Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- RESEARCH HIGHLIGHT
- 23 August 2021

Health benefits of fermented foods
- Karen O’Leary 0
Karen O’Leary is an Associate Research Analysis Editor with Nature Medicine.
You can also search for this author in PubMed Google Scholar
It is well known that diet influences the gut microbiome, which in turn can affect host health. Microbiome changes caused by ‘westernized’ diets are linked to increases in inflammation, BMI and other markers of poor health, whereas high-fiber and fermented foods are associated with health benefits including reduced risk of certain diseases.
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Rent or buy this article
Prices vary by article type
Prices may be subject to local taxes which are calculated during checkout
doi: https://doi.org/10.1038/d41591-021-00053-1
Related Articles
Melding microbiome and nutritional science with early child development
The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk
A framework for microbiome science in public health

- Inflammation
- Clinical trials
Multimodal decoding of human liver regeneration
Article 01 MAY 24

Metabolic rewiring promotes anti-inflammatory effects of glucocorticoids
Article 10 APR 24
IL-10 constrains sphingolipid metabolism to limit inflammation
Article 21 FEB 24

Clinical trial links oncolytic immunoactivation to survival in glioblastoma
Article 18 OCT 23

A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity
Article 23 NOV 21

Two chemoattenuated PfSPZ malaria vaccines induce sterile hepatic immunity
Article 30 JUN 21
Dad’s gut microbes matter for pregnancy health and baby’s growth
News & Views Forum 01 MAY 24

Exploring the lung microbiome’s role in disease
Outlook 17 APR 24

Gut bacteria break down cholesterol — hinting at probiotic treatments
News 02 APR 24
Faculty Positions at the Center for Machine Learning Research (CMLR), Peking University
CMLR's goal is to advance machine learning-related research across a wide range of disciplines.
Beijing, China
Center for Machine Learning Research (CMLR), Peking University
Faculty Positions at SUSTech Department of Biomedical Engineering
We seek outstanding applicants for full-time tenure-track/tenured faculty positions. Positions are available for both junior and senior-level.
Shenzhen, Guangdong, China
Southern University of Science and Technology (Biomedical Engineering)
Southeast University Future Technology Institute Recruitment Notice
Professor openings in mechanical engineering, control science and engineering, and integrating emerging interdisciplinary majors
Nanjing, Jiangsu (CN)
Southeast University
Staff Scientist
A Staff Scientist position is available in the laboratory of Drs. Elliot and Glassberg to study translational aspects of lung injury, repair and fibro
Maywood, Illinois
Loyola University Chicago - Department of Medicine
W3-Professorship (with tenure) in Inorganic Chemistry
The Institute of Inorganic Chemistry in the Faculty of Mathematics and Natural Sciences at the University of Bonn invites applications for a W3-Pro...
53113, Zentrum (DE)
Rheinische Friedrich-Wilhelms-Universität
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
REVIEW article
Fermented foods as a dietary source of live organisms.

- Department of Food Science and Technology, University of Nebraska—Lincoln, Lincoln, NE, United States
The popularity of fermented foods and beverages is due to their enhanced shelf-life, safety, functionality, sensory, and nutritional properties. The latter includes the presence of bioactive molecules, vitamins, and other constituents with increased availability due to the process of fermentation. Many fermented foods also contain live microorganisms that may improve gastrointestinal health and provide other health benefits, including lowering the risk of type two diabetes and cardiovascular diseases. The number of organisms in fermented foods can vary significantly, depending on how products were manufactured and processed, as well as conditions and duration of storage. In this review, we surveyed published studies in which lactic acid and other relevant bacteria were enumerated from the most commonly consumed fermented foods, including cultured dairy products, cheese, fermented sausage, fermented vegetables, soy-fermented foods, and fermented cereal products. Most of the reported data were based on retail food samples, rather than experimentally produced products made on a laboratory scale. Results indicated that many of these fermented foods contained 10 5−7 lactic acid bacteria per mL or gram, although there was considerable variation based on geographical region and sampling time. In general, cultured dairy products consistently contained higher levels, up to 10 9 /mL or g. Although few specific recommendations and claim legislations for what constitutes a relevant dose exist, the findings from this survey revealed that many fermented foods are a good source of live lactic acid bacteria, including species that reportedly provide human health benefits.
Introduction
Fermentation has long been used to preserve and enhance the shelf-life, flavor, texture, and functional properties of food ( Hutkins, 2018 ). More recently, the consumption of fermented foods containing live microorganisms has emerged as an important dietary strategy for improving human health ( Marco et al., 2017 ). In general, lactic acid bacteria (LAB) from several genera, including Lactobacillus, Streptococcus , and Leuconostoc are predominant in fermented foods, but other bacteria as well as yeast and fungi also contribute to food fermentations. Commercially-produced fermented foods also frequently serve as carriers for probiotic bacteria. Despite this interest and the potential public health benefits of these foods, there is still considerable confusion about which fermented foods actually contain live microorganisms, as well as understanding the role of these microbes on the gut microbiome ( Slashinski et al., 2012 ).
Nonetheless, yogurt and other cultured dairy products are generally perceived by consumers as good sources of live and health-promoting organisms ( Panahi et al., 2016 ). Moreover, in a survey of 335 adults, yogurt was the main food associated with probiotic bacteria ( Stanczak and Heuberger, 2009 ). However, the actual concept of fermentation is evidently not so familiar—a survey of 233 college students attending Brescia University College in London, Ontario revealed that nearly two-thirds were unfamiliar with the term “fermented dairy products,” and about the same percent were unsure that several cultured dairy products were fermented ( Hekmat and Koba, 2006 ).
That a particular food or beverage is produced by fermentation does not necessarily indicate that it contains live microorganisms. Bread, beer, wine, and distilled alcoholic beverages require yeasts for fermentation, but the production organisms are either inactivated by heat (in the case of bread and some beers) or are physically removed by filtration or other means (in the case of wine and beer). Moreover, many fermented foods are heat-treated after fermentation to enhance food safety or to extend shelf-life. Thus, fermented sausages are often cooked after fermentation, and soy sauce and sauerkraut and other fermented vegetables are made shelf-stable by thermal processing. Some products, such as many of the commercial pickles and olives, are not fermented at all, but rather are placed into brines containing salt and organic acids. Even non-thermally processed fermented foods may yet contain low levels of live or viable organisms simply due to inhospitable environmental conditions that reduce microbial populations over time. It is important to note, however, that the absence of live microbes in the final product does not preclude a positive functional role. For example, food fermentation microbes may produce vitamins or other bioactive molecules in situ or inactivate anti-nutritional factors and yet be absent at the time of consumption.
Labeling Live Microbes in Fermented Foods and Beverages
Yogurt, kefir, and other cultured dairy product manufacturers have long promoted the presence of live cultures. Indeed, the “live and active” seal was created by the National Yogurt Association (NYA), for yogurt products in the United States containing at least 100 million cells or cfu per gram at the time of manufacture ( Frye and Kilara, 2016 ). According to the NYA, the “live and active” seal refers only to yogurt cultures, and specifically to the two species that comprise such cultures, Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus . However, frozen yogurt, kefir and other cultured dairy products also claim the presence of live and active cultures, even though the microorganisms may be different than those found in yogurt. In the U.S., there is no regulatory requirement to state microbial levels, thus these label declarations are strictly voluntary.
In contrast, in other regions, the number of live microbes present in yogurt and other cultured dairy products must satisfy regulatory requirements. For example, according to the CODEX standards for fermented milk products, the minimum number of starter culture bacteria in yogurt is 10 7 cfu per g (CODEX STAN 243-2003). If other organisms are indicated on the label, they must be present at 10 6 cfu per g. Nonetheless, in Europe, to make a claim for yogurt containing live cultures for improving lactose digestion, the European Food Safety Agency requires a minimum of 10 8 cfu per g of live bacteria ( EFSA Panel on Dietetic Products, Nutrition and Allergies, 2010 ). In contrast, in Australia and New Zealand, a minimum of only 10 6 cfu per g is required ( Commonwealth of Australia Gazette, 2015 ).
For many years, cultured dairy products were the only fermented foods that included label declarations regarding the presence of live microorganisms. Label declarations on sauerkraut or kimchi or miso, had, until recently, been rare. The popularity of artisan-style fermented foods ( Johnson, 2016 ) and interest in their health properties ( Marco et al., 2017 ) has led more manufacturers to inform consumers, via food labels, that their products contain live microorganisms. In some cases, the species in these types of foods have been identified and then compared to label claims ( Yeung et al., 2002 ; Scourboutakos et al., 2017 ). However, to our knowledge, data on the actual levels of live microorganisms in most fermented retail products has not readily been reported or summarized in an organized form. Therefore, consumers, despite their interest in probiotics and functional fermented foods ( Linares et al., 2017 ), have had little access to this useful information.
Survey Design
The purpose of this study, therefore, was to survey the scientific literature and identify published papers in which the number of live microorganisms in a range of fermented foods was reported. Included were so-called western-fermented foods such as yogurt, cheese, and sausage, as well as soy-based and cereal-based fermented foods that are widely consumed in other regions ( Tamang et al., 2016 ). We then organized and summarized the quantitative data from those reports. Our interest was focused on those reports in which foods were obtained from retail locations or were made under manufacturing conditions. Thus, reports describing results from experimentally-produced fermented foods on a laboratory or pilot scale were excluded, in part because they do not reflect commercial processing, distribution, and storage conditions as do retail products. A large number of the reports in the literature in which levels of microbes in fermented foods were described were of this sort. In addition, many reports have analyzed the importance of microbial food safety and hygienic conditions of fermented food products and have reported the presence of spoilage microorganisms or food pathogens. However, the organisms responsible for fermentation and that are commonly present in the finished products were the focus of this current study.
Search Criteria
Scientific articles were chosen that satisfied specific parameters relevant to our stated goals. Specifically, our database search (Google Scholar, WorldCat, Scopus, and PubMed) focused on those studies that enumerated microorganisms exclusively in fermented food products. Keywords for these searches included, but were not limited to, the type of fermented food analyzed and, “commercially produced,” “commercial product,” “enumerated,” “lactic acid bacteria,” “microbial characterization,” “probiotic,” and “culture.” Food products that served only as vehicles for delivery of probiotic microorganisms were not included. Thus, studies that reported counts for frozen yogurt were included, but studies on ice cream containing probiotic microorganisms were not. In general, results were only included for commercial products, bought at retail locations, or those experimentally-produced under industrial manufacturing conditions. Thus, strictly experimental products (e.g., made in a laboratory or under small experimental-scale conditions) were not considered. The only exceptions were for products for which little or no data from retail or industrially manufactured sources was available. In those cases, lab- or pilot-scale-produced products were included, provided they were made using traditional manufacturing methods. No restrictions for date, location, or language were applied.
Data Reporting
For most products, quantitative data relied on cultural methods using well-established types of differential, selective, and general purpose media, as well as appropriate incubation conditions. LAB were the main group described, although other bacterial groups were occasionally reported. Some studies reported single microbial counts, whereas other reported ranges. Although papers reported counts either as log or as actual values, all of the data described in this review are shown as logs. For some products, values were estimated from graphs or figures. When products were held for shelf-life or aging studies, the counts from multiple times points are shown. Otherwise, single time-point data was reported. The region or origin of product manufacture was also noted.
General Survey Results
Approximately 400 published studies were reviewed in which fermented foods were characterized for the presence of live microorganisms. However, about three-fourths were excluded and not used in our results. Several excluded studies focused on development of selective methods for distinguishing between different species of LAB, determining ratios (e.g., cocci-to-rods in yogurt), or for enumerating only probiotics organisms. Although most studies reported data based on traditional plating methods, many of the more recent studies reported abundance data (i.e., 16S rRNA-based community sequencing). Because the latter 16S-based methods also detect non-viable cells, these studies were excluded unless total counts were also reported. Ultimately, more than 140 studies were included in our survey. Although the literature from which the results were assembled covers a 50 year period and a range of different regions and methodologies, the results are remarkably consistent. As summarized below, nine groups of fermented foods were reviewed in this survey. These included yogurt and other cultured dairy products, cheese, fermented meats, fermented vegetables, traditional fermented Asian products, fermented cereals, beer, and fermented tea (Kombucha).
Yogurt and Other Cultured Dairy Products
Studies were conducted for retail or commercially manufactured yogurts and other cultured dairy products obtained in the U.S., Australia, Spain, France, Norway, Greece, Argentina, and South Africa (Table 1 ). All of the yogurts examined contained the yogurt culture organisms, S. thermophilus and L. delbrueckii subsp. bulgaricus , at levels ranging from < 10 4 to 10 9 cfu/g or ml. In general, counts for S. thermophilus were somewhat higher than for L. delbrueckii subsp. bulgaricus . In several studies, other microorganisms, including Bifidobacterium spp. and Lactobacillus spp., were also enumerated. Levels of the latter ranged from undetectable (< 10 cfu/g) to 10 8 cfu/g. The addition of these probiotic bacteria did not appear to have any effect on levels of the yogurt culture organisms. Although most studies reported counts at only a single time point, other studies reported initial counts as well as at a second time point, usually considered end-of-shelf-life. In such cases, counts were generally similar at both time points (>10 6 cfu/g), provided samples were stored at refrigeration temperatures ( Hamann and Marth, 1984 ).
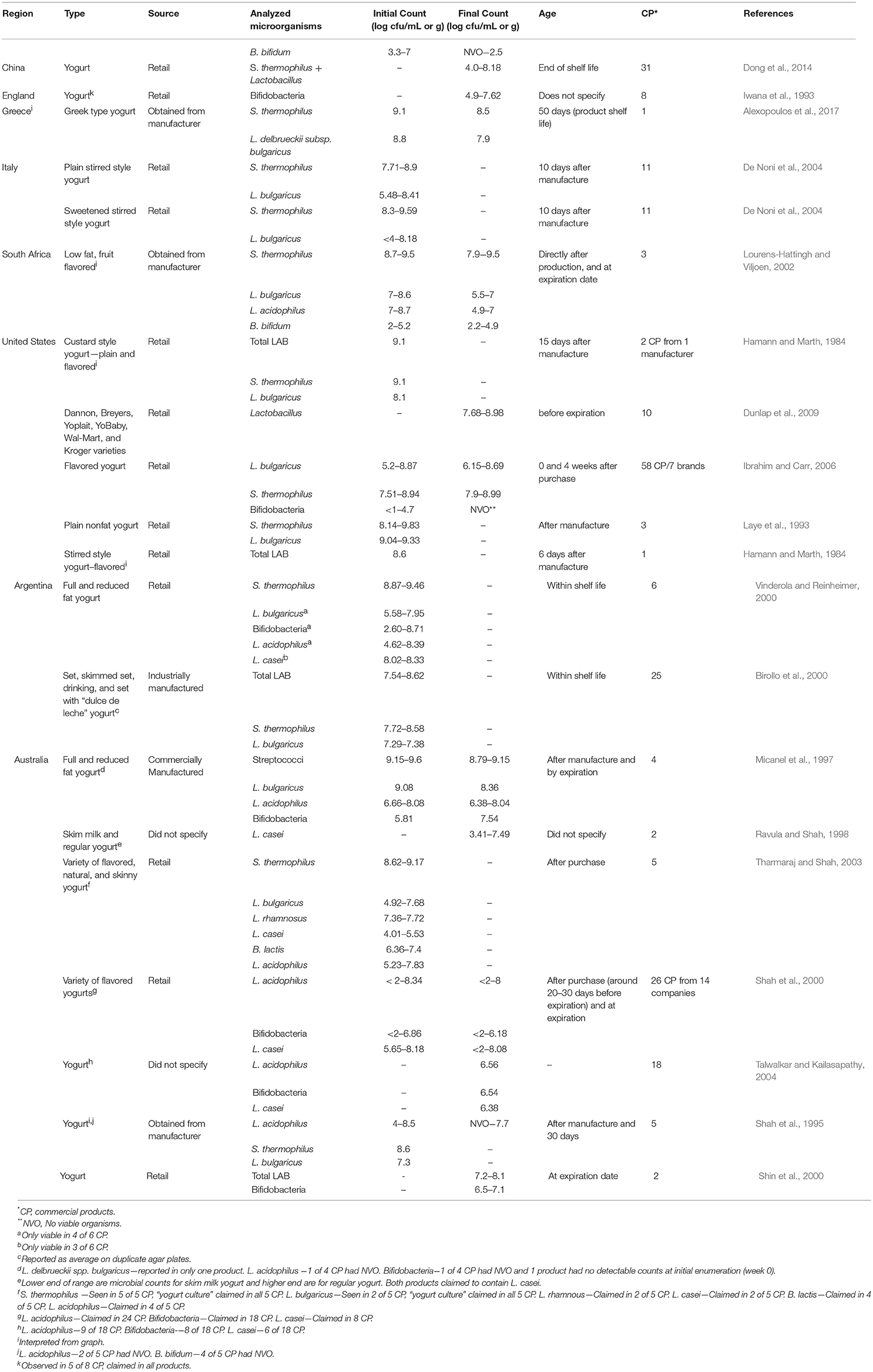
Table 1 . Organisms in commercial yogurt products by region.
In addition to fresh yogurt, frozen yogurt was also examined for bacteria. Results from several studies indicates that when these products were assessed for the relevant yogurt LAB, levels were generally similar to fresh yogurt, with counts ranging from 10 4 to 10 9 cfu/g. The stability of lactic cultures in frozen yogurt during long-term storage at freezer temperature (-23 C) has also been studied ( Lopez et al., 1998 ). In general, LAB ( S. thermophilus and L. delbrueckii subsp. bulgaricus ) survived beyond the designated shelf-life period (1 year), with less than a 0.5 log reduction for most samples.
The number and type of live microorganisms in other cultured dairy products have also been reported (Table 2 ). These include kefir, cultured buttermilk and simply “fermented milk.” As for other cultured dairy products, populations of LAB were in the 10 5 –10 9 cfu/g range.
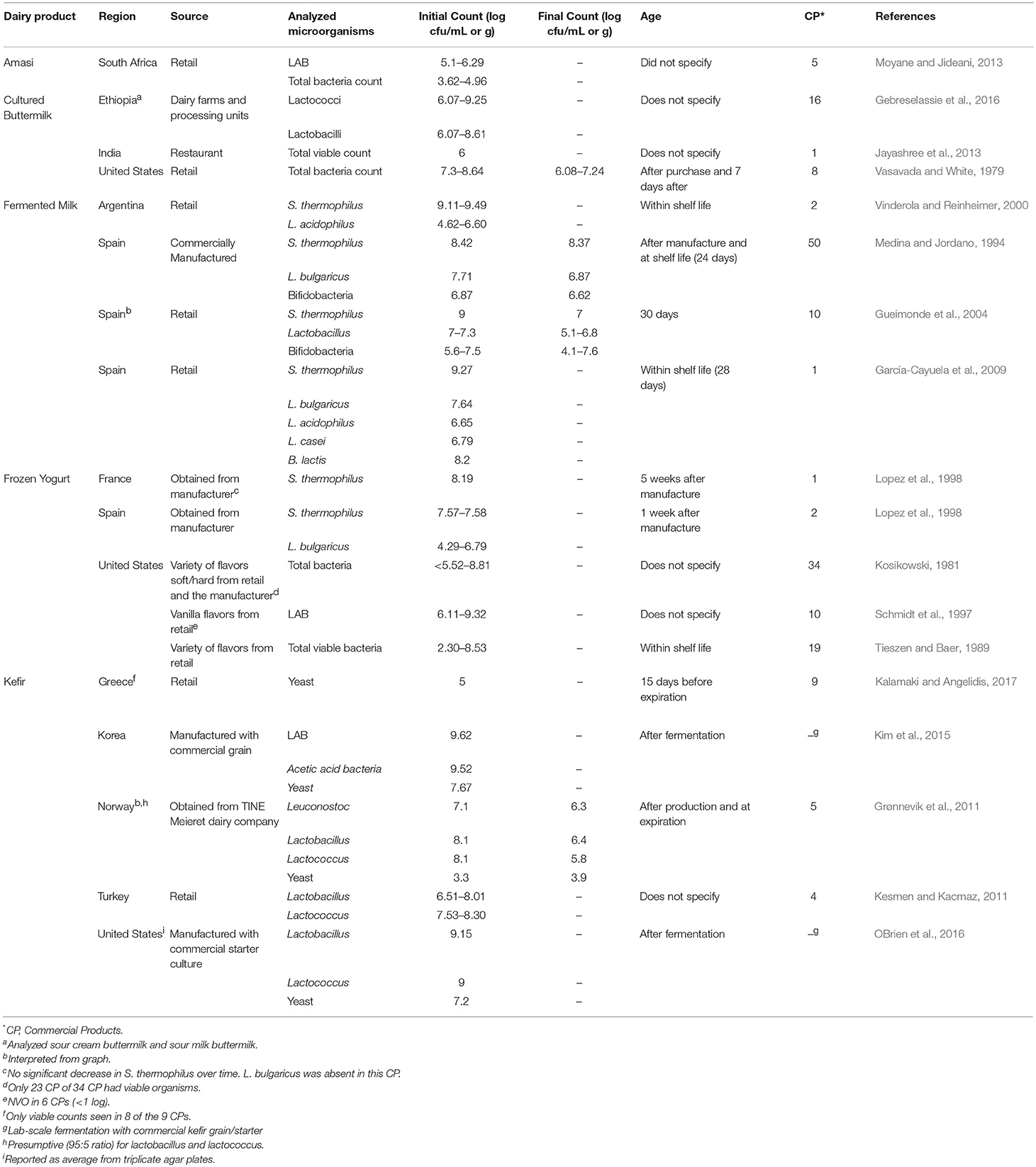
Table 2 . Organisms in commercial cultured dairy products separated by product.
Although considerable microbiological data for cheese exists, most of these reports are concerned with microorganisms having public health or cheese quality implications. Still, levels of lactic acid and related bacteria were reported for more than 30 types of cheese from 18 countries including the United States, Italy, France, Germany, Mexico, Ireland, and South Africa (Table 3 ). Many papers reported the microorganisms as mesophilic streptococci, lactococci, and lactobacilli or as thermophilic streptococci and lactobacilli. Others reported total microorganisms and total LAB. For most products, only one time period was recorded (usually the most aged sample). Microbial counts ranged from undetectable (< 10 3 cfu/g) to 10 9 cfu/g, with the highest levels found in Tilsit cheese (typically aged 2–4 months). In contrast, Grana Padano aged 1 year, Parmesan aged greater than 1 year, and Swiss Gruyere aged greater than 1 year all showed no detectable microorganisms (< 10 3 cfu/g). As noted for other products, the methods used by the investigators may have influenced the reported data. Thus, enumeration of selected organisms (e.g., S. thermophilus ) was only possible if the appropriate medium and growth conditions were used.
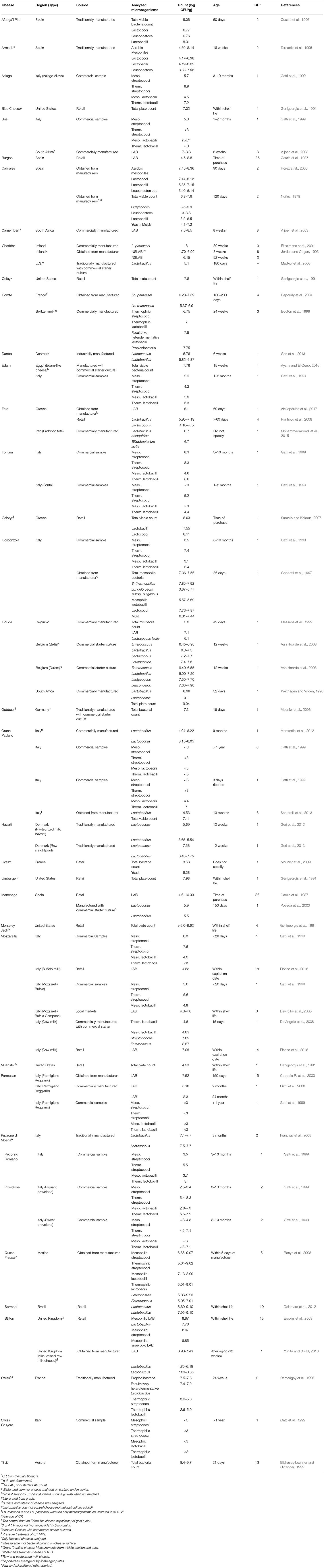
Table 3 . Organisms in commercial cheese separated by product.
Fermented Meats
Microbial counts for fermented sausages are shown in Table 4 . In general, samples were either obtained from retail, directly from manufacturers, or were produced via industrial conditions. Most samples were from the United States, Spain, Portugal, and Italy and were composed of pork and/or beef. The levels of microorganisms (LAB and total) ranged from undetectable (< 10 2 cfu/g) to 10 10 cfu/g. Data were reported as either within the product shelf life or after ripening or maturation of the sausage. Counts of viable microorganisms in sausages from the United States were generally lower (< 10 7 cfu/g) compared to sausages from other countries. In particular, LAB levels were all < 10 6 cfu/g. In contrast, several of the European sausages contained high levels of LAB (>10 8 cfu/g.). European sausages were more often artisan sausages from smaller manufacturers, although similar microorganisms are used in comparison to sausages from the United States.
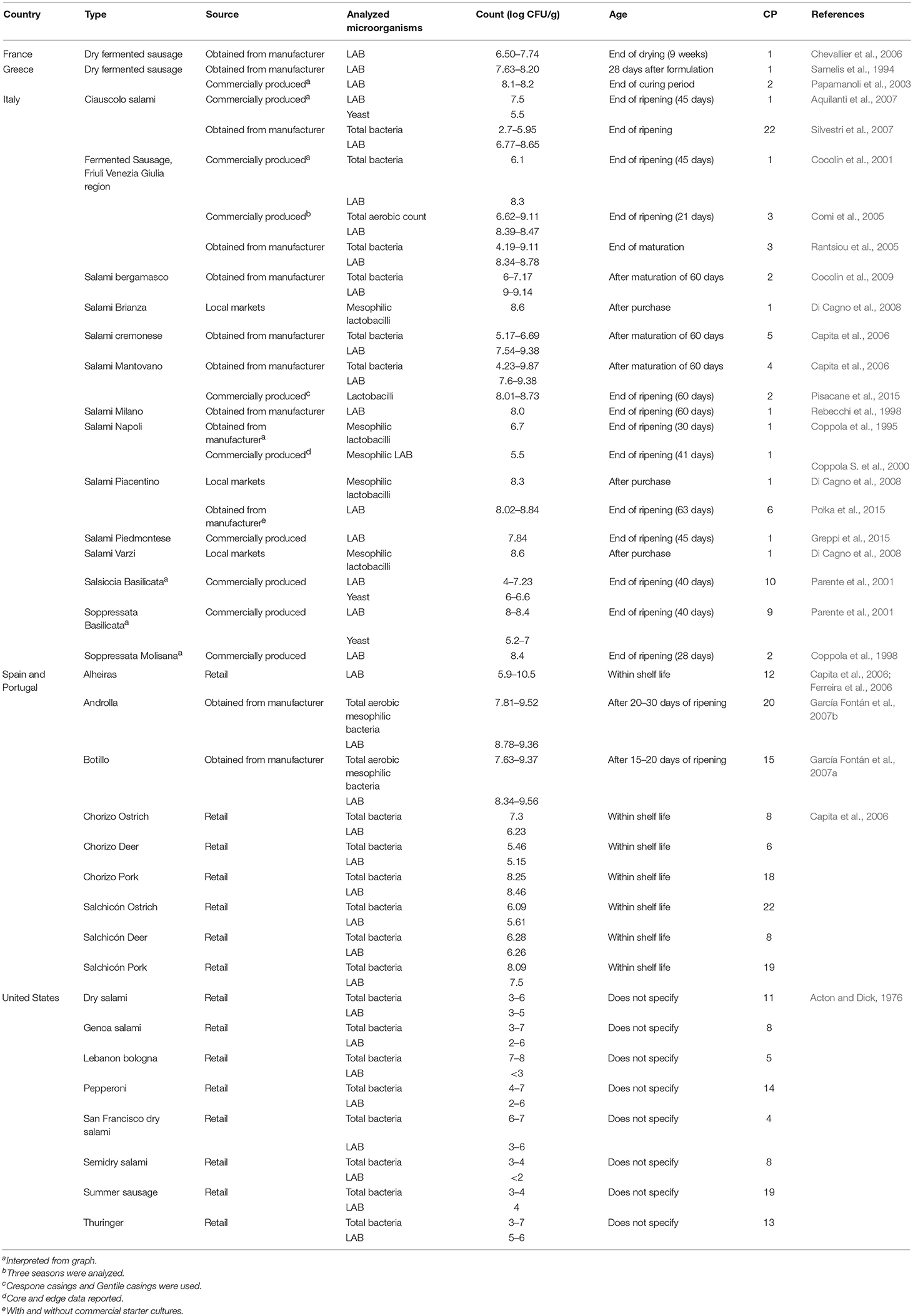
Table 4 . Organisms in commercial sausage products by region.
Fermented Vegetables
Microbial counts for fermented vegetables, including sauerkraut, olives, mustard pickles, pickles, and kimchi are summarized in Table 5 . Fermented cucumbers products were also considered (listed as pickles). Laboratory-manufactured products, using industrial or traditional practices, were included due to the lack of literature on fermented vegetables from retail sources.
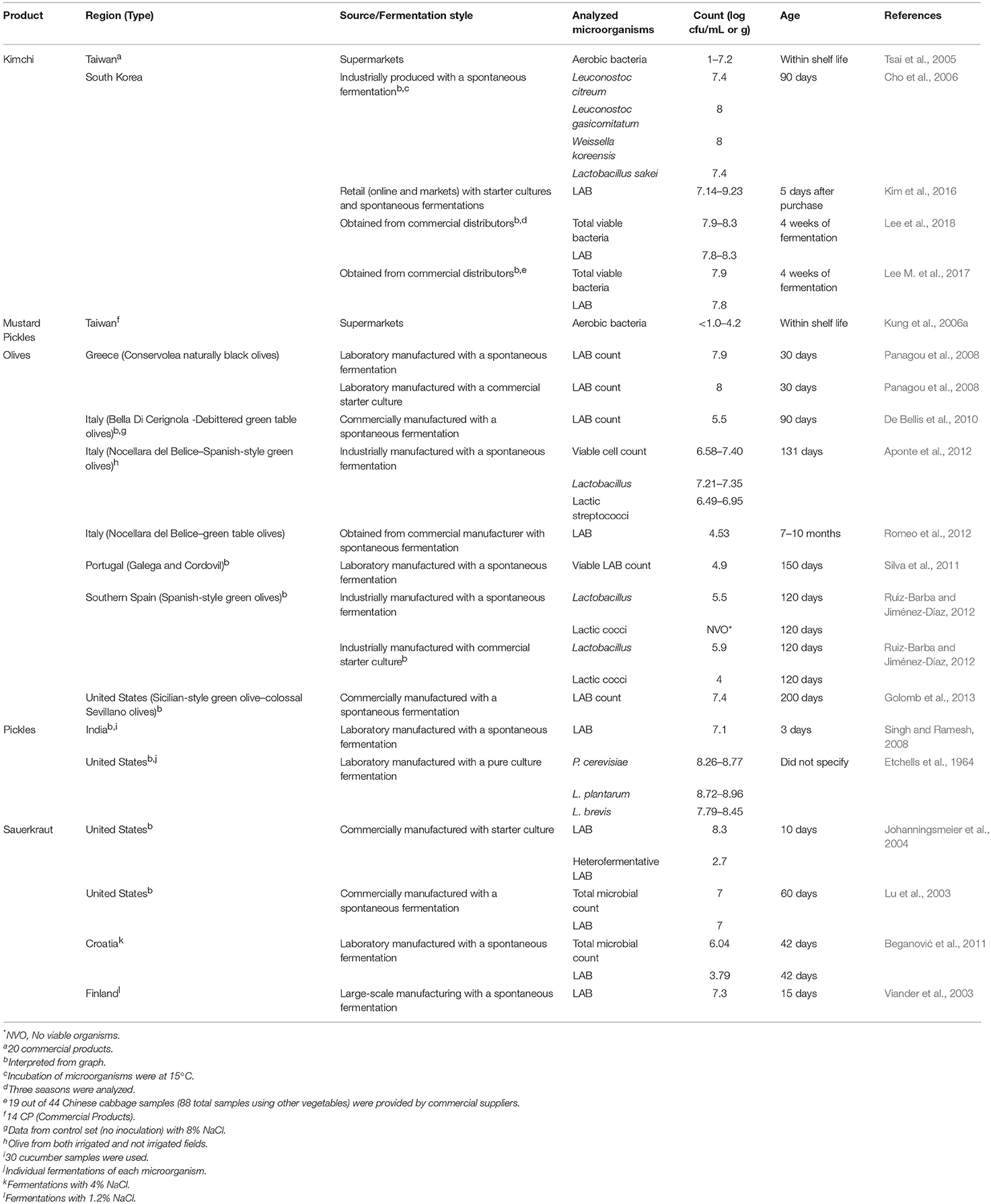
Table 5 . Organisms in fermented vegetables separated by product.
Microbial counts for sauerkraut were generally reported as LAB with counts ranging from 10 3 to 10 8 cfu/g. Reported samples were for sauerkraut originating from the United States, Finland, and Croatia. Levels of LAB and Lactobacillus were reported for olives produced in Italy, Greece, Portugal, Spain, and the United States. These products contained 10 4 to 10 8 cfu/g and were between 30 and 200 days.
Other products for which quantitative data were reported included mustard pickles and kimchi from Taiwan and pickled cucumbers from China, India, and the United States. Microbial counts ranged from undetectable (< 10 1 ) to 10 8 cfu/g. For several of these products, levels of species (e.g., Lactobacillus plantarum, Lactobacillus brevis , and Pediococcus cerevisiae ) were reported. Species of Leuconostoc, Weissella and Lactobacillus were also reported for Korean kimchi, where they were generally present between 10 7 and 10 8 cfu/g.
Traditional Asian Fermented Products
Another group of fermented foods that contain lactic acid bacteria and other bacteria are those products traditionally manufactured in Asia and that rely on grain or legume substrates. One important difference in the fermentation of these food products compared to other fermented foods is the reliance on fungal enzymes to convert complex carbohydrates to simple sugars. Aerobic conditions are another unique characteristic used in various parts of the fermentation process. Data were collected for several products, including miso, tempeh, fish sauce, and fermented fish (Table 6 ). Similar to the fermented vegetables, there were few reports on products from retail sources. Therefore, laboratory manufactured products made using industrial or traditional practices were included. In general, aerobic bacteria counts of miso ranged from 10 2 to 10 7 cfu/g. Similar bacterial counts were reported for fish sauce. LAB counts for tempeh and fermented fish were between 10 3 to 10 7 cfu/g with fermented fish being at the lower end of the range.
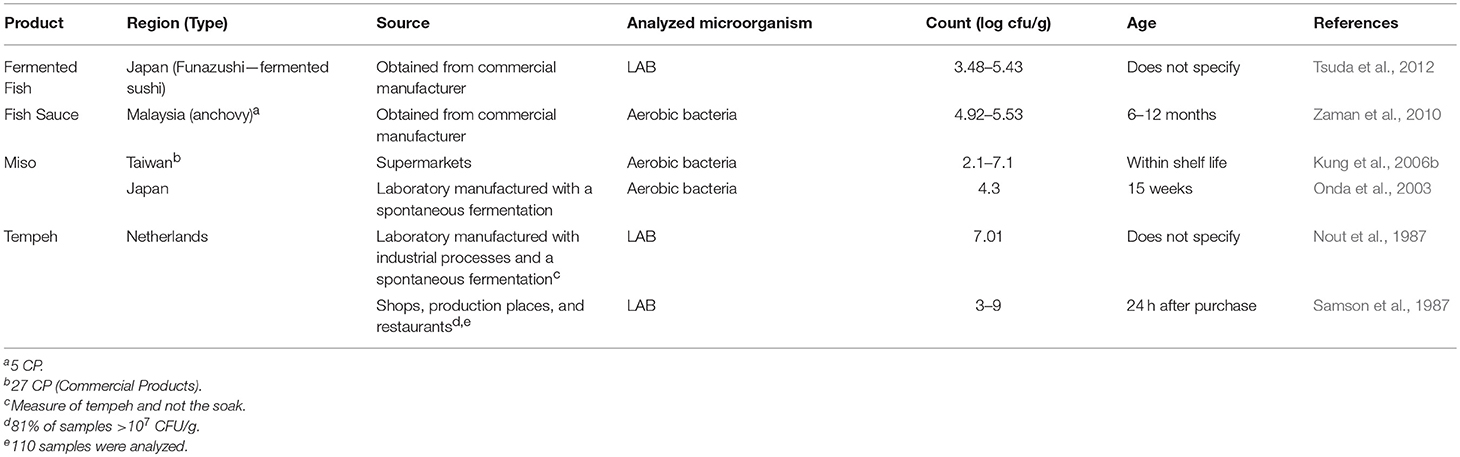
Table 6 . Organisms present in traditional Asian fermented products separated by product.
Fermented Cereals
Fermented porridges and gruels are widely consumed in many African countries. Here, studies were reported from Burkina Faso, Uganda, Ghana, Benin, Tanzania, and Mexico (Table 7 ). These cereals were made using pearl millet, millet, sorghum, and maize as starting grains. In general, the cereals contained LAB and mesophilic aerobic bacteria with a range of 10 5 to 10 9 cfu/g.
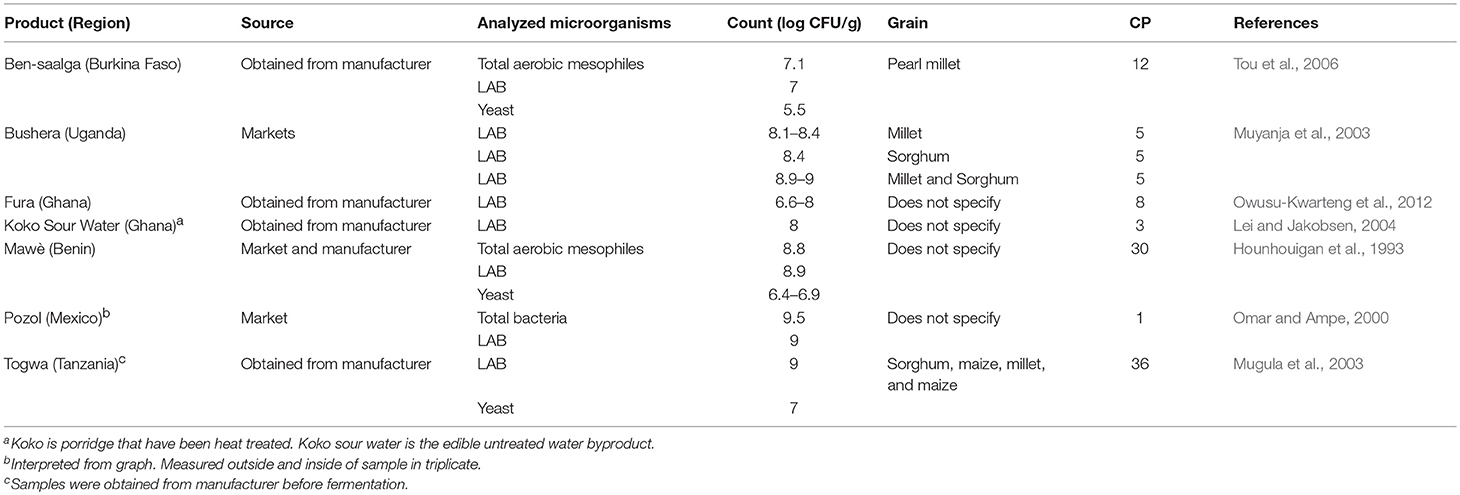
Table 7 . Organisms in commercial fermented cereals from Africa and Mexico.
Several sour beer products from Belgium, such as lambic and gueuze, were included in the survey (Table 8 ). LAB counts were reported for these products, ranging from 10 2 to 10 5 cfu/g. The age of the products reported in the table refers to the longest time the beer was left to age. This maximum aging time was found to range from 40 days to 5 years across the different products.
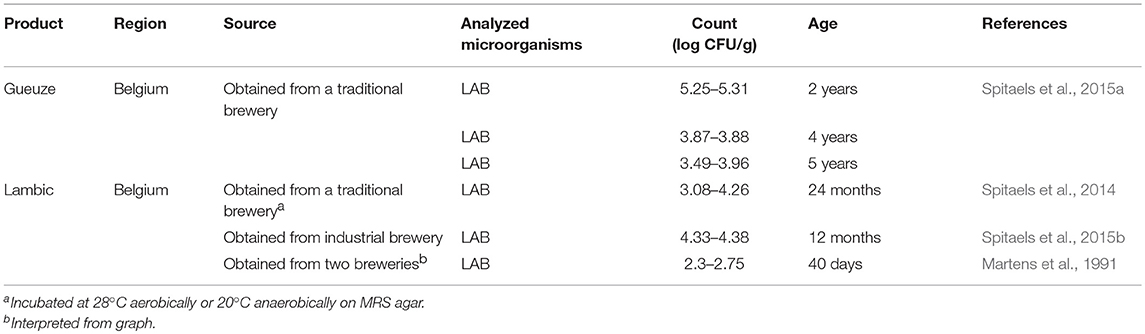
Table 8 . Organisms in commercial sour beer products.
Fermented Tea (Kombucha)
Kombucha is a fermented beverage made from sweetened tea to which a specialized culture is added. The latter is comprised of a s ymbiotic c ulture o f b acteria and y east or SCOBY, normally within a cellulose-type membrane. Bacteria commonly found in kombucha include the acetic acid bacteria belonging to the genera, Acetobacter, Gluconacetobacter , and Gluconobacter , as well as LAB. Most of the yeasts associated with kombucha are species of Saccharomyces , although other yeast genera may also be present ( Teoh et al., 2004 ; Coton et al., 2017 ). While this product is now widely consumed, and manufacturers promote the presence of live microorganisms on product labels, there are few published data on the levels of microbes present in retail products. One recent study reported both bacterial and yeast counts for two kombucha products that were produced under industrial manufacturing conditions ( Coton et al., 2017 ). In general, acetic acid bacteria levels ranged from 10 6 to 10 7 cfu/mL at the end of the fermentation, and similar counts were reported for LAB and total aerobic bacteria. Total yeast counts of about 10 7 cfu/mL were also reported.
Food-Associated Microbes Travel and Interact in the Gut
The human gastrointestinal tract is home to more than 10 12 microbes. This diverse ecosystem provides protection against pathogens, extracts nutrients from dietary components, and modulates the immune system ( Lozupone et al., 2013 ). The gut microbiota is also very stable, although several factors, including exposure to antibiotics, stress, and disease can disrupt this community, leading to dysbiosis ( Sommer et al., 2017 ). The ability of diet and dietary components to modulate the gastrointestinal microbiota, redress dysbiosis, and enhance human health is now well- established ( David et al., 2014 ; Graf et al., 2015 ; Sonnenburg and Bäckhed, 2016 ).
Among the food components known to influence the composition of the microbiota are fermentable fibers and prebiotics that enrich for particular members of the gut microbiota. Another route by which the gastrointestinal microbiota may be modulated is via consumption of probiotics—live microbes consumed at a dose sufficient to provide beneficial effects ( Hill et al., 2014 ). Probiotics, however, are temporary members of the microbiome and rarely persist more than a few days ( Tannock, 2003 ; Derrien and van Hylckama Vlieg, 2015 ; Zhang et al., 2016 ).
Perhaps the easiest and most common way to introduce potentially beneficial microbes to the gastrointestinal tract is via consumption of microbe-containing foods, and fermented foods and beverages, in particular. Like many probiotics, many microbes associated with fermented foods may also have the capacity to survive digestion, reach the gastrointestinal tract, and ultimately provide similar health benefits ( Marco et al., 2017 ). When consumed regularly, these fermentation-associated microbes form what some researchers have called the “transient microbiome” ( Derrien and van Hylckama Vlieg, 2015 ).
In general, the microorganisms present in fermented foods and beverages originate via one of two ways. For so-called natural or spontaneous fermented foods, the microorganisms are autochthonous and are naturally present in the raw material or manufacturing environment. To survive fermentation and processing, the LAB, yeasts, and any other microorganisms present in the finished product must manage a range of selective and competitive pressures, including salt, organic acids, ethanol, anaerobiosis, and low pH. Many of the fermented foods reviewed in this survey, including fermented cereals, sauerkraut, kimchi, and other fermented vegetables, and fermented soy-based products are made by natural fermentation. In addition, many wines and even some fermented sausages and beers are made in this manner.
Other fermented foods rely on the addition of a starter cultures. Cultured dairy products, cheese, and fermented sausages are commonly made using starter cultures. When cultures are used, their selection is based on the performance characteristics specific to the product. In addition, the incubation temperature during fermentation and the nutrient content are usually well-suited to the needs of the microorganisms. In many cases, the culture is added at such high inoculum levels, there would be little competition from other organisms. Collectively, most food fermentation microorganisms are well-adapted to the food environment.
In contrast, once the organisms present in fermented foods are consumed, they become foreign or allochthonous to the gastrointestinal tract. In most cases, they lack the physiological and biochemical resources to compete in this ecological niche. If they survive transit, they do not become stable members of this community ( Zhang et al., 2016 ). Nonetheless, the presence of food fermentation-associated microorganisms in the GI tract, even if they are just “passing through,” is now well-documented ( Lee et al., 1996 ; Walter et al., 2001 ; Dal Bello et al., 2003 ; David et al., 2014 ; Derrien and van Hylckama Vlieg, 2015 ; Zhang et al., 2016 ; Lisko et al., 2017 ).
Evidence of Health Benefits Associated with Fermented Foods
The evidence for the potential health benefits of fermented foods is based on numerous epidemiological as well as clinical reports (reviewed in Marco and Golomb, 2016 ; Kok and Hutkins, in press ). In general, epidemiological studies have shown that consumption of fermented foods is associated with improvements of health status or reductions in disease risk. For example, yogurt-rich diets were associated with a reduced risk of metabolic syndrome in older Mediterranean adults ( Babio et al., 2015 ). A similar finding was reported in another large cohort study that showed cultured milk consumption reduced the risk of bladder cancer ( Larsson et al., 2008 ). Yogurt consumption has also been associated with reduced weight gain ( Mozaffarian et al., 2011 ). Epidemiological data also suggests that consumption of other fermented foods may be correlated to beneficial health outcomes. Consumption of kimchi and other fermented vegetables, for example, correlated with reduced incidence of asthma and atopic dermatitis in Korean adults ( Park and Bae, 2016 ; Kim et al., 2017 ). Reduced risks of type 2 diabetes and high blood pressure among Japanese adults was associated with consumption of fermented soybean foods rich in phytoestrogens and bioactive peptides ( Kwon et al., 2010 ; Nozue et al., 2017 ). In contrast, the large European Prospective Investigation into Cancer and Nutrition cohort study from the Netherlands reported no association between fermented foods consumption and overall mortality ( Praagman et al., 2015 ).
Although many human clinical studies have assessed the effects of probiotic-containing fermented foods on health biomarkers, fewer randomized controlled trials (RCT) have considered fermented foods alone. Nonetheless, several reports provide evidence that fermented foods, such as kimchi, fermented soy products, and yogurt, can improve relevant biomarkers. For example, kimchi consumption improved fasting blood glucose and other metabolic syndrome symptoms in overweight and obese adults ( Kim et al., 2011 ), and similar improvements were observed in healthy adults ( Choi et al., 2013 ). Consumption of a fermented soybean paste also improved plasma triglyceride levels in obese adults ( Lee Y. et al., 2017 ). Perhaps the strongest evidence is for yogurt and improved lactose tolerance, due to in vivo expression and release of β-galactosidase by the yogurt culture microbes, S. thermophilus and L. delbrueckii subsp. bulgaricus ( Kolars et al., 1984 ; Martini et al., 1987 ; Pelletier et al., 2001 ; Savaiano, 2014 ). This is the only approved health claim approved by the European Food Safety Authority ( EFSA Panel on Dietetic Products, Nutrition and Allergies, 2010 ).
As noted previously, some fermented foods could impart health benefits even in the absence of live microorganisms in the finished products. For example, in sour dough bread manufacture, LAB may express phytase enzymes that degrade phytates and therefore enhance mineral absorption ( Nuobariene et al., 2015 ). In the manufacture of red wine, ethanol produced early in the fermentation enhances extraction of polyphenolic compounds from the grape skins. Fermented foods may also contain vitamins and other bioactive molecules produced in situ from microbial metabolism that are not present in the original food. Recently, Saubade et al. (2017) noted that folic acid deficiency is a global health problem and suggested that fermented foods could be a food-based alternative for delivering folic acid to at-risk populations. Although some LAB are able to produce modest levels of folate ( Leblanc et al., 2011 ), amounts produced in foods may be too low to be reach required levels ( Saubade et al., 2017 ). Thus, selection of over-producing strains, as well as combining strains with non-LAB may be necessary to enhance production of this vitamin in foods.
If present, fermentation-derived microorganisms, despite their transient nature, may yet have the potential to influence gut microbiota diversity, structure, and function ( Zhang et al., 2016 ). Notably, they may also affect health due to their ability to out-compete pathogens for resources, produce short chain fatty acids from available carbohydrates, secrete anti-microbial agents, contribute to immune homeostasis, and produce vitamins, in situ ( Derrien and van Hylckama Vlieg, 2015 ).
The Number of Fermentation-Associated Microbes Depends on Region and Product Age
In this survey, we reviewed the literature for studies that included quantitative data on microorganisms present in commercial fermented food products. To our knowledge, this is the first time that there has been a compilation of the hundreds of previous studies that enumerated microbes in fermented foods from retail samples or commercial products. In general, most of the products for which data were available contained at least 10 6 cells/mL or g. However, there was considerable variation depending on product age and region, and several relevant bacterial species or groups were present at less than that amount.
Although regular consumption of yogurt is often included in dietary guidelines ( Smug et al., 2014 ), recommendations for other fermented foods rarely exist ( Chilton et al., 2015 ). Likewise, to our knowledge, there are few guidelines for what constitutes a minimum dose of live microorganisms. The one exception is the yogurt health claim for “improved lactose tolerance” that was approved in 2010 by the European Food Safety Authority ( EFSA Panel on Dietetic Products, Nutrition and Allergies, 2010 ). The claim states that yogurt should contain at least 10 8 cfu live starter microorganisms per gram- the same count the NYA requires for the “live and active” seal, as noted above.
Even in the absence of a seal or stamp, many commercial yogurt products, as well as kefir, fermented vegetables, and miso, also provide numerical information on their labels. Recently, Derrien and van Hylckama Vlieg (2015 ) suggested that consumption of 10 10 cells would be necessary to induce an effect on the microbiota and host health. This could be achieved by consuming 100 g of fermented food containing 10 8 cells/g.
According to the results reported in this survey, many commercial fermented food products would be close to meeting this requirement (Figure 1 ). However, several caveats are relevant. First, there was a wide range of reported microbial counts (over several logs) within the various product groups. Some products also reported total LAB, whereas other reported specific genera or species or as thermophilic or mesophilic. Second, for most products, enumeration relied on standard cultural methods for LAB (including medium and incubation conditions), which may have under-estimated more fastidious species. This can be attributed to the high stress conditions of fermented products that can occasionally lead to injured microorganisms that are viable but not culturable.
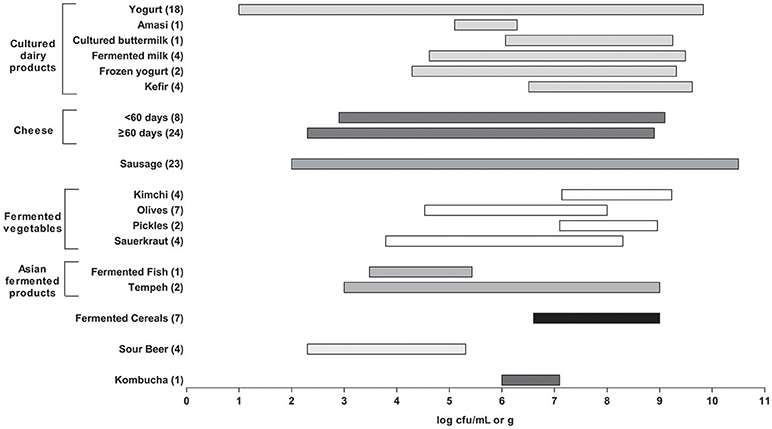
Figure 1 . Summary of lactic acid bacteria (LAB) counts in all fermented foods as reported in Tables 1–8 . The bar plots represents a range (minimum to maximum) of counts found across the studies surveyed. The number of studies used here for each fermented food is shown in brackets. Products were excluded if they had no viable counts or when LAB counts were not reported. For yogurt, initial counts were used for products that had counts for more than one timepoint. For cheese, the products were divided by aging time (60 days) and were excluded if aging time was not reported.
Finally, the age or time at which the products were analyzed also varied considerably. In general, “fresher” products had higher numbers. These would include yogurt and cultured dairy products, as well as kimchi, sauerkraut, and other fermented vegetables. The counts from the cheeses also varied widely, with the longer aged cheeses (e.g., Parmesan, Grana) consistently having the lowest counts.
Recommendation of Fermented Foods as Part of Dietary Guidelines
In many cultures, fermented foods containing live microorganisms are consumed on a regular or even daily basis ( Hutkins, 2018 ). Based on the data reported in this survey, consumption of fermented foods would not only provide important macronutrients, they could also deliver large numbers of potentially beneficial microorganisms to the gastrointestinal tract. For example, if Korean kimchi contains 10 8 lactic acid bacteria per g (Table 5 ), and given per capita consumption of kimchi is estimated at 100 g per person per day, then the daily consumption of live microbes from kimchi alone would be 10 10 . Likewise, in the Netherlands, where yogurt consumption is also around 100 g per day, similar levels of microbes (i.e., 10 10 cfu per day) would be ingested. These are the doses noted above that can influence the gut microbiota and provide a potential health benefit ( Derrien and van Hylckama Vlieg, 2015 ).
Recently, the concept of “shared core benefits” was introduced to explain how and why phylogenetically related organisms could deliver similar health benefits ( Sanders et al., 2018 ). Thus, although the microbes in fermented foods cannot, by definition, be considered probiotic, many of them are evolutionarily highly related to probiotic organisms, and they often share the same molecular mechanisms responsible for health-promoting properties in probiotic organisms. The application of various omic approaches to understand functional properties of fermentation-derived microbes will also likely reveal new attributes relevant to the health benefits these microbes may provide ( Macori and Cotter, 2018 ).
In part, this is why several prominent groups have recommended that health care professionals should promote fermented foods containing live microbes as part of public health policy ( Ebner et al., 2014 ; Sanders et al., 2014 ; Chilton et al., 2015 ; Bell et al., 2017 ; Hill et al., 2017 ). In particular, including fermented foods in dietary guidelines for specific populations has also been recommended. For example, Bell et al. (2018) recently suggested fermented foods should be introduced to children early in life and incorporated into their everyday meal plans. In addition, regular consumption of fermented foods could be especially important for low income, resource-challenged communities that are disproportionally susceptible to gastrointestinal infections ( Kort et al., 2015 ).
Author Contributions
SR, CK, and RH each contributed 30% to data collection. MH contributed 10% to data collection. SR, CK, and RH wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was funded by the National Dairy Council and facilitated by the International Scientific Association for Probiotics and Prebiotics. We thank Mary Ellen Sanders for her helpful comments.
Acton, J. C., and Dick, R. L. (1976). Composition of some commercial dry sausages. J. Food Sci. 41, 971–972. doi: 10.1111/j.1365-2621.1976.tb00768_41_4.x
CrossRef Full Text | Google Scholar
Alexopoulos, A., Plessas, S., Kourkoutas, Y., Stefanis, C., Vavias, S., Voidarou, C., et al. (2017). Experimental effect of ozone upon the microbial flora of commercially produced dairy fermented products. Int. J. Food Microbiol. 246, 5–11. doi: 10.1016/j.ijfoodmicro.2017.01.018
PubMed Abstract | CrossRef Full Text | Google Scholar
Aponte, M., Blaiotta, G., La Croce, F., Mazzaglia, A., Farina, V., Settanni, L., et al. (2012). Use of selected autochthonous lactic acid bacteria for Spanish-style table olive fermentation. Food Microbiol. 30, 8–16. doi: 10.1016/j.fm.2011.10.005
Aquilanti, L., Santarelli, S., Silvestri, G., Osimani, A., Petruzzelli, A., and Clementi, F. (2007). The microbial ecology of a typical Italian salami during its natural fermentation. Int. J. Food Microbiol. 120, 136–145. doi: 10.1016/j.ijfoodmicro.2007.06.010
Ayana, I. A. A. A., and El-Deeb, A. M. (2016). Quality enhancement of Edam-like cheese made from goat's milk. Am. J. Food Technol. 11, 44–53. doi: 10.3923/ajft.2016.44.53
Babio, N., Becerra-Tomas, N., Martinez-Gonzalez, M. A., Corella, D., Estruch, R., Ros, E., et al. (2015). Consumption of yogurt, low-fat milk, and other low-fat dairy products is associated with lower risk of metabolic syndrome incidence in an elderly Mediterranean population. J. Nutr. 145, 2308–2316. doi: 10.3945/jn.115.214593
Beganović, J., Pavunc, A. L., Gjuračić, K., Špoljarec, M., Šušković, J., and Kos, B. (2011). Improved sauerkraut production with probiotic strain Lactobacillus plantarum L4 and Leuconostoc mesenteroides LMG 7954. J. Food Sci. 76, 124–129. doi: 10.1111/j.1750-3841.2010.02030.x
Bell, V., Ferrão, J., and Fernandes, T. (2017). Nutritional guidelines and fermented food frameworks. Foods 6, 1–17. doi: 10.3390/foods6080065
Bell, V., Ferrão, J., and Fernandes, T. (2018). Fermented food guidelines for children. J. Pediatr. Pediatr. Med. 2, 1–4.
Google Scholar
Birollo, G. A., Reinheimer, J. A., and Vinderola, C. G. (2000). Viability of lactic acid microflora in different types of yoghurt. Food Res. Int. 33, 799–805. doi: 10.1016/S0963-9969(00)00101-0
Bouton, Y., Guyot, P., and Grappin, R. (1998). Preliminary characterization of microflora of Comté cheese. J. Appl. Microbiol. 85, 123–131. doi: 10.1046/j.1365-2672.1998.00476.x
Capita, R., Llorente-Marigómez, S., Prieto, M., and Alonso-Calleja, C. (2006). Microbiological profiles, pH, and titratable acidity of chorizo and salchichón (two Spanish dry fermented sausages) manufactured with ostrich, deer, or pork meat. J. Food Prot. 69, 1183–1189. doi: 10.4315/0362-028X-69.5.1183
Chevallier, I., Ammor, S., Laguet, A., Labayle, S., Castanet, V., Dufour, E., et al. (2006). Microbial ecology of a small-scale facility producing traditional dry sausage. Food Control 17, 446–453. doi: 10.1016/j.foodcont.2005.02.005
Chilton, S. N., Burton, J. P., and Reid, G. (2015). Inclusion of fermented foods in food guides around the world. Nutrients 7, 390–404. doi: 10.3390/nu7010390
Cho, J., Lee, D., Yang, C., Jeon, J., Kim, J., and Han, H. (2006). Microbial population dynamics of kimchi, a fermented cabbage product. FEMS Microbiol. Lett. 257, 262–267. doi: 10.1111/j.1574-6968.2006.00186.x
Choi, I. H., Noh, J. S., Han, J.-S., Kim, H. J., Han, E.-S., and Song, Y. O. (2013). Kimchi, a fermented vegetable, improves serum lipid profiles in healthy young adults: randomized clinical trial. J. Med. Food 16, 223–229. doi: 10.1089/jmf.2012.2563
Cocolin, L., Dolci, P., Rantsiou, K., Urso, R., Cantoni, C., and Comi, G. (2009). Lactic acid bacteria ecology of three traditional fermented sausages produced in the North of Italy as determined by molecular methods. Meat Sci. 82, 125–132. doi: 10.1016/j.meatsci.2009.01.004
Cocolin, L., Manzano, M., Cantoni, C., and Comi, G. (2001). Denaturing gradient gel electrophoresis analysis of the 16S rRNA gene V1 region to monitor dynamic changes in the bacterial population during fermentation of Italian sausages. Appl. Environ. Microbiol. 67, 5113–5121. doi: 10.1128/AEM.67.11.5113-5121.2001
Comi, G., Urso, R., Iacumin, L., Rantsiou, K., Cattaneo, P., Cantoni, C., et al. (2005). Characterisation of naturally fermented sausages produced in the North East of Italy. Meat Sci. 69, 381–382. doi: 10.1016/j.meatsci.2004.08.007
Commonwealth of Australia Gazette (2015). Australia New Zealand Food Standards Code, Amendment No. 154-2015. Commonwealth of Australia Gazette No. FSC 96.
Coppola, R., Giagnacovo, B., lorizzo, M., and Grazia, L. (1998). Characterization of lactobacilli involved in the ripening of soppressata molisana, a typical southern Italy fermented sausage. Food Microbiol. 15, 347–353. doi: 10.1006/fmic.1997.0179
Coppola, R., Marconi, E., Rossi, F., and Dellaglio, F. (1995). Artisanal production of Naples-type salami: chemical and microbiological aspects. Ital. J. Food Sci. 1, 57–61.
Coppola, R., Nanni, M., Iorizzo, M., Sorrentino, A., Sorrentino, E., Chiavari, C., et al. (2000). Microbiological characteristics of Parmigiano Reggiano cheese during the cheesemaking and the first months of the ripening. Lait 80, 479–490. doi: 10.1051/lait:2000139
Coppola, S., Mauriello, G., Aponte, M., Moschetti, G., and Villani, F. (2000). Microbial succession during ripening of Naples-type salami, a southern Italian fermented sausage. Meat Sci. 56, 321–329. doi: 10.1016/S0309-1740(00)00046-2
Coton, M., Pawtowski, A., Taminiau, B., Burgaud, G., Deniel, F., Coulloumme-Labarthe, L., et al. (2017). Unraveling microbial ecology of industrial-scale Kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol. Ecol. 93:fix048. doi: 10.1093/femsec/fix048
Cuesta, P., Fernández-Garcia, E., González de Llano, D., Montilla, A., and Rodriguez, A. (1996). Evolution of the microbiological and biochemical characteristics of Afuega'l Pitu cheese during ripening. J. Dairy Sci. 79, 1693–1698.
Dal Bello, F., Walter, J., Hammes, W. P., and Hertel, C. (2003). Increased complexity of the species composition of lactic acid bacteria in human feces revealed by alternative incubation condition. Microb. Ecol. 45, 455–463. doi: 10.1007/s00248-003-2001-z
David, L. A., Maurice, C. F., Carmody, R. N., Gootenberg, D. B., Button, J. E., Wolfe, B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. doi: 10.1038/nature12820
De Angelis, M., de Candia, S., Calasso, M. P., Faccia, M., Guinee, T. P., Simonetti, M. C., et al. (2008). Selection and use of autochthonous multiple strain cultures for the manufacture of high-moisture traditional Mozzarella cheese. Int. J. Food Microbiol. 125, 123–132. doi: 10.1016/j.ijfoodmicro.2008.03.043
De Bellis, P., Valerio, F., Sisto, A., Lonigro, S. L., and Lavermicocca, P. (2010). Probiotic table olives: microbial populations adhering on olive surface in fermentation sets inoculated with the probiotic strain Lactobacillus paracasei IMPC2.1 in an industrial plant. Int. J. Food Microbiol. 140, 6–13. doi: 10.1016/j.ijfoodmicro.2010.02.024
De Noni, I., Pellegrino, L., and Masotti, F. (2004). Survey of selected chemical and microbiological characteristics of (plain or sweetened) natural yoghurts from the Italian market. Lait 84, 421–433. doi: 10.1051/lait:2004020
Delamare, A. P. L., de Andrade, C. C. P., Mandelli, F., Chequeller De Almeida, R., and Echeverrigaray, S. (2012). Microbiological, physico-chemical and sensorial characteristics of Serrano, an artisanal Brazilian cheese. Food Nutr. Sci. 3, 1068–1075. doi: 10.4236/fns.2012.38142
Demarigny, Y., Beuvier, E., Dasen, A., and Duboz, G. (1996). Influence of raw milk microflora on the characteristics of Swiss-type cheeses. I. Evolution of microflora during ripening and characterization of facultatively heterofermentative lactobacilli. Lait 76, 371–387. doi: 10.1051/lait:1996428
Depouilly, A., Dufrene, F., Beuvier, É., and Berthier, F. (2004). Genotypic characterisation of the dynamics of the lactic acid bacterial population of Comté cheese. Lait 84, 155–167. doi: 10.1051/lait:2003036
Derrien, M., and van Hylckama Vlieg, J. E. T. (2015). Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends Microbiol. 23, 354–366. doi: 10.1016/j.tim.2015.03.002
Devirgiliis, C., Caravelli, A., Coppola, D., Barile, S., and Perozzi, G. (2008). Antibiotic resistance and microbial composition along the manufacturing process of Mozzarella di Bufala Campana. Int. J. Food Microbiol. 128, 378–384. doi: 10.1016/j.ijfoodmicro.2008.09.021
Di Cagno, R., Chaves Lòpez, C., Tofalo, R., Gallo, G., De Angelis, M., Paparella, A., et al. (2008). Comparison of the compositional, microbiological, biochemical and volatile profile characteristics of three Italian PDO fermented sausages. Meat Sci. 79, 223–235. doi: 10.1016/j.meatsci.2007.09.006
Dong, Y. P., Chen, Q., Cui, S. H., and Li, F. Q. (2014). Enumeration, Genetic characterization and antimicrobial susceptibility of lactobacillus and streptococcus isolates from retail yoghurt in Beijing, China. Biomed. Env. Sci 27, 740–748. doi: 10.3967/bes2014.109
Dunlap, B. S., Yu, H., and Elitsur, Y. (2009). The probiotic content of commercial yogurts in West Virginia. Clin. Pediatr. 48, 522–527. doi: 10.1177/0009922809331802
Ebner, S., Smug, L. N., Kneifel, W., Salminen, S. J., and Sanders, M. E. (2014). Probiotics in dietary guidelines and clinical recommendations outside the European Union. World J. Gastroenterol. 20, 16095–16100. doi: 10.3748/wjg.v20.i43.16095
EFSA Panel on Dietetic Products Nutrition and Allergies. (2010). Scientific opinion on the substantiation of health claims related to live yoghurt cultures and improved lactose digestion. EFSA J. 8:1763. doi: 10.2903/j.efsa.2010.1763
CrossRef Full Text
Eliskases-Lechner, F., and Ginzinger, W. (1995). The bacterial flora of surface-ripened cheeses with special regard to coryneforms. Lait 75, 571–584. doi: 10.1051/lait:1995644
Ercolini, D., Hill, P. J., and Dodd, C. E. R. (2003). Bacterial community structure and location in Stilton cheese. Appl. Environ. Microbiol. 69, 3540–3548. doi: 10.1128/AEM.69.6.3540-3548.2003
Etchells, J. L., Costilow, R. N., Anderson, T. E., and Bell, T. A. (1964). Pure culture fermentation of brined cucumbers. Appl. Microbiol. 12, 523–535.
PubMed Abstract | Google Scholar
Ferreira, V., Barbosa, J., Vendeiro, S., Mota, A., Silva, F., Monteiro, M. J., et al. (2006). Chemical and microbiological characterization of alheira: a typical Portuguese fermented sausage with particular reference to factors relating to food safety. Meat Sci. 73, 570–575. doi: 10.1016/j.meatsci.2006.02.011
Fitzsimons, N. A., Cogan, T. M., Condon, S., and Beresford, T. (2001). Spatial and temporal distribution of non-starter lactic acid bacteria in Cheddar cheese. J. Appl. Microbiol. 90, 600–608. doi: 10.1046/j.1365-2672.2001.01285.x
Flórez, A. B., María López-Díaz, T., Alvarez-Martín, P., and Mayo, B. (2006). Microbial characterisation of the traditional Spanish blue-veined Cabrales cheese: identification of dominant lactic acid bacteria. Eur. Food Res. Technol 223, 503–508. doi: 10.1007/s00217-005-0230-8
Franciosi, E., Settanni, L., Carlin, S., Cavazza, A., and Poznanski, E. (2008). A factory-scale application of secondary adjunct cultures selected from lactic acid bacteria during Puzzone di Moena cheese ripening. J. Dairy Sci. 91, 2981–2991. doi: 10.3168/jds.2007-0764
Frye, C. P., and Kilara, A. (2016). “Regulations for product standards and labeling,” in Dairy Processing and Quality Assurance , eds R. C. Chandan, A. Kilara, and N. P. Shah (Chichester: John Wiley & Sons, Ltd), 152–177.
García Fontán, M. C., Lorenzo, J. M., Martínez, S., Franco, I., and Carballo, J. (2007a). Microbiological characteristics of Botillo, a Spanish traditional pork sausage. LWT Food Sci. Technol. 40, 1610–1622. doi: 10.1016/j.lwt.2006.10.007
García Fontán, M. C., Lorenzo, J. M., Parada, A., Franco, I., and Carballo, J. (2007b). Microbiological characteristics of “androlla,” a Spanish traditional pork sausage. Food Microbiol. 24, 52–58. doi: 10.1016/j.fm.2006.03.007
García-Cayuela, T., Tabasco, R., Peláez, C., and Requena, T. (2009). Simultaneous detection and enumeration of viable lactic acid bacteria and bifidobacteria in fermented milk by using propidium monoazide and real-time PCR. Int. Dairy J. 19, 405–409. doi: 10.1016/j.idairyj.2009.02.001
Garcia, M. C., Otero, A., Garcia, M. L., and Moreno, B. (1987). Microbiological quality and composition of two types of Spanish sheep's milk cheeses (Manchego and Burgos varieties). J. Dairy Res. 54, 551–557.
Gatti, M., Fornasari, M. E., Mucchetti, G., Addeo, F., and Neviani, E. (1999). Presence of peptidase activities in different varieties of cheese. Lett. Appl. Microbiol. 28, 368–372. doi: 10.1046/j.1365-2672.1999.00541.x
Gatti, M., Lindner, J. D. D., De Lorentiis, A., Bottari, B., Santarelli, M., Bernini, V., et al. (2008). Dynamics of whole and lysed bacterial cells during Parmigiano-Reggiano cheese production and ripening. Appl. Environ. Microbiol. 74, 6161–6167. doi: 10.1128/AEM.00871-08
Gebreselassie, N., Abay, F., and Beyene, F. (2016). Biochemical and molecular identification and characterization of lactic acid bacteria and yeasts isolated from Ethiopian naturally fermented buttermilk. J. Food Sci. Technol. 53, 184–196. doi: 10.1007/s13197-015-2049-z
Genigeorgis, C., Carniciu, M., Dutulescu, D., and Farver, T. B. (1991). Growth and survival of Listeria monocytogenes in market cheeses stored at 4 to 30°C. J. Food Prot. 54, 662–668. doi: 10.4315/0362-028X-54.9.662
Gobbetti, M., Burzigotti, R., Smacchi, E., Corsetti, A., and De Angelis, M. (1997). Microbiology and Biochemistry of Gorgonzola cheese during ripening. Int. Dairy J. 7, 519–529. doi: 10.1016/S0958-6946(97)00047-2
Golomb, B. L., Morales, V., Jung, A., Yau, B., Boundy-Mills, K. L., and Marco, M. L. (2013). Effects of pectinolytic yeast on the microbial composition and spoilage of olive fermentations. Food Microbiol. 33, 97–106. doi: 10.1016/j.fm.2012.09.004
Gori, K., Ryssel, M., Arneborg, N., and Jespersen, L. (2013). Isolation and identification of the microbiota of Danish farmhouse and industrially produced surface-ripened cheeses. Microb. Ecol. 65, 602–615. doi: 10.1007/s00248-012-0138-3
Graf, D., Di Cagno, R., Fåk, F., Flint, H. J., Nyman, M., Saarela, M., et al. (2015). Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 26:26164. doi: 10.3402/mehd.v26.26164
Greppi, A., Ferrocino, I., La Storia, A., Rantsiou, K., Ercolini, D., and Cocolin, L. (2015). Monitoring of the microbiota of fermented sausages by culture independent rRNA-based approaches. Int. J. Food Microbiol. 212, 67–75. doi: 10.1016/j.ijfoodmicro.2015.01.016
Grønnevik, H., Falstad, M., and Narvhus, J. A. (2011). Microbiological and chemical properties of Norwegian kefir during storage. Int. Dairy J. 21, 601–606. doi: 10.1016/j.idairyj.2011.01.001
Gueimonde, M., Delgado, S., Mayo, B., Ruas-Madiedo, P., Margolles, A., and De Los Reyes-Gavil, C. G. (2004). Viability and diversity of probiotic Lactobacillus and Bifidobacterium populations included in commercial fermented milks. Food Res. Int. 37, 839–850. doi: 10.1016/j.foodres.2004.04.006
Hamann, W. T., and Marth, E. H. (1984). Survival of Streptococcus thermophilus and Lactobacillus bulgaricus in commercial and experimental yogurts. J. Food Prot. 47, 781–786. doi: 10.4315/0362-028X-47.10.781
Hekmat, S., and Koba, L. (2006). Fermented dairy products: knowledge and consumption. Can. J. Diet. Pract. Res. 67, 199–201. doi: 10.3148/67.4.2006.199
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document: the International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Hill, D., Sugrue, I., Arendt, E., Hill, C., Stanton, C., and Ross, R. P. (2017). Recent advances in microbial fermentation for dairy and health. F1000Res. 6:751. doi: 10.12688/f1000research.10896.1
Hounhouigan, D. J., Nout, M. J. R., Nago, C. M., Houben, J. H., and Rombouts, F. M. (1993). Composition and microbiological and physical attributes of mawè a fermented dough from Benin. Int. J. Food Sci. Technol. 28, 513–517. doi: 10.1111/j.1365-2621.1993.tb01300.x
Hutkins, R. W. (2018). Microbiology and Technology of Fermented Foods, 2nd Edn . Hoboken, NJ: Wiley.
Ibrahim, S. A., and Carr, J. P. (2006). Viability of bifidobacteria in commercial yogurt products in North Carolina during refrigerated storage. Int. J. Dairy Technol. 59, 272–277. doi: 10.1111/j.1471-0307.2006.00282.x
Iwana, H., Masuda, H., Fujisawa, T., Suzuki, H., and Mitsuoka, T. (1993). Isolation and identification of Bifidobacterium spp. in commercial yogurts sold in Europe. Bifidobact. Microflora 12, 39–45. doi: 10.12938/bifidus1982.12.1_39
Jayashree, S., Pushpanathan, M., Rajendhran, J., and Gunasekaran, P. (2013). Microbial diversity and phylogeny analysis of buttermilk, a fermented milk product, employing 16S rRNA-based pyrosequencing. Food Biotechnol. 27, 213–221. doi: 10.1080/08905436.2013.811084
Johanningsmeier, S. D., Fleming, H. P., and Breidt, F. Jr. (2004). Malolactic activity of lactic acid bacteria during sauerkraut fermentation. J. Food Sci. 69, M222–M227. doi: 10.1111/j.1365-2621.2004.tb09891.x
Johnson, A. J. (2016). Artisanal food microbiology. Nat. Microbiol. 1:16039. doi: 10.1038/nmicrobiol.2016.39
Jordan, K. N., and Cogan, T. M. (1993). Identification and growth of non-starter lactic acid bacteria in Irish Cheddar cheese. Irish J. Agric. Food Res. 32, 47–55.
Kalamaki, M. S., and Angelidis, A. S. (2017). Isolation and molecular identification of yeasts in Greek kefir. Int. J. Dairy Technol. 70, 261–268. doi: 10.1111/1471-0307.12329
Kesmen, Z., and Kacmaz, N. (2011). Determination of lactic microflora of kefir grains and kefir beverage by using culture-dependent and culture-independent methods. J. Food Sci. 76, M276–M283. doi: 10.1111/j.1750-3841.2011.02191.x
Kim, D. H., Chon, J. W., Kim, H., Kim, H. S., Choi, D., Hwang, D. G., et al. (2015). Detection and enumeration of lactic acid bacteria, acetic acid bacteria and yeast in kefir grain and milk using quantitative real-time PCR. J. Food Saf. 35, 102–107. doi: 10.1111/jfs.12153
Kim, E. K., An, S. Y., Lee, M. S., Kim, T. H., Lee, H. K., Hwang, W. S., et al. (2011). Fermented kimchi reduces body weight and improves metabolic parameters in overweight and obese patients. Nutr. Res. 31, 436–443. doi: 10.1016/j.nutres.2011.05.011
Kim, H.-Y., Bong, Y.-J., Jeong, J.-K., Lee, S., Kim, B.-Y., and Park, K.-Y. (2016). Heterofermentative lactic acid bacteria dominate in Korean commercial kimchi. Food Sci. Biotechnol. 25, 541–545. doi: 10.1007/s10068-016-0075-x
Kim, H. J., Ju, S., and Park, Y. K. (2017). Kimchi intake and atopic dermatitis in Korean aged 19-49 years: The Korea National Health and Nutrition Examination Survey 2010-2012. Asia Pac. J. Clin. Nutr. 26, 914–922. doi: 10.6133/apjcn.022017
Kok, C., and Hutkins, R. W. (in press). Yogurt other fermented foods as a source of health-promoting bacteria. Nutr. Rev .
Kolars, J. C., Michael, D. L., Aouji, M., and Savaiano, D. A. (1984). Yogurt - an autodigesting source of lactose. N. Engl. J. Med. 310, 1–3. doi: 10.1056/NEJM198401053100101
Kort, R., Westerik, N., Mariela Serrano, L., Douillard, F. P., Gottstein, W., Mukisa, I. M., et al. (2015). A novel consortium of Lactobacillus rhamnosus and Streptococcus thermophilus for increased access to functional fermented foods. Microb. Cell Fact. 14:195. doi: 10.1186/s12934-015-0370-x
Kosikowski, F. V. (1981). Properties of commercial flavored frozen yogurts. J. Food Prot. 44, 853–856. doi: 10.4315/0362-028X-44.11.853
Kung, H. F., Lee, Y. H., Teng, D. F., Hsieh, P. C., Wei, C. I., and Tsai, Y. H. (2006a). Histamine formation by histamine-forming bacteria and yeast in mustard pickle products in Taiwan. Food Chem. 99, 579–585. doi: 10.1016/j.foodchem.2005.08.025
Kung, H. F., Tsai, Y. H., and Wei, C. I. (2006b). Histamine and other biogenic amines and histamine-forming bacteria in miso products. Food Chem. 101, 351–356. doi: 10.1016/j.foodchem.2005.12.057
Kwon, D. Y., Daily, J. W. III., Kim, H. J., and Park, S. (2010). Antidiabetic effects of fermented soybean products on type 2 diabetes. Nutr. Res. 30, 1–13. doi: 10.1016/j.nutres.2009.11.004
Larsson, S. C., Andersson, S., Johansson, J. E., and Wolk, A. (2008). Cultured milk, yogurt, and dairy intake in relation to bladder cancer risk in a prospective study of Swedish women and men. Am. J. Clin. Nutr. 88, 1083–1087. doi: 10.1093/ajcn/88.4.1083
Laye, I., Karleskind, D., and Morr, C. V. (1993). Chemical, microbiological and sensory properties of plain nonfat yogurt. J. Food Sci. 58, 991–995. doi: 10.1111/j.1365-2621.1993.tb06096.x
Leblanc, J. G., Laiño, J. E., del Valle, M. J., Vannini, V., van Sinderen, D., Taranto, M. P., et al. (2011). B-group vitamin production by lactic acid bacteria - current knowledge and potential applications. J. Appl. Microbiol. 111, 1297–1309. doi: 10.1111/j.1365-2672.2011.05157.x
Lee, K. E., Cho, U. H., and Ji, G. E. (1996). Effect of kimchi intake on the composition of human large intestinal bacteria. Korean J. Food Sci. Technol. 28, 981–986.
Lee, M., Song, J. H., Jung, M. Y., Lee, S. H., and Chang, J. Y. (2017). Large-scale targeted metagenomics analysis of bacterial ecological changes in 88 kimchi samples during fermentation. Food Microbiol. 66, 173–183. doi: 10.1016/j.fm.2017.05.002
Lee, M., Song, J. H., Lee, S. H., Jung, M. Y., and Chang, J. Y. (2018). Effect of seasonal production on bacterial communities in Korean industrial kimchi fermentation. Food Control 91, 381–389. doi: 10.1016/j.foodcont.2018.04.023
Lee, Y., Cha, Y. S., Park, Y., and Lee, M. (2017). PPARγ2 C1431T polymorphism interacts with the antiobesogenic effects of Kochujang, a Korean fermented, soybean-based red pepper paste, in overweight/obese subjects: a 12-week, double-blind randomized clinical trial. J. Med. Food 20, 610–617. doi: 10.1089/jmf.2016.3911
Lei, V., and Jakobsen, M. (2004). Microbiological characterization and probiotic potential of koko and koko sour water, African spontaneously fermented millet porridge and drink. J. Appl. Microbiol. 96, 384–397. doi: 10.1046/j.1365-2672.2004.02162.x
Linares, D. M., Gómez, C., Renes, E., Fresno, J. M., Tornadijo, M. E., Ross, R. P., et al. (2017). Lactic acid bacteria and bifidobacteria with potential to design natural biofunctional health-promoting dairy foods. Front. Microbiol. 8:846. doi: 10.3389/fmicb.2017.00846
Lisko, D., Johnston, G., and Johnston, C. (2017). Effects of dietary yogurt on the healthy human gastrointestinal (GI) microbiome. Microorganisms 5:E6. doi: 10.3390/microorganisms5010006
Lopez, M. C., Medina, L. M., and Jordano, R. (1998). Survival of lactic acid bacteria in commercial frozen yogurt. J. Food Sci. 63, 706–708. doi: 10.1111/j.1365-2621.1998.tb15818.x
Lourens-Hattingh, A., and Viljoen, B. C. (2002). Survival of probiotic bacteria in South African commercial bio-yogurt. S. Afr. J. Sci. 98, 298–300. Available online at: http://hdl.handle.net/10520/EJC97483
Lozupone, C. A., Li, M., Campbell, T. B., Flores, S. C., Linderman, D., Gebert, M. J., et al. (2013). Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe 14, 329–339. doi: 10.1016/j.chom.2013.08.006
Lu, Z., Breidt, F., Plengvidhya, V., and Fleming, H. P. (2003). Bacteriophage ecology in commercial sauerkraut fermentations. Appl. Environ. Microbiol. 69, 3192–3202. doi: 10.1128/AEM.69.6.3192-3202.2003
Macori, G., and Cotter, P. D. (2018). Novel insights into the microbiology of fermented dairy foods. Curr. Opin. Biotechnol. 49, 172–178. doi: 10.1016/j.copbio.2017.09.002
Madkor, S. A., Tong, P. S., and El Soda, M. (2000). Ripening of Cheddar cheese with added attenuated adjunct cultures of lactobacilli. J. Dairy Sci. 83, 1684–1691. doi: 10.3168/jds.S0022-0302(00)75037-5
Marco, M. L., and Golomb, B. L. (2016). Fermented foods, Lactobacillus, and health. Microbe 11, 349–354. doi: 10.1128/microbe.11.349.1
Marco, M. L., Heeney, D., Binda, S., Cifelli, C. J., Cotter, P. D., Foligné, B., et al. (2017). Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 44, 94–102. doi: 10.1016/j.copbio.2016.11.010
Martens, H., Dawoud, E., and Verachtert, H. (1991). Wort enterobacteria and other microbial populations involved during the first month of lambic fermentation. J. Insitute Brew. 97, 435–439. doi: 10.1002/j.2050-0416.1991.tb01082.x
Martini, M. C., Bollweg, G. L., Levitt, M. D., and Savaiano, D. A. (1987). Lactose digestion by yogurt beta-galactosidase: influence of pH and microbial cell integrity. Am. J. Clin. Nutr. 45, 432–436. doi: 10.1093/ajcn/45.2.432
Medina, L. M., and Jordano, R. (1994). Survival of constitutive microflora in commercially fermented milk containing bifidobacteria during refrigerated storage. J. Food Prot. 57, 731–733. doi: 10.4315/0362-028X-57.8.731
Messens, W., Estepar-Garcia, J., Dewettinck, K., and Huyghebaert, A. (1999). Proteolysis of high-pressure-treated Gouda cheese. Int. Dairy J. 9, 775–782. doi: 10.1016/S0958-6946(99)00152-1
Micanel, N., Haynes, I. N., and Playne, M. J. (1997). Viability of probiotic cultures in commercial Australian yogurts. Aust. J. Dairy Technol. 52, 24–27.
Mohammadmoradi, S., Javidan, A., Kordi, J., and Goudarzi, M. H. (2015). Comparing the effect of ultra-filtered feta cheese and yoghurt as probiotic carriers on lipid profile: a double blinded randomized controlled trial. Med. J. Nutr. Metab. 8, 27–36. doi: 10.3233/MNM-140026
Monfredini, L., Settanni, L., Poznanski, E., Cavazza, A., and Franciosi, E. (2012). The spatial distribution of bacteria in Grana-cheese during ripening. Syst. Appl. Microbiol. 35, 54–63. doi: 10.1016/j.syapm.2011.07.002
Mounier, J., Goerges, S., Gelsomino, R., Vancanneyt, M., Vandemeulebroecke, K., Hoste, B., et al. (2006). Sources of the adventitious microflora of a smear-ripened cheese. J. Appl. Microbiol. 101, 668–681. doi: 10.1111/j.1365-2672.2006.02922.x
Mounier, J., Monnet, C., Jacques, N., Antoinette, A., and Irlinger, F. (2009). Assessment of the microbial diversity at the surface of Livarot cheese using culture-dependent and independent approaches. Int. J. Food Microbiol. 133, 31–37. doi: 10.1016/j.ijfoodmicro.2009.04.020
Moyane, J. N., and Jideani, A. I. O. (2013). The physicochemical and sensory evaluation of commercial sour milk (amasi) products. Afr. J. Food Sci. 7, 56–62. doi: 10.5897/AJFS12.089
Mozaffarian, D., Hao, T., Rimm, E. B., Willett, W. C., and Hu, F. B. (2011). Changes in diet and lifestyle and long- term weight gain in women and men. N. Engl. J. Med. 364, 2392–2404. doi: 10.1056/NEJMoa1014296
Mugula, J. K., Nnko, S. A. M., Narvhus, J. A., and Sørhaug, T. (2003). Microbiological and fermentation characteristics of togwa, a Tanzanian fermented food. Int. J. Food Microbiol. 80, 187–199. doi: 10.1016/S0168-1605(02)00141-1
Muyanja, C. M. B. K., Narvhus, J. A., Treimo, J., and Langsrud, T. (2003). Isolation, characterisation and identification of lactic acid bacteria from bushera: a Ugandan traditional fermented beverage. Int. J. Food Microbiol. 80, 201–210. doi: 10.1016/S0168-1605(02)00148-4
Nout, M. J. R., de Dreu, M. A., Zuurbier, A. M., and Bonants-van Laarhoven, T. M. G. (1987). Ecology of controlled soyabean acidification for tempe manufacture. Food Microbiol. 4, 165–172. doi: 10.1016/0740-0020(87)90032-3
Nozue, M., Shimazu, T., Sasazuki, S., Charvat, H., Mori, N., Mutoh, M., et al. (2017). Fermented soy product intake is inversely associated with the development of high blood pressure: the Japan public health center-based prospective study. J. Nutr. 147, 1749–1756. doi: 10.3945/jn.117.250282
Nuñez, M. (1978). Microflora of cabrales cheese: changes during maturation. J. Dairy Res. 45, 501–508. doi: 10.1017/S0022029900016721
Nuobariene, L., Cizeikiene, D., Gradzeviciute, E., Hansen, Å. S., Rasmussen, S. K., Juodeikiene, G., et al. (2015). Phytase-active lactic acid bacteria from sourdoughs: isolation and identification. LWT Food Sci. Technol. 63, 766–772. doi: 10.1016/j.lwt.2015.03.018
OBrien, K., Aryana, K., Prinyawiwatkul, W., Carabante Ordonez, K., and Boeneke, C. (2016). Short communication: the effects of frozen storage on the survival of probiotic microorganisms found in traditionally and commercially manufactured kefir. J. Dairy Sci. 99, 7043–7048. doi: 10.3168/jds.2015-10284
Omar, N. B., and Ampe, F. (2000). Microbial community dynamics during production of the Mexican fermented maize dough pozol. Appl. Environ. Microbiol. 66, 3664–3673. doi: 10.1128/AEM.66.9.3664-3673.2000
Onda, T., Yanagida, F., Tsuji, M., Shinohara, T., and Yokotsuka, K. (2003). Time series analysis of aerobic bacterial flora during miso fermentation. Lett. Appl. Microbiol. 37, 162–168. doi: 10.1046/j.1472-765X.2003.01371.x
Owusu-Kwarteng, J., Akabanda, F., Nielsen, D. S., Tano-Debrah, K., Glover, R. L. K., and Jespersen, L. (2012). Identification of lactic acid bacteria isolated during traditional fura processing in Ghana. Food Microbiol. 32, 72–78. doi: 10.1016/j.fm.2012.04.010
Panagou, E. Z., Schillinger, U., Franz, C. M. A. P., and Nychas, G. J. E. (2008). Microbiological and biochemical profile of cv. conservolea naturally black olives during controlled fermentation with selected strains of lactic acid bacteria. Food Microbiol. 25, 348–358. doi: 10.1016/j.fm.2007.10.005
Panahi, S., Fernandez, M., Marette, A., and Tremblay, A. (2016). Yogurt, diet quality and lifestyle factors. Eur. J. Clin. Nutr. 71, 573–579. doi: 10.1038/ejcn.2016.214
Papamanoli, E., Tzanetakis, N., Litopoulou-Tzanetaki, E., and Kotzekidou, P. (2003). Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci. 65, 859–867. doi: 10.1016/S0309-1740(02)00292-9
Parente, E., Martuscelli, M., and Gardini, F. (2001). Evolution of microbial populations and biogenic amine production in dry sausages produced in Southern Italy. J. Appl. Microbiol. 90, 882–891. doi: 10.1046/j.1365-2672.2001.01322.x
Park, S., and Bae, J. H. (2016). Fermented food intake is associated with a reduced likelihood of atopic dermatitis in an adult population (Korean National Health and Nutrition Examination Survey 2012-2013). Nutr. Res. 36, 125–133. doi: 10.1016/j.nutres.2015.11.011
Pelletier, X., Laure-Boussuge, S., and Donazzolo, Y. (2001). Hydrogen excretion upon ingestion of dairy products in lactose- intolerant male subjects : importance of the live flora. Eur. J. Clin. Nutr. 55, 509–512. doi: 10.1038/sj.ejcn.1601169
Pisacane, V., Callegari, M. L., Puglisi, E., Dallolio, G., and Rebecchi, A. (2015). Microbial analyses of traditional Italian salami reveal microorganisms transfer from the natural casing to the meat matrix. Int. J. Food Microbiol. 207, 57–65. doi: 10.1016/j.ijfoodmicro.2015.04.029
Pisano, M. B., Scano, P., Murgia, A., Cosentino, S., and Caboni, P. (2016). Metabolomics and microbiological profile of Italian mozzarella cheese produced with buffalo and cow milk. Food Chem. 192, 618–624. doi: 10.1016/j.foodchem.2015.07.061
Połka, J., Rebecchi, A., Pisacane, V., Morelli, L., and Puglisi, E. (2015). Bacterial diversity in typical Italian salami at different ripening stages as revealed by high-throughput sequencing of 16S rRNA amplicons. Food Microbiol. 46, 342–356. doi: 10.1016/j.fm.2014.08.023
Poveda, J. M., Sousa, M. J., Cabezas, L., and McSweeney, P. L. H. (2003). Preliminary observations on proteolysis in Manchego cheese made with a defined-strain starter culture and adjunct starter ( Lactobacillus plantarum ) or a commercial starter. Int. Dairy J. 13, 169–178. doi: 10.1016/S0958-6946(02)00150-4
Praagman, J., Dalmeijer, G. W., van der Schouw, Y. T., Soedamah-Muthu, S. S., Monique Verschuren, W. M., Bas Bueno-de-Mesquita, H., et al. (2015). The relationship between fermented food intake and mortality risk in the European prospective investigation into cancer and nutrition-Netherlands cohort. Br. J. Nutr. 113, 498–506. doi: 10.1017/S0007114514003766
Rantsiou, K., Urso, R., Dolci, P., Comi, G., and Cocolin, L. (2008). Microflora of feta cheese from four Greek manufacturers. Int. J. Food Microbiol. 126, 36–42. doi: 10.1016/j.ijfoodmicro.2008.04.031
Rantsiou, K., Urso, R., Iacumin, L., Cantoni, C., Cattaneo, P., Comi, G., et al. (2005). Culture-dependent and -independent methods to investigate the microbial ecology of Italian fermented sausages. Appl. Environ. Microbiol. 71, 1977–1986. doi: 10.1128/AEM.71.4.1977-1986.2005
Ravula, R. R., and Shah, N. P. (1998). Selective enumeration of Lactobacillus casei from yogurts and fermented milk drinks. Biotechnol. Tech. 12, 819–822. doi: 10.1023/A:1008829004888
Rebecchi, A., Crivori, S., Sarra, P. G., and Cocconcelli, P. S. (1998). Physiological and molecular techniques for the study of bacterial community development in sausage fermentation. J. Appl. Microbiol. 84, 1043–1049. doi: 10.1046/j.1365-2672.1998.00442.x
Renye, J. A., Somkuti, G. A., Vallejo-Cordoba, B., Van Hekken, D. L., and Gonzalez-Cordova, A. F. (2008). Characterization of the microflora isolated from Queso fresco made from raw and pasteurized milk. J. Food Saf. 28, 59–75. doi: 10.1111/j.1745-4565.2007.00095.x
Romeo, F. V., Piscopo, A., Mincione, A., and Poiana, M. (2012). Quality evaluation of different typical table olive preparations (cv Nocellara del Belice). Grasas y Aceites 63, 19–25. doi: 10.3989/gya.058511
Ruiz-Barba, J. L., and Jiménez-Díaz, R. (2012). A novel Lactobacillus pentosus -paired starter culture for Spanish-style green olive fermentation. Food Microbiol. 30, 253–259. doi: 10.1016/j.fm.2011.11.004
Samelis, J., and Kakouri, A. (2007). Microbial and safety qualities of PDO Galotyri cheese manufactured at the industrial or artisan scale in Epirus, Greece. Ital. J. Food Sci. 19, 81–90.
Samelis, J., Stavropoulos, S., Kakouri, A., and Metaxopoulos, J. (1994). Quantification and characterization of microbial populations associated with naturally fermented Greek dry salami. Food Microbiol. 11, 447–460. doi: 10.1006/fmic.1994.1050
Samson, R. A., Van Kooij, J. A., and De Boer, E. (1987). Microbiological quality of commercial tempeh in the Netherlands. J. Food Prot. 50, 92–94. doi: 10.4315/0362-028X-50.2.92
Sanders, M. E., Benson, A., Lebeer, S., Merenstein, D. J., and Klaenhammer, T. R. (2018). Shared mechanisms among probiotic taxa: implications for general probiotic claims. Curr. Opin. Biotechnol. 49, 207–216. doi: 10.1016/j.copbio.2017.09.007
Sanders, M. E., Lenoir-Wijnkoop, I., Salminen, S., Merenstein, D. J., Gibson, G. R., Petschow, B. W., et al. (2014). Probiotics and prebiotics: prospects for public health and nutritional recommendations. Ann. N. Y. Acad. Sci. 1309, 19–29. doi: 10.1111/nyas.12377
Santarelli, M., Bottari, B., Lazzi, C., Neviani, E., and Gatti, M. (2013). Survey on the community and dynamics of lactic acid bacteria in Grana Padano cheese. Syst. Appl. Microbiol. 36, 593–600. doi: 10.1016/j.syapm.2013.04.007
Saubade, F., Hemery, Y. M., Guyot, J. P., and Humblot, C. (2017). Lactic acid fermentation as a tool for increasing the folate content of foods. Crit. Rev. Food Sci. Nutr. 57, 3894–3910. doi: 10.1080/10408398.2016.1192986
Savaiano, D. A. (2014). Lactose digestion from yogurt: mechanism and relevance. Am. J. Clin. Nutr. 99, 1251S−1255S. doi: 10.3945/ajcn.113.073023
Schmidt, K. A., Kim, J., and Jeon, I. J. (1997). Composition of carbohydrates and concentration of β-galactosidase of commercial frozen yogurt. J. Food Qual. 20, 349–358. doi: 10.1111/j.1745-4557.1997.tb00478.x
Scourboutakos, M. J., Franco-Arellano, B., Murphy, S. A., Norsen, S., Comelli, E. M., and L'Abbé, M. R. (2017). Mismatch between probiotic benefits in trials versus food products. Nutrients 9:E400. doi: 10.3390/nu9040400
Shah, N. P., Ali, J. F., and Ravula, R. R. (2000). Populations of Lactobacillus acidophilus, Bifidobacterium spp., and Lactobacillus casei in commercial fermented milk products. Biosci. Microflora 19, 35–39. doi: 10.12938/bifidus1996.19.35
Shah, N. P., Lankaputhra, W. E. V., Britzb, M. L., and Kyle, W. S. A. (1995). Survival of Lactobacillus acidophilus and Bifidobacterium bifidum in commercial yoghurt during refrigerated storage. Int. Dairy J. 5, 515–521. doi: 10.1016/0958-6946(95)00028-2
Shin, H. S., Lee, J. H., Pestka, J. J., and Ustunol, Z. (2000). Viability of bifidobacteria in commercial dairy products during refrigerated storage. J. Food Prot. 63, 327–331. doi: 10.4315/0362-028X-63.3.327
Silva, T., Reto, M., Sol, M., Peito, A., Peres, C. M., Peres, C., et al. (2011). Characterization of yeasts from Portuguese brined olives, with a focus on their potentially probiotic behavior. LWT Food Sci. Technol. 44, 1349–1354. doi: 10.1016/j.lwt.2011.01.029
Silvestri, G., Santarelli, S., Aquilanti, L., Beccaceci, A., Osimani, A., Tonucci, F., et al. (2007). Investigation of the microbial ecology of Ciauscolo, a traditional Italian salami, by culture-dependent techniques and PCR-DGGE. Meat Sci. 77, 413–423. doi: 10.1016/j.meatsci.2007.04.015
Singh, A. K., and Ramesh, A. (2008). Succession of dominant and antagonistic lactic acid bacteria in fermented cucumber: insights from a PCR-based approach. Food Microbiol. 25, 278–287. doi: 10.1016/j.fm.2007.10.010
Slashinski, M. J., McCurdy, S. A., Achenbaum, L. S., Whitney, S. N., and McGuire, A. L. (2012). “Snake-oil,” “quack medicine,” and “industrially cultured organisms:” biovalue and the commercialization of human microbiome research. BMC Med. Ethics 13:28. doi: 10.1186/1472-6939-13-28
Smug, L. N., Salminen, S., Sanders, M. E., and Ebner, S. (2014). Yoghurt and probiotic bacteria in dietary guidelines of the member states of the European Union. Benef. Microbes 5, 61–66. doi: 10.3920/BM2013.0050
Sommer, F., Anderson, J. M., Bharti, R., Raes, J., and Rosenstiel, P. (2017). The resilience of the intestinal microbiota influences health and disease. Nat. Microbiol. 15, 630–638. doi: 10.1038/nrmicro.2017.58
Sonnenburg, J. L., and Bäckhed, F. (2016). Diet–microbiota interactions as moderators of human metabolism. Nature 535, 56–64. doi: 10.1038/nature18846
Spitaels, F., Kerrebroeck, S., Snauwaert, I., Aerts, M., Landschoot, A., et al. (2015a). Microbiota and metabolites of aged bottled gueuze beers converge to the same composition. Food Microbiol. 47, 1–11. doi: 10.1016/j.fm.2014.10.004
Spitaels, F., Wieme, A. D., Janssens, M., Aerts, M., Daniel, H. M., Van Landschoot, A., et al. (2014). The microbial diversity of traditional spontaneously fermented lambic beer. PLoS ONE 9:e95384. doi: 10.1371/journal.pone.0095384
Spitaels, F., Wieme, A. D., Janssens, M., Aerts, M., Van Landschoot, A., De Vuyst, L., et al. (2015b). The microbial diversity of an industrially produced lambic beer shares members of a traditionally produced one and reveals a core microbiota for lambic beer fermentation. Food Microbiol. 49, 23–32. doi: 10.1016/j.fm.2015.01.008
Stanczak, M., and Heuberger, R. (2009). Assessment of the knowledge and beliefs regarding probiotic use. Am. J. Heal. Educ. 40, 207–211. doi: 10.1080/19325037.2009.10599095
Talwalkar, A., and Kailasapathy, K. (2004). Comparison of selective and differential media for the accurate enumeration of strains of Lactobacillus acidophilus, Bifidobacterium spp. and Lactobacillus casei complex from commercial yoghurts. Int. Dairy J. 14, 143–149. doi: 10.1016/S0958-6946(03)00172-9
Tamang, J. P., Watanabe, K., and Holzapfel, W. H. (2016). Review: diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 7:377. doi: 10.3389/fmicb.2016.00377
Tannock, G. W. (2003). Probiotics: time for a dose of realism. Curr. Issues Intest. Microbiol. 4, 33–42. doi: 10.3920/BM2016.0140
Teoh, A. L., Heard, G., and Cox, J. (2004). Yeast ecology of Kombucha fermentation. Int. J. Food Microbiol. 95, 119–126. doi: 10.1016/j.ijfoodmicro.2003.12.020
Tharmaraj, N., and Shah, N. P. (2003). Selective enumeration of Lactobacillus delbrueckii ssp. bulgaricus, Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacteria, Lactobacillus casei, Lactobacillus rhamnosus , and Propionibacteria. J. Dairy Sci. 86, 2288–2296. doi: 10.3168/jds.S0022-0302(03)73821-1
Tieszen, K. M., and Baer, R. J. (1989). Composition and microbiological quality of frozen yogurts. Cult. Dairy Prod. J. 24, 11–14.
Tornadijo, M., Fresno, J., Bernardo, A., Martin Sarmiento, R., and Carballo, J. (1995). Microbiological changes throughout the manufacturing and ripening of a Spanish goat's raw milk cheese (Armada variety). Lait 75, 551–570.
Tou, E. H., Guyot, J. P., Mouquet-Rivier, C., Rochette, I., Counil, E., Traoré, A. S., et al. (2006). Study through surveys and fermentation kinetics of the traditional processing of pearl millet ( Pennisetum glaucum ) into ben-saalga, a fermented gruel from Burkina Faso. Int. J. Food Microbiol. 106, 52–60. doi: 10.1016/j.ijfoodmicro.2005.05.010
Tsai, Y. H., Kung, H. F., Lin, Q. L., Hwang, J. H., Cheng, S. H., Wei, C. I., et al. (2005). Occurrence of histamine and histamine-forming bacteria in kimchi products in Taiwan. Food Chem. 90, 635–641. doi: 10.1016/j.foodchem.2004.04.024
Tsuda, H., Kubota, K., Matsumoto, T., and Ishimi, Y. (2012). Isolation and identification of Lactic Acid Bacteria in traditional fermented sushi, Funazushi, from Japan. Food Sci. Technol. Res. 18, 77–82. doi: 10.3136/fstr.18.77
Van Hoorde, K., Verstraete, T., Vandamme, P., and Huys, G. (2008). Diversity of lactic acid bacteria in two Flemish artisan raw milk Gouda-type cheeses. Food Microbiol. 25, 929–935. doi: 10.1016/j.fm.2008.06.006
Vasavada, P. C., and White, C. H. (1979). Quality of commercial buttermilk. J. Dairy Sci. 62, 802–806. doi: 10.3168/jds.S0022-0302(79)83329-9
Viander, B., Aki, M. M., and Palva, A. (2003). Impact of low salt concentration, salt quality on natural large-scale sauerkraut fermentation. Food Microbiol. 20, 391–395. doi: 10.1016/S0740-0020(02)00150-8
Viljoen, B. C., Khoury, A. R., and Hattingh, A. (2003). Seasonal diversity of yeasts associated with white-surface mould-ripened cheeses. Food Res. Int. 36, 275–283. doi: 10.1016/S0963-9969(02)00169-2
Vinderola, C. G., and Reinheimer, J. A. (2000). Enumeration of Lactobacillus casei in the presence of L. acidophilus bifidobacteria and lactic starter bacteria in fermented dairy products. Int. Dairy, J. 10, 271–275. doi: 10.1016/S0958-6946(00)00045-5
Walter, J., Hertel, C., Tannock, G. W., Lis, C. M., Munro, K., and Hammes, W. P. (2001). Detection of Lactobacillus, Pediococcus, Leuconostoc, and Weissella species in human feces by using group-specific PCR primers and denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 67, 2578–2585. doi: 10.1128/AEM.67.6.2578-2585.2001
Welthagen, J. J., and Viljoen, B. C. (1998). Yeast profile in Gouda cheese during processing and ripening. Int. J. Food Microbiol. 41, 185–194. doi: 10.1016/S0168-1605(98)00042-7
Yeung, P. S. M., Sanders, M. E., Kitts, C. L., Cano, R., and Tong, P. S. (2002). Species-specific identification of commercial probiotic strains. J. Dairy Sci. 85, 1039–1051. doi: 10.3168/jds.S0022-0302(02)74164-7
Yunita, D., and Dodd, C. E. R. (2018). Microbial community dynamics of a blue-veined raw milk cheese from the United Kingdom. J. Dairy Sci. 101, 4923–4935. doi: 10.3168/jds.2017-14104
Zaman, M. Z., Bakar, F. A., Selamat, J., and Bakar, J. (2010). Occurrence of biogenic amines and amines degrading bacteria in fish sauce. Czech J. Food Sci. 28, 440–449. doi: 10.17221/312/2009-CJFS
Zhang, C., Derrien, M., Levenez, F., Brazeilles, R., Ballal, S. A., Kim, J., et al. (2016). Ecological robustness of the gut microbiota in response to ingestion of transient food-borne microbes. ISME J. 10, 2235–2245. doi: 10.1038/ismej.2016.13
Keywords: fermented foods, live microbes, lactic acid bacteria, health benefits, probiotics
Citation: Rezac S, Kok CR, Heermann M and Hutkins R (2018) Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol . 9:1785. doi: 10.3389/fmicb.2018.01785
Received: 13 May 2018; Accepted: 17 July 2018; Published: 24 August 2018.
Reviewed by:
Copyright © 2018 Rezac, Kok, Heermann and Hutkins. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert Hutkins, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Fermented-food diet increases microbiome diversity, decreases inflammatory proteins, study finds
Stanford researchers discover that a 10-week diet high in fermented foods boosts microbiome diversity and improves immune responses.
July 12, 2021 - By Janelle Weaver

Stanford researchers found that eating a diet high in fermented foods such as kimchi increases the diversity of gut microbes, which is associated with improved health. Nungning20/Shutterstock
A diet rich in fermented foods enhances the diversity of gut microbes and decreases molecular signs of inflammation, according to researchers at the Stanford School of Medicine .
In a clinical trial, 36 healthy adults were randomly assigned to a 10-week diet that included either fermented or high-fiber foods. The two diets resulted in different effects on the gut microbiome and the immune system.
Eating foods such as yogurt, kefir, fermented cottage cheese, kimchi and other fermented vegetables, vegetable brine drinks, and kombucha tea led to an increase in overall microbial diversity, with stronger effects from larger servings. “This is a stunning finding,” said Justin Sonnenburg , PhD, an associate professor of microbiology and immunology. “It provides one of the first examples of how a simple change in diet can reproducibly remodel the microbiota across a cohort of healthy adults.”
In addition, four types of immune cells showed less activation in the fermented-food group. The levels of 19 inflammatory proteins measured in blood samples also decreased. One of these proteins, interleukin 6, has been linked to conditions such as rheumatoid arthritis, Type 2 diabetes and chronic stress.
“Microbiota-targeted diets can change immune status, providing a promising avenue for decreasing inflammation in healthy adults,” said Christopher Gardner , PhD, the Rehnborg Farquhar Professor and director of nutrition studies at the Stanford Prevention Research Center . “This finding was consistent across all participants in the study who were assigned to the higher fermented food group.”

Justin Sonnenburg
Microbe diversity stable in fiber-rich diet
By contrast, none of these 19 inflammatory proteins decreased in participants assigned to a high-fiber diet rich in legumes, seeds, whole grains, nuts, vegetables and fruits. On average, the diversity of their gut microbes also remained stable. “We expected high fiber to have a more universally beneficial effect and increase microbiota diversity,” said Erica Sonnenburg , PhD, a senior research scientist in basic life sciences, microbiology and immunology. “The data suggest that increased fiber intake alone over a short time period is insufficient to increase microbiota diversity.”
The study published online July 12 in Cell. Justin and Erica Sonnenburg and Christopher Gardner are co-senior authors. The lead authors are Hannah Wastyk , a PhD student in bioengineering, and former postdoctoral scholar Gabriela Fragiadakis, PhD, who is now an assistant professor of medicine at UC-San Francisco.
A wide body of evidence has demonstrated that diet shapes the gut microbiome, which can affect the immune system and overall health. According to Gardner, low microbiome diversity has been linked to obesity and diabetes.
“We wanted to conduct a proof-of-concept study that could test whether microbiota-targeted food could be an avenue for combatting the overwhelming rise in chronic inflammatory diseases,” Gardner said.
The researchers focused on fiber and fermented foods due to previous reports of their potential health benefits. While high-fiber diets have been associated with lower rates of mortality, the consumption of fermented foods can help with weight maintenance and may decrease the risk of diabetes, cancer and cardiovascular disease.

Erica Sonnenburg
The researchers analyzed blood and stool samples collected during a three-week pre-trial period, the 10 weeks of the diet, and a four-week period after the diet when the participants ate as they chose.
The findings paint a nuanced picture of the influence of diet on gut microbes and immune status. On one hand, those who increased their consumption of fermented foods showed similar effects on their microbiome diversity and inflammatory markers, consistent with prior research showing that short-term changes in diet can rapidly alter the gut microbiome. On the other hand, the limited change in the microbiome within the high-fiber group dovetails with the researchers’ previous reports of a general resilience of the human microbiome over short time periods.
Designing a suite of dietary and microbial strategies
The results also showed that greater fiber intake led to more carbohydrates in stool samples, pointing to incomplete fiber degradation by gut microbes. These findings are consistent with other research suggesting that the microbiome of people living in the industrialized world is depleted of fiber-degrading microbes.
“It is possible that a longer intervention would have allowed for the microbiota to adequately adapt to the increase in fiber consumption,” Erica Sonnenburg said. “Alternatively, the deliberate introduction of fiber-consuming microbes may be required to increase the microbiota’s capacity to break down the carbohydrates.”
In addition to exploring these possibilities, the researchers plan to conduct studies in mice to investigate the molecular mechanisms by which diets alter the microbiome and reduce inflammatory proteins. They also aim to test whether high-fiber and fermented foods synergize to influence the microbiome and immune system of humans. Another goal is to examine whether the consumption of fermented food decreases inflammation or improves other health markers in patients with immunological and metabolic diseases, and in pregnant women and older individuals.

Christopher Gardner
“There are many more ways to target the microbiome with food and supplements, and we hope to continue to investigate how different diets, probiotics and prebiotics impact the microbiome and health in different groups,” Justin Sonnenburg said.
Other Stanford co-authors are Dalia Perelman, health educator; former graduate students Dylan Dahan, PhD, and Carlos Gonzalez, PhD; graduate student Bryan Merrill; former research assistant Madeline Topf; postdoctoral scholars William Van Treuren, PhD, and Shuo Han, PhD; Jennifer Robinson, PhD, administrative director of the Community Health and Prevention Research Master’s Program and program manager of the Nutrition Studies Group; and Joshua Elias, PhD.
Researchers from Chan-Zuckerberg Biohub also contributed to the study.
The work was supported by donations to the Center for Human Microbiome Research; Paul and Kathy Klingenstein; the Hand Foundation; Heather Buhr and Jon Feiber; Meredith and John Pasquesi; the National Institutes of Health (grant T32 AI 7328-29); a Stanford Dean’s Postdoctoral Fellowship; a National Science Foundation Graduate Student Fellowship; and seed funding from the Institute for Immunity, Transplantation and Infection and from the Sean N. Parker Center for Allergy and Asthma Research.
- Janelle Weaver
About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Hope amid crisis
Psychiatry’s new frontiers

Advertisement
Advancing Fermented Food Products: Exploring Bioprocess Technologies and Overcoming Challenges
- Published: 30 December 2023
Cite this article

- Sudarsini B 1 ,
- Venkateswarulu T. C 1 ,
- Krupanidhi S 1 ,
- Sumalatha B 2 &
- Indira M 1
359 Accesses
Explore all metrics
The fermented foods have been a part of the human diet in both traditional and modern cultures. Fermented food products are those produced through the addition of microbes such as yeast and bacteria. Each fermented food has distinct population of microorganisms, acts as probiotics, and produces a variety of metabolites and bioactive compounds. The physical and chemical changes in fermented food are due to the presence of probiotics and prebiotics, resulting in improved quality and long-term stability of the product. The positive health benefits have been investigated intensively, and the consolidated data are presented in this review. Once ingested, the beneficial bacteria colonize in the gut microbiome, produce bioactive compounds, and protect the human body from pathogenic microorganisms in various ways. The purpose of this paper is to present various types of fermented foods and beverages, probiotics, and prebiotics present in the foods, bioprocess technologies used for processing of fermented foods, nutritional quality of the fermented foods, and the influence of fermented foods on gut microbiome and health. Therefore, the benefits of fermented foods and beverages on gut microbiome should be studied and pursued to promote good health.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others
An overview of fermentation in the food industry - looking back from a new perspective

Fermented food/beverage and health: current perspectives

Microbial Fermentation in Food Preservation
Data availability.
No data were used for the research described in the article.
Abaci, N., Deniz, F. S. S., & Orhan, I. E. (2022). Kombucha–an ancient fermented beverage with desired bioactivities: A narrowed review. Food Chemistry: X, 14 , 100302.
PubMed CAS Google Scholar
Abo Bakr, T. M. (2020). Microwave applications in food processing: An overview. Alexandria Journal of Food Science and Technology, 17 (2), 11–22.
Article Google Scholar
Achi, O. K., & Asamudo, N. U. (2019). Cereal-based fermented foods of Africa as functional foods. In Bioactive molecules in food , 1527–1558.
Afzaal, M., Saeed, F., Anjum, F., Waris, N., Husaain, M., Ikram, A., . . . Suleria, H. (2021). Nutritional and ethnomedicinal scenario of koumiss: a concurrent review. Food Science & Nutrition , 9 (11), 6421–6428.
Agregan, R., Munekata, P. E., Zhang, W., Zhang, J., Pérez-Santaescolástica, C., & Lorenzo, J. M. (2021). High-pressure processing in inactivation of Salmonella spp. in food products. Trends in Food Science & Technology, 107 , 31–37.
Article CAS Google Scholar
Ahnan-Winarno, A. D., Cordeiro, L., Winarno, F. G., Gibbons, J., & Xiao, H. (2021). Tempeh: A semicentennial review on its health benefits, fermentation, safety, processing, sustainability, and affordability. Comprehensive Reviews in Food Science and Food Safety, 20 (2), 1717–1767.
Article PubMed CAS Google Scholar
Aiello, F., Restuccia, D., Spizzirri, U. G., Carullo, G., Leporini, M., & Loizzo, M. R. (2020). Improving Kefir bioactive properties by functional enrichment with plant and agro-food waste extracts. Fermentation, 6 (3), 83.
Akaike, M., Miyagawa, H., Kimura, Y., Terasaki, M., Kusaba, Y., Kitagaki, H., & Nishida, H. (2020). Chemical and bacterial components in sake and sake production process. Current Microbiology, 77 , 632–637.
Akkoyun, Y., & Arslan, S. (2020). The impact of quinoa flour on some properties of ayran. Food Science & Nutrition, 8 (10), 5410–5418.
Altemimi, A., Aziz, S. N., Al-HiIphy, A. R., Lakhssassi, N., Watson, D. G., & Ibrahim, S. A. (2019). Critical review of radio-frequency (RF) heating applications in food processing. Food Quality and Safety, 3 (2), 81–91.
Amara, A. A., & Shibl, A. (2015). Role of Probiotics in health improvement, infection control and disease treatment and management. Saudi Pharmaceutical Journal, 23 (2), 107–114.
Anal, A. K. (2019). Quality ingredients and safety concerns for traditional fermented foods and beverages from Asia: A review. Fermentation, 5 (1), 8.
Anastasova, L., Ivanovska, T. P., Petkovska, R., & Petrusevska-Tozi, L. (2018). Concepts, benefits and perspectives of functional dairy food products. Macedonian Pharmaceutical Bulletin, 64 (2), 73–83.
Antolak, H., Piechota, D., & Kucharska, A. (2021). Kombucha tea—a double power of bioactive compounds from tea and Symbiotic Culture of Bacteria and Yeasts (SCOBY). Antioxidants, 10 (10), 1541.
Article PubMed PubMed Central CAS Google Scholar
Arjun, J., Verma, A. K., & Prasad, S. B. (2014). Method of preparation and biochemical analysis of local tribal wine Judima: An indigenous alcohol used by Dimasa tribe of North Cachhar Hills District of Assam. India. International Food Research Journal, 21 (2), 463–470.
Google Scholar
Arshad, R. N., Abdul-Malek, Z., Munir, A., Buntat, Z., Ahmad, M. H., Jusoh, Y. M., . . . Aadil, R. M. (2020). Electrical systems for pulsed electric field applications in the food industry: an engineering perspective. Trends in Food Science & Technology , 104 , 1–13.
Arshad, R. N., Abdul-Malek, Z., Roobab, U., Munir, M. A., Naderipour, A., Qureshi, M. I., . . . Aadil, R. M. (2021). Pulsed electric field: a potential alternative towards a sustainable food processing. Trends in Food Science & Technology , 111 , 43–54.
Ashaolu, T. J., & Reale, A. (2020). A holistic review on Euro-Asian lactic acid bacteria fermented cereals and vegetables. Microorganisms, 8 (8), 1176.
Bakry, A. M., & Campelo, P. H. (2018). Mini-review on functional characteristics of viili and manufacturing process. Journal of Food Biotechnology Research , 2 (7).
Baliyan, N., Dindhoria, K., Kumar, A., Thakur, A., & Kumar, R. (2021). Comprehensive substrate-based exploration of probiotics from undistilled traditional fermented alcoholic beverage ‘Lugri’. Frontiers in Microbiology , 12 .
Bankar, R., Desale, R. J., Bhosale, S., & Chougale, C. (2021). Storage study of bael & whey protein enriched nutritional whey beverage. The Pharma Innovation Journal, 10 (8), 171–176.
Bansal, S., Mangal, M., Sharma, S. K., & Gupta, R. K. (2016). Non-dairy based probiotics: A healthy treat for intestine. Critical Reviews in Food Science and Nutrition, 56 (11), 1856–1867.
Barukcic, I., Jakopovic, K. L., & Bozanic, R. (2019). Whey and buttermilk—neglected sources of valuable beverages. In Natural Beverages , 209–242, Academic Press.
Baruzzi, F., Quintieri, L., Caputo, L., Cocconcelli, P., Borcakli, M., Owczarek, L., . . . Morea, M. (2016). Improvement of Ayran quality by the selection of autochthonous microbial cultures. Food Microbiology , 60 , 92–103.
Baschali, A., Tsakalidou, E., Kyriacou, A., Karavasiloglou, N., & Matalas, A. L. (2017). Traditional low-alcoholic and non-alcoholic fermented beverages consumed in European countries: A neglected food group. Nutrition Research Reviews, 30 (1), 1–24.
Basumatary, T. K., Terangpi, R., Brahma, C., & Roy, P. (2014). Jou: The traditional drink of the Boro tribe of Assam and North East India. Journal of Scientific & Innovative Research, 3 (2), 239–243.
Behera, S. S., El Sheikha, A. F., Hammami, R., & Kumar, A. (2020). Traditionally fermented pickles: How the microbial diversity associated with their nutritional and health benefits. Journal of Functional Foods, 70 , 103971.
Bhatt, K., Vaidya, D., Kaushal, M., Gupta, A., Soni, P., Arya, P., . . . Sharma, C. (2020). Microwaves and radio waves: in food processing and preservation. International Journal of Current Microbiology and Applied Sciences , 9 (9), 118–131.
Bishop, P., Pitts, E. R., Budner, D., & Thompson-Witrick, K. A. (2022). Kombucha: Biochemical and microbiological impacts on the chemical and flavor profile. Food Chemistry Advances , 100025.
Bisht, B., Bhatnagar, P., Gururani, P., Kumar, V., Tomar, M. S., Sinhmar, R., . . . Kumar, S. (2021). Food irradiation: effect of ionizing and non-ionizing radiations on preservation of fruits and vegetables–a review. Trends in Food Science & Technology , 114 , 372–385.
Borah, D., Gogoi, T., Sarma, J., Borah, P. J., Gohain, B., Mili, C., . . . Tangjang, S. (2021). Compendium of plants used for preparation of traditional alcoholic beverages by four major ethnic communities of Assam, northeast India. Biodiversitas Journal of Biological Diversity , 22 (4), 2019–2031.
Bourrie, B. C., Willing, B. P., & Cotter, P. D. (2016). The microbiota and health promoting characteristics of the fermented beverage kefir. Frontiers in Microbiology , 647.
Cantabrana, I., Perise, R., & Hernández, I. (2015). Uses of Rhizopus oryzae in the kitchen. International Journal of Gastronomy and Food Science, 2 (2), 103–111.
Celekli, A., Alslibi, Z. A., & Üseyin Bozkurt, H. (2019). Influence of incorporated Spirulina platensis on the growth of microflora and physicochemical properties of ayran as a functional food. Algal Research, 44 , 101710.
Chacha, J. S., Zhang, L., Ofoedu, C. E., Suleiman, R. A., Dotto, J. M., Roobab, U., . . . Guiné, R. P. (2021). Revisiting non-thermal food processing and preservation methods—action mechanisms, pros and cons: a technological update (2016–2021). Foods , 10 (6), 1430.
Chavan, R. S., Shraddha, R. C., Kumar, A., & Nalawade, T. (2015). Whey based beverage: Its functionality, formulations, health benefits and applications. Journal of Food Processing & Technology, 6 (10), 1–8.
CAS Google Scholar
Chen, M., Ye, X., Shen, D., & Ma, C. (2019). Modulatory effects of gut microbiota on constipation: The commercial beverage Yakult shapes stool consistency. Journal of Neuro Gastroenterology and Motility, 25 (3), 475.
Choi, J. S., Seo, H. J., Lee, Y. R., Kwon, S. J., Moon, S. H., Park, S. M., & Sohn, J. H. (2014). Characteristics and in vitro anti-diabetic properties of the Korean rice wine, makgeolli fermented with Laminaria japonica . Preventive Nutrition and Food Science, 19 (2), 98–107.
Chonan, O. (2021). Yakult’s research activities: inheritance and practice of Shirota-ism. Nature Portfolio , 1–6.
Cuvas-Limon, R. B., Nobre, C., Cruz, M., Rodriguez-Jasso, R. M., Ruíz, H. A., Loredo-Treviño, A., . . . Belmares, R. (2021). Spontaneously fermented traditional beverages as a source of bioactive compounds: an overview. Critical Reviews in Food Science and Nutrition , 61 (18), 2984–3006.
Das, S., & Tamang, J. P. (2021). Changes in microbial communities and their predictive functionalities during fermentation of toddy, an alcoholic beverage of India. Microbiological Research, 248 , 126769.
Das, T. K., Pradhan, S., Chakrabarti, S., Mondal, K. C., & Ghosh, K. (2022). Current status of probiotic and related health benefits. Applied Food Research, 2 (1), 100185.
Datta, A., Pal, A., & Bandyopadhyay, A. (2016). A study on the effect of habitual consumption of Madhuca longifolia drinks on the prevalence of diabetes and dyslipidemia among Santhal tribals. International Journal of Basic & Clinical Pharmacology, 5 (3), 1108–1111.
Dehghani, N., Tafvizi, F., & Jafari, P. (2021). Cell cycle arrest and anti-cancer potential of probiotic Lactobacillus rhamnosus against HT-29 cancer cells. BioImpacts: BI, 11 (4), 245–252.
Dimidi, E., Cox, S. R., Rossi, M., & Whelan, K. (2019). Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients, 11 (8), 1806.
Djeni, T. N., Kouame, K. H., Ake, F. D., Amoikon, L. S., Dje, M. K., & Jeyaram, K. (2020). Microbial diversity and metabolite profiles of palm wine produced from three different palm tree species in Côte d’Ivoire. Scientific Reports, 10 (1), 1715.
Durack, J., & Lynch, S. V. (2019). The gut microbiome: Relationships with disease and opportunities for therapy. Journal of Experimental Medicine, 216 (1), 20–40.
Duraiswamy, A., Jebakani, S., Selvaraj, S., Pramitha, L., Selvaraj, R., Sheriff, S. K., . . . Rathinamoorthy, S. (2022). Genetic manipulation of anti-nutritional factors in major crops for a sustainable diet in future. Frontiers in Plant Science , 13 .
Dutta, M. (2022). JUDIMA: A traditional brew of assam to earn the GI tag. Food and Scientific Reports, 3 (4), 19–21.
Dwivedi, A., Priyadarshini, A., & Induar, S. (2022). Mahua ( Madhuca longifolia ) flower and its application in food industry: A review. International Journal of Chemical Studies, 10 (1), 80–84.
El Sheikha, A. F. (2018). Molecular techniques and lactic acid-fermented fruits and vegetables. Molecular Techniques in Food Biology: Safety, Biotechnology, Authenticity and Traceability, 12 , 285–308.
Elhalis, H., Cox, J., Frank, D., & Zhao, J. (2021). The role of wet fermentation in enhancing coffee flavor, aroma and sensory quality. European Food Research and Technology, 247 , 485–498.
Enujiugha, V. N., & Badejo, A. A. (2017). Probiotic potentials of cereal-based beverages. Critical Reviews in Food Science and Nutrition, 57 (4), 790–804.
Farag, M. A., Jomaa, S. A., Abd El-Wahed, A., El-Seedi, R., & H. (2020). The many faces of kefir fermented dairy products: Quality characteristics, flavour chemistry, nutritional value, health benefits, and safety. Nutrients, 12 (2), 346.
Faria-Oliveira, F., Diniz, R. H., Godoy-Santos, F., Piló, F. B., Mezadri, H., Castro, I. M., & Brandão, R. L. (2015). The role of yeast and lactic acid bacteria in the production of fermented beverages in South America. Food Production and Industry , 107–135.
Firouz, M. S., Farahmandi, A., & Hosseinpour, S. (2019). Recent advances in ultrasound application as a novel technique in analysis, processing and quality control of fruits, juices and dairy products industries: A review. Ultrasonics Sonochemistry, 57 , 73–88.
Gaggia, F., Baffoni, L., Galiano, M., Nielsen, D. S., Jakobsen, R. R., Castro-Mejía, J. L., . . . Di Gioia, D. (2018). Kombucha beverage from green, black and rooibos teas: a comparative study looking at microbiology, chemistry and antioxidant activity. Nutrients , 11 (1), 1.
Gambus, H., Mickowska, B., Bartoń, H., Augustyn, G., Zięć, G., Litwinek, D., . . . Berski, W. (2021). Health benefits of kvass manufactured from rye whole meal bread. Journal of Microbiology, Biotechnology and Food Sciences , 2021 , 34–39.
Ganzle, M. G. (2020). Food fermentations for improved digestibility of plant foods–an essential ex situ digestion step in agricultural societies. Current Opinion in Food Science, 32 , 124–132.
Gavahian, M., & Tiwari, B. K. (2020). Moderate electric fields and ohmic heating as promising fermentation tools. Innovative Food Science & Emerging Technologies, 64 , 102422.
Ghosh, K., Adak, A., Halder, S. K., & Mondal, K. C. (2021). Physicochemical characteristics and lactic acid bacterial diversity of an ethnic rice fermented mild alcoholic beverage. Haria. Frontiers in Sustainable Food Systems, 5 , 680738.
Ghosh, S., Sen, S. K., & Mondal, S. (2022). Ethnobotanical perspectives of Bakhar: An indigenous starter culture used to prepare traditionally fermented rice beverage in rural West Bengal. India. Journal of Ethnic Foods, 9 (1), 1–10.
Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., . . . Reid, G. (2017). Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews Gastroenterology & Hepatology , 14 (8), 491–502.
Gu, Q., Yin, Y., Yan, X., Liu, X., Liu, F., & McClements, D. J. (2022). Encapsulation of multiple probiotics, synbiotics, or nutrabiotics for improved health effects: a review. Advances in Colloid and Interface Science , 102781.
Gulliver, E. L., Young, R. B., Chonwerawong, M., D'Adamo, G. L., Thomason, T., Widdop, J. T., . . . Forster, S. C. (2022). The future of microbiome‐based therapeutics. Alimentary Pharmacology & Therapeutics , 56 (2), 192–208.
Guzik, P., Kulawik, P., Zając, M., & Migdał, W. (2022). Microwave applications in the food industry: An overview of recent developments. Critical Reviews in Food Science and Nutrition, 62 (29), 7989–8008.
Hebbar, K. B., Pandiselvam, R., Manikantan, M. R., Arivalagan, M., Beegum, S., & Chowdappa, P. (2018). Palm sap—quality profiles, fermentation chemistry, and preservation methods. Sugar Tech, 20 (6), 621–634.
Hinds, L. M., O’Donnell, C. P., Akhter, M., & Tiwari, B. K. (2019). Principles and mechanisms of ultraviolet light emitting diode technology for food industry applications. Innovative Food Science & Emerging Technologies, 56 , 102153.
Hossain, K. S., Amarasena, S., & Mayengbam, S. (2022). B vitamins and their roles in gut health. Microorganisms, 10 (6), 1168.
Indiarto, R., & Rezaharsamto, B. (2020). A review on ohmic heating and its use in food. International Journal of Scientific & Technology Research, 9 (2), 485–490.
Indira, M., John Babu, D., Chaitanya, M., & Kodali, V. P. (2015a). Isolation and characterization of bacteriocin producing lactic acid bacteria from curd. International Journal of Chem Tech Research, 8 (1), 388–396.
Indira, M., Venkateswarulu, T. C., Chakravarthy, K., Reddy, A. R., & Prabhakar, K. V. (2015b). Isolation and characterization of bacteriocin producing lactic acid bacteria from dairy effluent. Research Journal of Pharmacy and Technology, 8 (11), 1560–1565.
Indira, M., Venkateswarulu, T. C., Chakravarthy, K., Reddy, A. R., Babu, D. J., & Kodali, V. P. (2016). Morphological and biochemical characterization of exopolysaccharide producing bacteria isolated from dairy effluent. Journal of Pharmaceutical Sciences and Research, 8 (2), 88.
Indira, M., Venkateswarulu, T. C., Prabhakar, K. V., Peele, K. A., & Krupanidhi, S. (2018). Isolation and characterization of bacteriocin producing Enterococcus casseliflavus and its antagonistic effect on Pseudomonas aeruginosa . Karbala International Journal of Modern Science, 4 (4), 361–368.
Indira, M., Venkateswarulu, T. C., Abraham Peele, K., Nazneen Bobby, M., & Krupanidhi, S. (2019a). Bioactive molecules of probiotic bacteria and their mechanism of action: a review. 3 Biotech , 9 (8), 306.
Indira, M., Venkateswarulu, T. C., Peele, K. A., Prabhakar, K. V., & Krupanidhi, S. (2019b). Characterization of bacteriocin producing probiotic properties of Enterococcus casseliflavus MI001 isolated from curd sample. Current Trends in Biotechnology and Pharmacy, 13 (1), 64–71.
Jadhav, H. B., Annapure, U. S., & Deshmukh, R. R. (2021). Non-thermal technologies for food processing. Frontiers in Nutrition, 8 , 657090.
Article PubMed PubMed Central Google Scholar
James, A., & Wang, Y. (2019). Characterization, health benefits and applications of fruits and vegetable probiotics. CyTA-Journal of Food, 17 (1), 770–780.
Jan, B., Shams, R., Rizvi, Q. E. H., & Manzoor, A. (2021). Ohmic heating technology for food processing: A review of recent developments. Journal of Postharvest Technology, 9 (1), 20–34.
Jeong, S. G., Yang, J. E., Park, J. H., Ko, S. H., Choi, I. S., Kim, H. M., . . . Park, H. W. (2020). Gamma irradiation improves the microbiological safety and shelf-life of kimchi seasoning mixture. LWT , 134 , 110144.
Jiang, J., Zhang, M., Bhandari, B., & Cao, P. (2020). Current processing and packing technology for space foods: A review. Critical Reviews in Food Science and Nutrition, 60 (21), 3573–3588.
Johar, V., & Kumar, R. (2020). Mahua: A versatile Indian tree species. Journal of Pharmacognosy and Phytochemistry, 9 (6), 1926–1931.
Jovel, J., Dieleman, L. A., Kao, D., Mason, A. L., & Wine, E. (2018). The human gut microbiome in health and disease. Metagenomics , 197–213.
Kandasamy, S., Kavitake, D., & Shetty, P. H. (2018). Lactic acid bacteria and yeasts as starter cultures for fermented foods and their role in commercialization of fermented foods. In Innovations in technologies for fermented food and beverage industries , 25–52, Springer Cham.
Kannan, V., Rangarajan, V., Manjare, S. D., & Pathak, P. V. (2021). Microbial production of value-added products from cashew apples-an economical boost to cashew farmers. Journal of Pure and Applied Microbiology, 15 (4), 1816–1832.
Kato-Kataoka, A., Nishida, K., Takada, M., Suda, K., Kawai, M., Shimizu, K., . . . Rokutan, K. (2016). Fermented milk containing Lactobacillus casei strain Shirota prevents the onset of physical symptoms in medical students under academic examination stress. Beneficial Microbes , 7 (2), 153–156.
Kayitesi, E., Onojakpor, O., & Moyo, S. M. (2023). Highlighting the impact of lactic-acid-bacteria-derived flavours or aromas on sensory perception of African fermented cereals. Fermentation, 9 (2), 111.
Khakholary, S. (2020). Beer and its socio-cultural and religious importance in Ancient Egypt. PalArch’s Journal of Archaeology of Egypt/egyptology, 17 (7), 14382–14386.
Khapudang, R., Sharma, S., & Joshi, V. K. (2018). Indigenous fermented food products of Eastern and Western Himalayan Region: A review. International Journal of Food and Fermentation Technology, 8 (2), 119–139.
Khatun, L., & Ray, S. (2022). A comprehensive review on rice based fermented food and beverages in North East India. The Pharma Innovation Journal, 11 (2), 370–375.
Kiefer, A. M., Seney, C. S., Lambright, A. L., Cottrill, K. A., & Young, V. A. (2018). Makgeolli: Rapid production of an alcoholic beverage from the fermentation of rice. Journal of Microbiology & Biology Education, 19 (2), 19–22.
Knez, E., Kadac-Czapska, K., & Grembecka, M. (2023). Effect of fermentation on the nutritional quality of the selected vegetables and legumes and their health effects. Life, 13 (3), 655.
Kodali, V. P., Lingala, V. K., Karlapudi, A. P., Indira, M., Venkateswarulu, T. C., & John Babu, D. (2013). Biosynthesis and potential application of bacteriocins. Journal of Pure & Applied Microbiology, 7 , 2933–2945.
Kondybayev, A., Loiseau, G., Achir, N., Mestres, C., & Konuspayeva, G. (2021). Fermented mare milk product (Qymyz, Koumiss). International Dairy Journal, 119 , 105065.
Kori, R. K., & Gupta, D. (2018). Alcohol and its induced aura that imposed adverse effects on socio-economic, neurobehavioral and biological health in Indians. Alcohol , 3 (1).
Kusumoto, K. I., Yamagata, Y., Tazawa, R., Kitagawa, M., Kato, T., Isobe, K., & Kashiwagi, Y. (2021). Japanese traditional miso and koji making. Journal of Fungi, 7 (7), 579.
Ladokun, O. A., Oni, S., Umezurike, E. T., Durosinlorun, O. O., Arojojoye, O. A., Bamisaye, A., & Adeosun, A. M. (2022). Physicochemical properties, microbial profile, and antipyretic efficacy of some lactic fermented liquids obtained from some cereals. Applied Food Research, 2 (1), 100101.
Lee, M., Regu, M., & Seleshe, S. (2015). Uniqueness of Ethiopian traditional alcoholic beverage of plant origin, tella. Journal of Ethnic Foods, 2 (3), 110–114.
Leeuwendaal, N. K., Stanton, C., O’Toole, P. W., & Beresford, T. P. (2022). Fermented foods, health and the gut microbiome. Nutrients, 14 (7), 1527.
Li, K. J., Burton-Pimentel, K. J., Vergères, G., Feskens, E. J., & Brouwer-Brolsma, E. M. (2022). Fermented foods and cardiometabolic health: definitions, current evidence, and future perspectives. Frontiers in Nutrition , 2214.
Luo, C., & Deng, S. (2016). Viili as fermented food in health and disease prevention: A review study. Journal of Agricultural Science and Food Technology, 2 (7), 105–113.
Macwan, S. R., Dabhi, B. K., Parmar, S. C., & Aparnathi, K. D. (2016). Whey and its utilization. International Journal of Current Microbiology and Applied Sciences, 5 (8), 134–155.
Mannaa, M., Han, G., Seo, Y. S., & Park, I. (2021). Evolution of food fermentation processes and the use of multi-omics in deciphering the roles of the microbiota. Foods, 10 (11), 2861.
Marak, S. R., Sharma, D., & Sarma, H. (2021). Ethnic preparation of Chubitchi, an alcoholic beverage of the Garo tribe of Meghalaya: A sociocultural analysis. Journal of Ethnic Foods, 8 (1), 29.
Marco, M. L., Heeney, D., Binda, S., Cifelli, C. J., Cotter, P. D., Foligné, B., . . . Hutkins, R. (2017). Health benefits of fermented foods: microbiota and beyond. Current Opinion in Biotechnology , 44 , 94–102.
Marco, M. L., Sanders, M. E., Gänzle, M., Arrieta, M. C., Cotter, P. D., De Vuyst, L., . . . Hutkins, R. (2021). The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nature Reviews Gastroenterology & Hepatology , 18 (3), 196–208.
Markowiak, P., & Śliżewska, K. (2017). Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients, 9 (9), 1021.
Mashau, M. E., Maliwichi, L. L., & Jideani, A. I. O. (2021). Non-alcoholic fermentation of maize ( Zea mays ) in Sub-Saharan Africa. Fermentation, 7 (3), 158.
McNabney, S. M., & Henagan, T. M. (2017). Short chain fatty acids in the colon and peripheral tissues: A focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients, 9 (12), 1348.
Mehta, B. M. (2015). Nutritional and toxicological aspects of the chemical changes of food components and nutrients during heating and cooking. Handbook of Food Chemistry, 897 , 936.
Melini, F., Melini, V., Luziatelli, F., Ficca, A. G., & Ruzzi, M. (2019). Health-promoting components in fermented foods: An up-to-date systematic review. Nutrients, 11 (5), 1189.
Mengesha, Y., Tebeje, A., & Tilahun, B. (2022). A review on factors influencing the fermentation process of Teff ( Eragrostis teff ) and other cereal-based Ethiopian injera. International Journal of Food Science, 2022 , 4419955.
Mirzaei, R., Afaghi, A., Babakhani, S., Sohrabi, M. R., Hosseini-Fard, S. R., Babolhavaeji, K., . . . Karampoor, S. (2021). Role of microbiota-derived short-chain fatty acids in cancer development and prevention. Biomedicine & Pharmacotherapy , 139 , 111619.
Mishra, A., & Poonia, A. (2019). Mahua ( Madhuca longifolia ) flowers: Review on processing and biological properties. Nutrition & Food Science, 49 (6), 1153–1163.
Mishra, B. K., Balamurugan, N., Hati, S., & Paul, B. (2015). ‘Chubitchi’-The native fermented rice beverage of the Garos’ of West Garo Hills, Meghalaya. International Journal of Fermented Foods , 4 (1and2), 81–90.
Mishra, B. K., Hati, S., Das, S., & Brahma, J. (2019). Fermented rice beverage of north east India: A systematic review. International Journal of Fermented Foods, 8 (1), 41–56.
Mitmesser, S., & Combs, M. (2017). Prebiotics: Inulin and other oligosaccharides. In The Microbiota in Gastrointestinal Pathophysiology , 201–208, Academic Press.
Mota de Carvalho, N., Costa, E. M., Silva, S., Pimentel, L., Fernandes, T. H., & Pintado, M. E. (2018). Fermented foods and beverages in human diet and their influence on gut microbiota and health. Fermentation, 4 (4), 90.
Narzary, Y., Brahma, J., Brahma, C., & Das, S. (2016). A study on indigenous fermented foods and beverages of Kokrajhar, Assam. India. Journal of Ethnic Foods, 3 (4), 284–291.
Nath, N., Ghosh, S., Rahaman, L., Kaipeng, D. L., & Sharma, B. K. (2019). An overview of traditional rice beer of North-east India: Ethnic preparation, challenges and prospects. Indian Journal of Traditional Knowledge, 18 (4), 744–757.
Navdeep, J. P., Ramesh, V., Amit Kumar, S. S., & Dixit, A. (2023). Development of protein enriched whey-bael beverage and its evaluation for antioxidant potential. The Pharma Innovation Journal, 12 (1), 254–259.
Nazir, Y., Hussain, S. A., Abdul Hamid, A., & Song, Y. (2018). Probiotics and their potential preventive and therapeutic role for cancer, high serum cholesterol, and allergic and HIV diseases. BioMed Research International, 2018 , 3428437.
Nicolas, G. R., & Chang, P. V. (2019). Deciphering the chemical lexicon of host–gut microbiota interactions. Trends in Pharmacological Sciences, 40 (6), 430–445.
Nielsen, E. S., Garnås, E., Jensen, K. J., Hansen, L. H., Olsen, P. S., Ritz, C., . . . Nielsen, D. S. (2018). Lacto-fermented sauerkraut improves symptoms in IBS patients independent of product pasteurization–a pilot study. Food & Function , 9 (10), 5323–5335.
Nile, S. H. (2015). The nutritional, biochemical and health effects of makgeolli–a traditional Korean fermented cereal beverage. Journal of the Institute of Brewing, 121 (4), 457–463.
Nishida, H. (2021). Sake brewing and bacteria inhabiting sake breweries. Frontiers in Microbiology, 12 , 602380.
Niu, D., Zeng, X. A., Ren, E. F., Xu, F. Y., Li, J., Wang, M. S., & Wang, R. (2020). Review of the application of pulsed electric fields (PEF) technology for food processing in China. Food Research International, 137 , 109715.
Nkhata, S. G., Ayua, E., Kamau, E. H., & Shingiro, J. B. (2018). Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Science & Nutrition, 6 (8), 2446–2458.
Nogal, A., Valdes, A. M., & Menni, C. (2021). The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes, 13 (1), 1897212.
Nuraida, L. (2015). A review: Health promoting lactic acid bacteria in traditional Indonesian fermented foods. Food Science and Human Wellness, 4 (2), 47–55.
Okuda, M. (2019). Rice used for Japanese sake making. Bioscience, Biotechnology, and Biochemistry, 83 (8), 1428–1441.
Parada Venegas, D., De la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., . . . Hermoso, M. A. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Frontiers in Immunology , 277.
Park, K. Y., Kim, H. Y., & Jeong, J. K. (2017). Kimchi and its health benefits. In Fermented Foods in Health and Disease Prevention , 477–502.
Patel, P., Butani, K., Kumar, A., Singh, S., & Prajapati, B. G. (2023). Effects of fermented food consumption on non-communicable diseases. Foods, 12 (4), 687.
Patra, J. K., Das, G., Paramithiotis, S., & Shin, H. S. (2016). Kimchi and other widely consumed traditional fermented foods of Korea: A review. Frontiers in Microbiology, 7 , 1493.
Pegu, B. K., Sarmah, P., Yein, J., & Sanong, J. (2022). Study on a few traditional fermented food practices of Mising community of Dhemaji District, Upper Assam, India. Asian Journal of Biological and Life Sciences, 11 (3), 841.
Perera, C. O., & Alzahrani, M. A. J. (2021). Ultrasound as a pre-treatment for extraction of bioactive compounds and food safety: A review. LWT, 142 , 111114.
Petrova, P., & Petrov, K. (2017). Traditional cereal beverage boza fermentation technology, microbial content and healthy effects. In Fermented Foods , 284–305, CRC Press.
Pswarayi, F., & Ganzle, M. G. (2019). Composition and origin of the fermentation microbiota of mahewu, a Zimbabwean fermented cereal beverage. Applied and Environmental Microbiology, 85 (11), e03130-e3218.
Qiu, L., Zhang, M., Tang, J., Adhikari, B., & Cao, P. (2019). Innovative technologies for producing and preserving intermediate moisture foods: A review. Food Research International, 116 , 90–102.
Ramires, F. A., Bleve, G., De Domenico, S., & Leone, A. (2022). Combination of solid state and submerged fermentation strategies to produce a new jellyfish-based food. Foods, 11 (24), 3974.
Rawat, J. M., Pandey, S., Debbarma, P., & Rawat, B. (2021). Preparation of alcoholic beverages by tribal communities in the Indian Himalayan region: A review on traditional and ethnic consideration. Frontiers in Sustainable Food Systems, 5 , 672411.
Ray, M., Ghosh, K., Singh, S., & Mondal, K. C. (2016a). Folk to functional: An explorative overview of rice-based fermented foods and beverages in India. Journal of Ethnic Foods, 3 (1), 5–18.
Ray, S., Bagyaraj, D. J., Thilagar, G., & Tamang, J. P. (2016b). Preparation of Chyang, an ethnic fermented beverage of the Himalayas, using different raw cereals. Journal of Ethnic Foods, 3 (4), 297–299.
Ren, S., Wang, C., Chen, A., Bai, Z., Tian, Y., & Lv, W. (2023). Lactobacillus paracasei influences the gut-microbiota-targeted metabolic modulation of the immune status of diarrheal mice. Food & Function, 14 (9), 4368–4379.
Rezac, S., Kok, C. R., Heermann, M., & Hutkins, R. (2018). Fermented foods as a dietary source of live organisms. Frontiers in Microbiology, 9 , 1785.
Riaz Rajoka, M. S., Thirumdas, R., Mehwish, H. M., Umair, M., Khurshid, M., Hayat, H. F., . . . Barba, F. J. (2021). Role of food antioxidants in modulating gut microbial communities: novel understandings in intestinal oxidative stress damage and their impact on host health. Antioxidants , 10 (10), 1563.
Romulo, A., & Surya, R. (2021). Tempe: A traditional fermented food of Indonesia and its health benefits. International Journal of Gastronomy and Food Science, 26 , 100413.
Saeed, F., Afzaal, M., Shah, Y. A., Khan, M. H., Hussain, M., Ikram, A., . . . Khashroum, A. O. (2022). Miso: A traditional nutritious & health‐endorsing fermented product. Food Science & Nutrition , 10 (12), 4103–4111.
Sanders, M. E., Merenstein, D., Merrifield, C. A., & Hutkins, R. (2018). Probiotics for Human Use. Nutrition Bulletin, 43 (3), 212–225.
Sarkar, P., Dh, L. K., Dhumal, C., Panigrahi, S. S., & Choudhary, R. (2015). Traditional and ayurvedic foods of Indian origin. Journal of Ethnic Foods, 2 (3), 97–109.
Sarmah, P., Pegu, B. K., & Sanong, J. (2022). Documentation of traditional method of preparation and characterization of Judima, a traditional rice beer of Dimasa Tribe of Assam and its comparison with old preserved Judima. Biosciences Biotechnology Research Asia, 19 (4), 1133–1138.
Sehrawat, R., Kaur, B. P., Nema, P. K., Tewari, S., & Kumar, L. (2021). Microbial inactivation by high pressure processing: Principle, mechanism and factors responsible. Food Science and Biotechnology, 30 , 19–35.
Article PubMed Google Scholar
Senanayake, D., Torley, P. J., Chandrapala, J., & Terefe, N. S. (2023). Microbial fermentation for improving the sensory, nutritional and functional attributes of legumes. Fermentation, 9 (7), 635.
Sha, S. P., Jani, K., Sharma, A., Anupma, A., Pradhan, P., Shouche, Y., & Tamang, J. P. (2017). Analysis of bacterial and fungal communities in Marcha and Thiat, traditionally prepared amylolytic starters of India. Scientific Reports, 7 (1), 10967.
Shah, A. M., Tarfeen, N., Mohamed, H., & Song, Y. (2023). Fermented foods: Their health-promoting components and potential effects on gut microbiota. Fermentation, 9 (2), 118.
Sharma, R., Garg, P., Kumar, P., Bhatia, S. K., & Kulshrestha, S. (2020). Microbial fermentation and its role in quality improvement of fermented foods. Fermentation, 6 (4), 106.
Sharma, R., Rashidinejad, A., & Jafari, S. M. (2022). Application of spray dried encapsulated probiotics in functional food formulations. Food and Bioprocess Technology, 15 (10), 2135–2154.
Shimoga, G., & Kim, S. Y. (2021). Makgeolli - The traditional choice of korean fermented beverage from cereal: An overview on its composition and health benefits. Food Science and Technology, 42 , 1–10.
Siddeeg, A., Afzaal, M., Saeed, F., Ali, R., Shah, Y. A., Shehzadi, U., . . . Al-Farga, A. (2022). Recent updates and perspectives of fermented healthy super food sauerkraut: a review. International Journal of Food Properties , 25 (1), 2320–2331.
Silva, R., Rocha, R. S., Guimarães, J. T., Balthazar, C. F., Pimentel, T. C., Neto, R. P., . . . Cruz, A. G. (2020a). Advantages of using ohmic heating in Dulce de Leche manufacturing. Innovative Food Science & Emerging Technologies , 65(4) , 102475.
Silva, Y. P., Bernardi, A., & Frozza, R. L. (2020b). The role of short-chain fatty acids from gut microbiota in gut-brain communication. Frontiers in Endocrinology, 11 , 25.
Smanalieva, J., Iskakova, J., & Musulmanova, M. (2022). Milk-and cereal-based Kyrgyz ethnic foods. International Journal of Gastronomy and Food Science, 29 , 100507.
Soemarie, Y. B., Milanda, T., & Barliana, M. I. (2021). Fermented foods as probiotics: A review. Journal of Advanced Pharmaceutical Technology & Research, 12 (4), 335.
Soni, A., Smith, J., Thompson, A., & Brightwell, G. (2020). Microwave-induced thermal sterilization-a review on history, technical progress, advantages and challenges as compared to the conventional methods. Trends in Food Science & Technology, 97 , 433–442.
Speranza, B., Racioppo, A., Bevilacqua, A., Buzzo, V., Marigliano, P., Mocerino, E., . . . Sinigaglia, M. (2021). Innovative preservation methods improving the quality and safety of fish products: beneficial effects and limits. Foods , 10 (11), 2854.
Sujana, K., Abraham, K. P., Mikkili, I., & Prabhakar, K. V. (2013). Biochemical and molecular characterization of biofilm producing bacteria. International Journal of Pharma and Biosciences, 4 , 702–712.
Sun, W., Shahrajabian, M. H., & Lin, M. (2022). Research progress of fermented functional foods and protein factory-microbial fermentation technology. Fermentation, 8 (12), 688.
Surya, R., & Lee, A. G. Y. (2022). Exploring the philosophical values of kimchi and kimjang culture. Journal of Ethnic Foods, 9 (1), 1–14.
Susila, A. V., & Sangeeta, S. (2021). Decalepis hamiltonii (swallow root) as a potential antimicrobial agent against endodontic pathogens: An in vitro study. Journal of Operative Dentistry and Endodontics, 6 (1), 2.
Tamang, J. P. (2022). Dietary culture and antiquity of the Himalayan fermented foods and alcoholic fermented beverages. Journal of Ethnic Foods, 9 (1), 30.
Tamang, J. P., Shin, D. H., Jung, S. J., & Chae, S. W. (2016). Functional properties of microorganisms in fermented foods. Frontiers in Microbiology, 7 , 578.
Terpou, A., Papadaki, A., Lappa, I. K., Kachrimanidou, V., Bosnea, L. A., & Kopsahelis, N. (2019). Probiotics in food systems: Significance and emerging strategies towards improved viability and delivery of enhanced beneficial value. Nutrients, 11 (7), 1591.
Thursby, E., & Juge, N. (2017). Introduction to the human gut microbiota. Biochemical Journal, 474 (11), 1823–1836.
Tichoniuk, M. (2019). Novel packaging materials improving food quality and extending its shelf Life. Towaroznawcze Problemy Jakości, 1 , 21–35.
Tsafrakidou, P., Michaelidou, A. M., Biliaderis, G., & C. (2020). Fermented cereal-based products: Nutritional aspects, possible impact on gut microbiota and health implications. Foods, 9 (6), 734.
Upadhyaya, A., & Sonawane, S. K. (2022). Palmyrah palm and its products (neera, jaggery and candy)–a review on chemistry and technology. Applied Food Research , 100256.
Uzay, M., Öztürk, H. İ, Buzrul, S., & Maskan, M. (2021). A study on rheological properties, sensory evaluation and shelf life of ayran–shalgam mixtures. Journal of Food Science and Technology, 58 (7), 2479–2486.
Uzel, R. A. (2018). Microwave-assisted green extraction technology for sustainable food processing. Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing , 159 .
Vieira, C. P., Rosario, A. I. L., Lelis, C. A., Rekowsky, B. S. S., Carvalho, A. P. A., Rosário, D. K. A., . . . Conte-Junior, C. A. (2021). Bioactive compounds from kefir and their potential benefits on health: a systematic review and meta-analysis. Oxidative Medicine and Cellular Longevity , 2021, 9081738.
Vivek, K., Mishra, S., Pradhan, R. C., Nagarajan, M., Kumar, P. K., Singh, S. S., . . . Gowda, N. N. (2022). A comprehensive review on microencapsulation of probiotics: technology, carriers and current trends. Applied Food Research , 100248.
Voidarou, C., Antoniadou, M., Rozos, G., Tzora, A., Skoufos, I., Varzakas, T., . . . Bezirtzoglou, E. (2020). Fermentative foods: microbiology, biochemistry, potential human health benefits and public health issues. Foods , 10 (1), 69.
Wang, Z., Huang, J., Ma, S., Wang, X., Sun, B., Wang, F., . . . Bao, Q. (2021). Novel heating technologies to improve fermentation efficiency and quality in wheat products: a short review. Grain & Oil Science and Technology , 4 (2), 81–87.
Waters, D. M., Mauch, A., Coffey, A., Arendt, E. K., & Zannini, E. (2015). Lactic acid bacteria as a cell factory for the delivery of functional biomolecules and ingredients in cereal-based beverages: A review. Critical Reviews in Food Science and Nutrition, 55 (4), 503–520.
Wei, Q., Mei, J., & Xie, J. (2022). Application of electron beam irradiation as a non-thermal technology in seafood preservation. LWT, 169 , 113994.
Wong, B., Muchangi, K., Quach, E., Chen, T., Owens, A., Otter, D., . . . Kam, R. (2023). Characterization of Korean rice wine (makgeolli) prepared by different processing methods. Current Research in Food Science , 6 , 100420.
Wu, Y., Mu, R., Li, G., Li, M., & Lv, W. (2022). Research progress in fluid and semifluid microwave heating technology in food processing. Comprehensive Reviews in Food Science and Food Safety, 21 (4), 3436–3454.
Xiang, H., Sun-Waterhouse, D., Waterhouse, G. I., Cui, C., & Ruan, Z. (2019). Fermentation-enabled wellness foods: A fresh perspective. Food Science and Human Wellness, 8 (3), 203–243.
Yang, M., Liang, Z., Wang, L., Qi, M., Luo, Z., & Li, L. (2020). Microencapsulation delivery system in food industry—challenge and the way forward. Advances in Polymer Technology, 2020 , 7531810.
Yen, Y. F., & Chen, S. D. (2022). Influence of radio frequency heating on the pasteurization and drying of solid-state fermented Wolfiporia cocos products. Foods, 11 (12), 1766.
Yu, T., Niu, L., & Iwahashi, H. (2020). High-pressure carbon dioxide used for pasteurization in food industry. Food Engineering Reviews, 12 (3), 364–380.
Article PubMed Central CAS Google Scholar
Yu, Z., Su, Y., Zhang, Y., Zhu, P., Mei, Z., Zhou, X., & Yu, H. (2021). Potential use of ultrasound to promote fermentation, maturation, and properties of fermented foods: A review. Food Chemistry, 357 , 129805.
Yuan, S., Li, C., Zhang, Y., Yu, H., Xie, Y., Guo, Y., & Yao, W. (2021). Ultrasound as an emerging technology for the elimination of chemical contaminants in food: A review. Trends in Food Science & Technology, 109 , 374–385.
Yucesoy, D., & Ozen, B. (2013). Authentication of a Turkish traditional aniseed flavoured distilled spirit, raki. Food Chemistry, 141 (2), 1461–1465.
Zabat, M. A., Sano, W. H., Wurster, J. I., Cabral, D. J., & Belenky, P. (2018). Microbial community analysis of sauerkraut fermentation reveals a stable and rapidly established community. Foods, 7 (5), 77.
Zhang, J., Iqbal, A., Murtaza, A., Zhou, X., Xu, X., Pan, S., & Hu, W. (2021). Effect of high-pressure carbon dioxide on the browning inhibition of sugar-preserved orange peel. Journal of CO2 Utilization , 46 , 101467.
Zhang, K., Wu, W., & Yan, Q. (2020). Research advances on sake rice, koji, and sake yeast: A review. Food Science & Nutrition, 8 (7), 2995–3003.
Zheng, D., Liwinski, T., & Elinav, E. (2020). Interaction between microbiota and immunity in health and disease. Cell Research, 30 (6), 492–506.
Zommiti, M., Feuilloley, M. G., & Connil, N. (2020). Update of probiotics in human world: A nonstop source of benefactions till the end of time. Microorganisms, 8 (12), 1907.
Zuntar, I., Petric, Z., Bursać Kovačević, D., & Putnik, P. (2020). Safety of probiotics: Functional fruit beverages and nutraceuticals. Foods, 9 (7), 947.
Download references
Acknowledgements
The authors would like to acknowledge the Department of Biotechnology, School of Biotechnology and Pharmaceutical Sciences, Vignan’s Foundation for Science Technology and Research, for providing the platform to carry out the research work.
Author information
Authors and affiliations.
Department of Biotechnology, Vignan’s Foundation for Science Technology and Research, Vadlamudi, 522213, Guntur, Andhra Pradesh, India
Sudarsini B, Venkateswarulu T. C, Krupanidhi S & Indira M
Department of Chemical Engineering, Vignan’s Foundation for Science Technology and Research, Vadlamudi, 522213, Guntur, Andhra Pradesh, India
Sumalatha B
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: Sudarsini B and Indira M; writing and figure preparation: Sudarsini B and Indira M; review and editing: Venkateswarulu TC, Krupanidhi S, and Sumalatha B.
Corresponding author
Correspondence to Indira M .
Ethics declarations
Ethics approval.
Not applicable.
Consent to Participate
Informed consent was not applicable.
Conflict of Interest
The authors declare no competing interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
B, S., C, V.T., S, K. et al. Advancing Fermented Food Products: Exploring Bioprocess Technologies and Overcoming Challenges. Food Bioprocess Technol (2023). https://doi.org/10.1007/s11947-023-03287-8
Download citation
Received : 08 June 2023
Accepted : 04 December 2023
Published : 30 December 2023
DOI : https://doi.org/10.1007/s11947-023-03287-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fermented foods
- Gut microbiome
- Inflammation
- Metabolites
- Find a journal
- Publish with us
- Track your research
Fiber and fermented foods may aid microbiome, overall health

May 10, 2024 — It’s well-known that eating a diet rich in fiber and fermented foods fosters healthy digestion, but research suggest that these foods may offer additional health benefits.
According to an April 26 Harvard Health Publishing article, high-fiber foods such as vegetables and whole grains can help with weight control and lower levels of LDL, or “bad” cholesterol, and promote a healthy gut microbiome —which ultimately may help reduce inflammation linked to increased risk of developing chronic conditions including heart disease, type 2 diabetes, and some cancers. Fermented foods such as yogurt and kimchi contain probiotics and prebiotics—good bacteria and food for good bacteria—which help promote a healthy gut microbiome.
Individual fiber needs can vary. Eric Rimm , professor in the Departments of Epidemiology and Nutrition , suggested in the article that people focus on adding more fiber-rich foods to their diets rather than trying to track daily amounts. It’s ok to add fiber supplements, he said, but whole foods should be the primary source of dietary fiber.
There are no recommended daily amounts for probiotics or prebiotics. Rimm said that fermented foods are a better source of these nutrients than dietary supplements, which do not require FDA approval and are not guaranteed to contain the ingredients and benefits listed on their labels.
Read the Harvard Health Publishing article: How—and why—to fit more fiber and fermented food into your meals
Photo: iStock / courtneyk
Health benefits of fermented foods
Affiliations.
- 1 a Biruni University, Faculty of Health Sciences , Nutrition and Dietetics Department , İstanbul , Turkey.
- 2 b Gazi University, Faculty of Health Sciences , Nutrition and Dietetics Department , Ankara , Turkey.
- 3 c Gazi University, Faculty of Tourism , Department of Gastronomy and Culinary Art , Gölbaşı/Ankara , Turkey.
- PMID: 28945458
- DOI: 10.1080/10408398.2017.1383355
In the past, the beneficial effects of fermented foods on health were unknown, and so people primarily used fermentation to preserve foods, enhance shelf life, and improve flavour. Fermented foods became an important part of the diet in many cultures, and over time fermentation has been associated with many health benefits. Because of this, the fermentation process and the resulting fermented products have recently attracted scientific interest. In addition, microorganisms contributing to the fermentation process have recently been associated with many health benefits, and so these microorganisms have become another focus of attention. Lactic acid bacteria (LAB) have been some of the most studied microorganisms. During fermentation, these bacteria synthesize vitamins and minerals, produce biologically active peptides with enzymes such as proteinase and peptidase, and remove some non-nutrients. Compounds known as biologically active peptides, which are produced by the bacteria responsible for fermentation, are also well known for their health benefits. Among these peptides, conjugated linoleic acids (CLA) have a blood pressure lowering effect, exopolysaccharides exhibit prebiotic properties, bacteriocins show anti-microbial effects, sphingolipids have anti-carcinogenic and anti-microbial properties, and bioactive peptides exhibit anti-oxidant, anti-microbial, opioid antagonist, anti-allergenic, and blood pressure lowering effects. As a result, fermented foods provide many health benefits such as anti-oxidant, anti-microbial, anti-fungal, anti-inflammatory, anti-diabetic and anti-atherosclerotic activity. However, some studies have shown no relationship between fermented foods and health benefits. Therefore, this paper aims to investigate the health effects of fermented foods.
Keywords: Fermented food; anti-carcinogenic; bioactive peptides; cardiovascular disease; lactic acid bacteria.
Publication types
- Anti-Infective Agents / analysis
- Anti-Inflammatory Agents / analysis
- Antifungal Agents / analysis
- Antioxidants / analysis
- Atherosclerosis / prevention & control
- Cultured Milk Products / analysis
- Fermentation
- Fermented Foods* / analysis
- Health Promotion*
- Hypoglycemic Agents / analysis
- Lactobacillales / metabolism
- Meat Products
- Minerals / metabolism
- Vitamins / biosynthesis
- Anti-Infective Agents
- Anti-Inflammatory Agents
- Antifungal Agents
- Antioxidants
- Hypoglycemic Agents

Showbizz Daily (English)
Fermented foods: The easy and delicious way to boost your health
Posted: January 5, 2024 | Last updated: April 26, 2024

What are fermented foods?
Fermented foods have been a staple of diets worldwide for thousands of years. They're made by letting bacteria, yeast, or fungi work their magic on various foods and drinks, producing delicious tangy, sour, or fizzy results that scientists now say can boost health and even longevity.

What's good for the gut is good for you
The latest scientific research shows that eating fermented food can change the makeup of the ecosystem of trillions of bacteria, viruses and fungi living in your gut, which is collectively known as the gut microbiome.

A landmark study shows fermented foods reduce inflammation
A 2021 Stanford University study looked at how fermented foods affected healthy adults. Half of a group of 36 were told to eat more fiber-rich plant foods like fruit and veggies, while the other half started eating a lot of fermented foods. After ten weeks, the fermented food group saw big reductions in inflammatory proteins associated with diseases like diabetes and arthritis.
Higher levels of gut microbiome diversity
Unspuringly, the guts of the people eating fermented foods also began to host more diverse microbes. The same study found more fermented foods were associated with more biodiversity. However, it wasn't only that the specific probiotics consumed started growing in the intestines. Instead, the fermented foods seemed to recruit a whole array of different microbes to the gut.

Specific food studies
While there are tons of different fermented foods, and it's a fairly new realm of study, a 2022 meta-analysis published in the journal Nutrients highlighted some of the most solid scientific evidence around the specific health effects of fermented foods so far.

Yogurt is associated with a reduced risk of type 2 diabetes
A study that followed nearly 4,000 people for decades found that dairy products didn't impact the chances of developing type 2 diabetes. That's good news in itself for dairy lovers, but yogurt was even linked to a reduced risk of developing the disease.

Fermented dairy products are good for longevity
A study that followed more than 4,500 people for a decade found that fermented dairy products like cheese, kefir or yogurt were inversely associated with overall mortality. In other words, people who consumed them more died less (during the study period).

Fermented dairy could help prevent cardiovascular disease
Population studies from around the world have found that consuming fermented dairy foods like cheese and yogurt is associated with a reduced risk for cardiovascular disease, according to a 2015 paper in the British Journal of Nutrition.

Fermented milk is good for muscle soreness
A 2013 Japanese study found that a fermented milk product alleviated muscle soreness and related biological markers after high-intensity exercise in a group of healthy young men.

Kimchi was linked to anti-diabetic and anti-obesity effects
One study found fermented kimchi to be beneficial in a group of people with pre-diabetes, even compared to those who ate fresh kimchi. Another study found consuming fermented kimchi reduced body weight and boosted the metabolism in overweight and obese people.

Potential effects on your mental health
In recent years, scientists have found strong links between the gut biome and mental health and cognitive performance. While studies are few, a 45-person review found people eating more fiber, prebiotics (like oatmeal) and fermented foods reported feeling less stressed than a control group on a different diet. Another 2015 study found fermented food consumption was linked to less social anxiety.

Miso: Calmness, lower blood pressure and decreased heart rate
A 2020 Japanese study found that miso, a fermented soybean paste often used for soup, can lower blood pressure, decrease heart rate and calm nerve activity even though it has a high salt content. That's interesting because salt is generally known to provoke the opposite effects.

Natto: Linked to reduced blood clots, lower blood pressure and increased nutrient absorption
Natto is another soy-based fermented food rich in protein, vitamins and minerals. But most health benefits are found in the nattokinase enzyme produced during fermentation. Studies link it to reduced blood clots, lower blood pressure, increased bone strength, and increased nutrient absorption.

Tempeh: Another fermented soy product from Japan linked to longevity
After controlling for other diet components, hypertension, diabetes, smoking, alcohol intake and other factors, scientists looking at nearly 100,000 Japanese people for 15 years found that 10% of people who ate the most fermented soy like tempeh, miso and natto compared with those in the lowest one-fifth for fermented soy intake had a 10% lower risk of death.
Photo: Ella Olsson/Unsplash

Kombucha: Fewer studies but a promising fermented drink
Besides evidence to support the power of fermented foods in general, kombucha, a fermented tea, hasn't been deeply studied. According to the Mayo Clinic, some research suggests kombucha tea may support a healthy immune system and prevent constipation, but more evidence is needed.

Pickles and sauerkraut: Check to see if they're pasteurized
These two beloved staples are both packing fiber, but they can either come with the benefits of fermented foods… or not. As a rule, if you buy them un-refrigerated, they are probably pasteurized, a heating process that kills all the probiotics. If unpasteurized pickles and sauerkraut are unavailable at the store, you can make them at home.

Democratized health: DYI fermented food
Pretty much any whole food can be fermented in your home on the cheap. "What I like about fermented foods is that they democratize science. They don't really cost much, and you don't have to get them from some fancy store. You can do it yourself," John Cryan, a professor of anatomy and neuroscience at University College Cork, told BBC.

Olives: Fermented but commonly pasteurized
Olives are one of the oldest fermented foods in the Mediterranean area. While some traditionally cured olives are available, many are often pasteurized too. That means they won't all carry the same probiotic punch as seen with other fermented foods. However, they have still been linked to numerous health benefits.

Fermented breads like sourdough are also promising
Again, studies are really just getting started, but a 2022 experiment in mice showed potential health benefits of sourdough bread, such as cholesterol reduction, inflammation alleviation, and healthy gut microbiota maintenance when compared to non-fermented but similar white bread.
Photo: Vicky Ng/Unsplash

Beer is fermented. Does that mean it's a superfood?
Yes, beer is fermented, but that doesn't mean that guzzling pints is good for your health. Most beers are pasteurized, which kills all bacteria, including the beneficial probiotics. However, some contain live bacteria, and studies have shown moderate consumption could boost gut microbiota. However, that must be balanced with the negative effects of alcohol.
Photo: Timothy Dykes/Unsplash

What about wine?
Wine is also made by fermenting grapes, but sulfites can inhibit the growth of beneficial probiotics. A 2019 study by Kings College London found red wine (not white) was associated with increased gut microbiota diversity, but suggested that the high levels of polyphenols, not probiotics, could be responsible for much of wine's controversial health benefits when used in moderation.
Photo: Nacho Domínguez Argenta/Unsplash
More for You
Russia accidentally bombs its own people
'Immortal' Zelensky already escaped ten assassination attempts

20 cars not as good as you think

'Fraudulent applications': Philippines to tighten tourist visa rules for Chinese
Russian casualty figures have declined but are set for massive growth
Eurovision: Dutch entrant Joost Klein excluded from final
Kitchen items you should get rid of today
Mikel Obi returns to Chelsea
What the colors you wear reveal about your personality
China urged to watch out as US chases AI-driven F-16 fighter jets for air combat of the future
US announces 370 million euro package of weapons for Ukraine
Ranked: the largest engine made by every major car company
Which ultra-processed foods are the worst for your health?
Dortmund vs. Real Madrid: the Champions League final's first major controversy
The most luxurious casinos in the world

NY: PEOPLE: Celebrating 50 Years exhibition opening - 53098679
Why I defected to APC – Ex-Abia Speaker, Orji
‘If the Russians go through Ukraine, do you think they won't go to Europe?’ - on Odesa’s front line
The best modern Audis ever made
Liverpool: Jurgen Klopp admits he ‘regrets’ his use of one Reds star during his farewell season

IMAGES
VIDEO
COMMENTS
1. Introduction. Fermented foods are defined as "foods or beverages produced through controlled microbial growth, and the conversion of food components through enzymatic action" [].Many foods have historically undergone fermentation, including meat and fish, dairy, vegetables, soybeans, other legumes, cereals and fruits.
1. Introduction. Fermented foods have been a component of the human diet from ancient times. Among the earliest evidence for the deliberate application of fermentation has been found in pottery vessels discovered in China dating from 7000 BC that were used to ferment rice, honey, and fruit [].However, it is likely that inadvertent production and consumption of fermented foods significantly ...
Fermented foods have long been produced according to knowledge passed down from generation to generation and with no understanding of the potential role of the microorganism(s) involved in the process. ... The research papers which were admitted for analysis were published in the last three years, so as to obtain a very up-to-date systematic ...
In the present review the latest studies on fermented foods are summarized. Special attention has been paid on the health benefits of main fermented foods available nowadays, the principal bioactive compounds responsible for such properties as well as the future trends of research studies regarding their potentialities.
Fermented foods are defined as foods or beverages produced through controlled microbial growth, and the conversion of food components through enzymatic action. In recent years, fermented foods have undergone a surge in popularity, mainly due to their proposed health benefits. The aim of this review …
Although fermented foods have been consumed for thousands of years, a clear definition has been lacking. ... Box 2 Key conclusions of this consensus paper. ... Teagasc Food Research Centre ...
Special attention has been paid on the health benefits of main fermented foods available nowadays, the principal bioactive compounds responsible for such properties as well as the future trends of research studies regarding their potentialities. This review emphasizes the need of clinical evidence to ensure that fermented foods may entail a ...
A study shows how fermented foods such as keffir and kimchi enhance microbiome diversity and reduce markers of inflammation. It is well known that diet influences the gut microbiome, which in turn ...
Introduction. Fermentation has long been used to preserve and enhance the shelf-life, flavor, texture, and functional properties of food (Hutkins, 2018).More recently, the consumption of fermented foods containing live microorganisms has emerged as an important dietary strategy for improving human health (Marco et al., 2017).In general, lactic acid bacteria (LAB) from several genera, including ...
The potential for fermented foods to positively impact the gut. microbiome suggests that further research is requir ed on this topic in order to establish. whether fermented foods can in fact be ...
Among all food fermentations (e.g., Fermented Foods: Past, Present and Future 15. milk, meat, fi sh, vegetables, soya or fruits), cereal fermentations reach the. highest volume (Brandt 2014). The ...
Fermented foods have been a part of human diet for almost 10,000 years, and their level of diversity in the 21st century is substantial. The health benefits of fermented foods have been intensively investigated; identification of bioactive peptides and microbial metabolites in fermented foods that can positively affect human health has consolidated this interest.
Fermented foods and beverages are usually enzymatic transformations of nutrient components, including microbial organisms. ... The papers cited involving animal research have been carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments, or the ...
For thousands of years, humans have enjoyed the novel flavors, increased shelf-life, and nutritional benefits that microbes provide in fermented foods and beverages. Recent sequencing surveys of ferments have mapped patterns of microbial diversity across space, time, and production practices. But a mechanistic understanding of how fermented food microbiomes assemble has only recently begun to ...
A diet rich in fermented foods enhances the diversity of gut microbes and decreases molecular signs of inflammation, according to researchers at the Stanford School of Medicine. In a clinical trial, 36 healthy adults were randomly assigned to a 10-week diet that included either fermented or high-fiber foods.
So far, only pediosin and nisin have received official approvals as food additives, yet the research in that scientific field is ongoing and the future of bacteriocins in the food industry is promising . 5.2. Antibiotic Resistance ... Fermented foods and beverages are produced worldwide, and they are valued for their sensorial and nutritional ...
The food products produced by fermenting them with microorganisms like yeast and bacteria are called fermented food products (Rezac et al., 2018).The uniqueness of the texture, appearance, aroma, and taste are due to the formation of substituting products by breaking the components present in the food (Mehta, 2015).Nowadays, fermented food consumption becoming a health trend in modern society ...
Fermented foods and drinks derived from animals as well as plants play an important role in diets. These foods usually contain lactic acid bacteria (LAB) grown during fermentation, and these naturally contain compounds, including organic acids, ethanol, or antimicrobial compounds with the ability to inhibit spoilage organisms and pathogenic bacteria in fermented foods.
The first documents to report on fermented foods date back to 13,000 BC and are primarily mediated by spontaneous fermentation by autochthonous microorganisms in raw material [].Fermentation is defined as the chemical transformation of any organic matter through microbial metabolism and is mediated by myriad enzymes [].The key advantages of engineering microbial fermentation over multicellular ...
May 10, 2024 — It's well-known that eating a diet rich in fiber and fermented foods fosters healthy digestion, but research suggest that these foods may offer additional health benefits.. According to an April 26 Harvard Health Publishing article, high-fiber foods such as vegetables and whole grains can help with weight control and lower levels of LDL, or "bad" cholesterol, and promote ...
Feature papers represent the most advanced research with significant potential for high impact in the field. ... With an extensive review of potentially cognitively influential changes in fermented foods, this paper may promote microbial and biochemical changes in fermented foods in a comprehensive manner that helps to understand the overall ...
These microbes are either harmless or beneficial, except for B. cereus (can cause food poisoning at >10 6 CFU g −1). The fermented tea would appear to have some advantages over the green tea. However, the presence and density of B. cereus should be monitored.
Ethnic fermented foods of northeast India are classified into fermented soybean and non-soybean legume foods, ... This study was supported by the Agricultural Research Center funded by the Ministry of Food, Forestry, and Fisheries, Republic of Korea. ... Background paper on biodiversity significance of North East India for the study on natural ...
This Special Issue of Foods, titled "Current Research on Probiotics and Fermented Products", offers a comprehensive dive into the current state and future trajectory of this vibrant field. With three review papers and seven research articles, this collection illuminates the multifaceted roles of microorganisms in foods, health, and diseases ...
A review on fermented vegetables: Microbial community and potential upgrading strategy via inoculated fermentation ... National Engineering Research Center for Fruit and Vegetable Processing, Key Laboratory of Fruit and Vegetable Processing, Ministry of Agriculture and Rural Affairs, Beijing Key Laboratory for Food Non-thermal Processing, China ...
2. Classification of Major Types of Fermented Foods and Beverages. Fermentation involves the action of enzymes and catalysts derived from microorganisms such as bacteria, yeast, and moulds for the chemical transformation of the complex organic compounds in the substrate into simpler, bioactive, functional, and nutritious compounds [].There are several classification methods for fermented foods ...
As a result, fermented foods provide many health benefits such as anti-oxidant, anti-microbial, anti-fungal, anti-inflammatory, anti-diabetic and anti-atherosclerotic activity. However, some studies have shown no relationship between fermented foods and health benefits. Therefore, this paper aims to investigate the health effects of fermented ...
Microorganisms have a key role in the production process of YD because it is a fermented food but the effect of microorganisms on the volatile compounds of YD is also not currently clear. RESULTS. In this paper, aroma compounds and microbial diversity in different fermentation stages of YD were analyzed using gas chromatography-mass ...
E-tongue results showed an increase in sourness and decreases in bitterness and astringency in fermented broccoli. The results of HS-GC-IMS revealed that a total of 29 volatile compounds were detected in fermented broccoli. The content of six of 11 aldehydes detected in fermented broccoli increased significantly.
The latest scientific research shows that eating fermented food can change the makeup of the ecosystem of trillions of bacteria, viruses and fungi living in your gut, which is collectively known ...