Paper as a scaffold for cell cultures: Teaching an old material new tricks
- Prospective Article
- Published: 01 March 2018
- Volume 8 , pages 1–14, ( 2018 )

Cite this article

- Xinchen Wu 1 ,
- Sanika Suvarnapathaki 1 ,
- Kierra Walsh 2 &
- Gulden Camci-Unal 3
237 Accesses
32 Citations
4 Altmetric
Explore all metrics
Paper-based cell culture platforms have emerged as a promising approach for a myriad of biomedical applications, such as tissue engineering, disease models, cancer research, biotechnology, high-throughput testing, biosensing, and diagnostics. Paper enables the generation of highly flexible, biocompatible, inexpensive, porous, and three-dimensional (3D) constructs and devices. These systems have been used to culture mammalian cells, bacteria, algae, and fungi. Studies have shown that paper is an exceptional material for applications in life sciences, materials sciences, engineering, and medicine. Paper has been employed for creating biomimetic cell culture environments by folding or stacking it into the desired 3D shapes and structures. This review discusses the use of paper-based platforms for cellular applications and provides a diverse range of examples.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others
Fabrication of paper-based devices for in vitro tissue modeling
Quantifying oxygen in paper-based cell cultures with luminescent thin film sensors.

Paper-based in vitro tissue chip for delivering programmed mechanical stimuli of local compression and shear flow
M. Jamal, S.S. Kadam, R. Xiao, F. Jivan, T.M. Onn, R. Fernandes, T.D. Nguyen, and D.H. Gracias: Bio-origami hydrogel scaffolds composed of photocrosslinked PEG bilayers. Adv. Healthc. Mater. 2 , 1142–1150 (2013).
CAS Google Scholar
E.A. Peraza-Hernandez, D.J. Hartl, R.J. Malak Jr., and D.C. Lagoudas: Origami-inspired active structures: a synthesis and review. Smart Mater. Struct. 23 , 094001 (2014).
Google Scholar
J.E. Moreau, K. Anderson, J.R. Mauney, T. Nguyen, D.L. Kaplan, and M. Rosenblatt: Tissue-engineered bone serves as a target for metastasis of human breast cancer in a mouse model. Cancer Res. 67 , 10304–10308 (2007).
R. Derda, S.K. Tang, A. Laromaine, B. Mosadegh, E. Hong, M. Mwangi, A. Mammoto, D.E. Ingber, and G.M. Whitesides: Multizone paper platform for 3D cell cultures. PLoS ONE 6 , e18940 (2011).
G. Camci-Unal, D. Newsome, B.K. Eustace, and G.M. Whitesides: Fibroblasts enhance migration of human lung cancer cells in a paperbased coculture system. Adv. Healthc. Mater. 5 , 641–647 (2016).
G. Camci-Unal, A. Laromaine, E. Hong, R. Derda, and G.M. Whitesides: Biomineralization guided by paper templates. Sci. Rep. 6 , 27693 (2016).
F. Piraino, G. Camci-Unal, M.J. Hancock, M. Rasponi, and A. Khademhosseini: Multi-gradient hydrogels produced layer by layer flow and crosslinking in open microchannels. Lab. Chip 12 , 659–661 (2012).
M.J. Hancock, F. Piraino, G. Camci-Unal, M. Rasponi, and A. Khademhosseini: Anisotropic material synthesis by capillary flow in a fluid stripe. Biomaterials 32 , 6493–6504 (2011).
J. Hjortnaes, G. Camci-Unal, J.D. Hutcheson, S.M. Jung, F.J. Schoen, J. Kluin, E. Aikawa, and A. Khademhosseini: Directing valvular interstitial cell myofibroblast-like differentiation in a hybrid hydrogel platform. Adv. Healthc. Mater. 4 , 121–130 (2015).
H.-Y. Hsieh, G. Camci-Unal, T.-W. Huang, R. Liao, T.-J. Chen, A. Paul, F.-G. Tseng, and A. Khademhosseini: Gradient static-strain stimulation in a microfluidic chip for 3D cellular alignment. Lab. Chip 14 , 482–493 (2014).
D. Lantigua, Y.N. Kelly, B. Unal, and G. Camci-Unal: Engineered paperbased cell culture platforms. Adv. Healthc. Mater. 6 , 22 (2017).
M.C. Sapp, H.J. Fares, A.C. Estrada, and K.J. Grande-Allen: Multilayer three-dimensional filter paper constructs for the culture and analysis of aortic valvular interstitial cells. Acta Biomater. 13 , 199–206 (2015).
K. Ng, B. Gao, K.W. Yong, Y. Li, M. Shi, X. Zhao, Z. Li, X. Zhang, B. Pingguan-Murphy, H. Yang, and F. Xu: Paper-based cell culture platform and its emerging biomedical applications. Mater. Today 20 , 32–44 (2017).
L.E. Freed, G. Vunjak-Novakovic, R.J. Biron, D.B. Eagles, D.C. Lesnoy, S. K. Barlow, and R. Langer: Biodegradable polymer scaffolds for tissue engineering. Nat. Biotechnol. 12 , 689–693 (1994).
C.Y. Chan, P.-H. Huang, F. Guo, X. Ding, V. Kapur, J.D. Mai, P.K. Yuen, and T.J. Huang: Accelerating drug discovery via organs-on-chips. Lab. Chip 13 , 4697–4710 (2013).
H.-J. Park, S.J. Yu, K. Yang, Y. Jin, A.-N. Cho, J. Kim, B. Lee, H.S. Yang, S.G. Im, and S.-W. Cho: based bioactive scaffolds for stem cell-mediated bone tissue engineering. Biomaterials 35 , 9811–9823 (2014).
Y. Wang, T. Uemura, J. Dong, H. Kojima, J. Tanaka, and T. Tateishi: Application of perfusion culture system improves in vitro and in vivo osteogenesis of bone marrow-derived osteoblastic cells in porous ceramic materials. Tissue Eng. 9 , 1205–1214 (2003).
R. Agarwal and A.J. García: Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv. Drug Deliv. Rev. 94 , 53–62 (2015).
R. Derda, A. Laromaine, A. Mammoto, S.K. Tang, T. Mammoto, D.E. Ingber, and G.M. Whitesides: Supported 3D cell culture for tissuebased bioassays. Proc. Natl. Acad. Sci. USA 106 , 18457–18462 (2009).
B. Mosadegh, B.E. Dabiri, M.R. Lockett, R. Derda, P. Campbell, K.K. Parker, and G.M. Whitesides: Three-dimensional paper-based model for cardiac ischemia. Adv. Healthc. Mater. 3 , 1036–1043 (2014).
L. Wang, C. Xu, Y. Zhu, Y. Yu, N. Sun, X. Zhang, K. Feng, and J. Qin: Human induced pluripotent stem cell-derived beating cardiac tissues on paper. Lab. Chip 15 , 4283–4290 (2015).
GE Lifesciences: https://www.gelifesciences.com/ (accessed 12/2/2017).
B. Mosadegh, M.R. Lockett, K.T. Minn, K.A. Simon, K. Gilbert, S. Hillier, D. Newsome, H. Li, A.B. Hall, and D.M. Boucher: A paper-based invasion assay: Assessing chemotaxis of cancer cells in gradients of oxygen. Biomaterials 52 , 262–271 (2015).
G. Choi, D.J. Hassett, and S. Choi: A paper-based microbial fuel cell array for rapid and high-throughput screening of electricity-producing bacteria. Analyst 140 , 4277–4283 (2015).
A. Fraiwan, S. Mukherjee, S. Sundermier, H.-S. Lee, and S. Choi: A paperbased microbial fuel cell: Instant battery for disposable diagnostic devices. Biosens. Bioelectron. 49 , 410–414 (2013).
N.C. Martins, C.S. Freire, C.P. Neto, A.J. Silvestre, J. Causio, G. Baldi, P. Sadocco, and T. Trindade: Antibacterial paper based on composite coatings of nanofibrillated cellulose and ZnO. Colloids Surf. A 417 , 111–119 (2013).
M. Funes-Huacca, A. Wu, E. Szepesvari, P. Rajendran, N. Kwan-Wong, A. Razgulin, Y. Shen, J. Kagira, R. Campbell, and R. Derda: Portable selfcontained cultures for phage and bacteria made of paper and tape. Lab Chip 12 , 4269–4278 (2012).
F. Deiss, M.E. Funes-Huacca, J. Bal, K.F. Tjhung, and R. Derda: Antimicrobial susceptibility assays in paper-based portable culture devices. Lab Chip 14 , 167–171 (2014).
J.B. Stielow, L.A. Vaas, M. Göker, P. Hoffmann, and H.-P. Klenk: Charcoal filter paper improves the viability of cryopreserved filamentous ectomycorrhizal and saprotrophic Basidiomycota and Ascomycota. Mycologia 104 , 324–330 (2012).
C.-C. Chen, Y.-J. Liu, and D.-J. Yao: Paper-based device for separation and cultivation of single microalga. Talanta 145 , 60–65 (2015).
I. Bhattacharya, C. Ghayor, and F.E. Weber: The use of adipose tissuederived progenitors in bone tissue engineering-a review. Transfus. Med. Hemother. 43 , 336–343 (2016).
G.F. Petersen, B.J. Hilbert, G.D. Trope, W.H. Kalle, and P.M. Strappe: A paper-based scaffold for enhanced osteogenic differentiation of equine adipose-derived stem cells. Biotechnol. Lett. 37 , 2321–2331 (2015).
J.L. Patnaik, T. Byers, C. DiGuiseppi, D. Dabelea, and T.D. Denberg: Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 13 , R64 (2011).
J. Petsche Connell, G. Camci-Unal, A. Khademhosseini, and J.G. Jacot: Amniotic fluid-derived stem cells for cardiovascular tissue engineering applications. Tissue Eng. Part B Rev. 19 , 368–379 (2013).
G. Camci-Unal, N. Alemdar, N. Annabi, and A. Khademhosseini: Oxygenreleasing biomaterials for tissue engineering. ACS Biomater. Sci. Eng. 62 , 843–848 (2013).
N. Alemdar, J. Leijten, G. Camci-Unal, Hjortnaes, J. Ribas, A. Paul, P. Mostafalu, A.K. Gaharwar, Y. Qiu, and S. Sonkusale: Oxygen-generating photo-cross-linkable hydrogels support cardiac progenitor cell survival by reducing hypoxia-induced necrosis. ACS Biomater. Sci. Eng. 3 , 9 (2016).
H.T. Lynch, T.C. Smyrk, P. Watson, S.J. Lanspa, J.F. Lynch, P.M. Lynch, R.J. Cavalieri, and C.R. Boland: Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology 104 , 1535–1549 (1993).
H. Karlsson, M. Fryknäs, R. Larsson, and P. Nygren: Loss of cancer drug activity in colon cancer HCT-116 cells during spheroid formation in a new 3-D spheroid cell culture system. Exp. Cell Res. 318 , 1577–1585 (2012).
A.C. Luca, S. Mersch, R. Deenen, S. Schmidt, I. Messner, K.-L. Schäfer, S.E. Baldus, W. Huckenbeck, R.P. Piekorz, and W. T. Knoefel: Impact of the 3D microenvironment on phenotype, gene expression, and EGFR inhibition of colorectal cancer cell lines. PLoS ONE 8 , e59689 (2013).
B. Hong, P. Xue, Y. Wu, J. Bao, Y.J. Chuah, and Y. Kang: A concentration gradient generator on a paper-based microfluidic chip coupled with cell culture microarray for high-throughput drug screening. Biomed. Microdevices 18 , 21 (2016).
S. Wang, L. Ge, X. Song, J. Yu, S. Ge, J. Huang, and F. Zeng: based chemiluminescence ELISA: lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens. Bioelectron. 31 , 212–218 (2012).
K. Erickson, R.D. Braun, D. Yu, J. Lanzen, D. Wilson, D.M. Brizel, T.W. Secomb, J.E. Biaglow, and M.W. Dewhirst: Effect of longitudinal oxygen gradients on effectiveness of manipulation of tumor oxygenation. Cancer Res. 63 , 4705–4712 (2003).
T. Han, D. Kang, D. Ji, X. Wang, W. Zhan, M. Fu, H.-B. Xin, and J.-B. Wang: How does cancer cell metabolism affect tumor migration and invasion? Cell Adh. Migr. 7 , 395–403 (2013).
V. Cristini, H.B. Frieboes, R. Gatenby, S. Caserta, M. Ferrari, and J. Sinek: Morphologic instability and cancer invasion. Clin. Cancer Res. 11 , 6772–6779 (2005).
C. Carmona-Fontaine, V. Bucci, L. Akkari, M. Deforet, J.A. Joyce, and J.B. Xavier: Emergence of spatial structure in the tumor microenvironment due to the Warburg effect. Proc. Natl. Acad. Sci. USA 110 , 19402–19407 (2013).
M.W. Boyce, G.J. LaBonia, A.B. Hummon, and M.R. Lockett: Assessing chemotherapeutic effectiveness using a paper-based tumor model. Analyst 142 , 2819–2827 (2017).
M.W. Boyce, R.M. Kenney, A.S. Truong, and M.R. Lockett: Quantifying oxygen in paper-based cell cultures with luminescent thin film sensors. Anal. Bioanal. Chem. 408 , 2985–2992 (2016).
K. Chwalek, L.J. Bray, and C. Werner: Tissue-engineered 3D tumor angiogenesis models: potential technologies for anti-cancer drug discovery. Adv. Drug Deliv. Rev. 79 , 30–39 (2014). Prospective Article
K.F. Lei, and C.-H. Huang: Based microreactor integrating cell culture and subsequent immunoassay for the investigation of cellular phosphorylation. ACS Appl. Mater. Interfaces 6 , 22423–22429 (2014).
A.S. Truong, C.A. Lochbaum, M.W. Boyce, and M.R. Lockett: Tracking the invasion of small numbers of cells in paper-based assays with quantitative PCR. Anal. Chem. 87 , 11263–11270 (2015).
J. Zhang and J. Liu: Tumor stroma as targets for cancer therapy. Pharmacol. Ther. 137 , 200–215 (2013).
R.M. Bremnes, T. Dønnem, S. Al-Saad, K. Al-Shibli, S. Andersen, R. Sirera, C. Camps, I. Marinez, and L.-T. Busund: The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J.Thorac. Oncol. 6 , 209–217 (2011).
F. Deiss, A. Mazzeo, E. Hong, D.E. Ingber, R. Derda, and G.M. Whitesides: Platform for high-throughput testing of the effect of soluble compounds on 3D cell cultures. Anal. Chem. 85 , 8085–8094 (2013).
F. Deiss, W.L. Matochko, N. Govindasamy, E.Y. Lin, and R. Derda: Flowthrough synthesis on teflon-patterned paper to produce peptide arrays for cell-based assays. Angew. Chem. Int. Ed. 53 , 6374–6377 (2014).
S.P. Jackson, and J. Bartek: The DNA-damage response in human biology and disease. Nature 461 , 1071 (2009).
A.C. Begg, F.A. Stewart, and C. Vens: Strategies to improve radiotherapy with targeted drugs. Nat. Rev. Cancer 11 , 239 (2011).
G.C. Barnett, C.M. West, A.M. Dunning, R.M. Elliott, C.E. Coles, P.D. Pharoah, and N.G. Burnet: Normal tissue reactions to radiotherapy: towards tailoring treatment dose by genotype. Nat. Rev. Cancer 9 , 134 (2009).
K.A. Simon, B. Mosadegh, K.T. Minn, M.R. Lockett, M.R. Mohammady, D.M. Boucher, A.B. Hall, S.M. Hillier, T. Udagawa, B.K. Eustace, and G.M. Whitesides: Metabolic response of lung cancer cells to radiation in a paper-based 3D cell culture system. Biomaterials 95 , 47–59 (2016).
R.M. Kenney, M.W. Boyce, A.S. Truong, C.R. Bagnell, and M.R. Lockett: Real-time imaging of cancer cell chemotaxis in paper-based scaffolds. Analyst 141 , 661–668 (2016).
F.F. Tao, X. Xiao, K.F. Lei, and I.-C. Lee: Based cell culture microfluidic system. Biochip J. 2 , 97–104 (2015).
K.F. Lei, C.-H. Huang, and N.-M. Tsang: Impedimetric quantification of cells encapsulated in hydrogel cultured in a paper-based microchamber. Talanta 147 , 628–633 (2016).
G. Choi and S. Choi: Bacterial cell transportation in paper-based microfluidics. In Transducers (IEEE:Anchorage, Alaska, USA, 2015), pp. 1921–1924.
A.K. Yetisen, M.S. Akram, and C.R. Lowe: Based microfluidic point-of-care diagnostic devices. Lab. Chip 13 , 2210–2251 (2013).
R. Veerubhotla, A. Bandopadhyay, D. Das, and S. Chakraborty: Instant power generation from an air-breathing paper and pencil based bacterial bio-fuel cell. Lab. Chip 15 , 2580–2583 (2015).
L. Zhang, M. Zhou, D. Wen, L. Bai, B. Lou, and S. Dong: Small-size biofuel cell on paper. Biosens. Bioelectron. 35 , 155–159 (2012).
I. Shitanda, S. Kato, Y. Hoshi, M. Itagaki, and S. Tsujimura: Flexible and high-performance paper-based biofuel cells using printed porous carbon electrodes. Chem. Commun. 49 , 11110–11112 (2013).
U. Desselberger: Emerging and re-emerging infectious diseases. J. Infect. 40 , 3–15 (2000).
X. Cui and T. Boland: Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 30 , 6221–6227 (2009).
A.W. Martinez, S.T. Phillips, E. Carrilho, S.W. Thomas III, H. Sindi, and G.M. Whitesides: Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal. Chem. 80 , 3699–3717 (2008).
T. Srimongkon, T. Ishida, K. Igarashi, and T. Enomae: Development of a bacterial culture system using a paper platform to accommodate media and an ink-jet printing to dispense bacteria. Am. J. Biochem. Biotechnol. 10 , 81–87 (2014).
F. Marciano, D. Lima-Oliveira, N. Da-Silva, A. Diniz, E. Corat, and V. Trava-Airoldi: Antibacterial activity of DLC films containing TiO 2 nanoparticles. J.Colloid Interface Sci. 340 , 87–92 (2009).
L. Zhang, Y. Jiang, Y. Ding, N. Daskalakis, L. Jeuken, M. Povey, A. J. O’Neill, and D.W. York: Mechanistic investigation into antibacterial behaviour of suspensions of ZnO nanoparticles against E. coli. J.Nanopart. Res. 12 , 1625–1636 (2010).
R. Tankhiwale and S. Bajpai: Preparation, characterization and antibacterial applications of ZnO-nanoparticles coated polyethylene films for food packaging. Colloids Surf. B 90 , 16–20 (2012).
J.T. Seil and T.J. Webster: Reduced Staphylococcus aureus proliferation and biofilm formation on zinc oxide nanoparticle PVC composite surfaces. Acta Biomater. 7 , 2579–2584 (2011).
R. Brayner, R. Ferrari-Iliou, N. Brivois, S. Djediat, M.F. Benedetti, and F. Fiévet: Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 6 , 866–870 (2006).
G. Choi and S. Choi: Monitoring electron and proton diffusion flux through three-dimensional, paper-based, variable biofilm and liquid media layers. Analyst 140 , 5901–5907 (2015).
A. Azam, A.S. Ahmed, M. Oves, M.S. Khan, S.S. Habib, and A. Memic: Antimicrobial activity of metal oxide nanoparticles against gram-positive and gram-negative bacteria: a comparative study. Int. J. Nanomed. 7 , 6003 (2012).
S. Pal, Y.K. Tak, and J.M. Song: Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 73 , 1712–1720 (2007).
I. Sondi and B. Salopek-Sondi: Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for gram-negative bacteria. J.Colloid Interface Sci. 275 , 177–182 (2004).
I. Díez, P. Eronen, M. Österberg, M.B. Linder, O. Ikkala, and R.H. Ras: Functionalization of nanofibrillated cellulose with silver nanoclusters: fluorescence and antibacterial activity. Macromol. Biosci. 11 , 1185–1191 (2011).
S. Baruah, M. Jaisai, R. Imani, M.M. Nazhad, and J. Dutta: Photocatalytic paper using zinc oxide nanorods. Sci. Technol. Adv. Mater. 11 , 055002 (2010).
K. Ghule, A.V. Ghule, B.-J. Chen, and Y.-C. Ling: Preparation and characterization of ZnO nanoparticles coated paper and its antibacterial activity study. Green Chem. 8 , 1034–1041 (2006).
R.J. Pinto, P.A. Marques, C.P. Neto, T. Trindade, S. Daina, and P. Sadocco: Antibacterial activity of nanocomposites of silver and bacterial or vegetable cellulosic fibers. Acta Biomater. 5 , 2279–2289 (2009).
N.C. Martins, C.S. Freire, R.J. Pinto, S.C. Fernandes, C.P. Neto, A. J. Silvestre, J. Causio, G. Baldi, P. Sadocco, and T. Trindade: Electrostatic assembly of Ag nanoparticles onto nanofibrillated cellulose for antibacterial paper products. Cellulose 19 , 1425–1436 (2012).
R. Gottesman, S. Shukla, N. Perkas, L.A. Solovyov, Y. Nitzan, and A. Gedanken: Sonochemical coating of paper by microbiocidal silver nanoparticles. Langmuir 27 , 720–726 (2011).
M.J. Ryan and D. Smith: Cryopreservation and freeze-drying of fungi employing centrifugal and shelf freeze-drying. Methods Mol. Biol. 368 , 127–140 (2007).
A. Michaelsen, F. Pinzari, K. Ripka, W. Lubitz, and G. Piñar: Application of molecular techniques for identification of fungal communities colonising paper material. Int. Biodeterior. Biodegrad. 58 , 133–141 (2006).
A. Ben-Amotz, R. Fishler, and A. Schneller: Chemical composition of dietary species of marine unicellular algae and rotifers with emphasis on fatty acids. Mar. Biol. 95 , 31–36 (1987).
H.W. Yen, I.C. Hu, C.Y. Chen, S.H. Ho, D.J. Lee, and J.S. Chang: Microalgae-based biorefinery—from biofuels to natural products. Bioresour. Technol. 135 , 166–174 (2013).
W. Liu, N. Dechev, I.G. Foulds, R. Burke, A. Parameswaran, and E.J. Park: A novel permalloy based magnetic single cell micro array. Lab. Chip 9 , 2381–2390 (2009).
S. Lindstrom and H. Andersson-Svahn: Miniaturization of biological assays—overview on microwell devices for single-cell analyses. Biochim. Biophys. Acta 1810 , 308–316 (2011).
S. Khanna: Microbiota replacement therapies: innovation in gastrointestinal care. Clin. Pharmacol. Ther. 103 , 102–111 (2017).
Download references
Acknowledgments
This work was supported by the University of Massachusetts Lowell start-up funds.
Author information
Authors and affiliations.
Biomedical Engineering and Biotechnology Program, University of Massachusetts Lowell, One University Avenue, Lowell, MA, 01854, USA
Xinchen Wu & Sanika Suvarnapathaki
Department of Biological Sciences, University of Massachusetts Lowell, One University Avenue, Lowell, MA, 01854, USA
Kierra Walsh
Department of Chemical Engineering, University of Massachusetts Lowell, One University Avenue, Lowell, MA, 01854, USA
Gulden Camci-Unal
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Gulden Camci-Unal .
Rights and permissions
Reprints and permissions
About this article
Wu, X., Suvarnapathaki, S., Walsh, K. et al. Paper as a scaffold for cell cultures: Teaching an old material new tricks. MRS Communications 8 , 1–14 (2018). https://doi.org/10.1557/mrc.2018.8
Download citation
Received : 02 December 2017
Accepted : 05 January 2018
Published : 01 March 2018
Issue Date : March 2018
DOI : https://doi.org/10.1557/mrc.2018.8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Advertisement
- Find a journal
- Publish with us
- Track your research
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 29 January 2019
Nanoparticle administration method in cell culture alters particle-cell interaction
- Thomas L. Moore ORCID: orcid.org/0000-0002-7886-5245 1 ,
- Dominic A. Urban 1 ,
- Laura Rodriguez-Lorenzo 1 ,
- Ana Milosevic 1 ,
- Federica Crippa 1 ,
- Miguel Spuch-Calvar 1 ,
- Sandor Balog ORCID: orcid.org/0000-0002-4847-9845 1 ,
- Barbara Rothen-Rutishauser ORCID: orcid.org/0000-0002-7805-9366 1 ,
- Marco Lattuada 2 &
- Alke Petri-Fink 1 , 2
Scientific Reports volume 9 , Article number: 900 ( 2019 ) Cite this article
12k Accesses
61 Citations
10 Altmetric
Metrics details
- Nanobiotechnology
- Techniques and instrumentation
As a highly interdisciplinary field, working with nanoparticles in a biomedical context requires a robust understanding of soft matter physics, colloidal behaviors, nano-characterization methods, biology, and bio-nano interactions. When reporting results, it can be easy to overlook simple, seemingly trivial experimental details. In this context, we set out to understand how in vitro technique, specifically the way we administer particles in 2D culture, can influence experimental outcomes. Gold nanoparticles coated with poly(vinylpyrrolidone) were added to J774A.1 mouse monocyte/macrophage cultures as either a concentrated bolus, a bolus then mixed via aspiration, or pre-mixed in cell culture media. Particle-cell interaction was monitored via inductively coupled plasma-optical emission spectroscopy and we found that particles administered in a concentrated dose interacted more with cells compared to the pre-mixed administration method. Spectroscopy studies reveal that the initial formation of the protein corona upon introduction to cell culture media may be responsible for the differences in particle-cell interaction. Modeling of particle deposition using the in vitro sedimentation, diffusion and dosimetry model helped to clarify what particle phenomena may be occurring at the cellular interface. We found that particle administration method in vitro has an effect on particle-cell interactions (i.e. cellular adsorption and uptake). Initial introduction of particles in to complex biological media has a lasting effect on the formation of the protein corona, which in turn mediates particle-cell interaction. It is of note that a minor detail, the way in which we administer particles in cell culture, can have a significant effect on what we observe regarding particle interactions in vitro .
Similar content being viewed by others

The origin of heterogeneous nanoparticle uptake by cells
Understanding the Lipid and Protein Corona Formation on Different Sized Polymeric Nanoparticles


The impact of cell culture media on the interaction of biopolymer-functionalized gold nanoparticles with cells: mechanical and toxicological properties
Introduction.
The increasing use of engineered nanomaterials in consumer products, development as diagnostic tools and therapeutic vectors, and growing awareness regarding nano-sized pollutants and environmental toxicology has led to a significant push in nanoscience research. Despite the prolific research output, in particular in the fields of nanomedicine and nanotoxicology, there has been mixed success translating biomedical nanoparticles (NPs) from the lab into the clinic 1 , 2 , 3 , 4 , 5 . The challenges in bridging the gap between bench-top work and clinical success may stem from difficulty in conducting large-scale synthesis of NPs, our limited understanding of fundamental bio-nano interactions, lack of worldwide standardization in the field regarding NP characterization and biological assays, and widespread inter-laboratory differences in techniques and methodology leading to difficulty reproducing work 6 , 7 , 8 . Furthermore, variation in experimental design, protocols, controls, and execution may influence experimental outcomes and hinder inter-laboratory reproducibility.
As a prominent example of the difficulty in standardizing experiments where nanoparticles-cell interactions are addressed, we investigated whether in vitro administration, i.e., the literal way in which researchers add NPs to cells, influenced the outcome of NP-cell interaction studies (i.e. NP uptake and adsorption to the cell surface). Particle administration could influence a number of factors such as the local environment around NPs, the distribution of NPs within the experimental media. It is therefore expected that the way in which NPs are administered (e.g. as a homogeneous suspension or as a concentrated bolus) would influence particle-cell interactions. Moreover, this could influence inter-particle interactions, NP colloidal stability, or protein adsorption.
In vitro , the ideal case is to expose cells to a colloidally stable, homogenous dispersion of NPs. However, this is not always possible given certain experimental restrictions–for example live-cell imaging setups can be sensitive to minor fluctuations in temperature, which can cause a shift in the imaging focal plane. Therefore, NPs may need to be administered as a concentrated dose that dilutes to the desired experimental concentration. We hypothesized that NP administration in vitro would influence the observed NP-cell interactions. There is a large body of work investigating the effect of particle physico-chemical properties (e.g. size, shape, surface charge and functionalization, material, hydrophilicity/hydrophobicity, etc.) on cellular uptake of particles 9 , 10 , 11 , 12 , and these factors definitely influence particle-cell interaction. However, issues of reproducibility in science persist, and these may be caused by lack of reporting, non-standardized characterization of nanomaterials, variations in biological assays, etc 13 .
The effect of particle administration in vitro on particle-cell interaction could be mediated through, for example, an effect on NPs colloidal stability, NP-protein interactions, or changing NPs sedimentation velocity/fluid dynamics 14 . Figure 1a provides a schematic representation of how NPs may be administered via different means. More generally stated, there are widespread inter-laboratory differences in cell culture that, when unrecognized or unacknowledged, may hinder reproducibility and proper interpretation of results. In fact, there have been numerous initiatives to improve reproducibility and confidence in biomedical research 15 and particle administration may be a variable which has been unacknowledged to date.
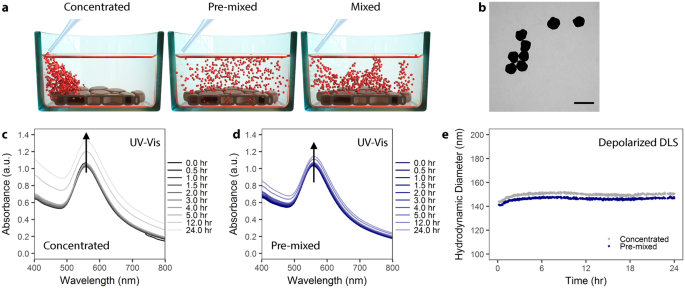
AuNP colloidal stability is independent of administration method. ( a ) Schematic showing the different administration method concepts. Particles can be administered to cells as a concentrated dose (left), a pre-mixed solution (center), or as a concentrated dose which is mixed via aspiration (right). ( b ) Transmission electron microscopy of citrate AuNP with shows a mean core diameter of 116 ± 12 nm (n = 289). Scale bar represents 200 nm. ( c ) UV-Vis spectra of PVP-coated AuNPs over 24 hr in cell culture media supplemented with 10% fetal bovine serum (FBS) following concentrated administration. ( d ) UV-Vis spectra of PVP-coated AuNPs administered via the pre-mixed method in cell culture media supplemented with 10% FBS. ( e ) Depolarized dynamic light scattering of PVP-coated AuNPs measured over 24 hr in complete cell culture media.
In the following work, we show that in vitro administration of NP can influence cellular uptake and NP-cell association. Moreover, we emphasize the critical influence of particle surface, and necessity for proper NP characterization. We utilized polyvinylpyrrolidone (PVP)-coated, 116 nm diameter gold nanoparticles (AuNP) as a model particle system to study the effects of in vitro administration on particle-cell interaction (Figs 1b and S1 ). AuNP were used because they are, from an analytical standpoint, simpler to detect. Moreover, due to surface plasmon effects we could qualitatively evaluate the formation of a protein corona around the NPs. We chose PVP as a stabilizing polymer because it is biocompatible, hydrophilic, and used commonly as a NP-stabilizing ligand 16 , 17 , 18 . Here, we favored the use of PVP 19 , 20 , 21 over a more common polymeric coating such as poly(ethylene glycol) (PEG) due to the tendency of PEG to minimize particle uptake 22 , 23 . 116 nm AuNP-PVP were administered to cells following three different administration paradigms: (1) NPs pre-mixed in complete cell culture media prior to cell exposure, (2) NPs administered as a concentrated dose to cells in media and then mixed via pipetting, or (3) NPs administered as a concentrated dose to cells in media without mixing (Fig. 1a ).
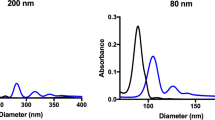
At this particular size, NP deposition and exposure to cells will be driven by particle sedimentation 24 . Thus the transport is simple, i.e. linear in time. Comparatively, transport of small NPs (i.e. <30 nm) is more heavily influenced by diffusion rather than sedimentation, and is non-linear in time 25 . Moreover, for larger NPs polydispersity is moderate and the influence of a non-instantaneous particle association to the cell is negligible, that is small particles may “diffuse away” if not immediately adherent to the cell surface while larger NPs “sit” on the cell. We anticipated that 116 nm AuNPs would emphasize any effects of pipetting on NP-cell interactions. Prior to in vitro cellular deposition and uptake studies, NPs were fully characterized in complete cell culture media via UV-Vis spectroscopy and depolarized dynamic light scattering (DDLS). NP dosimetry was simulated following the in vitro sedimentation, diffusion, and dosimetry (ISDD) model 26 , 27 , 28 , 29 . This model was further developed in order to account for administration-dependent interactions and model the observed phenomena. To fully understand this phenomenon, we also investigated 100 nm PVP-coated SiO 2 NP, and small, approximately 22 nm PVP-coated iron oxide NPs.
Results and Discussion
Nanoparticles are stable in complete cell culture medium.
Ensuring colloidal stability of NPs is not a trivial challenge when considering the behavior of NPs in complex biological media (e.g. blood or cell culture media containing serum). Upon introduction into media, macromolecules such as proteins and/or lipids will rapidly adsorb onto the NPs surface 30 , 31 , 32 , 33 . This event, coupled with the dynamic environment (i.e. high salt concentration, presence of electrolytes or other molecules, shifting pH), can result in a number of effects on NPs including colloidal destabilization and NP aggregation, competitive adsorption/desorption of macromolecules resulting in the loss of stabilizing ligands on the NP’s surface, and NP dissolution 14 , 34 . Therefore it is critical to characterize NPs in their relevant environment or media. Neglecting to consider these interactions may lead to difficulty or mistakes when interpreting data, or result in works wholly irreproducible by others 35 .
Colloidal stability of PVP-coated AuNP in 10% fetal bovine serum (FBS) supplemented, complete Dulbecco’s Modified Eagle’s Medium (cDMEM) was studied using UV-Vis spectroscopy and DDLS over 24 hr at 37 °C (Fig. 1c–e ). We added NPs to media via the pre-mixed or concentrated approaches in order to study potential NP aggregate formation due to the administration method. Figure 1c,d shows the evolution of the UV-Vis spectra of AuNPs and reveals that the NPs remain colloidally stable, as there is no significant peak broadening when administered via either the concentrated or pre-mixed method 36 , 37 . This colloidal stability was confirmed by DDLS (Fig. 1e ), demonstrating that PVP coating confers colloidal stability to NPs via steric repulsion, even under high salt conditions 38 .
Nanoparticle administration affects particle interaction with macrophages
We studied the effect of the NP administration methodology on cellular uptake by J774A.1 mouse monocyte/macrophages. Cells were exposed to AuNP-PVP via the concentrated, mixed, or pre-mixed methods for varying time points. J774A.1 cells were chosen as a model cell line because macrophages are phagocytic cells which readily uptake NPs 39 , 40 , 41 . These cells were therefore believed to give the most consistent conditions that would fulfill the ISDD model boundary conditions (i.e. the bottom of the cell culture dish is treated as a NP sink due to rapid particle uptake). At varying time points over 24 hr of exposure, AuNP-PVP uptake or deposition by J774A.1 cells was visualized by fluorescent and dark field hyperspectral microscopy (Fig. 2 ), and quantified via inductively coupled plasma-optical emission spectroscopy (ICP-OES). The deposition of AuNP-PVP following administration via the three methods showed that the fraction of the initial dose deposited for concentrated, mixed, and pre-mixed after 24 hr was 0.61, 0.63, and 0.30, respectively (Fig. 3a ). It is clear that the administration method influences cellular association of NPs even from the early time points of the experiment. However, it was unclear what drives this effect.
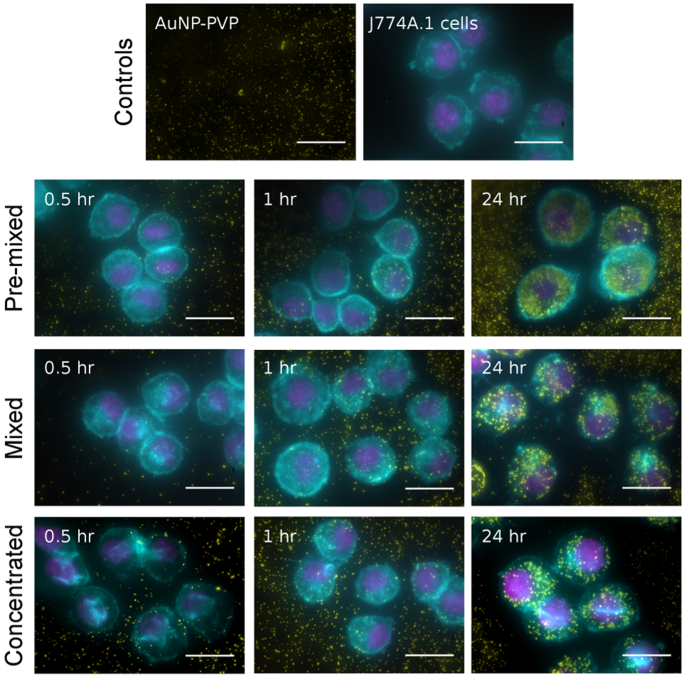
The deposition/uptake of AuNP-PVP (yellow) on J774A.1 cells was showed by overlaying enhanced darkfield microscopy images (AuNPs pseudo-colored yellow) onto fluorescent microscopy images of cells stained for actin (cyan) and cell nuclei (magenta). Scale bars represent 10 μm.
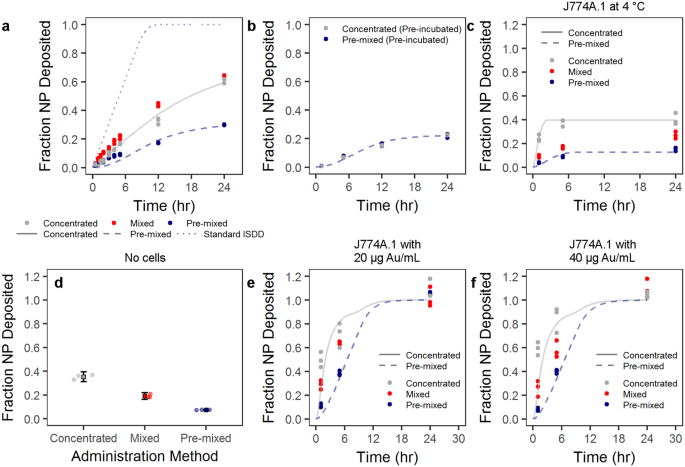
Particle cell interaction is dependent on administration method. ( a ) Particles administered as a concentrated, mixed, or pre-mixed dose showed variable uptake by J774A.1 mouse monocyte/macrophages as determined by ICP-OES. ( b ) This phenomenon was mitigated by first incubating the particle at a higher concentration in complete cell culture media, and then either adding as a concentrated dose or pre-mixing (pre-incubation). The trend in administration-dependent cellular interaction was conserved over various scenarios. ( c) Administration at 4 °C was conducted to reduce active cellular uptake. ( d ) Non-specific NP adsorption to the culture plate was tested by incubating particles in complete cell culture medium overnight. Circles represent single data points and boxes represent mean ± one standard deviation. ( e , f ) AuNP-PVP deposition at 20 and 40 μg Au/mL, respectively, was tested over 24 hr. Lines indicate modified ISDD fit of data with concentrated or pre-mixed assumptions, as well as the standard ISDD conditions.
To further investigate the potential effect of administration on the surface of the NP, we pre-incubated the NPs at an intermediate concentration (e.g. 700 μg Au/mL) in cDMEM prior to administration via the concentrated or pre-mixed methods (Fig. 3b ). By doing this pre-incubation any differences due to, for example protein adsorption, might be mitigate and the only difference between the two methods is thus physical. Indeed, it is apparent that the influence of administration was completely mitigated after the pre-incubation, thus indicating an effect of administration on the “biological identity” of the NPs.
It was interesting to see that the pre-mixed administration resulted in less particle deposition compared to the concentrated or mixed methods. In order to understand the results of these experiments better, we performed calculations based on the ISDD model (a full description of the calculations can be found in the Supporting Information).
The ISDD model makes several assumptions and sets boundary conditions regarding particle deposition and uptake: cells in the dish form a confluent layer, particles are stable or form “stable aggregates”, the bottom of the well-plate is treated as a particle sink (i.e. particles reaching this space are adsorbed or taken up by cells), and, importantly, that NPs are uniformly distributed in the cell culture media. Generally these are fair terms for an in vitro experiment, however as we show, these conditions are not always possible depending on the experiment. Thus, we altered the model to reflect some experimental differences. For one, we altered the boundary conditions so that the rate at which particles are uptaken by the cells is proportional to the concentration right outside the cells, and to the difference between the concentration of particles in the cells and a maximum concentration. This means that the rate at which particles can enter the cells depends on the particles surface and that cells can be saturated with cells. Moreover, the exists a stratified layer within the particle solution when adding via the concentrated method. Indeed, we observed that the particles formed a stratified layer when administered in the concentrated method (Fig. S2 ).
However, it is apparent that NP-cell interactions are affected by the way NPs are administered to cells. The environment surrounding NPs when introduced into complex biological media mediates this phenomenon, and concentration gradients in solution drive these protein-particle and inter-particle behaviors. This behavior may also be driven by the physico-chemical properties of NPs, which affect protein-protein, protein-NP, and NP-NP interactions. It was unclear whether cellular phenomena (e.g. active cellular uptake), NP concentration effects, or non-specific adsorption of AuNPs to the cell surface and well-plate bottom drove the differences in deposition and uptake for the AuNP-PVP. Therefore, we performed a number of experiments to observe whether active uptake, NP adsorption to the well plate, or NP concentration influenced our measurements. We investigated the role of active cellular uptake by observing AuNP-PVP uptake at a concentration of 70 μg Au/mL and at 4 °C (Fig. 3c ). By conducting the experiments at lower temperature, receptor-mediated (active) cellular uptake would be mitigated and any deposition should arise from non-specific NP adsorption to cells, well-plate sticking, or diffusion across the cellular membrane 42 , 43 . The trend remained that concentrated and mixed methods deposited more compared to pre-mixed. This trend appears to be a non-specific phenomenon, whereby administration method dictates the (non-specific) adsorption of NPs to cells or the well-plate surface. Figure 3d shows the fraction of NPs adsorbed onto a well-plate that did not contain any cells following incubation of AuNP-PVP overnight at 37 °C in cDMEM. A similar deposition trend (concentrated > mixed > pre-mixed) was observed, at values similar to the maximum deposition at 4 °C and 24 hr in the presence of cells.
Further experiments conducted at lower concentrations (20 and 40 μg Au/mL, 37 °C) showed the same trend where concentrated and mixed administration deposited more than the pre-mixed (Fig. 3e,f ). For both of these lower concentrations, the previous trend observed at earlier time points remained: Concentrated and mixed administration resulted in more AuNP-PVP deposition compared to the pre-mixed condition. However, at 24 hr it was shown that there were no differences between any of the three administration methods. These data show that regardless of NP concentration the deposition behavior is constant based on administration method. This indicates that the observed phenomenon is not linked to cellular behavior (e.g. active uptake), but is a product of the NP properties in solution.
By running the model for all the particles concentration (70, 40 and 20 μg Au/mL, 37 °C), it clearly appears that one at the highest concentration (70 μg Au/mL) there is a need to impose a maximum concentration of particles that the cells can uptake. In fact, these experiments are the only ones where after 24 hours only 30 to 60% of the particles have been taken up by the cells, compared to 100% for the lower concentrations. The conventional ISDD model, which imposes that all particles reaching the cells surface are taken up by them, substantially overestimates the uptake of particles in the case of 70 μg Au/mL. One can also observe, in the 20 and 40 μg Au/mL, that the standard ISDD model cannot account for the higher uptake of particles in concentrated case than in the premixed case. Since aggregation of particles has been ruled out, the only way to explain such a higher uptake is to consider a layering effect, where upon administration of a concentrated bolus of particles a thin layer with a concentration of particles about 10 times larger than that in the rest of the solution accumulates close to the cell surfaces and explains the faster increase in particles uptake compared to the ISDD model.
Initial protein adsorption drives administration-dependent particle uptake
In a previous work by Lesniak et al . 44 , it was shown that the presence of a protein corona can limit or slow particle adsorption to cells/surfaces in serum-free media. Thus, the protein corona can mediate the “adhesion” of particles on the cellular surface, and our observed difference in cellular deposition can therefore be supported by the variation of protein adsorption due to administration method. This hypothesis was investigated by following the change of extinction intensity in UV-Vis following different administration methods–higher protein adsorption was observed for concentrated compared to pre-mixed administration (Fig. 4a,b ).
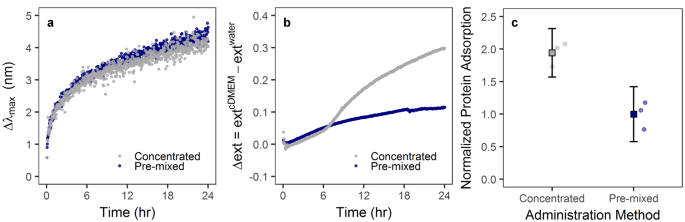
Particle introduction into complete cell culture media mediates protein adsorption to the particle surface. ( a ) UV-Vis analysis of AuNP-PVP over 24 hr showed a change in peak intensity wavelength. ( b ) UV-Vis analysis further showed a change in extinction intensity (Δext) dependent on administration method. These effects are indicative of protein adsorption to the nanoparticle surface. ( c ) Analysis of protein adsorption showed that, after 24 hr incubation in complete cell culture medium, AuNP-PVP administered via the concentrated route had an approximately 2-fold increase in protein adsorption compared to nanoparticles administered via the pre-mixed route.
Some studies have revealed that particle uptake was enhanced in the presence of serum proteins on polystyrene NPs 45 , 46 , and Prapainop et al . 47 also demonstrated that the coating layer on the NP surface can lead to misfolding of corona proteins, which then triggered NP uptake by specific cells that otherwise would not have done so. This protein structure modification may occur partially here because it has been demonstrated that some proteins in the presence of PVP showed higher refolding rate and aggregation-prone intermediates 48 , 49 . However, a slight red-shift of the LSPR band (max = 4–5 nm) was observed in AuNPs due to the formation of protein corona on particle surface (Fig. 4a ). A similar red-shift for BSA adsorption on polymer-coated gold nanorods was reported previously by Boulos et al . 50 . These spectroscopic observations were in agreement with the change of hydrodynamic size measured under the same conditions by DDLS (Fig. 1d ). The hydrodynamic size showed an increase of approximately 30 nm for AuNP-PVP, confirming the formation of protein corona, which might be composed of multiple protein layers. Most plasma proteins present a hydrodynamic diameter around 3–15 nm. Therefore, the protein corona in our case follows the model proposed by Simberg et al . 51 –a primary protein layer that adsorbs to the NP surface directly and a secondary layer that associates with the primary layer via protein-protein interactions. This multiple layers configuration may alter the activity of the proteins in the primary layer or modify the physiological response of the NPs, which may have an impact in the cellular uptake studies.
Interestingly, an increase in the extinction intensity over time was also observed (Fig. 4b ). This change of the extinction can also be attributed to protein adsorption on the NP surface, since the intensity also depends on changes in the complex dielectric function near the interface between the metal and the media. Nath and Chilkoti 52 and Shin et al . 53 demonstrated that the quantification of the protein adsorption/binding to AuNPs is comparatively facile using the measurement of extinction intensity. In these reports the intensity of maximum extinction increased as the increase in the concentration of protein bonded on gold surface. In addition, Nath and Chilkoti 52 reported that 12 nm diameter AuNPs can detect a refractive index change through the extinction intensity up to 24 nm away from the NP surface, whereas larger AuNPs can detect refractive index changes at a distance greater than 40 nm. Therefore, we can extract qualitative information about the amount of proteins adsorbed on NPs surface through the change of the extinction intensity upon incubating them in cDMEM.
The administration method-dependent response of AuNP-PVP, monitored in real time, is shown in Fig. 4b . The change of the extinction intensity was obtained with respect to the extinction in water (no presence of proteins). The change of the extinction profiles show a pronounced rise in the extinction intensity upon concentrated administration, while only a slight increase of the intensity for AuNP-PVP was detected upon pre-mixed administration. This finding demonstrates that the in vitro methodology can affect how many proteins are adsorbed on the NP surface, varying the physico-chemical properties of the NPs. In turn, this can have consequences on the NP-cell interaction. In order to support the extinction measurements, we added AuNP-PVP to cDMEM via the pre-mixed or concentrated method. After 24 hr, we centrifuged the AuNP-PVP and washed them in PBS. We estimated the concentration of protein adsorbed to the NP surfaces with SDS-PAGE and AgNO 3 staining using FIJI image analysis software to measure band density for every molecular weight at a fixed gold concentration (as determined by ICP-OES). Protein adsorption was compared by normalizing by the pre-mixed administration method (Fig. 4c ). Strikingly, protein adsorption with concentrated administration was 2-fold higher than with the pre-mixed method, even 24 hr after initial incubation. This indicates that initial administration has a lasting effect on particle biomolecular corona and subsequent cellular interaction.
Surface coating and size are critical factors mediating nanoparticle uptake
NP behavior in solution and with cells will be a factor of, among other things, the particle size, surface properties, and surface functionality. For example, PVP-coated AuNP will behave differently in solution compared to, for example, PEG-coated AuNP. Therefore, two AuNP formulations were synthesized as a control with amine-terminated PEG and amine-terminated poly(vinyl alcohol) (PVA) coatings. Both formulations showed colloidal stability over 24 hr in cDMEM independent of administration method (Fig. S3 ). In a control experiment, PVA-coated or PEG-coated AuNP with a core diameter of 116 nm were administered to J774A.1 mouse monocyte/macrophages via the pre-mixed and concentrated methods (Fig. 5a,d , respectively). We showed that there was no difference between the pre-mixed and mixed administration methods with PVA-coated or PEGylated AuNP. Moreover, there was significantly less NP uptake with PVA or PEG-coated AuNP compared to PVP-coated AuNP. The simulation of these data indicate that also in this case the standard ISDD boundary condition is not be able to account for the observed behavior. Only by imposing a substantial reduction in the rate of uptake of the particles the data can be properly simulated.
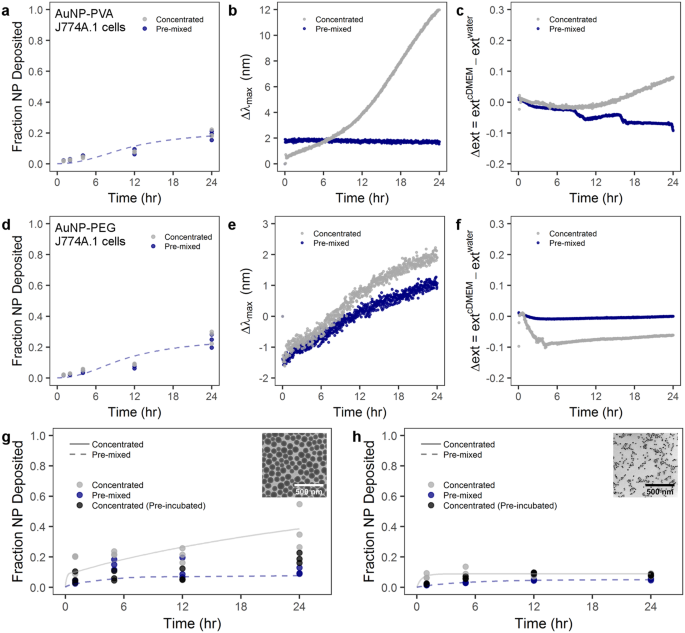
Administration method effect on particle-cell interaction is heavily dependent on particle material, size, and surface coating. Cellular uptake by J774A.1 murine cells and UV-Vis analysis of ( a – c ) PVA-coated AuNPs and ( d – f ) PEG-coated AuNPs. J774A.1 uptake of ( g ) PVP-coated SiO 2 NPs and ( h ) PVP-coated superparamagnetic iron oxide NPs. ( b , e ) LSPR band shift for PVA-coated and PEG-coated AuNPs, respectively, following the concentrated or pre-mixed administration. ( c , f ) Time-dependent change of the extinction intensity (Δext) with respect to the extinction in water at maximum for PVA-coated and PEG-coated AuNP, respectively. Lines indicate modified ISDD fit of data with concentrated or pre-mixed assumptions. Inset TEM micrographs show ( g ) PVP-coated SiO 2 and ( h ) PVP-coated superparamagnetic iron oxide NPs. Scale bars represent 500 nm.
This significant difference in uptake between PVP-coated AuNP and PEGylated or PVA-coated AuNP is explained by the “stealth” effect 22 , 54 , 55 , 56 . In essence, PEGylation reduces protein adsorption to NP and consequently NP are less prone to be recognized and phagocytosed by cells. Rather, the cells “see” water due to the mitigation of protein adsorption and the flexible nature of these hydrophilic polymer chains. This hypothesis was supported for the spectral evolution acquired by UV-Vis spectroscopy for PVA-coated and PEGylated AuNPs. Figure 5b,e shows no observable red-shifted of the LSPR band or increase of extinction intensity except in the case of AuNP-PVA(NH 2 ) with concentrated administration, where there is a noticeable variation in the maximum wavelength after of 8 hr. However, the hydrodynamic sizes of all NPs did not show any appreciable increase (Fig. S3 ), demonstrating that both polymer coatings prevent the protein adsorption within 24 hr. The variation in the spectral properties of AuNP-PVA(NH 2 ) can be likely attributed to hydrogen bonding and hydrophobic processes to enhance the colloidal stability in cDMEM, since the hydrodynamic size in water (232 nm) is 2-fold bigger than in cDMEM (136 nm).
In order to probe the effects of particle size and density, we studied PVP-coated SiO 2 and PVP-coated superparamagnetic iron oxide NPs (PVP-SPIONs) with core diameters of 99 ± 9 and 22 ± 1 nm, respectively (Fig. 5g,h inset TEM images and Fig. S4 ). The fraction of deposited SiO 2 NPs was determined by ICP-OES measurements, was similar to the AuNP-PVP case–concentrated administration resulted in greater NP deposition at 24 hr compared to pre-mixed administration (Fig. 5g ). Furthermore, by pre-incubating 100 nm PVP-coated SiO 2 at a higher concentration in cDMEM and then administering via the concentrated method (i.e. pre-incubation) there was no difference compared to the conventional pre-mixed case. However, size seems to play an important role, because size will affect the sedimentation behavior of particles 24 . The small, 22 nm PVP-SPIONs showed no difference between the concentrated, pre-mixed or concentrated with pre-incubation scenarios (Fig. 5h ). A similar protein study showed that for both PVP-coated SiO 2 NPs and PVP-SPIONs, the concentrated administration resulted in more protein adsorption (approximately 20 and 50% more, respectively) compared to pre-mixed administration at 24 hr after introduction to media. This presumably points to an interplay between protein adsorption onto particles, due to administration methods, and the particle size/density dictating sedimentation velocity (Fig. S5 ).
In order to advance our understanding of nanoparticle interactions with biological systems, it is pertinent that we fully understand how NPs behave in an in vitro environment including the application method and behavior in cDMEM over time. When trying to compare in vitro results between laboratories, there must be a comprehensive understanding of several key parameters: NP physico-chemical properties, colloidal stability in biological media, experimental design and implementation, and analytical methods. These factors are critical if we are to move forward in our understanding of nanotoxicology, particle-cell behavior, and use of particles for biomedical applications.
Here we have shown that a minor detail, the way in which NP are added to the cell culture, can have significant downstream effects in what we observe for the cellular interactions. Particles added as a concentrated “bolus” or as a bolus that is then mixed via pipetting, are more interacting with cells when compared to when NPs are pre-mixed to the desired concentration and then added to cells. The differences observed were also not attributable to active cellular mechanisms, but are more likely due to the initial introduction of NP to complex biological media. As a community, we should therefore make robust analytical efforts when interpreting in vitro data. This is of course non-trivial and requires a multidisciplinary approach. However, only with such precautions can we hope to gain a complete picture of NP-cell interactions.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Kamaly, N., Xiao, Z., Valencia, P. M., Radovic-Moreno, A. F. & Farokhzad, O. C. Targeted polymeric therapeutic nanoparticles: design, development and clinical translation. Chem. Soc. Rev. 41 , 2971–3010 (2012).
Article CAS Google Scholar
Venditto, V. J. & Szoka, F. C. Cancer nanomedicines: So many papers and so few drugs! Adv. Drug Deliv. Rev. 65 , 80–88 (2013).
Anselmo, A. C. & Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Rel. 190 , 15–28 (2014).
Tinkle, S. et al . Nanomedicines: Addressing the scientific and regulatory gap. Ann. N.Y. Acad. Sci. 1313 , 35–56 (2014).
Article ADS CAS Google Scholar
Min, Y., Caster, J. M., Eblan, M. J. & Wang, A. Z. Clinical translation of nanomedicine. Chem. Rev. 115 , 11147–11190 (2015).
Krug, H. F. & Wick, P. Nanotoxicology: An interdisciplinary challenge. Angew. Chem. - Intl. Ed. 50 , 1260–1278 (2011).
Krug, H. F. Nanosafety research-are we on the right track? Angew. Chem. - Intl. Ed. 53 , 12304–12319 (2014).
CAS Google Scholar
Henriksen-Lacey, M., Carregal-Romero, S. & Liz-Marzán, L. M. Current challenges towards in vitro cellular validation of inorganic nanoparticles. Bioconj. Chem . 212–221 (2016).
Zhang, L. W. & Monteiro-Riviere, N. A. Mechanisms of quantum dot nanoparticle cellular uptake. Toxicol. Sci. 110 , 138–155 (2009).
Yue, H. et al . Particle size affects the cellular response in macrophages. Eur. J. Pharm. Sci. 41 , 650–657 (2010).
Zhao, F. et al . Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 7 , 1322–1337 (2011).
Kinnear, C., Moore, T. L., Rodriguez-Lorenzo, L., Rothen-Rutishauser, B. & Petri-Fink, A. Form follows function: Nanoparticle shape and its implications for nanomedicine. Chem. Rev. 117 , 11476–11521 (2017).
Faria, M. et al . Minimum information reporting in bio–nano experimental literature. Nat. Nanotechnol. 13 , 777–785 (2018).
Moore, T. L. et al . Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 44 , 6287–6305 (2015).
Elliott, J. T. et al . Toward achieving harmonization in a nanocytotoxicity assay measurement through an interlaboratory comparison study. ALTEX 34 , 201–218 (2017).
Article Google Scholar
Kamada, H. et al . Antitumor activity of tumor necrosis factor-a conjugated with polyvinylpyrrolidone on solid tumors in mice. Cancer Res. 60 , 6416–6420 (2000).
CAS PubMed Google Scholar
Le Garrec, D. et al . Poly (N-vinylpyrrolidone)-block-poly (D, L-lactide) as a new polymeric solubilizer for hydrophobic anticancer drugs: in vitro and in vivo evaluation. J. Control. Rel. 99 , 83–101 (2004).
Stankus, D. P., Lohse, S. E., Hutchison, J. E. & Nason, J. A. Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environ. Sci. & Technol. 45 , 3238–3244 (2010).
Article ADS Google Scholar
Bihari, P. et al . Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part. Fibre Toxicol. 5 , 14 (2008).
Graf, C., Vossen, D. L. J., Imhof, A. & Van Blaaderen, A. A general method to coat colloidal particles with silica. Langmuir 19 , 6693–6700 (2003).
Fatisson, J., Quevedo, I. R., Wilkinson, K. J. & Tufenkji, N. Physicochemical characterization of engineered nanoparticles under physiological conditions: effect of culture media components and particle surface coating. Colloid. Surf. B: Biointer. 91 , 198–204 (2012).
Gref, R. et al . ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): Influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloid. Surf. B: Biointer. 18 , 301–313 (2000).
Walkey, C. D., Olsen, J. B., Guo, H., Emili, A. & Chan, W. C. W. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 134 , 2139–2147 (2012).
Cho, E. C., Zhang, Q. & Xia, Y. The effect of sedimentation and diffusion on cellular uptake of gold nanoparticles. Nat. Nanotechnol. 6 , 385–391 (2011).
Rodriguez-Lorenzo, L., Rothen-Rutishauser, B., Petri-Fink, A. & Balog, S. Nanoparticle Polydispersity Can Strongly Affect In Vitro Dose. Part. & Part. Syst. Charact. 32 , 321–333 (2015).
Teeguarden, J. G., Hinderliter, P. M., Orr, G., Thrall, B. D. & Pounds, J. G. Particokinetics in vitro : Dosimetry considerations for in vitro nanoparticle toxicity assessments. Toxicol. Sci. 95 , 300–312 (2007).
Hinderliter, P. M. et al . ISDD: A computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part. Fibre Toxicol. 7 , 36 (2010).
Cohen, J. M., Teeguarden, J. G. & Demokritou, P. An integrated approach for the in vitro dosimetry of engineered nanomaterials. Part. Fibre Toxicol. 11 , 20 (2014).
Thomas, D. G. et al . ISD3: A particokinetic model for predicting the combined effects of particle sedimentation, diffusion and dissolution on cellular dosimetry for in vitro systems. Part. Fibre Toxicol. 15 , 6 (2018).
Cedervall, T. et al . Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. 104 , 2050–2055 (2007).
Mahmoudi, M. et al . Protein-nanoparticle interactions: Opportunities and challenges. Chem. Rev. 111 , 5610–5637 (2011).
Docter, D. et al . The nanoparticle biomolecule corona: Lessons learned - challenge accepted? Chem. Soc. Rev. 44 , 6094–121 (2015).
Gebauer, J. S. et al . Impact of the nanoparticle–protein corona on colloidal stability and protein structure. Langmuir 28 , 9673–9679 (2012).
Larson, T. A., Joshi, P. P. & Sokolov, K. Preventing protein adsorption and macrophage uptake of gold nanoparticles via a hydrophobic shield. ACS Nano 6 , 9182–9190 (2012).
Plant, A. L., Locascio, L. E., May, W. E. & Gallagher, P. D. Improved reproducibility by assuring confidence in measurements in biomedical research. Nat. Methods 11 , 895–898 (2014).
Aili, D., Enander, K., Baltzer, L. & Liedberg, B. Assembly of polypeptide-functionalized gold nanoparticles through a heteroassociation- and folding-dependent bridging. Nano Lett. 8 , 2473–2478 (2008).
Amendola, V. & Meneghetti, M. Size evaluation of gold nanoparticles by UV–Vis spectroscopy. J. Phys. Chem. C 113 , 4277–4285 (2009).
Balog, S. et al . Characterizing nanoparticles in complex biological media and physiological fluids with depolarized dynamic light scattering. Nanoscale 7 , 5991–5997 (2015).
Wang, H., Wu, L. & Reinhard, B. M. Scavenger receptor mediated endocytosis of silver nanoparticles into J774A.1 macrophages is heterogeneous. ACS Nano 6 , 7122–7132 (2012).
Ahmad Khanbeigi, R. et al . The delivered dose: Applying particokinetics to in vitro investigations of nanoparticle internalization by macrophages. J. Control. Rel. 162 , 259–266 (2012).
Kuhn, D. A. et al . Different endocytotic uptake mechanisms for nanoparticles in epithelial cells and macrophages. Beilstein J. Nanotechnol. 5 , 1625–1636 (2014).
Chithrani, B. D. & Chan, W. C. W. Elucidating the mechanism of cellular uptake and removal of protein-coated gold nanoparticles of different sizes and shapes. Nano Lett. 7 , 1542–1550 (2007).
Silverstein, S. C., Steinman, R. M. & Cohn, Z. A. Endocytosis. Ann. Rev. Biochem. 46 , 669–722 (1977).
Lesniak, A. et al . Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J. Am. Chem. Soc. 135 , 1438–1444 (2013).
Ehrenberg, M. S., Friedman, A. E., Finkelstein, J. N., Oberdörster, G. & McGrath, J. L. The influence of protein adsorption on nanoparticle association with cultured endothelial cells. Biomater. 30 , 603–610 (2009).
Johnston, H. J. et al . Evaluating the uptake and intracellular fate of polystyrene nanoparticles by primary and hepatocyte cell lines in vitro . Toxicol. Appl. Pharmacol. 242 , 66–78 (2010).
Prapainop, K., Witter, D. P. & Wentworth, P. A chemical approach for cell-specific targeting of nanomaterials: Small molecule- initiated misfolding of nanoparticle corona proteins. J. Am. Chem. Soc. 134 , 4100–4103 (2012).
Jiang, Y., Yan, Y.-B. & Zhou, H.-M. Polyvinylpyrrolidone 40 assists the refolding of bovine carbonic anhydrase B by accelerating the refolding of the first molten globule intermediate. J. Biol. Chem. 281 , 9058–9065 (2006).
Treuel, L. et al . Quantifying the influence of polymer coatings on the serum albumin corona formation around silver and gold nanoparticles. J. Nanoparticle Res. 14 , 1102 (2012).
Boulos, S. P. et al . Nanoparticle–protein interactions: A thermodynamic and kinetic study of the adsorption of bovine serum albumin to gold nanoparticle surfaces. Langmuir 29 , 14984–14996 (2013).
Simberg, D. et al . Differential proteomics analysis of the surface heterogeneity of dextran iron oxide nanoparticles and the implications for their in vivo clearance. Biomater. 30 , 3926–3933 (2009).
Nath, N. & Chilkoti, A. Label-free biosensing by surface plasmon resonance of nanoparticles on glass: Optimization of nanoparticle size. Anal. Chem. 76 , 5370–5378 (2004).
Shin, Y.-B. et al . Analysis of recombinant protein expression using localized surface plasmon resonance (LSPR). Biosens. Bioelect. 22 , 2301–2307 (2007).
Owens, D. E. & Peppas, N. A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Intl. J. Pharm. 307 , 93–102 (2006).
Gilges, M., Kleemiss, M. H. & Schomburg, G. Capillary zone electrophoresis separations of basic and acidic proteins using poly(vinyl alcohol) coatings in fused silica capillaries. Anal. Chem. 66 , 2038–2046 (1994).
Park, J. et al . Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. Nat. Mater. 3 , 891–895 (2004).
Download references
Acknowledgements
The authors acknowledge support from the National Center of Competence in Research (NCCR) “Bio-inspired Materials” as well as the Adolphe Merkle Foundation.
Author information
Authors and affiliations.
Adolphe Merkle Institute, Université de Fribourg, Fribourg, 1700, Switzerland
Thomas L. Moore, Dominic A. Urban, Laura Rodriguez-Lorenzo, Ana Milosevic, Federica Crippa, Miguel Spuch-Calvar, Sandor Balog, Barbara Rothen-Rutishauser & Alke Petri-Fink
Chemistry Department, Université de Fribourg, Fribourg, 1700, Switzerland
Marco Lattuada & Alke Petri-Fink
You can also search for this author in PubMed Google Scholar
Contributions
T.L.M. and A.F. conceived the project and designed the experiments. T.L.M. synthesized SiO 2 NPs, conducted all cellular uptake experiments and analysed data. D.A.U. conducted ICP measurements. L.R.L. synthesized AuNPs, conducted DDLS and UV-Vis stability measurements and analysed spectra data. A.M. conducted protein adsorption experiments. F.C. synthesized SPIONs. M.S.C. coated, and characterized NPs. S.B. analysed DDLS data. M.L. developed and conducted theoretical modeling. The manuscript was written by T.L.M. and A.F. with contributions from L.R.L., B.R.R. and M.L. All co-authors read and approved the manuscript prior to submission.
Corresponding author
Correspondence to Alke Petri-Fink .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Moore, T.L., Urban, D.A., Rodriguez-Lorenzo, L. et al. Nanoparticle administration method in cell culture alters particle-cell interaction. Sci Rep 9 , 900 (2019). https://doi.org/10.1038/s41598-018-36954-4
Download citation
Received : 23 August 2018
Accepted : 09 November 2018
Published : 29 January 2019
DOI : https://doi.org/10.1038/s41598-018-36954-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.

IMAGES
VIDEO
COMMENTS
Cell culture is a very versatile tool in the investigation of basic scientific and translation research questions. The advantage of using cell lines in scientific research is their homogeneity and associated reproducibility in data generated. This chapter introduces the principles behind the setup of a cell culture lab and the guidelines that ...
Cell culture is a method for growing or maintaining cells in vitro under controlled conditions. ... New traceability and reporting standards aim to improve transparency in stem cell research ...
Cell culture is defined as a method to grow cells in a suitable physiological condition, including media, temperature, and potential of hydrogen (pH). Since the early last century, cell culture has been widely used for virus study, vaccine, gene function, and pharmaceutical productions. In basic research, cells are routinely used in studying ...
This paper reports culture conditions for the expansion of near-homogeneous populations of mouse Lgr5 + intestinal stem cells. These methods will enable the study of intestinal biology and ...
Furthermore, paper is advantageous concerning cell-based assays due to its reticulated structure, which offers a naturally 3D environment closer to the native cellular environment. ... This review highlights the fundamental role that cell culture methods play in life research and development, as well as recent discoveries and technological ...
Refresh cell culture. Nature Biomedical Engineering 5 , 783-784 ( 2021) Cite this article. Biomedical research needs upgraded standards for the monitoring, control and reporting of the ...
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications. ... Cell culture systems are one of ...
Research review paper. Mammalian cell culture for production of recombinant proteins: A review of the critical steps in their biomanufacturing. Author links open overlay panel Róisín O'Flaherty a, Adam Bergin a 1, Evangelia Flampouri a 1, Letícia Martins Mota a 1, Ismael Obaidi a b 1, Andrew Quigley a 1, Yongjing Xie a 1, Michael Butler a.
The unique features of paper-based system in supporting 3D cell culture enables it to be used for a range of studies, such as the development of normal or disease models in vitro by manipulating the physical or chemical properties of the paper to control nutrient and oxygen diffusion (Fig. 1) [9], [10].Such 3D cell culture models can also be used to study cell-drug interactions in a high ...
The culture method and cell characterizations will be summarized. Then the application of three-dimensional cell culture system is further explored, such as in the fields of drug screening, organoids and assembloids. Finally, the directions for future research of three-dimensional cell culture are stated briefly. 1.
Primary cell culture plays an important role in biomedical research. Changes in culturing conditions can regulate the biological behavior and performance of the cells, which can induce the cells into a more suitable state for use. Common methods include adding many kinds of regulating factors to the culture medium, such as cytokines, drugs, and ...
Paper-based cell culture platforms have emerged as a promising approach for a myriad of biomedical applications, such as tissue engineering, disease models, cancer research, biotechnology, high-throughput testing, biosensing, and diagnostics. Paper enables the generation of highly flexible, biocompatible, inexpensive, porous, and three-dimensional (3D) constructs and devices. These systems ...
1 Introduction. The influence of cell culture technology on human society has been immeasurable. Progress in biology in recent years, for example, has depended heavily on cell culture technology. 1 In addition, cell culture-based practical technologies have been developed in various areas, including the assessment of the efficacy and toxicity of new drugs, manufacture of vaccines and ...
Figure 2: 3D hydrogels for cell culture. ( a) 3D hydrogels can be engineered to present a more realistic microenvironment to cells. Hydrogel design variables are indicated. ( b) Mouse MSCs ...
General concept of cell culture is the prop agation o f cells or fibrob last or livin g. tissues in a defined media that conduciv e their growth. Shortly, a growth of cell. artificially known as ...
The present review provides a comprehensive outlook toward the possibilities of developing a functional in vitro liver tissue model on a paper platform. To this end, we first addressed the suitability of using a cellulose-based paper matrix as a substrate for cell culture applications. Then, we highlighted various patterning and substrate modification strategies that have been exploited to ...
It is demonstrated that low inoculation density promoted endothelial-to-hematopoietic transition, followed by improved hematopoietic progenitor formation and erythrocyte generation, and the low cell culture density will improve RBC generation from human PSCs. Human pluripotent stem cell (hPSC)-derived red blood cells (RBCs) possess great potential for compensating shortages in transfusion ...
As a highly interdisciplinary field, working with nanoparticles in a biomedical context requires a robust understanding of soft matter physics, colloidal behaviors, nano-characterization methods ...
Biosurface and Biotribology is an open access journal publishing papers focused on the surfaces and tribology associated with biological systems. Abstract It is a developed photosynthetic co-culture system to alleviate the hypoxia and hypoxia/reoxygenation (H/R)-injured human umbilical vein endothelial cells (HUVECs). ... LDH levels in cell ...
Dublin, May 10, 2024 (GLOBE NEWSWIRE) -- The "Global Cell Culture Market by Product (Consumables (Media, Sera, Reagents), Vessels (Roller Bottle, Cell... Global Cell Culture Research Report 2024 ...
Due to the lack of uniformed diagnosis criteria and the amount of ACTDs, it would be impossible to describe the cell culture types used in the research on all of them in one paper. So in this review, the authors have focused on few selected ACTDs, and the culture types used in research on them. These diseases are presented in Table 1.