- Open access
- Published: 26 July 2022

Effectiveness of aromatherapy for prevention or treatment of disease, medical or preclinical conditions, and injury: protocol for a systematic review and meta-analysis
- Sue E. Brennan ORCID: orcid.org/0000-0003-1789-8809 1 ,
- Steve McDonald 1 ,
- Melissa Murano 1 &
- Joanne E. McKenzie 1
Systematic Reviews volume 11 , Article number: 148 ( 2022 ) Cite this article
4 Citations
1 Altmetric
Metrics details
Aromatherapy — the therapeutic use of essential oils from plants (flowers, herbs or trees) to treat ill health and promote physical, emotional and spiritual well-being — is one of the most widely used natural therapies reported by consumers in Western countries. The Australian Government Department of Health (via the National Health and Medical Research Council) has commissioned a suite of independent evidence evaluations to inform the 2019-20 Review of the Australian Government Rebate on Private Health Insurance for Natural Therapies. This protocol is for one of the evaluations: a systematic review that aims to examine the effectiveness of aromatherapy in preventing and/or treating injury, disease, medical conditions or preclinical conditions.
Eligibility criteria : randomised trials comparing (1) aromatherapy (delivered by any mode) to no aromatherapy (inactive controls), (2) aromatherapy (delivered by massage) to massage alone or (3) aromatherapy to ‘gold standard’ treatments. Populations : any condition, pre-condition, injury or risk factor (excluding healthy participants without clearly identified risk factors). Outcomes : any for which aromatherapy is indicated.
Searches : Cochrane Central Register of Controlled Trials (CENTRAL), with a supplementary search of PubMed (covering a 6-month lag period for processing records in CENTRAL and records not indexed in MEDLINE), AMED and Emcare. No date, language or geographic limitations will be applied.
Data and analysis : screening by two authors, independently (records indexed by Aromatherapy or Oils volatile or aromatherapy in title; all full text) or one author (remaining records) with second author until 80% agreement. Data extraction and risk of bias assessment (ROB 2.0) will be piloted by three authors, then completed by a single author and checked by a second. Comparisons will be based on broad outcome categories (e.g. pain, emotional functioning, sleep disruption) stratified by population subgroups (e.g. chronic pain conditions, cancer, dementia) as defined in the analytic framework for the review. Meta-analysis or other synthesis methods will be used to combine results across studies. GRADE methods will be used to assess certainty of evidence and summarise findings.
Results of the systematic review will provide a comprehensive and up-to-date synthesis of evidence about the effectiveness of aromatherapy.
Systematic review registration
PROSPERO CRD42021268244
In 2015, the Australian Government conducted a Review of the Australian Government Rebate on Natural Therapies for Private Health Insurance (2015 Review). Underpinned by systematic reviews of evidence for each natural therapy, one of the findings from the 2015 Review was that there was no clear scientific evidence that aromatherapy was effective. This protocol for a systematic review of aromatherapy describes the methodology for one of a suite of independent evidence evaluations commissioned by the Australian Government Department of Health (the Department) via the National Health and Medical Research Council (NHMRC) to update the evidence and inform the Review of the Australian Government Rebate on Private Health Insurance for Natural Therapies 2019-20 (2019-20 Review) [ 1 ].
Aromatherapy is one of the most widely used natural therapies reported by consumers in Western countries. A systematic review of 89 surveys (97,222 participants), estimating the prevalence of complementary medicine (CM) use by consumers in the UK, found that aromatherapy was the third most popular CM from among 28 different therapies [ 2 ]. In Australia, a cross-sectional survey examining consultation with complementary therapists and use of complementary medicine products found that about half of all respondents (1016/2025 adults) used complementary medicines [ 3 , 4 ]. Aromatherapy oils were used by 11% of respondents ( N = 224/2019), and 3.9% of respondents had visited an aromatherapist ( N = 79/2019) [ 4 ]. Based on the average spending on complementary medicines reported in this survey, the study authors estimated the total expenditure on aromatherapy oils in Australia to be AUD 250 million in the previous 12 months (2016–2017) [ 3 ].
Description of the intervention
Aromatherapy is the therapeutic use of essential oils from plants (flowers, herbs or trees) to treat ill health and promote physical, emotional and spiritual well-being [ 1 , 5 , 6 ]. The name ‘aromatherapy’ suggests that treatments are delivered directly or indirectly through the olfactory system and that ‘aroma’ is central to therapeutic action. However, there are multiple modes of administration, and these include treatments intended to act through direct contact with the skin and inhalation into the lungs (rather than through an ‘aroma’ inhaled through the olfactory system). The inclusion of such therapies within the scope of aromatherapy practice has led some professional groups to suggest that a more apt description is ‘essential oil therapy’ [ 7 ].
Active ingredients and choice of essential oils
Although the scope of aromatherapy practice varies, the use of essential oils is central to all definitions [ 6 , 7 , 8 , 9 , 10 ]. Essential oils are volatile oils extracted using steam distillation or mechanical expression from aromatic plants [ 6 , 11 ]. While it is possible to extract essential oils using solvents (‘absolutes’) and to produce synthetic versions of some oils, aromatherapists generally consider that these are not true essential oils and are therefore unsuitable for therapeutic use [ 6 , 11 ].
Essential oils vary greatly in their molecular composition. This composition determines the aroma of each oil, and the pathways by which it is absorbed, distributed and metabolised to produce effects [ 6 , 11 ]. Aromatherapists tailor treatments to individual needs, selecting essential oils, and their mode of application, based on anticipated therapeutic properties for the targeted condition [ 1 , 6 ].
Mode of administration and dose
Inhalation through passive diffusion in the air (e.g. through mist or heat diffusers, steam vaporisation) and direct inhalation (e.g. individual inhalers, steam inhalation) can be used as the primary mode of administering essential oils. Topical application of diluted essential oils to the skin is also common [ 6 ]. The intention of topical application may be to produce local effects at the point of administration (e.g. to alleviate pain in a joint) and to mediate effects through inhalation (whether through the lungs or olfactory system) or through skin absorption. Massage is a common co-intervention used with topical application of essential oils. While massage may have a therapeutic effect when used independently of essential oils, it is generally described as an ‘integral’ part of aromatherapy treatment [ 7 ]. For topical application, essential oils are diluted in a carrier oil, usually vegetable or nut oil (e.g. sweet almond oil, grapeseed, jojoba oil) [ 12 ]. These carrier oils differ from essential oils in that they contain fatty acids, vitamins and minerals, and are believed to aid absorption of the essential oil through the skin [ 12 ].
Limiting the dose or concentration of essential oils is considered an important means of avoiding systemic toxicity or adverse effects, such as skin irritation or sensitivity [ 11 , 12 ]. The typical dose of essential oil used for therapeutic purposes varies depending on indication, and the oil and route of administration, but is generally in the range of a 2.5–5% dilution of essential oils for topical use [ 11 ]. Lower concentrations (i.e. higher dilutions) are recommended for some population groups, including women who are pregnant, children and people with conditions or receiving treatments/medications that may put them at greater risk of adverse effects (e.g. people with skin conditions or damage; people undergoing radiotherapy; people with asthma) [ 7 , 11 ].
Although other routes of administration are sometimes used, professional associations for aromatherapists in Australia, the UK, Canada and the USA have position statements recommending against ingestion of essential oils, internal use (on or near mucous membranes) and the use of undiluted essentials oils on the skin [ 7 , 8 , 9 ].
Practitioners of aromatherapy and regulation
Aromatherapy is practised by natural therapists, including aromatherapists, naturopaths and massage therapists. It is also an increasingly common professional education option for nurses, allied health professionals and those working in sectors such as palliative care.
Aromatherapy practice is not regulated by the Australian Health Practitioner Regulation National Law, which means there is no requirement for professional registration of practitioners of aromatherapy [ 13 , 14 ]. The International Aromatherapy and Aromatic Medicine Association (IAAMA) offers membership to aromatherapy practitioners in Australia who have completed accredited training through the National Quality Training Framework [ 15 ]. The IAAMA, and other associations for natural therapists in Australia, also set standards for practice and ethical conduct and have requirements for continuing professional education [ 15 , 16 ]. Some professional associations have safety guidelines and position statements aimed at preventing the use of contraindicated oils, unsafe therapies and treatments that are not widely accepted by the profession (for examples, see [ 7 , 8 , 9 , 10 ]).
In the 2016–2017 cross-sectional survey examining use of complementary medicine products, only a minority of those who reported therapeutic use of aromatherapy oil consulted a complementary medicine practitioner (12.5%) for a prescription, whereas self-prescription was common (43%) [ 3 ]. Indeed, part of the appeal of aromatherapy may be the accessibility of essential oils, which do not require a prescription. The Australian Government provides a safeguard for consumers by regulating essential oils intended for therapeutic use through the Therapeutic Goods Administration (TGA). However, most essential oils are designated as lower risk medicines, which means they are assessed by the TGA for quality and safety, but not effectiveness [ 17 ].
How aromatherapy might work
The research literature and guidance on aromatherapy describes multiple theories of how aromatherapy works (for examples, see [ 6 , 7 ]). This is perhaps unsurprising given that the exact mechanism by which aromatherapy brings about effects is likely to differ according to the molecular composition of the essential oil and the mode of administration. Similarly, the mechanism of action may vary across outcomes. For example, the mechanism(s) through which aromatherapy might relieve pain may be different from the mechanism for relieving nausea and vomiting [ 18 ]. If massage is used as a co-intervention, then the interaction between massage, the essential oil and the carrier oil may also influence the mechanism [ 6 , 12 ]. Research on these mechanisms comes predominantly from mainstream neurophysiological research on olfaction and pharmacological research. Some is specific to essential oils, but very little originates from literature on aromatherapy [ 6 ]. This research is comparatively recent, and evidence about the mechanisms of action for specific oils and modes of delivery is limited [ 6 , 19 ].
The prevailing description of how aromatherapy works — and one that aligns intuitively with the practice of aromatherapy — is that aromatherapy acts through the olfactory system. Volatile molecules in the aromatherapy oil (the odorant) interact with receptors in the nose, generating an electrical signal to the brain that triggers the perception of smell [ 6 , 19 , 20 ]. This perception includes responses initiated in the limbic system, which is involved in controlling memory and emotion, and through which odours are thought to produce effects on mood, alertness, mental stress, arousal and perceived health [ 6 ]. Biochemical or physiological pathways are likely to mediate the effects of essential oils applied to the skin, where either local or systemic effects may be possible depending on whether the active component diffuses through the skin [ 19 ]. Some of these effects are suggested to arise from antibacterial, anti-inflammatory and analgesic properties of essential oils [ 6 , 21 , 22 ].
Aromatherapy professional associations also describe a pathway involving an ‘energetic’ or spiritual response. Such mechanisms are described as a ‘vibrational interaction’ between the active component of the essential oil and ‘the energy flows within the body’ [ 7 ]. It is unclear whether this pathway relates to the disproven theory that posits a vibrational mechanism of olfaction in which the olfactory system detects molecular vibrations of odour molecules [ 7 , 20 , 23 ].
Description of conditions for which aromatherapy is used
Although texts on aromatherapy describe a breadth of clinical indications, aromatherapy is often used to treat symptoms of a condition and the side effects of treatment rather than the underlying condition. Examples include the use of aromatherapy to alleviate pain, symptoms of anxiety (that occur as a reaction to stress), low mood, sleep disturbance, behavioural disturbance, vomiting and nausea, and fatigue [ 6 , 24 , 25 , 26 , 27 ]. These indications align with the most commonly treated conditions reported in a 2015 survey completed by 36 practising aromatherapists in Australia [ 14 , 28 ]. Stress was the condition most frequently reported as ‘often treated’ (by 79% of aromatherapists). Musculoskeletal conditions associated with chronic pain were also frequently reported as often treated, especially neck (64% of aromatherapists), arthritis (54%), sciatica (42%) and knee pain (42%). Other conditions that were reported as ‘often treated’ were headache and migraine (66%), mental health conditions (40%), insomnia (47%), sports injury (27%), cancer (24%) and palliative care (21%).
There is a particular interest in using aromatherapy in circumstances where mainstream interventions may not provide satisfactory relief of symptoms, for example for people with unremitting chronic pain, cancer or advanced disease (not amenable to cure) [ 6 , 25 , 29 , 30 ]. Among people with cancer and advanced disease, aromatherapy is used as a form of supportive care to enhance physical and emotional well-being, in addition to alleviating specific symptoms [ 6 , 25 , 29 , 30 ]. In other cases, aromatherapy is used as an alternative or adjunctive therapy by those seeking to avoid pharmacological or invasive treatment. For example, aromatherapy has been used to ameliorate behavioural and sleep disturbances among people with dementia [ 24 ], to relieve pain during labour [ 31 ] and to treat postoperative nausea and vomiting [ 32 ]. These treatments may be delivered in a range of healthcare settings (primary, acute and subacute care), with varying levels of integration with conventional providers [ 33 ].
Because aromatherapy is often sought or prescribed for relief of symptoms, those receiving aromatherapy for the same indication may have very different underlying conditions (e.g. cancer, arthritis, chronic insomnia) or be undergoing different treatments (e.g. surgery, chemotherapy, minor procedures). Examining the effects of aromatherapy on outcomes for a particular condition may be of interest in some circumstances, but for many commonly treated symptoms or side effects, there is no clear clinical rationale for why the effects of aromatherapy would differ importantly by condition. Where this is the case, a broad synthesis across conditions addresses whether there is a consistent effect for the outcome of interest (benefit, little or no effect, harm), in addition to enabling exploration of whether the effect of aromatherapy differs by condition (e.g. smaller or larger effects).
Why it is important to do this review
This systematic review will inform the Australian Government’s Natural Therapies Review 2019-20, which is evaluating evidence of the clinical effectiveness of 16 therapies (including aromatherapy). The conclusion from the evidence evaluation conducted on aromatherapy for the 2015 Review was that ‘there was no clear evidence demonstrating efficacy of aromatherapy’ [ 34 ]. The evidence evaluation used overview methods, synthesising results from 20 systematic reviews published up to May 2013. Of the primary studies included in these systematic reviews ( N = 45), all but one were published prior to 2012. Since the completion of the original evidence evaluation, there has been substantial growth in published research on aromatherapy. A bibliometric analysis of scientific articles on aromatherapy found a steady increase in the number of primary studies and reviews from 1995 to 2014 [ 35 ]. Of the 549 research articles published in this period, a third ( N = 190) were published between 2012 and 2014. This finding marries with claims that there may be evidence available (either published in the last 5 years or not incorporated in systematic reviews at the time the overview was conducted) that may change the conclusions about the effects of aromatherapy [ 1 ]. In contrast to the 2015 aromatherapy evidence evaluation, this review will examine evidence from eligible primary studies published from database inception until the date of the last search for this systematic review.
The overall objective of this systematic review is to examine the evidence for the clinical effectiveness of aromatherapy in preventing and/or treating injury, disease, medical conditions or preclinical conditions [ 1 ]. The review will focus on outcomes (and underlying conditions) for which aromatherapy is commonly sought or prescribed in Australia, and which are relevant to the 2019-20 Review of the Private Health Insurance rebate. The specific objectives of the review follow (framed as questions). Examples of potentially relevant outcome domains and conditions are included to illustrate the breadth of questions to be addressed in the synthesis. These questions will be refined through a staged prioritisation process (‘ Methods ’ section, Fig. 1 ) to align with priorities for the 2019-20 Review, ensure a consistent approach across the evidence evaluations of natural therapies (where appropriate) and make best use of available evidence.
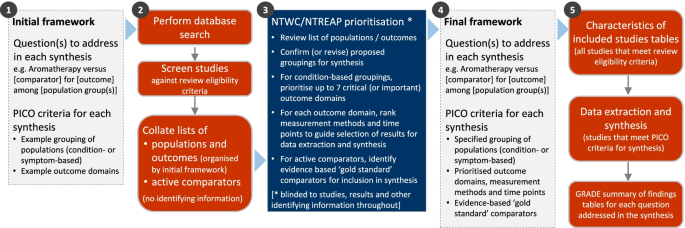
Staged approach for developing the analytic framework for this review
Primary objectives
What is the effect of aromatherapy compared to no aromatherapy (inactive controls) (see the section ‘ Types of interventions ’ — Comparisons) among people with any condition, pre-condition, injury or risk factor on outcomes for which aromatherapy is indicated? (for example, acute pain, emotional functioning and well-being, sleep disruption, behavioural disturbances, health-related quality of life)
What is the effect of aromatherapy plus massage compared to massage alone among people with any condition, pre-condition, injury or risk factor on outcomes for which aromatherapy is indicated? (examples as per objective 1)
Secondary objectives
What are the effects of aromatherapy for each underlying condition, pre-condition, injury or risk factor? (for example, effects on sleep disruption among people undergoing palliative care, people with chronic insomnia, people with chronic pain or people with dementia)
What are the effects of aromatherapy compared to evidence-based ‘gold standard’ treatments ? (see the section ‘ Types of interventions ’ — Comparisons)
What evidence exists examining the effects of aromatherapy compared to other active comparators? (i.e. not massage or a ‘gold standard’)
Methods reported in this protocol are based on the Cochrane Handbook for Systematic Reviews of Interventions [ 36 ]. The GRADE approach will be used to summarise and assess the certainty of evidence arising from this review (see the ‘ Data collection and analysis ’ section for details). GRADE methods are widely used in systematic reviews and guideline development to ensure a systematic, transparent and common approach to interpreting results [ 37 ]. The protocol is reported in accordance with the Preferred Reporting Items for Systematic review and Meta-Analyses Protocols (PRISMA-P) statement [ 38 , 39 ] with consideration given to the extensively updated guidance for reporting methods for systematic review in the Preferred Reporting Items for Systematic review and Meta-Analyses (PRISMA) 2020 statement [ 40 , 41 ]. The review has been prospectively registered on the International prospective register of systematic reviews (PROSPERO CRD42021268244 ).
The methods for this review are designed to accommodate the breadth of evidence about the effects of aromatherapy relevant to the 2019-20 Review and ensure a consistent approach with the other evidence evaluations of natural therapies (where appropriate). To achieve this, we will follow the staged approach summarised in Fig. 1 and elaborated in subsequent sections. We begin with an initial analytic framework (step 1) that will be refined through a prioritisation process. To facilitate this process, we will screen studies against the review eligibility criteria and compile an aggregate list of populations and outcomes, derived from the included studies and organised by the initial framework (step 2). No identifying information will be included (i.e. no study-level information, results, references, number of studies etc.). The NHMRC’s Natural Therapies Working Committee (NTWC) and the Department’s Natural Therapies Review Expert Advisory Panel (NTREAP) will review the list in order to prioritise outcomes and advise on the final framework for the synthesis (step 3), which will be finalised (step 4) prior to proceeding with the review (step 5).
Figure 2 shows the initial analytic framework for the review. Example populations and outcome domains are included to convey the breadth of the review, and illustrate possible population and outcome groups for synthesis. These are indicative and not intended to be exhaustive. The framework was informed by research on the outcomes (and underlying conditions) for which aromatherapy is commonly sought or prescribed in Australia, a scoping search of studies evaluating aromatherapy, the wider literature on aromatherapy and consideration of frameworks for classifying disease and outcomes [ 42 , 43 ]. Details for each population, intervention, comparator, outcomes (PICO) element follow (see the ‘ Criteria for considering studies for this review ’ section).
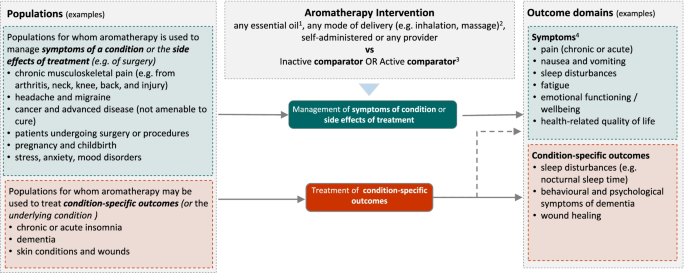
Initial analytic framework for the review. 1 Excluding oils considered unsafe for therapeutic use in humans. 2 Excluding ingestion, internal administration or undiluted application to the skin. 3 Synthesis limited to inactive, massage (when aromatherapy is delivered via massage) and evidence-based ‘gold standard’ treatments. 4 Symptoms relevant to each population group will vary
Criteria for considering studies for this review
Types of studies.
Randomised controlled trials (RCTs) are eligible for inclusion in the review (including individually and cluster randomised and cross-over trials).
Controlled trials in which the allocation sequence did not include a truly random element, was predictable or was not adequately concealed from investigators are eligible as long as there was an attempt to have some kind of ‘randomisation’ to groups. Examples include studies using methods for sequence generation based on alternation, dates (of birth or admission) and patient record numbers [ 44 ].
Non-randomised studies of interventions (NRSIs)
Studies described as ‘randomised trials’ or ‘controlled clinical trials’, but in which decisions about the allocation of participants to treatment groups were (1) made by clinicians or participants or (2) based on the availability of the intervention. These studies lack any ‘attempt’ at randomisation and, as such, are likely to be at high risk of selection bias whereby participants may be selected into groups based on factors that are prognostic of outcomes (which may introduce confounding). These studies will be treated as non-randomised studies.
Studies for which available reports have not been peer reviewed (grey literature)
The decision to exclude non-randomised studies was informed by scanning results from a scoping search of the Cochrane Central Register of Controlled Trials (CENTRAL) (see the ‘ Electronic searches ’ section) and results of a more limited search of PubMed using a resource on the National Institute of Health National Centre for Complementary and Integrative Health website [ 45 ]. The scoping search of CENTRAL retrieved in excess of 500 potentially eligible trials, from which we anticipate a high proportion (100–200) will meet eligibility criteria for the review. Given the likely size and breadth of the evidence base, and the proposed structure for the synthesis, any effect of aromatherapy on health outcomes should be detectable from randomised trials. The inclusion of NRSIs is unlikely to increase certainty of the results from a body of randomised trial evidence of this size or alter the conclusions of the review.
Date and language restrictions
There are no restrictions on publication date.
Potentially eligible studies published in languages other than English will not be included in the review, but will be listed according to whether they are likely to be eligible or whether eligibility cannot be determined (see the ‘ Selection of studies ’ section). The impact of excluding these studies will be considered in the assessment of bias due to missing results (see the ‘ Assessment of biases due to missing results ’ and ‘ Summary of findings tables and assessment of the certainty of the body of evidence ’ sections).
Types of participants
Studies involving participants with any disease, medical condition, injury or preclinical condition are eligible for the review. This includes healthy participants with clearly identified risk factors (e.g. biomedical, health behaviours or other). There are no restrictions on age.
We expect that studies will include participants that fall within broad population groups, such as those shown in Fig. 2 . These are indicative groups, included to illustrate the breadth of populations eligible for the review and possible groupings for synthesis. Decisions about which groups to include in the final analytic framework will be made through the prioritisation process (Fig. 1 ). This process may lead to changes and additions to the population groups (i.e. broader, narrower or new groups).
Basis for grouping
Because of the broad range of indications for aromatherapy (e.g. management of symptoms of a condition or side effects of treatment for a condition, versus treatment of an underlying condition), the basis of the population groups shown in the initial analytic framework varies. Some are grouped by symptom (e.g. chronic pain), some by treatment for an underlying condition (e.g. surgery and other procedures) and others by the underlying condition (e.g. chronic insomnia, dementia). The groupings are based on International Classification of Diseases 11th Revision (ICD-11) codes and encompass conditions identified in aromatherapy literature and the Practitioner Research and Collaboration Initiative (PRACI) survey as often treated by aromatherapists [ 14 , 28 ].
Participants who are otherwise healthy will be considered to have a clearly identified risk factor if participating in a trial aimed at prevention of a disease/condition for which their risk factor is an eligibility criterion (e.g. individuals with signs or symptoms of work-related anxiety who are confirmed to be at risk of developing a clinically diagnosed anxiety or fear related disorder).
Healthy populations seeking health improvement
Studies that include both healthy participants and participants eligible for the review will be included if separate data are available or a majority of participants meet the review eligibility criteria as per guidance in the Cochrane handbook [ 46 ]. For the latter, we will consider implications for the applicability of study findings in the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) assessment.
While studies involving any population will be included in the review (except for the specific exclusions above), if the number of eligible studies for synthesis is unmanageable, the synthesis may be limited to populations (conditions) most relevant to aromatherapy practice in Australia. Such decisions will be made through the prioritisation process (Fig. 1 ), guided by data about practice in the Australian context (e.g. practitioner or patient surveys that report reasons for use in Australia). Studies excluded from the synthesis will be included in an evidence inventory (objective 5).
Types of interventions
For the purpose of this review, aromatherapy is defined as ‘Administration of aromatherapy oils by inhalation, diluted topical use and massage’ [ 1 ].
Except for the specific exclusions below, aromatherapy treatments will be eligible irrespective of the type of essential oil, carrier or dispersant, mode of delivery or route of administration, whether self-administered or provided by a practitioner, the training or qualifications of the practitioner and the dose and duration of treatment. More details about each of these intervention features are considered under the ‘ Data extraction and management ’ section. See also Appendix 3, Additional file 1 .
Excluded therapies
In line with the recommendations from aromatherapy professional associations in Australia and internationally [ 7 , 8 , 9 , 10 ], we will exclude interventions in which an essential oil is:
Ingested or administered internally (e.g. oral, vaginal, rectal or other internal routes of administration)
Applied undiluted to the skin
Considered unsafe for therapeutic use in humans
Comparisons
Aromatherapy (delivered by any mode, including massage) versus any inactive comparator (placebo/sham, no intervention, wait list control, usual care)
Aromatherapy delivered by massage versus massage alone (this comparison is included to isolate the effects of aromatherapy)
Aromatherapy (delivered by any mode) versus evidence-based gold standard treatment(s) (see below for selection method)
Aromatherapy (delivered by any mode) versus other active comparators (for inclusion in evidence inventory only, not the synthesis — see below)
These comparisons will form the basis of separate syntheses (meta-analyses), each considering an outcome domain with studies grouped within by population group (where appropriate; see Fig. 2 for examples). Where a study includes multiple arms, with at least one eligible comparator (e.g. a placebo control arm), we will include the eligible comparison(s).
For comparison 3, evidence-based gold standard treatments will be identified through the prioritisation process (Fig. 1 , step 3). We will provide the NTWC and NTREAP with a list of active comparators identified from included studies (Fig. 1 , step 2). Studies with active comparators will not contribute to the synthesis except in the exceptional circumstance where the NTWC considers that the comparator intervention is an accepted, evidence-based ‘gold standard’ of care for the population in the studies, and there are studies suitable for conducting a synthesis (meta-analysis) (i.e. comparable PICO criteria, low risk of bias). These judgements will be made blinded to the studies and study results, to the fullest extent possible. For studies involving other active comparators, we will provide an inventory of available evidence, tabulating a brief description of the characteristics of PICO for each study.
In line with the main review objective, which is to examine the effects of aromatherapy rather than the comparative effects of different aromatherapy treatments, we will exclude head-to-head comparisons of aromatherapy from the review (see exceptions below). For example, we will exclude studies where the only comparator is:
Another essential oil or preparation of an essential oil (e.g. lavender versus ginger)
A different dilution or dose of the same essential oil
A different carrier of the same essential oil
A different mode of delivery of the same essential oil (e.g. two different modes of inhalation; inhalation versus massage)
Where the person administering the therapy has a different qualification, specialisation or skill level (e.g. aromatherapists versus other health professional; this includes comparisons of self-administration versus administration by a practitioner)
Or combinations of the above
Types of outcomes
Outcomes eligible for this review are those that align with the reasons why aromatherapy is sought by patients and prescribed by practitioners. In principle, this may include any patient-important outcome that helps elucidate the effects of aromatherapy on an underlying condition or its symptoms, recovery, rehabilitation or prevention of disease among people with specific risk factors or pre-conditions.
Example outcome domains are shown in Fig. 2 . Appendix 2 in Additional file 1 provides examples of specific outcomes within each domain, and populations to which the outcome domain may be relevant. Because aromatherapy is often used for the management of symptoms of a condition or side effects of treatments (anxiety, pain, nausea and vomiting), ‘symptoms’ are separated from ‘condition-based’ outcomes (the latter encompassing outcomes of relevance when aromatherapy is used to treat the underlying condition). The example outcome domains are intended to illustrate the breadth of outcomes likely to be important for understanding the effects of aromatherapy across a wide range of conditions, as identified from the PRACI survey of the conditions often treated by aromatherapists in Australia [ 14 , 28 ] and the wider literature on aromatherapy.
The initial grouping of broadly related outcomes within each domain (Fig. 2 ) is based on ICD-11 codes and the Core Outcome Measures in Effectiveness Trials (COMET) outcome taxonomy [ 42 , 43 ]. These systems provide a widely agreed and understood structure for categorising different outcomes and address the fact that there is not always a clear distinction between outcomes and conditions. For example, some outcome domains closely match the primary diagnosis for particular patients (e.g. insomnia, chronic pain, anxiety), but are a symptom or side effect of treatment for other conditions (e.g. cancer or surgery).
Prioritisation and selection of outcomes for summary and synthesis
Outcome prioritisation.
To accommodate the breadth of relevant outcomes, the outcome domains and population-specific outcomes for inclusion in the synthesis will be determined through the prioritisation process (Fig. 1 ).
To prioritise the most important outcomes for this review:
We will compile a list of specific outcomes from included studies and example outcome measures (without results or identification of studies).
Outcomes in the list will be categorised by the outcome domains and population groups in Fig. 2 . Outcomes that fall outside the proposed outcome domains will also be listed.
The NTWC will be asked to indicate whether each of the listed outcome domains (or specific outcomes) is critical, important or of limited importance for understanding the effects of aromatherapy on each population group. Only critical and important outcomes will be considered in the review.
Outcome selection
From each study, we will select only one outcome per outcome domain for data extraction (results), risk of bias assessment and inclusion in the summary and synthesis.
An initial hierarchy of population-specific outcomes and measures will be presented to the NTWC for discussion and approval (e.g. a hierarchy of pain outcomes and measures for osteoarthritis).
Where possible, the initial hierarchy will be based on outcome hierarchies used in published Cochrane reviews, systematic reviews of measures that provide evidence of the relevance and validity of measures, and core outcome sets.
We will also seek advice on the most relevant time point for outcome measurement. This is likely to be immediately post-intervention (end of the intervention period if multiple treatments). In some instances, the longest follow-up may be relevant (e.g. chronic pain).
The agreed hierarchy of population-specific outcomes measures and time points will be used to select the most relevant and valid measure of each outcome domain available from each study for inclusion in the synthesis.
Exclusions:
Experience of care (e.g. satisfaction)
Economic outcomes
Studies will not be excluded from the synthesis/reporting of results based on outcome, except where it is possible to confirm that the study did not measure an outcome eligible for the review (e.g. from a registry record or protocol).
Search methods for identification of studies
Electronic searches.
The primary source of studies will be the Cochrane Central Register of Controlled Trials (CENTRAL), the most comprehensive source of published and unpublished reports of randomised trials. Most CENTRAL records are derived from regular searches of bibliographic databases, such as MEDLINE, Embase and the Cumulative Index of Nursing and Allied Health Literature (CINAHL). Records from clinical trial registers (ClinicalTrials.gov and WHO International Clinical Trials Registry Platform [ICTRP]) and the specialised registers maintained by Cochrane groups also make up a substantial proportion of records in CENTRAL.
As part of Cochrane’s centralised search service, the major bibliographic databases and trial registers are searched monthly and, using a combination of automation and crowd screening, records deemed to be reports of randomised trials are added to CENTRAL [ 47 ]. In a recent evaluation, over 97% of studies included in Cochrane reviews were retrieved by Cochrane’s centralised search service [ 48 ]. Given the large volume of studies we anticipate will be eligible, we are confident that limiting our search to CENTRAL, with supplementary searches of PubMed and the Allied and Complementary Medicine Database (AMED), will capture a very high proportion of all relevant studies.
The proposed search strategy for CENTRAL includes the key thesaurus terms and text words for aromatherapy, as well as more peripheral terms, such as essential oils (see Appendix 1 in Additional file 1 ). The most commonly used essential oils are included as text words in their own right. This list of oils was compiled from (1) studies included in the overview of aromatherapy for the 2015 Review [ 34 ] and (2) the broader aromatherapy literature [ 6 , 21 , 22 , 24 , 25 , 26 , 27 , 31 ]. To ensure no commonly used essential oils were missing from the list, we examined a sample of 272 abstracts from a PubMed Clinical Query for aromatherapy (category: ‘Therapy’, scope: ‘Narrow’). We will not limit the search by language, year of publication or publication status.
Since there is a lag between when records are processed by Cochrane and when they appear in CENTRAL, we will run a search in PubMed for records added in the previous 6 months. In addition, to ensure we include records available in PubMed but which are not indexed in MEDLINE, we will search PubMed for all years, limited to the non-MEDLINE subset (see Appendix 1 in Additional file 1 ).
We will also search AMED and Emcare via Ovid as these databases are not ones that Cochrane searches centrally.
Scoping searches reveal that about 500 records in CENTRAL (excluding records from ClinicalTrials.gov or WHO ICTRP) are either indexed with the Medical Subject Headings (MeSH) or Emtree term Aromatherapy or have aromatherapy as a text word in the title. A further 1300 records are retrieved with the remaining search terms.
Searching other resources
We will screen studies provided by the public and key stakeholders (via the Department), NTREAP and NTWC for eligibility. Where these groups recommend particular systematic reviews, we will examine references for included studies to identify potentially eligible randomised trials.
We will ensure that all randomised trials included in the 2015 evidence evaluation for aromatherapy are considered for inclusion.
We will not examine the reference lists of included studies to identify additional trials (i.e. backward citation searching), nor will we conduct forwards citation searching (i.e. looking for studies that have cited included studies). Empirical studies assessing the value of reference checking (backward citation searching) as part of the systematic review process indicate that it is most useful for areas that are difficult to search electronically (new technologies, cross-disciplinary topics, complex interventions) or for which review authors aim to locate grey literature [ 49 ]. Forward citation searching is much less common in systematic reviews [ 50 ] and of questionable value [ 51 ]. Conducting forward citation searching for the large volume of aromatherapy studies we anticipate including in this review could generate thousands of additional records to screen, with little evidence that we would identify unique studies. This has significant time and cost implications [ 52 ]. We anticipate that our search is sufficiently sensitive that we are unlikely to miss important studies, and given the anticipated volume of eligible studies and breadth of the review question, it is unlikely that any missing studies would alter the findings of the review.
Data collection and analysis
Selection of studies.
Records from CENTRAL, PubMed and AMED will be imported into EndNote and duplicates removed. All remaining records will be imported into Covidence [ 53 ] for screening. Records submitted through the Department, NTREAP or NTWC will be screened to confirm that the type of study is eligible, then non-duplicate records will be imported into Covidence for screening alongside other studies.
We will pilot guidance for title and abstract screening on a sample of 50 records to ensure the eligibility criteria are being applied consistently by three reviewers (SB, MM, SM). If needed, we will amend the screening guidance (but not the eligibility criteria) to enhance consistency. We propose to split title and abstract screening into two phases. Phase 1 records (indexed with the thesaurus terms Aromatherapy or Oils volatile or with aromatherapy in the title) will be screened independently by at least two reviewers. Phase 2 (remaining records) will be screened by one reviewer, with a 10% random sample screened by a second reviewer (with further sampling if needed until 80% agreement is achieved). All records selected for full-text screening will be reviewed independently by two reviewers. Disagreements at either stage of screening will be resolved by consensus among members of the review team. Where disagreement cannot be resolved, advice will be sought from the NTWC (which will be provided with PICO characteristics for the de-identified study).
Studies confirmed as meeting the eligibility criteria, but for which results are not available in a published report, will be included in a list of ‘ongoing studies’.
The following will be included in a list of ‘studies awaiting classification’.
Studies that are only published as abstracts or for which a full report is not available (i.e. we will not seek further information from study authors to confirm eligibility)
Studies identified by, or submitted to, the review team after the date of the last search
Studies confirmed as likely to be eligible, but for which no English language translation of the full-text publication is available. Studies for which eligibility cannot be confirmed following translation of the title and abstract using Google Translate will be listed separately (Fig. 3 ).

Flowchart showing handling of studies in languages other than English (reproduced from NHMRC framework for natural therapies systematic reviews [ 54 ])
Studies that do not meet the eligibility criteria will be excluded and the reason for exclusion will be recorded at full-text screening. These studies will be included in a ‘Characteristics of excluded studies’ table in which the reason for exclusion is reported.
The search and study selection steps will be summarised in a PRISMA flow diagram.
For studies that originated from the call for evidence, NTREAP or NTWC, we will record and report exclusion decisions irrespective of whether the study was excluded during title and abstract screening or full-text review. We will document the flow of these studies through the review in the PRISMA flow chart and annotate tables with the source.
Dealing with duplicate and companion publications
Multiple publications to the same study (e.g. protocols, trial registry entries, trial reports) will be identified and linked at the data extraction stage in Covidence systematic review management software [ 53 ]. Each study will be given a unique identifier and all linked records cited in the final report. Records will be matched using trial registry numbers. Where these are not available, we will consider author names, trial name, trial location(s) and number of participants.
Data extraction and management
Study data will be collected and managed using REDCap electronic data capture tools hosted at Monash University [ 55 , 56 ]. Three authors (MM, SB or SM) will pre-test the data extraction and coding form on 3–5 studies (as needed to achieve consistent coding), purposefully selected from the included studies to cover the diversity of data types anticipated in the review. One author (SB) will review the extracted and coded data for completeness, accuracy and consistency. Where needed, advice will be sought from the clinical advisor (SG) and biostatistician (JM) to ensure data are extracted as planned. Revisions to the data extraction form and guidance will be made as required to maximise the quality and consistency of data collection.
For each included study, one review author (MM, SB or SM) will extract study characteristics and quantitative data using a pre-tested data extraction and coding form, with a 10% random sample extracted by a second author (with further sampling if needed until 80% agreement is achieved). For studies extracted by a single author, a second author (MM, SB or SM) will independently verify the quantitative data. Discrepancies will be resolved through discussion, and advice sought from the clinical advisor (SG) or biostatistician (JM) if agreement cannot be reached or for more complex scenarios.
We will extract information relating to the characteristics of included studies and results as follows.
Study identifiers and characteristics of the study design
Study references (multiple publications arising from the same study will be matched to an index reference; code as index paper, protocol, registry entry, results paper 1, 2, …)
Study name, location, commencement date and trial registration number
Study design (categorised as ‘individually randomised’, ‘cluster randomised’, ‘cross-over’ or ‘other’)
Funding sources and funder involvement in study
Financial and non-financial interests declared by investigators
Characteristics of each intervention group (including comparator groups)
Characteristics of the intervention structured by domains of the Template for Intervention Description and Replication (TIDieR) checklist [ 57 ] (see Appendix 3 in Additional file 1 for TIDieR domains, codes and an example of coding for aromatherapy)
Number of participants: randomised to each group, at follow-up for selected outcome, and included in analysis and reasons for loss to follow-up
Characteristics of participants
Participant eligibility criteria (verbatim)
Age (e.g. mean, median, range)
Population group: coded using categories specified in the final analytic framework for the review (e.g. chronic pain, headache and migraine, cancer and advanced disease (not amenable to cure), surgery or procedures, pregnancy and childbirth, chronic or insomnia, dementia, stress, anxiety and mood disorders)
Condition: specific underlying condition as described in study (e.g. haematological tumours; rheumatoid arthritis), including information about severity (if relevant)
Treatment/procedure: applies to studies in which aromatherapy is administered for the relief of symptoms or side effects of a treatment or procedure for an underlying condition (e.g. radiotherapy; bone marrow biopsy). May include pharmacological treatment, surgical, diagnostic or other procedures (as described in study, and coded using categories specified for the review e.g. pharmacological, surgery, minor or major non-surgical procedure)
Other characteristics of importance within the context of each study
Outcomes assessed and results
Outcomes measured (a list of all outcomes [noting primary outcome(s) for study], categorised according to the broad domains specified in the final analytic framework for the review, or as ‘other’ if none of the outcome domains applies)
For outcomes selected for inclusion in the summary and synthesis of results:
Outcome domain: categorised according to the broad domains specified in the final analytic framework for the review (e.g. pain, sleep disturbances, nausea and vomiting, emotional functioning/well-being, behavioural disturbances, cognitive functioning, fatigue, health-related quality of life)
° Outcome as described in the included study (verbatim or precis)
° Measurement method (e.g. Rotterdam Symptom Checklist, used to measure psychological and physical aspects of quality of life for people with cancer), information required to interpret the measure (scale range and direction, minimally important difference) and time point (exact, and time-frame categorised as ‘immediate’ or ‘longest follow-up’)
° Results including summary statistics by group (means and standard deviations, or number of events for cognitive outcomes that have been dichotomised, and sample size), estimates of intervention effect (e.g. mean differences (or adjusted mean differences), confidence intervals, t -values, p -values or risk ratios/odds ratios for binary outcomes)
° Data required to support risk of bias judgements (see the ‘ Assessment of risk of bias of included studies ’ section) [ 58 ]
Assessment of risk of bias of included studies
Assessment of risk of bias in rcts.
We will assess the risk of bias in included studies using the revised Cochrane ‘Risk of Bias’ tool (RoB 2) for randomised trials [ 44 , 58 ] for each critical (or important) outcome included in the synthesis. Our assessment will be based on the effect of assignment to the intervention .
RoB 2 addresses five domains:
Bias arising from the randomisation process
Bias due to deviations from intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
To promote concordance, the assessment will be piloted by three review authors (MM, SB, SMc) on 3–5 studies until consistent judgements are achieved across a range of scenarios. One review author (MM, SB or SMc) will then apply the tool to the selected results from each study following the RoB 2 guidance [ 44 ], and a second author will verify the assessments (SB or SMc). Supporting information and justifications for judgements for each domain (low, some concerns, high risk of bias) will be recorded. We will derive an overall summary of the risk of bias from each assessment, following the algorithm in the RoB 2 guidance [ 45 ]. Disagreement between review authors will be resolved through discussion, and a third review author (SB, SM or JM) will adjudicate where agreement cannot be reached. For cluster trials and cross-over trials, we will use the variant of the RoB 2 tool specific for the design [ 59 ].
When multiple effects of the intervention using different approaches are presented in the trial report, we will select one effect for inclusion in the meta-analysis and for risk of bias assessment. The selected effect will be chosen according to the following hierarchy, which orders the approaches from (likely) least to most biased for estimating the effect of assignment to the intervention : (1) the effect that corresponds to a full intention-to-treat analysis, where missing data have been multiply imputed, or a model-based approach has been used (e.g. likelihood-based analysis, inverse-probability weighting); (2) the effect corresponding to an analysis that adheres to intention-to-treat principles except that the missing outcome data are excluded; (3) the effect that corresponds to a full intention-to-treat analysis, where missing data have been imputed using methods that treat the imputed data as if they were observed (e.g. last observation carried forward, mean imputation, regression imputation, stochastic imputation); or (4) the effect that corresponds to an ‘as-treated’ or ‘per-protocol’ analysis, or an analysis from which eligible trial participants were excluded [ 58 , 59 ].
Measures of treatment effect
We anticipate that many of the outcomes will be continuous (e.g. pain, anxiety) and that varying measurement instruments will be used to measure the same underlying construct across the studies. For this reason, we will quantify the effects of aromatherapy using the standardised mean difference (SMD) (implementing the Hedges’ adjusted g version). In trials where a continuous measure has been dichotomised (e.g. a continuous pain scale is dichotomised into improvement or no improvement) and analysed as binary outcomes, we will re-express reported, or calculated, odds ratios as SMDs [ 60 ]. For dichotomous outcomes, we will quantify the effects of aromatherapy using risk ratios (RR). Given the wide range of conditions and outcomes in this review, it is not possible to specify specific thresholds for interpreting the size of the effect for each outcome. Given this, we plan to use Cohen’s guiding rules for SMDs where 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect [ 61 ]. Where a valid and reliable minimal important difference (MID) is available for a familiar measure of relevance to the population groups in the meta-analysis, we will re-express the SMD in units of the measure and interpret the effect in relation to the MID if feasible to do so [ 61 ]. For dichotomous outcomes, we will seek advice from the NTWC on interpreting the size of the effect (seeking agreement on a threshold for a small but important difference).
Unit of analysis issues
In this review, unit of analysis issues may arise from non-standard designs (cluster trials, cross-over trials) or from trials with more than two eligible intervention groups. In the following, we outline the methods for making adjustments when necessary. Any adjustments will be documented (e.g. assumed intra-cluster correlation and average cluster size). We will also report when necessary adjustments were unable to be made due to missing information.
For cluster randomised trials that have not appropriately accounted for correlation in observations within clusters, we will attempt a re-analysis. We will do this by inflating the variance of the intervention estimates by a design effect (DEFF). The DEFF is calculated from two quantities — an intra-cluster correlation (ICC) and the average cluster size. Estimates of ICC will be imputed from other cluster trials included in the review, where possible, or by using external estimates from empirical research (e.g. Bell [ 62 ]). The average cluster size will be calculated from reported information in the trial.
For cross-over trials where an appropriate paired analysis is not available, we will attempt to approximate a paired analysis by imputing missing statistics (e.g. correlation). Estimates of the missing statistics will be imputed from other cross-over trials included in the review, where possible, or by using external estimates from empirical research (e.g. Balk [ 63 ]).
For trials where more than one comparison from the same trial is eligible for inclusion in the same meta-analysis (e.g. lavender oil, ginger oil, control), we will combine intervention groups, where it makes sense to do so; otherwise, we will appropriately reduce the sample size so that the same participants do not contribute more than once.
Dealing with missing data
We will not contact trial authors to obtain missing information (e.g. study characteristics, description of conduct of the trial) or aggregate level statistics (e.g. missing standard deviations). However, we will attempt to calculate statistics necessary for meta-analysis using algebraic manipulation of reported statistics (e.g. computing the standard error for the treatment effect from a reported p -value). When standard deviations cannot be calculated from available statistics, but interquartile ranges or ranges are reported, we will use the formula in Wan et al. [ 64 ] to estimate approximate standard deviations. When neither of the above methods are possible, we will impute the standard deviation using the average standard deviation across trials included in the same meta-analysis that have used the same measurement tool. When means are missing, but medians are reported, we will use the formula in Wan et al. [ 64 ] to estimate approximate means.
Our approach for dealing with missing outcome data within the primary trials will be through sensitivity analyses, where trials judged to be at a high or unclear risk of bias will be excluded (see the ‘ Data synthesis ’ section). Risk of bias ‘due to missing outcome data’ is considered within the overall bias judgement for a trial.
Assessment of heterogeneity
We will assess statistical heterogeneity of the intervention effects visually by inspecting the overlap of confidence intervals on the forest plots, formally test for heterogeneity using the χ 2 test (using a significance level of α = 0.1), and quantify heterogeneity using the I 2 statistic [ 65 ].
Assessment of biases due to missing results
We will use a framework for assessing risk of bias due to missing results in which an assessment is made for each meta-analysis regarding the risk and potential impact of missing results from studies (termed ‘known-unknowns’) and the risk of missing studies (termed ‘unknown-unknowns’) [ 66 ]. We will use this framework to guide our assessments of whether there is ‘undetected’ or ‘suspected’ reporting bias for each of the comparisons in our GRADE assessment (see the ‘ Summary of findings tables and assessment of the certainty of the body of evidence ’ section).
In assessing ‘known-unknowns’, we will determine what trials meeting the inclusion criteria for the particular meta-analysis have missing results through examination of the publication’s methods section, trial registry entry (if available) and trial protocol (if available). We will make an assessment as to whether the missing result was potentially due the result itself (e.g. ‘not statistically significant’), and whether inclusion of the result could lead to a notable change in the meta-analysis (e.g. if the missing result is from a large trial). We will also assess the impact of missing results from studies reported in languages other than English that were judged as being likely to meet the eligibility criteria for each synthesis (see the ‘ Types of studies ’ and ‘ Selection of studies ’ sections).
In assessing ‘unknown-unknowns’, we will judge whether the trials not identified were likely to have results eligible for inclusion (e.g. for broad outcome domains such as ‘pain’, it is likely that for particular conditions, missing studies would have been eligible for inclusion). We will use funnel plots and contour-enhanced funnel plots to examine whether there is evidence of small-study effects [ 67 ]. If there is funnel plot asymmetry, we will undertake a sensitivity analysis to compare the combined effect estimated from the random-effects model (primary analysis) with that estimated from a fixed (common) effect model. If the random-effects estimate is importantly larger than the fixed-effect estimate, with no explanation for the difference (e.g. differences in clinical populations or intensity of the delivery of intervention between small and large trials, differences in risk of bias between small and large trials), then we will downgrade for ‘suspected’ reporting bias.
Data synthesis
- Meta-analysis
Separate comparisons will be set up based on outcome domains agreed in the final framework (see Fig. 2 and Appendix 2 in Additional file 1 for indicative groupings). These comparisons will be stratified by the population groups in the final framework, the basis for which may relate to symptoms (e.g. chronic pain), treatment for an underlying condition (e.g. patients undergoing surgery) or the underlying condition (e.g. chronic insomnia, dementia) (see Fig. 2 and the ‘ Types of participants ’ section for indicative groupings). This approach to structuring the meta-analysis will yield an overall estimate of the effect of aromatherapy for the outcome (review objectives 1, 2 and 4), as well as estimates within each population group (review objective 3). Subgroup analysis by population group will allow examination of whether these population groups explain any observed statistical heterogeneity in the intervention effects (see the ‘Subgroup analysis and investigation of heterogeneity’ section).
We will combine the effects using a random-effects meta-analysis model, since we expect there to be clinical and methodological diversity across the trials that may lead to statistical heterogeneity. These analyses will use the restricted maximum likelihood estimator (REML) of between trial heterogeneity variance and the Hartung-Knapp-Sidik-Jonkman confidence interval method.
Forest plots will be used to visually depict the intervention effect estimates and their confidence intervals. Forest plots will be stratified by condition and risk of bias (within population group).
Summary and synthesis when meta-analysis is not possible
Available effect estimates (95% confidence intervals, p -values), details of scales (direction and range), risk of bias assessments and intervention characteristics will be tabulated. Tables will be ordered by outcome domain, population group and risk of bias assessment.
For a particular comparison, if we are unable to analyse most of the effect estimates (due to incomplete reporting of effects and their variances, variability in the effect measures across the studies), we will consider alternative synthesis methods, such as calculating summary statistics of the effect estimates, combining p -values or vote counting based on the direction of effect [ 68 ]. Our choice of method will be determined by the available data (e.g. summary statistics if data permit; other methods if the data are more limited).
Subgroup analysis and investigation of heterogeneity
We will undertake a subgroup analysis to examine whether population group explains any observed statistical heterogeneity in the intervention effects (see Fig. 2 and the ‘ Types of participants ’ section for indicative population groupings and Appendix 2 in Additional file 1 for the subset of outcomes for which different population subgroups may be relevant). In addition, for the comparison aromatherapy versus inactive comparator, we will consider whether mode of delivery (massage or ‘other’) explains any observed statistical heterogeneity in the intervention effects.
Sensitivity analyses
We plan to undertake and report sensitivity analyses examining if the meta-analysis estimates are robust to the:
Meta-analysis model . In addition to fitting a random-effects model, we will fit fixed-effect models. This analysis will be undertaken to investigate the impact of any small-study effects.
Inclusion of trials judged to be at an overall high or unclear risk of bias. We will exclude trials judged to be at an overall high or unclear risk of bias.
Results of the sensitivity analyses will be tabulated, including the meta-analysis estimate (and its confidence interval), along with details of the original and sensitivity analysis assumptions.
Summary of findings tables and assessment of the certainty of the body of evidence
We will prepare GRADE summary of findings tables for each of the main comparisons, reporting results for critical and important outcome domains (up to seven). For each result, one author (SB) will use the GRADE approach to assess our confidence in where the effect lies relative to our threshold for a small effect (the certainty of evidence) (see the ‘ Measures of treatment effect ’ section). In accordance with detailed GRADE guidance [ 37 , 69 , 70 ], an overall GRADE of high, moderate, low or very low certainty will be reported for each result based on whether there are serious, very serious or no concerns in relation to each of the following domains.
Risk of bias. We will assess the overall risk of bias across all studies contributing to each synthesised result, considering the weight studies rated at high risk of bias contribute to the analysis. Serious or very serious concerns are more likely if studies at high risk of bias contribute considerable weight in the analysis and sensitivity analyses indicate that removing these studies changes the size of the effect (see the ‘Sensitivity analyses’ section).
Inconsistency. We will assess whether there is important, unexplained inconsistency in results across studies considering the overlap of confidence intervals (non-overlap indicating potentially important differences in direction or size of effect), statistical measures that quantify and test for heterogeneity (I 2 statistic, χ 2 test) and, where relevant, results of subgroup analyses (see the ‘ Assessment of heterogeneity ’ section). Where a result is based on a single study, inconsistency will not be rated.
Imprecision. We will assess whether the confidence interval for each pooled effect estimate is wide (e.g. including a small effect and little or no difference, which would lead to different interpretations) and, for large effects, whether the sample size meets the optimal information size (based on number of events for binary outcomes; sample size for continuous outcomes). In judging imprecision, we will use our threshold specified for a small effect (see the ‘ Measures of treatment effect ’ section).
Indirectness. We will assess whether there are important differences between the characteristics of studies included in each synthesis and the question we are seeking to address, such that the effects observed may not apply to our question (i.e. the applicability of the evidence). For example, differences between the interventions delivered and aromatherapy practice in Australia that are likely to influence the size of effect.
Publication bias. Our judgement of suspected publication bias will be based on assessment of bias due to missing results (see the ‘ Assessment of biases due to missing results ’ section). In these assessments, we will consider the potential impact on each synthesised result of excluding studies in languages other than English.
Upgrading domains (large effect size, dose response gradient, opposing plausible residual confounding). There is no precedent for rating up the evidence from randomised trials; however, in principle, these domains apply to any body of evidence so are included here for completeness.
Using GRADE decision rules, we will derive an overall GRADE for the certainty of evidence for each result included in the summary of findings table [ 70 ]. A result from a body of evidence comprised of randomised trials begins as ‘high’ certainty evidence (score = 4) and can be rated down (−1 or −2) for serious or very concerns on any GRADE domain that reduces confidence that aromatherapy has at least a small effect (as determined by the pre-specified thresholds) [ 61 , 69 , 70 ].
Summary of findings tables will be prepared using the GRADEpro GDT software [ 71 ]. The tables will include:
Estimates of the effects of aromatherapy reported as standardised mean differences, and for binary outcomes relative and absolute effects
The overall GRADE (rating of certainty) and an explanation of the reason(s) for rating down (or up) [ 68 ]
The study design(s), number of studies and number of participants contributing data
A plain language statement interpreting the evidence for each comparison and outcome, following GRADE guidance for writing informative statements [ 72 ].
We will present the four levels of certainty of evidence in summary of findings tables with the following symbols and interpretations.
High ( ⊕ ⊕ ⊕ ⊕ ): further research is very unlikely to change the confidence in the estimate of effect
Moderate ( ⊕ ⊕ ⊕ ⊝ ): further research is likely to have an important impact in the confidence in the estimate of effect
Low ( ⊕ ⊕ ⊝ ⊝ ): further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate
Very low ( ⊕ ⊝ ⊝ ⊝ ): any estimate of effect is very uncertain
Availability of data and materials
This is a protocol for a systematic review and does not contain any data. Requests for other material should be sent to the corresponding author.
Abbreviations
Allied and Complementary Medicine Database
Cochrane Central Register of Controlled Trials
Cumulative Index of Nursing and Allied Health Literature
- Complementary medicine
Core Outcome Measures in Effectiveness Trials
Design effect
Grading of Recommendations, Assessment, Development and Evaluation
International Aromatherapy and Aromatic Medicine Association
Intra-cluster correlation
International Classification of Diseases 11th Revision
International Clinical Trials Registry Platform
Medical Subject Headings
Minimal important difference
National Health and Medical Research Council
Non-randomised study of interventions
Natural Therapies Review Expert Advisory Panel
Natural Therapies Working Committee
Population, intervention, comparator, outcome
Practitioner Research and Collaboration Initiative
Preferred Reporting Items for Systematic review and Meta-Analyses
Preferred Reporting Items for Systematic review and Meta-Analyses Protocols
Randomised controlled trial
Restricted maximum likelihood estimator
Risk ratios
Standardised mean difference
Template for Intervention Description and Replication
Therapeutic Goods Administration
National Health and Medical Research Council. Statement of requirement: evidence evaluations for review of natural therapies (tranche two). 2020.
Google Scholar
Posadzki P, Watson LK, Alotaibi A, Ernst E. Prevalence of use of complementary and alternative medicine (CAM) by patients/consumers in the UK: systematic review of surveys. Clin Med (Lond). 2013;13(2):126–31. https://doi.org/10.7861/clinmedicine.13-2-126 .
Article Google Scholar
Harnett JE, McIntyre E, Steel A, Foley H, Sibbritt D, Adams J. Use of complementary medicine products: a nationally representative cross-sectional survey of 2019 Australian adults. BMJ Open. 2019;9(7):e024198. https://doi.org/10.1136/bmjopen-2018-024198 .
Article PubMed PubMed Central Google Scholar
Steel A, McIntyre E, Harnett J, Foley H, Adams J, Sibbritt D, et al. Complementary medicine use in the Australian population: results of a nationally-representative cross-sectional survey. Sci Rep. 2018;8(1):17325. https://doi.org/10.1038/s41598-018-35508-y .
Article CAS PubMed PubMed Central Google Scholar
National Health and Medical Research Council. Aromatherapy description developed in conversation with the National Health and Medical Research Council’s Natural Therapies Working Committee Chair and the Department of Health’s Natural Therapies Review Expert Advisory Panel (February 2020). 2020.
PDQ Integrative Alternative Complementary Therapies Editorial Board. Aromatherapy with essential oils (PDQ®): health professional version. PDQ cancer information summaries. Bethesda: National Cancer Institute (US); 2019.
International Federation of Professional Aromatherapists (IFPA). 2021. http://www.ifparoma.org/ . Accessed 4 Feb 2021.
Canadian Federation of Aromatherapists (CFA). About us. 2021. https://www.cfacanada.com/pages/about . Accessed 4 Feb 2021.
International Aromatherapy and Aromatic Medicine Association (IAAMA). About aromatherapy. 2021. https://www.iaama.org.au/about-aromatherapy.html . Accessed 4 Feb 2021.
National Association for Holistic Aromatherapy. About NAHA. 2021. https://naha.org/about/ . Accessed 4 Feb 2021.
Tisserand R, Young R. Essential oil safety: a guide for health care professionals. 2nd ed. Edinburgh: Elsevier Limited; 2014.
Orchard A, van Vuuren SF. Carrier oils in dermatology. Arch Dermatol Res. 2019;311(9):653–72. https://doi.org/10.1007/s00403-019-01951-8 .
Article PubMed Google Scholar
Australian Health Practitioner Regulatory Association: What we do. 2021. https://www.ahpra.gov.au/About-Ahpra/What-We-Do.aspx . Accessed 4 Feb 2021.
Steel A, Leach M, Wardle J, Sibbritt D, Schloss J, Diezel H, et al. The Australian complementary medicine workforce: a profile of 1,306 practitioners from the PRACI study. J Altern Complement Med. 2018;24(4):385–94. https://doi.org/10.1089/acm.2017.0206 .
International Aromatherapy & Aromatic Medicine Association. About IAAMA. 2021. https://www.iaama.org.au/about-iaama.html . Accessed 4 Feb 2021.
Australian Traditional Medicine Society. Australian Traditional Medicine Society (ATMS): about us. 2021. https://www.atms.com.au/about-us . Accessed 4 Feb 2021.
Therapeutic Goods Administration. An overview of the regulation of complementary medicines in Australia: Australian Government Department of Health; 2013. https://www.tga.gov.au/overview-regulation-complementary-medicines-australia . Accessed 4 Feb 2021
Sanger GJ, Andrews PLR. A history of drug discovery for treatment of nausea and vomiting and the implications for future research. Front Pharmacol. 2018;9:913. https://doi.org/10.3389/fphar.2018.00913 .
Koyama S, Heinbockel T. The effects of essential oils and terpenes in relation to their routes of intake and application. Int J Mol Sci. 2020;21(5):1558. https://doi.org/10.3390/ijms21051558 .
Article CAS PubMed Central Google Scholar
Block E. What’s that smell? A controversial theory of olfaction deemed implausible: The Conversation; 2015. https://theconversation.com/whats-that-smell-a-controversial-theory-of-olfaction-deemed-implausible-42449
Orchard A, van Vuuren S. Commercial essential oils as potential antimicrobials to treat skin diseases. Evid Based Complement Alternat Med. 2017;2017:4517971. https://doi.org/10.1155/2017/4517971 .
Peterfalvi A, Miko E, Nagy T, Reger B, Simon D, Miseta A, et al. Much more than a pleasant scent: a review on essential oils supporting the immune system. Molecules. 2019;24(24):4530. https://doi.org/10.3390/molecules24244530 .
Vosshall LB. Laying a controversial smell theory to rest. Proc Natl Acad Sci. 2015;112(21):6525–6. https://doi.org/10.1073/pnas.1507103112 .
Ball EL, Owen-Booth B, Gray A, Shenkin SD, Hewitt J, McCleery J. Aromatherapy for dementia. Cochrane Database Syst Rev. 2020;(8). https://doi.org/10.1002/14651858.CD003150.pub3 .
Candy B, Armstrong M, Flemming K, Kupeli N, Stone P, Vickerstaff V, et al. The effectiveness of aromatherapy, massage and reflexology in people with palliative care needs: a systematic review. Palliat Med. 2020;34(2):179–94. https://doi.org/10.1177/0269216319884198 .
Hines S, Steels E, Chang A, Gibbons K. Aromatherapy for treatment of postoperative nausea and vomiting. Cochrane Database Syst Rev. 2018;(3). https://doi.org/10.1002/14651858.CD007598.pub3 .
Stea S, Beraudi A, De Pasquale D. Essential oils for complementary treatment of surgical patients: state of the art. Evid Based Complement Alternat Med. 2014;2014:726341. https://doi.org/10.1155/2014/726341 .
Steel A. PRACI study: unpublished data; 2021.
Armstrong M, Flemming K, Kupeli N, Stone P, Wilkinson S, Candy B. Aromatherapy, massage and reflexology: a systematic review and thematic synthesis of the perspectives from people with palliative care needs. Palliat Med. 2019;33(7):757–69. https://doi.org/10.1177/0269216319846440 .
Steel A, Schloss J, Diezel H, Palmgren PJ, Maret JB, Filbet M. Complementary medicine visits by palliative care patients: a cross-sectional survey. BMJ Support Palliat Care. 2020:bmjspcare-2020-002269. https://doi.org/10.1136/bmjspcare-2020-002269 .
Smith CA, Collins CT, Crowther CA. Aromatherapy for pain management in labour. Cochrane Database Syst Rev. 2011;(7). https://doi.org/10.1002/14651858.CD009215 .
Johnson JR, Rivard RL, Griffin KH, Kolste AK, Joswiak D, Kinney ME, et al. The effectiveness of nurse-delivered aromatherapy in an acute care setting. Complement Ther Med. 2016;25:164–9. https://doi.org/10.1016/j.ctim.2016.03.006 .
Steel A, Schloss J, Leach M, Adams J. The naturopathic profession in Australia: a secondary analysis of the Practitioner Research and Collaboration Initiative (PRACI). Complement Ther Clin Pract. 2020;40:101220. https://doi.org/10.1016/j.ctcp.2020.101220 .
Green S, Hill M, Kim M, Kramer S, McDonald S, McKenzie J, et al. Evaluation of evidence about the effectiveness of aromatherapy: an overview of systematic reviews, Report prepared for the National Health and Medical Research Council by Cochrane Australia: Monash University; 2014.
Koo M. A bibliometric analysis of two decades of aromatherapy research. BMC Res Notes. 2017;10(1):46. https://doi.org/10.1186/s13104-016-2371-1 .
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020): Cochrane; 2020. www.training.cochrane.org/handbook
Schünemann HJ, Brozek J, Guyatt G, Oxman AD, editors. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Hamilton: McMaster University; 2013.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. https://doi.org/10.1186/2046-4053-4-1 .
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. https://doi.org/10.1136/bmj.g7647 .
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71 .
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. https://doi.org/10.1136/bmj.n160 .
Dodd S, Clarke M, Becker L, Mavergames C, Fish R, Williamson PR. A taxonomy has been developed for outcomes in medical research to help improve knowledge discovery. J Clin Epidemiol. 2018;96:84–92. https://doi.org/10.1016/j.jclinepi.2017.12.020 .
International statistical classification of diseases and related health problems (11th ed). World Health Organisation; 2019. https://www.who.int/classifications/classification-of-diseases . Accessed 15 Jan 2021.
Higgins JPT, Savovic J, Page MJ, Sterne JAC. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). 2019. https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2 . Accessed 8 Feb 2021.
Evidence-based medicine: literature reviews. National Centre for Complementary and Integrative Health, National Insitute of Health; 2022. https://www.nccih.nih.gov/health/providers/litreviews . Accessed 20 Sept 2021.
McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnson RV, Thomas J. Chapter 3: defining the criteria for including studies and how they will be grouped for the synthesis. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Welch V, editors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester: Wiley; 2019.
How CENTRAL is created. Cochrane. 2021. https://www.cochranelibrary.com/central/central-creation . Accessed 7 Feb 2021.
Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane Centralised Search Service showed high sensitivity identifying randomized controlled trials: a retrospective analysis. J Clin Epidemiol. 2020;127:142–50. https://doi.org/10.1016/j.jclinepi.2020.08.008 .
Article CAS PubMed Google Scholar
Horsley T, Dingwall O, Sampson M. Checking reference lists to find additional studies for systematic reviews. Cochrane Database Syst Rev. 2011;(8):MR000026. https://doi.org/10.1002/14651858.MR000026.pub2 .
Briscoe S, Bethel A, Rogers M. Conduct and reporting of citation searching in Cochrane systematic reviews: a cross-sectional study. Res Synth Methods. 2020;11(2):169–80. https://doi.org/10.1002/jrsm.1355 .
Wright K, Golder S, Rodriguez-Lopez R. Citation searching: a systematic review case study of multiple risk behaviour interventions. BMC Med Res Methodol. 2014;14:73. https://doi.org/10.1186/1471-2288-14-73 .
Cooper C, Booth A, Britten N, Garside R. A comparison of results of empirical studies of supplementary search techniques and recommendations in review methodology handbooks: a methodological review. Syst Rev. 2017;6(1):234. https://doi.org/10.1186/s13643-017-0625-1 .
Covidence systematic review software. Melbourne: Veritas Health Innovation. www.covidence.org . Accessed 20 Sept 2021.
National Health and Medical Research Council (NHMRC). Draft framework for protocols for systematic reviews of randomised controlled trials and non-randomised studies of interventions. Canberra: National Health and Medical Research Council (NHMRC); 2020.
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. https://doi.org/10.1016/j.jbi.2019.103208 .
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. https://doi.org/10.1016/j.jbi.2008.08.010 .
Hoffmann T, Glasziou P, Barbour V, Macdonald H. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;1687:1–13. https://doi.org/10.1136/bmj.g1687 .
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. https://doi.org/10.1136/bmj.l4898 .
Higgins JPT, Eldridge SM, Li T. Chapter 23: including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al., editors. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020): Cochrane; 2020. www.training.cochrane.org/handbook .
Chinn S. A simple method for converting an odds ratio to effect size for use in meta-analysis. Stat Med. 2000;19(22):3127–31. https://doi.org/10.1002/1097-0258 .
Schünemann HJ, Vist GE, Higgins J, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Welch V, editors. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020): Cochrane; 2019. www.training.cochrane.org/handbook .
Bell ML, McKenzie JE. Designing psycho-oncology randomised trials and cluster randomised trials: variance components and intra-cluster correlation of commonly used psychosocial measures. Psychooncology. 2013;22(8):1738–47. https://doi.org/10.1002/pon.3205 .
Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical assessment of within-arm correlation imputation in trials of continuous outcomes. Methods research report, Prepared by the Tufts Evidence-based Practice Center under Contract No. 290-2007-10055-I. AHRQ Publication No. 12(13)-EHC141-EF. Rockville: Agency for Healthcare Research and Quality; 2012. https://www.ncbi.nlm.nih.gov/books/NBK115797/
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. https://doi.org/10.1186/1471-2288-14-135 .
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.org/10.1002/sim.1186 .
Page M, Higgins J, Sterne J. Chapter 13: assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020); 2020. www.training.cochrane.org/handbook .
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol. 2008;61(10):991–6. https://doi.org/10.1016/j.jclinepi.2007.11.010 .
McKenzie JE, Brennan SE. Chapter 12: synthesizing and presenting findings using other methods. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Welch V, editors. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020): Cochrane; 2020. https://training.cochrane.org/handbook .
Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol. 2017;87(Epub 2017 May 18):4–17. https://doi.org/10.1016/j.jclinepi.2017.05.006 .
Schünemann HJ, Higgins J, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence. In: Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Welch V, editors. Cochrane handbook for systematic reviews of interventions version 6.1 (updated September 2020): Cochrane; 2020. https://training.cochrane.org/handbook .
GRADEpro GDT: GRADEpro guideline development tool. McMaster University and Evidence Prime; 2021. www.gradepro.org . Accessed 20 Sept 2021.
Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, et al. Improving GRADE Evidence Tables part 3: guidance for useful GRADE certainty in the evidence judgments through explanatory footnotes. J Clin Epidemiol. 2016. https://doi.org/10.1016/j.jclinepi.2015.12.006 .
Download references
Acknowledgements
We are grateful to the staff of the ONHMRC for their advice and feedback on the draft protocol and for contributions to text describing the context for 2019-20 Review of Natural Therapies. We thank the NTWC and the NTREAP for their feedback on the draft protocol. We thank John Liman, Senior Software Engineer at Helix, Monash University, for his assistance in developing our data extraction tools in REDCap and Sally Green for contributions to the funding application.
This review was commissioned and funded by the Australian Government Department of Health via the NHMRC under Official Order 2020-21P030 to update the evidence underpinning the 2015 Review of the Australian Government Rebate on Natural Therapies for Private Health Insurance (2015 Review) by the Department of Health (Department). The design and conduct of the review will be done in consultation with the Office of NHMRC (ONHMRC), the NHMRC’s Natural Therapies Working Committee (the Committee) and Department’s Natural Therapies Review Expert Advisory Panel (NTREAP). The NHMRC contracted independent methodological experts to undertake peer review of the protocol and the review report. SB, SM and MM are staff of Cochrane Australia which is funded by the Australian Government through the NHMRC. JEM is supported by an NHMRC Career Development Fellowship (1143429).
Author information
Authors and affiliations.
School of Public Health and Preventive Medicine, Monash University, 553 St Kilda Road, Melbourne, VIC, 3004, Australia
Sue E. Brennan, Steve McDonald, Melissa Murano & Joanne E. McKenzie
You can also search for this author in PubMed Google Scholar
Contributions
Design of the SR and writing of the original draft protocol was led by SB (overall), SM (search methods and study selection) and JM (analysis plan and question specification). Development of the data extraction tools in REDCap was performed by MM. All authors provided critical review and editing of drafts of the protocol. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Sue E. Brennan .
Ethics declarations
Ethics approval and consent to participate.
Not required.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix 1..
Database search strategies. Appendix 2. Example outcome domain. Appendix 3. TIDieR domains and example of application in aromatherapy systematic review.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Brennan, S.E., McDonald, S., Murano, M. et al. Effectiveness of aromatherapy for prevention or treatment of disease, medical or preclinical conditions, and injury: protocol for a systematic review and meta-analysis. Syst Rev 11 , 148 (2022). https://doi.org/10.1186/s13643-022-02015-1
Download citation
Received : 26 May 2022
Accepted : 01 July 2022
Published : 26 July 2022
DOI : https://doi.org/10.1186/s13643-022-02015-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Aromatherapy
- Essential oil therapy
- Volatile oils
- Natural therapies
- Complementary and alternative medicine
- Integrative medicine
- Supportive therapy
- Systematic review
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
Essential Oils and Health
Affiliations.
- 1 Campbell University School of Osteopathic Medicine, Lillington, NC.
- 2 Reproductive and Developmental Biology Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC.
- PMID: 32607090
- PMCID: PMC7309671
Essential oils (EOs) have risen in popularity over the past decade. These oils function in society as holistic integrative modalities to traditional medicinal treatments, where many Americans substitute EOs in place of other prescribed medications. EOs are found in a multitude of products including food flavoring, soaps, lotions, shampoos, hair styling products, cologne, laundry detergents, and even insect repellents. EOs are complex substances comprised of hundreds of components that can vary greatly in their composition depending upon the extraction process by the producer or the origin of the plant. Thus, making it difficult to determine which pathways in the body are affected. Here, we review the published research that shows the health benefits of EOs as well as some of their adverse effects. In doing so, we show that EOs, as well as some of their individual components, possess antimicrobial, antiviral, antibiotic, anti-inflammatory, and antioxidant properties as well as purported psychogenic effects such as relieving stress, treating depression, and aiding with insomnia. Not only do we show the health benefits of using EOs, but we also indicate risks associated with their use such as their endocrine disrupting properties leading to the induction of premature breast growth in young adolescents. Taken together, there are many positive and potentially negative risks to human health associated with EOs, which make it important to bring awareness to all their known effects on the human body.
Keywords: Endocrine disruptors; anti-inflammatory; antimicrobial; gynecomastia; prepubertal; psychological.
Copyright ©2020, Yale Journal of Biology and Medicine. This work is written in part by US Government employees and is in the public domain in the US.
Publication types
- Research Support, N.I.H., Intramural
- Aromatherapy / methods*
- Medicine, Traditional / methods
- Oils, Volatile* / adverse effects
- Oils, Volatile* / pharmacology
- Risk Assessment
- Oils, Volatile
Grants and funding
- ZIA ES070065/ImNIH/Intramural NIH HHS/United States
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 15 August 2017
The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells
- Andrea Puškárová 1 ,
- Mária Bučková 1 ,
- Lucia Kraková 1 ,
- Domenico Pangallo 1 &
- Katarína Kozics 2
Scientific Reports volume 7 , Article number: 8211 ( 2017 ) Cite this article
146 Citations
21 Altmetric
Metrics details
- Cell biology
- Environmental microbiology
Six essential oils (from oregano, thyme, clove, lavender, clary sage, and arborvitae) exhibited different antibacterial and antifungal properties. Antimicrobial activity was shown against pathogenic ( Escherichia coli , Salmonella typhimurium , Yersinia enterocolitica , Staphylococcus aureus , Listeria monocytogenes , and Enterococcus faecalis ) and environmental bacteria ( Bacillus cereus , Arthrobacter protophormiae , Pseudomonas fragi ) and fungi ( Chaetomium globosum, Penicillium chrysogenum , Cladosporium cladosporoides , Alternaria alternata , and Aspergillus fumigatus) . Oregano, thyme, clove and arborvitae showed very strong antibacterial activity against all tested strains at both full strength and reduced concentrations. These essential oils showed different fungistatic and fungicidal activities when tested by direct application and in the vapor phase. The genotoxic effects of these oils on HEL 12469 human embryo lung cells were evaluated using an alkaline comet assay for the first time, revealing that none of the oils induced significant DNA damage in vitro after 24 h. This study provides novel approaches for assessing the antimicrobial potential of essential oils in both direct contact and the vapor phase and also demonstrates the valuable properties of the phenol-free arborvitae oil. These results suggest that all the tested essential oils might be used as broad-spectrum anti-microbial agents for decontaminating an indoor environment.
Similar content being viewed by others

Cytotoxicity, early safety screening, and antimicrobial potential of minor oxime constituents of essential oils and aromatic extracts

Essential oil composition and antimicrobial potential of aromatic plants grown in the mid-hill conditions of the Western Himalayas

Chemical composition and biological activity of Peucedanum dhana A. Ham essential oil
Introduction.
Essential oils (EOs) are products derived from aromatic plants which contain around 20–60 components at quite different concentrations 1 . Their most common constituents are terpenes, aromatic and aliphatic compounds (especially alcohols, esters, ethers, aldehydes, ketones, lactones, phenols and phenol ethers) 1 .
EOs from Origanum vulgare L., Thymus vulgaris L., Salvia sclarea L., and Lavandula angustifolia Mill. belonging to the Lamiaceae family have been used for their medicinal properties 1 for centuries; they possess antibacterial, antifungal 2 , 3 , 4 , 5 , antioxidant, anti-inflammatory 6 , 7 and analgesic properties 7 . Clove EO from Eugenia caryophyllata L. ( Myrtaceae ) has shown antibacterial, antifungal, anti-oxidant 8 and anti-inflammatory effects 9 . EO from Thuja plicata ( Cupressaceae ) has been tested for antimicrobial 10 , 11 and insecticidal activity 12 .
The antibacterial properties of EOs have been observed in several studies 1 , 13 , 14 . Most of the studies have examined the direct effect of EOs on a range of microorganisms. For example several Gram-negative and Gram-positive bacteria are sensitive to various EOs 2 , 3 , 14 , 15 , 16 , showing clear zones on agar assays in which the tested EO inhibits the growth of a particular microorganism. Some studies also determined the minimal inhibition and minimal bactericidal concentrations in liquid medium 11 , 17 .
However, EOs can also exist in a potentially highly bioactive vapor phase, and some EOs have shown antimicrobial activity that does not require direct contact with the EO 18 , 19 , 20 , 21 . The vapor phase seems especially effective against fungi, and a number of studies have shown that EOs are more effective antifungals in the vapor state than in the liquid 20 , 21 , 22 . One possible explanation for this behavior is that the lipophilic molecules responsible for at least part of the activity might associate in the aqueous phase to form micelles, thereby suppressing their attachment to the organism, whereas the vapor phase allows free attachment 22 . In this situation, the observed antimicrobial activity arising from the easily volatilized components would result from a combination of the direct exposure to the vapor and the indirect exposure mediated by agar medium which absorbed the vapor 23 . Moreover, fungal strains tend to grow more on the agar surface than bacteria, and therefore would be more exposed to the vapor while the bacteria would be more strongly affected by the EO components that accumulated in the substrate.
EOs with biocidal activity were used to develop alternative disinfection strategies for indoor environments or in the food industry, on contaminated surfaces and equipment in food processing environments 15 , 24 , 25 , 26 . The ability of some EOs to prevent the formation of Listeria monocytogenes 15 and Salmonella enterica 26 biofilm on stainless steel surfaces has previously been demonstrated.
Although EOs were applied in the past to successfully treat a variety of diseases and to preserve health, they have been used more frequently for a greater variety of applications in recent years, including drugs, crop protectants, food additives, aromatherapy, and others. The resulting increase in human exposure as a consequence of this expanded usage therefore requires a careful re-assessment of their toxicity and genotoxicity on the level of mammalian cells 27 . The potential toxic effects of plant extracts, including EOs, on humans should not be underestimated. The mutagenicity of many plant extracts and their possible genotoxicity 28 , 29 , 30 , 31 , 32 have been evaluated previously. There are several studies examining the genotoxic properties of EOs 29 , 33 , 34 , 35 , but there is not nearly enough information about the potential risk of sensitization when using EOs.
The purpose of this study was to determine the antimicrobial properties of six EOs ( O . vulgare , T . vulgaris , S . sclarea , L . angustifolia , E . caryophyllata and T . plicata ) against clinical and food-borne bacterial pathogens and as well as several environmental bacterial and fungal strains. The antifungal properties of the vapor phase of these EOs were also investigated. Our in vitro trials determined the concentrations of EOs needed to reliably prevent the growth of pathogenic and environmental microorganisms. Finally, in this paper we also report the first in vitro results on the cytotoxic and genotoxic activities of these EOs in human embryo lung cells (HEL 12469).
Antibacterial activity of essential oils
The in vitro antibacterial activity of six EOs against bacterial strains from both clinical and environmental origins (both Gram-positive and Gram-negative bacteria) was assayed using the disc diffusion method by measuring inhibition zone diameters (Fig. 1 ). All EOs tested showed antibacterial effects based on these inhibition zones (*p < 0.05; **p < 0.01; ***p < 0.001). Origanum vulgare (OR) and Thymus vulgaris (TY) EOs were extremely effective on all tested bacteria, with inhibition zones ranging from 26–54 mm. The differences in the measured inhibition halos of OR (p = 0.000457), TY (p = 0.000457) and Lavandula angustifolia (LA; p = 0.0117) on Staphylococus aureus were statistically different from the control. Interestingly, OR and TY produced inhibition halos much larger than those of chloramphenicol, suggesting that they are more active than this antibiotic. Eugenia caryophyllata (clove; CL) and Thuja plicata (arborvitae; AR) EOs exhibited a lower degree of bacterial growth inhibition than OR and TY, while the greatest inhibition observed was caused by AR against Yersinia enterocolitica (p < 0.01). Environmental bacterial strains were much more sensitive to chloramphenicol than clinical strains; no significant difference in susceptibility was found between Gram-negative and Gram-positive bacteria. LA and Salvia sclarea (SA) EOs were both less active against all bacteria, with inhibition zones ranging from 8–14 mm.
Antimicrobial potential of EOs. Results for the agar diffusion assay performed on the six clinical bacterial strains and three environmental bacterial strains are shown. Chloramphenicol (30 μg/disc) was used as a positive control. Each bar of the chart shows the mean of the inhibitory zone obtained for each EO analyzed (1) Staphyloccocus aureus , (2) Listeria monocytogenes , (3) Enterococcus fecalis , (4) Escherichia coli , (5) Salmonella typhimurium , (6) Yersinia enterolitica , (7) Bacillus cereus , (8) Arthrobacter protophormiae , (9) Pseudomonas fragi . Data are represented by means ± 1 SD of 3 independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001 indicate statistically significant differences compared to the control (Student’s t -test).
Preliminary screening revealed that the OR, TY, CL, and AR EOs were the most effective against all tested bacteria; therefore, additional the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) assays were performed with these four EOs. MIC and MBC assays were performed using a broth microdilution method in 96-well strip tubes covered with strip-caps. The results obtained from these assays are shown in Table 1 . These antibacterial assays revealed that OR has a very strong activity (MIC 0.025%, MBC 0.025–0.05%) together with TY (MIC 0.025–0.125%, MBC 0.05–0.125%) while the CL and AR EOs had less antibacterial activity (MIC 0.05–0.125%, MBC 0.125–0.5%). All four EOs inhibited the growth of both clinical and environmental Gram-positive ( S . aureus , L . monocytogenes , E . faecalis , B . cereus , and A . protophormiae) and Gram-negative bacteria ( E . coli , S . typhimurium , Y . enterocolitica , and P . fragi ).
Antifungal activity of essential oils
A disc diffusion assay was performed to determine the sensitivity of five fungal strains to the six EOs by measuring the inhibition zone diameters (in mm). Our goal was to determine whether the different EOs had similar inhibition effects on several different fungal strains ( Cladosporium cladosporoides , Alternaria alternata , Aspergillus fumigatus , Chaetomium globosum and Penicillium chrysogenum ). All tested EOs at concentrations of 75, 50, 25, 10 and 5% (w/v) showed antifungal activity, inhibiting the mycelial growth (Figs 2 and 3 ). The LA and SA EOs exhibited a lower level of inhibition.
Antifungal activity of arborvitae and oregano EOs against Chaetomium globosum and Penicilium chrysogenum . The effects of different concentrations (75%, 50%, 25%, 10% – left plates and 5% – right plates) of EOs dissolved in DMSO are shown. The disc-diffusion assay reveals total growth inhibition after treatment with 75%, 50%, 25%, and 10% (w/v) EOs.
Detailed view of the inhibition of fungal growth and fungal sporulation in Chaetomium globosum after treatment with 5% arborvitae EO dissolved in DMSO. Arrows indicate inhibition of fungal growth (gray arrow IG) and inhibition of fungal sporulation (red arrow IS).
The MIC and minimal fungicidal concentrations (MFC) of the OR, TY, CL, and AR EOs against Ch . globosum, P . chrysogenum , C . cladosporoides , A . alternata , and A . fumigatus are summarized in Table 2 . The greatest antifungal activity against all tested strains was exhibited by OR, which had MICs of 0.01% and 0.025% and MFCs of 0.025%, 0.05% and 0.075%. TY EO, despite being efficient against all tested fungal strains, appeared to have no fungicidal activity against P . chrysogenum, C . cladosporoides and A . fumigatus (Table 2 ). CL also had no fungicidal activity against A . alternata and P . chrysogenum . Overall, OR, AR, TY and CL were effective as fungicidal agents but their efficiency varied from strain to strain (Table 2 ). The fungicidal effect was confirmed when sub-culturing the tested fungi from the agar dilution assays into fresh malt extract broth (MEB) without EO resulted in no further mycelial growth or resumption of spore germination. LA and SA EOs had no antifungal activity against any tested fungal strain.
Volatile vapor of essential oils
The efficacy of OR, TY, CL, AR, LA, and SA EOs in the vapor phase against Ch . globosum, P . chrysogenum , C . cladosporoides , A . alternata , and A . fumigatus was investigated. The volatile vapor of 0.005% EOs exhibited only a fungistatic effect on the tested fungi while the volatile vapor of 0.075% OR, TY, CL and AR completely inhibited the mycelial growth of all tested fungal strains (Fig. 4 ) and were also revealed to have a fungicidal effect after the re-inoculation of inhibited fungal mycelial plugs into fresh malt extract agar (MEA) and fresh MEB. Exceptionally, however, P . chrysogenum and A . fumigatus treated with CL volatile vapor (0.075%) continued to grow in fresh MEB after re-inoculation, meaning that CL had only a fungistatic effect on these strains.
Mycelial growth inhibition of thyme essential oil vapor at different concentrations against Chaetomium globosum , Aspergillus fumigatus and Penicillium chrysogenum on a Malt Extract agar plate. ( A ) control, ( B ) 0.005%, C: 0.075% (w/v) at dose levels of 1 µL/mL air space.
The volatile vapor of LA and SA at 0.075% concentration completely inhibited the growth of all tested fungi except A . alternata , but had no fungicidal properties against any of them. The vapor phase of TY and AR were more effective against P . chrysogenum , C . cladosporoides and A . fumigatus than in the liquid phase.
Cytotoxic and DNA-damaging effects of essential oils
The MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was used to determine the cytotoxic effects on HEL 12469 cells of a 24 h exposure of different concentrations of EOs (0.0025–1.0 µL/mL). Figure 5 summarizes the results: IC 50 values (the median inhibitory concentrations that cause approximately 50% cell death) were 0.058 µL/mL for OR, 0.15 µL/mL for AR and TY, 0.23 µL/mL for CL, 0.28 µL/mL for LA, and 0.45 µL/mL for SA; IC 20 values (the median inhibitory concentrations that cause approximately 20% cell death) were 0.026 µL/mL for OR, 0.10 µL/mL for AR, 0.085 µL/mL for TY, 0.13 µL/mL for CL, 0.23 µL/mL for LA, and 0.41 µL/mL for SA.
Cytotoxicity or viability of human HEL 12469 cells. The effects of a 24 h treatment of different concentrations of EOs (0.0025–1.0 µL/mL) are shown. Data are represented means ± 1 SD of 3 independent experiments.
Further studies examined the genotoxic effects of these EOs, which were assessed at IC~ 10–40 . Single-cell gel electrophoresis (SCGE; also known as comet assay) was used to determine the level of DNA single-strand breaks in HEL 12469 cells. Only one of the EOs, 0.2 µl/ml of AR, induced a significantly different level of DNA breaks than those observed in the untreated control cells, (**p < 0.01) (Fig. 6 ).
The levels of DNA single-strand breaks in HEL 12469 cells pre-treated with EOs for 24 h. As positive control, hydrogen peroxide (300 μM) was used. Data are represented means ± 1 SD of 3 independent experiments. *p < 0.05; **p < 0.01; ***p < 0.001 indicate statistically significant differences compared to untreated control cells (ANOVA test).
The antimicrobial efficacy of a given EO depends on its chemical composition, perhaps especially its phenolic components 1 , 14 . In our study, therefore, we selected the EOs which are well-known for its high content phenols (OR, TY, CL), EOs with lower phenol content (LA and SA), and a phenol-free EO (AR) 11 , 36 , 37 . This last was chosen based on previous studies on the susceptibility of various bacteria and fungi to cedar leaf EO 36 and on our own preliminary experiments (data not shown). This particular AR EO was obtained from heart-wood and contained mainly tropolones 37 .
The pathogenic bacteria selected for this study were chosen based on previous findings on the power of some EOs to inhibit some human pathogens 2 , 4 , 17 , 38 . The environmental bacterial and fungal strains were chosen to be representative of airborne contaminants which our group has isolated from indoor environments (unpublished data).
The measured inhibition halos of OR, TY, CL, AR, LA, SA indicated that all of EOs are effective against bacteria. The OR and TY used in this study were even more effective than the antibiotic chloramphenicol. Our results are in accordance with a previous study showing that the inhibitory halos produced by the EOs of Eugenia caryophyllata , Origanum vulgare and Thymus vulgaris were larger than those produced by ciprofloxacin 38 .
The low antibacterial activity of the LA and SA EOs may be due to the relatively low phenol content of these EOs: their main components are, respectively, alcohols 39 and esters 40 . This is consistent with another study on the antimicrobial efficiency of the EO 16 from Salvia officinalis , which reported a very low antibacterial activity for 1,8-cineole against S . aureus , B . subtilis , and E . coli . In the present study, CL EO, which is known for its high eugenol 41 content (a phenolic compound) was not found to be the most active EO against the microorganisms tested in the disc-diffusion assay. It is possible that the sample we used had a lower concentration of the relevant compounds; it has previously been shown that the EOs of plants belonging to the same species, but collected from different places can exhibit different antimicrobial activity 42 .
It should be noted that the disc-diffusion method is limited by the hydrophobic nature of most EOs, which prevents their uniform diffusion through the agar medium. Therefore, most researchers prefer liquid medium methods 43 . The EOs of OR, TY, CL and AR exhibited strong antimicrobial activity against all microorganisms in liquid medium, as it has been previously described 3 , 16 . It is also quite interesting that no bacterial strain tested was resistant to any of the EOs studied.
Some studies have reported that EOs tend to act more strongly on Gram-positive than Gram-negative bacteria 2 , 3 , 4 , 44 , presumably due to differences in cell wall composition 44 . There is no general rule with respect to Gram sensitivity: the literature reports many conflicting studies showing that some Gram-negative strains are more sensitive than some Gram-positive ones to certain EOs 21 , 36 , 38 . For example, Preuss et al . 45 found that origanum EO is lethal to E . coli and Klebsiella pneumoniae . Origanum syriacum L., Thymus syriacus Boiss. and Syzygium aromaticum L. EOs were effective against the Gram-negative bacteria E . coli O157:H7, Y . enterocolitica O9, Proteus spp., and K . pneumoniae 46 . Our results have also shown that some Gram-negative bacteria ( E . coli , S . typhimurium , Y . enterocolitica , and P . fragi) are sensitive to OR, TY, CL, AR.
Antifungal activity of EOs was determined by direct contact assay and also we tested their antifungal properties in the vapor phase. In a previous study, MICs of thyme red, clove, sage and lavender, for aspergilli and penicillin, ranged from 0.125% to 1% after 3 and 7 days 19 . Our results revealed that all tested fungal strains showed higher susceptibility (MICs of OR, TY, CL and AR EOs ranged from 0.01% - 0.075%). The volatile components of OR, TY, CL and AR showed fungicidal activity while the LA and SA vapors demonstrated fungistatic activity. These results are very different from the direct contact assay, where LA and SA were unsuccessful in inhibiting the growth of the tested strains. There is growing evidence that EOs in the vapor phase are more effective against fungi than in the liquid phase 18 , 19 , 20 , 21 , 22 , 47 . Thyme EOs vapours have been shown to be effective against Aspergillus sp. and Penicillium sp. 19 . The high antifungal activity of vapour evidenced in our study is in accordance with previous finding 48 , which showed that thyme and clove oils were more effective in vapour state against A . flavus .
AR vapors displayed comparable results to OR, TY and CL in spite of its lack of phenolic compounds and its high concentration of monoterpens, which are normally considered to be less effective antimicrobial substances 14 . According to 14 , antimicrobial activity can generally be classified in the following order: phenols > aldehydes > ketones > alcohols > esters > hydrocarbons. The link between the most abundant constituent type and the antimicrobial activity is somewhat variable; for example, Inouye et al . 22 reported that alcohol-containing EOs are more active than ketone-containing EOs against Trichophyton mentagrophytes .
Most EOs are safe and free of adverse side effects when used properly 49 . The most important safety factor for EOs is their dosage. EOs have shown antitumor activity both in vitro and in vivo and low toxicity 50 , 51 . On the other hand, it has also been shown that high concentrations of some EOs contributed to harmful changes in the body 52 .
We have demonstrated the EOs do have cytotoxic effect, but only at higher concentrations on HEL 12469 human embryonic lung cells under in vitro conditions. The effect of a 24 h treatment with a given EO on the viability of HEL cells was dependent on its concentration. IC 50 values declined in the order SA > LA > AR = TY > CL > OR. In other cell lines (human hepatocarcinoma cell line HepG2, human keratinocytes HaCaT, human melanoma cell line HMB-2), the cytotoxic effect of LA EO was detected at a slightly higher concentration ~ IC 50 -0.4 µL/mL, which might be explained by differences in cell sensitivity 53 . Finally, LA at high concentration was genotoxic to peripheral human lymphocytes 54 and to human monocyte THP-1 cells 55 .
In our experiments the EC 50 value for all EOs was >20 µg/mL (for example: IC 50 SA - 0.45 µl / ml = 0.351 mg/ml) indicating that none of them were cytotoxic based on the criteria set by the National Cancer Institute 56 , which state that only natural substances with EC 50 < 20 µg/mL are considered cytotoxic.
The level of DNA single-strand breaks induced in HEL 12469 cells by these EOs was determined using a Comet assay. Treatment with most EOs alone did not induce any significant increase in DNA strand breaks over the untreated control cells; the single exception was the highest concentration of AR EO examined (0.2 µL/mL). Similarly, it was recently shown that plant extracts of S . officinalis and T . vulgaris did not induce DNA damage in HepG2 cells or primary rat hepatocytes 57 , 58 .
Conclusions
This study provides a broad range of information about the biological activities of EOs. It determined the biocidal efficiency of six EOs (from OR, TY, CL, AR, LA and SA) against five different fungal and nine different bacterial strains. In order to verify the potential risk of EOs to human cells, the cytotoxicity and genotoxicity of each of these EOs on human HEL lung cells was assessed for the first time. Of the six EOs studied, OR, TY, CL, and AR were highly effective against all bacterial strains tested. LA and SA exhibited no antifungal activity by direct contact, but did show a fungistatic effect in the vapor phase. OR, TY, and AR exhibited important fungicidal activity against all strains tested; CL showed fungicidal activity against most strains, but only a fungistatic effect on P . chrysogenum and A . fumigatus .
The assayed EOs are not considered cytotoxic as judged by the criteria set by the National Cancer Institute and appeared not to damage the DNA of HEL cells.
The data reported in this study show that EOs might provide an alternative way to fight microbial contamination and that they can be considered safe for humans at relatively low concentrations. Generally, it is possible to recommend the use of EOs for various environmental disinfection strategies, but only after accurate in vitro trials, such as those described in this investigation.
Materials and Methods
Essential oils.
The commercially available EOs used in this work were OR from O . vulgare L., TY from T . vulgaris L., CL from E . caryophyllata L., LA from L . angustifolia Mill., SA from S . sclarea L., and AR from T . plicata Donn. (all from doTERRA, Pleasant Grove, USA). A GC/MS analysis was provided by the producer, who guaranteed the chemical composition of each EO. The EOs were stored in amber glass vials and sampled using sterile pipet tips to minimize contamination and oxygen exposure. Since the EOs varied in density, each of the EOs was weighed to determine the volume that comprised 10 mg. This amount was used in testing as the full-strength (100%) concentration and was then serially diluted in dimethyl sulfoxide (DMSO; Sigma-Aldrich Co., USA).
Microbial strains and growth conditions
The EO antimicrobial activities were investigated against different clinical and food-borne bacterial pathogens: S . aureus (FRIC 418), L . monocytogenes (FRIC 270), E . faecalis (FRIC 282); E . coli (FRIC 375), S . typhimurium (FRIC 305), and Y . enterocolitica (FRIC 30); environmental bacterial strains from our own collection were also examined, including B . cereus , P . fragi , and A . protophormiae .
The fungal strains used in this study ( Ch . globosum , P . chrysogenum , C . cladosporioides , A . alternata , A . fumigatus ) were air-borne isolates from our laboratory collection. The bacterial strains were kept frozen in stock cultures at −80 °C in cryovials, and the fungal cultures were stored at 4 °C and subcultured once a month. Prior to the inoculation of the strains with EOs, the bacteria were grown at 28 or 37 °C (depending on the type of microorganism) for 12–18 h on Luria–Bertani agar (LBA) for environmental bacteria or Mueller Hinton agar (MHA) for pathogenic bacteria. The fungal strains were grown at 26 °C on Malt Extract Agar (MEA).
Cell culture
HEL 12469 human embryo lung cells (Human embryonic lung fibroblast; ECACC 94101201), were cultivated in Eagle’s Minimum Essential Medium (MEM) supplemented with 10% fetal calf serum (FCS), 1% non-essential amino acids and antibiotics (streptomycin 50 μg/mL and penicillin 50 U/mL) 50 . Cell lines were cultured in a humidified atmosphere of 5% CO 2 at 37 °C. The chemicals and media used for cell cultivation were purchased from Gibco BRL (Paisley, UK).
Screening for antibacterial activity
A disc-diffusion assay was used to determine the growth inhibition of bacteria by EOs. A single colony from an overnight bacterial culture plate was seeded into 5 mL of an appropriate pre-warmed growth medium broth (LBB or MHB). Culture tubes were shaken at 300 rpm and 37 °C until the 600 nm absorbance of the growth solution was greater than 1.0. Using a sterile swab, cultures were spread evenly onto pre-warmed 37 °C agar plates. Sterile filter paper discs (6 mm Ø Whatman No.1) were gently pressed onto the surface of the agar plates, and EOs were then pipetted onto the discs. Each EO was tested at 100% strength, and at various dilutions (5, 10, 25, 50, and 75%) in DMSO. A pure DMSO control was included with each test to ensure that microbial growth was not inhibited by DMSO itself. Chloramphenicol (30 μg/disc; Sigma-Aldrich, USA) was used as a positive control. Plates were then inverted and incubated for approximately 24 h at 37 °C and the diameter of the inhibition zones was measured in mm, including the diameter of the disc. The sensitivity was classified according to Ponce et al . 59 as follows: not sensitive for a diameter less than 8 mm, sensitive for a diameter of 9–14 mm, very sensitive for a diameter of 15–19 mm, and extremely sensitive for a diameter larger than 20 mm. Each test was performed in three replicates.
Evaluation of MIC and MBC in liquid medium
The MIC and MBC of each EO was determined using a broth microdilution method in 96-well strip tubes with transparent strip-caps according to Poaty et al . 17 with modification. Bacterial suspensions were adjusted to a final concentration of 10 6 CFU/mL in MHB. One hundred microliters of MHB containing 5% DMSO was distributed into the wells of the micro titer plates. EOs (10 µL) were added to these wells at a range of concentrations, from stock solutions 0.05, 0.10, 0.50, 1.0, 2.5, 5.0, and 10% (w/v). For each dilution, the same volume as the full-strength sample was added. One hundred microliters of bacterial suspension was finally added to each. The plates were incubated at 37 °C for 24 h. MIC was determined after adding 40 µL of 0.2 mg/mL ρ-iodonitrotetrazolium violet (INT; Sigma-Aldrich, USA), followed by incubation at 37 °C for 30 min. MIC was determined as the lowest concentration of EO that inhibited visible growth of the tested microorganism. Growth of bacterial cells in each of the wells was verified by color change. When bacterial growth occurred (absence of inhibition), the INT changed from clear to purple. Wells with DMSO alone were used as controls. MBC is the lowest concentration of EO that results in microbial death. It was determined by subculturing from wells that exhibited no color change to sterile MHA plates that do not contain the test EO. The plates were then incubated at 37 °C for 24 h.
Screening for antifungal activity
Fungal suspensions were prepared according to De Lira Mota et al . 60 by washing the surface of the MEA slant culture with 5 mL of sterile saline and shaking the suspensions for 5 min. The resulting mixture of sporangiospores and hyphal fragments was withdrawn and transferred to a sterile tube. After heavy particles were allowed to settle for 3–5 min, the upper suspension was collected and vortexed for 15 s. Final conidia suspensions were adjusted using a Neubauer’s chamber to 10 6 conidia per mL. 300 µL of each fungal suspension were applied to MEA plates. Filter paper discs (6 mm Ø Whatman No.1) were placed on the agar surface of the Petri dishes and each EO, dissolved in DMSO at different concentrations (75, 50, 25, 10, 5%) was individually added. For each dilution, the same volume as the full-strength sample was placed on the sterile disc. Discs impregnated with 10 µL of DMSO, nystatin (50 µg/mL) and cycloheximide (50 µg/mL) (all Sigma-Aldrich, USA) were used as controls. Petri dishes were incubated at 26 °C for 5 days. Inhibition zone diameters were measured in mm. An inhibition zone larger than 1 mm was taken to indicate a positive effect.
Determination of fungistatic and fungicidal activities
The procedure reported by Thompson 61 was used to determine whether a given EO possessed only a fungistatic effect or if it also had fungicidal activity. Different concentrations (10 µL) of each stock EO solution, ranging from 0.50, 1.0, 2.5, 5.0, 10, 25, 50 and 75% (w/v), were prepared by mixing various quantities of a given EO in DMSO (v/v) with 10 mL molten MEA; this mixture was then poured into sterile Petri dishes. The center of each solidified medium was inoculated upside down with 6-mm square mycelial plugs cut from the periphery of 7-day-old cultures. Positive controls were simultaneously run with DMSO and without EO. After incubation of the plates for 7 days at 26 °C, those fungal plugs that did not show any growth were transferred to fresh MEA plates without EO for an additional 7 days at 26 °C to determine which concentration of each EO had a fungicidal effect. The lowest concentration of each EO that completely prevented visible fungal growth and allowed a revival of fungal growth during the transfer experiment was considered the MIC for that EO. This effect was identified as fungistatic. The concentration unfavorable for growth revival during the transfer experiment was taken as the MFC and this effect was identified as fungicidal. Seven days after reinoculation, the inhibited fungal mycelial plugs were once again reinoculated into fresh MEB without EO to see if their growth revived. Microscopic observations were carried out to investigate fungal cell growth after 5 days incubation at 26 °C. No growth was taken to confirm again the fungicidal activity and also to suggest a possible sporocidal effect.

Antifungal activity of vapor phase of essential oils
In order to determine the fungistatic or fungicidal activity of volatized EOs, 6 mm squares of growing fungal mycelia were taken from the margin of the active growth area of fungal colonies and placed onto MEA plates. EOs from stock solutions (5% and 75%) at dose levels of 1 µL/1 mL air space were placed on the inner surface of the Petri dish lid; controls with DMSO and without EO were also prepared. The plates were sealed with parafilm to prevent vapor leakage and were incubated inverted for 7 days at 26 °C. The radial mycelial growth of the fungus was then checked.
Transfer experiments for determining the fungistatic or fungicidal activity of EO vapors were carried out by replacing the Petri dish lid with a new, untreated one and incubating in an inverted orientation for an additional 7 days at 26 °C. The effect was identified as fungistatic if growth was observed after the new incubation period, and fungicidal if no growth was observed 62 . The effect was also confirmed by reinoculating the inhibited fungal mycelial plugs into fresh MEB without EO.
Cytotoxicity of essential oils
Exponentially growing HEL 12469 cells cultured in complete MEM were seeded onto 96 well plates (density of 2 × 10 4 cells/well) and later incubated in the presence or absence (negative control) of 0.0025–1.0 µL/mL EO for 24 h to test for cytotoxicity using the MTT assay 58 . The MTT test is a colorimetric method for measuring the activity of the mitochondrial enzymes that reduce MTT, a yellow tetrazole, to purple formazan. This reduction takes place only when reductase enzymes are active, and therefore conversion is often used as a measure of viable (living) cells. In our experiments, after incubation with EO, HEL 12469 cells were washed with fresh MEM and 100 μL of complete MEM medium and 50 μL of 1 mg/mL MTT solution was added followed by a 4 h incubation. The MTT solution was then replaced with 100 μL of DMSO and the plates were placed on an orbital shaker for 30 min to completely dissolve the formazan crystals. At least 4 parallel wells were used for each sample. Absorbance at 540 nm was measured using an xMark™ Microplate Spectrophotometer (Bio-Rad Laboratories, Inc.) and the background absorbance at 690 nm was subtracted.
Genotoxicity of essential oils
HEL 12469 cells were seeded into a series of Petri dishes (1 × 10 6 cells, Ø = 60 mm) and cultured in MEM. Cells were then exposed to different EO concentrations (0.0025–1.0 µL/mL) for 24 h; cells with no treatment were used as an intact control. After the treatment, the cells were washed, trypsinized, re-suspended in a fresh culture medium and the level of DNA lesions was detected using the single cell gel electrophoresis (SCGE), also known as comet assay (alkaline). The procedure of Singh et al . 63 was used with minor modifications suggested by Slameňová et al . 64 . In brief, 2 × 10 4 treated and control HEL 12469 cells were embedded in 0.75% low melting point (LMP) agarose. This cell suspension was spread as a single layer on a base layer of 1% normal melting point (NMP) agarose in PBS on microscopic slides and covered with cover slips. As positive control, hydrogen peroxide (300 μM) was used. After solidification of the gel, the cover slips were removed and placed in lysis solution (2.5 M NaCl, 100 mM Na 2 EDTA, 10 mM Tris-HCl, pH 10 and 1% Triton X-100, at 4 °C) for 1 h to remove cellular proteins and membranes. The slides were transferred to an electrophoresis box and immersed in an alkaline solution (300 mM NaOH, 1 mM Na 2 EDTA, pH > 13). After 40 min unwinding time, a voltage of 25 V (0.6 V/cm) was applied for 30 min at 4 °C. The slides were then neutralized with 3 × 5 min washes with Tris-HCl (0.4 M, pH 7.4), and stained with ethidium bromide (EtBr, 5 µg/mL; Sigma Chemical Company, St. Louis, MO). EtBr-stained nucleoides were examined with a Zeiss Imager Z2 fluorescence microscope with computerized image analysis (Metafer 3.6, Meta Systems GmbH, Altlussheim, Germany). The percentage of DNA in the tail was used as a parameter for estimating the number of DNA strand breaks. One hundred comets were scored for each sample in one electrophoresis run.
Statistical analysis
The data are given as means of 3 to 5 experiments ± one standard deviation (SD). The differences between the given groups were tested for statistical significance using Student’s t -test (*p < 0.05; **p < 0.01; ***p < 0.001) 50 . Because the antibacterial activity datasets were normally distributed, the independent samples t -test was performed to test for significant differences between groups. Differences between more than two groups were assessed by one way analysis of variance (ANOVA) followed by the Bonferroni test if equal variances were assumed or Tamhane’s test if equal variances were not assumed 50 . Differences with p < 0.05 are considered to be statistically significant.
Bakkali, F., Averbeck, S., Averbeck, D. & Idaomar, M. Biological effects of essential oils - a review. Food Chem. Toxicol. 46 , 446–475 (2008).
Article CAS PubMed Google Scholar
Fournomiti, M. et al. Antimicrobial activity of essential oils of cultivated oregano ( Origanum vulgare ), sage ( Salvia officinalis ),and thyme ( Thymus vulgaris ) against clinical isolates of Escherichia coli , Klebsiella oxytoca , and Klebsiella pneumoniae . Microb . Ecol . Health Dis . 26 , 23289–23295 (2015).
Lambert, R. J. W., Skandamis, P. N., Coote, P. & Nychas, G. J. E. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl.Microbiol. 91 , 453–462 (2001).
Mith, H. et al . Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci Nutr. 2 (4), 403–416 (2014).
Article CAS PubMed PubMed Central Google Scholar
Yuce, E., Yildirim, N., Yildirim, N. C., Paksoy, M. Y. & Bagci, E. Essential oil composition, antioxidant and antifungal activities of Salvia sclarea L. from Munzur Valley in Tunceli, Turkey. Cell. Mol. Biol. 60 (2), 1–5 (2014).
CAS PubMed Google Scholar
Cavanagh, H. M. A. & Wilkinson, J. M. Biological activities of lavender essential oil. Phytother. Res. 16 (4), 301–308 (2002).
Hajhashemi, V., Ghannadi, A. & Sharif, B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 89 (1), 67–71 (2003).
Article PubMed Google Scholar
Vanin, A. B. et al . Antimicrobial and antioxidant activities of clove essential oil and eugenyl acetate produced by enzymatic esterification. Applied biochemistry and biotechnology 174 (4), 1286–1298 (2014).
Taher, Y. A. et al . Experimental evaluation of anti-inflammatory, antinociceptive and antipyretic activities of clove oil in mice.Libyan. J Med. 10 (1), 28685 (2015).
Google Scholar
Johnston, W. H., Karchesy, J. J., Constantine, G. H. & Craig, A. M. Antimicrobial activity of some Pacific Northwest woods against anaerobic bacteria and yeast. Phytotherapy Research. 15 (7), 586–588 (2001).
Tsiri, D. et al . Chemosystematic value of essential oil compostion of Thuja species cultivated in Poland-Antimicrobial activity. Molecules 14 , 4707–4715 (2009).
Pavela, R. Insecticidal activity of some essential oils against larvae of Spodoptera littoralis. Fitoterapia. 76 (7), 691–696 (2005).
Burt, S. Essential oils: their antibacterial properties and potential applications in foods a review. Int. J. Food Microbiol. 94 , 223–53 (2004).
Kalemba, D. & Kunicka, A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 10 , 813–829 (2003).
De Oliveira, M. M. M., Brugnera, D. F., Cardoso, M. G., Alves, E. & Piccol, R. H. Disinfectant action of Cymbopogon sp. essential oils in different phases of biofilm formation by Listeria monocytogenes on stainless steel surface. Food Control 21 , 549–553 (2010).
Article Google Scholar
Mitić-Ćulafić, D., Vuković-Gačić, B., Knežević-Vukčević, J., Stanković, S. & Simić, D. Comparative study on the antibacterial activity of volatiles from sage ( Salvia officinalis L.). Arch . Biol . Sci . Belgrade 57 , 173–178 (2005).
Poaty, B., Lahlah, J., Porqueres, F. & Bouafif, H. Composition, antimicrobial and antioxidant activities of seven essential oils from the North American boreal forest. World J. Microbiol. Biotechnol. 31 , 907–919 (2015).
Inouye, S., Abe, S., Yamaguchi, H. & Asakura, M. Comparative study of antimicrobial and cytotoxic effects of selected essential oils by gaseous and solution contacts. Int. J. Aromather. 13 , 33–41 (2003).
Tullio, V. et al . Antifungal activity of essential oils against filamentous fungi determined by broth microdilution and vapour contact methods. J. Appl. Microbiol. 102 , 1544–1550 (2007).
Tyagi, A. K. & Malik, A. Liquid and vapour-phase antifungal activities of selected essential oils against Candida albicans : microscopic observations and chemical characterization of Cymbopogon citratus . BMC Complement. Altern. Med. 10 , 1–11 (2010).
Tyagi, A. K. & Malik, A. Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem. 126 , 228–235 (2011).
Article CAS Google Scholar
Inouye, S., Uchida, K. & Abe, S. Vapor activity of 72 essential oils against a Trichophyton mentagrophytes . J. Infect. Chemother. 12 , 210–216 (2006).
Bergkvist, T.P. Antimicrobial activity of four volatile essential oils. Master thesis in Pharmacy, Charles Sturt University, Goteborg, Sweden. (2007).
Chia, T. W. R., Goulter, R. M., McMeekin, T., Dykes, G. A. & Fegan, N. Attachment of different Salmonella serovars to materials commonly used in a poultry processing plant. Food Microbiology 26 , 853–859 (2009).
Jun, W. et al . Microbial biofilm detection on food contact surfaces by macro-scale fluorescence imaging. J. Food Eng. 99 , 314–322 (2010).
Valeriano, C. et al . The sanitizing action of essential oil-based solutions against Salmonella enterica serotype Enteritidis S64 biofilm formation on AISI 304 stainless steel. Food Control 25 , 673–677 (2012).
Slamenova, D., Horvathova, E., Kovacikova, Z., Kozics, K. & Hunakova, L. Essential rosemary oil protects testicular cells against DNA-damaging effects of H 2 O 2 and DMNQ. Food Chem. 129 , 64–70 (2011).
Basaran, A. A., Yu, T., Plewa, M. J. & Anderson, D. An investigation of some Turkish herbal medicines in Salmonella typhimurium and in the Comet assay in human lymphocytes. Teratog. Carcinog. Mutagen. 16 , 125–138 (1996).
Llana-Ruiz-Cabello, M. et al . In vitro toxicological evaluation of essential oils and their main compounds used in active food packaging: a review. Food Chem. Toxicol. 81 , 9–27 (2015).
Ruiz-Pérez, N. J. et al . Antimycotic Activity and Genotoxic Evaluation of Citrus sinensis and Citrus latifolia Essential Oils. Sci. Reports 6 , 253–271 (2016).
Schimmer, O., Kruger, A., Paulini, H. & Haefele, F. An evaluation of 55 commercial plant extracts in the Ames mutagenicity test. Pharmazie 49 , 448–451 (1994).
Sturchio, E. et al . Molecular and structural changes induced by essential oils treatments in Vicia faba roots detected by genotoxicity testing. J. Toxicol. Environ. Health A. 79 (4), 143–152 (2016).
Ortiz, C., Morales, L., Sastre, M., Haskins, W. E. & Matta, J. Cytotoxicity and genotoxicity assessment of Sandalwood essential oil in human breast cell lines MCF-7 and MCF-10A. Evid . Based Complement . Alternat . Med . (2016).
Navarra, M. et al . Effects of bergamot essential oil and its extractive fractions on SH‐SY5Y human neuroblastoma cell growth. J. Pharm. Pharmacol. 67 , 1042–1053 (2015).
Sinha, S., Jothiramajayam, M., Ghosh, M. & Mukherjee, A. Evaluation of toxicity of essential oils palmarosa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem. Toxicol. 68 , 71–77 (2014).
Hudson, J., Kuo, M. & Vimalanathan, S. The Antimicrobial Properties of Cedar Leaf ( Thuja plicata ) Oil; A Safe and Efficient Decontamination Agent for Buildings. Int. J. Environ. Res. Public Health 8 , 4477–4487 (2011).
Daniels, C. R. & Russell, J. H. Analysis of western redcedar (Thuja plicata Donn) heartwood components by HPLC as a possible screening tool for trees with enhanced natural durability. J. Chromatogr. Sci. 45 (5), 281–285 (2007).
Maida, I. et al . Exploring the anti- Burkholderia cepacia complex activity of essential oils: a preliminary analysis. Evid . Based Complement . Altern . Med . vol. 2014, pp. 10 (2014).
Shellie, R., Mondello, L., Marriott, P. & Dugo, G. Characterisation of lavender essential oils by using gas chromatography–mass spectrometry with correlation of linear retention indices and comparison with comprehensive two-dimensional gas chromatography. J. Chromatogr. A 970 (1), 225–234 (2002).
Cai, J., Lin, P., Zhu, X. & Su, Q. Comparative analysis of clary sage (S. sclarea L.) oil volatiles by GC–FTIR and GC–MS. Food chem. 99 (2), 401–407 (2006).
Politeo, O., Jukic, M. & Milos, M. Comparison of chemical composition and antioxidant activity of glycosidically bound and free volatiles from clove (Eugenia caryophyllata Thunb.). J. food biochem. 34 (1), 129–141 (2010).
Sarac, N. & Ugur, A. Antimicrobial activities and usage in folkloric medicine of some Lamiaceae species growing in Mugla, Turkey. EurAsia J. BioSci. 4 , 28–37 (2007).
Bilia, A.R., Santomauro, F., Sacco, C., Bergonzi, M.C. & Donato, R. Essential Oil of Artemisia annua L. An Extraordinary Component with Numerous Antimicrobial Properties. Evid . Based Complement . Altern . Med . Vol 2014 , 7 (2014).
Ratledge, C. & Wilkinson, S.G. An overview of microbial lipids in Microbial Lipids (eds. Ratledge, C. & Wilkinson S.G.) vol. 1. 3–22 (Academic Press, 1988).
Preuss, H. G., Echard, B., Enig, M., Brook, I. & Elliott, T. B. Minimum inhibitory concentrations of herbal essential oils and monolaurin for gram-positive and gram-negative bacteria. Mol. Cell Biochem. 272 , 29–34 (2005).
Al-Mariri, A. & Safi, M. In vitro antibacterial activity of several plant extracts and oils against some gram-negative bacteria. Iran. J. Med. Sci. 39 (1), 36–43 (2014).
PubMed PubMed Central Google Scholar
Lopez, P., Sanchez, C., Batile, R. & Nerin, C. Solid- and vapour-phase antimicrobial activities of six essential oils: susceptibility of selected foodborne bacterial and fungal strains. J. Agric. Food Chem. 53 , 6939–6946 (2005).
Suhr, K. I. & Nielsen, P. V. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J. Appl. Microbiol. 94 (4), 665–674 (2003).
Edris, A. E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother. Res. 21 (4), 308–323 (2007).
Russo, A. et al . Comparative phytochemical profile and antiproliferative activity on human melanoma cells of essential oils of three Lebanese Salvia species. Ind. Crop. Prod. 83 , 492–499 (2016).
Sertel, S., Eichhorn, T., Plinkert, P. K. & Efferth, T. Cytotoxicity of Thymus vulgaris essential oil towards human oral cavity squamous cell carcinoma. Anticancer Res. 31 , 81–87 (2011).
Maistro, E. L., Mota, S. F., Lima, E. B., Bernardes, B. M. & Goulart, F. C. Genotoxicity and mutagenicity of Rosmarinus officinalis (Labiatae) essential oil in mammalian cells in vivo . Genet. Mol. Res. 9 (4), 2113–2122 (2010).
Kozics et al. Antioxidant potential of essential oil from Lavandula angustifolia in in vitro and ex vivo cultured liver cells. Neoplasma (doi: 10.4149/neo_2017_401 ) in press .
Di Sotto, A., Mazzanti, G., Carbone, F., Hrelia, P. & Maffei, F. Genotoxicity of lavender oil, linalyl acetate, and linalool on human lymphocytes in vitro . Environ. Mol. Res. 52 (1), 69–71 (2011).
Huang, M. Y., Liao, M. H., Wang, Y. K., Huang, Y. S. & Wen, H. C. Effect of essential oil on LPS-stimulated inflammation. Am. J. Chin. Med. 40 (4), 845–859 (2012).
Geran, R. I., Greenberg, N. H., Macdonald, M. M., Schumacher, A. M. & Abbott, B. J. Protocols for screening chemical agents and natural products against animal tumours and other biological systems. Cancer Chemother. Rep. 3 , 59–61 (1972).
Horvathova, E. et al . Enriching the drinking water of rats with extracts of Salvia officinalis and Thymus vulgaris increases their resistance to oxidative stress. Mutagenesis 31 , 51–59 (2016).
Kozics, K. et al . Effects of Salvia officinalis and Thymus vulgaris on oxidant-induced DNA damage and antioxidant status in HepG2 cells. Food Chem. 141 , 2198–2206 (2013).
Ponce, A. G., Fritz, R., Del Valle, C. E. & Roura, S. I. Antimicrobial activity of essential oils on native microbial population of organic Swiss chard. Lebensm. Wiss. Technol. 36 , 679–684 (2003).
De Lira Mota, K. S. et al . Antifungal Activity of Thymus vulgaris L. Essential Oil and Its Constituent Phytochemicals against Rhizopus oryzae : Interaction with Ergosterol. Molecules 17 , 14418–14433 (2012).
Thompson, D. P. Fungitoxic activity of essential oil components on food storage fungi. Mycologia 81 , 151–153 (1989).
Feng, W., Chen, J., Zheng, X. & Liu, Q. Thyme oil to control Alternaria alternata In vitro and in vivo as fumigant and contact treatments. Food Control 22 , 78–81 (2011).
Singh, N. P., McCoy, M. T., Tice, R. R. & Schneider, E. L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 175 (1), 184–191 (1988).
Slameňová, D. et al . Detection of MNNG-induced DNA lesions in mammalian cells; validation of comet assay against DNA unwinding technique, alkaline elution of DNA and chromosomal aberrations. Mutation Research. DNA Repair. 383 , 243–252 (1997).
Download references
Acknowledgements
This study was funded by VEGA projects no. 2/0061/17 “Innovative disinfection strategies: the essential oils effect on microflora and materials of cultural heritage objects” and no. 2/0027/16 “Antioxidative, anticarcinogen and photoprotective effects of the essential oil from lavender in vitro ”. We are very grateful to Dr. Jacob Bauer for the English revision of the text.
Author information
Authors and affiliations.
Institute of Molecular Biology, Slovak Academy of Sciences, Dúbravská cesta 21, 84551, Bratislava, Slovakia
Andrea Puškárová, Mária Bučková, Lucia Kraková & Domenico Pangallo
Cancer Research Institute, Biomedical Research Center, Slovak Academy of Sciences, Dúbravská cesta 9, 84505, Bratislava, Slovakia
Katarína Kozics
You can also search for this author in PubMed Google Scholar
Contributions
A. Puškárová, M. Bučková and L. Kraková performed the antibacterial and antifungal analysis. K. Kozics was responsible for the cytotoxicity and genotoxicity assays. D. Pangallo critically revised the manuscript. A. Puškárová wrote the article. A. Puškárová, M. Bučková, and D. Pangallo participated in drafting the article. All authors discussed the results and commented on the manuscript.
Corresponding author
Correspondence to Andrea Puškárová .
Ethics declarations
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Puškárová, A., Bučková, M., Kraková, L. et al. The antibacterial and antifungal activity of six essential oils and their cyto/genotoxicity to human HEL 12469 cells. Sci Rep 7 , 8211 (2017). https://doi.org/10.1038/s41598-017-08673-9
Download citation
Received : 29 November 2016
Accepted : 12 July 2017
Published : 15 August 2017
DOI : https://doi.org/10.1038/s41598-017-08673-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Lavandula angustifolia mill. for a suitable non-invasive treatment against fungal colonization on organic-media cultural heritage.
- M. C. Sorrentino
- S. Pacifico
Heritage Science (2024)
Centaurea triumfetii essential oil chemical composition, comparative analysis, and antimicrobial activity of selected compounds
- Ivana Carev
- Andrea Gelemanović
- Gianni Prosseda
Scientific Reports (2023)
Lamiaceae Family Plants: One of the Potentially Richest Sources of Antimicrobials
- N. Zh. Sahakyan
Pharmaceutical Chemistry Journal (2023)
Synergistic Effect and Time-Kill Evaluation of Eugenol Combined with Cefotaxime Against Staphylococcus aureus
- Jullietta Lady
- Agustina D. R. Nurcahyanti
Current Microbiology (2023)
Amoebicidal activity of cationic carbosilane dendrons derived with 4-phenylbutyric acid against Acanthamoeba griffini and Acanthamoeba polyphaga trophozoites and cysts
- P. López-Barona
- C. Verdú-Expósito
- I. Heredero-Bermejo
Scientific Reports (2022)
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Microbiology newsletter — what matters in microbiology research, free to your inbox weekly.
SYSTEMATIC REVIEW article
Efficacy of essential oils in pain: a systematic review and meta-analysis of preclinical evidence.

- 1 Pharmacotechnology Documentation and Transfer Unit, Section of Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, Rende, Italy
- 2 Regional Center for Serious Brain Injuries, S. Anna Institute, Crotone, Italy
- 3 Laboratory of Chemical Pharmacology, Faculty of Pharmaceutical Sciences, Daiichi University of Pharmacy, Fukuoka, Japan
- 4 Center for Supporting Pharmaceutical Education, Faculty of Pharmaceutical Sciences, Daiichi University of Pharmacy, Fukuoka, Japan
- 5 Department of Physiology and Anatomy, Faculty of Pharmaceutical Sciences, Tohoku Medical and Pharmaceutical University, Sendai, Japan
- 6 Section of Preclinical and Translational Pharmacology, Department of Pharmacy, Health and Nutritional Sciences, University of Calabria, Rende, Italy
- 7 Department of Health Sciences, University “Magna Graecia” of Catanzaro, Catanzaro, Italy
- 8 School of Hospital Pharmacy, University “Magna Graecia” of Catanzaro, Catanzaro, Italy
Background: The demand for essential oils (EOs) has been steadily growing over the years. This is mirrored by a substantial increase in research concerned with EOs also in the field of inflammatory and neuropathic pain. The purpose of this present systematic review and meta-analysis is to investigate the preclinical evidence in favor of the working hypothesis of the analgesic properties of EOs, elucidating whether there is a consistent rational basis for translation into clinical settings.
Methods: A literature search has been conducted on databases relevant for medical scientific literature, i.e., PubMed/MEDLINE, Scopus, and Web of Science from database inception until November 2, 2020, following the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) criteria for systematic reviews and meta-analyses.
Results: The search was conducted in order to answer the following PICOS (participants/population, interventions, comparisons, outcomes, and study design) question: are EOs efficacious in reducing acute nociceptive pain and/or neuropathic pain in mice experimental models? The search retrieved 2,491 records, leaving 954 studies to screen after the removal of duplicates. The title and abstract of all 954 studies were screened, which left 127 records to evaluate in full text. Of these, 30 articles were eligible for inclusion.
Conclusion: Most studies (27) assessed the analgesic properties of EOs on acute nociceptive pain models, e.g. the acetic acid writhings test, the formalin test, and the hot plate test. Unfortunately, efficacy in neuropathic pain models, which are a more suitable model for human conditions of chronic pain, had fewer results (only three studies). Moreover, some methodologies raised concerns in terms of the risk of bias. Therefore, EOs with proven efficacy in both types of pain were corroborated by methodologically consistent studies, like the EO of bergamot, which should be studied in clinical trials to enhance the translational impact of preclinical modeling on clinical pain research.
1 Introduction
1.1 rationale.
Essential oils (EOs) containing components in exact proportion contributing synergically to the whole plant effect, have been used in traditional medicine for centuries since The Divine Farmer’s Materia Medica , the first text of Chinese Traditional Medicine, representing a form of combinatorial medicine ( Li and Weng, 2017 ). The search for natural and green products is constantly increasing the use of essential oils and the demand for these products from developing countries. There has been a remarkable increase in the import of EOs by the European market from 2011–2018 (Eurostat) and it is estimated that the demand for essential oils in the global market will grow by 7.5% from 2020 to 2027 ( GVR, 2020 ). These data are mirrored by the steady increase of research on EOs that pave the way for the development of these products.
Identifying the year 1880 as this field emerged ( Wood and Reichut, 1880 ), we found a remarkable increase in publications concerned with EOs up to 2020 ( Figures 1A,B ) (see also ( Scuteri et al., 2017a )). EOs have shown several beneficial properties, many of which concern the treatment of neurologic diseases, mood disturbances, and pain. Modulation of the γ-aminobutyric acid (GABA) neurotransmission and blockade of neuronal voltage-gated sodium channels (Na + channels) as well as activity on serotonergic neurotransmission are proposed as mechanisms involved in the action of EOs endowed with anxiolytic and anti-nociceptive properties like bergamot essential oil (BEO) ( Rombolà et al., 2017 ; Scuteri et al., 2018a ; Scuteri et al., 2019a ; Scuteri et al., 2019b ; Rombolà et al., 2019 ; Rombola et al., 2020 ), lavender essential oil (LEO) ( Lopez et al., 2017 ), and melissa (lemon balm) ( Abuhamdah et al., 2008 ; Awad et al., 2009 ). The cholinergic system is targeted by extracts of plants as sage ( Perry et al., 2000 ; Savelev et al., 2003 ; Savelev et al., 2004 ), ginkgo ( Stein et al., 2015 ; Zhang et al., 2018 ), and lemon balm ( Dastmalchi et al., 2009 ; Guginski et al., 2009 ), showing therapeutic potential for diseases like dementia. The gathered evidence shows the potential benefits of EOs in the treatment of pain in fragile patients for whom several drugs can be more harmful, e.g. in aging or chronic neurologic diseases such as dementia ( Achterberg et al., 2020 ). Pain is associated with mood disturbances ( Evans, 1987 ; Husebo et al., 2011 ) influenced by aging ( Hamm and Knisely, 1985 ; Scuteri et al., 2020a ) and neuropathology ( Scherder et al., 2003 ) and its treatment represents a field of strong inappropriateness in patients suffering from Alzheimer’s disease. ( Scuteri et al., 2017b ; Scuteri et al., 2018b ; Achterberg et al., 2020 ; Scuteri et al., 2020f ). Therefore, aromatherapy represents a fundamental tool for the safer handling of pain.
FIGURE 1 . Research in the field of essential oils (EOs) over the years. (A,B) Increase of research in the field of essential oils (EOs). (A) A PubMed advanced search using the key word “essential oils” combined with the dates of publication from 1880 to present through the Boolean operator AND has retrieved an increase from 106 to 17,212 (date of last search November 19, 2020) of results. The first interval “1880–1950” is wider because no great amount of research in this field has been detected up until the 1950s. (B) Data are presented per year of publication based on search query “essential oils” (date of last search November 25, 2020). Modified from ( Scuteri et al., 2017a ).
Despite a large amount of continuously growing research on EOs, a real translation of aromatherapy into clinical settings and the treatment of pain has not occurred. Research efforts have aimed to discover the mechanisms at the root of the analgesic activity of EOs, often focusing on the single components commonly present in different plant oils e.g., linalool, limonene, pinene, eugenol, and cinnamal. For instance, linalool, limonene, and pinene contribute to the anxiolytic and antidepressant properties of some EOs (see ( Lizarraga-Valderrama, 2020 )). In particular, some natural components of plants have been suggested as possible candidates for an analgesic action in neuropathic pain ( Quintans et al., 2014 ). However, the strongest effect of EOs is due to the whole phytocomplex made up of various plant components that need to be present in a precise ratio to exert the so called entourage effect ( Ribeiro, 2018 ). Definite combination of the constituents of EOs is necessary, but further studies are needed to highlight the exact active composition for each EO. The EOs of the species Citrus contain volatile components (85–99%), most abundantly terpenoids, and a non-volatile fraction including coumarins i.e. bergapten inducing phototoxicity ( Zaynoun et al., 1977 ). Thus, the EO of bergamot has been deprived of bergapten ( Bagetta et al., 2010 ), but is still endowed with its characteristics. The EO of bergamot can modulate the synaptic level of glutamate and this occurs when it is used as a bergapten-free fraction ( Morrone et al., 2007 ). Hence, a mixture of monoterpene hydrocarbons within the volatile fraction may be responsible for bergamot analgesic activity since glutamate is significantly involved in the pain descending pathway due to metabotropic glutamate receptors mGluR7 and mGluR8 ( Boccella et al., 2020 ). The novel phytocannabinoid cannabidihexol, with the terpenophenolic core of cannabidiol and Δ9-tetrahydrocannabinol, has proven to significantly reduce the late phase of the formalin test at low doses in C57BL/6J mice ( Linciano et al., 2020 ). Cannabidiol oil has been demonstrated to reduce traumatic brain injury-induced allodynia ( Belardo et al., 2019 ). Certain EOs have been proven to have enhanced efficacy if combined: e.g., peppermint and caraway oil are significantly effective on post-inflammatory visceral hyperalgesia only when used in combination ( Adam et al., 2006 ). Likewise, the route of administration and the time of exposure can influence the effects of EOs ( Scuteri et al., 2018a ; Koyama and Heinbockel, 2020 ). Moreover, some EOs are efficacious in a preclinical setting ( Sarmento-Neto et al., 2015 ), but often only in a definite model of pain, usually acute e.g. the acetic acid-induced writhings, that does not find a significant counterpart in clinic. Furthermore, EOs are often administered as gavage or for inhalation not always allowing an exact determination of the dose.
Clinical trials in aromatherapy are few, small and methodologically limited, hence it is not always possible to draw rigorous conclusions, particularly in dementia. As recently demonstrated in a Cochrane systematic review by Ball et al. (2020) , the design, reporting and consistency of outcome measurement have been identified as the weakest points and need to be improved in the future. Thus, despite accumulating preclinical and clinical evidence for EOs ( Scuteri et al., 2020d ) and nutraceuticals ( Scuteri et al., 2020e ) in lots of forms and supplements, which have been studied in several neurodegenerative conditions, a sound rationale for their clinical use, especially in treating chronic pain ( Lakhan et al., 2016 ), has not yet emerged.
2.1 Objectives
The present systematic review and meta-analysis aimed to verify the working hypothesis that EOs have analgesic properties by investigating preclinical evidence in favor of the latter, to understand whether there is a consistent rational basis for clinical translation. For this purpose, the objective was to assess the efficacy of EOs in preclinical models of both nociceptive and neuropathic pain through the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) ( Liberati et al., 2009 ; Moher et al., 2009 ) criteria for systematic reviews and meta-analyses. The systematic review and meta-analysis focuses on the following PICOS (participants/population, interventions, comparisons, outcomes, and study design) question: are EOs efficacious in reducing acute nociceptive pain and/or neuropathic pain in mice experimental models? In particular, this work aimed at evaluating:
analgesic effectiveness (outcome);
of EOs with a known composition (interventions), and not single components or extracts, administered intraperitoneally (i.p.) or subcutaneously (s.c.) to allow determination of the exact dose and reproducibility;
in male mice subjected to nociceptive or neuropathic pain models (participants/population);
with respect to providing a vehicle or other treatments (comparators);
in studies designed according to legislation to minimize the suffering of animals (study design).
To the best of our knowledge, this is the first meta-analysis of preclinical studies on the analgesic effects of EOs interventions in models of both nociceptive and neuropathic pain.
2.2 Protocol
The search strategy and extraction of data to answer to PICOS question followed the PRISMA ( Liberati et al., 2009 ; Moher et al., 2009 ) criteria. Due to the nature of preclinical animal intervention systematic review and meta-analysis, the latter aims at investigating the consistency of the body of evidence for clinical translation without an outcome of clear human relevance. For this reason, it has not been registered in the International prospective register of systematic reviews PROSPERO. However, statistically analyzing basic research independent studies testing the same hypothesis with comparable parameters can: determine its consistency allowing to study that phenomenon in a larger sample surmounting the issues concerned with small sample sizes; correct confounders; improve reproducibility ( Editorial, 2016 ). Thus, a systematic review and meta-analysis is fundamental to establish a real possible clinical translation of a preclinically studied effect since it can highlight whether it has been consistently proven with the most reliable human disease modelled approach. Two independent researchers ran the search in agreement with the previously established protocol and inclusion and exclusion criteria, including double-checking the retrieved results, and any conflicts found by them were resolved by a third author.
2.3 Eligibility Criteria
2.3.1 inclusion criteria.
The analysis included studies assessing the antinociceptive effect of EOs, administered i. p. or s. c. to allow determination of the exact dose and reproducibility, with a known percentage of components on male mice subjected to nociceptive or neuropathic pain models. Compliance with animal welfare regulations was an inclusion criterion of the utmost importance. The studies included needed to be designed according to legislation to minimize animal suffering. Either acute nociceptive or neuropathic pain models are included. Independently of the model used, the outcome of the study had to be antinociception for eligibility.
2.3.2 Exclusion Criteria
Studies on species different from mice or any strains and female gender were not eligible. The use of different species and genders would not allow comparison and the number of papers examining pain in non-rodent species is very small. Papers in which extracts or single plant components are used were excluded. Studies that did not consider ethics were excluded. Narrative or systematic reviews and meta-analysis, in vitro studies, abstracts and congress communications, proceedings, editorials, book chapters, and studies not published in English and not available in full text were not eligible.
2.4 Information Sources
A literature search was performed on PubMed/MEDLINE, Scopus, and Web of Science. Embase could not be searched as it was not freely/institutionally available. No restriction of publication date was applied and databases were searched for records matching the search strings used from their inception. The date of the last search was November 2, 2020. After the elimination of duplicate records, the first screening evaluated the title and abstract, and then the full text was assessed to define inclusion in qualitative and/or in quantitative synthesis.
2.5 Search Strategy
The following search terms and modifications were used as key words in combination: essential oils, pain, animal pain models, antinociceptive activity, allodynia, Von Frey (‘s test), hyperalgesia, Hargreaves (‘test), hot plate, capsaicin test, formalin test, tail flick test, acetone test, complete Freund's adjuvant, streptozocin, chemotherapy(-induced), oxaliplatin, cisplatin, paclitaxel, docetaxel, vincristine, vinblastine, eribulin, bortezomib, thalidomide, neuropathy, mice.
2.6 Data Collection Process
The eligibility of the studies was assessed independently by two authors to minimize the risk of excluding relevant records. The references list of the articles was examined to extend and refine the search. A complete consensus was reached and no relevant conflicts were raised. The selection process is illustrated in the PRISMA flow diagram ( Figure 2 ).
FIGURE 2 . Literature search and screening of retrieved records. PRISMA flow diagram ( Moher et al., 2009 ) of the selection process of the studies eligible for qualitative and quantitative synthesis.
2.7 Synthesis, Risk of Bias, and Statistical Analysis
A systematic and narrative synthesis of the results, according to the Cochrane Consumers and Communication Review Group guidelines ( Ryan, 2019 http://cccrg.cochrane.org , March 13, 2019 (accessed DATE).) was carried out. The risk of bias (internal validity) and the quality of the studies was assessed by two independent researchers through tools specific to preclinical animal studies like the Systematic Review Center for Laboratory Animal Experimentation (SYRCLE’s) risk of bias (RoB) tool ( Hooijmans et al., 2014 ) and the Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) checklist for study quality ( Macleod et al., 2004 ). Any discrepancies were resolved through consensus or with the help of a third author.
Meta-analyses were conducted using Cochrane Review Manager 5.3 (RevMan5.3; Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration). A minimum of five articles per outcome measure was required according to the systematic review protocol for animal intervention studies by SYRCLE. When the tests included in articles were multiple and performed at different times and doses, only the most significant time point for pain development and progression in the specific model was considered for meta-analysis and only data related to the most efficacious dose were included. Studies expressing the analgesic outcome in a comparable way were included in the meta-analysis. Data available and comparable, but not expressed with the same measure of effect size as proportional reduction of outcome in treated animals relative to untreated controls were converted in mean and standard deviation to allow statistical comparison. Data not available and not extractable from graphs using digital ruler software, e.g., PlotDigitizer 2.6.9, were excluded from quantitative analysis. The Higgins I 2 value was calculated to assess the heterogeneity of studies ( Higgins and Thompson, 2002 ). Differences were presented as risk ratios (RR) including 95% confidence intervals (CI), using a random effect model ( DerSimonian and Kacker, 2007 ) to manage the eventual heterogeneity of the studies and to assess intra- and inter-study variation. Publication bias was assessed through Egger’s linear regression test to measure funnel plot asymmetry, adjusted through the “trim and fill” method ( Egger et al., 1997 ; Duval and Tweedie, 2000 ; Sterne and Egger, 2001 ).
3.1 Selection Process and Data Collection
The search retrieved 2,491 results from databases and there were no results from additional searches. The records were screened for duplicates, leaving 954 studies. Title and abstract screening led to an initial exclusion of narrative or systematic reviews and meta-analysis, in vitro studies, abstracts and congress communications, proceedings, editorials, book chapters. This left 127 records in full text. Among these, two had to be excluded because the text was in Chinese ( Li et al., 1991 ; Chen et al., 2011 ) and one was excluded because it was written in Spanish ( Do Nascimento Silva et al., 2018 ). After full text screening, 30 studies were included in qualitative analysis: 40 studies were not available in full text and 54 were excluded because they did not meet inclusion criteria because of species used, route of administration, composition, or lack of compliance with animal welfare regulations. For instance, the study by Ali et al. (2012) in which the EO of Nepeta pogonosperma Jamzad et Assadi was proven to have significant efficacy at different doses in the tail-flick and formalin test in Wistar rats was therefore not eligible. Among the records included in qualitative analysis, eight were included in quantitative synthesis, reporting comparable outcomes and the exact number of animals used. The process of literature search and screening was illustrated in the PRISMA flow diagram ( Moher et al., 2009 ) in Figure 2 .
3.2 Qualitative Synthesis
The data obtained from the 46 studies included in the qualitative analysis were grouped according to Cochrane Consumers and Communication Review Group guidelines ( Ryan, 2019 http://cccrg.cochrane.org , March 13, 2019 (accessed DATE).). These groups were based on the experimental pain model used in 1) EOs showing analgesia in nociceptive models, and 2) EOs with analgesic properties in neuropathic pain. The majority (27/30) of the studies used an acute nociceptive model. Studies providing a range and not an exact number of animals per group were not considered eligible for quantitative analysis. Studies expressing the analgesic outcome in a not comparable manner to the majority were excluded from the meta-analysis. The main characteristics of the studies with reference to the PICOS question are reported in Tables 1 , 2 .
TABLE 1 . Main characteristics of the studies included showing the efficacy of EOs in nociceptive models.
TABLE 2 . Main characteristics of the studies included showing efficacy of EOs in neuropathic models.
3.2.1 Essential Oils Endowed With Efficacy in Acute Nociceptive Models
Based on the obtained results, several EOs showed analgesic activity in acute nociceptive tests like the acetic acid writhings test, hot-plate test, and the formalin test, with the latter very useful since it includes features of both peripheral and central pain. In the study by Anaya-Eugenio et al. (2016) the EO of artemisia ludoviciana Nutt (Asteraceae) exerted dose-dependent antinociceptive activity in the hot-plate and the formalin test. It was less potent than the reference drug morphine and antagonism studies have revealed that it was inhibited by the non-selective opioid receptor antagonist naloxone. Inula britannica L (Asteraceae) has shown analgesia in the acetic acid writhings test, in the formalin test, in the tail-flick, and the glutamate test ( Zarei et al., 2018 ). This effect is reversed by naloxone and potentiated by l -arginine, therefore all the studies performed with negative and positive controls highlighted the involvement of the opioid system and NO pathway ( Zarei et al., 2018 ).
The EO of Myrcia pubiflora DC., Myrtaceae ( Andrade et al., 2012 ) has demonstrated analgesic efficacy in the acetic acid writhings test and the formalin test, but not in the hot plate test. From the same family, the EO of Eugenia candolleana DC (Myrtaceae) reduced the number of writhings and licking behavior in the second phase of the formalin test in a dose-dependent manner (only at the dose of 100 mg/kg in the first phase, but not the nociceptive reaction in the hot-plate test ( Guimaraes et al., 2009 )).
Clove bud oil (Eugenia caryophyllata, Myrtaceae) significantly reduced formalin-induced pain behavior but affected tail-flick response in a variable way ( Halder et al., 2012 ). The study by Bae et al. (2020) considered basil for its i. p. administration and demonstrated analgesic properties linked to action on δ- and µ-opioid pathways. Moreover, it provides orofacial antinociception at high doses ( Venâncio et al., 2011 ). Aristolochia trilobata L. demonstrated strong analgesia in the formalin test and was comparable to morphine in the acetic acid test ( Quintans et al., 2017 ).
The EO of Croton conduplicatus Kunth (Euphorbiaceae) has shown efficacy ( de Oliveira Júnior et al., 2017 ; de Oliveira et al., 2018 ): in the acetic acid test; on the formalin‐induced nociceptive behavior at all the doses and in both phases, with effect antagonized by naloxone; on nociception in term of latency time at the highest dose (50 ( deOliveira et al., 2018 ) and 100 ( de Oliveira Júnior et al., 2017 ) mg/kg) in the hot‐plate test. In particular, the mechanism of action of this EO has been proposed to be influenced by ATP-sensitive K+ channels, opioid and cholinergic systems ( de Oliveira Júnior et al., 2017 ; de Oliveira et al., 2018 ).
Croton cordiifolius Baill (Euphorbiaceae) also had effective results in acetic acid, formalin, and glutamate but not the capsaicin test. This antinociceptive effect was independent on naloxone ( Nogueira et al., 2015 ). Croton adamantinus Müll. Arg. showed a strongly effective comparison with morphine in reducing licking and was more efficacious than indomethacin in decreasing abdominal contortions ( Ximenes et al., 2013 ). Of the study by Hajhashemi et al. (2009) only the experiments using the EO i. p. and on mice could be included in the analysis, showing the effectiveness of Heracleum persicum to be almost comparable to indomethacin in the reduction of the number of writhings.
In the study by Jahandar et al. (2018) only the experiments performed on mice were considered. Pycnocycla bashagardiana (Apiaceae) has not proven analgesic but anti-inflammatory properties. In another study by Ulku Karabay–Yavasoglu et al. (2006) only experiments with the formalin test in mice were considered. The EO of Satureja thymbra L (Lamiaceae) was demonstrated to have antinociceptive efficacy in both the early and late (also at a lower dose) phases of the formalin test ( Ulku Karabay–Yavasoglu et al., 2006 ).
In the study by Katsuyama et al. (2015) the EO of bergamot (Citrus bergamia Risso) demonstrated significant dose-dependent analgesia in both phases of the formalin test, only when administered in the ipsilateral hindpaw and antagonized by naloxone hydrochloride and methiodide (not able to cross the blood brain barrier), suggesting the involvement of peripheral opioid mechanisms. This was earlier observed in the capsaicin test in which it also enhanced morphine analgesia ( Sakurada et al., 2011 ).
Neroli (Citrus aurantium L.) significantly increases reaction time (at 40 mg/kg) in the hot-plate test and significantly decreased the number of writhings in the study by Khodabakhsh et al. (2015) , with the latter effect potentiated by L-nitro arginine methyl ester ( l -NAME). In the study by Khalid et al. (2011) the EO of Zingiber zerumbet (L.) Smith, dose-dependent and comparable to acetylsalicylic acid, inhibited the nociceptive response to capsaicin, acetic acid, glutamate, and phorbol 12-myristate 13-acetate (PMA). Eucalyptus EO has significantly reduced licking time in the second phase of the formalin test in the study by Lee et al. (2019) , and this effect was mediated by the opioid system. It also reduced the number of writhings in a dose-dependent manner but did not display activity on thermal hyperalgesia ( Lee et al., 2019 ). In the study by Lima et al. (2012) the EO of Chrysopogon zizanioides L (Roberty, Poaceae) produced antinociception similar to morphine in the acetic acid test, and this effect was partially reversed by naloxone. Moreover, it reduced the licking time in the second phase of the formalin test, but it did not demonstrate any effects in the Hargreaves’ test.
A common trait is the presence of antiinflammatory analgesia devoid of thermal anti-hyperalgesic effect. The EO of Zhumeria majdae Rech. F. and Wendelbo (Lamiaceae) has displayed dose-related antinociceptive properties in the acetic acid and in the hot-plate test ( Miraghazadeh et al., 2015 ). Chamaecyparis obtuse has also shown analgesia in the writhings and in the formalin, but not in the hot-plate test ( Park et al., 2015 ). Furthermore, in the study by Mishra et al. (2010) Senecio rufinervis D.C. (Asteraceae) produced significant and dose-dependent inhibition of writhes and thermal hyperalgesia. In the study by Sharif et al. (2020) Tanacetum balsamita (Compositae) presented an anti-hyperalgesic effect. The antinociceptive properties exerted by Xylopia laevigata (Annonaceae) in the acetic acid and in the formalin test have not proven dependency on opioid pathways ( Queiroz et al., 2014 ). The antinociceptive effect of Bunium persicum (Boiss.) is reversed by naloxone and attenuated by chlorpheniramine and cimetidine ( Zendehdel et al., 2015 ), thus confirming the complex neuromodulation and the involvement of histamine in nociception ( Hayashi et al., 2020 ). The main features of the studies on EOs analgesia in nociceptive models are summarized in Table 1 .
3.2.2 Essential Oils Endowed With Efficacy in Neuropathic Models
Studies assessing the analgesic properties of EOs in neuropathic pain models are fundamental because these painful conditions are the most appropriate to model chronic neuropathic pain in humans. In the study by Komatsu et al. (2018) the EO of bergamot (Citrus bergamia Risso) was demonstrated to reduce partial sciatic nerve ligation (PSNL)-induced mechanical allodynia on the seventh post-operative day, in which it peaks ( Kusunose et al., 2010 ). In the study by Kuwahata et al. (2013) the EO of bergamot increased mechanical thresholds dose-dependently and significantly at a dose of 20 μg/paw ( Kuwahata et al., 2013 ). Moreover, this anti-allodynic effect is stronger than that of comparable doses of morphine, of which the EO of bergamot enhances the activity ( Kuwahata et al., 2013 ), and it was reversed by naloxone methiodide, peripherally μ-opioid receptor preferring antagonist, β-funaltrexamine hydrochloride, selective μ-opioid receptor antagonist, and β-endorphin antiserum, but not by the non-selective δ-opioid receptor antagonist naltrindole and by the selective κ-opioid receptor antagonist nor-binaltorphimine. Importantly, the study by Hamamura et al. (2020) in which the EO of bergamot was administered s. c. with an osmotic pump to allow a continuous delivery devoid of smell during PSNL, demonstrated that the anti-allodynic effect of this EO is systemic and does not depend on olfactory stimulation. In this study ( Hamamura et al., 2020 ) the increase of planar activity during the light period induced by PSNL, with the maximum effect at the seventh post-operative day and like allodynia, was shown to be abolished by continuously administered EO. This effect is antagonized by naloxone hydrochloride. Observation lasting 14 days with a theoretical duration of the osmotic pump of one week can mimic administration during chronic pain. The main features of the studies on EOs anti-allodyinic properties are summarized in Table 2 .
3.3 Risk of Bias Assessment
The studies included in the qualitative analysis were assessed for methodological quality according to the SYRCLE’s RoB tool ( Hooijmans et al., 2014 ) and the CAMARADES checklist ( Macleod et al., 2004 ; Hooijmans et al., 2014 ; Suokas et al., 2014 ), based on the Cochrane RoB ( Sterne et al., 2019 ). These items comprise all the possible forms of bias. 1) Selection bias–sequence generation (allocation sequence able to produce comparable groups). 2) Selection bias–baseline characteristics (comparable and not adjusted for confounders in the analysis). 3) Selection bias–allocation concealment (during the enrollment). 4) Performance bias–random housing and randomization during the study. 5) Performance bias–blinding of investigators during the study. 6) Detection bias–random outcome assessment. 7) Detection bias–blinding of outcome assessors. 8) Attrition bias (animals eventually excluded from outcome assessment). 9) Reporting bias–reports free of selective outcome reporting. Finally, 10) other sources of bias: lack of evidence of induced pain using the selected behavioral outcome measure before EO administration and examination (i.e., sham procedure), clear description of methods with number of animals used, attention to circadian regulation for behavioral studies, use of the same observer for behavioral tests, use of control and positive and negative control drugs, sample size calculation, statement of conflict of interest, statement of compliance with animal welfare regulations and attention to ethics.
In terms of the two items regarding selection bias, no study reported the method of allocation and, even though they conducted baseline measures, none of the studies describe how experimental groups were composed to ensure homogeneity and consistency. Only the study by Lima and collaborators ( Lima et al., 2012 ) in which mice with baseline latencies of more than 10 s, and studies by de Oliveira Júnior and colleagues ( de Oliveira Júnior et al., 2017 ; de Oliveira et al., 2018 ) of more than 20 s, at the hot-plate were excluded from the experiments.
As reported in Table 1 , five studies ( Guimaraes et al., 2009 ; Andrade et al., 2012 ; Lima et al., 2012 ; de Oliveira Júnior et al., 2017 ; de Oliveira et al., 2018 ) adopted random housing of mice. The paper by Khodabakhsh et al. reported no randomization of mice but only of rats, which are not included in this systematic review and meta-analysis ( Khodabakhsh et al., 2015 ). Mice were tested in a randomized order in studies by Sakurada and collaborators and Katsuyama et al., 2015 ( Sakurada et al., 2011 ; Katsuyama et al., 2015 ). In the study by Bae et al. (2020) mice were randomly assigned to groups. The study by Khalid et al. (2011) used a blind, randomized design. Mice were randomly assigned to groups and experiments were performed in a blind manner in the study by Quintans and coworkers ( Quintans et al., 2017 ). Moreover, in the study by Ximenes et al. (2013) , the observation was conducted by a blind observer, but the number of animals used for behavioral testing was not reported, only for histological assays. Otherwise, the number of animals per group was reported, but studies that provided a range and not an exact number were not considered eligible for quantitative analysis. Attrition and reporting biases cannot be assessed from the full text of the included studies. Importantly, sham procedure and the certainty of exact execution of the pain model is present only in studies on allodynia, i.e., the studies by Hamamura et al. (2020) , Komatsu et al. (2018a) , and Kuwahata et al. (2013) .
Attention to the circadian rhythm in behavioral testing was reported by the following studies: ( Andrade et al., 2012 ; Quintans et al., 2017 ; Guimaraes et al., 2009 ; Halder et al., 2012 ; Katsuyama et al., 2015 ; Khodabakhsh et al., 2015 ; Miraghazadeh et al., 2015 ; Sakurada et al., 2011 ; Sharif et al., 2020 ; Zarei et al., 2018 ; Zendehdel et al., 2015 ; Komatsu et al., 2018b ; Kuwahata et al., 2013 ). All the studies used control and positive and negative modulators. Importantly, multiple controls were used in the following studies ( Katsuyama et al., 2015 ; Komatsu et al., 2018b ; Kuwahata et al., 2013 ; Sakurada et al., 2011 ). Behavioral testing was conducted by the same observers in the following studies ( de Oliveira Júnior et al., 2017 ; de Oliveira et al., 2018 ; Venãncio et al., 2011 ). Sample size calculation was not reported and the conflict of interest statement is present only in eight studies ( Queiroz et al., 2014 ; Nogueira et al., 2015 ; de Oliveira Júnior et al., 2017 ; de Oliveira et al., 2018 ; Jahandar et al., 2018 ; Zarei et al., 2018 ; Lee et al., 2019 ; Hamamura et al., 2020 ). This could be due to the lack of requirement of these aspects in journals in the last few years. A statement of compliance with animal welfare regulations is reported in all the studies since it is an inclusion criterion. Moreover, six studies ( Ulku Karabay–Yavasoglu et al., 2006 ; Venâncio et al., 2011 ; Queiroz et al., 2014 ; Khodabakhsh et al., 2015 ; Miraghazadeh et al., 2015 ; Sharif et al., 2020 ) also stated that they used each mouse only once, thus proving particular attention to animal welfare. Importantly, only the study by Hamamura et al. (2020) reported acclimatization to lighting conditions for one week and that an observation period of 14 days can model examination during chronic pain.
3.4 Meta-Analysis
This meta-analysis comprises eight studies for a total of 140 mice. The studies were considered comparable when the analgesic outcome was expressed as mean ± standard error of the mean (SEM) since these measures could be converted for meta-analysis in mean and standard deviation (SD). Moreover, only studies reporting the exact number of animals per group were included in quantitative analysis. Studies investigating the same pain model were considered. The formalin test pain model was chosen since it provides a biphasic nociceptive response. Due to the sensitization processes occurring during the second phase, the study on mechanical allodynia expressed has been included ( Komatsu et al., 2018b ). The results of the forest plot favor the analgesic efficacy of EO (Mean difference MD −59.77; 95% CI (−93.32) - (−26.22); I 2 = 94%; p < 0.00001; Figure 3 ), but need to be carefully examined because of the extremely high heterogeneity, which is also confirmed by the asymmetry of the funnel plot analysis standing for high risk of publication bias, small studies and high differences in study precision.
FIGURE 3 . Forest plot for EOs-induced analgesia. The results of the meta-analysis favor the efficacy of the EOs, but they are affected by high heterogeneity (Mean difference MD −59.77; 95% CI (−93.32) - (−26.22); I 2 = 94%; p < 0.00001).
4 Discussion
Interest in the use of EOs and aromatherapy has been continuously growing during the last few decades in parallel with preclinical research. However, in spite of all this effort of preclinical research, it is necessary to establish whether there is a strong rationale for the clinical use of EOs. This issue is even more controversial in the field of pain relief since the use of aromatherapy could reduce the dose of painkillers endowed with serious side effects, particularly in under studied areas of neuropathic pain, like opioids ( Morrone et al., 2017 ; Scuteri et al., 2020b ). Alternative pain treatments could increase time in treatment before the loss of efficacy. This is relevant to fragile populations, e.g., patients suffering from dementia, who are often undertreated compared to cognitively intact counterparts, more so during the Sars-CoV2 pandemic ( Scuteri et al., 2020c ).
This systematic review and meta-analysis assesses the efficacy of EOs in preclinical models of acute inflammatory nociception and neuropathic pain to understand if there is a rational basis for clinical translation. Several EOs from multiple families were found to be efficacious, in particular, croton and bergamot EOs have been extensively studied. It is noteworthy that 27 out of the 30 studies included in the qualitative analysis were only performed on acute pain models like writhings and the hot plate test. These tests are very useful since they are easy to conduct and provide fast results, but they do not resemble clinical pain conditions. Taking this into account, the quantitative analysis only includes studies on formalin test, which is more similar to clinical conditions due to its biphasic nature, and the only study on mechanical allodynia that could compare to the other seven included.
All these studies included in this review have a different experimental design and most of them present serious concerns in terms of selection, performance, and detection biases. Most studies do not adhere to the guidelines for Animal Research: Reporting In Vivo Experiments (ARRIVE), which are fundamental for accurate in vivo preclinical research ( Rice et al., 2013 ). Another methodological aspect responsible for bias in the meta-analysis is that control groups were often used in more than one experiment, and studies including multiple comparisons can introduce errors. Thus, this systematic review and meta-analysis points to the importance of appropriate in vivo modeling to enhance the translational impact of pain research. Future research is necessary to improve the methodological quality and homogeneity of studies.
The results of the meta-analysis highlighted the efficacy of EOs in preclinical pain, but these data are downgraded due to the high heterogeneity of the studies. In particular, the analyzed EOs present the analgesic efficacy required by the recommendations of the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) ( Turk et al., 2003 ), according to which a decrease in pain is defined as clinically meaningful if it accounts for a 30% to 36% reduction. However, this is referred to chronic pain and this systematic review and meta-analysis have found that only the EO of bergamot had proven efficacy both in nociceptive and in neuropathic pain models. Moreover, it was also studied for 14 days, an experimental setting suitable for modeling chronic pain ( Hamamura et al., 2020 ).
Another important issue is that the consolidated data come from hypothesis-generating completely original preclinical studies and that they are then confirmed by hypothesis-driven studies ( Mikolajewicz and Komarova, 2019 ). In this case, the EO of bergamot was confirmed to have strong analgesic properties in some of the most used and reliable models of inflammatory pain, i.e., formalin and capsaicin test in different experiments, sharing with most EO mechanisms involving opioid neurotransmission, and also in the PSNL. To the best of our knowledge, this is the first meta-analysis of preclinical studies on the analgesic effects of EOs and its working hypothesis was verified for bergamot EO, which could represent an important pharmacological tool for pain management in clinical settings. Along with clinical translations, more efforts are required to standardize in vivo preclinical studies in the field of pain research to allow for consistent research able to elucidate the mechanisms responsible for the analgesic properties of EOs.
Data Availability Statement
The original contributions presented in the study are included in the article.
Author Contributions
DS, GB, TS, SS, and MTC. conceived the study. All Authors participated in preparation and read and approved the final manuscript.
DS is a post-doc recipient of a research grant salary as part of the research project (Tutor: GB) “Pharmacoepidemiology of drugs used in the treatment of neuropsychiatric symptoms and pain in people aged (over 65) with dementia” funded by Calabria Region (POR Calabria FESR-FSE 2014/2020—Linea B) Azione 10.5.12.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abuhamdah, S., Huang, L., Elliott, M. S., Howes, M. J., Ballard, C., Holmes, C., et al. (2008). Pharmacological profile of an essential oil derived from Melissa officinalis with anti-agitation properties: focus on ligand-gated channels. J. Pharm. Pharmacol. 60 (3), 377–384. doi:10.1211/jpp.60.3.0014
PubMed Abstract | CrossRef Full Text | Google Scholar
Achterberg, W., Lautenbacher, S., Husebo, B., Erdal, A., and Herr, K. (2020). Pain in dementia. Pain Rep. 5, e803. doi:10.1097/PR9.0000000000000803
Adam, B., Liebregts, T., Best, J., Bechmann, L., Lackner, C., Neumann, J., et al. (2006). A combination of peppermint oil and caraway oil attenuates the post-inflammatory visceral hyperalgesia in a rat model. Scand. J. Gastroenterol. 41 (2), 155–160. doi:10.1080/00365520500206442
Ali, T., Javan, M., Sonboli, A., and Semnanian, S. (2012). Evaluation of the antinociceptive and anti-inflammatory effects of essential oil of Nepeta pogonosperma Jamzad et Assadi in rats. Daru 20, 48. doi:10.1186/2008-2231-20-48
Anaya-Eugenio, G. D., Rivero-Cruz, I., Bye, R., Linares, E., and Mata, R. (2016). Antinociceptive activity of the essential oil from Artemisia ludoviciana. J. Ethnopharmacol 179, 403–411. doi:10.1016/j.jep.2016.01.008
Andrade, G. S., Guimaraes, A. G., Santana, M. T., Siqueira, R. S., Passos, L. O., Machado, S. M. F., et al. (2012). Phytochemical screening, antinociceptive and anti-inflammatory effects of the essential oil of Myrcia pubiflora in mice. Revista Brasileira De Farmacognosia 22 (1), 181–188. doi:10.1590/s0102-695x2011005000205
CrossRef Full Text | Google Scholar
Awad, R., Muhammad, A., Durst, T., Trudeau, V. L., and Arnason, J. T. (2009). Bioassay-guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity. Phytother Res. 23 (8), 1075–1081. doi:10.1002/ptr.2712
Bae, A. H., Kim, G., Seol, G. H., Lee, S. B., Lee, J. M., Chang, W., et al. (2020). Delta- and mu-opioid pathways are involved in the analgesic effect of Ocimum basilicum L in mice. J. Ethnopharmacol 250, 112471. doi:10.1016/j.jep.2019.112471
Bagetta, G., Morrone, L. A., Rombolà, L., Amantea, D., Russo, R., Berliocchi, L., et al. (2010). Neuropharmacology of the essential oil of bergamot. Fitoterapia 81 (6), 453–461. doi:10.1016/j.fitote.2010.01.013
Ball, E. L., Owen-Booth, B., Gray, A., Shenkin, S. D., Hewitt, J., and McCleery, J. (2020). Aromatherapy for dementia. Cochrane Database Syst. Rev. 8, CD003150. doi:10.1002/14651858.CD003150.pub3
Belardo, C., Iannotta, M., Boccella, S., Rubino, R. C., Ricciardi, F., Infantino, R., et al. (2019). Oral cannabidiol prevents allodynia and neurological dysfunctions in a mouse model of mild traumatic brain injury. Front. Pharmacol. 10, 352. doi:10.3389/fphar.2019.00352
Boccella, S., Marabese, I., Guida, F., Luongo, L., Maione, S., and Palazzo, E. (2020). The modulation of pain by metabotropic glutamate receptors 7 and 8 in the dorsal striatum. Curr. Neuropharmacol. 18 (1), 34–50. doi:10.2174/1570159X17666190618121859
Chen, Y., Zhao, Y. Y., Wang, X. Y., Liu, J. T., Huang, L. Q., and Peng, C. S. (2011). [GC-MS analysis and analgesic activity of essential oil from fresh rhizoma of Cyperus rotundus]. Zhong Yao Cai 34 (8), 1225–1229.
PubMed Abstract Google Scholar
Dastmalchi, K., Ollilainen, V., Lackman, P., Boije af Gennäs, G., Dorman, H. J., Järvinen, P. P., et al. (2009). Acetylcholinesterase inhibitory guided fractionation of Melissa officinalis L. Bioorg. Med. Chem. 17 (2), 867–871. doi:10.1016/j.bmc.2008.11.034
de Oliveira Júnior, R. G., Ferraz, C. A. A., Silva, J. C., de Oliveira, A. P., Diniz, T. C., E Silva, M. G., et al. (2017). Antinociceptive effect of the essential oil from Croton conduplicatus Kunth (euphorbiaceae). Molecules 22 (6), 900. doi:10.3390/molecules22060900
de Oliveira, R. G., Ferraz, C. A. A., Silva, J. C., Teles, R. B. D., Silva, M. G., Diniz, T. C., et al. (2018). Neuropharmacological effects of essential oil from the leaves of Croton conduplicatus Kunth and possible mechanisms of action involved. J. Ethnopharmacology 221, 65–76. doi:10.1016/j.jep.2018.04.009
DerSimonian, R., and Kacker, R. (2007). Random-effects model for meta-analysis of clinical trials: an update. Contemp. Clin. Trials 28 (2), 105–114. doi:10.1016/j.cct.2006.04.004
Do Nascimento Silva, A., Bomfim, H. F., Magalhães, A. O., Da Rocha, M. L., and Lucchese, A. M. (2018). Chemical composition and antinociceptive activity of essential oil from myrcia rostrata dc. (myrtaceae) in animal models. Quimica Nova 41 (9), 982–988. doi:10.21577/0100-4042.20170274
Duval, S., and Tweedie, R. (2000). Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56 (2), 455–463. doi:10.1111/j.0006-341x.2000.00455.x
Editorial. (2016). Meta-analysis in basic biology. Nat. Methods 13 (12), 959. doi:10.1038/nmeth.4102
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315 (7109), 629–634. doi:10.1136/bmj.315.7109.629
Evans, L. K. (1987). Sundown syndrome in institutionalized elderly. J. Am. Geriatr. Soc. 35 (2), 101–108. doi:10.1111/j.1532-5415.1987.tb01337.x
Guginski, G., Luiz, A. P., Silva, M. D., Massaro, M., Martins, D. F., Chaves, J., et al. (2009). Mechanisms involved in the antinociception caused by ethanolic extract obtained from the leaves of Melissa officinalis (lemon balm) in mice. Pharmacol. Biochem. Behav. 93 (1), 10–16. doi:10.1016/j.pbb.2009.03.014
Guimaraes, A. G., Melo, M. S., Bonfim, R. R., Passos, L. O., Machado, S. M. F., Ribeiro, A. D., et al. (2009). Antinociceptive and anti-inflammatory effects of the essential oil of Eugenia candolleana DC., Myrtaceae, on mice. Revista Brasileira De Farmacognosia 19 (4), 883–887. doi:10.1590/s0102-695x2009000600016
GVR (2020). Report No.: 978-1-68038-549-6. 1-153. Essential oils market size, share & trends analysis report by application (food & beverages, spa & relaxation), by product (orange, peppermint), by sales channel, and segment forecasts, 2020–2027.
Google Scholar
Hajhashemi, V., Sajjadi, S. E., and Heshmati, M. (2009). Anti-inflammatory and analgesic properties of Heracleum persicum essential oil and hydroalcoholic extract in animal models. J. Ethnopharmacol 124 (3), 475–480. doi:10.1016/j.jep.2009.05.012
Halder, S., Mehta, A. K., Mediratta, P. K., and Sharma, K. K. (2012). Acute effect of essential oil of Eugenia caryophyllata on cognition and pain in mice. Naunyn Schmiedebergs Arch. Pharmacol. 385 (6), 587–593. doi:10.1007/s00210-012-0742-2
Hamamura, K., Katsuyama, S., Komatsu, T., Scuteri, D., Bagetta, G., Aritake, K., et al. (2020). Behavioral effects of continuously administered bergamot essential oil on mice with partial sciatic nerve ligation. Front. Pharmacol. 11, 1310. doi:10.3389/fphar.2020.01310
Hamm, R. J., and Knisely, J. S. (1985). Environmentally induced analgesia: an age-related decline in an endogenous opioid system. J. Gerontol. 40 (3), 268–274. doi:10.1093/geronj/40.3.268
Hayashi, T., Watanabe, C., Katsuyama, S., Agatsuma, Y., Scuteri, D., Bagetta, G., et al. (2020). Contribution of histamine to nociceptive behaviors induced by intrathecally administered cholecystokinin-8. Front. Pharmacol. 11, 590918. doi:10.3389/fphar.2020.590918
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Hooijmans, C. R., Rovers, M. M., de Vries, R. B., Leenaars, M., Ritskes-Hoitinga, M., and Langendam, M. W. (2014). SYRCLE's risk of bias tool for animal studies. BMC Med. Res. Methodol. 14 (1), 43. doi:10.1186/1471-2288-14-43
Husebo, B. S., Ballard, C., Sandvik, R., Nilsen, O. B., and Aarsland, D. (2011). Efficacy of treating pain to reduce behavioural disturbances in residents of nursing homes with dementia: cluster randomised clinical trial. BMJ 343, d4065. doi:10.1136/bmj.d4065
Jahandar, F., Asgarpanah, J., Najafizadeh, P., and Mousavi, Z. (2018). Anti-inflammatory activity and chemical composition of Pycnocycla bashagardiana fruit’s essential oil in animal models. Iran J. Basic Med. Sci. 21 (2), 188–193. doi:10.22038/ijbms.2017.20860.5426
Jun, Y. S., Kang, P., Min, S. S., Lee, J. M., Kim, H. K., and Seol, G. H. (2013). Effect of eucalyptus oil inhalation on pain and inflammatory responses after total knee replacement: a randomized clinical trial. Evid. Based Complement. Alternat Med. 2013, 502727. doi:10.1155/2013/502727
Katsuyama, S., Otowa, A., Kamio, S., Sato, K., Yagi, T., Kishikawa, Y., et al. (2015). Effect of plantar subcutaneous administration of bergamot essential oil and linalool on formalin-induced nociceptive behavior in mice. Biomed. Res. 36 (1), 47–54. doi:10.2220/biomedres.36.47
Khalid, M. H., Akhtar, M. N., Mohamad, A. S., Perimal, E. K., Akira, A., Israf, D. A., et al. (2011). Antinociceptive effect of the essential oil of Zingiber zerumbet in mice: possible mechanisms. J. Ethnopharmacol 137 (1), 345–351. doi:10.1016/j.jep.2011.05.043
Khodabakhsh, P., Shafaroodi, H., and Asgarpanah, J. (2015). Analgesic and anti-inflammatory activities of Citrus aurantium L. blossoms essential oil (neroli): involvement of the nitric oxide/cyclic-guanosine monophosphate pathway. J. Nat. Med. 69 (3), 324–331. doi:10.1007/s11418-015-0896-6
Komatsu, T., Katsuyama, S., Uezono, Y., Sakurada, C., Tsuzuki, M., Hamamura, K., et al. (2018a). Possible involvement of the peripheral Mu-opioid system in antinociception induced by bergamot essential oil to allodynia after peripheral nerve injury. Neurosci. Lett. 686, 127–132. doi:10.1016/j.neulet.2018.08.053
Komatsu, T., Katsuyama, S., Uezono, Y., Sakurada, C., Tsuzuki, M., Hamamura, K., et al. (2018b). Possible involvement of the peripheral Mu-opioid system in antinociception induced by bergamot essential oil to allodynia after peripheral nerve injury. Neurosci. Lett. 686, 127–132. doi:10.1016/j.neulet.2018.08.053
Koyama, S., and Heinbockel, T. (2020). The effects of essential oils and terpenes in relation to their routes of intake and application. Int. J. Mol. Sci. 21 (5), 1558. doi:10.3390/ijms21051558
Kusunose, N., Koyanagi, S., Hamamura, K., Matsunaga, N., Yoshida, M., Uchida, T., et al. (2010). Molecular basis for the dosing time-dependency of anti-allodynic effects of gabapentin in a mouse model of neuropathic pain. Mol. Pain 6, 83. doi:10.1186/1744-8069-6-83
Kuwahata, H., Komatsu, T., Katsuyama, S., Corasaniti, M. T., Bagetta, G., Sakurada, S., et al. (2013). Peripherally injected linalool and bergamot essential oil attenuate mechanical allodynia via inhibiting spinal ERK phosphorylation. Pharmacol. Biochem. Behav. 103 (4), 735–741. doi:10.1016/j.pbb.2012.11.003
Lakhan, S. E., Sheafer, H., and Tepper, D. (2016). The effectiveness of aromatherapy in reducing pain: a systematic review and meta-analysis. Pain Res. Treat. 2016, 8158693. doi:10.1155/2016/8158693
Lee, G., Park, J., Kim, M. S., Seol, G. H., and Min, S. S. (2019). Analgesic effects of eucalyptus essential oil in mice. Korean J. Pain 32 (2), 79–86. doi:10.3344/kjp.2019.32.2.79
Li, F. S., and Weng, J. K. (2017). Demystifying traditional herbal medicine with modern approach. Nat. Plants 3 (8), 17109. doi:10.1038/nplants.2017.109
Li, W., Chen, Y., Wang, X., and Qu, S. (1991). [Pharmacological studies on the volatile oil isolated from the leaves of Pinus pumila (Pall.) Regel]. Zhongguo Zhong Yao Za Zhi 16 (3), 172–192.
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLos Med. 6, e1000100. doi:10.1371/journal.pmed.1000100
Lima, G. M., Quintans-Júnior, L. J., Thomazzi, S. M., Almeida, E. M. S. A., Melo, M. S., Serafini, M. R., et al. (2012). Phytochemical screening, antinociceptive and anti-inflammatory activities of Chrysopogon zizanioides essential oil. Braz. J. Pharmacognosy 22 (2), 443–450. doi:10.1590/S0102-695X2012005000002
Linciano, P., Citti, C., Russo, F., Tolomeo, F., Laganà, A., Capriotti, A. L., et al. (2020). Identification of a new cannabidiol n-hexyl homolog in a medicinal cannabis variety with an antinociceptive activity in mice: cannabidihexol. Sci. Rep. 10 (1), 22019. doi:10.1038/s41598-020-79042-2
Lizarraga-Valderrama, L. R. (2020). Effects of essential oils on central nervous system: focus on mental health. Phytother Res. [Epub ahead of print]. doi:10.1002/ptr.6854
López, V., Nielsen, B., Solas, M., Ramírez, M. J., and Jäger, A. K. (2017). Exploring pharmacological mechanisms of lavender (lavandula angustifolia) essential oil on central nervous system targets. Front. Pharmacol. 8, 280. doi:10.3389/fphar.2017.00280
Macleod, M. R., O’Collins, T., Howells, D. W., and Donnan, G. A. (2004). Pooling of animal experimental data reveals influence of study design and publication bias. Stroke 35 (5), 1203–1208. doi:10.1161/01.STR.0000125719.25853.20
Mikolajewicz, N., and Komarova, S. V. (2019). Meta-analytic methodology for basic research: a practical guide. Front. Physiol. 10, 203. doi:10.3389/fphys.2019.00203
Miraghazadeh, S. G., Shafaroodi, H., and Asgarpanah, J. (2015). Analgesic and antiinflammatory activities of the essential oil of the unique plant Zhumeria majdae. Nat. Prod. Commun. 10 (4), 669–672. doi:10.1590/s2175-97902019000217011
Mishra, D., Bisht, G., Mazumdar, P. M., and Sah, S. P. (2010). Chemical composition and analgesic activity of Senecio rufinervis essential oil. Pharm. Biol. 48 (11), 1297–1301. doi:10.3109/13880209.2010.491083
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Group, P. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLos Med. 6, e1000097. doi:10.1371/journal.pmed.1000097
Morrone, L. A., Rombolà, L., Pelle, C., Corasaniti, M. T., Zappettini, S., Paudice, P., et al. (2007). The essential oil of bergamot enhances the levels of amino acid neurotransmitters in the hippocampus of rat: implication of monoterpene hydrocarbons. Pharmacol. Res. 55 (4), 255–262. doi:10.1016/j.phrs.2006.11.010
Morrone, L. A., Scuteri, D., Rombolà, L., Mizoguchi, H., and Bagetta, G. (2017). Opioids resistance in chronic pain management. Curr. Neuropharmacol 15 (3), 444–456. doi:10.2174/1570159X14666161101092822
Neves, I. A., Rezende, S. R. F., Kirk, J. M., Pontes, E. G., de Carvalho, M., and Gamble, A. (2017). Composition and larvicidal activity of essential oil of Eugenia candolleana DC. (MYRTACEAE) against Aedes aegypti . Rev. Virtual Quim. 9 (6), 2305–2315. doi:10.21577/1984-6835.20170138
Nogueira, Lde. M., Da Silva, M. R., Dos Santos, S. M., De Albuquerque, J. F., Ferraz, I. C., de Albuquerque, T. T., et al. (2015). Antinociceptive effect of the essential oil obtained from the leaves of croton cordiifolius baill. (Euphorbiaceae) in mice. Evid. Based Complement. Alternat Med. 2015, 620865. doi:10.1155/2015/620865
Park, Y., Jung, S. M., Yoo, S. A., Kim, W. U., Cho, C. S., Park, B. J., et al. (2015). Antinociceptive and anti-inflammatory effects of essential oil extracted from Chamaecyparis obtusa in mice. Int. Immunopharmacol 29 (2), 320–325. doi:10.1016/j.intimp.2015.10.034
Perry, N. S., Houghton, P. J., Theobald, A., Jenner, P., and Perry, E. K. (2000). In-vitro inhibition of human erythrocyte acetylcholinesterase by salvia lavandulaefolia essential oil and constituent terpenes. J. Pharm. Pharmacol. 52 (7), 895–902. doi:10.1211/0022357001774598
Queiroz, J. C., Antoniolli, A. R., Quintans-Júnior, L. J., Brito, R. G., Barreto, R. S., Costa, E. V., et al. (2014). Evaluation of the anti-inflammatory and antinociceptive effects of the essential oil from leaves of xylopia laevigata in experimental models. Sci. World J. 2014, 816450. doi:10.1155/2014/816450
Quintans, J. S., Alves, R. D., Santos, D. A., Serafini, M. R., Alves, P. B., Costa, E. V., et al. (2017). Antinociceptive effect of Aristolochia trilobata stem essential oil and 6-methyl-5-hepten-2yl acetate, its main compound, in rodents. Z. Naturforsch C J. Biosci. 72 (3-4), 93–97. doi:10.1515/znc-2016-0053
Quintans, J. S., Antoniolli, A. R., Almeida, J. R., Santana-Filho, V. J., and Quintans-Júnior, L. J. (2014). Natural products evaluated in neuropathic pain models - a systematic review. Basic Clin. Pharmacol. Toxicol. 114 (6), 442–450. doi:10.1111/bcpt.12178
Ribeiro, S. (2018). “Whole organisms or pure compounds? Entourage effect versus drug specificity,” in Plant medicines, healing and psychedelic science . Editors B. Labate, and C. Cavnar (Cham: Springer ).
Rice, A. S. C., Morland, R., Huang, W., Currie, G. L., Sena, E. S., and Macleod, M. R. (2013). Transparency in the reporting of in vivo pre-clinical pain research: the relevance and implications of the ARRIVE (Animal Research: reporting in Vivo Experiments) guidelines. Scand. J. Pain 4 (2), 58–62. doi:10.1016/j.sjpain.2013.02.002
Rombolà, L., Tridico, L., Scuteri, D., Sakurada, T., Sakurada, S., Mizoguchi, H., et al. (2017). Bergamot essential oil attenuates anxiety-like behaviour in rats. Molecules 22 (4), 614. doi:10.3390/molecules22040614
Rombolà, L., Scuteri, D., Adornetto, A., Straface, M., Sakurada, T., Sakurada, S., et al. (2019). Anxiolytic-like effects of bergamot essential oil are insensitive to flumazenil in rats. Evid. Based Complement. Alternat Med. 2019 , 2156873. doi:10.1155/2019/2156873
Rombolà, L., Scuteri, D., Watanabe, C., Sakurada, S., Hamamura, K., Sakurada, T., et al. (2020). Role of 5-HT1A receptor in the anxiolytic-relaxant effects of bergamot essential oil in rodent. Int. J. Mol. Sci. 21 (7), 2597. doi:10.3390/ijms21072597
Ryan, R. (2019). Cochrane Consumers and Communication Review Group: data synthesis and analysis. Available at: http://cccrg.cochrane.org (Accessed March 13, 2019).
Sakurada, T., Mizoguchi, H., Kuwahata, H., Katsuyama, S., Komatsu, T., Morrone, L. A., et al. (2011). Intraplantar injection of bergamot essential oil induces peripheral antinociception mediated by opioid mechanism. Pharmacol. Biochem. Behav. 97 (3), 436–443. doi:10.1016/j.pbb.2010.09.020
Sarmento-Neto, J. F., do Nascimento, L. G., Felipe, C. F., and de Sousa, D. P. (2015). Analgesic potential of essential oils. Molecules 21 (1), E20. doi:10.3390/molecules21010020
Savelev, S., Okello, E., Perry, N. S., Wilkins, R. M., and Perry, E. K. (2003). Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 75 (3), 661–668. doi:10.1016/s0091-3057(03)00125-4
Savelev, S. U., Okello, E. J., and Perry, E. K. (2004). Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother Res. 18 (4), 315–324. doi:10.1002/ptr.1451
Scherder, E. J., Sergeant, J. A., and Swaab, D. F. (2003). Pain processing in dementia and its relation to neuropathology. Lancet Neurol. 2 (11), 677–686. doi:10.1016/s1474-4422(03)00556-8
Scuteri, D., Morrone, L. A., Rombolà, L., Avato, P. R., Bilia, A. R., Corasaniti, M. T., et al. (2017a). Aromatherapy and aromatic plants for the treatment of behavioural and psychological symptoms of dementia in patients with alzheimer's disease: clinical evidence and possible mechanisms. Evid. Based Complement. Alternat Med. 2017, 9416305. doi:10.1155/2017/9416305
Scuteri, D., Piro, B., Morrone, L. A., Corasaniti, M. T., Vulnera, M., and Bagetta, G. (2017b). The need for better access to pain treatment: learning from drug consumption trends in the USA. Funct. Neurol. 22 (4), 229–230. doi:10.11138/fneur/2017.32.4.229
Scuteri, D., Crudo, M., Rombolà, L., Watanabe, C., Mizoguchi, H., Sakurada, S., et al. (2018a). Antinociceptive effect of inhalation of the essential oil of bergamot in mice. Fitoterapia 129, 20–24. doi:10.1016/j.fitote.2018.06.007
Scuteri, D., Garreffa, M. R., Esposito, S., Bagetta, G., Naturale, M. D., and Corasaniti, M. T. (2018b). Evidence for accuracy of pain assessment and painkillers utilization in neuropsychiatric symptoms of dementia in Calabria region, Italy. Neural Regen. Res. 13 (9), 1619–1621. doi:10.4103/1673-5374.237125
Scuteri, D., Rombolà, L., Morrone, L. A., Bagetta, G., Sakurada, S., Sakurada, T., et al. (2019a). Neuropharmacology of the neuropsychiatric symptoms of dementia and role of pain: essential oil of bergamot as a novel therapeutic approach. Int. J. Mol. Sci. 20 (13), 3327. doi:10.3390/ijms20133327
Scuteri, D., Rombolá, L., Tridico, L., Mizoguchi, H., Watanabe, C., Sakurada, T., et al. (2019b). Neuropharmacological properties of the essential oil of bergamot for the clinical management of pain-related BPSDs. Curr. Med. Chem. 26 (20), 3764–3774. doi:10.2174/0929867325666180307115546
Scuteri, D., Berliocchi, L., Rombolà, L., Morrone, L. A., Tonin, P., Bagetta, G., et al. (2020a). Effects of aging on formalin-induced pain behavior and analgesic activity of gabapentin in C57BL/6 mice. Front. Pharmacol. 11, 663. doi:10.3389/fphar.2020.00663
Scuteri, D., Mantovani, E., Tamburin, S., Sandrini, G., Corasaniti, M. T., Bagetta, G., et al. (2020b). Opioids in post-stroke pain: a systematic review and meta-analysis. Front. Pharmacol. [Epub ahead of print]. doi:10.3389/fphar.2020.587050
Scuteri, D., Matamala-Gomez, M., Bottiroli, S., Corasaniti, M. T., De Icco, R., Bagetta, G., et al. (2020c). Pain assessment and treatment in dementia at the time of coronavirus disease COVID-19. Front. Neurol. 11, 890. doi:10.3389/fneur.2020.00890
Scuteri, D., Rombolà, L., Morrone, L. A., Monteleone, D., Corasaniti, M. T., Sakurada, T., et al. (2020d). “Exploitation of aromatherapy in dementia-impact on pain and neuropsychiatric symptoms,” in The neuroscience of dementia: diagnosis and management in dementia . Editors V. R. Preedy, and C. R. Martin (San Diego: Academic Press ), 713–726.
Scuteri, D., Rombolà, L., Watanabe, C., Sakurada, S., Corasaniti, M. T., Bagetta, G., et al. (2020e). Impact of nutraceuticals on glaucoma: a systematic review. Prog. Brain Res. 257, 141–154. doi:10.1016/bs.pbr.2020.07.014
Scuteri, D., Vulnera, M., Piro, B., Bossio, R. B., Morrone, L. A., Sandrini, G., et al. (2020f). Pattern of treatment of behavioural and psychological symptoms of dementia and pain: evidence on pharmacoutilization from a large real-world sample and from a centre for cognitive disturbances and dementia. Eur. J. Clin. Pharmacol. [Epub ahead of print]. doi:10.1007/s00228-020-02995-w
Sharif, M., Najafizadeh, P., Asgarpanah, J., and Mousavi, Z. (2020). In vivo analgesic and anti-inflammatory effects of the essential oil from Tanacetum balsamita L. Braz. J. Pharm. Sci. 56, e18357. doi:10.1590/s2175-97902019000418357
Sofi, P. A., Zeerak, N. A., and Singh, P. (2009). Kala zeera (Bunium persicum Bioss.): a Kashmirian high value crop. Turkish J. Biol. 33, 249–258. doi:10.3906/biy-0803-18
Stein, C., Hopfeld, J., Lau, H., and Klein, J. (2015). Effects of ginkgo biloba extract EGb 761, donepezil and their combination on central cholinergic function in aged rats. J. Pharm. Pharm. Sci. 18 (4), 634–646. doi:10.18433/j3wc8v
Sterne, J. A., and Egger, M. (2001). Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J. Clin. Epidemiol. 54 (10), 1046–1055. doi:10.1016/s0895-4356(01)00377-8
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Sulaiman, M. R., Tengku Mohamad, T. A., Shaik Mossadeq, W. M., Moin, S., Yusof, M., Mokhtar, A. F., et al. (2010). Antinociceptive activity of the essential oil of Zingiber zerumbet. Planta Med. 76 (2), 107–112. doi:10.1055/s-0029-1185950
Suokas, A. K., Sagar, D. R., Mapp, P. I., Chapman, V., and Walsh, D. A. (2014). Design, study quality and evidence of analgesic efficacy in studies of drugs in models of OA pain: a systematic review and a meta-analysis. Osteoarthr Cartil 22 (9), 1207–1223. doi:10.1016/j.joca.2014.06.015
Todorova, M., Trendafilova, A., Ivanova, V., Danova, K., and Dimitrov, D. (2017). Essential oil composition of Inula britannica L. from Bulgaria. Nat. Prod. Res. 31 (14), 1693–1696. doi:10.1080/14786419.2017.1285295
Turk, D. C., Dworkin, R. H., Allen, R. R., Bellamy, N., Brandenburg, N., Carr, D. B., et al. (2003). Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain 106 (3), 337–345. doi:10.1016/j.pain.2003.08.001
Ulku Karabay-Yavasoglu, N., Baykan, S., Ozturk, B., Apaydin, S., and Tuglular, I. (2006). Evaluation of the antinociceptive and anti-inflammatory activities of Satureja thymbra. L. Essential oil. Pharm. Biol. 44 (8), 585–591. doi:10.1080/13880200600896827
Venâncio, A. M., Marchioro, M., Estavam, C. S., Melo, M. S., Santana, M. T., Onofre, A. S. C., et al. (2011). Ocimum basilicum leaf essential oil and (-)-linalool reduce orofacial nociception in rodents: a behavioral and electrophysiological approach. Braz. J. Pharmacognosy 21 (6), 1043–1051. doi:10.1590/S0102-695X2011005000147
Wood, H. C., and Reichut, E. T. (1880). Note on the action upon the circulation of certain volatile oils. J. Physiol. 2, 446. doi:10.1113/jphysiol.1880.sp000073
Ximenes, R. M., De Morais Nogueira, L., Cassundé, N. M., Jorge, R. J., Dos Santos, S. M., Magalhães, L. P., et al. (2013). Antinociceptive and wound healing activities of Croton adamantinus Müll. Arg. essential oil. J. Nat. Med. 67 (4), 758–764. doi:10.1007/s11418-012-0740-1
Zarei, M., Mohammadi, S., and Komaki, A. (2018). Antinociceptive activity of Inula britannica L. and patuletin: in vivo and possible mechanisms studies. J. Ethnopharmacol 219, 351–358. doi:10.1016/j.jep.2018.03.021
Zaynoun, S. T., Johnson, B. E., and Frain-Bell, W. (1977). A study of oil of bergamot and its importance as a phototoxic agent. I. Characterization and quantification of the photoactive component. Br. J. Dermatol. 96 (5), 475–482. doi:10.1111/j.1365-2133.1977.tb07149.x
Zendehdel, M., Torabi, Z., and Hassanpour, S. (2015). Antinociceptive mechanisms of Bunium persicum essential oil in the mouse writhing test: role of opioidergic and histaminergic systems. Veterinarni Medicina 60 (2), 63–70. doi:10.17221/7988-VETMED
Zhang, L., Li, D., Cao, F., Xiao, W., Zhao, L., Ding, G., et al. (2018). Identification of human acetylcholinesterase inhibitors from the constituents of EGb761 by modeling docking and molecular dynamics simulations. Comb. Chem. High Throughput Screen. 21 (1), 41–49. doi:10.2174/1386207320666171123201910
Keywords: essential oils, pain models, inflammatory pain, neuropathic pain, chronic pain, systematic review, meta-analysis
Citation: Scuteri D, Hamamura K, Sakurada T, Watanabe C, Sakurada S, Morrone LA, Rombolà L, Tonin P, Bagetta G and Corasaniti MT (2021) Efficacy of Essential Oils in Pain: A Systematic Review and Meta-Analysis of Preclinical Evidence. Front. Pharmacol. 12:640128. doi: 10.3389/fphar.2021.640128
Received: 10 December 2020; Accepted: 18 January 2021; Published: 01 March 2021.
Reviewed by:
Copyright © 2021 Scuteri, Hamamura, Sakurada, Watanabe, Sakurada, Morrone, Rombolà, Tonin, Bagetta and Corasaniti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giacinto Bagetta, [email protected] ; Laura Rombolà, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
March 7, 2020
Do Essential Oils Work? Here’s What Science Says
Every time you turn around someone is suggesting aromatherapy. Essential oils are a $1 billion industry, but are they effective?
By Everyday Einstein Sabrina Stierwalt

Madeleine Steinbach Getty Images

Your friend suggests that you use a lotion infused with peppermint essential oil to help combat your nausea. Your coworker insists that he has never slept so well since starting to sprinkle a little lavender oil on his pillow at night. Last year alone consumers in the United States spent $1 billion on essential oil products and is expected to exceed $11 billion by the year 2022. But what does the research say? Do essential oils really work?
What is aromatherapy?
On supporting science journalism.
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Essential oils are oils, typically fragrant ones, that have been extracted from the roots, flowers, leaves, or seeds of plants using steam or applied pressure. The qualifier “essential” refers to the fact that the oil contains the “essence” of the plant (i.e. the natural chemicals that provide a distinct odor or flavor). In the practice of aromatherapy, these oils—once diluted—are applied to the skin, smelled, dabbed on a pillow or in a bath, or heated so that their aroma is dispersed into the air. Some soaps and lotions can also be made with essential oils and used as aromatherapy products.
The use of essential oils is cross-cultural and dates back thousands of years. Many know the story of frankincense being offered as one of the gifts of the Magi. Even if you haven’t purchased an essential oil roller or diffuser, chances are you may have used them anyway. Vick’s Vaporub , typically rubbed on the chest as a cough suppressant, contains the essential eucalyptus, cedarleaf, and nutmeg oils (among others) suspended in petroleum jelly.
Do essential oils and aromatherapy work?
The National Institute of Health provides a thorough summary via the US National Library of Medicine of research conducted into the efficacy of essential oils . Currently, there is no evidence-backed research showing any illnesses that can be cured through the use of essential oils or the practice of aromatherapy. The results on the other possible benefits of essential oils as, for example, mood elevators or stress relievers, are more mixed. But most are still inconclusive.
One of the scientific studies that have revealed positive results from essential oils involves patients with dementia. Although, contrary to common lore, drinking a tablespoon of fish oil every day won’t likely stave off dementia , there is evidence that balm from lemon oil reduces agitation in patients with dementia according to a study in the Journal of Clinical Psychiatry.
There are other proven success stories for essential oils, such as the treatment of acne with tea tree oil and the treatment of alopecia areata or hair loss with oils like thyme, rosemary, lavender and cedarwood.
Research into the use of essential oils found in citrus fruits is particularly intriguing due to their natural antibacterial qualities. For example, citrus oil, particularly when combined with Dead Sea salts, was shown to inhibit bacterial growth in mice and act as an anti-inflammatory agent. The citrus essential oil bergamot could help fight the growth of common causes of food poisoning like listeria, e coli, and staphylococcus.
However, most of these studies have not yet extended to clinical trials, meaning there is still much more work to do before essential oils would be potentially prescribed by physicians. Given the strong public interest in essential oils, whether it be to target things other medicines have so far failed to fix (like migraines, anxiety, stress, insomnia, and memory) or to control what goes into their medicine cabinet without a prescription, more research into the possible benefits of essential oils is clearly worthwhile.
There are very few noted side effects associated with the use of essential oils, although in the US they do not require approval from the FDA . One exception is the estrogen-like effects noted for lavender and tea tree oils which have been linked to breast enlargement in pre-pubescent boys when applied over long periods of time.
So if you’re looking to relieve stress, adding a few drops of diluted essential oils to a warm bath probably doesn’t hurt. But before you spend $40 on a 15-mL bottle, you might want to try a scented candle first.
»Continue reading “Do Essential Oils Work? Here’s What Science Says” on QuickAndDirtyTips.com
The Leaf Essential Oils of Neolitsea vuquangensis : A Rich Resource of β-( E )-Ocimene
- Published: 23 May 2024
Cite this article

- Duong Quang Huan 1 ,
- Nguyen Quang Hop 1 ,
- Do Thi Lan Huong 2 ,
- Do Ngoc Dai 3 ,
- Nguyen Ngoc Linh 4 &
- Ninh The Son 5 , 6
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
L. D. Linh, P. H. Ban, T. M. Hoi, L. T. Huong, and I. A. Ogunwande, J. Essent. Oil-Bear. Plants , 21 , 1257 (2018).
Article CAS Google Scholar
Y. Cao, Z. L. Gao, G. Z. Su, X. L. Yu, P. F. Tu, and X. Y. Chai, Chem. Biodiv ., 12 , 1443 (2015).
R. C. Padalia, C. S. Chanotiya, B. C. Thakuri, and C. S. Mathela, Nat. Prod. Commun ., 2 , 591 (2017).
Google Scholar
Y. C. Su, K. P. Hsu, E. I. C. Wang, and C. L. Ho, Nat. Prod. Commun ., 8 , 531 (2013).
CAS PubMed Google Scholar
W. J. Yoon, J. Y. Moon, J. Y. Kanga, G. O. Kim, N. H. Lee, and C. G. Hyun, Nat. Prod. Commun ., 5 , 1311 (2010).
C. Mitsuyuki, S. Tagane, N. V. Ngoc, H. T. Binh, S. Suddee, S. Rueanguea, H. Toyama, K. Mase, C. J. Yang, A. Naiki, and T. Yahara, Acta Phytotax. Geobot ., 69 , 161 (2018).
L. T. Huong, L. N. Sam, D. N. Dai, P. V. Ty, and N. T. Son, J. Essent. Oil-Bear. Plants , 6 , 1221 (2022).
Article Google Scholar
L. T. Huong, N. T. Son, L. N. Sam, P. N. Minh, N. D. Luyen, N. T. Hao, and D. N. Dai, Nat. Prod. Commun ., 17 , 1 (2022).
N. T. Son, L. T. Anh, D. T. T. Thuy, N. D. Luyen, and T. T. Tuyen, Natl. Acad. Sci. Lett ., 46 , 71 (2023).
N. T. Hao, L. T. Anh, D. T. T. Thuy, N. D. Luyen, T. T. Tuyen, N. M. Ha, and N. T. Son, Chem. Nat. Compd ., 59 , 591 (2023).
J. G. Shyu, C. K. Hsu, K. P. Hsu, M. L. Yang, L. Y. Wei, H. T. Ho, and C. L. Ho, Nat. Prod. Commun ., 18 , 1 (2023).
T. D. Thang, D. N. Dai, T. H. Thai, and I. A. Ogunwande, Rec. Nat. Prod ., 7 , 192 (2013).
CAS Google Scholar
N. T. Viet, L. N. Sam, L. T. Huong, D. N. Dai, and I. A. Ogunwande, Chem. Nat. Compd ., 59 , 334 (2023).
T. D. Tavares, J. C. Antunes, J. Padrao, A. I. Ribeiro, A. Zille, T. P. Amorim, F. Ferreira, and H. P. Felgueiras, Antibiotics , 9 , 314 (2020).
Article CAS PubMed PubMed Central Google Scholar
D. T. M. Chau, N. T. Chung, L. T. Huong, N. H. Hung, I. A. Ogunwande, D. N. Dai, and W. N. Setzer, Plants , 9 , 606 (2020).
Download references
Acknowledgment
This research was funded by Hanoi Pedagogical University 2 foundation for Sciences and Technology Development via grant No. HPU2.2023-UT-05.
Author information
Authors and affiliations.
Faculty of Chemistry, Hanoi Pedagogical University 2 (HPU2), Nguyen Van Linh, Xuanhoa, Phucyen, Vinhphuc, Vietnam
Duong Quang Huan & Nguyen Quang Hop
Faculty of Biology and Agriculture, HPU2, Nguyen Van Linh, Xuanhoa, Phucyen, Vinhphuc, Vietnam
Do Thi Lan Huong
Faculty of Agriculture, Forestry and Fishery, Nghe An University of Economics, 51 Ly Tu Trong, Vinh, Nghean, Vietnam
Do Ngoc Dai
Faculty of Pharmacy, Thanh Do University, Kim Chung, Hoai Duc, Hanoi, 10000, Vietnam
Nguyen Ngoc Linh
Institute of Chemistry, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Caugiay, Hanoi, Vietnam
Ninh The Son
Department of Chemistry, Graduate University of Science and Technology, 18 Hoang Quoc Viet, Caugiay, Hanoi, Vietnam
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Ninh The Son .
Additional information
Published in Khimiya Prirodnykh Soedinenii , No. 3, May–June, 2024, pp. 490–492.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Huan, D.Q., Hop, N.Q., Huong, D.T.L. et al. The Leaf Essential Oils of Neolitsea vuquangensis : A Rich Resource of β-( E )-Ocimene. Chem Nat Compd (2024). https://doi.org/10.1007/s10600-024-04382-8
Download citation
Received : 03 August 2023
Published : 23 May 2024
DOI : https://doi.org/10.1007/s10600-024-04382-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Find a journal
- Publish with us
- Track your research

Avocados or “alligator pears” are known for their creamy smooth flesh and bumpy skin. They are a popular food across many cultures. Perhaps best known as the star ingredient in guacamole, they are versatile and prepared in an array of dishes, or simply eaten plain with a spoon. Although not sweet, avocados are botanically classified as a fruit with a large berry and single center pit, grown from the Persea americana tree. They are believed to have originated in Mexico or Central America, with Mexico being the leading producer worldwide. [1]
Their nutrition profile makes them a staple in various healthful meal plans. Avocados are a good source of fiber, and contain more fat (the good kind) than carbohydrate, so are popular on lower-carbohydrate diets such as with diabetes. Their heart-friendly fats do not increase blood cholesterol, which can provide satisfaction on a traditional cholesterol-lowering diet that is often low in fat and cholesterol. It is one the highest-fat plant foods, making it a popular inclusion in vegan and vegetarian diets. The slightly earthy but neutral flavor of avocados works well in sauces, salad dressings, sandwiches, baked goods, salads, and grain dishes to add richness.
- Fat (mostly monounsaturated 67%)
- Fiber (mostly insoluble but also soluble)
- Carotenoids (lutein, zeaxanthin)
A whole medium avocado contains about 240 calories, 13 grams carbohydrate, 3 grams protein, 22 grams fat (15 grams monounsaturated, 4 grams polyunsaturated, 3 grams saturated), 10 grams fiber, and 11 milligrams sodium. Along with their low sodium levels, avocados contain no cholesterol.
Avocados and Health
Avocados contain several nutrients including carotenoids, monounsaturated fats , potassium , and fiber that have been associated with a reduced risk of chronic diseases, especially when included as part of a balanced nutritious diet . The nutritional profile of avocados fits well with healthful dietary patterns such as the Mediterranean and DASH diets. Published health research on avocados is largely funded by avocado industry groups; the research cited below attempts to include non-industry-funded studies.
The primary type of monounsaturated fat in avocados is from oleic acid, the main fatty acid in olive oil. Some studies show a reduction in LDL cholesterol when replacing other types of fat in the diet with avocados. [2] As with all plant foods, avocados are cholesterol-free. However, they contain phytosterols, or plant sterols, that have a similar chemical structure to cholesterol but are poorly absorbed in the intestines and therefore may interfere with cholesterol absorption. According to the American Heart Association, phytosterols have been found to reduce total and LDL cholesterol levels in the body by lowering cholesterol absorption. [3] Avocados are also rich in potassium, a mineral that helps to regulate blood pressure by maintaining normal levels of fluid inside of cells and helping muscles to contract. Potassium also works as an electrolyte that sends electrical signals in the heart to create a steady heartbeat.
A large cohort of almost 69,000 women and 42,000 men were followed for 30 years to see if long-term avocado intake affected risk of cardiovascular disease (CVD). [4] The authors adjusted for diet and lifestyle factors associated with CVD to pinpoint the specific effect of avocados. Compared with those who didn’t eat avocados, those who ate two or more servings a week (1 serving = ½ avocado) had a 16% lower risk of CVD and a 21% lower risk of heart disease. The benefit was especially strong when swapping fat-filled foods like whole-milk dairy (butter, cheese, yogurt), processed meats (hot dogs, bacon, sausage), and eggs with an equal serving of avocado. However, there were similar heart benefits when swapping high-fat plant foods like nuts or olive oil with avocado.
Avocados are a good source of fiber, low in total carbohydrate, and rich in monounsaturated fats. They have a low glycemic index (GI) of about 40; low glycemic foods have a rating of 55 or less. Low GI foods are less likely to cause surges in blood glucose. Large epidemiological studies have found that replacing saturated fats with unsaturated (monounsaturated and polyunsaturated) fats can improve insulin sensitivity and reduce the risk of type 2 diabetes. [5] These nutritional components are favorable for the prevention and control of type 2 diabetes .
Avocados contain plant chemicals called carotenoids. Two types of these fat-soluble carotenoids, lutein and zeaxanthin, are found in the human eye and protect the eye from ultraviolet light damage from the sun. A diet rich in these carotenoids is associated with a lower risk of macular degeneration and cataracts. [6]
The fibers in avocados act as prebiotics (food for beneficial bacteria in the intestines) and have been found to improve the diversity of microflora in the colon. [1] These bacteria digest and break down fibers into short chain fatty acids , which are actively researched for their role in chronic disease prevention.
Depending on the variety, avocados may be round or pear-shaped, green or black, and small or large. The skin is typically bumpy. The flesh when ripe is smooth and buttery. They are a climacteric fruit, which continues to ripen after harvesting. The Hass avocado is the most common type, available year-round.
If you are planning to use an avocado immediately after purchase, choose a ripe one with dark green or almost black skin. It should yield to pressure when squeezed. Avocados with light green skin that are very firm are unripe and will need to sit a few days before eating. If the avocado has dark shriveled skin, dents, or spots of mushy flesh, it may be overripe and unpleasant to eat.
Avocado oil is extracted from the flesh of pressed avocados. It can replace other liquid cooking oils and has a very high smoke point of almost 500°F. Avocado oil is often compared with olive oil because they are both rich in the fatty acid, oleic acid, but avocado oil has a more neutral flavor. [7] It can also be used to make a homemade salad dressing: whisk or blend together ¼ cup avocado oil, 2 tablespoons Dijon mustard, and 4 teaspoons balsamic or apple cider vinegar; add additional low-sodium spices like black pepper or garlic powder as desired.
Avocados are often sold with hard, unripe flesh, which will ripen in 2-3 days. You can leave the fruit at room temperature, or place in direct sunlight to speed ripening. You can also place the avocado sealed in a paper bag with a banana ; the ethylene gases in the banana will speed ripening. When ripe, avocados will feel slightly soft when squeezed. The flesh of avocados is infamous for quickly turning brown once exposed to air, called enzymatic browning. Although unappetizing to see, the brown flesh is perfectly edible. Still, there are tips to slow or reduce browning after cutting into an avocado:
- Cover the flesh with lemon or lime juice.
- Wrap tightly with plastic wrap or place in a sealed airtight container and store in the refrigerator to reduce oxygen exposure.
- Store an avocado half with some sliced onion in a sealed airtight container; the sulfur compounds in the onion help preserve the avocado.
Removing an avocado pit isn’t as challenging as you might think. Although a popular method is to stab a knife into the pit of an avocado half and cleanly remove it, this carries the potential danger of stabbing your hand! Instead, place your index and middle finger on the flesh on each side of the pit, placing your thumb behind the avocado on the skin; push into the center with your thumb until the pit pops out. From there, slice, dice, or mash the flesh as desired to be used in recipes.
The monounsaturated fat in avocados is stable in high heat and can be used not only in cooking but also in baking. Pureed avocado can be substituted for butter or oil in baking recipes, using a 1:1 ratio (1 cup butter = 1 cup avocado).

- Diced and sprinkled into salads, soups, tacos, or whole grains
- Blended into smoothies to thicken and add richness
- Mashed as a spread on sandwiches and crackers
- Mashed onto whole grain breakfast toast, sprinkled with blueberries and ground flaxseeds or hemp seeds
- Sliced and rolled into maki sushi
- Cut in half, drizzled with a squeeze of lemon or lime juice, and eaten with a spoon as a snack
Did You Know?
- One serving of a medium avocado (half the fruit) has more potassium than a medium banana , 487 mg potassium versus 422 mg potassium, respectively.
- A ripe mashed avocado is sometimes used as a facial mask due its high content of hydrating oils and vitamin E .
- Bhuyan DJ, Alsherbiny MA, Perera S, Low M, Basu A, Devi OA, Barooah MS, Li CG, Papoutsis K. The odyssey of bioactive compounds in avocado (Persea americana) and their health benefits. Antioxidants . 2019 Oct;8(10):426.
- Schoeneck M, Iggman D. The effects of foods on LDL cholesterol levels: A systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutrition, Metabolism and Cardiovascular Diseases . 2021 May 6;31(5):1325-38.
- Lichtenstein AH, Deckelbaum RJ. Stanol/sterol ester–containing foods and blood cholesterol levels: a statement for healthcare professionals from the nutrition committee of the council on nutrition, physical activity, and metabolism of the American Heart Association. Circulation . 2001 Feb 27;103(8):1177-9.
- Pacheco LS, Li Y, Rimm EB, Manson JE, Sun Q, Rexrode K, Hu FB, Guasch-Ferré M. Avocado Consumption and Risk of Cardiovascular Disease in US Adults. Journal of the American Heart Association . 2022 Mar 30:e024014. Author disclosure: Dr. Pacheco collaborated in the Hass Avocado Board-funded trial Effects of Avocado Intake on the Nutritional Status of Families during 2016 to 2019 as a graduate student researcher, but the present study was not supported or endorsed by the Hass Avocado Board.
- Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Progress in lipid research . 2009 Jan 1;48(1):44-51.
- Wu J, Cho E, Willett WC, Sastry SM, Schaumberg DA. Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA ophthalmology . 2015 Dec 1;133(12):1415-24.
- Cervantes-Paz B, Yahia EM. Avocado oil: Production and market demand, bioactive components, implications in health, and tendencies and potential uses. Comprehensive Reviews in Food Science and Food Safety . 2021 Jul;20(4):4120-58.
Last reviewed April 2022
Terms of Use
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Medicines (Basel)

Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review
Wissal dhifi.
1 UR Ecophysiologie Environnementale et Procédés Agroalimentaires, Institut Supérieur de Biotechnologie de Sidi Thabet, BiotechPole de Sidi Thabet, Université de la Manouba, Ariana 2020, Tunisia; rf.oohay@2002d_lassiw
Sana Bellili
2 LR11-ES31 Laboratory of Biotechnology and Valorisation of Bio-GeoRessources (BVBGR), Higher Institute of Biotechnology of Sidi Thabet (ISBST), Biotechpole Sidi Thabet, University of Manouba, Ariana 2020, Tunisia; rf.liamtoh@anas-leb (S.B.); [email protected] (S.J.); [email protected] (N.B.)
3 Faculté des Sciences de Bizerte, Jarzouna-Bizerte, Université de Carthage, Carthage 7021, Tunisia
Sabrine Jazi
Nada bahloul, wissem mnif.
4 Faculty of Sciences and Arts in Balgarn, PO BOX 60 Balgarn, University of Bisha, Sabt Al Alaya 61985, Saudi Arabia
This review covers literature data summarizing, on one hand, the chemistry of essential oils and, on the other hand, their most important activities. Essential oils, which are complex mixtures of volatile compounds particularly abundant in aromatic plants, are mainly composed of terpenes biogenerated by the mevalonate pathway. These volatile molecules include monoterpenes (hydrocarbon and oxygenated monoterpens), and also sesquiterpenes (hydrocarbon and oxygenated sesquiterpens). Furthermore, they contain phenolic compounds, which are derived via the shikimate pathway. Thanks to their chemical composition, essential oils possess numerous biological activities (antioxidant, anti-inflammatory, antimicrobial, etc…) of great interest in food and cosmetic industries, as well as in the human health field.
1. Introduction
The attraction of medicinal and aromatic plants is continuously growing due to increasing consumers demand and interest in these plants for culinary, medicinal, and other anthropogenic applications.
As consumers are becoming more and more informed about issues of food, health, and nutrition, they are also becoming aware of the benefits and potential applications of medicinal and aromatic plants and their metabolites. These plants produce a large variety of secondary metabolites; among them, essential oils.
Despite their rich and complex composition, the use of essential oils remains wide and limited to the cosmetics and perfumery domains. It is worthy to develop a better understanding of their chemistry and the biological properties of these extracts and their individual components for new and valuable applications in human health, agriculture, and the environment. Essential oils could be exploited as effective alternatives or complements to synthetic compounds of the chemical industry, without inducing the same secondary effects.
2. Definition of Essential Oils
The term essential oil dates back to the sixteenth century and derives from the drug Quinta essentia , named by Paracelsus von Hohenheim of Switzerland [ 1 ]. Essential oils or “essences” owe their name to their flammability. Numerous authors have attempted to provide a definition of essential oils. The French Agency for Normalization: Agence Française de Normalisation (AFNOR) gives the following definition (NF T 75-006): “The essential oil is the product obtained from a vegetable raw material, either by steam distillation or by mechanical processes from the epicarp of Citrus, or “dry”” distillation. The essential oil is then separated from the aqueous phase by physical means [ 2 ]. This definition encompasses products obtained always from vegetable raw material, but using other extraction methods, such as using non-aqueous solvents or cold absorption. Thus, we can define four types of products [ 3 ].
Essential oils are soluble in alcohol, ether, and fixed oils, but insoluble in water. These volatile oils are generally liquid and colorless at room temperature. They have a characteristic odor, are usually liquid at room temperature and have a density less than unity, with the exception of a few cases (cinnamon, sassafras, and vetiver). They have a refractive index and a very high optical activity. These volatile oils contained in herbs are responsible for different scents that plants emit. They are widely used in the cosmetics industry, perfumery, and also aromatherapy. The latter is intended as a therapeutic technique including massage, inhalations, or baths using these volatile oils. The last key will serve as chemical signals allowing the plant to control or regulate its environment (ecological role): attraction of pollinating insects, repellent to predators, inhibition of seed germination, or communication between plants (emission signals chemically signaling the presence of herbivores, for example). Moreover, EOs also possesses antifungal or insecticide and deterrent activities. All parts of aromatic plants may contain essential oils as follows:
- Flowers, of course, including: orange, pink, lavender, and the (clove) flower bud or (ylang-ylang) bracts,
- Leaves, most often, including: eucalyptus, mint, thyme, bay leaf, savory, sage, pine needles, and tree underground organs, e.g., roots (vetiver),
- Rhizomes (ginger, sweet flag),
- Seeds (carvi, coriander),
- Fruits, including: fennel, anise, Citrus epicarps,
- Wood and bark, including: cinnamon, sandalwood, rosewood.
3. Chemistry of Essential Oils
Essential oils are produced by various differentiated structures, especially the number and characteristics of which are highly variable. Essential oils are localized in the cytoplasm of certain plant cell secretions, which lies in one or more organs of the plant; namely, the secretory hairs or trichomes, epidermal cells, internal secretory cells, and the secretory pockets. These oils are complex mixtures that may contain over 300 different compounds [ 4 ]. They consist of organic volatile compounds, generally of low molecular weight below 300. Their vapor pressure at atmospheric pressure and at room temperature is sufficiently high so that they are found partly in the vapor state [ 5 , 6 ]. These volatile compounds belong to various chemical classes: alcohols, ethers or oxides, aldehydes, ketones, esters, amines, amides, phenols, heterocycles, and mainly the terpenes. Alcohols, aldehydes, and ketones offer a wide variety of aromatic notes, such as fruity ((E)-nerolidol), floral (Linalool), citrus (Limonene), herbal (γ-selinene), etc.
Furthermore, essential oil components belong mainly to the vast majority of the terpene family ( Figure 1 ). Many thousands of compounds belonging to the family of terpenes have so far been identified in essential oils [ 7 ], such as functionalized derivatives of alcohols (geraniol, α-bisabolol), ketones (menthone, p -vetivone) of aldehydes (citronellal, sinensal), esters (γ-tepinyl acetate, cedryl acetate), and phenols (thymol).

Structures of some terpenes.
Essential oils also contain non-terpenic compounds biogenerated by the phenylpropanoids pathway, such as eugenol, cinnamaldehyde, and safrole.
Biogenetically, terpenoids and phenylpropanoids have different primary metabolic precursors and are generated through different biosynthetic routes ( Figure 2 ). The pathways involved in terpenoids are the mevalonate and mevalonate-independent (deoxyxylulose phosphate) pathways, whereas phenylpropanoids originate through the shikimate pathway [ 8 , 9 ]. Some authors have reviewed the biosynthetic pathways of terpenoids and phenylpropanoids, respectively, the enzymes and enzyme mechanisms involved, and information about genes encoding for these enzymes [ 8 , 9 ].

Biosynthesis pathways of monoterpenes and sesquiterpenes.
Essential oils have a very high variability of their composition, both in qualitative and quantitative terms. Various factors are responsible for this variability and can be grouped into two categories:
- Intrinsic factors related to the plant, and interaction with the environment (soil type and climate, etc.) and the maturity of the plant concerned, even at harvest time during the day,
- Extrinsic factors related to the extraction method and the environment.
The factors that determine essential oil yield and composition are numerous. In some cases, it is difficult to isolate these factors from each other as they are interrelated and influence each other. These parameters include the seasonal variations, plant organ, and degree of maturity of the plant, geographic origin, and genetics [ 10 , 11 , 12 ].
Several techniques are used for the trapping of volatiles from aromatic plants. The most often used device is the circulatory distillation apparatus described by Cocking and Middleton [ 13 ] introduced in the European Pharmacopoeia and several other pharmacopoeias. This device consists of a heated round-bottom flask into which the chopped plant material and water are placed and which is connected to a vertical condenser and a graduated tube, for the volumetric determination of the oil. At the end of the distillation process, the essential oil is separated from the water phase for further investigations. The length of distillation depends on the plant material to be investigated. It is usually fixed to 3–4 h.
A further improvement was the development of a simultaneous distillation–solvent extraction device by Likens and Nickerson in 1964 [ 14 ]. The device permits continuous concentration of volatiles during hydrodistillation in one step using a closed-circuit distillation system.
4. Biological Activities of Essential Oils
4.1. antibacterial activity.
The antimicrobial properties of essential oils and of their constituents have been considered [ 15 , 16 ] and the mechanism of action has been studied in detail [ 17 ]. An important feature of essential oils are their hydrophobicity, which allows them to partition into lipids of the cell membrane of bacteria, disrupting the structure, and making it more permeable [ 18 ]. This can then cause leakage of ions and other cellular molecules [ 19 , 20 , 21 , 22 ]. Although a certain amount of leakage of bacterial cells can be tolerated without loss of viability, greater loss of cell contents or critical output of molecules and ions can lead to cell death [ 23 ].
EOs and/or their constituents can have a single target or multiple targets of their activity. For instance, trans-cinnamaldehyde can inhibit the growth of Escherichia coli and Salmonella typhimirium without disintegrating the OM or depleting intracellular ATP. Similar to thymol and carvacrol, trans-cinnamaldehyde likely gains access to the periplasm and deeper portions of the cell [ 24 ]. Carvone is also ineffective against the OM and does not affect the cellular ATP pool [ 25 ].
It has been reported that EOs containing mainly aldehydes or phenols, such as cinnamaldehyde, citral, carvacrol, eugenol, or thymol were characterized by the highest antibacterial activity, followed by EOs containing terpene alcohols. Other EOs, containing ketones or esters, such as β-myrcene, α-thujone, or geranyl acetate, had much weaker activity, while volatile oils containing terpene hydrocarbons were usually inactive [ 26 , 27 ].
Generally, essential oils characterized by a high level of phenolic compounds, such as carvacrol, eugenol, and thymol, have important antibacterial activities [ 17 , 26 , 28 ].
These compounds are responsible for the disruption of the cytoplasmic membrane, the driving force of protons, electron flow, active transport, and also coagulation of cell contents [ 18 , 23 , 29 ].
The chemical structure of essential oils affects their mode of action concerning their antibacterial activity [ 28 ]. The importance of the presence of hydroxyl group in the phenolic compounds, such as carvacrol and thymol, was confirmed [ 22 , 28 , 30 ]. However, the relative position of the phenolic hydroxyl group on the ring does not appear to influence the intensity of the antibacterial activity.
The action of thymol against Bacillus cereus , Staphylococcus aureus , and Pseudomonas aeruginosa appears to be comparable to that of carvacrol, for example [ 17 , 22 ]. However, carvacrol and thymol act differently against Gram-positive and Gram-negative species [ 28 ]. Thymol, eugenol, and carvacrol have an antimicrobial effect against a broad spectrum of bacteria: Escherichia coli , Bacillus cereus , Listeria monocytogenes , Salmonella enterica , Clostridium jejuni , Lactobacillus sake , Staphylococcus aureus , and Helicobacter pyroli [ 31 , 32 ]. Other families of compounds also have valuable antibacterial properties: certain alcohols, aldehydes, and ketones, monoterpene (geraniol, linalol, menthol, terpineol, thujanol, myrcenol, citronelîaî, neral, thujone, camphor, carvone, etc.), phenylpropanes (cinnamaldehyde), and monoterpenes (γ-terpinene, p -cymene). Among these compounds, carvacrol is the most active. Known to be non-toxic, it is used as a preservative and food flavoring in drinks, sweets, and other preparations.
It is important to mention that essential oils are more active against Gram-positive than Gram-negative bacteria [ 33 , 34 , 35 , 36 , 37 ]. The latter are less susceptible to the action of essential oils with the outer membrane surrounding the cell wall that restricts the diffusion of hydrophobic compounds through its lipopolysaccharide film [ 36 ]. Furthermore, the antibacterial activity of essential oils related to their chemical composition, the proportions of volatile molecules, and their interactions [ 28 , 33 , 37 ].
An additive effect is observed when the combination is equal to the sum of the individual effects. Antagonism is observed when the effect of one or both compounds is less important when they are tested together than when used individually [ 38 ].
A synergistic effect is observed when the combination of substances is greater than the sum of the individual effects [ 39 ]. Some studies have shown that the use of the whole essential oil provides an effect which is greater than that of the major components used together [ 40 ]. This suggests that minor components are essential for activity and may have a synergistic effect.
It has been reported additive and synergistic effects of the combinations of 1,8-cineole and aromadendrene against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) and Enterococcus faecalis by using checkerboard and time-kill assays, respectively [ 41 ].The combined effects of plant volatile oils and benzoic acid derivatives against L. monocytogenes and S. enteritidis are considered as synergistic since the combined components allowed ≥log10 higher inhibition than the sum of the inhibitory effects of the components used separately [ 42 ]. Increased antifungal effects were caused by combinations (1:5, 1:7, and 1:9) of essential oils of S. aromaticum (clove) and Rosmarinus officinalis against C. albicans [ 43 ]. Moreover, Lambert et al. (2001) [ 17 ] reported that, combined, carvacrol and thymol showed additive effects against S. aureus and P. aeruginosa by using half-fold dilutions within the Bioscreen plat.
Two hypotheses have been proposed to explain synergistic effects of cinnamaldehyde/thymol or cinnamaldehyde/carvacrol against S. typhimurium : proving, on one hand, that thymol or carvacrol could increase the permeability of the cytoplasmic membrane, and probably enable cinnamaldehyde to be more easily transported into the cell, and, on the other hand, that thymol or carvacrol could increase the number, size, or duration of the existence of the pores created by the binding of cinnamaldehyde to proteins in the cell membrane [ 44 ]. These facts justify a synergistic effect achieved when these two components are used in combination. Mechanisms of interaction that produced antagonistic effects were less studied [ 45 ].
In addition, essential oils have also revealed to be effective on the inhibition of growth and reduction in numbers of the more serious foodborne pathogens, such as Salmonella spp., E. coli O157:H7, and Listeria monocytogenes [ 42 ].
4.2. Antioxidant Activity
Numerous studies have demonstrated the antioxidant properties of essential oils. The antioxidant potential of an essential oil depends on its composition. It is well established that phenolics and secondary metabolites with conjugated double bonds usually show substantial antioxidative properties [ 46 ]. Most of the essential oils are dominated by oxygenated monoterpenes such as alcohols ( Achillea filipendulina ), aldehydes ( Galagania fragrantissima ), ketones ( Anethum graveolens , Artemisia rutifolia , Hyssopus seravschanicus , Mentha longifolia , and Ziziphora clinopodioides ), and esters ( Salvia sclarea ). Artemisia absinthium and Artemisia scoparia predominantly contain monoterpene hydrocarbons, whereas phenolic terpenoids, such as thymol or carvacrol, characterize Origanum tyttanthum and Mentha longifolia EOs, which would explain why both plants exhibited generally the strongest antioxidant activity. Thymol and carvacrol, which are predominant in Origanum tyttanthum , are also responsible for the antioxidant activity of several other essential oils, such as Mentha longifolia and Thymus serpyllus [ 47 ].
The essential oils of cinnamon, nutmeg, clove, basil, parsley, oregano, and thyme are characterized by the most important antioxidant properties [ 43 ]. Thymol and carvacrol are the most active compounds. Their activity is related to their phenolic structure. These phenolic compounds have redox properties and, thus, play an important role in neutralizing free radicals and also in peroxide decomposition [ 40 ]. The antioxidant activity of essential oils is also due to certain alcohols, ethers, ketones, aldehydes, and monoterpenes: linalool, 1,8-CineoIe, geranial/neral, citronellal, isomenthone, menthone, and some monoterpenes: α-Terpinene, β-Terpinene and α-Terpinolene [ 43 ].
Essential oils with important scavenging capacity of free radicals may play an important role in some disease prevention, such as brain dysfunction, cancer, heart disease, and immune system decline. In fact, these diseases may result from cellular damage caused by free radicals [ 43 , 44 ].
EOs have shown their action as hepatoprotective agents in ageing polyunsaturated fatty acids mammals and it has been proved that they possess a beneficial impact upon the PUFAs, in particular the long chain C20 and C22 acids [ 48 ]. Moreover, essential oils being able to scavenge free radicals may also play an important role in some disease prevention, such as brain dysfunction, cancer, heart disease, and immune system decline [ 49 ].
Sharififar et al. (2011) [ 50 ] evaluated the antioxidant activity of Zataria multiflora Boiss. (Lamiaceae) essential oil in rats. Antioxidant activity was measured by the test of 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical inhibition and inhibition of lipid peroxidation by measuring the index of thiobarbituric acid reactive substances (TBARs). Three doses of 100, 200, and 400 μL/kg were administered to animals by intra gastric intubation (i.g) routh for 10 days. The blood was collected in eleventh day through direct puncture and the liver was rapidly excised. The histopathology studies of the animals were compared to animals in butylated hydroxyl toluene (BHT) group. The authors reported that all Zataria multiflora oils ZMO tested doses were able to scavenge DPPH radical ( p < 0.05). Moreover, ZMO decreased TBARs in a dose-dependent manner. No alteration in liver function test LFT enzymes or changes in histopathology of the liver was considered in ZMO treated groups. The results indicated that ZMO might be used in human healthy and food industry.
According to Manjamalai and Grace [ 51 ], essential oil of Wedelia chinensis (Osbeck) increases both the level of catalase and glutathione peroxidase in the lung and liver tissues, whereas in the serum the level of catalase decreased on the 22nd day (2.32 ± 0.016 Lung tissue 6.47 ± 0.060 liver tissue, 0.94 ± 0.007 serum). Furthermore, the level of Glutathione Peroxidase GPx in the liver (the range) was found to be decreased in the EO-treated group compared to the cancer-induced group and control group, whereas the level of GPx in the lung tissue was found to be low (76.2 ± 1.66).
4.3. Anti-Inflammatory Activity
Inflammation is a normal protective response induced by tissue injury or infection and functions to combat invaders in the body (microorganisms and non-self cells) and to remove dead or damaged host cells. The inflammatory response induces an increase of permeability of endothelial lining cells and influxes of blood leukocytes into the interstitium, oxidative burst, and release of cytokines, such as interleukins and tumor necrosis factor-α (TNF-α). It also stimulates the activity of several enzymes (oxygenases, nitric oxide synthases, peroxidases, etc.), as well as the arachidonic acid metabolism. Recently, essential oils have been used in clinical settings to treat inflammatory diseases, such as rheumatism, allergies, or arthritis [ 45 ]. Melaleuca alternifolia EO was reported to have a considerable anti-inflammatory activity [ 46 , 47 , 48 ].This activity is correlated with its major compound: α-terpineol [ 49 ]. The active compounds act by inhibiting the release of histamine or reducing the production of inflammation mediators. Geranium essential oil is another example [ 45 ]. Linalool and linalyl acetate showed anti-inflammatory activity on oedema of paw-induced mouse carrageenan [ 50 ]. Yoon et al. [ 52 ] reported that the oils of Torreya nucifera Siebold et Zucc. oil, mainly constituted by limonene, δ-3-carene, and α-pinene, have an inhibitory effect on COX-2, thus inducing a significant inhibitory effect on prostaglandin (PGE2) production. Furthermore, 1,8-cineole, present in many essential oils, was reported as an inhibitor of leukotrienes (LTB4) and PGE2, biogenerated both from pathways of arachidonic acid metabolism [ 52 ].
The anti-inflammatory activity of essential oils may be attributed not only to their antioxidant activities but also to their interactions with signaling cascades involving cytokines and regulatory transcription factors, and on the expression of pro-inflammatory genes. Essential oils, therefore, represent a new option in the treatment of inflammatory diseases.
4.4. Cancer Chemoprotective Activity
The varied therapeutic potential of essential oils attracted, in recent years, the attention of researchers for their potential activity against cancer. They and their volatile constituents of the studies target the discovery of new anticancer natural products [ 41 ]. Essential oils would act in the prevention of cancer, as well as at its removal. It is well known that certain foods, such as garlic and turmeric, are good sources of anticancer agents [ 53 ]. Garlic essential oil is a source of sulfur compounds recognized for their preventive effect against cancer [ 54 , 55 ]. Diallylsulfide, diallyldisulfide, and diallyltrisulfide are examples. According to Wu et al. [ 56 ], these compounds activate, in rats, the enzymes involved in the detoxification process of hepatic phase 1 (disintegration of chemical bonds that link carcinogenic toxins to each other) and phase 2 (bonds to toxins released detoxifying enzymes, such as glutathione S -transferase).
Metabolism happens mainly in the liver, the body’s largest internal organ. The portal vein carries blood from the small intestine directly to the liver. Sixty percent of liver tissue is made up of hepatic cells. More chemical processes happen in these than in any other group of cells in the body. Phase 1 metabolism involves chemical reactions, such as oxidation (most common), reduction, and hydrolysis. There are three possible results of phase 1 metabolism. The drug becomes completely inactive. In other words, the metabolites are pharmacologically inactive. One or more of the metabolites are pharmacologically active, but less so than the original drug. The original substance is not pharmacologically active, but one of its metabolites is. The original substance is called a prodrug.
Phase 2 metabolism involves reactions that chemically change the drug or phase 1 metabolites into compounds that are soluble enough to be excreted in urine. In these reactions, the molecule (drug or metabolite) is attached to an ionisable grouping. This is called conjugation and the product is called a conjugate. Metabolites formed in phase 2 are unlikely to be pharmacologically active. Some drugs undergo either phase 1 or phase 2 metabolism, but most undergo phase 1 metabolism followed by phase 2 metabolism.
Another example is myristicin, an allylbenzene present on a certain essential oil, especially that of nutmeg ( Myristica fragrans ). This molecule is known to activate glutathione S -transferase in mice [ 57 ] and inhibit carcinogenesis induced by benzo(a)pyrene in the lungs of mice [ 58 ]. Recently, it has been discovered that myristicin induces apoptosis in neuroblastoma (SK-N-SH) in humans [ 58 ]. There are other volatile compounds that showed a cytotoxic activity against various cancer cell lines [ 43 ]. Geraniol decreases the resistance of colon cancer cells (TC118) to 5-fluorouracil, an anticancer agent. Therefore, geraniol enhances this inhibitory effect of tumour growth 5-fluorouracil [ 59 , 60 ]. The essential oil of balsam fir and α-Humulene, showed significant anticancer activity in several cell lines and low toxicity to healthy cells [ 61 ].
In addition, anticancer activity of d -limonene, the main component of Citrus essential oil has been proven, especially at the level of stomach cancer and liver [ 62 ]. The α-Bisabolol, an abundant sesquiterpene alcohol in chamomile essential oil ( Matricaria ), has an antigliomale activity [ 63 ]. Many essential oils have a cytotoxic activity namely Melissa officinalis [ 64 ], Melaleuca alternifolia [ 65 ], Artemisia annua [ 66 ], and Comptonia peregrina [ 67 ].
4.5. Cytotoxicity
Due to their complex chemical composition, essential oils have no specific cellular ligands [ 21 ]. As lipophilic mixtures, they are able to cross the cell membrane and degrade the layers of polysaccharides, phospholipids and fatty acids, and permeabilize. This cytotoxicity appears to include such membrane damage. In bacteria, the membrane permeabilization is associated with the loss of ions and the reduction of the membrane potential, the collapse of the proton pump and the depletion of the ATP pool [ 22 , 68 , 69 , 70 ]. Essential oils may coagulate the cytoplasm [ 17 ] and damage lipids and proteins [ 22 , 40 ]. Damage to the wall and the cell membrane can lead to the leakage of macromolecules and lysis [ 17 , 20 , 71 ].
In addition, essential oils change membrane fluidity, which becomes abnormally permeable, resulting in a leakage of radicals, cytochrome C, the Ca 2+ ions, and proteins, like in the case of oxidative stress. This permeabilization of the outer and inner membranes causes cell death by apoptosis and necrosis [ 72 , 73 ]. Ultrastructural alteration of the cell can be observed at a plurality of compartments [ 52 , 74 , 75 ]. The interruption of the viral envelope herpes simplex virus HSV by essential oils can also be observed by electron microscopy [ 76 ]. The induction of membrane damage was also confirmed by an analysis showing that microtubule Saccharomyces cerevisiae genes involved in the biosynthesis of ergosterol, the absorption of sterols, lipid metabolism, the structure and function of cell wall cellular detoxification, and transport are affected by treatment with α-terpinene [ 77 ].
Recent work on the yeast Saccharomyces cerevisiae , has shown that the cytotoxicity of some essential oils based on the ability to form colonies differs significantly in relation to their chemical composition. Generally, essential oil cytotoxicity mainly correlates to the presence of phenols, alcohols, and monoterpene aldehydes [ 78 , 79 ]. The cytotoxic properties of essential oils are of great importance because they assume their use not only against certain human pathogens and animal parasites, but also in the preservation of agricultural and marine products against microbial attack. Indeed, some components of essential oils are effective against a variety of microorganisms as bacteria [ 80 ], viruses [ 81 ], fungi [ 77 , 82 , 83 , 84 ], protozoa [ 85 ], parasites [ 86 , 87 , 88 ], mites, and others.
In addition, α-humulene shows cytotoxicity against breast cancer cells in vitro. α-humulene was reported to be responsible for cytotoxicity (CI 50 55 mM) [ 89 ]. It induced a dose- and time-dependent decrease in cellular glutathione (GSH) content and an increase in reactive oxygen species (ROS) production.
Furthermore, Zeytinoglu et al. [ 90 ], focusing on the effects of carvacrol, one of the main compounds in the EO of oregano, on the DNA synthesis of N -ras transformed mouse myoblast CO25 cells, finding that this monoterpenic phenol was able to inhibit the DNA synthesis in the growth medium and ras-activating medium, which contained dexamethasone. They proposed that it may be valuable in cancer therapy because of its growth inhibition of myoblast cells, even after activation of mutated N -ras-oncogene.
The EO of the Anonaceae Xylopia aethiopica (Ethiopian pepper), a plant grown in Nigeria, showed, at a concentration of 5 mg/mL, a cytotoxic effect in the carcinoma cell line (Hep-2) [ 91 ].
Moreover, Yu et al. [ 92 ] tested the essential oil of the rhizome of the Aristolochiaceae Aristolochia mollissima for its cytotoxicity on four human cancer cell lines (ACHN, Bel-7402, Hep G2, HeLa). The rhizome oil possessed a significantly greater cytotoxic effect on these cell lines than the oil extracted from the aerial plant.
Linalool inhibited only moderate cell proliferation; however, in subtoxic concentrations potentiates doxorubicin-induced cytotoxicity and proapoptotic effects in both cell lines, MCF7 WT and MCF7 AdrR. This monoterpene improves the therapeutic index in the management of breast cancer, especially multidrug resistance (MDR) tumors [ 93 ].
An in vitro cytotoxicity assay indicated that the EO of Cyperus rotundus (Cyperaceae) characterized by the predominance of cyperene, α-Cyperone, isolongifolen-5-one, rotundene, and cyperorotundene, was very effective against L1210 leukemia cells, which correlates with significantly increased apoptotic DNA fragmentation [ 94 ].
4.6. Allelopathic Activity
According to the International Allelopathy Society (IAS), allelopathy was defined in 1996 as “The science that studies any process involving secondary metabolites produced by plants, algae, bacteria and fungi that influences the growth and development of agricultural and biological systems”. Allelopathic interactions derive from the production of secondary metabolites. The secondary metabolites are synthesized for a wide range defense by plant and microorganisms. The secondary metabolites involved are called allelochemicals [ 95 ].
Volatile oils and their constituents are being explored for weed and pest management, and are viewed as an important source of lead molecules in agriculture [ 96 ]. Bioactive terpenoids constitute an important part of the defensive mechanisms of a large number of organisms and represent a fairly untapped source of active compounds of potential use both in the agricultural field [ 97 ]. In fact, a large number of highly phytotoxic allelochemicals are derived from the terpenoid pathway [ 98 ] and the phytotoxicity of essential oils has been investigated [ 98 , 99 , 100 , 101 ]. The allelopathic activity of Melaleuca alternifolia (Maiden and Betche) Cheel (tea tree) essential oil was investigated by Angelini et al., [ 101 ] against Trichoderma harzianum , which is a fungal contaminant that causes extensive losses in the cultivation of Pleurotus species. This essential oil has, in vitro, an allelopathic ability to control Trichoderma harzianum . The antifungal activity of M. alternifolia essential oil and antagonist activities between Pleurotus species against three T. Harzianum strains were studied in dual-culture experiments done with different concentrations.
Santos et al. [ 102 ] reported that leaves’ and rhizomes’ EOs caused a decrease in dry matter. They also reported a reduction of shoot length in lettuce seedlings. Evaluating the effect of these EOs on the germination and vigor of the lettuce seedlings, they noticed a reduction of these parameters and concluded that rhizomes’ oil caused a greater reduction in all of the variables than the oil from the leaves.
Portulaca oleracea seeds’ germination and growth were significantly decreased by the treatment with rosemary EO [ 103 ]. These authors reported that a concentration of 1000 ppm of this oil, rosemary decreased Portulaca oleracea seed germination to 76 percent. They also noted that Artemisia and lavender essential oils have strong allelopathic effects and prevents weed germination and growth of Portulaca oleracea , which would be a promising result in the organic cultivation of crops to be followed, and it can be used in the production of herbicides with natural origin.
Furthermore, de Oliveira et al. [ 104 ] reported that Callistemon viminalis EO affected the growth of lettuce seedlings and caused a reduction in the length of shoots and the root system. This reduction was proportional to the EO concentration.
The results of the research of Saad and Abdelgaleil [ 105 ] revealed a correlation between EOs chemical composition and their effects on germination and seedling growth. It was reported that the most active compounds belonged to the groups of ketones and alcohols and were followed by the group of aldehydes and phenols [ 106 ]. Moreover, Kotan et al. [ 107 ] suggested that, in general, a potent phytotoxic activity of plant EOs is correlated to a high amount of oxygenated monoterpenes.
Almost all the effective oils had high percentages of oxygenated monoterpenes and this was in agreement with previous work of de Almeida et al. and Vokou et al. [ 108 , 109 ].
Dudai et al. [ 103 ] reported that monoterpenes act on seeds at very low levels. In particular, among the Lamiaceae family, many species release phytotoxic monoterpenes that hinder the development of herbaceous species, including pinene, limonene, p -Cymene, and 1,8-cineole [ 101 ]. Moreover, it is well known that monoterpenes in the essential oils have phytotoxic effects that may cause anatomical and physiological changes in plant seedlings leading to accumulation of lipid globules in the cytoplasm, reduction in some organelles such as mitochondria, possibly due to inhibition of DNA synthesis or disruption of membranes surrounding mitochondria and nuclei [ 110 , 111 ]. Since the continued use of synthetic herbicides may threaten sustainable agricultural production and result in serious ecological and environmental problems, essential oils with allelopatic properties could be exploited as in alternative strategies leading to the development of biodegradable and non-toxic compounds [ 112 ].
4.7. Repellent and Insecticidal Activity
Essential oils constitute a rich bank of structurally-diverse compounds with a variety of insecticidal and repellent mechanisms. Numerous studies have demonstrated that these compounds, as well as their parent blends, possess biological activity capable of eliciting adverse effects in arthropod pests. Several factors affecting the commercialization of plant essential oil extracts as repellents include regulatory requirements, intellectual property value, biological activity, product performance, and product quality [ 113 ].
The toxic effect of essential oils was not only suitable for granary insects but also for flying insects: Gaultheria (Ericaceae) and Eucalyptus (Myrtaceae) oils exhibited very high killing power on insects, such as the rice weevil Sitophilus oryzae , the beetles Callosobruchus chinensis (Coleoptera: Bruchidae) and S. paniceum , and also on M. domestica [ 114 ]. Actually, the activities of essential oils on species are manifold. Mentha, Lavandula (Lamiaceae), or Pinus (Pinaceae) essential oils were noted for their toxicity against Myzus persicae (Homoptera: Aphididae) and the greenhouse white fly Trialeurodes vaporariorum (Homoptera: Aleyrodidae), as well as the Colorado beetle Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) and the pear bug Stephanitis pyri (Hymenoptera: Stephanidae) [ 115 ].
Commonly, essential oils can be inhaled, ingested, or skin-absorbed by insects. The fumigant toxicity of essential oils and their main components, the volatile monoterpenes, has been described [ 116 ]. Insects were also very sensitive to topical applications Sitophilus zea-mais (Coleoptera: Curculionidae), Tribolium castaneum and Prostephanus truncatus (Coleoptera: Bostrychidae) reacted to citrus (Rutacae) essential oils. Pediculus capitis (Anoplura: Pediculidae), Anopheles funestus (Diptera: Culicidae), Cimex lectularius (Hemiptera: Cimicidae), and Periplaneta orientalis (Dictyoptera: Blattidae) were killed by contact with Eucalyptus saligna (Myrtaceae) oil within 2 to 30 min.
Essential oils belonging to plants in the citronella genus ( Poaceae ) are commonly used as ingredients of plant-based mosquito repellents, mainly Cymbopogon nardus , which is sold in Europe and North America in commercial preparations [ 117 ].
5. Conclusions
Thanks to their numerous biological activities, essential oils have to be valorized via several domains, mainly human health, green chemistry, and sustainable agriculture. However, numerous investigations should be carried out on their mode of action and their probable toxicological effects in order to optimize their potential uses.
Acknowledgments
This study was co-funded by LR11-ES31 Biotechnologie et Valorisation des Bio-Géo Ressources, and by UR Ecophysiologie Environnementale et Procédés Agroalimentaires; Institut Supérieur de Biotechnologie de Sidi Thabet, BiotechPole de Sidi Thabet, Université de la Manouba, Tunisie. Thanks are also expressed to Sharif Mohammad Shahidullah from Department of English, Faculty of Sciences and Arts in Balgarn, University of Bisha, Saudi Arabia for her contribution in the correction of English language. Wissem Mnif dedicates this work to the soul of his dear brother the architect “Mohamed Soufiene Mnif” died 11 July 2016. Rest in peace, dear brother. You are in my heart and your soul will guide me. You are with us forever.

Author Contributions
Wissal Dhifi and Wissem Mnif conceived and designed the paper. Wissal Dhifi, Sana Bellili, Sabrine Jazi and Nada Bahloul wrote the paper. Wissem Mnif re-viewed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.

IMAGES
VIDEO
COMMENTS
This review paper focuses on essential oils and their applications by employing essential oils as a natural preservative that are suitable to be used in food preservation, pharmaceutical ...
Essential oils (EOs) have been used for treatment of various ailments since ancient times and have gained popularity over the years. Safety and efficacy of EOs have been proved by several clinical trials. ... there is a need to explore new therapeutic agents and conduct further clinical research on traditional medicines obtained from various ...
Detailed insight on the antiviral action of essential oils still requires more research. Essential oils might interfere with virion envelopment, which is designed for entry into human host cells, synthesis of viral proteins, inhibition of the early gene expression process, glycosylation process of viral proteins, and inhibition of virus ...
Journal metrics Editorial board. Journal of Essential Oil Research ( JEOR) is the major forum for the publication of essential oil research and analysis. Each issue includes studies performed on the chemical composition of some of the 20,000 aromatic plants known in the plant kingdom. JEOR is devoted entirely to all phases of research from ...
Essential oils are a mixture of saturated and unsaturated hydrocarbons, alcohol, aldehydes, esters, ethers, ketones, oxides phenols and terpenes, ... This work was funded by the Deanship of Scientific Research, King Abdulaziz University, Jeddah under grant number (G-1436-156-590). One of the authors "Aftab Ahmad" therefore, acknowledges ...
Their pharmacological profile includes antimicrobial, anti-inflammatory, antitumor, antioxidant activities, among others [ 10, 12, 13, 14 ]. In view of this, in this review, relevant knowledge of the chemistry of essential oils is presented and their main mechanisms of action related to organic and infectious disorders are discussed. 2.
Comparative study on chemical characteristics of essential oils and genetic characteristics of true Cinnamon (Cinnamomum zeylanicum Blume) and three wild Cinnamon species of Sri Lanka. Hasitha Dhananjaya Weeratunge, Galbada Arachchige Sirimal Premakumara, Egodage Dilip de Silva & Witharanage Wasana Prasadini Rodrigo. Pages: 234-246.
Essential oils (EOs) are concentrated, extremely volatile aromatic compounds traditionally used for their therapeutic properties [].These oils are typically extracted from the leaves, stems, flowers, roots, and other parts of a plant using methods such as steam distillation, cold pressing, or solvent extraction, and they contain the natural fragrance and essence of the plant from which they ...
Aromatherapy — the therapeutic use of essential oils from plants (flowers, herbs or trees) to treat ill health and promote physical, emotional and spiritual well-being — is one of the most widely used natural therapies reported by consumers in Western countries. The Australian Government Department of Health (via the National Health and Medical Research Council) has commissioned a suite of ...
This chapter explores the latest advancements and applications of essential oils, focusing on evidence-based research and practical insights. Beginning with an introduction to essential oils ...
Essential oils (EOs) have risen in popularity over the past decade. These oils function in society as holistic integrative modalities to traditional medicinal treatments, where many Americans substitute EOs in place of other prescribed medications. EOs are found in a multitude of products including food flavoring, soaps, lotions, shampoos, hair ...
Essential oils (EOs) are colourless liquids, mostly aromatic and naturally occurring volatile organic compounds throughout all regions of the plant such as seeds, flowers, peel, stem, bark and the whole plants (Bhavaniramya et al., 2019).These are commonly included as medical products, perfumes, cosmetics and dietary supplements in different countries.
This review covers literature data summarizing, on one hand, the chemistry of essential oils and, on the other hand, their most important activities. Essential oils, which are complex mixtures of volatile compounds particularly abundant in aromatic plants, are mainly composed of terpenes biogenerated by the mevalonate pathway. These volatile molecules include monoterpenes (hydrocarbon and ...
Article. Chemical analyses and antioxidant activity of the essential oils from baccharis dracunculifolia DC. In Southern Brazil. Maíra Maciel Tomazzoli, Wanderlei Do Amaral, Roger Raupp Cipriano, Andreza Cerioni Belniaki, Jéssica de Cássia Tomasi, Beatriz Helena Lameiro Noronha Sales Maia & Cícero Deschamps. Published online: 25 Apr 2024.
Research in the field of essential oils (EOs) over the years.(A,B) Increase of research in the field of essential oils (EOs).(A) A PubMed advanced search using the key word "essential oils" combined with the dates of publication from 1880 to present through the Boolean operator AND has retrieved an increase from 106 to 17,212 (date of last search November 19, 2020) of results.
Six essential oils (from oregano, thyme, clove, lavender, clary sage, and arborvitae) exhibited different antibacterial and antifungal properties. ... in this paper we also report the first in ...
The importance of essential oils and their components in the industrial sector is attributed to their chemical characteristics and their application in the development of products in the areas of cosmetology, food, and pharmaceuticals. ... Feature papers represent the most advanced research with significant potential for high impact in the ...
FIGURE 1.Research in the field of essential oils (EOs) over the years.(A,B) Increase of research in the field of essential oils (EOs).(A) A PubMed advanced search using the key word "essential oils" combined with the dates of publication from 1880 to present through the Boolean operator AND has retrieved an increase from 106 to 17,212 (date of last search November 19, 2020) of results.
Essential oils are a rich source of numerous bioactive constituents, showcasing a wide range of beneficial properties such as antioxidative, antibacterial, antifungal, anticancer, antimicrobial ...
Essential oils are oils, typically fragrant ones, that have been extracted from the roots, flowers, leaves, or seeds of plants using steam or applied pressure. The qualifier "essential" refers ...
DOI: 10.1016/j.procbio.2024.05.021 Corpus ID: 270029392; Essential Oils Pharmacological Activity: Chemical Markers, Biogenesis, Plant Sources, and Commercial Products @article{Mohammed2024EssentialOP, title={Essential Oils Pharmacological Activity: Chemical Markers, Biogenesis, Plant Sources, and Commercial Products}, author={Hamdoon A. Mohammed and G. Sulaiman and Riaz A. Khan and Ali Z. Al ...
In the paper entitled "Exploring the anti-Burkholderia cepacia complex activity of essential oils: a preliminary analysis," I. Maida et al. have checked the ability of the essential oils extracted from six different aromatic plants to inhibit the growth of 18 known species of the Burkholderia cepacia complex (Bcc), an opportunistic human ...
Essential oils are concentrated liquids of complex mixtures of volatile compounds and can be extracted from several plant organs. Essential oils are a good source of several bioactive compounds, which possess antioxidative and antimicrobial properties. In addition, some essential oils have been used as medicine.
Neolitsea vuquangensis Mitsuyuki & Yahara was identified as a new species in 2018 [].This species is a medium tree with scaly terminal buds, scales with dense golden yellow hairs, light golden yellow young branchlets, and alternate leaves [].N. vuquangensis is now available in Vietnam [].For the first time to our knowledge, the current paper reports chemical compositions in its leaf oils, and ...
Lemon oil is known to: Reduce anxiety and depression. Reduce pain. Ease nausea. Kill bacteria. A study also states that aromatherapy of essential oils like lemon oil might improve the cognitive ...
This research aims to ameliorate the oxidation and storage stability of Prosopis juliflora Methyl Ester (PJME) using the Rancimat method (EN14112). Agricultural waste-derived essential oils (AWEOs), such as Citrus Aurantifolia L. (CA), Curcuma longa L. (CL), and Eucalyptus camaldulensis Dehn (EC), were used as natural antioxidants alongside the commonly used synthetic antioxidant TBHQ.
The promising antimicrobial activity of EOs has encouraged researchers to use them along with nanomaterials, essential oils of other plants, essential oil components, and antibiotics as potential antimicrobial agents. Encapsulation simultaneously increases the antimicrobial potency of essential oils by controlled/sustained release and ...
Potassium. Magnesium. Carotenoids (lutein, zeaxanthin) A whole medium avocado contains about 240 calories, 13 grams carbohydrate, 3 grams protein, 22 grams fat (15 grams monounsaturated, 4 grams polyunsaturated, 3 grams saturated), 10 grams fiber, and 11 milligrams sodium. Along with their low sodium levels, avocados contain no cholesterol.
Essential oils belonging to plants in the citronella genus ... Wissal Dhifi and Wissem Mnif conceived and designed the paper. Wissal Dhifi, Sana Bellili, Sabrine Jazi and Nada Bahloul wrote the paper. ... Progress in Essential Oil Research: 16th International Symposium on Essential Oils. De Walter de Gruyter; Berlin, Germany: 1986. pp. 429 ...