- Skip to main content
- Keyboard shortcuts for audio player

This experimental drug could change the field of cancer research

Sacha Pfeiffer

Jonaki Mehta

The new treatment is categorized as immunotherapy. skaman306/Getty Images hide caption
The new treatment is categorized as immunotherapy.
A tiny group of people with rectal cancer just experienced something of a scientific miracle: their cancer simply vanished after an experimental treatment.
In a very small trial done by doctors at New York's Memorial Sloan Kettering Cancer Center, patients took a drug called dostarlimab for six months. The trial resulted in every single one of their tumors disappearing. The trial group included just 18 people, and there's still more to be learned about how the treatment worked. But some scientists say these kinds of results have never been seen in the history of cancer research.
Dr. Hanna Sanoff of the University of North Carolina's Lineberger Comprehensive Cancer Center joined NPR's All Things Considered to outline how this drug works and what it could mean for the future of cancer research. Although she was not involved with the study, Dr. Sanoff has written about the results.
This interview has been lightly edited
On her first reaction to the results: I mean, I am incredibly optimistic. Like you said in the introduction, we have never seen anything work in 100 percent of people in cancer medicine.
On how the drug works to treat cancer: This drug is one of a class of drugs called immune checkpoint inhibitors. These are immunotherapy medicines that work not by directly attacking the cancer itself, but actually getting a person's immune system to essentially do the work. These are drugs that have been around in melanoma and other cancers for quite a while, but really have not been part of the routine care of colorectal cancers until fairly recently.
On the kinds of side effects patients experienced: Very, very few in this study - in fact, surprisingly few. Most people had no severe adverse effects at all.
On how this study could be seen as 'practice-changing': Our hope would be that for this subgroup of people - which is only about five percent to 10 percent of people who have rectal cancer - if they can go on and just get six months of immunotherapy and not have any of the rest of this - I don't even know the word to use. Paradigm shift is often used, but this really absolutely is paradigm-shifting.
On why the idea of being able to skip surgery for cancer treatment is so revolutionary: In rectal cancer, this is part of the conversation we have with someone when they're diagnosed. We are very hopeful for being able to cure you, but unfortunately, we know our treatments are going to leave you with consequences that may, in fact, be life-changing. I have had patients who, after their rectal cancer, have barely left the house for years - and in a couple of cases, even decades - because of the consequences of incontinence and the shame that's associated with this.
On next steps for the drug: What I'd really like us to do is get a bigger trial where this drug is used in a much more diverse setting to understand what the real, true response rate is going to be. It's not going to end up being 100 percent. I hope I bite my tongue on that in the future, but I can't imagine it will be 100 percent. And so when we see what the true response rate is, that's when I think we can really do this all the time.
This piece was reported by Sacha Pfeiffer, produced by Jonaki Mehta and edited by Kathryn Fox. It was adapted for the web by Manuela Lopez Restrepo.
- cancer treatment
Treatment Research
In a new study in mice, researchers showed they could enhance radiation therapy by boosting levels of the BAMBI protein in MDSC immune cells in the tumor microenvironment. After radiation, T cells flooded into the tumor and killed tumors elsewhere in the body.
In a clinical trial, people being treated for cancer who participated in virtual mind–body fitness classes were less likely to be hospitalized, and had shorter stays when they were hospitalized, than people who did not take the classes.
NCI’s James H. Doroshow, M.D., reflects on the accomplishments of NCI-MATCH, a first-of-its-kind precision medicine cancer trial, and gives an overview of three new successor trials: ComboMATCH, MyeloMATCH, and iMATCH.
A new study, conducted largely in mice, may help explain why a currently used molecular marker—called mismatch repair deficiency—doesn’t always work to predict which patients will respond to immunotherapies called immune checkpoint inhibitors.
New approach may increase the effectiveness of T-cell-based immunotherapy treatments against solid tumors.
A cancer-infecting virus engineered to tamp down a tumor’s ability to suppress the immune system shrank tumors in mice, a new study shows. The modified oncolytic virus worked even better when used along with an immune checkpoint inhibitor.
Despite recommendations, a new analysis shows few people with cancer undergo germline testing to learn if their cancer may have been caused by gene changes inherited from a parent. Germline testing can help doctors determine the best treatments for a patient and help identify people whose family members may be at higher risk of cancer.
ComboMATCH will consist of numerous phase 2 cancer treatment trials that aim to identify promising drug combinations that can advance to larger, more definitive clinical trials.
A new study has compared three formulations of an mRNA vaccine designed to treat cancers caused by human papillomavirus (HPV) infections. All three vaccines showed promise in mice.
Researchers have identified a mechanism by which cancer cells develop specific genetic changes needed to become resistant to targeted therapies. They also showed that this process, called non-homologous end-joining (NHEJ), can potentially be disrupted.
For some people with cancer, is 6 months of immunotherapy the only treatment they might ever need? Or 4 weeks of immunotherapy followed by minor surgery? Results from several small clinical trials suggest these scenarios may be bona fide possibilities.
Two research teams have developed ways of overcoming barriers that have limited the effectiveness of CAR T-cell therapies, including engineering ways to potentially make them effective against solid tumors like pancreatic cancer and melanoma.
In people with cancer treated with immune checkpoint inhibitors, a rare, but often fatal, side effect is inflammation in the heart, called myocarditis. Researchers have now identified a potential chief cause of this problem: T cells attacking a protein in heart cells called α-myosin.
Researchers have modified a chemo drug, once abandoned because it caused serious gut side effects, so that it is only triggered in tumors but not normal tissues. After promising results in mice, the drug, DRP-104, is now being tested in a clinical trial.
Two research teams have developed a treatment approach that could potentially enable KRAS-targeted drugs—and perhaps other targeted cancer drugs—flag cancer cells for the immune system. In lab studies, the teams paired these targeted drugs with experimental antibody drugs that helped the immune system mount an attack.
Inflammation is considered a hallmark of cancer. Researchers hope to learn more about whether people with cancer might benefit from treatments that target inflammation around tumors. Some early studies have yielded promising results and more are on the horizon.
NCI researchers are developing an immunotherapy that involves injecting protein bits from cytomegalovirus (CMV) into tumors. The proteins coat the tumor, causing immune cells to attack. In mice, the treatment shrank tumors and kept them from returning.
FDA has approved the combination of the targeted drugs dabrafenib (Tafinlar) and trametinib (Mekinist) for nearly any type of advanced solid tumor with a specific mutation in the BRAF gene. Data from the NCI-MATCH trial informed the approval.
People with cancer who take immunotherapy drugs often develop skin side effects, including itching and painful rashes. New research in mice suggests these side effects may be caused by the immune system attacking new bacterial colonies on the skin.
Researchers have developed tiny “drug factories” that produce an immune-boosting molecule and can be implanted near tumors. The pinhead-sized beads eliminated tumors in mice with ovarian and colorectal cancer and will soon be tested in human studies.
Women are more likely than men to experience severe side effects from cancer treatments such as chemotherapy, targeted therapy, and immunotherapy, a new study finds. Researchers hope the findings will increase awareness of the problem and help guide patient care.
Research to improve CAR T-cell therapy is progressing rapidly. Researchers are working to expand its use to treat more types of cancer and better understand and manage its side effects. Learn how CAR T-cell therapy works, which cancers it’s used to treat, and current research efforts.
Experts say studies are needed on how to best transition telehealth from a temporary solution during the pandemic to a permanent part of cancer care that’s accessible to all who need it.
Removing immune cells called naive T cells from donated stem cells before they are transplanted may prevent chronic graft-versus-host disease (GVHD) in people with leukemia, a new study reports. The procedure did not appear to increase the likelihood of patients’ cancer returning.
A specific form of the HLA gene, HLA-A*03, may make immune checkpoint inhibitors less effective for some people with cancer, according to an NCI-led study. If additional studies confirm the finding, it could help guide the use of these commonly used drugs.
The success of mRNA vaccines for COVID-19 could help accelerate research on using mRNA vaccine technology to treat cancer, including the development of personalized cancer vaccines.
Aneuploidy—when cells have too many or too few chromosomes—is common in cancer cells, but scientists didn’t know why. Two new studies suggest that aneuploidy helps the cells survive treatments like chemotherapy and targeted therapies.
New research suggests that fungi in the gut may affect how tumors respond to cancer treatments. In mice, when bacteria were eliminated with antibiotics, fungi filled the void and impaired the immune response after radiation therapy, the study found.
FDA has approved belumosudil (Rezurock) for the treatment of chronic graft-versus-host disease (GVHD). The approval covers the use of belumosudil for people 12 years and older who have already tried at least two other therapies.
In lab studies, the antibiotic novobiocin showed promise as a treatment for cancers that have become resistant to PARP inhibitors. The drug, which inhibits a protein called DNA polymerase theta, will be tested in NCI-supported clinical trials.
A drug called avasopasem manganese, which has been found to protect normal tissues from radiation therapy, can also make cancer cells more vulnerable to radiation treatment, a new study in mice suggests.
While doctors are familiar with the short-term side effects of immune checkpoint inhibitors, less is known about potential long-term side effects. A new study details the chronic side effects of these drugs in people who received them as part of treatment for melanoma.
Cholesterol-lowering drugs known as PCSK9 inhibitors may improve the effectiveness of cancer immune checkpoint inhibitors, according to studies in mice. The drugs appear to improve the immunotherapy drugs’ ability to find tumors and slow their growth.
Researchers have developed a nanoparticle that trains immune cells to attack cancer. According to the NCI-funded study, the nanoparticle slowed the growth of melanoma in mice and was more effective when combined with an immune checkpoint inhibitor.
A comprehensive analysis of patients with cancer who had exceptional responses to therapy has revealed molecular changes in the patients’ tumors that may explain some of the exceptional responses.
Researchers are developing a new class of cancer drugs called radiopharmaceuticals, which deliver radiation therapy directly and specifically to cancer cells. This Cancer Currents story explores the research on these emerging therapies.
FDA has recently approved two blood tests, known as liquid biopsies, that gather genetic information to help inform treatment decisions for people with cancer. This Cancer Currents story explores how the tests are used and who can get the tests.
Cancer cells with a genetic feature called microsatellite instability-high (MSI-high) depend on the enzyme WRN to survive. A new NCI study explains why and reinforces the idea of targeting WRN as a treatment approach for MSI-high cancer.
Efforts to contain the opioid epidemic may be preventing people with cancer from receiving appropriate prescriptions for opioids to manage their cancer pain, according to a new study of oncologists’ opioid prescribing patterns.
The gene-editing tool CRISPR is changing the way scientists study cancer, and may change how cancer is treated. This in-depth blog post describes how this revolutionary technology is being used to better understand cancer and create new treatments.
FDA’s approval of pembrolizumab (Keytruda) to treat people whose cancer is tumor mutational burden-high highlights the importance of genomic testing to guide treatment, including for children with cancer, according to NCI Director Dr. Ned Sharpless.
Patients with acute graft-versus-host disease (GVHD) that does not respond to steroid therapy are more likely to respond to the drug ruxolitinib (Jakafi) than other available treatments, results from a large clinical trial show.
NCI is developing the capability to produce cellular therapies, like CAR T cells, to be tested in cancer clinical trials at multiple hospital sites. Few laboratories and centers have the capability to make CAR T cells, which has limited the ability to test them more broadly.
An experimental drug may help prevent the chemotherapy drug doxorubicin from harming the heart and does so without interfering with doxorubicin’s ability to kill cancer cells, according to a study in mice.
In people with blood cancers, the health of their gut microbiome appears to affect the risk of dying after receiving an allogeneic hematopoietic stem cell transplant, according to an NCI-funded study conducted at four hospitals across the globe.
A novel approach to analyzing tumors may bring precision cancer medicine to more patients. A study showed the approach, which analyzes gene expression using tumor RNA, could accurately predict whether patients had responded to treatment with targeted therapy or immunotherapy.
Bone loss associated with chemotherapy appears to be induced by cells that stop dividing but do not die, a recent study in mice suggests. The researchers tested drugs that could block signals from these senescent cells and reverse bone loss in mice.
Some experts believe that proton therapy is safer than traditional radiation, but research has been limited. A new observational study compared the safety and effectiveness of proton therapy and traditional radiation in adults with advanced cancer.
In people with cancer, the abscopal effect occurs when radiation—or another type of localized therapy—shrinks a targeted tumor but also causes untreated tumors in the body to shrink. Researchers are trying to better understand this phenomenon and take advantage of it to improve cancer therapy.
An experimental drug, AMG 510, that targets mutated forms of the KRAS protein completely shrank tumors in cancer mouse models and data from a small clinical trial show that it appears to be active against different cancer types with a KRAS mutation.
Researchers have engineered an oncolytic virus to kill cancer cells and boost the immune response against tumors. In a new study, the virus provided T cells around tumors with a hormone they need for their own cell-killing functions.
FDA has approved entrectinib (Rozlytrek) for the treatment of children and adults with tumors bearing an NTRK gene fusion. The approval also covers adults with non-small cell lung cancer harboring a ROS1 gene fusion.
A new NCI-supported study showed that altering cancer cell metabolism by feeding mice a diet very low in the nutrient methionine improved the ability of chemotherapy and radiation therapy to shrink tumors.
An NCI-funded clinical trial is testing the immunotherapy drug nivolumab (Opdivo) in people who have advanced cancer and an autoimmune disease, such as rheumatoid arthritis, lupus, or multiple sclerosis, who are often excluded from such trials.
Researchers have identified a protein called CD24 that may be a new target for cancer immunotherapy. The protein is a ‘don’t eat me’ signal that prevents immune cells called macrophages from engulfing and eating cells.
Injecting cells undergoing necroptosis, a form of cell death, into tumors in mice kickstarted an immune response against the tumors, researchers have found. When combined with immunotherapy, the treatment was effective at eliminating tumors in mice.
Researchers have identified proteins that may play a central role in transforming T cells from powerful destroyers to depleted bystanders that can no longer harm cancer cells. The findings could lead to strategies for boosting cancer immunotherapies.
Did you know that NCI supports clinical trials of new treatments for pet dogs with cancer? Learn more about NCI’s comparative oncology studies and how they may also help people with cancer.
Researchers have discovered a potential way to turn on one of the most commonly silenced tumor-suppressor proteins in cancer, called PTEN. They also found a natural compound, I3C, that in lab studies could flip the on switch.
New findings from a clinical trial suggest that a single dose of radiation therapy may control painful bone metastases as effectively as multiple lower doses of radiation therapy.
The expanding use of cancer immunotherapy has revealed a variety of side effects associated with this treatment approach. Researchers are now trying to better understand how and why these side effects occur and develop strategies for better managing them.
The investigational immunotherapy drug bintrafusp alfa (also called M7824), a bifunctional fusion protein, shrank the tumors of some patients with advanced HPV-related cancers, according to results from a phase 1 clinical trial.
A new study provides insight into how cancer immunotherapy works and suggests ways to enhance the treatment’s effectiveness. The NCI-led study, published in Science, examined the effect of high potassium levels on T cells.
Pain is a common and much-feared symptom among people with cancer and long-term survivors. As more people survive cancer for longer periods, there is a renewed interest in developing new, nonaddictive approaches for managing their chronic pain.
- Scientific Advisors
- Our Approach
- Our Process
- Case Studies
- Frequently Asked Questions
- Testimonials
Care is just a click away. Contact us to learn more:
- Terms & Conditions
Diagnosis and treatment of cancer — Case Studies
Case Study 1 : A patient came to us while on Nexavar for large B cell lymphoma. After an initial response to the drug, the cancer had started to accelerate. We analyzed her cancer tumor and found that while the data supported the use of Nexavar, they also supported the addition of Irinotecan. When Irinotecan was added to her treatment, the tumor stabilized - and remains stable today. We also identified a second mechanism that points to specific clinical trial agents, should the disease start to progress.
Case Study 2 : A male in his 50s was diagnosed with metastatic melanoma. When we examined his tumor, we found two distinct actionable hypotheses. The scientifically stronger one was based on an upregulation of the CDK-2 pathway (combined with a deletion of PKC-eta, a gene that inhibits CDK-2), suggesting a investigational drug (Flavopiridol) that was accruing for a Phase II trial in melanoma. A somewhat weaker hypothesis (fewer corroborating pieces of evidence) was for the SRC and the LCK pathway, which are targets of dasatanib. The patient is currently responding well to his existing therapy, but these approaches remain as a “plan B” in the event that his current treatment stops working.
Case Study 3 : A 54-year old male suffering from malignant melanoma approached us. He was being treated by a top melanoma physician/researcher at UCLA. He had previously failed 5 different therapies, and because he had a BRAF mutation, he had enrolled in the PLX4032 trial that had produced excellent, if short term, results for patients with this mutation. This patient responded to the drug for only 3 months, after which time the tumor progressed and he discontinued the trial. He was then placed in a trial for a MEK inhibitor (GSK1120212), to which he responded briefly. With the support of the physician, we were invited to perform an analysis.
Our analysis of the patient’s tumor suggested that a drug approved for kidney cancer, Sutent, might be appropriate in this case. The patient’s physician put him on Sutent, and within 2-3 days his abdomen, which had been grossly extended, shrank back to normal size, and the patient was able to eat again. The patient’s sister told us: “Your analysis changed the therapy the oncologist gave my brother. He praised it for the well-thought through proposal and the unusual thoroughness and depth of the report. I only wished that we had engaged you earlier so that he could have started Sutent sooner."
Case Study 4 : A male in his 60s with metastatic Non-Small Cell Lung Carcinoma (NSCLC) came to us while partially responding to Avastin. Our analysis suggested that his cancer was driven by two primary pathways: VEGF and PDGF. VEGF is Avastin’s target, which explained the partial response. He was not being treated with a drug targeting PDGF. This suggested that Sutent (which targets both pathways) may be suitable alone or in combination with the Avastin, or Gleevec (which targets PDGF) may be suitable to add to Avastin. Interestingly, while the data indicated a problem with EGFR (a third pathway commonly activated in NSCLC), our analysis concluded that this was not the driving force behind his cancer because there was minimal activity in the rest of the pathway. After presenting this information, we learned that the patient had not responded to Tarceva (a drug that targets EGFR) despite having an EGFR mutation and KRAS unmutated – the personalized medicine “gold standard” that predicts a positive response to Tarceva.
*From our previous incarnation as the Personalized Oncology Services division of CollabRx.
Contact us to learn whether GeneKey can be helpful to you.
PERSPECTIVE article
Case report: end-stage recurrent glioblastoma treated with a new noninvasive non-contact oncomagnetic device.

- 1 Kenneth R. Peak Brain and Pituitary Tumor Treatment Center, Department of Neurosurgery, Houston Methodist Neurological Institute, Houston, TX, United States
- 2 Department of Neurosurgery, Houston Methodist Research Institute, Houston, TX, United States
- 3 Department of Neurosurgery, Weill Cornell Medical College, New York, NY, United States
Alternating electric field therapy has been approved for glioblastoma (GBM). We have preclinical evidence for anticancer effects in GBM cell cultures and mouse xenografts with an oscillating magnetic field (OMF) generating device. Here we report OMF treatment of end-stage recurrent glioblastoma in a 53-year-old man who had undergone radical surgical excision and chemoradiotherapy, and experimental gene therapy for a left frontal tumor. He experienced tumor recurrence and progressive enlargement with leptomeningeal involvement. OMF for 5 weeks was well tolerated, with 31% reduction of contrast-enhanced tumor volume and reduction in abnormal T2-weighted Fluid-Attenuated Inversion Recovery volume. Tumor shrinkage appeared to correlate with treatment dose. These findings suggest a powerful new noninvasive therapy for glioblastoma.
Introduction
For glioblastoma (GBM), the most common malignant tumor of the brain in adults, treatment outcome remains dismal. In over 40 years median survival has only shown modest improvement ( 1 ), and standard of care treatment often has negative impact on quality of life ( 2 ). Treatment including radiation and chemotherapy takes a heavy toll. Frequently patients cannot tolerate the completion of the prescribed chemotherapy cycles. Thus, there is a great unmet need for a completely different therapeutic approach with better outcome and less toxicity.
A new FDA-approved treatment involving electric fields alternating at 200 kHz called Optune™ therapy is now available for recurrent GBM as monotherapy and in combination with temozolomide for newly diagnosed GBM ( 3 , 4 ). It is also being tested in clinical trials for other cancers. Its hypothesized mechanism of action involves disruption of tubulin dimers, mitotic spindles, and cell division by electric field-induced dipole alignment and dielectrophoresis ( 5 ). It has a modest effect on survival, increasing median overall survival by 0.6 month in recurrent GBM ( 3 ), and in newly diagnosed GBM by 31% ( 4 ). Even this modest effect is encouraging for patients.
It has been shown that electromagnetic fields (EMF) produce anticancer effects in vitro ( 6 , 7 ). We have conducted preclinical experiments with a new noninvasive wearable device known as an Oncomagnetic device that generates oscillating magnetic fields (OMF) by rotating strong permanent magnets ( 8 , 9 ). The OMF generating components (oncoscillators) of the device can be attached to a helmet and treatment with the device does not require shaving the head. Using the oncoscillators of the device and specially devised patterns of magnet rotations we have produced strong selective anticancer effects in patient derived GBM and xenografted mouse models without causing adverse effects on cultured normal cells and normal mice ( 10 – 12 ). The mechanism of action of OMF differs from Optune™ and involves disruption of the electron transport in the mitochondrial respiratory chain causing elevation of reactive oxygen species and caspase-dependent cancer cell death ( 10 – 12 ).
Here we report evidence of treatment response in the first patient to ever receive this therapy with an untreatable left frontal GBM, treated with a wearable Oncomagnetic device in an FDA-approved Expanded Access Program.
Case Description
The patient is a 53-year-old man who first presented with altered mental status in May 2018. Imaging studies documented a large tumor in the left frontal lobe extending across the midline into the right frontal lobe, with diffuse and extensive infiltration through the corpus callosum. There was mass effect and severe edema. He was taken to the operating room on June 4, 2018, where he underwent left frontal craniotomy and radical excision of the tumor. The tumor was histopathologically confirmed as GBM. At the time of the surgery, the excision extended across the midline into the right frontal lobe. He was enrolled in a herpes simplex virus-thymidine kinase gene therapy program and received viral injection during surgery per protocol. In addition, per protocol, and as standard of care, he received concomitant radiation therapy and chemotherapy with temozolomide.
In August 2019, the patient presented with an area of contrast enhancement on MRI scan along the left ventricle. At first this was thought to be a treatment effect. This area progressively enlarged. Evaluations done before OMF treatment initiation on January 16, March 3, and April 15, 2020, demonstrated a clear recurrence. The tumor abutted the ventricle and there was evidence of leptomeningeal spread. The patient had already had radiation therapy and chemotherapy and the tumor was now progressing. The presence of leptomeningeal disease portends poor outcome, with median survival of 3.5 to 3.9 months ( 13 ).
Because of inadequacy of any standard of care options he was enrolled in an FDA-approved Expanded Access Program (EAP) for compassionate use treatment with the Oncomagnetic device. He signed an informed consent on April 15, 2020. The EAP study was carried out under a protocol approved by the Houston Methodist Research Institute Institutional Review Board.
Oncomagnetic Device
The Oncomagnetic device consists of 3 oncoscillators securely attached to an acrylonitrile butadiene styrene helmet and connected to a microprocessor-based electronic controller operated by a rechargeable battery ( Figure 1 ). Further details regarding the device are given in the Supplementary Appendix . Based on a finite element model-based calculation of the spread of the field and the size and magnetization of the rotated diametrically magnetized neodymium magnets, we estimated that the combined effective field (at least 1 mT in strength) of the 3 oncoscillators covered the entire brain, including the upper part of the brain stem.
Figure 1 Oncomagnetic Device. (A) Device helmet with 3 oncoscillators securely attached to it. The oncoscillators are connected to a controller box powered by a rechargeable battery. (B) The patient wearing the device helmet with three oncoscillators attached.
Oscillating Magnetic Field Treatment
The treatment consists of intermittent application of an OMF that needs to be generated by rotating permanent magnets in a specific frequency profile and timing pattern to be effective. The patient received this treatment initially in the Peak Center clinic under the supervision of the treating physician and the Principal Investigator (DSB) of this study for the first 3 days. The dose was escalated over this period as follows. On the first day, the treatment was for 2 hours with a 5-min break between the first and the second hour. On the second and third days, it was increased to 2 and 3 2-hour sessions, respectively, with 1-hour breaks between the sessions. The patient’s spouse was trained in the use and care of the device on these days. After this initial supervised phase, the treatment was continued at home unsupervised with the same regimen as on the third day, above. The spouse was instructed to maintain a daily log of the conduct and progress of treatment, and any observed treatment and adverse effects.
Clinical Evaluations and Neuroimaging
The patient was evaluated clinically by the treating physician on each of the 3 days that he received treatment in the clinic and 7, 16, 30 and 44 days after initiation of treatment. Magnetic Resonance Imaging (MRI) scans were done on Days 1, 3, 7, 16, 30 and 44. The Day 1 scan was done before initiation of treatment. All other scans were done after treatment initiation. The treatment was paused on Day 37 because of an unfortunate but unrelated severe closed head injury (CHI). MRI scans were done on a Siemens Magnetom Terra 7T scanner. MRI scans included T1 magnetization prepared rapid gradient echo scans with and without gadolinium contrast, and T2-weighted Fluid-Attenuated Inversion Recovery (FLAIR), T2-weighted Turbo Spin Echo, Diffusion Weighted Imaging, Susceptibility Weighted Imaging, proton Magnetic Resonance spectroscopy and Diffusion Tensor Imaging scans. Treatment effect on contrast-enhanced tumor (CET) was evaluated according to the response assessment in neuro-oncology (RANO) criteria for clinical trials ( 14 ). In addition, an automated software-based method developed in house was used to objectively calculate the CET volume (see below and Supplementary Appendix ).
Data Analysis
Post-contrast T1 anatomical and T2-FLAIR MRI scans at each of the 6 time points were used to determine changes in contrast-enhanced tumor (CET) volume and non-enhanced tumor infiltration, respectively, before and after initiation of treatment. Information on image processing, data normalization and plotting are given in the Supplementary Appendix . Values obtained from pre-treatment clinical scans taken at 2 time points over 3 months before enrollment of the patient were also plotted on the same graph. Because this is a single patient case report, we could not perform any meaningful statistical analysis. However, to obtain a semi-quantitative assessment of the significance of the trend seen with treatment, we analyzed the changes in CET volume using Bayesian logic, given the observed increasing trend at two pre-treatment time points. Accordingly, we assumed that the chance of increase, decrease and no change in the rate of tumor growth was the same at each time point after treatment initiation to calculate the probability of a decrease at each post-treatment initiation time point.
The patient received OMF treatment with the Oncomagnetic device for 36 days. The treatment regimen was changed at various times during this period based on the caregiver reports and clinical findings, as described below.
Clinical Findings
After the initial 3 days of supervised treatment, the patient was seen again by the treating physician in the outpatient clinic on Day 7 from the start of treatment. Because of inattention at baseline, the patient was having difficulty with the length of treatment sessions. They were reduced to 2 hours/day Monday through Friday with Saturday and Sunday off. The Day 16 clinical examination revealed that he was tolerating the treatment sessions well, so they were increased to a total of 3 hours/day (in one-hour increments with 5 min breaks) Monday through Friday and the weekends off. On Day 30 visit, the patient reported headaches related to transient hypertension for which he was taking medication. The treating physician increased blood pressure medication (Valsartan) with improvement. The treatment was paused on Day 36 because of a closed head injury from a fall. Whether the fall was related to the treatment in any way is uncertain. It is worth noting, however, that the patient had experienced several falls before initiation of treatment. At the last follow-up on Day 44 the patient was admitted to the inpatient unit for evaluation of closed head injury and underwent detailed assessment. There were no serious adverse events reported during treatment. The patient’s caregivers reported subjective improvement in speech and cognitive function.
MRI Findings
Evaluation of the T1 post-contrast clinical MRI scans obtained before initiation of treatment showed progression in accordance with the RANO criteria ( Figure 2A ). All scans acquired during treatment showed stable disease, according to these criteria ( Figure 2A ). To obtain an objective quantitative assessment of the CET volume we used an automated MATLAB software-based script. This analysis showed marked changes in CET volume with treatment. Figure 2B shows a plot of the CET volume as a function of time before and after initiation of treatment. It reveals that there was substantial growth of the tumor volume over the 3 months before the treatment. Within the first 3 days of treatment the trend is reversed with the volume steeply decreasing by ~10% on Day 7 and then less steeply by 31% on Day 30. Based on a Bayesian-type assessment of the probability of a decrease in CET volume at each post-treatment initiation time point, the decrease at Day 30 is statistically significant at P = 0.036. The treatment was paused on Day 37. After the pause we see another trend reversal and an increase in CET volume on Day 44.
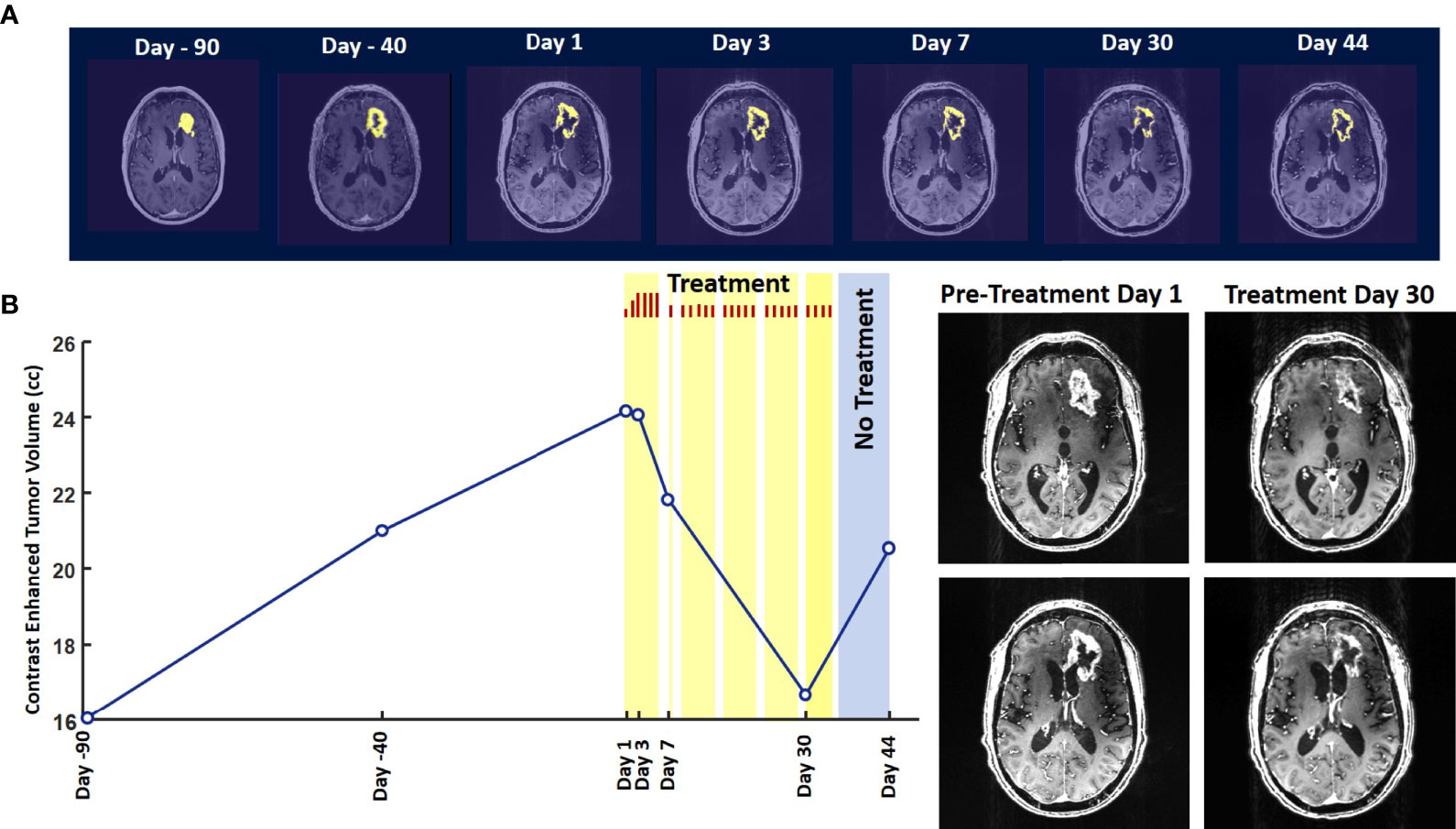
Figure 2 Change in Contrast-Enhanced Tumor Volume. (A) T1-weighted axial post-contrast scans showing the contrast-enhanced tumor (CET) highlighted with an overlayed automated computer program-generated light-yellow mask at different time points (B) Left – A graph showing the change in CET volume over time. The treatment times and durations are shown as red bars and light-yellow highlights. The long pause in treatment is shown as a light-blue highlight. Right – T1-weighted axial post-contrast scans showing CET at two levels along the dorso-ventral axis at Day 1 before treatment and Day 30 of treatment.
The T2-FLAIR data in Figure 3A show changes in enhanced intensity volume of 1 – 11% over time. The decreases in volume are greater after a 3-day pause in treatment on Day 7 and after an 8-day pause on Day 44. These decreases are likely due to reduction in treatment-related cerebral edema and/or reduction in non-contrast enhancing tumor infiltration. The patient died ~3 months after cessation of treatment from the CHI. A brain only autopsy showed a resection cavity in the left frontal lobe (6.0 x 5.0 x 3.5 cm) and recurrent/residual glioblastoma with associated treatment effect (see Figures 3B–E ). Residual/recurrent high-grade glioma was present, including foci of densely cellular tumor, focal microvascular proliferation, and necrosis ( Figure 3C ). In addition, there was prominent treatment effect with pallor and rarefaction of white matter ( Figure 3D ), reactive astrocytosis, infarct-like necrosis ( Figure 3E ) and bizarre nuclear atypia within residual tumor cells. Additional features of treatment effect included dystrophic calcifications ( Figure 3E ).
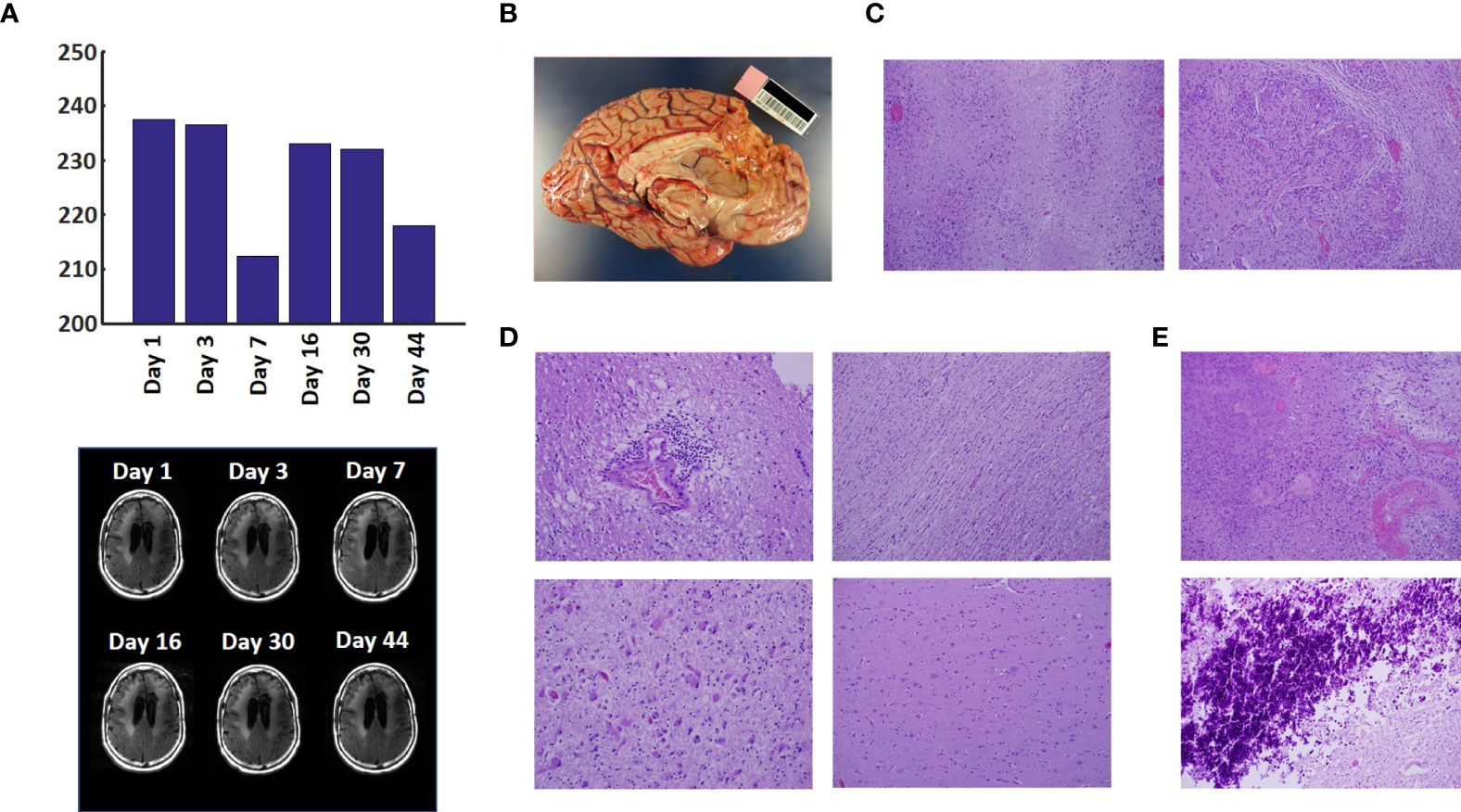
Figure 3 Variation in Enhanced Intensity Volumes in T2-FLAIR MRI Scans and Autopsy Findings. (A) Top – Bar plots of the volumes of T2-FLAIR intensity enhancement in the whole brain at different time points. Overall, there was up to 11% decrease in T2 FLAIR volume over the course of treatment. Bottom – Representative T2-FLAIR images are shown. (B) Left hemisphere of the brain, examined grossly, showing no tumor mass. (C) Photomicrographs of the left cortex showing bland necrosis, residual tumor, and microvascular proliferation with thick-walled vessels. (D) Top left – Microscopic field of the left cingulate cortex showing a focus of rarefied, perivascular inflammation. Bottom left – Cortical field showing rarefied parenchyma and residual tumor cells, enlarged with treatment-type effect that can be seen in GBM. Top right – Micrographic field of the corpus callosum showing thinned, rarefied white matter tract. Bottom right – Field showing relatively uninvolved contralateral (right) cortex. (E) Top – Micrographic field in the left cortex showing infarct-like necrosis (left), tumor (right), and fibrin thrombus (lower right). Bottom – Left cortical field showing necrotic tissue with dystrophic calcification.
The findings of this study indicate that Oncomagnetic device-based OMF therapy is well tolerated by a patient who has end-stage recurrent GBM with leptomeningeal involvement and has no other available effective treatment options. They also demonstrate a clinically significant reduction in CET volume with reductions in non-enhanced tumor volume and/or edema in T2-FLAIR scans. The temporal profile of changes in CET volume also suggests a correlation with the treatment dose and the presence or absence of treatment. When the treatment dose was higher (6 hours/day for 4 days) we see a tumor volume reduction rate of 2.32 cm 3 /day. When it was lower (2 hours/day for 9 days and 3 hours/day for 18 days) the reduction is 1.03 cm 3 /day. Moreover, when the treatment was paused for 8 days the decreasing trend reversed and the CET volume increased, instead. Assuming that the ~1.03 cm 3 /day decreasing trend had continued until the treatment was paused, we can estimate that the CET volume grew at the rate of 1.26 cm 3 /day during the pause. Despite the apparent correlation it is possible that the treatment response is independent of the short-term changes in the treatment dose.
To our knowledge, there is no report in the literature of a noninvasive treatment-related shrinkage of CET volume of GBM at a rate comparable to that seen in this study. One published report on Optune™ therapy has reported that the time course of change in tumor volume in MRI scans shows a ~15% reduction over ~3 months ( 15 ). Besides Optune™, the other type of treatment approved by the FDA and recommended as a standard in National Comprehensive Cancer Network guidelines for recurrent GBM is the anti-vascular endothelial growth factor (VEGF) monoclonal antibody, Bevacizumab ( 16 , 17 ). Bevacizumab treatment response of reduction in tumor volume on MRI scans has been reported to be lower than is observed in the present study ( 18 ). Furthermore, while anti-VEGF drugs in general have mild toxicity profiles and two Phase II trials have shown anti-tumor efficacy ( 19 , 20 ), a subsequent Phase III trial did not show a significant increase in overall survival ( 21 – 23 ).
Noninvasive Oncomagnetic device based OMF therapy appears to be a safe and efficacious new modality of treatment against GBM that potentially has many advantages over existing treatments. The present report has the limitation of the treatment being conducted in only a single patient so far. Extending it to more patients in research studies would provide additional information regarding safety and efficacy.
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Houston Methodist Research Institute Institutional Review Board. The patient/participant provided their written informed consent to participate in this study. Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SH and DB designed the study and drafted the manuscript. SH designed the device used in the study, supervised its construction and testing and quantitively analyzed the imaging data. DB provided medical care to the study subject, supervised the delivery of device treatment, and conducted his clinical assessments. SH, MS, and DB designed the device treatment protocol and interpreted the findings. LN constructed and tested the device and provided device treatment to the study subject. All authors contributed to the article and approved the submitted version.
This work was supported by a grant from the Translational Research Initiative of the Houston Methodist Research Institute to SH and DB, and by Donna and Kenneth Peak, the Kenneth R. Peak Foundation, the John S. Dunn Foundation, the Taub Foundation, the Blanche Green Fund of the Pauline Sterne Wolff Memorial Foundation, the Kelly Kicking Cancer Foundation, the Gary and Marlee Swarz Foundation, the Methodist Hospital Foundation, and the Veralan Foundation. The John S. Dunn Foundation also supports the Distinguished Professorship of MS.
Conflict of Interest
SH, MS, and DB are listed as inventors on a U.S. patent application filed by Houston Methodist Hospital for the device used in this report.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the patient for graciously volunteering to be a research subject in this study and the rest of his family for supporting him. We appreciate the assistance of Dr. Matthew Cykowski, MD, Department of Pathology and Genomic Medicine, who provided pathologic description and images. We thank Blessy S. John and Alvin Saldon for aiding in device construction.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.708017/full#supplementary-material
1. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med (2005) 352:987–96. doi: 10.1056/NEJMoa043330
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Henriksson R, Asklund T, Poulsen HS. Impact of Therapy on Quality of Life, Neurocognitive Function and Their Correlates in Glioblastoma Multiforme: A Review. J Neurooncol (2011) 104:639–46. doi: 10.1007/s11060-011-0565-x
3. Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. NovoTTF-100A Versus Physician’s Choice Chemotherapy in Recurrent Glioblastoma: A Randomised Phase III Trial of a Novel Treatment Modality. Eur J Cancer (2012) 48:2192–202. doi: 10.1016/j.ejca.2012.04.011
4. Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA (2017) 318:2306–16. doi: 10.1001/jama.2017.18718
5. Tuszynski JA, Wenger C, Friesen DE, Preto J. An Overview of Sub-Cellular Mechanisms Involved in the Action of TTFields. Int J Environ Res Public Health 13 (2016) 13:1–23. doi: 10.3390/ijerph13111128
CrossRef Full Text | Google Scholar
6. Saliev T, Begimbetova D, Masoud AR, Matkarimov B. Biological Effects of non-Ionizing Electromagnetic Fields: Two Sides of a Coin. Prog Biophys Mol Biol (2019) 141:25–36. doi: 10.1016/j.pbiomolbio.2018.07.009
7. Jimenez H, Blackman C, Lesser G, Debinski W, Chan M, Sharma S, et al. Use of non-Ionizing Electromagnetic Fields for the Treatment of Cancer. Front Biosci (Landmark Ed) (2018) 23:284–97. doi: 10.2741/4591
8. Helekar SA, Convento S, Nguyen L, John BS, Patel A, Yau JM, et al. The Strength and Spread of the Electric Field Induced by Transcranial Rotating Permanent Magnet Stimulation in Comparison With Conventional Transcranial Magnetic Stimulation. J Neurosci Methods (2018) 309:153–60. doi: 10.1016/j.jneumeth.2018.09.002
9. Helekar SA, Voss HU. Transcranial Brain Stimulation With Rapidly Spinning High-Field Permanent Magnets. IEEE Access (2016) 4:2520–8. doi: 10.1109/ACCESS.2016.2568739
10. Helekar S, Sharpe M, Pichumani K, Ijare O, Nguyen L, Baskin D. CTNI-48. Novel Treatment of End Stage Recurrent Glioblastoma Treated With a Noninvasive Oncomagnetic Device Using Oscillating Magnetic Fields – a New and Powerful Noninvasive Therapy. Neuro-Oncol (2020) 22:ii53–3. doi: 10.1093/neuonc/noaa215.214
11. Helekar S, Hambarde S, Baskin D, Sharpe M. EXTH-13. Potent Anticancer Effects of a New Wearable Noninvasive Oncomagnetic Device: Cellular Mechanisms of Action. Neuro-Oncol (2020) 22:ii89–9. doi: 10.1093/neuonc/noaa215.367
12. Hambarde S, Sharpe M, Baskin D, Helekar S. CBIO-07. Cell Death Induced by an Oscillating Magnetic Field in Patient Derived Glioblastoma Cells is Mediated by Reactive Oxygen Species. Neuro-Oncol (2020) 22:ii17–7. doi: 10.1093/neuonc/noaa215.067
13. Andersen BM, Miranda C, Hatzoglou V, DeAngelis LM, Miller AM. Leptomeningeal Metastases in Glioma: The Memorial Sloan Kettering Cancer Center Experience. Neurology (2019) 92:e2483–91. doi: 10.1212/WNL.0000000000007529
14. Wen PY, Chang SM, Van den Bent MJ, Vogelbaum MA, Macdonald DR, Lee EQ. Response Assessment in Neuro-Oncology Clinical Trials. J Clin Oncol (2017) 35:2439–49. doi: 10.1200/JCO.2017.72.7511
15. Robins HI, Nguyen HN, Field A, Howard S, Salamat S, Deming DA. Molecular Evolution of a Glioblastoma Controlled With Tumor Treating Fields and Concomitant Temozolomide. Front Oncol (2018) 8:451. doi: 10.3389/fonc.2018.00451
16. Kreisl TN, Zhang W, Odia Y, Shih JH, Butman JA, Hammoud D, et al. A Phase II Trial of Single-Agent Bevacizumab in Patients With Recurrent Anaplastic Glioma. Neuro Oncol (2011) 13:1143–50. doi: 10.1093/neuonc/nor091
17. Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab Alone and in Combination With Irinotecan in Recurrent Glioblastoma. J Clin Oncol (2009) 27:4733–40. doi: 10.1200/JCO.2008.19.8721
18. Daniels D, Guez D, Last D, Hoffmann C, Nass D, Talianski A, et al. Early Biomarkers From Conventional and Delayed-Contrast Mri to Predict the Response to Bevacizumab in Recurrent High-Grade Gliomas. AJNR Am J Neuroradiol (2016) 37:2003–9. doi: 10.3174/ajnr.A4866
19. Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, et al. Bevacizumab Plus Irinotecan in Recurrent Glioblastoma Multiforme. J Clin Oncol (2007) 25:4722–9. doi: 10.1200/JCO.2007.12.2440
20. Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA, et al. Phase II Trial of Bevacizumab and Irinotecan in Recurrent Malignant Glioma. Clin Cancer Res (2007) 13:1253–9. doi: 10.1158/1078-0432.CCR-06-2309
21. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab Plus Radiotherapy-Temozolomide for Newly Diagnosed Glioblastoma. N Engl J Med (2014) 370:709–22. doi: 10.1056/NEJMoa1308345
22. Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and Bevacizumab in Progressive Glioblastoma. N Engl J Med (2017) 377:1954–63. doi: 10.1056/NEJMoa1707358
23. Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N Engl J Med (2014) 370:699–708. doi: 10.1056/NEJMoa1308573
Keywords: magnetic resonance imaging, contrast enhanced tumor, compassionate use treatment, radiation-type tumor necrosis 2, oscillating magnetic fields
Citation: Baskin DS, Sharpe MA, Nguyen L and Helekar SA (2021) Case Report: End-Stage Recurrent Glioblastoma Treated With a New Noninvasive Non-Contact Oncomagnetic Device. Front. Oncol. 11:708017. doi: 10.3389/fonc.2021.708017
Received: 11 May 2021; Accepted: 21 June 2021; Published: 22 July 2021.
Reviewed by:
Copyright © 2021 Baskin, Sharpe, Nguyen and Helekar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: David S. Baskin, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

Clinical trials: A significant part of cancer care
Share this:.
Editor's note: May is National Cancer Research Month.
By Mayo Clinic staff
A cancer diagnosis is an emotional experience. Learning that you have cancer can create feelings of hopelessness, fear and sadness. This is especially true if your cancer is advanced or available treatments are unable to stop or slow its growth.
"Often, when patients are diagnosed with cancer , they feel hopeless and scared. Clinical trials are one way patients can be proactive. They can make a choice in how their care is going to be," says Matthew Block, M.D., Ph.D. , a Mayo Clinic medical oncologist.
Cancer clinical trials help physician-scientists test new and better ways to control and treat cancer. During a clinical trial, participants receive specific interventions, and researchers determine if those interventions are safe and effective. Interventions studied in clinical trials might be new cancer drugs or new combinations of drugs, new medical procedures, new surgical techniques or devices, new ways to use existing treatments, and lifestyle or behavior changes.
Clinical trials provide access to potential treatments under investigation, giving options to people who otherwise may face limited choices. "Clinical trials open the door to a new hope that maybe we can fight their cancer back and give them a better quality of life," says Geoffrey Johnson, M.D., Ph.D. , a Mayo Clinic radiologist, nuclear medicine specialist and co-chair of the Mayo Clinic Comprehensive Cancer Center Experimental and Novel Therapeutics Disease Group.
You will receive cancer treatment if you participate in a clinical trial. "I think one common misperception about clinical trials is that if you enter a clinical trial, you may not get treatment (receive a placebo). And that's actually very much not true. Most clinical trials are looking at one treatment compared to another treatment," says Judy C. Boughey, M.D. , a Mayo Clinic surgical oncologist, chair of Breast and Melanoma Surgical Oncology at Mayo Clinic in Rochester, Minnesota, and chair of the Mayo Clinic Comprehensive Cancer Center Breast Cancer Disease Group.
"I think one common misperception about clinical trials is that if you enter a clinical trial, you may not get treatment (receive a placebo). And that's actually very much not true. Most clinical trials are looking at one treatment compared to another treatment." Judy C. Boughey, M.D.
Watch this video to hear the experiences of people who have participated in cancer clinical trials and to hear Drs. Block, Johnson and Boughey discuss the importance of clinical trials in cancer care:
Clinical trials are a significant part of cancer care at Mayo Clinic Comprehensive Cancer Center. Cancer care teams work together across specialties to make sure the right clinical trials are available to serve the needs of people with cancer who come to Mayo Clinic.
"We are very particular in how we select the clinical trials that we have available for patients," says Dr. Boughey. "We want to have the best trials available for our patients. Some of the clinical trials are evaluating drugs — we are so excited about those drugs, but we can't prescribe those drugs for patients without having that trial. And so we will actually fight to try to get that trial open here to have it available as an opportunity for our patients."
If you choose to participate in a clinical trial, you will continue to receive cancer care. "For most patients that we evaluate, there's always the standard of care treatment option for those patients. And then, in many situations, there's also a clinical trial that the patient can participate in," says Dr. Boughey.
People who participate in clinical trials help make new and better cancer care available for future patients. The treatments available for cancer patients today exist because of the clinical trial participants of yesterday. "We couldn't advance medicine if it wasn't for people volunteering for trials. And the promise from our side is to say we're not going to put patients on trials or offer trials for them to consider unless we think there's a good chance that they'll get a benefit or that society at large will get a benefit," says Dr. Johnson.
"We couldn't advance medicine if it wasn't for people volunteering for trials. And the promise from our side is to say we're not going to put patients on trials or offer trials for them to consider unless we think there's a good chance that they'll get a benefit or that society at large will get a benefit." Geoffrey Johnson, M.D., Ph.D.
Participating in a clinical trial may give you access to cutting-edge treatment, improve your quality of life and extend your time with loved ones.
"It's definitely worth reaching out to your healthcare provider and asking, 'What clinical trials could I be a potential candidate for?'" says Dr. Boughey. "And remember, you can ask this of your surgical oncologist, your medical oncologist, your radiation oncologist, or any of the physicians you're seeing because there are trials in all disciplines. There are also ongoing trials that require the collection of tissue or the donation of blood. They can also be important in trying to help future generations as we continue to work to end cancer."
Participating in a clinical trial is an important decision with potential risks and benefits. Explore these FAQ about cancer clinical trials, and ask your care team if a clinical trial might be right for you.
Learn more about cancer clinical trials and find a clinical trial at Mayo Clinic.
Join the Cancer Support Group on Mayo Clinic Connect , an online community moderated by Mayo Clinic for patients and caregivers.
Read these articles about people who have participated in clinical trials at Mayo Clinic:
- A silent tumor, precancerous polyps and the power of genetic screening
- Mayo Clinic’s DNA study reveals BRCA1 mutations in 3 sisters, prompts life-changing decisions
Read more articles about Mayo Clinic cancer research made possible by people participating in clinical trials.
Related Posts

Drs. Terry Burns and Alfredo Quiñones-Hinojosa discuss brain tumors called gliomas and the hope being offered by new technology and clinical trials.

Dr. Sujay Vora is studying a new approach to glioblastoma treatment that is improving health outcomes and quality of life for elderly people like Richard Casper.

Dr. S. John Weroha discusses new treatments and research that are helping more people survive ovarian cancer.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Urol Case Rep
- v.30; 2020 May
Complete remission with immunotherapy: Case report of a patient with metastatic bladder cancer to the humerus
D. abdelhakam.
a Department of Cancer Biology, Mayo Clinic, Jacksonville, FL, USA
b Department of Clinical Pathology, Faculty of Medicine, Ainshams University, Cairo, Egypt
c Department of Urology, Mayo Clinic, Jacksonville, FL, USA
d Department of Radiology, Mayo Clinic, Jacksonville, FL, USA
e Department of Pathology and Laboratory Medicine, Mayo Clinic, Jacksonville, FL, USA
J.A. Copland
f Division of Hematology-Oncology, Mayo Clinic, Jacksonville, FL, USA
Bladder cancer is the sixth most common malignancy in the United States. Cisplatin combination regimens are first line therapy for patients with metastatic urothelial bladder cancer who are eligible candidates and no treatments have shown to improve outcome compared to chemotherapy for the past 20 years. Significant advances were made in past 2–3 years and the most significant was the introduction of checkpoints inhibitors in bladder cancer treatment. We present a patient diagnosed with metastatic urothelial carcinoma who progressed while on cisplatin/gemcitabine chemotherapy in the form of oligometastasis to the bone. He has achieved a durable complete response with atezolizumab.
Introduction
The management of urothelial cancer had been challenging due to multiple co-morbidities and the few available treatment options. Over the past few years, significant therapeutic advances were achieved through better understanding of the molecular mechanisms involved in pathogenesis and treatment. We present a case of a male patient diagnosed with metastatic urothelial cancer (mUC) who had developed complete response after treatment with atezolizumab.
Case presentation
A 53 year old male presented with gross hematuria. Further workup including cysto-urethroscopy, bilateral ureteroscopy, right ureteral tumor biopsy, right ureteral tumor laser ablation, and right ureteral stent placement was done. Pathology showed high-grade transitional cell carcinoma of his right ureteral tumor. He then underwent robotic-assisted laparoscopic right radical nephroureterectomy with right extended pelvic lymphadenectomy, cystoscopy, and stent extraction. Initial oncology consultation was done to discuss adjuvant chemotherapy but surveillance was favored. Three months later, CT scans showed polypoid filling defect in the right side of the bladder posteriorly and laterally suspicious for recurrent UC. A month later he underwent cysto-urethroscopy, transurethral resection of bladder tumor, left ureteroscopy, left ureteral tumor biopsy and transurethral resection of the prostate. Pathology showed evidence of a recurrence around his right ureteral stump with muscle wall invasion. He received neoadjuvant Cisplatin/Gemcitabine (GC) chemotherapy for 4 cycles. After completion of treatment, a metastatic lesion in the right proximal humerus was detected on his bone scan and a biopsy done was consistent with mUC ( Fig. 1 ). Between December 2016 and February 2017 the patient received 4 cycles of GC chemotherapy. In March 2017, no significant interval change in the biopsy-proven right proximal humeral diaphyseal metastatic lesion was detected by MRI ( Fig. 2 a, b). In addition, follow-up bone scans revealed a focus of increased radiotracer activity in the right proximal humerus consistent with patient's known metastatic disease. The patient was started on atezolizumab in April 2017, as second-line treatment. After 4 cycles, restaging scans were done and the patient was considered to have a stable disease with continued response to atezolizumab. April 2019, MR right humerus demonstrated no residual osseous tumor ( Fig. 2 c,d). The bone scan showed no suspicious foci of increased radiotracer activity to suggest new osteoblastic skeletal metastasis. Last dose of atezolizumab was in May 2019 where the patient completed 2 years of treatment. After discussion he decided to discontinue treatment and had to be followed up every 3 months, last time was August 2019 the patient is still in complete remission ( Fig. 3 ).

(a) Bone biopsy showing microscopic features of urothelial cancer, (b) Positive staining p63 urothelial carcinoma, and (c) Positive stain cytokeratin 7.

Coronal T2 weighted images (a) show increased signal in diaphysis of the right humerus, (b) Axial subtraction MR images (post contrast– precontrast T1-weighted images) before treatment (March 2017), (c) shows the resolved lesion after therapy (d) show interval resolution of enhancement after treatment (April 2019).

Whole body anterior and posterior images (a) dated April 2017 shows focal increased radiotracer activity in the right proximal humerus. Image (b) post-treatment whole body anterior and posterior images show resolution of focal increased radiotracer activity in the right proximal humerus.
Cisplatin combination regimens are the standard first line therapy for mUC of the bladder and urinary tract in patients who are cisplatin candidates. 1 The overall response rates (ORRs) are 60–70% with cisplatin-based chemotherapy and an overall survival of 14–15 months. 2 Cisplatin related toxicity makes a large proportion of patients ineligible to this treatment option. The advent of checkpoint inhibition has changed the treatment landscape in this disease; given the significant percentage of patients who have progressive disease on platinum-based therapy or who are cisplatin ineligible.
PD-1/PD-L1 are members of the Ig superfamily. PD-L1/PD-1 interaction leads to T-cell exhaustion and impaired cytotoxic activity, thus inhibiting the immune-response and allowing tumors to grow unchecked. Blocking this inhibitory pathway can help overcome one of the cancer evasive mechanisms allowing for increased immune surveillance. 3 Immunotherapy has emerged as a novel option for patients with various advanced cancers who had limited treatment options and poor outcomes. Checkpoint inhibitors (CPIs) are approved by FDA in several solid tumors. These agents are well tolerated, and may even show durable complete responses (CR) offering a novel therapeutic approach in the treatment of diseases that lacks significant options especially in metastatic settings.
FDA approved five immunotherapy agents for use in the treatment of patients with locally advanced or mUC since May 2016 including atezolizumab, pembrolizumab, nivolumab durvalumab and avelumab. Of these, only atezolizumab and pembrolizumab are approved as first-line therapy for patients ineligible to receive cisplatin chemotherapy, while all the agents are approved in previously treated patients. 4
Atezolizumab (Tecentriq) is an Fc-engineered, monoclonal antibody that targets PD-L1, inhibiting the interaction with PD-1 and B7.1 receptors. Approval of the drug by FDA in 2016 for use in patients with advanced or mUC of the bladder who progressed during or after a platinum-based regimen based on the results of the Phase (II) IMvigor 210 study, administered as a 1200 mg IV infusion on day 1 of each 3-week cycle. The primary end point of the study was over all response rate which was 14.8% at a median follow-up of 14.4 months. CR was seen in 5.5% (17 patients). Treatment-related adverse effects included fatigue (52%), nausea (25%), anorexia (26%), and constipation (21%). Atezolizumab was also evaluated in the front-line setting for metastatic patients who were cisplatin ineligible and was approved by the FDA in April 2017. 4 - 5
Clinical trials involving CPIs in UC included a fraction of patients who achieved durable CR but results from most of the trials are over limited treatment duration. This case presents a patient with mUC who progressed while on cisplatin/gemcitabine chemotherapy in the form of oligometastasis to the bone. The patient manifested an obvious clinical response to atezolizumab in the form of stable disease followed by CR in a two years period of treatment without any major adverse effects and is still in complete remission after discontinuation of treatment.
Our case is a presentation of mUC in complete remission on immunotherapy, in addition to the promising results of ongoing clinical trials demonstrating that immunotherapy is a realistic hope for patients in different settings of this disease. Moreover, it can be a treatment option offered to patients ineligible to platinum therapy due to its overall acceptable safety profile. Ongoing studies are working on prediction of patient's response, as well combination strategies able to increase the number of responders and to improve clinical outcomes. The treatment of UC is rapidly evolving and immunotherapy revolution has just begun.
Declaration of competing interest
The authors have stated that they have no conflicts of interest.
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Stanford Medicine delivers first FDA-approved cell-based therapy for solid tumors
The FDA recently approved the first cell-based therapy — widely used in treating blood cancers — for solid tumors. Stanford Medicine treated the first patient with advanced melanoma.
May 6, 2024 - By Krista Conger
Allison Betof Warner speaks with a patient who received CAR-T therapy for melanoma. Stanford Medicine
In March, Stanford Medicine became the first center in the nation to treat a patient with metastatic melanoma using a new cell-based therapy approved by the Food and Drug Administration. The therapy, which offers hope for people with advanced melanoma whose cancers have resisted immunotherapies, is the first cell-based therapy approved by the FDA to treat solid tumors.
Cell-based therapies — in which immune cells are collected from a patient and treated or genetically modified to enhance their cancer-fighting ability — are not new. Chimeric antigen receptor T-cell therapies, or CAR-T therapies, have been used for several years to fight blood cancers such as leukemias.
The new treatment, which uses immune cells harvested from a patient’s tumor called tumor-infiltrating lymphocytes, exploits the body’s own natural cancer-fighting ability. Once the tumor-infiltrating lymphocytes, or T cells, are harvested, they are encouraged in the laboratory to multiply into billions of cancer-fighting cells, then returned to the patient about a month later.
“These cells are naturally existing T cells that target multiple aspects of the existing tumor,” said assistant professor of medicine Allison Betof Warner , MD, PhD. “Before now, there was no approved therapy for people with melanoma whose cancers had progressed after immunotherapy and/or targeted therapy. Now we can bring the promise of cell therapy to these patients.”
In contrast to tumor-infiltrating lymphocytes, which are not genetically modified, CAR-T therapies use genetic engineering to modify a patient’s T cells to recognize and respond to a specific molecule or molecules on the surfaces of cancer cells. Doing so requires researchers to predict which molecules will serve as the best targets — easier for blood cancers than for solid tumors.
“We are very excited to move cell-based therapies beyond blood cancers,” said David Miklos , MD, PhD, professor of medicine and chief of bone marrow transplantation and cellular therapy. “This has been a long time coming, but now we have a new standard of care for these patients.”
Stanford Medicine is one of fewer than 30 centers around the country offering the treatment.
The therapy, called lifileucel and commercially known as Amtagvi, is marketed by San Carlos-based Iovance Biotherapeutics.
Clinical trials of lifileucel for metastatic melanoma that worsened while on standard treatment found that about 30% of 153 patients who received the therapy had their tumors shrink or disappear. Of those, 40% experienced no progression of their cancers for at least 18 months after the one-time infusion.
A quarter-century of research
Tumor-infiltrating lymphocyte therapy was first conceived of in the late 1980s by Steven Rosenberg, MD, PhD, at the National Cancer Institute. In the intervening decades, he and others optimized the treatment and conducted clinical trials exploring its promise. In October, Rosenberg was awarded the National Medal of Technology and Innovation, in part for his role in bringing tumor-infiltrating lymphocyte therapies to the clinic.
For the treatment, surgeons first remove a sample of the tumor and collect any T cells it contains. These cells have already infiltrated and started to fight the tumor, presumably by recognizing a variety of molecules on the surfaces of the cancer cells. These T cells are grown in the laboratory for about one month in the presence of a growth-promoting immune molecule called IL-2.
IL-2 stimulates the cells to divide repeatedly, generating an army of billions. While the cells are growing in the laboratory, the patient undergoes chemotherapy to deplete existing lymphocytes — creating a biological niche for the newly energized cells to further expand when they are returned to the patient.
“Normally, when you remove a tumor from a patient with cancer, you throw it away,” Miklos said. “But these trials have shown that the lymphocytes that tumor contains are gold. Your body is already trying to fight these cancers. We’re just helping it out.”
After the cells are returned, the patient is given several doses of IL-2 to further encourage the expansion of the cancer-fighting cells.
The technique works particularly well with melanomas, which are flush with lymphocytes. But Betof Warner and Miklos feel that improvements in the cell-expansion technique may make it useful even for tumors such as lung and colorectal cancers that tend to have fewer infiltrating immune cells — a class of tumor known as “immune cold.”
Researchers worldwide are now conducting clinical trials of tumor-infiltrating lymphocytes in other solid cancers including non-small cell lung cancer, advanced colorectalm and breast cancer, while other trials are investigating the effect of combining lifileucel with immunotherapy as a first-line treatment for advanced melanoma.
Not every person with metastatic melanoma will qualify for the therapy under the terms of the FDA approval. People need to be relatively healthy with good heart, kidney, and lung function and able to withstand the preparative immune-depleting chemotherapy given before the infusion. They also must have seen their cancers worsen while on immunotherapies or targeted treatment that are currently the standard of care for these cancers. Betof Warner estimates that, of the 8,000 to 10,000 or so people diagnosed with advanced melanomas each year in the United States, about 2,000 people will qualify if patients are identified appropriately and referred to authorized treatment centers.
The researchers are also investigating which molecules the tumor-infiltrating lymphocytes are targeting, with the hopes of making even more targeted treatments.
“We study the tumor that we remove from the patient, as well as the cells we return to the patient and compare that with the patient’s clinical response,” Betof Warner said. “Eventually we hope to predict and improve the responses based on the lymphocytes found in the tumor. Are they healthy? What molecules on the cancer cells are they targeting? Although there is more to learn, we are excited to have another option, another line of treatment for these advanced cancers.”
See also: The promise of TIL Therapy

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Hope amid crisis
Psychiatry’s new frontiers

Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- NEWS FEATURE
- 30 April 2024
Do cutting-edge CAR-T-cell therapies cause cancer? What the data say
- Cassandra Willyard 0
Cassandra Willyard is a science journalist in Madison, Wisconsin.
You can also search for this author in PubMed Google Scholar
A composite microscope image of engineered T cells (grey) attacking a breast cancer cell. Credit: Steve Gschmeissner/Science Photo Library
You have full access to this article via your institution.
US drug regulators dropped a bombshell in November 2023 when they announced an investigation into one of the most celebrated cancer treatments to emerge in decades. The US Food and Drug Administration (FDA) said it was looking at whether a strategy that involves engineering a person’s immune cells to kill cancer was leading to new malignancies in people who had been treated with it.
Bruce Levine, an immunologist at the University of Pennsylvania Perelman School of Medicine in Philadelphia who helped to pioneer the approach known as chimeric antigen receptor (CAR) T-cell therapy, says he didn’t hear the news until a reporter asked him for comments on the FDA’s announcement.
“Better get smart about it quick,” he remembers thinking.
Although the information provided by the FDA was thin at the time, the agency told reporters that it had observed 20 cases in which immune-cell cancers known as lymphomas had developed in people treated with CAR T cells. Levine, who is a co-inventor of Kymriah, the first CAR-T-cell therapy to be approved, started jotting down questions. Who were these patients? How many were there? And what other drugs had they received before having CAR-T-cell therapy?

How to supercharge cancer-fighting cells: give them stem cell skills
The FDA has since documented more cases. As of 25 March, the agency had received 33 reports of such lymphomas among some 30,000 people who had been treated. It now requires all CAR-T therapies to carry a boxed warning on the drug’s packaging, which states that such cancers have occurred. And the European Medicines Agency has launched its own investigation. But many of the questions that Levine had in November remain unanswered. It is unclear how many, if any, of the observed cancers came directly from the manipulations made to the CAR T cells. A lot of cancer therapies carry a risk of causing secondary malignancies, and the treated individuals had received other therapies. As Crystal Mackall, a paediatric oncologist who heads the cancer immunotherapy programme at Stanford University in California, puts it: “Do you have a smoking gun?”
Scientists are now racing to determine whether the cellular therapy is driving these cancers or contributing in some way to their development. From the data available so far, the secondary cancers seem to be a rare phenomenon, and the benefits of CAR T cells still outweigh the risks for most prospective recipients. But it’s an important puzzle to solve so that researchers can improve and expand the use of these engineered cells in medicine. CAR-T-cell treatments were once reserved for people who had few other options for therapy. But the FDA has approved several of these treatments as a relatively early, second-line option for lymphoma and multiple myeloma. And some companies are working to expand the therapy’s repertoire to solid tumours , autoimmune diseases , ageing , HIV and more .
Aric Hall, a haematologist at the University of Wisconsin–Madison, says that despite the enthusiasm for CAR-T therapy, the technology is still new. “I used to joke that for the first ten years there were more review articles about CAR T than there were patients who had been treated by CAR T products,” he says. He adds that the risks might be rare, but as CAR-T therapy moves into a bigger pool of patients who aren’t desperately ill, the calculus could change. “The problem is rare risks become a bigger deal when patients have better options.”
Vector safety
Throughout the development of these blockbuster therapies , researchers had reason to think that CAR T cells could become cancerous. CAR-T therapies are personalized — created from a person’s immune cells. Their T cells are extracted and then genetically modified in the laboratory to express a chimeric antigen receptor — or CAR — a protein that targets the T cell to specific cells that they will kill. T cells sporting these receptors are made to multiply and grow in the lab, and physicians then infuse them back into the individual, where they start battling cancer cells. The six CAR-T-cell therapies currently approved in the United States and Europe target antigens on the immune system’s B cells, so they work only against B-cell malignancies — leukaemias, lymphomas and multiple myeloma. But researchers are aiming to develop CAR-T therapies that work on other kinds of cancer, and for other conditions.
The genetic engineering is the step that creates a risk of malignancy. All six FDA-approved CAR-T therapies rely on a retrovirus — typically a lentivirus, such as HIV, or a gammaretrovirus — to ferry the genetic information into the cell. Scientists remove the parts of the viral genome that allow the virus to replicate, making room for the gene they want the virus vector to carry. Once inside a cell, the virus inserts the gene for the CAR into the cell’s genome. But there isn’t a good way to control exactly where the gene goes. If it slips in near a gene that can promote cancer development and activates it, or if it deactivates a tumour-suppressing gene, that boosts the risk of causing a T-cell cancer (see ‘CAR-T concerns’).
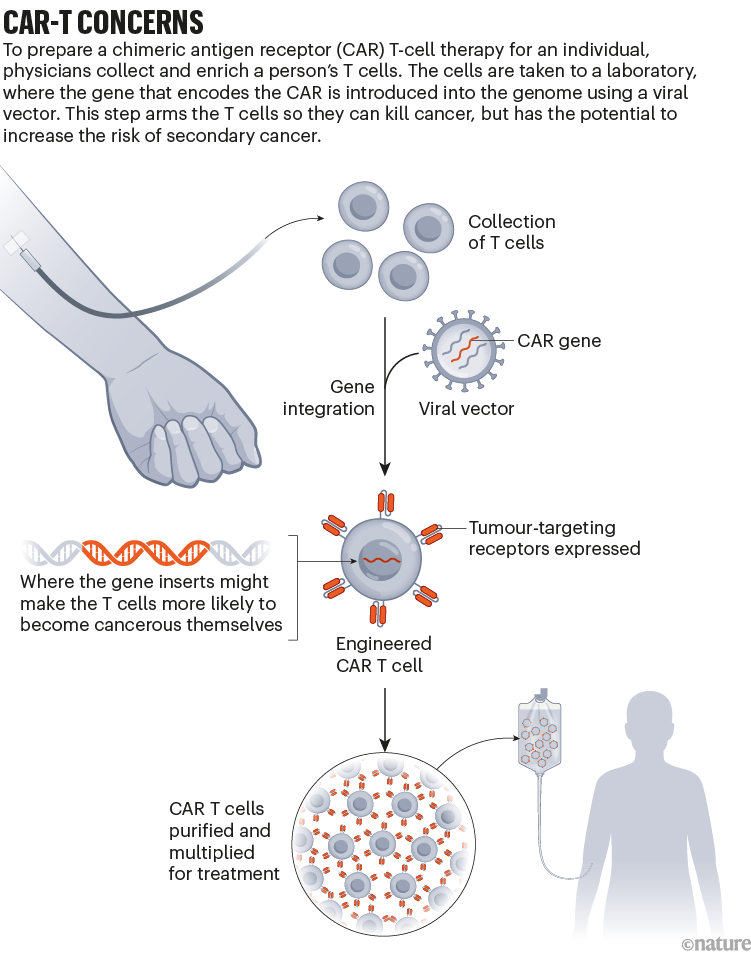
This phenomenon, known as insertional mutagenesis, is a risk with most gene therapies . About 20 years ago, for example, groups in London and Paris treated 20 infants who had severe combined immunodeficiency syndrome (SCID) with a gene therapy that used a retrovirus. The therapy worked for most participants, but the retrovirus switched on cancer genes in some. That activation led to leukaemia in five of the participants ; four recovered and one died.
As a result, scientists have reworked the vectors to make them safer, ensuring that their genes don’t recombine, for example. The FDA recommends that CAR-T products undergo testing to prove that the vectors cannot replicate. “The scrutiny we’ve been under has been tremendous,” says Hans-Peter Kiem, an oncologist at the Fred Hutchinson Cancer Center in Seattle, Washington, who has studied viral vectors for decades. Many felt confident about using viral vectors in CAR-T therapies, because T cells are difficult to prod towards malignancy, says Marco Ruella, a haematologist at the Perelman School of Medicine. “Truly the general feeling was that, in T cells, lenti- and retroviruses are extremely safe.”
Search for the smoking gun
When the FDA issued its warning in November, it wasn’t clear what specific reports had prompted the agency to act, or whether the link was causal. Levine recruited some of the biggest names in CAR-T therapies to co-write a commentary on the matter and discuss some of the questions he still had 1 . “I felt — we felt — that it was important to say, ‘Well, let’s take a step back for a minute and see what we really know,’” he says.
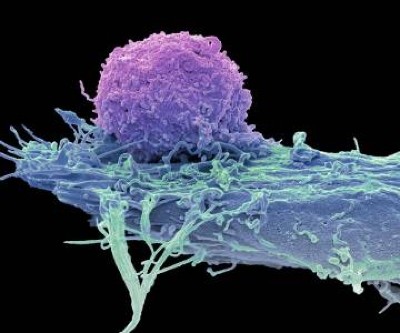
Turbocharged CAR-T cells melt tumours in mice — using a trick from cancer cells
In January, the FDA released more information. In an article in the New England Journal of Medicine , Peter Marks and Nicole Verdun at the FDA’s Center for Biologics Evaluation and Research in Silver Spring, Maryland, revealed that the agency had received 22 reports of leukaemia out of more than 27,000 people treated with various CAR-T therapies 2 . In three secondary cancers that were sequenced, the agency found that the cancerous T cells contained the CAR gene, “which indicates that the CAR-T product was most likely involved in the development of the T-cell cancer”, the authors wrote.
According to Paul Richards, a spokesperson for the FDA, 11 further reports of secondary cancer have since come in, as of 25 March. None of the extra cases has been confirmed as having the CAR gene, but neither are any of the cases so far definitively CAR-negative, Richards said in an e-mail. In many instances, the agency didn’t have a sample of the secondary cancer to analyse; in others, the genomic analysis isn’t yet complete. He adds that certain reports, specifically those positive for the CAR gene, “strongly suggest” that T-cell cancer should be considered a risk of the therapy.
But even when the CAR gene is present, proving causality can be tricky. In one case study, researchers in Australia described 3 a 51-year-old man who had been treated for multiple myeloma with a CAR-T therapy. The treatment was part of a clinical trial of Carvykti, made by Legend Biotech in Somerset, New Jersey, in partnership with the drug giant Johnson & Johnson. The treatment worked to clear his cancer, but five months later he developed an unusual, fast-growing bump on his nose. A biopsy revealed that it was T-cell lymphoma. When the team examined the cancerous cells, they found the gene for the CAR wedged into the regulatory region of a gene called PBX2 .
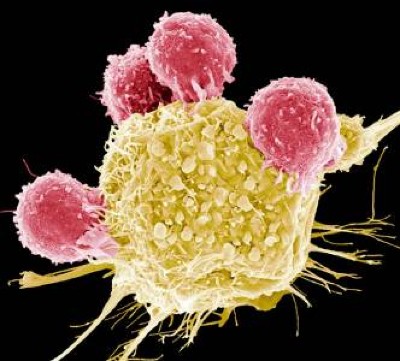
Cutting-edge CAR-T cancer therapy is now made in India — at one-tenth the cost
The finding is provocative, Mackall says, but still not a smoking gun, in her opinion. The researchers found that the cancer cells also carried a mutation often seen in lymphomas, and the person had a genetic variant that put him at increased risk of developing cancer, even without the CAR insertion. It’s likely that the cells harvested to create the therapy contained some pre-cancerous T cells, says Piers Blombery, a haematologist at the Peter MacCallum Cancer Centre in Melbourne, Australia, who leads the diagnostic lab that assessed the tumour samples. Now, the team is looking at samples taken before the therapy to determine whether that’s the case.
Other people who have received Carvykti have developed secondary cancers, too. The FDA’s initial warning focused on T-cell malignancies. But long-term follow-up of participants who’d been in an early trial of Carvykti revealed that 10 out of 97 people developed either myelodysplastic syndrome (a kind of pre-leukaemia) or acute myeloid leukaemia (see go.nature.com/3q8vrym ). Nine of them died. As a result, in December 2023, Legend Biotech added language to its boxed warning for Carvykti about the risk of secondary blood cancers.
Craig Tendler, head of oncology clinical development and global medical affairs at Johnson & Johnson Innovative Medicine in Raritan, New Jersey, says that the company looked for the CAR gene in cancer cells from these individuals, but didn’t find it. When the researchers looked at samples taken before the trial participants received treatment, they found pre-malignant cells with the same genetic make-up as the cancer cells. “So, it is likely that, in many of these cases, the prior therapies for multiple myeloma may have already predisposed these patients to secondary malignancy,” Tendler says. Then, it’s possible that the prolonged immune suppression related to the CAR-T treatment process nudged the cells to become cancerous.

Can autoimmune diseases be cured? Scientists see hope at last
When Ruella first saw the FDA warning, he immediately thought back to a 64-year-old man he had treated who, in 2020, developed a T-cell lymphoma 3 months after receiving CAR-T therapy for a B-cell lymphoma. Ruella and his colleagues identified the CAR gene in the biopsy taken from the man’s lymph node 4 . But it was at such low levels that it seemed unlikely it had integrated into the cancer cells, Ruella says. Instead, the genes could have come from CAR T cells that just happened to be circulating through that lymph node at the time the biopsy was taken. “We thought this was just an accidental finding,” Ruella says.
But after Ruella saw the FDA’s warning, he decided to revisit the case. He and his colleagues went back to a blood sample taken before the person received CAR-T therapy. The team assessed whether T cells with the same T-cell receptor as the lymphoma cells were present before treatment. They were, suggesting that the seeds of the lymphoma pre-dated the therapy. (The low number of cells made further analysis difficult.) Ruella adds that it’s possible the CAR-T treatment produced an inflammatory environment that allowed such seeds to become cancerous. “So this is not something that appears magically out of nowhere,” Ruella says.
Rare outcome
The good news is that these secondary cancers — CAR-driven or not — seem to be rare. After the FDA warning, Ruella and his colleagues also looked back at the files of people who had been treated with commercial CAR-T products at the University of Pennsylvania. Between January 2018 and November 2023, the centre treated 449 individuals who had leukaemias, lymphomas or multiple myeloma with CAR-T therapies 4 . Sixteen patients (3.6%) went on to develop a secondary cancer. But most of those were solid tumours, not the kind of cancer one would expect to come directly from the treatment. Only five of the treated patients developed blood cancers, and only one of those developed a T-cell cancer.
At the Mayo Clinic in Phoenix, Arizona, haematologist Rafael Fonseca and his colleagues also wondered whether the incidence of secondary cancers in people who had received CAR-T therapy differed from the incidence in those with the same cancers who had not. They combed through a data set containing medical records from 330 million people to find individuals who had been newly diagnosed with multiple myeloma or diffuse large B-cell lymphoma between 2018 and 2022. They then looked at how many of them developed T-cell lymphomas. The prevalence didn’t differ drastically from the 22 cases out of 27,000 people that the FDA had reported. The researchers published their findings on the online newsletter platform Substack (see go.nature.com/3u97s38 ). “We wanted to get it out as soon as possible because of the timeliness of what was going on,” Fonseca says.

The race to supercharge cancer-fighting T cells
Since the FDA’s warning, Hall has started talking about the possibility of secondary cancers to individuals who are contemplating CAR-T therapy. He presents it as a real risk, but a rare one — and explains that it is dwarfed by the risk posed by their current cancer. “For my late-stage myeloma patient, the main risk is that the CAR T doesn’t work and they die of their myeloma,” he says. Mackall and others agree. “I don’t think anyone believes that this will change practice in any way at the current time,” she adds. “Most cancer therapies can cause cancer. This is one of the paradoxes of our business.”
But what about other diseases? Researchers have already tested CAR T cells as a therapy for the autoimmune condition lupus , with impressive results 5 . And more clinical trials of these therapies for other autoimmune diseases are likely to follow. If most of the secondary cancers seen in people treated with CAR T cells are related to the litany of treatments they received beforehand, people with these conditions might not all have the same risk. But even if the therapy is driving some cancers, many say the benefits might still be worth the risk. “Autoimmune diseases are not benign diseases,” said Marks in response to an audience question at an industry briefing in January (see go.nature.com/3jpk6qj ). “Anyone who’s ever known somebody who’s had lupus cerebritis or lupus nephritis will know that those are potentially lethal diseases.”
CAR T cells also hold promise as a treatment for HIV infection, and a trial to test this idea kicked off in 2022 . Researchers are also studying how CAR T cells could be used as a way to curb rejection of transplanted kidneys, or to clear out zombie-like senescent cells that have been implicated in ageing. The possibilities are continuously expanding.
As for whether the benefit of CAR-T therapy outweighs the risk of secondary cancers for these other indications, only time will tell.
Nature 629 , 22-24 (2024)
doi: https://doi.org/10.1038/d41586-024-01215-0
Levine, B. L. et al. Nature Med. 30 , 338–341 (2024).
Article PubMed Google Scholar
Verdun, N. & Marks, P. N . Engl. J. Med. 390 , 584–586 (2024).
Harrison, S. J. et al. Blood 142 (Suppl. 1), 6939 (2023).
Article Google Scholar
Ghilardi, G. et al. Nature Med . https://doi.org/10.1038/s41591-024-02826-w (2024).
Müller, F. et al. N. Engl. J. Med. 390 , 687–700 (2024).
Download references
Reprints and permissions
Related Articles

- Health care
Decoding the interplay between genetic and non-genetic drivers of metastasis
Review Article 15 MAY 24

Engineered CD47 protects T cells for enhanced antitumour immunity
Article 15 MAY 24

Mapping genotypes to chromatin accessibility profiles in single cells
Article 08 MAY 24

Neglecting sex and gender in research is a public-health risk
Comment 15 MAY 24
Interpersonal therapy can be an effective tool against the devastating effects of loneliness
Correspondence 14 MAY 24

Is the Internet bad for you? Huge study reveals surprise effect on well-being
News 12 MAY 24

Senior Research Assistant in Human Immunology (wet lab)
Senior Research Scientist in Human Immunology, high-dimensional (40+) cytometry, ICS and automated robotic platforms.
Boston, Massachusetts (US)
Boston University Atomic Lab
Postdoctoral Fellow in Systems Immunology (dry lab)
Postdoc in systems immunology with expertise in AI and data-driven approaches for deciphering human immune responses to vaccines and diseases.
Global Talent Recruitment of Xinjiang University in 2024
Recruitment involves disciplines that can contact the person in charge by phone.
Wulumuqi city, Ürümqi, Xinjiang Province, China
Xinjiang University
Tenure-Track Assistant Professor, Associate Professor, and Professor
Westlake Center for Genome Editing seeks exceptional scholars in the many areas.
Westlake Center for Genome Editing, Westlake University
Faculty Positions at SUSTech School of Medicine
SUSTech School of Medicine offers equal opportunities and welcome applicants from the world with all ethnic backgrounds.
Shenzhen, Guangdong, China
Southern University of Science and Technology, School of Medicine
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Great Diseases
- Pre-College Programs
- Undergraduate Internships
- Resources for Building Inclusivity in Science
- Publications
Center for Science Education at Tufts University
- The Great Diseases
- Request Access
- Student Portal
- Online Courses
- Partnerships
- Infectious Diseases (ID)
- Neurological Disorders (ND)
- Metabolic Disease (MD)
- Cancer (CA)
- Order Printing
- - Online Courses
- - Webinars
- - Workshops
- - Partnerships
- - Community
- - COVID-19
- - Infectious Diseases (ID)
- - Neurological Disorders (ND)
- - Metabolic Disease (MD)
- - Cancer (CA)
- - Order Printing
Case Studies
Case study 1 — barbara’s dilemma: radiation therapy.
In this study, students must wrestle with the unknowns of science and medicine. After being diagnosed with breast cancer, a patient receives conflicting treatment suggestions from two different doctors. Students must evaluate the pros and cons to each treatment option to arrive at their own suggestion for the patient. In the lesson, students with no background in cancer will learn the very basics of what cancer is and how DNA repair proteins play a role in radiation treatment.
Download Case Study 1 here .
Case Study 2 — Fighting brain cancer with polio virus
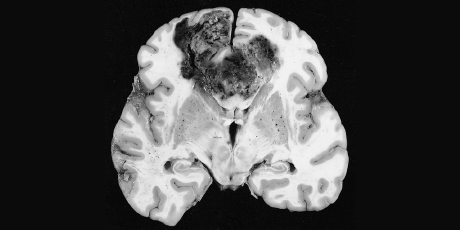
Glioblastomas are a very deadly type of brain cancer and the most common type of cancerous brain tumor in adults. Traditional therapies for cancer are surgery to remove the tumor, or using chemicals or radiation to destroy the tumor cells. These have not been very successful for glioblastomas because it is very hard to kill only the tumor cells in the brain, without damaging or destroying nearby normal cells. It is generally agreed that a goal for cancer treatment is to create drugs or treatments that can selectively target only the cancer cells, without harming the normal cells. The viral therapy described in this case study does just that. The virus is modified to enter and replicate within glioblastoma cells. Once inside, it replicates and sends a signal to the immune system. The cancer cells infected with this virus are now targets for immune destruction. This treatment is currently in phase I trials for safety. In this study, students will design and interpret experiments to evaluate the efficacy of this treatment.
Download Case Study 2 here .
Case Study 3 — Human papillomavirus (HPV) and cancer
Human papillomavirus (HPV) is the most common sexually-transmitted infection in the U.S. Infection is usually cleared by the immune system but can lead to cervical cancer, genital warts, and other cancers. A vaccine administered prior to the first sexual encounter can prevent infection; screening can detect abnormal cells so they can be treated before developing into cancer. In this study, students make predictions and compare these predictions to actual data. They then synthesize different data sets to construct an explanation for current Pap screening recommendations.
Download Case Study 3 here .
Case Study 4 — Smoking and cancer

In this study, students are introduced to the various types of studies that have contributed to establishing a definitive link between smoking and lung cancer. The case focuses on design features of one type of study—a population study. Students will design a study, then discuss aspects of certain design decisions. Finally, students will have the opportunity to re-design and predict results from their study. At the end, students compare their predicted results to actual data collected over the last century.
Download Case Study 4 here .

Disclaimer | Non-Discrimination | Privacy | Terms for Creating and Maintaining Sites
- Biomarker-Driven Lung Cancer
- HER2-Positive Breast Cancer
- Chronic Lymphocytic Leukemia
- Small Cell Lung Cancer
- Renal Cell Carcinoma

- CONFERENCES
- PUBLICATIONS
Case 1: 72-Year-Old Woman With Small Cell Lung Cancer

EP: 1 . Case 1: 72-Year-Old Woman With Small Cell Lung Cancer
Ep: 2 . case 1: extensive-stage small cell lung cancer background, ep: 3 . case 1: impower133 trial in small cell lung cancer, ep: 4 . case 1: caspian trial in extensive-stage small cell lung cancer, ep: 5 . case 1: biomarkers in small cell lung cancer, ep: 6 . case 1: small cell lung cancer in the era of immunotherapy.

EP: 7 . Case 2: 67-Year-Old Woman With EGFR+ Non–Small Cell Lung Cancer
Ep: 8 . case 2: biomarker testing for non–small cell lung cancer, ep: 9 . case 2: egfr-positive non–small cell lung cancer, ep: 10 . case 2: flaura study for egfr+ metastatic nsclc, ep: 11 . case 2: egfr+ nsclc combination therapies.

EP: 12 . Case 2: Treatment After Progression of EGFR+ NSCLC

EP: 13 . Case 3: 63-Year-Old Man With Unresectable Stage IIIA NSCLC
Ep: 14 . case 3: molecular testing in stage iii nsclc, ep: 15 . case 3: chemoradiation for stage iii nsclc, ep: 16 . case 3: pacific trial in unresectable stage iii nsclc, ep: 17 . case 3: standard of care in unresectable stage iii nsclc, ep: 18 . case 3: management of immune-related toxicities in stage iii nsclc.
Mark Socinski, MD: Thank you for joining us for this Targeted Oncology ™ Virtual Tumor Board ® focused on advanced lung cancer. In today’s presentations my colleagues and I will review three clinical cases. We will discuss an individualized approach to treatment for each patient, and we’ll review key clinical trial data that impact our decisions. I’m Dr. Mark Socinski from the AdventHealth cancer institute in Orlando, Florida. Today I’m joined by Dr Ed Kim, a medical oncologist from the Levine Cancer Institute in Charlotte, North Carolina; Dr Brendon Stiles, who is a thoracic surgeon from the Weill Cornell Medical Center in New York ; and Dr Tim Kruser, radiation oncologist from Northwestern Medicine Feinberg School of Medicine in Chicago. Thank you all for joining me today. We’re going to move to the first case, which is a case of small cell lung cancer. I’m going to ask Dr Kim to do the presentation.
Edward Kim, MD: Thanks, Mark. It’s my pleasure to walk us through the first case, which is small cell lung cancer. This is a case with a 72-year-old woman who presents with shortness of breath, a productive cough, chest pain, some fatigue, anorexia, a recent 18-pound weight loss, and a history of hypertension. She is a schoolteacher and has a 45-pack-a-year smoking history; she is currently a smoker. She is married, has 2 kids, and has a grandchild on the way. On physical exam she had some dullness to percussion with some decreased-breath sounds, and the chest x-ray shows a left hilar mass and a 5.4-cm left upper-lobe mass. CT scan reveals a hilar mass with a bilateral mediastinal extension. Negative for distant metastatic disease. PET scan shows activity in the left upper-lobe mass with supraclavicular nodal areas and liver lesions, and there are no metastases in the brain on MRI. The interventional radiographic test biopsy for liver reveals small cell, and her PS is 1. Right now we do have a patient who has extensive-stage small cell lung cancer. Unfortunately, it’s what we found. It’s very common to see this with liver metastases.
Transcript edited for clarity.

FDA Approval Marks Amivantamab's Milestone in EGFR+ NSCLC
In this episode, Joshua K. Sabari, MD, discusses the FDA approval of amivantamab plus chemotherapy as a first-line treatment for patients with EGFR exon 20 insertion mutation-positive non-small cell lung cancer.

Nogapendekin Alfa Plus Checkpoint Inhibition Improves Survival in NSCLC
Following its recent FDA approval in non-muscle-invasive bladder cancer, nogapendekin alfa has also shown overall survival benefits in addition to checkpoint inhibitor therapy in patients with non-small cell lung cancer.

Lisberg Discusses Dato-DXd's Role in Advanced Lung Cancer Care
In this episode of Targeted Talks, Aaron Lisberg, MD, discusses results from the phase 3 TROPION-Lung01 study of datopotamab in advanced or metastatic non–small cell lung cancer.

Emphasizing the Need for NGS in Advanced NSCLC to Determine Treatment
During a Case-Based Roundtable® event, Joshua Sabari, MD, led a discussion on the need for next-generation sequencing to determine treatment in patients with EGFR-positive advanced non–small cell lung cancer in the first article of a 2-part series.

Biomarker Testing Paves the Way for Better Targeted Therapies in NSCLC
At a live virtual event, Edward S. Kim, MD, MBA, discussed the evolving landscape of biomarker testing before making treatment decisions for patients with early-stage non–small cell lung cancer (NSCLC).
2 Commerce Drive Cranbury, NJ 08512
609-716-7777

Comparative effectiveness of first-line systemic treatments for metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis
- Research Article
- Published: 15 May 2024
Cite this article

- Jiahuan Ai ORCID: orcid.org/0009-0006-5349-0869 1 na1 ,
- Liuying Jian 1 na1 ,
- Xiaoqin Wen 1 ,
- Xiaotong Huo 1 ,
- Xuanyi Yang 1 ,
- Jie Jiang 1 &
- Tiantian Zhang 1 , 2
No head-to-head trials had been performed to estimate the relative effectiveness of poly ADP-ribose polymerase inhibitor (PARPi) and androgen receptor signaling inhibitor (ARSi) in the first-line treatment for metastatic castration-resistant prostate cancer (mCRPC). We aimed to perform a systematic review and network meta-analysis to evaluate the comparative effectiveness of various systemic treatment agents for patients with mCRPC.
A comprehensive literature search was conducted for abstracts and full-text articles from the database’s inception through April 27, 2023. The study concentrated on assessing radiographic progression-free survival (rPFS) for both overall and homologous recombination repair mutation (HRRm) population, with overall survival (OS) as the secondary measure. Under the Bayesian framework, the overall effect was pooled using the fixed-effects model in base case analysis. Scenario analysis using restricted mean survival time (RMST) methods was performed to test the robustness of the results.
Nine studies with 6,830 patients and 8 unique treatment options were included. Network meta-analysis demonstrated that talazoparib in combination with enzalutamide (TALA + ENZA; overall population, hazard ratio [HR], 0.20; 95% credible interval [CrI]: 0.16–0.26; RMST, 3.51; 95% confidence interval [CI] 2.46–4.60; HRRm population, HR, 0.15; 95% CrI: 0.09–0.23; RMST, 4.14; 95% CI 2.84–5.39) was superior to other treatments in the first-line setting in terms of rPFS. The results of Bayesian framework and RMST models showed consistent efficacy ranks. When extrapolated to overall survival benefit, within the Bayesian framework, olaparib plus abiraterone acetate and prednisone (OLAP + AAP) achieved the highest OS benefit for the overall population, which was not statistically significant when compared to TALA + ENZA. However, TALA + ENZA achieved the highest OS benefit at 3 years by applying RMST.
Conclusions
We suggest that talazoparib in combination with enzalutamide is probably a preferred treatment agent for the overall population and HRRm patients with mCRPC. Given the limitations of network framework and the modeling assumptions undertaken to finalize the analyses, results should be cautiously interpreted.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
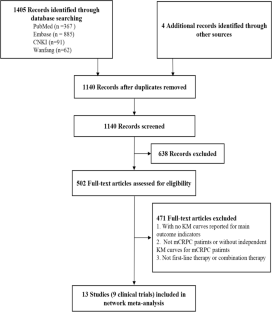
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
Abiraterone acetate plus prednisone
Androgen-deprivation therapy
Apalutamide
- Androgen receptor signaling inhibitor
Bicalutamide
Blinded independent central review
Best support care
Bone sialoprotein
Confidence interval
Credible interval
DNA damage response and repair
Enzalutamide
Food and Drug Administration
Hazard ratio
Homologous recombination repair mutation
Heat shock protein 90
Investigator
Individual patient data
Kaplan–Meier
- Metastatic castration-resistant prostate cancer
Mismatch repair
National Comprehensive Cancer Network
Non-homologous end-joining
- Network meta-analysis
Overall survival
Open Science Framework
Osteopontin
Poly ADP-ribose polymerase inhibitor
Proportional hazard
Preferred Reporting Items for Systematic Reviews and Meta-analyses
International Prospective Register of Systematic
Randomized controlled trial
Radiographic progression-free survival
Stereotactic ablative radiotherapy
Talazoparib
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492 .
Article PubMed Google Scholar
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 CANCERS in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. https://doi.org/10.3322/caac.21660 .
Article CAS PubMed Google Scholar
Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–32. https://doi.org/10.3322/caac.21338 .
Ma C, Ye D, Li C, Zhou F, Yao X, Zhang S, et al. Epidemiologic characteristics of prostate cancer and analysis of first-line endocrine therapy in advanced stage. Chin J Surg. 2008;12:921–5.
Google Scholar
Zhu Y. Expert consensus on the diagnosis and treatment of denervation-resistant prostate cancer in China. Chin Surg Miscell J. 2016;54(7):481–4.
Attard G, Murphy L, Clarke NW, Sachdeva A, Jones C, et al. Abiraterone acetate plus prednisolone with or without enzalutamide for patients with metastatic prostate cancer starting androgen deprivation therapy: final results from two randomised phase 3 trials of the STAMPEDE platform protocol. Lancet Oncol. 2023;24(5):443–56. https://doi.org/10.1016/S1470-2045(23)00148-1 .
Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr, Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–20. https://doi.org/10.1056/NEJMoa041318 .
Pritchard CC, Mateo J, Walsh MF, De Sarkar N, Abida W, Beltran H, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443–53. https://doi.org/10.1056/NEJMoa1603144 .
Article CAS PubMed PubMed Central Google Scholar
Shah S, Rachmat R, Enyioma S, Ghose A, Revythis A, et al. BRCA mutations in prostate cancer: assessment, implications and treatment considerations. Int J Mol Sci. 2021;22(23):12628. https://doi.org/10.3390/ijms222312628 .
Boussios S, Rassy E, Shah S, Ioannidou E, Sheriff M, et al. Aberrations of DNA repair pathways in prostate cancer: a cornerstone of precision oncology. Expert Opin Ther Targets. 2021;25(5):329–33. https://doi.org/10.1080/14728222.2021.1951226 .
Yang S, Liu X. Progress of drug therapy for metastatic denervation-resistant prostate cancer. J Med Res. 2022;51(11):4–9.
Agarwal N, Azad AA, Carles J, Fay AP, Matsubara N, Heinrich D, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023;S0140–6736(23):01055–63. https://doi.org/10.1016/S0140-6736(23)01055-3 .
Article Google Scholar
Clarke NW, Armstrong AJ, Thiery-Vuillemin A, Oya M,Shore ND, Procopio G, et al. (2023) Final pre-specified overall survival in PROpel: abiraterone and olaparib versus abiraterone and placebo as first-line therapy for metastatic castration-resistant prostate cancer. ASCO GU. https://ascopubs.org/doi/abs/ https://doi.org/10.1200/JCO.2023.41.6_suppl.LBA16 .
Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023;41(18):3339–51. https://doi.org/10.1200/JCO.22.01649 .
Wei Y, Zhang T, He Y, Efstathiou E, Attard G, Olmos D, et al. Preliminary efficacy and safety study of fluzoparib in the treatment of metastatic desmoplasia-resistant prostate cancer. Chin J Cancer. 2021;31(07):561–6. https://doi.org/10.19401/j.cnki.1007-3639.2021.07.001 .
Article CAS Google Scholar
Ghose A, Moschetta M, Pappas-Gogos G, Sheriff M, Boussios S. Genetic aberrations of DNA repair pathways in prostate cancer: translation to the clinic. Int J Mol Sci. 2021;22(18):9783. https://doi.org/10.3390/ijms22189783 .
Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152–60. https://doi.org/10.1016/S1470-2045(14)71205-7 .
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424–33. https://doi.org/10.1056/NEJMoa1405095 .
Saad F, Efstathiou E, Attard G, Flaig TW, Franke F, Goodman OB Jr, et al. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2021;22(11):1541–59. https://doi.org/10.1016/S1470-2045(21)00402-2 .
Penson DF, Armstrong AJ, Concepcion R, Agarwal N, Olsson C, Karsh L, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE Trial. J Clin Oncol. 2016;34(18):2098–106. https://doi.org/10.1200/JCO.2015.64.9285 .
Higano CS, Cheng HH. Poly-ADP ribose polymerase inhibitor and androgen receptor signaling inhibitor for all comers for first-line treatment of metastatic castration-resistant prostate cancer: is gene sequencing out? Curr Opin Urol. 2023;33(5):396–403. https://doi.org/10.1097/MOU.0000000000001114 .
NCCN (2022) The NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer(version 1.2023) [EB/OL]. http://www.nccn.org .
National Health Commission of the People's Republic of China. Guidelines for the diagnosis and treatment of prostate cancer [EB/OL]. http://www.nhc.gov.cn/yzygj/s7659/202204/a0e67177df1f439898683e1333957c74/files/64eb7728ee494e299a77846fff09840e.pdf .
Pan J, Zhao J, Ni X, Gan H, Wei Y, Wu J, et al. The prevalence and prognosis of next-generation therapeutic targets in metastatic castration-resistant prostate cancer. Mol Oncol. 2022;16(22):4011–22.
Saxby H, Boussios S, Mikropoulos C. Androgen receptor gene pathway upregulation and radiation resistance in oligometastatic prostate cancer. Int J Mol Sci. 2022;23(9):4786. https://doi.org/10.3390/ijms23094786 .
Saxby H, Mikropoulos C, Boussios S. An update on the prognostic and predictive serum biomarkers in metastatic prostate cancer. Diagnostics (Basel). 2020;10(8):549. https://doi.org/10.3390/diagnostics10080549 .
Fang M, Nakazawa M, Antonarakis ES, Li C. Efficacy of Abiraterone and enzalutamide in pre- and postdocetaxel castration-resistant prostate cancer: a trial-level meta-analysis. Prostate Cancer. 2017;2017:8560827. https://doi.org/10.1155/2017/8560827 .
Iannantuono GM, Chandran E, Floudas CS, et al. Efficacy and safety of PARP inhibitors in metastatic castration-resistant prostate cancer: a systematic review and meta-analysis of clinical trials. Cancer Treat Rev. 2023;120: 102623. https://doi.org/10.1016/j.ctrv.2023.102623 .
Hutton B, Salanti G, Caldwell DM, Choo-Wosoba H, Butera G, Roselli M, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–84. https://doi.org/10.7326/M14-2385 .
Ai J (2023) Comparative effectiveness of poly ADP-ribose polymerase inhibitor and androgen receptor signaling inhibitor in the first-line treatment for metastatic castration-resistant prostate cancer: a network meta-analysis. Accessed Sep 7, 2023. https://osf.io/axpuk .
Ai J. Comparative effectiveness of poly ADP-ribose polymerase inhibitor and androgen receptor signaling inhibitor in the first-line treatment for metastatic castration-resistant prostate cancer: a network meta-analysis. Accessed Sep 26, 2023. https://www.crd.york.ac.uk/prospero
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. https://doi.org/10.1136/bmj.d5928 .
Article PubMed PubMed Central Google Scholar
Chen J, Zhang Y, Zhang X, Zhao J, Ni Y, Zhu S, et al. Comparison of systemic treatments for metastatic castration-resistant prostate cancer after docetaxel failure: a systematic review and network meta-analysis. Front Pharmacol. 2022;12:789319. https://doi.org/10.3389/fphar.2021.789319 .
Pak K, Uno H, Kim DH, Tian L, Kane RC, Takeuchi M, et al. Interpretability of cancer clinical trial results using restricted mean survival time as an alternative to the hazard ratio. JAMA Oncol. 2017;3(12):1692–6. https://doi.org/10.1001/jamaoncol.2017.2797 .
Lombardi P, Filetti M, Falcone R, Di Bidino R, Iacovelli R, Ciccarese C, et al. New first-line immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and network meta-analysis. Cancer Treat Rev. 2022;106:102377. https://doi.org/10.1016/j.ctrv.2022.102377 .
Fisher D (2019) IPDMETAN: Stata module for performing two-stage IPD meta-analysis. Stata Software Components. Available at: https://econpapers.repec.org/software/bocbocode/S457785.htm .
Therneau GTM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–26.
Guyot P, Ades AE, Ouwens MJ, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. https://doi.org/10.1186/1471-2288-12-9 .
Petit C, Blanchard P, Pignon JP, Lueza B. Individual patient data network meta-analysis using either restricted mean survival time difference or hazard ratios: is there a difference? A case study on locoregionally advanced nasopharyngeal carcinomas. Syst Rev. 2019;8(1):96. https://doi.org/10.1186/s13643-019-0984-x .
Guyatt G, Oxman AD, Akl EA et al. GRADE guidelines: 1. Introduction GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64: 383–94.
Pu YS, Ahn H, Han W, Huang SP, Wu HC, Ma L, et al. Enzalutamide in chemotherapy-naïve metastatic castration-resistant prostate cancer: an Asian multiregional. Random Study Adv Ther. 2022;39(6):2641–56. https://doi.org/10.1007/s12325-022-02140-2 .
Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71(2):151–4. https://doi.org/10.1016/j.eururo.2016.07.032 .
Oya M, Armstrong AJ, Thiery-Vuillemin A, Shore N, Procopio G, Arslan C, et al. (2022) Biomarker analysis and updated results from the Phase III PROpel trial of abiraterone (abi) and olaparib (ola) vs abi and placebo (pbo) as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). ESMO 2022. https://d27mnwjqm5ztsa.cloudfront.net/4d4d150a-bf20-4c76-945a-ce2a670c3c99/031898bb-a199-4394-b7fa-aa1c6f7a6a2a/031898bb-a199-4394-b7fa-aa1c6f7a6a2a_source__v.pdf .
Rathkopf DE, Smith MR, de Bono JS, Logothetis CJ, Shore ND, de Souza P, et al. Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol. 2014;66(5):815–25. https://doi.org/10.1016/j.eururo.2014.02.056 .
Shore ND, Chowdhury S, Villers A, Klotz L, Siemens DR, Phung D, et al. Efficacy and safety of enzalutamide versus bicalutamide for patients with metastatic prostate cancer (TERRAIN): a randomised, double-blind, phase 2 study. Lancet Oncol. 2016;17(2):153–63. https://doi.org/10.1016/S1470-2045(15)00518-5 .
Armstrong AJ, Lin P, Tombal B, Saad F, Higano CS, Joshua AM, et al. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naïve metastatic castration-resistant prostate cancer from the PREVAIL Trial. Eur Urol. 2020;78(3):347–57. https://doi.org/10.1016/j.eururo.2020.04.061 .
Fizazi K, Azad A, Matsubara N, Carles J, Fay AP, Giorgi UD, et al. TALAPRO-2: Phase 3 study of talazoparib (TALA) + enzalutamide (ENZA) versus placebo (PBO) + ENZA as first-line (1L) treatment for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) harboring homologous recombination repair (HRR) gene alterations. https://doi.org/10.1200/JCO.2023.41.16_suppl.5004 .
Download references
Authors' Contributions
JA and TZ had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. The work reported in the paper has been performed by the authors, unless clearly specified in the text. Conception and design: JA, TZ. Acquisition, analysis, or interpretation of data: JA, LJ, XW. Drafting of the manuscript: JA, XH. Critical revision of the manuscript for important intellectual content: XY, JJ. Statistical analysis: JA, LJ, XH. Obtained funding: TZ. Administrative, technical, or material support: JJ, TZ. Supervision: TZ.
This work was supported by the National Natural Science Foundation of China (grant numbers 72274079). Role of the sponsor: The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Jiahuan Ai and Liuying Jian contributed equally to this work.
Authors and Affiliations
College of Pharmacy/Southern Institute of Pharmacoeconomics and Health Technology Assessment, Jinan University, Guangzhou, 510632, China
Jiahuan Ai, Liuying Jian, Xiaoqin Wen, Xiaotong Huo, Xuanyi Yang, Jie Jiang & Tiantian Zhang
Guangzhou Huabo Biopharmaceutical Research Institute, Guangzhou, 510010, China
Tiantian Zhang
You can also search for this author in PubMed Google Scholar
Corresponding authors
Correspondence to Jie Jiang or Tiantian Zhang .
Ethics declarations
Conflict of interest.
The authors declare that they have no conflict of interest.
Ethical approval
This manuscript is based entirely on the analysis of previously published data. As such, this study does not involve any direct research with human participants or animals, and therefore, it does not require ethical approval.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (DOCX 1016 KB)
Rights and permissions.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Ai, J., Jian, L., Wen, X. et al. Comparative effectiveness of first-line systemic treatments for metastatic castration-resistant prostate cancer: a systematic review and network meta-analysis. Clin Transl Oncol (2024). https://doi.org/10.1007/s12094-024-03506-4
Download citation
Received : 05 March 2024
Accepted : 26 April 2024
Published : 15 May 2024
DOI : https://doi.org/10.1007/s12094-024-03506-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- First-line treatment
- PARP inhibitor
- Effectiveness
- Find a journal
- Publish with us
- Track your research

IMAGES
VIDEO
COMMENTS
Increasing Mortality in Korean Patients With Breast Cancer: High Mortality Rate in Elderly Breast Cancer Population Due to Suboptimal Treatment and Other Diseases, Cancer Control, 28 ...
Dr. Mathew S. Lopes: A 65-year-old woman was transferred to this hospital because of chest pain. Six months before the current presentation, the patient presented to a hospital affiliated with ...
This study demonstrates how to responsibly generate RWE for treatment decisions. We validate our approach by demonstrating that we can uncover an existing adjuvant chemotherapy (ACT) guideline for stage II and III colon cancer (CC)—which came about using both data from randomized controlled trials and expert consensus—solely using RWD.
In a very small trial done by doctors at New York's Memorial Sloan Kettering Cancer Center, patients took a drug called dostarlimab for six months. The trial resulted in every single one of their ...
Methods. A case study approach was used because it allowed us to examine the complexity of cancer management from the perspective of one person's case as interpreted by multiple people, retaining its holistic and meaningful characteristics while being studied 17 answering how and why questions. 18 Interviews from four participants presented multiple perspectives of the same interested topic ...
Presentation of Case. Dr. Christopher T. Chen: A 34-year-old woman was evaluated in the oncology clinic of this hospital for the management of relapsed, metastatic intrahepatic cholangiocarcinoma ...
This subtype of breast cancer is known for being aggressive, having a high probability of distant recurrence after adjuvant therapy, and progressing quickly on palliative chemotherapy treatment in the metastatic setting. [2,3] Patients with metastatic TNBC have a poor prognosis, with a median overall survival of 13.3 months with treatment. [3]
This Case Study describes a patient with multiple relapses of atypical HLH that became refractory to treatment with numerous immunosuppressive agents. As HLH is driven by CD52 + T cells and ...
The earliest evidence of cancer treatment can be traced ... of the two first-in-class inhibitors of the mutant kinase KRAS G12C was a milestone in the decades-long efforts to study and treat ...
New research suggests that fungi in the gut may affect how tumors respond to cancer treatments. In mice, when bacteria were eliminated with antibiotics, fungi filled the void and impaired the immune response after radiation therapy, the study found. FDA Approves Belumosudil to Treat Chronic Graft-Versus-Host Disease.
Diagnosis and treatment of cancer — Case Studies The following are overviews of some notable recent cases* that exemplify our approach. In all cases, we were able to identify unexpected actionable hypotheses - ideas that pointed to FDA-approved therapies that an oncologist could prescribe or to clinical trials that a patient could enter. These hypotheses are strongly supported by our ...
Systemic treatment for breast cancer is determined by the hormonal and biological status of the tumor. A study by Avisar et al. showed that 56% of cases of male breast cancer showed overexpression of HER2 . This patient had a HER2-positive invasive ductal carcinoma of the breast (3+).
Case Report: We report here a case of a very young woman with diagnosis of early-stage lung adenocarcinoma harboring EML4-ALK rearrangement; she underwent radical surgery and adjuvant chemotherapy according to the pathologic stage. Potential risk factors for lung cancer in our patient are discussed and clinico-pathologic features and outcomes of lung cancer in the young population compared to ...
Current Problems in Cancer: Case Reports is an international, peer-reviewed open access Journal publishing oncology case reports. It aims to share awareness of groundbreaking cases that give insight into redefining concepts in cancer. Our topics are comprehensive, encompassing new understandings of cancer diagnosis, tumor biology, and treatment ...
Together, this study showed that using ex vivo CRISPR-edited, PD-1-ablated, patient-derived T cells for cancer treatment is clinically feasible and generally safe. This finding echoes the ...
The patient received this treatment initially in the Peak Center clinic under the supervision of the treating physician and the Principal Investigator (DSB) of this study for the first 3 days. The dose was escalated over this period as follows. On the first day, the treatment was for 2 hours with a 5-min break between the first and the second hour.
Lessons for the future. "This case is unusual, not only because of the multimodal approach but also the age of the patient," explains Dr. Kamath. "It underscores the unfortunate rise of young-onset colorectal cancer. Thirty years ago, the median age at onset of colorectal cancer was 72 years. It has fallen to 66 years, and we are seeing ...
1268 n engl j med 381;13 nejm.orgSeptember 26, 2019 The new england journal of medicine Presentation of Case Dr. Mathew S. Lopes: A 65-year-old woman was transferred to this hospital because of ...
revealed a tumor on the left lower lobe with pleural effusion, and thoracic puncture cytology indicated lung adenocarcinoma. Interventions: Four cycles of carboplatin and docetaxel chemotherapy reduced the size of the tumor; however, it increased in size after 8 months, and re-challenge chemotherapy (RC) with the same drugs was performed. Repeated RC controlled disease activity for 6 years ...
Cancer Therapy Advisor offers the latest cancer management and treatment info, cancer research news, and more for oncologists and medical professionals.
And that's actually very much not true. Most clinical trials are looking at one treatment compared to another treatment." Judy C. Boughey, M.D. Watch this video to hear the experiences of people who have participated in cancer clinical trials and to hear Drs. Block, Johnson and Boughey discuss the importance of clinical trials in cancer care:
Carol Edwards, a 39 year-old premenopausal woman, had a screening mammogram which revealed an abnormality in the right breast. She had no palpable masses on breast exam. A mammographically localized surgical biopsy was done and revealed a small (0.9 cm) grade III infiltrating ductal carcinoma with some associated ductal carcinoma-in-situ (DCIS ...
Over the past few years, significant therapeutic advances were achieved through better understanding of the molecular mechanisms involved in pathogenesis and treatment. We present a case of a male patient diagnosed with metastatic urothelial cancer (mUC) who had developed complete response after treatment with atezolizumab.
The new treatment, which uses immune cells harvested from a patient's tumor called tumor-infiltrating lymphocytes, exploits the body's own natural cancer-fighting ability. Once the tumor-infiltrating lymphocytes, or T cells, are harvested, they are encouraged in the laboratory to multiply into billions of cancer-fighting cells, then ...
Steven Rosenberg, chief of surgery at the National Cancer Institute, helped to develop the therapy and published the first successful results in a 2010 study for the treatment of lymphoma.
In one case study, researchers in Australia described 3 a 51-year-old man who had been treated for multiple myeloma with a CAR-T therapy. The treatment was part of a clinical trial of Carvykti ...
Case Study 1 — Barbara's dilemma: radiation therapy. In this study, students must wrestle with the unknowns of science and medicine. After being diagnosed with breast cancer, a patient receives conflicting treatment suggestions from two different doctors. Students must evaluate the pros and cons to each treatment option to arrive at their ...
During a Case-Based Roundtable® event, Joshua Sabari, MD, led a discussion on the need for next-generation sequencing to determine treatment in patients with EGFR-positive advanced non-small cell lung cancer in the first article of a 2-part series.
Prostate cancer represents one of the most prevalent malignancies of the male genitourinary system, with an incidence rate ranking 2nd among male cancer diagnoses, and is the second most common malignancy and the fifth leading cause of fatal cancers in men [].In 2020, 1,414,259 cases of morbidity and 375,304 deaths occurred globally [].With population aging and improving life expectancy, the ...