- Introduction
- Conclusions
- Article Information
BMI indicates body mass index; SES, socioeconomic status.
a Variables smoking status, SES, drinking pattern, former drinker bias only, occasional drinker bias, median age, and gender were removed.
b Variables race, diet, exercise, BMI, country, follow-up year, publication year, and unhealthy people exclusion were removed.
eAppendix. Methodology of Meta-analysis on All-Cause Mortality and Alcohol Consumption
eReferences
eFigure 1. Flowchart of Systematic Search Process for Studies of Alcohol Consumption and Risk of All-Cause Mortality
eTable 1. Newly Included 20 Studies (194 Risk Estimates) of All-Cause Mortality and Consumption in 2015 to 2022
eFigure 2. Funnel Plot of Log-Relative Risk (In(RR)) of All-Cause Mortality Due to Alcohol Consumption Against Inverse of Standard Error of In(RR)
eFigure 3. Relative Risk (95% CI) of All-Cause Mortality Due to Any Alcohol Consumption Without Any Adjustment for Characteristics of New Studies Published between 2015 and 2022
eFigure 4. Unadjusted, Partially Adjusted, and Fully Adjusted Relative Risk (RR) of All-Cause Mortality for Drinkers (vs Nondrinkers), 1980 to 2022
eTable 2. Statistical Analysis of Unadjusted Mean Relative Risk (RR) of All-Cause Mortality for Different Categories of Drinkers for Testing Publication Bias and Heterogeneity of RR Estimates From Included Studies
eTable 3. Mean Relative Risk (RR) Estimates of All-Cause Mortality Due to Alcohol Consumption up to 2022 for Subgroups (Cohorts Recruited 50 Years of Age or Younger and Followed up to 60 Years of Age)
Data Sharing Statement
- Errors in Figure and Supplement JAMA Network Open Correction May 9, 2023

See More About
Sign up for emails based on your interests, select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Get the latest research based on your areas of interest.
Others also liked.
- Download PDF
- X Facebook More LinkedIn
Zhao J , Stockwell T , Naimi T , Churchill S , Clay J , Sherk A. Association Between Daily Alcohol Intake and Risk of All-Cause Mortality : A Systematic Review and Meta-analyses . JAMA Netw Open. 2023;6(3):e236185. doi:10.1001/jamanetworkopen.2023.6185
Manage citations:
© 2024
- Permissions
Association Between Daily Alcohol Intake and Risk of All-Cause Mortality : A Systematic Review and Meta-analyses
- 1 Canadian Institute for Substance Use Research, University of Victoria, Victoria, British Columbia, Canada
- 2 Department of Psychology, University of Portsmouth, Portsmouth, Hampshire, United Kingdom
- Correction Errors in Figure and Supplement JAMA Network Open
Question What is the association between mean daily alcohol intake and all-cause mortality?
Findings This systematic review and meta-analysis of 107 cohort studies involving more than 4.8 million participants found no significant reductions in risk of all-cause mortality for drinkers who drank less than 25 g of ethanol per day (about 2 Canadian standard drinks compared with lifetime nondrinkers) after adjustment for key study characteristics such as median age and sex of study cohorts. There was a significantly increased risk of all-cause mortality among female drinkers who drank 25 or more grams per day and among male drinkers who drank 45 or more grams per day.
Meaning Low-volume alcohol drinking was not associated with protection against death from all causes.
Importance A previous meta-analysis of the association between alcohol use and all-cause mortality found no statistically significant reductions in mortality risk at low levels of consumption compared with lifetime nondrinkers. However, the risk estimates may have been affected by the number and quality of studies then available, especially those for women and younger cohorts.
Objective To investigate the association between alcohol use and all-cause mortality, and how sources of bias may change results.
Data Sources A systematic search of PubMed and Web of Science was performed to identify studies published between January 1980 and July 2021.
Study Selection Cohort studies were identified by systematic review to facilitate comparisons of studies with and without some degree of controls for biases affecting distinctions between abstainers and drinkers. The review identified 107 studies of alcohol use and all-cause mortality published from 1980 to July 2021.
Data Extraction and Synthesis Mixed linear regression models were used to model relative risks, first pooled for all studies and then stratified by cohort median age (<56 vs ≥56 years) and sex (male vs female). Data were analyzed from September 2021 to August 2022.
Main Outcomes and Measures Relative risk estimates for the association between mean daily alcohol intake and all-cause mortality.
Results There were 724 risk estimates of all-cause mortality due to alcohol intake from the 107 cohort studies (4 838 825 participants and 425 564 deaths available) for the analysis. In models adjusting for potential confounding effects of sampling variation, former drinker bias, and other prespecified study-level quality criteria, the meta-analysis of all 107 included studies found no significantly reduced risk of all-cause mortality among occasional (>0 to <1.3 g of ethanol per day; relative risk [RR], 0.96; 95% CI, 0.86-1.06; P = .41) or low-volume drinkers (1.3-24.0 g per day; RR, 0.93; P = .07) compared with lifetime nondrinkers. In the fully adjusted model, there was a nonsignificantly increased risk of all-cause mortality among drinkers who drank 25 to 44 g per day (RR, 1.05; P = .28) and significantly increased risk for drinkers who drank 45 to 64 and 65 or more grams per day (RR, 1.19 and 1.35; P < .001). There were significantly larger risks of mortality among female drinkers compared with female lifetime nondrinkers (RR, 1.22; P = .03).
Conclusions and Relevance In this updated systematic review and meta-analysis, daily low or moderate alcohol intake was not significantly associated with all-cause mortality risk, while increased risk was evident at higher consumption levels, starting at lower levels for women than men.
The proposition that low-dose alcohol use protects against all-cause mortality in general populations continues to be controversial. 1 Observational studies tend to show that people classified as “moderate drinkers” have longer life expectancy and are less likely to die from heart disease than those classified as abstainers. 2 Systematic reviews and meta-analyses of this literature 3 confirm J-shaped risk curves (protective associations at low doses with increasing risk at higher doses). However, mounting evidence suggests these associations might be due to systematic biases that affect many studies. For example, light and moderate drinkers are systematically healthier than current abstainers on a range of health indicators unlikely to be associated with alcohol use eg, dental hygiene, exercise routines, diet, weight, income 4 ; lifetime abstainers may be systematically biased toward poorer health 5 ; studies fail to control for biases in the abstainer reference group, in particular failing to remove “sick quitters” or former drinkers, many of whom cut down or stop for health reasons 2 ; and most studies have nonrepresentative samples leading to an overrepresentation of older White men. Adjustment of cohort samples to make them more representative has been shown to eliminate apparent protective associations. 6 Mendelian randomization studies that control for the confounding effects of sociodemographic and environmental factors find no evidence of cardioprotection. 7
We published 2 previous systematic reviews and meta-analyses that investigated these hypotheses. The first of these focused on all-cause mortality, 8 finding negligible reductions in mortality risk with low-volume alcohol use when study-level controls were introduced for potential bias and confounding, such as the widespread practice of misclassifying former drinkers and/or current occasional drinkers as abstainers (ie, not restricting reference groups to lifetime abstainers). 8 Our alcohol and coronary heart disease (CHD) mortality meta-analysis of 45 cohort studies 9 found that CHD mortality risk differed widely by age ranges and sex of study populations. In particular, young cohorts followed up to old age did not show significant cardio-protection for low-volume use. Cardio-protection was only apparent among older cohorts that are more exposed to lifetime selection biases (ie, increasing numbers of “sick-quitters” in the abstainer reference groups and the disproportionate elimination of drinkers from the study sample who had died or were unwell).
The present study updates our earlier systematic review and meta-analysis for all-cause mortality and alcohol use, 8 including studies published up to July 2021 (ie, 6.5 years of additional publications). The study also investigated the risk of all-cause mortality for alcohol consumption according to (1) median ages of the study populations (younger than 56 years or 56 years and older), replicating the methods of Zhao et al 9 ; (2) the sex distribution of the study populations, and (3) studies of cohorts recruited before a median age of 51 years of age and followed up in health records until a median age of at least 60 years (ie, with stricter rules to further minimize lifetime selection biases). Because younger cohorts followed up to an age at which they may experience heart disease are less likely to be affected by lifetime selection biases, 9 we hypothesized that such studies would be less likely to show reduced mortality risks for low-volume drinkers. Finally, we reran the analyses using occasional drinkers (<1 drink per week) as the reference, for whom physiological health benefits are unlikely. Occasional drinkers are a more appropriate reference group, given evidence demonstrating that lifetime abstainers may be biased toward ill health. 10
The present study updates the systematic reviews and meta-analyses described above 8 by including studies published up to July 2021 to investigate whether the risk differed for subgroups. The study protocol was preregistered on the Open Science Framework. 11 Inclusion criteria, search strategy, study selection, data extraction, and statistical analytical methods of the study are summarized in later sections (see eAppendix in Supplement 1 for more details).
The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses ( PRISMA ) reporting guideline. 12 The review sought cohort studies of all-cause mortality and alcohol consumption. We identified all potentially relevant articles published up to July 31, 2021, regardless of language, by searching PubMed and Web of Science, through reference list cross-checking of previous meta-analyses (eFigure 1 in Supplement 1 ). There were 87 studies identified by Stockwell et al. 8 After inclusion of 20 new studies meeting inclusion criteria, there were a total of 107 cohort studies (eTable 1 in Supplement 1 ). 13 - 32
Three coders (J. Z., F. A., and J. C.) reviewed all eligible studies to extract and code data independently from all studies fulfilling the inclusion criteria. Data extracted included (1) outcome, all-cause mortality; (2) measures of alcohol consumption; (3) study characteristics, including cohort ages at recruitment and follow-up; (4) types of misclassification error of alcohol consumers and abstainers; (5) controlled variables in individual studies. Alcoholic drinks were converted into grams per day according to country-specific definitions if not otherwise defined. 33 , 34
We also assessed publication bias, heterogeneity, and confounding of covariates that might potentially affect the association of interest using several statistical approaches. 35 - 41 Relative risk (RR), including hazard ratios or rate ratios, were converted to natural log-transformed formats to deal with skewness. Publication bias was assessed through visual inspection of the funnel plot of log-RR of all-cause mortality due to alcohol consumption against the inverse standard error of log-RR 42 and Egger’s linear regression method. 36 We also plotted forest graphs of log-RR of all-cause mortality for any level of drinking to assess heterogeneity among studies. 42 The between-study heterogeneity of RRs were assessed using Cochran Q 37 and the I 2 statistic. 38 If heterogeneity was detected, mixed-effects models were used to obtain the summarized RR estimates. Mixed-effects regression analyses were performed in which drinking groups and control variables were treated as fixed-effects with a random study effect because of significant heterogeneity. 43
All analyses were weighted by the inverse of the estimated variance of the natural log relative risk. Variance was estimated from reported standard errors, confidence intervals, or number of deaths. The weights for each individual study were created using the inverse variance weight scheme and used in mixed regression analysis to get maximum precision for the main results of the meta-analysis. 42 In comparison with lifetime abstainers, the study estimated the mean RR of all-cause mortality for former drinkers (ie, now completely abstaining), current occasional (<9.1 g per week), low-volume (1.3-24.0 g per day), medium-volume (25.0-44.0 g per day), high-volume (45.0-64.0 g) and highest-volume drinkers (≥65.0 grams per day). The analyses adjusted for the potential confounding effects of study characteristics including the median age and sex distribution of study samples, drinker biases, country where a study was conducted, follow-up years and presence or absence of confounders. Analyses were also repeated using occasional drinkers as the reference group. We used t tests to calculate P values, and significance was set at .05. All statistical analyses were performed using SAS version 9.4 (SAS Institute) and the SAS MIXED procedure was used to model the log-transformed RR. 44 Data were analyzed from September 2021 to August 2022.
There were 724 estimates of the risk relationship between level of alcohol consumption and all-cause mortality from 107 unique studies 13 - 32 , 45 - 131 , including 4 838 825 participants and 425 564 deaths available for the analysis. Table 1 describes the sample characteristics of the metadata. Of 39 studies 13 , 15 , 18 , 21 , 23 - 26 , 29 , 31 , 45 - 47 , 49 , 50 , 52 - 54 , 57 - 59 , 62 , 64 , 70 , 80 , 81 , 85 , 87 , 91 , 94 , 96 , 100 , 104 , 107 , 118 , 124 , 125 , 127 , 130 reporting RR estimates for men and women separately, 33 14 , 17 , 48 , 51 , 61 , 63 , 66 , 68 , 69 , 72 , 76 , 79 , 83 , 84 , 86 , 88 , 90 , 92 , 93 , 97 , 98 , 101 , 103 , 105 , 109 - 111 , 113 - 115 , 119 , 120 , 128 were for males only, 8 16 , 65 , 73 , 99 , 102 , 108 , 112 , 123 for females only, and 30 13 , 19 - 22 , 26 - 30 , 32 , 55 , 56 , 67 , 71 , 74 , 75 , 77 , 78 , 82 , 84 , 89 , 95 , 106 , 116 , 117 , 121 , 122 , 126 , 129 for both sexes. Twenty-one studies 13 , 17 , 19 , 21 , 22 , 26 , 27 , 45 - 58 (220 risk estimates) were free from abstainer bias (ie, had a reference group of strictly defined lifetime abstainers). There were 50 studies 14 - 16 , 18 , 20 , 23 - 25 , 29 , 59 - 99 (265 risk estimates) with both former and occasional drinker bias; 28 studies 28 , 30 - 32 , 100 - 122 , 130 (177 risk estimates) with only former drinker bias; and 8 studies 123 - 129 , 131 (62 risk estimates) with only occasional drinker bias.
Unadjusted mean RR estimates for most study subgroups categorized by methods/sample characteristics showed markedly or significantly higher RRs for alcohol consumers as a group vs abstainers. Exceptions were for studies with less than 10 years of follow-up and those with some form of abstainer bias ( Table 1 ). Bivariable analyses showed that mortality risks for alcohol consumers varied considerably according to other study characteristics, such as quality of the alcohol consumption measure, whether unhealthy individuals were excluded at baseline, and whether socioeconomic status was controlled for ( Table 1 ).
No evidence of publication bias was detected either by inspection of symmetry in the funnel plot of log-RR estimates and their inverse standard errors (eFigure 2 in Supplement 1 ) or by Egger linear regression analysis (eTable 2 in Supplement 1 , all P > .05 for each study group). Significant heterogeneity was observed across studies for all drinking categories confirmed by both the Q statistic ( Q 723 = 5314.80; P < .001) and I 2 estimates (all >85.87%). (See eFigure 3 in Supplement 1 for forest plot of unadjusted risk estimates of mortality risks for the 20 newly identified studies).
Pooled unadjusted estimates (724 observations) showed significantly higher risk for former drinkers (RR, 1.22; 95% CI, 1.11-1.33; P = .001) and significantly lower risk for low-volume drinkers (RR, 0.85; 95% CI, 0.81-0.88; P = .001) compared with abstainers as defined in the included studies ( Table 2 ; eFigure 4 in Supplement 1 ). In the fully adjusted model, mortality RR estimates increased for all drinking categories, becoming nonsignificant for low-volume drinkers (RR, 0.93; 95% CI, 0.85-1.01; P = .07), occasional drinkers (>0 to <1.3 g of ethanol per day; RR, 0.96; 95% CI, 0.86-1.06; P = .41), and drinkers who drank 25 to 44 g per day (RR, 1.05; 95% CI, 0.96-1.14; P = .28). There was a significantly increased risk among drinkers who drank 45 to 64 g per day (RR, 1.19; 95% CI, 1.07-1.32; P < .001) and 65 or more grams (RR, 1.35; 95% CI, 1.23-1.47; P < .001). The Figure shows the changes in RR estimates for low-volume drinkers when removing each covariate from the fully adjusted model. In most cases, removing study-level covariates tended to yield lower risk estimates from alcohol use.
Table 2 presents the RR estimates when occasional drinkers were the reference group. In fully adjusted models, higher though nonsignificant mortality risks were observed for both abstainers and medium-volume drinkers (RR, 1.04; 95% CI, 0.94-1.16; P = .44 and RR, 1.09; 95% CI, 0.96-1.25; P = .19, respectively). There were significantly elevated risks for both high and higher volume drinkers (RR, 1.24; 95% CI, 1.07-1.44; P = .004 and RR, 1.41; 95% CI, 1.23-1.61; . P = 001, respectively).
As hypothesized, there was a significant interaction between cohort age and mortality risk ( P = .02; F 601 = 2.93) and so RR estimates for drinkers were estimated in analyses stratified by median age of the study populations at enrollment ( Table 3 ). In unadjusted and partially adjusted analyses, older cohorts displayed larger reductions in mortality risk associated with low-volume consumption than younger cohorts. However, in fully adjusted analyses with multiple covariates included for study characteristics, these differences disappeared. Younger cohorts also displayed greater mortality risks than older cohorts at higher consumption levels. Among studies in which participants were recruited at age 50 years or younger and followed up to age 60 years (ie, there was likely reduced risk of lifetime selection bias) higher RR estimates were observed for all drinking groups vs lifetime abstainers. These differences were significant in all drinking groups except low-volume drinkers (eTable 3 in Supplement 1 ).
Across all levels of alcohol consumption, female drinkers had a higher RR of all-cause mortality than males ( P for interaction = .001). As can be seen in Table 4 , all female drinkers had a significantly increased mortality risk compared with female lifetime nondrinkers (RR, 1.22; 95% CI, 1.02-1.46; P = .03). Compared with lifetime abstainers, there was significantly increased risk of all-cause mortality among male drinkers who drank 45 to 64 g per day (RR, 1.15; 95% CI, 1.03-1.28; P = .01) and drank 65 or more (RR, 1.34; 95% CI, 1.23-1.47; P < .001), and among female drinkers who drank 25 to 44 g per day (RR, 1.21; 95% CI, 1.08-1.36; P < .01), 45 to 64 g (RR, 1.34; 95% CI, 1.11-1.63; P < .01) and 65 or more grams (RR, 1.61; 95% CI, 1.44-1.80; P = .001).
In fully adjusted, prespecified models that accounted for effects of sampling, between-study variation, and potential confounding from former drinker bias and other study-level covariates, our meta-analysis of 107 studies found (1) no significant protective associations of occasional or low-volume drinking (moderate drinking) with all-cause mortality; and (2) an increased risk of all-cause mortality for drinkers who drank 25 g or more and a significantly increased risk when drinking 45 g or more per day.
Several meta-analytic strategies were used to explore the role of abstainer reference group biases caused by drinker misclassification errors and also the potential confounding effects of other study-level quality covariates in studies. 2 Drinker misclassification errors were common. Of 107 studies identified, 86 included former drinkers and/or occasional drinkers in the abstainer reference group, and only 21 were free of both these abstainer biases. The importance of controlling for former drinker bias/misclassification is highlighted once more in our results which are consistent with prior studies showing that former drinkers have significantly elevated mortality risks compared with lifetime abstainers.
In addition to presenting our fully adjusted models, a strength of the study was the examination of the differences in relative risks according to unadjusted and partially adjusted models, including the effect of removing individual covariates from the fully adjusted model. We found evidence that abstainer biases and other study characteristics changed the shape of the risk relationship between mortality and rising alcohol consumption, and that most study-level controls increased the observed risks from alcohol, or attenuated protective associations at low levels of consumption such that they were no longer significant. The reduced RR estimates for occasional or moderate drinkers observed without adjustment may be due to the misclassification of former and occasional drinkers into the reference group, a possibility which is more likely to have occurred in studies of older cohorts which use current abstainers as the reference group. This study also demonstrates the degree to which observed associations between consumption and mortality are highly dependent on the modeling strategy used and the degree to which efforts are made to minimize confounding and other threats to validity.
It also examined risk estimates when using occasional drinkers rather than lifetime abstainers as the reference group. The occasional drinker reference group avoids the issue of former drinker misclassification that can affect the abstainer reference group, and may reduce confounding to the extent that occasional drinkers are more like low-volume drinkers than are lifetime abstainers. 2 , 8 , 132 In the unadjusted and partially adjusted analyses, using occasional drinkers as the reference group resulted in nonsignificant protective associations and lower point estimates for low-volume drinkers compared with significant protective associations and higher point estimates when using lifetime nondrinkers as the reference group. In the fully adjusted models, there were nonsignificant protective associations for low-volume drinkers whether using lifetime abstainers or occasional drinkers as the reference group, though this was only a RR of 0.97 for the latter.
Across all studies, there were few differences in risk for studies when stratified by median age of enrollment above or below age 56 years in the fully adjusted analyses. However, in the subset of studies who enrolled participants aged 50 years or younger who were followed for at least 10 years, occasional drinkers and medium-volume drinkers had significantly increased risk of mortality and substantially higher risk estimates for high- and higher-volume consumption compared with results from all studies. This is consistent with our previous meta-analysis for CHD, 9 in which younger cohorts followed up to older age did not show a significantly beneficial association of low-volume consumption, while older cohorts, with more opportunity for lifetime selection bias, showed marked, significant protective associations.
Our study also found sex differences in the risk of all-cause mortality. A larger risk of all-cause mortality for women than men was observed when drinking 25 or more grams per day, including a significant increase in risk for medium-level consumption for women that was not observed for men. However, mortality risk for mean consumption up to 25 g per day were very similar for both sexes.
A number of limitations need to be acknowledged. A major limitation involves imperfect measurement of alcohol consumption in most included studies, and the fact that consumption in many studies was assessed at only 1 point in time. Self-reported alcohol consumption is underreported in most epidemiological studies 133 , 134 and even the classification of drinkers as lifetime abstainers can be unreliable, with several studies in developed countries finding that the majority of self-reported lifetime abstainers are in fact former drinkers. 135 , 136 If this is the case, the risks of various levels of alcohol consumption relative to presumed lifetime abstainers are underestimates. Merely removing former drinkers from analyses may bias studies in favor of drinkers, since former drinkers may be unhealthy, and should rightly be reallocated to drinking groups according to their history. However, this has only been explored in very few studies. Our study found that mortality risk differed significantly by cohort age and sex. It might be that the risk is also higher for other subgroups, such as people living with HIV, 137 a possibility future research should investigate.
The number of available studies in some stratified analyses was small, so there may be limited power to control for potential study level confounders. However, the required number of estimates per variable for linear regression can be much smaller than in logistic regression, and a minimum of at least 2 estimates per variable is recommended for linear regression analysis, 138 suggesting the sample sizes were adequate in all models presented. It has been demonstrated that a pattern of binge (ie, heavy episodic) drinking removes the appearance of reduced health risks even when mean daily volume is low. 139 Too few studies adequately controlled for this variable to investigate its association with different outcomes across studies. Additionally, our findings only apply to the net effect of alcohol at different doses on all-cause mortality, and different risk associations likely apply for specific disease categories. The biases identified here likely apply to estimates of risk for alcohol and all diseases. It is likely that correcting for these biases will raise risk estimates for many types of outcome compared with most existing estimates.
This updated meta-analysis did not find significantly reduced risk of all-cause mortality associated with low-volume alcohol consumption after adjusting for potential confounding effects of influential study characteristics. Future longitudinal studies in this field should attempt to minimize lifetime selection biases by not including former and occasional drinkers in the reference group, and by using younger cohorts (ie, age distributions that are more representative of drinkers in the general population) at baseline.
Accepted for Publication: February 17, 2023.
Published: March 31, 2023. doi:10.1001/jamanetworkopen.2023.6185
Correction: This article was corrected on May 9, 2023, to fix errors in the Figure and Supplement.
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2023 Zhao J et al. JAMA Network Open .
Corresponding Author: Jinhui Zhao, PhD, Canadian Institute for Substance Use Research, University of Victoria, PO Box 1700 STN CSC, Victoria, BC V8Y 2E4, Canada ( [email protected] ).
Author Contributions: Drs Zhao and Stockwell had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Zhao, Stockwell, Naimi, Churchill, Sherk.
Acquisition, analysis, or interpretation of data: Zhao, Stockwell, Naimi, Clay.
Drafting of the manuscript: Zhao, Stockwell, Clay.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Zhao, Churchill.
Obtained funding: Zhao, Stockwell, Sherk.
Administrative, technical, or material support: Zhao, Stockwell, Naimi.
Supervision: Zhao, Stockwell, Naimi.
Conflict of Interest Disclosures: Dr Stockwell reported receiving personal fees from Ontario Public Servants Employees Union for expert witness testimony and personal fees from Alko outside the submitted work. Dr Sherk reported receiving grants from Canadian Centre on Substance Use and Addiction (CCSA) during the conduct of the study. No other disclosures were reported.
Funding/Support: This study was partly funded by the CCSA as a subcontract for a Health Canada grant to develop guidance for Canadians on alcohol and health.
Role of the Funder/Sponsor: Health Canada had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. CCSA staff conducted a preliminary search to identify potentially relevant articles but did not participate in decisions about inclusion/exclusion of studies, coding, analysis, interpretation of results or approving the final manuscript.
Data Sharing Statement: See Supplement 2 .
Additional Contributions: We gratefully acknowledge contributions by Christine Levesque, PhD (CCSA), and Nitika Sanger, PhD (CCSA), who conducted a preliminary literature search for potentially relevant articles. We also acknowledge the leadership of Drs Catherine Paradis, PhD (CCSA), and Peter Butt, MD (University of Saskatchewan), who cochaired the process of developing Canada’s new guidance on alcohol and health, a larger project which contributed some funds for the work undertaken for this study. We are grateful to Fariha Alam, MPH (Canadian Institute for Substance Use and Research), for her help coding the studies used in this study. None of them received any compensation beyond their normal salaries for this work.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Open access
- Published: 13 November 2019
Evidence-based models of care for the treatment of alcohol use disorder in primary health care settings: protocol for systematic review
- Susan A. Rombouts 1 ,
- James Conigrave 2 ,
- Eva Louie 1 ,
- Paul Haber 1 , 3 &
- Kirsten C. Morley ORCID: orcid.org/0000-0002-0868-9928 1
Systematic Reviews volume 8 , Article number: 275 ( 2019 ) Cite this article
7222 Accesses
3 Citations
Metrics details
Alcohol use disorder (AUD) is highly prevalent and accounts globally for 1.6% of disability-adjusted life years (DALYs) among females and 6.0% of DALYs among males. Effective treatments for AUDs are available but are not commonly practiced in primary health care. Furthermore, referral to specialized care is often not successful and patients that do seek treatment are likely to have developed more severe dependence. A more cost-efficient health care model is to treat less severe AUD in a primary care setting before the onset of greater dependence severity. Few models of care for the management of AUD in primary health care have been developed and with limited implementation. This proposed systematic review will synthesize and evaluate differential models of care for the management of AUD in primary health care settings.
We will conduct a systematic review to synthesize studies that evaluate the effectiveness of models of care in the treatment of AUD in primary health care. A comprehensive search approach will be conducted using the following databases; MEDLINE (1946 to present), PsycINFO (1806 to present), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL) (1991 to present), and Embase (1947 to present).
Reference searches of relevant reviews and articles will be conducted. Similarly, a gray literature search will be done with the help of Google and the gray matter tool which is a checklist of health-related sites organized by topic. Two researchers will independently review all titles and abstracts followed by full-text review for inclusion. The planned method of extracting data from articles and the critical appraisal will also be done in duplicate. For the critical appraisal, the Cochrane risk of bias tool 2.0 will be used.
This systematic review and meta-analysis aims to guide improvement of design and implementation of evidence-based models of care for the treatment of alcohol use disorder in primary health care settings. The evidence will define which models are most promising and will guide further research.
Protocol registration number
PROSPERO CRD42019120293.
Peer Review reports
It is well recognized that alcohol use disorders (AUD) have a damaging impact on the health of the population. According to the World Health Organization (WHO), 5.3% of all global deaths were attributable to alcohol consumption in 2016 [ 1 ]. The 2016 Global Burden of Disease Study reported that alcohol use led to 1.6% (95% uncertainty interval [UI] 1.4–2.0) of total DALYs globally among females and 6.0% (5.4–6.7) among males, resulting in alcohol use being the seventh leading risk factor for both premature death and disability-adjusted life years (DALYs) [ 2 ]. Among people aged 15–49 years, alcohol use was the leading risk factor for mortality and disability with 8.9% (95% UI 7.8–9.9) of all attributable DALYs for men and 2.3% (2.0–2.6) for women [ 2 ]. AUD has been linked to many physical and mental health complications, such as coronary heart disease, liver cirrhosis, a variety of cancers, depression, anxiety, and dementia [ 2 , 3 ]. Despite the high morbidity and mortality rate associated with hazardous alcohol use, the global prevalence of alcohol use disorders among persons aged above 15 years in 2016 was stated to be 5.1% (2.5% considered as harmful use and 2.6% as severe AUD), with the highest prevalence in the European and American region (8.8% and 8.2%, respectively) [ 1 ].
Effective and safe treatment for AUD is available through psychosocial and/or pharmacological interventions yet is not often received and is not commonly practiced in primary health care. While a recent European study reported 8.7% prevalence of alcohol dependence in primary health care populations [ 4 ], the vast majority of patients do not receive the professional treatment needed, with only 1 in 5 patients with alcohol dependence receiving any formal treatment [ 4 ]. In Australia, it is estimated that only 3% of individuals with AUD receive approved pharmacotherapy for the disorder [ 5 , 6 ]. Recognition of AUD in general practice uncommonly leads to treatment before severe medical and social disintegration [ 7 ]. Referral to specialized care is often not successful, and those patients that do seek treatment are likely to have more severe dependence with higher levels of alcohol use and concurrent mental and physical comorbidity [ 4 ].
Identifying and treating early stage AUDs in primary care settings can prevent condition worsening. This may reduce the need for more complex and more expensive specialized care. The high prevalence of AUD in primary health care and the chronic relapsing character of AUD make primary care a suitable and important location for implementing evidence-based interventions. Successful implementation of treatment models requires overcoming multiple barriers. Qualitative studies have identified several of those barriers such as limited time, limited organizational capacity, fear of losing patients, and physicians feeling incompetent in treating AUD [ 8 , 9 , 10 ]. Additionally, a recent systematic review revealed that diagnostic sensitivity of primary care physicians in the identification of AUD was 41.7% and that only in 27.3% alcohol problems were recorded correctly in primary care records [ 11 ].
Several models for primary care have been created to increase identification and treatment of patients with AUD. Of those, the model, screening, brief interventions, and referral to specialized treatment for people with severe AUD (SBIRT [ 12 ]) is most well-known. Multiple systematic reviews exist, confirming its effectiveness [ 13 , 14 , 15 ], although implementation in primary care has been inadequate. Moreover, most studies have looked primarily at SBIRT for the treatment of less severe AUD [ 16 ]. In the treatment of severe AUD, efficacy of SBIRT is limited [ 16 ]. Additionally, many patient referred to specialized care often do not attend as they encounter numerous difficulties in health care systems including stigmatization, costs, lack of information about existing treatments, and lack of non-abstinence-treatment goals [ 7 ]. An effective model of care for improved management of AUD that can be efficiently implemented in primary care settings is required.
Review objective
This proposed systematic review will synthesize and evaluate differential models of care for the management of AUD in primary health care settings. We aim to evaluate the effectiveness of the models of care in increasing engagement and reducing alcohol consumption.
By providing this overview, we aim to guide improvement of design and implementation of evidence-based models of care for the treatment of alcohol use disorder in primary health care settings.
The systematic review is registered in PROSPERO international prospective register of systematic reviews (CRD42019120293) and the current protocol has been written according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) recommended for systematic reviews [ 17 ]. A PRISMA-P checklist is included as Additional file 1 .
Eligibility criteria
Criteria for considering studies for this review are classified by the following:
Study design
Both individualized and cluster randomized trials will be included. Masking of patients and/or physicians is not an inclusion criterion as it is often hard to accomplish in these types of studies.
Patients in primary health care who are identified (using screening tools or by primary health care physician) as suffering from AUD (from mild to severe) or hazardous alcohol drinking habits (e.g., comorbidity, concurrent medication use). Eligible patients need to have had formal assessment of AUD with diagnostic tools such as Diagnostic and Statistical Manual of Mental Disorders (DSM-IV/V) or the International Statistical Classification of Diseases and Related Health Problems (ICD-10) and/or formal assessment of hazardous alcohol use assessed by the Comorbidity Alcohol Risk Evaluation Tool (CARET) or the Alcohol Use Disorders Identification test (AUDIT) and/or alcohol use exceeding guideline recommendations to reduce health risks (e.g., US dietary guideline (2015–2020) specifies excessive drinking for women as ≥ 4 standard drinks (SD) on any day and/or ≥ 8 SD per week and for men ≥ 5 SD on any day and/or ≥ 15 SD per week).
Studies evaluating models of care for additional diseases (e.g., other dependencies/mental health) other than AUD are included when they have conducted data analysis on the alcohol use disorder patient data separately or when 80% or more of the included patients have AUD.
Intervention
The intervention should consist of a model of care; therefore, it should include multiple components and cover different stages of the care pathway (e.g., identification of patients, training of staff, modifying access to resources, and treatment). An example is the Chronic Care Model (CCM) which is a primary health care model designed for chronic (relapsing) conditions and involves six elements: linkage to community resources, redesign of health care organization, self-management support, delivery system redesign (e.g., use of non-physician personnel), decision support, and the use of clinical information systems [ 18 , 19 ].
As numerous articles have already assessed the treatment model SBIRT, this model of care will be excluded from our review unless the particular model adds a specific new aspect. Also, the article has to assess the effectiveness of the model rather than assessing the effectiveness of the particular treatment used. Because identification of patients is vital to including them in the trial, a care model that only evaluates either patient identification or treatment without including both will be excluded from this review.
Model effectiveness may be in comparison with the usual care or a different treatment model.
Included studies need to include at least one of the following outcome measures: alcohol consumption, treatment engagement, uptake of pharmacological agents, and/or quality of life.
Solely quantitative research will be included in this systematic review (e.g., randomized controlled trials (RCTs) and cluster RCTs). We will only include peer-reviewed articles.
Restrictions (language/time period)
Studies published in English after 1 January 1998 will be included in this systematic review.
Studies have to be conducted in primary health care settings as such treatment facilities need to be physically in or attached to the primary care clinic. Examples are co-located clinics, veteran health primary care clinic, hospital-based primary care clinic, and community primary health clinics. Specialized primary health care clinics such as human immunodeficiency virus (HIV) clinics are excluded from this systematic review. All studies were included, irrespective of country of origin.
Search strategy and information sources
A comprehensive search will be conducted. The following databases will be consulted: MEDLINE (1946 to present), PsycINFO (1806 to present), Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials (CENTRAL) (1991 to present), and Embase (1947 to present). Initially, the search terms will be kept broad including alcohol use disorder (+synonyms), primary health care, and treatment to minimize the risk of missing any potentially relevant articles. Depending on the number of references attained by this preliminary search, we will add search terms referring to models such as models of care, integrated models, and stepped-care models, to limit the number of articles. Additionally, we will conduct reference searches of relevant reviews and articles. Similarly, a gray literature search will be done with the help of Google and the Gray Matters tool which is a checklist of health-related sites organized by topic. The tool is produced by the Canadian Agency for Drugs and Technologies in Health (CADTH) [ 20 ].
See Additional file 2 for a draft of our search strategy in MEDLINE.
Data collection
The selection of relevant articles is based on several consecutive steps. All references will be managed using EndNote (EndNote version X9 Clarivate Analytics). Initially, duplicates will be removed from the database after which all the titles will be screened with the purpose of discarding clearly irrelevant articles. The remaining records will be included in an abstract and full-text screen. All steps will be done independently by two researchers. Disagreement will lead to consultation of a third researcher.
Data extraction and synthesis
Two researchers will extract data from included records. At the conclusion of data extraction, these two researchers will meet with the lead author to resolve any discrepancies.
In order to follow a structured approach, an extraction form will be used. Key elements of the extraction form are information about design of the study (randomized, blinded, control), type of participants (alcohol use, screening tool used, socio-economic status, severity of alcohol use, age, sex, number of participants), study setting (primary health care setting, VA centers, co-located), type of intervention/model of care (separate elements of the models), type of health care worker (primary, secondary (co-located)), duration of follow-up, outcome measures used in the study, and funding sources. We do not anticipate having sufficient studies for a meta-analysis. As such, we plan to perform a narrative synthesis. We will synthesize the findings from the included articles by cohort characteristics, differential aspects of the intervention, controls, and type of outcome measures.
Sensitivity analyses will be conducted when issues suitable for sensitivity analysis are identified during the review process (e.g., major differences in quality of the included articles).
Potential meta-analysis
In the event that sufficient numbers of effect sizes can be extracted, a meta-analytic synthesis will be performed. We will extract effect sizes from each study accordingly. Two effect sizes will be extracted (and transformed where appropriate). Categorical outcomes will be given in log odds ratios and continuous measures will be converted into standardized mean differences. Variation in effect sizes attributable to real differences (heterogeneity) will be estimated using the inconsistency index ( I 2 ) [ 21 , 22 ]. We anticipate high degrees of variation among effect sizes, as a result moderation and subgroup-analyses will be employed as appropriate. In particular, moderation analysis will focus on the degree of heterogeneity attributable to differences in cohort population (pre-intervention drinking severity, age, etc.), type of model/intervention, and study quality. We anticipate that each model of care will require a sub-group analysis, in which case a separate meta-analysis will be performed for each type of model. Small study effect will be assessed with funnel plots and Egger’s symmetry tests [ 23 ]. When we cannot obtain enough effect sizes for synthesis or when the included studies are too diverse, we will aim to illustrate patterns in the data by graphical display (e.g., bubble plot) [ 24 ].
Critical appraisal of studies
All studies will be critically assessed by two researchers independently using the Revised Cochrane risk-of-bias tool (RoB 2) [ 25 ]. This tool facilitates systematic assessment of the quality of the article per outcome according to the five domains: bias due to (1) the randomization process, (2) deviations from intended interventions, (3) missing outcome data, (4) measurement of the outcome, and (5) selection of the reported results. An additional domain 1b must be used when assessing the randomization process for cluster-randomized studies.
Meta-biases such as outcome reporting bias will be evaluated by determining whether the protocol was published before recruitment of patients. Additionally, trial registries will be checked to determine whether the reported outcome measures and statistical methods are similar to the ones described in the registry. The gray literature search will be of assistance when checking for publication bias; however, completely eliminating the presence of publication bias is impossible.
Similar to article selection, any disagreement between the researchers will lead to discussion and consultation of a third researcher. The strength of the evidence will be graded according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach [ 26 ].
The primary outcome measure of this proposed systematic review is the consumption of alcohol at follow-up. Consumption of alcohol is often quantified in drinking quantity (e.g., number of drinks per week), drinking frequency (e.g., percentage of days abstinent), binge frequency (e.g., number of heavy drinking days), and drinking intensity (e.g., number of drinks per drinking day). Additionally, outcomes such as percentage/proportion included patients that are abstinent or considered heavy/risky drinkers at follow-up. We aim to report all these outcomes. The consumption of alcohol is often self-reported by patients. When studies report outcomes at multiple time points, we will consider the longest follow-up of individual studies as a primary outcome measure.
Depending on the included studies, we will also consider secondary outcome measures such as treatment engagement (e.g., number of visits or pharmacotherapy uptake), economic outcome measures, health care utilization, quality of life assessment (physical/mental), alcohol-related problems/harm, and mental health score for depression or anxiety.
This proposed systematic review will synthesize and evaluate differential models of care for the management of AUD in primary health care settings.
Given the complexities of researching models of care in primary care and the paucity of a focus on AUD treatment, there are likely to be only a few studies that sufficiently address the research question. Therefore, we will do a preliminary search without the search terms for model of care. Additionally, the search for online non-academic studies presents a challenge. However, the Gray Matters tool will be of guidance and will limit the possibility of missing useful studies. Further, due to diversity of treatment models, outcome measures, and limitations in research design, it is possible that a meta-analysis for comparative effectiveness may not be appropriate. Moreover, in the absence of large, cluster randomized controlled trials, it will be difficult to distinguish between the effectiveness of the treatment given and that of the model of care and/or implementation procedure. Nonetheless, we will synthesize the literature and provide a critical evaluation of the quality of the evidence.
This review will assist the design and implementation of models of care for the management of AUD in primary care settings. This review will thus improve the management of AUD in primary health care and potentially increase the uptake of evidence-based interventions for AUD.
Availability of data and materials
Not applicable.
Abbreviations
Alcohol use disorder
Alcohol Use Disorders Identification test
Canadian Agency for Drugs and Technologies in Health
The Comorbidity Alcohol Risk Evaluation
Cochrane Central Register of Controlled Trials
Diagnostic and Statistical Manual of Mental Disorders
Human immunodeficiency virus
10 - International Statistical Classification of Diseases and Related Health Problems
Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols
Screening, brief intervention, referral to specialized treatment
Standard drinks
World Health Organization
WHO. Global status report on alcohol and health: World health organization; 2018.
The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016. a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. 2018;5(12):987–1012.
Article Google Scholar
WHO. Global strategy to reduce the harmful use of alcohol: World health organization; 2010.
Rehm J, Allamani A, Elekes Z, Jakubczyk A, Manthey J, Probst C, et al. Alcohol dependence and treatment utilization in Europe - a representative cross-sectional study in primary care. BMC Fam Pract. 2015;16:90.
Morley KC, Logge W, Pearson SA, Baillie A, Haber PS. National trends in alcohol pharmacotherapy: findings from an Australian claims database. Drug Alcohol Depend. 2016;166:254–7.
Article CAS Google Scholar
Morley KC, Logge W, Pearson SA, Baillie A, Haber PS. Socioeconomic and geographic disparities in access to pharmacotherapy for alcohol dependence. J Subst Abus Treat. 2017;74:23–5.
Rehm J, Anderson P, Manthey J, Shield KD, Struzzo P, Wojnar M, et al. Alcohol use disorders in primary health care: what do we know and where do we go? Alcohol Alcohol. 2016;51(4):422–7.
Le KB, Johnson JA, Seale JP, Woodall H, Clark DC, Parish DC, et al. Primary care residents lack comfort and experience with alcohol screening and brief intervention: a multi-site survey. J Gen Intern Med. 2015;30(6):790–6.
McLellan AT, Starrels JL, Tai B, Gordon AJ, Brown R, Ghitza U, et al. Can substance use disorders be managed using the chronic care model? review and recommendations from a NIDA consensus group. Public Health Rev. 2014;35(2).
Storholm ED, Ober AJ, Hunter SB, Becker KM, Iyiewuare PO, Pham C, et al. Barriers to integrating the continuum of care for opioid and alcohol use disorders in primary care: a qualitative longitudinal study. J Subst Abus Treat. 2017;83:45–54.
Mitchell AJ, Meader N, Bird V, Rizzo M. Clinical recognition and recording of alcohol disorders by clinicians in primary and secondary care: meta-analysis. Br J Psychiatry. 2012;201:93–100.
Babor TF, Ritson EB, Hodgson RJ. Alcohol-related problems in the primary health care setting: a review of early intervention strategies. Br J Addict. 1986;81(1):23–46.
Kaner EF, Beyer F, Dickinson HO, Pienaar E, Campbell F, Schlesinger C, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev. 2007;(2):Cd004148.
O'Donnell A, Anderson P, Newbury-Birch D, Schulte B, Schmidt C, Reimer J, et al. The impact of brief alcohol interventions in primary healthcare: a systematic review of reviews. Alcohol Alcohol. 2014;49(1):66–78.
Bertholet N, Daeppen JB, Wietlisbach V, Fleming M, Burnand B. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med. 2005;165(9):986–95.
Saitz R. ‘SBIRT’ is the answer? Probably not. Addiction. 2015;110(9):1416–7.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Bmj. 2015;350:g7647.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. Jama. 2002;288(14):1775–9.
Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, part 2. Jama. 2002;288(15):1909–14.
CADTH. Grey Matters: a practical tool for searching health-related grey literature Internet. 2018 (cited 2019 Feb 22).
Higgins JPT. Thompson SG. Quantifying heterogeneity in a meta-analysis. 2002;21(11):1539–58.
Google Scholar
Higgins JPT, Thompson SG, Deeks JJ. Altman DG. Measuring inconsistency in meta-analyses. 2003;327(7414):557–60.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical research ed). 1997;315(7109):629–34.
Higgins JPT, López-López JA, Becker BJ, Davies SR, Dawson S, Grimshaw JM, et al. Synthesising quantitative evidence in systematic reviews of complex health interventions. BMJ Glob Health. 2019;4(Suppl 1):e000858–e.
Higgins, J.P.T., Sterne, J.A.C., Savović, J., Page, M.J., Hróbjartsson, A., Boutron, I., Reeves, B., Eldridge, S. (2016). A revised tool for assessing risk of bias in randomized trials. In: Chandler, J., McKenzie, J., Boutron, I., Welch, V. (editors). Cochrane methods. Cochrane database of systematic reviews, 10 (Suppl 1). https://doi.org/10.1002/14651858.CD201601 .
Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html ).
Download references
Acknowledgements
There is no dedicated funding.
Author information
Authors and affiliations.
Discipline of Addiction Medicine, Central Clinical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
Susan A. Rombouts, Eva Louie, Paul Haber & Kirsten C. Morley
NHMRC Centre of Research Excellence in Indigenous Health and Alcohol, Central Clinical School, Faculty of Medicine and Health, University of Sydney, Sydney, NSW, Australia
James Conigrave
Drug Health Services, Royal Prince Alfred Hospital, Camperdown, NSW, Australia
You can also search for this author in PubMed Google Scholar
Contributions
KM and PH conceived the presented idea of a systematic review and meta-analysis and helped with the scope of the literature. KM is the senior researcher providing overall guidance and the guarantor of this review. SR developed the background, search strategy, and data extraction form. SR and EL will both be working on the data extraction and risk of bias assessment. SR and JC will conduct the data analysis and synthesize the results. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Kirsten C. Morley .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1..
PRISMA-P 2015 Checklist.
Additional file 2.
Draft search strategy MEDLINE. Search strategy.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
Rombouts, S.A., Conigrave, J., Louie, E. et al. Evidence-based models of care for the treatment of alcohol use disorder in primary health care settings: protocol for systematic review. Syst Rev 8 , 275 (2019). https://doi.org/10.1186/s13643-019-1157-7
Download citation
Received : 25 March 2019
Accepted : 13 September 2019
Published : 13 November 2019
DOI : https://doi.org/10.1186/s13643-019-1157-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Model of care
- Primary health care
- Systematic review
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Search Menu
- Author Guidelines
- Submission Site
- Open Access
- About Alcohol and Alcoholism
- About the Medical Council on Alcohol
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Dispatch Dates
- Contact the MCA
- Journals on Oxford Academic
- Books on Oxford Academic

Editors-in-Chief
Dr Giancarlo Colombo
Dr Lorenzo Leggio
Publish with Alcohol and Alcoholism
Supportive and international Editorial Board, rigorous and constructive peer review, open access options, and much more. Learn why your research is a perfect fit for Alcohol and Alcoholism .
Featured Content
Advanced access, high cited articles, latest posts on x.

The liver collection
Research highlights related to ALD and AUD.
Browse the papers

10 Reasons to Publish with Alcohol and Alcoholism
Learn more about why your impactful research is the perfect fit for Alcohol and Alcoholism .
Read more here

Promote Your Article
Have you published an article? What should you do now? Read our top tips on promoting your work to reach a wider audience and ensure your work makes an impact.
Find out more

Explore the archive
Alcohol and Alcoholism offers free online access to all content older than 12 months back to Jan 1st 1996.

Trending articles
Discover the Alcohol and Alcoholism articles that your peers and the public are talking about, and the news stories they have been featured in.
Join global conversations

Committee on Publication Ethics (COPE)
This journal is a member of and subscribes to the principles of the Committee on Publication Ethics (COPE)
publicationethics.org

ISAJE (International Society of Addiction Journal Editors)
Alcohol and Alcoholism is an ISAJE Member Journal. ISAJE is a not-for-profit organization supporting journal editors, authors and reviewers who work in the addiction field.

Looking for a place to publish?
Alcohol and Alcoholism welcomes submissions, publishing papers on the biomedical, psychological, and sociological aspects of alcoholism and alcohol research.
To gain more information please see the Instructions to Authors page.

Recommend to your library
Fill out our simple online form to recommend Alcohol and Alcoholism to your library.
Recommend now

Open Access options for authors
Alcohol and Alcoholism welcomes submissions from Authors wishing to submit open access papers.

The International Impacts of Alcohol Use Disorder and Nicotine and Tobacco
Explore a collaborative collection showcasing crucial research into the global impacts of alcohol use disorder and nicotine and tobacco use.
Related titles
- Recommend to your Library
Affiliations
- Online ISSN 1464-3502
- Copyright © 2024 Medical Council on Alcohol and Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 14 May 2024
A burden of proof study on alcohol consumption and ischemic heart disease
- Sinclair Carr ORCID: orcid.org/0000-0003-0421-3145 1 ,
- Dana Bryazka 1 ,
- Susan A. McLaughlin 1 ,
- Peng Zheng 1 , 2 ,
- Sarasvati Bahadursingh 3 ,
- Aleksandr Y. Aravkin 1 , 2 , 4 ,
- Simon I. Hay ORCID: orcid.org/0000-0002-0611-7272 1 , 2 ,
- Hilary R. Lawlor 1 ,
- Erin C. Mullany 1 ,
- Christopher J. L. Murray ORCID: orcid.org/0000-0002-4930-9450 1 , 2 ,
- Sneha I. Nicholson 1 ,
- Jürgen Rehm 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 ,
- Gregory A. Roth 1 , 2 , 13 ,
- Reed J. D. Sorensen 1 ,
- Sarah Lewington 3 &
- Emmanuela Gakidou ORCID: orcid.org/0000-0002-8992-591X 1 , 2
Nature Communications volume 15 , Article number: 4082 ( 2024 ) Cite this article
1066 Accesses
1 Altmetric
Metrics details
- Cardiovascular diseases
- Epidemiology
- Risk factors
Cohort and case-control data have suggested an association between low to moderate alcohol consumption and decreased risk of ischemic heart disease (IHD), yet results from Mendelian randomization (MR) studies designed to reduce bias have shown either no or a harmful association. Here we conducted an updated systematic review and re-evaluated existing cohort, case-control, and MR data using the burden of proof meta-analytical framework. Cohort and case-control data show low to moderate alcohol consumption is associated with decreased IHD risk – specifically, intake is inversely related to IHD and myocardial infarction morbidity in both sexes and IHD mortality in males – while pooled MR data show no association, confirming that self-reported versus genetically predicted alcohol use data yield conflicting findings about the alcohol-IHD relationship. Our results highlight the need to advance MR methodologies and emulate randomized trials using large observational databases to obtain more definitive answers to this critical public health question.
Similar content being viewed by others

Alcohol consumption and risks of more than 200 diseases in Chinese men
Alcohol intake and the risk of chronic kidney disease: results from a systematic review and dose–response meta-analysis

Association of change in alcohol consumption with cardiovascular disease and mortality among initial nondrinkers
Introduction.
It is well known that alcohol consumption increases the risk of morbidity and mortality due to many health conditions 1 , 2 , with even low levels of consumption increasing the risk for some cancers 3 , 4 . In contrast, a large body of research has suggested that low to moderate alcohol intake – compared to no consumption – is associated with a decreased risk of ischemic heart disease (IHD). This has led to substantial epidemiologic and public health interest in the alcohol-IHD relationship 5 , particularly given the high prevalence of alcohol consumption 6 and the global burden of IHD 7 .
Extensive evidence from experimental studies that vary short-term alcohol exposure suggests that average levels of alcohol intake positively affect biomarkers such as apolipoprotein A1, adiponectin, and fibrinogen levels that lower the risk of IHD 8 . In contrast, heavy episodic drinking (HED) may have an adverse effect on IHD by affecting blood lipids, promoting coagulation and thus thrombosis risk, and increasing blood pressure 9 . With effects likely to vary materially by patterns of drinking, alcohol consumption must be considered a multidimensional factor impacting IHD outcomes.
A recent meta-analysis of the alcohol-IHD relationship using individual participant data from 83 observational studies 4 found, among current drinkers, that – relative to drinking less than 50 g/week – any consumption above this level was associated with a lower risk of myocardial infarction (MI) incidence and consumption between >50 and <100 g/week was associated with lower risk of MI mortality. When evaluating other subtypes of IHD excluding MI, the researchers found that consumption between >100 and <250 g/week was associated with a decreased risk of IHD incidence, whereas consumption greater than 350 g/week was associated with an increased risk of IHD mortality. Roerecke and Rehm further observed that low to moderate drinking was not associated with reduced IHD risk when accompanied by occasional HED 10 .
The cohort studies and case-control studies (hereafter referred to as ‘conventional observational studies’) used in these meta-analyses are known to be subject to various types of bias when used to estimate causal relationships 11 . First, neglecting to separate lifetime abstainers from former drinkers, some of whom may have quit due to developing preclinical symptoms (sometimes labeled ‘sick quitters’ 12 , 13 ), and to account for drinkers who reduce their intake as a result of such symptoms may introduce reverse causation bias 13 . That is, the risk of IHD in, for example, individuals with low to moderate alcohol consumption may be lower when compared to IHD risk in sick quitters, not necessarily because intake at this level causes a reduction in risk but because sick quitters are at higher risk of IHD. Second, estimates can be biased because of measurement error in alcohol exposure resulting from inaccurate reporting, random fluctuation in consumption over time (random error), or intentional misreporting of consumption due, for example, to social desirability effects 14 (systematic error). Third, residual confounding may bias estimates if confounders of the alcohol-IHD relationship, such as diet or physical activity, have not been measured accurately (e.g., only via a self-report questionnaire) or accounted for. Fourth, because alcohol intake is a time-varying exposure, time-varying confounding affected by prior exposure must be accounted for 15 . To date, only one study that used a marginal structural model to appropriately adjust for time-varying confounding found no association between alcohol consumption and MI risk 16 . Lastly, if exposure to a risk factor, such as alcohol consumption, did not happen at random – even if all known confounders of the relationship between alcohol and IHD were perfectly measured and accounted for – the potential for unmeasured confounders persists and may bias estimates 11 .
In recent years, the analytic method of Mendelian randomization (MR) has been widely adopted to quantify the causal effects of risk factors on health outcomes 17 , 18 , 19 . MR uses single nucleotide polymorphisms (SNPs) as instrumental variables (IVs) for the exposure of interest. A valid IV should fulfill the following three assumptions: it must be associated with the risk factor (relevance assumption); there must be no common causes of the IV and the outcome (independence assumption); and the IV must affect the outcome only through the exposure (exclusion restriction or ‘no horizontal pleiotropy’ assumption) 20 , 21 . If all three assumptions are fulfilled, estimates derived from MR are presumed to represent causal effects 22 . Several MR studies have quantified the association between alcohol consumption and cardiovascular disease 23 , including IHD, using genes known to impact alcohol metabolism (e.g., ADH1B/C and ALDH2 24 ) or SNP combinations from genome-wide association studies 25 . In contrast to the inverse associations found in conventional observational studies, MR studies have found either no association or a harmful relationship between alcohol consumption and IHD 26 , 27 , 28 , 29 , 30 , 31 .
To advance the knowledge base underlying our understanding of this major health issue – critical given the worldwide ubiquity of alcohol use and of IHD – there is a need to systematically review and critically re-evaluate all available evidence on the relationship between alcohol consumption and IHD risk from both conventional observational and MR studies.
The burden of proof approach, developed by Zheng et al. 32 , is a six-step meta-analysis framework that provides conservative estimates and interpretations of risk-outcome relationships. The approach systematically tests and adjusts for common sources of bias defined according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria: representativeness of the study population, exposure assessment, outcome ascertainment, reverse causation, control for confounding, and selection bias. The key statistical tool to implement the approach is MR-BRT (meta-regression—Bayesian, regularized, trimmed 33 ), a flexible meta-regression tool that does not impose a log-linear relationship between the risk and outcome, but instead uses a spline ensemble to model non-linear relationships. MR-BRT also algorithmically detects and trims outliers in the input data, takes into account different reference and alternative exposure intervals in the data, and incorporates unexplained between-study heterogeneity in the uncertainty surrounding the mean relative risk (RR) curve (henceforth ‘risk curve’). For those risk-outcome relationships that meet the condition of statistical significance using conventionally estimated uncertainty intervals (i.e., without incorporating unexplained between-study heterogeneity), the burden of proof risk function (BPRF) is derived by calculating the 5th (if harmful) or 95th (if protective) quantile risk curve – inclusive of between-study heterogeneity – closest to the log RR of 0. The resulting BPRF is a conservative interpretation of the risk-outcome relationship based on all available evidence. The BPRF represents the smallest level of excess risk for a harmful risk factor or reduced risk for a protective risk factor that is consistent with the data, accounting for between-study heterogeneity. To quantify the strength of the evidence for the alcohol-IHD relationship, the BPRF can be summarized in a single metric, the risk-outcome score (ROS). The ROS is defined as the signed value of the average log RR of the BPRF across the 15th to 85th percentiles of alcohol consumption levels observed across available studies. The larger a positive ROS value, the stronger the alcohol-IHD association. For ease of interpretation, the ROS is converted into a star rating from one to five. A one-star rating (ROS < 0) indicates a weak alcohol-IHD relationship, and a five-star rating (ROS > 0.62) indicates a large effect size and strong evidence. Publication and reporting bias are evaluated with Egger’s regression and by visual inspection with funnel plots 34 . Further conceptual and technical details of the burden of proof approach are described in detail elsewhere 32 .
Using the burden of proof approach, we systematically re-evaluate all available eligible evidence from cohort, case-control, and MR studies published between 1970 and 2021 to conservatively quantify the dose-response relationship between alcohol consumption and IHD risk, calculated relative to risk at zero alcohol intake (i.e., current non-drinking, including lifetime abstinence or former use). We pool the evidence from all conventional observational studies combined, as well as individually for all three study designs, to estimate mean IHD risk curves. Based on patterns of results established by previous meta-analyses 4 , 35 , we also use data from conventional observational studies to estimate risk curves by IHD endpoint (morbidity or mortality) and further by sex, in addition to estimating risk curves for MI overall and by endpoint. We follow PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines 36 through all stages of this study (Supplementary Information section 1 , Fig. S1 and Tables S1 and S2 ) and comply with GATHER (Guidelines on Accurate and Transparent Health Estimates Reporting) recommendations 37 (Supplementary Information section 2 , Table S3 ). The main findings and research implications of this work are summarized in Table 1 .
We updated the systematic review on the dose-response relationship between alcohol consumption and IHD previously conducted for the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 1 . Of 4826 records identified in our updated systematic review (4769 from databases/registers and 57 by citation search and known literature), 11 were eligible based on our inclusion criteria and were included. In total, combined with the results of the previous systematic reviews 1 , 38 , information from 95 cohort studies 26 , 27 , 29 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 27 case-control studies 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , and five MR studies 26 , 27 , 28 , 29 , 31 was included in our meta-analysis (see Supplementary Information section 1 , Fig. S1 , for the PRISMA diagram). Details on the extracted effect sizes, the design of each included study, underlying data sources, number of participants, duration of follow-up, number of cases and controls, and bias covariates that were evaluated and potentially adjusted for can be found in the Supplementary Information Sections 4 , 5 , and 6 .
Table 2 summarizes key metrics of each risk curve modeled, including estimates of mean RR and 95% UI (inclusive of between-study heterogeneity) at select alcohol exposure levels, the exposure level and RR and 95% UI at the nadir (i.e., lowest RR), the 85th percentile of exposure observed in the data and its corresponding RR and 95% UI, the BPRF averaged at the 15th and 85th percentile of exposure, the average excess risk or risk reduction according to the exposure-averaged BPRF, the ROS, the associated star rating, the potential presence of publication or reporting bias, and the number of studies included.
We found large variation in the association between alcohol consumption and IHD by study design. When we pooled the results of cohort and case-control studies, we observed an inverse association between alcohol at average consumption levels and IHD risk; that is, drinking average levels of alcohol was associated with a reduced IHD risk relative to drinking no alcohol. In contrast, we did not find a statistically significant association between alcohol consumption and IHD risk when pooling results from MR studies. When we subset the conventional observational studies to those reporting on IHD by endpoint, we found no association between alcohol consumption and IHD morbidity or mortality due to large unexplained heterogeneity between studies. When we further subset those studies that reported effect size estimates by sex, we found that average alcohol consumption levels were inversely associated with IHD morbidity in males and in females, and with IHD mortality in males but not in females. When we analyzed only the studies that reported on MI, we found significant inverse associations between average consumption levels and MI overall and with MI morbidity. Visualizations of the risk curves for morbidity and mortality of IHD and MI are provided in Supplementary Information Section 9 (Figs. S2a –c, S3a –c, and S4a–c ). Among all modeled risk curves for which a BPRF was calculated, the ROS ranged from −0.40 for MI mortality to 0.20 for MI morbidity. In the Supplementary Information, we also provide details on the RR and 95% UIs with and without between-study heterogeneity associated with each 10 g/day increase in consumption for each risk curve (Table S10 ), the parameter specifications of the model (Tables S11 and S12 ), and each risk curve from the main analysis estimated without trimming 10% of the data (Fig. S5a–l and Table S13 ).
Risk curve derived from conventional observational study data
The mean risk curve and 95% UI were first estimated by combining all evidence from eligible cohort and case-control studies that quantified the association between alcohol consumption and IHD risk. In total, information from 95 cohort studies and 27 case-control studies combining data from 7,059,652 participants were included. In total, 243,357 IHD events were recorded. Thirty-seven studies quantified the association between alcohol consumption and IHD morbidity only, and 44 studies evaluated only IHD mortality. The estimated alcohol-IHD association was adjusted for sex and age in all but one study. Seventy-five studies adjusted the effect sizes for sex, age, smoking, and at least four other covariates. We adjusted our risk curve for whether the study sample was under or over 50 years of age, whether the study outcome was consistent with the definition of IHD (according to the International Classification of Diseases [ICD]−9: 410-414; and ICD-10: I20-I25) or related to specified subtypes of IHD, whether the outcome was ascertained by self-report only or by at least one other measurement method, whether the study accounted for risk for reverse causation, whether the reference group was non-drinkers (including lifetime abstainers and former drinkers), and whether effect sizes were adjusted (1) for sex, age, smoking, and at least four other variables, (2) for apolipoprotein A1, and (3) for cholesterol, as these bias covariates were identified as significant by our algorithm.
Pooling all data from cohort and case-control studies, we found that alcohol consumption was inversely associated with IHD risk (Fig. 1 ). The risk curve was J-shaped – without crossing the null RR of 1 at high exposure levels – with a nadir of 0.69 (95% UI: 0.48–1.01) at 23 g/day. This means that compared to individuals who do not drink alcohol, the risk of IHD significantly decreases with increasing consumption up to 23 g/day, followed by a risk reduction that becomes less pronounced. The average BPRF calculated between 0 and 45 g/day of alcohol intake (the 15th and 85th percentiles of the exposure range observed in the data) was 0.96. Thus, when between-study heterogeneity is accounted for, a conservative interpretation of the evidence suggests drinking alcohol across the average intake range is associated with an average decrease in the risk of IHD of at least 4% compared to drinking no alcohol. This corresponds to a ROS of 0.04 and a star rating of two, which suggests that the association – on the basis of the available evidence – is weak. Although we algorithmically identified and trimmed 10% of the data to remove outliers, Egger’s regression and visual inspection of the funnel plot still indicated potential publication or reporting bias.
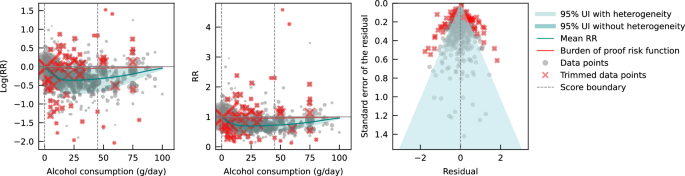
The panels show the log(relative risk) function, the relative risk function, and a modified funnel plot showing the residuals (relative to 0) on the x-axis and the estimated standard error that includes the reported standard error and between-study heterogeneity on the y-axis. RR relative risk, UI uncertainty interval. Source data are provided as a Source Data file.
Risk curve derived from case-control study data
Next, we estimated the mean risk curve and 95% UI for the relationship between alcohol consumption and IHD by subsetting the data to case-control studies only. We included a total of 27 case-control studies (including one nested case-control study) with data from 60,914 participants involving 16,892 IHD cases from Europe ( n = 15), North America ( n = 6), Asia ( n = 4), and Oceania ( n = 2). Effect sizes were adjusted for sex and age in most studies ( n = 25). Seventeen of these studies further adjusted for smoking and at least four other covariates. The majority of case-control studies accounted for the risk of reverse causation ( n = 25). We did not adjust our risk curve for bias covariates, as our algorithm did not identify any as significant.
Evaluating only data from case-control studies, we observed a J-shaped relationship between alcohol consumption and IHD risk, with a nadir of 0.65 (0.50–0.85) at 23 g/day (Fig. 2 ). The inverse association between alcohol consumption and IHD risk reversed at an intake level of 61 g/day. In other words, alcohol consumption between >0 and 60 g/day was associated with a lower risk compared to no consumption, while consumption at higher levels was associated with increased IHD risk. However, the curve above this level is flat, implying that the association between alcohol and increased IHD risk is the same between 61 and 100 g/day, relative to not drinking any alcohol. The BPRF averaged across the exposure range between the 15th and 85th percentiles, or 0–45 g/day, was 0.87, which translates to a 13% average reduction in IHD risk across the average range of consumption. This corresponds to a ROS of 0.14 and a three-star rating. After trimming 10% of the data, no potential publication or reporting bias was found.
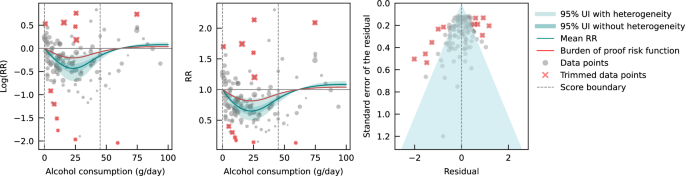
The panels show the log(relative risk) function, the relative risk function, and a modified funnel plot showing the residuals (relative to 0) on the x-axis and the estimated standard deviation that includes the reported standard deviation and between-study heterogeneity on the y-axis. RR relative risk, UI uncertainty interval. Source data are provided as a Source Data file.
Risk curve derived from cohort study data
We also estimated the mean risk curve and 95% UI for the relationship between alcohol consumption and IHD using only data from cohort studies. In total, 95 cohort studies – of which one was a retrospective cohort study – with data from 6,998,738 participants were included. Overall, 226,465 IHD events were recorded. Most data were from Europe ( n = 43) and North America ( n = 33), while a small number of studies were conducted in Asia ( n = 14), Oceania ( n = 3), and South America ( n = 2). The majority of studies adjusted effect sizes for sex and age ( n = 76). Fifty-seven of these studies also adjusted for smoking and at least four other covariates. Out of all cohort studies included, 88 accounted for the risk of reverse causation. We adjusted our risk curve for whether the study outcome was consistent with the definition of IHD or related to specified subtypes of IHD, and whether effect sizes were adjusted for apolipoprotein A1, as these bias covariates were identified as significant by our algorithm.
When only data from cohort studies were evaluated, we found a J-shaped relationship between alcohol consumption and IHD risk that did not cross the null RR of 1 at high exposure levels, with a nadir of 0.69 (0.47–1.01) at 23 g/day (Fig. 3 ). The shape of the risk curve was almost identical to the curve estimated with all conventional observational studies (i.e., cohort and case-control studies combined). When we calculated the average BPRF of 0.95 between the 15th and 85th percentiles of observed alcohol exposure (0–50 g/day), we found that alcohol consumption across the average intake range was associated with an average reduction in IHD risk of at least 5%. This corresponds to a ROS of 0.05 and a two-star rating. We identified potential publication or reporting bias after 10% of the data were trimmed.
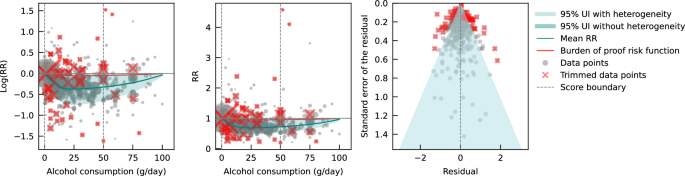
Risk curve derived from Mendelian randomization study data
Lastly, we pooled evidence on the relationship between genetically predicted alcohol consumption and IHD risk from MR studies. Four MR studies were considered eligible for inclusion in our main analysis, with data from 559,708 participants from China ( n = 2), the Republic of Korea ( n = 1), and the United Kingdom ( n = 1). Overall, 22,134 IHD events were recorded. Three studies used the rs671 ALDH2 genotype found in Asian populations, one study additionally used the rs1229984 ADH1B variant, and one study used the rs1229984 ADH1B Arg47His variant and a combination of 25 SNPs as IVs. All studies used the two-stage least squares (2SLS) method to estimate the association, and one study additionally applied the inverse-variance-weighted (IVW) method and multivariable MR (MVMR). For the study that used multiple methods to estimate effect sizes, we used the 2SLS estimates for our main analysis. Further details on the included studies are provided in Supplementary Information section 4 (Table S6 ). Due to limited input data, we elected not to trim 10% of the observations. We adjusted our risk curve for whether the endpoint of the study outcome was mortality and whether the associations were adjusted for sex and/or age, as these bias covariates were identified as significant by our algorithm.
We did not find any significant association between genetically predicted alcohol consumption and IHD risk using data from MR studies (Fig. 4 ). No potential publication or reporting bias was detected.
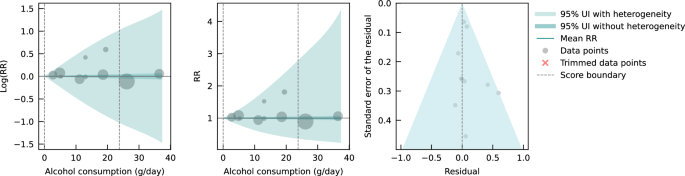
As sensitivity analyses, we modeled risk curves with effect sizes estimated from data generated by Lankester et al. 28 using IVW and MVMR methods. We also used effect sizes from Biddinger et al. 31 , obtained using non-linear MR with the residual method, instead of those from Lankester et al. 28 in our main model (both were estimated with UK Biobank data) to estimate a risk curve. Again, we did not find a significant association between genetically predicted alcohol consumption and IHD risk (see Supplementary Information Section 10 , Fig. S6a–c and Table S14 ). To test for consistency with the risk curve we estimated using all included cohort studies, we also pooled the conventionally estimated effect sizes provided in the four MR studies. We did not observe an association between alcohol consumption and IHD risk due to large unexplained heterogeneity between studies (see Supplementary Information Section 10 , Fig. S7, and Table S14 ). Lastly, we pooled cohort studies that included data from China, the Republic of Korea, and the United Kingdom to account for potential geographic influences. Again, we did not find a significant association between alcohol consumption and IHD risk (see Supplementary Information Section 10 , Fig. S8, and Table S14 ).
Conventional observational and MR studies published to date provide conflicting estimates of the relationship between alcohol consumption and IHD. We conducted an updated systematic review and conservatively re-evaluated existing evidence on the alcohol-IHD relationship using the burden of proof approach. We synthesized evidence from cohort and case-control studies combined and separately and from MR studies to assess the dose-response relationship between alcohol consumption and IHD risk and to compare results across different study designs. It is anticipated that the present synthesis of evidence will be incorporated into upcoming iterations of GBD.
Our estimate of the association between genetically predicted alcohol consumption and IHD runs counter to our estimates from the self-report data and those of other previous meta-analyses 4 , 35 , 158 that pooled conventional observational studies. Based on the conservative burden of proof interpretation of the data, our results suggested an inverse association between alcohol and IHD when all conventional observational studies were pooled (alcohol intake was associated with a reduction in IHD risk by an average of at least 4% across average consumption levels; two-star rating). In evaluating only cohort studies, we again found an inverse association between alcohol consumption and IHD (alcohol intake was associated with a reduction in IHD risk by an average of at least 5% at average consumption levels; two-star rating). In contrast, when we pooled only case-control studies, we estimated that average levels of alcohol consumption were associated with at least a 13% average decrease in IHD risk (three-star rating), but the inverse association reversed when consumption exceeded 60 g/day, suggesting that alcohol above this level is associated with a slight increase in IHD risk. Our analysis of the available evidence from MR studies showed no association between genetically predicted alcohol consumption and IHD.
Various potential biases and differences in study designs may have contributed to the conflicting findings. In our introduction, we summarized important sources of bias in conventional observational studies of the association between alcohol consumption and IHD. Of greatest concern are residual and unmeasured confounding and reverse causation, the effects of which are difficult to eliminate in conventional observational studies. By using SNPs within an IV approach to predict exposure, MR – in theory – eliminates these sources of bias and allows for more robust estimates of causal effects. Bias may still occur, however, when using MR to estimate the association between alcohol and IHD 159 , 160 . There is always the risk of horizontal pleiotropy in MR – that is, the genetic variant may affect the outcome via pathways other than exposure 161 . The IV assumption of exclusion restriction is, for example, violated if only a single measurement of alcohol consumption is used in MR 162 ; because alcohol consumption varies over the life course, the gene directly impacts IHD through intake at time points other than that used in the MR analysis. To date, MR studies have not succeeded in separately capturing the multidimensional effects of alcohol intake on IHD risk (i.e., effects of average alcohol consumption measured through frequency-quantity, in addition to the effects of HED) 159 because the genes used to date only target average alcohol consumption that encompasses intake both at average consumption levels and HED. In other words, the instruments used are not able to separate out the individual effects of these two different dimensions of alcohol consumption on IHD risk using MR. Moreover, reverse causation may occur through cross-generational effects 160 , 163 , as the same genetic variants predispose both the individual and at least one of his or her parents to (increased) alcohol consumption. In this situation, IHD risk could be associated with the parents’ genetically predicted alcohol consumption and not with the individual’s own consumption. None of the MR studies included accounted for cross-generational effects, which possibly introduced bias in the effect estimates. It is important to note that bias by ancestry might also occur in conventional observational studies 164 . In summary, estimates of the alcohol-IHD association are prone to bias in all three study designs, limiting inferences of causation.
The large difference in the number of available MR versus conventional observational studies, the substantially divergent results derived from the different study types, and the rapidly developing field of MR clearly argue for further investigation of MR as a means to quantify the association between alcohol consumption and IHD risk. Future studies should investigate non-linearity in the relationship using non-linear MR methods. The residual method, commonly applied in non-linear MR studies such as Biddinger et al. 31 , assumes a constant, linear relationship between the genetic IV and the exposure in the study population; a strong assumption that may result in biased estimates and inflated type I error rates if the relationship varies by population strata 165 . However, by log-transforming the exposure, the relationships between the genetic IV and the exposure as expressed on a logarithmic scale may be more homogeneous across strata, possibly reducing the bias effect of violating the assumption of a constant, linear relationship. Alternatively, or in conjunction, the recently developed doubly ranked method, which obviates the need for this assumption, could be used 166 . Since methodology for non-linear MR is an active field of study 167 , potential limitations of currently available methods should be acknowledged and latest guidelines be followed 168 . Future MR studies should further (i) employ sensitivity analyses such as the MR weighted median method 169 to relax the exclusion restriction assumption that may be violated, as well as applying other methods such as the MR-Egger intercept test; (ii) use methods such as g-estimation of structural mean models 162 to adequately account for temporal variation in alcohol consumption in MR, and (iii) attempt to disaggregate the effects of alcohol on IHD by dimension in MR, potentially through the use of MVMR 164 . General recommendations to overcome common MR limitations are described in greater detail elsewhere 159 , 163 , 170 , 171 and should be carefully considered. With respect to prospective cohort studies used to assess the alcohol-IHD relationship, they should, at a minimum: (i) adjust the association between alcohol consumption and IHD for all potential confounders identified, for example, using a causal directed acyclic graph, and (ii) account for reverse causation introduced by sick quitters and by drinkers who changed their consumption. If possible, they should also (iii) use alcohol biomarkers as objective measures of alcohol consumption instead of or in addition to self-reported consumption to reduce bias through measurement error, (iv) investigate the association between IHD and HED, in addition to average alcohol consumption, and (v) when multiple measures of alcohol consumption and potential confounders are available over time, use g-methods to reduce bias through confounding as fully as possible within the limitations of the study design. However, some bias – due, for instance, to unmeasured confounding in conventional observational and to horizontal pleiotropy in MR studies – is likely inevitable, and the interpretation of estimates should be appropriately cautious, in accordance with the methods used in the study.
With the introduction of the Moderate Alcohol and Cardiovascular Health Trial (MACH15) 172 , randomized controlled trials (RCTs) have been revisited as a way to study the long-term effects of low to moderate alcohol consumption on cardiovascular disease, including IHD. In 2018, soon after the initiation of MACH15, the National Institutes of Health terminated funding 173 , reportedly due to concerns about study design and irregularities in the development of funding opportunities 174 . Although MACH15 was terminated, its initiation represented a previously rarely considered step toward investigating the alcohol-IHD relationship using an RCT 175 . However, while the insights from an RCT are likely to be invaluable, the implementation is fraught with potential issues. Due to the growing number of studies suggesting increased disease risk, including cancer 3 , 4 , associated with alcohol use even at very low levels 176 , the use of RCTs to study alcohol consumption is ethically questionable 177 . A less charged approach could include the emulation of target trials 178 using existing observational data (e.g., from large-scale prospective cohort studies such as the UK Biobank 179 , Atherosclerosis Risk in Communities Study 180 , or the Framingham Heart Study 181 ) in lieu of real trials to gather evidence on the potential cardiovascular effects of alcohol. Trials like MACH15 can be emulated, following the proposed trial protocols as closely as the observational dataset used for the analysis allows. Safety and ethical concerns, such as those related to eligibility criteria, initiation/increase in consumption, and limited follow-up duration, will be eliminated because the data will have already been collected. This framework allows for hypothetical trials investigating ethically challenging or even untenable questions, such as the long-term effects of heavy (episodic) drinking on IHD risk, to be emulated and inferences to broader populations drawn.
There are several limitations that must be considered when interpreting our findings. First, record screening for our systematic review was not conducted in a double-blinded fashion. Second, we did not have sufficient evidence to estimate and examine potential differential associations of alcohol consumption with IHD risk by beverage type or with MI endpoints by sex. Third, despite using a flexible meta-regression tool that overcame several limitations common to meta-analyses, the results of our meta-analysis were only as good as the quality of the studies included. We were able, however, to address the issue of varying quality of input data by adjusting for bias covariates that corresponded to core study characteristics in our analyses. Fourth, because we were only able to include one-sample MR studies that captured genetically predicted alcohol consumption, statistical power may be lower than would have been possible with the inclusion of two-sample MR studies, and studies that directly estimated gene-IHD associations were not considered 23 . Finally, we were not able to account for participants’ HED status when pooling effect size estimates from conventional observational studies. Given established differences in IHD risk for drinkers with and without HED 35 and the fact that more than one in three drinkers reports HED 6 , we would expect that the decreased average risk we found at moderate levels of alcohol consumption would be attenuated (i.e., approach the IHD risk of non-drinkers) if the presence of HED was taken into account.
Using the burden of proof approach 32 , we conservatively re-evaluated the dose-response relationship between alcohol consumption and IHD risk based on existing cohort, case-control, and MR data. Consistent with previous meta-analyses, we found that alcohol at average consumption levels was inversely associated with IHD when we pooled conventional observational studies. This finding was supported when aggregating: (i) all studies, (ii) only cohort studies, (iii) only case-control studies, (iv) studies examining IHD morbidity in females and males, (v) studies examining IHD mortality in males, and (vi) studies examining MI morbidity. In contrast, we found no association between genetically predicted alcohol consumption and IHD risk based on data from MR studies. Our confirmation of the conflicting results derived from self-reported versus genetically predicted alcohol use data highlights the need to advance methodologies that will provide more definitive answers to this critical public health question. Given the limitations of randomized trials, we advocate using advanced MR techniques and emulating target trials using observational data to generate more conclusive evidence on the long-term effects of alcohol consumption on IHD risk.
This study was approved by the University of Washington IRB Committee (study #9060).
The burden of proof approach is a six-step framework for conducting meta-analysis 32 : (1) data from published studies that quantified the dose-response relationship between alcohol consumption and ischemic heart disease (IHD) risk were systematically identified and obtained; (2) the shape of the mean relative risk (RR) curve (henceforth ‘risk curve’) and associated uncertainty was estimated using a quadratic spline and algorithmic trimming of outliers; (3) the risk curve was tested and adjusted for biases due to study attributes; (4) unexplained between-study heterogeneity was quantified, adjusting for within-study correlation and number of studies included; (5) the evidence for small-study effects was evaluated to identify potential risks of publication or reporting bias; and (6) the burden of proof risk function (BPRF) – a conservative interpretation of the average risk across the exposure range found in the data – was estimated relative to IHD risk at zero alcohol intake. The BPRF was converted to a risk-outcome score (ROS) that was mapped to a star rating from one to five to provide an intuitive interpretation of the magnitude and direction of the dose-response relationship between alcohol consumption and IHD risk.
We calculated the mean RR and 95% uncertainty intervals (UIs) for IHD associated with levels of alcohol consumption separately with all evidence available from conventional observational studies and from Mendelian randomization (MR) studies. For the risk curves that met the condition of statistical significance when the conventional 95% UI that does not include unexplained between-study heterogeneity was evaluated, we calculated the BPRF, ROS, and star rating. Based on input data from conventional observational studies, we also estimated these metrics by study design (cohort studies, case-control studies), and by IHD endpoint (morbidity, mortality) for both sexes (females, males) and sex-specific. For sex-stratified analyses, we only considered studies that reported effect sizes for both females and males to allow direct comparison of IHD risk across different exposure levels; however, we did not collect information about the method each study used to determine sex. We also estimated risk curves for myocardial infarction (MI), overall and by endpoint, using data from conventional observational studies. As a comparison, we also estimated each risk curve without trimming 10% of the input data. We did not consider MI as an outcome or disaggregate findings by sex or endpoint for MR studies due to insufficient data.
With respect to MR studies, several statistical methods are typically used to estimate the associations between genetically predicted exposure and health outcomes (e.g., two-stage least squares [2SLS], inverse-variance-weighted [IVW], multivariable Mendelian randomization [MVMR]). For our main analysis synthesizing evidence from MR studies, we included the reported effect sizes estimated using 2SLS if a study applied multiple methods because this method was common to all included studies. In sensitivity analyses, we used the effect sizes obtained by other MR methods (i.e., IVW, MVMR, and non-linear MR) and estimated the mean risk curve and uncertainty. We also pooled conventionally estimated effect sizes from MR studies to allow comparison with the risk curve estimated with cohort studies. Due to limited input data from MR studies, we elected not to trim 10% of the observations. Furthermore, we estimated the risk curve from cohort studies with data from countries that corresponded to those included in MR studies (China, the Republic of Korea, and the United Kingdom). Due to a lack of data, we were unable to estimate a risk curve from case-control studies in these geographic regions.
Conducting the systematic review
In step one of the burden of proof approach, data for the dose-response relationship between alcohol consumption and IHD risk were systematically identified, reviewed, and extracted. We updated a previously published systematic review 1 in PubMed that identified all studies evaluating the dose-response relationship between alcohol consumption and risk of IHD morbidity or mortality from January 1, 1970, to December 31, 2019. In our update, we additionally considered all studies up to and including December 31, 2021, for eligibility. We searched articles in PubMed on March 21, 2022, with the following search string: (alcoholic beverage[MeSH Terms] OR drinking behavior[MeSH Terms] OR “alcohol”[Title/Abstract]) AND (Coronary Artery Disease[Mesh] OR Myocardial Ischemia[Mesh] OR atherosclerosis[Mesh] OR Coronary Artery Disease[TiAb] OR Myocardial Ischemia[TiAb] OR cardiac ischemia[TiAb] OR silent ischemia[TiAb] OR atherosclerosis Outdent [TiAb] OR Ischemic heart disease[TiAb] OR Ischemic heart disease[TiAb] OR coronary heart disease[TiAb] OR myocardial infarction[TiAb] OR heart attack[TiAb] OR heart infarction[TiAb]) AND (Risk[MeSH Terms] OR Odds Ratio[MeSH Terms] OR “risk”[Title/Abstract] OR “odds ratio”[Title/Abstract] OR “cross-product ratio”[Title/Abstract] OR “hazards ratio”[Title/Abstract] OR “hazard ratio”[Title/Abstract]) AND (“1970/01/01”[PDat]: “2021/12/31”[PDat]) AND (English[LA]) NOT (animals[MeSH Terms] NOT Humans[MeSH Terms]). Studies were eligible for inclusion if they met all of the following criteria: were published between January 1, 1970, and December 31, 2021; were a cohort study, case-control study, or MR study; described an association between alcohol consumption and IHD and reported an effect size estimate (relative risk, hazard ratio, odds ratio); and used a continuous dose as exposure of alcohol consumption. Studies were excluded if they met any of the following criteria: were an aggregate study (meta-analysis or pooled cohort); utilized a study design not designated for inclusion in this analysis: not a cohort study, case-control study, or MR study; were a duplicate study: the underlying sample of the study had also been analyzed elsewhere (we always considered the analysis with the longest follow-up for cohort studies or the most recently published analysis for MR studies); did not report on the exposure of interest: reported on combined exposure of alcohol and drug use or reported alcohol consumption in a non-continuous way; reported an outcome that was not IHD or a composite outcome that included but was not limited to IHD, or outcomes lacked specificity, such as cardiovascular disease or all-cause mortality; were not in English; and were animal studies. All screenings of titles and abstracts of identified records, as well as full texts of potentially eligible studies, and extraction of included studies, were done by a single reviewer (SC or HL) independently. If eligible, studies were extracted for study characteristics, exposure, outcome, adjusted confounders, and effect sizes and their uncertainty. While the previous systematic review only considered cohort and case-control studies, our update also included MR studies. We chose to consider only ‘one-sample’ MR studies, i.e., those in which genes, risk factors, and outcomes were measured in the same participants, and not ‘two-sample’ MR studies in which two different samples were used for the MR analysis so that we could fully capture study-specific information. We re-screened previously identified records for MR studies to consider all published MR studies in the defined time period. We also identified and included in our sensitivity analysis an MR study published in 2022 31 which used a non-linear MR method to estimate the association between genetically predicted alcohol consumption and IHD. When eligible studies reported both MR and conventionally estimated effect sizes (i.e., for the association between self-reported alcohol consumption and IHD risk), we extracted both. If studies used the same underlying sample and investigated the same outcome in the same strata, we included the study that had the longest follow-up. This did not apply when the same samples were used in conventional observational and MR studies, because they were treated separately when estimating the risk curve of alcohol consumption and IHD. Continuous exposure of alcohol consumption was defined as a frequency-quantity measure 182 and converted to g/day. IHD was defined according to the International Classification of Diseases (ICD)−9, 410-414, and ICD-10, I20-I25.
The raw data were extracted with a standardized extraction sheet (see Supplementary Information Section 3 , Table S4 ). For conventional observational studies, when multiple effect sizes were estimated from differently adjusted regression models, we used those estimated with the model reported to be fully adjusted or the one with the most covariates. In the majority of studies, alcohol consumption was categorized based on the exposure range available in the data. If the lower end of a categorical exposure range (e.g., <10 g/day) of an effect size was not specified in the input data, we assumed that this was 0 g/day. If the upper end was not specified (e.g., >20 g/day), it was calculated by multiplying the lower end of the categorical exposure range by 1.5. When the association between alcohol and IHD risk was reported as a linear slope, the average consumption level in the sample was multiplied by the logarithm of the effect size to effectively render it categorical. From the MR study which employed non-linear MR 31 , five effect sizes and their uncertainty were extracted at equal intervals across the reported range of alcohol exposure using WebPlotDigitizer. To account for the fact that these effect sizes were derived from the same non-linear risk curve, we adjusted the extracted standard errors by multiplying them by the square root of five (i.e., the number of extracted effect sizes). Details on data sources are provided in Supplementary Information Section 4 .
Estimating the shape of the risk-outcome relationship
In step two, the shape of the dose-response relationship (i.e., ‘signal’) between alcohol consumption and IHD risk was estimated relative to risk at zero alcohol intake. The meta-regression tool MR-BRT (meta-regression—Bayesian, regularized, trimmed), developed by Zheng et al. 33 , was used for modeling. To allow for non-linearity, thus relaxing the common assumption of a log-linear relationship, a quadratic spline with two interior knots was used for estimating the risk curve 33 . We used the following three risk measures from included studies: RRs, odds ratios (ORs), and hazard ratios (HRs). ORs were treated as equivalent to RRs and HRs based on the rare outcome assumption. To counteract the potential influence of knot placement on the shape of the risk curve when using splines, an ensemble model approach was applied. Fifty component models with random knot placements across the exposure domain were computed. These were combined into an ensemble by weighting each model based on model fit and variation (i.e., smoothness of fit to the data). To prevent bias from outliers, a robust likelihood-based approach was applied to trim 10% of the observations. Technical details on estimating the risk curve, use of splines, the trimming procedure, the ensemble model approach, and uncertainty estimation are described elsewhere 32 , 33 . Details on the model specifications for each risk curve are provided in Supplementary Information section 8 . We first estimated each risk curve without trimming input data to visualize the shape of the curve, which informed knot placement and whether to set a left and/or right linear tail when data were sparse at low or high exposure levels (see Supplementary Information Section 10 , Fig. S5a–l ).
Testing and adjusting for biases across study designs and characteristics
In step three, the risk curve was tested and adjusted for systematic biases due to study attributes. According to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) criteria 183 , the following six bias sources were quantified: representativeness of the study population, exposure assessment, outcome ascertainment, reverse causation, control for confounding, and selection bias. Representativeness was quantified by whether the study sample came from a location that was representative of the underlying geography. Exposure assessment was quantified by whether alcohol consumption was recorded once or more than once in conventional observational studies, or with only one or multiple SNPs in MR studies. Outcome ascertainment was quantified by whether IHD was ascertained by self-report only or by at least one other measurement method. Reverse causation was quantified by whether increased IHD risk among participants who reduced or stopped drinking was accounted for (e.g., by separating former drinkers from lifetime abstainers). Control for confounding factors was quantified by which and how many covariates the effect sizes were adjusted for (i.e., through stratification, matching, weighting, or standardization). Because the most adjusted effect sizes in each study were extracted in the systematic review process and thus may have been adjusted for mediators, we additionally quantified a bias covariate for each of the following potential mediators of the alcohol-IHD relationship: body mass index, blood pressure, cholesterol (excluding high-density lipoprotein cholesterol), fibrinogen, apolipoprotein A1, and adiponectin. Selection bias was quantified by whether study participants were selected and included based on pre-existing disease states. We also quantified and considered as possible bias covariates whether the reference group was non-drinkers, including lifetime abstainers and former drinkers; whether the sample was under or over 50 years of age; whether IHD morbidity, mortality, or both endpoints were used; whether the outcome mapped to IHD or referred only to subtypes of IHD; whether the outcome mapped to MI; and what study design (cohort or case-control) was used when conventional observational studies were pooled. Details on quantified bias covariates for all included studies are provided in Supplementary Information section 5 (Tables S7 and S8 ). Using a Lasso approach 184 , the bias covariates were first ranked. They were then included sequentially, based on their ranking, as effect modifiers of the ‘signal’ obtained in step two in a linear meta-regression. Significant bias covariates were included in modeling the final risk curve. Technical details of the Lasso procedure are described elsewhere 32 .
Quantifying between-study heterogeneity, accounting for heterogeneity, uncertainty, and small number of studies
In step four, the between-study heterogeneity was quantified, accounting for heterogeneity, uncertainty, and small number of studies. In a final linear mixed-effects model, the log RRs were regressed against the ‘signal’ and selected bias covariates, with a random intercept to account for within-study correlation and a study-specific random slope with respect to the ‘signal’ to account for between-study heterogeneity. A Fisher information matrix was used to estimate the uncertainty associated with between-study heterogeneity 185 because heterogeneity is easily underestimated or may be zero when only a small number of studies are available. We estimated the mean risk curve with a 95% UI that incorporated between-study heterogeneity, and we additionally estimated a 95% UI without between-study heterogeneity as done in conventional meta-regressions (see Supplementary Information Section 7 , Table S10 ). The 95% UI incorporating between-study heterogeneity was calculated from the posterior uncertainty of the fixed effects (i.e., the ‘signal’ and selected bias covariates) and the 95% quantile of the between-study heterogeneity. The estimate of between-study heterogeneity and the estimate of the uncertainty of the between-study heterogeneity were used to determine the 95% quantile of the between-study heterogeneity. Technical details of quantifying uncertainty of between-study heterogeneity are described elsewhere 32 .
Evaluating potential for publication or reporting bias
In step five, the potential for publication or reporting bias was evaluated. The trimming algorithm used in step two helps protect against these biases, so risk curves found to have publication or reporting bias using the following methods were derived from data that still had bias even after trimming. Publication or reporting bias was evaluated using Egger’s regression 34 and visual inspection using funnel plots. Egger’s regression tested for a significant correlation between residuals of the RR estimates and their standard errors. Funnel plots showed the residuals of the risk curve against their standard errors. We reported publication or reporting bias when identified.
Estimating the burden of proof risk function
In step six, the BPRF was calculated for risk-outcome relationships that were statistically significant when evaluating the conventional 95% UI without between-study heterogeneity. The BPRF is either the 5th (if harmful) or the 95th (if protective) quantile curve inclusive of between-study heterogeneity that is closest to the RR line at 1 (i.e., null); it indicates a conservative estimate of a harmful or protective association at each exposure level, based on the available evidence. The mean risk curve, 95% UIs (with and without between-study heterogeneity), and BPRF (where applicable) are visualized along with included effect sizes using the midpoint of each alternative exposure range (trimmed data points are marked with a red x), with alcohol consumption in g/day on the x-axis and (log)RR on the y-axis.
We calculated the ROS as the average log RR of the BPRF between the 15th and 85th percentiles of alcohol exposure observed in the study data. The ROS summarizes the association of the exposure with the health outcome in a single measure. A higher, positive ROS indicates a larger association, while a negative ROS indicates a weak association. The ROS is identical for protective and harmful risks since it is based on the magnitude of the log RR. For example, a mean log BPRF between the 15th and 85th percentiles of exposure of −0.6 (protective association) and a mean log BPRF of 0.6 (harmful association) would both correspond to a ROS of 0.6. The ROS was then translated into a star rating, representing a conservative interpretation of all available evidence. A star rating of 1 (ROS: <0) indicates weak evidence of an association, a star rating of 2 (ROS: 0–0.14) indicates a >0–15% increased or >0–13% decreased risk, a star rating of 3 (ROS: >0.14–0.41) indicates a >15–50% increased or >13–34% decreased risk, a star rating of 4 (ROS: >0.41–0.62) indicates a >50–85% increased or >34–46% decreased risk, and a star rating of 5 (ROS: >0.62) indicates a >85% increased or >46% decreased risk.
Statistics & reproducibility
The statistical analyses conducted in this study are described above in detail. No statistical method was used to predetermine the sample size. When analyzing data from cohort and case-control studies, we excluded 10% of observations using a trimming algorithm; when analyzing data from MR studies, we did not exclude any observations. As all data used in this meta-analysis were from observational studies, no experiments were conducted, and no randomization or blinding took place.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The findings from this study were produced using data extracted from published literature. The relevant studies were identified through a systematic literature review and can all be accessed online as referenced in the current paper 26 , 27 , 28 , 29 , 31 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 . Further details on the relevant studies can be found on the GHDx website ( https://ghdx.healthdata.org/record/ihme-data/gbd-alcohol-ihd-bop-risk-outcome-scores ). Study characteristics of all relevant studies included in the analyses are also provided in Supplementary Information Section 4 (Tables S5 and S6 ). The template of the data collection form is provided in Supplementary Information section 3 (Table S4 ). The source data includes processed data from these studies that underlie our estimates. Source data are provided with this paper.
Code availability
Analyses were carried out using R version 4.0.5 and Python version 3.10.9. All code used for these analyses is publicly available online ( https://github.com/ihmeuw-msca/burden-of-proof ).
Bryazka, D. et al. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet 400 , 185–235 (2022).
Article Google Scholar
World Health Organization. Global Status Report on Alcohol and Health 2018 . (World Health Organization, Geneva, Switzerland, 2019).
Bagnardi, V. et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose–response meta-analysis. Br. J. Cancer 112 , 580–593 (2015).
Article CAS PubMed Google Scholar
Wood, A. M. et al. Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet 391 , 1513–1523 (2018).
Article PubMed PubMed Central Google Scholar
Goel, S., Sharma, A. & Garg, A. Effect of alcohol consumption on cardiovascular health. Curr. Cardiol. Rep. 20 , 19 (2018).
Article PubMed Google Scholar
Manthey, J. et al. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. Lancet 393 , 2493–2502 (2019).
Vos, T. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396 , 1204–1222 (2020).
Brien, S. E., Ronksley, P. E., Turner, B. J., Mukamal, K. J. & Ghali, W. A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ 342 , d636 (2011).
Rehm, J. et al. The relationship between different dimensions of alcohol use and the burden of disease—an update. Addiction 112 , 968–1001 (2017).
Roerecke, M. & Rehm, J. Irregular heavy drinking occasions and risk of ischemic heart disease: a systematic review and meta-analysis. Am. J. Epidemiol. 171 , 633–644 (2010).
Hernan, M. A. & Robin, J. M. Causal Inference: What If . (CRC Press, 2023).
Marmot, M. Alcohol and coronary heart disease. Int. J. Epidemiol. 13 , 160–167 (1984).
Shaper, A. G., Wannamethee, G. & Walker, M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet 332 , 1267–1273 (1988).
Davis, C. G., Thake, J. & Vilhena, N. Social desirability biases in self-reported alcohol consumption and harms. Addict. Behav. 35 , 302–311 (2010).
Mansournia, M. A., Etminan, M., Danaei, G., Kaufman, J. S. & Collins, G. Handling time varying confounding in observational research. BMJ 359 , j4587 (2017).
Ilomäki, J. et al. Relationship between alcohol consumption and myocardial infarction among ageing men using a marginal structural model. Eur. J. Public Health 22 , 825–830 (2012).
Lawlor, D. A., Harbord, R. M., Sterne, J. A. C., Timpson, N. & Davey Smith, G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27 , 1133–1163 (2008).
Article MathSciNet PubMed Google Scholar
Burgess, S., Timpson, N. J., Ebrahim, S. & Davey Smith, G. Mendelian randomization: where are we now and where are we going? Int. J. Epidemiol. 44 , 379–388 (2015).
Sleiman, P. M. & Grant, S. F. Mendelian randomization in the era of genomewide association studies. Clin. Chem. 56 , 723–728 (2010).
Davies, N. M., Holmes, M. V. & Davey Smith, G. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362 , k601 (2018).
de Leeuw, C., Savage, J., Bucur, I. G., Heskes, T. & Posthuma, D. Understanding the assumptions underlying Mendelian randomization. Eur. J. Hum. Genet. 30 , 653–660 (2022).
Sheehan, N. A., Didelez, V., Burton, P. R. & Tobin, M. D. Mendelian randomisation and causal inference in observational epidemiology. PLoS Med. 5 , e177 (2008).
Van de Luitgaarden, I. A. et al. Alcohol consumption in relation to cardiovascular diseases and mortality: a systematic review of Mendelian randomization studies. Eur. J. Epidemiol. 1–15 (2021).
Edenberg, H. J. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res. Health 30 , 5–13 (2007).
PubMed PubMed Central Google Scholar
Gelernter, J. et al. Genome-wide association study of maximum habitual alcohol intake in >140,000 U.S. European and African American veterans yields novel risk loci. Biol. Psychiatry 86 , 365–376 (2019).
Article CAS PubMed PubMed Central Google Scholar
Millwood, I. Y. et al. Conventional and genetic evidence on alcohol and vascular disease aetiology: a prospective study of 500 000 men and women in China. Lancet 393 , 1831–1842 (2019).
Au Yeung, S. L. et al. Moderate alcohol use and cardiovascular disease from Mendelian randomization. PLoS ONE 8 , e68054 (2013).
Article ADS PubMed PubMed Central Google Scholar
Lankester, J., Zanetti, D., Ingelsson, E. & Assimes, T. L. Alcohol use and cardiometabolic risk in the UK Biobank: a Mendelian randomization study. PLoS ONE 16 , e0255801 (2021).
Cho, Y. et al. Alcohol intake and cardiovascular risk factors: a Mendelian randomisation study. Sci. Rep. 5 , 18422 (2015).
Article ADS CAS PubMed PubMed Central Google Scholar
Holmes, M. V. et al. Association between alcohol and cardiovascular disease: Mendelian randomisation analysis based on individual participant data. BMJ 349 , g4164 (2014).
Biddinger, K. J. et al. Association of habitual alcohol intake with risk of cardiovascular disease. JAMA Netw. Open 5 , e223849–e223849 (2022).
Zheng, P. et al. The Burden of Proof studies: assessing the evidence of risk. Nat. Med. 28 , 2038–2044 (2022).
Zheng, P., Barber, R., Sorensen, R. J., Murray, C. J. & Aravkin, A. Y. Trimmed constrained mixed effects models: formulations and algorithms. J. Comput. Graph. Stat. 30 , 544–556 (2021).
Article MathSciNet Google Scholar
Egger, M., Smith, G. D., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315 , 629–634 (1997).
Roerecke, M. & Rehm, J. Alcohol consumption, drinking patterns, and ischemic heart disease: a narrative review of meta-analyses and a systematic review and meta-analysis of the impact of heavy drinking occasions on risk for moderate drinkers. BMC Med. 12 , 182 (2014).
Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 10 , 89 (2021).
Stevens, G. A. et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. PLoS Med. 13 , e1002056 (2016).
Griswold, M. G. et al. Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 392 , 1015–1035 (2018).
Albert, C. M. et al. Moderate alcohol consumption and the risk of sudden cardiac death among US male physicians. Circulation 100 , 944–950 (1999).
Arriola, L. et al. Alcohol intake and the risk of coronary heart disease in the Spanish EPIC cohort study. Heart 96 , 124–130 (2010).
Bazzano, L. A. et al. Alcohol consumption and risk of coronary heart disease among Chinese men. Int. J. Cardiol. 135 , 78–85 (2009).
Bell, S. et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ 356 , j909 (2017).
Bergmann, M. M. et al. The association of pattern of lifetime alcohol use and cause of death in the European prospective investigation into cancer and nutrition (EPIC) study. Int. J. Epidemiol. 42 , 1772–1790 (2013).
Beulens, J. W. J. et al. Alcohol consumption and risk for coronary heart disease among men with hypertension. Ann. Intern. Med. 146 , 10–19 (2007).
Bobak, M. et al. Alcohol, drinking pattern and all-cause, cardiovascular and alcohol-related mortality in Eastern Europe. Eur. J. Epidemiol. 31 , 21–30 (2016).
Boffetta, P. & Garfinkel, L. Alcohol drinking and mortality among men enrolled in an American Cancer Society prospective study. Epidemiology 1 , 342–348 (1990).
Britton, A. & Marmot, M. Different measures of alcohol consumption and risk of coronary heart disease and all-cause mortality: 11-year follow-up of the Whitehall II Cohort Study. Addiction 99 , 109–116 (2004).
Camargo, C. A. et al. Moderate alcohol consumption and risk for angina pectoris or myocardial infarction in U.S. male physicians. Ann. Intern. Med. 126 , 372–375 (1997).
Chang, J. Y., Choi, S. & Park, S. M. Association of change in alcohol consumption with cardiovascular disease and mortality among initial nondrinkers. Sci. Rep. 10 , 13419 (2020).
Chiuve, S. E. et al. Light-to-moderate alcohol consumption and risk of sudden cardiac death in women. Heart Rhythm 7 , 1374–1380 (2010).
Colditz, G. A. et al. Moderate alcohol and decreased cardiovascular mortality in an elderly cohort. Am. Heart J. 109 , 886–889 (1985).
Dai, J., Mukamal, K. J., Krasnow, R. E., Swan, G. E. & Reed, T. Higher usual alcohol consumption was associated with a lower 41-y mortality risk from coronary artery disease in men independent of genetic and common environmental factors: the prospective NHLBI Twin Study. Am. J. Clin. Nutr. 102 , 31–39 (2015).
Dam, M. K. et al. Five year change in alcohol intake and risk of breast cancer and coronary heart disease among postmenopausal women: prospective cohort study. BMJ 353 , i2314 (2016).
Degerud, E. et al. Associations of binge drinking with the risks of ischemic heart disease and stroke: a study of pooled Norwegian Health Surveys. Am. J. Epidemiol. 190 , 1592–1603 (2021).
de Labry, L. O. et al. Alcohol consumption and mortality in an American male population: recovering the U-shaped curve–findings from the normative Aging Study. J. Stud. Alcohol 53 , 25–32 (1992).
Doll, R., Peto, R., Boreham, J. & Sutherland, I. Mortality in relation to alcohol consumption: a prospective study among male British doctors. Int. J. Epidemiol. 34 , 199–204 (2005).
Dyer, A. R. et al. Alcohol consumption and 17-year mortality in the Chicago Western Electric Company study. Prev. Med. 9 , 78–90 (1980).
Ebbert, J. O., Janney, C. A., Sellers, T. A., Folsom, A. R. & Cerhan, J. R. The association of alcohol consumption with coronary heart disease mortality and cancer incidence varies by smoking history. J. Gen. Intern. Med. 20 , 14–20 (2005).
Ebrahim, S. et al. Alcohol dehydrogenase type 1 C (ADH1C) variants, alcohol consumption traits, HDL-cholesterol and risk of coronary heart disease in women and men: British Women’s Heart and Health Study and Caerphilly cohorts. Atherosclerosis 196 , 871–878 (2008).
Friedman, L. A. & Kimball, A. W. Coronary heart disease mortality and alcohol consumption in Framingham. Am. J. Epidemiol. 124 , 481–489 (1986).
Fuchs, F. D. et al. Association between alcoholic beverage consumption and incidence of coronary heart disease in whites and blacks: the Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 160 , 466–474 (2004).
Garfinkel, L., Boffetta, P. & Stellman, S. D. Alcohol and breast cancer: a cohort study. Prev. Med. 17 , 686–693 (1988).
Gémes, K. et al. Alcohol consumption is associated with a lower incidence of acute myocardial infarction: results from a large prospective population-based study in Norway. J. Intern. Med. 279 , 365–375 (2016).
Gigleux, I. et al. Moderate alcohol consumption is more cardioprotective in men with the metabolic syndrome. J. Nutr. 136 , 3027–3032 (2006).
Goldberg, R. J., Burchfiel, C. M., Reed, D. M., Wergowske, G. & Chiu, D. A prospective study of the health effects of alcohol consumption in middle-aged and elderly men. The Honolulu Heart Program. Circulation 89 , 651–659 (1994).
Goldberg, R. J. et al. Lifestyle and biologic factors associated with atherosclerotic disease in middle-aged men. 20-year findings from the Honolulu Heart Program. Arch. Intern. Med. 155 , 686–694 (1995).
Gordon, T. & Doyle, J. T. Drinking and coronary heart disease: the Albany Study. Am. Heart J. 110 , 331–334 (1985).
Gun, R. T., Pratt, N., Ryan, P., Gordon, I. & Roder, D. Tobacco and alcohol-related mortality in men: estimates from the Australian cohort of petroleum industry workers. Aust. N.Z. J. Public Health 30 , 318–324 (2006).
Harriss, L. R. et al. Alcohol consumption and cardiovascular mortality accounting for possible misclassification of intake: 11-year follow-up of the Melbourne Collaborative Cohort Study. Addiction 102 , 1574–1585 (2007).
Hart, C. L. & Smith, G. D. Alcohol consumption and mortality and hospital admissions in men from the Midspan collaborative cohort study. Addiction 103 , 1979–1986 (2008).
Henderson, S. O. et al. Established risk factors account for most of the racial differences in cardiovascular disease mortality. PLoS ONE 2 , e377 (2007).
Hippe, M. et al. Familial predisposition and susceptibility to the effect of other risk factors for myocardial infarction. J. Epidemiol. Community Health 53 , 269–276 (1999).
Ikehara, S. et al. Alcohol consumption and mortality from stroke and coronary heart disease among Japanese men and women: the Japan collaborative cohort study. Stroke 39 , 2936–2942 (2008).
Ikehara, S. et al. Alcohol consumption, social support, and risk of stroke and coronary heart disease among Japanese men: the JPHC Study. Alcohol. Clin. Exp. Res. 33 , 1025–1032 (2009).
Iso, H. et al. Alcohol intake and the risk of cardiovascular disease in middle-aged Japanese men. Stroke 26 , 767–773 (1995).
Jakovljević, B., Stojanov, V., Paunović, K., Belojević, G. & Milić, N. Alcohol consumption and mortality in Serbia: twenty-year follow-up study. Croat. Med. J. 45 , 764–768 (2004).
PubMed Google Scholar
Keil, U., Chambless, L. E., Döring, A., Filipiak, B. & Stieber, J. The relation of alcohol intake to coronary heart disease and all-cause mortality in a beer-drinking population. Epidemiology 8 , 150–156 (1997).
Key, T. J. et al. Mortality in British vegetarians: results from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford). Am. J. Clin. Nutr. 89 , 1613S–1619S (2009).
Kitamura, A. et al. Alcohol intake and premature coronary heart disease in urban Japanese men. Am. J. Epidemiol. 147 , 59–65 (1998).
Kivelä, S. L. et al. Alcohol consumption and mortality in aging or aged Finnish men. J. Clin. Epidemiol. 42 , 61–68 (1989).
Klatsky, A. L. et al. Alcohol drinking and risk of hospitalization for heart failure with and without associated coronary artery disease. Am. J. Cardiol. 96 , 346–351 (2005).
Kono, S., Ikeda, M., Tokudome, S., Nishizumi, M. & Kuratsune, M. Alcohol and mortality: a cohort study of male Japanese physicians. Int. J. Epidemiol. 15 , 527–532 (1986).
Kunutsor, S. K. et al. Self-reported alcohol consumption, carbohydrate deficient transferrin and risk of cardiovascular disease: the PREVEND prospective cohort study. Clin. Chim. Acta 520 , 1–7 (2021).
Kurl, S., Jae, S. Y., Voutilainen, A. & Laukkanen, J. A. The combined effect of blood pressure and C-reactive protein with the risk of mortality from coronary heart and cardiovascular diseases. Nutr. Metab. Cardiovasc. Dis. 31 , 2051–2057 (2021).
Larsson, S. C., Wallin, A. & Wolk, A. Contrasting association between alcohol consumption and risk of myocardial infarction and heart failure: two prospective cohorts. Int. J. Cardiol. 231 , 207–210 (2017).
Lazarus, N. B., Kaplan, G. A., Cohen, R. D. & Leu, D. J. Change in alcohol consumption and risk of death from all causes and from ischaemic heart disease. BMJ 303 , 553–556 (1991).
Lee, D.-H., Folsom, A. R. & Jacobs, D. R. Dietary iron intake and Type 2 diabetes incidence in postmenopausal women: the Iowa Women’s Health Study. Diabetologia 47 , 185–194 (2004).
Liao, Y., McGee, D. L., Cao, G. & Cooper, R. S. Alcohol intake and mortality: findings from the National Health Interview Surveys (1988 and 1990). Am. J. Epidemiol. 151 , 651–659 (2000).
Licaj, I. et al. Alcohol consumption over time and mortality in the Swedish Women’s Lifestyle and Health cohort. BMJ Open 6 , e012862 (2016).
Lindschou Hansen, J. et al. Alcohol intake and risk of acute coronary syndrome and mortality in men and women with and without hypertension. Eur. J. Epidemiol. 26 , 439–447 (2011).
Makelä, P., Paljärvi, T. & Poikolainen, K. Heavy and nonheavy drinking occasions, all-cause and cardiovascular mortality and hospitalizations: a follow-up study in a population with a low consumption level. J. Stud. Alcohol 66 , 722–728 (2005).
Malyutina, S. et al. Relation between heavy and binge drinking and all-cause and cardiovascular mortality in Novosibirsk, Russia: a prospective cohort study. Lancet 360 , 1448–1454 (2002).
Maraldi, C. et al. Impact of inflammation on the relationship among alcohol consumption, mortality, and cardiac events: the health, aging, and body composition study. Arch. Intern. Med. 166 , 1490–1497 (2006).
Marques-Vidal, P. et al. Alcohol consumption and cardiovascular disease: differential effects in France and Northern Ireland. The PRIME study. Eur. J. Cardiovasc. Prev. Rehabil. 11 , 336–343 (2004).
Meisinger, C., Döring, A., Schneider, A., Löwel, H. & KORA Study Group. Serum gamma-glutamyltransferase is a predictor of incident coronary events in apparently healthy men from the general population. Atherosclerosis 189 , 297–302 (2006).
Merry, A. H. H. et al. Smoking, alcohol consumption, physical activity, and family history and the risks of acute myocardial infarction and unstable angina pectoris: a prospective cohort study. BMC Cardiovasc. Disord. 11 , 13 (2011).
Miller, G. J., Beckles, G. L., Maude, G. H. & Carson, D. C. Alcohol consumption: protection against coronary heart disease and risks to health. Int. J. Epidemiol. 19 , 923–930 (1990).
Mukamal, K. J., Chiuve, S. E. & Rimm, E. B. Alcohol consumption and risk for coronary heart disease in men with healthy lifestyles. Arch. Intern. Med. 166 , 2145–2150 (2006).
Ng, R., Sutradhar, R., Yao, Z., Wodchis, W. P. & Rosella, L. C. Smoking, drinking, diet and physical activity-modifiable lifestyle risk factors and their associations with age to first chronic disease. Int. J. Epidemiol. 49 , 113–130 (2020).
Onat, A. et al. Moderate and heavy alcohol consumption among Turks: long-term impact on mortality and cardiometabolic risk. Arch. Turkish Soc. Cardiol. 37 , 83–90 (2009).
Google Scholar
Pedersen, J. Ø., Heitmann, B. L., Schnohr, P. & Grønbaek, M. The combined influence of leisure-time physical activity and weekly alcohol intake on fatal ischaemic heart disease and all-cause mortality. Eur. Heart J. 29 , 204–212 (2008).
Reddiess, P. et al. Alcohol consumption and risk of cardiovascular outcomes and bleeding in patients with established atrial fibrillation. Can. Med. Assoc. J. 193 , E117–E123 (2021).
Article CAS Google Scholar
Rehm, J. T., Bondy, S. J., Sempos, C. T. & Vuong, C. V. Alcohol consumption and coronary heart disease morbidity and mortality. Am. J. Epidemiol. 146 , 495–501 (1997).
Renaud, S. C., Guéguen, R., Schenker, J. & d’Houtaud, A. Alcohol and mortality in middle-aged men from eastern France. Epidemiology 9 , 184–188 (1998).
Ricci, C. et al. Alcohol intake in relation to non-fatal and fatal coronary heart disease and stroke: EPIC-CVD case-cohort study. BMJ 361 , k934 (2018).
Rimm, E. B. et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 338 , 464–468 (1991).
Roerecke, M. et al. Heavy drinking occasions in relation to ischaemic heart disease mortality– an 11-22 year follow-up of the 1984 and 1995 US National Alcohol Surveys. Int. J. Epidemiol. 40 , 1401–1410 (2011).
Romelsjö, A., Allebeck, P., Andréasson, S. & Leifman, A. Alcohol, mortality and cardiovascular events in a 35 year follow-up of a nationwide representative cohort of 50,000 Swedish conscripts up to age 55. Alcohol Alcohol. 47 , 322–327 (2012).
Rostron, B. Alcohol consumption and mortality risks in the USA. Alcohol Alcohol. 47 , 334–339 (2012).
Ruidavets, J.-B. et al. Patterns of alcohol consumption and ischaemic heart disease in culturally divergent countries: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). BMJ 341 , c6077 (2010).
Schooling, C. M. et al. Moderate alcohol use and mortality from ischaemic heart disease: a prospective study in older Chinese people. PLoS ONE 3 , e2370 (2008).
Schutte, R., Smith, L. & Wannamethee, G. Alcohol - The myth of cardiovascular protection. Clin. Nutr. 41 , 348–355 (2022).
Sempos, C., Rehm, J., Crespo, C. & Trevisan, M. No protective effect of alcohol consumption on coronary heart disease (CHD) in African Americans: average volume of drinking over the life course and CHD morbidity and mortality in a U.S. national cohort. Contemp. Drug Probl. 29 , 805–820 (2002).
Shaper, A. G., Wannamethee, G. & Walker, M. Alcohol and coronary heart disease: a perspective from the British Regional Heart Study. Int. J. Epidemiol. 23 , 482–494 (1994).
Simons, L. A., McCallum, J., Friedlander, Y. & Simons, J. Alcohol intake and survival in the elderly: a 77 month follow-up in the Dubbo study. Aust. N.Z. J. Med. 26 , 662–670 (1996).
Skov-Ettrup, L. S., Eliasen, M., Ekholm, O., Grønbæk, M. & Tolstrup, J. S. Binge drinking, drinking frequency, and risk of ischaemic heart disease: a population-based cohort study. Scand. J. Public Health 39 , 880–887 (2011).
Snow, W. M., Murray, R., Ekuma, O., Tyas, S. L. & Barnes, G. E. Alcohol use and cardiovascular health outcomes: a comparison across age and gender in the Winnipeg Health and Drinking Survey Cohort. Age Ageing 38 , 206–212 (2009).
Song, R. J. et al. Alcohol consumption and risk of coronary artery disease (from the Million Veteran Program). Am. J. Cardiol. 121 , 1162–1168 (2018).
Streppel, M. T., Ocké, M. C., Boshuizen, H. C., Kok, F. J. & Kromhout, D. Long-term wine consumption is related to cardiovascular mortality and life expectancy independently of moderate alcohol intake: the Zutphen Study. J. Epidemiol. Community Health 63 , 534–540 (2009).
Suhonen, O., Aromaa, A., Reunanen, A. & Knekt, P. Alcohol consumption and sudden coronary death in middle-aged Finnish men. Acta Med. Scand. 221 , 335–341 (1987).
Thun, M. J. et al. Alcohol consumption and mortality among middle-aged and elderly U.S. adults. N. Engl. J. Med. 337 , 1705–1714 (1997).
Tolstrup, J. et al. Prospective study of alcohol drinking patterns and coronary heart disease in women and men. BMJ 332 , 1244–1248 (2006).
Wannamethee, G. & Shaper, A. G. Alcohol and sudden cardiac death. Br. Heart J. 68 , 443–448 (1992).
Wannamethee, S. G. & Shaper, A. G. Type of alcoholic drink and risk of major coronary heart disease events and all-cause mortality. Am. J. Public Health 89 , 685–690 (1999).
Wilkins, K. Moderate alcohol consumption and heart disease. Health Rep. 14 , 9–24 (2002).
Yang, L. et al. Alcohol drinking and overall and cause-specific mortality in China: nationally representative prospective study of 220,000 men with 15 years of follow-up. Int. J. Epidemiol. 41 , 1101–1113 (2012).
Yi, S. W., Yoo, S. H., Sull, J. W. & Ohrr, H. Association between alcohol drinking and cardiovascular disease mortality and all-cause mortality: Kangwha Cohort Study. J. Prev. Med. Public Health 37 , 120–126 (2004).
Younis, J., Cooper, J. A., Miller, G. J., Humphries, S. E. & Talmud, P. J. Genetic variation in alcohol dehydrogenase 1C and the beneficial effect of alcohol intake on coronary heart disease risk in the Second Northwick Park Heart Study. Atherosclerosis 180 , 225–232 (2005).
Yusuf, S. et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet 395 , 795–808 (2020).
Zhang, Y. et al. Association of drinking pattern with risk of coronary heart disease incidence in the middle-aged and older Chinese men: results from the Dongfeng-Tongji cohort. PLoS ONE 12 , e0178070 (2017).
Augustin, L. S. A. et al. Alcohol consumption and acute myocardial infarction: a benefit of alcohol consumed with meals? Epidemiology 15 , 767–769 (2004).
Bianchi, C., Negri, E., La Vecchia, C. & Franceschi, S. Alcohol consumption and the risk of acute myocardial infarction in women. J. Epidemiol. Community Health 47 , 308–311 (1993).
Brenner, H. et al. Coronary heart disease risk reduction in a predominantly beer-drinking population. Epidemiology 12 , 390–395 (2001).
Dorn, J. M. et al. Alcohol drinking pattern and non-fatal myocardial infarction in women. Addiction 102 , 730–739 (2007).
Fan, A. Z., Ruan, W. J. & Chou, S. P. Re-examining the relationship between alcohol consumption and coronary heart disease with a new lens. Prev. Med. 118 , 336–343 (2019).
Fumeron, F. et al. Alcohol intake modulates the effect of a polymorphism of the cholesteryl ester transfer protein gene on plasma high density lipoprotein and the risk of myocardial infarction. J. Clin. Investig. 96 , 1664–1671 (1995).
Gaziano, J. M. et al. Moderate alcohol intake, increased levels of high-density lipoprotein and its subfractions, and decreased risk of myocardial infarction. N. Engl. J. Med. 329 , 1829–1834 (1993).
Genchev, G. D., Georgieva, L. M., Weijenberg, M. P. & Powles, J. W. Does alcohol protect against ischaemic heart disease in Bulgaria? A case-control study of non-fatal myocardial infarction in Sofia. Cent. Eur. J. Public Health 9 , 83–86 (2001).
CAS PubMed Google Scholar
Hammar, N., Romelsjö, A. & Alfredsson, L. Alcohol consumption, drinking pattern and acute myocardial infarction. A case referent study based on the Swedish Twin Register. J. Intern. Med. 241 , 125–131 (1997).
Hines, L. M. et al. Genetic variation in alcohol dehydrogenase and the beneficial effect of moderate alcohol consumption on myocardial infarction. N. Engl. J. Med. 344 , 549–555 (2001).
Ilic, M., Grujicic Sipetic, S., Ristic, B. & Ilic, I. Myocardial infarction and alcohol consumption: a case-control study. PLoS ONE 13 , e0198129 (2018).
Jackson, R., Scragg, R. & Beaglehole, R. Alcohol consumption and risk of coronary heart disease. BMJ 303 , 211–216 (1991).
Kabagambe, E. K., Baylin, A., Ruiz-Narvaez, E., Rimm, E. B. & Campos, H. Alcohol intake, drinking patterns, and risk of nonfatal acute myocardial infarction in Costa Rica. Am. J. Clin. Nutr. 82 , 1336–1345 (2005).
Kalandidi, A. et al. A case-control study of coronary heart disease in Athens, Greece. Int. J. Epidemiol. 21 , 1074–1080 (1992).
Kaufman, D. W., Rosenberg, L., Helmrich, S. P. & Shapiro, S. Alcoholic beverages and myocardial infarction in young men. Am. J. Epidemiol. 121 , 548–554 (1985).
Kawanishi, M., Nakamoto, A., Konemori, G., Horiuchi, I. & Kajiyama, G. Coronary sclerosis risk factors in males with special reference to lipoproteins and apoproteins: establishing an index. Hiroshima J. Med. Sci. 39 , 61–64 (1990).
Kono, S. et al. Alcohol intake and nonfatal acute myocardial infarction in Japan. Am. J. Cardiol. 68 , 1011–1014 (1991).
Mehlig, K. et al. CETP TaqIB genotype modifies the association between alcohol and coronary heart disease: the INTERGENE case-control study. Alcohol 48 , 695–700 (2014).
Miyake, Y. Risk factors for non-fatal acute myocardial infarction in middle-aged and older Japanese. Fukuoka Heart Study Group. Jpn. Circ. J. 64 , 103–109 (2000).
Oliveira, A., Barros, H., Azevedo, A., Bastos, J. & Lopes, C. Impact of risk factors for non-fatal acute myocardial infarction. Eur. J. Epidemiol. 24 , 425–432 (2009).
Oliveira, A., Barros, H. & Lopes, C. Gender heterogeneity in the association between lifestyles and non-fatal acute myocardial infarction. Public Health Nutr. 12 , 1799–1806 (2009).
Romelsjö, A. et al. Abstention, alcohol use and risk of myocardial infarction in men and women taking account of social support and working conditions: the SHEEP case-control study. Addiction 98 , 1453–1462 (2003).
Schröder, H. et al. Myocardial infarction and alcohol consumption: a population-based case-control study. Nutr. Metab. Cardiovasc. Dis. 17 , 609–615 (2007).
Scragg, R., Stewart, A., Jackson, R. & Beaglehole, R. Alcohol and exercise in myocardial infarction and sudden coronary death in men and women. Am. J. Epidemiol. 126 , 77–85 (1987).
Tavani, A., Bertuzzi, M., Gallus, S., Negri, E. & La Vecchia, C. Risk factors for non-fatal acute myocardial infarction in Italian women. Prev. Med. 39 , 128–134 (2004).
Tavani, A. et al. Intake of specific flavonoids and risk of acute myocardial infarction in Italy. Public Health Nutr. 9 , 369–374 (2006).
Zhou, X., Li, C., Xu, W., Hong, X. & Chen, J. Relation of alcohol consumption to angiographically proved coronary artery disease in chinese men. Am. J. Cardiol. 106 , 1101–1103 (2010).
Yang, Y. et al. Alcohol consumption and risk of coronary artery disease: a dose-response meta-analysis of prospective studies. Nutrition 32 , 637–644 (2016).
Zheng, J. et al. Recent developments in Mendelian randomization studies. Curr. Epidemiol. Rep. 4 , 330–345 (2017).
Mukamal, K. J., Stampfer, M. J. & Rimm, E. B. Genetic instrumental variable analysis: time to call Mendelian randomization what it is. The example of alcohol and cardiovascular disease. Eur. J. Epidemiol. 35 , 93–97 (2020).
Verbanck, M., Chen, C.-Y., Neale, B. & Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 50 , 693–698 (2018).
Shi, J. et al. Mendelian randomization with repeated measures of a time-varying exposure: an application of structural mean models. Epidemiology 33 , 84–94 (2022).
Burgess, S., Swanson, S. A. & Labrecque, J. A. Are Mendelian randomization investigations immune from bias due to reverse causation? Eur. J. Epidemiol. 36 , 253–257 (2021).
Davey Smith, G., Holmes, M. V., Davies, N. M. & Ebrahim, S. Mendel’s laws, Mendelian randomization and causal inference in observational data: substantive and nomenclatural issues. Eur. J. Epidemiol. 35 , 99–111 (2020).
Burgess, S. Violation of the constant genetic effect assumption can result in biased estimates for non-linear mendelian randomization. Hum. Hered. 88 , 79–90 (2023).
Tian, H., Mason, A. M., Liu, C. & Burgess, S. Relaxing parametric assumptions for non-linear Mendelian randomization using a doubly-ranked stratification method. PLoS Genet. 19 , e1010823 (2023).
Levin, M. G. & Burgess, S. Mendelian randomization as a tool for cardiovascular research: a review. JAMA Cardiol. 9 , 79–89 (2024).
Burgess, S. et al. Guidelines for performing Mendelian randomization investigations: update for summer 2023. Wellcome Open Res. 4 , 186 (2019).
Bowden, J., Davey Smith, G., Haycock, P. C. & Burgess, S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40 , 304–314 (2016).
Holmes, M. V., Ala-Korpela, M. & Smith, G. D. Mendelian randomization in cardiometabolic disease: challenges in evaluating causality. Nat. Rev. Cardiol. 14 , 577–590 (2017).
Labrecque, J. A. & Swanson, S. A. Interpretation and potential biases of Mendelian randomization estimates with time-varying exposures. Am. J. Epidemiol. 188 , 231–238 (2019).
Spiegelman, D. et al. The Moderate Alcohol and Cardiovascular Health Trial (MACH15): design and methods for a randomized trial of moderate alcohol consumption and cardiometabolic risk. Eur. J. Prev. Cardiol. 27 , 1967–1982 (2020).
DeJong, W. The Moderate Alcohol and Cardiovascular Health Trial: public health advocates should support good science, not undermine it. Eur. J. Prev. Cardiol. 28 , e22–e24 (2021).
National Institutes of Health. NIH to End Funding for Moderate Alcohol and Cardiovascular Health Trial https://www.nih.gov/news-events/news-releases/nih-end-funding-moderate-alcohol-cardiovascular-health-trial (2018).
Miller, L. M., Anderson, C. A. M. & Ix, J. H. Editorial: from MACH15 to MACH0 – a missed opportunity to understand the health effects of moderate alcohol intake. Eur. J. Prev. Cardiol. 28 , e23–e24 (2021).
Anderson, B. O. et al. Health and cancer risks associated with low levels of alcohol consumption. Lancet Public Health 8 , e6–e7 (2023).
Au Yeung, S. L. & Lam, T. H. Unite for a framework convention for alcohol control. Lancet 393 , 1778–1779 (2019).
Hernán, M. A. & Robins, J. M. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol. 183 , 758–764 (2016).
Sudlow, C. et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12 , e1001779 (2015).
The ARIC Investigators. The Atherosclerosis Risk in Communit (ARIC) study: design and objectives. Am. J. Epidemiol. 129 , 687–702 (1989).
Mahmood, S. S., Levy, D., Vasan, R. S. & Wang, T. J. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet 383 , 999–1008 (2014).
Gmel, G. & Rehm, J. Measuring alcohol consumption. Contemp. Drug Probl. 31 , 467–540 (2004).
Guyatt, G. H. et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336 , 924–926 (2008).
Tibshirani, R. Regression shrinkage and selection via the lasso. J. R. Stat. Soc. Ser. B 58 , 267–288 (1996).
Biggerstaff, B. J. & Tweedie, R. L. Incorporating variability in estimates of heterogeneity in the random effects model in meta‐analysis. Stat. Med. 16 , 753–768 (1997).
Download references
Acknowledgements
Research reported in this publication was supported by the Bill & Melinda Gates Foundation [OPP1152504]. S.L. has received grants or contracts from the UK Medical Research Council [MR/T017708/1], CDC Foundation [project number 996], World Health Organization [APW No 2021/1194512], and is affiliated with the NIHR Oxford Biomedical Research Centre. The University of Oxford’s Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU) is supported by core grants from the Medical Research Council [Clinical Trial Service Unit A310] and the British Heart Foundation [CH/1996001/9454]. The CTSU receives research grants from industry that are governed by University of Oxford contracts that protect its independence and has a staff policy of not taking personal payments from industry. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. The funders of the study had no role in study design, data collection, data analysis, data interpretation, writing of the final report, or the decision to publish.
Author information
Authors and affiliations.
Institute for Health Metrics and Evaluation, University of Washington, Seattle, WA, USA
Sinclair Carr, Dana Bryazka, Susan A. McLaughlin, Peng Zheng, Aleksandr Y. Aravkin, Simon I. Hay, Hilary R. Lawlor, Erin C. Mullany, Christopher J. L. Murray, Sneha I. Nicholson, Gregory A. Roth, Reed J. D. Sorensen & Emmanuela Gakidou
Department of Health Metrics Sciences, School of Medicine, University of Washington, Seattle, WA, USA
Peng Zheng, Aleksandr Y. Aravkin, Simon I. Hay, Christopher J. L. Murray, Gregory A. Roth & Emmanuela Gakidou
Clinical Trial Service Unit & Epidemiological Studies Unit, Nuffield Department of Population Health, University of Oxford, Oxford, Oxfordshire, UK
Sarasvati Bahadursingh & Sarah Lewington
Department of Applied Mathematics, University of Washington, Seattle, WA, USA
Aleksandr Y. Aravkin
Institute for Mental Health Policy Research, Centre for Addiction and Mental Health, Toronto, ON, Canada
Jürgen Rehm
Campbell Family Mental Health Research Institute, Centre for Addiction and Mental Health, Toronto, ON, Canada
Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
Department of Psychiatry, University of Toronto, Toronto, ON, Canada
Faculty of Medicine, Institute of Medical Science (IMS), University of Toronto, Toronto, ON, Canada
World Health Organization / Pan American Health Organization Collaborating Centre, Centre for Addiction and Mental Health, Toronto, ON, Canada
Center for Interdisciplinary Addiction Research (ZIS), Department of Psychiatry and Psychotherapy, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany
Institute of Clinical Psychology and Psychotherapy, Technische Universität Dresden, Dresden, Germany
Division of Cardiology, Department of Medicine, University of Washington, Seattle, WA, USA
Gregory A. Roth
You can also search for this author in PubMed Google Scholar
Contributions
S.C., S.A.M., S.I.H., and E.C.M. managed the estimation or publications process. S.C. wrote the first draft of the manuscript. S.C. had primary responsibility for applying analytical methods to produce estimates. S.C. and H.R.L. had primary responsibility for seeking, cataloging, extracting, or cleaning data; designing or coding figures and tables. S.C., D.B., S.B., E.C.M., S.I.N., J.R., and R.J.D.S. provided data or critical feedback on data sources. S.C., D.B., P.Z., A.Y.A., S.I.N., and R.J.D.S. developed methods or computational machinery. S.C., D.B., P.Z., S.B., S.I.H., E.C.M., C.J.L.M., S.I.N., J.R., R.J.D.S., S.L., and E.G. provided critical feedback on methods or results. S.C., D.B., S.A.M., S.B., S.I.H., C.J.L.M., J.R., G.A.R., S.L., and E.G. drafted the work or revised it critically for important intellectual content. S.C., S.I.H., E.C.M., and E.G. managed the overall research enterprise.
Corresponding author
Correspondence to Sinclair Carr .
Ethics declarations
Competing interests.
G.A.R. has received support for this manuscript from the Bill and Melinda Gates Foundation [OPP1152504]. S.L. has received grants or contracts from the UK Medical Research Council [MR/T017708/1], CDC Foundation [project number 996], World Health Organization [APW No 2021/1194512], and is affiliated with the NIHR Oxford Biomedical Research Centre. The University of Oxford’s Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU) is supported by core grants from the Medical Research Council [Clinical Trial Service Unit A310] and the British Heart Foundation [CH/1996001/9454]. The CTSU receives research grants from industry that are governed by University of Oxford contracts that protect its independence and has a staff policy of not taking personal payments from industry. All other authors declare no competing interests.
Peer review
Peer review information.
Nature Communications thanks Shiu Lun Au Yeung, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information, peer review file, reporting summary, source data, source data, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions

About this article
Cite this article.
Carr, S., Bryazka, D., McLaughlin, S.A. et al. A burden of proof study on alcohol consumption and ischemic heart disease. Nat Commun 15 , 4082 (2024). https://doi.org/10.1038/s41467-024-47632-7
Download citation
Received : 14 June 2023
Accepted : 08 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1038/s41467-024-47632-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

The NIAAA is the lead agency for U.S. research on the causes, consequences, prevention and treatment of alcohol use disorder and alcohol-related problems.
National Institute on Alcohol Abuse and Alcoholism (NIAAA)
Major research initiatives.
Addressing alcohol-related issues—from basic science to clinical studies.
Extramural Research: Research at Grantee Institutions
Primary areas of research, funding opportunities, and staff listings for Extramural Research Divisions.
Intramural Research: Research in NIAAA Labs
Organization, primary areas of research, and staff listings for Intramural Research Labs.

Guidance and policies for scientists pursuing alcohol research.

NIAAA’s research priorities for the next five years.

NIAAA’s peer-reviewed scientific journal.
Research on factors that compel youth to begin and continue drinking.
Research on medications in development for treatment of alcohol use disorder.
The NIAAA data archive is a data repository that houses and shares human subjects data generated by NIAAA-funded research.
Resources include biological specimens, animals, data, materials, tools, or services made available to any qualified investigato r to accelerate alcohol-related research in a cost-effective manner.
Current and potential alcohol research investigators and trainees are encouraged to subscribe to our new email list to receive NIAAA information and updates relevant to the research community. To sign up, enter your contact information below.
Sign Up for Email Updates
niaaa.nih.gov
An official website of the National Institutes of Health and the National Institute on Alcohol Abuse and Alcoholism

Binge drinking is a growing public health crisis − a neurobiologist explains how research on alcohol use disorder has shifted
Assistant Professor of Biology, Biomedical Engineering and Pharmacology, Penn State
Disclosure statement
Nikki Crowley receives funding from The National Institutes of Health, The Brain and Behavior Research Foundation, and the Penn State Huck Institutes of the Life Sciences endowment funds.
Penn State provides funding as a founding partner of The Conversation US.
View all partners
With the new Amy Winehouse biopic “Back to Black ” in U.S. theaters as of May 17, 2024, the late singer’s relationship with alcohol and drugs is under scrutiny again. In July 2011, Winehouse was found dead in her flat in north London from “death by misadventure” at the age of 27. That’s the official British term used for accidental death caused by a voluntary risk.
Her blood alcohol concentration was 0.416%, more than five times the legal intoxication limit in the U.S. – leading her cause of death to be later adjusted to include “alcohol toxicity” following a second coroner’s inquest.
Nearly 13 years later, alcohol consumption and binge drinking remain a major public health crisis , not just in the U.K. but also in the U.S.
Roughly 1 in 5 U.S. adults report binge drinking at least once a week, with an average of seven drinks per binge episode . This is well over the amount of alcohol thought to produce legal intoxication, commonly defined as a blood alcohol concentration over 0.08% – on average, four drinks in two hours for women, five drinks in two hours for men.
Among women, days of “heavy drinking” increased 41% during the COVID-19 pandemic compared with pre-pandemic levels , and adult women in their 30s and 40s are rapidly increasing their rates of binge drinking , with no evidence of these trends slowing down. Despite efforts to comprehend the overall biology of substance use disorders, scientists’ and physicians’ understanding of the relationship between women’s health and binge drinking has lagged behind.
I am a neurobiologist focused on understanding the chemicals and brain regions that underlie addiction to alcohol . I study how neuropeptides – unique signaling molecules in the prefrontal cortex , one of the key brain regions in decision-making, risk-taking and reward – are altered by repeated exposure to binge alcohol consumption in animal models.
My lab focuses on understanding how things like alcohol alter these brain systems before diagnosable addiction, so that we can better inform efforts toward both prevention and treatment.
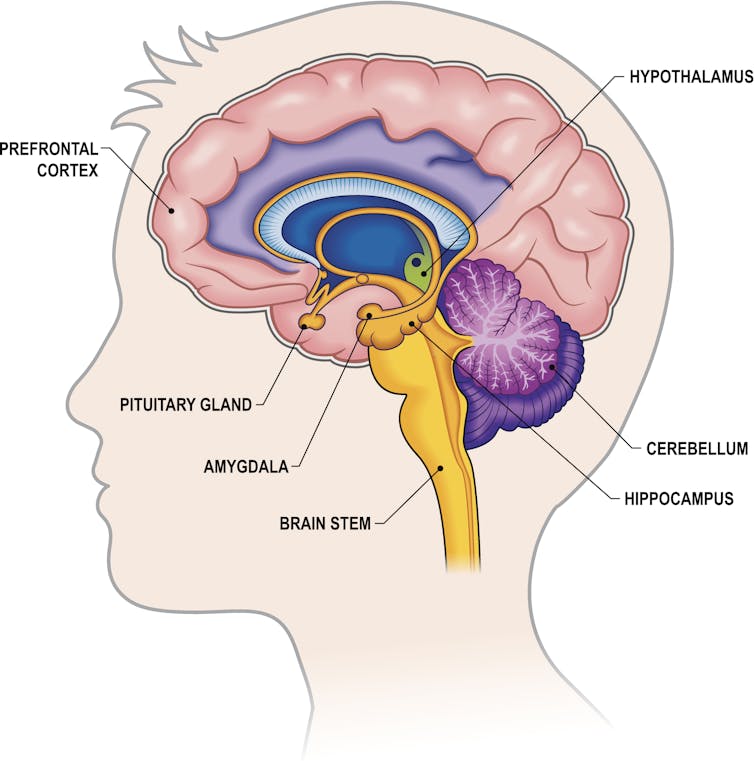
The biology of addiction
While problematic alcohol consumption has likely occurred as long as alcohol has existed, it wasn’t until 2011 that the American Society of Addiction Medicine recognized substance addiction as a brain disorder – the same year as Winehouse’s death. A diagnosis of an alcohol use disorder is now used over outdated terms such as labeling an individual as an alcoholic or having alcoholism.
Researchers and clinicians have made great strides in understanding how and why drugs – including alcohol, a drug – alter the brain. Often, people consume a drug like alcohol because of the rewarding and positive feelings it creates, such as enjoying drinks with friends or celebrating a milestone with a loved one. But what starts off as manageable consumption of alcohol can quickly devolve into cycles of excessive alcohol consumption followed by drug withdrawal.
While all forms of alcohol consumption come with health risks, binge drinking appears to be particularly dangerous due to how repeated cycling between a high state and a withdrawal state affect the brain. For example, for some people, alcohol use can lead to “ hangxiety ,” the feeling of anxiety that can accompany a hangover.
Repeated episodes of drinking and drunkenness, coupled with withdrawal, can spiral, leading to relapse and reuse of alcohol. In other words, alcohol use shifts from being rewarding to just trying to prevent feeling bad.
It makes sense. With repeated alcohol use over time, the areas of the brain engaged by alcohol can shift away from those traditionally associated with drug use and reward or pleasure to brain regions more typically engaged during stress and anxiety .
All of these stages of drinking, from the enjoyment of alcohol to withdrawal to the cycles of craving, continuously alter the brain and its communication pathways . Alcohol can affect several dozen neurotransmitters and receptors , making understanding its mechanism of action in the brain complicated.
Work in my lab focuses on understanding how alcohol consumption changes the way neurons within the prefrontal cortex communicate with each other. Neurons are the brain’s key communicator, sending both electrical and chemical signals within the brain and to the rest of your body.
What we’ve found in animal models of binge drinking is that certain subtypes of neurons lose the ability to talk to each other appropriately. In some cases, binge drinking can permanently remodel the brain. Even after a prolonged period of abstinence, conversations between the neurons don’t return to normal .
These changes in the brain can appear even before there are noticeable changes in behavior . This could mean that the neurobiological underpinnings of addiction may take root well before an individual or their loved ones suspect a problem with alcohol.
Researchers like us don’t yet fully understand why some people may be more susceptible to this shift, but it likely has to do with genetic and biological factors, as well as the patterns and circumstances under which alcohol is consumed.
Women are forgotten
While researchers are increasingly understanding the medley of biological factors that underlie addiction, there’s one population that’s been largely overlooked until now: women.
Women may be more likely than men to have some of the most catastrophic health effects caused by alcohol use, such as liver issues, cardiovascular disease and cancer . Middle-aged women are now at the highest risk for binge drinking compared with other populations.
When women consume even moderate levels of alcohol, their risk for various cancers goes up, including digestive, breast and pancreatic cancer , among other health problems – and even death. So the worsening rates of alcohol use disorder in women prompt the need for a greater focus on women in the research and the search for treatments.
Yet, women have long been underrepresented in biomedical research.
It wasn’t until 1993 that clinical research funded by the National Institutes of Health was required to include women as research subjects. In fact, the NIH did not even require sex as a biological variable to be considered by federally funded researchers until 2016. When women are excluded from biomedical research, it leaves doctors and researchers with an incomplete understanding of health and disease, including alcohol addiction.
There is also increasing evidence that addictive substances can interact with cycling sex hormones such as estrogen and progesterone . For instance, research has shown that when estrogen levels are high, like before ovulation, alcohol might feel more rewarding , which could drive higher levels of binge drinking. Currently, researchers don’t know the full extent of the interaction between these natural biological rhythms or other unique biological factors involved in women’s health and propensity for alcohol addiction.

Looking ahead
Researchers and lawmakers are recognizing the vital need for increased research on women’s health. Major federal investments into women’s health research are a vital step toward developing better prevention and treatment options for women.
While women like Amy Winehouse may have been forced to struggle both privately and publicly with substance use disorders and alcohol, the increasing focus of research on addiction to alcohol and other substances as a brain disorder will open new treatment avenues for those suffering from the consequences.
For more information on alcohol use disorder, causes, prevention and treatments, visit the National Institute on Alcohol Abuse and Alcoholism .
- Amy Winehouse
- Binge drinking
- Neurobiology
- Intoxication
- Alcohol consumption
- Alcohol use
- Alcohol use disorder
- COVID-19 pandemic

Compliance Lead

Lecturer / Senior Lecturer - Marketing

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health

Lecturer/Senior Lecturer, Earth System Science (School of Science)

Alcohol and Your Brain: The Latest Scientific Insights
Want to protect your brain here's what you need to know about alcohol consumption..
Posted March 18, 2024 | Reviewed by Devon Frye
- What Is Alcoholism?
- Find a therapist to overcome addiction
- Transient memory loss, “blackouts,” and hangovers related to alcohol consumption are brain health risks.
- Alcohol use disorder (alcoholism) is a risk factor for developing dementia.
- Heavy or excessive alcohol consumption is dangerous to the brain for a number of reasons.
- The impact of mild to moderate alcohol consumption (1-3 drinks a day) on brain function is less clear.

Depending on who you ask, you might be told to drink a few glasses of red wine a day or to avoid alcohol altogether. The reasons for such recommendations are many, but, by and large, they tend to stem from a study someone read about or saw reported in the news.
So why is it so hard to know whether alcohol is good or bad for us—especially for our brains? In this post, we’ll explore the current science and some practical ideas on how to approach the topic.
What Is Alcohol Anyway?
When people talk about drinking “alcohol,” they’re almost always referring to the consumption of ethanol. Ethanol is a natural product that is formed from the fermentation of grains, fruits, and other sources of sugar. It’s found in a wide range of alcoholic beverages including beer, wine, and spirits like vodka, whiskey, rum, and gin.
Evidence for human consumption of alcohol dates back over 10,000 years. Consumption of alcohol has and continues to serve major roles in religious and cultural ceremonies around the world. But unlike most food products, in the last century, alcohol has been wrapped up in nearly perpetual controversy over its moral effects and health implications.
How Does Alcohol Impact the Brain?
As anyone who’s consumed alcohol knows, ethanol can directly influence brain function. Ethanol is classified as a “depressant” because it has a generally slowing effect on brain activity through activation of γ-aminobutyric acid (GABA) pathways.
In an acute sense, consumption of alcohol can lead to uninhibited behavior, sedation, lapses in judgment, and impairments in motor function. At higher levels, the effects can progress to coma and even death.
The Known Brain-Damaging Effects of Excess Alcohol
There is no debate here: Excessively high levels of alcohol consumption over short periods of time are toxic and potentially deadly, specifically because of its effects on the brain.
One critical fact to understand about the overall and brain-specific effects of alcohol is that the entirety of the debate around the risk/benefit ratio concerns mild to moderate alcohol consumption. As it relates to the effects of high amounts of alcohol on the body and brain, the research is consistent: It’s a very bad choice.
High amounts of alcohol use are causal risk factors in the development of disease in the heart, liver, pancreas, and brain (including the brains of children in utero). In fact, 1 in 8 deaths in Americans aged 20-64 is attributable to alcohol use. When it comes to adults, excessive alcohol use can cause multiple well-defined brain issues ranging from short-term confusion to dementia .
What Is “Excessive” or “High” Alcohol Use?
Key to the nuance in the conversation about alcohol use are definitions. Across the board, “excessive” or “high” alcohol use is linked to worse overall and brain health outcomes. So what does that mean?
While definitions can be variable, one way to look at this is the consumption of 4 or more drinks on an occasion (for women) and 5 or more for men. Additionally, excess alcohol is defined as drinking more than 8 drinks a week (women) and 15 a week (men), or consuming alcohol if you are pregnant or younger than age 21.
Beyond this, by definition, consuming enough alcohol to cause a “brownout,” “blackout,” hangover, or other overt brain symptomatology is evidence that the alcohol you’ve consumed is creating problems in your brain. Alcohol use disorder (or alcoholism ) is also a clear issue for the brain. It has been linked to a higher risk for dementia, especially early-onset dementia in a study of 262,000 adults, as well as to smaller brain size .
Is There a “Safe” Amount of Alcohol for the Brain?
In a highly publicized article from Nature Communications , researchers looked at brain imaging data from nearly 37,000 middle-aged to older adults and cross-referenced their brain scans with their reported alcohol consumption. The findings were profound: People who drank more alcohol had smaller brains, even in people drinking only one or two alcoholic beverages a day.

Conversely, other recent data suggest a lower risk for dementia in people consuming a few alcoholic beverages a day. This includes a 2022 study showing that in around 27,000 people, consuming up to 40 grams of alcohol (around 2.5 drinks) a day was linked to a lower risk for dementia versus abstinence in adults over age 60. A much larger study of almost 4 million people in Korea noted that mild to moderate alcohol consumption was linked to a lower risk for dementia compared to non-drinking.
How Do We Make Sense of This Data?
When it comes to the bottom line as it relates to alcohol consumption and brain health, the data are rather solid on some fronts, and a bit less so on others. There’s also the potential for confounding variables, including the fact that many people like to drink alcohol to enjoy and enhance social bonds (which we know are beneficial for the brain). Here’s a summary of what the most recent research is telling us.
- Experiencing transient memory loss, “blackouts,” or hangovers related to alcohol consumption is overt evidence of threats to brain health.
- The impact of mild to moderate alcohol consumption (1-3 drinks a day) on brain function is less clear, but it seems unreasonable to start alcohol use for brain health.

Austin Perlmutter, M.D. , is a board-certified internal medicine physician and the co-author of Brain Wash .
- Find a Therapist
- Find a Treatment Center
- Find a Psychiatrist
- Find a Support Group
- Find Online Therapy
- United States
- Brooklyn, NY
- Chicago, IL
- Houston, TX
- Los Angeles, CA
- New York, NY
- Portland, OR
- San Diego, CA
- San Francisco, CA
- Seattle, WA
- Washington, DC
- Asperger's
- Bipolar Disorder
- Chronic Pain
- Eating Disorders
- Passive Aggression
- Personality
- Goal Setting
- Positive Psychology
- Stopping Smoking
- Low Sexual Desire
- Relationships
- Child Development
- Self Tests NEW
- Therapy Center
- Diagnosis Dictionary
- Types of Therapy

At any moment, someone’s aggravating behavior or our own bad luck can set us off on an emotional spiral that threatens to derail our entire day. Here’s how we can face our triggers with less reactivity so that we can get on with our lives.
- Emotional Intelligence
- Gaslighting
- Affective Forecasting
- Neuroscience
- Signs of Addiction
Addiction Research
Discover the latest in addiction research, from the neuroscience of substance use disorders to evidence-based treatment practices. reports, updates, case studies and white papers are available to you at hazelden betty ford’s butler center for research..

Why do people become addicted to alcohol and other drugs? How effective is addiction treatment? What makes certain substances so addictive? The Butler Center for Research at the Hazelden Betty Ford Foundation investigates these and other questions and publishes its scientific findings in a variety of alcohol and drug addiction research papers and reports. Research topics include:
- Evidence-based treatment practices
- Addiction treatment outcomes
- Addiction, psychiatry and the brain
- Addictive substances such as prescription opioids and heroin
- Substance abuse in youth/teens, older adults and other demographic groups such as health care or legal professionals
These research queries and findings are presented in the form of updates, white papers and case studies. In addition, the Butler Center for Research collaborates with the Recovery Advocacy team to study special-focus addiction research topics, summarized in monthly Emerging Drug Trends reports. Altogether, these studies provide the latest in addiction research for anyone interested in learning more about the neuroscience of addiction and how addiction affects individuals, families and society in general. The research also helps clinicians and health care professionals further understand, diagnose and treat drug and alcohol addiction. Learn more about each of the Butler Center's addiction research studies below.
Research Updates
Written by Butler Center for Research staff, our one-page, topic-specific summaries discuss current research on topics of interest within the drug abuse and addiction treatment field.
View our most recent updates, or view the archive at the bottom of the page.
Patient Outcomes Study Results at Hazelden Betty Ford
Trends and Patterns in Cannabis Use across Different Age Groups
Alcohol and Tobacco Harm Reduction Interventions
Harm Reduction: History and Context
Racial and Ethnic Health Disparities and Addiction
Psychedelics as Therapeutic Treatment
Sexual and Gender Minority Youth and SUDs
Health Care Professionals and Mental Health
Grief and Addiction
Helping Families Cope with Addiction
Emerging Drug Trends Report and National Surveys
Shedding New Light on America’s No. 1 Health Problem
In collaboration with the University of Maryland School of Public Health and with support from the Butler Center for Research, the Recovery Advocacy team routinely issues research reports on emerging drug trends in America. Recovery Advocacy also commissions national surveys on attitudes, behaviors and perspectives related to substance use. From binge drinking and excessive alcohol use on college campuses, to marijuana potency concerns in an age of legalized marijuana, deeper analysis and understanding of emerging drug trends allows for greater opportunities to educate, inform and prevent misuse and deaths.
Each drug trends report explores the topic at hand, documenting the prevalence of the problem, relevant demographics, prevention and treatment options available, as well as providing insight and perspectives from thought leaders throughout the Hazelden Betty Ford Foundation.
View the latest Emerging Drug Trends Report:
Pediatricians First Responders for Preventing Substance Use
- Clearing Away the Confusion: Marijuana Is Not a Public Health Solution to the Opioid Crisis
- Does Socioeconomic Advantage Lessen the Risk of Adolescent Substance Use?
- The Collegiate Recovery Movement Is Gaining Strength
- Considerations for Policymakers Regarding Involuntary Commitment for Substance Use Disorders
- Widening the Lens on the Opioid Crisis
- Concerns Rising Over High-Potency Marijuana Use
- Beyond Binging: “High-Intensity Drinking”
View the latest National Surveys :
- College Administrators See Problems As More Students View Marijuana As Safe
College Parents See Serious Problems From Campus Alcohol Use
- Youth Opioid Study: Attitudes and Usage
About Recovery Advocacy
Our mission is to provide a trusted national voice on all issues related to addiction prevention, treatment and recovery, and to facilitate conversation among those in recovery, those still suffering and society at large. We are committed to smashing stigma, shaping public policy and educating people everywhere about the problems of addiction and the promise of recovery. Learn more about recovery advocacy and how you can make a difference.
Evidence-Based Treatment Series
To help get consumers and clinicians on the same page, the Butler Center for Research has created a series of informational summaries describing:
- Evidence-based addiction treatment modalities
- Distinctive levels of substance use disorder treatment
- Specialized drug and alcohol treatment programs
Each evidence-based treatment series summary includes:
- A definition of the therapeutic approach, level of care or specialized program
- A discussion of applicability, usage and practice
- A description of outcomes and efficacy
- Research citations and related resources for more information
View the latest in this series:
Motivational Interviewing
Cognitive Behavioral Therapy
Case Studies and White Papers
Written by Hazelden Betty Ford Foundation researchers and clinicians, case studies and white papers presented by the Butler Center for Research provide invaluable insight into clinical processes and complex issues related to addiction prevention, treatment and recovery. These in-depth reports examine and chronicle clinical activities, initiatives and developments as a means of informing practitioners and continually improving the quality and delivery of substance use disorder services and related resources and initiatives.
- What does it really mean to be providing medication-assisted treatment for opioid addiction?
Adolescent Motivational Interviewing
Peer Recovery Support: Walking the Path Together
Addiction and Violence During COVID-19
The Brain Disease Model of Addiction
Healthcare Professionals and Compassion Fatigue
Moving to Trauma-Responsive Care
Virtual Intensive Outpatient Outcomes: Preliminary Findings
Driving Under the Influence of Cannabis
Vaping and E-Cigarettes
Using Telehealth for Addiction Treatment
Grandparents Raising Grandchildren
Substance Use Disorders Among Military Populations
Co-Occurring Mental Health and Substance Use Disorders
Women and Alcohol
Prescription Rates of Opioid Analgesics in Medical Treatment Settings
Applications of Positive Psychology to Substance Use Disorder
Substance Use Disorders Among Legal Professionals
Factors Impacting Early Alcohol and Drug Use Among Youths
Animal-Assisted Therapy for Substance Use Disorders
Prevalence of Adolescent Substance Misuse
Problem Drinking Behaviors Among College Students
The Importance of Recovery Management
Substance Use Factors Among LGBTQ individuals
Prescription Opioids and Dependence
Alcohol Abuse Among Law Enforcement Officers
Helping Families Cope with Substance Dependence
The Social Norms Approach to Student Substance Abuse Prevention
Drug Abuse, Dopamine and the Brain's Reward System
Women and Substance Abuse
Substance Use in the Workplace
Health Care Professionals: Addiction and Treatment
Cognitive Improvement and Alcohol Recovery
Drug Use, Misuse and Dependence Among Older Adults
Emerging Drug Trends
Does Socioeconomic Advantage Lessen the Risk of Adolescent Substance Use
The Collegiate Recovery Movement is Gaining Strength
Involuntary Commitment for Substance Use Disorders
Widening the Lens of the Opioid Crisis
Beyond Binge Drinking: High Intensity Drinking
High Potency Marijuana
National Surveys
College Administrators See Problems as More Students View Marijuana as Safe
Risky Opioid Use Among College-Age Youth
Case Studies/ White Papers
What does it really mean to be providing medication-assisted treatment for opioid addiction
Are you or a loved one struggling with alcohol or other drugs? Call today to speak confidentially with a recovery expert. Most insurance accepted.
Harnessing science, love and the wisdom of lived experience, we are a force of healing and hope for individuals, families and communities affected by substance use and mental health conditions..

How Binge Drinking Shifted Research On Alcohol Use Disorders
W ith the new Amy Winehouse biopic "Back to Black " in U.S. theaters as of May 17, 2024, the late singer's relationship with alcohol and drugs is under scrutiny again. In July 2011, Winehouse was found dead in her flat in north London from "death by misadventure" at the age of 27. That's the official British term used for accidental death caused by a voluntary risk.
Her blood alcohol concentration was 0.416%, more than five times the legal intoxication limit in the U.S. – leading her cause of death to be later adjusted to include "alcohol toxicity" following a second coroner's inquest.
Nearly 13 years later, alcohol consumption and binge drinking remain a major public health crisis , not just in the U.K. but also in the U.S.
Roughly 1 in 5 U.S. adults report binge drinking at least once a week, with an average of seven drinks per binge episode . This is well over the amount of alcohol thought to produce legal intoxication, commonly defined as a blood alcohol concentration over 0.08% – on average, four drinks in two hours for women, five drinks in two hours for men.
Among women, days of "heavy drinking" increased 41% during the COVID-19 pandemic compared with pre-pandemic levels , and adult women in their 30s and 40s are rapidly increasing their rates of binge drinking , with no evidence of these trends slowing down. Despite efforts to comprehend the overall biology of substance use disorders, scientists' and physicians' understanding of the relationship between women's health and binge drinking has lagged behind.
I am a neurobiologist focused on understanding the chemicals and brain regions that underlie addiction to alcohol . I study how neuropeptides – unique signaling molecules in the prefrontal cortex , one of the key brain regions in decision-making, risk-taking and reward – are altered by repeated exposure to binge alcohol consumption in animal models.
My lab focuses on understanding how things like alcohol alter these brain systems before diagnosable addiction, so that we can better inform efforts toward both prevention and treatment.
Signaling molecules in the prefrontal cortex are altered by repeated exposure to excessive alcohol consumption in animal models. jambojam/iStock via Getty Images
The Biology Of Addiction
While problematic alcohol consumption has likely occurred as long as alcohol has existed, it wasn't until 2011 that the American Society of Addiction Medicine recognized substance addiction as a brain disorder – the same year as Winehouse's death. A diagnosis of an alcohol use disorder is now used over outdated terms such as labeling an individual as an alcoholic or having alcoholism.
Researchers and clinicians have made great strides in understanding how and why drugs – including alcohol, a drug – alter the brain. Often, people consume a drug like alcohol because of the rewarding and positive feelings it creates, such as enjoying drinks with friends or celebrating a milestone with a loved one. But what starts off as manageable consumption of alcohol can quickly devolve into cycles of excessive alcohol consumption followed by drug withdrawal.
While all forms of alcohol consumption come with health risks, binge drinking appears to be particularly dangerous due to how repeated cycling between a high state and a withdrawal state affect the brain. For example, for some people, alcohol use can lead to " hangxiety ," the feeling of anxiety that can accompany a hangover.
Repeated episodes of drinking and drunkenness, coupled with withdrawal, can spiral, leading to relapse and reuse of alcohol. In other words, alcohol use shifts from being rewarding to just trying to prevent feeling bad.
It makes sense. With repeated alcohol use over time, the areas of the brain engaged by alcohol can shift away from those traditionally associated with drug use and reward or pleasure to brain regions more typically engaged during stress and anxiety .
All of these stages of drinking, from the enjoyment of alcohol to withdrawal to the cycles of craving, continuously alter the brain and its communication pathways . Alcohol can affect several dozen neurotransmitters and receptors , making understanding its mechanism of action in the brain complicated.
Work in my lab focuses on understanding how alcohol consumption changes the way neurons within the prefrontal cortex communicate with each other. Neurons are the brain's key communicator, sending both electrical and chemical signals within the brain and to the rest of your body.
What we've found in animal models of binge drinking is that certain subtypes of neurons lose the ability to talk to each other appropriately. In some cases, binge drinking can permanently remodel the brain. Even after a prolonged period of abstinence, conversations between the neurons don't return to normal .
These changes in the brain can appear even before there are noticeable changes in behavior . This could mean that the neurobiological underpinnings of addiction may take root well before an individual or their loved ones suspect a problem with alcohol.
Researchers like us don't yet fully understand why some people may be more susceptible to this shift, but it likely has to do with genetic and biological factors, as well as the patterns and circumstances under which alcohol is consumed.
Work in the author's lab explores how alcohol use can alter the way neurons communicate in the prefrontal cortex brain region. Estrogen receptors are labeled in purple and receptors for somatostatin, a key regulatory hormone, in blue. Victora Nudell
Women are Forgotten
While researchers are increasingly understanding the medley of biological factors that underlie addiction, there's one population that's been largely overlooked until now: women.
Women may be more likely than men to have some of the most catastrophic health effects caused by alcohol use, such as liver issues, cardiovascular disease and cancer . Middle-aged women are now at the highest risk for binge drinking compared with other populations.
When women consume even moderate levels of alcohol, their risk for various cancers goes up, including digestive, breast and pancreatic cancer , among other health problems – and even death. So the worsening rates of alcohol use disorder in women prompt the need for a greater focus on women in the research and the search for treatments.
Yet, women have long been underrepresented in biomedical research.
It wasn't until 1993 that clinical research funded by the National Institutes of Health was required to include women as research subjects. In fact, the NIH did not even require sex as a biological variable to be considered by federally funded researchers until 2016. When women are excluded from biomedical research, it leaves doctors and researchers with an incomplete understanding of health and disease, including alcohol addiction.
There is also increasing evidence that addictive substances can interact with cycling sex hormones such as estrogen and progesterone . For instance, research has shown that when estrogen levels are high, like before ovulation, alcohol might feel more rewarding , which could drive higher levels of binge drinking. Currently, researchers don't know the full extent of the interaction between these natural biological rhythms or other unique biological factors involved in women's health and propensity for alcohol addiction.
Middle-aged women are at the highest risk for some of the most severe health consequences of binge drinking. Peter Dazeley/The Image Bank via Getty Images
Looking Ahead
Researchers and lawmakers are recognizing the vital need for increased research on women's health. Major federal investments into women's health research are a vital step toward developing better prevention and treatment options for women.
While women like Amy Winehouse may have been forced to struggle both privately and publicly with substance use disorders and alcohol, the increasing focus of research on addiction to alcohol and other substances as a brain disorder will open new treatment avenues for those suffering from the consequences.
For more information on alcohol use disorder, causes, prevention and treatments, visit the National Institute on Alcohol Abuse and Alcoholism .
Nikki Crowley is an Assistant Professor of Biology, Biomedical Engineering and Pharmacology at Penn State. This article is republished from The Conversation under a Creative Commons license . Read the original article .


Take a road trip to Harford County, MD

6/28: It's Happening with Snooki & Joey

How to reinvest in your small business

What to know about plant-based diets

- Health News
- Children's Health
- Entertainment
- Food & Drink
- Restaurants
- Family-Friendly
- Performances
- Fantasy Football
- Staff / Contributors
- Legal / Privacy
© 2024 WWB Holdings, LLC. All rights reserved
- Google Plus
More Health:
- Healthy Eating
- Mental Health
May 17, 2024
- Binge drinking is a growing public health crisis − here's how research on alcohol use disorder has shifted
The act of consuming several drinks within a short time frame can permanently remodel the brain.
All forms of alcohol consumption come with health risks, but binge drinking appears to be particularly dangerous due to how repeated cycling between a high state and a withdrawal state affect the brain.
With the new Amy Winehouse biopic "Back to Black " in U.S. theaters as of May 17, 2024, the late singer's relationship with alcohol and drugs is under scrutiny again. In July 2011, Winehouse was found dead in her flat in north London from "death by misadventure" at the age of 27. That's the official British term used for accidental death caused by a voluntary risk.
Her blood alcohol concentration was 0.416%, more than five times the legal intoxication limit in the U.S. – leading her cause of death to be later adjusted to include "alcohol toxicity" following a second coroner's inquest.
Nearly 13 years later, alcohol consumption and binge drinking remain a major public health crisis , not just in the U.K. but also in the U.S.
Roughly 1 in 5 U.S. adults report binge drinking at least once a week, with an average of seven drinks per binge episode . This is well over the amount of alcohol thought to produce legal intoxication, commonly defined as a blood alcohol concentration over 0.08% – on average, four drinks in two hours for women, five drinks in two hours for men.
Among women, days of "heavy drinking" increased 41% during the COVID-19 pandemic compared with pre-pandemic levels , and adult women in their 30s and 40s are rapidly increasing their rates of binge drinking , with no evidence of these trends slowing down. Despite efforts to comprehend the overall biology of substance use disorders, scientists' and physicians' understanding of the relationship between women's health and binge drinking has lagged behind.
I am a neurobiologist focused on understanding the chemicals and brain regions that underlie addiction to alcohol . I study how neuropeptides – unique signaling molecules in the prefrontal cortex , one of the key brain regions in decision-making, risk-taking and reward – are altered by repeated exposure to binge alcohol consumption in animal models.
My lab focuses on understanding how things like alcohol alter these brain systems before diagnosable addiction, so that we can better inform efforts toward both prevention and treatment.
The biology of addiction
While problematic alcohol consumption has likely occurred as long as alcohol has existed, it wasn't until 2011 that the American Society of Addiction Medicine recognized substance addiction as a brain disorder – the same year as Winehouse's death. A diagnosis of an alcohol use disorder is now used over outdated terms such as labeling an individual as an alcoholic or having alcoholism.
Researchers and clinicians have made great strides in understanding how and why drugs – including alcohol, a drug – alter the brain. Often, people consume a drug like alcohol because of the rewarding and positive feelings it creates, such as enjoying drinks with friends or celebrating a milestone with a loved one. But what starts off as manageable consumption of alcohol can quickly devolve into cycles of excessive alcohol consumption followed by drug withdrawal.
While all forms of alcohol consumption come with health risks, binge drinking appears to be particularly dangerous due to how repeated cycling between a high state and a withdrawal state affect the brain. For example, for some people, alcohol use can lead to " hangxiety ," the feeling of anxiety that can accompany a hangover.
Repeated episodes of drinking and drunkenness, coupled with withdrawal, can spiral, leading to relapse and reuse of alcohol. In other words, alcohol use shifts from being rewarding to just trying to prevent feeling bad.
It makes sense. With repeated alcohol use over time, the areas of the brain engaged by alcohol can shift away from those traditionally associated with drug use and reward or pleasure to brain regions more typically engaged during stress and anxiety .
All of these stages of drinking, from the enjoyment of alcohol to withdrawal to the cycles of craving, continuously alter the brain and its communication pathways . Alcohol can affect several dozen neurotransmitters and receptors , making understanding its mechanism of action in the brain complicated.
Work in my lab focuses on understanding how alcohol consumption changes the way neurons within the prefrontal cortex communicate with each other. Neurons are the brain's key communicator, sending both electrical and chemical signals within the brain and to the rest of your body.
What we've found in animal models of binge drinking is that certain subtypes of neurons lose the ability to talk to each other appropriately. In some cases, binge drinking can permanently remodel the brain. Even after a prolonged period of abstinence, conversations between the neurons don't return to normal .
These changes in the brain can appear even before there are noticeable changes in behavior . This could mean that the neurobiological underpinnings of addiction may take root well before an individual or their loved ones suspect a problem with alcohol.
Researchers like us don't yet fully understand why some people may be more susceptible to this shift, but it likely has to do with genetic and biological factors, as well as the patterns and circumstances under which alcohol is consumed.
Women are forgotten
While researchers are increasingly understanding the medley of biological factors that underlie addiction, there's one population that's been largely overlooked until now: women.
Women may be more likely than men to have some of the most catastrophic health effects caused by alcohol use, such as liver issues, cardiovascular disease and cancer . Middle-aged women are now at the highest risk for binge drinking compared with other populations.
When women consume even moderate levels of alcohol, their risk for various cancers goes up, including digestive, breast and pancreatic cancer , among other health problems – and even death. So the worsening rates of alcohol use disorder in women prompt the need for a greater focus on women in the research and the search for treatments.
Yet, women have long been underrepresented in biomedical research.
It wasn't until 1993 that clinical research funded by the National Institutes of Health was required to include women as research subjects. In fact, the NIH did not even require sex as a biological variable to be considered by federally funded researchers until 2016. When women are excluded from biomedical research, it leaves doctors and researchers with an incomplete understanding of health and disease, including alcohol addiction.
There is also increasing evidence that addictive substances can interact with cycling sex hormones such as estrogen and progesterone . For instance, research has shown that when estrogen levels are high, like before ovulation, alcohol might feel more rewarding , which could drive higher levels of binge drinking. Currently, researchers don't know the full extent of the interaction between these natural biological rhythms or other unique biological factors involved in women's health and propensity for alcohol addiction.
Looking ahead
Researchers and lawmakers are recognizing the vital need for increased research on women's health. Major federal investments into women's health research are a vital step toward developing better prevention and treatment options for women.
While women like Amy Winehouse may have been forced to struggle both privately and publicly with substance use disorders and alcohol, the increasing focus of research on addiction to alcohol and other substances as a brain disorder will open new treatment avenues for those suffering from the consequences.
Nikki Crowley , Assistant Professor of Biology, Biomedical Engineering and Pharmacology, Penn State
This article is republished from The Conversation under a Creative Commons license. Read the original article .
Nikki Crowley, Penn State
Health Videos

Listen to your head…but listen to your gut, too

How socioeconomic factors shape health outcomes in minority communities
Just in.
- Lung cancer is the deadliest of all cancers, and screening could save many lives − if more people could access it
- Pa. native Sabrina Carpenter sings her hit songs, joins a sketch during 'SNL' debut
- Pro-Palestinian encampment set up on Drexel University campus
- Northern Liberties Night Market returns with food trucks and beer gardens
- Demonstration erupts at Penn after pro-Palestinian protesters attempt to occupy academic building
Waiting to remove life support may provide a clearer picture of a patient's outlook, study shows
- Why it's important to pay attention to expiration dates on medications
- For older adults, a broken hip is often more deadly than cancer, study shows
- Stranded in the ER, seniors await hospital care and suffer avoidable harm
- RFK Jr. says a worm ate his brain. A local doctor explains how that can happen
- Combining high intensity exercise with time-restricted eating may boost weight loss, study finds
Must Read
Development
Developers plan Northeast Philly marina with restaurants near Tacony-Palmyra Bridge

Health Technology
New DBS device for Parkinson's patients

Adult Health

Food & Drink
The Elephant, an espresso martini bar, opens in Center City

Jason Kelce joins 'Monday Night Countdown,' 2027 Super Bowl coverage, ESPN confirms

Sailors will hit the Delaware River for the first Philadelphia Cup Regatta in 8 years

- About PhillyVoice.com
- About aHealthierPhilly
The contents of this website, such as text, graphics, images, and other material contained on this website, are for informational purposes only and do not constitute medical advice.
a healthier philly is sponsored by Independence Blue Cross, the leading health insurance organization in Southeastern Pennsylvania, serving nearly 2.5 million people in the region, providing health news and related information that leads to a more informed, healthier life.
a healthier philly and its health-related information resources are not a substitute for the medical advice, diagnosis, and treatment that patients receive from their physicians or health care providers and are not meant to be the practice of medicine, the practice of nursing, or to carry out any professional health care advice or service in the state where you live. Nothing in this website is meant to be used for medical or nursing diagnosis or professional treatment.
Always seek the advice of your physician or other licensed health care provider. Always consult your health care provider before beginning any new treatment, or if you have any questions regarding your health condition. You should not disregard medical advice, or delay seeking medical advice, because of something you read on this site. In the event of a medical emergency, call a doctor or 911 immediately.
This website does not recommend or endorse any specific tests, physicians, procedures, opinions, or other information that may be mentioned on this website. Descriptions of, references to, or links to other products, publications, or services does not imply endorsement of any kind. Reliance on any information provided by this website is solely at your own risk.
Although we try to keep the information on the site as accurate as possible, a healthier philly disclaims any warranty concerning its accuracy, timeliness and completeness of content, and any other warranty, express or implied, including warranties of merchantability or fitness for a particular purpose. a healthier philly also reserves the right to temporarily or permanently discontinue this website, any page or any functionality at any time and without any notice.
The website and its content are provided on an “as is” basis.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- J Family Med Prim Care
- v.9(1); 2020 Jan
Alcohol consumption in India– An epidemiological review
V. m. anantha eashwar.
1 Department of Community Medicine, Saveetha Medical College and Hospital, Thandalam, Chennai, Tamil Nadu, India
2 Department of Community Medicine, Sree Balaji Medical College and Hospital, Chrompet, Chennai, Tamil Nadu, India
S. Gopalakrishnan
One of the most important products of global addiction demand is an alcoholic beverage. In developing countries like India, alcohol consumption tends to be a major problem because of the various socio-cultural practices across the nation, different alcohol policies and practices across the various states, lack of awareness of alcohol-related problems among the community, false mass media propaganda about alcohol use, various alcohol drinking patterns among the alcohol consumers and the emergence of social drinking as a habit because of the widespread urbanisation across the country. Stringent alcohol policies are needed across the various states to reduce alcohol consumption, and alcohol consumers have to be educated about the various harmful effects of alcohol consumption and the effects it can have on their mind, body and soul. This review article focuses on the burden of alcohol consumption in context with its various harmful effects on the mind and body with a note on the alcohol policies in the country.
Introduction
The term alcohol refers to 'ethyl alcohol'. It is consumed as an alcoholic beverage in diluted concentrations of absolute (i.e., 100%) ethyl alcohol. There are various types of alcoholic beverages that are consumed around the world. One standard alcoholic beverage corresponds to 10 g of absolute alcohol. The quantity differs among the types of alcoholic beverages. The most commonly used alcoholic beverages are beer, wine, whiskey, rum, vodka, gin and brandy and locally brewed beverages like arrack and toddy. Alcohol consumption becomes a problem when the individual engages in problematic drinking pattern that puts him at the risk of developing adverse health events.[ 1 ] The various drinking patterns of alcohol consumption[ 2 , 3 , 4 , 5 ] are given in Table 1 .
Various patterns of alcohol consumption
WHO: World Health Organization
Global prevalence of alcohol consumption
According to recent data published by the World Health Organization (WHO), the total per capita consumption of alcohol by individuals above 15 years of age is 6.2 L of pure alcohol per year, which equals 13.5 g of pure alcohol per day. However, there is a wide variation between the WHO regions and member states. Nearly 5.1% of the global burden of disease is attributable to alcohol consumption, and it causes nearly 3.3 million deaths every year.[ 1 ]
Alcohol consumption and its associated factors in various parts of India
Alcohol consumption practices vary across different parts of India because of various socio-cultural diversity and difference in laws governing individual States within India. Table 2 shows the prevalence of alcohol consumption in various parts of India and the associated factors governing alcohol use.[ 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 ]
Disease burden because of alcohol consumption
Alcohol use disorder (AUD) (which includes a level that's is sometimes called alcoholism) is a pattern of alcohol use that involves problems controlling your drinking, being preoccupied with alcohol, continuing to use alcohol even when it causes problems, having to drink more to get the same effect or having withdrawal symptoms when you rapidly decrease or stop drinking. The prevalence of AUDs is highest in Europe (7.5%) and the lowest among eastern Mediterranean regions, which includes Afghanistan, Bahrain and Egypt. Globally, 50% of the deaths caused by liver cirrhosis, 30% of the deaths because of oral and pharyngeal cancers, 22% of the deaths caused by inter-personal violence, 22% of the deaths because of self-harm, 15% of the deaths caused by traffic injuries, 12% of the deaths because of tuberculosis (TB) and 12% of the deaths caused by liver cancer were attributed to alcohol consumption.[ 1 ]
The 12-month prevalence of AUDs in India in the year 2010 was 2.6% and that of alcohol dependence was 2.1%. In 2012, 33.1% of all the road traffic accident deaths were attributable to drunk and driving. The National Mental Health Survey of India 2015–16 found the prevalence of AUDs to be 9% in adult men. In India, the alcohol-attributable fraction (AAF) of all cause deaths was found to be 5.4%. Around 62.9% of all the deaths due to liver cirrhosis were attributable to alcohol use.[ 19 ]
Medical consequences of alcohol use
When alcoholic beverages are consumed, alcohol gets absorbed from the stomach and small intestine. It is distributed through blood circulation to every organ in the body. The alcohol gets absorbed by the liver at a rapid pace and excreted through the kidneys, which accounts for 95% to 98% of the alcohol consumed.[ 5 ] In a study done by Gururaj et al ., it was concluded that, because of the increase in the alcohol consumption occurring all over the country, the hospital admission rates because of alcohol consumption were also increasing with 20% to 30% of admissions because of direct or indirect problems caused because of alcohol consumption.[ 20 ]
The various medical complications because of alcohol consumption are:
- Gastrointestinal (GI) complications: The direct effect of alcohol on the lining of the stomach can lead to acute gastritis and present as vomiting, usually associated with heavy drinking. Repeated damage can lead to hyperacidity leading to peptic ulcer disease. Alcohol is one of the most important reasons for haemorrhagic gastritis. The most common complication of long-term alcohol is alcoholic liver disease (ALD)[ 5 ]
- Cancer : Drinking as few as 1.5 drinks per day increases a woman's risk of breast cancer 1.4-fold. For both the genders, four drinks per day increase the risk for oral and oesophageal cancers by approximately three-fold and rectal cancers by 1.5 fold.[ 5 ] In a study done by Bangardi et al ., it was found that alcohol most strongly increased the risk of cancers of the pharynx, oral cavity, oesophagus and larynx[ 21 ]
- Changes in the genitourinary system : Acutely, modest doses of ethanol can not only increase sexual drive but can also lead to decrease in the erectile capacity in men. Even in the absence of liver impairment, significant minority of chronic alcoholic men show irreversible testicular atrophy with shrinkage of seminiferous tubules, resulting in decrease in ejaculate volume and a low sperm count.[ 22 ] In a study done by Chandra et al ., it was found that there was disproportionately high association of alcohol abuse with high-risk sexual behaviour and HIV infection[ 23 ]
- Muscular changes : Between one-half and two-thirds of alcoholics can have skeletal muscle weakness caused by the acute alcoholic myopathy, which may improve with abstinence, but it is not fully cured. Effects of alcohol consumption on the skeletal system can include lower bone density.[ 22 ] In a study done by Venkat et al ., it was found that those who suffer from chronic alcoholism suffered from avascular necrosis of the femoral head and reduced bone density[ 24 ]
- Neurological complications : The short-term effects of alcohol consumption that can get relieved after stopping alcohol consumption include blackouts, blurred vision, impaired memory and slower reaction times.[ 25 ] In a study done by Peng et al ., it was found that chronic alcohol use can lead to the development of alcoholic tremors, myopathy, Wernicke's encephalopathy and cerebellar degeneration[ 26 ]
In a case-control study of completed suicides in Bangalore done by Gururaj, it was found that alcohol consumption was a major risk factor for suicide with nearly a 25 times increase among the alcohol users. Suicide rates among women increased by nearly six times who were a spouse of alcohol abusers.[ 20 ] A study done by Vijayakumar et al ., in Chennai, found that suicide rates were higher among alcohol users as compared with non-users.[ 28 ]
Social consequences of alcohol use
Alcohol consumption not only affects the individuals but also his family members get affected in one way or the other. The person in an intoxicated state may indulge in domestic violence with his family members; may exhaust the savings of the family, which can negatively affect the education of his children, and the children of alcoholic fathers will have strained relationship with their family members, which can affect their psychological wellbeing. In a study done by Gururaj et al ., in Bangalore, it was found that emotionally abusing the spouse was found to be 2.5 times more common among persons who consume alcohol, 23.3% of the users physically abused their spouse and 7.8% of them physically abused their spouse resulting in injuries.[ 29 ] In a study done by Markowitz et al ., domestic violence was reported by 20% of women and husband's practice of alcohol consumption was reported by them as the most significant cause for domestic violence.[ 30 ]
Impact of alcohol use on economic and family finances
The economic impact of alcohol consumption plays a major role in families belonging to lower socio-economic strata. In a study done by Bonu et al ., it was found that there was an empirical association found between the use of alcohol and tobacco and impoverishment through borrowing and selling off assets in distress because of hospitalisation.[ 30 ]
In a study done by Benegal et al ., it was found that alcohol-dependent persons spent more money than they earned, they were forced to take loans to spend for their expenses related to alcohol consumption, on an average, 12.2 working days were lost to the habit and around 60% of the families were financially supported by the income from other family members.[ 31 ] In a study done by Ramanan et al ., half of the persons who consume alcoholic beverages had strained relations with their family members especially their spouse and children.[ 12 ]
Road traffic accidents
One of the major problem of alcohol consumption are road traffic accidents which occur due to driving vehicles under the influence of alcoholic beverages. Both developing and developed countries report high rates of road traffic accidents because of alcohol consumption.[ 5 ]
In a study conducted by the National Institute of Mental Health and Neurosciences (NIMHANS) in 12 major hospitals of Bangalore city, it was found that nearly 28% of injuries because of road traffic accidents were directly attributable to alcohol. The roadside survey revealed that nearly up to 40% of the drivers were under the influence of alcohol.[ 31 , 32 ] In a study done by Aditya et al ., it was found that 20% of the fatal road traffic accidents were because of alcohol use. The blood alcohol concentration (BAC) of 38% of those alcohol users were above the permissible limits.[ 27 ] In a study done by Gururaj it was found that alcohol abuse was reported in over 20% of the traumatic brain injuries.[ 33 ] According to the latest data released by the National Crime Records Bureau (2015), Tamil Nadu recorded the highest number of drunk and driving accidents in the country.[ 34 ] In a study done by Korlakunta et al ., high-risk behaviour was more common among alcohol-dependent individuals with road traffic accidents being the most frequently observed.[ 35 ]
Legal problems because of alcohol consumption
Another important area where complications arise because of alcohol abuse is legal problems. Crimes that are committed following alcohol intoxication include sexual/physical assault, rape, exploitation of women in commercial sex work and homicide. According to the National Crime Records Bureau of India, the different crimes that are related to alcohol consumption fall under four major acts namely, the Prohibition Act, Gambling Act, Psychotropic Substance Act and Excise Act. However, the major reason because of which the public nuisance created because of alcohol abuse goes unnoticed is that those crimes are classified under petty crime and they largely go unrecognised or they may get overlooked.[ 29 ]
Benefits of alcohol consumption
There are many studies that have pointed out that drinking alcohol in moderate amounts is good for the heart as they help in preventing coronary artery diseases (CADs). However, individual susceptibility plays a major role in the protective benefits of alcohol consumption. The American Heart Association (AHA) states that 'it is not possible to predict in which people alcoholism will become a problem' and advice not to consume alcohol for the benefits it may carry.[ 36 ]
In a multi-centre study done in India, it was found that even light or occasional consumption of alcohol might increase the risk of CAD. So, the benefits of alcohol consumption may not be true for Indians at least.[ 37 ]
Alcohol policy in India
Although the prohibition of alcohol use is encouraged in the constitution of India, alcohol policy is a state subject. States are having full control of alcohol-related legislation, excise rates and the production, distribution and sale of alcohol. Newly independent India, which was born post-independence, retained alcohol prohibition until mid-1960s, and by 1970, only the state of Gujarat had a complete alcohol prohibition policy.[ 29 ] In Bihar, there is complete prohibition of alcohol use since 4 April 2016. However, following a year after the ban, trade of illicit liquor flourished along the borders, as the neighbouring states have no prohibition on alcohol. In addition, there seems to be illicit trade of narcotic drugs as people have begun to look for other substances for addiction.[ 38 ]
Another controversial 'Dry State' is Manipur, where the prohibition of alcohol consumption is in force since 1991, but scheduled castes (SCs) and scheduled tribes (STs) were allowed to brew their traditional liquor. In 2002, the government lifted the ban of alcohol in some districts in Manipur. Manipur is now popularly called 'Wettest Dry State'. Government is now looking to remove the prohibition act, as illicit liquor use, deaths because of methanol poisoning and substance abuse are on the rise.[ 39 ] The major reason states experience fluctuation on the alcohol prohibition at the policy level is that it generates nearly 15% to 20% of their revenue from alcohol taxation, contributing a significant amount to the state treasury.[ 40 ] In states like Gujarat, where complete prohibition is in force, the rich have continued access to alcoholic beverages and the lower class and poor people resort to illegal brewing of alcohol with increase in deaths because of methanol poisoning.[ 39 ] In countries like United States of America (USA) increased taxation on alcoholic beverages has been used to reduce alcohol consumption. In India, those measures will not work, as the alcohol consumers have easy access to illicit liquor and substances. Other laws related to the regulation of alcohol-use like hours of sale, drunken driving and sale to minors are regularly breached.[ 41 ] Legal drinking age is the minimum age after which a person is allowed to buy alcohol. The legal age in different states in India is given in Table 3 .[ 42 ]
Legal drinking age in various states in the country[ 42 ]
Drunken driving (Motor Vehicle Act)
When a person consumes an alcoholic beverage, there is a rise in BAC because of which there is gradual and progressive loss of driving ability because of increase in the reaction time, overconfidence, degraded muscle coordination, impaired concentration and decreased auditory and visual acuity. This is known as drunken driving. There are laws to govern drunken driving in India. The BAC limits are fixed at 0.03%. As per the Motor Vehicle Act, any person whose BAC values are found to be more than this limit are booked under the first offence and may be fined about INR 2,000 to 10,000 and/or he or she may face a maximum of 6 months to 4 years imprisonment.[ 43 ]
Alcohol advertisements
As per the Cable Television Network (Regulation) Amendment Bill, advertising of alcoholic beverages was banned in India. Still, private channels are often permitting alcohol companies to advertise using surrogate means like using brand names for soda or water or music. However, as the target audience is moving from watching television to mobile phones, liquor companies have now begun to invest in online video marketing.[ 43 , 44 ]
Alcohol prohibition in Tamil Nadu
Before independence, the Madras Abkari Act, imposed in the year 1886, enacted strict rules and regulations that prohibited the local manufacturing of alcoholic beverages and confined it to the central distilleries, where excise duty was paid to the government before being sold in the market. This favoured foreign liquors resulted in anti-alcohol agitation by Indian freedom movements like Swadeshi and Non-Cooperation Movement. In 1937, alcohol prohibition was imposed in Salem district, which was later extended throughout the presidency.[ 45 ] After independence, Tamil Nadu continued liquor ban until 1971. After 1971, the then chief minister (CM) lifted the prohibition of alcoholic beverages. Again, in 1981, the CM during that period closed down all arrack and toddy shops but left the Indian-made foreign liquor untouched. That was the time when it was noticed that, whenever alcohol prohibition was imposed, illegal sales of toddy and arrack and consumption of methanol would rise, resulting in loss of many lives; thus, the ban would be lifted.[ 6 ] In 2002, the retail sale of alcohol was brought under government control. A panel of five IAS officers governs it. It has nearly 6,800 retail alcohol outlets across the state.[ 46 ] As of now, no steps are being taken by the government on the sale and consumption of alcoholic beverages in the state.
Primary care intervention for alcohol-related problems
In developing countries like India, primary care physicians are the first contact of patients with the healthcare system. It is a major platform for screening to identify at-risk individuals and diagnose AUDs. As recommended by the WHO, the AUDs Identification Test (AUDIT), for use in a primary care setting, is a validated screening tool. It can be used to identify alcohol consumers who are harmful/hazardous drinkers and alcohol-dependent individuals.[ 2 , 47 , 48 ] Systematic reviews and randomised controlled trials (RCTs) have demonstrated that brief intervention in primary care setting by one-to-one counselling can help at-risk drinkers and those with mild alcohol-related problems.[ 49 ] Based on evidence, primary care management of alcohol-related problems include three core steps, namely, counselling the patient on the ill-effects of alcohol and, if necessary, prescribing medications like disulfiram and connecting with the patients by organizing treatment programs and forming support groups. If necessary, they have to refer the patient to higher centres for further care and management.[ 50 , 51 , 52 , 53 ]
Alcohol consumption is emerging as a major public health problem in India. Multi-centric scientific community-based research studies have to be conducted in various individual states to understand the problem better. Various policymakers, media, professionals and society have to be educated about the consequences of chronic alcohol through sensitisation programmes and health education campaigns. There is a dire need for rational alcohol control policy with specific objectives like alcohol taxation, production and promotion policy.
Financial support and sponsorship
Conflicts of interest.
There are no conflicts of interest.

IMAGES
VIDEO
COMMENTS
Neurobiological models of addiction focus on the brain reward and stress system dysfunction that contributes to the development and maintenance of alcohol use disorder, that is, the "addiction cycle" (15, 16). The alcohol and addiction research domain criteria (AARDoC) , which have been operationalized in the addictions neuroclinical ...
Alcohol is a very easily available source of addiction, which is one of the main reasons why it remains a serious threat to the community. There is a huge variety that is available as far as alcoholic drinks are concerned. Alcohol is also one of the cheaply accessible means of addiction; this explains why alcoholism is so prevalent.
This systematic review and meta-analysis evaluates the association between daily alcohol intake and risk of all-cause mortality. ... Addiction. 2012;107(7):1246-1260. doi: ... Many papers in epidemiology, including this paper, may be much more practical if physicians could tell patients that certain behaviors will shorten their life ...
It is well recognized that alcohol use disorders (AUD) have a damaging impact on the health of the population. According to the World Health Organization (WHO), 5.3% of all global deaths were attributable to alcohol consumption in 2016 [].The 2016 Global Burden of Disease Study reported that alcohol use led to 1.6% (95% uncertainty interval [UI] 1.4-2.0) of total DALYs globally among females ...
Only a small percent of individuals with alcohol use disorder contribute to the greatest societal and economic costs ().For example, in the 2015 National Survey on Drug Use and Health survey (total n = 43,561), a household survey conducted across the United States, 11.8% met criteria for an alcohol use disorder (n = 5124) ().Of these 5124 individuals, 67.4% (n = 3455) met criteria for a mild ...
Alcohol, Clinical and Experimental Research provides direct access to the most significant and current research findings on the nature and management of alcoholism and alcohol-related disorders. Read Virtual Issues on key topics in the field of Addiction ... the recipients of the ACER Journal Award for Outstanding Paper by an Early Career ...
Alcohol and Alcoholism welcomes submissions, publishing papers on the biomedical, psychological, and sociological aspects of alcoholism and alcohol research. To gain more information please see the Instructions to Authors page.
While most of our knowledge on the effects of alcohol on the brain and cognitive outcomes is based on research in adults, several recent reviews have examined the effects of alcohol on the brain ...
The numbers for substance use disorders are large, and we need to pay attention to them. Data from the 2018 National Survey on Drug Use and Health suggest that, over the preceding year, 20.3 million people age 12 or older had substance use disorders, and 14.8 million of these cases were attributed to alcohol.When considering other substances, the report estimated that 4.4 million individuals ...
Alcohol use disorders consist of disorders characterised by compulsive heavy alcohol use and loss of control over alcohol intake. Alcohol use disorders are some of the most prevalent mental disorders globally, especially in high-income and upper-middle-income countries; and are associated with high mortality and burden of disease, mainly due to medical consequences, such as liver cirrhosis or ...
We also estimated the mean risk curve and 95% UI for the relationship between alcohol consumption and IHD using only data from cohort studies. In total, 95 cohort studies - of which one was a ...
The growing scale of alcoholism, as well as its multidimensional consequences, justify the need for an in--depth analysis of this phenomenon. Harmful alcohol use and dependence is a risk factor ...
Abstract. Alcohol consumption is known to be an addiction that provides negative outcomes mainly on health, excessive drinking of alcohol brings adverse effects on human health, also on activities ...
Alcohol Research Resource (R24 and R28) Awards. Resources include biological specimens, animals, data, materials, tools, or services made available to any qualified investigato r to accelerate alcohol-related research in a cost-effective manner. Current and potential alcohol research investigators and trainees are encouraged to subscribe to our ...
Research on the treatment of alcoholism has gained significant ground over the past 40 years. Studies such as the National Institute on Alcohol Abuse and Alcoholism's Project MATCH, which examined the prospect of tailoring treatments for particular people to better suit their needs, and Project COMBINE, which examined in-depth, cognitive-behavioral therapy and medical management, helped ...
Abstract. Alcohol abuse is reflected as a major public health concern in worldwide. It impaired many areas of life, including familial, vocational, psychological, legal, social, or physical ...
When women are excluded from biomedical research, it leaves doctors and researchers with an incomplete understanding of health and disease, including alcohol addiction.
Alcohol and drug use can lead to poor decision making, like breaking the law, sexual abuse, getting in fights, etc. Of the respondents, 92.4% were white and the average age was 22.3 years. This study found that a little more than 68% reported using alcohol and/or drugs during the past year.
Once established, alcohol addiction—hereafter equated with alcoholism—is a chronic relapsing disorder in which alcohol use becomes compulsive, i.e., continues despite negative consequences ().Understanding the transition from controlled to compulsive alcohol use is a critical challenge for addiction research.
High amounts of alcohol use are causal risk factors in the development of disease in the heart, liver, pancreas, and brain (including the brains of children in utero). In fact, 1 in 8 deaths in ...
The authors are entirely responsible for the research and results reported in this paper, and their position or opinions do not necessarily represent those of the University of Miami, the National Institute on Alcohol Abuse and Alcoholism, or the National Institute on Drug Abuse. ... Alcohol Research and Health. 2003; 27 (2):181-185. [PMC ...
The Butler Center for Research at the Hazelden Betty Ford Foundation investigates these and other questions and publishes its scientific findings in a variety of alcohol and drug addiction research papers and reports. Research topics include: These research queries and findings are presented in the form of updates, white papers and case studies.
Theoretical framework. Recovery Capital (RC) was used as a guiding framework to explore students' recovery experiences. Since its inception over 20 years ago (Granfield & Cloud, Citation 1999), RC has become a guiding policy and research framework in Canada and the USA, and recently applied to understand students' recovery experiences (Hennessy et al., Citation 2022; Park et al., Citation ...
In conclusion alterations in PL synaptic activity were strongly associated with individual addiction scores, indicating their role as potential markers of the behavioral manifestations linked to AUD psychopathology. The transition to alcohol use disorder (AUD) involves persistent neuroadaptations in executive control functions primarily regulated by the medial prefrontal cortex. However, the ...
Roughly 1 in 5 U.S. adults report binge drinking at least once a week, with an average of seven drinks per binge episode. This is well over the amount of alcohol thought to produce legal ...
Addiction treatment services research assumes that people with substance use disorders have a range of problems, degrees of severity, resources, and strengths. This is featured in substantial work with alcohol [ 98 ] and drug treatment services [ 99 ].
Roughly 1 in 5 U.S. adults report binge drinking at least once a week, with an average of seven drinks per binge episode. While all forms of alcohol consumption come with health risks, binge ...
The 12-month prevalence of AUDs in India in the year 2010 was 2.6% and that of alcohol dependence was 2.1%. In 2012, 33.1% of all the road traffic accident deaths were attributable to drunk and driving. The National Mental Health Survey of India 2015-16 found the prevalence of AUDs to be 9% in adult men.