in the U.S. Journal of Health Economics 33:43–56. https://doi.org/10.1016/j.jhealeco.2013.10.006.
- Ledford, H. 2020. CRISPR gene therapy shows promise against blood diseases. Nature 588(7838):383. https://doi.org/10.1038/d41586-020-03476-x.
- Levine, A. D. 2012. State stem cell policy and the geographic preferences of scientists in a contentious emerging field. Science and Public Policy 39(4):530–541. https://doi.org/10.1093/scipol/scs038.
- Little, M.-T., and R. Storb. 2002. History of haematopoietic stem-cell transplantation. Nature Reviews: Cancer 2(3):231–238. https://doi.org/10.1038/nrc748.
- Liu, A. 2021. Bluebird Bio hits pause on rollout for ill-fated gene therapy Zynteglo as trial flags 2 cancer cases. Fierce Pharma, February 16. Available at: https://www.fiercepharma.com/pharma/bluebird-bio-halts-selling-ill-fated-gene-therapy-zynteglo-as-trial-flags-2-cancer-cases (accessed January 25, 2023).
- Lomax, G. P., A. Torres, and M. T. Millan. 2020. Regulated, reliable, and reputable: Protect patients with uniform standards for stem cell treatments. Stem Cells Translational Medicine 9(5):547–553. https://doi.org/10.1002/sctm.19-0377.
- Mahmoudi, S., L. Xu, and A. Brunet. 2019. Turning back time with emerging rejuvenation strategies. Nature Cell Biology 21(1):32–43. https://doi.org/10.1038/s41556-018-0206-0.
- Maeda, D., T. Yamaguchi, T. Ishizuka, M. Hirata, K. Takekita, and D. Sato. 2015. Regulatory frameworks for gene and cell therapies in Japan. Advances in Experimental Medicine and Biology 871:147–162. https://doi.org/10.1007/978-3-319-18618-4_8.
- Mannick, J. A., H. L. Lochte Jr, C. A. Ashley, E. D. Thomas, and J. W. Ferrebee. 1960. Autografts of bone marrow in dogs after lethal total-body radiation. Blood 127(17):2047. https://doi.org/10.1182/blood-2016-01-696476.
- Marchant, G. E., K. W. Abbott, and B. Allenby. 2014. Innovative governance models for emerging technologies. Cheltenham, UK: Edward Elgar Pub.
- Mason, C., and P. Dunnill. 2008. A brief definition of regenerative medicine. Regenerative Medicine 3(1):1–5. https://doi.org/ 10.2217/17460751.3.1.1.
- Master, Z., W. Fu, D. Paciulli, and D. Sipp. 2017. Industry responsibilities in tackling direct-to-consumer marketing of unproven stem cell treatments. Clinical Pharmacology and Therapeutics 102(2):177–179. https://doi.org/10.1002/cpt.704.
- Mathé, G., J. L. Amiel, L. Schwarzenberg, A. Cattan, and M. Schneider. 1965. Adoptive immunotherapy of acute leukemia: Experimental and clinical results. Cancer Research 25(9):1525–1531.
- Mathews, D. J. H. 2017. When emerging biomedical technologies converge or collide. In J. Illes (ed.), Neuroethics: Anticipating the future. Oxford Academic: United Kingdom. https://doi.org/10.1093/oso/9780198786832.003.0001.
- Mathews, K. R. W., and E. H. Yang. 2019. Politics and policies guiding human embryo research in the United States. Available at: https://www.bakerinstitute.org/research/politics-and-policies-guiding-human-embryo-research-united-states (accessed January 25, 2023).
- Maus, M. V. and S. Nikiforow. 2017. The why, what, and how of the new FACT standards for immune effector cells. Journal for Immunotherapy of Cancer 5(36). https://doi.org/10.1186/s40425-017-0239-0.
- McCann, S., and R. P. Gale. 2018. A brief history of bone marrow transplantation. Advances in Cell and Gene Therapy 1(1):e8. https://doi.org/https://doi.org/10.1002/acg2.8.
- McDonald, G. B., B. M. Sandmaier, M. Mielcarek, M. Sorror, S. A. Pergam, G.-S. Cheng, S. Hingorani, M. Boeckh, M. D. Flowers, S. J. Lee, F. R. Appelbaum, R. Storb, P. J. Martin, H. J. Deeg, G. Schoch, and T. A. Gooley. 2020. Survival, nonrelapse mortality, and relapse-related mortality after allogeneic hematopoietic cell transplantation: Comparing 2003–2007 versus 2013–2017 cohorts. Annals of Internal Medicine 172(4):229–239. https://doi.org/10.7326/M19-2936.
- Mertens, J., D. Reid, S. Lau, Y. Kim, and F. H. Gage. 2018. Aging in a dish: iPSC-derived and directly induced neurons for studying brain aging and age-related neurodegenerative diseases. Annual Review of Genetics 52:271–293. https://doi.org/10.1146/annurev-genet-120417-031534.
- Murdoch, B., A. R. Marcon, and T. Caulfield. 2020. The law and problematic marketing by private umbilical cord blood banks. BMC Medical Ethics 21(1):52. https://doi.org/10.1186/s12910-020-00494-2.
- Murdoch, B., A. Zarzeczny, and T. Caulfield. 2018. Exploiting science? A systematic analysis of complementary and alternative medicine clinic websites’ marketing of stem cell therapies. BMJ Open 8(2):e019414. http://dx.doi.org/10.1136/bmjopen-2017-019414.
- Murugan, V. 2009. Embryonic stem cell research: A decade of debate from Bush to Obama. The Yale Journal of Biology and Medicine 82(3):101–103. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2744932 (accessed September 6, 2022).
- National Academies of Sciences, Engineering, and Medicine (NASEM). 2019. Framework for addressing ethical dimensions of emerging and innovative biomedical technologies: A synthesis of relevant National Academies reports. Washington, DC: The National Academies Press. https://doi.org/10.17226/25491.
- National Bioethics Advisory Commission (NBCA). 1999. Ethical issues in human stem cell research: Report and recommendations of the National Bioethics Advisory Commission. Rockville, MD. Available at: https://hdl.handle.net/1805/23 (accessed September 6, 2022).
- National Institutes of Health (NIH). 2013. Statement by NIH Director Francis S. Collins, M.D., Ph.D., on Supreme Court’s decision regarding stem cell case. Available at: https://www.nih.gov/about-nih/who-we-are/nih-director/statements/statement-nih-director-francis-s-collins-md-phd-supreme-courts-decision-regarding-stem-cell-case (accessed September 6, 2022).
- NIH. 2009. NIH guidelines for human stem cell research. Available at: https://stemcells.nih.gov/research-policy/guidelines-for-human-stem-cell-research (accessed September 6, 2022).
- NIH. 1994. Report of the Human Embryo Research Panel. National Institutes of Health, Bethesda, MD. Available at: https://repository.library.georgetown.edu/bitstream/handle/10822/559352/human_embryo_vol_1.pdf?sequence=1&isAllowed=y (accessed September 6, 2022).
- The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research (NCPHSBBR). 1979. The Belmont Report: Ethical principles and guidelines for the protection of human subjects of research. Available at: https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html (accessed September 5, 2022).
- Navarro Negredo, P., R. W. Yeo, and A. Brunet. 2020. Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell 27(2):202–223. https://doi.org/10.1016/j.stem.2020.07.002.
- The New York Times. 2019. The 1619 Project. Available at: https://www.nytimes.com/interactive/2019/08/14/magazine/1619-america-slavery.html (accessed September 6, 2022).
- The Nobel Prize. 2012. Shinya Yamanaka Nobel lecture. Available at: https://www.nobelprize.org/prizes/medicine/2012/yamanaka/lecture (accessed September 6, 2022).
- The Nobel Prize. 2009. Carol W. Greider biographical. Available at: https://www.nobelprize.org/prizes/medicine/2009/greider/biographical (accessed September 7, 2022).
- The Nobel Prize. 1990. E. Donnall Thomas, facts. Available at: https://www.nobelprize.org/prizes/medicine/1990/thomas/facts/ (accessed September 6, 2022).
- The Nobel Prize. 1980. Jean Dausset Nobel lecture. Available at: https://www.nobelprize.org/prizes/medicine/1980/dausset/lecture (accessed September 6, 2022).
- Ocampo, A., P. Reddy, P. Martinez-Redondo, A. Platero-Luengo, F. Hatanaka, T. Hishida, M. Li, D. Lam, M. Kurita, E. Beyret, T. Araoka, E. Vazquez-Ferrer, D. Donoso, J. L. Roman, J. Xu, C. Rodriguez Esteban, G. Nuñez, E. Nuñez Delicado, J. M. Campistol, I. Guillen, P. Guillen, and J. C. Izpisua Belmonte. 2016. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell 167(7):1719–1733. https://doi.org/10.1016/j.cell.2016.11.052.
- Online Catalog of Human Genes and Genetic Disorders (OMIM). n.d.a. #603903 sickle cell anemia. Available at: https://www.omim.org/entry/603903 (accessed September 6, 2022).
- OMIM. n.d.b. #219700 cystic fibrosis; CF. Available at: https://www.omim.org/entry/219700 (accessed September 6, 2022).
- OMIM. n.d.c. #310200 muscular dystrophy; Duchenne type; DMD. Available at: https://www.omim.org/entry/310200 (accessed September 6, 2022).
- Osgood, E. E., M. C. Riddle, and T. J. Mathews. 1939. Aplastic anemia treated with daily transfusions and intravenous marrow; case report. Annals of Internal Medicine 13(2):357–367. https://doi.org/10.7326/0003-4819-13-2-357.
- Oudshoorn, M., A. van Leeuwen, H. G. vd Zanden, and J. J. van Rood. 1994. Bone marrow donors worldwide: A successful exercise in international cooperation. Bone Marrow Transplant 14(1):3–8.
- Pauling, L., H. A. Itano, S. J. Singer, and I. C. Wells. 1949. Sickle cell anemia, a molecular disease. Science 110(2865):543–548. https://doi.org/10.1126/science.110.2865.543.
- Pearce, D. A. 2020. We need better regulation of stem cell therapies, especially rogue clinics. Stat, February 6. Available at: https://www.statnews.com/2020/02/06/rogue-stem-cell-clinics-better-regulation (accessed September 6, 2022).
- Personal communication from ISSCR Leadership. n.d.
- Petersdorf, E. W. 2010. The World Marrow Donor Association: 20 years of international collaboration for the support of unrelated donor and cord blood hematopoietic cell transplantation. Bone Marrow Transplantation 45(5):807–810. https://doi.org/10.1038/bmt.2010.10.
- Pew Research Center. 2019. FDA’s framework for regulating regenerative medicine will improve oversight: Further action needed to facilitate development of safe, effective treatments. Available at: https://www.pewtrusts.org/en/research-and-analysis/reports/2019/10/17/fdas-framework-for-regulating-regenerative-medicine-will-improve-oversight (accessed September 6, 2022).
- Pidala, J., J. Kim, M. Schell, S. J. Lee, R. Hillgruber, V. Nye, E. Ayala, M. Alsina, B. Betts, R. Bookout, H. F. Fernandez, T. Field, F. L. Locke, T. Nishihori, J. L. Ochoa, L. Perez, J. Perkins, J. Shapiro, C. Tate, M. Tomblyn, and C. Anasetti. 2013. Race/ethnicity affects the probability of finding an HLA-A, -B, -C and -DRB1 allele-matched unrelated donor and likelihood of subsequent transplant utilization. Bone Marrow Transplantation 48(3):346–350. https://doi.org/10.1038/bmt.2012.150.
- Pinto, V. M., M. Poggi, R. Russo, A. Giusti, and G. L. Forni. 2019. Management of the aging beta-thalassemia transfusion-dependent population—The Italian experience. Blood Reviews 38:100594. https://doi.org/10.1016/j.blre.2019.100594.
- Prabhakar, H., C. Haywood Jr., and R. Molokie. 2010. Sickle cell disease in the United States: Looking back and forward at 100 years of progress in management and survival. American Journal of Hematology 85(5):346–353. https://doi.org/10.1002/ajh.21676.
- President’s Council on Bioethics. 2005. White paper: Alternative sources of pluripotent stem cells. Available at: https://bioethicsarchive.georgetown.edu/pcbe/reports/white_paper/index.html (accessed September 6, 2022).
- Quine, W. E. 1896. Chairman’s address. JAMA 26(21):1012–1016. https://doi.org/10.1001/jama.1896.02430730014001c.
- Rabb, H. S. 1999. Letter to Harold Varmus, MD: Federal funding for research involving human pluripotent stem cells. Available at: https://profiles.nlm.nih.gov/spotlight/mv/catalog/nlm:nlmuid-101584926X407-doc (accessed September 6, 2022).
- Regenberg, A. C., L. A. Hutchinson, B. Schanker, and D. J. Mathews. 2009. Medicine on the fringe: Stem cell‐based interventions in advance of evidence. Stem Cells 27(9):2312–2319. https://doi.org/10.1002/stem.132.
- Rekers, P. E., M. P. Coulter, and S. L. Warren. 1950. Effect of transplantation of bone marrow into irradiated animals. Archives of Surgery 60(4):635–667. https://doi.org/10.1001/archsurg.1950.01250010656001.
- Rickham, P. P. 1964. Human experimentation. Code of ethics of the world medical association. Declaration of Helsinki. British Medical Journal 2(5402):177. https://doi.org/10.1136/bmj.2.5402.177.
- Robertson, J. A. 2010. Embryo stem cell research: Ten years of controversy. Journal of Law, Medicine & Ethics 38(2):191–203. https://doi.org/10.1111/j.1748-720X.2010.00479.x.
- Sacchi, N., P. Costeas, L. Hartwell, C. K. Hurley, C. Raffoux, A. Rosenmayr, H. Greinix, and the Quality Assurance and Clinical Working Groups of the World Marrow Donor Association. 2008. Haematopoietic stem cell donor registries: World Marrow Donor Association recommendations for evaluation of donor health. Bone Marrow Transplantation 42(1):9–14. https://doi.org/10.1038/bmt.2008.76.
- Sagonowsky, E. 2020. New sickle cell drugs from Novartis, GBT need big discounts: ICER draft. Fierce Pharma, January 27. Available at: https://www.fiercepharma.com/pharma/new-sickle-cell-disease-drugs-from-novartis-global-blood-therapeutics-need-big-discounts (accessed September 7, 2022).
- Scott, R. B. 1970. Health care priority and sickle cell anemia. JAMA 214(4):731–734. https://doi.org/10.1001/jama.1970.03180040039008.
- Scudellari, M. 2016. How iPS cells changed the world. Nature 534(7607):310–312. https://doi.org/10.1038/534310a.
- Shamblott, M. J., J. Axelman, S. Wang, E. M. Bugg, J. W. Littlefield, P. J. Donovan, P. D. Blumenthal, G. R. Huggins, and J. D. Gearhart. 1998. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proceedings of the National Academy of Sciences 95(23):13726–13731. https://doi.org/10.1073/pnas.95.23.13726.
- Shearer, W. T., B. H. Lubin, M. S. Cairo, L. D. Notarangelo, Section on Hematology/Oncology, Section on Allergy and Immunology, J. Hord, G. Crouch, G. Hale, J. Harper, J. Lipton, Z. Rogers, E. Werner, E. C. Matsui, S. L. Abramson, C. Dinakar, A.-M. Irani, T. A. Mahr, J. S. Kim, M. Pistiner, and J. Wang. 2017. Cord blood banking for potential future transplantation. Pediatrics 140(5):e20172695. https://doi.org/10.1542/peds.2017-2695.
- Sherkow, J. S. 2017. CRISPR, patents, and the public health. Yale Journal of Biology and Medicine 90:667–672. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5733839/ (accessed September 6, 2022).
- Shyntum, Y., and E. Kalkreuter. 2009. Stem cell patents—reexamination/litigation—the last 5 years. Tissue Engineering: Part B Reviews 15(1):87–90. https://doi.org/10.1089/ten.teb.2008.0456.
- Smith, L. A., S. O. Oyeku, C. Homer, and B. Zuckerman. 2006. Sickle cell disease: A question of equity and quality. Pediatrics 117(5):1763–1770. https://doi.org/10.1542/peds.2005-1611.
- Spehar, K., A. Pan, and I. Beerman. 2020. Restoring aged stem cell functionality: Current progress and future directions. Stem Cells 38(9):1060–1077. https://doi.org/10.1002/stem.3234.
- Stamatoyannopoulos, G., W. G. Wood, T. Papayannopoulou, and P. E. Nute. 1975. A new form of hereditary persistence of fetal hemoglobin in blacks and its association with sickle cell trait. Blood 46(5):683–692. Available at: https://www.sciencedirect.com/science/article/pii/S0006497120742205?via%3Dihub (accessed September 6, 2022).
- Stein, R. 2020. 1st Patients to get CRISPR gene-editing treatment continue to thrive. NPR Morning Edition, December 15. Available at: https://www.npr.org/sections/health-shots/2020/12/15/944184405/1st-patients-to-get-crispr-gene-editing-treatment-continue-to-thrive (accessed September 6, 2022).
- Svendsen, C. N. 2013. Back to the future: How human induced pluripotent stem cells will transform regenerative medicine. Human Molecular Genetics 22(R1):R32–R38. https://doi.org/10.1093/hmg/ddt379.
- Sun, J., J. Allison, C. McLaughlin, L. Sledge, B. Waters-Pick, S. Wease, and J. Kurtzberg. 2010. Differences in quality between privately and publicly banked umbilical cord blood units: A pilot study of autologous cord blood infusion in children with acquired neurologic disorders. Transfusion 50(9):1980–1987. https://doi.org/10.1111/j.1537-2995.2010.02720.x.
- Tachibana, M., P. Amato, M. Sparman, N. M. Gutierrez, R. Tippner-Hedges, H. Ma, E. Kang, A. Fulati, H. S. Lee, H. Sritanaudomchai, K. Masterson, J. Larson, D. Eaton, K. Sadler-Fredd, D. Battaglia, D. Lee, D. Wu, J. Jensen, P. Patton, S. Gokhale, R. L. Stouffer, D. Wolf, and S. Mitalipov. 2013. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell 153(6):1228–1238. https://doi.org/10.1016/j.cell.2013.05.006.
- Takahashi, K., and S. Yamanaka. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676. https://doi.org/10.1016/j.cell.2006.07.024.
- Taylor, P. L., R. A. Barker, K. G. Blume, E. Cattaneo, A. Colman, H. Deng, H. Edgar, I. J. Fox, C. Gerstle, L. S. Goldstein, K. A. High, A. Lyall, R. Parkman, F. J. Pitossi, E. D. Prentice, H. M. Rooke, D. A. Sipp, A. Srivastava, S. Stayn, G. K. Steinberg, A. J. Wagers, and I. L. Weissman. 2010. Patients beware: Commercialized stem cell treatments on the web. Cell Stem Cell 7(1):43–49. https://doi.org/10.1016/J.STEM.2010.06.001.
- Thomas, E. D. 2005. Bone marrow transplantation from the personal viewpoint. International Journal of Hematology 81(2):89–93. https://doi.org/10.1532/ijh97.04197.
- Thomas, E. D. 1999. A history of haemopoietic cell transplantation. British Journal of Haematology 105(2):330–339. https://doi.org/10.1111/j.1365-2141.1999.01337.x.
- Thomas, E. D., C. D. Buckner, J. E. Sanders, T. Papayannopoulou, C. Borgna-Pignatti, P. De Stefano, K. M. Sullivan, R. A. Clift, and R. Storb. 1982. Marrow transplantation for thalassaemia. The Lancet 320(8292): 227–229. https://doi.org/10.1016/s0140-6736(82)90319-1.
- Thomas, E. D., H. L. Lochte, Jr., J. H. Cannon, O. D. Sahler, and J. W. Ferrebee. 1959. Supralethal whole body irradiation and isologous marrow transplantation in man. Journal of Clinical Investigation 38(10 Pt 1–2):1709–1716. https://doi.org/10.1172/JCI103949.
- Thomson, J. A., J. Itskovitz-Eldor, S. S. Shapiro, M. A. Waknitz, J. J. Swiergiel, V. S. Marshall, and J. M. Jones. 1998. Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147. https://doi.org/10.1126/science.282.5391.1145.
- Todd, K. 2017. Hope for to-marrow: The status of paid peripheral blood stem cell donation under the national organ transplant act. Journal of Law and the Biosciences 4(2):412–423. https://doi.org/10.1093/jlb/lsx027.
- Turner, L., and P. Knoepfler. 2016. Selling stem cells in the USA: Assessing the direct-to-consumer industry. Cell Stem Cell 19(2):154–157. https://doi.org/10.1016/j.stem.2016.06.007.
- U.S. Code § 262. Regulation of biological products. Available at: https://www.law.cornell.edu/uscode/text/42/262 (accessed September 7, 2022).
- Van Rood, J. J., and M. Oudshoorn. 2008. Eleven million donors in bone marrow donors worldwide! Time for reassessment? Bone Marrow Transplantation 41(1):1–9. https://doi.org/10.1038/sj.bmt.1705866.
- Verginer, L. and M. Riccaboni. 2021. Stem cell legislation and its impact on the geographic preferences of stem cell researchers. Eurasian Business Review 11:163–189. https://doi.org/10.1007/s40821-021-00182-0.
- Vermylen, C., E. Fernandez Robles, J. Ninane, and G. Cornu. 1988. Bone marrow transplantation in five children with sickle cell anaemia. Lancet 1(8600):1427–1428. https://doi.org/10.1016/s0140-6736(88)92239-8.
- Wailoo, K. 2017. Sickle cell disease—a history of progress and peril. New England Journal of Medicine 376(9):805–807. https://doi.org/10.1056/NEJMp1700101.
- Wailoo, K., and S. Pemberton. 2006. The troubled dream of genetic medicine: ethnicity and innovation in Tay-Sachs, cystic fibrosis, and sickle cell disease. Baltimore, MD: Johns Hopkins University Press.
- Wan, W., and L. McGinley. 2019. FDA wins groundbreaking case against for-profit stem cell company. The Washington Post, June 4. Available at: https://www.washingtonpost.com/health/fda-wins-groundbreaking-case-against-for-profit-stem-cell-company/2019/06/03/498373fa-864e-11e9-98c1-e945ae5db8fb_story.html (accessed September 6, 2022).
- Watson, J., A. W. Stahman, and F. P. Bilello. 1948. The significance of the paucity of sickle cells in newborn Negro infants. Obstetrical & Gynecological Survey 3(6):819–820. Available at: https://journals.lww.com/obgynsurvey/citation/1948/12000/the_significance_of_the_paucity_of_sickle_cells_in.22.aspx (accessed September 6, 2022).
- White House. 2009. Executive Order 13505. Removing barriers to responsible scientific research involving human stem cells. Available at: https://obamawhitehouse.archives.gov/the-press-office/removing-barriers-responsible-scientific-research-involving-human-stem-cells (accessed September 6, 2022).
- World Marrow Donor Association (WMDA). 2022. WMDA scientific publications & recommendations. Available at: https://share.wmda.info/pages/viewpage.action?pageId=322174994 (accessed September 7, 2022).
- WMDA. 2021. Total number of donors and cord blood units. Available at: https://statistics.wmda.info (accessed September 6, 2022).
- Yawn, B. P., G. R. Buchanan, A. N. Afenyi-Annan, S. K. Ballas, K. L. Hassell, A. H. James, L. Jordan, S. M. Lanzkron, R. Lottenberg, W. J. Savage, P. J. Tanabe, R. E. Ware, M. H. Murad, J. C. Goldsmith, E. Ortiz, R. Fulwood, A. Horton, and J. John-Sowah. 2014. Management of sickle cell disease: Summary of the 2014 evidence-based report by expert panel members. JAMA 312(10):1033–1048. https://doi.org/10.1001/jama.2014.10517.
- Yu, J., M. A. Vodyanik, K. Smuga-Otto, J. Antosiewicz-Bourget, J. L. Frane, S. Tian, J. Nie, G. A. Jonsdottir, V. Ruotti, R. Stewart, Slukvin, II, and J. A. Thomson. 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science 318(5858):1917–1920. https://doi.org/10.1126/science.1151526.
- Zarzeczny, A., T. Caulfield, U. Ogbogu, P. Bell, V. A. Crooks, K. Kamenova, Z. Master, C. Rachul, J. Snyder, M. Toews, and S. Zoeller. 2014. Professional regulation: a potentially valuable tool in responding to “stem cell tourism.” Stem Cell Reports 3(3):379–384. https://doi.org/10.1016/j.stemcr.2014.06.016.
- Zhou, X., Y. Hong, H. Zhang, and X. Li. 2020. Mesenchymal stem cell senescence and rejuvenation: Current status and challenges. Frontiers of Cell Development and Biology 8:364. https://doi.org/10.3389/fcell.2020.00364.
https://doi.org/10.31478/202311d
Suggested Citation
Mathews, D., A. Abernethy, E. Chaikof, R. A. Charo, G. Q. Daley, J. Enriquez, S. Gottlieb, J. Kahn, R. D. Klausner, S. Tavazoie, R. Fabi, A. C. Offodile II, J. S. Sherkow, R. D. Sullenger, E. Freiling, and C. Balatbat. 2023. Regenerative Medicine: Case Study for Understanding and Anticipating Emerging Science and Technology. NAM Perspectives. Discussion Paper, National Academy of Medicine, Washington, DC. https://doi.org/10.31478/202311d .
Author Information
Debra Mathews, PhD, MA, is Associate Director for Research and Programs at the Johns Hopkins Berman Institute of Bioethics and Professor, Department of Genetic Medicine at the Johns Hopkins University School of Medicine. Amy Abernethy, MD, PhD, is President of Product Development and Chief Medical Officer at Verily. Elliot Chaikof, MD, PhD, is Chair, Department of Surgery and Surgeon-in-Chief at Beth Israel Deaconess Medical Center, and Johnson and Johnson Professor of Surgery at Harvard Medical School. R. Alta Charo, JD, is Principal, Alta Charo Consulting, LLC and Warren P. Knowles Professor Emerita of Law and Bioethics at University of Wisconsin-Madison. George Q. Daley, MD, PhD, is Dean of the Faculty of Medicine and Caroline Shields Walker Professor of Medicine at Harvard Medical School. Juan Enriquez, MBA, is Managing Director at Excel Venture Management. Scott Gottlieb, MD, is Senior Fellow at the American Enterprise Institute. Jeffrey Kahn, PhD, is Andreas C. Dracopoulos Director and Robert Henry Levi and Ryda Hecht Levi Professor of Bioethics and Public Policy at Johns Hopkins Berman Institute of Bioethics, and Professor, Department of Health Policy and Management, Johns Hopkins Bloomberg School of Public Health. Richard D. Klausner, MD, is Founder and Board Chair at Lyell Immunopharma. Sohail Tavazoie, MD, PhD, is Leon Hess Profesor and Senior Attending Physician at The Rockefeller University. Rachel Fabi, PhD, is Associate Professor, Center for Bioethics and Humanities at SUNY Upstate Medical University. Anaeze C. Offodile II, MD, MPH, is Chief Strategy Officer at Memorial Sloan Kettering Cancer Center. Jacob S. Sherkow, JD, MA, is Professor of Law at the Illinois College of Law, Professor of Medicine at the Carle Illinois College of Medicine, Professor at the European Union Center, and Affiliate of the Carl R. Woese Institute for Genomic Biology at the University of Illinois. Rebecca D. Sullenger, BSPH, is a medical student at the Duke University School of Medicine. Emma Freiling, BA, is a Research Associate at the National Academy of Medicine. Celynne Balatbat, BA, was the Special Assistant to the NAM President at the National Academy of Medicine while this paper was authored.
Acknowledgments
This manuscript benefitted from the thoughtful input of Guillermo Ameer, Northwestern University; and Kavita Shah Arora, University of North Carolina at Chapel Hill
Conflict-of-Interest Disclosures
Amy Abernethy reports personal fees from Verily/Alphabet, relationships with Georgiamune and EQRx, and personal investments in Iterative Health and One Health, outside the submitted work. Elliot Chaikof reports grants from the National Institutes of Health, outside the submitted work. George Q. Daley reports holding equity from Redona Therapeutics and from iTCells, outside the submitted work. Juan Enriquez reports investments with Excel Venture Management, outside the submitted work; investments in various life science technologies, including leading-edge brain technologies, and co-authoring a book on the impact of emerging brain technologies. Scott Gottlieb reports personal fees from Pfizer, Inc, Illumina, Inc, Aetion, Tempus Labs, National Resilience, Inc, Cell-Carta, Parker Institute for Cancer Immunotherapy, Mount Sinai Health System, New Enterprise Associates, and American Enterprise Institute outside the submitted work. Sohail Tavazoie reports personal fees from Inspirna, outside the submitted work. Jacob S. Sherkow reports employment with the University of Illinois, grants from National Institutes of Health, and personal fees from Expert Consulting services, outside the submitted work.
Correspondence
Questions or comments should be directed to Debra Mathews at [email protected].
The views expressed in this paper are those of the authors and not necessarily of the authors’ organizations, the National Academy of Medicine (NAM), or the National Academies of Sciences, Engineering, and Medicine (the National Academies). The paper is intended to help inform and stimulate discussion. It is not a report of the NAM or the National Academies. Copyright by the National Academy of Sciences. All rights reserved.
Join Our Community
Sign up for nam email updates.
Neurology and Neurosurgery
- Study finds stem cell therapy is safe and may benefit people with spinal cord injuries
May 23, 2024
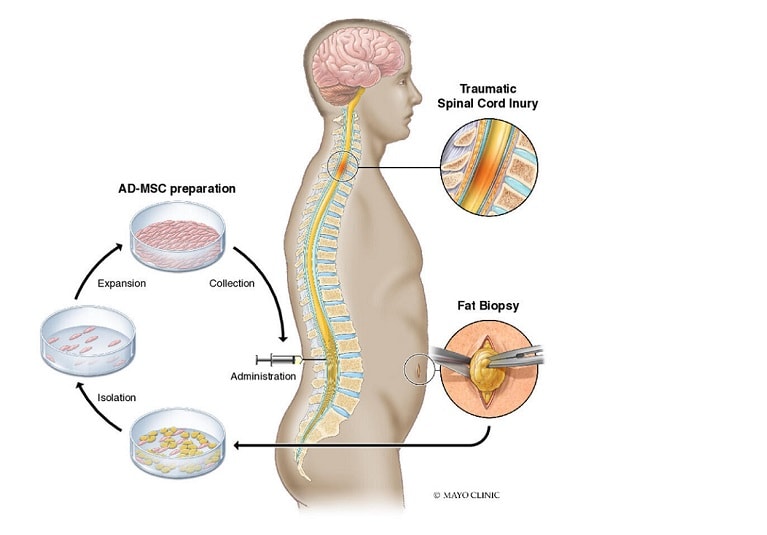
Mayo Clinic researchers have demonstrated the safety and potential benefit of stem cell regenerative medicine therapy for patients with subacute and chronic spinal cord injury.
The results of the phase 1 Clinical Trial of Autologous Adipose-Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury, known as CELLTOP, were published in Nature Communications.
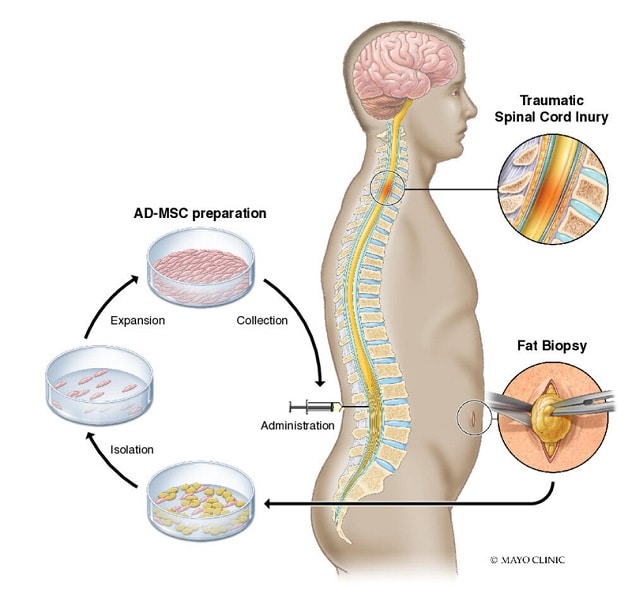
Illustration shows the process of fat harvest via biopsy, adipose-derived mesenchymal stem cells (AD-MSC) preparation and administration of treatment.
All trial participants had experienced traumatic spinal injury classified as grade A or B on the American Spinal Injury Association Impairment Scale (AIS). Stem cell treatment was initiated on average 11 months after injury. Participants were evaluated over a two-year period.
Key findings:
- Stem cells were successfully manufactured, and products were delivered to all 10 enrolled participants.
- No serious adverse effects occurred among any participants. The most commonly reported side effects were headache and musculoskeletal pain, which resolved with over-the-counter treatment.
- Seven participants demonstrated improvement, with each moving up at least one AIS grade.
As reported earlier in Mayo Clinic Proceedings, the first participant in the phase 1 trial was a superresponder who, after stem cell therapy, saw significant improvements in the function of his upper and lower extremities.
"Future research may show whether stem cells in combination with other therapies could be part of a new paradigm of treatment to improve outcomes for patients," says Mohamad Bydon, M.D. , a neurosurgeon at Mayo Clinic in Rochester, Minnesota, and the first author of both studies. "Not every patient who receives stem cell treatment is going to be a superresponder. One objective in our future studies is to delineate the optimal treatment protocols and understand why patients respond differently."
Dr. Bydon notes that stem cells' mechanism of action isn't fully understood. The researchers are analyzing changes in participants' MRI and cerebrospinal fluid to identify avenues for potential regeneration. Work is also underway on a larger, controlled trial of stem cell regenerative therapy.
"For years, treatment of spinal cord injury has been limited to stabilization surgery and physical therapy," Dr. Bydon says. "Many historical textbooks state that this condition does not improve. We have seen findings in recent years that challenge prior assumptions. This research is a step forward toward the ultimate goal of improving treatments for patients."
For more information
Bydon M, et al. Intrathecal delivery of adipose-derived mesenchymal stem cells in traumatic spinal cord injury: Phase I trial . Nature Communications. 2024;15:2201.
Bydon M, et al. CELLTOP clinical trial: First report from a phase I trial of autologous adipose tissue-derived mesenchymal stem cells in the treatment of paralysis due to traumatic spinal cord injury . Mayo Clinic Proceedings. 2020;95:406.
Refer a patient to Mayo Clinic.
Receive Mayo Clinic news in your inbox.
- Medical Professionals
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.

Viterbi Conversations in Ethics

Stem Cells: A Case for the Use of Human Embryos in Scientific Research
Embryonic stem cells have immense medical potential. While both their acquisition for and use in research are fraught with controversy, arguments against their usage are rebutted by showing that embryonic stem cells are not equivalent to human lives. It is then argued that not using human embryos is unethical. Finally, an alternative to embryonic stem cells is presented.
INTRODUCTION
Embryonic stem cells have the potential to cure nearly every disease and condition known to humanity. Stem cells are nature’s Transformers. They are small cells that can regenerate indefinitely, waiting to transform into a specialized cell type such as a brain cell, heart cell or blood cell [1]. Most stem cells form during the earliest stages of human development, immediately when an embryo is formed. These cells, known as embryonic stem cells (ESCs), eventually develop into every single type of cell in the body. As the embryo develops, adult stem cells (ASCs) replace these all-powerful embryonic stem cells. ASCs can only become a number of different cells within their potency. This limited application means an adult mesenchymal stem cell cannot become a neural cell.
By harnessing the unique ability of embryonic stem cells to transform into functional cells, scientists can develop treatments for a number of diseases and injuries, according to the California Institute for Regenerative Medicine, a private organization which awards grants for stem cell research [1]. For example, scientists at the Cleveland Clinic converted ESCs into heart muscle cells and injected them into patients who suffered from heart attacks. The cells continued to grow and helped the patients’ hearts recover [2].
With this enormous potential to cure devastating diseases, including heart failure, spinal cord injuries and Alzheimer’s disease, governments and research organizations have the moral imperative to support and encourage embryonic stem cell research. President Barack Obama signed an executive order in 2009 loosening federal funding restrictions on stem cell research, saying, “We will aim for America to lead the world in the discoveries it one day may yield.” [3]. The National Institute of Health and seven state governments, including California, Maryland and New York, followed Obama’s lead by creating programs that offered over $5 billion in funding and other incentives to scientists and research institutions for stem cell research [4].
A MIRACLE CURE
Scientists believe that harnessing the capability of embryonic stem cells will unlock the cure for countless diseases. “I am very excited about embryonic stem cells,” said Dr. Dieter Egli, professor of developmental cell biology at Columbia University. “They will lead to unprecedented discoveries that will transform life. I have no doubt about it.” [5]. The results thus far are inspiring. In 2016, Kris Boesen, a 21-year-old college student from Bakersfield, California, suffered a severe spinal cord injury in a car accident that left him paralyzed from the neck down. In a clinical trial conducted by Dr. Charles Liu at the University of Southern California Keck School of Medicine, Boesen was injected with 10 million embryonic stem cells that transformed into nerve cells [6]. Three months after the treatment, Boesen regained the use of his arms and hands. He could brush his teeth, operate a motorized wheelchair, and live more independently. “All I’ve wanted from the beginning was a fighting chance,” he said. The power of stem cells made his wish possible [6].
Embryonic stem cell treatments may also cure type 1 diabetes. Type 1 diabetes, which affects 42 million worldwide, is an autoimmune disorder that results in the destruction of insulin-producing beta cells found in the pancreas [7]. ViaCyte, a company in San Diego, California, is developing an implant that contains replacement beta cells originating from embryonic stem cells [7]. The implant will preserve or replace the original beta cells to protect them from the patient’s immune system [7]. The company believes that if successful, this strategy will effectively cure type 1 diabetes. Patients with the disease will no longer have to closely monitor their blood sugar levels and inject insulin [7]. ViaCyte projects that an experimental version of this implant will become available by 2020 [7].
Ultimately, scientists believe they will grow complex organs using stem cells within the next decade [8]. Over 115,000 people in the United States need a life-saving organ donation, and an average of 20 people die every day due to the lack of available organs for transplant, according to the American Transplant Foundation [9]. Three-dimensional printing of entire organs derived from stem cells holds the most promise for solving the organ shortage crisis [8]. Researchers at the University of California, San Diego have successfully printed part of a functional liver [8]. While the printed liver is not ready for transplant, it still performs the functions of a normal liver. This has helped scientists reduce the need for often cruel and unethical animal testing. The scientists expose drugs to the printed liver and observe how it reacts. The liver’s response closely mimics that of a human being’s and no living animals are harmed in the process [8].
HUMAN CELLS OR HUMAN LIFE?
Research using embryonic stems cells provides an unprecedented understanding of human development and the potential to cure devastating diseases. However, stem cell research has generated controversy among religious organizations such as the Catholic Church as well as the “pro-life” movement [3]. That is because scientists harvest stem cells from embryos donated by fertility clinics. Opponents of embryonicstem cell research equate the destruction of an embryo to the murder of an innocent human being [10]. Pope Benedict XVI said that harvesting stem cells is “not only devoid of the light of God but is also devoid of humanity” [3]. However, this view does not reflect a reasonable understanding and interpretation of basic biology. Researchers typically harvest embryonic stem cells from an embryo five days after fertilization [1]. At this stage, the entire embryo consists of less than 250 cells, smaller than the tip of a pin. Of these cells, only 30 are embryonic stem cells, which cannot perform any human function [11]. For comparison, an adult has more than 72 trillion cells, each with a specialized function [3]. Therefore, this microscopic blob of cells in no way represents human life.
With no functional cells, there exist no characteristics of a human being. Fundamentalist Christians believe that the presence or absence of a heartbeat signifies the beginning and end of a human life [10]. However, at this stage there is no heart, not even a single heart cell [10]. Some contend that brain activity, or the ability to feel, defines a human being. Michael Gazzaniga, president of the Cognitive Neuroscience Institute at the University of California, Santa Barbara, explains in his book, The Ethical Brain, that the “fertilized egg is a clump of cells with no brain.” [12]. There is no brain nor nerve cells that could allow this cellular object to interact with its environment [12]. The only uniquely human feature of embryonic cells at this stage is that they contain human DNA. This means that a 5-day-old human embryo is effectively no different than the Petri dishes of human cells that have grown in laboratories for decades with no controversy or opposition. Therefore, if the cluster of cells in the earliest stage of a human embryo is considered a “human life,” a growing plate of skin cells must also be considered “human life.” Few would claim that a Petri dish of human cells is morally equivalent to a living human or any other animal. Why, then, would a microscopic collection of embryonic cells have the same moral status as an adult human?
The status of the human embryo comes from its potential to turn into a fully grown human being. However, the potential of this entity to become an individual does not logically mean that it has the same status as an individual who can think and feel. If this were true, virtually every cell grown in a laboratory would be subject to the same controversy. This is because scientists have developed technology to convert an ordinary cell such as a skin cell into an embryo [10]. Although this requires a laboratory with special conditions, the normal development of a human being also requires special conditions in the womb of the mother. Therefore, almost any cell could be considered a potential individual, so it is illogical to conclude that a cluster of embryonic cells deserves a higher moral status.
THE FATE OF UNUSED EMBRYOS
Hundreds of thousands of embryos are destroyed each year in a process known as in vitro fertilization (IVF), a popular procedure that helps couples have children [13]. Society has an ethical obligation to use these discarded embryos to make medical advancements rather than simply throw them in the trash for misguided ideological and religious reasons as opponents of embryonic stem cell research desire.
With IVF, a fertility clinician harvests sperm and egg cells from the parents and creates an embryo in a laboratory before implanting it in the woman’s womb. However, creating and implanting a single embryo is expensive and often leads to unsuccessful implantation. Instead, the clinician typically creates an average of seven embryos and selects the healthiest few to implant [13].
This leaves several unused embryos for every one implanted. The couple can pay a fee to preserve the unused embryos by freezing them or can donate them to another family. Otherwise, they are slated for destruction [14]. A 2011 study in the “Journal of the American Society for Reproductive Medicine” found that 19 percent of the unused embryos are discarded and only 3 percent are donated for scientific research [14]. Many of these embryos could never grow into a living person given the chance because they are not healthy enough to survive past early stages of development [14]. If a human embryo is already destined for destruction or has no chance of survival, scientists have the ethical imperative to use these embryos to research and develop medical treatments that could save lives. The modern version of the Hippocratic oath states, “I will apply, for the benefit of the sick, all measures which are required [to heal]” [10]. Republican Senator Orrin Hatch of Utah supports the pro-life movement, which recognizes early embryos as human individuals. However, even he favors using the leftover embryos for the greater good. “The morality of the situation dictates that these embryos, which are routinely discarded, be used to improve and save lives. The tragedy would be in not using these embryos to save lives when the alternative is that they would be discarded.” [3]
ALTERNATIVES TO EMBRYONIC STEM CELLS
Although scientists have used embryonic stem cells (ESCs) for promising treatments, they are not ideal, and scientists hope to eliminate the need for them. Primarily, ESCs come from an embryo with different DNA than the patient who will receive the treatment, meaning they are not autologous. ESCs are not necessarily compatible with everyone and could cause the immune system to reject the treatment [11]. The most promising alternative to ESCs are known as induced pluripotent stem cells. In 2008, scientists discovered a way to reprogram human skin cells to embryonic stem cells [15]. Scientists easily obtained these cells from a patient’s skin, converted them into the desired cell type, then transplanted them into the diseased organ without risk of immune rejection [15]. This eliminates any ethical concerns because no embryos are harvested or destroyed in the process. However, induced stem cells have their own risks. Recent studies have shown that they can begin growing out of control and turn into cancer [3]. Several of the first clinical trials with induced stem cells, including one aimed at curing blindness by regenerating a patient’s retinal cells, were halted because potentially cancerous mutations were detected [3].
Scientists believe that induced stem cells created in a laboratory will one day completely replace embryonic stem cells harvested from human embryos. However, the only way to create perfect replicas of ESCs is to thoroughly understand their structure and function. Scientists still do not completely understand how ESCs work. Why does a stem cell sometimes become a nerve cell, sometimes become a heart cell and other times regenerate to produce another stem cell? How can we tell a stem cell what type of cell to become? To develop a viable alternative to ESCs, scientists must first answer these questions with experiments on ESCs from human embryos. Therefore, extensive embryonic stem cell research today will eliminate the need for embryonic stem cells in the future.
The Biomedical Engineering Society Code of Ethics calls upon engineers to “use their knowledge, skills, and abilities to enhance the safety, health and welfare of the public.” [16] Stem cell research epitomizes this. Stem cells hold the cure for numerous diseases ranging from spinal cord injuries to organ failure and have the potential to transform modern medicine. Therefore, the donation of human embryos to scientific research falls within most conventional ethical frameworks and should be allowed with minimal restriction.
Because of widespread ignorance about the science behind stem cells, ill-informed opposition has prevented scientists from receiving the funding and support they need to save millions of lives. For example, George W. Bush’s religious opposition to stem cell research resulted in a 2001 law severely limiting government funding for such research [3]. Although most opponents of stem cell research compare the destruction of a human embryo to the death of a living human, the biology of these early embryos is no more human than a plate of skin cells in a laboratory. Additionally, all embryos sacrificed for scientific research would otherwise be discarded and provide no benefit to society. If society better understood the process and potential of embryonic stem cell research, more people would surely support it.
Within the next decade, stem cells will likely provide simple cures for diseases that are currently untreatable, such as Alzheimer’s disease and organ failure [1]. As long as scientists receive support for embryonic stem cell research, stem cell therapies will become commonplace in clinics and hospitals around the world. Ultimately, the fate of this new medical technology lies in the hands of the public, who must support propositions that will continue to allow and expand the impact of embryonic stem cell research.
By Jonathan Sussman, Viterbi School of Engineering, University of Southern California
ABOUT THE AUTHOR
At the time of writing this paper, Jonathan Sussman was a senior at the University of Southern California studying biomedical engineering with an emphasis in biochemistry. He was an undergraduate research assistant in the Graham Lab investigating proteomics of cancer cells and was planning to attend an MD/PhD program.
[1] “Stem Cell Information”, Stem Cell Basics , 2016. [Online]. Available at: https://stemcells.nih.gov/info/basics/3.htm [Accessed 11 Oct. 2018].
[2] Cleveland Clinic, “Stem Cell Therapy for Heart Disease | Cleveland Clinic”, 2017. [Online]. Available at: https://my.clevelandclinic.org/health/diseases/17508-stem-cell-therapy-for-heart-disease [Accessed 14 Oct. 2018].
[3] B. Lo and L. Parham, “Ethical Issues in Stem Cell Research”, Endocrine Reviews , 30(3), pp.204-213, 2009.
[4] G. Gugliotta, “Why Many States Now Have Stem Cell Research Programs”, 2015. [Online]. Available at: http://www.governing.com/topics/health-human-services/last-decades-culture-wars-drove-some-states-to-fund-stem-cell-research.html [Accessed 14 Oct. 2018].
[5] D. Cyranoski, “How human embryonic stem cells sparked a revolution”, Nature Journal , 2018. [Online]. Available at: https://www.nature.com/articles/d41586-018-03268-4 [Accessed 11 Oct. 2018].
[6] K. McCormack, “Young man with spinal cord injury regains use of hands and arms after stem cell therapy”, The Stem Cellar, 2016. [Online]. Available at: https://blog.cirm.ca.gov/2016/09/07/young-man-with-spinal-cord-injury-regains-use-of-hands-and-arms-after-stem-cell-therapy/ [Accessed 11 Oct. 2018].
[7] A. Coghlan, “First implants derived from stem cells to ‘cure’ type 1 diabetes”, New Scientist , 2017. [Online]. Available at: https://www.newscientist.com/article/2142976-first-implants-derived-from-stem-cells-to-cure-type-1-diabetes/ [Accessed 11 Oct. 2018].
[8] C. Scott, “University of California San Diego’s 3D Printed Liver Tissue May Be the Closest We’ve Gotten to a Real Printed Liver”, 3DPrint.com | The Voice of 3D Printing / Additive Manufacturing , 2018. [Online]. Available at: https://3dprint.com/118932/uc-san-diego-3d-printed-liver/ [Accessed 11 Oct. 2018].
[9] American Transplant Foundation, “Facts and Myths about Transplant”. [Online]. Available at: https://www.americantransplantfoundation.org/about-transplant/facts-and-myths/ [Accessed 11 Oct. 2018].
[10] A. Siegel, “Ethics of Stem Cell Research”, Stanford Encyclopedia of Philosophy , 2013. [Online]. Available at: https://plato.stanford.edu/entries/stem-cells/ [Accessed 11 Oct. 2018].
[11] I. Hyun, “Stem Cells – The Hastings Center”, The Hastings Center , 2018. [Online]. Available at: https://www.thehastingscenter.org/briefingbook/stem-cells/ [Accessed 11 Oct. 2018].
[12] M. Gazzaniga, “The Ethical Brain”, New York: Harper Perennial , 2006.
[13] M. Bilger, “Shocking Report Shows 2.5 Million Human Beings Created for IVF Have Been Killed | LifeNews.com”, LifeNews , 2016. [Online]. Available at: https://www.lifenews.com/2016/12/06/shocking-report-shows-2-5-million-human-beings-created-for-ivf-have-been-killed/ [Accessed 11 Oct. 2018].
[14] Harvard Gazette, “Stem cell lines created from discarded IVF embryos”, 2008. [Online]. Available at: https://news.harvard.edu/gazette/story/2008/01/stem-cell-lines-created-from-discarded-ivf-embryos/ [Accessed 11 Oct. 2018].
[15] K. Murray, “Could we make babies from only skin cells?”, CNN, 2017. [Online]. Available at: https://www.cnn.com/2017/02/09/health/embryo-skin-cell-ivg/index.html [Accessed 11 Oct. 2018].
[16] Biomedical Engineering Society, “Biomedical Engineering Society Code of Ethics”, 2004. [Online]. Available at: https://www.bmes.org/files/CodeEthics04.pdf [Accessed 11 Oct. 2018].
- Open access
- Published: 26 February 2019

Stem cells: past, present, and future
- Wojciech Zakrzewski 1 ,
- Maciej Dobrzyński 2 ,
- Maria Szymonowicz 1 &
- Zbigniew Rybak 1
Stem Cell Research & Therapy volume 10 , Article number: 68 ( 2019 ) Cite this article
568k Accesses
861 Citations
53 Altmetric
Metrics details
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation. Quality control and teratoma formation assays are important procedures in assessing the properties of the stem cells tested. Derivation methods and the utilization of culturing media are crucial to set proper environmental conditions for controlled differentiation. Among many types of stem tissue applications, the use of graphene scaffolds and the potential of extracellular vesicle-based therapies require attention due to their versatility. The review is summarized by challenges that stem cell therapy must overcome to be accepted worldwide. A wide variety of possibilities makes this cutting edge therapy a turning point in modern medicine, providing hope for untreatable diseases.
Stem cell classification
Stem cells are unspecialized cells of the human body. They are able to differentiate into any cell of an organism and have the ability of self-renewal. Stem cells exist both in embryos and adult cells. There are several steps of specialization. Developmental potency is reduced with each step, which means that a unipotent stem cell is not able to differentiate into as many types of cells as a pluripotent one. This chapter will focus on stem cell classification to make it easier for the reader to comprehend the following chapters.
Totipotent stem cells are able to divide and differentiate into cells of the whole organism. Totipotency has the highest differentiation potential and allows cells to form both embryo and extra-embryonic structures. One example of a totipotent cell is a zygote, which is formed after a sperm fertilizes an egg. These cells can later develop either into any of the three germ layers or form a placenta. After approximately 4 days, the blastocyst’s inner cell mass becomes pluripotent. This structure is the source of pluripotent cells.
Pluripotent stem cells (PSCs) form cells of all germ layers but not extraembryonic structures, such as the placenta. Embryonic stem cells (ESCs) are an example. ESCs are derived from the inner cell mass of preimplantation embryos. Another example is induced pluripotent stem cells (iPSCs) derived from the epiblast layer of implanted embryos. Their pluripotency is a continuum, starting from completely pluripotent cells such as ESCs and iPSCs and ending on representatives with less potency—multi-, oligo- or unipotent cells. One of the methods to assess their activity and spectrum is the teratoma formation assay. iPSCs are artificially generated from somatic cells, and they function similarly to PSCs. Their culturing and utilization are very promising for present and future regenerative medicine.
Multipotent stem cells have a narrower spectrum of differentiation than PSCs, but they can specialize in discrete cells of specific cell lineages. One example is a haematopoietic stem cell, which can develop into several types of blood cells. After differentiation, a haematopoietic stem cell becomes an oligopotent cell. Its differentiation abilities are then restricted to cells of its lineage. However, some multipotent cells are capable of conversion into unrelated cell types, which suggests naming them pluripotent cells.
Oligopotent stem cells can differentiate into several cell types. A myeloid stem cell is an example that can divide into white blood cells but not red blood cells.
Unipotent stem cells are characterized by the narrowest differentiation capabilities and a special property of dividing repeatedly. Their latter feature makes them a promising candidate for therapeutic use in regenerative medicine. These cells are only able to form one cell type, e.g. dermatocytes.
Stem cell biology
A blastocyst is formed after the fusion of sperm and ovum fertilization. Its inner wall is lined with short-lived stem cells, namely, embryonic stem cells. Blastocysts are composed of two distinct cell types: the inner cell mass (ICM), which develops into epiblasts and induces the development of a foetus, and the trophectoderm (TE). Blastocysts are responsible for the regulation of the ICM microenvironment. The TE continues to develop and forms the extraembryonic support structures needed for the successful origin of the embryo, such as the placenta. As the TE begins to form a specialized support structure, the ICM cells remain undifferentiated, fully pluripotent and proliferative [ 1 ]. The pluripotency of stem cells allows them to form any cell of the organism. Human embryonic stem cells (hESCs) are derived from the ICM. During the process of embryogenesis, cells form aggregations called germ layers: endoderm, mesoderm and ectoderm (Fig. 1 ), each eventually giving rise to differentiated cells and tissues of the foetus and, later on, the adult organism [ 2 ]. After hESCs differentiate into one of the germ layers, they become multipotent stem cells, whose potency is limited to only the cells of the germ layer. This process is short in human development. After that, pluripotent stem cells occur all over the organism as undifferentiated cells, and their key abilities are proliferation by the formation of the next generation of stem cells and differentiation into specialized cells under certain physiological conditions.
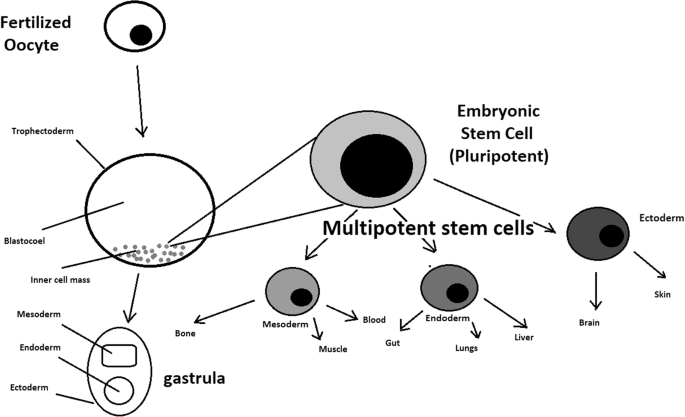
Oocyte development and formation of stem cells: the blastocoel, which is formed from oocytes, consists of embryonic stem cells that later differentiate into mesodermal, ectodermal, or endodermal cells. Blastocoel develops into the gastrula
Signals that influence the stem cell specialization process can be divided into external, such as physical contact between cells or chemical secretion by surrounding tissue, and internal, which are signals controlled by genes in DNA.
Stem cells also act as internal repair systems of the body. The replenishment and formation of new cells are unlimited as long as an organism is alive. Stem cell activity depends on the organ in which they are in; for example, in bone marrow, their division is constant, although in organs such as the pancreas, division only occurs under special physiological conditions.
Stem cell functional division
Whole-body development.
During division, the presence of different stem cells depends on organism development. Somatic stem cell ESCs can be distinguished. Although the derivation of ESCs without separation from the TE is possible, such a combination has growth limits. Because proliferating actions are limited, co-culture of these is usually avoided.
ESCs are derived from the inner cell mass of the blastocyst, which is a stage of pre-implantation embryo ca. 4 days after fertilization. After that, these cells are placed in a culture dish filled with culture medium. Passage is an inefficient but popular process of sub-culturing cells to other dishes. These cells can be described as pluripotent because they are able to eventually differentiate into every cell type in the organism. Since the beginning of their studies, there have been ethical restrictions connected to the medical use of ESCs in therapies. Most embryonic stem cells are developed from eggs that have been fertilized in an in vitro clinic, not from eggs fertilized in vivo.
Somatic or adult stem cells are undifferentiated and found among differentiated cells in the whole body after development. The function of these cells is to enable the healing, growth, and replacement of cells that are lost each day. These cells have a restricted range of differentiation options. Among many types, there are the following:
Mesenchymal stem cells are present in many tissues. In bone marrow, these cells differentiate mainly into the bone, cartilage, and fat cells. As stem cells, they are an exception because they act pluripotently and can specialize in the cells of any germ layer.
Neural cells give rise to nerve cells and their supporting cells—oligodendrocytes and astrocytes.
Haematopoietic stem cells form all kinds of blood cells: red, white, and platelets.
Skin stem cells form, for example, keratinocytes, which form a protective layer of skin.
The proliferation time of somatic stem cells is longer than that of ESCs. It is possible to reprogram adult stem cells back to their pluripotent state. This can be performed by transferring the adult nucleus into the cytoplasm of an oocyte or by fusion with the pluripotent cell. The same technique was used during cloning of the famous Dolly sheep.
hESCs are involved in whole-body development. They can differentiate into pluripotent, totipotent, multipotent, and unipotent cells (Fig. 2 ) [ 2 ].
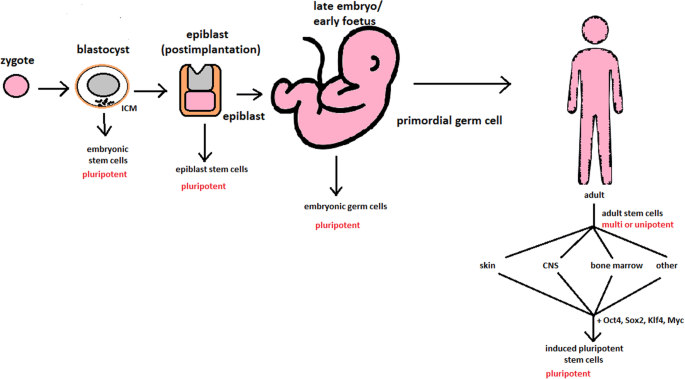
Changes in the potency of stem cells in human body development. Potency ranges from pluripotent cells of the blastocyst to unipotent cells of a specific tissue in a human body such as the skin, CNS, or bone marrow. Reversed pluripotency can be achieved by the formation of induced pluripotent stem cells using either octamer-binding transcription factor (Oct4), sex-determining region Y (Sox2), Kruppel-like factor 4 (Klf4), or the Myc gene
Pluripotent cells can be named totipotent if they can additionally form extraembryonic tissues of the embryo. Multipotent cells are restricted in differentiating to each cell type of given tissue. When tissue contains only one lineage of cells, stem cells that form them are called either called oligo- or unipotent.
iPSC quality control and recognition by morphological differences
The comparability of stem cell lines from different individuals is needed for iPSC lines to be used in therapeutics [ 3 ]. Among critical quality procedures, the following can be distinguished:
Short tandem repeat analysis—This is the comparison of specific loci on the DNA of the samples. It is used in measuring an exact number of repeating units. One unit consists of 2 to 13 nucleotides repeating many times on the DNA strand. A polymerase chain reaction is used to check the lengths of short tandem repeats. The genotyping procedure of source tissue, cells, and iPSC seed and master cell banks is recommended.
Identity analysis—The unintentional switching of lines, resulting in other stem cell line contamination, requires rigorous assay for cell line identification.
Residual vector testing—An appearance of reprogramming vectors integrated into the host genome is hazardous, and testing their presence is a mandatory procedure. It is a commonly used procedure for generating high-quality iPSC lines. An acceptable threshold in high-quality research-grade iPSC line collections is ≤ 1 plasmid copies per 100 cells. During the procedure, 2 different regions, common to all plasmids, should be used as specific targets, such as EBNA and CAG sequences [ 3 ]. To accurately represent the test reactions, a standard curve needs to be prepared in a carrier of gDNA from a well-characterized hPSC line. For calculations of plasmid copies per cell, it is crucial to incorporate internal reference gDNA sequences to allow the quantification of, for example, ribonuclease P (RNaseP) or human telomerase reverse transcriptase (hTERT).
Karyotype—A long-term culture of hESCs can accumulate culture-driven mutations [ 4 ]. Because of that, it is crucial to pay additional attention to genomic integrity. Karyotype tests can be performed by resuscitating representative aliquots and culturing them for 48–72 h before harvesting cells for karyotypic analysis. If abnormalities are found within the first 20 karyotypes, the analysis must be repeated on a fresh sample. When this situation is repeated, the line is evaluated as abnormal. Repeated abnormalities must be recorded. Although karyology is a crucial procedure in stem cell quality control, the single nucleotide polymorphism (SNP) array, discussed later, has approximately 50 times higher resolution.
Viral testing—When assessing the quality of stem cells, all tests for harmful human adventitious agents must be performed (e.g. hepatitis C or human immunodeficiency virus). This procedure must be performed in the case of non-xeno-free culture agents.
Bacteriology—Bacterial or fungal sterility tests can be divided into culture- or broth-based tests. All the procedures must be recommended by pharmacopoeia for the jurisdiction in which the work is performed.
Single nucleotide polymorphism arrays—This procedure is a type of DNA microarray that detects population polymorphisms by enabling the detection of subchromosomal changes and the copy-neutral loss of heterozygosity, as well as an indication of cellular transformation. The SNP assay consists of three components. The first is labelling fragmented nucleic acid sequences with fluorescent dyes. The second is an array that contains immobilized allele-specific oligonucleotide (ASO) probes. The last component detects, records, and eventually interprets the signal.
Flow cytometry—This is a technique that utilizes light to count and profile cells in a heterogeneous fluid mixture. It allows researchers to accurately and rapidly collect data from heterogeneous fluid mixtures with live cells. Cells are passed through a narrow channel one by one. During light illumination, sensors detect light emitted or refracted from the cells. The last step is data analysis, compilation and integration into a comprehensive picture of the sample.
Phenotypic pluripotency assays—Recognizing undifferentiated cells is crucial in successful stem cell therapy. Among other characteristics, stem cells appear to have a distinct morphology with a high nucleus to cytoplasm ratio and a prominent nucleolus. Cells appear to be flat with defined borders, in contrast to differentiating colonies, which appear as loosely located cells with rough borders [ 5 ]. It is important that images of ideal and poor quality colonies for each cell line are kept in laboratories, so whenever there is doubt about the quality of culture, it can always be checked according to the representative image. Embryoid body formation or directed differentiation of monolayer cultures to produce cell types representative of all three embryonic germ layers must be performed. It is important to note that colonies cultured under different conditions may have different morphologies [ 6 ].
Histone modification and DNA methylation—Quality control can be achieved by using epigenetic analysis tools such as histone modification or DNA methylation. When stem cells differentiate, the methylation process silences pluripotency genes, which reduces differentiation potential, although other genes may undergo demethylation to become expressed [ 7 ]. It is important to emphasize that stem cell identity, together with its morphological characteristics, is also related to its epigenetic profile [ 8 , 9 ]. According to Brindley [ 10 ], there is a relationship between epigenetic changes, pluripotency, and cell expansion conditions, which emphasizes that unmethylated regions appear to be serum-dependent.
hESC derivation and media
hESCs can be derived using a variety of methods, from classic culturing to laser-assisted methodologies or microsurgery [ 11 ]. hESC differentiation must be specified to avoid teratoma formation (see Fig. 3 ).
Spontaneous differentiation of hESCs causes the formation of a heterogeneous cell population. There is a different result, however, when commitment signals (in forms of soluble factors and culture conditions) are applied and enable the selection of progenitor cells
hESCs spontaneously differentiate into embryonic bodies (EBs) [ 12 ]. EBs can be studied instead of embryos or animals to predict their effects on early human development. There are many different methods for acquiring EBs, such as bioreactor culture [ 13 ], hanging drop culture [ 12 ], or microwell technology [ 14 , 15 ]. These methods allow specific precursors to form in vitro [ 16 ].
The essential part of these culturing procedures is a separation of inner cell mass to culture future hESCs (Fig. 4 ) [ 17 ]. Rosowski et al. [ 18 ] emphasizes that particular attention must be taken in controlling spontaneous differentiation. When the colony reaches the appropriate size, cells must be separated. The occurrence of pluripotent cells lasts for 1–2 days. Because the classical utilization of hESCs caused ethical concerns about gastrulas used during procedures, Chung et al. [ 19 ] found out that it is also possible to obtain hESCs from four cell embryos, leaving a higher probability of embryo survival. Additionally, Zhang et al. [ 20 ] used only in vitro fertilization growth-arrested cells.
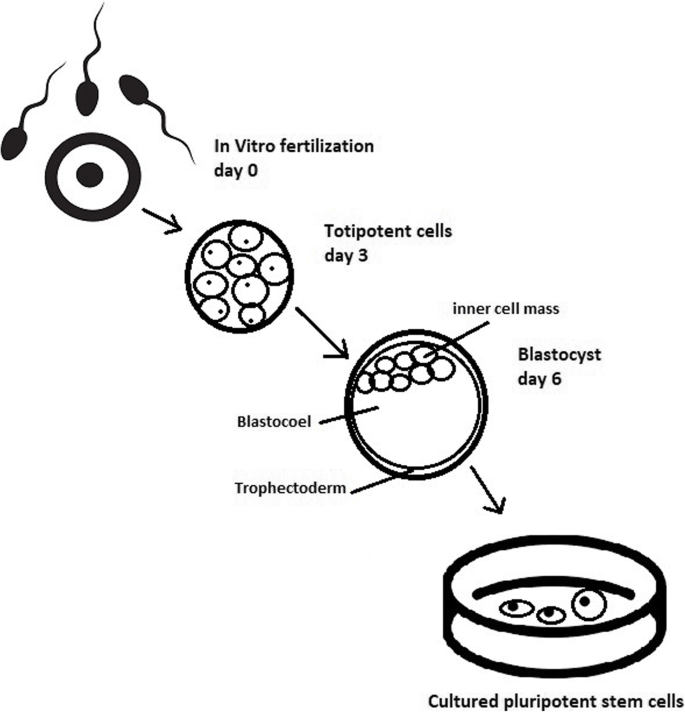
Culturing of pluripotent stem cells in vitro. Three days after fertilization, totipotent cells are formed. Blastocysts with ICM are formed on the sixth day after fertilization. Pluripotent stem cells from ICM can then be successfully transmitted on a dish
Cell passaging is used to form smaller clusters of cells on a new culture surface [ 21 ]. There are four important passaging procedures.
Enzymatic dissociation is a cutting action of enzymes on proteins and adhesion domains that bind the colony. It is a gentler method than the manual passage. It is crucial to not leave hESCs alone after passaging. Solitary cells are more sensitive and can easily undergo cell death; collagenase type IV is an example [ 22 , 23 ].
Manual passage , on the other hand, focuses on using cell scratchers. The selection of certain cells is not necessary. This should be done in the early stages of cell line derivation [ 24 ].
Trypsin utilization allows a healthy, automated hESC passage. Good Manufacturing Practice (GMP)-grade recombinant trypsin is widely available in this procedure [ 24 ]. However, there is a risk of decreasing the pluripotency and viability of stem cells [ 25 ]. Trypsin utilization can be halted with an inhibitor of the protein rho-associated protein kinase (ROCK) [ 26 ].
Ethylenediaminetetraacetic acid ( EDTA ) indirectly suppresses cell-to-cell connections by chelating divalent cations. Their suppression promotes cell dissociation [ 27 ].
Stem cells require a mixture of growth factors and nutrients to differentiate and develop. The medium should be changed each day.
Traditional culture methods used for hESCs are mouse embryonic fibroblasts (MEFs) as a feeder layer and bovine serum [ 28 ] as a medium. Martin et al. [ 29 ] demonstrated that hESCs cultured in the presence of animal products express the non-human sialic acid, N -glycolylneuraminic acid (NeuGc). Feeder layers prevent uncontrolled proliferation with factors such as leukaemia inhibitory factor (LIF) [ 30 ].
First feeder layer-free culture can be supplemented with serum replacement, combined with laminin [ 31 ]. This causes stable karyotypes of stem cells and pluripotency lasting for over a year.
Initial culturing media can be serum (e.g. foetal calf serum FCS), artificial replacement such as synthetic serum substitute (SSS), knockout serum replacement (KOSR), or StemPro [ 32 ]. The simplest culture medium contains only eight essential elements: DMEM/F12 medium, selenium, NaHCO 3, l -ascorbic acid, transferrin, insulin, TGFβ1, and FGF2 [ 33 ]. It is not yet fully known whether culture systems developed for hESCs can be allowed without adaptation in iPSC cultures.
Turning point in stem cell therapy
The turning point in stem cell therapy appeared in 2006, when scientists Shinya Yamanaka, together with Kazutoshi Takahashi, discovered that it is possible to reprogram multipotent adult stem cells to the pluripotent state. This process avoided endangering the foetus’ life in the process. Retrovirus-mediated transduction of mouse fibroblasts with four transcription factors (Oct-3/4, Sox2, KLF4, and c-Myc) [ 34 ] that are mainly expressed in embryonic stem cells could induce the fibroblasts to become pluripotent (Fig. 5 ) [ 35 ]. This new form of stem cells was named iPSCs. One year later, the experiment also succeeded with human cells [ 36 ]. After this success, the method opened a new field in stem cell research with a generation of iPSC lines that can be customized and biocompatible with the patient. Recently, studies have focused on reducing carcinogenesis and improving the conduction system.
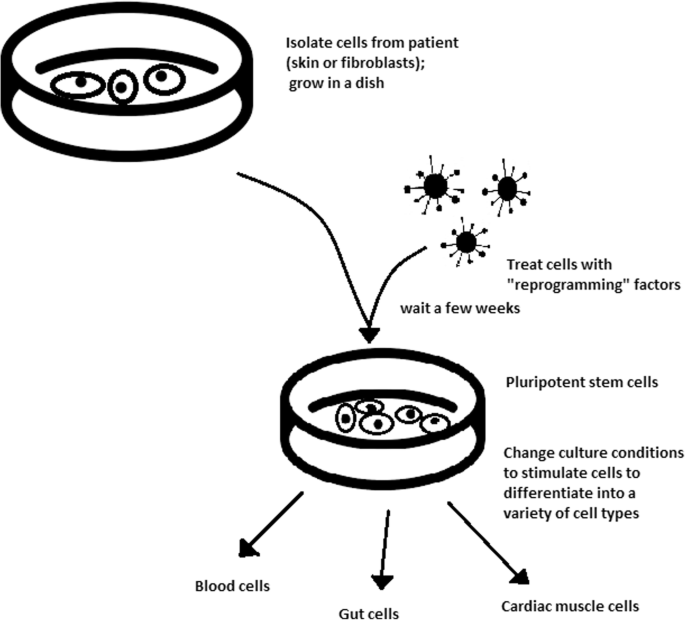
Retroviral-mediated transduction induces pluripotency in isolated patient somatic cells. Target cells lose their role as somatic cells and, once again, become pluripotent and can differentiate into any cell type of human body
The turning point was influenced by former discoveries that happened in 1962 and 1987.
The former discovery was about scientist John Gurdon successfully cloning frogs by transferring a nucleus from a frog’s somatic cells into an oocyte. This caused a complete reversion of somatic cell development [ 37 ]. The results of his experiment became an immense discovery since it was previously believed that cell differentiation is a one-way street only, but his experiment suggested the opposite and demonstrated that it is even possible for a somatic cell to again acquire pluripotency [ 38 ].
The latter was a discovery made by Davis R.L. that focused on fibroblast DNA subtraction. Three genes were found that originally appeared in myoblasts. The enforced expression of only one of the genes, named myogenic differentiation 1 (Myod1), caused the conversion of fibroblasts into myoblasts, showing that reprogramming cells is possible, and it can even be used to transform cells from one lineage to another [ 39 ].
Although pluripotency can occur naturally only in embryonic stem cells, it is possible to induce terminally differentiated cells to become pluripotent again. The process of direct reprogramming converts differentiated somatic cells into iPSC lines that can form all cell types of an organism. Reprogramming focuses on the expression of oncogenes such as Myc and Klf4 (Kruppel-like factor 4). This process is enhanced by a downregulation of genes promoting genome stability, such as p53. Additionally, cell reprogramming involves histone alteration. All these processes can cause potential mutagenic risk and later lead to an increased number of mutations. Quinlan et al. [ 40 ] checked fully pluripotent mouse iPSCs using whole genome DNA sequencing and structural variation (SV) detection algorithms. Based on those studies, it was confirmed that although there were single mutations in the non-genetic region, there were non-retrotransposon insertions. This led to the conclusion that current reprogramming methods can produce fully pluripotent iPSCs without severe genomic alterations.
During the course of development from pluripotent hESCs to differentiated somatic cells, crucial changes appear in the epigenetic structure of these cells. There is a restriction or permission of the transcription of genes relevant to each cell type. When somatic cells are being reprogrammed using transcription factors, all the epigenetic architecture has to be reconditioned to achieve iPSCs with pluripotency [ 41 ]. However, cells of each tissue undergo specific somatic genomic methylation. This influences transcription, which can further cause alterations in induced pluripotency [ 42 ].
Source of iPSCs
Because pluripotent cells can propagate indefinitely and differentiate into any kind of cell, they can be an unlimited source, either for replacing lost or diseased tissues. iPSCs bypass the need for embryos in stem cell therapy. Because they are made from the patient’s own cells, they are autologous and no longer generate any risk of immune rejection.
At first, fibroblasts were used as a source of iPSCs. Because a biopsy was needed to achieve these types of cells, the technique underwent further research. Researchers investigated whether more accessible cells could be used in the method. Further, other cells were used in the process: peripheral blood cells, keratinocytes, and renal epithelial cells found in urine. An alternative strategy to stem cell transplantation can be stimulating a patient’s endogenous stem cells to divide or differentiate, occurring naturally when skin wounds are healing. In 2008, pancreatic exocrine cells were shown to be reprogrammed to functional, insulin-producing beta cells [ 43 ].
The best stem cell source appears to be the fibroblasts, which is more tempting in the case of logistics since its stimulation can be fast and better controlled [ 44 ].
- Teratoma formation assay
The self-renewal and differentiation capabilities of iPSCs have gained significant interest and attention in regenerative medicine sciences. To study their abilities, a quality-control assay is needed, of which one of the most important is the teratoma formation assay. Teratomas are benign tumours. Teratomas are capable of rapid growth in vivo and are characteristic because of their ability to develop into tissues of all three germ layers simultaneously. Because of the high pluripotency of teratomas, this formation assay is considered an assessment of iPSC’s abilities [ 45 ].
Teratoma formation rate, for instance, was observed to be elevated in human iPSCs compared to that in hESCs [ 46 ]. This difference may be connected to different differentiation methods and cell origins. Most commonly, the teratoma assay involves an injection of examined iPSCs subcutaneously or under the testis or kidney capsule in mice, which are immune-deficient [ 47 ]. After injection, an immature but recognizable tissue can be observed, such as the kidney tubules, bone, cartilage, or neuroepithelium [ 30 ]. The injection site may have an impact on the efficiency of teratoma formation [ 48 ].
There are three groups of markers used in this assay to differentiate the cells of germ layers. For endodermal tissue, there is insulin/C-peptide and alpha-1 antitrypsin [ 49 ]. For the mesoderm, derivatives can be used, e.g. cartilage matrix protein for the bone and alcian blue for the cartilage. As ectodermal markers, class III B botulin or keratin can be used for keratinocytes.
Teratoma formation assays are considered the gold standard for demonstrating the pluripotency of human iPSCs, demonstrating their possibilities under physiological conditions. Due to their actual tissue formation, they could be used for the characterization of many cell lineages [ 50 ].
Directed differentiation
To be useful in therapy, stem cells must be converted into desired cell types as necessary or else the whole regenerative medicine process will be pointless. Differentiation of ESCs is crucial because undifferentiated ESCs can cause teratoma formation in vivo. Understanding and using signalling pathways for differentiation is an important method in successful regenerative medicine. In directed differentiation, it is likely to mimic signals that are received by cells when they undergo successive stages of development [ 51 ]. The extracellular microenvironment plays a significant role in controlling cell behaviour. By manipulating the culture conditions, it is possible to restrict specific differentiation pathways and generate cultures that are enriched in certain precursors in vitro. However, achieving a similar effect in vivo is challenging. It is crucial to develop culture conditions that will allow the promotion of homogenous and enhanced differentiation of ESCs into functional and desired tissues.
Regarding the self-renewal of embryonic stem cells, Hwang et al. [ 52 ] noted that the ideal culture method for hESC-based cell and tissue therapy would be a defined culture free of either the feeder layer or animal components. This is because cell and tissue therapy requires the maintenance of large quantities of undifferentiated hESCs, which does not make feeder cells suitable for such tasks.
Most directed differentiation protocols are formed to mimic the development of an inner cell mass during gastrulation. During this process, pluripotent stem cells differentiate into ectodermal, mesodermal, or endodermal progenitors. Mall molecules or growth factors induce the conversion of stem cells into appropriate progenitor cells, which will later give rise to the desired cell type. There is a variety of signal intensities and molecular families that may affect the establishment of germ layers in vivo, such as fibroblast growth factors (FGFs) [ 53 ]; the Wnt family [ 54 ] or superfamily of transforming growth factors—β(TGFβ); and bone morphogenic proteins (BMP) [ 55 ]. Each candidate factor must be tested on various concentrations and additionally applied to various durations because the precise concentrations and times during which developing cells in embryos are influenced during differentiation are unknown. For instance, molecular antagonists of endogenous BMP and Wnt signalling can be used for ESC formation of ectoderm [ 56 ]. However, transient Wnt and lower concentrations of the TGFβ family trigger mesodermal differentiation [ 57 ]. Regarding endoderm formation, a higher activin A concentration may be required [ 58 , 59 ].
There are numerous protocols about the methods of forming progenitors of cells of each of germ layers, such as cardiomyocytes [ 60 ], hepatocytes [ 61 ], renal cells [ 62 ], lung cells [ 63 , 64 ], motor neurons [ 65 ], intestinal cells [ 66 ], or chondrocytes [ 67 ].
Directed differentiation of either iPSCs or ESCs into, e.g. hepatocytes, could influence and develop the study of the molecular mechanisms in human liver development. In addition, it could also provide the possibility to form exogenous hepatocytes for drug toxicity testing [ 68 ].
Levels of concentration and duration of action with a specific signalling molecule can cause a variety of factors. Unfortunately, for now, a high cost of recombinant factors is likely to limit their use on a larger scale in medicine. The more promising technique focuses on the use of small molecules. These can be used for either activating or deactivating specific signalling pathways. They enhance reprogramming efficiency by creating cells that are compatible with the desired type of tissue. It is a cheaper and non-immunogenic method.
One of the successful examples of small-molecule cell therapies is antagonists and agonists of the Hedgehog pathway. They show to be very useful in motor neuron regeneration [ 69 ]. Endogenous small molecules with their function in embryonic development can also be used in in vitro methods to induce the differentiation of cells; for example, retinoic acid, which is responsible for patterning the nervous system in vivo [ 70 ], surprisingly induced retinal cell formation when the laboratory procedure involved hESCs [ 71 ].
The efficacy of differentiation factors depends on functional maturity, efficiency, and, finally, introducing produced cells to their in vivo equivalent. Topography, shear stress, and substrate rigidity are factors influencing the phenotype of future cells [ 72 ].
The control of biophysical and biochemical signals, the biophysical environment, and a proper guide of hESC differentiation are important factors in appropriately cultured stem cells.
Stem cell utilization and their manufacturing standards and culture systems
The European Medicines Agency and the Food and Drug Administration have set Good Manufacturing Practice (GMP) guidelines for safe and appropriate stem cell transplantation. In the past, protocols used for stem cell transplantation required animal-derived products [ 73 ].
The risk of introducing animal antigens or pathogens caused a restriction in their use. Due to such limitations, the technique required an obvious update [ 74 ]. Now, it is essential to use xeno-free equivalents when establishing cell lines that are derived from fresh embryos and cultured from human feeder cell lines [ 75 ]. In this method, it is crucial to replace any non-human materials with xeno-free equivalents [ 76 ].
NutriStem with LN-511, TeSR2 with human recombinant laminin (LN-511), and RegES with human foreskin fibroblasts (HFFs) are commonly used xeno-free culture systems [ 33 ]. There are many organizations and international initiatives, such as the National Stem Cell Bank, that provide stem cell lines for treatment or medical research [ 77 ].
Stem cell use in medicine
Stem cells have great potential to become one of the most important aspects of medicine. In addition to the fact that they play a large role in developing restorative medicine, their study reveals much information about the complex events that happen during human development.
The difference between a stem cell and a differentiated cell is reflected in the cells’ DNA. In the former cell, DNA is arranged loosely with working genes. When signals enter the cell and the differentiation process begins, genes that are no longer needed are shut down, but genes required for the specialized function will remain active. This process can be reversed, and it is known that such pluripotency can be achieved by interaction in gene sequences. Takahashi and Yamanaka [ 78 ] and Loh et al. [ 79 ] discovered that octamer-binding transcription factor 3 and 4 (Oct3/4), sex determining region Y (SRY)-box 2 and Nanog genes function as core transcription factors in maintaining pluripotency. Among them, Oct3/4 and Sox2 are essential for the generation of iPSCs.
Many serious medical conditions, such as birth defects or cancer, are caused by improper differentiation or cell division. Currently, several stem cell therapies are possible, among which are treatments for spinal cord injury, heart failure [ 80 ], retinal and macular degeneration [ 81 ], tendon ruptures, and diabetes type 1 [ 82 ]. Stem cell research can further help in better understanding stem cell physiology. This may result in finding new ways of treating currently incurable diseases.
Haematopoietic stem cell transplantation
Haematopoietic stem cells are important because they are by far the most thoroughly characterized tissue-specific stem cell; after all, they have been experimentally studied for more than 50 years. These stem cells appear to provide an accurate paradigm model system to study tissue-specific stem cells, and they have potential in regenerative medicine.
Multipotent haematopoietic stem cell (HSC) transplantation is currently the most popular stem cell therapy. Target cells are usually derived from the bone marrow, peripheral blood, or umbilical cord blood [ 83 ]. The procedure can be autologous (when the patient’s own cells are used), allogenic (when the stem cell comes from a donor), or syngeneic (from an identical twin). HSCs are responsible for the generation of all functional haematopoietic lineages in blood, including erythrocytes, leukocytes, and platelets. HSC transplantation solves problems that are caused by inappropriate functioning of the haematopoietic system, which includes diseases such as leukaemia and anaemia. However, when conventional sources of HSC are taken into consideration, there are some important limitations. First, there is a limited number of transplantable cells, and an efficient way of gathering them has not yet been found. There is also a problem with finding a fitting antigen-matched donor for transplantation, and viral contamination or any immunoreactions also cause a reduction in efficiency in conventional HSC transplantations. Haematopoietic transplantation should be reserved for patients with life-threatening diseases because it has a multifactorial character and can be a dangerous procedure. iPSC use is crucial in this procedure. The use of a patient’s own unspecialized somatic cells as stem cells provides the greatest immunological compatibility and significantly increases the success of the procedure.
Stem cells as a target for pharmacological testing
Stem cells can be used in new drug tests. Each experiment on living tissue can be performed safely on specific differentiated cells from pluripotent cells. If any undesirable effect appears, drug formulas can be changed until they reach a sufficient level of effectiveness. The drug can enter the pharmacological market without harming any live testers. However, to test the drugs properly, the conditions must be equal when comparing the effects of two drugs. To achieve this goal, researchers need to gain full control of the differentiation process to generate pure populations of differentiated cells.
Stem cells as an alternative for arthroplasty
One of the biggest fears of professional sportsmen is getting an injury, which most often signifies the end of their professional career. This applies especially to tendon injuries, which, due to current treatment options focusing either on conservative or surgical treatment, often do not provide acceptable outcomes. Problems with the tendons start with their regeneration capabilities. Instead of functionally regenerating after an injury, tendons merely heal by forming scar tissues that lack the functionality of healthy tissues. Factors that may cause this failed healing response include hypervascularization, deposition of calcific materials, pain, or swelling [ 84 ].
Additionally, in addition to problems with tendons, there is a high probability of acquiring a pathological condition of joints called osteoarthritis (OA) [ 85 ]. OA is common due to the avascular nature of articular cartilage and its low regenerative capabilities [ 86 ]. Although arthroplasty is currently a common procedure in treating OA, it is not ideal for younger patients because they can outlive the implant and will require several surgical procedures in the future. These are situations where stem cell therapy can help by stopping the onset of OA [ 87 ]. However, these procedures are not well developed, and the long-term maintenance of hyaline cartilage requires further research.
Osteonecrosis of the femoral hip (ONFH) is a refractory disease associated with the collapse of the femoral head and risk of hip arthroplasty in younger populations [ 88 ]. Although total hip arthroplasty (THA) is clinically successful, it is not ideal for young patients, mostly due to the limited lifetime of the prosthesis. An increasing number of clinical studies have evaluated the therapeutic effect of stem cells on ONFH. Most of the authors demonstrated positive outcomes, with reduced pain, improved function, or avoidance of THA [ 89 , 90 , 91 ].
Rejuvenation by cell programming
Ageing is a reversible epigenetic process. The first cell rejuvenation study was published in 2011 [ 92 ]. Cells from aged individuals have different transcriptional signatures, high levels of oxidative stress, dysfunctional mitochondria, and shorter telomeres than in young cells [ 93 ]. There is a hypothesis that when human or mouse adult somatic cells are reprogrammed to iPSCs, their epigenetic age is virtually reset to zero [ 94 ]. This was based on an epigenetic model, which explains that at the time of fertilization, all marks of parenteral ageing are erased from the zygote’s genome and its ageing clock is reset to zero [ 95 ].
In their study, Ocampo et al. [ 96 ] used Oct4, Sox2, Klf4, and C-myc genes (OSKM genes) and affected pancreas and skeletal muscle cells, which have poor regenerative capacity. Their procedure revealed that these genes can also be used for effective regenerative treatment [ 97 ]. The main challenge of their method was the need to employ an approach that does not use transgenic animals and does not require an indefinitely long application. The first clinical approach would be preventive, focused on stopping or slowing the ageing rate. Later, progressive rejuvenation of old individuals can be attempted. In the future, this method may raise some ethical issues, such as overpopulation, leading to lower availability of food and energy.
For now, it is important to learn how to implement cell reprogramming technology in non-transgenic elder animals and humans to erase marks of ageing without removing the epigenetic marks of cell identity.
Cell-based therapies
Stem cells can be induced to become a specific cell type that is required to repair damaged or destroyed tissues (Fig. 6 ). Currently, when the need for transplantable tissues and organs outweighs the possible supply, stem cells appear to be a perfect solution for the problem. The most common conditions that benefit from such therapy are macular degenerations [ 98 ], strokes [ 99 ], osteoarthritis [ 89 , 90 ], neurodegenerative diseases, and diabetes [ 100 ]. Due to this technique, it can become possible to generate healthy heart muscle cells and later transplant them to patients with heart disease.
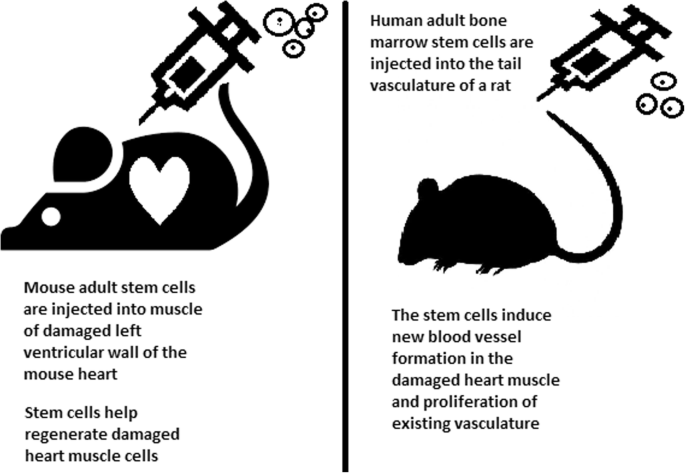
Stem cell experiments on animals. These experiments are one of the many procedures that proved stem cells to be a crucial factor in future regenerative medicine
In the case of type 1 diabetes, insulin-producing cells in the pancreas are destroyed due to an autoimmunological reaction. As an alternative to transplantation therapy, it can be possible to induce stem cells to differentiate into insulin-producing cells [ 101 ].
Stem cells and tissue banks
iPS cells with their theoretically unlimited propagation and differentiation abilities are attractive for the present and future sciences. They can be stored in a tissue bank to be an essential source of human tissue used for medical examination. The problem with conventional differentiated tissue cells held in the laboratory is that their propagation features diminish after time. This does not occur in iPSCs.
The umbilical cord is known to be rich in mesenchymal stem cells. Due to its cryopreservation immediately after birth, its stem cells can be successfully stored and used in therapies to prevent the future life-threatening diseases of a given patient.
Stem cells of human exfoliated deciduous teeth (SHED) found in exfoliated deciduous teeth has the ability to develop into more types of body tissues than other stem cells [ 102 ] (Table 1 ). Techniques of their collection, isolation, and storage are simple and non-invasive. Among the advantages of banking, SHED cells are:
Guaranteed donor-match autologous transplant that causes no immune reaction and rejection of cells [ 103 ]
Simple and painless for both child and parent
Less than one third of the cost of cord blood storage
Not subject to the same ethical concerns as embryonic stem cells [ 104 ]
In contrast to cord blood stem cells, SHED cells are able to regenerate into solid tissues such as connective, neural, dental, or bone tissue [ 105 , 106 ]
SHED can be useful for close relatives of the donor
Fertility diseases
In 2011, two researchers, Katsuhiko Hayashi et al. [ 107 ], showed in an experiment on mice that it is possible to form sperm from iPSCs. They succeeded in delivering healthy and fertile pups in infertile mice. The experiment was also successful for female mice, where iPSCs formed fully functional eggs .
Young adults at risk of losing their spermatogonial stem cells (SSC), mostly cancer patients, are the main target group that can benefit from testicular tissue cryopreservation and autotransplantation. Effective freezing methods for adult and pre-pubertal testicular tissue are available [ 108 ].
Qiuwan et al. [ 109 ] provided important evidence that human amniotic epithelial cell (hAEC) transplantation could effectively improve ovarian function by inhibiting cell apoptosis and reducing inflammation in injured ovarian tissue of mice, and it could be a promising strategy for the management of premature ovarian failure or insufficiency in female cancer survivors.
For now, reaching successful infertility treatments in humans appears to be only a matter of time, but there are several challenges to overcome. First, the process needs to have high efficiency; second, the chances of forming tumours instead of eggs or sperm must be maximally reduced. The last barrier is how to mature human sperm and eggs in the lab without transplanting them to in vivo conditions, which could cause either a tumour risk or an invasive procedure.
Therapy for incurable neurodegenerative diseases
Thanks to stem cell therapy, it is possible not only to delay the progression of incurable neurodegenerative diseases such as Parkinson’s disease, Alzheimer’s disease (AD), and Huntington disease, but also, most importantly, to remove the source of the problem. In neuroscience, the discovery of neural stem cells (NSCs) has nullified the previous idea that adult CNS were not capable of neurogenesis [ 110 , 111 ]. Neural stem cells are capable of improving cognitive function in preclinical rodent models of AD [ 112 , 113 , 114 ]. Awe et al. [ 115 ] clinically derived relevant human iPSCs from skin punch biopsies to develop a neural stem cell-based approach for treating AD. Neuronal degeneration in Parkinson’s disease (PD) is focal, and dopaminergic neurons can be efficiently generated from hESCs. PD is an ideal disease for iPSC-based cell therapy [ 116 ]. However, this therapy is still in an experimental phase ( https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4539501 /). Brain tissue from aborted foetuses was used on patients with Parkinson’s disease [ 117 ]. Although the results were not uniform, they showed that therapies with pure stem cells are an important and achievable therapy.
Stem cell use in dentistry
Teeth represent a very challenging material for regenerative medicine. They are difficult to recreate because of their function in aspects such as articulation, mastication, or aesthetics due to their complicated structure. Currently, there is a chance for stem cells to become more widely used than synthetic materials. Teeth have a large advantage of being the most natural and non-invasive source of stem cells.
For now, without the use of stem cells, the most common periodontological treatments are either growth factors, grafts, or surgery. For example, there are stem cells in periodontal ligament [ 118 , 119 ], which are capable of differentiating into osteoblasts or cementoblasts, and their functions were also assessed in neural cells [ 120 ]. Tissue engineering is a successful method for treating periodontal diseases. Stem cells of the root apical areas are able to recreate periodontal ligament. One of the possible methods of tissue engineering in periodontology is gene therapy performed using adenoviruses-containing growth factors [ 121 ].
As a result of animal studies, dentin regeneration is an effective process that results in the formation of dentin bridges [ 122 ].
Enamel is more difficult to regenerate than dentin. After the differentiation of ameloblastoma cells into the enamel, the former is destroyed, and reparation is impossible. Medical studies have succeeded in differentiating bone marrow stem cells into ameloblastoma [ 123 ].
Healthy dental tissue has a high amount of regular stem cells, although this number is reduced when tissue is either traumatized or inflamed [ 124 ]. There are several dental stem cell groups that can be isolated (Fig. 7 ).
Localization of stem cells in dental tissues. Dental pulp stem cells (DPSCs) and human deciduous teeth stem cells (SHED) are located in the dental pulp. Periodontal ligaments stem cells are located in the periodontal ligament. Apical papilla consists of stem cells from the apical papilla (SCAP)
Dental pulp stem cell (DPSC)
These were the first dental stem cells isolated from the human dental pulp, which were [ 125 ] located inside dental pulp (Table 2 ). They have osteogenic and chondrogenic potential. Mesenchymal stem cells (MSCs) of the dental pulp, when isolated, appear highly clonogenic; they can be isolated from adult tissue (e.g. bone marrow, adipose tissue) and foetal (e.g. umbilical cord) [ 126 ] tissue, and they are able to differentiate densely [ 127 ]. MSCs differentiate into odontoblast-like cells and osteoblasts to form dentin and bone. Their best source locations are the third molars [ 125 ]. DPSCs are the most useful dental source of tissue engineering due to their easy surgical accessibility, cryopreservation possibility, increased production of dentin tissues compared to non-dental stem cells, and their anti-inflammatory abilities. These cells have the potential to be a source for maxillofacial and orthopaedic reconstructions or reconstructions even beyond the oral cavity. DPSCs are able to generate all structures of the developed tooth [ 128 ]. In particular, beneficial results in the use of DPSCs may be achieved when combined with other new therapies, such as periodontal tissue photobiomodulation (laser stimulation), which is an efficient technique in the stimulation of proliferation and differentiation into distinct cell types [ 129 ]. DPSCs can be induced to form neural cells to help treat neurological deficits.
Stem cells of human exfoliated deciduous teeth (SHED) have a faster rate of proliferation than DPSCs and differentiate into an even greater number of cells, e.g. other mesenchymal and non-mesenchymal stem cell derivatives, such as neural cells [ 130 ]. These cells possess one major disadvantage: they form a non-complete dentin/pulp-like complex in vivo. SHED do not undergo the same ethical concerns as embryonic stem cells. Both DPSCs and SHED are able to form bone-like tissues in vivo [ 131 ] and can be used for periodontal, dentin, or pulp regeneration. DPSCs and SHED can be used in treating, for example, neural deficits [ 132 ]. DPSCs alone were tested and successfully applied for alveolar bone and mandible reconstruction [ 133 ].
Periodontal ligament stem cells (PDLSCs)
These cells are used in periodontal ligament or cementum tissue regeneration. They can differentiate into mesenchymal cell lineages to produce collagen-forming cells, adipocytes, cementum tissue, Sharpey’s fibres, and osteoblast-like cells in vitro. PDLSCs exist both on the root and alveolar bone surfaces; however, on the latter, these cells have better differentiation abilities than on the former [ 134 ]. PDLSCs have become the first treatment for periodontal regeneration therapy because of their safety and efficiency [ 135 , 136 ].
Stem cells from apical papilla (SCAP)
These cells are mesenchymal structures located within immature roots. They are isolated from human immature permanent apical papilla. SCAP are the source of odontoblasts and cause apexogenesis. These stem cells can be induced in vitro to form odontoblast-like cells, neuron-like cells, or adipocytes. SCAP have a higher capacity of proliferation than DPSCs, which makes them a better choice for tissue regeneration [ 137 , 138 ].
Dental follicle stem cells (DFCs)
These cells are loose connective tissues surrounding the developing tooth germ. DFCs contain cells that can differentiate into cementoblasts, osteoblasts, and periodontal ligament cells [ 139 , 140 ]. Additionally, these cells proliferate after even more than 30 passages [ 141 ]. DFCs are most commonly extracted from the sac of a third molar. When DFCs are combined with a treated dentin matrix, they can form a root-like tissue with a pulp-dentin complex and eventually form tooth roots [ 141 ]. When DFC sheets are induced by Hertwig’s epithelial root sheath cells, they can produce periodontal tissue; thus, DFCs represent a very promising material for tooth regeneration [ 142 ].
Pulp regeneration in endodontics
Dental pulp stem cells can differentiate into odontoblasts. There are few methods that enable the regeneration of the pulp.
The first is an ex vivo method. Proper stem cells are grown on a scaffold before they are implanted into the root channel [ 143 ].
The second is an in vivo method. This method focuses on injecting stem cells into disinfected root channels after the opening of the in vivo apex. Additionally, the use of a scaffold is necessary to prevent the movement of cells towards other tissues. For now, only pulp-like structures have been created successfully.
Methods of placing stem cells into the root channel constitute are either soft scaffolding [ 144 ] or the application of stem cells in apexogenesis or apexification. Immature teeth are the best source [ 145 ]. Nerve and blood vessel network regeneration are extremely vital to keep pulp tissue healthy.
The potential of dental stem cells is mainly regarding the regeneration of damaged dentin and pulp or the repair of any perforations; in the future, it appears to be even possible to generate the whole tooth. Such an immense success would lead to the gradual replacement of implant treatments. Mandibulary and maxillary defects can be one of the most complicated dental problems for stem cells to address.
Acquiring non-dental tissue cells by dental stem cell differentiation
In 2013, it was reported that it is possible to grow teeth from stem cells obtained extra-orally, e.g. from urine [ 146 ]. Pluripotent stem cells derived from human urine were induced and generated tooth-like structures. The physical properties of the structures were similar to natural ones except for hardness [ 127 ]. Nonetheless, it appears to be a very promising technique because it is non-invasive and relatively low-cost, and somatic cells can be used instead of embryonic cells. More importantly, stem cells derived from urine did not form any tumours, and the use of autologous cells reduces the chances of rejection [ 147 ].
Use of graphene in stem cell therapy
Over recent years, graphene and its derivatives have been increasingly used as scaffold materials to mediate stem cell growth and differentiation [ 148 ]. Both graphene and graphene oxide (GO) represent high in-plane stiffness [ 149 ]. Because graphene has carbon and aromatic network, it works either covalently or non-covalently with biomolecules; in addition to its superior mechanical properties, graphene offers versatile chemistry. Graphene exhibits biocompatibility with cells and their proper adhesion. It also tested positively for enhancing the proliferation or differentiation of stem cells [ 148 ]. After positive experiments, graphene revealed great potential as a scaffold and guide for specific lineages of stem cell differentiation [ 150 ]. Graphene has been successfully used in the transplantation of hMSCs and their guided differentiation to specific cells. The acceleration skills of graphene differentiation and division were also investigated. It was discovered that graphene can serve as a platform with increased adhesion for both growth factors and differentiation chemicals. It was also discovered that π-π binding was responsible for increased adhesion and played a crucial role in inducing hMSC differentiation [ 150 ].
Therapeutic potential of extracellular vesicle-based therapies
Extracellular vesicles (EVs) can be released by virtually every cell of an organism, including stem cells [ 151 ], and are involved in intercellular communication through the delivery of their mRNAs, lipids, and proteins. As Oh et al. [ 152 ] prove, stem cells, together with their paracrine factors—exosomes—can become potential therapeutics in the treatment of, e.g. skin ageing. Exosomes are small membrane vesicles secreted by most cells (30–120 nm in diameter) [ 153 ]. When endosomes fuse with the plasma membrane, they become exosomes that have messenger RNAs (mRNAs) and microRNAs (miRNAs), some classes of non-coding RNAs (IncRNAs) and several proteins that originate from the host cell [ 154 ]. IncRNAs can bind to specific loci and create epigenetic regulators, which leads to the formation of epigenetic modifications in recipient cells. Because of this feature, exosomes are believed to be implicated in cell-to-cell communication and the progression of diseases such as cancer [ 155 ]. Recently, many studies have also shown the therapeutic use of exosomes derived from stem cells, e.g. skin damage and renal or lung injuries [ 156 ].
In skin ageing, the most important factor is exposure to UV light, called “photoageing” [ 157 ], which causes extrinsic skin damage, characterized by dryness, roughness, irregular pigmentation, lesions, and skin cancers. In intrinsic skin ageing, on the other hand, the loss of elasticity is a characteristic feature. The skin dermis consists of fibroblasts, which are responsible for the synthesis of crucial skin elements, such as procollagen or elastic fibres. These elements form either basic framework extracellular matrix constituents of the skin dermis or play a major role in tissue elasticity. Fibroblast efficiency and abundance decrease with ageing [ 158 ]. Stem cells can promote the proliferation of dermal fibroblasts by secreting cytokines such as platelet-derived growth factor (PDGF), transforming growth factor β (TGF-β), and basic fibroblast growth factor. Huh et al. [ 159 ] mentioned that a medium of human amniotic fluid-derived stem cells (hAFSC) positively affected skin regeneration after longwave UV-induced (UVA, 315–400 nm) photoageing by increasing the proliferation and migration of dermal fibroblasts. It was discovered that, in addition to the induction of fibroblast physiology, hAFSC transplantation also improved diseases in cases of renal pathology, various cancers, or stroke [ 160 , 161 ].
Oh [ 162 ] also presented another option for the treatment of skin wounds, either caused by physical damage or due to diabetic ulcers. Induced pluripotent stem cell-conditioned medium (iPSC-CM) without any animal-derived components induced dermal fibroblast proliferation and migration.
Natural cutaneous wound healing is divided into three steps: haemostasis/inflammation, proliferation, and remodelling. During the crucial step of proliferation, fibroblasts migrate and increase in number, indicating that it is a critical step in skin repair, and factors such as iPSC-CM that impact it can improve the whole cutaneous wound healing process. Paracrine actions performed by iPSCs are also important for this therapeutic effect [ 163 ]. These actions result in the secretion of cytokines such as TGF-β, interleukin (IL)-6, IL-8, monocyte chemotactic protein-1 (MCP-1), vascular endothelial growth factor (VEGF), platelet-derived growth factor-AA (PDGF-AA), and basic fibroblast growth factor (bFGF). Bae et al. [ 164 ] mentioned that TGF-β induced the migration of keratinocytes. It was also demonstrated that iPSC factors can enhance skin wound healing in vivo and in vitro when Zhou et al. [ 165 ] enhanced wound healing, even after carbon dioxide laser resurfacing in an in vivo study.
Peng et al. [ 166 ] investigated the effects of EVs derived from hESCs on in vitro cultured retinal glial, progenitor Müller cells, which are known to differentiate into retinal neurons. EVs appear heterogeneous in size and can be internalized by cultured Müller cells, and their proteins are involved in the induction and maintenance of stem cell pluripotency. These stem cell-derived vesicles were responsible for the neuronal trans-differentiation of cultured Müller cells exposed to them. However, the research article points out that the procedure was accomplished only on in vitro acquired retina.
Challenges concerning stem cell therapy
Although stem cells appear to be an ideal solution for medicine, there are still many obstacles that need to be overcome in the future. One of the first problems is ethical concern.
The most common pluripotent stem cells are ESCs. Therapies concerning their use at the beginning were, and still are, the source of ethical conflicts. The reason behind it started when, in 1998, scientists discovered the possibility of removing ESCs from human embryos. Stem cell therapy appeared to be very effective in treating many, even previously incurable, diseases. The problem was that when scientists isolated ESCs in the lab, the embryo, which had potential for becoming a human, was destroyed (Fig. 8 ). Because of this, scientists, seeing a large potential in this treatment method, focused their efforts on making it possible to isolate stem cells without endangering their source—the embryo.
Use of inner cell mass pluripotent stem cells and their stimulation to differentiate into desired cell types
For now, while hESCs still remain an ethically debatable source of cells, they are potentially powerful tools to be used for therapeutic applications of tissue regeneration. Because of the complexity of stem cell control systems, there is still much to be learned through observations in vitro. For stem cells to become a popular and widely accessible procedure, tumour risk must be assessed. The second problem is to achieve successful immunological tolerance between stem cells and the patient’s body. For now, one of the best ideas is to use the patient’s own cells and devolve them into their pluripotent stage of development.
New cells need to have the ability to fully replace lost or malfunctioning natural cells. Additionally, there is a concern about the possibility of obtaining stem cells without the risk of morbidity or pain for either the patient or the donor. Uncontrolled proliferation and differentiation of cells after implementation must also be assessed before its use in a wide variety of regenerative procedures on living patients [ 167 ].
One of the arguments that limit the use of iPSCs is their infamous role in tumourigenicity. There is a risk that the expression of oncogenes may increase when cells are being reprogrammed. In 2008, a technique was discovered that allowed scientists to remove oncogenes after a cell achieved pluripotency, although it is not efficient yet and takes a longer amount of time. The process of reprogramming may be enhanced by deletion of the tumour suppressor gene p53, but this gene also acts as a key regulator of cancer, which makes it impossible to remove in order to avoid more mutations in the reprogrammed cell. The low efficiency of the process is another problem, which is progressively becoming reduced with each year. At first, the rate of somatic cell reprogramming in Yamanaka’s study was up to 0.1%. The use of transcription factors creates a risk of genomic insertion and further mutation of the target cell genome. For now, the only ethically acceptable operation is an injection of hESCs into mouse embryos in the case of pluripotency evaluation [ 168 ].
Stem cell obstacles in the future
Pioneering scientific and medical advances always have to be carefully policed in order to make sure they are both ethical and safe. Because stem cell therapy already has a large impact on many aspects of life, it should not be treated differently.
Currently, there are several challenges concerning stem cells. First, the most important one is about fully understanding the mechanism by which stem cells function first in animal models. This step cannot be avoided. For the widespread, global acceptance of the procedure, fear of the unknown is the greatest challenge to overcome.
The efficiency of stem cell-directed differentiation must be improved to make stem cells more reliable and trustworthy for a regular patient. The scale of the procedure is another challenge. Future stem cell therapies may be a significant obstacle. Transplanting new, fully functional organs made by stem cell therapy would require the creation of millions of working and biologically accurate cooperating cells. Bringing such complicated procedures into general, widespread regenerative medicine will require interdisciplinary and international collaboration.
The identification and proper isolation of stem cells from a patient’s tissues is another challenge. Immunological rejection is a major barrier to successful stem cell transplantation. With certain types of stem cells and procedures, the immune system may recognize transplanted cells as foreign bodies, triggering an immune reaction resulting in transplant or cell rejection.
One of the ideas that can make stem cells a “failsafe” is about implementing a self-destruct option if they become dangerous. Further development and versatility of stem cells may cause reduction of treatment costs for people suffering from currently incurable diseases. When facing certain organ failure, instead of undergoing extraordinarily expensive drug treatment, the patient would be able to utilize stem cell therapy. The effect of a successful operation would be immediate, and the patient would avoid chronic pharmacological treatment and its inevitable side effects.
Although these challenges facing stem cell science can be overwhelming, the field is making great advances each day. Stem cell therapy is already available for treating several diseases and conditions. Their impact on future medicine appears to be significant.
After several decades of experiments, stem cell therapy is becoming a magnificent game changer for medicine. With each experiment, the capabilities of stem cells are growing, although there are still many obstacles to overcome. Regardless, the influence of stem cells in regenerative medicine and transplantology is immense. Currently, untreatable neurodegenerative diseases have the possibility of becoming treatable with stem cell therapy. Induced pluripotency enables the use of a patient’s own cells. Tissue banks are becoming increasingly popular, as they gather cells that are the source of regenerative medicine in a struggle against present and future diseases. With stem cell therapy and all its regenerative benefits, we are better able to prolong human life than at any time in history.
Abbreviations
Basic fibroblast growth factor
Bone morphogenic proteins
Dental follicle stem cells
Dental pulp stem cells
Embryonic bodies
Embryonic stem cells
Fibroblast growth factors
Good Manufacturing Practice
Graphene oxide
Human amniotic fluid-derived stem cells
Human embryonic stem cells
Human foreskin fibroblasts
Inner cell mass
Non-coding RNA
Induced pluripotent stem cells
In vitro fertilization
Knockout serum replacement
Leukaemia inhibitory factor
Monocyte chemotactic protein-1
Fibroblasts
Messenger RNA
Mesenchymal stem cells of dental pulp
Myogenic differentiation
Osteoarthritis
Octamer-binding transcription factor 3 and 4
Platelet-derived growth factor
Platelet-derived growth factor-AA
Periodontal ligament stem cells
Rho-associated protein kinase
Stem cells from apical papilla
Stem cells of human exfoliated deciduous teeth
Synthetic Serum Substitute
Trophectoderm
Vascular endothelial growth factor
Transforming growth factors
Sukoyan MA, Vatolin SY, et al. Embryonic stem cells derived from morulae, inner cell mass, and blastocysts of mink: comparisons of their pluripotencies. Embryo Dev. 1993;36(2):148–58
Larijani B, Esfahani EN, Amini P, Nikbin B, Alimoghaddam K, Amiri S, Malekzadeh R, Yazdi NM, Ghodsi M, Dowlati Y, Sahraian MA, Ghavamzadeh A. Stem cell therapy in treatment of different diseases. Acta Medica Iranica. 2012:79–96 https://www.ncbi.nlm.nih.gov/pubmed/22359076 .
Sullivan S, Stacey GN, Akazawa C, et al. Quality guidelines for clinical-grade human induced pluripotent stem cell lines. Regenerative Med. 2018; https://doi.org/10.2217/rme-2018-0095 .
Amps K, Andrews PW, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat. Biotechnol. 2011; 29 (12):1121–44.
Google Scholar
Amit M, Itskovitz-Eldor J. Atlas of human pluripotent stem cells: derivation and culturing. New York: Humana Press; 2012.
Ludwig TE, Bergendahl V, Levenstein ME, Yu J, Probasco MD, Thomson JA. Feeder-independent culture of human embryonic stem cells. Nat Methods. 2006;3:637–46.
CAS PubMed Google Scholar
Kang MI. Transitional CpG methylation between promoters and retroelements of tissue-specific genes during human mesenchymal cell differentiation. J. Cell Biochem. 2007;102:224–39.
Vaes B, Craeye D, Pinxteren J. Quality control during manufacture of a stem cell therapeutic. BioProcess Int. 2012;10:50–5.
Bloushtain-Qimron N. Epigenetic patterns of embryonic and adult stem cells. Cell Cycle. 2009;8:809–17.
Brindley DA. Peak serum: implications of serum supply for cell therapy manufacturing. Regenerative Medicine. 2012;7:809–17.
Solter D, Knowles BB. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci U S A. 1975;72:5099–102.
CAS PubMed PubMed Central Google Scholar
Hoepfl G, Gassmann M, Desbaillets I. Differentiating embryonic stem cells into embryoid bodies. Methods Mole Biol. 2004;254:79–98 https://doi.org/10.1385/1-59259-741-6:079 .
Lim WF, Inoue-Yokoo T, Tan KS, Lai MI, Sugiyama D. Hematopoietic cell differentiation from embryonic and induced pluripotent stem cells. Stem Cell Res Ther. 2013;4(3):71. https://doi.org/10.1186/scrt222 .
Article CAS PubMed PubMed Central Google Scholar
Mohr JC, de Pablo JJ, Palecek SP. 3-D microwell culture of human embryonic stem cells. Biomaterials. 2006;27(36):6032–42. https://doi.org/10.1016/j.biomaterials.2006.07.012 .
Article CAS PubMed Google Scholar
Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of the visceral yolk sac, blood islands, and myocardium. J Embryol Exp Morphol. 1985;87:27–45.
Kurosawa HY. Methods for inducing embryoid body formation: in vitro differentiation system of embryonic stem cells. J Biosci Bioeng. 2007;103:389–98.
Heins N, Englund MC, Sjoblom C, Dahl U, Tonning A, Bergh C, Lindahl A, Hanson C, Semb H. Derivation, characterization, and differentiation of human embryonic stem cells. Stem Cells. 2004;22:367–76.
Rosowski KA, Mertz AF, Norcross S, Dufresne ER, Horsley V. Edges of human embryonic stem cell colonies display distinct mechanical properties and differentiation potential. Sci Rep. 2015;5:Article number:14218.
PubMed Google Scholar
Chung Y, Klimanskaya I, Becker S, Li T, Maserati M, Lu SJ, Zdravkovic T, Ilic D, Genbacev O, Fisher S, Krtolica A, Lanza R. Human embryonic stem cell lines generated without embryo destruction. Cell Stem Cell. 2008;2:113–7.
Zhang X, Stojkovic P, Przyborski S, Cooke M, Armstrong L, Lako M, Stojkovic M. Derivation of human embryonic stem cells from developing and arrested embryos. Stem Cells. 2006;24:2669–76.
Beers J, Gulbranson DR, George N, Siniscalchi LI, Jones J, Thomson JA, Chen G. Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc. 2012;7:2029–40.
Ellerström C, Hyllner J, Strehl R. single cell enzymatic dissociation of human embryonic stem cells: a straightforward, robust, and standardized culture method. In: Turksen K, editor. Human embryonic stem cell protocols. Methods in molecular biology: Humana Press; 2009. p. 584.
Heng BC, Liu H, Ge Z, Cao T. Mechanical dissociation of human embryonic stem cell colonies by manual scraping after collagenase treatment is much more detrimental to cellular viability than is trypsinization with gentle pipetting. Biotechnol Appl Biochem. 2010;47(1):33–7.
Ellerstrom C, Strehl R, Noaksson K, Hyllner J, Semb H. Facilitated expansion of human embryonic stem cells by single-cell enzymatic dissociation. Stem Cells. 2007;25:1690–6.
Brimble SN, Zeng X, Weiler DA, Luo Y, Liu Y, Lyons IG, Freed WJ, Robins AJ, Rao MS, Schulz TC. Karyotypic stability, genotyping, differentiation, feeder-free maintenance, and gene expression sampling in three human embryonic stem cell lines deri. Stem Cells Dev. 2004;13:585–97.
Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–6.
Nie Y, Walsh P, Clarke DL, Rowley JA, Fellner T. Scalable passaging of adherent human pluripotent stem cells. 2014. https://doi.org/10.1371/journal.pone.0088012 .
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7.
Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cellsexpress an immunogenic nonhuman sialic acid. Nat. Med. 2005;11:228–32.
Smith AG, Heath JK, Donaldson DD, Wong GG, Moreau J, Stahl M, Rogers D. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336(6200):688–90. https://doi.org/10.1038/336688a0 .
Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK. Feeder-free growth of undifferentiated human embryonic stem cells. Nature Biotechnol. 2001;19:971–4. https://doi.org/10.1038/nbt1001-971 .
Article CAS Google Scholar
Weathersbee PS, Pool TB, Ord T. Synthetic serum substitute (SSS): a globulin-enriched protein supplement for human embryo culture. J. Assist Reprod Genet. 1995;12:354–60.
Chen G, Gulbranson DR, Hou Z, Bolin JM, Ruotti V, Probasco MD, Smuga-Otto K, Howden SE, Diol NR, Propson NE, Wagner R, Lee GO, Antosiewicz-Bourget J, Teng JM, Thomson JA. Chemically defined conditions for human iPSC derivation and culture. Nat. Methods. 2011;8:424–9.
Sommer CA, Mostoslavsky G. Experimental approaches for the generation of induced pluripotent stem cells. Stem Cell Res Ther. 2010;1:26.
PubMed PubMed Central Google Scholar
Takahashi K, Yamanaka S. Induced pluripotent stem cells in medicine and biology. Development. 2013;140(12):2457–61 https://doi.org/10.1242/dev.092551 .
Shi D, Lu F, Wei Y, et al. Buffalos ( Bubalus bubalis ) cloned by nuclear transfer of somatic cells. Biol. Reprod. 2007;77:285–91. https://doi.org/10.1095/biolreprod.107.060210 .
Gurdon JB. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. Development. 1962;10:622–40 http://dev.biologists.org/content/10/4/622 .
CAS Google Scholar
Kain K. The birth of cloning: an interview with John Gurdon. Dis Model Mech. 2009;2(1–2):9–10. https://doi.org/10.1242/dmm.002014 .
Article PubMed Central Google Scholar
Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;24(51(6)):987–1000.
Quinlan AR, Boland MJ, Leibowitz ML, et al. Genome sequencing of mouse induced pluripotent stem cells reveals retroelement stability and infrequent DNA rearrangement during reprogramming. Cell Stem Cell. 2011;9(4):366–73.
Maherali N, Sridharan R, Xie W, Utika LJ, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R, Plath K, Hochedlinger K. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70.
Ohi Y, Qin H, Hong C, Blouin L, Polo JM, Guo T, Qi Z, Downey SL, Manos PD, Rossi DJ, Yu J, Hebrok M, Hochedlinger K, Costello JF, Song JS, Ramalho-Santos M. Incomplete DNA methylation underlines a transcriptional memory of somatic cells in human IPS cells. Nat Cell Biol. 2011;13:541–9.
Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32 https://doi.org/10.1038/nature07314 .
Hilfiker A, Kasper C, Hass R, Haverich A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: is there a future for transplantation? Langenbecks Arch Surg. 2011;396:489–97.
Zhang Wendy, Y., de Almeida Patricia, E., and Wu Joseph, C. Teratoma formation: a tool for monitoring pluripotency in stem cell research. StemBook, ed. The Stem Cell Research Community . June 12, 2012. https://doi.org/10.3824/stembook.1.53.1 .
Narsinh KH, Sun N, Sanchez-Freire V, et al. Single cell transcriptional profiling reveals heterogeneity of human induced pluripotent stem cells. J Clin Invest. 2011;121(3):1217–21.
Gertow K, Przyborski S, Loring JF, Auerbach JM, Epifano O, Otonkoski T, Damjanov I, AhrlundRichter L. Isolation of human embryonic stem cell-derived teratomas for the assessment of pluripotency. Curr Protoc Stem Cell Biol . 2007, Chapter 1, Unit 1B 4. 3: 1B.4.1-1B.4.29.
Cooke MJ, Stojkovic M, Przyborski SA. Growth of teratomas derived from human pluripotent stem cells is influenced by the graft site. Stem Cells Dev. 2006;15(2):254–9.
Przyborski SA. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells. 2005;23:1242–50.
Tannenbaum SE, Turetsky TT, Singer O, Aizenman E, Kirshberg S, Ilouz N, Gil Y, Berman-Zaken Y, Perlman TS, Geva N, Levy O, Arbell D, Simon A, Ben-Meir A, Shufaro Y, Laufer N, Reubinoff BE. Derivation of xeno-free and GMP-grade human embryonic stem cells- platforms for future clinical applications. PLoS One. 2012;7:e35325.
Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12:243–52.
Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2007;60(2):199–214. https://doi.org/10.1016/j.addr.2007.08.036 .
Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116–29.
Rao TP, Kuhl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–806.
Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–714.
Efthymiou AG, Chen G, Rao M, Chen G, Boehm M. Self-renewal and cell lineage differentiation strategies in human embryonic stem cells and induced pluripotent stem cells. Expert Opin Biol Ther. 2014;14:1333–44.
Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDRþembryonic-stem-cell-derived population. Nature. 2008;453:524–8.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52. https://doi.org/10.1038/nbt1393 .
Vallier L, Reynolds D, Pedersen RA. Nodal inhibits differentiation of human embryonic stem cells along the neuroectodermal default pathway. Dev Biol. 2004;275:403–21.
Burridge PW, Zambidis ET. Highly efficient directed differentiation of human induced pluripotent stem cells into cardiomyocytes. Methods Mol Biol. 2013;997:149–61.
Cai J, Zhao Y, Liu Y, Ye F, Song Z, Qin H, Meng S, Chen Y, Zhou R, Song X, Guo Y, Ding M, Deng H. Directed differentiation of human embryonic stem cells into functional hepatic cells. Hepatology. 2007;45:1229–39.
Takasato M, Er PX, Becroft M, Vanslambrouck JM, Stanley EG, Elefanty AG, Little MH. Directing human embryonic stem cell differentiation towards a renal lineage generates a selforganizing kidney. Nat Cell Biol. 2014;16:118–26.
Huang SX, Islam MN, O’Neill J, Hu Z, Yang YG, Chen YW, Mumau M, Green MD, VunjakNovakovic G, Bhattacharya J, Snoeck HW. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol. 2014;32:84–91.
Kadzik RS, Morrisey EE. Directing lung endoderm differentiation in pluripotent stem cells. Cell Stem Cell. 2012;10:355–61.
Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–97.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9.
Oldershaw RA, Baxter MA, Lowe ET, Bates N, Grady LM, Soncin F, Brison DR, Hardingham TE, Kimber SJ. Directed differentiation of human embryonic stem cells toward chondrocytes. Nat Biotechnol. 2010;28:1187–94.
Jun Cai, Ann DeLaForest, Joseph Fisher, Amanda Urick, Thomas Wagner, Kirk Twaroski, Max Cayo, Masato Nagaoka, Stephen A Duncan. Protocol for directed differentiation of human pluripotent stem cells toward a hepatocyte fate. 2012. DOI: https://doi.org/10.3824/stembook.1.52.1 .
Frank-Kamenetsky M, Zhang XM, Bottega S, Guicherit O, Wichterle H, Dudek H, Bumcrot D, Wang FY, Jones S, Shulok J, Rubin LL, Porter JA. Small-molecule modulators of hedgehog signaling: identification and characterization of smoothened agonists and antagonists. J Biol. 2002;1:10.
Oshima K, Shin K, Diensthuber M, Peng AW, Ricci AJ, Heller S. Mechanosensitive hair celllike cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–16.
Osakada F, Jin ZB, Hirami Y, Ikeda H, Danjyo T, Watanabe K, Sasai Y, Takahashi M. In vitro differentiation of retinal cells from human pluripotent stem cells by small-molecule induction. J Cell Sci. 2009;122:3169–79.
Kshitiz PJ, Kim P, Helen W, Engler AJ, Levchenko A, Kim DH. Control of stem cell fate and function by engineering physical microenvironments. Intergr Biol (Camb). 2012;4:1008–18.
Amps K, Andrews PW, Anyfantis G, Armstrong L, Avery S, Baharvand H, Baker J, Baker D, Munoz MB, Beil S, Benvenisty N, Ben-Yosef D, Biancotti JC, Bosman A, Brena RM, Brison D, Caisander G, Camarasa MV, Chen J, ChiaoE CYM, Choo AB, Collins D, et al. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nat Biotechnol. 2011;29:1132–44.
Nukaya D, Minami K, Hoshikawa R, Yokoi N, Seino S. Preferential gene expression and epigenetic memory of induced pluripotent stem cells derived from mouse pancreas. Genes Cells. 2015;20:367–81.
Murdoch A, Braude P, Courtney A, Brison D, Hunt C, Lawford-Davies J, Moore H, Stacey G, Sethe S, Procurement Working Group Of National Clinical H, E. S. C. F, National Clinical H, E. S. C. F. The procurement of cells for the derivation of human embryonic stem cell lines for therapeutic use: recommendations for good practice. Stem Cell Rev. 2012;8:91–9.
Hewitson H, Wood V, Kadeva N, Cornwell G, Codognotto S, Stephenson E, Ilic D. Generation of KCL035 research grade human embryonic stem cell line carrying a mutation in HBB gene. Stem Cell Res. 2016;16:210–2.
Daley GQ, Hyun I, Apperley JF, Barker RA, Benvenisty N, Bredenoord AL, Breuer CK, Caulfield T, Cedars MI, Frey-Vasconcells J, Heslop HE, Jin Y, Lee RT, Mccabe C, Munsie M, Murry CE, Piantadosi S, Rao M, Rooke HM, Sipp D, Studer L, Sugarman J, et al. Setting global standards for stem cell research and clinical translation: the 2016 ISSCR guidelines. Stem Cell Rep. 2016;6:787–97.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–76. https://doi.org/10.1016/j.cell.2006.07.024 .
Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X, Bourque G, George J, Leong B, Liu J, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40.
Menasche P, Vanneaux V, Hagege A, Bel A, Cholley B, Cacciapuoti I, Parouchev A, Benhamouda N, Tachdjian G, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Guillemain R, SuberbielleBoissel C, Tartour E, Desnos M, Larghero J. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J. 2015;36:2011–7.
Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, Hubschman JP, Davis JL, Heilwell G, Spirn M, Maguire J, Gay R, Bateman J, Ostrick RM, Morris D, Vincent M, Anglade E, Del Priore LV, Lanza R. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–16.
Ilic D, Ogilvie C. Concise review: human embryonic stem cells-what have we done? What are we doing? Where are we going? Stem Cells. 2017;35:17–25.
Rocha V, et al. Clinical use of umbilical cord blood hematopoietic stem cells. Biol Blood Marrow Transplant. 2006;12(1):34–4.
Longo UG, Ronga M, Maffulli N. Sports Med Arthrosc 17:112–126. Achilles tendinopathy. Sports Med Arthrosc. 2009;17:112–26.
Tempfer H, Lehner C, Grütz M, Gehwolf R, Traweger A. Biological augmentation for tendon repair: lessons to be learned from development, disease, and tendon stem cell research. In: Gimble J, Marolt D, Oreffo R, Redl H, Wolbank S, editors. Cell engineering and regeneration. Reference Series in Biomedical Engineering. Cham: Springer; 2017.
Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–34.
Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14:177–82.
Li R, Lin Q-X, Liang X-Z, Liu G-B, et al. Stem cell therapy for treating osteonecrosis of the femoral head: from clinical applications to related basic research. Stem Cell Res Therapy. 2018;9:291 https://doi.org/10.1186/s13287-018-1018-7 .
Gangji V, De Maertelaer V, Hauzeur JP. Autologous bone marrow cell implantation in the treatment of non-traumatic osteonecrosis of the femoral head: five year follow-up of a prospective controlled study. Bone. 2011;49(5):1005–9.
Zhao D, Cui D, Wang B, Tian F, Guo L, Yang L, et al. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone. 2012;50(1):325–30.
Sen RK, Tripathy SK, Aggarwal S, Marwaha N, Sharma RR, Khandelwal N. Early results of core decompression and autologous bone marrow mononuclear cells instillation in femoral head osteonecrosis: a randomized control study. J Arthroplast. 2012;27(5):679–86.
Lapasset L, Milhavet O, Prieur A, Besnard E, Babled A, Aït-Hamou N, Leschik J, Pellestor F, Ramirez JM, De Vos J, Lehmann S, Lemaitre JM. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011;25:2248–53.
Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–8.
Petkovich DA, Podolskiy DI, Lobanov AV, Lee SG, Miller RA, Gladyshev VN. Using DNA methylation profiling to evaluate biological age and longevity interventions. Cell Metab. 2017;25:954–60 https://doi.org/10.1016/j.cmet.2017.03.016 .
Gerontology, Rejuvenation by cell reprogramming: a new horizon in. Rodolfo G. Goya, Marianne Lehmann, Priscila Chiavellini, Martina Canatelli-Mallat, Claudia B. Hereñú and Oscar A. Brown. Stem Cell Res Therapy . 2018, 9:349. https://doi.org/10.1186/s13287-018-1075-y .
Ocampo A, Reddy P, Martinez-Redondo P, Platero-Luengo A, Hatanaka F, Hishida T, Li M, Lam D, Kurita M, Beyret E, Araoka T, Vazquez-Ferrer E, Donoso D, Roman JLXJ, Rodriguez-Esteban C, Nuñez G, Nuñez Delicado E, Campistol JM, Guillen I, Guillen P, Izpisua. In vivo amelioration of age-associated hallmarks by partial reprogramming. Cell. 2016;167:1719–33.
de Lázaro I, Cossu G, Kostarelos K. Transient transcription factor (OSKM) expression is key towards clinical translation of in vivo cell reprogramming. EMBO Mol Med. 2017;9:733–6.
Sun S, Li ZQ, Glencer P, Cai BC, Zhang XM, Yang J, Li XR. Bringing the age-related macular degeneration high-risk allele age-related maculopathy susceptibility 2 into focus with stem cell technology. Stem Cell Res Ther. 2017;8:135 https://doi.org/10.1186/s13287-017-0584-4 .
Liu J. Induced pluripotent stem cell-derived neural stem cells: new hope for stroke? Stem Cell Res Ther. 2013;4:115 https://doi.org/10.1186/scrt326 .
Shahjalal HM, Dayem AA, Lim KM, Jeon TI, Cho SG. Generation of pancreatic β cells for treatment of diabetes: advances and challenges. Stem Cell ResTher. 2018;9:355 https://doi.org/10.1186/s13287-018-1099-3 .
Kroon E, Martinson LA, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26;443–52.
Arora V, Pooja A, Munshi AK. Banking stem cells from human exfoliated deciduous teeth. J Clin Pediatr Dent. 2009;33(4):289–94.
Mao JJ. Stem cells and the future of dental care. New York State Dental J. 2008;74(2):21–4.
Reznick, Jay B. Continuing education: stem cells: emerging medical and dental therapies for the dental Professional. Dentaltown Magazine . 2008, pp. 42–53.
Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells. 2008;26(7):1787–95.
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, Smith A. Dental pulp tissue engineering with stem cells from exfoliated. J Endod. 2008;34(8):962–9.
Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell. 2011;146(4):519–32. https://doi.org/10.1016/j.cell.2011.06.052 .
Sadri-Ardekani H, Atala A. Testicular tissue cryopreservation and spermatogonial stem cell transplantation to restore fertility: from bench to bedside. Stem Cell ResTher. 2014;5:68 https://doi.org/10.1186/scrt457 .
Zhang Q, Xu M, Yao X, Li T, Wang Q, Lai D. Human amniotic epithelial cells inhibit granulosa cell apoptosis induced by chemotherapy and restore the fertility. Stem Cell Res Ther. 2015;6:152 https://doi.org/10.1186/s13287-015-0148-4 .
Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19:672–82. https://doi.org/10.1038/cr.2009.56 .
Dantuma E, Merchant S, Sugaya K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell ResTher. 2010;1:37 https://doi.org/10.1186/scrt37 .
Wang Q, Matsumoto Y, Shindo T, Miyake K, Shindo A, Kawanishi M, Kawai N, Tamiya T, Nagao S. Neural stem cells transplantation in cortex in a mouse model of Alzheimer’s disease. J Med Invest. 2006;53:61–9. https://doi.org/10.2152/jmi.53.61 .
Article PubMed Google Scholar
Moghadam FH, Alaie H, Karbalaie K, Tanhaei S, Nasr Esfahani MH, Baharvand H. Transplantation of primed or unprimed mouse embryonic stem cell-derived neural precursor cells improves cognitive function in Alzheimerian rats. Differentiation. 2009;78:59–68. https://doi.org/10.1016/j.diff.2009.06.005 .
Byrne JA. Developing neural stem cell-based treatments for neurodegenerative diseases. Stem Cell ResTher. 2014;5:72. https://doi.org/10.1186/scrt461 .
Awe JP, Lee PC, Ramathal C, Vega-Crespo A, Durruthy-Durruthy J, Cooper A, Karumbayaram S, Lowry WE, Clark AT, Zack JA, Sebastiano V, Kohn DB, Pyle AD, Martin MG, Lipshutz GS, Phelps PE, Pera RA, Byrne JA. Generation and characterization of transgene-free human induced pluripotent stem cells and conversion to putative clinical-grade status. Stem Cell Res Ther. 2013;4:87. https://doi.org/10.1186/scrt246 .
Peng J, Zeng X. The role of induced pluripotent stem cells in regenerative medicine: neurodegenerative diseases. Stem Cell ResTher. 2011;2:32. https://doi.org/10.1186/scrt73 .
Wright BL, Barker RA. Established and emerging therapies for Huntington’s disease. 2007;7(6):579–87 https://www.ncbi.nlm.nih.gov/pubmed/17896994/579-87 .
Lin NH, Gronthos S, Bartold PM. Stem cells and periodontal regeneration. Aust Dent J. 2008;53:108–21.
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–55.
Ramseier CA, Abramson ZR, Jin Q, Giannobile WV. Gene therapeutics for periodontal regenerative medicine. Dent Clin North Am. 2006;50:245–63.
Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. OrthodCraniofac Res. 2005;8:191–9.
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein. J Dent Res. 2004;83:590–5.
Hu B, Unda F, Bopp-Kuchler S, Jimenez L, Wang XJ, Haikel Y, et al. Bone marrow cells can give rise to ameloblast-like cells. J Dent Res. 2006;85:416–21.
Liu Y, Liu W, Hu C, Xue Z, Wang G, Ding B, Luo H, Tang L, Kong X, Chen X, Liu N, Ding Y, Jin Y. MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells. 2011;29(11):1804–16. https://doi.org/10.1002/stem.728 .
Raspini G, Wolff J, Helminen M, Raspini G, Raspini M, Sándor GK. Dental stem cells harvested from third molars combined with bioactive glass can induce signs of bone formation in vitro. J Oral Maxillofac Res. 2018;9(1):e2. Published 2018 Mar 31. https://doi.org/10.5037/jomr.2018.9102 .
Christodoulou I, Goulielmaki M, Devetzi M, Panagiotidis M, Koliakos G, Zoumpourlis V. Mesenchymal stem cells in preclinical cancer cytotherapy: a systematic review. Stem Cell Res Ther. 2018;9(1;336). https://doi.org/10.1186/s13287-018-1078-8 .
Bansal R, Jain A. Current overview on dental stem cells applications in regenerative dentistry. J Nat Sci Biol Med. 2015;6(1):29–34. https://doi.org/10.4103/0976-9668.149074 .
Article PubMed PubMed Central Google Scholar
Edgar Ledesma-Martínez, Víctor Manuel Mendoza-Núñez, Edelmiro Santiago-Osorio. Mesenchymal stem cells derived from dental pulp: a review. Stem Cells Int . 2016, 4,709,572, p. doi: https://doi.org/10.1155/2016/4709572 ].
Grzech-Leśniak K. Making use of lasers in periodontal treatment: a new gold standard? Photomed Laser Surg. 2017;35(10):513–4.
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003;100(10):5807–12. https://doi.org/10.1073/pnas.0937635100 .
Yasui T, Mabuchi Y, Toriumi H, Ebine T, Niibe K, Houlihan DD, Morikawa S, Onizawa K, Kawana H, Akazawa C, Suzuki N, Nakagawa T, Okano H, Matsuzaki Y. Purified human dental pulp stem cells promote osteogenic regeneration. J Dent Res. 2016;95(2):206–14. https://doi.org/10.1177/0022034515610748 .
Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res. 2014;78:16–20. https://doi.org/10.1016/j.neures.2013.10.010 .
d’Aquino R, De Rosa A, Lanza V, Tirino V, Laino L, Graziano A, Desiderio V, Laino G, Papaccio G. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;12, PMID: 19908196:75–83.
Wang L, Shen H, Zheng W, Tang L, Yang Z, Gao Y, Yang Q, Wang C, Duan Y, Jin Y. Characterization of stem cells from alveolar periodontal ligament. Tissue Eng. Part A. 2011;17(7–8):1015–26. https://doi.org/10.1089/ten.tea.2010.0140 .
Iwata T, Yamato M, Zhang Z, Mukobata S, Washio K, Ando T, Feijen J, Okano T, Ishikawa I. Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol. 2010;37(12):1088–99. https://doi.org/10.1111/j.1600-051X.2010.01597.x .
Chen F-M, Gao L-N, Tian B-M, Zhang X-Y, Zhang Y-J, Dong G-Y, Lu H, et al. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: a randomized clinical trial. Stem Cell Res Ther. 2016;7:33. https://doi.org/10.1186/s13287-016-0288-1 .
Bakopoulou A, Leyhausen G, Volk J, Tsiftsoglou A, Garefis P, Koidis P, Geurtsen W. Comparative analysis of in vitro osteo/odontogenic differentiation potential of human dental pulp stem cells (DPSCs) and stem cells from the apical papilla (SCAP). Arch Oral Biol. 2011;56(7):709–21. https://doi.org/10.1016/j.archoralbio.2010.12.008 .
Han C, Yang Z, Zhou W, Jin F, Song Y, Wang Y, Huo N, Chen L, Qian H, Hou R, Duan Y, Jin Y. Periapical follicle stem cell: a promising candidate for cementum/periodontal ligament regeneration and bio-root engineering. Stem Cells Dev. 2010;19(9):1405–15. https://doi.org/10.1089/scd.2009.0277 .
Luan X, Ito Y, Dangaria S, Diekwisch TG. Dental follicle progenitor cell heterogeneity in the developing mouse periodontium. Stem Cells Dev. 2006;15(4):595–608. https://doi.org/10.1089/scd.2006.15.595 .
Handa K, Saito M, Tsunoda A, Yamauchi M, Hattori S, Sato S, Toyoda M, Teranaka T, Narayanan AS. Progenitor cells from dental follicle are able to form cementum matrix in vivo. Connect Tissue Res. 2002;43(2–3):406–8 PMID: 12489190.
Guo W, Chen L, Gong K, Ding B, Duan Y, Jin Y. Heterogeneous dental follicle cells and the regeneration of complex periodontal tissues. Tissue Engineering. Part A. 2012;18(5–6):459–70 https://doi.org/10.1089/ten.tea.2011.0261 .
Bai, Yudi et al. Cementum- and periodontal ligament-like tissue formation by dental follicle cell sheets co-cultured with Hertwig’s epithelial root sheath cells. Bone. 2011, 48, Issue 6, pp. 1417–1426, https://doi.org/10.1016/j.bone.2011.02.016 .
Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. 2008, 34, pp. 962–969.
Dobie K, Smith G, Sloan AJ, Smith AJ. Effects of alginate, hydrogels and TGF-beta 1 on human dental pulp repair in vitro. Connect Tissue Res 2. 2002;43:387–90.
Friedlander LT, Cullinan MP, Love RM. Dental stem cells and their potential role in apexogenesis and apexification. Int Endod J. 2009;42:955–62.
Cai J, Zhang Y, Liu P, Chen S, Wu X, Sun Y, Li A, Huang K, Luo R, Wang L, Liu Y, Zhou T, Wei S, Pan G, Pei D, Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen (Lond). July 30, 2013, 2(1), pp. 6, doi: https://doi.org/10.1186/2045-9769-2-6 .
Craig J. Taylor, Eleanor M. Bolton, and J. Andrew Bradley 2011 Aug 12 and https://doi.org/10.1098/rstb.2011.0030 ], 366(1575): 2312–2322. [doi:. Immunological considerations for embryonic and induced pluripotent stem cell banking,. Philos Trans R SocLond B Biol Sci. 2011, 366(1575), pp. 2312–2322, doi: https://doi.org/10.1098/rstb.2011.0030 .
T.R. Nayak, H. Andersen, V.S. Makam, C. Khaw, S. Bae, X.F. Xu, P.L.R. Ee, J.H. Ahn, B.H. Hong, G. Pastorin, B. Ozyilmaz, ACS Nano, 5 (6) (2011), pp. 4. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells,. ACS Nano. 2011, pp. 4670–4678.
Lee WC, Lim C, Shi H, Tang LAL, Wang Y, Lim CT, Loh KP. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano. 2011;5(9):7334–41.
Kenry LWC, Loh KP, Lim CT. When stem cells meet graphene: opportunities and challenges in regenerative medicine. Biomaterials. 2018;155:236–50.
Yuan A, Farber EL, Rapoport AL, Tejada D, Deniskin R, Akhmedov NB, et al. Transfer of microRNAs by embryonic stem cell microvesicles. 2009. 2009, 4(3), p. https://doi.org/10.1371/journal.pone . 0004722.
Oh, Myeongsik, et al. Exosomes derived from human induced pluripotent stem cells ameliorate the aging of skin fibroblasts. Int. J. Mol. Sci. 2018, 19(6), p. 1715.
Ramirez MI. et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881–906.
Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–9.
Mateescu B, et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—an ISEV position paper. J. Extracell. Vesicles. 2017;6(1). https://doi.org/10.1080/20013078.2017.1286095 .
Nawaz M, et al. Extracellular vesicles: evolving factors in stem cell biology. Stem Cells Int. 2016;2016:17. Article ID 1073140.
Helfrich, Y.R., Sachs, D.L. and Voorhees, J.J. Overview of skin aging and photoaging. Dermatol. Nurs. 20, pp. 177–183, https://www.ncbi.nlm.nih.gov/pubmed/18649702 .
Julia Tigges, Jean Krutmann, Ellen Fritsche, Judith Haendeler, Heiner Schaal, Jens W. Fischer, Faiza Kalfalah, Hans Reinke, Guido Reifenberger, Kai Stühler, Natascia Ventura, Sabrina Gundermann, Petra Boukamp, Fritz Boege. The hallmarks of fibroblast ageing, mechanisms of ageing and development, 138, 2014, Pages 26–44. 2014, 138, pp. 26–44, ISSN 0047–6374, https://doi.org/10.1016/j.mad.2014.03.004 .
Huh MI, Kim MS, Kim HK, et al. Effect of conditioned media collected from human amniotic fluid-derived stem cells (hAFSCs) on skin regeneration and photo-aging. Tissue Eng Regen Med. 2014;11:171 https://doi.org/10.1007/s13770-014-0412-1 .
Togel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31.
Liu J, Han G, Liu H, et al. Suppression of cholangiocarcinoma cell growth by human umbilical cord mesenchymal stem cells: a possible role of Wnt and Akt signaling. PLoS One. 2013;8:e62844.
Oh M, et al. Promotive effects of human induced pluripotent stem cell-conditioned medium on the proliferation and migration of dermal fibroblasts. Biotechnol. Bioprocess Eng. 2017;22:561–8.
Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PloS One. 2008;3:e1886.
Bae J-S, Lee S-H, Kim J-E, Choi J-Y, Park R-W, Park JY, Park H-S, Sohn Y-S, Lee D-S, Lee EB. βig-h3 supports keratinocyte adhesion, migration, and proliferation through α3β1 integrin. Biochem. Biophys. Res. Commun. 2002;294:940–8.
Zhou B-R, Xu Y, Guo S-L, Xu Y, Wang Y, Zhu F, Permatasari F, Wu D, Yin Z-Q, Luo D. The effect of conditioned media of adipose-derived stem cells on wound healing after ablative fractional carbon dioxide laser resurfacing. BioMed Res. Int. 2013;519:126.
Peng Y, Baulier E, Ke Y, Young A, Ahmedli NB, Schwartz SD, et al. Human embryonic stem cells extracellular vesicles and their effects on immortalized human retinal Müller cells. PLoS ONE. 2018, 13(3), p. https://doi.org/10.1371/journal.pone.019400 .
Harris MT, Butler DL, Boivin GP, Florer JB, Schantz EJ, Wenstrup RJ. Mesenchymal stem cells used for rabbit tendon repair can form ectopic bone and express alkaline phosphatase activity in constructs. J Orthop Res. 2004;22:998–1003.
Mascetti VL, Pedersen RA. Human-mouse chimerism validates human stem cell pluripotency. Cell Stem Cell. 2016;18:67–72.
Gandia C, Armiñan A, García-Verdugo JM, Lledó E, Ruiz A, Miñana MD, Sanchez-Torrijos J, Payá R, Mirabet V, Carbonell-Uberos F, Llop M, Montero JA, Sepúlveda P. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells. 2007;26(3):638–45.
Perry BC, Zhou D, Wu X, Yang FC, Byers MA, Chu TM, Hockema JJ, Woods EJ, Goebel WS. Collection, cryopreservation, and characterization of human dental pulp-derived mesenchymal stem cells for banking and clinical use. Tissue Eng Part C Methods. 2008;14(2):149–56.
Garcia-Olmo D, Garcia-Arranz M, Herreros D, et al. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–23.
de Mendonça CA, Bueno DF, Martins MT, Kerkis I, Kerkis A, Fanganiello RD, Cerruti H, Alonso N, Passos-Bueno MR. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg. 2008;19(1):204–10.
Seo BM, Sonoyama W, Yamaza T, Coppe C, Kikuiri T, Akiyama K, Lee JS, Shi S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008;14(5):428–34.
Abbas, Diakonov I., Sharpe P. Neural crest origin of dental stem cells. Pan European Federation of the International Association for Dental Research (PEF IADR). 2008, Vols. Seq #96 - Oral Stem Cells.
Kerkis I, Ambrosio CE, Kerkis A, Martins DS, Gaiad TP, Morini AC, Vieira NM, Marina P, et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs. J Transl Med. 2008;6:35.
Xianrui Yang, Li Li, Li Xiao, Donghui Zhang. Recycle the dental fairy’s package: overview of dental pulp stem cells. Stem Cell Res Ther . 2018, 9, 1, 1. https://doi.org/10.1186/s13287-018-1094-8 .
Wang J, Wang X, Sun Z, Wang X, Yang H, Shi S, Wang S. Stem cells from human-exfoliated deciduous teeth can differentiate into dopaminergic neuron-like cells. Stem Cells Dev. 2010;19:1375–83.
Wang J, et al. The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly (L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater. 2010;6(10):3856–63.
Davies OG, Cooper PR, Shelton RM, Smith AJ, Scheven BA. A comparison of the in vitro mineralisation and dentinogenic potential of mesenchymal stem cells derived from adipose tissue, bone marrow and dental pulp. J Bone Miner Metab. 2015;33:371–82.
Huang GT-J, Shagramanova K, Chan SW. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. J Endod. 2006;32:1066–73.
Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone. 2001;29(6):532–9.
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–30.
Nuti N, Corallo C, Chan BMF, Ferrari M, Gerami-Naini B. Multipotent differentiation of human dental pulp stem cells: a literature review. Stem Cell Rev Rep. 2016;12:511–23.
Ferro F, et al. Dental pulp stem cells differentiation reveals new insights in Oct4A dynamics. PloS One. 2012;7(7):e41774.
Conde MCM, Chisini LA, Grazioli G, Francia A, Carvalho RVd, Alcázar JCB, Tarquinio SBC, Demarco FF. Does cryopreservation affect the biological properties of stem cells from dental tissues? A systematic review. Braz Dent J. 2016;1210(6):633-40. https://doi.org/10.1590/0103-6440201600980 .
Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, Menditti D, De Rosa A, Carinci F, Laino G. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208:319–25.
Alge DL, Zhou D, Adams LL, et. al. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4(1):73–81.
Jo Y-Y, Lee H-J, Kook S-Y, Choung H-W, Park J-Y, Chung J-H, Choung Y-H, Kim E-S, Yang H-C, Choung P-H. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007;13:767–73.
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–5.
Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005;20:1394–402.
Zainal A, Shahrul H, et al. In vitro chondrogenesis transformation study of mouse dental pulp stem cells. Sci World J. 2012;2012:827149.
Wei X, et al. Expression of mineralization markers in dental pulp cells. J Endod. 2007;33(6):703–8.
Dai J, et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials. 2012;33(31):7699–711.
Vasandan AB, et al. Functional differences in mesenchymal stromal cells from human dental pulp and periodontal ligament. J Cell Mol Med. 2014;18(2):344–54.
Werle SB, et al. Carious deciduous teeth are a potential source for dental pulp stem cells. Clin Oral Investig. 2015;20:75–81.
Nemeth CL, et al. Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG-GelMA-HA hydrogels. Tissue Eng A. 2014;20(21–22):2817–29.
Paino F, Ricci G, De Rosa A, D’Aquino R, Laino L, Pirozzi G, et al. Ecto-mesenchymal stem cells from dental pulp are committed to differentiate into active melanocytes. Eur. Cell Mater. 2010;20:295–305.
Ferro F, Spelat R, Baheney CS. Dental pulp stem cell (DPSC) isolation, characterization, and differentiation. In: Kioussi C, editor. Stem cells and tissue repair. Methods in molecular biology (methods and protocols): Humana Press. 2014;1210.
Ishkitiev N, Yaegaki K, Imai T, Tanaka T, Nakahara T, Ishikawa H, Mitev V, Haapasalo M. High-purity hepatic lineage differentiated from dental pulp stem cells in serum-free medium. J Endod. 2012;38:475–80.
Download references
Acknowledgements
Not applicable.
This work is supported by Wrocław Medical University in Poland.
Availability of data and materials
Please contact author for data requests.
Author information
Authors and affiliations.
Department of Experimental Surgery and Biomaterials Research, Wroclaw Medical University, Bujwida 44, Wrocław, 50-345, Poland
Wojciech Zakrzewski, Maria Szymonowicz & Zbigniew Rybak
Department of Conservative Dentistry and Pedodontics, Krakowska 26, Wrocław, 50-425, Poland
Maciej Dobrzyński
You can also search for this author in PubMed Google Scholar
Contributions
WZ is the principal author and was responsible for the first draft of the manuscript. WZ and ZR were responsible for the concept of the review. MS, MD, and ZR were responsible for revising the article and for data acquisition. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Wojciech Zakrzewski .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Zakrzewski, W., Dobrzyński, M., Szymonowicz, M. et al. Stem cells: past, present, and future. Stem Cell Res Ther 10 , 68 (2019). https://doi.org/10.1186/s13287-019-1165-5
Download citation
Published : 26 February 2019
DOI : https://doi.org/10.1186/s13287-019-1165-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Differentiation
- Pluripotency
- Induced pluripotent stem cell (iPSC)
- Stem cell derivation
- Growth media
- Tissue banks
- Tissue transplantation
Stem Cell Research & Therapy
ISSN: 1757-6512
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Clinical Trials
Stem cell therapy.
Displaying 75 studies
The purpose of this study is to assess the effectiveness and safety of Cx601, adult allogeneic expanded adipose-derived stem cells (eASC), for the treatment of complex perianal fistula(s) in patients with Crohn's disease over a period of 24 weeks and a follow-up period up to 52 weeks.
The purpose of this study is to evaluate the safety of using unlicensed cord blood units from the National Cord Blood Program in unrelated patients needing stem cell transplants, by carefully documenting all infusion-related problems.
The purpose of this study is to collect human menstrual blood at the time of gynecology visits in order to conduct future studies on the isolation and characterization of human menstrual blood and endometrial stem cells to better individualize treatment for abnormal uterine bleeding (AUB) and study the therapeutic properties of human menstrual-derived Mesenchymal Stem Cells (MSCs).
The purpose of this study is to collect fat and blood vessel wall tissue for processing into adult stem cells and then test those cells for specific biological markings.
The purpose of this study is to assess optimal dosing frequency, effectiveness and safety of adipose-derived autologous mesenchymal stem cells delivered into the spinal fluid of patients with multipe system atrophy (MSA).
Multiple system atrophy (MSA) is a rare, rapidly progressive, and invariably fatal neurological condition characterized by autonomic failure, parkinsonism, and/or ataxia. There is no available treatment to slow or halt disease progression.
The purpose of this study is to explore patients’ perceptions using educational interventions to debunk or prebunk misinformation of advertisements about unproven stem cell interventions (SCIs).
The purpose of this study is to assess the safety and tolerability of intravenously delivered mesenchymal steml cells (MSC) in one of two fixed dosing regimens at two time points in patients with chronic kidney disease.
The purpose of this study to test the feasibility and safety for autologous (from your own body) skin cells that are manufactured into stem cells of cardiac lineage to be delivered into the heart muscle to determine if those stem cells will strengthen the heart muscle and can be used as an additional treatment for the management of congenital heart disease.
The purpose of this study is to assess the effectiveness and safety of Cx601, adult allogeneic expanded adipose-derived stem cells (eASC), for the treatment of complex perianal fistula(s) in patients with Crohn's disease over a period of 24 weeks and a follow-up period up to 52 weeks.
The purpose of this study is to evaluate the long-term safety of a single dose of darvadstrocel in participants with Crohn's disease (CD) and complex perianal fistula by evaluation of adverse events (AEs), serious adverse events (SAEs), and adverse events of special interest (AESIs).
The aim of this study is to measure the differences in quality of life and mood of hematopoietic stem cell transplant (HCT) patients and their caregivers staying at a hospital hospitality house (HHH), such as the Gift of Life Transplant House, the Help in Healing Home, and the Gabriel House of Care versus staying at a hotel/rental apartment or house. The goal is to investigate if staying in a HHH, with its different environment and support systems and programs, has a positive impact on the quality of life (QOL) and mood of patients undergoing a HCT and their caregivers.
The purpose of this study is to assess the safety and feasibility of mesenchymal stem cells therapy in patients with advanced chronic obstructive pulmonary disease.
The purpose of this study is to evaluate the pharmacokinetics (PK), safety and tolerability of pegcetacoplan in patients with TA-TMA.
The purpose of this study is to determine the safety and feasibility of allogeneic, culture-expanded BM-MSCs in subjects with painful facet joint arthropathy.
The purpose of this study is to produce, using current Good Manufacturing Practices (cGMPs), a bank of 50 primary fibroblast cell lines from skin biopsies obtained by consenting donors who meet 21 CFR 1271 donor eligibility criteria, and to use fibroblasts in the cell bank generated in aim 1 to produce new induced pluripotent stem cell lines using Good Manufacturing Practices (cGMPs). These iPSC lines will then be screened to identify those with optimal characteristics for treatment purposes, as well as for the potential generation of transplantable tissues and therapeutics for chronic disease.
The purpose of this study is to determine determine the safety of intraspinal delivery of mesenchymal stem cells (MSCs) to the cerebral spinal fluid of patients with Amyotrophic Lateral Sclerosis (ALS) using a dose-escalation study.
This study aims to evaluate the safety of local delivery of AMSCs for recurrent GBM by noting the incidence of adverse events, as well as radiological and clinical progression.
To assess the preliminary efficacy of local delivery of AMSCs for recurrent GBM by comparing the clinical, survival, progression, and radiographic outcomes from patients enrolled in our study to historical controls from our institution.
The purpose of this trial is to compare the treatment strategy of Autologous Hematopoietic Stem Cell Transplantation (AHSCT) to the treatment strategy of Best Available Therapy (BAT) for treatment-resistant relapsing multiple sclerosis (MS). Participants will be randomized at a 1 to 1 (1:1) ratio. All participants will be followed for 72 months after randomization (Day 0, Visit 0).
The purpose of this study is to evaluate the effectiveness of ibrutinib in reducing the incidence of NIH moderate/severe chronic GVHD.
To determine the safety and toxicity of intra-arterial infused autologous adipose derived mesenchymal stromal (stem) cells in patients with vascular occlusive disease of the kidney.
The objective of this study is to evaluate the safety and feasibility of autologous mononuclear cells (MNC) collected from bone marrow (BM) delivered into the myocardium of the right ventricle of subjects with Ebstein anomaly undergoing surgical Ebstein repair. Additionally, the potential cardiovascular benefits will also be evaluated. This add-on procedure is anticipated to pose little risk to the subject and has the potential to foster a new strategy that leverages the regenerative capacity of individuals with congenital heart disease during the surgically mandated Ebstein repair.
To assess the safety and feasibility of mesenchymal stem cells therapy in patients with transplant related bronchiolitis obliteran syndrome (BOS).
This phase I/II trial studies the side effects and best dose of oncolytic measles virus encoding thyroidal sodium iodide symporter (MV-NIS) infected mesenchymal stem cells and to see how well it works in treating patients with recurrent ovarian cancer. Mesenchymal stem cells may be able to carry tumor-killing substances directly to ovarian cancer cells.
The purpose of this study is to assess the safety and tolerability of intra-arterially delivered mesenchymal stem/stromal cells (MSC) to a single kidney in one of two fixed doses at two time points in patients with progressive diabetic kidney disease.
Diabetic kidney disease, also known as diabetic nephropathy, is the most common cause of chronic kidney disease and end-stage kidney failure requiring dialysis or kidney transplantation. Regenerative, cell-based therapy applying MSCs holds promise to delay the progression of kidney disease in individuals with diabetes mellitus. Our clinical trial will use MSCs processed from each study participant to test the ...
The purpose of the present study is to investigate the safety and efficacy of a single intrathecal injection of autologous, culture expanded AD-MSCs specifically in subjects with severe traumatic SCI when compared to patients undergoing physical therapy.
The overall goal of this study is to determine the safety and feasibility of infusing adipose-derived mesenchymal stem cells directly into the artery of renal allografts with biopsy-proven rejection in order to reduce inflammation detected in the graft. We contend that future studies will show that administering immunomodulatory cells directly into the allograft will be more effective and safer than the current approaches of delivering massive doses of systemic immunosuppression.
Study participation involves receiving mesenchymal stem cells (MSC), created from the adipose tissue (body fat) of a donor, and infused into the main artery of a transplanted ...
The purpose of this study is to assess the safety of autologous mesenchymal stromal (stem) cell transfer using a biomatrix (the Gore Fistula Plug) to treat perianal fistula.
The purpose of this study is to collect, convert and bank blood cells from healthy volunteers into stem cells (iPSCs) at a current good manufacturing practice (cGMP) facility within the Discovery and Innovation building on the Mayo FLorida campus. After comprehensive validation, we will bank those cGMP-iPSCs as a resource available to Mayo Clinic investigators and also to outside investigators as appropriate. Those bio-specimens could be unique resources to develop new protocols for production of clinical grade iPSC-derived cells, cell-derived products such as extracellular vesicles, and tissues to support Investigational New Drug (IND) and related clinical trials.
To compare the effect of senolytic drugs on cellular senescence, physical ability or frailty, and adipose tissue-derived MSC functionality in patients with chronic kidney disease. Primary Objectives: To assess the efficacy of a single 3-day treatment regimen with dasatinib and quercetin (senolytic drugs) on clearing senescent adipose-derived MSC in patients with CKD. To assess the efficacy of a single 3-day treatment regimen with dasatinib and quercetin (senolytic drugs) on improving adipose-derived MSC functionality in patients with CKD. Secondary Objective: To assess the short-term effect of a single 3-day treatment regimen with dasatinib and quercetin (senolytic drugs) on ...
The investigators propose to study the safety of autologous mesenchymal stromal cell transfer using a biomatrix (the Gore Bio-A Fistula Plug) in a Phase I study using a single dose of 20 million cells. 20 patients (age 12 to 17 years) with Crohns perianal fistulas will be enrolled. Subjects will undergo standard adjuvant therapy including drainage of infection and placement of a draining seton. Six weeks post placement of the draining seton, the seton will be replaced with the MSC loaded Gore fistula plug as per current clinical practice. The subjects will be subsequently followed for fistula response and closure ...
The purpose of this study is to test the safety of this novel cell, combination- based regenerative therapy for use in patients with symptomatic focal cartilage defects of the knee.
This study aims to evaluate the safety of intramyocardial delivery of autologous umbilical cord blood-derived mononuclear cells during Fontan surgical palliation and measure surrogate markers of myocardial protection within a non-randomized study design to obtain prospective data from treatment and control populations.
The purpose of this study is to engage a cohort of patients who are avid information seekers about stem cells to assess their beliefs, online information sources and their credibility, and views on the credibility and persuasiveness of advertisements and warning messages available on the internet; we will use this data along with health behavior theories to develop communication messages aimed at inoculating patients against misinformation, correcting misconceptions, and providing evidence-based information about stem cell procedures.
Group 1: The primary purpose of this study is to evaluate the safety and tolerability of an autologous dendritic cells (DC) vaccine delivered by intra-tumoral injection in patients with primary liver cancer treated with high-dose conformal external beam radiotherapy (EBRT).
Group 2: The primary purpose of this study is to estimate the progression-free survival rate at 2 years post-registration to see if treatment is efficacious compared to historical data
The purpose of this study is to determine the safety and efficacy of intrathecal treatment delivered to the cerebrospinal fluid (CSF) of mesenchymal stem cells in ALS patients every 3 months for a total of 4 injections over 12 months. Mesenchymal stem cells (MSCs) are a type of stem cell that can be grown into a number of different kinds of cells. In this study, MSCs will be taken from the subject's body fat and grown. CSF is the fluid surrounding the spine. The use of mesenchymal stem cells is considered investigational, which means it has not been approved by ...
This study is an extension to re-treat partial and non-responders from the previously approved Phase 1 MCS-AFP protocols IRB #12-009716 (Crohn's Disease perianal fistulas) and 15-003200 (cryptoglandular perianal fistulas).
The investigators propose to study the safety of autologous mesenchymal stromal cell transfer using a biomatrix (the Gore® Bio-A®; Fistula Plug) in a Phase I study using a single dose of 20 million cells. Twenty adult patients (age 18 years or older) with refractory, complicated perianal fistulizing Crohn's disease will be enrolled. Subjects will undergo standard adjuvant therapy including drainage of infection and placement of a draining seton with continuation of pre-existing anti-Crohn's therapy. Six weeks post placement of the draining seton, the seton will be replaced with the MSC loaded Gore® Bio-A® fistula plug as per current clinical practice. ...
In this proposal, we will generate hiPSCs from AA patients and use our TREE-based approaches to introduce AA-associated variants into isogenic hiPSCs. In turn, we will use these isogenic hiPSC lines in a 3-D cortical model to address the following hypothesis-testing questions: (1) Does the presence of specific ABCA7 variants modulate disease-related phenotypes in a hiPSC-based system? (2) Are the risk modifying effects of the ABCA7 variants mediated through cell-autonomous or non-autonomous mechanisms? (3) Do these ABCA7 variants exert their effects through modulation of Aβ processing, secretion, and uptake? (4) What is the effect of these ABCA7 variants ...
The purpose of this study is to determine the safety and practical treatment use of STEM cells collected from a patient's own fat tissue, expanded in laboratory culture, and injected to treat symptoms of mild to severe knee osteoarthritis.
The purpose of this study is to assess the safety and effectiveness of a Stem cell transfer using a biomatrix (The Gore Fistula Plug) in patients with persistent symptoms of post-surgical gastrointestinal leaks despite current standard radiologic and endoscopic treatments. The subjects will be followed for fistula response and closure for 18 months. This is an autologous product (derived from the patient) and used only for the same patient.
The purpose of this study is to determine whether AVB-114 compared to standard of care treatment is effective in inducing remission of the treated complex perianal fistula in subjects with Crohn’s Disease. It also aims to assess clinical and radiologic components of fistula remission, safety of treatment, disease activity, patient Quality of Life, and patient care journey, between AVB-114 and standard of care treatment.
The purpose of this study is to assess neurodevelopmental and psychosocial outcomes (i.e., executive function, social cognition, psychosocial adjustment, adaptive skills) in children with hypoplastic left heart syndrome (HLHS) who underwent right-ventricle-directed delivery of autologous umbilical cord derived mononuclear cells during staged cardiac surgical palliation, and to compare their outcomes to a matched sample of children with HLHS who did not receive autologous umbilical cord derived mononuclear cells during surgery.
The purpose of this study is to assess the safety, tolerability, optimal dosing, effectiveness signals reflecting kidney repair, and markers of mesenchymal stem cells (MSC) function that relate to response to allogenenic adipose tissue-derived MSC in patients with Chronic Kidney Disease (CKD).
Will injection(s) of autologous culture-expanded AMSCs be safe and efficacious for treatment of painful Hip OA, and if so, which dosing regimen is most effective?
The purpose of this study is to determine the safety of using an autologous mesenchymal stromal cell (MSC) coated fistula plug in people with fistulizing Crohn's disease. Autologous means these cells to coat the plug come from the patient.
This study will evaluate the safety of intramuscular administration of PLX-R18 (allogenetic ex-vivo explanded placental adherent stromal cells) in subjects who have with incomplete hematopoietic recovery after hematopoietic stem cell transplantation.
The purpose of this study is to evaluate the safety and effectiveness of CD34+ cell intracoronary injections for treating coronary endothelial dysfunction (CED).
The objective of this study is to generate a panel of iPSCs from 30 subjects who do not have a personal history of major neuropsychiatric disorders.
State-of-the-art induced pluripotent stem cells (iPSC) technology has become a powerful biomedical research tool and it clearly holds great potential for application to neuropsychiatric research.
Ulcerative Colitis (UC) is a chronic inflammatory disease affecting the mucosal lining of the colon and rectum and the incidence is increasing, but the etiology remains unknown. Patients may require a proctocolectomy due to refractory disease. Prior to an operation, UC is treated with antibiotic therapy, immunomodulatory therapy and immunosuppressive agents. While there is an increasing number of approved biologics for the treatment of UC, there are many patients that still suffer from refractory disease. Thus, alternative mechanisms of therapy are desperately needed.
Treatments that have the potential to reduce mucosal inflammation could alleviate the pathology of luminal UC. This trial ...
The purpose of this study is to determine the success of mesenchymal stem cells, developed from the patient's own fat tissue, for reducing hemodialysis arteriovenous fistula failure when applied during the time of surgical creation.
The purpose of this study is to collect adipose tissue from patients undergoing elective surgery, or from healthy volunteers, test the donors to assure that they comply with all regulatory aspects required of healthy donors, expand and test mesenchymal stromal cells (MSC), and bank them for future use.
The current proposal aims to test the feasibility of immune function analysis for Tai Chi Easy (TCE) intervention in multiple myeloma (MM) patients undergoing autologous stem cell transplantation (ASCT) with concurrent exploration of health related quality of life (HRQOL).
The purpose of this study is to evaluate quality of life over time in patients treated with CAR-T therapy compared with autologous and allogeneic stem cell transplant.
This phase Ib/II trial studies how well dendritic cell therapy after cryosurgery in combination with pembrolizumab works in treating patients with stage III-IV melanoma that cannot be removed by surgery. Vaccines made from a person's white blood cells mixed with tumor proteins may help the body build an effective immune response to kill tumor cells. Cryosurgery, also known as cryoablation or cryotherapy, kills tumor cells by freezing them. Monoclonal antibodies, such as pembrolizumab, may block tumor growth in different ways by targeting certain cells. Giving dendritic cell therapy after cryosurgery in combination with pembrolizumab may work better in treating patients ...
The purpose of this study is to determine the effectiveness of MB-CART2019.1 cells administered following a conditioning lymphodepletion regimen in diffuse large B cell lymphoma (DLBCL) subjects who failed at least two lines of therapy as measured by objective response rate (ORR) at one month.
This is a double-blind, sham-controlled clinical study to evaluate the safety and feasibility of AMI MultiStem therapy in subjects who have had a heart attack (Non-ST elevation MI).
The purpose of this study is to evaluate safety, tolerability, pharmacokinetics, and effectiveness of SER-155 in adults undergoing hematopoietic stem cell transplantation to reduce the risk of infection and graft vs. host disease.
The purpose of this study is to compare the efficacy and safety of maribavir to valganciclovir for the treatment of cytomegalovirus (CMV) infection in asymptomatic hematopoietic stem cell transplant recipients.
The purpose of this study is to determine the safety of using an autologous mesenchymal stromal cell (MSC) coated fistula plug in people with rectovaginal fistulizing Crohn's disease. Autologous means that these cells that coat the plug come from you. You will be in this study for two years. There is potential to continue to monitor your progress with lifelong regular visits as part of your standard of care. All study visits take place at Mayo Clinic and Rochester, MN. The study visit schedule is as follows: Visit 1 (Week -6) - Screening visit: exam under anesthesia and surgery to ...
The purpose of this study is to evaluate the cellular composition of PRP produced by the Arthrex Angel System.
The purpose of this study is evaluate the safety of allogeneic adipose derived mesenchymal stem cell (AMSC) use during hemodialysis arteriovenous fistula and arterial bypass creation and its effectiveness on improving access maturation and primary anastomotic patency.
The purpose of this study is to evaluate the side effects of vaccine therapy in treating patients with glioblastoma that has come back. Vaccines made from a person's white blood cells mixed with tumor proteins from another person's glioblastoma tumors may help the body build an effective immune response to kill tumor cells. Giving vaccine therapy may work better in treating patients with glioblastoma.
The purpose of this trial is to evaluate the cosmetic role of novel anti-aging regenerative skin care product, human platelet extract (HPE), on skin rejuvenation.
Skin aging is a natural part of human aging process caused by intrinsic and extrinsic factors, such as genetics, cellular metabolism, chronic light exposure and other toxins. Cosmetological care for facial skin aging includes daily skin care, correct sun protection and aesthetic non-invasive procedures. 80 participants over the age of 40 years with moderate photoaging (dyschromic facial skin with fine lines and wrinkles) will be recruited from Mayo Clinic Center for Aesthetic Medicine and ...
The purpose of this study is to collect adiopose tissue to derive mesenchymal stem cells.
Although survivorship recommendations have been developed in areas such as lymphoma and stem cell transplant, the long-term effects of CAR-T therapy are unknown. In addition, relatively little is known about the psychosocial impact of CAR-T on survivors and their caregivers. Due to the intensive nature of CAR-T treatment and its unique side effects, including neurotoxicity in the acute setting and infections and financial burden in the long-term setting, a longitudinal study that assesses these issues in a quantitative and qualitative fashion is required. Consideration of both patient and caregiver needs is important for the provision of appropriate and ...
The study aims to characterize patient factors, such as pre-existing comorbidities, cancer type and treatment, and demographic factors, associated with short- and long-term outcomes of COVID-19, including severity and fatality, in cancer patients undergoing treatment. The study also is aimed to describe cancer treatment modifications made in response to COVID-19, including dose adjustments, changes in symptom management, or temporary or permanent cessation. Lastely, evaluate the association of COVID-19 with cancer outcomes in patient subgroups defined by clinico-pathologic characteristics.
The purpose of this study is to compare standard-dose combination chemotherapy to high-dose combination chemotherapy and stem cell transplant in treating patients with germ cell tumors that have returned after a period of improvement or did not respond to treatment. Drugs used in chemotherapy, such as paclitaxel, ifosfamide, cisplatin, carboplatin, and etoposide, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Giving chemotherapy before a stem cell transplant stops the growth of cancer cells by stopping them from dividing or killing them. Giving ...
The purpose of this study is to assess the feasibility and safety of delivering adipose mesenchymal stem cells (AMSCs) to kidney allografts.
The purpose of this study is to assess the safety, effectiveness, and overall benefit of FCR001 cell therapy in de novo living donor renal transplantation.
This randomized phase III trial studies rituximab after stem cell transplant and to see how well it works compared with rituximab alone in treating patients with in minimal residual disease-negative mantle cell lymphoma in first complete remission. Monoclonal antibodies, such as rituximab, may interfere with the ability of cancer cells to grow and spread. Giving chemotherapy before a stem cell transplant helps kill any cancer cells that are in the body and helps make room in the patient's bone marrow for new blood-forming cells (stem cells) to grow. After treatment, stem cells are collected from the patient's blood and stored. ...
The purpose of this research study is to evaluate a treatment regimen called IRD which will be given to participants after their stem cell transplant in an effort to help prolong the amount of time the participants are disease-free after transplant. IRD is a three-drug regimen consisting of ixazomib, lenalidomide (also called Revlimid), and dexamethasone. After 4 cycles of IRD, the participants will be randomized to receive maintenance therapy either with ixazomib or lenalidomide. 09/23/2019: Upon review of the interim analysis that suggested inferior progression-free survival in the ixazomib maintenance arm, there will be no further randomizations into the ...
The primary objective of the United States Food and Drug Administration (FDA) for this study is to demonstrate non-inferiority in subjects who received an allogeneic BMT for subjects randomized to Rezafungin for Injection compared to subjects randomized to the standard antimicrobial regimen (SAR) for fungal-free survival at Day 90 (±7 days).
The primary objective of the European Medicines Agency (EMA) for this study is to demonstrate superiority in subjects who received an allogeneic BMT randomized to Rezafungin for Injection compared to subjects randomized to the SAR for fungal-free survival at Day 90 (±7 days).
This randomized phase III trial studies ibrutinib to see how well it works compared to placebo when given before and after stem cell transplant in treating patients with diffuse large B-cell lymphoma that has returned after a period of improvement (relapsed) or does not respond to treatment (refractory). Before transplant, stem cells are taken from patients and stored. Patients then receive high doses of chemotherapy to kill cancer cells and make room for healthy cells. After treatment, the stem cells are then returned to the patient to replace the blood-forming cells that were destroyed by the chemotherapy. Ibrutinib is a ...
The primary purpose of this study is to identify the therapeutic effect of Adipose-Induced Regeneration (AIR) in radiation-induced skin injury of post-mastectomy breast cancer patients.
Mayo Clinic Footer
- Request Appointment
- About Mayo Clinic
- About This Site
Legal Conditions and Terms
- Terms and Conditions
- Privacy Policy
- Notice of Privacy Practices
- Notice of Nondiscrimination
- Manage Cookies
Advertising
Mayo Clinic is a nonprofit organization and proceeds from Web advertising help support our mission. Mayo Clinic does not endorse any of the third party products and services advertised.
- Advertising and sponsorship policy
- Advertising and sponsorship opportunities
Reprint Permissions
A single copy of these materials may be reprinted for noncommercial personal use only. "Mayo," "Mayo Clinic," "MayoClinic.org," "Mayo Clinic Healthy Living," and the triple-shield Mayo Clinic logo are trademarks of Mayo Foundation for Medical Education and Research.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Stem Cell Reports
- v.17(6); 2022 Jun 14
100 plus years of stem cell research—20 years of ISSCR
Urban lendahl.
1 Department of Cell and Molecular Biology, Karolinska Institutet, 17177 Stockholm, Sweden
Associated Data
The International Society for Stem Cell Research (ISSCR) celebrates its 20 th anniversary in 2022. This review looks back at some of the key developments in stem cell research as well as the evolution of the ISSCR as part of that field. Important discoveries from stem cell research are described, and how the improved understanding of basic stem cell biology translates into new clinical therapies and insights into disease mechanisms is discussed. Finally, the birth and growth of ISSCR into a leading stem cell society and a respected voice for ethics, advocacy, education and policy in stem cell research are described.
In this Perspective by Urban Lendahl, the history of stem cell research from discoveries in the late 1800s to the most recent developments is described. The Perspective also discusses the progress in clinical translation of stem cell research discoveries. Finally, the 20-year history of ISSCR as well as the society’s role in research, ethics, advocacy, education, and policy is described.
Stem cell research—the early years
Stem cells are defined by the ability to self-renew and to produce differentiated cells, but what could be considered the starting point of this research field? A defining moment is difficult to identify precisely, but an important conceptual prerequisite for stem cell research, and in fact for all cell biology, was the development of the cell theory in the mid-1800s by Rudolf Virchow, Rudolf Remak, and Theodor Schwann and the realization that all cells are derived from other cells through cell division – “ omnis cellula a cellula” ( Virchow, 1858 ). The first descriptions of the word stem cell also date back to the mid-1800s. Ernst Haeckel used the term “ Stammzellen” in 1868, but originally in a more phylogenetic context, to denote a unicellular organism from which multicellular organisms developed. In 1877 he extended its use to the fertilized egg, in line with his concept of “ontogeny recapitulates phylogeny” ( Haeckel, 1877 ) (see Figure 1 for a timeline of some of the key discoveries in stem cell research). Theodor Heinrich Boveri and Valentin Häcker used stem cell as a term for cells giving rise to the germ line, thus expanding its use to cell types other than the fertilized egg cell. Häcker also made the important observation that cell division in the crustacean Cyclops led to one cell remaining as a stem cell while the other cell differentiated ( Haecker, 1892 )—an early observation of asymmetric cell division. Boveri characterized cells giving rise to germ cells and somatic cells and referred to them as stem cells (for review see Maehle, 2011 ). After the initial use of stem cells referring to the germ line, Alexander Maximow, Wera Dantschakoff, and Artur Pappenheim started using the term stem cell in the context of hematopoiesis to denote cells producing the different types of cells in the blood ( Dantschakoff, 1908 ; Maximow, 1909 ; Pappenheim, 1896 ).

Time axis for 25 major discoveries in stem cell research
Stem cell research milestones—lessons from four organ systems
Progress in stem cell research was reported from many frontiers during the 1900s. In the pre-molecular era, transplantation experiments in amphibians yielded new insights into the role of communication between various organs and tissues for cellular differentiation ( Spemann and Mangold, 1924 ). The development of transgenic technologies, more advanced cell culturing techniques and decoding of genomes from various species further fueled progress in our understanding of stem cells and their various differentiation trajectories. A detailed account of this era is beyond the scope of this review, and I will instead focus on discussing some of the landmark discoveries in stem cell research in four mammalian organs—the hematopoietic system, the skin, the CNS, and the intestine—as each of these organs has provided important concepts for the field at large and revealed opportunities for clinical translation and understanding of disease processes.
The hematopoietic system
As discussed above, Pappenheim, Maximow, and Dantschkoff introduced the stem cell concept for hematopoiesis, and Pappenheim, in fact, produced a scheme for a hematopoietic stem cell hierarchy not too distant from the version that is agreed upon today ( Ramalho-Santos and Willenbring, 2007 ). It was, however, extensively discussed whether there was a single or more than one type of stem cells for the blood, and there were two camps, dualists and unitarians, with different views on this subject. The unitarian Ernst Neumann observed that hematopoiesis takes place in the bone marrow and suggested that one cell can give rise to all the different blood cells ( Neumann, 1868 ), whereas Paul Ehrlich, for example, advocated separate origins for the different types of cells in the blood ( Ramalho-Santos and Willenbring, 2007 ).
This issue took considerable time to resolve, as hematopoietic stem cells amount to less than 0.01% of all bone marrow cells. An initial landmark discovery in hematopoietic research, and for stem cell research in general, was the first functional assay to quantitate hematopoietic stem cells (or at least closely related multipotent progenitors). This was accomplished by James Till and Ernest McCulloch, who, on the basis of the discovery that bone marrow cells could be successfully transplanted to an irradiated mouse host ( Ford et al., 1956 ), demonstrated that hematopoietic stem cells were endowed with self-renewing as well as multi-lineage differentiation capacities ( Becker et al., 1963 ; Till and McCulloch, 1961 ). Irving Weissman’s laboratory, building on the Till and McCulloch discoveries, combined the recently invented fluorescence-activated cell sorting (FACS) technology with the use of novel monoclonal antibodies and a negative selection strategy, to provide the first set of molecular markers (Thy1.1 lo -Sca-1 hi -Lin –/lo ) that gave high enrichment of hematopoietic stem cells. As few as 30 such cells were sufficient for 50% survival in transplanted lethally irradiated mice and reconstituted all hematopoietic cell types ( Spangrude et al., 1988 ). Several observations, however, suggested that the Thy1.1 lo -Sca-1 hi -Lin –/lo cells remained heterogeneous and that further subdivision of the cells resolved them into long-term self-renewing, short-term self-renewing, and non-self-renewing multipotent progenitors ( Morrison and Weissman, 1994 ). Subsequent work further refined the molecular definition of hematopoietic stem cells ( Kiel et al., 2005 ), but several lines of evidence, including analysis of their epigenetic state and dormancy, suggested that the hematopoietic stem cell pool is molecularly heterogeneous ( Foudi et al., 2009 ; Oguro et al., 2013 ; Wilson et al., 2008 ; Yu et al., 2016 ).
Based on the success in transplanting bone marrow in mice ( Ford et al., 1956 ), it was realized early on that transplantation of hematopoietic stem cells could have huge medical potential, providing an opportunity to replace an ailing or cancerous human hematopoietic system. In 1957, Donnell Thomas and colleagues performed the first allogeneic (from a genetically different individual) bone marrow transplantation in humans ( Thomas et al., 1957 ). Although the first six patients died within 100 days, there were indications of a “take” of the donor bone marrow cells, demonstrating that the concept as such was viable. Georges Mathé a few years later realized that the immunological reaction of the grafted cells toward the cells in the recipient host could be harnessed as a means to help rid the body of remaining cancerous cells, and this controlled graft-versus-host reaction saved patients with relapsing lymphoblastic leukemia that had been transplanted by mixed-donor bone marrow cells ( Mathé et al., 1965 ). The range of diseases that could be treated was gradually extended, and allogeneic transfer was used to replace the hematopoietic system in patients suffering from severe combined immunodeficiency (SCID) and Wiskott-Aldrich syndrome ( Bach et al., 1968 ; Gatti et al., 1968 ). Following these early pioneering discoveries, further progress in what is now referred to as allogeneic hematopoietic stem cell transfer (allo-HSCT) has been made with regard to immunological matching and immunosuppression, and allo-HSCT is today used routinely in the clinic. To improve the donor base, efforts have been made to establish international registries of unrelated donors, such as the World Marrow Donor Association ( Lown et al., 2014 ). Furthermore, the repertoire of donor cell sources has been expanded to include, for example, umbilical cord blood cells, which, however, take longer to reconstitute the hematopoietic system (see Cieri et al., 2021 for review).
The brain and spinal cord constitute the CNS and arise from stem cells in the embryonic neuroectoderm. Induction of neuroectoderm by the underlying mesoderm was initially demonstrated by Hilde Mangold and Hans Spemann through transplantation experiments in amphibians ( Spemann and Mangold, 1924 ). Retroviral lineage-tracing experiments revealed the existence of embryonic neural stem cells giving rise to both neurons and glial cells ( Price and Thurlow, 1988 ; Turner and Cepko, 1987 ), and a similar lineage bipotentiality was observed for cultured neural stem cells ( Cattaneo and McKay, 1990 ; Davis and Temple, 1994 ). A few years later, it was demonstrated that radial glial cells, previously assumed to have more structural roles, served as neural stem cells in the embryonic brain ( Malatesta et al., 2000 ; Noctor et al., 2001 ). How neural stem cells proceed to acquire specific neuronal identities and the role of transcription factors and morphogens in this process was elucidated by the late Tom Jessell and colleagues ( Briscoe et al., 1999 ; Liem et al., 1997 ).
The question whether stem cells persisted in the adult brain was a thornier question and a matter of considerable debate for many years. Wilhelm His, in fact, observed cells with mitotic figures near the ventricles of the adult human brain almost 150 years ago ( Breunig et al., 2011 ; His, 1874 ), supporting that cell division indeed took place. This notion was, however, disputed by many leading contemporary neurobiologists, including Ramón y Cajal, who remained skeptical and argued that “nothing changed after development” ( Breunig et al., 2011 ; Takagi, 2016 ). In the 1960s, the technique to label dividing cells with tritiated thymidine in vivo was developed ( Smart and Leblond, 1961 ). Joseph Altman used this technique to identify cell divisions in the adult rat subventricular zone (SVZ) and dentate gyrus and to show that dividing cells born in the postnatal SVZ migrated along a rostral migratory stream to the olfactory region ( Altman, 1961 , 1969 ; Altman and Das, 1965 ). Adult neurogenesis was not confined only to mammals but was also reported in canary birds, in which new neurons are generated yearly in association with song behavior ( Goldman and Nottebohm, 1983 ). An indication that adult neurogenesis occurs in humans was provided by Fred Gage and the late Peter Eriksson, who identified label-retaining cells in post mortem brains from patients who had received bromodeoxyuridine (BrdU) for diagnostic purposes in conjunction with tumor therapy ( Eriksson et al., 1998 ). The precise location of the adult CNS stem cells near the ventricles of the brain was for some time a matter of debate ( Doetsch et al., 1999 ; Johansson et al., 1999 ), but the current model holds that stem cells with primary cilia (type B cells) give rise to transient-amplifying cells (type C cells), which subsequently become neuroblasts (type A cells). Adult CNS stem cells are largely quiescent ( Doetsch et al., 1999 ), but a certain amount of cellular turnover in the human brain has been demonstrated by Jonas Frisén and colleagues, who developed a unique technique to birth-date cells based on their cellular carbon-14 content, taking advantage of the “spike” in atmospheric carbon-14 levels resulting from above-ground nuclear testing in the early 1960s ( Spalding et al., 2005 , 2013 ). The extent to which there is adult neurogenesis in specific human brain regions, such as the dentate gyrus of the hippocampus, is, however, still intensely debated, and reports arguing for ( Boldrini et al., 2018 ; Spalding et al., 2013 ) or against ( Franjic et al., 2022 ; Sorrells et al., 2018 ) adult hippocampal neurogenesis have been presented (for review see Kempermann et al., 2018 ; Paredes et al., 2018 ).
The history of stem cells in the skin is strongly centered around one scientist, Howard Green, who not only pioneered skin transplantation in the clinic but also worked out important cellular principles and trained a cadre of today’s leading epithelial biology scientists. Howard Green started his research in the early 1970s by studying teratomas (as many other scientists did at the time; see below) and in these studies he noted that epithelial cells formed colonies in cell culture and that their ability to expand was enhanced by culturing them on feeder cells ( Rheinwald and Green, 1975 ). Next, he developed procedures for detaching the cultured epithelial sheets ( Green et al., 1979 ) and succeeded in transplanting them to mice ( Banks-Schlegel and Green, 1980 ). In a landmark study, Green transplanted two patients with third-degree burn injuries with autologous skin grafts ( O’Connor et al., 1981 ), which represented the first step toward a life-saving therapy for patients with severe burn injuries. Skin transplantation is nowadays well established in the clinic and has more recently been combined with gene correction techniques: a patient with junctional epidermolysis bullosa was grafted with skin in which the LAMB3 gene was inserted to provide correct expression of laminin-332 ( Hirsch et al., 2017 ).
Skin constitutes approximately 15% of body weight and is composed of two layers (epidermis and dermis), as well as hair follicles, sweat glands, and sebaceous glands. Epidermal stem cells are located in the basal layer ( Barrandon and Green, 1987 ) and balance self-renewal and production of keratinocytes that progress through the upper, suprabasal layers, eventually ending up as dead squames, which are shed from the stratum corneum (for review see Blanpain and Fuchs, 2009 ). Differentiation is accompanied by a coordinated change in keratin expression to appropriately adapt the cytoskeleton of the keratinocytes to their position in the epidermis ( Fuchs and Green, 1978 , 1980 ). The choice between self-renewal and differentiation of the epidermal stem cells is, at least in part, controlled by asymmetric cell division and the angle of the cleavage plane ( Lechler and Fuchs, 2005 ; Smart, 1970 ). Regulation of clone size and distribution of clones derived from individual epidermal stem cells and whether clone expansion involves an intermediate transient-amplifying cell population are topics that have been intensely studied in the mouse. Models based on stochastic events, neutral drift, or distinct stem cell pools and lineages have been proposed ( Clayton et al., 2007 ; Gomez et al., 2013 ; Jones and Watt, 1993 ; Mascré et al., 2012 ; for review see Rognoni and Watt, 2018 ).
In addition to the stem cells residing in the basal layer, the hair follicles contain molecularly distinct stem cell populations positioned at different locations of the hair follicle, including the bulge region and the sebaceous gland ( Cotsarelis et al., 1990 ; Jensen et al., 2009 ; Tumbar et al., 2004 ). While bulge stem cells normally give rise only to the hair follicle, lineage tracing and transplantation experiments revealed that they can contribute to both hair and epidermis ( Blanpain et al., 2004 ; Claudinot et al., 2005 ). A specific feature of the hair follicle stem cells is that they need to tune their activity to the hair cycle, which switches between a resting (telogen), a regenerative (anagen), and a destructive (catagen) phase ( Blanpain and Fuchs, 2009 ). Fgf18 and BMP6 produced from differentiating cell progeny play key roles for maintaining quiescence ( Hsu et al., 2011 ), while SHH produced from transient-amplifying cells activates the stem cells ( Hsu et al., 2014 ). In the normal, non-injured state, the various stem cell populations give rise to distinct subsets of differentiated cells ( Jensen et al., 2009 ; Page et al., 2013 ), but in response to injury, all stem cell populations give rise to epidermal cells ( Aragona et al., 2017 ; Donati et al., 2017 ; Ge et al., 2017 ; Park et al., 2017 ). This “all hands on deck” stem cell contribution to epidermal cells upon injury is likely important to rapidly heal an epidermal wound. Interestingly, the response of epidermal stem cells may be tuned by previous experiences, such as acute inflammation ( Naik et al., 2017 ).
The intestine is a hostile environment for cells, and in line with this, there is a very rapid cell turnover, with an average life span of only a few days for intestinal cells. The intestine is composed of two principal categories of cells: absorptive (enterocytes and M cells) and secretory (goblet, Paneth, tuft, and enteroendocrine cells) cells, and both categories originate from intestinal stem cells ( Beumer and Clevers, 2021 ). The tiny crypt-base columnar (CBC) cells, localized at the bottom of the intestinal crypts between the much larger Paneth cells, were first proposed as the enigmatic crypt stem cell ( Cheng and Leblond, 1974 ). Later, Chris Potten proposed that cells located at position +4 (counting from the crypt base) rather than the CBC cells constituted the “real” stem cell ( Potten et al., 2002 ), which remained the dominant view for years. In an early version of genetic lineage tracing, cell progeny born at or near the crypt base were demonstrated to migrate away from the crypts along the crypt-villus axis ( Winton et al., 1988 ). The discovery that the crypt-base columnar cells express Lgr5 provided definitive, lineage-tracing-based evidence in support of CBCs as the crypt stem cell ( Barker et al., 2007 ). An interesting feature of the Lgr5 + intestinal stem cells is that they constantly divide and show high telomerase activity ( Sato et al., 2009 ; Schepers et al., 2011 ), which sets them apart from quiescent stem cells in many other organs. Lineage tracing of the intestinal stem cells by virtue of their Lgr5 expression has provided insights into clonal distribution of differentiated cell progeny ( Barker et al., 2007 ), and neutral drift and competition between the stem cells eventually leads to clonality in the crypts ( Lopez-Garcia et al., 2010 ; Snippert et al., 2010 ). More recently, a slow-dividing Lgr5 + cell population has been identified, which normally gives rise to Paneth and enteroendocrine cells, but upon injury can generate all intestinal cell types ( Buczacki et al., 2013 ). An intriguing finding is that more differentiated intestinal cell types can revert to become intestinal stem cells upon injury ( van Es et al., 2012 ; Jadhav et al., 2017 ; Tetteh et al., 2016 ), thus helping to replenish the intestinal stem cell pool.
The stem cell niche
Stem cells do not function in splendid isolation; they are, in fact, highly dependent on interactions with surrounding cells and tissues, which constitute the stem cell niche. Ray Schofield launched the concept of a stem cell “niche” in the hematopoietic system ( Schofield, 1978 ), and considerable progress has since then been made in terms of characterizing the hematopoietic stem cell niche. In the 1970s, Michael Dexter and colleagues showed that stromal cells were important for culturing of hematopoietic stem cells in vitro ( Dexter et al., 1977 ). Next, osteoblasts in the bone marrow were considered to be the important niche cells ( Calvi et al., 2003 ; Zhang et al., 2003 ), but results from subsequent studies have instead revealed that hematopoietic stem cells reside near sinusoidal blood vessels in the bone marrow ( Kiel et al., 2005 , 2007 ; Sugiyama et al., 2006 ), suggesting that the endothelial or paravascular cells provide the important niche signals and that the osteoblasts exert more indirect effects. Indeed, leptin receptor-positive stromal cells, together with endothelial cells, produce factors such as stem cell factor (SCF) and Cxcl12, which are critical for stem cell maintenance ( Ding and Morrison, 2013 ; Ding et al., 2012 ; for review see Comazzetto et al., 2021 ; Morrison and Scadden, 2014 ). Furthermore, the late Paul Frenette demonstrated that the sympathetic nervous systems provided signals for hematopoietic stem cell mobilization ( Katayama et al., 2006 ). Hematopoietic stem cell maintenance is also influenced by various types of immune cells, such as granulocytes and monocytes, located at specific sites in the bone marrow ( Hérault et al., 2017 ; Zhang et al., 2021 ), as well as by stress conditions ( Severe et al., 2019 ), offering mechanisms by which changes in overall physiological status can be sensed and influence hematopoietic stem cell activity.
In the adult brain, stem cells are primarily located in the subventricular zone and the hippocampus, and progress has been made in decoding their niches. In the subventricular zone, the stem cells reside in pinwheel-like niche structures near the brain vasculature and cerebrospinal fluid ( Mirzadeh et al., 2008 ; Shen et al., 2008 ; Tavazoie et al., 2008 ), while neural stem cells in the hippocampus are positioned close to the inner granule cell layer in the dentate gyrus ( Sun et al., 2015 ). How changes in niche composition, for example with regard to nutrient sensing, contributes to the cognitive decline observed during aging is an emerging research area (for review see Navarro Negredo et al., 2020 ). Other, somewhat less intuitive, and longer-range niche components in the brain are the meninges, which are membranous structures circumscribing the brain, and the choroid plexus, the major source of cerebrospinal fluid production. Both the meninges and the choroid plexus produce factors that influence neural stem cells, such as CCL2, CXCL12, and retinoic acid ( Belmadani et al., 2015 ; Radakovits et al., 2009 ; Siegenthaler et al., 2009 ; Silva-Vargas et al., 2016 ). The microenvironment for oligodendrocyte progenitor cells has been shown to stiffen with age in the brain, which contributes to an age-related decline in oligodendrocyte production ( Segel et al., 2019 ).
In the skin, the epidermal stem cells in fact contribute to shaping their own niche by producing the extracellular matrix on which they sit in the basal layer ( Blanpain and Fuchs, 2009 ). The extracellular matrix also provides a reference point for the stem cell division plane, dictating the balance between self-renewal and differentiation (see above). External stimuli can affect the niche to regulate epidermal stem cell activity, and such stimuli include mechanical stretching of the skin ( Aragona et al., 2020 ; Fiore et al., 2020 ), as well as stress and activity in the sympathetic innervation to the skin ( Shwartz et al., 2020 ; Zhang et al., 2020 ). Furthermore, alterations in the epidermal stem cell secretome modulate surrounding lymphatic capillaries, which constitute part of the epidermal stem cell niche ( Gur-Cohen et al., 2019 ). For hair follicle stem cells, the dermal papilla, located adjacent to the bottom of the hair follicle, is a driver of stem cell activation, and immune cells (T cells and macrophages) also influence hair follicle stem cells ( Ali et al., 2017 ; Castellana et al., 2014 ).
Paneth cells, i.e., the cells intercalated between the Lgr5 + stem cells, constitute an important niche component for intestinal stem cells ( Sato et al., 2011 ). If Paneth cells are experimentally ablated, they can be replaced by other cells, such as enteroendocrine cells, that take over the niche function ( van Es et al., 2019 ; for review see McCarthy et al., 2020 ). Paneth cell replacement, along with replacement of ailing or lost intestinal stem cells by more differentiated cells (see above), provides important mechanisms for safeguarding intestinal cell turnover and contributes to making the intestine quite resilient to injury. More “long-range” niche signals provided to the intestinal stem cells have also been identified, with mesenchymal cells (a.k.a. myoepithelial cells or telocytes) in the vicinity of the crypts and villi providing secreted factors such as Wnt, R-spondin, and BMP-inhibitors ( Beumer and Clevers, 2021 ). Analysis of stem cell niches in different organs is a very active research field, and further progress is expected regarding the response of the niches to altered physiological conditions, injury, and age, and to shed light on how stem cells themselves contribute to the niche ( Fuchs and Blau, 2020 ; Gola and Fuchs, 2021 ).
The quest for cellular rejuvenation and pluripotency
In addition to understanding the underpinning mechanisms of stem cell maintenance and differentiation, there was a parallel interest in learning whether the phenotype of a differentiated cell could in some way be reversed, leading to “rejuvenation” of a mature cell. This was first explored by asking whether a differentiated cell nucleus could revert to a more immature state if transferred to an enucleated, undifferentiated cell. The idea of nuclear transfer was already contemplated by Hans Spemann, but it was Robert Briggs and Thomas King who showed that such an experiment was technically possible, by demonstrating that nuclei from frog blastula transplanted into enucleated frog eggs gave rise to tadpoles ( Briggs and King, 1952 ). John Gurdon, using the Briggs and King somatic cell nuclear transfer (SCNT) technology, then provided the first demonstration that tadpoles could be produced after transplantation of a cell nucleus from a differentiated adult frog cell ( Gurdon, 1962 ). Following this pioneering report, SCNT was established in mammalian species, including sheep ( Wilmut et al., 1997 ) and mice, where a combination of SCNT and gene therapy could correct a genetic defect in the nuclear donor strain ( Rideout et al., 2002 ). SCNT has also found novel medical uses, for example in the mitochondrial replacement technique (MRT), an emerging strategy to correct mitochondrial disease in humans. MRT rests on a combination of in vitro fertilization techniques originally pioneered by Patrick Steptoe and Robert Edwards ( Steptoe et al., 1971 ) and SNCT and is used as a means for mothers carrying severe mtDNA mutations to have genetically related children ( Cohen et al., 2020 ). Technically, by transferring the male and female pronuclei from a fertilized egg from parents with mitochondrial disease by pronuclear DNA transfer (PNT) into an enucleated donor zygote, or alternatively transferring the metaphase II spindle complex from the mother’s oocyte into an enucleated donor oocyte, the faulty mitochondria from the mother are replaced with those from the donor zygote or oocyte (see also below under the ISSCR guidelines).
To learn to culture the most undifferentiated cells in vitro represented another Holy Grail for stem cell research, as it was expected to give insights into cellular pluripotency and how such a state could be maintained. It was argued that pluripotent cells should reside in the inner cell mass of the blastocyst but possibly also in teratomas and teratocarcinomas, tumors containing a bewildering variety of differentiated cell types and tissue, suggesting the existence of highly undifferentiated stem cells in these tumors (for review see Solter, 2006 ). Leroy Stevens and Clarence Cook Little showed that the propensity for developing testicular teratomas, which normally is very low in mice, was elevated in a specific mouse strain, the 129-strain ( Stevens and Little, 1954 ). This opened new vistas for gaining insights into this tumor type, and teratocarcinomas from the 129-strain could be propagated in the abdominal cavity of mice ( Kleinsmith and Pierce, 1964 ). Subcutaneous transplantation of single cells from the ascites fluid contributed to a variety of tissues ( Kleinsmith and Pierce, 1964 ), revealing that multipotent cells (referred to as embryonal carcinoma cells) could be identified experimentally. Ralph Brinster advanced the transplantation paradigm further by demonstrating that transfer of embryonal carcinomas cells into the early mouse blastocyst resulted in chimeric offspring ( Brinster, 1974 ).
The question, however, remained whether cells that could give rise to all cell types in an animal, i.e., totally pluripotent cells, could be identified and harnessed in vitro . Gail Martin and Martin Evans managed to culture inner-cell mass-derived cells (referred to as embryonic stem [ES] cells) and produce teratocarcinomas upon transplantation ( Evans and Kaufman, 1981 ; Martin, 1981 ). Three years later, Evans and colleagues demonstrated that ES cells could yield germline chimeric mice ( Bradley et al., 1984 ). ES cell lines were next generated from non-human primates ( Thomson et al., 1995 ) and humans ( Shamblott et al., 1998 ; Thomson et al., 1998 ).
The SCNT experiments discussed above demonstrated that a differentiated cell nucleus could be rejuvenated if placed in an appropriate juvenile cellular environment; but would it be possible to revert an intact differentiated cell into an undifferentiated, pluripotent state? For some time, this remained more of a thought experiment, and the route to inducing pluripotency in a cell was viewed to be very complex, if not impossible. The molecular decoding of ES cells, however, provided insights into factors required to maintain the pluripotent state in the culture dish ( Chambers et al., 2003 ; Nichols et al., 1998 ). This information was used to test combinations of such factors, and the discovery that expression of only a very small set of transcriptional regulators (Oct4, Sox2, cMyc, and Klf4) was sufficient to convert a differentiated mouse fibroblast into an induced pluripotent stem cell (iPS cell) came as a surprise to the field ( Takahashi and Yamanaka, 2006 ). The generation of human iPS cells was published a year later ( Takahashi et al., 2007 ; Yu et al., 2007 ). It was also soon demonstrated that provision of a small set of transcriptional regulators could drive a direct conversion of one type of differentiated cell into another, without proceeding through the pluripotent state. In this way, β-cells, oligodendrocytes, and neurons were produced from other differentiated cell types by direct lineage conversion ( Vierbuchen et al., 2010 ; Yang et al., 2013 ; Zhou et al., 2008 ; for review see Falk et al., 2021 ). The notion that a direct lineage conversion could be obtained by expression of specific combinations of transcription factors was also in line with a classical observation by the late Harold Weintraub that expression of MyoD was sufficient to convert fibroblasts (10T1/2 cells) into myoblasts ( Lassar et al., 1986 ).
Protocols were developed to steer ES and iPS cell differentiation toward specific differentiated cell fates, and when combined with the introduction of disease-specific mutations into the genome of the pluripotent cells, new light was shed on the molecular basis for monogenic diseases, such as amyotrophic lateral sclerosis, Parkinson’s disease, and long QT syndrome (see Soldner and Jaenisch, 2018 for review). Most of the early protocols, however, relied on culturing the cells as a flat two-dimensional (2D) monolayer, and it made intuitive sense that three-dimensional (3D) culturing of cells would more closely recapitulate the in vivo situation and thus be superior to 2D culturing. There was, therefore, an interest in exploring whether ES and iPS cells could be not only differentiated but guided toward forming more complex mini-organs, called organoids, when cultured in 3D (see Lancaster and Knoblich, 2014 for review). When ES or iPS cells were allowed to proceed through an embryoid body-like state, recapitulating early embryo development, they revealed signs of self-organization into organ-like structures. Pioneering research by the late Yoshiki Sasai yielded retinal and brain organoids ( Eiraku et al., 2008 , 2011 ), and subsequent work by Jürgen Knoblich’s and Sasai’s research groups demonstrated that brain organoids with advanced anatomical organization could be generated and that brain disease-specific features could be mimicked in the organoids ( Kadoshima et al., 2014 ; Lancaster et al., 2013 ).
Adult-tissue stem cells from various epithelial structures turned out to be an alternative cellular source for organoid generation. A breakthrough in this area was the discovery that intestinal Lgr5 + stem cells (see above) gave rise to organoids with many features of the intestinal crypt ( Sato et al., 2009 ) and that such organoids engrafted successfully when transplanted to the mouse intestine ( Yui et al., 2012 ). Organ-specific organoids have now been generated from Lgr5 + stem cells from most other organs, including liver ( Huch et al., 2013 ). Organoids not only shed light on principles for organ generation but are increasingly used to study disease mechanisms, for example in infectious disease research, where the effects of Zika virus exposure have been studied in brain organoids ( Qian et al., 2016 ), and various organoid systems have rapidly been adapted for COVID-19 research (see Geurts et al., 2021 for review). The effects of specific disease mutations have been explored in organoid systems, including mutations causing microcephaly ( Lancaster et al., 2013 ), CFTR ( Dekkers et al., 2013 ) and liver diseases such as alpha1-antitrypsin and Alagille syndrome ( Huch et al., 2015 ). Although most examples of disease modeling in organoids still come from monogenic diseases, organoids from patients with genetically more complex, non-monogenic diseases, such as biliary atresia, have also unveiled disease-specific phenotypes ( Babu et al., 2020 ). Finally, organoids are increasingly used to unravel the molecular basis for various types of cancer and to explore personalized-medicine approaches for cancer therapy ( Kastner et al., 2021 ). As will be discussed later, we can envisage the generation of increasingly more complex organoid systems, and analysis of interactions between organoids and specific cell types, such as immune cells. To grow larger organoids will at some point likely require vascularization, and blood vessel organoids have recently been generated ( Wimmer et al., 2019 ).
An interesting recent offshoot from the organoid tree is the development of blastoids, blastocyst-like structures that have spurred a new research field referred to as synthetic embryology ( Li et al., 2022 ). Blastoids were first produced from mouse cells, where an assembly of ES cells and trophoblast stem cells produced blastocyst-like structures ( Rivron et al., 2018 ). More recently, human blastoids have been developed that undergo lineage specification in the order expected of blastocysts and that can attach to endometrial cells ( Kagawa et al., 2022 ; Yanagida et al., 2021 ; Yu et al., 2021 ).
Toward clinical translation of stem cell research
Given the vast body of basic stem cell research, how rapid is the transition of this information to clinical applications in the different organ systems? Progress is made on many fronts, but the extent of clinical translation differs between organ systems. Allo-HSCT from bone marrow, peripheral blood, or cord blood is well established and currently used routinely in the clinic to treat hematological malignancies or congenital immunodeficiencies such as β -thalassemia, Fanconi anemia, sickle cell anemia, acute and chronic leukemia, non-Hodgkin’s lymphoma, and Hodgkin’s disease ( Ferrari et al., 2021 ). There is also considerable progress in developing gene correction strategies for autologous hematopoietic stem/progenitor cells. Adenosine deaminase deficiency (ADA), an immune-deficiency disorder, was cured by adding the ADA gene into a patient’s bone marrow cells and peripheral blood lymphocytes, providing long-term immune system restoration ( Bordignon et al., 1995 ). However, the use of retroviral vectors for gene corrections in hematopoietic cells initially resulted in aberrant viral integrations leading to T cell acute lymphoblastic leukemia ( Howe et al., 2008 ), For a long time, the risk of tumor development cast a shadow over the entire gene therapy field, but improvement in viral vectors, for example through the use of self-inactivating gammaretroviral or lentiviral vectors, have enhanced efficacy and led to safer therapies ( Ferrari et al., 2021 ). Direct gene editing, rather than virus-based gene insertions, is an interesting avenue to explore, and successful correction of the mutated IL2RG gene in hematopoietic stem cells from a SCID patient has been reported ( Genovese et al., 2014 ). CRISPR-Cas9 gene modification strategies are being developed but are not yet clinically approved ( Ferrari et al., 2021 ).
In contrast to skin transplantation and allo-HSCT, where therapies are well established in the clinic, cell replacement therapies for brain diseases are still a work in progress. Parkinson’s disease (PD) has long been an attractive candidate for stem cell therapy because a specific cell type, the A9 nigral neurons providing dopaminergic innervation to striatum, are lost in PD. Furthermore, the efficacy of dopaminergic agents such as L-DOPA and levodopa declines after a few years and can cause side effects, notably dyskinesia (involuntary movements). Important steps toward PD cell therapy included proof-of-principle for survival of dopaminergic neuronal grafts in rats ( Olson and Seiger, 1973 ) and development of a PD-mimicking rat model where dopaminergic neurons in the nigrostriatal system were chemically depleted by 6-hydroxydopamine (6-OHDA) ( Ungerstedt, 1968 ). It was next reported that transplantation of fetal dopaminergic grafts improved outcome in the rat 6-OHDA model ( Björklund et al., 1980 ; Freed et al., 1981 ; Perlow et al., 1979 ). A study using adrenal medullary tissue grafted into two PD patients ( Backlund et al., 1985 ) spurred open-label trials of human fetal ventral mesencephalic allografts ( Lindvall et al., 1989 ), which showed some evidence of clinical success and cell survival, based on PET imaging. This was followed by a series of further open-label studies and then two double-blind NIH-funded placebo-controlled studies in the US, which gave conflicting results regarding the extent of improvement, if any, that was seen in transplanted patients. The analysis was also complicated by inclusion of patients with different disease severity, the use of differing amounts of the transplanted tissue, and different levels of immunosuppression ( Barker et al., 2015 ). It was also noted that Lewy body formation was observed in some of the grafts ( Kordower et al., 2008 ; Li et al., 2008 ), indicating that the pathology may spread from host tissue to the graft.
The finding that some PD patients having received fetal grafts showed some long-term improvements ( Kefalopoulou et al., 2014 ) was encouraging, but the use of fetal tissue is ethically problematic, and the supply of tissue is limited. Therefore, in vitro differentiation of ES and iPS cells along the dopaminergic neuron trajectory has been intensely pursued as an alternative source of transplantable cells. Protocols for differentiation of dopaminergic, tyrosine hydroxylase-positive neurons were established by several groups, and the realization that dopaminergic neurons were derived from floor plate cells ( Bonilla et al., 2008 ; Ono et al., 2007 ) led to considerably improved protocols and differentiation efficiency ( Chambers et al., 2009 ; Kriks et al., 2011 ) as well as successful outcomes on transplantation of the resulting cells into animal models ( Kikuchi et al., 2017 ; Kirkeby et al., 2017 ; see Kim et al., 2020 for review).
With the data from fetal and ES/iPS transplantations at hand, clinical translation is now pursued in different projects and consortia, including GForce-PD, an international consortium to advance and harmonize stem cell-derived dopamine cell transplant therapies for PD ( Barker et al., 2015 ). The first PD patients have recently been grafted with iPS-derived neurons ( Schweitzer et al., 2020 ; Takahashi, 2020 ; see also Kim et al., 2020 ; Parmar and Björklund, 2020 for review). Transplantations have recently started or will soon start also at other centers and consortia, including a Cambridge-Lund study (STEM-PD), Kyoto University ( Takahashi, 2020 ), and Sloan Kettering ( Kim et al., 2020 ). It will be interesting to learn how effective stem cell-based therapies will be for PD patients across the various consortia and how such therapies will compare with other therapies for advanced PD, such as deep brain stimulation (DBS) ( Barker et al., 2021 ).
Transplantation of different retinal cell types is an interesting avenue to restore vision for patients with certain eye diseases, such as dry age-related macular degeneration (AMD). AMD is accompanied by progressive loss of retinal pigment epithelial (RPE) cells, which are crucial for survival of the overlying photoreceptor cells. As is the case for PD, fetal transplants have been performed to replace lost cells, paving the way for stem cell-based approaches ( Algvere et al., 1997 ). That study also highlighted the importance of immunosuppression despite the fact that the eye is considered somewhat immunoprivileged. Robust xeno-free, defined, and scalable differentiation protocols for RPE and photoreceptor cells have been developed and have shown promise in animal models ( Osakada et al., 2008 ; Vaajasaari et al., 2011 ; McGill et al., 2017 ; Plaza Reyes et al., 2020 ; Ribeiro et al., 2021 ). The first transplantation of ES cell-derived RPE cells in humans was carried out in 2012 in patients with dry AMD and Stargardt disease, a disease leading to macular degeneration in younger individuals ( Schwartz et al., 2012 ). In a subsequent larger study, there was visual improvement in half of the patients but also some significant side effects, such as cataract and inflammation ( Schwartz et al., 2015 ). The first autologous iPSC-based cell therapy trial for any disease was performed in 2014 aiming to treat neovascular AMD ( Mandai et al., 2017 ) and was later followed by an alternative strategy with banked HLA-matched allogeneic iPSCs to reduce the need of immunosuppression ( Sugita et al., 2020 ). An alternative strategy to minimize immunological rejection has recently been reported through removal of HLA class I and II ( Petrus-Reurer et al., 2020 ). Although transplantation of PSC-derived RPE cells shows promise for potentially halting further progression of disease, restoration of vision will ultimately also require replacement of lost photoreceptors.
In type 1 diabetes, β-cells in the pancreas are lost, and although whole pancreas or islet transplantation provides relief from hypoglycemia, donor tissue is in limited supply ( Krentz et al., 2021 ), making type 1 diabetes a candidate for cell therapy-based approaches. A first-generation protocol for ES or iPS cell differentiation to β-cells initially yielded mixed cellular phenotypes and no glucose-responding cells ( D’Amour et al., 2005 ). Advanced protocols produced human β-cells that turned out to be glucose responding and insulin secreting but only after transplantation and further maturation in mice ( Kroon et al., 2008 ). The company ViaCyte conducted a phase 1/2 clinical trial using these immature endocrine cells held in an encapsulation device, and although the cells were tolerated after transplantation, no evidence of insulin production was reported ( Henry et al., 2018 ). The next step was to create holes in the encapsulation device to allow nutrients and oxygen exchange, and with this modification, now requiring the administration of immunosuppressants, a few of the 15 patients receiving the cells and device have shown evidence of insulin production (stimulated C-peptide levels) ( Ramzy et al., 2021 ). The company Vertex, after having acquired Semma therapeutics, has taken a different approach. By use of stem cell-derived islets that are fully differentiated and mature ( Pagliuca et al., 2014 ), positive results in blood glucose control and therapeutic levels of insulin production have been reported from the first patient transplanted with such cells (VX880) into the liver, again along with immunosuppressants.
Myocardial infarction leads to muscle loss and formation of fibrotic tissue, and there are currently no functional therapies to replace lost or ailing cardiomyocytes. Moreover, the heart appears to be one of the organs lacking a robust endogenous adult stem cell pool ( Senyo et al., 2013 ). For two decades, various types of adult cells have therefore been transplanted to improve post-infarction heart function, but with rather modest success (see Murry and MacLellan, 2020 for review). Hopes have since been pinned instead on cell therapy using in vitro -engineered cardiomyocytes. Initially, it may have been thought that development of such therapies would be rather straightforward, given that ES cells easily could be differentiated into beating cardiomyocytes in the culture dish, an in vitro differentiation paradigm that has been used for disease-modeling of different channelopathies ( Giacomelli et al., 2020 ). The in vitro -differentiated cardiomyocytes are, however, thus far immature and do not exhibit all the properties of a fully differentiated cardiomyocyte ( Karbassi et al., 2020 ). This has hampered clinical testing, but engraftment in animal models provides reason for cautious optimism. Survival and engraftment of cardiomyocytes have been demonstrated in rats and guinea pigs ( Riegler et al., 2015 ; Weinberger et al., 2016 ), and improved cardiac function following transplantation has been demonstrated in experimentally infarcted macaques ( Liu et al., 2018 ) and pigs ( Romagnuolo et al., 2019 ). Furthermore, microvascular grafts have been tested and shown to improve perfusion in infarcted rat hearts ( Redd et al., 2019 ), and patches of iPS-derived cardiomyocytes, smooth muscle cells, and endothelial cells to experimentally infarcted pig hearts resulted in improved ventricular function ( Gao et al., 2018 ). The transplantation of ES/iPS-derived cardiomyocytes to pigs and non-human primates has however also caused arrythmias, presumably as a consequence of the transplanted patch acting as an ectopic pacemaker ( Liu et al., 2018 ; Romagnuolo et al., 2019 ). Although a first attempt to transplant human ES cell-derived cardiomyocytes to a patient has been reported ( Menasché et al., 2015 ), the arrythmias observed in animal experiments currently represent a serious concern, which will require further research to address.
The International Society for Stem Cell Research
Early in the 21 st century, the technology to generate human ES cells was established, and there was a debate in several countries, not least in the US, on how the use of, and generation of, novel, ES cell lines should be regulated. These questions were also discussed among stem cell scientists, but there was no organized international forum for exchanging ideas, showcasing the latest research results, and discussing the ethical implications of stem cells and their use in future therapy development. As a response to this, Leonard Zon started the International Society for Stem Cell Research (ISSCR), with the ambition to promote the science in the field but also to engage in public outreach and communication, advocacy, and policy—a choice that may seem obvious now but was not obvious then.
The overarching mission of ISSCR is the promotion of excellence in stem cell science and in translation of research for the benefit of human health. In 2003, ISSCR organized its first Annual Meeting, which since then has been an integral part of ISSCR’s activities (for a timeline of milestone events in ISSCR, see Figure 2 ). The Annual Meetings have grown in size from around 500 participants during the first years to 3,000–4,000 participants from over 60 countries in recent years (see Figure S1 for a list of the Annual Meetings). The majority of the Annual Meetings have been held in the US but also span the globe, and to widen its geographical footprint and to complement the large-scale Annual Meeting with a more intimate meeting format, ISSCR launched so-called International Symposia (see Figure S1 for a list of all International Symposia). The first International Symposium was held in Shanghai in 2008, and over the years 18 physical and two virtual International Symposia, which accommodate 250–500 participants, have been held in nine countries. In 2015, the Workshop on Clinical Translation, as a part of the Annual Meeting, was established to broaden the interface to the translational and clinical community. Along similar lines, the “Stem Cells Clinical Trials: Practical Advice for Physicians and Ethics/Institutional Review Boards” was published in 2018.

Time axis for ISSCR milestones
In 2006, ISSCR took a bold step by publishing a first set of Guidelines for Stem Cell Research and Clinical Translation (hereafter called the Guidelines). Recognizing the need to lay out principles for how stem cell research should be conducted in an ethically sound way and with high research integrity, ISSCR published the first set of Guidelines in 2006. New versions of the Guidelines appeared in 2008, 2016, and 2021, and the focus of each version reflects where the field of stem cell research stood at the time and what technologies were emerging. The 2006 edition of the Guidelines thus placed a strong focus on human ES cells, whereas later versions have incorporated recommendations, for example, for iPS cell, organoid, blastoid, editing of the human genome, and embryo research and for interspecies chimera research. The Guidelines have been proactive in providing recommendations for emerging fields, for example by including recommendations for mitochondrial replacement techniques (MRT), a technology currently allowed only in the UK, in the 2021 edition. By providing principled recommendations for how research and its application should be conducted, and whether some of the currently widely accepted regulatory frameworks should be updated or altered, the Guidelines has, inevitably in some cases, stirred a debate in the stem cell community. One such example is the suggestion to modify the so-called 14-day limit for research on human embryos, which dates back to the 1980s ( McLaren, 1984 ) and is enshrined in law in more than 10 countries ( Cavaliere, 2017 ). The notion that human embryos could be sustained in vitro for up to 13 days after fertilization ( Deglincerti et al., 2016 ; Shahbazi et al., 2016 ) triggered an interest in revisiting the 14-day limit ( Hyun et al., 2021 ; McCully, 2021 ). The 2021 Guidelines calls for a broader discussion on extending the time limit for embryos in culture beyond 14 days under special circumstances and with appropriate oversight ( Lovell-Badge et al., 2021 ; Master et al., 2021 ). The call to open discussion on the 14-day rule has been challenged by some scientists ( Green et al., 2021 ; Johnston et al., 2021 ). Another area of discussion regards ISSCR’s position on editing of the human germ line. Genomic editing for the germ line is currently prohibited in most countries, and the 2021 Guidelines agrees that clinical application should be prohibited at this time but recommends that research be supported. This has led to a debate, as some hold the view that the germ line should be sacrosanct and spared from genome-editing exercises altogether ( Baylis, 2021 ).
As stem cell research and the marketing of unproven stem cell therapies entered the public’s awareness, ISSCR developed information about stem cell research directly for the general public. In 2008, ISSCR published the “Patient Handbook,” which in 12 languages provides answers to frequently asked questions about stem cell therapy and has since been updated. Two years later, in 2010, another public education initiative was launched, when the “A Closer Look at Stem Cells” Website was introduced, an award-winning initiative to provide easy-to-grasp information about many aspects of stem cell biology and its impact on human health, including fact-based information on stem cell therapies and so-called unproven (a.k.a. “snake oil”) stem cell-based therapies. ISSCR has been very active in terms of advocacy, notably by launching a policy and advocacy program in 2015, and by informing about the risks of unproven therapies. ISSCR members have on several occasions testified on behalf of ISSCR before governing bodies around the globe to support and defend important scientific principles and have provided expert testimony on issues such as ES cell and human fetal research, MRT, and unproven therapies.
In 2013, ISSCR took another major step, when the first issue of the journal Stem Cell Reports was published. Stem Cell Reports , which is published by Cell Press, Elsevier, has established itself as a leading journal in the field, currently publishing more than 200 articles per year and with a journal impact factor of 7.7 (2020). During its first 20 years, ISSCR has furthermore established several awards to recognize important scientific discoveries or other outstanding achievements by stem cell scientists (for a complete list of the ISSCR awards and awardees, see Figure S2 ). The Anne McLaren Memorial Award was established in 2008, followed by the Outstanding Young Investigator Award in 2009 (since 2018 called the Dr. Susan Lim Award for Outstanding Young Investigator), and the McEwen Award for Innovation (since 2018 called the ISSCR Award for Innovation) the Public Service Award and the Ernest McCulloch Memorial Lecture were established in 2010. In 2016, the Tobias Award Lecture was established, followed by the ISSCR Achievement Award and the Momentum Award in 2020 Figure S2 .
ISSCR, like many other societies, was hit by the COVID-19 pandemic, and the 2020 and 2021 Annual Meetings needed to be switched from a physical to a digital meeting format. ISSCR, however, rapidly managed to gain the necessary expertise in arranging virtual meetings. The newly learned skills in digital content creation and meeting organization will also be useful going forward into the post-pandemic era, where a mix of real-life, hybrid, and virtual meetings will likely be the norm for ISSCR and many other societies. The ability to create professional virtual content is also increasingly used by ISSCR to produce courses, workshops, and activities such as ISSCR Digital. This contributes to a more dynamic, “year-round active” society, an improvement over the traditionally strong focus on the Annual Meeting with relatively few other activities spaced out throughout the rest of the year.
Concluding remarks
During its first 20 years, ISSCR has made remarkable progress and established itself as a trusted voice for stem cell research, ethics, policy, and advocacy, and is providing an expanding portfolio of scientific meetings. ISSCR has also helped to call out unproven cell therapies and importantly published guidelines for how to conduct stem cell research with scientific and ethical integrity. With the current pace of progress in stem cell research, it will be interesting to see what new topics will be presented at future ISSCR meetings and addressed in new editions of the Guidelines. I, however, rest assured that ISSCR will be able to handle these tasks in a scholarly and wise manner. I wish ISSCR the best of luck in these future endeavors and a happy 20 th birthday!
Conflicts of interest
U.L. holds a research grant from Merck KGaA but no personal remuneration. U.L. is a member of the Editorial Board of Stem Cell Reports .
Acknowledgments
I thank several past and present members of the ISSCR Executive Board and members of the ISSCR staff for valuable comments on the manuscript text; all errors are my own. I apologize that not all work can be cited because of space limitations. Work in the author’s laboratory is supported by the Swedish Research Council and the Swedish Cancer Society.
Supplemental information can be found online at https://doi.org/10.1016/j.stemcr.2022.04.004 .
Supplemental information
- Algvere P.V., Berglin L., Gouras P., Sheng Y., Kopp E.D. Transplantation of RPE in age-related macular degeneration: observations in disciform lesions and dry RPE atrophy. Graefes Arch. Clin. Exp. Ophthalmol. 1997; 235 :149–158. [ PubMed ] [ Google Scholar ]
- Ali N., Zirak B., Rodriguez R.S., Pauli M.L., Truong H.A., Lai K., Ahn R., Corbin K., Lowe M.M., Scharschmidt T.C., et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell. 2017; 169 :1119–1129.e11. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Altman J. Are new neurons formed in the brains of adult mammals? Science. 1961; 135 :1127–1128. [ PubMed ] [ Google Scholar ]
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J. Comp. Neurol. 1969; 137 :433–457. [ PubMed ] [ Google Scholar ]
- Altman J., Das G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965; 124 :319–335. [ PubMed ] [ Google Scholar ]
- Aragona M., Dekoninck S., Rulands S., Lenglez S., Mascré G., Simons B.D., Blanpain C. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 2017; 8 :14684. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Aragona M., Sifrim A., Malfait M., Song Y., van Herck J., Dekoninck S., Gargouri S., Lapouge G., Swedlund B., Dubois C., et al. Mechanisms of stretch-mediated skin expansion at single-cell resolution. Nature. 2020; 584 :268–273. doi: 10.1038/s41586-020-2555-7. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Babu R.O., Lui V.C.H., Chen Y., Yiu R.S.W., Ye Y., Niu B., Wu Z., Zhang R., Yu M.O.N., Chung P.H.Y., et al. Beta-amyloid deposition around hepatic bile ducts is a novel pathobiological and diagnostic feature of biliary atresia. J. Hepatol. 2020; 73 :1391–1403. [ PubMed ] [ Google Scholar ]
- Bach F.H., Albertini R.J., Joo P., Anderson J.L., Bortin M.M. Bone-marrow transplantation in a patient with the Wiskott-Aldrich syndrome. Lancet. 1968; 2 :1364–1366. [ PubMed ] [ Google Scholar ]
- Backlund E.O., Granberg P.O., Hamberger B., Knutsson E., Mårtensson A., Sedvall G., Seiger Å., Olson L. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J. Neurosurg. 1985; 62 :169–173. [ PubMed ] [ Google Scholar ]
- Banks-Schlegel S., Green H. Formation of epidermis by serially cultivated human epidermal cells transplanted as an epithelium to athymic mice. Transplantation. 1980; 29 :308–313. [ PubMed ] [ Google Scholar ]
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007; 449 :1003–1007. [ PubMed ] [ Google Scholar ]
- Barker R.A., Drouin-Ouellet J., Parmar M. Cell-based therapies for Parkinson disease-past insights and future potential. Nat. Rev. Neurol. 2015; 11 :492–503. [ PubMed ] [ Google Scholar ]
- Barker R.A., Björklund A., Frucht S.J., Svendsen C.N. Stem cell-derived dopamine neurons: will they replace DBS as the leading neurosurgical treatment for Parkinson’s disease? J. Parkinsons Dis. 2021; 11 :909–917. [ PubMed ] [ Google Scholar ]
- Barrandon Y., Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl. Acad. Sci. U S A. 1987; 84 :2302–2306. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Baylis F. ISSCR guidelines fudge heritable human-genome editing. Nature. 2021; 594 :333. [ PubMed ] [ Google Scholar ]
- Becker A.J., Mcculloch E.A., Till J.E. Spleen colonies derived from transplanted mouse marrow cells. Nature. 1963; 197 :452–454. [ PubMed ] [ Google Scholar ]
- Belmadani A., Ren D., Bhattacharyya B.J., Rothwangl K.B., Hope T.J., Perlman H., Miller R.J. Identification of a sustained neurogenic zone at the dorsal surface of the adult mouse hippocampus and its regulation by the chemokine SDF-1. Hippocampus. 2015; 25 :1224–1241. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Beumer J., Clevers H. Cell fate specification and differentiation in the adult mammalian intestine. Nat. Rev. Mol. Cell Biol. 2021; 22 :39–53. [ PubMed ] [ Google Scholar ]
- Björklund A., Dunnett S.B., Stenevi U., Lewis M.E., Iversen S.D. Reinnervation of the denervated striatum by substantia nigra transplants: functional consequences as revealed by pharmacological and sensorimotor testing. Brain Res. 1980; 199 :307–333. [ PubMed ] [ Google Scholar ]
- Blanpain C., Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009; 10 :207–217. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Blanpain C., Lowry W.E., Geoghegan A., Polak L., Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004; 118 :635–648. [ PubMed ] [ Google Scholar ]
- Boldrini M., Fulmore C.A., Tartt A.N., Simeon L.R., Pavlova I., Poposka V., Rosoklija G.B., Stankov A., Arango V., Dwork A.J., et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell. 2018; 22 :589–599. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bonilla S., Hall A.C., Pinto L., Attardo A., Götz M., Huttner W.B., Arenas E. Identification of midbrain floor plate radial glia-like cells as dopaminergic progenitors. Glia. 2008; 56 :809–820. [ PubMed ] [ Google Scholar ]
- Bordignon C., Notarangelo L.D., Nobili N., Ferrari G., Casorati G., Panina P., Mazzolari E., Maggioni D., Rossi C., Servida P., et al. Gene therapy in peripheral blood lymphocytes and bone marrow for ADA- immunodeficient patients. Science. 1995; 270 :470–475. [ PubMed ] [ Google Scholar ]
- Bradley A., Evans M., Kaufman M.H., Robertson E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature. 1984; 309 :255–256. [ PubMed ] [ Google Scholar ]
- Breunig J.J., Haydar T.F., Rakic P. Neural stem cells: historical perspective and future prospects. Neuron. 2011; 70 :614–625. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Briggs R., King T.J. Transplantation of living nuclei from blastula cells into enucleated frogs’ eggs. Proc. Natl. Acad. Sci. U S A. 1952; 38 :455–463. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brinster R.L. The effect of cells transferred into the mouse blastocyst on subsequent development. J. Exp. Med. 1974; 140 :1049–1056. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Briscoe J., Sussel L., Serup P., Hartigan-O’Connor D., Jessell T.M., Rubenstein J.L.R., Ericson J. Homeobox gene Nkx2.2 and specification of neuronal identity by graded Sonic hedgehog signalling. Nature. 1999; 398 :622–627. [ PubMed ] [ Google Scholar ]
- Buczacki S.J.A., Zecchini H.I., Nicholson A.M., Russell R., Vermeulen L., Kemp R., Winton D.J. Intestinal label-retaining cells are secretory precursors expressing lgr5. Nature. 2013; 495 :65–69. [ PubMed ] [ Google Scholar ]
- Calvi L.M., Adams G.B., Weibrecht K.W., Weber J.M., Olson D.P., Knight M.C., Martin R.P., Schipani E., Divieti P., Bringhurst F.R., et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003; 425 :841–846. [ PubMed ] [ Google Scholar ]
- Castellana D., Paus R., Perez-Moreno M. Macrophages contribute to the cyclic activation of adult hair follicle stem cells. PLoS Biol. 2014; 12 :e1002002. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cattaneo E., McKay R. Proliferation and differentiation of neuronal stem cells regulated by nerve growth factor. Nature. 1990; 347 :762–765. [ PubMed ] [ Google Scholar ]
- Cavaliere G. A 14-day limit for bioethics: the debate over human embryo research. BMC Med. Ethics. 2017; 18 :38. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003; 113 :643–655. [ PubMed ] [ Google Scholar ]
- Chambers S.M., Fasano C.A., Papapetrou E.P., Tomishima M., Sadelain M., Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 2009; 27 :275–280. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cheng H., Leblond C.P. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am. J. Anat. 1974; 141 :537–561. [ PubMed ] [ Google Scholar ]
- Cieri N., Maurer K., Wu C.J. 60 years young: the evolving role of allogeneic hematopoietic stem cell transplantation in cancer immunotherapy. Cancer Res. 2021; 81 :4373–4384. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Claudinot S., Nicolas M., Oshima H., Rochat A., Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl. Acad. Sci. U S A. 2005; 102 :14677–14682. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Clayton E., Doupé D.P., Klein A.M., Winton D.J., Simons B.D., Jones P.H. A single type of progenitor cell maintains normal epidermis. Nature. 2007; 446 :185–189. [ PubMed ] [ Google Scholar ]
- Cohen I.G., Adashi E.Y., Gerke S., Palacios-Gonzaacutelez C., Ravitsky V. The regulation of mitochondrial replacement techniques around the world. Annu. Rev. Genomics Hum. Genet. 2020; 21 :565–586. [ PubMed ] [ Google Scholar ]
- Comazzetto S., Shen B., Morrison S.J. Niches that regulate stem cells and hematopoiesis in adult bone marrow. Dev. Cell. 2021; 56 :1848–1860. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cotsarelis G., Sun T.-T., Lavker R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990; 61 :1329–1337. [ PubMed ] [ Google Scholar ]
- D’Amour K.A., Agulnick A.D., Eliazer S., Kelly O.G., Kroon E., Baetge E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005; 23 :1534–1541. [ PubMed ] [ Google Scholar ]
- Dantschakoff W. Untersuchungen über die Entwickelung des Blutes und Bindegewebes bei den Vögeln’ Anat. Hefte. 1908; 37 :471–509. [ Google Scholar ]
- Davis A.A., Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994; 372 :263–266. [ PubMed ] [ Google Scholar ]
- Deglincerti A., Croft G.F., Pietila L.N., Zernicka-Goetz M., Siggia E.D., Brivanlou A.H. Self-organization of the in vitro attached human embryo. Nature. 2016; 533 :251–254. [ PubMed ] [ Google Scholar ]
- Dekkers J.F., Wiegerinck C.L., de Jonge H.R., Bronsveld I., Janssens H.M., de Winter-De Groot K.M., Brandsma A.M., de Jong N.W.M., Bijvelds M.J.C., Scholte B.J., et al. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat. Med. 2013; 19 :939–945. [ PubMed ] [ Google Scholar ]
- Dexter T.M., Allen T.D., Lajtha L.G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J. Cell Physiol. 1977; 91 :335–344. [ PubMed ] [ Google Scholar ]
- Ding L., Morrison S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013; 495 :231–235. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ding L., Saunders T.L., Enikolopov G., Morrison S.J. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012; 481 :457–462. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Doetsch F., Caille I., Lim D.A., Garcı J.M., Alvarez-buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999; 97 :703–716. [ PubMed ] [ Google Scholar ]
- Donati G., Rognoni E., Hiratsuka T., Liakath-Ali K., Hoste E., Kar G., Kayikci M., Russell R., Kretzschmar K., Mulder K.W., et al. Wounding induces dedifferentiation of epidermal Gata6 + cells and acquisition of stem cell properties. Nat. Cell Biol. 2017; 19 :603–613. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M., Wataya T., Nishiyama A., Muguruma K., Sasai Y. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008; 3 :519–532. [ PubMed ] [ Google Scholar ]
- Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T., Sasai Y. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 2011; 472 :51–58. [ PubMed ] [ Google Scholar ]
- Eriksson P.S., Perrfilieva E., BJörk-Eriksson T., Alborn A.-M., Nordborg C., Peterson D.A., Gage F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998; 4 :1313–1317. [ PubMed ] [ Google Scholar ]
- Evans M.J., Kaufman M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981; 292 :154–156. [ PubMed ] [ Google Scholar ]
- Falk S., Han D., Karow M. Cellular identity through the lens of direct lineage reprogramming. Curr. Opin. Genet. Dev. 2021; 70 :97–103. [ PubMed ] [ Google Scholar ]
- Ferrari G., Thrasher A.J., Aiuti A. Gene therapy using haematopoietic stem and progenitor cells. Nat. Rev. Genet. 2021; 22 :216–234. [ PubMed ] [ Google Scholar ]
- Fiore V.F., Krajnc M., Quiroz F.G., Levorse J., Pasolli H.A., Shvartsman S.Y., Fuchs E. Mechanics of a multilayer epithelium instruct tumour architecture and function. Nature. 2020; 585 :433–439. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ford C.E., Hamerton J.L., Barnes D.W.H., Loutit J.F. Cytological identification of radiation-chimæras. Nature. 1956; 177 :452–454. [ PubMed ] [ Google Scholar ]
- Foudi A., Hochedlinger K., van Buren D., Schindler J.W., Jaenisch R., Carey V., Hock H. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat. Biotechnol. 2009; 27 :84–90. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Franjic D., Skarica M., Ma S., Arellano J.I., Tebbenkamp A.T.N., Choi J., Xu C., Li Q., Morozov Y.M., Andrijevic D., et al. Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells. Neuron. 2022; 110 :452–469.e14. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Freed W.J., Morihisa J.M., Spoor E., Hoffer B.J., Olson L., Seiger A., Wyatt R.J. Transplanted adrenal chromaffin cells in rat brain reduce lesion-induced rotational behaviour. Nature. 1981; 292 :351–352. [ PubMed ] [ Google Scholar ]
- Fuchs E., Blau H.M. Tissue stem cells: architects of their niches. Cell Stem Cell. 2020; 27 :532–556. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fuchs E., Green H. The expression of keratin genes in epidermis and cultured epidermal cells. Cell. 1978; 15 :887–897. [ PubMed ] [ Google Scholar ]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980; 19 :1033–1042. [ PubMed ] [ Google Scholar ]
- Gao L., Gregorich Z.R., Zhu W., Mattapally S., Oduk Y., Lou X., Kannappan R., Borovjagin A.v., Walcott G.P., Pollard A.E., et al. Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation. 2018; 137 :1712–1730. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gatti R.A., Meuwissen H.J., Allen H.D., Hong R., Good R.A. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968; 2 :1366–1369. [ PubMed ] [ Google Scholar ]
- Ge Y., Gomez N.C., Adam R.C., Nikolova M., Yang H., Verma A., Lu C.P.J., Polak L., Yuan S., Elemento O., et al. Stem cell lineage infidelity drives wound repair and cancer. Cell. 2017; 169 :636–650.e14. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Genovese P., Schiroli G., Escobar G., di Tomaso T., Firrito C., Calabria A., Moi D., Mazzieri R., Bonini C., Holmes M.C., et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature. 2014; 510 :235–240. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Geurts M.H., van der Vaart J., Beumer J., Clevers H. The organoid platform: promises and challenges as tools in the fight against COVID-19. Stem Cell Rep. 2021; 16 :412–418. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Giacomelli E., Meraviglia V., Campostrini G., Cochrane A., Cao X., van Helden R.W.J., Krotenberg Garcia A., Mircea M., Kostidis S., Davis R.P., et al. Human-iPSC-Derived cardiac stromal cells enhance maturation in 3D cardiac microtissues and reveal non-cardiomyocyte contributions to heart disease. Cell Stem Cell. 2020; 26 :862–879.e11. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gola A., Fuchs E. Environmental control of lineage plasticity and stem cell memory. Curr. Opin. Cell Biol. 2021; 69 :88–95. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Goldman S.A., Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc. Natl. Acad. Sci. U S A. 1983; 80 :2390–2394. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gomez C., Chua W., Miremadi A., Quist S., Headon D.J., Watt F.M. The interfollicular epidermis of adult mouse tail comprises two distinct cell lineages that are differentially regulated by Wnt, Edaradd, and Lrig1. Stem Cell Rep. 2013; 1 :19–27. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Green H., Kehinde O., Thomas J. Growth of cultured human epidermal cells into multiple epithelia suitable for grafting. Proc. Natl. Acad. Sci. U S A. 1979; 76 :5665–5668. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Green R.M., West M.D., Hayflick L. Don’t abandon 14-day limit on embryo research. Nature. 2021; 594 :333. [ PubMed ] [ Google Scholar ]
- Gur-Cohen S., Yang H., Baksh S.C., Miao Y., Levorse J., Kataru R.P., Liu X., de la Cruz-Racelis J., Mehrara B.J., Fuchs E. Stem cell–driven lymphatic remodeling coordinates tissue regeneration. Science. 2019; 366 :1218–1225. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gurdon J. The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J. Embryol. Exp. Morphol. 1962; 10 :622–640. [ PubMed ] [ Google Scholar ]
- Haeckel E. Leipzig, W. Engelmann; 1877. Anthropogenie oder Entwickelungsgeschichte des Menschen. [ Google Scholar ]
- Haecker V. Die Kerntheilungsvorgänge bei der Mesoderm- und Entodermbildung von Cyclops. Arch. Mikrosk. Anat. 1892; 39 :556–581. [ Google Scholar ]
- Henry R.R., Pettus J., Wilenxky J., Shapiro A.M.J., Senior P.A., Roep B., Wang R., Kroon E.J., Scott M., D’Amour K., et al. Initial clinical evaluation of VC-01TM combination product—a stem cell–derived islet replacement for type 1 diabetes (T1D) Diabetes. 2018; 67 :138-OR. [ Google Scholar ]
- Hérault A., Binnewies M., Leong S., Calero-Nieto F.J., Zhang S.Y., Kang Y.A., Wang X., Pietras E.M., Chu S.H., Barry-Holson K., et al. Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. Nature. 2017; 544 :53–58. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hirsch T., Rothoeft T., Teig N., Bauer J.W., Pellegrini G., de Rosa L., Scaglione D., Reichelt J., Klausegger A., Kneisz D., et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017; 551 :327–332. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- His W. Engelmann; 1874. Unsere Körperform und das Physiologische Problem ihrer Entstehung. Briefe an einen Befreundeten Naturforscher. [ Google Scholar ]
- Howe S.J., Mansour M.R., Schwarzwaelder K., Bartholomae C., Hubank M., Kempski H., Brugman M.H., Pike-Overzet K., Chatters S.J., de Ridder D., et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Invest. 2008; 118 :3143–3150. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hsu Y.C., Pasolli H.A., Fuchs E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell. 2011; 144 :92–105. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hsu Y.C., Li L., Fuchs E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell. 2014; 157 :935–949. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Huch M., Dorrell C., Boj S.F., van Es J.H., Li V.S.W., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M.J., et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 2013; 494 :247–250. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Huch M., Gehart H., Boxtel R., van Hamer K., Blokzijl F., Verstegen M.M., Ellis E., Wenum M.V., Fuchs S.A., Ligt J.D., et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell. 2015; 160 :299–312. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hyun I., Bredenoord A.L., Briscoe J., Klipstein S., Tan T. Human embryo research beyond the primitive streak. Science. 2021; 371 :998–1000. [ PubMed ] [ Google Scholar ]
- Jadhav U., Saxena M., O’Neill N.K., Saadatpour A., Yuan G.C., Herbert Z., Murata K., Shivdasani R.A. Dynamic reorganization of chromatin accessibility signatures during dedifferentiation of secretory precursors into Lgr5+ intestinal stem cells. Cell Stem Cell. 2017; 21 :65–77. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jensen K.B., Collins C.A., Nascimento E., Tan D.W., Frye M., Itami S., Watt F.M. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009; 4 :427–439. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Johansson C.B., Momma S., Clarke D.L., Risling M., Lendahl U., Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999; 96 :25–34. [ PubMed ] [ Google Scholar ]
- Johnston J., Baylis F., Greele H.T. ISSCR: grave omission of age limit for embryo research. Nature. 2021; 594 :495. [ PubMed ] [ Google Scholar ]
- Jones P.H., Watt F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993; 73 :713–724. [ PubMed ] [ Google Scholar ]
- Kadoshima T., Sakaguchi H., Nakano T., Soen M., Ando S., Eiraku M., Sasai Y. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl. Acad. Sci. U S A. 2014; 110 :20284–20289. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kagawa H., Javali A., Khoei H.H., Sommer T.M., Sestini G., Novatchkova M., Scholte op Reimer Y., Castel G., Bruneau A., Maenhoudt N., et al. Human blastoids model blastocyst development and implantation. Nature. 2022; 601 :600–605. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Karbassi E., Fenix A., Marchiano S., Muraoka N., Nakamura K., Yang X., Murry C.E. Cardiomyocyte maturation: advances in knowledge and implications for regenerative medicine. Nat. Rev. Cardiol. 2020; 17 :341–359. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kastner C., Hendricks A., Deinlein H., Hankir M., Germer C.T., Schmidt S., Wiegering A. Organoid models for cancer research—from bed to bench side and back. Cancers. 2021; 13 :4812. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Katayama Y., Battista M., Kao W.M., Hidalgo A., Peired A.J., Thomas S.A., Frenette P.S. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006; 124 :407–421. [ PubMed ] [ Google Scholar ]
- Kefalopoulou Z., Politis M., Piccini P., Mencacci N., Bhatia K., Jahanshahi M., Widner H., Rehncrona S., Brundin P., Björklund A., et al. Long-term clinical outcome of fetal cell transplantation for Parkinson disease: two case reports. JAMA Neurol. 2014; 71 :83–87. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kempermann G., Gage F.H., Aigner L., Song H., Curtis M.A., Thuret S., Kuhn H.G., Jessberger S., Frankland P.W., Cameron H.A., et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell. 2018; 23 :25–30. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kiel M.J., Yilmaz Ö.H., Iwashita T., Yilmaz O.H., Terhorst C., Morrison S.J. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005; 121 :1109–1121. [ PubMed ] [ Google Scholar ]
- Kiel M.J., Radice G.L., Morrison S.J. Lack of evidence that hematopoietic stem cells depend on N-Cadherin-Mediated adhesion to osteoblasts for their maintenance. Cell Stem Cell. 2007; 1 :204–217. [ PubMed ] [ Google Scholar ]
- Kikuchi T., Morizane A., Doi D., Magotani H., Onoe H., Hayashi T., Mizuma H., Takara S., Takahashi R., Inoue H., et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 2017; 548 :592–596. [ PubMed ] [ Google Scholar ]
- Kim T.W., Koo S.Y., Studer L. Pluripotent stem cell therapies for Parkinson disease: present challenges and future opportunities. Front. Cell Dev. Biol. 2020; 8 :729. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kirkeby A., Nolbrant S., Tiklova K., Heuer A., Kee N., Cardoso T., Ottosson D.R., Lelos M.J., Rifes P., Dunnett S.B., et al. Predictive markers guide differentiation to improve graft outcome in clinical translation of hESC-based therapy for Parkinson’s disease. Cell Stem Cell. 2017; 20 :135–148. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kleinsmith L.J., Pierce G.B. Multipotentiality of single embryonal carcinoma cells. Cancer Res. 1964; 24 :1544–1551. [ PubMed ] [ Google Scholar ]
- Kordower J.H., Chu Y., Hauser R.A., Freeman T.B., Olanow C.W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008; 14 :504–506. [ PubMed ] [ Google Scholar ]
- Krentz N.A.J., Shea L.D., Huising M.O., Shaw J.A.M. Restoring normal islet mass and function in type 1 diabetes through regenerative medicine and tissue engineering. Lancet Diabetes Endocrinol. 2021; 9 :708–724. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kriks S., Shim J.W., Piao J., Ganat Y.M., Wakeman D.R., Xie Z., Carrillo-Reid L., Auyeung G., Antonacci C., Buch A., et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011; 480 :547–551. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kroon E., Martinson L.A., Kadoya K., Bang A.G., Kelly O.G., Eliazer S., Young H., Richardson M., Smart N.G., Cunningham J., et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008; 26 :443–452. [ PubMed ] [ Google Scholar ]
- Lancaster M.A., Knoblich J.A. Organogenesisin a dish: modeling development and disease using organoid technologies. Science. 2014; 345 :283–292. [ PubMed ] [ Google Scholar ]
- Lancaster M.A., Renner M., Martin C.A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., Knoblich J.A. Cerebral organoids model human brain development and microcephaly. Nature. 2013; 501 :373–379. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lassar A.B., Paterson B.M., Weintraub H. Transfection of a DNA locus that mediates the conversion of 10T12 fibroblasts to myoblasts. Cell. 1986; 47 :649–656. [ PubMed ] [ Google Scholar ]
- Lechler T., Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005; 437 :275–280. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Li J.Y., Englund E., Holton J.L., Soulet D., Hagell P., Lees A.J., Lashley T., Quinn N.P., Rehncrona S., Björklund A., et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008; 14 :501–503. [ PubMed ] [ Google Scholar ]
- Li R., Zhong C., Izpisua Belmonte J.C. Time matters: human blastoids resemble the sequence of blastocyst development. Cell. 2022; 185 :581–584. [ PubMed ] [ Google Scholar ]
- Liem K.F., Tremml G., Jessell T.M. A role for the roof plate and its resident TGFβ-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997; 91 :127–138. [ PubMed ] [ Google Scholar ]
- Lindvall O., Rehncrona S., Brundin P., Gustavii B., Åstedt B., Widner H., Lindholm T., Björklund A., Leenders K.L., Rothwell J.C., et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. Arch. Neurol. 1989; 46 :615–631. [ PubMed ] [ Google Scholar ]
- Liu Y.W., Chen B., Yang X., Fugate J.A., Kalucki F.A., Futakuchi-Tsuchida A., Couture L., Vogel K.W., Astley C.A., Baldessari A., et al. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat. Biotechnol. 2018; 36 :597–605. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lopez-Garcia C., Klein A.M., Simons B.D., Winton D.J. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010; 330 :822–825. [ PubMed ] [ Google Scholar ]
- Lovell-Badge R., Anthony E., Barker R.A., Bubela T., Brivanlou A.H., Carpenter M., Charo R.A., Clark A., Clayton E., Cong Y., et al. ISSCR guidelines for stem cell research and clinical translation: the 2021 update. Stem Cell Rep. 2021; 16 :1398–1408. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lown R.N., Philippe J., Navarro W., van Walraven S.M., Philips-Johnson L., Fechter M., Pawson R., Bengtsson M., Beksac M., Field S., et al. Unrelated adult stem cell donor medical suitability: recommendations from the world marrow donor association clinical working group committee. Bone Marrow Transplant. 2014; 49 :880–886. [ PubMed ] [ Google Scholar ]
- Maehle A.-H. Ambiguous cells: the emergence of the stem cell concept in the nineteenth and twentieth centuries. Notes Rec. R. Soc. Lond. 2011; 65 :359–378. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Malatesta P., Hartfuss E., Götz M. Isolation of radial glial cells by fluorescent-activated cell sorting reveals a neuronal lineage. Development. 2000; 127 :5253–5263. [ PubMed ] [ Google Scholar ]
- Mandai M., Kurimoto Y., Takahashi M. Autologous induced stem-cell–derived retinal cells for macular degeneration. N. Engl. J. Med. 2017; 377 :792–793. [ PubMed ] [ Google Scholar ]
- Martin G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. U S A. 1981; 78 :7634–7638. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mascré G., Dekoninck S., Drogat B., Youssef K.K., Brohée S., Sotiropoulou P.A., Simons B.D., Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012; 489 :257–262. [ PubMed ] [ Google Scholar ]
- Master Z., Lovell-Badge R., Knoppers B. ISSCR upholds right to science. Nature. 2021; 595 :494. [ PubMed ] [ Google Scholar ]
- Mathé G., Amiel J.L., Schwarzenberg L., Cattan A., Schneider M., Devries M.J., Tubiana M., Lalanne C., Binet J.L., Papiernik M., et al. Successful allogenic bone marrow transplantation in man: chimerism, induced specific tolerance and possible anti-leukemic effects. Blood. 1965; 25 :179–196. [ PubMed ] [ Google Scholar ]
- Maximow A. Der Lymphozyt als gemeinsame Stammzelle der verschiedenen Blutelemente in der embryonalen Entwicklung und im postfetalen Leben der Säugetiere. Fol. Hematol. 1909; 8 :125–134. [ Google Scholar ]
- McCarthy N., Kraiczy J., Shivdasani R.A. Cellular and molecular architecture of the intestinal stem cell niche. Nat. Cell Biol. 2020; 22 :1033–1041. [ PubMed ] [ Google Scholar ]
- McCully S. The time has come to extend the 14-day limit. J. Med. Ethics. 2021; 47 :e66. [ PubMed ] [ Google Scholar ]
- McGill T.J., Bohana-Kashtan O., Stoddard J.W., Andrews M.D., Pandit N., Rosenberg-Belmaker L.R., Wiser O., Matzrafi L., Banin E., Reubinoff B., et al. Matzrafi, L., Banin, E., Reubinoff, B., et al. (2017). Long-term efficacy of GMP grade xeno-free hESC-derived RPE cells following transplantation. Transl. Vis. Sci. Technol. 2017; 6 :17. doi: 10.1167/tvst.6.3.17. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- McLaren A. Where to draw the line? Proc. R. Inst. 1984; 56 :101–121. [ Google Scholar ]
- Menasché P., Vanneaux V., Hagège A., Bel A., Cholley B., Cacciapuoti I., Parouchev A., Benhamouda N., Tachdjian G., Tosca L., et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur. Heart J. 2015; 36 :2011–2017. [ PubMed ] [ Google Scholar ]
- Mirzadeh Z., Merkle F.T., Soriano-Navarro M., Garcia-Verdugo J.M., Alvarez-Buylla A. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008; 3 :265–278. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Morrison S.J., Scadden D.T. The bone marrow niche for haematopoietic stem cells. Nature. 2014; 505 :327–334. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Morrison S.J., Weissman I.L. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994; 1 :661–673. [ PubMed ] [ Google Scholar ]
- Murry C.E., MacLellan W.R. Stem cells and the heart - the road ahead. Science. 2020; 367 :854–855. [ PubMed ] [ Google Scholar ]
- Naik S., Larsen S.B., Gomez N.C., Alaverdyan K., Sendoel A., Yuan S., Polak L., Kulukian A., Chai S., Fuchs E. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature. 2017; 550 :475–480. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Navarro Negredo P., Yeo R.W., Brunet A. Aging and rejuvenation of neural stem cells and their niches. Cell Stem Cell. 2020; 27 :202–223. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Neumann E. Über die bedeutung de knochenmarkers für die blutbildung. Zentralbl. Med. Wissensch. 1868; 6 :689. [ Google Scholar ]
- Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., Schöler H., Smith A. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998; 95 :379–391. [ PubMed ] [ Google Scholar ]
- Noctor S.C., Flint A.C., Weissman T.A., Dammerman R.S., Kriegstein A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001; 409 :714–720. [ PubMed ] [ Google Scholar ]
- O’Connor N.E., Mulliken J.B., Banks-Schlegel S., Kehinde O., Green H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981; 317 :75–78. [ PubMed ] [ Google Scholar ]
- Oguro H., Ding L., Morrison S.J. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013; 13 :102–116. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Olson L., Seiger Å. Development and growth of immature monoamine neurons in rat and man in situ and following intraocular transplantation in the rat. Brain Res. 1973; 62 :353–360. [ PubMed ] [ Google Scholar ]
- Ono Y., Nakatani T., Sakamoto Y., Mizuhara E., Minaki Y., Kumai M., Hamaguchi A., Nishimura M., Inoue Y., Hayashi H., et al. Differences in neurogenic potential in floor plate cells along an anteroposterior location: midbrain dopaminergic neurons originate from mesencephalic floor plate cells. Development. 2007; 134 :3213–3225. [ PubMed ] [ Google Scholar ]
- Osakada F., Ikeda H., Mandai M., Wataya T., Watanabe K., Yoshimura N., Akaike A., Sasai Y., Takahashi M. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat. Biotechnol. 2008; 26 :215–224. [ PubMed ] [ Google Scholar ]
- Page M.E., Lombard P., Ng F., Göttgens B., Jensen K.B. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013; 13 :471–482. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pagliuca F.W., Millman J.R., Gürtler M., Segel M., van Dervort A., Ryu J.H., Peterson Q.P., Greiner D., Melton D.A. Generation of functional human pancreatic β cells in vitro. Cell. 2014; 159 :428–439. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pappenheim A. Über entwickelung and ausbildung der erythroblasten. Virchows Arch. A Pathol. Anat. 1896; 145 :587–643. [ Google Scholar ]
- Paredes M.F., Sorrells S.F., Cebrian-Silla A., Sandoval K., Qi D., Kelley K.W., James D., Mayer S., Chang J., Auguste K.I., et al. Does adult neurogenesis persist in the human Hippocampus? Cell Stem Cell. 2018; 23 :780–781. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Park S., Gonzalez D.G., Guirao B., Boucher J.D., Cockburn K., Marsh E.D., Mesa K.R., Brown S., Rompolas P., Haberman A.M., et al. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat. Cell Biol. 2017; 19 :155–163. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Parmar M., Björklund A. From skin to brain: a Parkinson’s disease patient transplanted with his own cells. Cell Stem Cell. 2020; 27 :8–10. [ PubMed ] [ Google Scholar ]
- Perlow M.J., Freed W.J., Hoffer B.J., Seiger A., Olson L., Wyatt R.J. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979; 204 :643–647. [ PubMed ] [ Google Scholar ]
- Petrus-Reurer S., Winblad N., Kumar P., Gorchs L., Chrobok M., Wagner A.K., Bartuma H., Lardner E., Aronsson M., Plaza Reyes Á., et al. Generation of retinal pigment epithelial cells derived from human embryonic stem cells lacking human leukocyte antigen class I and II. Stem Cell Rep. 2020; 14 :648–662. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Plaza Reyes A., Petrus-Reurer S., Padrell Sánchez S., Kumar P., Douagi I., Bartuma H., Aronsson M., Westman S., Lardner E., André H., et al. Identification of cell surface markers and establishment of monolayer differentiation to retinal pigment epithelial cells. Nat. Commun. 2020; 11 :1609. doi: 10.1038/s41467-020-15326-5. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Potten C.S., Owen G., Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 2002; 115 :2381–2388. [ PubMed ] [ Google Scholar ]
- Price J., Thurlow L. Cell lineage in the rat cerebral cortex: a study using retroviral-mediated gene transfer. Development. 1988; 104 :473–482. [ PubMed ] [ Google Scholar ]
- Qian X., Nguyen H.N., Song M.M., Hadiono C., Ogden S.C., Hammack C., Yao B., Hamersky G.R., Jacob F., Zhong C., et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell. 2016; 165 :1238–1254. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Radakovits R., Barros C.S., Belvindrah R., Patton B., Müller U. Regulation of radial glial survival by signals from the meninges. J. Neurosci. 2009; 29 :7694–7705. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ramalho-Santos M., Willenbring H. On the origin of the term “stem cell” Cell Stem Cell. 2007; 1 :35–38. [ PubMed ] [ Google Scholar ]
- Ramzy A., Thompson D.M., Ward-Hartstonge K.A., Ivison S., Cook L., Garcia R.v., Loyal J., Kim P.T.W., Warnock G.L., Levings M.K., et al. Implanted pluripotent stem-cell-derived pancreatic endoderm cells secrete glucose-responsive C-peptide in patients with type 1 diabetes. Cell Stem Cell. 2021; 28 :2047–2061.e5. [ PubMed ] [ Google Scholar ]
- Redd M.A., Zeinstra N., Qin W., Wei W., Martinson A., Wang Y., Wang R.K., Murry C.E., Zheng Y. Patterned human microvascular grafts enable rapid vascularization and increase perfusion in infarcted rat hearts. Nat. Commun. 2019; 10 :584. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rheinwald J.G., Green H. Formation of a keratinizing epithelium in culture by a cloned cell line derived from a teratoma. Cell. 1975; 6 :317–330. [ PubMed ] [ Google Scholar ]
- Ribeiro J., Procyk C.A., West E.L., Pearson R.A., Gonzalez-cordero A., Ali R.R., Ribeiro J., Procyk C.A., West E.L., Hara-wright M.O., et al. Restoration of visual function in advanced disease after transplantation of purified human pluripotent stem cell-derived cone photoreceptors. Cell Rep. 2021; 35 :109022. doi: 10.1016/j.celrep.2021.109022. [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Rideout W.M., Hochedlinger K., Kyba M., Daley G.Q., Jaenisch R. Correction of a genetic defect by nuclear transplantation and combined cell and gene therapy. Cell. 2002; 109 :17–27. [ PubMed ] [ Google Scholar ]
- Riegler J., Tiburcy M., Ebert A., Tzatzalos E., Raaz U., Abilez O.J., Shen Q., Kooreman N.G., Neofytou E., Chen V.C., et al. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circ. Res. 2015; 117 :720–730. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rivron N.C., Frias-Aldeguer J., Vrij E.J., Boisset J.C., Korving J., Vivié J., Truckenmüller R.K., van Oudenaarden A., van Blitterswijk C.A., Geijsen N. Blastocyst-like structures generated solely from stem cells. Nature. 2018; 557 :106–111. [ PubMed ] [ Google Scholar ]
- Rognoni E., Watt F.M. Skin cell heterogeneity in development, wound healing, and cancer. Trends Cell Biol. 2018; 28 :709–722. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Romagnuolo R., Masoudpour H., Porta-Sánchez A., Qiang B., Barry J., Laskary A., Qi X., Massé S., Magtibay K., Kawajiri H., et al. Human embryonic stem cell-derived cardiomyocytes regenerate the infarcted pig heart but induce ventricular tachyarrhythmias. Stem Cell Rep. 2019; 12 :967–981. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009; 459 :262–265. [ PubMed ] [ Google Scholar ]
- Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011; 469 :415–418. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schepers A.G., Vries R., van den Born M., van de Wetering M., Clevers H. Lgr5 intestinal stem cells have high telomerase activity and randomly segregate their chromosomes. EMBO J. 2011; 30 :1104–1109. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978; 4 :7–25. [ PubMed ] [ Google Scholar ]
- Schwartz S.D., Hubschman J.P., Heilwell G., Franco-Cardenas V., Pan C.K., Ostrick R.M., Mickunas E., Gay R., Klimanskaya I., Lanza R. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012; 379 :713–720. [ PubMed ] [ Google Scholar ]
- Schwartz S.D., Regillo C.D., Lam B.L., Eliott D., Rosenfeld P.J., Gregori N.Z., Hubschman J.-P., Davis J.L., Heilwell G., Spirn M., et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 2015; 385 :509–516. [ PubMed ] [ Google Scholar ]
- Schweitzer J.S., Song B., Herrington T.M., Park T.-Y., Lee N., Ko S., Jeon J., Cha Y., Kim K., Li Q., et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson’s disease. N. Engl. J. Med. 2020; 382 :1926–1932. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Segel M., Neumann B., Hill M.F.E., Weber I.P., Viscomi C., Zhao C., Young A., Agley C.C., Thompson A.J., Gonzalez G.A., et al. Niche stiffness underlies the ageing of central nervous system progenitor cells. Nature. 2019; 573 :130–134. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Senyo S.E., Steinhauser M.L., Pizzimenti C.L., Yang V.K., Cai L., Wang M., Wu T.D., Guerquin-Kern J.L., Lechene C.P., Lee R.T. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013; 493 :433–436. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Severe N., Karabacak N.M., Gustafsson K., Baryawno N., Courties G., Kfoury Y., Kokkaliaris K.D., Rhee C., Lee D., Scadden E.W., et al. Stress-induced changes in bone marrow stromal cell populations revealed through single-cell protein expression mapping. Cell Stem Cell. 2019; 25 :570–583. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shahbazi M.N., Jedrusik A., Vuoristo S., Recher G., Hupalowska A., Bolton V., Fogarty N.M.E., Campbell A., Devito L.G., Ilic D., et al. Self-organization of the human embryo in the absence of maternal tissues. Nat. Cell Biol. 2016; 18 :700–708. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shamblott M.J., Axelman J., Wang S., Bugg E.M., Littlefield J.W., Donovan P.J., Blumenthal P.D., Huggins G.R., Gearhart J.D. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl. Acad. Sci. U S A. 1998; 95 :13726–13731. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shen Q., Wang Y., Kokovay E., Lin G., Chuang S.M., Goderie S.K., Roysam B., Temple S. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 2008; 3 :289–300. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shwartz Y., Gonzalez-Celeiro M., Chen C.L., Pasolli H.A., Sheu S.H., Fan S.M.Y., Shamsi F., Assaad S., Lin E.T.Y., Zhang B., et al. Cell types promoting goosebumps form a niche to regulate hair follicle stem cells. Cell. 2020; 182 :578–593. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Siegenthaler J.A., Ashique A.M., Zarbalis K., Patterson K.P., Hecht J.H., Kane M.A., Folias A.E., Choe Y., May S.R., Kume T., et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009; 139 :597–609. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Silva-Vargas V., Maldonado-Soto A.R., Mizrak D., Codega P., Doetsch F. Age-dependent niche signals from the choroid plexus regulate adult neural stem cells. Cell Stem Cell. 2016; 19 :643–652. [ PubMed ] [ Google Scholar ]
- Smart I.H.M. Variation in the plane of cell cleavage during the process of stratification in the mouse epidermis. Br. J. Dermatol. 1970; 82 :276–282. [ PubMed ] [ Google Scholar ]
- Smart I., Leblond C.P. Evidence for division and trans- formations of neuroglia cells in the mouse brain, as derived from radioautography after injection of thymidine-H3. J. Comp. Neurol. 1961; 116 :349–367. [ Google Scholar ]
- Snippert H.J., van der Flier L.G., Sato T., van Es J.H., van den Born M., Kroon-Veenboer C., Barker N., Klein A.M., van Rheenen J., Simons B.D., et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010; 143 :134–144. [ PubMed ] [ Google Scholar ]
- Soldner F., Jaenisch R. Stem cells, genome editing, and the path to translational medicine. Cell. 2018; 175 :615–632. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Solter D. From teratocarcinomas to embryonic stem cells and beyond: a history of embryonic stem cell research. Nat. Rev. Genet. 2006; 7 :319–327. [ PubMed ] [ Google Scholar ]
- Sorrells S.F., Paredes M.F., Cebrian-Silla A., Sandoval K., Qi D., Kelley K.W., James D., Mayer S., Chang J., Auguste K.I., et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature. 2018; 555 :377–381. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Spalding K.L., Bhardwaj R.D., Buchholz B.A., Druid H., Frisén J. Retrospective birth dating of cells in humans. Cell. 2005; 122 :133–143. [ PubMed ] [ Google Scholar ]
- Spalding K.L., Bergmann O., Alkass K., Bernard S., Salehpour M., Huttner H.B., Boström E., Westerlund I., Vial C., Buchholz B.A., et al. Dynamics of hippocampal neurogenesis in adult humans. Cell. 2013; 153 :1219–1227. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Spangrude G.J., Heimfeld S., Weissman I.L. Purification and characterization of mouse hematopoietic stem cells. Science. 1988; 241 :58–62. [ PubMed ] [ Google Scholar ]
- Spemann H., Mangold H. Über induktion von embryonalanlagen durch implantation artfremder organisatoren. Arch. Mikrosk. Anat. Entwicklungsmech. 1924; 100 :599–638. [ Google Scholar ]
- Steptoe P.C., Edwards R.G., Purdy J.M. Human blastocysts grown in culture. Nature. 1971; 229 :132–133. [ PubMed ] [ Google Scholar ]
- Stevens L.C., Little C.C. Spontaneous testicular teratomas in an inbred strain of mice. Proc. Natl. Acad. Sci. U S A. 1954; 40 :1080–1087. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sugita S., Mandai M., Hirami Y., Takagi S., Maeda T., Fujihara M., Matsuzaki M., Yamamoto M., Iseki K., Hayashi N., et al. HLA-matched allogeneic IPS cells-derived rpe transplantation for macular degeneration. J. Clin. Med. 2020; 9 :2217. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sugiyama T., Kohara H., Noda M., Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006; 25 :977–988. [ PubMed ] [ Google Scholar ]
- Sun G.J., Zhou Y., Ito S., Bonaguidi M.A., Stein-O’brien G., Kawasaki N.K., Modak N., Zhu Y., Ming G.L., Song H. Latent tri-lineage potential of adult hippocampal neural stem cells revealed by Nf1 inactivation. Nat. Neurosci. 2015; 18 :1722–1724. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Takagi Y. History of neural stem cell research and its clinical application. Neurol. Med. Chir. 2016; 56 :110–124. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Takahashi J. iPS cell-based therapy for Parkinson’s disease: a Kyoto trial. Regen. Ther. 2020; 13 :18–22. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126 :663–676. [ PubMed ] [ Google Scholar ]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131 :861–872. [ PubMed ] [ Google Scholar ]
- Tavazoie M., van der Veken L., Silva-Vargas V., Louissaint M., Colonna L., Zaidi B., Garcia-Verdugo J.M., Doetsch F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 2008; 3 :279–288. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Tetteh P.W., Basak O., Farin H.F., Wiebrands K., Kretzschmar K., Begthel H., van den Born M., Korving J., de Sauvage F., van Es J.H., et al. Replacement of lost lgr5-positive stem cells through plasticity of their enterocyte-lineage daughters. Cell Stem Cell. 2016; 18 :203–213. [ PubMed ] [ Google Scholar ]
- Thomas E.D., Lochte H.L., Jr., Lu W.C., Ferrebee J.W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 1957; 257 :951–957. [ PubMed ] [ Google Scholar ]
- Thomson J.A., Kalishman J., Golos T.G., Durning M., Harris C.P., Becker R.A., Hearn J.P. Isolation of a primate embryonic stem cell line. Proc. Natl. Acad. Sci. U S A. 1995; 92 :7844–7848. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., Jones J.M. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282 :1145–1147. [ PubMed ] [ Google Scholar ]
- Till J.E., McCulloch E.A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 1961; 14 :213–222. [ PubMed ] [ Google Scholar ]
- Tumbar T., Guasch G., Greco V., Blanpain C., Lowry W.E., Rendl M., Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004; 303 :359–363. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Turner D.L., Cepko C.C. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987; 328 :131–136. [ PubMed ] [ Google Scholar ]
- Ungerstedt U. 6-Hydroxy-Dopamine induced degeneration of central monoamine neurons. Eur. J. Pharmacol. 1968; 5 :107–110. [ PubMed ] [ Google Scholar ]
- Vaajasaari H., Ilmarinen T., Juuti-Uusitalo K., Rajala K., Onnela N., Narkilahti S., Suuronen R., Hyttinen J., Uusitalo H., Skottman H. Toward the defined and xeno-free differentiation of functional human pluripotent stem cell-derived retinal pigment epithelial cells. Mol. Vis. 2011; 17 :558–575. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- van Es J.H., Sato T., van de Wetering M., Lyubimova A., Yee Nee A.N., Gregorieff A., Sasaki N., Zeinstra L., van den Born M., Korving J., et al. Dll1 + secretory progenitor cells revert to stem cells upon crypt damage. Nat. Cell Biol. 2012; 14 :1099–1104. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- van Es J.H., Wiebrands K., López-Iglesias C., van de Wetering M., Zeinstra L., van den Born M., Korving J., Sasaki N., Peters P.J., van Oudenaarden A., et al. Enteroendocrine and tuft cells support Lgr5 stem cells on Paneth cell depletion. Proc. Natl. Acad. Sci. U S A. 2019; 116 :26599–26605. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Vierbuchen T., Ostermeier A., Pang Z.P., Kokubu Y., Südhof T.C., Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010; 463 :1035–1041. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Virchow R.L.C. Hirschwald; 1858. Die Cellularpathologie in Ihrer Begründung auf Physiologische und Pathologische Gewebelehre. [ Google Scholar ]
- Weinberger F., Breckwoldt K., Pecha S., Kelly A., Geertz B., Starbatty J., Yorgan T., Cheng K.H., Lessmann K., Stolen T., et al. Cardiac repair in Guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci. Transl. Med. 2016; 8 :363ra148. [ PubMed ] [ Google Scholar ]
- Wilmut I., Schnieke A.E., McWhir J., Kind A.J., Campbell K.H.S. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997; 385 :810–813. [ PubMed ] [ Google Scholar ]
- Wilson A., Laurenti E., Oser G., van der Wath R.C., Blanco-Bose W., Jaworski M., Offner S., Dunant C.F., Eshkind L., Bockamp E., et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008; 135 :1118–1129. [ PubMed ] [ Google Scholar ]
- Wimmer R.A., Leopoldi A., Aichinger M., Wick N., Hantusch B., Novatchkova M., Taubenschmid J., Hämmerle M., Esk C., Bagley J.A., et al. Human blood vessel organoids as a model of diabetic vasculopathy. Nature. 2019; 565 :505–510. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Winton D.J., Blount M.A., Ponder B.A.J. A clonal marker induced by mutation in mouse intestinal epithelium. Nature. 1988; 333 :463–466. [ PubMed ] [ Google Scholar ]
- Yanagida A., Spindlow D., Nichols J., Dattani A., Smith A., Guo G. Naive stem cell blastocyst model captures human embryo lineage segregation. Cell Stem Cell. 2021; 28 :1016–1022. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yang N., Zuchero J.B., Ahlenius H., Marro S., Ng Y.H., Vierbuchen T., Hawkins J.S., Geissler R., Barres B.A., Wernig M. Generation of oligodendroglial cells by direct lineage conversion. Nat. Biotechnol. 2013; 31 :434–439. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007; 318 :1917–1920. [ PubMed ] [ Google Scholar ]
- Yu V.W.C., Yusuf R.Z., Oki T., Wu J., Saez B., Wang X., Cook C., Baryawno N., Ziller M.J., Lee E., et al. Epigenetic memory underlies cell-autonomous heterogeneous behavior of hematopoietic stem cells. Cell. 2016; 167 :1310–1322. [ PubMed ] [ Google Scholar ]
- Yu L., Wei Y., Duan J., Schmitz D.A., Sakurai M., Wang L., Wang K., Zhao S., Hon G.C., Wu J. Blastocyst-like structures generated from human pluripotent stem cells. Nature. 2021; 591 :1–7. [ PubMed ] [ Google Scholar ]
- Yui S., Nakamura T., Sato T., Nemoto Y., Mizutani T., Zheng X., Ichinose S., Nagaishi T., Okamoto R., Tsuchiya K., et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5 + stem cell. Nat. Med. 2012; 18 :618–623. [ PubMed ] [ Google Scholar ]
- Zhang J., Niu C., Ye L., Huang H., He X., Tong W.-G., Ross J., Haug J., Johnson T., Feng J.Q., et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003; 425 :837–841. [ PubMed ] [ Google Scholar ]
- Zhang B., Ma S., Rachmin I., He M., Baral P., Choi S., Gonçalves W.A., Shwartz Y., Fast E.M., Su Y., et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature. 2020; 577 :676–681. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhang J., Wu Q., Johnson C.B., Pham G., Kinder J.M., Olsson A., Slaughter A., May M., Weinhaus B., D’Alessandro A., et al. In situ mapping identifies distinct vascular niches for myelopoiesis. Nature. 2021; 590 :457–462. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhou Q., Brown J., Kanarek A., Rajagopal J., Melton D.A. In vivo reprogramming of adult pancreatic exocrine cells to b -cells. Nature. 2008; 455 :627–632. [ PMC free article ] [ PubMed ] [ Google Scholar ]

Developmental Biology and Stem Cell
The Department of Genetics and Genome Sciences has a strong focus on developmental and stem cell biology. We use a variety of cell and animal models to study the impact of genetic and epigenetic aberrations on normal development and their contribution to disease.
The advent of embryonic and induced pluripotent stem cell technology has enabled temporal access to disease-relevant cells and tissues. Many labs throughout the department are using pluripotent stem cell technology to define the mechanisms underlying normal development and a spectrum of disorders including the labs of Tony Wynshaw-Boris (autism and microcephaly), Ann Harris (cystic fibrosis), Paul Tesar (multiple sclerosis and other myelin disorders), Ashleigh Schaffer (neurogenetic disorders), Fulai Jin (regulation of pluripotency), Helen Miranda (motor neuron disorders), Yan Li (diabetes), and Peter Scacheri (CHARGE syndrome).
Mouse models and advancements in genome engineering such as CRISPR/Cas9 are being used to study endocrine disorders in David Buchner ’s lab, cystic fibrosis in the labs of Mitch Drumm , Craig Hodges , and Ron Conlon , social behavioral disorders in Tony Wynshaw-Boris ’ lab, and neurogenetic disorders in the labs of Ashleigh Schaffer and Paul Tesar .
The department also has a longstanding interest in germ cell biology and current efforts are focused on germline ovarian stem cell biology led by Helen Salz , spermatogenesis led by Shih-Hsing Leir and Ann Harris , and ovarian insufficiency led by David Buchner .
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Research Highlight
- Published: 10 August 2023
Studying human embryo development with E-assembloids
- Janet Rossant 1
Cell Research volume 33 , pages 737–738 ( 2023 ) Cite this article
1893 Accesses
13 Altmetric
Metrics details
- Multipotent stem cells
You have full access to this article via your institution.
Stem cell-based models of early human embryo development can provide important insights into key stages of human pregnancy. In a recent Cell Research study, based on data obtained from their detailed analysis of gene expression in intact human embryo cultures, Ai and colleagues document the formation of stem cell ‘E-assembloids’ that mimic morphogenetic events of early human post-implantation development in vitro .
In vitro fertilization (IVF) has become a standard form of reproductive technology worldwide: it is estimated that 3% of babies born in China each year are produced by IVF. Yet we still have very little understanding of why IVF only succeeds in 40% of cases and why early pregnancy loss remains a persistent problem worldwide. IVF allows access to early human embryos in the first days of their development up to the blastocyst stage. The blastocyst marks the stage when the progenitors of the fetus itself become separate from the outer trophoblast and hypoblast cells that will go on to make the placenta and yolk sac membranes. After this stage, the embryo implants into the mother’s uterus. The next key stages of development, including amnion and yolk sac formation, gastrulation, and placental initiation, all take place out of reach of experimental observation or intervention. There is an urgent need to understand these key stages in terms of both fundamental understanding of human biology and insights into improving IVF success, preventing early pregnancy loss, and exploring the onset of congenital defects and developmental origins of adult disease.
Many groups worldwide are tackling this problem in different ways, based on direct study of human embryos, comparative study of non-human primate embryos and generation of stem cell-based models of human development. In a paper recently published in Cell Research , 1 Ai et al. make use of their previously published 3D culture system for human blastocysts 2 to develop a detailed single-cell transcriptomic profile of the human embryo in culture over the early implantation period. By probing this dataset they were able to identify signaling pathways that drive extraembryonic lineage formation from human pluripotent stem cells. They then aggregated naïve hESCs with cells treated with BMP that provide a ‘signaling nest’ for the pluripotent cells. Interactions between the two cell types and a careful time course of application of Wnt, BMP, and Nodal agonists and antagonists resulted in the formation of E-assembloids that could grow and develop in culture for up to 9 days. The E-assembloids contained an outer layer of hypoblast-like cells enclosing the epiblast structures derived from the ES cells. Importantly, morphological events typical of the early post-implantation stages of development were observed, albeit not always as coherently as in the embryo itself. The bilaminar disc structure of the epiblast, and the formation of the amniotic and yolk sac cavities were all observed to occur, along with extraembryonic mesenchyme. Initiation of primitive streak formation and germ cell development were not so easily recognizable, although single-cell RNA sequencing analysis of the E-assembloids did indicate some possible candidate cells. This study provides strong pilot data supporting the relevance of these structures to normal development, based on the authors’ own careful comparative analysis of cell profiles from human embryos themselves. Further improvements in the culture system and in defining the different starting cell components should enhance the efficiency and reproducibility of the E-assembloid approach over time.
These E-assembloids join a now-growing list of stem cell-derived models aimed at modeling these early human post-implantation stages in vitro. Some of these, like the E-assembloids, combine epiblast progenitors with extraembryonic endoderm/hypoblast-like cells, 3 , 4 which seem to be the key components for initiating cavitation and morphogenesis within the epiblast. Surprisingly, perhaps, the inclusion of trophoblast-like cells does not seem to be necessary to initiate differentiation of the post-implantation epiblast towards gastrulation. Two papers did include both trophoblast and hypoblast-like cells in the mix, 5 , 6 but in one case the putative trophoblast cells failed to maintain trophoblast gene expression profiles, 5 making the role of trophoblast uncertain. The second study did propose a key role for trophoblast derivatives for full embryonic development. 6 However, at this early stage in the research, we still lack clarity on all the key components needed for full organization of these embryo-like structures into true embryo models. Every protocol is different from the next: the starting cell state often differs, and the culture conditions are far from fully defined.
While there has been media attention on some of these models proclaiming them as ‘synthetic embryos’, it is important to point out that none of the stem cell embryo models produced to date are in any way complete replicas of a functioning embryo. And, indeed, that is not and should not be the goal of the experiments. The goal is to provide tractable model systems to explore specific aspects of early human development and translate that knowledge into improved IVF and better pregnancy outcomes. There are many exciting challenges ahead in this field.
Ai, Z. et al. Cell Res. https://doi.org/10.1038/s41422-023-00846-8 (2023).
Article PubMed PubMed Central Google Scholar
Xiang, L. et al. Nature 577 , 537–542 (2020).
Article CAS PubMed Google Scholar
Pedroza, M. et al. Nature https://doi.org/10.1038/s41586-023-06354-4 (2023).
Article PubMed Google Scholar
Liu, L. et al. Cell https://doi.org/10.1016/j.cell.2023.07.018 (2023).
Weatherbee, B. A. T. et al. Nature https://doi.org/10.1038/s41586-023-06368-y (2023).
Oldak, B. et al. bioRxiv https://doi.org/10.1101/2023.06.14.544922 (2023).
Download references
Author information
Authors and affiliations.
The Gairdner Foundation and the Hospital for Sick Children, Toronto, ON, Canada
Janet Rossant
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Janet Rossant .
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Rossant, J. Studying human embryo development with E-assembloids. Cell Res 33 , 737–738 (2023). https://doi.org/10.1038/s41422-023-00863-7
Download citation
Published : 10 August 2023
Issue Date : October 2023
DOI : https://doi.org/10.1038/s41422-023-00863-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies

IMAGES
VIDEO
COMMENTS
Stem cell case studies Read our stem cell case studies to discover how umbilical cord cells have been used to treat conditions such as leukaemia, stroke, brain injury and autism. Since 1988, cord blood stem cells have been used to treat a growing number of diseases and disorders.
The most notable case involved the injection of a cell ... and hepatocellular carcinoma. 57 Although preclinical studies have shown that stem cell ... has revolutionized stem cell research ...
Since stem cells were first discovered, researchers have identified distinct stem cell populations in different organs and with various functions, converging on the unique abilities of self ...
However, these studies were either limited regarding the type of stem cell used, such as multipotent stem cells only 33, or their search was conducted using only one database (mostly ...
Despite the controversy associated with its efficacy and ongoing research, stem cell therapy is one of the few available interventions attempted for ALS. 8 - 10 The purpose of this case report is to highlight the effects that marketing of interventions such as SCT have on the decision making of desperate patients with progressive neurological ...
Stem cells began their role in modern regenerative medicine in the 1950's with the first bone marrow transplantation occurring in 1956. Stem cell therapies are at present indicated for a range of clinical conditions beyond traditional origins to treat genetic blood diseases and have seen substantial success.
Interview with Dr. Helen Blau on stem cells in the treatment of disease. 9m 12s Download. The derivation of induced pluripotent stem cells (iPSCs) has revolutionized stem-cell research (see the ...
Case Study: Regenerative Medicine. Regenerative medicine as a field is quite broad but is generally understood to focus on the regeneration, repair, and replacement of cells, tissues, and organs to restore function (Mason and Dunnill, 2008). ... Ethical issues in human stem cell research: Report and recommendations of the National Bioethics ...
"Future research may show whether stem cells in combination with other therapies could be part of a new paradigm of treatment to improve outcomes for patients," says Mohamad Bydon, M.D., a neurosurgeon at Mayo Clinic in Rochester, Minnesota, and the first author of both studies. "Not every patient who receives stem cell treatment is going to be ...
Lessons learned from pioneering neural stem cell studies. Stem Cell Rep. 2017; 8: 191-193. Abstract; Full Text; Full Text PDF; ... In the case where the cell therapy product (biologic) requires a non-cleared 510(k), a non-PMA device for delivery or it is integrated into the product, the FDA designates the cell therapy and delivery device be ...
The ethical implications of stem cell research are often described in terms of risks, side effects, safety, and therapeutic value, which are examples of so-called hard impacts. Hard impacts are typically measurable and quantifiable. To understand the broader spectrum of ethical implications of stem cell research on science and society, it is ...
Stem Cell Research & Therapy (2023) Despite many reports of putative stem-cell-based treatments in genetic and degenerative disorders or severe injuries, the number of proven stem cell therapies ...
An international peer-reviewed journal, it publishes high-quality open access research articles with a special emphasis on basic, translational and clinical research into stem cell therapeutics and regenerative therapies, including animal models and clinical trials. The journal also provides reviews, viewpoints, commentaries, reports and methods.
Embryonic stem cells have immense medical potential. While both their acquisition for and use in research are fraught with controversy, arguments against their usage are rebutted by showing that embryonic stem cells are not equivalent to human lives. It is then argued that not using human embryos is unethical. Finally, an alternative to embryonic stem cells is presented.
Stem cell-based therapies. Stem cell-based therapies are defined as any treatment for a disease or a medical condition that fundamentally involves the use of any type of viable human stem cells including embryonic stem cells (ESCs), iPSCs and adult stem cells for autologous and allogeneic therapies ().Stem cells offer the perfect solution when there is a need for tissue and organ ...
In recent years, stem cell therapy has become a very promising and advanced scientific research topic. The development of treatment methods has evoked great expectations. This paper is a review focused on the discovery of different stem cells and the potential therapies based on these cells. The genesis of stem cells is followed by laboratory steps of controlled stem cell culturing and derivation.
Stem-cell research articles from across Nature Portfolio. Stem-cell research is the area of research that studies the properties of stem cells and their potential use in medicine. As stem cells ...
Stem Cell Coated Fistula Plug in Patients With Crohn's RVF Rochester, MN; Rochester, MN. The purpose of this study is to determine the safety of using an autologous mesenchymal stromal cell (MSC) coated fistula plug in people with rectovaginal fistulizing Crohn's disease. Autologous means that these cells that coat the plug come from you.
Stem Cell Research is dedicated to publishing high-quality manuscripts focusing on the biology and applications of stem cell research.Submissions to Stem Cell Research, may cover all aspects of stem cells, including embryonic stem cells, tissue-specific stem cells, cancer stem cells, developmental studies, genomics and translational research. Special focus of SCR is on mechanisms of ...
Stem cells articles from across Nature Portfolio. Atom. RSS Feed. Stem cells are cells that have the capacity to self-renew by dividing and to develop into more mature, specialised cells. Stem ...
In this Perspective by Urban Lendahl, the history of stem cell research from discoveries in the late 1800s to the most recent developments is described. The Perspective also discusses the progress in clinical translation of stem cell research discoveries. Finally, the 20-year history of ISSCR as well as the society's role in research, ethics ...
The Department of Genetics and Genome Sciences has a strong focus on developmental and stem cell biology. We use a variety of cell and animal models to study the impact of genetic and epigenetic aberrations on normal development and their contribution to disease. ... Biomedical Research Building 2109 Adelbert Rd Cleveland , OH 44106 ...
The SkillsCenter is a fully stocked laboratory organized with distinct "skills bays" (e.g., buffer station, centrifuge station, DNA and protein gel station, etc.) that students can reserve through an online resource calendar. Proctors staff the lab during normal work hours and provide students with on-site support.
Stem cell-based models of early human embryo development can provide important insights into key stages of human pregnancy. In a recent Cell Research study, based ... 6 but in one case the ...