Cookies on this website
We use cookies to ensure that we give you the best experience on our website. If you click 'Accept all cookies' we'll assume that you are happy to receive all cookies and you won't see this message again. If you click 'Reject all non-essential cookies' only necessary cookies providing core functionality such as security, network management, and accessibility will be enabled. Click 'Find out more' for information on how to change your cookie settings.

- Accessibility

HERC staff and DPhils had their annual Away-Day at the beautiful Worcester College, Oxford. Meet the HERC Team!


The Nuffield Department of Population Health has a full-time three year DPhil programme intended to train new researchers in Population Health.
HERC and COVID-19
Despite the practical challenges we are all facing, several members of the HERC team have been able to use their skills and experience to contribute to the scientific evidence base on COVID-19.

Genomic Technology
HERC is involved in analysing the economic implications in several studies in the area of genomic technologies. These studies span numerous disease areas including cancers, cardiovascular disease and infectious diseases within hospitals and use a range of health economics tools, especially micro-costing, modelling and discrete choice experiments

Our purpose is to contribute through our research to health and healthcare in the UK and internationally. We examine the economic aspects of health and disease, the costs and benefits of prevention and treatment, and the design and evaluation of health systems. Of particular importance in all areas is the rigour and innovativeness of the research methods we use.
The Health Economics Research Centre (HERC)
HERC was established by the University of Oxford in 1996. Our aim is to contribute to health and healthcare in the UK and internationally, by conducting research on economic aspects of health and disease, the costs and benefits of prevention and treatment, and the design and evaluation of health systems.
Watch videos of our staff, students and alumni on the HERC@25 page.
Latest publications
Altunkaya J. et al, (2024), The Lancet Diabetes and Endocrinology
POUWELS K. et al, (2024), Nature Communications
Klepac P. et al, (2024), Trans R Soc Trop Med Hyg
ROOPE L. et al, (2024), Communications Medicine
Mendelson M. et al, (2024), The Lancet
HERC & Oxford NIHR BRC - RESEARCH TRAINING Bursary Scheme
Since 2015, HERC has been in collaboration with the Research Education and Training Group at the Oxford NIHR Biomedical Research Council (Oxford NIHR BRC) and has supported over 50 funded places to attend our Health Economics Short Courses.
For more information on how to apply for these bursaries, please visit the Oxford NIHR BRC website

Latest news
Early blood glucose control for people with type 2 diabetes is crucial for reducing complications and prolonging life
17 May 2024
Does workforce explain the relationship between funding and patient experience? A mediation analysis of primary care data in England
20 February 2024
Assessing the relationship between coverage of essential health services and poverty levels in low- and middle-income countries
14 February 2024
Duration of protective immunity following COVID-19 vaccination of individuals with underlying health conditions: A rapid review
Rationing in an Era of Multiple Tight Constraints: Is Cost-Utility Analysis Still Fit for Purpose?
12 February 2024
Helping parents to help their children: a digital intervention for child anxiety problems
6 February 2024
2023-2024 Programme: HERC Short Courses in Health Economics

HERC offers a varied programme of short courses designed for health economists, health care professionals, academic researchers, students and clinical investigators at different stages in their careers.
Online Integrating Economic Evaluation into Clinical Trials : 25 - 26 June 2024 Registration now open. Please visit here for full details.
Classroom Applied Method of Cost-Effectiveness Analysis : 08 - 10 July 2024 Registration now open. Please visit here for full details
Online Introduction to Health Economic Evaluation : 24 - 25 September 2024 Registration is now open. Please visit here for full details.
Online Inequality in health and health care: theoretical and empirical considerations: 06 - 08 November Registration now open. Please visit here for full details.
Classroom Understanding and Predicting Choice Behaviour in Health: Preference Elicitation and Analysis : Next dates will be scheduled soon . Please visit here for full details on course structure and to register on waiting list.
For full information on all of the above courses, please visit https://www.herc.ox.ac.uk/herc-short-courses
Founded in 1996
30+ Researchers
Over 1000 course participants
12000+ followers on social media
HERC Data Privacy Statement
Herc twitter, forthcoming herc events, the health economic and policy seminar series.
Wednesday, 03 April 2024 to Saturday, 31 May 2025
COVID-19-related catastrophic health expenditure and multidimensional poverty in Ghana: further discussions 20 years after implementing a social health insurance policy
Thursday, 30 May 2024, 2pm to 3pm
The History of Health Economics
Thursday, 27 June 2024, 11am to 12.30pm
Selected pages
Research design support for nhs researchers, current vacancies at herc, study with us, herc newsletters, herc presentations, social media.
- Visit the Gateway
- Visit the Alliance
- Visit HDR UK Futures
The Health Data Research Innovation Gateway provides a common entry point to discover and request access to UK health datasets. Users can search for health data tools, research projects, publications and collaborate via a community forum. Find out more
We are the national institute for health data science.
Our mission is to unite the UK's health data to enable discoveries that improve people's lives.
Our Research Priorities
Five UK-wide Research Driver programmes are focusing on areas where data science has great potential to improve public health, prevent people from becoming unwell, and enhance patient care.
The Alliance marks its fifth anniversary
The UK Health Data Research Alliance is marking five remarkable years of progress and commitment to maximising the benefits of health data research for all.
CVD-COVID-UK / COVID-IMPACT
CVD-COVID-UK aims to understand the relationship between COVID-19 and cardiovascular diseases such as heart attack, heart failure, stroke, and blood clots in the lungs through analyses of de-identified, linked, nationally collated healthcare datasets across the four nations of the UK.
Health Data Science Black Internship Programme
As the UK’s national institute for health data research, we are helping to transform the prospects of talented Black health data scientists in the UK by providing opportunities to flourish in STEM careers through our health data research Black Internship Programme.
HDR UK Futures
HDR UK Futures is a free and flexible virtual learning platform which lets you learn from national and international experts however and whenever suits you.
Ambitious “rising stars” programme to explore cardiovascular disease, cancer and other complex conditions
The Health Data Research UK (HDR UK) Big Data for Complex Disease Driver Programme has awarded six prestigious fellowships, with a total of £1.8 million funding.

New insights to tackle major global health data research challenges
Hdr uk and ga4gh form a strategic partnership to unite genomic and health data.

Impact at HDR UK
Our vision is that every patient, clinical trial, biomedical discovery and public health policy benefits from the use of large scale data and advanced analytics.

Research Driver Programmes
Five UK-wide Research Driver Programmes will harness the power of large-scale data to deliver ground-breaking scientific impacts and infrastructure innovation.

Case Studies
Case study examples of how we have enabled research across the UK.

Discover Data on the Gateway
The Health Data Research Innovation Gateway (the 'Gateway') search engine or 'portal' provides a common entry point for researchers and innovators to discover and request access to health...

Health Data Research Hubs
The Health Data Research Hubs provide a rich toolkit of healthcare datasets, infrastructure and expertise that enable users to identify, access, understand and use data to improve people’s lives.

UK Health Data Research Alliance
The UK Health Data Research Alliance (the 'Alliance') is an independent alliance of leading healthcare and research organisations united to establish best practice for the ethical use of UK health...
Subscribe to our Mailing List
Join our community and keep up-to-date on matters related to health data science!
Data Saves Lives
Harnessing data and technology to improve health
The Health eResearch Centre is a world-leading digital health network that unites and provides data-intensive research and education across Northern England and beyond.
At the Health eResearch Centre (HeRC) we bring together a multi-disciplinary team of researchers, developers and clinicians to understand more about how under-used health data can be re-purposed to improve health. Working on over forty research projects we are combining the latest technology with innovative, mathematical models to deliver better services across the UK. #datasaveslives
- Privacy Statement

- Research and Development
The NIHR HealthTech Research Centre

Technological innovations have the potential to revolutionise healthcare services for everyone. The NIHR HealthTech Research Centre in Sustainable Innovation (HRC) is a partnership between the Royal Devon University Healthcare NHS Foundation Trust and the University of Exeter, designed to streamline the development pipeline from prototype to commercial product.
The HRC is one of 14 newly established research centres across England with the objective of driving innovation in health technology. Our purpose is to work with businesses to support the development of medical devices, diagnostics and digital technologies, accelerating the regulatory approvals process and smoothing the adoption pathway into the NHS and other healthcare institutions.
Innovation in action
More About us
Patients front and centre
We use patient and physician feedback to improve usability, effectiveness and adoption leading to better products and an evidence base that aims to speed up the regulatory approval process.

Our core themes
The work of the HRC focuses on four core themes, which include projects focused on:
- Diagnostics and biomarkers
- Data-led research, ai and digital innovation
- Rehabilitation and frailty
- Sustainable innovation
A track record of innovation
Exeter Hip Stem: This hip replacement project, pioneered by the Royal Devon and the University of Exeter has transformed the lives of more than 2M patients worldwide.
Type 1 Diabetes Genetic Risk Score (T1D-GRS): We developed a simple, inexpensive and robust genetic test to predict and classify type 1 diabetes with 97% accuracy.
Capture and Recycling of Waste Volatile Anaesthetic Agents: Sagetech Medical approached us to supervise and support the validation work required to progress an innovation that would recycle anaesthetic gases
Areas of expertise

Clinical staff
We have access to some of the best clinicians in their fields with specialist interests across all areas of medical research.

Patient and Public Involvement and Engagement (PPIE)
By adopting a patient-centred approach, we place patients at the heart of HealthTech Design and Development.

Healthcare technical solutions
We co-develop and evaluate data algorithms, AI, Apps and sensor technology (on person and in environments) for use in clinical and community settings.

Infrastructure & facilities
We have systems and partnerships in place to quickly evaluate new technologies including; the NIHR Exeter Biomedical Research Centre , the NIHR Applied Research Collaboration South West Peninsula (PenARC), the Clinical Research Network South West Peninsula , the NIHR Exeter Clinical Research Facility and the VSIM and Gillings centre.

Collaboration and partnerships
We work with local, national and international companies as well as regulatory bodies, NHS health and care organisations, charities and development partners
Navigating the regulatory landscape
We use our combined expertise to support the journey through regulation, reducing the time to target, collect and present evidence.

Work with us
If you are looking for access to the best health technology research infrastructure, clinical and academic expertise and a fast track route through the regulatory approvals process, then get in touch by emailing [email protected]
Get in contact
Welcome to the temporary page for the NIHR HealthTech Research Centre, South West Peninsula.
Get in touch by emailing [email protected]
Download a brochure
Helpful websites
Last updated: May 09, 2024.
Our site uses cookies to help give you a better experience. By continuing to use it you consent to the use of cookies as set out in our privacy policy.
- Health Research Classification System
- UK Health Research Analysis
Public Health Research
In 2006, the major funders of public health research in the UK came together under the auspices of the UKCRC to develop a coordinated approach to improving the UK public health research environment. The findings of the UKCRC Public Health Research Strategic Planning Group (SPG) are documented in a report, Strengthening Public Health Research in the UK.
The outcome of the SPG was a commitment of over £20m by a consortium of eight funding partners to create UKCRC Public Health Research Centres of Excellence in 2008.
Since 2008, partners in the UKCRC have invested £37 million in a network of six Public Health Centres of Excellence to increase infrastructure, build academic capacity in public health research in the UK and provide a platform to engage with policy and practice.
The final report shows how 10-years of collaborative work by these centres – based in Edinburgh, Belfast, Newcastle, Nottingham, Cambridge and Cardiff – has exceeded expectations of what was though possible back in 2008.
The UKCRC Centres have expanded the pool of early-career researchers and nutured their talent while creating new opportunities to work across academia, policy and practice. Researchers have gone on to expand their networks and advance their careers, securing fellowships and lectureships, winnning awards and promotion. The UKCRC centres have been a strong magnet for leveraging significant additional funding to increase the volume and quality of public health health research.
The centres have helped change the way we think about how to align research with the needs of policymakers and practitioners to improve health at a local level.
At the national level, the impact has been wide-reaching, with evidence from centre research programmes influencing the government sugar tax, encouraging healthy transport policies, providing guidance on physical activity, promoting health in schols and playing a leading role in government policy on tobacco smoking and vaping.
Centre collaborations and acdemic-policy partnerships have changed the public health landscape, paving the way for ambitious new prevention intitiatives, like the UK Preventation Research Partnership.
The funding Partners contributing to this joint initiative are:
- British Heart Foundation
- Cancer Research UK
- National Institute of Health Research
- Economic and Social Research Council
- Medical Research Council
- Health and Social Care Research and Development Office, Northern Ireland
- National Institute for Social Care and Health Research (Welsh Assembly Government)
- Wellcome Trust
The six UKCRC Public Health Research Centres of Excellence are:
- Centre for Diet and Activity Research (CEDAR)
- Centre of Excellence for Public Health Northern Ireland
- Centre for Development and Evaluation of Complex Interventions for Public Health (DECIPHer)
- Centre for Translational Research in Public Health (Fuse)
- UK Centre for Tobacco Control and Alcohol Studies (UKCTAS)
- The Scottish Collaboration for Public Health Research and Policy
Useful Links
- UKCRC Public Health Research Centres of Excellence Final Report 2018
- Strengthening Public Health Research in the UK
- Executive Summary: Strengthening Public Health Research in the UK
Cookies on GOV.UK
We use some essential cookies to make this website work.
We’d like to set additional cookies to understand how you use GOV.UK, remember your settings and improve government services.
We also use cookies set by other sites to help us deliver content from their services.
You have accepted additional cookies. You can change your cookie settings at any time.
You have rejected additional cookies. You can change your cookie settings at any time.
- Entering and staying in the UK
- Visas and entry clearance
- Work and investor visas
- UKRI endorsement: employing or hosting institutions (Global Talent visa)
- UK Visas and Immigration
UKRI list of approved research organisations
Updated 10 May 2024

© Crown copyright 2024
This publication is licensed under the terms of the Open Government Licence v3.0 except where otherwise stated. To view this licence, visit nationalarchives.gov.uk/doc/open-government-licence/version/3 or write to the Information Policy Team, The National Archives, Kew, London TW9 4DU, or email: [email protected] .
Where we have identified any third party copyright information you will need to obtain permission from the copyright holders concerned.
This publication is available at https://www.gov.uk/government/publications/ukri-endorsement-employing-or-hosting-institutions-global-talent-visa/ukri-list-of-approved-research-organisations
Approved research organisations
UKRI accepts these UK organisations are able to employ or host an applicant who holds, or provides critical contributions to work supported by, an award or grant from an endorsed funder:
- Advanced Forming Research Centre (AFRC)
- Advanced Manufacturing Research Centre (AMRC)
- Advanced Research and Invention Agency
- Agricultural Engineering Precision Innovation Centre
- Agriculture and Horticulture Development Board
- Agri-Food and Biosciences Institute
- Agrimetrics
- Alan Turing Institute
- All NHS Trusts, Hospitals, Boards and GP Practises
- All UK Higher education institutions
- Animal and Plant Health Agency (APHA)
- Anthony Nolan
- Armagh Observatory
- Arts Council England
- Arts Council Wales
- Atomic Weapons Establishment
- Babraham Institute
- Beatson Institute for Cancer Research
- BirdLife International
- British Broadcasting Corporation (BBC)
- British Film Institute
- British Institute of International and Comparative Law
- British Library
- British Museum
- British Trust for Ornithology
- Building Research Establishment Ltd (BRE)
- Butterfly Conservation
- CAB International
- Cambridge Arctic Shelf Programme
- Cambridge Crystallographic Data Centre
- Campden BRI
- Cell and Gene Therapy Catapult
- Centre for Crop Health and Protection
- Centre for Environment, Fisheries and Aquaculture Science (Cefas)
- Centre for Innovation Excellence in Livestock
- Chatham House (Royal Institute of International Affairs)
- Clinical Practice Research Datalink (subsidiary of MHPRA)
- Compound Semiconductor Applications Catapult
- Connected Places Catapult
- Defence Science and Technology Laboratory
- Diamond Light Source Ltd
- Digital Catapult
- Earlham Institute
- Earthwatch Institute
- EMBL - European Bioinformatics Institute
- Energy Systems Catapult
- English Heritage
- Environment Agency
- European Marine Energy Centre (EMEC)
- European Synchrotron Radiation Facility (ESRF)
- FERA Science Ltd
- Forest Research
- Fraunhofer UK Research Ltd
- Genomics England
- Glass Futures
- Health and Safety Executive
- Health and Safety Laboratory (HSL)
- Health Data Research UK (HDR UK)
- Historic Buildings and Monuments Commission for England
- Historic Environment Scotland
- Historic Royal Palaces
- HR Wallingford Group Ltd
- UK Hydrographic Office
- Imperial War Museum
- Institute for Fiscal Studies
- Institute of Development Studies
- Institute of Occupational Medicine
- Instruct-ERIC
- Intellectual Property Office
- International Institute for Environment and Development
- Isaac Newton Group
- John Innes Centre
- Joint Astronomy Centre
- Joint Nature Conservation Committee (JNCC)
- LGC Ltd [footnote 1]
- London Institute of Mathematical Sciences (LIMS)
- Malaria Consortium (UK)
- Manufacturing Technology Centre (MTC)
- Marine Biological Association
- Marine Management Organisation
- Marine Scotland Science
- Medicines and Healthcare products Regulatory Agency (subsidiary of MHPRA)
- Medicines Discovery Catapult
- Moredun Research Institute
- MRC Centre for Macaques
- MRC Harwell Institute
- MRC Laboratory of Medical Sciences
- MRC Laboratory of Molecular Biology
- Museum of London Archaeology
- National Archives
- National Botanical Gardens of Wales
- National Centre for Atmospheric Science
- National Centre for Earth Observation
- National Centre for Social Research
- National Composites Centre
- National Engineering Laboratory
- National Foundation for Educational Research
- National Galleries of Scotland
- National Gallery
- National Innovation Centre for Ageing (NICA)
- National Institute for Biological Standards and Control (NIBSC)
- National Institute for Biological Standards and Control (subsidiary of MHPRA)
- National Institute of Agricultural Botany
- National Institute of Economic and Social Research
- National Institute of Health and Care Excellence
- National Library of Scotland
- National Library of Wales
- National Manufacturing Institute Scotland (NMIS)
- National Maritime Museum
- National Museum Wales
- National Museums Liverpool
- National Museums of Scotland
- National Nuclear Laboratory
- National Oceanography Centre
- National Physical Laboratory
- National Portrait Gallery
- Natural England
- Natural History Museum
- Natural Resources Wales
- NERC British Antarctic Survey
- NERC British Geological Survey
- Net Zero Technology Centre
- Northern Ireland Environment Agency
- Nuclear AMRC
- Office of Health Economics (OHE)
- Office for National Statistics (ONS)
- Offshore Renewable Energy Catapult
- Ordnance Survey
- Organic Research Centre
- Overseas Development Institute (ODI)
- Plymouth Marine Laboratory
- Public Health Wales
- Quadram Institute Bioscience
- RAND Europe Community Interest Company
- Rosalind Franklin Institute
- Rothamsted Research
- Royal Armouries
- Royal Botanic Gardens - Edinburgh
- Royal Botanic Gardens - Kew
- Royal Commission on the Ancient and Historical Monuments of Wales
- Royal Society for the Protection of Birds
- Royal United Services Institute for Defence and Security Studies
- Satellite Applications Catapult
- SCI Foundation
- Science and Advice for Scottish Agriculture
- Science Museum Group
- Scottish Association for Marine Sciences
- Scottish Environment Protection Agency
- Sightsavers
- Sir Alister Hardy Foundation for Ocean Science
- Sir John Soane’s Museum
- Smith Institute
- Sport England
- Sports Council Wales
- STFC - Laboratories
- Tavistock Institute of Human Relations
- The Aga Khan University (International) in the United Kingdom
- The Faraday Institution
- The Francis Crick Institute
- The James Hutton Institute
- The Met Office
- The National Trust
- The Pirbright Institute
- The Resolution Foundation
- The Sainsbury Laboratory
- The Square Kilometre Array Organisation (SKA)
- The Welding Institute
- Transport Research Laboratory Limited
- UK Astronomy Technology Centre
- UK Centre for Ecology and Hydrology
- UK Dementia Research Institute
- UK Health Security Agency
- UK Space Agency
- Unlimit Health
- Vaccines Manufacturing and Innovation Centre UK Ltd
- Veterinary Medicines Directorate (VMD)
- Victoria and Albert Museum
- Wallace Collection
- Warwick Manufacturing Group HVM Catapult (WMG)
- Wellcome Trust Sanger Institute
- World Conservation Monitoring Centre WCMC
- Young Foundation
- Zoological Society of London, Institute of Zoology
LGC Ltd (formerly Laboratory of Government Chemist) including the National Measurement Laboratory for Chemical and Biological Measurements, Office of the Government Chemist, and MHRA’s Official Medicines Control Laboratory (OMCL) for chemical testing and the British Pharmacopoeia (BP) Commission Laboratory. ↩
Is this page useful?
- Yes this page is useful
- No this page is not useful
Help us improve GOV.UK
Don’t include personal or financial information like your National Insurance number or credit card details.
To help us improve GOV.UK, we’d like to know more about your visit today. Please fill in this survey (opens in a new tab) .
You are using an outdated browser. Please upgrade your browser to improve your experience and security.
Centre For Health Research and Education
An independent healthcare company bridging the policy and practice gap in public health for global cancer prevention.

We are an independent healthcare solutions company with global cancer prevention ambitions. We work with organisations across the world to design and implement practical and scalable health interventions. We use innovative approaches for a sustainable, positive impact on peoples’ lives.
At CHRE, we work with organisations and funding bodies from across the world on global cancer prevention projects. Due to the potential scale and global impact of our work, we are currently focusing on reducing the harms from tobacco and separately, childhood obesity prevention.
Latest from CHRE
International women’s day 2022 #breakthebias.
On International Women’s Day 2022, CHRE’s India team launched a campaign for women tobacco users - smokers as well as oral tobacco users- to demand attention and support for tobacco cessation from their healthcare professionals.

Please sign up to view details of The Smoke-Free UK Project
Mr Mrs Miss Ms Other
United Kingdom United States Canada Mexico Afghanistan Albania Algeria American Samoa Andorra Angola Anguilla Antigua and Barbuda Argentina Armenia Armenia Aruba Australia Austria Azerbaijan Azerbaijan Bahamas Bahrain Bangladesh Barbados Belarus Belgium Belize Benin Bermuda Bhutan Bolivia Bonaire Bosnia and Herzegovina Botswana Bouvet Island (Bouvetoya) Brazil British Indian Ocean Territory (Chagos Archipelago) British Virgin Islands Brunei Darussalam Bulgaria Burkina Faso Burundi Cambodia Cameroon Cape Verde Cayman Islands Central African Republic Chad Chile China Christmas Island Cocos (Keeling) Islands Colombia Comoros Congo Congo Cook Islands Costa Rica Cote d'Ivoire Croatia Cuba Curaçao Cyprus Cyprus Czech Republic Denmark Djibouti Dominica Dominican Republic Ecuador Egypt El Salvador Equatorial Guinea Eritrea Estonia Ethiopia Falkland Islands (Malvinas) Faroe Islands Fiji Finland France French Guiana French Polynesia French Southern Territories Gabon Gambia Georgia Germany Ghana Gibraltar Greece Greenland Grenada Guadeloupe Guam Guatemala Guernsey Guinea Guinea-Bissau Guyana Haiti Heard Island and McDonald Islands Holy See (Vatican City State) Honduras Hong Kong Hungary Iceland India Indonesia Iran Iraq Ireland Isle of Man Israel Italy Jamaica Japan Jersey Jordan Kazakhstan Kazakhstan Kenya Kiribati Korea Korea Kuwait Kyrgyz Republic Lao People's Democratic Republic Latvia Lebanon Lesotho Liberia Libyan Arab Jamahiriya Liechtenstein Lithuania Luxembourg Macao Macedonia Madagascar Malawi Malaysia Maldives Mali Malta Marshall Islands Martinique Mauritania Mauritius Mayotte Micronesia Moldova Monaco Mongolia Montenegro Montserrat Morocco Mozambique Myanmar Namibia Nauru Nepal Netherlands Netherlands Antilles New Caledonia New Zealand Nicaragua Niger Nigeria Niue Norfolk Island Northern Mariana Islands Norway Oman Pakistan Palau Palestinian Territory Panama Papua New Guinea Paraguay Peru Philippines Pitcairn Islands Poland Portugal Puerto Rico Qatar Reunion Romania Russian Federation Rwanda Saint Barthelemy Saint Helena Saint Kitts and Nevis Saint Lucia Saint Martin Saint Pierre and Miquelon Saint Vincent and the Grenadines Samoa San Marino Sao Tome and Principe Saudi Arabia Senegal Serbia Seychelles Sierra Leone Singapore Sint Maarten (Netherlands) Slovakia (Slovak Republic) Slovenia Solomon Islands Somalia South Africa South Georgia & S. Sandwich Islands Spain Sri Lanka Sudan Suriname Svalbard & Jan Mayen Islands Swaziland Sweden Switzerland Syrian Arab Republic Taiwan Tajikistan Tanzania Thailand Timor-Leste Togo Tokelau Tonga Trinidad and Tobago Tunisia Turkey Turkey Turkmenistan Turks and Caicos Islands Tuvalu U.S. Virgin Islands U.S. Minor Outlying Islands Uganda Ukraine United Arab Emirates Uruguay Uzbekistan Vanuatu Venezuela Vietnam Wallis and Futuna Western Sahara Yemen Zambia Zimbabwe
Please see the Privacy Policy and T&Cs : by entering your details, you are agreeing to our T&Cs.

NIHR awards HealthTech Research Centre Network £5m to advance health innovations
by Jen Brogan | 21st May 2024 | News

The National Institute for Health and Care Research (NIHR) has awarded an NIHR HealthTech Research Centre (HRC) Network £5m to provide national coordination and leadership for NIHR HRCs to advance health innovations.
Hosted by Sheffield Teaching Hospitals NHS Foundation Trust from 1 September 2024, the network will support the development, evaluation and adoption of innovative health and care technology.
In November 2023, the NIHR announced £42m in funding to support 14 new NIHR HRCs to work with businesses to support the development of medical devices, diagnostics and digital technologies to allow people to better monitor their health, make earlier diagnoses and improve the management of conditions such as cancer, dementia, cardiovascular and respiratory disease.
The HRCs are located in leading NHS organisations across England, including Guy’s and St Thomas’ and King’s College London’s HRC for cardiovascular and respiratory medicine, announced in April.
The newly launched network aims to support “the UK’s ability to develop, test and deliver… new technological solutions” to support “patients, the public and the health and care system,” while “attracting support from the commercial sector,” said professor Mike Lewis, scientific director, innovation, NIHR.
It will work with the 12 HRCs to provide strategic collaboration with health and care research systems; coordination and leadership of cross-HRC initiatives; proactive support for industry to work HRCs; and a focal point to facilitate links with national partners, including the Medicines and Healthcare products Regulatory Agency, the National Institute for Health and Care Excellence and NHS England.
In addition, the network will help to build capacity for the development and evaluation of health technologies, patient and public involvement, engagement and participation, as well as identifying and addressing health inequalities.
Professor Wendy Tindale, director, NIHR HRC Network and NIHR HRC in long term conditions, commented: “Working in partnership with all 14 HRCs will be key to delivering the network’s ambitions in transforming UK HealthTech into a thriving ecosystem of industry, academia, NHS and stakeholders, collaborating to bring innovative health technologies more rapidly to patients.”
Related posts

BHF awards £35m funding to nine UK universities for cardiovascular disease
May 29, 2024

NHS and NICE announce plan to adopt innovative medical technologies for patients

LifeArc and ALS TDI announce partnership to develop new clinical tools for ALS and MND
May 28, 2024

MRC awards two medical research units at University of Cambridge £30m in funding
News alerts, latest content.

Contact details
PharmaTimes Media Ltd. Mansard House Church Road Little Bookham Leatherhead Surrey KT23 3JG
E: [email protected] E: [email protected] T: +44 (0)20 7240 6999 F: +44 (0)20 7240 4479

Research innovation, collaboration and capacity building to improve public health practice – ASM 2024

The NIHR School for Public Health Research held its tenth Annual Scientific Meeting (ASM) 16–17 th May hosted by PHRESH at the Edgbaston Park Hotel and Conference Centre in Birmingham.
More than 190 delegates attended including researchers, public partners and public health professionals. The meeting had more than 60 speakers.
There were insightful talks from six distinguished keynote speakers Justin Varney , Leah Silva and Liz Green , Vanessa Pinfold , Nason Maani and Cassey Muir .
Delegates also heard from the School’s research programmes about project progress and results so far. To watch these see: Children, young people and families ; Public mental health ; Healthy places, healthy planet ; and Health inequalities .

Capacity building
ASM 2024 featured 21 lightning talks from SPHR trainees and 22 posters from interns, PhD, pre and postdoctoral fellows and the SPHR’s three networks .
The poster and talk winners were:
- Best oral prize: Daniel Mutanda (pre-doctoral fellow) and Rukun Khalaf (PhD student)
- Runner up oral prize: Helen Hoyle (TDP award holder)
- Best poster prize: Anamika Basu ( PhD student)
- Best inclusive poster: Oscar Sharples (Intern)
- Runner up poster: Susannah Tooze (PhD student)

Thank you to everyone who took the time to provide a nomination and congratulations to all those who received a nomination . The winners that were announced at the ASM 2024 were:
Winner: Lorna Hatch
Runner-up: Lauren Cross
Winner: The Twinkle Twinkle Arti team. Find out about the Twinkle Twinkle Arti Children’s book
Director’s Special Award
New for 2024 was the Director’s Special Award for those who have added value to the School and gone above and beyond. The winner of this award was Daniel Mutanda for organising the successful Systems Thinking Webinar Series .
Live drawings
Inspired by the diversity of topics at the ASM, Dr Chloe Asker created live drawings for each of the research programme presentations.
Loving the diversity of the topics covered at #ASMSPHR2024 ! All the talks have been incredible & so engaging. So inspiring in fact, that I’ve been creating some live drawings of the proceedings! (Pls note that I’m new to this!!) pic.twitter.com/Vf6yZtEwwA — Dr Chloe Asker (@chloeasker) May 16, 2024

Watch the recordings
The recordings from the day can now be watched on YouTube, the timestamps in the video descriptions form chapters you can use to navigate between the sessions.

The School would like to thank PHRESH for hosting the ASM 2024
- University of Bristol
- University of Cambridge
- University of Exeter
- Imperial College London
- London School of Hygiene and Tropical Medicine
- PHRESH – Public Health RESearch for Health Consortium
- University of Sheffield
- Directorate
- Management Group
- Executive Group
- Advisory Board
- Research Review Panel (RRP)
- Equality, diversity and inclusion
- Children, young people & families programme
- Public mental health programme
- Places & communities programme
- Changing behaviour at population level theme
- Health inequalities theme
- Efficient & equitable public health systems theme
- Alcohol programme
- Ageing well programme
- Health inequalities: communities in control programme
- What is impact?
- Our approach to impact
- Case Studies
- Training team
- Our Research Network (ResNet)
- Development opportunities
- Training news and events
- PhD students
- Pre-doctoral fellows
- Post-doctoral fellows
- NIHR Academy
- Public involvement
- Public health professionals
- What is public involvement?
- What can you expect?
- How you can get involved
- Public involvement news & events
- Useful tools & resources
- Our work & how you can use it
- How we can work with you
- Public health professionals news & events
- Public Health Practice Evaluation Scheme
- Research briefings
- Resources for public health professionals
NIHR School for Public Health Research William Leech Building, Newcastle University, Newcastle upon Tyne, Tyne and Wear NE2 4HH [email protected]
We use cookies to ensure that we give you the best experience on our website. If you continue to use this site we will assume that you are happy with it.
The browser you are using is no longer supported and for that reason you will not get the best experience when using our website.
You currently have JavaScript disabled in your web browser, please enable JavaScript to view our website as intended.
University of Leicester heart research receives £7 million funding boost
Professor André Ng
The British Heart Foundation (BHF) has awarded £3 million to the University of Leicester to support its world-class cardiovascular disease research over the next five years.
The University of Leicester has pledged to match the award from the BHF, with additional funding from the University Hospitals of Leicester, taking the total investment in cardiovascular disease research in Leicester to around £7 million.
Funding will support the University to expand its ground-breaking studies into the causes of common and rare cardiovascular diseases, as well as refining appropriate treatments and interventions.
Professor André Ng, Professor of Cardiac Electrophysiology and Consultant Cardiologist and Head of Department of Cardiovascular Sciences at the University of Leicester, who led the team in the competitive bid, said: “We are thrilled and truly honoured to receive this award which reflects the hard work and dedication of all our researchers and support staff past and present who tirelessly strive to make advancements in cardiovascular science.
“We are committed to translating innovative research into tangible benefits for patients. This funding will allow us to expand the fantastic work being done here to turbocharge our efforts towards impactful research output.
“A major focus will be to raise the next generation of talented cardiovascular researchers and advance cutting-edge science for the benefits of our patients. We will build a collaborative community that brings researchers working across different disease areas and non-medical disciplines to drive forward lifesaving breakthroughs.”
Funding for the £3 million BHF award comes from the charity’s highly competitive Research Excellence Awards scheme. It will support researchers at the University of Leicester to:
- Continue to use genetics and related technologies to identify causes of common and rare cardiovascular disease and to predict who is at most risk of developing disease for targeting preventative strategies.
- Develop, test and refine new ways of cardiovascular treatments for patients and increase access and find out which treatments are best for different people.
- Determine how cardiovascular diseases and other common conditions are associated with each other and help identify who might benefit from interventions that target more than one condition at a time, and the combinations of treatments that different patients might require, particularly working with different ethnic groups to identify which treatments are best for each group.
Professor Bryan Williams, Chief Scientific and Medical Officer at the British Heart Foundation, said: “We’re delighted to continue to support research at the University of Leicester addressing the biggest challenges in cardiovascular disease. This funding recognises the incredible research already happening in Leicester and will help to cement its status as a global leader in the field.
Pro Vice-Chancellor Professor Tom Robinson, Head of the College of Life Sciences and Dean of Medicine at the University, said: “This match-funded award is further evidence of the strength of our ground-breaking cardiovascular bench-to-bedside research and builds on the recognition of the REF2021 Clinical Medicine Unit of Assessment, where we were placed second, and our NIHR Biomedical Research Centre.
“Around 7.6 million people in the UK are living with heart and circulatory diseases in the UK so it’s our hope that our work, in collaboration with our major NHS partner, the University Hospitals of Leicester NHS Trust, can make a real difference to the lives of others.”
University Vice-Chancellor, Professor Nishan Canagarajah, said: “I’m delighted that the research Professor Ng is undertaking alongside colleagues will be further boosted by this fantastic award. This BHF award is a testament to the global impact we are making here in Leicester in developing treatments for heart disease across all ethnicities.”
In 2019 the University received a £1 million Accelerator Award from the BHF to develop its cardiovascular research programme. The funding has supported research that is already laying the foundations for future breakthroughs including the discovery of genetic determinants for cell lifespan and the development of cardiovascular disease, as well as demonstrating the clinical value of a polygenic risk score to improve the prediction of cardiovascular disease.
Work has also taken place to develop novel data analysis tools for the prediction of lethal arrhythmias exploiting cutting edge engineering and artificial intelligence techniques. In addition, researchers have improved understanding of heart valve and diabetes related heart disease, demonstrating the long-term prognostic importance of imaging detected myocardial fibrosis as well as microvascular dysfunction. All these advances are aimed at developing better personalised therapeutic strategies.
- Awards and prizes
- Cardiovascular
Related stories
Exciting placebo research to be unveiled at literary event, new digital tool aims to reduce diagnosis delays in those with chronic breathlessness, innovative leicester centre spearheads international initiative, student nurse clinches prestigious award, international lifetime achievement award in empathy presented at pioneering educational course, exercise programmes benefit a wide range of long-term health conditions.
Internet Explorer is no longer supported by Microsoft. To browse the NIHR site please use a modern, secure browser like Google Chrome, Mozilla Firefox, or Microsoft Edge.

NIHR HealthTech Research Centres - Stage 1 Application Guidance

Published: 18 August 2022
Version: v1.0 August 2022
Introduction
The mission of the National Institute for Health and Care Research (NIHR) is to improve the health and wealth of the nation through research. NIHR delivers against this mission through six core workstreams set out in Best Research for Best Health - the next chapter .
The NIHR is launching a new, two-stage, open competition to designate and fund NIHR HealthTech Research Centres (HRCs) in England. This scheme replaces the current NIHR Medtech and In Vitro Diagnostic Cooperative (MIC) scheme.
A total of up to £45 million over five years is available for this round of NIHR HRC funding from 1 April 2024. Eligible NHS organisations may submit one application.
HealthTech refers to any technology, device or digital solution designed to improve health and care. It includes medical devices, diagnostics (including in vitro diagnostics) and digital health technologies.
Please see our frequently asked questions document for a full list of published questions. In addition, we will provide answers to any additional queries submitted through our online form which will be open until 19 October 2022.
NOTE: Applications for Stage 1 are now closed.
The aims of the NIHR HealthTech Research Centres are to:
- catalyse innovation in the development of new HealthTech for areas of unmet need, areas of high disease burden, and/or improve efficiencies in the health and care system.
- provide expertise to evaluate innovations and generate appropriate evidence to support uptake into the health and care system. End-user engagement should be central to design and testing to ensure products are fit for the intended purpose or setting (patients, the public or the health and care system).
- work collaboratively with the wider adoption landscape to improve efficiencies in translating research into benefits for patients and the public, the health and care system and for wider economic gain.
- increase the system's capacity to work at the interface between industry, academia and the health and care system through training and support for those managing the commercialisation process. This will include increasing the understanding of market access requirements to improve the uptake of innovations.
- build capacity and expertise in methodological approaches (e.g. health economics, human factors, care pathway assessments etc) to generate robust evidence.
- contribute to regional and national economic growth through supporting UK-based SMEs and encouraging inward investment and trade by supporting companies to bring innovations to market in the UK and other regulated jurisdictions.
HRCs will be centres of excellence that support the development of HealthTech and the generation of appropriate evidence to support necessary approvals and enable effective implementation of innovations to improve care and the health and care system.
Each NIHR HRC will be led by a Director with proven experience of leadership in developing and implementing novel technologies in the health and care system e.g. NHS, public health, and/or a social care setting. NIHR HRC funding will enable a range of collaborating partners and stakeholders to be brought together to generate a programme of work, leading to the generation of high-quality follow-on research projects. This will include engaging and working alongside multidisciplinary teams of health and care professionals, researchers, patients and the public, industry, regulators, NHS commissioners and Integrated Care Systems to identify needs and issues from a front-line service and patient/public (including carers and service users) perspective.
NIHR HRCs will use their extensive knowledge of the innovation pipeline to primarily support industry partners navigate the health and care landscape, including the regulatory requirements for market access to increase the chance of adoption. When supporting academically led innovations, there will be an expectation of future commercialisation. Innovations supported by the NIHR HRCs should seek to address areas where there is demonstrable unmet need from the health and care system. NIHR HRCs should support growth of local/regional innovation clusters while ensuring their expertise remains accessible to UK and global companies, working as part of the NIHR to remove geographical barriers to research and generating evidence that has the potential to have a national impact.
Each NIHR HRC will be expected to have access to a range of relevant expertise to support generation of evidence on safety, validity, utility, cost effectiveness, care pathway benefits and ‘real-life’ applicability (real-world evidence) of HealthTech innovations.
While the focus of the remit of activity within an NIHR HRC funded by the award will be the development of collaborations, the NIHR HRC scheme will also fund relevant pilot/proof of concept studies. These pilot/proof of concept studies should aim to support further larger scale research projects, including aspects such as full development, health economics and care pathway assessment. NIHR HRCs are expected to leverage additional research funding, including from other public, charity and industry research funding sources to undertake full scale studies.
To support the development and adoption of products, NIHR HRCs will be expected to work closely with the relevant parts of the health and care system including the Medicines and Healthcare products Regulatory Agency (MHRA), National Institute for Health and Care Excellence (NICE), NHS England, Academic Health Science Networks (AHSNs) and the NHS Innovation Service.
Please note, NIHR HRCs will be expected to be responsive to support national health and care priorities. Priorities within the health and care system change, and NIHR HRCs will need to be responsive to emerging needs and situations can arise (nationally and globally) which require access to research, research expertise and resource at short notice. NIHR HRCs will be expected to prioritise resource towards addressing national health and care priorities at the request of the Department for Health and Social Care (DHSC).
Skills and Workforce Development
NIHR HRCs will have a remit to support the development of a highly skilled research workforce to support the development of HealthTech and meet growing demands in this area. This should include skills development in areas such as methodology, health economics, human factors, care pathway assessments and commercialisation. NHS organisations will be expected to demonstrate how they will support skills and workforce development, through the provision of both theoretical and practical training. Consideration should be given to providing support across the career development pathway for staff at all levels from early training into continuous professional development to build capacity and expertise within the NIHR HRC.
Eligibility Criteria
All NHS organisations in England that can demonstrate a substantial portfolio of HealthTech research are eligible and may submit one application. NIHR HRC designation will be awarded to a single NHS organisation.
The NHS organisation may identify additional NHS organisations and/or universities who would form part of the designated NIHR HRC and would work with the contracted NHS organisation to deliver its programme of work. These organisations should have specified roles in the application. NIHR HRCs are encouraged to work with NHS organisations and/or universities that bring additional strength and depth to the HRCs workplan and build research capacity and expertise across the country.
In addition, the NIHR HRCs may collaborate with other non-NHS organisations non-NHS organisations (care providers or organisations from the independent sector providing health and care services) or Universities and can pass funding to them via an appropriate mechanism, such as a subcontract.
Funding Available
A total of up to £45 million is available for the NIHR HRC scheme over a five-year period (starting 1 April 2024). The number of Centres and the amount of funding allocated to each NIHR HRC has not been predetermined however funding is expected to be n o more than £3 million per centre for the five-year period. Funding will be informed by the scale, nature and quality of the research activity to be conducted by that Centre.
Competition Process and Timetable
Stage 1: selection criteria.
At Stage 1 an Independent Selection Committee will review the applications and make recommendations to DHSC on which applicants should be invited submit a Stage 2 application. The information provided in the Stage 1 application will be assessed against the following selection criteria:
- the strength of the strategic plan ; including a commitment to collaborative working to support industry;
- the depth and breadth of high-quality HealthTech research;
- existing capacity and capability to support the development and commercialisation of HealthTech innovations.
Stage 2: Applicants invited to Stage 2 will be provided with further guidance. The selection criteria for Stage 2 are provided for information in Annex 3.
Annex 1: outline of the stage 1 application form, applications must be submitted electronically via the research management system (rms). the following information will be requested: 1. details of the proposed nihr hrc.
Please use the following format NIHR- Name of the NHS Organisation- HRC
2. Director(s) of the proposed NIHR HRC
Please provide the name(s) of the proposed Director(s).
3. Please provide details of the host NHS organisation
Please select the name of the host NHS organisation using the drop-down menu.
4. Please provide details of any other partners
Please list any additional NHS organisations that will be formal partners in the NIHR HRC. Formal agreement from these partners will be sought at Stage 2.
Please note: NIHR HRC designation will be awarded to a single NHS organisation. The NHS organisation may identify additional NHS organisations and/or universities who would form part of the designated NIHR HRC and would work with the contracted NHS organisation to deliver its programme of work. These organisations should have specified roles and should be listed in this section of the application.
In addition, the NIHR HRCs may collaborate with non-NHS organisations (care providers or organisations from the independent sector providing health and care services) or Universities and can pass funding to them via an appropriate mechanism, such as a subcontract. Please refer to these organisations in the application but do not list them as formal partners in this section.
5. Overall funding requested
Please provide an indicative total cost for the proposed NIHR HRC, this value should be within 5% of the final costs that will be submitted in a Stage 2 application. A full breakdown of costs will be required at Stage 2.
6. Proposed Director(s) leadership and expertise (500 words)
Please summarise the proposed Director(s) leadership and expertise in developing and implementing novel technologies in a health and care system. This should include:
- The organisational leadership experience of the Director(s), including examples of leadership at a local, regional and/or national/international level;
- Evidence of their experience in progressing HealthTech products through the innovation pathways and supporting commercial partners and/or the commercialisation of innovations;
- A statement highlighting the Director’s commitment to, and experience of, improving research culture and equality, diversity and inclusivity in the research workforce; and
- Up to 3 (including URL) that outline the Directors expertise in developing or implementing HealthTech research.
Note: Doesn’t necessarily have to be a peer reviewed publication e.g. if the Director has been a major contributor to NICE guidelines that would be accepted.
7. Expertise in translating HealthTech research
7.1 Please provide a brief description of the cross-cutting expertise which will underpin the activities of the NIHR HRC. (500 words)
Please describe the approach and strategy of the proposed NIHR HRC for working with industry, including examples of key strategic industry partnerships already in place that are directly relevant to the proposed Themes (500 words)
7.2 Please provide up to three case studies which highlight previous successes in working with industry to move HealthTech products along the innovation pipeline towards adoption. The case study should demonstrate how the NHS organisation and industry work together as partners, fully utilising both sets of unique expertise, and the benefit industry receives in working with the NHS. The information presented should be understandable to both lay and expert members of the committee. Text should be written in plain English without use of jargon and any technical terms should be explained. (400 words per case study)
8. Outline Strategic Plan
8.1 In plain English, briefly summarise the vision, scientific rationale/context and goals of the proposed NIHR HRC. (500 words)
8.2 Please provide a description of the proposed NIHR HRC. Please include the areas to be addressed as a set of ‘Themes’ and how the themes will dovetail into a coherent Centre. The plan should set out how the Centre will meet the published aims of the scheme. The NHS organisation’s approach to improvements in research culture and plans to support equality, diversity and inclusion across the proposed activities should be clearly explained. (1500 words)
9. Table of NIHR HRC Themes
Please list the Themes that will be included in the proposal.
For each Theme please provide:
- the name of the Theme Lead(s) and co-Theme lead (where applicable); and
- a short title.
Please note: The organisation and ORCID number for the Directors, lead and co-leads for each theme will be taken from their portal account ('Manage my Details' section) which should be updated prior to submission.
PPIE and training should not be included as individual themes but should be embedded within the research themes.
10. Theme Summary (400 words per theme)
Please provide a short abstract for each Theme highlighting the areas of need to be addressed and the potential outcomes of benefit to patients, the public, and the health and care system. Outline the expertise and track record of the proposed team in this area.
Annex 2: Stage 2 - Financial Information
1. the purpose of nihr healthtech research centre (hrc) funding.
The purpose of the funding is to meet the NHS research infrastructure costs incurred by the NIHR HRC in carrying out an approved programme of HealthTech research.
Our expectation is that NIHR HRC funding will fund a team of staff who will enable a range of collaborating partners and stakeholders to be brought together to generate a programme of work, leading to the generation of high-quality follow-on research projects supported by other research funders (e.g. research councils, charities, industry or other NIHR funding streams e.g. i4i, HTA and EME) and/or in collaboration with industry.
Funding awards will be made to the designated NHS organisation, but it is permissible for funds to flow to other organisations that are formally part of the NIHR HRC, via a suitable mechanism such as a subcontract.
2. The financial plan
The financial plan should provide a breakdown of the NHS Research Infrastructure direct costs, reasonable NHS indirect costs and eligible NHS Support Costs and for which funding is being requested to carry out the proposed work plan.
It is important to undertake a thorough, realistic and accurate costing. You must provide a clear and full justification for all major resources. You must also ensure that you include all costs, including those required to secure good research management and governance. In all cases, the value for money of the proposal will be an important selection criterion.
3. Supporting Information
Prior to completing the finance section of the application it is important applicants have a good understanding of the Attributing costs of health and social care Research and Development (AcoRD) guidance .
The AcoRD guidance clarifies the distinction between research costs, NHS support costs and NHS treatment costs associated with non-commercial research studies/programmes:
We strongly recommend that applicants familiarise themselves with these definitions, and consult AcoRD Annex A and AcoRD Annex B as well as the NIHR webpage on Excess Treatment costs
Please note the following:
- Applicants need to separate eligible direct, indirect research costs as well as NHS support costs. The finance form is formatted to allow applicants to separate these costs. Guidance on how to complete the finance form is provided within the finance section of the application.
- Further itemisation of costs and explanation of calculation methods may be requested to support the application if required.
- Applications should be costed at current (2022/23) prices.
- We expect standard NHS accounting policy and guidance to be followed (as set out in the NHS Finance Manual) in determining the appropriate costs to be charged to this Research Infrastructure Award.
- Where necessary applications are expected to have appropriate NHS, university, commercial and other partner input into the finance section.
- The NIHR will not support any costs incurred, prior to, or following the completion date of the infrastructure award.
- Years should be calculated starting from the start date of the proposed award i.e. 1 April 2024. Once an award has been made DHSC will require host organisations to provide regular financial statements regarding the use of funds provided under the NIHR funding scheme. DHSC reserves the right to send independent auditors to the NHS organisation to confirm the actual use of funds.
- Payments will only be made to the contracted organisation who will take responsibility for distributing any funds to any Partner(s).
- Appropriate research project agreement and/or sub-contracts must be put in place for any element of the work programme that is to be paid to another organisation.
4. Information for Different Types of Organisations
4.1 nhs organisations.
Up to 100% of direct and indirect research costs as well as NHS support costs incurred by NHS organisations will be funded.
4.2 Universities
NIHR HRC funding will fund up to 100% of direct research costs for universities. NIHR HRC funding does not pay indirect costs for universities.
4.3 Commercial organisations
Up to 100% of direct and indirect research costs will be funded for commercial organisations or consultancies. Commercial organisation indirect costs need to demonstrate value for money. The NIHR reserves the right to set limits on indirect costs charged.
4.4 Other partner organisations
Up to 100% of direct and indirect research costs will be funded for partner organisations (local authorities, charities, non-governmental organisations, etc.). Other partner organisation indirect costs need to demonstrate value for money. The NIHR reserves the right to set limits on indirect costs charged.
5. Eligible Costs
5.1 direct costs, 5.1.1. overview of direct costs.
Direct costs are eligible research infrastructure costs that will be incurred by the NHS organisation in carrying out the proposed work programme for the NIHR HRC. These costs should be recorded and supported by an appropriate audit trail.
Eligible direct costs are listed below.
5.1.2 Annual costs of staff posts and salaries
The NIHR HRC award will reimburse the time of staff engaged in the NIHR HRC’s work programme. Salaries may be sought for core NIHR HRC research, research support or other staff, required to work full or part-time on the NIHR HRC’s research programme.
Newly established posts should be created with an NHS organisation as the employer. It is permissible for staff to be employed by university partners named on the application where justified, but NIHR will not fund the associated indirect costs for these staff.
All staff members working on the NIHR HRC award must be costed at FY22/23 prices, based on current salary scales and increments. Where staff will be recruited for the proposed NIHR HRC, please provide the estimated annual salary using current rates of pay and build in any known annual increments. Nationally or locally agreed pay increases should be excluded.
Please note that annual increments should be based on the Agenda for Change pay arrangements as applicable on 1 April 2021. Trusts will not be able to claim the costs of pay awards retrospectively.
5.1.3 Travel, subsistence and conference fees
Travel costs
Enter the total cost of travel for all anticipated journeys. If travel is by car, apply your institution’s mileage rates (this should not exceed HMRC approved mileage allowance payments, which is 45p per mile for the first 10,000 miles and 25p thereafter). Travel by the most economic means possible is encouraged; NIHR funding schemes do not usually fund first class travel. Only a reasonable level of international travel will be considered.
Subsistence
Subsistence covers accommodation, where necessary, and any meals associated with the travel but excluding alcoholic beverages.
Conference fees
UK conference attendance is supported where justified. Costs associated with international conference attendance should be individually stated and fully justified.
5.1.4 Equipment
There is no DHSC capital funding available through the NIHR HRC funding scheme. Purchase or lease costs for essential items of equipment as well as maintenance and related costs not included as part of estates can be included. Only purchase costs for pieces of equipment up to £5,000, excluding VAT, will be considered. Pieces of equipment costing more than £5,000 to purchase will need to be leased.
Items of equipment valued at £250 or more must be itemised separately; however, grouping the same type of equipment is permitted. Costs of computers are normally restricted to a maximum of £1000 each excluding VAT. A statement of justification must be included in the relevant ‘Justification of Costs’ section for any purchase above this limit.
Equipment must exclude VAT, but if the organisation incurring the cost is not VAT registered and cannot claim back VAT on cost items, then it would have to enter the gross value of a cost item (including VAT) on the financial plan. You will need to seek advice from the organisation that the piece of equipment is purchased from regarding its VAT status.
The cost of equipment maintenance contracts should be included in this section.

5.1.5 Consumables
This section includes non-reusable items specific to the NIHR HRC’s work plan. Please itemise and describe the requirements fully. These items should be research specific, not just general office costs which should be covered by indirect costs.
5.1.6 Patient and public involvement and engagement (PPIE)
Offering members of the public payment for their time, skill and expertise is considered good practice in structuring and operating the proposed NIHR HRCs. Please itemise and describe fully all costs to support the delivery of the NIHR HRCs patient and public involvement and engagement strategy. This will include:
- Costs to support novel involvement and engagement mechanisms such as community engagement, digital engagement or other models that broaden reach.
- Payments to recognise time, skills and expertise contributed by public members.
- All out of pocket expenses incurred by public members in supporting the NIHR HRCs PPIE activities. Equal opportunities for involvement are facilitated if expenses are covered. Members of the public should not end up financially worse off for providing a public service.
NIHR has produced guidance to help staff supporting reach identify and calculate costs of public involvement in their research facing activities.
Note: Costs of staff posts to support the delivery of the PPIE strategy should be included in the Staff Posts and Salaries and Annual Costs of Staff Posts tabs. Costs for PPIE activities should be included in the research themes. Costs for cross cutting PPIE leadership e.g. the PPIE Lead should be included in the CORE costs.
5.1.7 Open Access Costs
This includes any associated with Open Access Publishing. Please review the NIHR Open Access Policy and the NIHR Open Access publications funding guidance .
For NIHR Infrastructure awards , including the NIHR HRCs, Open Access costs should be budgeted and earmarked by applicants at application stage. Costs for Open Access must be entered and reported as a separate item on the finance form. Contractors are expected to manage Open Access funding equitably, transparently, and in accordance with the Open Access policy throughout the duration of the award.
5.1.8 Dissemination Costs
Any costs associated with presentation or dissemination (excluding Open Access costs, travel and subsistence costs) can be included here. All events must be run at the lowest possible cost, with minimal catering.
5.1.9 Other direct costs
These are costs that have been not identified elsewhere but are specifically attributable to the research infrastructure. For example, external consultancy costs, software licensing, PhD tuition fees and advertising costs.
Please note that external consultants must not be people who are already employed by any NHS organisations, equivalent non-NHS settings, or organisations from the independent sector providing NHS services and other universities, who will be conducting research activities via an appropriately justified subcontract. If they are, any costs should be entered as direct costs in the ‘Staff Posts and Salaries’ and ‘Annual Costs of Staff Posts’ sections.
5.2 Indirect Costs
NIHR HRC awards will fund legitimate and reasonable, indirect costs for the NHS, commercial and other partner organisations. This will include the proportion of the costs of accommodation in the NHS used for the NIHR HRC's work, and an appropriate proportion of HR, payroll, and finance costs. Please seek advice from your finance department about the appropriate cost for this section. Total indirect costs must be fully justified, outlining the rate charged.
NIHR will not meet indirect costs incurred by universities involved in delivering the work of the NIHR HRC.
All indirect costs need to demonstrate value for money. The NIHR reserves the right to set limits on indirect costs charged.
5.3 NHS Support Costs
NIHR HRC awards will fund the NHS Support Costs that are integral to the proposed NIHR HRC’s work programme, and these costs should be included in the application.
NHS Support Costs are the additional patient care costs associated with the proposed work programme of the NIHR HRC, which would end once the R&D activity in question has stopped, even if the patient care service involved continues to be provided. These might cover items such as staff time to recruit and consent patients, or additional activities which will not form part of the on-going intervention.
For single centre non-commercial research, any NHS support costs should be met through the NIHR HRC award.
It should be noted that there are other NIHR funding schemes used to support the cost of NHS infrastructure for research within the NHS ( e.g . NIHR Clinical Research Network), and that NHS infrastructure and support costs associated with work outside the scope of the NIHR HRC award should not be included within the application.
The NHS Support Costs should be separated into staff costs and other (non-staff) costs (including pharmacy, pathology and imaging).
For the following, the appropriate NHS Support costs, or equivalent for non-NHS settings, should be sought through the NIHR Clinical Research Network (NIHR CRN) for studies which meet the NIHR CRN Portfolio eligibility criteria:
- NIHR HRC-led research studies within third party collaborating NHS, or equivalent non-NHS setting, site ( e. not a formal site of the host NHS organisation, or not formally subcontracted by the host NHS organisation for the purposes of the NIHR HRC);
- Research funded by NIHR’s non-commercial research partners (for example UKRI, medical research charities) conducted within the NIHR HRC’s work programme; and
- Research funded by NIHR research programmes (for example HS&DR, i4i and Health Protection Research Units).
6. Ineligible Costs
The funding is not intended to meet NHS Treatment costs associated with the research programme of the NIHR HRC award.
Funding will not be provided for university laboratories or infrastructure, or to meet the costs of animal research, or costs of audits of practice and service evaluations. Please refer to the UK Policy Framework for Health and Social Care Research for further details.
NIHR HRC awards will not fund indirect costs for universities.
Equipment costing more than £5,000 will not be funded via the NIHR HRC award.
No capital funding is available through this award. Research activities should be undertaken within existing facilities or planned facilities for which there is confirmed funding.
London School of Hygiene & Tropical Medicine
Addressing impact of climate change on malaria and neglected tropical diseases: a call for research and action.

LSHTM researchers, Professor Chris Drakeley, Dr. Rachel Lowe and Isabel Byrne from the Malaria Centre and the Centre on Climate Change and Planetary Health (CCCPH), are part of the World Health Organization’s Task Team on Climate Change, Neglected Tropical Diseases (NTDs), and Malaria, in partnership with Reaching the Last Mile (RLM).
A scoping review, published in the Transactions of the Royal Society of Tropical Medicine & Hygiene, explores the effects of climate change on malaria and 20 NTDs, and potential mitigation and adaptation strategies. Analysing 42,693 articles, the review reveals insufficient understanding of the actual and potential impacts of human-induced climate changes on malaria and NTDs.
The review identified significant research gaps regarding the influence of climate change on diseases other than malaria, dengue, and chikungunya. Too often, research has focused on low-disease burden countries with high-quality healthcare, perpetuating a lack of comprehensive understanding.
This represents a growing emergency for historically underserved communities that continue to be overlooked at the intersection of climate and these diseases. The poorest populations, already disproportionately affected by malaria and NTDs, are likely to experience even greater hardships.
Isabel Byrne, a Research Fellow and a member of both the Malaria Centre and CCCPH, stated: “The impact of climate change on global health is increasingly evident, with profound implications for malaria and NTDs. This major WHO review has revealed enormous gaps in our understanding of how our rapidly changing climate will affect these diseases - or how to best mitigate and prevent them.”
The review calls for a greater research focus on mitigation and adaptation strategies to safeguard global health gains against NTDs and malaria and urges that the research agenda be reimagined soon, driven by scientists from populations most vulnerable to the impacts of climate change.
Publication
Klepac P, Hsieh JL, Ducker CL, Assoum M, Booth M, Byrne I, Dodson S, Martin DL, Turner CMR, van Daalen KR, Abela B, Akamboe J, Alves F, Brooker SJ, Ciceri-Reynolds K, Cole J, Desjardins A, Drakeley C, Ediriweera DS, Ferguson NM, Gabrielli AF, Gahir J, Jain S, John MR, Juma E, Kanayson P, Deribe K, King JD, Kipingu AM, Kiware S, Kolaczinski J, Kulei WJ, Laizer TL, Lal V, Lowe R, Maige JS, Mayer S, McIver L, Mosser JF, Nicholls RS, Nunes-Alves C, Panjwani J, Parameswaran N, Polson K, Radoykova HS, Ramani A, Reimer LJ, Reynolds ZM, Ribeiro I, Robb A, Sanikullah KH, Smith DRM, Shirima GG, Shott JP, Tidman R, Tribe L, Turner J, Vaz Nery S, Velayudhan R, Warusavithana S, Wheeler HS, Yajima A, Abdilleh AR, Hounkpatin B, Wangmo D, Whitty CJM, Campbell-Lendrum D, Hollingsworth TD, Solomon AW, Fall IS. Climate change, malaria and neglected tropical diseases: a scoping review . The Royal Society of Tropical Medicine and Hygiene, 2024
Our postgraduate taught courses provide health practitioners, clinicians, policy-makers, scientists and recent graduates with a world-class qualification in public and global health.
If you are coming to LSHTM to study a distance learning programme (PG Cert, PG Dip, MSc or individual modules) starting in 2024, you may be eligible for a 5% discount on your tuition fees.
These fee reduction schemes are available for a limited time only.
- Study with us
- Research and impact
- News and events
Sign up for our newsletter
Subscribe to our monthly newsletter and get all the latest research news, views, videos and event listings from the School.
Subscribe to RSS feed
- Open access
- Published: 16 May 2024
Experiences of UK clinical scientists (Physical Sciences modality) with their regulator, the Health and Care Professions Council: results of a 2022 survey
- Mark McJury 1
BMC Health Services Research volume 24 , Article number: 635 ( 2024 ) Cite this article
Metrics details
In healthcare, regulation of professions is an important tool to protect the public. With increasing regulation however, professions find themselves under increasing scrutiny. Recently there has also been considerable concern with regulator performance, with high profile reports pointing to cases of inefficiency and bias. Whilst reports have often focused on large staff groups, such as doctors, in the literature there is a dearth of data on the experiences of smaller professional groups such Clinical Scientists with their regulator, the Health and Care Professions Council.
This article reports the findings of a survey from Clinical Scientists (Physical Sciences modality) about their experiences with their regulator, and their perception of the quality and safety of that regulation.
Between July–October 2022, a survey was conducted via the Medical Physics and Engineering mail-base, open to all medical physicists & engineers. Questions covered typical topics of registration, communication, audit and fitness to practice. The questionnaire consisted of open and closed questions. Likert scoring, and thematic analysis were used to assess the quantitative and qualitative data.
Of 146 responses recorded, analysis was based on 143 respondents. Overall survey sentiment was significantly more negative than positive, in terms of regulator performance (negative responses 159; positive 106; significant at p < 0.001). Continuous Professional Development audit was rated median 4; other topics were rated as neutral (fitness to practice, policies & procedures); and some as poor (value).
Conclusions
The Clinical Scientist (Physical Sciences) professional registrants rated the performance of their regulator more negatively than other reported assessments (by the Professional Standards Authority). Survey respondents suggested a variety of performance aspects, such as communication and fitness to practice, would benefit from improvement. Indications from this small dataset, suggest a larger survey of HCPC registrants would be useful.
Peer Review reports
In Healthcare, protection of patients and the public is a core principle. Part the framework of protections, includes regulation of professions [ 1 ]. This aims to mitigate risks such as the risk from bogus practitioners – insufficiently trained people acting as fully-trained professional practitioners, see Fig. 1 .
Recent UK media report on a bogus healthcare practitioner [ 2 ]
Regulation of professions ensures that titles (e.g. Doctor, Dentist, Clinical Scientist) are protected in law. The protected title means someone may only use that title, if they are on the national register, managed by the regulator – the Health and Care Professions Council (HCPC). It is a criminal offence to use a protected title if you are not entitled to do so [ 3 ]. There are a large number of regulators in healthcare – see Table 1 . Most of the regulators manage a register for one profession, except the HCPC which regulates 15 professions.
To be included on the register, a candidate must meet the regulators criteria for knowledge and training, and a key element to remain, is to show evidence of continuous professional development (CPD). Being on the register ensures that a practitioner has met the appropriate level of competence and professional practice.
For many healthcare workers, being on the HCPC register is a compulsory requirement to be appointable to a post. They must pay the necessary annual fees, and abide by the policies drawn-up by the regulator, and generally professions have no choice of regulator – these are statutory bodies, setup by government.
Recently, there has been considerable public dissatisfaction with the activity & performance of some regulators, notably Ofwat [ 4 ], and Ofgem [ 5 ]. Healthcare workers should expect a high level of professionalism, efficiency, and integrity from a regulator, as the regulator’s performance directly affects staff and public safety.
In terms of the regulation of UK Clinical Scientists, there is a dearth of data regarding experiences with the HCPC and views on the quality of regulation provided.
Findings are reported here from a 2022 survey of Medical Physicists and Engineers (one of the 16 job roles or ‘modalities’ under the umbrella of Clinical Scientist). The research aim was to assess experiences, and the level of ‘satisfaction’ with the regulator. For the remainder of this report, the term Clinical Scientist will be taken to mean Clinical Scientist (Medical Physicist/Engineer). The survey was designed to gather & explore data about opinions and experiences regarding several key aspects of how the HCPC performs its role, and perception of the quality & safety of regulation delivered.
A short survey questionnaire was developed, with questions aimed to cover the main regulatory processes, including registration & renewal, CPD audit, and fitness-to-practice. There were also questions relating more generally to HCPC’s performance as an organisation, e.g. handling of personal data. Finally, participants were asked to rate the HCPC’s overall performance and what they felt was the ‘value’ of regulation. The survey questions are listed in the Supplementary file along with this article.
Questions were carefully worded and there was a balance of open and closed questions. A five-point Likert score was used to rate closed questions. The survey was anonymous, and the questions were not compulsory, allowing the responders to skip irrelevant or difficult questions. The survey also aimed to be as short & concise as possible, to be a minimal burden to busy clinical staff & hopefully maximise response rate. There were a small number of questions at the start of the survey, to collect basic demographics on the respondents (role, grade, UK nation etc.).
The survey was advertised on the online JISC-hosted UK Medical Physics and Engineering (UKMPE) mail-base. This offered convenient access for the majority of Clinical Scientists. The survey was advertised twice, to allow for potential work absence, holiday/illness etc. It was active from the end of July 2002 until October 2022, when responses appeared to saturate.
The data is a combination of quantitative rating scores, and qualitative text responses. This allows a mixed-methods approach to data analysis, combining quantitative assessment of the Likert scoring, and (recursive) thematic analysis of the free-text answers [ 6 ]. Thematic analysis is a standard tool, and has been reported as a useful & appropriate for assessing experiences, thoughts, or behaviours in a dataset [ 7 ]. The survey questions addressed the main themes, but further themes were identified using an inductive, data-driven approach. Qualitative data analysis (QDA) was performed using NVivo (QSR International).
Two survey questions attempted to obtain an overall perception of HCPC’s performance: the direct one (Q12), and a further question’Would you recommend HCPC as a regulator…?’. This latter question doesn’t perhaps add anything more, and in fact a few respondents suggested it was a slightly awkward question, given professions do not have a choice of regulator – so that has been excluded from the analysis.
Study conduct was performed in accordance with relevant guidelines and regulations [ 8 , 9 ]. Before conducting the survey of Clinical Scientists, the survey was sent to their professional body, the Institute of Physics and Engineering in Medicine (IPEM). The IPEM Professional Standards Committee reviewed the survey questions [ 10 ]. Written informed consent was obtained from participants.
Data analysis
Data was collected via an MS form, in a single excel sheet and stored on a secure network drive. The respondents were anonymised, and the data checked for errors. The data was then imported into NVivo v12.
Qualitative data was manually coded for themes, and auto-coded for sentiment. An inductive approach was used to develop themes.
The sample size of responses allowed the use of simple parametric tests to establish the level of statistical significance.
Survey demographics
A total of 146 responses were collected. Two respondents noted that they worked as an HCPC Partner (a paid role). They were excluded from the analysis due to potential conflict of interest. One respondent’s responses were all blank aside from the demographic data, so they were also excluded from further analysis.
Analysis is based on 143 responses, which represents ~ 6% of the UK profession [ 11 ]. It is arguable whether it is representative of the profession at this proportion of response – but these responses do offer the only sizeable pool of data currently available. The survey was aimed at those who are on the statutory register as they are most likely to have relevant interactions & experiences of the HCPC, but a small number of responses were also received from Clinical Technologists (Medical Technical Officers-MTOs) and Engineers (CEs) and these have been included in the analysis. Figure 2 shows the breakdown in respondents, by nation.
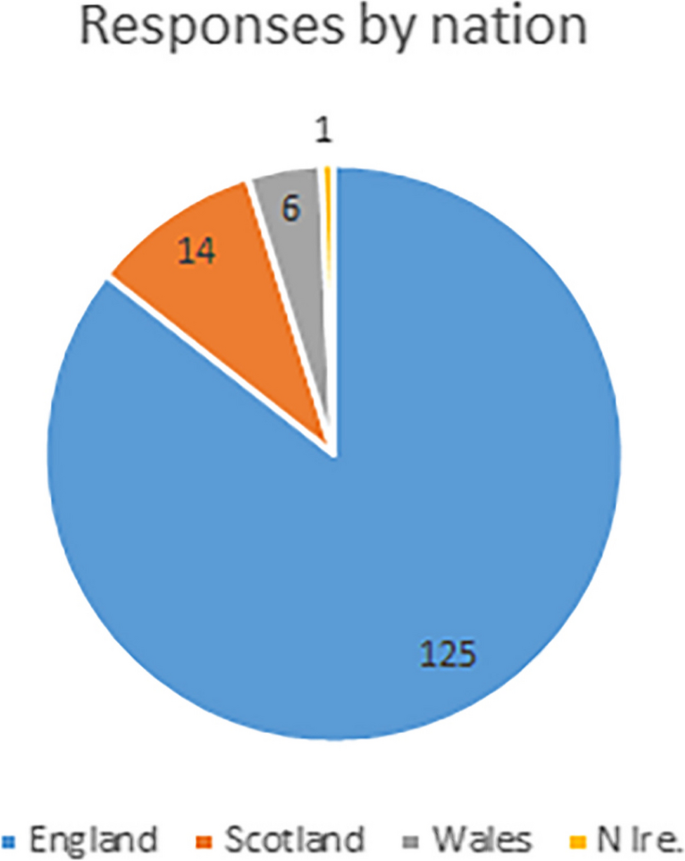
Proportion of respondents, by nation
Of the respondents, 91% are registered Clinical Scientists, and would therefore have a broad range of experience with HCPC and its processes. Mean time on the register was 12 yrs. Respondents show a large range in seniority, and their roles are shown in Fig. 3 (CS-Clinical Scientist; CE-Clinical Engineer; MTO-Medical Technical Officer/Technician; CS-P are those working in private healthcare settings, so not on Agenda for Change (AfC) pay bands).
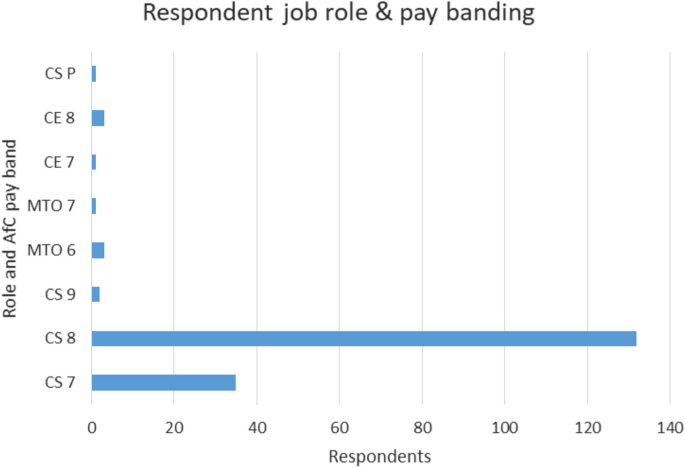
Breakdown in respondents, by role and pay banding
These data can be compared with the most recent HCPC ‘snapshot’ of the CS registrants (find here: Registrants by profession snapshot—1967 to 2019 | ( https://www.hcpc-uk.org/resources/data/2019/registrant-snapshot/ )).
The perception of overall regulator performance, can be assessed in two ways – one interview question directly asked for a rating score, and the overall survey sentiment also offers additional insight.
The score for overall performance was a median of 3 (mean 2.7; response rate 90%) which suggests neutral satisfaction.
Respondents were not asked directly to explain this overall performance rating – themes were extracted from the questionnaire as a whole.
The auto-coded sentiment scores generated in the NVivo software are shown in Table 2 . There is a significantly stronger negative sentiment than positive for HCPC performance – moderate, strong and total sentiment scores are all higher for negative sentiment. The normal test for a single proportion (109), shows the negative and positive sentiment differences have statistical significance with p < 0.001. Whilst the PSA assessment of HCPC performance in 2022–23 shows 100% performance for 4 out of 5 assessment areas, survey data here from regulated professionals suggests considerably less satisfaction with HCPC. This raises associated questions about the relevance and validity of PSA assessment.
A large number of respondents seem to question the value of regulation. Whilst many accepted the value for it in terms of protecting the safety of the public, many questioned its relevance & benefit to themselves. Many respondents also queried the payment model where although the main beneficiaries of regulation are the public & the employer, it is the registrants actually pay the fees for registration. There was very little mention in survey responses, of benefit in terms of protected-title. These issues were amalgamated into Theme 1— Value of regulation , with the two sub-themes Value in monetary terms (value-for-money) and Value in professional terms (benefit and relevance to the individual professional) (see Table 3 ).
In the survey, several aspects of HCPC organisational performance were scored – handling of personal data, registration and renewal, engagement with the profession, audit, and the quality and usefulness of HCPC policies. These formed Theme 2 and its sub-themes.
A third theme Registrant competence and vulnerability , was developed to focus on responses to questions related to the assessment of registrant competence and Fitness To Practice (FTP) processes.
Finally, the survey also directly asked respondents if they could suggest improvements which would have resulted in higher scoring for regulation quality and performance. These were grouped into Theme 4.
Theme 1 – Value of regulation
Value in monetary terms.
The Likert score for value-for-money was a median of 2 (mean 2.3; response rate 100%) which suggests dissatisfaction. This is one of the few survey questions to elicit a 100% response rate – a clear signal of its importance for registrants.
There was a high number of responses suggesting fees are too expensive (and a significantly smaller number suggesting good value). This ties in with some respondents explaining that the ‘benefit’ from registration is mainly for the employer (an assurance of high quality, well-trained staff). Several respondents point to little ‘tangible’ benefit for registrants and query whether the payment model is fair and if the employer should pay registrant fees.
“Expensive fees for what appears to be very little support.” Resp094
“It seems that I pay about £100 per year to have my name written on a list. It is unclear to me what the HCPC actually does in order to justify such a high fee.” Resp014
“I get, quite literally, nothing from it. It’s essentially a tax on work.” Resp008
Several respondents suggested that as registration was mandated by the employer, it was in essence an additional ‘tax’ on their employment, which was highlighted previously by Unison [ 12 ]. A comparator for payment model, are the checks preformed on potential staff who will be working with children and vulnerable adults. In general, these ‘disclosure’ checks are paid for by the employer, however the checks are not recurrent cost for each individual, but done once at recruitment.
Value in professional terms & relevance
This was not a direct question on the questionnaire, but emerged consistently in survey responses. Aside from value-for-money, the value of regulation can also refer to more general benefit and relevance for a professional, for example in protecting a professional title or emphasising the importance of a role. Many respondents commented, in relation to the ‘value’ of regulation, about the relevance of the HCPC to them and their job/role.
The largest number of responses highlighted the lack of clarity about HCPC’s role, and also to note its lack of relevance felt by a significant proportion of respondents.
“Not sure I have seen any value in my registration except that it is a requirement for my role” Resp017
“I really fail to understand what (sic) the benefits of registration.” Resp018
“They do not promote the profession. I see no evidence of supporting the profession. I pay to have the title and I am not aware of any other benefits.” Resp038
Theme 2 – HCPC performance
Communication & handling data.
The survey questionnaire did not have a specific question relating to communication, therefore no specific Likert scores are available. Rather, communication was a sub-theme which emerged in survey responses. The response numbers related to positive (1) and negative experiences (50) clearly suggest an overall experience of poor communication processes (and statistically significant at p < 0.001 for a normal proportion test).
One respondent noted they had ‘given up’ trying to communicate with HCPC electronically. Several respondents also noted issues with conventional communication—letters from HCPC going to old addresses, or being very slow to arrive.
“…I have given up on contacting by electronic means.” Resp134
When trying to renew their registration, communication with HCPC was so difficult that two respondents noted they raised a formal complaint.
A number of respondents noted that when they eventually got through to the HCPC, staff were helpful, so the main communication issue may relate to insufficiently resourced lines of communication (phones & email) or the need for a more focussed first point of contact e.g. some form of helpdesk or triaging system.
“Recently long wait to get through to speak to someone… Once through staff very helpful.” Resp126
This topic overlaps with the next (Processing Registration & renewals) in that both involve online logins, website use etc.
Security & data handling was rated as neutral (median 3, mean 3.4; response rate 91%). Although responses were balanced in terms of satisfaction, a significant number noted a lack of knowledge about HCPC processes. There are almost equal proportions of respondents reporting no issues, some problems with handling of personal data, or insufficient knowledge to express an opinion.
Registration and renewal
The score for processing registrations & renewals, was a median of 4 (mean 3.5; response rate 92%) which suggests modest satisfaction.
The overall rating also suggests that the issues may have been experienced by a comparative minority of registrants and that for most, renewal was straightforward.
“They expected people to call their phone number, which then wasn’t picked up. They didn’t reply to emails except after repeated attempts and finally having to resort to raising a complaint.” Resp023
“Difficult to get a timely response. Difficult to discuss my situation with a human being…” Resp044
Although the Likert score is positive, the themes in responses explaining the rating, are more mixed. Many respondents mentioned either having or knowing others who had issues with registration renewal, and its online processes including payments. A few respondents mentioned that the process was unforgiving of small errors. One respondent, for example, missed ticking a box on the renewal form, was removed from the register and experienced significant difficulties (poor communication with HCPC) getting the issue resolved.
Some respondents noted issues related to a long absence from work (e.g. maternity/illness etc.) causing them to miss registration deadlines – for some, this seems to have resulted in additional fees to renew registration. It seems rather easy for small errors (on either side) to result in registrants being removed from the register. For registrants, this can have very serious consequences and it can then be difficult and slow to resolve this, sometimes whilst on no pay. There have also been other reported instances of renewal payment collection errors [ 13 ].
“I had been off work… and had missed their renewal emails…I was told that there would be no allowances for this situation, and I would have to pay an additional fee to re-register…” Resp139.
Some respondents raised the issue of exclusion – certain staff groups not being included on the register—such as Clinical Technologists and Clinical Engineers. This desire for inclusion, also points to a perception of value in being on the register. One respondent raised an issue of very difficult and slow processing of registration for a candidate from outside the UK.
“Staff member who qualified as medical physicist abroad…has had a dreadful, drawn out and fruitless experience.” Resp135
Overall, many respondents noted difficulties in renewing registration and issues with HCPC’s online processes. Some of these issues (e.g. website renewal problems) may have been temporary and are now resolved, but others (e.g. available routes for registration) remain to be resolved.
Audit process & policies
In the survey, 12% respondents reported having been audited by HCPC regarding their CPD (response rate 97%). This is well above the level of 2.5% of each profession, which HCPC aims to review at each renewal [ 14 ], and similar values reported by some professional bodies [ 15 ]. The participants seem representative, although two respondents mentioned their perception of low audit rates. Data on CPD audit is available here: https://www.hcpc-uk.org/about-us/insights-and-data/cpd/cpd-audit-reports/
Respondents rated the process of being audited as a median of 4 (mean 3.7), which is the joint highest score on the survey, pointing to satisfaction with the process. From the responses, the overall perception could be summed up as straight-forward, but time-consuming. Without regular record-keeping, unfortunately most audits will be time-consuming – the HCPC more so, as it is not an annual audit, but covers the two preceding years.
Some respondents did find the process not only straight-forward, but also useful (related to feedback received). However, responses regarding feedback were mixed, with comments on both good, and poor feedback from HCPC.
“Not difficult but quite long-winded” Resp008
“Very stressful and time consuming” Resp081
“While it was a lot of work the process seemed very thorough and well explained.” Resp114
The HCPC’s policies & procedures were rated as a median of 3 (mean 3.2; response rate 98%). This neutral score could suggest a mixture of confidence in HCPC practise. This score may also reflect the fact that the majority of respondents had either not read, or felt they had no need to read the policies, and so are largely unfamiliar with them.
The reasons for this lack of familiarity are also explained by some respondents – four commented that the policies & procedures are rather too generic/vague. Three respondents noted that they felt the policies were not sufficiently relevant to their clinical roles to be useful. This may be due to the policies being written at a level to be applicable to registrants from all 16 modalities – and perhaps a limitation of the nature of HCPC as a very large regulator. Familiarity seemed mainly to be restricted to policies around registration, and CPD. There were slightly lower response levels for positive sentiment (6), than negative sentiment (9).
“I’ve never had cause to read them.” Resp115
“Detached from the real clinical interface for our professions…” Resp083
HCPC split their policies into ‘corporate’- which relate to organisational issues (e.g. equality & diversity; find them here: Our policies and procedures | ( https://www.hcpc-uk.org/about-us/corporate-governance/freedom-of-information/policies/#:~:text=Our%20main%20policies%20and%20procedures%201%20Customer%20feedback,scheme%20...%207%20Freedom%20of%20Information%20Policy%20 )) and those more relevant to professions (e.g. relating to the register; find them here: Resources | ( https://www.hcpc-uk.org/resources/?Query=&Categories=76 )).
One respondent noted not only that the policies were ‘as you might expect’, but felt the policies were less demanding than those from other similar bodies such as the CQC ( https://www.cqc.org.uk/publications ).
“…Other regulatory bodies (such as the CQC for example) have policies and procedures that are a lot more challenging to comply with.” Resp022
Theme 3 – Registrant competence and vulnerability
In this survey, 3.5% (5/143) of respondents noted some involvement with the HCPC’s Fitness to Practice service. These interactions were rated at a median of 3 (mean 2.8) suggesting neutral sentiment.
Firstly, we can immediately see the level of interaction with the FTP team is very small. CS registrants represent approx. 2% of HCPC registrants, and the level of CS referrals to FTP in 2020–21 was 0.2% [ 16 ].
The data is a very small sample, but responses vary strongly, so it is worth digging a little further into the granularity of individual responses. Response scores were 1, 1, 2, 5, 5 – which are mainly at the extremes of the rating spectrum. The majority of respondents described poor experiences with the FTP team: errors, a process which was ‘extremely prolonged’, involved slow/poor communication, and processes which were ‘entirely opaque’.
“It is slow, the process was badly managed… and the system was entirely opaque,” Resp37
“They were hard to contact and I didn't feel they listened…no explanation, apology or assurance it would not happen again. It left my colleague disillusioned and me very angry on their behalf…” Resp044
Some respondents commented that the team were not only difficult to contact, but also didn’t seem to listen. At the end of a process which involved errors from HCPC, one respondent noted were ‘no explanation, apologies or assurance that it would not happen again’, leaving the registrant ‘disillusioned’. These experiences do not fit with the HCPC’s stated goal to be a compassionate regulator, see Fig. 4 . Arguably it is more difficult to change a culture of behaviour and beliefs, than to publish a corporate goal or statement of vision.
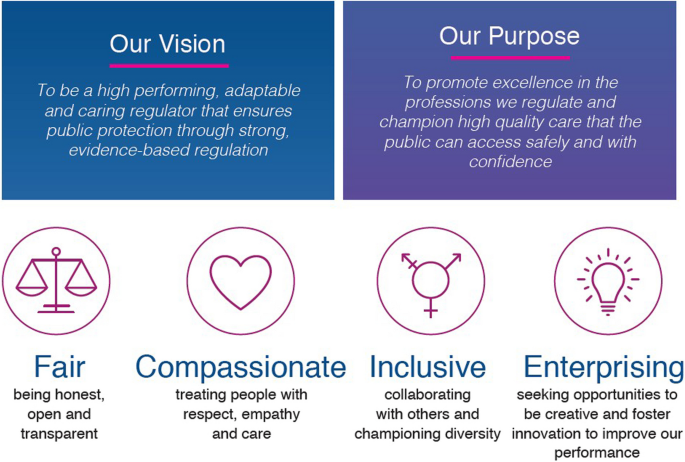
HCPC’s vision statement & purpose [ 17 ]
Some survey respondents have noted the necessity of regulation for our profession.
“Ultimately I am very grateful that I can register as a professional.” Resp024
Theme 4 – Suggestions for improved regulation
Following the question relating to overall performance, respondents were invited to suggest things which might improve their rating for HCPC’s performance and value. These suggestions were also combined with those which appeared in earlier survey responses.
Although we are in a current cost-of-living crisis, responses did not query simply high absolute cost of fees, but also queried the value/benefit of HCPC regulation for registrants. Many responses expressed doubt as to the added value & relevance of HCPC registration for them. They seem to point to a desire for more tangible benefit from their fees. Perhaps, given the costs and levels of scrutiny, registrants want some definite benefit to balance the scales .
“Cost less and do more for the people who are on the register.” Resp089
“Vastly reduced cost. Employer paying registrant fees.” Resp074
A significant number of responses pointed out that the main benefits of registration are for the public, and for employers – but that it is the registrants who pay for registration. Many queries why this should be, and whether there should be a different payment model, where for example employers pay.
Similarly, some respondents felt that the HCPC’s unusual position of regulating a large swathe of healthcare professions was not necessarily helpful for their profession or others.
Communication and response times are obviously an issue of concern for registrants, and improvements are needed based on the low satisfaction levels reported here. This is also linked to a wish for increased engagement with the CS profession.
“Engagement with the workforce, specialism specific development, reduced fees” Resp025
Some responses suggested they would be comforted by increased accountability / governance of HCPC including improved FTP efficiency.
“More accountability to registrants” Resp130
Finally, improvement in terms of additional registration routes for Engineers & Technical staff were also suggested. It may be damaging to work-place moral, if two professionals doing roles of a similar nature are not being governanced is the same way and if there is not parity of their gross salary due to mandatory professional fees & reductions.
Value-for-money : This will vary between individuals depending on many variables, such as upbringing & environment, salary, lifestyle priorities, political persuasion, and so on. However, many of these factors should balance in a large sample. In general, it can be suggestive of satisfaction (or lack of) with a service. The score here suggesting dissatisfaction, echoes with other reports on HCPC’s spending, and financial irregularities [ 18 , 19 ].
In the survey findings, respondents have voiced dissatisfaction with registration value for money. In fact, HCPC’s registration fees are not high when compared to the other healthcare professions regulators. Table 1 shows data from 2021–22 for regulator annual registration fees. However, the HCPC has risen from having the lowest regulator fees in 2014–5, to its current position (9 th of 13) slightly higher in the table. Perhaps more concerning than the absolute level of fees, are when large increases are proposed [ 12 , 20 , 21 , 22 ].
However, fees have regularly increased to current figure of £196.48 for a two-year cycle. During a consultation process in 2018, the Academy for Healthcare Clinical Scientists (AHCS) wrote an open letter to the HCPC, disputing what they felt was a disproportionate fee increase [ 23 ]. Further fee rises have also been well above the level of inflation at the time.
HCPC expenditure (which is linked to registration fees) has arguably been even more controversial than fee increases – noted by several respondents. A freedom of information (FOI) request in 2016 showed HCPC’s spending of £17,000 for their Christmas party [ 18 ] – which amounts to just over £76 per person. This cost was close to the annual registration fee (at that time) for registrants.
In 2019, regulation of social workers in England moved from HCPC, to Social Work England. This resulted in a loss of over 100,000 registrants, and a loss in registration fee income. HCPC raised fees to compensate, but a freedom of information (FoI) request in 2020 [ 18 ] showed that even though there was an associated lowering in workload associated with the loss of 100 k registrants, the HCPC had no redundancies, suggesting the loss of income was compensated mainly by the fees increase.
Inherent value & relevance
One of HCPC’s aims is to promote ‘the value of regulation’ [ 24 ]. However, not only is there dissatisfaction with value-for-money, the second highest response suggests a lack of inherent value (or benefit) from regulation to the individual registrant. In some ways, there is a lack of balance – registrants are under increasing scrutiny, but feel there is little direct benefit, to provide balance.
This also suggests that HCPC’s aim or message is not getting through to the CS profession. It’s not clear what the HCPC 2021–22 achieved milestone – ‘Embedded our registrant experiences research into employee learning and development and inductions’ has actually achieved.
A large number of responses pointed to the lack of clarity about HCPC’s role, and also to note its lack of relevance for respondents. Some of this is understandable – until recently, many CS registrants will have little interaction with HCPC. They would typically get one email reminder each year to renew their registration and pay those fees, and hear little else from the HCPC. That is beginning to change, and HCPC have recently begun to send more regular, direct emails/updates to registrants.
However, for many registrants, the HCPC appears not to be clearly communicating its role, or the relevance/importance of regulation. As mentioned above, this also links in to previous mentions of the lack of any tangible benefit for registrants. Some note little more relevance other than the mandatory aspects of regulation.
Finally, relevance is also queried in relation to the limited access for some professional groups to a professional register. The current situation of gaps in registration for some groups, results in two situations – firstly, for Clinical Scientists and Clinical Engineers/Technologists, one group has to compulsorily pay a fee to be allowed/approved to do their job and the other does not; also, the public are routinely helped and assisted by Clinical Scientists and Clinical Engineers/Technologists – but only one group is regulated to ensure public safety.
HCPC Communication
This was highlighted by respondents as often poor. Recently in the media, there has been a concern raised by The College of Paramedics (CoP) about communications issues with HCPC—changes to the HCPC policy on the use of social media [ 25 ]. They raised particular concerns about the use of social media content and ‘historical content’ in the context of investigations of fitness-to practice.
There have previously been some concerns raised on the UKMPE mail-base regarding handling of personal data, and lack of efficiency in addressing the issue [ 26 ]. Several messages detailed HCPC communicating unencrypted registrant passwords in emails and sending personal data to the incorrect registrant. Some on the forum noted that they had reported this problem over a period of several years to HCPC, suggesting HCPC’s response to these serious issues was extremely slow. Several responses noted these previous issues.
Registration processes
Although responses here show some satisfaction, there have been reports in the media of significant issues with registration (such as removing registrants from the register in error) with associated impact for patients and the public [ 27 , 28 ]. Similarly, there were reports on the UKMPE mail-base of significant issues with registration renewals being problematic [ 26 ]. In Scotland, NHS.net email accounts ceased to be supported in July-Sept 2020 and the associated lack of access to email accounts and messages used for HCPC communication and registration, caused a major issue in registration renewal. This coincided with COVID lockdowns and a period of unusually difficult communication with HCPC. If NHS staff lose registration (irrespective of the reason), respondents noted that some Human Resources (HR) departments were quick to suspend staff from work, and in some cases withhold pay. That spike in difficulties is likely the cause of the most common responses suggesting issues with a complicated process.
In safe-guarding public safety, a key task for a healthcare regulator is assessing the competence of registrants. This is done via a small set of related activities. Registrants must return regular evidence of CPD, and these are audited for 2.5% registrants. This process is simple and routine, and as seen in Theme 2 responses here suggest registrants are reasonably satisfied with this process.
More formal and in-depth competence assessment happens when a complaint is raised against a registrant, either by a work colleague/management, a member of the public or occasionally by the HCPC itself. The process is complex, lengthy and can end in a registrant attending a court hearing [ 29 ].
It is usual for registrants to continue in their normal job during FTP investigations – effectively the public remains at risk from a registrant if their competence is eventually proven to be below the regulators standards, so there is a need for investigations to be efficient both in timeliness, and outcome.
Obviously, being under investigation can be highly stressful, and has the potential for the registrant to be ‘struck off’ the register, and lose their job if registration is mandated (e.g. NHS posts). There are many reports of the process & experience either provoking or increasing underlying mental health challenges [ 30 , 31 , 32 ]. Along with efficiency, a regulator needs to behave compassionately. Investigations of highly-skilled professionals engaging in complex work activities, is also necessarily complex and requires a high degree of knowledge and experience from the regulator’s investigational panel.
The Professional Standards Authority (PSA) regulate the HCPC, and publish annual reviews of their performance ( https://www.professionalstandards.org.uk/publications/performance-reviews ) (see Table 4 ). HCPC performance as reported by PSA, seems to be generally higher than noted by survey respondents here. For 2022–23, aside from one area, the HCPC has scored 100% for performance, which seems at odds with these survey responses [ 33 ]. The FTP team is notable in repeatedly performing very poorly compared to most other sections of the HCPC (even though the majority of the HCPC budget goes to FTP activity, see Fig. 4 ). The HCPC Annual Report 2018–9 [ 34 ] highlighted the completion of the first phase of the Fitness-To-Practice Improvement Plan. This delivered “A root and branch review of this regulatory function… a restructure, tightened roles and processes and the introduction of a new Threshold Policy”, but this seems to have no impact on the performance reported by the PSA for the next few years shown in Table 4 . However, the most recent data does suggest improvement, and HCPC continues to develop FTP team practice [ 17 ].
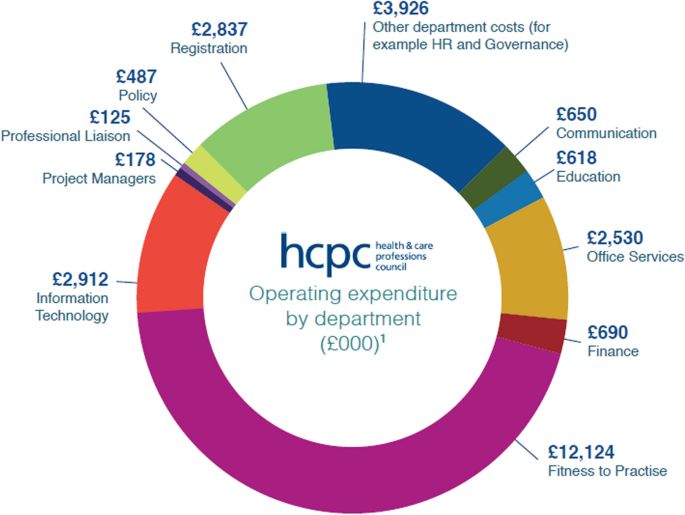
HCPC expenditure for the year 2020–21 [ 17 ]
There are other reports of poor experiences with this team [ 35 , 36 ], and in one report the FTP team’s processes have been noted as being rather inhumane [ 35 ].
Regulation is an important part of public protection, but how effectively it is managed & enforced is also a concern, given it involves increased scrutiny of registrants. A topical comparator is the current dissatisfaction by a large section of the public about several other government regulators allowing seemingly poor performance to go unchecked [ 4 , 5 ].
It is arguable, that registrants remain on the register as long as the HCPC allows them. Several respondents in this survey noted being removed from the register through HCPC administrative error. Removal could also happen through poor judgement/decision-making – the FTP team handle large numbers of very complex investigational cases – 1603 concluded cases for the year 2021–22 and 1024 hearings [ 16 ]. Every justice system is subject to a level of error – guilty parties can be erroneously ‘cleared’, and vice-versa. It is essential therefore, that policies & procedures relating to FTP are fit for purpose—that the FTP team work effectively and humanely, and that there is genuine & effective governance of HCPC to ensure accountability. In this survey, some respondents seem to be saying that currently this seems not to be the case.
It might have been anticipated that the greatest concern is costs, especially in the current cost-of-living crisis. The recent HCPC consultation to increase fees [ 37 ] seems particularly tone-deaf and has caused concern across the professions [ 21 , 22 ].
Above findings show respondents are interested in lower fees, but also increased benefit for their fees. Some respondents pointed out that whilst registrants pay for registration, benefit is mainly for the public and employers. The HCPC is a statutory body, its funding model will have been designed/decided upon by government, and may be unlikely to change. However, there are a variety of potential regulation models [ 38 ], and so change is possible. A review of the financial model for regulation may be welcome.
Regulator size
Some aspects of HCPC performance, policies, and distribution of spending, is related to the nature of it being the largest and only multi-professional regulator in the healthcare sector. Data from the HCPC suggests (see Fig. 5 ) that the majority of spending relates to FTP activity. Data also points to Clinical Scientists having very low levels of FTP investigation compared to others in HCPC [ 16 ]. This suggests that a significant proportion of CS registrant fees are used to investigate other professions. It’s possible (perhaps simplistically) that if, like many other healthcare professions such as doctors & dentists who’s regulator is concerned only with that single profession, if CSs were regulated separately, their registrant fees may be reduced. This model of single-profession regulation may also mitigate against other disadvantages of the HCPC’s practice, such as the ‘generic’ policies aiming to apply to a pool of 15 professions.
Although there is a very low level of data for this topic, the concerned raised by registrants are serious in nature. There also seems to be issues in handling of complaints related to this service and advocacy for registrants. Certainly, there is a clear governance path via PSA, to the Health Secretary. However, this does not offer a route for individual complaints to be raised and addressed. Unlike complaints from the public in other areas, there is no recourse to an ombudsman for registrants. The only option for individual registrants, is the submission of a formal complaint to the HCPC itself, which is dealt with internally. Comments from survey respondents suggest this process does not guarantee satisfaction. Indeed, one of the respondents who mentioned submitting a complaint, made it clear they remained unhappy with HCPC’s response. Overall, there seems to be a lack of clear & effective advocacy for registrants.
“…the HCPC’s stance appeared to be guilty until proven innocent… At no point did I feel the HCPC cared that their (sic) was an individual involved....” Resp044.
FTP processes affect a comparatively small number of CS registrants, compared to other professions. However, it seems clear that the majority of those who have interacted with the FTP team have had poor experiences, and respondents have suggested improvements are needed. The reason for FTP investigations, is protection of staff and the public. If processes are slow, and investigations prolonged, or decisions flawed, the public may be exposed to increased levels of risk, as healthcare practitioners who may be lacking in competence continue to practice. The data in Table 4 shows concerning but improving trends in FTP performance levels.
Limitations
There are two main limitations to this work. Firstly, due to time constraints, there was no pilot work done when designing the survey questionnaire. This may have helped, as noted earlier, a few responses pointed to some awkwardness with one survey question. Although no pilot work was done, the questionnaire was reviewed by the IPEM Professional Standards Committee, as noted in the Acknowledgements section.
The other obvious limitation is the low response rate (~ 6% of UK Medical Physicists). Circulation of the survey was performed via the only online forum for the profession currently available. The survey was advertised multiple times to ensure visibility to staff who may have missed it initially due to leave etc. However, the forum does reach 100% of the profession, and some addressees may have filters set to send specific posts to junk folders etc. The professional body IPEM declined to offer support in circulating the survey (believing the issues involved would affect/be of interest only to a small minority of members.)
The low response rate also has a particular impact on the pool of responses relating to FTP issues, which inherently affect low numbers of registrants.
However, the importance of some of the findings here (e.g. expressed dissatisfaction with regulation in terms of value; the poor experience of some members with the Registration, Communication and FTP teams) and the low sample surveyed, both justify the need for a larger follow-on survey, across all of Clinical Science.
In Healthcare, regulation of professions is a key aspect of protecting the public. However, to be effective, regulation must be performed professionally, impartially, and associated concerns or complaints investigated efficiently and respectfully.
This report presents findings from a survey aimed at collecting a snap-shot of the experiences of Clinical Scientists with their regulator, and their perception of the quality and safety of that regulation performance.
Overall survey sentiment scores showed a significantly more negative responses than positive. Survey comments relate not only to current issues, but to previous problems and controversial issues [ 18 , 26 ]. It seems that some respondents have at some point lost confidence and trust in the HCPC, and survey responses suggest there has not been enough engagement and work done by HCPC to repair and rebuild this trust.
In the midst of a cost of living crisis, costs are a large concern for many. The HCPC fees are neither the highest not lowest amongst the healthcare regulators. Spending is transparent, and details can be found in any of the HCPC’s annual reports.
A repeating sub-theme in responses, was a lack of tangible value for the registrant, and that the employer should pay the costs of registration, where registration is mandated by the job.
Many respondents have suggested that they feel there should be more proactive engagement from HCPC with the profession. Most respondents were not familiar with or felt the HCPC policies are relevant/important to them.
Survey data showed moderate satisfaction with registration processes for the majority of respondents. Some respondents also noted a lack of registration route for engineering & technical healthcare staff. CPD processes also achieved a score indicating registrant satisfaction. This generated the highest ratings in the survey. Communication scored poorly and many respondents suggests there needs to be improved levels of communication in terms of response times and access to support.
The CS profession experiences low levels of interaction with the FTP service. However, those interactions which were recorded in the survey, show some poor experiences for registrants. There also seems to be a lack of advocacy/route for complaints about HCPC from individual registrants. There may need to be more engagement between registrants and their professional body regarding HCPC performance, and more proactivity from the stake-holder, IPEM.
Some of the findings reported here relate to important issues, but the survey data are based on a low response rate. A larger survey across all of Clinical Science is being planned.
Availability of data and materials
To protect confidentiality of survey respondents, the source data is not available publicly, but are available from the author on reasonable request.
Abbreviations
Agenda for Change
Academy for Healthcare Clinical Scientists
Continuous professional development
Clinical Engineer
Clinical Scientist
College of Paramedics
Clinical Technologist
Freedom of Information
Fitness-to-practice
Health and Care Professions Council
Human resources
Institute of Physics and Engineering in Medicine
Joint Information Systems Committee
Medical Technical Officer
Professional Standards Authority
Professional Standards Committee
Qualitative data analysis
UK Medical Physics and Engineering
Professional Standards Authority. Professional healthcare regulation in the UK. https://www.professionalstandards.org.uk/news-and-blog/blog/detail/blog/2018/04/10/professional-healthcare-regulation-explained#:~:text=Regulation%20is%20simply%20a%20way,may%20face%20when%20receiving%20treatment . Accessed 26 Jul 2023
Evening Standard. Bogus surgeon treated hundreds. https://www.standard.co.uk/hp/front/bogus-surgeon-treated-hundreds-6326549.html . Accessed 26 Jul 2023.
HCPC . About registration: protected titles. http://www.hcpc-uk.org/aboutregistration/protectedtitles/ . Accessed 27 Jul 23.
The Guardian. Public patience is wearing thin. Ofwat must wield the big stick | Nils Pratley | https://www.theguardian.com/business/nils-pratley-on-finance/2022/dec/08/public-patience-is-wearing-thin-ofwat-must-wield-the-big-stick . Accessed 19 Jul 2023.
TrustPilot. Reviews of Ofgem. Ofgem Reviews | Read Customer Service Reviews of ofgem.com (trustpilot.com). Accessed 19 Jul 2023.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Article Google Scholar
Kiger ME, Varpio L. Thematic analysis of qualitative data: AMEE Guide No. 131. Med Teach. 2020;42(8):846–54.
Article PubMed Google Scholar
Declaration of Helsinki. 2013. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ . Accessed 12 Sept 2023.
UK Data Protection Act. 2018. https://www.gov.uk/data-protection . Accessed 15 Sept 2023.
Rowbottom C. Private communication on behalf of the IPEM Professional Standards Committee; 2022.
IPEM Workforce Team. Clinical scientist & engineer workforce data. Personal communication. 2022.
Unison. HCPC fee increase is an unjustified ‘tax on practising.’ https://www.unison.org.uk/news/press-release/2019/02/hcpc-fee-increase-unjustified-tax-practising/ . Accessed 27 Jul 2023.
HCPC. Direct debit collection errors. https://www.hcpc-uk.org/news-and-events/news/2020/early-direct-debit-collections/?dm_i=2NJF,141CO,7C0ZNI,4A8IE,1 . Accessed 27 Jul 23.
HCPC. CPD audit rates. https://www.hcpc-uk.org/cpd/cpd-audits/ . Accessed 21 Jul 2023.
IPEM. CPD audit rates. https://www.ipem.ac.uk/your-career/cpd-career-development/cpd-audit/ . Accessed 21 Jul 2023.
HCPC. Fitness to practice annual report 2020–21. https://www.hcpc-uk.org/about-us/insights-and-data/ftp/fitness-to-practise-annual-report-2020-21/ . Accessed 23 Jul 2023.
HCPC. Annual report and accounts, 2020–21. https://www.hcpc-uk.org/resources/reports/2022/annual-report-and-accounts-2020-21/ . Accessed 19 Jul 2023.
Wikipedia. The health and care professions council. https://en.wikipedia.org/wiki/Health_and_Care_Professions_Council . Accessed 2 Jul 23.
HCPC. Annual report 2005–06. https://www.hcpc-uk.org/resources/reports/2006/annual-report-2005-06/ . Accessed 19 Jul 2023.
British Dental Association. BDA very disappointed by HCPC decision to raise registration fees by 18%. https://www.bda.uk.com/resource/bda-very-disappointed-by-hcpc-decision-to-raise-registration-fees-by-18.html . Accessed 27 Jul 2023.
British Psychological Society. HCPC fees consultation – share your views. https://www.bps.org.uk/news/hcpc-fee-consultation-share-your-views . Accessed 27 Jul 23.
IBMS. IBMS response to the HCPC registration fees consultation. https://www.ibms.org/resources/news/ibms-response-to-hcpc-registration-fees-consultation/ . Accessed 17 Jul 23.
Association of HealthCare Scientists. Open letter to HCPC. https://www.ahcs.ac.uk/wp-content/uploads/2018/11/HCPC-Open-Letter.pdf . Accessed 27 Jul 23.
HCPC. Corporate plan 2022–23. https://www.hcpc-uk.org/resources/reports/2022/hcpc-corporate-plan-2022-23/ . Accessed 23 Jul 2023.
College of Paramedics. Our formal response to the HCPC consultation. https://collegeofparamedics.co.uk/COP/News/2023/Our%20formal%20response%20to%20the%20HCPC%20consultation.aspx . Accessed 27 Jul 23.
JISC Mail - MPE mailbase. JISCMail - Medical-physics-engineering list at www.jiscmail.ac.uk . Accessed 19 July 2023.
The Guardian. Thousands miss out on treatment as physiotherapists are taken off UK register. https://www.theguardian.com/society/2022/may/14/thousands-miss-out-on-treatment-as-physiotherapists-are-struck-off-uk-register . Accessed 27 Jul 2023.
HSJJobs.com. https://www.hsjjobs.com/article/thousands-of-clinicians-unable-to-work-after-registration-blunder . Accessed 27 Jul 2023.
HCPC. How we investigate. https://www.hcpc-uk.org/concerns/how-we-investigate/ . Accessed 21 Nov 2023.
Sirriyeh R, Lawton R, Gardner P, Armitage G. Coping with medical error: a systematic review of papers to assess the effects of involvement in medical errors on healthcare professionals’ psychological well-being. Br Med J Qual Saf. 2010;19:6.
Google Scholar
Bourne T, Wynants L, Peters M, van Audenhove C, Timmerman D, van Calster B, et al. The impact of complaints procedures on the welfare, health and clinical practise of 7926 doctors in the UK: a cross-sectional survey. BMJ Open. 2015;5:e006687.
Article PubMed PubMed Central Google Scholar
Jones-Berry S. Suicide risk for nurses during fitness to practice process. Ment Health Pract. 2016;19:8.
Professional Standards Authority. HCPC performance review 2022–23. https://www.professionalstandards.org.uk/publications/performance-review-detail/periodic-review-hcpc-2022-23 . Accessed 25 Jul 2023
HCPC. Annual report and accounts, 2018–19. https://www.hcpc-uk.org/resources/reports/2019/hcpc-annual-report-and-accounts-2018-19/ . Accessed 19 Jul 2023.
Maben J, Hoinville L, Querstret D, Taylor C, Zasada M, Abrams R. Living life in limbo: experiences of healthcare professionals during the HCPC fitness to practice investigation process in the UK. BMC Health Serv Res. 2021;21:839–54.
Leigh J, Worsley A, Richard C, McLaughlin K. An analysis of HCPC fitness to practise hearings: fit to practise or fit for purpose? Ethics Soc Welfare. 2017;11(4):382–96.
HCPC. Consultation changes to fees. https://www.hcpc-uk.org/news-and-events/consultations/2022/consultation-on-changes-to-fees/ . Accessed 27 Jul 23
Department of Health. Review of the regulation of public health professions. London: DoH; 2010.
Download references
Acknowledgements
The author wishes to kindly acknowledge the input of Dr Carl Rowbottom (IPEM Professional Standards Committee), in reviewing the survey questions. Thanks also to Dr Nina Cockton for helpful advice on ethics and recruitment issues.
There were no sources of funding required for this work.
Author information
Authors and affiliations.
University of Glasgow, Level 2, ICE Building, Queen Elizabeth University Hospital Campus, 1345 Govan Road, Glasgow, G51 4TF, UK
Mark McJury
You can also search for this author in PubMed Google Scholar
Contributions
All work to collect, analyse & publish this survey, are the work of the author Dr Mark McJury.
Corresponding author
Correspondence to Mark McJury .
Ethics declarations
Ethics approval and consent to participate.
As this study relates to low risk, survey data, formal ethics committee approval is not required (exemption obtained from NHSGGC REC04 REC Officer Dr Judith Godden [email protected]). As the survey responses were from members of a professional body (The Institute of Medical Physics and Engineering in Medicine (IPEM) it was consulted. Its Professional Standards Committee (PSC) reviewed the survey and raised no objections. The survey questions were assessed for bias and approved unchanged (acknowledged in the manuscript). Written informed consent was obtained from all participants in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
The survey questionnaire has been provided as a supplementary file.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
McJury, M. Experiences of UK clinical scientists (Physical Sciences modality) with their regulator, the Health and Care Professions Council: results of a 2022 survey. BMC Health Serv Res 24 , 635 (2024). https://doi.org/10.1186/s12913-024-10956-7
Download citation
Received : 06 September 2023
Accepted : 05 April 2024
Published : 16 May 2024
DOI : https://doi.org/10.1186/s12913-024-10956-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Regulation of professions
- Clinical scientists
- Medical physicists
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]

Funding opportunity: Centre in Climate Change and Health Full Stage (invite only)
Last updated: 31 August 2023 - see all updates
Apply for funding to establish a centre in climate change and health.
This centre will be a world-leading centre of excellence carrying out interdisciplinary, cutting-edge, and impactful research to address major challenges at the interface of climate and health research.
You must have already submitted an outline proposal and been shortlisted at the outline panel. You must be based at a UK research organisation eligible for UK Research and Innovation (UKRI) funding.
The full economic cost (FEC) of your project can be between £5 million and £9.7 million. We will fund 80% of the FEC.
Funding is available for up to five years.
Who can apply
This funding opportunity is only open to you if you submitted an outline proposal and have subsequently been shortlisted and invited to submit a full proposal. Any uninvited proposals will be rejected.
Standard UKRI eligibility rules apply. UKRI has updated the Individual Eligibility Policy and has introduced new role types for funding opportunities being run on UKRI’s new Funding Service
For full details, visit UKRI Eligibility Guidance .
This funding opportunity is being administered by ESRC on behalf of UKRI. Standard ESRC eligibility rules for project co-leads apply.
It is likely that successful applications will be led by experienced researchers who are internationally recognised, with proven ability to deliver a large-scale research project successfully and lead an investment of this nature. The amount of time committed to the grant by the project lead and project co-lead must be costed into the proposal, but very small fractions of project co-lead time should be avoided. Our standard research funding rules would apply for staff engaged in more than one ESRC grant (see ESRC Research Funding Guide ).
International Applicants
The inclusion of international project co-leads in proposals is permitted. Academic researchers (at PhD or equivalent status) must be from established overseas research organisations of comparable standing to UKRI-eligible UK research organisations to be listed as international project co-leads under this funding opportunity. You should read project co-lead (international) policy guidance for details of eligible organisations and costs.
International collaboration is not limited to project co-lead but may also involve partnerships to develop international datasets, promote knowledge exchange and international impact, and enhance social science conceptual development on an international scale.
Business, third sector or government body project co-leads
We welcome inter-institutional applications and strongly encourage collaboration to fulfil the aims of the centre. Partnerships are encouraged with non-HEI organisations and across third sector, business and the public sector, either as project partners or as project co-lead.
We will fund all justified costs associated with international and UK business, civil society or government bodies project co-leads at 100% FEC. However, these combined costs must not exceed 30% of the full 100% FEC cost of the grant.
Equality, diversity and inclusion
We are committed to achieving equality of opportunity for all funding applicants. We encourage applications from a diverse range of researchers.
We support people to work in a way that suits their personal circumstances. This includes:
- career breaks
- support for people with caring responsibilities
- flexible working
- alternative working patterns
Find out more about equality, diversity and inclusion at UKRI .
What we're looking for
We are looking for a centre that will find solutions to address the challenges that climate change poses to population health, in a way that enhances both environmental and health outcomes.
The centre will bring together the right people, disciplines, institutions, and infrastructure to deliver impact within a five-year timeframe. The centre will form part of a portfolio of investments under the UKRI securing better health, ageing and wellbeing strategic theme, as set out in the UKRI strategy 2022 to 2027 .
Please note that we will allow for a 10% deviation, either an increase or decrease, in the 100% full economic cost of proposals between the outline and full proposal stages. However please note that the maximum limit of £9.7 million, and minimum limit of £5 million at 100% full economic cost remains in force for all proposals.
Disruptions to Earth’s natural systems, caused by human activity, are already having a detrimental impact on our health and wellbeing. Climate change has wide ranging impacts on the environment including:
- biodiversity and habitat loss
- disruption to food systems
- water scarcity
- extremes of temperature
These in turn are having direct and indirect effects on aspects of human health, including nutrition, mental health, and diseases (both infectious and non-communicable).
Climate change and human health do not exist in a vacuum and are part of broader complex systems and relationships. This is central to the concept of planetary health, which describes how the health of humans and other living organisms are inextricably linked and how these in turn depend on Earth systems that sustain life. The issue of intergenerational justice is pertinent here as well: that is, what we owe to future generations.
The centre will accelerate understanding of the links between climate change and human health across the life course. It will form an evidence base and develop solutions which will propel decision makers towards sustainable, transformational action within the lifespan of the award and beyond.
We know that adaptation, decarbonisation and mitigation strategies designed to address climate change can impact human health in various ways. Moreover, health interventions can have positive or negative impacts on environmental quality.
This centre will take account of co-effects when targeting solutions to climate change and health challenges. For example, the potential for climate change mitigation and adaptation strategies to have positive and detrimental impacts on health or the environment. A key focus of the centre should be on interventions to address upstream determinants (including mitigation, adaptation, and resilience), rather than down-stream individual-level factors.
The drivers of climate change and human health are embedded in complex systems, relationships, and boundaries. The centre will identify how these often-intersecting drivers affect both climate and health (for example, environmental factors such as biodiversity loss or land use change, culture, economic, technological, legal, and political systems).
The centre should apply systems thinking and methods, to ensure that solutions are developed and delivered with a deep understanding of the mosaic of factors at play.
This approach calls for input from a variety of disciplinary backgrounds. We encourage applications that integrate perspectives from across the disciplinary remits of the research councils involved in this funding opportunity, to bring different partners and sectors together to co-develop solutions. There should be meaningful links to policy makers and end-users.
Areas in scope
In your proposal you should describe the challenge your centre would address. The centre should identify a range of questions relevant to an overarching challenge, rather than narrowly focusing on one specific topic. You should describe how the centre would adopt interdisciplinary and whole systems approaches.
We provide some broad examples of areas in scope below, but they are not exhaustive, and we encourage you to think creatively about potential challenges and the impacts that could be achieved.
Vulnerability to the effects of climate change on health including understanding biological mechanisms is in scope. For this centre we are interested in population-level variation in these impacts, for example, increased vulnerabilities to the effects of climate change in particular population groups, and how mitigations might have a protective effect.
Those most at risk may include, but are not exclusive to, individuals and groups at vulnerable stages of the life course, with established disease or those disadvantaged by inequalities. Effects on animals are only eligible for inclusion where relevant to human health (for example, on nutrition).
Also in scope are the wider factors that influence individual and group health behaviours, and how these are influenced by the social, physical, technological, political and economic environment. How can we use this understanding to shape ethical solutions to mitigate climate change impacts on health and to support resilient communities?
This could include a consideration of legal frameworks, the built environment, and of cultural, heritage and environmental assets (for example, museums, historic monuments, rivers, parks).
Proposals may consider new ways to use data, machine learning and technology to improve our understanding of climate change and its risks to health, and novel technology solutions to address these risks. This could include challenges for technology integration and may incorporate a consideration of how best to re-engineer complex healthcare systems challenges to deliver early intervention for climate-related health risks.
The centre could include a focus on food and nutrition, providing links are made through to impacts on both human health and the environment. This could include developing a better understanding of the impact of climate change on nutrition security across the food system.
It might also encompass an exploration of the link between climate change and the health and nutrition of soils, crops, and livestock as well as the health of consumers (including access to quality food).
As the impacts of climate change are not experienced equally across society, the centre should include health, social and environmental inequality as a cross-cutting theme. Meaningful co-creation with affected communities across the research lifecycle is strongly encouraged, and relevant costs to support this should be included.
The centre will work in partnership with a variety of stakeholders to ensure the research outputs drive forward and deliver benefits to both the environment and health.
The centre should be primarily focused on the UK. A place-based approach may be acceptable, for example with a focus on a geographically defined community, if justified by the research proposed. Where this approach is taken, potential for wider scale up and applicability must be demonstrated.
Proposals can include an international element where justified, including learning from international approaches and demonstrating leadership through applying UK learning elsewhere, but proposals focused entirely outside the UK are out of scope. International project co-leads are eligible.
We expect to fund a single centre through this funding opportunity.
The research will address a gap that is not being addressed elsewhere that can be delivered within a five-year timeframe.
Research challenges
You must demonstrate that your centre will deliver the following:
High-quality, interdisciplinary, internationally recognised research findings to address unanswered questions about a defined challenge to our society, economy or both
In this case, the centre will need to clearly articulate how the proposed programme of interdisciplinary research addresses challenges climate change poses to health in a way that promises co-benefits to both the environment and health.
You should include details of any innovative research methods that you intend to develop and use, including in evaluating the impact of potential solutions.
Significant economic and societal impact, demonstrating that the centre is responding to its specified challenge.
Impact should be a major consideration throughout the scoping of a proposal, and during and beyond the lifetime of a centre.
Impact should be multisectoral, with evidence of user engagement from inception throughout all stages of the planned timeframe for the award.
Centres should consider other investments in terms of adding value to their centres, for example maximising opportunities through impact acceleration accounts.
You must include a logic model in the Approach section demonstrating the changes the centre will bring about to respond to the challenge, and how and why your programme of research will bring about those changes.
Further resources to support the development of a logic model can be found under ‘Supporting Documents’ in the ‘Additional information’ section.
The resources needed to become a centre of excellence that adds value to the wider community. This will include developing the people, producing the data and creating the infrastructure needed to respond to their specified challenge, as well as making use of existing datasets and infrastructure.
Studentships
Up to three associated studentships may also be included in this application. They must be based in a doctoral training partnership (DTP) or centre for doctoral training (CDT) eligible to receive studentships from the research council most closely aligned to the primary discipline of the studentship(s). Further information can also be found in our postgraduate funding guide . For each studentship that you intend to fund or part fund from UK Research and Innovation (UKRI) funds, please provide details of the number of studentships and the PhD topics to be undertaken.
Please provide the following information for each studentship being requested in full or partly supported through UKRI funds. (If they are not being funded by UKRI funds there is no requirement to provide this information):
- proposed start and end date of the studentship(s)
- duration of each studentship in years
- name of the student(s), if known at the point of application
- name of main supervisor
- details of the accredited subject area in which the student will be based
- confirmation that the DTP/CDT director supports the proposed studentship arrangements
- a summary statement of the PhD topic(s) to be undertaken and a justification for the length of the programme of study
- a clear statement of how this is independent from, but will add value to, the principal research objectives set out in the application
Associated studentships linked to a grant are designed to add value to the proposed research outlined in the application, while providing a clear opportunity for a distinct and independent course of enquiry for the student. Through being embedded within a high-quality research team, they should offer the student an opportunity to develop their substantive research skills, alongside broader professional development. The main research grant project should still be viable without the studentship and should have distinct objectives that are not reliant upon the studentship
Up to three studentships can be applied for on any single application. Studentships may only be linked to grants that are for three years or more. The student(s) must be located within a UKRI accredited DTP or CDT and studying through an accredited subject area. The project lead or project co-leads approved to act as a primary supervisors for PhD students must also be based within a DTP or CDT. The costs associated with the studentship should be included within the costs table and justified in the Resources and cost justification section.
Career and skills development
You should clearly articulate your plans for career development.
UKRI is a signatory to the Concordat to Support the Career Development of Researchers, and the Technician Commitment, through these UKRI commits to support the professional and career development of researchers and technicians through its funding opportunities. You are encouraged to consider both leadership development and capacity building in your plans.
Leadership development skills should be considered at all career stages to equip researchers in the centre with the leadership skills needed to be able to design, lead and deliver large and complex or interdisciplinary projects and teams.
Research leadership should go beyond project management to include a capacity to enthuse, ignite and sustain an intellectual vision that is inclusive, flexible and open to challenge. The report fit for the future: research leadership matters gives insight into the skills related to research leadership at different career stages and some preliminary suggestions for how those skills might be supported across the career life-course.
You should also demonstrate how you plan to build capacity among decision makers to use evidence as well as building skills in academia, policy and industry engagement among research staff and technicians at all career levels, from PhD students to early and mid-career academics to established professors.
There is a significant opportunity to better integrate health and environment data to explore the intersection and develop interventions.
Centres should maximise the use of relevant existing data resources in the first instance, as well as (where appropriate) producing data that responds to their proposed challenge and is of value to the wider community.
Data collection and management should be in accordance with the ESRC research data policy . UKRI funds a range of data infrastructures that are available and free to use for all bona fide researchers (subject to appropriate data sharing considerations).
Management and structure
You should consider the structure of your proposed centre to ensure it can successfully deliver the objectives of the funding opportunity, whether through a consortium approach or single institution.
The centre should span a range of distinct disciplines and we also encourage the inclusion of different organisations within each proposal. Partnerships with non-higher education institution organisations across government, industry and civil society are also encouraged, where appropriate.
You should consider existing funded research, including from UKRI, to ensure that the centres’ research objectives are novel. The centre will be required to collaborate and engage with a wide range of stakeholders, including existing relevant research activity. This should evolve over time, but might include the transforming UK food systems programme , clean air programme , mobilising community assets to tackle health inequalities and the UK Prevention Research Partnership . Your proposal should not duplicate current and previously funded research.
We encourage applications from individuals with diverse backgrounds and experiences, either as project lead or project co-leads.
You must include a management plan in the Approach section, demonstrating:
- how you will provide leadership across the collaborators involved in the application
- how the management of the centre and its activities will be carried out, including details of project management and administration resource
You should also include details of any advisory group that will be appointed to oversee the development of the centre.
You are also expected to indicate your plans for monitoring progress against your logic model, and any plans for self-evaluation throughout the lifetime of the award.
The successful centre will be allocated an ESRC investment manager who will work with their centre to agree a monitoring and evaluation plan in the starting phase of the award.
You may propose a title for this centre.
- Organisational support
We will be looking for evidence of long-term strategic and financial institutional commitment to the proposed centre, above the required 20% (as we fund at 80% FEC), which should be detailed in the Organisational Support section. This should be through the provision of grant-associated parallel activities. Examples include but are not limited to:
- studentships
- summer schools
- refurbishment of facilities for the centre
- provision of equipment
- administration
- new lectureships
We do not require an institutional letter of support for the full stage of the centres competition. By submitting your proposal to us, you are confirming that your institution is supportive of and committed to your centre.
Research ethics
You must ensure that the proposed research will be carried out to a high ethical standard.
You must clearly state how any potential ethical, safeguarding and health and safety issues have been considered and will be addressed, ensuring that all necessary ethical approval is in place and all risks are minimised before the award commences.
All proposals, including those involving animal and human participants, must state how they will comply with relevant UKRI policies and the ESRC framework for research ethics . If your proposal involves animals, you must read and refer to the UKRI position statement on the use of animals in research .
Equality, diversity and inclusion (EDI)
Promoting EDI is an integral part of our vision to deliver new knowledge and an enriched, healthier, more sustainable and resilient society and culture, and to contribute to a more prosperous economy.
You are expected to demonstrate throughout your proposal how you will consider EDI during the centre’s lifetime.
Environmental sustainability
We recognise that we must embed environmental sustainability in everything we do.
You are expected to consider the environmental impact of the centre’s activities and to put in place actions that encourage sustainability and mitigate any risk of environmental harm.
How to apply
We are running this funding opportunity on the new UKRI Funding Service. You cannot apply on the Joint Electronic Submissions (Je-S) system.
The project lead is responsible for completing the application process on the Funding Service, but we expect all team members and project partners to contribute to the application.
Only the lead research organisation can submit an application to UKRI.
- Follow the link to the Funding Service in the email sent from the ESRC Centres mailbox.
- Confirm you are the project lead.
- Sign in or create a Funding Service account. To create an account, select your organisation, verify your email address, and set a password. If your organisation is not listed, email [email protected]
- Answer questions directly in the text boxes. You can save your answers and come back to them, or work offline and return to copy and paste your answers. All questions and assessment criteria are listed in the ‘How to apply’ section on this Funding finder page.
- Send the completed application to your research office for checking. They will return it to you if it needs editing
- Your research office will submit the completed and checked application to UKRI.
Watch our research office webinars about the new UKRI Funding Service .
We must receive your application by 7 November 2023 at 4:00pm UK time.
You will not be able to apply after this time.
You should ensure you are aware of and follow any internal institutional deadlines that may be in place.
Processing personal data:
ESRC as part of UKRI, will need to collect some personal information to manage your funding service account and the registration of your funding applications.
We will handle personal data in line with UK data protection legislation and manage it securely. For more information, including how to exercise your rights, read our privacy notice .
Outcomes publication:
ESRC, as part of UKRI, will publish the outcomes of this funding opportunity at What ESRC has funded – UKRI
If your application is successful, some personal information will be published via the UKRI Gateway to Research .
UKRI Funding Service: section guidance
In plain English, provide a summary that can be sent to potential reviewers to determine if your proposal is within their field of expertise.
This summary may be made publicly available on external facing websites, so please ensure it can be understood by a variety of readers, for example:
- opinion-formers
- policymakers
- the general public
- the wider research community.
Guidance for writing a summary
Succinctly describe your proposed work in terms of:
- its context
- the challenge the project addresses and how it will be applied to this
- its aims and objectives
- its potential applications and benefits.
Word count: 550
List the key members of your team and assign them roles from the following:
- project lead (PL)
- project co-lead UK (PcL)
- project co-lead (International) (PcL (I))
- grant manager
- professional enabling staff
- research and Innovation Associate
- visiting researcher
Only list one individual as project lead.
Find out more about UKRI’s new grant roles and eligibility .
Section: Vision
Question: What are you hoping to achieve with your proposed work?
What the assessors are looking for in your response
explain how your proposed work:
- meets a clearly defined challenge that is critical to the future of our society or the economy, or both
- is of excellent quality and importance within or beyond the field(s) or area(s)
- has the potential to advance current understanding, generates new knowledge, thinking or discovery within or beyond the field or area
- is timely given current trends, context and needs
- impacts world-leading research, society, the economy or the environment
Within the Vision section we also expect you to:
- identify the potential direct or indirect benefits and who the beneficiaries might be
Within this section you can also:
- demonstrate elements of your responses in visual form if relevant.
- use images sparingly and only to convey important information that cannot easily be put into words
- insert each new image onto a new line
- provide a descriptive legend for each image immediately underneath it (this counts towards your word limit)
Files must be:
- In JPEG, JPG, JPE, JFI, JIF, JFIF, PNG, GIF, BMP or WEBP format
- Be smaller than 8MB
Word count: 1,000
Section: Approach
Question: How are you going to deliver your proposed work?
Explain how you have designed your approach so that it:
- Is interdisciplinary, bringing together the right disciplines to respond to your proposed challenge.
- is effective and appropriate to achieve your objectives
- is feasible, and comprehensively identifies any risks to delivery and how they will be managed
- if applicable, uses a clear and transparent methodology
- makes use of existing data where relevant and justifies the collection of any new data in line with the challenge area
- if applicable, summarises the previous work and describes how this will be built upon and progressed
- will maximise translation of outputs into outcomes and impacts
- ensures that impact is multisectoral with evidence of user engagement from inception throughout all stages of the planned timeframe for the award. This should be backed up by a clear logic model demonstrating the changes the centre will bring about to respond to the challenge.
- describes how your, and if applicable your team’s, research environment (in terms of the place, its location, and relevance to the project) will contribute to the success of the work
- Demonstrates evidence that issues relating to equality, diversity, inclusion have been considered throughout your approach.
Within the Approach section we also expect you to:
- demonstrate access to the appropriate services, facilities, infrastructure, or equipment to deliver the proposal
- provide a detailed and comprehensive project plan including milestones and timelines in the form of a Gantt chart or similar
- include a detailed and appropriate plan for how you will acquire and manage data
Images embedded within must be:
- in JPEG, JPG, JPE, JFI, JIF, JFIF, PNG, GIF, BMP or WEBP format
- be smaller than 8MB
Word count: 4,000
Section: Governance
Question: How will you manage the centre to successfully deliver its objectives?
Explain how the proposed centre will be managed, demonstrating that your centre:
- will be effectively governed, including details about advisory groups
- will be effectively and inclusively managed, demonstrated by a clear management plan
- has clear leadership team roles and responsibilities
- will manage and encourage partnerships with non-HEI organisations across government, industry and civil society
- has plans for monitoring your progress against your logic model (included in the Approach section), as well as self-evaluation throughout the lifetime of your award
Section: Capacity building
Question: What do you think the capacity-building needs associated with this research challenge are and what is your approach to address them?
What the assessors are looking for in your response:
Explain your approach to and plans for building capability, including how you will:
- support careers and capacity building, in line with the challenge area
- demonstrate how you will enhance equality, diversity and inclusion across career stages and job roles in your Centre
- demonstrate how you will support all career stages, pathways and types
- demonstrate how you will add value by convening and aligning existing training activity across the UK
- demonstrate how you will share good practice in skills and career development
- explain what the skills needs are in the challenge area in context of activities already on offer either within participating research organisations or nationally and justify how you are going to address them
- identify your intended training, careers and capacity building outcomes, actions to achieve these, and the relevant timescales, success criteria and evidence for each outcome.
Within this section we also expect you to:
- Proposed start and end date of the studentship(s)
- Duration of each studentship in years
- Name of the student(s), if known at the point of application
- Name of main supervisor
- Details of the accredited subject area in which the student will be based
- Confirmation that the DTP/CDT Director supports the Proposed studentship arrangements
- A summary statement of the PhD topic(s) to be undertaken and a justification for the length of the programme of study
- A clear statement of how this is independent from, but will add value to, the principal research objectives set out in the application
- A letter of support from the DTP Director should be submitted with this application. A letter should be within one side of A4 per student.
Word count: 2,000
Section: References
Question: List the references you’ve used to support your application.
Ensure your application is a self-contained description. You can provide hyperlinks to relevant publications or online resources. However, assessors are not obliged to access the information they lead to or consider it in their assessment of your application. You must not include links to web resources in order to extend your application. If linking to web resources, to ensure the information’s integrity is maintained include, where possible, persistent identifiers such as digital object identifiers.
Section: Applicant and team capability to deliver
Question: Why are you the right individual or team to successfully deliver the proposed work?
Evidence of how you, and if relevant your team, have:
- the relevant experience (appropriate to career stage) to deliver the proposed work
- the right balance of skills and expertise to cover the proposed work
- the appropriate leadership and management skills to deliver the work and your approach to develop others
- contributed to developing a positive research environment and wider community
The word count for this section is 3,000 words, 2,000 words to be used for R4RI modules and, if necessary, a further 1,000 words for Additions.
Use the Résumé for Research and Innovation (R4RI) format to showcase the range of relevant skills you, and if relevant your team (investigators, researchers, other (technical) staff for example research software engineers, data scientists and so on, and partners), have and how this will help to deliver the proposed work. You can include individuals’ specific achievements but only choose past contributions that best evidence their ability to deliver this work.
Complete this section using the R4RI module headings listed below. You should use each heading once and include a response for the whole team, see the UKRI guidance on R4RI . You should consider how to balance your answer, and emphasise where appropriate the key skills each team member brings:
- contributions to the generation of new ideas, tools, methodologies, or knowledge
- the development of others and maintenance of effective working relationships
- contributions to the wider research and innovation community
- contributions to broader research or innovation users and audiences and towards wider societal benefit
Additions: Provide any further details relevant to your application. This section is optional and can be up to 1,000 words. You should not use it to describe additional skills, experiences or outputs, but any factors that provide context for the rest of your R4RI (for example, details of career breaks if you wish to disclose them).
You should complete this as a narrative and you should avoid CV type format.
Word count 3,000
Section: Your organisation’s support
Question: Provide details of support from your research organisation.
Provide a statement of support, no more than two sides of A4, from your research organisation detailing why the proposed work is needed. This should include details of any matched funding that will be provided to support the activity and any additional support that might add value to the work.
The committee will be looking for a strong statement of commitment from your research organisation.
We recognise that in some instances, this information may be provided by the Research Office, the Technology Transfer Office (TTO) or equivalent, or a combination of both.
You must also include the following details:
- a significant person’s name and their position, from the TTO or Research Office, or both
- office address or web link
Upload details are provided within the service on the actual application.
Word count: 10
Section: Project partners
Question: Provide details about any project partners’ contributions using the template provided.
Download and complete the Project partner contributions template (DOCX, 52KB) . Paste the completed table into the funding service.
Each letter or email you provide should:
- confirm the partner’s commitment to the project
- clearly explain the value, relevance, and possible benefits of the work to them
- describe any additional value that they bring to the project
- be no more than one side of A4
Save letters or emails of support from each partner in a single PDF no bigger than 8MB. Unless specially requested, please do not include any personal data within the attachment.
For the file name, use the unique funding service number the system gives you when you create an application, followed by the words ‘Project partner’.
If the attachment does not meet these requirements, the application will be rejected.
The Funding Service will provide document upload details when you apply. If you do not have any project partners, you will be able to indicate this in the Funding Service.
Ensure you have prior agreement from project partners so that, if you are offered funding, they will support your project as indicated in the contributions template.
For audit purposes, UKRI requires formal collaboration agreements to be put in place if an award is made.
Do not provide letters of support from host and project co-leads’ research organisations.
Word count: 1,500
Section: Facilities
Question: Does your proposed research require the support and use of a facility?
If not, enter N/A into the text box, mark this section as complete and move on to the next section.
If you will need to use a facility (including access to, and use of data, infrastructure and resources) you should follow your proposed facility’s normal access request procedures. Where prior agreement is required, ensure you obtain their agreement that, should you be offered funding, they will support the use of their facility on your project. ESRC encourages the use of secondary and linked datasets.
In the text box below, for each requested facility you should provide:
- the name of facility, copied and pasted from facility information list (DOCX, 35KB)
- the proposed usage or costs, or costs per unit where indicted on that list
- confirmation you have their agreement where required
Do not put the facility contact details in your response.
Word count: 250
Section: Data management
Question: How will you manage and share data collected or acquired through the proposed research?
Provide a data management plan which should clearly detail how you will comply with ESRC’s published Research Data Policy , which includes detailed guidance notes.
If you are not generating new data as part of your grant application, you are not required to complete this section. Please enter ‘N/A’ in the text box, mark this section as complete and move to the next question.
We recognise the importance of research data quality and provenance. Research data generated by ESRC-funded research must be well-managed by the grant holder to enable their data to be exploited to the maximum potential for further research.
Using the text box below you should:
- describe how you will publish your research findings ,
- demonstrate that you comply with ESRC’s Research Data Policy and ESRC Framework for Research Ethics . This should include confirmation that existing datasets have been reviewed and why currently available datasets are inadequate for the proposed research. You should cover any legal and ethical considerations of collecting, releasing or storing the data, including consent, confidentiality, anonymisation, security and other ethical issues.
- explain how data collected, generated or acquired through the proposed research (such as primary input into research and first order results of that research) will be managed, including planning for the research through the life cycle of the award until data is accepted for archiving by the UK Data Service (UKDS). See the importance of managing and sharing data on the UKDS website for further information. Detailed advice on what assessors are looking for in your response can also be found on the UKDS site . We expect you to provide a summary of the points provided.
- critically consider any challenges to data sharing (e.g., copyright or data confidentiality), with possible solutions discussed to optimise data sharing. Most data collected, generated or acquired as a result of economic and social research can be successfully archived and shared. However, some research data are more sensitive than others. It is a responsibility of the grant holders to consider all issues related to confidentiality, ethics, security and copyright before initiating the research.
Word count: 500
Section: Ethics and responsible research and innovation (RRI)
Question: What are the ethical or RRI implications and issues relating to the proposed work? If you do not think that the proposed work raises any ethical or RRI issues, explain why.
Demonstrate that you have identified and evaluated the relevant ethical or responsible research and innovation considerations, and how you will manage them.
All proposals have to comply with the ESRC Framework for Research Ethics which includes guidance for applicants and links to related web resources.
All necessary ethical approvals must be in place before the project commences, but do not need to have been secured at the time of application.
If you are generating new data as part of your project, you should complete the Data Management question and should cover ethical considerations relating to data in your response.
If you are not generating new data and have not completed the Data Management question you should address any legal or ethical considerations relating to your use of data here.
Additional sub-questions (to be answered only if appropriate) relating to research involving:
- human participants
Section: Research involving the use of animals
Question: Does your proposed research involve the use of vertebrate animals or other organisms covered by the Animals Scientific Procedures Act?
If not, enter ‘N/A’ into the text box, mark this section as complete and do the same for the next question.
If you are proposing research that requires using animals, write ‘Yes’ in the text box. Then, download and complete the animal research question template (DOCX, 74KB) , which contains all the questions relating to research using vertebrate animals or other Animals (Scientific Procedures) Act 1986 regulated organisms. Then, save it as a PDF and upload to your application. Unless specifically requested, do not include any personal data within the attachment.
Section: Conducting research with animal overseas
Question: Will any of the proposed animal research be conducted overseas?
If not, enter ‘N/A’ in the text box, mark as complete and move to the next question.
If you are proposing to conduct overseas research, it must be conducted in accordance with welfare standards consistent with those in the UK, as per Responsibility in the Use of Animals in Bioscience Research , on page 14.
You should also ensure all named applicants in the UK and overseas are aware of this requirement and provide a statement below to confirm that:
- all named applicants are aware of the requirements and have agreed to abide by them
- this overseas research will be conducted in accordance with welfare standards consistent with the principles of UK legislation
- the expectation set out in ‘ Responsibility in the Use of Animals in Bioscience Research’ will be applied and maintained
- appropriate national and institutional approvals are in place.
Overseas studies proposing to use non-human primates, cats, dogs, equines or pigs, will be assessed during NC3Rs review of research proposals. The required information should be provided by completing the template from the question ‘Research Involving the use of animals’.
For studies involving other species listed below, you should select the relevant checklist or checklists from the list below, complete it and save it as a PDF and use the file upload feature to attach. If you need to complete more than one checklist, you should merge them into a single document and then save it as a PDF before uploading it:
- Additional questions on the use of rodents overseas (DOCX 49.1KB)
- Additional questions on the use of rabbits overseas (DOCX 49.2KB)
- Additional questions on the use of sheep overseas (DOCX 50.9KB)
- Additional questions on the use of goats overseas (DOCX 47.3KB)
- Additional questions on the use of pigs overseas (DOCX 51.4KB)
- Additional questions on the use of cattle overseas (DOCX 57.0KB)
- Additional questions on the use of Xenopus laevis and Xenopus tropicalis overseas (DOCX 57.2KB)
Word count: 700
Section: Research involving human participation
Question: Will the project involve the use of human subjects or their personal information?
If not, enter ‘N/A’ into the text box, mark this section as complete and move on to the next section.
If you are proposing research that requires the involvement of human subjects, provide the name of any required approving body and whether approval is already in place. Then, justify the number and the diversity of the participants involved, as well as any procedures.
Provide details of any areas of substantial or moderate severity of impact.
Word count: 700
Section: Research involving human tissues or biological samples
Question: Does your proposed research involve the use of human tissues, or biological samples?
If you’re answering ‘yes’, provide the name of any required approving body and whether approval is already in place.
You should justify the use of human tissue or biological samples specifying the nature and quantity of the material to be used and its source.
Section: Resources and cost justification
Question: What will you need to deliver your proposed work and how much will it cost?
Justify the application’s more costly resources, in particular:
- project staff
- significant travel for field work or collaboration (but not regular travel between collaborating organisations or to conferences)
- any equipment that will cost more than £10,000
- any consumables beyond typical requirements, or that are required in exceptional quantities
- all facilities and infrastructure costs
- all resources that have been costed as ‘Exceptions’
Assessors are not looking for detailed costs or a line-by-line breakdown of all project resources. Overall, they want you to demonstrate how the resources you anticipate needing for your proposed work:
- are comprehensive, appropriate, and justified
- represent the optimal use of resources to achieve the intended outcomes
- maximise potential outcomes and impacts
Additionally, where relevant you should explain:
- support for activities to either increase impact, for public engagement, knowledge exchange or to support responsible innovation
- support for access to facilities, infrastructure or procurement of equipment
- support for preserving, long-term storage, or sharing of data
- support from your organisation or partner organisations and how that enhances value for money
- support for activities outsourced to a third party (such as consultancy or social surveys)
- support for project co-leads under our international, business and third sector eligibility policies
- evidence that environmental sustainability has been considered and reflected in your proposed resource and justified appropriately
For detailed guidance on eligible costs please see the ESRC Research Funding Guide .
How we will assess your application
Assessment process.
This is the second stage of the assessment process, and proposals have already been shortlisted by an outline panel.
The full stage proposals will be assessed using the following process:
Peer Review
Full stage proposals will be subject to external review. You will have an opportunity to respond to reviewer comments before the proposals are discussed by an assessment panel
Assessment Panel
Proposals will be shortlisted at an assessment panel in March 2024
If shortlisted you will be invited to interview in April 2024, after which funding decisions will be made. You will be notified of decisions in May 2024.
The primary assessment criteria are those under the Vision section, however panel members will be guided to take account of all the assessment criteria in deciding which proposals to recommend for funding.
We will give feedback with the outcome of your application
Principles of assessment
We support the San Francisco declaration on research assessment and recognise the relationship between research assessment and research integrity.
Find out about UKRI principles of assessment and decision making .
We reserve the right to modify the assessment process as needed.
Assessment criteria – full stage
The criteria we will assess your application against can be found in the How to apply section under ‘What the assessors are looking for in your response’.
Assessors will refer to criteria under these headings only:
- Applicant and team capability to deliver
- Capacity-building
- Resources and cost justification
- Ethics and Responsible Research and Innovation (RRI)
Contact details
Get help with your application.
For help on costings and writing your application, contact your research office. Allow enough time for your organisation’s submission process.
Ask about this funding opportunity
Email: [email protected]
Phone: 01793 547490
Our phone lines are open Monday-Thursday 8:30am to 5:00pm and Friday 8:30am to 4:30pm
Questions about eligibility
Read UKRI’s research organisation and applicant eligibility guidance.
Sensitive information
If you, or a key team member, need to tell us something you wish to remain confidential, email the Funding Service helpdesk on [email protected] You must include in the subject line: <ESRC Centres, sensitive info, Funding Service application number>
Typical examples of confidential information include:
- applicant is unavailable until a certain date (for example due to parental leave)
- declaration of interest
- additional information about eligibility to apply that would not be appropriately shared in the Applicant and team capability section
- conflict of interest for UKRI to consider in reviewer or panel participant selection
For information about how UKRI handles personal data, see UKRI’s privacy notice .
Additional info
Supporting documents.
Copy web page from the centre in climate change and health outline stage (PDF, 246KB)
Equality impact assessment (PDF, 345KB)
Logic model guidance (PDF, 101KB)
- 31 August 2023 Word count added to the 'Ethics and responsible research and innovation (RRI)' section in the 'How to apply' section.
- 31 July 2023 Opening date amended from 28 July 2023 to 4 August 2023; closing date amended from 31 October 2023 to 7 November 2023.
This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services .

- Faculty of Arts, Humanities and Cultures
- School of Fine Art, History of Art and Cultural Studies
Sardines — BA Fine Art Degree Show 2024
School of Fine Art, History of Art and Cultural Studies event Thursday 6 June 2024 - Sunday 16 June 2024

- Date : Thursday 6 June 2024, 10:00 – 18:00
- Location : Fine Art, History of Art and Cultural Studies
- Interval : Every day
- Cost : Free
- Download : Outlook, iCal
Sardines showcases the works of 43 artists from the University of Leeds who have navigated their academic journey in busy shared studios and collaborative conditions.
Through a diverse array of mediums and expressions, the exhibition reveals the paths these artists are taking from their mutual experiences as students to the world beyond university.
The artists behind Sardines have formed a tight, vibrant community of individuals through creating art together. They have collectively curated an exhibition that playfully celebrates the ways of making and the range of experiences that come from working side by side within shared spaces.
Artworks are united under one roof through the student-led curation of this group show, whilst exploring diverse themes such as satire, protest, femininity, surveillance, extended realities, play and identity.
Sardines offers a chance for you to see what this generation of artists care about and where they are headed. From provocative paintings to immersive installations, no two artworks are alike yet all represent small fish ready to dive into the big pond.
Join us to witness the future of art as these emerging artists prepare to make a big splash.

Abby Colclough • Alex Neish • Amelia Bird • Annabel Adern • Ava Da Costa Freeman • Charlotte Aldred • Charlotte Hullah • Daisy Grange • Danni Yao • Ed Green • Eleanor Marshall • Elizabeth Wilkinson • Ella Georgiou • Emilia Bryant • Eris Guilliam • Fergus Thomas • Florence Bennett • Francesca Scott • Georgia Bennett • Georgia Jones • Hannah Guy • Ho Pao • Imogen Climo • Jazmin Camilleri • Jessica Bills • Jiayun Li • Jun Rui Lo • Livia Garrod • Lucas Mitchell • Mathilda Doubleday • Mia Jones • Michael Grayshon • Millie Porritt • Naomi Marchbank • Niamh Warren • Nina Jurewicz • Rhiannon Piper • Ruth Dodgson • Shuyuan Yang • Skye Davies • Tiago Beltrão • Wufei Fan • Zara Sajovic
Find out more about the artists.
Opening times
The exhibition is open daily from 6 to 16 June, 10am to 6pm.
Sardines launches with a Private View on Thursday 6 June, 6 to 9pm. If you would like to attend, please RSVP to [email protected]
School of Fine Art, History of Art and Cultural Studies University of Leeds University Road Leeds LS2 9JT
How to find us.
More information
See the Sardines website for further details about the BA Fine Art Degree Show and the artists taking part.
Follow the show on Instagram @sardinesshow
Find out more about the School of Fine Art, History of Art and Cultural Studies.
Designs by final year BA Fine Art students at the University of Leeds.
Related events
See all School of Fine Art, History of Art and Cultural Studies events
Pre-sessional study at Leeds student panel
School of Fine Art, History of Art and Cultural Studies - Thursday 30 May 2024
Make your Masters a reality with funding
School of Fine Art, History of Art and Cultural Studies - Wednesday 5 June 2024
Symposium: What is Furniture History?
School of Fine Art, History of Art and Cultural Studies - Thursday 6 June 2024

IMAGES
COMMENTS
Journals, publications and data. Explore NIHR-supported research projects, their findings and data from our range of resources: Sign up for news, announcements, events and more. The nation's largest funder of health and care research, providing the people, facilities and technology for research to thrive.
The Medical Research Council (MRC) provides funding to research institutes, units and centres across the UK. MRC has a mission to support research and training with the aim of maintaining and improving human health. To address important scientific opportunities and health needs, and when stand-alone grant support alone is insufficient, our ...
Website. www .nihr .ac .uk. The National Institute for Health and Care Research ( NIHR) is the British government's major funder of clinical, public health, social care and translational research. [3] With a budget of over £1.2 billion in 2020-21, [4] its mission is to "improve the health and wealth of the nation through research". [5]
The Medical Research Council (MRC) provides funding to the research institutes, units and centres listed below. Institutes. Laboratory of Molecular Biology; Laboratory of Medical Sciences; Health Data Research UK; UK Dementia Research Institute; The Francis Crick Institute; Centres and Units Infections and Immunity Board. Units:
The Medical Research Council (MRC) is responsible for co-coordinating and funding medical research in the United Kingdom.It is part of United Kingdom Research and Innovation (UKRI), which came into operation 1 April 2018, and brings together the UK's seven research councils, Innovate UK and Research England. UK Research and Innovation is answerable to, although politically independent from ...
NIHR Biomedical Research Centres (BRCs) bring together academics and clinicians to translate early scientific breakthroughs into potential new treatments, diagnostics and health technologies. The NIHR has awarded nearly £800m in funding to facilities across England, creating an environment where experimental medicine can thrive.
The Global Health Research Centres programme will hold its first funding call in 2020. See the dates for our funding calls. Call 1 - Non-communicable diseases (2020) Call 1 will fund and designate NIHR Global Health Research Centres in non-communicable diseases. Call 1 was launched on 14 October 2020. The centres awarded funding was announced ...
The Health Economics Research Centre (HERC) HERC was established by the University of Oxford in 1996. Our aim is to contribute to health and healthcare in the UK and internationally, by conducting research on economic aspects of health and disease, the costs and benefits of prevention and treatment, and the design and evaluation of health ...
BHF Data Science Centre; Data Saves Lives: Mental Health; HDR Global; Health Data Research Hubs; Population Research UK; The Sudlow Review; Study and Train. Study; Train; ... The Health Data Research UK (HDR UK) Big Data for Complex Disease Driver Programme has awarded six prestigious fellowships, with a total of £1.8 million funding. ...
The Health eResearch Centre is a world-leading digital health network that unites and provides data-intensive research and education across Northern England and beyond. At the Health eResearch Centre (HeRC) we bring together a multi-disciplinary team of researchers, developers and clinicians to understand more about how under-used health data ...
Last updated: 31 March 2022. This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services. By harnessing health and biomedical data in the UK, Health Data Research UK (HDR UK) will develop and apply cutting edge data.
The NIHR HealthTech Research Centre in Sustainable Innovation (HRC) is a partnership between the Royal Devon University Healthcare NHS Foundation Trust and the University of Exeter, designed to streamline the development pipeline from prototype to commercial product. About us. The HRC is one of 14 newly established research centres across ...
The findings of the UKCRC Public Health Research Strategic Planning Group (SPG) are documented in a report, Strengthening Public Health Research in the UK. The outcome of the SPG was a commitment of over £20m by a consortium of eight funding partners to create UKCRC Public Health Research Centres of Excellence in 2008.
The Centre for Mental Health Research carries out collaborative research to improve the quality of mental health care. We conduct high quality research that aims to improve the delivery and experience of mental health care across a range of service settings and contexts. At the heart of this research is people with lived experience of using ...
Babraham Institute. Beatson Institute for Cancer Research. BirdLife International. British Broadcasting Corporation (BBC) British Film Institute. British Institute of International and Comparative ...
Directory of research centres at City, University of London. Our centres produce world-leading research in a wide range of fields. ... Department of Health Services Research and Management. Health Services Management at City. ... (Bayes CCE) is a leading nonprofit and philanthropy centre in the UK. School: Bayes Business School . Previous page ...
The NIHR has awarded almost £42 million (£41,790,690) to establish 14 new centres across England that will drive life-changing research into health technologies. From 1 April 2024, the 14 new NIHR HealthTech Research Centres (HRCs) will work with businesses to support the development of medical devices, diagnostics and digital technologies.
HSR UK and the Health Foundation Join Forces to Foster Inclusivity and Opportunity in Health and Care Services Research. HSR UK is delighted to announce an exciting partnership with the Health Foundation, a leading independent charitable o…. More. Viewpoint 22 Feb 2024 Bringing Health and Care Workforce Research into Practice. Viewpoint 19 ...
We are an independent healthcare solutions company with global cancer prevention ambitions. We work with organisations across the world to design and implement practical and scalable health interventions. We use innovative approaches for a sustainable, positive impact on peoples' lives. At CHRE, we work with organisations and funding bodies ...
The National Institute for Health and Care Research (NIHR) has awarded an NIHR HealthTech Research Centre (HRC) Network £5m to provide national coordination and leadership for NIHR HRCs to advance health innovations. ... "Working in partnership with all 14 HRCs will be key to delivering the network's ambitions in transforming UK HealthTech ...
They will generate research to improve the health of communities across the UK, reduce health inequalities, and develop and evaluate effective, long-lasting and environmentally sustainable interventions. Units and centres. MRC supports the following units and centres, which study the various determinants of population health:
The NIHR School for Public Health Research held its tenth Annual Scientific Meeting (ASM) 16-17th May hosted by PHRESH at the Edgbaston Park Hotel and Conference Centre in Birmingham. More than 190 delegates attended including researchers, public partners and public health professionals. The meeting had more than 60 speakers. There were insightful talks from six distinguished keynote ...
The British Heart Foundation (BHF) has awarded £3 million to the University of Leicester to support its world-class cardiovascular disease research over the next five years. The University of Leicester has pledged to match the award from the BHF, with additional funding from the University Hospitals of Leicester, taking the total investment in ...
The aims of the NIHR HealthTech Research Centres are to: catalyse innovation in the development of new HealthTech for areas of unmet need, areas of high disease burden, and/or improve efficiencies in the health and care system. provide expertise to evaluate innovations and generate appropriate evidence to support uptake into the health and care ...
LSHTM researchers, Professor Chris Drakeley, Dr. Rachel Lowe and Isabel Byrne from the Malaria Centre and the Centre on Climate Change and Planetary Health (CCCPH), are part of the World Health Organization's Task Team on Climate Change, Neglected Tropical Diseases (NTDs), and Malaria, in partnership with Reaching the Last Mile (RLM).
Background In healthcare, regulation of professions is an important tool to protect the public. With increasing regulation however, professions find themselves under increasing scrutiny. Recently there has also been considerable concern with regulator performance, with high profile reports pointing to cases of inefficiency and bias. Whilst reports have often focused on large staff groups, such ...
UK Collaboratorium for Research in Infrastructure and Cities. Learn how we're supporting work to develop a world-class national infrastructure capability. This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services.
Introduction. Improving the provision of, and engagement with, community services for people affected by dementia remains both a national health goal in the UK and a global one (ADI, Citation 2022; DHSC, Citation 2022; WHO, Citation 2017).With the number of people with dementia predicted to grow significantly between now and 2050 (Luengo-Fernandez & Landeiro, Citation 2023), this is a pressing ...
Apply for funding to establish a centre in climate change and health. This centre will be a world-leading centre of excellence carrying out interdisciplinary, cutting-edge, and impactful research to address major challenges at the interface of climate and health research. ... You must be based at a UK research organisation eligible for UK ...
If you would like to attend, please RSVP to [email protected]. Venue. School of Fine Art, History of Art and Cultural Studies University of Leeds University Road Leeds LS2 9JT. How to find us. More information. See the Sardines website for further details about the BA Fine Art Degree Show and the artists taking part. Follow the show on ...