Medical Writing
"The Ultimate Guide to Medical Writing" is a comprehensive handbook that offers a step-by-step approach to writing clear and effective medical documents. It covers all aspects of medical writing, from preparing for a writing project to publishing a manuscript. With helpful tips and practical examples, this guide is a must-have for anyone involved in medical communication. Read less


Recommended
More related content, similar to medical writing, similar to medical writing ( 20 ), recently uploaded, recently uploaded ( 20 ).
- 1. The Ultimate Guide to M edical Writing
- 2. Medical writing is an integral part of the medical field that provides detailed and accurate information related to treatments, research, and other topics that are related to healthcare. It enables healthcare professionals to understand the complexities of the medical field resulting in making better decisions for their patients. Medical writing also involves well-structured scientific documents such as clinical research documents, content for healthcare websites, health magazines, journals, and news. The field of medical science is a very complex and highly specialized field that requires a deep understanding and strong knowledge of medical terminology and the ability to communicate complex topics in a clear, concise manner. Medical writing is a demanding field, but it is also rewarding and provides an important service to the medical community. Thus, making their work easier.
- 3. Types of Medical Writing There are several types of medical writing as mentioned below: 01 The medical writers draft content for clinics documentations. Clinical documentation consists of detailing a medical treatment, medical trial, and or clinical test. This can help the healthcare sector find the details of the patient in just a few clicks, resulting in more efficiency and productivity. Clinical documentation Scientific Publications In the scientific publications sector, the writing includes research-based writing, medical writers work with physicians and other researchers to write and edit manuscripts for reviewed journals. It also has inclusions like posters, oral presentations for medical conferences, and abstracts. Summaries of conference sessions are also generated that include news and features for a professional audience. 02
- 4. As per the growing industry of research and development in creating new medical devices, there comes a requirement to draft the documentation for the medical device. In this area, the medical writers work along with the research and development team and create a draft in a way that explains everything about the medical device. They even help in creating marketing materials for them, press releases, and other required things in a way that can be understood by the public. 03 Medical device documentation This area of medical writing includes the writer focusing on the documents at various stages of a clinical trial. It includes writing regulatory documents like protocols, investigator brochures (IB), and informed consent forms (ICF). Once the trial ends, a clinical study report (CSR) and common technical document (CTD) are required While writing in this area a writer needs to be aware of all the regulations of the country for which he/she is writing the content. The document should transmit the required information accurately and transparently to the target audience. 04 Regulatory writing
- 5. The study and practice of communicating promotional health information such as health education, public health campaigns, and between doctor-patient. In this area, medical writers focus on writing information in a way where it can influence and improve health literacy and personal health choices. Health communication is considered a unique niche as it allows professionals to communicate strategies and inform the public to improve their health. But as the information is directly reaching the public so the communication should be refined and accurate. Also, it should let people know how to avoid specific health risks. 05 Health Communications 06 Drug and medication documentation In medical writing there comes writing and drafting information on the drugs and medications from the start of the development itself. It is important that the data that is being drafted with the information about the drug should be accurate and medical writers keep this in mind. They know that critical information has to be kept in a way that is easy to understand.
- 6. In this area, Medical writers services focus on writing promotional material for marketing. In this, thewriters streamline the process of press releases, media advisories, and other marketing material to help the healthcare organization. 07 Promotional Writing Grantsmanship is a document written to grant proposals for funding scientific research. The organizations thatwork in this range from healthcare and academic institutions to private foundations, professional associations, and patient advocacy groups. Grantsmanship 08
- 7. Benefits of Medical Writing High-quality medical writing As medical writing experts are the experts in this field,they will be able to deliver high-quality content for you.Their content will be accurate and concise content. They can also help in generating a lot of other marketing material for you with the help of which you can generate leads and let the audience know about you. Increased credibility and accuracy Medical writing can help in increasing credibility and accuracy for healthcare organizations and professionals because they provide accurate and up-to-date information about a particular product. Thus, they can help to build trust and confidence in credibility and accuracy. Medical writing also provides detailed reports on clinical trials and research studies.The writers also help in creating press releases and media advisories that are professional, which can help to increase an organization's professional credibility in the eyes of the public.
- 8. Challenges of Medical Writing Ensuring accuracy and compliance To ensure accuracy and compliance, writers have to make sure thatthey are up todatewith thetrends and the information related to the research or product they are writing information about. It is quite challenging for them to do so and it requires a lot of focus and dedication to making it work. Strict timelines Then it comes tothetimelines. Writing content thatis accurate and of high quality under strict timelines is quite stressful. But medical writing ensures thatthecontent is delivered on time without any errors. They are habitual in performing under tight deadlines making them experts in the domain.
- 9. How can medical writing help healthcare professionals? Medical writing can help the healthcare industry because they ensure that the medical documents are accurate, timely, and compliant with regulatory guidelines. Being stressed over tight deadlines is also solved with the help of medical writing. The experts write the content within the bounds of regulatory guidelines and help ensure that patients receive the best care.
- 10. we have learned about medical writing. Medical writing is a service where complex medical information is accurate, ethical, and effective. Medical writing can help the healthcare industry because the experts will write the content within the bounds of regulatory guidelines. There are different types of medical writing such as clinical documentation, Scientific publications, medical device documentation, regulatory writing, health communications, drug and medication documentation, promotional writing, and grantsmanship. Every area has its importance and it is mandatory for the information to be accurate and up-to-date. The benefits that medical writing offers are that it saves time and cost, you get high-quality medical writing, and it helps in increasing the credibility and accuracy of the written material. But in order to ensure that everything is competent and true to the facts there are some challenges that are faced by medical writing such as ensuring that the information they are providing is accurate and compliant. Secondly, working under tight deadlines and giving positive results. Conclusion

Medical Writing
A Guide for Clinicians, Educators, and Researchers
- © 2018
- Latest edition
- Robert B. Taylor 0
Oregon Health & Science University, Portland, USA
You can also search for this author in PubMed Google Scholar
- A comprehensive and accessible guide to writing for all medical professions across all specialties
- Specific guidelines for review articles, case reports, books and book chapters, grant proposals, research proposals, and clinical study reports
- Fully updated to include coverage of open access journal, peer review fraud, parachute studies, sharing clinical data, and more.
55k Accesses
3 Citations
8 Altmetric
This is a preview of subscription content, log in via an institution to check access.
Access this book
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Other ways to access
Licence this eBook for your library
Institutional subscriptions
Table of contents (12 chapters)
Front matter, getting started in medical writing.
Robert B. Taylor
Basic Writing Skills
Medical writing: getting started and getting finished, technical issues in medical writing, what’s special about medical writing, how to write a review article, case reports, editorials, letters to the editor, book reviews, and other publication models, writing book chapters and books, how to write a research protocol, how to write a grant proposal, how to write a report of a research study, getting your writing published, back matter.
- medical writing
- peer review
- grant proposal
- medical language
About this book
This book is a clear and comprehensive guide that assists readers in translating observations, ideas, and research into articles, reports, or book chapters ready for publication. For both researchers and practicing physicians, skills in medical writing are essential. Dr. Robert B. Taylor, a distinguished leader in academic medicine, uses a clear, conversational style throughout this book to emphasize the professional and personal enrichment that writing can bring. The text includes in depth instructions for writing and publishing: review articles, case reports, editorials and letters to the editor, book reviews, book chapters, reference books, research protocols, grant proposals, and research reports. This third edition is additionally fully updated to include the intricacies of medical writing and publishing today, with new coverage of: open access, pay to publish and predatory journals, peer review fraud, publication bias, parachute studies, public domain images, and phantom authors. Loaded with practical information, tips to help achieve publication, and real world examples, Medical Writing can improve skills for clinicians, educators, and researchers, whether they are new to writing or seasoned authors.
Authors and Affiliations
Oregon health & science university, portland, usa, about the author.
Robert B. Taylor, M.D.
Department of Family Medicine
Oregon Health & Science University School of Medicine
Portland, Oregon, USA
Bibliographic Information
Book Title : Medical Writing
Book Subtitle : A Guide for Clinicians, Educators, and Researchers
Authors : Robert B. Taylor
DOI : https://doi.org/10.1007/978-3-319-70126-4
Publisher : Springer Cham
eBook Packages : Medicine , Medicine (R0)
Copyright Information : Springer International Publishing AG 2018
Softcover ISBN : 978-3-319-70125-7 Published: 09 January 2018
eBook ISBN : 978-3-319-70126-4 Published: 13 December 2017
Edition Number : 3
Number of Pages : XVI, 420
Number of Illustrations : 8 b/w illustrations
Topics : General Practice / Family Medicine , Primary Care Medicine , Medicine/Public Health, general
- Publish with us
Policies and ethics
- Find a journal
- Track your research
The Importance of Medical Writing in Clinical Research
Other pages on the topic:
We'll deliver straight to your inbox.
Key Learnings contained in this article:
Clinical research plays a crucial role in advancing healthcare and improving patient outcomes. It involves meticulous planning, rigorous data collection and analysis, and effective communication of findings. One key aspect of this communication is medical writing, which serves as the bridge between complex scientific research and its translation into meaningful, accessible information for various stakeholders.
Understanding the role of medical writing in clinical research
At its core, medical writing can be defined as the process of transforming intricate scientific data into coherent, evidence-based documents. Its purpose extends far beyond simply presenting findings – it encompasses the ability to distill complex medical information into clear, concise, and organized content.
In the realm of clinical research, medical writing serves as a vital tool for communicating research protocols, regulatory documents, manuscripts, and data analysis reports. It requires a deep understanding of scientific concepts, as well as the ability to convey them effectively to diverse audiences, including researchers, healthcare professionals, regulatory authorities, and even the general public.
Defining medical writing and its purpose
Medical writing involves crafting various types of documents related to clinical research. These can range from research proposals and study protocols to regulatory submissions and scientific publications. The purpose of medical writing is to ensure clarity, accuracy, and accessibility of information, fostering a shared understanding among different stakeholders.
The relationship between medical writing and clinical research
Medical writing is intertwined with every stage of the clinical research process. It begins with the development of research proposals, where medical writers play a crucial role in articulating research objectives, justification, and methodology. As the research progresses, medical writers collaborate with researchers to create detailed study protocols, which outline the study design, patient selection criteria, data collection methods, and statistical analysis plans.
However, the role of medical writing goes beyond the mere creation of documents. Medical writers also contribute significantly to the ethical considerations of clinical research. They ensure that the research is conducted in accordance with regulatory guidelines and ethical principles, safeguarding the rights and well-being of study participants.
During data analysis, medical writers facilitate the interpretation and presentation of the findings, transforming raw data into comprehensive reports that convey the significance and implications of the research outcomes. They also contribute to drafting manuscripts and presentations for submission to scientific journals and conferences, ensuring adherence to strict guidelines and standards.
Moreover, medical writers are instrumental in bridging the gap between researchers and the wider scientific community. They play a crucial role in disseminating research findings to healthcare professionals, policymakers, and the general public. By translating complex scientific concepts into accessible language, medical writers facilitate the understanding and application of research outcomes in clinical practice.
In conclusion, medical writing is an indispensable component of clinical research. It not only involves the creation of various documents but also encompasses ethical considerations and effective communication. Medical writers are essential in ensuring the accuracy, clarity, and accessibility of scientific information, ultimately contributing to the advancement of medical knowledge and improving patient care.
The integral components of effective medical writing
Effective medical writing involves a combination of skills, including scientific knowledge, linguistic expertise, and an understanding of regulatory frameworks. It requires careful attention to detail, clarity, and organization to convey complex information accurately and succinctly.
The art of conveying complex medical information
Translating intricate scientific concepts into accessible language is at the heart of medical writing. Skilled medical writers employ clear and concise prose, along with appropriate visual aids, to enhance comprehension and engagement. They strike a balance between providing comprehensive information and avoiding overwhelming the reader with technical jargon.
For instance, when explaining complex medical procedures, medical writers often use diagrams and illustrations to simplify the process. These visual aids not only make the information more digestible but also help readers visualize the steps involved, making it easier for them to understand and retain the knowledge.
Furthermore, medical writers understand the importance of storytelling in conveying medical information effectively. By presenting real-life case studies and patient stories, they humanize the data and create a connection between the reader and the subject matter. This approach not only makes the information more relatable but also increases the reader's engagement and interest in the topic.
Ensuring accuracy and compliance in medical writing
Precision and accuracy are fundamental requirements for medical writing in clinical research. Medical writers meticulously review and verify the data, ensuring that it aligns with the objectives of the study and the intended message. They meticulously fact-check, cite references, and adhere to ethical guidelines to maintain the integrity of the information presented.
Moreover, medical writers are well aware of the significance of peer review in the medical field. Before publication, their work undergoes a rigorous evaluation process by experts in the respective field. This peer review not only ensures the accuracy and validity of the information but also helps in identifying any potential gaps or areas that require further clarification.
In addition, medical writers must be well-versed in regulatory requirements to ensure compliance with relevant guidelines and standards, such as Good Clinical Practice (GCP) and the International Committee of Medical Journal Editors (ICMJE) guidelines. This adherence to regulatory protocols is essential for maintaining the credibility and validity of the research.
Furthermore, medical writers play a crucial role in ensuring transparency and disclosure of conflicts of interest. They are responsible for disclosing any financial or non-financial relationships that may influence the content of their work. This transparency helps readers assess the potential biases and make informed decisions based on the information presented.
The impact of medical writing on clinical trial success
Medical writing significantly influences the success of clinical trials, both in terms of design and execution. Through their expert contribution, medical writers enhance the quality and credibility of research, ultimately leading to better outcomes and improved patient care.
How medical writing influences trial design and execution
During the initial stages of clinical trial design, medical writers collaborate with researchers and stakeholders to develop comprehensive study protocols that encompass every aspect of the trial. This includes defining the target population, outlining the study procedures, and selecting appropriate endpoints and statistical analyses.
By leveraging their writing skills and scientific knowledge, medical writers ensure that study protocols are clear, ethically sound, and aligned with regulatory requirements. This clarity in trial design minimizes errors, enhances patient safety, and improves the validity and reliability of study results.
Medical writing's role in data interpretation and presentation
Data interpretation and presentation are vital aspects of medical writing that contribute to the overall success of clinical trials. Medical writers collaborate closely with researchers to analyze and summarize the data, highlighting key findings and interpreting their significance in the context of the study objectives.
They craft compelling narratives that convey the therapeutic potential and implications of the research. With their expertise, medical writers ensure that the data is presented in a manner that is understandable to both scientific and non-scientific audiences, enabling informed decision-making and facilitating widespread dissemination of knowledge.
The ethical implications of medical writing in clinical research
Ethics and integrity are foundational principles in the field of medical writing. Ensuring the ethical conduct of research and maintaining transparency in scientific communication are essential for upholding public trust and advancing medical knowledge.
Upholding integrity and transparency through medical writing
Medical writers play a critical role in upholding the principles of integrity and transparency in clinical research. They ensure that research findings are accurately represented and disclosed, thereby mitigating the risk of biased or misleading reporting. By adhering to ethical guidelines and industry standards, medical writers contribute to maintaining the highest ethical standards in scientific communication.
The consequences of poor medical writing practices
Poor medical writing can have far-reaching consequences for clinical research. Inaccurate or ambiguous reporting may lead to misinterpretation of results, compromising patient safety and potentially impeding the progress of scientific knowledge. Similarly, non-compliance with guidelines and regulatory requirements can result in delayed publication, rejection of manuscripts, or even legal implications.
It is therefore imperative to prioritize the development and enhancement of medical writing skills, while fostering a culture of quality and accountability within the realm of clinical research.
The future of medical writing in clinical research
As clinical research continues to evolve, so does the role of medical writing. Advancements in technology, shifts in research methodologies, and the increasing complexity of healthcare demand constant adaptation and innovation in the field of medical writing.
Emerging trends and advancements in medical writing
In the digital age, medical writers are harnessing technologies such as data visualization, interactive graphics, and multimedia tools to enhance the presentation of scientific data. These innovations not only improve comprehension but also enable researchers to engage with their audience more effectively.
Additionally, the rise of open access publishing, preprint servers, and social media platforms has revolutionized the way scientific findings are disseminated. Medical writers are at the forefront of this new era, enabling rapid and widespread access to research outcomes while maintaining the highest standards of quality and credibility.
The evolving challenges and opportunities for medical writers
The field of medical writing faces various challenges and opportunities in the ever-evolving landscape of clinical research. Technological advancements may streamline the writing process, but they also require medical writers to continuously update their skills to adapt to the changing landscape.
Furthermore, the demand for transparency and increased scrutiny on research integrity necessitates medical writers to stay abreast of evolving regulations and guidelines. Adhering to these standards ensures the ethical and accurate dissemination of research, enhancing the credibility of medical writing as an integral component of clinical research.
Medical writing in clinical research is a vital component that facilitates effective communication and translation of scientific findings. It plays a pivotal role in ensuring clarity, accuracy, and accessibility of information, while adhering to ethical guidelines and regulatory standards.
As the field continues to evolve, medical writers must embrace emerging trends and advancements while upholding the highest standards of integrity. By doing so, they contribute to the success of clinical trials, play a crucial role in driving advancements in healthcare, and ultimately improve patient outcomes.
Therapeutic

Introduction to Medical Writing
by ClinSkill | Nov 30, 2019 | Medical Writing | 0 comments

In this post we shall provide an Introduction to Medical Writing, its types and scope in today’s medical world and the in the clinical research industry.
Table of Contents
Medical writing is defined as communicating clinical and scientific data for a varied audience. This audience may include physicians, nurses, allied health professionals, pharmaceutical companies, scientists and even the general public.
Despite the variety of readers, medical writing is an activity which involves writing scientific information in a logical and precise manner.
According to Silvia Rogers, Phd. its main purpose is to record data obtained through research.This research could include that of journals, websites, peer reviewed articles, books etc.
Medical writing vs Literary writing
Medical writing differs from literary writing.
‘Literary writing is an art based on principles of personal style, fiction, and originality.’ It’s success largely depend on the creative imagination of the author.
However medical writing is often considered a craft as it requires certain skills, to communicate scientific data in a logical manner. It involves little room for creativity and builds on skills required for effective communication of scientific data.
History of Medical writing
The history of medical writing can be divided into three ages namely:
Age I – when medicine and writing became linked. Ancient medical texts have recorded beliefs and traditions of various medical institutions of the past.
Age II – when medical physicians became authors and began writing and spreading information through their literature. Medicine was now not only recorded but written and exchanged.
Age III – when writing included scientific development. This development led to all experimentation and evidence to be published and made available to the public.
Age IV – when medical writing finally became more sophisticated and required incorporating multimedia, sounds and pictures.
Age III ushered in a new era for medical writing. During this period the first scientific journal was published in the early 1600’s. Following this Louis Pasteur established his IMRD (Introduction, Methods, Results, Discussion). This was an introduction to formatting and publishing all scientific experimentation and discourse.Style manuals and books were prescribed for publishing scientific material. Publication became an integral final step of research. Any research that was not published was void. During and after World War II medical writers were no longer physicians.
Age IV is the period post 2000. At this stage evidence based medicine came to be established. With the advent of new technology, reporting research and its interpretation required more accuracy and analysis. Medical literature now came to include sounds, pictures, videos, graphs, charts and various new formats to make it more vibrant. Patient and physician education materials became more detailed and informative.
Types of Medical writing
Medical writing is broadly divided into two namely educational or academic medical writing and marketing medical writing.
Educational or academic medical writing
This is usually taken up by health professionals involves writing about various diseases and clinical conditions. Most health practitioners, present research and findings about varied clinical situations in journals, articles and books. With their science backgrounds, they learn to write about medicine. This forms the bulk of academic medical writing.
Marketing medical writing
This type of writing is often taken by graduates in journalism or literature graduates and involves writing about development of drugs and creating content for pharmaceutical companies. With their literary background, they learn medicine as they write.
Regulatory writing
This writing includes preparing a range of documents for regulatory submissions, including protocols and final reports for clinical trials, and clinical expert reports. They may also involve preparation of manuscripts for publication in medical journals. This is usually required by pharmaceutical companies to get their products registered with international regulatory authorities.
As discussed earlier, this flow chart explains various writing materials prepared in medical writing.
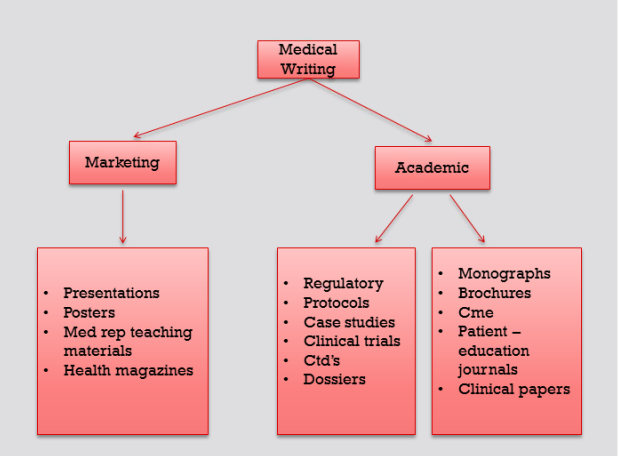
Marketing medical literature often includes presentation, posters, teaching materials for medical representatives, health magazines, etc.
Regulatory writing for pharmaceutical companies, includes preparing protocols, dossiers, case studies, clinical trial reports and common technical documents (CTD’s).
Academic medical literature also involves preparing continuing medical education materials, brochures, newsletters, journals, scientific papers and peer reviewed articles. The list is endless.
Qualifications and Skills
The qualifications for medical writing are varied. However, a degree in a life science would be extremely beneficial.
As of 2004, 65% of medical writers at the American Medical Writers’ Association had a masters’ or doctorate degree. And almost 50 % of them had a science background.
Apart from the qualifications, certain skills need to be developed and honed. These include good writing and word processing skills. As a writer it is important to ensure literature that is free from grammatical errors.
The ability to communicate effectively with others sometimes over long distances becomes necessary and is often a challenge for new writers.
Marketing any medical information requires thorough research and attention to small details, either in print or media. Most medical writing assignments are adherent to specific time frames. Being oriented to a deadline and fulfilling them is a skill that needs to be mastered.
Informal Medical training
In an effort to train oneself in medical writing, its important to be in the know of new medical and pharmaceutical developments by subscribing and reading newly published medical literature. Most medical reports involve statistics , understanding basic statistics can help especially while preparing reports and protocols.
Various institutions require different writing style guides and formats. Familiarize yourself with the different styles namely APA, Harvard etc. Update yourself with FDA and other medical regulating bodies and their guidelines. Using computer software with ease is an advantage to medical writers, updating skills in computers can help you become more efficient.
Characteristics of Good Medical writing
A good medical writer should be :
Thoroughly researched: Any article, presentation or medical content should be researched well with the most recent representation of the content and its findings as far as its possible.
Accurate: It should be accurate and precise so that readers can arrive at a conclusion after what they have read. Logically organized: Since most sciences are based on logic it is important for writers to demonstrate a logical train of thought as they write.
Readable: It should be easy to read without any complicated language or terms. The content written must reflect a coherent thought process.
Credible: Good medical writing should also be written convincingly in terms of concept, style and language so that readers find it credible.
Efficient: By improving skills it can be written efficiently using all the tools of moderns technology so as to present scientific data.
Transparent: One of the most important characteristics is transparency. Good medical content is transparent and simple not confusing and vague.
Medical writing Organizations
Currently there are four medical writing organizations that train and organize lectures and seminars for medical writers. They provide forums or platforms for medical writers to learn from each other. India also now has an organization of its own namely the Indian Medical Writers’ Association (IMWA).
The others being the American Medical Writers’ Association (AMWA), European Medical Writers’ Association (EMWA) and Australasian Medical Writers’ Association (AMWA).
Scope of Medical writing
For medical writers today the scope is vast. Depending on your qualifications and skills medical writers can take up a role as any of those mentioned below:
- Public Affairs or Public communications associate
- Publications specialist, Coordinator, Manager or Director
- Regulatory Affairs or regulatory compliance associate
- Science writer, Science Editor
- Technical writer, Technical editor
- Web Content Administrator
As one gains experience, the position becomes more specialized and focused. Publications specialists, Regulatory Affairs Associates and Medical editors are writers who have spent years on the job.
One might begin asking where are medical writers needed?
Medical writers are now needed at advertising agencies, contract research organizations, government agencies, hospitals and other health care centers. Since its conception the medical writing field has been constantly growing with more and more institutions looking to hire them.
Many pharmaceutical companies, medical schools and associations also need medical writers to help market drugs and report risk/ benefits of the same.
Biotechnology companies and medical diagnostic companies also rely on medical literature to create awareness regarding breakthroughs in medical technology.
With media now encompassing the television, radio and internet the scope of medical writing has increased by leaps and bounds thus making it a vibrant and dynamic field to work in.
Sub-Specialisation in Medical writing
Depending on one skill and interest one can develop writing in a particular division thus gaining experience and expertise on the subject.
Regulatory writing can be subdivided into preparing documents for regulatory submission of drugs, case narratives, clinical study reports, periodic update safety reports, patient information leaflets value dossiers and investigative brochures.
You can focus your attention on creating content only for websites, journals, or brand development of different drugs.
Future of Medical writing
In the future medical writing is likely to become more specialized. As communication techniques, become more sophisticated, it will allow more specialization.
Also as more research comes to be published, new formats for print and electronic coding may come into place thus formalizing the way medical literature is prepared and written.
Since the medical writing is growing exponentially, it would become necessary to establish programs that could teach and mentor students in the field. With the sub-specialization of science and medicine, medical writers will increasingly be pressured to focus on diversifying into a particular subject or format.
Want to explore a career in Medical writing? Join our Diploma in Medical Writing program and kick-start a career in Medical and Scientific writing.
Already completed a program in Medical writing. Enhance your expertise on the subject by subscribing to our Diploma in Medical Writing course to get access to a wealth of knowledge on the subject on our learning portal.
You may be interested in…

Diploma in Medical Writing

Diploma in Medical Journalism
Submit a comment cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.

ICMR School of Public Health
NIeCer 103: Scientific Writing in Health Research for health and allied sciences
A self paced online certificate course
Any current or potential health researchers (undergraduate, post-graduate medical professionals, (medical, dental, AYUSH) and allied health professionals (nursing, health related degrees), PhD. scholars and faculty of medical, dental and AYUSH streams.
Self-Paced; enroll and complete the course at any time
- Video lectures
- Presentation slides
- Further readings
Attempting all assignments is mandatory. Participants who obtain a minimum score of 50% in each assignment will be awarded an e-certificate of completion. e-certificate will be sent to the registered email id of successful participants on the last working of every month.
Indian Council of Medical Research [ICMR], National Institute of Epidemiology [NIE], has been offering online programs on bio-medical and public health research- the ‘ N IE- I CMR e - Cer tificate’ – NIeCer courses since 2016. The third in this series, NIeCer 103: Scientific Writing in Health Research, is a self-paced course on scientific writing for health researchers. Communicating research findings to the scientific community is the responsibility of every researcher. Our course will explain the fundamental concepts in drafting a scientific manuscript to effectively communicate research findings. It will provide an overview of the steps and principles involved in organizing a manuscript logically and sequentially within a structure. It will also introduce writing styles and formatting guidelines to be adhered to while drafting a scientific manuscript. We expect this course to be useful to individuals interested in improving their scientific writing skills and enhancing their publication profile.
Course faculty
All the instructors are faculty members for the two-year Master’s level public health training [MPH] programme at the ICMR School of Public Health of the ICMR-National Institute of Epidemiology, Chennai, India. Besides the Masters’ level programme, NIE has been conducting MSc in Biostatistics, PG diploma and various short-term training programmes in public health/epidemiology and biostatistics. ICMR-NIE offers PhD in Epidemiology, Biostatistics and Microbiology in association with other Universities. The faculty members have been conducting epidemiological/public health research and have rich experience in publishing high impact factor, national and international journals.
Dr. Tarun Bhatnagar
Scientist F
Dr. Tarun Bhatnagar heads ICMR - School of Public health. His area of interest is causality, epidemiology methods and emerging infectious diseases
Dr. P. Manickam
Dr. P. Manickam is physician in Siddha medicine and an epidemiologist by post-graduate & doctoral training and through practice. He heads the division of Online Courses and coordinates the development and running of health research related online courses at ICMR-NIE. He serves as core faculty in public health training programmes of the Institute and his focus areas are capacity building in public health, outbreak science, surveillance and response to public health emergencies/at mass gatherings, and clinical research in traditional medicine.
Dr. Lokesh S.
Scientist E
Prof (Dr) S Lokesh is an Internal Medicine expert working as Scientist-E, he has over 15 years of experience in patient care, teaching and research. His area of interest is Clinical Trials and has over 40 publications in indexed medical journals, 2 copyrights and 2 provisional patents.
Dr. R. Archana
Dr. Archana Ramalingam did her undergrad (MBBS) and postgrad (MD, Community Medicine) education from JIPMER, Puducherry. She has over ten years of research experience and has worked at renowned intuitions like MAMC and ILBS, New Delhi. She has recently joined at ICMR-National Institute of Epidemiology as a Scientist-E. She has over 40 publications to her credit in journals of repute. She works mainly in Non-communicable diseases epidemiology, Chronic liver disease epidemiology, and medical education.

Dr. Jeyashree K.
Scientist D
Dr. Jeyashree Kathiresan is a public health physician with a postgraduate degree (M.D.) in Community Medicine. She has a decade of experience in mentoring medical undergraduates, public health scholars and postgraduates in India and operational researchers in South East Asia and Latin America. While working on providing essential pieces of evidence for tuberculosis control in India, her ultimate goal is to see an equitable distribution in health across all income categories and genders.
Dr. P. Ganeshkumar
Dr. P. Ganeshkumar is a trained community physician with 14 years of professional experience in health system research, public health training and response to public health emergencies. He has published nearly 40 peer-reviewed papers in national/ international journals.
Dr. Rizwan S. A.
Dr. Rizwan is a Community Medicine specialist with more than 10 years of experience in the field of health research. He has published about 110 research articles in peer-reviewed national and international journals of high impact. He currently serves as Scientist - D in ICMR National Institute of Epidemiology.
Dr. Sirshendu C.
Dr. Sirshendu C. s a Community Medicine specialist with more than 10 years of experience in the public health. He has published about 15 research articles. His area of interest is maternal and child health, ethics in biomedical research, and medical education.
Dr. M. Sendhilkumar
Scientist C
Dr.M.Sendhilkumar did the Master of Public Health from SRMIST, Chennai, and has more than 9 years of experience in public health and epidemiology fields. He had worked previously on many national and international projects and published research papers in peer-reviewed journals covering public health and Ayush domains. He is working as a Project scientist- C in ICMR-National Institute of Epidemiology and focusing on public health surveillance, mass gathering and Ayush.
Teaching Assistant
Dr. Joshua Chadwick Scientist - B
Course Layout
Course content
- ● The Argument matrix
- ● How to set the background of your article, write rationale and objective(s)
- ● Referencing using Zotero
- ● How to write 'Methods' section?
- ● Designing effective tables and graphs
- ● Writing the 'Results' section
- ● Data sharing policy
- ● How to write a 'Discussion' Section?
- ● How to write a title, abstract and list keywords?
- ● Additional information
- ● Publication ethics
- ● Choosing the right journal for publication
- ● Responding to peer-review
- ● Scientific writing style
Assignment submission
- ● All lectures will have online assignments in the form of multiple-choice questions (MCQs).
- ● ● Attempting all assignments is mandatory
Certificate
- ● Attempting all assignments is mandatory. Participants who obtain a minimum score of 50% in each assignment will be awarded an e-certificate of completion. e-certificate will be sent to the registered email id of successful participants on the last working day of every month.
Click here to enroll
Note: Name as entered during enrollment will appear in the e-certificate
e-certificate will be sent to the registered email id of successful participants on the last working day of every month.

MEDICAL WRITING FOR REGULATORY SUBMISSION IN CLINICAL RESEARCH AND ITS CHALLENGES
Nov 28, 2017
50 likes | 64 Views
Regulatory medical writing in clinical trials requires medical writers to possess sufficient knowledge of the regulatory guidelines of concerned authorities of specific countries and needs to have dedication and commitment to handle large volumes of regulatory data. A professional regulatory writer needs to have sufficient understanding of the drug development process to determine the important documents that need to be written and submitted for regulatory submissions
Share Presentation
- regulatory writing
- medical writing
- regulatory requirements
- regulatory medical writing
- global regulatory writing services

Presentation Transcript
Medical writing for regulatory submission in clinical research and its challenges Medical writing for regulatory submission in clinical research and its challenges Regulatory medical writing in clinical trials requires medical writers to possess sufficient knowledge of the regulatory guidelines of concerned authorities of specific countries and needs to have dedication and commitment to handle large volumes of regulatory data. A professional regulatory writer needs to have sufficient understanding of the drug development process to determine the important documents that need to be written and submitted for regulatory submissions. Regulatory submissions challenges in clinical trials Regulatory Writing and Publishing poses many challenges for the medical writers in the writing and development of critical documents like Clinical Study Report, Investigator’s Brochure, and clinical trial protocol development and in the preparation of documents for FDA meetings and briefings. The Clinical Study Report (CSR) is a critical document that provides an integrated report comprising the clinical and statistical description of the investigational study of therapeutic or prophylactic drugs in a single report with relevant tables, figures, and appendices. A medical writer will face challenges in understanding the guidelines and statutory requirements and also developing suitable document template that covers all current regulatory requirements. Investigator’s Brochure is an essential regulatory document that provides an overview of the clinical and non-clinical findings of the trial study and is primarily used as an investigator guide to assessing the risks and benefits of the product under investigation. The major challenge commonly faced by the regulatory medical writer in the preparation of Investigator’s brochure include © 2017-2018 All Rights Reserved, No part of this document should be modified/used without prior consent Medical Writing Experts™ -A Unit of Guires Solutions Pvt Ltd. INDIA: Nungambakkam, Chennai, 600 034. UK: The Portergate, Ecclesall Road, Sheffield, S11 8NX. Email:[email protected], Web:www.medicalwritingexperts.com.
Need for being concise with suitable presentation styles. Ascertaining the appropriate length of the document Completeness and readability challenges Time management Preparing briefing documents for FDA meetings is another major challenge faced by the regulatory medical writers as it involves extensive writing relating to new products description, clinical pharmacology, mechanism of action, pharmacokinetics, clinical review on its efficacy, safety, Benefit-Risk summary, and assessment. Clinical Trial Protocol Development is a complicated process that involves proper planning and diverse document requirements during the pre-clinical and clinical stage as specified by the regulatory authority, which includes Animal studies relating to safety and toxicology Common Technical Document (CTD) Stability studies Development of full protocol and trial document formats Good knowledge of regulatory requirements Obtaining informed consents from the participants of the study Regulatory medical writers need to have an adequate understanding of the important activities involved in Clinical Trials and Good Clinical Practice, which may present them challenges like large time requirements to develop high-quality medical contents specific to the target audience. Also, Pre-clinical and scientific reports pose challenges to the regulatory medical writers as these reports need to have accurate facts, statistical data, relevant tables, and figures. © 2017-2018 All Rights Reserved, No part of this document should be modified/used without prior consent Medical Writing Experts™ -A Unit of Guires Solutions Pvt Ltd. INDIA: Nungambakkam, Chennai, 600 034. UK: The Portergate, Ecclesall Road, Sheffield, S11 8NX. Email:[email protected], Web:www.medicalwritingexperts.com.
Comprehensive Regulatory Writing Services International regulatory writing firms can offer immense assistance to the companies conducting clinical research trials by helping them in the writing, editing, organising and the compilation of broad range of essential medical and scientific documentation like Clinical Development Plans (CSP) Clinical Study Reports (CSR) Documents relating toInvestigational New Drug Applications (NDAs) Investigator’s Brochures Benefit and Risk Assessment reports FDA meeting documents and briefings Thus, it is advisable for the healthcare, pharmaceutical, biotechnology, medical device, CROs companies to entrust these complex regulatory works to a global regulatory writing services team of experts, to meet the various challenges like stringent regulatory body requirements, multiple agencies approval prerequisites and timely submission of essential documents. © 2017-2018 All Rights Reserved, No part of this document should be modified/used without prior consent Medical Writing Experts™ -A Unit of Guires Solutions Pvt Ltd. INDIA: Nungambakkam, Chennai, 600 034. UK: The Portergate, Ecclesall Road, Sheffield, S11 8NX. Email:[email protected], Web:www.medicalwritingexperts.com.
- More by User

pre-clinical and clinical regulatory overview
Outline I. New Drug Review Process: First Principles II. FDA Model of Serial Evaluation:Investigational New Drug (IND) .... Pre-Clinical studies .... Clinical Trials Phases I ? IV New Drug Application (NDA)Post-Marketing Surveillance III. Life Science Reviewers: Roles/Opportunities Pharmacology Physiology Toxicology PathologyMolecular Biology Chemistry Statistical ScienceVeterinary Med9447
536 views • 19 slides

Clinical research = Daily medical practice
Session 21 Practical interpretation of regulatory requirements for documenting the clinical trial for CRAs and CRCs creating the “squeaky clean” file Ruth Ann Nylen, PhD. Clinical research = Daily medical practice.
953 views • 69 slides

Good Clinical Practices Regulatory Guidelines for the Conduct of Clinical research
Good Clinical Practices Regulatory Guidelines for the Conduct of Clinical research. Introduction to Research Pharmacy Services Javier Palacios, R.Ph. Sponsored by the Research Committee of the Medical Dental Staff at UHS. Learning Objectives. Define Good Clinical Practice and
363 views • 10 slides

Medicare Submission for Pre-Approval in Clinical Research
Medicare Submission for Pre-Approval in Clinical Research. Ronda Sharp, R.N., B.S.N., C.C.R.C. Clinical Research Facilitator Methodist Research Institute. History of Pre-Approval Process. At Methodist Research Institute, we have been submitting for pre-approval for IDE trials since 1999.
429 views • 24 slides

Using Electronic Medical Records Systems for Clinical Research: Benefits and Challenges
Using Electronic Medical Records Systems for Clinical Research: Benefits and Challenges . Prakash M. Nadkarni. Introduction. Opportunities Availability of clinical, financial and administrative data in electronic form Challenges Using EMR Software for research operations
521 views • 29 slides

Medical research and clinical trials
Medical research and clinical trials. Lukáš Prudil Masaryk University Brno, Czech Republic. Medical research milestones. 1750 : Voltaire states: "The art of medicine consists of amusing the patient while nature cures the disease."
841 views • 67 slides

Medical Research and Clinical Trials
Medical Research and Clinical Trials. Rels 300 / Nurs 330 31 October 2013. What is a clinical trial?. a research study designed to evaluate a new drug or medical therapy; e.g., compare a new drug or treatment with a commonly used (older) drug or treatment;
388 views • 24 slides

Clinical Research in South Africa - Ethical and Regulatory Processes
NATIONAL HEALTH RESEARCH ETHICS COUNCIL. Clinical Research in South Africa - Ethical and Regulatory Processes . Professor A Dhai Deputy Chair National Health Research Ethics Council Director – Steve Biko Centre of Bioethics, University of the Witwatersrand, Johannesburg.
341 views • 13 slides

Policy and regulatory challenges for CCS
Policy and regulatory challenges for CCS. Dr Paul Zakkour, ERM Energy & Climate Change Services, UK International Workshop on CCS in the Power Sector: R&D Priorities for India. Delhi 23 rd January 2008. Overview. Policy :
369 views • 22 slides

Writing Clinical Research Protocols, Part 2: Writing and Editing Clinical Research Protocols
Writing Clinical Research Protocols, Part 2: Writing and Editing Clinical Research Protocols. Jonathan McCall, BA Editor DCRI Communications. Writing Clinical Research Protocols. Writing Clinical Research Protocols. General Considerations Parts of the Protocol Good Writing Matters
748 views • 51 slides

Regulatory Submission Process
Regulatory Submission Process. Clinical Study Application: Submit an appointment request Fill one of the three templates: BE / BA study Phases I-III Study Phase IV ( Observational) Study. Regulatory Submission Process. Complete the application by submitting all the required Documents :
841 views • 23 slides

Diabetes Clinical and Translational Research: Rewards and Challenges
Diabetes Clinical and Translational Research: Rewards and Challenges. Ruth S. Weinstock MD PhD Medical Director, Joslin Diabetes Center and Clinical Research Unit Professor of Medicine and Chief, Endocrinology, Diabetes and Metabolism SUNY Upstate Medical University Syracuse NY
251 views • 12 slides

Pediatric Clinical Research A Regulatory and Everyday Perspective
Pediatric Clinical Research A Regulatory and Everyday Perspective. Linda DiMeglio, MD, MPH; Lucy Miller, RN, BSN, CCRP; Jody Harland, MS, CIP Department of Pediatrics, IU School of Medicine Shawn Axe, CIP Research Compliance Administration, IUPUI. Objectives . To understand:
513 views • 39 slides

Medical Decision-Making and Clinical Research
Medical Decision-Making and Clinical Research. Class Format. 5-10 minute quiz. Class Format. 5-10 minute quiz on readings Interactive. Class Format. 5-10 minute quiz on readings Interactive Didactics, prn. Class Format. 5-10 minute quiz on readings Interactive Didactics, prn
490 views • 40 slides

Clinical Data management and its Types in Clinical Research
Clinical Research is a most knowledge intensive and interesting field in pharmaceutical industry. The billion dollar industry is demanding the employment opportunities of qualified and trained professionals in clinical research sector.
45 views • 3 slides

420 views • 40 slides

General Surgery Medical Billing and its Challenges
General surgery comprises a large number of surgical procedures u2013 from kidney surgery, pancreas, thoracic and abdominal surgery, liver transplantation, gastrointestinal (GI) tract surgery, to elective surgery and breast surgery.
65 views • 5 slides

Clinical trial protocol writing: Challenges and Guidelines
u2022The clinical study protocol is defined as the procedures by which clinical research is conducted u2022A clinical study protocol should provide a clear clinical study design to meet the objective of the clinical trial u2022A defined protocol must address the proposed medical question and protect the safety and rights of the clinical trial participant/patients To Continue Reading : https://bit.ly/2W5AgGu Contact Us: Website : https://bit.ly/33Fwsye Email us: [email protected] Whatsapp: 91 9884350006
37 views • 3 slides

Medical writing and Clinical data management
Candidates looking for the Best medical writing programs can enroll in our academy to get more knowledge about the course. After the course completion, we also provide placement assistance in top MNCs. We solemnly pledge to offer quality training for the Good clinical data management certificate online for the aspirants. Our team of well-teaching experts hosts the training session practically.
13 views • 7 slides
Clinical Imaging Submission
Clinical Images are photographic depictions of the patientu2019s body - such as an injury, skin lesion or body fluid - or an image of a pathology report, diagnostic image, or medication. It should not exceed more than 5 figures with a description, not exceeding 300 words. Generally, no references and citations are required here. If necessary, only three references can be allowed. Clinical Images Submission: Clinical Imaging Submission is a relevant keyword of clinical image submission, clinical imaging submission, clinical imaging journal, journal of clinical imaging, imaging of journal etc. Jour
6 views • 2 slides
Clinical Images Submission
Clinical Images are photographic depictions of the patientu2019s body - such as an injury, skin lesion or body fluid - or an image of a pathology report, diagnostic image, or medication. It should not exceed more than 5 figures with a description, not exceeding 300 words. Generally, no references and citations are required here. If necessary, only three references can be allowed. Clinical Images Submission: Clinical Imaging Submission is a relevant keyword of clinical image submission, clinical imaging submission, clinical imaging journal, journal of clinical imaging, imaging of journal etc.
5 views • 2 slides
2 views • 2 slides
- VIRTUAL CAMPUS

- No products in the cart.
Effective Medical Writing & Data Presentation
Book a session.
- Description
- Programme agenda
- Competency framework
Testimonials
About this course, reasons to attend, what's included.
- Documents and materials related to this course are included
- Globally recognised certificates awarded after test completion
- This course has been granted PharmaTrain Recognition
Course schedule
- Two Day face-to-face training: 09h00-17h00
Course Description
Programme highlight.
- The basics of medical writing and communication
- The problem statement
- How to guide the reader through the text
- Accurate vocabulary (avoiding ‘pharma speak’, ethically correct wording, accurate wording)
- How can I be clearly synthetic?
- Using guidelines to simplify your work
- Organizing your writing projects
- Contents and flow of the different sections of scientific documents and their abstracts
- Designing and describing effective figures and tables
- Effectively communicating with the audience
Learning objectives
- Craft clear, concise, and compliant clinical reports: Apply guidelines and tailor writing for regulators, researchers, and patients.
- Transform data into impactful narratives: Utilize captivating visuals and storytelling techniques to communicate complex findings effectively.
- Present clinical research with confidence and persuasion: Develop engaging presentations and master delivery skills to drive action.
- Communicate ethically and responsibly: Understand and apply ethical principles in data reporting and authorship.
- Stay ahead of the curve: Remain informed about evolving trends and best practices in clinical communication.
- Empower stakeholders through clear communication: Enhance research impact, foster collaboration, and improve patient understanding
Who should attend
Anyone who needs to write clinical trial documents like the protocol, investigator brochure (IB), scientific articles, presentations for congresses, etc. needing to be shared with the stakeholders.
Competencies
This course covers competencies that are part of the ECCRT Competency Framework
- Scientific Concepts & Research Design (0)
- Ethical & Participants Safety Considerations (0)
- Investigational Product Development and Regulation (0)
- Clinical Studies Operations (GCPs or ISO 14155) (0)
- Study and Site Management (0)
- Data Management and Informatics (0)
- Leadership and Professionalism (0)
- Communication (2)
- Teamwork (0)
- Business acumen (0)

Mr. Andrea Rossi
No testimonial yet
Privacy Overview
User registration, reset password.
What is Medical Writing?

Changing market dynamics, expiring patents, and depleted product pipelines are all exerting pressure on pharmaceutical companies to outsource non-core activities in an effort to control costs and enhance drug-development cycle time. One of these activities is medical writing—an area that, although not at the core of pharmaceutical operations, has become increasingly critical as regulatory agencies have adopted more elaborate review measures that require substantial amounts of documentation.
Medical writing involves creating well-structured scientific documents that include clinical research documents, content for healthcare websites, health magazines, journals and news. Medical writing can be classified into two types: Regulatory medical writing and Educational medical writing.
Regulatory medical writing involves writing documents for regulatory agencies seeking approval for devices, drugs and biologics. This includes clinical trial data, regulatory submission documents, post approval documents, etc.
Educational medical writing is writing documents about devices, biologics and medicine, specifically for general studies
Explore the digital path forward
Explore our Digital Offerings
Get HCLTech Insights and Updates delivered to your inbox

- Preferences

Medical Writing in Regulatory Submission for Clinical Research - PowerPoint PPT Presentation

Medical Writing in Regulatory Submission for Clinical Research
The major challenge commonly faced by the regulatory medical writer in the preparation of investigator’s brochure include need for being concise with suitable presentation styles ascertaining the appropriate length of the document completeness and readability challenges time management – powerpoint ppt presentation.
- the guidelines and statutory requirements and also developing suitable document template that covers all current regulatory requirements.
- Investigators Brochure is an essential regulatory document that provides an overview of the clinical and non-clinical findings of the trial study and is primarily used as an investigator guide to assessing the risks and benefits of the product under investigation. The major challenge commonly faced by the regulatory medical writer in the preparation of Investigators brochure include
- Need for being concise with suitable presentation styles
- Ascertaining the appropriate length of the document
- Completeness and readability challenges
- Time management
- Preparing briefing documents for FDA meetings is another major challenge faced by the regulatory medical writers as it involves extensive writing relating to new products description, clinical pharmacology, mechanism of action, pharmacokinetics, clinical review on its efficacy, safety, Benefit-Risk summary, and assessment.
- Clinical Trial Protocol Development is a complicated process that involves proper planning and diverse document requirements during the pre-clinical and clinical stage as specified by the regulatory authority, which includes
- Animal studies relating to safety and toxicology
- Common Technical Document (CTD)
- Stability studies
- Development of full protocol and trial document formats
- Good knowledge of regulatory requirements
- Obtaining informed consents from the participants of the study
- Regulatory medical writers need to have an adequate understanding of the important activities involved in Clinical Trials and Good Clinical Practice, which may present them challenges like large time requirements to develop high-quality medical contents specific to the target audience.
- Comprehensive Regulatory Writing Services
- International regulatory writing firms can offer immense assistance to the companies conducting clinical research trials by helping them in the writing, editing, organising and the compilation of broad range of essential medical and scientific documentation like
- Clinical Development Plans (CSP)
- Clinical Study Reports (CSR)
- Documents relating to Investigational New Drug Applications (NDAs)
- Investigators Brochures
- Benefit and Risk Assessment reports
- FDA meeting documents and briefings
- Thus, it is advisable for the healthcare, pharmaceutical, biotechnology, medical device, CROs companies to entrust these complex regulatory works to a global regulatory writing services team of experts, to meet the various challenges like stringent regulatory body requirements, multiple agencies approval prerequisites and timely submission of essential documents.
- About Pepgra
- Pepgra is a quality-driven Contract Research Organisation (CRO) comprising advanced regulatory writers capable of delivering clinical study protocols and study reports in complete compliance with the ICH GCP guidelines. Pepgra offers complete assistance to the pharmaceutical and medical device companies
PowerShow.com is a leading presentation sharing website. It has millions of presentations already uploaded and available with 1,000s more being uploaded by its users every day. Whatever your area of interest, here you’ll be able to find and view presentations you’ll love and possibly download. And, best of all, it is completely free and easy to use.
You might even have a presentation you’d like to share with others. If so, just upload it to PowerShow.com. We’ll convert it to an HTML5 slideshow that includes all the media types you’ve already added: audio, video, music, pictures, animations and transition effects. Then you can share it with your target audience as well as PowerShow.com’s millions of monthly visitors. And, again, it’s all free.
About the Developers
PowerShow.com is brought to you by CrystalGraphics , the award-winning developer and market-leading publisher of rich-media enhancement products for presentations. Our product offerings include millions of PowerPoint templates, diagrams, animated 3D characters and more.

An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- GMS J Med Educ
- v.34(1); 2017

Language: English | German
Accepted standards on how to give a Medical Research Presentation: a systematic review of expert opinion papers
Anerkannte standards zum halten medizinischer vorträge: eine systematische Übersicht publizierter experteneinschätzungen, christine blome.
1 University Medical Center Hamburg-Eppendorf (UKE), Institute for Health Services Research in Dermatology and Nursing (IVDP), German Center for Health Services Research in Dermatology (CVderm), Hamburg, Germany
Hanno Sondermann
Matthias augustin.
Background: This systematic review aimed to extract recommendations from expert opinion articles on how to give a medical research presentation on a scientific conference and to determine whether the experts agree on what makes an effective or poor presentation.
Methods: Presentation-related terms were searched within article titles listed in PubMed, restricting the search to English-language articles published from January 1975 to July 2015. Recommendations were extracted from the articles, grouped by content, and analyzed for frequency. Ninety-one articles were included. Among 679 different recommendations, 29 were given in more than 20% of articles each. The five most frequent recommendations were to keep slides simple, adjust the talk to the audience, rehearse, not read the talk from slides or a manuscript, and make eye contact.
Results: No article gave advice that was the complete opposite of the 29 most frequent recommendations with the exception of whether a light or dark background should be used for slides.
Conclusions: Researchers should comply with these widely accepted standards to be perceived as effective presenters.
Zusammenfassung
Hintergrund: Ziel dieser systematischen Übersichtsarbeit war es, aus publizierten Expertenstellungnahmen Empfehlungen zur Vorgehensweise bei medizinischen Präsentationen auf wissenschaftlichen Fachtagungen zu extrahieren und abzuleiten, ob Experten in der Frage übereinstimmen, was eine gute oder schlechte Präsentation ausmacht.
Methoden: Präsentationsbezogene Schlagwörter wurden in den Titeln englischsprachiger, in PubMed geführter und zwischen Januar 1975 und Juli 2015 erschienener Artikel gesucht. Aus den gefundenen Expertenartikeln wurden Empfehlungen extrahiert, inhaltlich gruppiert und nach Häufigkeit ausgewertet. Einundneunzig Artikel wurden eingeschlossen. Von insgesamt 679 unterschiedlichen Empfehlungen fanden sich 29 jeweils in mindestens 20% der Artikel. Die fünf häufigsten Empfehlungen lauteten: Einfache Folien verwenden; die Zuhörerschaft kennen; Augenkontakt halten; die Präsentation üben; nicht von Folien oder Manuskript ablesen.
Ergebnisse: In keinem Artikel wurde eine Empfehlung gegeben, die das klare Gegenteil einer der 29 häufigsten Empfehlungen darstellten, bis auf die Frage, ob ein heller oder dunkler Folienhintergrund verwendet werden sollte.
Schlussfolgerung: Wissenschaftler sollten sich an die hier gefundenen, weithin akzeptierten Empfehlungen halten, damit ihre Präsentationen positiv wahrgenommen werden.
1. Introduction
Some presentations at medical conferences are easy to follow, engaging, and even inspire changes in the way patients are treated or scientific work is conducted. Conversely, others induce the audience to check their mobile phones or take a nap because they are so difficult to concentrate on.
What exactly makes great medical research presentations great? Empirical or even experimental data on this question are scarce [ 1 ], [ 2 ], [ 3 ], [ 4 ]. However, more than 80 authors of expert opinion articles have described what they believe a medical presenter should or should not do. The aim of this review was to extract all recommendations from these articles and determine whether the experts agree on what makes a medical research presentation either effective or poor.
Parts of this study were obtained from a previous dissertation by Sondermann, 2014 [ 5 ].
Presentation-related terms were searched within the titles of articles listed in PubMed, restricting the search to English-language articles published from January 1975 to July 2015. The search terms were:
(scientific[ti] AND presentation*[ti]) OR (conference[ti] AND presentation*[ti]) OR (oral[ti] AND presentation*[ti]) OR (research[ti] AND presentation*[ti]) OR (scientific[ti] AND meeting*[ti]) OR (public[ti] AND speaking[ti]) OR (public[ti] AND speech[ti]) OR (Power[ti] AND Point[ti]) OR PowerPoint[ti] OR (scientific[ti] AND talk*[ti]) OR lecturing[ti] OR lectures[ti] OR (scientific[ti] AND conference*[ti]) OR (medical[ti] AND presentation*[ti]) OR (paper[ti] AND presentation*[ti]) AND "1975/01/01"[PDAT]:"2015/07/31"[PDAT] AND English[lang]
The bibliographies of eligible articles were reviewed for further references.
We included expert opinion articles and editorials that provided advice on how to give a medical research presentation at scientific conferences. We excluded articles exclusively referring to lectures to students, continued medical education, or health care management.
Recommendations were extracted from each article, including both direct (e.g., “You should…”) and indirect recommendations (e.g., “Remember the audience’s time (…) should not be abused by presentation of uninteresting preliminary material” [ 6 ]). Mere suggestions were not extracted; these were typically signaled by words such as “consider.” We also excluded recommendations on abstract writing, use of outdated technology (e.g., diapositives), radiologic images (for being too specific), and technical aspects (e.g., choice of software).
Differently worded advice from two authors was regarded as the same recommendation if equal in content (e.g., “initially, rehearse alone” [ 7 ] and “initially, practice the talk alone” [ 8 ]). Similar recommendations were grouped into more general but still concrete advice. For example, “limit the number of lines on a slide to six” [ 9 ] and “no more than seven lines per slide” [ 10 ] were grouped into “limit the number of lines per slide.” Finally, we determined the frequency of recommendations, counting those given in two articles by the same first author only once.
The PubMed search delivered 4,140 hits, 91 of which met the inclusion criteria [ 6 ], [ 7 ], [ 8 ], [ 9 ], [ 10 ], [ 11 ], [ 12 ], [ 13 ], [ 14 ], [ 15 ], [ 16 ], [ 17 ], [ 18 ], [ 19 ], [ 20 ], [ 21 ], [ 22 ], [ 23 ], [ 24 ], [ 25 ], [ 26 ], [ 27 ], [ 28 ], [ 29 ], [ 30 ], [ 31 ], [ 32 ], [ 33 ], [ 34 ], [ 35 ], [ 36 ], [ 37 ], [ 38 ], [ 39 ], [ 40 ], [ 41 ], [ 42 ], [ 43 ], [ 44 ], [ 45 ], [ 46 ], [ 47 ], [ 48 ], [ 49 ], [ 50 ], [ 51 ], [ 52 ], [ 53 ], [ 54 ], [ 55 ], [ 56 ], [ 57 ], [ 58 ], [ 59 ], [ 60 ], [ 61 ], [ 62 ], [ 63 ], [ 64 ], [ 65 ], [ 66 ], [ 67 ], [ 68 ], [ 69 ], [ 70 ], [ 71 ], [ 72 ], [ 73 ], [ 74 ], [ 75 ], [ 76 ], [ 77 ], [ 78 ], [ 79 ], [ 80 ], [ 81 ], [ 82 ], [ 83 ], [ 84 ], [ 85 ], [ 86 ], [ 87 ], [ 88 ], [ 89 ], [ 90 ], [ 91 ], [ 92 ], [ 93 ], [ 94 ], [ 95 ], [ 96 ]. Of the 91 articles, 63 were from the medical field and 28 from related fields such as nursing. We found 3 to 103 different recommendations in each article, totaling 3,135 recommendations. Identification of identical recommendations and grouping similar ones resulted in 679 different recommendations. Of these, 349 were given in only one article each; for example, “remain in the hall from the start of the session until your talk” [ 94 ].
The most frequent advice, given in 62.9% of articles, was to keep slides simple. In particular, authors stated that one should not overload slides or include too much detail, but use clear, concise, simply designed visuals instead. Simplicity of visuals was also the subject of 5 of the 29 most frequent recommendations (see Table 1 (Tab. 1) ), including limiting the number of lines per slide (42.9%) and number of words per line (28.6%), using simple tables and graphs (34.1%), using animations carefully (27.5%), and putting phrases, not sentences, on slides (24.2%).

The second most frequent advice, to know one’s audience (52.7%), referred to who the audience is (e.g., profession, size, age, education), what they already know of the topic, or why they are there (i.e., what their expectations, attitudes, and interests are). Authors advised adjusting the presentation accordingly instead of using canned talks.
Making eye contact was the third most frequent advice (46.2%). This was specified by some authors as making eye contact with many or all persons, making eye contact with persons in all sections of the audience, or making continuous eye contact.
Rehearsal of the presentation was recommended in 44.0% of the articles. In addition, one-third of the articles advised rehearsal in front of other persons. Taken together, 56.0% of the articles gave at least one of these two recommendations. Timing the presentation beforehand – recommended by 38.5% – can ensure that the presenter will stick to the allotted time, an advice given by 40.7%. Further advice calling for thorough preparation was to know one’s topic “like the back of one’s hand” (31.9%), to develop an objective for the talk (28.6%), to and prepare for questions (20.9%). All equipment should be tested beforehand (27.5%).
When delivering the presentation, one should not read the talk from either slides or a manuscript (44.0%). For this purpose (and for simplicity) slides should contain words or phrases instead of complete sentences (24.2%).
The presenter should vary the presentation of his or her voice instead of speaking monotonously (29.7%), not speak too fast (24.2%), face the audience (23.1%), and show some enthusiasm, excitement, or energy (20.9%). To enhance understanding, one should keep the presentation clear and simple (26.4%), be logical (23.1%), and end with a summary (26.4%). The number of slides should be limited (27.5%); most articles specified one slide per minute (n=7, 7.7%).
The slides should be readable (42.9%), referring to both text and visuals. This was probably also the reason for recommending large font sizes (this advice was not included in the 29 most frequent recommendations, however; n=18, 19.8%). Authors generally disagreed regarding the exact size to be used, which ranged from 18 to 32 points; a font size of 24 points was recommended most frequently (n=8, 8.8%).
Authors agreed that the slide design should be consistent throughout the presentation (20.9%) and that contrasting colors should be used (20.9%). Most authors recommended using a dark background (26.4%), while only few recommended using a light background (n=3, 3.3%), arguing that this makes slides easier to read [ 15 ], [ 46 ]; one paper [ 89 ] recommended light background for charts and graphs, but not for text slides (without giving reasons).
None of the included articles gave advice that was the complete opposite of these 29 most frequent recommendations (except for the light versus dark background). However, limiting advice was occasionally given, such as not to practice too much in order to save some enthusiasm [ 62 ] or not to exceed >10% of the original time [ 19 ]. Authors also disagreed on a few topics that did not make it to the 29 most frequent recommendations, including whether clipart or cartoons should be included, whether using a pointer is recommended, and whether information should be added sequentially on a slide.
4. Conclusions
This review extracted recommendations from 91 expert opinion articles on how to give a medical research presentation. We found a high degree of concordance among authors, with 29 recommendations given in more than one-fifth of articles each and very little explicit discordance.
Our findings are limited by the fact that we restricted the literature search to one database and to article titles (without the latter, our search would have yielded 195,766 hits). Nevertheless, we included 91 articles on the presentation of medical research and thus considerably more than two previous reviews, which included 9 expert opinion articles on podium presentations each [ 97 ], [ 98 ].
In addition, the distinction between what authors meant to be recommendations versus mere suggestions was a matter of interpretation; the same is true for decisions on whether recommendations were similar enough to be grouped.
The fact that many authors recommend a behavior does not necessarily mean it will indeed be effective. This can be tested in experimental studies that systematically vary a presenter’s behavior. As in clinical studies, the outcome of interest would need to be defined, which is rarely done in expert opinion articles. We propose as “presenter-relevant outcomes” a) to induce learning effects (i.e., comprehension and retention [ 99 ]), b) to change attitudes, c) to interest and entertain, and d) to improve the presenter’s reputation (e.g., by appearing competent).
To our knowledge, experimental studies have only been done for presentations other than medical research presentations. Surprisingly, the recommendation given most often in this study, “keep your slides simple”, has not been supported with regard to the amount of text on a slide (an aspect also related to further recommendations, like “limit the number of lines per slide”, “limit the number of words per line”, and “put phrases, not sentences, on slides”). A number of studies in students did not find significant differences in retention of information after presentations with concise slides as compared to presentations with more detailed slides [ 100 ], [ 101 ], [ 102 ], as would have been expected by cognitive load theory. This theory states that information will not be encoded adequately if the capacity of our working memory is overloaded [ 103 ], [ 104 ], for example when trying to understand detailed slides and at the same time listen to the presenter. These surprising findings underline the necessity of experimental research on presentation techniques. However, simple slides have been found to be more effective with regard to a different aspect: that is, whether they include pictures not related to the content of the talk. Here, recall was better in students who attended a presentation using slides with irrelevant pictures [ 105 ].
The third most frequent advice, to make eye contact, was found to be effective in one study: Not only did students consider a speaker who made eye contact to be more credible and his talk to be more comprehensible, but they actually learned more as indicated by a subsequent multiple-choice test [ 102 ]. In this study, the “eye contact” condition also differed from the control condition in that the presentation was more lively (recommendation no. 13: “vary your voice“) and in that the presenter did not read from written text only but also made colloquial interjections (recommendation no. 5: “do not read the talk from slides or a manuscript”).
It is quite possible that empirical studies will contradict the advice found in this opinion-based study. For example, there is reason to assume that dark backgrounds (recommended by 24 experts as compared to 3 experts recommending light background) may have disadvantages. For example, they may require dimming the lights so that the audience can read the slides, which in turn may lead to reduced levels of attention due to increased tiredness.
In addition, findings from previous studies may not be generalizable to medical conference presentations where the audience may differ in important aspects from students (which have been the subjects of many of the experiments [ 106 ]) – for example with regard to their reasons for attendance and their prior knowledge of the topic. Future experimental studies should therefore investigate whether the recommendations found in this study are indeed effective, looking at different audiences and contexts, and focusing also on rarely explored aspects related to the preparation of the presentation, like adjustment of the talk to the specific audience (recommendation no. 2) and rehearsal (recommendation no. 4).
Probably one of the main reasons that a particular piece of advice was given in the expert opinion papers is that the authors believed that many presenters did not yet follow it. The 29 most frequent recommendations can thus be interpreted as the 29 most common mistakes made by conference presenters. Most of them appear to be common sense and are generally well known [ 99 ]; therefore, why are flaws so common, even in senior presenters [ 98 ]? Researchers may be unwilling to invest time in thorough preparation [ 107 ], or perhaps they have competing interests such as drawing the audience’s attention away from themselves or using slides as a memory aid [ 104 ]. However, if presenters want their talk to be inspiring and practice-changing, they should adhere to the agreed advice found in this review.
Future experimental studies should investigate the effectiveness of the recommendations found in this opinion-based review.
Funding sources
The authors have no funding sources to declare.
Authors' contributions
CB conceived of the study, participated in its design, conduction, and analysis, and drafted the manuscript. HS participated in the study design, conduction, and analysis and helped draft the manuscript. MA participated in the study design. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.

IMAGES
VIDEO
COMMENTS
Medical writing in clinical research. Mar 24, 2018 • Download as PPTX, PDF •. 15 likes • 5,762 views. PatelHemangini2. Subject Covered include: understanding the work of medical writers; core writing and editing skills. Education. 1 of 23. Download now. MEDICAL WRITING IN.
Medical Communications ('med comms') is the generation of written, audiovisual, oral or online materials dealing with medicine and healthcare. Medical communications agencies provide consultancy services to the pharmaceutical industry to help raise awareness of medicines via education and promotion, (Moon, 2015).
Narrative writing is an integral part of medical writing services. The purpose of writing patient narratives is to provide a concise summary of identified/specific adverse events (AEs) occurring in a patient to conclude causal relationship between the investigational drug and AE. These narratives are submitted along with the clinical study ...
ÐÏ à¡± á> þÿ þÿÿÿþÿÿÿù ú û ü ý þ ÿ ÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿÿ
Medical writing also involves well-structured scientific documents such as clinical research documents, content for healthcare websites, health magazines, journals, and news. The field of medical science is a very complex and highly specialized field that requires a deep understanding and strong knowledge of medical terminology and the ability ...
A clinician should continuously strive to increase knowledge by reviewing and critiquing papers, thoughtfully considering how to integrate new data into practice. This is the essence of evidence-based medicine (EBM).[1] When new clinical queries arise, one should seek answers in the published literature. The ability to read a scientific or medical manuscript remains vitally important ...
ÐÏ à¡± á> þÿ ³ § þÿÿÿ¯ ° ± ² ú € ú ý ...
• Understand more about the art of medical writing, includ-ing motivation, conceptualization, composition, and frustrations • Know how to use different models of medical writing, such as the review article, report of clinical research, and more • Recognize how to get a manuscript published • Realize that writing can be fun
Medical writing in clinical research is a vital component that facilitates effective communication and translation of scientific findings. It plays a pivotal role in ensuring clarity, accuracy, and accessibility of information, while adhering to ethical guidelines and regulatory standards. As the field continues to evolve, medical writers must ...
Introduction to Medical Writing. Medical writing is defined as communicating clinical and scientific data for a varied audience. This audience may include physicians, nurses, allied health professionals, pharmaceutical companies, scientists and even the general public. Despite the variety of readers, medical writing is an activity which ...
Interpretation and presentation of research data - writing scientific documents involves review and interpretation of research data, presentation of those data in text, tables, and graphs, and developing logical discussion and conclusions as to what the data means. Medical writers must have sufficient knowledge of the research topic, and ...
Indian Council of Medical Research [ICMR], National Institute of Epidemiology [NIE], has been offering online programs on bio-medical and public health research- the 'NIE-ICMR e-Certificate' - NIeCer courses since 2016. The third in this series, NIeCer 103: Scientific Writing in Health Research, is a self-paced course on scientific writing for health researchers.
Regulatory medical writing in clinical trials requires medical writers to possess sufficient knowledge of the regulatory guidelines of concerned authorities of specific countries and needs to have dedication and commitment to handle large volumes of regulatory data. A professional regulatory writer needs to have sufficient understanding of the drug development process to determine the ...
The concept of lean and mean medical writing is yet evolving and is yet to be globally accepted by the pharmaceutical companies, academics, and scientific communities. The lean and mean medical writing involves a lot of precision around presentation of huge data in a limited volume, with focused data analysis and presentation.
Join our Effective Medical Writing & Data Presentation course and unlock the power to communicate clinical research with impact. This in-depth, interactive program is designed for researchers, medical writers, and healthcare professionals who want to elevate their communication skills in the realm of clinical research.
Background. Timely and complete reporting of the results of clinical trials is an ethical imperative []; it helps to efficiently disseminate research findings and eliminate duplicative effort thereby reducing waste in research funding [], enables researchers to develop more up-to-date study hypotheses and allows clinicians and patients to judge the benefits or risks of different therapies.
Medical writing involves creating well-structured scientific documents that include clinical research documents, content for healthcare websites, health magazines, journals and news. Medical writing can be classified into two types: Regulatory medical writing and Educational medical writing. Regulatory medical writing involves writing documents ...
Protocol writing allows the researcher to review and critically evaluate the published literature on the interested topic, plan and review the project steps and serves as a guide throughout the investigation. The proposal is an inevitable document that enables the researcher to monitor the progress of the project [ 5 ].
clinical Research courses help you Become the best Medical Writer - The clinical research industry has seen an increasing demand due for medical writing has gone up significantly in the last few years. The reasons for this massive demand are innumerable research studies being conducted today in the biomedical field; development of new drugs and ...
Background: This systematic review aimed to extract recommendations from expert opinion articles on how to give a medical research presentation on a scientific conference and to determine whether the experts agree on what makes an effective or poor presentation. Methods: Presentation-related terms were searched within article titles listed in PubMed, restricting the search to English-language ...