
Irritable Bowel Syndrome News
Top headlines, latest headlines.
- Effective Dietary Treatment in IBS
- Fiber Discovery Could Shape Better Gut Health
- Irritable Bowel Syndrome: Low Bacterial ...
- Wearable Device Reveals IBS-Related Changes
- Treating Gut Pain
- Gravity May Cause Irritable Bowel Syndrome?
- Bloating Common Issue Among Americans
- Inactive Inflammatory Bowel Disease and ...
- IBS: Specific Diets Less Important Than Expected
- Treating IBS Pain With Help of Tarantula Venom?
Earlier Headlines
Wednesday, july 27, 2022.
- Histamine-Producing Gut Bacteria Can Trigger Chronic Abdominal Pain
- LATEST NEWS
- Health & Medicine
- Diseases & Conditions
- Alzheimer's Research
- Amyotrophic Lateral Sclerosis
- Attention Deficit Disorder
- Back and Neck Pain
- Birth Defects
- Bladder Disorders
- Blood Clots
- COVID and SARS
- Cervical Cancer
- Bladder Cancer
- Multiple Myeloma
- Pancreatic Cancer
- Brain Tumor
- Colon Cancer
- Breast Cancer
- Ovarian Cancer
- Lung Cancer
- Mesothelioma
- Skin Cancer
- Prostate Cancer
- Cerebral Palsy
- Chikungunya
- Chronic Fatigue Syndrome
- Cold and Flu
- Crohn's Disease
- Cystic Fibrosis
- Dengue Fever
- Down Syndrome
- Eating Disorder Research
- Encephalitis
- Epilepsy Research
- Erectile Dysfunction
- Fibromyalgia
- Gastrointestinal Problems
- HIV and AIDS
- Headache Research
- Hearing Loss
- Heart Health
- Cholesterol
- Stroke Prevention
- Heart Disease
- Hormone Disorders
- Hypertension
- Infectious Diseases
- Insomnia Research
- Irritable Bowel Syndrome
- Kidney Disease
- Liver Disease
- Lung Disease
- Lyme Disease
- Mental Health Research
- Multiple Sclerosis Research
- Mumps, Measles, Rubella
- Muscular Dystrophy
- Osteoporosis
- Parkinson's Research
- Prostate Health
- Restless Leg Syndrome
- Sickle Cell Anemia
- Sleep Disorder Research
- Thyroid Disease
- Triglycerides
- Tuberculosis
- Medical Topics
- Accident and Trauma
- Alternative Medicine
- Birth Control
- Bone and Spine
- Chronic Illness
- Controlled Substances
- Dietary Supplements and Minerals
- Epigenetics
- Food Additives
- Foodborne Illness
- Foot Health
- Gene Therapy
- Health Policy
- Human Biology
- Immune System
- Joint Health
- Medical Imaging
- Nervous System
- Pain Control
- Personalized Medicine
- Pharmacology
- Psychology Research
- Wounds and Healing
- PHYSICAL/TECH
- ENVIRONMENT
- SOCIETY & EDUCATION
- Charge Your Laptop in a Minute?
- Caterpillars Detect Predators by Electricity
- 'Electronic Spider Silk' Printed On Human Skin
- Engineered Surfaces Made to Shed Heat
- Innovative Material for Sustainable Building
- Human Brain: New Gene Transcripts
- Epstein-Barr Virus and Resulting Diseases
- Origins of the Proton's Spin
- Symbiotic Bacteria Communicate With Plants
- Birdsong and Human Voice: Same Genetic Blueprint
Trending Topics
Strange & offbeat.

Study at Cambridge
About the university, research at cambridge.
- For Cambridge students
- For our researchers
- Business and enterprise
- Colleges and Departments
- Email and phone search
- Give to Cambridge
- Museums and collections
- Events and open days
- Fees and finance
- Postgraduate courses
- How to apply
- Fees and funding
- Postgraduate events
- International students
- Continuing education
- Executive and professional education
- Courses in education
- How the University and Colleges work
- Visiting the University
- Annual reports
- Equality and diversity
- A global university
- Public engagement
Large-scale genetic study reveals new clues for the shared origins of irritable bowel syndrome and mental health disorders
- Research home
- About research overview
- Animal research overview
- Overseeing animal research overview
- The Animal Welfare and Ethical Review Body
- Animal welfare and ethics
- Report on the allegations and matters raised in the BUAV report
- What types of animal do we use? overview
- Guinea pigs
- Equine species
- Naked mole-rats
- Non-human primates (marmosets)
- Other birds
- Non-technical summaries
- Animal Welfare Policy
- Alternatives to animal use
- Further information
- Funding Agency Committee Members
- Research integrity
- Horizons magazine
- Strategic Initiatives & Networks
- Nobel Prize
- Interdisciplinary Research Centres
- Open access
- Energy sector partnerships
- Podcasts overview
- S2 ep1: What is the future?
- S2 ep2: What did the future look like in the past?
- S2 ep3: What is the future of wellbeing?
- S2 ep4 What would a more just future look like?
- Research impact

An international study of more than 50,000 people with irritable bowel syndrome (IBS) has revealed that IBS symptoms may be caused by the same biological processes as conditions such as anxiety. The research highlights the close relationship between brain and gut health and paves the way for development of new treatments.
Although IBS occurs more frequently in those who are prone to anxiety, we don’t believe that one causes the other – our study shows these conditions have shared genetic origins Miles Parkes
IBS is a common condition worldwide, affecting around 1 in 10 people and causing a wide range of symptoms including abdominal pain, bloating and bowel dysfunction that can significantly affect people’s lives. Diagnosis is usually made after considering other possible conditions (such as Crohn’s disease or bowel cancer), with clinical tests coming back ‘normal’. The condition often runs in families and is also more common among people who are prone to anxiety. The causes of IBS are not well understood, but an international team of researchers has now identified several genes that provide clues into the origins of IBS.
The research team, including more than 40 institutions and coordinated by scientists in UK and Spain, looked at genetic data from 40,548 people who suffer with IBS from the UK Biobank and 12,852 from the Bellygenes initiative (a world-wide study aiming to identify genes linked to IBS) and compared them to 433,201 people without IBS (controls), focusing on individuals of European ancestry. The findings were repeated with de-identified data from the genomics company 23andMe Inc., provided by customers who have consented to research, by comparing 205,252 people with IBS to 1,384,055 controls.
The results showed that overall, heritability of IBS (how much your genes influence the likelihood of developing a particular condition) is quite low, indicating the importance of environmental factors such as diet, stress and patterns of behaviour that may also be shared in the family environment.
However, six genetic differences (influencing the genes NCAM1, CADM2, PHF2/FAM120A, DOCK9, CKAP2/TPTE2P3 and BAG6) were more common in people with IBS than in controls. As IBS symptoms affect the gut and bowel, it would be expected that genes associated with increased risk of IBS would be expressed there – but this is not what the researchers found. Instead, most of the altered genes appear to have more clear-cut roles in the brain and possibly the nerves which supply the gut, rather than the gut itself.
Researchers also looked for overlap between susceptibility to IBS and other physical and mental health conditions. They found that the same genetic make-up that puts people at increased risk of IBS also increases the risk for common mood and anxiety disorders such as anxiety, depression, and neuroticism, as well as insomnia. However, the researchers stress that this doesn’t mean that anxiety causes IBS symptoms or vice versa.
Study co-senior investigator and consultant gastroenterologist Professor Miles Parkes from the University of Cambridge explained: “IBS is a common problem, and its symptoms are real and debilitating. Although IBS occurs more frequently in those who are prone to anxiety, we don’t believe that one causes the other – our study shows these conditions have shared genetic origins, with the affected genes possibly leading to physical changes in brain or nerve cells that in turn cause symptoms in the brain and symptoms in the gut.”
The study also found that people with both IBS and anxiety were more likely to have been treated frequently with antibiotics during childhood. The study authors hypothesise that repeated use of antibiotics during childhood might increase the risk of IBS (and perhaps anxiety) by altering the ‘normal’ gut flora (healthy bacteria that normally live in the gut) which in turn influence nerve cell development and mood.
Current treatments for IBS vary widely and include dietary changes, prescription medications targeting the gut or brain, or behavioural interventions. Lead author Chris Eijsbouts from the University of Oxford suggests that discovering genes which contribute to IBS may aid in the development of new treatments in the long term. He said: "Even genetic changes that have only subtle effects on IBS can provide clues about pathways to target therapeutically. Unlike the individual genetic changes themselves, drugs targeting the pathways they tell us about may have a considerable impact on the condition, as we know from other disease areas."
Co-senior investigator Dr Luke Jostins from the University Oxford commented: “We anticipate that future research will build on our discoveries, both by investigating the target genes identified and exploring the shared genetic risk across conditions to improve understanding of the disordered brain-gut interactions which characterise IBS.”
“IBS represents a remarkable challenge for genetic studies. These initial findings have been long awaited, and finally tell us this type of research is worth the struggle,” added Ikerbasque Professor Mauro D’Amato from CIC bioGUNE, co-senior investigator and coordinator of the Bellygenes initiative.
This research received funding and support from National Institute for Health Research (NIHR) Biomedical Research Centres in Cambridge, Oxford, Nottingham and Manchester. Further funding and support was received from the Wellcome Trust, the Li Ka Shing Foundation and the Kennedy Trust for Rheumatology Research in the UK, and the Spanish Ministry of Economy and Competitiveness (Instituto Salud Carlos III), the Health Department of the Basque Government and the Swedish Research Council (Vetenskapsradet).
Reference Eijsbouts, C et al. Genome-wide analysis of 53,400 people with irritable bowel syndrome highlights shared genetic pathways with mood and anxiety disorders. Nature Genetics; 5 Nov 2021; DOI: 10.1038/s41588-021-00950-8
Adapted from a press release by the National Institute for Health Research

Read this next

One in two children with ADHD experience emotional problems, study finds

“I feel like I’m Alice in Wonderland”: nightmares and ‘daymares’ could be early warning signs of autoimmune disease

Over 20,000 people join search for new dementia treatments

Study unpicks why childhood maltreatment continues to impact on mental and physical health into adulthood
3D image showing irritable bowel syndrome
Credit: Scientific Animations
Search research
Sign up to receive our weekly research email.
Our selection of the week's biggest Cambridge research news sent directly to your inbox. Enter your email address, confirm you're happy to receive our emails and then select 'Subscribe'.
I wish to receive a weekly Cambridge research news summary by email.
The University of Cambridge will use your email address to send you our weekly research news email. We are committed to protecting your personal information and being transparent about what information we hold. Please read our email privacy notice for details.
- Mental health
- Spotlight on neuroscience
- Miles Parkes
- School of Clinical Medicine
Related organisations
- National Institute for Health Research (NIHR)
- NIHR Cambridge Biomedical Research Centre
- Li Ka Shing Foundation
- Kennedy Trust for Rheumatology Research
Connect with us

© 2024 University of Cambridge
- Contact the University
- Accessibility statement
- Freedom of information
- Privacy policy and cookies
- Statement on Modern Slavery
- Terms and conditions
- University A-Z
- Undergraduate
- Postgraduate
- Cambridge University Press & Assessment
- Research news
- About research at Cambridge
- Spotlight on...
Recent advances in the treatment of irritable bowel syndrome
Affiliations.
- 1 Department of Medical Sciences, University of Turin, Turin, Italy
- 2 Institute of Biostructure and Bioimaging, National Research Council, Molecular Biotechnology Center, Turin, Italy
- 3 Gastroenterology and Endoscopy Unit, Cardinal Massaia Hospital, Asti, Italy
- 4 Unit of Gastroenterology, Molinette-SGAS Hospital, Turin, Italy
- 5 Institute of Biostructure and Bioimaging, National Research Council, Molecular Biotechnology Center, Turin, Italy; Unit of Gastroenterology, Molinette-SGAS Hospital, Turin, Italy. [email protected]
- PMID: 34463082
- DOI: 10.20452/pamw.16067
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder which presents with abdominal pain and altered bowel habits. It affects about 20% of the general population, mainly women, and has a considerable impact on the quality of life and health care costs. Four different entities of IBS have been identified: IBS with constipation (IBS‑ C), IBS with diarrhea (IBS D), IBS with a mixed pattern of constipation and diarrhea, and unclassified IBS. Although the precise pathogenesis of IBS remains unclear, its multifactorial nature is evident and includes environmental and host factors. Management of patients with this disease is challenging and a personalized approach is required. A strong, reassuring physician‑ patient relationship is crucial, followed by patient education, dietary advice, and stress reduction. For nonresponding patients, the therapeutic approach may include nonpharmacological therapies and / or pharmacotherapy. The choice of pharmacological treatment is based on the predominant symptom and a prespecified time point should be planned for effectiveness evaluation and dose adjustment. In patients with IBS‑ D, the therapeutic options include mainly antibiotics, such as rifaximin, peripheral opioid agonists, mixed opioid agonists / antagonists, bile acid sequestrants, and antagonists of serotonin 5‑ hydroxytryptamine type 3 receptors. Bulking agents and osmotic laxatives represent the first line therapy for IBS‑ C, while lubiprostone and linaclotide should be reserved for difficult to treat patients. The involvement of gastrointestinal microbiota constitutes a fascinating field of exploration as it offers the potential to be modulated by the use of probiotics, prebiotics, synbiotics as well as fecal microbiota transplantation. This review offers an updated overview on the recent advances in the treatment of IBS.
Publication types
- Abdominal Pain
- Constipation
- Irritable Bowel Syndrome* / therapy
- Quality of Life
Digestive Diseases
- Current and future treatments for irritable bowel syndrome associated with diarrhea
Sept. 05, 2015

Irritable bowel syndrome (IBS) is a multifactorial disorder marked by recurrent abdominal pain or discomfort and altered bowel function. It affects between 10 and 20 percent of people in the developed world, about one-third of whom have IBS associated with diarrhea (IBS-D).
Certain factors that alter gastrointestinal function can contribute to IBS symptoms, including stress, prior gastroenteritis, changes in the gut microbiome, and bile acids and short-chain fatty acids, which may stimulate serotonin (5-HT) release and increase colonic permeability and motility.
Still, the underlying cause of IBS in many cases remains unknown. Michael Camilleri, M.D. , of Mayo Clinic in Rochester, Minn., says the ultimate goal "is a better understanding of the mechanisms behind this syndrome so we can foster individualized, specific treatment for IBS patients." So far, that goal remains unrealized.
The only drug currently approved for IBS-D is alosetron, a 5-HT3 antagonist that may relieve abdominal pain and slow colonic and small bowel transit. Alosetron was withdrawn from the market for safety reasons in 2000 and was reintroduced in 2002 with a more restricted indication. Today, incidence rates of adverse events, including ischemic colitis and complications of constipation, are similar to those before the drug was withdrawn.
Non-IBS medications for IBS-D
Given the limited number of drugs marketed specifically for IBS-D, other medications are often used to treat symptoms. They include:
This synthetic mu-opioid agonist decreases intestinal transit while increasing intestinal water and ion absorption. In a small, placebo-controlled study, loperamide improved pain, stool consistency, urgency and overall subjective response, but it must be carefully titrated for individual patients to avoid constipation.
Bile acid binders
Roughly 30 percent of people with IBS-D have diagnosed bile acid malabsorption, and for this subset of patients, bile acid sequestration may relieve the cholerrheic effect of bile acids. Some evidence suggests that certain genetic variants may influence response to the bile sequestrant colesevelam, a medication that may be preferable to cholestyramine.
Antidepressants
Tricyclic agents such as amitriptyline and imipramine were initially prescribed to IBS patients with significant depression. Today, they are frequently used to treat patients with severe or refractory IBS symptoms and may have analgesic and neuromodulatory benefits in addition to their psychotropic effects. In one trial, nearly 70 percent of patients receiving 10 mg of amitriptyline experienced a complete loss of IBS symptoms compared with 28 percent of those on placebo.
Of increasing interest in many gastrointestinal disorders, single or combination probiotics have been investigated for IBS-D in several small trials. In these studies, bloating and distension improved but not diarrhea.
Mast cell stabilizers and 5-aminosalicylic acid (5-ASA)
Gastroenteritis precedes IBS-D in about 25 percent of people. Two anti-inflammatory agents have been used for this subset of patients: mast cell stabilizers such as disodium cromoglycate and ketotifen, and 5-ASA, which has shown mixed results for IBS-D in four small trials.
New drugs for IBS-D
Currently under development or in clinical trials, these drugs are more likely than others to play a role in the future management of IBS-D.
Serotonin synthesis inhibitors
LX-1031 is a tryptophan hydroxylase inhibitor that reduces local 5-HT synthesis and 5-hydroxyindoleacetic acid (5-HIAA) excretion. Unlike previous 5-HT inhibitors, LX-1031 does not cross the blood-brain barrier, thereby reducing the risk of depression and central nervous system disorders. A randomized, placebo-controlled phase II clinical trial in 155 patients showed reductions in urinary 5-HIAA and blood 5-HT as well as improvements in pain and stool consistency.
In two placebo-controlled, parallel-group studies of 1,000 patients with IBS-D, this selective 5-HT3 antagonist increased self-reported global assessment of relief of IBS symptoms. Constipation occurred in roughly 5 percent of participants — less than the rate observed with alosetron.
Spherical carbon adsorbent
AST-120 is a preparation consisting of spherical carbon particles that adsorb bacterial toxins, inflammatory mediators and bile acid products and prevent them from entering systemic circulation. In a phase II randomized, controlled eight-week trial of AST-120 in 115 patients, improvements in pain and bloating were short-lived and there was no significant improvement in stool consistency.
Benzodiazepine receptor modulator
The benzodiazepine receptor modulator dextofisopam binds to benzodiazepine receptors in the brain, not the GI tract, without a sedating effect. In animal studies, it exhibited the potential to reduce colonic motility and visceral sensitivity in response to stress. Further studies are needed to determine the mechanism of action, safety and efficacy in humans.
Peripheral k-agonist
Asimadoline, a kappa-opioid agonist, is being evaluated in clinical trials. So far, it has shown a good safety profile and reduced pain, urgency and stool frequency in IBS-D patients.
In spite of ongoing studies, Dr. Camilleri says several challenges must be met in order to achieve therapeutic advances, including "significant advances in research to understand the pathophysiology and clinical phenotyping of diverse patients with IBS-D, interest and investment by the pharmaceutical companies to develop the next generation of compounds, and greater definition of study endpoints by regulatory agencies to identify a clear path for approval and marketing of those medications."
For more information
Camilleri M. Current and future pharmacological treatments for diarrhea-predominant irritable bowel syndrome . Expert Opinion on Pharmacotherapy. 2013;14:1151.
Receive Mayo Clinic news in your inbox.
Related content.

- Medical Professionals
Your gift holds great power – donate today!
Make your tax-deductible gift and be a part of the cutting-edge research and care that's changing medicine.
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, global research trends in irritable bowel syndrome: a bibliometric and visualized study.

- 1 Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2 Department of Gastroenterology, Xiyuan Hospital, China Academy of Traditional Chinese Medical Sciences, Beijing, China
- 3 National Clinical Research Center for Chinese Medicine Cardiology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 4 Xiyuan Hospital, Traditional Chinese Medicine Research Institute of Spleen and Stomach Diseases, China Academy of Chinese Medical Sciences, Beijing, China
Background: There are about 10–23% of adults worldwide suffering from irritable bowel syndrome (IBS). Over the past few decades, there are many aspects of uncertainty regarding IBS leading to an ongoing interest in the topic as reflected by a vast number of publications, whose heterogeneity and variable quality may challenge researchers to measure their scientific impact, to identify collaborative networks, and to grasp actively researched themes. Accordingly, with help from bibliometric approaches, our goal is to assess the structure, evolution, and trends of IBS research between 2007 and 2022.
Methods: The documents exclusively focusing on IBS from 2007 to 2022 were retrieved from the Science Citation Index Expanded of the Web of Science Core Collection. The annual productivity of IBS research, and the most prolific countries or regions, authors, journals and resource-, intellectual- and knowledge-sharing in IBS research, as well as co-citation analysis of references and keywords were analyzed through Microsoft Office Excel 2019, CiteSpace, and VOSviewer.
Results: In total, 4,092 publications were reviewed. The USA led the list of countries with the most publications (1,226, 29.96%). Mayo Clinic contributed more publications than any other institution (193, 4.71%). MAGNUS SIMREN stood out as the most active and impactful scholar with the highest number of publications and the greatest betweenness centrality value. The most high-yield journal in this field was Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society (275, 6.72%). Gastroenterology had the most co-citations (3,721, 3.60%). Keywords with the ongoing strong citation bursts were chromogranin A, rat model, peptide YY, gut microbiota, and low-FODMAP diet, etc.
Conclusion: Through bibliometric analysis, we gleaned deep insight into the current status of literature investigating IBS for the first time. These findings will be useful to scholars interested in understanding the key information in the field, as well as identifying possible research frontiers.
Introduction
Associated with abdominal pain, bloating, and altered bowel habits, irritable bowel syndrome (IBS) is a chronic, cyclical and relapsing functional bowel disorder ( 1 ). The global prevalence of IBS is currently estimated at 15%, and IBS symptoms occur in about 10–20% of Westerners ( 2 – 4 ). Irrespective of bowel habit, diagnoses of IBS have traditionally been made by using the Rome diagnostic criteria, which is a symptom-based diagnostic standard that is being updated to Rome IV criteria ( 5 ). Further subtypes of IBS include diarrhea-predominant IBS (IBS-D), constipation-predominant IBS (IBS-C), mixed type of IBS with both diarrhea and constipation (IBS-M), and unclassified IBS ( 6 , 7 ). A key challenge that has faced IBS research to date has been the pathophysiology, which is thought to be multifactorial ( 8 ). There are still no satisfactory treatments for patients with IBS because of its complex pathogenesis. Currently, there is an emphasis on symptomatic management; yet, it involves multiple medications and fails to address the underlying complex pathogenesis of the disease, with approximately one-third of patients failing to respond ( 9 – 12 ).
As a result of the multiple and persistent symptoms of IBS, it contributes to a decline in quality of life, high absenteeism, and high socioeconomic burden. It has been estimated that between 8.5 and 21.6 days a year are taken off work due to IBS. There are approximately 3.6 million physician office visits related to IBS every year, resulting in healthcare costs of more than $30 billion ( 13 – 15 ).
There are many aspects of uncertainty regarding IBS, leading to an ongoing interest in the field as reflected by the huge amount of literature. Thus, it is difficult to characterize the evolution of knowledge components, the current body of knowledge, and the research trends.
The bibliometric analysis utilizes mathematical and statistical methods and involves the use of a series of defined metrics to evaluate the structure, productivity, progress, quality, impact and inter-connectivity of scientific work ( 16 , 17 ). One way to accurately capture and integrate data from disparate sources of heterogeneous information is through a knowledge map, which visualizes the connections between complex data silos ( 18 ). Furthermore, key authors, institutions and countries as well as the structure of scientific collaboration networks can be identified. However, there are few bibliometric studies on IBS research. In this context, the present study aims to use a bibliometric approach to identify, evaluate and visualize all literature published on IBS since 2007 regarding quantitative, semiqualitative, and chronological elements of data collected.
Materials and Methods
Source of the data and search strategy.
The search was performed on the Science Citation Index Expanded of the Web of Science Core Collection (WoSCC) of Clarivate Analytics. All searches were conducted on the same day, February 1, 2022. The literature search was completed by two authors independently for identifying IBS-related publications with the following search strategy: TOPIC:[(adaptive colitis) OR (colon spasm) OR (functional bowel disease) OR (irritable bowel) OR (irritable colon) OR (membranous colitis) OR (mucous colitis) OR (spastic colitis) OR (spastic colon) OR (spastic bowel) OR (functional colonic disease) OR (colon irritable) OR (colon neurosis) OR (bowel neurosis) OR (functional colopathy) OR (functional colonopathy) OR (chronic catarrhal colitis) OR (colica mucosa) OR (colonic enterospasm) OR (dyskinesia of the colon) OR (dyssynergia of the colon) OR (functional enterocolonopathy) OR (Glarry enteritis) OR (glutinous diarrhea) OR (intestinal croup) OR (irritable gut syndrome) OR (lienteric diarrhea) OR (membranous catarrh of the intestine) OR (mucomembranous colic) OR (myxoneurosis) OR (nervous diarrhea) OR (neurogenic mucous) OR (non-specific diarrhea) OR (tubular diarrhea) OR (unhappy colon) OR (unstable colon)] AND Language:(English). Additionally, articles and reviews containing at least one search term in the “title” were included since the aim was to obtain the academic research on the topic of interest. However, the “TOPIC” search enables the inclusion of a considerable amount of off-topic publications with the search terms in abstract, author keywords and keywords plus. The retrieval time was from February 1, 2007 until February 1, 2022. The bibliographic records were collected and saved in plain text. Ultimately, these documents were imported into CiteSpace and VOSviewer for analysis.
Data Analysis
CiteSpace ( 19 ), which is a freely available Java-based software package developed by Professor Chaomei Chen at Drexel University, was applied to (1) perform co-occurrence analysis; (2) visualize key features of literature, such as authors, countries or regions, organizations, and keywords; (3) perform a co-citation analysis of references; (4) depict timeline view of keywords; and (5) capture keywords and references with strong citation bursts. VOSviewer ( 20 ), which is a free software tool based on the Java environment developed by Nees Jan van Eck and Ludo Waltman from Leiden University, was used for creating clusters of keywords. Microsoft Excel 2019 was used to demonstrate the amount of scientific literature published annually.
Publication Output
A total of 4,092 publications were identified, including 3,299 articles (80.62%) and 793 reviews (19.37%). The number of publications per year since 2007 is shown in Figure 1 . There is an overall trend of increased output of scientific research over time, which falls into two stages. The first phase from 2007 to 2016 exhibited a growing trend despite the decrease in 2010, 2013, 2015, and 2016. As the second stage has progressed, the number of documents has increased from 270 in 2016 to 344 in 2019, to 424 in 2021. The volume of papers published during the last 6 years (2016–2021) accounted for 49.82% of all publications.
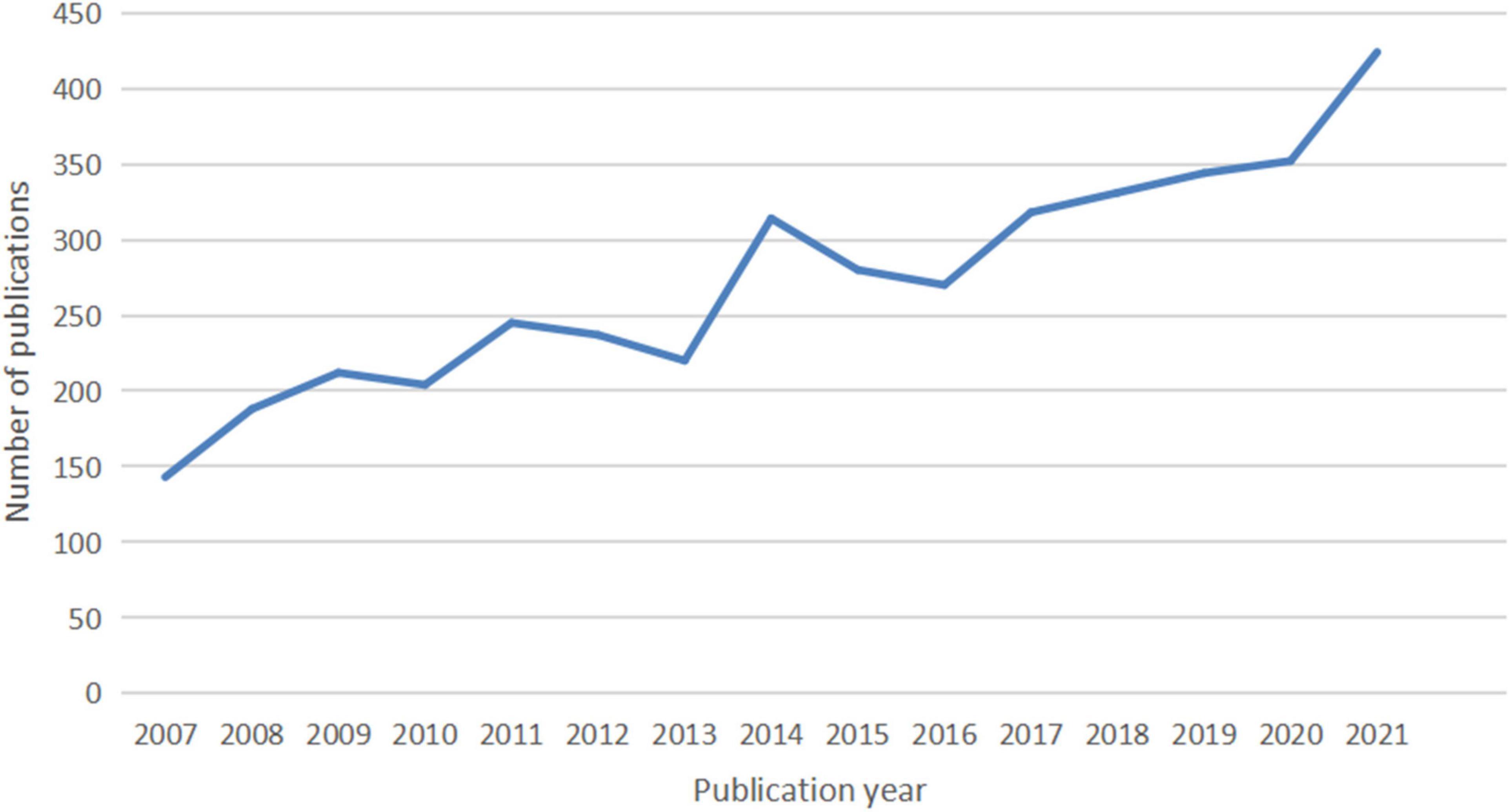
Figure 1. The number of articles published annually in IBS research.
Countries or Regions and Institutions Analysis
In total, IBS articles were published by 407 institutions from 116 countries or regions. As shown in Table 1 , the top 10 countries and institutions are listed. The United Stateswas the leading country in the field, which had an overwhelmingly higher number of publications (1,226, 29.96%). China ranked second (577, 14.10%). In third place is England (421, 10.28%).
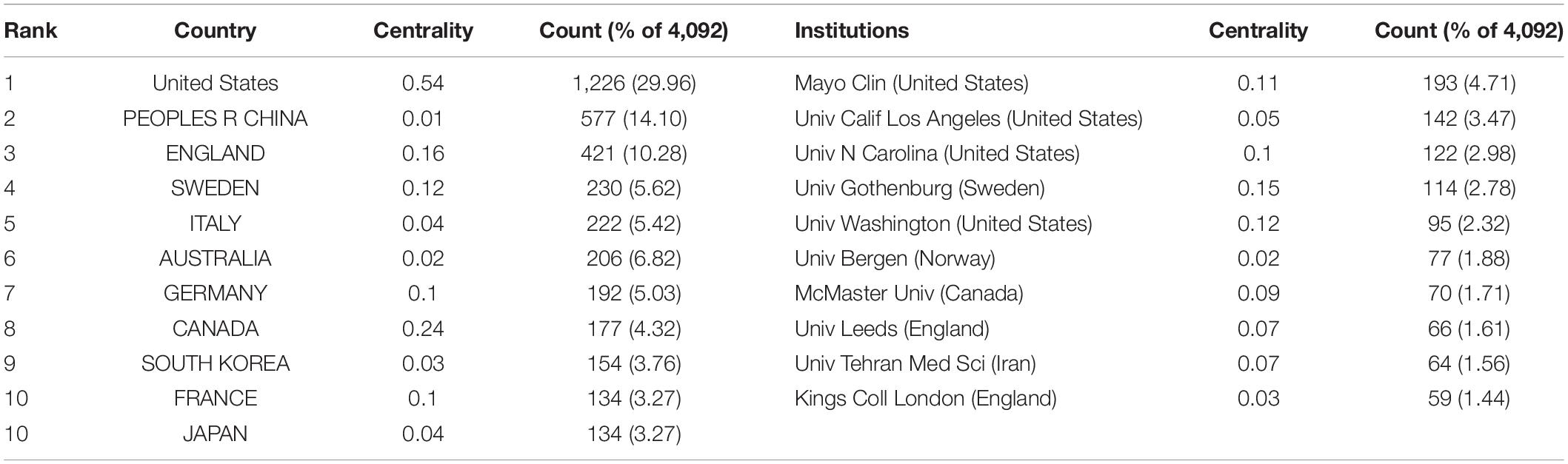
Table 1. The top 10 countries or regions and institutions involved in IBS research.
With regard to contributions of institutions, the majority of the top 10 prolific institutions were from the USA (40%) and England (20%). Among them, Mayo Clin contributed the most publications (193, 4.71%), followed by Univ Calif Los Angeles (142, 3.47%) and Univ N Carolina (122, 2.98%).
In addition, the USA ranked first by the betweenness centrality value (0.54), followed by Canada (0.24), and England (0.16). Univ Gothenburg ranked first by the betweenness centrality value (0.15), followed by Univ Washington (0.12) and Mayo Clin (0.11).
Figure 2 shows the collaboration among the countries or regions. In the map, each node represents a country or territory. The radius of a node increases with its contribution to the research on IBS. The links between nodes represent the collaboration, whereby their thicknesses are proportional to the intensity of the collaboration. A node’s betweenness centrality is calculated in order to identify the node that lies between two or more large groups of nodes. In a network, a node with a betweenness centrality value of more than 0.1 (i.e., one interconnected with more than 10% of the other nodes) exerts substantial influence over others because more information passes through that node. A node with a high betweenness centrality value is marked with a purple ring, while a red ring denotes a burst.
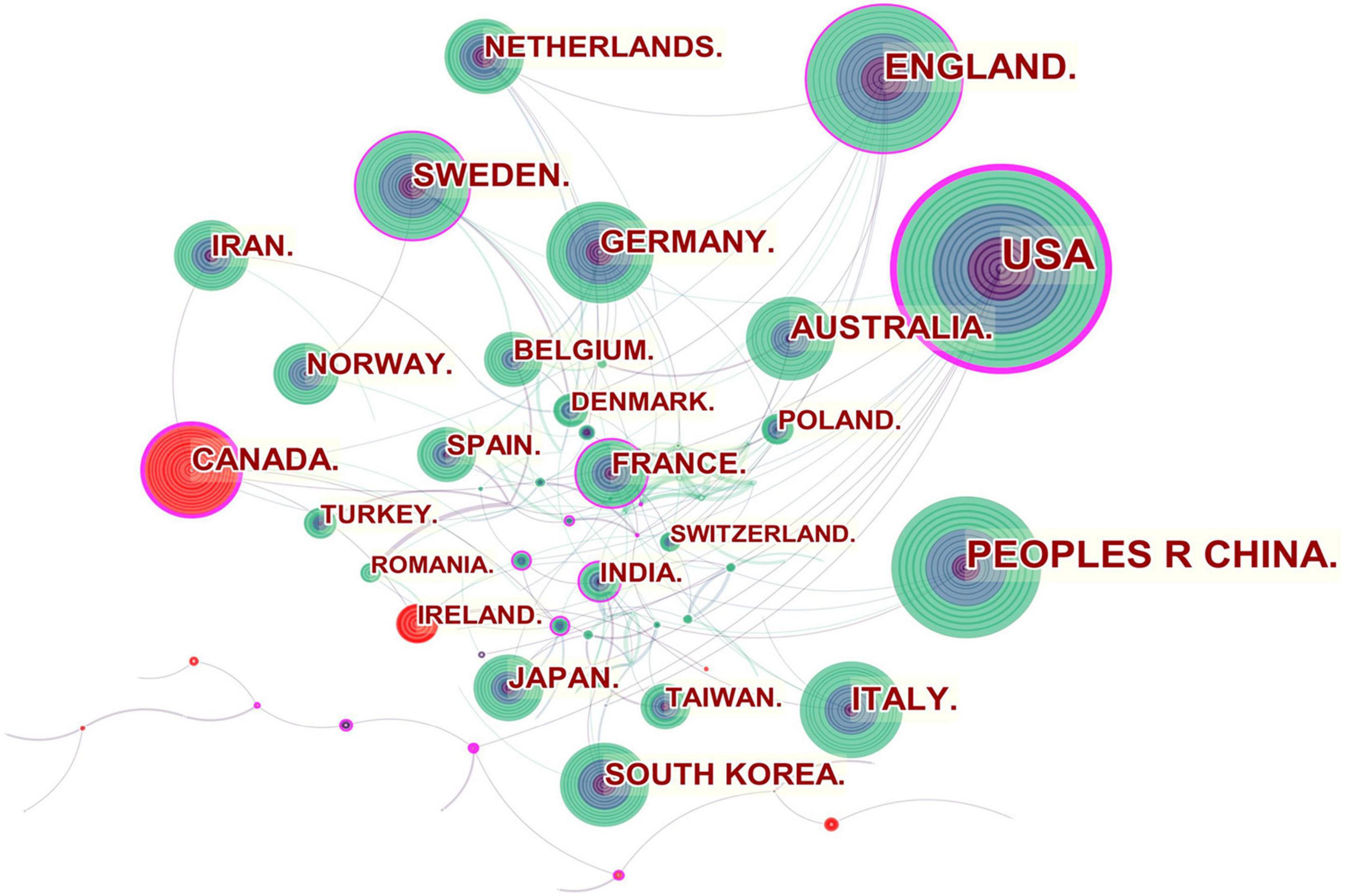
Figure 2. Network of countries and regions engaged in IBS research.
The United States, Canada, England, Sweden, France and India were referred to as central countries for the network owing to their cooperation occurring worldwide. For example, the United States, which possessed the broadest scientific collaboration, worked intensively with Australia, Peru, Israel, Sweden, Canada, Netherlands, Russia, Japan, and South Korea. Canada had close cooperation with Iran, England, the United States, South Africa, France, Argentina, Mexico, and Ireland. The main collaborators with England were the Netherlands, Germany, Australia, New Zealand, Scotland, Jordan, Palestine, Pakistan and Switzerland. Strong bursts were detected for Canada and Ireland.
In the institutional collaboration network shown in Figure 3 , the landmark nodes included Univ Gothenburg, Univ Washington, Mayo Clin, Univ N Carolina, and Univ Nottingham, signifying that they partnered extensively with academic organizations across the globe. The main institutions that collaborated with Univ Gothenburg were Karolinska Inst, Univ N Carolina, Univ North Carolina Chapel Hill, Univ Copenhagen, Sahlgrens Univ Hosp, Sabbatsbergs Hosp, AstraZeneca R&D, Katholieke Univ Leuven, and Univ Leuven. Univ Washington collaborated actively with Keimyung Univ, Ewha Womans Univ, Fred Hutchinson Canc Res Ctr, Broad Inst MIT and Harvard, Harvard Med Sch, Brigham and Womens Hosp, Mayo Clin, Grp Hlth Cooperat Puget Sound, and Campbell Univ. Mayo Clin cooperated frequently with Harvard Med Sch, Baylor Coll Med, Univ Complutense, Univ Sydney, Broad Inst MIT and Harvard, Univ Washington, Montefiore Med Ctr, and Brigham and Womens Hosp. Teheran Univ Med Sci, Univ Calif Los Angeles, Mayo Clin, Univ Washington, McMaster Univ, Univ Manchester, and Cedars Sinai Med Ctr were detected with strong bursts.
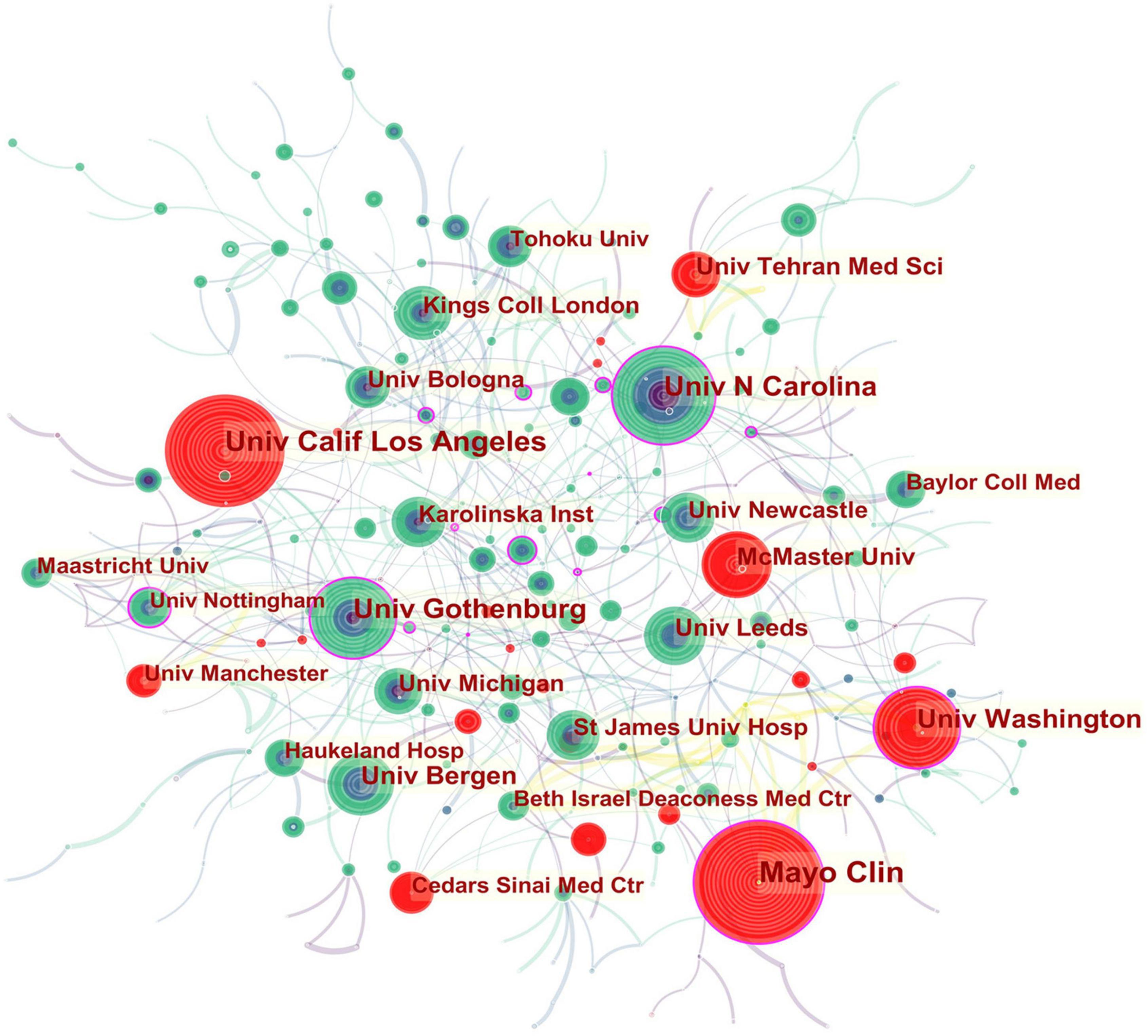
Figure 3. Network of institutions engaged in IBS research.
In all, 423 authors contributed to the IBS studies. As shown in Table 2 , IBS articles were mostly published by authors affiliated with institutions in America (308). MAGNUS SIMREN contributed the most articles (87, 2.12%), followed by MICHAEL CAMILLERI (75, 1.83%), and ALEXANDER C FORD (68, 1.66%). The top authors by the betweenness centrality value were MAGNUS SIMREN (0.13), EMERAN A MAYER (0.12), MICHAEL CAMILLERI (0.09), and NICHOLAS J TALLEY (0.09).
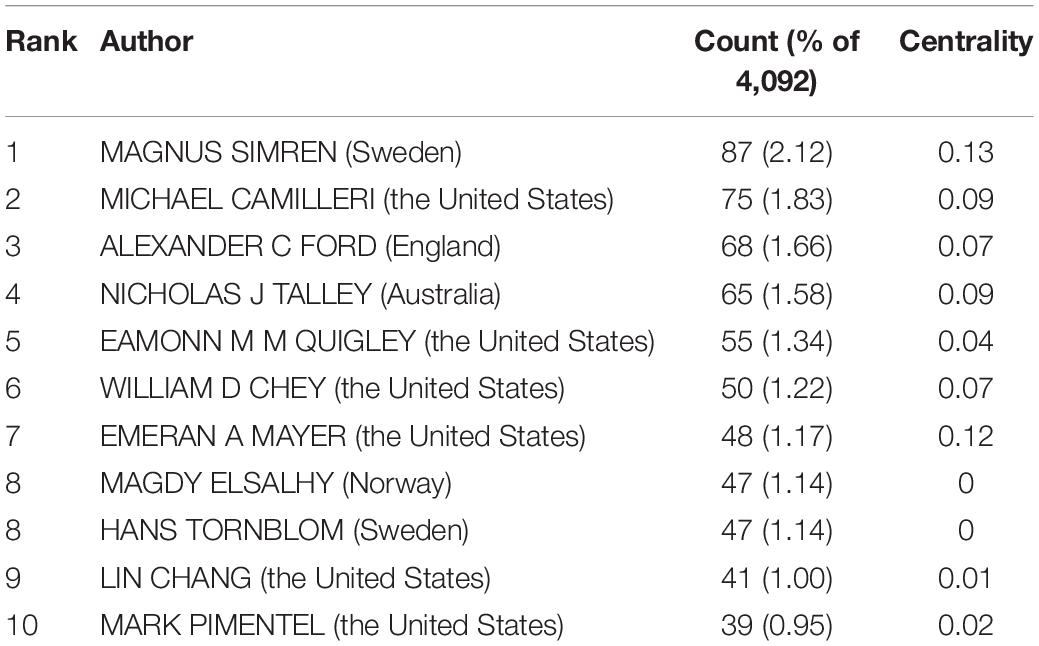
Table 2. The top 10 authors of IBS research.
From the author’s collaboration network, which is presented in Figure 4 , MAGNUS SIMREN and EMERAN A MAYER were located at a central position in the collaboration network. Active collaborations were seen among MAGNUS SIMREN, GUY BOECKXSTAENS (Belgium), LENA OHMAN (Sweden), GISELA RINGSTROM (Sweden), IRIS POSSERUD (Sweden), HANS TORNBLOM (Sweden), EVA JAKOBSSON UNG (Sweden), STINE STORSRUD (Sweden), OLAFUR S PALSSON (the United States), and HASSE ABRAHAMSSON (Sweden). EMERAN A MAYER had close communication with BEATE NIESLER (Germany), BRUCE NALIBOFF (the United States), WENDY SHIH (the United States), ANGELA P PRESSON (Germany), ARPANA GUPTA (the United States), JENNIFER S LABUS (the United States), GUY BOECKXSTAENS (Belgium), and KIRSTEN TILLISCH (the United States).
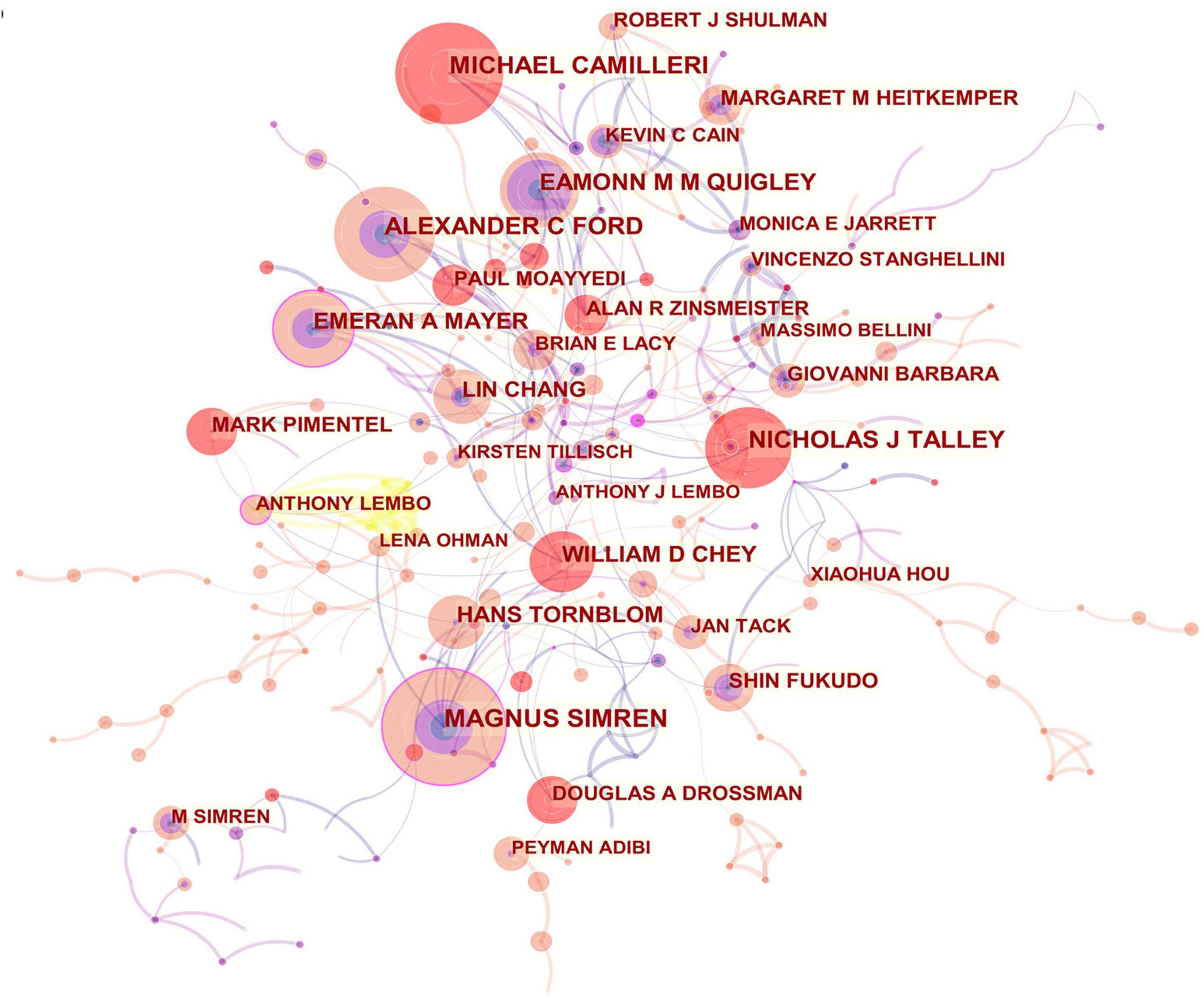
Figure 4. Network of authors in IBS research.
Journals and Co-cited Academic Journals
Publications pertaining to IBS research were found in 799 journals. Below is a brief summary of the 10 most prolific journals as shown in Table 3 . Among them, Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society published the highest number of articles (275, 6.72%), followed by World journal of gastroenterology (171, 4.17%), and Alimentary pharmacology and therapeutics (166, 4.05%). Gastroenterology with the highest impact factor (IF) of 22.682, published 76 articles (1.85%), ranked ninth for the total number of scientific articles. While the journal with the lowest IF of 3.067 was BMC gastroenterology , which ranked eighth with 78 articles (1.90%).
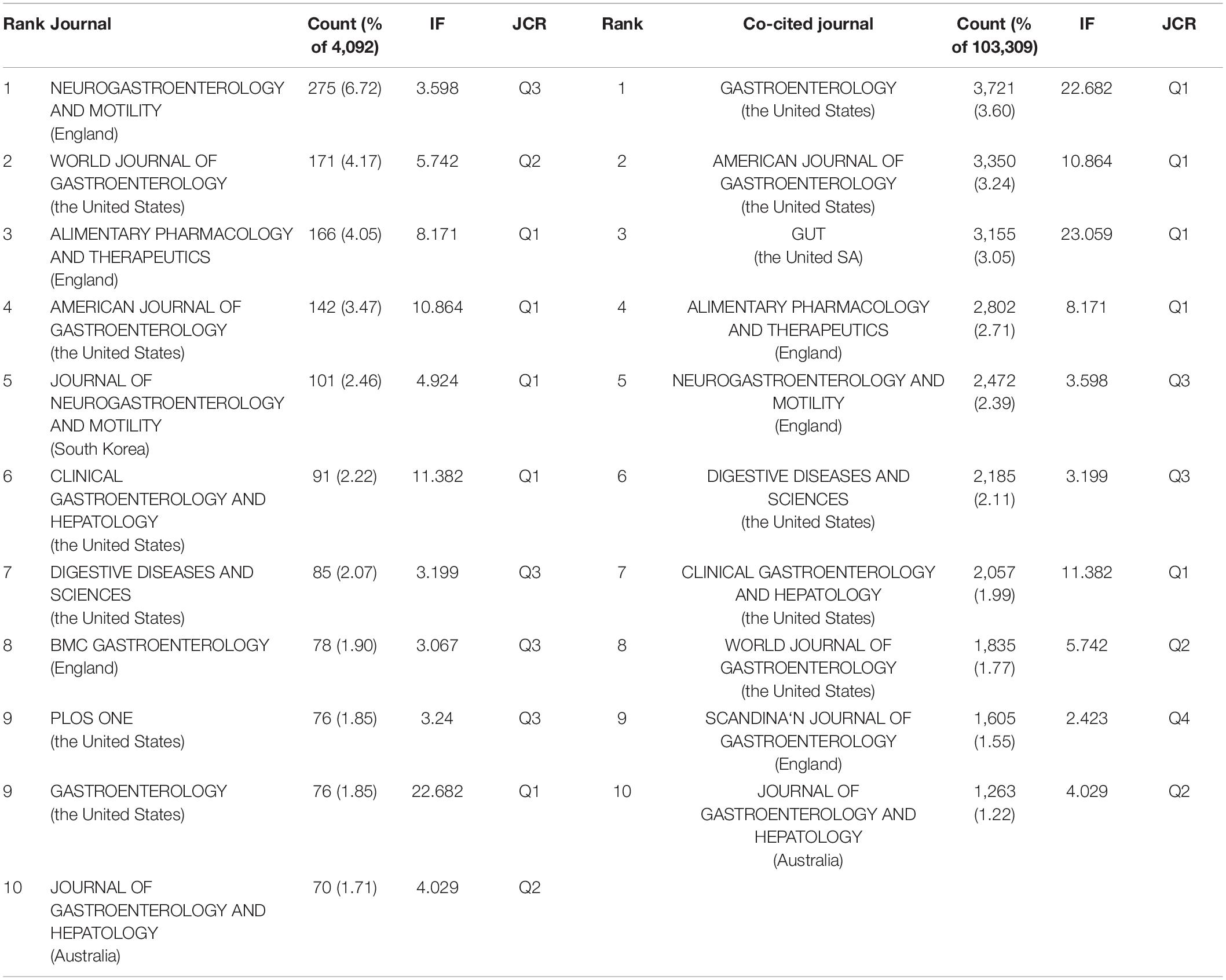
Table 3. Top 10 journal and top 10 co-cited journals in IBS research.
When two or more documents are cited simultaneously by a third paper, the former is termed as “co-cited” ( 21 ). Due to the scientific and objective nature of the co-citation analysis, subjects have been expanded from papers to authors, journals, and disciplines. The frequency at which the documents of two journals are cited together by the documents of another journal is called journal co-citation ( 22 , 23 ). The papers that published research in IBS were co-cited by 1,394 scholarly journals. As shown in Table 3 , Gastroenterology had the most co-citations (3,721, 3.60%), followed by The American journal of gastroenterology (3,350, 3.24%), and Gut (3,155, 3.05%).
There is a concurrence of Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society , World journal of gastroenterology , Alimentary pharmacology and therapeutics , The American journal of gastroenterology , Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association , Digestive diseases and sciences , Gastroenterology , and Journal of gastroenterology and hepatology in the prolific journals and highly co-cited ones.
Co-cited References and References With Citati on Bursts
In the 4,092 IBS publications, there were 1,386 references co-cited. Table 4 provides a list of the top 10 co-cited references. Of the eleven documents, five were published in Gastroenterology , two were published in The New England journal of medicine , one was published in Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association , one was published in JAMA , one was published in The American journal of gastroenterology , and the last one was from Nature reviews. Disease primers . Among them, Mearin et al. ( 24 ) published an article, entitled “ Bowel Disorders ” in Gastroenterology , which was the most frequently co-cited and ranked first (425), followed by “ Functional bowel disorders ”, written by Longstreth et al. ( 7 ) in Gastroenterology (266), “ Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis ,” authored by Lovell and Ford et al. ( 3 ) in Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association (246), and “ Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV ,” published by Drossman ( 25 ) in Gastroenterology (205).
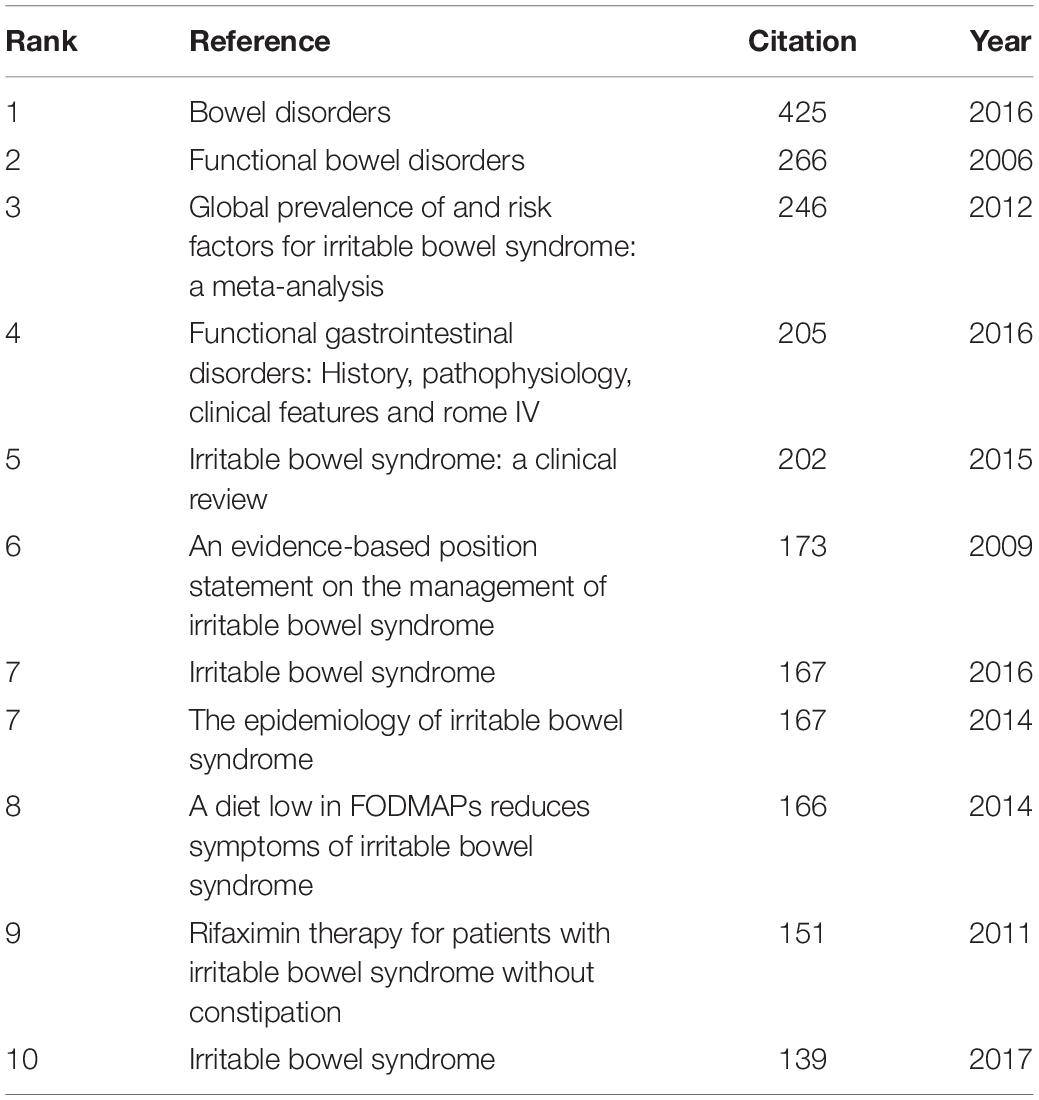
Table 4. Top 10 co-cited references in IBS research.
As shown in Table 5 , the highest-ranked co-cited references by the betweenness centrality value were published from 2008 to 2019. Of the nine references, two were published in Gut , two were published in Alimentary pharmacology and therapeutics , two were published in The American journal of gastroenterology , one was published in World journal of gastroenterology , and the other two were from Gastroenterology and Gut , respectively.
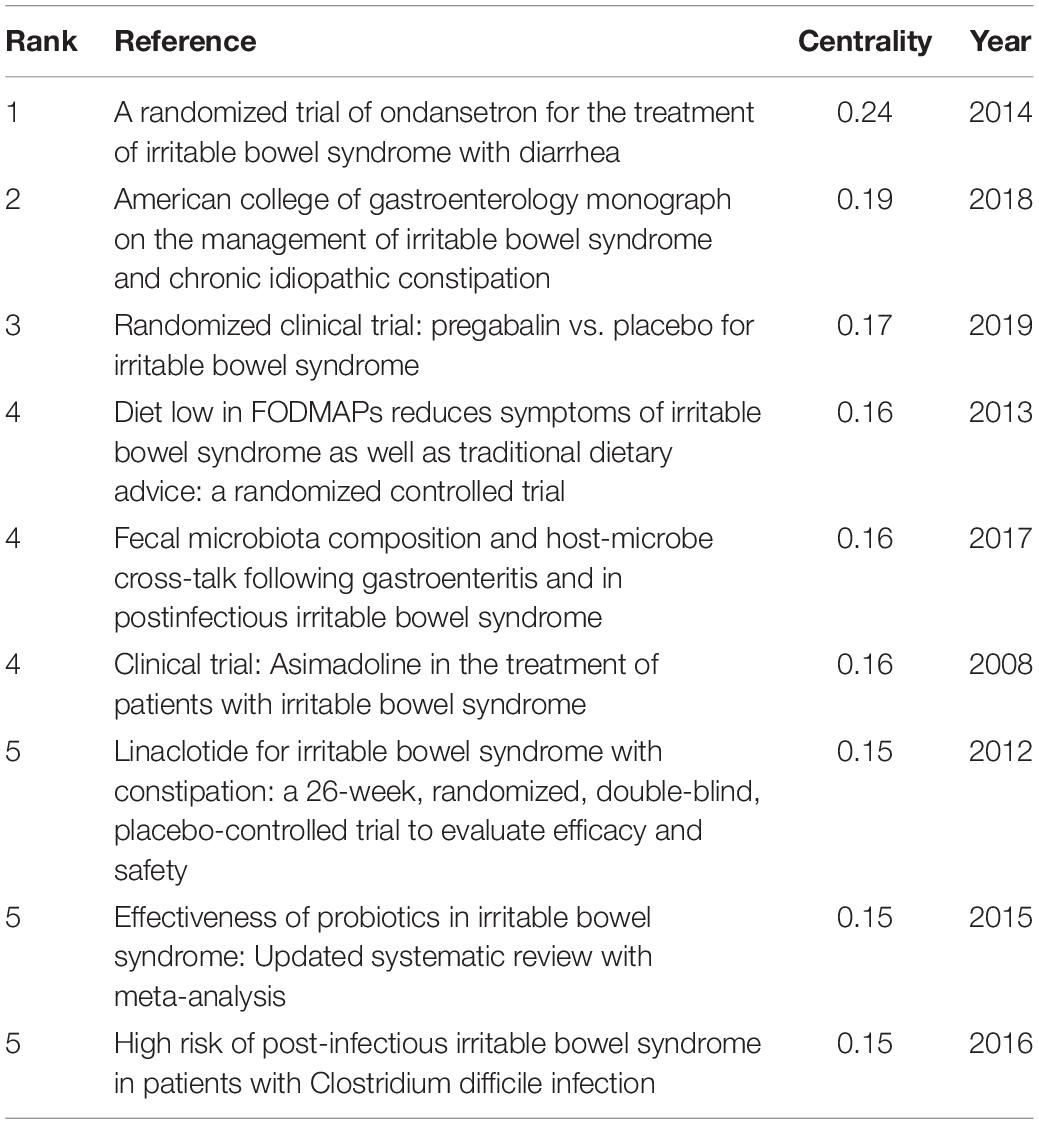
Table 5. Top 5 co-cited references with the highest betweenness centrality in IBS research.
In order to identify literature that has received a lot of attention from peers, the burst detection strategy was applied to publications cited at an increasingly fast rate. In Figure 5 , strong citation bursts for 25 references are shown. Year denotes when the article was published. Strength represents the citation strength. The length of the line corresponds to the period from 2007 to 2022, in which the red segment indicates the time interval of citation bursts. The strongest citation burst was the article entitled “ Functional bowel disorders ” published in Gastroenterology by Longstreth et al. ( 7 ) with a citation burst lasting from 2007 to 2011 (119.61), followed by “ Bowel Disorders ” published by Mearin et al. ( 24 ) with a citation burst spanning from 2017 to 2022 (114.93), and “ Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis ,” published in Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association by Lovell and Ford et al. ( 3 ), which showed a citation burst from 2012 to 2021 (87.08). Those references whose citation bursts ended in 2021 or later deserve special consideration ( 2 , 3 , 24 – 34 ).
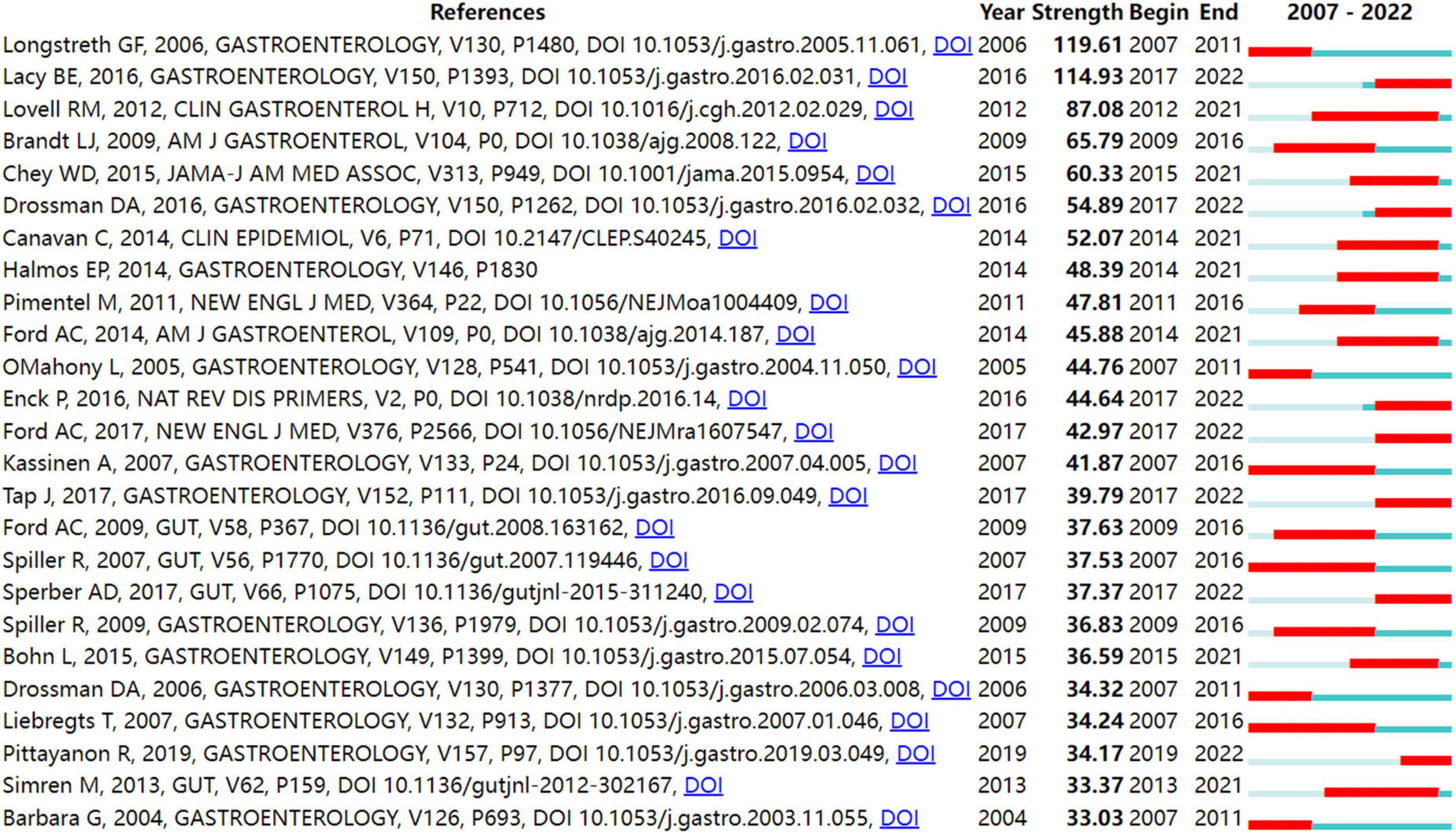
Figure 5. Top 25 references with strong citation bursts in IBS research.
Keywords Analysis
Keyword co-occurrence analysis is derived from the concept of citation coupling as well as co-citation in bibliometrics ( 35 , 36 ). That is, when two keywords that reflect the core research contents of an article appear in the same document, it is considered that there exist the relationship between the two terms. The higher the number of co-occurrences of two terms, the closer their relationship is. A map of keywords co-occurrence is generated based on the frequency of appearance for paired keywords. One of the common methods of identifying hot topics in bibliometrics was co-occurrence analysis of keywords. In the present study, keywords were extracted from 4,092 publications. After excluding irrelevant keywords and merging those with the same semantic meaning, 773 keywords were identified.
Figure 6 shows the map of keywords with highly co-occurrence frequencies that VOSviewer analyzed. Keywords were stratified into four clusters: clinical trials related to IBS (green cluster), post-infectious IBS (purple cluster), the role of the altered composition of intestinal microbiota in IBS (dark blue cluster), pathophysiological mechanisms of IBS (red cluster), IBS or IBS-like symptoms (light blue cluster), and pharmacological and non-pharmacological treatments for IBS (yellow cluster).
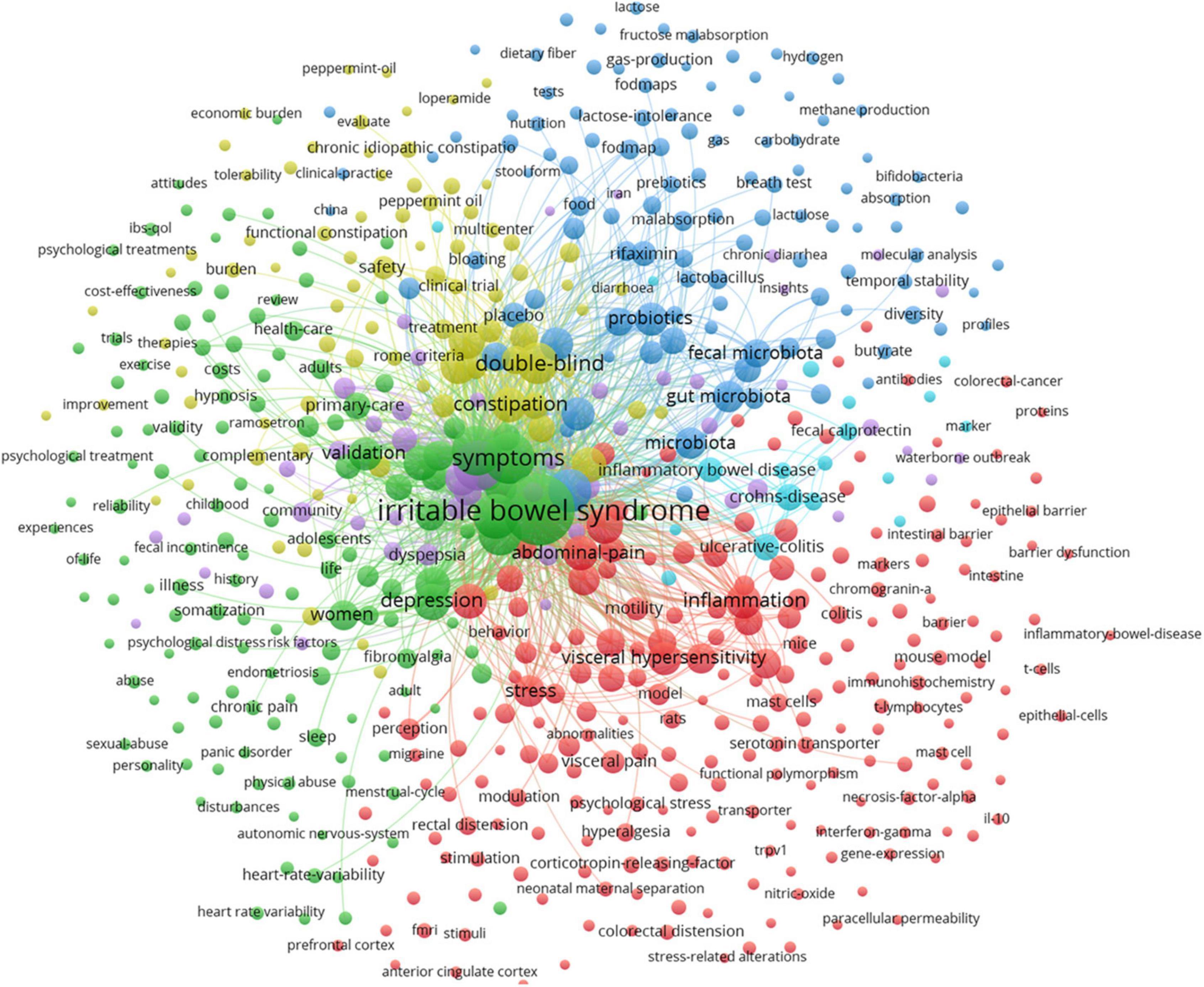
Figure 6. Map of keyword clustering with a minimum of 5 occurrences in IBS.
In Figure 7 , the keywords co-occurrence was visualized in chronologic order. The year placed at the top of the view corresponds to the earliest year when each keyword appeared. Each node in the map represents a keyword. Co-occurrences of keywords are represented by the links.
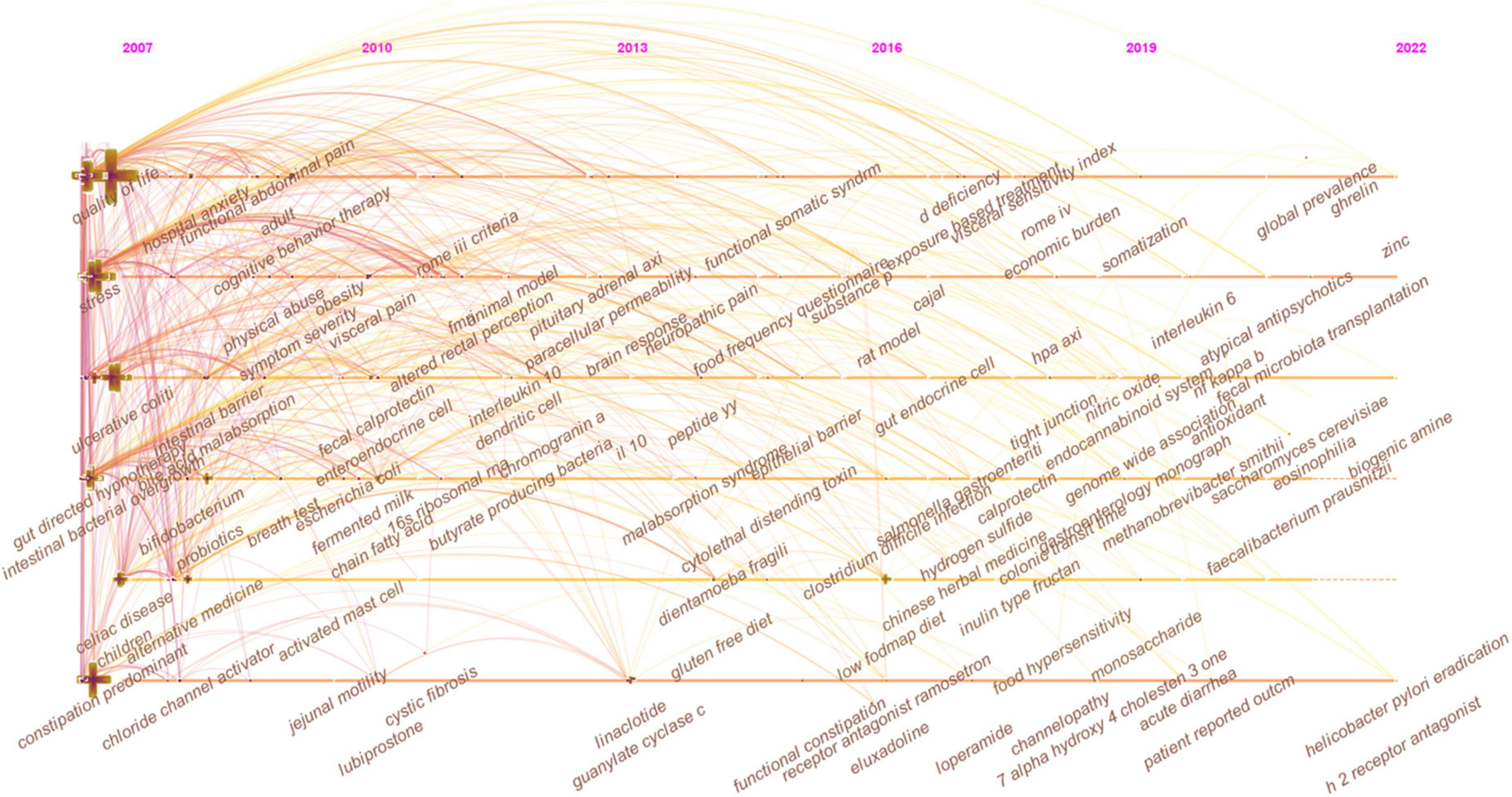
Figure 7. The timeline view of keywords in IBS research.
As shown in Figure 8 , closely related keywords were grouped into different clusters. A cluster is assigned a tag number, and the smaller the number, the more keywords comprise the cluster. The following 10 blocks were presented: #0 visceral pain; #1 primary care; #2 prevalence; #3 small intestinal bacterial overgrowth; #4 diversity, #5 immunohistochemistry; #6 neonatal maternal separation; #7 dyspepsia; #8 food allergy; and #9 serotonin transporter.
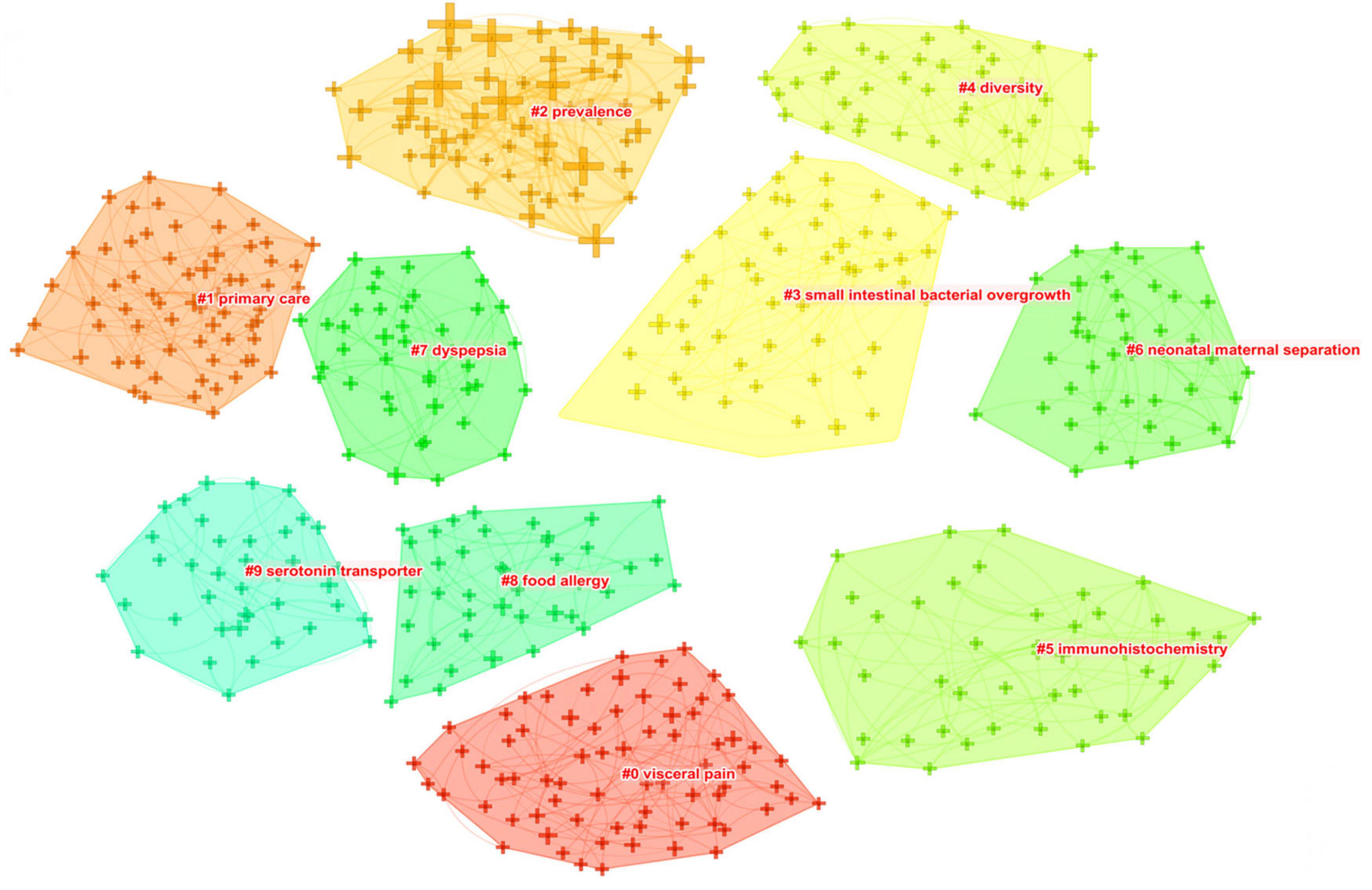
Figure 8. The keyword clustering knowledge map of IBS research.
Table 6 presents the meaningful keywords with high frequency in IBS research. The most frequent keywords were symptom (1,020, 0.01), clinical trial (888, 0.1), quality of life (854, 0.01), epidemiology (819, 0.05), pain (566, 0), gut microbiota (518, 0.05), management (431, 0), hypersensitivity (424, 0.02), efficacy (297, 0.01), and constipation predominant IBS (276, 0.02).
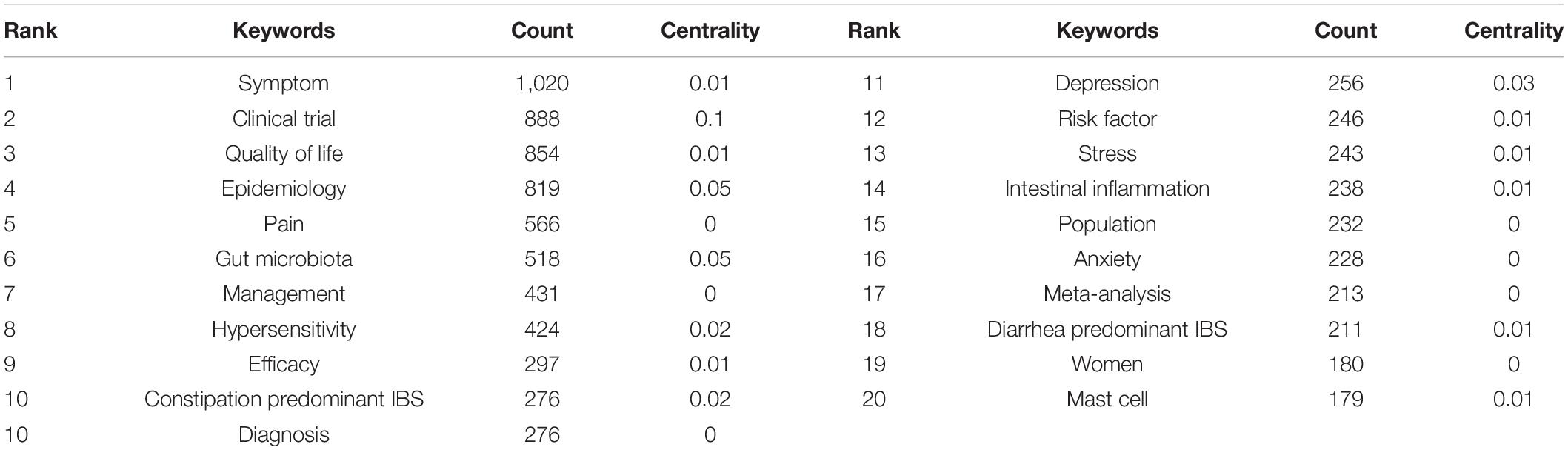
Table 6. Top 20 keywords with the highest count in IBS research.
Strong citation bursts are considered indicators of research frontiers within a particular period of time since the number of citations and occurrences of those terms have surged (19). Figure 9 shows the keywords with strong citation bursts. Some of them exhibited ongoing strong citation bursts, including chromogranin A, rat model, peptide YY (PYY), and gut microbiota, etc.
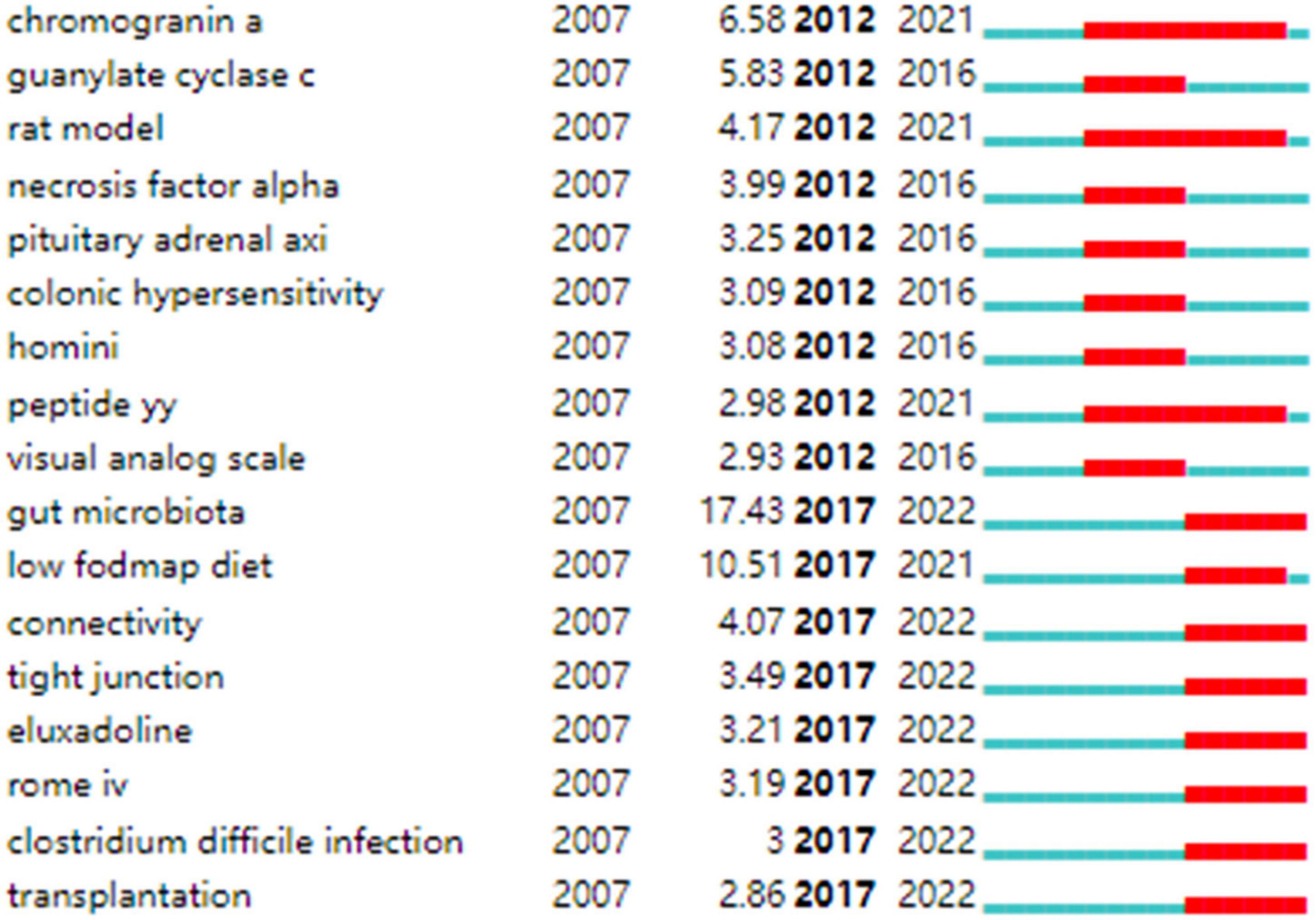
Figure 9. Keywords with strong citation bursts in IBS research.
General Information
The number of academic publications is an important reflection of research activity. As shown in Figure 1 , a total of 2,039 documents were published from 2016 to 2021, a high-yield and rapid growth stage in the field, which has increased significantly compared with the previous 6 years, with 1,500 documents, from 2010 to 2015. Accordingly, it seems possible that the field is about to enter its golden period in the next few years.
Table 1 shows that the highest yielding countries are mostly based in Europe (England, Sweden, Italy, Germany, and France), Asia (China, South Korea, Japan), and North America (the United States and Canada). Any subject with a betweenness centrality value surpassing 0.1 is considered influential in the network. Thus, the highly productive countries in Europe and North America contributed the most impactful research in the field of IBS. Among the high-yield institutions, the top three ranked institutions were in the United States, while there was only one research institution in Asia (Iran). In addition, American and Swedish institutions, including Mayo Clin, Univ N Carolina, Univ Gothenburg, and Univ Washington were prominent in the field with considerable academic influence, given their high volume of publications as well as a high betweenness centrality value. Therefore, the United States and Sweden dominated in research quality and productivity; however, research capacities in Asian regions were generally weak. In the United States, for example, research capacity is likely related to overwhelming support in terms of research, the diversity of researchers with an interest in this field, a wealth of environments well-equipped for research, and the greater availability of a well-trained workforce. In addition, due to the strength of the economy of the United States, significant financial resources are made available to researchers, and scientists enjoy enhanced mobility ( 37 , 38 ). Functional gastrointestinal disorders such as IBS are linked to dysbiosis, and the symptoms triggered is often caused by episodes that affect the microbiome in an environment where the emotional context and enteric nervous system are in synergy ( 39 ). Further, in 2013, the United States launched an innovative program on the gut microbiota-brain axis ( 40 ), which also has led to a surge in publications related to IBS.
As shown in Figure 2 , in general, collaboration exerts positive effects on scientific output, and cooperative research results are of high scientific quality with high academic impacts, particularly those related to transnational collaboration. North American and European countries, which played an influential role in the IBS research, developed cooperative partnerships worldwide. However, collaborations in Asian countries tended to be intra-continental phenomena. It is possible that scientific advances in IBS research in Asian countries were plagued by less transnational cooperation and academic exchange. Notably, Canada and Ireland, which were detected with strong bursts, revealed high scholarly activity over a brief period.
In Figure 3 , collaborations and partnerships among institutions mostly occurred within North America and Europe. Even though some Asian countries have contributed substantially to publications counts, they have not formed a cooperative network, which further confirms that IBS research in Asia lacked intercontinental collaboration. Teheran Univ Med Sci, Univ Calif Los Angeles, Mayo Clin, Univ Washington, McMaster Univ, Univ Manchester, and Cedars Sinai Med Ctr, which exhibited strong bursts, witnessed a large increase in recent publications.
In Table 2 and Figure 4 , the productive authors were mainly from European and North American countries. Swedish and American researchers wielded major influence in IBS research, which also demonstrates the outstanding performance and leading roles of the United States and Sweden in the field. Instead, the academic impacts of Asian scholars were minor. Besides, the overall cooperation and communication still centered on European and American scholars. Hence, Asian nations, including China, South Korea, Japan, India, and Iran, are urged to follow the international pattern of fostering scientific cooperation while raising scientific output, which is directly linked to greater research quality and fortified scientific capability. In fact, a collaborative research project might lead to the participation of experts from various fields, which has been interpreted as positive evidence regarding its impact on the quality of research. As shown in Table 2 , these prolific gastroenterologists who are also likely to initiate collaborations and in most cases provide the central funding or resource support in their community clusters, have interests in neurology, nutrition, and endocrinology (e.g., ALEXANDER C FORD, EMERAN A MAYER, MAGDY ELSALHY, and others). Moreover, the highest-ranked scholar by the betweenness centrality value was MAGNUS SIMREN, indicating that his academic attainments earned him great credibility among peers and had considerable influence in the field. Scholarly contributions from EMERAN A MAYER also occupied an eminent position.
According to Table 3 , IBS research has been published largely in journals from Western countries that specialize in gastroenterology. Studies of high quality and well-designed design are the evidence base for IBS research, as the top prolific journals are typically found in Q1 or Q2. Journals with high co-citations are referred to as mainstream journals, to which researchers are dedicating great attention. Likewise, highly co-cited journals were issued in Western countries, which were classified as Q1 or Q2. This finding enhances the perception of strengthening the construction of scholarly periodicals, especially in Asian nations, for the generation of high-quality scientific outcomes and the dissemination of knowledge in the IBS field. Moreover, Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society , World journal of gastroenterology , Alimentary pharmacology and therapeutics , The American journal of gastroenterology , Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association , Digestive diseases and sciences , Gastroenterology , and Journal of gastroenterology and hepatology were deemed core journals in the field with high publications and co-citations. In addition to serving as reliable references for IBS-related manuscripts, they can also be taken into consideration when submitting manuscripts.
Knowledge Base
Co-cited references are publications that have been cited together by other publications, and are viewed as a knowledge base for a particular field of study. As shown in Table 4 , most literature published in high-impact journals between 2006 and 2017 were reviews or articles describing the epidemiology, risk factors, diagnosis, clinical features, pathophysiology, and management of IBS ( 1 , 3 , 4 , 7 , 24 – 26 , 30 , 41 ). In addtion, Halmos et al. ( 27 ) published the eighth co-cited paper in Gastroenterology in 2014; this study showed that low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP) diet (LFD) which consisted in reducing the intake of poorly absorbed short-chain carbohydrates, such as lactose or fructo-oligosaccharides, improved gastrointestinal symptoms in IBS. Another co-cited article was published in The New England journal of medicine by Pimentel et al. ( 42 ). The researchers conducted two large Phase III trials of rifaximin in patients with non-constipated IBS, which demonstrated significant relief from symptoms of IBS such as bloating, abdominal pain, and watery or loose stools.
In Table 5 , the top 5 co-cited references with the highest betweenness centrality value, which were considered key in defining the intellectual base of IBS, revolved around (1) therapies that target visceral pain modulation, including 5-hydroxytryptamine 3 (5-HT 3 ) receptor antagonist ( 43 ), the blocker of α2δ subunit on voltage-dependent calcium channels ( 44 ), and peripheral diaryl acetamide kappa-opioid receptor agonist ( 45 ); (2) therapies that increase intestinal secretion for IBS-C, such as agonist of the guanylate cyclase C receptor ( 46 ); (3) treatment targeting the intestinal microbiota ( 47 ); (4) non-pharmacological measure such as dietary modifications ( 32 ); (4) characterization of the intestinal microbiota in especially post-infectious IBS ( 48 ); and (5) risk factors for post-infectious IBS ( 49 ).
As can be seen in Table 6 , the extant studies included in the analysis have primarily addressed IBS-C and IBS-D. The potential explanation for this trend may be that these subtypes are more prevalent. In a recent meta-analysis involving 6,756 participants, it has been reported that, using the Rome IV criteria, the global prevalence for IBS-D is 1.4%, followed by 1.3% for IBS-C, 1.1% for IBS-M, and 0.5% for IBS-U ( 50 ). In spite of the use of the Bristol stool form scale in Rome IV to categorize patients with IBS into subtypes, which results in a lower proportion of patients meeting the criteria for IBS-M or IBS-U, these individuals still comprised more than one third of the patients with IBS according to this meta-analysis ( 50 ). At present, however, there are no licensed therapeutics for use in these patients, which represents a significant unmet need. Therefore, enhanced research is needed.
Additionally, from Figure 7 , in which “depressive symptoms,” “psychological distress,” “FODMAP,” “dysbiosis,” “microbiota,” “visceral hypersensitivity,” “functional magnetic resonance imaging (fMRI),” and “cortex,” are included in each cluster, this demonstrates that there has been a proliferation of research into brain-gut-microbiota (BGM) axis within global IBS research. We can infer that the IBS field will undergo a paradigm shift with the involvement of experts in psychiatry, neurology, microbiology, nutrition, and imaging.
Hot Topics and Frontiers
Figure 5 shows the top 25 references with the strongest bursts of citations, whose research topics scholars followed closely over the past fifteen years. Among them, fourteen references ( 2 , 3 , 24 – 34 ) whose citation bursts continued to 2021 or later have attracted considerable interest from the scientific community, thus reflecting the hot topics and emerging trends in IBS research.
In Figure 7 , the evolution of research topics was identified. In the early years from 2007 to 2013, IBS research began to focus on (1) overlap syndrome; (2) 16S ribosomal RNA gene sequencing; (2) fMRI, glucose breath test, and lactulose breath test; (3) IBS-like symptoms; (4) fibromyalgia, menstrual cycle, endometriosis, and chronic fatigue syndrome; (5) acute gastroenteritis, ischemic colitis, antibiotic-associated diarrhea, Inflammatory bowel disease (IBD), and celiac disease; (6) idiopathic constipation and obstructed defecation syndrome; (7) somatization, sexual abuse, physical abuse, and post-traumatic stress disorder; (8) gluten-free diet; (9) cingulate cortex, dorsal horn neurons, and prefrontal cortex; (10) serine protease activity, lactoferrin, and short chain fatty acids (SCFAs); (11) colonic fermentation, hydrogen sulfide, and methane production; (12) colonic hypersensitivity, altered rectal perception, allodynia, and neuropathic pain; (13) intestinal bacterial overgrowth; (14) butyrate-producing bacteria, lactic acid bacteria, Escherichia coli , Blastocystis hominis , Lactobacillus rhamnosus GG, Lactobacillus reuteri , and Lactobacillus plantarum 299v ; (15) brain-gut axis and hypothalamic-pituitary-adrenal axis; (16) corticotropin-releasing hormone and cortisol; (17) PYY, cholecystokinin, and glutamine; (18) neonatal maternal separation; (19) enteroendocrine cells (EECs), dendritic cells, and T lymphocytes; (20) 5-hydroxytryptamine (5-HT) transporter, cannabinoid receptor, calcium channel, estrogen receptor-β, fibrosis transmembrane conductance regulator, and chloride channel activator; (21) E-cadherin; and (22) tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-10, and IL-1β.
From 2013 to 2016, the field focused on (1) early life stress, panic disorder, alexithymia, functional somatic syndrome; (2) collagenous colitis and malabsorption syndrome; (3) functional connectivity, anterior cingulate cortex, and catecholamine; (4) Dientamoeba fragilis and Bifidobacterium infantis 35624; (5) fecal microbiota transplantation (FMT); (6) visceral hyperalgesia and substance P; (7) interstitial cells of Cajal; (8) low-grade inflammation, paracellular permeability, and oxidative stress; and (9) δ opioid receptor and ion transport.
From 2016 to 2022, researchers turned to research on (1) food intake disorder, Brugada syndrome, and channelopathy; (2) Salmonella gastroenteritis and Clostridium difficile infection; (3) uroguanylin, fecal calprotectin, and heat-stable enterotoxin; (4) duodenal microbiome; (5) LFD; (6) water-avoidance stress; (7) bile acid metabolism; (8) Faecalibacterium prausnitzii ( F. prausnitzii ), Akkermansia muciniphila , Methanobrevibacter smithii , and Saccharomyces cerevisiae ; (9) glucagon-like peptide 1, brain derived neurotrophic factor, ghrelin, and neuropeptide Y; (10) eosinophilia and mast cell; and (11) IL-6, nitric oxide, nuclear factor kappa B (NF-κB), and guanylyl cyclase C.
In addition, keywords with the ongoing strong citation bursts shown in Figure 9 were used to identify the hot issues within the field. Of these, gut microbiota is the keyword with the strongest citation burst. Given the hot topics are not separated, but influential and interrelated to each other. We discussed these key hot topics in IBS research under the most popular researched “gut microbiota” framework, highlighting their interrelated aspects as follows:
FODMAP are short-chain carbohydrates that are not readily absorbed in the small intestine, increasing water delivery into the lumen due to osmotic action causing diarrhea. FODMAPs act as a prebiotic for gas-producing bacteria, Clostridium, in the large intestine, increasing gas production ( 51 ). Luminal distension, in turn, is worsened. Among the metabolites fermented from FODMAP are SCFAs like butyrate, acetate, and propionate, as well as carbon dioxide and hydrogen. The presence of these metabolites might also affect microbial colonic environments and IBS symptoms ( 52 , 53 ). The effects of butyrate on visceral sensitivity were demonstrated in healthy volunteers ( 54 ). Through stimulation of 5-HT release from the intestinal mucosa, SCFAs initiate high-amplitude propagated colonic contractions, accelerating intestinal transit ( 55 ).
Over the last decade, research has shown that the FODMAP-restricted diet may be a safe and effective dietary intervention ( 56 ). Several studies have been conducted to conclude that an LFD is effective in relieving overall IBS symptoms and behaves either with non-inferiority or superiority with respect to other comparators ( 27 , 57 , 58 ). The consumption of an LFD has been found to improve symptoms in more than half of IBS patients ( 59 ). However, these trials have been focused on showing their short-term effectiveness, and long-term studies still need to be carried out.
FODMAP restrictions may decrease levels of prebiotics, including fructo-oligosaccharides, galacto-oligosaccharides, and fibers, which are utilized by host microorganisms in a health-enhancing manner. In the end, this results in a reduced amount of highly beneficial bacteria and decreased production of SCFAs that are beneficial to colonocytes. Some studies have consistently reported the effect of an LFD leading to a reduction in Bifidobactrium ( 60 – 63 ), which is believed to be associated with a worse symptom profile, though no studies have yet investigated the detrimental effects of lower bifidobacteria resulting from LFD on long-term health. Twenty-seven IBS patients and six healthy participants were studied with an LFD or a typical Australian diet and researchers found Clostridium Cluster IV and F. prausnitzii levels were reduced in comparison to controls, with the latter known for anti-inflammatory properties due to its ability to produce butyrate, which regulates T helper 17 and T regulatory cells ( 62 ). An impaired level of F. prausnitzii could potentially harm the integrity of the intestinal mucous barrier, which results in dysbiotic microbes causing IBD ( 64 ). In a controlled, single-blind study with forty IBS patients (twenty on an LFD and twenty on a high FODMAP diet) for 3 weeks, researchers found that the LFD increased the richness and diversity of Actinobacteria ( 65 ). A recent review reports changes in gut microbiota composition after an LFD, such as a lower abundance of Bifidobacterium or Bifidobacteriaceae, Lactobacillaceae, Propionibacteriaceae, Clostridium cluster IV , F. prausnitzii , and an increased abundance of Bilophila wadsworthia , Clostridiales family XIII incertae sedis, and Porphyromonas IV .
In conclusion, mixed results were found in research conducted on the effects of LFD on gut microbiota and its metabolites. Inconsistencies between studies may be related to heterogeneity in LFD study designs, and different sample collection, storage, and analysis methodologies. In addition, feces analysis, however, does not reliably present the actual picture of the gastrointestinal tract. Metagenomics, transcriptomics, proteomics, and metabolomics can be more informative.
Another challenge with LFD is that only 50% of IBS patients report symptomatic improvement on an LFD. In an effort to optimize patient selection most likely to respond to the LFD, and to avoid unnecessary dietary restrictions in those less likely to respond, the potential cause of non-response is being investigated in greater detail. The question of whether baseline colonization of microbiota can predict symptomatic response to the diet is receiving increasing attention. In a clinical trial, thirty-one patients with IBS were randomly assigned to follow the LFD and thirty patients to follow National Institute for Health and Care Excellence dietary advice, and over 4 weeks, researchers found the dysbiosis index was higher in non-responders to the diet than in responders ( 63 ). Among responders, Bacteroides stercoris , Pseudomonas , Acinetobacter , Desulfitispora , Parabacteroides , Bacillus , Salmonella ( Citrobacter , Cronobacter , Enterobacter ), Corea , Ruminococcus gnavus , Clostridium , Firmicutes ( Clostridia ), and Streptococcus were lower at baseline ( 63 ).
In another study, 61 IBS adult patients followed the LFD for 4 weeks, with 52% of responders having different microbial composition at baseline when compared with 48% of non-responders ( 66 ). Bacteroides fragilis , Acinetobacter , Ruminiclostridium , Streptococcus , and Eubacterium were revealed to be higher in the responder group compared to the non-responder group and Clostridia/Negativicutes/Bacilli, Actinomycetales , Anaerotruncus, Clostridiales and Shigella / Escherichia were found to be lower in responders than non-responders at baseline ( 66 ). In both studies, the results regarding microbiota differences were noteworthy; it turned out that the results differed even though the intervention, selection criteria, and microbiota test were the same. Only one genus, Streptococcus was identified common in the studies but revealed opposing trends, indicating there is certainly much yet to learn about how symptom response to an LFD could be predicted by fecal bacterial profiles. In addition, fecal volatile organic compounds may serve as predictors of response to the LFD ( 67 ). Hence, gaps of interest include a deeper understanding of how an LFD affects the gut microbiota and research into diagnostic indicators such as bacterial markers and fecal metabolites to help to identify those likely to benefit from this specific intervention.
Enteroendocrine Cells
It has been suggested that cellular components in the gastrointestinal mucosa contribute to IBS pathogenesis; much attention has been devoted to EECs now. The colonic glands are defined by the presence of serotonin-containing (enterochromaffin) cells, peptide YY (PYY)-, oxyntomodulin (enteroglucagon)-containing L cells, pancreatic polypeptide (PP)- and somatostatin-producing cells ( 68 ). Kyösola et al. ( 69 ) and Verity et al. ( 70 ) were among the first to study EECs in the intestinal mucosa of patients with IBS who reported increased numbers of EECs in rectal biopsies from these patients. However, this topic that has felt static for years begins to move.
Chromogranin A, a common marker for EECs, is a granin secreted from secretory granules of all the different types of EECs ( 71 , 72 ). Patients with IBS have a lower density of chromogranin A in their duodenum and colon than healthy subjects, suggesting that their EECs are generally less dense ( 73 ). These abnormalities probably contribute to IBS pathophysiology because low-density EECs are characterized by subsequent low levels of certain hormones, and dysmotility of the gut, visceral hypersensitivity, and abnormal secretion may result from this in patients with IBS ( 74 ). Low cell densities of Musashi 1 (a marker for stem cells and their early progenitors) and neurogenin 3 (a marker for EEC progenitor) in the small and large intestines of IBS patients indicate that intestinal stem cells are low in clonogenic activity and differentiate slowly into endocrine cells ( 75 , 76 ). Thus, it is proposed that the abnormal behavior of stem cells remains a possible cause of the low density of EECs ( 77 ).
Intestinal stem cells are a possible avenue through which factors contributing to the pathophysiology of IBS exert their effects. Specifically, the diet we consume is thought to act as a prebiotic, therefore stimulating certain species of bacteria to grow. In turn, the bacteria ferment the diet, releasing by-products that affect the stem cells and their progeny in a way that reduces their numbers and causes a low differentiation into endocrine cells, which finally results in low density in EECs and the development of IBS symptoms. However, gastrointestinal EECs interact and communicate with each other in complicated ways; it is reported that the higher densities of gastrin-producing cells and lower somatostatin-producing cells observed in the antrum of IBS patients cannot be explained by abnormal stem cells like those that are seen in the small and large intestines, given that the densities of Musashi-1-positive cells don’t differ between IBS patients and healthy controls in the stomach ( 78 ).
Furthermore, it has been established that diet interacts with EECs. This has been supported by the finding that in the stomach and colon of IBS patients, EECs detected by chromogranin A increase toward the values seen in healthy controls following an LFD, probably due to the changes in gastrin-, enterochromaffin-, ghrelin-, and somatostatin-secreting cells in the stomach and enterochromaffin cells and PYY containing L-cells in the colon ( 79 – 82 ).
Moreover, the gut microbiota is able to interact with EECs. FMT is shown to affect the densities of EECs in the duodenum and colon ( 83 , 84 ). To investigate the mechanisms behind the restoration of EECs after receiving FMT, in a study by Mazzawi et al. ( 85 ), patients reported improvements in their IBS symptoms in parallel with changes in their EECs density 3 weeks after FMT. In fact, the changes in the density of EECs do not appear to be caused by an alteration in the stem cells or their early progenitors, rather they may be due to changes in the differentiation progeny, as observed by neurogenin 3 ( 85 ).
PYY which has been captured with a strong citation burst in our study is a hotly researched gut-derived hormone in IBS pathophysiology. This peptide along with enteroglucagon and glucagon-like peptide 1 is co-produced from L cells. The density of EECs, including PYY cells, in the colon and rectum, are lower in IBS patients when compared to healthy controls ( 86 , 87 ). In this way, the presence of low amounts of PYY and low densities of PYY cells in the large intestine will impair the release of PYY, contributing to the dysmotility that is associated with IBS in that this hormone inhibits gastric and pancreatic secretion, delays the emptying of the stomach, and increases water and electrolyte absorption ( 88 ). Moreover, inferred from the fact that PYY modulates 5-HT release, which regulates visceral sensitivity, the low PYY concentration could indirectly contribute to IBS symptoms of visceral hypersensitivity ( 89 ). It has been reported that the consumption of LFD increases the PYY cell density to the normal level in IBS patients and improves the symptoms of IBS as well ( 82 ); in a similar manner, SCFAs, one of the fermentation products of intestinal microbes, have been reported to promote gene expression and stimulate the production of PYY ( 90 ). Hence, it is possible to restore PYY abnormalities by modifying the diet or the microbiota in IBS, and a PYY receptor stimulator could also be helpful for the treatment of IBS.
Taken together, the optimism in research on the links between diet, gut microbiota, stem cells, and EECs permits us to believe that the knowledge of stem cells and EECs (with hormones) will lead to their use in therapeutics and help to elucidate the mechanisms underlying improved symptoms and non-response following FMT and diet modification.
Tight Junctions
While providing nutrients and water to the body, the intestinal barrier protects internal organs from bacteria, luminal antigens, and luminal pro-inflammatory factors ( 91 ). In several disease conditions, the intestinal barrier dysfunction causes bacteria, endotoxins, and other inflammatory mediators to proliferate. In the case of IBS, increased intestinal permeability was highly variable, with 2–62% showing increased permeability compared to 0–15% in controls ( 92 ). The access of noxious substances to submucosa is largely prevented by a network of tight junctions (TJs), adherens junctions, and desmosomes within the intestinal epithelium. A physiological condition allows only water and electrolytes to penetrate the epithelium on the paracellular level. Paracellular permeability is regulated principally by TJs, a network of proteins found at the apex of epithelial lateral membranes, including claudins (CLDN), occludin, junctional adhesion molecule-A, zonula occludens (ZO), etc. A loss of TJs can allow entry of antigenic macromolecules, lipids, peptides from microbes, and even microbes through the epithelium ( 93 ). As a result, the mucosal immune system is over-stimulated, which has been linked to visceral hypersensitivity and symptom generation in IBS.
In IBS, the mechanisms responsible for modulating TJ expression and assembly are complex. It has been suggested that the intestinal microbial changes are a potential driver. When compared with conventional mice, CLDN-1 and occludin levels were higher in germ-free mice with lower paracellular uptake of a standard probe ( 94 ). Based on this, it appears that commensal microbiota affects colonic TJ proteins and paracellular permeability. In germ-free rodents, De Palma et al. ( 95 ) examined the role of the microbiome in regulating intestinal permeability and found that the colonic barrier was disrupted following gavage of fecal slurry from IBS-D. Studies suggest that patients with IBS, particularly those with post-infectious IBS (PI-IBS), exhibit higher levels of fecal proteolytic activity ( 96 ). Dysbiosis-derived proteases contribute to TJ disruption in IBS through the activation of a protease-activated receptor pathway ( 97 ). A study reports that Escherichia coli Nissle 1917 can mitigate the increase in paracellular permeability associated with supernatants obtained from patients with IBS ( 98 ). Furthermore, the activation of toll-like receptor 4 (TLR4)/myeloid differentiation factor 88 (MyD88)/NF-κB pathway in IBS-D is linked to imbalanced inflammatory cytokine expression, finally affecting gastrointestinal motility, secretion, and re-absorption as well as increasing intestinal sensitivity ( 99 , 100 ). More specifically, TLR4 and MyD88 are involved in pro-inflammatory signaling induced by bacterial lipopolysaccharide, whose activation may induce the expression of IFN-γ and TNF-α. In this way, intestinal epithelial barrier function is compromised by IFN-γ and TNF-α, which regulate the organization of several TJ proteins, such as ZO-1, CLDN-1, CLDN-4, and occludin ( 101 ). Therefore, blocking LPS-mediated signaling can be beneficial in protecting against the disruption of gut barriers. The TLR4-MyD88-transforming growth factor β-activated kinase1-NF-kB pathway is induced by wogonin to suppress inflammatory response and the down-regulation of CLDN-1 and ZO-1 in Caco-2 cells ( 102 ). IBS-D rats treated with QingHuaZhiXie prescription, a Chinese herbal compound prescription, showed that occludin, CLDN-1 and ZO-1 expression were restored in colon tissue by inhibiting the TLR4/MyD88/NF-κB pathway, which is accompanied by the improved symptoms of diarrhea and intestinal hypersensitivity ( 103 ).
In addition to the role of bacteria and their structural components in regulating TJ proteins, there may be different ways in which the microbial metabolites play a role. Butyrate, for instance, stimulates adenosine monophosphate-activated protein kinase, resulting in the assembly of TJ proteins which are important for intestinal barrier integrity repair ( 104 ). In response to sodium butyrate treatment, the motif-specific promoter region of CLDN-1 interacts with transcription factor specificity protein 1 to increase the transcription of CLDN-1 ( 105 ). It has been demonstrated that 6-formylindolo (3,2-b) carbazole, a tryptophan ligand, acts by activating the aryl hydrocarbon receptor and prevents TNF-α/IFN-γ-induced decrease in transepithelial electrical resistance and disruption of TJ proteins ( 106 ). Furthermore, polyamines, bile acid metabolites, conjugated fatty acids, and polyphenolic derivatives are examples of microbial metabolites that significantly affect TJ proteins ( 101 ).
Increased intestinal permeability has been observed in 37–62% of patients with IBS-D and 16–50% of patients with PI-IBS ( 92 ). IBS-C studies, however, showed the same level of permeability as controls ( 92 ). The rectosigmoid and descending colon represent the most extensively studied parts of the bowel in IBS, where TJ proteins are still highly heterogeneous. In addition, microbiota abundance and composition are not only related to a particular region within the digestive system, but also to the place where they are sampled. Also, in IBS, remissions are mixed with periods of symptoms escalating. Hence, to identify microbial changes that are missed with cross-sectional sampling, longitudinal sampling strategies, multiple time-point samples, a post-intervention follow-up, and a washout period for cross-over studies are needed; similarly, to better understand target TJ proteins in IBS, it may prove beneficial to sample longitudinally different areas of the gut in the same volunteers.
Even though some promising findings have been made, the intricate relationship between altered microbiota composition with microbial metabolites and TJ proteins modulation should be further explored, particularly in IBS-D and PI-IBS subtypes.
Neuroimaging and Gut Microbiota
Usually, IBS is a medical label used for medically unexplained gastrointestinal symptoms, but it may also reflect disturbances of the BGM axis ( 8 ). Since changes in the composition or functions of gut microbiota are known to affect human behavior and brain physiology, and dysbiosis is often presented by IBS patients, a great deal of attention has been paid to the role that gut microbiota play in this interaction ( 107 ). There is no adequate understanding of how gut microbiota signaling to the brain in humans works, but this process appears to be either modulated by microbial interactions with the host or diet, producing neuroactive compounds that can send signals to the brain via afferent vagal pathways or humoral channels or mediated by bacterial metabolites, which regulates immune function and cytokine production with downstream effects on brain functions through the regulation of neuroinflammation ( 108 ).
The use of neuroimaging as a non-invasive tool to explore the mechanisms of these pathways can be useful in addition to measuring the bioactives. In a new perspective on disorders of the BGM-axis, brain networks (brain connectome) and networks of gut cells and microbiota (gut connectome) are integrated, leading to a significant increase in studies that have combined gut microbiota and neuroimaging to investigate IBS pathophysiology ( 109 ).
Besides identifying structural and functional changes in specific brain regions, neuroimaging studies largely focus on brain connectivity using techniques such as diffusion MRI or fMRI recordings coupled with topological networks ( 110 ). The study by Labus et al. ( 111 ) examined the relationship between gut microbiota and brain structure among IBS patients. An increase in Clostridium members was significantly associated with a larger volume of subcortical areas (putamen, caudate nucleus, nucleus accumbens) and lower insula and prefrontal cortex volumes among the IBS participants ( 111 ). They also focused on Clostridiales, and examined its association with brain function and gastrointestinal sensorimotor function in another study ( 112 ). Lachnospiraceae incertae sedis , Clostridium XIVa and Coprococcus , all within the order Clostridiales, were found to be associated with gastrointestinal sensorimotor function in healthy controls, but not in IBS ( 112 ). Within the subcortical regions, Clostridium XIVa negatively correlated with putamen connectivity, as did Coprococcus and caudate nuclei, which were both related to improved gastrointestinal sensorimotor function ( 112 ). However, in IBS, there was a positive association between Clostridium XIVa and the putamen, the caudate nucleus, and the thalamus connectivity, suggesting that these subcortical areas may be affected by Clostridium XIVa and Coprococcus , and this may contribute to visceral hypersensitivity and pain in IBS ( 112 ). Osadchiy et al. ( 113 ) also reported on fecal metabolites and resting state fMRI. Histidine, glycine, glutamate, spermidine, and anserine variations were significantly correlated with changes in left dorsal part of the posterior cingulate gyrus to the left putamen ( 113 ). In addition, variations in histidine, tryptophan, uracil, 2-deoxyuridine, thymidine, and succinate were related to changes in the right superior frontal gyrus to right putamen. Aberrant tryptophan signaling may be responsible for this interaction in IBS patients ( 113 ). In fact, tryptophan is an important precursor of 5-HT. Several studies have suggested that tryptophan may be an essential amino acid in IBS because 5-HT is important for secretion, absorption, and intestinal transit, as well as mood, pain, and cognitive functions ( 114 ). Recently, Jacobs JP et al. ( 115 ) have shown that among IBS patients, as compared to non-responders, those who responded to cognitive behavioral therapy had increased levels of several members of the Clostridiales order as well as decreased levels of Bacteriodales. Responders showed a reduction in connectivity across multiple cortical networks including sensorimotor, default mode, salience, and emotion regulation networks following treatment, and these brain changes occurred in conjunction with a conversion to Bacteroides-predominant microbiota ( 115 ).
Microbiota together with non-invasive techniques that assess brain function, such as fMRI, have shed light on some aspects of the BGM-axis. The Bergen BGM-study ( 116 ), among others, provides an excellent example of multimodal and interdisciplinary clinical studies designed to verify directionality and causality in the BGM-axis in IBS.
Fecal Microbiota Transplantation
FMT involves the application of a fecal solution from a healthy contributor into the gut of a receiver that is aimed at restoring a dysfunctional microbial composition to a healthy one, and so to improve the function of the gut microbiota. In addition to its commonly recommended use in Clostridioides difficile infection, FMT has also recently gained attention due to the strong evidence linking dysbiosis to IBS pathogenesis ( 117 ). No consensus exists regarding FMT procedure, different routes of FMT delivery (e.g., colonoscopy, nasogastric tube, enema, and oral capsules), the types of formulations (frozen, dried and fresh), and the number and type of donors were examined. Each of these modes of treatment has had varying degrees of clinical success.
According to a meta-analysis of 267 IBS patients, colonoscopic FMT treatment was effective, nasogastric tube treatment was marginally beneficial, and oral capsules failed to deliver benefits ( 118 ). The results of another study investigating the effectiveness of FMT via colonoscopy for the treatment of IBS patients with diarrhea or diarrhea and constipation showed that clinically significant improvement of symptoms was observed in 65% of patients undergoing FMT after 3 months, compared to 43% of control subjects receiving their own feces ( 119 ). In patients with frozen FMT, as opposed to fresh transplants, better results were obtained ( 119 ). In addition, other studies suggest that fresh or frozen donated stool may provide benefits, while capsulized FMT could be harmful ( 118 , 120 ). El-Salhy et al. ( 121 ) conducted a study evaluating the effects of two different doses (30 and 60 g) of FMT. There was a response in 23.6% of the patients who received a placebo, 76.9% who got 30 g FMT, and 89.1% who got 60 g FMT, suggesting a dose-dependent response ( 121 ). Holvoet et al. ( 122 ) suggested a microbiota modulation strategy through FMT could benefit the subgroups with severe bloating and flatulence, in whom there is the most profound disruption to gut microbial composition. FMT also appears to be dependent on the donor, so having a superdonor available would be crucial to ensuring the success of treatment ( 123 ). In addition, having a greater diversity of microbes pre-FMT makes it more likely that the individual will respond positively to FMT ( 122 ). Increasing microbial diversity would seem counterintuitive if FMT succeeded; possibly other transplanted components were responsible for its efficacy.
A Danish trial by Halkjær et al. ( 124 ) presented results that conflicted with the reporting of beneficial effects. During this randomized, double-blind, and placebo-controlled trial, 52 patients with moderate-to-severe IBS were randomized to FMT capsules or placebo for 12 days, with a follow up of 6 months. Although FMT altered gut microbiota composition, patients in the placebo group experienced greater relief of symptoms after 3 months than those in the treatment group. The authors state that altered gut microbiota does not suffice to obtain clinical improvement in IBS ( 121 ). In this regard, it remains to be seen if altered microbiota composition, function, and abundance contribute to rather than result from IBS.
FMT has been accompanied by sparse long-term follow-up data in people with IBS, but it must be stressed that it is not without risks. A study found 20% of the FMT group experienced side effects vs. 2% of the autologous FMT group, including two patients with diverticulitis in the FMT group and none with diverticulitis in the patients with autologous feces ( 121 ). FMT, as currently practiced, is only investigated in a research setting, and the available data on its potential effects is not sufficient to make any conclusive conclusions. Before the FMT can be made available as an openly available treatment option, more large-scale clinical trials are necessary.
Based on the hot topics outlined, we know that there has been a marked improvement in knowledge regarding IBS in recent years, with an increasing understanding of the BGM axis as well as potentially effective therapeutic options. Since IBS is defined as one of disorders of the gut-brain interaction, psychological aspects of IBS require attention.
An estimated 44 and 25% of IBS patients seen in gastroenterology clinics with anxiety and depression constitute the significant cohort of psychiatric comorbidity, respectively ( 125 ). The proposed biopsychological model of IBS implies that gut microbiota influence anxiety and depression secondarily (bottom-up model) and that psychological factors themselves lead to gut microbiota reconfigurations (top-down model) ( 126 ). To date, however, the mechanisms underpinning these psychological comorbidities remain unresolved.
It is interesting to note that most IBS patients with comorbid anxiety and depression manifest gastrointestinal symptoms prior to presenting with psychiatric symptoms ( 127 ) and as described by our analysis, the current landscape of IBS research also appears to primarily focus on the bottom-up approach, with efforts to delineate the roles of gut microbiota in dysregulation of the hypothalamic-pituitary-adrenal axis, the crosstalk between gut microbiota and the host’s immune system (e.g., microbe-associated molecular patterns), the increased intestinal permeability in the setting of inflammation, and the involvement of microbial metabolites, such as SCFAs and neuroactive molecules, in gut-brain communication. The presence of neuroinflammation associated with low-grade intestinal and systemic inflammation in IBS seems to contribute to psychiatric comorbidity ( 128 – 130 ), despite there still being a limited understanding of the nature of this entity that sits at the intersection between IBS, depression, and anxiety. In fact, in few studies, gut microbiota signatures associated with psychiatric comorbidity in IBS have been evaluated ( 95 , 131 , 132 ). In these studies, at least, it appears likely that comorbid patients cluster differently from patients with IBS, depression, or anxiety alone. Research efforts should be intensified to uncover why certain microbiota alterations result in IBS in some cases while in other cases leading to IBS with psychological disorders.
It has also been established but recently further demonstrated in recent preclinical studies that stress, a major contributor to the development of IBS and depression in later life, can also affect the composition and function of the gut microbiota ( 133 , 134 ). BGM axis is a bidirectional pathway; however, research on top-down hypothesis in this subset of patients appears to be lagging.
In this study, based on the 4,092 documents on IBS research retrieved from the WOSCC from 2007 to 2022, we conducted a bibliometric analysis of the knowledge structure, active research topics as well as emerging trends of IBS research. Overall, scientific production showed an upward trend. The United States and Sweden remained dominant in the IBS field with a high number of publications, great scholarly impact, and broad collaboration network in terms of authorship, (intra- and inter-) nationally and institutionally. MAGNUS SIMREN was the predominant contributor to the field with a high academic impact. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society , World journal of gastroenterology , Alimentary pharmacology and therapeutics , The American journal of gastroenterology , Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association , Digestive diseases and sciences , Gastroenterology , and Journal of gastroenterology and hepatology were deemed core journals in the field with high publications and co-citations. In addition, this study identified the LFD, EECs, tight junctions, neuroimaging and gut microbiota, and FMT as the research foci in the IBS field.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
XT, FW, and BZ led the team and were responsible for all aspects of the project. TZ, XM, and WT contributed to the methods, data acquisition, results, and interpretation. WT, JZ, and YW participated in designing and writing the manuscript. TZ, BZ, WT, and JZ revised this manuscript critically for important intellectual content. XT gave final approval of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (No. 81830118), the National Natural Science Foundation of China (No. 81804089), the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No: ZYYCXTD-C-202010), and Special Fund for Basic Scientific Research Business of Central Public Welfare Scientific Research Institute (No. ZZ13-YQ-003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, et al. Irritable bowel syndrome. Nat Rev Dis Primers. (2016) 2:16014. doi: 10.1038/nrdp.2016.14
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. (2017) 66:1075–82. doi: 10.1136/gutjnl-2015-311240
3. Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. (2012) 10:712–21.e4. doi: 10.1016/j.cgh.2012.02.029
4. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. (2014) 6:71–80. doi: 10.2147/CLEP.S40245
5. Schmulson MJ, Drossman DA. What Is New in Rome IV. J Neurogastroenterol Motil. (2017) 23:151–63. doi: 10.5056/jnm16214
6. Spiller R, Aziz Q, Creed F, Emmanuel A, Houghton L, Hungin P, et al. Clinical Services Committee of The British Society of Gastroenterology. Guidelines on the irritable bowel syndrome: mechanisms and practical management. Gut. (2007) 56:1770–98. doi: 10.1136/gut.2007.119446
7. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. (2006) 130:1480–91. doi: 10.1053/j.gastro.2005.11.061
8. Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The Microbiome and Irritable Bowel Syndrome - A Review on the Pathophysiology, Current Research and Future Therapy. Front Microbiol. (2019) 10:1136. doi: 10.3389/fmicb.2019.01136
9. Schoenfeld PS. Advances in IBS 2016: A Review of Current and Emerging Data. Gastroenterol Hepatol (N Y). (2016) 12(8 Suppl. 3):1–11.
Google Scholar
10. Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. (2014) 20:6759–73. doi: 10.3748/wjg.v20.i22.6759
11. Thompson WG. The treatment of irritable bowel syndrome. Aliment Pharmacol Ther. (2002) 16:1395–406. doi: 10.1046/j.1365-2036.2002.01312.x
12. Devanarayana NM, Rajindrajith S. Irritable bowel syndrome in children: Current knowledge, challenges and opportunities. World J Gastroenterol. (2018) 24:2211–35. doi: 10.3748/wjg.v24.i21.2211
13. Maxion-Bergemann S, Thielecke F, Abel F, Bergemann R. Costs of irritable bowel syndrome in the UK and US. Pharmacoeconomics. (2006) 24:21–37. doi: 10.2165/00019053-200624010-00002
14. Kwan AC, Hu WH, Chan YK, Yeung YW, Lai TS, Yuen H. Prevalence of irritable bowel syndrome in Hong Kong. J Gastroenterol Hepatol. (2002) 17:1180–6. doi: 10.1046/j.1440-1746.2002.02871.x
15. Cash BD, Lacy BE, Rao T, Earnest DL. Rifaximin and eluxadoline - newly approved treatments for diarrhea-predominant irritable bowel syndrome: what is their role in clinical practice alongside alosetron? Expert Opin Pharmacother. (2016) 17:311–22. doi: 10.1517/14656566.2016.1118052
16. Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000 - 2014). Expert Opin Biol Ther. (2014) 14:1295–317. doi: 10.1517/14712598.2014.920813
17. Thomson Reuters. Using Bibliometrics: A Guide to Evaluating Research Performance with Citation Data. White Paper. Toronto, CA: Thomson Reuters (2008).
18. Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. (2004) 101(Suppl. 1):5303–10. doi: 10.1073/pnas.0307513100
19. Chen C. CiteSpace II: Detecting and Visualizing Emerging Trends and Transient Patterns in Scientific Literature. J Am Soc Inf Sci Technol (2006) 57:359–77. doi: 10.1002/asi.20317
CrossRef Full Text | Google Scholar
20. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
21. Boyack KW, Klavans R. Co-Citation Analysis, Bibliographic Coupling, and Direct Citation: Which Citation Approach Represents the Research Front Most Accurately? J Am Soc Inf Sci Technol (2010) 61:2389–404. doi: 10.1002/asi.21419
22. Tsay M-Y, Xu H, Wu C-W. Journal Co-Citation Analysis of Semiconductor Literature. Scientometrics (2003) 57:7–25. doi: 10.1023/a:1023667318934
23. Trujillo CM, Long TM. Document co-citation analysis to enhance transdisciplinary research. Sci Adv. (2018) 4:e1701130. doi: 10.1126/sciadv.1701130
24. Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, et al. Bowel disorders. Gastroenterology. (2016) 150:1393–407. doi: 10.1053/j.gastro.2016.02.031
25. Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features and Rome IV. Gastroenterology. (2016). 150:1262–79. doi: 10.1053/j.gastro.2016.02.032
26. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. JAMA. (2015) 313:949–58. doi: 10.1001/jama.2015.0954
27. Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. (2014) 146:67–75.e5. doi: 10.1053/j.gastro.2013.09.046
28. Ford AC, Moayyedi P, Lacy BE, Lembo AJ, Saito YA, Schiller LR, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. (2014) 109(Suppl. 1):S2–26. doi: 10.1038/ajg.2014.187
29. Spiller R, Major G. IBS and IBD - separate entities or on a spectrum? Nat Rev Gastroenterol Hepatol. (2016) 13:613–21. doi: 10.1038/nrgastro.2016.141
30. Ford AC, Lacy BE, Talley NJ. Irritable Bowel Syndrome. N Engl J Med. (2017) 376:2566–78. doi: 10.1056/NEJMra1607547
31. Tap J, Derrien M, Törnblom H, Brazeilles R, Cools-Portier S, Doré J, et al. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology. (2017) 152:111–23.e8. doi: 10.1053/j.gastro.2016.09.049
32. Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, Törnblom H, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. (2015) 149:1399–407.e2. doi: 10.1053/j.gastro.2015.07.054
33. Pittayanon R, Lau JT, Yuan Y, Leontiadis GI, Tse F, Surette M, et al. Gut Microbiota in Patients With Irritable Bowel Syndrome-A Systematic Review. Gastroenterology. (2019) 157:97–108. doi: 10.1053/j.gastro.2019.03.049
34. Simrén M, Barbara G, Flint HJ, Spiegel BM, Spiller RC, Vanner S, et al. Rome Foundation Committee. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut. (2013) 62:159–76. doi: 10.1136/gutjnl-2012-302167
35. Li H, An H, Wang Y, Huang J, Gao X. Evolutionary Features of Academic Articles Co-Keyword Network and Keywords Co-Occurrence Network: Based on Two-Mode Affiliation Network. Physica A: Stat Mechanics Its Appl (2016) 450:657–69. doi: 10.1016/j.physa.2016.01.017
36. Lee P-C, Su H-N. Investigating the Structure of Regional Innovation System Research Through Keyword Co-Occurrence and Social Network Analysis. Innovation (2010) 12:26–40. doi: 10.5172/impp.12.1.26
37. Philipson L. Medical research activities, funding, and creativity in Europe: comparison with research in the United States. JAMA. (2005) 294:1394–8. doi: 10.1001/jama.294.11.1394
38. Fontanarosa PB, DeAngelis CD, Hunt N. Medical research–state of the science. JAMA. (2005) 294:1424–5. doi: 10.1001/jama.294.11.1424
39. Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol Stress. (2017) 7:124–36. doi: 10.1016/j.ynstr.2017.03.001
40. Wang HX, Wang YP. Gut Microbiota-brain Axis. Chin Med J (Engl). (2016) 129:2373–80. doi: 10.4103/0366-6999.190667
41. American College of Gastroenterology Task Force on Irritable Bowel Syndrome, Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, et al. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. (2009) 104(Suppl. 1):S1–35. doi: 10.1038/ajg.2008.122
42. Pimentel M, Lembo A, Chey WD, Zakko S, Ringel Y, Yu J, et al. TARGET Study Group. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med. (2011) 364:22–32. doi: 10.1056/NEJMoa1004409
43. Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. (2014) 63:1617–25. doi: 10.1136/gutjnl-2013-305989
44. Saito YA, Almazar AE, Tilkes KE, Choung RS, Van Norstrand MD, Schleck CD, et al. Randomised clinical trial: pregabalin vs placebo for irritable bowel syndrome. Aliment Pharmacol Ther. (2019) 49:389–97. doi: 10.1111/apt.15077
45. Mangel AW, Bornstein JD, Hamm LR, Buda J, Wang J, Irish W, et al. Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther. (2008) 28:239–49. doi: 10.1111/j.1365-2036.2008.03730.x
46. Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. (2012) 107:1702–12. doi: 10.1038/ajg.2012.254
47. Didari T, Mozaffari S, Nikfar S, Abdollahi M. Effectiveness of probiotics in irritable bowel syndrome: Updated systematic review with meta-analysis. World J Gastroenterol. (2015) 21:3072–84. doi: 10.3748/wjg.v21.i10.3072
48. Jalanka-Tuovinen J, Salojärvi J, Salonen A, Immonen O, Garsed K, Kelly FM, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut. (2014) 63:1737–45. doi: 10.1136/gutjnl-2013-305994
49. Wadhwa A, Al Nahhas MF, Dierkhising RA, Patel R, Kashyap P, Pardi DS, et al. High risk of post-infectious irritable bowel syndrome in patients with Clostridium difficile infection. Aliment Pharmacol Ther. (2016) 44:576–82. doi: 10.1111/apt.13737
50. Oka P, Parr H, Barberio B, Black CJ, Savarino EV, Ford AC. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5:908-917. doi: 10.1016/S2468-1253(20)30217-X
CrossRef Full Text Epub 2020 Jul 20. Erratum Lancet Gastroenterol Hepatol. (2020) 5:e8. | Google Scholar
51. Rodiño-Janeiro BK, Vicario M, Alonso-Cotoner C, Pascua-García R, Santos JA. Review of Microbiota and Irritable Bowel Syndrome: Future in Therapies. Adv Ther. (2018) 35:289–310. doi: 10.1007/s12325-018-0673-5
52. Muir JG, Gibson PR. The Low FODMAP Diet for Treatment of Irritable Bowel Syndrome and Other Gastrointestinal Disorders. Gastroenterol Hepatol (N Y). (2013) 9:450–2.
53. Murray K, Wilkinson-Smith V, Hoad C, Costigan C, Cox E, Lam C, et al. Differential effects of FODMAPs (fermentable oligo-, di-, mono-saccharides and polyols) on small and large intestinal contents in healthy subjects shown by MRI. Am J Gastroenterol. (2014) 109:110–9. doi: 10.1038/ajg.2013.386
54. Vanhoutvin SA, Troost FJ, Kilkens TO, Lindsey PJ, Hamer HM, Jonkers DM, et al. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil. (2009) 21:952–e76. doi: 10.1111/j.1365-2982.2009.01324.x
55. Hill P, Muir JG, Gibson PR. Controversies and Recent Developments of the Low-FODMAP Diet. Gastroenterol Hepatol (N Y). (2017) 13:36–45.
56. Gibson PR. History of the low FODMAP diet. J Gastroenterol Hepatol. (2017) 32(Suppl. 1):5–7. doi: 10.1111/jgh.13685
57. Staudacher HM, Whelan K, Irving PM, Lomer MC. Comparison of symptom response following advice for a diet low in fermentable carbohydrates (FODMAPs) versus standard dietary advice in patients with irritable bowel syndrome. J Hum Nutr Diet. (2011) 24:487–95. doi: 10.1111/j.1365-277X.2011.01162.x
58. Chumpitazi BP, Cope JL, Hollister EB, Tsai CM, McMeans AR, Luna RA, et al. Randomised clinical trial: gut microbiome biomarkers are associated with clinical response to a low FODMAP diet in children with the irritable bowel syndrome. Aliment Pharmacol Ther. (2015) 42:418–27. doi: 10.1111/apt.13286
59. Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. (2017) 66:1517–27. doi: 10.1136/gutjnl-2017-313750
60. Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, et al. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. (2012) 142:1510–8. doi: 10.3945/jn.112.159285
61. Staudacher HM, Whelan K. Altered gastrointestinal microbiota in irritable bowel syndrome and its modification by diet: probiotics, prebiotics and the low FODMAP diet. Proc Nutr Soc. (2016) 75:306–18. doi: 10.1017/S0029665116000021
62. Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. (2015) 64:93–100. doi: 10.1136/gutjnl-2014-307264
63. Bennet SMP, Böhn L, Störsrud S, Liljebo T, Collin L, Lindfors P, et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut. (2018) 67:872–81. doi: 10.1136/gutjnl-2016-313128
64. Cao Y, Shen J, Ran ZH. Association between Faecalibacterium prausnitzii Reduction and Inflammatory Bowel Disease: A Meta-Analysis and Systematic Review of the Literature. Gastroenterol Res Pract. (2014) 2014:872725. doi: 10.1155/2014/872725
65. McIntosh K, Reed DE, Schneider T, Dang F, Keshteli AH, De Palma G, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017 Jul;66(7):1241-1251. doi: 10.1136/gutjnl-2015-311339
PubMed Abstract | CrossRef Full Text Epub 2016 Mar 14. Erratum Gut. (2019) 68:1342. | Google Scholar
66. Valeur J, Småstuen MC, Knudsen T, Lied GA, Røseth AG. Exploring Gut Microbiota Composition as an Indicator of Clinical Response to Dietary FODMAP Restriction in Patients with Irritable Bowel Syndrome. Dig Dis Sci. (2018) 63:429–36. doi: 10.1007/s10620-017-4893-3
67. Rossi M, Aggio R, Staudacher HM, Lomer MC, Lindsay JO, Irving P, et al. Volatile Organic Compounds in Feces Associate With Response to Dietary Intervention in Patients With Irritable Bowel Syndrome. Clin Gastroenterol Hepatol. (2018) 16:385–91.e1. doi: 10.1016/j.cgh.2017.09.055
68. Sandström O, el-Salhy M. Human rectal endocrine cells and aging. Mech Ageing Dev. (1999) 108:219–26. doi: 10.1016/s0047-6374(99)00015-9
69. Kyösola K, Penttilä O, Salaspuro M. Rectal mucosal adrenergic innervation and enterochromaffin cells in ulcerative colitis and irritable colon. Scand J Gastroenterol. (1977) 12:363–7. doi: 10.3109/00365527709180942
70. Verity MA, Mellinkoff SM, Frankland M, Greipel M. Serotonin content and argentaffin and Paneth cell changes in ulcerative colitis. Gastroenterology. (1962) 43:24–31.
71. Gunawardene AR, Corfe BM, Staton CA. Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol. (2011) 92:219–31. doi: 10.1111/j.1365-2613.2011.00767.x
72. Rindi G, Leiter AB, Kopin AS, Bordi C, Solcia E. The “normal” endocrine cell of the gut: changing concepts and new evidences. Ann N Y Acad Sci. (2004) 1014:1–12. doi: 10.1196/annals.1294.001
73. El-Salhy M, Seim I, Chopin L, Gundersen D, Hatlebakk JG, Hausken T. Irritable bowel syndrome: the role of gut neuroendocrine peptides. Front Biosci (Elite Ed). (2012) 4:2783–800. doi: 10.2741/e583
74. El-Salhy M, Gundersen D, Gilja OH, Hatlebakk JG, Hausken T. Is irritable bowel syndrome an organic disorder? World J Gastroenterol. (2014) 20:384–400. doi: 10.3748/wjg.v20.i2.384
75. El-Salhy M, Hausken T, Gilja OH, Hatlebakk JG. The possible role of gastrointestinal endocrine cells in the pathophysiology of irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. (2017) 11:139–48. doi: 10.1080/17474124.2017.1269601
76. Jenny M, Uhl C, Roche C, Duluc I, Guillermin V, Guillemot F, et al. Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. (2002) 21:6338–47. doi: 10.1093/emboj/cdf649
77. El-Salhy M. Possible role of intestinal stem cells in the pathophysiology of irritable bowel syndrome. World J Gastroenterol. (2020) 26:1427–38. doi: 10.3748/wjg.v26.i13.1427
78. El-Salhy M, Hausken T, Hatlebakk JG. Density of Musashi-1-positive stem cells in the stomach of patients with irritable bowel syndrome. Mol Med Rep. (2020) 22:3135–40. doi: 10.3892/mmr.2020.11412
79. Mazzawi T, Gundersen D, Hausken T, El-Salhy M. Increased gastric chromogranin A cell density following changes to diets of patients with irritable bowel syndrome. Mol Med Rep. (2014) 10:2322–6. doi: 10.3892/mmr.2014.2498
80. Mazzawi T, Gundersen D, Hausken T, El-Salhy M. Increased chromogranin a cell density in the large intestine of patients with irritable bowel syndrome after receiving dietary guidance. Gastroenterol Res Pract. (2015) 2015:823897. doi: 10.1155/2015/823897
81. Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Effect of dietary management on the gastric endocrine cells in patients with irritable bowel syndrome. Eur J Clin Nutr. (2015) 69:519–24. doi: 10.1038/ejcn.2014.151
82. Mazzawi T, Hausken T, Gundersen D, El-Salhy M. Dietary guidance normalizes large intestinal endocrine cell densities in patients with irritable bowel syndrome. Eur J Clin Nutr. (2016) 70:175–81. doi: 10.1038/ejcn.2015.191
83. Mazzawi T, Lied GA, Sangnes DA, El-Salhy M, Hov JR, Gilja OH, et al. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS One. (2018) 13:e0194904. doi: 10.1371/journal.pone.0194904
84. Mazzawi T, Hausken T, El-Salhy M. Changes in colonic enteroendocrine cells of patients with irritable bowel syndrome following fecal microbiota transplantation. Scand J Gastroenterol. (2022) 1–5. doi: 10.1080/00365521.2022.2036809 [Epub ahead of print].
85. Mazzawi T, El-Salhy M, Lied GA, Hausken T. The Effects of Fecal Microbiota Transplantation on the Symptoms and the Duodenal Neurogenin 3, Musashi 1, and Enteroendocrine Cells in Patients With Diarrhea-Predominant Irritable Bowel Syndrome. Front Cell Infect Microbiol. (2021) 11:524851. doi: 10.3389/fcimb.2021.524851
86. El-Salhy M, Gundersen D, Hatlebakk JG, Gilja OH, Hausken T. Abnormal rectal endocrine cells in patients with irritable bowel syndrome. Regul Pept. (2014) 188:60–5. doi: 10.1016/j.regpep.2013.11.005
87. El-Salhy M, Gundersen D, Ostgaard H, Lomholt-Beck B, Hatlebakk JG, Hausken T. Low densities of serotonin and peptide YY cells in the colon of patients with irritable bowel syndrome. Dig Dis Sci. (2012) 57:873–8. doi: 10.1007/s10620-011-1948-8
88. Vona-Davis LC, McFadden DW. NPY family of hormones: clinical relevance and potential use in gastrointestinal disease. Curr Top Med Chem. (2007) 7:1710–20. doi: 10.2174/156802607782340966
89. Kojima S, Tohei A, Ikeda M, Anzai N. An Endogenous Tachykinergic NK2/NK3 Receptor Cascade System Controlling the Release of Serotonin from Colonic Mucosa. Curr Neuropharmacol. (2015) 13:830–5. doi: 10.2174/1570159x13666150825220524
90. Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, et al. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. (2008) 295:E1160–6. doi: 10.1152/ajpendo.90637.2008
91. Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. (2012) 489:231–41. doi: 10.1038/nature11551
92. Hanning N, Edwinson AL, Ceuleers H, Peters SA, De Man JG, Hassett LC, et al. Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review. Therap Adv Gastroenterol. (2021) 14:1756284821993586. doi: 10.1177/1756284821993586
93. Berkes J, Viswanathan VK, Savkovic SD, Hecht G. Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut. (2003) 52:439–51. doi: 10.1136/gut.52.3.439
94. Hayes CL, Dong J, Galipeau HJ, Jury J, McCarville J, Huang X, et al. Commensal microbiota induces colonic barrier structure and functions that contribute to homeostasis. Sci Rep. (2018) 8:14184. doi: 10.1038/s41598-018-32366-6
95. De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, Jury J, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. (2017) 9:eaaf6397. doi: 10.1126/scitranslmed.aaf6397
96. Barbaro MR, Fuschi D, Cremon C, Carapelle M, Dino P, Marcellini MM, et al. Escherichia coli Nissle 1917 restores epithelial permeability alterations induced by irritable bowel syndrome mediators. Neurogastroenterol Motil. (2018) 30:e13388. doi: 10.1111/nmo.13388
97. Edogawa S, Edwinson AL, Peters SA, Chikkamenahalli LL, Sundt W, Graves S, et al. Serine proteases as luminal mediators of intestinal barrier dysfunction and symptom severity in IBS. Gut. (2020) 69:62–73. doi: 10.1136/gutjnl-2018-317416
98. Maharshak N, Huh EY, Paiboonrungruang C, Shanahan M, Thurlow L, Herzog J, et al. Enterococcus faecalis Gelatinase Mediates Intestinal Permeability via Protease-Activated Receptor 2. Infect Immun. (2015) 83:2762–70. doi: 10.1128/IAI.00425-15
99. He X, Cui LH, Wang XH, Yan ZH, Li C, Gong SD, et al. Modulation of inflammation by toll-like receptor 4/nuclear factor-kappa B in diarrhea-predominant irritable bowel syndrome. Oncotarget . (2017) 8:113957–65. doi: 10.18632/oncotarget.23045
100. Gribar SC, Anand RJ, Sodhi CP, Hackam DJ. The role of epithelial Toll-like receptor signaling in the pathogenesis of intestinal inflammation. J Leukoc Biol. (2008) 83:493–8. doi: 10.1189/jlb.0607358
101. Ghosh S, Whitley CS, Haribabu B, Jala VR. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell Mol Gastroenterol Hepatol. (2021) 11:1463–82. doi: 10.1016/j.jcmgh.2021.02.007
102. Wang W, Xia T, Yu X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro . Inflamm Res. (2015) 64:423–31. doi: 10.1007/s00011-015-0822-0
103. Huang H, Zhao P, Xi M, Li F, Ji L. Mechanism of QingHuaZhiXie Prescription Regulating TLR4-IECs Pathway in the Intervention of Diarrhea Predominant Irritable Bowel Syndrome. Evid Based Complement Alternat Med. (2021) 2021:5792130. doi: 10.1155/2021/5792130
104. Peng L, Li ZR, Green RS, Holzman IR, Lin J. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J Nutr. (2009) 139:1619–25. doi: 10.3945/jn.109.104638
105. Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. (2012) 57:3126–35. doi: 10.1007/s10620-012-2259-4
106. Yu M, Wang Q, Ma Y, Li L, Yu K, Zhang Z, et al. Aryl Hydrocarbon Receptor Activation Modulates Intestinal Epithelial Barrier Function by Maintaining Tight Junction Integrity. Int J Biol Sci. (2018) 14:69–77. doi: 10.7150/ijbs.22259
107. Casén C, Vebø HC, Sekelja M, Hegge FT, Karlsson MK, Ciemniejewska E, et al. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther. (2015) 42:71–83. doi: 10.1111/apt.13236
108. Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol. (2018) 6:133–48. doi: 10.1016/j.jcmgh.2018.04.003
109. Mayer EA, Labus J, Aziz Q, Tracey I, Kilpatrick L, Elsenbruch S, et al. Role of brain imaging in disorders of brain-gut interaction: a Rome Working Team Report. Gut. (2019) 68:1701–15. doi: 10.1136/gutjnl-2019-318308
110. Kano M, Grinsvall C, Ran Q, Dupont P, Morishita J, Muratsubaki T, et al. Resting state functional connectivity of the pain matrix and default mode network in irritable bowel syndrome: a graph theoretical analysis. Sci Rep. (2020) 10:11015. doi: 10.1038/s41598-020-67048-9
111. Labus JS, Hollister EB, Jacobs J, Kirbach K, Oezguen N, Gupta A, et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome. (2017) 5:49. doi: 10.1186/s40168-017-0260-z
112. Labus JS, Osadchiy V, Hsiao EY, Tap J, Derrien M, Gupta A, et al. Evidence for an association of gut microbial Clostridia with brain functional connectivity and gastrointestinal sensorimotor function in patients with irritable bowel syndrome, based on tripartite network analysis. Microbiome. (2019) 7:45. doi: 10.1186/s40168-019-0656-z
113. Osadchiy V, Mayer EA, Gao K, Labus JS, Naliboff B, Tillisch K, et al. Analysis of brain networks and fecal metabolites reveals brain-gut alterations in premenopausal females with irritable bowel syndrome. Transl Psychiatry. (2020) 10:367. doi: 10.1038/s41398-020-01071-2
114. Mawe GM, Hoffman JM. Serotonin signalling in the gut–functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013 Aug;10(8):473-86. doi: 10.1038/nrgastro.2013.105
PubMed Abstract | CrossRef Full Text Epub 2013 Jun 25. Erratum Nat Rev Gastroenterol Hepatol. (2013) 10:564. | Google Scholar
115. Jacobs JP, Gupta A, Bhatt RR, Brawer J, Gao K, Tillisch K, et al. Cognitive behavioral therapy for irritable bowel syndrome induces bidirectional alterations in the brain-gut-microbiome axis associated with gastrointestinal symptom improvement. Microbiome. (2021) 9:236. doi: 10.1186/s40168-021-01188-6
116. Berentsen B, Nagaraja BH, Teige EP, Lied GA, Lundervold AJ, Lundervold K, et al. Study protocol of the Bergen brain-gut-microbiota-axis study: A prospective case-report characterization and dietary intervention study to evaluate the effects of microbiota alterations on cognition and anatomical and functional brain connectivity in patients with irritable bowel syndrome. Medicine (Baltimore). (2020) 99:e21950. doi: 10.1097/MD.0000000000021950
117. Goldenberg SD, Merrick B. The role of faecal microbiota transplantation: looking beyond Clostridioides difficile infection. Ther Adv Infect Dis. (2021) 8:2049936120981526. doi: 10.1177/2049936120981526
118. Ianiro G, Eusebi LH, Black CJ, Gasbarrini A, Cammarota G, Ford AC. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment Pharmacol Ther. (2019) 50:240–8. doi: 10.1111/apt.15330
119. Johnsen PH, Hilpüsch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. (2018) 3:17–24. doi: 10.1016/S2468-1253(17)30338-2
120. Xu D, Chen VL, Steiner CA, Berinstein JA, Eswaran S, Waljee AK, et al. Efficacy of Fecal Microbiota Transplantation in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis. Am J Gastroenterol. (2019) 114:1043–50. doi: 10.14309/ajg.0000000000000198
121. El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. (2020) 69:859–67. doi: 10.1136/gutjnl-2019-319630
122. Holvoet T, Joossens M, Vázquez-Castellanos JF, Christiaens E, Heyerick L, Boelens J, et al. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients With Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-term Results From a Placebo-Controlled Randomized Trial. Gastroenterology. (2021) 160:145–57.e8.
123. Wilson BC, Vatanen T, Cutfield WS, O’Sullivan JM. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front Cell Infect Microbiol. (2019) 9:2. doi: 10.3389/fcimb.2019.00002
124. Halkjær SI, Christensen AH, Lo BZS, Browne PD, Günther S, Hansen LH, et al. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. (2018) 67:2107–15. doi: 10.1136/gutjnl-2018-316434
125. Midenfjord I, Polster A, Sjövall H, Törnblom H, Simrén M. Anxiety and depression in irritable bowel syndrome: Exploring the interaction with other symptoms and pathophysiology using multivariate analyses. Neurogastroenterol Motil. (2019) 31:e13619. doi: 10.1111/nmo.13619
126. Stasi C, Rosselli M, Bellini M, Laffi G, Milani S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: the top-down and the bottom-up model. J Gastroenterol. (2012) 47:1177–85. doi: 10.1007/s00535-012-0627-7
127. Koloski NA, Jones M, Talley NJ. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment Pharmacol Ther. (2016) 44:592–600. doi: 10.1111/apt.13738
128. Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, et al. Increased plasma concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disord. (1995) 34:301–9. doi: 10.1016/0165-0327(95)00028-l
129. Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry. (2013) 3:e249. doi: 10.1038/tp.2013.27
130. Burns G, Carroll G, Mathe A, Horvat J, Foster P, Walker MM, et al. Evidence for Local and Systemic Immune Activation in Functional Dyspepsia and the Irritable Bowel Syndrome: A Systematic Review. Am J Gastroenterol. (2019) 114:429–36. doi: 10.1038/s41395-018-0377-0
131. Kurokawa S, Kishimoto T, Mizuno S, Masaoka T, Naganuma M, Liang KC, et al. The effect of fecal microbiota transplantation on psychiatric symptoms among patients with irritable bowel syndrome, functional diarrhea and functional constipation: An open-label observational study. J Affect Disord. (2018) 235:506–12. doi: 10.1016/j.jad.2018.04.038
132. Liu Y, Zhang L, Wang X, Wang Z, Zhang J, Jiang R, et al. Similar Fecal Microbiota Signatures in Patients With Diarrhea-Predominant Irritable Bowel Syndrome and Patients With Depression. Clin Gastroenterol Hepatol. (2016) 14:1602–11.e5. doi: 10.1016/j.cgh.2016.05.033
133. Cryan JF, O’Riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, et al. The Microbiota-Gut-Brain Axis. Physiol Rev. (2019) 99:1877–2013. doi: 10.1152/physrev.00018.2018
134. Tannock GW, Savage DC. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun. (1974) 9:591–8. doi: 10.1128/iai.9.3.591-598.1974
Keywords : irritable bowel syndrome, VOSviewer, CiteSpace, bibliometrics, hot topics, trends
Citation: Zhang T, Ma X, Tian W, Zhang J, Wei Y, Zhang B, Wang F and Tang X (2022) Global Research Trends in Irritable Bowel Syndrome: A Bibliometric and Visualized Study. Front. Med. 9:922063. doi: 10.3389/fmed.2022.922063
Received: 17 April 2022; Accepted: 09 June 2022; Published: 27 June 2022.
Reviewed by:
Copyright © 2022 Zhang, Ma, Tian, Zhang, Wei, Zhang, Wang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beihua Zhang, [email protected] ; Fengyun Wang, [email protected] ; Xudong Tang, [email protected]
† These authors have contributed equally to this work and share first authorship
This article is part of the Research Topic
Global Excellence in Gastroenterology: Europe
- AGA Journals
- AGA University
- AGA Research Foundation
- AGA Community
- AGA Job Board
- Create Account

Clinical Guidance
Our clinical guidelines and updates help you make the best evidence-based decisions for your patients.
- Library AGA’s guidelines, practice updates and care pathways in one place.
- Guideline Toolkits Comprehensive resources for managing diseases – Crohn’s disease now available.

Journals & Publications
Latest research and ideas from the GI field.
- Gastroenterology The premier journal in GI.
- Clinical Gastroenterology and Hepatology (CGH) The go-to resource in clinical GI.
- Cellular and Molecular Gastroenterology and Hepatology (CMGH) Impactful digestive biology research.
- Techniques and Innovations in Gastrointestinal Endoscopy (TIGE) Cutting-edge advances in GI endoscopy.
- Gastro Hep Advances Open access GI and hepatology journal.
- GI & Hepatology News AGA’s official newspaper.
- The New Gastroenterologist Insights for fellows and early career GIs.

Meetings & Learning
Earn CME, MOC and improve your skills.
- AGA University Your hub for the best in GI education – AGA Postgraduate Course, Tech Summit and more.
- Digestive Disease Week® The most prestigious GI meeting.
- Crohn's & Colitis Congress® The premier meeting on IBD.
- Maintenance of Certification Resources for maintaining certification.
- DDSEP® The leading self-assessment tool for GI.
- Inside Scope Podcast An AGA podcast with bite-sized education.

More than 16,000 professionals worldwide call AGA their professional home.
- Join AGA Join our diverse mix of professionals.
- Renew Membership Continue to receive exclusive benefits and discounts.
- Benefits Unrivaled by any other GI organization.
- Membership Directory Contact other AGA members.
- Recognition Awards We honor our esteemed members.
- Initiatives & Programs Advancing the science and practice of GI.
- Get Involved with AGA Help us achieve a world free from digestive diseases.
- Advocacy & AGA PAC Advancing public policies that support gastroenterology.

Practice Resources
Tools to maximize efficiency and help you deliver high-quality care.
- Practice Tools Cutting-edge resources to improve your patient care.
- New Technology & Techniques The latest innovations in GI.
- Quality & Performance Measures Support to meet reporting requirements.
- Reimbursement Tools to understand policies and advocate for reimbursement.
- GI Patient Center By specialists, for patients.

Research & Awards
Funding opportunities and other initiatives advancing discovery.
- Research Awards More than $2 million in annual research funding.
- Registries & Studies Data to support new techs and treatments.
- Gut Microbiome One of GI’s most promising areas of research.
- AGA Research Foundation Funding the future of gastroenterology.

Fellows & Early Career
Resources designed for early career gastroenterologists.
- Resources Resources for every stage of your career.
- Fellowship Match Information for programs and candidates.
- AGA GTE® The first training exam for GI programs and fellows.
- Mentoring Connect with prospective mentors.
- Job Board Find your next opportunity.
- June 21, 2022
New AGA guidelines: A targeted approach to IBS-C and IBS-D treatment
Join us for a webinar on each of the guidelines
Please join the guideline authors for Gastro Bites on IBS-C and IBS-D. During each of the 30-minute webinars, they will explain the guidance in greater detail and answer your questions about applying these guidelines in your practice.
- Register for Gastro Bites: Irritable bowel syndrome with constipation (IBS-C) taking place on Thursday, July 7 at noon EDT
- Register for Gastro Bites: Irritable bowel syndrome with diarrhea (IBS-D) taking place on Thursday, July 21 at noon EDT
Additional Resources
- IBS-C guideline
- IBS-D guideline
- Spotlight (one-page infographic)
- Clinical decision support tool
AGA clinical guidance
Find the latest evidence-based recommendations for treating your patients.

4930 Del Ray Avenue, Bethesda, MD 20814 301-654-2055
Connect with aga.
© American Gastroenterological Association

- Privacy Overview
- Strictly Necessary Cookies
- Cookie Policy
This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Strictly Necessary Cookie should be enabled at all times so that we can save your preferences for cookie settings.
If you disable this cookie, we will not be able to save your preferences. This means that every time you visit this website you will need to enable or disable cookies again.
More information about our Cookie Policy
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Gastroenterol Hepatol (N Y)
- v.11(4 Suppl 2); 2015 Apr
New and Emerging Treatment Options for Irritable Bowel Syndrome
Brian e. lacy.
Professor of Medicine Geisel School of Medicine at Dartmouth Chief of Gastroenterology and Hepatology Dartmouth-Hitchcock Medical Center Lebanon, New Hampshire
William D. Chey
Timothy T. Nostrant Professor of Medicine Division of Gastroenterology Director, GI Physiology Laboratory Co-director, Michigan Bowel Control Program University of Michigan Health System Ann Arbor, Michigan
Anthony J. Lembo
Associate Professor of Medicine Harvard Medical School Boston, Massachusetts
Irritable bowel syndrome (IBS) is a functional gastrointestinal disorder associated with abdominal pain, diarrhea, constipation, or a mix of symptoms. The pathophysiology of IBS is not completely understood but appears to involve genetics, the gut microbiome, immune activation, altered intestinal permeability, and brain-gut interactions. There is no gold standard for diagnosis. Several sets of symptom-based guidelines exist. Treatment strategies for IBS may include both nonpharmacologic and pharmacologic approaches. Lifestyle modifications that aim to improve exercise, sleep, diet, and stress may be warranted. Recent data suggest that a gluten-free diet and a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) may benefit some patients. For patients with diarrhea-predominant IBS, treatment options include the synthetic peripheral μ -opioid receptor agonist loperamide, antispasmodic agents, antidepressants, serotonin 5-HT 3 antagonists, and the gut-specific antibiotic rifaximin. Ongoing research is evaluating the use of probiotics. For patients with constipation-predominant IBS, therapeutic strategies may include dietary fiber, laxatives, and the prosecretory agents lubiprostone and linaclotide. Research is continuing to optimize the use of available agents and evaluating new approaches to further improve the care of patients with IBS.
Current Insights into the Pathophysiology of Irritable Bowel Syndrome
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder that may cause abdominal pain or discomfort, diarrhea, constipation, and related symptoms. IBS is common, with prevalence reaching more than 20% in some countries ( Figure 1 ). 1 In western countries, rates of IBS are higher in women than in men. 1 Only a fraction of affected individuals—between 25% and 50%—seek medical care for their symptoms. 1

Worldwide prevalence of irritable bowel syndrome.
IBS is categorized into subgroups based on the dominant bowel-related symptom(s). These subgroups include IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), and IBS with mixed or alternating diarrhea and constipation (IBS-M). 2 The IBS subgroups are defined based on stool consistency, which has been shown to correlate with constipation and diarrhea better than stool frequency. 2 Stool consistency is also a better surrogate for colon transit, with hard or lumpy stools predicting slow transit and loose or watery stools predicting more rapid transit ( Table 1 ). 3 Identifying the relevant subgroup is important for selecting the appropriate diagnostic tests and treatment strategies.
Accuracy of Stool Form in Predicting Delayed Transit
BSFS, Bristol Stool Form Scale; CTT, colonic transit time; ROM, radio-opaque marker; WGT, whole-gut transit; WGTT, whole-gut transit time.
Data from Saad RJ et al. Am J Gastroenterol . 2010;105(2):403-411. 3
Although an IBS diagnosis including subtype is based upon symptoms present at the time of evaluation, the majority of patients experience some change in their symptoms over time, most often from IBS-C or IBS-D to IBS-M. Less frequently, patients alternate between IBS-C and IBS-D.
Comorbidities are common in IBS and can affect both the GI tract and other systems. A substantial proportion of patients fulfill criteria for IBS and also have symptoms suggestive of other functional GI disorders, including functional chest pain, heartburn, dyspepsia, and/or abdominal pain. 4 Patients with IBS are also more likely than the general population to have other pain-related disorders, including migraine headache, fibromyalgia, and chronic pelvic pain. 5 The presence of GI manifestations and global pain disorders suggests a shared pathophysiology, perhaps as a consequence of altered pain processing. Depression and anxiety are also more common in IBS patients and influence their illness experience. 5
IBS is a costly disorder that has tremendous consequences on a societal level. Patients with IBS tend to utilize more health care services than the general population, as measured by nearly any parameter (eg, outpatient office visits, diagnostic testing, and costs attributable to over-the-counter or prescription medications). 6 In the United States, estimated annual direct costs attributable to IBS exceed $10 billion. 7 Estimated indirect costs, which exceed $20 billion, are primarily due to missed work—either absenteeism or presenteeism (reduced productivity while at work). 7
Pathophysiology of Irritable Bowel Syndrome
The pathophysiology of IBS is not fully understood. When the pathophysiology of IBS was first considered in the 1950s and 1960s, the symptoms were largely attributed to psychiatric illness. In the 1960s, the focus shifted toward abnormalities in GI motility as a cause for IBS. 8 In the 1970s and 1980s, the concept of visceral hypersensitivity was proposed, suggesting that IBS patients might perceive any of a number of stimuli differently, leading to GI symptoms. 9 In the 1990s, the concept of the brain-gut axis and related abnormalities gained popularity. 10 In the past 10 years, there have been tremendous advances in our understanding of how these different factors might fit together in the pathophysiology of IBS. 11
Genetics, the microbiome, immune activation, and altered intestinal permeability may all contribute to the pathogenesis of IBS, 11 and a biopsychosocial model has been proposed to account for the influence of interactions between the brain and the gut. 12 Factors early in life that may contribute to the development of IBS include impaired family dynamics (eg, abuse or maternal deprivation), acute GI infection, and, potentially, the use of systemic antibiotics. 11 These factors may predispose individuals to the development of abnormalities in enteric nerve dysfunction, motility, visceral sensation, and brain-gut interactions, as well as mentalhealth disorders. Later in life, environmental triggers may contribute to the development, or at least the exacerbation, of symptoms in patients with underlying abnormalities in motility, function, and sensation. In susceptible individuals, stimuli such as food or stress can be important triggers for the abdominal pain and altered bowel habits characteristic of IBS.
Recently, there has been a significant focus on the role of the gut microbiome in the pathophysiology of IBS. 13 When considering the role of the microbiota, it is helpful to recognize the significant presence of bacteria in the human body. The number of bacteria far exceeds that of the host somatic cells. At least 500 to 1000 species of bacteria have been identified in the human body. Moreover, 60% of the fecal biomass consists of bacteria.
The microbiome plays a critically important role in the normal development and function of the GI tract. Animals raised in a germ-free environment exhibit abnormal GI tract development and functioning. 14 A variety of factors influence the gut microbiome, including genetics, diet, exposure to GI pathogens, and medications ( Figure 2 ). 15 The effects of antibiotics on the microbiome are well known, and other medications may also have an impact.

Long-term dietary changes can alter the gut microbiome.
Various alterations in the microbiome have been identified in patients with IBS, who show qualitative and quantitative differences, differences in distribution within the GI tract, and a lack of microbial diversity compared with people without IBS. 16 - 20 The clinical relevance of these early observations, and their role in the cause vs effects of IBS, are not yet known.
Multiple studies have shown an association between IBS and small intestinal bacterial overgrowth (SIBO). 21 There are substantial limitations in the sensitivity and specificity of the breath tests used to detect SIBO. 20 Nonetheless, these findings are useful for exploring the hypothesis that patients with IBS may have abnormalities in the microbiome, not only in the colon but also in the small bowel. Supporting this hypothesis are studies suggesting that the microbiome in the small bowel of IBS patients may be altered both quantitatively and qualitatively. 16
Emerging evidence also suggests that intestinal permeability is impaired in patients with IBS, lending possible credence to the concept of the “leaky gut.” 22 Some patients with IBS demonstrate markers of increased immune activation, including higher mast cell concentrations and activation, altered immune markers, and elevated levels of cytokines, including tumor necrosis factor—α. 23
Patients who have experienced severe acute infectious gastroenteritis (bacterial, viral, or parasitic) are at increased risk of developing long-term IBS symptoms, a condition known as postinfectious IBS. 24 , 25 Perhaps most clinically relevant today are the differences in the natural history of patients who develop IBS after an infection. IBS is typically a chronic condition, but when it develops after an infection, symptoms will resolve in approximately two-thirds of patients in 5 to 6 years. 26 Thus, it is important to inform patients with postinfectious IBS that they are likely to improve over time, regardless of the interventions offered. At present, there is no evidence to suggest that one therapy is better than another for postinfectious IBS. In the future, however, there may be therapies specifically for postinfectious IBS that affect immune activation or the microbiome.
Multiple factors may contribute to the development of IBS, including a genetic predisposition in the presence of dysbiosis (abnormalities in the microbiome); environmental triggers, such as stress; psychologic disorders; diet; medications; and infections. Together, these factors may lead to alterations in intestinal permeability that permit increased antigen presentation, promoting immune activation. These events could lead to altered sensation and functioning of the GI tract, causing the symptoms of IBS. It has been hypothesized that systemic chemokines and cytokines may lead to the extraintestinal symptoms, such as fatigue, joint pain, and skin rash, often reported by patients with IBS. 27
Diagnostic Strategies in Irritable Bowel Syndrome
IBS is a complex disorder, and a gold standard for diagnosis does not exist. The diagnosis continues to perplex health care providers, due to several factors: symptoms are nonspecific, patients are heterogeneous, and the evaluation criteria vary across specialties and health care providers. Different beliefs regarding the etiology of IBS have led to inconsistent diagnostic strategies. Guidelines continue to change as new evidence is added to an ever-expanding database.
Currently, IBS is defined by symptoms—abdominal pain, bloating, constipation, and diarrhea—in the absence of obvious morphologic or biochemical abnormalities. These symptoms lead to a broad differential diagnosis. Nevertheless, when diagnostic criteria are fulfilled and alarm features are absent, the need for diagnostic testing should be minimal.
The Importance of a Confident Diagnosis
In an international survey of 1966 adult patients diagnosed with IBS, the diagnosis was made a mean 6.6 years (±9.7 years) after symptoms began. 1 Confirmation of a positive diagnosis of IBS is important for several reasons. A diagnosis can reassure patients about the nature of their symptoms and avoid unnecessary testing, procedures, and surgeries. 2 Patients with IBS are more likely to undergo unnecessary cholecystectomy, appendectomy, and hysterectomy than matched controls. 3 An accurate and timely diagnosis will also allow clinicians to present the management options and initiate treatment. Persistent symptoms can reduce work productivity ( Figure 3 ) and further increase the economic burden of this prevalent disorder. Lastly, a diagnosis can help patients feel confident about future treatment decisions and optimize the use of health care resources.

Impact of irritable bowel syndrome on various aspects of life, as reported in a survey of 261 patients.
The symptoms of IBS are nonspecific. Disorders that can mimic IBS include inflammatory bowel disease (IBD), celiac disease, lactose intolerance, microscopic colitis, malabsorptive disorders, GI infections, dietary intolerances, and hormonal disturbances. The first textbook of American gastroenterology, published in 1944, advocated a diagnosis of exclusion. 4 An assumption underlying this strategy was that a battery of normal test 4 would reassure the patient that nothing was wrong. Unfortunately, the use of extensive testing proved to be expensive and misguided. In a study of patients who underwent colonoscopy for their GI symptoms, a normal result did not allay patients’ fears that they were suffering from a severe organic illness. 5 In addition, invasive tests (eg, colonoscopy) place the patient at some risk.
For the diagnosis of IBS, many gastroenterologists use the definition provided by the American College of Gastroenterology: abdominal pain with disordered defecation. 6 This definition captures the key clinical IBS characteristic of abdominal pain while highlighting the changes in bowel habits. It has not yet been validated.
More detailed guidelines are also available, including the Manning criteria 7 and the Rome criteria. 8 None are ideal. The Rome III diagnostic criteria require recurrent abdominal pain/discomfort at least 3 days per month over 3 months, with symptom onset at least 6 months before diagnosis and the presence of at least 2 of the following symptoms: improvement of pain/ discomfort with defecation, onset associated with a change in stool frequency, or onset associated with a change in stool appearance. IBS subtyping is based on the predominant stool pattern using the Bristol Stool Form Scale. 9 The Rome III criteria are commonly used in research studies but not in clinical practice.
Diagnostic Tests
Bristol stool form scale.
The Bristol Stool Form Scale was developed in the 1990s in the Bristol Royal Infirmary in England. 9 The authors described 7 types of stool:
- Type 1: Separate hard lumps, like nuts (hard to pass).
- Type 2: Sausage-shaped, but lumpy.
- Type 3: Like a sausage, but with cracks on its surface.
- Type 4: Like a sausage or snake, smooth and soft.
- Type 5: Soft blobs with clear-cut edges (passed easily).
- Type 6: Fluffy pieces with ragged edges, a mushy stool.
- Type 7: Watery, no solid pieces, entirely liquid.
Stool types 3 and 4 are considered normal. Constipation is indicated by types 1 and 2. Diarrhea is indicated by types 6, 7, and (to some degree) 5. When the Bristol Stool Form Scale was first published, the authors postulated that it could be used as a surrogate marker for colon transit, but that concept has since been challenged. Nonetheless, the Bristol scale provides a convenient way for patients to describe their bowel habits, and it is routinely used in clinical trials. In IBS-C patients, more than 25% of bowel movements are types 1 or 2. In patients with IBS-D, more than 25% are types 6 or 7. In patients with the mixed subtype of alternating constipation and diarrhea, more than 25% of bowel movements are types 1 or 2, and more than 25% are types 6 or 7.
Blood Tests
If a patient presents with symptoms thought to represent IBS, meets Rome III criteria, and does not have any warning signs on history or examination, then routine serologic tests are not recommended. Systematic reviews show that patients with IBS symptoms are not more likely to have an organic disorder (eg, Crohn’s disease, ulcerative colitis, colorectal cancer, and thyroid disease) than healthy controls. 10 , 11 In clinical practice, however, health care providers routinely order a complete blood count and often a C-reactive protein (CRP) test or erythrocyte sedimentation rate (ESR) test, especially in patients with diarrhea-predominant symptoms. A recent meta-analysis has shown that this practice appears to be reasonable, as it may distinguish IBS patients from those with an organic cause for their symptoms. 12 These results must be confirmed in a large prospective study. However, given the ease, safety, and relatively low cost of this practice, it will likely be incorporated into future guidelines.
The decision to evaluate IBS patients for celiac disease is controversial. In 2009, recommendations from the American College of Gastroenterology Task Force on IBS included the use of celiac serology testing in patients with IBS. 6 More recent data, however, have shown that the prevalence of celiac disease among patients with nonconstipated IBS (0.41%) was similar to that in the general population (0.44%). 13 Decision analytic models have demonstrated that serologic screening for celiac disease is cost-effective as long as the prevalence exceeds 1%. 14 Based on these findings, routine serologic screening for celiac disease in patients with persistent IBS symptoms—primarily diarrhea or significant bloating—is currently recommended.
No single biomarker, or panel of biomarkers, can be used to confidently make the diagnosis of IBS. Some biomarkers, however, can provide insight into the nature of a patient’s symptoms. Calprotectin, a protein released by white blood cells, was evaluated as a marker to distinguish IBS from organic intestinal disease. 15 Patients with organic disease (n=263) or IBS (n=339) underwent measurement of fecal calprotectin, serologic testing of CRP and ESR, and assessment of small intestine permeability. Abnormal fecal calprotectin detected organic disease with a sensitivity of 89% and a specificity of 79%. An abnormal calprotectin test had an odds ratio (OR) for organic disease of 27.8 (95% CI, 17.6-43.7), much higher than that for elevated CRP (OR, 4.2; 95% CI, 2.9-6.1 [ P <.0001]) or ESR (OR, 3.2; 95% CI, 2.2-4.6 [ P <.0001]). Abnormal intestinal permeability detected small intestinal disease (organic disease) with a sensitivity of 63% and a specificity of 87%. Therefore, abnormal fecal calprotectin, abnormal small intestinal permeability, and an elevated CRP and ESR can distinguish organic from nonorganic intestinal disease.
The utility of fecal lactoferrin, along with fecal calprotectin and CRP, was evaluated in a study of patients with IBD or IBS. 16 Patients with ulcerative colitis or Crohn’s disease and active inflammation showed significantly higher levels of lactoferrin, calprotectin, and CRP compared with patients who had inactive inflammation or IBS. The authors reported an overall diagnostic accuracy in IBD patients of 80% for high fecal lactoferrin and calprotectin and 64% for high CRP. Another study investigating the utility of fecal lactoferrin as a marker of inflammation also found significantly higher levels in IBD patients compared with IBS patients and healthy controls. 17 High levels of lactoferrin could distinguish active IBD from IBS and healthy controls (grouped together) at a sensitivity of 67%, a specificity of 96%, a positive predictive value of 87%, and a negative predictive value of 86.8%.
A widely reported study evaluated the utility of distinguishing IBS patients from non-IBS patients by measuring a panel of 10 biomarkers. 18 In this study, the sensitivity and specificity of the biomarker algorithm for differentiating IBS from non-IBS was 50% and 88%, respectively. The positive predictive value was 81%, and the negative predictive value was 64%.
Stool Tests
Several different stool studies are available for clinicians who suspect that their patient’s symptoms represent an organic disorder of the colon. Stool studies are useful for patients with liquid/watery/loose diarrheal stool to distinguish an infection or inflammation from IBS with diarrhea. However, since infection and inflammation cause diarrhea, the absence of diarrhea (ie, formed stools) effectively excludes clinically significant inflammation or an infection. Stool examination for bacterial infections, ova, and parasites may be useful in patients with acute diarrhea (< 4 weeks in duration) but not chronic diarrhea (>4 weeks). (IBS patients fit into the latter category.) In patients with symptoms thought to represent IBS with diarrhea, results from a complete blood count and CRP can effectively differentiate IBS from IBD; no stool tests are necessary if both are normal. When there is still concern that IBD is causing the patient’s symptoms, and serologic tests for celiac disease are negative, then stool studies for fecal leukocytes and fecal calprotectin are reasonable. If these test results are negative—and the patient has normal results on a complete blood count and CRP—the diagnosis of IBD is extremely unlikely.
Breath Tests
When patients with IBS report symptoms of bloating, clinicians often consider whether underlying SIBO might be the cause. Objective testing can be performed using either lactulose or glucose as the substrate. Although lactulose breath tests are the most commonly employed, glucose breath tests may be less likely to produce false-positive results. 19 Both tests can be used to measure hydrogen and methane production and overproduction, which is seen in patients with SIBO.
Lactose intolerance seems to be slightly more prevalent in IBS patients. 6 In one study, lactose intolerance, as demonstrated by lactose breath testing, was present in approximately 38% of subjects with IBS symptoms and 26% of controls. 6 It is difficult to prove that lactose maldigestion causes IBS symptoms; self-reporting of symptoms thought secondary to lactose intolerance has not proved reliable. A trial of dietary exclusion is less expensive than a hydrogen breath test. Other carbohydrates, such as fructose and sucrose, can exacerbate IBS symptoms, although the clinical utility of routinely testing for these dietary intolerances is unknown. 20 , 21
Colonoscopic Testing
A large prospective study evaluated the use of colonoscopy and rectosigmoid biopsies in patients with suspected non-constipated IBS. 22 The only findings significantly more common in the IBS population were mucosal erythema or ulceration ( Table 2 ). In the control population, polyps and diverticulosis were significantly more common than in patients with suspected IBS. Mucosal erythema or ulceration was significantly less common in the control group. On histologic examination, patients with suspected IBS had a lower prevalence of adenomas (7.7% vs 26.1%; P <.0001) compared with controls. 22 In the IBS group, the overall rate of microscopic colitis was 1.5%; the rate was slightly higher, at 2.3%, among older patients (≥45 years). Therefore, in patients with diarrhea or alternating bowel movements, colonoscopy should include careful inspection of the terminal ileum to exclude IBD, and colonic biopsies should be performed to exclude microscopic colitis. This study highlights the fact that structural abnormalities are not more common among patients with nonconstipated IBS compared with the healthy population. The findings support previous guidelines stating that routine colonic imaging is not recommended in the evaluation of IBS in patients younger than 50 years with typical symptoms and no alarm features. 6
Colonoscopic Findings in IBS Patients Vs Controls
IBS, irritable bowel syndrome; NS, not significant.
Data from Chey WD et al. Am J Gastroenterol . 2010;105(4):859-865. 22
Radiologic Tests
Clinicians frequently ask whether radiologic imaging is required to make or confirm the diagnosis of IBS. Multiple studies from the 1980s and 1990s demonstrated that routine radiologic imaging is not helpful for the diagnosis of IBS. 6 Imaging may be appropriate for IBS patients with warning signs suggesting other maladies, physical abnormalities, or abnormal results on laboratory tests.
Current and Emerging Therapies for Irritable Bowel Syndrome
Although a variety of nonpharmacologic approaches and pharmacologic agents are available for IBS symptoms, management of these patients begins with reassurance, education, and lifestyle modification. In addition, clinicians should build a strong, therapeutic relationship with their patients, which has been shown to improve clinical outcomes. 1 For example, in a 6-week study involving 262 IBS patients, those randomized to an “augmented” patient-practitioner relationship—characterized by warmth, empathy, active listening, and a positive outlook—reported significantly greater reduction in IBS symptoms and improvement in quality of life than patients assigned to a “limited” patient-practitioner relationship. 2
Lifestyle Modifications
Lifestyle modifications, such as increased exercise, have been shown to improve IBS symptoms. In a study of IBS patients, those randomized to physical activity (20-60 minutes of moderate to vigorous exertion 3 times a week for 12 weeks) reported significant reduction in IBS symptoms compared with patients randomized to usual care. 3
Other important lifestyle factors include adequate sleep and stress management. A detailed dietary history can often provide insight into possible triggers of IBS symptoms; the most common triggers include foods containing lactose or high concentrations of fructose, fatty foods, and inadequate or excessive amounts of fiber. 4 Recently, there has been substantial focus on the role of 2 dietary triggers: gluten and fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs).
Studies of Dietary Strategies
Multiple studies have shown that a gluten-free diet can improve IBS symptoms, at least in some patients. Mayo Clinic researchers conducted a 4-week, randomized controlled trial evaluating the efficacy of a gluten-free diet in patients with IBS-D and without celiac disease. 5 After 4 weeks, bowel movement frequency was reduced in patients randomized to a gluten-free diet as compared with patients randomized to a diet containing gluten ( P =.04; Figure 4 ); this effect was more significant in patients with the HLA-DQ2 or HLA-DQ8 genes. 5 In another study, reintroduction of gluten to IBS patients who had previously responded to a gluten-free diet was associated with an increase in symptoms compared with patients who remained gluten-free. 6 These studies and others suggest that at least a subset of IBS patients might benefit from a diet with no gluten or a reduced amount.

Frequency of BMs in patients randomized to a diet with or without gluten. BM, bowel movement; BSFS, Bristol Stool Form Scale; GCD, gluten-containing diet; GFD, gluten-free diet.
Studies evaluating the low-FODMAP diet have shown that abdominal and bowel symptoms improve in some patients. Recently, a randomized, controlled crossover study compared a low-FODMAP diet to a standard Australian diet in 30 patients with IBS who were naive to prior dietary manipulations. 7 Patients met the Rome III criteria, and they did not have celiac disease. The mean age was 41 years, and 70% were women. During this study, all meals were provided, and standardized questionnaires were used to report daily symptoms. Patients rated their daily symptoms using a visual analog scale ranging from 0 to 100 (with a lower score meaning fewer symptoms). Among patients following the low-FODMAP diet, global IBS symptom scores were nearly halved as compared with the placebo group (22.8 vs44.9; P <.001). Individual symptoms of bloating and abdominal pain were also significantly better for patients on the low-FODMAP diet. The greatest symptom improvement occurred within the first 7 days in most patients; no differences were noted among IBS subtypes. Patients following the low-FODMAP diet had a significant improvement in IBS symptoms ( P <.001). 7 Stool consistency was improved in patients with all IBS subtypes. Improvement in stool frequency was reported only in patients with IBS-D. It should be noted that a FODMAP diet is fairly complicated and may be difficult for patients to initiate on their own. Consultation with a nutritionist is generally recommended. When an initial trial on a low-FODMAP diet is completed, patients should systematically reintroduce the eliminated foods to see if any can be linked to symptoms.
After appropriate lifestyle modifications have been incorporated, the next step for IBS patients with ongoing symptoms involves the use of pharmacologic treatments. In general, these treatments are directed at the predominant symptom dictated by the underlying or primary bowel pattern: constipation or diarrhea. Patients with IBS-M may require treatment for both symptoms.
Pharmacologic Strategies for Irritable Bowel Syndrome With Diarrhea
In patients with diarrhea-predominant IBS, loperamide, a synthetic peripheral μ-opioid receptor agonist that decreases colon transit and increases water absorption, is often recommended. Studies of loperamide in the treatment of IBS have shown improvements in stool consistency and frequency, but more limited effects on abdominal pain and other abdominal symptoms. 8 , 9 In a double-blind, placebo-controlled study in 60 patients with IBS, loperamide significantly improved stool frequency and consistency in patients with painless diarrhea. 8 In a smaller double-blind, placebo-controlled trial of patients with IBS-D, loperamide significantly improved stool consistency, pain, and urgency. 9 Loperamide is associated with constipation, and therefore the initial dose is low (1-2 mg/day) and then titrated up or down as needed to improve bowel function.
Antispasmodics
Antispasmodic agents are another common therapy in patients with IBS. This class of medication reduces contractions in the bowel, potentially improving abdominal pain and cramps, particularly in patients with pain that is postprandial or episodic. 10 The antispasmodic dicyclomine was shown to reduce IBS symptoms in a systematic review of randomized controlled trials. 11 Most antispasmodics available in the United States are anticholinergic agents. Although they are generally most beneficial in patients with IBS-D, low or intermittent use may also be effective in patients with IBS-C or IBS-M.
Peppermint oil is an antispasmodic that exerts its effects by reducing the influx of calcium in smooth muscle cells. A recent meta-analysis found peppermint oil to be significantly superior to placebo for global improvement of IBS symptoms and improvement in abdominal pain. 12 Peppermint oil is generally well tolerated, with the most common side effect being heartburn. A slow-release peppermint oil is now available in the United States and may reduce side effects.
Antidepressants
Antidepressants are commonly used in IBS-D. A recent meta-analysis confirmed the efficacy of antidepressants, including tricyclic antidepressants (TCAs), in the treatment of IBS symptoms. 13 A double-blind, randomized controlled trial in 50 patients with IBS-D found that low-dose amitriptyline (10 mg nightly) was associated with a significant reduction in the incidence of loose stool and feeling of incomplete defecation at 2 months compared with placebo ( P <.05). 14 The anticholinergic effects of TCAs can cause constipation, particularly at high doses, and therefore these agents are better suited for patients with IBS-D. However, similar to antispasmodics, TCAs can be effective and tolerated at lower doses in patients with IBS-C.
Other types of antidepressants, including selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors, are also frequently used in patients with IBS. Because selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors can be associated with diarrhea, they tend to be used more in patients with IBS-C.
5-HT 3 Receptor Antagonists
Alosetron Women with refractory IBS-D symptoms that are severe and unresponsive to other agents may be candidates for the serotonin 5-HT 3 antagonist alosetron. In a study from 2000, alosetron reduced abdominal pain and discomfort (the primary endpoint), stool frequency, and urgency in women with IBS-D. 15 Alosetron was initially approved by the US Food and Drug Administration (FDA) at a dose of 1 mg twice daily but was removed from the market in 2000 following reports of serious complications, such as constipation and ischemic colitis. 16 Alosetron was subsequently reintroduced under a risk management plan with a lower recommended starting dose of 0.5 mg twice daily and a narrower indication: women with severe IBS-D who had an inadequate response to conventional therapy. 17 Since the reintroduction of alosetron, serious outcomes from constipation and ischemic colitis appear to have been mitigated. 18
Ondansetron The 5-HT 3 antagonist ondansetron is used for the prevention of nausea and vomiting associated with cancer chemotherapy, radiation therapy, and surgery. It is not FDA-approved for use in IBS, but it appears to be effective. In a recent randomized, double-blind, placebo-controlled crossover study of patients with IBS-D, ondansetron met the primary endpoint of improvement in average stool consistency in the last 2 weeks of treatment ( P <.001; Figure 5 ). In addition, ondansetron was significantly more effective than placebo as assessed by improvements in urgency ( P <.001), frequency ( P <.001), bloating ( P =.002), and symptom severity ( P =.001). 19 There were no reports of ischemic colitis in this study.

Time course for stool consistency during treatment periods in a randomized, double-blind, crossover study of ondansetron.
The gut microbiota has been explored as a target of treatment in IBS, given its importance in the development of symptoms in some patients. Approaches include the use of antibiotics and probiotics. The best-studied antibiotic in IBS is rifaximin, an oral, gut-specific antibiotic. Rifaximin is minimally absorbed by the GI tract.
TARGET 1 and 2 (Rifaximin 3 Times/Day [TID] for Non-Constipation Irritable Bowel Syndrome [IBS]) were large, placebo-controlled, multicenter trials evaluating rifaximin at 550 mg 3 times per day. Treatment was administered for 2 weeks. Afterward, patients were evaluated for response during a 4-week follow-up period. The primary endpoint was the proportion of patients who had adequate relief of global IBS symptoms for at least 2 of the 4 weeks during the primary evaluation period (weeks 3 through 6).
The primary endpoint was met by significantly more patients in the rifaximin group than the placebo group in both TARGET 1 (40.8% vs 31.2%; P =.01) and TARGET 2 (40.6% vs 32.2%; P =.03). 20 Rifaximin was significantly more effective than placebo in the proportion of patients with adequate relief of bloating over the same time period in TARGET 1 (39.5% vs 28.7%; P =.005) and TARGET 2 (41.0% vs 31.9%; P =.02). 20 Other outcomes, including improvements in abdominal pain and stool consistency, were also significantly better in the rifaximin arm. The efficacy of rifaximin appeared to be durable; the improvement over placebo persisted during the 10-week drug-free follow-up period ( Figure 6 ). Adverse event rates were similar with rifaximin and placebo. 20 Specifically, rifaximin does not appear to be associated with enteric infections, such as Clostridium difficile, or with clinically significant bacterial resistance. A meta-analysis of 18 clinical trials of rifaximin in IBS found the overall number needed to treat to be 10.2. 21

Patients with adequate relief of global IBS symptoms in TARGET 1 and TARGET 2 during the 10-week follow-up period after cessation of treatment with rifaximin or placebo. IBS, irritable bowel syndrome.
Most recently, the randomized, placebo-controlled TARGET 3 study evaluated the efficacy and safety of repeat treatment with rifaximin in 636 patients with IBS-D who had previously responded to open-label rifaximin but developed recurrent symptoms during the 18-week observation period. 22 This study used an endpoint recommended by the FDA for IBS-D, which is a composite endpoint consisting of the percentage of patients who experienced improvement during at least 2 of 4 weeks in both abdominal pain (defined as ≥30% decrease from baseline in mean weekly pain score) and stool consistency (defined as ≥50% decrease from baseline in the number of days per week with bowel movements matching type 6 or 7 on the Bristol Stool Form Scale). This endpoint differed from that used in the TARGET 1 andTARGET 2 trials. Efficacy with retreatment was similar to that in the rifaximin-naive patients, with response rates of 33% compared with 25% for placebo ( P =.02), and is consistent with standards in the FDA Guidance for Clinical Evaluation of IBS drugs. 23 Notably, the symptom severity inpatients starting rifaximin retreatment was lower than it had been before the initial course of rifaximin. However, these findings indicate the efficacy of rifaximin even when given as a second course. Based on these results, re-treating patients with IBS-D who responded to an initial course of rifaximin but relapsed may be appropriate.
Probiotics are a potential treatment option for patients with IBS. The potential benefit of probiotics is thought to occur through a modification of the gut bacterial microbiome that may improve mucosal immunity and restore the gut barrier function. The best-studied probiotic to date is Bifidobacteria infantis, which in 2 large, placebo-controlled trials improved abdominal symptoms, including bloating and bowel function. 24 , 25 A 2014 meta-analysis that included multiple probiotics, many administered as combinations, found evidence to support their use in IBS. 26 However, the optimal approach in regard to which individual species, strains, or combinations to use, and at what dose and for what duration, remains unknown.
Bile Acid Binders
Bile acid binders are another potential treatment option for IBS-D. Studies have shown that a substantial proportion of IBS-D patients have evidence ofbile acid malabsorption. 27 , 28 In a systematic review of 1223 patients with IBS-D, bile acid malabsorption was mild in 26%, moderate in 32%, and severe in 10%. 29 Pharmacodynamic studies have shown that bile acid levels are associated with stool frequency and consistency, fecal fat, and colonic transit. 30 In a small, randomized, double-blind, placebo-controlled trial of patients with IBS-D, the bile acid sequestrant colesevelam hydrochloride decreased transit in the ascending colon and improved ease of stool passage and stool consistency compared with placebo. 31 Treatment with colesevelam hydrochloride delayed emptying of the ascending colon by an average of 4 hours compared with placebo. 31
Emerging Therapies for Irritable Bowel Syndrome With Diarrhea
Several new and promising therapies are on the horizon for the treatment of IBS-D. The one that is furthest in development is eluxadoline, a mixed μ-opioid receptor agonist and δ-opioid receptor antagonist. Eluxadoline (at doses of 75 mg and 100 mg) was evaluated in 2 randomized, double-blind, phase 3 clinical trials of patients with IBS-D. This study used the FDA- recommended composite endpoint based on simultaneous daily improvement in abdominal pain and stool consistency, which was evaluated from weeks 1 through 12 (FDA responder endpoint) and weeks 1 through 26 (European Medicines Agency responder endpoint). Eluxadoline was significantly more effective than placebo in daily improvement in abdominal pain and stool consistency for at least 50% of the days during weeks 1 through 12 of treatment (P <.005). 32 Eluxadoline had a greater effect on bowel-related symptoms than on abdominal pain. Pancreatitis and sphincter of Oddi dysfunction have been reported, particularly in patients without a gallbladder (eg, with a history of cholecystectomy or agenesis of the gallbladder) or those who abuse alcohol.
Therapeutic Strategies for Irritable Bowel Syndrome With Constipation
The first-line therapy for most patients with IBS-C involves over-the-counter laxatives and dietary fiber. Although these approaches improve constipation-related symptoms, few have been evaluated in clinical trials of patients with IBS-C. In a randomized trial of 139 patients with IBS-C, polyethylene glycol (PEG) 3350 was more effective than placebo as assessed by spontaneous bowel movements (the primary endpoint), responder rates, stool consistency, and straining. 33 However, changes in abdominal discomfort and pain were similar with PEG and placebo after 4 weeks. 33 Whether PEG can improve global symptoms in IBS-C therefore remains unclear. A systematic review and meta-analysis showed that fiber improved overall IBS symptoms in a relatively small proportion of patients. 34 Subgroup analysis showed that the benefits were mainly associated with soluble fibers (eg, psyllium) rather than insoluble fibers (eg, bran). 34
Currently, 2 prosecretory agents are available for the treatment of IBS-C. Lubiprostone is a chloride channel type 2 activator. By opening chloride channels, lubiprostone causes an influx of chloride, sodium, and water into the lumen, thereby increasing intestinal transit and improving bowel function. Recent evidence suggests that lubiprostone may also activate the cystic fibrosis transmembrane conductance regulator channel, perhaps stimulating muscle contraction through prostaglandin E1 receptors. 35 Two large, randomized, placebo-controlled trials evaluated lubiprostone. The primary endpoint was overall response, which was based on weekly assessments of IBS symptom relief provided by patients via an electronic diary. The trials demonstrated a significantly higher overall response with lubiprostone vs placebo (17.9% vs 10.1%; P =.0001) in patients with IBS-C ( Figure 7 ). 36 The efficacy of lubiprostone also appears to be long-lasting; a 52-week extension study showed continued improvement in overall response, as reported by patients, for up to 13 months. 37 Lubiprostone is FDA-approved for the treatment of women with IBS-C at a dose of 8 μg twice daily.

Overall response in patients with IBS-C in 2 randomized, placebo-controlled trials comparing lubiprostone vs placebo. IBS-C, irritable bowel syndrome with constipation.
Linaclotide is a 14-amino acid peptide that acts on the guanylate cyclase C receptor located on the luminal surface of intestinal epithelial cells, resulting in the activation of the cystic fibrosis transmembrane conductance regulator and subsequent chloride secretion. In 2 randomized, controlled, phase 3 trials, linaclotide was significantly more effective than placebo at improving bowel and abdominal symptoms in patients with IBS-C. 38 , 39 Both trials used the FDA’s primary composite endpoint for IBS-C (improvement of ≥30% in average daily worst abdominal pain score and increase by≥1 complete spontaneous bowel movement from baseline [same week] for at least 50% of weeks assessed) and 3 other primary endpoints, based on improvements in abdominal pain and complete spontaneous bowel movements for 9 of 12 weeks. In a 12-week study of 800 patients, the FDA endpoint was met by 33.6% of linaclotide-treated patients compared with 21.0% of placebo-treated patients ( P <.0001). 38 Another study randomized 804 IBS-C patients to linaclotide or placebo for 26 weeks. The FDA endpoint was met by 33.7% of linaclotide-treated patients vs 13.9% of placebo-treated patients ( P <.0001). 39 Linaclotide is FDA-approved for men and women with IBS-C at a dose of 290 Kg once daily.
Emerging Therapies for Irritable Bowel Syndrome With Constipation
Bile acid modulators.
Elobixibat is a minimally absorbed ileal bile acid transporter inhibitor. It reduces the active ileal reabsorption of bile acids, resulting in a net increase of bile acids entering the colon, which increases the transit of the colon. In studies of patients with chronic constipation, elobixibat improved the number of bowel movements, loosened stool consistency, and decreased straining. 40 , 41 In a double-blind, placebo-controlled study of 36 women with functional constipation, elobixibat significantly accelerated overall colonic transit ( P =.059). 40 A phase 2 study of patients with chronic idiopathic constipation used a primary endpoint of change in the number of spontaneous bowel movements during week 1 compared with baseline. The mean increase in spontaneous bowel movements for week 1 was 1.7 for placebo vs 2.5, 4.0, and 5.4 for elobixibat at doses of 5 mg, 10 mg ( P <.002), and 15 mg ( P <.001), respectively. 41 Studies in patients with IBS-C have yet to be conducted.

Guanylate Cyclase-C Receptor Agonist
Like linaclotide, plecanatide activates the guanylyl cyclase C receptor in the GI tract, leading to intracellular secretion of chloride and extracellular antinociceptive effects via excretion of cyclic guanosine monophosphate. Preliminary results from a 12-week, double-blind, placebo-controlled trial found improvement in IBS-C symptoms, including abdominal pain and bowel habits, particularly with the 3.0 mg and 9.0 mg doses. The FDA composite endpoint for IBS-C was reached by 41.9% of patients in the 3.0 mg group, 40% in the 9.0 mg group, and 24.7% in the placebo group. 42
Sodium Reuptake Inhibitors
Tenapanor is a selective inhibitor of the Na+/H+ antiport protein, which is a sodium transporter on the surface of the intestinal epithelia. By reducing the absorption of sodium, tenapanor increases water in the intestines. A recent phase 2b clinical trial reported improvement in symptoms in 371 IBS-C patients. 43 The primary endpoint—overall rate of patients with complete spontaneous bowel movements— was 60.7% with tenapanor vs 33.7% with placebo ( P <.001).
New and Emerging Treatment Options for Irritable Bowel Syndrome: Q&A
G&H Are specific probiotics useful for patients with IBS?
William D. Chey, MD There is no recommendation for a specific probiotic. A 2014 meta-analysis by the American College of Gastroenterology Task Force on the Management of Functional Bowel Disorders appeared to find benefit with probiotics when considered as a whole. 1 However, evidence did not support the use of an individual probiotic species. The initial data on B infantis are still considered to be some of the highest quality, most positive results for probiotics. 2 However, these findings have not been replicated. The combination VSL#3 strain showed benefit in a study from 2005. 3
Clinicians tend to consider using B infantis in patients with very mild symptoms. However, in a randomized controlled trial in 275 patients with mild abdominal pain or bloating, probiotics were not superior to placebo. 4 Perhaps the probiotics were ineffective, or perhaps it was an issue of the wrong population being studied, as patients with minimal symptoms are not likely to have dramatic improvement. Overall, the idea of universal probiotics for IBS is not evidence-based. Although probiotics seem to offer some benefit as a whole, we unfortunately cannot make a specific recommendation for one probiotic over another.
Brian E. Lacy, PhD, MD There is still much to learn about probiotics. Many of my IBS patients have spent significant amounts of money trying different over-the-counter probiotics. Most of these products have never been tested; they probably do not even make it to the colon. My message is “buyer beware.”
William D. Chey, MD If we think about the diversity of the microbiome between individuals, it does not make sense that one probiotic would work for everybody. The concept of manipulating the microbiome is one of the most promising new ways in which to treat patients with IBS, but there is still much to learn.
G&H What is the role of diets?
Brian E. Lacy, PhD, MD As Dr Lembo discussed, many patients are interested in the effect of diet on IBS. Between 60% to 70% of IBS patients report a worsening of symptoms after meals, 50% to 70% believe that they are intolerant to various foods, and more than 70% believe that foods are the etiologic basis for their symptoms. 5 - 11 Although a number of different diets are now employed, data supporting their use are limited. Frequently used diets include a very-low carbohydrate diet, a gluten-free diet, and a low-FODMAP diet.
One small study evaluated the impact of a very low— carbohydrate diet on GI symptoms in patients with IBS-D. 12 Among the 13 patients who completed the study, all met the primary endpoint: adequate relief of IBS symptoms for at least 2 of the 4 weeks. Significant improvements were noted in stool frequency and consistency ( P <.001 for each) and abdominal pain ( P =.007). Although the exact mechanism is unknown, restricting carbohydrates may improve IBS symptoms because these nutrients are broken down in the colon, thereby creating intestinal gas and increasing the osmotic load, which leads to diarrhea.
A low-gluten diet may improve IBS symptoms, according to the results of 2 recent trials. A double-blind, placebo-controlled rechallenge dietary study enrolled patients who had reported previous improvement in IBS symptoms when following a gluten-free diet. 13 This study evaluated whether the addition of gluten (2 bread slices and 1 muffin per day) to a gluten-free diet would impact symptoms. More patients in the gluten group reported that their symptoms were not controlled (68% vs 40%; P =.001). These patients also experienced worse abdominal pain ( P =.016), bloating ( P =.031), overall symptoms ( P =.047), and fatigue ( P =.001). Dr Lembo discussed a study in which a glutenfree diet was associated with reduced stool frequency, particularly in patients with the HLA-DQ2 or DQ8 genes. 14 Gluten also increased small bowel permeability, an effect heightened in patients with these genes.
The idea behind the low-FODMAP diet is that restricting foods that ferment in the GI tract should improve symptoms of gas, bloating, diarrhea, and urgency. Dr Lembo described results of the recent Australian study. 15 Among patients following the low-FODMAP diet, global IBS symptoms were nearly halved as compared with the placebo group. The greatest symptom improvement occurred within the first 7 days in most patients; no differences were noted among IBS subtypes. Three caveats to this study are the crossover design, the use of a “typical” Australian diet, and the small sample size. That being said, this most recent study adds further evidence to the use of a low-FODMAP diet in clinical practice.
G&H What is the role of antibiotics in the treatment of IBS?
Anthony J. Lembo, MD Rifaximin is by far the best-studied antibiotic in IBS, and it has several appealing properties. First, rifaximin is gut-specific; its predominant effect is intraluminal, and it has limited systemic availability. Second, it does not significantly alter the microbiota of the GI tract. 16 It does not tend to cause diarrhea or other superinfections such as C difficile. 7
There have been smaller studies showing some efficacy with neomycin. 18 However, neomycin is associated with more side effects than rifaximin, so it is less ideal. Systemic antibiotics have not been well studied as a treatment for IBS, and I generally do not recommend them. In contrast, single-agent rifaximin has demonstrated significantly greater efficacy over placebo as assessed by relief of global IBS symptoms and abdominal bloating. 19 Moreover, based on the TARGET 3 trial, retreatment with rifaximin in previously responding patients shows a similar efficacy of approximately 10% over placebo, suggesting that repeated treatments are also effective, with no significant adverse effects. 16
Brian E. Lacy, PhD, MD That is an important message. Some IBS patients, especially those with IBS-D or IBS-M, will do better on a gut-selective antibiotic. It can be dangerous to treat all IBS patients with broad-spectrum antibiotics, such as ciprofloxacin and metronidazole.
William D. Chey, MD It is also important to keep in mind the potential consequences of administering repeated courses of systemically absorbed antibiotics to our IBS patients, who are often young women. Although a proportion of patients will respond to antibiotics, symptoms typically recur within 3 to 6 months, and patients may therefore require multiple courses. I would caution gastroenterologists and primary care doctors against giving young women repeated courses of systemically absorbed antibiotics.
G&H What is the role of breath testing for IBS-D?
William D. Chey, MD As Dr Lacy alluded to, breath testing is a confusing topic. Abnormal results on a glucose hydrogen breath test and, perhaps to a lesser degree, a lactu-lose hydrogen breath test, provide some indication that the patient might have SIBO and would therefore likely benefit from antibiotics. The downside of breath testing is its lack of accuracy. 20 Lactulose hydrogen breath testing is sensitive because lactulose passes all the way through the small intestine and almost always reaches the colon. In fact, the lactulose hydrogen breath test was developed to measure oral-fecal transit time. Lactulose is absorbed in the small intestine in patients with SIBO. Therefore, although the lactulose hydrogen breath test is sensitive, and will pick up bacterial overgrowth anywhere in the small bowel, it is nonspecific. Studies now show that in some people, particularly IBS-D patients with rapid transit, lactulose will progress to the colon within 15 to 30 minutes of oral ingestion. 21 The notion that an early peak can be used to predict the presence of bacterial overgrowth is not true.
Glucose-based testing has the opposite issue. Because glucose is avidly absorbed by various transporters in the proximal small bowel, an oral glucose load is completely absorbed by the mid jejunum. Therefore, an increase in hydrogen or methane production indicates that fermentation is occurring somewhere in the foregut, proximal to the mid jejunum. It may be occurring in the small bowel or in the stomach. The problem is that a lot of bacterial overgrowth likely occurs in the distal small bowel, where it will not be picked up with glucose. Therefore, the glucose hydrogen breath test is more specific than the lactulose hydrogen breath test, but it is undoubtedly less sensitive. Personally, I use glucose hydrogen breath testing because it makes me feel more comfortable about the diagnosis. But I acknowledge that this use is debatable.
Anthony J. Lembo, MD I would emphasize that no studies have adequately evaluated the association between treatment response and results of breath testing.
G&H What are some areas of future research?
Anthony J. Lembo, MD The future is very promising. In the past 10 years, we have come closer to an understanding of the IBS pathophysiology. The last few years have seen interesting advancements in the understanding of the microbiota and other areas.
Brian E. Lacy, PhD, MD I agree. The past decade has seen some remarkable accomplishments in the management of IBS. There is a better understanding of IBS etiology, and we now recognize the importance of prior infections and the concept of postinfectious IBS. Initial forays have been made into understanding the interactions of the gut microbiome with both the enteric nervous system and central system, and we are starting to appreciate how these interactions contribute to the pathophysiology of IBS. In addition, treatment has moved beyond fiber. Options now include exercise, diet, probiotics, antibiotics, chloride channel activators, guanylate cyclase activators, serotonergic agents, antidepressants, and a number of behavioral interventions, including hypnotherapy and cognitive behavioral therapy. 22 , 23 Despite these gains, however, there is still much to learn about the etiology and pathophysiology of IBS. Many patients remain symptomatic.
The diagnostic criteria for IBS are currently being revised. The Rome IV guidelines should be available in the spring of 2016. Although previous iterations of the Rome criteria have been used to guide clinical research, it will be important to ensure the validity of these new criteria not just in research studies, but also in everyday practice. As a part of this review process, it will also be important to determine whether the criteria used to subtype IBS patients help predict response to therapy. Research will need to focus on the relative roles of intestinal permeability, immune activation, and the gut microbiome in the pathophysiology of IBS. It will be important to determine how these factors relate to intestinal motility, visceral hypersensitivity, and central hypersensitiv-ity—critical components of IBS symptom generation. Further research is needed into the role of biomarkers for the diagnosis of IBS. In addition, determination of the utility of these biomarkers in guiding treatment will be essential.
In terms of treatment, it will be necessary to identify predictors of response (eg, genetic factors, psychologic factors, gut microbiome factors) for IBS patients and to define whether specific IBS subtypes are more likely to respond to one intervention or another based on these factors. Further studies are needed to identify diets that may help improve symptoms and to determine whether specific diets are best for specific IBS subtypes. Patients and providers are well aware of the temporal relationship between food ingestion and symptom development in IBS patients. A better understanding of this relationship is important, as it may translate into new treatment options for IBS patients with significant postprandial distress. Bloating is an important symptom for many IBS patients. The pathophysiology is still not well understood, although recent experimental studies using diet, probiotics, and antibiotics have shed some light on this complex phenomenon. Further research in this area is needed, especially given the fact that no medication is FDA-approved for the treatment of bloating. Lastly, the need to better understand the role of the gut microbiome cannot be understated, as it may turn out to be the critical factor in symptom generation, and thus the best target to improve patients’ symptoms.
Biographies
Slide library.

For a free electronic download of these slides, please direct your browser to the following web address: http://www.gastroenterologyandhepatology.net
Supported through funding from Salix Pharmaceuticals, Inc.
Disclaimer: Funding for this monograph has been provided by Salix Pharmaceuticals, Inc. Support of this monograph does not imply the supporter’s agreement with the views expressed herein. Every effort has been made to ensure that drug usage and other information are presented accurately; however, the ultimate responsibility rests with the prescribing physician. Gastro-Hep Communications, Inc., the supporter, and the participants shall not be held responsible for errors or for any consequences arising from the use of information contained herein. Readers are strongly urged to consult any relevant primary literature. No claims or endorsements are made for any drug or compound at present under clinical investigation.
Disclosure: Dr Chey is a consultant for Ardelyx, AstraZeneca, Asubio, Furiex, Forest, Ironwood, Nestle, Prometheus, Salix, SK, Sucampo, and Takeda. He has received research grants from Ironwood, Nestle, and Prometheus.
Disclosure: Dr Lacy is a member of the Scientific Advisory Boards of Forest, Ironwood, Prometheus, Salix, and Takeda.
Disclosure: Dr Lembo is a member of the Advisory Boards of Ironwood Pharmaceuticals, Prometheus, Salix, and Forest Laboratories (now Actavis). He is a consultant for Forest, GI Health Foundation, Ironwood Pharmaceutical, Prometheus, and Salix.
Disclosures: Dr Chey is a consultant for Ardelyx, AstraZeneca, Asubio, Furiex, Forest, Ironwood, Nestle, Prometheus, Salix, SK, Sucampo, and Takeda. He has received researchgrantsfrom IroMwood, Nestle, and Prometheus. Dr Lacy is a member of the Scientific Advisory Boards of Forest, Ironwood, Prometheus, Salix, and Takeda. Dr Lembo is a member of the Advisory Boards of Iromwood Pharmaceutical, Prometheus, and Salix. He is a consultant for Forest, GI Health Foundation, Ironwood Pharmaceutical, Prometheus, and Salix.
Contributor Information
Brian E. Lacy, Professor of Medicine Geisel School of Medicine at Dartmouth Chief of Gastroenterology and Hepatology Dartmouth-Hitchcock Medical Center Lebanon, New Hampshire.
William D. Chey, Timothy T. Nostrant Professor of Medicine Division of Gastroenterology Director, GI Physiology Laboratory Co-director, Michigan Bowel Control Program University of Michigan Health System Ann Arbor, Michigan.
Anthony J. Lembo, Associate Professor of Medicine Harvard Medical School Boston, Massachusetts.
FDA approves two new drugs for irritable bowel syndrome
Two new drugs for IBS – rifaximin, an antibiotic, and eluxadoline, an antagonist and agonist of the δ and µ opioid receptors, respectively – have been approved for use in the United States.
CNRI / Science Photo Library
The US Food and Drug Administration (FDA) has approved two new treatments for patients with irritable bowel syndrome with diarrhoea (IBS-D).
Rifaximin (marketed as Xifaxan by Salix Pharmaceuticals) is an antibiotic, and eluxadoline (marketed as Viberzi by Actavis) is an antagonist and agonist of the δ and µ opioid receptors, respectively. Eluxadoline has been approved as a first-line treatment for the condition. Rifaximin is already available as an antibiotic in Europe for other conditions.
Anton Emmanuel, a gastroenterologist at University College Hospital in London, says rifaximin treatment for IBS-D is based on the theory that some people with the condition have an imbalance of bacteria in the gut. “This is a controversial theory,” he says. Some researchers in the United States believe that all patients with IBS have this imbalance. “In the UK and Sweden we’ve found it to be closer to around 15–20% of patients,” says Emmanuel.
The FDA considered two phase III studies, in which 1,258 patients were given rifaximin or placebo. In the four weeks after treatment, 40.7% of the patients given rifaximin and 31.7% of the placebo patients had relief of IBS symptoms ( P <0.001).
Rifaximin is currently used to treat recurring overt hepatic encephalopathy, in which the liver is unable to detoxify blood and which can result from infections in the gut. It is used in this instance because it remains mostly in the digestive tract and is not absorbed into the blood stream, thereby minimising side effects.
But Emmanuel raises the question about how long patients can be treated as “the effect of these antibiotics inevitably starts to wear off”. The recommended treatment course for IBS-D is three tablets daily for two weeks. If there is a recurrence, patients can be retreated up to two times. “It’s quite intrusive for the patient,” adds Emmanuel.
The most common side effects of rifaximin are nausea and an increase in the liver enzyme alanine aminotransferase. Another recognised side effect, as with other antibiotics, is Clostridium difficile infection.
Eluxadoline, the other drug to be licensed by the FDA for IBS-D, has mixed opioid receptor activity and is described by its manufacturer Actavis as a first-in-class drug.
“Antagonism of the δ opioid receptor is supposed to decrease the pain associated with IBS-D. And agonism of the µ opioid receptor slows down contractions of the bowel, working on diarrhoea,” says Emmanuel.
The FDA has granted eluxadoline a first-line licence, even though it has not undergone direct comparison trials with existing treatments, such as loperamide. Emmanuel says he “certainly wouldn’t use it first-line”.
In two phase III trials, involving 2,425 patients, eluxadoline was more effective than placebo at simultaneously reducing abdominal pain and improving stool consistency over 26 weeks of treatment.
The most common side effects in patients treated with eluxadoline include constipation, nausea and abdominal pain. In more serious cases, the drug can cause spasm in the sphincter of Oddi, located in a shared portion of the bile and pancreatic ducts, which can lead to pancreatitis. It is therefore not recommended for patients with a history of bile duct obstruction, pancreatitis, severe liver impairment, or severe constipation and for people who drink more than three alcoholic drinks per day.
In Europe, drug companies must carry out a study in children before gaining a licence from the European Medicines Agency (EMA), unless a waiver has been obtained. Actavis has had a paediatric investigation plan for 6–18 year olds approved by the EMA but has also been granted a deferral, meaning that it does not need to complete the study before applying for a licence in adults. The manufacturer has not yet submitted the medicine for approval.
You may also be interested in

MHRA approvals miss targets by more than 100 days, data show

Just out of reach: why the UK must rethink its barriers to novel medicines

DHSC to clarify to MPs that medical cannabis licensing will include clinical trials

Current IBS Studies: Help Advance Research
Here are ways that you can help advance research – in person or from the comfort of your home. Take part in these studies.
A Randomized, Double-Blind, Technology-Enabled Trial to Evaluate the Impact of a Multi-Strain Synbiotic (DS-01) on Metagenomic Stability and Metabolic Output of the Gut Microbiota.
Purpose of Study: This study aims to assess the impact of multi-strain consortia of 24 commensal organisms across 12 species with extensive strain-specific in vivo data, assessing a range of gastrointestinal symptoms without negatively altering the naive gut microbiota. High-throughput shotgun DNA sequencing will provide an opportunity for ‘-omics’-based analyses of the gut microbiota, which can be augmented by the metabolite profiles resulting from total microbial activity in the gut. Since many of these metabolites are bioeffector molecules acting upon the host, such analysis can provide a direct measure of the consequences of microbial activity in the gut and provide a novel integrated data set for patients with IBS. Recruited subjects will also use a smart-phone application to report day to day gastrointestinal symptoms, a patient-centric hallmark of this chronic gut condition.
Sponsor: Beth Israel Deaconess Medical Center
Condition : Irritable bowel Syndrome-Constipation (IBS-C) and Irritable Bowel Syndrome-Mixed (IBS-M)
Participation:
- Patient must be willing and able to give informed assent/ consent for participation in the study (see Section 15.2).
- Patient must be willing and able (in the PI’s opinion) to comply with all study requirements.
- Patient must be a premenopausal female aged 18 and older.
- Patient must have a documented history of IBS that is not completely controlled by current IBS drugs.
- Patient must have a score of ≥150 on the IBS-SSS at screening.
- Patient must have no clinically relevant (in the judgment of the PI) abnormal blood laboratory levels at screening or randomization.
- The clinician will assess eligibility as per the Rome IV criteria (Recurrent abdominal pain or discomfort at least 1 day/week in the last 3 months associated with two or more of the following: Improvement with defecation. Onset associated with a change in frequency of stool).
Study Contact: For more information, call Vivian Cheng, MS, MPH at (617)-667-0682, or Email: [email protected]
Added April 2021
Nutrition counseling study for irritable bowel syndrome.
Purpose of study : This study involves free nutrition sessions with a skilled IBS-specializing dietitian.
Sponsor: UCLA Oppenheimer Family Center for Neurobiology of Stress
Participation : Eligible male and female individuals ages 18 and older with a diagnosis of IBS with diarrhea or diarrhea with constipation (IBS-D or IBS-M). To participate, individuals must not have tried the low FODMAP diet before.
Contact : For more information, visit www.uclacns.org/patients/clinical-research or call (310) 206-1656. You may also email Nafessa Islam at [email protected]
Verified May 2018
Irritable Bowel Syndrome and Control Volunteers: Diet Challenge
Purpose of study : The purpose of this study is to study the relationship between the bile acids, short chain fatty acids and bacteria within the intestines. The hypothesis is that changes in the bacterial composition of the stool are associated with the differences in bile acids and short chain fatty acids in patients having irritable bowel syndrome compared to healthy individuals.
Sponsor: Indiana University and NIDDK
Participation : Patients with IBS, ages 18-65 fulfilling Rome IV criteria and asymptomatic controls with no prior history of GI disease or symptoms.
Participants should be on a stable and consistent diet regimen and should not be following an extreme diet intervention such as gluten-free or a low FODMAP diet at the time of study participation.
Contact : For more information, visit clinicaltrials.gov/
Contact: Tonya Hamilton 317-278-9296 [email protected] Contact: Anita Gupta 317-948-9227 ext 3179489227 [email protected]
Added February 2020
Lactobacillus LB as Treatment for Irritable Bowel Syndrome With Predominance of Diarrhea (IBS-D)
Purpose of study : The combination of Lactobacillus fermentum and Lactobacillus delbrueckii (Lactobacillus LB) has proven to be effective and safe for treatment of acute diarrhea in children. Also, a clinical trial in adult patients with chronic diarrhea, showed a reduction in the number of daily stools. However, the evidence is not enough regarding the efficacy and safety of Lactobacillus LB for treatment of patients with irritable bowel syndrome with predominance of diarrhea (IBS-D).
Sponsor: Hospital General de Mexico
Participation : Patients meeting Rome IV criteria for IBS-D, without specific medical treatment for the last 4 weeks prior to inclusion in this study.
Contact : For more information, visit https://clinicaltrials.gov/ or email [email protected]
Purpose of study : The purpose of this study is to examine brain networks at rest in chronic pain conditions compared to healthy controls.
Participation : Men and women between the ages of 18 and 55 who are diagnosed with IBS, right handed, not pregnant and no significant neurological or psychological medical history.
About the study: Participation involves a screening visit, an MRI and one stool sample.
Participants will be compensated up to $100 and get a digital picture of your brain.
Contact : For more information, visit www.uclacns.org/patients/clinical-research or call (310) 206-8545. You can also email Nafeesa Islam at [email protected]
Added May 2018
A Longitudinal Study to Identify IBS Phenotypes Using Fecal Microbiota and Hydrogen Breath Testing
Purpose of study : To provide treatment to patients with IBS-D.
Sponsor: University of Michigan and Michigan Institute for Clinical and Health Research (MICHR)
Participation : Men and women over the age of 18 diagnosed with IBS-D. Prior colonoscopy or sigmoidoscopy within the past 2 years with random colon biopsies to exclude the presence of microscopic colitis.
About the study: Patients will receive either rifaximin or low FODMAP dietary intervention.
Contact : For more information, visit https://clinicaltrials.gov/ct2/ or contact Dr. Allen Lee at (734) 936-9454, [email protected]
Participants Sought for Study on Complementary Approaches to the Treatment of IBS
Purpose of study: The purpose of this study is to find a complementary treatment to help Irritable Bowel Syndrome (IBS) patients in need of relief.
About The Study: The length of this study will be three weeks long, with a short online intervention everyday to help individuals deal with their body’s reactions to their environments. Participants will have additional surveys to assess their overall state at the beginning of the study, at the end of the three week intervention, and again at six weeks for a follow-up.
Participation: Eligible individuals between the ages of 18 – 65 years of age who are experiencing pain or discomfort associated with their gut. Cannot be smokers or have an inflammatory bowel disease.
How to Sign Up: http://bit.ly/IBS_study
There will be a $15 Target gift card for first 30 participants upon completion of study.
Study Contact: Jenna N. Ray, Health Psychologist. Phone: (919) 257 – 7291, Email: [email protected]
Verified August 2017
Canadian IBS and IBD Patients Needed for IMAGINE (Inflammation, Microbiome, and Alimentation: Gastro-Intestinal and Neuropsychiatric Effects) Study
Purpose of study: The overall aim of IMAGINE Network is to understand the interactions between diet-microbiome-host and find new therapies for the treatment of IBS, IBD and associated psychiatric disorders.
Develop innovative therapies (changes in diet, probiotics, fecal transplants or antibiotics) to improve IBD, IBS and mental health Improve outcomes of existing therapies through the assessment of diet-microbiome-host interactions Develop strategies to optimize current therapies to target those who will most benefit from medication as well as identify those in whom medication can be safely discontinued with significant personal benefit and cost savings to the Canadian healthcare system
About The Study: Transform the management of IBD and IBS and associated mental health issues with these disorders.
Sponsor: The IMAGINE (Inflammation, Microbiome, and Alimentation: Gastro-Intestinal and Neuropsychiatric Effects) Chronic Disease Network involves 17 hospitals/universities and 75 researchers across Canada who will study the interactions between the inflammation, microbiome, diet and mental health in patients with inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS). The IMAGINE Network is one of five chronic disease networks in the SPOR (Strategy for Patient Oriented Research) initiative of CIHR (Canadian Institutes of Health Research).
Participation: Persons from across Canada are being invited to participate in the IMAGINE Network. You may be eligible to participate in this study if you have been diagnosed by your physician with Irritable Bowel Syndrome (IBS), Inflammatory Bowel Disease (IBD), including ulcerative colitis or Crohn’s disease or are a healthy individual without gastrointestinal symptoms.
You will not be eligible to participate if you have any of the following criteria:
Age under 4 years. Past gastrointestinal bypass surgery or major bowel resections unrelated to Crohn’s disease. Major concurrent illness such as chronic kidney disease, chronic liver disease other than primary sclerosing cholangitis, chronic immune disease unrelated to IBD. If you have an active eating disorder such anorexia nervosa or bulimia. If you cannot communicate in either French or English.
Due to the specific nature of this research, we require that participants have not had major gastrointestinal surgery (e.g. Roux en y, bowel resection), do not have additional disease(s) that might affect ability to participate (e.g. decompensated liver disease), prescription or non-prescription drug use that is known to cause gastrointestinal (GI) symptoms (e.g. chronic antibiotic use), fad diets or eating disorders that may cause GI symptoms.
Study Contact: Aida Fernandes, Executive Director [email protected] or visit http://imaginespor.com/participate-in-research/
Added May 2020
An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Entire Site
- Research & Funding
- Health Information
- About NIDDK
- Digestive Diseases
- Irritable Bowel Syndrome (IBS)
- Clinical Trials
- Español
Clinical Trials for Irritable Bowel Syndrome
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and other components of the National Institutes of Health (NIH) conduct and support research into many diseases and conditions, including digestive diseases.
What are clinical trials for IBS?
Clinical trials—and other types of clinical studies —are part of medical research and involve people like you. When you volunteer to take part in a clinical study, you help doctors and researchers learn more about disease and improve health care for people in the future.
Researchers are studying many aspects of irritable bowel syndrome (IBS), such as
- the relationship between the colon microbiome and IBS symptoms
- genetic and neurological factors related to IBS
- the development of IBS after an acute gastrointestinal infection
Find out if clinical studies are right for you .
Watch a video of NIDDK Director Dr. Griffin P. Rodgers explaining the importance of participating in clinical trials.
What clinical studies for IBS are looking for participants?
You can view a filtered list of clinical studies on IBS that are federally funded, open, and recruiting at www.ClinicalTrials.gov . You can expand or narrow the list to include clinical studies from industry, universities, and individuals; however, the NIH does not review these studies and cannot ensure they are safe. Always talk with your health care provider before you participate in a clinical study.
What have we learned about IBS from NIDDK-funded research?
The NIDDK has supported many research projects to learn more about IBS. For example, an NIDDK-supported clinical trial found that a home-based version of cognitive behaviorally therapy led to significant and lasting improvement in IBS symptoms.
This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), part of the National Institutes of Health. NIDDK translates and disseminates research findings to increase knowledge and understanding about health and disease among patients, health professionals, and the public. Content produced by NIDDK is carefully reviewed by NIDDK scientists and other experts.
The NIDDK would like to thank: Lin Chang, M.D., David Geffen School of Medicine, University of California Los Angeles
- News, Analysis & Insights on Nutrition, Supplements, and Health
Enbiosis finds microbiome-based personalized diet more effective than low FODMAP in IBS
20-May-2024 - Last updated on 20-May-2024 at 13:42 GMT
- Email to a friend
The study, published in The American Journal of Gastroenterology , compared the efficacy of a personalized diet (PD) provided by UK based biotechnology company Enbiosis to that of a low FODMAP diet (LFD), on different sub-types of irritable bowel syndrome (IBS), with "exciting" results, according to the firm.
The PD provided by Enbiosis was based on the analysis of trillions of gut bacteria to provide personalized food and supplement advice.
This diet showed a substantial effect in improving symptom severity scores (SSS) and quality of life (QOL) scores across various subtypes of IBS but most notably showed substantial effect in alleviating symptoms in in the IBS-D (diarrhea) subtype.
While the PD intervention led to significant improvements for all subtypes, the LFD intervention showed significant improvements only for the IBS-C and IBS-D subtypes.
Beyza Hilal Ermiş, R&D nutrition specialist at Enbiosis Biotechnology, said the significant improvements in the PD group included: IBS severity and quality of life scores, anxiety and depression levels, microbiome diversity, and increased levels of beneficial bacteria like Faecalibacterium prausnitzii (notable for its anti-inflammatory and gut health properties).
"The results were truly remarkable and exceeded our expectations," she told NutraIngredients.
Conversely, the LFD intervention did not exhibit similar positive effects on gut microbiome parameters, especially in means of alpha diversity.
"These observations suggest that the PD intervention may have a broader impact on gut health, potentially contributing to its effectiveness in improving symptoms and quality of life in IBS patients," the paper stated.
Conducted and authored by researchers in Turkey, including two Enbiosis scientists, this was the first randomized controlled trial to assess a microbiome based personalized diet with an active comparator.
Study details
The study recruited 121 patients and assigned 70 to the PD group and 51 to the LFD group. IBS subtypes, demographics, symptom severity (IBS-SSS), anxiety, depression and quality of life (IBS-QOL) were evaluated. Both interventions spanned six weeks. The trial's primary outcome was the within-individual difference in IBS-SSS compared between intervention groups.
For the primary outcome, there was a change in IBS-SSS of -112.7 for those in the PD group vs -99.9 for those in the LFD group (p: 0.29).
Significant improvement occurred in IBS-SSS scores, frequency, abdominal distension and life interference in both groups. Additionally, there were significant improvements in anxiety levels and IBS-QOL scores for both groups.
Importantly, PD was effective in reducing IBS SSS scores across all IBS subtypes IBS-C, IBS-D and IBS-M subtypes, while LFD exhibited comparable improvements in IBS-C and IBS-M.
PD intervention significantly improved IBS-QOL scores for all subtypes, while the LFD did so for the IBS-C and IBS-D.
Notably, PD intervention led to significant microbiome diversity shifts and taxa alterations compared to LFD.
The authors noted that by prioritizing microbiome diversity and health, they aimed to provide a more comprehensive and targeted therapeutic strategy for individuals with IBS, potentially yielding long-term benefits beyond symptom relief
"This research highlights a significant milestone in IBS management," the firm noted. "Tailored dietary approaches, such as that provided by Enbiosis, offer a comprehensive strategy for improving the long-term health of patients with IBS. Extending beyond symptom relief, AI-powered personalized nutrition offers a means of optimizing microbial gut communities and improving overall health outcomes. Ultimately, personalized nutrition powered by AI has the potential to revolutionize the way we approach IBS care.”
Source: The American Journal of Gastroenterology doi: 10.14309/ajg.0000000000002862 "A Multicenter Randomized Controlled Trial Of Microbiome-Based Artifıcial Intelligence-Assisted Personalized Diet Vs Low Fodmap Diet: A Novel Approach for the Management of Irritable Bowel Syndrome" Authors: Tunali. V., et al
- NMN Insights Uthever® NMN Whitepaper Available Now Effepharm Ltd | Download Technical / White Paper
- Perfect skin radiance & uniformity Robertet Health & Beauty | Download Product Brochure
- Nextida: Precision where it matters Rousselot | Download Product Brochure
- Harness the power of algae for omega-3 innovation dsm-firmenich | Download Insight Guide
- Well-being from Mediterranean Nutrition Science™ Esserre Pharma | Download Product Brochure
- Optimize Nutritional Value with DuraLipo™ INNOBIO Corporation Limited | Download Technical / White Paper
Upcoming supplier webinars
- 20 Jun 2024 Thu Proteins for all: essential in sports from amateur to professional levels Ingredia SA
Upcoming editorial webinars
- 19 Jun 2024 Wed Webinar Women's health
On-demand webinars
- How to reduce crosslinking and ensure fast dissolution in soft caps
- How does Keratin hydrolysate improve Skin, hair, and nails beauty?
- Mitigating safety: Challenges in pet food manufacturing Webinar
- Premium categories: Does the humanization trend still have legs? Webinar
- Sustainable sourcing: Limiting pet industry impact on ecosystems Webinar
- Mining the microbiome: Balance is everything Webinar

NutraIngredients
- Advertise with us
- Press Releases – Guidelines
- Contact the Editor
- Report a technical problem
- Subscription Benefits
- Why Register
- Whitelist our newsletters
- Editorial Calendar
- Event Calendar
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 15 April 2020
Global burden of irritable bowel syndrome: trends, predictions and risk factors
- Christopher J. Black ORCID: orcid.org/0000-0001-5449-3603 1 , 2 &
- Alexander C. Ford 1 , 2
Nature Reviews Gastroenterology & Hepatology volume 17 , pages 473–486 ( 2020 ) Cite this article
7704 Accesses
247 Citations
226 Altmetric
Metrics details
- Epidemiology
- Irritable bowel syndrome
Irritable bowel syndrome (IBS) is one of the most common disorders of gut–brain interaction worldwide, defined according to patterns of gastrointestinal symptoms as described by the Rome diagnostic criteria. However, these criteria, developed with reference to research conducted largely in Western populations, might be limited in their applicability to other countries and cultures. Epidemiological data show a wide variation in the prevalence of IBS globally and more rigorous studies are needed to accurately determine any differences that might exist between countries as well as the potential explanations. The effects of IBS on the individual, in terms of their quality of life, and on health-care delivery and society, in terms of economic costs, are considerable. Although the magnitude of these effects seems to be comparable between nations, their precise nature can vary based on the existence of societal and cultural differences. The pathophysiology of IBS is complex and incompletely understood; genetics, diet and the gut microbiome are all recognized risk factors, but the part they play might be influenced by geography and culture, and hence their relative importance might vary between countries. This Review aims to provide an overview of the burden of IBS in a global context, to discuss future implications for the care of people with IBS worldwide, and to identify key areas for further research.
Irritable bowel syndrome (IBS) is one of the most common disorders of gut–brain interaction, estimated to affect around 1 in 10 people globally.
Prevalence rates appear to differ between countries, but the magnitude of the effect of IBS, in terms of cost and quality of life, seems comparable around the world.
The pathophysiology of IBS is complex and the role of risk factors such as genetics, diet and the microbiome might operate differently, dependent on geography.
As developing countries increasingly adopt a Western diet and lifestyle, we might see a corresponding increase in IBS prevalence rates, a trend that might also reflect increasing awareness of the condition.
Even if prevalence rates remain unchanged, projections of global population growth alone indicate that there will be many more people living with IBS worldwide.
Well-designed and adequately funded research, which is multi-cultural in design and encourages global collaboration, is needed to further advance our understanding of IBS and promote optimized patient care.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
195,33 € per year
only 16,28 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others

Irritable bowel syndrome and mental health comorbidity — approach to multidisciplinary management
The neurobiology of irritable bowel syndrome
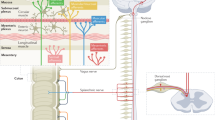
Immune activation in irritable bowel syndrome: what is the evidence?
Lovell, R. M. & Ford, A. C. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin. Gastroenterol. Hepatol. 10 , 712–721.e4 (2012).
PubMed Google Scholar
Canavan, C., West, J. & Card, T. Review article: the economic impact of the irritable bowel syndrome. Aliment. Pharmacol. Ther. 40 , 1023–1034 (2014).
CAS PubMed Google Scholar
Gwee, K. A. Irritable bowel syndrome in developing countries – a disorder of civilization or colonization? Neurogastroenterol. Motil. 17 , 317–324 (2005).
Danivat, D., Tankeyoon, M. & Sriratanaban, A. Prevalence of irritable bowel syndrome in a non-Western population. Br. Med. J. 296 , 1710 (1988).
CAS Google Scholar
Gwee, K. A., Wee, S., Wong, M. L. & Png, D. J. The prevalence, symptom characteristics, and impact of irritable bowel syndrome in an asian urban community. Am. J. Gastroenterol. 99 , 924–931 (2004).
Xiong, L. S. et al. A population-based epidemiologic study of irritable bowel syndrome in South China: stratified randomized study by cluster sampling. Aliment. Pharmacol. Ther. 19 , 1217–1224 (2004).
Corsetti, M., Tack, J., Attara, G. & Sewell, M. IBS global impact report 2018: uncovering the true burden of irritable bowel syndrome (IBS) on people’s lives. GI Society https://badgut.org/wp-content/uploads/IBS-Global-Impact-Report.pdf (2018).
Mearin, F. et al. Bowel disorders. Gastroenterology 150 , 1393–1407.e5 (2016).
Google Scholar
Sperber, A. D. et al. Conducting multinational, cross-cultural research in the functional gastrointestinal disorders: issues and recommendations. A Rome foundation working team report. Aliment. Pharmacol. Ther. 40 , 1094–1102 (2014).
Schmulson, M. et al. A four-country comparison of healthcare systems, implementation of diagnostic criteria, and treatment availability for functional gastrointestinal disorders: a report of the Rome foundation working team on cross-cultural, multinational research. Neurogastroenterol. Motil. 26 , 1368–1385 (2014).
Drossman, D. A. Rome IV Functional Gastrointestinal Disorders: Disorders of Gut-Brain Interaction Vol. 1 Ch. 7 (Rome Foundation, 2016).
Palsson, O., Heymen, S. & Whitehead, W. Abdominal pain versus abdominal discomfort: implications for diagnostic assessment of irritable bowel syndrome (IBS) [abstract P0405]. United European Gastroenterol. J. 2 , A243 (2014).
Ghoshal, U. C. et al. Epidemiological and clinical profile of irritable bowel syndrome in India: report of the Indian Society of Gastroenterology Task Force. Indian J. Gastroenterol. 27 , 22–28 (2008).
Francisconi, C. F. et al. Multicultural aspects in functional gastrointestinal disorders (FGIDs). Gastroenterology 150 , 1344–1354.e2 (2016).
Gwee, K. A., Lu, C. L. & Ghoshal, U. C. Epidemiology of irritable bowel syndrome in Asia: something old, something new, something borrowed. J. Gastroenterol. Hepatol. 24 , 1601–1607 (2009).
Holtmann, G. J. & Talley, N. J. Inconsistent symptom clusters for functional gastrointestinal disorders in Asia: is Rome burning? Gut 67 , 1911–1915 (2018).
Longstreth, G. F. et al. Functional bowel disorders. Gastroenterology 130 , 1480–1491 (2006).
Black, C. J., Yiannakou, Y., Houghton, L. A. & Ford, A. C. Epidemiological, clinical, and psychological characteristics of individuals with self-reported irritable bowel syndrome based on the Rome IV vs Rome III criteria. Clin. Gastroenterol. Hepatol. 18 , 392–398.e2 (2020).
Sperber, A. D. et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut 66 , 1075–1082 (2017).
Sperber, A. et al. Global study of the epidemiology of functional gastrointestinal disorders. ResearchGate https://www.researchgate.net/project/Global-study-of-the-epidemiology-of-the-functional-gastrointestinal-disorders (2019).
Makharia, G. K. et al. Prevalence of irritable bowel syndrome: a community based study from northern India. J. Neurogastroenterol. Motil. 17 , 82–87 (2011).
PubMed PubMed Central Google Scholar
Chang, F. Y. et al. Prevalence of functional gastrointestinal disorders in Taiwan: questionnaire-based survey for adults based on the Rome III criteria. Asia Pac. J. Clin. Nutr. 21 , 594–600 (2012).
Krogsgaard, L. R., Engsbro, A. L. & Bytzer, P. The epidemiology of irritable bowel syndrome in Denmark. A population-based survey in adults ≤50 years of age. Scand. J. Gastroenterol. 48 , 523–529 (2013).
Matsumoto, S. et al. Relationship between overactive bladder and irritable bowel syndrome: a large-scale internet survey in Japan using the overactive bladder symptom score and Rome III criteria. BJU Int. 111 , 647–652 (2013).
Perveen, I., Rahman, M. M., Saha, M., Rahman, M. M. & Hasan, M. Q. Prevalence of irritable bowel syndrome and functional dyspepsia, overlapping symptoms, and associated factors in a general population of Bangladesh. Indian J. Gastroenterol. 33 , 265–273 (2014).
Cai, S. T. et al. Overlap of gastroesophageal reflux disease and functional bowel disorders in the general Chinese rural population. J. Dig. Dis. 16 , 395–399 (2015).
Le Pluart, D. et al. Functional gastrointestinal disorders in 35,447 adults and their association with body mass index. Aliment. Pharmacol. Ther. 41 , 758–767 (2015).
Rasmussen, S. et al. Overlap of symptoms of gastroesophageal reflux disease, dyspepsia and irritable bowel syndrome in the general population. Scand. J. Gastroenterol. 50 , 162–169 (2015).
Kanazawa, M. et al. Abdominal bloating is the most bothersome symptom in irritable bowel syndrome with constipation (IBS-C): a large population-based Internet survey in Japan. Biopsychosoc. Med. 10 , 19 (2016).
Althaus, A. et al. Determinants and frequency of irritable bowel syndrome in a German sample. Z. Gastroenterol. 54 , 217–225 (2016).
Siah, K. T., Wong, R. K., Chan, Y. H., Ho, K. Y. & Gwee, K. A. Prevalence of irritable bowel syndrome in Singapore and its association with dietary, lifestyle, and environmental factors. J. Neurogastroenterol. Motil. 22 , 670–676 (2016).
Long, Y. et al. Prevalence and risk factors for functional bowel disorders in South China: a population based study using the Rome III criteria. Neurogastroenterol. Motil. 29 , e12897 (2017).
Ghoshal, U. C. & Singh, R. Frequency and risk factors of functional gastro-intestinal disorders in a rural Indian population. J. Gastroenterol. Hepatol. 32 , 378–387 (2017).
Saha, M. et al. Irritable bowel syndrome: prevalence and dietary factors in the sylhet district of Bangladesh. Mymensingh Med. J. 27 , 82–88 (2018).
Torres, M. J. et al. Food consumption and dietary intakes in 36,448 adults and their association with irritable bowel syndrome: Nutrinet-Sante study. Therap. Adv. Gastroenterol. 11 , 1756283x17746625 (2018).
Schauer, B. et al. Irritable bowel syndrome, mental health, and quality of life: data from a population-based survey in Germany (SHIP-Trend-0). Neurogastroenterol. Motil. 31 , e13511 (2019).
Iri, R. et al. Epidemiological study of irritable bowel syndrome and its related factors in Sanandaj from 2013 to 2014: a population-based study. Sci. J. Kurd. Univ. Med. Sci. 22 , 61–71 (2017).
Keshteli, A. H., Dehestani, B., Daghaghzadeh, H. & Adibi, P. Epidemiological features of irritable bowel syndrome and its subtypes among Iranian adults. Ann. Gastroenterol. 28 , 253–258 (2015).
Liu, C. et al. Prevalence of and risk factors for irritable bowel syndrome in community residents in Nanning. World Chin. J. Digestol. 22 , 5365–5370 (2014).
Miwa, H. Life style in persons with functional gastrointestinal disorders-large-scale internet survey of lifestyle in Japan. Neurogastroenterol. Motil. 24 , 464–471, e217 (2012).
Gomez Alvarez, D. F. et al. Prevalence of irritable bowel syndrome and associated factors according to the Rome III diagnostic criteria in a general population in Colombia [Spanish]. Gastroenterol. Hepatol. 32 , 395–400 (2009).
Khoshkrood-Mansoori, B. et al. Irritable bowel syndrome: a population based study. J. Gastrointestin. Liver Dis. 18 , 413–418 (2009).
Lee, S. et al. Irritable bowel syndrome is strongly associated with generalized anxiety disorder: a community study. Aliment. Pharmacol. Ther. 30 , 643–651 (2009).
Grubic, P. et al. Irritable bowel syndrome in Croatia. Coll. Antropol. 38 , 565–570 (2014).
Sperber, A. D., Shvartzman, P., Friger, M. & Fich, A. A comparative reappraisal of the Rome II and Rome III diagnostic criteria: are we getting closer to the ‘true’ prevalence of irritable bowel syndrome? Eur. J. Gastroenterol. Hepatol. 19 , 441–447 (2007).
Palsson, O. S., Whitehead, W., Tornblom, H., Sperber, A. D. & Simren, M. Prevalence of Rome IV functional bowel disorders among adults in the United States, Canada, and the United Kingdom. Gastroenterology 158 , 1262–1273.e3 (2020).
Lovell, R. M. & Ford, A. C. Effect of gender on prevalence of irritable bowel syndrome in the community: systematic review and meta-analysis. Am. J. Gastroenterol. 107 , 991–1000 (2012).
Gwee, K. A., Ghoshal, U. C. & Chen, M. Irritable bowel syndrome in Asia: pathogenesis, natural history, epidemiology, and management. J. Gastroenterol. Hepatol. 33 , 99–110 (2018).
Wigington, W. C., Johnson, W. D. & Minocha, A. Epidemiology of irritable bowel syndrome among African Americans as compared with whites: a population-based study. Clin. Gastroenterol. Hepatol. 3 , 647–653 (2005).
Kang, J. Y. Systematic review: the influence of geography and ethnicity in irritable bowel syndrome. Aliment. Pharmacol. Ther. 21 , 663–676 (2005).
Ho, K. Y., Kang, J. Y. & Seow, A. Prevalence of gastrointestinal symptoms in a multiracial Asian population, with particular reference to reflux-type symptoms. Am. J. Gastroenterol. 93 , 1816–1822 (1998).
Rajendra, S. & Alahuddin, S. Prevalence of irritable bowel syndrome in a multi-ethnic Asian population. Aliment. Pharmacol. Ther. 19 , 704–706 (2004).
Qumseya, B. J. et al. Irritable bowel syndrome in middle-aged and elderly Palestinians: its prevalence and effect of location of residence. Am. J. Gastroenterol. 109 , 723–739 (2014).
Sperber, A. D. et al. Rates of functional bowel disorders among Israeli Bedouins in rural areas compared with those who moved to permanent towns. Clin. Gastroenterol. Hepatol. 3 , 342–348 (2005).
Klooker, T. K. et al. Exposure to severe wartime conditions in early life is associated with an increased risk of irritable bowel syndrome: a population-based cohort study. Am. J. Gastroenterol. 104 , 2250–2256 (2009).
Gralnek, I. M., Hays, R. D., Kilbourne, A., Naliboff, B. & Mayer, E. A. The impact of irritable bowel syndrome on health-related quality of life. Gastroenterology 119 , 654–660 (2000).
Frank, L. et al. Health-related quality of life associated with irritable bowel syndrome: comparison with other chronic diseases. Clin. Ther. 24 , 675–689 (2002).
Singh, P. et al. Patients with irritable bowel syndrome-diarrhea have lower disease-specific quality of life than irritable bowel syndrome-constipation. World J. Gastroenterol. 21 , 8103–8109 (2015).
Buono, J. L., Carson, R. T. & Flores, N. M. Health-related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health Qual. Life Outcomes 15 , 35 (2017).
Ballou, S. et al. Effects of irritable bowel syndrome on daily activities vary among subtypes based on results from the IBS in America survey. Clin. Gastroenterol. Hepatol. 17 , 2471–2478.e36 (2019).
Bushnell, D. M., Martin, M. L., Ricci, J. F. & Bracco, A. Performance of the EQ-5D in patients with irritable bowel syndrome. Value Health 9 , 90–97 (2006).
Drossman, D. A. et al. A focus group assessment of patient perspectives on irritable bowel syndrome and illness severity. Dig. Dis. Sci. 54 , 1532–1541 (2009).
Lacy, B. E. et al. IBS patients’ willingness to take risks with medications. Am. J. Gastroenterol. 107 , 804–809 (2012).
Drossman, D. A. et al. International survey of patients with IBS: symptom features and their severity, health status, treatments, and risk taking to achieve clinical benefit. J. Clin. Gastroenterol. 43 , 541–550 (2009).
Canavan, C., West, J. & Card, T. Change in quality of life for patients with irritable bowel syndrome following referral to a gastroenterologist: a cohort study. PLoS One 10 , e0139389 (2015).
Weerts, Z. Z. R. M. et al. Reduction in IBS symptom severity is not paralleled by improvement in quality of life in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 31 , e13629 (2019).
Spiegel, B. M. et al. Clinical determinants of health-related quality of life in patients with irritable bowel syndrome. Arch. Intern. Med. 164 , 1773–1780 (2004).
World Health Organization. WHOQOL: measuring quality of life (WHO, 2019).
Kanazawa, M. et al. Patients and nonconsulters with irritable bowel syndrome reporting a parental history of bowel problems have more impaired psychological distress. Dig. Dis. Sci. 49 , 1046–1053 (2004).
Kanazawa, M. et al. Translation and validation of a Japanese version of the irritable bowel syndrome-quality of life measure (IBS-QOL-J). Biopsychosoc. Med. 1 , 6 (2007).
Park, J. M. et al. Cross-cultural validation of irritable bowel syndrome quality of life in Korea. Dig. Dis. Sci. 51 , 1478–1484 (2006).
Schmulson, M. et al. Further validation of the IBS-QOL: female Mexican IBS patients have poorer quality of life than females from North Carolina. Dig. Dis. Sci. 52 , 2950–2955 (2007).
Zhang, F., Xiang, W., Li, C. Y. & Li, S. C. Economic burden of irritable bowel syndrome in China. World J. Gastroenterol. 22 , 10450–10460 (2016).
Muller-Lissner, S. A. & Pirk, O. Irritable bowel syndrome in Germany. A cost of illness study. Eur. J. Gastroenterol. Hepatol. 14 , 1325–1329 (2002).
Nellesen, D., Yee, K., Chawla, A., Lewis, B. E. & Carson, R. T. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J. Manag. Care Pharm. 19 , 755–764 (2013).
Flacco, M. E. et al. Costs of irritable bowel syndrome in European countries with universal healthcare coverage: a meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 23 , 2986–3000 (2019).
Tack, J. et al. Economic burden of moderate to severe irritable bowel syndrome with constipation in six European countries. BMC Gastroenterol. 19 , 69 (2019).
Akehurst, R. L. et al. Health-related quality of life and cost impact of irritable bowel syndrome in a UK primary care setting. Pharmacoeconomics 20 , 455–462 (2002).
Hungin, A. P., Whorwell, P. J., Tack, J. & Mearin, F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment. Pharmacol. Ther. 17 , 643–650 (2003).
Frandemark, A., Tornblom, H., Jakobsson, S. & Simren, M. Work productivity and activity impairment in irritable bowel syndrome (IBS): a multifaceted problem. Am. J. Gastroenterol. 113 , 1540–1549 (2018).
Cash, B., Sullivan, S. & Barghout, V. Total costs of IBS: employer and managed care perspective. Am. J. Manag. Care 11 (Suppl. 1), S7–S16 (2005).
Leong, S. A. et al. The economic consequences of irritable bowel syndrome: a US employer perspective. Arch. Intern. Med. 163 , 929–935 (2003).
Poulsen, C. H. et al. Irritable bowel symptoms, use of healthcare, costs, sickness and disability pension benefits: a long-term population-based study. Scand. J. Public. Health 47 , 867–875 (2019).
Wong, R. K. et al. Partner burden in irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 11 , 151–155 (2013).
Saito, Y. A. et al. Familial aggregation of irritable bowel syndrome: a family case-control study. Am. J. Gastroenterol. 105 , 833–841 (2010).
Waehrens, R., Ohlsson, H., Sundquist, J., Sundquist, K. & Zoller, B. Risk of irritable bowel syndrome in first-degree, second-degree and third-degree relatives of affected individuals: a nationwide family study in Sweden. Gut 64 , 215–221 (2015).
Levy, R. L. et al. Irritable bowel syndrome in twins: heredity and social learning both contribute to etiology. Gastroenterology 121 , 799–804 (2001).
Bengtson, M. B., Ronning, T., Vatn, M. H. & Harris, J. R. Irritable bowel syndrome in twins: genes and environment. Gut 55 , 1754–1759 (2006).
Mohammed, I., Cherkas, L. F., Riley, S. A., Spector, T. D. & Trudgill, N. J. Genetic influences in irritable bowel syndrome: a twin study. Am. J. Gastroenterol. 100 , 1340–1344 (2005).
Jin, D. C. et al. Regulation of the serotonin transporter in the pathogenesis of irritable bowel syndrome. World J. Gastroenterol. 22 , 8137–8148 (2016).
CAS PubMed PubMed Central Google Scholar
Zhu, Y., Zheng, G. & Hu, Z. Association between SERT insertion/deletion polymorphism and the risk of irritable bowel syndrome: a meta-analysis based on 7039 subjects. Gene 679 , 133–137 (2018).
Garcia-Etxebarria, K. et al. Increased prevalence of rare sucrase-isomaltase pathogenic variants in irritable bowel syndrome patients. Clin. Gastroenterol. Hepatol. 16 , 1673–1676 (2018).
Verstraelen, T. E., Ter Bekke, R. M., Volders, P. G., Masclee, A. A. & Kruimel, J. W. The role of the SCN5A-encoded channelopathy in irritable bowel syndrome and other gastrointestinal disorders. Neurogastroenterol. Motil. 27 , 906–913 (2015).
Bonfiglio, F. et al. A GWAS meta-analysis from 5 population-based cohorts implicates ion channel genes in the pathogenesis of irritable bowel syndrome. Neurogastroenterol. Motil. 30 , e13358 (2018).
Bonfiglio, F. et al. Female-specific association between variants on chromosome 9 and self-reported diagnosis of irritable bowel syndrome. Gastroenterology 155 , 168–179 (2018).
Norcliffe-Kaufmann, L., Slaugenhaupt, S. A. & Kaufmann, H. Familial dysautonomia: History, genotype, phenotype and translational research. Prog. Neurobiol. 152 , 131–148 (2017).
Komuro, H. et al. Corticotropin-releasing hormone receptor 2 gene variants in irritable bowel syndrome. PLoS One 11 , e0147817 (2016).
Sato, N. et al. Corticotropin-releasing hormone receptor 1 gene variants in irritable bowel syndrome. PLoS One 7 , e42450 (2012).
Monsbakken, K. W., Vandvik, P. O. & Farup, P. G. Perceived food intolerance in subjects with irritable bowel syndrome–etiology, prevalence and consequences. Eur. J. Clin. Nutr. 60 , 667–672 (2006).
Buscail, C. et al. Western dietary pattern is associated with irritable bowel syndrome in the french nutrinet cohort. Nutrients 9 , 986 (2017).
PubMed Central Google Scholar
Eswaran, S. Low FODMAP in 2017: lessons learned from clinical trials and mechanistic studies. Neurogastroenterol. Motil. 29 , e13055 (2017).
Hill, P., Muir, J. G. & Gibson, P. R. Controversies and recent developments of the low-FODMAP diet. Gastroenterol. Hepatol. 13 , 36–45 (2017).
Catassi, C. et al. The overlapping area of non-celiac gluten sensitivity (NCGS) and wheat-sensitive irritable bowel syndrome (IBS): an update. Nutrients 9 , 1268 (2017).
Dionne, J. et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low FODMAPs diet in treating symptoms of irritable bowel syndrome. Am. J. Gastroenterol. 113 , 1290–1300 (2018).
Zhou, Q. et al. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut 68 , 996–1002 (2019).
Parker, T. J. et al. Irritable bowel syndrome: is the search for lactose intolerance justified? Eur. J. Gastroenterol. Hepatol. 13 , 219–225 (2001).
Varju, P. et al. Lactose intolerance but not lactose maldigestion is more frequent in patients with irritable bowel syndrome than in healthy controls: a meta-analysis. Neurogastroenterol. Motil. 31 , e13527, https://doi.org/10.1111/nmo.13527 (2018).
Article CAS PubMed PubMed Central Google Scholar
Fritscher-Ravens, A. et al. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology 157 , 109–118.e5 (2019).
Chong, P. P. et al. The microbiome and irritable bowel syndrome-a review on the pathophysiology, current research and future therapy. Front. Microbiol. 10 , 1136 (2019).
Kassinen, A. et al. The fecal microbiota of irritable bowel syndrome patients differs significantly from that of healthy subjects. Gastroenterology 133 , 24–33 (2007).
Parthasarathy, G. et al. Relationship between microbiota of the colonic mucosa vs feces and symptoms, colonic transit, and methane production in female patients with chronic constipation. Gastroenterology 150 , 367–379.e1 (2016).
Tap, J. et al. Identification of an Intestinal microbiota signature associated with severity of irritable bowel syndrome. Gastroenterology 152 , 111–123.e8 (2017).
Enck, P. et al. Irritable bowel syndrome. Nat. Rev. Dis. Primers 2 , 16014 (2016).
Quigley, E. M. M. Gut microbiome as a clinical tool in gastrointestinal disease management: are we there yet? Nat. Rev. Gastroenterol. Hepatol. 14 , 315–320 (2017).
Halmos, E. P. et al. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 64 , 93–100 (2015).
Pimentel, M. et al. Antibiotic treatment of constipation-predominant irritable bowel syndrome. Dig. Dis. Sci. 59 , 1278–1285 (2014).
Ianiro, G. et al. Systematic review with meta-analysis: efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 50 , 240–248 (2019).
El-Salhy, M., Hatlebakk, J. G., Gilja, O. H., Brathen Kristoffersen, A. & Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut https://doi.org/10.1136/gutjnl-2019-319630 (2019).
Article PubMed Google Scholar
Ford, A. C. et al. Efficacy of prebiotics, probiotics, and synbiotics in irritable bowel syndrome and chronic idiopathic constipation: systematic review and meta-analysis. Am. J. Gastroenterol. 109 , 1547–1561 (2014).
Ford, A. C., Harris, L. A., Lacy, B. E., Quigley, E. M. M. & Moayyedi, P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 48 , 1044–1060 (2018).
Deschasaux, M. et al. Depicting the composition of gut microbiota in a population with varied ethnic origins but shared geography. Nat. Med. 24 , 1526–1531 (2018).
Yatsunenko, T. et al. Human gut microbiome viewed across age and geography. Nature 486 , 222–227 (2012).
Mueller, S. et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: a cross-sectional study. Appl. Environ. Microbiol. 72 , 1027–1033 (2006).
Vangay, P. et al. US immigration westernizes the human gut microbiome. Cell 175 , 962–972.e10 (2018).
Spiller, R. & Garsed, K. Postinfectious irritable bowel syndrome. Gastroenterology 136 , 1979–1988 (2009).
Card, T. et al. Post-infectious IBS: defining its clinical features and prognosis using an internet-based survey. United European Gastroenterol. J. 6 , 1245–1253 (2018).
Neal, K. R., Hebden, J. & Spiller, R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: postal survey of patients. BMJ 314 , 779–782 (1997).
Thabane, M., Kottachchi, D. T. & Marshall, J. K. Systematic review and meta-analysis: the incidence and prognosis of post-infectious irritable bowel syndrome. Aliment. Pharmacol. Ther. 26 , 535–544 (2007).
Wadhwa, A. et al. High risk of post-infectious irritable bowel syndrome in patients with Clostridium difficile infection. Aliment. Pharmacol. Ther. 44 , 576–582 (2016).
Ghoshal, U. C. & Rahman, M. M. Post-infection irritable bowel syndrome in the tropical and subtropical regions: Vibrio cholerae is a new cause of this well-known condition. Indian J. Gastroenterol. 38 , 87–94 (2019).
Neal, K. R., Barker, L. & Spiller, R. C. Prognosis in post-infective irritable bowel syndrome: a six year follow up study. Gut 51 , 410–413 (2002).
Schwille-Kiuntke, J. et al. Postinfectious irritable bowel syndrome: follow-up of a patient cohort of confirmed cases of bacterial infection with Salmonella or Campylobacter. Neurogastroenterol. Motil. 23 , e479–e488 (2011).
Rodriguez, L. A. & Ruigomez, A. Increased risk of irritable bowel syndrome after bacterial gastroenteritis: cohort study. BMJ 318 , 565–566 (1999).
Zanini, B. et al. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am. J. Gastroenterol. 107 , 891–899 (2012).
Marshall, J. K., Thabane, M., Borgaonkar, M. R. & James, C. Postinfectious irritable bowel syndrome after a food-borne outbreak of acute gastroenteritis attributed to a viral pathogen. Clin. Gastroenterol. Hepatol. 5 , 457–460 (2007).
Hanevik, K. et al. Irritable bowel syndrome and chronic fatigue 6 years after giardia infection: a controlled prospective cohort study. Clin. Infect. Dis. 59 , 1394–1400 (2014).
Wensaas, K. A. et al. Irritable bowel syndrome and chronic fatigue 3 years after acute giardiasis: historic cohort study. Gut 61 , 214–219 (2012).
Svendsen, A. T., Bytzer, P. & Engsbro, A. L. Systematic review with meta-analyses: does the pathogen matter in post-infectious irritable bowel syndrome? Scand. J. Gastroenterol. 54 , 546–562 (2019).
Klem, F. et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: a systematic review and meta-analysis. Gastroenterology 152 , 1042–1054.e1 (2017).
Rahman, M. M. et al. Long-term gastrointestinal consequences are frequent following sporadic acute infectious diarrhea in a tropical country: a prospective cohort study. Am. J. Gastroenterol. 113 , 1363–1375 (2018).
Parida, P. K. et al. A prospective study on incidence, risk factors, and validation of a risk score for post-infection irritable bowel syndrome in coastal eastern India. Indian J. Gastroenterol. 38 , 134–142 (2019).
Youn, Y. H. et al. Long-term clinical course of post-infectious irritable bowel syndrome after shigellosis: a 10-year follow-up study. J. Neurogastroenterol. Motil. 22 , 490–496 (2016).
Center for Disease Control and Prevention. Foodborne illness and germs (CDC, 2019).
GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 17 , 909–948 (2017).
Zamani, M., Alizadeh-Tabari, S. & Zamani, V. Systematic review with meta-analysis: the prevalence of anxiety and depression in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 50 , 132–143 (2019).
Van Oudenhove, L. et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointestinal disorders. Gastroenterology 150 , 1355–1367.e2 (2016).
Koloski, N. A. et al. The brain-gut pathway in functional gastrointestinal disorders is bidirectional: a 12-year prospective population-based study. Gut 61 , 1284–1290 (2012).
Quigley, E. M. M. The gut-brain axis and the microbiome: clues to pathophysiology and opportunities for novel management strategies in irritable bowel syndrome (IBS). J. Clin. Med. 7 , 6 (2018).
Koloski, N. A., Jones, M. & Talley, N. J. Evidence that independent gut-to-brain and brain-to-gut pathways operate in the irritable bowel syndrome and functional dyspepsia: a 1-year population-based prospective study. Aliment. Pharmacol. Ther. 44 , 592–600 (2016).
World Health Organization. Mental disorders affect one in four people (WHO, 2019).
Baxter, A. J. et al. Challenging the myth of an “epidemic” of common mental disorders: trends in the global prevalence of anxiety and depression between 1990 and 2010. Depress. Anxiety 31 , 506–516 (2014).
Steel, Z. et al. The global prevalence of common mental disorders: a systematic review and meta-analysis 1980-2013. Int. J. Epidemiol. 43 , 476–493 (2014).
Baxter, A. J., Scott, K. M., Vos, T. & Whiteford, H. A. Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol. Med. 43 , 897–910 (2013).
Khoury, C. K. et al. Increasing homogeneity in global food supplies and the implications for food security. Proc. Natl Acad. Sci. USA 111 , 4001–4006 (2014).
GlobalData. EpiCast report: irritable bowel syndrome — Epidemiology forecast to 2026 (GlobalData, 2017).
PopulationPyramid.net. Population of the World in 2020. PopulationPyramid.net https://www.populationpyramid.net/world/2020/ (2019).
PopulationPyramid.net. Population of the World 2040. PopulationPyramid.net https://www.populationpyramid.net/world/2040/ (2019).
Davies, S. C. Annual report of the Chief Medical Officer 2015, on the state of the public’s health, baby boomers: fit for the future (Department of Health and Social Care, 2016).
Blue Cross Blue Shield. The health of millennials. bcbs https://www.bcbs.com/the-health-of-america/reports/the-health-of-millennials (2019).
Anderson, N. B. Stress in America: paying with our health (American Psychological Association, 2015).
Bupa. Stress leaves Generation X old before their time. Bupa https://www.bupa.co.uk/newsroom/ourviews/stress-leaves-generation (2016).
Black, C. J. & Ford, A. C. Rational investigations in irritable bowel syndrome. Frontline Gastroenterol. 11 , 140–147 (2019).
Lacy, B. E., Ford, A. C. & Talley, N. J. Quality of care and the irritable bowel syndrome: is now the time to set standards? Am. J. Gastroenterol. 113 , 167–169 (2018).
Chang, L. et al. Functional bowel disorders: a roadmap to guide the next generation of research. Gastroenterology 154 , 723–735 (2018).
International Confederation of Dietetic Associations. Dieticians around the world: their education and their work (ICDA, 2008).
Choudhry, F. R., Mani, V., Ming, L. C. & Khan, T. M. Beliefs and perception about mental health issues: a meta-synthesis. Neuropsychiatr. Dis. Treat. 12 , 2807–2818 (2016).
Beck, A., Nadkarni, A., Calam, R., Naeem, F. & Husain, N. Increasing access to cognitive behaviour therapy in low and middle income countries: a strategic framework. Asian J. Psychiatr. 22 , 190–195 (2016).
Download references
Author information
Authors and affiliations.
Leeds Gastroenterology Institute, St. James’s University Hospital, Leeds, UK
Christopher J. Black & Alexander C. Ford
Leeds Institute of Medical Research at St. James’s, University of Leeds, Leeds, UK
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed equally to all aspects of this manuscript.
Corresponding author
Correspondence to Alexander C. Ford .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Peer review information.
Nature Reviews Gastroenterology & Hepatology thanks S. Fukudo, W. Whitehead and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions.
Reprints and permissions
About this article
Cite this article.
Black, C.J., Ford, A.C. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol 17 , 473–486 (2020). https://doi.org/10.1038/s41575-020-0286-8
Download citation
Accepted : 03 March 2020
Published : 15 April 2020
Issue Date : August 2020
DOI : https://doi.org/10.1038/s41575-020-0286-8
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Work-related problems and the psychosocial characteristics of individuals with irritable bowel syndrome: an updated literature review.
- Nagisa Sugaya
BioPsychoSocial Medicine (2024)
Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis
- María R. Aburto
- John F. Cryan
Nature Reviews Gastroenterology & Hepatology (2024)
Factors Underlying the Difference in Response to Fecal Microbiota Transplantation Between IBS Patients with Severe and Moderate Symptoms
- Magdy El-Salhy
- Jan Gunnar Hatlebakk
Digestive Diseases and Sciences (2024)
Hydrogen Sulfide Producers Drive a Diarrhea-Like Phenotype and a Methane Producer Drives a Constipation-Like Phenotype in Animal Models
- Maria J. Villanueva-Millan
- Gabriela Leite
- Mark Pimentel
Engagement in GI Behavioral Health Is Associated with Reduced Portal Messages, Phone Calls, and ED Visits
- Brian J. Arizmendi
- Meredith R. Craven
- Jessica K. Salwen-Deremer
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Alzheimer's disease & dementia
- Arthritis & Rheumatism
- Attention deficit disorders
- Autism spectrum disorders
- Biomedical technology
- Diseases, Conditions, Syndromes
- Endocrinology & Metabolism
- Gastroenterology
- Gerontology & Geriatrics
- Health informatics
- Inflammatory disorders
- Medical economics
- Medical research
- Medications
- Neuroscience
- Obstetrics & gynaecology
- Oncology & Cancer
- Ophthalmology
- Overweight & Obesity
- Parkinson's & Movement disorders
- Psychology & Psychiatry
- Radiology & Imaging
- Sleep disorders
- Sports medicine & Kinesiology
- Vaccination
- Breast cancer
- Cardiovascular disease
- Chronic obstructive pulmonary disease
- Colon cancer
- Coronary artery disease
- Heart attack
- Heart disease
- High blood pressure
- Kidney disease
- Lung cancer
- Multiple sclerosis
- Myocardial infarction
- Ovarian cancer
- Post traumatic stress disorder
- Rheumatoid arthritis
- Schizophrenia
- Skin cancer
- Type 2 diabetes
- Full List »
share this!
May 24, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
reputable news agency
Rates of severe multiple drug intolerance syndrome up in fibromyalgia, IBS
by Elana Gotkine
Patients with fibromyalgia and irritable bowel syndrome (IBS) have increased rates of severe multiple drug intolerance syndrome (MDIS), according to a study published in the May issue of the Journal of Allergy and Clinical Immunology: In Practice .
Alicia A. Alvarez, M.D., from Sarasota Memorial Hospital in Florida, and colleagues conducted a retrospective chart review to examine the prevalence of MDIS in patients diagnosed with fibromyalgia or IBS. Patients who had been seen at a large academic center were identified and matched to controls seen within the same timeframe by exact birthdate and sex.
The researchers found that compared with controls, patients with fibromyalgia and IBS were 12 and three times more likely to have severe MDIS, respectively.
In both groups, severe MDIS was associated with polypharmacy. Across all participants, opiates were the most frequently reported drug intolerance. Patients with IBS more often reported gastrointestinal symptoms as adverse reactions , while those with fibromyalgia did not report pain or behavioral changes as adverse reactions more often.
"Further evaluation is needed to understand which patients with fibromyalgia or IBS are at risk of developing MDIS and whether this can be prevented, possibly by reducing polypharmacy," the authors write.
Copyright © 2024 HealthDay . All rights reserved.
Explore further
Feedback to editors

New technique detects novel biomarkers for kidney diseases with nephrotic syndrome
May 25, 2024

In experiments, mice get ill from raw milk carrying bird flu virus
Understanding a broken heart—study finds link between stress and recurrent heart failure

Genetic cause of rare childhood immune disorders discovered

New surgical tool moves tiny bioparticles with robotics and acoustic energy
The link between defective autophagy and pancreatitis could point to new treatments

Possible association between tattoos and lymphoma revealed

Walkability in neighborhoods linked to health, study of siblings shows

Scientists uncover new treatment pathway for rare 'spider web' childhood brain tumors
How COVID-19 'breakthrough' infections alter your immune cells
Related stories.

Green eyeglasses reduce pain-related anxiety in fibromyalgia patients, study shows
Oct 24, 2022
Does insulin resistance cause fibromyalgia?
May 7, 2019

New data align with EULAR recommendations for fibromyalgia management
Jun 3, 2022

Irritable bowel syndrome patients found to suffer higher rates of fibromyalgia and chronic fatigue syndrome
Jan 9, 2024

App helps manage fibromyalgia through acceptance and commitment therapy
Nov 22, 2023

Understanding opioids in fibromyalgia
Jun 2, 2023
Recommended for you

Colon cancers are rising among the young: New study outlines the warning signs
How air pollution affects the digestive system
Study reveals right atrium changes in cardiovascular diseases
New global targets proposed to reduce AMR-linked deaths and improve access to essential antibiotics
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
New gut calming discovery to bring relief to IBS sufferers

Dr Jenny Bailey, CEO of University of Bristol spin out Ferryx.
Newswise — The discovery of a strain of bacteria shown to reduce inflammation in the intestine caused by irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) could bring relief to millions of sufferers after being turned into an innovative natural food supplement by University of Bristol biotech spin-out Ferryx .
Treatment options for individuals with IBS and IBD are limited and while probiotics are popular, there are currently no conventional probiotics that can function to alleviate symptoms during active flare-ups or if someone is stressed.
The discovery of FX856, a friendly strain of live bacteria, which has shown in animal models with inflammatory bowel disease to survive and thrive during periods of active inflammation delaying disease onset and reducing symptoms, led the Bristol team to create Ferrocalm .
The gut-calming solution, developed over 10 years’ R&D at the University of Bristol, contains FX856 and aims to reduce symptoms such as stomach cramps, bloating, diarrhoea and constipation that people suffer during active flare-ups of IBS, IBD and Crohn’s and ulcerative colitis.
Currently approved for use as a food supplement, Ferrocalm will undergo clinical trials in patients with inflammatory bowel disease in 2024 to test efficacy as a pharmaceutical treatment.
Ferrocalm is the brainchild of Dr Jenny Bailey, a Bristol graduate and award-winning CEO entrepreneur of Ferryx, who has spent 15 years researching how gut inflammation develops to help find a natural solution to improve quality of life for people who suffer from IBS and other gut conditions.
Dr Jenny Bailey, CEO of Ferryx, said: “The launch of Ferrocalm is the culmination of ten years’ of scientific research, all with the aim of helping people to take control of their condition, alleviate their symptoms, and live the life they want to lead using safe, effective bacterial products that prevent and treat inflammatory illness. If we can help one person to feel better, enabling them to go to an event that they would otherwise have missed, take part in sports, go to work, or simply enjoy time with family and friends, then I will be happy. We are already seeing some great results and we look forward to helping many more people achieve relief from gut conditions.”
Dr Tristan Cogan, CTO and Co-founder of FerryX and Senior Lecturer in Infectious Diseases at the University of Bristol Vet School, added: “Both IBS and IBD are lifelong conditions with no cure that can have a significant impact on a sufferer’s day-to-day life.Treatment is mainly based on suppression of symptoms, often with numerous side effects and trials of probiotics in this disease have frequently produced disappointing results as they are rapidly outcompeted during active inflammation or stress. Our discovery of this specific bacteria strain showed promising results in studies including delayed onset of colitis and reduced clinical signs of the disease.”
Ferrocalm is available from www.ferrocalm.com where one month’s supply (30 capsules) costs £19.99 and two months’ supply (60 capsules) is currently available at the discounted rate of £29.98.
Further information
Ferryx is a University of Bristol spin-out biotech company which specialises in scientific innovation in treatment of inflammatory diseases. Formed by CEO Dr Jenny Bailey and Tristan Cogan (CTO), Ferryx researches and produces live biotherapeutic products for human and veterinary use. Incorporated in 2019, the company was the 2020 runner up in the University of Bristol New Enterprise scheme funding awards, and the winners of the Techspark SPARKies ‘Good’ award. Ferryx received investment of £305k to further develop their innovative gut treatments and was named as one of Tech South West’s Scaleup Ones to Watch in February 2023.
Credit: Ferryx (all images)
FX856, a friendly strain of live bacteria: https://fluff.bris.ac.uk/fluff/u1/ficmc/7N7jtD_eC9DNZweOCWHL6gBO7/
https://fluff.bris.ac.uk/fluff/u2/ficmc/AgUZVvHITIhcPCgxYMcHYwBOf/
Dr Jenny Bailey:
https://fluff.bris.ac.uk/fluff/u4/ficmc/TNLzqFHhgB0I_v2PUl4CLQBOb/
Dr Tristan Cogan:
https://fluff.bris.ac.uk/fluff/u3/ficmc/hadAoFu_pEriH_9V6st6ewBOB/
Issued by the University of Bristol Media Team
MEDIA CONTACT
Type of article.
- Experts keyboard_arrow_right Expert Pitch Expert Query Expert Directory
- Journalists
- About keyboard_arrow_right Member Services Accessibility Statement Newswise Live Invoice Lookup Services for Journalists Archived Wires Participating Institutions Media Subscribers Sample Effectiveness Reports Terms of Service Privacy Policy Our Staff Contact Newswise
- Blog FAQ Help
Food & Function
Research progress in the treatment of inflammatory bowel disease with natural polysaccharides and related structure–activity relationships.

* Corresponding authors
a Affiliated Hospital, Changchun University of Chinese Medicine, Changchun, China E-mail: [email protected]
b College of Pharmacy, Changchun University of Chinese Medicine, Changchun, China
Inflammatory bowel disease (IBD) comprises a group of highly prevalent and chronic inflammatory intestinal tract diseases caused by multiple factors. Despite extensive research into the causes of the disease, IBD's pathogenic mechanisms remain unclear. Moreover, side effects of current IBD therapies restrict their long-term clinical use. In contrast, natural polysaccharides exert beneficial anti-IBD effects and offer advantages over current anti-IBD drugs, including enhanced safety and straightforward isolation from abundant and reliable sources, and thus may serve as components of functional foods and health products for use in IBD prevention and treatment. However, few reviews have explored natural polysaccharides with anti-IBD activities or the relationship between polysaccharide conformation and anti-IBD biological activity. Therefore, this review aims to summarize anti-IBD activities and potential clinical applications of polysaccharides isolated from plant, animal, microorganismal, and algal sources, while also exploring the relationship between polysaccharide conformation and anti-IBD bioactivity for the first time. Furthermore, potential mechanisms underlying polysaccharide anti-IBD effects are summarized, including intestinal microbiota modulation, intestinal inflammation alleviation, and intestinal barrier protection from IBD-induced damage. Ultimately, this review provides a theoretical foundation and valuable insights to guide the development of natural polysaccharide-containing functional foods and nutraceuticals for use as dietary IBD therapies.

- This article is part of the themed collection: Food & Function Review Articles 2023
Article information
Download citation, permissions.
J. Chen, Y. Gao, Y. Zhang and M. Wang, Food Funct. , 2024, Advance Article , DOI: 10.1039/D3FO04919A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page .
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page .
Read more about how to correctly acknowledge RSC content .
Social activity
Search articles by author.
This article has not yet been cited.
Advertisements

Mediterranean diet could help people with irritable bowel symptoms
N ew research looking at the benefits of a Mediterranean diet for people with irritable bowel syndrome (IBS) has yielded surprising results.
Not only did the diet, rich in fruits, vegetables and legumes, improve the mental health of the study participants, but their gastrointestinal symptoms improved, as well.
Dr. Heidi Staudacher, National Health and Medical Research Council Emerging Leadership Fellow at Deakin University's Food & Mood Center, said it was common for people with IBS to avoid some of the foods important in a Mediterranean diet as they are known to trigger a worsening of symptoms.
"Previously we had an understanding that foods such as legumes, certain whole grains and onion, can worsen gut symptoms in some people," Dr. Staudacher said.
"This research suggests there might be a new way to help reduce the burden of IBS symptoms that doesn't focus on cutting out foods that are known to be important for good health."
Dr. Staudacher's research , published in Alimentary Pharmacology & Therapeutics , measured outcomes of 59 people over six weeks who were either following the Mediterranean diet via counseling from a dietitian or eating their usual diet (control group).
"Previous research has shown the Mediterranean diet improves depressive symptoms and we wanted to see whether this type of diet was possible for people with IBS, and also whether it would improve both depressive and gut symptoms in people with IBS," Dr. Staudacher said.
"Many people with IBS also have mental health problems like anxiety and depression. Given the known gut-brain connection, it is plausible that if we can improve people's mental health this might then lead to improvements in the gut symptoms that people with IBS live with."
The study found:
- 83% of participants on the Mediterranean diet had a reduction in their IBS-SSS score (gut symptom severity score) throughout the trial compared with only 37% in the control group,
- Depressive symptoms were lower in the Mediterranean diet compared with controls at the end of the study. This is in line with other research using a Mediterranean diet in people with depression,
- Somewhat surprisingly, gastrointestinal symptoms were also lower in the Mediterranean diet group compared with controls.
"These findings suggest we may be able to look beyond current dietary advice for people with IBS and encourage a broadly health-promoting diet to help manage their symptoms. We now urgently need to conduct a bigger study that compares a Mediterranean diet to a better control diet to give us more clarity about its effect on gut and psychological symptoms," Dr. Staudacher said.
"Dietitians will also need to be involved, to help people increase high fiber and high FODMAP foods gradually into their diets to avoid triggering gut symptoms."
More information: Heidi M. Staudacher et al, Clinical trial: A Mediterranean diet is feasible and improves gastrointestinal and psychological symptoms in irritable bowel syndrome, Alimentary Pharmacology & Therapeutics (2023). DOI: 10.1111/apt.17791
Provided by Deakin University

Advertisement
Supported by
Ozempic Cuts Risk of Chronic Kidney Disease Complications, Study Finds
A major clinical trial showed such promising results that the drug’s maker halted it early.
- Share full article

By Dani Blum
Dani Blum has reported on Ozempic and similar drugs since 2022.
Semaglutide, the compound in the blockbuster drugs Ozempic and Wegovy , dramatically reduced the risk of kidney complications, heart issues and death in people with Type 2 diabetes and chronic kidney disease in a major clinical trial, the results of which were published on Friday. The findings could transform how doctors treat some of the sickest patients with chronic kidney disease, which affects more than one in seven adults in the United States but has no cure.
“Those of us who really care about kidney patients spent our whole careers wanting something better,” said Dr. Katherine Tuttle, a professor of medicine at the University of Washington School of Medicine and an author of the study. “And this is as good as it gets.” The research was presented at a European Renal Association meeting in Stockholm on Friday and simultaneously published in The New England Journal of Medicine .
The trial, funded by Ozempic maker Novo Nordisk, was so successful that the company stopped it early . Dr. Martin Holst Lange, Novo Nordisk’s executive vice president of development, said that the company would ask the Food and Drug Administration to update Ozempic’s label to say it can also be used to reduce the progression of chronic kidney disease or complications in people with Type 2 diabetes.
Diabetes is a leading cause of chronic kidney disease, which occurs when the kidneys don’t function as well as they should. In advanced stages, the kidneys are so damaged that they cannot properly filter blood. This can cause fluid and waste to build up in the blood, which can exacerbate high blood pressure and raise the risk of heart disease and stroke, said Dr. Subramaniam Pennathur, the chief of the nephrology division at Michigan Medicine.
The study included 3,533 people with kidney disease and Type 2 diabetes, about half of whom took a weekly injection of semaglutide, and half of whom took a weekly placebo shot.
Researchers followed up with participants after a median period of around three and a half years and found that those who took semaglutide had a 24 percent lower likelihood of having a major kidney disease event, like losing at least half of their kidney function, or needing dialysis or a kidney transplant. There were 331 such events among the semaglutide group, compared with 410 in the placebo group.
People who received semaglutide were much less likely to die from cardiovascular issues, or from any cause at all, and had slower rates of kidney decline.
Kidney damage often occurs gradually, and people typically do not show symptoms until the disease is in advanced stages. Doctors try to slow the decline of kidney function with existing medications and lifestyle modifications, said Dr. Melanie Hoenig, a nephrologist at Beth Israel Deaconess Medical Center who was not involved with the study. But even with treatment, the disease can progress to the point that patients need dialysis, a treatment that removes waste and excess fluids from the blood, or kidney transplants.
The participants in the study were extremely sick — the severe complications seen in some study participants are more likely to occur in people the later stages of chronic kidney disease, said Dr. George Bakris, a professor of medicine at the University of Chicago Medicine and an author of the study. Most participants in the trial were already taking medication for chronic kidney disease.
For people with advanced kidney disease, in particular, the findings are promising. “We can help people live longer,” said Dr. Vlado Perkovic, a nephrologist and renal researcher at the University of New South Wales, Sydney, and another author of the study.
While the data shows clear benefits, even the researchers studying drugs like Ozempic aren’t sure how, exactly, they help the kidneys. One leading theory is that semaglutide may reduce inflammation, which exacerbates kidney disease.
And the results come with several caveats: Roughly two-thirds of the participants were men and around two-thirds were white — a limitation of the study, the authors noted, because chronic kidney disease disproportionately affects Black and Indigenous patients. The trial participants taking semaglutide were more likely to stop the drug because of gastrointestinal issues, which are common side effects of Ozempic.
Doctors said they wanted to know whether the drug might benefit patients who have kidney disease but not diabetes, and some also had questions about the potential long-term risks of taking semaglutide.
Still, the results are the latest data to show that semaglutide can do more than treat diabetes or drive weight loss. In March, the F.D.A. authorized Wegovy for reducing the risk of cardiovascular issues in some patients. And scientists are examining semaglutide and tirzepatide, the compound in the rival drugs Mounjaro and Zepbound, for a range of other conditions , including sleep apnea and liver disease.
If the F.D.A. approves the new use, it could drive even more demand for Ozempic, which has faced recurrent shortages .
“I think it’s a game changer,” Dr. Hoenig said, “if I can get it for my patients.”
Dani Blum is a health reporter for The Times. More about Dani Blum
A Close Look at Weight-Loss Drugs
Supplement Stores: GNC and the Vitamin Shoppe are redesigning displays and taking other steps to appeal to people who are taking or are interested in drugs like Ozempic and Wegovy.
Senate Investigation: A Senate committee is investigating the prices that Novo Nordisk charges for Ozempic and Wegovy, which are highly effective at treating diabetes and obesity but carry steep price tags.
A Company Remakes Itself: Novo Nordisk’s factories work nonstop turning out Ozempic and Wegovy , but the Danish company has far bigger ambitions.
Transforming a Small Danish Town: In Kalundborg, population under 17,000, Novo Nordisk is making huge investments to increase production of Ozempic and Wegovy.
Ozempic’s Inescapable Jingle: The diabetes drug has become a phenomenon, and “Oh, oh, oh, Ozempic!” — a takeoff of the Pilot song “Magic” — has played a big part in its story.
More From Forbes
Inflation is already soaking the rich 1%, new research shows.
- Share to Facebook
- Share to Twitter
- Share to Linkedin
HUNGARY - JUNE 28: A Woman Lighting Her Cigarette With A Bill Of A Billion Pengos, As If It Was ... [+] Merely A Piece Of Paper, On June 28, 1946, While Hungary Was Going Through A Galloping Inflation. (Photo by Keystone-France/Gamma-Keystone via Getty Images)
The recent inflationary surge is squeezing households, but not likely in the way you think. It’s hitting the wealthy disproportionately, new research shows.
Inflation peaked at 9.1% in June 2022, and has recently dropped to 3.4% in April, according to data collated by TradingEconomics . However, the inflation rate is still way higher than it was in the five years through 2020 when the Consumer Price index grew at a rate that hovered around 2% and sometimes went negative.
In many ways that stability was good for the economy. But the jump in inflation after the pandemic has hit everyone in the wallet, experts say. However, it’s also true some groups got hit harder than others.
“Virtually all households are hurt by inflation,” states a recent working paper distributed by the National Bureau of Economic Research titled ‘ Inflation’s Impact on American Households .’ “But richer households typically face larger percentage losses thanks to their higher share of asset income relative to labor income.”
Put simply, wealthier people tend to get more from their dividends or interest on investments rather than from salaries. The impact of inflation on the former (dividends and interest) is far greater.
“Inflation’s net taxation is highly progressive with the richest 1 percent experiencing nearly 2.6 times the average percentage decline in lifetime spending as those in the bottom quintile,” the paper states. It was authored by David Altig, Alan J. Auerbach, Erin F. Eidschun, Laurence J. Kotlikoff, and Victor Yifan Ye.
The 83 Best Memorial Day Sales According To Our Deal Savvy Editors
The best beers in canada according to the canadian brewing awards, nyt ‘strands’ #84 hints, spangram and answers for sunday, may 26th.
The authors use an example of someone in the top quartile of income would see a drop in inflation-adjusted spending of 9.84% if inflation jumped from zero to 10% per annum. However, someone in the 75th percentile would see a drop of 4.83% in spending, on average, according to the paper’s analysis.
The downside for the economy is richer people invest far more money into the economy which helps creates growth and creates jobs. If the rich feel squeezed fiscally then, rightly or wrongly, the rest of us suffer. Investment is a key variable in determining growth and most economists believe that without foreign and domestic investing any economy will suffer.
Findings from the paper also suggest that the inflation-tax (its tax because it robs people of the spending power of their savings) turns out to be a progressive tax. That means that the rich are hurt far more than the poor by this un-legislated hit to their wealth.
“Even were inflation fully anticipated and, thus, financially neutral, it would have a major and quite progressive impact, via the fiscal system, on the average level and distribution of Americans’ lifetime spending,” the report states. Put another way, the poorer you are the less inflation hurts your net worth. And it works vice versa also.
That’s something that the White House administration may find desirable at the moment given its leftward political leaning. However, what will happen to the economy if nominal in tax rate increases are added to the mix? How bad will that be for the U.S.

- Editorial Standards
- Reprints & Permissions
Join The Conversation
One Community. Many Voices. Create a free account to share your thoughts.
Forbes Community Guidelines
Our community is about connecting people through open and thoughtful conversations. We want our readers to share their views and exchange ideas and facts in a safe space.
In order to do so, please follow the posting rules in our site's Terms of Service. We've summarized some of those key rules below. Simply put, keep it civil.
Your post will be rejected if we notice that it seems to contain:
- False or intentionally out-of-context or misleading information
- Insults, profanity, incoherent, obscene or inflammatory language or threats of any kind
- Attacks on the identity of other commenters or the article's author
- Content that otherwise violates our site's terms.
User accounts will be blocked if we notice or believe that users are engaged in:
- Continuous attempts to re-post comments that have been previously moderated/rejected
- Racist, sexist, homophobic or other discriminatory comments
- Attempts or tactics that put the site security at risk
- Actions that otherwise violate our site's terms.
So, how can you be a power user?
- Stay on topic and share your insights
- Feel free to be clear and thoughtful to get your point across
- ‘Like’ or ‘Dislike’ to show your point of view.
- Protect your community.
- Use the report tool to alert us when someone breaks the rules.
Thanks for reading our community guidelines. Please read the full list of posting rules found in our site's Terms of Service.

IMAGES
VIDEO
COMMENTS
Amitriptyline Helps Relieve IBS Symptoms. Oct. 16, 2023 — Amitriptyline can improve symptoms of irritable bowel syndrome in patients seen in GP surgeries, new research has found. The cheap and ...
An international study of more than 50,000 people with irritable bowel syndrome (IBS) has revealed that IBS symptoms may be caused by the same biological processes as conditions such as anxiety. The research highlights the close relationship between brain and gut health and paves the way for development of new treatments.
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder which presents with abdominal pain and altered bowel habits. It affects about 20% of the general population, mainly women, and has a considerable impact on the quality of life and health care costs. ... National Research Council, Molecular Biotechnology Center ...
New research published in the journal Cellular and Molecular Gastroenterology and Hepatology sheds light on disease mechanisms common to irritable bowel syndrome (IBS) and cardiovascular diseases ...
Introduction. Irritable bowel syndrome (IBS) is a prevalent disorder that greatly reduces patients' quality of life (QOL) and adversely affects the medical economy [].A recent epidemiological survey using the Rome IV criteria revealed that the prevalence of IBS in the general population globally is 4.1% [].In Japan, the prevalence of IBS is 2.2% but that of functional bowel disorders is 25.2 ...
Irritable bowel syndrome is considered a functional bowel disorder with no known organic cause. Latest Research and Reviews Serine proteases and metalloproteases are highly increased in irritable ...
A total of 24,845 respondents reported current abdominal symptoms meeting standard diagnostic criteria (DHQ Rome III, Fig. 1) at the time of the survey, providing a point prevalence of IBS of 14.5 ...
Genetic predisposition contributes to disease pathophysiology in irritable bowel syndrome (IBS). This Review provides a comprehensive overview of genetic research in IBS and discusses new concepts ...
The only drug currently approved for IBS-D is alosetron, a 5-HT3 antagonist that may relieve abdominal pain and slow colonic and small bowel transit. Alosetron was withdrawn from the market for safety reasons in 2000 and was reintroduced in 2002 with a more restricted indication. Today, incidence rates of adverse events, including ischemic ...
Irritable bowel syndrome (IBS) is a common chronic disorder of gut-brain interaction, characterised by the presence of abdominal pain in association with a change in stool form or frequency.1 Some people experience predominantly constipation (IBS-C), some mostly diarrhoea (IBS-D), and others a mixture of the two (IBS-M). IBS is common, estimated to affect 5% of the global population at any ...
Introduction. Associated with abdominal pain, bloating, and altered bowel habits, irritable bowel syndrome (IBS) is a chronic, cyclical and relapsing functional bowel disorder ().The global prevalence of IBS is currently estimated at 15%, and IBS symptoms occur in about 10-20% of Westerners (2-4).Irrespective of bowel habit, diagnoses of IBS have traditionally been made by using the Rome ...
Bethesda, MD (June 21, 2022) — New treatment guidelines released today in Gastroenterology outline a personalized approach for treating patients with approved drug treatments for irritable bowel syndrome (IBS) with constipation (IBS-C) or IBS with diarrhea (IBS-D). IBS is one of the most common disorders of both intestines, affecting up to 35 million Americans.
In fact, irritable bowel syndrome, or I.B.S., is a real problem causing real symptoms, no matter how hard its sufferers may wish it gone. But unlike an infection or tumor, I.B.S. is what medicine ...
The American Gastroenterological Association (AGA) is the trusted voice of the GI community. AGA publishes top-tier journals, guidelines and patient education ...AGA has published new treatment guidelines in Gastroenterology providing a personalized approach for treating your patients with approved drug treatments for irritable bowel syndrome (IBS) with constipation (IBS-C) or IBS with ...
For patients with diarrhea-predominant IBS, treatment options include the synthetic peripheral μ -opioid receptor agonist loperamide, antispasmodic agents, antidepressants, serotonin 5-HT 3 antagonists, and the gut-specific antibiotic rifaximin. Ongoing research is evaluating the use of probiotics.
The US Food and Drug Administration (FDA) has approved two new treatments for patients with irritable bowel syndrome with diarrhoea (IBS-D). Rifaximin (marketed as Xifaxan by Salix Pharmaceuticals) is an antibiotic, and eluxadoline (marketed as Viberzi by Actavis) is an antagonist and agonist of the δ and µ opioid receptors, respectively.
Irritable Bowel Syndrome and Control Volunteers: Diet Challenge. Purpose of study: The purpose of this study is to study the relationship between the bile acids, short chain fatty acids and bacteria within the intestines.The hypothesis is that changes in the bacterial composition of the stool are associated with the differences in bile acids and short chain fatty acids in patients having ...
Dietary interventions could be considered as an initial treatment for patients with IBS, although more research is needed for personalized treatment plans. ... According to a new study, evidence ...
The NIDDK has supported many research projects to learn more about IBS. For example, an NIDDK-supported clinical trial found that a home-based version of cognitive behaviorally therapy led to significant and lasting improvement in IBS symptoms. This content is provided as a service of the National Institute of Diabetes and Digestive and Kidney ...
The finding paves the way for more efficient treatment of irritable bowel syndrome and other food intolerances. ... a gastroenterologist at KU Leuven and lead author of the new research. "With ...
New research suggests that a microbiome-based, AI-powered personalized diet can optimize microbial gut communities and improve health outcomes for IBS patients more effectively than a low FODMAP diet. The study, published in The American Journal of Gastroenterology , compared the efficacy of a personalized diet (PD) provided by UK based ...
Irritable bowel syndrome (IBS) is one of the most common disorders of gut-brain interaction worldwide, defined according to patterns of gastrointestinal symptoms as described by the Rome ...
The researchers found that compared with controls, patients with fibromyalgia and IBS were 12 and three times more likely to have severe MDIS, respectively. In both groups, severe MDIS was ...
The gut-calming solution, developed over 10 years' R&D at the University of Bristol, contains FX856 and aims to reduce symptoms such as stomach cramps, bloating, diarrhoea and constipation that ...
Irritable Bowel Syndrome (IBS), ... not only methodologically validates the causal relationship between depression-mediated gut microbiota and the risk of IBS but also offers new insights for further advancements in IBS research. Future studies should prioritize on interpreting the dynamic interplay between gut microbiota, depression, and IBS ...
Inflammatory bowel disease (IBD) comprises a group of highly prevalent and chronic inflammatory intestinal tract diseases caused by multiple factors. Despite extensive research into the causes of the disease, IBD's pathogenic mechanisms remain unclear. Moreover, side effects of current IBD therapies restrict their Food &; Function Review Articles 2023
New research looking at the benefits of a Mediterranean diet for people with irritable bowel syndrome (IBS) has yielded surprising results. Not only did the diet, rich in fruits, vegetables and ...
Still, the results are the latest data to show that semaglutide can do more than treat diabetes or drive weight loss. In March, the F.D.A. authorized Wegovy for reducing the risk of cardiovascular ...
It's hitting the wealthy disproportionately, new research shows. Inflation peaked at 9.1% in June 2022, and has recently dropped to 3.4% in April, according to data collated by TradingEconomics .