
- < Previous
Home > ETD > OPEN_ACCESS_DISSERTATIONS > 1697

Open Access Dissertations
Quantifying human heat stress in working environments, and their relationship to atmospheric dynamics, due to global climate change.
Jonathan R. Buzan , Purdue University
Date of Award
Degree type.
Dissertation
Degree Name
Doctor of Philosophy (PhD)
Earth, Atmospheric, and Planetary Sciences
Committee Chair
Matthew Huber
Committee Member 1
H. Jay Melosh
Committee Member 2
Committee member 3.
Daniel Chavas
Committee Member 4
Thomas W. Hertel
Committee Member 5
Keith Oleson
Heat stress is a global issue that crosses socioeconomic status. Heat stress leads to reduced worker capacity on seasonal scales, and weekly to sub-daily timescales, incapacitation, morbidity, and mortality. This dissertation focuses on 2 distinct parts: quantification methods of heat stress, and heat stress applications. Quantification methods of heat stress Chapters 1–3 focus on historical analysis of heat stress. Chapter 1 is a detailed assessment of previous work in heat stress—methods, history, and future research outlook. Chapter 2 focuses on the implementation and quantification of a battery of heat stress metrics within the global circulation model framework. The ultimate outcome is a Fortran module, the HumanIndexMod [1], that may be run independently on individual datasets, or used with the Community Earth System Model 1, Community Land Model Version 5 (released February 2018 w/HumanIndexMod). Chapter 3 is an analysis of a battery of heat stress metrics with the focus on showing their differences in global circulation models, and thermodynamic predictability and scalability. Heat stress applications Chapters 4 and 5 focus on applications for physical impact modeling and economic outcomes. Chapter 4 quantifies labor impacts from heat stress due to the covariance or temperature, humidity, and radiation. My predictions of labor productivity losses from heat stress are amenable to Integrated Assessment Modeling. Chapter 5 is a preliminary economic impacts analysis–a 1st order sensitivity perturbation study for labor impacts–which will guide a flagship application for the Purdue University Big Idea Project, GLASS: Global to Local Analysis of Systems Sustainability. My labor productivity losses from heat stress will become a boundary condition for a series of sensitivity assessments intended to inform the policy making process to help achieve the United Nations Sustainability Development Goals.
Recommended Citation
Buzan, Jonathan R., "Quantifying Human Heat Stress in Working Environments, and Their Relationship to Atmospheric Dynamics, Due to Global Climate Change" (2018). Open Access Dissertations . 1697. https://docs.lib.purdue.edu/open_access_dissertations/1697
Since March 13, 2022
Advanced Search
- Notify me via email or RSS
- Purdue Libraries
- Purdue University Press Open Access Collections
Links for Authors
- Policies and Help Documentation
- Collections
- Disciplines
Home | About | FAQ | My Account | Accessibility Statement
Privacy Copyright
Advertisement
Physiological and molecular insights on wheat responses to heat stress
- Published: 20 September 2021
- Volume 41 , pages 501–518, ( 2022 )
Cite this article

- Milan Kumar Lal 1 , 2 ,
- Rahul Kumar Tiwari 1 , 2 ,
- Vijay Gahlaut 3 ,
- Vikas Mangal 1 ,
- Awadhesh Kumar 4 ,
- Madan Pal Singh 2 ,
- Vijay Paul 2 ,
- Sudhir Kumar 2 ,
- Brajesh Singh 1 &
- Gaurav Zinta ORCID: orcid.org/0000-0002-5503-8618 3 , 5
52 Citations
5 Altmetric
Explore all metrics
Increasing temperature is a key component of global climate change, affecting crop growth and productivity worldwide. Wheat is a major cereal crop grown in various parts of the globe, which is affected severely by heat stress. The morphological parameters affected include germination, seedling establishment, source-sink activity, leaf area, shoot and root growth. The physiological parameters such as photosynthesis, respiration, leaf senescence, water and nutrient relation are also affected by heat. At the cellular level, heat stress leads to the generation of reactive oxygen species that disrupt the membrane system of thylakoid, chloroplast and plasma membrane. The deactivation of the photosystem, reduction in photosynthesis and inactivation of rubisco affect the production of photoassimilates and their allocation. This ultimately affects anthesis, grain filling, size, number and maturity of wheat grains, which hamper crop productivity. The interplay of various systems comprising antioxidants and hormones plays a crucial role in imparting heat stress tolerance in wheat. Thus, implementation of various omics technologies could foster in-depth insights on heat stress effects, eventually devising heat stress mitigation strategies by conventional and modern breeding to develop heat-tolerant wheat varieties. This review provides an integrative view of heat stress responses in wheat and also discusses approaches to develop heat-tolerant wheat varieties.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
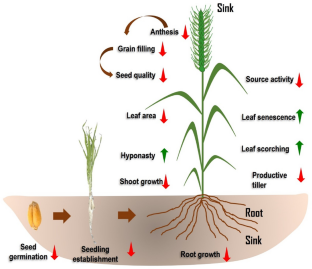
source activity is decreased. This lead to having various morphological changes in both the vegetative (reduction in productive tillers) and reproductive part (reduction in anthesis) of the plant. Heat stress also affects leaf senescence and scorching by increasing their incidence. Due to the decrease in leaf area under heat stress, there is a reduction in the amount of photoassimilates produces which further hampers the shoot as well as root growth. Ultimately the reproductive part of the plant is highly affected due to its sensitivity against heat stress. Thus grain filling and seed qualities are affected which leads to a reduction in the production and productivity of wheat under heat stress. The upward arrow means increase, and downward arrow means decrease
Similar content being viewed by others

Understanding the Mechanism of High-Temperature Stress Effect and Tolerance in Wheat

Ecophysiology and Response of Plants Under High Temperature Stress
Heat stress in wheat: adaptation strategies.
Abou-Elwafa SF, Shehzad T (2021) Genetic diversity, GWAS and prediction for drought and terminal heat stress tolerance in bread wheat ( Triticum aestivum L.). Genet Resour Crop Evol 68:711–728. https://doi.org/10.1007/s10722-020-01018-y
Article CAS Google Scholar
Acuña-Galindo MA, Mason RE, Subramanian NK, Hays DB (2015) Meta-analysis of wheat QTL regions associated with adaptation to drought and heat stress. Crop Sci 55:477–492. https://doi.org/10.2135/cropsci2013.11.0793
Article Google Scholar
Afzal I, Akram MW, Rehman HU et al (2020) Moringa leaf and sorghum water extracts and salicylic acid to alleviate impacts of heat stress in wheat South African. J Bot 129:169–174. https://doi.org/10.1016/j.sajb.2019.04.009
Ahammed GJ, Xu W, Liu A, Chen S (2018) COMT1 silencing aggravates heat stress-induced reduction in photosynthesis by decreasing chlorophyll content, photosystem II activity, and electron transport efficiency in tomato. Front Plant Sci 9:998. https://doi.org/10.3389/fpls.2018.00998
Article PubMed PubMed Central Google Scholar
Akter N, Rafiqul Islam M (2017) Heat stress effects and management in wheat. A review. Agron Sustain Dev 37:37. https://doi.org/10.1007/s13593-017-0443-9
Alghabari F, Shafqat W, Ahmad M et al (2019) Heat stress and plant development: role of sulphur metabolites and management strategies. Acta Agric Scand Sect B Soil Plant Sci 69:1–11. https://doi.org/10.1080/09064710.2019.1569715
Almeselmani M, Viswanathan PSD, Deshmukh PS, Chinnusamy V (2012) Effects of prolonged high temperature stress on respiration, photosynthesis and gene expression in wheat ( Triticum aestivum L.) varieties differing in their thermotolerance. Plant Stress 6:25–32
Google Scholar
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396. https://doi.org/10.1104/pp.106.082040
Article CAS PubMed PubMed Central Google Scholar
Asthir B (2015) Protective mechanisms of heat tolerance in crop plants. J Plant Interact 10:202–210
Asthir B, Rai PK, Bains NS, Sohu VS (2012) Genotypic variation for high temperature tolerance in relation to carbon partitioning and grain sink activity in wheat. Am J Plant Sci 3:381–390. https://doi.org/10.4236/ajps.2012.33046
Backhausen JE, Scheibe R, Ahmad I et al (2014) Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO 2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Plant Physiol Biochem 30:963–967. https://doi.org/10.1007/s11738-009-0415-z
Barnabás B, Jäger K, Fehér A (2008) The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ 31:11–38. https://doi.org/10.1111/j.1365-3040.2007.01727.x
Article CAS PubMed Google Scholar
Begcy K, Weigert A, Egesa A, Dresselhaus T (2018) Compared to Australian Cultivars, European summer wheat ( Triticum aestivum ) overreacts when moderate heat stress is applied at the pollen development stage. Agronomy 8:99. https://doi.org/10.3390/agronomy8070099
Bennett D, Reynolds M, Mullan D et al (2012) Detection of two major grain yield QTL in bread wheat ( Triticum aestivum L.) under heat, drought and high yield potential environments. Theor Appl Genet 125:1473–1485. https://doi.org/10.1007/s00122-012-1927-2
Article PubMed Google Scholar
Berry J, Bjorkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Berz J, Simm S, Schuster S et al (2019) Heatster: a database and web server for identification and classification of heat stress transcription factors in plants. Bioinform Biol Insights. https://doi.org/10.1177/1177932218821365
Brestic M, Zivcak M, Kalaji HM et al (2012) Photosystem II thermostability in situ: Environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol Biochem 57:93–105. https://doi.org/10.1016/J.PLAPHY.2012.05.012
Brestic M, Zivcak M, Kunderlikova K, Allakhverdiev SI (2016) High temperature specifically affects the photoprotective responses of chlorophyll b-deficient wheat mutant lines. Photosynth Res 130:251–266. https://doi.org/10.1007/s11120-016-0249-7
Brestic M, Zivcak M, Hauptvogel P et al (2018) Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynth Res 136:245–255. https://doi.org/10.1007/s11120-018-0486-z
Buttar ZA, Wu SN, Arnao MB et al (2020) Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 9:1–17. https://doi.org/10.3390/plants9070809
Carmo-Silva E, Scales JC, Madgwick PJ, Maj P (2015) Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ 38:1817–1832. https://doi.org/10.1111/pce.12425
Casaretto JA, El-kereamy A, Zeng B et al (2016) Expression of OsMYB55 in maize activates stress-responsive genes and enhances heat and drought tolerance. BMC Genom. https://doi.org/10.1186/s12864-016-2659-5
Caverzan A, Casassola A, Brammer SP (2016) Antioxidant responses of wheat plants under stress. Genet Mol Biol 39:1–6. https://doi.org/10.1590/1678-4685-GMB-2015-0109
Chaudhary C, Sharma N, Khurana P (2021) Decoding the wheat awn transcriptome and overexpressing TaRca1β in rice for heat stress tolerance. Plant Mol Biol 105:133–146. https://doi.org/10.1007/s11103-020-01073-0
Chauhan H, Khurana N, Agarwal P, Khurana P (2011) Heat shock factors in rice (Oryza sativa L.): genome-wide expression analysis during reproductive development and abiotic stress. Mol Genet Genom 286:171–187. https://doi.org/10.1007/s00438-011-0638-8
Chen X, Zhang W, Zhang B et al (2011) Phosphoproteins regulated by heat stress in rice leaves. Proteome Sci. https://doi.org/10.1186/1477-5956-9-37
Cheng W, Sakai H, Yagi K, Hasegawa T (2010) Combined effects of elevated [CO 2 ] and high night temperature on carbon assimilation, nitrogen absorption, and the allocations of C and N by rice ( Oryza sativa L.). Agric Meteorol 150:1174–1181. https://doi.org/10.1016/j.agrformet.2010.05.001
Chovancek E, Zivcak M, Botyanszka L et al (2019) Transient heat waves may affect the photosynthetic capacity of susceptible wheat genotypes due to insufficient photosystem I Photoprotection. Plants 8:282. https://doi.org/10.3390/plants8080282
Article CAS PubMed Central Google Scholar
Cochard H, Venisse J-S, Barigah TS et al (2007) Putative role of aquaporins in variable hydraulic conductance of leaves in response to light. Plant Physiol 143:122–133
Comastri A, Janni M, Simmonds J et al (2018) Heat in wheat: exploit reverse genetic techniques to discover new alleles within the Triticum durum shsp26 family. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01337
Cossani CM, Reynolds MP (2012) Physiological traits for improving heat tolerance in wheat. Plant Physiol 160:1710–1718. https://doi.org/10.1104/pp.112.207753
Crafts-Brandner SJ, Salvucci ME (2000) Rubisco activase constrains the photosynthetic potential of leaves at high temperature and CO 2 . Proc Natl Acad Sci USA 97:13430–13435. https://doi.org/10.1073/pnas.230451497
Crafts-Brandner SJ, Salvucci ME (2002) Sensitivity of photosynthesis in a C 4 plant, maize, to heat stress. Plant Physiol 129:1773–1780. https://doi.org/10.1104/pp.002170
Degen GE, Orr DJ, Carmo-Silva E (2021) Heat-induced changes in the abundance of wheat Rubisco activase isoforms. New Phytol 229:1298–1311. https://doi.org/10.1111/nph.16937
Dhindsa RS, Plumb-dhindsa P, Thorpe TA (1981) Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32:93–101. https://doi.org/10.1093/jxb/32.1.93
Djanaguiraman M, Prasad PVV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48:999–1007. https://doi.org/10.1016/j.plaphy.2010.09.009
Djanaguiraman M, Boyle DL, Welti R et al (2018) Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol. https://doi.org/10.1186/s12870-018-1263-z
Djanaguiraman M, Narayanan S, Erdayani E, Prasad PVV (2020) Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol 20:1–12. https://doi.org/10.1186/s12870-020-02479-0
Dubey R, Pathak H, Chakrabarti B et al (2020) Impact of terminal heat stress on wheat yield in India and options for adaptation. Agric Syst. https://doi.org/10.1016/j.agsy.2020.102826
El-Sarag EI, Ismaeil RIM (2013) Evaluation of some bread wheat cultivars productivity as affected by sowing dates and water stress in semi-arid region. Asian J Crop Sci 5:167–178. https://doi.org/10.3923/ajcs.2013.167.178
Fahad S, Adnan M, Hassan S, et al (2019) Rice responses and tolerance to high temperature. In: Advances in rice research for abiotic stress tolerance. Elsevier, pp 201–224
Farooq M, Bramley H, Palta JA, Siddique KHM (2011) Heat stress in wheat during reproductive and grain-filling phases. CRC Crit Rev Plant Sci 30:491–507. https://doi.org/10.1080/07352689.2011.615687
Farooq M, Hussain M, Siddique KHM (2014) Drought stress in wheat during flowering and grain-filling periods. CRC Crit Rev Plant Sci 33:331–349. https://doi.org/10.1080/07352689.2014.875291
Fleitas MC, Mondal S, Gerard GS et al (2020) Identification of CIMMYT spring bread wheat germplasm maintaining superior grain yield and quality under heat-stress. J Cereal Sci. https://doi.org/10.1016/j.jcs.2020.102981
Frey FP, Urbany C, Hüttel B et al (2015) Genome-wide expression profiling and phenotypic evaluation of European maize inbreds at seedling stage in response to heat stress. BMC Genom. https://doi.org/10.1186/s12864-015-1282-1
Fu YB (2015) Understanding crop genetic diversity under modern plant breeding. Theor Appl Genet 128:2131–2142
Fu J, Momčilović I, Clemente TE et al (2008) Heterologous expression of a plastid EF-Tu reduces protein thermal aggregation and enhances CO 2 fixation in wheat ( Triticum aestivum ) following heat stress. Plant Mol Biol 68:277–288. https://doi.org/10.1007/s11103-008-9369-6
Gahlaut V, Baranwal VK, Khurana P (2018) miRNomes involved in imparting thermotolerance to crop plants. 3 Biotech 8:1–19
Gahlaut V, Samtani H, Khurana P (2020) Genome-wide identification and expression profiling of cytosine-5 DNA methyltransferases during drought and heat stress in wheat ( Triticum aestivum ). Genomics 112:4796–4807. https://doi.org/10.1016/j.ygeno.2020.08.031
Gardiner LJ, Quinton-Tulloch M, Olohan L et al (2015) A genome-wide survey of DNA methylation in hexaploid wheat. Genome Biol 16:273. https://doi.org/10.1186/s13059-015-0838-3
Gaur PM, Samineni S, Thudi M et al (2019) Integrated breeding approaches for improving drought and heat adaptation in chickpea ( Cicer arietinum L.). Plant Breed 138:389–400
Gifford RM (2003) Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct Plant Biol 30:171–183. https://doi.org/10.1071/FP02083
González-Schain N, Dreni L, Lawas LMF et al (2016) Genome-wide transcriptome analysis during anthesis reveals new insights into the molecular basis of heat stress responses in tolerant and sensitive rice varieties. Plant Cell Physiol 57:57–68. https://doi.org/10.1093/pcp/pcv174
Gourdji SM, Mathews KL, Reynolds M et al (2013) An assessment of wheat yield sensitivity and breeding gains in hot environments. Proc R Soc B Biol Sci. https://doi.org/10.1098/rspb.2012.2190
Gupta NK, Agarwal S, Agarwal VP et al (2013) Effect of short-term heat stress on growth, physiology and antioxidative defence system in wheat seedlings. Acta Physiol Plant 35:1837–1842. https://doi.org/10.1007/s11738-013-1221-1
Gupta OP, Mishra V, Singh NK et al (2015) Deciphering the dynamics of changing proteins of tolerant and intolerant wheat seedlings subjected to heat stress. Mol Biol Rep 42:43–51. https://doi.org/10.1007/s11033-014-3738-9
Gupta PK, Balyan HS, Sharma S, Kumar R (2020) Genetics of yield, abiotic stress tolerance and biofortification in wheat ( Triticum aestivum L.). Theor Appl Genet 133:1569–1602
Haider S, Iqbal J, Naseer S et al (2021) Molecular mechanisms of plant tolerance to heat stress: current landscape and future perspectives. Plant Cell Rep 1:1–25. https://doi.org/10.1007/S00299-021-02696-3
Hall AE (2010) Breeding for heat tolerance. Plant breeding reviews. Wiley, Oxford, pp 129–168
Chapter Google Scholar
Haque MS, Kjaer KH, Rosenqvist E et al (2014) Heat stress and recovery of photosystem II efficiency in wheat ( Triticum aestivum L.) cultivars acclimated to different growth temperatures. Environ Exp Bot 99:1–8. https://doi.org/10.1016/J.ENVEXPBOT.2013.10.017
Hasanuzzaman M, Nahar K, Alam MM et al (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684. https://doi.org/10.3390/ijms14059643
Hauvaux M (1993) Rapid photosynthetic adaptation to heat stress triggered in potato leaves by moderately elevated temperatures. Plant Cell Environ 16:461–467. https://doi.org/10.1111/j.1365-3040.1993.tb00893.x
Hossain A, Teixeira da Silva JA, Lozovskaya MV, Zvolinsky VP (2012) High temperature combined with drought affect rainfed spring wheat and barley in South-Eastern Russia: I. Phenology and growth. Saudi J Biol Sci 19:473–487. https://doi.org/10.1016/j.sjbs.2012.07.005
Hu Z, Song N, Zheng M et al (2015) Histone acetyltransferase GCN 5 is essential for heat stress-responsive gene activation and thermotolerance in Arabidopsis. Plant J 84:1178–1191. https://doi.org/10.1111/tpj.13076
Hurkman WJ, Vensel WH, Tanaka CK et al (2009) Effect of high temperature on albumin and globulin accumulation in the endosperm proteome of the developing wheat grain. J Cereal Sci 49:12–23. https://doi.org/10.1016/j.jcs.2008.06.014
IPCC (2013) Climate change 2013: the physical science basis. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, New York, pp 1535. https://doi.org/10.1017/CBO9781107415324 .
Ivanov AG, Velitchkova MY, Allakhverdiev SI, Huner NPA (2017) Heat stress-induced effects of photosystem I: an overview of structural and functional responses. Photosynth Res 133:17–30. https://doi.org/10.1007/s11120-017-0383-x
Jaganathan D, Ramasamy K, Sellamuthu G et al (2018) CRISPR for crop improvement: an update review. Front Plant Sci. https://doi.org/10.3389/fpls.2018.00985
Jamil M, Ali A, Gul A et al (2019) Genome-wide association studies of seven agronomic traits under two sowing conditions in bread wheat. BMC Plant Biol 19:149. https://doi.org/10.1186/s12870-019-1754-6
Janda T, Khalil R, Tajti J et al (2019) Responses of young wheat plants to moderate heat stress. Acta Physiol Plant 41:1–8. https://doi.org/10.1007/s11738-019-2930-x
Janni M, Gullì M, Maestri E et al (2020) Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J Exp Bot 71:3780–3802
Khan MIR, Iqbal N, Masood A et al (2013) Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Signal Behav. https://doi.org/10.4161/psb.26374
Klimenko SB, Peshkova AA, Dorofeev NV (2006) Nitrate reductase activity during heat shock in winter wheat. J Stress Physiol Biochem 2:50–55
Kong L, Liu Y, Wang X, Chang C (2020) Insight into the role of epigenetic processes in abiotic and biotic stress response in wheat and barley. Int J Mol Sci 21:1480. https://doi.org/10.3390/ijms21041480
Kosová K, Vítámvás P, Prášil IT, Renaut J (2011) Plant proteome changes under abiotic stress—contribution of proteomics studies to understanding plant stress response. J Proteom 74:1301–1322
Kothari A, Lachowiec J (2021) Roles of brassinosteroids in mitigating heat stress damage in cereal crops. Int J Mol Sci 22:1–15. https://doi.org/10.3390/ijms22052706
Kumar RR, Goswami S, Sharma SK et al (2012) Protection against heat stress in wheat involves change in cell membrane stability, antioxidant enzymes, osmolyte, H 2 O 2 and transcript of heat shock protein. Int J Plant Physiol Biochem 4:83–91. https://doi.org/10.5897/ijppb12.008
Kumar RR, Pathak H, Sharma SK et al (2015) Novel and conserved heat-responsive microRNAs in wheat ( Triticum aestivum L.). Funct Integr Genom 15:323–348. https://doi.org/10.1007/s10142-014-0421-0
Kumar RR, Singh K, Ahuja S et al (2019a) Quantitative proteomic analysis reveals novel stress-associated active proteins (SAAPs) and pathways involved in modulating tolerance of wheat under terminal heat. Funct Integr Genom 19:329–348. https://doi.org/10.1007/s10142-018-0648-2
Kumar RR, Tasleem M, Jain M et al (2019b) Nitric oxide triggered defense network in wheat: augmenting tolerance and grain-quality related traits under heat-induced oxidative damage. Environ Exp Bot 158:189–204. https://doi.org/10.1016/j.envexpbot.2018.11.016
Kumar RR, Dubey K, Arora K et al (2021) Characterizing the putative mitogen-activated protein kinase (MAPK) and their protective role in oxidative stress tolerance and carbon assimilation in wheat under terminal heat stress. Biotechnol Rep 29:e00597. https://doi.org/10.1016/j.btre.2021.e00597
Kumari A, Hemantaranjan A (2019) Mitigating effects of 24-epibrassinolide on heat stress damage by shifting biochemical and antioxidant defense mechanisms in wheat ( Triticum aestivum L.) at pre-flowering stage and post-flowering stage. J Pharmacogn Phytochem 8:1157–1161
CAS Google Scholar
Law RD, Crafts-Brandner SJ (1999) Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol 120:173–181. https://doi.org/10.1104/pp.120.1.173
Li X, Lawas LMF, Malo R et al (2015) Metabolic and transcriptomic signatures of rice floral organs reveal sugar starvation as a factor in reproductive failure under heat and drought stress. Plant Cell Environ 38:2171–2192. https://doi.org/10.1111/pce.12545
Li L, Mao X, Wang J et al (2019) Genetic dissection of drought and heat-responsive agronomic traits in wheat. Plant Cell Environ 42:2540–2553. https://doi.org/10.1111/pce.13577
Liu Z, Xin M, Qin J et al (2015) Temporal transcriptome profiling reveals expression partitioning of homeologous genes contributing to heat and drought acclimation in wheat ( Triticum aestivum L.). BMC Plant Biol 15:152. https://doi.org/10.1186/s12870-015-0511-8
Liu B, Asseng S, Wang A et al (2017) Modelling the effects of post-heading heat stress on biomass growth of winter wheat. Agric Meteorol 247:476–490. https://doi.org/10.1016/j.agrformet.2017.08.018
Liu C, Sukumaran S, Claverie E et al (2019) Genetic dissection of heat and drought stress QTLs in phenology-controlled synthetic-derived recombinant inbred lines in spring wheat. Mol Breed. https://doi.org/10.1007/s11032-019-0938-y
Lu Y, Li R, Wang R et al (2017) Comparative proteomic analysis of flag leaves reveals new insight into wheat heat adaptation. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01086
Macabuhay A, Houshmandfar A, Nuttall J et al (2018) Can elevated CO 2 buffer the effects of heat waves on wheat in a dryland cropping system? Environ Exp Bot 155:578–588. https://doi.org/10.1016/j.envexpbot.2018.07.029
Machado S, Paulsen GM (2001) Combined effects of drought and high temperature on water relations of wheat and sorghum. Plant Soil 233:179–187
Mangelsen E, Kilian J, Harter K et al (2011) Transcriptome analysis of high-temperature stress in developing barley caryopses: early stress responses and effects on storage compound biosynthesis. Mol Plant 4:97–115. https://doi.org/10.1093/mp/ssq058
Martínez-Ballesta MC, López-Pérez L, Muries B et al (2009) Climate change and plant water balance: the role of aquaporins–a review. Climate change, intercropping pest control and beneficial microorganisms. . Springer, pp 71–89
Mathur S, Agrawal D, Jajoo A (2014) Photosynthesis: response to high temperature stress. J Photochem Photobiol B Biol 137:116–126. https://doi.org/10.1016/j.jphotobiol.2014.01.010
Maulana F, Ayalew H, Anderson JD et al (2018) Genome-wide association mapping of seedling heat tolerance in winter wheat. Front Plant Sci. https://doi.org/10.3389/fpls.2018.01272
Mendanha T, Rosenqvist E, Hyldgaard B, Ottosen CO (2018) Heat priming effects on anthesis heat stress in wheat cultivars ( Triticum aestivum L.) with contrasting tolerance to heat stress. Plant Physiol Biochem 132:213–221. https://doi.org/10.1016/j.plaphy.2018.09.002
Mohammadi M, Karimizadeh RA, Naghavi MR et al (2009) Selection of bread wheat genotypes against heat and drought tolerance based on chlorophyll content and stem reserves. J Agric Soc Sci 5:119–122
Nahar K, Ahamed KU, Fujita M (2010) Phenological variation and its relation with yield in several wheat ( Triticum aestivum L.) cultivars under normal and late sowing mediated heat stress condition. Not Sci Biol 2:51–56
Narayanan S, Prasad PVV, Welti R (2016) Wheat leaf lipids during heat stress: II. Lipids experiencing coordinated metabolism are detected by analysis of lipid co-occurrence. Plant Cell Environ 39:608–617. https://doi.org/10.1111/pce.12648
Narayanan S, Prasad PVV, Welti R (2018) Alterations in wheat pollen lipidome during high day and night temperature stress. Plant Cell Environ 41:1749–1761. https://doi.org/10.1111/pce.13156
Neuwald AF, Aravind L, Spouge JL, Koonin EV (1999) Assembly, operation, and disassembly of protein complexes AAA+: a class of chaperone-like atpases associated with the assembly, operation, and disassembly of protein complexes. Genome Res 9:27–43. https://doi.org/10.1101/gr.9.1.27
Nishiyama Y, Allakhverdiev SI, Murata N (2011) Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol Plant 142:35–46
Niu Y, Xiang Y (2018) An overview of biomembrane functions in plant responses to high-temperature stress. Front Plant Sci 9:915
Ohama N, Sato H, Shinozaki K, Yamaguchi-Shinozaki K (2017) Transcriptional regulatory network of plant heat stress response. Trends Plant Sci 22:53–65. https://doi.org/10.1016/j.tplants.2016.08.015
Paliwal R, Röder MS, Kumar U et al (2012) QTL mapping of terminal heat tolerance in hexaploid wheat ( Triticum aestivum L.). Theor Appl Genet 125:561–575. https://doi.org/10.1007/s00122-012-1853-3
Parry MAJ, Reynolds M, Salvucci ME et al (2011) Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J Exp Bot 62:453–467. https://doi.org/10.1093/jxb/erq304
Patel D, Franklin KA (2009) Temperature-regulation of plant architecture. Plant Signal Behav 4:577–579
Perdomo JA, Capó-Bauçà S, Carmo-Silva E, Galmés J (2017) Rubisco and rubisco activase play an important role in the biochemical limitations of photosynthesis in rice, wheat, and maize under high temperature and water deficit. Front Plant Sci 8:490. https://doi.org/10.3389/fpls.2017.00490
Portis AR (2003) Rubisco activase—Rubisco’s catalytic chaperone. Photosynth Res 75:11–27
Poudel PB, Poudel MR (2020) Heat stress effects and tolerance in wheat: a review. J Biol Today’s World 9:1–6
Prasad PVV, Boote KJ, Vu JCV, Allen LH (2004) The carbohydrate metabolism enzymes sucrose-P synthase and ADG-pyrophosphorylase in phaseolus bean leaves are up-regulated at elevated growth carbon dioxide and temperature. Plant Sci 166:1565–1573. https://doi.org/10.1016/j.plantsci.2004.02.009
Prasad PVV, Pisipati SR, Momčilović I, Ristic Z (2011) Independent and combined effects of high temperature and drought stress during grain filling on plant yield and chloroplast EF-Tu expression in spring wheat. J Agron Crop Sci 197:430–441. https://doi.org/10.1111/j.1439-037X.2011.00477.x
Qin D, Wu H, Peng H et al (2008) Heat stress-responsive transcriptome analysis in heat susceptible and tolerant wheat ( Triticum aestivum L.) by using Wheat Genome Array. BMC Genomics. https://doi.org/10.1186/1471-2164-9-432
Ragupathy R, Ravichandran S, Mahdi MSR et al (2016) Deep sequencing of wheat sRNA transcriptome reveals distinct temporal expression pattern of miRNAs in response to heat, light and UV. Sci Rep 6:1–15. https://doi.org/10.1038/srep39373
Rai KK, Pandey N, Rai SP (2019) Salicylic acid and nitric oxide signaling in plant heat stress. Physiol Plant 168:ppl.12958. https://doi.org/10.1111/ppl.12958
Rangan P, Furtado A, Henry R (2020) Transcriptome profiling of wheat genotypes under heat stress during grain-filling. J Cereal Sci. https://doi.org/10.1016/j.jcs.2019.102895
Ravichandran S, Ragupathy R, Edwards T et al (2019) Microrna-guided regulation of heat stress response in wheat. BMC Genom 20:1–16. https://doi.org/10.1186/s12864-019-5799-6
Rennenberg H, Loreto F, Polle A et al (2006) Physiological responses of forest trees to heat and drought. Plant Biol 8:556–571
Rezaei EE, Siebert S, Manderscheid R et al (2018) Quantifying the response of wheat yields to heat stress: the role of the experimental setup. F Crop Res 217:93–103
Rochaix J-D (2011) Assembly of the photosynthetic apparatus. Plant Physiol 155:1493–1500. https://doi.org/10.1104/pp.110.169839
Roy S, Arora A, Chinnusamy V, Singh VP (2017) Endogenous reduced ascorbate: an indicator of plant water deficit stress in wheat. Indian J Plant Physiol 22:365–368. https://doi.org/10.1007/s40502-017-0308-x
Rumeau D, Peltier G, Cournac L (2007) Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ 30:1041–1051. https://doi.org/10.1111/j.1365-3040.2007.01675.x
Sairam RK, Srivastava GC, Saxena DC (2000) Increased antioxidant activity under elevated temperatures: a mechanism of heat stress tolerance in wheat genotypes. Biol Plant 43:245–251
Salvucci ME, Crafts-Brandner SJ (2004) Mechanism for deactivation of Rubisco under moderate heat stress. Physiol Plant 122:513–519. https://doi.org/10.1111/j.1399-3054.2004.00419.x
Sattar A, Sher A, Ijaz M et al (2020) Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS One 15:e0232974. https://doi.org/10.1371/journal.pone.0232974
Scafaro AP, De Vleesschauwer D, Bautsoens N et al (2019) A single point mutation in the C-terminal extension of wheat Rubisco activase dramatically reduces ADP inhibition via enhanced ATP binding affinity. J Biol Chem 294:17931–17940. https://doi.org/10.1074/jbc.RA119.010684
Sehgal A, Sita K, Siddique KHM et al (2018) Drought or/and heat-stress effects on seed filling in food crops: impacts on functional biochemistry, seed yields, and nutritional quality. Front Plant Sci 9:1–19. https://doi.org/10.3389/fpls.2018.01705
Shah MH, Paulsen GM (2005) Injury to photosynthesis and productivity from interaction between high temperature and drought during maturation of wheat. Asian J Plant Sci 4:67–74
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:1–26. https://doi.org/10.1155/2012/217037
Sharma DK, Torp AM, Rosenqvist E et al (2017) QTLs and potential candidate genes for heat stress tolerance identified from the mapping populations specifically segregating for Fv/Fm in wheat. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01668
Shirdelmoghanloo H, Taylor JD, Lohraseb I et al (2016) A QTL on the short arm of wheat ( Triticum aestivum L) chromosome 3B affects the stability of grain weight in plants exposed to a brief heat shock early in grain filling. BMC Plant Biol. https://doi.org/10.1186/s12870-016-0784-6
Sing S (2009) Variation in physiological traits for thermotolerance in wheat. Indian J Plant Physiol 14:407–412
Spilde LA (1989) Influence of seed size and test weight on several agronomic traits of barley and hard red spring wheat. J Prod Agric 2:169. https://doi.org/10.2134/jpa1989.0169
Spreitzer RJ, Salvucci ME (2002) Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annu Rev Plant Biol 53:449–475
Stocker TF, Qin D, Plattner GK et al (2014) Climate change 2013—the physical science basis. Cambridge University Press, Cambridge
Stone PJ, Nicolas ME, Stone P, Nicolas M (1994) Wheat cultivars vary widely in their responses of grain yield and quality to short periods of post-anthesis heat stress. Aust J Plant Physiol 21:887–900. https://doi.org/10.1071/PP9940887
Tadesse W, Suleiman S, Tahir I et al (2019) Heat-tolerant QTLs associated with grain yield and its components in spring bread wheat under heat-stressed environments of Sudan and Egypt. Crop Sci 59:199–211. https://doi.org/10.2135/cropsci2018.06.0389
Talukder ASMHM, McDonald GK, reGill GS, (2014a) Effect of short-term heat stress prior to flowering and early grain set on the grain yield of wheat. F Crop Res 160:54–63. https://doi.org/10.1016/j.fcr.2014.01.013
Talukder SK, Babar MA, Vijayalakshmi K et al (2014b) Mapping QTL for the traits associated with heat tolerance in wheat ( Triticum aestivum L.). BMC Genet. https://doi.org/10.1186/s12863-014-0097-4
Tewari AK, Tripathy BC (1998) Temperature-stress-induced impairment of chlorophyll biosynthetic reactions in cucumber and wheat. Plant Physiol 117:851–858
Thomason K, Babar MA, Erickson JE et al (2018) Comparative physiological and metabolomics analysis of wheat ( Triticum aestivum L.) following post-anthesis heat stress. PLoS One. https://doi.org/10.1371/journal.pone.0197919
Tian X, Wang F, Zhao Y et al (2020) Heat shock transcription factor A1b regulates heat tolerance in wheat and Arabidopsis through OPR3 and jasmonate signalling pathway. Plant Biotechnol J 18:1109. https://doi.org/10.1111/PBI.13268
Tiwari RK, Lal MK, Kumar R et al (2020a) Mechanistic insights on melatonin mediated drought stress mitigation in plants. Physiol Plant. https://doi.org/10.1111/ppl.13307
Tiwari RK, Lal MK, Naga KC et al (2020b) Emerging roles of melatonin in mitigating abiotic and biotic stresses of horticultural crops. Sci Hortic (Amst) 272:109592. https://doi.org/10.1016/j.scienta.2020.109592
ur Rehman A, Habib I, Ahmad N, et al (2009) Screening wheat germplasm for heat tolerance at terminal growth stage. Plant Omics 2:9–19
Upreti KK, Sharma M (2016) Role of plant growth regulators in abiotic stress tolerance 2. Springer. https://doi.org/10.1007/978-81-322-2725-0_2
Valluru R, Davies WJ, Reynolds MP, Dodd IC (2016) Foliar abscisic acid-to-ethylene accumulation and response regulate shoot growth sensitivity to mild drought in wheat. Front Plant Sci 7:461. https://doi.org/10.3389/fpls.2016.00461
Vijayalakshmi K, Fritz AK, Paulsen GM et al (2010) Modeling and mapping QTL for senescence-related traits in winter wheat under high temperature. Mol Breed 26:163–175. https://doi.org/10.1007/s11032-009-9366-8
Viswanathan C, Khanna-Chopra R (2001) Effect of heat stress on grain growth, starch synthesis and protein synthesis in grains of wheat ( Triticum aestivum L.) varieties differing in grain weight stability. J Agron Crop Sci 186:1–7. https://doi.org/10.1046/j.1439-037x.2001.00432.x
Wachter RM, Henderson JN (2015) Photosynthesis: rubisco rescue. Nat Plants 1:1–2
Wahid A (2007) Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane ( Saccharum officinarum ) sprouts. J Plant Res 120:219–228. https://doi.org/10.1007/s10265-006-0040-5
Wang X, Dinler BS, Vignjevic M et al (2015) Physiological and proteome studies of responses to heat stress during grain filling in contrasting wheat cultivars. Plant Sci 230:33–50. https://doi.org/10.1016/j.plantsci.2014.10.009
Wang QL, Chen JH, He NY, Guo FQ (2018a) Metabolic reprogramming in chloroplasts under heat stress in plants. Int J Mol Sci 19(3):849
Article PubMed Central Google Scholar
Wang X, Xu Y, Hu Z, Xu C (2018b) Genomic selection methods for crop improvement: current status and prospects. Crop J 6:330–340. https://doi.org/10.1016/j.cj.2018.03.001
Wei Y, Hu W, Wang Q et al (2017) Identification, transcriptional and functional analysis of heat-shock protein 90s in banana ( Musa acuminata L.) highlight their novel role in melatonin-mediated plant response to Fusarium wilt. J Pineal Res. https://doi.org/10.1111/jpi.12367
Weis E (1981) Reversible heat-inactivation of the Calvin cycle: a possible mechanism of the temperature regulation of photosynthesis. Planta 151:33–39. https://doi.org/10.1007/BF00384234
Wu B, Qiao J, Wang X et al (2021) Factors affecting the rapid changes of protein under short-term heat stress. BMC Genom 22:1–11. https://doi.org/10.1186/s12864-021-07560-y
Xin M, Wang Y, Yao Y et al (2010) Diverse set of microRNAs are responsive to powdery mildew infection and heat stress in wheat ( Triticum aestivum L). BMC Plant Biol 10:123. https://doi.org/10.1186/1471-2229-10-123
Yan K, Chen P, Shao H et al (2013) Dissection of photosynthetic electron transport process in sweet sorghum under heat stress. PLoS One 8:e62100. https://doi.org/10.1371/journal.pone.0062100
Yousuf PY, Abd_Allah EF, Nauman M, et al (2017) Responsive Proteins in Wheat Cultivars with Contrasting Nitrogen Efficiencies under the Combined Stress of High Temperature and Low Nitrogen. Genes (Basel) 8:356. doi: https://doi.org/10.3390/genes8120356
Zhang X, Zhou Q, Wang X et al (2016) Physiological and transcriptional analyses of induced post-anthesis thermo-tolerance by heat-shock pretreatment on germinating seeds of winter wheat. Environ Exp Bot 131:181–189. https://doi.org/10.1016/J.ENVEXPBOT.2016.08.002
Zhang X, Högy P, Wu X, Schmid I, Wang X, Schulze WX, Jiang D, Fangmeier A (2018) Physiological and proteomic evidence for the interactive effects of post-anthesis heat stress and elevated CO 2 on wheat. Proteomics. https://doi.org/10.1002/pmic.201800262
Zhao H, Dai T, Jing Q et al (2007) Leaf senescence and grain filling affected by post-anthesis high temperatures in two different wheat cultivars. Plant Growth Regul 51:149–158
Download references
The funding has been received form Council of Scientific and Industrial Research (CSIR), India (Grant No. MLP-201); and Indian Council of Agricultural Research (ICAR). This manuscript represents CSIR-IHBT publication number 4897.
Author information
Authors and affiliations.
ICAR-Central Potato Research Institute, Shimla, Himachal Pradesh, India
Milan Kumar Lal, Rahul Kumar Tiwari, Vikas Mangal & Brajesh Singh
ICAR-Indian Agricultural Research Institute, New Delhi, India
Milan Kumar Lal, Rahul Kumar Tiwari, Madan Pal Singh, Vijay Paul & Sudhir Kumar
Division of Biotechnology, CSIR-Institute of Himalayan Bioresource Technology, Palampur, Himachal Pradesh, India
Vijay Gahlaut & Gaurav Zinta
ICAR-National Rice Research Institute, Cuttack, Odisha, India
Awadhesh Kumar
Academy of Scientific and Innovative Research (AcSIR), Ghaziabad, Uttar Pradesh, India
Gaurav Zinta
You can also search for this author in PubMed Google Scholar
Contributions
MKL, RKT, VG and GZ: Conceptualization and writing – original draft preparation; VM, AK: Writing – original draft preparation, revision of the manuscript, figures and table; MPS, VP, SK, BS, and GZ: Supervision, writing–review and editing. All authors read and approved the manuscript.
Corresponding authors
Correspondence to Brajesh Singh or Gaurav Zinta .
Ethics declarations
Conflict of interest.
The authors declare there are no conflicts of interests or competing interests.
Additional information
Communicated by Manzer H. Siddiqui.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Milan Kumar Lal, Rahul Kumar Tiwari and Vijay Gahlaut contributed equally to this work
Rights and permissions
Reprints and permissions
About this article
Lal, M.K., Tiwari, R.K., Gahlaut, V. et al. Physiological and molecular insights on wheat responses to heat stress. Plant Cell Rep 41 , 501–518 (2022). https://doi.org/10.1007/s00299-021-02784-4
Download citation
Received : 24 May 2021
Accepted : 07 September 2021
Published : 20 September 2021
Issue Date : March 2022
DOI : https://doi.org/10.1007/s00299-021-02784-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Heat stress
- Photosynthesis
- Climate change
- Find a journal
- Publish with us
- Track your research

- eScholar Home
- Faculty of Science
- Doctoral Dissertations
Cognitive function during exertional heat stress assessed using traditional and serious game technology

Collections
- Doctoral Dissertations [71]
- Electronic Theses and Dissertations [1478]

Department of Nutrition, Exercise and Sports
- Calendar archive
- PhD defence: Environme...
PhD defence: Environmental heat stress and motor-cognitive performance
Jacob feder piil.

Introduction
As the world warms, heat waves will become longer and happen more often. This is concerning for manual laborers because during heat waves, work performance gets worse and more work-site accidents happen. Although these real-world findings are well-documented, results from laboratory studies have been inconsistent, with multiple reports showing that heat improves, worsens, or has no effect on cognitive performance.
These inconsistent findings are likely due to the variable study protocols, the lack of familiarization to these protocols, and the lack of other potential compounding variables during testing. Therefore, the present thesis first aimed to develop a new cognitive testing method, which could detect reductions in cognitive-motor ability caused by heat stress. Next, this method was used to test whether dehydration and solar radiation worsened, or if heat acclimation protected against, the reductions in motor-cognitive performance caused by the heat.
Applying the new assessment method, the effects of: 1) moderate [1°C] and severe [≥2°C] changes in core temperature alone or combined with, 2) dehydration levels of 2% body mass loss, or 3) solar radiation applied to the head, and 4) moderate (14 days) and prolonged (28 days) heat acclimation, were explored.
Moderate increases in core temperature in hot-dry environments do not impair motor-cognitive performance, however, severe increases in core temperature reduced motor-cognitive performance by a lot. Further, motor-cognition was much worse when the participants were dehydrated. Direct solar radiation exposure of the head, combined with moderate increases in core temperature, caused reductions in motor-cognition that were as bad as the reductions caused by severe changes in core temperature without solar radiation. Finally, heat acclimation did not protect against the reductions in cognitive performance caused by heat stress.
Conclusions
The new testing method was both sensitive and reliable enough to detect reductions in motor-cognition caused by heat stress and that dehydration and solar-radiation make the reductions in motor-cognition worse, but that heat acclimation does not protect against these reductions.
Together, these findings help to explain why real-world and laboratory-based studies are in disagreement concerning whether heat affects cognitive performance and identify other environmental and internal risks to address in order to protect worker wellbeing.
2020, 138 pages.
15 April 2020, 14:00
Digital defence.
Professor Bente Kiens (chair), University of Copenhagen, Department of Nutrition, Exercise and Sports, Denmark.
Research Associate Professor Jason Lee Kai Wei National University Singapore, Singapore.
Professor Craig Crandall Texas Health Presbyterian Hospital Dallas, USA.
Professor Lars Nybo, Department of Nutrition, Exercise and Sports, University of Copenhagen, Denmark.
Time: 15 Apr. 2020, 14:00
Place: Digital defence
Organizer: Department of Nutrition, Exercise and Sports, University of Copenhagen
The effect of exercise and heat stress on indirect blood markers of gastrointestinal damage in well-trained populations
- Alice Wallett
Student thesis : Doctoral Thesis
File : application/pdf, 3.99 MB
Type : Full Text
https://doi.org/10.26191/jp7v-0b49

Monitoring heat strain during work in challenging environments
Individuals working in physically demanding occupations can experience high levels of heat strain due to the physical demands of the job, harsh environmental conditions and/or the wearing of personnel protective equipment (PPE). The ability to monitor body core temperature (Tc) could reduce the risk of heat illness. The main purpose of this thesis was to examine non-invasive methods of Tc measurement. Studies 1, 2 and 3 investigated the validity of insulated skin temperature (Tis) as a non-invasive measure of Tc, in emergency service (ES) and military personnel. In Study 1, a model including Tis and micro-climate temperature (Tmc) was developed to predict rectal temperature (Tre) for ES personnel wearing PPE. The resulting standard error of the estimate (SEE=0.20 °C) was within the a-priori pre-defined SEE limit (0.20 °C), providing encouragement for the further investigation of Tis as a surrogate measure of Tc. Studies 2 and 3 sought to determine the validity of Tis in predicting Tre in military personnel, wearing PPE in temperate conditions (Study 2) and in a desert environment (Study 3). Although the SEE was outside the acceptable SEE in Study 2 (0.22 °C), the sensitivity (97 %) for predicting Tre values over 38.5 °C provided scope for a military application for Tis to predict Tc. In Study 3, Tis could not predict Tre in a desert environment, with simulated solar radiation directly affecting Tis and invalidating the prediction (SEE = 0.29 °C). Study 4 involved a series of experiments performed under six conditions in a thermal chamber with two clothing types, to determine whether the addition of other physiological and/or environmental factors might improve the prediction of Tre. A model including Tis and Tmc resulted in an SEE of 0.26 °C; with heart rate (HR) and work significantly reducing the SEE (0.23 °C) (p<0.05). Although the SEE achieved in the validation (0.27 °C) was larger than in Studies 1 and 2, these results provide novel information regarding the measures that explain the variance when predicting Tre in a wide range of heat stress conditions. To our knowledge this is the most detailed analysis of Tc prediction based on non-invasive sensors, with the inclusion of all the parameters that are likely to be relevant. The main conclusion from the work thus far was that it is unlikely a reliable prediction of Tc can be achieved, using Tis, for validity under different types of heat stress conditions. However, predictions of Tre for more specific conditions using Tis are achievable. Having collected a vast amount of data on participants demonstrating high levels of heat strain, it was considered valuable to analyse the drop-outs in more detail. More specifically the goal of Study 5 was to see whether measurements of individual heat strain (Tc, HR) and the combination of these in the Physiological Strain Index (PSI) had predictive power for individual drop-out. There were no differences in PSI between individuals who stopped from heat exhaustion (HE) (7.9 ± 0.8) and those who completed the trial (C) (8.3 ± 0.9). The only differences between these two groups were rate of rise of Tre, (C 0.03 ± 0.01 °C min-1 and; HE 0.04 ± 0.01 °C min-1), chest temperature (Tchest) (C 38.1 ± 1.0 °C and; HE 39.0 ± 0.6 °C) and the temperature gradient between Tchest and Tre (C 1.04 ± 1.07 °C, and; HE -0.05 ± 0.59 °C) (p<0.05); It was therefore concluded that PSI did not provide a good personal heat strain measure that would predict tolerance of the individual. In conclusion, Tis (with Tmc) is promising as a non-invasive measure of Tre, in ES and military personnel wearing fully-encapsulated PPE, based on the resulting SEE and sensitivity and specificity. With the current methodology, it is not valid in conditions with a solar load. The addition of HR and work together improve the prediction of Tre. The PSI does not enable identification of individuals who are approaching heat exhaustion, requiring the inclusion of other physiological responses which determine tolerance.
- Design and Creative Arts
Rights holder
Publication date, ethos persistent id, supervisor(s), qualification name, qualification level, this submission includes a signed certificate in addition to the thesis file(s).
- I have submitted a signed certificate
Administrator link
- https://repository.lboro.ac.uk/account/articles/9356780
Usage metrics

- Design not elsewhere classified

- Reference Manager
- Simple TEXT file
People also looked at
Original research article, thermal stress-related physiological, behavioral, and serum biochemical responses in indigenous pigs adapted to eastern himalayan region.

- Division of Animals and Fisheries Sciences, ICAR Research Complex for NEH Region, Umiam, Shillong, Meghalaya, India
Introduction: The current study was carried out to investigate the effect of micro-environmental variations on physiological, behavioral, and serum biochemical parameters of indigenous (Niang Megha), Hampshire, and crossbred (75% Hampshire X 25% Niang Megha).
Methods: Rectal temperature (T R ), skin surface temperature (T SS ), respiration rate (RR), and heart rate (HR) were recorded at 0,900 and 1,600 h weekly once for 2 months for each season in grower pigs of each genotype. CCTV video cameras were utilized to observe the behavioral changes. Five milliliters of blood samples was collected to estimate different biochemical parameters.
Results: Season affected ( p < 0.05) all physiological parameters which generally increased during summer except T R and RR of indigenous pig. T R , T SS , RR, and HR were significantly ( p < 0.05) higher for Hampshire than for indigenous and crossbred in the summer season. The frequency and behavioral activities to heat loss or to conserve heat such as shivering and wallowing were lower except for physical activity that was higher at all times in indigenous pigs. Seasonal variations influenced metabolic activity and serum activity of alkaline phosphatase (ALP) and alanine transaminase (ALT), which rose in summer in all genotypes. Serum ALP and thyroxine (T 4 ) were significantly ( p < 0.05) higher for indigenous pig in both the seasons. The insulin level was significantly ( p < 0.05) higher in indigenous pigs with no significant difference between Hampshire and crossbred in summer whereas there was significant difference among the genotypes in winter. However, superoxide dismutase (SOD) showed no significant difference in the study. Indigenous pigs had the lowest serum cortisol concentrations, whereas Hampshire had the highest.
Conclusion: The current study's findings on several parameters of three different genotypes suggest that indigenous pigs in this region are more adaptable to the region's changing climatic conditions.
Introduction
The Eastern Himalayas are one of the most biologically rich areas on the Earth. In India, the Northeastern Hill (NEH) region represents the Eastern Himalayan region and has a total geographical area covering ~2,62,379 sq. km. area and lies between 21°34′-29°50′ N latitudes and 87°32′-97°52′ E longitudes in the Eastern Himalayan hill region ( 1 ). This region is well-known for the diverse culture of human races and home to a large number of ethnic people ( 2 ), providing a diverse range of habitats to about 225 tribes in India, out of 450 in the country ( 3 ). Agriculture is the primary source of income for the majority of the rural population of this region (85%), who conducts mixed farming, with livestock accounting for 18% of the value of output from the agricultural sector ( 4 ). Moreover, the livestock sector plays a major role in the socioeconomic development in this region, and it is the major source of livelihood for 20 million people, especially women ( 5 ), also an important contributor to sustainable food security for many nations, particularly in low-income areas and marginal habitats that are unsuitable for crop production ( 6 ). Among all the livestock, pig husbandry is one of the popular livestock and total pig population is mainly dominated by local non-descript pigs (65–75%) in the region ( 7 ). Indigenous pig breeds bear unique features such as better heat tolerance, disease resistance, good maternal qualities, early sexual maturity ( 8 ), and good quality bristles ( 9 ) compared with exotic and crossbreds. The body coat color of indigenous pigs of this region is predominantly black in color and has long and dense hairs extending from wither to the hindquarter.
Climate change is a major worldwide challenge and leads to various environmental stresses that have an impact on the production of livestock ( 10 ). Animals can adapt to climatic stressors by different types of adaptive mechanisms including genetic or biological adaptation, phenotypic or physiological adaptation, acclimatization, and habituation ( 11 ). However, the response mechanisms that ensure survival are detrimental to performance and productivity ( 12 ). The physiological changes when exposed to environmental stress can be measured by the variation in respiration rate, rectal temperature, heart rate, and skin surface temperature ( 13 ). Increased respiration or panting increases airflow and evaporation of water from the lungs and hence releases additional heat ( 14 ). The physiological response of the animal to its internal and external environment is also reflected in the blood profiles. Changes in hemato-biochemical parameters are useful methods for determining the degree of stress caused by environmental and dietary factors ( 15 ) and adaptability to given environmental conditions of an animal ( 16 ). Metabolic hormones affecting thermogenesis can also be estimated as the physiological index of environmental adaptation of an animal ( 17 ). Similarly, pigs exhibit natural behavioral responses to thermal stress, which include huddling together and shivering when cold, seeking shade, wallowing in water, and changing from diurnal to nocturnal feeding times when heat stressed during the day ( 18 ). Pigs have a thick subcutaneous adipose tissue layer and fewer sweat glands; therefore, pigs control their body temperature by behavioral thermoregulation instead of sweating ( 19 ). To enhance heat dissipation, they increase direct contact with cool surfaces ( 20 ), modifying their lying position, increased excretion, and wallowing in their excreta ( 21 ). According to anecdotal evidence, indigenous pigs of this region are better adapted to harsh climatic conditions and subsistence farming, but there is little scientific evidence to back up the statement. Indigenous breeds are more thermotolerant than crossbred and purebred animals in terms of the adaptation potential of the livestock species ( 22 ). Indigenous breeds that have evolved in tropical and subtropical regions have a higher adaptive capacity to such stress than exotic breeds. The South African indigenous Windsnyer pigs had better thermoregulatory mechanisms than the large white pigs of the temperate region in the semiarid climate of South Africa ( 23 ). Indian breeds of cattle such as Bos indicus perform well as compared to exotic cattle such as Bos taurus under stressful tropical environments ( 24 ). These are some examples of unique adaptive characteristics of the indigenous breeds, which evolved in stressful tropical environments enabling them to survive adverse environments. Therefore, it was hypothesized that indigenous pigs (Niang Megha) have better thermoregulatory mechanisms under changing climatic conditions of this region than exotic or crossbred pigs.
The objective of this study was set out to investigate the physiological, behavioral, and serum biochemical changes in response to thermal stress challenges in indigenous, crossbred, and exotic breeds of pigs.
Materials and methods
Location of the study.
The present study was carried out in a pig farm of ICAR Research Complex for NEH Region, Umiam, Meghalaya. The study site is located at 25° 41′ 21″ N latitude and 91° 55′ 25″ E longitude with an altitude of 1,010 m above the mean sea level which falls in humid subtropical high rainfall area and receives rain in the range from 2,239 mm to 2,953 mm annually. In this region, the hottest months are usually July and August, while the coldest months are December and January.
Meteorological measurements
The meteorological data were collected from the Division of System Research and Engineering (DSRE) of the institute during the experimental months. The temperature humidity index (THI) was calculated for each season using the formula, THI = 0.8T+ (RHT-14.4)/100 + 46.4 ( 25 ). The thermoneutral zone for a pig is between 18 and 20°C ( 26 ). The THI value ≤ 75 means no stress, 75 to 78 stressful, and ≥78 extreme stress ( 27 ). The THI value of 79–83 is considered danger, and exceeding this causes severe stress and death ( 28 ).
Experimental animals and experimental design
Two experiments were conducted separately for summer (July–August) and winter (December–January) to study the effect of heat stress (summer) and cold stress (winter) on three genotypes of pigs, namely, indigenous (Niang Megha), Hampshire, and crossbred (having 75% Hampshire and 25% Niang Megha inheritance). Niang Megha is a registered indigenous pig breed of Meghalaya in India (accession no.: INDIA_PIG_1300_NIANGMEGHA_09002), with a small body size at maturity, having dense and long hairs and known for being better adapted to its native climatic conditions. Crossbred pig used in the present study is a crossbred pig variety called “Lumsniang” developed in the ICAR Research Complex for the Northeastern Hill Region, Meghalaya, for better adaptability and performance in the hill ecosystem of the Eastern Himalaya region. Six grower pigs (5–6 months) from each genetic group were selected for each experiment. The experimental animals were maintained in the pen system of housing and fed with balanced concentrate mesh feed two times daily and drinking water was provided ad libitum throughout the period. The experimental pigs were maintained under the standard and uniform managemental conditions. The animals were dewormed on a routine basis and regularly vaccinated. The welfare of the pigs was protected, and the study was approved by the Institutional Animal Ethics Committee (IAEC).
Physiological responses to summer and winter
Physiological parameters including rectal temperature (T R ), skin surface temperature (T SS ), heart rate (HR), and respiration rate (RR) were recorded at 0,900 and 1,600 h weekly once for 2 months for each season, and the mean values were considered. Rectal temperature was recorded by inserting a clinical digital thermometer into the rectum of the pigs with proper restraining to avoid stress. The measurement for skin surface temperature was taken at the lumbar region of the pig using an infrared thermal imager Testo 875i (Testo India Pvt. Ltd., Pune, India). The emissivity of 0.95 was taken as the standard emissivity of the pig body surface in the study. Heart rate was determined by counting the number of heartbeats per minute using a stethoscope. Respiration rate was taken when the pig was at the resting phase by visual observation of flank movement to detect breaths per minute. All the parameters were taken by two trained personnel for all the experimental animals to avoid variation between individuals.
Behavioral response to summer and winter
Behavioral mechanisms associated with heat and cold stress were observed in summer and winter, respectively. The pigs were monitored for different lying positions, such as huddling, shivering, standing, wallowing, and physical activity, by using CCTV video cameras. A total of six CCTV video cameras were used for this study. The thermoregulatory behavior patterns of each genetic group were recorded for 60 days in both the seasons. The duration (min) and frequency (%) for each activity during 24 h (from 6.00 a.m. until 6.00 a.m. of the next day) for 8 days were taken weekly once (for 8 weeks) in each season from pre-recorded videos for each animal.
Serum biochemical changes in response to summer and winter
Blood samples were collected from all the experimental animals separately at 15-day interval during two experimental seasons. Five milliliters of blood samples was aseptically collected before feeding in the morning by venipuncture of the anterior vena cava using sterilized plastic disposable syringes from each animal. The collected samples were immediately transferred to a sterile serum separator vacutainer tube (Becton Dickinson, Franklin, USA), allowed to clot, and then centrifuged at 3,000 rpm for 15 min at room temperature. During blood collection, pigs were handled very carefully to avoid handling stress, and the experimental animals were restrained in the ventro-dorsal position. The collected sera samples were stored at −20°C until further analysis. To assess the metabolic and serum biochemical changes in grower pigs in response to heat stress (summer) and cold stress (winter), commercially available kits were used to analyze the following parameters: alkaline phosphatase (ALP), insulin, thyroxine (T4), superoxide dismutase (SOD), acid phosphatase, alanine transaminase (ALT), and cortisol. All the parameters were estimated as per the manufacturer's instruction.
Statistical analysis
The data on physiological, behavioral, and serum biochemical parameters in different genetic groups and between the seasons were analyzed by comparing the means through multivariate analysis of variance (ANOVA). The data on behavioral activities were first transformed using log 10 to normalize it before analysis. The mean differences of different genetic groups for different parameters and between the seasons were tested for statistical significance (at a 5% level of significance) by Duncan's multiple range test (DMRT) using SPSS version 22.0 statistical software.
The maximum and minimum temperature, relative humidity, and temperature humidity index for the study period are presented in Table 1 . The present study recorded a maximum THI of 93–96 and a minimum THI of 73–79 during the summer months. Similarly, a maximum THI of 83–87 and a minimum THI of 53–63 were recorded during winter. The highest temperature, relative humidity, and THI were recorded in the months of June (temp. 29.3°C, RH 86%, THI 95.89) and July (temp. 29.70°C, RH 88.90 %, THI 96.47) during the study period. The lowest temperature, relative humidity, and THI were recorded in January (temp. 5.50°C, RH 44.6%, THI 53.11) and February (temp. 8°C, RH 40.8%, THI 55.92).
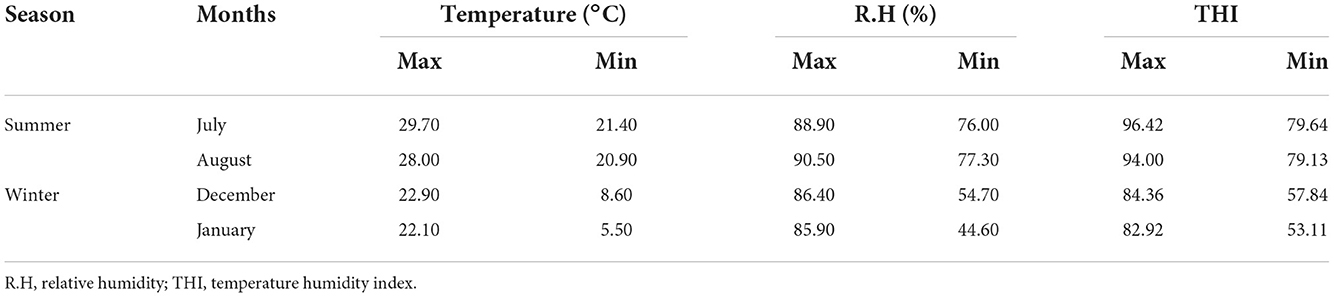
Table 1 . Maximum and minimum temperature, relative humidity, and THI recorded during experimental period.
Physiological measurements
Physiological measurements of different genetic groups during summer and winter are presented in Table 2 . In the summer season, indigenous pigs had significantly lower ( p < 0.05) T R with no significant difference between Hampshire and crossbreed pigs. Skin surface temperature was significantly higher ( p < 0.05) in Hampshire with no significant difference between indigenous and crossbred pigs. There were significant differences in RR and HR among the genetic groups. Indigenous pigs had significantly lower ( p < 0.05) RR and HR followed by crossbred pigs and the highest was recorded in Hampshire.
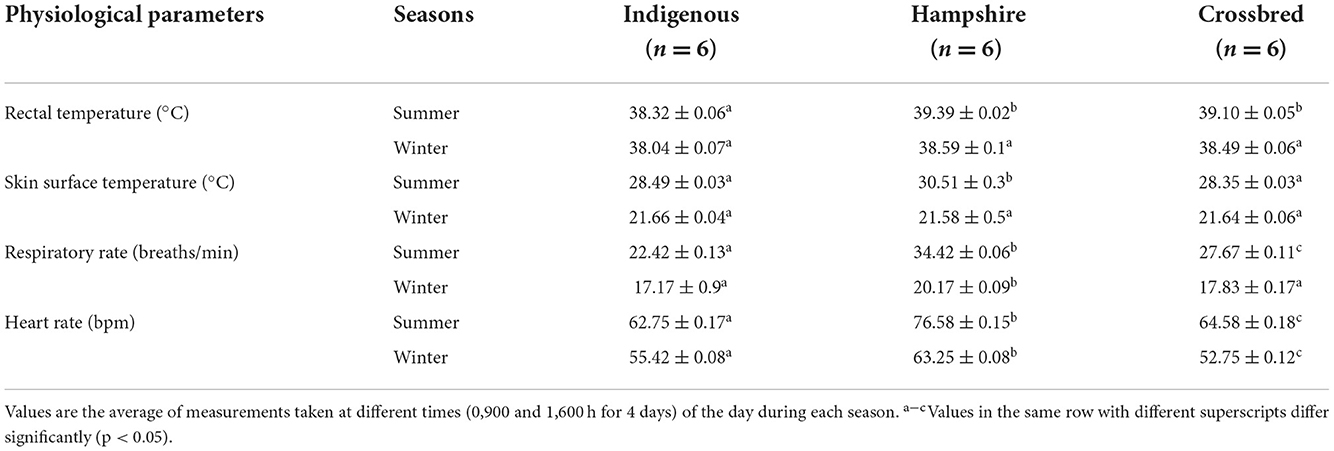
Table 2 . Means ± SE for physiological parameters of indigenous, Hampshire, and crossbred pigs during the study period.
In the winter season, there were no significant differences in T R and T SS among the genetic groups. Although RR was significantly higher ( p < 0.05) in Hampshire, but had no significant ( p < 0.05) difference between indigenous and crossbred pigs. Heart rate had a significant ( p < 0.05) difference among the genetic groups. Hampshire had significantly higher ( p < 0.05) HR followed by indigenous, and the lowest was recorded in crossbred pigs.
Behavioral mechanisms response to summer and winter
Different behavioral mechanisms were observed among the genetic groups when exposed to heat and cold stresses in the experimental animals ( Table 3 ). Hampshire showed significantly ( p < 0.05) higher duration and frequency of lateral and half-lateral lying positions in both the seasons followed by crossbred and indigenous pigs, while the duration and frequency of sternal lying were significantly ( p < 0.05) higher in indigenous pigs followed by crossbred and Hampshire pigs. Similarly, indigenous pigs showed higher duration and frequency of standing and physical activity in both the seasons. Wallowing behavior was observed only in the summer season, while huddling and shivering behavior were observed in the winter season in all the genetic groups. Wallowing and shivering thermogenesis behavior were observed significantly ( p < 0.05) highest in Hampshire followed by crossbred pigs during heat and cold stresses, respectively, whereas huddling behavior was observed significantly ( p < 0.05) highest in indigenous pigs followed by crossbred pigs during cold stress.
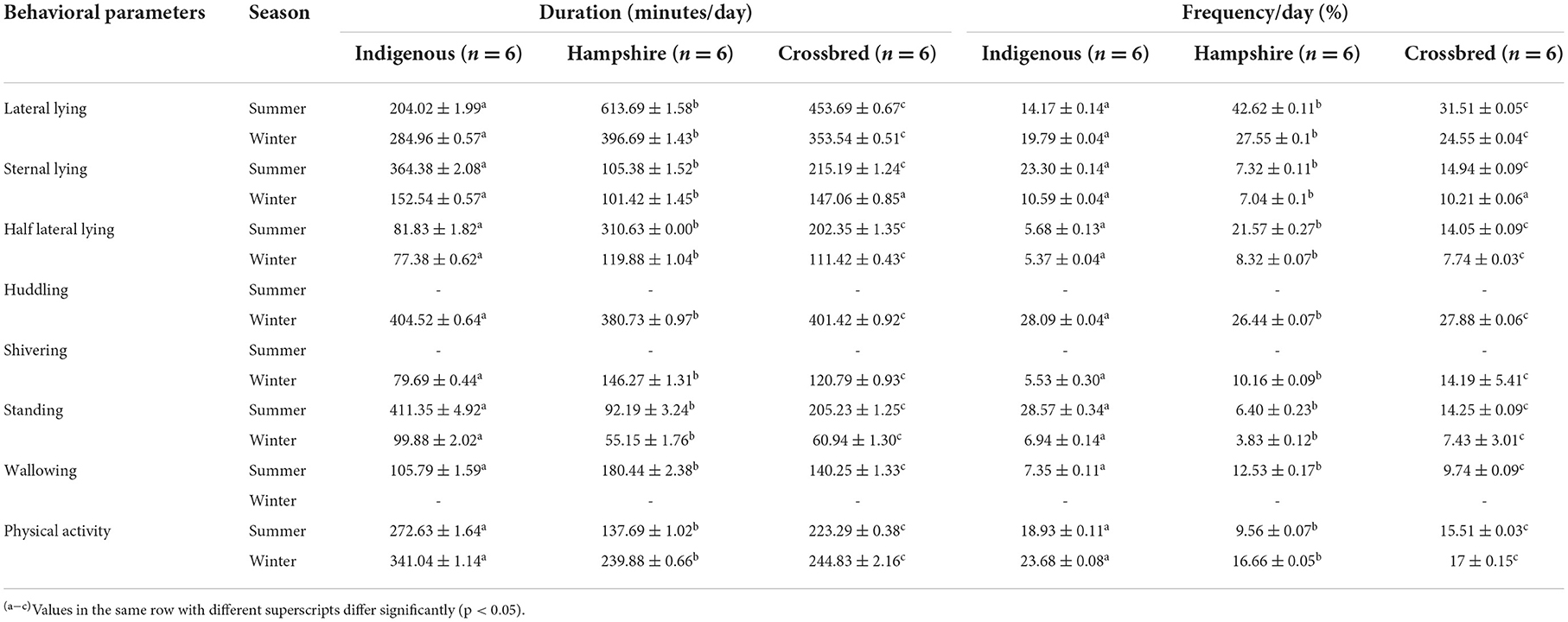
Table 3 . Means ± SE for behavioral parameters of indigenous, Hampshire, and crossbred pigs in response to heat and cold stress observed under a CCTV video camera during the experimental period.
Serum biochemical response to summer and winter
The serum biochemical responses to heat and cold stresses are presented in Table 4 . The result revealed that during heat stress, the serum concentrations of alkaline phosphatase (ALP) and thyroxine (T 4 ) were significantly ( p < 0.05) higher in indigenous pigs followed by crossbred and Hampshire. Among different genetic groups, the serum insulin level was significantly ( p < 0.05) higher in indigenous pigs; however, there was no significant difference between crossbred and Hampshire. The serum concentrations of alanine transaminase (ALT) differed significantly ( p < 0.05) among different genetic groups. It was significantly ( p < 0.05) higher in crossbred followed by Hampshire and indigenous pigs. The activity of superoxide dismutase (SOD) and acid phosphatase showed no significant difference among the genetic groups during heat stress. The cortisol level was significantly ( p < 0.05) lowest in indigenous pigs followed by crossbred and highest in Hampshire during heat stress.
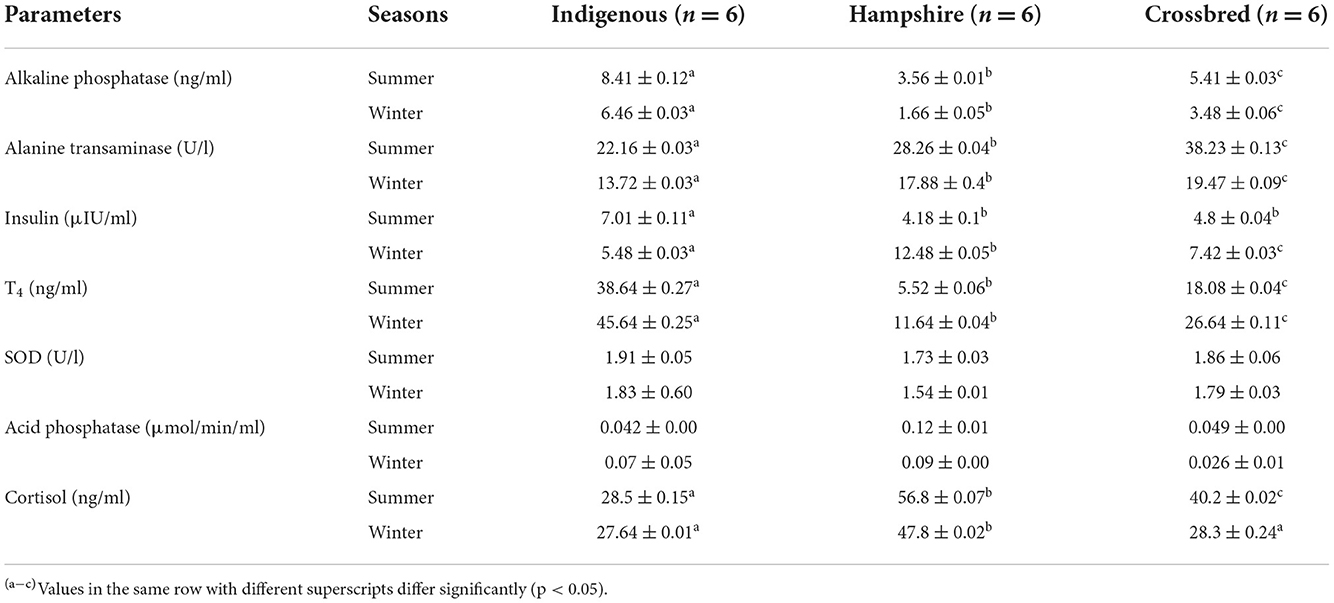
Table 4 . Means ± SE of indigenous, Hampshire, and crossbred pigs for serum biochemical parameters during the study period.
Similarly, during cold stress the serum concentrations of alkaline phosphatase (ALP) and thyroxine (T 4 ) were significantly ( p < 0.05) higher in indigenous pigs followed by crossbred and Hampshire. The serum insulin levels and ALT concentrations were significantly ( p < 0.05) different among the genetic groups. The insulin levels were recorded highest in Hampshire followed by crossbred and indigenous pigs; however, the ALT concentrations were recorded highest in crossbred pigs followed by Hampshire and indigenous pigs. No significant difference in SOD and acid phosphatase was observed among the genetic groups. The cortisol level was significantly ( p < 0.05) higher in Hampshire with no significant difference between indigenous and crossbred pigs.
It is important to know that pigs are susceptible to heat and cold stresses. The present study revealed that the microclimate conditions of the present study location cause heat stress and cold stress in pigs during the summer and winter seasons, respectively. The maximum and minimum THI values during the summer season were above the established normal THI values of 75 or less for pigs ( 27 ). The THI values observed in the current study were those that have previously been reported to cause extreme stress in pigs and may even be fatal ( 27 , 28 ). The primary response of animals under thermal stress is an increase in respiration rate, rectal temperature, and heart rate ( 29 ). Rectal temperature is a delayed indicator of heat stress only responding when the temperature is over 27°C or the THI is >80 ( 30 , 31 ). In the present study, Hampshire and crossbred pigs had higher T R during heat stress than indigenous pigs. Given that an increase in T R is an indication of heat stress, higher T R in Hampshire and crossbred pigs may indicate that they are more susceptible to heat stress. This is confirmed by the previous study, where pigs exposed to heat stress had a higher rectal temperature ( 32 ). While lower TR in indigenous pigs suggest that indigenous pigs might have a better thermoregulatory mechanism to dissipate heat from the body. T R for indigenous and exotic pigs under heat stress, however, did not differ according to some studies ( 23 , 33 ). During heat stress, Hampshire pigs were found to have higher T SS than indigenous and crossbred pigs, which may indicate that they are more susceptible to heat stress. Elevated T SS with an increase in ambient temperature was reported in pigs ( 23 ). Furthermore, exposure of ruminants to high environmental temperature also increased skin temperature ( 34 ), documented in Nguni and Boran cattle breeds ( 35 ) and Osmanabadi goats ( 36 ). This higher T SS might be directly attributable to the vasodilatation of the skin capillary bed, which would enhance blood now to the cutaneous blood vessels, allowing more efficient heat transfer to the surroundings and sensible heat loss ( 36 ). The fact that indigenous and crossbred pigs had lower T SS may be related to their long hair and high hair density, which serve as insulation and reduce heat loss, as demonstrated by Silanikove ( 30 ). Stress triggers the hypothalamus, which enhances respiratory activity to speed up heat escape from the body through respiratory evaporation ( 37 ). In animals, there is a correlation between respiration rate and the surrounding temperature and microenvironments ( 28 ). Thus, when animals are exposed to high ambient temperatures, they have an increased rate of respiration and perspiration ( 31 ). However, it was well-documented that porcine sweat glands are non-functional ( 19 ). Respiration rate is the first sign of heat stress and can be affected by temperatures as low as 21.3°C ( 31 ) or a THI of 73 ( 30 ). In the present study, RR of indigenous and crossbred pigs was within the normal range, that is, 15–30 breaths per minute as reported by Silanikove ( 30 ); however, it was higher than the normal range during heat stress in Hampshire, suggesting the susceptibility to heat stress. Among the genetic groups, higher RR in Hampshire and crossbred pigs during heat stress in the study could be the mechanism to dissipate more heat from the body by evaporating to the surrounding. Our finding of lower respiratory rate of indigenous pigs when exposed to heat stress corroborates with the finding of Moyo ( 38 ) in indigenous Windsnyer pigs of South Africa, which might be due to better adaptability of indigenous pigs to the agroclimatic condition of the region. An increase in RR was also reported in pigs exposed to high heat load ( 23 , 39 ) and various cattle breeds ( 40 ). Heart rate is a stress marker that can be changed in response to thermal stress. Hampshire had higher HR than indigenous and crossbred pigs during heat stress, and this could be an attempt to dissipate excess heat to its surroundings by increasing blood flow to its peripheral tissues. A similar observation was earlier observed and documented by Madzimure et al. ( 23 ) who reported higher HR in large white than in indigenous Windsnyer pig in South Africa. Similar findings were also reported in sheep breeds reared in the Indian semiarid regions ( 41 ) and in other farm animals ( 36 ).
In the winter season, the temperature reaches a minimum of 5.5°C in the study location, which is below the lower limit of the thermoneutral zone (18–20°C) for a pig ( 26 ). Although pigs are susceptible to cold stress, there was not much variation in the physiological parameters during cold stress among the genetic groups. Pigs have a thick subcutaneous adipose tissue layer, which is attributed to maintain their normal physiological parameters during cold stress. Previous research has shown that when pigs are exposed to low temperature, they may increase heat production through muscular shivering thermogenesis ( 42 ), conserve heat through changing posture to reduce the body surface exposed to cold ( 43 ), build nests, huddling together, and select favorable microhabitats ( 42 , 44 ). Similarly, on exposure to cold temperature, increased physical activity, huddling, and shivering were observed in the present study. Increased physical activity and huddling were observed highest in indigenous pigs followed by crossbred and Hampshire, while shivering was observed highest in Hampshire followed by crossbred pigs. Similarly, huddling together in cold temperature was observed in indigenous pigs (Windsnyer and Kolbroek) of South Africa ( 38 ). Increased physical activity was reported in large white ( 38 ) and crossbred (large white x Pietrain) pigs ( 45 ), which is contrary to the present study observation where it is highest in indigenous pigs.
Less time in standing and sitting and more time in lying postures in comparison with control during the cooling phase after the pigs are exposed to high temperature are reported in cooled sow ( 46 ). Similarly, more time in lying postures was observed during heat stress than cold stress and the highest lying behavior was observed in Hampshire followed by crossbred in the entire present study. Heat-stressed sows have been observed to show reduced standing posture ( 47 ) and consistently increase lying postures, especially lying laterally ( 48 ). Similarly, less standing time and more laterally lying posture were observed during heat stress, and the highest was observed in Hampshire followed by crossbred in the present study. Lying laterally appears to be a strategical posture when pigs suffer from heat stress, which is due to the skin surface in contact with the floor, which is greater than any other posture, enhancing heat loss through conduction ( 48 ). Reduced overall activity is one of the behavioral strategies used to help animals cope with heat stress. Physical activity was significantly reduced in heat stress in all the genotypes. Among the genetic groups, physical activity was observed highest in indigenous followed by crossbred than in Hampshire in both the seasons. Wallowing and standing behavior is one of the adaptive behavioral mechanisms to dissipate heat and reduce heat stress. When the heat load increased, animals spent more time standing to maximize heat dissipation by increasing the surface area of the skin exposed to air or wind flow ( 49 ). Standing behavior was observed highest in indigenous pigs, whereas wallowing behavior was highest in Hampshire. Pigs wallow for a variety of reasons, including cooling and protection from solar radiation and preventing attacks from insects ( 50 ). Wallowing allows pigs to lose heat more effectively during hot weather than sweating as well as during cold conditions; mud acts as insulation, allowing them to keep their body warm ( 14 ). However, wallowing behavior was not observed in all groups during the cold season in the present study as they are kept in an intensive system of rearing. Similar to previous findings, in this present study as well-different behavioral responses to conserve heat or dissipate heat during the cold and hot seasons were observed. In this study, indigenous pigs exhibited increased physical activity to generate body heat and huddled together in an attempt to conserve heat. On the contrary, Hampshire pigs attempt to increase heat production by shivering thermogenesis. Similarly, indigenous pigs showed the increased standing time to dissipate heat, while Hampshire and crossbred pigs presented a higher time of wallowing and reduced physical activity to reduce heat production. The fact that Hampshire and crossbred pigs spent more time in lying and wallowing could indicate that they were attempting to cool down to a comfortable temperature through evaporative cooling. However, physical activity was significantly higher and wallowing behavior was significantly lower in indigenous pigs followed by crossbred pigs. These behavioral responses indicate better homeostasis and adaptability of indigenous and crossbred pigs than Hampshire.
This study demonstrates that most of the metabolic and serum biochemical activities were influenced by thermal stress. Some metabolic enzymes increase their activity when exposed to high ambient temperatures. Among other enzymes, alanine aminotransferase (ALT) is one of the important metabolic enzymes that increased its activity when exposed to stress as frequently reported by several researchers. The increased activity of these enzymes was reported in goats ( 51 ), sheep ( 52 ), and pig ( 53 , 54 ) during exposure to heat stress. A similar finding was observed in the present study. According to Banerjee et al. ( 55 ), an increase in the activity of this enzyme is related to the higher adaptive capability of the animals to cope with heat stress. It was also reported that ALT activity increases during stress ( 56 ), such as increased after severe exercise in humans ( 57 ) and restraint in rats ( 58 ). The enzyme activity of ALT was observed highest in Hampshire followed by crossbred pigs, suggesting that Hampshire pigs are more susceptible to stress than indigenous and crossbred pigs of this region. Acid phosphatase (ACP) and alkaline phosphatase (ALP) are two key enzymes involved in animal metabolism. The levels of these enzymes are generally low in heat-stressed animals, which could be attributed to a metabolic shift in the animals ( 51 ). Several investigations have reported a decrease in ALP activity in heat-stressed animals ( 53 , 59 ), including pigs ( 60 ), which is contradictory to our present finding. Why ALP activity is higher during summer than winter in the present study is not known. It is thought that reduced ALP activity is attributed to reduce the functioning of the liver during heat stress exposure ( 61 ). The present observation revealed that the activity of serum ALP was highest in indigenous followed by crossbred pigs and lowest in Hampshire during the study periods. However, acid phosphatase did not differ significantly among genotypes in the present study. The above observations could suggest that indigenous pigs and crossbred are more resilient and have better adaptability to different climatic conditions than Hampshire.
The blood insulin concentration directly reflects the energy status of the animal to sustain production under extreme environmental conditions ( 62 ). An increase in basal and stimulated circulating insulin has been reported in a variety of species ( 63 ), including pigs ( 64 ), during heat stress. Ironically, Sejian et al. ( 17 ) reported a significant reduction in the insulin level after thermal exposure in goats. Similarly, we observed a higher level of insulin in indigenous pigs, which is inconsistent with the earlier findings ( 63 , 64 ) and lower level of insulin in Hampshire and crossbred pigs ( 17 ) during heat stress. This difference in the level of insulin could be due to their nutritional status. A reduction in the level of insulin in Hampshire and crossbred pigs could be due to heat stress and resulted in reduced feed intake, as reported by Sejian et al. ( 65 ), and there was a significant reduction in the insulin level when thermal stress was combined with restricted feeding. Our findings also showed that exposure to a cold environment significantly reduced the level of insulin in indigenous pigs, but significantly increased in Hampshire and crossbred pigs. In rats, cold exposure decreased insulin secretion ( 66 ), stimulating the insulin signaling pathways in the brown adipose tissues for utilizing energy for thermogenesis ( 67 ). However, the increased level of insulin in Hampshire and crossbred pigs when exposed to a cold environment could be due to increased feed intake and elevated glucose concentration in the body, which stimulates the release of insulin.
The thyroid gland is extremely sensitive to temperature changes in the environment ( 68 ). Proper thyroid gland function and thyroid hormone activity are thought to be essential for domestic animals to maintain productive performance ( 69 ). Increased secretion of thyroid hormone increases body metabolism and hence heat production ( 28 ). Various researchers have reported a decrease in thyroid hormone (T 4 and T 3 ) blood concentrations in diverse species when exposed to heat stress ( 70 , 71 ) including pigs ( 28 ). The serum concentrations of T 4 and T 3 were higher in cold stress than in heat stress conditions ( 52 ). The previous findings support the finding of the present study in that the serum concentration of T 4 was shown to be significantly lower during heat stress. Reduced concentrations of circulating T 3 and T 4 have been reported in heifers ( 72 ), sheep ( 73 ), and goat ( 69 ), suggestive of an attempt to reduce metabolic rate and thus metabolic heat production. Overall, during the study periods, indigenous pigs demonstrated a significantly higher T 4 serum concentration than either of the other genotypes. The lower level of T 4 in Hampshire and crossbred pigs might indicate that they are more prone to cold and heat stresses than that in indigenous pigs. When an animal is exposed to heat, reduced food intake and metabolism slow down, which leads to negative energy balance and results in hypofunction of the thyroid gland ( 74 ). Consequently, Sejian et al. ( 17 ) reported a decrease in the level of thyroid hormones (T 3 and T 4 ) in stressed animals. In the present study, higher thyroid hormone (T 4 ) secretion may be indicative of the superior adaptive capability of indigenous pigs to the different climatic conditions of this region.
Antioxidants, such as SOD and glutathione peroxidase, can efficiently eliminate reactive oxygen species (ROS) from the intracellular environment by detoxifying them ( 75 ). During hot and cold climate, there is an increase in the rate of reactive oxygen species (ROS) production leading to the activation of antioxidant enzymes to scavenge the ROS ( 76 ). Some researchers have also observed higher activity of SOD as a marker of oxidative stress in various species during hot ambient temperature ( 77 ). However, lower activity of SOD during heat stress pig was documented by Yang et al. ( 78 ). Kataria and Kataria ( 76 ) reported significantly higher activity of SOD during hot and cold environments as compared to moderate environment. However, in the present study, the enzymatic activity of SOD did not show a significant alteration among the genotypes during thermal stress.
The detection of cortisol is one of the most widely used methods to assess stress in animals. It has proven to be a major stress hormone and a reliable indicator of stress ( 79 ). An increase in circulating cortisol concentrations caused by the activation of the hypothalamic–pituitary–adrenal axis is one of the most frequent and general responses of an animal to stressful situations ( 30 ). An increase in serum cortisol levels is related to the increase in adrenocortical activity, a characteristic linked to the activation of the autonomic nervous system by stress ( 80 ). Therefore, higher serum cortisol concentrations in Hampshire during the study periods suggest that they are more vulnerable to heat and cold stresses. It was also observed that there was a significant increase in serum cortisol concentrations during summer in crossbred pigs, suggestive of heat stress. Similarly, higher cortisol level in heat-stressed pig was reported from arid tracts in Rajasthan, India ( 81 ). However, indigenous pigs of this region maintained the serum cortisol concentrations during thermal stress. The findings indicate that the climatic conditions of this region are stressful to Hampshire and crossbred pigs than indigenous pigs of this region.
The results suggest that indigenous pigs had better adaptability to heat and cold stresses than Hampshire and crossbred pigs. The superior tolerance to thermal stress of indigenous pigs was associated with an ability to maintain their physiological and behavioral activities. Behavioral activities to heat loss or conserve heat such as shivering and wallowing were lower in indigenous pigs, but higher physical activity during thermal stress. The better adaptability is also related to higher metabolic activity, which is shown by higher T 4 activity and lower serum cortisol concentrations. Also, the better adaptability of indigenous pigs might be due to their unique anatomical features such as small body size, short legs, and long and dense bristles. Further detailed studies need to be carried out for a better understanding of the thermoregulatory mechanism of indigenous pigs and identifying the gene responsible for the resilient traits and developed adaptation signature that will be useful for the mainstreaming in the breeding program under changing climatic conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Institutional Animal Ethics Committee (IAEC), ICAR Research Complex for NEH Region, Umiam, Meghalaya, India.
Author contributions
Conception, design of study, and interpretation were done by KG. Data collection and analysis were performed by CG and NSS. The manuscript was drafted by CG, NSS, and KG. Critical revision of the manuscript was done by NMS. All authors contributed to the manuscript revision and have read and approved the final manuscript.
This research was funded by the Indian Council of Agricultural Research (ICAR) under the National Innovation on Climate Resilient Agriculture (NICRA) project.
Acknowledgments
The authors thankfully acknowledge the National Innovation on Climate Resilient Agriculture (NICRA) project, Indian Council of Agricultural Research (ICAR), for providing funding and facilities to conduct the research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mao AA, Hynniewta TM, Sanjappa M. Plant wealth of Northeast India with reference to ethnobotany. Indian J Tradit Knowl . (2009) 8:96–103. Available online at: http://www.niscair.res.in/sciencecommunication/researchjournals/rejour/ijtk/ijtk0.asp
Google Scholar
2. Chakraborty R, De B, Devanna N, Sen S. North-East India an ethnic storehouse of unexplored medicinal plants. J Nat Prod Plant Resour. (2012) 2:143–52. Available online at: http://scholarsresearchlibrary.com/archive.html
3. Chatterjee S, Saikia A, Dutta P, Ghosh D, Pangging G, Goswami, AK. Background paper on “Biodiversity significance of North East India” for the study on natural resources, water and environment nexus for development and growth in North Eastern India. In: WWF-India, New Delhi , (2006). p. 1–71.
4. Kumar A, Staal S, Elumalai K, Singh DK. Livestock sector in North-Eastern region of India: an appraisal of performance. Agric Econ Res Rev. (2007) 20:255–72.
5. Borah M, Halim RA. Dynamics and performance of livestock and poultry sector in India: a temporal analysis. J Acad Ind Res. (2014) 3:1–9.
6. Godber OF, Wall R. Livestock and food security: vulnerability to population growth and climate change. Glob Change Biol. (2014) 20:3092–102. doi: 10.1111/gcb.12589
PubMed Abstract | CrossRef Full Text | Google Scholar
7. 20th The Livestock Census. Department of Animal Husbandry, Dairying and Fisheries, Ministry of Agriculture, New Delhi (2019).
8. Karunakaran M, Mondal M, Rajarajan K, Karmakar HD, Bhat BP, Das J, et al. Early puberty in local Naga boar of India: assessment through epididymal spermiogram and in vivo pregnancy. Anim Reprod Sci. (2009) 111:112–9. doi: 10.1016/j.anireprosci.2008.02.009
9. Mohan NH, Debnath S, Mahapatra RK, Nayak LK, Baruah S, Das A, et al. Tensile properties of hair fibres obtained from different breeds of pigs. Biosyst Eng. (2014) 119:35–43. doi: 10.1016/j.biosystemseng.2014.01.003
CrossRef Full Text | Google Scholar
10. Theusme C, Avendaño-Reyes L, Macías-Cruz U, Correa-Calderón A, García-Cueto RO, Mellado M, et al. Climate change vulnerability of confined livestock systems predicted using bioclimatic indexes in an arid region of México. Sci Total Environ. (2021) 751:141779. doi: 10.1016/j.scitotenv.2020.141779
11. Gaughan JB. Basic Principles Involved in Adaption of Livestock to Climate Change. In Environmental Stress and Amelioration in Livestock Production . Berlin, Heidelberg: Springer (2012). p. 245–61.
12. Pragna P, Sejian V, Soren NM, Bagath M, Krishnan G, Beena V, et al. Summer season induced rhythmic alterations in metabolic activities to adapt to heat stress in three indigenous (Osmanabadi, Malabari and Salem Black) goat breeds. Biol Rhythm Res. (2018) 49:551–65. doi: 10.1080/09291016.2017.1386891
13. Mirkena T, Duguma G, Haile A, Tibbo M, Okeyo AM, Wurzinger M, et al. Genetics of adaptation in domestic farm animals: a review. Livest Sci. (2010) 132:1–2. doi: 10.1016/j.livsci.2010.05.003
14. McGlone JJ. Managing heat stress in the outdoor pig breeding herd. In: Ina Symposium on Outdoor Pig Production in Brazil (1999).
15. Mmereole FUC. The effects of replacing groundnut cake with rubber seed meal on the haematological and serological indices of broilers. Int J Poult Sci. (2008) 7:622–4. doi: 10.3923/ijps.2008.622.624
16. Sejian V, Singh AK, Sahoo A, Naqvi SM. Effect of mineral mixture and antioxidant supplementation on growth, reproductive performance and adaptive capability of m alpura ewes subjected to heat stress. J Anim Physiol Anim Nutr. (2014) 98:72–83. doi: 10.1111/jpn.12037
17. Sejian V, Maurya VP, Naqvi SM. Adaptive capability as indicated by endocrine and biochemical responses of Malpura ewes subjected to combined stresses (thermal and nutritional) in a semi-arid tropical environment. Int J Biometeorol. (2010) 54:653–61. doi: 10.1007/s00484-010-0341-1
18. Ingram DL, Legge KF. Variations in deep body temperature in the young unrestrained pig over the 24-hour period. J Physiol. (1970) 210:989–98. doi: 10.1113/jphysiol.1970.sp009253
19. Zumbach B, Misztal I, Tsuruta S, Sanchez JP, Azain M, Herring W, et al. Genetic components of heat stress in finishing pigs: parameter estimation. J Anim Sci. (2008) 86:2076–81. doi: 10.2527/jas.2007-0282
20. Algers BM, Sanaa T, Nunes B, Wechsler HAM, Spoolder MC, Meunier-Salaun LJ. Scientific report on animal health and welfare aspects of different housing and husbandry systems for adult breeding boars, pregnant, farrowing sows and weaned piglets. EFSA J. (2007) 572:1–107. doi: 10.2903/j.efsa.2007.572
21. Huynh TT, Aarnink AJ, Truong CT, Kemp B, Verstegen MW. Effects of tropical climate and water cooling methods on growing pigs' responses. Livest Sci. (2006) 104:278–91. doi: 10.1016/j.livsci.2006.04.029
22. Rashamol VP, Sejian V, Bagath M, Krishnan G, Archana PR, Bhatta R. Physiological adaptability of livestock to heat stress: an updated review. J Anim Behav Biometeorol. (2020) 6:62–71. doi: 10.31893/2318-1265jabb.v6n3p62-71
23. Madzimure J, Chimonyo M, Zander KK, Dzama K. Diurnal heat-related physiological and behavioural responses in South African indigenous gilts. J Arid Environ. (2012) 87:29–34. doi: 10.1016/j.jaridenv.2012.05.010
24. Niyas PA, Chaidanya K, Shaji S, Sejian V, Bhatta R. Adaptation of livestock to environmental challenges. J Vet Sci Med Diagn. (2015) 4:2. doi: 10.4172/2325-9590.1000162
25. Mader TL, Davis MS, Brown-Brandl T. Environmental factors influencing heat stress in feedlot cattle. J Anim Sci. (2006) 84:712–9. doi: 10.2527/2006.843712x
26. Wathes C, Whittemore C. Environmental management of pigs. In:Whittemore CT, Kyriazakis I, , editors. Whittemore's Science and Practice of Pig Production . 3rd ed. Oxford: Blackwell Publishing (2006). p. 533–92. doi: 10.1002/9780470995624.ch17
27. Lamoureux VS. Current Research in Animal Physiology, 1st Edn . New York, NY: Apple Academic Press (2012). p. 1–318. doi: 10.1201/b12225
28. Pathak PK, Roychoudhury R, Saharia J, Borah MC, Dutta DJ, Bhuyan R, et al. Impact of seasonal thermal stress on physiological and blood biochemical parameters in pigs under different dietary energy levels. Trop Anim health Prod. (2018) 50:1025–32. doi: 10.1007/s11250-018-1526-6
29. Das R, Sailo L, Verma N, Bharti P, Saikia J, Kumar R. Impact of heat stress on health and performance of dairy animals: a review. Vet world. (2016) 9:260. doi: 10.14202/vetworld.2016.260-268
30. Silanikove N. Effects of heat stress on the welfare of extensively managed domestic ruminants. Livest Prod Sci. (2000) 67:1–8. doi: 10.1016/S0301-6226(00)00162-7
31. Lorschy ML. Definitions of Ambient Temperature Requirements for Pigs: A Review . Council Research News-Prairie Swine Centre Inc. (2005).
32. Huynh TT, Aarnink AJ, Verstegen MW, Gerrits WJ, Heetkamp MJ, Kemp B, et al. Effects of increasing temperatures on physiological changes in pigs at different relative humidities. J Anim Sci. (2005) 83:1385–96. doi: 10.2527/2005.8361385x
33. Renaudeau D, Huc E, Noblet J. Acclimation to high ambient temperature in Large White and Caribbean Creole growing pigs. J Anim Sci. (2007) 85:779–90. doi: 10.2527/jas.2006-430
34. Sejian V, Bhatta R, Gaughan JB, Dunshea FR, Lacetera N. Adaptation of animals to heat stress. Animal. (2018) 12:s431–44. doi: 10.1017/S1751731118001945
35. Katiyatiya CL, Bradley G, Muchenje V. Thermotolerance, health profile and cellular expression of HSP90AB1 in Nguni and Boran cows raised on natural pastures under tropical conditions. J Therm Biol. (2017) 69:85–94. doi: 10.1016/j.jtherbio.2017.06.009
36. Shilja S, Sejian V, Bagath M, Mech A, David CG, Kurien EK, et al. Adaptive capability as indicated by behavioral and physiological responses, plasma HSP70 level, and PBMC HSP70 mRNA expression in Osmanabadi goats subjected to combined (heat and nutritional) stressors. Int J Biometeorol. (2016) 60:1311–23. doi: 10.1007/s00484-015-1124-5
37. Fukushi I, Yokota S, Okada Y. The role of the hypothalamus in modulation of respiration. Respir Physiol Neurobiol . (2019) 265:172–9. doi: 10.1016/j.resp.2018.07.003
38. Moyo D. Comparison of Febrile Responses, Thermoregulation and Skin Morphology in the Local Kolbroek, Windsnyer and Exotic Large White Breeds of Pigs in South Africa (Doctoral dissertation). University of the Witwatersrand, Faculty of Health Sciences, Johannesburg (2017).
39. Seibert JT, Graves KL, Hale BJ, Keating AF, Baumgard LH, Ross JW. Characterizing the acute heat stress response in gilts: I. Thermoregulatory and production variables. J Anim Sci. (2018) 96:941–9. doi: 10.1093/jas/skx036
40. Valente ÉEL, Chizzotti ML, Oliveira CVR, Galvão MC, Domingues SS, Rodrigues AC, et al. Intake, physiological parameters and behavior of Angus and Nellore bulls subjected to heat stress. Semina: Ciências Agrárias. (2015) 36:4565–74. doi: 10.5433/1679-0359.2015v36n6Supl2p4565
41. Rathwa SD, Vasava AA, Pathan MM, Madhira SP, Patel YG, Pande AM. Effect of season on physiological, biochemical, hormonal, and oxidative stress parameters of indigenous sheep. Vet world. (2017) 10:650. doi: 10.14202/vetworld.2017.650-654
42. Berg F, Gustafson U, Andersson L. The uncoupling protein 1 gene (UCP1) is disrupted in the pig lineage: a genetic explanation for poor thermoregulation in piglets. PLoS Genet. (2006) 8:e129. doi: 10.1371/journal.pgen.0020129
43. Hayne SM, Tennessen T, Anderson DM. The responses of growing pigs exposed to cold with varying amounts of straw bedding. Can J Anim Sci. (2000) 80:539–46. doi: 10.4141/A00-003
44. Olczak K, Nowicki J, Klocek C. Pig behaviour in relation to weather conditions-a review. Ann Anim Sci. (2015) 15:601. doi: 10.1515/aoas-2015-0024
45. Quiniou N, Noblet J, Van Milgen J, Dubois S. Modelling heat production and energy balance in group-housed growing pigs exposed to low or high ambient temperatures. Br J Nutri. (2001) 85:97–106. doi: 10.1079/BJN2000217
46. Parois SP, Cabezón FA, Schinckel AP, Johnson JS, Stwalley RM, Marchant-Forde JN. Effect of floor cooling on behavior and heart rate of late lactation sows under acute heat stress. Front Vet Sci. (2018) 5:223. doi: 10.3389/fvets.2018.00223
47. Canaday DC, Salak-Johnson JL, Visconti AM, Wang X, Bhalerao K, Knox RV. Effect of variability in lighting and temperature environments for mature gilts housed in gestation crates on measures of reproduction and animal well-being. J Anim Sci. (2013) 91:1225–36. doi: 10.2527/jas.2012-5733
48. Muns R, Malmkvist J, Larsen ML, Sorensen D, Pedersen LJ. High environmental temperature around farrowing induced heat stress in crated sows. J Anim Sci. (2016) 94:377–84. doi: 10.2527/jas.2015-9623
49. Tucker CB, Rogers AR, Schütz KE. Effect of solar radiation on dairy cattle behaviour, use of shade and body temperature in a pasture-based system. Appl Anim Behav Sci. (2008) 109:141–54. doi: 10.1016/j.applanim.2007.03.015
50. Bracke MB. Review of wallowing in pigs: description of the behaviour and its motivational basis. Appl Anim Behav Sci. (2011) 132:1–3. doi: 10.1016/j.applanim.2011.01.002
51. Gupta M, Kumar S, Dangi SS, Jangir BL. Physiological, biochemical and molecular responses to thermal stress in goats. Int J Livest Res. (2013) 3:27–38. doi: 10.5455/ijlr.20130502081121
52. Nazifi S, Saeb M, Rowghani E, Kaveh K. The influences of thermal stress on serum biochemical parameters of Iranian fat-tailed sheep and their correlation with triiodothyronine (T3), thyroxine (T4) and cortisol concentrations. Comp Clin Path. (2003) 12:135–9. doi: 10.1007/s00580-003-0487-x
53. Mayengbam P, Tolenkhomba TC. Seasonal variation of hemato-biochemical parameters in indigenous pig: Zovawk of Mizoram. Vet World. (2015) 8:732. doi: 10.14202/vetworld.2015.732-737
54. Pourouchottamane R, Pankaj PK, Banik S, Naskar S, Venkatsubramanian V, Ramana DB. Effect of micro-environmental variations on biomolecular profile and performance of pig. J Agrometerol. (2013) 15:1–6.
55. Banerjee D, Upadhyay RC, Chaudhary UB, Kumar R, Singh S, Ashutosh Das TK, et al. Seasonal variations in physio-biochemical profiles of Indian goats in the paradigm of hot and cold climate. Biol Rhythm Res. (2015) 46:221–36. doi: 10.1080/09291016.2014.984999
56. Luo Z, Zhu W, Guo Q, Luo W, Zhang J, Xu W, et al. Weaning induced hepatic oxidative stress, apoptosis, and aminotransferases through MAPK signaling pathways in piglets. Oxid Med Cell Longev. (2016) 2016:4768541. doi: 10.1155/2016/4768541
57. Mashiko T, Umeda T, Nakaji S, Sugawara K. Effects of exercise on the physical condition of college rugby players during summer training camp. Br J Sports Med. (2004) 38:186–90. doi: 10.1136/bjsm.2002.004333
58. Arakawa H, Kodama H, Matsuoka N, Yamaguchi I. Stress increases plasma enzyme activity in rats: differential effects of adrenergic and cholinergic blockades. J Pharmacol Exp Ther. (1997) 280:1296–303.
PubMed Abstract | Google Scholar
59. Pearce SC, Gabler NK, Ross JW, Escobar J, Patience JF, Rhoads RP, et al. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J Anim Sci. (2013) 91:2108–18. doi: 10.2527/jas.2012-5738
60. Wen X, Wu W, Fang W, Tang S, Xin H, Xie J, et al. Effects of long-term heat exposure on cholesterol metabolism and immune responses in growing pigs. Livest Sci. (2019) 230:103857. doi: 10.1016/j.livsci.2019.103857
61. Hooda OK, Singh G. Effect of thermal stress on feed intake, plasma enzymes and blood biochemicals in buffalo heifers. Indian J Anim Nutr. (2010) 27:122–7.
62. Sejian V, Srivastava RS, Varshney VP. Pineal-adrenal relationship: modulating effects of glucocorticoids on pineal function to ameliorate thermal-stress in goats. Asian-Austr J Anim Sci. (2008) 21:988–94. doi: 10.5713/ajas.2008.70482
63. Baumgard LH, Rhoads Jr RP. Effects of heat stress on postabsorptive metabolism and energetics. Annu Rev Anim Biosci. (2013) 1:311–37. doi: 10.1146/annurev-animal-031412-103644
64. Victoria Sanz Fernandez M, Johnson JS, Abuajamieh M, Stoakes SK, Seibert JT, Cox L, et al. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol Rep. (2015) 3:e12315. doi: 10.14814/phy2.12315
65. Sejian V, Srivastava RS, Varshney VP. Effect of thermal stress on endocrine profile and phagocytosis index in goats. Indian Vet J. (2010) 87:11567. Available online at: http://www.indvetjournal.com
66. Gasparetti AL, de Souza CT, Pereira-da-Silva M, Oliveira RL, Saad MJ, Carneiro EM, et al. Cold exposure induces tissue-specific modulation of the insulin-signalling pathway in Rattus norvegicu s . J Physiol . (2003) 552:149–62. doi: 10.1113/jphysiol.2003.050369
67. Wang X, Wahl R. Responses of the insulin signaling pathways in the brown adipose tissue of rats following cold exposure. PLoS ONE. (2014) 9:e99772. doi: 10.1371/journal.pone.0099772
68. Rasouli A, Nouri M, Khajeh GH, Rasekh A. The influences of seasonal variations on thyroid activity and some biochemical parameters of cattle. Iranian J Vet Res. (2004) 5:1383–91.
69. Todini L. Thyroid hormones in small ruminants: effects of endogenous, environmental and nutritional factors. Animal . (2007)1:997–1008. doi: 10.1017/S1751731107000262
70. Salem MH, Elsherbiny AA, Khalil MH, Yousef MK. Diurnal and seasonal rhythm in plasma-cortisol, triiodothyronine and thyronine as affected by the wool coat in barki sheep. Indian J Anim Sci. (1991) 61:946–51.
71. Abdel-Samee AM. Heat adaptability of growing Bedouin goats in Egypt. Der Tropenlandwirt-J Agric Trop Subtrop. (1996) 97:137–47.
72. Pereira AM, Baccari F, Titto EA, Almeida JA. Effect of thermal stress on physiological parameters, feed intake and plasma thyroid hormones concentration in Alentejana, Mertolenga, Frisian and Limousine cattle breeds. Int J Biometeorol. (2008) 52:199–208. doi: 10.1007/s00484-007-0111-x
73. Indu S, Sejian V, Kumar D, Pareek A, Naqvi SM. Ideal proportion of roughage and concentrate for Malpura ewes to adapt and reproduce in a semi-arid tropical environment. Trop Anim Health Prod. (2015) 47:1487–95. doi: 10.1007/s11250-015-0889-1
74. McManus C, Paludo GR, Louvandini H, Gugel R, Sasaki LC, Paiva SR. Heat tolerance in Brazilian sheep: physiological and blood parameters. Trop Anim Health Prod. (2009) 41:95–101. doi: 10.1007/s11250-008-9162-1
75. Dizdaroglu M, Olinski R, Doroshow JH, Akman SA. Modification of DNA bases in chromatin of intact target human cells by activated human polymorphonuclear leukocytes. Cancer Res. (1993) 53:1269–72.
76. Kataria AK, Kataria N. Evaluation of oxidative stress in sheep affected with peste des petits ruminants. J Stress Physiol Biochem. (2012) 8:72–7.
77. Sakatani M, Balboula AZ, Yamanaka K, Takahashi M. Effect of summer heat environment on body temperature, estrous cycles and blood antioxidant levels in Japanese Black cow. Anim Sci J. (2012) 83:394–402. doi: 10.1111/j.1740-0929.2011.00967.x
78. Yang P, Hao Y, Feng J, Lin H, Feng Y, Wu X, et al. The expression of carnosine and its effect on the antioxidant capacity of longissimus dorsi muscle in finishing pigs exposed to constant heat stress. Asian-Austr J Anim Sci. (2014) 27:1763. doi: 10.5713/ajas.2014.14063
79. Sadoul B, Geffroy B. Measuring cortisol, the major stress hormone in fishes. J Fish Biol. (2019) 94:540–55. doi: 10.1111/jfb.13904
80. Rocha LM, Devillers N, Maldague X, Kabemba FZ, Fleuret J, Guay F, et al. Validation of anatomical sites for the measurement of infrared body surface temperature variation in response to handling and transport. Anim. (2019) 9:425. doi: 10.3390/ani9070425
81. Kataria N, Kataria AK. Alterations in prolactin and cortisol levels in heat stressed pigs from arid tracts in India. Porc Res. (2013) 3:4–8.
Keywords: adaptation, behavior, indigenous, pig, thermal stress, response
Citation: Govindasamy K, Gonmei C, Singh NS and Singh NM (2022) Thermal stress-related physiological, behavioral, and serum biochemical responses in indigenous pigs adapted to Eastern Himalayan region. Front. Vet. Sci. 9:1034635. doi: 10.3389/fvets.2022.1034635
Received: 01 September 2022; Accepted: 14 November 2022; Published: 15 December 2022.
Reviewed by:
Copyright © 2022 Govindasamy, Gonmei, Singh and Singh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kadirvel Govindasamy, velvet.2007@rediffmail.com
This article is part of the Research Topic
Behavior and Heat Stress

IMAGES
VIDEO
COMMENTS
Heat stress is a global issue that crosses socioeconomic status. Heat stress leads to reduced worker capacity on seasonal scales, and weekly to sub-daily timescales, incapacitation, morbidity, and mortality. This dissertation focuses on 2 distinct parts: quantification methods of heat stress, and heat stress applications. Quantification methods of heat stress Chapters 1-3 focus on historical ...
e heat is on Doctoral Dissertation Series 2018:61 9 789177 535379 ISBN 978-91-7753-537-9 ... ISRN LUTMDN/TMAT-1028-SE The heat is on Evaluation of workplace heat stress under a changing climate KARIN LUNDGREN KOWNACKI ERGONOMICS AND AEROSOL TECHNOLOGY | DESIGN SCIENCES LUND UNIVERSITY ... My PhD research has been a journey of a lifeti me ...
Thermal indices and occupational heat stress: a systematic review and meta-analysis. In: Effects of Heat on Behavioral and Physiological Mechanisms of the Human Thermoregulatory System during Rest, Exercise, and Work (PhD Thesis). Thessaly, Greece: University of Greece, chapt 6, 2020. Google Scholar; 58.
Each QTL explains approximately 4.5-19.3% phenotypic variance, and a combination of the superior haplotype of three QTLs on chromosomes 1B, 5A, and 6D can improve the genetic effect of heat tolerance compared with a single locus (Mason et al. 2011). Pinto et al. (2010) also identified 16 QTLs associated with heat stress adaptive traits using ...
The overall effects of heat stress on morphological parameters of wheat are illustrated in Fig. 1. The temperature range for wheat seed germination is from 4 to 37 °C, where 12 °C to 25 °C is the optimal temperature range, however high temperature up to 40 °C adversely affects photosynthesis (Spilde 1989 ).
Abstract and Figures. Wheat production requires at least ~ 2.4% increase per year rate by 2050 globally to meet food demands. However, heat stress results in serious yield loss of wheat worldwide ...
heat stress was higher in Janz (33%) than CM9-6Y (15%), followed by Krichauff (17%) and CM9-4Y (20%). ... dissertation both of my supervisors have spent many hours revising written material and editing my publications and their great efforts and assistance have been much appreciated but ... friendship during my PhD tenure.
Molecular Studies on Bread Wheat (Triticum aestivum L.) for Drought Stress Tolerance. December 2019. Thesis for: PhD Thesis. Advisor: Prof. Hamdy Emara, Prof Ahmed Nower, Prof, Khaled Salem and ...
These data 1349 indicate that the reduction in V ̇ O2max under heat stress is associated with a rise in whole-body temperature, 1350 rather than the prevailing ambient conditions per se. Red ...
The assessment of the risk of human exposure to heat is a topic as relevant today as a century ago. The introduction and use of heat stress indices and models to predict and quantify heat stress and heat strain has helped to reduce morbidity and mortality in industrial, military, sports, and leisure activities dramatically.
Effective heat stress management requires adequate understanding of occupational heat stress risks and adaptation policies. ... This manuscript is part of the PhD thesis of Victor Fannam Nunfam. We thank the study participants for willingness and informed consent during the study. We recognise the role of Christopher Nyarko, an official of the ...
Thesis title: COGNITIVE FUNCTION DURING EXERTIONAL HEAT STRESS ASSESSED USING TRADITIONAL AND SERIOUS GAME TECHNOLOGY The undersigned certify that the student has presented his thesis, that the thesis is acceptable in form and content and that a satisfactory knowledge of the field covered by the thesis was demonstrated by
This is the second in a Series of two papers on heat and health. Introduction. The first paper in this Series outlined the negative health effects of human heat stress, and the greater health risk associated with the increased exposure to extreme heat and hot weather projected with climate change. 1 In many countries, extended bouts of very hot weather (ie, heatwaves) are already responsible ...
Thus, the current thesis aimed to evaluate the effects of exercise-induced heat stress on cognitive function in firefighters using the CANTAB testing battery and a recently developed serious game simulating the decision making tasks required of firefighters in a two-storey residential fire while walking on a treadmill.
PhD defence: Environmental heat stress and motor-cognitive performance Jacob Feder Piil PhD thesis. Introduction. As the world warms, heat waves will become longer and happen more often. This is concerning for manual laborers because during heat waves, work performance gets worse and more work-site accidents happen.
The results of this thesis suggest that: 1) well-trained and elite athletes experience transient perturbations in blood markers of gastrointestinal damage when exercising in the heat; 2) maximal repeated-sprint exercise in the heat acutely increases gastrointestinal damage and immune activation compared to sprinting in the cool; and 3) LCHF ...
However, in heat stress regime hybrids with best performance include BKR-02 x V00183, BKR-02 x SH-02, V00183 x Ch-86, SH-02 x BKR-02 and SH-02 x Ch-86. Similarly F1 hybrids as mentioned above registered good performance for various physio-morphological traits confirming the validity of techniques involved for determination of high yielding ...
Revisiting summer infertility in the boar: impact of heat stress on the quality and DNA integrity of spermatozoa, and its mitigation by antioxidant therapy Thesis submitted by Santiago T. Peña Jr. DVM, MTVSc In January 2018 for the degree of Doctor of Philosophy College of Public Health, Medical and Veterinary Sciences James Cook University
Heat stress in dairy calves: Insights on importance, methodology and abatement PhD Thesis Mikolt Bakony 2021. Supervisors Dr. László Könyves, PhD. supervisor University of Veterinary Medicine Department of Animal Hygiene, Herd Health and Mobile Clinic Dr. Levente Kovács PhD.
Individuals working in physically demanding occupations can experience high levels of heat strain due to the physical demands of the job, harsh environmental conditions and/or the wearing of personnel protective equipment (PPE). The ability to monitor body core temperature (Tc) could reduce the risk of heat illness. The main purpose of this thesis was to examine non-invasive methods of Tc ...
3.2 The proportion of four protein fractions. The relative distribution of four protein fractions, that is, albumins, globulins, glutelins and prolamins in seeds of different genotypes, is given in Figures 2-5.The relative proportion of albumins and globulins was shown to decrease while glutelin and prolamins increase at high temperatures or under heat stress.
neering courses in the areas of thermal stresses, directed MS and PhD thesis and dissertation research projects, including studies of thermal stresses in the thermal shroud of a space environmental simulation chamber, and conducted continuing education courses involving thermal stress applications for practicing engineers for more than 3 decades.
IntroductionThe current study was carried out to investigate the effect of micro-environmental variations on physiological, behavioral, and serum biochemical parameters of indigenous (Niang Megha), Hampshire, and crossbred (75% Hampshire X 25% Niang Megha).MethodsRectal temperature (TR), skin surface temperature (TSS), respiration rate (RR), and heart rate (HR) were recorded at 0,900 and 1,600 ...