Advertisement

Urban forest invertebrates: how they shape and respond to the urban environment
- Open access
- Published: 19 May 2022
- Volume 25 , pages 1589–1609, ( 2022 )
Cite this article
You have full access to this open access article

- D. Johan Kotze ORCID: orcid.org/0000-0003-4211-4420 1 ,
- Elizabeth C. Lowe ORCID: orcid.org/0000-0003-3792-3178 2 ,
- J. Scott MacIvor ORCID: orcid.org/0000-0002-2443-8192 3 ,
- Alessandro Ossola ORCID: orcid.org/0000-0002-0507-6026 2 , 4 , 7 ,
- Briony A. Norton ORCID: orcid.org/0000-0001-9354-5904 5 ,
- Dieter F. Hochuli ORCID: orcid.org/0000-0002-6673-4475 6 ,
- Luis Mata ORCID: orcid.org/0000-0002-4142-8666 7 ,
- Marco Moretti ORCID: orcid.org/0000-0002-5845-3198 8 ,
- Sara A. Gagné 9 ,
- I. Tanya Handa ORCID: orcid.org/0000-0002-7272-031X 10 ,
- Therésa M. Jones ORCID: orcid.org/0000-0002-5300-0018 11 ,
- Caragh G. Threlfall ORCID: orcid.org/0000-0002-4197-8588 12 &
- Amy K. Hahs ORCID: orcid.org/0000-0003-0163-6732 7
11k Accesses
18 Citations
Explore all metrics
Invertebrates comprise the most diversified animal group on Earth. Due to their long evolutionary history and small size, invertebrates occupy a remarkable range of ecological niches, and play an important role as “ecosystem engineers” by structuring networks of mutualistic and antagonistic ecological interactions in almost all terrestrial ecosystems. Urban forests provide critical ecosystem services to humans, and, as in other systems, invertebrates are central to structuring and maintaining the functioning of urban forests. Identifying the role of invertebrates in urban forests can help elucidate their importance to practitioners and the public, not only to preserve biodiversity in urban environments, but also to make the public aware of their functional importance in maintaining healthy greenspaces. In this review, we examine the multiple functional roles that invertebrates play in urban forests that contribute to ecosystem service provisioning, including pollination, predation, herbivory, seed and microorganism dispersal and organic matter decomposition, but also those that lead to disservices, primarily from a public health perspective, e.g., transmission of invertebrate-borne diseases. We then identify a number of ecological filters that structure urban forest invertebrate communities, such as changes in habitat structure, increased landscape imperviousness, microclimatic changes and pollution. We also discuss the complexity of ways that forest invertebrates respond to urbanisation, including acclimation, local extinction and evolution. Finally, we present management recommendations to support and conserve viable and diverse urban forest invertebrate populations into the future.
Similar content being viewed by others
When is a forest a forest forest concepts and definitions in the era of forest and landscape restoration.

Urban biodiversity: State of the science and future directions
Species richness and ecological connectivity of the mammal communities in urban and peri-urban areas at Mexico City
Avoid common mistakes on your manuscript.
Introduction
Terrestrial invertebrates, including insects, mites, spiders, millipedes, centipedes, snails and earthworms amongst many others, comprise the most diversified animal group on Earth, accounting for as much as 80% of all known terrestrial animal species (Zhang 2011 ). They are characterised by the lack of an internal bone skeleton, instead showing a remarkable breadth in body plans ranging from hard exoskeletons (e.g., insects, particularly beetles) to hydrostatic skeletons (e.g., earthworms). Invertebrates occupy a vast range of ecological niches and microhabitats across terrestrial ecosystems and play an important role in structuring networks of mutualistic and antagonistic ecological interactions in almost all terrestrial ecosystems (Ings et al. 2009 ), thus contributing to key ecosystem services including pollination, nutrient recycling and pest control (Noriega et al. 2018 ). For these reasons alone, terrestrial invertebrates have long been recognised as ‘the little things that run the world’ (Wilson 1987 ).
Given their role in physically shaping the environment in which they live, and their capacity to change the availability of resources for other species, invertebrates can be considered as “ecosystem engineers” (Jones et al. 1994 ). While some invertebrates are intentional engineers that directly alter their environment (e.g., ants and termites who create a network of above- and belowground nests), other species are accidental engineers that modify habitat indirectly or as a by-product of their activities (e.g., earthworms that create temporary tunnels as they move through the soil and provide additional nutrients in the form of excretions).
Here we focus on the role of invertebrates in shaping urban forests and in providing key ecosystem functions and underlying services. We define urban forests as self-regenerating communities with a tree canopy that exist as remnant patches of formerly contiguous habitat now surrounded by urban areas, as well as communities composed of planted trees, such as those in gardens, parks and along streets (see Alvey 2006 ). Urban forests in their most complex form are composed of an upper tree canopy, one or more understory layers, a groundcover layer and soil layers (Jim 2017 ). They are also one of the dominant types of natural areas in cities ‒ by one estimate, remnant forests account for 68% of parkland area across the most populous cities in the USA (Pregitzer et al. 2021 ). There is a large amount of variation in the structure and species composition of urban forests, ranging from structurally complex native forest patches to highly simplified systems dominated by a few exotic species (Threlfall et al. 2016 ). In many contexts, urban forests constitute novel ecosystems (sensu Hobbs et al. 2006 ), where plants and animals that do not share an evolutionary history now co-exist, including many invasive species. Urban forests provide a plethora of benefits to citizens, including environmental ecosystem services, i.e., the regulation of air, water, soil and climate, the provision of habitat and other goods and services (Roeland et al. 2019 ) and cultural ecosystem services, including physical, psychological and social health benefits and economic development (Nesbitt et al. 2017 ). Invertebrates play a critical role in the functioning of urban forests and therefore in providing these benefits to urban inhabitants.
In this review, we describe the many roles invertebrates play in structuring and maintaining the functioning of urban forests and argue for the conservation of urban forests and their invertebrates in the Anthropocene, the current epoch characterised by climate change, urban densification and habitat loss, and the apparent mass loss of invertebrate biomass worldwide (see Hallmann et al. 2017 ; Sánchez-Bayo and Wyckhuys 2019 ; Cardoso et al. 2020 ; Harvey et al. 2020 , but see Macgregor et al. 2019 ; Crossley et al. 2020 ). The review covers four broad subject areas related to urban forest invertebrates, with the aim of generating a compendium of evidence to be used in the study, planning and management of urban forests. The subject areas are: 1) the diversity and complexity of invertebrate communities in urban forests with a focus on their roles in the provisioning of various ecosystem services and their contributions to people (Díaz et al. 2018 ); 2) the major ecological filters affecting species assemblages in urban areas; 3) our current understanding of how urban forest invertebrates respond to these filters and the consequences of these responses for ecosystem service provisioning; and 4) how we can incorporate invertebrates into urban design and management to deliver healthier and more taxonomically and functionally resilient urban forests for the future. This review focuses on evidence in the literature of forest invertebrate community composition and change in human-modified landscapes.
Functional roles of invertebrates in urban forests
Urban forests contain terrestrial and aquatic systems that support invertebrates and their complex and varied life cycle requirements (Wilbur 1980 ). Our focus is primarily on terrestrial and semi-aquatic invertebrates that occupy the different strata within urban forests, from belowground, to ground level, to understory, sub-canopy and canopy. Terrestrial invertebrates contribute to an array of ecosystem functions (Scudder 2009 ), which translate into a multitude of services for humans (Prather et al. 2013 ), but also disservices (Dunn 2010 ), collectively termed nature’s contribution to people (NCP) (Díaz et al. 2018 ). In this section, we explore the contributions of invertebrates to urban forests and how these forests support invertebrates performing diverse functional roles, recognising that some species may perform different and multiple functions depending on life cycle stage and that their functions in an ecosystem may change over the course of their life. For instance, both holometabolous (complete metamorphosis) and hemimetabolous (partial metamorphosis) insects can experience remarkable ecological niche shifts while transitioning between larval/nymph and adult life stages, e.g., from herbivorous caterpillars to pollinating butterfly and moth adults, or from predacious aquatic nymph to predacious aerial dragonflies.
Pollination
Pollination refers to the exchange of genetic material between plants via reproduction and is a critical process in the ongoing recruitment of new generations for many plant species. Urban forests are a significant habitat resource for pollinators, which primarily include bees (Anthophila), flies (Diptera), and butterflies and moths (Lepidoptera). For example, wild bee communities in remnant forests are stratified vertically in the forest canopy (Urban-Mead et al. 2021 ) and contain unique species unable to persist in surrounding built-up areas (Harrison et al. 2018 ; Landsman et al. 2019 ). Similarly, urban parks that contain patches of remnant forest host more butterfly species, including woodland specialist species, than parks that contain only planted vegetation (e.g., Kitahara and Fujii 1997 ). However, planted trees (including exotics) can also be important to pollinators (Buchholz and Kowarik 2019 ), confirmed by the barcoding of pollen sampled from four bee species in five different EU cities (Müller 2021 ). Additionally, pollinators supported by urban forests provide pollination services both within these forests and to surrounding urban and rural habitats.
Urban forests provide significant nesting resources for social and solitary wild bees. For instance, social bumblebees will forage in private or community gardens, but queens construct nests in the less-disturbed soils of urban parks and forest edges (McFrederick and LeBuhn 2006 ). Many cavity-nesting solitary bees nest in logs, snags and stumps, and some, for instance leaf-cutting bees, collect leaves from a variety of trees and shrubs to partition their brood cells in the nest (MacIvor 2016 ). Many bees, such as species of the genus Xylocopa , depend on dead wood for nesting and are potentially limited by these resources in cities, which are found nearly exclusively in remnant urban forests. More generally, many pollinators rely on a variety of urban land covers to complete their complex life cycles, depending on remnant forest for nesting and flower-rich urban greenspaces for foraging. Consequently, ensuring adequate nesting resources in urban forests will improve pollination in nearby urban greenspaces where it is valued (e.g., in residential and community gardens).
In degraded urban forests, many weedy herbaceous species may be present, which often provide foraging resources for generalist pollinators, including non-native honeybees (Threlfall et al. 2015 ) that might interact with native bees of conservation concern (Colla and MacIvor 2017 ). However, weeds can have extended flowering periods, or flower at different times than native plants, expanding the foraging season for many groups of pollinators or potentially ‘filling the gap’ brought about by climate warming if flowering and fruiting phenologies shift and thereby create periods of low resource availability (Sherry et al. 2007 ). It is therefore important to value and appropriately manage a range of urban forest types, even those perceived as lower quality.
Urban forests also contain many tree and shrub species required by moths and butterflies for oviposition and subsequent offspring development, and the structure of the forest resource in the landscape is important for these taxa. Hardy and Dennis ( 1999 ) showed that the proportion of forest in the urban matrix was positively correlated with butterfly diversity. Similarly, Kurylo et al. ( 2020 ) found that butterfly species richness increased with tree cover across the urban matrix, and Lintott et al. ( 2014 ) found that moth diversity in urban forests increased in larger, older, and less fragmented patches.
Predation is the mechanisms through which populations of more abundant species are regulated by complex top-down trophic interactions. Invertebrate predator–prey interactions are ubiquitous on the forest floor (epigaeic stratum), with the main taxa involved including carabid and rove beetles (Carabidae, Staphylinidae), ants (Formicidae) and spiders (Araneae). These predators exert top-down control on the epigaeic and edaphic (soil) invertebrate communities, including members of their own guild (i.e., intra-guild predation), thus contributing an important top-down ecological process (predation) that structures communities (Niemelä 1993 ; Vidal and Murphy 2018 ). Invertebrate community structure in urban landscapes is, however, different from that in rural landscapes, with a general trend of predacious groups shifting towards smaller-sized species (see Merckx et al. 2018 ), species capable of flight (Niemelä and Kotze 2009 ) and thermophilic species (Piano et al. 2017 ). These differences are in line with the general processes operating in urban landscapes, including habitat fragmentation and degradation and the urban heat-island effect. Furthermore, for the largely predacious carabid beetle taxon, Kotze et al. ( 2012 ) argued that due to a long history of urban forest fragmentation, forest specialist species have all but disappeared from boreal cities, like Helsinki, although some remain in highly specialized habitats in the city, such as bogs (Noreika et al. 2015 ).
Research on the effects of the apparent decoupling of interactions between different trophic levels in urban forests (see Samways et al. 2010 ) is needed to evaluate the functional importance of this dominant epigaeic predatory guild. A non-urban example illustrates the complex effects of epigaeic predators on ecosystem processes: Lawrence and Wise ( 2000 ) showed that the removal of spiders from the forest floor in a secondary oak-hickory-maple forest in Madison County, Kentucky, USA, resulted in increased densities of springtails (Collembola). Yet, rather than an increase in the rate of litter decomposition due to a greater number of springtails, the authors later reported lower decomposition rates in the absence of spiders due, in part, to the release of mesopredators of other potentially important decomposer groups, such as mites (Acari) or flies (Diptera) (Lawrence and Wise 2004 ).
Urban pest populations often flourish when resources such as food or habitat are increased or novel community structures result in decreases in competition and/or predation (Robinson 1996 ). Changes in the climate of urban areas – as well as a lack of natural enemies in the case of exotic species – can facilitate pest outbreaks (Meineke et al. 2013 ) and associated economic consequences (Kovacs et al. 2010 ), such as the northward expansion by the emerald ash borer ( Agrilus planipennis ) into Canadian cities and towns (Herms and McCullough 2014 ) and the hemlock woolly adelgid ( Adelges tsugae ) across the Northeastern USA (Paradis et al. 2008 ). Arthropod pests such as some species of mosquitoes (Culicidae), cockroaches and termites (Blattodea) and beetles (Coleoptera) require extensive management in cities because they threaten stored products, public health or building structures (Rust 2009 ). In urban parks and forests, arthropod pests can damage native vegetation (Ciceoi et al. 2017 ) through elevated levels of herbivory (Christie and Hochuli 2005 ), or negatively affect native animals through predation or competition. However, arthropod pests may be subject to top-down control in urban areas, as evidenced by decreased foliage loss in large cities across Europe as a result of elevated bird predation (Kozlov et al. 2017 ). Increases in urban forest pests are also of concern as they can spread to nearby, more natural landscapes, as was shown for the Asian long-horned beetle, Anoplophora glabripennis (Dodds and Orwig 2011 ).
As indicated above, biological control has the potential to regulate arthropod pests in urban forests, thereby reducing the need for pesticides or other control agents and potentially lowering monetary costs in the long term (Olkowski et al. 1976 ; Kenis et al. 2017 ). The success of biological control in urban areas relies on diverse source populations of natural enemies, resource accessibility and the ability of these organisms to permeate through and persist in the urban matrix (Shrewsbury and Leather 2012 ; Frey et al. 2018 ). For example, urban vegetation fragments can be an important source for biological control agents such as spiders (Lowe et al. 2018 ) and parasitoids (Fenoglio et al. 2013 ), and can increase the diversity of predator communities in nearby urban gardens (Vergnes et al. 2012 ). Increasing supplementary resources for natural enemies within the urban matrix can also increase biological control services (Ellis et al. 2005 ; Egerer et al. 2018 ). However, biological control can be hard to achieve in urban areas as arthropod predator communities are often disrupted, limiting their ability to counter pest populations (Meineke et al. 2014 ; Gardiner and Harwood 2017 ).
Herbivory is the process through which the energy plants capture from the sun is transferred to the next level of organisms, and is therefore an essential process for life on Earth. Invertebrate herbivores are a taxonomically diverse and speciose ecological group, dominated by juvenile and adult stages of moths and butterflies (Lepidoptera), beetles (Coleoptera), bugs (Hemiptera), flies (Diptera) and grasshoppers and crickets (Orthoptera). Some are specialist feeders on certain host plants, while others have the capacity to feed on a wide array of hosts (Forister et al. 2019 ). The sheer diversity and abundance of insect herbivores in urban forests make the interactions between plants and insects a key driver in productivity and nutrient cycling (Hawlena et al. 2012 ).
Collectively, invertebrate herbivores in urban forests are not a homogenous functional group as they employ an extraordinary array of strategies to consume plant material (Strong et al. 1984 ). This variation in foraging strategy has equally varied impacts on plants. For example, herbivory can result in substantial reductions in photosynthetic area, the destruction of reproductive structures such as flowers or seeds and, in some instances, can promote disease if invertebrates themselves are disease vectors (e.g., Dutch Elm Disease, Ophiostoma ulmi and O. novo-ulmi ), or if their herbivory creates entry points for pathogens. In an urban context, herbivory, when out of control (e.g., gypsy moth infestations in Eastern North America [Moeller et al. 1977 ; Schultz and Baldwin 1982 ]), defoliates trees and impacts recreation and the overall appreciation of urban forests (see also the “ Disservices ” section). Therefore, overabundant invertebrate herbivores in urban forests are typically perceived as pests, particularly when the extent of defoliation is severe and the health of the urban forest is compromised (Raupp et al. 2010 ).
The engineering role of herbivorous insects is most apparent during population outbreaks that threaten the persistence of key plant species, especially when outbreaks interact with other disturbances such as fire (Parker et al. 2006 ; Halofsky et al. 2020 ). A range of factors may contribute to elevated levels of herbivorous insects and thus herbivory in urban forests, such as loss of key predators (Hochuli and Threlfall 2018 ) or parasitoids (Peralta et al. 2011 ; Nelson and Forbes 2014 ), changes in landscape structure and configuration (Fenoglio et al. 2012 ; Rossetti et al. 2017 ) and microclimate (Meineke et al. 2013 ; Dale and Frank 2017 ). Mechanisms driving the population ecology of insect herbivores remain a key frontier in identifying how their impacts in urban forests can be assessed (see “ Invertebrate responses to urban environments ” section) and managed (see “ Managing urban forests for invertebrates ” section).
Dispersal of seeds and microorganisms
As plants and microbes are sessile, their main mechanism for movement into new locations is through the dispersal of seeds, spores and other propagules. While some ant species are known for playing an important role in seed dispersal in urban forests (Thompson and Mclachlan 2007 ), there is emerging evidence that seeds are also dispersed by other insect taxa such as hornets ( Vespa spp.) (Chen et al. 2017 ), crickets (Grylloidea) (Suetsugu 2020 ) and dung beetles (Scarabaeoidea) (Milotić et al. 2019 ). Indeed, there are many examples where plants have co-evolved with invertebrates to such an extent that plants develop specialised structures that enable dispersal by specific taxa (e.g., the elaiosomes on seeds of Acacia spp. that enable dispersal by ants). Yet, such ant-seed dispersal relationships can be disrupted in urban areas, as evidenced by elevated rates of seed dispersal after the restoration of ant communities via urban forest restoration efforts in Sydney, Australia (Lomov et al. 2009 ). Additionally, invertebrates assist with the movement of fungal spores, bacteria and other microorganisms through intentional (e.g., transporting fruiting bodies of fungi) or incidental means (e.g., through digestion and excretion or via surface adhesion) (Bray and Wickings 2019 ). For instance, some beetles act as transport for fungi, moving and injecting significant quantities and diversity of spores into dead wood and thus improving decomposition and accelerating the creation of hollows that provide habitat for other organisms (Seibold et al. 2019 ). The movement and foraging of invertebrate taxa such as earthworms (Grant 1983 ; Milcu et al. 2006 ), ants (Beattie and Culver 1982 ; Christian and Stanton 2004 ; Rowles and O’Dowd 2009 ) and dung beetles (deCastro-Arrazola et al. 2020 ) not only facilitate seed dispersal (and fungal dispersal, see next section) but may be important mediators of germination success and seedling recruitment by protecting seeds from predation and locating seeds in nutrient-rich microsites. Although seed dispersal in urban areas can be a significant driver of urban plant community composition, this interaction remains poorly understood (Cheptou et al. 2008 ; Johnson et al. 2018 ). Supporting urban forest invertebrate communities that provide seed and microorganism dispersal could be critical for the species and genetic diversity of urban organisms.
Organic matter decomposition and soil development
The decomposition of organic matter closes the nutrient cycle loop in urban forests by reducing the accumulation of dead material and returning nutrients back to the soil to become available to plants once again. There are many soil- and litter-dwelling invertebrates who perform these important functions. Macro-detritivores (e.g., earthworms, woodlice and millipedes) break down leaf litter into smaller pieces (comminution) making it accessible to micro-detritivores (e.g., springtails, oribatid mites) and bacteria and fungi (David and Handa 2010 ; Ossola et al. 2017 ). Estimates across various biomes and ecosystems (not including urban forests) show that the presence of complex decomposer communities, including macro-detritivores and their predators, can accelerate both carbon and nitrogen loss on average by 11% (Handa et al. 2014 ). Studies in urban habitats remain scarce and are much needed, but recent studies have confirmed the importance of soil faunal community complexity for litter decomposition in both urban gardens (Tresch et al. 2019a ) and urban forests (Meyer et al. 2020 ).
Some invertebrates burrow into the soil but feed on the forest floor (e.g., anecic earthworms), which allows for the incorporation of organic detritus and nutrients from the surface deep into the soil profile, while promoting soil gas exchange and water infiltration (Ossola et al. 2015a ). In fire-prone urban ecosystems, the removal of large quantities of plant litter from forests by detritivorous invertebrates can decrease fuel loads and fire risk for neighbouring communities (Buckingham et al. 2015 ). An increase in detritivore species richness significantly enhances the process of decomposition in urban greenspaces and urban forests, as shown in urban gardens in Switzerland (Tresch et al. 2019a , b ) and in urban forests in Melbourne, Australia (Ossola et al. 2016 ), despite the latter being dominated by exotic species from Europe. The dominance of exotic detritivore species, however, is not uncommon and numerous species are now ubiquitous in cities worldwide due to trade and the movement of soil and plant material (Tóth et al. 2020 ). For example, historic anthropogenic disturbance, over a century old, best explained the intensity of exotic earthworm invasion in a north-eastern North American peri-urban forest (Beauséjour et al. 2014 ). Exotic detritivorous earthworms in North American forests change plant species composition by favouring non-native plants and reducing the cover of native species (Craven et al. 2017 ) and by reducing the diversity and density of soil invertebrates (Ferlian et al. 2017 ).
Decomposing dead wood, including snags/stags (standing dead trees), old roots and fallen branches, is another important forest resource (e.g., Thorn et al. 2020 ), but not always assessed in urban forest management (Korhonen et al. 2020 ). Wood decomposition is a long process occurring in different parts of a tree and at different stages of its life, thus providing nursery and refuge resources (i.e., a habitat tree, see Bauerle and Nothdurft 2011 ) to many taxa and from different trophic levels. For example, dead wood can provide important habitat to springtail (Collembola) communities (Raymond-Leonard et al. 2020 ). Habitat trees and tree related microhabitats are also particularly important to saproxylic invertebrates, especially jewel beetles (Buprestidae), long-horned beetles (Cerambycidae) and bark beetles (Scolytinae) (Speight 1989 ; Grove 2002 ; Kraus et al. 2016 ) whose larval stage can last up to five years. A specific example is the European stag beetle ( Lucanus cervus ), which often occurs in warm urban deciduous forests (Harvey et al. 2011 ). Saproxylic beetles are key actors in ecosystem processes such as wood decomposition and nutrient cycling (Dajoz 2000 ), and their richness, community composition and genetic diversity depend mainly on tree species identity, decay stage, wood size and volume (Schiegg 2000 ; Brin et al. 2011 ) and distribution (Horák 2011 , 2018 ), as well as on the connectivity and management regime of old trees and woody debris (Vandekerkhove et al. 2013 ). Old trees and woody debris are a critical resource for this group of invertebrates, however these elements are often missing from urban forests due to public safety concerns and aesthetical preferences (Hauru et al. 2014 ; Le Roux et al. 2014 ), threatening the persistence of these animals and the functions they perform.
Many saproxylic invertebrates feed on nectar and pollen as adults, thus the distribution and configuration of floral feeding resources (meadows, flowering bushes and trees) outside urban forests are complementary (e.g., Colding 2007 ) to maintain viable populations within urban forests (Matteson and Langellotto 2010 ). Since saproxylic invertebrates are generally not highly mobile, such floral resources should be in close proximity to decaying wood in urban forests, or should be well connected through green corridors providing feeding resources and resting places (see also the “ Pollination ” section).
Disservices
While biodiversity and nature offer many benefits to people, they can also give rise to negative interactions or consequences that can be considered “disservices”. Some examples include property damage by termites or other wood boring insects (e.g., Xylocopa ), entomophobia (fear of insects) and major outbreaks of pests, both medical and economic. One of the disservices with the most direct consequences for humans occurs when invertebrates transmit diseases that pose a significant risk to public health (Lyytimäki et al. 2008 ). Arthropod-borne diseases are of significant concern in urban landscapes (LaDeau et al. 2015 ), with key groups being mosquitoes (Culicidae) (Lourenço-de-Oliviera et al. 2004 ; Rochlin et al. 2016 ; Murdock et al. 2017 ; Goodman et al. 2018 ) and ticks (Acari) (Maupin et al. 1991 ; Stafford and Magnarelli 1993 ; Frank et al. 1998 ; Uspensky 2017 ). The latter rely on vertebrate hosts also being present in forests; therefore, understanding how the interactions between host, tick, and pathogen are affected by characteristics of the urban environment is essential for reducing public health risk (Ostfeld and Keesing 2017 ). For example, Krystosik et al. ( 2020 ) conducted a systematic review and found that solid waste associated with urban landscapes provided a breeding ground for zoonotic disease hosts (often mammals) and invertebrate transmission vectors. Given the potential of public health risks to shape perceptions and management of urban forests, it is vital that risks be assessed and compared against the benefits that these forests provide to nature and humans alike.
Filters acting on urban forest invertebrates
To understand community assembly of urban forest invertebrates and associated ecosystem functions, we must consider how the urban environment acts as an ecological filter of invertebrate traits (Brousseau et al. 2018 ; Fournier et al. 2020 ). Filters determining urban pools of species act at different temporal and spatial scales. They include both biophysical and biogeographical constraints, as well as broad-scale human factors such as human-mediated species dispersal at a global scale (Swan et al. 2011 ; Aronson et al. 2016 ). Beyond such regional considerations, once a species arrives, they must initially survive the local urban environment to become established. Survival will depend on traits that influence morphology, phenology, physiology and behaviour, enabling individuals to overcome the range of dispersal, abiotic and biotic filters at play in urban landscapes (Brousseau et al. 2018 ). These include urban landscape configuration, development history and human activity and decision-making, as well as interactions among multiple species (Aronson et al. 2016 ). For example, invertebrates in urban forests face abiotic filters such as higher temperatures resulting from heat islands (Arnfield 2003 ; but see Ziter et al. 2019 ) or pollutants such as noise, light or chemicals (Halfwerk and Slabbekoorn 2015 ) compared to non-urban forests.
At the bottom strata, soil-dwelling invertebrates in cities are confronted with a soil matrix of diverse origins that may include rubble or other non-native parent material (Pickett et al. 2011 ). These soils may also exhibit alkaline conditions associated with cement, may be compacted and sealed under impervious surfaces, have contaminants such as salt and heavy metals and be subject to major disruptions such as litter removal (Pickett et al. 2011 ; Szlavecz et al. 2018 ). Such soils are associated with changes in the abundance and composition of belowground invertebrate communities (Santorufo et al. 2012 ) and the composition of aboveground communities (e.g. Do et al. 2014 ). Altered soil conditions may also lead to invertebrate communities with particular traits that enable survival. For example, in a study of Collembola under urban trees in Italy, species most tolerant to filters such as low organic matter and high heavy metal concentrations were smaller, pigmented, sexually reproducing and had a well-developed jumping apparatus (Santorufo et al. 2014 ).
Biotic filters may have equally important, if not greater effects on invertebrate communities (see Kraft et al. 2015 ). The high proportion of non-native plant species in urban areas results in novel resources available to urban forest invertebrates and may influence trophic and non-trophic interactions in these ecosystems (Valentine et al. 2020 ). For instance, non-native trees as sources of organic matter for invertebrates have the potential to influence the community assembly of collembolans (Raymond-Leonard et al. 2018 ) and mites (Malloch et al. 2020 ), and ultimately, litter decomposition rates (Makkonen et al. 2012 ; but see Finerty et al. 2016 ).
Cities are thus home to novel community assemblages, including non-native organisms introduced by humans. Indeed, urban areas can be entry points for invasions, for instance for carabid beetles (Spence and Spence 1988 ) and earthworms (Hendrix et al. 2008 ). Introduced plants may be intentionally selected for the purposes of urban planning, landscaping or other cultural activities by which they provide ecosystem services or disservices; but plant and invertebrate species may also arrive in cities unintentionally (Padayachee et al. 2017 ). For example, the harlequin ladybird ( Harmonia axyridis ) was introduced outside of its native range as a biological control agent, but has now spread to urban areas over several continents (Brown et al. 2011 ). The following paragraphs explore in further detail how various filters may shape invertebrate communities and their associated ecosystem functions.
Changes in habitat structure and vegetation simplification
Invertebrates inhabiting urban forests can be highly sensitive to changes in habitat structure and composition. For instance, leaf litter and wood detritus are both habitat and trophic resources for detritivores and saproxylic organisms, respectively. As such, the removal of leaf litter and dead wood from urban forests can obliterate communities of these specialised invertebrates (Siitonen 2001 ; Vandekerkhove et al. 2013 ; Ossola et al. 2016 ). In highly frequented forests, soil trampling by humans can cause the reduction of burrows and suitable microhabitats on the forest floor, which negatively affects the cover of understorey forest vegetation (Hamberg et al. 2008 ). This alters carabid beetle assemblages compared to less trampled areas, yet the responses of individual species may vary, as many forest specialist species have already been lost from urban forests (Kotze et al. 2012 ). Similarly, when soil becomes compacted or sealed with impervious surfaces, this limits nesting resources for burrowing organisms.
Vegetation structure simplification can lead to a more simplified invertebrate community (Threlfall et al. 2017 ; Mata et al. 2021 ). Often, changes in vegetation structure occur as a result of development, management (e.g., mowing), or through invasive species proliferation in city parks (Kühn and Klotz 2006 ; Cadotte et al. 2017 ). The latter has been shown to result in a decline in soil micro-invertebrate richness and abundance along an urbanization gradient in Toronto, Canada (Malloch et al. 2020 ). However, the invasion of urban forests by exotic tree species can accelerate species turnover without decreasing invertebrate richness or abundance (Buchholz et al. 2015 ). Taxa most affected by the simplification of vegetation structure are phytophagous insects dependent on host plants; for example, butterfly caterpillars feeding on leaves, or bees feeding on pollen and nectar (Bernays and Chapman 1994 ). Particularly vulnerable are those species that form obligate associations and mutualistic relationships with plants or other invertebrates. For instance, in some urban forests in eastern Australia, the larvae of the imperial hairstreak butterfly ( Jalmenus evagoras ) feed on a limited set of tree species within the genus Acacia and form a mutualistic relationship with a few ant species of the genus Iridomyrmex that receive nutrient-rich secretions from the larvae in exchange for the protection they provide.
Urban forests often have a history of management that includes the planting of trees that resist urban pollutants and other anthropogenic stressors (Roy et al. 2012 ). Urban afforestation efforts are typically accompanied by the management of herbaceous, often invasive, plants, including by mowing, herbicide application and physical removal (Oldfield et al. 2013 ). Management interventions such as these, or their absence, influence the type and number of invertebrates in urban forests, which have both positive and negative impacts on the particular ecosystem services invertebrates provide. For example, some ant and butterfly species that are adapted to sparse and more open forests might be impacted by changes in plant communities that occur through plant succession, shrub encroachment or plant invasion (Ossola et al. 2015b ). On the other hand, excessive mowing drastically reduces the number of flowers, reducing invertebrate diversity (Watson et al. 2020 ), while mown parks and urban grasslands result in lower invertebrate abundance compared to un-mown vegetation (Garbuzov et al. 2015 ; Norton et al. 2019 ).
Microclimatic changes
Changes in urban habitat structure can affect microclimatic conditions, and thus invertebrate diversity and ecological processes (Ossola et al. 2016 ). Microclimates available to urban forest invertebrates are influenced by landscape-level changes (e.g., temperature and windflow; Arnfield 2003 ) as well as the structure and composition of the forest itself. The interior areas of larger forests are buffered against higher temperatures and drier conditions (and edge effects in general) in the surrounding built-up environment (Chang et al. 2007 ; Chow et al. 2011 ) and potentially serve as a refuge for edge-sensitive species. However, edge environments and their associated increases in solar radiation, wind and lower moisture levels are common given the very fragmented nature of urban greenspaces. Habitats close to urban forest edges typically support different communities compared to areas away from edges (Kotze et al. 2012 ). The extent to which the composition and richness of invertebrate communities change is related to the contrast of the forest edge compared to built infrastructure or non-forest vegetation (Noreika and Kotze 2012 ; Soga et al. 2013 ; Davis and Gagné 2018 ). The interior areas of urban forests are also subject to disturbance, such as trampling, that can lead to dramatic changes in habitat structure and microclimatic conditions similar to those at edges. Notably, forest paths have very different conditions from adjacent vegetation, with trails and roadways leading to more open vegetation cover (Lehvävirta et al. 2006 ) that create movement corridors (or ‘flyways’) for some aerial insects (e.g., Papilio butterflies; Esaki 1949 ) and compressed leaf litter and soils (Duffey 1975 ) that can reduce abundances of carabid beetles adapted to more humid conditions (Lehvävirta et al. 2006 ; Kotze et al. 2012 ).
Forest management can also have important effects on microclimate. Many urban tree stands, e.g., along roadsides or in parks with mown lawns, are actively managed to avoid an understory from developing (Jorgensen et al. 2002 ) leading to very dry and sun-exposed conditions similar to those in grasslands, and subsequent changes in species composition (Norton 2011 ). Urban forest management can also have the opposite effect. For instance, the suppression of fire in built-up areas (Kareiva et al. 2007 ) and a reduction in historical coppicing (Rackham 2008 ) that result in a more closed canopy, change the composition of spider communities where species that are dependent on open vegetation decline in number (Košulič et al. 2016 ). Additionally, the loss of dead wood, leaf litter and tree hollows in urban forests, particularly those managed for public amenity, reduces microclimates available to invertebrates. These are habitats that provide moist and cooler refuge from higher temperatures (Scheffers et al. 2014 ) and their loss in urban environments might be detrimental to taxa that depend on these conditions (Sebek et al. 2013 ).
Anthropogenic sensory pollution mainly comes in three forms: acoustic noise, artificial light and chemical substances (Halfwerk and Slabbekoorn 2015 ), all of which may have varied effects on organisms and communities, including the exclusion of maladapted traits, the alteration of acoustic and visual communication or an increase in fitness through adaptation (Swaddle et al. 2015 ; Henneken and Jones 2017 ). The effects of light pollution on invertebrates are the most obvious, ranging from a few insects circling a light in the home, to hundreds stuck in the grille or windshield of an automobile and even thousands or more at street lamps, e.g., during termite nuptial flights. However, while light pollution attracts some insects, it also repels other more photophobic species (see examples in Firebaugh and Haynes 2019 ). Larger urban forest patches may buffer against the effects of light pollution, but this becomes compromised as fragmentation creates ever smaller forest patches in urban environments (Villarroya-Villalba et al. 2021 ). Consequently, photophobic invertebrates may become locally extinct, while those attracted to light may disperse to a suboptimal environment where they are exposed to elevated predation, physical harm (automobile collisions) and unfavourable habitat conditions.
Artificial light can also have behavioural effects on invertebrates that use light for communication, like fireflies (Lampyridae). Fireflies inhabit ecologically diverse habitats, from wetlands, grasslands, forests to urban parks, but have lost much of this habitat due to urbanisation (Lewis et al. 2020 ). Owens et al. ( 2018 ) showed that male Aquatica ficta fireflies emit brighter signals with decreased frequency when exposed to artificial light at wavelengths below 533 nm, demonstrating light signal plasticity in these fireflies. Firebaugh and Haynes ( 2019 ) showed that fireflies lured to artificial light were less likely to engage in courtship dialogues, arguing that these light-polluted areas act as demographic traps. Recent evidence on moths also suggests that artificial light has the potential to disrupt chemical communication resulting in reduced mating opportunities (van Geffen et al. 2015a ). The increasing global presence of artificial light at night is linked to shifts and declines in invertebrate diversity (Knop et al. 2017 ; Grubisic et al. 2018 ) and related ecosystem processes (e.g., pollination), which may spill over into diurnal insect communities active during the day (Knop et al. 2017 ).
Little is known about the effects of anthropogenic noise on invertebrates, and even less so in urban forests. In their review, Morley et al. ( 2014 ) identified two studies on invertebrates and noise that found: 1) positive correlations between noise levels and both call frequency and chorusing in the cicada Cryptotympana takasagona in urban parks, and 2) a greater low-frequency component in the songs of male grasshoppers ( Chorthippus biguttulus ) from noisy roadsides compared to paired quiet areas. In addition, a study by Davis et al. ( 2018 ) showed that monarch butterfly ( Danaus plexippus ) larvae exposed to roadside noise for two hours experienced a significant increase in heart rate, which was interpreted as a stress response. Yet, longer exposure to continuous traffic noise did not elevate heart rates at the end of larval development, suggesting desensitisation. Anthropogenic noise (here compressor noise at a natural gas field in New Mexico, USA) has been shown to decrease the abundances of various arthropod taxa collected with pitfall traps (velvet ants and wolf spiders), while some taxa showed no effect and leafhoppers increased (Bunkley et al. 2017 ).
Chemical disturbances in urban landscapes arise from both indirect sources (e.g., microplastics and heavy metals) and deliberate applications (e.g., pesticides). The effects of active ingredients in some pesticides (e.g., neonicotinoids) have been well documented (Chagnon et al. 2015 ; van der Sluijs et al. 2015 ) and led to their ban in areas such as the European Union. In an urban forest context, chemical contamination is likely to be present either as a legacy from past land-uses (e.g., asbestos or heavy metals), current practices (e.g., the direct spraying of or unintentional drift from pesticides, as well as industrial and vehicular emissions), or from novel sources such as microplastics and nanoparticles. While the effects of these first two sources on invertebrates have been relatively well studied (Eggleton 2020 ), the impacts of novel sources of chemical pollution (e.g., per- and polyfluoroalkyl substances, PFAS) are still largely unknown. Yet regardless of the source, the overwhelming picture is that the presence of chemical contaminants has played a major role in contributing to the massive declines in insect abundance and diversity over the past 20 years (Forister et al. 2019 ), and even in interfering with the cognitive ability, i.e., learning and memory of honeybees that may have significant consequences for the vital ecosystem service of pollination (Leonard et al. 2019 ).
Invertebrate responses to urban environments
In the following section, we look at some of the consequences of the filters discussed above for urban forest invertebrates.
Persistence vs. local extinction
Habitat destruction, reduction, fragmentation and transformation act synergistically with urbanisation-derived threats (New 2009 ; Kotze et al. 2011 ), such as the heat island and wind tunnel effects and light, noise and chemical pollution (New 2015 ). Indeed, many studies have shown the negative relationship between urbanisation and invertebrate species richness (McKinney 2008 ; Faeth et al. 2011 ; Mata et al. 2014 ; Fenoglio et al. 2020 ; Piano et al. 2020 ) and trait composition change (Merckx et al. 2018 ; Fournier et al. 2020 ). While these studies explored response patterns at the community level, recent studies are increasingly highlighting that the response of invertebrates to urbanisation processes and filters are species- and trait-specific – that is, some species and trait values tend to be absent in extreme environmental conditions, whereas others are present across a wider environmental gradient (Magura et al. 2013 ; Mata et al. 2014 , 2017 ; Threlfall et al. 2017 ). Species-specific mechanisms driving the responses of invertebrates to urban filters are poorly understood. Specialist species interact with a narrow subset of mutualistic and prey species, and may be acutely susceptible to local extinctions (Dunn 2005 ; Kotze et al. 2011 ). For example, specialist insect herbivores, such as the weevil Cydmaea dorsalis , the leafhopper Pogonella bispinus , the lacebug Radinacantha tasmanica and the plant louse Acizzia keithi that depend on specific plant species are unlikely to persist if one or more urban filters result in the local extirpation of their host plant (Moir et al. 2011 ).
Adaptation and rapid evolution
The urban filters discussed above have the capacity to drive evolutionary as well as phenotypic change in urban-dwelling populations (Johnson and Munshi-South 2017 ; Alberti 2015 ; Alberti et al. 2017a , b ; Hopkins et al. 2018 ). To date, the majority of studies assessing adaptation to urban environments are vertebrate and plant focussed (e.g., Kark et al. 2007 ; McDonnell and Hahs 2015 ), with little research on invertebrates in urban forests. However, increasing evidence indicates that invertebrates may show comparable biological responses along an urban–rural cline (Altermatt and Ebert 2016 ; Kotze et al. 2011 ; Eggenberger et al. 2019 ). For instance, arthropods in urban environments exhibit increased intraspecific variation in morphology (Weller and Ganzhorn 2004 ; Magura et al. 2006 ; Lowe et al. 2014 ; Eggenberger et al. 2019 ), key life history traits (Miyashita 1990 ; Lowe et al. 2016 ) and behaviour (Kralj-Fišer and Schneider 2012 ) compared to their rural counterparts. In addition, chemical analyses reveal the presence of human food in the diet of urban ants (Penick et al. 2015 ).
More recently, the focus has been on exploring the impact of specific anthropogenic stressors on physiological tolerance and behaviour within urban environments, but findings linked to urban forests are scarce. Such studies reveal an increased tolerance for heat in urban ants (Angilletta et al. 2007 ; Diamond et al. 2018 ) and carabid beetles (Piano et al. 2017 ), and higher frequencies in the courtship signals of grasshoppers inhabiting roadside verges (Lampe et al. 2012 ). Light at night disrupts physiological processes and biological timing (Hopkins et al. 2018 ), as well as ecosystem functions, such as pollination, carried out by nocturnal and diurnal communities (Knop et al. 2017 ). Experimental laboratory studies reveal dramatic ecological variation in invertebrates linked directly to the presence of light at night (Durrant et al. 2018 ; Willmott et al. 2018 ; van Geffen et al. 2015a , b ; van Geffen et al. 2014 ). The impact of light at night is species-specific and the observed patterns may not reveal the true nature of the ecological trade-offs faced. For example, in spiders, the presence of artificial light at night may disrupt biological timing, affecting growth and development under a resource-controlled laboratory environment (Willmott et al. 2018 ), but this may be offset by increased nocturnal prey capture rates, as measured in natural environments (Lowe et al. 2014 , 2016 ; Willmott et al. 2019 ).
Although there are examples of invertebrates that have undergone contemporary evolution to adapt to human activity, there are few documented examples of invertebrate evolution being a direct effect of landscape change (Johnson and Munshi-South 2017 ). Van’t Hof et al. ( 2016 ) demonstrated that industrial pollution accumulating on urban trees in nineteenth century Britain led to greater predation on light-coloured peppered moths ( Biston betularia ), and so populations underwent mutation for greater melanism. In another study, Jha ( 2015 ) showed that gene flow in a bumblebee species ( Bombus vosnesenskii ) was inhibited by impervious surfaces that replaced natural areas. Reduced flight-to-light behaviour of individuals of the small ermine moth ( Yponomeuta cagnagella ) from urban (artificial light at night) compared to rural (natural light at night) regions suggests adaptation (Altermatt and Ebert 2016 ). However, there is limited understanding of how fragmentation via the alteration or removal of urban forests and other greenspaces restricts gene flow and genetic variability in invertebrate species, and whether affected populations are more susceptible to future urbanisation and global change (Santangelo et al. 2018 ). Novel habitats generated as a result of urbanisation may also increase gene flow for some ubiquitous urban species and pests (Miles et al. 2018 ), including those that exploit and further damage urban forests. In general, more work is needed to understand how landscape changes in urban areas impact the evolution of urban invertebrates (Miles et al. 2019 ), both to mitigate evolutionary resistance in pests and foster gene flow within native species populations.
Acknowledging the complexity of responses
There are some general patterns and trends in the responses of urban forest invertebrates to increasing urbanisation that are consistently observed across the diversity of taxa and species. However, there is also an equally diverse array of complex responses that are highly influenced by local context and the specific components of a particular system (see Fournier et al. 2020 ). For example, at the local scale, insectivorous birds, as well as invertebrate predators, parasites and pathogenic fungi, play an important top-down role in regulating insect numbers in urban tree canopies. Within remnant forests in Sydney, Australia, where small insectivorous birds have been lost from the urban environment, herbivorous insects have been released from predation resulting in higher levels of herbivory (Hochuli et al. 2004 ). In contrast, recent work from multiple cities in Eastern Europe has found that insectivorous birds are more likely to move into cities, resulting in declines in the density of insects and reduced levels of herbivory (Kozlov et al. 2017 ). As such, in some cities, urban forests in highly urbanised settings may show enhanced defoliation, whereas in others the impacts of herbivorous insects are diminished through antagonistic trophic interactions. However, changes in bottom-up interactions may be equally at play. Around the world, urban forests are overwhelmingly dominated by very few species (Paquette et al. 2021 ). Such a simplification of leaf litter traits may lead to a simplification of associated invertebrate consumers. Recent studies in natural forests or plantations have shown a consistent covariation of leaf litter traits and detritivore feeding traits ranging from mesofauna such as springtails (Raymond-Leonard et al. 2019 ) to detritivorous macrofauna such as millipedes and isopods (Brousseau et al. 2019 ).
The diversity of invertebrates themselves, and their complex life cycles that include reliance on multiple terrestrial and aquatic habitat types, also mean that it is challenging to identify a common framework that applies across all cities and contexts, and as such, idiosyncratic responses to urbanisation are likely to be common.
Managing urban forests for invertebrates
Based on what we have learned from the responses of invertebrates to the urban environment in general, here we make a series of recommendations aimed at maintaining and enhancing invertebrates in urban forests. We believe that the evidence of impacts in urban environments, even if not specific to forests, is sufficient to formulate measures to mitigate the impacts described above. We recommend that the protection and promotion of urban forest invertebrate diversity be a two-pronged approach that seeks to 1) enhance resources for invertebrates, whilst 2) reducing exposure to urban threats (Fig. 1 ).
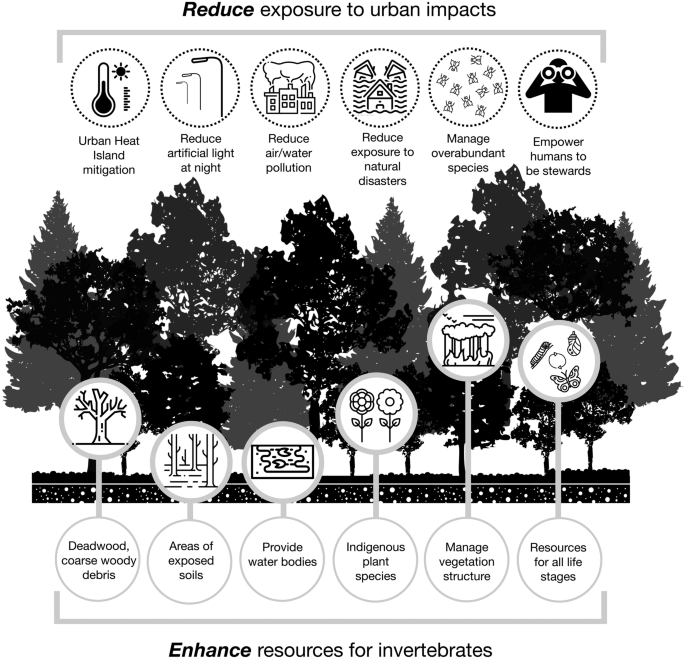
Actions to promote the presence and diversity of invertebrates in urban forests. Invertebrate communities are enhanced by maintaining or increasing habitat complexity through the addition of dead and decaying wood, providing areas of exposed soil and clean water bodies, promoting native and indigenous plant species across all strata of the forest and ensuring resources for all life stages. At the same time, actions that reduce deleterious effects of the city include mitigating the urban heat island effect, reducing light, air, water and soil pollution, and managing overabundant, often pest, species. By involving the public in these actions, people are empowered to be stewards of urban forests and the invertebrates they contain
Enhancing resources for invertebrates requires actions such as:
Maintaining leaf litter cover and dead and decaying wood to promote a speciose detritivore and saproxylic community (Siitonen 2001 ; Ossola et al. 2016 ). Dead wood is a limiting resource in urban environments (Harper et al. 2005 ; Sebek et al. 2013 ; Le Roux et al. 2014 ; Korhonen et al. 2020 ) but an important refuge from, for instance, increased temperatures (Scheffers et al. 2014 ). These resources can be reintroduced by actively adding piles of dead wood of different species, sizes and decay stages (Gaston et al. 2005 ) and in certain configurations to show intent (the ‘cues to care’ concept by Nassauer 1995 ; Li and Nassauer 2020 ) together with information posts to explain the benefits of dead wood, changing management to support natural regeneration of dead wood features (Sebek et al. 2013 ), or introducing artificial structures that mimic these features (Goldingay and Stevens 2009 ). However, it should be noted that the latter strategy is limited in its benefit as it does not provide feeding resources for target taxa.
Maintain areas of exposed soils and soils that have not been overly compacted or otherwise altered by human activities to provide habitat for invertebrates with a soil-dwelling (e.g., earthworms, ants or beetles) or soil-nesting (e.g., some bees and wasps) life stage. Additionally, limiting the sealing and compaction of soils by reducing human use to a small number of trails or areas will benefit ground beetles, ground-nesting bees, and other epigeic and endogeic groups (Cane et al. 2006 ; Lehvävirta et al. 2006 ; Galli et al. 2015 ).
Protect, restore and create water bodies , including natural and human-made permanent or semi-permanent standing or running sources of water, for invertebrates with aquatic life stages. These species can be further supported through the presence of vegetation and natural surfaces (e.g., sand or mud) and minimal macro, micro and chemical pollutants (Forister et al. 2019 ).
Promote indigenous plant species (Mata et al. 2021 ), and undertake management practices that support them, to ensure that food resources are provided in a form that is accessible to co-evolved native invertebrates. For example, open cup flowers are an essential resource if we wish to support short-tongued native bee species in southeastern Australia’s urban forests (Threlfall et al. 2015 ), while the maintenance or establishment of a native forest understory layer will benefit the detritivore community, as well as provide habitat and resources for bees, beetles, and wasps among others (Ossola et al. 2016 ; Threlfall et al. 2017 ; Mata et al. 2021 ). Note that we are not advocating for the exclusion of exotic, ornamental plant species, which also provide important resources for many urban forest invertebrates, but do promote native plant species that are important for co-evolved invertebrate-plant relationships.
Provide resources for all life stages to support a complex assemblage of invertebrates (see Hauck and Weisser 2015 ) that require a diversity of habitat types (e.g., dragonflies depend on aquatic and terrestrial habitats to complete their life cycle and aerial moths develop from soil-dwelling larvae) and food sources (e.g., herbivorous caterpillars or xylophagous beetle larvae become nectar-feeding butterflies and beetles, respectively). It is also important to consider that threats and degrading processes may change as organisms proceed through their life cycle, and to manage habitat accordingly. Furthermore, the length of different life stages should be considered when managing dead wood ‒ saproxylic beetles such as the European stag beetle ( Lucanus cervus ) spend up to five years in decaying roots.
Maintain or increase vegetation structural complexity to provide a wide range of food, shelter and other resources that support the invertebrates that feed directly on plant leaves, nectar, flowers, seeds and fruits, and the predatory invertebrates that feed on them. For public managers, the benefits of increasing vegetation structural complexity in urban forests may be public preference for the resulting naturalistic character this creates (Heyman 2012 ; Harris et al. 2018 ), although this may not be a universal phenomenon. Public support for increasing the complexity of an urban forest may be enhanced by co-management programmes that engage with stakeholders to collaboratively achieve common management goals (Tsuchiya et al. 2013 ), as illustrated by the co-management approach to woodlands in the neighbourhood of Sletten, Denmark (Fors et al. 2018 ).
While the previous recommendations highlight the positive actions than can enhance urban forests for invertebrates, the following mitigation actions will additionally benefit them by reducing the impact of broader threats and disturbances (Fig. 1 ). These actions include:
Reduce chemical pollutants by reducing inputs at the source, and by prioritising non-chemical control of pests (e.g., biological control (Kenis et al. 2017 )) through Integrated Pest Management.
Reduce the urban heat island effect and detrimental disturbances through landscape-scale planning, site-scale design, and appropriate management practices. For example, large greenspaces provide cooling benefits and offer a refuge where invertebrates can reduce their exposure to the urban heat island effect (Ziter et al. 2019 ). Site and landscape design can also provide local refuges where invertebrates can shelter during disturbances, as can management practices such as creating mosaics of different age stands.
Reduce light pollution by: (i) maintaining unlit areas; (ii) reducing the duration of lighting; (iii) minimising the ‘trespass’ of light through improved design; (iv) changing the intensity of lighting; (v) adjusting the spectral composition of lighting (Gaston et al. 2012 ); and (vi) use new technology and lighting systems (Goddard et al. 2021 ). While night lighting is an important amenity for people, including for their perceived safety, there is also strong evidence that the associated loss of darkness interrupts natural circadian rhythms with negative consequences for human physical and mental health (Cho et al. 2015 ).
Reduce exposure to natural disasters such as catastrophic bushfires or destructive flooding by distributing areas of urban forest across the landscape, and providing areas where invertebrates can retreat to safety (or repopulate landscapes) such as upland areas in floodplains, or reducing the risk of catastrophic fire through cultural burning and Indigenous land management practices (McKemey et al. 2019 ).
Manage overabundant invasive species and pests to help prevent completely disrupting urban forest ecosystems by altering invertebrate, vertebrate and plant communities and ecological processes, as has happened with extremely successful invaders, such as Argentine and yellow crazy ants ( Linepithema humile , Anoplolepis gracilipes ) (Silverman and Brightwell 2008 ). As urban areas are also often primary sites for biological invasions, monitoring invertebrates can complement biosecurity efforts and allow a more rapid response to emerging pests (Hendrix et al. 2008 ). Supporting diverse invertebrate communities can help reduce the incidence and rate of invasions by providing a broader range of potential natural enemies (Jones and Leather 2012 ; Gaudon and Smith 2020 ).
Empower humans to become stewards of ecosystems, through actions such as engaging citizens and other urban stakeholders in the sustainable management of pest species (Lowe et al. 2019 ) and the conservation of wild, forested land in cities. Creative ways to engage and empower people to uphold conservation could include public demonstrations, art installations, guided walks, or education programmes. This, in turn, will help reduce the growing disconnect between people and nature, and generate stronger support for the other actions outlined in this section.
Research gaps and future research
While we know quite a lot about invertebrates in urban environments, several gaps still remain in our knowledge of these organisms in urban forests. To effectively manage and conserve diverse communities of urban forest invertebrates, we need to build a stronger understanding of how they survive, persist and respond to densifying urban landscapes. For a broader perspective on a research agenda for urban biodiversity, see Knapp et al. ( 2021 ).
Some of the important questions for future research on invertebrates in urban forests, but also in urban environments in general, include:
At what rate are forest invertebrate numbers, biomass and species declining and how strong is the evidence for a sliding baseline of invertebrate numbers based on the experiences of the people who are doing the assessments? This requires long-term research on population sizes, biomass and species richness in multiple cities and biogeographic areas (Hallmann et al. 2017 ; Macgregor et al. 2019 ; Sánchez-Bayo and Wyckhuys 2019 ), and an understanding of the pitfalls in these long-term studies (Didham et al. 2020 ).
What are the implications of current and future actions on forest invertebrates and the ability of urban areas to support them under climate extremes (e.g., intensified storm events, droughts and heatwaves) and intentional, e.g., the planting of street trees, and unintentional changes in vegetation (Ossola et al. 2020 )?
How do forest invertebrates respond to different types of disturbance events (e.g., catastrophic wildfires versus Indigenous cultural burning practices) and what are the underlying mechanisms behind these responses (Erenler et al. 2020 ; Filazzola et al. 2021 )?
What are the metapopulation and metacommunity structures and dynamics present in urban forest invertebrate communities? What can highly fragmented, isolated and dynamic patches of forests in cities reveal with respect to theories on species and community occupancy (Turrini and Knop 2015 )?
What are the effects of city history and historical landscapes (legacy effects) on contemporary invertebrate communities (e.g., Lindborg and Eriksson 2004 ; du Toit et al. 2016 for plants; Ossola et al. 2021 )? Furthermore, how do invertebrate communities change over time in different urban spatial contexts and are priority effects, i.e., the impacts on a community based on the order or timing of species arrival (Fukami 2015 ), important in this process?
What are the pre-existing adaptations that enable invertebrates to persist in cities now and in the future, and what are the eco-evolutionary responses that are emerging in invertebrates in response to current and future urban filters and pressures (Alberti 2015 )? What are the potential consequences of these changes on future urban forest invertebrate assemblages and their ability to deliver critical ecosystem services?
To what extent do cities act as a conservation refuge for endangered and vulnerable species that might struggle to survive in human-dominated peri-urban landscapes, such as those affected by intensive agriculture, forest logging, or desertification (Hall et al. 2017 )?
Invertebrates play a key role in supporting healthy urban environments for people, as reflected in their diverse and varied contributions to the functioning of urban forest ecosystems. While many environmental filters are similar in the urban milieu across the globe, individual responses to these filters are highly variable, reflecting the diversity and complexity of invertebrate ecology and life cycles. Given the current concern of an acute loss of invertebrate biomass, abundance and diversity, the conservation of forests in urban environments, and the invertebrates that support key ecosystem processes and underlying services, is even more critical. Delivering healthier and more resilient urban systems into the future requires urgent action to enhance the role of invertebrates as ecosystem engineers of urban forests. This can be achieved by actively promoting access to essential urban forest resources for a broad variety of organisms, combined with management actions to reduce the negative impacts of urban environments, such as habitat loss and air, noise and light pollution. Our success in safeguarding ecosystem functions and processes in the face of an ever-densifying human population truly does rely on ‘the little things that run the world’.
Availablity of data and material
Code availability.
Alberti M (2015) Eco-evolutionary dynamics in an urbanizing planet. Trends Ecol Evol 30:114–126
Article Google Scholar
Alberti M, Correa C, Marzluff JM, Hendry AP, Palkovacs EP, Gotanda KM, Hunt VM, Apgar TM, Zhou Y (2017a) Global urban signatures of phenotypic change in animal and plant populations. PNAS 114:8951–8956
Article CAS Google Scholar
Alberti M, Marzluff J, Hunt VM (2017b) Urban driven phenotypic changes: empirical observations and theoretical implications for eco-evolutionary feedback. Philos Trans R Soc B 372:20160029
Altermatt F, Ebert D (2016) Reduced flight-to-light behaviour of moth populations exposed to long-term urban light pollution. Biol Lett 12:20160111
Alvey AA (2006) Promoting and preserving biodiversity in the urban forest. Urban For Urban Green 5:195–201
Angilletta MJ, Wilson RS Jr, Niehaus AC, Sears MW, Navas CA, Ribeiro PL (2007) Urban physiology: city ants possess high heat tolerance. PLoS ONE 2(2):e258
Arnfield AJ (2003) Two decades of urban climate research: a review of turbulence, exchanges of energy and water, and the urban heat island. Int J Climatol 23:1–26
Aronson MFJ, Nilon CH, Lepczyk CA, Parker TS, Warren PS, Cilliers SS, Goddard MA, Hahs AK, Herzog C, Katti M, La Sorte FA, Williams NSG, Zipperer W (2016) Hierarchical filters determine community assembly of urban species pools. Ecology 97:2952–2963
Bauerle H, Nothdurft A (2011) Spatial modeling of habitat trees based on line transect sampling and point pattern reconstruction. Can J For Res 41:715–727
Beattie AJ, Culver DC (1982) Inhumation: How ants and other invertebrates help seeds. Nature 297:627
Beauséjour R, Handa IT, Lechowicz MJ, Gilbert B, Vellend M (2014) Historical anthropogenic disturbances influence patterns of non-native earthworm and plant invasions in a temperate primary forest. Biol Invasions 17:1267–1281
Bernays EA, Chapman RF (1994) Host-plant selection by phytophagous insects. Chapman & Hall, New York
Book Google Scholar
Bray N, Wickings K (2019) The roles of invertebrates in the urban soil microbiome. Front Ecol Evol Urban Ecol 7:359
Brin A, Bouget C, Brustel H, Jactel H (2011) Diameter of downed woody debris does matter for saproxylic beetle assemblages in temperate oak and pine forests. J Insect Conserv 15:653–669
Brousseau P-M, Gravel D, Handa IT (2018) On the development of a predictive functional trait approach for studying terrestrial arthropods. J Anim Ecol 87:1209–1220
Brousseau PM, Gravel D, Handa IT (2019) Traits of litter-dwelling temperate forest arthropod predators and detritivores covary spatially with traits of their resources. Ecology 100:e02815
Brown PMJ, Thomas CE, Lombaert E, Jeffries DL, Estoup A, Handley LJL (2011) The global spread of Harmonia axyridis (Coleoptera: Coccinellidae): distribution, dispersal and routes of invasion. Biocontrol 52:623–641
Buchholz S, Kowarik I (2019) Urbanisation modulates plant-pollinator interactions in invasive vs. native plant species. Sci Rep 9:6375
Buchholz S, Tietze H, Kowarik I, Schirmel J (2015) Effects of a major tree invader on urban woodland arthropods. PLoS ONE 10(9):e0137723
Buckingham S, Murphy N, Gibb H (2015) The effects of fire severity on macroinvertebrate detritivores and leaf litter decomposition. PLoS ONE 10(4):e0124556
Bunkley JP, McClure CJW, Kawahara AY, Francis CD, Barber JR (2017) Anthropogenic noise changes arthropod abundances. Ecol Evol 7:2977–2985
Cadotte MW, Yasui SLE, Livingstone S, MacIvor JS (2017) Are urban systems beneficial, detrimental, or indifferent for biological invasion? Biol Invasions 19:3489–3503
Cane JH, Minckley RL, Kervin LJ, Roulston TAH, Williams NM (2006) Complex responses within a desert bee guild (Hymenoptera: Apiformes) to urban habitat fragmentation. Ecol Appl 16:632–644
Cardoso P, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, Fukushima CS, Gaigher R, Habel JC, Hallmann CA, Hill MJ, Hochkirch A, Kwak ML, Mammola S, Noriega JA, Orfinger AB, Pedraza F, Pryke JS, Roque FO, Settele J, Simaika JP, Stork NE, Suhling F, Vorster C, Samways MJ (2020) Scientists’ warning to humanity on insect extinctions. Biol Conserv 242:108426
Chagnon M, Kreutzweiser D, Mitchell EAD, Morrissey CA, Noome DA, van der Sluijs JP (2015) Risks of large-scale use of systemic insecticides to ecosystem functioning and services. Environ Sci Pollut Res 22:119–134
Chang C-R, Li M-H, Chang S-D (2007) A preliminary study on the local cool-island intensity of Taipei city parks. Landsc Urban Plan 80:386–395
Chen G, Wang ZW, Qin Y, Sun WB (2017) Seed dispersal by hornets: An unusual insect-plant mutualism. J Integr Plant Biol 59:792–796
Cheptou PO, Carrue O, Rouifed S, Cantarel A (2008) Rapid evolution of seed dispersal in an urban environment in the weed Crepis sancta . PNAS 105:3796–3799
Cho Y, Ryu SH, Lee BR, Kim KH, Lee E, Choi J (2015) Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int 32:1294–1310
Chow WTL, Pope RL, Martin CA, Brazel AJ (2011) Observing and modeling the nocturnal park cool island of an arid city: Horizontal and vertical impacts. Theor Appl Climatol 103:197–211
Christian CE, Stanton ML (2004) Cryptic consequences of a dispersal mutualism: Seed burial, elaiosome removal, and seed-bank dynamics. Ecology 85:1101–1110
Christie FJ, Hochuli DF (2005) Elevated levels of herbivory in urban landscapes: are declines in tree health more than an edge effect? Ecol Soc 10:10.
Ciceoi R, Gutue C, Gutue M, Roşca I (2017) Current status of pests associated with urban vegetation in Bucharest area. Acta Zool Bulg Suppl 9:181–190
Google Scholar
Colding J (2007) ‘Ecological land-use complementation’ for building resilience in urban ecosystems. Landsc Urban Plan 81:46–55
Colla SR, MacIvor JS (2017) Questioning public perception, conservation policy, and recovery actions for honeybees in North America. Conserv Biol 31:1202–1204
Craven D, Thakur M, Cameron E, Frelich L, Beauséjour R, Blair R, Blossey B, Burtis J, Choi A, Dávalos A, Fahey TJ, Fisichelli NA, Gibson K, Handa IT, Hopfensperger K, Loss SR, Nuzzo V, Maerz JC, Sackett T, Scharenbroch BC, Smith SM, Vellend M, Umek LG, Eisenhauer N (2017) The unseen invaders: introduced earthworms as drivers of change in plant communities in North American forests. Glob Change Biol 23:1065–1074
Crossley MS, Meier AR, Baldwin EM, Berry LL, Crenshaw LC, Hartman GL, Lagos-Kutz D, Nochols DH, Patel K, Varriano S, Snyder WE, Moran MD (2020) No net insect abundance and diversity declines across US Long Term Ecological Research sites. Nat Ecol Evol 4:1368–1376
Dajoz R (2000) Insects and forests: the role and diversity of insects in the forest environment. Intercept Lavoisier, Paris
Dale AG, Frank SD (2017) Warming and drought combine to increase pest insect fitness on urban trees. PLoS ONE 12:e0173844
David JF, Handa IT (2010) The ecology of saprophagous macroarthropods (millipedes, woodlice) in the context of global change. Biol Rev 85:881–895
Davis DE, Gagné SA (2018) Boundaries in ground beetle (Coleoptera: Carabidae) and environmental variables at the edges of forest patches with residential developments. PeerJ 6:e4226
Davis AK, Schroeder H, Yeager I, Pearce J (2018) Effects of simulated highway noise on heart rates of larval monarch butterflies, Danaus plexippus : implications for roadside habitat suitability. Biol Lett 14:20180018
deCastro-Arrazola I, Hortal J, Noriega JA, Sánchez-Piñero F (2020) Assessing the functional relationship between dung beetle traits and dung removal, burial, and seedling emergence. Ecology 101:e03138
Diamond SE, Chick LD, Perez A, Strickler SA, Zhao C (2018) Evolution of plasticity in the city: urban acorn ants can better tolerate more rapid increases in environmental temperature. Conserv Physiol 6:coy030
Díaz S, Pascual U, Stenseke M, Martín-López B, Watson RT, Molnár Z, Hill R, Chan KMA, Baste IA, Brauman KA, Polasky S, Church A, Lonsdale M, Larigauderie A, Leadley PW, van Oudenhoven APE, van der Plaat F, Schröter M, Lavorel S, Aumeeruddy-Thomas Y, Bukvareva E, Davies K, Demissew S, Erpul G, Failler P, Guerra CA, Hewitt CL, Keune H, Lindley S, Shirayama Y (2018) Assessing nature’s contributions to people. Science 359:270–272
Didham RK, Basset Y, Collins CM, Leather SR, Littlewood NA, Menz MHM, Müller J, Packer L, Saunders ME, Schönrogge K, Stewart AJA, Yanoviak SP, Hassall C (2020) Interpreting insect declines: seven challenges and a way forward. Insect Conserv Divers 13:103–114
Do Y, Lineman M, Joo GJ (2014) Carabid beetles in green infrastructures: the importance of management practices for improving the biodiversity in a metropolitan city. Urban Ecosyst 17:661–673
Dodds KJ, Orwig DA (2011) An invasive urban forest pest invades natural environments –Asian longhorned beetle in northeastern US hardwood forests. Can J for Res 41:1729–1742
Duffey E (1975) The effects of human trampling on the fauna of grassland litter. Biol Conserv 7:255–274
Dunn RR (2005) Modern insect extinctions, the neglected majority. Conserv Biol 19:1030–1036
Dunn RR (2010) Global mapping of ecosystem disservices: the unspoken reality that nature sometimes kills us. Biotropica 42:555–557
Durrant J, Botha LM, Green MP, Jones TM (2018) Artificial light at night prolongs juvenile development time in the black field cricket, Teleogryllus commodus . J Exp Zool Part B 330:225–233
du Toit MJ, Kotze DJ, Cilliers SS (2016) Landscape history, time lags and drivers of change: urban natural grassland remnants in Potchefstroom, South Africa. Landsc Ecol 31:2133–2150
Egerer MH, Liere H, Bichier P, Philpott SM (2018) Cityscape quality and resource manipulation affect natural enemy biodiversity in and fidelity to urban agroecosystems. Landsc Ecol 33:985–998
Eggenberger H, Frey D, Pellissier L, Ghazoul J, Fontana S, Moretti M (2019) Urban bumblebees are smaller and more phenotypically diverse than their rural counterparts. J Anim Ecol 88:1522–1533
Eggleton P (2020) The state of the world’s insects. Annu Rev Environ Resour 45:61–82
Ellis JA, Walter AD, Tooker JF, Ginzel MD, Reagel PF, Lacey ES, Bennett AB, Grossman EM, Hanks LM (2005) Conservation biological control in urban landscapes: Manipulating parasitoids of bagworm (Lepidoptera: Psychidae) with flowering forbs. Biol Control 34:99–107
Erenler HE, Gillman MP, Ollerton J (2020) Impact of extreme events on pollinator assemblages. Curr Opin Insect Sci 38:34–39
Esaki T (1949) Records on Papilio flyways. J Lepid Soc 3:62
Faeth SH, Bang C, Saari S (2011) Urban biodiversity: patterns and mechanisms. Ann NY Acad Sci 1223:69–81
Fenoglio MS, Rossetti MR, Videla M (2020) Negative effects of urbanization on terrestrial arthropod communities: A meta-analysis. Glob Ecol Biogeogr 29:1412–1429
Fenoglio MS, Srivastava D, Valladares G, Cagnolo L, Salvo A (2012) Forest fragmentation reduces parasitism via species loss at multiple trophic levels. Ecology 93:2407–2420
Fenoglio MS, Videla M, Salvo A, Valladares G (2013) Beneficial insects in urban environments: Parasitism rates increase in large and less isolated plant patches via enhanced parasitoid species richness. Biol Conserv 164:82–89
Ferlian O, Eisenhauer N, Aguirrebengoa M, Camara M, Ramirez-Rojas I, Santos F, Tanalgo K, Thakur MP (2017) Invasive earthworms erode soil biodiversity: A meta-analysis. J Anim Ecol 87:162–172
Filazzola A, Matter SF, MacIvor JS (2021) The direct and indirect effects of extreme climate events on insects. Sci Total Environ 769:145161
Finerty GE, de Bello F, Bílá K, Berg MP, Dias ATC, Pezzatti GB, Moretti M (2016) Exotic or not, leaf trait dissimilarity modulates the effect of dominant species on mixed litter decomposition. J Ecol 104:1400–1409
Firebaugh A, Haynes KJ (2019) Light pollution may create demographic traps for nocturnal insects. Basic Appl Ecol 34:118–125
Forister ML, Pelton EM, Black SH (2019) Declines in insect abundance and diversity: we know enough to act now. Conserv Sci Pract 8:e80
Fors H, Nielsen AB, Konijnendijk van den Bosch CC, Jansson M (2018) From borders to ecotones – private-public co-management of urban woodland edges bordering private housing. Urban for Urban Green 30:46–55
Fournier B, Frey D, Moretti M (2020) The origin of urban communities: From the regional species pool to community assemblages in cities. J Biogeogr 47:615–629
Frank DH, Fish D, Moy FH (1998) Landscape features associated with lyme disease risk in a suburban residential environment. Landsc Ecol 13:27–36
Frey D, Vega K, Zellweger F, Ghazoul J, Hansen D, Moretti M (2018) Predation risk shaped by habitat and landscape complexity in urban environments. J Appl Ecol 55:2343–2353
Fukami T (2015) Historical contingency in community assembly: Integrating niches, species pools, and priority effects. Annu Rev Ecol Evol Syst 46:1–23
Galli L, Bonacchi A, Capurro M, Conti I, Crovetto F, Ferrari C, Conti FD, Menta C (2015) Assessment of the impact of trampling on soil Arthropoda in a Mediterranean habitat. Acta Societatis Zoologicae Bohemicae 79:193–198
Garbuzov M, Fensome KA, Ratnieks FL (2015) Public approval plus more wildlife: twin benefits of reduced mowing of amenity grass in a suburban public park in Saltdean, UK. Insect Conserv Divers 8:107–119
Gardiner MM, Harwood JD (2017) Influence of heavy metal contamination on urban natural enemies and biological control. Curr Opin Insect Sci 20:45–53
Gaston KJ, Smith RM, Thompson K, Warren PH (2005) Urban domestic gardens II: experimental tests of methods for increasing biodiversity. Biodivers Conserv 14:395–413
Gaston KJ, Davies TW, Bennie J, Hopkins J (2012) Reducing the ecological consequences of night-time light pollution: options and developments. J Appl Ecol 49:1256–1266
Gaudon JM, Smith SM (2020) Augmentation of the native North American natural enemies for the biological control of the introduced emerald ash borer in central Canada. Biocontrol 65:71–79
Goddard MA et al (2021) A global horizon scan of the future impacts of robotics and autonomous systems on urban ecosystems. Nat Ecol Evol 5:219–230
Goldingay RL, Stevens JR (2009) Use of artificial tree hollows by Australian birds and bats. Wildl Res 36:81–97
Goodman H, Egizi A, Fonseca DM, Leisnham PT, LaDeau SL (2018) Primary blood-hosts of mosquitoes are influenced by social and ecological conditions in a complex urban landscape. Parasites Vectors 11:218
Grant JD (1983) The activities of earthworms and the fates of seeds. In: Satchell JE (ed) Earthworm Ecology: From Darwin to Vermiculture. Springer, Dordrecht, pp 107–122
Chapter Google Scholar
Grove SJ (2002) The influence of forest management history on the integrity of the saproxylic beetle fauna in an Australian lowland tropical rainforest. Biol Conserv 104:149–171
Grubisic M, van Grunsven RHA, Kyba CCM, Manfrin A, Hölker F (2018) Insect declines and agroecosystems: does light pollution matter? Ann Appl Biol 173:180–189
Halfwerk W, Slabbekoorn H (2015) Pollution going multimodal: the complex impact of the human-altered sensory environment on animal perception and performance. Biol Lett 11:20141051
Hall DM, Camilo GR, Tonietto RK, Ollerton J, Ahrné K, Arduser M, Ascher JS, Baldock KCR, Fowler R, Frankie G, Goulson D, Gunnarsson B, Hanley ME, Jackson JI, Langellotto G, Lowenstein D, Minor ES, Philpott SM, Potts SG, Sirohi MH, Spevak EM, Stone GN, Threlfall CG (2017) The city as a refuge for insect pollinators. Conserv Biol 31:24–29
Hallmann CA, Sorg M, Jongejans E, Siepel H, Hofland N, Schwan H, Stenmans W, Müller A, Sumser H, Hörren T, Goulson D, de Kroon H (2017) More than 75 percent decline over 27 years in total flying insect biomass in protected areas. PLoS ONE 12(10):e0185809
Halofsky JE, Peterson DL, Harvey BJ (2020) Changing wildfire, changing forests: The effects of climate change on fire regimes and vegetation in the Pacific Northwest, USA. Fire Ecol 16:4
Hamberg L, Lehvävirta S, Malmivaara-Lämsä M, Rita H, Kotze DJ (2008) The effects of habitat edges and trampling on understorey vegetation in urban forests in Helsinki, Finland. Appl Veg Sci 11:81–96
Handa IT, Aerts R, Berendse F, Berg MP, Bruder A, Butenschoen O, Chauvet E, Gessner MO, Jabiol J, Makkonen M, McKie BG, Malmqvist B, Peeters ETHM, Scheu S, Schmid B, van Ruijven J, Vos VCA, Hättenschwiler S (2014) Consequences of biodiversity loss for litter decomposition across biomes. Nature 509:218–221
Hardy PB, Dennis RL (1999) The impact of urban development on butterflies within a city region. Biodivers Conserv 8:1261–1279
Harper MJ, McCarthy MA, van der Ree R (2005) The abundance of hollow-bearing trees in urban dry sclerophyll forest and the effect of wind on hollow development. Biol Conserv 122:181–192
Harris V, Kendal D, Hahs AK, Threlfall CG (2018) Green space context and vegetation complexity shape people’s preferences for urban public parks and residential gardens. Landsc Res 43:150–162
Harrison T, Gibbs J, Winfree R (2018) Forest bees are replaced in agricultural and urban landscapes by native species with different phenologies and life-history traits. Glob Change Biol 24:287–296
Harvey DJ, Gange AC, Hawes CJ, Rink M (2011) Bionomics and distribution of the stag beetle, Lucanus cervus (L.) across Europe. Insect Conserv Divers 4:23–38
Harvey JA, Heinen R, Armbrecht I, Basset Y, Baxter-Gilbert JH, Bezemer TM, de Kroon H (2020) International scientists formulate a roadmap for insect conservation and recovery. Nat Ecol Evol 4:174–176
Hauck TE, Weisser WW (2015) AAD Animal-Aided Design © . Bei der Projektleitung, Freising (ISBN 978–3–00–047519–1)
Hauru K, Koskinen S, Kotze DJ, Lehvävirta S (2014) The effects of decaying logs on the aesthetic experience and acceptability of urban forests – Implications for forest management. Landsc Urban Plan 123:114–123
Hawlena D, Strickland MS, Bradford MA, Schmitz OJ (2012) Fear of predation slows plant-litter decomposition. Science 336:1434–1438
Hendrix PF, Callaham MA Jr, Drake JM, Huang C-Y, James SW, Snyder BA, Zhang W (2008) Pandora’s box contained bait: the global problem of introduced earthworms. Annu Rev Ecol Evol Syst 39:593–613
Henneken J, Jones TM (2017) Pheromones based sexual selection in a rapidly changing world. Curr Opin Insect Sci 24:84–88
Herms DA, McCullough DG (2014) Emerald ash borer invasion of North America: history, biology, ecology, impacts, and management. Annu Rev Entomol 59:13–30
Heyman E (2012) Analysing recreational values and management effects in an urban forest with the visitor-employed photography method. Urban for Urban Green 11:267–277
Hobbs RJ, Arico S, Aronson J, Baron JS, Bridgewater P, Cramer VA, Epstein PR, Ewel JJ, Klink CA, Lugo AE, Norton D, Ojima D, Richardson DM, Sanderson EW, Valladares F, Vilà M, Zamora R, Zobel M (2006) Novel ecosystems: theoretical and management aspects of the new ecological world order. Glob Ecol Biogeogr 15:1–7
Hochuli DF, Gibb H, Burrows SE, Christie FJ (2004) Ecology of Sydney’s urban fragments: has fragmentation taken the sting out of insect herbivory? In: Lunney D, Burgin S (eds) Urban wildlife: More than meets the eye. Royal Zoological Society of New South Wales, Mosman NSW, pp 63–69
Hochuli DF, Threlfall CG (2018) Planning for protection: Promoting pest suppressing urban landscapes through habitat management. In: Ossola A, Niemelä J (eds) Urban biodiversity: From research to practice. Routledge, London, UK, pp 54–70
Hopkins GR, Gaston KJ, Visser ME, Elgar MA, Jones TM (2018) Artificial light at night as a driver of evolution across urban–rural landscapes. Front Ecol Environ 16:472–479
Horák J (2011) Response of saproxylic beetles to tree species composition in a secondary urban forest area. Urban for Urban Green 10:213–222
Horák J (2018) The Role of urban environments for saproxylic insects. In: Ulyshen MD (ed) Saproxylic Insects. Springer, Singapore, pp 835–846
Ings TC, Montoya JM, Bascompte J, Blüthgen N, Brown L, Dormann CF, Edwards F, Figueroa D, Jacob U, Jones JI, Lauridsen RB, Ledger ME, Lewis HM, Olesen JM, van Veen F, Warren PH, Woodward G (2009) Ecological networks – beyond food webs. J Anim Ecol 78:253–269
Jha S (2015) Contemporary human-altered landscapes and oceanic barriers reduce bumble bee gene flow. Mol Ecol 24:993–1006
Jim CY (2017) Conservation and creation of urban woodlands. In: Tan PY, Jim CY (eds) Greening cities: forms and functions. Springer, Singapore, pp 307–330
Johnson AL, Borowy D, Swan CM (2018) Land use history and seed dispersal drive divergent plant community assembly patterns in urban vacant lots. J Appl Ecol 55:451–460
Johnson MT, Munshi-South J (2017) Evolution of life in urban environments. Science 358:eaam8327
Jones CG, Lawton JH, Shachak M (1994) Organisms as ecosystem engineers. Oikos 68:373–386
Jones EL, Leather SR (2012) Invertebrates in urban areas: A review. Eur J Entomol 109:463–478
Jorgensen A, Hitchmough J, Calvert T (2002) Woodland spaces and edges: their impact on perception of safety and preference. Landsc Urban Plan 60:135–150
Kareiva P, Watts S, McDonald R, Boucher T (2007) Domesticated nature: shaping landscapes and ecosystems for human welfare. Science 316:1866–1869
Kark S, Iwaniuk A, Schalimtzek A, Banker E (2007) Living in the city: can anyone become an ‘urban exploiter’? J Biogeogr 34:638–651
Kenis M, Hurley BP, Hajek AE, Cock MJW (2017) Classical biological control of insect pests of trees: facts and figures. Biol Invasions 19:3401–3417
Kitahara M, Fujii K (1997) An island biogeographical approach to the analysis of butterfly community patterns in newly designed parks. Res Popul Ecol 39:23–35
Knapp S, Aronson MFJ, Carpenter E, Herrera-Montes A, Jung K, Kotze DJ, La Sorte FA, Lepczyk CA, MacGregor-Fors I, MacIvor JS, Moretti M, Nilon CH, Piana MR, Rega Brodsky CC, Salisbury A, Threlfall CG, Trisos C, Williams NSG, Hahs AK (2021) A research agenda for urban biodiversity in the global extinction crisis. BioScience 71:268–279
Knop E, Zoller L, Ryser R, Gerpe C, Hörler M, Fontaine C (2017) Artificial light at night as a new threat to pollination. Nature 548:206–209
Korhonen A, Siitonen J, Kotze DJ, Immonen A, Hamberg L (2020) Stand characteristics and dead wood in urban forests: Potential biodiversity hotspots in managed boreal landscapes. Landsc Urban Plan 201:103855
Košulič O, Michalko R, Hula V (2016) Impact of canopy openness on spider communities: implications for conservation management of formerly coppiced oak forests. PLoS ONE 11(2):e0148585
Kotze DJ, Lehvävirta S, Koivula M, O’Hara RB, Spence JR (2012) Effects of habitat edges and trampling on the distribution of ground beetles (Coleoptera, Carabidae) in urban forests. J Insect Conserv 16:883–897
Kotze DJ, Venn S, Niemela J, Spence J (2011) Effects of urbanization on the ecology and evolution of arthropods. In: Niemela J (ed) Urban ecology, patterns processes and applications. Oxford University Press, New York, pp 159–166
Kovacs KF, Haight RG, McCullough DG, Mercader RJ, Siegert NW, Liebhold AM (2010) Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecol Econ 69:569–578
Kozlov MV, Lanta V, Zverev V, Rainio K, Kunavin MA, Zvereva EL (2017) Decreased losses of woody plant foliage to insects in large urban areas are explained by bird predation. Glob Change Biol 23:4354–4364
Kraft NJB, Adler PB, Godoy O, James EC, Fuller S, Levine JM (2015) Community assembly, coexistence and the environmental filtering metaphor. Funct Ecol 29:592–599
Kralj-Fišer S, Schneider JM (2012) Individual behavioural consistency and plasticity in an urban spider. Anim Behav 84:197–204
Kraus D, Bütler R, Krumm F, Lachat T, Larrieu L, Mergner U, Paillet Y, Rydkvist T, Schuck A, Winter S (2016) Catalogue of tree microhabitats – Reference field list. https://informar.eu/tree-microhabitats
Krystosik A, Njoroge G, Odhiambo L, Forsyth JE, Mutuku F, LaBeaud AD (2020) Solid wastes provide breeding sites, burrows, and food for biological disease vectors, and urban zoonotic reservoirs: a call to action for solutions-based research. Front Public Health 7:405
Kurylo JS, Threlfall CG, Parris KM, Ossola A, Williams NSG, Evans KL (2020) Butterfly richness and abundance along a gradient of imperviousness and the importance of matrix quality. Ecol Appl 30:e02144
Kühn I, Klotz S (2006) Urbanization and homogenization–comparing the floras of urban and rural areas in Germany. Biol Conserv 127:292–300
LaDeau SL, Allan BF, Leisnham PT, Levy MZ (2015) The ecological foundations of transmission potential and vector-borne disease in urban landscapes. Funct Ecol 29:889–901
Lampe U, Schmoll T, Franzke A, Reinhold K (2012) Staying tuned: grasshoppers from noisy roadside habitats produce courtship signals with elevated frequency components. Funct Ecol 26:1348–1354
Landsman AP, Ladin ZS, Gardner D, Bowman JL, Shriver G, D’Amico V, Delaney DA (2019) Local landscapes and microhabitat characteristics are important determinants of urban–suburban forest bee communities. Ecosphere 10:e02908
Lawrence KL, Wise DH (2000) Spider predation on forest-floor Collembola and evidence for indirect effects on decomposition. Pedobiologia 44:33–39
Lawrence KL, Wise DH (2004) Unexpected indirect effect of spiders on the rate of litter disappearance in a deciduous forest. Pedobiologia 48:149–157
Lehvävirta S, Kotze DJ, Niemelä J, Mäntysaari M, O’Hara B (2006) Effects of fragmentation and trampling on carabid beetle assemblages in urban woodlands in Helsinki, Finland. Urban Ecosyst 9:13–26
Leonard RJ, Pettit TJ, Irga P, McArthur C, Hochuli DF (2019) Acute exposure to urban air pollution impairs olfactory learning and memory in honeybees. Ecotoxicology 28:2056–2062
Le Roux DS, Ikin K, Lindenmayer DB, Blanchard W, Manning AD, Gibbons P (2014) Reduced availability of habitat structures in urban landscapes: Implications for policy and practice. Landsc Urban Plan 125:57–64
Lewis SM, Wong CH, Owens ACS, Fallon C, Jepsen S, Thancharoen A, Wu C, De Cock R, Novák M, López-Palafox T, Choo V, Reed JM (2020) A global perspective on firefly extinction threats. Bioscience 70:157–167
Li J, Nassauer JI (2020) Cues to care: a systematic analytical review. Landsc Urban Plan 201:103821
Lindborg R, Eriksson O (2004) Historical landscape connectivity affects present plant species diversity. Ecology 85:1840–1845
Lintott PR, Bunnefeld N, Fuentes-Montemayor E, Minderman J, Blackmore LM, Goulson D, Park KJ (2014) Moth species richness, abundance and diversity in fragmented urban woodlands: implications for conservation and management strategies. Biodivers Conserv 23:2875–2829
Lomov B, Keith DA, Hochuli DF (2009) Linking ecological function to species composition in ecological restoration: Seed removal by ants in recreated woodland. Austral Ecol 34:751–760
Lourenço-de-Oliveira R, Castro MG, Braks MA, Lounibos LP (2004) The invasion of urban forest by dengue vectors in Rio de Janeiro. J Vector Ecol 29:94–100
Lowe EC, Latty T, Webb CE, Whitehouse MEA, Saunders ME (2019) Engaging urban stakeholders in the sustainable management of arthropod pests. J Pest Sci 92:987–1002
Lowe EC, Threlfall CG, Wilder SM, Hochuli DF (2018) Environmental drivers of spider community composition at multiple scales along an urban gradient. Biodivers Conserv 27:829–852
Lowe EC, Wilder SM, Hochuli DF (2014) Urbanisation at multiple scales is associated with larger size and higher fecundity of an orb-weaving spider. PLoS ONE 9(8):e105480
Lowe EC, Wilder SM, Hochuli DF (2016) Persistence and survival of the spider Nephila plumipes in cities: do increased prey resources drive the success of an urban exploiter? Urban Ecosyst 19:705–720
Lyytimäki J, Petersen LK, Normander B, Bezák P (2008) Nature as a nuisance? Ecosystem services and disservices to urban lifestyle. Environ Sci 5:161–172
Macgregor CJ, Williams JH, Bell JR, Thomas CD (2019) Moth biomass increases and decreases over 50 years in Britain. Nat Ecol Evol 3:1645–1649
MacIvor JS (2016) DNA barcoding to identify leaf preference of leafcutting bees. Royal Soc Open Sci 3:150623
Magura T, Nagy D, Tóthmérész B (2013) Rove beetles respond heterogeneously to urbanization. J Insect Conserv 17:715–724
Magura T, Tóthmérész B, Lövei GL (2006) Body size inequality of carabids along an urbanisation gradient. Basic Appl Ecol 7:472–482
Makkonen M, Berg MP, Handa IT, Hättenschwiler S, van Ruijven J, van Bogedom P, Aerts R (2012) Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett 15:1033–1041
Malloch B, Tatsumi S, Seibold S, Cadotte MW, MacIvor JS (2020) Urbanization and plant invasion alter the structure of litter microarthropod communities. J Anim Ecol 89:2496–2507
Mata L, Andersen AN, Morán-Ordóñez A, Hahs AK, Backstrom A, Ives CD, Bickel D, Duncan D, Palma E, Thomas F, Cranney K, Walker K, Shears I, Semeraro L, Malipatil M, Moir ML, Plein M, Porch N, Vesk PA, Smith TR, Lynch Y (2021) Indigenous plants promote insect biodiversity in urban greenspaces. Ecol Appl 31:e02309
Mata L, Goula M, Hahs AK (2014) Conserving insect assemblages in urban landscapes: accounting for species-specific responses and imperfect detection. J Insect Conserv 18:885–894
Mata L, Threlfall CG, Williams NSG, Hahs AK, Malipatil M, Stork NE, Livesley SJ (2017) Conserving herbivorous and predatory insects in urban green spaces. Sci Rep 7:40970
Matteson KC, Langellotto GA (2010) Determinates of inner city butterfly and bee species richness. Urban Ecosyst 13:333–347
Maupin GO, Fish D, Zultowsky J, Campos EG, Piesman J (1991) Landscape ecology of lyme disease in a residential area of Westchester County, New York. Am J Epidemiol 133:1105–1113
McDonnell MJ, Hahs AK (2015) Adaptation and adaptedness of organisms to urban environments. Annu Rev Ecol Evol Syst 46:261–280
McFrederick QS, LeBuhn G (2006) Are urban parks refuges for bumble bees Bombus spp. (Hymenoptera: Apidae)? Biol Conserv 129:372–382
McKemey MB, Patterson ML, Rangers B, Ens EJ, Reid N, Hunter JT, Costello O, Ridges M, Miller C (2019) Cross-cultural monitoring of a cultural keystone species informs revival of Indigenous burning of country in South-Eastern Australia. Hum Ecol 47:893–904
McKinney M (2008) Effects of urbanization on species richness: a review of plants and animals. Urban Ecosyst 11:161–176
Meineke EK, Dunn RR, Frank SD (2014) Early pest development and loss of biological control are associated with urban warming. Biol Lett 10:20140586
Meineke EK, Dunn RR, Sexton JO, Frank SD (2013) Urban warming drives insect pest abundance on street trees. PLoS ONE 8(3):e59687
Merckx T, Souffreau C, Kaiser A, Baardsen LF, Backeljau T, Bonte D, Brans KI, Cours M, Dahirel M, Debortoli N, De Wolf K, Engelen JMT, Fontaneto D, Gianuca AT, Govaert L, Hendrickx F, Higuti J, Lens L, Martens K, Matheve H, Matthysen E, Piano E, Sablon R, Schön I, Van Doninck K, De Meester L, Van Dyck H (2018) Body-size shifts in aquatic and terrestrial urban communities. Nature 558:113–116
Meyer S, Rusterholz H-P, Salamon J-A, Baur B (2020) Leaf litter decomposition and litter fauna in urban forests: Effect of the degree of urbanisation and forest size. Pedobiologia 78:150609
Milcu A, Schumacher J, Scheu S (2006) Earthworms ( Lumbricus terrestris ) affect plant seedling recruitment and microhabitat heterogeneity. Funct Ecol 20:261–268
Miles LS, Breitbart ST, Wagner HH, Johnson MT (2019) Urbanization shapes the ecology and evolution of plant-arthropod herbivore interactions. Front Ecol Evol 7:310
Miles LS, Dyer RJ, Verrelli BC (2018) Urban hubs of connectivity: contrasting patterns of gene flow within and among cities in the Western black widow spider. Proc Royal Soc B 285:20181224
Milotić T, Baltzinger C, Eichberg C, Eycott AE, Heurich M, Müller J, Noriega JA, Menendez R, Stadler J, Ádám R, Bargmann T, Bilger I, Buse J, Calatayud J, Ciubuc C, Boros G, Jay-Robert P, Kruus M, Merivee E, Miessen G, Must A, Ardali E, Preda E, Rahimi I, Rohwedder D, Rose R, Slade EM, Somay L, Tahmasebi P, Ziani S, Hoffmann M (2019) Functionally richer communities improve ecosystem functioning: Dung removal and secondary seed dispersal by dung beetles in the Western Palaearctic. J Biogeogr 46:70–82
Miyashita T (1990) Decreased reproductive rate of the spider Nephila clavata , inhabiting small woodlands in urban areas. Ecol Res 5:341–351
Moeller GH, Marler R, McCay RE, White WB (1977) Economic Analysis of the Gypsy Moth Problem in the Northeast: III. Impacts on homeowners and managers of recreation areas. Forest Service, US Department of Agriculture, Northeastern Forest Experiment Station (No. 360)
Moir ML, Vesk PA, Brennan KE, Keith DA, McCarthy MA, Hughes L (2011) Identifying and managing threatened invertebrates through assessments of coextinction risk. Conserv Biol 25:787–796
Morley EL, Jones G, Radford AN (2014) The importance of invertebrates when considering the impacts of anthropogenic noise. Proc Royal Soc B 281:20132683
Murdock CC, Evans MV, McClanahan TD, Miazgowicz KL, Tesla B (2017) Fine-scale variation in microclimate across an urban landscape shapes variation in mosquito population dynamics and the potential of Aedes albopictus to transmit arboviral disease. PLoS Negl Trop Dis 11:e0005640
Müller MS (2021) Feeding behavior of urban bees - A study on the feeding behavior of solitary bees in urban areas across five European cities. Department of Evolutionary Biology and Environmental Studies, University of Zurich (MSc thesis)
Nassauer JI (1995) Messy ecosystems, orderly frames. Landsc J 14:161–169
Nelson AE, Forbes AA (2014) Urban land use decouples plant-herbivore-parasitoid interactions at multiple spatial scales. PLoS ONE 9:e102127
Nesbitt L, Hotte N, Barron S, Cowan J, Sheppard SRJ (2017) The social and economic value of cultural ecosystem services provided by urban forests in North America: A review and suggestions for future research. Urban for Urban Green 25:103–111
New TR (2009) Insect species conservation. Cambridge University Press, Cambridge
New TR (2015) Insect conservation and urban environments. Springer, Cham
Niemelä J (1993) Interspecific competition in ground-beetle assemblages (Carabidae): what have we learned? Oikos 66:325–335
Niemelä J, Kotze DJ (2009) Carabid beetle assemblages along urban to rural gradients: A review. Landsc Urban Plan 92:65–71
Noriega JA, Hortal J, Azcárate FM, Berg MP, Bonada N, Briones MJI, Del Toro I, Goulson D, Ibanez S, Landis DA, Moretti M, Potts SG, Slade EM, Stout JC, Ulyshen MD, Wackers FL, Woodcock BA, Santos AMC (2018) Research trends in ecosystem services provided by insects. Basic Appl Ecol 26:8–23
Noreika N, Kotze DJ (2012) Forest edge contrasts have a predictable effect on the spatial distribution of carabid beetles in urban forests. J Insect Conserv 16:867–881
Noreika N, Pajunen T, Kotze DJ (2015) Urban mires as hotspots of epigaeic arthropod diversity. Biodivers Conserv 24:2991–3007
Norton BA (2011) The sanitisation of urban ecosystems: simplification of the ground layer in eucalypt woodlands and the effects on arthropod communities. Science Botany, The University of Melbourne (PhD thesis)
Norton BA, Bending GD, Clark R, Corstanje R, Dunnett N, Evans KL, Grafius DR, Gravestock E, Grice SM, Harris JA, Hilton S, Hoyle H, Lim E, Mercer TG, Pawlett M, Pescott OL, Richards JP, Southon GE, Warren PH (2019) Urban meadows as an alternative to short mown grassland: effects of composition and height on biodiversity. Ecol Appl 29:e01946
Oldfield EE, Warren RJ, Felson AJ, Bradford MA (2013) Challenges and future directions in urban afforestation. J Appl Ecol 50:1169–1177
Olkowski W, Olkowski H, van den Bosch R, Hom R (1976) Ecosystem management: A framework for urban pest control. Bioscience 26:384–389
Ossola A, Aponte C, Hahs AK, Livesley SJ (2017) Contrasting effects of urban habitat complexity on metabolic functional diversity and composition of litter and soil bacterial communities. Urban Ecosyst 20:595–607
Ossola A, Cadenasso ML, Meineke EK (2021) Valuing the role of time in urban ecology. Front Ecol Evol. https://doi.org/10.3389/fevo.2021.620620
Ossola A, Hahs AK, Livesley SJ (2015a) Habitat complexity influences fine scale hydrological processes and the incidence of stormwater runoff in managed urban ecosystems. J Environ Manag 159:1–10
Ossola A, Hahs AK, Nash MA, Livesley SJ (2016) Habitat complexity enhances comminution and decomposition processes in urban ecosystems. Ecosystems 19:927–941
Ossola A, Hoeppner JM, Burley H, Gallagher RV, Beaumont LJ, Leishman MR (2020) The Global Urban Tree Inventory: A database of the diverse tree flora that inhabits the world’s cities. Glob Ecol Biogeogr 29:1907–1914
Ossola A, Nash M, Christie F, Hahs A, Livesley S (2015b) Urban habitat complexity affects species richness but not environmental filtering of morphologically-diverse ants. PeerJ 3:e1356
Ostfeld RS, Keesing F (2017) Is biodiversity bad for your health? Ecosphere 8:e01676
Owens ACS, Meyer-Rochow VB, Yang E-C (2018) Short- and medium-wavelength artificial light influences the flash signals of Aqutica ficta fireflies (Coleoptera: Lampyridae). PLoS ONE 13(2):e0191576
Padayachee AL, Irlich UM, Faulkner KT, Gaertner M, Procheş S, Wilson JRU, Rouget M (2017) How do invasive species travel to and through urban environments? Biol Invasions 19:3557–3570
Paquette A, Sousa-Silva R, Maure F, Cameron E, Belluau M, Messier C (2021) Praise for diversity: a functional approach to reduce risks in urban forests. Urban for Urban Green 62:127157
Paradis A, Elkinton J, Hayhoe K, Buonaccorsi J (2008) Role of winter temperature and climate change on the survival and future range expansion of the hemlock woolly adelgid ( Adelges tsugae ) in eastern North America. Mitig Adapt Strateg Glob Chang 13:541–554
Parker TJ, Clancy KM, Mathiasen RL (2006) Interactions among fire, insects and pathogens in coniferous forests of the interior western United States and Canada. Agric for Entomol 8:167–189
Penick CA, Savage AM, Dunn RR (2015) Stable isotopes reveal links between human food inputs and urban ant diets. Proc Royal Soc B 282:20142608
Peralta G, Fenoglio MS, Salvo A (2011) Physical barriers and corridors in urban habitats affect colonisation and parasitism rates of a specialist leaf miner. Ecol Entomol 36:673–679
Piano E, De Wolf K, Bona F, Bonte D, Bowler DE, Isaia M, Lens L, Merckx T, Mertens D, Van Kerckvoorde M, De Meester L, Hendrickx F (2017) Urbanization drives community shifts towards thermophilic and dispersive species at local and landscape scales. Glob Change Biol 23:2554–2564
Piano E, Souffreau C, Merckx T, Baardsen LF, Backeljau T, Bonte D, Brans KI, Cours M, Dahirel M, Debortoli N, Decaestecker E, De Wolf K, Engelen JMT, Fontaneto D, Gianuca AT, Govaert L, Hanashiro FTT, Higuti J, Lens L, Martens K, Matheve H, Matthysen E, Pinseel E, Sablon R, Schön I, Stoks R, Van Doninck K, Van Dyck H, Vanormelingen P, Van Wichelen J, Vyverman W, De Meester L, Hendrickx F (2020) Urbanization drives cross-taxon declines in abundance and diversity at multiple scales. Glob Change Biol 26:1196–1211
Pickett ST, Cadenasso ML, Grove JM, Boone CG, Groffman PM, Irwin E, Kaushal SS, Marshall V, McGrath BP, Nilon CH, Poyat RV, Szlavecz K, Troy A, Warren P (2011) Urban ecological systems: Scientific foundations and a decade of progress. J Environ Manage 92:331–362
Prather CM, Pelini SL, Laws A, Rivest E, Woltz M, Bloch CP, Del Toro I, Ho CK, Kominoski J, Newbold TAS, Parsons S, Joern A (2013) Invertebrates, ecosystem services and climate change. Biol Rev 88:327–348
Pregitzer CC, Charlop-Powers S, Bradford MA (2021) Natural area forests in US cities: Opportunities and challenges. J Forest 119:141–151
Raupp MJ, Shrewsbury PM, Herms DA (2010) Ecology of herbivorous arthropods in urban landscapes. Annu Rev Entomol 55:19–38
Rackham O (2008) Ancient woodlands: modern threats. New Phytol 180:571–586
Raymond-Leonard LJ, Bouchard M, Handa IT (2020) Dead wood provides habitat for springtails across a latitudinal gradient of forests in Quebec Canada. For Ecol Manag 472:118237
Raymond-Léonard LJ, Gravel D, Handa IT (2019) A novel set of traits to describe Collembola mouthparts: Taking a bite out of the broad chewing mandible classification. Soil Biol Biochem 138:107608
Raymond-Leonard LJ, Gravel D, Reich PB, Handa IT (2018) Springtail community structure is influenced by functional traits but not biogeographic origin of leaf litter in soils of novel forest ecosystems. Proc Royal Soc B 285:20180647
Robinson WH (1996) Urban entomology: insect and mite pests in the human environment. Chapman & Hall
Rochlin I, Faraji A, Ninivaggi DV, Barker CM, Kilpatrick AM (2016) Anthropogenic impacts on mosquito populations in North America over the past century. Nat Commun 7:13604
Roeland S, Moretti M, Amorim JH, Branquinho C, Fares S, Morelli F, Niinemets U, Paoletti E, Pinho P, Sgrigna G, Stojanovski V, Tiwary A, Sicard P, Calfapietra C (2019) Towards an integrative approach to evaluate the environmental ecosystem services provided by urban forest. J for Res 30:1981–1996
Rossetti MR, Tscharntke T, Aguilar R, Batáry P (2017) Responses of insect herbivores and herbivory to habitat fragmentation: A hierarchical meta-analysis. Ecol Lett 20:264–272
Rowles AD, O’Dowd DJ (2009) New mutualism for old: indirect disruption and direct facilitation of seed dispersal following Argentine ant invasion. Oecologia 158:709–716
Roy S, Byrne J, Pickering C (2012) A systematic quantitative review of urban tree benefits, costs, and assessment methods across cities in different climatic zones. Urban for Urban Green 11:351–363
Rust MK (2009) Urban habitats. In Encyclopedia of Insects, 2nd edn. Academic Press, pp 1025–1027
Samways MJ, McGeoch MA, New TR (2010) Insect Conservation. A Handbook of Approaches and Methods. Oxford University Press, UK
Sánchez-Bayo F, Wyckhuys KAG (2019) Worldwide decline of the entomofauna: A review of its drivers. Biol Conserv 232:8–27
Santangelo JS, Rivkin LR, Johnson MT (2018) The evolution of city life. Proc Royal Soc B 285:20181529
Santorufo L, Cortet J, Arena C, Goudon R, Rakoto A, Morel JL, Maisto G (2014) An assessment of the urban environment on collembolan communities in soils using taxonomy and trait based approaches. App Soil Ecol 78:48–56
Santorufo L, Van Gestel CA, Rocco A, Maisto G (2012) Soil invertebrates as bioindicators of urban soil quality. Environ Pollut 161:57–63
Scheffers BR, Edwards DP, Diesmos A, Williams SE, Evans TA (2014) Microhabitats reduce animal’s exposure to climate extremes. Glob Change Biol 20:495–503
Schiegg K (2000) Effects of dead wood volume and connectivity on saproxylic insect species diversity. Écoscience 7:290–298
Schultz JC, Baldwin IT (1982) Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science 217:149–151
Scudder G (2009) The importance of insects. In: Foottit RG, Adler PH (eds) Insect biodiversity– Science and society. Wiley-Blackwell, Chichester, United Kingdom
Sebek P, Altman J, Platek M, Cizek L (2013) Is active management the key to the conservation of saproxylic biodiversity? Pollarding promotes the formation of tree hollows. PLoS ONE 8:e60456
Seibold S, Müller J, Baldrian P, Cadotte MW, Štursová M, Biedermann PHW, Krah F-S, Bässler C (2019) Fungi associated with beetles dispersing from dead wood - Let’s take the beetle bus! Fungal Ecol 39:100–108
Sherry RA, Zhou X, Gu S, Arnone JA III, Schimel DS, Verburg PS, Wallace LL, Luo Y (2007) Divergence of reproductive phenology under climate warming. PNAS 104:198–202
Shrewsbury PM, Leather SR (2012) Using biodiversity for pest suppression in urban landscapes: Biodiversity and insect pests. John Wiley & Sons, Ltd, pp 293–308
Siitonen J (2001) Forest management, coarse woody debris and saproxylic organisms: Fennoscandian boreal forests as an example. Ecol Bull 49:11–41
Silverman J, Brightwell RJ (2008) The argentine ant: Challenges in managing an invasive unicolonial pest. Annu Rev Entomol 53:231–252
Soga M, Kanno N, Yamaura Y, Koike S (2013) Patch size determines the strength of edge effects on carabid beetle assemblages in urban remnant forests. J Insect Conserv 17:421–428
Speight MCD (1989) Saproxylic invertebrates and their conservation. Council of Europe, Strasburg, p 81
Spence JR, Spence DH (1988) Of ground beetles and men: Introduced species and the synanthropic fauna of Western Canada. Mem Ent Soc Can 144:151–168
Stafford KC, Magnarelli LA (1993) Spatial and temporal patterns of Ixodes scapularis (Acari: Ixodidae) in Southeastern Connecticut. J Med Entomol 30:762–771
Strong DR, Lawton JH, Southwood R (1984) Insects on plants: Community patterns and mechanisms. Blackwell Scientific, Oxford
Suetsugu K (2020) A novel seed dispersal mode of Apostasia nipponica could provide some clues to the early evolution of the seed dispersal system in Orchidaceae. Evol Lett 4:457–464
Swaddle JP, Francis CD, Barber JR, Cooper CB, Kyba CCM, Dominoni DM, Shannon G, Aschehoug E, Goodwin SE, Kawahara AY, Luther D, Spoelstra K, Voss M, Longcore T (2015) A framework to assess evolutionary responses to anthropogenic light and sound. Trends Ecol Evol 30:550–560
Swan CM, Pickett STA, Szlavecz K, Warren P, Willey KT (2011) Biodiversity and community composition in urban ecosystems: Coupled human, spatial, and metacommunity processes. In: Niemelä J, Breuste JH, Guntenspergen G, McIntyre NE, Elmqvist T, James P (eds) Urban Ecology: Patterns, Processes, and Application. Oxford University Press, pp 179–186
Szlavecz K, Yesilonis I, Pouyat R (2018) Soil as a foundation to urban biodiversity. In: Ossola A, Niemelä J (eds) Urban biodiversity- from research to practice. Routledge Taylor & Francis Group
Thompson B, Mclachlan SM (2007) The effects of urbanization on ant communities and myrmecochory in Manitoba, Canada. Urban Ecosyst 10:43–52
Thorn S, Seibold S, Leverkus AB, Michler T, Müller J, Noss RF, Stork N, Vogel S, Lindenmayer DB (2020) The living dead: acknowledging life after tree death to stop forest degradation. Front Ecol Environ 18:505–512
Threlfall CG, Mata L, Mackie JA, Hahs AK, Stork NE, Williams NS, Livesley SJ (2017) Increasing biodiversity in urban green spaces through simple vegetation interventions. J Appl Ecol 54:1874–1883
Threlfall CG, Ossola A, Hahs AK, Williams NSG, Wilson L, Livesley SJ (2016) Variation in vegetation structure and composition across urban green space types. Front Ecol Evol Urban Ecology 4:66
Threlfall CG, Walker K, Williams NSG, Hahs AK, Mata L, Stork N, Livesley SJ (2015) The conservation value of urban green space habitats for Australian native bee communities. Biol Conserv 187:240–248
Tóth Z, Szlavecz K, Schmidt DJE, Hornung E, Setälä H, Yesilonis ID, Kotze DJ, Dombos M, Pouyat R, Mishra S, Cilliers S, Yarwood S, Csuzdi C (2020) Earthworm assemblages in urban habitats across biogeographical regions. App Soil Ecol 151:103530
Tresch S, Frey D, Le Bayon R-C, Zanetta A, Rasche F, Fliessbach A, Moretti M (2019a) Litter decomposition driven by soil fauna, plant diversity and soil management in urban gardens. Sci Total Environ 658:1614–1629
Tresch S, Frey D, Le Bayon R-C, Mäder P, Stehle B, Fliessbach A, Moretti M (2019b) Direct and indirect effects of urban gardening on aboveground and belowground diversity influencing soil multifunctionality. Sci Rep 9:9769
Tsuchiya K, Okuro T, Takeuchi K (2013) The combined effects of conservation policy and co-management alter the understory vegetation of urban woodlands: a case study in the Tama Hills area, Japan. Landsc Urban Plan 110:87–98
Turrini T, Knop E (2015) A landscape ecology approach identifies important drivers of urban biodiversity. Glob Change Biol 21:1652–1667
Urban-Mead KR, Muñiz P, Gillung J, Espinoza A, Fordyce R, van Dyke M, McArt SH, Danforth BN (2021) Bees in the trees: Diverse spring fauna in temperate forest edge canopies. For Ecol Manage 482:118903
Uspensky IV (2017) Blood-sucking ticks (Acarina, Ixodoidea) as an essential component of the urban environment. Entomol Rev 97:941–969 (Zoologicheskii Zhurnal 96:871–898 (in Russian))
Valentine L, Ramalho C, Mata L, Craig M, Kennedy P, Hobbs R (2020) Novel resources: opportunities for and risks to species conservation. Front Ecol Environ 18:558–566
Vandekerkhove K, Thomaes A, Jonsson BG (2013) Connectivity and fragmentation: island biogeography and metapopulation applied to old-growth elements. In: Kraus D, Krumm F (eds) Integrative approaches as an opportunity for the conservation of forest biodiversity. European Forest Institute, pp 104–115
van der Sluijs JP, Amaral-Rogers V, Belzunces LP, Bijleveld van Lexmond MFIJ, Bonmatin J-M, Chagnon M, Downs CA, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke C, Liess M, Long E, McField M, Mineau P, Mitchell EAD, Morrissey CA, Noome DA, Pisa L, Settele J, Simon-Delso N, Stark JD, Tapparo A, Van Dyck H, van Praagh J, Whitehorn PR, Wiemers M (2015) Conclusions of the Worldwide Integrated Assessment on the risks of neonicotinoids and fipronil to biodiversity and ecosystem functioning. Environ Sci Pollut Res 22:148–154
van Geffen KG, Groot AT, Van Grunsven RHA, Donners M, Berendse F, Veenendaal EM (2015a) Artificial night lighting disrupts sex pheromone in a noctuid moth. Ecol Entomol 40:401–408
van Geffen KG, van Eck E, de Boer RA, van Grunsven RHA, Salis L, Berendse F, Veenendaal EM (2015b) Artificial light at night inhibits mating in a Geometrid moth. Insect Conserv Divers 8:282–287
van Geffen KG, van Grunsven RHA, Ruijven J, Berendse F, Veenendaal EM (2014) Artificial light at night causes diapause inhibition and sex-specific life history changes in a moth. Ecol Evol 4:2082–2089
Van’t Hof AE, Campagne P, Rigden DJ, Yung CJ, Lingley J, Quail MA, Hall N, Darby AC, Saccheri IJ (2016) The industrial melanism mutation in British peppered moths is a transposable element. Nature 534:102–105
Vergnes A, Viol IL, Clergeau P (2012) Green corridors in urban landscapes affect the arthropod communities of domestic gardens. Biol Conserv 145:171–178
Vidal MC, Murphy SM (2018) Bottom-up vs. top-down effects on terrestrial insect herbivores: a meta-analysis. Ecol Lett 21:138–150
Villarroya-Villalba L, Casenelles-Abella J, Moretti M, Pinho P, Samson R, Van Mensel A, Chiron F, Zellweger F, Obrist MK (2021) Response of bats and nocturnal insects to urban green areas in Europe. Basic Appl Ecol 51:59–70
Watson CJ, Carignan-Guillemette L, Turcotte C, Maire V, Proulx R (2020) Ecological and economic benefits of low-intensity urban lawn management. J Appl Ecol 57:436–446
Weller B, Ganzhorn JU (2004) Carabid beetle community composition, body size, and fluctuating asymmetry along an urban-rural gradient. Basic Appl Ecol 5:193–201
Wilbur HM (1980) Complex life cycles. Annu Rev Ecol Syst 11:67–93
Willmott NJ, Henneken J, Selleck CJ, Jones TM (2018) Artificial light at night alters life history in a nocturnal orb-web spider. PeerJ 6:e5599
Willmott NJ, Henneken J, Elgar MA, Jones TM (2019) Guiding lights: foraging responses of juvenile nocturnal orb-weavers to the presence of artificial light at night. Ethology 125:289–297
Wilson EO (1987) The little things that run the world - The importance and conservation of invertebrates. Conserv Biol 1:344–346
Zhang Z (2011) Animal biodiversity: An introduction to higher-level classification and taxonomic richness. Zootaxa 3148:7–12
Ziter CD, Pedersen EJ, Kucharik CJ, Turner MG (2019) Scale-dependent interactions between tree canopy cover and impervious surfaces reduce daytime urban heat during summer. PNAS 116:7575–7580
Download references
Acknowledgements
Icons in Fig. 1 are licenced through creative commons by Noun Project creators: Luis Prado (US), H Alberto Gongora (CO), Eucalyp, Insticon (IE), Free Fair & Healthy, Poultry National Park, Priyanka (IN), Maxicons (TH), wahyakup (ID), Sundar Yadhav (IN), Made (AU), Creative Mania (IN), supalerk laipawat (TH), Aline Escobar (MX).
Open Access funding provided by University of Helsinki including Helsinki University Central Hospital. CGT was supported by the Clean Air and Urban Landscapes Hub, funded by the Australian Government’s National Environmental Science Program and an Australian Research Council Discovery Early Career Researcher Fellowship (DE200101226).
Author information
Authors and affiliations.
Faculty of Biological and Environmental Sciences, Ecosystems and Environment Research Programme, University of Helsinki, Niemenkatu 73, 15140, Lahti, Finland
D. Johan Kotze
School of Natural Sciences, Macquarie University, Sydney, NSW, 2109, Australia
Elizabeth C. Lowe & Alessandro Ossola
Department of Biological Sciences, University of Toronto Scarborough, 1265 Military Trail, Toronto, ON, Canada
J. Scott MacIvor
Department of Plant Sciences, University of California, Davis, 95616, USA
Alessandro Ossola
Environmental Sustainability Research Centre, University of Derby, Derby, DE22 1GB, UK
Briony A. Norton
School of Life and Environmental Sciences, The University of Sydney, Sydney, NSW, 2006, Australia
Dieter F. Hochuli
School of Ecosystem and Forest Sciences, The University of Melbourne, 500 Yarra Blvd, Richmond, VIC, 3121, Australia
Alessandro Ossola, Luis Mata & Amy K. Hahs
Biodiversity and Conservation Biology, Swiss Federal Research Institute WSL, Zurcherstrasse 111, 8903, Birmensdorf, Switzerland
Marco Moretti
Department of Geography and Earth Sciences, University of North Carolina at Charlotte, 9201 University City Blvd., Charlotte, NC, 28223, USA
Sara A. Gagné
Institut des sciences de l’environnement, Université du Québec à Montréal, C.P. 8888, succursale centre-ville, Montréal, Québec, H3C 3P8, Canada
I. Tanya Handa
The School of BioSciences, The University of Melbourne, Victoria, 3010, Australia
Therésa M. Jones
Caragh G. Threlfall
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed substantially to the writing of this review.
Corresponding author
Correspondence to D. Johan Kotze .
Ethics declarations
Ethics approval, concent to participate, consent for publication.
The authors give concent to publish this work in Urban Ecosystems.
Conflicts of interest/Competing interests
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Kotze, D.J., Lowe, E.C., MacIvor, J.S. et al. Urban forest invertebrates: how they shape and respond to the urban environment. Urban Ecosyst 25 , 1589–1609 (2022). https://doi.org/10.1007/s11252-022-01240-9
Download citation
Accepted : 27 April 2022
Published : 19 May 2022
Issue Date : December 2022
DOI : https://doi.org/10.1007/s11252-022-01240-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Biodiversity
- Disturbance
- Ecosystem services
- Functional groups
- Life stages
- Ecological networks
- Urban ecology
- Find a journal
- Publish with us
- Track your research
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

The economic importance of the biodiversity of the invertebrates fauna in the corn culture soil in Copşa Mică (Sibiu County) Romania

The goal of our researches is in bringing the scientific arguments of the necessity of including the biologic parameters, mainly of the invertebrates in the soil, in the evaluation studies of the impact upon the environment and the national strategies of monitoring of the soils quality. If the chemical analysis measure the quantity of the polluters, the invertebrates in the soil, especially the insects, reflect intensively the anthropologic influences, emphasizing the intensifications or inhibitions of their activity under the stress conditions. The study upon the invertebrates’ fauna was carried on in Copşa Mică area (Sibiu County) in the corn agricultural ecosystem. The properties of the soil in this area are strongly changed by the industrial activity as a result of an accumulation of great quantities of heavy metals (lead, cadmium). The researches in this area are a part of a greater study upon the invertebrates’ fauna in the corn culture soil of the Sibiu County, researches tha...
Related Papers
Sustainability
Timea Gabor
Industrial activities in the Baia Mare, Romania area have generated strong pollution, and the impact on soil quality in the neighboring areas of the city remains unclear. The aim of the research is to investigate samples of soil from Baia Mare in order to determine the quality of the soil and also the ecological risk of the soil. This study presents among the first studies using the ecological risk assessment methodology on the soil from the Baia Mare area and aims to serve as scientific support for future studies and research. Evaluation of the soil quality state was performed by determining the physical-chemical characteristics of the soil (pH, texture, structure, and concentration of metals). Evaluation of the anthropic activity from the studied areas was conducted by determining the pollution indices: Cf; Cd, PLI, Er, and PERI. The results of this study indicate that the surface soils have very high concentrations of metals (Cd: 3.5–14.4 mg kg−1; Cu: 9.4–361.5 mg kg−1; Pb: 29.7–...
Iuliana Antonie
The invertebrates constitute important indicators in the appreciation of the soil. A first step in this respect is constituted by the analysis of the heavy metals in the corn fields in the Axente Sever and Copsa Micaƒ Area. Methods of researching: collecting the samples in the soil by using the agricultural drill; the determination of the heavy metals by spectroscopy of atomic absorption, using the spectrometer of atomic absorption ContrAA 700. As a result of the researches the average concentration of lead and cadmium in the soil of the agricultural ecosystems from the studied area is still high.
Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Horticulture
WILHEMINA ASARE
The results showed diverse patterns of Pb, Cd, Zn, Co, Cu, Ni, Mn, Cr and Sn, in case of Pb in all areas exceeded the M.L.A (average 32.59 mg/kg while M.L.A. = 20 mg/kg), other elements shows high concentration that exceed the M.L.A. for Ferneziu and Săsar area. In the case of plant material also records exceedances of the M.L.A for Ferneziu and Săsar area, but in the Dura area there were no overtaking of M.L.A.
Marin Senila , A. Michnea , M. Jelea
AES Bioflux
Roxana Elena Ionete
An investigation of soils is performed in order to establish the impact of heavy metal pollution near an industrial power plant. The use of synthetic products (e.g. pesticides, paints, batteries, industrial waste and land application of industrial or domestic sludge) can result in heavy metal contamination of soils. Heavy metals also occur naturally, but rarely at toxic levels. Soil samples from different depth were drawn. Heavy metals such as Pb, Zn, Cu and Mn were analysed to establish the level of contamination relative to maximum limits enforced by environmental protection agencies. In addition, a measurement for the total organic carbon (TOC) concentration is accomplished.
Oltenia Journal for Studies in Natural Sciences
C. M . Cismasiu
Natural ecosystems have a complex structure, being, in most cases, made up of heterogeneous populations of organisms. These ecosystems are generally characterized by distinct spatial features, as a result of their geographical position and the specificity of layering processes, and temporal features, as a result of the successional patterns of the existing populations. All populations of organisms that occupy a specific habitat establish different types of interactions, both among themselves and with the abiotic environment. These types of interactions are highly influenced by the dynamics of natural phenomena, but, also, by the anthropogenic interventions. The present paper is an overview of the main research studies carried out in the Romanian sector of the Danube, which revealed the importance of the Danubian-Carpathian region in preserving natural biodiversity. The investigations were conducted in several areas from the Danube River, the Danube Delta, Tulcea County, and the rivers present in Oltenia Plain, at different periods of time. Different issues are addressed, especially related to the presence and distribution of gastropod species, the concentrations of heavy metals in soils and in the shells of some gastropods species, but, also, the dynamics of several physical-chemical and microbiological parameters, such as the total microbial biomass, numerical density of physiological groups of microorganisms and the intensity of extracellular enzymatic activity. Other issues presented in this paper are the results of microbiological research carried out in the contaminated industrial areas.
Wafaa Osman
Human activities can have dramatic impacts on animal populations around urban areas with heavy metal contamination being a primary cause of hazardous effects. Insects as residents of ecosystems are especially susceptible to heavy metal contamination and have the potential to serve as indicators for environmental stresses .To better understand the effect of heavy metals pollution on terrestrial insect, the detection of different heavy metals was investigated. The following metals cadmium (Cd), copper (Cu), zinc (Zn) and lead (Pb) were found and their concentrations were estimated in soil samples from either polluted or reference site. As the Cd concentration was significantly high in the polluted site, its concentration in the tissues of the studied insect Blaps polycresta (Coleoptera:Tenebrionidae), was investigated as well as the antioxidant defense system and lipid peroxidation biomarkers. The results of insect's tissues in polluted site showed a significant decrease in the activity of antioxidant enzymes, glutathione S-transferase (GST), superoxide dismutase (SOD), catalase (CAT), and reduction in the level of glutathione (GSH). In addition, there was a significant decrease in the total protein content. On the other hand a significant increase of transaminases (AST, ALT), and lipid peroxidation (LPO) levels was found. In conclusion, insect can be considered as a good bioindicator species for environmental heavy metals pollution especially by cadmium that accumulates in soft tissues and has deleterious effects.
Pedobiologia
Emmanuel Lapied
biologie.uvt.ro
Eniko Bereczki
Food Science and Quality Management
Joseph Anikwe
RELATED PAPERS
Express Logistic
Lainy Kholilah
Fadhilah Samhabib
2018 IEEE International Conference on Consumer Electronics (ICCE)
Soheeb Khan
BAR. Brazilian Administration Review
Valeska Guimaraes
Analytical Sciences: X-ray Structure Analysis Online
nurhan gümrükçüoğlu
Clinical Endocrinology
Mehmet Sargin
The American Journal of Cardiology
maria de la cruz
Harry Grainger
The International Journal of Climate Change: Impacts and Responses
Sonia Murshed
Journal of Clinical Gastroenterology
Pierre Boulanger
IEEE Transactions on Computer-Aided Design of Integrated Circuits and Systems
Jason (Jingsheng) Cong
Physics of Plasmas
Juan Gustavo Wouchuk Schmidt
International Journal of Advanced Trends in Computer Science and Engineering
Sanjith S L
Jurnal Abdidas
Nikson Sitindaon
Durmuş Ali Bircan
American Journal of Theoretical and Applied Statistics
Malaria Journal
Berhanu Erko
Korean Journal of Soil Science and Fertilizer
Kyung-Hwa Han
Linda Rhodes
Toplum ve Bilim
Biriz Berksoy
Revista de la Sociedad Argentina de Diabetes
Maria de Lujan Calcagno
International Journal of Global Warming
Hakan Oğuz DEMİR
Physical Review C
Bill Briscoe
Jayson Bayogo
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024
- Open supplemental data
- Reference Manager
- Simple TEXT file
People also looked at
Original research article, invertebrates in science communication: confronting scientists’ practices and the public’s expectations.

- 1 Museum of New Zealand Te Papa Tongarewa, Wellington, New Zealand
- 2 Department of Animal Ecology, Netherlands Institute of Ecology (NIOO-KNAW), Wageningen, Netherlands
- 3 Centre for Science in Society, Victoria University of Wellington, Wellington, New Zealand
- 4 Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, Brazil
- 5 Escola Politécnica, Universidade de São Paulo, São Paulo, Brazil
Good science communication should give the public the tools to make informed decisions and take action, which can be particularly important for nature conservation. The crisis in invertebrate conservation might be rooted in public prejudices against invertebrate animals, which are perceived as the unpopular 97% of Earth’s animal biodiversity. As such, how we approach science communication regarding those animals might yet play a critical role in their conservation. Given how specialized a topic invertebrate biology is, a large part of its communication fall to scientists. Here, we surveyed both scientists and members of the public about the former’s approaches and assumptions and the latter’s interest and expectations regarding invertebrate science communication, confronting the results of each survey. Our findings show that scientists and the public are only tangentially aligned; there is plenty of ground scientists and communicators need to pay attention to and explore better in order to achieve more meaningful and balanced science communication. Among other findings, topics and approaches that could be used to greater effect include (depending on age groups of the audience) history, folklore, pop culture, and pathology. Our results have unveiled some issues in science communication of invertebrates and are thus a good first approach to start defining the way forward.
Introduction
Invertebrates are animals that neither have nor develop a vertebral column, that is, a spine or backbone derived from a precursor structure called notochord. They are not a “natural” lineage in evolutionary terms, but, unnoticed to most of us, this group contains over 97% of all animal species known to science. To those unfamiliar with their astounding diversity, complex structures, and the many evolutionary reasons behind them, invertebrates might come across as ugly, bizarre, or even repulsive creatures (e.g., Bjerke and Østdahl, 2004 ; Batt, 2009 ; Cardoso et al., 2011 ; Czekanski-Moir and Rundell, 2020 ). Only few of them are expressive or charismatic enough for most people to bond with and, with little to no empathy involved, discussing some invertebrate-related topics becomes an uncomfortable moment for a good deal of the general public ( Kellert, 1993b ; Batt, 2009 ).
Even so, being as abundant as they are and forming the majority of the planet’s biota, the ecological importance of these animals is unmistakable. Invertebrates often serve as indicator species to monitor ecological change, and scientists use them as models to keep track of ecosystem health and integrity, evaluate habitat restoration, and evaluate the effects of pollution and contamination in 7 out of 10 studies ( Siddig et al., 2016 ), besides being model organisms for groundbreaking research in biomedicine, genetics and neurobiology (e.g., Jennings, 2011 ; Wilson-Sanders, 2011 ; Balogun et al., 2018 ; Gelperin, 2019 ). Furthermore, many current environmental issues involve invertebrates in some way, including some relating to economy (e.g., fisheries, farming) and public health (e.g., pharmaceuticals, mosquito-borne diseases) (e.g., Losey and Vaughan, 2006 ; Anderson et al., 2011 ; Reumont et al., 2014 ; Carmichael et al., 2015 ; Berenbaum, 2017 ; Madau et al., 2020 ). As concerns about mass extinctions and biodiversity loss increase, the fact that the majority of losses will be invertebrate species is seldom acknowledged in mainstream media/discourse ( New, 1993 ).
While the general public (used here in a broad sense in which non-scientists are the vast majority of the audience, though we acknowledge the existence of many “publics;” Horst et al., 2017 ; Berentson-Shaw, 2018 ) usually receives scientific information from television and the internet, the role of museums and outreach activities by scientists has been increasing again in importance in the last decades ( McComas et al., 2008 ). Furthermore, the once-perceived gap between scientists and the public has decreased, largely due to the former being active on social media platforms such as Twitter ( Schiffman, 2012 ; Reeve and Partridge, 2017 ). While scientists remain a small fraction of the total science communication framework, they are an important one ( Weber and Word, 2001 ; Bowater and Yeoman, 2012 ). In particular, for a topic as specialized as invertebrate biology, which may seem inaccessible or even irrelevant to non-experts (including non-expert science communicators), it is arguable that effective outreach initiatives can be largely relegated to scientists themselves. In fact, insufficient and/or inappropriate science communication has been identified as an impediment to proper conservation of invertebrate animals ( Black et al., 2001 ; Cardoso et al., 2011 ).
According to Burns et al. (2003) : Figure 1, science communication is defined as “the use of appropriate skills, media, activities, and dialogue to produce one or more of the following personal responses to science: awareness […], enjoyment […], interest […], opinion-forming […], understanding […].” Science communication is pivotal in helping the public understand current issues so that they are capable of making informed decisions and taking action (e.g., Treise and Weigold, 2002 ). Given the weight that public opinion can have in conservation efforts, animal rights, and other policy issues (e.g., Brockington et al., 2006 ; Cardoso et al., 2011 ), it is of utmost importance that the public is reasonably aware of invertebrate biodiversity, ecology, and life history. For example, it has been argued that the “crisis” of low support for invertebrate conservation is rooted in public prejudices against these animals, which prevents sufficient funding for conservation efforts focused on them ( New, 1993 ; Knight, 2008 ).
A key point in public engagement with science (PES) literature is that the audience needs to “connect” with the topic, linking it to something already valued or prioritized by them and thus, giving it more personal relevance (e.g., Nisbet and Scheufele, 2009 ). Therefore, how we approach science communication related to typically unappealing animals such as invertebrates is a critical point. As some authors have argued (e.g., Berentson-Shaw, 2018 ), science communication has long been based largely on untested processes and practices and assumptions of what the public wants. As such, more research and trials on methodology and effectiveness is needed.
Given that, in broad terms, public perception can affect nature conservation and that science communication regarding environmentally-important invertebrates can be often relegated to scientists, we investigated the two sides of this coin. In this study, we have taken a comparative approach to investigating effective science communication practices about invertebrates, drawing on the perspectives of both scientists and members of the public. Using this approach, we aimed to interrogate scientists’ assumptions about lay-audiences, and to reveal what the public believes to be effective science communication. Our data is based on two separate questionnaires that were distributed, respectively, to invertebrate scientists around the world and to members of the public in New Zealand. The first questionnaire aimed to uncover the approaches practicing scientists use in science communication when dealing with the “unpopular 97%” of Earth’s animal biodiversity. The second questionnaire investigated the public’s understanding and awareness of invertebrate animals, how they engage with science communication related to this subject, and their interests and expectations.
Part 1: Surveying Scientists
For part one of the research project, we prepared a questionnaire with 19 questions, of which nine are related to personal information (Survey 1, Questions 1.1–1.9; see Supplementary Material ) and nine pertain to science communication (Q1.10–1.18). The final question (Q1.19) asked simply whether respondents wanted to be informed of our results. Not all questions were mandatory, for two reasons: 1) respondents might not be comfortable sharing personal information (e.g., name, ethnicity) or personal experience; 2) some questions depend on a positive reply in preceding questions. Due to the nature of the subject, different types of questions (e.g., open-ended or close-ended, including rating scales and multiple choice) were used depending on the circumstance.
We shared our questionnaire with subscribers of email lists in the areas of Systematics, Paleontology, Evolution, Malacology, and Entomology. We targeted the following six email lists: CONCH-L, Entomo-L, MolluscaList, Taxacom, EvolDir, PaleoNet. These lists attract mostly researchers and university-level students, but also include enthusiasts from all walks of life. Furthermore, these lists have a worldwide scope, even though the English language greatly filters participation. The questionnaire remained open for two weeks in late May and early June 2018.
Part 2: Surveying the Public
Our second questionnaire was entirely anonymous, comprising 21 questions (Survey 2; see Supplementary Material ). In accordance with the Human Ethics Approval (HEC27046), the first question (Q2.1) confirmed that the person read the information sheet explaining the project and conditions for anonymity. The next seven questions were related to personal information (Q2.2–2.8), as above. The following questions functioned to assess participants’ general knowledge of (Q2.9–2.12) and interest in (Q2.13–2.18) invertebrate animals. We used non-scientific names for organisms in these questions, and presented participants with images of animals representing the groups. The final questions (Q2.19–2.21) pertained to science communication, with Q2.21 in particular being open-ended, encouraging the responders to comment on recent personal experiences.
The survey was carried out in person during February and early March 2019 in three public spaces in the city of Wellington, New Zealand: the Museum of New Zealand Te Papa Tongarewa (henceforth “Te Papa”); Zealandia Ecosanctuary (formerly known as the Karori Wildlife Sanctuary); and Otari-Wilton’s Bush. Furthermore, the questionnaire was made available online and advertised by Te Papa’s blog and Facebook and Twitter profiles, being available for the same period as the in-person survey. Our only criteria for participants in the survey was that they were over the age of 16 and did not work as scientists or science communicators. It should be noted that Te Papa is not a natural history museum; while it does have a nature exhibition, its exhibitions also encompass History, Anthropology, Culture and Art, which together have more space and visibility than natural history.
Analyses were performed in R version 4.0.2 ( R Core Team, 2020 ). Unless otherwise stated, we used a Pearson’s chi-squared test to compare the distribution of the responses grouped into various categories (gender, continent, scientist vs. public, etc.). In some instances, the expected frequencies were too low for a correct chi-square approximation, so the p -values were obtained via Monte Carlo simulation with 1,000,000 iterations (thus, results of those simulations are presented with no degrees of freedom). For the open-ended questions (Q1.18 and Q2.21), we used an online word cloud generator ( Davies, 2018 ) to highlight the most common topics and to help guiding our analysis.
For part 1, we investigated if the answers depended on the continent (Europe, North America, or Other), gender (Female or Male) or age group (in the case if Q1.10) of the respondent. We also tested whether different approaches (Q1.16) were also applied to different age groups. Due to the low expected frequencies of responses in some of the response/category combinations, we had to lump South America, Africa and Asia into a single category “Other” and exclude the gender category “Prefer not to say”.
For part 2 we also investigated whether the answers depended on gender, age group or the nationality (New Zealand or overseas) of the respondent. For questions Q2.9 (know what is an invertebrate), Q2.11 (what proportion of animals are invertebrates) and Q2.13 (do you think invertebrates are interesting), we converted the responses into 1 (Q2.9/Q2.13 “yes” or Q2.11 the correct answer 95%) and 0 (other responses). We then used generalized linear models with logit-link and a Binomial error distribution to test if the probability of a positive/correct answer depended on the sex, age and nationality of the respondent (included as explanatory variables). To define the minimal model, we performed backwards model selection, dropping non-significant terms in each step, the statistics for each term were obtained at the point of exclusion of the term from the model (full results are presented in the Supplementary Material ). In all other cases, we used a Pearson’s chi-squared test as explained above. Because Q2.15, Q2.17 and Q2.19 accepted multiple answers, we summed all answers for each category even if the same person contributed to more than one category. Finally, we did not analyze Q2.12 (do you think invertebrates are important) because we only had only 2 respondents who answered “maybe” while all others answered “yes.”
Finally, to confront the answers from Parts 1 and 2, we compared the responses of the public and the scientists to evaluate if the public’s preference would match the scientists’ strategies. For the questions about topics (Q1.11 vs. Q2.16), approaches (Q1.15 vs. Q2.18) and hands-on activities (Q1.17 vs. Q2.17), we employed a similar approach as when analyzing the responses relative to location, age and sex of the respondent and compared the distribution of the responses with a Pearson's chi-squared test using “scientist” or “public” as the sets of data. When comparing topics and approaches we simply used the same datasets as in previous analyses. However, when comparing the preferred type of hands-on activities of the public and scientists, the sets of questions differed: scientists responded how often they would employ each activity type, while the public chose one or more preferred activity type. To compare both sets of data, we pooled all responses from the public into each of the six activities and, in the case of the scientists, we only used the activities where answers were from “always” or “often” categories.
Results and Discussion
Part 1: scientists.
Responses of scientists were in general independent from the gender or location of the respondent with a few exceptions (see below).

Respondents
A total of 210 respondents completed the first questionnaire, of which 54% were male and 45% female (Q1.3). The vast majority of respondents shared their names (Q1.1, ∼78%) and gave us their email addresses to hear about our results (Q1.19, ∼61%). Most respondents were American (∼34%), followed by Australians (∼9%) and British and Canadians (∼7% each) (Q1.4, Supplementary Table S1 ). Unfortunately, the pool of researchers reached by our questionnaire was not diverse (Q1.5, optional question), with ∼90% of respondents identifying as white/Caucasian.
We asked respondents to fill in the name of their institution or workplace (Q1.6), thus, to analyze the answers in a meaningful way we had to classify them in categories that are simultaneously straightforward and unambiguous. The categories applied were: university, museum, research institute, governmental body, NGO, private sector, and independent researcher. As expected, universities and museums dominated the answers (Supplementary Material: Supplementary Figure S1A ). Regarding the place where respondents presently work (Q1.7, Supplementary Table S2 ), the majority were located in the United States (40%), followed by Australia (∼11.5%) and the United Kingdom (∼6%). Even though we aimed for a worldwide audience by targeting email list subscribers, we clearly did not achieve that, given the dominance of English-speaking countries in the answers and the scarce representation of other world-leading countries in scientific research (e.g., Germany).
We classified the diverse answers given to Q1.8 ( Supplementary Figure S1A ), which asked about jobs/positions, in the following categories: professor, researcher, curator, technician, student (graduate or undergraduate), post-doctoral researcher, and educator. Some minor categories (retired, independent researcher, citizen scientist, and manager) could overlap somewhat among themselves or with the previous categories, but their proportions were so low ( Supplementary Figure S1B ) that it does not affect the overall landscape significantly. As expected, most respondents were researchers or professors (∼25% each), but there was also a significant number of graduate students involved as well (∼17%), which is in line with previous research (e.g., Andrews et al., 2005 ). This diversity of academic positions (and career stages) included in the survey responses was also reflected by the age groups (Q1.3, Supplementary Figure S1C ).
Since most respondents were expected to be zoologists, we asked them to specify the group in which they specialize Q1.9 ( Supplementary Table S3 ). The majority of respondents were malacologists (∼33%), followed by entomologists (∼19%) and carcinologists and arachnologists (∼7% each). Since there are arguably many more researchers specializing in arthropods than other invertebrate phyla, we consider that malacologists were simply more prone to answer the questionnaire because the lead author, who shared the questionnaire in the email lists, is a known malacologist. Furthermore, we expected only researchers directly working with invertebrates would respond, so it was a pleasant surprise that ∼6% of respondents were botanists or studied a vertebrate group.
Biodiversity
We asked the respondents to nominate groups of organisms that, in their experience, tend to fascinate the public (Q1.12, Supplementary Table S4 ). As expected, the lepidopterans (butterflies and moths) ranked highest (∼12.5%), which are arguably the most aesthetically pleasing of invertebrates. Cephalopods (octopuses, squids, etc.) also ranked at 12.5%, which was surprising at first sight. However, cephalopods have starred in several popular science books in recent years, as well as receiving increased (social) media attention due to their intelligence, communication and camouflage abilities, which arguably makes them charismatic and of public interest. The next group, crustaceans (crabs, lobsters, etc.), only amounted to half the value of the former groups (6.5%). These were followed by cnidarians (jellyfish and corals), hymenopterans (bees, ants, wasps), and, rather unexpectedly (given they are extinct), trilobites (∼6% each).
Some invertebrate taxa count with a remarkable fossil record that include unique lineages now completely extinct, such as trilobites and ammonoids. When questioned whether their public prefers living or fossil animals (Q1.13), most respondents answered living animals (∼39%), although a good portion (26%) reported that they thought public is equally interested in both. Only a few (∼13%) thought the public prefer fossil invertebrates, but many respondents (22%) were unsure.
We also asked the respondents to list three species that are particularly good at capturing the public’s attention (Q1.14). Given the astounding diversity of invertebrates, we were expecting a myriad of species. Our results, however, indicated some interesting points. About half the answers pointed at general groups (down to genus level). Most species (∼34% of total answers) were indeed mentioned only once, while a few (∼8% of total answers) appear at least twice. Interestingly, the following species were consistently cited: the monarch butterfly Danaus plexippus , octopuses of the genus Octopus (especially O. vulgaris ), the European honey bee Apis mellifera , the giant squid Architeuthis dux , and the nautiluses (genus Nautilus , in particular Nautilus pompilius ). These had 2–3% of the “votes” each, which, given the broad array of species, was very surprising to us.
It is reasonably straightforward to understand the appeal of these species: they are all relatively familiar to the public, but have an extra “something,” which is different for each of them. The monarch is an amazing example of long-distance migration, a feat usually reserved to birds in science education. Octopuses are becoming recognized for their remarkable intelligence and are especially popular on the internet. The honey bee is an important species for humans; moreover, they have been repeatedly featured on the news due to their population decay (e.g., Seitz et al., 2016 ). The giant squid is notable for being the largest invertebrate on the planet; it also has an eerie air of mystery, since it was this species that gave rise to the legend of the Kraken ( Salvador and Tomotani, 2014 ). The interest in nautiluses is a little harder to understand and is probably aesthetic, related to their beautiful and large shells; furthermore, nautiluses’ shells are often presented to the public as mathematically “perfect” golden spirals, which is fallacious ( Peterson, 2005 ).
Other species commonly mentioned (<1%) in the answers to Q1.14 were: hissing cockroaches (genus Gromphadorhina ), terror shrimps ( Anomalocaris ), bumblebees ( Bombus ), cone snails ( Conus ), peacock spiders ( Maratus ), and giant octopuses ( Enteroctopus ).
Science Communication: Frequency and Topics
Regarding the events in which respondents engaged in science communication during the three months prior to the questionnaire (Q1.10), most were involved in one or two events, although a high percentage reported involvement in seven or more separate activities ( Supplementary Figure S2 ). The number of science communication events was higher in North America than in other continents ( Supplementary Figure S3 ; χ 2 (NA, n = 210) = 22.84, p = 0.01). However, it was independent of gender or age of the respondent ( Supplementary Table S5 ), contrary to some previous works surveying a broader array of scientists (e.g., Andrews et al., 2005 ).
The most common overarching topic addressed by respondents in their science communication activities was biodiversity (Q1.11, Figure 1 ). While conservation and evolution were also commonly used topics, the economic and medical importance of species was addressed far less ( Figure 1 ). This could be due to many invertebrates being negatively perceived by the public due to their relationship with diseases and agricultural damage ( Kellert, 1993b ). The choice of topic was independent of the gender or location of the respondent ( Supplementary Table S6 ).
FIGURE 1 . Comparison between public interest and how often scientists use the typical five overarching topics during their science communication/outreach activities (Q2.16 and Q1.11, respectively). (A) biodiversity; (B) conservation; (C) economical applications; (D) evolution; (E) pathology.
Science Communication: Approaches and Practices
It has been argued that, when it comes to species that invoke feelings of disgust (as is the case with many invertebrate animals), greater knowledge of a particular species correlates with a more positive attitude toward it (e.g., Prokop et al., 2008 ; Prokop et al., 2009 ). Evidence for this relationship between knowledge and attitude is not sound, however, and research based on this model tends to focus only on one particular species or issue (e.g., Prokop et al., 2008 ; Prokop et al., 2009 ; Prokop et al., 2010 ). In fact, this “deficit model” of science communication has been repeatedly challenged in recent years, with some academics claiming that socio-cultural factors pertaining to a person’s background were stronger indicators of their attitudes toward particularly contentious scientific issues than their level of scientific literacy or knowledge ( Salmon et al., 2017 ; Berentson-Shaw, 2018 ). When lacking motivation to learn or pay attention, people fall back to mental shortcuts and emotion, typically in detriment of actual knowledge ( Bubela et al., 2009 ).
Therefore, effective science communication is not necessarily about addressing information deficits in public knowledge, but rather, it is closely tied to the manner in which scientists approach science communication and engage the public ( Bubela et al., 2009 ; Besley and Tanner, 2011 ; Salmon et al., 2017 ). Linking the new information to something important to the public, especially as part of a narrative or story, is deemed the most efficient way to communicate science ( Dahlstrom, 2014 ; Berentson-Shaw, 2018 ). Furthermore, the use of narratives to convey research is linked to faster and better comprehension and to greater recall by the public ( Berentson-Shaw, 2018 ).
The approaches that respondents used to start communication was addressed in Q1.15 ( Figure 2 ). The main approaches used by respondents were the pleasing aesthetics of some invertebrate groups and the perceived amazing feats the animals are capable of (>60% in both cases, considering the ‘always’ or ‘often’ answers). “Disturbing” facets of the animals’ biology are also commonly employed (>50%), while topics linked to the humanities and arts are scarcely used (<20% in all cases; Figure 2 ). This result was partially echoed by responses to the open-ended Q1.18 in which respondents reported common “tricks” to engage the audience: surprising stories about unpopular and quirky species were sometimes alluded to, as well as using image magnification and/or models (see also Q1.17 below) to show what the small specimens really look like up close. While responses were independent of the continent of the respondent, more females reported to employ the strategy of pointing to amazing feats ( χ 2 (NA, n = 210) = 19.51, p < 0.05) and/or weird/disturbing facts ( χ 2 (NA, n = 210) = 14.82, p < 0.05) of invertebrates than males ( Supplementary Figures S4A,B ; Supplementary Table S7 ).
FIGURE 2 . Comparison between public interest and how often scientists use the different approaches to engage the public (Q2.18 and Q1.15, respectively). (A) aesthetics; (B) amazing feats; (C) archaeology/history; (D) beneficial species for humankind; (E) folklore/myths, (F) harmful species; (G) pop culture; (H) weird/disturbing facts.
The common thought that scientists need to overcome the public’s “disgust” for invertebrates by pointing out how important these animals are for human welfare (e.g., Kellert, 1993a ; Kellert, 1993b ) was not reflected in the respondents’ answers. However, the answers to Q1.11 and Q1.15 ( Figures 1 , 2 , respectively) show that respondents do tend to prioritize the beneficial aspects of invertebrates over the harmful ones, but this topic still plays a secondary role in regard to other aspects of the fauna, such as aesthetics and natural history.
Finally, the public is not a homogenous entity and different approaches might be required for different sections of the public, or publics ( Dietz et al., 2002 ; Berentson-Shaw, 2018 ). Since age is the major factor in separating distinct generations and values, some of the approaches described above (from Q1.15) might be preferred when communicating with certain age groups. This issue was addressed in Q1.16. Indeed, the age group distribution was dependent on the approach in question ( χ 2 (42, n = 5,279) = 205.45, p < 0.05) with certain approaches being more commonly applied to a younger audience ( Supplementary Figure S5 ). Approaches using pop culture and amazing or disturbing facts are preferred when dealing with younger audiences (up to 35 years old), while history/archaeology and beneficial or harmful effects are preferred when dealing with older audiences (36 years old or more).
Hands-on activities are often used to aid science communication ( van Dijck, 2003 ). In Q1.17, respondents shared the types of activities they used and how often they did so (Q1.17; Figure 3 ; Supplementary Figure S6 ). The answers show that preserved museum specimens are the most commonly employed prop (>25%), but, curiously, the equally readily-available fossil specimens are less often used (∼15%). Live specimens are also uncommon, but this is more easily understandable, especially in the typical urban settings of most museums and universities. Overall, the need for hands-on activities was also prioritized by several respondents in the open-ended Q1.18 (see below). Again, the responses did not depend on the continent of the respondent, but, interestingly, more males reported to use fossils than females ( χ 2 (4, n = 210) = 15.841, p < 0.05) ( Supplementary Figure S4C ; Supplementary Table S8 ), a possible reflection of the persistent gender bias among paleontologists ( Plotnick et al., 2014 ).
FIGURE 3 . Comparison between the public’s preferred hands-on activities and scientists’ use of such activities (Q2.20 and Q1.17, respectively). The barplots indicate the proportion of times the public selected each activity and the proportion of times the scientists mentioned each activity as either often or always used.
Science Communication: Personal Experience
Approximately 67% of respondents answered the optional Q1.18, where we asked for personal stories about (un)successful experiences with science communication involving invertebrates. Although analyzing these answers is largely a qualitative task, we generated a word cloud ( Supplementary Figure S7 ) in order to readily identify the most prominent keywords that appeared. As a result, we identified some recurrent topics that warrant further discussion.
Firstly, the interaction of the public with actual scientists seem to be a key factor in the assumed experience of the former (by the latter). The responses suggested a belief that having the chance to see a scientist at work is more inspiring than receiving information “second handed” via an educator. Even if most scientists lack training as educators (and thus might have problems to keep the audience fully captivated), they believe they compensate by showing the public their passion for their research subjects. However, it is known that outreach activities are viewed by many scientists as a form of volunteer work that happens on top of their main responsibilities and in spite of their busy schedules (e.g., Andrews et al., 2005 ). As such, it might pay off to start implementing dedicated positions and career paths focusing on accurate and efficient science communication in research institutions.
Secondly, it was pointed out that most outreach programs are undertaken irregularly and address a broad audience that includes people of all ages. However, the importance of programs directed at children was repeatedly raised, as they are seemingly more open to new information. Several respondents, therefore, reported working closely with schools and local groups on a regular basis. The need for more regular programs for adults and teenagers was also raised. It has been shown that well-structured outreach programs can greatly benefit the public (e.g., Metz et al., 2018 ).
A further important point relates to indoor vs. outdoor activities. Most outreach events happen indoors, such as talks (both formal and informal, such as Pint of Science; https://pintofscience.com/ ), museum tours (including “open days” and back-of-house tours), workshops and fairs. However, several respondents argued in favor of field trips and experiencing nature first-hand, especially for children. Some of the reported outdoor activities were also linked to citizen science projects. In fact, there are some anecdotal reports of outdoor activities being linked to more positive attitudes toward invertebrates (e.g., Fančovičová and Prokop, 2011 ; Silva and Minor, 2017 ).
Hands-on activities and contact with live animals were also deemed essential. Such interactive “do-it-yourself” activities were reported to be a great tool to reach the public and pave the way to deeper learning (supported by van Dijck, 2003 ); moreover, the powerful emotions of discovering something by oneself was been raised by some respondents. A further possibility for greater engagement is virtual hands-on activities, which were touched upon by just a few respondents, despite being already in practice in many museum exhibitions. In these, the public interacts with a purpose-built software (such as a virtual lab or a game); virtual reality is an even newer tool for this purpose. These have been shown to be as effective as traditional approaches, although the research comparing outcomes is still laden with controversial results ( Brinson, 2015 ).
Several respondents recounted frustrating interactions with journalists, such as instances where they had been misquoted, or their research was poorly presented in news articles. Some of these respondents claimed that journalists tend to underestimate the capabilities of their readership, and over-simplify scientific content. These frustrations with journalists are long-standing and well documented ( Ashwell, 2016 ), and have been exacerbated by the decline of print news media as a whole. Declines in staff, time and resources inevitably affect the quality of science reporting, and dedicated science journalists have become a rarity in even the largest media outlets ( Ashwell, 2016 ).
Conversely, several respondents talked about speaking on the radio and on podcasts, and almost all of these experiences were perceived as positive and successful examples of public outreach. Our preliminary results suggest that invertebrate scientists, at least, perceive these media as more effective and successful than written articles based on brief interviews or press releases, because they have a greater degree of control over the presentation of their research. Similarly, these scientists seem to perceive their own articles, blog posts and books to be more successful tools for public outreach than articles written by journalists.
As expected, many respondents praised the new realm of science communication that has been unveiled by blogs and social media platforms such as Twitter and YouTube. This new outlet allows for a closer, more regular, and hopefully more transparent, communication between scientists and the public ( Reeve and Partridge, 2017 ). Effective use of social media can greatly raise the public’s awareness and nurture interest in science, especially for fields such as Entomology ( Lessard et al., 2017 ). Furthermore, the public is always keen on visual representations ( Trumbo, 1999 ) and such platforms can offer high definition images and videos, alongside compelling infographics and interactive experiences. However, some respondents cautioned that social media, while helpful, should not replace in-person outreach activities. Even so, other respondents commented on the importance of visual representations for putting people in “contact” with animals from faraway places that they may otherwise never get to know.
A few respondents advocated for showing people the local biodiversity of where they live, both living and fossil. Learning about the importance of one’s region to scientific research seems to be a meaningful starting point for science communication. Furthermore, one respondent reported that an outdoor field activity can be a very powerful tool to bring together groups with conflicting interests in the region, such as local farmers, environmental NGOs, government officials and attorneys.
Finally, some topics appeared only once or twice among the answers, but we consider them worthwhile of receiving more attention. 1) One arachnologist was involved in helping people with arachnophobia to deal with their condition by making them understand spider biology and ecology. This goes beyond the exposure therapy that is typically used, where the patient is simply presented with live animals until they are desensitized ( Öst, 1989 ). Since several invertebrates other than spiders can cause a similar reaction, this partnership between medicine/psychology and invertebrate zoology might yet prove important. 2) One respondent talked about using field excursions to showcase habitats in need of protection and to raise money for conservation. Given the current popularity of online crowdfunding initiatives, this could be a helpful path for NGOs and academic societies to explore. Some initiatives, including citizen science projects, are already starting to tap this potential ( Jones et al., 2017 ; Gallo-Cajiao et al., 2018 ). 3) Two respondents reported that a good way to entice the public and make them perceive the importance of invertebrates is to link these animals to the usual “relatable” fauna. One malacologist provided an example of the decrease in songbird populations caused by the decline of land snails, which are a source of calcium for the former. 4) One respondent spoke of using pop culture to start the conversation and another respondent, a paleontologist, also followed this line, connecting the monsters from the Pokémon franchise to actual fossils. The link between pop culture and conservation in science communication is a strong point and is starting to be explored in the literature ( Dorward et al., 2017 ; Salvador, 2017 ; Patterson and Barratt, 2019 ). In fact, circa 20% of our respondents (Q1.15, Figure 2G ) reported using pop culture as a starting point in their science communication.
General Discussion
As vehemently argued by Berentson-Shaw (2018) , science communication still remains based almost exclusively on assumptions. These include assumptions of how communicators should approach it and also of what the public wants or expects. That author called for more focused research on the methodologies of science communication and the effectiveness of their outcome.
Unsurprisingly, the literature regarding zoological science communication is very scarce and usually restricted to reports of specific local activities or surveys of the public’s attitude toward one group of animals or another (e.g., Kellert, 1993b ; Prokop et al., 2010 ; Pontes-da-Silva et al., 2016 ; Silva and Minor, 2017 ). Typically, these works do not focus on communication strategies regarding invertebrate animals, but rather only offer general advice based on punctual findings. For instance, Kellert (1993a) ; Kellert (1993b) simply stated that communicators need to counter people’s disgust toward invertebrates by pointing out how important these animals are for human welfare and survival.
Therefore, there is still a large gap in the literature on how to actually address these matters in activities other than writing and some of the more recent works, while helpful, are overly specific. For instance, Lessard et al. (2017) proposed a framework for promoting museums (in specific entomological collections) via social media, but it does not stray far from simple marketing strategy of counting online interactions. Institutions also expect their scientists to participate in outreach activities but offer them little training, guidance and support ( Andrews et al., 2005 ).
Part 2: Public
The second survey was designed and delivered after we had received and analyzed the results of the first, in an attempt to benchmark and compare the assumptions and beliefs of invertebrate scientists about the science communication with the public, with public responses on the same topic. For pragmatic reasons, this second survey was carried out in only one country (where the lead author is based at the national museum) rather than internationally. While the results of the two surveys cannot therefore be directly compared, we believe there is merit to this approach. This is reinforced by the first survey not indicating substantive differences in response by geographical region.
Furthermore, it should be acknowledged that, while the survey was conducted in public spaces, those are places that attracts a portion of the public that is already more inclined toward science, nature or cultural activities. As such, our sample might not be representative of a broader and less scientifically-interested or literate public, which can bias our result. Still, the strong interest New Zealanders in general have in nature and conservation ( Craig et al., 2000 ) might alleviate this bias a bit.
The second survey had a total of 197 respondents, of which 60% were conducted in person (the remaining 40% were online answers). As expected, most respondents were New Zealand citizens (Q2.2, ∼57%), but a significantly large proportion of respondents reported as female (Q2.5, ∼65%), and only ∼33% reporting as male. This same exact proportion of female respondents (∼65%) is maintained when analyzing in-person and online answers separately. The possibility of gender bias existing in survey responses has long been recognized (e.g., Groves et al., 1992 ), but scarcely investigated. With the advent of online surveys, this subject is starting to be re-examined, with controversial results: some studies reported greater number of female respondents (e.g., Yetter and Capaccioli, 2010 ; Saleh and Bista, 2017 ); others reported more male respondents (e.g., McCabe et al., 2006 ); while some did not report any gender effect (e.g., Fan and Yan, 2010 ).
Regarding education level (Q2.6), the majority of the respondents held a university degree (∼65%; Supplementary Table S9 ); included in this number are those respondents who have a post-graduate degree (∼17% of total). This was expected given the age demographics we targeted and the distribution of the respondents’ age groups (Q2.7; Supplementary Figure S8 ). However, our study group was heavily skewed toward older people, with over 40% of respondents being over 50 years old ( Supplementary Figure S8 ). This abnormality in the age distribution of respondents heavily affected and maybe dictated the overall patterns of the answers, so we also analyzed the answers considering the age groups.
In total, 93 respondents (∼47%) shared some meaningful experiences of science communication they had in our final open-ended question (Q2.21).
Gauging Knowledge of Invertebrates
When questioned whether they knew what an invertebrate animal was (Q2.9; Supplementary Table S13 ), ∼79% answered “yes,” while ∼7% answered “no” and ∼14% answered “maybe.” Those who answered “no” were presented with a brief explanation of what these animals are and a series of photographs (extracted from the taxa’s Wikipedia entries) exemplifying the main groups. Those who answered “yes” or “maybe” were then asked to name three invertebrate animals (Q2.10).
Most respondents gave consistent answers to Q2.10 ( Supplementary Table S10 ), with only very few naming vertebrates and protozoans ( Supplementary Table S11 ). The most common mistake were snakes, cited as being invertebrates in ∼2.6% of the answers. The vast majority of people answered Q2.10 with broad categories such as “squid”, with very few naming a single species (e.g., colossal squid). The most mentioned types of animals were: snails (∼11%), worms (∼10%), crabs (∼7.5%), jellyfish (∼6.2%), and hymenopterans (bees, ants, wasps) and spiders (∼5.2% each). The term “worms” appears typically undefined; the most typical representatives are earthworms, of course, but the term could refer to several others groups, like other annelids, flatworms, round worms, velvet worms, etc. A few respondents were more specific when naming their worms ( Supplementary Table S10 ).
However, when taking the more inclusive groups together (Classes or Phyla), insects were the most usually given as examples (∼20%). They are followed by gastropods (snails and slugs; ∼16%), crustaceans (crabs, lobsters, etc.; ∼11.5%), “worms” (∼10.0%), and cnidarians (jellyfish and corals) and cephalopods (∼7% each). That is partially expected, as insects are the most diverse group of animals. However, the high number of snails and slugs was surprising, given that these animals are not such a common and visible feature of most people’s environments.
Respondents were then asked to indicate which proportion of animal species they thought were invertebrates (Q2.11; Supplementary Table S14 ). Most people’s answers (∼36%) were spot on, indicating that invertebrates make up almost the whole biodiversity of our planet, with ∼33.5% giving a more conservative answer of three-quarters ( Supplementary Figure S9 ). Even so, when asked whether they thought invertebrates were important for ecosystems and the environment (Q2.12), the answer was a resounding yes (∼99%); the remaining 1% were uncertain. However, it is almost certain that the answers to Q2.12 are biased, given that the respondents were facing a questionnaire entirely about invertebrates. This question should be tested in the future under different circumstances.
Sources and Frequency
The respondents were asked to whom they would turn to if they wanted to learn more about invertebrates (Q2.15; Supplementary Figure S10 , Supplementary Table S16 ). Most people reported they would look for documentaries (∼40.5%), but many listed scientists and university professors (∼27.5%). Journalists and school teachers would be in low demand: respectively, ∼6 and ∼4.5%. A reasonable number of people chose the “Other” option (∼7%) and then listed online search engines. Those engines will likely point them to content provided by one of the former options, such as Wikipedia pages, but there is now a growing problem: the number of misinformation online is increasing and becoming more prominent due to flawed algorithms (e.g., Lewis, 2018 ; DiResta, 2019 ).
A related question involved which type of media/communication they prefer to use in order to learn about invertebrates (Q2.19; Supplementary Figure S11 ). TV and documentaries are people’s first option (∼23.5%), in line with the answers to Q2.15 above. Respondents’ second option were museums, zoos, and aquaria (∼21%); this number is very close to that of the first option, re-affirming the importance these time-honored institutions still play in our society (e.g., Ballantyne and Packer, 2016 ; Packer and Ballantyne, 2010 ). The third option were internet articles and blog posts (∼17%), which surprisingly outnumbered internet videos (∼13.5%). Books (∼7%) and newspaper and magazine article (∼6.5%) appear to be clearly less important.
When questioned how often respondents engaged with each channel of information, the responses were largely unsurprising (Q2.20; Supplementary Figure S12 ). Most daily or weekly interactions were with internet articles or blogs (∼66%), newspapers and magazines (∼44%), internet videos (∼41%) and TV documentaries (∼36%). Books and museums (including zoos and aquaria) were typically sources used on a monthly basis or just occasionally. The majority of respondents never engaged in workshops/symposia (∼54%), citizen science projects (∼62%) or learned societies (∼75%). Unsurprisingly (e.g., van Deursen and Helsper, 2015 ), age played a role in the frequency that respondents used online videos (but not online articles or blogs): older audiences (over 50) rely less on videos ( χ 2 (NA, n = 197) = 36.59, p < 0.05; Supplementary Figure S14E ).
The answers to open-ended question Q2.21 were in line with the results above ( Supplementary Figure S13 ). Many respondents mentioned TV documentaries, specifically citing those by Sir David Attenborough, and YouTube as sources for information and entertainment. Several people also showed concern for the reported worldwide decline of insects that became news earlier in 2018. This is a good example of why trustworthy science communication is important. The news of this decline reported by the media were based on a scientific article and branded “Insectageddon” for impact. That particular article received backlash from the scientific community on Twitter in a matter of hours, showing that its methodology was flawed and cautioning about the results (the critique was later published by Thomas et al., 2019 ). The media, however, neither reported that backlash, nor corrected its published pieces, leaving the public misinformed.
When asked if they thought invertebrates were interesting (Q2.13; Supplementary Table S15 ), most people answered “yes” (∼86%), with some being uncertain (∼12%). As for Q2.12 (see above), the answers to this question are likely biased. In any event, the respondents were then asked to indicate which groups of invertebrate animals they find interesting, choosing from a list (Q2.14). Perhaps not surprisingly given the attention these creatures have been receiving in (social) media, cephalopods came out in first place (∼16.7%; Supplementary Table S12 ). The surprise lies in the second place: cnidarians (jellyfish and corals; ∼15.7%), which the science community does not think are relatable. It is possible that the beauty (and/or danger) of some jellyfish species played a major role here. These are followed by the three main arthropod groups: insects (∼15.3%), crustaceans (crabs, lobsters, etc.; ∼14.3%) and arachnids (spiders, scorpions, etc.; ∼12.5%). Snails and other non-cephalopod mollusks are only the seventh place (∼6.4%), even though they are the most readily-given example of invertebrate animals, as shown above (Q2.10). The answers to the open-ended question Q2.21 reflected the above to a certain extent, indicating that people are most often intrigued or interested in the oceans and its fauna, with cephalopods and jellyfish being specifically mentioned in some cases. Though it has been argued that public interest and attitude toward certain taxa could be linked to previous knowledge (or lack thereof) about the animals (e.g., Prokop et al., 2008 ; Prokop et al., 2009 ), as argued above, evidence in support of that claim is tenuous at best and the “deficit model” of science communication is widely challenged (e.g., Salmon et al., 2017 ).
Conservation was reported to be the most interesting overarching topic (Q2.16; Figure 1 ; Supplementary Table S17 ). Evolution and biodiversity were also noted as typically engaging (>70% of ‘extremely’ or ‘very interesting’ answers), while economic applications and pathology showed more widespread answers. Pathology, in particular, was the least interesting topic. The strong interest in conservation is likely a cultural phenomenon, since New Zealanders are known to have a significant concern for these matters ( Craig et al., 2000 ). Several answers to the open-ended question Q2.21 also mention concerns with conservation, climate change and biodiversity loss.
Regarding more specific topics and approaches (Q2.18; Figure 2 ; Supplementary Table S19 ), respondents reported more interest in “amazing feats” that animals are capable of (∼86% found this ‘extremely’ or ‘very interesting’), “beneficial” and “harmful” species (∼74% each), “aesthetics” (∼72%), and “weird facts” (∼70%). “Folklore/myths” and “history/archaeology” received less reported interest (∼55% each) and “pop culture” even less (∼30%). However, age played a major role here ( Supplementary Figures S14A–D ). Younger audiences (50 years or less) reported a larger interest in “folklore/myths” ( χ 2 (NA, n = 197) = 19.86, p = 0.01) and, rather expectedly, in “pop culture” ( χ 2 (NA, n = 197) = 19.75, p = 0.01). There was also an effect of gender, with female respondents being more interested in “amazing feats” ( χ 2 (NA, n = 197) = 16.36, p < 0.05) and “aesthetics” ( χ 2 (NA, n = 197) = 14.72, p < 0.05).
The public was also asked what type of hands-on activities they enjoy the most (Q2.17; Figure 3 ; Supplementary Table S18 ). Live animals were a clear first choice (∼28%), but field trips were also in high demand (∼23%). It should be noted, however, that the interest in field trips might also be a cultural bias of more nature-oriented New Zealanders and the kind of tourists the country attracts ( Craig et al., 2000 ), given that previous reports elsewhere have been more mixed (e.g., Bixler et al., 1994 ; Bixler and Floyd, 1997 ; Bixler and Floyd, 1999 ). Fossils and preserved museum specimens also seem to be relatively welcomed (∼14.5% each), but models do not seem to be enjoyed (∼8%).
It is widely recognized that the public is not homogenous ( Dietz et al., 2002 ; Berentson-Shaw, 2018 ), but there are means to minimize or circumvent this problem. In the first place, we must recognize that we cannot reach all members of the public with our science topics ( Berentson-Shaw, 2018 ). Therefore, we focused our questionnaire on those people who already have some interest in nature and biology and are therefore most likely to be interested in the topic (i.e., visitors to Te Papa or the two reserves, as well as Te Papa’s social media followers). Furthermore, by focusing on this subset, the issue of having a small sample size was attenuated.
Our results also emphasized the importance that the internet has for people searching for information. It has become much easier for the public to find information; there are plenty of good sources online (e.g., Wikipedia), but the amount of misleading or false information (especially on YouTube and social media) is unfortunately even greater. Natural history documentaries are also clearly another important source of information, and seem to be largely the primary and most trusted source for the respondents.
Comparing the Surveys
One of the most interesting aspects of this research was the comparison between what scientists thought the public wants with what the public reported. To explore this, we compared the answers to pairs of matching questions: Q1.12 vs. Q2.14; Q1.11 vs. Q2.16; Q1.15 vs. Q2.18; Q1.17 vs. Q2.17.
For the first pair of questions (Q1.12 vs. Q2.14), we identified some agreement about which animals are apparently most interesting to the public: cephalopods. However, the remaining taxa are not well-aligned. Scientists think the public likes lepidopterans (butterflies and moths; Supplementary Table S4 ), while our public respondents did not find those animals that interesting ( Supplementary Table S10 ). Instead, they reported being more interested on cnidarians, especially jellyfish (as discussed above), and crustaceans. There are substantially less scientists working on cnidarians than on insects, which might explain why the collective of scientists did not rank the former high on their priority list ( Supplementary Table S4 ).
The distribution of the responses of the public and the scientists differed in all other cases ( Table 1 ), with the exception of the use of pop culture (Q1.15 vs. Q2.18; see below).
TABLE 1 . Results of the Pearson's chi-squared tests comparing the distribution of answers of scientists (Part 1: Q1.11, Q1.15, and Q1.17) and the public (Part 2: Q.2.16, Q21 Q2.18, and Q2.20). Each line represents a different test. Significant results are shown in bold font.
Regarding the overarching topics in invertebrate science communication (Q1.11 vs. Q2.16; Figure 1 ), we compared the frequency which the scientists address each topic with the general interest the public reported. There is much more public interest in Conservation, Evolution, Economy and even Pathology, than the scientists acknowledge. On the contrary, there is less public interest in Biodiversity than what is perceived by scientists, thought that might be due to a mismatch of the scientific definition of that term and public knowledge.
For the more specific topics and approaches (Q1.15 vs. Q2.18; Figure 2 ), we likewise compared the frequency which the scientists address each with the degree of interest the public reported. The only topics in agreement is Pop Culture. There is much more public interest than acknowledged by scientists in the other topics. Remarkably, there is a large public interest in topics related to History/Archaeology and to folklore/myths, which are rarely touched upon by scientists. Understandably, the latter topics are outside the experience or interest of most scientists and will rarely be addressed; nevertheless, if scientists were willing to make more use of these topics, they could get a good response from their public.
For the hands-on activities (Q1.17 vs. Q2.17; Figure 3 ), scientists’ expectations were reasonably aligned with public interested. It was expected that there would be a large public interest in live animals, but those are not typically easy for scientists to procure or arrange, even though they do recognize the public’s preference (Q1.19). Hands-on activities with animals also have the benefit of reducing fear and disgust toward the animals, which can be helpful for the public image of most invertebrates (e.g., Randler et al., 2012 ). The public is also very interested in field trips, which are considered by scientists as the best hands-on activity possible and shown to positively shape environmental attitudes (e.g., Neiman and Ades, 2014 ). On the other extreme, models of animals, which are typically a low priority for scientists, are also the least favorite of the public.
It should be kept in mind, however, that the scientists surveyed here represent an international assemblage (though mostly white and from anglophone countries: United States, United Kingdom and Australia), while the surveyed public is largely composed of New Zealanders. Even though New Zealanders are recognized as a more environmentally-minded public ( Craig et al., 2000 ), we could potentially expect similar answers from the public in those other anglophone countries. However, our findings are potentially not immediately transferable to the public from other countries.
Our questionnaire has shed some light on the types of personal responses to science (as defined by Burns et al., 2003 ; see Introduction ) regarding the difficult subject of invertebrate animals, and we hope our results will inform both scientist-communicators and science communicators alike. Even though the focus of our initial survey was on researchers (who are mostly responsible from communicating invertebrate biology), effective communication can (and should) be done not only by scientists, but also by mediators and even members of the public ( Burns et al., 2003 ).
It is widely acknowledged that scientists prioritize educating the public and correcting misinformation, while largely ignoring communication that builds trust and resonance with the public ( Dudo and Besley, 2016 ). However, this is hardly attuned to the public’s interests, so communicators need to understand the public’s values and priorities to successfully transmit their message ( Berentson-Shaw, 2018 ). Science communication is not simply a one-way, top-down process, but rather it should be an ongoing dialogue between communicators and the public ( Miller, 2001 ; Davies, 2008 ; Salmon et al., 2017 ). Even so, the onus is not entirely on scientists and communicators: the gaps in public knowledge actually do exist and is potentially a symptom of a still ineffective science curriculum in all levels of education (e.g., Moore, 1990 ; Smith, 2010 ; Waldrop, 2015 ). One of our respondents, a member of the public, even told us on Q2.21: “I learned what an invertebrate is!”
While our sample of public and scientists were not as diverse as we wanted, they are still useful to help us to draw some conclusions about the scientists vs. public perception. In particular, even though the public was very much biased toward a nature-friendly audience, the responses were still highly contrasting to the scientist’s expectation. Thus, we can speculate that responses would be even more contrasting if a wider public was surveyed.
Our study assessed both sides of the story, searching for what is in agreement between scientists (internationally) and the (largely New Zealand) public and what is discrepant. We discovered that very few things are in fact aligned ( Figures 1 , 2 ), so there is still plenty of opportunity for invertebrate scientists to learn and improve on their science communication efforts. Further studies could focus on investigating these “conflicts” in more detail, in order to fine-tune ways to address them. However, our results point toward topics and approaches that, in general, could be better explored, such as folklore, pop culture and pathology, considering the appropriate age groups of the audience. It will be of utmost importance to understand better the role that age plays on the public’s interests and also to investigate peculiarities of publics from different countries and different social media platforms. Finally, our results bring clear indications ( Figure 3 ) of aspects where managers of museums, universities and other institutions could start to allocate a larger budget.
While our study deals only with a limited aspect of invertebrate science communication, we believe its results will be informative and useful for communicators and educators. Furthermore, as we cannot answer all questions and solve all issues in a single paper, we hope this contribution will serve as a starting point for future research in the area.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Human Ethics Committee, Victoria University of Wellington. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
RS conceptualized and led the project and writing of the manuscript. All authors helped in delineating the surveys and writing the manuscript. KO’D conducted the in-person survey. JT and BT were responsible for data clean-up and analysis.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are very grateful to all the people who took their time to answer our questionnaire and share their experiences; this research would not have been possible without them. Our sincere thanks to Te Papa’s staff for the help during the survey, especially to Claire Gibb, Jack Fisher and Rachael Hockridge; to Matt Scott, the Center for Science in Society and the Human Ethics Committee at Victoria University of Wellington for their support and feedback; and to the three reviewers for constructive comments and suggestions. RS acknowledges the bequest of Bruce Fraser Hazelwood and Te Papa; KO’D received a Summer Research Scholarship from VUW and Te Papa.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2021.606416/full#supplementary-material .
Anderson, S. C., Flemming, J. M., Watson, R., and Lotze, H. K. (2011). Rapid global expansion of invertebrate fisheries: trends, drivers, and ecosystem effects. PLoS One 6 (3), e14735. doi:10.1371/journal.pone.0014735
PubMed Abstract | CrossRef Full Text | Google Scholar
Andrews, E., Weaver, A., Hanley, D., Shamatha, J., and Melton, G. (2005). Scientists and public outreach: participation, motivations, and impediments. J. Geosci. Edu. 53 (3), 281–293. doi:10.5408/1089-9995-53.3.281
CrossRef Full Text | Google Scholar
Ashwell, D. J. (2016). The challenges of science journalism: the perspectives of scientists, science communication advisors and journalists from New Zealand. Public Understanding Sci. 25 (3), 379–393. doi:10.1177/0963662514556144
Ballantyne, R., and Packer, R. (2016). Visitors’ perceptions of the conservation education role of zoos and aquariums: implications for the provision of learning experiences. Visitor Stud. 19, 193–210. doi:10.1080/10645578.2016.1220185
Balogun, W. G., Cobham, A. E., Amin, A., and Seeni, A. (2018). Using invertebrate model organisms for neuroscience research and training: an opportunity for Africa. Metab. Brain Dis. 33, 1431–1441. doi:10.1007/s11011-018-0250-2
Batt, S. (2009). Human attitudes towards animals in relation to species similarity to humans: a multivariate approach. Biosci. Horizons 2 (2), 180–190. doi:10.1093/biohorizons/hzp021
Berenbaum, M. R. (2017). Communicating about science communication: a brief entomological history. Ann. Entomol. Soc. America 110 (5), 435–438. doi:10.1093/aesa/sax060
Berentson-Shaw, J. (2018). A matter of fact: talking truth in a post-truth world . Wellington: BWB , 192.
Besley, J. C., and Tanner, A. H. (2011). What science communication scholars think about training scientists to communicate. Sci. Commun. 33 (2), 239–263. doi:10.1177/1075547010386972
Bixler, R. D., Carlisle, C. L., Hammitt, W. E., and Floyd, M. F. (1994). Observed fears and discomforts among urban students on field trips to wildland areas. J. Environ. Edu. 26, 24–33. doi:10.1080/00958964.1994.9941430
Bixler, R. D., and Floyd, M. F. (1999). Hands on or hands off? Disgust sensitivity and preference for environmental education activities. J. Environ. Edu. 30 (3), 4–11. doi:10.1080/00958969909601871
Bixler, R. D., and Floyd, M. F. (1997). Nature is scary, disgusting, and uncomfortable. Environ. Behav. 29 (4), 443–467. doi:10.1177/001391659702900401
Bjerke, T., and Østdahl, T. (2004). Animal-related attitudes and activities in an urban population. Anthrozoös 17, 109–129. doi:10.2752/089279304786991783
Black, S. H., Shepard, M., and Allen, M. M. (2001). Endangered invertebrates: the case for greater attention to invertebrate conservation. Endangered Species Update 18, 42–50.
Google Scholar
Bowater, L., and Yeoman, K. (2012). Science communication: a practical guide for scientists . Chichester: Wiley-Blackwell , 384.
Brinson, J. R. (2015). Learning outcome achievement in non-traditional (virtual and remote) versus traditional (hands-on) laboratories: a review of the empirical research. Comput. Edu. 87, 218–237. doi:10.1016/j.compedu.2015.07.003
Brockington, D., Igoe, J., and Schmidt-Soltau, K. (2006). Conservation, human rights, and poverty reduction. Conservation Biol. 20, 250–252. doi:10.1111/j.1523-1739.2006.00335.x
Bubela, T., Nisbet, M. C., Borchelt, R., Brunger, F., Critchley, C., Einsiedel, E., et al. (2009). Science communication reconsidered. Nat. Biotechnol. 27 (6), 514–518. doi:10.1038/nbt0609-514
Burns, T. W., O’Connor, D. J., and Stocklmayer, S. M. (2003). Science communication: a contemporary definition. Public Underst Sci. 12, 183–202. doi:10.1177/09636625030122004
Cardoso, P., Erwin, T. L., Borges, P. A. V., and New, T. R. (2011). The seven impediments in invertebrate conservation and how to overcome them. Biol. Conservation 144 (11), 2647–2655. doi:10.1016/j.biocon.2011.07.024
Carmichael, R. H., Bottom, M. L., Shin, P. K. S., and Cheung, S. G. (2015). Changing global perspectives on horseshoe crab biology, conservation and management . New York, NY: Springer , 599.
Craig, J., Anderson, S., Clout, M., Creese, B., Mitchell, N., Ogden, J., et al. (2000). Conservation issues in New Zealand. Annu. Rev. Ecol. Syst. 31, 61–78. doi:10.1146/annurev.ecolsys.31.1.61
Czekanski-Moir, J. E., and Rundell, R. J. (2020). Endless forms most stupid, icky, and small: the preponderance of noncharismatic invertebrates as integral to a biologically sound view of life. Acad. Practive Ecol. Evol. 10 (23), 12638–12649. doi:10.1002/ece3.6892
Dahlstrom, M. F. (2014). Using narratives and storytelling to communicate science with nonexpert audiences. PNAS 111 (Suppl. 4), 13614–13620. doi:10.1073/pnas.1320645111
Davies, J. (2018). World cloud generator Available at: https://www.jasondavies.com/wordcloud/ (Accessed May 7, 2019)
Davies, S. R. (2008). Constructing communication: talking to scientists about talking to the public. Sci. Commun. 29 (4), 413–434. doi:10.1177/1075547008316222
Dietz, T., Kalof, L., and Stern, P. C. (2002). Gender, values, and environmentalism. Social Sci. Q 83 (1), 353–364. doi:10.1111/1540-6237.00088
DiResta, R. (2019). How Amazon’s algorithms curated a dystopian bookstore. Wired . Available from: https://www.wired.com/story/amazon-and-the-spread-of-health-misinformation/ , A19, May, 3 (Accessed May 7, 2019)
Dorward, L. J., Mittermeier, J. C., Sandbrook, C., and Spooner, F. (2017). Pokémon Go: benefits, costs, and lessons for the conservation movement. Conservation Lett. 10 (1), 160–165. doi:10.1111/conl.12326
Dudo, A., and Besley, J. C. (2016). Scientists’ prioritization of communication objectives for public engagement. PLoS One 11 (2), e0148867. doi:10.1371/journal.pone.0148867
Fan, W., and Yan, Z. (2010). Factors affecting response rates of the web survey: a systematic review. Comput. Hum. Behav. 26, 132–139. doi:10.1016/j.chb.2009.10.015
Fančovičová, J., and Prokop, P. (2011). Plants have a chance: outdoor educational programmes alter students’ knowledge and attitudes towards plants. Environ. Edu. Res. 17, 537–551. doi:10.1080/13504622.2010.545874
Gallo‐Cajiao, E., Archibald, C., Friedman, R., Steven, R., Fuller, R. A., Game, E. T., et al. (2018). Crowdfunding biodiversity conservation. Conservation Biol. 32 (6), 1426–1435. doi:10.1111/cobi.13144
Gelperin, A. (2019). “Recent trends in invertebrate neuroscience,” in The oxford handbook of invertebrate neurobiology . Editor J. H. Byrne (New York: Oxford University Press ), 1–57.
Groves, R. M., Cialdini, R. B., and Couper, M. P. (1992). Understanding the decision to participate in a survey. Public Opin. Q. 56, 475–495. doi:10.1086/269338
Horst, M., Davies, S. R., and Irwin, A. (2017). “Reframing science communication,” in The handbook of science and technology studies . 4th Edn, Editors U. Felt, R. Fouché, C. A. Miller, and L. Smith-Doerr (Cambridge: MIT Press ), 881–907.
Jennings, B. H. (2011). Drosophila —a versatile model in biology & medicine. Biomaterials 14 (5), 190–195. doi:10.1016/s1369-7021(11)70113-4
Jones, B. L., Unsworth, R. K. F., McKenzie, L. J., Yoshida, R. L., and Cullen-Unsworth, L. C. (2017). Crowdsourcing conservation: the role of citizen science in securing a future for seagrass. Mar. Pollut. Bull. 134, 210–215. doi:10.1016/j.marpolbul.2017.11.005
Kellert, S. R. (1993a). Attitudes, knowledge, and behavior to-ward wildlife among the industrial superpowers: United States, Japan, and Germany. J. Soc. Issues 49, 53–69. doi:10.1111/j.1540-4560.1993.tb00908.x
Kellert, S. R. (1993b). Values and perceptions of invertebrates. Conservation Biol. 7, 845–855. doi:10.1046/j.1523-1739.1993.740845.x
Knight, A. J. (2008). “Bats, snakes and spiders, Oh my!” How aesthetic and negativistic attitudes, and other concepts predict support for species protection. J. Environ. Psychol. 28, 94–103. doi:10.1016/j.jenvp.2007.10.001
Lessard, B. D., Whiffin, A. L., and Wild, A. L. (2017). A guide to public engagement for entomological collections and natural history museums in the age of social media. Ann. Entomol. Soc. America 110 (5), 467–479. doi:10.1093/aesa/sax058
Lewis, P. (2018). “Fiction is outperforming reality’: how YouTube’s algorithm distorts truth. The Guardian . Available at: https://www.theguardian.com/technology/2018/feb/02/how-youtubes-algorithm-distorts-truth , A18, February, 2 (Accessed May 7, 2019)
Losey, J. E., and Vaughan, M. (2006). The economic value of ecological services provided by insects. Bioscience 56, 311–323. doi:10.1641/0006-3568(2006)56[311:tevoes]2.0.co;2
Madau, F. A., Arru, B., Furesi, R., and Pulina, P. (2020). Insect farming for feed and food production from a circular business model perspective. Sustainability 12, 5418. doi:10.3390/su12135418
McCabe, S. E., Couper, M. P., Cranford, J. A., and Boyd, C. J. (2006). Comparison of Web and mail surveys for studying secondary consequences associated with substance use: evidence for minimal mode effects. Addict. Behaviors 31, 162–168. doi:10.1016/j.addbeh.2005.04.018
McComas, K. A., Arvai, J. L., and Besley, J. C. (2008). “Linking public participation and decision making through risk communication,” in Handbook of crisis and risk communication . Editors R. L. Heath, and D. H. O’Hair (New York: Routledge ), 364–385.
Metz, C. J., Downes, S., and Metz, M. J. (2018). The in’s and out’s of science outreach: assessment of an engaging new program. Adv. Physiol. Edu. 42 (3), 487–492. doi:10.1152/advan.00085.2018
Miller, S. (2001). Public understanding of science at the crossroads. Public Understanding Sci. 10 (1), 115–120. doi:10.1088/0963-6625/10/1/308
Moore, R. (1990). What’s wrong with science education & how do we fix it? Am. Biol. Teach. 52 (6), 330–337. doi:10.2307/4449128
Neiman, Z., and Ades, C. (2014). Contact with nature: effects of field trips on pro-environmental knowledge, intentions and attitudes. Ciênc. Educ. 20 (4), 889–902. doi:10.1590/1516-73132014000400008
New, T. R. (1993). Angels on a pin: dimensions of the crisis in invertebrate conservation. Am. Zool 33, 623–630. doi:10.1093/icb/33.6.623
Nisbet, M. C., and Scheufele, D. A. (2009). What’s next for science communication? Promising directions and lingering distractions. Am. J. Bot. 96 (10), 1767–1778. doi:10.3732/ajb.0900041
Öst, L.-G. (1989). One-session treatment for specific phobias. Behav. Res. Ther. 27 (1), 1–7. doi:10.1016/0005-7967(89)90113-7
Packer, J., and Ballantyne, R. (2010). The role of zoos and aquariums in education for a sustainable future. New Dir. Adult Cont. Edu. 2010 (127), 25–34. doi:10.1002/ace.378
Patterson, T., and Barratt, S. (2019). Playing for the planet—how video games can deliver for people and the environment . Arendal: UN Environment/GRID-Arendal , 23
Peterson, I. (2005). Sea shell spirals. Sci. News Soc. Sci. Public . Available at: https://www.sciencenews.org/article/sea-shell-spirals (Accessed June 27, 2018)
Plotnick, R. E., Stigall, A. L., and Stefanescu, I. (2014). Evolution of paleontology: long-term gender trends in an earth-science discipline. Gsat . 24 (11), 44–45. doi:10.1130/gsatg219gw.1
Pontes-da-Silva, E., Pacheco, M. L. T., Pequeno, P. A. C. L., Franklin, E., and Kaefer, I. L. (2016). Attitudes towards scorpions and frogs: a survey among teachers and students from schools in the vicinity of an Amazonian protected area. J. Ethnobiol. 36, 395–411. doi:10.2993/0278-0771-36.2.395
Prokop, P., Kubiatko, M., and Fančovičová, J. (2008). Slovakian pupils’ knowledge of, and attitudes toward, birds. Anthrozoös 21, 221–235. doi:10.2752/175303708x332035
Prokop, P., Kubiatko, M., and Fančovičová, J. (2009). Vampires are still alive: slovakian students’ attitudes toward bats. Anthrozoös 22, 19–30. doi:10.2752/175303708x390446
Prokop, P., Tolarovičová, A., Camerik, A. M., and Peterková, V. (2010). High school students’ attitudes towards spiders: a cross‐cultural comparison. Int. J. Sci. Edu. 32, 1665–1688. doi:10.1080/09500690903253908
R Core Team (2020). R: a language and environment for statistical computing. R foundation for statistical computing, Vienna. Available at: https://www.R-project.org/ (Accessed June 22, 2020)
Randler, C., Hummel, E., and Prokop, P. (2012). Practical work at school reduces disgust and fear of unpopular animals. Soc. Anim. 20, 61–74. doi:10.1163/156853012x614369
Reeve, M. A., and Partridge, M. (2017). The use of social media to combat research-isolation. Ann. Entomol. Soc. America 110 (5), 449–456. doi:10.1093/aesa/sax051
Reumont, B. V., Campbell, L., and Jenner, R. (2014). Quo vadis venomics? A roadmap to neglected venomous invertebrates. Toxins 6, 3488–3551. doi:10.3390/toxins6123488
Saleh, A., and Bista, K. (2017). Examining factors impacting online survey response rates in educational research: perceptions of graduate students. J. MultiDisciplinary Eval. 13 (29), 63–74.
Salmon, R. A., Priestley, R. K., and Goven, J. (2017). The reflexive scientist: an approach to transforming public engagement. J. Environ. Stud. Sci. 7 (1), 53–68. doi:10.1007/s13412-015-0274-4
Salvador, R. B. (2017). The unexplored potential of video games for animal conservation. Tentacle 25, 3–5.
Salvador, R. B., and Tomotani, B. M. (2014). The Kraken: when myth encounters science. Hist. Cienc. Saude-manguinhos 21, 971–994. doi:10.1590/s0104-59702014000300010
Schiffman, D. S. (2012). Twitter as a tool for conservation education and outreach: what scientific conferences can do to promote live-tweeting. J. Environ. Stud. Sci. 2 (3), 257–262. doi:10.1007/s13412-012-0080-1
Seitz, N., Traynor, K. S., Steinhauer, N., Rennich, K., Wilson, M. E., Ellis, D., et al. (2016). A national survey of managed honey bee 2014–2015 annual colony losses in the USA. J. Apicultural Res. 54, 291–304. doi:10.1080/00218839.2016.1153294
Siddig, A. A. H., Ellison, A. M., Ochs, A., Villar-Leeman, C., and Lau, M. K. (2016). How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in Ecological Indicators. Ecol. Indicators 60, 223–230. doi:10.1016/j.ecolind.2015.06.036
Silva, A., and Minor, E. S. (2017). Adolescents’ experience and knowledge of, and attitudes toward, bees: implications and recommendations for conservation. Anthrozoös 30, 19–32. doi:10.1080/08927936.2017.1270587
Smith, M. U. (2010). Current status of research in teaching and learning evolution: II. pedagogical issues. Sci. Educ. 19 (6–8), 539–571. doi:10.1007/s11191-009-9216-4
Thomas, C. D., Jones, T. H., and Hartley, S. E. (2019). “Insectageddon”: a call for more robust data and rigorous analyses. Glob. Change Biol. 25 (6), 1891–1892. doi:10.1111/gcb.14608
Treise, D., and Weigold, M. F. (2002). Advancing science communication: a survey of science communicators. Sci. Commun. 23 (3), 310–322. doi:10.1177/107554700202300306
Trumbo, J. (1999). Visual literacy and science communication. Sci. Commun. 20 (4), 409–425. doi:10.1177/1075547099020004004
van Deursen, A., and Helsper, E. (2015). A nuanced understanding of Internet use and non-use amongst older adults. Eur. J. Commun. 30 (2), 171–187. doi:10.1177/0267323115578059
van Dijck, J. (2003). After the “Two Cultures”: toward a “(multi)cultural” practice of science communication. Sci. Commun. 25 (2), 177–190. doi:10.1177/1075547003259540
Waldrop, M. M. (2015). The science of teaching science. Nature 523, 272–274. doi:10.1038/523272a
Weber, J. R., and Word, C. S. (2001). The communication process as evaluative context: what do nonscientists hear when scientists speak? Scientists and nonscientists benefit by recognizing that attempts at mutual influence, multiple frames of reference, and “objective” information in science communication are not neutral but evaluated with other social influences. BioScience 51 (6), 487–495. doi:10.1641/0006-3568(2001)051[0487:tcpaec]2.0.co;2
Wilson-Sanders, S. E. (2011). Invertebrate models for biomedical research, testing, and education. Inst. Lab. Anim. Res. J. 52 (2), 126–152. doi:10.1093/ilar.52.2.126
Yetter, G., and Capaccioli, K. (2010). Differences in responses to web and paper surveys among school professionals. Behav. Res. Methods 42 (1), 266–272. doi:10.3758/BRM.42.1.266
Keywords: conservation, hands-on activities, outreach, public engagement, social media
Citation: Salvador RB, Tomotani BM, O’Donnell KL, Cavallari DC, Tomotani JV, Salmon RA and Kasper J (2021) Invertebrates in Science Communication: Confronting Scientists’ Practices and the Public’s Expectations. Front. Environ. Sci. 9:606416. doi: 10.3389/fenvs.2021.606416
Received: 14 September 2020; Accepted: 28 January 2021; Published: 09 March 2021.
Reviewed by:
Copyright © 2021 Salvador, Tomotani, O’Donnell, Cavallari, Tomotani, Salmon and Kasper. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rodrigo B. Salvador, [email protected]

Why Are Invertebrates Important?
Invertebrates, a diverse group of animals lacking a backbone, play significant ecological roles and possess various economic and cultural importance. Understanding the importance of these organisms is crucial for maintaining ecosystem functioning and promoting conservation efforts.
This article aims to explore the reasons why invertebrates are important. Firstly, we will examine their ecological roles, such as pollination and plant reproduction, decomposition and nutrient cycling, and their significance as food sources for other organisms.
Secondly, we will discuss the economic value that invertebrates hold through their use in agriculture, medicine, and industry.
Thirdly, we will delve into the cultural significance and symbolism attributed to certain invertebrate species across different societies.
Lastly, we will highlight the need for conservation measures to protect these creatures due to their vulnerability to habitat loss and environmental changes.
By comprehensively understanding the importance of invertebrates, we can facilitate informed decision-making regarding their preservation while ensuring the sustainability of ecosystems they inhabit.

Ecological Roles of Invertebrates
Invertebrates play crucial ecological roles through their diverse interactions with other organisms. They serve as decomposers, pollinators, and predators.
As decomposers, invertebrates contribute to the functioning of ecosystems by breaking down organic matter and recycling nutrients. They help maintain soil fertility and nutrient cycling.
Many invertebrates also act as pollinators, facilitating reproduction in flowering plants. They ensure the production of fruits and seeds, which is important for maintaining biodiversity and supporting food webs.
Invertebrates participate in predator-prey dynamics, regulating populations of other organisms. They help maintain balance within ecosystems.
Some invertebrates engage in habitat engineering activities, such as building burrows or creating physical structures. These activities enhance ecosystem structure and function.
Overall, the ecological roles played by invertebrates are essential for the stability and sustainability of ecosystems.
Pollination and Plant Reproduction
Pollination and plant reproduction heavily rely on the active involvement of various organisms within ecosystems. Pollinators, such as bees, birds, butterflies, and bats, play a crucial role in transferring pollen from the male reproductive structures (anthers) to the female reproductive structures (stigma) of flowers. This transfer of pollen facilitates fertilization and subsequent seed production in plants. Without pollinators, many plant species would struggle to reproduce successfully, leading to reduced genetic diversity and potentially impacting ecosystem stability.
The relationship between plants and their pollinators is often mutually beneficial. Pollinators obtain nectar or other rewards from flowers while inadvertently transferring pollen in the process. As they move from flower to flower in search of resources, they facilitate cross-pollination among different individuals within a plant population, increasing genetic variation.
In addition to facilitating reproduction for individual plants, pollinators also contribute to ecosystem functioning by promoting biodiversity. By enabling the reproduction of various plant species, they support diverse habitats that provide food sources for other organisms. Furthermore, pollinator-dependent crops are essential for human food security and economic well-being.
Therefore, understanding the importance of pollinators in plant reproduction is crucial for conserving biodiversity and ensuring sustainable ecosystems.
Decomposition and Nutrient Cycling
Decomposition, a vital process in ecosystems, involves the breakdown of organic matter by microorganisms and contributes to nutrient cycling.
In this process, invertebrates play crucial roles. They act as decomposers by feeding on dead plant and animal material, accelerating the decomposition process.
Invertebrates such as worms, beetles, and flies break down complex organic compounds into simpler forms that can be easily absorbed by plants and utilized for growth.
Additionally, these organisms help to release nutrients locked within dead organic matter back into the environment. This nutrient recycling is essential for maintaining soil fertility and supporting plant growth.
Furthermore, invertebrates also contribute to the physical breakdown of organic matter through their burrowing activities, which improves soil structure and aeration.
Overall, invertebrates’ involvement in decomposition processes facilitates nutrient cycling and plays a pivotal role in sustaining healthy ecosystems.
Invertebrates as Food Sources
Insects and other small invertebrates serve as valuable sources of food for a wide range of animals, including birds, bats, and amphibians. These creatures play a crucial role in maintaining the balance of ecosystems by serving as intermediate links in the food chain.
Moreover, they also contribute to nutrient cycling by consuming organic matter and breaking it down into simpler compounds.
In addition to their ecological importance, invertebrates have proven to be valuable bioindicators. Their presence or absence can indicate the health of an ecosystem and provide insight into environmental conditions.
Furthermore, certain invertebrates are utilized in medical research due to their unique physiological characteristics that make them suitable models for studying human diseases. By studying these organisms, scientists can gain a better understanding of biological processes and develop potential treatments for various ailments.
Economic Importance of Invertebrates
Arthropods, such as bees and butterflies, contribute significantly to the global economy through their role in crop pollination. Invertebrate agriculture plays a crucial role in providing food for both humans and animals.
Insects like ants and beetles help decompose organic matter, enhancing soil fertility. Additionally, invertebrates have immense economic value in the field of medical research. For example, horseshoe crabs are used for testing bacterial contamination in medical devices and vaccines due to their unique immune system.
Furthermore, spiders produce silk that has numerous industrial applications, including manufacturing bulletproof vests and surgical sutures. The economic importance of invertebrates extends beyond these examples as they also serve as indicators of environmental health and play integral roles in various ecosystems.
Understanding and conserving these creatures is vital for maintaining ecological balance and sustaining our global economy.
Cultural Significance and Symbolism
Cultural significance and symbolism can be observed in the various ways that invertebrates are represented and revered in different cultures around the world.
In many cultural traditions, certain invertebrate species hold symbolic meanings and associations. For example, butterflies are often seen as symbols of transformation and rebirth due to their life cycle from caterpillar to butterfly. Similarly, spiders are sometimes associated with creativity and weaving, as their intricate webs symbolize the interconnectedness of life.
These symbolic representations of invertebrates can be found in various forms of artistic expression such as paintings, sculptures, and traditional crafts.
In addition to their symbolic value, invertebrates also play a role in cultural practices like insect-based cuisine or medicinal uses derived from specific species.
Therefore, understanding the cultural significance and symbolism attributed to invertebrates provides insights into human perceptions and relationships with these organisms throughout history.

Conservation and Protection of Invertebrates
The preservation of diverse invertebrate species is crucial for maintaining the ecological balance and biodiversity of ecosystems. Invertebrates play a key role in various ecological processes such as nutrient cycling, pollination, and decomposition. However, they are facing significant threats due to invertebrate habitat loss and the decline of certain species. This poses a serious concern as numerous invertebrates are already listed as endangered or at risk of extinction.
In order to combat these challenges, conservation efforts focus on protecting and restoring invertebrate habitats through the establishment of protected areas, implementing habitat restoration programs, and promoting sustainable agricultural practices. These measures aim to safeguard both individual species and the overall functioning of ecosystems by ensuring the survival of diverse invertebrate populations.
Bryan Harding is a member of the American Society of Mammalogists and a member of the American Birding Association. Bryan is especially fond of mammals and has studied and worked with them around the world. Bryan serves as owner, writer, and publisher of North American Nature.

Invertebrates

The little things that run the world
Invertebrates -animals without a backbone- have been accorded little attention in science, considering how incredibly biodiverse they are and their key role in maintaining the healthy functioning of our ecosystems.
6.1 million species of insects
We have an inkling of the global importance of invertebrates but have merely scratched the surface of their complex evolutionary history, ecological roles, interactions and behaviour.
It has been estimated that there are seven million terrestrial arthropod species on Earth consisting of 6.1 million species of insects, 1.5 million of which are beetles. Tropical forests hold a majority of these species, yet few such places have been adequately sampled for alpha diversity, and there remains even more uncertainty about beta diversity.
From an ecological point of view, we’re interested in the functions they have
Invertebrates have important roles in the functioning of ecosystems: nutrient cycling, pollination, and herbivory. We need to better understand these complex interactions to predict how they are likely to change in the face of a rapidly changing climate. As we move into an era of increased pressure on old-growth habitats and biodiversity, it is imperative that we understand how changes to invertebrate communities, and the extinction of species, affect ecosystems and those dependant on them.
Digg deeper
The Future of Tropical Invertebrate Research , a special issue published by @Biotropica, dives into the latest science on invertebrate and highlights priorities for tropical invertebrate research: conservation and sustainability (Stone et al., 2020), technology and methods (Raine et al. 2020), biogeography and ecosystem functioning (França et al., 2020) and highlight why they are important (Dahlsjö et al., 2020; Kitching et al., 2020). These studies add to our general understanding of the dynamics of tropical ecosystems. They also provide powerful tools for monitoring and responding to environmental change.
- Special Issue: The Future of Tropical Invertebrate Research , Edited by Cecilia Dahlsjö, and Roger Kitching, Biotropica, 52: 203-395, 2020.
- Dahlsjö, CAL, et al., Tropical terrestrial invertebrates—Where to from here?, Biotropica, 52: 391– 394, 2020.
- Raine, EH, Mikich, SB, Lewis, OT, Slade, EM. Linking dung beetle‐mediated functions to interactions in the Atlantic Forest: Sampling design matters, Biotropica, 52: 215– 220, 2020.
- Kitching, RL, et al. Invertebrates and the complexity of tropical ecosystems, Biotropica, 52: 207– 214, 2020.
- Stone, MJ, et al. Recovery of decomposition rates and decomposer invertebrates during rain forest restoration on disused pasture, Biotropica, 52: 230– 241, 2020.
- França, FM, et al. El Niño impacts on human‐modified tropical forests: Consequences for dung beetle diversity and associated ecological processes, Biotropica, 52: 252– 262, 2020.
Stressed beetles take less crap!

Let’s dig a little deeper into the wonderful world of Invertebrates. Drs. Filipe França and Joice Ferreira explore the role of dung beetles in rainforests. They find that dung beetles help rainforests regrow, but that extreme drought and wildfires in the Amazon are killing them off.
Dung beetles are hard shelled scarabs that live on every continent except Antarctica. They play a very important role in maintaining healthy ecosystems: they recycle feces, suppress parasites that could otherwise harm people and animals, spread seeds and nutrients in the soil. Dung beetles are also a good indicator of how healthy an ecosystem is. They are one of the first to suffer when their habitat is struggling. In tropical forests, for example, stress caused by environmental disturbances causes dung beetles to gain body fat and work less. Less beetles means less seed dispersal. This means slower re-growth of the forest after a big disturbance, and sometimes not enough regrowth before the next big disturbance hits the forest (França and Ferreira, 2020).
“Since 2010, we have collected and studied over 14,000 dung beetles from 98 different species in the vast and still wild interior of Brazil’s Santarém region, a remote corner of the Amazon forest – part of a long-term project with the Sustainable Amazon Network,” describe Drs. Filipe França and Joice Ferreira. “Most recently, we studied dung beetles to assess the Amazon’s recovery from the intense drought and forest fires of 2015 and 2016, extreme climatic events brought on by the most severe El Niño on record. (…) our study reveals that both forest fires and drought are far more damaging than previously thought.”
Tapirs, monkeys, ants, bees, beetles, and wasps are also powerful seed dispersers that suffer from environmental stress. “From our field sites deep in the Amazon, we are rooting for all the little creeping and crawling creatures that keep the world running.” (França, FM, et al., 2020).
Other recent stories

- Subject List
- Take a Tour
- For Authors
- Subscriber Services
- Publications
- African American Studies
- African Studies
- American Literature
- Anthropology
- Architecture Planning and Preservation
- Art History
- Atlantic History
- Biblical Studies
- British and Irish Literature
- Childhood Studies
- Chinese Studies
- Cinema and Media Studies
- Communication
- Criminology
- Environmental Science
- Evolutionary Biology
- International Law
- International Relations
- Islamic Studies
- Jewish Studies
- Latin American Studies
- Latino Studies
- Linguistics
- Literary and Critical Theory
- Medieval Studies
- Military History
- Political Science
- Public Health
- Renaissance and Reformation
- Social Work
- Urban Studies
- Victorian Literature
- Browse All Subjects
How to Subscribe
- Free Trials
In This Article Expand or collapse the "in this article" section Freshwater Invertebrate Ecology
Introduction, general overviews.
- Habitats and Distributions
- Trophic Relationships
- Secondary Production
- Biological Assessment
- Conservation
Related Articles Expand or collapse the "related articles" section about
About related articles close popup.
Lorem Ipsum Sit Dolor Amet
Vestibulum ante ipsum primis in faucibus orci luctus et ultrices posuere cubilia Curae; Aliquam ligula odio, euismod ut aliquam et, vestibulum nec risus. Nulla viverra, arcu et iaculis consequat, justo diam ornare tellus, semper ultrices tellus nunc eu tellus.
- Bacterial Diversity in Freshwater
- Biodiversity and Ecosystem Functioning
- Conservation Biology
- Stream Ecology
- Terrestrial Insect Ecology
- Trophic Levels
Other Subject Areas
Forthcoming articles expand or collapse the "forthcoming articles" section.
- Abundance Biomass Comparison Method
- Modeling and Data Analysis in Movement Ecology
- Sclerochronology
- Find more forthcoming articles...
- Export Citations
- Share This Facebook LinkedIn Twitter
Freshwater Invertebrate Ecology by Matt R. Whiles LAST REVIEWED: 24 April 2023 LAST MODIFIED: 26 August 2013 DOI: 10.1093/obo/9780199830060-0132
The field of freshwater invertebrate ecology has developed for well over a century now. Ecologists studying freshwater invertebrates have made numerous significant contributions to both applied and theoretical facets of the field of ecology, as well as to other aspects of the biological sciences. Human interest in this particular group of organisms stems from their important roles in freshwater food webs, their link to the production of focal management groups such as fishes and waterfowl, and their roles in the transmission of some of the most important human diseases on the planet, such as Malaria and Schistosomiasis. Freshwater invertebrates are also increasingly used by resource managers for biological assessment of freshwater habitat integrity, and some, such as Daphnia (Cladocera: Daphniidae) and Chironomus tentans (Diptera: Chironomidae), are model organisms for toxicological studies. Along with their applied significance, freshwater invertebrate populations and communities can make excellent models for basic ecological studies because they are present in virtually every freshwater habitat on the planet and, in many cases, a given assemblage presents tremendous diversity in forms and functions. Currently, there is a great deal of interest among freshwater ecologists on the ecological roles of freshwater invertebrates, including how they influence ecosystem processes and functions such as decomposition, primary production, and nutrient cycling, and how those with amphibious life cycles can link aquatic and terrestrial habitats through energy and nutrient subsidies.
Hynes 1970a , a classic book on general stream ecology, moved the field of freshwater invertebrate ecology forward through careful synthesis of existing information and progressive thinking on research directions, particularly on the ecological roles of stream invertebrates. That same year, Hynes 1970b provided a synthesis of many aspects of freshwater invertebrate ecology in a now decades-old review that is still highly cited. Many of the concepts in both of Hynes’s classic works are evident in one of the most highly cited pieces of literature in freshwater ecology, the river continuum concept in Vannote, et al. 1980 , which presents a conceptual model of stream continua, including how the relative importance of invertebrate functional feeding groups changes predictably from headwaters to large rivers. Resh and Rosenberg 1984 , the classic edited book The Ecology of Aquatic Insects that is now out of print and hard to find, provides a synthesis of many aspects of freshwater invertebrate ecology and served as one of the primary resources on the topic for many years. More recent books, including Ward 1992 and Williams and Feltmate 1992 , provide more updated summaries and syntheses, but are also becoming dated. Although brief, Cummins, et al. 2008 provides a relatively up-to-date overview of aquatic insect ecology. Many limnology and freshwater ecology textbooks provide general information on freshwater invertebrate ecology. Dodds and Whiles 2010 , a textbook on freshwater ecology, includes numerous sections on freshwater invertebrates. Among the freshwater invertebrates, aquatic insects and planktonic crustaceans such as copepods and cladocerans are often the foci of ecological studies, and this bias is evident in the literature.
Cummins, Kenneth W., Richard W. Merritt, and Martin B. Berg. 2008. Ecology and distribution of aquatic insects. In An introduction to the aquatic insects of North America . 4th ed. By Kenneth W. Cummins, Richard W. Merritt, and Martin B. Berg, 105–122. Dubuque, IA: Kendall/Hunt.
This book chapter provides a brief but very informative and up-to-date overview of aquatic insect ecology, with information summarized in easily interpreted tables and figures. Many other chapters in this edited book include information on various aspects of aquatic invertebrate ecology, but it is obviously focused on the insect groups.
Dodds, Walter K., and Matt R. Whiles. 2010. Freshwater ecology: Concepts and environmental applications of limnology . 2d ed. Amsterdam and Boston: Academic Press.
An intermediate-level general freshwater ecology text, this book includes numerous chapters and sections covering various aspects of freshwater invertebrate ecology. Much of the information presented here is from the ecosystem perspective, including the roles of aquatic invertebrates in ecosystem processes.
Hynes, H. B. N. 1970a. The ecology of running waters . Toronto: Univ. of Toronto Press.
A classic that helped define and guide the field of stream ecology, this book includes numerous chapters and sections focused on the ecology of invertebrates and other organisms that inhabit streams. Hynes’s progressive, holistic views are evident throughout. This is a must-have for anyone studying freshwater ecology.
Hynes, H. B. N.. 1970b. The ecology of stream insects. Annual Review of Entomology 15:25–42.
DOI: 10.1146/annurev.en.15.010170.000325
A classic in the field by one of the most influential freshwater ecologists, this review has both summarized the state of knowledge and moved the field forward because of the author’s progressive views of freshwater invertebrates and ecology in general. While the article is focused on insects, much of the information is relevant to other groups. Available online for purchase or by subscription.
Resh, Vincent H., and David M. Rosenberg, eds. 1984. The ecology of aquatic insects . New York: Praeger.
Although focused on insects, this edited book served for years as a foundation for anyone studying freshwater invertebrate ecology. Parts are now dated, but overall, the book still provides a wealth of information, with chapters by some of the most influential people in the field at the time. It is out of print and now becoming hard to find.
Vannote, Robin W., G. Wayne Minshall, Kenneth W. Cummins, James R. Sedell, and Colbert E. Cushing. 1980. The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37:130–137.
DOI: 10.1139/f80-017
One of the most influential, highly cited pieces of literature in freshwater ecology, this brief article lays out a conceptual model of stream ecosystem structure and function along longitudinal continua, from headwaters to large rivers. Ecological roles of invertebrate functional groups, and how they change along the continuum, are central. Available online for purchase or by subscription.
Ward, James V. 1992. Aquatic insect ecology . Vol. 1. Biology and habitat . New York: Wiley.
This book, which provides a thorough and detailed overview of many aspects of aquatic insect ecology, is more focused on evolutionary ecology than many other works on the topic.
Williams, Dudley D., and Blair W. Feltmate. 1992. Aquatic insects . Wallingford, UK: CAB International.
A book that provides a wealth of information on basic and applied aspects of aquatic insect ecology. Sections range from explorations of how studies of aquatic insects are increasingly used to address basic ecological concepts and theory, to use of aquatic invertebrates for biological assessment of freshwater habitats.
back to top
Users without a subscription are not able to see the full content on this page. Please subscribe or login .
Oxford Bibliographies Online is available by subscription and perpetual access to institutions. For more information or to contact an Oxford Sales Representative click here .
- About Ecology »
- Meet the Editorial Board »
- Accounting for Ecological Capital
- Adaptive Radiation
- Agroecology
- Allelopathy
- Allocation of Reproductive Resources in Plants
- Animals, Functional Morphology of
- Animals, Reproductive Allocation in
- Animals, Thermoregulation in
- Antarctic Environments and Ecology
- Anthropocentrism
- Applied Ecology
- Approaches and Issues in Historical Ecology
- Aquatic Conservation
- Aquatic Nutrient Cycling
- Archaea, Ecology of
- Assembly Models
- Benthic Ecology
- Biodiversity, Dimensionality of
- Biodiversity, Marine
- Biodiversity Patterns in Agricultural Systms
- Biogeochemistry
- Biological Chaos and Complex Dynamics
- Biological Rhythms
- Biome, Alpine
- Biome, Boreal
- Biome, Desert
- Biome, Grassland
- Biome, Savanna
- Biome, Tundra
- Biomes, African
- Biomes, East Asian
- Biomes, Mountain
- Biomes, North American
- Biomes, South Asian
- Braun, E. Lucy
- Bryophyte Ecology
- Butterfly Ecology
- Carson, Rachel
- Chemical Ecology
- Classification Analysis
- Coastal Dune Habitats
- Coevolution
- Communicating Ecology
- Communities and Ecosystems, Indirect Effects in
- Communities, Top-Down and Bottom-Up Regulation of
- Community Concept, The
- Community Ecology
- Community Genetics
- Community Phenology
- Competition and Coexistence in Animal Communities
- Competition in Plant Communities
- Complexity Theory
- Conservation Genetics
- Coral Reefs
- Darwin, Charles
- Dead Wood in Forest Ecosystems
- Decomposition
- De-Glaciation, Ecology of
- Dendroecology
- Disease Ecology
- Drought as a Disturbance in Forests
- Early Explorers, The
- Earth’s Climate, The
- Eco-Evolutionary Dynamics
- Ecological Dynamics in Fragmented Landscapes
- Ecological Education
- Ecological Engineering
- Ecological Forecasting
- Ecological Informatics
- Ecological Relevance of Speciation
- Ecology, Introductory Sources in
- Ecology, Microbial (Community)
- Ecology of Emerging Zoonotic Viruses
- Ecology of the Atlantic Forest
- Ecology, Stochastic Processes in
- Ecosystem Ecology
- Ecosystem Engineers
- Ecosystem Multifunctionality
- Ecosystem Services
- Ecosystem Services, Conservation of
- Elton, Charles
- Endophytes, Fungal
- Energy Flow
- Environmental Anthropology
- Environmental Justice
- Environments, Extreme
- Ethics, Ecological
- European Natural History Tradition
- Evolutionarily Stable Strategies
- Facilitation and the Organization of Communities
- Fern and Lycophyte Ecology
- Fire Ecology
- Fishes, Climate Change Effects on
- Flood Ecology
- Foraging Behavior, Implications of
- Foraging, Optimal
- Forests, Temperate Coniferous
- Forests, Temperate Deciduous
- Freshwater Invertebrate Ecology
- Genetic Considerations in Plant Ecological Restoration
- Genomics, Ecological
- Geographic Range
- Gleason, Henry
- Grazer Ecology
- Greig-Smith, Peter
- Gymnosperm Ecology
- Habitat Selection
- Harper, John L.
- Harvesting Alternative Water Resources (US West)
- Heavy Metal Tolerance
- Heterogeneity
- Himalaya, Ecology of the
- Host-Parasitoid Interactions
- Human Ecology
- Human Ecology of the Andes
- Human-Wildlife Conflict and Coexistence
- Hutchinson, G. Evelyn
- Indigenous Ecologies
- Industrial Ecology
- Insect Ecology, Terrestrial
- Invasive Species
- Island Biogeography Theory
- Island Biology
- Keystone Species
- Kin Selection
- Landscape Dynamics
- Landscape Ecology
- Laws, Ecological
- Legume-Rhizobium Symbiosis, The
- Leopold, Aldo
- Lichen Ecology
- Life History
- Literature, Ecology and
- MacArthur, Robert H.
- Mangrove Zone Ecology
- Marine Fisheries Management
- Marine Subsidies
- Mass Effects
- Mathematical Ecology
- Mating Systems
- Maximum Sustainable Yield
- Metabolic Scaling Theory
- Metacommunity Dynamics
- Metapopulations and Spatial Population Processes
- Microclimate Ecology
- Multiple Stable States and Catastrophic Shifts in Ecosyste...
- Mutualisms and Symbioses
- Mycorrhizal Ecology
- Natural History Tradition, The
- Networks, Ecological
- Niche Versus Neutral Models of Community Organization
- Nutrient Foraging in Plants
- Ocean Sprawl
- Oceanography, Microbial
- Odum, Eugene and Howard
- Ordination Analysis
- Organic Agriculture, Ecology of
- Paleoecology
- Paleolimnology
- Parental Care, Evolution of
- Pastures and Pastoralism
- Patch Dynamics
- Patrick, Ruth
- Phenotypic Plasticity
- Phenotypic Selection
- Philosophy, Ecological
- Phylogenetics and Comparative Methods
- Physics, Ecology and
- Physiological Ecology of Nutrient Acquisition in Animals
- Physiological Ecology of Photosynthesis
- Physiological Ecology of Water Balance in Terrestrial Anim...
- Physiological Ecology of Water Balance in Terrestrial Plan...
- Plant Blindness
- Plant Disease Epidemiology
- Plant Ecological Responses to Extreme Climatic Events
- Plant-Insect Interactions
- Polar Regions
- Pollination Ecology
- Population Dynamics, Density-Dependence and Single-Species
- Population Dynamics, Methods in
- Population Ecology, Animal
- Population Ecology, Plant
- Population Fluctuations and Cycles
- Population Genetics
- Population Viability Analysis
- Populations and Communities, Dynamics of Age- and Stage-St...
- Predation and Community Organization
- Predation, Sublethal
- Predator-Prey Interactions
- Radioecology
- Reductionism Versus Holism
- Religion and Ecology
- Remote Sensing
- Restoration Ecology
- Ricketts, Edward Flanders Robb
- Seed Ecology
- Serpentine Soils
- Shelford, Victor
- Simulation Modeling
- Socioecology
- Soil Biogeochemistry
- Soil Ecology
- Spatial Pattern Analysis
- Spatial Patterns of Species Biodiversity in Terrestrial En...
- Spatial Scale and Biodiversity
- Species Distribution Modeling
- Species Extinctions
- Species Responses to Climate Change
- Species-Area Relationships
- Stability and Ecosystem Resilience, A Below-Ground Perspec...
- Stoichiometry, Ecological
- Sustainable Development
- Systematic Conservation Planning
- Systems Ecology
- Tansley, Sir Arthur
- Terrestrial Nitrogen Cycle
- Terrestrial Resource Limitation
- Territoriality
- Theory and Practice of Biological Control
- Thermal Ecology of Animals
- Tragedy of the Commons
- Transient Dynamics
- Tropical Humid Forest Biome
- Urban Ecology
- Urban Forest Ecology
- Vegetation Classification
- Vegetation Mapping
- Vicariance Biogeography
- Weed Ecology
- Wetland Ecology
- Whittaker, Robert H.
- Wildlife Ecology
- Privacy Policy
- Cookie Policy
- Legal Notice
- Accessibility
Powered by:
- [66.249.64.20|195.158.225.230]
- 195.158.225.230
Economic Importance of Fish | Vertebrates | Chordata | Zoology
Fishes form one of the most important group of vertebrates for man, influencing his life in various ways. Millions of human beings suffer due to hunger and malnutrition, and fishes form a rich source of food and provide a means to tide over the nutritional difficulties of man. In addition to serving as an important item of food, fishes provide several byproducts to us. Fish have considerable economic importance are useful as well as harmful to man.
Useful Fishes :
1. Fish as Human Food:
Fish have formed an important item of human diet. Nearly all fish freshwater and marine are edible and have been an important source of protein, fat and vitamins A and D since time immemorial. In most fishes, the flesh is white, contains about 13 to 20% of protein and has a food value of 300 to 1600 calories per pound.
According to Pottinger and Baldwin, fish meat contains atleast 5 of the essential amino acids. Besides this, it contains vitamin A and D together with large amount of phosphorus present in it. Important marine fishes include salmon, cod, halibut, herring, eels, tuna, mackerel, and sardines. Important freshwater food fishes are cat fish, trout, bass, perch and mullet. Even eggs of certain fishes, such as Russian sturgeon are eaten as cavier.
ADVERTISEMENTS:
The major food fishes of India include Labeo, Catla, Cirrhina, Mystus Wallago, Notopterus, Ophiocepholus, etc. The cartilaginous sharks, skates and rays are also used as human food in several countries. They are eaten by poorer classes of people living along the sea coasts of India and Sri Lanka. The canned meat of sharks is sold commercially under the name of gray fish. In south-east Asian countries, shark fins are dried and boiled to get a gelatinous material used as soups.
Fresh fish meat is usually cooked for human consumption. However, large quantities are refrigerated, salted, smoked, canned or pickled. Today, fisheries of the world carry on business worth several hundred thousand rupees annually and also provide employment to thousands of people.
2. Fish as Food of Cattle:
The scrap from canneries, as well as entire fishes that are not relished by man, are dried and ground in mill. This is called fish meal and is used as artificial food for poultry, pig and cattle. Fish meal is produced in several states like Maharashtra, Andhra Pradesh, Tamil Nadu, Bengal and Kerala chiefly from sardines, meckerels, ribbon fish, etc. The fish is first cooked in large pots containing sufficient quantity of water, on fire or on steam.
The cooked material is then pressed to remove moisture and dried in the sun on suitable platforms. The resultant product is then stored, and if preserved in airtight containers after sterilisation retains its nutritive value for a long time.
The fish meal contains about 60% protein and high percentage of calcium phosphate so that it is very valuable for cattle and poultry. The manufacture of fish meal can be undertaken as a cottage industry requiring little expenditure.
3. Fish Manure:
Fish that are unfit for human consumption are used to prepare fish manure for the fields. During peak season, when there is a large supply of fish, or they are landed in spoiled condition, they are sun dried on the beach. The dried fish is ground and converted to manure, which contains a high percentage of nitrogen and phosphate.
Mostly sardines are used for preparing fish meal and the waste material forms the fish guano. It contains 8.9% nitrogen and phosphate, and when mixed with soil, forms a rich fertiliser for plants. It is several times richer than ordinary cattle manure.
4. Fish Oils:
The most important fishery by-product is that of the fish oil, which is of two kinds- body oil and liver oil. The oil extracted from the whole body of the fish is called fish body oil, while that obtained from liver of certain fish is called the fish liver oil. Liver oil contains vitamin A and D, while the body oil contains them in traces.
The refined oil from the liver of fishes has a medicinal use, being the source of vitamin A and D. The body oil from fish has many uses, such as in painting, varnishing, soap, candle, leather and steel industries. For preparing body oil, fish are boiled in large quantity of water. Oil is removed quickly and washed in boiling salt water.
Liver oil is prepared from the liver of several species, including sharks and rays. Oil is extracted from the liver soon after the fish are caught to avoid action of enzymes. Liver is cut into small pieces and boiled in sufficient quantity of water. Oil is skimmed from the surface of water and sent for purification. Liver oil contains 55-75% fat, 5-10% protein and the rest water. It is of considerable medicinal value.
5. Fish Skin and Leather:
The skin of several fishes like the sharks and rays are used for making polishing and smoothing material. Shark skin leather is of some commercial importance in the manufacture of many useful articles such as shoes and hand bags, etc. In Japan, lantern are prepared from the skin of puffer fishes (e.g., Tetrodon). Some tribal people used skins of puffer and porcupine fishes {e.g., Diodon) for war helmets.
Crude skins of sharks and rays are used by carpenters and metal workers. Shark skin tanned with placoid scales on it is called shagreen. It has been used as an abrasive for polishing wood and ivory and also for covering jewel boxes, fine books and sword handles.
6. Fish Glue:
Liquid glues are prepared from skin, head and other trimmings of certain fishes. This glue has an adhesiveness of great power for paper, wood, leather and glass.
7. Isinglass:
It is a gelatinous product obtained from the air-bladders of certain fishes such as sturgeons, carps, perches, salmons, cat fishes, cods, etc. The isinglass is a shining powder and is used for clearing wine, beer, making edible jelly and in the preparation of adhesive material.
The air-bladder is removed from fish, washed in cold water and flattened by beating it on a piece of wood. The bladder is then dried in the sun, and is exported for the preparation of isinglass. The finest quality of isinglass is obtained from Russia.
8. Fish Fin:
The fins of sharks are exported to China where they are used for preparing soup.
9. Medicines and Disease Control:
The refined oils extracted from livers of cods and sharks are rich in vitamins A and D. Pituitary glands yield important extracts for medicines. Fishes like top minnows {Gambusia affinis), Trichogaster, Chela, Puntius, Barilius, Danio, Colisa, Rasbora, Esomus, Ambassis, Aplocheilus, Barbus, Panchax, etc., feed voraciously on mosquito larvae.
These larvicidal fishes are propagated and distributed widely into ponds, lakes and tanks to destroy mosquito that transmit malaria, yellow fever and other dreadful diseases of tropical countries. Certain fishes and their bye-products contribute to useful Ayurvedic and Unani medicines for treatment of duodenal ulcers, skin diseases, night blindness, general weakness, loss of appetite, colds, coughs, bronchitis, ashthma, tuberculosis, etc.
10. Sports and Recreation:
Sport fishing by individuals and fishing parties is a popular recreation of million of people, as well as a source of food, all over the world. The most commonly hunted fishes are the freshwater perch and trout and the marine tarpon. However, some of the best game fish is most famous for sport that it provides, but its flesh is not palatable.
Many people’s hobby is to cultivate certain tropical fishes as pets. Both native as well as foreign fishes are displayed in home aquaria for their beauty and graceful movements. Common aquarium fishes are gold fish (Carassius auratus), angel fish (Pterophyllum), sword tail guppy (Xiphophorus), minnow (Gambusia affinis), siamese fighter (Betta splendens), paradise fish (Macropodus), Hemigrammus, Aphyocharax, loach (Botid), Trichogaster, Tilapia, etc. Goldfish cultured and not found in nature and the Japanese have produced their several curious artificial varieties. Pet shops now-a-days stock many kinds of fishes for hobbyists and scientists.
11. Fancy Articles:
Scales of garpike (Lepidosteus) are used for jewelry and novelties. From scales of some fish is secured a pigment whose water suspension is known as pearl essence. It is used in the manufacture of artificial pearl in Europe, especially in France.
12. Scientific Study:
Fishes have considerable use as experimental animals, especially in the fields of Genetics, Embryology, Animal Behaviour and Pharmacology. Certain fishes such as Latimeria and dipnoans have anatomical features of great zoological interest. Fishes like dogfish (Scoliodori), perch (Perca) and carp (Labeo), etc., are dissected for anatomical study in zoological laboratories. Researches in ichthyology are conducted for the benefit of fisheries and mankind.
13. Industries:
As the fish forms a rich source of food, millions of people are engaged in fishing industry and depends on fisheries for their livelihood in various ways. Besides those who directly catch the fish for marketing, there are equally large number of people engaged in subsidiary industries like refrigeration, preservation, canning, and in the manufacture of fish products and by-products.
Harmful Fishes :
1. Destructive:
All the cartilaginous fishes are predaceous and feed chiefly on large quantities of crabs, lobsters, squids and other valuable marine animals. They cause great harm by destroying eggs, young and even adults of our food fishes. Sharks are extremely dangerous in the sea and injure fisherman and damage their nets, steal baits, devour captured fish and drive away squids and fishes to be netted. The damage done by these sharks amounts to several hundred thousand rupees.
2. Injurious:
Some larger sharks and sword fish may capsize small boats and injure or even kill fisherman. All sharks, small or big, are a menace to bathers and skin divers in shallow waters. The pirhanas of Central and South America have very strong teeth and are dangerous even to man.
Some fishes like cartilaginous electric ray (Torpedo) have electric organs which give powerful shocks to swimmers and fishermen. An extreme case of specialisation is the bony electric eel (Electrophorus electricus) of the Amazon. It is nearly 1.5 metres long and the postero-ventral four-fifth part of the body is occupied by the electric organ. It can generate electricity up to 600 volts in potential with a maximum output of 1000 watts. Carnivorous fishes eat away the larvae of useful insects.
3. Poisonous:
Poisonous glands are found in many cartilaginous fishes such as sting ray (Trygon) and eagle ray (Myliobatis). They can inflict painful wounds, sometimes fatal by the poisonous stings or spines. Poisonous glands are also found in Squalus (dogfish), Heterodontos, Chimaera, Diodon (porcupine fish), Tetrodon (globe fish), scorpion fish, etc. These are also capable of causing wounds by their spines or spiny opercula. Flesh of some tropical fishes (e.g., Tetrodon) is also poisonous and may prove fatal to man.
Related Articles:
- Rohu Fish: Origin, Economic Importance | Vertebrates | Chordata | Zoology
- Economic Importance of Mammals | Vertebrates | Chordata | Zoology
- Economic Importance of Protozoa | Zoology
- Economic Importance of Protozoa | Microorganisms | Zoology

IMAGES
VIDEO
COMMENTS
Invertebrates appeared very early on (Precambrian) and nowadays dominate the diversity of life on Earth. With the appearance and intensifying activities of humans, many invertebrate species are threatened by extinction. Our decisions, policies, and conservation actions will determine the fate of invertebrates.
Mar 1, 2020 • Download as PPTX, PDF •. 3 likes • 7,099 views. A. Amir Amir. Economic importance of invertebrates. Science. 1 of 13. Download now. Economic importance of invertebrates - Download as a PDF or view online for free.
Ecological importance and invertebrate conservation. T. R. New and A. L. Yen. The immense ecological importance of invertebrate animals is discussed in relation to using ecological values in setting priorities for their conservation. The spectrum of values, from taxa that we know perform vital ecological roles to taxa that may be ecologically ...
The goal of our researches is in bringing the scientific arguments of the necessity of including the biologic parameters, mainly of the invertebrates in the soil, in the evaluation studies of the ...
(a) Audition in invertebrates. Although audition is currently documented in detail in relatively few invertebrate species [22,23], the ability to detect sound has evolved multiple times in the insects alone, resulting in a diversity of auditory structures that can be found on nearly any segment of the body and with sensitivities anywhere between 10s of Hz to over 100 kHz [24,25].
Making up over 92% of life in our oceans, marine invertebrates inhabit every zone in the water column, with contributions ranging from ecosystem functioning to socioeconomic development. Compared to charismatic species, marine invertebrates are often underrepresented in IUCN reports and national conservation efforts. Because of this, as climate change intensifies in conjunction with increasing ...
Freshwater invertebrates can have economic importance in the biomonitoring of water quality in lakes and rivers, in terms of both the resulting decisions (e.g., consequent interventions to improve water quality or abandonment of projects) and the size of the industry that monitors freshwater invertebrates (Carter and Resh, 2001).
Invertebrates comprise the most diversified animal group on Earth. Due to their long evolutionary history and small size, invertebrates occupy a remarkable range of ecological niches, and play an important role as "ecosystem engineers" by structuring networks of mutualistic and antagonistic ecological interactions in almost all terrestrial ecosystems. Urban forests provide critical ...
Important issues facing insect conservation are discussed, namely, key threatening processes, threats to habitats and ecological communities, the importance of maintaining insect interactions, the value of vegetation remnants in agricultural ecosystems andThe importance of community participation, and recommendations for the conservation management of invertebrates and their habitats.
The invertebrates constitute important indicators in the appreciation of the soil. A first step in this respect is constituted by the analysis of the heavy metals in the corn fields in the Axente Sever and Copsa Micaƒ Area. ... Scientific Papers Series Management, Economic Engineering in Agriculture and Rural Development Vol. 14, Issue 3, 2014 ...
Good science communication should give the public the tools to make informed decisions and take action, which can be particularly important for nature conservation. The crisis in invertebrate conservation might be rooted in public prejudices against invertebrate animals, which are perceived as the unpopular 97% of Earth's animal biodiversity. As such, how we approach science communication ...
Semantic Scholar extracted view of "Economic Aspects of Freshwater Invertebrates" by V. Resh et al. ... Semantic Scholar's Logo. Search 218,217,193 papers from all fields of science. Search. Sign In Create Free Account. DOI: 10.1016/B978--12-385026-3.00006-1; ... Ecological and Economic Importance of Trichoptera ( Aquatic Insect ) T. Prommi. ...
Invertebrates, a diverse group of animals lacking a backbone, play significant ecological roles and possess various economic and cultural importance. Understanding the importance of these organisms is crucial for maintaining ecosystem functioning and promoting conservation efforts. This article aims to explore the reasons why invertebrates are important. Firstly, we will examine their ...
Invertebrates have important roles in the functioning of ecosystems: nutrient cycling, pollination, and herbivory. We need to better understand these complex interactions to predict how they are likely to change in the face of a rapidly changing climate. As we move into an era of increased pressure on old-growth habitats and biodiversity, it is ...
Invertebrates in the Everglades have received little attention relative to vertebrate species; Trexler and Loftus (2016) found only 20 papers focused on freshwater invertebrate ecology in the ...
Making up over 92% of life in our oceans, marine invertebrates inhabit every zone in the water column, with contributions ranging from ecosystem functioning to socioeconomic development.
General Overviews. Hynes 1970a, a classic book on general stream ecology, moved the field of freshwater invertebrate ecology forward through careful synthesis of existing information and progressive thinking on research directions, particularly on the ecological roles of stream invertebrates.That same year, Hynes 1970b provided a synthesis of many aspects of freshwater invertebrate ecology in ...
The importance of invertebrates when considering the impacts of anthropogenic noise. Anthropogenic noise is now recognized as a major global pollutant. Rapidly burgeoning research has identified impacts on individual behaviour and physiology through to community disruption.
Invertebrates are a diverse group of animals that make up the majority of the animal kingdom and encompass a wide array of species with varying adaptations and characteristics. Invertebrates are found in nearly all of the world's habitats, including aquatic, marine, and terrestrial environments. There are many misconceptions about invertebrate sentience, welfare requirements, the need for ...
importance of invertebrates to sustaining plant communities. Invertebrates are important in the soil for aeration, decomposition of dead plant material to create an organic layer and consequent release of nutrients which are then available for living plants, and assisting with formation of soil structure. In the absence of leaf litter feeders our
importance for the broader landscape, by maintaining water quality by processing organic matter, providing key food items for ecologically-important fish species, and representing an ... A comprehensive checklist of aquatic invertebrates from Nyika iii) A scientific paper on the key factors structuring the freshwater macroinvertebrate ...
1.3 Explain ONE importance of the development of a coelom. (2) 1.4 Write down the names of the phyla that display the characteristic represented by C but not the characteristic represented by D. (2) 1.5 Describe ONE way in which the coelom of annelids and arthropods are different. (2)
Notwithstanding this general trend of chromosome-scale conservation, ALGs have been obliterated by extensive genome rearrangements in certain groups, most notably including Clitellata (oligochaetes and leeches), a group of easily overlooked invertebrates that is of tremendous ecological, agricultural and economic importance (Charles 2019 ...
Fishes form one of the most important group of vertebrates for man, influencing his life in various ways. Millions of human beings suffer due to hunger and malnutrition, and fishes form a rich source of food and provide a means to tide over the nutritional difficulties of man. In addition to serving as an important item of food, fishes provide several byproducts to us. Fish have considerable ...