- Download PDF
- CME & MOC
- Share X Facebook Email LinkedIn
- Permissions

Ulcerative Colitis in Adults : A Review
- 1 IBD Edinburgh Unit, Western General Hospital, Edinburgh, Scotland
- 2 Department of Gastroenterology and Hepatology, Reina Sofía University Hospital, Córdoba, Spain
- 3 Division of Gastroenterology and Hepatology, Departments of Medicine and Community Health Sciences, University of Calgary, Calgary, Alberta, Canada
- Preliminary Communication Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis Samuel P. Costello, MBBS; Patrick A. Hughes, PhD; Oliver Waters, MBBS; Robert V. Bryant, MScR; Andrew D. Vincent, PhD; Paul Blatchford, PhD; Rosa Katsikeros, BSc; Jesica Makanyanga, MBChB; Melissa A. Campaniello, BSc; Chris Mavrangelos, BSc; Carly P. Rosewarne, PhD; Chelsea Bickley, BSc; Cian Peters, MS; Mark N. Schoeman, PhD; Michael A. Conlon, PhD; Ian C. Roberts-Thomson, PhD; Jane M. Andrews, PhD JAMA
- JAMA Clinical Guidelines Synopsis Ulcerative Colitis in Adults Laura R. Glick, MD; Adam S. Cifu, MD; Lauren Feld, MD JAMA
- Original Investigation Induction Therapy With Olamkicept vs Placebo for Active Ulcerative Colitis Shenghong Zhang, MD; Baili Chen, MD; Bangmao Wang, MD; Hong Chen, MD; Yan Li, MD; Qian Cao, MD; Jie Zhong, MD; Ming-Jium Shieh, MD; Zhihua Ran, MD; Tongyu Tang, MD; Ming Yang, MS; Beibei Xu, MD; Qiang Wang, MD; Yunjie Liu, MD; Lijia Ma, MD; Xiaolin Wang, MS; Nan Zhang, PhD; Su Zhang, MD; Wenyu Guo, MD; Liang Huang, MS; Stefan Schreiber, MD; Minhu Chen, MD JAMA
- JAMA Patient Page Patient Information: Ulcerative Colitis Rebecca Voelker, MSJ JAMA
Importance Ulcerative colitis (UC) is a chronic inflammatory condition of the colon, with a prevalence exceeding 400 per 100 000 in North America. Individuals with UC have a lower life expectancy and are at increased risk for colectomy and colorectal cancer.
Observations UC impairs quality of life secondary to inflammation of the colon causing chronic diarrhea and rectal bleeding. Extraintestinal manifestations, such as primary sclerosing cholangitis, occur in approximately 27% of patients with UC. People with UC require monitoring of symptoms and biomarkers of inflammation (eg, fecal calprotectin), and require colonoscopy at 8 years from diagnosis for surveillance of dysplasia. Risk stratification by disease location (eg, Montreal Classification) and disease activity (eg, Mayo Score) can guide management of UC. First-line therapy for induction and maintenance of remission of mild to moderate UC is 5-aminosalicylic acid. Moderate to severe UC may require oral corticosteroids for induction of remission as a bridge to medications that sustain remission (biologic monoclonal antibodies against tumor necrosis factor [eg, infliximab], α4β7 integrins [vedolizumab], and interleukin [IL] 12 and IL-23 [ustekinumab]) and oral small molecules that inhibit janus kinase (eg, tofacitinib) or modulate sphingosine-1-phosphate (ozanimod). Despite advances in medical therapies, the highest response to these treatments ranges from 30% to 60% in clinical trials. Within 5 years of diagnosis, approximately 20% of patients with UC are hospitalized and approximately 7% undergo colectomy. The risk of colorectal cancer after 20 years of disease duration is 4.5%, and people with UC have a 1.7-fold higher risk for colorectal cancer compared with the general population. Life expectancy in people with UC is approximately 80.5 years for females and 76.7 years for males, which is approximately 5 years shorter than people without UC.
Conclusions and Relevance UC affects approximately 400 of every 100 000 people in North America. An effective treatment for mild to moderate UC is 5-aminosalicylic acid, whereas moderate to severe UC can be treated with advanced therapies that target specific inflammation pathways, including monoclonal antibodies to tumor necrosis factor, α4β7 integrins, and IL-12 and IL-23 cytokines, as well as oral small molecule therapies targeting janus kinase or sphingosine-1-phosphate.
Read More About
Gros B , Kaplan GG. Ulcerative Colitis in Adults : A Review . JAMA. 2023;330(10):951–965. doi:10.1001/jama.2023.15389
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
Ulcerative Colitis With an Unexpected Cause
— can the immune response to covid-19 trigger inflammatory bowel disease.
by Kate Kneisel , Contributing Writer, MedPage Today March 1, 2022

A 50-year-old male patient presented to an outpatient clinic in the spring of 2020 with fever and dyspnea; he told clinicians that the symptoms had persisted for the past 3 days.
Physical examination findings included a fever of 37.8°C (100°F), respiratory rate of 24 breaths/min, and heart rate of 105 beats/min. There was no organomegaly, and the patient was a non-smoker.
Initial laboratory test findings included:
- White blood cell count: 6.4 × 109/L
- C-reactive protein (CRP): 4.6 mg/L
- Ferritin: 162 ng/mL
- D-dimer: 842 ng/mL
Findings of a polymerase chain reaction (PCR) test for SARS-CoV-2 were negative. However, the patient's wife and two children had positive PCR test results; and the patient's CT chest scan revealed diffuse ground-glass opacities consistent with viral pneumonia. Clinicians diagnosed him with COVID-19, and he was started on a now-debunked 7-day regimen of hydroxychloroquine and azithromycin. Once he was clinically stable, he was released with instructions to return for a follow-up assessment.
He returned several weeks later with bloody diarrhea, which he explained had come on about 2 weeks after he completed COVID-19 treatment. Stool analysis revealed 10 to 12 erythrocytes and five to six leukocytes. However, there was no evidence of amoebas or Clostridium difficile A+B. Complete blood count and CRP were within normal ranges. Clinicians prescribed treatment with ciprofloxacin, metronidazole, and probiotics.
On follow-up assessment 1 week later, the patient reported no improvement in symptoms. His stool calprotectin level was 1800 μg/g (normal range: 0-50 μg/g). Endoscopy revealed a diffuse, micro-ulcerated, granulated appearance that clinicians noted continued uninterrupted from the dentate line to the sigmoid colon, as well as distortion of the submucosal vascularization.
Based on presumed diagnoses of infectious colitis and ulcerative colitis, biopsies were taken. Pathology findings included mucin loss and distortion in the colonic glands, as well as evidence of polymorphonuclear leukocytes (PMNL) and plasma cell infiltration. Clinicians also noted cryptitis and a crypt abscess between the glands; no granulomatous or specific micro-organisms were detected.
The patient was diagnosed with ulcerative colitis, which clinicians believed had been triggered by COVID-19. The patient was prescribed treatment with 5-aminosalicylic acid (5-ASA) therapy, initiated orally and by enema. After 3 days of this drug therapy, his bloody diarrhea and other symptoms resolved.
On testing, the patient's anti-SARS-CoV-2 antibodies were found to be IgG positive and IgM weak positive. A subsequent CT scan revealed significant improvement from the initial findings and evidence of a sequela lesion.
Clinicians presenting this case – which they believe is the second documentation of ulcerative colitis with COVID-19 in the literature – made the report "to show that COVID-19 can appear with other organ pathologies, in addition to upper and lower respiratory tract complaints."
The group noted that initial reports of COVID-19 from China around the time this patient was diagnosed focused only on its respiratory manifestations, so the absence of reports of diarrhea or other gastrointestinal complaints may have "led to under-recognition of these symptoms."
They noted that several studies have since reported the involvement of other organs and diarrhea symptoms. For example, a single-center study of 95 COVID-19 patients admitted to the hospital found GI symptoms in 61% (n=58) of patients overall. Of those symptomatic patients, about 12% were symptomatic on admission, and the remaining 49% developed symptoms (primarily elevated bilirubin and, to a lesser extent, diarrhea) during hospitalization, possibly aggravated by various medications, including antibiotics, researchers reported.
Those researchers found "no statistically significant difference in the general demographics or clinical outcomes between patients with and without GI symptoms." Of the 58 patients with GI manifestations , impaired hepatic function occurred in about 31% during hospitalization, compared with only 1% who were affected on initial presentation.
The next most common symptom, diarrhea (two to 10 loose or watery stools a day) was noted in 24% overall, followed by anorexia and nausea, each affecting 18%. Vomiting affected just 4% of patients.
The researchers noted that antibiotic treatment was associated with development of diarrhea ( P =0.034) and elevated bilirubin levels ( P =0.028) during hospitalization, effects that were not noted with antiviral treatment. Importantly, of the 11 patients with GI symptoms only, 12% had no evidence of COVID-19 pneumonia on imaging, that paper stated.
Authors of the current case report noted that while "COVID-19 RNA can be detected by PCR tests in the stool after respiratory samples become negative in some infected patients," it is not known how long the COVID-19 virus can remain viable in the stool.
They referred to a recent study conducted at China's Wuhan Inflammatory Bowel Disease (IBD) Center which suggested that the prompt measures taken to prevent the spread of the virus may explain why none of the 318 registered IBD patients developed COVID-19. While another case series noted diarrhea in just 3% to 5% of patients, authors of the current case report wrote that "clinicians have begun to question the prevalence of IBD as a symptom of COVID-19," citing another report in which 31% of 84 patients with COVID-19 pneumonia had diarrhea.
Case authors pointed to the other report of COVID-19 and ulcerative colitis in which a 19-year-old female from Italy "presented with fever, vomiting, bloody diarrhea, and loss of taste and smell ... a positive PCR test but no CT evidence of pneumonia, and contrast enhancement in the ileum and colon." She recovered fully, returned a negative PCR test, and was diagnosed with ulcerative colitis following an ultrasound of the small bowel on day 16, with no evidence of COVID-19 in stool samples.
Likewise, case authors noted that their patient also tested negative for COVID-19, despite CT evidence of diffuse ground-glass opacities, "the most common manifestation of COVID-19"; he also developed GI symptoms after finishing treatment for COVID-19, which improved on CT.
While noting that their patient's clinical picture was not compatible with ischemic colitis, case authors advised clinicians to also "consider ischemic colitis in the differential diagnosis of antibiotic-induced colitis." Regarding the latter, the group noted that while "late-onset antibiotic-induced colitis can occur on rare occasions," that did not apply in the current case, given his lack of symptoms for 2 weeks after antibiotic treatment, and the absence of amoeba and C. difficile toxins in stool analyses.
In this case, authors noted that their patient's clinical parameters, the presence of bloody diarrhea in the absence of a toxic condition (such as ischemia or necrosis), endoscopic and pathological findings, plus his "very rapid response to 5-ASA treatment for ulcerative colitis, and the onset of complaints after recovery from COVID-19" suggest his ulcerative colitis may have been due to an immune response triggered by COVID-19.
The group explained that the etiology of ulcerative colitis is unknown -- the disease may be induced by inflammation triggered by any condition. That their patient had no history of GI complaints; developed bloody diarrhea and abdominal pain shortly after the onset of COVID-19 symptoms; and had imaging and pathology findings compatible with ulcerative colitis "might suggest that the disease could be triggered by COVID-19," the group noted.
The high levels of angiotensin-converting enzyme-2 (ACE-2) and transmembrane serine protease required for the COVID-19 virus to enter cells are expressed by human intestines, they noted, citing emerging data suggesting the virus's effect on the GI system and liver may also be associated with hepatic cells' expression of ACE-2, "a major receptor for gastrointestinal epithelial cells and COVID-19."
Case authors observed that, while little is known about COVID-19 and inflammatory bowel disease (IBD), "the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) ... recently recommended reducing corticosteroid therapy and maintaining thiopurines and biologics." In 2021, that group also released consensus recommendations regarding SARS-CoV-2 vaccination for IBD patients.
Given the dynamic course seen in COVID-19 and the increasing range of clinical symptoms being reported, "there is an urgent need to properly determine the clinical features of COVID-19," case authors wrote. They acknowledged that while the lack of PCR investigations in stool or tissue samples was a limitation in this case, there was considerable evidence to suggest that the patient did experience COVID-19.
They concluded by urging further study of the association between COVID-19 and IBD, especially ulcerative colitis, and COVID-19 testing in patients presenting with gastrointestinal complaints.
![case study ulcerative colitis patient author['full_name']](https://clf1.medpagetoday.com/media/images/author/KKneisel_188.jpg)
Kate Kneisel is a freelance medical journalist based in Belleville, Ontario.
Disclosures
The case report authors noted no conflicts of interest.
Primary Source
Turkish Journal of Gastroenterology
Source Reference: Aydın MF, Taşdemir H "Ulcerative colitis in a COVID-19 patient: A case report" Turk J Gastroenterol 2021; DOI: 10.5152/tjg.2021.20851.

- Earn HPCSA and SACNASP CPD Points
- I have spots and my skin burns
- A case of a 10 year old boy with a 3 week history of diarrhoea, vomiting and cough
- A case of fever and general malaise
- A case of persistant hectic fever
- A case of sudden rapid neurological deterioration in an HIV positive 27 year old female
- A case of swollen hands
- An unusual cause of fulminant hepatitis
- Case of a right axillary swelling
- Case of giant wart
- Case of recurrent meningitis
- Case of repeated apnoea and infections in a premature infant
- Case of sudden onset of fever, rash and neck pain
- Doctor, my sister is confused
- Eight month old boy with recurrent infections
- Enlarged Testicles
- Failure to thrive despite appropriate treatment
- Right Axillary Swelling
- Severe anaemia in HIV positive child
- The case of a floppy infant
- Two year old with spiking fevers and depressed level of consciousness
- 17 year old male with fever and decreased level of consciousness
- 3 TB Vignettes
- A 10 year old girl with a hard palate defect
- A case of decreased joint function, fever and rash
- Keep up while the storm is raging
- Fireworks of autoimmunity from birth
- My hands swell and are painful
- My eyes cross at twilight
- A case of a 3 month old infant with bloody urine and stools
- A case of scaly annular plaques
- Case of eye injury and decreased vision
- My head hurts and I cannot speak?
- TB or not TB: a confusing case
- A 7 year old with severe muscle weakness and difficulty walking
- Why can I not walk today?
- 14 year old with severe hip pain
- A 9 year old girl presents with body swelling, shortness of breath and backache
- A sudden turn of events after successful therapy
- Declining CD4 count, despite viral suppression?
- Defaulted treatment
- 25 year old female presents with persistent flu-like symptoms
A case of persistent bloody diarrhoea
- I’ve been coughing for so long
- A case of acute fever, rash and vomiting
- Adverse event following routine vaccination
- A case of cough, wasting and lymphadenopathy
- A case of lymphadenopathy and night sweats
- Case of enlarged hard tongue
- A high risk pregnancy
- A four year old with immunodeficiency
- Young girl with recurrent history of mycobacterial disease
- Immunodeficiency and failure to thrive
- Case of recurrent infections
- An 8 year old boy with recurrent respiratory infections
- 4 year old boy with recurrent bacterial infections
- Is this treatment failure or malnutrition
- 1. A Snapshot of the Immune System
- 2. Ontogeny of the Immune System
- 3. The Innate Immune System
- 4. MHC & Antigen Presentation
- 5. Overview of T Cell Subsets
- 6. Thymic T Cell Development
- 7. gamma/delta T Cells
- 8. B Cell Activation and Plasma Cell Differentiation
- 9. Antibody Structure and Classes
- 10. Central and Peripheral Tolerance
- Immuno-Mexico 2024 Introduction
- Modulation of Peripheral Tolerance
- Metabolic Adaptation to Pathologic Milieu
- T Cell Exhaustion
- Suppression in the Context of Disease
- Redirecting Cytotoxicity
- Novel Therapeutic Strategies
- ImmunoInformatics
- Grant Writing
- Introduction to Immuno-Chile 2023
- Core Modules
- Gut Mucosal Immunity
- The Microbiome
- Gut Inflammation
- Viral Infections and Mucosal Immunity
- Colorectal Cancer
- Inflammatory Bowel Disease
- Equity, Diversity, Inclusion in Academia
- Immuno-India 2023 Introduction
- Principles of Epigenetic Regulation
- Epigenetics Research in Systems Immunology
- Epigenetic (De)regulation in Non-Malignant Diseases
- Epigenetic (De)regulation in Immunodeficiency and Malignant Diseases
- Immunometabolism and Therapeutic Applications of Epigenetic Modifiers
- Immuno-Morocco 2023 Introduction
- Cancer Cellular Therapies
- Cancer Antibody Therapies
- Cancer Vaccines
- Immunobiology of Leukemia & Therapies
- Immune Landscape of the Tumour
- Targeting the Tumour Microenvironment
- Flow Cytometry
- Immuno-Zambia 2022 Introduction
- Immunity to Viral Infections
- Immunity to SARS-CoV2
- Basic Immunology of HIV
- Immunity to Tuberculosis
- Immunity to Malaria
- Immunity to Schistosomiasis
- Immunity to Helminths
- Equity, Diversity and Inclusion in Academia
- Immuno-Argentina 2022 Introduction
- Dendritic Cells
- Trained Innate Immunity
- Gamma-Delta T cells
- Natural Killer Cell Memory
- Innate Immunity in Viral Infections
- Lectures – Innate Immunity
- T cells and Beyond
- Lectures – Cellular Immunity
- Strategies for Vaccine Design
- Lectures – Humoral Immunity
- Lectures – Vaccine development
- Lectures – Panel and Posters
- Immuno-Cuba 2022 Introduction
- Poster and Abstract Examples
- Immuno-Tunisia 2021 Introduction
- Basics of Anti-infectious Immunity
- Inborn Errors of Immunity and Infections
- Infection and Auto-Immunity
- Pathogen-Induced Immune Dysregulation & Cancer
- Understanding of Host-Pathogen Interaction & Applications (SARS-CoV-2)
- Day 1 – Basics of Anti-infectious Immunity
- Day 2 – Inborn Errors of Immunity and Infections
- Day 3 – Infection and Auto-immunity
- Day 4 – Pathogen-induced Immune Dysregulation and Cancer
- Day 5 – Understanding of Host-Pathogen Interaction and Applications
- Student Presentations
- Roundtable Discussions
- Orientation Meeting
- Poster Information
- Immuno-Colombia Introduction
- Core Modules Meeting
- Overview of Immunotherapy
- Check-Points Blockade Based Therapies
- Cancer Immunotherapy with γδ T cells
- CAR-T, armored CARs and CAR-NK therapies
- Anti-cytokines Therapies
- Tumor-infiltrating Lymphocytes (TIL)
- MDSC Promote Tumor Growth and Escape
- Immunological lab methods for patient’s follow-up
- Student Orientation Meeting
- Lectures – Week 1
- Lectures – Week 2
- Research Project
- Closing and Social
- Introduction to Immuno-Algeria 2020
- Hypersensitivity Reactions
- Immuno-Algeria Programme
- Online Lectures – Week 1
- Online Lectures – Week 2
- Student Presentations – Week 1
- Student Presentations – Week 2
- Introduction to Immuno-Ethiopia 2020
- Neutrophils
- Leishmaniasis – Transmission and Epidemiology
- Leishmaniasis – Immune Responses
- Leishmaniasis – Treatment and Vaccines
- Immunity to Helminth Infections
- Helminth immunomodulation on co-infections
- Malaria Vaccine Progress
- Immunity to Fungal Infections
- How to be successful scientist
- How to prepare a good academic CV
- Introduction to Immuno-Benin
- Immune Regulation in Pregnancy
- Immunity in infants and consequence of preeclampsia
- Schistosome infections and impact on Pregnancy
- Infant Immunity and Vaccines
- Regulation of Immunity & the Microbiome
- TGF-beta superfamily in infections and diseases
- Infectious Diseases in the Global Health era
- Immunity to Toxoplasma gondii
- A. melegueta inhibits inflammatory responses during Helminth Infections
- Host immune modulation by Helminth-induced products
- Immunity to HIV
- Immunity to Ebola
- Immunity to TB
- Genetic susceptibility in Tuberculosis
- Plant Extract Treatment for Diabetes
- Introduction to Immuno-South Africa 2019
- Models for Testing Vaccines
- Immune Responses to Vaccination
- IDA 2019 Quiz
- Introduction to Immuno-Jaipur
- Inflammation and autoinflammation
- Central and Peripheral Tolerance
- Autoimmunity and Chronic Inflammatory Diseases
- Autoimmunity & Dysregulation
- Novel Therapeutic strategies for Autoimmune Diseases
- Strategies to apply gamma/delta T cells for Immunotherapy
- Immune Responses to Cancer
- Tumour Microenvironment
- Cancer Immunotherapy
- Origin and perspectives of CAR T cells
- Metabolic checkpoints regulating immune responses
- Transplantation
- Primary Immunodeficiencies
- Growing up with Herpes virus
- Introduction to IUIS-ALAI-Mexico-ImmunoInformatics
- Introduction to Immunization Strategies
- Introduction to Immunoinformatics
- Omics Technologies
- Computational Modeling
- Machine Learning Methods
- Introduction to Immuno-Kenya
- Viruses hijacking host immune responses
- IFNs as 1st responders to virus infections
- HBV/HCV & Hepatocellular Carcinoma
- Cytokines as biomarkers for HCV
- HTLV & T cell Leukemia
- HCMV and Cancers
- HPV and Cancers
- EBV-induced Oncogenesis
- Adenoviruses
- KSHV and HIV
- Ethics in Cancer Research
- Sex and gender in Immunity
- Introduction to Immuno-Iran
- Immunity to Leishmaniasis
- Breaking Tolerance: Autoimmunity & Dysregulation
- Introduction to Immuno-Morocco
- Cancer Epidemiology and Aetiology
- Pathogens and Cancer
- Immunodeficiency and Cancer
- Introduction to Immuno-Brazil
- 1. Systems Vaccinology
- 2. Vaccine Development
- 3. Adjuvants
- 4. DNA Vaccines
- 5. Mucosal Vaccines
- 6. Vaccines for Neurodegenerative Diseases
- Introduction to Immuno-Gambia
- Immuno-Gambia Photos
- 1. Infant Immunity and Vaccines
- 2. Dendritic Cells
- 3. Conventional T Cells
- 4. gamma/delta T Cells
- 5. Immunity to Viral Infections
- 6. Immunity to Helminth Infections
- 7. Immunity to TB
- 8. Immunity to Malaria
- 9. Flow Cytometry
- Introduction to Immuno-South Africa
- 1. Introduction to Immunization Strategies
- 2. Immune Responses to Vaccination
- 3. Models for Testing Vaccines
- 4. Immune Escape
- 5. Grant Writing
- Introduction to Immuno-Ethiopia
- 1. Neutrophils
- 3. Exosomes
- 5. Immunity to Leishmania
- 6. Immunity to HIV
- 7. Immunity to Helminth Infections
- 8. Immunity to TB
- 9. Grant Writing
- Introduction to ONCOIMMUNOLOGY-MEXICO
- ONCOIMMUNOLOGY-MEXICO Photos
- 1. Cancer Epidemiology and Etiology
- 2. T lymphocyte mediated immunity
- 3. Immune Responses to Cancer
- 4. Cancer Stem Cells and Tumor-initiating cells.
- 5. Tumor Microenvironment
- 6. Pathogens and Cancer
- 7. Cancer Immunotherapy
- 8. Flow cytometry approaches in cancer
- Introduction to the Immunology Course
- Immuno-Tunisia Photo
- 1. Overview of the Immune System
- 2. Role of cytokines in Immunity
- 3. Tolerance and autoimmunity
- 4. Genetics, Epigenetics and immunoregulation
- 5. Microbes and immunoregulation
- 6. Inflammation and autoinflammation
- 7. T cell mediated autoimmune diseases
- 8. Antibody-mediated autoimmune diseases
- Introduction to the Immunology Symposium
- Immuno-South Africa Photo
- 1. Antibody Generation by B cells
- 2. Mucosal Immunity
- 3. Immunity to TB
- 4. Immunity to Malaria
- 5. Immunity to HIV
- 6. Defining a Biomarker
- 7. Grant Writing Exercise
- Immuno-Colombia Photo
- 1. Overview of Complement
- 2. Transplantation
- 3. Immune Regulation in Pregnancy
- 4. Breaking Tolerance: Autoimmunity & Dysregulation
- 5. Mucosal Immunity & Immunopathology
- 6. Regulation of Immunity & the Microbiome
- 7. Epigenetics & Modulation of Immunity
- 8. Primary Immunodeficiencies
- 9. Anti-tumour Immunity
- 10. Cancer Immunotherapy
- Introduction
- Immune Cells
- NCDs and Multimorbidity
- Mosquito Vector Biology
- Vaccines and Other Interventions
- Autoimmunity
- Career Development
- SUN Honours Introduction
- A Snapshot of the Immune System
- Ontogeny of the Immune System
- The Innate Immune System
- MHC & Antigen Presentation
- Overview of T Cell Subsets
- B Cell Activation and Plasma Cell Differentiation
- Antibody Structure and Classes
- Cellular Immunity and Immunological Memory
- Infectious Diseases Immunology
- Vaccinology
- Mucosal Immunity & Immunopathology
- Central & Peripheral Tolerance
- Epigenetics & Modulation of Immunity
- T cell and Ab-mediated autoimmune diseases
- Immunology of COVID-19 Vaccines
- 11th IDA 2022 Introduction
- Immunity to COVID-19
- Fundamentals of Immunology
- Fundamentals of Infection
- Integrating Immunology & Infection
- Infectious Diseases Symposium
- EULAR Symposium
- Thymic T Cell Development
- Immune Escape
- Genetics, Epigenetics and immunoregulation
- AfriBop 2021 Introduction
- Adaptive Immunity
- Fundamentals of Infection 2
- Fundamentals of Infection 3
- Host pathogen Interaction 1
- Host pathogen Interaction 2
- Student 3 minute Presentations
- 10th IDA 2021 Introduction
- Day 1 – Lectures
- Day 2 – Lectures
- Day 3 – Lectures
- Day 4 – Lectures
- Afribop 2020 Introduction
- WT PhD School Lectures 1
- EULAR symposium
- WT PhD School Lectures 2
- Host pathogen interaction 1
- Host pathogen interaction 2
- Bioinformatics
- Introduction to VACFA Vaccinology 2020
- Overview of Vaccinology
- Basic Principles of Immunity
- Adverse Events Following Immunization
- Targeted Immunization
- Challenges Facing Vaccination
- Vaccine Stakeholders
- Vaccination Questions Answered
- Malaria Vaccines
- IDA 2018 Introduction
- Vaccine Development
- Immune Escape by Pathogens
- Immunity to Viral Infections Introduction
- Flu, Ebola & SARS
- Antiretroviral Drug Treatments
- Responsible Conduct in Research
- Methods for Enhancing Reproducibility
- 6. B Cell Activation and Plasma Cell Differentiation
- 7. Antibody Structure and Classes
- CD Nomenclature
- 1. Transplantation
- 2. Central & Peripheral Tolerance
- 8. Inflammation and autoinflammation
- 9. T cell mediated autoimmune diseases
- 10. Antibody-mediated autoimmune diseases
- 1. Primary Immunodeficiencies
- Cancer Stem Cells and Tumour-initiating Cells
- 6. Tolerance and Autoimmunity
- Discovery of the Thymus as a central immunological organ
- History of Immune Response
- History of Immunoglobulin molecules
- History of MHC – 1901 – 1970
- History of MHC – 1971 – 2011
- SAIS/Immunopaedia Webinars 2022
- Metabolic control of T cell differentiation during immune responses to cancer
- Microbiome control of host immunity
- Shaping of anti-tumor immunity in the tumor microenvironment
- The unusual COVID-19 pandemic: the African story
- Immune responses to SARS-CoV-2
- Adaptive Immunity and Immune Memory to SARS-CoV-2 after COVID-19
- HIV prevention- antibodies and vaccine development (part 2)
- HIV prevention- antibodies and vaccine development (part 1)
- Immunopathology of COVID 19 lessons from pregnancy and from ageing
- Clinical representation of hyperinflammation
- In-depth characterisation of immune cells in Ebola virus
- Getting to the “bottom” of arthritis
- Immunoregulation and the tumor microenvironment
- Harnessing innate immunity from cancer therapy to COVID-19
- Flynn Webinar: Immune features associated natural infection
- Flynn Webinar: What immune cells play a role in protection against M.tb re-infection?
- JoAnne Flynn: BCG IV vaccination induces sterilising M.tb immunity
- IUIS-Immunopaedia-Frontiers Webinar on Immunology taught by P. falciparum
- COVID-19 Cytokine Storm & Paediatric COVID-19
- Immunothrombosis & COVID-19
- Severe vs mild COVID-19 immunity and Nicotinamide pathway
- BCG & COVID-19
- COVID-19 Vaccines
- Antibody responses and serology testing
- Flow Cytometry Part 1
- Flow Cytometry Part 2
- Flow Cytometry Part 3
- Lateral Flow
- Diagnostic Tools
- Diagnostic Tests
- HIV Life Cycle
- ARV Drug Information
- ARV Mode of Action
- ARV Drug Resistance
- Declining CD4 count
- Ambassador of the Month – 2024
- North America
- South America
- Ambassador of the Month – 2023
- Ambassador of the Month – 2022
- The Day of Immunology 2022
- AMBASSADOR SCI-TALKS
- The Day of Immunology 2021
- Ambassador of the Month – 2021
- Ambassador of the Month-2020
- Ambassador of the Month – 2019
- Ambassador of the Month – 2018
- Ambassador of the Month – 2017
- Immuno-South Africa 2024
- COLLABORATIONS
We will not share your details

Patient presentation
Differential diagnosis, examination, investigations, special investigations, final outcome.
Patient is a 22 year old female who presented to the surgery department of a tertiary level hospital having been referred from a private clinic, with a two month history of severe abdominal cramps, persistent bloody and mucoid diarrhoea, weight loss and tiredness.
Acknowledgement This case study was kindly provided by Dr Monica Mercer from Immunopaedia
2 months ago: Symptoms began with abdominal cramps and an intense urge to pass stool after every meal. Her symptoms rapidly worsened with passage of stool becoming more frequent. Within two days she was passing persistently watery diarrhoea mixed with fresh blood and mucous. She was seen by her general practitioner who treated her for gastritis.
One week later she collapsed at home and was admitted to hospital for investigations. She was discharged two days later without a diagnosis.
1 month ago: Symptoms persisted and she experienced diarrhoea and vomiting after eating or drinking, which lasted for 10 days. She was admitted to hospital for rehydration and further investigations. No conclusive diagnosis was made.
Currently: Patient is passing 10-20 liquid stools per day. Diarrhoea is mucoid and bloody. Occurs day and night. Patient complains of malaise, lethargy and anorexia. She has lost 8 kg in the past 2 months.
No past surgical history No significant medical history
Family history: Mother – type 2 Diabetes Mellitus No other family members with chronic disease
No known allergies
- Cryptosporidium,
- Shigella,
- salmonella,
- E.coli,
- Campylobacter,
- Clostridium difficile
- If HIV positive consider- MAC, Isospera beli, cryptosporidium, TB
- Functional bowel syndromes e.g. irritable bowel syndrome (IBS)
- Malabsorbtion
- Coeliac disease
- Inflammatory bowel disease (IBD)
Thin ill looking young woman, conscious and alert, in obvious discomfort.
Vitals : Heart rate: 80bpm Respiratory rate: 18 bpm Blood pressure: 120/70 Temperature: 37˚C
Pale mucous membranes
Abdominal examination: Guarding and tenderness noted in the left iliac fossa and hypogastrium.
No results available from previous admissions. All results are from current admission.
Abdominal X-ray: No toxic megacolon
Gastroscopy Report: Oesophagus and gastro- oesopahageal junction were normal. Stomach mucosa was intact and normal. No gastritis, ulceration or blood was noted. Cardia was normal. Pylorus and duodenum normal.
Colonoscopy report: Very friable mucosa. Extensive ulceration with pseudopolyps, involving the rectum, entire sigmoid and left colon up to the transverse colon. Multiple biopsies of the colonic tissue were taken for histological analysis.
Histological Findings: Pathology is limited to the mucosa and submucosa. Intense infiltration of the mucosa and submucosa with neutrophils and crypt abscesses, lamina propria with lymphoid aggregates, plasma cells, mast cells and eosinophils, and shortening and branching of the crypts.
What is the Diagnosis?
Ulcerative Colitis, which is a chronic disease associated with diffuse mucosal inflammation of the colon, giving rise to significant morbidity and recurrent symptoms of intermittent bloody diarrhea, rectal urgency and tenesmus. Patients also present with fever, anemia, fatigue, weight loss, loss of appetite, loss of body fluids and nutrients, skin lesions, joint pain, and failure to grow. The latter is specifically seen in children. About half of the people diagnosed with ulcerative colitis have mild symptoms (Ulcerative colitis, no date). Onset of symptoms typically occurs between 15 and 40 years of age, with a second peak in incidence between 50 and 80 years of age.
Ulcerative colitis is closely related to another inflammatory intestinal condition called Crohn’s disease, which can lead to chronic inflammation in any part of the gastrointestinal tract. Together, these two conditions are collectively referred to as inflammatory bowel disease, or IBD. (Ulcerative colitis, no date).
Men and women are equally likely to develop ulcerative colitis. Extraintestinal manifestations may occur in up to 25% of patients. These include osteoporosis in 15%, oral ulcerations in 10%, arthritis in 5% to 10%, primary sclerosing cholangitis in 3%, uveitis in 0.5% to 3%, pyoderma gangrenosum in 0.5% to 2.0%, deep venous thrombosis in 0.3% and pulmonary embolism in 0.2%. Current cigarette smoking is associated with a reduction in the risk for ulcerative colitis, but former smokers have a higher risk of developing ulcerative colitis vs never smokers. Although the exact cause of ulcerative colitis is still unknown, there is strong evidence that primary dysregulation of the mucosal immune system causes an excessive immunologic response to normal microflora. Other contributing factors to ulcerative colitis are taken to be both genetic and environmental in nature (Richards, 2019).
What is the pathogenesis of ulcerative colitis?
What gene associations occur in ulcerative colitis?
The array of genetic polymorphisms associated with UC would point to the likelihood of abnormalities in the epithelial barrier contributing to the onset of this condition, one hypothesis supporting the presence of an epithelial cell defect that initiates the disease under pressure from the colonic microbiome (figure 4) (Fuss and Strober, 2015).
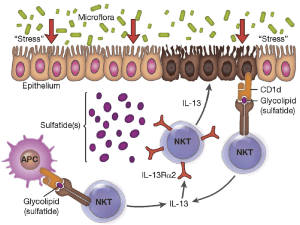
Figure 4:” Proposed mechanism of immune-mediated inflammation in UC. Inflammation in UC is initiated by release of glycolipid antigen(s) arising from genetically impaired epithelial cells under stress from exposure to components of the gut microbiome. These antigens are presented to and stimulate NK T cells in the context of CD1 on the surface of epithelial cells or on lamina propria dendritic cells. The NK T cells so stimulated cause epithelial cell damage by direct cytotoxic activity via interaction with CD1d loaded with glycolipid on the epithelial cell surface. Alternatively, the NK T cells cause epithelial apoptosis by release of IL-13 that then causes epithelial damage. Interleukin-13 also enhances inflammation by interacting with IL-13Rα2 on NK T cells, thereby inducing further NK T cell cytotoxic activity. Finally, epithelial ulceration resulting from these processes allows entry of bacterial components into the lamina propria that stimulates secondary inflammatory reactions.” Source: (Fuss and Strober, 2015)
What are Peyer’s patches?
Peyer’s patches are aggregations of lymphoid tissue, made up of lymphoid follicles located in the lamina propria of the mucosa. In adults, B lymphocytes are seen to predominate in the follicles’ germinal centers. T lymphocytes are found in the zones between follicles. With the lumen exposed to to the external environment, there are large numbers of potentially pathogenic microorganisms present. Peyer’s patches therefore carry out immune surveillance containing macrophages, dendritic cells, B-lymphocytes, and T-lymphocytes. The lymphoid tissue is covered by a special epithelium that contains specialized cells called M cells which sample antigen directly from the lumen and deliver it to antigen-presenting cells. These cells then pass to the mesenteric lymph nodes where the immune response is amplified.
How do you grade the severity of the disease?
Ulcerative Colits disease severity (based on Truelove and Witt classification):
- Symptoms Mild Severe Fulminant
- Stools per day 6 >10
- Hematochaezia Intermittent Frequent Continuous
- Temperature Normal >37.5 C
- Pulse Normal >90
- Haemoglobin Normal <75% of normal Transfusion
- ESR 30mm/hr
Download images for case
Ulcerative colitis.
Treatment and management: On admission patient was rehydrated and given Solucortef 100 mg IMI tds. She continued to pass 10 stools the following day.
Day 3: Patient continued to experience diarrhoea and unable to tolerate food or water. Transfused with 2 units of packed cells Prescribed: Asacol 1.2g po, tds ( mesalazine ) Asacol suppository PR bds Morphine 15mg IMI PRN Flagyl 500mg tds
Day 6: Patient has continued to experience diarrhoea of watery, bloody stools. Abdominal pain has decreased and abdomen is soft and undistended. It was decided to continue medical management for a further 7 days, with the addition of: Cyclosporine 80mg IVI, infused over 2hrs Losec 20 mg po daily ( omeprazol ) Slow K rider IVI bds Slow Magnesium IVI daily Clexane 40 mg S/C daily ( enoxaparin )
Day 13: It was decided that medical management had failed as no relief of symptoms was achieved. Surgical management was therefore required.
A laparoscopic total colectomy and ileostomy was performed. Three months post surgery the patient is scheduled to return for ileal-anal pouch surgery, to eliminate the need to wear a bag.
- Ulcerative colitis (no date) MedicineNet. Available at: https://www.medicinenet.com/ulcerative_colitis/article.htm (Accessed: November 14, 2022).
- Richards, M. (2019) Immunopathogenesis of Ulcerative Colitis. USA: Maureen Richards Immunology & Microbiology: YouTube channel. Available at: https://www.youtube.com/watch?v=FnahtfSmP60 .
- Fuss, I. J. and Strober, W. (2015) “Ulcerative Colitis,” in Mucosal Immunology. Elsevier, pp. 1573–1612.
Multiple Choice Questions
Earn 1 hpcsa or 0.25 sacnasp cpd points – online quiz.

© 2004 - 2024 Immunopaedia.org.za Sitemap - Privacy Policy - Cookie Policy - PAIA - Terms & Conditions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License .

- CLINICAL CASES
- ONLINE COURSES
- AMBASSADORS
- TREATMENT & DIAGNOSTICS
Tofacitinib for Biologic-Experienced Hospitalized Patients With Acute Severe Ulcerative Colitis: A Retrospective Case-Control Study
Affiliations.
- 1 Department of Internal Medicine, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, Michigan; Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan; Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, Michigan. Electronic address: [email protected].
- 2 Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan.
- 3 Department of Medicine, St Joseph Mercy Ann Arbor Hospital, Ypsilanti, Michigan.
- 4 Department of Internal Medicine, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, Michigan; Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan.
- 5 Department of Internal Medicine, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, Michigan.
- 6 Department of Pharmacy Services, Michigan Medicine, Ann Arbor, Michigan.
- 7 Department of Internal Medicine, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, Michigan; Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan; Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, Michigan.
- 8 Department of Internal Medicine, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, Michigan; Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan; Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, Michigan; Michigan Integrated Center for Health Analytics and Medical Prediction (MiCHAMP), Ann Arbor.
- 9 Department of Internal Medicine, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, Michigan; Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan; Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, Michigan; VA Center for Clinical Management Research, VA Ann Arbor Health Care System, Ann Arbor, Michigan.
- 10 Department of Internal Medicine, Division of Gastroenterology and Hepatology, Michigan Medicine, Ann Arbor, Michigan; Department of Internal Medicine, Michigan Medicine, Ann Arbor, Michigan; Institute for Healthcare Policy and Innovation, University of Michigan, Ann Arbor, Michigan; Michigan Integrated Center for Health Analytics and Medical Prediction (MiCHAMP), Ann Arbor; VA Center for Clinical Management Research, VA Ann Arbor Health Care System, Ann Arbor, Michigan.
- PMID: 34048936
- PMCID: PMC8760630
- DOI: 10.1016/j.cgh.2021.05.038
Background & aims: Despite rescue therapy, more than 30% of patients with acute severe ulcerative colitis (ASUC) require colectomy. Tofacitinib is a rapidly acting Janus kinase inhibitor with proven efficacy in ulcerative colitis. Tofacitinib may provide additional means for preventing colectomy in patients with ASUC.
Methods: A retrospective case-control study was performed evaluating the efficacy of tofacitinib induction in biologic-experienced patients admitted with ASUC requiring intravenous corticosteroids. Tofacitinib patients were matched 1:3 to controls according to gender and date of admission. Using Cox regression adjusted for disease severity, we estimated the 90-day risk of colectomy. Rates of complications and steroid dependence were examined as secondary outcomes.
Results: Forty patients who received tofacitinib were matched 1:3 to controls (n = 113). Tofacitinib was protective against colectomy at 90 days compared with matched controls (hazard ratio [HR], 0.28, 95% confidence interval [CI], 0.10-0.81; P = .018). When stratifying according to treatment dose, 10 mg three times daily (HR, 0.11; 95% CI, 0.02-0.56; P = .008) was protective, whereas 10 mg twice daily was not significantly protective (HR, 0.66; 95% CI, 0.21-2.09; P = .5). Rate of complications and steroid dependence were similar between tofacitinib and controls.
Conclusions: Tofacitinib with concomitant intravenous corticosteroids may be an effective induction strategy in biologic-experienced patients hospitalized with ASUC. Prospective trials are needed to identify the safety, optimal dose, frequency, and duration of tofacitinib for ASUC.
Keywords: Colectomy; Tofacitinib; Ulcerative Colitis.
Copyright © 2021 AGA Institute. Published by Elsevier Inc. All rights reserved.
Publication types
- Research Support, N.I.H., Extramural
- Biological Products*
- Case-Control Studies
- Colitis, Ulcerative* / drug therapy
- Colitis, Ulcerative* / surgery
- Piperidines
- Prospective Studies
- Pyrimidines
- Retrospective Studies
- Biological Products
- tofacitinib
Grants and funding
- R01 DK118154/DK/NIDDK NIH HHS/United States
- R01 DK124779/DK/NIDDK NIH HHS/United States
- T32 DK062708/DK/NIDDK NIH HHS/United States
- Case Report
- Open access
- Published: 10 October 2012
Ulcerative colitis in a Nigerian girl: A case report
- Idowu O Senbanjo 1 , 6 ,
- Kazeem A Oshikoya 2 , 3 ,
- Charles A Onyekwere 4 ,
- Fatimah B Abdulkareem 5 &
- Olisamedua F Njokanma 1
BMC Research Notes volume 5 , Article number: 564 ( 2012 ) Cite this article
11k Accesses
7 Citations
Metrics details
Ulcerative colitis (UC) is uncommon in the tropics and sub-tropics. We report a case of UC in a 7 year old girl whose parents were both Nigerians. This report is to alert healthcare professionals in sub-Saharan Africa that UC is not a rare health problem, especially in children.
Case presentation
The patient presented with frequent passage of blood stained stool, abdominal pain and significant weight loss. The diagnosis was entertained after she was investigated for common causes of chronic diarrhea in our setting and the findings were negative. The patient symptoms abated after she was commenced on steroid therapy.
Under-diagnosis and misdiagnosis may account for a dearth of information on UC in African children.
Ulcerative colitis (UC) is a chronic inflammatory disease of unknown aetiology, localized to the colon and spares the upper gastrointestinal tract[ 1 ]. The inflammation is characteristically remitting and relapsing[ 2 ].
The prevalence of UC varies across geographical zones and from one country to another[ 3 ]. In North America, the prevalence varies from 37.5 to 238 per 100000 people[ 4 ]. The disease is less common in children than adults[ 2 ]. In patients with UC, 20% are younger than 20 years of age, 4% are children aged less than 5 years and 1% are infants[ 3 , 5 ]. Although, UC can occur at any age, the incidence peak in age group 15–25 years and in 55–65 years[ 6 ]. The first paediatric case was reported in 1923 by Helmholz[ 7 ], thereafter, several other cases have been reported in children[ 8 – 10 ]. UC usually exists in isolation and together with Crohn’s disease (CD) and indeterminate colitis (IC) constitutes a genetically, immunologically and histopathologically heterogeneous group of inflammatory bowel disorders called inflammatory bowel disease (IBD)[ 11 ]. Very rarely are these diseases diagnosed in the same patient[ 12 ]. However, a rare case of UC co-exiting with CD had been reported in an adult[ 13 ].
Many familial cases have been reported in the United States, yet no simple Mendelian genetic mechanism has been able to explain its transmission in those with associated family history of the disease[ 2 ]. The prevalence of UC is highest in Europe and America among Caucasians and Ashkenazic Jews and lowest in black Americans and in African countries and Japan[ 1 ]. Although, cases of UC in African-American children living in the United States of America have been reported[ 14 ], none has been reported in Africa. The rarity of the disease in Africa may limit the experience of clinicians in its diagnosis and management, especially in children. This case is therefore reported to create an awareness of UC among paediatric age group and to discuss the challenges facing the diagnosis and management of the disease in a resource poor country.
A 7 year old girl presented to the paediatric gastroenterology clinic at the Lagos State University Teaching Hospital (LASUTH), Ikeja with a history of prolonged diarrhoea of 10 weeks that progressed to frank haematochezia 2 weeks later. She also presented with abdominal pain weight loss of over 8 weeks duration. Stool was initially watery, not offensive or mucoid. Bowel motions were about 10 times per day. There was no vomiting, fever, jaundice, mouth ulcer or joint pains. The abdominal pain was crampy, diffusely localized to the umbilical and supra-pubic regions. It was neither aggravated nor relieved by any known factors. Pain did not radiate elsewhere nor, disturb the patient from sleep, associate with tenesmus or abdominal distension. The symptoms were however associated with a significant weight loss despite good appetite and adequate feeding. There were no associated respiratory and urinary symptoms. The past medical history was remarkable in the sense that she had initially presented to a general hospital where she was investigated and treated for dysentery with a course of metronidazole, co-trimoxazole and hyoscine bromide for 6 weeks without any appreciable improvement. There was no history or signs of past abdominal surgery. Patient is the first of three children to both monogamous parents. The parents are Nigerians and there was no history of similar illness in any member of the family.
On examination, she was afebrile, anicteric, mildly pale, weighed 19kg, not irritable or in respiratory distress, not dehydrated or had peripheral oedema. There was no peripheral lymphadenopathy, skin desquamation or skin discolorations. The mucous membranes and nails were normal. Mild tenderness was elicited in the peri-umbillical region but no palpable abdominal mass, hepatomegaly or splenomegaly. Rectal examination was painful, no palpable rectal mass. The rectum appeared to be narrowed and the examination finger was stained with frank blood.
The patient was admitted and investigated for causes of lower gastrointestinal bleeding. The investigations revealed Hb of 10g/dL, white blood cell count of 19,400/mm 3 with neutrophil differential of 61%, lymphocyte-32% and monocyte-7%. The ESR was elevated to 34mm/hr and serum protein significantly reduced with hypoalbuminaemia of 21g/dL. The liver function test and electrolyte with urea were essentially normal. The stool and urine cultures yielded no growth after 48 hours of incubation. No eggs, ova or intestinal parasites were seen on stool microscopy. Patient was commenced on a high protein diet and all antibiotics discontinued for 10 days. While the symptoms persisted, barium enema was requested which showed dilatation of the sigmoid and descending colon in association with persistent narrowing of the rectum and effacement of the mucosal pattern that was replaced by thumb printing appearances (Figure 1 ). These findings were suggestive of UC. Colonoscopy and rectal biopsy were performed later. The colonoscopy showed inflammatory changes extending from the anal opening up to the visible part of the descending colon. The bowel mucosa was erythemous and oedematous, with effaced vascular pattern. Tissue biopsy was granular and friable. The histology of the rectal tissue biopsy confirmed UC as shown in Figure 2 .
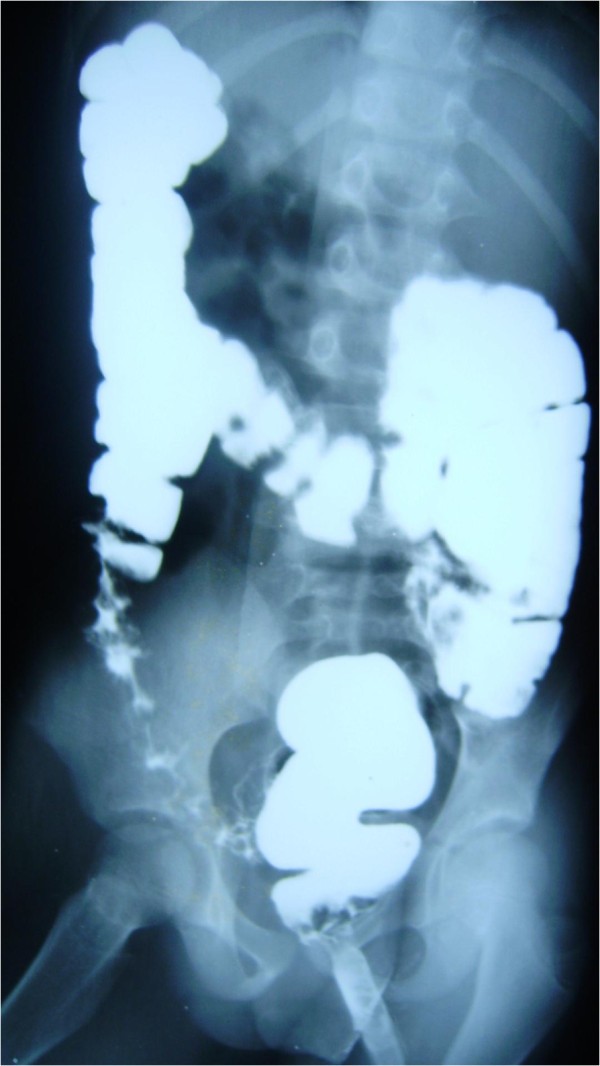
Barium enema showed dilatation of the sigmoid and descending colon, persistent narrowing of the rectum with effacement of the mucosal pattern that was replaced by thumb printing appearances.
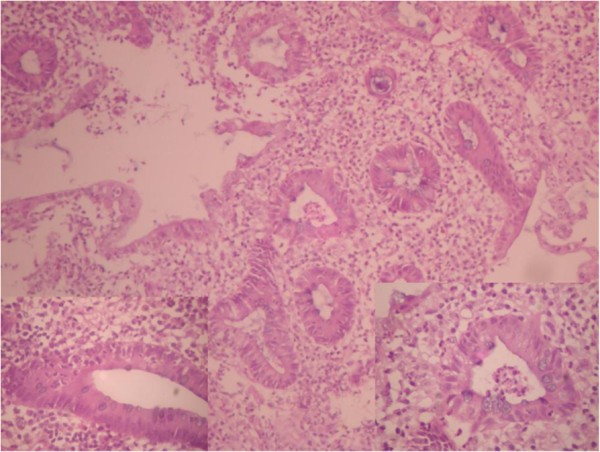
Photomicrograph of UC (rectal mucosa) showing intense inflammation with disordered crypts and evidence of cryptitis and crypt abscess. H & E (magnification x 200).
Patient was commenced on sulfasalazine 50mg/kg/day in two divided doses and gradually increased to 60mg/kg/day after a week treatment as it was well tolerated. Patient was in remission until 6 months follow up. However, she defaulted from the clinic for about 6 months but continued taking her medications at home for another 5 months. She presented again with bleeding diarrhoea after stopping her medications for about a month. Sulfasalazine was re-commenced at the same dose plus prednisolone 1 mg/kg/day in two divided doses. Patient responded very well to the new regime and now in remission. She is being followed at the outpatient clinic every 4 weeks.
UC is known to affect children and adults globally. However, it is less common in Africa probably due to under-diagnosis, misdiagnosis or low racial distribution. Few cases of UC in adults have been reported in South Africa[ 15 ], Uganda[ 16 ] and Sudan[ 17 ]. Lack of reported cases in African children therefore underscores the importance of this current case report.
A major challenge in the management of UC in developing countries is making an accurate diagnosis. Our patient was presumptively treated for amoebic dysentery at a general hospital. In spite of persistent symptoms after 6 weeks of antibiotic therapy, UC was not suspected by her physician. Thus, a high index of suspicion may be required for early diagnosis of UC which should be considered as a differential diagnosis of blood stained chronic diarrhoeal diseases in children.
Presently, there is no permanent medical cure for UC[ 1 , 2 ]. The general goals of treatment in children are to control symptoms of the disease with minimal adverse effects of the medicines used and to achieve normal functioning of the patient[ 1 , 2 ]. A multidisciplinary approach has been suggested for effective management of UC in children[ 2 ]. Patient should be treated and followed up jointly by a team consisting of a paediatric gastroenterologist, paediatric surgeon, child psychiatrist, clinical psychologist and social worker. The intensity of treatment is dependent on the severity of the disease[ 1 ]. Less than 5% of children with UC may present predominantly with extraintestinal manifestations, such as growth failure; arthropathy; dermatological, genitourinary or pulmonary manifestations; coagulopathy; or liver disease[ 1 , 2 ]. However, none of these symptoms was manifested in the patient. Based on the symptoms and signs of bloody diarrhoea, abdominal cramps, urgency to defecate, abdominal tenderness, weight loss and mild anemia at presentation, and colonoscopy with histologic findings, the patient was diagnosed of moderate UC[ 2 ]. Mild to moderate UC is usually treated on an outpatient basis. Admission becomes inevitable upon failure of maximal outpatient therapy or progression to severe disease[ 1 , 2 ].
The patient was managed on outpatient basis after initial investigation and stabilization on admission. She was commenced on paediatric medical regimen for UC consisting of low residue diet, antimotility and sulfasalazine (a first line medicine)[ 1 , 2 ]. Sulfasalazine is known to treat UC effectively and prevents recurrence[ 1 , 2 ]. Its prolonged use, even during remission, has been recommended in children[ 1 ]. However, hypersensitivity adverse reaction to the sulfa component of sulfasalazine is a major limitation to its use as a first line medicine which may occur in 10-20% of patients[ 2 ]. Fortunately, the medicine was well tolerated by the patient. On rare occasions, sulfasalazine can exacerbate the symptoms and signs of UC which may prompt patient to self discontinue the medication. On the contrary, there was a tremendous improvement in the patient following the use of sulfasalazine. The medication was self discontinued as both the patient and the parents felt a permanent cure had been achieved after 11 months of remission. Lack of adequate counseling and psychosocial support might have contributed to the poor drug compliance exhibited by the patient at a later stage of treatment. The role of a clinical psychologist is to promote the psychological wellbeing of the patient and enable her to adjust to her daily normal life activities. The multidisciplinary approach to managing this type of patients is important and equally necessary when managing other chronic childhood illness. Unfortunately, the number of clinical psychologists and social workers in Nigeria is just a handful and are confined to academic institutions and tertiary health care facility.
Prednisolone is a second line medicine that was included in the treatment when the patient re-presented to our hospital. The use was justified by the slow response to sulfasalazine. However, both medicines were able to abate the symptoms and signs of the recurrent UC without any adverse effects. Corticosteroids are known to control acute flares of UC effectively but less effective at maintaining long term remission[ 2 , 18 ]. The numerous adverse effects of prolong use of corticosteroids also preclude their maintenance use during UC remission. We, therefore plan to taper off the dose of prednisolone over time and maintain the patient on sulfasalazine after achieving a prolonged remission.
Clinic default and poor medication compliance is a common problem of children with chronic diseases[ 19 ]. Poor follow-up has been reported in South African adults with UC[ 14 ]. Further default and poor medication compliance may put the patient at risks of progressing to fulminant colitis or becoming refractory to medical therapy[ 1 , 2 ]. Approximately 5-10% of such patients may require acute surgical intervention[ 2 , 20 ]. Surgery should, however, be seen as a complementary to medical therapy and as a means of preventing complications[ 20 ].
This is the first time UC is reported in an African child. Under-diagnosis and misdiagnosis may have accounted for lack of reports on this subject from Africa. Ingenuity may therefore be required for early diagnosis. UC should be suspected in childhood bloody chronic diarrhoeal diseases and patient should be investigated as such. Optimal management is required to achieve long term remission on medical therapy with minimal adverse effects.
A written informed consent was obtained from the patient’s legal guardian for publication of this case report and any accompanying images.
Hyams JS: Inflammatory bowel disease. Nelson textbook of pediatrics. Edited by: Kliegman RM, Behrman RE, Jenson HB, Stanton B. 2007, Philadelphia: Saunders Elsevier, 1575-1585. 8
Google Scholar
Markowitz JE, Mamula P, Baldassano R, Piccoli DA, Dancel LD: Ulcerative Colitis. eMedicine Specialties. 2009, Available on http://emedicine.medscape.com/article/930146-overview (Accessed Feb 2010),
Cosnes J, Gower-Rousseau C, Seksik P, Cortot A: Epidemiology and Natural History of Inflammatory Bowel Diseases. Gastroenterology. 2011, 140: 1785-1794. 10.1053/j.gastro.2011.01.055.
Article PubMed Google Scholar
Jacobsen BA, Fallingborg J, Rasmussen HH, Nielsen KR, Drewes AM, Puho E, Nielsen GL, Sørensen HT: Increase in incidence and prevalence of inflammatory bowel disease in northern Denmark: a population-based study, 1978–2002. Eur J Gastroenterol Hepatol. 2006, 18: 601-606. 10.1097/00042737-200606000-00005.
Kappelman MD, Graud RJ: Does inflammatory bowel disease develop in infants?. Inflamm Bowel Dis. 2009, 15: 1438-1447.
Article Google Scholar
Kim SC, Ferry GD: Inflammatory bowel disease in pediatric and adolescent patients: Clinical, therapeutic and psychosocial considerations. Gastroenterology. 2004, 126: 1550-1560. 10.1053/j.gastro.2004.03.022.
Helmholz HF: Chronic ulcerative colitis in childhood. Am J Dis Child. 1923, 26: 418-
Davidson S: Infatilism in ulcerative colitis. Arch Int Med. 1939, 64: 1187-10.1001/archinte.1939.00190060056004.
King RC, Linden AE, Pollard HM: Chronic ulcerative colitis in childhood. Arch Dis Child. 1959, 34: 257-10.1136/adc.34.175.257.
Article PubMed CAS PubMed Central Google Scholar
Rukunuzzaman M, Karim AB: Ulcerative colitis in infancy. Saudi J Gastroenterol. 2011, 17: 414-417. 10.4103/1319-3767.87185.
Article PubMed PubMed Central Google Scholar
Vucelic B: Inflammatory bowel diseases: controversies in the use of diagnostic procedure. Dig Dis. 2009, 27: 269-277. 10.1159/000228560.
McDermott FT, Pihl EA, Kemp DR, Polglase AL: Coexisting Crohn’s disease and ulcerative colitis: report of a case. Dis Colon Rectum. 1982, 25: 600-602. 10.1007/BF02564179.
Article PubMed CAS Google Scholar
Chen GI, Saibil F, Morava-Protzner I: Two for one: Coexisting ulcerative colitis and Crohn's disease. Can J Gaastroenterol. 2002, 16: 29-34.
Ogunbi SO, Ransom JA, Sullivan K, Schoen BT, Gold BD: Inflammatory bowel disease in African-American children living in Georgia. J Pediatr. 1998, 133: 103-13S7. 10.1016/S0022-3476(98)70187-8.
Segal I: Ulcerative colitis in a developing country of Africa: The Baragwanath experience of the first 46 patients. Int J Coloreact Dis. 1988, 3: 222-225. 10.1007/BF01660719.
Article CAS Google Scholar
Billinghurst JR, Welchman JM: Idiopathic ulcerative colitis in the African: a report of four cases. Brit Med J. 1966, 1: 211-213. 10.1136/bmj.1.5481.211.
Khalifa SE, Mudawi HM, Fedail SS: Presentation and management outcome of inflammatory bowel disease in Sudan. Trop Gastroenterol. 2005, 26: 194-196.
PubMed CAS Google Scholar
Turner D: Severe acute ulcerative colitis: the pediatric perspective. Dig Dis. 2009, 27: 322-326. 10.1159/000228568.
Lilleyman JS, Lennard L: Non-compliance with oral chemotherapy in childhood leukaemia. BMJ. 1996, 313: 1219-1220. 10.1136/bmj.313.7067.1219.
Andersson P, Soderholm JD: Surgery in ulcerative colitis: indication and timing. Dig Dis. 2009, 27: 335-340. 10.1159/000228570.
Download references
Author information
Authors and affiliations.
Department of Paediatrics and Child Health, Lagos State University College of Medicine, PMB 21266, Ikeja, Lagos State, Nigeria
Idowu O Senbanjo & Olisamedua F Njokanma
Pharmacology Department, Lagos State University College of Medicine, PMB 21266, Ikeja, Lagos State, Nigeria
Kazeem A Oshikoya
Academic Division of Child Health, Medical School (University of Nottingham), Derbyshire Children’s Hospital, Uttoxeter Road, Derby, DE22 3DT, UK
Department of Medicine, Lagos State University College of Medicine, PMB 21266, Ikeja, Lagos State, Nigeria
Charles A Onyekwere
Gastrointesinal/Hepato-pathology Unit, Morbid Anatomy Department, College of Medicine, University of Lagos, P.M.B. 12003, Idi-Araba, Lagos, Nigeria
Fatimah B Abdulkareem
Paediatrics Gastroenterology, Hepatology and Nutrition Unit, Department of Paediatrics and Child Health, Lagos State University Teaching Hospital, Ikeja, Lagos, Nigeria
Idowu O Senbanjo
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Idowu O Senbanjo .
Additional information
Competing interest, authors' contributions.
IOS was the Pediatric gastroenterologist that managed the patient, conceived and designed the report. KAO participated in the management of the patient and the design of the report. CAO carried out the colonoscopy and biopsy assisted by IOS. FBA carried out the histopathologic evaluation of specimens and interpreted the patient samples. OFN guided and provided essential comments during production of the manuscipt. All the authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Authors’ original file for figure 1
Authors’ original file for figure 2, rights and permissions.
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License ( http://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Senbanjo, I.O., Oshikoya, K.A., Onyekwere, C.A. et al. Ulcerative colitis in a Nigerian girl: A case report. BMC Res Notes 5 , 564 (2012). https://doi.org/10.1186/1756-0500-5-564
Download citation
Received : 14 May 2012
Accepted : 01 October 2012
Published : 10 October 2012
DOI : https://doi.org/10.1186/1756-0500-5-564
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ulcerative colitis
BMC Research Notes
ISSN: 1756-0500
- Submission enquiries: [email protected]
- General enquiries: [email protected]

- Nutrition counsellor
- Food Allergy
- gastroenterology
- Heptologist In Delhi
- Liver transplant
- Case Study: Severe Ulcerative Colitis
Final diagnosis: Ulcerative Colitis
Patient: Female, 27-year-old
Ulcerative colitis signs and Symptoms: bloody diarrhoea, abdominal tenderness and crampy abdominal pain, and weight loss
Speciality: Gastroenterology and hepatology
Causes, symptoms, and treatment of severe ulcerative colitis
Ulcerative colitis is a chronic inflammatory disease of the colon and rectum. Going by the severe ulcerative colitis definition, it does not affect the upper gastrointestinal tract. It causes inflammation and ulcers in the digestive tract. It affects the inner lining of the large intestine and rectum. The symptoms of the disease develop over time; they do not appear suddenly. Although the disease can occur at any age, it is less common in children. ulcerative colitis symptoms in females
Case Review
In this case study on ulcerative colitis, a twenty seven-year-old girl was brought to the clinic with a history of prolonged diarrhoea that had lasted ten weeks and was progressive. The patient presented frequent passage of stool with small amounts of blood, abdominal pain and noticeable weight loss. She also reported bowel movements about ten times per day. On reviewing the patient, it was observed that there was fever, vomiting, jaundice, joint pains or mouth ulcers. She was suffering from crampy abdominal pain, which was neither relieved nor aggravated by any known factor. The symptoms were associated with noticeable weight loss despite a good appetite and sufficient diet. No other urinary or respiratory symptoms were reported. On physical examination, she was pale, weighed 19 kgs and was neither irritable nor dehydrated. The rectum appeared to be narrowed, and the examination finger had stains of blood. These findings suggested the presence of ulcerative colitis.
Case Discussion
Ulcerative colitis affects adults and children globally. Currently, there is an ulcerative colitis cure for the disease. The goal of the treatment is to control the symptoms with the least possible side effects of the medicines prescribed and enable the patient to function normally. A comprehensive approach is suggested for effective treatment and management of ulcerative colitis. She should be treated and followed up by a team consisting of a gastroenterologist, paediatrician and hepatologist. The line of treatment for severe ulcerative colitis treatment depends on the severity of the infection. The signs of abdominal cramps, bloody diarrhoea, the urgency to defecate but an inability to do so, weight loss, and abdominal tenderness were indicative of mild ulcerative colitis. Medicines were prescribed to reduce inflammation and patient-reported reduction of symptoms after a continual treatment. In some instances of acute colitis ulcerative colitis complications, the patient may require surgery. However, it is complementary to the medical procedures and is advised only for preventing complications.
Clinical Symptoms
Ulcerative colitis, an inflammatory bowel disease, causes inflammation and sores in the digestive tract. The symptoms can vary depending on the location of the inflammation and its severity. The symptoms do not happen overnight but usually develop over time. The inflammation damages the inner lining of the large intestine (colon) and rectum. Some common symptoms include diarrhoea often accompanied with pus or blood, pain in the abdomen, pain in the rectum area, rectal bleeding, or blood with stool, having a feeling to defecate but not being able to despite the urgency. In severe cases, the patient may experience fatigue, weight loss and fever.
The patient had mild to moderate symptoms with bleeding, pain, and problems in passing stool.
The type of treatment depends on the reasons behind ulcerative colitis. In most cases, the treatment option includes symptomatic care, medicines to regulate bowel movement. In some cases, patients who have acute colitis may need IV fluid to restore fluid balance. Blood tests and stool tests were advised to confirm the diagnosis of ulcerative colitis. Primarily, the treatment involves medications that reduce inflammation. The medicines that work on some people may not work for another person usually, as it takes some time to identify medicines that help relieve the symptoms. As drugs have side effects, the risks and benefits were weighed before prescribing the medicines and supporting disease management. When medicines do not seem to be effective, then surgery is an option. The process involves the removal of the rectum and colon. Though disease management is a challenge, making lifestyle and diet changes can help control the symptoms. Though there is no evidence that what you eat causes ulcerative colitis, certain foods are known to aggravate the symptoms and flare-ups. Here are some general suggestions for a severe ulcerative colitis diet:
- Restrict dairy products intake: Often, problems like diarrhoea, gas, and abdominal pain can improve by limiting or eliminating the dairy products.
- Eat small meals: The symptoms often abate by consuming five to six smaller meals than two to three larger ones.
- Keep hydrated: It is best to drink as much water as possible. Consuming drinks that contain caffeine can worsen the symptoms.
In this case, the patient was put on medicines as it was a mild case of ulcerative colitis. After a few months of therapy, the patient reported relief in the symptoms. She was advised to make lifestyle changes as complementary to medicine.
With routine check-ups and adequate treatment, ulcerative colitis can be cured. If you or someone you know is experiencing any of the symptoms above, consult the gastro & liver clinic Patna Bihar. It is best to consult gastroenterologists online free or online gastroenterologist doctors. Excellent medical assistance is available in several cities, including best physician in Jammu city, max hospital liver specialist, nor gastro liver clinic Gurgaon, the best doctor in Patna for stomach, best female gynaecologist in Jhansi, liver cirrhosis specialist doctor in India, and gastro surgeon in Delhi
1. Is the colon the same as the large intestine?
Yes, ulcerative colitis is the inflammation of the large intestine or the colon.
2. What is the most primary symptom of ulcerative colitis?
Rectal bleeding is a significant symptom. However, other symptoms presented with the disease are diarrhoea and cramping abdominal pain.
3. Can antibiotics help in curing ulcerative colitis?
Antibiotics cannot help in the cure of ulcerative antibiotics. They only help in the management of the disease.
Privacy Preference Center
Privacy preferences, nutrition counselling.
Nutrition counseling is the assessment of an individual’s dietary intake after which, they are helped set achievable goals and taught various ways of maintaining these goals. The nutrition counselor provides information, educational materials, support and follow-up care to help an individual make and maintain the needed dietary changes for problems like obesity.
Obesity/ Food allergy
I assist people dealing with weight-related health problems by evaluating the health risks and help in obesity management. I also help patients manage various food allergies.
As a hepatologist, I specialize in the treatment of liver disorders, pancreas, gallbladder, hepatitis C, jaundice and the biliary tree. I also see patients suffering from pancreatitis, liver cancers alcoholic cirrhosis and drug induced liver disease(DILI), which has affected the liver.
Gastroenterology
As a gastroenterologist, my primary focus is the overall health of the digestive system. I treat everything from acid reflux to ulcers, IBS, IBD: Crohns disease and ulcerative colitis, and colon cancer.
Endoscopy is a nonsurgical procedure to examine a person’s digestive tract. It is carried out with an endoscope, a flexible tube with a light and camera attached to it so that the doctor can see pictures of the digestive tract on a color TV monitor.
Medical Gastroenterology
Gastroenterology is a specialty that evaluates the entire alimentary tract from the mouth to anus and involves studying the diseases of the pancreas.
Liver Transplant Services
A liver transplant is a surgical procedure that removes a patient’s non-functioning liver and replaces it with a healthy liver from a deceased donor or a portion of healthy liver from a living donor. It is reserved as a treatment option for people who have significant complications due to end-stage chronic liver disease or in case of sudden failure of a previously healthy liver.

Hypertrophic osteoarthropathy in poorly treated ulcerative colitis
Citation, doi, disclosures and case data.
At the time the case was submitted for publication Domenico Nicoletti had no financial relationships to ineligible companies to disclose.
Presentation
Ulcerative colitis, not well-treated with mesalazine and steroids. Onset of pain to the forearms and lower limbs, in particular wrists and ankles.
Patient Data
Forearms and lower limbs.
Diffuse cortical thickening of the diaphysis of the femurs of the tibiae and fibulas as well as of the radius and ulna diaphysis with enlargement of the bones and periosteal new bone formation , always on both sides . Bilateral gonarthrosis and coxarthrosis.
Abdominal Ultrasound
Wall thickening of the entire colon with loss of multilayer pattern and marked Doppler signal associated with “comb sign” with parallel mesenteric vessels as a sign of severe inflammation (Limberg Score 4). It is accompanied by creeping fa t and ulcers .
Histologically report of colonoscopic biopsies
The mucosal surface is irregular, with edema, interstitial hemorrhage, and inflammatory exudate in the lamina propria. In the mucosa, there is also interstitial infiltration of lymphocytes, plasma cells, eosinophils, and neutrophils, with erosions and exudate of neutrophils from crypt abscesses. Findings correlated with active ulcerative colitis with transmucosal distribution, with an abnormally high density of neutrophils, lymphocytes, and plasma cells in the lamina propria with mucosal erosions and ulceration.
Note the soft-tissue clubbing of the terminal phalanges of the hands with speculated periostosis of the distal radius and ulna on both sides.
Detail of the speculated periostosis on the lower parts of the tibia and fibula on both sides. The same finding occurs at the level of the radius and ulna, distally. There's also smooth cortical thickening in the diaphysis of the tibia and fibula of both legs, especially the tibiae.
Case Discussion
Non-small cell lung cancer (NSCLC) is the most common cause of clubbing and hypertrophic osteoarthropathy .
Other conditions associated with clubbing are cardiovascular diseases such as congenital cyanotic heart disease and infective endocarditis or gastrointestinal diseases such as cirrhosis , primary sclerosing cholangitis , Crohn's disease and ulcerative colitis .
Finger clubbing is usually bilateral and symmetrical. Rare cases of unidigital clubbing are described in sarcoidosis .
Imaging technologists : TSRM Nunzio Bianco, TSRM Simone Pasini
- 1 Oppenheimer D & Jones H. Hypertrophic Osteoarthropathy of Chronic Inflammatory Bowel Disease. Skeletal Radiol. 1982;9(2):109-13. doi:10.1007/BF00360493 - Pubmed
- 2 Furfaro F, Dal Buono A, Allocca M et al. Bowel Ultrasound in Inflammatory Bowel Disease: How Far in the Grayscale? Life (Basel). 2021;11(7):649. doi:10.3390/life11070649 - Pubmed
- 3 Tohtz S & Putzier M. [Secondary Hypertrophic Osteoarthropathy in Colitis Ulcerosa--A Case Demonstration]. Z Orthop Ihre Grenzgeb. 2004;142(4):486-8. doi:10.1055/s-2004-822822 - Pubmed
1 public playlist includes this case
- Arthritides by Troy Zanger
Related Radiopaedia articles
- Crohn disease
- Finger clubbing
- Hypertrophic osteoarthropathy
- Infective endocarditis
- Non-small cell lung cancer
- Primary sclerosing cholangitis
- Sarcoidosis
- Ulcerative colitis
Promoted articles (advertising)
How to use cases.
You can use Radiopaedia cases in a variety of ways to help you learn and teach.
- Add cases to playlists
- Share cases with the diagnosis hidden
- Use images in presentations
- Use them in multiple choice question
Creating your own cases is easy.
- Case creation learning pathway
ADVERTISEMENT: Supporters see fewer/no ads
By Section:
- Artificial Intelligence
- Classifications
- Imaging Technology
- Interventional Radiology
- Radiography
- Central Nervous System
- Gastrointestinal
- Gynaecology
- Haematology
- Head & Neck
- Hepatobiliary
- Interventional
- Musculoskeletal
- Paediatrics
- Not Applicable
Radiopaedia.org
- Feature Sponsor
- Expert advisers

- Open access
- Published: 21 May 2024
Mogroside V reduced the excessive endoplasmic reticulum stress and mitigated the Ulcerative colitis induced by dextran sulfate sodium in mice
- Yue-Rong Tan 1 na1 ,
- Si-Yang Shen 1 na1 ,
- Xin-Yi Li 1 ,
- Peng-Fei Yi 1 , 2 ,
- Ben-Dong Fu 1 , 2 &
- Lu-Yuan Peng 1 , 2
Journal of Translational Medicine volume 22 , Article number: 488 ( 2024 ) Cite this article
Metrics details
Ulcerative colitis (UC) is an idiopathic, chronic inflammatory condition of the colon, characterized by repeated attacks, a lack of effective treatment options, and significant physical and mental health complications for patients. The endoplasmic reticulum (ER) is a vital intracellular organelle in maintaining cellular homeostasis. Endoplasmic reticulum stress (ERS) is induced when the body is exposed to adverse external stimuli. Numerous studies have shown that ERS-induced apoptosis plays a vital role in the pathogenesis of UC. Mogroside V (MV), an active ingredient of Monk fruit, has demonstrated excellent anti-inflammatory and antioxidant effects. In this study, we investigated the therapeutic effects of MV on dextran sulfate sodium (DSS)-induced UC and its potential mechanisms based on ERS. The results showed that MV exerted a protective effect against DSS-induced UC in mice as reflected by reduced DAI scores, increased colon length, reduced histological scores of the colon, and levels of pro-inflammatory cytokines, as well as decreased intestinal permeability. In addition, the expression of ERS pathway including BIP, PERK, eIF2α, ATF4, CHOP, as well as the apoptosis-related protein including Caspase-12, Bcl-2 and Bax, was found to be elevated in UC. However, MV treatment significantly inhibited the UC and reversed the expression of inflammation signaling pathway including ERS and ERS-induced apoptosis. Additionally, the addition of tunicamycin (Tm), an ERS activator, significantly weakened the therapeutic effect of MV on UC in mice. These findings suggest that MV may be a therapeutic agent for the treatment of DSS-induced UC by inhibiting the activation of the ERS-apoptosis pathway, and may provide a novel avenue for the treatment of UC.
Introduction
Ulcerative colitis (UC) is an idiopathic, chronic inflammatory disorder, which starts distally and can gradually extend proximally through the whole colon [ 1 ]. Clinical manifestations typically include bloody diarrhoea, frequency, abdominal pain, fatigue and fecal incontinence, which is increasing in incidence and prevalence [ 2 ]. Although UC was originally prevalent in North America and Europe, its incidence has been gradually increasing in developing country such as China in recent years [ 3 ], and it tends to affect young adults, imposing a heavy societal burden [ 4 ]. From initial use of traditional anti-inflammatory drugs, such as sodium aminosalicylate [ 5 ] and corticosteroids, to the introduction of biologic agents, such as immunosuppressants, anti-tumor necrosis factor alpha, and anti-IL-17 classes [ 6 ], there has been a growing understanding of UC treatment. However, issues such as hormone dependence, adverse reactions, and drug resistance to these treatments persist, and the objective of developing a safe and effective treatment remains a significant challenge [ 7 ]. Furthermore, the high cost of treatment remains unresolved. Consequently, it is imperative to identify a novel therapeutic strategy.
The intestinal barrier serves as an important protective barrier against external pathogens and harmful substances [ 8 ]. Although the pathogenesis of UC is not yet fully understood, the occurrence of UC appears to be related to disruption in the intestinal mucosa barriers and resultant perturbations of the gut microbiota [ 9 ]. When the intestinal barrier is compromised, intestinal permeability increases, and exogenous toxins or bacteria can more easily enter the body, leading to the development of UC [ 10 ]. The endoplasmic reticulum (ER) functions as a quality-control organelle for protein homeostasis, or “proteostasis”. The accumulation of misfolded proteins in the ER leads to endoplasmic reticulum stress (ERS), resulting in activation of the unfolded protein response (UPR) that aims to restore protein homeostasis [ 11 ]. ERS and defects in UPR signaling are emerging as key contributors to a growing list of human diseases, including diabetes, liver diseases, neurodegeneration, and cancer [ 12 ]. Several studies have shown that the ERS pathway plays a crucial role in maintaining the integrity of the intestinal mucosal barrier [ 13 ]. Excessive ERS signaling or persistent UPR activation can lead to apoptotic pathways [ 14 ], intestinal epithelial cell death, abnormal secretion of intestinal mucosal barrier proteins, and activation of pro-inflammatory responses in the intestine, leading to UC [ 15 ]. Modulation of ERS has emerged as a new therapeutic strategy in the treatment of UC.
Traditional Chinese medicine (TCM) is used as complementary and alternative therapies all over the world for the treatment of various diseases. Due to its multi-targeted mode of action and few side effects, it has unique advantages for treating chronic diseases, including definite curative effects, effective maintenance, recurrence rate reduction, and only minor toxic side effects [ 16 ]. With the continuous development of theoretical research in TCM, TCM has shown great progress in the research of treating UC [ 17 ]. Mogroside V (MV) is a sweet chemical extracted from the medicinal and food source of Siraitia grosvenorii, and has been used to prepare specially flavoured foods [ 18 ]. It has demonstrated pharmacological effects such as antioxidant and anti-tumour properties [ 19 ], and is commonly used as a cough suppressant and lung humectant [ 20 ]. Previous studies have shown that mogroside V can alleviate oxidative damage [ 21 ]. However, the efficacy of MV in treating UC remains unclear. Therefore, this study aimed to investigate the therapeutic effects of MV on dextran sulfate sodium-induced UC and explore its underlying mechanisms based on the ERS, to provide potential therapeutic drugs for the prevention and treatment of UC.
Materials and methods
The MV (purity > 99%) was purchased from Chengdu Must Biotechnology Co., Ltd. (Chengdu, China). Female C57BL/6J mice were obtained from the Experimental Animal Centre of Baiqiu’en School of Medicine, Jilin University (Jilin, China).
Animals and experimental design
Female C57BL/6J mice weighing between 20 and 22 g were housed in a temperature-controlled room at 24 ± 2 °C with a 12-hour light/dark cycle. The mice were allowed to acclimatize for one week before grouping based on similar body weight for the experiment. All animal procedures were conducted following the provisions of the Guide for the Care and Use of Laboratory Animals of Jilin University and were approved by the Animal Ethics Committee of Jilin University. All procedures requiring anesthesia were performed under isoflurane.
To determine the therapeutic effect of MV for treatment of UC, female C57BL/6J mice were randomly divided into five groups ( n = 6) including control group, model group and DSS + MV (25, 50, 100 mg/kg) groups. Except for the control group, the mice in the other groups drank 3% DSS freely for 7 days according to the previous report [ 22 ]. Meanwhile, mice in the control and model groups were orally administered deionized water. Mice in the DSS + MV groups were orally administered MV dissolved in deionized water (25, 50 and 100 mg/kg). (1) control group: normal drinking water plus saline by gavage; (2) model group: 3% DSS (molecular weight: 36,000–50,000, MP Biomedical, Morgan Irvine, CA, USA) added to drinking water and saline by gavage; (3) DSS low-dose MV: 3% DSS was added to the mice’s drinking water and an equal amount of low-concentration MV (25 mg/kg BW) was added by gavage; (4) DSS medium-dose MV: 3% DSS was added to the drinking water of mice and an equal amount of medium-concentration MV (50 mg/kg BW) was administered by gavage; (5) DSS high-dose MV: Mice were given 3% DSS in their drinking water and an equal amount of high-concentration MV (100 mg/kg BW) by gavage.
To investigate whether MV exerts its therapeutic effect on murine colitis through the ERS pathway, female C57BL/6J mice were divided into five groups, each consisting of six mice. The groups were as follows: (1) control group: normal drinking water plus saline by gavage; (2) model group: 3% DSS added to drinking water and saline by gavage; (3) DSS high-dose MV group: 3% DSS was added to mice drinking water, and an equal amount of high-concentration MV (100 mg/kg BW) was administered by gavage; (4) DSS high-dose MV and 4-PBA group: 3% DSS was added to the drinking water of mice, and an equal amount of high-concentration MV (100 mg/kg BW) and 4-phenylbutyric acid (4-PBA, MCE USA, 500 mg/kg BW) were administered by gavage; (5) DSS high-dose MV and Tm group: Mice were given 3% DSS in their drinking water and equal amounts of high concentrations of MV (100 mg/kg BW) and tunicamycin (Tm, Aladdin, Shanghai, China, 1 mg/kg BW) by gavage.
DAI and histopathological inflammation score
The Disease Activity Index (DAI) is a scoring system that evaluates the severity of colitis by assessing weight loss, stool texture, and stool bleeding. The scoring for each aspect is as follows: weight loss rate (0: none, 1: 1–5%, 2: 6–10%, 3: 11–20%, 4: >20%); stool texture (0: normal, 1: loose, 2: semi-formed, 3: loose stool, 4: watery diarrhea); and stool bleeding (0: no bleeding, 1: positive occult blood, 2: blood in stool, 3: perianal bleeding, 4: bloody diarrhea) [ 23 ].
The pathological histological score is based on the following three aspects: the degree of epithelial damage and ulcer formation, the extent of ulcer depth, and the degree of inflammatory cell infiltration. The scoring details for each aspect are as follows: degree of injury and ulceration (0: none, 1: slight erosion, 2: diffuse erosion, 3: ulceration); depth of ulceration (0: none, 1: submucosal, 2: muscular layer, 3: plasma layer); degree of inflammatory cell infiltration (0: none, 1: mild, confined to the mucosa, 2: moderate, reaching the muscular layer, 3: severe, reaching the plasma layer) [ 24 ].
Intestinal permeability assay
Intestinal permeability in mice was measured using non-absorbable FITC-dextran (4 kDa). Mice were fasted for 8 h prior to the experiment. FITC-dextran was prepared at a concentration of 50 mg/mL and administered by gavage at a dose of 0.1 mL/mouse. Blood samples were collected from the eyes 4 h later, and the supernatant was collected after centrifugation. A standard curve was generated by preparing a 20 µg/mL solution of FITC-dextran and successively diluting it in the following ratios: 10 µg/mL, 5 µg/mL, 2.5 µg/mL, 1.25 µg/mL, 0.625 µg/mL, and 0.3125 µg/mL. The samples to be tested were diluted with PBS (1/2 dilution) and 100 µL of the solution was added to each well. The absorbance was measured using an excitation wavelength of 480 nm and a measuring wavelength of 520 nm. The standard curve was plotted, and the concentration of the samples was converted according to the standard curve.
RNA extraction and qRT-PCR
Total RNA was extracted from the samples using Trizol reagent (PolymerMei, China) following the manufacturer’s instructions. Subsequently, cDNA was synthesized using a reverse transcription kit (PolymerMei, China). qRT-PCR was performed using specific primers, the sequences of which are provided in Table 1 .
Histological analysis
To investigate the pathological changes in mouse colon tissue, 4% PFA-fixed paraffin-embedded colon tissue sections were prepared and subjected to hematoxylin-eosin (H&E) staining and Alcian blue-Schiff’s periodate (AB-PAS) staining. Positive staining for adhesion proteins was quantitatively analyzed using IPWIN60. Immunohistochemical staining for anti-ZO-1 (from Sevilla, Wuhan, China) was also performed on mouse colon tissues.
Western blot analysis
Proteins were extracted from mouse colon tissues through homogenization and lysis using pre-cooled RIPA lysis buffer. Protein content was determined using the BCA protein assay kit (Thermo Fisher, USA). Protein samples containing 30 µg of total protein were collected, separated by SDS-PAGE, and transferred to PVDF membranes (Thermo Fisher, USA). The membranes were blocked with 5% BSA for 4 h at room temperature, followed by incubation at 4 °C with specific primary antibodies including ZO-1 (1:1000), Occludin (1:2000), Claudin-1 (1:1000), BIP (1:1000), PERK (1:1000), ATF4 (1: 1000), eIF2α (1:1000), CHOP (1:1000), Bcl-2 (1:1000), Bax (1:1000), Caspase-12 (1:1000), and GAPDH (1:1000). The membranes were then incubated with HRP-conjugated secondary antibodies (1:5000) for 2 h at room temperature. Finally, spots were visualized using an enhanced chemiluminescence immunoblotting detector (Mona, USA) and quantified using ImageJ software (National Institute of Mental Health, USA).
Data analysis
Data analysis was performed using the mean and standard deviation. Differences between the two groups were determined using a T-test with SPSS 17.0 statistical software (Chicago, IL, USA). Differences between groups were also analyzed using one-way ANOVA or two-way ANOVA with GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA, USA). Statistical significance was defined as P < 0.05.

MV treatment alleviated the severity of UC in mice
The recordings of the mice’s weight loss showed that the mice on the DSS-containing diet began losing weight on day 4, and the weight loss increased over time. However, MV treatment obviously slowed the DSS-induced weight loss in the mice (Fig. 1 A). Mice in the MV-treated group had lower DAI scores compared to the model group, with a more pronounced difference on day 7 (Fig. 1 B). In the model group, the colon length was significantly shorter, swollen, and the contents were indeterminate and celiac-like, compared to the control group. However, MV treatment significantly reversed the shortening of the colon in a dose-dependent manner, and the colon length and faecal morphology in the MV group were similar to the control group (Fig. 1 C and D). Histological analysis of colon in DSS group displayed extensive ulcerative damage, loss of glandular crypts, extensive loss of goblet cell, and infiltration of inflammatory cells. In contrast, an increased number of goblet cells, a general recovery of crypt glands, and a small amount of inflammatory infiltration were seen in the colon sections of the mice after MV treatment (Fig. 1 E and F). Furthermore, MV treatment significantly reduced the secretion of proinflammatory cytokine IL-1β and TNF-α, improved the release of anti-inflammatory cytokine IL-2 in colonic tissues induced by DSS (Fig. 1 G-I).
Effect of MV on the severity of UC mice. ( A ) the changes of body weight. ( B ) DAI scores. ( C ) representative images of colons. ( D ) colon lengths. ( E ) histopathologic examination of colon. ( F ) histological score. The secretion of cytokines of ( G ) TNF-α, ( H )IL-1β, ( I ) IL-2. Values are expressed as mean ± SEM. # p and ## p represents a significant difference compared with the control group. * p and ** p represent a significant difference compared with the DSS group
MV repaired the intestinal barrier in UC mice
Intestinal permeability is a crucial indicator of the response to intestinal mucosal injury and the assessment of intestinal barrier function. In this study, we estimated the expression of tight junction proteins ZO-1, Occludin, and Claudin-1 was detected by western blot and qRT-PCR, the results showed that the expression of ZO-1, Occludin, and Claudin-1 was significantly reduced in the DSS group compared with the control group. After MV treatment, its expression level was significantly restored (Fig. 2 A-G). Moreover, the immunohistochemical staining showed that the expression of ZO-1 in goblet cells was significantly reduced in the DSS group, while the expression of ZO-1 was enhanced with the increase of MV treatment concentration (Fig. 2 H). In addition, intestinal permeability by measuring serum FITC-dextran levels. The serum FITC-dextran level of mice in the model group was significantly higher than that of the blank group. However, MV treatment effectively reduced level of serum FITC-dextran (Fig. 2 I).
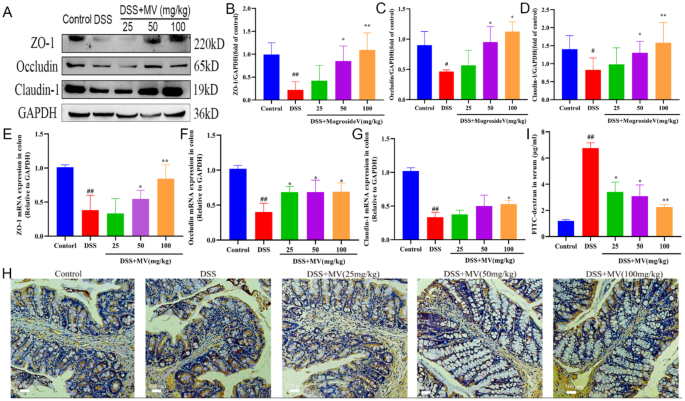
Effect of MV on the intestinal permeability. ( A ) representative western blotting of ZO-1, Occludin, Claudin-1. GAPDH was determined as the loading control. Protein expression quantification of ( B ) ZO-1, ( C ) Occludin and ( D ) Claudin-1. The relative mRNA expression of ( E ) ZO-1, ( F ) Occludin and ( G ) Claudin-1. ( H ) Immunohistochemical staining results of ZO-1. ( I ) the serum FITC level of mice. Values are expressed as mean ± SEM. # p and ## p represents a significant difference compared with the control group. * p and ** p represent a significant difference compared with the DSS group
MV inhibited the ERS-UPR signal pathway activation in UC mice
ERS is the result of the accumulation of unfolded and misfolded proteins in the ER. The UPR acts as a highly conserved signaling mechanism that enables cells to respond to and calm ERS. In this study, we investigated the protein and mRNA levels of ERS -UPR signal pathway to elevate the regulatory effect of MV on ERS by western blot and qRT-PCR. The results showed that DSS treatment significantly increased the protein and mRNA expression of ERS-UPR signal pathway including BIP, PERK, eIF2α, ATF4 and CHOP when compared to control group. However, administration of MV obviously inhibited the expression of ERS-UPR signal pathway especially in protein levels (Fig. 3 ).
Effect of MV on the ERS-UPR signal pathway. ( A ) representative western blotting of ERS-UPR signal pathway. GAPDH was determined as the loading control. Protein expression quantification of ( B ) BIP, ( C ) PERK, ( D ) eIF2α, ( E ) ATF4, ( F ) CHOP. And the relative mRNA expression of ( G ) BIP, ( H ) PERK, ( I ) eIF2α, ( J ) ATF4, ( K ) CHOP. Values are expressed as mean ± SEM. # p and ## p represents a significant difference compared with the control group. * p and ** p represent a significant difference compared with the DSS group
MV inhibited the ERS-associated apoptosis in UC mice
Prolonged ERS can activate chop proteins in the UPR process, leading to ERS-associated apoptosis and the onset of mitochondria-dependent pathway apoptosis. To examine the effects of MV on ERS-induced apoptosis, we investigated the changes in the ratio of the ERS-specific apoptotic protein Caspase-12 and apoptosis-regulating protein family Bcl-2 and Bax by qRT-PCR and western blot. The results showed that the expression of Caspase-12 was significantly increased and the ratio of Bcl-2/Bax was significantly reduced in DSS group when compared to the control group. However, MV treatment significantly inhibited the expression of Caspase-12 and improved the ratio of Bcl-2/Bax to restrain the ERS-induced apoptosis (Fig. 4 ).
Effect of MV on the ERS-associated apoptosis pathway. ( A ) representative western blotting of Bax, Bcl-2 and Caspase-12. GAPDH was determined as the loading control. Protein expression quantification of ( B ) Bcl-2/Bax ratio, ( C ) Caspase-12. And the relative mRNA expression of ( D ) Bcl-2/Bax ratio, ( E ) Caspase-12. Values are expressed as mean ± SEM. # p and ## p represent a significant difference compared with the control group. * p and ** p represent a significant difference compared with the DSS group
Treatment with Tm reversed the effect of MV on DSS-induced ERS-UPR signal pathway in UC mice
To investigate whether the therapeutical effect of MV on UC was related to the ERS pathway, we administered ERS inhibitor, 4-PBA, and activator, Tm, to DSS mice at high doses of MV and observed their effects on UC mice. The results displayed that treatment with MV significantly inhibited the expression of BIP, PERK, eIF2α, ATF4 and CHOP induced by DSS. However, inhibition of ERS by Tm significantly weakened the inhibiting effect of MV on ERS-UPR pathway. In contrast, the expression of BIP, PERK, eIF2α, ATF4 and CHOP in the colonic tissue of DSS + MV + 4-PBA group was showing no significant difference compared to DSS + MV group. These results suggested that inhibition of ERS alleviate the inhibiting effect of MV on ERS-UPR signal pathway (Fig. 5 ).
Effect of 4-PBA and Tm on the MV inhibiting ERS. ( A ) representative western blotting of ERS-UPR signal pathway. GAPDH was determined as the loading control. Protein expression quantification of ( B ) BIP, ( C ) PERK, ( D ) eIF2α, ( E ) ATF4, ( F ) CHOP. And the relative mRNA expression of ( G ) BIP, ( H ) PERK, ( I ) eIF2α, ( J ) ATF4, ( K ) CHOP. Values are expressed as mean ± SEM. n.s. was considered to have no significant difference. * p < 0.05 and ** p < 0.01 represent a significant difference
Activation of ERS reversed the effect of MV on DSS-induced ERS-associated apoptotic in UC mice
We further investigated the effects of inhibition and activation of ERS by 4-PBA and Tm on apoptotic proteins associated with the MV treatment group. Compared to the DSS + MV group, the DSS + MV + Tm group was observed to reverse the decrease in Caspase-12 protein expression, eliminate the therapeutic effect of MV, and further upregulate the Bcl-2/Bax ratio. Meanwhile, 4-PBA induced ERS inhibition had no significant effect on the regulatory effect of MV on ERS associated apoptosis. These results suggest that inhibition of ERS reversed the effect of MV on DSS-induced ERS-associated apoptotic in UC mice (Fig. 6 ).
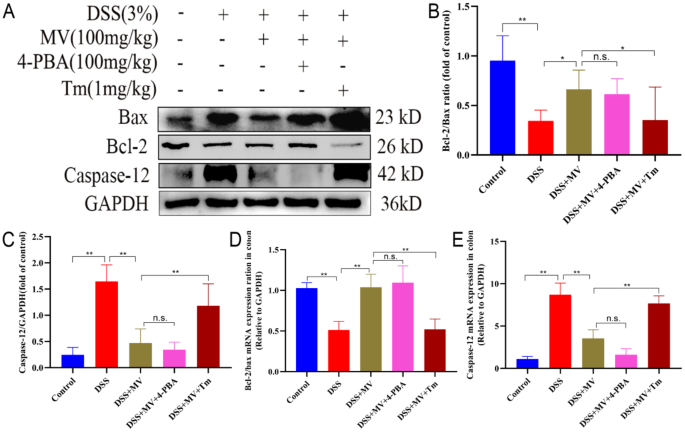
Effect of 4-PBA and Tm on the MV restraining ERS-related apoptosis. ( A ) representative western blotting of Bax, Bcl-2 and Caspase-12. GAPDH was determined as the loading control. Protein expression quantification of ( B ) Bcl-2/Bax ratio, ( C ) Caspase-12. And the relative mRNA expression of ( D ) Bcl-2/Bax ratio, ( E ) Caspase-12. Values are expressed as mean ± SEM. n.s. was considered to have no significant difference. * p < 0.05 and ** p < 0.01 represent a significant difference
Activation of ERS reversed the effect of MV on the severity of UC
The results showed that the addition of Tm counteracted the therapeutic effect of MV, with a similar degree of weight loss as the model group and a relatively higher DAI score, the shorter colon length and heavier histopathological damage than MV group. On the other hand, the application of 4-PBA had no obvious influence on the MV alleviating the severity of UC (Fig. 7 ).
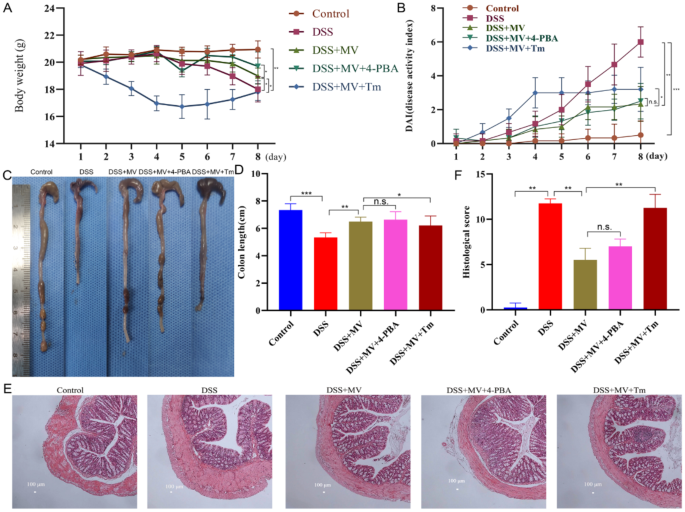
Effect of 4-PBA and Tm on the MV reducing the severity of UC mice. ( A ) the changes of body weight. ( B ) DAI scores. ( C ) representative images of colons. ( D ) colon lengths. ( E ) histopathologic examination of colon. ( F ) histological score. Values are expressed as mean ± SEM. n.s. was considered to have no significant difference. * p < 0.05 and ** p < 0.01 represent a significant difference
Activation of ERS reversed the effect of MV on the intestinal barrier
Results of the intestinal permeability test indicated that Tm treatment significantly weakened the effects of repairing the intestinal barrier of MV showing by increased serum FITC-dextran levels, reduced expression of ZO-1, Occludin and Claudin-1. Conversely, the 4-PBA treatment had no significant difference from DSS + MV group (Fig. 8 ).
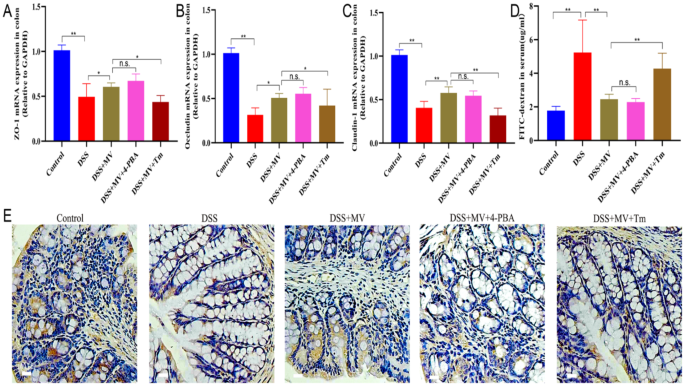
Effect of 4-PBA and Tm on the MV improving the intestinal barrier of UC mice. The relative mRNA expression of ( A ) ZO-1, ( B ) Occludin and ( C ) Claudin-1. ( D ) the serum FITC level of mice. ( E ) Immunohistochemical staining results of ZO-1. Values are expressed as mean ± SEM. n.s. was considered to have no significant difference. * p < 0.05 and ** p < 0.01 represent a significant difference
UC is a chronic, nonspecific inflammatory bowel disease that has been classified by the World Health Organization as a modern refractory disease. It has a long course and is challenging to cure. Recent years have witnessed a growing trend of applying TCM to UC owing to their low toxicity and side-effects relative to western medicine [ 16 ]. In this study, MV, a natural pharmaceutical monomer with anti-inflammatory and antioxidant effects, was found to exhibit good therapeutic effects at concentrations ranging from 25 to 100 mg/kg, which significantly increased with increasing concentrations. Inflammatory factors can cause damage to intestinal epithelial cells, increased intestinal permeability, and inflammatory cell infiltration, and their levels are significantly elevated during the onset and progression of colitis [ 25 , 26 ]. Treatment with MV significantly reduced the releases of proinflammatory factors TNF-α, IL-1β, and increased the levels of anti-inflammatory factors IL-2 in mice with colitis, indicating its good anti-inflammatory effects. Moreover, the expression of tight junction protein ZO-1, Claudin-1, and Occludin in intestinal mucosal epithelial cells decreased, causing an increase in intestinal permeability [ 27 ], which leads to the entry of pathogenic microorganisms and harmful substances into the intestinal mucosa, activating immune cells to produce inflammatory responses and further aggravating the development of colitis [ 28 ]. However, treatment with MV restored the expression of the tight junction protein ZO-1, Claudin-1, and Occludin and reduced the permeability of the intestinal mucosa, thereby restoring intestinal barrier function.
ERS plays a crucial role in the pathogenesis and development of colitis [ 14 , 29 ]. The enhanced and dysregulated ERS response in intestinal tissues of colitis patients is closely associated with various pathophysiological processes, including intestinal mucosal barrier damage, inflammatory cell infiltration, and apoptotic cell death [ 30 , 31 ]. Therefore, recent studies have focused on modulating the ERS pathway to alleviate the onset and progression of colitis [ 32 , 33 ]. Given the critical role of ERS in the pathogenesis of colitis, this study aimed to investigate the mechanism by which MV alleviates the symptoms of DSS-induced UC mice via the ERS pathway. Furthermore, we examined the effects of the ERS inhibitor 4-PBA and the ERS activator Tm on the treatment of UC [ 34 , 35 ]. Our results revealed that the ERS pathway was activated in colitis mice. Treatment with MV reduced the major protein expression of the PERK-ATF4-eIF2α-CHOP pathway, thereby alleviating intestinal tissue damage. Furthermore, MV treatment led to reduced Caspase-12 protein expression and downregulation of the Bcl-2/Bax ratio, ultimately decreasing intestinal cell apoptosis. Treatment with the ERS inhibitor 4-PBA attenuated the intracellular ERS response and promoted normal protein folding, thereby enhancing the cytoprotective effect of MV. Conversely, treatment with the ERS activator Tm exacerbated ERS and reversed the therapeutic effect of MV.
Recently, nano-traditional Chinese medicine have been a promising strategy in consideration of their large specific surface area, strong targeting capability, good sustained-release effect [ 36 ]. The green synthesis of nanoparticles is a promising and environmentally friendly approach and the natural reagents such as herbs, bacteria, and fungi are used in the green synthesis of nanomaterials. Green nanomaterials is highly beneficial compared to traditional methods due to its low cost, and safety for the environment and human health [ 37 ]. Some studies have reported that quercetin supramolecular nanoribbons, nanoparticles from Platycladi Cacumen Carbonisata and the rhei radix rhizoma-based carbon dots ameliorates the UC in mice [ 38 , 39 , 40 ]. MV is expected to be developed into green nanomaterials for the treatment of UC in the future.
In conclusion, our study suggests that MV can alleviate the symptoms of DSS-induced UC mice by modulating the ERS pathway, reducing the apoptosis of intestinal cells, maintaining the integrity of the intestinal barrier, and alleviating the damage of intestinal tissues. These findings not only provide a reference for the development of the ERS pathway as a new target for the treatment of colitis but also present a novel idea and strategy for clinical treatment. Therefore, MV has the potential to be an effective drug for the treatment of colitis, with broad application prospects.
Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606–19.
Article PubMed Google Scholar
Segal JP, LeBlanc JF, Hart AL. Ulcerative colitis: an update. Clin Med (Lond). 2021;21:135–9.
da Silva BC, Lyra AC, Rocha R, Santana GO. Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J Gastroenterol. 2014;20:9458–67.
Article PubMed PubMed Central Google Scholar
Gettler K, Levantovsky R, Moscati A, Giri M, Wu Y, Hsu NY, Chuang LS, Sazonovs A, Venkateswaran S, Korie U, et al. Common and rare variant prediction and penetrance of IBD in a Large, multi-ethnic, Health System-based Biobank Cohort. Gastroenterology. 2021;160:1546–57.
Article CAS PubMed Google Scholar
Ala M, Jafari RM, Nematian H, Shadboorestan A, Dehpour AR. Sodium Selenite modulates IDO1/Kynurenine, TLR4, NF-κB and Bcl2/Bax Pathway and mitigates Acetic Acid-Induced Colitis in Rat. Cell Physiol Biochem. 2022;56:24–35.
Ahmed W, Galati J, Kumar A, Christos PJ, Longman R, Lukin DJ, Scherl E, Battat R. Dual biologic or small molecule therapy for treatment of inflammatory bowel disease: a systematic review and Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:e361–79.
Bots SJ, Parker CE, Brandse JF, Löwenberg M, Feagan BG, Sandborn WJ, Jairath V, D’Haens G. Vande Casteele N: anti-drug antibody formation against biologic agents in inflammatory bowel disease: a systematic review and Meta-analysis. BioDrugs. 2021;35:715–33.
Article CAS PubMed PubMed Central Google Scholar
Foerster EG, Mukherjee T, Cabral-Fernandes L, Rocha JDB, Girardin SE, Philpott DJ. How autophagy controls the intestinal epithelial barrier. Autophagy. 2022;18:86–103.
Feuerstein JD, Moss AC, Farraye FA. Ulcerative Colitis. Mayo Clin Proc. 2019;94:1357–73.
Dong L, Xie J, Wang Y, Jiang H, Chen K, Li D, Wang J, Liu Y, He J, Zhou J, et al. Mannose ameliorates experimental colitis by protecting intestinal barrier integrity. Nat Commun. 2022;13:4804.
Marciniak SJ, Chambers JE, Ron D. Pharmacological targeting of endoplasmic reticulum stress in disease. Nat Rev Drug Discov. 2022;21:115–40.
Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–94.
Gundamaraju R, Vemuri R, Chong WC, Myers S, Norouzi S, Shastri MD, Eri R. Interplay between Endoplasmic Reticular Stress and Survivin in Colonic Epithelial Cells. Cells 2018, 7.
Qi Z, Chen L. Endoplasmic reticulum stress and Autophagy. Adv Exp Med Biol. 2019;1206:167–77.
Ren MT, Gu ML, Zhou XX, Yu MS, Pan HH, Ji F, Ding CY. Sirtuin 1 alleviates endoplasmic reticulum stress-mediated apoptosis of intestinal epithelial cells in ulcerative colitis. World J Gastroenterol. 2019;25:5800–13.
Liu Y, Li BG, Su YH, Zhao RX, Song P, Li H, Cui XH, Gao HM, Zhai RX, Fu XJ, Ren X. Potential activity of traditional Chinese medicine against Ulcerative colitis: a review. J Ethnopharmacol. 2022;289:115084.
Wang M, Fu R, Xu D, Chen Y, Yue S, Zhang S, Tang Y. Traditional Chinese medicine: a promising strategy to regulate the imbalance of bacterial flora, impaired intestinal barrier and immune function attributed to ulcerative colitis through intestinal microecology. J Ethnopharmacol. 2024;318:116879.
Sun Z, Lü B, Feng Y. [Synthetic biology for the synthesis of mogroside V - a review]. Sheng Wu Gong Cheng Xue Bao. 2020;36:2017–28.
CAS PubMed Google Scholar
Chen J, Jiao D, Li Y, Jiang C, Tang X, Song J, Chen Q. Mogroside V inhibits hyperglycemia-induced Lung Cancer cells metastasis through reversing EMT and damaging Cytoskeleton. Curr Cancer Drug Targets. 2019;19:885–95.
Liu Y, Wang J, Guan X, Yu D, Huangfu M, Dou T, Zhou L, Wang L, Liu G, Li X, et al. Mogroside V reduce OVA-induced pulmonary inflammation based on lung and serum metabolomics. Phytomedicine. 2021;91:153682.
Sui L, Yan K, Zhang H, Nie J, Yang X, Xu CL, Liang X. Mogroside V alleviates oocyte meiotic defects and quality deterioration in Benzo(a)pyrene-Exposed mice. Front Pharmacol. 2021;12:722779.
Li MX, Li MY, Lei JX, Wu YZ, Li ZH, Chen LM, Zhou CL, Su JY, Huang GX, Huang XQ, Zheng XB. Huangqin decoction ameliorates DSS-induced ulcerative colitis: role of gut microbiota and amino acid metabolism, mTOR pathway and intestinal epithelial barrier. Phytomedicine. 2022;100:154052.
Wei Y, Liang Y, Lin H, Dai Y, Yao S. Autonomic nervous system and inflammation interaction in endometriosis-associated pain. J Neuroinflammation. 2020;17:80.
Mohammed Vashist N, Samaan M, Mosli MH, Parker CE, MacDonald JK, Nelson SA, Zou GY, Feagan BG, Khanna R, Jairath V. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev. 2018;1:Cd011450.
PubMed Google Scholar
Shah SC, Itzkowitz SH. Colorectal Cancer in inflammatory bowel disease: mechanisms and management. Gastroenterology. 2022;162:715–e730713.
Friedrich M, Pohin M, Powrie F. Cytokine networks in the pathophysiology of inflammatory bowel disease. Immunity. 2019;50:992–1006.
Kuo WT, Zuo L, Odenwald MA, Madha S, Singh G, Gurniak CB, Abraham C, Turner JR. The tight Junction protein ZO-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology. 2021;161:1924–39.
Catanzaro D, Rancan S, Orso G, Dall’Acqua S, Brun P, Giron MC, Carrara M, Castagliuolo I, Ragazzi E, Caparrotta L, Montopoli M. Boswellia serrata preserves intestinal epithelial barrier from oxidative and inflammatory damage. PLoS ONE. 2015;10:e0125375.
Gao H, He C, Hua R, Guo Y, Wang B, Liang C, Gao L, Shang H, Xu JD. Endoplasmic reticulum stress of Gut Enterocyte and Intestinal diseases. Front Mol Biosci. 2022;9:817392.
Stengel ST, Fazio A, Lipinski S, Jahn MT, Aden K, Ito G, Wottawa F, Kuiper JWP, Coleman OI, Tran F, et al. Activating transcription factor 6 mediates inflammatory signals in intestinal epithelial cells upon endoplasmic reticulum stress. Gastroenterology. 2020;159:1357–e13741310.
Adolph TE, Niederreiter L, Blumberg RS, Kaser A. Endoplasmic reticulum stress and inflammation. Dig Dis. 2012;30:341–6.
Long Y, Zhao Y, Ma X, Zeng Y, Hu T, Wu W, Deng C, Hu J, Shen Y. Endoplasmic reticulum stress contributed to inflammatory bowel disease by activating p38 MAPK pathway. Eur J Histochem 2022, 66.
Solà Tapias N, Denadai-Souza A, Rolland-Fourcade C, Quaranta-Nicaise M, Blanpied C, Marcellin M, Edir A, Rolland C, Cirillo C, Dietrich G, et al. Colitis linked to endoplasmic reticulum stress induces trypsin activity affecting epithelial functions. J Crohns Colitis. 2021;15:1528–41.
Zeng M, Sang W, Chen S, Chen R, Zhang H, Xue F, Li Z, Liu Y, Gong Y, Zhang H, Kong X. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol Lett. 2017;271:26–37.
Liu C, Zhou B, Meng M, Zhao W, Wang D, Yuan Y, Zheng Y, Qiu J, Li Y, Li G, et al. FOXA3 induction under endoplasmic reticulum stress contributes to non-alcoholic fatty liver disease. J Hepatol. 2021;75:150–62.
Wei D, Yang H, Zhang Y, Zhang X, Wang J, Wu X, Chang J. Nano-traditional Chinese medicine: a promising strategy and its recent advances. J Mater Chem B. 2022;10:2973–94.
Gupta D, Boora A, Thakur A, Gupta TK. Green and sustainable synthesis of nanomaterials: recent advancements and limitations. Environ Res. 2023;231:116316.
Sun W, Chen Y, Wang L, Wang Z, Liu S, Zhang M, Liu Y, Li Q, Zhang H. Gram-scale preparation of quercetin supramolecular nanoribbons for intestinal inflammatory diseases by oral administration. Biomaterials. 2023;295:122039.
Zhang ML, Liu YH, Qu HH. Protective effect of nanoparticles from Platycladi Cacumen Carbonisata on 2,4,6-Trinitrobenzene Sulfonic Acid (TNBS)-Induced colitis in rats. J Biomed Nanotechnol. 2022;18:422–34.
Zhang Y, Zhao J, Zhao Y, Bai X, Chen Y, Liu Y, Zhang Y, Kong H, Qu H, Zhao Y. The Rhei radix rhizoma-based carbon dots ameliorates dextran sodium sulphate-induced ulcerative colitis in mice. Artif Cells Nanomed Biotechnol. 2023;51:180–91.
Download references
This work was supported by Scientific research project of Education Department of Jilin Province (No. JJKH20231202KJ) and the National Natural Science Foundation of China (No. 31972724).
Author information
Yue-Rong Tan and Si-Yang Shen contributed equally to this article.
Authors and Affiliations
College of Veterinary Medicine, Jilin University, No. 5333 Xi’an Road, Changchun, Jilin, 130062, China
Yue-Rong Tan, Si-Yang Shen, Xin-Yi Li, Peng-Fei Yi, Ben-Dong Fu & Lu-Yuan Peng
State Key Laboratory for Diagnosis and Treatment of Severe Zoonotic Infectious Diseases, Jilin University, No. 5333 Xi’an Road, Changchun, Jilin, 130062, China
Peng-Fei Yi, Ben-Dong Fu & Lu-Yuan Peng
You can also search for this author in PubMed Google Scholar
Contributions
Conceptualization: YRT, LYP, and BDF. Data curation: LYP. Investigation: YRT, XYL and SYS. Writing – original draft: YRT. Writing – review and editing: PFY, LYP and BDF. Funding acquisition: BDF and LYP. All authors read and approved the final manuscript. Revising the manuscript: SYS and XYL.
Corresponding authors
Correspondence to Ben-Dong Fu or Lu-Yuan Peng .
Ethics declarations
Competing interest.
The authors declare no competing financial interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Tan, YR., Shen, SY., Li, XY. et al. Mogroside V reduced the excessive endoplasmic reticulum stress and mitigated the Ulcerative colitis induced by dextran sulfate sodium in mice. J Transl Med 22 , 488 (2024). https://doi.org/10.1186/s12967-024-05285-6
Download citation
Received : 19 February 2024
Accepted : 08 May 2024
Published : 21 May 2024
DOI : https://doi.org/10.1186/s12967-024-05285-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Mogroside V
- Ulcerative colitis
- Endoplasmic reticulum stress
Journal of Translational Medicine
ISSN: 1479-5876
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Health Conditions
- Health Products
Procedure primer: Colonoscopy for ulcerative colitis

A doctor may order a colonoscopy to help diagnose ulcerative colitis (UC), check for complications of UC, or see whether the condition is progressing.
UC is a type of inflammatory bowel disease that causes inflammation and lesions in the large intestine, including the rectum, colon, or both.
A doctor may also order a colonoscopy to check for signs of colorectal cancer, which affects people with UC at higher rates than people without the condition.
The American College of Gastroenterology recommends that people who have UC that extends beyond the rectum get a colonoscopy to check for signs of colorectal cancer every 1–3 years, beginning 8 years after diagnosis.
The procedure primer below shows what a colonoscopy involves.
Last medically reviewed on May 20, 2024
- Uncategorized
How we reviewed this article:
- Colonoscopy. (n.d.). https://www.asahq.org/madeforthismoment/preparing-for-surgery/procedures/colonoscopy/
- Lynch WD, et al. (2023). Ulcerative colitis. https://www.ncbi.nlm.nih.gov/books/NBK459282/
- Meseeha M, et al. (2023). Colon polyps. https://www.ncbi.nlm.nih.gov/books/NBK430761/
- Rao Q. (2024). Personal interview.
- Rubin DT, et al. (2019). ACG clinical guideline: Ulcerative colitis in adults. https://journals.lww.com/ajg/fulltext/2019/03000/acg_clinical_guideline__ulcerative_colitis_in.10.aspx
- Stauffer CM, et al. (2023). https://www.ncbi.nlm.nih.gov/books/NBK559274/
Share this article
Latest news
- Immunotherapy before and after surgery may improve lung cancer survival rates
- Obstructive sleep apnea during REM stage linked to memory decline
- Unique brain circuits may be linked to eating habits, weight loss
- Counting steps or logging minutes? What is the best way to measure exercise?
- Wegovy weight loss benefits sustained for 4 years, doubling prior estimates
Related Coverage
A person with ulcerative colitis may need surgery if their symptoms cannot be managed by medication.
Lialda is a prescription drug that treats ulcerative colitis. Find out about possible interactions with alcohol, other drugs, supplements, foods, and…
The inflammation associated with ulcerative colitis may also link to eye conditions, such as scleritis and uveitis. Here is what to know about UC and…
Ulcerative colitis may be associated with a number of sexual issues, such as low libido, pain during sex, and erectile dysfunction.
Get the facts on cost and Omvoh, how generics compare with brand names, what financial assistance may be available, and more.
- Open access
- Published: 14 May 2024
The development of probiotics and prebiotics therapy to ulcerative colitis: a therapy that has gained considerable momentum
- Jing Guo 1 ,
- Liping Li 1 ,
- Yue Cai 2 &
- Yongbo Kang 1
Cell Communication and Signaling volume 22 , Article number: 268 ( 2024 ) Cite this article
344 Accesses
1 Altmetric
Metrics details
Ulcerative colitis (UC) is increasingly common, and it is gradually become a kind of global epidemic. UC is a type of inflammatory bowel disease (IBD), and it is a lifetime recurrent disease. UC as a common disease has become a financial burden for many people and has the potential to develop into cancer if not prevented or treated. There are multiple factors such as genetic factors, host immune system disorders, and environmental factors to cause UC. A growing body of research have suggested that intestinal microbiota as an environmental factor play an important role in the occurrence and development of UC. Meanwhile, evidence to date suggests that manipulating the gut microbiome may represent effective treatment for the prevention or management of UC. In addition, the main clinical drugs to treat UC are amino salicylate and corticosteroid. These clinical drugs always have some side effects and low success rate when treating patients with UC. Therefore, there is an urgent need for safe and efficient methods to treat UC. Based on this, probiotics and prebiotics may be a valuable treatment for UC. In order to promote the wide clinical application of probiotics and prebiotics in the treatment of UC. This review aims to summarize the recent literature as an aid to better understanding how the probiotics and prebiotics contributes to UC while evaluating and prospecting the therapeutic effect of the probiotics and prebiotics in the treatment of UC based on previous publications.
Introduction
Ulcerative colitis (UC) is a chronic non-specific intestinal inflammatory disease [ 1 ]. UC becomes an important health problem, because it’s high morbidity. Especially in newly industrialized countries [ 2 ]. Research shows that the incidence of UC is 10 to 20 patients per 100,000 people every year [ 3 ]. UC often presents with recurrent attacks. And the inflammatory of UC will become a factor of colon cancer in the long run [ 4 ]. The pathogenic factors of UC are sophisticated, it is related to intestinal microbiota, immune function of the body (For example, UC is closely related with Th2 cells) [ 5 ], genetic factor and environment factor (e.g. life-style, dietary habits) and so on [ 6 ]. wherein, intestinal microbiota is one of the most important factor that arise UC [ 7 ]. Therefore, we can use probiotics to regulate the intestinal flora in the treatment of UC [ 8 , 9 ]. A growing body of research has shown that probiotics and prebiotics can bring about remission the symptoms of UC improving intestinal mucosal homeostasis, ameliorating the intestinal microbiota environment, regulating the body’s immune function. Therefore, probiotics and prebiotics may be a very safe and efficient treatment for UC. At the same time, it can greatly reduce the financial burden of patients. Furthermore, New techniques have made it possible to attempt systematic studies of probiotics prebiotics, which can provide more specific information about their functions and pathological variations. This review summarizes cutting-edge research on probiotics and prebiotics treatment for UC, existing issues in probiotics treatment and prebiotics therapy, the future of probiotics and prebiotics, and microbial therapeutics.
Pathogenesis of UC
Ulcerative colitis is a chronic inflammatory disorder of the gastrointestinal tract. It is characterized by a progressive decline in health. UC is marked by inflammation of the mucosal lining, usually confined to the colon and rectum [ 10 ]. The pathogenesis of UC is closely related to a variety of factors, such as genetics and environment [ 11 ]. Statistically, genetics can only explain 7.5% of the variation in disease and has little predictive power for phenotype. Therefore, it has limited clinical application. Examples of loci associated with increased susceptibility to UC including genes associated with barrier function and human leukocyte antigen, such as HNF4A and CDH1 [ 12 , 13 ]. Environment plays an important role in the development of UC. Such as, living condition, hygiene, diet, etc. While UC is mainly due to immune dysfunction and intestinal barrier dysfunction. Colonic epithelial cells (colonocytes), as the first line of defense of the gut immune system, are closely related to the pathogenesis of the UC. Research findings, the expression of peroxisome proliferator-activated receptor γ (PPAR γ) is reduced in the colonocytes in patients with UC. And the reduced expression of PPAR γ, which is a nuclear receptor that downregulates inflammation, will stimulate an inflammatory cascade responses through a series of immune responses, leading to the production of large quantities of inflammatory factors [ 14 ]. Also, when certain genes in the intestinal epithelium are functionally deficient, it may lead to disruption of the intestinal barrier function [ 10 ]. The deficiency or malfunction of various immune cells and the abnormal expression of cytokines, which play an important signaling function, can also lead to inflammation, which, if prolonged, can lead to the development of UC. The intestinal immune system also involves the intrinsic and adaptive immunity [ 15 ], involving a variety of immune cells and molecules and others. If dendritic cells abundantly express Toll-like receptors (TLR) which can recognize pathogen pattern receptors, this will leads to the activation of several inflammatory signaling pathway, such as NF-κB [ 16 ] and MAPK pathway, triggering an inflammatory response. The production of large amounts of pro-inflammatory factors affects the differentiation of immune cells such as T cell differentiation towards subpopulation. For example, massive activation of Th2 cells leads to high expression of IL-13, which induces apoptosis of epithelial cells and disrupts the integrity of mucosal barrier [ 17 , 18 ]. Other T helper cells also play an important role in UC. And some research suggest that Breg deficiency may also associated with UC [ 19 ]. The damage of the intestinal mucosal barrier is also an important causative factor in UC. Intestinal secretory dysfunction such as decreased secretion of antimicrobial peptides and mucus layer, or structural defects of intestinal barrier including occludin, ZO-1, ZO-2 and so on. It has been found that the disruption of human gut microbiota, the largest collection of microbes within the body [ 20 ], is critical in the progression of UC, but the specific mechanism is not yet clear.
The role of gut microflora in UC
Gut microflora lives on intestinal mucosal and forms bacterial layer. Thus, there is a strong and complex relationship between gut microbiota and gut. Intestinal dysbacteriosis can leads to a decrease in intestinal defense function and immune regulatory function. Furthermore, the decrease of the body immune function and an increase in associated pathogenic factors leading to the intestinal mucosal invasion or exacerbates the gastrointestinal diseases [ 6 ]. Recently, a large number of studies have shown that alterations of intestinal microbiota can play an important role in the occurrence and development of UC. Meanwhile, some studies have shed light on UC subjects exhibiting alterations in the relative abundance of “beneficial” and potentially “harmful” bacteria compared to healthy subjects. The existence of a link between UC and the gut microbiota was indicated based on studies in animals and patients with UC. Changes of gut microbiota together with their-derived products and metabolites account for the important factors to promote UC occurrence. Here, the possible mechanisms of microbiome-gut action in promoting UC occurrence are discussed as well as outlined in Fig. 1 .
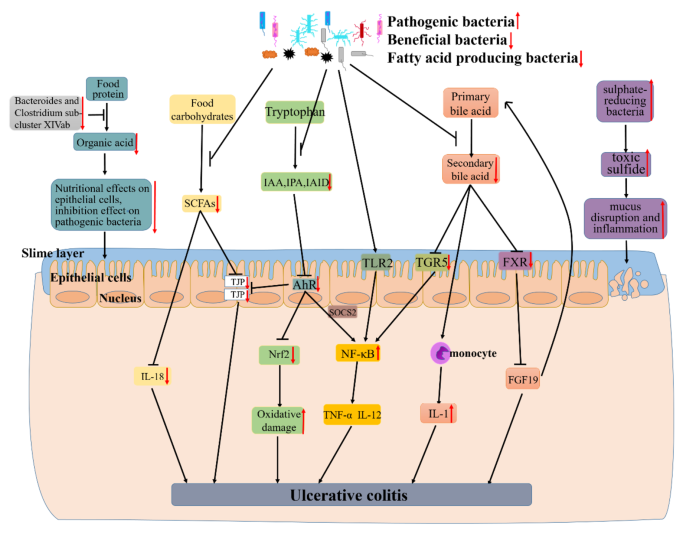
The mechanism of UC caused by dysbiosis of gut microbiota. Research findings, the decline of certain beneficial bacteria inhibits the conversion of food protein into organic acid which can nourish epithelial cells and inhibit pathogenic bacteria. Firmicutes as a major producer of butyrate (a kind of SCFAs), its decline leads to lower intestinal SCFAs. Leading the decreased secretion of epithelial repair cytokine interleukin-18, reduced the integrity of epithelial cells, and inhibited goblet cells secrete mucin and modification of tight junctions. And the decline of some gut microbiota also can lead to a decrease of indoles and their derivatives (e.g., IAA, IPA and IAID) which is produced by tryptophan. Thereby reducing the activation of AhR, a member of the activation of PER-ARNT-SIM (PAS) superfamily of transcription factors. The activation of AhR can inhibited the expression of NF-κB in a manner dependent on suppressor of cytokine signaling 2 (SOCS2). And AhR can also maintains the integrity of intestinal barrier activation by increasing the expressions of intestinal tight junction protein (TJPs) or activating the AhR-Nrf2 pathway. All of these effects were reversed due to the decrease of IAA, IPA or IAID. Thus lead to the increase of inflammatory factors (e.g., TNF-α and IL-17) and oxidative damage. Other researchers found that certain pathogenic bacteria such as Bacteroides (B.) fragilis and capsular lipopolysaccharide A can activate NF-κB signaling pathway and promote the secretion of inflammatory factors. The gut microbiota dysbiosis can also lead to the decreased synthesis of secondary bile acid. And secondary acid act as high-affinity ligands for TGR5 and FXR, its decline can promote NF-κB activation to synthesize inflammatory and the expression of proinflammatory cytokines secreted by monocyte and downregulate the expression of FGF19 and promote the synthesis of bile acids thus increasing its toxicity effect on tissues. As an intestinal pathogen, the increase of sulphate-reducing bacteria leads to cell disintegration and inflammatory via toxic sulfide. All of these can lead to the occurrence and development of UC.
A large number of studies have shown that patients with UC have a decrease in the bacterial diversity of gut microbiota [ 21 ]. Animal study results indicate a close association between gut microbiota and UC. Li et al. found that Firmicutes and Proteobacteria increased, whereas Bacteroidetes decreased in UC rats. And Lactobacillus , Lachnospiraceae_NK4A136_group , Prevotella_9 and Bacteroides were dominant genera in the model group [ 22 ]. Consistent with animal studies, the existence of a link between UC and the gut microbiota was indicated based on studies in patients with UC. Guo et al. also found that the abundance of Bacteroides and Clostridium sub-cluster XIVab as well as the concentration of organic acids significantly decrease by comparing with healthy individuals [ 23 ]. Similarly, Mizoguchi et al. shown that UC patients harbored relatively more abundant Actinobacteria, Proteobacteria and Tenericutes [ 24 ]. A comparison between UC and healthy individuals differed in the composition and diversity of the microbiota, with an upward trend in the Clostridium cluster IX and a decreased Clostridium cluster XIVa in patients with UC [ 25 ]. Consistent with the above results, there is a reduced amounts of bacterial groups from the Clostridium cluster XIVa , and the levels of Bacteroidetes was increased [ 26 ].
In addition, Kotlowski et al. found that the numbers of Escherichia coli were high in the rectal tissue of patients with UC [ 27 ]. By comparing with healthy controls, Xu et al. showed that the inflamed mucosa had more Proteobacteria (e.g. Escherichia–Shigella ) and fewer Firmicutes (e.g., Enterococcus ) [ 28 ]. As demonstrated by Schwiertz et al., Patients with active UC have lower cell counts of Bifidobacterium than healthy controls [ 29 ]. Another study found that the sulfate-reducing bacteria which is the dominant microflora in UC, it may proliferate with the release of toxic sulfide [ 30 ].
Recently, Verma et al. shown that during the active and remission stages of UC cases, the proportions of Bacteroides , Eubacterium , and Lactobacillus spp. are decrease [ 31 ]. Similarly, in another analysis of mucosa-associated flora in UC patients, it was learned that UC patients contained proportionally less Firmicutes , and correspondingly more Bacteroidetes [ 32 ]. Tahara et al. demonstrated that Fusobacterium nucleatum is common which is isolate from human intestinal biopsy from UC, compared to healthy controls [ 33 ].
In keeping with these results, Machiels et al. found that there is a decrease of the Roseburia hominis and Faecalibacterium prausnitzii in patients with UC [ 34 ]. Lepage et al. demonstrated that patients with UC are characterized by more Actinobacteria and Proteobacteria and less bacteria from the Lachnospiraceae and Ruminococcaceae families [ 35 ]. Likewise, a significant reduction was found on the UC mucosa compared with the non-IBD controls, that is levels of Clostridium clostridioforme , the Eubacterium rectale group, Faecalibacterium prausnitzii , Bifidobacteria , Lactobacilli , and Clostridium butyricum [ 36 ]. Consistent with the above results of this study, patients with UC in remission compared to that of controls, there is a loss of Bacteroides , Escherichia , Eubacterium , Lactobacillus , and Ruminococcus spp [ 37 ].
Recently, Hu et al. [ 38 ] found that the decreased of the dominant bacteria that digest food carbohydrates to short chain fatty acid (SCFA) lead to the reduce of intestinal barrier integrity (for example, the decrease of TJPs in colon). Guo et al. [ 23 ] also found that SCFAs can affect the secretion of the epithelial repair cytokine interleukin-18. And they found that the decreased of Bacteroides and Clostridium sub-cluster XIVab leading to the decrease of organic acid, which reduces the trophic effect of organic acid to epithelial cells and the inhibitory effect on pathogenic bacteria [ 39 ]. Agus et al. [ 40 ] found that the reduced of certain intestinal flora inhibited the conversion of tryptophan to indole and its derivatives, and AhR as a receptor of indole and its derivatives, its activation will reduced, thereby inhibiting the intestinal TJP and AhR-Nrf2 pathway, leading to the reduced of intestinal barrier integrity and increased oxidative stress [ 41 ]. Rothhammer et al. [ 42 ] demonstrated that the reduce of AhR can promote the activation of NF-κB pathway in a manner dependent on suppressor of cytokine signaling 2 (SOCS2), then increase the expression of a number of inflammatory factors, including TNF-α and IL-12 et al. It is reported that some bacteria regulate the secretion of TNF-α and IL-12 by activating the NF-κB pathway through TLR2 receptor [ 43 ]. Iracheta et al. [ 44 ] found that primary bile acid are converted to secondary bile acid by gut microorganisms after being secreted into gut through a series of reactions, and that a decline of these gut microorganisms leads to a decrease of secondary bile acid. The decrease of secondary bile acid, which act as high-affinity ligands for TGR5 and FXR, leads to a decreased activation of TGF5 and FXR. The inhibitory effect of TGR5 on NF-κB is reduced, thereby promoting the activation of NF-κB. Reduced activation of FXR down-regulates the expression of FGF19, then its inhibitory effect to hepatic bile acid is declined, leading to a further increase of bile acid and exacerbating the development of inflammation [ 45 ]. And the decrease of secondary bile acids promote the secretion of pro-inflammatory factors by monocytes [ 46 ]. Figliuolo et al. [ 47 ] found that the increase of sulphate-reducing bacteria lead to an increase of toxic sulfide, which cause the disruption of gut epithelial cell and increase intestinal inflammatory.
Taken together, these results provide further insights into a role for gut microbiota in the pathogenesis of UC and might potentially serve as guidance for the interventions of UC by manipulating gut microbiota.
Research advances existing challenges IBD treatment
At present, there are many various treatment methods for IBD. Conventional treatment is the use of pharmacotherapy, including aminosalicylates, corticosteroids (CSs), immunomodulators (e.g., thiopurines (TPs), methotrexate (MTX), and calcineurin inhibitors), and biologics (e.g., pro-inflammatory cytokine inhibitors and integrin antagonists). Surgical resection and other methods including apheresis therapy, antibiotics, probiotics and prebiotics can also be used for treatment [ 48 ]. However, the side effects and high reccurence rate of these substances and methods limit there application. For example, research found, although aminosalicylates have been used in the treatment of IBD for the past 80 years, its efficacy remains controversial. And its mild side effects include diarrhea, nausea, abdominal pain, flatulence and others [ 49 ]. Severe cases can lead to infertility and anemia. CSs inhibits the transcription of certain inflammatory factors [ 50 ] and regulate the expression of certain anti-inflammatory genes [ 51 ] through certain signaling pathways. And it has many side effects, including diabetes mellitus, hypertension, venous thromboembolism (VTE), etc [ 52 ].. Some patients may also have dependence on this medication [ 53 ]. TPs inhibits intestinal inflammatory response by regulating T cell proliferation and activation. But TPs can cause side effects such as liver damage [ 54 ] and gastrointestinal intolerance [ 55 ]. MTS excerts its effects also by downregulating inflammatory factors. But it can cause adverse reactions such as fatigue, diarrhea, pneumonia and rash [ 51 ]. Calcineurin inhibitors also supresses inflammatory responses by interfering with signaling pathways. The incidence of side effects of calcineurin inhibitors is high, including renal function damage, hyperkalemia and infectious diseases and so on [ 56 ]. Anti-TNF therapy will inhibit the secretion of pro-inflammatory factor TNF-α. Anti-IL-12/23 therapy works by inhibiting the production of pro-inflammatory factor IL-12 and IL-23 by antigen-presenting cells. Anti-integrin therapy inhibits the accumulation of white blood cells in intestinal and alleviates intestinal inflammatory. But these biological agents are expensive and many patients may experience unresponsive and intolerant states. Therefore, it is urgent to study effective and safe methods to treat UC.
In the recent years, regulating gut microbiota has become a hot topic in the treatment of UC. Therefore, as a promising method for treating IBD, probiotics act as live microorganisms have therapeutic effects on IBD which is caused by intestinal ecological disorders and other reasons. The treatment of IBD can be achieved through its antioxidant effects [ 57 ], the regulatory effect on gut microbiota [ 58 ], anti-inflammatory effect [ 59 ], the promotion effect to intestinal barrier integrity [ 60 ] and so on. As an indigestible food ingredient, prebiotics can also be used to treat or alleviate UC by regulating the redox system, immune system, etc. It can also selectively regulate colon microbiota, for example, enhancement of beneficial intestinal bacteria and inhibition of the growth of pathogenic microorganisms. All of these suggests that probiotics and prebiotics have a lot of room to develop as new form of treatment.
Effect and mechanism of probiotics and prebiotics in treating UC
Probiotics are nonpathogenic living microorganisms which, when administered in adequate amounts, have been shown to confer health benefits to the host and regulate intestinal microecological balance. Probiotics are widely used in medical application to prevent or treat many diseases, such as obesity [ 61 ], hepatocellular Carcinoma [ 62 ], autoimmune hepatitis [ 63 ], diabetic retinopathy [ 64 ], and alcoholic liver disease [ 65 ] and so on. The therapeutic effects of probiotics on UC have also been confirmed in animals and humans (Tables 1 and 2 ). Thus, therapeutic interventions with probiotics may offer new treatment for UC. Here, the possible effects and mechanisms of probiotics in the treatment of UC are summarized in Fig. 2 .
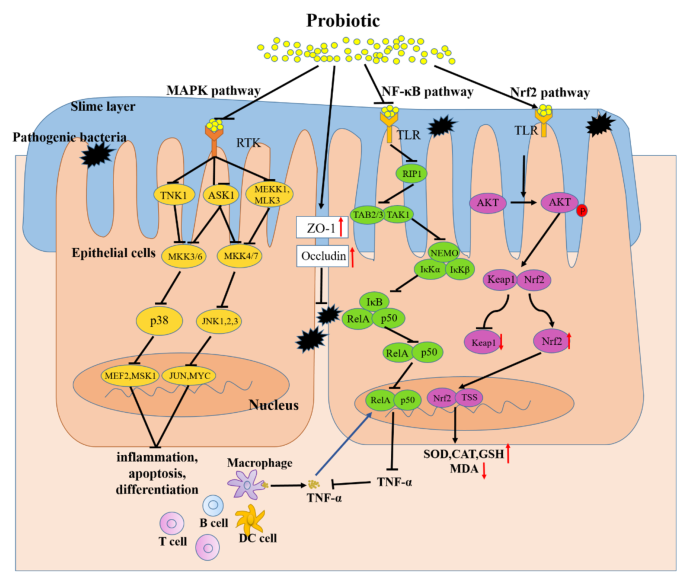
The potential mechanism of probiotics in alleviating Ulcerative Colitis (UC). Probiotics that enter the gut can bind with corresponding receptors (e.g. PTK) which are on the intestinal epithelial cells, then inhibit its stimulation to MAPKKK (e.g. TNK1, ASK1, MEKK1, MLK3), further suppress the activation of MAPKK (e.g. MKK3/6, MKK4/7) which are activated by MAPKKK, thereby inhibiting the activation of MAPK (e.g. p38, JNK1,2,3). Blocking the transcription factor transcribe of relevant genes (e.g. Cyclin D1, Raf). Finally, inhibition the inflammatory, apoptosis, and differentiation activated by this pathway. Meanwhile, probiotics protect the intestinal barrier by increasing the levels of tight junction proteins of ZO-1 and Occludin between intestinal epithelial cells, preventing the invasion of pathogenic microorganisms. In addition, probiotics can bind with its receptors (e.g. TLR) on the intestinal epithelial cells, inhibiting the activation of adaptor protein (e.g. RIP1) and suppressing the recruitment of TAB/TAK complex, thereby inhibiting the ubiquitination degradation of IκB by ubiquitinatingNEMO. Prevents the release of NF-κB proteins (RelA/p50) to nucleus. Ultimately inhibits the transcription of proinflammatory factors (e.g. TNF-β) and reduces the promotion effect of TNFα releasing by macrophages to this pathway. Meanwhile, probiotics act on intestinal epithelial cells-associated receptors (e.g. TLR), then phosphorylate AKT, and inhibit the degradation of Nrf2. Nrf2 enters the nucleus and promotes the expression of a range of cytoprotective genes (e.g. SOD, CAT, GSH).
Probiotics therapy
Experimental studies.
Convincing evidence from animal studies indicate that probiotics treatment can relieve UC (Table 1 ). Wu et al. [ 66 ] found that the use of Bifidobacterium longum CCFM1206 to treat Dextran-Sulfate-Sodium (DSS) induced Colitis mice will promotes the conversion of Glucoraphanin (GRP) to sulforaphane (SFN). SFN help to upregulate the Nrf2 signaling pathway and inhibit the NF-κB activity, which can ameliorate DSS-induced colitis. The result also indicated that the intervention of B.longum CCFM1206 could relieve the dysbiosis of intestinal microbiota. That is, promoted the proportion of Alistipes , Bifidobacterium , Blautia and Lachnospiraceae NK4A136 group and inhibited the proportion of Acinetobacter , and Lachnospiraceae A2 in the gut. Similar study, Han et al. [ 67 ] demonstrated that Bifidobacterium infantis enhances genetic stability by maintaining the balance of gut flora to increase anaphase-promoting complex subunit 7 (APC7) expression in colonic tissues, changing gut flora such as an increase in B.infantis . Then reducing DSS-induced colonic inflammation. Consistent with the above results, Fu et al. [ 68 ] found that Bacteroides xylanisolvens AY11-1 regulate the intestinal microbiota through the efficient degradation of alginate, improving the dysbiosis of intestinal ecology and promoting the growth of beneficial bacteria, for example, the increase of Blautia spp and Prevotellaceae UCG-001. Then ameliorated the symptoms of DSS-induced UC in mice. Wang et al. [ 69 ] revealed that the administration of probiotic Companilactobacillus crustorum MN047 in DSS-induced UC mice resulted in the expression of tight junctions, and down-regulation of pro-inflammatory and chemokine expression. It was also found that an increase of goblet cells, MUCs, TFF3, and TJs in the probiotic group, which demonstrated that the treat with CCMN could enhance the gut barrier function. And confirmed by fecal microbiota transplantation (FMT), the mechanisms of CCMN alleviating UC were partly due to its modulation to gut microbiota. The result showed that an increase in Bacteroidaceae and Burkholderiaceae and a decrease in Akkermansiaceae and Eggerthellaceae . Hu et al. [ 70 ] also found that Selenium-enriched Bifidobacterium longum DD98 administration alleviated the symptoms caused by DSS, inhibited the expression of the pro-inflammatory cytokines, decreased the level of oxidative stress, promoted the expression of tight junction proteins, inhibited the activation of toll-like receptor 4 (TLR4), and regulated the gut flora. They found that after the treatment of Se-B. longum DD98, the phylum of Bacteroidetes decreased and the phylum of Firmicutes increased. All of the above can be effective attenuated DSS-induced colitis in mice. In another study, the results of Han et al.’s [ 71 ] study of Lacticaseibacillus rhamnosus Hao9 in DSS-induced UC mice showed that the use of Hao9 attenuated weight loss which is caused by DSS, lowered DAI scores, attenuated colonic damage and inflammatory infiltrates and promoted the growth of Faecalibaculum and Romboutsia in the gut. The researcher attributed the observed effects of Hao9 on UC to its ability to inhibit lipopolysaccharide-induced intestinal IκB activation of mice. Consistent with the above results, Huang et al. [ 72 ] also showed that Lactobacillus paracasei R3 supplementation improved the general symptoms of murine colitis, attenuated inflammatory cell infiltration and more. And it was showed that the imbalance of Treg/Th17 cell in the intestinal inflammation caused by DSS was restored after treatment with L.p R3. Similarly, Xu et al. [ 73 ] investigated the effect of Saccharomyces boulardii and its postbiotics on DSS-induced UC in mice, showing that both S. boulardii elements and its postbiotics could significantly alleviate weight loss, reduce colonic tissue damage, regulate the balance of pro/anti-inflammatory cytokines in serum and colon, promote the expression of colonic tight junction proteins, and regulate the stability of intestinal microecology in mice. Changing in the bacterial flora were characterized by a significant increase in Turcibacter at the genus level, which collectively attenuate DSS-induced colitis. Komaki et al. [ 74 ] administered Lactococcus lactis subsp.lactis JCM5850 to mices with colitis induced by DSS and found that moderate amounts of L. lactis had a mitigating effect on colitis. In keeping with these results, Hizay et al. [ 75 ] also found that Lactobacillus acidophilus reduces abnormally high levels of serotonin in colon tissue in acetic acid-induced UC and relieves inflammation in intestinal tissue. As with the results above, Gao et al. [ 76 ] made Saccharomyces boulardii into suspension, observing its effect on DSS induced colitis in mice. The results suggested that S. boulardii can alleviate the clinical symotoms of colitis in mice exposed to DSS and the histological lesions. And it was found that the mechanism of S. boulardii to treat UC is inhibite nuclear transcription factor kappa B (NF-κB) and activate nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway. As demonstrated by He [ 77 ] et al., Enterococcus faecium administration prevented DSS-induced intestinal inflammation and intestinal flora dysbiosis and particially repaired the damage to intestinal mucosal barrier and tight junctions. The modulatory effect on intestinal flora was characterized by an increase in Butyricicoccus sp., Lactobacillus sp., and Bifidobacterium sp. and a decrease in Ochrobactrum sp. and Acinetobacter sp. .
By studying the effects of tetrapeptide from maize (TPM) and probiotic (5 Lactobacillus strains: L.animalis- BA12, L.bulgaricus- LB42, L.paracasei- LC86, L.casei- LC89 and L.plantarum- LP90) in mice with DSS-induced UC, Li et al. [ 78 ] found that it could reduce the level of oxidative stress, attenuate the loss of kidney and colon, and regulate the intestinal flora to alleviate the inflammatory effects of UC. Wherein, the modulation effect to gut microbiota in manifested as an increase in Muribaculaceae, Alistipes, Ligilactobacillus and Lactobacillus . Recently, Shang et al. [ 79 ] reported that Bifidobacterium bifidum H3-R2 can effectively alleviate of pathogenesis by inhibiting inflammatory signaling, maintaining intestinal ecological homeostasis, and protecting colonic integrity. B.bifidum H3-R2 administration similarly affected the composition of gut microbiota, showing that B.bifidum H3-R2 caused a significant increase in the abundance of Bifidobacterium and Lactobacillus and a decrease in Enterobacter , Enterococcus and Streptococcus . Chen et al. [ 80 ] also discovered Lactobacillus fermentum ZS40 could inhibit DSS-induced mice colon shortening, colon damage, and intestinal wall thickening. It does so by inhibiting the activation of NF-κB and MAPK signaling pathways, and ultimately relieved inflammation.
To sum up, these results provide important clues for the design and use of more effective probiotic agents to treat UC and may provide new insights into the mechanisms by which host-microbe interactions confer the protective effect. And probiotics as additionally supplemented active micro-organisms, may have better value in clinical applications as drugs in the future [ 81 ].
Clinical studies
There are many contributing factors to UC, but much evidence suggests a strong link between host gut microbes and the treatment of UC pathogenesis, and suggests that mediation of gut microbes is the key to treating UC. Probiotics have been shown to alleviate UC by altering the composition of the gut microbiota and many other ways. A growing number of clinical trials have also demonstrated the therapeutic effects of probiotics in UC (Table 2 ). As early as 2010, Hegazy et al.’s [ 82 ] study showed that administration of probiotics ( Lactobacillus delbruekii and L. fermentum ) not only decreased the NF-κB DNA binding activity, but also reduced the accumulation of leukocytes, and down-regulated levels of pro-inflammatory factors, and thereby ameliorated the severity of the colitis. Similarly, in order to study the long-term effect of probiotics on UC, Palumbo et al. [ 83 ] conducted a clinical study and the results of the study showed that patients in the probiotics ( L. salivarius , L.acidophilus and B.bifidus strain BGN4) treatment group had better outcomes which is reflected through MMDAI. Thus, the use of probiotics may enhance the anti-inflammatory effect. Similar results, Bjarnason et al. [ 84 ] tried to prove the impact of the probiotic Symprove (including Lactobacillus rhamnosus NCIMB 30,174, Lactobacillus plantarum NCIMB 30,173, Lactobacillus acidophilus NCIMB 30,175 and Enterococcus faecium NCIMB 30,176) which contains four naturally occurring bacterial strain for this experiment. Research showed that Symprove are associated with reduced intestinal inflammation in UC patients. In line with these results, Tsuda et al. [ 85 ] gave patients with moderate to severe UC treated with BIO-THREE (containing Streptococcus faecalisa T-110, Clostridium butyricum TO-A and Bacillus mesentericus TO-A). Researchers found that the treated with BIO-THREE were able to improve clinical and endoscopic examinations in about half of UC patients who were intolerant to conventional therapy. And its intake improved intestinal microflora, the main change may be an increase in bifidobacteria. After a six-week study, Agraib et al. [ 86 ] found that patients in the probiotic (containing nine Lactobacillus and five Bifidobacterium species) group had higher levels of anti-inflammatoty and better clinical symptoms compared with the placebo group. Groeger et al. [ 87 ] demonstrated that Bifidobacterium infantis 35,624 achieved palliate effect to UC primarily by reducing intestinal inflammatory biomarkers (e.g. CRP, TNF-α, IL-6). In 2021, the study conducted by Gu et al. [ 88 ] revealed that Akkermansia muciniphila activate aryl hydrocarbon receptor (AhR) signaling, inhibite Kyn pathway (KP) activation, and restore the down-regulation of anti-inflammatory factors through increasing the levels of indoleacetic acid (IAA) and indole acrylic acid (IA) in the tryptophan (Trp) metabolic pathway. Similarly, the mitigation effect of probiotics (containing L.casei Zhang, L.plantarum P-8 and B.animalis subsp. lactis V9) was demonstrated in a trail by Chen et al. [ 89 ] in the treatment of UC. And the researchers found that the probiotic group had more beneficial bacteria, such as Eubacterium ramulus , Pediococcus pentosaceus , Bacteroides fragilis and Weissella cibaria .
All in all, these clinical studies have shown that the effectiveness of treating UC patients with probiotics is increasingly being proven. Above all, probiotics intervention might be a potentially effective approach in the treatment of UC by restoration of gut microbiota. Meanwhile, therapies that may most efficiently bring the disease under control are still being sought.
Prebiotics therapy
Prebiotics are selectively fermentable, non-digestible oligosaccharides, or ingredients. They function to accelerate beneficial bacterial growth and suppress harmful bacterial growth, thus adjusting the balance of gut microbiota. In addition, they can lead to the production of SCFAs, regulate immune response, control gene expression in bacterial cells, and improve absorption of micronutrients. And prebiotics are used to treat a wide variety of disease, such as obesity [ 90 ], chronic enteritis [ 91 ], skin disease [ 92 ] and autism spectrum disorder [ 93 ]. The therapeutic effects of prebiotics on UC have also been confirmed in animal and humans (Tables 3 and 4 ). Thus, Prebiotics can be used as a novel dietary management approach for UC. Here, the possible effects and mechanisms of prebiotics in the treatment of UC are summarized in Fig. 3 .
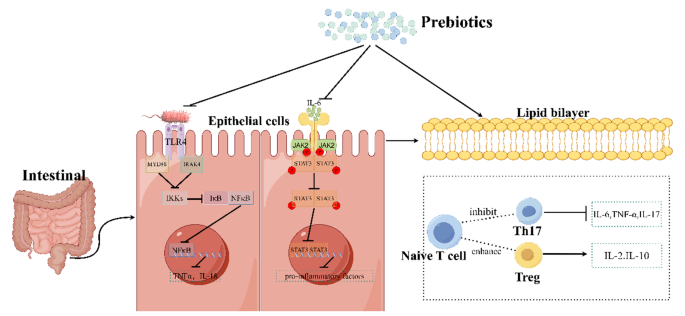
The mechanism of prebiotics in alleviating Ulcerative Colitis in Mice. It was found that the mechanism of prebiotics alleviate UC is probably through inhibiting of the TLR4/NF-κB signaling pathway, the JAK2/STAT3 signaling pathway, and regulating the ratio of T cell subsets. Firstly, prebiotics inhibit the activation effect of lipopolysaccharides from Gram-positive bacteria on TLR4 receptors, thereby inhibiting NF-κB from being released into nucleus and thus reducing the transcription of pro-inflammatory factors. Secondly, prebiotics can inhibit the activation of cytokine receptors by IL-6, thus suppress the entry of STAT3 into the nucleus and likewise inhibit its production of pro-inflammatory factors. Thirdly, prebiotics can inhibit of the conversion of naive T cells into Th17 cells and promote of their conversion into Treg cells, causing an increase of the expression of anti-inflammatory. (This mechanism diagram was drawn by Figdraw ( https://www.figdraw.com ))
Convincing evidence from animal studies indicate that prebiotics treatment can relieve UC. Koleva et al. [ 94 ] showed that fructo-oligosaccharides (FOS) promoted Bifidobacterium spp. and inulin and FOS can all decrease Clostridium cluster XI in rats, while Bifidobacterium spp. and Clostridium cluster XI correlated negatively and positively, respectively, to chronic intestinal inflammation. That is, both this two fructans inhibited intestinal inflammation. Hoentjen et al. [ 95 ] also orally administered a prebiotic combination of chicory-derived long-chain inulin-type fructans and short-chain inulin fraction oligofructose to HLA-B27 transgenic rats and found that this prebiotic can significantly reduce colitis and demonstrated that this effect was not only related to the gut microbiota, but also to immunomodulatory effects. They found that the prebiotic can promote the increase of bifidobacteria and endogenous lactobacilli. In immunomodulation, for example, it is possible to increase TGF-β in cecum. Wang et al. [ 96 ] allowed C57BL/6 mice with UC to receive oral administration of stachyose which is a prebiotic that traditionally extracted from plants for a period of time, and demonstrated the effect of stachyose on the recovery of body weight and found that it can reduced colonic tissue damage, lowered the level of pro-inflammatory cytokines, and restored the dysbiosis of the intestinal microbiota imblance (reduce the abundance of Escherichia_Shigella , Parabacteroides , Romboutsia and Turicibacter and raise the abundance of Alistipes and Roseburia ). In the study of Lunken et al. [ 97 ], they used an adoptive T-cell transfer mice model of colitis to examine the effects of enriching exclusive enteral nutrition (EEN) with inulin-type fructans (IN) (ENN IN) on colitis and found that a less deterioration of the mucus layer, increased butyrate production, and the expansion of anti-inflammatory T-cell subsets, including IL-10 producing Foxp3 + Tregs. And they also found an increased relative abundance of beneficial microbes ( Bifidobacterium spp. and Anaerostipes caccae ) and an reduced relative abundance of potentially pathogenic microbes ( Escherichia Shigella spp.). All of these results continue to prove the benefits of prebiotics in UC. Li et al. [ 98 ] established the DSS-induced mice model of colitis by evaluating the therapeutic effects of prebiotics high-substituted hydroxypropyl cellulose (HHPC) and low-substituted hydroxypropyl cellulose (LHPC) on UC, and the results confirmed that these two prebiotics dose-dependently ameliorated the inflammation in colitis mice, inhibited pro-inflammatory cytokine and regulated the balance of intestinal flora, including increased the relative abundance of Bacteroides and Alloprevotella genus and reduced the relative abundance of Firmicutes. Kanauchi et al. [ 99 ] investigated the effect of Germinated barley foodstuff (GBF), a prebiotic product, on the gut environment and found that it can inhibited the expression of STAT3 and NF-κB, thereby reducing the inflammatory response of the epithelium.
In summary, these animal experiments have showed the good effect of prebiotic therapy alone or in combination to UC. This provides a new direction in the clinical treatment of UC.
Many clinical studies have demonstrated the benefits of prebiotics for people with UC. Oligofructose and Inulin as the oligosaccharide fraction of Raftilose and the oligosaccharide fraction of Raftiline, which was obtained by the extraction of chicory roots, Gibson et al. [ 100 ] have demonstrated the stimulatory effect of these two substances on intestinal bifibacteria, which is a bacterium thought to be beneficial to health through clinical experiment and reduced some pathogenic bacteria that can produce toxins or hydrolyzed proteins, including bacteroides, clostridia, and fusobacteria. Vulevic et al. [ 101 ] found that Galactooligosaccharides (GOSs) promoted the population of beneficial bacteria, especially bifidobacteria and lactobacilli, and reduced numbers of less beneficial bacteria (bacteroides, the C. histolyticum group, E. coli , and Desulfovibrio spp.), and also enhanced the immune response and reduced the production of pro-inflammatory factors. Similarly. Casellas et al. [ 102 ] demonstrated that oligofructose-enriched inulin reduced intestinal inflammation by measuring fecal calprotectin levels in patients. Faghfoori et al. [ 103 ] administrated germinated barley foodstuff (GBF) to patients with UC and showed that GBF were able to reduce serum levels of pro-inflammatory including IL-6, IL-8,TNF-α. As demonstrated by Mitsuyama et al. [ 104 ], by determining the changes of microorganisms in the feces of patients with UC after four weeks of oral administration of GBF, the results proved that prebiotics can increase the concentration of fecal Bifidobacterium and Eubacterium limosum and increase the concentration of colonic butyrate, which is a source of energy for epithelium. And decreased the presence of Bacteroides .
Ryan et al. [ 105 ] conducted in vitro and in vivo experiments and demonstrated the promoting effect of \( {2}^{{\prime }}\) -fucosyllactose ( \( {2}^{{\prime }}\) -FL) which is a prebiotic human milk oligosaccharide on butyric acid producers, including Bifidobacterium , Clostridium cluster XIVa and Roseburia spp. Butyric acid, on the other hand, as a kind of SCFA, can inhibit the inflammatory response. In this study, they also found a significant increase in fecal Faecalibacterium prausnitzii , Anaerotruncus colihominis , and Pseudoflavonifractor species. Consistent with the above results, Suzuki et al. [ 106 ] tested the effectiveness of Bifidogenic growth stimulator (BGS) which is a prebiotic preparation produced by Propionibacterium freudenreichii isolated from Swiss cheese in patients with UC and found that it can selectively stimulated the activation of Bifidobacteria , which not only produced butyrate to nourish colonocytes and inhibited cytokine production and activation of NF-κB pathway, but also improved the balance of the intestinal microflora to maintain intestinal mucosal integrity and prevented intestinal damage. In the clinical study by Li et al. [ 107 ], they demonstrated the potential of Xylo-oligosaccharide (XOS) to alleviate microecological dysbiosis in patients with UC by measuring the effect of XOS on the intestinal flora. They found that XOS promotes the proliferation of Bifidobacteria , which produces a variety of organic acids and inhibits the growth of harmful bacteria by altering their metabolites.
In conclusion, these clinical studies demonstrated the palliative effects of prebiotics on UC, showing that prebiotics hold promise as primary or adjunctive maintenance therapy for UC.
Concluding remarks
UC as a common disease has become a financial burden for many people and has the potential to develop into cancer if not prevented or treated. Therefore, it is important to identify and intervene in a timely manner. The pathogenesis of UC is complex, that’s why it’s important to find a reliable treatment. There is a strong and complex relationship between gut microbiota and gut. Crucially, growing evidence strongly suggests that the gut microbiota plays a pivotal role in intestinal defense function, immune regulatory function, inflammatory responses, as a result, the development and progression of UC. Meanwhile, mechanistic studies have demonstrated these particular species of intestinal commensal bacteria capable of playing either a protective or pathogenic role in UC development. Traditional treatment methods come with a lot of side effects. And probiotics and prebiotics emerge as a new therapeutic modality to modulate the gut microbiota. Based on these, numerous animal and clinical studies have shown that regulating gut microbiota may be an effective strategy to treat UC.
Probiotics being able to confer notable health benefits by modulating the composition of gut microbiota and restoring the physiological bacterial flora. However, while an increasing number of studies have pointed to the therapeutic effects of probiotics on UC, the available data in this field remain limited and the relevant scientific work is still in its early stages. Thus, further research is still necessary. Firstly, due to the complex relationship between gut microbiota and UC, in order to better use probiotics to treat UC, it is necessary to further study the mechanism of intestinal flora affecting the occurrence and development of UC through more animal and clinical experiments. Secondly, we need to know how these probiotics regulate gut microbiota or how they function in the intestinal and what factors contribute to their long-run stability in both health and disease. Changes in certain pathway molecules can be probed to determine the specific mechanism of probiotic treats UC. Meanwhile, in the study of probiotics in the treatment of UC, we should pay more attention to the etiology and pathogenesis. Based on this, the composition and metabolites of probiotics should be of great concern. In particularly, it should be thoroughly studied for their antioxidant effects, anti-inflammatory properties, maintenance of the intestinal homeostasis, regulation of mucosal immune homeostasis, and so on. Some key probiotic components and metabolites may be highly effective postbiotic in the treatment of UC. Thirdly, most medications for the treatment of UC have many adverse effects. Meanwhile, probiotics have great potential as drugs to treat UC. Therefore, it may be more cost-efficient to invest more effort in probiotics than in developing new anti-inflammatory drugs. Fourth, in order to provide more effective probiotics to clinical, we can study the beneficial gut microbiota of healthy humans to dig out more and better probiotics. At the same time, it is necessary to search for the most effective probiotic compositions for the treatment of UC. Fifth, more clinical rationalized trials should be carried out to determine whether probiotics is safe and effective in the treatment of UC. Furthermore, because the composition of the gut microbiota is related to region, ethnicity, and diet, it is necessary to study large samples of people in different regions. Sixth, we must figure out the route of administration of the probiotics as well as the dosage, to ensure the probiotics will maximize the benefits in patient’s body under safe administration. Seventh, in order to make it easier and more convenient for patients to use probiotics, such as how to keep probiotics maintain highly active in some way and make it easier for patients to take, we should further explore the production and preservation of probiotics. Last but not least, to accepted by patients as a reliable treatment, it should be clarified for which patients a particular probiotic is effective, or which is preferable for a single probiotic or a blend of strains. So, there are still many problems it faces. In the future, probiotic therapy may be a potentially useful approach for UC, but research in this area has just started.
Prebiotics offer an exciting new approach to dietary management of gastrointestinal disorders including UC. It has been accepted as a dietary food ingredient that helps to nourish gut microbes, which can improve health and prevent UC. But while many studies to date have demonstrated the beneficial effects of prebiotics in UC, it still faces numerous challenges. Now many studies have the limitation of too small a sample size or lack of a control group, so the evidence for a significant effect of prebiotics is still lacking. The dosage of prebiotics is also a question to be confirmed, if too high a dosage will lead to tolerance, or if a higher dosage of prebiotics will produce better results when well tolerated. With so many types of prebiotics available, it is also deserving of further study as to which prebiotics have better results for which type of UC patients. Although a large number of in vitro and in vivo experiments have confirmed the positive effects of prebiotics, there is still a need for more clinical trials or animal experiments to further evaluate their specific effects.The specific mechanism by which we found that prebiotics alleviate UC remains unclear. It’s worth exploring further. In-depth experiments are needed to further elucidate the role of prebiotics in patients with UC, whether it is their own structure or their metabolites that play a role. And to meet the needs of consumers, new strategies for cost-effective and efficient prebiotics can be developed. Prebiotics, as a food-sourced ingredient for the treatment of UC, offer a new clinical direction, and it is important to study its good effects and side effects as clearly as possible. Therefore, in any case, the prospect of the application of prebiotics in UC is worthy of attention and expectation.
Certainly, in order to gain wider acceptance and recognition for probiotics and prebiotics to treat UC, further research is urgently required.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Hijova E, Soltesova A. Effects of probiotics and prebiotics in ulcerative colitis. Bratislava Med J. 2013;114(09):540–3.
Article CAS Google Scholar
Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. The Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21 St Century: a systematic review of Population-Based studies. Gastroenterology. 2017;152(5):S970–1.
Article Google Scholar
Iheozor-Ejiofor Z, Kaur L, Gordon M, Baines PA, Sinopoulou V, Akobeng AK. Probiotics for maintenance of remission in ulcerative colitis. Cochrane Database of Systematic Reviews. 2020;3(3):CD007443.
Mladenova D, Kohonen-Corish MRJ. Mouse models of inflammatory bowel disease - insights into the mechanisms of inflammation-associated Colorectal Cancer. Vivo. 2012;26(4):627–46.
CAS Google Scholar
Li J, Ueno A, Fort Gasia M, Luider J, Wang T, Hirota C, et al. Profiles of Lamina Propria T Helper Cell subsets discriminate between Ulcerative Colitis and Crohnʼs Disease. Inflamm Bowel Dis. 2016;22(8):1779–92.
Article PubMed Google Scholar
Shen Z-H, Zhu C-X, Quan Y-S, Yang Z-Y, Wu S, Luo W-W, et al. Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol. 2018;24(1):5–14.
Article CAS PubMed PubMed Central Google Scholar
Yao SY, Zhao ZX, Wang WJ, Liu XL. Bifidobacterium Longum: Protection against Inflammatory Bowel Disease. Journal of Immunology Research. 2021:8030297.
Zou JF, Shen YM, Chen MJ, Zhang ZM, Xiao SW, Liu C, et al. Lizhong decoction ameliorates ulcerative colitis in mice via modulating gut microbiota and its metabolites. Appl Microbiol Biotechnol. 2020;104(13):5999–6012.
Article CAS PubMed Google Scholar
Rather IA, Majumder R, Alshammari FH, Park JG, Bajpai VK. Ulcerative colitis and probiotics: an overview. Pak J Pharm Sci. 2016;29(5):1877–80.
CAS PubMed Google Scholar
Peyrin-Biroulet L, Sandborn W, Sands BE, Reinisch W, Bemelman W, Bryant RV, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324–38.
Kushkevych I, Martinkova K, Vitezova M, Rittmann SKR. Intestinal microbiota and perspectives of the Use of Meta-Analysis for comparison of Ulcerative Colitis studies. J Clin Med. 2021;10(3):462.
Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119–24.
Barrett JC, Lee JC, Lees CW, Prescott NJ, Anderson CA, Phillips A, et al. Genome-wide association study of ulcerative colitis identifies three new susceptibility loci, including the < i > HNF4A region. Nat Genet. 2009;41(12):1330–U99.
Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380(9853):1606–19.
Sands BE, Kaplan GG. therapeutic review the role of tnfα α in ulcerative colitis.2007;47(8):930-41.
Zhang FX, Kirschning CJ, Mancinelli R, Xu XP, Jin Y, Faure E, et al. Bacterial lipopolysaccharide activates nuclear factor-kappab through interleukin-1 signaling mediators in cultured human dermal endothelial cells and mononuclear phagocytes. J Biol Chem. 1999;274(12):7611–4.
Selin KA, Hedin CRH, Villablanca EJ. Immunological networks defining the heterogeneity of Inflammatory Bowel diseases. J Crohns Colitis. 2021;15(11):1959–73.
Article PubMed PubMed Central Google Scholar
Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, et al. Interleukin-13 is the Key Effector Th2 Cytokine in Ulcerative Colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129(2):550–64.
Zhu QG, Rui K, Wang SJ, Tian J. Advances of Regulatory B cells in Autoimmune diseases. Front Immunol. 2021;12:12:592914.
Dave M, Higgins PD, Middha S, Rioux KP. The human gut microbioma current knowledge, challenges, and future directions. Translational Res. 2012;160(4):246–57.
He XL, Gao J, Peng L, Hu TT, Wan Y, Zhou MJ, et al. Bacterial O-GlcNAcase genes abundance decreases in ulcerative colitis patients and its administration ameliorates colitis in mice. Gut. 2021;70(10):1872–83.
Li BL, Du PL, Du Y, Zhao DY, Cai YR, Yang Q et al. Luteolin alleviates inflammation and modulates gut microbiota in ulcerative colitis rats. Life Sci. 2021;269:269:119008.
Guo XY, Liu XJ, Hao JY. Gut microbiota in ulcerative colitis: insights on pathogenesis and treatment. J Dig Dis. 2020;21(3):147–59.
Mizoguchi E, Tong M, Li X, Wegener Parfrey L, Roth B, Ippoliti A et al. A Modular Organization of the Human Intestinal Mucosal Microbiota and its Association with Inflammatory Bowel Disease. PLoS ONE. 2013;8(11):e80702.
Andoh A, Ida S, Tsujikawa T, Benno Y, Fujiyama Y. Terminal restriction fragment polymorphism analyses of fecal microbiota in five siblings including two with ulcerative colitis. Clin J Gastroenterol. 2009;2(5):343–5.
Fuentes S, Rossen NG, van der Spek MJ, Hartman JHA, Huuskonen L, Korpela K, et al. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 2017;11(8):1877–89.
Kotlowski R, Bernstein CN, Sepehri S, Krause DO. High prevalence of Escherichia coli belonging to the B2 + D phylogenetic group in inflammatory bowel disease. Gut. 2007;56(5):669–75.
Xu J, Chen N, Wu Z, Song Y, Zhang Y, Wu N et al. 5-Aminosalicylic acid alters the gut bacterial microbiota in patients with Ulcerative Colitis. Front Microbiol. 2018;9:1274.
Schwiertz A, Jacobi M, Frick J-S, Richter M, Rusch K, Köhler H. Microbiota in Pediatric Inflammatory Bowel Disease. J Pediatr. 2010;157(2):240–e41.
Khalil NA, Walton GE, Gibson GR, Tuohy KM, Andrews SC. In vitrobatch cultures of gut microbiota from healthy and ulcerative colitis (UC) subjects suggest that sulphate-reducing bacteria levels are raised in UC and by a protein-rich diet. Int J Food Sci Nutr. 2013;65(1):79–88.
Verma R, Verma AK, Ahuja V, Paul J. Real-Time Analysis of Mucosal Flora in patients with inflammatory bowel disease in India. J Clin Microbiol. 2010;48(11):4279–82.
Walker AW, Sanderson JD, Churcher C, Parkes GC, Hudspith BN, Rayment N et al. High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11:7.
Tahara T, Shibata T, Kawamura T, Okubo M, Ichikawa Y, Sumi K, et al. Fusobacterium detected in Colonic Biopsy and Clinicopathological features of Ulcerative Colitis in Japan. Dig Dis Sci. 2014;60(1):205–10.
Machiels K, Joossens M, Sabino J, De Preter V, Arijs I, Eeckhaut V, et al. A decrease of the butyrate-producing speciesRoseburia hominisandFaecalibacterium prausnitziidefines dysbiosis in patients with ulcerative colitis. Gut. 2014;63(8):1275–83.
Lepage P, Häsler R, Spehlmann ME, Rehman A, Zvirbliene A, Begun A, et al. Twin study indicates loss of Interaction between Microbiota and Mucosa of patients with Ulcerative Colitis. Gastroenterology. 2011;141(1):227–36.
Fite A, Macfarlane S, Furrie E, Bahrami B, Cummings JH, Steinke DT, et al. Longitudinal analyses of gut mucosal microbiotas in Ulcerative Colitis in Relation to Patient Age and Disease Severity and Duration. J Clin Microbiol. 2013;51(3):849–56.
Ott SJ, Plamondon S, Hart A, Begun A, Rehman A, Kamm MA, et al. Dynamics of the Mucosa-Associated Flora in Ulcerative Colitis patients during remission and clinical relapse. J Clin Microbiol. 2008;46(10):3510–3.
Hu Y, Chen Z, Xu C, Kan S, Chen D. Disturbances of the gut microbiota and microbiota-derived metabolites in Inflammatory Bowel Disease. Nutrients. 2022;14(23):5140.
Andreopoulou M, Tsiouris V, Georgopoulou I. Effects of organic acids on the gut ecosystem and on the performance of broiler chickens. J Hellenic Veterinary Med Soc. 2017;65(4):289.
Agus A, Planchais J, Sokol H. Gut microbiota regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe. 2018;23(6):716–24.
Singh R, Chandrashekharappa S, Bodduluri SR, Baby BV, Hegde B, Kotla NG et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 2019;10(1):89.
Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. 2016;22(6):586–97.
Q W, RM M, BA C, M C-D, KJ Z. D G, - A bacterial carbohydrate links innate and adaptive responses through Toll-like. D– 2985109r. (– 0022-1007 (Print)):- 2853-63.
A I-V CDC, K JPAA. K, L A, - FXR and TGR5 Agonists Ameliorate Liver Injury, Steatosis, and Inflammation After. D– 101695860. (– 2471-254X (Electronic)):- 1379-91.
Agus A, Clément K, Sokol H. Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut: J Br Soc Gastroenterol. 2021;70(6):1174–82.
Wahlstrom A, Kovatcheva-Datchary P, Stahlman M, Backhed F, Marschall H-U. Crosstalk between bile acids and gut microbiota and its impact on farnesoid receptor signalling. Dig Dis. 2017;35(3):246–50.
Figliuolo VR, Coutinho-Silva R, Lara Melo Coutinho CM. Contribution of sulfate-reducing bacteria to homeostasis disruption during intestinal inflammation. Life Sci. 2018;215:145–51.
Cai Z, Wang S, Li J. Treatment of inflammatory bowel disease: a Comprehensive Review. Front Med (Lausanne). 2021;8:765474.
Tremblay L, Pineton de Chambrun G, De Vroey B, Lavogiez C, Delaporte E, Colombel JF, et al. Stevens-Johnson syndrome with sulfasalazine treatment: report of two cases. J Crohns Colitis. 2011;5(5):457–60.
Hayashi R, Wada H, Ito K, Adcock IM. Effects of glucocorticoids on gene transcription. Eur J Pharmacol. 2004;500(1–3):51–62.
Lichtenstein GR, Abreu MT, Cohen R, Tremaine W, American Gastroenterological A. American Gastroenterological Association Institute technical review on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130(3):940–87.
Curkovic I, Egbring M, Kullak-Ublick GA. Risks of inflammatory bowel disease treatment with glucocorticosteroids and aminosalicylates. Dig Dis. 2013;31(3–4):368–73.
Chhaya V, Saxena S, Cecil E, Subramanian V, Curcin V, Majeed A, et al. Steroid dependency and trends in prescribing for inflammatory bowel disease - a 20-year national population-based study. Aliment Pharmacol Ther. 2016;44(5):482–94.
D’Haens G. Systematic review: second-generation vs. conventional corticosteroids for induction of remission in ulcerative colitis. Aliment Pharmacol Ther. 2016;44(10):1018–29.
Jharap B, Seinen ML, de Boer NK, van Ginkel JR, Linskens RK, Kneppelhout JC, et al. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. 2010;16(9):1541–9.
Herrlinger KR, Barthel DN, Schmidt KJ, Buning J, Barthel CS, Wehkamp J, et al. Infliximab as rescue medication for patients with severe ulcerative/indeterminate colitis refractory to tacrolimus. Aliment Pharmacol Ther. 2010;31(9):1036–41.
Moura FA, de Andrade KQ, Dos Santos JCF, Araujo ORP, Goulart MOF. Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biol. 2015;6:617–39.
Hui L, Wei-Hsien L, Hanying Z, Haotian F, Wen Z, Wei-Lian H et al. Bifidobacterium lactis BL-99 protects mice with osteoporosis caused by colitis via gut inflammation and gut microbiota regulation. Food & Function. 2022;13(3):1482-1494.
Aghamohammad S, Sepehr A, Miri ST, Najafi S, Pourshafie MR, Rohani M. The role of combining Probiotics in Preventing and Controlling inflammation: a focus on the anti-inflammatory and Immunomodulatory effects of Probiotics in an in vitro model of IBD. Can J Gastroenterol Hepatol. 2022:2045572.
White R, Atherly T, Guard B, Rossi G, Wang C, Mosher C, et al. Randomized, controlled trial evaluating the effect of multi-strain probiotic on the mucosal microbiota in canine idiopathic inflammatory bowel disease. Gut Microbes. 2017;8(5):451–66.
Kang Y, Cai Y. The development of probiotics therapy to obesity: a therapy that has gained considerable momentum. Hormones. 2018;17(2):141–51.
Kang Y, Cai Y, Yang Y. The gut Microbiome and Hepatocellular Carcinoma: implications for early diagnostic biomarkers and Novel therapies. Liver Cancer. 2022;11(2):113–25.
Li LP, Kang YB. The gut Microbiome and Autoimmune Hepatitis: implications for early diagnostic biomarkers and Novel therapies. Molecular Nutrition & Food Research; 2023;67(24):e2300043.
Cai Y, Kang YB. Gut microbiota and metabolites in diabetic retinopathy: insights into pathogenesis for novel therapeutic strategies. Biomed Pharmacother. 2023;164:114994.
Liu HX, Kang X, Yang XD, Yang H, Kuang XY, Ren P, et al. Compound Probiotic ameliorates Acute Alcoholic Liver Disease in mice by modulating gut microbiota and maintaining intestinal barrier. Probiotics Antimicrob Proteins. 2023;15(1):185–201.
Wu J, Cui S, Tang X, Zhang Q, Jin Y, Zhao J, et al. Bifidobacterium longum CCFM1206 promotes the Biotransformation of Glucoraphanin to Sulforaphane that contributes to amelioration of Dextran-Sulfate-Sodium-Induced Colitis in mice. J Agric Food Chem. 2023;71(2):1100–12.
Han T, Hu X, Li K, Zhang D, Zhang Y, Li J. Bifidobacterium infantis maintains Genome Stability in Ulcerative Colitis via regulating anaphase-promoting Complex Subunit 7. Front Microbiol. 2021;12:761113.
Fu T, Wang Y, Ma M, Dai W, Pan L, Shang Q et al. Isolation of Alginate-degrading Bacteria from the human gut microbiota and Discovery of Bacteroides xylanisolvens AY11-1 as a Novel Anti-colitis Probiotic Bacterium. Nutrients. 2023;15(6):1352.
Wang T, Shi C, Wang S, Zhang Y, Wang S, Ismael M, et al. Protective effects of Companilactobacillus Crustorum MN047 against Dextran Sulfate Sodium-Induced Ulcerative Colitis: a fecal microbiota transplantation study. J Agric Food Chem. 2022;70(5):1547–61.
Hu Y, Jin X, Gao F, Lin T, Zhu H, Hou X et al. Selenium-enriched Bifidobacterium longum DD98 effectively ameliorates dextran sulfate sodium-induced ulcerative colitis in mice. Front Microbiol. 2022;13:955112.
Han M, Liao W, Si X, Bai C, Gai Z. Protective effects of lacticaseibacillus rhamnosus Hao9 on dextran sulphate sodium-induced ulcerative colitis in mice. J Appl Microbiol. 2022;133(3):2039–49.
Huang J, Yang Z, Li Y, Chai X, Liang Y, Lin B et al. Lactobacillus paracasei R3 protects against dextran sulfate sodium (DSS)-induced colitis in mice via regulating Th17/Treg cell balance. J Translational Med. 2021;19(1):356.
Xu X, Wu J, Jin Y, Huang K, Zhang Y, Liang Z. Both Saccharomyces boulardii and its postbiotics alleviate Dextran Sulfate Sodium-Induced Colitis in mice, Association with modulating inflammation and intestinal microbiota. Nutrients. 2023;15(6):1484.
Komaki S, Haque A, Miyazaki H, Matsumoto T, Nakamura S. Unexpected effect of probiotics by Lactococcus lactis subsp. lactis against colitis induced by dextran sulfate sodium in mice. J Infect Chemother. 2020;26(6):549–53.
Hizay A, Dag K, Oz N, Comak-Gocer EM, Ozbey-Unlu O, Ucak M et al. Lactobacillus acidophilus regulates abnormal serotonin availability in experimental ulcerative colitis. Anaerobe. 2023;80:102710.
Gao H, Li Y, Sun J, Xu H, Wang M, Zuo X, et al. Saccharomyces Boulardii ameliorates Dextran Sulfate Sodium-Induced Ulcerative Colitis in mice by regulating NF-κB and Nrf2 signaling pathways. Oxidative Med Cell Longev. 2021;2021:1–14.
Google Scholar
He W, Ni W, Zhao J, M’Koma A. Enterococcus faecium alleviates Gut Barrier Injury in C57BL/6 mice with Dextran Sulfate Sodium-Induced Ulcerative Colitis. Gastroenterol Res Pract. 2021;2021:1–9.
Li ZG, Zhang S, Xu L, Fang XX, Wan YZ, Yu DH, et al. A tetrapeptide from maize combined with probiotics exerted strong anti-inflammatory effects and modulated gut microbiota in DSS-induced colitis mice. Food Funct. 2022;13(24):12602–18.
Shang JC, Yang S, Tang ZX, Chen YH, Duan BF, Meng XC. Bifidobacterium bifidum H3-R2 and its Molecular Communication within the context of Ulcerative Colitis. J Agric Food Chem. 2022;70(37):11678–88.
Chen ZX, Yi L, Pan YN, Long XY, Mu JF, Yi RK et al. Lactobacillus fermentum ZS40 ameliorates inflammation in mice with Ulcerative Colitis Induced by Dextran Sulfate Sodium. Front Pharmacol. 2021;12:700217.
Ren Z, Hong Y, Huo Y, Peng L, Lv H, Chen J et al. Prospects of probiotic adjuvant drugs in clinical treatment. Nutrients. 2022;14(22):4723.
Hegazy SK. Effect of probiotics on pro-inflammatory cytokines and NF-κB activation in ulcerative colitis. World J Gastroenterol. 2010;16(33):4145-51.
Palumbo VD, Romeo M, Gammazza AM, Carini F, Damiani P, Damiano G, et al. The long-term effects of probiotics in the therapy of ulcerative colitis: a clinical study. Biomedical Papers-Olomouc. 2016;160(3):372–7.
Bjarnason I, Sission G, Hayee B. A randomised, double-blind, placebo-controlled trial of a multi-strain probiotic in patients with asymptomatic ulcerative colitis and Crohn’s disease. Inflammopharmacology. 2019;27(3):465–73.
Tsuda Y, Yoshimatsu Y, Aoki H, Nakamura K, Irie M, Fukuda K, et al. Clinical effectiveness of probiotics therapy (BIO-THREE) in patients with ulcerative colitis refractory to conventional therapy. Scand J Gastroenterol. 2009;42(11):1306–11.
Agraib LM, Yamani MI, Tayyem R, Abu-Sneineh AT, Rayyan YM. Probiotic supplementation induces remission and changes in the immunoglobulins and inflammatory response in active ulcerative colitis patients: a pilot, randomized, double-blind, placebo-controlled study. Clin Nutr ESPEN. 2022;51:83–91.
Groeger D, O’Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, et al. Bifidobacterium infantis35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2014;4(4):325–39.
Gu ZY, Pei WL, Shen YH, Wang LJ, Zhu J, Zhang Y, et al. Akkermansia muciniphila and its outer protein Amuc_1100 regulates tryptophan metabolism in colitis. Food Funct. 2021;12(20):10184–95.
Chen P, Xu HY, Tang H, Zhao FY, Yang CC, Kwok LY, et al. Modulation of gut mucosal microbiota as a mechanism of probiotics-based adjunctive therapy for ulcerative colitis. Microb Biotechnol. 2020;13(6):2032–43.
Carnahan S, Balzer A, Panchal SK, Brown L. Prebiotics in obesity. Panminerva Med. 2014;56(2):165–75.
Looijer-van Langen MA, Dieleman LA. Prebiotics in chronic intestinal inflammation. Inflamm Bowel Dis. 2009;15(3):454–62.
Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71(4):814–21.
Sanlier N, Kocabas S. The effect of probiotic, prebiotic and gut microbiota on ASD: a review and future perspectives. Crit Rev Food Sci Nutr. 2023;63(15):2319–30.
Koleva PT, Valcheva RS, Sun X, Ganzle MG, Dieleman LA. Inulin and fructo-oligosaccharides have divergent effects on colitis and commensal microbiota in HLA-B27 transgenic rats. Br J Nutr. 2012;108(9):1633–43.
Hoentjen F, Welling GW, Harmsen HJM, Zhang X, Snart J, Tannock GW, et al. Reduction of colitis by prebiotics in HLA-B27 transgenic rats is associated with microflora changes and immunomodulation. Inflamm Bowel Dis. 2005;11(11):977–85.
Wang C, Bai J, Wang B, Yu L, Tian F, Zhao J, et al. Stachyose modulates gut microbiota and alleviates DSS-induced ulcerative colitis in mice. Food Sci Hum Wellness. 2023;12(6):2211–20.
Lunken GR, Tsai K, Schick A, Lisko DJ, Cook L, Vallance BA, et al. Prebiotic enriched exclusive Enteral Nutrition suppresses colitis via gut microbiome modulation and expansion of anti-inflammatory T cells in a mouse model of colitis. Cell Mol Gastroenterol Hepatol. 2021;12(4):1251–66.
Li X, Lv H, Shi F, Song J, Zhang Z. The potential therapeutic effects of hydroxypropyl cellulose on acute murine colitis induced by DSS. Carbohydr Polym. 2022;289:119430.
Kanauchi O, Serizawa I, Araki Y, Suzuki A, Andoh A, Fujiyama Y, et al. Germinated barley foodstuff, a prebiotic product, ameliorates inflammation of colitis through modulation of the enteric environment. J Gastroenterol. 2003;38(2):134.
Gibson GR, Beatty ER, Wang X, Cummings JH, Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of Bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108(4):975– 82.
Vulevic J, Drakoularakou A, Yaqoob P, Tzortzis G, Gibson GR. Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. Am J Clin Nutr. 2008;88(5):1438–46.
Casellas F, Borruel N, Torrejon A, Varela E, Antolin M, Guarner F, et al. Oral oligofructose-enriched inulin supplementation in acute ulcerative colitis is well tolerated and associated with lowered faecal calprotectin. Aliment Pharmacol Ther. 2007;25(9):1061–7.
Faghfoori Z, Navai L, Shakerhosseini R, Somi MH, Nikniaz Z, Norouzi MF. Effects of an oral supplementation of germinated barley foodstuff on serum tumour necrosis factor-alpha, interleukin-6 and– 8 in patients with ulcerative colitis. Ann Clin Biochem. 2011;48(Pt 3):233–7.
Mitsuyama K, Saiki T, Kanauchi O, Iwanaga T, Sata M. Treatment of ulcerative colitis with germinated barley foodstuff feeding: a pilot study. Aliment Pharmacol Ther. 1998;12(12):1225–30.
Ryan JJ, Monteagudo-Mera A, Contractor N, Gibson GR. Impact of 2’-Fucosyllactose on Gut Microbiota Composition in Adults with Chronic Gastrointestinal Conditions: Batch Culture Fermentation Model and Pilot Clinical Trial Findings. Nutrients. 2021;13(3):938.
Suzuki A, Mitsuyama K, Koga H, Tomiyasu N, Masuda J, Takaki K, et al. Bifidogenic growth stimulator for the treatment of active ulcerative colitis: a pilot study. Nutrition. 2006;22(1):76–81.
Li Z, Li Z, Zhu L, Dai N, Sun G, Peng L, et al. Effects of Xylo-Oligosaccharide on the gut microbiota of patients with Ulcerative Colitis in Clinical Remission. Front Nutr. 2021;8:778542.
Download references
Acknowledgements
Not applicable.
This work was supported by Shanxi Province Natural Science Foundation (Grant No. 202203021221182), Science Research Start-up Fund for Doctor of Shanxi Medical University (Grant No. XD1807), Science Research Start-up Fund for Doctor of Shanxi Province (Grant No.SD1807), Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (Grant No. 2019L0425), and Shanxi Province Science Foundation for Youths (Grant No. 201901D211314).
Author information
Authors and affiliations.
Department of microbiology and immunology, School of Basic Medical Sciences, Shanxi Medical University, Taiyuan, Shanxi, China
Jing Guo, Liping Li & Yongbo Kang
Faculty of Life science and Technology, Kunming University of Science and Technology, Kunming, Yunnan, China
You can also search for this author in PubMed Google Scholar
Contributions
Jing Guo wrote the main manuscript text with the help of Liping Li, Xinwei Huang and Yongbo Kang. All authors reviewed the manuscript.
Corresponding author
Correspondence to Yongbo Kang .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Guo, J., Li, L., Cai, Y. et al. The development of probiotics and prebiotics therapy to ulcerative colitis: a therapy that has gained considerable momentum. Cell Commun Signal 22 , 268 (2024). https://doi.org/10.1186/s12964-024-01611-z
Download citation
Received : 10 October 2023
Accepted : 10 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s12964-024-01611-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Ulcerative colitis
- Gut microflora
- Inflammation
Cell Communication and Signaling
ISSN: 1478-811X
- General enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Am J Case Rep
- PMC10640891

Upadacitinib as a Rescue Therapy in Acute Severe Ulcerative Colitis: A Case Report and Review of the Literature
Patient: Female, 14-year-old
Final Diagnosis: Ulcerative colitis
Symptoms: Diarrhea
Clinical Procedure: Colonoscopy
Specialty: Gastroenterology and Hepatology
Unusual or unexpected effect of treatment
Background:
Ulcerative colitis (UC) is a chronic immune-mediated disease of the colon. The mainstay of treatment to achieve and maintain remission is 5-aminosalicylic acid (5-ASA). At least 20% of patients with UC experience an acute severe ulcerative colitis (ASUC) flare, requiring aggressive early intervention to prevent complications. The first-line treatment of ASUC is intravenous steroids followed by infliximab or cyclosporin in patients for whom steroids fail. Refractory disease failing medical therapy and warranting surgery is common. Lately, Janus kinase (JAK) inhibitors, such as tofacitinib, filgotinib, and upadacitinib, have been licensed for moderate-to-severe UC in adults. Nevertheless, the safety and efficacy of upadacitinib in ASUC has not yet been established.
Case Report:
We report a case of an 18-year-old woman with 4-year history of severe UC. Both infliximab and adalimumab treatments failed, despite the concurrent use of azathioprine, and she was reliant on steroids. Moreover, tofacitinib failed after 1 year of therapy. She was admitted as a case of ASUC. Flexible sigmoidoscopy confirmed severe pancolitis. Finally, she was treated effectively with oral upadacitinib 45 mg given once daily. She went into full clinical, biochemical, and steroid-free remission in 60 days and endoscopic remission at 180 days.
Conclusions:
This case report features the potential safety and efficacy of upadacitinib in adults with ASUC. Larger trials are required to confirm the efficacy and safety in patients admitted with ASUC.
Ulcerative colitis (UC) is a chronic immune-mediated disorder of the colon and rectum with no definite cure. It is believed to be the result of an interface between the immune system, environment, and intestinal microbiome in genetically predis-posed individuals. Abdominal pain, rectal bleeding, and diarrhea are the most common symptoms of the disease. Early adulthood, ranging between 15 and 30 years of age, displays the highest incidence of UC. Prolonged treatment is usually required to maintain the disease in remission; however, choosing the appropriate therapy depends on the severity of the disease [ 1 ]. For mild-to-moderate disease, 5-aminosalicylic acid (5-ASA) and azathioprine are advised. Acute exacerbations of the disease, up to this time, are mainly treated with steroids. Regarding moderate-to-severe UC in adults, biologics, for example tumor necrosis factor inhibitor (anti-TNF), specifically vedolizumab, and ustekinumab, are used [ 2 ]. At least 20% of patients with UC will develop acute severe ulcerative colitis (ASUC), a life-threatening medical emergency, in the course of their illness, requiring early and aggressive intervention and hospitalization to prevent complications [ 3 ]. According to the Truelove and Witts criteria, ASUC is defined as a combination of ≥6 bloody stools with at least 1 of the following; pulse rate >90 beats per min, temperature >37.8°C, hemoglobin level of 30 mm/h, erythrocyte sedimentation rate (ESR) >30 mm/h, or C-reactive protein (CRP) level >30 mg/L [ 4 ]. The therapeutic options for ASUC are more limited, with corticosteroids remaining the cornerstone of therapy for such flares, followed by infliximab or cyclosporine when corticosteroid treatment fails [ 3 ]. Additionally, tofacitinib, a Janus kinase (JAK) inhibitor, demonstrated promising results as a rescue therapy for adults with ASUC in whom the usual therapy with either infliximab or cyclosporin failed [ 5 ]. Upadacitinb, another JAK inhibitor, has been licensed for treating adults with moderate-to-severe UC; however, its safety and efficacy in ASUC has not yet been well established. In this paper, we present an 18-year-old woman, dependent on steroid treatment, who responded clinically, biochemically, and endoscopically to oral upadacitinib 45 mg once daily. She went into steroid-free remission and avoided proctocolectomy, which was offered for her as a salvage therapy. This case features the potential safety and efficacy of upadacitinib for treating ASUC.
Case Report
A 18-year-old woman with a known case of severe UC with a 4-year duration was admitted for a flare. At the time of diagnosis in December 2019, at the age of 15 years, she presented with severe UC that was steroid-resistant. She started induction therapy with infliximab (10 mg/kg) and showed a resolution of her symptoms. For about 6 months, she was maintained in remission using both infliximab 10 mg/kg every 8 weeks and azathioprine 2.5 mg/kg. She was steroid free, and her body mass index (BMI) improved from 17.3 kg/m 2 at the time of diagnosis to 19.3 kg/m 2 on therapy. However, in June 2020, she was readmitted for an exacerbation of her disease, with a BMI of 17.3 kg/m 2 once again. As per protocol, infectious workup was conducted and came back negative for Clostridium difficile and cytomegalovirus colitis. The diagnosis of severe pancolitis (Mayo 3, involvement of the entire colon) was confirmed by flexible sigmoidoscopy. Further workup assessing her infliximab drug level and antibodies was performed. Results came back as an undetectable infliximab drug level and a high level of antibodies against infliximab (30 AU/mL, normal reference range <5 AU/mL), presenting a picture of secondary non-response because of immunogenicity. Clinical remission was achieved after starting her on a tapering course of prednisone 40 mg. To maintain remission, which was achieved for 9 months, infliximab was substituted for adalimumab 40 mg every 2 weeks subcutaneously with azathioprine (2.5 mg/kg). Unfortunately, in March 2021, at the age of 17 years, the patient presented with another relapse. The diagnosis of severe pancolitis, involvement of the entire colon, was established by flexible sigmoidoscopy. Once more, her infectious workup was negative. Based on therapeutic drug monitoring, her dose of adalimumab was boosted to 40 mg weekly and ultimately to 80 mg weekly, given her drug level was sub-standard (5 ug/mL) and owing to the presence of antibodies (5 AU/mL). Another prednisone 40 mg tapering course was offered, although complete wean off was not possible, as the patient developed rapid relapse once the prednisone dose reached 15 mg/day. Her adalimumab level at the time was therapeutic (12 ug/mL); however, adalimumab antibodies reached 20 (reference range <10 AU/ mL). Her laboratory test results revealed the following: white blood cell count 4.6 (reference range 3.9-10×10 9 /L), microcytic anemia (hemoglobin 81 g/L and mean corpuscular volume 71.6 fL), platelet count of 362 000 mcL, and hypoalbuminemia (albumin 28 g/L). Her inflammatory markers were as follows: CRP level of 110 mg/L and ESR of 74 mm/hr. A trial of a total exclusive enteral nutrition diet was offered to the patient by our dietician, with no success. At this stage, our colorectal surgeons advised for proctocolectomy; however, it was refused by the patient’s parents (BMI was still at 17.3 kg/m 2 ).
Other medical alternatives on an off-label use were discussed with the patient’s parents, owing to the complexity of her case. Since both vedolizumab and ustekinumab are not approved for pediatrics with UC, her medical insurance company declined them. Tofacitinib, a pan-JAK inhibitor approved for adults with UC and pediatric patients with juvenile idiopathic arthritis, was offered as a possible salvage therapy. It was explained to the patient and her family that it is not yet licensed for pediatrics with UC, but they agreed to give it a trial. Informed consent explaining its benefits, risks, adverse effects, and complications
was obtained from the patient and her parents, since she was under 18, and the drug was funded to the patient by a patient funding organization. Her lipid profile and cardiopulmo-nary status were both assessed and were normal, confirming the presence of no contraindications to try tofacitinib. More importantly, regarding her varicella zoster status, it was confirmed that she had varicella zoster infection when she was 5 years old and she was vaccinated fully up to her age. Oral tofacitinib 5 mg given twice daily was started and clinical remission was accomplished. She was able to taper off steroids with no recurrence of her symptoms. Clinical, biochemical, and endoscopic remission was achieved at 9 months. Her follow-up CRP level was 5 mg/L, and fecal calprotectin level declined from >1000 ug/g to 110 ug/g. Her BMI improved to 21.2 kg/m 2 . Remarkably, clinical and endoscopic remissions were sustained for 12 months.
A year later, at the age of 18 years, in November 2022, she was admitted to the hospital with ASUC. She presented with abdominal pain associated with 9 episodes of bloody diarrhea per day. Her laboratory test results on admission showed a hemoglobin level of 95 g/L and CRP level of 75 mg/L, and all infectious and stool workups were negative. She underwent sigmoidoscopy to confirm the diagnosis, which revealed severe pancolitis (Mayo 3, involvement of the entire colon) with numerous pseudopolyps ( Figure 1A, 1B ). Intravenous methylprednisolone was started and the tofacitinib dose was increased in an attempt to control the flare (10 mg 3 times daily).

( A ) The rectum before starting upadacitinib, with severe ulcerations, loss of vascularity, pseudopolyps, and deformed lumen. ( B ) The sigmoid colon before starting upadacitinib, with severe ulcerations, loss of vascularity, pseudopolyps,and deformed lumen. ( C ) The rectum after 6 months of upadacitinib, with no ulcerations except for mild erythema. ( D ) The sigmoid after 6 months of upadacitinib, with no ulcerations.
Three days after admission and treatment, the patient was still passing 9 bloody diarrhea per day and her CRP level remained elevated, at 55 mg/L. The patient did not want to receive cyclosporine owing to its adverse effects and her previous azathioprine failure history. Once more, the patient and her parents were advised to do colectomy, but refused surgery. Other salvage medical options were searched for as per the request of the patient and the patient’s parents. Upadacitinib 45 mg orally once daily was started after a thorough informed consent, discussing the risks, benefits, adverse effects, and off-label use. As early as 3 days after the start of treatment with upadacitinib, her bowel movement improved to 3 bloody diarrhea per day, and she was switched to oral prednisone. Seven days after therapy, she was passing 2 watery, non-bloody bowel movements per day, and her CRP level improved to 25 mg/L. On discharge, she was given upadacitinib 45 mg orally once daily and a prednisone 40-mg tapering course. On her 60-day follow-up appointment, she was remarkably doing well, with normal bowel movements, a BMI of 22.2 kg/m 2 , CRP level of 5 mg/L, and fecal calprotectin level of 181 ug/g. To maintain the patient in remission, the upadacitinib dose was reduced to 30 mg orally once daily. Six months later, she underwent colonoscopy to confirm endoscopic remission, which revealed restored healthy mucosa throughout the colon (Mayo 0), with mild loss of erythema in the sigmoid colon (Mayo 1). Biopsies obtained from the colon revealed dormant chronic colitis in the rectum ( Figure 1C, 1D ).
In this case, we used upadacitinib, a once-daily oral selective JAK1 inhibitor, for an 18-year-old woman with ASUC flare with a loss of response history to tofacitinib, a pan-JAK inhibitor, after 1 year of therapy. No studies up to this time have thoroughly elucidated the use of upadacitinib in ASUC; however, given its established effectiveness in tofacitinib-experienced adult patients with moderate-to-severe UC, we decided to offer it to our patient after exhausting all therapeutic options [ 6 – 8 ]. Clinical improvement was evident as early as 1 week after starting treatment, and clinical/biochemical remission was seen at week 8, avoiding proctocolectomy.
With regards to the treatment of ASUC, which is considered a life-threatening medical emergency requiring hospitalization, it is worth mentioning the current management recommendations among the adult population, which includes the use of steroids as the first-line therapy, followed by rescue therapy with either cyclosporin or infliximab in patients for whom steroid treatment failed after a period of 3 to 5 days [ 3 ]. The choice of using either cyclosporin or infliximab depends solely on physician preference and their availability, as there is no difference in efficacy between the 2 agents, according to the CySIF and CONSTRUCT trials [ 9 , 10 ]. It is imperative to not forget the importance of starting patients on thromboprophylaxis, as inflammatory bowel disease is considered an independent risk factor for thromboembolism [ 11 ]. Surgery conducted in a 3-step approach, starting with subtotal colectomy and loop ileostomy, followed by ileal pouch anal anastomosis, and finally ileostomy reversal, remains the last resort when medical therapy fails, as the failure rate remains high, reaching 20% to 40%, demonstrating the important role of colorectal surgeons and the importance of involving them in the management of ASUC early in the course [ 12 , 13 ].
In contrast to the use of upadacitinib to treat ASUC, its effective and safe use in treating moderate-to-severe UC has been well established in several studies. The comprehensible phase 2b U-ACHIEVE induction (UC1) trial established great evidence about upadacitinib efficacy in treating adults with moderate-to-severe UC who developed insufficient response, loss of response, or intolerance to steroids, immunosuppressive agents, and/or biologics. The aforementioned study has shown a positive dose-response relationship between multiple upadacitinib doses, 7.5 mg, 15 mg, 30 mg, and 45 mg once daily, at inducing remission, the primary outcome, compared with placebo at 8 weeks [ 6 ]. Studies showed that the symptomatic relief from UC symptoms was evident as early as 1 to 3 days after starting treatment with upadacitinib [ 14 ]. Similarly, our patient reported improvement in her symptoms 3 days after starting on upadacitinib 45 mg orally once daily, avoiding colectomy once again, which was excluded according to her wishes.
Upadacitinib is licensed as a therapy for adults with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, and non-radiographic axial spondyloarthritis, with its efficacy and safety being well elaborated in these disease entities [ 15 , 16 ]. Despite the dose of upadacitinib, 45 mg once daily, being higher than the induction dose indicated for diseases other than inflammatory bowel disease, it established the secondary outcome of endoscopic improvement and remission, displaying a prime benefit-risk profile, high efficacy, and no significant increase in clinically related safety events in comparison with lower doses, which was confirmed in the U-ACCOMPLISH (UC2) phase 3 trial, promoting its selection as the optimal induction dose. A total of 451 patients from the previous trials who attained clinical remission after an 8-week induction therapy with upadacitinib 45 mg once daily were randomly assigned to obtain a maintenance dose of upadacitinib of either 15 mg, 30 mg, or placebo once daily for a duration of 52 weeks in the U-ACHIEVE maintenance (UC3) trial. The principal outcome of the UC3 study, clinical remission, clinical response, and endoscopic and histological improvement at 52 weeks, was achieved by 63 (42%) of 149 patients receiving upadacitinib 15 mg once daily, 80 (52%) of 154 receiving upadacitinib 30 mg once daily, and 18 (12%) of 149 receiving placebo (adjusted treatment difference of 30.7% [95% CI 21.7–39.8] for upadacitinib 15 mg vs placebo, P <0.0001; and 39.0% [29.7–48.2] for upadacitinib 30 mg vs placebo, P <0.0001) [ 17 ]. After these thorough trials, the Food and Drug Administration (FDA) and European Medicines Agency licensed the drug for the treatment of adults with moderate-to-severe UC.
In addition to the previous major trials, a prospective real-world single-center analysis of 18 patients with UC in whom treatment with tofacitinib and at least 2 biologics had failed concluded that upadacitinib is an effective treatment for moderate-to-severe UC. The study established this after close follow-up of those patients after starting upadacitinib, confirming clinical, steroid-free, and biochemical remission at 8 weeks [ 8 ]. In our case, we accomplished clinical, steroid-free, and biochemical remission at 8 weeks after starting the patient on upadacitinib 45 mg orally once daily and endoscopic remission at 180 days after the start of treatment.
As the rates of colectomy in adults with ASUC remain high, there is an ongoing need to evaluate novel medical therapies that could delay surgical intervention and increase survival without the need for surgery [ 18 ]. A systemic review including 148 adult patients assessing tofacitinib, a JAK inhibitor, as a rescue therapy for treating adults with ASUC in whom steroid, infliximab, or cyclosporin treatment failed and who were deemed to require colectomy, demonstrated high short-term colectomy-free survival among such patients. The study displayed a promising outcome for tofacitinib as a rescue therapy for adults with ASUC [ 5 ]. Similarly, the results were confirmed by another systemic review published in June 2023 that incorporated 2 observational studies, 7 case series, and 5 case reports, with a total of 134 adult patients with ASUC who were treated with tofacitinib. The study reported a colectomy-free rate of 79.9% (95% CI 73.1–86.7) at 90 days and a colectomy-free rate of 71.6% (95% CI 64–79.2) at 6 months. Once again, the study established the effectiveness of tofacitinib in ASUC [ 23 ].
Due to the complexity of JAK signaling, healthcare providers should consider the detrimental effects related to the use of JAK inhibitors, as they are quite common. These include headache, nausea, arthralgia, increased low-density lipoprotein levels, infections, mainly varicella zoster, thrombosis, creatine phosphokinase elevation, neutropenia/lymphopenia, and acne [ 17 , 19 , 20 ]. Our patient had her vaccination against shingles, her lipid profile was normal during her regular checkups, and she showed no adverse effects specific to the drug throughout treatment.
In 2021, after the results of a randomized control trial studying adults with rheumatoid arthritis on methotrexate, comparing tofacitinib with an TNF inhibitor, the FDA updated the record of adverse effects linked to the use of tofacitinib, adding to it the elevated risk of cardiovascular events, malignancy, thrombosis, and death. This was done after revealing a higher risk of acquiring the aforementioned risks in patients receiving tofacitinib. Therefore, physicians should educate patients thoroughly about these detrimental events and advise them to carefully outweigh the risks and benefits when considering tofacitinib. Upadacitinib safety concerns were not studied in depth compared with those of tofacitinib; however, since upadacitinib descends from the same drug class as tofacitinib and shares the same mechanism of action, it was deemed necessary for the FDA to include upadacitinib in this update with the presumption of it having the same adverse effects. The inclusion criteria of the above-mentioned trial was age 50 years or older and a minimum of 1 cardiovascular risk factor, including smoking, hypertension, diabetes mellitus, and previous heart attack [ 21 , 22 ]. Since our patient was younger than 50 years and had no cardiovascular risk factors, the above-mentioned adverse effects were less feared. Based on our case, upadacitinib 45 mg orally once daily monotherapy, similar to tofacitinib, revealed promising results to support the role of upadacitinib in achieving clinical response and remission in adults with ASUC, avoiding the need for the ultimate cure, proctocolectomy, and its sequelae.
Conclusions
Upadacitinib, showing promising outcomes in ASUC, can be considered as a salvage therapy on an off-label use for adults with ASUC for whom biologic treatment failed. Therefore, randomized controlled trials are required to determine its efficacy and safety.
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:

IMAGES
VIDEO
COMMENTS
CMV colitis may complicate underlying ulcerative colitis 8-15; this occurs in up to one third of patients with glucocorticoid-refractory disease. 9 The majority of cases represent reactivation ...
Case Report: Medical Management of Acute Severe Ulcerative Colitis. Sudheer Kumar Vuyyuru, DM, 1,2 Vipul Jairath, MD, PhD, 1,2,3 Jurij Hanžel, MD, PhD, 2,4 Christopher Ma, MD, MPH, 2,5 and Brian G. Feagan, MD 1,2,3. A 29-year-old female patient presented 5 years ago with bloody diarrhea, fecal urgency, and crampy abdominal pain. A colonoscopy ...
Source Reference: Cathomas M, et al "Herpes simplex virus colitis mimicking acute severe ulcerative colitis: A case report and review of the literature" J Surg Case Rep 2023; DOI: 10.1093/jscr ...
This month: A noteworthy case study. A 13-year-old girl with severe ulcerative colitis (UC) presented for assessment due to worsening symptoms. Two years earlier she had been diagnosed with severe ...
Introduction. Ulcerative colitis (UC) was first described in mid-1800s.1 It is an idiopathic, chronic inflammatory disorder of the colonic mucosa that commonly involves the rectum and may extend in a proximal and continuous fashion to involve other parts of the colon.2 The disease typically affects individuals in the second or third decade of life with hallmark clinical symptoms of bloody ...
Ulcerative colitis (UC), 1 of the 2 major forms of inflammatory bowel disease (IBD), is a chronic inflammatory condition of the colon. 1, 2 In North America, the prevalence of UC in 2023 is estimated to be 0.4% of the population, 3, 4 which represents approximately 1.5 million people living with UC in North America.
Ulcerative colitis is an idiopathic inflammatory condition of the colon that results in diffuse friability and superficial erosions on the colonic wall associated with bleeding. It is the most common form of inflammatory bowel disease worldwide. It characteristically involves inflammation restricted to the mucosa and submucosa of the colon. Typically, the disease starts in the rectum and ...
IBD Interactive Cases. IBD Interactive Cases provide you with real-word adult or pediatric cases on a given topic using an interactive PowerPoint format. They are developed and peer reviewed by our Nurse & Advanced Practice Provider Committee. Click on each link to view and download an interactive case.
In addition, 20% of patients with ulcerative colitis start with severe crises at diagnosis. In our study of 202 patients with inflammatory bowel disease at the Hospital Pablo Tobon Uribe, 23.1% of patients with ulcerative colitis showed severe activity (2). The mortality rate in these individuals in the UK is 2.8%.
Source Reference: Aydın MF, Taşdemir H "Ulcerative colitis in a COVID-19 patient: A case report" Turk J Gastroenterol 2021; DOI: 10.5152/tjg.2021.20851. Share on Facebook. Opens in a new tab or ...
Patient presentation. Patient is a 22 year old female who presented to the surgery department of a tertiary level hospital having been referred from a private clinic, with a two month history of severe abdominal cramps, persistent bloody and mucoid diarrhoea, weight loss and tiredness. Acknowledgement. This case study was kindly provided by Dr ...
Tofacitinib is a rapidly acting Janus kinase inhibitor with proven efficacy in ulcerative colitis. Tofacitinib may provide additional means for preventing colectomy in patients with ASUC. Methods: A retrospective case-control study was performed evaluating the efficacy of tofacitinib induction in biologic-experienced patients admitted with ASUC ...
This activity is intended for gastroenterologists, nurse practitioners, and physician assistants who care for patients with moderate to severe ulcerative colitis (UC). The goal of this activity is for learners to be better able to select advanced UC therapies that allow for optimization of patient outcomes as measured by objective histologic ...
Background Ulcerative colitis (UC) is uncommon in the tropics and sub-tropics. We report a case of UC in a 7 year old girl whose parents were both Nigerians. This report is to alert healthcare professionals in sub-Saharan Africa that UC is not a rare health problem, especially in children. Case presentation The patient presented with frequent passage of blood stained stool, abdominal pain and ...
Ulcerative colitis is a lifelong inflammatory disease affecting the rectum and colon to a variable extent. In 2023, the prevalence of ulcerative colitis was estimated to be 5 million cases around the world, and the incidence is increasing worldwide. Ulcerative colitis is thought to occur in people with a genetic predisposition following environmental exposures; gut epithelial barrier defects ...
P HARMACEUTICAL RESEARCH. www.wjcmpr.com ISSN: 2582-0222. Case report on ulcerative colitis in 16 year girl. MD.Salma, Y. Siva, J.Bhargava Narendra. Department of Pharmacy Practice, QIS College of ...
Ulcerative colitis is associated with various extracolonic manifestations. These include uveitis, pyoderma gangrenosum, pleuritis, erythema nodosum, ankylosing spondylitis, and spondyloarthropathies. Reportedly, 6.2% of patients with inflammatory bowel disease have a major extraintestinal manifestation. Uveitis is the most common, with an ...
In this case study on ulcerative colitis, a twenty seven-year-old girl was brought to the clinic with a history of prolonged diarrhoea that had lasted ten weeks and was progressive. The patient presented frequent passage of stool with small amounts of blood, abdominal pain and noticeable weight loss.
Wall thickening of the entire colon with loss of multilayer pattern and marked doppler signal associated with "comb sign" with parallel mesenteric vessels as a sign of severe inflammation (Limberg Score 4). It is accompanied by creeping fat and ulcers.. Histologically report of colonoscopic biopsies. The mucosal surface is irregular, with edema, interstitial hemorrhage, and inflammatory ...
In a recent study conducted at the Wuhan Inflammatory Bowel ... In this report, we present a case of ulcerative colitis diagnosed following the treatment of COVID-19 pneumonia. CASE PRESENTATION. A 50-year-old male patient presented to the internal diseases outpatient clinic on April 16, 2020, with complaints of fever and shortness of breath ...
Previous metabolomic studies have found that l-arginine levels were significantly reduced in both UC patients and dextran sodium sulfate (DSS)-induced colitis mouse models. 15-17 However, dietary supplementation with l-arginine alleviated the symptoms of DSS-induced colitis, possibly due to its immunoregulatory properties. 18 Specifically, l ...
Ulcerative colitis (UC) is an idiopathic, chronic inflammatory condition of the colon, characterized by repeated attacks, a lack of effective treatment options, and significant physical and mental health complications for patients. The endoplasmic reticulum (ER) is a vital intracellular organelle in maintaining cellular homeostasis. Endoplasmic reticulum stress (ERS) is induced when the body ...
Vedolizumab Safety During Pregnancy and Lactation in a Patient with Ulcerative Colitis: A Case Report. Fernanda Patrícia Jeronymo Pinto 1 Department of Internal Medicine, São Paulo State University (Unesp ... and infant outcome: a prospective multicentre study. J Crohns Colitis. 2022;16:1808-1815. PMID: 35708729. doi:10.1093/ecco-jcc ...
A doctor may order a colonoscopy to help diagnose ulcerative colitis (UC), check for complications of UC, or see whether the condition is progressing. UC is a type of inflammatory bowel disease ...
In a retroactive, 24-month study, researchers compared key drivers of medical costs in a SonarMD engaged population of 495 patients with IBD from three New Jersey-based medical practices against a ...
Further, 47.3% and bio-naïve and 43.4% of bio-failed patients on mirikizumab achieved clinical remission at week 52 according to the Crohn's Disease Activity Index (CDAI), versus 26.5% and 12.4 ...
Ulcerative colitis (UC) is increasingly common, and it is gradually become a kind of global epidemic. UC is a type of inflammatory bowel disease (IBD), and it is a lifetime recurrent disease. UC as a common disease has become a financial burden for many people and has the potential to develop into cancer if not prevented or treated. There are multiple factors such as genetic factors, host ...
Ulcerative colitis (UC) is a chronic immune-mediated disease of the colon. The mainstay of treatment to achieve and maintain remission is 5-aminosalicylic acid (5-ASA). At least 20% of patients with UC experience an acute severe ulcerative colitis (ASUC) flare, requiring aggressive early intervention to prevent complications.