Music & How It Impacts Your Brain, Emotions
Music is a common phenomenon that crosses all borders of nationality, race, and culture. A tool for arousing emotions and feelings, music is far more powerful than language. An increased interest in how the brain processes musical emotion can be attributed to the way in which it is described as a “language of emotion” across cultures. Be it within films, live orchestras, concerts or a simple home stereo, music can be so evocative and overwhelming that it can only be described as standing halfway between thought and phenomenon.
But why exactly does this experience of music distinctly transcend other sensory experiences? How is it able to evoke emotion in a way that is incomparable to any other sense?
Music can be thought of as a type of perceptual illusion, much the same way in which a collage is perceived. The brain imposes structure and order on a sequence of sounds that, in effect, creates an entirely new system of meaning. The appreciation of music is tied to the ability to process its underlying structure — the ability to predict what will occur next in the song. But this structure has to involve some level of the unexpected, or it becomes emotionally devoid.
Skilled composers manipulate the emotion within a song by knowing what their audience’s expectations are, and controlling when those expectations will (and will not) be met. This successful manipulation is what elicits the chills that are part of any moving song.
Music, though it appears to be similar to features of language, is more rooted in the primitive brain structures that are involved in motivation, reward and emotion. Whether it is the first familiar notes of The Beatles’ “Yellow Submarine,” or the beats preceding AC/DC’s “Back in Black,” the brain synchronizes neural oscillators with the pulse of the music (through cerebellum activation), and starts to predict when the next strong beat will occur. The response to ‘groove’ is mainly unconscious; it is processed first through the cerebellum and amygdala rather than the frontal lobes.
Music involves subtle violations of timing and, because we know through experience that music is not threatening, these violations are ultimately identified by the frontal lobes as a source of pleasure. The expectation builds anticipation, which, when met, results in the reward reaction.
More than any other stimulus, music has the ability to conjure up images and feelings that need not necessarily be directly reflected in memory. The overall phenomenon still retains a certain level of mystery; the reasons behind the ‘thrill’ of listening to music is strongly tied in with various theories based on synesthesia.
When we are born, our brain has not yet differentiated itself into different components for different senses – this differentiation occurs much later in life. So as babies, it is theorized that we view the world as a large, pulsing combination of colors and sounds and feelings, all melded into one experience – ultimate synesthesia. As our brains develop, certain areas become specialized in vision, speech, hearing, and so forth.
Professor Daniel Levitin, a neuroscientist and composer, unpacks the mystery of the emotion in music by explaining how the brain’s emotional, language and memory centers are connected during the processing of music – providing what is essentially a synesthetic experience. The extent of this connection is seemingly variable among individuals, which is how certain musicians have the ability to create pieces of music which are brimming with emotional quality, and others simply cannot. Be it classics from the Beatles and Stevie Wonder or fiery riffs from Metallica and Led Zeppelin, the preference for a certain type of music has an effect on its very experience. It could be this heightened level of experience in certain people and musicians that allows them to imagine and create music that others simply cannot, painting their very own sonic image.
Last medically reviewed on May 17, 2016

Read this next
Sleep deprivation, stress, or underlying health conditions can lead to an inability to focus. Self-help techniques can help improve your concentration.
Positive thinking is an essential practice to improve your overall health and well-being. Discover how to incorporate positive thinking into your…
Dreaming about babies can hold different meanings for everyone. Although theories vary, biological and psychological factors may influence your dreams.
If you're seeking to boost your concentration, practicing mindfulness, chewing gum, and brain games are just a few techniques to try. Learn how they…
Creating a schedule and managing stress are ways to make your days go by faster. Changing your perception of time can also improve your overall…
Experiencing unwanted and difficult memories can be challenging. But learning how to replace negative memories with positive ones may help you cope.
Engaging in brain exercises, like sudoku puzzles and learning new languages, enhances cognitive abilities and improves overall well-being.
There are many reasons why spider dreams may occur, like unresolved feelings or chronic stress. Learning how to interpret your dream may help you cope.
Tornado dreams are manifestations of the subconscious mind that may indicate various interpretations, such as personal fears or major life changes.
Work burnout occurs due to chronic stress and other factors, such as long work hours or toxic workplace culture. But help is available for you to cope.
Greater Good Science Center • Magazine • In Action • In Education
How Many Emotions Can Music Make You Feel?
The “Star-Spangled Banner” stirs pride. Ed Sheeran’s “The Shape of You” sparks joy. And “ooh là là!” best sums up the seductive power of George Michael’s “Careless Whispers.”
UC Berkeley researchers have surveyed more than 2,500 people in the United States and China about their emotional responses to these and thousands of other songs from genres including rock, folk, jazz, classical, marching band, experimental, and heavy metal.
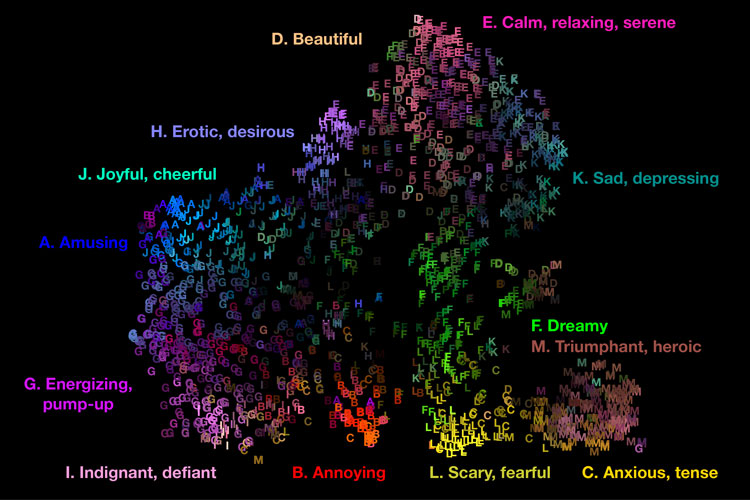
The upshot? The subjective experience of music across cultures can be mapped within at least 13 overarching feelings: amusement, joy, eroticism, beauty, relaxation, sadness, dreaminess, triumph, anxiety, scariness, annoyance, defiance, and feeling pumped up.

“Imagine organizing a massively eclectic music library by emotion and capturing the combination of feelings associated with each track. That’s essentially what our study has done,” said study lead author Alan Cowen, a UC Berkeley doctoral student in neuroscience.
The findings were published recently in the journal Proceedings of the National Academy of Sciences .
“We have rigorously documented the largest array of emotions that are universally felt through the language of music,” said study senior author Dacher Keltner, a UC Berkeley professor of psychology and Greater Good Science Center founding director.
Cowen and fellow researchers have translated the data into an interactive audio map where visitors can move their cursors to listen to any of thousands of music snippets to find out, among other things, if their emotional reactions match how people from different cultures respond to the music.
Potential applications for these research findings range from informing psychological and psychiatric therapies designed to evoke certain feelings to helping music streaming services like Spotify adjust their algorithms to satisfy their customers’ audio cravings or set the mood.
While both U.S. and Chinese study participants identified similar emotions—such as feeling fear when hearing the Jaws movie score—they differed on whether those emotions made them feel good or bad.
“People from different cultures can agree that a song is angry, but can differ on whether that feeling is positive or negative,” said Cowen, noting that positive and negative values, known in psychology parlance as “valence,” are more culture-specific.
Across cultures, study participants mostly agreed on general emotional characterizations of musical sounds, such as anger, joy, and annoyance. But their opinions varied on the level of “arousal,” which refers in the study to the degree of calmness or stimulation evoked by a piece of music.
How they conducted the study
For the study, more than 2,500 people in the United States and China were recruited online. First, these volunteers scanned thousands of videos on YouTube for music evoking a variety of emotions. From those, the researchers built a collection of audio clips to use in their experiments.
Next, nearly 2,000 study participants in the United States and China each rated some 40 music samples based on 28 different categories of emotion, as well as on a scale of positivity and negativity, and for levels of arousal.
Using statistical analyses, the researchers arrived at 13 overall categories of experience that were preserved across cultures and found to correspond to specific feelings, such as “depressing” or “dreamy.”
To ensure the accuracy of these findings in a second experiment, nearly 1,000 people from the United States and China rated over 300 additional Western and traditional Chinese music samples that were specifically intended to evoke variations in valence and arousal. Their responses validated the 13 categories.
Vivaldi’s “Four Seasons” made people feel energized. The Clash’s “Rock the Casbah” pumped them up. Al Green’s “Let’s Stay Together” evoked sensuality, and Israel (Iz) Kamakawiwoʻole’s “Somewhere over the Rainbow” elicited joy.
Meanwhile, heavy metal was widely viewed as defiant and, just as its composer intended, the shower scene score from the movie Psycho triggered fear.
Researchers acknowledge that some of these associations may be based on the context in which the study participants had previously heard a certain piece of music, such as in a movie or YouTube video. But this is less likely the case with traditional Chinese music, with which the findings were validated.
Cowen and Keltner previously conducted a study in which they identified 27 different human emotions , in response to visually evocative YouTube video clips. For Cowen, who comes from a family of musicians, studying the emotional effects of music seemed like the next logical step.
“Music is a universal language, but we don’t always pay enough attention to what it’s saying and how it’s being understood,” Cowen said. “We wanted to take an important first step toward solving the mystery of how music can evoke so many nuanced emotions.”
This article was originally published on Berkeley News . Read the original article .
About the Author

Yasmin Anwar
Yasmin Anwar is a Media Relations Representative at UC Berkeley.
You May Also Enjoy

How Music Bonds Us Together

Five Ways Music Can Make You Healthier

Why Do Some People Love Sad Music?

Why We Love Music

Five Ways Music Can Make You a Better Person

Four Ways Music Strengthens Social Bonds
- Search Menu
- Sign in through your institution
- Advance articles
- Author Guidelines
- Submission Site
- Open Access Options
- Preparing your manuscript
- COPE guidelines for peer review
- Fair Editing and Peer Review
- Promoting your article
- About Music Therapy Perspectives
- About the American Music Therapy Association
- Editorial Board
- Advertising and Corporate Services
- Self-Archiving Policy
- Dispatch Dates
- Journals on Oxford Academic
- Books on Oxford Academic

- < Previous
Understanding the Influence of Music on Emotions: A Historical Review
- Article contents
- Figures & tables
- Supplementary Data
Kimberly Sena Moore, Understanding the Influence of Music on Emotions: A Historical Review, Music Therapy Perspectives , Volume 35, Issue 2, October 2017, Pages 131–143, https://doi.org/10.1093/mtp/miw026
- Permissions Icon Permissions
Music has long been thought to influence human emotions. There is significant interest among researchers and the public in understanding music-induced emotions; in fact, a common motive for engaging with music is its emotion-inducing capabilities ( Juslin & Sloboda, 2010). Traditionally, the influence of music on emotions has been described as dichotomous. The Greeks viewed it as either mimesis , a representation of an external reality, or catharsis , a purification of the soul through an emotional experience ( Cook & Dibben, 2010). This type of dichotomous viewpoint has persisted under various labels, such as formalist versus absolutist, and referential versus expressionist ( Meyer, 1956). However, these perspectives all emerged from musicology. Outside musicology, the scientific study of emotions was intermittent and, until recently, references to music’s effect on emotions were rare ( Sloboda & Juslin, 2010). Since the 1990s, there has been increased interest in studying music-induced emotions, particularly in psychology ( Juslin & Sloboda, 2010). This interest extends to the music therapy profession as well. For example, a professional music therapist in the United States is required to be able to develop and implement music therapy experiences designed to focus on emotion-related treatment goals, such as the ability to empathize, and the client’s overall affect, mood, and emotions ( Certification Board for Music Therapists [CBMT], 2015), and must apply knowledge of music-based emotional responses ( American Music Therapy Association [AMTA], 2013). Given the increased interest in psychology and the clinical implications for the music therapist, it seems timely to analyze and reflect on how the understanding of music-induced emotions has evolved in order to support current and future research and clinical practice. As current understanding is built upon prior knowledge, a historical review can serve to examine previous directions and help inform future study ( Hanson-Abromeit & Davis, 2007). Thus, the purpose of this inquiry was to provide a historical overview of prominent theories of music and emotion and connect them to current understanding. More specifically, the objectives were:
Email alerts
Citing articles via.
- Recommend to your Library
Affiliations
- Online ISSN 2053-7387
- Copyright © 2024 American Music Therapy Association
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
ORIGINAL RESEARCH article
Emotional responses to music: shifts in frontal brain asymmetry mark periods of musical change.

- 1 School of Psychological Sciences, Monash University, Melbourne, VIC, Australia
- 2 Institute for Systematic Musicology, University of Hamburg, Hamburg, Germany
- 3 Monash Biomedical Imaging, Monash University, University of Newcastle, Newcastle, NSW, Australia
- 4 Centre for Positive Psychology, Graduate School of Education, University of Melbourne, Melbourne, VIC, Australia
Recent studies have demonstrated increased activity in brain regions associated with emotion and reward when listening to pleasurable music. Unexpected change in musical features intensity and tempo – and thereby enhanced tension and anticipation – is proposed to be one of the primary mechanisms by which music induces a strong emotional response in listeners. Whether such musical features coincide with central measures of emotional response has not, however, been extensively examined. In this study, subjective and physiological measures of experienced emotion were obtained continuously from 18 participants (12 females, 6 males; 18–38 years) who listened to four stimuli—pleasant music, unpleasant music (dissonant manipulations of their own music), neutral music, and no music, in a counter-balanced order. Each stimulus was presented twice: electroencephalograph (EEG) data were collected during the first, while participants continuously subjectively rated the stimuli during the second presentation. Frontal asymmetry (FA) indices from frontal and temporal sites were calculated, and peak periods of bias toward the left (indicating a shift toward positive affect) were identified across the sample. The music pieces were also examined to define the temporal onset of key musical features. Subjective reports of emotional experience averaged across the condition confirmed participants rated their music selection as very positive, the scrambled music as negative, and the neutral music and silence as neither positive nor negative. Significant effects in FA were observed in the frontal electrode pair FC3–FC4, and the greatest increase in left bias from baseline was observed in response to pleasurable music. These results are consistent with findings from previous research. Peak FA responses at this site were also found to co-occur with key musical events relating to change, for instance, the introduction of a new motif, or an instrument change, or a change in low level acoustic factors such as pitch, dynamics or texture. These findings provide empirical support for the proposal that change in basic musical features is a fundamental trigger of emotional responses in listeners.
Introduction
One of the most intriguing debates in music psychology research is whether the emotions people report when listening to music are ‘real.’ Various authorities have argued that music is one of the most powerful means of inducing emotions, from Tolstoy’s mantra that “music is the shorthand of emotion,” to the deeply researched and influential reference texts of Leonard Meyer (“Emotion and meaning in music”; Meyer, 1956 ) and Juslin and Sloboda (“The Handbook of music and emotion”; Juslin and Sloboda, 2010 ). Emotions evolved as a response to events in the environment which are potentially significant for the organism’s survival. Key features of these ‘utilitarian’ emotions include goal relevance, action readiness and multicomponentiality ( Frijda and Scherer, 2009 ). Emotions are therefore triggered by events that are appraised as relevant to one’s survival, and help prepare us to respond, for instance via fight or flight. In addition to the cognitive appraisal, emotions are also widely acknowledged to be multidimensional, yielding changes in subjective feeling, physiological arousal, and behavioral response ( Scherer, 2009 ). The absence of clear goal implications of music listening, or any need to become ‘action ready,’ however, challenges the claim that music-induced emotions are real ( Kivy, 1990 ; Konecni, 2013 ).
A growing body of ‘emotivist’ music psychology research has nonetheless demonstrated that music does elicit a response in multiple components, as observed with non-aesthetic (or ‘utilitarian’) emotions. The generation of an emotion in subcortical regions of the brain (such as the amygdala) lead to hypothalamic and autonomic nervous system activation and release of arousal hormones, such as noradrenaline and cortisol. Sympathetic nervous system changes associated with physiological arousal, such as increased heart rate and reduced skin conductance, are most commonly measured as peripheral indices of emotion. A large body of work now illustrates, under a range of conditions and with a variety of music genres, that emotionally exciting or powerful music impacts on these autonomic measures of emotion (see Bartlett, 1996 ; Panksepp and Bernatzky, 2002 ; Hodges, 2010 ; Rickard, 2012 for reviews). For example, Krumhansl (1997) recorded physiological (heart rate, blood pressure, transit time and amplitude, respiration, skin conductance, and skin temperature) and subjective measures of emotion in real time while participants listened to music. The observed changes in these measures differed according to the emotion category of the music, and was similar (although not identical) to that observed for non-musical stimuli. Rickard (2004) also observed coherent subjective and physiological (chills and skin conductance) responses to music selected by participants as emotionally powerful, which was interpreted as support for the emotivist perspective on music-induced emotions.
It appears then that the evidence supporting music evoked emotions being ‘real’ is substantive, despite no obvious goal implications, or need for action, of this primarily aesthetic stimulus. Scherer and Coutinho (2013) have argued that music may induce a particular ‘kind’ of emotion – aesthetic emotions – that are triggered by novelty and complexity, rather than direct relevance to one’s survival. Novelty and complexity are nonetheless features of goal relevant stimuli, even though in the case of music, there is no significance to the listener’s survival. In the same way that secondary reinforcers appropriate the physiological systems of primary reinforcers via association, it is possible then that music may also hijack the emotion system by sharing some key features of goal relevant stimuli.
Multiple mechanisms have been proposed to explain how music is capable of inducing emotions (e.g., Juslin et al., 2010 ; Scherer and Coutinho, 2013 ). Common to most theories is an almost primal response elicited by psychoacoustic features of music (but also shared by other auditory stimuli). Juslin et al. (2010) describe how the ‘brain stem reflex’ (from their ‘BRECVEMA’ theory) is activated by changes in basic acoustic events – such as sudden loudness or fast rhythms – by tapping into an evolutionarily ancient survival system. This is because these acoustic events are associated with events that do in fact signal relevance for survival for real events (such as a nearby loud noise, or a rapidly approaching predator). Any unexpected change in acoustic feature, whether it be in pitch, timbre, loudness, or tempo, in music could therefore fundamentally be worthy of special attention, and therefore trigger an arousal response ( Gabrielsson and Lindstrom, 2010 ; Juslin et al., 2010 ). Huron (2006) has elaborated on how music exploits this response by using extended anticipation and violation of expectations to intensify an emotional response. Higher level music events – such as motifs, or instrumental changes – may therefore also induce emotions via expectancy. In seminal work in this field, Sloboda (1991) asked participants to identify music passages which evoked strong, physical emotional responses in them, such as tears or chills. The most frequent musical events coded within these passages were new or unexpected harmonies, or appoggiaturas (which delay an expected principal note), supporting the proposal that unexpected musical events, or substantial changes in music features, were associated with physiological responses. Interestingly, a survey by Scherer et al. (2002) rated musical structure and acoustic features as more important in determining emotional reactions than the listener’s mood, affective involvement, personality or contextual factors. Importantly, because music events can elicit emotions via both expectation of an upcoming event and experience of that event, physiological markers of peak emotional responses may occur prior to, during or after a music event.
This proposal has received some empirical support via research demonstrating physiological peak responses to psychoacoustic ‘events’ in music (see Table 1 ). On the whole, changes in physiological arousal – primarily, chills, heart rate or skin conductance changes – coincided with sudden changes in acoustic features (such as changes in volume or tempo), or novel musical events (such as entry of new voices, or harmonic changes).
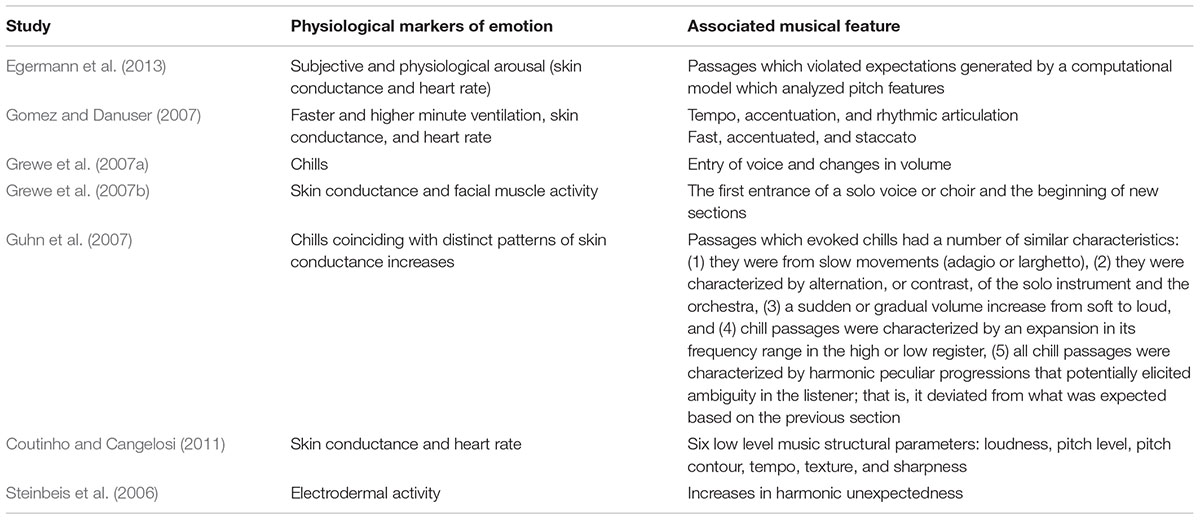
TABLE 1. Music features identified in the literature to be associated with various physiological markers of emotion.
Supporting evidence for the similarity between music-evoked emotions and ‘real’ emotions has also emerged from research using central measures of emotional response. Importantly, brain regions associated with emotion and reward have been shown to also respond to emotionally powerful music. For instance, Blood and Zatorre (2001) found that pleasant music activated the dorsal amygdala (which connects to the ‘positive emotion’ network comprising the ventral striatum and orbitofrontal cortex), while reducing activity in central regions of the amygdala (which appear to be associated with unpleasant or aversive stimuli). Listening to pleasant music was also found to release dopamine in the striatum ( Salimpoor et al., 2011 , 2013 ). Further, the release was higher in the dorsal striatum during the anticipation of the peak emotional period of the music, but higher in the ventral striatum during the actual peak experience of the music. This is entirely consistent with the differentiated pattern of dopamine release during craving and consummation of other rewarding stimuli, e.g., amphetamines. Only one group to date has, however, attempted to identify musical features associated with central measures of emotional response. Koelsch et al. (2008a) performed a functional MRI study with musicians and non-musicians. While musicians tended to perceive syntactically irregular music events (single irregular chords) as slightly more pleasant than non-musicians, these generally perceived unpleasant events induced increased blood oxygen levels in the emotion-related brain region, the amygdala. Unexpected chords were also found to elicit specific event related potentials (ERAN and N5) as well as changes in skin conductance ( Koelsch et al., 2008b ). Specific music events associated with pleasurable emotions have not yet been examined using central measures of emotion.
Davidson and Irwin (1999) , Davidson (2000 , 2004 ), and Davidson et al. (2000) , have demonstrated that a left bias in frontal cortical activity is associated with positive affect. Broadly, a left bias frontal asymmetry (FA) in the alpha band (8–13 Hz) has been associated with a positive affective style, higher levels of wellbeing and effective emotion regulation ( Tomarken et al., 1992 ; Jackson et al., 2000 ). Interventions have been demonstrated to shift frontal electroencephalograph (EEG) activity to the left. An 8-week meditation training program significantly increased left sided FA when compared to wait list controls ( Davidson et al., 2003 ). Blood et al. (1999) observed that left frontal brain areas were more likely to be activated by pleasant music than by unpleasant music. The amygdala appears to demonstrate valence-specific lateralization with pleasant music increasing responses in the left amygdala and unpleasant music increasing responses in the right amygdala ( Brattico, 2015 ; Bogert et al., 2016 ). Positively valenced music has also been found to elicit greater frontal EEG activity in the left hemisphere, while negatively valenced music elicits greater frontal activity in the right hemisphere ( Schmidt and Trainor, 2001 ; Altenmüller et al., 2002 ; Flores-Gutierrez et al., 2007 ). The pattern of data in these studies suggests that this frontal lateralization is mediated by the emotions induced by the music, rather than just the emotional valence they perceive in the music. Hausmann et al. (2013) provided support for this conclusion via mood induction through a musical procedure using happy or sad music, which reduced the right lateralization bias typically observed for emotional faces and visual tasks, and increased the left lateralization bias typically observed for language tasks. A right FA pattern associated with depression was found to be shifted by a music intervention (listening to 15 min of ‘uplifting’ popular music previously selected by another group of adolescents) in a group of adolescents ( Jones and Field, 1999 ). This measure therefore provides a useful objective marker of emotional response to further identify whether specific music events are associated with physiological measures of emotion.
The aim in this study was to examine whether: (1) music perceived as ‘emotionally powerful’ and pleasant by listeners also elicited a response in a central marker of emotional response (frontal alpha asymmetry), as found in previous research; and (2) peaks in frontal alpha asymmetry were associated with changes in key musical or psychoacoustic events associated with emotion. To optimize the likelihood that emotions were induced (that is, felt rather than just perceived), participants listened to their own selections of highly pleasurable music. Two validation hypotheses were proposed to confirm the methodology was consistent with previous research. It was hypothesized that: (1) emotionally powerful and pleasant music selected by participants would be rated as more positive than silence, neutral music or a dissonant (unpleasant) version of their music; and (2) emotionally powerful pleasant music would elicit greater shifts in frontal alpha asymmetry than control auditory stimuli or silence. The primary novel hypothesis was that peak alpha periods would coincide with changes in basic psychoacoustic features, reflecting unexpected or anticipatory musical events. Since music-induced emotions can occur both before and after key music events, FA peaks were considered associated with music events if the music event occurred within 5 s before to 5 s after the FA event. Music background and affective style were also taken into account as potential confounds.
Materials and Methods
Participants.
The sample for this study consisted of 18 participants (6 males, 12 females) recruited from tertiary institutions located in Melbourne, Australia. Participants’ ages ranged between 18 and 38 years ( M = 22.22, SD = 5.00). Participants were excluded if they were younger than 17 years of age, had an uncorrected hearing loss, were taking medication that may impact on mood or concentration, were left-handed, or had a history of severe head injuries or seizure-related disorder. Despite clearly stated exclusion criteria, two left handed participants attended the lab, although as the pattern of their hemispheric activity did not appear to differ to right-handed participants, their data were retained. Informed consent was obtained through an online questionnaire that participants completed prior to the laboratory session.
Online Survey
The online survey consisted of questions pertaining to demographic information (gender, age, a left-handedness question, education, employment status and income), music background (MUSE questionnaire; Chin and Rickard, 2012 ) and affective style (PANAS; Watson and Tellegen, 1988 ). The survey also provided an anonymous code to allow matching with laboratory data, instructions for attending the laboratory and music choices, and explanatory information about the study and a consent form.
Peak Frontal Asymmetry in Alpha EEG Frequency Band
The physiological index of emotion was measured using electroencephalography (EEG). EEG data were recorded using a 64-electrode silver-silver chloride (Ag-AgCl) EEG elastic Quik-cap (Compumedics) in accordance with the international 10–20 system. Data are, however, analyzed and reported from midfrontal sites (F3/F4 and FC3/FC4) only, as hemispheric asymmetry associated with positive and negative affect has been observed primarily in frontal cortex ( Davidson et al., 1990 ; Tomarken et al., 1992 ; Dennis and Solomon, 2010 ). Further spatial exploration of data for structural mapping purposes was beyond of the scope of this paper. In addition, analyses were performed for the P3–P4 sites as a negative control ( Schmidt and Trainor, 2001 ; Dennis and Solomon, 2010 ). All channels were referenced to the mastoid electrodes (M1–M2). The ground electrode was situated between FPZ and FZ and impedances were kept below 10 kOhms. Data were collected and analyzed offline using the Compumedics Neuroscan 4.5 software.
Subjective Emotional Response
The subjective feeling component of emotion was measured using ‘EmuJoy’ software ( Nagel et al., 2007 ). This software allows participants to indicate how they feel in real time as they listen to the stimulus by moving the cursor along the screen. The Emujoy program utilizes the circumplex model of affect ( Russell, 1980 ) where emotion is measured in a two dimensional affective space, with axes of arousal and valence. Previous studies have shown that valence and arousal account for a large portion of the variation observed in the emotional labeling of musical (e.g., Thayer, 1986 ), as well as linguistic ( Russell, 1980 ) and picture-oriented ( Bradley and Lang, 1994 ) experimental stimuli. The sampling rate was 20 Hz (one sample every 50 ms), which is consistent with recommendations for continuous monitoring of subjective ratings of emotion ( Schubert, 2010 ). Consistent with Nagel et al. (2007) , the visual scale was quantified as an interval scale from -10 to +10.
Music Stimuli
Four music stimuli—practice, pleasant, unpleasant, and neutral—were presented throughout the experiment. Each stimulus lasted between 3 and 5 min in duration. The practice stimulus was presented to familiarize participants with the Emujoy program and to acclimatize participants to the sound and the onset and offset of the music stimulus (fading in at the start and fading out at the end). The song was selected on the basis that it was likely to be familiar to participants, positive in affective valence, and containing segments of both arousing and calming music—The Lion King musical theme song, “ The circle of life. ”
The pleasant music stimulus was participant-selected. This option was preferred over experimenter-selected music as participant-selected music was considered more likely to induce robust emotions ( Thaut and Davis, 1993 ; Panksepp, 1995 ; Blood and Zatorre, 2001 ; Rickard, 2004 ). Participants were instructed to select a music piece that made them, “experience positive emotions (happy, joyful, excited, etc.) – like those songs you absolutely love or make you get goose bumps.” This song selection also had to be one that would be considered a happy song by the general public. That is, it could not be sad music that participants enjoyed. While previous research has used both positively and negatively valenced music to elicit strong experiences with music, in the current study, we limited the music choices to those that expressed positive emotions. This decision was made to reduce variability in EEG responses arising from perception of negative emotions and experience of positive emotions, as EEG can be sensitive to differences in both perception and experience of emotional valence. The music also had to be alyrical 1 —music with unintelligible words, for example in another language or skat singing, were permitted—as language processing might conceivably elicit different patterns of hemisphere activation solely as a function of the processing of vocabulary included in the song. [It should be noted that there are numerous mechanisms by which a piece of music might induce an emotion (see Juslin and Vastfjall, 2008 ), including associations with autobiographical events, visual imagery and brain stem reflexes. Differentiating between these various causes of emotion was, however, beyond the scope of the current study.]
The unpleasant music stimulus was intended to induce negative emotions. This was a dissonant piece produced by manipulating the participant’s pleasant music stimulus and was achieved using Sony Sound Forge© 8 software. This stimulus consisted of three versions of the song played simultaneously— one shifted a tritone down, one pitch shifted a whole tone up, and one played in reverse (adapted from Koelsch et al., 2006 ). The neutral condition was an operatic track, La Traviata, chosen based upon its neutrality observed in previous research ( Mitterschiffthaler et al., 2007 ).
The presentation of music stimuli was controlled by the experimenter via the EmuJoy program. The music volume was set to a comfortable listening level, and participants listened to all stimuli via bud earphones (to avoid interference with the EEG cap).
Prior to attending the laboratory session, participants completed the anonymously coded online survey. Within 2 weeks, participants attended the EEG laboratory at the Monash Biomedical Imaging Centre. Participants were tested individually during a 3 h session. An identification code was requested in order to match questionnaire data with laboratory session data.
Participants were seated in a comfortable chair and were prepared for fitting of the EEG cap. The participant’s forehead was cleaned using medical grade alcohol swabs and exfoliated using NuPrep exfoliant gel. Participants were fitted with the EEG cap according to the standardized international 10/20 system ( Jasper, 1958 ). Blinks and vertical/horizontal movements were recorded by attaching loose electrodes from the cap above and below the left eye, as well as laterally on the outer canthi of each eye. The structure of the testing was explained to participants and was as follows (see Figure 1 ):
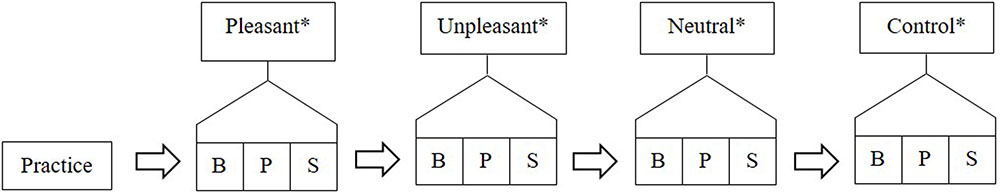
FIGURE 1. Example of testing structure with conditions ordered; pleasant, unpleasant, neutral, and control. B, baseline; P, physiological recording; and S, subjective rating. ∗ These stimuli were presented to participants in a counter balanced order.
The testing comprised four within-subjects conditions: pleasant, unpleasant, neutral, and control. Differing only in the type of auditory stimulus presented, each condition consisted of:
(a) Baseline recording (B)—no stimulus was presented during the baseline recordings. These lasted 3 min in duration and participants were asked to close their eyes and relax.
(b) Physiological recording (P)—the stimulus (depending on what condition) was played and participants were asked to have their eyes closed and to just experience the sounds.
(c) Subjective rating (S)—the stimulus was repeated, however, this time participants were asked to indicate, with eyes open, how they felt as they listened to the same music on the computer screen using the cursor and the EmuJoy software.
At every step of each condition, participants were guided by the experimenter (e.g., “I’m going to present a stimulus to you now, it may be music, something that sounds like music, or it could be nothing at all. All I would like you to do is to close your eyes and just experience the sounds”). Before the official testing began, the participant was asked to practice using the EmuJoy program in response to the practice stimulus. Participants were asked about their level of comfort and understanding with regards to using the EmuJoy software; experimentation did not begin until participants felt comfortable and understood the use of EmuJoy. Participants were reminded of the distinction between rating emotions felt vs. emotions perceived in the music; the former was encouraged throughout relevant sections of the experiment. After this, the experimental procedure began with each condition being presented to participants in a counterbalanced fashion. All procedures in this study were approved by the Monash University Human Research Ethics Committee.
EEG Data Analysis for Frontal Asymmetry
Electroencephalograph data from each participant was visually inspected for artifacts (eye movements and muscle artifacts were manually removed prior to any analyses). EEG data were also digitally filtered with a low-pass zero phase-shift filter set to 30 Hz and 96 dB/oct. All data were re-referenced to mastoid processes. The sampling rate was 1250 Hz and eye movements were controlled for with automatic artifact rejection of >50 μV in reference to VEO. Data were baseline corrected to 100 ms pre-stimulus period. EEG data were aggregated for all artifact-free periods within a condition to form a set of data for the positive music, negative music, neutral, and the control.
Chunks of 1024 ms were extracted for analyses using a Cosine window. A Fast Fourier Transform (FFT) was applied to each chunk of EEG permitting the computation of the amount of power at different frequencies. Power values from all chunks within an epoch were averaged (see Dumermuth and Molinari, 1987 ). The dependent measure that was extracted from this analysis was power density (μV 2 /Hz) in the alpha band (8–13 Hz). The data were log transformed to normalize their distribution because power values are positively skewed ( Davidson, 1988 ). Power in the alpha band is inversely related to activation (e.g., Lindsley and Wicke, 1974 ) and has been the measure most consistently obtained in studies of EEG asymmetry ( Davidson, 1988 ). Cortical asymmetry [ln(right)–ln(left)] was computed for the alpha band. This FA score provides a simple unidimensional scale representing relative activity of the right and left hemispheres for an electrode pair (e.g., F3 [left]/F4 [right]). FA scores of 0 indicate no asymmetry, while scores greater than 0 putatively are indicative of greater left frontal activity (positive affective response) and scores below 0 are indicative of greater right frontal activity (negative affective response), assuming that alpha is inversely related to activity ( Allen et al., 2004 ). Peak FA periods at the FC3/FC4 site were also identified across each participant’s pleasant music piece for purposes of music event analysis. FA (difference between left and right power densities) values were ranked from highest (most asymmetric, left biased) to lowest using spectrograms (see Figure 2 for an example). Due to considerable inter-individual variability in asymmetry ranges, descriptive ranking was used as a selection criterion instead of an absolute threshold or statistical difference criterion. The ranked FA differences were inspected and those that were clearly separated from the others (on average six peaks were clearly more asymmetric than the rest of the record) were selected for each individual as their greatest moments of FA. This process was performed by two raters (authors H-AA and NR), with 100% interrater reliability, so no further analysis was performed/considered necessary required to rank the FA peaks.
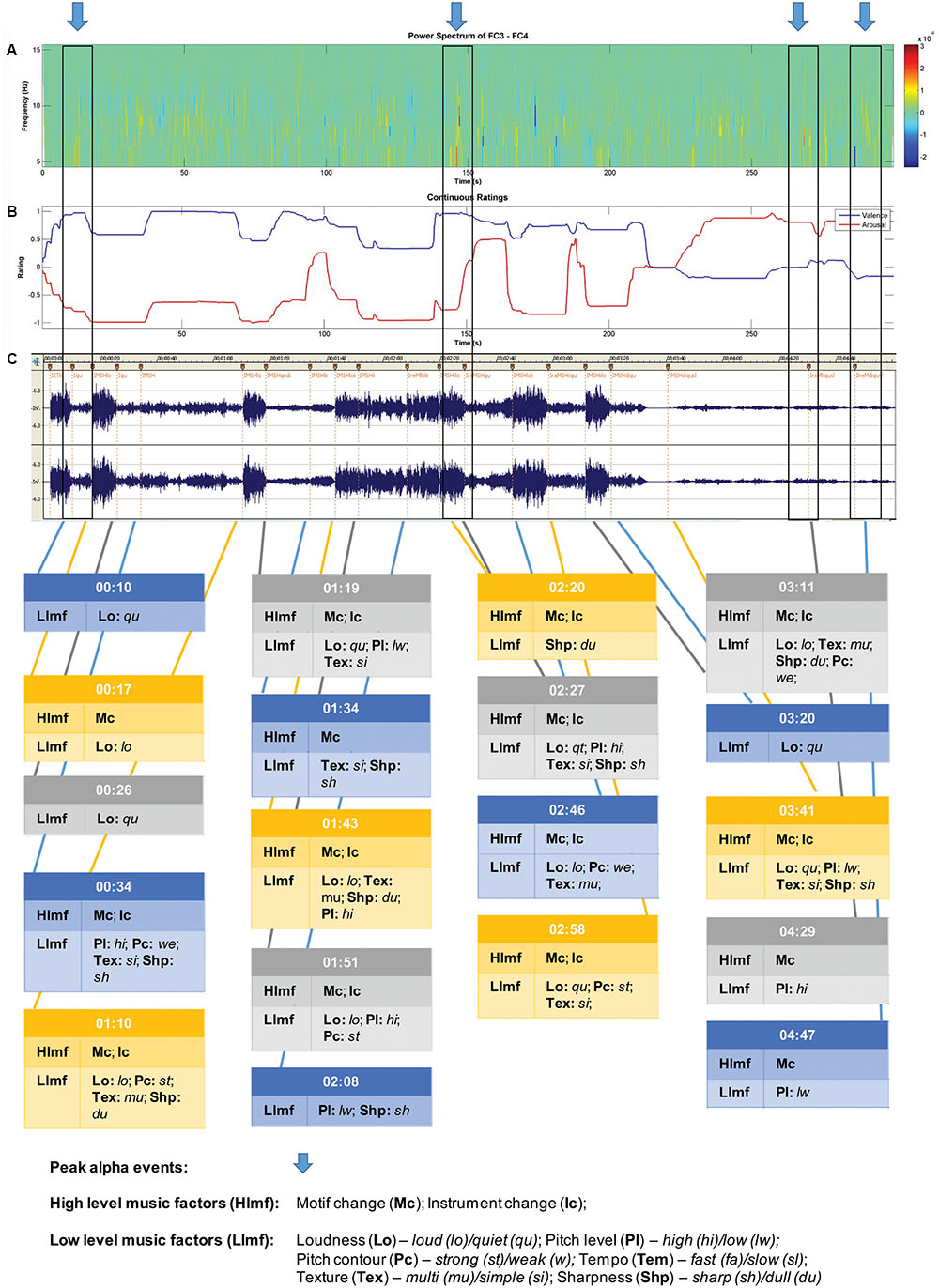
FIGURE 2. Sample data for participant 4 – music selection: The Four Seasons: Spring; Antonio Vivaldi. Recording: Karoly Botvay, Budapest Strings, Cobra Entertainment). (A) EEG alpha band spectrogram; (B) subjective valence and arousal ratings; and (C) music feature analysis.
Music Event Data Coding
A subjective method of annotating each pleasant music piece with temporal onsets and types of all notable changes in musical features was utilized in this study. Coding was performed by a music performer and producer with postgraduate qualifications in systematic musicology. A decision was made to use subjective coding as it has been successfully used previously to identify significant changes in a broad range of music features associated with emotional induction by music ( Sloboda, 1991 ). This method was framed within a hierarchical category model which contained both low-level and high-level factors of important changes. First, each participant’s music piece was described by annotating the audiogram, noting the types of music changes at respective times. Secondly, the low-level factor model utilized by Coutinho and Cangelosi (2011) was applied to assign the identified music features deductively to changes within six low-level factors: loudness, pitch level, pitch contour, tempo, texture, and sharpness. Each low-level factor change was coded as a change toward one of the two anchors of the feature. For example, if a modification was marked in terms of loudness with ‘loud,’ it described an increase in loudness of the current part compared to the part before (see Table 2 ).
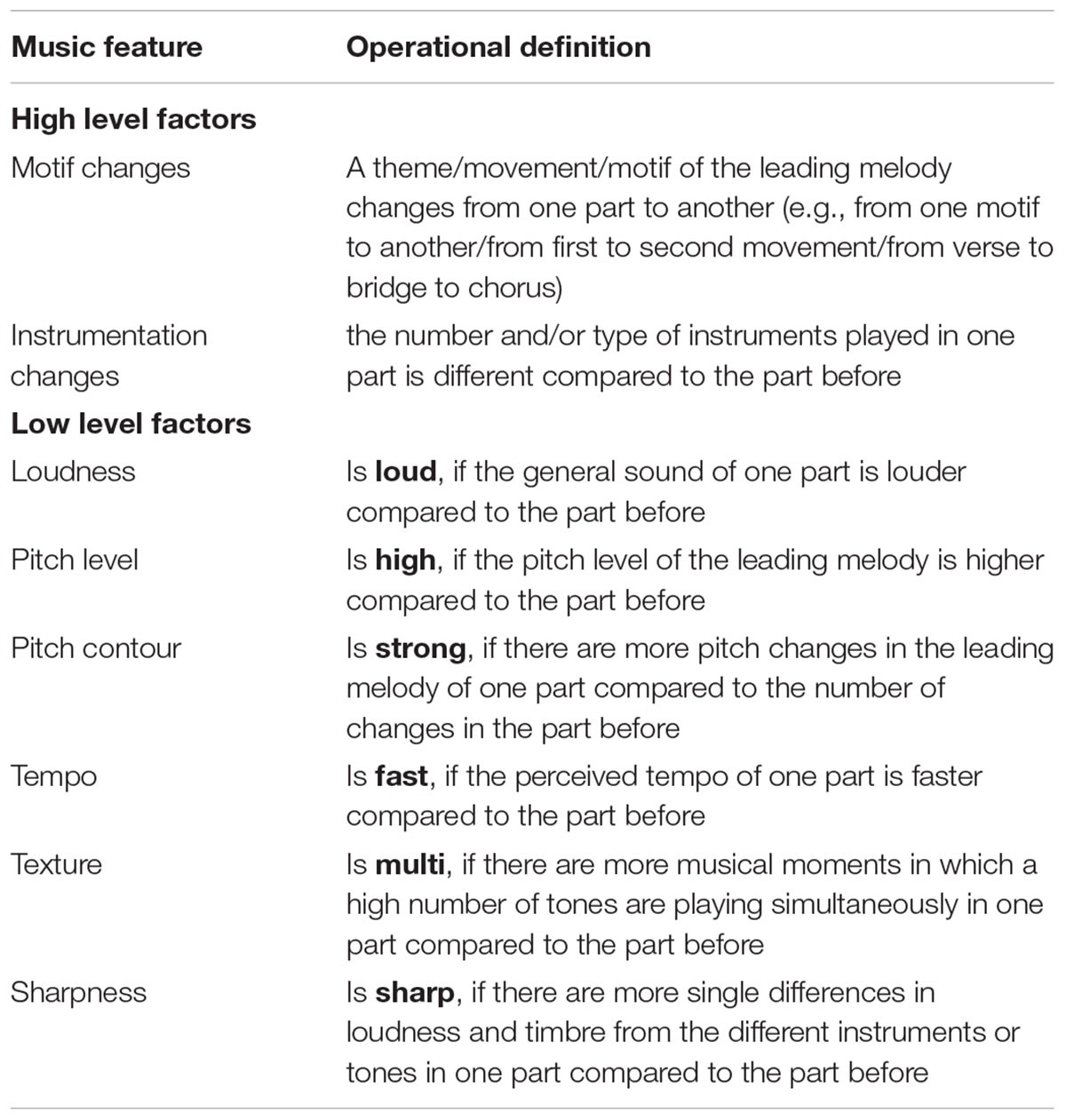
TABLE 2. Operational definitions of high and low level musical features investigated in the current study.
Due to the high variability of the analyzed musical pieces from a musicological perspective – including the genre, which ranged from classical and jazz to pop and electronica – every song had a different frequency of changes in terms of these six factors. Hence, we applied a third step of categorization which led to a more abstract layer of changes in musical features that included two higher-level factors: motif changes and instrument changes. A time point in the music is marked with ‘motif change’ if the theme, movement or motif of the leading melody change from one part to the next one. The factor ‘instrument change’ can be defined as an increase or decrease of the number of playing instruments or as a change of instruments used within the current part.
Data were scored and entered into PASW Statistics 18 for analyses. No missing data or outliers were observed in the survey data. Bivariate correlations were run between potential confounding variables – Positive affect negative affect schedule (PANAS), and the Music use questionnaire (MUSE) – and FA to determine if they were potential confounds, but no correlations were observed.
A sample of data obtained for each participant is shown in Figure 2 . For this participant, five peak alpha periods were identified (shown in blue arrows at top). Changes in subjective valence and arousal across the piece are shown in the second panel, and then the musicological analysis in the final section of the figure.
Subjective Ratings of Emotion – Averaged Emotional Responses
A one-way analysis of variance (ANOVA) was conducted to compare mean subjective ratings of emotional valence. Kolmogorov–Smirnov tests of normality indicated that distributions were normal for each condition except the subjective ratings of the control condition D (9) = 0.35, p < 0.001. Nonetheless, as ANOVAs are robust to violations of normality when group sizes are equal ( Howell, 2002 ), parametric tests were retained. No missing data or outliers were observed in the subjective rating data. Figure 3 below shows the mean ratings of each condition.
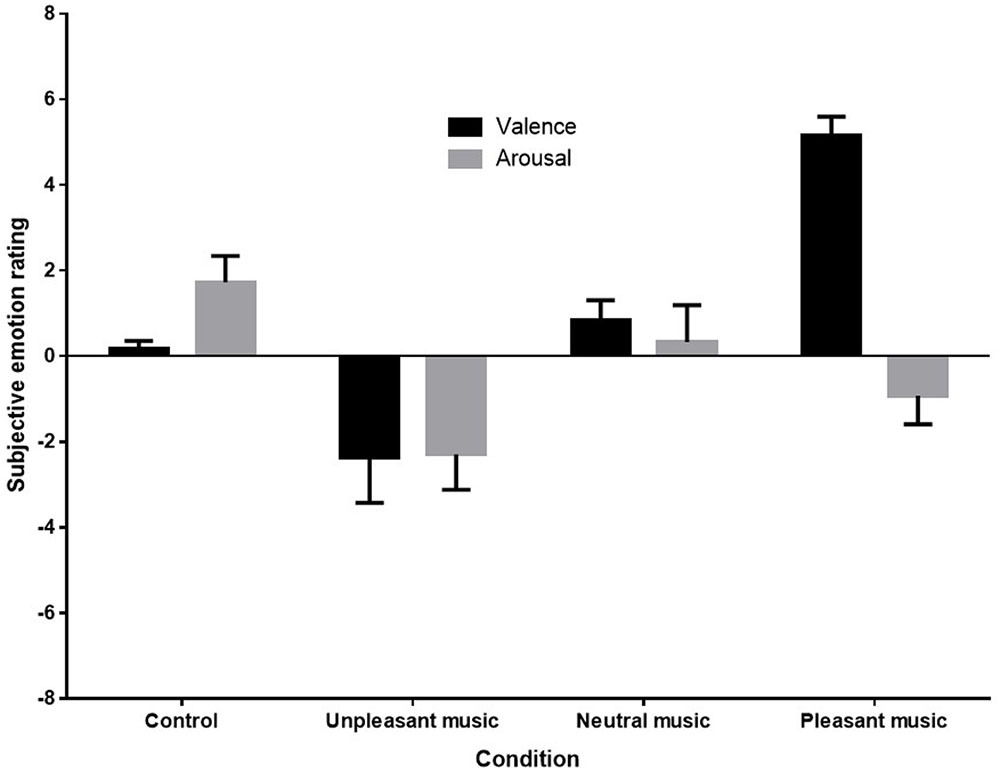
FIGURE 3. Mean subjective emotion ratings (valence and arousal) for control (silence), unpleasant (dissonant), neutral, and pleasant (self-selected) music conditions.
Figure 3 shows that both the direction and magnitude of subjective emotional valence differed across conditions, with the pleasant condition rated very positively, the unpleasant condition rated negatively, and the control and neutral conditions rated as neutral. Arousal ratings appeared to be reduced in response to unpleasant and pleasant music. (Anecdotal reports from participants indicated that in addition to being very familiar with their own music, participants recognized the unpleasant piece as a dissonant manipulation of their own music selection, and were therefore familiar with it also. Several participants noted that this made the piece even more unpleasant to listen to for them.)
Sphericity was met for the arousal ratings, but not for valence ratings, so a Greenhouse-Geisser correction was made for analyses on valence ratings. A one-way repeated measures ANOVA revealed a significant effect of stimulus condition on valence ratings, F (1.6,27.07) = 23.442, p < 0.001, η p 2 = 0.58. Post hoc contrasts revealed that the mean subjective valence rating for the unpleasant music was significantly lower than for the control F (1,17) = 5.59, p = 0.030, η p 2 = 0.25, and the mean subjective valence rating for the pleasant music was significantly higher than for the control condition, F (1,17) = 112.42, p < 0.001, η p 2 = 0.87. The one-way repeated measures ANOVA for arousal ratings also showed a significant effect for stimulus condition, F (3,51) = 5.20, p = 0.003, η p 2 = 0.23. Post hoc contrasts revealed that arousal ratings were significant reduced by both unpleasant, F (1,17) = 10.11, p = 0.005, η p 2 = 0.37, and pleasant music, F (1,17) = 6.88, p = 0.018, η p 2 = 0.29, when compared with ratings for the control.
Aim 1: Can Emotionally Pleasant Music Be Detected by a Central Marker of Emotion (FA)?
Two-way repeated measures ANOVAs were conducted on the FA scores (averaged across baseline period, and averaged across condition) for each of the two frontal electrode pairs, and the control parietal site pair. The within-subjects factor included the music condition (positive, negative, neutral, and control) and time (baseline and stimulus). Despite the robustness of ANOVA to assumptions, caution should be taken in interpreting results as both the normality and sphericity assumptions were violated across each electrode pair. Where sphericity was violated, a Greenhouse–Geisser correction was applied. Asymmetry scores above two were considered likely a result of noisy or damaged electrodes (62 points out of 864) and were omitted as missing data which were excluded pairwise. Two outliers were identified in the data and were replaced with a score ±3.29 standard deviations from the mean.
A signification time by condition interaction effect was observed at the FC3/FC4 site, F (2.09,27.17) = 3.45, p = 0.045, η p 2 = 0.210, and a significant condition main effect was observed at the F3/F4 site, F (2.58,21.59) = 3.22, p = 0.039, η p 2 = 0.168. No significant effects were observed at the P3/P4 site [time by condition effect, F (1.98,23.76) = 2.27, p = 0.126]. The significant interaction at FC3/FC4 is shown in Figure 4 .
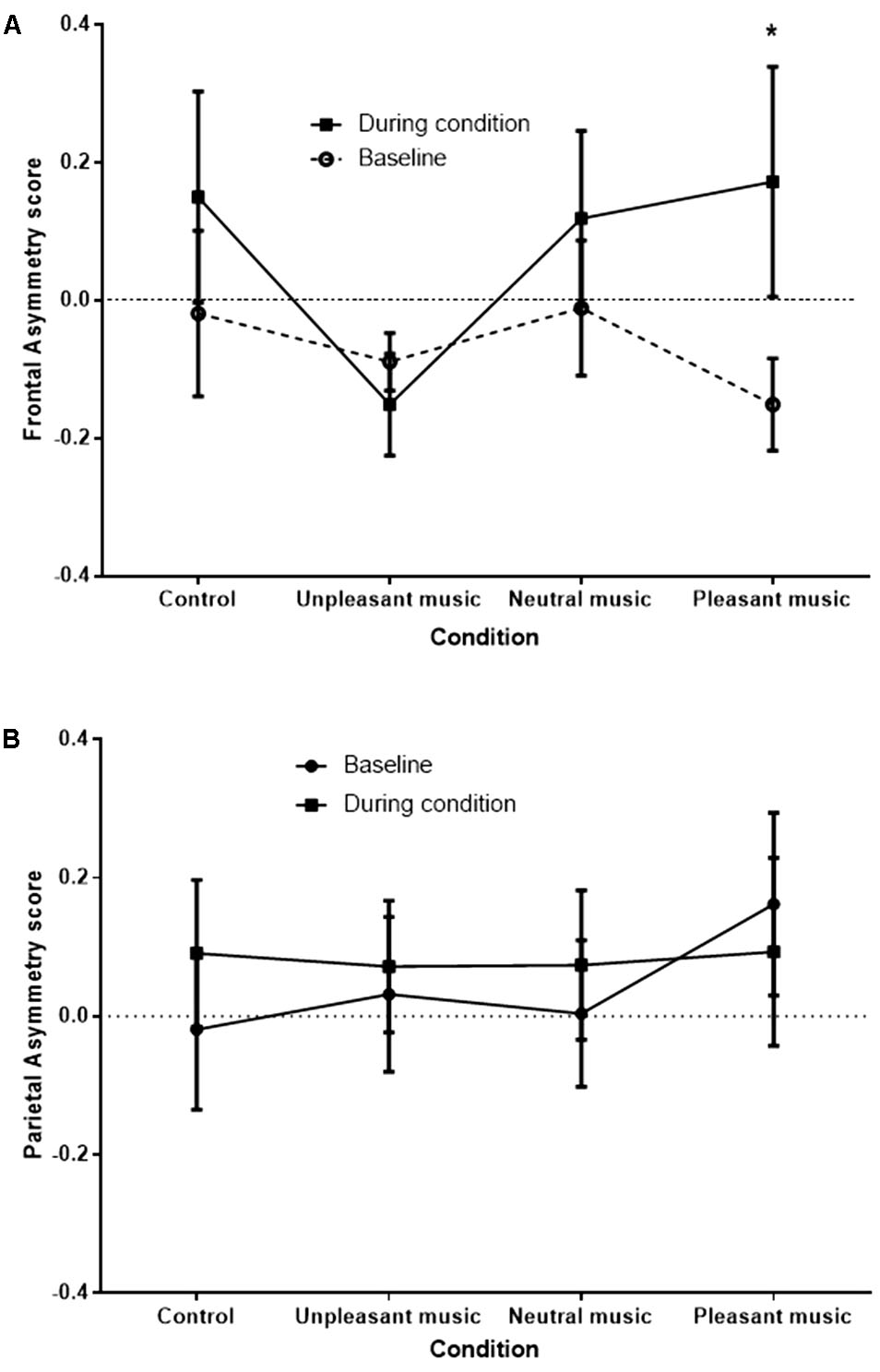
FIGURE 4. FC3/FC4 (A) and P3/P4 (B) (control) asymmetry score at baseline and during condition, for each condition. Asymmetry scores of 0 indicate no asymmetry. Scores >0 indicate left bias asymmetry (and positive affect), while scores <0 indicate right bias asymmetry (and negative affect). ∗ p < 0.05.
The greatest difference between baseline and during condition FA scores was for the pleasant music, representative of a positive shift in asymmetry from the right hemisphere to the left when comparing the baseline period to the stimulus period. Planned simple contrasts revealed that when compared with the unpleasant music condition, only the pleasant music condition showed a significant positive shift in FA score, F (1,13) = 6.27, p = 0.026. Positive shifts in FA were also apparent for control and neutral music conditions, although not significantly greater than for the unpleasant music condition [ F (1,13) = 2.60, p = 0.131, and F (1,13) = 3.28, p = 0.093], respectively.
Aim 2: Are Peak FA Periods Associated with Particular Musical Events?
Peak periods of FA were identified for each participant, and the sum varied between 2 and 9 ( M = 6.5, SD = 2.0). The music event description was then examined for presence or absence of coded musical events within a 10 s time window of (5 s before to 5 s after) the peak FA time-points. Across all participants, 106 peak alpha periods were identified, 78 of which (74%) were associated with particular music events. The type of music event coinciding with peak alpha periods is shown in Table 3 . A two-step cluster analysis was also performed to explore natural groupings of peak alpha asymmetry events that coincided with distinct combinations (2 or more) of musical features. A musical feature was to be deemed a salient characteristic of a cluster if present in at least 70% of the peak alpha events within the same cluster.
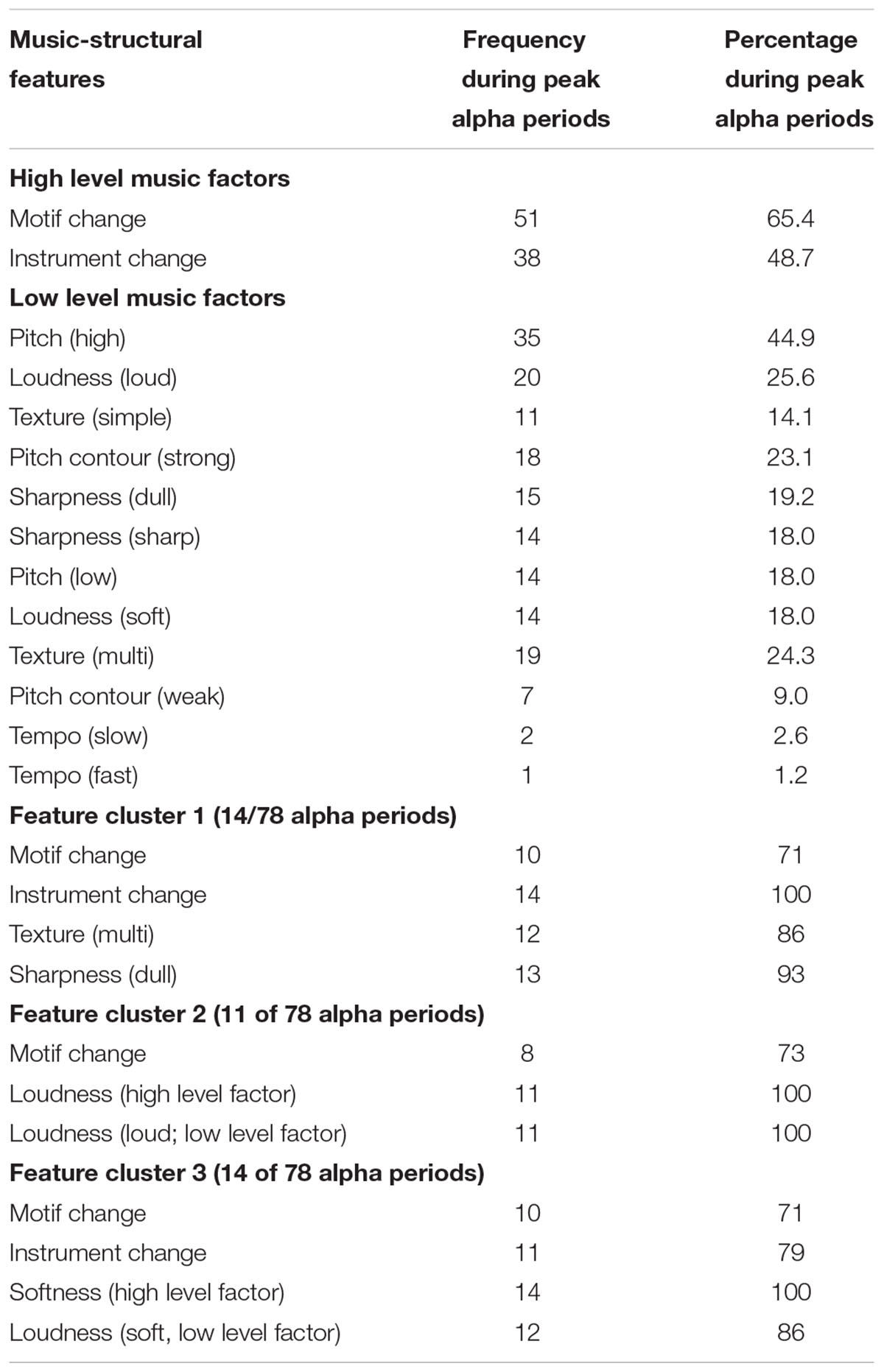
TABLE 3. Frequency and percentages of musical features associated with a physiological marker of emotion (peak alpha FA). High level, low level, and clusters of music features are distinguished.
Table 3 shows that, considered independently, the most frequent music features associated with peak alpha periods were primarily high level factors (changes in motif and instruments), with the addition of one low level factor (pitch). In exploring the data for clusters of peak alpha events associated with combinations of musical features, a four cluster solution was found to successfully group approximately half (53%) of the events into groups with identifiable patterns. This equated to 3 separate clusters characterized by distinct combinations of musical features, with the remaining half (47%) deemed unclassifiable as higher factor solutions provided no further differentiation.
In the current study, a central physiological marker (alpha FA) was used to investigate the emotional response of music selected by participants to be ‘emotionally powerful’ and pleasant. Musical features of these pieces were also examined to explore associations between key musical events and central physiological markers of emotional responding. The first aim of this study was to examine whether pleasant music elicited physiological reactions in this central marker of emotional responding. As hypothesized, pleasant musical stimuli elicited greater shifts in FA than did the control auditory stimulus, silence or an unpleasant dissonant version of each participant’s music. This finding confirmed previous research findings and demonstrated that the methodology was robust and appropriate for further investigation. The second aim was to examine associations between key musical features (affiliated with emotion), contained within participant-selected musical pieces, and peaks in FA. FA peaks were commonly associated with changes in both high and low level music features, including changes in motif, instrument, loudness and pitch, supporting the hypothesis that key events in music are marked by significant physiological changes in the listener. Further, specific combinations of individual musical features were identified that tended to predict FA peaks.
Central Physiological Measures of Responding to Musical Stimuli
Participants’ subjective valence ratings of music were consistent with expectations; control and neutral music were both rated neutrally, while unpleasant music was rated negatively and pleasant music was rated very positively. These findings are consistent with previous research indicating that music is capable of eliciting strong felt positive affective reports ( Panksepp, 1995 ; Rickard, 2004 ; Juslin et al., 2008 ; Zenter et al., 2008 ; Eerola and Vuoskoski, 2011 ). The current findings were also consistent with previous negative subjective ratings (unpleasantness) by participants listening to the dissonant manipulation of musical stimuli ( Koelsch et al., 2006 ). It is not entirely clear why arousal ratings were reduced by both the unpleasant and pleasant music. The variety of pieces selected by participants means that both relaxing and stimulating pieces were present in these conditions, although overall, the findings suggest that listening to music (regardless of whether pleasant or unpleasant) was more calming than silence for this sample. In addition, as both pieces were likely to be familiar (as participants reported that they recognized the dissonant manipulations of their own piece), familiarity could have reduced the arousal response expected for unpleasant music.
As hypothesized, FA responses from the FC3/FC4 site were consistent with subjective valence ratings, with the largest shift to the left hemisphere observed for the pleasant music condition. While not statistically significant, the small shifts to the left hemisphere during both control and neutral music conditions, and the small shift to the right hemisphere during the unpleasant music condition, indicate the trends in FA were also consistent with subjective valence reports. These findings support previous research findings on the involvement of the left frontal lobe in positive emotional experiences, and the right frontal lobe in negative emotional experiences ( Davidson et al., 1979 , 1990 ; Fox and Davidson, 1986 ; Davidson and Fox, 1989 ; Tomarken et al., 1990 ). The demonstration of these effects in the FC3/FC4 site is consistent with previous findings ( Davidson et al., 1990 ; Jackson et al., 2003 ; Travis and Arenander, 2006 ; Kline and Allen, 2008 ; Dennis and Solomon, 2010 ), although meaningful findings are also commonly obtained from data collected from the F3/F4 site (see Schmidt and Trainor, 2001 ; Thibodeau et al., 2006 ), which was not observed in the current study. The asymmetry findings also verify findings observed in response to positive and negative emotion induction by music ( Schmidt and Trainor, 2001 ; Altenmüller et al., 2002 ; Flores-Gutierrez et al., 2007 ; Hausmann et al., 2013 ). Importantly, no significant FA effect was observed in the control P3/P4 sites, which is an area not implicated in emotional responding.
Associations between Musical Features and Peak Periods of Frontal Asymmetry
Individual musical features.
Several individual musical features coincided with peak FA events. Each of these musical features occurred in over 40% of the total peak alpha asymmetry events identified throughout the sample and appear to be closely related to changes in musical structure. These included changes in motif and instruments (high level factors), as well as pitch (low level factor). Such findings are in line with previous studies measuring non-central physiological measures of affective responding. For example, high level factor musical features such as instrument change, specifically changes and alternations between orchestra and solo piece instruments have been cited to coincide with chill responses ( Grewe et al., 2007b ; Guhn et al., 2007 ). Similarly, pitch events have been observed in previous research to coincide with various physiological measures of emotional responding including skin conductance and heart rate ( Coutinho and Cangelosi, 2011 ; Egermann et al., 2013 ). In the current study, instances of high pitch were most closely associated with physiological reactions. These findings can be explained through Juslin and Sloboda’s (2010 ) description of the activation of a ‘brain stem reflex’ in response to changes in basic acoustic events. Changes in loudness and high pitch levels may trigger physiological reactions on account of being psychoacoustic features of music that are shared with more primitive auditory stimuli that signal relevance for survival to real events.
Changes in instruments and motif, however, may be less related to primitive auditory stimuli and stimulate physiological reactions differently. Motif changes have not been observed in previous studies yet appeared most frequently throughout the peak alpha asymmetry events identified in the sample. In music, motif has been described as “...the smallest structural unit possessing thematic identity” ( White, 1976 , p. 26–27) and exists as a salient and recurring characteristic musical fragment throughout a musical piece. Within the descriptive analysis of the current study, however, a motif can be understood in a much broader sense (see definitions in Table 2 ). Due to the broad musical diversity of the songs selected by participants, the term motif change emerged as most appropriate description to cover high level structural changes in all the different musical pieces (e.g., changes from one small unit to another in a classic piece and changes from a long repetitive pattern to a chorus in an electronic dance piece). Changes in such a fundamental musical feature, as well as changes in instrument, are likely to stimulate a sense of novelty and add complexity, and possibly unexpectedness (i.e., features of goal oriented stimuli), to a musical piece. This may therefore also recruit the same neural system which has evolved to yield an emotional response, which in this study, is manifest in the greater activation in the left frontal hemisphere compared to the right frontal hemisphere. Many of the other musical features identified, however, did not coincide frequently with peak FA events. While peripheral markers of emotion, such as skin conductance and heart rate changes, are likely to respond strongly to basic psychoacoustic events associated with arousal, it is likely that central markers such as FA are more sensitive to higher level musical events associated with positive affect. This may explain why motif changes were a particularly frequent event associated with FA peaks. Alternatively, some musical features may evoke emotional and physiological reactions only when present in conjunction with other musical features. It is recognized that an objective method of low level music feature identification would also be useful in future research to validate the current findings relating to low level psychoacoustic events. A limitation of the current study, however, was that the coding of both peak FA events and music events was subjective, which limits both replicability and objectivity. It is recommended future research utilize more objective coding techniques including statistical identification of peak FA events, and formal psychoacoustic analysis (such as achieved using software tools such as MIR Toolbox or PsySound). While an objective method of detecting FA events occurring within a specific time period after a music event is also appealing, the current methodology operationalized synchrony of FA and music events within a 10 s time window to include mechanisms of anticipation as well as experience of the event. Future research may be able to provide further distinction between these emotion induction mechanisms by applying different time windows to such analyses.
Feature Clusters of Musical Feature Combinations
Several clusters comprising combinations of musical features were identified in the current study. A number of musical events which on their own did not coincide with FA peaks did nonetheless appear in music event clusters that were associated with FA peaks. For example, feature cluster 1 consists of motif and instrument changes—both individually considered to coincide frequently with peak alpha asymmetry events—as well as texture (multi) and sharpness (dull). Changes in texture and sharpness, individually, were observed to occur in only 24.3 and 19.2% of the total peak alpha asymmetry events, respectively. After exploring the data for natural groupings of musical events that occurred during peak alpha asymmetry scores, however, texture and sharpness changes appeared to occur frequently in conjunction with motif changes and instrument changes. Within feature cluster 1, texture and sharpness occurred in 86 and 93% of the peak alpha asymmetry periods. This suggests that certain musical features, like texture and sharpness, may lead to stronger emotional responses in central markers of physiological functioning when presented concurrently with specific musical events as compared to instances where they are present in isolation.
An interesting related observation is the specificity with which these musical events can combine to form a cluster. While motif and instrument changes occurred often in conjunction with texture (multi) and sharpness (dull) during peak alpha asymmetry events, both also occurred distinctly in conjunction with dynamic changes in volume (high level factor) and softness (low level factor) in a separate feature cluster. While both the texture/sharpness and loudness change/softness combinations frequently occur with motif and instrument changes, they appear to do so in a mutually exclusive manner. This suggests a high level of complexity and specificity with which musical features may complement one another to stimulate physiological reactions during musical pieces.
The current findings extend previous research which has demonstrated that emotionally powerful music elicits changes in physiological, as well as subjective, measures of emotion. This study provides further empirical support for the emotivist theory of music and emotion which proposes that if emotional responses to music are ‘real,’ then they should be observable in physiological indices of emotion ( Krumhansl, 1997 ; Rickard, 2004 ). The pattern of FA observed in this study is consistent with that observed in previous research in response to positive and negative music ( Blood et al., 1999 ; Schmidt and Trainor, 2001 ), and non-musical stimuli ( Fox, 1991 ; Davidson, 1993 , 2000 ). However, the current study utilized music which expressed and induced positive emotions only, whereas previous research has also included powerful emotions induced by music expressing negative emotions. It would be of interest to replicate the current study with a broader range of powerful music to determine whether FA is indeed a marker of emotional experience, or a mixture of emotion perception and experience.
The findings also extend those obtained in studies which have examined musical features associated with strong emotional responses. Consistent with the broad consensus in this research, strong emotional responses often coincide with music events that signal change, novelty or violated expectations ( Sloboda, 1991 ; Huron, 2006 ; Steinbeis et al., 2006 ; Egermann et al., 2013 ). In particular, FA peaks were found to be associated in the current sample’s music selections with motif changes, instrument changes, dynamic changes in volume, and pitch, or specific clusters of music events. Importantly, however, these conclusions are limited by the modest sample size, and consequently by the music pieces selected. Further research utilizing a different set of music pieces may identify a quite distinct pattern of music features associated with FA peaks. In sum, these findings provide empirical support for anticipation/expectation as a fundamental mechanism underlying music’s capacity to evoke strong emotional responses in listeners.
Ethics Statement
This study was carried out in accordance with the recommendations of the National Statement on Ethical Conduct in Human Research, National Health and Medical Research Council, with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Monash University Standing Committee for Ethical Research on Humans.
Author Contributions
H-AA conducted the experiments, contributed to the design and methods of the study, analysis of data and preparation of all sections of the manuscript. NR contributed to the design and methods of the study, analysis of data and preparation of all sections the manuscript, and provided oversight of this study. JH conducted the musicological analyses of the music selections, and contributed to the methods and results sections of the manuscript. BP performed the analyses of the EEG recordings and contributed to the methods and results sections of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
- ^ One participant only chose music with lyrical content; the experimenter confirmed with this participant that the language (Italian) was unknown to them.
Allen, J., Coan, J., and Nazarian, M. (2004). Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biol. Psychol. 67, 183–218. doi: 10.1016/j.biopsycho.2004.03.007
PubMed Abstract | CrossRef Full Text | Google Scholar
Altenmüller, E., Schürmann, K., Lim, V. K., and Parlitz, D. (2002). Hits to the left, flops to the right: different emotions during listening to music are reflected in cortical lateralisation patterns. Neuropsychologia 40, 2242–2256. doi: 10.1016/S0028-3932(02)00107-0
Bartlett, D. L. (1996). “Physiological reactions to music and acoustic stimuli,” in Handbook of Music Psychology , 2nd Edn, ed. D. A. Hodges (San Antonio, TX: IMR Press), 343–385.
Google Scholar
Blood, A. J., and Zatorre, R. J. (2001). Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Natl. Acad. Sci. U.S.A. 98, 11818–11823. doi: 10.1073/pnas.191355898
Blood, A. J., Zatorre, R. J., Bermudez, P., and Evans, A. C. (1999). Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nat. Neurosci. 2, 382–387. doi: 10.1038/7299
Bogert, B., Numminen-Kontti, T., Gold, B., Sams, M., Numminen, J., Burunat, I., et al. (2016). Hidden sources of joy, fear, and sadness: explicit versus implicit neural processing of musical emotions. Neuropsychologia 89, 393–402. doi: 10.1016/j.neuropsychologia.2016.07.005
Bradley, M. M., and Lang, P. J. (1994). Measuring emotion: the self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry 25, 49–59. doi: 10.1016/0005-7916(94)90063-9
CrossRef Full Text | Google Scholar
Brattico, E. (2015). “From pleasure to liking and back: bottom-up and top-down neural routes to the aesthetic enjoyment of music,” in Art, Aesthetics and the Brain , eds M. Nadal, J. P. Houston, L. Agnati, F. Mora, and C. J. CelaConde (Oxford, NY: Oxford University Press), 303–318. doi: 10.1093/acprof:oso/9780199670000.003.0015
Chin, T. C., and Rickard, N. S. (2012). The Music USE (MUSE) questionnaire; an instrument to measure engagement in music. Music Percept. 29, 429–446. doi: 10.1525/mp.2012.29.4.429
Coutinho, E., and Cangelosi, A. (2011). Musical emotions: predicting second-by-second subjective feelings of emotion from low-level psychoacoustic features and physiological measurements. Emotion 11, 921–937. doi: 10.1037/a0024700
Davidson, R. J. (1988). EEG measures of cerebral asymmetry: conceptual and methodological issues. Int. J. Neurosci. 39, 71–89. doi: 10.3109/00207458808985694
Davidson, R. J. (1993). “The neuropsychology of emotion and affective style,” in Handbook of Emotion , eds M. Lewis and J. M. Haviland (New York, NY: The Guildford Press), 143–154.
Davidson, R. J. (2000). Affective style, psychopathology, and resilience. Brain mechanisms and plasticity. Am. Psychol. 55, 1196–1214. doi: 10.1037/0003-066X.55.11.1196
Davidson, R. J. (2004). Well-being and affective style: neural substrates and biobehavioural correlates. Philos. Trans. R. Soc. 359, 1395–1411. doi: 10.1098/rstb.2004.1510
Davidson, R. J., Ekman, P., Saron, C. D., Senulis, J. A., and Friesen, W. V. (1990). Approach-withdrawal and cerebral asymmetry: emotional expression and brain physiology: I. J. Pers. Soc. Psychol. 58, 330–341. doi: 10.1037/0022-3514.58.2.330
Davidson, R. J., and Fox, N. A. (1989). Frontal brain asymmetry predicts infants’ response to maternal separation. J. Abnorm. Psychol. 98, 127–131. doi: 10.1037/0021-843X.98.2.127
Davidson, R. J., and Irwin, W. (1999). The functional neuroanatomy of emotion and affective style. Trends Cogn. Sci. 3, 11–21. doi: 10.1016/S1364-6613(98)01265-0
Davidson, R. J., Jackson, D. C., and Kalin, N. H. (2000). Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol. Bull. 126, 890–909. doi: 10.1037/0033-2909.126.6.890
Davidson, R. J., Kabat-Zinn, J., Schumacher, J., Rosenkranz, M., Muller, D., Santorelli, S. F., et al. (2003). Alterations in brain and immune function produced by mindfulness meditation. Psychosom. Med. 65, 564–570. doi: 10.1097/01.PSY.0000077505.67574.E3
Davidson, R. J., Schwartz, G. E., Saron, C., Bennett, J., and Goleman, D. J. (1979). Frontal versus parietal EEG asymmetry during positive and negative affect. Psychophysiology 16, 202–203.
Dennis, T. A., and Solomon, B. (2010). Frontal EEG and emotion regulation: electrocortical activity in response to emotional film clips is associated with reduced mood induction and attention interference effects. Biol. Psychol. 85, 456–464. doi: 10.1016/j.biopsycho.2010.09.008
Dumermuth, G., and Molinari, L. (1987). “Spectral analysis of EEG background activity,” in Handbook of Electroencephalography and Clinical Neurophysiology: Methods of Analysis of Brain Electrical and Magnetic Signals , Vol. 1, eds A. S. Gevins and A. Remond (Amsterdam: Elsevier), 85–130.
Eerola, T., and Vuoskoski, J. K. (2011). A comparison of the discrete and dimensional models of emotion in music. Psychol. Music 39, 18–49. doi: 10.1093/scan/nsv032
Egermann, H., Pearce, M. T., Wiggins, G. A., and McAdams, S. (2013). Probabilistic models of expectation violation predict psychophysiological emotional responses to live concert music. Cogn. Affect. Behav. Neurosci. 13, 533–553. doi: 10.3758/s13415-013-0161-y
Flores-Gutierrez, E. O., Diaz, J.-L., Barrios, F. A., Favila-Humara, R., Guevara, M. A., del Rio-Portilla, Y., et al. (2007). Metabolic and electric brain patterns during pleasant and unpleasant emotions induced by music masterpieces. Int. J. Psychophysiol. 65, 69–84. doi: 10.1016/j.ijpsycho.2007.03.004
Fox, N. A. (1991). If it’s not left, it’s right: electroencephalogram asymmetry and the development of emotion. Am. Psychol. 46, 863–872. doi: 10.1037/0003-066X.46.8.863
Fox, N. A., and Davidson, R. J. (1986). Taste-elicited changes in facial signs of emotion and the asymmetry of brain electrical activity in human newborns. Neuropsychologia 24, 417–422. doi: 10.1016/0028-3932(86)90028-X
Frijda, N. H., and Scherer, K. R. (2009). “Emotion definition (psychological perspectives),” in Oxford Companion to Emotion and the Affective Sciences , eds D. Sander and K. R. Scherer (Oxford: Oxford University Press), 142–143.
Gabrielsson, A., and Lindstrom, E. (2010). “The role of structure in the musical expression of emotions,” in Handbook of Music and Emotion: Theory, Research, Applications , eds P. N. Juslin and J. A. Sloboda (New York, NY: Oxford University Press), 367–400.
Gomez, P., and Danuser, B. (2007). Relationships between musical structure and psychophysiological measures of emotion. Emotion 7, 377–387. doi: 10.1037/1528-3542.7.2.377
Grewe, O., Nagel, F., Kopiez, R., and Altenmüller, E. (2007a). Emotions over time: synchronicity and development of subjective, physiological, and facial affective reactions to music. Emotion 7, 774–788.
PubMed Abstract | Google Scholar
Grewe, O., Nagel, F., Kopiez, R., and Altenmüller, E. (2007b). Listening to music as a re-creative process: physiological, psychological, and psychoacoustical correlates of chills and strong emotions. Music Percept. 24, 297–314. doi: 10.1525/mp.2007.24.3.297
Guhn, M., Hamm, A., and Zentner, M. (2007). Physiological and musico-acoustic correlates of the chill response. Music Percept. 24, 473–484. doi: 10.1525/mp.2007.24.5.473
Hausmann, M., Hodgetts, S., and Eerola, T. (2013). Music-induced changes in functional cerebral asymmetries. Brain Cogn. 104, 58–71. doi: 10.1016/j.bandc.2016.03.001
Hodges, D. (2010). “Psychophysiological measures,” in Handbook of Music and Emotion: Theory, Research and Applications , eds P. N. Juslin and J. A. Sloboda (New York, NY: Oxford University Press), 279–312.
Howell, D. C. (2002). Statistical Methods for Psychology , 5th Edn. Belmont, CA: Duxbury.
Huron, D. (2006). Sweet Anticipation: Music and the Psychology of Expectation. Cambridge, MA: MIT Press.
Jackson, D. C., Malmstadt, J. R., Larson, C. L., and Davidson, R. J. (2000). Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology 37, 515–522. doi: 10.1111/1469-8986.3740515
Jackson, D. C., Mueller, C. J., Dolski, I., Dalton, K. M., Nitschke, J. B., Urry, H. L., et al. (2003). Now you feel it now you don’t: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychol. Sci. 14, 612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x
Jasper, H. H. (1958). Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr. Clin. Neurophysiol. 10, 370–375. doi: 10.1016/0013-4694(58)90053-1
Jones, N. A., and Field, T. (1999). Massage and music therapies attenuate frontal EEG asymmetry in depressed adolescents. Adolescence 34, 529–534.
Juslin, P. N., Liljestrom, S., Vastfjall, D., Barradas, G., and Silva, A. (2008). An experience sampling study of emotional reactions to music: listener, music, and situation. Emotion 8, 668–683. doi: 10.1037/a0013505
Juslin, P. N., Liljeström, S., Västfjäll, D., and Lundqvist, L. (2010). “How does music evoke emotions? Exploring the underlying mechanisms,” in Music and Emotion: Theory, Research and Applications , eds P. N. Juslin and J. A. Sloboda (Oxford: Oxford University Press), 605–642.
Juslin, P. N., and Sloboda, J. A. (eds) (2010). Handbook of Music and Emotion: Theory, Research and Applications. New York, NY: Oxford University Press.
Juslin, P. N., and Vastfjall, D. (2008). Emotional responses to music: the need to consider underlying mechanisms. Behav. Brain Sci. 31, 559–621. doi: 10.1017/S0140525X08005293
Kivy, P. (1990). Music Alone; Philosophical Reflections on the Purely Musical Experience. London: Cornell University Press.
Kline, J. P., and Allen, S. (2008). The failed repressor: EEG asymmetry as a moderator of the relation between defensiveness and depressive symptoms. Int. J. Psychophysiol. 68, 228–234. doi: 10.1016/j.ijpsycho.2008.02.002
Koelsch, S., Fritz, T., and Schlaugh, G. (2008a). Amygdala activity can be modulated by unexpected chord functions during music listening. Neuroreport 19, 1815–1819. doi: 10.1097/WNR.0b013e32831a8722
Koelsch, S., Fritz, T., von Cramon, Y., Muller, K., and Friederici, A. D. (2006). Investigating emotion with music: an fMRI study. Hum. Brain Mapp. 27, 239–250. doi: 10.1002/hbm.20180
Koelsch, S., Kilches, S., Steinbeis, N., and Schelinski, S. (2008b). Effects of unexpected chords and of performer’s expression on brain responses and electrodermal activity. PLOS ONE 3:e2631. doi: 10.1371/journal.pone.0002631
Konecni, V. (2013). Music, affect, method, data: reflections on the Carroll versus Kivy debate. Am. J. Psychol. 126, 179–195. doi: 10.5406/amerjpsyc.126.2.0179
Krumhansl, C. L. (1997). An exploratory study of musical emotions and psychophysiology. Can. J. Exp. Psychol. 51, 336–352. doi: 10.1037/1196-1961.51.4.336
Lindsley, D. B., and Wicke, J. D. (1974). “The electroencephalogram: autonomous electrical activity in man and animals,” in Bioelectric Recording Techniques , eds R. Thompson and M. N. Patterson (New York, NY: Academic Press), 3–79.
Meyer, L. B. (1956). “Emotion and meaning in music,” in Handbook of Music and Emotion: Theory, Research and Applications , eds P. N. Juslin and J. A. Sloboda (Oxford: Oxford University Press), 279–312.
Mitterschiffthaler, M. T., Fu, C. H. Y., Dalton, J. A., Andrew, C. M., and Williams, S. C. R. (2007). A functional MRI study of happy and sad affective states induced by classical music. Hum. Brain Mapp. 28, 1150–1162. doi: 10.1002/hbm.20337
Nagel, F., Kopiez, R., Grewe, O., and Altenmuller, E. (2007). EMuJoy: software for continuous measurement of perceived emotions in music. Behav. Res. Methods 39, 283–290. doi: 10.3758/BF03193159
Panksepp, J. (1995). The emotional sources of ‘chills’ induced by music. Music Percept. 13, 171–207. doi: 10.2307/40285693
Panksepp, J., and Bernatzky, G. (2002). Emotional sounds and the brain: the neuro-affective foundations of musical appreciation. Behav. Process. 60, 133–155. doi: 10.1016/S0376-6357(02)00080-3
Rickard, N. S. (2004). Intense emotional responses to music: a test of the physiological arousal hypothesis. Psychol. Music 32, 371–388. doi: 10.1177/0305735604046096
Rickard, N. S. (2012). “Music listening and emotional well-being,” in Lifelong Engagement with Music: Benefits for Mental Health and Well-Being , eds N. S. Rickard and K. McFerran (Hauppauge, NY: de Sitter), 207–238.
Russell, J. A. (1980). A circumplex model of affect. J. Soc. Psychol. 39, 1161–1178. doi: 10.1037/h0077714
Salimpoor, V. N., Benovoy, M., Larcher, K., Dagher, A., and Zatorre, R. J. (2011). Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat. Neurosci. 14, 257–264. doi: 10.1038/nn.2726
Salimpoor, V. N., van den Bosch, I., Kovacevic, N., McIntosh, A. R., Dagher, A., and Zatorre, R. J. (2013). Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science 340, 216–219. doi: 10.1126/science.1231059
Scherer, K. R. (2009). Emotions are emergent processes: they require a dynamic computational architecture. Philos. Trans. R. Soc. Ser. B 364, 3459–3474. doi: 10.1098/rstb.2009.0141
Scherer, K. R., and Coutinho, E. (2013). “How music creates emotion: a multifactorial process approach,” in The Emotional Power of Music , eds T. Cochrane, B. Fantini, and K. R. Scherer (Oxford: Oxford University Press). doi: 10.1093/acprof:oso/9780199654888.003.0010
Scherer, K. R., Zentner, M. R., and Schacht, A. (2002). Emotional states generated by music: an exploratory study of music experts. Music. Sci. 5, 149–171. doi: 10.1177/10298649020050S106
Schmidt, L. A., and Trainor, L. J. (2001). Frontal brain electrical activity (EEG) distinguishes valence and intensity of musical emotions. Cogn. Emot. 15, 487–500. doi: 10.1080/02699930126048
Schubert, E. (2010). “Continuous self-report methods,” in Handbook of Music and Emotion: Theory, Research and Applications , eds P. N. Juslin and J. A. Sloboda (Oxford: Oxford University Press), 223–224.
Sloboda, J. (1991). Music structure and emotional response: some empirical findings. Psychol. Music 19, 110–120. doi: 10.1177/0305735691192002
Steinbeis, N., Koelsch, S., and Sloboda, J. (2006). The role of harmonic expectancy violations in musical emotions: evidence from subjective, physiological, and neural responses. J. Cogn. Neurosci. 18, 1380–1393. doi: 10.1162/jocn.2006.18.8.1380
Thaut, M. H., and Davis, W. B. (1993). The influence of subject-selected versus experimenter-chosen music on affect, anxiety, and relaxation. J. Music Ther. 30, 210–233. doi: 10.1093/jmt/30.4.210
Thayer, J. F. (1986). Multiple Indicators of Affective Response to Music. Doctoral Dissertation, New York University, New York, NY.
Thibodeau, R., Jorgsen, R. S., and Kim, S. (2006). Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. J. Abnorm. Psychol. 115, 715–729. doi: 10.1037/0021-843X.115.4.715
Tomarken, A. J., Davidson, R. J., and Henriques, J. B. (1990). Resting frontal brain asymmetry predicts affective responses to films. J. Pers. Soc. Psychol. 59, 791–801. doi: 10.1037/0022-3514.59.4.791
Tomarken, A. J., Davidson, R. J., Wheeler, R. E., and Doss, R. C. (1992). Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. J. Pers. Soc. Psychol. 62, 676–687. doi: 10.1037/0022-3514.62.4.676
Travis, F., and Arenander, A. (2006). Cross-sectional and longitudinal study of effects of transcendental meditation practice on interhemispheric frontal asymmetry and frontal coherence. Int. J. Neurosci. 116, 1519–1538. doi: 10.1080/00207450600575482
Watson, D., and Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: the PANAS scales. J. Pers. Soc. Psychol. 54, 1063–1070. doi: 10.1037/0022-3514.54.6.1063
White, J. D. (1976). The Analysis of Music. Duke, NC: Duke University Press.
Zenter, M., Grandjean, D., and Scherer, K. R. (2008). Emotions evoked by the sound of music: characterization, classification, and measurement. Emotion 8, 494–521. doi: 10.1037/1528-3542.8.4.494
Keywords : frontal asymmetry, subjective emotions, pleasurable music, musicology, positive and negative affect
Citation: Arjmand H-A, Hohagen J, Paton B and Rickard NS (2017) Emotional Responses to Music: Shifts in Frontal Brain Asymmetry Mark Periods of Musical Change. Front. Psychol. 8:2044. doi: 10.3389/fpsyg.2017.02044
Received: 08 November 2016; Accepted: 08 November 2017; Published: 04 December 2017.
Reviewed by:
Copyright © 2017 Arjmand, Hohagen, Paton and Rickard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nikki S. Rickard, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- Architecture and Design
- Asian and Pacific Studies
- Business and Economics
- Classical and Ancient Near Eastern Studies
- Computer Sciences
- Cultural Studies
- Engineering
- General Interest
- Geosciences
- Industrial Chemistry
- Islamic and Middle Eastern Studies
- Jewish Studies
- Library and Information Science, Book Studies
- Life Sciences
- Linguistics and Semiotics
- Literary Studies
- Materials Sciences
- Mathematics
- Social Sciences
- Sports and Recreation
- Theology and Religion
- Publish your article
- The role of authors
- Promoting your article
- Abstracting & indexing
- Publishing Ethics
- Why publish with De Gruyter
- How to publish with De Gruyter
- Our book series
- Our subject areas
- Your digital product at De Gruyter
- Contribute to our reference works
- Product information
- Tools & resources
- Product Information
- Promotional Materials
- Orders and Inquiries
- FAQ for Library Suppliers and Book Sellers
- Repository Policy
- Free access policy
- Open Access agreements
- Database portals
- For Authors
- Customer service
- People + Culture
- Journal Management
- How to join us
- Working at De Gruyter
- Mission & Vision
- De Gruyter Foundation
- De Gruyter Ebound
- Our Responsibility
- Partner publishers

Your purchase has been completed. Your documents are now available to view.
Music and Emotions
- Jenefer Robinson
Ever since Plato people have thought that there is an especially intimate relationship between music and the emotions, but in fact there are several such relationships. In this essay I explain how music can express emotions and arouse emotions. And although strictly speaking, music cannot represent emotions, it can tell psychological stories that lend themselves to expressive interpretations. As a philosopher, my main aim is to analyze these different relationships between emotion and music, but I also illustrate my arguments with an array of musical examples.
Some people have claimed that music can represent the passions. According to the Baroque doctrine of Affektenlehre , different movements of a suite or concerto should ›represent‹ distinct emotional states such as gaiety or melancholy. The emotion ›represented‹ was often a principal means of unifying the movement. Some Baroque composers also wrote ›character pieces‹ that portray different characters or temperaments, sometimes illustrating that of their friends or the notabilities of the day.
But ›representation‹ in music is not strictly representation at all. A picture can identify a specific person or thing or event, but with some minor exceptions music without the aid of a title or program or the words of a song cannot do this. All it can do is present qualities, including emotion qualities such as »cheerful« and »melancholy«, that may or may not be attributed to or characterize some specific individual.
In the Romantic era, it became a commonplace that music can express emotions, whether the emotions of a character or protagonist in the music or the emotions of the composer himself. Some theorists believe that musical expressiveness is a matter of the listener's experiencing music as resembling expressive human gestures such as vocal intonations and expressive movements and behavior. On this view when we say that a piece of music is expressive of sadness, we are not saying that there is anybody around who is actually expressing any sadness. It's just that the music is experienced as sounding like or moving like a person who is sad. Others believe that when we hear music as expressive of emotion, we hear or imagine an agent or persona in the music, the ›owner‹ of the states expressed. Even some ›pure‹ instrumental music – especially some music from the Romantic era – can be heard as containing a persona who is expressing emotions. My own view is that expressing emotion in music in the full Romantic sense should be thought of as in essentials very much like the expression of emotion in ordinary life: it is primarily something that a composer or a persona in the music does or achieves, rather than primarily something detected or experienced by listeners.
Finally, I turn to the question of whether and how music can arouse emotions in its listeners. In Book III of The Republic , Plato argued that the musical mode known as the »Lydian« mode should be banned from the education of future governors of the state on the grounds that it makes people lascivious and lazy, whereas the Dorian mode should be encouraged because it makes people brave and virtuous. There is now ample evidence that Plato was right to think that music affects the emotions of its listeners. There are several ways in which it does this. As Peter Kivy has remarked, listeners often get pleasure from the beauty and clever craftsmanship of a well-constructed piece of music. Leonard Meyer has shown how having certain emotions is a mode of understanding certain music. Thus when listening to a piece in sonata form, we might feel anxiety at the delayed return of the tonic, bewilderment when the keys modulate further and further from the tonic and relief when finally the tonic returns. Another way in which music arouses emotions is by getting us to respond sympathetically to emotions expressed in the music by the composer, or his surrogate in the music. Finally, there is good evidence that music arouses emotions and moods in a more direct bodily way as well, influencing the autonomic system and the motor activity of listeners. These various mechanisms of emotional arousal often function simultaneously so as to produce powerful, complex, ambiguous emotional states.
- X / Twitter
Supplementary Materials
Please login or register with De Gruyter to order this product.
Journal and Issue
Articles in the same issue.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 29 March 2022
Music in the brain
- Peter Vuust ORCID: orcid.org/0000-0002-4908-735X 1 ,
- Ole A. Heggli ORCID: orcid.org/0000-0002-7461-0309 1 ,
- Karl J. Friston ORCID: orcid.org/0000-0001-7984-8909 2 &
- Morten L. Kringelbach ORCID: orcid.org/0000-0002-3908-6898 1 , 3 , 4
Nature Reviews Neuroscience volume 23 , pages 287–305 ( 2022 ) Cite this article
26k Accesses
105 Citations
278 Altmetric
Metrics details
- Neuroscience
Music is ubiquitous across human cultures — as a source of affective and pleasurable experience, moving us both physically and emotionally — and learning to play music shapes both brain structure and brain function. Music processing in the brain — namely, the perception of melody, harmony and rhythm — has traditionally been studied as an auditory phenomenon using passive listening paradigms. However, when listening to music, we actively generate predictions about what is likely to happen next. This enactive aspect has led to a more comprehensive understanding of music processing involving brain structures implicated in action, emotion and learning. Here we review the cognitive neuroscience literature of music perception. We show that music perception, action, emotion and learning all rest on the human brain’s fundamental capacity for prediction — as formulated by the predictive coding of music model. This Review elucidates how this formulation of music perception and expertise in individuals can be extended to account for the dynamics and underlying brain mechanisms of collective music making. This in turn has important implications for human creativity as evinced by music improvisation. These recent advances shed new light on what makes music meaningful from a neuroscientific perspective.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Microdosing with psilocybin mushrooms: a double-blind placebo-controlled study

Mapping model units to visual neurons reveals population code for social behaviour

Volatile working memory representations crystallize with practice
Zatorre, R. J., Chen, J. L. & Penhune, V. B. When the brain plays music: auditory–motor interactions in music perception and production. Nat. Rev. Neurosci. 8 , 547–558 (2007). A seminal review of auditory–motor coupling in music .
Article CAS PubMed Google Scholar
Koelsch, S. Toward a neural basis of music perception–a review and updated model. Front. Psychol. 2 , 110 (2011).
Article PubMed PubMed Central Google Scholar
Maes, P. J., Leman, M., Palmer, C. & Wanderley, M. M. Action-based effects on music perception. Front. Psychol. 4 , 1008 (2014).
Koelsch, S. Brain correlates of music-evoked emotions. Nat. Rev. Neurosci. 15 , 170–180 (2014). In this review, the author shows how music engages phylogenetically old reward networks in the brain to evoke emotions, and not merely subjective feelings .
Vuust, P. & Witek, M. A. Rhythmic complexity and predictive coding: a novel approach to modeling rhythm and meter perception in music. Front. Psychol. 5 , 1111 (2014).
Friston, K. The free-energy principle: a unified brain theory? Nat. Rev. Neurosci. 11 , 127–138 (2010). This review posits that several global brain theories may be unified by the free-energy principle .
Koelsch, S., Vuust, P. & Friston, K. Predictive processes and the peculiar case of music. Trends Cogn. Sci. 23 , 63–77 (2019). This review focuses specifically on predictive coding in music .
Article PubMed Google Scholar
Meyer, L. Emotion and Meaning in Music (Univ. of Chicago Press, 1956).
Lerdahl, F. & Jackendoff, R. A Generative Theory of Music (MIT Press, 1999).
Huron, D. Sweet Anticipation (MIT Press, 2006). In this book, Huron draws on evolutionary theory and statistical learning to propose a general theory of musical expectation .
Hansen, N. C. & Pearce, M. T. Predictive uncertainty in auditory sequence processing. Front. Psychol. https://doi.org/10.3389/fpsyg.2013.01008 (2014).
Vuust, P., Brattico, E., Seppanen, M., Naatanen, R. & Tervaniemi, M. The sound of music: differentiating musicians using a fast, musical multi-feature mismatch negativity paradigm. Neuropsychologia 50 , 1432–1443 (2012).
Altenmüller, E. O. How many music centers are in the brain? Ann. N. Y. Acad. Sci. 930 , 273–280 (2001).
Monelle, R. Linguistics and Semiotics in Music (Harwood Academic Publishers, 1992).
Rohrmeier, M. A. & Koelsch, S. Predictive information processing in music cognition. A critical review. Int. J. Psychophysiol. 83 , 164–175 (2012).
Vuust, P., Dietz, M. J., Witek, M. & Kringelbach, M. L. Now you hear it: a predictive coding model for understanding rhythmic incongruity. Ann. N. Y. Acad. Sci. https://doi.org/10.1111/nyas.13622 (2018).
Vuust, P., Ostergaard, L., Pallesen, K. J., Bailey, C. & Roepstorff, A. Predictive coding of music–brain responses to rhythmic incongruity. Cortex 45 , 80–92 (2009).
Vuust, P. & Frith, C. Anticipation is the key to understanding music and the effects of music on emotion. Behav. Brain Res. 31 , 599–600 (2008). This is the foundation for the PCM model used in this Review .
Google Scholar
Garrido, M. I., Sahani, M. & Dolan, R. J. Outlier responses reflect sensitivity to statistical structure in the human brain. PLoS Comput. Biol. 9 , e1002999 (2013).
Article CAS PubMed PubMed Central Google Scholar
Lumaca, M., Baggio, G., Brattico, E., Haumann, N. T. & Vuust, P. From random to regular: neural constraints on the emergence of isochronous rhythm during cultural transmission. Soc. Cogn. Affect. Neurosci. 13 , 877–888 (2018).
Quiroga-Martinez, D. R. et al. Musical prediction error responses similarly reduced by predictive uncertainty in musicians and non-musicians. Eur. J. Neurosci. https://doi.org/10.1111/ejn.14667 (2019).
Article Google Scholar
Koelsch, S., Schröger, E. & Gunter, T. C. Music matters: preattentive musicality of the human brain. Psychophysiology 39 , 38–48 (2002).
Koelsch, S., Schmidt, B.-h & Kansok, J. Effects of musical expertise on the early right anterior negativity: an event-related brain potential study. Psychophysiology 39 , 657–663 (2002).
Lumaca, M., Dietz, M. J., Hansen, N. C., Quiroga-Martinez, D. R. & Vuust, P. Perceptual learning of tone patterns changes the effective connectivity between Heschl’s gyrus and planum temporale. Hum. Brain Mapp. 42 , 941–952 (2020).
Lieder, F., Daunizeau, J., Garrido, M. I., Friston, K. J. & Stephan, K. E. Modelling trial-by-trial changes in the mismatch negativity. PLoS Comput. Biol. 9 , e1002911 (2013).
Wacongne, C., Changeux, J. P. & Dehaene, S. A neuronal model of predictive coding accounting for the mismatch negativity. J. Neurosci. 32 , 3665–3678 (2012).
Kiebel, S. J., Garrido, M. I. & Friston, K. J. Dynamic causal modelling of evoked responses: the role of intrinsic connections. Neuroimage 36 , 332–345 (2007).
Feldman, H. & Friston, K. J. Attention, uncertainty, and free-energy. Front. Hum. Neurosci. 4 , 215 (2010).
Cheung, V. K. M. et al. Uncertainty and surprise jointly predict musical pleasure and amygdala, hippocampus, and auditory cortex activity. Curr. Biol. 29 , 4084–4092 e4084 (2019). This fMRI study ties uncertainty and surprise to musical pleasure .
McDermott, J. H. & Oxenham, A. J. Music perception, pitch, and the auditory system. Curr. Opin. Neurobiol. 18 , 452–463 (2008).
Thoret, E., Caramiaux, B., Depalle, P. & McAdams, S. Learning metrics on spectrotemporal modulations reveals the perception of musical instrument timbre. Nat. Hum. Behav. 5 , 369–377 (2020).
Siedenburg, K. & McAdams, S. Four distinctions for the auditory “wastebasket” of timbre. Front. Psychol. 8 , 1747 (2017).
Bendor, D. & Wang, X. The neuronal representation of pitch in primate auditory cortex. Nature 436 , 1161–1165 (2005).
Zatorre, R. J. Pitch perception of complex tones and human temporal-lobe function. J. Acoustical Soc. Am. 84 , 566–572 (1988).
Article CAS Google Scholar
Warren, J. D., Uppenkamp, S., Patterson, R. D. & Griffiths, T. D. Separating pitch chroma and pitch height in the human brain. Proc. Natl Acad. Sci. USA 100 , 10038–10042 (2003). Using fMRI data, this study shows that pitch chroma is represented anterior to the primary auditory cortex, and pitch height is represented posterior to the primary auditory cortex .
Rauschecker, J. P. & Scott, S. K. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat. Neurosci. 12 , 718–724 (2009).
Leaver, A. M., Van Lare, J., Zielinski, B., Halpern, A. R. & Rauschecker, J. P. Brain activation during anticipation of sound sequences. J. Neurosci. 29 , 2477–2485 (2009).
Houde, J. F. & Chang, E. F. The cortical computations underlying feedback control in vocal production. Curr. Opin. Neurobiol. 33 , 174–181 (2015).
Lee, Y. S., Janata, P., Frost, C., Hanke, M. & Granger, R. Investigation of melodic contour processing in the brain using multivariate pattern-based fMRI. Neuroimage 57 , 293–300 (2011).
Janata, P. et al. The cortical topography of tonal structures underlying Western music. Science 298 , 2167–2170 (2002).
Saffran, J. R., Aslin, R. N. & Newport, E. L. Statistical learning by 8-month-old infants. Science 274 , 1926–1928 (1996).
Saffran, J. R., Johnson, E. K., Aslin, R. N. & Newport, E. L. Statistical learning of tone sequences by human infants and adults. Cognition 70 , 27–52 (1999).
Krumhansl, C. L. Perceptual structures for tonal music. Music. Percept. 1 , 28–62 (1983).
Margulis, E. H. A model of melodic expectation. Music. Percept. 22 , 663–714 (2005).
Temperley, D. A probabilistic model of melody perception. Cogn. Sci. 32 , 418–444 (2008).
Pearce, M. T. & Wiggins, G. A. Auditory expectation: the information dynamics of music perception and cognition. Top. Cogn. Sci. 4 , 625–652 (2012).
Sears, D. R. W., Pearce, M. T., Caplin, W. E. & McAdams, S. Simulating melodic and harmonic expectations for tonal cadences using probabilistic models. J. N. Music. Res. 47 , 29–52 (2018).
Näätänen, R., Gaillard, A. W. & Mäntysalo, S. Early selective-attention effect on evoked potential reinterpreted. Acta Psychol. 42 , 313–329 (1978).
Näätänen, R., Paavilainen, P., Rinne, T. & Alho, K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin. Neurophysiol. 118 , 2544–2590 (2007). This classic review covers three decades of MMN research to understand auditory perception .
Wallentin, M., Nielsen, A. H., Friis-Olivarius, M., Vuust, C. & Vuust, P. The Musical Ear Test, a new reliable test for measuring musical competence. Learn. Individ. Differ. 20 , 188–196 (2010).
Tervaniemi, M. et al. Top-down modulation of auditory processing: effects of sound context, musical expertise and attentional focus. Eur. J. Neurosci. 30 , 1636–1642 (2009).
Burunat, I. et al. The reliability of continuous brain responses during naturalistic listening to music. Neuroimage 124 , 224–231 (2016).
Burunat, I. et al. Action in perception: prominent visuo-motor functional symmetry in musicians during music listening. PLoS ONE 10 , e0138238 (2015).
Article PubMed PubMed Central CAS Google Scholar
Alluri, V. et al. Large-scale brain networks emerge from dynamic processing of musical timbre, key and rhythm. Neuroimage 59 , 3677–3689 (2012). A free-listening fMRI study showing brain networks involved in perception of distinct acoustical features of music .
Halpern, A. R. & Zatorre, R. J. When that tune runs through your head: a PET investigation of auditory imagery for familiar melodies. Cereb. Cortex 9 , 697–704 (1999).
Herholz, S. C., Halpern, A. R. & Zatorre, R. J. Neuronal correlates of perception, imagery, and memory for familiar tunes. J. Cogn. Neurosci. 24 , 1382–1397 (2012).
Pallesen, K. J. et al. Emotion processing of major, minor, and dissonant chords: a functional magnetic resonance imaging study. Ann. N. Y. Acad. Sci. 1060 , 450–453 (2005).
McPherson, M. J. et al. Perceptual fusion of musical notes by native Amazonians suggests universal representations of musical intervals. Nat. Commun. 11 , 2786 (2020).
Helmholtz H. L. F. On the Sensations of Tone as a Physiological Basis for the Theory of Music (Cambridge Univ. Press, 1954).
Vassilakis, P. N. & Kendall, R. A. in Human Vision and Electronic Imaging XV . 75270O (International Society for Optics and Photonics, 2010).
Plomp, R. & Levelt, W. J. M. Tonal consonance and critical bandwidth. J. Acoustical Soc. Am. 38 , 548–560 (1965).
McDermott, J. H., Schultz, A. F., Undurraga, E. A. & Godoy, R. A. Indifference to dissonance in native Amazonians reveals cultural variation in music perception. Nature 535 , 547–550 (2016). An ethnomusicology study showing that consonance preference may be absent in people with minimal exposure to Western music .
Mehr, S. A. et al. Universality and diversity in human song. Science https://doi.org/10.1126/science.aax0868 (2019).
Patel, A. D., Gibson, E., Ratner, J., Besson, M. & Holcomb, P. J. Processing syntactic relations in language and music: an event-related potential study. J. Cogn. Neurosci. 10 , 717–733 (1998). This classic study compares responses to syntactic incongruities in both language and Western tonal music .
Janata, P. The neural architecture of music-evoked autobiographical memories. Cereb. Cortex 19 , 2579–2594 (2009).
Maess, B., Koelsch, S., Gunter, T. C. & Friederici, A. D. Musical syntax is processed in Broca’s area: an MEG study. Nat. Neurosci. 4 , 540–545 (2001).
Koelsch, S. et al. Differentiating ERAN and MMN: an ERP study. Neuroreport 12 , 1385–1389 (2001). Using EEG, the authors show that ERAN and MMN reflect different cognitive mechanisms .
Loui, P., Grent-‘t-Jong, T., Torpey, D. & Woldorff, M. Effects of attention on the neural processing of harmonic syntax in Western music. Cogn. Brain Res. 25 , 678–687 (2005).
Koelsch, S., Fritz, T., Schulze, K., Alsop, D. & Schlaug, G. Adults and children processing music: an fMRI study. Neuroimage 25 , 1068–1076 (2005).
Tillmann, B., Janata, P. & Bharucha, J. J. Activation of the inferior frontal cortex in musical priming. Ann. N. Y. Acad. Sci. 999 , 209–211 (2003).
Garza-Villarreal, E. A., Brattico, E., Leino, S., Ostergaard, L. & Vuust, P. Distinct neural responses to chord violations: a multiple source analysis study. Brain Res. 1389 , 103–114 (2011).
Leino, S., Brattico, E., Tervaniemi, M. & Vuust, P. Representation of harmony rules in the human brain: further evidence from event-related potentials. Brain Res. 1142 , 169–177 (2007).
Sammler, D. et al. Co-localizing linguistic and musical syntax with intracranial EEG. Neuroimage 64 , 134–146 (2013).
Loui, P., Wessel, D. L. & Hudson Kam, C. L. Humans rapidly learn grammatical structure in a new musical scale. Music. Percept. 27 , 377–388 (2010).
Loui, P., Wu, E. H., Wessel, D. L. & Knight, R. T. A generalized mechanism for perception of pitch patterns. J. Neurosci. 29 , 454–459 (2009).
Cheung, V. K. M., Meyer, L., Friederici, A. D. & Koelsch, S. The right inferior frontal gyrus processes nested non-local dependencies in music. Sci. Rep. 8 , 3822 (2018).
Haueisen, J. & Knosche, T. R. Involuntary motor activity in pianists evoked by music perception. J. Cogn. Neurosci. 13 , 786–792 (2001).
Bangert, M. et al. Shared networks for auditory and motor processing in professional pianists: evidence from fMRI conjunction. Neuroimage 30 , 917–926 (2006).
Baumann, S. et al. A network for audio-motor coordination in skilled pianists and non-musicians. Brain Res. 1161 , 65–78 (2007).
Lahav, A., Saltzman, E. & Schlaug, G. Action representation of sound: audiomotor recognition network while listening to newly acquired actions. J. Neurosci. 27 , 308–314 (2007).
Bianco, R. et al. Neural networks for harmonic structure in music perception and action. Neuroimage 142 , 454–464 (2016).
Eerola, T., Vuoskoski, J. K., Peltola, H.-R., Putkinen, V. & Schäfer, K. An integrative review of the enjoyment of sadness associated with music. Phys. Life Rev. 25 , 100–121 (2018).
Huron, D. M. D. The harmonic minor scale provides an optimum way of reducing average melodic interval size, consistent with sad affect cues. Empir. Musicol. Rev. 7 , 15 (2012).
Huron, D. A comparison of average pitch height and interval size in major-and minor-key themes: evidence consistent with affect-related pitch prosody. 3 , 59-63 (2008).
Juslin, P. N. & Laukka, P. Communication of emotions in vocal expression and music performance: different channels, same code? Psychol. Bull. 129 , 770 (2003).
Fritz, T. et al. Universal recognition of three basic emotions in music. Curr. Biol. 19 , 573–576 (2009).
London, J. Hearing in Time: Psychological Aspects of Musical Meter (Oxford Univ. Press, 2012).
Honing, H. Without it no music: beat induction as a fundamental musical trait. Ann. N. Y. Acad. Sci. 1252 , 85–91 (2012).
Hickok, G., Farahbod, H. & Saberi, K. The rhythm of perception: entrainment to acoustic rhythms induces subsequent perceptual oscillation. Psychol. Sci. 26 , 1006–1013 (2015).
Yabe, H., Tervaniemi, M., Reinikainen, K. & Näätänen, R. Temporal window of integration revealed by MMN to sound omission. Neuroreport 8 , 1971–1974 (1997).
Andreou, L.-V., Griffiths, T. D. & Chait, M. Sensitivity to the temporal structure of rapid sound sequences — an MEG study. Neuroimage 110 , 194–204 (2015).
Jongsma, M. L., Meeuwissen, E., Vos, P. G. & Maes, R. Rhythm perception: speeding up or slowing down affects different subcomponents of the ERP P3 complex. Biol. Psychol. 75 , 219–228 (2007).
Graber, E. & Fujioka, T. Endogenous expectations for sequence continuation after auditory beat accelerations and decelerations revealed by P3a and induced beta-band responses. Neuroscience 413 , 11–21 (2019).
Brochard, R., Abecasis, D., Potter, D., Ragot, R. & Drake, C. The “ticktock” of our internal clock: direct brain evidence of subjective accents in isochronous sequences. Psychol. Sci. 14 , 362–366 (2003).
Lerdahl, F. & Jackendoff, R. An overview of hierarchical structure in music. Music. Percept. 1 , 229–252 (1983).
Large, E. W. & Kolen, J. F. Resonance and the perception of musical meter. Connect. Sci. 6 , 177–208 (1994).
Large, E. W. & Jones, M. R. The dynamics of attending: how people track time-varying events. Psychol. Rev. 106 , 119–159 (1999).
Cutietta, R. A. & Booth, G. D. The influence of metre, mode, interval type and contour in repeated melodic free-recall. Psychol. Music 24 , 222–236 (1996).
Smith, K. C. & Cuddy, L. L. Effects of metric and harmonic rhythm on the detection of pitch alterations in melodic sequences. J. Exp. Psychol. 15 , 457–471 (1989).
CAS Google Scholar
Palmer, C. & Krumhansl, C. L. Mental representations for musical meter. J. Exp. Psychol. 16 , 728–741 (1990).
Einarson, K. M. & Trainor, L. J. Hearing the beat: young children’s perceptual sensitivity to beat alignment varies according to metric structure. Music. Percept. 34 , 56–70 (2016).
Large, E. W., Herrera, J. A. & Velasco, M. J. Neural networks for beat perception in musical rhythm. Front. Syst. Neurosci. 9 , 159 (2015).
Nozaradan, S., Peretz, I., Missal, M. & Mouraux, A. Tagging the neuronal entrainment to beat and meter. J. Neurosci. 31 , 10234–10240 (2011).
Nozaradan, S., Peretz, I. & Mouraux, A. Selective neuronal entrainment to the beat and meter embedded in a musical rhythm. J. Neurosci. 32 , 17572–17581 (2012).
Nozaradan, S., Schonwiesner, M., Keller, P. E., Lenc, T. & Lehmann, A. Neural bases of rhythmic entrainment in humans: critical transformation between cortical and lower-level representations of auditory rhythm. Eur. J. Neurosci. 47 , 321–332 (2018).
Lenc, T., Keller, P. E., Varlet, M. & Nozaradan, S. Neural and behavioral evidence for frequency-selective context effects in rhythm processing in humans. Cereb. Cortex Commun. https://doi.org/10.1093/texcom/tgaa037 (2020).
Jacoby, N. & McDermott, J. H. Integer ratio priors on musical rhythm revealed cross-culturally by iterated reproduction. Curr. Biol. 27 , 359–370 (2017).
Hannon, E. E. & Trehub, S. E. Metrical categories in infancy and adulthood. Psychol. Sci. 16 , 48–55 (2005).
Hannon, E. E. & Trehub, S. E. Tuning in to musical rhythms: infants learn more readily than adults. Proc. Natl Acad. Sci. USA 102 , 12639–12643 (2005).
Vuust, P. et al. To musicians, the message is in the meter pre-attentive neuronal responses to incongruent rhythm are left-lateralized in musicians. Neuroimage 24 , 560–564 (2005).
Grahn, J. A. & Brett, M. Rhythm and beat perception in motor areas of the brain. J. Cogn. Neurosci. 19 , 893–906 (2007). This fMRI study investigates participants listening to rhythms of varied complexity .
Toiviainen, P., Burunat, I., Brattico, E., Vuust, P. & Alluri, V. The chronnectome of musical beat. Neuroimage 216 , 116191 (2019).
Chen, J. L., Penhune, V. B. & Zatorre, R. J. Moving on time: brain network for auditory-motor synchronization is modulated by rhythm complexity and musical training. J. Cogn. Neurosci. 20 , 226–239 (2008).
Levitin, D. J., Grahn, J. A. & London, J. The psychology of music: rhythm and movement. Annu. Rev. Psychol. 69 , 51–75 (2018).
Winkler, I., Haden, G. P., Ladinig, O., Sziller, I. & Honing, H. Newborn infants detect the beat in music. Proc. Natl Acad. Sci. USA 106 , 2468–2471 (2009).
Phillips-Silver, J. & Trainor, L. J. Feeling the beat: movement influences infant rhythm perception. Science 308 , 1430–1430 (2005).
Cirelli, L. K., Trehub, S. E. & Trainor, L. J. Rhythm and melody as social signals for infants. Ann. N. Y. Acad. Sci. https://doi.org/10.1111/nyas.13580 (2018).
Cirelli, L. K., Einarson, K. M. & Trainor, L. J. Interpersonal synchrony increases prosocial behavior in infants. Dev. Sci. 17 , 1003–1011 (2014).
Repp, B. H. Sensorimotor synchronization: a review of the tapping literature. Psychon. Bull. Rev. 12 , 969–992 (2005).
Repp, B. H. & Su, Y. H. Sensorimotor synchronization: a review of recent research (2006-2012). Psychon. Bull. Rev. 20 , 403–452 (2013). This review, and Repp (2005), succinctly covers the field of sensorimotor synchronization .
Zarco, W., Merchant, H., Prado, L. & Mendez, J. C. Subsecond timing in primates: comparison of interval production between human subjects and rhesus monkeys. J. Neurophysiol. 102 , 3191–3202 (2009).
Honing, H., Bouwer, F. L., Prado, L. & Merchant, H. Rhesus monkeys ( Macaca mulatta ) sense isochrony in rhythm, but not the beat: additional support for the gradual audiomotor evolution hypothesis. Front. Neurosci. 12 , 475 (2018).
Hattori, Y. & Tomonaga, M. Rhythmic swaying induced by sound in chimpanzees (Pan troglodytes). Proc. Natl Acad. Sci. USA 117 , 936–942 (2020).
Danielsen, A. Presence and Pleasure. The Funk Grooves of James Brown and Parliament (Wesleyan Univ. Press, 2006).
Madison, G., Gouyon, F., Ullen, F. & Hornstrom, K. Modeling the tendency for music to induce movement in humans: first correlations with low-level audio descriptors across music genres. J. Exp. Psychol. Hum. Percept. Perform. 37 , 1578–1594 (2011).
Stupacher, J., Hove, M. J., Novembre, G., Schutz-Bosbach, S. & Keller, P. E. Musical groove modulates motor cortex excitability: a TMS investigation. Brain Cogn. 82 , 127–136 (2013).
Janata, P., Tomic, S. T. & Haberman, J. M. Sensorimotor coupling in music and the psychology of the groove. J. Exp. Psychol. 141 , 54 (2012). Using a systematic approach, this multiple-studies article shows that the concept of groove can be widely understood as a pleasurable drive towards action .
Witek, M. A. et al. A critical cross-cultural study of sensorimotor and groove responses to syncopation among Ghanaian and American university students and staff. Music. Percept. 37 , 278–297 (2020).
Friston, K., Mattout, J. & Kilner, J. Action understanding and active inference. Biol. Cybern. 104 , 137–160 (2011).
Longuet-Higgins, H. C. & Lee, C. S. The rhythmic interpretation of monophonic music. Music. Percept. 1 , 18 (1984).
Sioros, G., Miron, M., Davies, M., Gouyon, F. & Madison, G. Syncopation creates the sensation of groove in synthesized music examples. Front. Psychol. 5 , 1036 (2014).
Witek, M. A., Clarke, E. F., Wallentin, M., Kringelbach, M. L. & Vuust, P. Syncopation, body-movement and pleasure in groove music. PLoS ONE 9 , e94446 (2014).
Kowalewski, D. A., Kratzer, T. M. & Friedman, R. S. Social music: investigating the link between personal liking and perceived groove. Music. Percept. 37 , 339–346 (2020).
Bowling, D. L., Ancochea, P. G., Hove, M. J. & Tecumseh Fitch, W. Pupillometry of groove: evidence for noradrenergic arousal in the link between music and movement. Front. Neurosci. 13 , 1039 (2019).
Matthews, T. E., Witek, M. A. G., Heggli, O. A., Penhune, V. B. & Vuust, P. The sensation of groove is affected by the interaction of rhythmic and harmonic complexity. PLoS ONE 14 , e0204539 (2019).
Matthews, T. E., Witek, M. A., Lund, T., Vuust, P. & Penhune, V. B. The sensation of groove engages motor and reward networks. Neuroimage 214 , 116768 (2020). This fMRI study shows that the sensation of groove engages both motor and reward networks in the brain .
Vaquero, L., Ramos-Escobar, N., François, C., Penhune, V. & Rodríguez-Fornells, A. White-matter structural connectivity predicts short-term melody and rhythm learning in non-musicians. Neuroimage 181 , 252–262 (2018).
Zatorre, R. J., Halpern, A. R., Perry, D. W., Meyer, E. & Evans, A. C. Hearing in the mind’s ear: a PET investigation of musical imagery and perception. J. Cogn. Neurosci. 8 , 29–46 (1996).
Benadon, F. Meter isn’t everything: the case of a timeline-oriented Cuban polyrhythm. N. Ideas Psychol. 56 , 100735 (2020).
London, J., Polak, R. & Jacoby, N. Rhythm histograms and musical meter: a corpus study of Malian percussion music. Psychon. Bull. Rev. 24 , 474–480 (2017).
Huron, D. Is music an evolutionary adaptation? Ann. N. Y. Acad. Sci. 930 , 43–61 (2001).
Koelsch, S. Towards a neural basis of music-evoked emotions. Trends Cogn. Sci. 14 , 131–137 (2010).
Eerola, T. & Vuoskoski, J. K. A comparison of the discrete and dimensional models of emotion in music. Psychol. Music. 39 , 18–49 (2010).
Lonsdale, A. J. & North, A. C. Why do we listen to music? A uses and gratifications analysis. Br. J. Psychol. 102 , 108–134 (2011).
Juslin, P. N. & Laukka, P. Expression, perception, and induction of musical emotions: a review and a questionnaire study of everyday listening. J. N. Music. Res. 33 , 217–238 (2004).
Huron, D. Why is sad music pleasurable? A possible role for prolactin. Music. Sci. 15 , 146–158 (2011).
Brattico, E. et al. It’s sad but I like it: the neural dissociation between musical emotions and liking in experts and laypersons. Front. Hum. Neurosci. 9 , 676 (2015).
PubMed Google Scholar
Sachs, M. E., Damasio, A. & Habibi, A. Unique personality profiles predict when and why sad music is enjoyed. Psychol. Music https://doi.org/10.1177/0305735620932660 (2020).
Sachs, M. E., Habibi, A., Damasio, A. & Kaplan, J. T. Dynamic intersubject neural synchronization reflects affective responses to sad music. Neuroimage 218 , 116512 (2020).
Juslin, P. N. & Vastfjall, D. Emotional responses to music: the need to consider underlying mechanisms. Behav. Brain Sci. 31 , 559–575 (2008). Using a novel theoretical framework, the authors propose that the mechanisms that evoke emotions from music are not unique to music .
Rickard, N. S. Intense emotional responses to music: a test of the physiological arousal hypothesis. Psychol. Music. 32 , 371–388 (2004).
Cowen, A. S., Fang, X., Sauter, D. & Keltner, D. What music makes us feel: at least 13 dimensions organize subjective experiences associated with music across different cultures. Proc. Natl Acad. Sci. USA 117 , 1924–1934 (2020).
Argstatter, H. Perception of basic emotions in music: culture-specific or multicultural? Psychol. Music. 44 , 674–690 (2016).
Stevens, C. J. Music perception and cognition: a review of recent cross-cultural research. Top. Cogn. Sci. 4 , 653–667 (2012).
Pearce, M. Cultural distance: a computational approach to exploring cultural influences on music cognition. in Oxford Handbook of Music and the Brain Vol. 31 (Oxford Univ. Press, 2018).
van der Weij, B., Pearce, M. T. & Honing, H. A probabilistic model of meter perception: simulating enculturation. Front. Psychol. 8 , 824 (2017).
Kringelbach, M. L. & Berridge, K. C. Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn. Sci. 13 , 479–487 (2009).
Blood, A. J. & Zatorre, R. J. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proc. Natl Acad. Sci. USA 98 , 11818–11823 (2001). This seminal positron emission tomography study shows that the experience of musical chills correlates with activity in the reward system .
Salimpoor, V. N. & Zatorre, R. J. Complex cognitive functions underlie aesthetic emotions: comment on “From everyday emotions to aesthetic emotions: towards a unified theory of musical emotions” by Patrik N. Juslin. Phys. Life Rev. 10 , 279–280 (2013).
Salimpoor, V. N. et al. Interactions between the nucleus accumbens and auditory cortices predict music reward value. Science 340 , 216–219 (2013).
Salimpoor, V. N., Benovoy, M., Larcher, K., Dagher, A. & Zatorre, R. J. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat. Neurosci. 14 , 257–262 (2011).
Salimpoor, V. N., Benovoy, M., Longo, G., Cooperstock, J. R. & Zatorre, R. J. The rewarding aspects of music listening are related to degree of emotional arousal. PLoS ONE 4 , e7487 (2009).
Mas-Herrero, E., Zatorre, R. J., Rodriguez-Fornells, A. & Marco-Pallares, J. Dissociation between musical and monetary reward responses in specific musical anhedonia. Curr. Biol. 24 , 699–704 (2014).
Martinez-Molina, N., Mas-Herrero, E., Rodriguez-Fornells, A., Zatorre, R. J. & Marco-Pallares, J. Neural correlates of specific musical anhedonia. Proc. Natl Acad. Sci. USA 113 , E7337–E7345 (2016).
Gebauer, L. K., M., L. & Vuust, P. Musical pleasure cycles: the role of anticipation and dopamine. Psychomusicology 22 , 16 (2012).
Shany, O. et al. Surprise-related activation in the nucleus accumbens interacts with music-induced pleasantness. Soc. Cogn. Affect. Neurosci. 14 , 459–470 (2019).
Gold, B. P., Pearce, M. T., Mas-Herrero, E., Dagher, A. & Zatorre, R. J. Predictability and uncertainty in the pleasure of music: a reward for learning? J. Neurosci. 39 , 9397–9409 (2019).
Swaminathan, S. & Schellenberg, E. G. Current emotion research in music psychology. Emot. Rev. 7 , 189–197 (2015).
Madison, G. & Schiölde, G. Repeated listening increases the liking for music regardless of its complexity: implications for the appreciation and aesthetics of music. Front. Neurosci. 11 , 147 (2017).
Corrigall, K. A. & Schellenberg, E. G. Liking music: genres, contextual factors, and individual differences. in Art, Aesthetics, and the Brain (Oxford Univ. Press, 2015).
Zentner, A. Measuring the effect of file sharing on music purchases. J. Law Econ. 49 , 63–90 (2006).
Rentfrow, P. J. & Gosling, S. D. The do re mi’s of everyday life: the structure and personality correlates of music preferences. J. Pers. Soc. Psychol. 84 , 1236–1256 (2003).
Vuust, P. et al. Personality influences career choice: sensation seeking in professional musicians. Music. Educ. Res. 12 , 219–230 (2010).
Rohrmeier, M. & Rebuschat, P. Implicit learning and acquisition of music. Top. Cogn. Sci. 4 , 525–553 (2012).
Münthe, T. F., Altenmüller, E. & Jäncke, L. The musician’s brain as a model of neuroplasticity. Nat. Rev. Neurosci. 3 , 1–6 (2002). This review highlights how professional musicians represent an ideal model for investigating neuroplasticity .
Habibi, A. et al. Childhood music training induces change in micro and macroscopic brain structure: results from a longitudinal study. Cereb. Cortex 28 , 4336–4347 (2018).
Schlaug, G., Jancke, L., Huang, Y., Staiger, J. F. & Steinmetz, H. Increased corpus callosum size in musicians. Neuropsychologia 33 , 1047–1055 (1995).
Baer, L. H. et al. Regional cerebellar volumes are related to early musical training and finger tapping performance. Neuroimage 109 , 130–139 (2015).
Kleber, B. et al. Voxel-based morphometry in opera singers: increased gray-matter volume in right somatosensory and auditory cortices. Neuroimage 133 , 477–483 (2016).
Gaser, C. & Schlaug, G. Brain structures differ between musicians and non-musicians. J. Neurosci. 23 , 9240–9245 (2003). Using a morphometric technique, this study shows a grey matter volume difference in multiple brain regions between professional musicians and a matched control group of amateur musicians and non-musicians .
Sluming, V. et al. Voxel-based morphometry reveals increased gray matter density in Broca’s area in male symphony orchestra musicians. Neuroimage 17 , 1613–1622 (2002).
Palomar-García, M.-Á., Zatorre, R. J., Ventura-Campos, N., Bueichekú, E. & Ávila, C. Modulation of functional connectivity in auditory–motor networks in musicians compared with nonmusicians. Cereb. Cortex 27 , 2768–2778 (2017).
Schneider, P. et al. Morphology of Heschl’s gyrus reflects enhanced activation in the auditory cortex of musicians. Nat. Neurosci. 5 , 688–694 (2002).
Bengtsson, S. L. et al. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 8 , 1148–1150 (2005).
Zamorano, A. M., Cifre, I., Montoya, P., Riquelme, I. & Kleber, B. Insula-based networks in professional musicians: evidence for increased functional connectivity during resting state fMRI. Hum. Brain Mapp. 38 , 4834–4849 (2017).
Kraus, N. & Chandrasekaran, B. Music training for the development of auditory skills. Nat. Rev. Neurosci. 11 , 599–605 (2010).
Koelsch, S., Schröger, E. & Tervaniemi, M. Superior pre-attentive auditory processing in musicians. Neuroreport 10 , 1309–1313 (1999).
Münte, T. F., Kohlmetz, C., Nager, W. & Altenmüller, E. Superior auditory spatial tuning in conductors. Nature 409 , 580 (2001).
Seppänen, M., Brattico, E. & Tervaniemi, M. Practice strategies of musicians modulate neural processing and the learning of sound-patterns. Neurobiol. Learn. Mem. 87 , 236–247 (2007).
Guillot, A. et al. Functional neuroanatomical networks associated with expertise in motor imagery. Neuroimage 41 , 1471–1483 (2008).
Bianco, R., Novembre, G., Keller, P. E., Villringer, A. & Sammler, D. Musical genre-dependent behavioural and EEG signatures of action planning. a comparison between classical and jazz pianists. Neuroimage 169 , 383–394 (2018).
Vuust, P., Brattico, E., Seppänen, M., Näätänen, R. & Tervaniemi, M. Practiced musical style shapes auditory skills. Ann. N. Y. Acad. Sci. 1252 , 139–146 (2012).
Bangert, M. & Altenmüller, E. O. Mapping perception to action in piano practice: a longitudinal DC-EEG study. BMC Neurosci. 4 , 26 (2003).
Li, Q. et al. Musical training induces functional and structural auditory-motor network plasticity in young adults. Hum. Brain Mapp. 39 , 2098–2110 (2018).
Herholz, S. C., Coffey, E. B. J., Pantev, C. & Zatorre, R. J. Dissociation of neural networks for predisposition and for training-related plasticity in auditory-motor learning. Cereb. Cortex 26 , 3125–3134 (2016).
Putkinen, V., Tervaniemi, M. & Huotilainen, M. Musical playschool activities are linked to faster auditory development during preschool-age: a longitudinal ERP study. Sci. Rep. 9 , 11310–11310 (2019).
Putkinen, V., Tervaniemi, M., Saarikivi, K., Ojala, P. & Huotilainen, M. Enhanced development of auditory change detection in musically trained school-aged children: a longitudinal event-related potential study. Dev. Sci. 17 , 282–297 (2014).
Jentschke, S. & Koelsch, S. Musical training modulates the development of syntax processing in children. Neuroimage 47 , 735–744 (2009).
Chobert, J., François, C., Velay, J. L. & Besson, M. Twelve months of active musical training in 8-to 10-year-old children enhances the preattentive processing of syllabic duration and voice onset time. Cereb. Cortex 24 , 956–967 (2014).
Moreno, S. et al. Musical training influences linguistic abilities in 8-year-old children: more evidence for brain plasticity. Cereb. Cortex 19 , 712–723 (2009).
Putkinen, V., Huotilainen, M. & Tervaniemi, M. Neural encoding of pitch direction is enhanced in musically trained children and is related to reading skills. Front. Psychol. 10 , 1475 (2019).
Wong, P. C., Skoe, E., Russo, N. M., Dees, T. & Kraus, N. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat. Neurosci. 10 , 420–422 (2007).
Virtala, P. & Partanen, E. Can very early music interventions promote at-risk infants’ development? Ann. N. Y. Acad. Sci. 1423 , 92–101 (2018).
Flaugnacco, E. et al. Music training increases phonological awareness and reading skills in developmental dyslexia: a randomized control trial. PLoS ONE 10 , e0138715 (2015).
Fiveash, A. et al. A stimulus-brain coupling analysis of regular and irregular rhythms in adults with dyslexia and controls. Brain Cogn. 140 , 105531 (2020).
Schellenberg, E. G. Correlation = causation? music training, psychology, and neuroscience. Psychol. Aesthet. Creat. Arts 14 , 475–480 (2019).
Sala, G. & Gobet, F. Cognitive and academic benefits of music training with children: a multilevel meta-analysis. Mem. Cogn. 48 , 1429–1441 (2020).
Saffran, J. R. Musical learning and language development. Ann. N. Y. Acad. Sci. 999 , 397–401 (2003).
Friston, K. The free-energy principle: a rough guide to the brain? Trends Cogn. Sci. 13 , 293–301 (2009).
Pearce, M. T. Statistical learning and probabilistic prediction in music cognition: mechanisms of stylistic enculturation. Ann. N. Y. Acad. Sci. 1423 , 378–395 (2018).
Article PubMed Central Google Scholar
Novembre, G., Knoblich, G., Dunne, L. & Keller, P. E. Interpersonal synchrony enhanced through 20 Hz phase-coupled dual brain stimulation. Soc. Cogn. Affect. Neurosci. 12 , 662–670 (2017).
Konvalinka, I. et al. Frontal alpha oscillations distinguish leaders from followers: multivariate decoding of mutually interacting brains. Neuroimage 94C , 79–88 (2014).
Novembre, G., Mitsopoulos, Z. & Keller, P. E. Empathic perspective taking promotes interpersonal coordination through music. Sci. Rep. 9 , 12255 (2019).
Wolpert, D. M., Ghahramani, Z. & Jordan, M. I. An internal model for sensorimotor integration. Science 269 , 1880–1882 (1995).
Patel, A. D. & Iversen, J. R. The evolutionary neuroscience of musical beat perception: the action simulation for auditory prediction (ASAP) hypothesis. Front. Syst. Neurosci. 8 , 57 (2014).
Sebanz, N. & Knoblich, G. Prediction in joint action: what, when, and where. Top. Cogn. Sci. 1 , 353–367 (2009).
Friston, K. J. & Frith, C. D. Active inference, communication and hermeneutics. Cortex 68 , 129–143 (2015). This article proposes a link between active inference, communication and hermeneutics .
Konvalinka, I., Vuust, P., Roepstorff, A. & Frith, C. D. Follow you, follow me: continuous mutual prediction and adaptation in joint tapping. Q. J. Exp. Psychol. 63 , 2220–2230 (2010).
Wing, A. M. & Kristofferson, A. B. Response delays and the timing of discrete motor responses. Percept. Psychophys. 14 , 5–12 (1973).
Repp, B. H. & Keller, P. E. Sensorimotor synchronization with adaptively timed sequences. Hum. Mov. Sci. 27 , 423–456 (2008).
Vorberg, D. & Schulze, H.-H. Linear phase-correction in synchronization: predictions, parameter estimation, and simulations. J. Math. Psychol. 46 , 56–87 (2002).
Novembre, G., Sammler, D. & Keller, P. E. Neural alpha oscillations index the balance between self-other integration and segregation in real-time joint action. Neuropsychologia 89 , 414–425 (2016). Using dual-EEG, the authors propose alpha oscillations as a candidate for regulating the balance between internal and external information in joint action .
Keller, P. E., Knoblich, G. & Repp, B. H. Pianists duet better when they play with themselves: on the possible role of action simulation in synchronization. Conscious. Cogn. 16 , 102–111 (2007).
Fairhurst, M. T., Janata, P. & Keller, P. E. Leading the follower: an fMRI investigation of dynamic cooperativity and leader-follower strategies in synchronization with an adaptive virtual partner. Neuroimage 84 , 688–697 (2014).
Heggli, O. A., Konvalinka, I., Kringelbach, M. L. & Vuust, P. Musical interaction is influenced by underlying predictive models and musical expertise. Sci. Rep. 9 , 1–13 (2019).
Heggli, O. A., Cabral, J., Konvalinka, I., Vuust, P. & Kringelbach, M. L. A Kuramoto model of self-other integration across interpersonal synchronization strategies. PLoS Comput. Biol. 15 , e1007422 (2019).
Heggli, O. A. et al. Transient brain networks underlying interpersonal strategies during synchronized action. Soc. Cogn. Affect. Neurosci. 16 , 19–30 (2020). This EEG study shows that differences in interpersonal synchronization are reflected by activity in a temporoparietal network .
Patel, A. D. Music, Language, and the Brain (Oxford Univ. Press, 2006).
Molnar-Szakacs, I. & Overy, K. Music and mirror neurons: from motion to ‘e’motion. Soc. Cogn. Affect. Neurosci. 1 , 235–241 (2006).
Beaty, R. E., Benedek, M., Silvia, P. J. & Schacter, D. L. Creative cognition and brain network dynamics. Trends Cogn. Sci. 20 , 87–95 (2016).
Limb, C. J. & Braun, A. R. Neural substrates of spontaneous musical performance: an FMRI study of jazz improvisation. PLoS ONE 3 , e1679 (2008).
Liu, S. et al. Neural correlates of lyrical improvisation: an FMRI study of freestyle rap. Sci. Rep. 2 , 834 (2012).
Rosen, D. S. et al. Dual-process contributions to creativity in jazz improvisations: an SPM-EEG study. Neuroimage 213 , 116632 (2020).
Boasen, J., Takeshita, Y., Kuriki, S. & Yokosawa, K. Spectral-spatial differentiation of brain activity during mental imagery of improvisational music performance using MEG. Front. Hum. Neurosci. 12 , 156 (2018).
Berkowitz, A. L. & Ansari, D. Generation of novel motor sequences: the neural correlates of musical improvisation. Neuroimage 41 , 535–543 (2008).
Loui, P. Rapid and flexible creativity in musical improvisation: review and a model. Ann. N. Y. Acad. Sci. 1423 , 138–145 (2018).
Beaty, R. E. The neuroscience of musical improvisation. Neurosci. Biobehav. Rev. 51 , 108–117 (2015).
Vuust, P. & Kringelbach, M. L. Music improvisation: a challenge for empirical research. in Routledge Companion to Music Cognition (Routledge, 2017).
Norgaard, M. Descriptions of improvisational thinking by artist-level jazz musicians. J. Res. Music. Educ. 59 , 109–127 (2011).
Kringelbach, M. L. & Deco, G. Brain states and transitions: insights from computational neuroscience. Cell Rep. 32 , 108128 (2020).
Deco, G. & Kringelbach, M. L. Hierarchy of information processing in the brain: a novel ‘intrinsic ignition’ framework. Neuron 94 , 961–968 (2017).
Pinho, A. L., de Manzano, O., Fransson, P., Eriksson, H. & Ullen, F. Connecting to create: expertise in musical improvisation is associated with increased functional connectivity between premotor and prefrontal areas. J. Neurosci. 34 , 6156–6163 (2014).
Pinho, A. L., Ullen, F., Castelo-Branco, M., Fransson, P. & de Manzano, O. Addressing a paradox: dual strategies for creative performance in introspective and extrospective networks. Cereb. Cortex 26 , 3052–3063 (2016).
de Manzano, O. & Ullen, F. Activation and connectivity patterns of the presupplementary and dorsal premotor areas during free improvisation of melodies and rhythms. Neuroimage 63 , 272–280 (2012).
Beaty, R. E. et al. Robust prediction of individual creative ability from brain functional connectivity. Proc. Natl Acad. Sci. USA 115 , 1087–1092 (2018).
Daikoku, T. Entropy, uncertainty, and the depth of implicit knowledge on musical creativity: computational study of improvisation in melody and rhythm. Front. Comput. Neurosci. 12 , 97 (2018).
Belden, A. et al. Improvising at rest: differentiating jazz and classical music training with resting state functional connectivity. Neuroimage 207 , 116384 (2020).
Arkin, C., Przysinda, E., Pfeifer, C. W., Zeng, T. & Loui, P. Gray matter correlates of creativity in musical improvisation. Front. Hum. Neurosci. 13 , 169 (2019).
Bashwiner, D. M., Wertz, C. J., Flores, R. A. & Jung, R. E. Musical creativity “revealed” in brain structure: interplay between motor, default mode, and limbic networks. Sci. Rep. 6 , 20482 (2016).
Przysinda, E., Zeng, T., Maves, K., Arkin, C. & Loui, P. Jazz musicians reveal role of expectancy in human creativity. Brain Cogn. 119 , 45–53 (2017).
Large, E. W., Kim, J. C., Flaig, N. K., Bharucha, J. J. & Krumhansl, C. L. A neurodynamic account of musical tonality. Music. Percept. 33 , 319–331 (2016).
Large, E. W. & Palmer, C. Perceiving temporal regularity in music. Cogn. Sci. 26 , 1–37 (2002). This article proposes an oscillator-based approach for the perception of temporal regularity in music .
Cannon, J. J. & Patel, A. D. How beat perception co-opts motor neurophysiology. Trends Cogn. Sci. 25 , 137–150 (2020). The authors propose that cyclic time-keeping activity in the supplementary motor area, termed ‘proto-actions’, is organized by the dorsal striatum to support hierarchical metrical structures .
Keller, P. E., Novembre, G. & Loehr, J. Musical ensemble performance: representing self, other and joint action outcomes. in Shared Representations: Sensorimotor Foundations of Social Life Cambridge Social Neuroscience (eds Cross, E. S. & Obhi, S. S.) 280-310 (Cambridge Univ. Press, 2016).
Rao, R. P. & Ballard, D. H. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 2 , 79–87 (1999).
Clark, A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behav. Brain Sci. 36 , 181–204 (2013).
Kahl, R. Selected Writings of Hermann Helmholtz (Wesleyan Univ. Press, 1878).
Gregory, R. L. Perceptions as hypotheses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 290 , 181–197 (1980).
Gibson, J. J. The Ecological Approach to Visual Perception (Houghton Mifflin, 1979).
Fuster, J. The Prefrontal Cortex Anatomy, Physiology and Neuropsychology of the Frontal Lobe (Lippincott-Raven, 1997).
Neisser, U. Cognition and Reality: Principles and Implications of Cognitive Psychology (W H Freeman/Times Books/ Henry Holt & Co, 1976).
Arbib, M. A. & Hesse, M. B. The Construction of Reality (Cambridge Univ. Press, 1986).
Cisek, P. & Kalaska, J. F. Neural mechanisms for interacting with a world full of action choices. Annu. Rev. Neurosci. 33 , 269–298 (2010).
Isomura, T., Parr, T. & Friston, K. Bayesian filtering with multiple internal models: toward a theory of social intelligence. Neural Comput. 31 , 2390–2431 (2019).
Friston, K. & Frith, C. A duet for one. Conscious. Cogn. 36 , 390–405 (2015).
Hunt, B. R., Ott, E. & Yorke, J. A. Differentiable generalized synchronization of chaos. Phys. Rev. E 55 , 4029–4034 (1997).
Ghazanfar, A. A. & Takahashi, D. Y. The evolution of speech: vision, rhythm, cooperation. Trends Cogn. Sci. 18 , 543–553 (2014).
Wilson, M. & Wilson, T. P. An oscillator model of the timing of turn-taking. Psychon. Bull. Rev. 12 , 957–968 (2005).
Download references
Acknowledgements
Funding was provided by The Danish National Research Foundation (DNRF117). The authors thank E. Altenmüller and D. Huron for comments on early versions of the manuscript.
Author information
Authors and affiliations.
Center for Music in the Brain, Aarhus University and The Royal Academy of Music (Det Jyske Musikkonservatorium), Aarhus, Denmark
Peter Vuust, Ole A. Heggli & Morten L. Kringelbach
Wellcome Centre for Human Neuroimaging, University College London, London, UK
Karl J. Friston
Department of Psychiatry, University of Oxford, Oxford, UK
Morten L. Kringelbach
Centre for Eudaimonia and Human Flourishing, Linacre College, University of Oxford, Oxford, UK
You can also search for this author in PubMed Google Scholar
Contributions
The authors contributed equally to all aspects of this article.
Corresponding author
Correspondence to Peter Vuust .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Nature Reviews Neuroscience thanks D. Sammler and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Patterns of pitched sounds unfolding over time, in accordance with cultural conventions and constraints.
The combination of multiple, simultaneously pitched sounds to form a chord, and subsequent chord progressions, a fundamental building block of Western music. The rules of harmony are the hierarchically organized expectations for chord progressions.
The structured arrangement of successive sound events over time, a primary parameter of musical structure. Rhythm perception is based on the perception of duration and grouping of these events and can be achieved even if sounds are not discrete, such as amplitude-modulated sounds.
Mathematically, the expected values or means of random variables.
The ability to extract statistical regularities from the world to learn about the environment.
In Western music, the organization of melody and harmony in a hierarchy of relations, often pointing towards a referential pitch (the tonal centre or the tonic).
A predictive framework governing the interpretation of regularly recurring patterns and accents in rhythm.
The output of a model generating outcomes from their causes. In predictive coding, the prediction is generated from expected states of the world and compared with observed outcomes to form a prediction error.
The subjective experience accompanying a strong expectation that a particular event will occur.
An enactive generalization of predictive coding that casts both action and perception as minimizing surprise or prediction error (active inference is considered a corollary of the free-energy principle).
A quantity used in predictive coding to denote the difference between an observation or point estimate and its predicted value. Predictive coding uses precision-weighted prediction errors to update expectations that generate predictions.
Expectations of musical events based on prior knowledge of regularities and patterns in musical sequences, such as melodies and chords.
Expectations of specific events or patterns in a familiar musical sequence.
Short-lived expectations that dynamically shift owing to the ongoing musical context, such as when a repeated musical phrase causes the listener to expect similar phrases as the work continues.
The inverse variance or negative entropy of a random variable. It corresponds to a second-order statistic (for example, a second-order moment) of the variable’s probability distribution or density. This can be contrasted with the mean or expectation, which constitutes a first-order statistic (for example, a first-order moment).
(MMN). A component of the auditory event-related potential recorded with electroencephalography or magnetoencephalography related to a change in different sound features such as pitch, timbre, location of the sound source, intensity and rhythm. It peaks approximately 110–250 ms after change onset and is typically recorded while participants’ attention is distracted from the stimulus, usually by watching a silent film or reading a book. The amplitude and latency of the MMN depends on the deviation magnitude, such that larger deviations in the same context yield larger and faster MMN responses.
(fMRI). A neuroimaging technique that images rapid changes in blood oxygenation levels in the brain.
In the realm of contemporary music, a persistently repeated pattern played by the rhythm section (usually drums, percussion, bass, guitar and/or piano). In music psychology, the pleasurable sensation of wanting to move.
The perceptual correlate of periodicity in sounds that allows their ordering on a frequency-related musical scale.
Also known as tone colour or tone quality, the perceived sound quality of a sound, including its spectral composition and its additional noise characteristics.
The pitch class containing all pitches separated by an integer number of octaves. Humans perceive a similarity between notes having the same chroma.
The contextual unexpectedness or surprise associated with an event.
In the Shannon sense, the expected surprise or information content (self-information). In other words, it is the uncertainty or unpredictability of a random variable (for example, an event in the future).
(MEG). A neuroimaging technique that measures the magnetic fields produced by naturally occurring electrical activity in the brain.
A very small electrical voltage generated in the brain structures in response to specific events or stimuli.
Psychologically, consonance is when two or more notes sound together with an absence of perceived roughness. Dissonance is the antonym of consonance. Western listeners consider intervals produced by frequency ratios such as 1:2 (octave), 3:2 (fifth) or 4:3 (fourth) as consonant. Dissonances are intervals produced by frequency ratios formed from numbers greater than 4.
Stereotypical patterns consisting of two or more chords that conclude a phrase, section or piece of music. They are often used to establish a sense of tonality.
(EEG). An electrophysiological method that measures electrical activity of the brain.
A method of analysing steady-state evoked potentials arising from stimulation or aspects of stimulation repeated at a fixed rate. An example of frequency tagging analysis is shown in Fig. 1c .
A shift of rhythmic emphasis from metrically strong accents to weak accents, a characteristic of multiple musical genres, such as funk, jazz and hip hop.
In Aristotelian ethics, refers to a life well lived or human flourishing, and in affective neuroscience, it is often used to describe meaningful pleasure.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Vuust, P., Heggli, O.A., Friston, K.J. et al. Music in the brain. Nat Rev Neurosci 23 , 287–305 (2022). https://doi.org/10.1038/s41583-022-00578-5
Download citation
Accepted : 22 February 2022
Published : 29 March 2022
Issue Date : May 2022
DOI : https://doi.org/10.1038/s41583-022-00578-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Improved emotion differentiation under reduced acoustic variability of speech in autism.
- Mathilde Marie Duville
- Luz María Alonso-Valerdi
- David I. Ibarra-Zarate
BMC Medicine (2024)
Decoding predicted musical notes from omitted stimulus potentials
- Tomomi Ishida
- Hiroshi Nittono
Scientific Reports (2024)
Exploring the neural underpinnings of chord prediction uncertainty: an electroencephalography (EEG) study
- Kentaro Ono
- Ryohei Mizuochi
- Shigeto Ymawaki
Spatiotemporal brain hierarchies of auditory memory recognition and predictive coding
- G. Fernández-Rubio
- M. L. Kringelbach
Nature Communications (2024)
Enhancing music rhythmic perception and performance with a VR game
- Matevž Pesek
- Matija Marolt
Virtual Reality (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Front Neurosci
Music-Evoked Emotions—Current Studies
Hans-eckhardt schaefer.
1 Tübingen University, Institute of Musicology, Tübingen, Germany
2 Institute of Functional Matter and Quantum Technology, Stuttgart University, Stuttgart, Germany
Associated Data
The present study is focused on a review of the current state of investigating music-evoked emotions experimentally, theoretically and with respect to their therapeutic potentials. After a concise historical overview and a schematic of the hearing mechanisms, experimental studies on music listeners and on music performers are discussed, starting with the presentation of characteristic musical stimuli and the basic features of tomographic imaging of emotional activation in the brain, such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), which offer high spatial resolution in the millimeter range. The progress in correlating activation imaging in the brain to the psychological understanding of music-evoked emotion is demonstrated and some prospects for future research are outlined. Research in psychoneuroendocrinology and molecular markers is reviewed in the context of music-evoked emotions and the results indicate that the research in this area should be intensified. An assessment of studies involving measuring techniques with high temporal resolution down to the 10 ms range, as, e.g., electroencephalography (EEG), event-related brain potentials (ERP), magnetoencephalography (MEG), skin conductance response (SCR), finger temperature, and goose bump development (piloerection) can yield information on the dynamics and kinetics of emotion. Genetic investigations reviewed suggest the heredity transmission of a predilection for music. Theoretical approaches to musical emotion are directed to a unified model for experimental neurological evidence and aesthetic judgment. Finally, the reports on musical therapy are briefly outlined. The study concludes with an outlook on emerging technologies and future research fields.
Introduction
Basic discussions of music center about questions such as: What actually is music? How can we understand music? What is the effect of music on human beings? Music is described as multidimensional and researchers have categorized it by its arousal properties (relaxing/calming vs. stimulating), emotional quality (happy, sad, peaceful), and structural features (as, e.g., tempo, tonality, pitch range, timbre, rhythmic structure) (Chanda and Levitin, 2013 ). One can ask the question how to recognize and describe the concretely beautiful in music. Efforts have been undertaken to answer this question (Eggebrecht, 1991 ), e.g., by discussing the beauty of the opening theme of the second movement of Mozart's piano concerto in d minor (KV 466). In this formal attempt to transform music into a descriptive language, particular sequences of tones and rhythmical structures have been tentatively ascribed to notions such as “flattering” or “steady-firm” (Eggebrecht, 1991 ). From the viewpoint of a composer, Mozart himself obviously was aware of the attractiveness of this beauty-component in music, stating that his compositions should be “…angenehm für die Ohren…” of the audience “…natürlich ohne in das Leere zu fallen…” (…pleasing for the ear… (of the audience) …naturally without falling into the shallow…) (see Eggebrecht, 1991 ). In modern and contemporary music, however, formal attempts of understanding are useless because form and self-containedness are missing (Zender, 2014 ). Thus, in atonality and in the emancipation of noise, a tonal center is absent, by simultaneous appearance of different rhythmic sequences the regular meter is demolished, and in aleatory music the linear order of musical events is left open.
A few earlier comments on the understanding of the interplay between music and man may be quoted here: “…there is little to be gained by investigation of emotion in music when we have little idea about the true fundamental qualities of emotion” (Meyer, 1956 ). “…music is so individual that attempts to provide a systematic explanation of the interaction might well be ultimately fruitless—there may be no systematic explanation of what happens when individuals interact with music” (Waterman, 1996 ). “Die Qualitäten und die Inhalte ihrer (der Komponisten) Musik zu beschreiben ist unmöglich. Eben deshalb werden sie in Klang gefasst, weil sie sonst nicht erfahrbar sind” (To describe the qualities and content of their (of the composers) music is impossible. Exactly for this reason they are expressed in musical sound, otherwise they are not communicable) (Maurer, 2014 ). Some historical comments on music-evoked emotions are compiled in section Historical Comments on the Impact of Music on People of this study.
The advent of brain-imaging technology with high spatial resolution (see principles section Experimental Procedures for Tomographic Imaging of Emotion in the Brain) gave new impact to interdisciplinary experimental research in the field of music-evoked emotions from the physiological and molecular point of view. With the broader availability of magnetic resonance imaging (MRI, first demonstrated in 1973; Lauterbur, 1973 ) and positron emission tomography (PET, first demonstrated 1975; Ter-Pogossian, 1975 ) since about two decades for studying both music listeners and performing musicians, a wealth of music-evoked brain activation data has been accomplished which is discussed in section Experimental Results of Functional (tomographic) Brain Imaging (fMRI, PET) together with psychoendocrinological and molecular markers. Due to the refinement of the more phenomenological measuring techniques, such as electroencephalography (EEG) and magnetoencephalography [MEG, section Electro- and Magnetoencephalography (EEG, MEG)], skin conductance response and finger temperature measurements (section Skin Conductance Response (SCR) and Finger Temperature) as well as goose bump development (section Goose Bumps—Piloerection), emotions can be measured with high temporal resolution. Genetic studies of musical heredity are reported in section Is There a Biological Background for the Attractiveness of Music?—Genomic Studies and recent theoretical approaches of musical emotions in section Towards a Theory of Musical Emotions. Some therapeutic issues of music are discussed in section Musical Therapy for Psychiatric or Neurologic Impairments and Deficiencies in Music Perception prior to the remarks concluding this study with an outlook. A brief outline of the psychological discussion of music-evoked emotion is given in the online Supplementary Material section.
Historical comments on the impact of music on people
The effects of music on man have been considered phenomenologically from antiquity to the nineteenth century mainly from the medical point of view according to Kümmel ( 1977 ) which will be preferentially referred to in the brief historical comments of the present section.
The only biblical example of a healing power of music refers to King Saul (~1,000 BC) who was tormented by an evil spirit and relief came to him when David played the lyre (1. Sam. 16, 14-23). In Antiquity, Pythagoras (~570-507 BC) was said to substantially affect the souls of people by diatonic, chromatic, or enharmonic tunes (see Kümmel, 1977 ). Platon (428-348 BC) in his Timaios suggested for the structure of the soul the same proportions of the musical intervals which are characteristic for the trajectories of the celestial bodies (see Kümmel, 1977 ). This concept of a numeral order of music and its effect on man was transferred to the Middle Ages, e.g., by Boethius (480-525). The Greek physician Asklepiades (124-60 BC) was said to have used music as a remedy for mental illness where the application of the Phrygian mode was considered to be particularly adequate for brightening up depressive patients. Boethius emphasized that music has to be correlated to the category of “moralitas” because of its strong effect on individuals. In his treatise De institutione musica he stated that “…music is so naturally united with us that we cannot be free from it even if we so desired….” Since the ninth century, music took a strong position in the medicine of the Arabic world and the musician was an assisting professional of the physician. According to Arabic physicians, music for therapeutic purposes should be “pleasant,” “dulcet,” “mild,” “lovely,” “charming,” and in the course of the assimilation of the Arabic medicine, the Latin West took over the medical application of music. Johannes Tinctoris (1435-1511) listed 20 effects of music, such as, e.g., that music banishes unhappiness, contributes to a cheerful mood, and cures diseases. In addition, music was supposed to delay aging processes. Agrippa von Nettesheim (1486-1535) was convinced that music can maintain physical health and emboss a moral behavior. He discusses in his treatise De occulta philosophia (Agrippa von Nettesheim, 1992 ) the powerful and prodigious effects of music. From his list of 20 different musical effects—adapted to the sequence of effects established by Johannes Tinctoris (1435-1511) (Schipperges, 2003 ) a brief selection should be presented here:
- (1) Musica Deum delectat
- (7) Musica tristitiam repellit
- (13) Musica homines laetificat
- (14) Musica aegrotos sanat
- (17) Musica amorem allicit etc.
These effects could be translated into nowadays notions as religiosity (1), depression (7), joy (13), therapy (14), and sexuality (17).
Agrippa points out the alluring effects of music on unreasoning beasts: “…ipsas quoque bestias, serpentes, volucres, delphines, ad auditum suae modulationis provocat…magna vis est musica” (It stirs the very beasts, even serpents, birds and dolphins, to want to hear its melody…great is the power of music).
The physician of Arnstadt, Johann Wittich (1537-1598) summarized the requirement for good health concisely: “Das Hertz zu erfrewen/und allen Unmuht zu wenden/haben sonderliche große Krafft diese fünff Stück (To rejoice the heart/ and reverse all discontent/five things have particularly great power):
- Gottes Wort (The word of God).
- Ein gutes Gewissen (A clear conscience).
- Die Musica (Music).
- Ein guter Wein (good wine).
- Ein vernünftig Weib (A sensible wife).”
René Descartes (1596-1650) formulated a fairly detailed view of the effects of music: The same music which stimulates some people to dancing may move others to tears. This exclusively depends on the thoughts which are aroused in our memory. In the medical encyclopedia of Bartolomeo Castelli of 1682 it is stated that music is efficient for both the curing of diseases and for maintaining health. A famous historical example for a positive impact of music on mental disorders is the Spanish King Philipp V (1683-1746) who—due to his severe depressions—stopped signing official documents and got up from his bed only briefly and only by night. In 1737, his wife Elisabeth Farnese (1692-1766, by the way a descendant of Pope Paul III and Emperor Karl V) appointed the famous Italian castrato singer Carlo Broschi Farinelli (1705-1782) to Madrid. Over 10 years, Farinelli performed every night (in total 3,600 times) four arias in order to banish the black melancholia from the kings mind until the king himself “…die Musik lernet…” (…learns music…) (see Kümmel, 1977 ). With his singing, Farinelli succeeded in agitating the king to partial fulfillment of his governmental duties and an occasional appearance in the governmental council. The king's favorite aria was Quell' usignolo with a difficult coloratura part (see Figure Figure1) 1 ) of Geminiano Giacomelli's (1692-1740) opera Merope (1734).

Extract from the aria Quell' usignolo of Geminiano Giacomelli's (1692-1740) opera Merope (1734) sung by Carlo Broschi Farinelli (1705-1782) for Philipp V (1683-1746), king of Spain (Haböck, 1923 ). Reprinted with permission from Haböck ( 1923 ) © 1923 Universal Edition.
The widely known Goldberg Variationen composed by J. S. Bach in 1740 may be considered, as reported by Bach biographer J. N. Forkel (1749-1818), as therapeutic music. H. C. von Keyserlingk, a Russian diplomat, asked Bach for “…einige Clavierstücke für seinen Adlatus Johann Gottlieb Goldberg,…die so sanften und etwas munteren Charakters wären, daß er dadurch in seinen schlaflosen Nächten ein wenig aufgeheitert werden könnte…” (… a number of clavier pieces for his personal assistant J. G. Goldberg…which should be of such gentle and happy character that he be somewhat cheered in his sleepless nights…). Bach chose a variations composition because of the unchanged basic harmony, although he initially had regarded a piece of this technique as a thankless task (see Kümmel, 1977 ).
In 1745 the medicine professor E. A. Nicolai (1722-1802) of Jena University started to report on more physical observations: “… wenn man Musik höre richten sich die Haare …in die Höhe, das Blut bewegt sich von aussen nach innen, die äusseren Teile fangen an kalt zu werden, das Herz klopft geschwinder und man hohlt etwas langsamer und tiefer Athem” (…when one hears music the hair stands on end (see section Goose Bumps—Piloerection), the blood is withdrawn from the surface, the outer parts begin to cool, the heart beats faster, and one breathes somewhat slower and more deeply). The French Encyclopédie of 1765 listed the diseases for which music was to be employed therapeutically: Pathological anxieties, the bluster of mental patients, gout pain, melancholia, epilepsy, fever, and plague. The physician and composer F. A. Weber (1753-1806) of Heilbronn, Germany assessed in 1802 the health effects of music more reluctantly: “Nur in Übeln aus der Klasse der Nervenkrankheiten läßt sich von…der Musik etwas Gedeihliches erhoffen. Vollständige Impotenz ist durch Musik nicht heilbar…Allein als Erwärmungsmittel erkaltender ehelicher Zärtlichkeit mag Musik vieles leisten” (Only in afflictions of the class of nervous diseases can …something profitable be expected from music. Complete impotence is not curable by music. …But as a means of rekindling marital tenderness music may achieve considerable results). The French psychiatrist J. E. D. Esquirol (1772-1840, see Charland, 2010 ) started to perform numerous experiments with the application of music to single patients or to groups. He, however, stated that the effect of music was transient and disappeared when the music ended. This change of thinking is also visible in the essay by Eduard Hanslick (1825-1904) Vom musikalisch Schönen (1854): “Die körperliche Wirkung der Musik ist weder an sich so stark, noch so sicher, noch von psychischen und ästhetischen Voraussetzungen so unabhängig, noch endlich so willkürlich behandelbar, dass sie als wirkliches Heilmittel in Betracht kommen könnte” (The physical effect of music is as such neither sufficiently strong, consistent, free from psychic and aesthetic preconditions nor freely usable as to allow its use as a real medical treatment).
With the rise of the experimental techniques of natural sciences in the medicine of the late nineteenth century, the views, patterns, and notions as determined by musical harmony began to take a backseat. It should be mentioned here that skepticism with regard to the effects of music arose in early times. In the third century Quintus Serenus declared the banishing of fever by means of vocals as pure superstition. In 1650 Athanasius Kircher wrote: “Denn dass durch (die Musik) ein Schwindsüchtiger, ein Epileptiker oder ein Gicht-Fall…geheilt werden können, halte ich für unmöglich.” (For I hold it for impossible that a consumptive, an epileptic or a gout sufferer …could be cured by music).
The mechanisms of hearing
Sound waves are detected by the ear and converted into neural signals which are sent to the brain. The ear has three divisions: The external, the middle, and the inner ear (see Figure Figure2A). 2A ). The sound waves vibrate the ear drum which is connected to the ear bones (malleus, incus, and stapes) in the middle ear that mechanically carry the sound waves to the frequency-sensitive cochlea (35 mm in length, Figure Figure2B) 2B ) with the basilar membrane in the inner ear. Here, making use of the cochlear hair cells (organ of Corti), the sound waves are converted into neural signals which are passed to the brain via the auditory nerve (Zenner, 1994 ). For each frequency, there is a region of maximum stimulation, or resonance region, on the basilar membrane. The spatial position x along the basilar membrane of the responding hair cells and the associated neurons determine the primary sensation of the pitch. A change in frequency of a pure tone causes a shift of the position of the activated region. This shift is then interpreted as a change in pitch (see Roederer, 2008 ) effect and laser studies allowed for a precise measurement of the movement of the basilar membrane (see Roederer, 2008 ).

Anatomy of the ear. Reprinted with permission from William E. Brownell © 2016. (B) Components of the inner ear. Reprinted with permission from © 2016 Encyclopedia Britannica. (C) Confocal micrographs of rat auditory hair cells. Scale bar: 1 μm. The protein myosin XVa is localized to the stereocilia tips (Rzadzinska et al., 2004 ). Reprinted with permission from Rzadzinska et al. ( 2004 ) © 2016 Bechara Kachar.
The cochlear hair cells assist in relaying sound to the brain. The about 20,000 hair cells in the human ear are covered by stereocilia (see Figure Figure2C), 2C ), giving them a hairy look. The stereocilia of the hair cell, which is sitting on the basilar membrane, are the primary structures used in sound transduction. With acoustic stimulation, the stereocilia bend which causes a signal that goes to the auditory nerve (see Figure Figure2A) 2A ) and eventually to the auditory cortex allowing sound to be processed by the brain.
At loudest sound the bending amplitude of the stereocilia is about their diameter of 200 nm (a nanometer nm is a millionth of a mm) and at auditory threshold the movement is about 1 nm or, in the order of the diameter of small molecules (Fettiplace and Hackney, 2006 ), i.e., close to the thermal equilibrium fluctuations of the Brownian motion in the surrounding lymphatic liquid (Roederer, 2008 ).
The bending of the stereocilia initiates an uptake of potassium ions (K + ) which in turn opens voltage-dependent calcium ion (Ca + ) channels. This causes neurotransmitter release at the basal end of the hair cell, eliciting an action potential in the dendrites of the auditory nerve (Gray, 0000 ).
The action speed of the hair cells is incredibly high to satisfy the amazing demands for speed in the auditory system. Signal detection and amplification must be preferentially handled by processes occurring within one hair cell. The acoustic apparatus cannot afford the “leisurely pace” of the nervous system that works on a time scale of several milliseconds or more.
Specific experimental techniques for studying musical emotion and discussion of the results
Emotionally relevant musical stimuli.
Emotional relevance of music is ascribed, e.g., to enharmonic interchange, starting of a singing voice, the climax of a crescendo, a downward quint, or in general a musically unexpected material (Spitzer, 2003, 2014 ). Four musical parameters for the activation of emotions appear to be particularly prominent in the literature (Kreutz et al., 2012 ): musical tempo, consonance, timbre, and loudness. Musical tempo could influence cardiovascular dynamics. The category of consonance could be associated with activation in the paralimbic and cortical brain areas (Blood and Zatorre, 2001 ) whereas dissonances containing partials with non-integer (irrational) frequency ratios may give rise to a sensation of roughness. The loudness or the physical sound pressure seems to be of relevance to psychoneuroendocrinological responses to music. Thus, crescendo leads to specific modulation of cardiovascular activity (see Kreutz et al., 2012 ), such as musical expectancy and tension (Koelsch, 2014 ). Musical sounds are often structured in time, space, and intensity. Several structural factors in music give rise to musical tension: consonance or dissonance, loudness, pitch, and timber can modulate tension. Sensory consonance and dissonance are already represented in the brainstem (Tramo et al., 2001 ) and modulate activity in the amygdala.
The stability of a musical structure also contributes to tension, such as a stable beat or its perturbation (for example, by an accelerando or a ritardando, syncopations, off-beat phrasings, etc.) (Koelsch, 2014 ). The stability of a tonal structure in tonal music also contributes to tension. Moving away from the tonal center creates tension and returning to it evokes relaxation. Figure Figure3 3 illustrates how the entropy of the frequency of the occurrence of tones and chords determines the stability of a tonal structure and thus the ease, or the difficulty, of establishing a tonal center. Additionally, the extent of a structural context contributes to tension. Figure Figure3 3 shows the probabilities of certain chords following other chords in Bach chorales. The red bars indicate that after a dominant the next chord is most likely to be a tonic. The uncertainty of the predictions for the next chord (and thus the entropy of the probability distribution for the next chord) is low during the dominant, intermediate during the tonic, and relatively high during the submediant. Progressive tones and harmonies thus create an entropic flux that gives rise to constantly changing (un)certainties of predictions. The increasing complexity of regulations, and thus the increase of entropic flux, requires an increasing amount of knowledge about the musical regularities to make precise predictions about upcoming events. Tensions emerge from the suspense about whether a prediction proves true (Koelsch, 2014 ). Tensions and release may be important for a religious chorale as metaphors for sin and redemption (Koelsch, 2014 ).

This graph shows the context-dependent bigram probabilities for the corpus of Bach chorales. Blue bars show probabilities of chord functions following the tonic (I), green bars following the submediant (vi), and red bars following a dominant (V). The probability for, e.g., a tonic (I) following a dominant (V) is high, the entropy is low (Koelsch, 2014 ). Reprinted with permission from Koelsch ( 2014 ) © 2014 Nature Publishing Group.
Tension can be further modulated by a structural breach. The emotional effects of the violations of predictions, which can be treated in analogy to the free energy of a system (Friston and Friston, 2013 ) includes surprise. Irregular unexpected chord functions, with rating of felt tensions, evoke skin conductance responses, activity changes in the amygdala and the orbitofrontal cortex while listening to a piece of classical piano music (see Koelsch, 2014 ).
Anticipatory processes can also be evoked by structural cues, for example by a dominant in a Bach chorale with a high probability being followed by a tonic (see Figure Figure3), 3 ), or a dominant seventh chord which has a high probability for being followed by a tonic, thus evoking the anticipation of release. Such anticipation of relaxation might envolve dopaminergic activity in the dorsal striatum (Koelsch, 2014 ).
Another effect arising from music is emotional contagion. Music can trigger psychological processes that reflect emotion: “happy” music triggers the zygomatic muscle for smiling, together with an increase in skin conductance and breathing rate, whereas “sad” music activates the corrugator muscle. Interestingly, there seems to be an acoustic similarity between expression of emotion in Western music and affective prosody (see Koelsch, 2014 ).
Experimental procedures for tomographic imaging of emotion in the brain
Magnetic resonance imaging (mri) and functional magnetic resonance imaging (fmri).
Magnetic resonance imaging (see Reiser et al., 2008 ) can show anatomy and in some cases function (fMRI). Studies on the molecular level have been reported recently (Xue et al., 2013 ; Liu et al., 2014 ). In a magnetic resonance scanner (Figure (Figure4A) 4A ) the magnetic moments of the hydrogen nuclei (protons) are aligned (Figure (Figure4A) 4A ) by a strong external magnetic field (usually 1.5 Tesla) that is generated in a superconducting coil cooled by liquid helium. Magnetic resonance of the proton magnetic moments—a quantum mechanical phenomenon—can be initiated by exciting the proton spin system to precession resonance (Figure (Figure4A) 4A ) by means of radio-frequency (RF) pulses of some milliseconds duration. This gives rise to a voltage signal with the resonance frequency ω 0 (Larmor frequency) which decays with the relaxation times T1 (longitudinal or spin-lattice relaxation time) and T2 (transversal or spin-spin relaxation time) which are characteristic for different chemical surroundings (see Figure Figure4B 4B ).

(A) Principles of magnetic resonance tomography (Birbaumer and Schmidt, 2010 ). (a) The patient is moved into the center of the MRI scanner. (b) A strong homogeneous magnetic field aligns the magnetic moments of the protons in in the patient's body. (c) An RF-pulse excites the proton magnetic moments to precession which gives rise to an alternating voltage signal in the detector. (d) After the switching-off the RF-pulse the proton magnetic moments relax to the initial orientation. The relaxation times (see B ) are measured. Reprinted with permission from Birbaumer and Schmidt ( 2010 ) © 2010 Springer. (B) Nuclear magnetic relaxation times T1 (top) and T2 (bottom) of hydrogen nuclei for various biological materials (Schnier and Mehlhorn, 2013 ). Reprinted with permission from Schnier and Mehlhorn ( 2013 ) © 2013 Phywe Systeme. (C) Spatial encoding of the local magnetic resonance information (Birbaumer and Schmidt, 2010 ). Due to a slicing (left) and finally a three-dimensional structuring (right) by means of gradient fields, the resonance frequency and the relaxation times can be assigned to a particular pixel. Reprinted with permission from Birbaumer and Schmidt ( 2010 ) © 2010 Springer.
A necessary condition for image generation is the exact information about the magnetic resonance signal's spatial origin. This spatial information is generated by additional site-dependent magnetic fields, called magnetic field gradients, along the three spatial axes. Due to these field gradients—much smaller in magnitude than the homogeneous main field—the magnetic field is grid-like (see Figure Figure4C) 4C ) slightly different in each volume element (voxel). As a consequence, the application of an RF pulse with the frequency ω' excites only the nuclear magnetic moment ensemble in voxels where the Larmor frequency ω 0 —given by the local magnetic field strength—matches the resonance condition. The signal intensity which is determined by the number of nuclear spins and the relaxation times characteristic for the particular tissue (Figure (Figure4B) 4B ) is assigned in this spatial encoding procedure to an element (pixel) in the three-dimensional image. The MRI scanner (Figure (Figure4A) 4A ) comprising the homogeneous magnetic field, the RF systems, and the gradient fields is controlled by a computer including fast Fourier-transform algorithms for frequency analysis.
Functional magnetic resonance imaging (fMRI) is based on the effect that in the case of activation of neurons by, e.g., musical stimuli, an oxygen (O 2 )-enrichment occurs in oxyhemoglobin which gives rise to an enhancement of the relaxation time T2 (Birbaumer and Schmidt, 2010 ) of the protons of this molecule and an enhancement of the magnetic resonance signal. This effect which enables active brain areas to be imaged is called BOLD (blood oxygen level dependent) effect.
By an increase of the magnetic field strength, the signal-to-noise ratio and thereby the spatial resolution can be enhanced.
Positron emission tomography (PET)
PET imaging is based on the annihilation of positrons with electrons of the body. The positrons are emitted from proton-rich radioactive atomic nuclei (see Table Table1) 1 ) which are embedded in specific biomolecules (Figure (Figure5A). 5A ). The positron-electron annihilation process gives rise to two high-energy (0.511 MeV) annihilation photons (Figure (Figure5B) 5B ) which can be monitored by radiation detectors around the body of the patient and thereby identify the site of the radioactive element. In a PET camera or PET scanner many detectors are implemented (Figure (Figure5B) 5B ) allowing for tomographic imaging with good spatial resolution of about 4 mm.
PET isotopes produced by high energy protons in a cyclotron accelerator.
see http://en.wikipedia.org/wiki/Positron_emission_tomography ; downloaded 22.12. 14 .

(A) Chemical formulae of two compounds doped with the positron emitters 18 F (left. http://de.wikipedia.org/wiki/Fluordesoxyglucose ; 19.12.14) and 11 C (right; http://www.ncbi.nlm.nih.gov/books/NBK23614/ 19.12.14) for PET scans. (B) Principles of positron emission tomography (PET). Left: A positron is emitted from a radioactive nucleus and annihilated with electrons of the tissue emitting two colinear annihilation photons which are monitored by radiation detectors and checked for coincidence. Right: Multi-detector PET scanner taking images (slices) of the concentration of positron emitting isotopes in the brain and thereby measuring the emotional activity of brain sections (Birbaumer and Schmidt, 2010 ). Reprinted with permission from Birbaumer and Schmidt ( 2010 ) © 2010 Springer.
Making use of fluorodeoxyglucose ( 18 F-FDG) doped with the radioactive fluorine isotope 18 F (Figure (Figure5A), 5A ), the local sugar metabolism in neurologically activated areas of the brain can be monitored (Figure (Figure5B). 5B ). After injection of 18 F-FDG into a patient, a PET scanner (Figure (Figure5B) 5B ) can form a three-dimensional image of the 18 F-FDG concentration in the body. For specifically probing molecular changes in postsynaptic monoamine receptors such as the dopamine receptor D 2 and the serotonin receptor 5-HT 2A , 11 C-N-methyl-spiperone (11C-MNSP, Figure Figure5A) 5A ) doped with the positron-emitting carbon isotope 11 C can be used. It should be pointed out here that the combination of MRI/PET (Bailey et al., 2014 ) represents an innovative imaging modality.
Experimental results of functional (tomographic) brain imaging (fMRI, PET)
Movements during listening to music.
Music is a universal feature of human societies, partly owing to its power to evoke strong emotions and influence moods. Understanding of neural correlates of music-evoked emotions has been invaluable for the understanding of human emotions (Koelsch, 2014 ).
Functional neuroimaging studies on music and emotion, such as fMRI and PET (see Figure Figure6A) 6A ) show that music can modulate the activity in brain structures that are known to be crucially involved in emotion, such as the amygdala and nucleus accumbens (NAc). The nucleus accumbens plays an important role in the mesolimbic system generating pleasure, laughter, reward but also fear, aggression, impulsivity, and addiction. The mesolimbic system is additionally intensely involved in emotional learning processes. Drugs can in this system effectuate the release of the neurotransmitter dopamine (Figure (Figure6B). 6B ). Neurotransmitters such as dopamine, serotonin, adrenaline, noradrenaline, or acetylcholine are biochemicals (see Figure 6B) which diffuse across a chemical synapse, bind to a postsynaptic receptor opening a sodium ion (Na + ) channel to transfer the excitation of a neuron to the neighboring neuron.

(A) Neural correlates of music-evoked emotions. A meta-analysis of brain-imaging studies that shows neural correlates of music-evoked emotions. A meta-analysis is a statistical analysis of a lager set of the analyses of earlier data. The meta -analysis indicates clusters of activities derived from numerous studies (for references see Koelsch, 2014 ) in the amygdala (SF, LB), the hippocampal formation (a), the left caudate nucleus with a maximum in the nucleus accumbens (NAc, b), pre-supplementary motor area (SMA), rostral cingulated zone (RCZ), orbifrontal cortex (OFC), and mediodorsal thalamus (MD, c), as well as in auditory regions (Heschls gyrus HG) and anterior superior temporal gyrus (aSTG, d). Additional limbic and paralimbic brain areas may contribute to music-evoked emotions. For details see Koelsch ( 2014 ). Reprinted with permission from Koelsch ( 2014 ) © 2014 Nature Publishing Group. (B) Structural formula of dopamine ( http://de.wikipedia.org/wiki/Dopamin ) downloaded19.12.14.
A meta-analysis of functional neuroimaging studies (fMRI, PET) of music-evoked emotions is shown in Figure Figure6A, 6A , including studies of music of intense pleasure, consonant or dissonant music, happy or sad music, joy- or fear-evoking music, muzak, expectancy violations, and music-evoked tension (for references see Koelsch, 2014 ).
In response to music, changes of the activity of the amygdala, the hippocampus, the right central striatum, the auditory cortex, the pre-supplementary motor area, the cingulate cortex, and the orbitofrontal cortex are observed (Figure (Figure6A). 6A ). In the following, the role of the amygdala, the nucleus accumbens and the hippocampus in music-evoked emotion is briefly discussed in more detail.
The amygdala is central in the emotion network and can regulate and modulate this network. It processes emotions such as happiness, anxiety, anger, annoyance, and, additionally assesses the impression of facial expression and thereby contributes to communication, social behavior, and memory (Kraus and Canlon, 2012 ). It, moreover, releases a number of neurotransmitters such as dopamine and serotonin, and effectuates reflexes such as being scared (Kraus and Canlon, 2012 ). The amygdala receives input from the central auditory system (Kraus and Canlon, 2012 ) and the sensory systems, and its pathways to the hypothalamus affect the sympathetic neuronal system for the release of hormones via the hypothalamus-pituitary-adrenal (HPA)-axis but also the parasympathetic neuronal system (Kraus and Canlon, 2012 ). The hormone cortisol and the neuropeptide endorphine have been observed in musical tasks 20 years ago (see Kreutz et al., 2012 ).
Fear conditioning is mediated by synaptic plasticity in the amygdala (Koelsch et al., 2006 ). It may affect the auditory cortex and its plasticity (learning) by a thalamus-amygdala-cullicular feedback circuit (Figure (Figure7A). 7A ). Neuronal pathways between the hippocampus and the amygdala allow for a direct interaction of emotion and declarative verbally describable memory and vice versa (Koelsch et al., 2006 ).

(A) Main pathways underlying autonomic and muscular responses to music. The cortex (AC) also projects to the orbifrontal cortex (OFC) and the cingulated cortex (projections not shown). Moreover, the amygdala (AMYG), the OFC and the cingulated cortex send numerous projections to the hypothalamus (not shown) and thus also exert influence on the endocrine system. ACC, anterior cingulate cortex; CN, cochlear nuclei; IC, inferior colliculus; M1, primary motor cortex; MCC, middle cingulate cortex; MGB, medial geniculate body; NAc, nucleus accumbens; PMC, premotor cortex; RCZ, rostral cingulated zone; VN, vestibular nuclei (Koelsch, 2014 ). Reprinted with permission from Koelsch ( 2014 ) © 2014 Nature Publishing Group. (B) Hippocampus. Reprinted with permission from Annie Krusznis © 2016.
The superficial amygdala is sensitive to faces, sounds, and music that is perceived as pleasant or joyful. Functional connections between the superficial amygdala, the nucleus accumbens (Figure (Figure7A), 7A ), and the mediodorsal thalamus are stronger during joy-evoking music than during fear-evoking music. The laterobasal amygdala shows activity changes during joyful or sad music. The connection of the amygdala to the hypothalamus affects the sympathetic neuronal system for the release of corticosteroid hormones via the HPS-axis and also affects the parasympathetic neural system (Kraus and Canlon, 2012 ). Functional magnetic resonance imaging (fMRI) (Koelsch et al., 2006 ) evidenced music-induced activity changes in the amygdala, ventral striatum and the hippocampal formation without the experience of “chills.” The study compared the brain responses of joyful dance-tunes by A. Dvorak and J. S. Bach (Figure (Figure8) 8 ) played by professional musicians with responses to electronically manipulated dissonant (unpleasant) variations of these tunes. Unpleasant music induced increases of the blood-oxygen-level dependent (BOLD) signals in the amygdala and the hippocampus in contrast to pleasant music giving rise to BOLD decreases in these structures. In a PET experiment (Blood and Zatorre, 2001 ) the participants' favorite CD music was used in order to induce “chills” or “shivers down the spine.” Increased chill intensity was observed in brain regions ascribed to reward and emotion such as the nucleus accumbens (NAc), in the anterior cingulate cortex (ACC) and the orbitofrontal cortex (see Figure Figure7A). 7A ). Decreases of the blood flow were observed in the amygdala and the anterior hippocampal formation with increasing chill intensity.

Joyful instrumental dance-tunes of major-minor tonal music by Dvorak ( 1955 ) and Bach ( 1967 ) used from commercially available CDs as pleasant stimuli in Koelsch et al. ( 2006 ). Reprinted with permission from Bach ( 1967 ) © 1967 Bärenreiter.
These observations demonstrated the modulation of the activities of the brain core structures ascribed to emotion processing by music. Furthermore, they gave direct support to the phenomenological efforts in music-therapeutic approaches for the treatment of disorders such as depression and anxiety because these disorders are partly ascribed to dysfunctions of the amygdala and presumably of the hippocampus (Koelsch and Stegemann, 2012 ) (see section Musical Therapy for Psychiatric or Neurologic Impairments and Deficiencies in Music Perception).
Nucleus accumbens (NAc)
The activities observed by functional neuroimaging in this brain section (see Figure Figure7A) 7A ) are initiated by “musical frissons,” involving experiences of shivers or goose bumps. This brain section is sensitive to primary rewards (food, drinks, or sex), consuming the rewards, and to addiction. This shows that music-evoked pleasure is associated with the activation of a phylogenetically old reward network that functions to ensure the survival of the individual and the species. The network seems to be functionally connected with the auditory cortex: while listening to music the functional connectivity between the nucleus accumbens and the auditory cortex predicts whether individuals will decide to buy a song (Salimpoor et al., 2013 ).
A PET study on musical frissons (Blood and Zatorre, 2001 ) making use of the radioactive marker 11 C-raclopride to measure the release of the neurotransmitter dopamine at synapses indicated that neural activity in the ventral and dorsal striatum involves increased dopamine availability, probably released by dopaminergic neurons in the ventral tegmental area (VTA). This indicates that music-evoked pleasure is associated with activation of the mesolimbic dopaminergic reward pathway.
Hippocampus
A number of studies on music-evoked emotions has reported activity changes in the hippocampus (see Figure Figure7B), 7B ), in striking contrast to the monetary or erotic rewards which do not activate the hippocampus (see Koelsch, 2014 ). This suggests that music-evoked emotions are not related to reward alone. Hippocampal activity was associated in some studies with music-evoked tenderness, peacefulness, joy, frissons or sadness and both, positive or negative emotions (for references see Koelsch, 2014 ). There is mounting evidence that the hippocampus is involved in emotion due to its role in the hippothalamus-pituitary-adrenal (HPA) axis stress response. The hippocampus appears to be involved in music-evoked positive emotions that have endocrine effects (see section Psychoneuroendocrinology—Neuroendocrine and Immunological Markers) associated with a reduction of emotional stress effectuated by a lowering of the cortisol (C 21 H 30 O 5 ) level which controls the carbon hydrate, fat, and protein metabolisms.
Another emotional function of the hippocampus in humans, beyond stress regulation, is the formation and maintenance of social attachments, such as, e.g., love. The evocation of attachment-related neurological activities by music appears to confirm the phenomenologically observed social functions of music establishing, maintaining, and strengthening social attachments. In this sense, music is directly related to the fulfillment of basic human needs, such as contact and communication, social cohesion and attachment (Koelsch, 2014 ). Some researchers even speculate that the strengthening of inter-individual attachments could have been an important adaptive function of music in the evolution of humans (Koelsch, 2014 ).
The prominent task of the hippocampal-auditory system is the long-term auditive memory. The downloading from the music memory activates the hippocampus predominantly on the right hemisphere (Watanabe et al., 2008 ). The hippocampus is, due to its projections to the amygdala, also involved in the emotional processing of music (Mitterschiffthaler et al., 2007 ). fMRI studies show an activation of the right hippocampus and the amygdala by sad music but not by happy or neutral music (Koelsch et al., 2006 ). Functional neuroimaging studies investigated how music influences and interacts with the processing of visual information (see Koelsch, 2014 ). These studies show that a combination of films or images with music expressing joy, fear, or surprise increase BOLD responses in the amygdala or the hippocampus (see Koelsch, 2014 ).
The hippocampus finds projections from the frontal, temporal and parietal lobes, as well as from the parahippocampal and the perirhinal cortices. The amygdala can modify the information storage processes of the hippocampus but, inversely, the reactions generated in the amygdala by external stimuli can be influenced by the hippocampus. These synergetic effects can contribute to the long-term storage of emotional events which is supported by the plasticity of the two units, enabling the acquisition of experience.
The degree of overlap between music-evoked emotions and so-called everyday emotions remains to be specified. Some musical emotions may appear in everyday life, such as surprise or joy. Some emotions are sought in music because they might be rare in everyday life, such as transcendence or wonder and some so-called moral emotions of everyday life, such as shame or guilt are lacking in music (Koelsch, 2014 ).
The molecular level of music-evoked neural processes can be achieved by making use of PET scans employing biomolecules doped with radioactive positron emitters. By using 11 C-N-methyl-spiperone ( 11 C-NMSP, see Figure Figure5A) 5A ) as an antagonist binding the postsynaptic dopamine receptor 2 (D 2 ) and the serotonin receptor 5-hydroxytriptamine2A (5-HT 2A , see Figure Figure9A), 9A ), acute changes of these neurotransmitter receptors in response to frightening music could be demonstrated (Zhang et al., 2012 ). Thus, the binding of 11 C-NMSP directly reflects the postsynaptic receptor level. Because the antagonist 11 C-NMSP binds predominantly D 2 in the striatum and 5-HT 2A in the cortex the antagonist can be used to map these receptors directly and simultaneously in the same individual (Watanabe, 2012 ). It is hypothesized (Zhang et al., 2012 ) that emotional processing of fear is mediated by the D 2 and the 5-HT 2A receptors. Frightening music is reported (Zhang et al., 2012 ) to rapidly arouse emotions in listeners that mimic those from actual life-threatening experiences.

(A) 5-hydroxytryptamine (serotonin) receptor 2A (5-HT 2A ), G protein coupled; diameter of the protein alpha-helix ~0.5 nm https://en.wikipedia.org/wiki/5-HT2A_receptor downloaded 4. 10. 2016. (B) PET images showing decrease in 11 C-NMSP binding clusters (arrows) in a subject listening to frightening music: right caudate head, right frontal subgirus, and right anterior cingulated (A); left lateral globus pallidus and left caudate body (B); right anterior cingulated (C); and right superior temporal gyrus, right claustrum, and right amygdala. (D) (Zhang et al., 2012 ). Reprinted with permission from Zhang et al. ( 2012 ) © 2012 SNMMI. (C) PET images showing increase in 11C-NMSP binding clusters (arrows) in a subject listening to frightening music: right frontal lobe and middle frontal gyrus (A); right fusiform gyrus and right middle occipital gyrus (B); right superior occipital gyrus, right middle occipital gyrus (C); and left middle temporal gyrus (D) (Zhang et al., 2012 ). Reprinted with permission from Zhang et al. ( 2012 ) © 2012 SNMMI.
However, studies of the underlying mechanisms for perceiving danger created by music are limited. The musical stimulus in the investigations on frightening music (Zhang et al., 2012 ) discussed here was selected from the Japanese horror film Ju-On which is widely accepted as one of the scariest and most influential movies ever made (Shimizu, 2004 ). The film music (see The Grudge theme song https://www.youtube.com/watch?v=1dqjXyIu02s ) has been composed by Shiro Sato.
For the PET scans (see Figures 9B,C ) 11 C-NMSP-activities of 740 MBq (20 mCi) were used. In the course of frightening music significant decreases in 11 C-NMSP binding was observed in the limbic and paralimbic brain regions in four clusters (Figure (Figure9B): 9B ): In the right caudate head, the right frontal subgyral region, and the right anterior cingulate region (A); the left lateral globus pallidus and left caudate body (B); the right anterior cingulate region (C); and the right superior temporal gyrus, right claustrum, and right amygdala (D). Increased 11 C-NMSP accumulation (Figure (Figure9C) 9C ) was found in the cerebral cortex, in the right frontal lobe and the middle frontal gyrus (A); the right fusiform gyrus and the right middle occipital gyrus (B); the right superior occipital gyrus, the right middle occipital gyrus, and the superior occipital gyrus (C); and the left middle temporal gyrus (D).
The decrease in the caudate nucleus in response to frightening music indicates that frightening music triggers a downregulation of postsynaptic D 2. This suggests that the caudate nucleus is involved in a wide range of emotional processes evoked by music (Zhang et al., 2012 ). The finding that the 11 C-NMSP binding decreases significantly (Figure (Figure9B) 9B ) during frightening music demonstrates the musical triggering of the monoamine receptors in the amygdala. It is assumed (Zhang et al., 2012 ) that changes of 11 C-NMSP binding (Figures 9B,C ) mainly reflect 5-HT 2A levels in the cortex, where 5-HT 2A overdensity is thought to be involved in the pathogenesis of depression (Eison and Mullins, 1996 ).
It should be additionally pointed out that the 11 C-NMSP PET study (Zhang et al., 2012 ) found the right hemisphere to have superiority in the processing of auditory stimuli and the defense reaction.
Movements of performing musicians
Brain activation of professional classical singers has been monitored by fMRI during overt singing and imagined singing of an Italian aria (Kleber et al., 2007 ). Overt singing (Figure 10A ) involved bilateral primary (A1) and secondary sensorimotor areas (SMA) and auditory cortices with Broca's and Wernike's areas but also areas associated with speech and language.

(A) Overt singing. The activation maps show activations of the bilateral sensorimotor cortex and the cerebellum, the bilateral auditory cortex, Broca's and Wernicke's areas, medulla, thalamus, and ventral striatum but also ACC and insula were activated. Coordinates of cuts are given above each slice (Kleber et al., 2007 ). Reprinted with permission from Kleber et al. ( 2007 ) © 2007 Elsevier. (B) Mental rehearsal of singing (imaginary singing). Activation of typical imagery regions such as sensorimotor areas (SMA), premotor cortex areas, thalamus, basal ganglia, and cerebellum. Areas processing emotions showed intense activation (ACC and insula, hippocampus, amygdala, and ventrolateral prefrontal cortex). Coordinates of cuts are given above each slice (Kleber et al., 2007 ). Reprinted with permission from Kleber et al. ( 2007 ) © 2007 Elsevier.
Activation in the gyri of Heschl occurred in both hemispheres, together with the subcortical motor areas (cerebellum, thalamus, medulla and basal ganglia) and slight activation in areas of emotional processing (anterior cingulate cortex, anterior insula). Imagined singing (Figure 10B ) effectuated cerebral activation centered in fronto-parietal areas and bilateral primary and secondary sensorimotor areas. No activation was found in the primary auditory cortex or in the auditory belt area. Regions processing emotion showed intense activation (anterior cingulate cortex—ACC, insula, hippocampus, and amygdala).
Performing music in one's mind is a technique commonly used by professional musicians to rehearse. Composers write music regardless of the presence of a musical instrument, as, e.g., Mozart or Schubert did (see Kleber et al., 2007 ). Singing of classical music involves technical-motor and emotional engagement in order to communicate artistic, emotional, and semantic aspects of the song. A tight regulation of pitch, meter, and rhythm as well as an increased sound intensity and vocal range, vibrato and a dramatic expression of emotion are indispensible. Motor aspects of these requirements are reflected in a fine laryngeal motor control and a high involvement of the thoracic muscles during singing. The aria used in this study (Kleber et al., 2007 ) comprises text, rhythm, and melody which make the bilateral activation of A1 plausible.
For the study of music-evoked emotions during performing in the fMRI scanner the bel canto aria Caro mio ben by Tommaso Giordani (1730-1806) has been used (Kleber et al., 2007 ).
Interestingly, most areas involved in motor processing were activated both during overt singing and imaginary singing, a finding that may demonstrate the significance of imagined rehearsal. The basal ganglia which were active in both overt and imaginary singing may be involved in the modulation of the voice. The overt singing task activated only the ACC and the insula which were both also activated during imaginary singing. The ACC is involved in the recall of emotions (Kleber et al., 2007 )—a capability which is important for both overt and imaginary performance. The activation of the insula seems to reflect the intensity of the emotion. The amygdala which was only activated by imagined singing is known to be involved in passive avoidance or approach tasks. This is reported (Kleber et al., 2007 ) to be consistent with the observation that the amygdala was not active during overt singing. Imagined singing activated a large fronto-parietal network, indicating increased involvement of working memory processes during mental imagery which in turn may indicate that imagined singing is less automatized than overt singing (Kleber et al., 2007 ). Areas processing emotions showed also enhanced activation during imagined singing which may reflect increased emotional recall during this task.
An overview of the sensory-motor control of the singing voice has been given based on fMRI research of somatosensory and auditory feedback processing during singing in comparison to theoretical models (Zarate, 2013 ).
Movement organization that enables skilled piano performance has been recently reviewed, including the advances in diagnosis and therapy of movement disorders (Furuya and Altenmüller, 2013 ).
Psychoneuroendocrinology—neuroendocrine and immunological markers
Psychoneuroendocrinology (PNE) aims at the study of the musical experiences leading to hormonal changes in the brain and the body. These effects may be similar to those effectuated by pharmacological substances. In addition to investigating psychiatric illnesses and syndromes, PNE investigates more positive experiences such as the neurobiology of love (see Kreutz et al., 2012 ). In contrast to the neuronal system which transmits its messages by electrical signals, the endocrinal system makes use of biomolecules, such as hormones in order to communicate with the target organs which are equipped with specific receptors for these hormones (see Birbaumer and Schmidt, 2010 ).
For considering the neuroendocrine and immunological molecular markers which could be released during music-evoked emotion, the three interrelated systems regulating hormonal stress responses should be briefly introduced:
The hypothalamic-pituitary-adrenocortical axis (HPA). This axis is initiated by a stimulus in the brain area of the hypothalamus giving rise to the release of the corticotropin releasing factor (CRF) which in turn leads to the release of adrenocorticotropic hormone (ACTH) and beta-endorphin from the pituitary into the circulation. ACTH then stimulates the synthesis and release of cortisol and of testosterone from the adrenal cortex.
Beta-endorphin (see Figure Figure11) 11 ) is a hormone where increased concentration levels are associated with situative stress. Delivering special relaxation music to coronary patients leads to significant decrease of beta-endorphin concentration with a simultaneous reduction of blood pressure, anxiety and worry. Music therapy can also be effective before and during surgeries in operating theaters, again due to a reduction of the beta-endorphin level (see Kreutz et al., 2012 ).

Neuroendocrine and immunological molecular markers released during music- evoked emotion (see Kreutz et al., 2012 ). The molecular masses are given in kDa = 1.66 × 10 −24 kg. http://en.wikipedia.org/wiki/Beta-endorphin#mediaviewer/File:Betaendorphin.png ; http://de.wikipedia.org/wiki/Cortisol ; http://de.wikipedia.org/wiki/Testosteron ; http://de.wikipedia.org/wiki/Prolaktin ; http://de.wikipedia.org/wiki/Oxytocin ; http://en.wikipedia.org/wiki/Immunoglobulin_A downloads 20.12.2014.
Cortisol (see Figure Figure11) 11 ) is a hormone where high levels of concentration are associated with psychological and physiological stresses. Listening to classical choral, meditative, or folk music significantly reduces the cortisol level, however, increases have been detected for listeners exposed to Techno (see Kreutz et al., 2012 ). Individual differences were evidenced in listening experiments where music students responded with increases and biology students with decreases of the cortisol levels. Changes of the cortisol concentration can also be induced by actively singing. In clinical context, exposure to music has been shown to reduce cortisol levels during medical treatment. In gender studies cortisol reductions were found in females in contrast to males, exhibiting increases. Little is known about the sustainability of these effects over a longer period of time (see Kreutz et al., 2012 ).
Testosterone (see Figure Figure11), 11 ), a sex hormone, appears to be of particular relevance to music. Darwin ( 1871 ; see Kreutz et al., 2012 ) suggests music as originating from sexual selection. Female composers showed above average and male composers below average testosterone levels which has initiated discussions whether physiologically androgynous individuals are on a higher level of creativity.
Secretory immunoglobulin A (sIgA; see Figure Figure11) 11 ) is an antibody considered as a molecular marker of the local immune system in the respiratory tract and as a first line of defense against bacterial and viral infections. High levels of sIgA may exert positive effects and low levels may be characteristic for chronic stress. Significant increases of sIgA concentrations were observed in response to listening to relaxation music or musak. Increases of the sIgA concentration were observed from rehearsal to public performance of choral singers (Kreutz et al., 2012 ).
Another study investigated the concentration of prolactin (see Figure Figure11) 11 ) while listening to music of Hans-Werner Henze. The concentration of prolactin which is a hormone with important regulatory functions during pregnancy decreased in response to Henze (Kreutz et al., 2012 ).
It should be summarized that the neuroendocrine changes reflecting the psychophysiological processes in response to music appear to be complex but might promise favorable effects with respect to health implications deserving enhanced research activities.
The simpatho-adrenomedullary system is part of the sympathetic nervous system executing fight and flight responses. By, e.g., stress activation, norepinephrine is released. Sympathetic enervations of the medulla of the adrenal glands give rise to the secretion of the catecholamines (dopamine, epinephrine, norepinephrine). Since this works by nervous operation of the adreanal gland it responds much faster than the HPA which is regulated by hormonal processes.
The endogeneous opioid system is related to the HPA axis and can influence the ACTH and cortisol levels in the blood (see Kreutz et al., 2012 ). None of these three responses is specific to one kind of challenge and the response delays vary to a great deal.
There is an increasing interest in PNE research for studying musical behavior due to the increasing specificity of neuroendocrinological research technologies. It is likely that musical behaviors significantly influence neurotransmitter processes.
Whether music processing can be associated with the processing of, e.g., linguistic sound is a matter of debate (Kreutz et al., 2012 ). However, functional imaging brain studies suggest that the perception of singing is different of the perception of speech since singing evokes stronger activations in the subcortical regions which are associated with emotional processing (see Kreutz et al., 2012 ).
Experiments are suggested (Chanda and Levitin, 2013 ) that aim to uncover the connection between music, the neurochemical changes in the following health domains
- Reward, motivation, and pleasure,
- Stress and arousal,
- Immunity, and
- Social affiliation,
and the neurochemical systems
- Dopamine and opioids,
- Cortisol, adrenocorticotropic hormone (ACTH)
- Serotonin, and
- And the “love” drug oxytocin (see Figure Figure11 11 ).
Electro- and magnetoencephalography (EEG, MEG)
Electroencephalography (eeg) and event-related brain potentials (erp).
This technique yields valuable information on the brain—behavior relationship on much shorter time scales (ms) than tomography, however, with limited spatial information.
Measurements of electrical potentials are performed making use of an array of voltage probes on the scalp. The EEG arises due to electrical potential oscillations in the brain, i.e., by excitatory postsynaptic potentials. Cortical afferences of the thalamus activate the apical dendrities (see Figure Figure12). 12 ). Compensating extracellular electrical currents (Figure (Figure12) 12 ) generate measurable potentials on the scalp with characteristic oscillations in the frequency range of about 4–15 Hz (Birbaumer and Schmidt, 2010 ). Event-related brain potentials (ERPs) are of particular interest in the present context of considering music-evoked emotions (Neuhaus, 2013 ). By synchronized averaging of many measurements, the ERPs are extracted from noise showing a sequence of characteristic components which can be ascribed to separate phases of cognitive processes. Slow negative potentials (100–600 ms) are thought to be generated by cortical cholinergic synapses with high synchronization of pulses at the apical dendrites (see Figure Figure12). 12 ). Positive potentials may be due to a decrease of the synchronization of the thalamic activity (Birbaumer and Schmidt, 2010 ).

Negative surface slow brain potentials on the skalp are generated by extracellular currents (red dashed arrows) which arise due to the electrical activation of apical dendrites by thalamocortical afferences (Birbaumer and Schmidt, 2010 ). Reprinted with permission from Birbaumer and Schmidt ( 2010 ) © 2010 Springer.
The interpretation of single ERP components as correlates of processing specific information is on a phenomenological stage. Up to 300 ms the components are ascribed to unconscious (autonomous) processing. Changes of consciousness can be attributed to components from 300 ms and higher (Birbaumer and Schmidt, 2010 ).
An impressive neurocognitive approach to musical form perception has been presented recently by ERP studies (Neuhaus, 2013 ). The study investigates the listeners' chunking abilities of two eight-measure theme types AABB and ABAB for pattern similarity (AA) and pattern contrast (AB). In the experiments a theme type of eight measures in length (2+2+2+2), often found in the Classical and Romantic periods, was used. In addition to behavioral rating considerations, ERP measurements were performed while non-musicians listened. The advantage of ERP, compared to the more direct neuroimaging techniques such as PET and fMRI, is the good time resolution in range of about 10 ms.
The experiments were performed on 20 students without musical training. The tunes were presented in various transpositions so that the tonality has not to be considered as an independent parameter. Each melody of the AABB or ABAB form types used the harmonic scheme tonic—dominant—tonic. The melodies with an average duration of 10.8 s and form part length of 2.7 s were presented from a programmable keyboard with a tempo of 102.4 BPM. The brain activity was measured making use of 59 Ag/AgCl electrodes with an impedance below 5 Ω.
In the behavioral studies the sequence ABAB is more often assessed as non-sequential than the sequence AABB. The tendency to recognize chunk form parts was high with the two following aspects coinciding: Rhythmic contrasts in A and B and when the melodic contour was upward- downward.
In grand average ERPs, an anterior negative shift N300 for immediate AA sequences as well as for non-immediate repetitions ABA or ABAB of similar form parts was observed suggesting pattern matching at phrase onsets based on rhythmical similarity. In the discussion of the grand average the most interesting feature is the negative shift in the time range 300–600 ms with a maximum in the fronto-central brain. This is ascribed to recognition of pattern similarity at phrase onsets with exactly the same rhythmical structure. The maximum amplitudes measured in the frontal parts of the brain suggest that non-expert listeners use the frontal part working memory for musical pattern recognition processes.
Magnetoencephalography (MEG)
Weak magnetic fields which can be detected on the scalp are generated by the electrical currents in the brain (Figure 13A ). By measuring these magnetic fields by a highly sensitive detector (Figure 13B ), a tomographic image (MEG) of the brain activities can be reconstructed. The brain comprises about 2 × 10 10 cells and about 10 14 synapses. The dendritic current in the cell (see Figure 13A ) generally flows perpendicular to the cortex (Figure 13A ). In the case of the sulcus, this gives rise to a magnetic field in parallel to the scalp which is suggested to be detected outside when about 100,000 cells contribute, e.g., in the auditory cortex, with a spatial resolution of about 2–3 mm (Vrba and Robinson, 2001 ).

(A) Origin of the MEG signal. (a) Coronal section of the human brain with the cortex in dark color. The electrical currents flow roughly perpendicular to the cortex. (b) In the convoluted cortex with the sulci and gyri the currents flow either radially or tangentially (c) or radially (d) in the head. (e) The magnetic fields generated by the tangential currents can be detected outside the head (Vrba and Robinson, 2001 ). Reprinted with permission from Vrba and Robinson ( 2001 ) © 2001 Elsevier. (B) (a) Magnetoencephalography facility containing 150 magnetic field sensors. (b) SQUIDs (superconducting quantum interference devices) and sensors immersed for cooling in liquid helium contained in a Dewar vessel (cross section) (Birbaumer and Schmidt, 2010 ). Reprinted with permission from Birbaumer and Schmidt ( 2010 ) © 2010 Springer. (C) Cortical stimulation by pure and piano tones . Left : Medial–lateral coordinates are shown for single equivalent current dipoles fitted to the field patterns evoked by pure sine tones and piano tones in control subjects. The inset defines the coordinate system of the head. Right : Equivalent current dipoles (ECD) shift toward the sagittal midline along the medial–lateral coordinate as a function of the frequency of the tone. Ant–post, anterior–posterior; med–lat, medial–lateral; inf–sup, inferior–superior (Pantev et al., 1998 ). Reprinted with permission from Pantev et al. ( 1998 ) © 2001 Nature Publishing Group.
The brain magnetic fields (10 −13 Tesla) are much smaller than the earth magnetic field (6.5 × 10 −5 Tesla) and much smaller than the urban magnetic noise (10 −6 Tesla) (Vrba and Robinson, 2001 ). The only detectors resolving these small fields are superconducting quantum interference devices (SQUIDs) based on the Josephson effect (see Figure 13B ). The SQUIDs are coupled to the brain magnetic fields using combinations of superconducting coils called flux transformers (primary sensors, see Figure 13B ).
One of the most successful methods for noise elimination is the use of synthetic higher-order gradiometers. A number of approaches is available for image reconstruction of the MEG signals. Present MEG systems incorporate several hundred sensors in a liquid helium helmet array (see Figure 13B ).
By MEG scanning, neuronal activation in the brain can be monitored locally (Vrba and Robinson, 2001 ). Acoustic stimuli are processed in the auditory cortex by neurons that are aggregated into “tonotopic” maps according to their specific frequency tunings (see Pantev et al., 1998 ). In the auditory cortex, the tonotopic representation of the cortical sources corresponding to tones with different spectral content distributes along the medial-lateral axis of the supratemporal lane (see Figure 13C , left), with the medial-lateral center of the cortical activation shifting toward the sagittal midline with increasing frequency (see Figure 13C , right). This shift is less pronounced for a piano tone than for a pure sine tone. In this study, it could be additionally shown that dipole moments for piano tones are enhanced by about 25% in musicians compared with control subjects who had never played an instrument (Pantev et al., 1998 ). In the evaluation of the MEG data, for each evoked magnetic field a single equivalent current dipole (ECD) of about 50 nA was derived by a fit. From that a contribution of ~150,000 dendrites to this magnetic field can be estimated (Pantev et al., 1998 ). The coordinates of the dipole location were calculated satisfying the requirements of an anatomical distance of the ECD to the midsagittal plane of >2 cm and an inferior-superior value of >2 cm.
Skin conductance response (SCR) and finger temperature
In a study of the relationship of the temporal dynamics of emotion and the verse-chorus form of five popular “heartbreak” songs, the listeners' skin conductance responses (SCR; Figure 14A ) and finger temperatures (Figure 14B ) were used to infer levels of arousal and relaxation, respectively (Tsai et al., 2014 ). The passage preceding the chorus and the entrance of the chorus evoked two significant skin conductance responses (see Figure 14A ). These two responses may reflect the arousal associated with the feelings of “wanting” and “liking,” respectively. Brain-imaging studies have shown that pleasurable music activates the listeners' reward system and serves as an abstract reward (Blood and Zatorre, 2001 ). The decrease of the finger temperature (Figure 14A ) within the first part of the songs indicated negative emotions in the listeners, whereas the increases of the finger temperature within the second part may reflect a release of negative emotions. These findings may demonstrate the rewarding nature of the chorus and the cathartic effects associated with the verse-chorus form of heart-break songs.

(A) The median curve of the skin conductance response (SCR) amplitude around the entrance of the chorus. The first downbeat was set to t = 0 s (Tsai et al., 2014 ). The two peaks are ascribed to the two closely related phases of listening experience: anticipatory “wanting” and hedonic “liking” of rewards. Reprinted with permission from Tsai et al. ( 2014 ) © 2014 Sage. (B) The u-shaped time-dependence of the finger temperatures of the listeners during presentation of the five songs. The end of the first chorus (see full dots) devides each song into two parts with a decrease of the finger temperature in the first part and an increase in the second part (Tsai et al., 2014 ). Reprinted with permission from Tsai et al. ( 2014 ) © 2014 Sage. The symbols *** and * indicate that the two peaks are significantly larger than the control data.
Goose bumps—piloerection
The most common psychological elicitors of piloerection or chills are moving music passages, or scenes in movies, plays, or books (see Benedek and Kaernbach, 2011 ). Other elicitors may be heroic or nostalgic moments, or physical contact with other persons. In Charles Darwin's seminal work on The expression of emotions in Man and Animals (1872), he already acknowledged that “…hardly any expressive movement is so general as the involuntary erection of the hairs…” (Darwin, 1872 ). Musical structures for triggering goose bumps or chills are considered to be crescendos, unexpected harmonies, or the entry of a solo voice, a choir, or a an additional instrument. It thus was concluded that piloerection may be a useful indicator which marks individual peaks in emotional arousal. Recently optical measuring techniques have been developed for monitoring and analyzing chills by means of piloerection (Benedek et al., 2010 ).
Additional experimental studies had shown that chills gave rise to higher skin conduction, increased heart and respiratory rates, and an enhancement of skin temperature (see Benedek and Kaernbach, 2011 ). Positron emission tomography correlated to musical chills showed a pattern typical for processes involved in reward, euphoria, and arousal, including ventral striatum, midbrain, amygdala, orbitofrontal cortex, and ventral medial prefrontal cortex (see Benedek and Kaernbach, 2011 ).
In the studies of piloerection as an objective and direct means of monitoring music-evoked emotion, music pieces ranging from 90 s (theme of Pirates of the Caribbean ) to 300 s ( The Scientist ). Film audio tracks ( Knocking on Heavens Door, Dead Poets Society ) ranging from 141 to 148 s were employed. All musical stimuli were averaged to the same root mean square power (RMS), so that they featured equal average power.
Half of the musical stimuli ( My Heart will go on by Celine Dion, Only Time by Enya, and film tracks of Armageddon and Braveheart ) was pre-selected by the experimenter and half, with stronger stimulation, was self-selected by the 50 participants. The stimuli were presented via closed Beyerdynamic DT 770 PRO head-phones (Heilbronn, Germany) at an average sound pressure level of 63 dB. The procedure was approved by the Ethics Committee of the German Psychological Society (Benedek and Kaernbach, 2011 ). The sequence of a measurement is depicted in Figure 15A .

(A) Time-dependence of the relative piloerection intensity of a single experiment, including a baseline period (30 s), stimulus description (20 s) and stimulus presentation (variable duration). The initial stable level of piloerection intensity indicates no visible piloerection. In this experiment, piloerection occurs shortly after the onset of stimulus presentation; after some time it fades away. The asterisk marks the first detected onset of piloerection. This time is used for the short-term physiological response (Benedek and Kaernbach, 2011 ). Reprinted with permission from Benedek and Kaernbach ( 2011 ) © 2011 Elsevier. (B) Procedure of piloerection quantification without (top row) and with visible piloerection (bottom row). From B (bottom) a two-dimensional spatial Fourier transform is computed (C, shown for the frequency range ±1.13 mm −1 ) which is converted to a one-dimensional spectrum of spatial frequency. The maximum spectral power in the 0.23–0.75 mm −1 range (D) is considered as a correlate of the piloerection intensity (Benedek et al., 2010 ). Reprinted with permission from Benedek et al. ( 2010 ) © 2010 Wiley. (C) Time dependence of the short-term response of physiological measurements for a time slot of −15 s to +15 s around the first onset of piloerection. Dark bars indicate significant deviations from zero, white bars indicate non-significant deviations. ISCR-integrated skin conductance response, SCL-skin conductance level, HR-heart rate, PVA-pulse volume amplitude, RR-respiration rate, RD- respiration depth (Benedek and Kaernbach, 2011 ). Reprinted with permission from Benedek and Kaernbach ( 2011 ) © 2011 Elsevier.
The formation of piloerection on the forearm was monitored by a video scanner with a sampling rate of 10 Hz, with simultaneous measurements of the skin conductance response and the increased heart and respiratory rates. By means of the Gooselab software the spatial Fourier transform (Figure 15B ) of a video scan (Figure 15B ) is derived which is a measure of the intensity of piloerection.
Piloerection could not always be detected objectively when indicated by the participant and was sometimes detected without an indication by the participant.
Piloerection starts with the onset of music (Figure 15A ), then increases with a time constant of ~20 s and then fades off (time constant about 10 s). An analysis of the time constants of piloerection and of the kinetics of the simultaneously monitored physiological reactions (Figure 15C ), should provide us with specific information on the neuronal and muscular processes contributing. This has not been discussed up to now. In the physiological quantities (Figure 15C ) studied simultaneously with piloerection, a significant increase in skin conductance response, in heart rate, and in respiration depth has been observed. This demonstrates that a number of subsystems of the sympathetic neuronal system can be activated by music and that in particular listening to film sound tracks initiates a physiological state of intense arousal (Benedek and Kaernbach, 2011 ). Based on the experimental studies of piloerection and physiological quantities (Benedek and Kaernbach, 2011 ), two models of piloerection are discussed (Benedek and Kaernbach, 2011 ): On the one hand, it had been argued that the appearance of piloerection may mark a peak in emotional arousal (see Grewe et al., 2009 ). On the other hand, the psychobiological model (Panksepp, 1995 ) conceives emotional piloerection as an evolutionary relic of thermoregulatory response to an induced sensation of coldness and links it with the emotional quality of sadness (separation call hypothesis) (Panksepp, 1995 ). By comparing the physiological patterns of the two approaches to the experimental results, the authors (Benedek and Kaernbach, 2011 ) favor the separation call hypothesis (Panksepp, 1995 ) to the hypothesis of peak arousal (Grewe et al., 2009 ).
Is there a biological background for the attractiveness of music?—genomic studies
In a recent genomic study, the correlation of the frequency of the listening to music and the availability of the arginine vasopressin receptor 1A (AVPR1A) gene or haplotype (with a length of 1,472 base pairs) has been investigated. A haplotype is a collection of particular d eoxyribonucleic acid (DNA) sequences in a cluster of tightly-linked genes on a chromosome that are likely to be inherited together. In this sense, a haplotype is a group of genes that a progeny inherits from one parent [ http://en.wikipedia.org/wiki/Haplotype ]. The AVPR1A gene encodes for a receptor molecule amino peptide that mediates the influence of the arginine vasopressin (AVP) hormone in the brain which plays an important role in memory and learning [ http://en.wikipedia.org/wiki/Haplotype ]. AVPR1A has been shown to modulate the social cognition and behavior, including social bonding and altruism in humans (Wallum et al., 2008 ). However, in contrast to that, the AVPR1A gene has also been referred to as the “ruthlessness gene” (Hopkin, 2008 ).
Recently an association of the AVPR1A gene with musical aptitude and with creativity in music, e.g., composing and arranging of music, has been reported (see Ukkola-Vuoti et al., 2011 ). In this study (Ukkola-Vuoti et al., 2011 ) a total of 31 Finnish families with 437 family members (mean age 43 years) participated. The musical aptitude of the individuals was tested by means of the Karma test. In this test, which does not depend on training in music, musical aptitude is defined as the ability of auditory structuring (Karma, 2007 ). In addition, the individual frequency of music listening was registered. Genomic DNA was extracted from peripheral blood of the individuals for the determination of the AVPR1A gene. The AVPR1A gene showed strongest association with current active music listening which is defined as attentive listening to music, including attending concerts. No dependence of the musical aptitude was discovered. These results appear to indicate a biological background for the attractiveness of music. The association with the AVPR1A gene suggests that listening to music is related to the neural pathways affecting attachment behavior and social communication (Ukkola-Vuoti et al., 2011 ).
Towards a theory of musical emotions
In a recent overview (Juslin, 2013 ) aimed at a unified theory of musical emotions, a framework is suggested that tries to explain both the everyday emotions and aesthetic emotions, and yields some outlines for future research. This model comprises eight mechanisms for emotion by music—referred to as BRECVEMA: Brain stem reflexes, Rhythmic entrainment, Evaluative conditioning, Contagion, Visual imagery, Episodic memory, Musical expectancy, and Aesthetic judgment. The first seven mechanisms (BRECVEM) arousing the everyday emotions, are each correlated (see Juslin, 2013 ) to the evolutionary order, the survival value of the brain functions, the information focus, the mental representation, the key brain regions identified experimentally, the cultural impact, the ontogenetic development, the induced effect, the temporal focus of the effect, the induction speed, the degree of volitional influence, the availability of consciousness, and the dependence of musical structure.
Of particular significance is the addition of a mechanism corresponding to aesthetic judgments of music, in order to better account for typical appreciation emotions such as admiration and awe.
Aesthetic judgments have not received much attention in psychological research to date (Juslin, 2013 ) since aesthetic and stylistic norms and ideas change over time in society. Though it may be difficult to characterize aesthetic judgments, some preliminaries are offered (Juslin, 2013 ) as to how a psychological theory of aesthetic judgment in music experience might look like.
Some pieces of music will invite an aesthetic attitude of the listener due to perceptual inputs by sensory impressions, due to more knowledge-based cognitive inputs, or due to emotional inputs. Some criteria that may underlie listeners' aesthetic judgments of music are suggested (Juslin, 2013 ) such as beauty, wittiness, originality, taste, sublimity, expression, complexity, use as art, artistic skill, emotion arousal, message, representation, and artistic intention. Certain criteria such as expression, emotional arousal, originality, skill, message, or beauty were considered as more important than others (see Figure 16A ) and different listeners tend to focus on different criteria (see Figure 16B ). With its multi-level framework of everyday emotions and aesthetic judgment, the study (Juslin, 2013 ) might help to explain the occurrence of mixed emotions such as bitter-sweet combinations of joy and melancholy.

(A) Mean values and standard errors for listeners' ratings of criteria for aesthetic value of music. (B) Individual ratings of criteria for aesthetic value of music by four subjects (see Juslin, 2013 ). Reprinted with permission from Juslin ( 2013 ) © 2013 Elsevier.
This discussion suggests (Juslin, 2013 ) that researchers have to elaborate specific experimental paradigms that reliably arouse specific emotions in listeners through each of the mechanisms mentioned, including the empirical exploration of candidate-criteria for aesthetic value, similarly to what has been performed for various BRECVEM mechanisms. Empirical research so far has primarily focused on the beauty criterion (see Juslin, 2013 ). Developments of hypotheses for the criteria such as style appreciation, neural correlates of perceived expressivity in music performances, or perceptual correlates of novelty appear feasible (Juslin, 2013 ). An additional possibility could be the use of a neurochemical interference strategy (Chanda and Levitin, 2013 ; Juslin, 2013 ). It has been shown that blocking of a specific class of amino acid receptors in the amygdala can interfere with the acquisition of evaluative conditioning (see Juslin, 2013 ) discussed within BRECVEM. Interactions between BRECVEM mechanisms and aesthetic judgments have yet to be investigated.
Musical therapy for psychiatric or neurologic impairments and deficiencies in music perception
Mounting evidence indicates that making music or listening to music activates a multitude of brain structures involved in cognitive, sensorimotor, and emotional processing (see Koelsch and Stegemann, 2012 ). The present knowledge on the neural correlates of music-evoked emotions and their health-related autonomic, endocrinological, and immunological effects could be used as a starting point for high-quality investigations of the beneficial effects of music on psychological and physiological health (Koelsch and Stegemann, 2012 ).
Music-evoked emotions can give rise to autonomic and endocrine responses as well as to motoric expression of motion (facial expression). The evidence that music improves health and well-being through the engagement of neurochemical systems for (i) reward, motivation and pleasure; (ii) stress and arousal; (iii) immunity; and (iv) social affiliation has been reviewed (Chanda and Levitin, 2013 ). From these observations, criteria for the potential use of music in therapy should be derived.
Dysfunctions and structural abnormalities in, e.g., the amygdala, hippocampus, thalamus, nucleus accumbens, caudate, and cingulate cortex are characteristic in pychiatric and neurological disorders, such as depression, anxiety, stress disorder, Parkinson's disease, schizophrenia, and neurodegenerative diseases. The findings that music can change the activity in these structures should encourage high-quality studies (see Koelsch, 2014 ) of the neural correlates of the therapeutic effects of music in order to provide convincing evidence for these effects (Drevets et al., 2008 ; Maratos et al., 2008 ; Omar et al., 2011 ). The activation of the amygdala and the hippocampal formation by musical chills as demonstrated in PET scans (Blood and Zatorre, 2001 ) may give direct support to the phenomenological efforts in music-therapeutic approaches for the treatment of disorders such as depression and anxiety because these disorders are partly ascribed to dysfunctions of the amygdala and presumably of the hippocampus (Koelsch and Stegemann, 2012 ).
Another condition in which music should have therapeutic effects is autism spectrum disorder (ASD). Functional MRI studies show (Caria et al., 2011 ) that individuals with ASD exhibit relatively intact perception and processing of music-evoked emotions despite their deficit in the ability to understand emotions in non-musical social communication (Lai et al., 2012 ). Active music therapy can be used to develop communication skills since music involves communication capabilities (Koelsch, 2014 ).
With regard to neurodegenerative disorders, some patients with Alzheimer's disease (AD) have almost preserved memory of musical information for, e.g., familiar or popular tunes. Learning of sung lyrics might lead to better retention of words in AD patients and anxiety levels of these patients can be reduced with the aid of music. Because of colocalization of memory functions and emotion in the hippocampus, future studies are suggested to more specifically investigate how music is preserved in AD patients and how it can ameliorate AD effects (Cuddy et al., 2012 ) and other neurodegenerative diseases such as Parkinson's disease (Nombela et al., 2013 ). In addition, music-therapeutical efforts for cancer (Archie et al., 2013 ) or stroke (Johansson, 2012 ) have been reported.
Music has been shown to be effective for the reduction of worries and anxiety (Koelsch and Stegemann, 2012 ) as well as for pain relief in clinical settings with, however, minor effects compared to analgesic drugs (see Koelsch, 2014 ). Deficiencies in music perception are reported for patients with cerebral degeneration or damage (Koelsch, 2014 ). Recognition of music expressing joy, sadness, anger, or fear is impaired in patients with frontotemporal lobar degeneration or damage of the amygdala (Koelsch, 2014 ). Patients with lesions in the hippocampus find dissonant music pleasant in contrast to healthy controls who find dissonance unpleasant. The degree of overlap between music-evoked emotions and so-called everyday emotions remains to be specified.
Conclusions and outlook
As shown by tomographic imaging (fMRI, PET), which exhibits a high spatial resolution, activation of various brain areas can be initiated by musical stimuli. Some of these areas can be correlated to particular functions such as motor or auditive functions activated by non-musical stimuli. In the case of fMRI, emotion processing is identified by the more general feature of local energy consumption. Imaging of emotional processing on a molecular level can be achieved by PET, where specific molecules such as 11 C-NMSP have been employed (Zhang et al., 2012 ) for a targeted investigation of synaptic activity (Zhang et al., 2012 ). A powerful combination of specific detection of molecules and tomographic imaging of the brain could arise from a future development of Raman tomography (Demers et al., 2012 ). Raman scattering provides specific information on the characteristic properties of molecules, such as vibrational or rotational modes.
Development of the technically demanding tomographic methods (fMRI, PET, MEG) for easy use would be highly desirable for the investigation of the emotions of performing musicians or even the astounding sensations of composers while composing, as, e.g., expressed by Ennio Morricone, composer of the music of the film Once upon a time in the West (Spiel mir das Lied vom Tod, 1968): “Vermutlich hat der Komponist, während er ein Stück schreibt, nicht mal die Kontrolle über seine eigenen Emotionen” (Morricone, 2014, Jun 1 ). (The composer, when witing a piece, is probably not even in control of his own emotions). Jörg Widmann, composer of the contemporary opera Babylon (2012), formulates: “Man gerät beim Schreiben in extreme Zustände, kann nicht schlafen, macht weiter in einer Art Rausch – und Rausch ist womöglich der klarste Zustand überhaupt.” (Widmann, 2014, August 20 ) (When composing one gets into extreme states, cannot sleep, continues in a sort of drunkenness—and drunkenness is perhaps the clearest possible state).
Future studies on a targeted molecular level may deepen the understanding of music-evoked emotion. Novel microscopy technologies for investigating single molecules are emerging. The rapid fusion of synaptic vesicles for neurotransmission after optical stimulation has been observed by cryo electron microscopy (Chemistry Nobel Prize 2017) with an electron energy of 200 keV where radiation damage appears tolerable and on a time scale of 15 ms (Watanabe et al., 2013 ) (see Figure 17A ). Radiation damage can be entirely suppressed by combining electron holography and coherent electron diffraction imaging in a low- energy (50–250 eV) lens-less electron microscope with a spatial resolution of 0.2 nm (Latychevskaia et al., 2015 ). Of particular interest is the in vivo optical imaging of neurons (see Figure 17B ) in the brain by STED (stimulated emission depletion) optical microscopy techniques (Chemistry Nobel Prize 2014) with a lateral resolution of 67 nm (Berning et al., 2012 ). The dynamics of the neuron spine morphology on a 7-min time scale (Figure 17B ) potentially reflect alterations in the connectivity in the neural network characteristic for learning processes, even in the adult brain.

(A) Representative cryo electron micrographs of fusing vesicles (see arrows) in mouse hippocampal synapses at 15 ms (c) and 30 ms (d) after light onset (Watanabe et al., 2013 ). Reprinted with permission from Watanabe et al. ( 2013 ) © 2013 Nature Publishing Group. (B) STED (stimulated emission depletion) microscopy in the molecular layer of the somatosensory cortex of a mouse with EYFP-labeled neurons. (A) Anesthetized mouse under the objective lens. (B) Projected volumes of dendritic and axonal structures reveal (C) temporal dynamics of spine morphology with (D) an approximately four-fold improved spatial resolution compared with diffraction limited imaging. The curve is three-pixel-wide line profile fitted to raw data with a Gaussian. Scale bars, 1 μm (Berning et al., 2012 ). Reprinted with permission from Berning et al. ( 2012 ) © 2012 AAAS.
In addition, neurochemical interference strategies could be promising for future research as discussed in section Musical Therapy for Psychiatric or Neurologic Impairments and Deficiencies in Music Perception. For example, blocking of a specific class of amino acid receptors in the amygdala can interfere with the acquisition of evaluative conditioning (Juslin, 2013 ). In fact, studies of the neurochemistry of music may be the next great frontier (Chanda and Levitin, 2013 ), particularly as researchers try to investigate claims about the effects of music on health, where neurochemical studies are thought to be more appropriate than neuroanatomical studies (Chanda and Levitin, 2013 ).
The number of reports on beneficial effects of music on reward, motivation, pleasure, stress, arousal, immunity and social affiliation is mounting and the following issues could have future impact (Chanda and Levitin, 2013 ): (i) Rigorously matched control conditions in postoperative or chronic pain trials, including controls such as speeches, TV, comedy recordings etc. (ii) Experiments to uncover the neurochemical basis of pleasure and reward, such as through the use of the opioid antagonist naloxone in order to discover whether musical pleasure is subserved by the same chemical system as other forms of pleasure (Chanda and Levitin, 2013 ). (iii) Experiments to uncover the connection between oxytoxin (see Figure Figure11), 11 ), group affiliation, and music (Chanda and Levitin, 2013 ). (iv) Investigation of the contribution of stress hormones, vasopressin, dopamine, and opioids in biological assays and pharmacological interventions together with neuroimaging (Chanda and Levitin, 2013 ).
The investigation of particular BRECVEM mechanisms (see section Musical Therapy for Psychiatric or Neurologic Impairments and Deficiencies in Music Perception) could be intensified through specific experiments. The interaction between BRECVEM mechanisms and aesthetic judgments has yet to be explored (Juslin, 2013 ). For an empirical exploration of candidate criteria for aesthetic judgment one has to map the characteristics of separate aesthetic criteria, as has been done with various BRECVEM mechanisms. Empirical research so far has focused on the beauty criterion (see Juslin, 2013 ) The more phenomenological measuring techniques such as encephalographic methods (EEG, MEG), skin conductance, and finger temperature or goose bump development characterized by a high time resolutions of 10 ms to 1 s are powerful tools for future observation of the dynamics and kinetics of emotional processing, where MEG can provide good time resolution together with moderate spatial resolution (Vrba and Robinson, 2001 ).
In addition to short-term studies, high-quality long-term studies would be desirable for the assessment of therapeutic efficacy over months in analogy to the year-long efforts of Carlo Farinelli for King Philipp V of Spain (see Section Historical Comments on the Impact of Music on People).
Author contributions
H-ES selected the topic, performed the literature retrieval, and wrote the manuscript.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer AF declared a shared affiliation, with no collaboration, with the author HS to the handling Editor.
Acknowledgments
The present study has been stimulated by a discussion with Hans-Christoph Rademann, Internationale Bachakademie Stuttgart. Continuous support of Thomas Schipperges, University of Tübingen is highly appreciated. The author is indebted to Christiane Neuhaus, University of Hamburg; Hans-Peter Zenner, University of Tübingen; Klaus Scheffler, Max Planck Institute of Biological Cybernetics and University of Tübingen; Hubert Preissl, Helmholtz Center Munich at the University of Tübingen; Boris Kleber, Sunjung Kim, and Julian Malcolm Clarke, University of Tübingen; and Bernd-Christoph Kämper and Ulrike Mergenthaler, University of Stuttgart for most competent discussions. Bettina Dietrich carefully read the manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2017.00600/full#supplementary-material
- Agrippa von Nettesheim H. C. (1992). De Occulta Philosophia , ed P. Compagni, Leiden: Vittoria. [ Google Scholar ]
- Archie P., Bruera E., Cohen L. (2013). Music-based intervention in palliative cancer care: a review of quantitative studies and neurobiological literature . Support. Care Cancer 21 , 2609–2624. 10.1007/s00520-013-1841-4 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Bach J. S. (1967). Neue Ausgabe sämtlicher Werke, Serie VII: Orchesterwerke Band 1. Kassel: Bärenreiter. [ Google Scholar ]
- Bailey D. L., Barthel H., Beuthin-Baumann B., Beyer T., Bisdas S., Boellaard R., et al.. (2014). Combined PET/MR: where are we now? Summary report of the second international workshop on PET/MR imaging April 8-12, 2013, Tübingen, Germany . Mol. Imaging Biol . 16 , 295–310. 10.1007/s11307-014-0725-4 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Benedek M., Kaernbach C. (2011). Physiological correlates and emotional specificity of human piloerection . Biol. Psychol. 86 , 320–329. 10.1016/j.biopsycho.2010.12.012 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Benedek M., Wilfling B., Lukas-Wolfbauer R., Katzur B. H., Kaernbach C. (2010). Objective and continuous measurement of piloerection . Psychophysiology 47 , 989–993. 10.1111/j.1469-8986.2010.01003.x [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Berning S., Willig K. I., Steffens H., Dibay P., Hell S. W. (2012). Nanoscopy in a living mouse brain . Science 335 , 551–551. 10.1126/science.1215369 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Birbaumer N., Schmidt R. F. (2010). Biologische Psychologie. Heidelberg: Springer-Verlag. [ Google Scholar ]
- Blood A. J., Zatorre R. J. (2001). Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion . Proc. Nat. Acad. Sci. U.S.A. 98 , 11818–11823. 10.1073/pnas.191355898 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Caria A., Venuti P., de Falco S. (2011). Functional and dysfunctional brain circuits underlying emotional processing of music in autism spectrum disorders . Cereb. Cortex 21 , 2838–2849. 10.1093/cercor/bhr084 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Chanda M. L., Levitin D. J. (2013). The neurochemistry of music . TrendsCogn. Sci. 17 , 179–193 10.1016/j.tics.2013.02.007 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Charland L. C. (2010). Reinstating the passions: arguments from the history of psychopathology , in The Oxford Handbook of Philosophy of Emotion , ed Goldie P. (Oxford: Oxford University Press; ), 237–259. [ Google Scholar ]
- Cuddy L. L., Duffin J. M., Gill S. S., Brown C. L., Sikka R., Vanstone A. D. (2012). Memories for melodies and lyrics in Alzheimer's disease . Music Percept . 29 , 479–491. 10.1525/mp.2012.29.5.479 [ CrossRef ] [ Google Scholar ]
- Darwin C. (1871). The Descent of Man and Selection in Relation to Sex . London: John Murray [ Google Scholar ]
- Darwin C. (1872). The Expression of Emotions in Man and Animals . London: John Murray. [ Google Scholar ]
- Demers J. L. H., Davis S. C., Pogue B. W., Morris M. D. (2012). Multichannel diffuse optical Raman tomography for bone characterization in vivo : a phantom study . Biomed. Optics Express 3 , 2299–2305. 10.1364/BOE.3.002299 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Drevets W. C., Price J. L., Furey M. L. (2008). Brain structure and functional abnormalities in mood disorders: implications for neurocircuitry models of depression . Brain Struct. Funct . 213 , 93–118. 10.1007/s00429-008-0189-x [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Dvorak A. (1955). Slavonic Dances, Edition Based on the Composers Manuscript . Prag: Artia Prag. [ Google Scholar ]
- Eggebrecht H. H. (1991). Musik im Abendland – Prozesse und Stationen vom Mittelalter bis zur Gegenwart. München: Piper. [ Google Scholar ]
- Eison A. S., Mullins U. L. (1996). Regulation of central 5-HT2A receptors: a review of in vivo studies . Behav. Brain Res. 73 , 177–181. 10.1016/0166-4328(96)00092-7 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Fettiplace R., Hackney C. M. (2006). The sensory and motor roles of auditory hair cells . Nat. Rev. Neurosci. 7 , 19–29. 10.1038/nrn1828 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Friston K. J., Friston D. A. (2013). A free energy formulation of music performance and perception - Helmholtz revisited , in Sound-Perception-Performance , ed Bader R. (Heidelberg: Springer; ), 43–69. [ Google Scholar ]
- Furuya S., Altenmüller (2013). Flexibility of movement organization in piano performance . Front. Hum. Neurosci. 7 :173. 10.3389/fnhum.2013.00173 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Gray L. Auditory System: Structure and Function in Neuroscience. Online-Electronic Textbook for the Neurosciences . The University of Texas Medical School; Available online at: http://neuroscience.uth.tmc.edu/s2/chapter12.html [ Google Scholar ]
- Grewe O., Kopiez R., Altenmüller E. (2009). The chill parameter: goose bumps and shivers as promising measures in emotion research . Music Percept. 27 , 61–74. 10.1525/mp.2009.27.1.61 [ CrossRef ] [ Google Scholar ]
- Haböck F. (1923). Die Gesangskunst der Kastraten. Erster Notenband: A. Die Kunst des Cavaliere Carlo Broschi Farinelli. B. Farinellis berühmte Arien . Wien: Universal Edition. [ Google Scholar ]
- Hopkin M. (2008). Ruthlessness gene' discovered . Nature News . [Epub ahead of print]. 10.1038/news.2008.738 [ CrossRef ] [ Google Scholar ]
- Johansson B. B. (2012). Multisensory stimulation in stroke rehabilitation . Front. Hum. Neurosci. [Epub ahead of print]. 6 :60. 10.3389/fnhum.2012.00060 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Juslin P. N. (2013). From everyday emotions to aesthetic emotions: towards a unified theory of musical emotions . Phys. Life Rev. 10 , 235–266. 10.1016/j.plrev.2013.05.008 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Karma K. (2007). Musical aptitude definition and measure validation: ecological validity can endanger the construct validity of musical aptitude tests . Psychomusicology 19 , 79–90. 10.1037/h0094033 [ CrossRef ] [ Google Scholar ]
- Kleber B., Birbaumer N., Veit R., Trevorrow T., Lotze M. (2007). Overt and imagined singing of an Italian aria . Neuroimage 36 , 889–900. 10.1016/j.neuroimage.2007.02.053 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koelsch S. (2014). Brain correlates of music-evoked emotion . Nat. Rev. Neurosci. 15 , 170–180. 10.1038/nrn3666 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koelsch S., Fritz T., Cramon D. Y. V., Müller K., Friederici A. D. (2006). Investigating emotion with music: an fMRI study . Hum. Brain Mapp. 27 , 239–250. 10.1002/hbm.20180 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Koelsch S., Stegemann T. (2012). The brain and positive biological effects in healthy and clinical populations , in Music, Health and Wellbeing , eds MacDonald R., Kreutz D., Mitchell L. (Oxford: Oxford University Press; ), 436–456. [ Google Scholar ]
- Kraus K. S., Canlon S. (2012). Neuronal connectivity and interactions between the auditory and the limbic systems . Hear. Res. 288 , 34–46. 10.1016/j.heares.2012.02.009 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Kreutz G., Murcia C. Q., Bongard S. (2012). Psychoneuroendocrine research on music and health: an overview , in Music, Health and Wellbeing , eds MacDonald R., Kreutz D., Mitchell L. (Oxford: Oxford University Press; ), 457–476. [ Google Scholar ]
- Kümmel W. F. (1977). Musik und Medizin – Ihre Wechselbeziehung in Theorie und Praxis von 800 bis 1800 . Freiburg: Verlag Alber. [ Google Scholar ]
- Lai G., Pantazatos S. P., Schneider H., Hirsch J. (2012). Neural systems for speech and song in autism . Brain 135 , 961–975. 10.1093/brain/awr335 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Latychevskaia T., Longchamp J.-N., Escher C., Fink H.-W. (2015). Holography and coherent diffraction with low-energy electrons: a route towards structural biology at the single molecule level . Ultramicroscopy . 159 , 395–402. 10.1016/j.ultramic.2014.11.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Lauterbur P. C. (1973). Image formation by induced local interactions: examples employing nuclear magnetic resonance . Nature 242 , 190–191. 10.1038/242190a0 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Liu C. H., Ren J., Liu C.-M., Liu P. K. (2014). Intracellular gene transcription factor protein-guided MRI by DNA aptamers in vivo . FASEB J. 28 , 464–473. 10.1096/fj.13-234229 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maratos A., Gold C., Wang X., Crawford M. (2008). Music therapy for depression . Cochrane Database Syst. Rev. 1 :CD004517 10.1002/14651858.CD004517.pub2 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Maurer B. (2014). Saitenweise – Neue Klangphänomene auf Streichinstrumenten und ihre Notation. Wiesbaden: Breitkopf and Härtel. [ Google Scholar ]
- Meyer L. B. (1956). Emotion and Meaning in Music . Chicago: The University of Chicago Press. [ Google Scholar ]
- Mitterschiffthaler M. T., Fu C. H., Dalton J. A., Andrew C. M., Williams S. C. (2007). A functional MRI study of happy and sad affective states evoked by classical music . Hum. Brain Mapp. 28 , 1150–1162. 10.1002/hbm.20337 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Morricone E. (2014, Jun 1). Besser werden . Sonntag Aktuell , p. 12. [ Google Scholar ]
- Neuhaus C. (2013). Processing musical form: behavioural and neurocognitive approaches . Mus. Sci. 17 , 109–127. 10.1177/1029864912468998 [ CrossRef ] [ Google Scholar ]
- Nombela C., Hughes L. E., Owen A. M., Grahn J. A. (2013). Into the groove: can rhythm influence Parkinson's disease? Neurosci. Biobehav. Rev. 37 , 2564–2570. 10.1016/j.neubiorev.2013.08.003 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Omar R., Henley S. M. D., Bartlett J. W., Hailstone J. C., Gordon E., Sauter D. A., et al.. (2011). The structural neuroanatomy of music emotion recognition: evidence from frontotemporal lobar degeneration , Neuroimage 56 , 1814–1861. 10.1016/j.neuroimage.2011.03.002 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Panksepp J. (1995). The emotional sources of, chills' induced by music . Music Percept. 13 , 171–207. 10.2307/40285693 [ CrossRef ] [ Google Scholar ]
- Pantev C., Osterveld R., Engelien A., Ross B., Roberts L. E., Hoke M. (1998). Increased auditory cortical representation in musicians . Nature 392 , 811–813. 10.1038/33918 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Reiser M. F., Semmler W., Hricak H. (eds.). (2008). Magnetic Resonance Tomography . Berlin; Heidelberg: Springer-Verlag. [ Google Scholar ]
- Roederer J. G. (2008). The Physics and Psychophysics of Music. An Introduction . New York, NY: Springer Science and Business. [ Google Scholar ]
- Rzadzinska A. K., Schneider M. E., Davis C., Riordan G. P., Kachar B. (2004). An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal . J. Cell Biol. 164 , 887–897. 10.1083/jcb.200310055 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Salimpoor V. N., van den Bosch I., Kovacevic N., McIntosh A. R., Dagher A., Zatorre R. J. (2013). Interactions between the nucleus accumbens and auditory cortices predict music reward value . Science 340 , 216–219. 10.1126/science.1231059 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Schipperges T. (2003). Wider die Musik. Untersuchungen zur Entdeckung der Musikfeindschaft als Idee im sechzehnten bis achtzehnten Jahrhundert mit Rückblicken auf die Tradition der effectus musicae und Ausblicken zu ihrem Weiterwirken, Habilitationsschrift 2000; Separatdruck . Zeitschrift für Religions- und Geistesgeschichte 55 , 205–226. 10.1163/157007303322146529 [ CrossRef ] [ Google Scholar ]
- Schnier F., Mehlhorn M. (2013). Magnetic Resonance Tomography. Göttingen: Phywe Systeme. [ Google Scholar ]
- Shimizu T. (2004). Ju-On (DVD) . Santa Monica, CA; Lionsgate Entertainment Corp.). The film music (see The Grudge theme song Available online at: https://www.youtube.com/watch?v=1dqjXyIu02s ). [ Google Scholar ]
- Spitzer M. (2003, 2014). Musik im Kopf . Stuttgart: Schattauer. [ Google Scholar ]
- Ter-Pogossian M. M., Phelps M. E., Hoffman E. J., Mullani N. A. (1975). A positron-emission transaxial tomograph for nuclear imaging (PETT) . Radiology 114 , 89–98. 10.1148/114.1.89 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Tramo M. J., Cariani P. A., Delgutte B., Braida L. D. (2001). Neurobiological foundations for the theory of harmony in Western tonal music , in The Biological Foundations of Music , Vol. 930 , ed Zatorre R. J., Peretz I. (New York, NY: Academy of Sciences; ), 92–116. [ PubMed ] [ Google Scholar ]
- Tsai C.-G., Chen R.-S., Tsai T.-S. (2014). The arousing and cathartic effects of popular heartbreak songs as revealed in the physiological responses of the listeners . Musicae Sci. 18 , 410–422. 10.1177/1029864914542671 [ CrossRef ] [ Google Scholar ]
- Ukkola-Vuoti L., Oikkonen J., Onkamo P., Karma K., Raijas P., Järvelä I. (2011). Association of the arginine vasopressin receptor 1A (AVPR1A) haplotypes with listening to music . J. Hum. Genet. 56 , 324–329. 10.1038/jhg.2011.13 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Vrba J., Robinson S. E. (2001). Signal processing in magnetoencephalography . Methods 25 , 249–271. 10.1006/meth.2001.1238 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Wallum K., Westberg L., Henningsson S., Neiderhiser J. M., Reiss D., Igl W., et al. (2008). Genetic variation in the vasopressin receptor 1A gene (AVPR1A) associates with pair bonding in humans . Proc. Nat. Acad. Sci. U.S.A. 105 , 14153–14156. 10.1073/pnas.0803081105 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Watanabe S., Rost B. R., Camacho-Perez M., Davis M. W., Söhl-Kielczynski B., Rosenmund C., et al.. (2013). Ultrafast endocytosis at mouse hippocampal synapses . Nature 504 , 242–247. 10.1038/nature12809 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Watanabe T., Yagishita S., Kikyo H. (2008). Memory of music: roles of right hippocampus and left inferior frontal gyrus . Neuroimage 39 , 483–491. 10.1016/j.neuroimage.2007.08.024 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Watanabe Y. (2012). New findings on the underlying neural mechanism of emotion induced by frightening music . J. Nucl. Med. 53 , 1497–1498. 10.2967/jnumed.112.109447 [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Waterman M. (1996). Emotional responses to music: implicit and explicit effects in listeners and performers . Psychol. Music 24 , 53–64. 10.1177/0305735696241006 [ CrossRef ] [ Google Scholar ]
- Widmann J. (2014, August 20). Der Rausch ist womöglich überhaupt der klarste Zustand . Der Standard , p. 24. [ Google Scholar ]
- Xue S., Qiao J., Pu F., Cameron M., Yang J. J. (2013). Design of a novel class of protein- based magnetic resonance imaging contrast agents for the molecular imaging of cancer biomarkers . Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol . 5 . 163–179. 10.1002/wnan.1205 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zarate S. M. (2013). The Neural control of singing . Front. Hum. Neurosci. 7 :237. 10.3389/fnhum.2013.00237 [ PMC free article ] [ PubMed ] [ CrossRef ] [ Google Scholar ]
- Zender Hans. (2014). Waches Hören – Über Musik . München: Carl Hanser Verlag. [ Google Scholar ]
- Zenner H.-P. (1994). Hören – Physiologie, Biochemie, Zell- und Neurobiologie. Stuttgart: Georg Thieme Verlag. [ Google Scholar ]
- Zhang Y., Chen Q. Z., Du F. L., Hu Y. N., Chao F. F., Tian M., et al.. (2012). Frightening music triggers rapid changes in brain monoamine receptors: a pilot PET study . J. Nucl. Med. 53 , 1573–1578. 10.2967/jnumed.112.106690 [ PubMed ] [ CrossRef ] [ Google Scholar ]

Essay on music and emotions
How can different types of music affect people’s emotions? Music has many different ways to affect people. In some ways, it is good for the body both physically and mentally. In other ways, people think it is nice to listen to. More detailed, music has personalities, which can express what people feel. There are many observations involving different ways to express human emotions. Emotions are very interesting things, especially when they involve music. Music can have many personalities, affect people’s emotions, and be used as therapy. If music reveals emotions, it is not a normal emotion like any other (Stecker 273). The expression in music can be considered a traditionalized phenomenon (Stecker 273). There are common questions about …show more content…
(www.bellaonline.com/articles/). And can slow down when there is softer music playing such as a lullaby (www.bellaonline.com/articles/). Rhythms of the music can adjust brain waves and breathing patterns (www.bellaonline.com/articles/). The vibrations from the music have an impact on the body, which can change peoples’ moods and bodily functions (www.bellaonline.com/articles/). The mind is greatly impacted by music by showing healthful changes (www.bellaonline.com/articles/). Doctors now use music for their patients’ treatments in order to help them stay healthy (www.bellaonline.com/articles/). Heart patients acquired the same benefits from listening to classical music for thirty minutes as they did from anti-anxiety medication (www.bellaonline.com/articles/). Musical therapy has been used to help people with heart problems, which worked quite effectively. (www.bellaonline.com/articles/). People who have had migraines frequently, were trained to use music and relaxing procedures to reduce their headaches. Studies have also shown that music helps students with their intelligence levels (www.bellaonline.com/articles/). A majority of students had higher test scores than others because they listened to Mozart before their exam. People who listened to classical music for an hour and a half while revising manuscripts increased their accuracy by 21% (www.bellaonline.com/articles) (Mish 725.). Mozart has a big impact on people
The Day You Stop Lookin Back Analysis
Very often, people have a hard time expressing their feelings through their own words. Music has a way of doing this for society. Based on the context of the music the person is listening to, friends and family are able to get a general

Essay about The mozart effect
- 3 Works Cited
Not only does music affect thought, but it also benefits health. Students usually study in quiet, relaxed surroundings while listening to serene music. Classical music can steady a fast heartbeat and a slower heartbeat induces relaxation. Exercise plays a critical role in maintaining good health, and relaxing music can be favorable to this. Music reduces muscle tension, resulting in a better work out. Scientists performed controlled studies using adult males who were around twenty-five years old. Blood samples were taken before and after treadmill running. The experiment found that with the presence of music, “heart rate, blood pressure, and lactate secretion in the brain were significantly lower” . The results proved that music
The Effect Of Music On The Brain And Its Functions
Music can have multiple positive effects on the brain. One such example is what is known as the Mozart Effect. The Mozart Effect is the change in brain
How Music Affects A Faster Heart Rate
On mindblowingfacts.org it states that different speeds of music can alter your heart rate as well as decreaseing your muscle tension. While I was doing my research I found many other examples of how music can affect yourself. Music can affect your stress level, happiness, or your look on life. Music is a very important thing in people’s life. It can calm people down, or make people happy, so it also affects our attitude and mood.
Music And Music
The auditory system is the most abundant in the human body, which is why listening to music can affect people. Many have said that music makes them feel better; some say that listening to music helps them learn; others, it motivates. While reading in the internet we found many interesting stories of how music has helped stroke patients learn how to talk again, how patients with Alzheimer could regain some of their memories, and that premature babies could gain weight while listening to music. When we read this, we couldn't help but wonder how can all of this happen with music?
Beethoven Seventh Symphony Analysis
att Carrol stated that “Music is the language of emotion, and emotions tend to be the same the world over, in spite of differences in social customs and language.” In other words, music is a fabulous expression of the human being no matter the culture or norms because it manages to immediately transmit different feelings and emotions that other forms of art may not transmit.
Effectiveness of Music Therapy Essay
- 8 Works Cited
Music is composed of sounds intertwined with melody and rhythm that can have powerful effects on a person. It can help people focus on tasks or calm the mind. Research has shown that music has beneficial effects on the mind, body, and health of a person. A journal article by Rastogi, Solanki, and Zafar (2013) refers, on the contrary, to:
Depression Affected By Music
When some people can connect with music, it can change a person’s whole perspective. Music for some people is something that can help them cope with problems like anger and depression. Also music can get some people through chores, a car ride, and boredom in general. Music can do a lot to a person’s mental state or body. So most times when people feel a certain way they turn to music to help them cope with how they’re feeling. For some people , music can be an emotional outlet for some people that are going through a hard time. Music is also something that can change a person’s emotion, when I say that I mean it can affect the mood and decrease depression.
Analysis : ' Audio Engineering '
Even now I notice how I constantly critique the music I listen to. It 's to the point where I can tell if the music an artist is using the beat effectively or not. This ability that I 've developed over time by listening to countless sounds and analyzing them. I believe music has the ability to convey all sorts of emotion. Whether the emotion is joy and happiness or sadness and despair through rhythms, harmonies and the lyrics music shows it. The effect that music can have on our emotions is greater than we realize, as it can bring people to tears or bursts of laughter.
Explain How Music Shows Emotions
Music shows emotions through the characteristics of music such as modality, musical contour, and tempo. These characteristics shape the type of emotions that are experienced by the listener. There is one theory that suggests music as a language, where there are different emotions that are brought across by different types of musical intervals. An example of this would be an augmented fourth which tends to expresses distress, while a major third usually expresses happiness.
Should Music Classes Be Mandatory In Middle And High School?
For many people, listening to music is part of their daily life. There are many different genres and a wide variety of people listening to them. However, what are the other effects of music besides pleasure? Much research has been done on this topic. Numerous people believe that it is beneficial for the human brain. Few believe that it is just a distraction. Much research has been done showing that the effects of music on the developing brain are only positive. Music classes should be mandatory in middle and high school because they can promote better grades, a positive attitude, and are beneficial to the developing brain.
The Influence Of Music On Emotions
Emotions do truly control our life. We act out of fear, love, happiness, hatred, jeoulosy, the list is almost endless. But music has a profound effect on all of them as I stated earlier. Levitin and I both realize it 's influence. So why do I always write about the influence of music on emotions. We ll looking at my first essay it is easy to see. I clearly state “ Since music is so psychologically important in my mind, I find it no problem to believe that I am an emotional listener” (personal essay pg 2). If I am an emotional listener why can I not be an emotional writer. The theme of emotions are universal since everyone has them (some we may argue do not). My writing has involved emotions from my first essay to the last. It is important to notice this because it says what is important to me. People write to get a personal theme out as a message to the world. My message has always mentioned emotions. This focus and dedication towards the theme of emotions now shows that it would be beneficial to write about in future classes.
The Healing Power of Music Essay
In order to understand how music can affect the body and mind, one needs to understand the composition of sound itself. Don Campbell describes it by
Music Vs. Classical Music
How often do you listen to music while you’re studying to try to make the task more entertaining? Students regularly listen to music while studying as a way to help them stay engaged in studying (Beentjes, Koolstra, & van der Voort, 1996). Well, based on what music you listen to, you may actually be hindering yourself rather than helping. When students listen to classical music while they are studying it has many beneficial effects. It has positive body influences, it activates the left and right hemispheres of the brain, slows heart rate, and lowers blood pressure. Students are always looking for effective ways to study and improve test scores and this is a viable option. With vast research on this topic, we should be using it to inform students instead of keeping them in the dark and leaving them to their own resources. Because research shows that listening to classical music while studying will improve test scores, memory and learning, and a decrease in anxiety, we should encourage all students to listen to classical music while they study.
Music Can Help Us Boost Our Abilities And Concentration
Music is as much expressive as normal human language. Since music carries much more powerful emotional charge than the real-life events, modern psychologists use it for the therapy. It can be explained by positive impact on the human nervous system. Emotions that rise during the process of listening to the music can be divided into two types - perceived and felt. This means that a person is able to understand the mood of a piece of music, even if he had never experienced such feelings in real life. So, when someone is in depression, happy music only makes it worse. On the contrary, sad music makes a person fell better.
Related Topics
Home / Essay Samples / Music / Pop Music / The Influence of Music On Mood
The Influence of Music On Mood
- Category: Music
- Topic: Pop Music
Pages: 1 (575 words)
- Downloads: -->
Using Music for Emotional Regulation
--> ⚠️ Remember: This essay was written and uploaded by an--> click here.
Found a great essay sample but want a unique one?
are ready to help you with your essay
You won’t be charged yet!
Concert Review Essays
Elvis Presley Essays
Just Walk on By Essays
Classical Music Essays
Taylor Swift Essays
Related Essays
We are glad that you like it, but you cannot copy from our website. Just insert your email and this sample will be sent to you.
By clicking “Send”, you agree to our Terms of service and Privacy statement . We will occasionally send you account related emails.
Your essay sample has been sent.
In fact, there is a way to get an original essay! Turn to our writers and order a plagiarism-free paper.
samplius.com uses cookies to offer you the best service possible.By continuing we’ll assume you board with our cookie policy .--> -->