Ohio State nav bar
The Ohio State University
- BuckeyeLink
- Find People
- Search Ohio State

Patient Case Presentation
Chief Complaint : Infertility
Background: Ms. L.C. is a 34 year old female presenting with concerns of infertility. She has been attempting a pregnancy over the past 16 months with no success. Patient reports that several times she thought she could be pregnant due to a cessation in her menses with accompanying constipation and some abdominal pain. Patient also reports pain that is more intense during menstruation, with “sharp and stabbing” characteristics that is not relieved by use of NSAIDs or hot compresses. The pain radiates from her lower abdominal area into her flanks, which she rates to be a 6 on a scale of 1-10. Patient reports her cycle can be irregular, with the length ranging up to 25-38 days or occasionally no period at all. She is concerned that her and her husband have not had enough intercourse for a pregnancy due to dyspareunia and general pelvic pain.

Figure 1. Photograph of Doctor and Patient (2014)
Past Medical History
- Menarche, age 10
- IUD in place, 26 to 32 years
- Pelvic mass, ruled to be non-cancerous, 30 years
- Most recent sexually transmitted infection (STI) screen negative for chlamydia, syphilis, and gonorrhea, 33 years
- Patient does not take any medications
- Patient has no history of pregnancy
Pertinent Family History
- Mother alive and healthy at 58 years of age
- Maternal grandmother passed from ovarian cancer at 42 years
- Sister, 28, also struggles with infertility
Pertinent Social History
- Patient reports stress and a decrease in sexual fulfillment from infertility concerns.
- Patient is monogamous with her primary partner.
- Patient does not drink alcohol or use recreational drugs.
- Patient follows a vegetarian diet and avoids dairy.
- Patient works as an architect for a small firm in Columbus, and occasionally travels domestically for work.
- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- Pathophysiology,...
Pathophysiology, diagnosis, and management of endometriosis
- Related content
- Peer review
- Andrew W Horne , professor of gynaecology and reproductive sciences 1 ,
- Stacey A Missmer , professor of obstetrics, gynaecology, and reproductive biology , adjunct professor of epidemiology 2 3
- 1 EXPPECT Edinburgh and MRC Centre for Reproductive Health, University of Edinburgh, Edinburgh, UK
- 2 Michigan State University, Grand Rapids, MI, USA
- 3 Harvard T.H. Chan School of Public Health, Boston, MA, USA
- Correspondence to: A W Horne andrew.horne{at}ed.ac.uk
Endometriosis affects approximately 190 million women and people assigned female at birth worldwide. It is a chronic, inflammatory, gynecologic disease marked by the presence of endometrial-like tissue outside the uterus, which in many patients is associated with debilitating painful symptoms. Patients with endometriosis are also at greater risk of infertility, emergence of fatigue, multisite pain, and other comorbidities. Thus, endometriosis is best understood as a condition with variable presentation and effects at multiple life stages. A long diagnostic delay after symptom onset is common, and persistence and recurrence of symptoms despite treatment is common. This review discusses the potential genetic, hormonal, and immunologic factors that lead to endometriosis, with a focus on current diagnostic and management strategies for gynecologists, general practitioners, and clinicians specializing in conditions for which patients with endometriosis are at higher risk. It examines evidence supporting the different surgical, pharmacologic, and non-pharmacologic approaches to treating patients with endometriosis and presents an easy to adopt step-by-step management strategy. As endometriosis is a multisystem disease, patients with the condition should ideally be offered a personalized, multimodal, interdisciplinary treatment approach. A priority for future discovery is determining clinically informative sub-classifications of endometriosis that predict prognosis and enhance treatment prioritization.
Introduction
Endometriosis is a chronic, inflammatory, gynecologic disease marked by the presence of endometrial-like tissue outside the uterus, which affects approximately 10% of women during their reproductive years—190 million women worldwide. 1 In many patients, it is associated with chronic painful symptoms and other comorbidities, including infertility. 2 The health burden of endometriosis includes chronic pain and significant lifetime costs of $27 855 per year per patient, 3 accumulating to annual healthcare costs for endometriosis of approximately $22bn in the US alone and £12.5bn in the UK in treatment, work loss, and healthcare costs. 4 Although more than 50% of adults diagnosed as having endometriosis report onset of severe pelvic pain during adolescence, 5 most young women with endometriosis do not receive timely treatment. Almost 60% of women will see three or more clinicians before a diagnosis of endometriosis is made after an average of seven years with symptoms. 6 Women with endometriosis lose on average 11 hours of work per week, similar to other chronic conditions including type 2 diabetes, Crohn’s disease, and rheumatoid arthritis. 7 Adolescents are at risk of having inadequately remediated symptoms during prime years for social development and life planning, 8 and women must be resilient against inadequately remediated symptoms and emerging comorbidities. Women, healthcare providers, and scientists would benefit from conceptualizing endometriosis as a condition that can affect the whole woman. This includes a better understanding of the risk of subsequent development of autoimmune disease, cancer, and cardiovascular disease and a whole health approach to monitoring and wellbeing. 9
This review is aimed at general practitioners and pediatric specialists who are most likely to interact with patients as signs and symptoms of endometriosis first emerge and from whom early attention and empiric treatment may dramatically shorten the burden; gynecology specialists for whom myths must be dispelled and who must be aware of state of the art knowledge about patient centered treatments; endometriosis specialists who care for women’s endometriosis associated symptoms across the life course; and clinical researchers and scientists who must be inspired to bring their expertise and creativity to answer the fundamental enigmas of endometriosis etiology, informative sub-phenotyping, and novel patient centered treatment.
In this review, we use the terms “woman” and “women.” However, it is important to note that endometriosis can affect all people assigned female at birth.
Sources and selection criteria
We searched PubMed for studies using the term “endometriosis.” We considered all peer reviewed studies published in the English language between 1 January 2010 and 28 February 2022. We also identified references from international guidelines on endometriosis published during this time period. We selected relevant publications outside this timeline on the basis of review of the bibliography. We predefined the priority of study selection for this review according to the level of the evidence (meta-analyses, systematic or scoping reviews, randomized controlled trials (RCTs), prospective cohort studies, case-control studies, cross sectional studies; a priori exclusion of case series and case reports), by sample size (we prioritized studies with larger sample size as well as studies providing precision statistics), by population sampling (we prioritized studies with more diverse populations or with declared sub-population design over narrow population samples), and publication date (we prioritized more recent studies).
Overall quality of evidence
Much of the knowledge on endometriosis is based on concepts in early stages of evidence development or on sparse literature. Many studies include single hospital or clinic population samples with small total sample sizes and disproportionately representing patients presenting with infertility compared with endometriosis associated pain. 10
Beyond the limitations of the existing literature, fundamental problems with the diagnosis of endometriosis must be overcome before we can adequately define endometriosis, its prevalence, biologically and clinically informative sub-phenotypes, and its response to treatment and long term prognosis. 11 The lack of a non-invasive diagnostic modality creates insurmountable diagnostic biases driven by characteristics of those patients who can and those who cannot access a definitive surgical or imaging diagnosis and at what point in their endometriosis journey the condition is diagnosed.
Ovarian endometrioma or deep endometriosis can be diagnosed through imaging if the patient is geographically, economically, and socially able to achieve referral to and evaluation from an experienced imaging specialist. 12 13 For women with superficial peritoneal disease, definitive diagnosis by means of surgical evaluation is limited to those with symptoms deemed sufficiently severe and life affecting and resistant to empiric treatment to justify the inherent risks of surgery. Even among patients with symptoms deemed to have enough of an effect to warrant referral for a surgical evaluation, stigma, 14 disbelief and misperceptions of pain or fertility that can be driven by racism or elitism, 15 and geographic and economic barriers to accessing endometriosis focused surgeons remain.
Beyond access to an appropriate, skilled physician, the wide range of symptoms associated with endometriosis—many of which are stigmatized or normalized 14 16 —reduces the likelihood of referral and increases time to referral to appropriate specialists. 5 6 11 17 The bias in diagnosis itself may be influenced by variations in clinical symptoms among different populations not adequately captured or appreciated by standard clinical definitions or may represent implicit bias in healthcare, leading to an alternate interpretation of the same symptoms affecting the likelihood of diagnosis. This delay to diagnosis affects patients directly, but it also results in most scientific studies capturing patients’ characteristics, biologic samples, and biomarker measurements far into the natural pathophysiologic progression of the disease. Moreover, studies from African and Asian countries are considerably under-represented compared with European and North American countries. 10 High quality studies from these regions and development of a sensitive non-invasive diagnostic tool might alter existing global prevalence and incidence estimates and may reveal a more comprehensive view of what early milieu, signs and symptoms, and long term health outcomes are truly attributable to endometriosis.
Definition, symptoms, and classification
Among women with the condition, endometriosis has a highly heterogeneous presentation of visualized endometriotic lesions, multisystem symptom presentation, and comorbid conditions ( fig 1 ).

Highly varied presentation of endometriosis
- Download figure
- Open in new tab
- Download powerpoint
Surgically visualized macro sub-phenotypes of endometriosis
Endometriosis is defined by the presence of endometrium-like epithelium and/or stroma (lesions) outside the endometrium and myometrium, usually with an associated inflammatory process. 18 Most endometriosis is found within the abdominal cavity, and it exists as three subtypes: superficial peritoneal endometriosis (accounting for around 80% of endometriosis), ovarian endometriosis (cysts or “endometrioma”), and deep endometriosis 1 19 ( box 1 ; fig 2 ). All forms of endometriosis can be found together, not solely as separate entities. Although not a subtype, endometriosis situated inside the bowel wall is termed “bowel endometriosis.” It mostly affects the rectosigmoid area, but lesions can also be found in other parts of the gastrointestinal system, including the appendix. Endometriosis involving the detrusor muscle and/or the bladder epithelium is termed “bladder endometriosis.” Extra-abdominal (replacing the older term “extra-pelvic”) endometriosis is used to describe any endometriosis lesions found outside of the abdomen (for example, thoracic endometriosis). 20 Iatrogenic endometriosis describes endometriosis thought to be arising from direct or indirect dissemination of endometrium following surgery (for example, cesarean scar endometriosis).
Nomenclature 18
Superficial peritoneal endometriosis.
Endometrium-like tissue lesions involving the peritoneal surface with multiple appearances
Ovarian endometriosis
Endometrium-like tissue lesions in the form of ovarian cysts containing endometrium-like tissue and dark blood stained fluid (endometrioma or “chocolate cysts”)
Deep endometriosis
Endometrium-like tissue lesions extending on or infiltrating the peritoneal surface (usually nodular, invading into adjacent structures, and associated with fibrosis)
Extra-abdominal endometriosis
Endometrium-like tissue outside the abdominal cavity (for example, thoracic, umbilical, brain endometriosis)
Iatrogenic endometriosis
Direct or indirect dissemination of endometrium following surgery (for example, cesarean scar endometriosis)

Surgical images of endometriosis sub-phenotypes
Adenomyosis is not a sub-phenotype of endometriosis, 21 although it is characterized by endometrial tissue surrounded by smooth muscle cells within the myometrium. 22 Symptoms include dysmenorrhea and heavy menstrual and/or abnormal uterine bleeding, 23 and a heterogeneous adenomyosis presentation is visualized with radiologic imaging or at hysterectomy that lacks an agreed terminology or classification system. 24 25 Evidence is emerging of tissue injury and repair mechanisms mediated by estradiol and inflammation. 25 26
Endometriosis associated symptoms
Endometriosis is often associated with a range of painful symptoms that include chronic pelvic pain (cyclical and non-cyclical), painful periods (dysmenorrhea), painful sex (dyspareunia), and pain on defecation (dyschezia) and urination (dysuria). 1 27 Their severity can range from mild to debilitating. Some women have no symptoms, others have episodic pelvic pain, and still others experience constant pain in multiple body regions. 28 A related observation is that some women transition between these categories, progressing from episodic and localized pain to that which is chronic, complex, and more difficult to treat. Furthermore, women with disease that is anatomically “severe” can have minimal symptoms and women with “minimal” evidence of endometriosis can have severe, life affecting symptoms. 1 19 In common with other chronic pain conditions, women with endometriosis often report experiencing fatigue and depression. Infertility is significantly more common in patients with endometriosis, with a doubling of risk compared with women without endometriosis. 2 Endometriosis is discovered in 30-50% of women who present for assisted reproductive treatment. 29 30
Endometriosis associated or high risk comorbidities
Endometriosis is certainly a multisystem condition, perhaps as a result of common pathogenesis or as a consequence of the chronic endogenous response to the presence of endometriotic lesions. 9 Although pelvic pain is the most common symptom of possible endometriosis, women with endometriosis also have a high risk of co-occurring or evolving multisite pain. 28 Patients with endometriosis have a higher risk of presentation with comorbid chronic pain conditions such as fibromyalgia, 31 32 33 migraines, 34 35 and also rheumatoid arthritis, 33 36 psoriatic arthritis, 37 and osteoarthritis. 36 38 Reports of back, bladder, or bowel pain are prevalent, 16 39 with dyschezia being potentially predictive of endometriosis. 40 Nearly 50% of women with bladder pain syndrome or interstitial cystitis have endometriosis. 41 42 Irritable bowel syndrome is a common co-occurring diagnosis that reinforces the importance of awareness of endometriosis among gastroenterologists. 43 44 45 These conditions may share a common cause, 46 they may arise together owing to shared environmental or genetic factors, and/or the occurrence of comorbid pain conditions could be due to changes in pain perception after repeated sensitization. 47 Research focused on disentangling the overlapping and independent pathways of these frequently co-occurring pain associated conditions is essential. 48 49
Women with endometriosis have a greater risk of presenting with other non-malignant gynecologic diseases, including uterine fibroids and adenomyosis. 50 51 They are also at greater risk of a subsequent diagnosis of malignancies, autoimmune diseases, early natural menopause, and cerebrovascular and cardiovascular conditions. 36 52 53 54 55 56 57 The hypothesized causal mechanisms for endometriosis discussed below are all thought to be enhanced by and/or result in chronic inflammation. Local and systemic chronic inflammation can directly activate afferent nociceptive fibers and promote pelvic pain, 58 although this does not entirely explain the heterogeneity in types and severity of painful symptoms that patients experience. Furthermore, endometriosis induced chronic inflammation and immune dysregulation may also contribute to the endometriosis associated subsequent risk of each of these comorbid conditions. 59 60
Although this multisystem effect reinforces the importance of knowledge of and attention to endometriosis from general practitioners and a myriad specialists for whole healthcare, the most prominent association, and the focus of the greatest volume of comorbidity research, is the elevated risk of ovarian cancer among women with endometriosis. A recent meta-analysis confirmed this association, 53 finding a nearly twofold greater relative risk of ovarian cancer among patients with endometriosis (summary relative risk (SRR) 1.93, 95% confidence interval 1.68 to 2.22; n=24 studies) that was strongest for clear cell (3.44, 2.82 to 4.42; n=5 studies) and endometrioid (2.33, 1.82 to 2.98; n=5 studies) histotypes. However, among these 24 studies, significant evidence existed of both heterogeneity across studies and publication bias (Egger’s and Begg’s P values <0.01). Clinicians need to reinforce that ovarian cancer is rare regardless of women’s endometriosis status 61 : the absolute lifetime risk in the general population is 1.3%, 62 and applying the risk estimate from the meta-analysis (SRR 1.9) gives an absolute lifetime risk for women with endometriosis of 2.5%, which is 1.2% higher than the absolute risk for women without endometriosis and still very low.
We should also recognize that coexisting gynecologic conditions such as adenomyosis and uterine fibroids, 50 as well as associations with endometrial cancer, 53 can be influenced by diagnostic biases and failure to distinguish between diagnoses in women undergoing hysterectomy and those in women with an intact uterus. 11 51 When attempting to infer a causal relation between endometriosis and other conditions, applying rigorous prospective temporality (rather than cross sectional co-occurrence) is particularly important for valid subsequent risk associations. 53 These studies need large study populations with well documented longitudinal data. A large impediment is the lack of routine, harmonized documentation of the characteristics of endometriosis and its absence from international classification of diseases coding. 63 64
Endometriosis classification systems
Several classification, staging, and reporting systems have been developed; 22 systems were published between 1973 and 2021. 65 The three most commonly used systems are the revised American Society for Reproductive Medicine (rASRM) classification (stages I-IV; where stage I is equivalent to “minimal” disease and stage 4 to “severe” disease), the ENZIAN (and newer #ENZIAN) classification, and the Endometriosis Fertility Index (EFI). 66 67 68 69 Many validation studies and reports on the implementation of the different systems have been published. The rASRM system (scored at surgery on the basis of the extent of visualized superficial peritoneal lesions, endometriomas, and adhesions) has been shown to have poor correlation with pain, 70 fertility outcomes, and prognosis, and the ENZIAN system (which additionally includes deep endometriosis) has been shown to have poor correlation with symptoms and infertility. 71 72 73 The EFI is a well validated clinical tool that predicts pregnancy rates after surgical staging of endometriosis, with ongoing evaluation to determine the predictive importance of the individual parameters included in the scoring algorithm as well as the effect of completeness of surgical treatment on pregnancy prediction. 74 Unfortunately, no international agreement exists on how to describe endometriosis or how to classify it. As most systems show no, or very little, correlation with patients’ symptoms and outcomes, this is further evidence of our lack of understanding of the physiology underlying the symptoms associated with endometriosis.
Epidemiology
The exact prevalence of endometriosis is unknown given diagnostic delays and barriers, and—perhaps consequently—it is extremely varied depending on the population and the indication for evaluation. A recent meta-analysis identified 69 studies describing the prevalence and/or incidence of endometriosis, among which 26 studies were general population samples, 17 were from regional/national hospitals or insurance claims systems, and the remaining 43 studies were conducted in single clinic or hospital settings. 10 The prevalence reported in general population studies ranged from 0.7% to 8.6%, whereas that reported in single clinic or hospital based studies ranged from 0.2% to 71.4%.
When defined by indications for diagnosis, the prevalence of endometriosis ranged from 15.4% to 71.4% among women with chronic pelvic pain, from 9.0% to 68.0% among women presenting with infertility, and from 3.7% to 43.3% among women undergoing tubal sterilization. Few studies have investigated the incidence and prevalence of endometriosis specifically among adolescents. The reported prevalence of visually confirmed endometriosis among adolescents with pelvic pain ranges from 25% to 100%, with an average of 49% among adolescents with chronic pelvic pain and 75% among those unresponsive to medical treatment. 75 The Ghiasi meta-analysis reported a decrease in recorded prevalence across the past 30 years. 10 Speculating, this may be due to more rapid and more ubiquitous embracing of empiric treatment of symptom, forgoing or delaying definitive imaging or surgical diagnosis, a patient centered approach that has been ratified by the most recent European endometriosis guideline. 13 This hypothesis is supported by a recent report from a large US health system’s electronic medical records database that observed a decline from 2006 through 2015 in incidence rates for endometriosis (from 30.2 per 10 000 person years in 2006 to 17.4 per 10 000 person years in 2015) but an increase in documentation of chronic pelvic pain diagnoses (from 3.0% to 5.6%). 76
Pathophysiology
Heritability and genetics.
Estimates from twin studies suggest 47-51% total heritability of endometriosis, with 26% estimated to be from genetic variation. 77 78 79 To date, nine genome-wide association studies have been reported. 59 The largest study so far, using 17 045 cases and 191 596 controls, has identified 19 single nucleotide polymorphisms, most of which were more strongly associated with rASRM stage III/IV, rather than stage I/II, explaining 1.75% of risk for endometriosis. 80 Consistent with other complex diseases with multifactorial origins, no high penetrance susceptibility genes for endometriosis have yet been identified. 62 The loci discovered to date are almost all located in intergenic regions that are known to play a role in the regulation of expression of target genes yet to be identified. The critical next steps in genetic discovery are to identify additional genes that reveal novel pathophysiological pathways and also emerge to better define the underpinnings of variation in symptoms (in particular, pain types and infertility and treatment response predictors) and also gene expression correlated with comorbid autoimmune, cancer, and cardiovascular conditions. 62
Reflux of endometrial tissue fragments/cells and protein rich fluid through the fallopian tubes into the pelvis during menstruation is considered the most likely explanation for why endometriotic lesions form within the peritoneal cavity, although this mechanism is not sufficient as nearly all women experience retrograde menstruation. 81 82 Additional postulated origins include celomic metaplasia and lymphatic and vascular metastasis. Scientific avenues exploring contributions of interacting endocrine, immunologic, proinflammatory, and proangiogenic processes are drawing curiosity and expertise from varied disciplines with application of state of the art technologies. 59 Retrograde menstruation of stem cells contributes to the establishment of endometriosis, 83 whereas bone marrow stem cells contribute to the continued growth of endometriosis lesions. 84 85 Bone marrow derived stem cells may be responsible for those cases of endometriosis outside of the abdominal cavity. 86
Studies exploring why lesions develop in some, but not all, women have detected changes in the endometrial tissue as well as in the peritoneal fluid and cells lining the cavity. Eutopic endometrial tissue has a significantly different immune profile in women with endometriosis compared with those without it. 87 However, the extent to which this inflammation is a cause or an effect of endometriosis remains unclear. Aberrant inflammation could have an effect on the development of endometriosis lesions and disease progression in various ways, including immune angiogenesis and immune-endocrine interaction. 59 60 Specifically, some of the proposed pathways include altered production of inflammatory cytokines by immune cells in lesions or by endometrial cells themselves involving decreased immune clearance of abnormal endometrial cells and consequent seeding and development of lesions, increased likelihood of adhesion to mesothelial cells due to pro-invasion inflammatory milieu, inflammation promoted proliferation of endometrial cells, and inflammation promoted reduction in apoptosis of endometrial cells. 88 Analysis of eutopic endometrium from women with endometriosis has identified altered expression of genes implicated in the inflammation/immune response, angiogenesis, and steroid responsiveness (progesterone “resistance”). 89 Shed menstrual tissue contains high concentrations of pro-inflammatory cytokines, proteases, and immune cells, all of which may influence the peritoneal microenvironment after reflux. Stem/progenitor cells have been identified in the endometrium and are thought to survive and implant onto the peritoneum, contributing to lesions. 83 Mesothelial cells line the pelvic peritoneal cavity, and changes in their function in women with endometriosis, including altered morphology and metabolism (switch to aerobic glycolysis) 90 and production of factors that promote immune cell recruitment and angiogenesis are all thought to favor survival and establishment of lesions. 91 Physiological hormonal fluctuations in women induce cyclical episodes of cell proliferation, inflammation, injury, and repair within lesions that favor fibroblast to myofibroblast differentiation and fibrosis.
Mechanisms of endometriosis associated pain
The development of a new blood supply and associated nerves (neuroangiogenesis) is considered key to the establishment of endometriotic lesions and the activation of peripheral pain pathways ( fig 3 ). 92 Sensory C, sensory Ad, cholinergic, and adrenergic nerve fibers have all been detected in lesions. Estrogens can promote crosstalk between immune cells and nerves within lesions, increasing expression of nociceptive ion channels such as the transient receptor potential cation channel subfamily V member 1. 93 Factors that promote inflammation and nerve growth, such as nerve growth factor, tumor necrosis factor α, and interleukin 1-β, are increased in the peritoneal fluid of women with endometriosis and may exacerbate a neuroinflammatory cascade. Consistent with other conditions associated with chronic pain, endometriosis is associated with unique, and sometimes disease specific, alterations in the peripheral and central nervous systems, including changes in the volume of regions of the brain and in brain biochemistry. 94 Increased risk of central sensitization may partially explain why approximately 30% of patients with endometriosis will develop chronic pelvic pain that is unresponsive to conventional treatments, including surgery. 95 Through this central process, patients can experience reduced pain thresholds, increased responsiveness and length of aftereffects to noxious stimuli, and expansion of the receptive field so that input from non-injured tissue may elicit pain. 46 47 Among endometriosis patients with central sensitization, the removal of the endometriotic lesions is unlikely to result in adequate pain remediation owing to continued activation of the central nervous system. 96 97 Thus, endometriosis associated pain does not neatly fall into one of the three main categories of chronic pain (that is, nociceptive, neuropathic, or nociplastic), 98 99 100 and it likely has a mixed pain phenotype or sits somewhere along a continuum of these pain phenotypes. For example, some patients have primarily nociceptive or neuropathic pain, others have primarily nociplastic pain, and the rest have a mixed phenotype with variable contributions of nociceptive, neuropathic, and nociplastic pain.

Pathophysiology of endometriosis: (1) potential factors contributing to endometriosis associated pain; (2) potential mechanisms of endometriosis associated infertility; (3) local factors involved in the development of an endometriosis lesion; (4) role of the eutopic endometrium in the development of an endometriosis lesion. CNS=central nervous system; PNS=peripheral nervous system
Mechanisms of endometriosis associated infertility
Endometriosis may impair fertility through multiple pathways, including peritoneal inflammation and endocrine derangements, which interfere with the follicular environment and consequently affect ovarian function and ultimately reduce oocyte competence. 101 Several studies of women undergoing in vitro fertilization have documented lower oocyte yield or ovarian reserve among women with endometriosis compared with those with other infertility diagnoses. 102 103 A recent study observed lower oocyte yield among endometrioma affected ovaries but not among the contralateral ovaries that were unaffected by endometriosis compared with unexposed ovaries from women with no evidence of endometriosis. 104 In addition, although unproven, anatomical distortion and adhesions caused by endometriosis, particularly in stage III-IV disease, seem likely to reduce the chance of natural conception.
No way to prevent endometriosis is known. Enhanced awareness, followed by early diagnosis and management, may slow or halt the natural progression of the disease and reduce the long term burden of painful symptoms, including possibly the risk of central sensitization, but no cure exists. Furthermore, the evidence for modifiable risk factors for endometriosis remains unacceptably sparse. 12 Critically needed are large scale longitudinal studies that can quantify modifiable exposures in girls and young women in the pre-diagnostic, and ideally the pre-symptomatic, window that are then explored further in humans, 105 106 107 as well as in experimental models, to determine the physiologic pathways defined by causal effects on the epigenome, transcriptome, proteome, and metabolome. To date, few risk factors have been robustly replicated in multiple populations, with the most consistently associated with endometriosis including müllerian anomalies, low birth weight and lean body size, early age at menarche, short menstrual cycles, and nulliparity. 1 11 Less research has supported associations with endocrine disrupting toxins including diethystilbestrol. 108
Clinical course
A critical aspect of care for women with endometriosis is that associated symptoms progress and recede over the life course, sometimes in response to treatment and sometimes with age or altered environment in pathways that we do not yet understand ( fig 4 ). For example, pain remediation is often a priority among adolescents, 109 whereas older women may be focused on fertility or on life affecting fatigue. 8 110 Furthermore, a long held belief that endometriosis and its symptoms do not occur in adolescents and end at menopause was erroneous. However, the years of perimenopause can be a time of increased pelvic pain, 111 112 with particular attention needing to be paid to symptom management that may include an unexpected return of pain in those patients for whom a treatment regimen had been successful during premenopause. 113 Clinicians need to focus across the life course on patient centered care, engaging in a dialogue to capture evolving symptomatology but also to collaborate on what symptoms are of most importance to the patient at this life stage. 110 Importantly, all that we believe we know about endometriosis is limited to the characteristics and natural history of those women who successfully obtain a diagnosis. To whatever extent asymptomatic or incidental findings have influenced the diagnostic population or to whatever extent health disparities or biases regarding symptom belief or access to pain or infertility care have limited or skewed those diagnosed, our elucidation of the true signs and symptoms and prognosis of endometriosis will evolve as care and access to it improves. 11

Endometriosis risk, establishment, and multisystem effects encompassing evolution across the life course
Diagnosis and monitoring
Although endometriosis has a highly variable presentation, steps can be recommended for decision making by general practitioners and gynecologists to approach a “working diagnosis” of probable endometriosis, implement treatment to remediate endometriosis associated symptoms, and consider multi-specialty collaboration for patient centered whole healthcare ( fig 5 ).

Flowchart for a step-by-step approach to patients with suspected endometriosis (adapted from flowcharts in the NICE 114 and ESHRE 13 endometriosis guidelines). *Imaging does not rule out endometriosis; if “negative” imaging but symptoms highly suggestive of endometriosis, consider “working diagnosis” of probable endometriosis. †General practitioners should monitor for emergence of signs of conditions associated with endometriosis and involve/refer to appropriate specialist (eg, gastroenterologist, cardiologist, rheumatologist, psychologist, oncologist). ‡Ideally within accredited specialist endometriosis center
“Red flag” symptoms and signs
The diagnosis of endometriosis should be considered in women (including girls aged 17 and under) presenting with one (or more) of the following symptoms or signs: chronic pelvic pain with or without cyclic flares, dysmenorrhea (affecting daily activities and quality of life), deep dyspareunia, cyclical gastrointestinal symptoms (particularly dyschezia), cyclical urinary symptoms (particularly hematuria or dysuria), or infertility in association with one (or more) of the preceding symptoms or signs. 114 Shoulder tip pain (pain under the shoulder blade), catamenial pneumothorax, cyclical cough/hemoptysis/chest pain, and cyclical scar swelling/pain can indicate endometriosis at extra-abdominal sites. 13 40 115 116 Fatigue is commonly reported by women with endometriosis. An abdomino-pelvic examination may help to identify ovarian and deep disease. 117
Diagnostic biomarkers
Many research studies and Cochrane reviews have assessed potential biomarkers for endometriosis, 118 119 120 with the ultimate goal of reducing the delay that exists in diagnosing endometriosis. Unfortunately, all of the candidates investigated to date have proven non-specific or unreliable, making them inappropriate for routine clinical use.
Imaging to diagnose endometriosis
Ultrasonography and magnetic resonance imaging (ideally two dimensional, T2 weighted sequences without fat suppression) can be used to diagnose endometriosis preoperatively, but the absence of findings on imaging does not exclude endometriosis, particularly superficial peritoneal disease. 121 122 Nevertheless, the ENDO Study enrolled 131 women from the general population who had not presented for gynecologic evaluation, among whom magnetic resonance imaging was used to diagnose endometriosis in 11%. 123 Although the sensitivity of transvaginal ultrasonography is maximized only for endometriomas, technological and training advances are improving detection of all sub-phenotypes of endometriotic lesions. 124 125 Saline infusion sonoPODography is a novel technique that may be able to diagnose superficial peritoneal endometriosis on ultrasonography, although it needs to be validated. 126
Laparoscopic diagnosis and appearance of endometriosis
In patients with suspected endometriosis, in whom imaging has shown no obvious pelvic pathology or for whom empirical treatment has been unsuccessful, laparoscopy is recommended for diagnosis. Laparoscopy for endometriosis should always involve a comprehensive exploration of the abdominal and pelvic contents. Histopathological confirmation is ideal; however, histologic definitions for endometriosis have remained stagnant for decades, with a lower than expected sensitivity, 12 particularly among younger women with endometriosis. 127 Superficial peritoneal endometriosis has been described as having a black (powder burn) or dark bluish appearance from the accumulation of blood pigments ( fig 2 ). 128 However, lesions can appear as white opacifications, red flame-like lesions, or yellow-brown patches in earlier, active stages of disease. 129 Ovarian endometriomas have a distinct morphology classically described as a “chocolate cysts,” containing old menstrual blood, necrotic fluid, and other poorly defined components that give their contents a dark brown appearance. Adhesions are often found in association with endometriomas and consist of fibrous scar tissue resulting from chronic inflammation. In many cases, endometriosis is present at the site of ovarian fixation. 130 Deep endometriosis appears as multifocal nodules and may infiltrate the surrounding viscera and peritoneal tissue. 131 Almost 40% of laparoscopies done for pelvic pain do not identify any pathology. 99 Clinicians should always consider other pelvic and non-pelvic visceral and somatic structures, as well as centrally mediated pain factors, that could be generating or contributing to the pain. 99
“Working diagnosis” of probable endometriosis
In women with a high suspicion of endometriosis, in whom imaging has not shown obvious pelvic pathology and a laparoscopy has not been done or is awaited, giving a “working diagnosis” of probable endometriosis and instigating early medical treatment without waiting for a more definitive diagnosis can be helpful. 13 114 132 133 This is an emerging concept for which some people use the terms “working” and “clinical” diagnosis interchangeably.
Delayed diagnosis
Endometriosis can occur at any age, with some patients reporting that pelvic pain symptoms arose at or soon after thelarche or menarche. Among women with endometriosis diagnosed in adulthood, nearly a fifth report that their symptoms began before age 20 and two thirds report onset before age 30. 5 The exact time of disease onset is unknown for endometriosis, as symptoms must emerge and be sufficiently life affecting to gain referral for definitive diagnosis. Furthermore, non-specific symptoms such as dysmenorrhea have often been treated with hormonal drugs without consideration of endometriosis, whereas the current recommendation is to be aware and consider a working diagnosis of probable endometriosis. Thus, varied non-specific symptomatology, normalization of pelvic pain, clinicians’ awareness of endometriosis, and economic and geographic access to care all contribute to a delay averaging seven years from symptom onset to surgical diagnosis. 1 5
Long term monitoring of endometriosis
Follow-up, including psychological support, should be considered in women with confirmed endometriosis, with renewed evaluation and a revised treatment plan if symptoms emerge, recur, or worsen over time. However, no evidence exists of benefit of regular long term monitoring (for example, imaging) for early detection of endometriotic lesion recurrence, complications, or malignant transformation, in the absence of complex ovarian masses or endometriosis with deep bowel effect. 134 135 Given growing evidence of risk of multisystem involving conditions ( fig 4 ), patient centered whole healthcare dictates that monitoring by general practitioners for emergence of signs and symptoms of mental health conditions, cardiovascular disease, immunologic and autoimmune disorders, gastrointestinal conditions, or multifocal pain conditions should be heightened and referral to a non-gynecologic specialist should be considered as needed.
Management of endometriosis associated pain
The growing recognition that endometriosis associated pain has a mixed pain phenotype (or occupies different points on a continuum) supports a personalized, multimodal, interdisciplinary treatment approach, 13 which might include surgical ablation/excision of lesions, analgesics, hormonal treatments, non-hormonal treatments including neuromodulators, and non-drug therapies (or a combination of the above). 1 The evidence supporting different surgical, pharmacologic, and non-pharmacologic approaches to treating endometriosis is examined below.
Surgical management of endometriosis associated pain
The most recent guidelines for endometriosis (for example, the National Institute for Health and Care Excellence (NICE), ESHRE) recommend surgery as a treatment option to reduce endometriosis associated pain. 13 114 However, only a limited number of RCTs have assessed pain outcomes after surgery (and most are small, offer little detail on endometriosis sub-phenotypes visualized at surgery, and have a follow-up period of less than 12 months). Furthermore, the authors of the most recent Cochrane review of surgery for endometriosis associated pain concluded that they were “uncertain of the effect of laparoscopic surgery on pain and quality of life” owing to the low quality of the available studies. 136 They included only two of the published RCTs (comparing surgical treatment of endometriosis with diagnostic laparoscopy alone) in their analysis of laparoscopic excision to improve pain and quality of life. 137 138 One trial of 16 participants experiencing pain associated with endometriosis assessed “overall pain” scores at 12 months (mean difference on 0-100 visual analog scale (VAS) 1.65, 95% confidence interval 1.11 to 2.19), and the other trial of 39 participants assessed quality of life at six months measured using the EuroQol-5D (mean difference 0.03, –0.12 to 0.18). The evidence of benefit for specific subtypes is discussed in more detail below.
Surgery for superficial peritoneal endometriosis
Little evidence shows that surgery to treat isolated superficial peritoneal endometriosis improves overall symptoms and quality of life. The uncertainty around surgical management of this subtype is compounded by the limited evidence to allow an informed selection of specific surgical modalities to remove the lesions (for example, laparoscopic ablation versus laparoscopic excision). 139 140
Surgery for ovarian endometriosis
To our knowledge, no RCTs have compared cystectomy versus no treatment in women with endometrioma and measured the effect on painful symptoms. Also, no published data indicate a threshold cyst size below which surgery may be safely withheld in the absence of suspicious features on imaging (surgery is the only means by which a tissue specimen can be obtained to rule out ovarian malignancy). Thus, surgical excision is generally considered the optimal treatment for ovarian endometriosis. Cystectomy, instead of drainage and coagulation, is the preferred surgical approach as it reduces recurrence of endometrioma and endometriosis associated pain. 141 Cystectomy should be chosen with caution for women who desire fertility, as a risk of fertility affecting diminished ovarian reserve exists, and a highly skilled conservative approach should be applied to minimize ovarian damage. 142
Surgery for deep endometriosis
Surgical treatment to completely excise deep disease is generally considered to be the treatment of choice. 143 144 Nevertheless, most of the studies that have reported improvements in quality of life following surgical excision of deep endometriosis (typically involving the bowel) have been done in small cohorts of women, usually from single centers, without a comparator arm, and this affects the precision and generalizability of the results. The largest multicenter prospective non-randomized study published to date reported the six, 12, and 24 month follow-up outcomes on nearly 5000 women undergoing laparoscopic excision of deep rectovaginal endometriosis. 143 This showed clinically and statistically significant reductions in premenstrual, menstrual, and non-cyclical pelvic pain, deep dyspareunia, dyschezia, low back pain, and bladder pain at 24 months (data from 524-560 participants for each symptom) with a corresponding improvement in quality of life (575 participants, median score on EuroQol-5D 76, 95% confidence interval 75 to 80). Although the results should be interpreted with caution, because data were missing for >70% of patients at 24 months, assigned score methods suggest that evidence of improvement remained statistically significant.
Hysterectomy for endometriosis
No RCTs on hysterectomy for the treatment of endometriosis associated pain have been done. Most published articles are retrospective case series, and only a few prospective studies have been reported. Hysterectomy (with or without oophorectomy) with removal of all visible endometriosis lesions should be reserved for women who no longer wish to conceive and who have not responded to more conservative management. Women with endometriosis should be informed that hysterectomy is not a “cure” for endometriosis and that it is best reserved for women with coexisting adenomyosis (which does occur inside the uterus) or for women with severe pain who have exhausted all other options to improve their symptoms. 145 Recent longitudinal studies have not found a benefit of bilateral oophorectomy for long term pain management. 145 146 Of note, BIPOC (black, indigenous, and people of color) women are more likely to have complications of hysterectomy, in part because they are more likely to undergo laparotomy rather than minimally invasive laparoscopy. 147 Women should be informed that hysterectomy is associated with long term morbidity, 148 including cardiovascular disease, 56 among those with and without surgically induced menopause. 149 150
Recurrence or progression of endometriosis after surgery
The reported recurrence rate of painful symptoms attributed to endometriosis is high, estimated as 21.5% at two years and 40-50% at five years. 146 151 However, although a purist’s definition of “endometriosis recurrence” calls for “second look” laparoscopy, it is most often diagnosed in the real world on the basis of recurrence of symptoms alone. In addition, no robust evidence exists to support an ordered progression of endometriotic lesions. In prospective studies of repeat surgeries, lesions progressed (in 29% of cases), regressed (in 42%), or were static (in 29%). 152 Surgical treatment of certain subtypes of endometriosis could also exacerbate painful symptoms. 153 154
Preoperative and postoperative hormone treatment
Preoperative hormone treatment has not been shown to improve the immediate outcome of surgery for pain, or reduce recurrence, in women with endometriosis. 155 A meta-analysis of 340 participants found that compared with surgery alone, postoperative hormone treatment of endometriosis reduced pelvic pain after 12 months (standardized mean difference on VAS −0.79, −1.02 to −0.56), but the evidence is very low quality. 155 Women with endometriosis who undergo hysterectomy with oophorectomy should be advised to start continuous combined hormone replacement therapy (HRT) for at least the first few years after surgery. 156 This may be changed later to estrogen alone, but this needs to be balanced with the theoretical risk of reactivation and malignant transformation of any residual endometriosis, which can occur many years later.
Pharmacologic management of endometriosis associated pain
Most women with suspected or known endometriosis use over-the-counter drugs, such as non-steroidal anti-inflammatory drugs (NSAIDs). However, the available evidence to support their use is scarce. The data on the benefit of NSAIDs are limited to one small RCT. 157 They can be useful as “breakthrough medication” in the management of a pain flare.
Hormonal treatments
Hormone treatments for endometriosis include combined contraceptives, progestogens, gonadotrophin releasing hormone (GnRH) agonists, GnRH antagonists, and aromatase inhibitors ( table 1 ). All of these hormone treatments (except the newer GnRH antagonists, which have not been so extensively studied) have been included in a multivariate network meta-analysis of the outcomes “menstrual pain” and “non-menstrual pelvic pain” (pain relief on VAS; total of 1680 participants). 114 All treatments led to a clinically significant reduction in pain on the VAS compared with placebo. The magnitude of this treatment effect is similar for all treatments, suggesting that little difference exists between them in their capacity to reduce pain. Furthermore, symptoms return after cessation of treatment and hormone treatments used to manage endometriosis all have side effects. In addition, although the contraceptive properties of the hormones may be welcome if the woman does not wish to become pregnant, they may be unwanted if fertility is desired.
Hormone treatments
- View inline
Non-hormonal treatments
Analgesic tricyclic antidepressants (for example, amitriptyline, nortriptyline), selective serotonin uptake inhibitors (for example, duloxetine) and anticonvulsants (for example, gabapentin and pregabalin) are sometimes used clinically in the treatment of endometriosis associated pain without a strong evidence base. 167 These “neuromodulatory drugs” differ from conventional analgesics, such as NSAIDs, in that they primarily affect the central nervous system’s modulation of pain rather than peripheral mediators of inflammation. However, in a recent RCT for the management of chronic pelvic pain (in the absence of endometriosis), gabapentin was not shown to be superior to placebo and was associated with dose limiting side effects. 168
Non-drug management of endometriosis associated pain
Pelvic physiotherapy.
An increasing number of women with endometriosis report anecdotal benefit from pelvic physiotherapy. Physiotherapists may support women with activity management (for example, exercises, pacing strategies, and goal setting) and/or use complementary approaches to manage their pelvic pain symptoms (for example, massage and trigger point release therapy). Establishing the independent benefit of standalone physiotherapy is difficult, because most studies have assessed it in combination with psychological and medical management. 169 Two small pilot studies assessed the outcome of manipulations and massage for relief of endometriosis associated pain specifically, but they included specific patient groups and need expansion and replication to support recommendations for care of endometriosis patients. 170 171
The most common psychologically based intervention for chronic pain is cognitive behavioral therapy (CBT). Most of the studies of CBT in women with endometriosis are of low quality, designed using different methods and based on different psychological frameworks (making separation of effects difficult). However, given that CBT has been evaluated across a spectrum of other chronic pain disorders and shown to be effective for developing pain coping strategies, 98 99 172 it should be integrated into individualized treatment plans when needed.
Dietary intervention
Diet has been postulated to affect symptoms of endometriosis. However, very few studies (all of limited quality) have evaluated the benefit of dietary interventions and their effect on endometriosis symptoms. Supplements, such as omega-3 polyunsaturated fatty acids (O-PUFAs), have been investigated as a way of reducing inflammation and pain in endometriosis. 173 174 In a recent review, decreased pain scores were observed in women with endometriosis after use of O-PUFAs, which were not seen in controls. 175 Clinicians should be aware that women with endometriosis have an increased risk of co-presenting with irritable bowel syndrome concomitant with endometriosis associated dyschezia. 44 Patients are not uncommonly referred for gastroenterology evaluation without consideration of potential endometriosis. 45
Treatment of endometriosis associated infertility
Hormonal/medical therapies.
No evidence exists of benefit of suppression of ovarian function in women with endometriosis associated infertility who wish to conceive. 176 Following surgery for endometriosis, women seeking pregnancy should not be treated with postoperative hormone suppression with the sole purpose of enhancing future pregnancy rates.
Surgery to increase chance of natural pregnancy
Moderate quality evidence from a Cochrane meta-analysis of three RCTs in a total of 528 participants shows that laparoscopic treatment (ablation or excision) of superficial peritoneal endometriosis increases viable intrauterine pregnancy rates compared with diagnostic laparoscopy only (odds ratio 1.89, 95% confidence interval 1.25 to 2.86). 136 We found no data on live birth rates, and the effect on ectopic pregnancy and miscarriage rates is unclear. No published RCTs have assessed fertility outcomes after surgery for ovarian or deep disease, and surgery is generally recommended only in the presence of painful symptoms. 13 Use of the Endometriosis Fertility Index to support decision making for the most appropriate option to achieve pregnancy after surgery (for example, women who may benefit from medically assisted reproduction) has been recently suggested. 13 68
Medically assisted reproduction
Low quality evidence shows that viable intrauterine pregnancy rates are increased in women with superficial peritoneal endometriosis if they undergo intrauterine insemination with ovarian stimulation, instead of expectant management or intrauterine insemination alone. In one RCT of 103 participants randomized either to ovarian stimulation with gonadotrophins and intrauterine insemination treatment or to expectant management, the live birth rate was 5.6 (95% confidence interval 1.18 to 17.4) times higher in the treated couples. 177 In women with ovarian or deep endometriosis, the benefit of ovarian stimulation with intrauterine insemination is unclear. No RCTs have evaluated the efficacy of assisted reproductive technology (ART) versus no intervention in women with endometriosis. Recommendations in guidelines suggesting that ART may be effective for endometriosis associated infertility have been based on meta-analyses of observational studies comparing the outcomes of ART in women with and without endometriosis. 13 178 179 Doing surgery before ART for infertility associated with superficial peritoneal endometriosis is not recommended, as the evidence suggesting benefit is based on a single retrospective study of low quality 180 (and is not supported by indirect evidence from multiple studies comparing outcomes in women with surgically treated endometriosis and those managed without surgery 179 ). Doing surgery for ovarian endometrioma before ART to improve live birth rates is also not recommended. Current evidence shows no benefit, and surgery is likely to have a negative effect on ovarian reserve. 181 182 In addition, no evidence shows that doing surgical excision of deep endometriosis before ART improves reproductive outcomes, and this should be reserved for women with concomitant painful symptoms.
Specialist endometriosis centers
Specialist centers were first formally proposed in 2006, 183 and this model of care has been successfully implemented in the UK and several other European countries such as Denmark, Germany, and France. 184 185 The role of specialist endometriosis centers should be to offer a coordinated, holistic, multidisciplinary, multimodal approach to women with complex symptoms of endometriosis that are experienced and evolve across the life course ( fig 5 ). Although relevant surgical expertise is important, the role of a center is not to focus solely on surgical treatment to eradicate lesions. Thus, a specialist center should offer an integrated service, including gynecologists, colorectal surgeons, urologists, endometriosis specialist nurses, pain medicine specialists, psychologists, physiotherapists, fertility specialists, and imaging experts.
Various national and international organizations have issued guidelines for the assessment and management of endometriosis. We reviewed and compared nine of these guidelines (including the recent 2022 update of the ESHRE guideline). 186 187 188 189 190 191 192 193 All of the guidelines recommend the combined oral contraceptive pill and progestogens for endometriosis associated pain, but they differ in the recommendations around “second line” medical treatments. All of the guidelines recommend laparoscopic surgery for the management of endometriosis associated pain, although some acknowledge the lack of evidence for surgery in the management of pain associated with superficial peritoneal endometriosis specifically. 13 114 No clear consensus exists regarding surgical treatment for endometriosis associated infertility, especially with regard to the management of an endometrioma before assisted reproduction.
Emerging diagnostic tools and treatments
Most endometriosis research studies to date have been underfunded and on a small scale, and have involved poorly defined populations of women and samples captured from those who receive a diagnosis well along in their endometriosis journey. However, real hope exists of a breakthrough in the development of a biomarker to diagnose endometriosis closer to emergence and earlier in its natural progression, and to predict response to treatment, owing to the establishment of globally harmonized endometriosis protocols for clinical data and human tissue collection. 105 106 107 The biomarker field will also hopefully benefit from new insights being gained from the study of serum microRNAs and metabolomics. 194 195 Preclinical studies of new non-hormonal medical treatments have offered insights by focusing on inflammation, pain, and metabolism as the platform for repurposing of drugs already approved for other conditions. 19 90 196 Increasing evidence also suggests that the “gut-brain axis” could be a novel therapeutic target for pain symptom relief in endometriosis. 197 Microbiomes likely play a role in the gut-brain axis, are associated with the spectrum of symptoms associated with endometriosis, and are an exciting putative therapeutic target. Lastly, although randomized evaluations of surgical interventions for endometriosis have been rare (and some interventions have been adopted without rigorous evaluation), we are witnessing important collaboration between research and surgical communities to conduct large scale, appropriate, and well designed trials (for example, PRE-EMPT ( https://www.birmingham.ac.uk/research/bctu/trials/womens/pre-empt/index.aspx ), REGAL ( https://w3.abdn.ac.uk/hsru/REGAL/Public/Public/index.cshtml ), ESPriT2 ( https://www.ed.ac.uk/centre-reproductive-health/esprit2 ), and DIAMOND ( https://w3.abdn.ac.uk/hsru/DIAMOND/Public/Public/index.cshtml )). Surgical trials are difficult to undertake successfully and pose practical and methodological challenges. However, the inherent value of a well conducted RCT to predict the outcomes and/or success rates of surgical treatments for endometriosis should not be overlooked.
Endometriosis is a prevalent, often life affecting condition that in most women emerges during adolescence and can evolve to include symptoms and conditions encompassing multiple systems. Endometriosis demands to be known, considered, and tackled by all practitioners—general and specialist—who treat female patients at all stages across the life course. Patient centered whole healthcare requires a dialog between a woman and her healthcare practitioners to monitor symptom remediation, persistence, or recurrence and to prioritize the focus of care—for example, fatigue remediation when sports participation is paramount, fertility when family building is desired, a revision of medical treatment during perimenopause, or early response to signs of cardiovascular changes. Stigma around menstrual health and chronic pain remain all too ubiquitous barriers to high quality healthcare. Awareness in the general public and among healthcare providers is essential.
Once their symptoms are acknowledged and treated, most patients with endometriosis do well. However, despite overcoming diagnostic delays and access to state of the art treatment, some experience persistence or progression of symptoms. Critical next steps for discovery include defining sub-phenotypes of endometriosis that classify patients into groups that are predictive of prognosis and the natural course of the condition and indicate selection of treatments most likely to be successful to restore high quality of life. We must also answer foundational questions that remain about the causes and natural progression of endometriosis that need expanded funding and attraction of multidisciplinary scientists from all areas of population and bench science. Recommendations to permit a “working diagnosis” of probable endometriosis are having an effect on patient centered care and faster symptom remediation. Through the work of endometriosis associations, non-governmental organizations, and the endometriosis community across the globe, awareness of endometriosis has increased in recent years, along with some increases in funding. We are early on the necessary trajectory, but the journey is gaining speed.
Questions for future research
What causes endometriosis?
Can a non-invasive screening tool be developed to aid the diagnosis of endometriosis?
What are the most effective ways of maximizing and/or maintaining fertility in women with confirmed or suspected endometriosis?
What are the most effective ways of managing the emotional, psychological, and/or fatigue related impact of living with endometriosis?
Can we predict the outcomes and/or success rates for surgical or medical treatments for endometriosis?
What are the most effective non-surgical ways of managing endometriosis related pain and/or symptoms?
Adapted from the James Lind Alliance “Top ten research priorities for endometriosis in the UK and Ireland” 198
Patient involvement
We consulted Emma Cox, chief executive of Endometriosis UK, a nationally recognized representative and voice of patients with endometriosis, in the development of this review, and she commented on the draft and final manuscript. No patients were involved directly in the preparation of this article.
Acknowledgments
In addition to invaluable insight provided by Emma Cox, we thank Naoko Sasamoto and Marzieh Ghiasi for early design of figures 1 and 4, which were further adapted by SAM for this review; Kevin Kuan for designing figure 3 in BioRender; and Dan Martin for contributing image 1 to figure 2.
Series explanation: State of the Art Reviews are commissioned on the basis of their relevance to academics and specialists in the US and internationally. For this reason they are written predominantly by US authors
Contributors: AWH and SAM contributed equally to the planning, analysis, and writing of the article. AWH is the guarantor.
Funding: AWH is supported by an MRC Centre Grant (MRC G1002033) and an NIHR Project Grant (NIHR129801).
Competing interests: We have read and understood BMJ policy on declaration of interests and declare the following interests: AWH’s institution (University of Edinburgh) has received payment for consultancy and grant funding from Roche Diagnostics to assist in the early development of a possible blood diagnostic biomarker for endometriosis. AWH has received grant funding from the MRC and NIHR for endometriosis research; he is a board member of the World Endometriosis Society and Society for Endometriosis and Uterine Disorders, is co-editor in chief of Reproduction and Fertility , has been a member of the NICE and ESHRE Endometriosis Guideline Groups, and is a trustee and medical adviser to Endometriosis UK. SAM has received payment for consultancy and grant funding from AbbVie, LLC, for population based research unrelated to product development and has received grant funding from the US National Institutes of Health, US Department of Defense, and the Marriott Family Foundations for endometriosis research. SAM is a board member of the World Endometriosis Society, World Endometriosis Research Foundation, American Society for Reproductive Medicine Endometriosis Special Interest Group, and the European Society for Human Reproduction and Embryology Special Interest Group on Endometriosis and Endometrial Disorders; a member of the Interdisciplinary Network on Female Pelvic Health of the Society for Women’s Health Research; and is a statistical advisory board member for Human Reproduction and field chief editor for Frontiers in Reproductive Health .
Provenance and peer review: Commissioned; externally peer reviewed.
- Zondervan KT ,
- Becker CM ,
- Prescott J ,
- Farland LV ,
- Tobias DK ,
- Soliman AM ,
- Simoens S ,
- Dunselman G ,
- Dirksen C ,
- Nnoaham KE ,
- Hummelshoj L ,
- Webster P ,
- World Endometriosis Research Foundation Global Study of Women’s Health consortium
- Stratton P ,
- Cleary SD ,
- Ballweg ML ,
- Missmer SA ,
- Agarwal SK ,
- Kulkarni MT ,
- Shafrir AL ,
- Taylor HS ,
- Adamson GD ,
- Diamond MP ,
- Heikinheimo O ,
- ESHRE Endometriosis Guideline Group
- DiVasta AD ,
- Vitonis AF ,
- Laufer MR ,
- Ballard K ,
- Tomassetti C ,
- Johnson NP ,
- Petrozza J ,
- International Working Group of AAGL, ESGE, ESHRE and WES
- Saunders PTK ,
- Andres MP ,
- Arcoverde FVL ,
- Souza CCC ,
- Fernandes LFC ,
- Halvorson LM
- Isaacson K ,
- Halvorson LM ,
- Giudice LC ,
- Vannuccini S ,
- Petraglia F ,
- Bourdon M ,
- Santulli P ,
- Jeljeli M ,
- Saunders PTK
- Williams C ,
- Bedaiwy MA ,
- Counsellor VS
- Eskenazi B ,
- Coloma JL ,
- Martínez-Zamora MA ,
- Collado A ,
- Larrosa Pardo F ,
- Bondesson E ,
- Schelin MEC ,
- Palmor MC ,
- Fourquet J ,
- Miller JA ,
- Shigesi N ,
- Kvaskoff M ,
- Kirtley S ,
- Harris HR ,
- Korkes KMN ,
- Costenbader KH ,
- Kennedy SH ,
- Jenkinson C ,
- World Endometriosis Research Foundation Women’s Health Symptom Survey Consortium
- Rodríguez MA ,
- Buchwald DS ,
- National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Urological Chronic Pelvic Pain
- Tirlapur SA ,
- Chaliha C ,
- Chiaffarino F ,
- Cipriani S ,
- Zimmerman LA ,
- Fadayomi AB ,
- Morotti M ,
- Pogatzki-Zahn EM ,
- Liedgens H ,
- IMI-PainCare PROMPT consensus panel
- Nunez-Badinez P ,
- Laux-Biehlmann A ,
- Gallagher CS ,
- Mäkinen N ,
- 23andMe Research Team
- Mahamat-Saleh Y ,
- Thombre Kulkarni M ,
- Shafrir A ,
- Degnan WJ 3rd . ,
- Rich-Edwards J ,
- Spiegelman D ,
- Forman JP ,
- Vincent K ,
- Taylor RN ,
- Symons LK ,
- Miller JE ,
- Rahmioglu N ,
- Morris AP ,
- WERF EPHect Working Group
- Whitaker LHR ,
- Vermeulen N ,
- Einarsson JI ,
- International working group of AAGL, ESGE, ESHRE and WES
- ↵ Revised American Society for Reproductive Medicine classification of endometriosis: 1996 . Fertil Steril 1997 ; 67 : 817 - 21 . doi: 10.1016/S0015-0282(97)81391-X pmid: 9130884 OpenUrl CrossRef PubMed Web of Science
- Tuttlies F ,
- Keckstein J ,
- Saridogan E ,
- Ulrich UA ,
- Schliep KC ,
- Mumford SL ,
- Peterson CM ,
- Shamiyeh A ,
- World Endometriosis Society Sao Paulo Consortium
- Borrelli GM ,
- Maheux-Lacroix S ,
- Nesbitt-Hawes E ,
- Janssen EB ,
- Rijkers AC ,
- Hoppenbrouwers K ,
- Meuleman C ,
- D’Hooghe TM
- Christ JP ,
- Schulze-Rath R ,
- Grafton J ,
- Treloar SA ,
- O’Connor DT ,
- O’Connor VM ,
- Pettersson HJ ,
- Svedberg P ,
- Nyholt DR ,
- ANZGene Consortium ,
- International Endogene Consortium ,
- Genetic and Environmental Risk for Alzheimer’s disease Consortium
- Sapkota Y ,
- Steinthorsdottir V ,
- iPSYCH-SSI-Broad Group
- Hammond MG ,
- Cousins FL ,
- Figueira PG ,
- Critchley HOD ,
- Babayev E ,
- Gajbhiye R ,
- McKinnon B ,
- Mortlock S ,
- Mueller M ,
- Montgomery G
- Saunders PT ,
- Greaves E ,
- Esnal-Zufiurre A ,
- Mechsner S ,
- Saunders PT
- As-Sanie S ,
- Harris RE ,
- Napadow V ,
- Coccia ME ,
- Rizzello F ,
- Palagiano A ,
- Scarselli G
- Sutton CJ ,
- Whitelaw N ,
- Khachikyan I ,
- Carrillo J ,
- Di Tucci C ,
- Di Feliciantonio M ,
- Senapati S ,
- Sammel MD ,
- Barnhart KT
- Carvalho LFP ,
- Fassbender A ,
- Buck Louis GM
- Gallagher JS ,
- Angioni S ,
- Gemmell LC ,
- Webster KE ,
- VanBuren WM ,
- Kuznetsov L ,
- Dworzynski K ,
- Overton C ,
- Guideline Committee
- Ballard KD ,
- Seaman HE ,
- de Vries CS ,
- Bonsignore L ,
- Samuels S ,
- Vercellini P
- Rouzier R ,
- Thomassin-Naggara I ,
- Nisenblat V ,
- Bossuyt PM ,
- Farquhar C ,
- Johnson N ,
- Moura APC ,
- Ribeiro HSAA ,
- Bernardo WM ,
- Buck Louis GM ,
- Hediger ML ,
- ENDO Study Working Group
- Guerriero S ,
- Condous G ,
- van den Bosch T ,
- Leonardi M ,
- Mestdagh W ,
- Stamatopoulos N ,
- Watkins JC ,
- Mettler L ,
- Schollmeyer T ,
- Lehmann-Willenbrock E ,
- Jansen RP ,
- Koninckx PR ,
- Adamyan L ,
- Wattiez A ,
- Barraclough K ,
- Chapron C ,
- Andreotti RF ,
- Vercellini P ,
- Sergenti G ,
- Frattaruolo MP ,
- Beebeejaun Y ,
- Bosteels J ,
- Jarrell J ,
- Mohindra R ,
- Taenzer P ,
- Daniels J ,
- DeSarno M ,
- Findley J ,
- Maouris P ,
- Shaltout MF ,
- Elsheikhah A ,
- BSGE Endometriosis Centres
- Bendifallah S ,
- Sandström A ,
- Johansson M ,
- Bäckström T ,
- Shakiba K ,
- McGill KM ,
- Orlando MS ,
- Luna Russo MA ,
- Richards EG ,
- Stewart EA ,
- Laughlin-Tommaso SK ,
- Weaver AL ,
- Hans Evers JL
- Schrepf AD ,
- Zakhari A ,
- Delpero E ,
- McKeown S ,
- Tomlinson G ,
- Choudhry AJ ,
- ↵ British Menopause Society. Tools for Clinicians: induced menopause in women with endometriosis. 2019. https://thebms.org.uk/publications/tools-for-clinicians/ .
- Kauppila A ,
- di Tucci C ,
- Achilli C ,
- Benedetti Panici P
- Kettner LO ,
- Lessey BA ,
- Tanimoto M ,
- Kusumoto T ,
- Arjona Ferreira JC ,
- Hewitt CA ,
- GaPP2 collaborative
- Nordling J ,
- Jaszczak P ,
- Deboute O ,
- Zacharopoulou C ,
- Valiani M ,
- Ghasemi N ,
- Bahadoran P ,
- Williams ACC ,
- Eccleston C
- Abokhrais IM ,
- Denison FC ,
- Nodler JL ,
- Collins JJ ,
- Fedorkow DM ,
- Vandekerckhove P
- Tummon IS ,
- Martin JS ,
- Gallos ID ,
- Coomarasamy A
- Opøien HK ,
- Fedorcsak P ,
- Nickkho-Amiry M ,
- Majumder K ,
- Edi-O’sagie E ,
- D’Hooghe T ,
- Hummelshoj L
- van Hanegem N ,
- van Kesteren P ,
- Kalaitzopoulos DR ,
- Samartzis N ,
- Kolovos GN ,
- Dunselman GA ,
- European Society of Human Reproduction and Embryology
- Collinet P ,
- Revel-Delhom C ,
- Buchweitz O ,
- German and Austrian Societies for Obstetrics and Gynecology
- Leyland N ,
- Laberge P ,
- Practice Committee of the American Society for Reproductive Medicine
- World Endometriosis Society Montpellier Consortium
- Moustafa S ,
- Mamillapalli R ,
- Nematian S ,
- Koscielniak M ,
- Williams L ,
- Salliss ME ,
- Mahnert ND ,
- Herbst-Kralovetz MM
- Endometriosis Priority Setting Partnership Steering Group (appendix)
- Search Menu
- Sign in through your institution
- Volume 2024, Issue 5, May 2024 (In Progress)
- Volume 2024, Issue 4, April 2024
- Bariatric Surgery
- Breast Surgery
- Cardiothoracic Surgery
- Colorectal Surgery
- Colorectal Surgery, Upper GI Surgery
- Gynaecology
- Hepatobiliary Surgery
- Interventional Radiology
- Neurosurgery
- Ophthalmology
- Oral and Maxillofacial Surgery
- Otorhinolaryngology - Head & Neck Surgery
- Paediatric Surgery
- Plastic Surgery
- Transplant Surgery
- Trauma & Orthopaedic Surgery
- Upper GI Surgery
- Vascular Surgery
- Author Guidelines
- Submission Site
- Open Access
- Reasons to Submit
- About Journal of Surgical Case Reports
- Editorial Board
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, case report, conflict of interest statement, consent for publication, abdominal wall endometriosis: a case report.
- Article contents
- Figures & tables
- Supplementary Data
Stefanos K Stefanou, Kostas Tepelenis, Christos K Stefanou, George Gogos-Pappas, Christos Tsalikidis, Konstantinos Vlachos, Abdominal wall endometriosis: a case report, Journal of Surgical Case Reports , Volume 2021, Issue 4, April 2021, rjab055, https://doi.org/10.1093/jscr/rjab055
- Permissions Icon Permissions
Abdominal wall endometriosis has an incidence of 0.3–1% of extrapelvic disease. Α 48-year-old female appeared in the emergency department with cellulitis in a lower midline incision. She had an endometrioma of the anterior abdominal wall removed 2 years ago. After 5 months, she underwent an open repair of an incisional hernia with a propylene mesh, which was unfortunately infected and removed 1 month later. Finally, in July 2019, she had her incisional hernia repaired with a biological mesh. Imaging modalities revealed a large mass below the umbilicus. Mass was punctured under ultrasound guidance. Cytology reported the recurrence of endometriosis. Pain and abdominal mass associating with menses were the two most typical symptoms. Wide local excision of the mass with at least 1 cm negative margins is the preferred treatment. Surgeons should maintain a high suspicion of the disease in reproductive women with circular pain, palpable abdominal mass and history of uterine-relating surgery.
Endometriosis is a common condition where endometrial cells, both glands and stroma, are found outside the womb [ 1 ]. Most often, endometriosis is located on the ovaries, fallopian tubes and tissue around the uterus and ovaries, whereas the extrapelvic disease is rare [ 2 ]. Abdominal wall endometriosis (AWE), the commonest site of extrapelvic disease, has an incidence of 0.03–1% [ 3 ]. Cause is not entirely clear, and several theories have been proposed about its pathogenesis [ 2 , 4 ]. The main symptom is a recurrent cyclic pain associated with menstruation [ 5 ]. Differential diagnosis encompasses hernias, abscesses, lipomas, desmoids tumors and malignancies [ 6 ].
A 48-year-old female patient visited the emergency department due to cellulitis in a lower midline incision. She had a tumor of the anterior abdominal wall removed at 2017, which turned out to be an endometrioma in the histological report. Six months later, she underwent an open repair of an incisional hernia with a polypropylene mesh. The mesh was placed on lay. One month after the surgery, the mesh was removed owing to infection. On July 2019, her incisional hernia was repaired with a biological mesh. From obstetric history, she had a caesarian delivery 18 years ago.
Abdominal examination disclosed a palpable hard mass below the umbilicus. The patient was admitted to the surgical department for observation, and antibiotics were commenced, particularly vancomycin. A computed tomography (CT) of the abdomen was obtained, which demonstrated a large oval mass 10.7 × 5.7 × 7.8 cm with rim enhancement and dense content, which was located below the umbilicus.
On the same day, percutaneous drainage of the mass was carried out. Macroscopically, the fluid seemed to be blood. Then, a small drainage catheter was left in place to drain the hematoma.
Inflammation of the anterior abdominal wall was subsided on Day 2, while the percutaneous drainage catheter was removed on Day 3. The culture was sterile, and the patient was discharged on Day 8 without complications. Cytology report evinced the diagnosis of AWE: the presence of endometrial glands and stroma.
Endometriosis is the presence of endometrial mucosa, both glands and stroma, outside the uterus [ 1 ]. Its incidence in the general population is 6–10% and it usually affects reproductive women. However, the exact incidence of endometriosis is difficult to capture as the diagnosis requires biopsy or visual identification of the endometrium during laparoscopy or laparotomy [ 7 ].
AWE was first reported by Meyer in 1903 [ 8 ]. It is the most common site of extrapelvic disease with an incidence of 0.03–1% [ 3 , 8 ]. Although the exact pathogenesis remains unclear, several theories have been proposed. Direct transplantation theory postulates that endometrial cells can be transported to the abdominal wall during surgery involving the uterine cavity, such as hysterectomy or cesarean delivery. Coelomic metaplasia theory suggests that cells in the mesothelial lining of the abdominal peritoneum can differentiate themselves into endometrial cells. It seems that hormonal and immunological factors stimulate this procedure. Finally, lymphatic and vascular metastasis theory proposes that endometrial cells enter circulation and are deposited at the abdominal wall [ 2 , 4 ].
The most frequent appearance is a cyclic pain associated with menstruation. Other symptoms include skin discoloration, bleeding, dysmenorrheal, dyspareunia and bowel or bladder symptoms, whereas some patients have no symptoms. Clinical examination reveals a palpable abdominal mass, which is usually immobile and painful. Even though pain, skin discoloration, bleeding and an increase in the size of the mass are linked with menses, only 57% of the patients exhibit cyclic symptoms [ 5 ].
Abdominal ultrasound is regarded as the first-line imaging modality for masses and mass-like lesions in the abdominal wall. AWE is depicted as a heterogeneous hypoechoic mass with irregular shape and indistinct margins. It is usually solid, though sometimes has a cystic appearance. Occasionally, vascularity can be seen on color Doppler imaging [ 6 , 9 ].
CT and magnetic resonance imaging (MRI) of the abdomen provide useful information for choosing the best method for closing the fascia defect during operation, as they reveal the extent of the disease and the involvement of the fascia of the rectus muscle. The imaging findings on CT encompass a solid soft-tissue heterogeneous mass with mild-to-moderate enhancement after the administration of intravenous contrast material. On MRI, it is portrayed as a hyperintense or isointense heterogeneous lesion on both T1- and T2-weighted images. Feeding vessels can be observed on occasion on both imaging modalities [ 6 , 9 ].
Fine needle aspiration under ultrasound guidance is an easy, inexpensive and accurate procedure to confirm the diagnosis of AWE. Caution should be taken to avoid the introduction of new implants at the puncture sites. In case of a limited amount of sample material, an additional biopsy may be necessary [ 6 , 8 ].
AWE needs to be distinguished preoperatively from hernias, abscesses, lipomas, desmoids tumors and malignancies. It is worth noting that the malignant transformation of endometriosis is rare, with an incidence of 1% of cases [ 9 ].
The treatment of choice for AWE is wide local excision of the mass with at least 1 cm negative margins [ 7 , 10 ]. Mesh reconstruction should be taken into account for patients with an abdominal mass ≥5 cm on ultrasound or with the involvement of the abdominal wall fascia and muscle [ 5 , 8 ]. The reported recurrence rate is approximately 5% [ 8 , 10 ]. Higher recurrence rates are associated with large lesions and involvement of the rectus muscle [ 10 ]. Medical treatment is ineffective in treating AWE. The recurrence rate is high, especially after the cessation of the drug [ 7 , 8 ].
Pain and abdominal mass associating with menses are the two most typical symptoms of AWE. Abdominal ultrasound is the first-line modality for the diagnosis of AWE, while CT and MRI disclose the extent of the disease and the involvement of rectus muscle. Wide local excision with negative margins of 1 cm at least is the preferred treatment. Surgeons should maintain a high suspicion of the disease in reproductive women with circular pain, palpable abdominal mass and history of uterine-relating surgery.
None declared.
Written informed consent was obtained from the patient prior to publication.
Blanco RG , Parithivel VS , Shah AK , Gumbs MA , Schein M , Gerst PH . Abdominal wall endometriomas . Am J Surg 2003 ; 185 : 596 – 8 .
Google Scholar
Gachabayov M , Horta R , Afanasyev D , Gilyazov T . Abdominal wall endometrioma: our experience in Vladimir, Russia . Niger Med J 2016 ; 57 : 329 .
Ecker AM , Donnellan NM , Shepherd JP , Lee TT . Abdominal wall endometriosis: 12 years of experience at a large academic institution . Am J Obstet Gynecol 2014 ; 211 : 363.e1 – 5 .
Gidwaney R , Badler RL , Yam BL , Hines JJ , Alexeeva V , Donovan V , et al. Endometriosis of abdominal and pelvic wall scars: multimodality imaging findings, pathologic correlation, and radiologic mimics . RadioGraphics 2012 ; 32 : 2031 – 43 .
Burney RO , Giudice LC . Pathogenesis and pathophysiology of endometriosis . Fertil Steril 2012 ; 98 : 511 – 9 .
Stratton P , Berkley KJ . Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications . Hum Reprod Update 2011 ; 17 : 327 – 46 .
Pachori G , Sharma R , Sunaria RK , Bayla T . Scar endometriosis: diagnosis by fine needle aspiration . J Cytol 2015 ; 32 : 65 – 7 .
Ding Y , Zhu J . A retrospective review of abdominal wall endometriosis in Shanghai, China . Int J Gynecol Obstet 2013 ; 121 : 41 – 4 .
Horton JD , Dezee KJ , Ahnfeldt EP , Wagner M . Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases . Am J Surg 2008 ; 196 : 207 – 12 .
Pados G , Tympanidis J , Zafrakas M , Athanatos D , Bontis JN . Ultrasound and MR-imaging in preoperative evaluation of two rare cases of scar endometriosis . Cases J 2008 ; 1 : 97 .
- abdominal mass
- endometriosis
- menstruation
- reproductive physiological process
- surgical mesh
- surgical procedures, operative
- diagnostic imaging
- surgery specialty
- abdominal wall
- hernia, incisional
- endometrioma
- abdominal wall, anterior
- wide local excision
Email alerts
Citing articles via, affiliations.
- Online ISSN 2042-8812
- Copyright © 2024 Oxford University Press and JSCR Publishing Ltd
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Published: 19 July 2018
Endometriosis
- Krina T. Zondervan 1 , 2 ,
- Christian M. Becker 1 ,
- Kaori Koga 3 ,
- Stacey A. Missmer 4 , 5 ,
- Robert N. Taylor 6 &
- Paola Viganò 7
Nature Reviews Disease Primers volume 4 , Article number: 9 ( 2018 ) Cite this article
21k Accesses
690 Citations
119 Altmetric
Metrics details
- Infertility
- Inflammation
- Reproductive disorders
- Reproductive techniques
Endometriosis is a common inflammatory disease characterized by the presence of tissue outside the uterus that resembles endometrium, mainly on pelvic organs and tissues. It affects ~5–10% of women in their reproductive years — translating to 176 million women worldwide — and is associated with pelvic pain and infertility. Diagnosis is reliably established only through surgical visualization with histological verification, although ovarian endometrioma and deep nodular forms of disease can be detected through ultrasonography and MRI. Retrograde menstruation is regarded as an important origin of the endometrial deposits, but other factors are involved, including a favourable endocrine and metabolic environment, epithelial–mesenchymal transition and altered immunity and inflammatory responses in genetically susceptible women. Current treatments are dictated by the primary indication (infertility or pelvic pain) and are limited to surgery and hormonal treatments and analgesics with many adverse effects that rarely provide long-term relief. Endometriosis substantially affects the quality of life of women and their families and imposes costs on society similar to those of other chronic conditions such as type 2 diabetes mellitus, Crohn’s disease and rheumatoid arthritis. Future research must focus on understanding the pathogenesis, identifying disease subtypes, developing non-invasive diagnostic methods and targeting non-hormonal treatments that are acceptable to women who wish to conceive.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
24,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
92,52 € per year
only 92,52 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
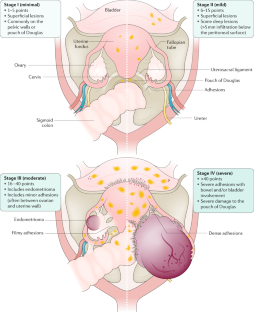
Similar content being viewed by others
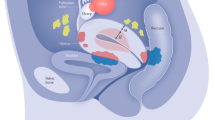
Rethinking mechanisms, diagnosis and management of endometriosis
Real world data on symptomology and diagnostic approaches of 27,840 women living with endometriosis

Endometriosis and chronic pelvic pain have similar impact on women, but time to diagnosis is decreasing: an Australian survey
Marsh, E. E. & Laufer, M. R. Endometriosis in premenarcheal girls who do not have an associated obstructive anomaly. Fertil. Steril. 83 , 758–760 (2005).
PubMed Google Scholar
Halme, J., Hammond, M. G., Hulka, J. F., Raj, S. G. & Talbert, L. M. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 64 , 151–154 (1984).
CAS PubMed Google Scholar
Horton, J. D., Dezee, K. J., Ahnfeldt, E. P. & Wagner, M. Abdominal wall endometriosis: a surgeon’s perspective and review of 445 cases. Am. J. Surg. 196 , 207–212 (2008).
Goldberg, J. & Davis, A. Extrapelvic endometriosis. Semin. Reprod. Med. 35 , 98–101 (2016).
Hirsch, M. et al. Diagnosis and management of endometriosis: a systematic review of international and national guidelines. BJOG 125 , 556–564 (2018).
American Society for Reproductive Medicine. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 67 , 817–821 (1997).
Google Scholar
Vercellini, P. et al. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum. Reprod. 22 , 266–271 (2006).
Nnoaham, K. E. et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil. Steril. 96 , 366–373.e8 (2011). This paper presents the largest multicentre prospective study to date describing the effects of endometriosis; in women undergoing their first laparoscopy, a significantly reduced physical (but not mental) HRQOL, which was associated with delay in diagnosis, was reported in those with endometriosis compared with endometriosis-free symptomatic and asymptomatic women .
PubMed PubMed Central Google Scholar
Peres, L. C. et al. Racial/ethnic differences in the epidemiology of ovarian cancer: a pooled analysis of 12 case-control studies. Int. J. Epidemiol. 47 , 460–472 (2018).
Eskenazi, B. & Warner, M. L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. North Am. 24 , 235–258 (1997).
Buck Louis, G. M. et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil. Steril. 96 , 360–365 (2011).
Janssen, E. B., Rijkers, A. C. M., Hoppenbrouwers, K., Meuleman, C. & D’Hooghe, T. M. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: a systematic review. Hum. Reprod. Update 19 , 570–582 (2013).
Zondervan, K. T., Cardon, L. R. & Kennedy, S. H. What makes a good case–control study? Hum. Reprod. 17 , 1415–1423 (2002).
Adamson, G. D., Kennedy, S. & Hummelshoj, L. Creating solutions in endometriosis: global collaboration through the World Endometriosis Research Foundation. J. Endometr. Pelvic Pain Disord. 2 , 3–6 (2010).
Gemmell, L. C. et al. The management of menopause in women with a history of endometriosis: a systematic review. Hum. Reprod. Update 23 , 481–500 (2017).
CAS PubMed PubMed Central Google Scholar
Leibson, C. L. et al. Incidence and characterization of diagnosed endometriosis in a geographically defined population. Fertil. Steril. 82 , 314–321 (2004).
Missmer, S. A. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am. J. Epidemiol. 160 , 784–796 (2004).
Nnoaham, K. E., Webster, P., Kumbang, J., Kennedy, S. H. & Zondervan, K. T. Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies. Fertil. Steril. 98 , 702–712.e6 (2012).
Missmer, S. A. et al. Reproductive history and endometriosis among premenopausal women. Obstet. Gynecol. 104 , 965–974 (2004).
Peterson, C. M. et al. Risk factors associated with endometriosis: importance of study population for characterizing disease in the ENDO Study. Am. J. Obstet. Gynecol. 208 , 451.e1–451.e11 (2013).
Prescott, J. et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum. Reprod. 31 , 1475–1482 (2016).
Buck Louis, G. M. et al. Women’s reproductive history before the diagnosis of incident endometriosis. J. Womens Health 25 , 1021–1029 (2016).
Farland, L. V. et al. History of breast feeding and risk of incident endometriosis: prospective cohort study. BMJ 358 , j3778 (2017).
Leeners, B., Damaso, F., Ochsenbein-Kölble, N. & Farquhar, C. The effect of pregnancy on endometriosis — facts or fiction? Hum. Reprod. Update 24 , 290–299 (2018).
Shah, D. K., Correia, K. F., Vitonis, A. F. & Missmer, S. A. Body size and endometriosis: results from 20 years of follow-up within the Nurses’ Health Study II prospective cohort. Hum. Reprod. 28 , 1783–1792 (2013).
Farland, L. V. et al. Associations among body size across the life course, adult height and endometriosis. Hum. Reprod. 32 , 1732–1742 (2017).
McCann, S. E. et al. Endometriosis and body fat distribution. Obstet. Gynecol. 82 , 545–549 (1993).
Rahmioglu, N. et al. Genome-wide enrichment analysis between endometriosis and obesity-related traits reveals novel susceptibility loci. Hum. Mol. Genet. 24 , 1185–1199 (2015).
de Ridder, C. M. et al. Body fat mass, body fat distribution, and plasma hormones in early puberty in females. J. Clin. Endocrinol. Metab. 70 , 888–893 (1990).
Cramer, D. W. The relation of endometriosis to menstrual characteristics, smoking, and exercise. JAMA 255 , 1904–1908 (1986).
Baron, J. A., La Vecchia, C. & Levi, F. The antiestrogenic effect of cigarette smoking in women. Am. J. Obstet. Gynecol. 162 , 502–514 (1990).
Ohtake, F., Fujii-Kuriyama, Y., Kawajiri, K. & Kato, S. Cross-talk of dioxin and estrogen receptor signals through the ubiquitin system. J. Steroid Biochem. Mol. Biol. 127 , 102–107 (2011).
Parazzini, F. Selected food intake and risk of endometriosis. Hum. Reprod. 19 , 1755–1759 (2004).
Trabert, B., Peters, U., De Roos, A. J., Scholes, D. & Holt, V. L. Diet and risk of endometriosis in a population-based case–control study. Br. J. Nutr. 105 , 459–467 (2010).
Missmer, S. A. et al. A prospective study of dietary fat consumption and endometriosis risk. Hum. Reprod. 25 , 1528–1535 (2010).
Savaris, A. L. & do Amaral, V. F. Nutrient intake, anthropometric data and correlations with the systemic antioxidant capacity of women with pelvic endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 158 , 314–318 (2011).
Mozaffarian, D. et al. Dietary intake of trans fatty acids and systemic inflammation in women. Am. J. Clin. Nutr. 79 , 606–612 (2004).
Lebovic, D. I., Mueller, M. D. & Taylor, R. N. Immunobiology of endometriosis. Fertil. Steril. 75 , 1–10 (2001).
Rier, S. E. et al. Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 21 , 433–441 (1993).
CAS Google Scholar
Smarr, M. M., Kannan, K. & Buck Louis, G. M. Endocrine disrupting chemicals and endometriosis. Fertil. Steril. 106 , 959–966 (2016).
Kvaskoff, M. et al. Endometriosis: a high-risk population for major chronic diseases? Hum. Reprod. Update 21 , 500–516 (2015). This article presents a meta-analysis of all data and a critical methodologic review suggesting that patients with endometriosis are at higher risk of ovarian and breast cancers, cutaneous melanoma, asthma and some autoimmune, cardiovascular and atopic diseases and are at decreased risk of cervical cancer .
Benagiano, G., Brosens, I. & Habiba, M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum. Reprod. Update 20 , 386–402 (2014).
Kunz, G. et al. Adenomyosis in endometriosis — prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum. Reprod. 20 , 2309–2316 (2005).
Pearce, C. L. et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case–control studies. Lancet Oncol. 13 , 385–394 (2012).
Kim, H. S., Kim, T. H., Chung, H. H. & Song, Y. S. Risk and prognosis of ovarian cancer in women with endometriosis: a meta-analysis. Br. J. Cancer 110 , 1878–1890 (2014).
Farland, L. V. et al. Endometriosis and the risk of skin cancer: a prospective cohort study. Cancer Causes Control 28 , 1011–1019 (2017).
Sinaii, N. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum. Reprod. 17 , 2715–2724 (2002).
Nielsen, N. M., Jorgensen, K. T., Pedersen, B. V., Rostgaard, K. & Frisch, M. The co-occurrence of endometriosis with multiple sclerosis, systemic lupus erythematosus and Sjogren syndrome. Hum. Reprod. 26 , 1555–1559 (2011).
Harris, H. R. et al. Endometriosis and the risks of systemic lupus erythematosus and rheumatoid arthritis in the Nurses’ Health Study II. Ann. Rheum. Dis. 75 , 1279–1284 (2015).
Mu, F. et al. Association between endometriosis and hypercholesterolemia or hypertensionnovelty and significance. Hypertension 70 , 59–65 (2017).
Nyholt, D. R. et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat. Genet. 44 , 1355–1359 (2012).
Borghese, B., Zondervan, K. T., Abrao, M. S., Chapron, C. & Vaiman, D. Recent insights on the genetics and epigenetics of endometriosis. Clin. Genet. 91 , 254–264 (2017).
Treloar, S. A. et al. Genomewide linkage study in 1,176 affected sister pair families identifies a significant susceptibility locus for endometriosis on chromosome 10q26. Am. J. Hum. Genet. 77 , 365–376 (2005).
Zondervan, K. T. et al. Significant evidence of one or more susceptibility loci for endometriosis with near-Mendelian inheritance on chromosome 7p13–15. Hum. Reprod. 22 , 717–728 (2006).
Rahmioglu, N., Montgomery, G. W. & Zondervan, K. T. Genetics of endometriosis. Womens Health 11 , 577–586 (2015).
Sapkota, Y. et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat. Commun. 8 , 15539 (2017).
Lee, S. H. et al. Estimation and partitioning of polygenic variation captured by common SNPs for Alzheimer’s disease, multiple sclerosis and endometriosis. Hum. Mol. Genet. 22 , 832–841 (2012).
Uimari, O. et al. Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum. Reprod. 32 , 780–793 (2017).
Lu, Y. et al. Shared genetics underlying epidemiological association between endometriosis and ovarian cancer. Hum. Mol. Genet. 24 , 5955–5964 (2015).
Painter, J. N. et al. Genetic overlap between endometriosis and endometrial cancer: evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Med. 7 , 1978–1987 (2018).
Sapkota, Y. et al. Analysis of potential protein-modifying variants in 9000 endometriosis patients and 150000 controls of European ancestry. Sci. Rep. 7 , 11380 (2017).
Visscher, P. M., Brown, M. A., McCarthy, M. I. & Yang, J. Five years of GWAS discovery. Am. J. Hum. Genet. 90 , 7–24 (2012).
Fung, J. N. et al. The genetic regulation of transcription in human endometrial tissue. Hum. Reprod. 32 , 1–12 (2017).
Becker, C. M. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: I. Surgical phenotype data collection in endometriosis research. Fertil. Steril. 102 , 1213–1222 (2014).
Vitonis, A. F. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: II. Clinical and covariate phenotype data collection in endometriosis research. Fertil. Steril. 102 , 1223–1232 (2014).
Rahmioglu, N. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonization Project: III. Fluid biospecimen collection, processing, and storage in endometriosis research. Fertil. Steril. 102 , 1233–1243 (2014).
Fassbender, A. et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: IV. Tissue collection, processing, and storage in endometriosis research. Fertil. Steril. 102 , 1244–1253 (2014).
Wiegand, K. C. et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 363 , 1532–1543 (2010).
Yamamoto, S., Tsuda, H., Takano, M., Tamai, S. & Matsubara, O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod. Pathol. 25 , 615–624 (2011).
Anglesio, M. S. et al. Cancer-associated mutations in endometriosis without cancer. N. Engl. J. Med. 376 , 1835–1848 (2017).
Kato, S., Lippman, S. M., Flaherty, K. T. & Kurzrock, R. The conundrum of genetic ‘drivers’ in benign conditions. J. Natl Cancer Inst. 108 , djw036 (2016).
Guo, S.-W. Epigenetics of endometriosis. Mol. Hum. Reprod. 15 , 587–607 (2009).
Wu, Y., Starzinski-Powitz, A. & Guo, S.-W. Trichostatin A, a histone deacetylase inhibitor, attenuates invasiveness and reactivates E-cadherin expression in immortalized endometriotic cells. Reprod. Sci. 14 , 374–382 (2007).
Dyson, M. T. et al. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PLoS Genet. 10 , e1004158 (2014).
Burney, R. O. et al. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol. Hum. Reprod. 15 , 625–631 (2009).
Saare, M. et al. Challenges in endometriosis miRNA studies — from tissue heterogeneity to disease specific miRNAs. Biochim. Biophys. Acta 1863 , 2282–2292 (2017).
Sampson, J. A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 14 , 422–469 (1927).
Vercellini, P. et al. Asymmetry in distribution of diaphragmatic endometriotic lesions: evidence in favour of the menstrual reflux theory. Hum. Reprod. 22 , 2359–2367 (2007).
D’Hooghe, T. M., Bambra, C. S., Raeymaekers, B. M. & Koninckx, P. R. Increased prevalence and recurrence of retrograde menstruation in baboons with spontaneous endometriosis. Hum. Reprod. 11 , 2022–2025 (1996).
Witz, C. A., Cho, S., Centonze, V. E., Montoya-Rodriguez, I. A. & Schenken, R. S. Time series analysis of transmesothelial invasion by endometrial stromal and epithelial cells using three-dimensional confocal microscopy. Fertil. Steril. 79 (Suppl. 1), 770–778 (2003).
Reis, F. M., Petraglia, F. & Taylor, R. N. Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum. Reprod. Update 19 , 406–418 (2013).
Sanchez, A. M. et al. The endometriotic tissue lining the internal surface of endometrioma: hormonal, genetic, epigenetic status, and gene expression profile. Reprod. Sci. 22 , 391–401 (2015).
Borghese, B., Zondervan, K. T., Abrao, M. S., Chapron, C. & Vaiman, D. Recent insights on the genetics and epigenetics of endometriosis. Clin. Genet. 91 , 254–264 (2016).
Meyer, R. Zur Frage der heterotopen Epithelwucherung, insbesondere des Peritonealepithels und in die Ovarien [German]. Virch Arch. Path. Anat. Phys. 250 , 595–610 (1924).
Ferguson, B. R., Bennington, J. L. & Haber, S. L. Histochemistry of mucosubstances and histology of mixed müllerian pelvic lymph node glandular inclusions. Evidence for histogenesis by müllerian metaplasia of coelomic epithelium. Obstet. Gynecol. 33 , 617–625 (1969).
Figueira, P. G. M., Abrão, M. S., Krikun, G. & Taylor, H. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann. NY Acad. Sci. 1221 , 10–17 (2011).
Du, H. & Taylor, H. S. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 25 , 2082–2086 (2007).
Gargett, C. E. & Masuda, H. Adult stem cells in the endometrium. Mol. Hum. Reprod. 16 , 818–834 (2010).
Matsuzaki, S. & Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum. Reprod. 27 , 712–721 (2012).
Somigliana, E. et al. Association rate between deep peritoneal endometriosis and other forms of the disease: pathogenetic implications. Hum. Reprod. 19 , 168–171 (2004).
Zheng, W. et al. Initial endometriosis showing direct morphologic evidence of metaplasia in the pathogenesis of ovarian endometriosis. Int. J. Gynecol. Pathol. 24 , 164–172 (2005).
Troncon, J. K. et al. Endometriosis in a patient with mayer-rokitansky-küster-hauser syndrome. Case Rep. Obstet. Gynecol. 2014 , 376231 (2014).
Taguchi, S., Enomoto, Y. & Homma, Y. Bladder endometriosis developed after long-term estrogen therapy for prostate cancer. Int. J. Urol. 19 , 964–965 (2012).
Halban, J. Hysteroadenosis metastatica. Zentralbl. Gyndkoi 7 , 387–391 (1925).
Mechsner, S. et al. Estrogen and progestogen receptor positive endometriotic lesions and disseminated cells in pelvic sentinel lymph nodes of patients with deep infiltrating rectovaginal endometriosis: a pilot study. Hum. Reprod. 23 , 2202–2209 (2008).
Gargett, C. E. et al. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol. Hum. Reprod. 20 , 591–598 (2014).
Witz, C. A. et al. Short-term culture of peritoneum explants confirms attachment of endometrium to intact peritoneal mesothelium. Fertil. Steril. 75 , 385–390 (2001).
Zeitoun, K. M. & Bulun, S. E. Aromatase: a key molecule in the pathophysiology of endometriosis and a therapeutic target. Fertil. Steril. 72 , 961–969 (1999).
Pellegrini, C. et al. The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil. Steril. 98 , 1200–1208 (2012).
Plante, B. J. et al. G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod. Sci. 19 , 684–693 (2012).
Han, S. J. et al. Estrogen receptor β modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 163 , 960–974 (2015).
Vercellini, P., Viganò, P., Somigliana, E. & Fedele, L. Endometriosis: pathogenesis and treatment. Nat. Rev. Endocrinol. 10 , 261–275 (2013). For this review, the best-quality evidence was selected to describe the performance of diagnostic tools and the effectiveness of approaches to address endometriosis-associated symptoms and infertility .
Patel, B. et al. Role of nuclear progesterone receptor isoforms in uterine pathophysiology. Hum. Reprod. Update 21 , 155–173 (2014).
Al-Sabbagh, M., Lam, E. W.-F. & Brosens, J. J. Mechanisms of endometrial progesterone resistance. Mol. Cell. Endocrinol. 358 , 208–215 (2012).
La Marca, A., Carducci Artenisio, A., Stabile, G., Rivasi, F. & Volpe, A. Evidence for cycle-dependent expression of follicle-stimulating hormone receptor in human endometrium. Gynecol. Endocrinol. 21 , 303–306 (2005).
Zondervan, K. et al. Beyond endometriosis genome-wide association study: from genomics to phenomics to the patient. Semin. Reprod. Med. 34 , 242–254 (2016).
Jiang, Y., Chen, L., Taylor, R. N., Li, C. & Zhou, X. Physiological and pathological implications of retinoid action in the endometrium. J. Endocrinol. 236 , R169–R188 (2018).
Orvis, G. D. & Behringer, R. R. Cellular mechanisms of Müllerian duct formation in the mouse. Dev. Biol. 306 , 493–504 (2007).
Vigano, P. et al. Time to redefine endometriosis including its pro-fibrotic nature. Hum. Reprod. 33 , 347–352 (2018).
Yu, J. et al. Endometrial stromal decidualization responds reversibly to hormone stimulation and withdrawal. Endocrinology 157 , 2432–2446 (2016).
Yin, X., Pavone, M. E., Lu, Z., Wei, J. & Kim, J. J. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J. Clin. Endocrinol. Metab. 97 , E35–E43 (2012).
Du, Y., Liu, X. & Guo, S.-W. Platelets impair natural killer cell reactivity and function in endometriosis through multiple mechanisms. Hum. Reprod. 32 , 1–17 (2017).
Lessey, B., Lebovic, D. & Taylor, R. Eutopic endometrium in women with endometriosis: ground zero for the study of implantation defects. Semin. Reprod. Med. 31 , 109–124 (2013).
Cominelli, A. et al. Matrix metalloproteinase-27 is expressed in CD163+/CD206+ M2 macrophages in the cycling human endometrium and in superficial endometriotic lesions. Mol. Hum. Reprod. 20 , 767–775 (2014).
Takebayashi, A. et al. Subpopulations of macrophages within eutopic endometrium of endometriosis patients. Am. J. Reprod. Immunol. 73 , 221–231 (2015).
Nisenblat, V. et al. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 5 , CD012179 (2016).
Wang, X.-Q. et al. The high level of RANTES in the ectopic milieu recruits macrophages and induces their tolerance in progression of endometriosis. J. Mol. Endocrinol. 45 , 291–299 (2010).
McKinnon, B. D., Kocbek, V., Nirgianakis, K., Bersinger, N. A. & Mueller, M. D. Kinase signalling pathways in endometriosis: potential targets for non-hormonal therapeutics. Hum. Reprod. Update 22 , 382–403 (2016).
Morotti, M., Vincent, K. & Becker, C. M. Mechanisms of pain in endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 209 , 8–13 (2017).
Berkley, K. J. The pains of endometriosis. Science 308 , 1587–1589 (2005).
Taylor, R. N. & Lebovic, D. I. in Yen and Jaffe’s Reproductive Endocrinology (eds Strauss, J. F. & Barbieri, R. L.) 565–585 (Saunders Elsevier, 2014). This book chapter discusses the diagnosis and management of endometriosis, with a particularly well-referenced review of theories of aetiology and pathogenesis
Gnecco, J. S. et al. Compartmentalized culture of perivascular stroma and endothelial cells in a microfluidic model of the human endometrium. Ann. Biomed. Eng. 45 , 1758–1769 (2017).
Sanchez, A. M. et al. The endometriotic tissue lining the internal surface of endometrioma. Reprod. Sci. 22 , 391–401 (2014).
Ryan, I. P., Schriock, E. D. & Taylor, R. N. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J. Clin. Endocrinol. Metab. 78 , 642–649 (1994).
Greaves, E., Critchley, H. O. D., Horne, A. W. & Saunders, P. T. K. Relevant human tissue resources and laboratory models for use in endometriosis research. Acta Obstet. Gynecol. Scand. 96 , 644–658 (2017).
Korch, C. et al. DNA profiling analysis of endometrial and ovarian cell lines reveals misidentification, redundancy and contamination. Gynecol. Oncol. 127 , 241–248 (2012).
Grümmer, R. Translational animal models to study endometriosis-associated infertility. Semin. Reprod. Med. 31 , 125–132 (2013). Given the ethical limitations of clinical studies, this is a comprehensive review of the use of animal models to investigate factors contributing to the fertility-compromising effects of endometriosis .
Bruner-Tran, K. L., McConaha, M. E. & Osteen, K. G. in Endometriosis. Science and Practice (eds Giudice, L. C., Evers, J. L. H. & Healy, D. L.) 270–283 (Wiley-Blackwell, 2012).
Mariani, M. et al. The selective vitamin D receptor agonist, elocalcitol, reduces endometriosis development in a mouse model by inhibiting peritoneal inflammation. Hum. Reprod. 27 , 2010–2019 (2012).
Fazleabas, A. in Endometriosis: Science and Practice (eds Giudice, L. C., Evers, J. L. & Healy, D. L.) 285–291 (Wiley-Blackwell, 2012).
Ngô, C. et al. Antiproliferative effects of anastrozole, methotrexate, and 5-fluorouracil on endometriosis in vitro and in vivo. Fertil. Steril. 94 , 1632–1638.e1 (2010).
Bruner-Tran, K. L., Osteen, K. G. & Duleba, A. J. Simvastatin protects against the development of endometriosis in a nude mouse model. J. Clin. Endocrinol. Metab. 94 , 2489–2494 (2009).
Pullen, N. et al. The translational challenge in the development of new and effective therapies for endometriosis: a review of confidence from published preclinical efficacy studies. Hum. Reprod. Update 17 , 791–802 (2011).
Greaves, E. et al. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am. J. Pathol. 184 , 1930–1939 (2014).
Stilley, J. A. W., Woods-Marshall, R., Sutovsky, M., Sutovsky, P. & Sharpe-Timms, K. L. Reduced fecundity in female rats with surgically induced endometriosis and in their daughters: a potential role for tissue inhibitors of metalloproteinase 11. Biol. Reprod. 80 , 649–656 (2009).
Zondervan, K. T. Familial aggregation of endometriosis in a large pedigree of rhesus macaques. Hum. Reprod. 19 , 448–455 (2004).
Lebovic, D. I. et al. Peroxisome proliferator-activated receptor-γ receptor ligand partially prevents the development of endometrial explants in baboons: a prospective, randomized, placebo-controlled study. Endocrinology 151 , 1846–1852 (2010).
D’Hooghe, T. M. Clinical relevance of the baboon as a model for the study of endometriosis. Fertil. Steril. 68 , 613–625 (1997).
Fazleabas, A. Progesterone resistance in a baboon model of endometriosis. Semin. Reprod. Med. 28 , 75–80 (2010).
D’Hooghe, T. M. et al. Nonhuman primate models for translational research in endometriosis. Reprod. Sci. 16 , 152–161 (2009).
Chapron, C. et al. Surgical complications of diagnostic and operative gynaecological laparoscopy: a series of 29,966 cases. Hum. Reprod. 13 , 867–872 (1998).
Chung, M. K., Chung, R. R., Gordon, D. & Jennings, C. The evil twins of chronic pelvic pain syndrome: endometriosis and interstitial cystitis. JSLS 6 , 311–314 (2002).
Redwine, D. B. Diaphragmatic endometriosis: diagnosis, surgical management, and long-term results of treatment. Fertil. Steril. 77 , 288–296 (2002).
Fedele, L. et al. Ileocecal endometriosis: clinical and pathogenetic implications of an underdiagnosed condition. Fertil. Steril. 101 , 750–753 (2014).
DiVasta, A. D., Vitonis, A. F., Laufer, M. R. & Missmer, S. A. Spectrum of symptoms in women diagnosed with endometriosis during adolescence versus adulthood. Am. J. Obstet. Gynecol. 218 , 324.e1–324.e11 (2018).
Ballard, K., Lane, H., Hudelist, G., Banerjee, S. & Wright, J. Can specific pain symptoms help in the diagnosis of endometriosis? A cohort study of women with chronic pelvic pain. Fertil. Steril. 94 , 20–27 (2010).
Becker, C. M., Gattrell, W. T., Gude, K. & Singh, S. S. Reevaluating response and failure of medical treatment of endometriosis: a systematic review. Fertil. Steril. 108 , 125–136 (2017).
Brawn, J., Morotti, M., Zondervan, K. T., Becker, C. M. & Vincent, K. Central changes associated with chronic pelvic pain and endometriosis. Hum. Reprod. Update 20 , 737–747 (2014).
Morotti, M., Vincent, K., Brawn, J., Zondervan, K. T. & Becker, C. M. Peripheral changes in endometriosis-associated pain. Hum. Reprod. Update 20 , 717–736 (2014).
Brosens, I., Gordts, S. & Benagiano, G. Endometriosis in adolescents is a hidden, progressive and severe disease that deserves attention, not just compassion. Hum. Reprod. 28 , 2026–2031 (2013).
Laufer, M. R., Sanfilippo, J. & Rose, G. Adolescent endometriosis: diagnosis and treatment approaches. J. Pediatr. Adolesc. Gynecol. 16 , S3–S11 (2003).
Davis, G. D., Thillet, E. & Lindemann, J. Clinical characteristics of adolescent endometriosis. J. Adolesc. Health 14 , 362–368 (1993).
Saraswat, L. et al. Pregnancy outcomes in women with endometriosis: a national record linkage study. BJOG 124 , 444–452 (2017).
Sanchez, A. M. et al. Is the oocyte quality affected by endometriosis? A review of the literature. J. Ovarian Res. 10 , 43 (2017).
Simón, C. et al. Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum. Reprod. 9 , 725–729 (1994).
Senapati, S., Sammel, M. D., Morse, C. & Barnhart, K. T. Impact of endometriosis on in vitro fertilization outcomes: an evaluation of the Society for Assisted Reproductive Technologies Database. Fertil. Steril. 106 , 164–171.e1 (2016).
Taniguchi, F. et al. Analysis of pregnancy outcome and decline of anti-Müllerian hormone after laparoscopic cystectomy for ovarian endometriomas. J. Obstet. Gynaecol. Res. 42 , 1534–1540 (2016).
Lessey, B. A. & Kim, J. J. Endometrial receptivity in the eutopic endometrium of women with endometriosis: it is affected, and let me show you why. Fertil. Steril. 108 , 19–27 (2017).
Miravet-Valenciano, J., Ruiz-Alonso, M., Gómez, E. & Garcia-Velasco, J. A. Endometrial receptivity in eutopic endometrium in patients with endometriosis: it is not affected, and let me show you why. Fertil. Steril. 108 , 28–31 (2017).
Díaz, I. et al. Impact of stage iii–iv endometriosis on recipients of sibling oocytes: matched case-control study. Fertil. Steril. 74 , 31–34 (2000).
Prapas, Y. et al. History of endometriosis may adversely affect the outcome in menopausal recipients of sibling oocytes. Reprod. Biomed. Online 25 , 543–548 (2012).
Burney, R. O. et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148 , 3814–3826 (2007).
Dunselman, G. A. J. et al. ESHRE guideline: management of women with endometriosis. Hum. Reprod. 29 , 400–412 (2014). This guideline discusses the management of general endometriosis .
Weijenborg, P. T. M., ter Kuile, M. M. & Jansen, F. W. Intraobserver and interobserver reliability of videotaped laparoscopy evaluations for endometriosis and adhesions. Fertil. Steril. 87 , 373–380 (2007).
Tuttlies, F. et al. ENZIAN-score, a classification of deep infiltrating endometriosis [German]. Zentralbl. Gynakol. 127 , 275–281 (2005).
Johnson, N. P. et al. World Endometriosis Society consensus on the classification of endometriosis. Hum. Reprod. 32 , 315–324 (2017).
Nisolle, M. & Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 68 , 585–596 (1997).
Fukuda, S. et al. Thoracic endometriosis syndrome: comparison between catamenial pneumothorax or endometriosis-related pneumothorax and catamenial hemoptysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 225 , 118–123 (2018).
Rousset-Jablonski, C. et al. Catamenial pneumothorax and endometriosis-related pneumothorax: clinical features and risk factors. Hum. Reprod. 26 , 2322–2329 (2011).
Redwine, D. B. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil. Steril. 72 , 310–315 (1999).
Hudelist, G. et al. Diagnostic accuracy of transvaginal ultrasound for non-invasive diagnosis of bowel endometriosis: systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 37 , 257–263 (2011).
Holland, T. K. et al. Ultrasound mapping of pelvic endometriosis: does the location and number of lesions affect the diagnostic accuracy? A multicentre diagnostic accuracy study. BMC Womens Health 13 , 43 (2013).
Noventa, M. et al. Ultrasound techniques in the diagnosis of deep pelvic endometriosis: algorithm based on a systematic review and meta-analysis. Fertil. Steril. 104 , 366–383.e2 (2015).
Bazot, M. et al. European Society of Urogenital Radiology (ESUR) guidelines: MR imaging of pelvic endometriosis. Eur. Radiol. 27 , 2765–2775 (2016).
Exacoustos, C., Manganaro, L. & Zupi, E. Imaging for the evaluation of endometriosis and adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 28 , 655–681 (2014).
Stratton, P. Diagnostic accuracy of laparoscopy, magnetic resonance imaging, and histopathologic examination for the detection of endometriosis. Fertil. Steril. 79 , 1078–1085 (2003).
Kennedy, S. et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 20 , 2698–2704 (2005).
Wykes, C. B., Clark, T. J. & Khan, K. S. REVIEW: Accuracy of laparoscopy in the diagnosis of endometriosis: a systematic quantitative review. BJOG 111 , 1204–1212 (2004).
Balasch, J. et al. Visible and non-visible endometriosis at laparoscopy in fertile and infertile women and in patients with chronic pelvic pain: a prospective study. Hum. Reprod. 11 , 387–391 (1996).
Lessey, B. A., Higdon, H. L., Miller, S. E. & Price, T. A. Intraoperative detection of subtle endometriosis: a novel paradigm for detection and treatment of pelvic pain associated with the loss of peritoneal integrity. J. Vis. Exp. https://doi.org/10.3791/4313 (2012).
Lue, J. R., Pyrzak, A. & Allen, J. Improving accuracy of intraoperative diagnosis of endometriosis: role of firefly in minimal access robotic surgery. J. Minim. Access Surg. 12 , 186–189 (2016).
Practice Committee of American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis. Fertil. Steril. 90 , S260–S269 (2008). This article is a guideline for the management of pelvic pain associated with endometriosis .
Duffy, J. M. N. et al. Laparoscopic surgery for endometriosis. Cochrane Database Syst. Rev. 4 , CD011031 (2014).
Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: a committee opinion. Fertil. Steril. 98 , 591–598 (2012). This paper is a guideline for the management of infertility associated with endometriosis .
Tanbo, T. & Fedorcsak, P. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 96 , 659–667 (2017).
Osuga, Y. et al. Role of laparoscopy in the treatment of endometriosis-associated infertility. Gynecol. Obstet. Invest. 53 , 33–39 (2002).
Hart, R. J., Hickey, M., Maouris, P., Buckett, W. & Garry, R. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.CD004992.pub2 (2005).
Benaglia, L. et al. Rate of severe ovarian damage following surgery for endometriomas. Hum. Reprod. 25 , 678–682 (2010).
Vercellini, P. et al. Effect of patient selection on estimate of reproductive success after surgery for rectovaginal endometriosis: literature review. Reprod. Biomed. Online 24 , 389–395 (2012).
Nyangoh Timoh, K., Ballester, M., Bendifallah, S., Fauconnier, A. & Darai, E. Fertility outcomes after laparoscopic partial bladder resection for deep endometriosis: retrospective analysis from two expert centres and review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 220 , 12–17 (2018).
Adamson, G. D. & Pasta, D. J. Endometriosis fertility index: the new, validated endometriosis staging system. Fertil. Steril. 94 , 1609–1615 (2010).
Werbrouck, E., Spiessens, C., Meuleman, C. & D’Hooghe, T. No difference in cycle pregnancy rate and in cumulative live-birth rate between women with surgically treated minimal to mild endometriosis and women with unexplained infertility after controlled ovarian hyperstimulation and intrauterine insemination. Fertil. Steril. 86 , 566–571 (2006).
National Institute for Health and Care Excellence. Fertility problems: assessment and treatment. NICE https://www.nice.org.uk/guidance/cg156/chapter/Recommendations#intrauterine-insemination (2013).
de Ziegler, D., Borghese, B. & Chapron, C. Endometriosis and infertility: pathophysiology and management. Lancet 376 , 730–738 (2010).
Steures, P. et al. Prediction of an ongoing pregnancy after intrauterine insemination. Fertil. Steril. 82 , 45–51 (2004).
Reindollar, R. H. et al. A randomized clinical trial to evaluate optimal treatment for unexplained infertility: the fast track and standard treatment (FASTT) trial. Fertil. Steril. 94 , 888–899 (2010).
Eijkemans, M. J. C. et al. Cost-effectiveness of ‘immediate IVF’ versus ‘delayed IVF’: a prospective study. Hum. Reprod. 32 , 999–1008 (2017).
Hamdan, M., Omar, S. Z., Dunselman, G. & Cheong, Y. Influence of endometriosis on assisted reproductive technology outcomes. Obstet. Gynecol. 125 , 79–88 (2015).
Benschop, L., Farquhar, C., van der Poel, N. & Heineman, M. J. Interventions for women with endometrioma prior to assisted reproductive technology. Cochrane Database Syst. Rev. 11 , CD008571 (2010).
de Ziegler, D. et al. Use of oral contraceptives in women with endometriosis before assisted reproduction treatment improves outcomes. Fertil. Steril. 94 , 2796–2799 (2010).
Donnez, J., García-Solares, J. & Dolmans, M.-M. Fertility preservation in women with ovarian endometriosis. Minerva Ginecol. https://doi.org/10.23736/S0026-4784.18.04229-6 (2018).
Somigliana, E. et al. Surgical excision of endometriomas and ovarian reserve: a systematic review on serum antimüllerian hormone level modifications. Fertil. Steril. 98 , 1531–1538 (2012).
Bianchi, P. H. M. et al. Extensive excision of deep infiltrative endometriosis before in vitro fertilization significantly improves pregnancy rates. J. Minim. Invasive Gynecol. 16 , 174–180 (2009).
Zullo, F. et al. Endometriosis and obstetrics complications: a systematic review and meta-analysis. Fertil. Steril. 108 , 667–672.e5 (2017).
Vigano, P., Corti, L. & Berlanda, N. Beyond infertility: obstetrical and postpartum complications associated with endometriosis and adenomyosis. Fertil. Steril. 104 , 802–812 (2015).
Donnez, J. & Squifflet, J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum. Reprod. 25 , 1949–1958 (2010).
Zanelotti, A. & Decherney, A. H. Surgery and endometriosis. Clin. Obstet. Gynecol. 60 , 477–484 (2017).
Olive, D. L. Medical therapy of endometriosis. Semin. Reprod. Med. 21 , 209–222 (2003).
Harada, T., Momoeda, M., Taketani, Y., Hoshiai, H. & Terakawa, N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil. Steril. 90 , 1583–1588 (2008).
Vercellini, P. et al. Treatment of symptomatic rectovaginal endometriosis with an estrogen–progestogen combination versus low-dose norethindrone acetate. Fertil. Steril. 84 , 1375–1387 (2005).
Harada, T. et al. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis — a randomized, double-blind, multicenter, controlled trial. Fertil. Steril. 91 , 675–681 (2009).
Casper, R. F. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil. Steril. 107 , 533–536 (2017).
Abou-Setta, A. M., Houston, B., Al-Inany, H. G. & Farquhar, C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst. Rev. 1 , CD005072 (2013).
Brown, J., Pan, A. & Hart, R. J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 12 , CD008475 (2010).
Sagsveen, M. et al. Gonadotrophin-releasing hormone analogues for endometriosis: bone mineral density. Cochrane Database Syst. Rev. 4 , CD001297 (2003).
Bedaiwy, M. A., Allaire, C. & Alfaraj, S. Long-term medical management of endometriosis with dienogest and with a gonadotropin-releasing hormone agonist and add-back hormone therapy. Fertil. Steril. 107 , 537–548 (2017).
Taylor, H. S. et al. Treatment of endometriosis-associated pain with elagolix, an oral GnRH antagonist. N. Engl. J. Med. 377 , 28–40 (2017).
Hornstein, M. D. An oral GnRH antagonist for endometriosis — a new drug for an old disease. N. Engl. J. Med. 377 , 81–83 (2017).
Bedaiwy, M. A., Alfaraj, S., Yong, P. & Casper, R. New developments in the medical treatment of endometriosis. Fertil. Steril. 107 , 555–565 (2017).
Shakiba, K., Bena, J. F., McGill, K. M., Minger, J. & Falcone, T. Surgical treatment of endometriosis. Obstet. Gynecol. 111 , 1285–1292 (2008).
Vercellini, P. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis: results of a randomized, controlled trial. Fertil. Steril. 68 , S3 (1997).
Meuleman, C. et al. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum. Reprod. Update 17 , 311–326 (2011).
Koga, K., Takamura, M., Fujii, T. & Osuga, Y. Prevention of the recurrence of symptom and lesions after conservative surgery for endometriosis. Fertil. Steril. 104 , 793–801 (2015).
Gao, X. et al. Health-related quality of life burden of women with endometriosis: a literature review. Curr. Med. Res. Opin. 22 , 1787–1797 (2006).
Simoens, S. et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 27 , 1292–1299 (2012). This prospective study calculates the average annual costs and HRQOL per woman with endometriosis-associated symptoms, showing it to be similar to other chronic conditions .
Jones, G., Kennedy, S., Barnard, A., Wong, J. & Jenkinson, C. Development of an endometriosis quality-of-life instrument. Obstet. Gynecol. 98 , 258–264 (2001). This study describes the only validated endometriosis-specific quality-of-life outcome tool developed, measuring endometriosis-related health status on five scales. The tool has since been shown to be sensitive to changes in symptoms, making it a useful tool in endometriosis-specific clinical trials .
Jones, G., Jenkinson, C. & Kennedy, S. Development of the short form endometriosis health profile questionnaire: the EHP-5. Qual. Life Res. 13 , 695–704 (2004).
Jones, G., Jenkinson, C. & Kennedy, S. Evaluating the responsiveness of the endometriosis health profile questionnaire: the EHP-30. Qual. Life Res. 13 , 705–713 (2004).
Hirsch, M. et al. Variation in outcome reporting in endometriosis trials: a systematic review. Am. J. Obstet. Gynecol. 214 , 452–464 (2016).
Khan, K. The CROWN Initiative: journal editors invite researchers to develop core outcomes in women’s health. BJOG 123 , 103–104 (2016).
van Nooten, F. E., Cline, J., Elash, C. A., Paty, J. & Reaney, M. Development and content validation of a patient-reported endometriosis pain daily diary. Health Qual. Life Outcomes 16 , 3 (2018).
Rogers, P. A. W. et al. Research priorities for endometriosis. Reprod. Sci. 24 , 202–226 (2017).
Butrick, C. W. Patients with chronic pelvic pain: endometriosis or interstitial cystitis/painful bladder syndrome? JSLS 11 , 182–189 (2007).
Sørlie, T. et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl Acad. Sci. USA 100 , 8418–8423 (2003).
Cancer Genome Atlas Network. et al. Comprehensive molecular portraits of human breast tumours. Nature 490 , 61–70 (2012).
Gupta, D. et al. Endometrial biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 4 , CD012165 (2016).
Nisenblat, V. et al. Combination of the non-invasive tests for the diagnosis of endometriosis. Cochrane Database Syst. Rev. 7 , CD012281 (2016).
Nisenblat, V., Bossuyt, P. M. M., Farquhar, C., Johnson, N. & Hull, M. L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2 , CD009591 (2016).
May, K. E. et al. Peripheral biomarkers of endometriosis: a systematic review. Hum. Reprod. Update 16 , 651–674 (2010).
May, K. E., Villar, J., Kirtley, S., Kennedy, S. H. & Becker, C. M. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum. Reprod. Update 17 , 637–653 (2011).
van der Zanden, M. & Nap, A. W. Knowledge of, and treatment strategies for, endometriosis among general practitioners. Reprod. Biomed. Online 32 , 527–531 (2016).
Seear, K. The etiquette of endometriosis: stigmatisation, menstrual concealment and the diagnostic delay. Soc. Sci. Med. 69 , 1220–1227 (2009).
Greene, R., Stratton, P., Cleary, S. D., Ballweg, M. L. & Sinaii, N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil. Steril. 91 , 32–39 (2009).
Horne, A. W., Saunders, P. T. K., Abokhrais, I. M. & Hogg, L. Top ten endometriosis research priorities in the UK and Ireland. Lancet 389 , 2191–2192 (2017).
Hans Evers, J. L. H. Is adolescent endometriosis a progressive disease that needs to be diagnosed and treated? Hum. Reprod. 28 , 2023 (2013).
Neal, D. M. & McKenzie, P. J. Putting the pieces together: endometriosis blogs, cognitive authority, and collaborative information behavior. J. Med. Libr. Assoc. 99 , 127–134 (2011).
Uno, S. et al. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat. Genet. 42 , 707–710 (2010).
Painter, J. N. et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat. Genet. 43 , 51–54 (2011).
Albertsen, H. M., Chettier, R., Farrington, P. & Ward, K. Genome-wide association study link novel loci to endometriosis. PLoS ONE 8 , e58257 (2013).
Steinthorsdottir, V. et al. Common variants upstream of KDR encoding VEGFR2 and in TTC39B associate with endometriosis. Nat. Commun. 7 , 12350 (2016).
Sobalska-Kwapis, M. et al. New variants near RHOJ and C2, HLA-DRA region and susceptibility to endometriosis in the Polish population — the genome-wide association study. Eur. J. Obstet. Gynecol. Reprod. Biol. 217 , 106–112 (2017).
Download references
Acknowledgements
R.N.T. acknowledges funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development through grants R01-HD33238, U54-HD37321, U54-HD55787, R01-HD55379, U01-HD66439 and R21-HD78818. S.A.M. is also affiliated with the Boston Center for Endometriosis, Boston Children’s Hospital and Brigham and Women’s Hospital and the Division of Adolescent and Young Adult Medicine, Department of Medicine, Boston Children’s Hospital and Harvard Medical School. The authors thank N. Moore (Oxford University Hospitals Foundation Trust, UK) for providing MRI images, D. Barber (Oxford Endometriosis CaRe Centre, UK) for providing ultrasonography pictures and J. Malzahn (Nuffield Department of Orthopaedics, Rheumatology and Musculoskeletal Sciences, University of Oxford, UK) for providing the picture of the histology slide in Fig. 5.
Reviewer information
Nature Reviews Disease Primers thanks I. Brosens, M. J. Canis, S. Ferrero, C. Nezhat, V. Remorgida, M. Simões Abrão and other anonymous referee(s) for the peer review of this work.
Author information
Authors and affiliations.
Oxford Endometriosis CaRe Centre, Nuffield Department of Women’s & Reproductive Health, University of Oxford, Oxford, UK
Krina T. Zondervan & Christian M. Becker
Wellcome Centre for Human Genetics, University of Oxford, Oxford, UK
Krina T. Zondervan
Department of Obstetrics and Gynecology, The University of Tokyo, Tokyo, Japan
Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA
Stacey A. Missmer
Department of Obstetrics, Gynecology and Reproductive Biology, College of Human Medicine, Michigan State University, Grand Rapids, MI, USA
Department of Obstetrics and Gynecology, University of Utah School of Medicine, Salt Lake City, UT, USA
Robert N. Taylor
Division of Genetics and Cell Biology, San Raffaele Scientific Institute, Milano, Italy
Paola Viganò
You can also search for this author in PubMed Google Scholar
Contributions
Introduction (K.T.Z.); Epidemiology (S.A.M.); Mechanisms/pathophysiology (R.N.T. and P.V.); Diagnosis, screening and prevention (C.M.B.); Management (K.K.); Quality of life (K.T.Z.); Outlook (K.T.Z.); Overview of the Primer (K.T.Z.).
Corresponding author
Correspondence to Krina T. Zondervan .
Ethics declarations
Competing interests.
K.T.Z. has received grant funding from the Wellcome Trust, Medical Research Council UK, the US NIH, the European Union and the World Endometriosis Research Foundation (WERF). She also has scientific collaborations with, and has received grant funding from, Bayer AG, MDNA Life Sciences, Roche Diagnostics and Volition Rx and has served as a scientific consultant to AbbVie and Roche Diagnostics. She is Secretary of the World Endometriosis Society (WES), the European Society of Human Reproduction and Embryology (ESHRE) Special Interest Group in Endometriosis and Endometrial Disorders and Wellbeing of Women, and she is Chair of the WES Research Directions Working Group. C.M.B. is a member of the independent data monitoring group for a clinical endometriosis trial by ObsEva. He has received research grants from Bayer AG, MDNA Life Sciences, Volition Rx and Roche Diagnostics as well as from Wellbeing of Women, Medical Research Council UK, the NIH, the UK National Institute for Health Research and the European Union. He is the current Chair of the Endometriosis Guideline Development Group of the ESHRE and was a co-opted member of the Endometriosis Guideline Group by the UK National Institute for Health and Care Excellence (NICE). K.K. has received grant funding from the Ministry of Education, Culture, Sports Science and Technology Japan, the Ministry of Health, Labour and Welfare Japan, Takeda Research Support and MSD. She has also served as a scientific consultant to Bayer AG. She is an ambassador of the WES and a member of the Guideline Development Group of the Japan Society of Obstetrics and Gynecology. S.A.M. has received grant funding from the NIH and the Marriott family foundations and has served as an adviser to and has scientific collaborations with AbbVie, Celmatix and Oratel Diagnostics. She is a treasurer of the WES, Secretary of the WERF, Chair of the American Society of Reproductive Medicine Endometriosis Special Interest Group and a member of the NIH Reproductive Medicine Network Data Safety and Monitoring Board. R.N.T. has received grant funding from Bayer AG, Ferring Research Institute, the NIH and Pfizer and has served as a scientific consultant or adviser to AbbVie, Allergan, the NIH, ObsEva SA and the Population Council. He is the immediate past honorary secretary of the WES. P.V. has received grant funding from Bayer AG and Merck Serono and has served as a scientific consultant to Ferring Pharmaceuticals and Roche Diagnostics. She is a board member of the WES.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
WERF Endometriosis Phenome and Biobanking Harmonisation Project: https://endometriosisfoundation.org/ephect/
Rights and permissions
Reprints and permissions
About this article
Cite this article.
Zondervan, K.T., Becker, C.M., Koga, K. et al. Endometriosis. Nat Rev Dis Primers 4 , 9 (2018). https://doi.org/10.1038/s41572-018-0008-5
Download citation
Published : 19 July 2018
DOI : https://doi.org/10.1038/s41572-018-0008-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Vitamin d and reproductive disorders: a comprehensive review with a focus on endometriosis.
- Pooya Farhangnia
- Morvarid Noormohammadi
- Ali-Akbar Delbandi
Reproductive Health (2024)
Impact of oil-based contrast agents in hysterosalpingography on fertility outcomes in endometriosis: a retrospective cohort study
- Yingqin Huang
Reproductive Biology and Endocrinology (2024)
Efficacy and safety of a novel pain management device, AT-04, for endometriosis-related pain: study protocol for a phase III randomized controlled trial
- Hiroshi Ishikawa
- Osamu Yoshino
Surge in endometriosis research after decades of underfunding could herald new era for women’s health
- Clare Watson
Nature Medicine (2024)
Predictors of partnership and sexual satisfaction and dyadic effects in couples affected by endometriosis and infertility
- Deborah van Eickels
- Maren Schick
- Beate Ditzen
Archives of Gynecology and Obstetrics (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 26 January 2022
Challenges of and possible solutions for living with endometriosis: a qualitative study
- Gabriella Márki 1 , 2 ,
- Dorottya Vásárhelyi 2 ,
- Adrien Rigó 2 ,
- Zsuzsa Kaló 2 ,
- Nándor Ács 3 &
- Attila Bokor 3
BMC Women's Health volume 22 , Article number: 20 ( 2022 ) Cite this article
11k Accesses
20 Citations
28 Altmetric
Metrics details
Endometriosis as a chronic gynecological disease has several negative effects on women’s life, thereby placing a huge burden on the patients and the health system. The negative impact of living with endometriosis (impaired quality of life, diverse medical experiences) is detailed in the literature, however, we know less about patients’ self-management, social support, the meaning of life with a chronic disease, and the needs of patients. To implement a proper multidisciplinary approach in practice, we need to have a comprehensive view of the complexity of endometriosis patients’ life and disease history.
Four focus group discussions were conducted between October 2014 and November 2015 by a team consisting of medical and psychological specialists. 21 women (age: 31.57; SD = 4.45) with surgical and histological confirmation of endometriosis were included in the study. Discussions were audiotaped and transcribed verbatim, and a 62,051-word corpus was analyzed using content analysis.
Four main themes emerged from the analysis: (1) the impact of endometriosis on quality of life, (2) medical experiences, (3) complementary and alternative treatments, and (4) different coping strategies in disease management. All themes were interrelated and highly affected by a lack of information and uncertainty caused by endometriosis. A supporting doctor-patient relationship, active coping, and social support were identified as advantages over difficulties. Finding the positive meaning of life after accepting endometriosis increased the possibility of posttraumatic growth. Furthermore, women’s needs were identified at all levels of the ecological approach to health promotion.
Conclusions
Our results highlight the need for multidisciplinary healthcare programs and interventions to find solutions to the difficulties of women with endometriosis. To achieve this goal, a collaboration of professionals, psychologists, and support organizations is needed in the near future.
Peer Review reports
Introduction
Endometriosis is a chronic inflammatory disease that is defined as the presence of endometrium-like tissues outside the uterus causing pain symptoms (dysmenorrhea, dyspareunia, chronic pelvic pain) and infertility [ 1 ]. This gynecological disease affects approximately 2–10% of the reproductive-aged and 50% of infertile women, and women with endometriosis have increased risk of obstetric outcome [ 2 ]. Because symptoms are not specific, the diagnostic delay is almost 8–10 years [ 3 ].
Endometriosis has a negative impact on health-related quality of life (HRQoL) [ 4 ]. Quantitative studies identified deterioration in physical wellbeing [ 5 ], psychological functioning [ 6 , 7 ], daily life activities and work productivity [ 8 , 9 ], social participation [ 10 ], quality of sexual life [ 11 ], and an increase in financial burden [ 12 ]. Decreased HRQoL has a negative feedback effect on endometriosis progression [ 13 , 14 ]. Furthermore, it is already known that pain is a major cause of these physical, psychosocial, emotional, and work-related difficulties among patients [ 15 , 16 , 17 ].
Previously published qualitative data demonstrated the negative impact of endometriosis on HRQoL and medical experiences but offered fewer findings of self-management, social support, femininity, the meaning of life with a chronic disease, and future directions and needs of patients. Therefore, this study aims to expand knowledge of (i) the difficulties women have when living with endometriosis and (ii) their opportunities and mechanisms for coping with the negative impact of the disease. We assume that by exploring these main areas we can help to develop health promotion strategies to reduce further negative effects on women's lives.
Study design, procedure, and data collection
This qualitative study is part of a comprehensive study on the psychosocial aspects of endometriosis conducted by Eötvös Loránd University in conjunction with Semmelweis University, Budapest, Hungary. Participants with surgical and histological confirmation of endometriosis from our previous study [ 18 ] were invited via e-mail to participate in exploratory focus group discussions, where participation was voluntary. The invitation explained the nature and details of the study. Four focus group discussions were conducted in the department room of the Institute of Psychology, Eötvös Loránd University between October 2014 and November 2015. Within the postpositivist qualitative paradigm [ 19 ] we followed the phenomenological approach to inquiry. Focus groups allow data to be collected through a group, where participants express their opinion more naturally and influence one another, thus it is more likely that new issues will be raised than in a one-to-one interview. Furthermore, focus groups allow the perceptions, emotions, and concerns of participants to be explored [ 20 , 21 ].
Focus group participants were asked to articulate their concerns and experiences on two topics: (i) living with endometriosis, (ii) disease-management, and experiences of medical or other treatments. All conversations were guided by the first author and trained assistant, who took field notes. Focus groups were audiotaped with their permission and transcribed verbatim by the assistant. Overall, there were 462 min of recording which were then transcribed into a 62,051-word corpus for the analysis.
The verbatim transcripts included typical or relevant non-verbal expressions (laughing, long pauses) that were confirmed by the assistant who made observations during focus group sessions. The basic element of analysis was the word. After checking the transcript (rereading the text while listening to the voice recording), the text was analyzed line by line using content analysis [ 22 , 23 ] in ATLAS.ti by two independent coders [ 24 ]. They discussed and compared collected codes from the data and after reaching consensus code groups, defined categories and created themes. Final themes and categories were checked against codes [ 22 ].
Sample characteristics
The study involved 21 patients diagnosed with endometriosis with a mean age of 31.57 (SD = 4.45). Participants (> 18 years) represented a homogenous group in terms of socio-economic background and ethnicity. On average, participants saw more than three gynecologists (range 1–15) and one alternative healer (range 0–4) before being diagnosed with endometriosis. The diagnostic delay was 2.05 years (SD = 3.32; range 0–12). Most participants (66.7%) had endometriosis-related symptoms by the time of the study. In all our participants, peritoneal endometriosis was observed (21/21, 100%), ovarian endometriosis was found in 11 patients (11/21, 52.3%) while deep infiltrating endometriosis affecting the rectum and/or the rectovaginal space was present in 6 cases (6/21, 28.6%). None of our patients had extrapelvic or abdominal wall endometriosis. Most patients of the whole sample used medical hormonal treatment at the time of the conversation. From the whole cohort, 18 (18/21, 85.7%) women received combined oral contraceptive therapy on a continuous regimen. We have observed no difference between the types of oral contraceptives since they were all combined pills containing dienogest and ethinylestradiol. Most of our participants (16/21) struggled to get pregnant, only one of them had a successful clinical pregnancy and delivery at the time of the conversation, further 23.8% of participants were undergoing in vitro fertilization (IVF). None of study participants had a comorbid psychiatric disorder.
Thematic analysis findings
Four main themes emerged from the analysis: (a) impact of endometriosis on quality of life, (b) medical experiences, (c) complementary and alternative treatments, (d) different coping strategies in disease-management (Table 1 ). Notably, that all themes were highly affected by a lack of information and uncertainty related to endometriosis, while all the emerged themes showed a dynamic connection between them and present the patients’ circular pathways (Fig. 1 ).
A model of the dynamic relationship of endometriosis-related themes and negative impacts
Theme 1: Impact of endometriosis on quality of life
Physical impacts.
Most women mentioned chronic pelvic pain, dysmenorrhea, and dyspareunia as leading symptoms of their endometriosis. Most of them reported that pain killers or special body positions did not significantly relieve pain.
It was never-ending, so I lived like this every day. I kneeled on the ground. I moved back and forth because it was not good any other way and yet I still held tightly onto my hair because I was in so much pain.
Psychological impacts
Besides the physical burden, participants also reported psychological consequences of endometriosis, namely anxiety, stress, and helplessness , and sometimes these were more confusing and annoying than the physical symptoms. One participant described how depressing it was to realize that she had lost 10 years of her life living in permanent pain without receiving the correct diagnosis and treatment. Feelings of loss and shame were also highlighted by participants. Uncertainty about the possible recurrence of the disease has been identified as a further stress factor in women who wanted to take an active role in their disease management. The negative emotional state negatively shaped their way of thinking, and subsumed their everyday lives.
You can’t do anything about the physical side anymore, but the psychological aspects, they leave their mark on you. I gave in to endometriosis, in fact, my whole life revolved around it. It made me bitter, and I realized after a while that I couldn’t think about anything else.
The psychosocial effects of endometriosis
This category includes common and cumulative effects of physical and psychological impacts. Endometriosis-related uncertainty had several negative impacts on women’s life. Families and friendships were affected by a lack of adequate information and a feeling of helplessness.
Friendships were ruined at that time. There was not one aspect of my life that was not affected by endometriosis. Within the family, you can release the stress that you cannot release anywhere else. It was common for me to cry during family dinners. Even friends who were supportive did not always understand what I was going through.
Intimate relationships were negatively affected by uncertainties. Participants mentioned that explaining the disease and giving reassurance to their husbands was difficult. Women agreed that a supportive partner can be the biggest source of help and support, but not every relationship was able to handle the burden of endometriosis.
It [endometriosis] cost me my marriage… At that time, we had already started in vitro fertilization. The first one ended up in colonic obstruction and I got a stoma for three months. Before the next round of IVF started my ex-husband said it was over for him.
Some women experienced sexual problems and the inconvenience of sharing their experiences of dyspareunia due to the normalizing reaction of society and health care providers. The non-sharable experiences led in two cases to sexual aversion, when “ sex was equal to pain”.
Besides dyspareunia and sexual dysfunctions, the most burden for most women were fertility problems. Participants stated that they pursued one of two options: some women insisted on childbearing and did not give up even after the defeats and inconveniences of IVF, because they thought it was worth the sacrifice; others re-evaluated pregnancy and went on to consider other options for motherhood.
We try and we hope, and I don’t know. I have learned a big lesson from this—that it would be nice to have a baby, but what if I can never have my own baby—because it could happen. Now I can say it out loud: it is okay, I can adopt a child or choose other options.
Female identity was negatively affected by infertility, sexual problems, and impersonal medical examinations. Repression and negative attitudes towards femininity have been mentioned as possible causes of their disease.
If you do not experience your femininity, it will come back to haunt you at some point.
Endometriosis and its treatment had a significant impact on participation in education and employment . Women mentioned sick-leave and semester deferral due to dysmenorrhea, as well as sleeping problems and surgery. The impact on employment usually depended on the boss and the flexibility of the workplace.
It can cause a lot of tension, finding where the line is between asking your boss to let you leave and be patient, or feeling that you are risking your job and tomorrow maybe you don’t have to go to work anymore.
The cost of gynecological consultations, medications, surgery, healthy nutrition, and further treatments caused a financial burden and required a considerable amount of time and energy .
The costs associated with endometriosis are so high; my family has an emergency budget just for this.
Theme 2: Medical experiences
Diagnostic delay.
Participants usually experienced that health professionals normalized symptoms of dysmenorrhea. It was not only normalization but physicians’ lack of adequate knowledge relating to endometriosis that caused misdiagnosis and diagnostic delay.
I went from doctor to doctor for seven years and I knew something was wrong because I could not conceive, so we were looking for the reason behind it. But a lot of doctors did not recognize the disease and that was the biggest problem.
Treatment of endometriosis
The option of pharmacological treatments was divisive among participants; most of them were concerned about side effects. Participants reported being fearful before surgery and stated that they were concerned about reproductive organs and intestinal involvement or getting stoma. Fear and uncertainty were pronounced concerning recovery and lack of information right after surgery. Participants reported that having a child was usually expressly recommended by gynecologists as a potential treatment option . These women often experienced medical and social pressure to have a baby, even if they did not feel ready to become mothers.
A woman can find herself in this trap. Although the gynecologist means well, saying you must have a child as soon as possible is such a burden on the woman. It is unbearable and impossible to process.
Infertility was a sensitive topic in every discussion. Participants who underwent assisted reproductive technology treatment described it as impersonal, physically, and mentally stressful, for men as well. Furthermore, the possible recurrence of endometriosis proved to be one of the biggest uncertainty factors, and it placed a huge burden on women.
Doctor-patient relationship
Most women agreed that having a good, reliable gynecologist specialized in endometriosis is one of the most essential factors in managing endometriosis. Many participants had negative experiences with doctors who were negligent or had insufficient professional knowledge of endometriosis, which increased diagnostic delay by several years. Women highlighted that healthcare professionals’ uncertainty led to mistrust, increased fear, and despondency, and caused them to go ‘doctor-shopping’ because they could not accept their doctor’s negligent attitude towards their symptoms or recommended treatment options. All the women agreed that physicians who reassured and informed them properly as a specialized professional in endometriosis engendered the most trust.
It was an odd experience, that even doctors can’t tell me what is wrong with me and what will make me feel better. So, you have to go until you find someone you can at least trust.
Theme 3: Complementary and alternative treatments
This theme includes women’s motivation towards all kinds of complementary and alternative treatments which may supplement or substitute medical treatments.
Lifestyle changes as treatment
Despite a lack of scientific evidence and findings of the positive effects of lifestyle change , women wanted to achieve better physical health and HRQoL and long-term recovery.
You have to be very conscious and responsible and need an incredible amount of time to develop this routine. I was exhausted and I wanted nothing more than to go to bed, but I knew if I did not prep my lunch for the next day, then I wasn’t going to have a [healthy] meal.
These women were given a great deal of contradictory information about their potential endometriosis diet . Those following a strict diet said it was like being a prisoner and they suffered because of the financial cost. When the diet was ineffective or too strict, women gave in and started to follow the needs of their bodies and developed a unique, personalized diet.
When I accidentally ate something, which was forbidden in the diet I would hate myself. Now I listen to my body, the things it likes or does not like.
Although it is difficult to find enough time and mobilize resources, all women agreed that physical activity is an essential part of managing the disease. Women were doing various sports (yoga, running, cycling, Zumba, swimming, intimate muscle training, and Pilates) regularly, but the efficacy of these sports was not specified during discussions.
Due to the unknown etiology of the disease, participants stated that they had thought about the psychosomatic, stress-related origin of endometriosis. Many women sought psychological help by using cognitive methods, schema therapy, EMDR (Eye Movement Desensitization and Reprocessing), stress management, autogenic training, meditation, and hypnosis to alleviate their symptoms.
I went to a psychologist, and I opened up about this stuff [dyspareunia]. She pointed out things that I could not see myself, and for some reason, I believe that if I defeat this misery the endometriosis will disappear, too.
Naturopathy and other methods
A wide range of naturopathic medicine (acupuncture, reflexology, Chinese medicine, Ayurveda, kinesiology, herbs) was mentioned in focus group discussions. When women find no answer in western medicine, they seek help through alternative treatments.
Then I decided to start taking a path which I normally would not take, as the path that I am currently on is not working.
Participants were not able to agree about the impact of the aforementioned methods, because each woman had a different view of those effects.
Theme 4: Different coping strategies in disease-management
Obtaining information.
One of the most important aspects of disease-management was obtaining reliable information. Women were motivated to access as much information as possible, however, it was the area with the most obstruction. Contradictory information increased the feeling of uncertainty (see Fig. 2 ). Insufficient information from health care professionals also increased uncertainty . Only some women felt that they were properly informed by their gynecologists, and many of them found that they had to drag the information out of their doctors. After the diagnosis, some participants were sent home to read about endometriosis on the internet .
When you are sent home to look into it on the internet, it’s like being thrown into the sea in order to teach you how to swim.
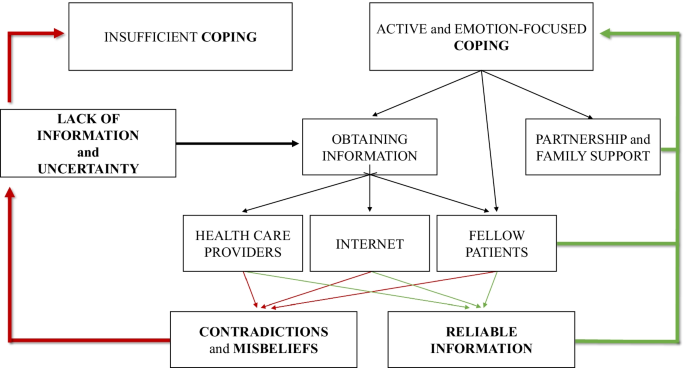
The model of sufficient and insufficient ways of coping with endometriosis
All women experienced that the internet is full of contradictions and misconceptions . Furthermore, destructive opinions, negative experiences, and rumors from fellow patients on blogs and online forums often confused them. Some women learned the most about endometriosis from fellow patients in the waiting room, where they were able to exchange experiences and inform one another. However, they also drew attention to the distress they had experienced.
We [fellow patients] can understand each other’s problems because we are in the same boat, but if I get no positive feedback and I can’t say anything to her that she needs, it’s not good.
Active control and emotion-focused coping
Women described a wide range of active and emotion-focused coping mechanisms they need to be able to use flexibly. In addition to obtaining information, most women avoided passivity and took control, assuming an active role and self-care in managing endometriosis. Some women stated that since changing their lifestyle, they have been able to live a full life. Others mentioned the importance of listening to the signs and needs of their bodies. Many participants coped with the difficulties and uncertainty of endometriosis by having a positive attitude , trying to find the positive aspects, and trying to remain optimistic.
Endometriosis taught me to take care of myself, and try to heal myself, to listen to my body and my inner voice, to look for methods that might help, and to find those which really help.
Social support
All women agreed on the importance of social support and support from their partners . “I cannot tell you how much it helps when he [male partner] stands by you.” They were able to cope with living with endometriosis, operations, and treatment thanks to the personal support of relatives and friends . Several participants mentioned the important role played by endometriosis community members, who can give support by sharing intimate experiences of endometriosis so that women do not have to face their problems alone.
It is always nice to get support from others who have experienced similar things and similar problems, so it feels good to talk about it. You are not alone.
Positive meaning of life after accepting endometriosis
Women described the difficulties, uncertainties, and lack of information surrounding endometriosis as pervasive features of their lives. Nevertheless, despite many difficulties and problems, women described a positive impact ( peace, patience, openness, personality development, and gratitude ) on their life after accepting their condition.
A whole new world has opened up before me. I am not saying that it is good to have endometriosis, but I have completely changed because of it. I would not be the same person if I had not gone through this. I improved as a person, and the journey is not over yet. I would not be as open towards people, I would not have these kinds of relationships, my family and my relationship would not be the same. I have a sense of purpose.
Possible responses to “do patients know what they need?”
Focus group discussions allowed women with endometriosis to demonstrate their desire to take an active role in the management of their disease and to express their needs and options for alleviating the difficulties and deficiencies. These suggestions allow us to understand the real needs of women with endometriosis and design a proper health promotion program.
Giving proper information from reliable sources could be one of the best ways of reducing uncertainty and increasing HRQoL. Participants highlighted the need for information about surgical results right after the postoperative wake-up, which would reduce postoperative stress, anxiety, and uncertainty.
Women suggested that diagnostic delay, the risk of misdiagnosis, and the normalization of dysmenorrhea could be reduced through more extensive training and by improving the specialist knowledge of a broader range of health care professionals and medical students in all related medical areas in relation to the recognition of endometriosis.
Almost every woman agreed that clinical or health psychologists are needed in hospitals to help cope with diagnosis and surgery and to process disease-management.
All women agreed on the importance of raising awareness of endometriosis by involving male partners, friends, and colleagues. Educating and informing men about endometriosis would have long-term advantages, as men could provide effective help and support to women with endometriosis. Women highlighted the fact that it could also be very stressful for men to be involved, and that because of many uncertainty factors there should be educational and supportive groups for men as well.
To prevent more severe conditions, participants agreed that awareness of and education about endometriosis is necessary from menarche. Preventive and educational programs relating to endometriosis in schools would help ensure the early diagnosis of future patients.
Furthermore, women stated that it is a social responsibility to increase publicity and awareness of endometriosis in society, and a campaign like that for breast cancer would help to educate all social groups.
In our study, we first report a mutual dynamic connection between the main endometriosis-related themes (HRQoL, medical experiences, complementary and alternative treatment, and coping strategies), and show that these areas are negatively influenced by the most prominent themes: uncertainty and lack of information. Exploring the connections between these themes will also help to understand patient pathways, which is essential for planning the long-term management of women with endometriosis.
Identified topics are comparable with previous findings [ 25 , 26 ], where negative impact on HRQoL and medical experience of endometriosis appeared as essential topics. Our results highlight that these themes are not independent of one another (see Figs. 1 , 2 ). Prolonged (pain)symptoms of endometriosis decrease quality of life, and direct women to health care, where patients can face a variety of different experiences. An inadequate doctor-patient relationship affects not only medical experiences and the physical condition of patients but also impairs adherence, compliance, and HRQoL. Ineffective medical attention or treatment affects women’s relationship with healthcare and leads them to use (non)evidence-based alternative treatments. Patients need active, emotion-focused coping strategies which are properly supported by positive medical experiences, reliable information, and effective social support. In their absence, patients may use inadequate coping options, which can have a negative impact on HRQoL. Lifestyle change as a potential coping and disease-management strategy [ 27 ] is an obvious opportunity for women to have control over one aspect of their condition. Nonetheless, the effectiveness of nonmedical treatments in endometriosis has not been sufficiently explored by evidence-based medicine [ 3 ]. Our results highlight the importance of finding a scientific response to women’s questions because failed attempts have a negative impact on prognosis, quality of life, and self-esteem [ 25 ].
Uncertainty and lack of information can have a direct impact on HRQoL, medical experiences, coping, and indirectly, on fertility as well [ 15 ]. The normalization and rejection of symptoms as a general problem impact the doctor-patient relationship before diagnosis and leads to diagnostic delay and eliminates the benefits of early diagnosis [ 3 ].
The lack of information at health care centers causes women to seek self-management strategies [ 15 , 28 , 29 ]. The lack of information causes women to seek self-management strategies. Women try to obtain information from various sources, but they come across a great deal of contradictory information, which needs to be dealt with. Studies identified that becoming assertive and taking control can be a potential coping mechanism before diagnosis and treatment [ 28 ], but there are fewer findings of how women cope with endometriosis and achieve an asymptomatic and fertile life after diagnosis. The women in our study used positive emotion-focused coping strategies to focus on the positive and optimistic aspects of their lives. Besides, problem-focused coping (versus non-adaptive focus on emotions) was found as an adaptive and assertive coping strategy that correlates with lower stress and depressive symptoms. [ 30 ]. On the other hand, catastrophizing is a negative cognitive and emotional coping response to pain [ 31 ] and enhances pain perception as a predictor among women with endometriosis [ 32 ]. Roomaney and Kagee [ 33 ] highlight—in line with our results—that both problem-focused and positive emotion-focused coping strategies can be helpful for women with endometriosis. A third means of coping is based on the help and support provided by personal relationships and endometriosis communities. Strong relationships were characterized by admiration for women’s courage, independence, and inner strength [ 34 , 35 ]. Self-help groups and endometriosis foundations can provide effective support to women from the individual (see reliable information; health promotion programs) [ 36 ] to society (see social awareness and publicity) [ 37 , 38 ].
In addition to negative consequences and needs, there were some interesting findings supporting the results of Facchin et al. [ 22 ] about finding the meaning of life with endometriosis. Women with positive emotion-focused coping strategies and a lower level of stress can accept the disease and find positive meaning in their lives from endometriosis. These results suggest the possibility of posttraumatic growth (PTG) in endometriosis. PTG is defined as the “positive psychological change experienced as a result of the struggle with highly challenging life circumstances” [ 39 ] (e.g. chronic disease as trauma or danger to health). Previous studies on women with chronic disease identified that PTG is negatively associated with age, depression, and stress, while positively associated with time since diagnosis, education, income, social support, mental HRQoL, self-efficacy, self-esteem, and optimism [ 40 , 41 , 42 , 43 , 44 ]. These characteristics show similarities with predictors of mental health quality in endometriosis [ 18 , 45 ], although to the author’s knowledge PTG in endometriosis patients has not been measured yet. The authors suggest that PTG may occur due to multiple health behavior changes which improve active coping and the patient’s sense of control [ 46 , 47 , 48 ]. Therefore, it is recommended that the possibility of PTG be explored in future endometriosis studies.
The authors acknowledge that are some limitations to the current study. Firstly, the study sample was low and consisted of participants with homogeneous demographic and disease characteristics. Secondly, we collected our data retrospectively. We asked women about their experiences about living with endometriosis without making differences in the pre- and post-operative period, because we wanted to collect all the affected areas in their life. Although there can be differences before and after the endometriosis surgery for example in the quality of sexual life [ 49 ]. These differences can be analyzed in further qualitative studies.
Thirdly, as endometriosis is a benign disorder, the primary objective of any treatment should be to alleviate symptoms, control progression, and improve quality of life. Laparoscopic surgery is the most widely accepted surgical approach in cases of peritoneal, ovarian, and deep infiltrating endometriosis (DIE) [ 3 , 50 , 51 ]. Peritoneal disease can be excised or vaporized using different energy sources, while ovarian endometriosis can be managed by cystectomy or ablation. According to the recent data the ovarian cystectomy may lead to the loss ovarian reserve [ 52 ]. The optimal type of colorectal resection in case of bowel DIE, whether conservative (shaving, disc resection) or radical technique (segmental bowel resection) has to be applied is under discussion [ 53 , 54 , 55 , 56 , 57 ]. It has been suggested that the conservative surgical therapy of colorectal DIE is associated with lower morbidity, however the unequivocal evidence supporting this hypothesis is still lacking. The external validity of present data regarding the surgical therapy of endometriosis should be investigated in future multicentric prospective randomized trials on a large cohort of patients. A clear limitation of our study is that we did not assess the impact of different surgical methods on the endometriosis related quality of life in our group of patients.
Further, as a result of the recruitment process predominantly women with active coping strategies and an optimistic attitude applied to take part in the study. Thirdly, the themes that emerged were facilitated by means of predetermined questions, and participants would have continued conversations in three areas. This may cause some limitations to the possible themes and topics of endometriosis discussed (e.g. symptoms, medical and surgical experiences).
Finally, coping strategies and PTG in endometriosis would have been identified by using appropriate questionnaires.
Uncertainty and lack of information about endometriosis as main challenges and difficulties have a significant impact on women’s life. The present findings indicate that cooperation between health care professionals, psychologists, and support organizations will be necessary for the future to provide care and find possible solutions to the needs of women living with endometriosis. Communication must be improved, and psychosocial problems need to be recognized by health care providers to ensure that empathetic care is provided. Having evidence-based answers about the efficiency of alternative and complementary therapies could decrease the uncertainty and lack of information. Furthermore, in order to reduce diagnostic delay, health care providers’ knowledge and society’s awareness of endometriosis should be improved in the near future. Health promotion programs and support groups should be managed to facilitate coping and posttraumatic growth in women with endometriosis. Achieving these recommendations would allow women to live an asymptomatic, fertile, and balanced life with endometriosis.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Infiltrating endometriosis
- Health-related quality of life
In vitro fertilization
Posttraumatic growth
Giudice LC, Kao LC. Endometriosis. The Lancet. 2004;364(9447):1789–99.
Article Google Scholar
Berlanda N, Alio W, Angioni S, Bergamini V, Bonin C, Boracchi P, et al. Impact of endometriosis on obstetric outcome after natural conception: a multicenter Italian study. Arch Gynecol Obstet; 2021.
Dunselman GAJ, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod Oxf Engl. 2014;29(3):400–12.
Article CAS Google Scholar
D’Alterio MN, Saponara S, Agus M, Laganà AS, Noventa M, Loi ES, et al. Medical and surgical interventions to improve the quality of life for endometriosis patients: a systematic review. Gynecol Surg. 2021;18(1):13.
De Graaff AA, D’Hooghe TM, Dunselman GAJ, Dirksen CD, Hummelshoj L, WERF EndoCost Consortium, et al. The significant effect of endometriosis on physical, mental and social wellbeing: results from an international cross-sectional survey. Hum Reprod Oxf Engl. 2013; 28(10):2677–85.
Pope CJ, Sharma V, Sharma S, Mazmanian D. A systematic review of the association between psychiatric disturbances and endometriosis. J Obstet Gynaecol Can JOGC J Obstet Gynecol Can JOGC. 2015;37(11):1006–15.
Smorgick N, Marsh CA, As-Sanie S, Smith YR, Quint EH. Prevalence of pain syndromes, mood conditions, and asthma in adolescents and young women with endometriosis. J Pediatr Adolesc Gynecol. 2013;26(3):171–5.
Article PubMed Google Scholar
Fourquet J, Gao X, Zavala D, Orengo JC, Abac S, Ruiz A, et al. Patients’ report on how endometriosis affects health, work, and daily life. Fertil Steril. 2010;93(7):2424–8.
Article PubMed PubMed Central Google Scholar
Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco NF, de Cicco NC, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96(2):366-373.e8.
Gilmour JA, Huntington A, Wilson HV. The impact of endometriosis on work and social participation. Int J Nurs Pract. 2008;14(6):443–8.
Barbara G, Facchin F, Buggio L, Somigliana E, Berlanda N, Kustermann A, et al. What is known and unknown about the association between endometriosis and sexual functioning: a systematic review of the literature. Reprod Sci Thousand Oaks Calif. 2017;1:1933719117707054.
Google Scholar
Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod Oxf Engl. 2012;27(5):1292–9.
Laganà AS, La Rosa VL, Rapisarda AMC, Valenti G, Sapia F, Chiofalo B, et al. Anxiety and depression in patients with endometriosis: impact and management challenges. Int J Womens Health. 2017;9:323–30.
Tariverdian N, Theoharides TC, Siedentopf F, Gutiérrez G, Jeschke U, Rabinovich GA, et al. Neuroendocrine-immune disequilibrium and endometriosis: an interdisciplinary approach. Semin Immunopathol. 2007;29(2):193–210.
Culley L, Law C, Hudson N, Denny E, Mitchell H, Baumgarten M, et al. The social and psychological impact of endometriosis on women’s lives: a critical narrative review. Hum Reprod Update. 2013;19(6):625–39.
Facchin F, Barbara G, Saita E, Mosconi P, Roberto A, Fedele L, et al. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J Psychosom Obstet Gynaecol. 2015;36(4):135–41.
Moradi M, Parker M, Sneddon A, Lopez V, Ellwood D. Impact of endometriosis on women’s lives: a qualitative study. BMC Womens Health. 2014;14:123.
Márki G, Bokor A, Rigó J, Rigó A. Physical pain and emotion regulation as the main predictive factors of health-related quality of life in women living with endometriosis. Hum Reprod. 2017;32(7):1432–8.
Ponterotto JG. Qualitative research in counseling psychology: A primer on research paradigms and philosophy of science. J Couns Psychol. 2005;52:126–36.
Krueger RA, Casey MA. Focus groups: A practical guide for applied research. Thousand Oaks: Sage Publications; 2014.
Morgan DL. The focus group guidebook, vol. 1. Thousand Oaks: Sage Publications; 1997.
Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77–101.
Joffe H, Yardley L. Content and thematic analysis. In: Research methods for clinical and health psychology. 2004th ed. California: Sage; 2004. p. 56–68.
Creswell JW. Qualitative research design and inquiry: choosing among five approaches. 2nd ed. Thousand Oaks: Sage Publications Inc.; 2007.
Facchin F, Saita E, Barbara G, Dridi D, Vercellini P. “Free butterflies will come out of these deep wounds”: A grounded theory of how endometriosis affects women’s psychological health. J Health Psychol. 2017;11:1359105316688952.
Young K, Fisher J, Kirkman M. Women’s experiences of endometriosis: a systematic review and synthesis of qualitative research. J Fam Plann Reprod Health Care. 2015;41(3):225–34.
Buggio L, Barbara G, Facchin F, Frattaruolo MP, Aimi G, Berlanda N. Self-management and psychological-sexological interventions in patients with endometriosis: strategies, outcomes, and integration into clinical care. Int J Womens Health. 2017;9:281–93.
Article CAS PubMed PubMed Central Google Scholar
Cox H, Henderson L, Andersen N, Cagliarini G, Ski C. Focus group study of endometriosis: struggle, loss and the medical merry-go-round. Int J Nurs Pract. 2003;9(1):2–9.
Cox H, Henderson L, Wood R, Cagliarini G. Learning to take charge: women’s experiences of living with endometriosis. Complement Ther Nurs Midwifery. 2003;9(2):62–8.
Donatti L, Ramos DG, de Andres MP, Passman LJ, Podgaec S. Patients with endometriosis using positive coping strategies have less depression, stress and pelvic pain. Einstein Sao Paulo Braz. 2017;15(1):65–70.
Sullivan MJL, Lynch ME, Clark AJ. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. Pain. 2005;113(3):310–5.
Martin CE, Johnson E, Wechter ME, Leserman J, Zolnoun DA. Catastrophizing: a predictor of persistent pain among women with endometriosis at 1 year. Hum Reprod Oxf Engl. 2011;26(11):3078–84.
Roomaney R, Kagee A. Coping strategies employed by women with endometriosis in a public health-care setting. J Health Psychol. 2016;21(10):2259–68.
Culley L, Law C, Hudson N, Mitchell H, Denny E, Raine-Fenning N. A qualitative study of the impact of endometriosis on male partners. Hum Reprod Oxf Engl. 2017;15:1–7.
Fernandez I, Reid C, Dziurawiec S. Living with endometriosis: the perspective of male partners. J Psychosom Res. 2006;61(4):433–8.
Shoebotham A, Coulson NS. Therapeutic affordances of online support group use in women with endometriosis. J Med Internet Res. 2016;18(5):e109.
Richard L, Potvin L, Kishchuk N, Prlic H, Green LW. Assessment of the integration of the ecological approach in health promotion programs. Am J Health Promot AJHP. 1996;10(4):318–28.
Article CAS PubMed Google Scholar
Facchin F, Saita E, Barbara G, Dridi D, Vercellini P. Free butterflies will come out of these deep wounds: A grounded theory of how endometriosis affects women’s psychological health. J Health Psychol. 2017;23:538–49.
Tedeschi RG, Calhoun LG. Posttraumatic growth: conceptual foundations and empirical evidence. Psychol Inq. 2004;15(1):1–18.
Barskova T, Oesterreich R. Post-traumatic growth in people living with a serious medical condition and its relations to physical and mental health: a systematic review. Disabil Rehabil. 2009;31(21):1709–33.
Danhauer SC, Case LD, Tedeschi R, Russell G, Vishnevsky T, Triplett K, et al. Predictors of posttraumatic growth in women with breast cancer. Psychooncology. 2013;22(12):2676–83.
Koutrouli N, Anagnostopoulos F, Griva F, Gourounti K, Kolokotroni F, Efstathiou V, et al. Exploring the relationship between posttraumatic growth, cognitive processing, psychological distress, and social constraints in a sample of breast cancer patients. Women Health. 2016;56(6):650–67.
Wang M-L, Liu J-E, Wang H-Y, Chen J, Li Y-Y. Posttraumatic growth and associated socio-demographic and clinical factors in Chinese breast cancer survivors. Eur J Oncol Nurs Off J Eur Oncol Nurs Soc. 2014;18(5):478–83.
Yeung NCY, Lu Q. Perceived stress as a mediator between social support and posttraumatic growth among Chinese American breast cancer survivors. Cancer Nurs. 2016;41:53.
Facchin F, Barbara G, Dridi D, Alberico D, Buggio L, Somigliana E, et al. Mental health in women with endometriosis: searching for predictors of psychological distress. Hum Reprod. 2017;32(9):1855–61.
Fong AJ, McDonough MH, Pila E, Sabiston CM. Posttraumatic growth in breast cancer survivors: the roles of physical activity and social support. J Exerc Mov Sport. 2017;49(1):165.
Hawkes AL, Pakenham KI, Chambers SK, Patrao TA, Courneya KS. Effects of a multiple health behavior change intervention for colorectal cancer survivors on psychosocial outcomes and quality of life: a randomized controlled trial. Ann Behav Med Publ Soc Behav Med. 2014;48(3):359–70.
Love C, Sabiston CM. Exploring the links between physical activity and posttraumatic growth in young adult cancer survivors. Psychooncology. 2011;20(3):278–86.
Di Donato N, Montanari G, Benfenati A, Monti G, Leonardi D, Bertoldo V, et al. Sexual function in women undergoing surgery for deep infiltrating endometriosis: a comparison with healthy women. J Fam Plann Reprod Health Care. 2015;41(4):278–83.
Redwine DB, Sharpe DR. Laparoscopic segmental resection of the sigmoid colon for endometriosis. J Laparoendosc Surg. 1991;1(4):217–20.
Bokor A, Hudelist G, Dobó N, Dauser B, Farella M, Brubel R, et al. Low anterior resection syndrome following different surgical approaches for low rectal endometriosis: a retrospective multicenter study. Acta Obstet Gynecol Scand. 2021;100(5):860–7.
Younis JS, Shapso N, Ben-Sira Y, Nelson SM, Izhaki I. Endometrioma surgery-a systematic review and meta-analysis of the effect on antral follicle count and anti-Müllerian hormone. Am J Obstet Gynecol. 2021; S0002-9378(21)00788-2.
Bokor A, Lukovich P, Csibi N, D’Hooghe T, Lebovic D, Brubel R, et al. Natural orifice specimen extraction during laparoscopic bowel resection for colorectal endometriosis: technique and outcome. J Minim Invasive Gynecol. 2018;25(6):1065–74.
Hudelist G, Aas-Eng MK, Birsan T, Berger F, Sevelda U, Kirchner L, et al. Pain and fertility outcomes of nerve-sparing, full-thickness disk or segmental bowel resection for deep infiltrating endometriosis—a prospective cohort study. Acta Obstet Gynecol Scand. 2018;97(12):1438–46.
Roman H, Bubenheim M, Huet E, Bridoux V, Zacharopoulou C, Daraï E, et al. Conservative surgery versus colorectal resection in deep endometriosis infiltrating the rectum: a randomized trial. Hum Reprod Oxf Engl. 2018;33(1):47–57.
Borghese G, Raimondo D, Esposti ED, Aru AC, Raffone A, Orsini B, et al. Preoperative ureteral stenting in women with deep posterior endometriosis and ureteral involvement: Is it useful? Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet. 2021.
Seracchioli R, Raimondo D, Arena A, Zanello M, Mabrouk M. Clinical use of endovenous indocyanine green during rectosigmoid segmental resection for endometriosis. Fertil Steril. 2018;109(6):1135.
Download references
Acknowledgements
The authors would like to thank Szilvia Kassai of the Institute of Psychology, Eötvös Loránd University, Budapest, Hungary, for her expert and methodological advice in relation to this study, and Boglárka Kristóf, a psychology student at Eötvös Loránd University, Budapest, Hungary, for her help with the pilot analysis.
Open access funding provided by Semmelweis University. There were no external sources of funding for this study. We acknowledge the significant contribution of the author’s workplaces, the Institute of Psychology, Eötvös Loránd University, Budapest 1064, Hungary and Department of Obstetrics and Gynecology, Semmelweis University, Budapest 1085, Hungary.
Author information
Authors and affiliations.
Doctoral School of Psychology, Eötvös Loránd University, Budapest, 1064, Hungary
Gabriella Márki
Institute of Psychology, Eötvös Loránd University, Izabella Street 46, Budapest, 1064, Hungary
Gabriella Márki, Dorottya Vásárhelyi, Adrien Rigó & Zsuzsa Kaló
Department of Obstetrics and Gynecology, Faculty of Medicine, Semmelweis University, Baross Street 27, Budapest, 1088, Hungary
Nándor Ács & Attila Bokor
You can also search for this author in PubMed Google Scholar
Contributions
G.M., A.R., N.Á., and A.B. were involved in designing this study. G.M., A.B., and N.Á. helped recruit participants. G.M. and D.V. corrected the database verbatim. G.M., D.V., Z.K., and A.R. were involved in the analysis and interpretation of data. This manuscript was drafted by G.M., V.D., Z.K., and A.R., and A.B. edited the article. All the authors have approved the final draft.
Corresponding author
Correspondence to Attila Bokor .
Ethics declarations
Ethical approval and consent to participate..
This study was approved by the Regional, Institutional Research and Ethics Committee of Semmelweis University, Budapest, Hungary (registration number TUKEB 60/2014), and the work was conducted in accordance with the tenets of the Declaration of Helsinki. Written informed consent was provided by all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Márki, G., Vásárhelyi, D., Rigó, A. et al. Challenges of and possible solutions for living with endometriosis: a qualitative study. BMC Women's Health 22 , 20 (2022). https://doi.org/10.1186/s12905-022-01603-6
Download citation
Received : 22 September 2021
Accepted : 14 January 2022
Published : 26 January 2022
DOI : https://doi.org/10.1186/s12905-022-01603-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Endometriosis
- Psychosocial impact
- Focus group
BMC Women's Health
ISSN: 1472-6874
- Submission enquiries: [email protected]
- General enquiries: [email protected]

SARINA SCHRAGER, MD, MS, JULIANNE FALLERONI, DO, MPH, AND JENNIFER EDGOOSE, MD, MPH
A more recent article on endometriosis is available.
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2013;87(2):107-113
Patient information : A handout on this topic is available at https://familydoctor.org/online/famdocen/home/women/reproductive/gynecologic/476.html .
Author disclosure: No relevant financial affiliations to disclose.
Endometriosis, which affects up to 10 percent of reproductive-aged women, is the presence of endometrial tissue outside of the uterine cavity. It is more common in women with pelvic pain or infertility ([corrected] 70 to 90 percent and 21 to 40 percent, respectively). Some women with endometriosis are asymptomatic, whereas others present with symptoms such as debilitating pelvic pain, dysmenorrhea, dyspareunia, and decreased fertility. Diagnosis of endometriosis in primary care is predominantly clinical. Initial treatment includes common agents used for primary dysmenorrhea, such as nonsteroidal anti-inflammatory drugs, combination estrogen/progestin contraceptives, or progestin-only contraceptives. There is some evidence that these agents are helpful and have few adverse effects. Referral to a gynecologist is necessary if symptoms persist or the patient is unable to become pregnant. Laparoscopy is commonly used to confirm the diagnosis before additional treatments are pursued. Further treatments include gonadotropin-releasing hormone analogues, danazol, or surgical removal of ectopic endometrial tissue. These interventions may control symptoms more effectively than initial treatments, but they can have significant adverse effects and limits on duration of therapy.
Endometriosis is defined as the presence of endometrial glandular and/or stromal cells outside of the uterine cavity. There are generally three distinct clinical presentations: endometrial implantation superficially on the peritoneum; endometrial lined ovarian cysts (chocolate cysts) or endometriomas; and endometriotic nodules (a complex, solid mass of endometrial, adipose, and fibromuscular tissue found between the rectum and vagina). 1 However, further laparoscopic studies of the peritoneum also have shown nonclassic lesions, such as clear vesicles, red vesicles, or microscopic disease. 2 Typically, ectopic endometrial tissue is found within the pelvis ( Table 1 ) . 3 Although reported in virtually all organ systems, extrapelvic deposition is exceedingly rare.
Endometriosis is generally considered a benign disease. However, several large cohort studies suggest that endometriosis is an independent risk factor for clear-cell carcinoma and endometrioid ovarian carcinoma. 4
The earliest and most widely accepted theory of endometriosis etiology is that refluxed menstrual tissue enters the pelvic peritoneal cavity and embeds into other intra-abdominal areas. 5 , 6 This theory is supported by the fact that the most commonly affected sites are closest to the fallopian tubes. 6 In addition, endometriosis often occurs in women with outflow obstruction, such as cervical stenosis, a transverse vaginal septum, and an imperforate hymen. 7
Although women with endometriosis have higher volumes of refluxed menstrual blood and endometrial tissue, 8 most women have some component of retrograde menstruation. The plasminogen activator inhibitor gene has been shown to increase the likelihood of endometrial implantation after retrograde menstruation. 6
The coelomic metaplasia theory of endometriosis proposes that the coelomic epithelium of the peritoneal cavity retains multipotential cells that can develop into endometriotic tissue. This explains rare cases of endometriosis in prepubertal girls, women with Müllerian agenesis, and men. 6 Another theory is that endometrial tissue can be transported to distant sites via lymphatic and vascular channels, which explains rare cases of extra-abdominal endometriosis. 6 Finally, newer research suggests an immunologic component to the development of endometriosis. Concentrations of macrophages, leptin, tumor necrosis factor-α, and interleukin-6 often are higher in the abdominal fluid of women with endometriosis. 6 , 9 , 10

Epidemiology
Endometriosis is an estrogen-dependent disease predominantly affecting reproductive-aged women, with the highest incidence among women 25 to 29 years of age. 11 The prevalence of endometriosis in the general population is difficult to accurately assess because some women with the disease have limited or no symptoms. Some studies suggest that it affects up to 10 percent of reproductive-aged women. 12 [corrected] Endometriosis is diagnosed in 21 to 40 percent of women with infertility 13 and in 70 to 90 percent of women with chronic pelvic pain. 14
In the United States, endometriosis is the third leading cause of gynecologic hospitalizations. 15 It is estimated that the disease leads to $2,801 in health care costs and $1,023 in lost productivity at work per patient annually. 16 In one nationwide survey, 50 percent of women with endometriosis reported spending entire days in bed over the previous 12 months because of the condition, with an average of 17.8 days spent in bed. 17
Risk Factors
It has been reported that the risk of endometriosis is six times higher in first-degree relatives of women with severe endometriosis. 9 However, a more recent case-control study showed that the familial impact on the incidence of endometriosis is not significant. 18
Early menarche and late menopause, which lead to increased exposure to menstruation, are commonly cited risk factors for endometriosis. 5 , 19 However, epidemiologic studies are equivocal as to whether these are true risk factors or findings associated with the disease itself. 20 Low body mass index 21 , 22 and higher caffeine or alcohol consumption 21 also are associated with an increased risk of endometriosis. Table 2 includes possible risk factors for the disease. 20 – 22 Oral contraceptives and regular exercise (i.e., more than four hours per week) may decrease the risk. 5 , 21
Clinical Presentation
The clinical presentation of endometriosis is highly variable and ranges from debilitating pelvic pain and infertility to no symptoms. Table 3 lists the symptoms and comorbidities that are associated with higher rates of endometriosis. 23 In a large case-control study in the United Kingdom, 73 percent of women with endometriosis reported dysmenorrhea, abdominal or pelvic pain, or menorrhagia, compared with 20 percent of women without the disease. 23 Many women with endometriosis present with nonspecific symptoms, such as chronic lower back pain or abdominal pain, which may delay diagnosis. Table 4 includes the differential diagnosis of common symptoms of endometriosis. 5 It takes an average of 11.7 years for endometriosis to be diagnosed in a woman with symptoms. 24
Similarly, objective physical examination findings are limited and nonspecific. Although many women with endometriosis will have normal examination findings, some will exhibit tenderness of the posterior fornix, limited motion of the uterus or ovaries, or an adnexal mass. Some women will have diffuse tenderness on pelvic examination. However, in women undergoing evaluation for infertility, uterosacral nodularity with associated tenderness is pathognomonic for endometriosis. 25
The diagnosis of endometriosis in primary care is initially clinical and based on history and physical examination findings. Histologic confirmation is usually achieved with the detection of extrauterine endometrial cells on laparoscopy. Less invasive diagnostic tests are being pursued. Transvaginal ultrasonography can reliably detect cystic endometriomas (89 percent sensitivity, 91 percent specificity) and is considered the imaging modality of choice, 26 , 27 although the test does not reliably detect smaller endometrial implants. The cancer antigen 125 assay has been extensively researched, but a large systematic review of 23 studies shows limited overall value in the diagnosis of endometriosis. The cancer antigen 125 level is often elevated in women with endometriosis, but its specificity for the disease is low. 28 Magnetic resonance imaging also is being explored, particularly for deeper rectosigmoid and ureteral infiltrating lesions, but it is not a standard diagnostic tool because of its low sensitivity. 29
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often the first-line treatment for endometriosis, followed by hormone therapy. Laparoscopy can be used to confirm the diagnosis before additional treatments are pursued; empiric therapy with another suppressive medication is also an option. Table 5 summarizes evidence-based therapies. 30 – 37 Figure 1 is an algorithm for treating endometriosis in primary care.
NONSTEROIDAL ANTI-INFLAMMATORY DRUGS
A Cochrane review evaluated NSAIDs in the treatment of endometriosis, but included only one randomized controlled trial (n = 24), which compared naproxen with placebo. 30 There was no difference in pain relief between naproxen and placebo, and there was no evidence that one NSAID is superior.
NSAIDs often are attempted first because they are beneficial in women with primary dysmenorrhea, are available over the counter, and are relatively safe. Although endometriosis is a condition of secondary dysmenorrhea, it seems reasonable to consider NSAIDs as a first-line treatment in women with suspected endometriosis.
COMBINATION ESTROGEN/PROGESTIN CONTRACEPTIVES
Combination oral contraceptives are more effective than placebo at reducing dysmenorrhea in women with endometriosis. 31 – 33 A double-blind, randomized controlled trial of 100 women with endometriosis demonstrated that low-dose combination oral contraceptives improved endometriosis pain compared with placebo. 32 Another study showed that combination oral contraceptives were less effective at six months compared with gonadotropin-releasing hormone (GnRH) analogues, although both significantly improved symptoms after 12 months. 38 Combination oral contraceptives have significantly fewer adverse effects than GnRH analogues.
A small, prospective, nonblinded, cohort study compared the ethinyl estradiol/etonogestrel vaginal ring (Nuvaring) with the norelgestromin/ethinyl estradiol transdermal patch (Ortho Evra) in patients with endometriosis. 39 Although continuous use of both treatments reduced pain, the ring was superior for dysmenorrhea. Patient satisfaction also seemed higher in patients using the ring. Continuous use of these treatments resulted in more breakthrough bleeding compared with cyclic use.
PROGESTERONE-ONLY CONTRACEPTIVES
Medroxyprogesterone (oral [Provera] or depot injection [Depo-Provera]) may improve symptoms of endometriosis compared with placebo. 33 Two trials comparing a lower-dose depot medroxyprogesterone (Depo-subQ Provera) with the GnRH analogue leuprolide (Lupron) showed comparable improvement in pain. 34 , 40 Both trials indicated that depot medroxyprogesterone resulted in less bone loss and hypoestrogenic adverse effects than leuprolide; however, depot medroxyprogesterone labels include U.S. Food and Drug Administration boxed warnings for bone loss. Two studies comparing dienogest (a new selective progestin that is not yet available in the United States) with GnRH analogues also showed comparable improvement in pain. 41 , 42
A small study showed that the etonogestrel subdermal implant (Implanon) was as effective as depot medroxyprogesterone for endometriosis pain. 43 Small nonrandomized studies have shown a possible improvement in endometriosis pain with the levonorgestrel-releasing intrauterine system (Mirena). 33 , 35
GONADOTROPIN-RELEASING HORMONE ANALOGUES
If NSAIDs and hormonal contraceptives are ineffective, the next step is treatment with a GnRH analogue such as leuprolide or goserelin (Zoladex). GnRH analogue therapy downregulates the pituitary, resulting in “medical menopause,” 44 and has been shown to improve pain in women with endometriosis. 36 However, the therapy causes adverse effects, such as hot flashes, night sweats, and possible bone loss, in many women. To mitigate the menopausal symptoms, reinitiating hormone therapy with low-dose estrogen and progestin is common.
Danazol, an androgen, is effective in the treatment of pelvic pain associated with endometriosis. 37 However, androgenic adverse effects, such as acne, hirsutism, and male pattern baldness, often limit its use. The drug has several U.S. Food and Drug Administration boxed warnings, including the risk of thrombosis and teratogenicity.
SURGICAL OPTIONS
Laparoscopic ablation of deposits and excision of endometriomas are options to relieve pain and treat infertility. Excision of endometriomas will more effectively improve pregnancy rates than a drainage and ablation technique, but there is little evidence on the success of surgical treatment in advanced disease. 26 , 45 If a woman with endometriosis does not desire future pregnancy and all medical treatments and conservative surgical therapies have been ineffective, a hysterectomy may be performed.
FUTURE TREATMENTS
Several medications are under evaluation for the treatment of endometriosis, including mifepristone (Mifeprex); aromatase inhibitors (i.e., letrozole [Femara], anastrozole [Arimidex], and exemestane [Aromasin]); Chinese herbal medications; gestrinone (a 19-nortestosterone derivative that has antiprogestational and antiestrogenic properties; not available in the United States); immunomodulators (i.e., pentoxifylline [Trental] and interferon); and selective estrogen receptor modulators. 33 Acupuncture may also be effective in the treatment of pain. 46
Managing Endometriosis-Related Infertility
Women with infertility due to endometriosis usually will undergo laparoscopy. In women with mild endometriosis diagnosed by laparoscopy, surgical treatment of lesions improves pregnancy rates compared with no treatment of lesions. 26 , 47
A Cochrane review evaluated 25 randomized controlled trials comparing oral contraceptives, progestins, and danazol with placebo to determine the effectiveness of temporary ovulation suppression for endometriosis-related infertility. 45 The review measured outcomes related to effects on subsequent fertility, such as live birth after 20 weeks' gestation and clinical pregnancy (evidenced by fetal heart motion and gestational sac) compared with adverse events (i.e., miscarriage, ectopic pregnancy, fetal abnormalities, adverse drug effects). The review showed that ovulation suppression had no effect on subsequent fertility compared with placebo.
Data Sources: We searched PubMed, the Cochrane Database of Systematic Reviews, the National Guideline Clearinghouse, and Clinical Evidence using the search terms endometriosis, etiology of endometriosis, epidemiology of endometriosis, treatment of endometriosis, and infertility associated with endometriosis. Search dates: December 2010 to January 2011.
Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268-279.
Eltabbakh GH, Bower NA. Laparoscopic surgery in endometriosis. Minerva Ginecol. 2008;60(4):323-330.
Jenkins S, Olive DL, Haney AF. Endometriosis: pathogenetic implications of the anatomic distribution. Obstet Gynecol. 1986;67(3):335-338.
Van Gorp T, Amant F, Neven P, Vergote I, Moerman P. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):349-371.
Farquhar C. Endometriosis. BMJ. 2007;334(7587):249-253.
Bedaiwy MA, Abdel-Aleem MA, Miketa A, Falcone T. Endometriosis: a critical appraisal of the advances and the controversies of a challenging health problem. Minerva Ginecol. 2009;61(4):285-298.
Olive DL, Henderson DY. Endometriosis and mullerian anomalies. Obstet Gynecol. 1987;69(3 pt 1):412-415.
Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64(2):151-154.
Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789-1799.
Harada T, Taniguchi F, Izawa M, et al. Apoptosis and endometriosis. Front Biosci. 2007;12:3140-3151.
Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160(8):784-796.
Wheeler JM. Epidemiology of endometriosis-associated infertility. J Reprod Med. 1989;34(1):41-46.
Strathy JH, Molgaard CA, Coulam CB, Melton LJ. Endometriosis and infertility: a laparoscopic study of endometriosis among fertile and infertile women. Fertil Steril. 1982;38(6):667-672.
Ozawa Y, Murakami T, Terada Y, et al.. Management of the pain associated with endometriosis: an update of the painful problems. Tohoku J Exp Med. 2006;210(3):175-188.
Velebil P, Wingo PA, Xia Z, Wilcox LS, Peterson HB. Rate of hospitalization for gynecologic disorders among reproductive-age women in the United States. Obstet Gynecol. 1995;86(5):764-769.
Simoens S, Hummelshoj L, D'Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13(4):395-404.
Kjerulff KH, Erickson BA, Langenberg PW. Chronic gynecological conditions reported by US women: findings from the National Health Interview Survey, 1984 to 1992. Am J Public Health. 1996;86(2):195-199.
Nouri K, Ott J, Krupitz B, Huber JC, Wenzl R. Family incidence of endometriosis in first-, second-, and third-degree relatives: case-control study. Reprod Biol Endocrinol. 2010;8:85.
Missmer SA, Hankinson SE, Spiegelman D, et al. Reproductive history and endometriosis among premenopausal women. Obstet Gynecol. 2004;104(5 pt 1):965-974.
Treloar SA, Bell TA, Nagle CM, Purdie DM, Green AC. Early menstrual characteristics associated with subsequent diagnosis of endometriosis. Am J Obstet Gynecol. 2010;202(6):534.e1-6.
Ozkan S, Murk W, Arici A. Endometriosis and infertility: epidemiology and evidence-based treatments. Ann N Y Acad Sci. 2008;1127:92-100.
Hediger ML, Hartnett HJ, Louis GM. Association of endometriosis with body size and figure. Fertil Steril. 2005;84(5):1366-1374.
Ballard KD, Seaman HE, de Vries CS, Wright JT. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—part 1. BJOG. 2008;115(11):1382-1391.
Hadfield R, Mardon H, Barlow D, Kennedy S. Delay in the diagnosis of endometriosis: a survey of women from the USA and the UK. Hum Reprod. 1996;11(4):878-880.
Matorras R, Rodríguez F, Pijoan JI, et al. Are there any clinical signs and symptoms that are related to endometriosis in infertile women?. Am J Obstet Gynecol. 1996;174(2):620-623.
Practice bulletin no. 114: management of endometriosis. Obstet Gynecol. 2010;116(1):223-236.
Alcázar JL, Laparte C, Jurado M, López-García G. The role of transvaginal ultrasonography combined with color velocity imaging and pulsed Doppler in the diagnosis of endometrioma. Fertil Steril. 1997;67(3):487-491.
Mol BW, Bayram N, Lijmer JG, et al. The performance of CA-125 measurement in the detection of endometriosis: a meta-analysis. Fertil Steril. 1998;70(6):1101-1108.
Brosens I, Puttemans P, Campo R, Gordts S, Kinkel K. Diagnosis of endometriosis: pelvic endoscopy and imaging techniques. Best Pract Res Clin Obstet Gynaecol. 2004;18(2):285-303.
Allen C, Hopewell S, Prentice A, Gregory D. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst Rev. ;2009(2):CD004753.
Davis L, Kennedy SS, Moore J, Prentice A. Modern combined oral contraceptives for pain associated with endometriosis. Cochrane Database Syst Rev. ;2007(3):CD001019.
Harada T, Momoeda M, Taketani Y, Hoshiai H, Terakawa N. Low-dose oral contraceptive pill for dysmenorrhea associated with endometriosis: a placebo-controlled, double-blind, randomized trial. Fertil Steril. 2008;90(5):1583-1588.
Ferrero S, Remorgida V, Venturini PL. Current pharmacotherapy for endometriosis. Expert Opin Pharmacother. 2010;11(7):1123-1134.
Schlaff WD, Carson SA, Luciano A, Ross D, Bergqvist A. Subcutaneous injection of depot medroxyprogesterone acetate compared with leuprolide acetate in the treatment of endometriosis-associated pain. Fertil Steril. 2006;85(2):314-325.
Lockhat FB, Emembolu JO, Konje JC. The efficacy, side-effects and continuation rates in women with symptomatic endometriosis undergoing treatment with an intra-uterine administered progestogen (levonorgestrel): a 3 year follow-up. Hum Reprod. 2005;20(3):789-793.
Brown J, Pan A, Hart RJ. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst Rev. ;2010(12):CD008475.
Selak V, Farquhar C, Prentice A, Singla A. Danazol for pelvic pain associated with endometriosis. Cochrane Database Syst Rev. ;2007(4):CD000068.
Ferrero S, Remorgida V, Venturini PL. Endometriosis. Clin Evid (Online) . August 13, 2010. http://clinical-evidence.bmj.com/ceweb/conditions/woh/0802/0802-get.pdf [login required]. Accessed February 15, 2011.
Vercellini P, Barbara G, Somigliana E, Bianchi S, Abbiati A, Fedele L. Comparison of contraceptive ring and patch for the treatment of symptomatic endometriosis. Fertil Steril. 2010;93(7):2150-2161.
Crosignani PG, Luciano A, Ray A, Bergqvist A. Subcutaneous depot medroxyprogesterone acetate versus leuprolide acetate in the treatment of endometriosis-associated pain. Hum Reprod. 2006;21(1):248-256.
Strowitzki T, Marr J, Gerlinger C, Faustmann T, Seitz C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: a 24-week, randomized, multicentre, open-label trial. Hum Reprod. 2010;25(3):633-641.
Harada T, Momoeda M, Taketani Y, et al. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis—a randomized, double-blind, multicenter, controlled trial. Fertil Steril. 2009;91(3):675-681.
Walch K, Unfried G, Huber J, et al. Implanon versus medroxyprogesterone acetate: effects on pain scores in patients with symptomatic endometriosis—a pilot study. Contraception. 2009;79(1):29-34.
de Ziegler D, Borghese B, Chapron C. Endometriosis and infertility: pathophysiology and management. Lancet. 2010;376(9742):730-738.
Hughes E, Brown J, Collins JJ, Farquhar C, Fedorkow DM, Vandekerckhove P. Ovulation suppression for endometriosis. Cochrane Database Syst Rev. ;2007(3):CD000155.
Rubi-Klein K, Kucera-Sliutz E, Nissel H, et al. Is acupuncture in addition to conventional medicine effective as pain treatment for endometriosis? A randomised controlled cross-over trial. Eur J Obstet Gynecol Reprod Biol. 2010;153(1):90-93.
Jacobson TZ, Duffy JM, Barlow D, Farquhar C, Koninckx PR, Olive D. Laparoscopic surgery for subfertility associated with endometriosis. Cochrane Database Syst Rev. ;2010(1):CD001398.
Continue Reading
More in AFP
More in pubmed.
Copyright © 2013 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
Case Study: Endometriosis or Hernia?
— a tricky diagnostic dilemma in a woman with groin pain.
by Kate Kneisel , Contributing Writer, MedPage Today

"Medical Journeys" is a set of clinical resources reviewed by doctors, meant for physicians and other healthcare professionals as well as the patients they serve. Each episode of this 12-part journey through a disease state contains both a physician guide and a downloadable/printable patient resource. "Medical Journeys" chart a path each step of the way for physicians and patients and provide continual resources and support, as the caregiver team navigates the course of a disease.
This month: A noteworthy case study.
A 33-year-old woman presented with pain in her left groin that radiated to her left thigh and worsened during menstruation. She told clinicians she had been experiencing these symptoms for the last 2 years, but was otherwise in good health. She noted that her periods were regular, with no severe menstrual cramps or pain, and neither did she have dyspareunia or any other symptoms that might be associated with endometriosis.
She had not undergone any abdominal or pelvic surgeries or gynecological interventions and was not on hormone therapy or contraceptives or any regular medications.
Physical Examination/Scanning
Physical examination identified a 1.5-cm left inguinal mass, which was adherent to the underlying tissue and tender on palpation. Clinicians performed an ultrasound of the abdomen and pelvis, which revealed a poorly defined speculated solid hypoechoic left inguinal mass, 1.6×1.4 cm in diameter. Ultrasound findings of the uterus and ovaries were unremarkable.
Computed tomography (CT) of her pelvis showed a central hypo-attenuation left inguinal mass 1.7×1.2 cm in diameter, and thickening of the left round ligament.

The scan did not reveal any other lesions, or signs of endometriosis, cancer, or inguinal lymphadenopathy. Given the patient's presenting symptoms, and findings of the physical and radiological examinations, clinicians considered left inguinal hernia as one of the differential diagnoses.
Surgical Findings
The patient underwent surgical examination of the left inguinal canal exploration, which revealed a mass measuring 1.5 cm attached to the round ligament and floor of the canal, which was completely excised along with a 0.5 cm margin. Surgeons repaired the floor of the inguinal canal and reinforced it with proline mesh. The patient had no complications from the procedure, recovered well, and was discharged in good condition.
The mass -- which macroscopically measured 3.5×3×1.5 cm and consisted of fibrous tissue with a cut section showing hemorrhagic areas -- was sent to histopathology for assessment, which revealed multiple foci of endometrial glands surrounded by endometrial stroma embedded within the fibrous tissue.
Post-Discharge
After her discharge, the patient attended surgery and gynecology outpatient clinics for follow-up. The consulting gynecologist reported that there was no need for additional surveillance imaging or postoperative hormonal therapy. Her symptoms did not reappear.
Rare and Mimics Other Common Conditions
Clinicians reporting this case of inguinal endometriosis note that because it is a rare clinical entity that mimics several other common inguinal conditions, a high index of suspicion is important for diagnosis before surgical treatment. This is especially crucial in cases involving a palpable inguinal mass, usually associated with cyclic changes in size and severity of pain.
Endometriosis, which affects an estimated 10% of women of reproductive age, typically manifests in intra-pelvic organs and peritoneum, although organs external to the pelvis may also be involved. The condition generally develops following pelvic surgical procedures, due to implantation of endometrial tissue, the authors added.
Inguinal endometriosis, however, is very rare, with only about 50 cases reported in the literature, the case authors noted. As such, "it is often misdiagnosed as other inguinal pathologies such as inguinal hernia, soft tissue tumors, and inguinal lymphadenopathy." Those cases have generally been addressed through surgery, and without the use of diagnostic imaging or biopsy.
First described in 1986, inguinal endometriosis is "characterized by the presence of endometrial stroma and glands in the extraperitoneal portion of the round ligament and in the surrounding connective and lymphatic tissues," explained the authors of a 2021 study of three such cases.
The condition tends to occur in women who have had several children and have undergone gynecological or obstetric surgery, the case authors said, noting that their review of the English literature identified just 29 cases of inguinal endometriosis affecting nulliparous women, as in this patient's case.
Affected women present with an inguinal swelling that is easily detected on palpation, along with cyclical pain and change in size. The authors cautioned that this periodic worsening of symptoms is a typical feature of endometriosis that is often missed during the initial assessment. Patients may also report having pain with menstruation and with intercourse, as well as a history of difficulty conceiving – symptoms suggestive of pelvic endometriosis.
Distinguishing Features
Features that may help distinguish inguinal from pelvic endometriosis include the presence of regular menstrual cycles, which the authors explained, "can be a misleading point in the clinical assessment ."
In addition, the right side is more likely to be affected in patients with inguinal endometriosis, presumably due to the presence of the sigmoid colon, which the case authors explained, "places pressure on the left inguinal area, acting as a preventive measure ." Only 13 cases of left-sided inguinal endometriosis have been reported in the literature to date, the group said.
The condition can present with symptoms common to various other inguinal conditions, such as inguinal hernia, hemangioma, lymphadenopathy, and hydrocele of canal of Nuck. The rarity, along with inconclusive results of imaging, make inguinal endometriosis very challenging to diagnose before surgery is performed, and the relative efficacy of the various imaging modalities in these cases has yet to be studied, the case authors noted.
Ultrasound imaging often shows "a hypoechoic unilocular or multilocular cyst that is difficult to distinguish from other inguinal region pathologies such as lymph nodes and simple cysts," although it may help rule out possible differential diagnoses, the group stated.
As was the case with this patient, CT does not always help confirm the diagnosis of inguinal endometriosis, but it can be used to exclude other possibilities, the authors noted. "Magnetic resonance imaging (MRI) is the most specific and sensitive imaging modality for the diagnosis of endometriosis in general," due to its ability to detect iron particles in the hemosiderin that is present in the endometrioma. On MRI, both inguinal and pelvic endometriosis show high intensity on T1-weighted images and hypointensity on T2-weighted images, and the generally atypical and non-specific MRI findings for endometriosis prevent a conclusive diagnosis of inguinal endometriosis.
The team referenced a case series of 20 inguinal endometriosis patients in which most had a mixed hyper- and hypointensity of both T1- and T2-weighted images (61.1% and 50%, respectively).
Although preoperative fine-needle aspiration cytology (FNAC) can be used to diagnose endometriosis, it is only rarely utilized because "most patients are treated surgically with a preoperative diagnosis of incarcerated inguinal hernia or other inguinal pathologies," and post-excision, histopathological evidence of endometrial glands and stroma from testing of the mass confirms the diagnosis, the authors said.
They noted that CT findings in their patient did not point to endometriosis, and because a possible inguinal hernia had not been ruled out, they did not use preoperative FNAC, which carried a risk of injuring the hernial sac.
Inguinal endometriosis – typically managed with radical surgery to reduce the chance of recurrence -- often exists concurrently with an inguinal hernia or hydrocele of canal of Nuck -- both of which may be treated surgically before endometriosis is diagnosed. This is why radical surgical resection is not done in most cases without evidence of recurrence on follow-up, the authors explained.
Recommendations
They advised that because inguinal endometriosis often occurs concomitantly with pelvic endometriosis, patients should be referred following surgery for a complete gynecological assessment. Patients with inguinal endometriosis who have clinical symptoms such as dysmenorrhea, dyspareunia, or infertility that suggest pelvic endometriosis should be assessed laparoscopically, the clinicians added.
Hormone therapy may be used in patients with concomitant inguinal and pelvic endometriosis, the authors stated, adding that its use in women with only inguinal endometriosis is more controversial, although it may be recommended as adjuvant postsurgical therapy to reduce the risk of recurrence.
Since their patient had no signs suggesting pelvic endometriosis, she received only gynecological follow-ups, without the need for diagnostic laparoscopy and hormonal therapy, the authors said.
Read previous installments of this series:
Part 1: Endometriosis: Understanding the Pathogenesis and Pathophysiology
Part 2: Diagnosing Endometriosis
Part 3: Managing Endometriosis: Research and Recommendations
![a case study of endometriosis author['full_name']](https://clf1.medpagetoday.com/media/images/author/KKneisel_188.jpg)
Kate Kneisel is a freelance medical journalist based in Belleville, Ontario.
Disclosures
The case report authors noted no conflicts of interest.
Primary Source
American Journal of Case Reports
Source Reference: AlSinan FM, et al "Inguinal endometriosis in a nulliparous woman mimicking an inguinal hernia: A case report with literature review" Am J Case Rep 2021; 22: e934564.
Case study of a rare form of endometriosis
Affiliation.
- 1 Obstetrics and Gynecology Department, Bucharest University Hospital.
- PMID: 23599823
- PMCID: PMC3624650
Endometriosis is a common, benign, chronic, estrogen-dependent disorder. The endometrial tissue implants itself outside the uterus and can be usually found in the pelvis or, in rare cases, it can be found nearly anywhere in the body. There are no pathognomonic symptoms of this disease, therefore, in some cases the tumors are incidentally discovered during surgery. Deep infiltrative endometriosis (DIE) is a rare form of this condition, which mostly affects the uterosacral ligaments, the rectovaginal space, and the upper third of the posterior vaginal wall, the bowel, and the urinary tract. We present the case of a 29-year-old pregnant female who was diagnosed with infiltrative endometriosis during the cesarean section at 38 weeks of gestation. The tumors involving the vesicouterine peritoneum had a tendency of infiltrating the urinary bladder, but the patient had been completely asymptomatic prior to this incidental discovery. As cited by literature, the discovery and management of urinary endometriosis, as well as that of other localizations of DIE, is not based on high-level evidence data, but rather on case-series reported by surgical teams working in different centers worldwide.
Keywords: infiltrating endometriosis; pregnancy; urinary bladder.
Publication types
- Case Reports
- Endometriosis / pathology*
- Endometriosis / surgery
- Peritoneum / pathology
- Peritoneum / surgery
- Uterus / pathology
- Uterus / surgery

A Case Study on Endometriosis
Endometriosis is a chronic reproduction condition that still remains a mystery to the medical community. This paper starts off by providing the background information on what endometriosis is, the etiology, and risk factors associated with the condition. Following the introduction is a case study on a 20 year old female who currently suffers from the condition herself. Based on Patient X’s life, the end of this paper focuses on the prognosis she has as far as living with the disease goes, and things she can change in her lifestyle to improve her symptoms.
qnk322f06z_version1_Endometriosis_Case_Study.docx
size: 24.6 KB | mime_type: application/vnd.openxmlformats-officedocument.wordprocessingml.document | date: 2015-04-16
- V1 published November 16, 2020
Collections
This resource is currently not in any collection.
Work History
Version 1 published.
November 16, 2020 23:39 by Scholarsphere 4 Migration
April 04, 2024 10:20 by [unknown user]
The Barriers That Adolescents and Young Adults with Endometriosis Experience in the United States: A Conceptual Review and Model
- Published: 17 May 2024
Cite this article

- Jenny Niedenfuehr ORCID: orcid.org/0000-0001-6096-5881 1 &
- Lindsey M. King ORCID: orcid.org/0000-0002-5986-954X 2
71 Accesses
1 Altmetric
Explore all metrics
Introduction
Endometriosis is a chronic disease that vastly impacts patients’ lives, especially those who do not know how to manage the disease, understand the treatment options, or find specialists who can provide the proper care. The aim of this review and conceptual model is to provide a public health foundation for physicians and health care providers to further understand the barriers to endometriosis treatment that young adults and adolescents with endometriosis (10–25 years of age) experience grounded by theory and constructs.
A search was performed on PubMed with mix of MesH headings and keywords such as “adolescent,” “Adult, Young,” “United States,” Endometriosis,” and “Barrier.” Papers were reviewed through an inclusion/exclusion process to reduce bias. All papers that were excluded had no relevance to endometriosis, young adults, and adolescents, or were not located in the United States.
Our conceptual model presents the individual factors within the social ecological model (SEM), belief model, social cognitive theory (SCT) (outcome expectations, observational learning), social support, theory of fundamental causes (stigma, racism and discrimination, the built environment, lack of health policies, high costs, lack of health literacy among patients, lack of knowledge among providers), experiences, and outcomes.
Conclusions
The conceptual model and critical review highlight the intertwined, multi-faceted barriers that patients face to endometriosis treatment at each level of the SEM and may serve as an excellent starting point for future research.
Policy Implications
There are no existing policies for endometriosis patients. This is the first conceptual model to include multiple public health theories in relation to endometriosis and can best guide policy makers, program development, public health interventions, and researchers in mitigating patient barriers.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions

Similar content being viewed by others

Impact of endometriosis on women’s lives: a qualitative study
Patient experiences of being advised by a healthcare professional to get pregnant to manage or treat endometriosis: a cross-sectional study

Challenges of and possible solutions for living with endometriosis: a qualitative study
Availability of data and materials.
The data that support this study will be shared upon reasonable request to the corresponding author.
Allchin, A., Chaplin, V., & Horwitz, J. (2019). Limiting access to lethal means: Applying the social ecological model for firearm suicide prevention. Injury Prevention, 25 (Suppl 1), i44–i48.
Article PubMed Google Scholar
As-Sanie, S., et al. (2019). Assessing research gaps and unmet needs in endometriosis. American Journal of Obstetrics and Gynecology, 221 (2), 86–94.
As-Sanie, S., et al. (2020). Healthcare utilization and cost burden among women with endometriosis by opioid prescription status in the first year after diagnosis: A retrospective claims database analysis. Journal of Medical Economics, 23 (4), 371–377.
Balogh, E.P., Miller, B.T., & Ball, J.R. (2015). Improving Diagnosis in Health Care in Improving Diagnosis in Health Care.
Bandura, A. (1977). Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review, 84 (2), 191–215.
Bandura, A. (2004). Health promotion by social cognitive means. Health Education & Behavior, 31 (2), 143–164.
Article Google Scholar
Basile, A., Failla, G., & Gozzo, C. (2021). Pelvic congestion syndrome. Seminars in Ultrasound, CT and MR, 42 (1), 3–12.
Bohn, J. A., et al. (2021). Stepwise approach to the management of endometriosis-related dysmenorrhea: A cost-effectiveness analysis. Obstetrics and Gynecology, 138 (4), 557–564.
Bougie, O., et al. (2019). Influence of race/ethnicity on prevalence and presentation of endometriosis: A systematic review and meta-analysis. British journal of obstetrics and gynaecology, 126 (9), 1104–1115.
Bougie, O., Nwosu, I., & Warshafsky, C. (2022). Revisiting the impact of race/ethnicity in endometriosis. Reprod Fertil, 3 (2), R34–R41.
Article PubMed PubMed Central Google Scholar
Brotto, L. A., et al. (2021). #ItsNotInYourHead: A social media campaign to disseminate information on provoked vestibulodynia. Archives of Sexual Behavior, 50 (1), 57–68.
Brown, N., et al. (2022). Teachers’ perceptions and experiences of menstrual cycle education and support in UK schools. Front Glob Womens Health, 3 ,
Bullo, S. (2020). “I feel like I’m being stabbed by a thousand tiny men”: The challenges of communicating endometriosis pain. Health (london, England), 24 (5), 476–492.
Carneiro, M. M., et al. (2020). Using social media to educate women and healthcare providers on endometriosis: Preliminary results. Jornal Brasileiro De Reproducao Assistida, 24 (1), 9–12.
Google Scholar
Cene, C. W., et al. (2016). A narrative review of patient and family engagement: The “foundation” of the medical “home.” Medical Care, 54 (7), 697–705.
Centers for Disease Control and Prevention. (2022, January 18). The social-ecological model: A framework for prevention. Retrieved May 16, 2022, from https://www.cdc.gov/violenceprevention/about/social-ecologicalmodel.html
Champion, V. L., et al. (2008). Health behavior and health education. Theory, Research, and Practice (Eds), : p. 45–65.
Chatman, D. L. (1976). Endometriosis in the black woman. American Journal of Obstetrics and Gynecology, 125 (7), 987–989.
Chen, C. X., et al. (2018). Reasons women do not seek health care for dysmenorrhea. Journal of Clinical Nursing, 27 (1–2), e301–e308.
PubMed Google Scholar
Cradock, A. L., & Duncan, D. (2014). The role of the built environment in supporting health behavior change. In K. A. Riekert, J. K. Ockene, & L. Pbert (Eds.), The handbook of health behavior change (pp. 437-462). Springer Publishing Company.
Culley, L., et al. (2013). The social and psychological impact of endometriosis on women ’ s lives: A critical narrative review . Human Reproduction Update, 19 (6), 625–639.
Darba, J., & Marsa, A. (2022). Economic implications of endometriosis: A review. PharmacoEconomics, 40 (12), 1143–1158.
De Graaff, A. A., et al. (2013). The significant effect of endometriosis on physical, mental and social wellbeing: Results from an international cross-sectional survey. Human Reproduction, 28 (10), 2677–2685.
Della Corte, L., et al. (2020). The burden of endometriosis on women’s lifespan: A narrative overview on quality of life and psychosocial wellbeing. International Journal of Environmental Research and Public Health , 17 (13).
DiVasta, A. D., et al. (2018). Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs adulthood. American Journal of Obstetrics and Gynecology, 218 (3), 24 e1-324 e11.
Doll, K. M., et al. (2020). Assessment of prediagnostic experiences of Black women with endometrial cancer in the United States. JAMA Network Open, 3 (5), e204954.
Dun, E.C., et al. (2015). Endometriosis in adolescents . Journal of the Society of Laparoscopic & Robotic Surgeons, 19 (2).
Earp, J. A., & Ennett, S. T. (1991). Conceptual models for health education research and practice. Health Education Research, 6 (2), 163–171.
Ellis, K., Munro, D., & Clarke, J. (2022). Endometriosis is undervalued: A call to action. Frontiers in Global Women’s Health, 3 ,
Facchin, F., et al. (2021). A woman’s worth: The psychological impact of beliefs about motherhood, female identity, and infertility on childless women with endometriosis. Journal of Health Psychology, 26 (7), 1026–1034.
Falcone, T., & Flyckt, R. (2018). Clinical management of endometriosis. Obstetrics and Gynecology, 131 (3), 557–571.
Fourquet, J., et al. (2019). Disparities in healthcare services in women with endometriosis with public vs private health insurance. American Journal of Obstetrics and Gynecology, 221 (6), 623.e1-623.e11.
Fuldeore, M., et al. (2015). Healthcare utilization and costs in women diagnosed with endometriosis before and after diagnosis: A longitudinal analysis of claims databases. Fertility and Sterility, 103 (1), 163–171.
Gallagher, J. S., et al. (2018). The impact of endometriosis on quality of life in adolescents. Journal of Adolescent Health, 63 (6), 766–772.
Gilmour, J. A., Huntington, A., & Wilson, H. V. (2008). The impact of endometriosis on work and social participation. International Journal of Nursing Practice, 14 (6), 443–448.
Glanz, K., Rimer, B. K., & Viswanath, K. V. (Eds.). (2015). Health behavior: Theory, research, and practice, 5th ed. , Hoboken, NJ, US: Jossey-Bass/Wiley. pp. xxv, 485.
González-Echevarría, A. M., et al. (2019). Impact of coping strategies on quality of life of adolescents and young women with endometriosis. Journal of Psychosomatic Obstetrics and Gynecology, 40 (2), 138–145.
Grant, M. J., & Booth, A. (2009). A typology of reviews: An analysis of 14 review types and associated methodologies. Health Information and Libraries Journal, 26 (2), 91–108.
Gubbels, A., et al. (2020). Adolescent endometriosis. Obstetrical & Gynecological Survey, 75 (8), 483–496.
Gupta, J., et al. (2018). How do adolescent girls and boys perceive symptoms suggestive of endometriosis among their peers? Findings from focus group discussions in New York City. British Medical Journal Open, 8 (6), e020657.
Hatchett, L., et al. (2011). Knowledge and perceptions of pelvic floor disorders among African American and Latina women. Female Pelvic Medicine & Reconstructive Surgery, 17 (4), 190–194.
Hennegan, J., et al. (2020). Measurement in the study of menstrual health and hygiene: A systematic review and audit. PLoS ONE, 15 (6).
Holt-Lunstad, J., & Uchino, B. N. (2015). Social support and health. health behavior: Theory, research, and practice (5th ed., pp. 183–204). Jossey-Bass/Wiley.
Horne, A. W., & Missmer, S. A. (2022). Pathophysiology, diagnosis, and management of endometriosis. British medical journal, 379 ,
Houston, D. E. (1984). Evidence for the risk of pelvic endometriosis by age, race and socioeconomic status. Epidemiologic Reviews, 6 , 167–191.
Hudson, N. (2022). The missed disease? Endometriosis as an example of ‘ undone science ’. Reprod Biomed Soc Online, 14 , 20–27.
Hunsche, E., et al. (2023). Endometriosis symptoms and their impacts on the daily lives of US women: Results from an interview study. International Journal of Womens Health, 15 , 893–904.
James, T.G., et al. (2021). Conceptual model of emergency department utilization among deaf and hard-of-hearing patients:A critical review. International Journal of Environmental Research and Public Health . 18(24).
Johnston, J. L., Reid, H., & Hunter, D. (2015). Diagnosing endometriosis in primary care: Clinical update. British Journal of General Practice, 65 (631), 101–102.
Kelder, S. H., Hoelscher, D., & Perry, C. L. (2015). How individuals, environments, and health behaviors interact: Social cognitive theory. Health behavior: Theory, research, and practice (5th ed., pp. 159–181). Jossey-Bass/Wiley.
Khan, N. A. J., et al. (2018). Slipping rib syndrome in a female adult with longstanding intractable upper abdominal pain. Case Reports in Medicine, 2018 , 7484560.
Krug, E. G., et al. (2002). The world report on violence and health. Lancet, 360 (9339), 1083–1088.
Lee, D. W., et al. (2016). Chronic pelvic pain arising from dysfunctional stabilizing muscles of the hip joint and pelvis. Korean Journal of Pain, 29 (4), 274–276.
Leonardi, M., et al. (2020). When to do surgery and when not to do surgery for endometriosis: A systematic review and meta-analysis. Journal of Minimally Invasive Gynecology, 27 (2), 390-407 e3.
Link, B. G., & Phelan, J. (1995). Social conditions as fundamental causes of disease. Journal of Health and Social Behavior, 80–94.
Maddern, J., et al. (2020). Pain in Endometriosis. Frontiers in Cellular Neuroscience, 14 , 590823.
Mandimika, C. L., et al. (2015). Racial disparities in knowledge of pelvic floor disorders among community-dwelling women. Female Pelvic Medicine & Reconstructive Surgery, 21 (5), 287–292.
Marki, G., et al. (2022). Challenges of and possible solutions for living with endometriosis: A qualitative study. BMC Women’s Health, 22 (1), 20.
Maroko, A. R., et al. (2021). Public restrooms, periods, and people experiencing homelessness: An assessment of public toilets in high needs areas of Manhattan, New York. PLoS ONE, 16 (6), e0252946.
Martin, C. E., et al. (2011). Catastrophizing: A predictor of persistent pain among women with endometriosis at 1 year. Human Reproduction, 26 (11), 3078–3084.
Martin, L. R., et al. (2005). The challenge of patient adherence. Therapeutics and Clinical Risk Management, 1 (3), 189–199.
PubMed PubMed Central Google Scholar
Matloobi, M., et al. (2022). Effect of sex education on sexual function and sexual quality of life in women with endometriosis: A quasi-experimental study. International Journal of Gynaecology and Obstetrics, 159 (3), 702–710.
Metzler, J. M., et al. (2022). Examining the influence on perceptions of endometriosis via analysis of social media posts: Cross-sectional study. JMIR Formative Research, 6 (3)
Miklovic, T. and V.C. Sieg. (2023). Ehlers-Danlos syndrome, in StatPearls, Treasure Island (FL)
Mirin, A. A. (2021). Gender disparity in the funding of diseases by the U.S. National Institutes of Health. Journal of Womens Health (Larchmt), 30 (7), 956–963.
Naliboff, B. D., et al. (2015). Widespread psychosocial difficulties in men and women with urologic chronic pelvic pain syndromes: Case-control findings from the multidisciplinary approach to the study of chronic pelvic pain research network. Urology, 85 (6), 1319–1327.
Ng, N., et al. (2020). Endometriosis and negative perception of the medical profession. Journal of Obstetrics and Gynaecology Canada, 42 (3), 248–255.
Nnoaham, K.E., et al. (2011). Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertility and Sterility, 96(2).
Orlando, M. S., et al. (2022). Racial and ethnic disparities in surgical care for endometriosis across the United States. American Journal of Obstetrics and Gynecology, 226 (6), 824 e1-824 e11.
Parasar, P., Ozcan, P., & Terry, K. L. (2017). Endometriosis: Epidemiology, diagnosis and clinical management. Current Obstetrics and Gynecology, 6 (1), 34–41.
Payne, L. A., et al. (2019). Experimental evaluation of central pain processes in young women with primary dysmenorrhea. Pain, 160 (6), 1421–1430.
Phelan, J. C., & Link, B. G. (2005). Controlling disease and creating disparities: A fundamental cause perspective. Journals of gerontology. Series B: psychological sciences and social sciences, 60 (2), 27–33.
Phelan, J. C., Link, B. G., & Tehranifar, P. (2010). Social conditions as fundamental causes of health inequalities: Theory, evidence, and policy implications. Journal of Health and Social Behavior, 51 (Suppl), S28-40.
Poleshuck, E. L., et al. (2009). Pain and depression in gynecology patients. Psychosomatics, 50 (3), 270–276.
Rosenstock, I.M. (1990). The health belief model: Explaining health behavior through expectancies .
Russell, S. L., et al. (2009a). Beliefs of women ’ s risk as research subjects: A four-city study examining differences by sex and by race/ethnicity . Journal of Women’s Health, 18 (2), 235–243.
Russell, S. L., et al. (2009b). Beliefs of women’s risk as research subjects: A four-city study examining differences by sex and by race/ethnicity. Journal of Women’s Health (2002), 18 (2), 235–243.
Saha, S., et al. (2013). Dysmenorrhea in women with Crohn ’ s disease: A case-control study . Inflammatory Bowel Diseases, 19 (7), 1463–1469.
Salmond, S., & Dorsen, C. (2022). Time to reflect and take action on health disparities and health inequities. Orthopaedic Nursing, 41 (2), 64–85.
Samani, E. N., et al. (2019). Micrometastasis of endometriosis to distant organs in a murine model. Oncotarget, 10 (23), 2282–2291.
Sampson, J. A. (1927). Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. The American Journal of Pathology, 3 (2), 93-110.43.
Sayer-Jones, K., & Sherman, K. A. (2023). My body...tends to betray me sometimes: A qualitative analysis of affective and perceptual body image in individuals living with endometriosis. International Journal of Behavioral Medicine, 30 (4), 543–554.
Schimmels, J., & Cunningham, L. (2021). How do we move forward with trauma-informed care? Journal Of Nursing Practice, 17 (4), 405–411.
Schneider, M. P., et al. (2020). Quality of life in adolescent and young adult women with dyspareunia and endometriosis. Journal of Adolescent Health, 67 (4), 557–561.
Schrager, S., et al. (2022). Adenomyosis: Diagnosis and management. American Family Physician, 105 (1), 33–38.
Sebert Kuhlmann, A., et al. (2019). Unmet menstrual hygiene needs among low-income women. Obstetrics and Gynecology, 133 (2), 238–244.
Shadbolt, N. A., Parker, M. A., & Orthia, L. A. (2013). Communicating endometriosis with young women to decrease diagnosis time. Health Promotion Journal of Australia, 24 (2), 151–154.
Shafrir, A.L., et al. (2022). Cohort profile: The endometriosis pain quality after surgical treatment (EndoQUEST) study . PLoS ONE, 17(6).
Shim, J. Y., & Laufer, M. R. (2020). Adolescent endometriosis: An update. Journal of Pediatric and Adolescent Gynecology, 33 (2), 112–119.
Shum, L. K., et al. (2018). Deep dyspareunia and sexual quality of life in women with endometriosis. Sexual Medicine, 6 (3), 224–233.
Sieberg, C. B., Lunde, C. E., & Borsook, D. (2020). Endometriosis and pain in the adolescent- striking early to limit suffering: A narrative review. Neuroscience and Biobehavioral Reviews, 108 , 866–876.
Simons-Morton, B., McLeroy, K., & Wendel, M. (2011). Behavior theory in health promotion practice and research.
Simpson, C. N., Lomiguen, C. M., & Chin, J. (2021). Combating diagnostic delay of endometriosis in adolescents via educational awareness: A systematic review. Cureus, 13 (5), e15143.
Sims, O.T., et al. (2021). Stigma and endometriosis: A brief overview and recommendations to improve psychosocial well-being and diagnostic delay. International Journal of Environmental Research and Public Health, 18(15).
Smith, D. L. (2008). Disparities in health care access for women with disabilities in the United States from the 2006 National Health Interview Survey. Disability and Health Journal, 1 (2), 79–88.
Smolarz, B., Szyllo, K., & Romanowicz, H. (2021). Endometriosis: Epidemiology, classification, pathogenesis, treatment and genetics (review of literature). International journal of molecular sciences, 22 (19).
Soliman, A. M., et al. (2018). Real-world evaluation of direct and indirect economic burden among endometriosis patients in the United States. Advances in Therapy, 35 (3), 408–423.
Soliman, A. M., et al. (2019). Health care utilization and costs associated with endometriosis among women with Medicaid insurance. Journal of Managed Care and Specialty Pharmacy, 25 (5), 566–572.
Soliman, A. M., Fuldeore, M., & Snabes, M. C. (2017). Factors associated with time to endometriosis diagnosis in the United States. Journal of Women’s Health, 26 (7), 788–797.
Sourial, S., Tempest, N., & Hapangama, D. K. (2014). Theories on the pathogenesis of endometriosis. International Journal of Reproductive Medicine, 2014 ,
Strzempko, Butt, & Chesla, C. (2007). Relational patterns of couples living with chronic pelvic pain from endometriosis. Qualitative Health Research, 17 (5), 571–85.
Sutcliffe, S., et al. (2015). Urological chronic pelvic pain syndrome flares and their impact: Qualitative analysis in the MAPP network. International Urogynecology Journal and Pelvic Floor Dysfunction, 26 (7), 1047–1060.
Syed, S. T., Gerber, B. S., & Sharp, L. K. (2013). Traveling towards disease: Transportation barriers to health care access. Journal of Community Health, 38 (5), 976–993.
Tabaac, A. R., et al. (2022). Prevalence of chronic pelvic pain by sexual orientation in a large cohort of young women in the United States. Journal of Sexual Medicine, 19 (6), 1012–1023.
Tan, S. S., & Goonawardene, N. (2017). Internet health information seeking and the patient-physician relationship: A systematic review. Journal of Medical Internet Research, 19 (1), e9.
Towne, J., et al. (2021). Health information in the era of social media: An analysis of the nature and accuracy of posts made by public Facebook pages for patients with endometriosis. Journal of Minimally Invasive Gynecology, 28 (9), 1637–1642.
Volker, C., & Mills, J. (2022). Endometriosis and body image: Comparing people with and without endometriosis and exploring the relationship with pelvic pain. Body Image, 43 , 518–522.
Weisberg, E., & Fraser, I. S. (2015). Contraception and endometriosis: Challenges, efficacy, and therapeutic importance. Open Access Journal of Contraception, 6 , 105–115.
Wilson, C., & Nelson, J. P. (2012). Exploring the patterns, practices, and experiences of military women who managed genitourinary symptoms in deployed settings. JOGNN - Journal of Obstetric, Gynecologic, and Neonatal Nursing, 41 (2), 293–302.
Zarbo, C., et al. (2018). Behavioral, cognitive, and emotional coping strategies of women with endometriosis: A critical narrative review. Archives of Women’s Mental Health, 21 (1), 1–13.
Download references
Author information
Authors and affiliations.
College of Public Health & Health Professions, University of Florida, Gainesville, FL, 32611, USA
Jenny Niedenfuehr
Department of Health Services Research, Management and Policy, College of Public Health & Health Professions, University of Florida, Gainesville, USA
Lindsey M. King
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Jenny Niedenfuehr .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix. PubMed search strategy
(Adolescent [MeSH] OR Adolescen* OR Youth* OR Teenag* OR Humans[MeSH] OR “Adult, Young” OR “Young Adults”) AND (“Young adult*” OR “Young Adult” [MeSH]) AND (“United States” [MeSH] OR “United State*” OR “United States/Epidemiology”[MeSH]) AND (Barrier* OR "Perceived Barrier*" OR Coping OR "Psychological Wellbeing" OR "Social Stigma" [MeSH] OR "Quality of Life"[MeSH] OR "Adaptation, Psychological"[MeSH] OR "Coping Strategies" OR “Social Stigma*” OR “Stigma, Social” OR “Interpersonal Relations” [MeSH] OR “Interpersonal Relation” OR “Partner Communication” OR “Social Relationship*” OR "Life Quality" OR "Health-Related Quality Of Life" OR “HRQQL” OR “Health Related Quality of Life” OR “Employment/Education”[MeSH] OR Employment [MeSH] OR Education [MeSH] OR “Coping Behavior” OR “Behavior, Coping” OR “Coping Skill*” OR “Burden of Illness” OR “Disease Burden” OR “Cost of Illness” [Mesh] OR “Mental Disorders” OR “Stress Disorders, Traumatic, Acute”[MeSH] OR “Patient Satisfaction” [MeSH] or Physician–Patient Relations* OR “Patient Satisfaction” OR “Perception*” OR "Perception"[Mesh])AND (Therapeutics [MeSH] OR “Treatment Effectiveness” OR Treatment* OR Therapy* OR Laparoscopy OR Laparoscopy [MeSH] OR Excision OR Physician[MeSH]) AND (Endometriosis[MeSH] OR "Endometriosis/epidemiology"[MeSH] OR "Endometriosis/physiopathology"[Mesh] OR “Endometriosis / psychology” OR Endometrio* OR Pelvic Pain [MeSH] OR Endometrio* OR Endometriosis OR Endometrioma* OR Adhesions OR “Peritoneal Endometriosis” OR Endometriosis/Therapy*[MeSH] OR “Deep Infiltrating Endometriosis” OR “Ovarian Endometriosis” OR “Women’s Health” [MeSH] OR “Chronic Pelvic Pain” OR “Gynecological Disease” OR “Gynecologic Disease*” OR “Pelvic Pain” OR “Pains, Pelvic” OR “Pelvic Pain*” OR “Endometriosis.”
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Niedenfuehr, J., King, L.M. The Barriers That Adolescents and Young Adults with Endometriosis Experience in the United States: A Conceptual Review and Model. Sex Res Soc Policy (2024). https://doi.org/10.1007/s13178-024-00972-x
Download citation
Accepted : 01 April 2024
Published : 17 May 2024
DOI : https://doi.org/10.1007/s13178-024-00972-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Endometriosis
- Adolescents
- Young adults
- United States
- Pelvic pain
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Am J Case Rep

Cutaneous Endometriosis: A Case Report and Review of the Literature
Rodolfo h. gonzalez.
1 Department of Obstetrics and Gynecology, Inova Fairfax Women’s Hospital Medical Campus, Fairfax, VA, USA
Minakshi Sardha Singh
Sara a. hamza.
2 Department of Obstetrics and Gynecology, Millennium Pregnancy and Gynecology Center, Reston, VA, USA
Patient: Female, 39-year-old
Final Diagnosis: Cutaneous endometriosis
Symptoms: Blood mixed fluid from left border of Cesarean scar mass • pain and discoloration around incision line
Medication: —
Clinical Procedure: Excision of the mass
Specialty: Obstetrics and Gynecology
Rare disease
Background:
Endometriosis is a unique entity described in ample literature as the decidualization of endometrial tissues under the influence of gynecological hormones outside the uterine cavity. The post-surgical presence of ectopic endometrial tissue on the skin is known as abdominal wall endometriosis, cutaneous endometriosis, or scar endometriosis. Iatrogenic implantation of detached endometrial tissues at the incision site is the most widely accepted theory for this rare monad. The unspecific scar endometriosis presentation makes it challenging to diagnose. Moreover, it can easily be confused with hematoma, hernia, lipoma, abscess, scar granuloma, and tumor. Here, we report and discuss a rare case of scar endometriosis with various available treatment modalities.
Case Report:
We delineate a case of a 39-year-old woman with abdominal wall cutaneous endometriosis. An “inverted T” incision opened the abdominal and uterine cavity as it was a problematic preterm breech in labor. After an uneventful postoperative and postpartum period, she presented with a painful, discolored nodular mass of approximately 3 cm in diameter at the left border of the cesarian scar, developed over 1.5 years, often accompanied by drainage of brownish discharge. Ultrasonography with color Doppler showed a hypoechoic lesion with internal vascularity, corroborated our preliminary diagnosis of scar endometriosis, which was further confirmed by surgical excision and histopathology.
Conclusions:
A proper surgical resection is the standard treatment line for scar endometriosis. However, patients need regular follow-up to look for recurrences, even after treatment. Further studies are recommended to establish factors associated with cutaneous endometriosis recurrence.
A proliferation of endometrial-like tissues outside of the uterus that bleeds and thickens with each menstrual cycle is acknowledged as endometriosis [ 1 ], affecting 10–15% of all potential childbearing women [ 1 , 2 ]. Patients with previous cesarean sections have an enormous impact on the incidence abdominal wall endometriosis. Transplantation and implantation of the endometrium during cesarean delivery are considered to promote scar endometriosis [ 3 ]. Cutaneous endometriosis is divided into primary cutaneous endometriosis and secondary cutaneous endometriosis [ 4 ]. Spontaneous change in specific tissues under unknown factors is considered the etiology for primary cutaneous endometriosis, with a reported incidence of 0.5–1% [ 5 ]. However, iatrogenic factors are responsible for secondary cutaneous endometriosis. The reported incidence of secondary cutaneous endometriosis is about 3.5% in patients who undergo gynecological surgery and about 0.8% in all women with a previous cesarean section [ 6 ]. In the gynecological literature, scar endometriosis accounts for 0.03% to 0.15% of all cases of endometriosis [ 7 ]. The varied presentation, such as pain, discoloration, and swelling around a Pfannenstiel skin incision, results in a superfluous course of action leading to a deferred diagnosis and exorbitant referrals. We describe a case of cutaneous endometriosis and present a literature review, which may help reduce the emotional and physical distress of patients.
Case Report
We report the rare case of a 39-year-old woman seen in consultation for a painful lower abdominal nodular mass with skin discolorations at and around the abdominal incision site. She was a healthy-looking woman who underwent an emergency cesarean section 1.5 years ago for preterm pregnancy with breech presentation in active labor. Because of a problematic preterm breech, incision on the skin, and the unformed lower uterine segment, we converted to an “inverted T” incision. After an uneventful hospital stay and unremarkable postpartum follow-up at 6 weeks, she started having pain on the left side of the incision after 4 months of the surgery. She indicated that the severity of pain and tenderness was constant and was 3 out of 10 on the pain scale on most of the days, but doubled around menses and followed a cyclic pattern every month for the previous few months, often accompanied by red-colored fluid coming from the incision site. Physical examination revealed a non-mobile, nodular, moderately pigmented area of approximately 2×3 cm at the incision’s left lateral border. Palpation of the mass exhibited exquisite point tenderness. After ruling out differentials, a preliminary diagnosis of cutaneous endometrioma was considered. It was further substantiated by soft-tissue ultrasound utilizing a linear high-frequency transducer with color Doppler evaluation. A 2×1.3×2.2 cm irregular hypoechoic solid mass partially projecting into subcutaneous tissues, with internal vascularity noticed in the area of palpable concern ( Figure 1 ).

The transabdominal imaging pelvic ultrasound showing an irregular hypoechoic solid mass of 2×1.3×2.2 cm, partially projecting into the subcutaneous tissues.
We planned to explore and resect the abdominal mass to confirm cutaneous endometriosis. Extensive fibrosis of abdominal tissue around the scar was noticed, which was excised entirely, including the nodular portion for histopathological examination. The final histopathology report revealed “endometriosis involving fibro-adipose tissue with dense fibrous scarring” ( Figure 2 ). Molecular biology studies showed that increased expression of estrogen receptors, increase in local growth factors, and staining with anti-CD 10 (classification determinant 10) can better demonstrate cutaneous endometriosis in the proliferative phase. In our case, we did not perform because of its confirmed histopathological findings and cost-effectiveness. The patient is in follow-up, and the stitch line has healed without any recurrence.

Hematoxylin and eosin photomicrograph ×20 magnification showed tissue surrounding benign endometrial glands and stroma consistent with endometriosis, showing hemorrhage at the center of the cystic space.
Endometriosis is a chronic pathology characterized by the presence of endometrial tissue outside the uterus. The most common implantation site is in the ovaries or tubes, but it can be located throughout the body. Endometriotic implants located in the skin are known as “cutaneous endometriosis.” Within this type, we distinguish primary cutaneous endometriosis without a history and secondary cutaneous endometriosis, which occurs after surgical operations. Since it is usually located in the scars of these interventions, it is also known as scar endometriosis.
The most common presentation of cutaneous endometriosis is a triad of non-malignant abdominal mass, recurring pain with menses, and previous history of abdominal surgery. The degree of pain and dimensions of scar endometriosis vary with the menstrual cycle [ 8 ]. The average reported duration between cesarean section surgery and the onset of symptoms is 3.7–4.5 years.
Established risk factors for abdominal wall endometriosis are low body mass index, nulliparity, early menarche, late meno-pause, and the presence of endometriosis in a first-degree relative. Khan et al [ 9 ] demonstrated that patients with abdominal wall endometriosis who had higher parity and body mass index tended to present with more cyclic pain than controls. However, our case was para 2 with an average body mass index. The pathogenesis of cutaneous endometriosis may be explained by the metaplasia theory, embryonic rest theory, or transport theory. Our case also suggested iatrogenic implantation of endometrial tissue that escaped through an emergency cesarean incision and seeded into the edge of the corresponding abdominal wall. Careful history taking and a diligent examination supported by conventional imaging are pivotal for preoperative diagnosis. However, only after excision, the concluding diagnosis is begotten. In our case, clinical presentation and experience confirmed the preliminary diagnosis.
A spectrum of differentials, such as infections at the scar site, abscess, stitch granuloma, keloid, hematoma, desmoid tumor, lymphadenopathy, and benign (neuroma) and malignant growths (melanoma), culminate in a high rate of misdiagnosis, leading to unnecessary procedures with increased distress among patients.
Most non-invasive diagnostic methods performed, such as ultrasound with or without color Doppler, computed tomography scan, and magnetic resonance imaging, may help in the divergent diagnosis but are often inconclusive. Ultrasound and computed tomography are indeterminate for the nature of the lesion. Moreover, contrast computed tomography and magnetic resonance imaging can discern hemorrhagic signals. High spatial resolution magnetic resonance imaging can be more helpful in localizing small endometriotic spots and better differentiate between planes of muscles and abdominal subcutaneous tissue [ 10 ]. Some authors described dermoscopy as a valuable, non-invasive, and economical emerging tool for diagnosing cutaneous endometriosis. It describes a homogenously red-pigmented area containing small red globular structures corresponding to irregular endometriotic glands using epiluminescence microscopy [ 11 ].
Fine-needle aspiration cytology is a valuable invasive diagnostic tool. Ultrasound-guided fine-needle biopsy is a valuable and economical technique, as assessed by Medeiros et al [ 12 ]. However, fine-needle aspiration in the diagnosis of scar endometriosis is controversial, as this may cause nucleation of the endometriotic tissue in new areas, further aggravating the condition [ 13 ].
The use of progestogens, oral contraceptive pills, and danazol provides partial relief of symptoms. Gonadotropin agonists provide fast pain relief but do not alter the disease [ 14 ]. Recurrence is often noticed in patients on hormonal treatment, requiring close follow-up and excision in case of failure. The reported postoperative recurrence rate is reported to be 1.5–9.1% [ 15 ]. Malignancy should be suspected in case of incessant cutaneous endometriosis. However, the details of malignant transfiguration of benign cutaneous endometriosis are unclear. The causes of malignant transformation of endometriosis are also unclear, but they appear to involve genetic, immunologic, and hormonal factors.
Cryoablation, intra-lesion alcohol injection, or wide local excision of the lesion were found to be helpful in some cases [ 16 , 17 ]. However, for diagnostic as well as curative purposes, surgical excision remains the most effective treatment for cutaneous endometriosis. Excision should include standard tissue 1 cm away from the solid endometriotic tissue and may require the use of a polypropylene mesh to prevent incisional hernia. The recurrence rate is low in patient who undergo surgical excision of the lesion. In a study conducted by Lopez-Soto et al [ 18 ], out of 33 women who underwent cutaneous endometriosis treatment, only 3 (9%) had a recurrence. Our patient is in follow-up, and no recurrence has been reported to date for the previous 5 months.
With an increase in the cesarean section rate, cases of cutaneous endometriosis could be prevented by following simple measures. When dry or wet, swabs are used to clean during or after a cesarean section; quick removal of these swabs from the operation site is suggested to prevent inoculation of the endometriotic tissue. Many obstetricians prefer abdominal compresses as a physical barrier between the subcutaneous tissue and the skin to protect the surgical margins from the excavated uterine cavity. Moreover, some surgeons avoid reusing surgical tools used earlier during the operative procedure, such as needle holders and forceps, and suture materials, while closing abdominal wall layers. Many preferred to irrigate the incisional site vigorously with a high saline jet before the abdominal closure to ensure clearing all dead space in the subcutaneous area [ 19 ].
Conclusions
The diagnosis of cutaneous endometriosis can be made with ultrasound, medical history, or examination, but the definitive diagnosis is by pathology. Surgical removal of the exogenous endometriotic tissue is a prompt treatment that can improve quality of life. The increased cesarean section rate has amplified the chances of finding cutaneous endometriosis. Therefore, education to raise awareness among obstetricians is required to prevent cutaneous endometriosis.
Acknowledgments
We thank Jennifer Leidy MD (Department of Surgical Pathology, Inova Central Lab, Fairfax) and Karan Lofti MD (Women’s Imaging Center, Reston) for providing histopathological and radiological assessments, respectively.
Department and Institution Where Work Was Done
Department of Obstetrics and Gynecology, Inova Fairfax Women’s Hospital and Millennium Pregnancy and Gynecology Center, Reston, VA, USA.
Declaration of Figures Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- Open access
- Published: 14 May 2024
Pregnancy-related complications in patients with endometriosis in different stages
- Khadijeh Shadjoo 1 ,
- Atefeh Gorgin 2 ,
- Narges Maleki 2 ,
- Arash Mohazzab 3 ,
- Maryam Armand 2 ,
- Atiyeh Hadavandkhani 2 ,
- Zahra Sehat 2 &
- Aynaz Foroughi Eghbal 4
Contraception and Reproductive Medicine volume 9 , Article number: 23 ( 2024 ) Cite this article
441 Accesses
1 Altmetric
Metrics details
Endometriosis is one of the most common and costly diseases among women. This study was carried out to investigate pregnancy outcomes in women with endometriosis because of the high prevalence of endometriosis in reproductive ages and its effect on pregnancy-related complications outcomes.
This was a cross-sectional study performed on 379 pregnant women with endometriosis who were referred to the endometriosis clinic of the Avicenna Infertility Treatment Center from 2014 to 2020. Maternal and neonatal outcomes were assessed for the endometriosis group and healthy mothers. The group with endometriosis was further divided into two groups: those who underwent surgery and those who either received medication alone or were left untreated before becoming pregnant. The analysis of the data was done using SPSS 18.
The mean age of the patients was 33.65 ± 7.9 years. The frequency of endometriosis stage ( P = 0.622) and surgery ( P = 0.400) in different age groups were not statistically significant. The highest rates of RIF and infertility were in stages 3 ( N = 46, 17.2%) ( P = 0.067), and 4 ( N = 129, 48.3%) ( P = 0.073), respectively, but these differences were not statistically different, and the highest rate of pregnancy with ART/spontaneous pregnancy was observed in stage 4 without significant differences ( P = 0.259). Besides, the frequency of clinical/ectopic pregnancy and cesarean section was not statistically different across stages ( P > 0.05). There is no significant relationship between endometriosis surgery and infertility ( P = 0.089) and RIF ( P = 0.232). Most of the people who had endometriosis surgery with assisted reproductive methods got pregnant, and this relationship was statistically significant ( P = 0.002) in which 77.1% ( N = 138) of ART and 63% ( N = 264) of spontaneous pregnancies were reported in patients with endometriosis surgery. The rate of live births (59.4%) was not statistically significant for different endometriosis stages ( P = 0.638). There was no stillbirth or neonatal death in this study. All cases with preeclampsia ( N = 5) were reported in stage 4. 66.7% ( N = 8) of the preterm labor was in stage 4 and 33.3% ( N = 4) was in stage 3 ( P = 0.005). Antepartum bleeding, antepartum hospital admission, preterm labor, gestational diabetes, gestational hypertension, abortion, placental complications and NICU admission were higher in stage 4, but this difference had no statistical difference.
Endometriosis is significantly correlated with infertility. The highest rates of RIF and infertility are observed in stages 3 and 4 of endometriosis. The rate of pregnancy with ART/spontaneous pregnancy, preterm labor, preeclampsia and pregnancy-related complications is higher in stage 4. Most of the people who had endometriosis surgery with assisted reproductive methods got significantly pregnant. Clinical/ectopic pregnancy, cesarean sections, and live birth were not affected by the endometriosis stages.
Introduction
The presence of endometrial-like glandular tissue, stroma, or endometrial tissue outside the uterine cavity is known as endometriosis, a chronic gynecological disease that affects 30 to 50% of infertile women [ 1 ]. Endometriosis commonly affects various parts of the female reproductive system, including the pelvic area, ovaries, posterior cul-de-sac, uterine ligaments, pelvic peritoneum, rectovaginal septum, cervix, vulva, vagina, as well as the intestines and urinary system. Endometriosis can cause symptoms like infertility, dysmenorrhea, and chronic pelvic inflammatory disease, which can worsen pain, dyspareunia, and painful bowel movements, ultimately lowering the quality of life for the affected woman [ 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 ]. Laparoscopic surgery is both the standard surgical procedure and the best treatment for endometriosis [ 14 ]. However, endometriosis remains a problematic issue due to its negative impact on ovarian reserve and the recurrence rate of 40–50% after 5 years of surgery [ 15 , 16 ]. Numerous studies have shown the negative effects of endometriosis on pregnancy, including the increase in preterm labor, placental abruption and cesarean delivery, preeclampsia, placental problems and postpartum hemorrhage, premature rupture of membranes (PROM), preterm birth, small for gestational age (SGA), NICU admission, neonatal mortality and morbidity, and hypertensive disorders of pregnancy (HDP) with low birth weight (LBW) [ 2 , 5 , 6 , 17 ]. Since the effects of endometriosis on the course of pregnancy are still controversial, this work aimed to first identify the negative effects of endometriosis on pregnancy and then determine whether laparoscopic surgery or other drug interventions before pregnancy were beneficial.
Materials and methods
This cross-sectional study was carried out on 379 pregnant women with a history of endometriosis and pregnancy who were referred to the endometriosis clinic of the Avicenna Infertility Treatment Center between January 2014 and January 2020. This study was approved by the Ethics Committee of Avicenna Infertility Treatment Center (IR.ACECR.AVICENNA.REC.1398.031) in accordance with the tents of the Declaration of Helsinki, and the patient’s oral and written consent was obtained to ensure that they participated in the study voluntarily. Specific means of identifying endometriosis were approved after laparoscopic surgery with pathologic confirmation, magnetic resonance imaging (MRI), ultrasound imaging, and clinically confirmed presence of symptoms. Exclusion criteria were less than 22 weeks of gestation at the time of delivery, fetal malformations, and incomplete medical files. Maternal and neonatal outcomes were assessed for the endometriosis group and healthy mothers. The group with endometriosis was further divided into two groups: those who underwent surgery and those who either received medication alone or were left untreated before becoming pregnant. A history of laparoscopic surgery or other surgeries and hormonal therapies (oral contraceptive pills, progestin, and gonadotropin-releasing hormone agonists) were obtained from the patient’s medical files. Maternal characteristics in this study included maternal age, parity, pre-pregnancy weight and BMI, pre-pregnancy blood pressure, chronic hypertension, diabetes mellitus (DM), cholestasis, and assisted reproductive technology (ART). Outcomes evaluated included gestational age, ectopic pregnancy, clinical pregnancy, mode of delivery, antepartum hemorrhage, antepartum hospitalization, preterm labor (< 37 weeks of gestation), labor dystocia, gestational diabetes mellitus (GDM), gestational hypertension, gestational cholestasis, placental abruption and placenta previa, PROM, and abortion. Neonatal characteristics included birth weight, height, SGA, stillbirth, neonatal death, and NICU admission.
Statistical analysis
The data were analyzed using SPSS 18. Normality was checked using the Kolmogorov-Smirnov test. Continuous variables with a normal distribution were summarized as mean and standard deviation and compared between the two groups using an independent t-test. Categorical variables were presented as frequency and percentage to be compared between the two groups using either the Fisher’s exact test or the chi-square ( x 2 ) test. The significance level was defined as p < 0.05.
During the study period, all 379 women with a mean age of 33.65 ± 7.9 years underwent treatment and were followed until a negative pregnancy test or the end of the pregnancy. The mean marriage duration was 9.72 ± 4.71 years. In this study, 16.1% of the people were in the age group of 25–30 years, 35.6% were in the age group of 30–35 years, and the rest (92.3%) belonged to the age group of more than 40 years. The age group with the highest number of surgeries for endometriosis is 35–40 years. The age group of 25–30 years experiences the highest incidence of stage 1 endometriosis, while the age group of 30–35 years has the highest occurrence of stage 2. Additionally, the age group of 30–35 years also has the highest number of individuals with stage 3, while the age group of 35–40 years has the highest number of people with stage 4 (Table 1 ). The majority of patients in stage 4 needed surgery (89.9%) (Table 2 ).
According to the information in Table 3 , the highest rate of RIF and infertility was in stage 3 ( N = 46, 17.2%) ( P = 0.067), and 4 ( N = 129, 48.3%) ( P = 0.073), respectively but these differences were not statistically significant. Also, the highest rate of pregnancy with ART/spontaneous pregnancy was observed in stage 4 without significant differences ( P = 0.259). Besides, the frequency of clinical/ectopic pregnancy and cesarean sections was not statistically different across stages ( P > 0.05) (Table 4 ).
There is no significant relationship between endometriosis surgery and infertility ( P = 0.089) and RIF ( P = 0.232). Most of the people who had endometriosis surgery with assisted reproductive methods got pregnant, and this relationship was statistically significant ( P = 0.002) in which 77.1% ( N = 138) of ART and 63% ( N = 264) of spontaneous pregnancies were reported in patients with endometriosis surgery (Table 3 ).
The rate of live births (59.4%) was not statistically significant by different endometriosis stages ( P = 0.638) (Table 5 ).
There was no stillbirth or neonatal death in this study. All cases with preeclampsia ( N = 5) were reported in stage 4. Additionally, 66.7% ( N = 8) of the preterm labor were in stage 4 and 33.3% ( N = 4) were in stage 3 in which this difference was statistically significant ( P = 0.005). Antepartum bleeding (70%), antepartum hospital admission (75.9%), preterm labor (66.7%), gestational diabetes (80%), gestational hypertension (85.7%), abortion (71.4%), placental complications (66.7%) and NICU admission (71%) were higher in stage 4 but this difference had no statistical difference (Table 6 ).
Women with endometriosis have lower fertility rates than ever before, but many of them are still able to give birth because of advancements in IVF and intracytoplasmic sperm injection (ICSI) technology. This cross-sectional research was conducted to examine maternal and neonatal outcomes in endometriosis patients with a history of pregnancy referred to the Avicenna Infertility Treatment Center between January 2014 and January 2020. Patients with endometriosis had a live birth rate of 54.9% Endometriosis is a common cause of infertility, and ART can help patients become pregnant. Despite these interventions, some studies have shown poor pregnancy outcomes in patients with endometriosis. Poor oocyte and embryo quality and impaired endometrial receptivity have been suggested as potential causes of poor clinical outcomes. Burghaus et al. Endometriosis risk factors have been identified as age at menarche, length of each menstrual cycle, length of menstrual years, number of pregnancies, miscarriages, and smoking [ 7 ].
Hardiman et al. concluded that premenstrual spotting lasting more than two days is significantly associated with endometriosis, with a higher predictive rate than painful menstruation and painful intercourse [ 8 ]. It may be more difficult to distinguish between the effects of endometriosis on pregnancy complications and the assisted reproductive process if many endometriosis-affected women use ART techniques during their pregnancies [ 9 ]. According to studies, there is no established association between endometriosis and preeclampsia, meaning that some studies report an increased risk of preeclampsia after endometriosis, while other research reports no change and other research reports a decreasing pattern [ 5 ].
Pérez-López et al. found a significant association between endometriosis and gestational diabetes mellitus [ 10 ]. Maggiore et al. found in 2016 that there is a significant connection between endometriosis and placenta previa. Furthermore, this association is not related to spontaneous insemination or laboratory-assisted reproductive techniques and occurs in both cases. In this context, fetal malformations and cesarean sections can be attributed to placenta previa [ 11 ]. There is a significant association between endometriosis, and cesarean sections and low birth weight in spontaneous fertilization, but no association has been found in ART pregnancies [ 6 ]. Also, Lim et al. found that women diagnosed with endometriosis exhibited a significantly higher incidence of unfavorable pregnancy outcomes in comparison to their counterparts who did not have endometriosis. These unfavorable outcomes associated with endometriosis encompassed preterm labor, preterm birth, preeclampsia, fetal growth restriction, placenta previa, placental abruption, stillbirth, antepartum, and postpartum bleeding. Furthermore, they also demonstrated an augmented risk of blood transfusion, uterine artery embolization, and cesarean hysterectomy in the group of women with endometriosis as opposed to the group without this condition [ 18 ]. Besides, Miura et al. disclosed that there was a heightened incidence of postpartum hemorrhage and placenta previa in the group diagnosed with endometriosis. Nonetheless, the other maternal and neonatal consequences exhibited no significant disparity among patients with/without endometriosis [ 19 ]. Borisova et al. reported that even though patients with endometriosis may achieve pregnancy after undergoing assisted reproductive technologies, they still face a significantly elevated risk of obstetric complications. These complications include, but are not limited to, miscarriage, preterm birth, preeclampsia, placental abnormalities, hemorrhage during labor, the birth of infants who are small for their gestational age, stillbirth, and a higher incidence of cesarean section. Furthermore, it is important to note that acute complications specific to endometriosis can manifest during pregnancy, and in most cases, surgical intervention becomes necessary to address this condition [ 20 ].
Based on the aforementioned studies, the findings of our study were consistent in the majority of respects, and the novelty of our investigation lies in the evaluation of various stages of endometriosis, which holds significance as a considerable number of patients seek the assistance of pertinent clinics during the final stages. Consequently, understanding the adverse effects at the stage of interest can provide clinicians with valuable insights into effectively addressing the patients’ status.
Endometriosis is significantly correlated with infertility. The highest rates of RIF and infertility are observed in stages 3 and 4 of endometriosis. The rate of pregnancy with ART/spontaneous pregnancy, preterm labor, preeclampsia, and pregnancy-related complications is higher in stage 4. Most of the people who had endometriosis surgery with assisted reproductive methods got significantly pregnant. Clinical/ectopic pregnancy, cesarean sections and live birth were not affected by endometriosis stages.
Data availability
The data used in this study can be send after formal and reasonable request to the corresponding author.
Kadivar M, Vafa A, Farahzadi A, Khani S. 6 years evaluation of prevalence of abdominal wall endometriosis in patients with definite histopathological diagnosis of endometriosis admitted in Rasool-Akram, Shariati and Atieh hospitals in Tehran. Razi J Med Sci. 2012;18:20–6.
Google Scholar
Mekaru K, Masamoto H, Sugiyama H, Asato K, Heshiki C, Kinjyo T, et al. Endometriosis and pregnancy outcome: are pregnancies complicated by endometriosis a high–risk group? Eur J Obstet Gynecol Reprod Biol. 2014;172:36–9. https://doi.org/10.1016/j.ejogrb.2013.10.024 .
Article PubMed Google Scholar
Williams HE, Barsky S, Storino W. Umbilical endometrioma (silent type). Arch Dermatol. 1976;112:1435–36.
Article CAS PubMed Google Scholar
Thomas EJ, Rock J, editors. Modern approaches to endometriosis. New York: Springer Science & Business Media; 2012.
Brosens IA, Sutter P, De, Hamerlynck T, Imeraj L, Yao Z, Cloke B, Brosens JJ, Dhont M. Endometriosis is associated with a decreased risk of pre-eclampsia. Hum Reprod. 2007;22:1725–9.
Lalani S, Choudhry AJ, Firth B, Bacal V, Walker M, Wen SW, Singh S, Amath A, Hodge M, Chen I. Endometriosis and adverse maternal, fetal and neonatal outcomes, a systematic review and meta-analysis. Hum Reprod. 2018;33(10):1854–65.
Article CAS PubMed PubMed Central Google Scholar
Burghaus S, Klingsiek P, Fasching P, Engel A, Haeberle L, Strissel P, et al. Risk factors for endometriosis in a German case–control study. Geburtshilfe Frauenheilkd. 2011;71:1073.
Hardiman P, Pillay OS, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003;361:1810–12.
Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, et al. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. 2016;22:104–15. https://doi.org/10.1093/humupd/dmv044 .
Pérez-López FR, Martínez-Domínguez SJ, Viñas A, Pérez-Tambo R, Lafita A, Lajusticia H, Chedraui P. Endometriosis and gestational diabetes mellitus risk: a systematic review and meta-analysis. Gynecol Endocrinol. 2017; 0:1–7. Informa UK Ltd.
Maggiore ULR, Ferrero S, Mangili G, Bergamini A, Inversetti A, Giorgione V, Viganò P, Candiani M. A systematic review on endometriosis during pregnancy: diagnosis, misdiagnosis, complications and outcomes. Hum Reprod Update. 2016;22:70–103.
Article Google Scholar
Kennedy S, Bergqvist A, Chapron C, D’Hooghe T, Dunselman G, Greb R, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20(10):2698–704.
Parasar P, Ozcan P, Terry KL. Endometriosis: epidemiology, diagnosis and clinical management. Curr Obstet Gynecol Rep. 2017;6(1):34–41.
Article PubMed PubMed Central Google Scholar
Catalano GF, Marana R, Caruana P, Muzzi L, Mancuso S. Laparoscopic versus microsurgery by laparotomy for excision of ovarian cysts in patients with moderate or severe endometriosis. J Am Assoc Gynecol Laparosc. 1996;3:267–70.
Dunselman GAJ, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, et al. ESHRE guideline: management of women with endometriosis. Hum Reprod. 2014;29(3):400–12.
Guo SW. Recurrence of endometriosis and its control. Hum Reprod Update. 2009;15(4):441–61.
Mayo M, Takafumi U, Kenji I, Jingwen W, Yoshinori M, Tomoko N-K, Satoko O… and, Tomomi K. Adverse effects of endometriosis on pregnancy: a case-control study. BMC Pregnancy Childbirth. 2019;19:373–80.
Lim HJ, Sun J, Min B, Song M, Kim TH, Kim BJ, Hwang KR, Lee TS, Jeon HW, Kim SM. Endometriosis and adverse pregnancy outcomes: a Nationwide Population-based study. J Clin Med. 2023;12(16):5392.
Miura M, Ushida T, Imai K, Wang J, Moriyama Y, Nakano-Kobayashi T, Osuka S, Kikkawa F, Kotani T. Adverse effects of endometriosis on pregnancy: a case-control study. BMC Pregnancy Childbirth. 2019;19:1–7.
Article CAS Google Scholar
Borisova AV, Konnon SRD, Tosto V, Gerli S, Radzinsky VE. Obstetrical complications and outcome in patients with endometriosis. J Matern Fetal Neonatal Med. 2022;35(14):2663–77.
Download references
Acknowledgements
We acknowledge all staffs who worked in Avicenna Fertility Center for their great help to perform this study.
Author information
Authors and affiliations.
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
Khadijeh Shadjoo
Infertility Clinic, Avicenna Research Institute, ACECR, Tehran, Iran
Atefeh Gorgin, Narges Maleki, Maryam Armand, Atiyeh Hadavandkhani & Zahra Sehat
Department of Epidemiology, School of Public Health, Iran University of Medical Sciences, Tehran, Iran
Arash Mohazzab
Urmia University of Medical Sciences, Urmia, Iran
Aynaz Foroughi Eghbal
You can also search for this author in PubMed Google Scholar
Contributions
KS and AG wrote the main manuscript text and NM, AM and AFE prepared the data. AM, MA, AHK and ZS analyzed the data and prepared their interpretation. All authors contributed in the writing of the draft. All authors reviewed the manuscript before submission.
Corresponding author
Correspondence to Khadijeh Shadjoo .
Ethics declarations
Ethics approval and consent to participate.
The research conducted in accordance with the tents of the Declaration of Helsinki. The present study was approved by the ethical committee of Avicenna Research Institute, Tehran, Iran. Written informed consent was obtained from all the participants (IR.ACECR.AVICENNA.REC.1398.031).
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Shadjoo, K., Gorgin, A., Maleki, N. et al. Pregnancy-related complications in patients with endometriosis in different stages. Contracept Reprod Med 9 , 23 (2024). https://doi.org/10.1186/s40834-024-00280-0
Download citation
Received : 11 February 2024
Accepted : 01 April 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s40834-024-00280-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Endometriosis
- Pregnancy complication
Contraception and Reproductive Medicine
ISSN: 2055-7426
- General enquiries: [email protected]

'Treating my endometriosis costs me more than $27,000 a year'
Bridget Armstrong estimates treating her endometriosis has cost her $82,000 in the last three years.
The 35-year-old was diagnosed in 2019 after years of experiencing excruciating pain, bleeding and bloating since her period began in her first year of high school.
Armstrong explained to 9news.com.au that the road to her diagnosis was not only lengthy, but one that had impacted her employment and financial security.
READ MORE: Australian celebrity chef Kylie Kwong to close restaurant
"I lost a job when I was studying at university because of the amount of sick leave I needed to take due to endometriosis," she told 9news.com.au.
"It took me 15 years to be diagnosed with significant stage three endometriosis in my uterus, arteries and around my bladder."
As cost of living pressures increase across all Australian households, Armstrong is noticing a significant blow to her hip pocket.
She estimates she's lost approximately $75,000 of income in the last three years due to her endometriosis in the form of unpaid sick leave or inability to work.
And because Armstrong can only work part-time around 59 per cent of her earnings are absorbed by her rent.
"The cost of medical imaging, the doctors appointments, even the cost of transport getting there, my Mirena, it all adds up," she said.
The best treatment for Armstrong's endometriosis is laparoscopic surgery, however this means Armstrong has to take two weeks off work to recover.
The surgery also leaves her hundreds of dollars out of pocket despite having private health insurance.
"I had to fork out for in $500 excess fees on top, I had to stay another three days because of complications," she said.
Armstrong said for her second surgery she had opted for a public hospital and while it did save her some money, she still had to pay hundreds of dollars for an MRI.
"You don't get anything back from that," she said.
Apart from surgery, there is only one class of medication to treat pelvic pain related to endometriosis - they include hormone treatments such as the contraceptive pill, implant or intrauterine device (IUD).
Newer, better contraceptive options that come with fewer side effects are not covered by the Pharmaceutical Benefits Scheme (PBS) and can cost up to triple the amount compared to their older counterparts.
Armstrong also lives with another chronic condition and as a result, she is unable to take oral contraception due to an increased risk of blood clotting, a less common side-effect associated with oestrogen.
Instead, she has to use a hormonal IUD called the Mirena, she said the device itself and the procedure cost her around $230 all up.
"Every year I am losing approximately $27,343 as a direct result of endometriosis," she said.
"This amount would be much higher if I had elected to have my second laparoscopic surgery in a private hospital.
"Even with private health insurance, the out-of-pocket expenses... are not possible for me."
READ MORE: Australia's public holidays for 2024 and 2025: Make the most of your annual leave
Mike Armour, an associate professor in reproductive health at Western Sydney University, told 9news.com.au there were significant direct and indirect costs associated with treating endometriosis and complex pelvic pain.
Armour's research has found that people with endometriosis and chronic pelvic pain spend about $400 a month on allied health, while a previous study he published in 2019 found endometriosis can cost a person up to $30,900 a per year.
"One of the things we can do is try to reduce those indirect costs such as supporting people at work, by allowing people to stay in work, not having them use all their hours, or sick leave that can help people potentially protect themselves against the cost of living," he said.
"The other thing is making sure health funds cover the common interventions of people with endometriosis.
"And governments need to be really mindful of any effective medications that need to be put on the PBS to help reduce costs."
The federal government last week announced $49.1 million in funding to assist women with endometriosis and other gynaecological conditions, including chronic pelvic pain and polycystic ovary syndrome (PCOS).
The investment will allow for extended consultation times and higher rebates for specialist gynaecologist appointments under the Medicare Benefits Schedule.
This means Medicare subsidies will lift to $168.60 for a minimum of 45 minutes during a longer initial gynaecologist consultation, compared to the usual rate of $95.60.
Medicare will almost double its cover for follow-up consultations increasing subsidies to $84.35 for a minimum of 45 minutes, compared to the usual rate of $48.05.
The government has also committed to providing 430,000 more services to help people across the country with complex gynaecological conditions.
Armstrong told 9news.com.au the federal government's latest investment into endometriosis and pelvic pain was "a step in the right direction".
"Anything that benefits the patient will make a difference," she said.
"I hope that GPs will also have access to the Endometriosis Living Guidelines so that they can be led by best practice evidence-based information."

- Type 2 Diabetes
- Heart Disease
- Digestive Health
- Multiple Sclerosis
- Diet & Nutrition
- Supplements
- Health Insurance
- Public Health
- Patient Rights
- Caregivers & Loved Ones
- End of Life Concerns
- Health News
- Thyroid Test Analyzer
- Doctor Discussion Guides
- Hemoglobin A1c Test Analyzer
- Lipid Test Analyzer
- Complete Blood Count (CBC) Analyzer
- What to Buy
- Editorial Process
- Meet Our Medical Expert Board
How SSRIs Affect Periods
- SSRIs and Periods
- Which Affect Periods More?
- Tapering Off of SSRIs
- Side Effects
- Other Factors
- Antidepressants and PMDD
Selective serotonin reuptake inhibitors (SSRIs) are a class of antidepressants that can affect menstrual (period) bleeding, with side effects such as missed periods or heavy bleeding. Examples of common SSRIs are Celexa (citalopram), Prozac (fluoxetine), Lexapro (escitalopram), and Zoloft (sertraline).
Scientists are still determining why and how SSRIs affect menstruation, but these drugs appear to have an impact on certain hormones.
In this article, learn more about how SSRIs affect periods and how to manage these changes.
Filmstax / Getty Images
Effect of SSRIs on Periods
SSRIs can affect your period in various ways; they can cause you to miss periods or have heavier bleeding than usual.
One study found that menstrual disorders were more common among people who take antidepressants (24.6%) compared to people who do not take antidepressants (12.2%).
Delayed or Absent Periods
Some people taking SSRIs may experience delayed or absent periods ( amenorrhea ).
Both Zoloft, whose main ingredient is sertraline, and Lexapro , a brand of escitalopram, have been associated with amenorrhea. However, most of the research on this topic comprises case studies of individuals, so it is hard to draw conclusions and seems to be a rare occurrence.
Researchers theorize that antidepressants are linked to amenorrhea due to the hormone prolactin , which helps regulate the menstrual cycle (among other functions).
SSRIs can cause abnormally high levels of prolactin ( hyperprolactinemia ) and lead to amenorrhea. However, the link between antidepressants and amenorrhea due to prolactin is weak; more research is needed.
Heavy Bleeding
You can also experience heavier-than-normal periods due to SSRIs.
Multiple case studies have linked Prozac , whose main ingredient is fluoxetine, with heavy menstrual bleeding. In these case reports, new heavy menstrual bleeding occurred while using fluoxetine but subsided when the medication was discontinued.
Intermenstrual Bleeding
There are also some reports of SSRIs and intermenstrual bleeding ( metrorrhagia ), which is vaginal bleeding that occurs outside the expected period.
One case study of a 34-year-old woman who started Zoloft found that she had sudden, mid-cycle vaginal bleeding on her third day of treatment. Her bleeding stopped within 24 hours of discontinuing Zoloft.
In another case study of a 54-year-old woman who was postmenopausal (had gone through menopause, the time in which menstrual periods have stopped for 12 straight months) and started Lexapro, she experienced heavy vaginal bleeding for a week, which subsided a few days after discontinuing Lexapro.
Types of SSRIs Most Likely to Affect Periods
Antidepressants affecting your period is a relatively uncommon occurrence, although additional large-group studies are needed to fully understand its impact, including the risks associated with each type of SSRI or serotonin-norepinephrine reuptake inhibitor (SNRI).
The most extensive study to date on SSRIs and abnormal bleeding was a 2012 study that examined 1,432 women, with a control group and an antidepressant group.
In that study, menstrual disorders were most associated with the following antidepressants :
- Effexor XR ( venlafaxine )
- A combination of the above with Remeron (mirtazapine)
Not everyone who takes SSRIs will experience a change in their periods. In the previous study, the incidence of antidepressant-induced menstrual disorders was 14.5%.
Coming Off SSRIs and Menstruation Effects
If you're experiencing abnormal uterine bleeding, consult with your prescribing healthcare provider. Coming off SSRIs may help resolve heavy bleeding or amenorrhea, as shown in various case studies.
However, suddenly quitting antidepressants can be dangerous. Always do this under the guidance of a healthcare provider who can offer alternative medications and instruction for the safest way to lower or discontinue your dosage.
Seek Help for Depression
If you think you may be in a position to harm yourself or someone else, call 911 or your local emergency number ASAP. There are also several treatment resources and support groups that can be sought through the Substance Abuse and Mental Health Services Administration (SAMHSA) National Hotline at 800-662-HELP (4357) .
If you are having suicidal thoughts, dial 988 to contact the 988 Suicide & Crisis Lifeline and connect with a trained counselor.
How to Manage SSRI Side Effects
SSRIs have side effects beyond affecting menstrual bleeding. These include:
- Blurred vision
- Diarrhea or constipation
- Loss of appetite
- Loss of libido
- Sleep problems
Most side effects subside after a few weeks of taking the medication. If you struggle to tolerate side effects, work with your healthcare provider. They may adjust your dosage, the time of day you take your medication, or the medication itself to reduce side effects.
Benefits vs. Drawbacks
When dealing with side effects from SSRIs, it's important to consider the benefits and drawbacks of what led you to take the medication in the first place. Some people take SSRIs to cope with premenstrual dysphoric disorder (PMDD) or other female reproductive conditions such as endometriosis .
Is It My Antidepressant or Something Else?
While it is important to consider the medication you take and its possible side effects, there may be another cause of changes in your periods.
Stress, weight loss, vitamins or supplements, contraceptive pills or devices, and various conditions, such as polycystic ovary syndrome (PCOS, in which ovaries produce an abnormally high amount of androgens) and adenomyosis (tissue normally lining the uterus grows into the muscular wall of the uterus), and more, can all affect your periods.
Antidepressants and PMDD
Premenstrual dysphoric disorder is a mood disorder that occurs during the two weeks leading up to your period, known as the luteal phase of the menstrual cycle. It is more severe than premenstrual syndrome (PMS), and symptoms include depression, mood swings, hopelessness, irritability, anxiety, and more.
Antidepressants are considered a first-line treatment for PMDD and more severe cases of PMS. Unlike treatment for general depression, antidepressants can be prescribed intermittently for PMDD and PMS. This means that you take the medication during the luteal phase but not during other phases, and it can help reduce side effects and withdrawal symptoms.
Antidepressants have been linked to abnormal uterine bleeding, including missed periods, heavy periods, or bleeding between periods. However, the research on this topic is sparse and primarily limited to case studies. It is, therefore, difficult to conclude how likely an SSRI is to have this side effect or what types of SSRI are more likely than others to have this side effect.
If you are taking an SSRI and experience menstrual abnormalities, be sure to let your healthcare provider know so they can make an appropriate assessment and recommendation.
National Health Service. Overview - selective serotonin reuptake inhibitors (ssris) . December 8, 2021.
U.S. Food & Drug Administration. Selective serotonin reuptake inhibitors (SSRIs) information . 12/23/2014.
Uguz F, Sahingoz M, Kose SA, et al. Antidepressants and menstruation disorders in women: a cross-sectional study in three centers . Gen Hosp Psychiatry . 2012;34(5):529-533. doi:10.1016/j.genhosppsych.2012.03.014
Selvaraj V, Hour S, Gunasekar P, Gray C, Smith JF. Escitalopram-induced amenorrhea and false positive urine pregnancy test . Korean J Fam Med . 2017;38(1):40-42. doi:10.4082/kjfm.2017.38.1.40
Ekinci N, Güneş S, Kalinli M, Ekinci Ö. Sertraline-related amenorrhea in an adolescent . Clin Neuropharmacol . 2019;42(3):99-100. doi:10.1097/wnf.0000000000000336
Park YM. Serum prolactin levels in patients with major depressive disorder receiving selective serotonin-reuptake inhibitor monotherapy for 3 months: a prospective study . Psychiatry Investig . 2017;14(3):368-371. doi:10.4306%2Fpi.2017.14.3.368
Fourman LT, Fazeli PK. Neuroendocrine causes of amenorrhea—an update . The Journal of Clinical Endocrinology & Metabolism . 2015;100(3):812-824. doi: 10.1210/jc.2014-3344
Zhuo C, Chen G, Lin C, et al. Risk-to-befit ratios of consecutive antidepressants for heavy menstrual bleeding in young women with bipolar disorder or major depressive disorder . Front Psychiatry . 2022;13:1012644. doi:10.3389/fpsyt.2022.1012644
Türkoğlu S, Türkoğlu G. Vaginal bleeding and hemorrhagic prepatellar bursitis in a preadolescent girl, possibly related to fluoxetine . Journal of Child and Adolescent Psychopharmacology . 2015;25(2):186-187. doi:10.1089/cap.2014.0124
Shaheen M. Fluoxetine induced menorrhagia . BJPsych Open . 2023;9(Suppl 1):S128. doi:10.1192%2Fbjo.2023.358
Asan Ö, Göka E. Intermenstrual vaginal bleeding due to sertraline treatment, a case report and review of the literature . PBS . 2019;(0):1. doi:10.5455/PBS.20181006062238
Yadav A, Bharat BS, Montrose S. Abnormal uterine bleed in a postmenopausal woman with the use of escitalopram . Cureus . 2022;14(3). doi:10.7759%2Fcureus.23432
National Health Service. Stopping or coming off antidepressants . August 3, 2021.
National Health Service. Side effects - selective serotonin reuptake inhibitors (ssris) . December 8, 2021.
Reilly TJ, Wallman P, Clark I, Knox CL, Craig MC, Taylor D. Intermittent selective serotonin reuptake inhibitors for premenstrual syndromes: a systematic review and meta-analysis of randomised trials . J Psychopharmacol . 2023;37(3):261-267. doi:10.1177%2F02698811221099645
Marjoribanks J, Brown J, O’Brien PMS, Wyatt K. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Gynaecology and Fertility Group, ed . Cochrane Database of Systematic Reviews . 2013. doi:10.1002/14651858.CD001396.pub3
By Sarah Bence, OTR/L Bence is an occupational therapist with a range of work experience in mental healthcare settings. She is living with celiac disease and endometriosis.
Can music education boost grades, attendance? A new case study suggests it might

A new case study that included hundreds of Tennessee public schools suggests that music education may be tied to better math and reading scores, along with better attendance and positive social, emotional and behavioral effects.
The study, titled "Face the Music: A Case Study for Expanding Music and Arts in Schools," was the result of a joint effort between the CMA Foundation and the Mr. Holland's Opus Foundation. It examined the challenges and benefits stemming from music and arts education across 589 schools and 23 districts in Tennessee, and also included feedback from music and arts teachers, students and lifelong learners.
“Music and arts education are playing a key role in helping students catch up after the pandemic, from helping with math and reading proficiency to coping with stress and trauma. The research is clear: arts and music classes are a must-have, not a nice-to-have," CMA Foundation Executive Director Tiffany Kerns said in a news release.
The foundations behind the study hope it will serve as a model for other states to boost music and arts education at the state and district level, along with gaining support from nonprofit organizations and philanthropy.
Here are key takeaways from the study.
Related: TN high school band gets surprise invite to perform at CMA Fest as director wins award
A connection between music, arts, math and reading
Data from the majority of districts in the study showed that students enrolled in music and arts programs demonstrated significantly higher proficiency rates in math and literacy. The majority of districts also saw better attendance rates from music and arts students, compared to schoolwide averages. These outcomes align with a handful of other studies. However, the case study report did note an important caveat: Most studies on music and arts participation don't prove it's the cause of improved academics.
Here's a look at the proficiency and attendance numbers the study revealed:
- Roughly 1-in-4 school districts reported 47% of students enrolled in music and arts programs showed reading proficiency, compared to an overall school average of 36%.
- Roughly 7-in-10 school districts reported 46% of students enrolled in music and arts programs excelled in math, compared to an overall school average of 35%.
- Roughly 3-in-4 districts reported increased attendance rates for students enrolled in music and arts programs. The study noted that schools where music and arts students outpaced the school average for attendance saw an average attendance boost of 12%.
Systemic barriers to music and arts education
The case study also outlined factors that hamper access to music and arts education for students and schools. They largely align with trends reported in the most recent National Arts Education Status Report and historical data from the Tennessee Arts Education Data Project, the study said.
The barriers for students identified in the study are:
- Participation requirements, including after-school attendance and prerequisites or auditions
- Housing insecurity
- Transportation challenges
- Participation fees
- Equipment costs
The study also showed that curriculum scheduling is a primary issue facing schools. That can disrupt the ability to offer sequential music and arts classes in every grade, allowing students to explore a variety of options and build upon their skills each year.
Recommendations for action
The study mapped out a series of recommendations for how everyone from parents and students to educators and lawmakers can take action to bolster music and arts education in Tennessee.
For school community members like parents, students and educators, that can range from simply attending music and arts events to show support to advocating for more funding for programs. The study also calls on school and state leaders to partner to fund music and arts education, train teachers and develop partnerships that help sustain those programs in schools. It also challenges philanthropic organizations to fund grants, promote advocacy and awareness and take other steps to partner with and support schools.
"Each of us can support a system of change to enhance music and arts education for a more well-rounded education for our students," the study stated.
A look at the demographics and schools
The demographics of the schools that participated were similar to the state of Tennessee as a whole, but they differed slightly when it came to the population of public school students statewide. Students in the study were:
- 16% Black or African American
- 9% Hispanic or Latino
- 2% other racial identities
- 24% socioeconomically disadvantaged
- 13% engaged in special education services
- 9% English language learners
- 2% experiencing homelessness or in foster care
- 1% identified as migrants or refugees
According to 2022-23 academic year data from the Tennessee Department of Education, here's how Tennessee schools demographics broke down:
- 24% Black and African American
- 14% Hispanic
- 3% other racial identities
- 30% economically disadvantaged
- 14% students with disabilities
- 8% English learners
- Less than 1% in foster care
- 2% experiencing homelessness
- Less than 1% identified as migrants
Here's the full list of districts that participated in the case study:
- Arlington Community Schools
- Bartlett City Schools
- Benton County Schools
- Chester County School District
- Clarksville-Montgomery County School System
- Coffee County School District
- Germantown Municipal School District
- Giles County School System
- Greene County Schools
- Hamilton County Schools
- Jackson-Madison County School District
- Knox County Schools
- Lincoln County Schools
- Marion County Schools
- Maury County Public Schools
- Metro Nashville Public Schools
- Paris Special School District
- Robertson County Schools
- Rutherford County Schools
- Tullahoma City Schools
- Weakley County Schools
- Williamson County School
- Wilson County Schools
The CMA Foundation is the philanthropic arm of the Country Music Association. More information, along with the foundation's extensive research and initiatives, can be found at cmafoundation.org . Learn more about the Mr. Holland's Opus Foundation at mhopus.org .
share this!
May 22, 2024
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
Beach erosion will make Southern California coastal living five times more expensive by 2050, study predicts
by Nina Raffio, University of Southern California
Rising sea levels and urban development are accelerating coastal erosion at an alarming rate in Southern California with significant ripple effects on the region's economy, a USC study reveals.
The study , published in Communications Earth & Environment , predicts that Southern California's coastal living costs will surge fivefold by 2050 as a direct result of beach erosion. This erosion will require more frequent and costly beach nourishment projects to maintain the state's treasured shorelines, consequently driving up the cost of living along the coast.
"Our study presents compelling evidence of the rapid deterioration of Southern California's coastal landscapes," said Essam Heggy, a geoscientist in the Ming Hsieh Department of Electrical and Computer Engineering/Electrophysics at the USC Viterbi School of Engineering and the study's corresponding author.
"The challenges facing Southern California mirror a growing threat shared by coastal communities worldwide. The environmental and economic implications of coastal erosion reach far beyond California's shores and demand interdisciplinary, global solutions," he said.
Coastal erosion: Cost of living sure to surge as sandy beaches disappear
To predict future changes along California's sandy coastlines, the researchers focused on the Gulf of Santa Catalina, which stretches over 150 miles from the Palos Verdes Peninsula in Los Angeles County to the northern tip of Baja California in Mexico.
They used a combination of historical and recent satellite images as well as advanced algorithms to analyze coastline movement and predict future erosion based on different trends and environmental factors.
The study predicts a tripling of erosion rates by 2050, increasing from an average of 1.45 meters per year to 3.18 meters by 2100. Consequently, the annual sand requirement for beach nourishment could triple by 2050, with costs rising fivefold due to the global increase in sand prices. This will exacerbate economic and logistical pressures on coastal communities.
Beach nourishment is adding sand to an eroded beach to rebuild it and create a wider barrier against waves and storms.
"Our investigation suggests that coastal problems start inland due to the rapid growth of cities along the coast, which compromise inland sediment replenishment of sandy beaches ," said Heggy, whose research focuses on understanding water evolution in Earth's arid environments.
"As our beaches shrink, the cost of maintaining them will rise. Finding innovative solutions is key to securing a sustainable future for our shores and local economies," he said.
Coastal erosion in California: A case study for a global problem
Coastal cities in Southern California and those in North Africa bordering the Mediterranean Sea face a common challenge: a semi-arid climate year-round coupled with the growing threats of rising sea levels and eroding shorelines.
A significant portion of Earth's landmass, roughly 41%, falls under arid or semi-arid classifications, and these areas support over a third of the global population.
To understand this global challenge, the researchers focused on two specific locations: Corona del Mar in Orange County, Calif.—an example of the typical Southern California coastline—and Hammamet North Beach in Tunisia. Both are densely populated and share similar climates, prone to increasing droughts, flash floods and unpredictable rainfall patterns. These characteristics mirror the challenges faced by countless coastal communities worldwide.
The findings showed that the average rate of shoreline retreat in these areas varies. In Southern California, beaches are receding between 0.75 and 1.24 meters per year. In Hammamet North Beach, the retreat rate ranges from 0.21 to about 4.49 meters annually.
"While beach nourishment can temporarily combat erosion, however, it presents significant challenges for developing countries," said Oula Amrouni, a sedimentologist at the National Institute of Marine Sciences and Technologies at the University of Carthage, Tunis, Tunisia, and one of the study's co-authors.
"The high cost of acquiring the right sand, with the specific grain size, quality and composition, and the technical complexity of extracting and laying it are major hurdles. Additionally, worsening erosion in previously stable areas compels more frequent nourishment projects, straining already limited budgets and leading to unplanned expenditures for many communities."
Journal information: Communications Earth & Environment
Provided by University of Southern California
Explore further
Feedback to editors

New fossils provide evidence for an 'Age of Monotremes'
9 hours ago

TESS finds intriguing world sized between Earth and Venus

NASA launches ground-breaking climate change satellite
May 25, 2024

Dyson spheres: Astronomers report potential candidates for alien structures, and evidence against their existence

You leave a 'microbe fingerprint' on every piece of clothing you wear—and it could help forensic scientists solve crimes

Saturday Citations: The cheapness horizon of electric batteries; the battle-worthiness of ancient armor; scared animals

Cosmic leap: NASA Swift satellite and AI unravel the distance of the farthest gamma-ray bursts

Scientists discover CO₂ and CO ices in outskirts of solar system

Charge your laptop in a minute? Supercapacitors can help; new research offers clues
New study discovers tiny target on RNA to short-circuit inflammation
Relevant physicsforums posts, adirondack mountains and earthquakes.
May 23, 2024
The Secrets of Prof. Verschure's Rosetta Stones
Mt. vesuvius 1944 eruption light show -- static electricity, can a glass of water be filled to its edge.
May 21, 2024
Pyramids built along dry river bed
May 18, 2024
A very puzzling rock or a pallasite / mesmosiderite or a nothing burger
May 8, 2024
More from Earth Sciences
Related Stories

California's beaches are eroding: An expert explains how to save them

'The perfect storm': Hurricane Idalia leaves Florida beaches vulnerable to future inclement weather
Sep 13, 2023

Beach building is keeping the Atlantic Coast from going under
Jan 31, 2019

Coastal dunes are retreating as sea levels rise. Research reveals the accelerating rate of change
Mar 28, 2024

Shoreline model predicts long-term future of storm protection and sea-level rise
Apr 24, 2024

Sand study shows new data to help manage Southern California's shrinking beaches
Feb 19, 2024
Recommended for you

Heavy water: How melting ice sheets and pumped groundwater can lower local sea levels—and boost them elsewhere
May 24, 2024
Ambitious targets are needed to end ocean plastic pollution by 2100, analysis finds

Drought in the Brazil's Cerrado is the worst for at least seven centuries, study shows

Climate change will reduce streamflow in the upper Colorado river basin as groundwater levels fall, study finds

Study: While most of the world trusts climate scientists, a skeptical minority can lead to climate inaction
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter

IMAGES
COMMENTS
Patient reports stress and a decrease in sexual fulfillment from infertility concerns. Patient is monogamous with her primary partner. Patient does not drink alcohol or use recreational drugs. Patient follows a vegetarian diet and avoids dairy. Patient works as an architect for a small firm in Columbus, and occasionally travels domestically for ...
In this study, scar endometriosis was shown to be the more common variety of endometriosis, with 50% of cases predominantly developing at the lower segment cesarean section (LSCS) scar site. As a result, women with endometriosis are more likely to have miscarriages, and the quality of their embryos declines as a result. ... Case study of a rare ...
For patients where endometriosis is an incidental finding and patient has no symptoms of obstruction, hormonal therapy can be trailed . However, surgical intervention is recommended in symptomatic patients with intestinal endometriosis as demonstrated in our case. Studies have shown that there is significant improvement in patient symptoms.
Discussion. Endometriosis is the presence of endometrial gland tissues outside the uterine cavity. The most common sites are the pelvic peritoneum and pelvic organs. 1 Extra pelvic endometriosis is an uncommon entity and sites include the bowel, appendix, pleura and lung, abdominal wall, particularly the umbilicus and around surgical scars. . Endometriosis in the groin is rare. 2 The right ...
Endometriosis can have a profound impact on women's lives, including associated pain, infertility, decreased quality of life, and interference with daily life, relationships, and livelihood. The first step in alleviating these adverse sequelae is to diagnose the underlying condition. For many women, the journey to endometriosis diagnosis is long and fraught with barriers and misdiagnoses.
We searched PubMed for studies using the term "endometriosis." We considered all peer reviewed studies published in the English language between 1 January 2010 and 28 February 2022. ... (RCTs), prospective cohort studies, case-control studies, cross sectional studies; a priori exclusion of case series and case reports), by sample size (we ...
Endometriosis is a common disease affecting 5-10% of women of reproductive age globally. However, despite its prevalence, diagnosis is typically delayed by years, misdiagnosis is common, and delivery of effective therapy is prolonged. Identification and prompt treatment of endometriosis are essential and facilitated by accurate clinical diagnosis. Endometriosis is classically defined as a ...
Abdominal wall endometriosis has an incidence of 0.3-1% of extrapelvic disease. Α 48-year-old female appeared in the emergency department with cellulitis in a lower midline incision. She had an endometrioma of the anterior abdominal wall removed 2 years ago. After 5 months, she underwent an open repair of an incisional hernia with a ...
Endometriosis is a multifocal, chronic disease defined by extrauterine endometrial glands and stroma. This case report describes the author's experience of living with stage IV endometriosis, including a 10-year diagnostic delay, the impact on daily life, management, and treatment. The diagnostic delay for endometriosis averages between seven to nine years globally, which imparts significant ...
However, a US population-based case-control study found greater odds of endometriosis associated with higher fruit consumption (OR 1.5, 95% CI 1.2-2.3) and no association with red meat intake ...
ENDOMETRIOSIS A Clinical Study of 37 Cases GEORGE CRILE JR., M, . D. Endometriosis is define ads "th presence of endometriae tissu ilen ectopic locations. Endometria" tissu mae bye implantel on thde ovaries, on the pelvic peritoneum in, the umbilicus i,n the rectovaginal septum, or in laparotom scarsy I. functiont as endometrium under, -
Endometriosis is a chronic inflammatory disease that is defined as the presence of endometrium-like tissues outside the uterus causing pain symptoms (dysmenorrhea, dyspareunia, chronic pelvic pain) and infertility [].This gynecological disease affects approximately 2-10% of the reproductive-aged and 50% of infertile women, and women with endometriosis have increased risk of obstetric outcome [].
Pelvic Pain - Endometriosis Symptoms. A 27-year-old woman presented with severe dysmenorrhoea and pain with intercourse (dyspareunia). She also complained of bowel-related pain during menstruation.. Diagnosis. Laparoscopy revealed significant endometriosis behind the uterus (Pouch of Douglas) which extended right through to the vagina.. Outcome
Table 3 lists the symptoms and comorbidities that are associated with higher rates of endometriosis. 23 In a large case-control study in the United Kingdom, 73 percent of women with endometriosis ...
Endometriosis, which affects an estimated 10% of women of reproductive age, typically manifests in intra-pelvic organs and peritoneum, although organs external to the pelvis may also be involved ...
Deep infiltrative endometriosis (DIE) is a rare form of this condition, which mostly affects the uterosacral ligaments, the rectovaginal space, and the upper third of the posterior vaginal wall, the bowel, and the urinary tract. We present the case of a 29-year-old pregnant female who was diagnosed with infiltrative endometriosis during the ...
A Case Study on Endometriosis . Endometriosis is a chronic reproduction condition that still remains a mystery to the medical community. This paper starts off by providing the background information on what endometriosis is, the etiology, and risk factors associated with the condition. Following the introduction is a case study on a 20 year old ...
Endometriosis is a growth of endometrial. tissues outside the uterus, which is subjec t to the normal cyclic changes. The condition is. associated with infertility in 30-40% of women who leave ...
Cervical endometriosis is uncommon (incidence of 0.11-2.4%). 6 Only half of cervical endometriosis cases present with a clinically visible cervical lesion or vaginal bleeding while the remainder are diagnosed on histopathology. 7 When presenting as a mass, particularly with polypoid endometriosis, cervical endometriosis can mimic a cervical ...
Approximately 1 in 10 people with a uterus are reported to have endometriosis, affecting at least 190 million globally (Horne & Missmer, 2022).However, uncertainty remains regarding endometriosis frequency, prevalence, and incidence rates due to differences and variations in study design and population sampling (Smolarz et al., 2021).Endometriosis is a disease where endometrial-like lesions ...
Out of the included case studies, liver endometriosis only recurred in one person. There was only one report of a complication after surgery, which was a bile leak. There were no reports of severe ...
Discussion. Endometriosis is a chronic pathology characterized by the presence of endometrial tissue outside the uterus. The most common implantation site is in the ovaries or tubes, but it can be located throughout the body. Endometriotic implants located in the skin are known as "cutaneous endometriosis.".
The study included 457 women, of whom 228 had IBD, and 229 age-matched healthy controls. No differences were found in the use of contraceptives, infertility, and endometriosis. The risk of spontaneous and voluntary abortions was significantly higher in IBD patients than in healthy controls [odds ratio (OR) 2 and 3.62, respectively].
Endometriosis is one of the most common and costly diseases among women. This study was carried out to investigate pregnancy outcomes in women with endometriosis because of the high prevalence of endometriosis in reproductive ages and its effect on pregnancy-related complications outcomes. This was a cross-sectional study performed on 379 pregnant women with endometriosis who were referred to ...
Armour's research has found that people with endometriosis and chronic pelvic pain spend about $400 a month on allied health, while a previous study he published in 2019 found endometriosis can ...
In another case study of a 54-year-old woman who was postmenopausal (had gone through menopause, the time in which menstrual periods have stopped for 12 straight months) and started Lexapro, she experienced heavy vaginal bleeding for a week, which subsided a few days after discontinuing Lexapro.
The study, titled "Face the Music: A Case Study for Expanding Music and Arts in Schools," was the result of a joint effort between the CMA Foundation and the Mr. Holland's Opus Foundation.
The study, published in Communications Earth & Environment, predicts that Southern California's coastal living costs will surge fivefold by 2050 as a direct result of beach erosion.This erosion ...