Null hypothesis
Null hypothesis n., plural: null hypotheses [nʌl haɪˈpɒθɪsɪs] Definition: a hypothesis that is valid or presumed true until invalidated by a statistical test
Table of Contents

Null Hypothesis Definition
Null hypothesis is defined as “the commonly accepted fact (such as the sky is blue) and researcher aim to reject or nullify this fact”.
More formally, we can define a null hypothesis as “a statistical theory suggesting that no statistical relationship exists between given observed variables” .
In biology , the null hypothesis is used to nullify or reject a common belief. The researcher carries out the research which is aimed at rejecting the commonly accepted belief.
What Is a Null Hypothesis?
A hypothesis is defined as a theory or an assumption that is based on inadequate evidence. It needs and requires more experiments and testing for confirmation. There are two possibilities that by doing more experiments and testing, a hypothesis can be false or true. It means it can either prove wrong or true (Blackwelder, 1982).
For example, Susie assumes that mineral water helps in the better growth and nourishment of plants over distilled water. To prove this hypothesis, she performs this experiment for almost a month. She watered some plants with mineral water and some with distilled water.
In a hypothesis when there are no statistically significant relationships among the two variables, the hypothesis is said to be a null hypothesis. The investigator is trying to disprove such a hypothesis. In the above example of plants, the null hypothesis is:
There are no statistical relationships among the forms of water that are given to plants for growth and nourishment.
Usually, an investigator tries to prove the null hypothesis wrong and tries to explain a relation and association between the two variables.
An opposite and reverse of the null hypothesis are known as the alternate hypothesis . In the example of plants the alternate hypothesis is:
There are statistical relationships among the forms of water that are given to plants for growth and nourishment.
The example below shows the difference between null vs alternative hypotheses:
Alternate Hypothesis: The world is round Null Hypothesis: The world is not round.
Copernicus and many other scientists try to prove the null hypothesis wrong and false. By their experiments and testing, they make people believe that alternate hypotheses are correct and true. If they do not prove the null hypothesis experimentally wrong then people will not believe them and never consider the alternative hypothesis true and correct.
The alternative and null hypothesis for Susie’s assumption is:
- Null Hypothesis: If one plant is watered with distilled water and the other with mineral water, then there is no difference in the growth and nourishment of these two plants.
- Alternative Hypothesis: If one plant is watered with distilled water and the other with mineral water, then the plant with mineral water shows better growth and nourishment.
The null hypothesis suggests that there is no significant or statistical relationship. The relation can either be in a single set of variables or among two sets of variables.
Most people consider the null hypothesis true and correct. Scientists work and perform different experiments and do a variety of research so that they can prove the null hypothesis wrong or nullify it. For this purpose, they design an alternate hypothesis that they think is correct or true. The null hypothesis symbol is H 0 (it is read as H null or H zero ).
Why is it named the “Null”?
The name null is given to this hypothesis to clarify and explain that the scientists are working to prove it false i.e. to nullify the hypothesis. Sometimes it confuses the readers; they might misunderstand it and think that statement has nothing. It is blank but, actually, it is not. It is more appropriate and suitable to call it a nullifiable hypothesis instead of the null hypothesis.
Why do we need to assess it? Why not just verify an alternate one?
In science, the scientific method is used. It involves a series of different steps. Scientists perform these steps so that a hypothesis can be proved false or true. Scientists do this to confirm that there will be any limitation or inadequacy in the new hypothesis. Experiments are done by considering both alternative and null hypotheses, which makes the research safe. It gives a negative as well as a bad impact on research if a null hypothesis is not included or a part of the study. It seems like you are not taking your research seriously and not concerned about it and just want to impose your results as correct and true if the null hypothesis is not a part of the study.
Development of the Null
In statistics, firstly it is necessary to design alternate and null hypotheses from the given problem. Splitting the problem into small steps makes the pathway towards the solution easier and less challenging. how to write a null hypothesis?
Writing a null hypothesis consists of two steps:
- Firstly, initiate by asking a question.
- Secondly, restate the question in such a way that it seems there are no relationships among the variables.
In other words, assume in such a way that the treatment does not have any effect.
The usual recovery duration after knee surgery is considered almost 8 weeks.
A researcher thinks that the recovery period may get elongated if patients go to a physiotherapist for rehabilitation twice per week, instead of thrice per week, i.e. recovery duration reduces if the patient goes three times for rehabilitation instead of two times.
Step 1: Look for the problem in the hypothesis. The hypothesis either be a word or can be a statement. In the above example the hypothesis is:
“The expected recovery period in knee rehabilitation is more than 8 weeks”
Step 2: Make a mathematical statement from the hypothesis. Averages can also be represented as μ, thus the null hypothesis formula will be.
In the above equation, the hypothesis is equivalent to H1, the average is denoted by μ and > that the average is greater than eight.
Step 3: Explain what will come up if the hypothesis does not come right i.e., the rehabilitation period may not proceed more than 08 weeks.
There are two options: either the recovery will be less than or equal to 8 weeks.
H 0 : μ ≤ 8
In the above equation, the null hypothesis is equivalent to H 0 , the average is denoted by μ and ≤ represents that the average is less than or equal to eight.
What will happen if the scientist does not have any knowledge about the outcome?
Problem: An investigator investigates the post-operative impact and influence of radical exercise on patients who have operative procedures of the knee. The chances are either the exercise will improve the recovery or will make it worse. The usual time for recovery is 8 weeks.
Step 1: Make a null hypothesis i.e. the exercise does not show any effect and the recovery time remains almost 8 weeks.
H 0 : μ = 8
In the above equation, the null hypothesis is equivalent to H 0 , the average is denoted by μ, and the equal sign (=) shows that the average is equal to eight.
Step 2: Make the alternate hypothesis which is the reverse of the null hypothesis. Particularly what will happen if treatment (exercise) makes an impact?
In the above equation, the alternate hypothesis is equivalent to H1, the average is denoted by μ and not equal sign (≠) represents that the average is not equal to eight.
Significance Tests
To get a reasonable and probable clarification of statistics (data), a significance test is performed. The null hypothesis does not have data. It is a piece of information or statement which contains numerical figures about the population. The data can be in different forms like in means or proportions. It can either be the difference of proportions and means or any odd ratio.
The following table will explain the symbols:
P-value is the chief statistical final result of the significance test of the null hypothesis.
- P-value = Pr(data or data more extreme | H 0 true)
- | = “given”
- Pr = probability
- H 0 = the null hypothesis
The first stage of Null Hypothesis Significance Testing (NHST) is to form an alternate and null hypothesis. By this, the research question can be briefly explained.
Null Hypothesis = no effect of treatment, no difference, no association Alternative Hypothesis = effective treatment, difference, association
When to reject the null hypothesis?
Researchers will reject the null hypothesis if it is proven wrong after experimentation. Researchers accept null hypothesis to be true and correct until it is proven wrong or false. On the other hand, the researchers try to strengthen the alternate hypothesis. The binomial test is performed on a sample and after that, a series of tests were performed (Frick, 1995).
Step 1: Evaluate and read the research question carefully and consciously and make a null hypothesis. Verify the sample that supports the binomial proportion. If there is no difference then find out the value of the binomial parameter.
Show the null hypothesis as:
H 0 :p= the value of p if H 0 is true
To find out how much it varies from the proposed data and the value of the null hypothesis, calculate the sample proportion.
Step 2: In test statistics, find the binomial test that comes under the null hypothesis. The test must be based on precise and thorough probabilities. Also make a list of pmf that apply, when the null hypothesis proves true and correct.
When H 0 is true, X~b(n, p)
N = size of the sample
P = assume value if H 0 proves true.
Step 3: Find out the value of P. P-value is the probability of data that is under observation.
Rise or increase in the P value = Pr(X ≥ x)
X = observed number of successes
P value = Pr(X ≤ x).
Step 4: Demonstrate the findings or outcomes in a descriptive detailed way.
- Sample proportion
- The direction of difference (either increases or decreases)
Perceived Problems With the Null Hypothesis
Variable or model selection and less information in some cases are the chief important issues that affect the testing of the null hypothesis. Statistical tests of the null hypothesis are reasonably not strong. There is randomization about significance. (Gill, 1999) The main issue with the testing of the null hypothesis is that they all are wrong or false on a ground basis.
There is another problem with the a-level . This is an ignored but also a well-known problem. The value of a-level is without a theoretical basis and thus there is randomization in conventional values, most commonly 0.q, 0.5, or 0.01. If a fixed value of a is used, it will result in the formation of two categories (significant and non-significant) The issue of a randomized rejection or non-rejection is also present when there is a practical matter which is the strong point of the evidence related to a scientific matter.
The P-value has the foremost importance in the testing of null hypothesis but as an inferential tool and for interpretation, it has a problem. The P-value is the probability of getting a test statistic at least as extreme as the observed one.
The main point about the definition is: Observed results are not based on a-value
Moreover, the evidence against the null hypothesis was overstated due to unobserved results. A-value has importance more than just being a statement. It is a precise statement about the evidence from the observed results or data. Similarly, researchers found that P-values are objectionable. They do not prefer null hypotheses in testing. It is also clear that the P-value is strictly dependent on the null hypothesis. It is computer-based statistics. In some precise experiments, the null hypothesis statistics and actual sampling distribution are closely related but this does not become possible in observational studies.
Some researchers pointed out that the P-value is depending on the sample size. If the true and exact difference is small, a null hypothesis even of a large sample may get rejected. This shows the difference between biological importance and statistical significance. (Killeen, 2005)
Another issue is the fix a-level, i.e., 0.1. On the basis, if a-level a null hypothesis of a large sample may get accepted or rejected. If the size of simple is infinity and the null hypothesis is proved true there are still chances of Type I error. That is the reason this approach or method is not considered consistent and reliable. There is also another problem that the exact information about the precision and size of the estimated effect cannot be known. The only solution is to state the size of the effect and its precision.
Null Hypothesis Examples
Here are some examples:
Example 1: Hypotheses with One Sample of One Categorical Variable
Among all the population of humans, almost 10% of people prefer to do their task with their left hand i.e. left-handed. Let suppose, a researcher in the Penn States says that the population of students at the College of Arts and Architecture is mostly left-handed as compared to the general population of humans in general public society. In this case, there is only a sample and there is a comparison among the known population values to the population proportion of sample value.
- Research Question: Do artists more expected to be left-handed as compared to the common population persons in society?
- Response Variable: Sorting the student into two categories. One category has left-handed persons and the other category have right-handed persons.
- Form Null Hypothesis: Arts and Architecture college students are no more predicted to be lefty as compared to the common population persons in society (Lefty students of Arts and Architecture college population is 10% or p= 0.10)
Example 2: Hypotheses with One Sample of One Measurement Variable
A generic brand of antihistamine Diphenhydramine making medicine in the form of a capsule, having a 50mg dose. The maker of the medicines is concerned that the machine has come out of calibration and is not making more capsules with the suitable and appropriate dose.
- Research Question: Does the statistical data recommended about the mean and average dosage of the population differ from 50mg?
- Response Variable: Chemical assay used to find the appropriate dosage of the active ingredient.
- Null Hypothesis: Usually, the 50mg dosage of capsules of this trade name (population average and means dosage =50 mg).
Example 3: Hypotheses with Two Samples of One Categorical Variable
Several people choose vegetarian meals on a daily basis. Typically, the researcher thought that females like vegetarian meals more than males.
- Research Question: Does the data recommend that females (women) prefer vegetarian meals more than males (men) regularly?
- Response Variable: Cataloguing the persons into vegetarian and non-vegetarian categories. Grouping Variable: Gender
- Null Hypothesis: Gender is not linked to those who like vegetarian meals. (Population percent of women who eat vegetarian meals regularly = population percent of men who eat vegetarian meals regularly or p women = p men).
Example 4: Hypotheses with Two Samples of One Measurement Variable
Nowadays obesity and being overweight is one of the major and dangerous health issues. Research is performed to confirm that a low carbohydrates diet leads to faster weight loss than a low-fat diet.
- Research Question: Does the given data recommend that usually, a low-carbohydrate diet helps in losing weight faster as compared to a low-fat diet?
- Response Variable: Weight loss (pounds)
- Explanatory Variable: Form of diet either low carbohydrate or low fat
- Null Hypothesis: There is no significant difference when comparing the mean loss of weight of people using a low carbohydrate diet to people using a diet having low fat. (population means loss of weight on a low carbohydrate diet = population means loss of weight on a diet containing low fat).
Example 5: Hypotheses about the relationship between Two Categorical Variables
A case-control study was performed. The study contains nonsmokers, stroke patients, and controls. The subjects are of the same occupation and age and the question was asked if someone at their home or close surrounding smokes?
- Research Question: Did second-hand smoke enhance the chances of stroke?
- Variables: There are 02 diverse categories of variables. (Controls and stroke patients) (whether the smoker lives in the same house). The chances of having a stroke will be increased if a person is living with a smoker.
- Null Hypothesis: There is no significant relationship between a passive smoker and stroke or brain attack. (odds ratio between stroke and the passive smoker is equal to 1).
Example 6: Hypotheses about the relationship between Two Measurement Variables
A financial expert observes that there is somehow a positive and effective relationship between the variation in stock rate price and the quantity of stock bought by non-management employees
- Response variable- Regular alteration in price
- Explanatory Variable- Stock bought by non-management employees
- Null Hypothesis: The association and relationship between the regular stock price alteration ($) and the daily stock-buying by non-management employees ($) = 0.
Example 7: Hypotheses about comparing the relationship between Two Measurement Variables in Two Samples
- Research Question: Is the relation between the bill paid in a restaurant and the tip given to the waiter, is linear? Is this relation different for dining and family restaurants?
- Explanatory Variable- total bill amount
- Response Variable- the amount of tip
- Null Hypothesis: The relationship and association between the total bill quantity at a family or dining restaurant and the tip, is the same.
Try to answer the quiz below to check what you have learned so far about the null hypothesis.
Choose the best answer.
Send Your Results (Optional)

- Blackwelder, W. C. (1982). “Proving the null hypothesis” in clinical trials. Controlled Clinical Trials , 3(4), 345–353.
- Frick, R. W. (1995). Accepting the null hypothesis. Memory & Cognition, 23(1), 132–138.
- Gill, J. (1999). The insignificance of null hypothesis significance testing. Political Research Quarterly , 52(3), 647–674.
- Killeen, P. R. (2005). An alternative to null-hypothesis significance tests. Psychological Science, 16(5), 345–353.
©BiologyOnline.com. Content provided and moderated by Biology Online Editors.
Last updated on June 16th, 2022
You will also like...

Human Perception – Neurology
This tutorial investigates perception as two people can interpret the same thing differently. Know more about human perc..

Ecological Research: Measuring & Analysis
This lesson is about the methods used for ecological research, such as quadrat and transect sampling, canopy fogging, an..
Cell Structure
A typical eukaryotic cell is comprised of cytoplasm with different organelles, such as nucleus, endoplasmic reticulum, G..

The Gene Pool and Population Genetics
According to Charles Darwin's theory of natural selection, preferable genes are favored by nature in the gene pool, and ..

Population Regulation in an Ecosystem
With regard to the population size of a species and what factors may affect them, two factors have been defined. They ar..

The Dinosaurs
Dinosaurs represented a major turn in the evolutionary development of organisms on Earth. The first dinosaurs were presu..
Related Articles...

No related articles found
Null Hypothesis Definition and Examples
PM Images / Getty Images
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
In a scientific experiment, the null hypothesis is the proposition that there is no effect or no relationship between phenomena or populations. If the null hypothesis is true, any observed difference in phenomena or populations would be due to sampling error (random chance) or experimental error. The null hypothesis is useful because it can be tested and found to be false, which then implies that there is a relationship between the observed data. It may be easier to think of it as a nullifiable hypothesis or one that the researcher seeks to nullify. The null hypothesis is also known as the H 0, or no-difference hypothesis.
The alternate hypothesis, H A or H 1 , proposes that observations are influenced by a non-random factor. In an experiment, the alternate hypothesis suggests that the experimental or independent variable has an effect on the dependent variable .
How to State a Null Hypothesis
There are two ways to state a null hypothesis. One is to state it as a declarative sentence, and the other is to present it as a mathematical statement.
For example, say a researcher suspects that exercise is correlated to weight loss, assuming diet remains unchanged. The average length of time to achieve a certain amount of weight loss is six weeks when a person works out five times a week. The researcher wants to test whether weight loss takes longer to occur if the number of workouts is reduced to three times a week.
The first step to writing the null hypothesis is to find the (alternate) hypothesis. In a word problem like this, you're looking for what you expect to be the outcome of the experiment. In this case, the hypothesis is "I expect weight loss to take longer than six weeks."
This can be written mathematically as: H 1 : μ > 6
In this example, μ is the average.
Now, the null hypothesis is what you expect if this hypothesis does not happen. In this case, if weight loss isn't achieved in greater than six weeks, then it must occur at a time equal to or less than six weeks. This can be written mathematically as:
H 0 : μ ≤ 6
The other way to state the null hypothesis is to make no assumption about the outcome of the experiment. In this case, the null hypothesis is simply that the treatment or change will have no effect on the outcome of the experiment. For this example, it would be that reducing the number of workouts would not affect the time needed to achieve weight loss:
H 0 : μ = 6
- Null Hypothesis Examples
"Hyperactivity is unrelated to eating sugar " is an example of a null hypothesis. If the hypothesis is tested and found to be false, using statistics, then a connection between hyperactivity and sugar ingestion may be indicated. A significance test is the most common statistical test used to establish confidence in a null hypothesis.
Another example of a null hypothesis is "Plant growth rate is unaffected by the presence of cadmium in the soil ." A researcher could test the hypothesis by measuring the growth rate of plants grown in a medium lacking cadmium, compared with the growth rate of plants grown in mediums containing different amounts of cadmium. Disproving the null hypothesis would set the groundwork for further research into the effects of different concentrations of the element in soil.
Why Test a Null Hypothesis?
You may be wondering why you would want to test a hypothesis just to find it false. Why not just test an alternate hypothesis and find it true? The short answer is that it is part of the scientific method. In science, propositions are not explicitly "proven." Rather, science uses math to determine the probability that a statement is true or false. It turns out it's much easier to disprove a hypothesis than to positively prove one. Also, while the null hypothesis may be simply stated, there's a good chance the alternate hypothesis is incorrect.
For example, if your null hypothesis is that plant growth is unaffected by duration of sunlight, you could state the alternate hypothesis in several different ways. Some of these statements might be incorrect. You could say plants are harmed by more than 12 hours of sunlight or that plants need at least three hours of sunlight, etc. There are clear exceptions to those alternate hypotheses, so if you test the wrong plants, you could reach the wrong conclusion. The null hypothesis is a general statement that can be used to develop an alternate hypothesis, which may or may not be correct.
- Difference Between Independent and Dependent Variables
- Examples of Independent and Dependent Variables
- What Are Examples of a Hypothesis?
- What Is a Hypothesis? (Science)
- What 'Fail to Reject' Means in a Hypothesis Test
- What Are the Elements of a Good Hypothesis?
- Null Hypothesis and Alternative Hypothesis
- Scientific Hypothesis Examples
- What Is a Control Group?
- Understanding Simple vs Controlled Experiments
- Six Steps of the Scientific Method
- Scientific Method Vocabulary Terms
- Definition of a Hypothesis
- How to Conduct a Hypothesis Test
- Type I and Type II Errors in Statistics

Statistics Made Easy
How to Write a Null Hypothesis (5 Examples)
A hypothesis test uses sample data to determine whether or not some claim about a population parameter is true.
Whenever we perform a hypothesis test, we always write a null hypothesis and an alternative hypothesis, which take the following forms:
H 0 (Null Hypothesis): Population parameter =, ≤, ≥ some value
H A (Alternative Hypothesis): Population parameter <, >, ≠ some value
Note that the null hypothesis always contains the equal sign .
We interpret the hypotheses as follows:
Null hypothesis: The sample data provides no evidence to support some claim being made by an individual.
Alternative hypothesis: The sample data does provide sufficient evidence to support the claim being made by an individual.
For example, suppose it’s assumed that the average height of a certain species of plant is 20 inches tall. However, one botanist claims the true average height is greater than 20 inches.
To test this claim, she may go out and collect a random sample of plants. She can then use this sample data to perform a hypothesis test using the following two hypotheses:
H 0 : μ ≤ 20 (the true mean height of plants is equal to or even less than 20 inches)
H A : μ > 20 (the true mean height of plants is greater than 20 inches)
If the sample data gathered by the botanist shows that the mean height of this species of plants is significantly greater than 20 inches, she can reject the null hypothesis and conclude that the mean height is greater than 20 inches.
Read through the following examples to gain a better understanding of how to write a null hypothesis in different situations.
Example 1: Weight of Turtles
A biologist wants to test whether or not the true mean weight of a certain species of turtles is 300 pounds. To test this, he goes out and measures the weight of a random sample of 40 turtles.
Here is how to write the null and alternative hypotheses for this scenario:
H 0 : μ = 300 (the true mean weight is equal to 300 pounds)
H A : μ ≠ 300 (the true mean weight is not equal to 300 pounds)
Example 2: Height of Males
It’s assumed that the mean height of males in a certain city is 68 inches. However, an independent researcher believes the true mean height is greater than 68 inches. To test this, he goes out and collects the height of 50 males in the city.
H 0 : μ ≤ 68 (the true mean height is equal to or even less than 68 inches)
H A : μ > 68 (the true mean height is greater than 68 inches)
Example 3: Graduation Rates
A university states that 80% of all students graduate on time. However, an independent researcher believes that less than 80% of all students graduate on time. To test this, she collects data on the proportion of students who graduated on time last year at the university.
H 0 : p ≥ 0.80 (the true proportion of students who graduate on time is 80% or higher)
H A : μ < 0.80 (the true proportion of students who graduate on time is less than 80%)
Example 4: Burger Weights
A food researcher wants to test whether or not the true mean weight of a burger at a certain restaurant is 7 ounces. To test this, he goes out and measures the weight of a random sample of 20 burgers from this restaurant.
H 0 : μ = 7 (the true mean weight is equal to 7 ounces)
H A : μ ≠ 7 (the true mean weight is not equal to 7 ounces)
Example 5: Citizen Support
A politician claims that less than 30% of citizens in a certain town support a certain law. To test this, he goes out and surveys 200 citizens on whether or not they support the law.
H 0 : p ≥ .30 (the true proportion of citizens who support the law is greater than or equal to 30%)
H A : μ < 0.30 (the true proportion of citizens who support the law is less than 30%)
Additional Resources
Introduction to Hypothesis Testing Introduction to Confidence Intervals An Explanation of P-Values and Statistical Significance
Featured Posts

Hey there. My name is Zach Bobbitt. I have a Masters of Science degree in Applied Statistics and I’ve worked on machine learning algorithms for professional businesses in both healthcare and retail. I’m passionate about statistics, machine learning, and data visualization and I created Statology to be a resource for both students and teachers alike. My goal with this site is to help you learn statistics through using simple terms, plenty of real-world examples, and helpful illustrations.
2 Replies to “How to Write a Null Hypothesis (5 Examples)”
you are amazing, thank you so much
Say I am a botanist hypothesizing the average height of daisies is 20 inches, or not? Does T = (ave – 20 inches) / √ variance / (80 / 4)? … This assumes 40 real measures + 40 fake = 80 n, but that seems questionable. Please advise.
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Join the Statology Community
Sign up to receive Statology's exclusive study resource: 100 practice problems with step-by-step solutions. Plus, get our latest insights, tutorials, and data analysis tips straight to your inbox!
By subscribing you accept Statology's Privacy Policy.
Have a thesis expert improve your writing
Check your thesis for plagiarism in 10 minutes, generate your apa citations for free.
- Knowledge Base
- Null and Alternative Hypotheses | Definitions & Examples
Null and Alternative Hypotheses | Definitions & Examples
Published on 5 October 2022 by Shaun Turney . Revised on 6 December 2022.
The null and alternative hypotheses are two competing claims that researchers weigh evidence for and against using a statistical test :
- Null hypothesis (H 0 ): There’s no effect in the population .
- Alternative hypothesis (H A ): There’s an effect in the population.
The effect is usually the effect of the independent variable on the dependent variable .
Table of contents
Answering your research question with hypotheses, what is a null hypothesis, what is an alternative hypothesis, differences between null and alternative hypotheses, how to write null and alternative hypotheses, frequently asked questions about null and alternative hypotheses.
The null and alternative hypotheses offer competing answers to your research question . When the research question asks “Does the independent variable affect the dependent variable?”, the null hypothesis (H 0 ) answers “No, there’s no effect in the population.” On the other hand, the alternative hypothesis (H A ) answers “Yes, there is an effect in the population.”
The null and alternative are always claims about the population. That’s because the goal of hypothesis testing is to make inferences about a population based on a sample . Often, we infer whether there’s an effect in the population by looking at differences between groups or relationships between variables in the sample.
You can use a statistical test to decide whether the evidence favors the null or alternative hypothesis. Each type of statistical test comes with a specific way of phrasing the null and alternative hypothesis. However, the hypotheses can also be phrased in a general way that applies to any test.
The null hypothesis is the claim that there’s no effect in the population.
If the sample provides enough evidence against the claim that there’s no effect in the population ( p ≤ α), then we can reject the null hypothesis . Otherwise, we fail to reject the null hypothesis.
Although “fail to reject” may sound awkward, it’s the only wording that statisticians accept. Be careful not to say you “prove” or “accept” the null hypothesis.
Null hypotheses often include phrases such as “no effect”, “no difference”, or “no relationship”. When written in mathematical terms, they always include an equality (usually =, but sometimes ≥ or ≤).
Examples of null hypotheses
The table below gives examples of research questions and null hypotheses. There’s always more than one way to answer a research question, but these null hypotheses can help you get started.
*Note that some researchers prefer to always write the null hypothesis in terms of “no effect” and “=”. It would be fine to say that daily meditation has no effect on the incidence of depression and p 1 = p 2 .
The alternative hypothesis (H A ) is the other answer to your research question . It claims that there’s an effect in the population.
Often, your alternative hypothesis is the same as your research hypothesis. In other words, it’s the claim that you expect or hope will be true.
The alternative hypothesis is the complement to the null hypothesis. Null and alternative hypotheses are exhaustive, meaning that together they cover every possible outcome. They are also mutually exclusive, meaning that only one can be true at a time.
Alternative hypotheses often include phrases such as “an effect”, “a difference”, or “a relationship”. When alternative hypotheses are written in mathematical terms, they always include an inequality (usually ≠, but sometimes > or <). As with null hypotheses, there are many acceptable ways to phrase an alternative hypothesis.
Examples of alternative hypotheses
The table below gives examples of research questions and alternative hypotheses to help you get started with formulating your own.
Null and alternative hypotheses are similar in some ways:
- They’re both answers to the research question
- They both make claims about the population
- They’re both evaluated by statistical tests.
However, there are important differences between the two types of hypotheses, summarized in the following table.
To help you write your hypotheses, you can use the template sentences below. If you know which statistical test you’re going to use, you can use the test-specific template sentences. Otherwise, you can use the general template sentences.
The only thing you need to know to use these general template sentences are your dependent and independent variables. To write your research question, null hypothesis, and alternative hypothesis, fill in the following sentences with your variables:
Does independent variable affect dependent variable ?
- Null hypothesis (H 0 ): Independent variable does not affect dependent variable .
- Alternative hypothesis (H A ): Independent variable affects dependent variable .
Test-specific
Once you know the statistical test you’ll be using, you can write your hypotheses in a more precise and mathematical way specific to the test you chose. The table below provides template sentences for common statistical tests.
Note: The template sentences above assume that you’re performing one-tailed tests . One-tailed tests are appropriate for most studies.
The null hypothesis is often abbreviated as H 0 . When the null hypothesis is written using mathematical symbols, it always includes an equality symbol (usually =, but sometimes ≥ or ≤).
The alternative hypothesis is often abbreviated as H a or H 1 . When the alternative hypothesis is written using mathematical symbols, it always includes an inequality symbol (usually ≠, but sometimes < or >).
A research hypothesis is your proposed answer to your research question. The research hypothesis usually includes an explanation (‘ x affects y because …’).
A statistical hypothesis, on the other hand, is a mathematical statement about a population parameter. Statistical hypotheses always come in pairs: the null and alternative hypotheses. In a well-designed study , the statistical hypotheses correspond logically to the research hypothesis.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
Turney, S. (2022, December 06). Null and Alternative Hypotheses | Definitions & Examples. Scribbr. Retrieved 27 May 2024, from https://www.scribbr.co.uk/stats/null-and-alternative-hypothesis/
Is this article helpful?

Shaun Turney
Other students also liked, levels of measurement: nominal, ordinal, interval, ratio, the standard normal distribution | calculator, examples & uses, types of variables in research | definitions & examples.

- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems
- Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books
Hypothesis Examples

A hypothesis is a prediction of the outcome of a test. It forms the basis for designing an experiment in the scientific method . A good hypothesis is testable, meaning it makes a prediction you can check with observation or experimentation. Here are different hypothesis examples.
Null Hypothesis Examples
The null hypothesis (H 0 ) is also known as the zero-difference or no-difference hypothesis. It predicts that changing one variable ( independent variable ) will have no effect on the variable being measured ( dependent variable ). Here are null hypothesis examples:
- Plant growth is unaffected by temperature.
- If you increase temperature, then solubility of salt will increase.
- Incidence of skin cancer is unrelated to ultraviolet light exposure.
- All brands of light bulb last equally long.
- Cats have no preference for the color of cat food.
- All daisies have the same number of petals.
Sometimes the null hypothesis shows there is a suspected correlation between two variables. For example, if you think plant growth is affected by temperature, you state the null hypothesis: “Plant growth is not affected by temperature.” Why do you do this, rather than say “If you change temperature, plant growth will be affected”? The answer is because it’s easier applying a statistical test that shows, with a high level of confidence, a null hypothesis is correct or incorrect.
Research Hypothesis Examples
A research hypothesis (H 1 ) is a type of hypothesis used to design an experiment. This type of hypothesis is often written as an if-then statement because it’s easy identifying the independent and dependent variables and seeing how one affects the other. If-then statements explore cause and effect. In other cases, the hypothesis shows a correlation between two variables. Here are some research hypothesis examples:
- If you leave the lights on, then it takes longer for people to fall asleep.
- If you refrigerate apples, they last longer before going bad.
- If you keep the curtains closed, then you need less electricity to heat or cool the house (the electric bill is lower).
- If you leave a bucket of water uncovered, then it evaporates more quickly.
- Goldfish lose their color if they are not exposed to light.
- Workers who take vacations are more productive than those who never take time off.
Is It Okay to Disprove a Hypothesis?
Yes! You may even choose to write your hypothesis in such a way that it can be disproved because it’s easier to prove a statement is wrong than to prove it is right. In other cases, if your prediction is incorrect, that doesn’t mean the science is bad. Revising a hypothesis is common. It demonstrates you learned something you did not know before you conducted the experiment.
Test yourself with a Scientific Method Quiz .
- Mellenbergh, G.J. (2008). Chapter 8: Research designs: Testing of research hypotheses. In H.J. Adèr & G.J. Mellenbergh (eds.), Advising on Research Methods: A Consultant’s Companion . Huizen, The Netherlands: Johannes van Kessel Publishing.
- Popper, Karl R. (1959). The Logic of Scientific Discovery . Hutchinson & Co. ISBN 3-1614-8410-X.
- Schick, Theodore; Vaughn, Lewis (2002). How to think about weird things: critical thinking for a New Age . Boston: McGraw-Hill Higher Education. ISBN 0-7674-2048-9.
- Tobi, Hilde; Kampen, Jarl K. (2018). “Research design: the methodology for interdisciplinary research framework”. Quality & Quantity . 52 (3): 1209–1225. doi: 10.1007/s11135-017-0513-8
Related Posts
9.1 Null and Alternative Hypotheses
The actual test begins by considering two hypotheses . They are called the null hypothesis and the alternative hypothesis . These hypotheses contain opposing viewpoints.
H 0 , the — null hypothesis: a statement of no difference between sample means or proportions or no difference between a sample mean or proportion and a population mean or proportion. In other words, the difference equals 0.
H a —, the alternative hypothesis: a claim about the population that is contradictory to H 0 and what we conclude when we reject H 0 .
Since the null and alternative hypotheses are contradictory, you must examine evidence to decide if you have enough evidence to reject the null hypothesis or not. The evidence is in the form of sample data.
After you have determined which hypothesis the sample supports, you make a decision. There are two options for a decision. They are reject H 0 if the sample information favors the alternative hypothesis or do not reject H 0 or decline to reject H 0 if the sample information is insufficient to reject the null hypothesis.
Mathematical Symbols Used in H 0 and H a :
H 0 always has a symbol with an equal in it. H a never has a symbol with an equal in it. The choice of symbol depends on the wording of the hypothesis test. However, be aware that many researchers use = in the null hypothesis, even with > or < as the symbol in the alternative hypothesis. This practice is acceptable because we only make the decision to reject or not reject the null hypothesis.
Example 9.1
H 0 : No more than 30 percent of the registered voters in Santa Clara County voted in the primary election. p ≤ 30 H a : More than 30 percent of the registered voters in Santa Clara County voted in the primary election. p > 30
A medical trial is conducted to test whether or not a new medicine reduces cholesterol by 25 percent. State the null and alternative hypotheses.
Example 9.2
We want to test whether the mean GPA of students in American colleges is different from 2.0 (out of 4.0). The null and alternative hypotheses are the following: H 0 : μ = 2.0 H a : μ ≠ 2.0
We want to test whether the mean height of eighth graders is 66 inches. State the null and alternative hypotheses. Fill in the correct symbol (=, ≠, ≥, <, ≤, >) for the null and alternative hypotheses.
- H 0 : μ __ 66
- H a : μ __ 66
Example 9.3
We want to test if college students take fewer than five years to graduate from college, on the average. The null and alternative hypotheses are the following: H 0 : μ ≥ 5 H a : μ < 5
We want to test if it takes fewer than 45 minutes to teach a lesson plan. State the null and alternative hypotheses. Fill in the correct symbol ( =, ≠, ≥, <, ≤, >) for the null and alternative hypotheses.
- H 0 : μ __ 45
- H a : μ __ 45
Example 9.4
An article on school standards stated that about half of all students in France, Germany, and Israel take advanced placement exams and a third of the students pass. The same article stated that 6.6 percent of U.S. students take advanced placement exams and 4.4 percent pass. Test if the percentage of U.S. students who take advanced placement exams is more than 6.6 percent. State the null and alternative hypotheses. H 0 : p ≤ 0.066 H a : p > 0.066
On a state driver’s test, about 40 percent pass the test on the first try. We want to test if more than 40 percent pass on the first try. Fill in the correct symbol (=, ≠, ≥, <, ≤, >) for the null and alternative hypotheses.
- H 0 : p __ 0.40
- H a : p __ 0.40
Collaborative Exercise
Bring to class a newspaper, some news magazines, and some internet articles. In groups, find articles from which your group can write null and alternative hypotheses. Discuss your hypotheses with the rest of the class.
As an Amazon Associate we earn from qualifying purchases.
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute Texas Education Agency (TEA). The original material is available at: https://www.texasgateway.org/book/tea-statistics . Changes were made to the original material, including updates to art, structure, and other content updates.
Access for free at https://openstax.org/books/statistics/pages/1-introduction
- Authors: Barbara Illowsky, Susan Dean
- Publisher/website: OpenStax
- Book title: Statistics
- Publication date: Mar 27, 2020
- Location: Houston, Texas
- Book URL: https://openstax.org/books/statistics/pages/1-introduction
- Section URL: https://openstax.org/books/statistics/pages/9-1-null-and-alternative-hypotheses
© Jan 23, 2024 Texas Education Agency (TEA). The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

- Foundations
- Write Paper
Search form
- Experiments
- Anthropology
- Self-Esteem
- Social Anxiety

Null Hypothesis
The null hypothesis, H 0 , is an essential part of any research design, and is always tested, even indirectly.
This article is a part of the guide:
- Research Hypothesis
- Defining a Research Problem
- Selecting Method
- Test Hypothesis
Browse Full Outline
- 1 Scientific Method
- 2.1.1 Null Hypothesis
- 2.1.2 Research Hypothesis
- 2.2 Prediction
- 2.3 Conceptual Variable
- 3.1 Operationalization
- 3.2 Selecting Method
- 3.3 Measurements
- 3.4 Scientific Observation
- 4.1 Empirical Evidence
- 5.1 Generalization
- 5.2 Errors in Conclusion
The simplistic definition of the null is as the opposite of the alternative hypothesis , H 1 , although the principle is a little more complex than that.
The null hypothesis (H 0 ) is a hypothesis which the researcher tries to disprove, reject or nullify.
The 'null' often refers to the common view of something, while the alternative hypothesis is what the researcher really thinks is the cause of a phenomenon.
An experiment conclusion always refers to the null, rejecting or accepting H 0 rather than H 1 .
Despite this, many researchers neglect the null hypothesis when testing hypotheses , which is poor practice and can have adverse effects.

Examples of the Null Hypothesis
A researcher may postulate a hypothesis:
H 1 : Tomato plants exhibit a higher rate of growth when planted in compost rather than in soil.
And a null hypothesis:
H 0 : Tomato plants do not exhibit a higher rate of growth when planted in compost rather than soil.
It is important to carefully select the wording of the null, and ensure that it is as specific as possible. For example, the researcher might postulate a null hypothesis:
H 0 : Tomato plants show no difference in growth rates when planted in compost rather than soil.
There is a major flaw with this H 0 . If the plants actually grow more slowly in compost than in soil, an impasse is reached. H 1 is not supported, but neither is H 0 , because there is a difference in growth rates.
If the null is rejected, with no alternative, the experiment may be invalid. This is the reason why science uses a battery of deductive and inductive processes to ensure that there are no flaws in the hypotheses.
Many scientists neglect the null, assuming that it is merely the opposite of the alternative, but it is good practice to spend a little time creating a sound hypothesis. It is not possible to change any hypothesis retrospectively, including H 0 .

Significance Tests
If significance tests generate 95% or 99% likelihood that the results do not fit the null hypothesis, then it is rejected, in favor of the alternative.
Otherwise, the null is accepted. These are the only correct assumptions, and it is incorrect to reject, or accept, H 1 .
Accepting the null hypothesis does not mean that it is true. It is still a hypothesis, and must conform to the principle of falsifiability , in the same way that rejecting the null does not prove the alternative.
Perceived Problems With the Null
The major problem with the H 0 is that many researchers, and reviewers, see accepting the null as a failure of the experiment . This is very poor science, as accepting or rejecting any hypothesis is a positive result.
Even if the null is not refuted, the world of science has learned something new. Strictly speaking, the term ‘failure’, should only apply to errors in the experimental design , or incorrect initial assumptions.
Development of the Null
The Flat Earth model was common in ancient times, such as in the civilizations of the Bronze Age or Iron Age. This may be thought of as the null hypothesis, H 0 , at the time.
H 0 : World is Flat
Many of the Ancient Greek philosophers assumed that the sun, moon and other objects in the universe circled around the Earth. Hellenistic astronomy established the spherical shape of the earth around 300 BC.
H 0 : The Geocentric Model: Earth is the centre of the Universe and it is Spherical
Copernicus had an alternative hypothesis , H 1 that the world actually circled around the sun, thus being the center of the universe. Eventually, people got convinced and accepted it as the null, H 0 .
H 0 : The Heliocentric Model: Sun is the centre of the universe
Later someone proposed an alternative hypothesis that the sun itself also circled around the something within the galaxy, thus creating a new H 0 . This is how research works - the H 0 gets closer to the reality each time, even if it isn't correct, it is better than the last H 0 .
- Psychology 101
- Flags and Countries
- Capitals and Countries
Martyn Shuttleworth (Feb 3, 2008). Null Hypothesis. Retrieved May 29, 2024 from Explorable.com: https://explorable.com/null-hypothesis
You Are Allowed To Copy The Text
The text in this article is licensed under the Creative Commons-License Attribution 4.0 International (CC BY 4.0) .
This means you're free to copy, share and adapt any parts (or all) of the text in the article, as long as you give appropriate credit and provide a link/reference to this page.
That is it. You don't need our permission to copy the article; just include a link/reference back to this page. You can use it freely (with some kind of link), and we're also okay with people reprinting in publications like books, blogs, newsletters, course-material, papers, wikipedia and presentations (with clear attribution).
Related articles
How to Write a Hypothesis
Statistical Hypothesis Testing
Hypothetico-Deductive Method
Testability
Want to stay up to date? Follow us!
Get all these articles in 1 guide.
Want the full version to study at home, take to school or just scribble on?
Whether you are an academic novice, or you simply want to brush up your skills, this book will take your academic writing skills to the next level.

Download electronic versions: - Epub for mobiles and tablets - For Kindle here - PDF version here
Save this course for later
Don't have time for it all now? No problem, save it as a course and come back to it later.
Footer bottom
- Privacy Policy

- Subscribe to our RSS Feed
- Like us on Facebook
- Follow us on Twitter

- school Campus Bookshelves
- menu_book Bookshelves
- perm_media Learning Objects
- login Login
- how_to_reg Request Instructor Account
- hub Instructor Commons
Margin Size
- Download Page (PDF)
- Download Full Book (PDF)
- Periodic Table
- Physics Constants
- Scientific Calculator
- Reference & Cite
- Tools expand_more
- Readability
selected template will load here
This action is not available.

2.2: Standard Statistical Hypothesis Testing
- Last updated
- Save as PDF
- Page ID 21580

- Luke J. Harmon
- University of Idaho
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
Standard hypothesis testing approaches focus almost entirely on rejecting null hypotheses. In the framework (usually referred to as the frequentist approach to statistics) one first defines a null hypothesis. This null hypothesis represents your expectation if some pattern, such as a difference among groups, is not present, or if some process of interest were not occurring. For example, perhaps you are interested in comparing the mean body size of two species of lizards, an anole and a gecko. Our null hypothesis would be that the two species do not differ in body size. The alternative, which one can conclude by rejecting that null hypothesis, is that one species is larger than the other. Another example might involve investigating two variables, like body size and leg length, across a set of lizard species 1 . Here the null hypothesis would be that there is no relationship between body size and leg length. The alternative hypothesis, which again represents the situation where the phenomenon of interest is actually occurring, is that there is a relationship with body size and leg length. For frequentist approaches, the alternative hypothesis is always the negation of the null hypothesis; as you will see below, other approaches allow one to compare the fit of a set of models without this restriction and choose the best amongst them.
The next step is to define a test statistic, some way of measuring the patterns in the data. In the two examples above, we would consider test statistics that measure the difference in mean body size among our two species of lizards, or the slope of the relationship between body size and leg length, respectively. One can then compare the value of this test statistic in the data to the expectation of this test statistic under the null hypothesis. The relationship between the test statistic and its expectation under the null hypothesis is captured by a P-value. The P-value is the probability of obtaining a test statistic at least as extreme as the actual test statistic in the case where the null hypothesis is true. You can think of the P-value as a measure of how probable it is that you would obtain your data in a universe where the null hypothesis is true. In other words, the P-value measures how probable it is under the null hypothesis that you would obtain a test statistic at least as extreme as what you see in the data. In particular, if the P-value is very large, say P = 0.94, then it is extremely likely that your data are compatible with this null hypothesis.
If the test statistic is very different from what one would expect under the null hypothesis, then the P-value will be small. This means that we are unlikely to obtain the test statistic seen in the data if the null hypothesis were true. In that case, we reject the null hypothesis as long as P is less than some value chosen in advance. This value is the significance threshold, α , and is almost always set to α = 0.05. By contrast, if that probability is large, then there is nothing “special” about your data, at least from the standpoint of your null hypothesis. The test statistic is within the range expected under the null hypothesis, and we fail to reject that null hypothesis. Note the careful language here – in a standard frequentist framework, you never accept the null hypothesis, you simply fail to reject it.
Getting back to our lizard-flipping example, we can use a frequentist approach. In this case, our particular example has a name; this is a binomial test, which assesses whether a given event with two outcomes has a certain probability of success. In this case, we are interested in testing the null hypothesis that our lizard is a fair flipper; that is, that the probability of heads p H = 0.5. The binomial test uses the number of “successes” (we will use the number of heads, H = 63) as a test statistic. We then ask whether this test statistic is either much larger or much smaller than we might expect under our null hypothesis. So, our null hypothesis is that p H = 0.5; our alternative, then, is that p H takes some other value: p H ≠ 0.5.
To carry out the test, we first need to consider how many "successes" we should expect if the null hypothesis were true. We consider the distribution of our test statistic (the number of heads) under our null hypothesis ( p H = 0.5). This distribution is a binomial distribution (Figure 2.1).
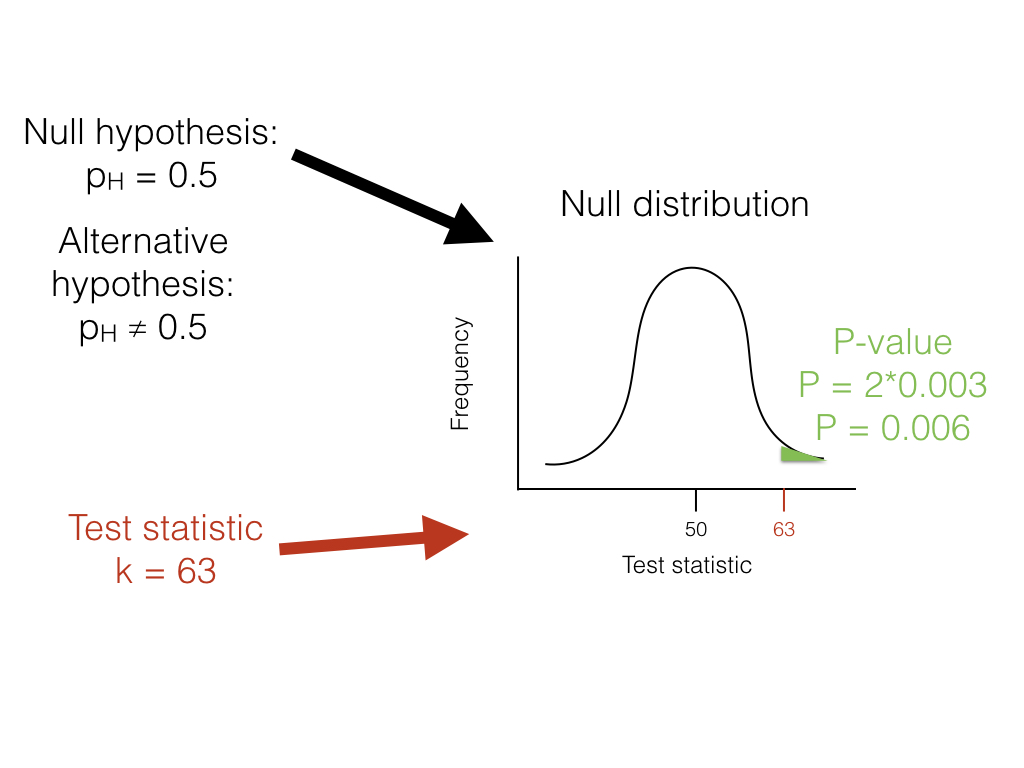
We can use the known probabilities of the binomial distribution to calculate our P-value. We want to know the probability of obtaining a result at least as extreme as our data when drawing from a binomial distribution with parameters p = 0.5 and n = 100. We calculate the area of this distribution that lies to the right of 63. This area, P = 0.003, can be obtained either from a table, from statistical software, or by using a relatively simple calculation. The value, 0.003, represents the probability of obtaining at least 63 heads out of 100 trials with p H = 0.5. This number is the P-value from our binomial test. Because we only calculated the area of our null distribution in one tail (in this case, the right, where values are greater than or equal to 63), then this is actually a one-tailed test, and we are only considering part of our null hypothesis where p H > 0.5. Such an approach might be suitable in some cases, but more typically we need to multiply this number by 2 to get a two-tailed test; thus, P = 0.006. This two-tailed P-value of 0.006 includes the possibility of results as extreme as our test statistic in either direction, either too many or too few heads. Since P < 0.05, our chosen α value, we reject the null hypothesis, and conclude that we have an unfair lizard.
In biology, null hypotheses play a critical role in many statistical analyses. So why not end this chapter now? One issue is that biological null hypotheses are almost always uninteresting. They often describe the situation where patterns in the data occur only by chance. However, if you are comparing living species to each other, there are almost always some differences between them. In fact, for biology, null hypotheses are quite often obviously false. For example, two different species living in different habitats are not identical, and if we measure them enough we will discover this fact. From this point of view, both outcomes of a standard hypothesis test are unenlightening. One either rejects a silly hypothesis that was probably known to be false from the start, or one “fails to reject” this null hypothesis 2 . There is much more information to be gained by estimating parameter values and carrying out model selection in a likelihood or Bayesian framework, as we will see below. Still, frequentist statistical approaches are common, have their place in our toolbox, and will come up in several sections of this book.
One key concept in standard hypothesis testing is the idea of statistical error. Statistical errors come in two flavors: type I and type II errors. Type I errors occur when the null hypothesis is true but the investigator mistakenly rejects it. Standard hypothesis testing controls type I errors using a parameter, α , which defines the accepted rate of type I errors. For example, if α = 0.05, one should expect to commit a type I error about 5% of the time. When multiple standard hypothesis tests are carried out, investigators often “correct” their P-values using Bonferroni correction. If you do this, then there is only a 5% chance of a single type I error across all of the tests being considered. This singular focus on type I errors, however, has a cost. One can also commit type II errors, when the null hypothesis is false but one fails to reject it. The rate of type II errors in statistical tests can be extremely high. While statisticians do take care to create approaches that have high power, traditional hypothesis testing usually fixes type I errors at 5% while type II error rates remain unknown. There are simple ways to calculate type II error rates (e.g. power analyses) but these are only rarely carried out. Furthermore, Bonferroni correction dramatically increases the type II error rate. This is important because – as stated by Perneger (1998) – “… type II errors are no less false than type I errors.” This extreme emphasis on controlling type I errors at the expense of type II errors is, to me, the main weakness of the frequentist approach 3 .
I will cover some examples of the frequentist approach in this book, mainly when discussing traditional methods like phylogenetic independent contrasts (PICs). Also, one of the model selection approaches used frequently in this book, likelihood ratio tests, rely on a standard frequentist set-up with null and alternative hypotheses.
However, there are two good reasons to look for better ways to do comparative statistics. First, as stated above, standard methods rely on testing null hypotheses that – for evolutionary questions - are usually very likely, a priori, to be false. For a relevant example, consider a study comparing the rate of speciation between two clades of carnivores. The null hypothesis is that the two clades have exactly equal rates of speciation – which is almost certainly false, although we might question how different the two rates might be. Second, in my opinion, standard frequentist methods place too much emphasis on P-values and not enough on the size of statistical effects. A small P-value could reflect either a large effect or very large sample sizes or both.
In summary, frequentist statistical methods are common in comparative statistics but can be limiting. I will discuss these methods often in this book, mainly due to their prevalent use in the field. At the same time, we will look for alternatives whenever possible.
If you're seeing this message, it means we're having trouble loading external resources on our website.
If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked.
To log in and use all the features of Khan Academy, please enable JavaScript in your browser.
AP®︎/College Statistics
Course: ap®︎/college statistics > unit 10.
- Idea behind hypothesis testing
Examples of null and alternative hypotheses
- Writing null and alternative hypotheses
- P-values and significance tests
- Comparing P-values to different significance levels
- Estimating a P-value from a simulation
- Estimating P-values from simulations
- Using P-values to make conclusions
Want to join the conversation?
- Upvote Button navigates to signup page
- Downvote Button navigates to signup page
- Flag Button navigates to signup page

Video transcript
- Math Article
Null Hypothesis

In mathematics, Statistics deals with the study of research and surveys on the numerical data. For taking surveys, we have to define the hypothesis. Generally, there are two types of hypothesis. One is a null hypothesis, and another is an alternative hypothesis .
In probability and statistics, the null hypothesis is a comprehensive statement or default status that there is zero happening or nothing happening. For example, there is no connection among groups or no association between two measured events. It is generally assumed here that the hypothesis is true until any other proof has been brought into the light to deny the hypothesis. Let us learn more here with definition, symbol, principle, types and example, in this article.
Table of contents:
- Comparison with Alternative Hypothesis
Null Hypothesis Definition
The null hypothesis is a kind of hypothesis which explains the population parameter whose purpose is to test the validity of the given experimental data. This hypothesis is either rejected or not rejected based on the viability of the given population or sample . In other words, the null hypothesis is a hypothesis in which the sample observations results from the chance. It is said to be a statement in which the surveyors wants to examine the data. It is denoted by H 0 .
Null Hypothesis Symbol
In statistics, the null hypothesis is usually denoted by letter H with subscript ‘0’ (zero), such that H 0 . It is pronounced as H-null or H-zero or H-nought. At the same time, the alternative hypothesis expresses the observations determined by the non-random cause. It is represented by H 1 or H a .
Null Hypothesis Principle
The principle followed for null hypothesis testing is, collecting the data and determining the chances of a given set of data during the study on some random sample, assuming that the null hypothesis is true. In case if the given data does not face the expected null hypothesis, then the outcome will be quite weaker, and they conclude by saying that the given set of data does not provide strong evidence against the null hypothesis because of insufficient evidence. Finally, the researchers tend to reject that.
Null Hypothesis Formula
Here, the hypothesis test formulas are given below for reference.
The formula for the null hypothesis is:
H 0 : p = p 0
The formula for the alternative hypothesis is:
H a = p >p 0 , < p 0 ≠ p 0
The formula for the test static is:
Remember that, p 0 is the null hypothesis and p – hat is the sample proportion.
Also, read:
Types of Null Hypothesis
There are different types of hypothesis. They are:
Simple Hypothesis
It completely specifies the population distribution. In this method, the sampling distribution is the function of the sample size.
Composite Hypothesis
The composite hypothesis is one that does not completely specify the population distribution.
Exact Hypothesis
Exact hypothesis defines the exact value of the parameter. For example μ= 50
Inexact Hypothesis
This type of hypothesis does not define the exact value of the parameter. But it denotes a specific range or interval. For example 45< μ <60
Null Hypothesis Rejection
Sometimes the null hypothesis is rejected too. If this hypothesis is rejected means, that research could be invalid. Many researchers will neglect this hypothesis as it is merely opposite to the alternate hypothesis. It is a better practice to create a hypothesis and test it. The goal of researchers is not to reject the hypothesis. But it is evident that a perfect statistical model is always associated with the failure to reject the null hypothesis.
How do you Find the Null Hypothesis?
The null hypothesis says there is no correlation between the measured event (the dependent variable) and the independent variable. We don’t have to believe that the null hypothesis is true to test it. On the contrast, you will possibly assume that there is a connection between a set of variables ( dependent and independent).
When is Null Hypothesis Rejected?
The null hypothesis is rejected using the P-value approach. If the P-value is less than or equal to the α, there should be a rejection of the null hypothesis in favour of the alternate hypothesis. In case, if P-value is greater than α, the null hypothesis is not rejected.
Null Hypothesis and Alternative Hypothesis
Now, let us discuss the difference between the null hypothesis and the alternative hypothesis.
Null Hypothesis Examples
Here, some of the examples of the null hypothesis are given below. Go through the below ones to understand the concept of the null hypothesis in a better way.
If a medicine reduces the risk of cardiac stroke, then the null hypothesis should be “the medicine does not reduce the chance of cardiac stroke”. This testing can be performed by the administration of a drug to a certain group of people in a controlled way. If the survey shows that there is a significant change in the people, then the hypothesis is rejected.
Few more examples are:
1). Are there is 100% chance of getting affected by dengue?
Ans: There could be chances of getting affected by dengue but not 100%.
2). Do teenagers are using mobile phones more than grown-ups to access the internet?
Ans: Age has no limit on using mobile phones to access the internet.
3). Does having apple daily will not cause fever?
Ans: Having apple daily does not assure of not having fever, but increases the immunity to fight against such diseases.
4). Do the children more good in doing mathematical calculations than grown-ups?
Ans: Age has no effect on Mathematical skills.
In many common applications, the choice of the null hypothesis is not automated, but the testing and calculations may be automated. Also, the choice of the null hypothesis is completely based on previous experiences and inconsistent advice. The choice can be more complicated and based on the variety of applications and the diversity of the objectives.
The main limitation for the choice of the null hypothesis is that the hypothesis suggested by the data is based on the reasoning which proves nothing. It means that if some hypothesis provides a summary of the data set, then there would be no value in the testing of the hypothesis on the particular set of data.
Frequently Asked Questions on Null Hypothesis
What is meant by the null hypothesis.
In Statistics, a null hypothesis is a type of hypothesis which explains the population parameter whose purpose is to test the validity of the given experimental data.
What are the benefits of hypothesis testing?
Hypothesis testing is defined as a form of inferential statistics, which allows making conclusions from the entire population based on the sample representative.

When a null hypothesis is accepted and rejected?
The null hypothesis is either accepted or rejected in terms of the given data. If P-value is less than α, then the null hypothesis is rejected in favor of the alternative hypothesis, and if the P-value is greater than α, then the null hypothesis is accepted in favor of the alternative hypothesis.
Why is the null hypothesis important?
The importance of the null hypothesis is that it provides an approximate description of the phenomena of the given data. It allows the investigators to directly test the relational statement in a research study.
How to accept or reject the null hypothesis in the chi-square test?
If the result of the chi-square test is bigger than the critical value in the table, then the data does not fit the model, which represents the rejection of the null hypothesis.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Maths related queries and study materials
Your result is as below
Request OTP on Voice Call
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 01 April 2023
PIEZO1 and PECAM1 interact at cell-cell junctions and partner in endothelial force sensing
- Eulashini Chuntharpursat-Bon ORCID: orcid.org/0000-0002-0767-6877 1 na2 ,
- Oleksandr V. Povstyan 1 na1 ,
- Melanie J. Ludlow 1 na1 ,
- David J. Carrier 1 , 2 ,
- Marjolaine Debant 1 ,
- Jian Shi 1 ,
- Hannah J. Gaunt 1 ,
- Claudia C. Bauer 1 ,
- Alistair Curd ORCID: orcid.org/0000-0002-3949-7523 3 ,
- T. Simon Futers ORCID: orcid.org/0000-0003-3810-190X 1 ,
- Paul D. Baxter 1 ,
- Michelle Peckham ORCID: orcid.org/0000-0002-3754-2028 3 , 4 ,
- Stephen P. Muench ORCID: orcid.org/0000-0001-6869-4414 2 , 4 ,
- Antony Adamson ORCID: orcid.org/0000-0002-5408-0013 5 ,
- Neil Humphreys 5 ,
- Sarka Tumova ORCID: orcid.org/0000-0003-2044-4998 1 ,
- Robin S. Bon ORCID: orcid.org/0000-0003-1733-3680 1 , 4 ,
- Richard Cubbon 1 ,
- Laeticia Lichtenstein ORCID: orcid.org/0000-0003-3900-786X 1 &
- David J. Beech ORCID: orcid.org/0000-0002-7683-9422 1 na2
Communications Biology volume 6 , Article number: 358 ( 2023 ) Cite this article
22 Citations
8 Altmetric
Metrics details
- Adherens junctions
- Mechanotransduction
Two prominent concepts for the sensing of shear stress by endothelium are the PIEZO1 channel as a mediator of mechanically activated calcium ion entry and the PECAM1 cell adhesion molecule as the apex of a triad with CDH5 and VGFR2. Here, we investigated if there is a relationship. By inserting a non-disruptive tag in native PIEZO1 of mice, we reveal in situ overlap of PIEZO1 with PECAM1. Through reconstitution and high resolution microscopy studies we show that PECAM1 interacts with PIEZO1 and directs it to cell-cell junctions. PECAM1 extracellular N-terminus is critical in this, but a C-terminal intracellular domain linked to shear stress also contributes. CDH5 similarly drives PIEZO1 to junctions but unlike PECAM1 its interaction with PIEZO1 is dynamic, increasing with shear stress. PIEZO1 does not interact with VGFR2. PIEZO1 is required in Ca 2+ -dependent formation of adherens junctions and associated cytoskeleton, consistent with it conferring force-dependent Ca 2+ entry for junctional remodelling. The data suggest a pool of PIEZO1 at cell junctions, the coming together of PIEZO1 and PECAM1 mechanisms and intimate cooperation of PIEZO1 and adhesion molecules in tailoring junctional structure to mechanical requirement.
Similar content being viewed by others
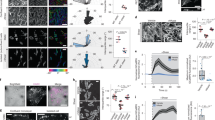
The spectrin cytoskeleton integrates endothelial mechanoresponses
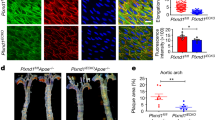
The guidance receptor plexin D1 is a mechanosensor in endothelial cells
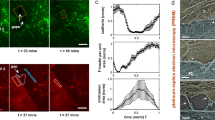
Feedback between mechanosensitive signaling and active forces governs endothelial junction integrity
Introduction.
The endothelium comprises a monolayer of endothelial cells at the inner surface of all arteries, veins, capillaries and lymphatics. A key function is to provide a selective barrier to the exchange of substances and cells between blood and tissue 1 , 2 . Endothelial cell permeability, transmembrane proteins and the structures between endothelial cells such as adherens junctions all contribute to the barrier 1 , 2 . These systems operate in the context of mechanical forces caused by the heartbeat, skeletal and smooth muscle-induced movements, gravity, interstitial pressure, cell-cell interactions and cell-matrix interactions 3 , 4 , 5 , 6 , 7 . The flow of blood and lymph have particular importance because of the shear stress they generate at the endothelial surface 3 , 4 , 8 . Shear stress varies in ferocity and orientation depending on vascular architecture and must be coordinated with other forces such as pulsatile circumferential strain 3 . There has been progress in understanding how these forces interact with the biological mechanisms but much remains opaque; especially regarding the sensing of local mechanical forces, the integration of such sensing with other endothelial mechanisms and the subcellular organisation of the sensing mechanisms.
PECAM1 (Platelet Endothelial Cell Adhesion Molecule 1 or CD31) is a candidate mediator of endothelial responses to shear stress 9 , even though its predominant localisation at adherens junctions hides most of it from shear stress 1 , 4 , 8 . CDH5 (Cadherin-5 or Vascular endothelial (VE) cadherin) is included in the PECAM1 hypothesis along with vascular endothelial growth factor receptor 2 (VGFR2), leading to the concept of a PECAM1 triad as the shear stress sensor 10 , 11 , 12 , 13 , 14 , 15 , 16 . PECAM1 and CDH5 are key endothelial cell adhesion molecules. They are single-pass membrane proteins that mediate cell contact and junctional integrity 2 , 17 , 18 , 19 , 20 , 21 . PECAM1 belongs to type-I membrane glycoprotein and immunoglobulin super-families 17 , 20 , 22 . It has an extracellular region containing immunoglobulin-like domains, a transmembrane region of one α-helix and a cytoplasmic region containing tyrosine regulatory motifs. It is often used to identify endothelial cells, though is also expressed and functional in leucocytes and platelets 17 , 20 . CDH5 belongs to a family of transmembrane Ca 2+ -dependent adhesion molecules 19 , 21 , 23 . Like PECAM1, it has an extracellular domain that mediates homotypic interactions and intracellular regulatory sites, which, in this case, bind β- or γ-catenin to promote cytoskeletal interaction 19 , 21 . CDH5 is likened to a biochemical Velcro and may also be a mechanical transducer 24 .
PIEZO1 protein was recognised later 25 , 26 , 27 , 28 and suggested as a mediator of endothelial responses to shear stress 29 , 30 , 31 , 32 . It assembles as trimers to form large Ca 2+ -permeable non-selective cationic channels, each with 114 (3×38) membrane-spanning segments 25 , 27 , 33 , 34 , 35 . These channels are exquisitely sensitive to activation by various mechanical forces 25 , 26 , 27 , 28 , 29 . Importantly in the context of endothelial cell and other cell biology, shear stress activates PIEZO1 channels 29 , 30 , 31 , 32 , 36 , 37 , 38 . PIEZO1 is strongly, although not uniquely, expressed in endothelial cells 26 , 29 . It is required for endothelial cell responses to force such as their alignment to the direction of fluid flow and the activation of endothelial nitric oxide synthase (NOS3) 29 , 30 , 32 . Shear stress-activated PIEZO1 channels are present in the apical endothelial membrane 38 , 39 and so they are ideally located to mediate the sensing of shear stress, albeit potentially via or amplified by intermediates of the glycocalyx 40 and spectrin cytoskeleton 41 .
The study described here was motivated by a desire to understand the apparently competing ideas for sensing of shear stress by the PECAM1 triad and PIEZO1, but this relationship is also important to investigate because of the relationship between PIEZO1 and endothelial cell-cell junctions, and thus the integrity of the endothelial cell monolayer and its permeability. Endothelial-specific PIEZO1 disruption in mice suppresses or enhances vascular permeability caused by excess vascular or alveolar pressures 42 , 43 . In mice, endothelial PIEZO1 is required for leucocyte diapedesis 44 , which is the process of leucocytes passing through the endothelial monolayer. In cultured mouse or human endothelial cells, stimulation of PIEZO1 by a small-molecule agonist (Yoda1) straightens CDH5 junctions 45 and PIEZO1 depletion inhibits stretch-evoked remodelling of endothelial cell adherens junctions 46 . These data suggest that PIEZO1 is an important regulator of cell-cell junctions in addition to having a role in sensing shear stress. In mechanistic interpretations of these and other such data, PIEZO1 is currently placed at the apical endothelial surface, signalling from a distance to cell-cell junctions and other mechanisms 1 , 47 . We agree with such a location of PIEZO1 channels 38 , 39 but hypothesise greater complexity.
Here we suggest a pool of PIEZO1 at adherens junctions, interactions of PIEZO1 with PECAM1 and CDH5 and roles of PIEZO1 in cell junction remodelling. First, to enable specific labelling of endogenous PIEZO1 and thus determination of its localisation in vivo, we engineered a mouse with a non-disruptive tag in PIEZO1.
Genetic engineering of mice enables insertion of a non-disruptive HA tag in native PIEZO1
For definitive localisation of endogenous PIEZO1, we genetically modified mice to encode a non-disruptive haemagglutinin (HA) tag in the C-terminal Extracellular Domain (CED) of native PIEZO1 (Supplementary Fig. 1 ). Activity of these PIEZO1 HA channels was recorded in endothelium of mesenteric artery where we previously showed the presence of wild-type PIEZO1 (PIEZO1 WT ) channels activated by fluid flow, membrane stretch or the PIEZO1 small-molecule agonist Yoda1 38 , 39 . The PIEZO1 HA channels studied in excised outside-out endothelial membrane patches are similar to PIEZO1 WT channels in their activation by fluid flow, unitary conductance and sensitivity to inhibition by gadolinium ions (Gd 3+ ), which non-specifically inhibit PIEZO1 channels 25 , 38 (Fig. 1a–c cf Fig. 1f–h, k ). There is basal activity of the PIEZO1 HA channels in the static (no-flow) condition (Fig. 1a, k ) as reported previously and shown independently here for PIEZO1 WT channels 38 , 39 (Fig. 1f, k ). Membrane potential recordings from multicellular endothelial fragments similarly obtained freshly from the arteries of PIEZO1 HA mice depolarise in response to fluid flow (Fig. 1d, e, k ), again similar to wild-type endothelium (Fig. 1i–k ) and as expected for PIEZO1 activity 38 , 39 . Consistent with similar properties of native PIEZO1 HA and PIEZO1 WT channels, PIEZO1 HA mice appear healthy and breed normally (Supplementary Fig. 2a, b ), in contrast to PIEZO1 knockouts, which are embryonic lethal 30 , 37 . Red blood cells (RBCs) are an abundant and readily purified cell type that expresses PIEZO1 48 , 49 and so they were used for PIEZO1 HA detection by western blotting, which reveals PIEZO1 HA protein of the expected mass (Supplementary Fig. 2c ), again similar to that of PIEZO1 WT 30 . The data suggest suitability of PIEZO1 HA mice for determining native localisation of PIEZO1.
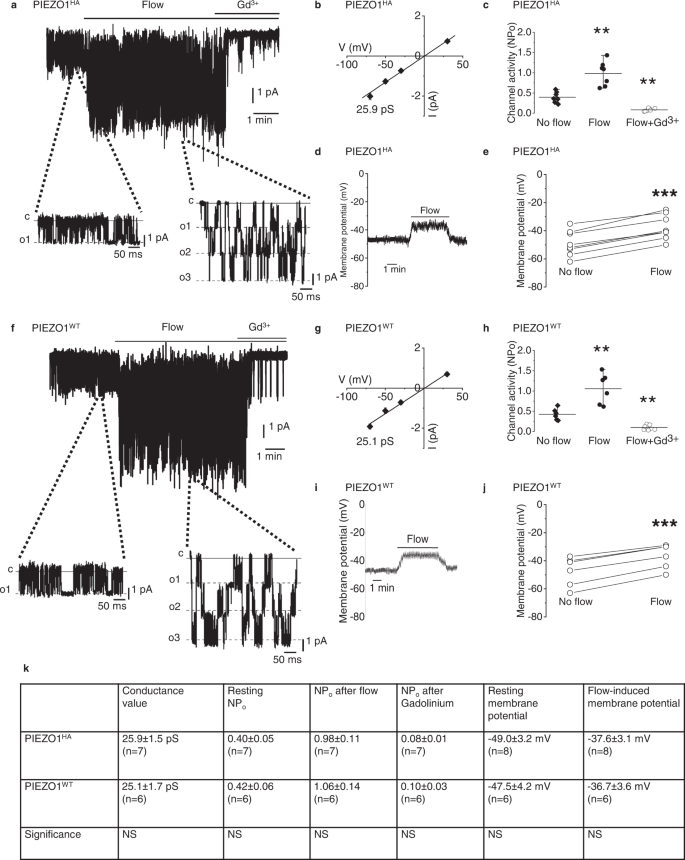
a Example current recording from an outside-out patch from freshly isolated endothelium of second-order mesenteric artery of PIEZO1 HA mouse. Holding potential, −70 mV. Fluid flow, 20 μl.s −1 . Gadolinium (III) ion (Gd 3+ ), 10 μM. Currents on expanded time-base are shown below in which c indicates the closed channel current level and o1, o2 and o3 the open channel current levels for simultaneous openings of up to 3 channels. b Mean ± s.e.mean unitary current amplitudes for flow-induced ion channel activity as in a plotted against holding voltage ( n = 7 independent recordings). The fitted line indicates unitary conductance of 25.9 pS. c Channel activity indicated by NP o (number of channels per patch × probability of channel opening) for experiments of the type exemplified in a for no flow and flow conditions with or without Gd 3+ . Individual data points for each experiment are represented by symbols, superimposed on which are the mean ± s.e.mean values (** P Flow = 0.0001703, ** P Flow+Gd3+ = 0.000165, n = 7 for each group). d Example trace of membrane potential measured from freshly isolated endothelium of second-order mouse mesenteric artery of PIEZO1 HA mouse. Fluid flow, 20 μl.s −1 . e Individual data points representing membrane potentials before and after flow as exemplified in d (*** P = 0.00000343) ( n = 7). Data points connected by a line were from the same recording. f Example current recording from an outside-out patch from freshly isolated endothelium of second-order mesenteric artery of PIEZO1 WT mouse. Holding potential, −70 mV. Fluid flow, 20 μl.s −1 . Gadolinium (III) ion (Gd 3+ ), 10 μM. g Mean ± s.e.mean unitary current amplitudes for flow-induced ion channel activity as in b plotted against holding voltage ( n = 6 independent recordings). The fitted line indicates unitary conductance of 25.9 pS. h Channel activity indicated by NP o (number of channels per patch × probability of channel opening) for experiments of the type exemplified in f for no flow and flow conditions with or without Gd 3+ . Individual data points for each experiment are represented by symbols, superimposed on which are the mean ± s.e.mean values (** P Flow = 0.000141, ** P Flow+Gd3+ = 0.000241, n = 6 for each group). i Example trace of membrane potential measured from freshly isolated endothelium of second-order mouse mesenteric artery of PIEZO1 WT mouse. Fluid flow, 20 μl.s −1 . j Individual data points representing membrane potentials before and after flow as exemplified in i ( P = 0.0000375, n = 6). Data points connected by a line were from the same recording. k Table of data comparing values from PIEZO1 HA mouse and PIEZO1 WT mouse, (mean ± s.e.m.). NS indicates no statistically significant difference between PIEZO1 HA and PIEZO1 WT . For the outside-out patch and membrane potential recordings, the external solution consisted of: 135 mM NaCl, 4 mM KCl, 2 mM CaCl 2 , 1 mM MgCl 2 , 10 mM glucose and 10 mM HEPES (titrated to pH 7.4 with NaOH). The patch pipette contained: 145 mM KCl, 1 mM MgCl 2 , 0.5 mM EGTA and 10 mM HEPES (titrated to pH 7.2 with KOH).
PIEZO1 HA expression pattern is similar to that of PECAM1 in endothelium
To explore PIEZO1 localisation, we studied the retina where the entire vascular tree is imaged in one sample (Supplementary Fig. 3 ). PIEZO1 HA is detected in retinal veins (Fig. 2a, b cf further data and controls shown in Supplementary Fig. 4a–e ). Close inspection shows PIEZO1 at areas of cell-cell contact where PECAM1 is predominantly expressed (+PECAM1) (Fig. 2a, c ). In non-junctional areas without PECAM1 (−PECAM1), the normalised fluorescence signal is close to 0.4 (Fig. 2c ), which is at or near the background values obtained from PIEZO1 WT tissues under similar conditions and the same microscope settings (Supplementary Fig. 4a–c, f–k ). PIEZO1 HA is not detected in retinal artery, although PIEZO1 may be in these arteries 50 but below the threshold for detection in our assay (Supplementary Fig. 4f–k ). PIEZO1 HA is detected in retinal intermediate and deep capillaries (Supplementary Fig. 4l–q ). The data suggest that PIEZO1 is at points of endothelial cell-cell contact with PECAM1.
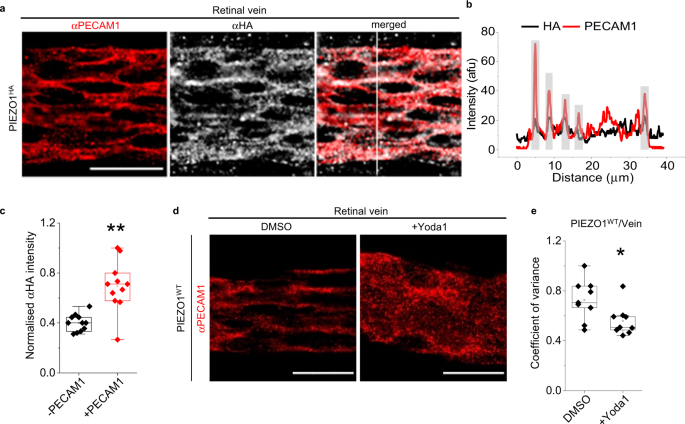
a Images for retinal vein of PIEZO1 HA mouse after immuno-staining with αPECAM1 (red) and αHA (grey). The image on the right side is a merged αPECAM1 and αHA image. Scale bar, 20 µm. b Line-intensity plot for the vertical scan line superimposed in the merged image of a . Grey highlighting indicates cell-cell junctions. c Box-plot quantification of image intensity for PIEZO1 HA retinal veins stained with αHA antibody, shown in arbitrary fluorescence units (afu) normalised to the background measurements in the same afu. ** P = 0.00127 for the comparison of the regions with (+) or without (−) PECAM1. Superimposed data points are average intensity of individual images ( N = 11). Data are for n = 3 independent experiments. d Data obtained after WT mice were infused for 30 min with standard bath solution (SBS) containing DMSO (the solvent for Yoda1) or 3 µM Yoda1. Representative images of d retinal vein immuno-stained with αPECAM1 antibody. Scale bar, 20 µm. Box-plots e show coefficients of variance calculated from scan lines that were vertical to a blood vessel oriented from left to right (* P = 0.02728 for Yoda1 cf DMSO in vein). Lower variance indicates less organised structure. n = 3 independent experiments and 3 replicates were used in each case. Superimposed data points are the coefficient of variance for individual images (DMSO, N = 9 and +Yoda1, N = 9).
Pharmacological activation of PIEZO1 disrupts PECAM1 structural organisation
To determine if PIEZO1 has functional implications for in situ PECAM1, we infused PIEZO1 channel small-molecule agonist Yoda1 51 for 30 min in vivo, using exsanguinated mice to minimise problems due to potential Yoda1 instability and plasma protein binding. Mice were then perfusion-fixed and retinal vasculature was stained. We showed previously that the effects of Yoda1 on mouse endothelial cells are abolished by endothelial-specific PIEZO1 deletion 38 . In the retina, Yoda1 disorders the pattern of PECAM1, notably in vein (Fig. 2d, e , Supplementary Fig. 5a ) but not artery (Supplementary Fig. 5b–d ). The venous specificity aligns with the venous localisation (Fig. 2a, c ), consistent with the idea that Yoda1 acts via PIEZO1. The data suggest that PIEZO1 is capable of regulating the organisation of PECAM1 at endothelial cell-cell junctions.
PIEZO1 channel function decreases when the abundance of PECAM1 increases
We considered the possibility of a two-way relationship between PIEZO1 and PECAM1, with PECAM1 affecting PIEZO1 activity. We studied human umbilical vein endothelial cells (HUVECs) in culture as a model endothelial cell system for mechanistic studies. As HUVECs increase in density (Fig. 3a–c ), they express more PECAM1 (Fig. 3d, e ) and there is more PECAM1 at cell-cell junctions (Fig. 3b ). There is simultaneously reduced PIEZO1-mediated Ca 2+ entry (Fig. 3f, g ). The data suggest that increased abundance of PECAM1 reduces PIEZO1 channel function.
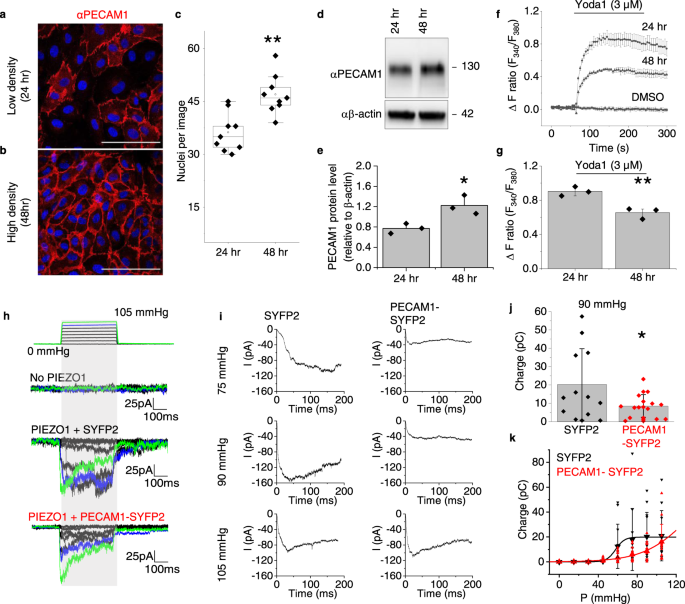
a – g HUVEC data. Representative of 3 independent experiments, confocal images at low a and high b cell density, 24 and 48 h after plating. Cells were stained with αPECAM1 (red) and DAPI (nuclei, blue). Scale bar, 100 µm. c For experiments of the type in a and b , box plots of the number of nuclei per field of view at the 24 and 48 h time-points ( n = 3, ** P = 0.00264). Superimposed data points are nuclei numbers for individual images (24 h, N = 9 and 4 h, N = 9). d Representative western blot and e quantification of the PECAM1 in such blots d normalised to β-actin (mean ± s.e. mean, n = 3, * P = 0.04216, two sample t-test). Superimposed data points are quantification of independent western blots. f , g As for a and b but fura2-based Ca 2+ measurement data. f Representative time-series traces showing effects of 3 μM Yoda1 at the two cell-culture time-points, compared with vehicle control DMSO (3 replicates per data point). g For data of the type in f , mean ± s.e.mean ( n = 3) for peak Ca 2+ signals evoked by Yoda1 (** P = 0.00705, two sample t-test). Superimposed data points are the mean intensity ratios for each independent repeat. h – k Data from outside-out patch recordings on T-Rex™-293 cells. h Example original current traces for empty cells without incorporation of PIEZO1 (No PIEZO1) or T-REx-293 cells with tetracycline-induced expression of human PIEZO1 after transient transfection with SYFP2 (PIEZO1 + SYFP2) or human PECAM1-SYFP2 (PIEZO1 + PECAM1-SYFP2). The holding potential was −80 mV and 200-ms positive pressure pulses were applied from 0 to 105 mmHg in 15 mmHg increments at intervals of 12 s as illustrated above the current traces (traces for 90 and 105 mmHg are highlighted in blue and green respectively). i Average PIEZO1 currents for cells transfected with SYFP2 ( n = 12) or PECAM1-SYFP2 ( n = 13) for each of the indicated pressure steps. j For the same type of data as h , electric charge compared at 90 mmHg for the two groups, suggesting statistically significant difference by t-test (* P = 0.01797). Superimposed individual data points for PIEZO1 + SYFP2 (black, n = 12) and PIEZO1 + PECAM1-SYFP2 (red, n = 15). k For the same type of data as h , mean ± S.D. and superimposed individual data points for PIEZO1 + SYFP2 (black, n = 11) and PIEZO1 + PECAM1-SYFP2 (red, n = 15), showing integrated electrical charge per pressure step plotted against pressure. F-test indicated statistically significant different between the pressure curves of the two conditions (*** P = 7.45 × 10 −8 ).
PECAM1 inhibits PIEZO1 mechanical sensitivity
To more directly test if PECAM1 inhibits PIEZO1 activity, we made patch-clamp recordings of PIEZO1 channel activity in modified HEK 293 cells engineered for conditional overexpression of human PIEZO1 (hPIEZO1 T-Rex™-293 cells) and transfected with PECAM1 tagged with a fluorescent marker protein Super Yellow Fluorescent Protein 2 (SYFP2) or the control, SYFP2 only. We used excised outside-out patches for data collection from the surface membrane and applied positive pressure steps to mechanically activate the channels, as previously reported 52 . As expected 52 , cells expressing PIEZO1 show prominent mechanically activated macroscopic currents (Fig. 3h , Supplementary Fig. 6 ). PECAM1 inhibits the PIEZO1 activity quantified as the total charge flow per pressure step up to 90 mmHg (Fig. 3i, j ), shifting the pressure-response curve to the right (Fig. 3k ). Inactivation is unaffected (Supplementary Fig. 6 ). Mouse PIEZO1 is similarly modulated (Supplementary Fig. 7 ). The data suggest that PECAM1 reduces the mechanical sensitivity of PIEZO1, consistent with the idea of a two-way relationship.
PECAM1 interacts with PIEZO1 N-terminus
N-terminal regions of PIEZO1 determine mechanical sensitivity 33 , 35 , 53 and so PECAM1 may interact with these regions. We tested if there is interaction between PIEZO1 and PECAM1 by coexpressing PECAM1 with Halo-tagged human PIEZO1 or Halo tag alone as a control. Using an antibody to the Halo Tag, PECAM1 co-precipitates with Halo-tagged PIEZO1, but not with the Halo tag alone, suggesting that PECAM1 and PIEZO1 interact (Supplementary Fig. 8a ). Proteins were also purified using GST-anti-GFP nanobodies 54 from HEK 293 cells coexpressing PECAM1 and green fluorescent protein (GFP)-tagged PIEZO1 (PIEZO1-GFP) and then purified by size-exclusion chromatography. There is copurification of PECAM1 with PIEZO1, along with the detached GST-anti-GFP nanobody, again suggesting interaction (Supplementary Fig. 8b ). We next generated HA-tagged PIEZO1 deletion constructs that retained the N-terminus (Supplementary Fig. 8c ). We did not delete the N-terminus because of its importance for surface trafficking 55 . The constructs were coexpressed with PECAM1 and precipitated using anti-HA antibody. PIEZO1 without HA was a control for non-specificity and did not precipitate but all HA-tagged PIEZO1 constructs precipitated, including the shortest N-terminal fragment T6 (Supplementary Fig. 8d ). The data suggest that PECAM1 inhibits PIEZO1 by interacting with its N-terminal regions.
Reconstituted PIEZO1 and PECAM1 are at cell-cell junctions and physically close
To investigate molecular details of PECAM1-PIEZO1 relationships in subcellular regions, we sought a host cell reconstitution system for high resolution light microscopy studies. African green monkey kidney COS-7 cells are such cells. They normally express little or no PIEZO1 or PECAM1, conferring a relatively null background. Exogenous PECAM1 was previously shown to naturally accumulate at COS-7 cell-cell junctions through diffusion trapping, suggesting suitability of these cells as a PECAM1 host 56 . The cells allow reconstitution of the PECAM1 triad and endothelial cell-like alignment to shear stress 11 . We therefore transfected human PECAM1 into COS-7 cells and labelled it with antibody targeted to PECAM1 extracellular N-terminus. HA was engineered into the human PIEZO1 C-terminal extracellular domain (CED) and transfected into COS-7 cells for specific labelling with the antibody targeted to HA. Cells were unpermeabilised, thereby allowing selective labelling of surface membrane proteins, and grown to confluence, so that they had cell-cell junctions. Transfection efficiency was optimised to minimise protein abundance while still enabling detection, resulting in only some cells being transfected and visualised.
Stimulated emission depletion (STED) microscopy was used for imaging at ~50 nm spatial resolution (Supplementary Fig. 9a–d ). PECAM1 is located in puncta concentrated at cell junctions (Supplementary Fig. 9b ). PIEZO1 HA puncta are slightly larger (Supplementary Fig. 9c ). Merged PECAM1 and PIEZO1 HA images show that the two puncta are close to each other, particularly at cell-cell junctions (Supplementary Fig. 9d ). We modelled the distribution of distances between PIEZO1 HA and PECAM1 with non-Gaussian distributions as described previously 57 , 58 (see also the Methods section). From the fitted distribution of distances between PIEZO1 HA particles and their nearest PECAM1 particles, we found frequent proximity averaging 34 nm and occasional proximity averaging 169 nm at cell-cell junctions (Supplementary Fig. 9e–h ). At non-junctional regions, models fitted poorly, resulting in parameter uncertainties greater than parameter estimates, but proximity of ~50 nm is inferred from inspection of the histogram (Supplementary Fig. 9i–k ). The data suggest close proximity of the reconstituted proteins at cell-cell junctions, consistent with the two proteins interacting.
PECAM1 drives PIEZO1 to cell-cell junctions
To investigate the PIEZO1-PECAM1 relationship in more detail, fluorescence lifetime imaging microscopy (FLIM) was used for quantification of Förster Resonance Energy Transfer (FRET), which occurs at distances of less than 10 nm. On PIEZO1 we engineered a donor fluorophore (mTurquoise2) and on PECAM1 an acceptor fluorophore, for which we used SYFP2. We inserted a linker between the target protein and fluorescent tag for both constructs. Expressed alone, PIEZO1-mTurquoise2 is primarily around nuclei (N) and in endoplasmic reticulum (ER) but not at cell-cell junctions (Fig. 4a ). By contrast, when coexpressed with PECAM1-SYFP2, the PIEZO1 enriches at points of cell-cell contact (Fig. 4b ). The data suggest that PECAM1 drives a pool of PIEZO1 to cell-cell junctions.
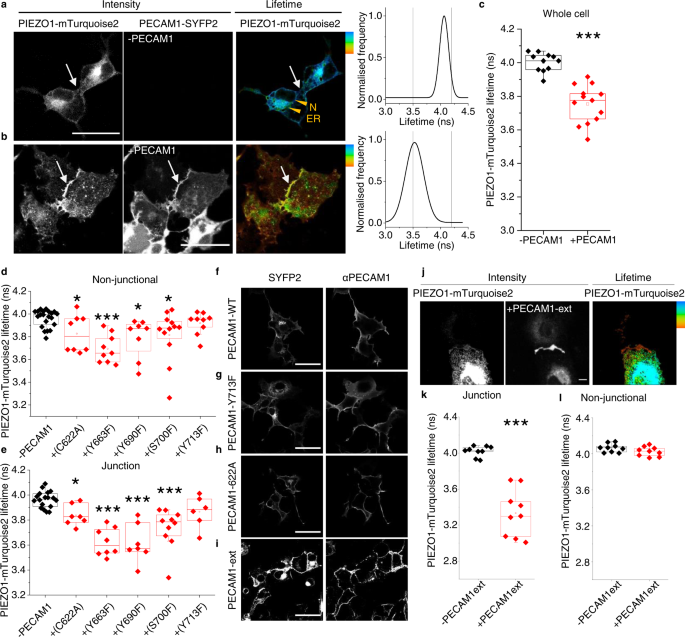
Data are for non-permeabilised COS-7 cells expressing human PIEZO1-mTurquoise2 with human PECAM1-SYFP2. a – l FRET/FLIM studies. a PIEZO1-mTurquoise2 alone. The intensity image in which white is high intensity and lifetime image calibrated to the rainbow scale (3.5–4.2 ns). The white arrows point to a region of cell-cell contact and the orange arrowheads nucleus (N) and endoplasmic reticulum (ER). The graph on the right is the lifetime distribution with the limits of the rainbow scale indicated by vertical lines. b Similar to a except PIEZO1-mTurquoise2 with PECAM1-SYFP2 (+PECAM1). a , b Scale bars, 50 µm. c Box plot presentation of summary data of the type shown in a and b , showing peak lifetime for the entire cell (Whole cell). n = 4 independent experiment repeats for PIEZO1-mTurquoise2 alone (−PECAM1, black) and PIEZO1-mTurquoise2 plus PECAM1-SYFP2 cells (+PECAM1, red). *** P = 3.16 ×10 −5 . The superimposed data points are average lifetimes for different images, −PECAM1 ( N = 11) and +PECAM1 ( N = 13). d – h Data are for COS-7 cells expressing PIEZO1-mTurquoise2 only (−PECAM1) or co-expressing PIEZO1-mTurquoise2 and PECAM1-SYFP2 in which PECAM1 was mutated at the indicated amino acid residue. Box plot presentations of FRET/FLIM peak lifetime data measured at: d Non-junctional (intracellular) regions (* P C622A = 0.0381,*** P Y663F = 5.98 ×10 −4 ; * P Y690F = 0.0431, * P S700F = 0.02136, P Y713F = 0.13532); e Cell-cell junction regions (* P C622A = 0.014, *** P Y663F = 3.56 ×10 −4 , *** P Y690F = 7.76 ×10 −4 , *** P S700F = 2.07 ×10 −4 , P Y713F = 0.1024). Data are for n = 3 independent experiment repeats. The superimposed data points are average lifetimes for different images, −PECAM1 ( N non junctional = 21 and N junctional = 18), +C622A ( N non junctional = 8 and N junctional = 7), +Y663F ( N non junctional = 9 and N junctional = 8), +Y690F ( N non junctional = 8 and N junctional = 7), +S700F ( N non junctional = 12 and N junctional = 10), +Y713F ( N non junctional = 9 and N junctional = 6). Confocal images showing sub cellular distribution of f wild-type (WT) PECAM1, g Y713F and h C622A as SYFP2 fluorescence and after immunostaining using antibody to PECAM1 extracellular domain (αPECAM1) in non-permeabilised cells. Images are representative of n = 3 independent experiments. Scale bars, 50 µm. i – l Data are for COS-7 cells expressing PIN-G-tagged N-terminal PECAM1-ex-SYFP2 alone (PECAM1-ex) or with PIEZO1-mTurquoise2. i Confocal image showing sub cellular distribution of PECAM1-ex. j Intensity images and lifetime image calibrated to the rainbow scale indicated at the top right corner (3.5–4.2 ns). Scale bar, 50 µm, applies to all images. Box plot presentations of FRET/FLIM peak lifetime data measured at: k Cell-cell junctions (*** P = 4.12 ×10 −4 ); l Non-junctional regions ( P = 0.18533). Data are for n = 3 independent experiment repeats. The superimposed data points are average lifetime values junctions (−PECAM1ext, N = 9 and +PECAM1ext, N = 9) and non-junctions (−PECAM1ext, N = 9 and +PECAM1ext, N = 9) from separate images.
Detected PIEZO1-PECAM1 proximity is similar to that of PIEZO1-PIEZO1
The fluorescence lifetime of PIEZO1-mTurquoise2 (expressed alone) peaks at ~4 ns (Fig. 4a, c ). When coexpressed with PECAM1-SYFP2, there is shortening to ~3.5 ns, suggesting that the two fluorophores are within 10 nm (Fig. 4b, c ), which is a distance lower than the width of one PIEZO1 channel (~20 nm) 33 . Comparable lifetime shortening occurs when PIEZO1-mTurquoise2 is co-expressed with PIEZO1-SYFP2 (Supplementary Fig. 10 ). PIEZO1 forms trimeric ion channels in which 3 PIEZO1s are physically bound 27 , 33 . Because of the comparable FRET of PIEZO1-PECAM1 and PIEZO1-PIEZO1, we suggest that PIEZO1 and PECAM1 interact as closely as two PIEZO1s.
Mutation of C-terminal residues in PECAM1 prevents or reduces PIEZO1 interaction
Because the FRET signal in the above PIEZO1-PECAM1 studies originated intracellularly (Fig. 4a–c ), we hypothesised importance of intracellular (C-terminal) regions of PECAM1. To test this hypothesis, 5 amino acid residues in the C-terminus of PECAM1 were mutated based on prior knowledge of PECAM1 (Supplementary Fig. 11 ). Wild-type PECAM1-SYFP2 and mutant PECAM1-SYFP2 were then co-expressed with PIEZO1-mTurquoise2 in COS-7 cells and lifetimes determined at non-junctional regions and junctions identified by accumulated PECAM1 (Fig. 4d, e , Supplementary Fig. 12a–e ). The Y713F mutation prevents FRET at both locations and is less able to drive PIEZO1 to junctions (Fig. 4d, e , Supplementary Fig. 12f, g ), while retaining normal abundance and localisation (Fig. 4g cf 4f ). FRET occurs with the other 4 mutants but is reduced for C622A and S700F, particularly in non-junctional regions (Fig. 4d ). There is normal abundance and localisation (shown for C622A in Fig. 4h ). The data suggest that C-terminal structure of PECAM1 and particularly Y713 influence the interaction of PECAM1 with PIEZO1.
PECAM1 N-terminus alone is sufficient for interaction
The disruption caused by Y713F could be explained by a direct role of PECAM1 C-terminus or a distance effect transmitted to its extracellular N-terminus. To specifically investigate the N-terminus, transmembrane and cytoplasmic regions of PECAM1 were replaced with plasma membrane-targeting sequence 59 . SYFP2 was engineered into the intracellular side, generating PECAM1-ex-SYFP2, which localises to plasma membrane as expected (Fig. 4i ). PIEZO1-mTurquoise2 and PECAM1-ex-SYFP2 enrich at cell-cell junctions (Fig. 4j ) and donor (mTurquoise2) lifetime is affected exclusively at cell-cell junctions (Fig. 4k, l ). Green fluorescent protein (GFP) trap was also used to bind PECAM1-SYFP2 (SYFP2 is a GFP variant) and test for coprecipitation of PIEZO1 co-expressed in HEK 293 cells. PIEZO1 is detected strongly when PECAM1-SYFP2 is coexpressed, suggesting precipitation that depends on PECAM1 (Supplementary Fig. 13 ). Consistent with a dominant role of N-terminal PECAM1, the Y713F mutation does not affect the precipitation (Supplementary Fig. 13 ). The data suggest that N-terminus of PECAM1 is sufficient for PIEZO1 localisation to junctions and its PECAM1 interaction.
Preference for hypoglycosylated PECAM1
PECAM1 migrates at multiple molecular masses and the isoform with the smallest mass coprecipitates with PIEZO1 (Supplementary Fig. 14a ). Post-translational hypoglycosylation of PECAM1 occurs at mature cell-cell junctions to improve the strength of transhomotypic interactions 60 , 61 . Treatment with N-Glycosidase F (PNGase F) confirms that the smallest molecular mass band is the hypoglycosylated state (Supplementary Fig. 14b ). The data suggest that PIEZO1 preferentially interacts with hypoglycosylated PECAM1, the species of mature junctions.
CDH5 also drives PIEZO1 to cell-cell junctions
To determine if the other components of the PECAM1 triad, CDH5 and VGFR2, couple with PIEZO1, we studied CDH5-mVenus and VGFR2-SYFP2 in COS-7 cells. mVenus and SYFP2 differ by one amino acid and have similar spectra. Like PECAM1, CDH5 drives PIEZO1 to cell-cell junctions (Fig. 5a ) and reduces the lifetime of the donor fluorophore (mTurquoise2), suggesting interaction (Fig. 5a, c , and Supplementary Fig. 15a–e ). VGFR2-SYFP2, however, lacks effect on PIEZO1 localisation (Fig. 5b cf Fig. 4a ) or mTurquoise2 lifetime (Fig. 5d ). Similar to the finding with PECAM1 (Supplementary Fig. 14 ), PIEZO1 preferentially coprecipitates with hypoglycosylated CDH5 (Supplementary Fig. 16 ). To investigate the relevance to the native proteins, we returned to retinal vascular studies. Staining of retinas of PIEZO1 HA mice shows colocalisation of endogenous CDH5 and PIEZO1 in situ in retinal vein (Fig. 5e–j ). The data suggest that CDH5, but not VGFR2, also drives PIEZO1 to cell-cell junctions and interacts with it.
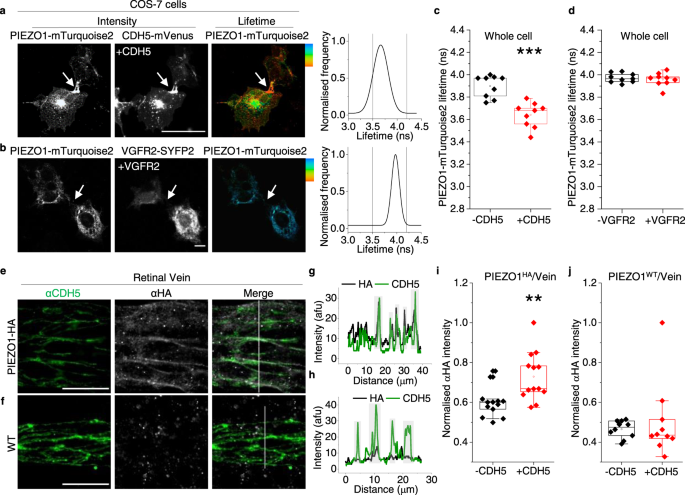
a – d FRET/FLIM images and analysis for COS-7 cells expressing a PIEZO1-mTurquoise2 plus CDH5-mVenus (CDH5-mVenus) or b PIEZO1-mTurquoise2 plus VGFR2-SYFP2. a , b Intensity images (white, high intensity), lifetime images calibrated to the rainbow scale indicated at the top corner (3.5–4.2 ns) and graphs of the lifetime distributions in which grey vertical lines indicate the rainbow scale limits. Scale bars, 50 µm, apply to all images. c , d Box plot summary peak whole cell lifetime data for the experiment types of a and b . 3 independent experimental repeats for PIEZO1-mTurquoise2 alone (-CDH5/VGFR2, black) and PIEZO1-mTurquoise2 plus CDH5-mVenus (*** P = 7.68 ×10 −4 ) or VGFR2-SYFP2 ( P = 0.72393) (+CDH5/VGFR2, red). Superimposed data points are average lifetime values for individual images (−CDH5, N = 9; +CDH5, N = 9; −VEFGR2, N = 9; +VGFR2, N = 9). e – j Images and image analysis for retinal veins of HA-PIEZO1 mice (PIEZO1 HA ) or wild-type (WT) mice (PIEZO1 WT ) immuno-stained with αCDH5 antibody (green) and αHA antibody (grey). e , f Representative images for αCDH5 and αHA staining with merger of these images to the right. Scale bars, 20 µm. g , h are the line-intensity (grey value) plots for the vertical scan lines superimposed on the Merge images of e and f . Green αCDH5, grey αHA. Light grey highlighting indicates cell-cell junctions. i , j Box-plot quantification of image intensity for e PIEZO1 HA or f PIEZO1 WT retinal veins stained with αHA, shown for junctional regions indicated by αCDH5 staining (+CDH5) and non-junctional regions (−CDH5) in arbitrary fluorescence units (AFU). The intensity of each image was normalised to the image background. ** P = 0.00347. Data are for n = 3 independent experiments. The superimposed data points are the average intensity for individual images, PIEZO1 HA (−CDH5, N = 13 and +CDH5, N = 13) PIEZO1 WT (−CDH5, N = 10 and +CDH5, N = 10).
Shear stress enhances CDH5 but not PECAM1 interaction
CDH5 is not equivalent to PECAM1 in the triad. It is recruited as an adaptor in response to shear stress 10 . We therefore investigated the effect of shear stress on PIEZO1-related FRET/FLIM signals in COS-7 cells. Preconditioning shear stress induced by fluid flow established a physiological cell condition prior to a static no-flow period. Then shear stress was applied again for 10 min or cells were retained in static condition (Fig. 6a–j ). Shear stress has no effect on the ability of PECAM1-SYFP2 to lower the lifetime of the donor fluorophore of PIEZO1 (Fig. 6a–e ) but increases the effect of CDH5-mVenus specifically at cell-cell junctions (Fig. 6f–j ). VGFR2-SYFP2 showed no FRET signal, with or without shear stress (Supplementary Fig. 17 ). The data suggest that shear stress increases PIEZO1’s interaction with CDH5 but not PECAM1, consistent with additional CDH5 being recruited in response to force.
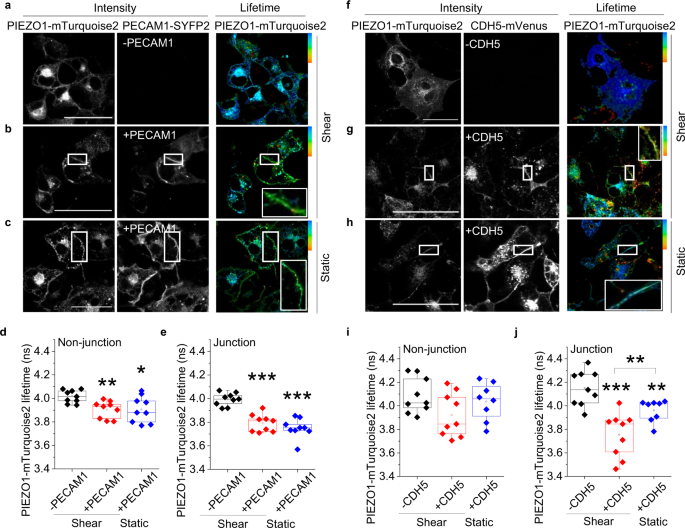
COS-7 cell data obtained by FRET/FLIM after expressing PIEZO1-mTurquoise2 with (+) or without (−): a – e PECAM1-SYFP2 (PECAM1); f – j CDH5-mVenus (CDH5). Prior to imaging, cells were preconditioned for 24 h with laminar shear stress (10 dyn.cm −2 ) followed by static condition for 30 min and then 10 min 10 dyn.cm −2 (shear) or continued static condition (static). For 3 independent experiments each, the box plots show the lifetimes for PIEZO1-mTurquoise2 at cell-cell junctions: e +PECAM1 *** P = 2.64 ×10 −4 shear and *** P = 1.37 ×10 −4 static, both compared with –PECAM1; j +CDH5 *** P = 2.63 ×10 −4 shear and ** P = 0.0079 static, both compared with –CDH5. +CDH5 shear cf + CDH5 static ** P = 0.00036 and the lifetimes for PIEZO1-mTurquoise2 at non junctional regions: d +PECAM1 ** P = 0.0021 shear and * P = 0.011 static, both compared with –PECAM1; i +CDH5 showed no significant differences to the –CDH5 under shear and static conditions. The superimposed data points are for individual junctions ( N = 9) and non-junctions ( N = 9) from separate images. P values are from Mann–Whitney tests with Bonferroni correction.
CDH5 lacks effect on PIEZO1 channel activity
CDH5 interacts with PIEZO1 and so we studied its effects on PIEZO1 channel activity using HEK 293 cell patch-clamp recording. However, no effects on PIEZO1 channel currents are evident (Supplementary Fig. 18 ). The data suggest that CDH5 lacks effect on PIEZO1 activity, despite its interaction.
Physiological PIEZO1 increases junction width and radial actin in endothelial cells
CDH5 is Ca 2+ regulated and so PIEZO1 channels could serve to regulate Ca 2+ locally and thereby link local mechanical force to adherens junction structure. Consistent with this hypothesis, force-induced junctional remodelling in HUVECs is associated with transient elevation of cytosolic Ca 2+ concentration sufficient for junctional remodelling 46 . We depleted and then reintroduced extracellular Ca 2+ to observe Ca 2+ regulated junction formation in confluent HUVECs (Fig. 7a ), testing the role of PIEZO1 by depleting it (but not PECAM1 or CDH5) using PIEZO1 targeted siRNA (Supplementary Fig. 19 ). In the PIEZO1-depleted group, there are thinner (tighter) junctions in the +calcium (post) condition (Fig. 7a–e , Supplementary Fig. 20 ). We also stained F-actin using phalloidin 568 (Supplementary Fig. 21a–c ) because cytoskeletal architecture is coordinated with junctional remodelling and affected by stretch 46 . In the PIEZO1-depleted condition, there are fewer F-actin peaks in cross-section, suggesting less radial actin (which spans focal adhesions) and more cortical actin (which coordinates with cell junctions). This is most apparent when extracellular Ca 2+ is returned after Ca 2+ depletion (+calcium (post) in Supplementary Fig. 21d–f ). The data suggest that PIEZO1 increases the width of junctions, consistent with the junctions being less tight and more able to remodel. Moreover, PIEZO1 promotes radial actin, which is also consistent with PIEZO1 facilitating cell and junctional remodelling (Fig. 7f, g ).
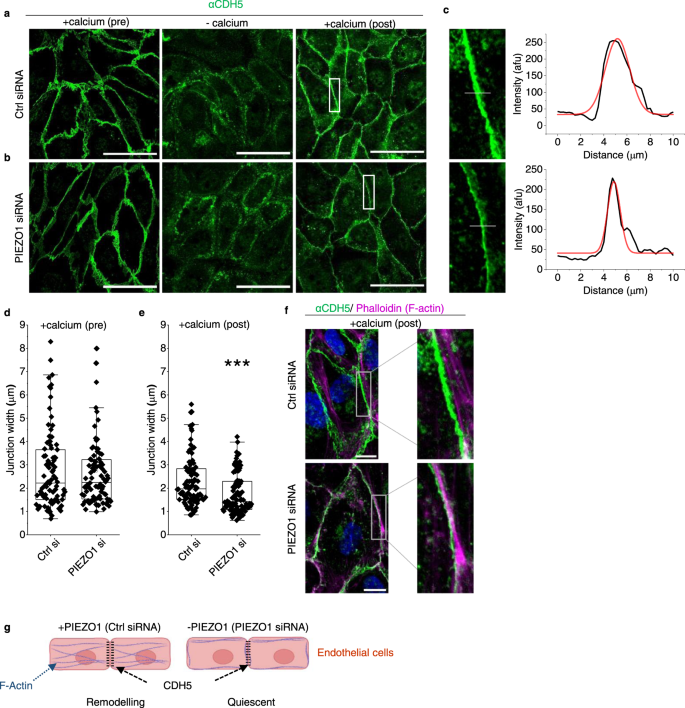
HUVEC data. a – f Cells cultured in a confluent monolayer treated with a control (Ctrl) or b PIEZO1 siRNA and subjected to extracellular Ca 2+ switch assay: pre-treatment (normal Ca 2+ ); −calcium (30 min Ca 2+ -depletion); and recovery (+calcium (post); 30 min after restoring Ca 2+ ). After treatments, cells were stained with anti-CDH5 antibody (αCDH5) (green). The scale bars are 100 µm. c Images are enlarged from the white boxes in a , b +calcium (post). To the right are line-intensity plots (black) with a Gaussian fit (red) used to calculate peak width at half maximum for the superimposed grey lines shown in the enlarged images. d , e As for a and b but showing box plots for +calcium pre-treatment ( n = 3, P = 0.92988) and +calcium (post) ( n = 3, *** P = 8.86242 ×10 −5 ) conditions. Superimposed data points are measurements from individual cell-cell junctions for +calcium (pre) (Ctrl si = 89, PIEZO1 si = 90) and +calcium (post) (Ctrl si = 87, PIEZO1 si = 88). f Enlarged and merged images of CDH5 (green, from a , b ) and F-actin (phalloidin) (magenta, from Supplementary Fig. 21a, b ) staining for the calcium switch recovery (+calcium (post); 30 min after restoring Ca 2+ ) conditions in Ctrl or PIEZO1 siRNA treated HUVECs. Scale bars are 25 µm g Schematic representation of F-actin and CDH5 with (+) and without (−) PIEZO1 or PIEZO1 activation by mechanical force, based on the data of a – f and Supplementary Fig. 21 . In the +PIEZO1 condition, there is suggested to be junctional remodelling with more radial actin and wider (less tight and more leaky) junctions.
Pharmacological activation of PIEZO1 causes radial actin collapse
External force was not applied in the studies of Fig. 7 and Supplementary Fig. 21 and so we assume PIEZO1 was activated only by physiological forces inherent to the cells and their substrate. Application of the small-molecule agonist of PIEZO1, Yoda1, near its concentration for 50% effect (3 μM) 62 causes substantial intracellular Ca 2+ elevation above basal levels of such cells, suggesting strong additional PIEZO1 activation (Supplementary Fig. 19a, b ). This concentration of Yoda1 strikingly disorganises F-actin structure (Supplementary Fig. 22 ). Something similar may have occurred when Yoda1 was infused in situ (Fig. 2d, e ).
From these results, we suggest connection of PIEZO1 and PECAM1 concepts in endothelial force sensing through protein-protein interaction and a pool of PIEZO1 at cell junctions in addition to the already established pool at the apical membrane 1 , 38 , 39 , 47 (Fig. 8 ). We show similarity of PIEZO1’s in vivo expression pattern to that of PECAM1, an established junctional protein. We show PIEZO1’s functional suppression when the amount of PECAM1 increases and that PECAM1 inhibits PIEZO1’s mechanical sensitivity, potentially through constraint of its N-terminus, the force sensing region. We show PIEZO1 and PECAM1 reconstitution at cell-cell junctions and that PECAM1 drives PIEZO1 to junctions. PECAM1 N-terminus is sufficient for interaction but intracellular C-terminal regions previously linked to sensing of shear stress also participate. CDH5, the other cell adhesion molecule of PECAM1’s triad, similarly drives PIEZO1 to junctions and interacts with it, in this case regulated by shear stress.
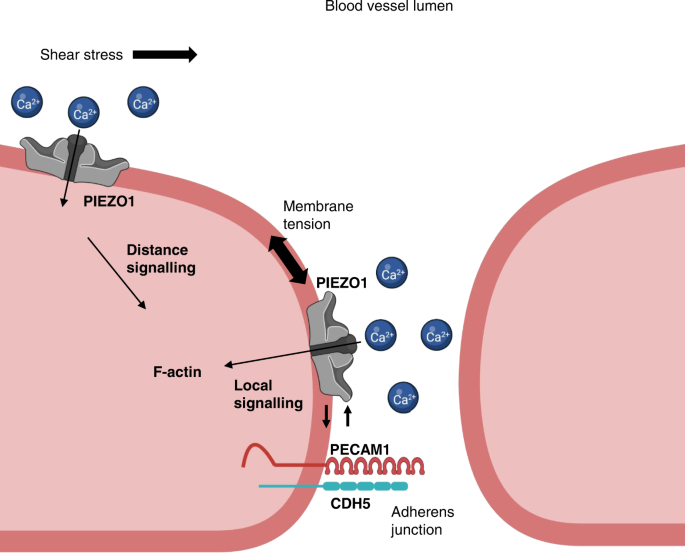
Sketch of part of two adjacent endothelial cells with a cell-cell junction in between. The junction is 15–30 nm in reality and so much narrower than apparent in the sketch. The junction includes adherens and tight junctions, but only the adherens junction is referred to here. Two pools of PIEZO1 channels are proposed. One pool is in the apical membrane of the endothelial cell and suggested to be particularly involved in sensing force as part of the shear stress sensing machinery. The other pool is in the adherens junction membrane of the endothelial cell and suggested to be particularly involved in sensing forces such as membrane tension as part of the adherens junction force sensing machinery. Force sensing is suggested to be mediated by PIEZO1 channels in both cases, leading to local and distance signalling to modulate endothelial cell function. Integration between the pools is envisaged to coordinate apical and junctional membrane events. PIEZO1 channels are Ca 2+ -permeable non-selective cation channels and so local intracellular Ca 2+ elevation and extracellular Ca 2+ depletion are likely when the channels open and this may contribute to regulation of nearby mechanisms, such as F-actin and the adhesion molecules PECAM1 and CDH5. We suggest also direct interaction between PIEZO1 and the adhesion molecules that is important both for localising PIEZO1 to junctions and regulating junctional structure once PIEZO1 is at the junctions. Negative feedback is suggested to occur from PECAM1 to PIEZO1 as junctional intensity increases to enable PIEZO1’s role in driving junctional remodelling to be suppressed once remodelling is complete and junctions need to return to a tighter, less leaky, state.
Determining specific roles of the junctional pool will be challenging but PIEZO1 channels are highly adapted to sensing increased membrane tension 52 , so we suggest a role of this PIEZO1 in detecting local tension in junctional membranes, coupling it to junctional structures via local Ca 2+ signalling. We show that PIEZO1 is required for Ca 2+ -dependent remodelling of junctions and associated actin cytoskeleton. Interaction of PIEZO1 with PECAM1 and CDH5 may be important in enabling such remodelling to happen efficiently. Whether both PIEZO1 and the adhesion molecules sense force is unknown, but we note the compelling evidence that PIEZO1 channels are direct and specific sensors of mechanical force, apparently having evolved for this purpose 25 , 26 , 27 , including in endothelial cells 30 , 38 , 39 , 63 . Therefore, without excluding mechanical detection by the PECAM1 triad, we suggest that critical force sensing arises at PIEZO1 channels and that they may confer force sensitivity on the triad.
Interaction between PIEZO1 and cell adhesion molecules could exist simply to enable PIEZO1 to reach junctions but functional interaction occurs too. We suggest negative feedback from PECAM1 to PIEZO1 when junctional density becomes high, serving to dampen PIEZO1’s remodelling role. In addition, we envisage Ca 2+ permeability of junctional PIEZO1 channels regulating the local cytosolic Ca 2+ concentration under the junctional membrane, thereby locally activating Ca 2+ -dependent mechanisms such as calpain to regulate junctional organisation 29 , 30 , 42 . Calpain is a known downstream mediator of PIEZO1 effects, regulator of cytoskeletal anchorage complexes and component of the endothelial shear stress sensing machinery 29 , 30 , 42 , 43 , 64 , 65 , 66 . PECAM1 is cleaved by calpain 67 . Apical PIEZO1 may be largely independent of junctional proteins such as PECAM1 because PECAM1 is primarily at junctions in endothelial cells. It is nevertheless important, detecting shear stress at the apical surface 38 , 39 and triggering distance signalling. The detection may involve intermediates 40 , 41 and signalling via Ca 2+ elevation, ATP release, G protein-coupled receptors (e.g., P2Y 2 receptors), phospholipase Cβ and other systems to coordinate apical shear stress with junctional structure and other events such as the production of nitric oxide 1 , 42 , 47 .
Ca 2+ -permeable channels regulate cytosolic Ca 2+ but depletion of local extracellular Ca 2+ could also be relevant here because of the restricted extracellular space of endothelial cell-cell junctions. A case has been made for local depletion of extracellular Ca 2+ at similarly-sized synaptic junctions 68 . Depletion of extracellular Ca 2+ in narrow diffusion-restricted spaces between endothelial cells, which have a width about the size of a PIEZO1 channel 33 (15–30 nm) 69 , may be sufficient to cause Ca 2+ to dissociate from ectodomains of PECAM1 and CDH5, a consequence of which is expected to be less transhomophilic interaction and weaker cell-cell contact, facilitating junction remodelling. CDH5 is an inherently Ca 2+ sensitive protein, belonging to an extended family of Ca 2+ binding adhesion molecules 21 . The Ca 2+ binding occurs in triplicates at multiple sites in the extracellular N-terminus and is important for the rigid crescent shape of the extracellular domain, the structure of X-dimer intermediate and opening of the A strand 21 . PECAM1 also contains extracellular Ca 2+ binding sites that are more restricted, but at a position associated with modulated homophilic binding affinity 22 , 70 . Such Ca 2+ binding sites are thought to be saturated at plasma Ca 2+ concentrations but the Ca 2+ affinity is relatively low and so the possibility exists for Ca 2+ unbinding, should extracellular Ca 2+ decline 68 . This supposition is encouraged by our finding that the extracellular domain of PECAM1 interacts with PIEZO1 at cell-cell junctions. Therefore, binding of the PECAM1 extracellular domain in the vicinity of the outer vestibule of the PIEZO1 channel could orchestrate a local sink-like effect in which Ca 2+ is efficiently drawn away from the PECAM1 extracellular domain. Consistent with this idea, our data point to preferential interaction of PIEZO1 with hypoglycosylated states of PECAM1 and CDH5, suggesting optimisation for mature junctions, which are achieved partly by posttranslational deglycosylation 60 , 61 .
We identified importance of tyrosine 713 (Y713) in PECAM1’s relationship with PIEZO1. Deletion of a domain containing this residue prevents tyrosine phosphorylation of PECAM1 in response to mechanical force 9 . This domain may also associate reversibly with the inner leaflet of the bilayer, controlling its phosphorylation 71 . The adjacent exon 13- and 15- encoded domains are the suggested points of interaction of PECAM1 with γ-catenin and β-catenin, conferring physical links to the cytoskeleton 72 . These findings point to additional mechanisms by which a PIEZO1-PECAM1 partnership could confer integration and regulate force sensing. Moreover, our data highlight the value of protein-protein interaction studies in situ in cells using techniques such as FRET/FLIM where the 3-dimensional subcellular architecture and localisation mechanisms of cells are retained. Our biochemical approaches that dissipated cell structure did not reveal an effect of mutating Y713.
CDH5’s association with PIEZO1 is enhanced by shear stress, suggesting that it has a more dynamic relationship with PIEZO1 than PECAM1. Evidence for diversity of PIEZO1 relationships with cell adhesion molecules is emerging. In epithelial cells, CDH1 interacts with PIEZO1. CDH1 has a strong enhancing effect on PIEZO1 channel function 73 . This study also reported an enhancing effect of CDH5 on PIEZO1 channel activity, albeit smaller than that of CDH1. The technique used for mechanical activation was ‘cell poking’ with a stylus, which may not be comparable with the membrane stretch applied in our experiments. Nevertheless, the data are consistent with our proposal that CDH5 does not inhibit PIEZO1 function.
PIEZO1 channels are also linked to NOTCH1 63 , which participates in cell-cell interaction through NOTCH ligands in endothelial cells and other cell types 74 . ADAM10 sheddase, which mediates the effect of PIEZO1 on NOTCH1 63 , cleaves CDH5 75 . Membrane-bound NOTCH1 signals to CDH5 via Rac1 to drive assembly of adherens junctions 76 . These observations suggest a broader role of PIEZO1 in endothelial cell-cell junctions beyond PECAM1 and CDH5 and add to an emerging picture of PIEZO1 channels as mechanical detectors with important roles in cell-cell interactions.
The endothelial response to shear stress may often occur alongside adaptive changes in endothelium such as cytoskeletal rearrangements and junction remodelling in diapedesis, endothelial remodelling, inflammation or other events. PIEZO1 is important here too, apparently existing as an adaptable mechanical force-sensing cassette in multiple subcellular compartments. PIEZO1 stimulation straightens CDH5 junctions and its depletion inhibits stretch-evoked remodelling 45 , 46 . PIEZO1 also has roles in focal adhesions 30 , 77 , to which radial actin is attached, consistent with a remodelling role of PIEZO1. Although our data and that of other investigators support roles for PIEZO1 as an enabler of junctional remodelling, whether this results in tighter or weaker junctions (less or more permeability) may depend on context as PIEZO1 depletion enhances or suppresses vascular permeability in vivo 42 , 43 .
We bring apparently competing ideas closer together but there remain major questions to answer about how endothelium responds to shear stress and other mechanical forces. We would like to know, for example, the first event or events when shear stress increases and the downstream consequences as the coordinated system response unfolds. We would like to know how these processes vary depending on the type of blood vessel or lymphatic, the context, the direction and chaos of the shear stress and the co-applied forces such as membrane tension and radial stretch. We are a long way from fully understanding. Instead, we know some molecules that are critical and a plethora of molecular changes that occur over time. What we lack is a time-series of events and their spatial orchestration. A key idea emerging from our work and that of others is that PIEZO1 channels are extremely rapid and sensitive responders to mechanical force sitting at the heart of this biology. Our hypothesis is that activation of these channels is a first or near-first event when force is applied at the endothelial surface or glycocalyx. This puts PECAM1 downstream. Consistent with this model, tyrosine phosphorylation of PECAM1 measured 5 min after the start of shear stress depends on PIEZO1 32 . However, we need much more sophisticated experiments to understand what happens. Our findings suggest an approach in which it would be necessary to measure in real-time the protein structural conformations of PIEZO1 and PECAM1 at apical and junctional membranes during shear stress. This would ideally be complemented by experiments incorporating specific disruption of the PIEZO1-PECAM1 interaction, thereby enabling testing of its role and the role of the proposed negative feedback of PECAM1 on PIEZO1.
Some of our conclusions are based on expression of tagged proteins in model cell systems, including non-endothelial cells so that we could utilise their technical advantages and reconstitute effects on null backgrounds for PIEZO1, PECAM1 and CDH5. Such technical approaches confer accuracy and reliability, are flexible, can be robustly tested and controlled and enable the relatively simple testing of the roles of specific amino acid residues through expression of mutants. However, there are potential limitations. The host cell type may not handle the exogenous proteins in the same way as the native cell. The experiments may involve overexpression (i.e., abnormally high protein expression that does not occur physiologically and which may cause effects that are not physiological). We took care to minimise the risk of abnormal expression, using transfection efficiencies that were just sufficient to enable detection of tagged constructs in natural cellular patterns. We also used multiple methods and cell types to validate our conclusions as each system has different limitations. The arising data provide a framework for designing future studies of such mechanisms in vivo, for example through the engineering of mice containing mutant or fluorescently-tagged endogenous proteins that can be studied in the native context of endothelial cells.
The suggested inhibitory effect of PECAM1 on PIEZO1 function is based both on our overexpression studies in HEK 293 cells and studies of the native proteins in endothelial cells under conditions of high PECAM1 abundance and junctional intensity. These studies independently point to the same conclusion and are consistent with a prior suggestion that degradation of PIEZO1 facilitates junctional normalisation after stretch-evoked remodelling 46 ; i.e., that PIEZO1 activity is enabled for remodelling but otherwise suppressed. The mechanism of this inhibitory effect of PECAM1 on PIEZO1 channel activity is unknown. It could relate to an effect of PECAM1 on membrane stiffness, local protein density or local lipid composition, although the effect was not reproduced by CDH5, which might be expected to similarly produce such disturbances. We successfully copurified PIEZO1 and PECAM1 overexpressed in HEK 293 cells, consistent with there being interaction, but this should be seen in the context of the central idea that there are two pools of PIEZO1 – one associated with and one not associated with PECAM1. We suggest that the associated pool is at cell-cell junctions, which are small but important. We investigated this pool in situ using FRET/FLIM and based on the arising data suggest stable association at cell-cell junctions of quiescent cell monolayers after junctions have formed. While the results of the HEK 293 cell experiments support our hypothesis, the value of this approach is limited in the context of such complex biology. Investigation at the site where the association occurs is especially important. Future structural studies may reveal details of the interaction that can then be used to understand it and enable its specific disruption in the native endothelial context.
We show that pharmacological activation of PIEZO1 (by the agonist Yoda1) disrupts PECAM1’s structural organisation and causes the collapse of radial actin. Our data suggest that Yoda1 can activate PIEZO1 substantially above its physiological activation levels, generating effects that are not necessarily physiologically relevant. In future studies, titrating Yoda1’s concentration down may lead to better mimicry of PIEZO1 activation by physiological force, although it may also be necessary to apply the Yoda1 in a localised and directional manner, as occurs with forces such as shear stress and membrane tension.
Our findings help to resolve an apparent contradiction in understanding how force is sensed by endothelium, placing PIEZO1 at the centre of an integrated concept across subcellular compartments of the endothelium. In the future, it will be interesting to investigate the mechanisms in more detail and elucidate their specific in vivo relevance. It will also be interesting to determine if relationships of PIEZO1 to PECAM1 and CDH5 extend to other cell adhesion molecules and other cell types. PIEZO1 is widely expressed 26 , 29 and there are numerous cell adhesion molecules in endothelial cells and other cell types 21 , 23 . Studies of CDH1 already support the idea of broader relevance and suggest general roles of adhesion molecules in tuning PIEZO1’s mechanical sensitivity and its relationship to cytoskeleton 73 .
COS-7 cells were from the American Type Culture Collection (ATCC) and maintained in Dulbecco’s Modified Eagle Media (DMEM) supplemented with 10% FCS, 2 mM L glutamine, 100 U ⋅ ml −1 penicillin, 100 µg ⋅ ml −1 streptomycin in 5% CO 2 , 95% air atmosphere. Cells were sub-cultured upon reaching a surface area density of 80–90% by detaching with 0.5% trypsin. T-REx-293 cells from Thermo Fisher Scientific engineered with tetracycline inducible expression of human PIEZO1 38 were maintained in DMEM supplemented with 10% FCS, 2 mM L-glutamine, 100 U ⋅ ml −1 penicillin, 100 µg ⋅ ml −1 streptomycin in 5% CO 2 , 95% air atmosphere. Cells were selected with zeocin (400 μg ⋅ ml −1 ) and blasticidin (5 μg ⋅ ml −1 ) and induced with tetracycline (100 ng ⋅ ml −1 ) for 24 h. T-REx-293 cells transfected with PECAM1 were generated by seeding at 70% confluency in a 6-well plate. Cells were transfected for 5 h with 500 ng PECAM1 plasmid and 0.3% Lipofectamine 2000 (Invitrogen). 48 h after selection with 5 μg ⋅ ml −1 blasticidin began, single cell lines stably expressing PECAM1 were isolated. HUVECs were cultured in EGM-2 growth medium supplemented with EGM-2 bullet kit (Lonza). Cells were maintained at 37 °C in a humidified atmosphere containing 5% CO 2 . T-REx-293 cells overexpressing mouse PIEZO1 were generated by seeding at 70% confluency in a 6-well plate. Cells were transfected for 5 h with 500 ng mPIEZO1 plasmid and 0.3% Lipofectamine 2000 (Invitrogen). 48 h after selection with 5 μg ⋅ ml −1 blasticidin began, single cell lines stably expressing mPIEZO1 were isolated.
Mouse breeding and husbandry
All animal use was authorised by the University of Leeds Animal Ethics Committee and The Home Office, UK. Animals were maintained in GM500 individually ventilated cages (Animal Care Systems) at 21 °C, 50–70% humidity, light/dark cycle 12/12 h on chow diet ad libitum and bedding of Pure’o Cell (Special Diet Services, Datesand Ltd, Manchester, UK). Genotypes were determined using real-time PCR with specific probes designed for each gene (Transnetyx, Cordova, TN).
Generation of HA-PIEZO1 mice C57BL/6J
Mice with PIEZO1-HA tag were generated by introducing an HA sequence between amino acids A2439 and D2440 by CRISPR-Cas9 (Fig. 1 Supplementary Fig. S1 ). A sgRNA was selected based on proximity to the target region and low off targeting potential ( catcgagctgcaggactgca -agg; ( https://www.sanger.ac.uk/htgt/wge/crispr/538567112 ), and a ssDNA repair template with the HA tag sequence and 60nt flanking homology arms was designed to facilitate integration of the HA tag sequence after Cas9 induced double strand break was synthesised (Integrated DNA technologies, with PAGE purification). sgRNA sequence was synthesised as an Alt-R crRNA (Integrated DNA Technologies) oligo and re-suspended in sterile Opti-MEM (Gibco) and annealed with tracrRNA (Integrated DNA Technologies) by combining crRNA (2.5 μg) with tracrRNA (5 μg) and heated to 95 °C. After annealing the complex, an equimolar amount was mixed with Cas9 recombinant protein (1500 ng) (NEB), the ssDNA repair template (final concentration 10 ng ⋅ μl −1 ) in Opti-MEM (total volume, 15 μl) and incubated (RT, 15 min). Mouse embryos were electroporated (Nepa21 electroporator, Sonidel) using AltR crRNA:tracrRNA:Cas9 complex (200 ng ⋅ μl −1 ; 200 ng ⋅ μl −1 ; 200 ng ⋅ μl −1 respectively) and ssDNA HDR template (500 ng ⋅ μl −1 ) 78 . Zygotes were cultured overnight and the resulting 2 cell embryos surgically implanted into the oviduct of day 0.5 post-coitum pseudopregnant mice. After birth and weaning genomic DNA extracted using REDExtract-N-Amp™ tissue PCR kit (Sigma) and used to genotype pups by PCR using primers cgactctaactatcccactcaac and atccctctgcagtactcacc , followed by Sanger sequencing of candidate pup1. Mice were bred to obtain homozygotes for HA-tag-PIEZO1 (PIEZO1 HA ).
DNA constructs and cloning
human PIEZO1-mTurquoise2 was sub-cloned from human PIEZO1-GFP 30 . pSYFP2-C1 79 (Addgene plasmid # 22878; http://n2t.net/addgene:22878 ; RRID:Addgene_22878) was a gift from Dorus Gadella. mTurquoise2-C1 80 (Addgene plasmid # 54842; http://n2t.net/addgene:54842 ; RRID:Addgene_54842) was a gift from Michael Davidson and Dorus Gadella. Human PECAM1 was obtained from Origene (TrueClone, SC119894). PIEZO1 and PECAM1 pcDNA6 templates were generated by inverse PCR with the Phusion® DNA polymerase (New England Bio Labs). mTurquoise2 and SYFP2 were incorporated to the PIEZO1/PECAM1 templates by overlap PCR using In-Fusion® HD cloning kit (Takara). Linkers were then attached between PIEZO1/PECAM1 and mTurquoise2/SFYP2 using In-Fusion® HD cloning kit (Takara). For PIEZO1/mTurquoise2 and PIEZO1/SYFP2 the linker was flanked by a BamH1 restriction. The linker on PECAM1/SYFP2 and PECAM1/mTurquoise2 was flanked by a HindIII restriction site. VGFR2-SYFP2 was sub-cloned from mEmerald-VGFR2-N1 (Addgene plasmid # 54298; http://n2t.net/addgene:54298 ; RRID:Addgene_54298) was a gift from Michael Davidson. When sequenced, mEmerald-VGFR2-N1 was found to contain a frameshifting CT insertion between residues T1303-A1304 and the single nucleotide polymorphisms (SNPs) V297I and H472Q. Site-directed mutagenesis was performed to remove the insertion and correct the SNPs. To facilitate cloning of VGFR2-SYFP2, a linker flanked by AgeI and SacII restriction sites was introduced into pcDNA™4/TO between EcoRI and XhoI restriction sites using Gibson Assembly® (New England Biolabs). The SYFP2 fluorophore was inserted downstream of the linker between SacII and XbaI restriction sites using pSYFP2‐C1. PCR products were assembled using Gibson Assembly® to insert VGFR2 upstream of the linker. The C-terminal fluorophore was removed from this construct using Gibson Assembly® to assemble VGFR2 and vector PCR products. mVenus-CDH5-N-10 (Addgene plasmid # 56340; http://n2t.net/addgene:56340 ; RRID:Addgene_56340) was a gift from Michael Davidson and was used as acquired. To generate C-terminal HA-tagged PIEZO1 the C-terminal GFP from the human PIEZO1-GFP construct 30 was first removed by ligating the KpnI and NotI restriction enzyme digested vector and PIEZO1 PCR product. This construct was subsequently used as a template to create full-length and truncated PIEZO1 expression vectors. PCR products covering (T1) full length PIEZO1 sequence to L2471, G2174, I2089, S1591 and A1128 were produced and inserted into the vector using Gibson Assembly®. PIEZO1 HA (CED). An HA-tag was also introduced between T2413 and C2414 located in the C-terminal extracellular domain. PECAM1 mutants were generated using the PECAM1-SYFP2 plasmid as a template. Mutagenesis was carried out using PrimeSTAR HS DNA polymerase (TaKaRa). The extracellular domain of PECAM1 containing SYFP2 and PIN-G was cloned from the PECAM1-SYFP2 plasmid using In-Fusion® HD cloning kit (Clonetech). The human PIEZO1-GFP construct used for co-purification was generated from PIEZO1-GFP 30 using round-the-horn PCR to insert a HRV3C protease cleavage site between PIEZO1 and the GFP tag. Halo tagged PIEZO1 was obtained from the Kazusa DNA Research Institute. pcDNA3_mouse PIEZO1_IRES_GFP, (Addgene plasmid # 80925; https://www.addgene.org/80925 ) a gift from A Patapoutian, was used as a template to clone the mouse PIEZO1 (mPIEZO1) coding sequence, into pcDNA™4/TO. Overlapping mouse PIEZO1 and pcDNA™4/TO PCR products (PrimeSTAR HS DNA Polymerase, TaKaRa) were assembled using Gibson Assembly (NEB). Constructs did not contain tetracycline operator sequences.
Paraformaldehyde fixation
Cells were fixed with 4% paraformaldehyde (10 min, RT) and washed with PBS 3 times for 5 min. Fixation was quenched with glycine (0.1 M in PBS, 10 min, RT) and cells were washed with PBS (3 times, 5 min, RT). Fixed cells were covered in PBS and stored at 4 °C. All fixation steps were carried out under low light and sterile condition. Samples were imaged on the same day or stored for a maximum of 24 h.
STED imaging
COS-7 cells were grown on coverslips placed inside 12-well plates. Wells were seeded with ~1 × 10 6 cells in 3 ml cell culture medium. Cells were transfected using FuGene with PIEZO1-HA and PECAM1-SYFP2 per well. After 48 h, cells were fixed and transferred to 12-well plates blocked with 1% BSA (in PBS) (15 min at RT). Primary antibodies against HA (rabbit anti-HA, Cell Signalling Technology, #3724, 1:800) and PECAM1 (mouse anti-PECAM1, 1:200) were diluted in 1% BSA/PBS and added to cells for 1 h at 37 °C. After incubation, cells were washed 3 times (5 min, RT) with PBS. Secondary antibodies against rabbit (anti-rabbit 580, Aberrior, 41367, 1:100) and mouse (anti-mouse STAR red, Aberrior, 52283, 1:100) diluted in PBS were added to the cells and incubated (30 min, RT). Cells were washed 3 times with PBS (5 min, RT) and mounted using ProLong® Gold antifade mountant (ThermoFisher, P36930). STED microscopy was performed using a 100x objective on the STEDYCON 2-colour STED imaging system (Abberior Instruments). Images were exported to FIJI 81 for final processing and assembly. Masks of junctional regions were generated manually using the polygon tool. Images were prepared for particle analysis by using the Gaussian Blur filter (Sigma radius = 0.015 µm in scaled units). Background subtraction was carried out using a sliding paraboloid with rolling ball radius of 3 pixels (0.06 µm). After applying the regional masks, the analysis particles tool was used to identify objects with areas between 0 and 50 pixels (0–0.02 µm 2 ) with circularity 0-1. Particle positions were saved in the ROI manager and applied to the raw images to calculate the centres of mass. In a Python script, for the centre of mass of every PIEZO1 particle, we found the distance to the centres of mass of all PECAM1 particles within 0.5 µm in X and Y. From those, we selected the nearest neighbour distance for inclusion in the histogram data. We fitted a parametric distance distribution to this distance histogram with curve_fit , a non-linear least-squares method in the scipy Python library 82 . The model distribution describes the distance between two 2D Gaussian distributions of localisations. Equivalently, this is the distribution of distances between localisations, where the errors on the localisation coordinates have a 2D Gaussian distribution. The sum of two such distributions was included in the model, which fitted the experimental cell-cell junction data well. The parameter estimates were allowed to vary freely, and results described a prominent proximal distribution of near neighbour distances and a secondary, more distal distribution. The equation used was:
(adapted from Churchman et al. 57 ), where:
DD ( r ) is the distribution of distances r from PECAM1 to PIEZO1 localisations
μ 1 and μ 2 are characteristic distances from PECAM1 to PIEZO1 localisations
σ 1 and σ 2 are the variances of the contributions associated with μ 1 and μ 2
A 1 and A 2 are the amplitudes of the contributions associated with μ 1 and μ 2
I o is the modified Bessel function of integer order zero.
The parameters of the distance distribution are found by least-squares fitting, which also outputs the covariance matrix for the fitted parameters. From this covariance matrix, confidence intervals for the parameters are calculated and we use 95% confidence intervals as described in Curd et al. 58 .
Red blood cell (RBC) membrane preparation and Western blotting
RBCs were lysed by incubation for 5 min in a 14× volume of hypotonic solution (46.2 mM NaCl, 0.6 mM HEPES) on ice. Membranes were pelleted by centrifugation at 18,407 × g for 30 min at 4 °C and proteins solubilised using detergent containing lysis buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 0.5 mM EDTA, 0.5% Nonidet P40 substitute, 0.1% glycerol containing protease inhibitor cocktail (Sigma)). Samples were loaded on 7% gels and resolved by electrophoresis. Proteins were transferred to PVDF membranes and labelled overnight with anti-HA (0.01 μg ⋅ ml −1 , Roche clone 3F10). Horse radish peroxidase donkey anti-rat secondary antibodies (1:10000, Jackson ImmunoResearch) and SuperSignal Femto detection reagents (Pierce) were used for visualisation.
Isolation of endothelium from mouse mesenteric artery
Endothelium was freshly isolated from second-order branches of mouse mesenteric arteries 38 . Dissected second-order mesenteric arteries were enzymatically digested in dissociation solution containing 126 mM NaCl, 6 mM KCl, 10 glucose, 11 mM HEPES, 1.2 mM MgCl 2 , 0.05 mM CaCl 2 (pH titrated to 7.2) plus 1 mg ⋅ ml −1 collagenase Type IA (Sigma) for 14 min at 37 °C and then triturated gently to release endothelium on a glass coverslips for recordings on the same day.
Patch-clamp recording from endothelium
Recordings were made at room temperature using an Axopatch-200B amplifier equipped with a Digidata 1550 A and pCLAMP 10.6 software (Molecular Devices, Sunnyvale, CA, USA). Endothelium was in a standard bath solution containing 135 mM NaCl, 4 mM KCl, 2 mM CaCl 2 , 1 mM MgCl 2 , 10 mM glucose and 10 mM HEPES (titrated to pH 7.4 using NaOH). Membrane potential recordings were made in zero current mode using heat-polished patch pipettes with tip resistances between 3 and 5 MΩ and containing amphotericin B (Sigma-Aldrich) as the perforating agent, added to a pipette solution containing: 145 mM KCl, 1 mM MgCl 2 , 0.5 mM EGTA and 10 mM HEPES (titrated to pH 7.2 using KOH). Outside-out membrane patch recordings were made in voltage-clamp mode. The tip resistances of recording pipettes were between 12 and 15 MΩ. Currents were sampled at 20 kHz and filtered at 2 kHz. The external solution was standard bath solution and the patch pipette contained: 145 mM KCl, 1 mM MgCl 2 , 0.5 mM EGTA and 10 mM HEPES (titrated to pH 7.2 using KOH). For application of fluid flow, endothelium or a membrane patch was manoeuvred to the exit of a capillary tube with tip diameter of 350 μm, out of which ionic (bath) solution flowed at 20 μl.s −1 .
Infusion of Yoda1 into mice
Male wild-type (C57BL/6J), 10–14 weeks old, were anaesthetised by intraperitoneal injection of ketamine hydrochloride (100 mg.kg −1 ) and xylazine hydrochloride (15 mg.kg −1 ). Mice were exsanguinated and then intravenously perfused in situ via portal vein for 10 min at 37 °C with standard bath solution (SBS) containing: 130 mM NaCl, 5 mM KCl, 1.2 mM MgCl 2 , 8 mM glucose, 10 mM HEPES, 1.5 mM CaCl 2 , then Yoda1 (3 µM in SBS, 30 min) or DMSO, followed by 4% PFA (10 min) at a flow rate of 1 ml.min −1 using a peristaltic pump (Watson-Marlow 505Di). Eyes were harvested for dissection and immunostaining.
PFA perfusion of PIEZO1 HA and wild-type mice
Male, 10–14 weeks old, C57BL/6J wild-type or PIEZO1 HA mice were anaesthetised under isoflurane (5% induction and 2% maintenance). Mice were perfused via the portal vein by syringe with PBS (10 ml), followed by 4% PFA (20 ml). Eyes were harvested for dissection and immunostaining.
Dissection and immunostaining of retinas
Dissection and immunostaining procedures were based on published protocols 83 . Eyes were placed in 4% PFA in PBS for 2 h on ice and washed three times with PBS prior to dissection of retinas. Permeabilisation and blocking of retinas was carried out using staining buffer (PBS pH 6.8, 0.5% Triton, 0.01% Na deoxycholate, 1% BSA, 0.02% NaN 3 with (mM) 0.1 CaCl 2 , 0.1 MgCl 2 and 0.1 MnCl 2 ) containing 2% goat serum (Agilent, CA, USA), overnight at 4 °C on an orbital shaker. Primary antibodies against PECAM1 (BD Pharmingen™, 550274, 1:100) and HA (Cell Signalling, mAB3724, 1:100) were diluted in a 1:1 solution of PBS:staining buffer and incubated overnight at 4 °C on an orbital shaker. Retinas were rinsed in PBS with 0.25 % Triton (6×, 15 min) at room temperature. Goat secondary antibodies (Invitrogen A21246 and A11006, 1:200) were diluted in a 1:1 solution of PBS:staining buffer and incubated overnight at 4 °C on an orbital shaker in the dark. Excess antibody was removed by washing with PBS containing 0.25% Triton (6×, 15 min) at room temperature (RT). Retinas were washed in PBS prior to making small quadrantic incisions to allow whole-mounting between a slide and coverslip using ProLong™ Gold (Invitrogen). Imaging was carried out on a LSM710 with 63× oil-immersion objective (Carl Zeiss Ltd.). Images were exported to FIJI for final processing and assembly. Linear adjust of brightness and contrast was applied to the entire image. For quantification of PIEZO1 HA intensities at the intracellular and junctional regions, masks of the junctions were generated from the corresponding PECAM1 image. The intensity value for each image was normalised to the background. Line profiles were determined using the ImageJ plot profile function. To calculate the coefficient of variance for PECAM1 staining with Yoda1 or DMSO treatment, line profile data for each image were extracted to OriginPro and normalised to minimum. The coefficient of variance for each trace was plotted.
FLIM sample preparation
For cells imaged under static conditions, 35-mm plastic cell culture dishes were plated with ~3 × 10 6 cells in 3 ml cell culture medium. After subculture, cells were transfected, using FuGene, with PIEZO1-mTurquoise. For co-transfections, acceptor-labelled constructs were added. After 48 h, cells were fixed with 4% paraformaldehyde. For flow experiments, cells (~1 × 10 6 in 200 µl medium) were plated onto the ibiTreat µSlide-I 0.8 Luer (Ibidi) and allowed to attach for 24 h. Transfections were carried out using Lipofectamine™ 3000. The medium in each slide was replaced with the transfection mixture in fresh medium and cells were incubated for 24 h. To culture and stimulate cells under flow conditions we used a system comprising an air pressure pump, pump control software, perfusion set (yellow/green) and fluidics unit (Ibidi). Cells were exposed to shear stress of 10 dyn.cm −2 for 24 h. Flow was stopped for a 30 min rest period after which cells were stimulated with flow (10 dyn.cm −2 , 10 min) and then fixed with 4% PFA.
FLIM microscopy
Intensity and FLIM images were obtained on an upright LSM710 (Carl Zeiss) microscope with a 40×/1.0 NA, water-dipping objective or 63×/1.40 Oil (Carl Zeiss). Acceptor intensity images were obtained with excitation at 512 nm using an Argon laser and registered on the Zeiss PMT detectors. Two-photon excitation was provided by Chameleon (Coherent) Ti:Sapphire laser tuned to 800 nm. FLIM emission events were recorded by an external detector (HPM-100, Becker & Hickl) attached to a commercial time-correlated single photon counting electronics module (Becker & Hickl) with a 480/40 (Chroma) emission filter. FLIM images were fitted using in SPCImage (Becker &Hickl). A single component incomplete multi-exponential model was used with a laser repetition time of 12.5 ns. Colour-coded lifetime maps and greyscale intensity images were exported from SPCImage. Acceptor intensity images were processed using FIJI. The histogram intensity weighted mean lifetimes for each image was generated by SPCImage 5.6; values were exported to OriginPro. The peak values were obtained by doing a Gaussian fit.
Immunostaining
COS-7 cells (~1 × 10 6 .ml −1 medium, 30 µl) were plated onto ibiTreat µSlide-VI 0.4 Luer (Ibidi) and allowed to attach for 24 h. Transfections were carried out using Lipofectamine™ 3000. After 48 h, cells were fixed and blocked with 1 % BSA (in PBS) for 15 min at RT. Primary antibodies against PECAM1 (mouse anti-PECAM1, clone JC70A, Dako1:200) were diluted in 1 % BSA/PBS and added to cells for 1 h at 37 °C. After incubation, cells were washed 3 times (5 min) with PBS. Secondary antibodies mouse (anti-mouse Alexa 594, Jackson Immuno Research, 1:300) diluted in PBS were added to the cells and incubated for 30 min at RT. Cells were washed 3 times with PBS (5 min) and stored in PBS at 4 °C. Samples were imaged on the same day or stored for a maximum of 24 h. Imaging was carried out on LSM710 (Carl Zeiss Ltd.) using a 40×/1.3 oil objective. Images were exported to FIJI for final processing and assembly.
Patch-clamp on cells overexpressing PIEZO1
T-REx-293 cells with tetracycline inducible overexpression of human PIEZO1 or T-REx-293 cells with overexpression of mouse PIEZO1 were seeded into a T25 tissue culture flask. After 24 h, transfections were carried out using Lipofectamine™ 3000. Cells were transfected with PECAM1-SYFP2 or SYFP2 (human PECAM1). Expression of PIEZO1 was induced by the addition of tetracycline (100 ng ⋅ ml −1 , 24 h). Cells expressing PIEZO1 with PECAM1-SYFP2 or SYFP2 were detached using trypsin and seeded onto coverslips. Macroscopic transmembrane ionic currents through outside-out patches were recorded using standard patch-clamp technique in voltage-clamp mode. Patch pipettes were fire-polished and had a resistance of 4–7 MΩ when filled with pipette solution. Symmetrical Na + (K + / Ca 2+ -free) solution of the following composition: NaCl 140. mM, HEPES 10 mM and EGTA 5 mM (pH 7.4, NaOH), was used for both, pipette and bath solutions, and the currents were recorded at −80 mV. All recordings were made with an Axopatch-200B amplifier (Axon Instruments, Inc., USA) equipped with Digidata 1550B and pClamp 10.6 software (Molecular Devices, USA) at room temperature. 200-ms pressure steps were applied directly to the patch pipette with an interval of 12 s and with an increment of 15 mmHg using High Speed Pressure Clamp HSPC-1 System (ALA Scientific Instruments, USA). Current records were filtered at 2 or 5 kHz and digitally acquired at 5 or 20 kHz.
Co-immunoprecipitation
500 μg of transiently transfected HEK 293 cell lysate (lysis buffer containing 0.5% Nonidet P40 substitute, 0.1% glycerol containing protease inhibitor cocktail (Sigma) and (mM) 10 Tris, 150 NaCl, 0.5 EDTA, at pH 7.4) was incubated with 1 μg anti-HA (Roche, clone 3F10) for at least 4 h at 4 °C prior to extraction overnight using Protein G agarose (Pierce). The beads were washed three times with ice-cold lysis buffer and bound proteins eluted using sample buffer (4× SB: 250 mM Tris pH 6.8, 8% SDS, 40% glycerol, 8% β-mercaptoethanol) and heating at 95 °C. Samples were loaded on 7% gels and resolved by electrophoresis. Proteins were transferred to PVDF membranes and labelled overnight with anti-HA (0.01 μg ⋅ ml −1 , Roche clone 3F10), anti-CDH5 (0.5 μg ⋅ ml −1 , R&D Systems; MAB9381), anti-PECAM1 (1:1000, Dako; clone JC70A) or anti-β-actin (200 ng ⋅ ml −1 , Santa Cruz). Horseradish peroxidase donkey anti-mouse, anti-rat, anti-goat secondary antibodies (1:10000, Jackson ImmunoResearch) and SuperSignal Femto detection reagents (Pierce) were used for visualisation. PNGase F (NEB) was used as per manufacturer’s instructions.
T-REx™-293 cells expressing tetracycline-inducible PIEZO1 were transfected in 6 well plates with PECAM1-SYFP2 or PECAM1-Y713F-SYFP2. HEK 293 cells and cells transfected with an empty vector served as controls. Following tetracycline induction, cells were lysed with lysis buffer (10 mM Tris-HCl pH7.5, 150 mM NaCl, 0.5 mM EDTA, 0.5% Nonidet P40 substitute) and centrifuged at 12,000 × g for 10 min. Supernatants were quantified, and 400 µg of protein was rotated with GFP-Trap Agarose for 2 h at 4 °C. For input samples, 30 µl of diluted supernatant was removed prior to addition of GFP-Trap Agarose. Following two washes, proteins were eluted with Novex™ Tris-Glycine SDS Sample Buffer (2x) containing 10% (v/v) β-mercaptoethanol at 50 °C for 10 min. Input samples were treated in the same way. Proteins were detected by western blotting (anti-PIEZO1 BEEC4, 1:1000; anti-GFP, Abcam ab1218; 1:5000). BEEC4 is a custom-designed anti-peptide ([C]-DLAKGGTVEYANEKHMLALA) antibody generated in rabbit and affinity-purified by Cambridge Biosciences.
Halo-Tag pulldowns
Halo-tagged PIEZO1 was pulled-down using HaloTag® Mammalian Pull-Down Systems (Promega) as per the manufacturer’s instructions. Briefly, Griptite™ 293 MSR cells (ThermoFisher) on a 6-well plate were transfected with Halo-PIEZO1 together with human PECAM1. Cells were collected using cold PBS and spun down for 5 min at 500 × g at 4 °C to collect the cell pellet. The pellet was frozen at −80 °C for at least 30 min. The pellet was then re-suspended using lysis buffer with 4 μl 50× protease inhibitors from the HaloTag® kit, and homogenised by passing through a 23 G needle 10 times. The homogenate was centrifuged at 14,000 × g for 5 min. The supernatant was rotated with Halo-resin for 45 min at room temperature so that protein complexes containing Halo-tag bound to the resin. Proteins were then eluted with by boiling with 4x Laemmli protein sample buffer (Bio-rad) and detected by western blotting (anti-Halo, 1:1000, Promega, mouse monoclonal and Proteintech PIEZO1 antibody).
Co-purification
HEK 293 cells, grown in FreeStyle TM 293 Expression Medium (12338018) to a density of 1 ×10 6 cells per ml, were PEI transfected with equal amounts of PIEZO1-GFP and PECAM1 DNA at a PEI to DNA ratio of 3:1 84 and the cells were harvested 72 h post transfection. All procedures following this point were carried out at 4 °C. Cell pellet from 2 L of culture was resuspended in 80 mL buffer (50 mM Tris pH 8, 150 mM NaCl, 1 mM EDTA, 10% glycerol) supplemented with complete protease inhibitor cocktail (Roche), 1 mM AEBSF, 1 mM PMSF. After sonication, the lysate was clarified by centrifugation at 2000 × g for 15 min and then supernatant ultra-centrifuged at 100,000 × g for 1 h at 4 °C. The membranes were collected and resuspended to a final concentration of 20 mg ⋅ ml −1 in buffer with protease inhibitors, as above, with the addition of 0.5% lauryl maltose neopentyl glycol (LMNG), 0.1% cholesterol hemisuccinate (CHS) and rotated at 4 °C for 2 h. Unsolubilised material was removed by ultra-centrifugation at 100,000 × g for 1 h at 4 °C. The supernatant was then incubated with pre-equilibrated GFP-nanobody coupled Sepharose resin 54 and incubated for 4 h. The resin was loaded onto a column and washed 4 times with 5 column volumes of buffer plus protease inhibitors with decreasing concentrations of LMNG: 0.2, 0.1, 0.01 and 0.005% LMNG. The GST-anti-GFP-nanobody with the protein complex bound was eluted form the GST-resin using 3 column volumes of elution buffer (buffer with 0.005% LMNG and 10 mM reduced L-glutathione) and concentrated to 500 µL by Vivaspin centrifugal filter (300 kDa MWCO). The sample was then loaded onto a Superose 6 increase column equilibrated with 50 mM Tris pH 8, 150 mM NaCl and 0.003 LMNG and fractions corresponding to PIEZO1/PECAM1 complex collected. Bolt TM LDS sample buffer with Bolt TM Sample reducing agent were added to a sample of the fractions (20 µL) and incubated at room temperature (RT) for 20 min before loading onto a Bolt TM 4–12% Bis-Tris gels (all Bolt TM buffer and gels from Invitrogen) and resolving by electrophoresis. The gels were then imaged for GFP fluorescence and Coomassie stained or transferred to PVDF and probed with anti-PECAM1 (1:1000, Dako; clone JC70A) or anti-GST (1:5000, GE Healthcare; 27457701) antibodies. Horse radish peroxidase goat anti-mouse and rabbit anti-goat secondary antibodies (1:5000, Jackson ImmunoResearch) and SuperSignal Femto detection reagents (Pierce) were used for visualisation.
Cell density experiments
HUVECs were seeded, 500,000 cells per well, into 6 well plates. After 24 or 48 h of growth cells were fixed with 4% PFA for immunostaining or lysed in RIPA buffer supplemented with PMSF, protease inhibitor mixture, and sodium orthovanadate (RIPA Lysis Buffer System, sc24948, Santa Cruz, Dallas, TX) for immunoblotting. All image processing was carried out in FIJI (imageJ). Nuclei counts were obtained from Hoechst images using the imageJ analyse particle function. Junction width was obtained from the αCDH5 immuno-stained images. FIJI line profiles were drawn perpendicular to junctions to generate line profiles. Each profile was fitted with a Gaussian distribution using OriginPro and the full width at half maximum for each peak was measured. F-actin distribution was determined from the phalliodin stained images. ImageJ was used to draw line profile across each cell perpendicular to the actin filaments. Using OriginPro the coefficient of variance for each line profile was calculated.
Fura2 calcium measurements
HUVECs were plated, 60,000 cells per well, into 96 well plates. After 24 h or 48 h of growth cells were incubated for 1 h in Standard Bath Solution (SBS, containing: 130 mM NaCl, 5 mM KCl, 8 mM D-glucose, 10 mM HEPES, 1.2 mM MgCl 2 , 1.5 mM CaCl 2 , pH 7.4) supplemented with 2 μM fura-2-AM (F1201, Molecular Probes, Eugene, OR) and 0.01% pluronic acid. Cells were then washed in SBS at room temperature for 30 min. Fura-2. Fluorescence (F) acquisition (excitation 340 and 380 nm; emission 510 nm) was performed on a Flexstation three microplate reader with SoftMax Pro 5.4.5 software (Molecular Devices). After 60 s of recording, Yoda1 (3 µM) or DMSO was injected. Ca 2+ entry was quantified after normalisation (ΔF340/380 = F340/380(t)-F340/380(t = 0)).
Calcium switch assay
HUVECs were transfected with siRNA using Opti-MEM I Reduced Serum Medium (31985070, ThermoFisher Scientific, Waltham, MA) and Lipofectamine 2000 (11668019, ThermoFisher Scientific). For transfection of cells in 6-well plates, a total of 50 nmol of PIEZO1 siRNA (Sigma-Aldrich, GCAAGUUCGUGCGCGGAUU[DT][DT]) or control siRNA (Dharmacon, L-001810) in 0.1 mL was added to 0.8 mL cell culture medium per well. Medium was changed after 4 h. After 48 h cells were placed in fresh medium for ~30 min before starting the calcium switch assay. Pre-treatment wells were fixed using 4% PFA for 10 min. Calcium depletion and recovery wells were washed with serum-free medium and incubated with serum free HUVEC culture medium containing 3 mM EGTA (Anaspec, ANA84097) for 30 min under culture conditions. Calcium depletion wells were fixed with 4% PFA for 10 min. Recovery wells were washed and covered with HUVEC culture medium containing serum and returned to the culture incubator for 30 min, followed by fixation with 4% PFA, 10 min. Junction thickness and F-actin distributions were calculated as for the cell density experiment.
Immuno-fluorescence and phalloidin staining of HUVECs
After fixation, cells were washed with PBS and permeabilised with 0.1% Triton X100 for 10 min. Blocking was carried out with 0.1% BSA for 15 min. Primary antibodies against CDH5 1:300 (Abcam, AB33168) and PECAM1 1:50 (Dako JC70A) were diluted in 0.1%BSA and added to cells overnight at 4 °C. 3 Washes with PBS for 5 min each were followed by incubation with secondary antibodies αrabbit 488, 1:200 (Jackson laboratories, 711-545-152), anti-mouse 647, 1:400 (Thermofisher, A21240) and phalloidin 568 (Cambridge Biosciences, 00044) in 1% BSA. Cells were incubated for 10 min in 1 μg ⋅ ml −1 Hoechst and washed with PBS.
Yoda1 treatment of HUVEC monolayers
HUVECs were seeded, 500,000 cells per well, into 6 well plates. After 48 h of growth cells were treated with either DMSO or 3 µM Yoda1 in serum-free medium and incubated for 30 min under culture conditions. Cells were fixed with 4% PFA for immunostaining. All image processing was carried out in FIJI (imageJ). F-actin distribution was determined from the phalliodin stained images. ImageJ was used to draw line profile across each cell perpendicular to the actin filaments. The coefficient of variance for each line profile was calculated using OriginPro.
Statistics and reproducibility
Statistical analysis of fluorescent lifetime values and intensity measurements was carried out using OriginPro. Normality test revealed that at least 1 dataset was not significantly drawn from a normally distributed population at the 0.05 level. Consequently, the Mann–Whitney Test was used to compare if two distributions are significantly different. For data containing 3 different conditions the Kruskal-Wallis Anova was used to check for variance, followed by use of the Mann–Whitney Test to compare data pairs. Each image was treated as an independent replicate, ordering effects were negligible. The number of experiments performed from different cell preparations or animals is defined as n. The study was aimed at deciphering a biological mechanism and so, in the absence of prior knowledge of this mechanism, power calculations were not considered to be applicable. For quantitative data, 3 independent repeats (n) were performed with a minimum of 3 technical repeats each. We selected numbers of independent repeats of experiments based on prior experience of studies of this type. Where multiple pairwise tests were performed on a single dataset, Bonferroni correction was applied. F-test was performed when comparing fitted data. The person performing the patch measurements was blinded to the constructs that had been transfected into the cells. All electrophysiological data were analysed and plotted using pClamp 10.6 and MicroCal Origin 2018 (OriginLab Corporation, USA) Software. Pressure-dependent curves were constructed in Origin and fitted with Boltzmann equation: y = A2 + (A1 − A2)/(1 + exp((x − x0)/dx)), where A1 and A2 are the minima and maxima, x0 the mid-point and dx the slope. For box plot graphs, box = 25%~75%, error bar range within 1.5 interquartile range, median line, □ mean, ♦ outliers (observations that are outside the error bars of the box plot).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Uncropped blots are available in Supplementary Fig. 23 . The source data behind the graphs in the paper are available in Supplementary Data 1 . The source data behind the graphs in the supplementary figures are available in Supplementary Data 2 . Supplementary Data 3 contains the table of primers.
Wettschureck, N., Strilic, B. & Offermanns, S. Passing the vascular barrier: endothelial signaling processes controlling extravasation. Physiol. Rev. 99 , 1467–1525 (2019).
Article CAS PubMed Google Scholar
Schmidt, E. P., Kuebler, W. M., Lee, W. L. & Downey, G. P. Adhesion molecules: master controllers of the circulatory system. Compr. Physiol. 6 , 945–973 (2016).
Article PubMed Google Scholar
Chiu, J. J. & Chien, S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91 , 327–387 (2011).
Baeyens, N., Bandyopadhyay, C., Coon, B. G., Yun, S. & Schwartz, M. A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Investig. 126 , 821–828 (2016).
Article PubMed PubMed Central Google Scholar
Gulino-Debrac, D. Mechanotransduction at the basis of endothelial barrier function. Tissue Barriers 1 , e24180 (2013).
Mammoto, T. & Ingber, D. E. Mechanical control of tissue and organ development. Development 137 , 1407–1420, https://doi.org/10.1242/dev.024166 (2010).
Article CAS PubMed PubMed Central Google Scholar
Oldenburg, J. & de Rooij, J. Mechanical control of the endothelial barrier. Cell Tissue Res. 355 , 545–555 (2014).
Baratchi, S. et al. Molecular sensors of blood flow in endothelial cells. Trends Mol. Med. 23 , 850–868 (2017).
Osawa, M., Masuda, M., Harada, N., Lopes, R. B. & Fujiwara, K. Tyrosine phosphorylation of platelet endothelial cell adhesion molecule-1 (PECAM-1, CD31) in mechanically stimulated vascular endothelial cells. Eur. J. Cell Biol. 72 , 229–237 (1997).
CAS PubMed Google Scholar
Conway, D. & Schwartz, M. A. Lessons from the endothelial junctional mechanosensory complex. F1000 Biol. Rep. 4 , 1 (2012).
Tzima, E. et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437 , 426–431 (2005).
Givens, C. & Tzima, E. Endothelial mechanosignaling: does one sensor fit all? Antioxid. Redox Signal. 25 , 373–388 (2016).
Fujiwara, K. Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. J. Intern. Med. 259 , 373–380 (2006).
Fujiwara, K., Masuda, M., Osawa, M., Kano, Y. & Katoh, K. Is PECAM-1 a mechanoresponsive molecule? Cell Struct. Funct. 26 , 11–17 (2001).
Harada, N., Masuda, M. & Fujiwara, K. Fluid flow and osmotic stress induce tyrosine phosphorylation of an endothelial cell 128 kDa surface glycoprotein. Biochem. Biophys. Res. Commun. 214 , 69–74 (1995).
Osawa, M., Masuda, M., Kusano, K. & Fujiwara, K. Evidence for a role of platelet endothelial cell adhesion molecule-1 in endothelial cell mechanosignal transduction: is it a mechanoresponsive molecule? J. Cell Biol. 158 , 773–785 (2002).
Privratsky, J. R. & Newman, P. J. PECAM-1: regulator of endothelial junctional integrity. Cell Tissue Res. 355 , 607–619 (2014).
Reglero-Real, N., Colom, B., Bodkin, J. V. & Nourshargh, S. Endothelial cell junctional adhesion molecules: role and regulation of expression in inflammation. Arteriosclerosis Thrombosis Vasc. Biol. 36 , 2048–2057 (2016).
Article CAS Google Scholar
Gavard, J. Endothelial permeability and VE-cadherin: a wacky comradeship. Cell Adhes. Migr. 8 , 158–164 (2014).
Article Google Scholar
Newman, P. J. & Newman, D. K. Signal transduction pathways mediated by PECAM-1: new roles for an old molecule in platelet and vascular cell biology. Arteriosclerosis Thrombosis Vasc. Biol. 23 , 953–964 (2003).
Brasch, J., Harrison, O. J., Honig, B. & Shapiro, L. Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol. 22 , 299–310 (2012).
Lertkiatmongkol, P., Liao, D., Mei, H., Hu, Y. & Newman, P. J. Endothelial functions of platelet/endothelial cell adhesion molecule-1 (CD31). Curr. Opin. Hematol. 23 , 253–259 (2016).
Colas-Algora, N. & Millan, J. How many cadherins do human endothelial cells express? Cell. Mol. Life Sci. CMLS 76 , 1299–1317 (2019).
Leckband, D. E. & de Rooij, J. Cadherin adhesion and mechanotransduction. Annu. Rev. Cell Dev. Biol. 30 , 291–315 (2014).
Coste, B. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330 , 55–60 (2010).
Murthy, S. E., Dubin, A. E. & Patapoutian, A. Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat. Rev. Mol. Cell Biol. 18 , 771–783 (2017).
Jiang, Y., Yang, X., Jiang, J. & Xiao, B. Structural designs and mechanogating mechanisms of the mechanosensitive Piezo channels. Trends Biochem Sci. 46 , 472–488 (2021).
Wu, J., Lewis, A. H. & Grandl, J. Touch, tension, and transduction - the function and regulation of Piezo ion channels. Trends Biochem. Sci. 42 , 57–71 (2017).
Beech, D. J. & Kalli, A. C. Force sensing by Piezo channels in cardiovascular health and disease. Arteriosclerosis Thrombosis Vasc. Biol. 39 , 2228–2239 (2019).
Li, J. et al. Piezo1 integration of vascular architecture with physiological force. Nature 515 , 279–282 (2014).
Albarran-Juarez, J. et al. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J. Exp. Med. 215 , 2655–2672 (2018).
Wang, S. et al. Endothelial cation channel PIEZO1 controls blood pressure by mediating flow-induced ATP release. J. Clin. Investig. 126 , 4527–4536 (2016).
Guo, Y. R. & MacKinnon, R. Structure-based membrane dome mechanism for Piezo mechanosensitivity. eLife 6 , e33660 (2017).
Saotome, K. et al. Structure of the mechanically activated ion channel Piezo1. Nature 554 , 481–486 (2018).
Zhao, Q. et al. Structure and mechanogating mechanism of the Piezo1 channel. Nature 554 , 487–492 (2018).
Maneshi, M. M., Ziegler, L., Sachs, F., Hua, S. Z. & Gottlieb, P. A. Enantiomeric Abeta peptides inhibit the fluid shear stress response of PIEZO1. Sci. Rep. 8 , 14267 (2018).
Ranade, S. S. et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl Acad. Sci. USA 111 , 10347–10352 (2014).
Rode, B. et al. Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat. Commun. 8 , 350 (2017).
Shi, J. et al. Sphingomyelinase disables inactivation in endogenous PIEZO1 channels. Cell Rep. 33 , 108225 (2020).
Tarbell, J. M., Simon, S. I. & Curry, F. R. Mechanosensing at the vascular interface. Annu Rev. Biomed. Eng. 16 , 505–532 (2014).
Mylvaganam, S. et al. The spectrin cytoskeleton integrates endothelial mechanoresponses. Nat. Cell Biol. 24 , 1226–1238 (2022).
Friedrich, E. E. et al. Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc. Natl Acad. Sci. USA 116 , 12980–12985 (2019).
Zhong, M. et al. Alveolar stretch activation of endothelial Piezo1 protects adherens junctions and lung vascular barrier. Am. J. Respir. Cell Mol. Biol. 62 , 168–177 (2020).
Wang, S. et al. Mechanosensation by endothelial PIEZO1 is required for leukocyte diapedesis. Blood 140 , 171–183 (2022).
Nonomura, K. et al. Mechanically activated ion channel PIEZO1 is required for lymphatic valve formation. Proc. Natl Acad. Sci. USA 115 , 12817–12822 (2018).
Miroshnikova, Y. A. et al. Calcium signaling mediates a biphasic mechanoadaptive response of endothelial cells to cyclic mechanical stretch. Mol. Biol. Cell 32 , 1724–1736 (2021).
Jin, Y. J. et al. Protein kinase N2 mediates flow-induced eNOS activation and vascular tone regulation. J. Clin. Investig. 131 , e145734 (2021).
Zarychanski, R. et al. Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood 120 , 1908–1915 (2012).
Evans, E. L. et al. RBCs prevent rapid PIEZO1 inactivation and expose slow deactivation as a mechanism of dehydrated hereditary stomatocytosis. Blood 136 , 140–144 (2020).
Harraz, O. F., Klug, N. R., Senatore, A. J., Hill-Eubanks, D. C. & Nelson, M. T. Piezo1 is a mechanosensor channel in central nervous system capillaries. Circ. Res. 130 , 1531–1546 (2022).
Syeda, R. et al. Chemical activation of the mechanotransduction channel Piezo1. eLife 4 , e07369 (2015).
Lewis, A. H. & Grandl, J. Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. eLife 4 , e12088 (2015).
De Vecchis, D., Beech, D. J. & Kalli, A. C. Molecular dynamics simulations of Piezo1 channel opening by increases in membrane tension. Biophys. J. 120 , 1510–1521 (2021).
Katoh, Y., Nozaki, S., Hartanto, D., Miyano, R. & Nakayama, K. Architectures of multisubunit complexes revealed by a visible immunoprecipitation assay using fluorescent fusion proteins. J. Cell Sci. 128 , 2351–2362 (2015).
Bae, C., Suchyna, T. M., Ziegler, L., Sachs, F. & Gottlieb, P. A. Human PIEZO1 ion channel functions as a split protein. PLoS One 11 , e0151289 (2016).
Albelda, S. M., Muller, W. A., Buck, C. A. & Newman, P. J. Molecular and cellular properties of PECAM-1 (endoCAM/CD31): a novel vascular cell-cell adhesion molecule. J. Cell Biol. 114 , 1059–1068 (1991).
Churchman, L. S., Flyvbjerg, H. & Spudich, J. A. A non-Gaussian distribution quantifies distances measured with fluorescence localization techniques. Biophys. J. 90 , 668–671 (2006).
Curd, A. P. et al. Nanoscale pattern extraction from relative positions of sparse 3D localizations. Nano Lett. 21 , 1213–1220 (2021).
McKeown, L., Robinson, P., Greenwood, S. M., Hu, W. & Jones, O. T. PIN-G–a novel reporter for imaging and defining the effects of trafficking signals in membrane proteins. BMC Biotechnol. 6 , 15 (2006).
Liwosz, A., Lei, T. & Kukuruzinska, M. A. N-glycosylation affects the molecular organization and stability of E-cadherin junctions. J. Biol. Chem. 281 , 23138–23149 (2006).
Nita-Lazar, M., Rebustini, I., Walker, J. & Kukuruzinska, M. A. Hypoglycosylated E-cadherin promotes the assembly of tight junctions through the recruitment of PP2A to adherens junctions. Exp. Cell Res. 316 , 1871–1884 (2010).
Parsonage, G. et al. Improved PIEZO1 agonism through 4-benzoic acid modification of Yoda1. Br. J. Pharmacol. , https://doi.org/10.1111/bph.15996 (2022).
Caolo, V. et al. Shear stress activates ADAM10 sheddase to regulate Notch1 via the Piezo1 force sensor in endothelial cells. eLife 9 , e50684 (2020).
McHugh, B. J. et al. Integrin activation by Fam38A uses a novel mechanism of R-Ras targeting to the endoplasmic reticulum. J. Cell Sci. 123 , 51–61 (2010).
Lebart, M. C. & Benyamin, Y. Calpain involvement in the remodeling of cytoskeletal anchorage complexes. FEBS J. 273 , 3415–3426 (2006).
Miyazaki, T., Honda, K. & Ohata, H. Requirement of Ca2+ influx- and phosphatidylinositol 3-kinase-mediated m-calpain activity for shear stress-induced endothelial cell polarity. Am. J. Physiol. Cell Physiol. 293 , C1216–C1225 (2007).
Naganuma, Y. et al. Cleavage of platelet endothelial cell adhesion molecule-1 (PECAM-1) in platelets exposed to high shear stress. J. Thrombosis Haemost. 2 , 1998–2008 (2004).
Kim, S. A., Tai, C. Y., Mok, L. P., Mosser, E. A. & Schuman, E. M. Calcium-dependent dynamics of cadherin interactions at cell-cell junctions. Proc. Natl Acad. Sci. USA 108 , 9857–9862 (2011).
Le Bihan, O., Decossas, M., Gontier, E., Gerbod-Giannone, M. C. & Lambert, O. Visualization of adherent cell monolayers by cryo-electron microscopy: A snapshot of endothelial adherens junctions. J. Struct. Biol. 192 , 470–477 (2015).
Jackson, D. E., Loo, R. O., Holyst, M. T. & Newman, P. J. Identification and characterization of functional cation coordination sites in platelet endothelial cell adhesion molecule-1. Biochemistry 36 , 9395–9404 (1997).
Paddock, C. et al. Residues within a lipid-associated segment of the PECAM-1 cytoplasmic domain are susceptible to inducible, sequential phosphorylation. Blood 117 , 6012–6023 (2011).
Ilan, N. & Madri, J. A. PECAM-1: old friend, new partners. Curr. Opin. Cell Biol. 15 , 515–524 (2003).
Wang, J. et al. Tethering Piezo channels to the actin cytoskeleton for mechanogating via the cadherin-beta-catenin mechanotransduction complex. Cell Rep. 38 , 110342 (2022).
Siebel, C. & Lendahl, U. Notch signaling in development, tissue homeostasis, and disease. Physiol. Rev. 97 , 1235–1294 (2017).
Schulz, B. et al. ADAM10 regulates endothelial permeability and T-Cell transmigration by proteolysis of vascular endothelial cadherin. Circ. Res 102 , 1192–1201 (2008).
Polacheck, W. J. et al. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature 552 , 258–262 (2017).
Yao, M. et al. Force- and cell state-dependent recruitment of Piezo1 drives focal adhesion dynamics and calcium entry. Sci. Adv. 8 , eabo1461 (2022).
Kaneko, T. Genome editing in mouse and rat by electroporation. Methods Mol. Biol. 1630 , 81–89 (2017).
Kremers, G. J., Goedhart, J., van Munster, E. B. & Gadella, T. W. Jr. Cyan and yellow super fluorescent proteins with improved brightness, protein folding, and FRET Forster radius. Biochemistry 45 , 6570–6580 (2006).
Goedhart, J. et al. Structure-guided evolution of cyan fluorescent proteins towards a quantum yield of 93. Nat. Commun. 3 , 751 (2012).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9 , 676–682 (2012).
Virtanen, P. et al. SciPy 1.0–fundamental algorithms for scientific computing in python. Nat. Methods 17 , 261–272 (2020).
Pitulescu, M. E., Schmidt, I., Benedito, R. & Adams, R. H. Inducible gene targeting in the neonatal vasculature and analysis of retinal angiogenesis in mice. Nat. Protoc. 5 , 1518–1534 (2010).
Portolano, N. et al. Recombinant protein expression for structural biology in HEK 293F suspension cells: a novel and accessible approach. J. Vis. Exp. , e51897, https://doi.org/10.3791/51897 (2014).
Download references
Acknowledgements
The study was supported by a Wellcome Investigator Award (110044/Z/15/Z) and British Heart Foundation Programme Grant (RG/17/11/33042) to D.J.B., a British Heart Foundation Intermediate Fellowship to J.S. (FS/17/2/32559), a British Heart Foundation PhD Studentship to H.J.G. (FS/14/22/30734), a BBSRC Grant (BB/S015787/1) to M.P./A.C., a Wellcome ISSF Discipline Hopping Fellowship (204825/Z/16/Z) to A.C. and a British Heart Foundation Project Grant to R.S.B. (PG/19/2/34084). We thank Sally Boxall and Ruth Hughes (Leeds Bio-Imaging Facility) and David Myers for microscopy technical support and Bing Hou and Deborah Linley for experimental data that encouraged elements of the study but which are not included in this article. We thank Lynn McKeown for the PIN-G construct. Figures 7 g and 8 were created partly using BioRender.com. For the purpose of Open Access, the authors have applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Author information
These authors contributed equally: Oleksandr V. Povstyan, Melanie J. Ludlow.
These authors jointly supervised this work: Eulashini Chuntharpursat-Bon, David J. Beech.
Authors and Affiliations
School of Medicine, University of Leeds, Leeds, LS2 9JT, UK
Eulashini Chuntharpursat-Bon, Oleksandr V. Povstyan, Melanie J. Ludlow, David J. Carrier, Marjolaine Debant, Jian Shi, Hannah J. Gaunt, Claudia C. Bauer, T. Simon Futers, Paul D. Baxter, Sarka Tumova, Robin S. Bon, Richard Cubbon, Laeticia Lichtenstein & David J. Beech
School of Biomedical Sciences, University of Leeds, Leeds, LS2 9JT, UK
David J. Carrier & Stephen P. Muench
School of Molecular and Cellular Biology, University of Leeds, Leeds, LS2 9JT, UK
Alistair Curd & Michelle Peckham
Astbury Centre for Structural Molecular Biology, University of Leeds, Leeds, LS2 9JT, UK
Michelle Peckham, Stephen P. Muench & Robin S. Bon
Faculty of Biology, Medicine and Health, University of Manchester, AV Hill Building, Manchester, M13 9PT, UK
Antony Adamson & Neil Humphreys
You can also search for this author in PubMed Google Scholar
Contributions
E.C.-B., D.J.C. and M.J.L. designed protein constructs. E.C.-B., M.J.L., D.J.C. and H.J.G. cloned protein constructs. E.C.-B. designed and performed microscopy experiments. O.V.P. performed patch-clamp experiments and analysed the data for PIEZO1 overexpression studies. M.J.L., S.T., M.D., C.C.B. designed and performed immunoprecipitation experiments. M.D. performed calcium recordings. D.J.C. performed co-purification studies. S.P.M. performed protein modelling and supervised co-purification studies. A.A. and N.H. genetically engineered mice. T.S.F. bred and maintained genetically engineered mice. J.S. performed patch-clamp studies on endothelium. R.C. advised and assisted on retinal staining experiments. L.L. performed in vivo perfusion and organ harvesting. E.C.-B., O.V.P., A.C., M.P., J.S., M.D. and D.J.B. analysed data. P.D.B. guided statistical analysis of imaging data. E.C.-B. and D.J.B. directed the study, made the figures and wrote the manuscript. D.J.B. conceptualised the study and supervised the project team. D.J.B., J.S. and R.S.B. generated funding.
Corresponding authors
Correspondence to Eulashini Chuntharpursat-Bon or David J. Beech .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Peer review
Peer review information.
Communications Biology thanks Irena Levitan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Marco Fritzsche and Manuel Breuer. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Peer review file, supplementary information-new, description of additional supplementary data, supplementary data 1, supplementary data 2, supplementary data 3, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Chuntharpursat-Bon, E., Povstyan, O.V., Ludlow, M.J. et al. PIEZO1 and PECAM1 interact at cell-cell junctions and partner in endothelial force sensing. Commun Biol 6 , 358 (2023). https://doi.org/10.1038/s42003-023-04706-4
Download citation
Received : 30 March 2022
Accepted : 14 March 2023
Published : 01 April 2023
DOI : https://doi.org/10.1038/s42003-023-04706-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
- Open access
- Published: 27 May 2024
Current status of community resources and priorities for weed genomics research
- Jacob Montgomery 1 ,
- Sarah Morran 1 ,
- Dana R. MacGregor ORCID: orcid.org/0000-0003-0543-0408 2 ,
- J. Scott McElroy ORCID: orcid.org/0000-0003-0331-3697 3 ,
- Paul Neve ORCID: orcid.org/0000-0002-3136-5286 4 ,
- Célia Neto ORCID: orcid.org/0000-0003-3256-5228 4 ,
- Martin M. Vila-Aiub ORCID: orcid.org/0000-0003-2118-290X 5 ,
- Maria Victoria Sandoval 5 ,
- Analia I. Menéndez ORCID: orcid.org/0000-0002-9681-0280 6 ,
- Julia M. Kreiner ORCID: orcid.org/0000-0002-8593-1394 7 ,
- Longjiang Fan ORCID: orcid.org/0000-0003-4846-0500 8 ,
- Ana L. Caicedo ORCID: orcid.org/0000-0002-0378-6374 9 ,
- Peter J. Maughan 10 ,
- Bianca Assis Barbosa Martins 11 ,
- Jagoda Mika 11 ,
- Alberto Collavo 11 ,
- Aldo Merotto Jr. ORCID: orcid.org/0000-0002-1581-0669 12 ,
- Nithya K. Subramanian ORCID: orcid.org/0000-0002-1659-7396 13 ,
- Muthukumar V. Bagavathiannan ORCID: orcid.org/0000-0002-1107-7148 13 ,
- Luan Cutti ORCID: orcid.org/0000-0002-2867-7158 14 ,
- Md. Mazharul Islam 15 ,
- Bikram S. Gill ORCID: orcid.org/0000-0003-4510-9459 16 ,
- Robert Cicchillo 17 ,
- Roger Gast 17 ,
- Neeta Soni ORCID: orcid.org/0000-0002-4647-8355 17 ,
- Terry R. Wright ORCID: orcid.org/0000-0002-3969-2812 18 ,
- Gina Zastrow-Hayes 18 ,
- Gregory May 18 ,
- Jenna M. Malone ORCID: orcid.org/0000-0002-9637-2073 19 ,
- Deepmala Sehgal ORCID: orcid.org/0000-0002-4141-1784 20 ,
- Shiv Shankhar Kaundun ORCID: orcid.org/0000-0002-7249-2046 20 ,
- Richard P. Dale 20 ,
- Barend Juan Vorster ORCID: orcid.org/0000-0003-3518-3508 21 ,
- Bodo Peters 11 ,
- Jens Lerchl ORCID: orcid.org/0000-0002-9633-2653 22 ,
- Patrick J. Tranel ORCID: orcid.org/0000-0003-0666-4564 23 ,
- Roland Beffa ORCID: orcid.org/0000-0003-3109-388X 24 ,
- Alexandre Fournier-Level ORCID: orcid.org/0000-0002-6047-7164 25 ,
- Mithila Jugulam ORCID: orcid.org/0000-0003-2065-9067 15 ,
- Kevin Fengler 18 ,
- Victor Llaca ORCID: orcid.org/0000-0003-4822-2924 18 ,
- Eric L. Patterson ORCID: orcid.org/0000-0001-7111-6287 14 &
- Todd A. Gaines ORCID: orcid.org/0000-0003-1485-7665 1
Genome Biology volume 25 , Article number: 139 ( 2024 ) Cite this article
40 Accesses
Metrics details
Weeds are attractive models for basic and applied research due to their impacts on agricultural systems and capacity to swiftly adapt in response to anthropogenic selection pressures. Currently, a lack of genomic information precludes research to elucidate the genetic basis of rapid adaptation for important traits like herbicide resistance and stress tolerance and the effect of evolutionary mechanisms on wild populations. The International Weed Genomics Consortium is a collaborative group of scientists focused on developing genomic resources to impact research into sustainable, effective weed control methods and to provide insights about stress tolerance and adaptation to assist crop breeding.
Each year globally, agricultural producers and landscape managers spend billions of US dollars [ 1 , 2 ] and countless hours attempting to control weedy plants and reduce their adverse effects. These management methods range from low-tech (e.g., pulling plants from the soil by hand) to extremely high-tech (e.g., computer vision-controlled spraying of herbicides). Regardless of technology level, effective control methods serve as strong selection pressures on weedy plants and often result in rapid evolution of weed populations resistant to such methods [ 3 , 4 , 5 , 6 , 7 ]. Thus, humans and weeds have been locked in an arms race, where humans develop new or improved control methods and weeds adapt and evolve to circumvent such methods.
Applying genomics to weed science offers a unique opportunity to study rapid adaptation, epigenetic responses, and examples of evolutionary rescue of diverse weedy species in the face of widespread and powerful selective pressures. Furthermore, lessons learned from these studies may also help to develop more sustainable control methods and to improve crop breeding efforts in the face of our ever-changing climate. While other research fields have used genetics and genomics to uncover the basis of many biological traits [ 8 , 9 , 10 , 11 ] and to understand how ecological factors affect evolution [ 12 , 13 ], the field of weed science has lagged behind in the development of genomic tools essential for such studies [ 14 ]. As research in human and crop genetics pushes into the era of pangenomics (i.e., multiple chromosome scale genome assemblies for a single species [ 15 , 16 ]), publicly available genomic information is still lacking or severely limited for the majority of weed species. Recent reviews of current weed genomes identified 26 [ 17 ] and 32 weed species with sequenced genomes [ 18 ]—many assembled to a sub-chromosome level.
Here, we summarize the current state of weed genomics, highlighting cases where genomics approaches have successfully provided insights on topics such as population genetic dynamics, genome evolution, and the genetic basis of herbicide resistance, rapid adaptation, and crop dedomestication. These highlighted investigations all relied upon genomic resources that are relatively rare for weedy species. Throughout, we identify additional resources that would advance the field of weed science and enable further progress in weed genomics. We then introduce the International Weed Genomics Consortium (IWGC), an open collaboration among researchers, and describe current efforts to generate these additional resources.
Evolution of weediness: potential research utilizing weed genomics tools
Weeds can evolve from non-weed progenitors through wild colonization, crop de-domestication, or crop-wild hybridization [ 19 ]. Because the time span in which weeds have evolved is necessarily limited by the origins of agriculture, these non-weed relatives often still exist and can be leveraged through population genomic and comparative genomic approaches to identify the adaptive changes that have driven the evolution of weediness. The ability to rapidly adapt, persist, and spread in agroecosystems are defining features of weedy plants, leading many to advocate agricultural weeds as ideal candidates for studying rapid plant adaptation [ 20 , 21 , 22 , 23 ]. The insights gained from applying plant ecological approaches to the study of rapid weed adaptation will move us towards the ultimate goals of mitigating such adaptation and increasing the efficacy of crop breeding and biotechnology [ 14 ].
Biology and ecological genomics of weeds
The impressive community effort to create and maintain resources for Arabidopsis thaliana ecological genomics provides a motivating example for the emerging study of weed genomics [ 24 , 25 , 26 , 27 ]. Arabidopsis thaliana was the first flowering plant species to have its genome fully sequenced [ 28 ] and rapidly became a model organism for plant molecular biology. As weedy genomes become available, collection, maintenance, and resequencing of globally distributed accessions of these species will help to replicate the success found in ecological studies of A. thaliana [ 29 , 30 , 31 , 32 , 33 , 34 , 35 ]. Evaluation of these accessions for traits of interest to produce large phenomics data sets (as in [ 36 , 37 , 38 , 39 , 40 ]) enables genome-wide association studies and population genomics analyses aimed at dissecting the genetic basis of variation in such traits [ 41 ]. Increasingly, these resources (e.g. the 1001 genomes project [ 29 ]) have enabled A. thaliana to be utilized as a model species to explore the eco-evolutionary basis of plant adaptation in a more realistic ecological context. Weedy species should supplement lessons in eco-evolutionary genomics learned from these experiments in A. thaliana .
Untargeted genomic approaches for understanding the evolutionary trajectories of populations and the genetic basis of traits as described above rely on the collection of genotypic information from across the genome of many individuals. While whole-genome resequencing accomplishes this requirement and requires no custom methodology, this approach provides more information than is necessary and is prohibitively expensive in species with large genomes. Development and optimization of genotype-by-sequencing methods for capturing reduced representations of newly sequence genomes like those described by [ 42 , 43 , 44 ] will reduce the cost and computational requirements of genetic mapping and population genetic experiments. Most major weed species do not currently have protocols for stable transformation, a key development in the popularity of A. thaliana as a model organism and a requirement for many functional genomic approaches. Functional validation of genes/variants believed to be responsible for traits of interest in weeds has thus far relied on transiently manipulating endogenous gene expression [ 45 , 46 ] or ectopic expression of a transgene in a model system [ 47 , 48 , 49 ]. While these methods have been successful, few weed species have well-studied viral vectors to adapt for use in virus induced gene silencing. Spray induced gene silencing is another potential option for functional investigation of candidate genes in weeds, but more research is needed to establish reliable delivery and gene knockdown [ 50 ]. Furthermore, traits with complex genetic architecture divergent between the researched and model species may not be amenable to functional genomic approaches using transgenesis techniques in model systems. Developing protocols for reduced representation sequencing, stable transformation, and gene editing/silencing in weeds will allow for more thorough characterization of candidate genetic variants underlying traits of interest.
Beyond rapid adaptation, some weedy species offer an opportunity to better understand co-evolution, like that between plants and pollinators and how their interaction leads to the spread of weedy alleles (Additional File 1 : Table S1). A suite of plant–insect traits has co-evolved to maximize the attraction of the insect pollinator community and the efficiency of pollen deposition between flowers ensuring fruit and seed production in many weeds [ 51 , 52 ]. Genetic mapping experiments have identified genes and genetic variants responsible for many floral traits affecting pollinator interaction including petal color [ 53 , 54 , 55 , 56 ], flower symmetry and size [ 57 , 58 , 59 ], and production of volatile organic compounds [ 60 , 61 , 62 ] and nectar [ 63 , 64 , 65 ]. While these studies reveal candidate genes for selection under co-evolution, herbicide resistance alleles may also have pleiotropic effects on the ecology of weeds [ 66 ], altering plant-pollinator interactions [ 67 ]. Discovery of genes and genetic variants involved in weed-pollinator interaction and their molecular and environmental control may create opportunities for better management of weeds with insect-mediated pollination. For example, if management can disrupt pollinator attraction/interaction with these weeds, the efficiency of reproduction may be reduced.
A more complete understanding of weed ecological genomics will undoubtedly elucidate many unresolved questions regarding the genetic basis of various aspects of weediness. For instance, when comparing populations of a species from agricultural and non-agricultural environments, is there evidence for contemporary evolution of weedy traits selected by agricultural management or were “natural” populations pre-adapted to agroecosystems? Where there is differentiation between weedy and natural populations, which traits are under selection and what is the genetic basis of variation in those traits? When comparing between weedy populations, is there evidence for parallel versus non-parallel evolution of weediness at the phenotypic and genotypic levels? Such studies may uncover fundamental truths about weediness. For example, is there a common phenotypic and/or genotypic basis for aspects of weediness among diverse weed species? The availability of characterized accessions and reference genomes for species of interest are required for such studies but only a few weedy species have these resources developed.
Population genomics
Weed species are certainly fierce competitors, able to outcompete crops and endemic species in their native environment, but they are also remarkable colonizers of perturbed habitats. Weeds achieve this through high fecundity, often producing tens of thousands of seeds per individual plant [ 68 , 69 , 70 ]. These large numbers in terms of demographic population size often combine with outcrossing reproduction to generate high levels of diversity with local effective population sizes in the hundreds of thousands [ 71 , 72 ]. This has two important consequences: weed populations retain standing genetic variation and generate many new mutations, supporting weed success in the face of harsh control. The generation of genomic tools to monitor weed populations at the molecular level is a game-changer to understanding weed dynamics and precisely testing the effect of artificial selection (i.e., management) and other evolutionary mechanisms on the genetic make-up of populations.
Population genomic data, without any environmental or phenotypic information, can be used to scan the genomes of weed and non-weed relatives to identify selective sweeps, pointing at loci supporting weed adaptation on micro- or macro-evolutionary scales. Two recent within-species examples include weedy rice, where population differentiation between weedy and domesticated populations was used to identify the genetic basis of weedy de-domestication [ 73 ], and common waterhemp, where consistent allelic differences among natural and agricultural collections resolved a complex set of agriculturally adaptive alleles [ 74 , 75 ]. A recent comparative population genomic study of weedy barnyardgrass and crop millet species has demonstrated how inter-specific investigations can resolve the signatures of crop and weed evolution [ 76 ] (also see [ 77 ] for a non-weed climate adaptation example). Multiple sequence alignments across numerous species provide complementary insight into adaptive convergence over deeper timescales, even with just one genomic sample per species (e.g., [ 78 , 79 ]). Thus, newly sequenced weed genomes combined with genomes available for closely related crops (outlined by [ 14 , 80 ]) and an effort to identify other non-weed wild relatives will be invaluable in characterizing the genetic architecture of weed adaptation and evolution across diverse species.
Weeds experience high levels of genetic selection, both artificial in response to agricultural practices and particularly herbicides, and natural in response to the environmental conditions they encounter [ 81 , 82 ]. Using genomic analysis to identify loci that are the targets of selection, whether natural or artificial, would point at vulnerabilities that could be leveraged against weeds to develop new and more sustainable management strategies [ 83 ]. This is a key motivation to develop genotype-by-environment association (GEA) and selective sweep scan approaches, which allow researchers to resolve the molecular basis of multi-dimensional adaptation [ 84 , 85 ]. GEA approaches, in particular, have been widely used on landscape-wide resequencing collections to determine the genetic basis of climate adaptation (e.g., [ 27 , 86 , 87 ]), but have yet to be fully exploited to diagnose the genetic basis of the various aspects of weediness [ 88 ]. Armed with data on environmental dimensions of agricultural settings, such as focal crop, soil quality, herbicide use, and climate, GEA approaches can help disentangle how discrete farming practices have influenced the evolution of weediness and resolve broader patterns of local adaptation across a weed’s range. Although non-weedy relatives are not technically required for GEA analyses, inclusion of environmental and genomic data from weed progenitors can further distinguish genetic variants underpinning weed origins from those involved in local adaptation.
New weeds emerge frequently [ 89 ], either through hybridization between species as documented for sea beet ( Beta vulgaris ssp. maritima) hybridizing with crop beet to produce progeny that are well adapted to agricultural conditions [ 90 , 91 , 92 ], or through the invasion of alien species that find a new range to colonize. Biosecurity measures are often in place to stop the introduction of new weeds; however, the vast scale of global agricultural commodity trade precludes the possibility of total control. Population genomic analysis is now able to measure gene flow between populations [ 74 , 93 , 94 , 95 ] and identify populations of origin for invasive species including weeds [ 96 , 97 , 98 ]. For example, the invasion route of the pest fruitfly Drosophila suzukii from Eastern Asia to North America and Europe through Hawaii was deciphered using Approximate Bayesian Computation on high-throughput sequencing data from a global sample of multiple populations [ 99 ]. Genomics can also be leveraged to predict invasion rather than explain it. The resequencing of a global sample of common ragweed ( Ambrosia artemisiifolia L.) elucidated a complex invasion route whereby Europe was invaded by multiple introductions of American ragweed that hybridized in Europe prior to a subsequent introduction to Australia [ 100 , 101 ]. In this context, the use of genomically informed species distribution models helps assess the risk associated with different source populations, which in the case of common ragweed, suggests that a source population from Florida would allow ragweed to invade most of northern Australia [ 102 ]. Globally coordinated research efforts to understand potential distribution models could support the transformation of biosecurity from perspective analysis towards predictive risk assessment.
Herbicide resistance and weed management
Herbicide resistance is among the numerous weedy traits that can evolve in plant populations exposed to agricultural selection pressures. Over-reliance on herbicides to control weeds, along with low diversity and lack of redundancy in weed management strategies, has resulted in globally widespread herbicide resistance [ 103 ]. To date, 272 herbicide-resistant weed species have been reported worldwide, and at least one resistance case exists for 21 of the 31 existing herbicide sites of action [ 104 ]—significantly limiting chemical weed control options available to agriculturalists. This limitation of control options is exacerbated by the recent lack of discovery of herbicides with new sites of action [ 105 ].
Herbicide resistance may result from several different physiological mechanisms. Such mechanisms have been classified into two main groups, target-site resistance (TSR) [ 4 , 106 ] and non-target-site resistance (NTSR) [ 4 , 107 ]. The first group encompasses changes that reduce binding affinity between a herbicide and its target [ 108 ]. These changes may provide resistance to multiple herbicides that have a common biochemical target [ 109 ] and can be effectively managed through mixture and/or rotation of herbicides targeting different sites of action [ 110 ]. The second group (NTSR), includes alterations in herbicide absorption, translocation, sequestration, and/or metabolism that may lead to unpredictable pleotropic cross-resistance profiles where structurally and functionally diverse herbicides are rendered ineffective by one or more genetic variant(s) [ 47 ]. This mechanism of resistance threatens not only the efficacy of existing herbicidal chemistries, but also ones yet to be discovered. While TSR is well understood because of the ease of identification and molecular characterization of target site variants, NTSR mechanisms are significantly more challenging to research because they are often polygenic, and the resistance causing element(s) are not well understood [ 111 ].
Improving the current understanding of metabolic NTSR mechanisms is not an easy task, since genes of diverse biochemical functions are involved, many of which exist as extensive gene families [ 109 , 112 ]. Expression changes of NTSR genes have been implicated in several resistance cases where the protein products of the genes are functionally equivalent across sensitive and resistant plants, but their relative abundance leads to resistance. Thus, regulatory elements of NTSR genes have been scrutinized to understand their role in NTSR mechanisms [ 113 ]. Similarly, epigenetic modifications have been hypothesized to play a role in NTSR, with much remaining to be explored [ 114 , 115 , 116 ]. Untargeted approaches such as genome-wide association, selective sweep scans, linkage mapping, RNA-sequencing, and metabolomic profiling have proven helpful to complement more specific biochemical- and chemo-characterization studies towards the elucidation of NTSR mechanisms as well as their regulation and evolution [ 47 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 ]. Even in cases where resistance has been attributed to TSR, genetic mapping approaches can detect other NTSR loci contributing to resistance (as shown by [ 123 ]) and provide further evidence for the role of TSR mutations across populations. Knowledge of the genetic basis of NTSR will aid the rational design of herbicides by screening new compounds for interaction with newly discovered NTSR proteins during early research phases and by identifying conserved chemical structures that interact with these proteins that should be avoided in small molecule design.
Genomic resources can also be used to predict the protein structure for novel herbicide target site and metabolism genes. This will allow for prediction of efficacy and selectivity for new candidate herbicides in silico to increase herbicide discovery throughput as well as aid in the design and development of next-generation technologies for sustainable weed management. Proteolysis targeting chimeras (PROTACs) have the potential to bind desired targets with great selectivity and degrade proteins by utilizing natural protein ubiquitination and degradation pathways within plants [ 125 ]. Spray-induced gene silencing in weeds using oligonucleotides has potential as a new, innovative, and sustainable method for weed management, but improved methods for design and delivery of oligonucleotides are needed to make this technique a viable management option [ 50 ]. Additionally, success in the field of pharmaceutical drug discovery in the development of molecules modulating protein–protein interactions offers another potential avenue towards the development of herbicides with novel targets [ 126 , 127 ]. High-quality reference genomes allow for the design of new weed management technologies like the ones listed here that are specific to—and effective across—weed species but have a null effect on non-target organisms.
Comparative genomics and genome biology
The genomes of weed species are as diverse as weed species themselves. Weeds are found across highly diverged plant families and often have no phylogenetically close model or crop species relatives for comparison. On all measurable metrics, weed genomes run the gamut. Some have smaller genomes like Cyperus spp. (~ 0.26 Gb) while others are larger, such as Avena fatua (~ 11.1 Gb) (Table 1 ). Some have high heterozygosity in terms of single-nucleotide polymorphisms, such as the Amaranthus spp., while others are primarily self-pollinated and quite homozygous, such as Poa annua [ 128 , 129 ]. Some are diploid such as Conyza canadensis and Echinochloa haploclada while others are polyploid such as C. sumetrensis , E. crus-galli , and E. colona [ 76 ]. The availability of genomic resources in these diverse, unexplored branches of the tree of life allows us to identify consistencies and anomalies in the field of genome biology.
The weed genomes published so far have focused mainly on weeds of agronomic crops, and studies have revolved around their ability to resist key herbicides. For example, genomic resources were vital in the elucidation of herbicide resistance cases involving target site gene copy number variants (CNVs). Gene CNVs of 5-enolpyruvylshikimate-3-phosphate synthase ( EPSPS ) have been found to confer resistance to the herbicide glyphosate in diverse weed species. To date, nine species have independently evolved EPSPS CNVs, and species achieve increased EPSPS copy number via different mechanisms [ 153 ]. For instance, the EPSPS CNV in Bassia scoparia is caused by tandem duplication, which is accredited to transposable element insertions flanking EPSPS and subsequent unequal crossing over events [ 154 , 155 ]. In Eleusine indica , a EPSPS CNV was caused by translocation of the EPSPS locus into the subtelomere followed by telomeric sequence exchange [ 156 ]. One of the most fascinating genome biology discoveries in weed science has been that of extra-chromosomal circular DNAs (eccDNAs) that harbor the EPSPS gene in the weed species Amaranthus palmeri [ 157 , 158 ]. In this case, the eccDNAs autonomously replicate separately from the nuclear genome and do not reintegrate into chromosomes, which has implications for inheritance, fitness, and genome structure [ 159 ]. These discoveries would not have been possible without reference assemblies of weed genomes, next-generation sequencing, and collaboration with experts in plant genomics and bioinformatics.
Another question that is often explored with weedy genomes is the nature and composition of gene families that are associated with NTSR. Gene families under consideration often include cytochrome P450s (CYPs), glutathione- S -transferases (GSTs), ABC transporters, etc. Some questions commonly considered with new weed genomes include how many genes are in each of these gene families, where are they located, and which weed accessions and species have an over-abundance of them that might explain their ability to evolve resistance so rapidly [ 76 , 146 , 160 , 161 ]? Weed genome resources are necessary to answer questions about gene family expansion or contraction during the evolution of weediness, including the role of polyploidy in NTSR gene family expansion as explored by [ 162 ].
Translational research and communication with weed management stakeholders
Whereas genomics of model plants is typically aimed at addressing fundamental questions in plant biology, and genomics of crop species has the obvious goal of crop improvement, goals of genomics of weedy plants also include the development of more effective and sustainable strategies for their management. Weed genomic resources assist with these objectives by providing novel molecular ecological and evolutionary insights from the context of intensive anthropogenic management (which is lacking in model plants), and offer knowledge and resources for trait discovery for crop improvement, especially given that many wild crop relatives are also important agronomic weeds (e.g., [ 163 ]). For instance, crop-wild relatives are valuable for improving crop breeding for marginal environments [ 164 ]. Thus, weed genomics presents unique opportunities and challenges relative to plant genomics more broadly. It should also be noted that although weed science at its core is an applied discipline, it draws broadly from many scientific disciplines such as, plant physiology, chemistry, ecology, and evolutionary biology, to name a few. The successful integration of weed-management strategies, therefore, requires extensive collaboration among individuals collectively possessing the necessary expertise [ 165 ].
With the growing complexity of herbicide resistance management, practitioners are beginning to recognize the importance of understanding resistance mechanisms to inform appropriate management tactics [ 14 ]. Although weed science practitioners do not need to understand the technical details of weed genomics, their appreciation of the power of weed genomics—together with their unique insights from field observations—will yield novel opportunities for applications of weed genomics to weed management. In particular, combining field management history with information on weed resistance mechanisms is expected to provide novel insights into evolutionary trajectories (e.g. [ 6 , 166 ]), which can be utilized for disrupting evolutionary adaptation. It can be difficult to obtain field history information from practitioners, but developing an understanding among them of the importance of such information can be invaluable.
Development of weed genomics resources by the IWGC
Weed genomics is a fast-growing field of research with many recent breakthroughs and many unexplored areas of study. The International Weed Genomics Consortium (IWGC) started in 2021 to address the roadblocks listed above and to promote the study of weedy plants. The IWGC is an open collaboration among academic, government, and industry researchers focused on producing genomic tools for weedy species from around the world. Through this collaboration, our initial aim is to provide chromosome-level reference genome assemblies for at least 50 important weedy species from across the globe that are chosen based on member input, economic impact, and global prevalence (Fig. 1 ). Each genome will include annotation of gene models and repetitive elements and will be freely available through public databases with no intellectual property restrictions. Additionally, future funding of the IWGC will focus on improving gene annotations and supplementing these reference genomes with tools that increase their utility.
The International Weed Genomics Consortium (IWGC) collected input from the weed genomics community to develop plans for weed genome sequencing, annotation, user-friendly genome analysis tools, and community engagement
Reference genomes and data analysis tools
The first objective of the IWGC is to provide high-quality genomic resources for agriculturally important weeds. The IWGC therefore created two main resources for information about, access to, or analysis of weed genomic data (Fig. 1 ). The IWGC website (available at [ 167 ]) communicates the status and results of genome sequencing projects, information on training and funding opportunities, upcoming events, and news in weed genomics. It also contains details of all sequenced species including genome size, ploidy, chromosome number, herbicide resistance status, and reference genome assembly statistics. The IWGC either compiles existing data on genome size, ploidy, and chromosome number, or obtains the data using flow cytometry and cytogenetics (Fig. 1 ; Additional File 2 : Fig S1-S4). Through this website, users can request an account to access our second main resource, an online genome database called WeedPedia (accessible at [ 168 ]), with an account that is created within 3–5 working days of an account request submission. WeedPedia hosts IWGC-generated and other relevant publicly accessible genomic data as well as a suite of bioinformatic tools. Unlike what is available for other fields, weed science did not have a centralized hub for genomics information, data, and analysis prior to the IWGC. Our intention in creating WeedPedia is to encourage collaboration and equity of access to information across the research community. Importantly, all genome assemblies and annotations from the IWGC (Table 1 ), along with the raw data used to produce them, will be made available through NCBI GenBank. Upon completion of a 1-year sponsoring member data confidentiality period for each species (dates listed in Table 1 ), scientific teams within the IWGC produce the first genome-wide investigation to submit for publication including whole genome level analyses on genes, gene families, and repetitive sequences as well as comparative analysis with other species. Genome assemblies and data will be publicly available through NCBI as part of these initial publications for each species.
WeedPedia is a cloud-based omics database management platform built from the software “CropPedia” and licensed from KeyGene (Wageningen, The Netherlands). The interface allows users to access, visualize, and download genome assemblies along with structural and functional annotation. The platform includes a genome browser, comparative map viewer, pangenome tools, RNA-sequencing data visualization tools, genetic mapping and marker analysis tools, and alignment capabilities that allow searches by keyword or sequence. Additionally, genes encoding known target sites of herbicides have been specially annotated, allowing users to quickly identify and compare these genes of interest. The platform is flexible, making it compatible with future integration of other data types such as epigenetic or proteomic information. As an online platform with a graphical user interface, WeedPedia provides user-friendly, intuitive tools that encourage users to integrate genomics into their research while also allowing more advanced users to download genomic data to be used in custom analysis pipelines. We aspire for WeedPedia to mimic the success of other public genomic databases such as NCBI, CoGe, Phytozome, InsectBase, and Mycocosm to name a few. WeedPedia currently hosts reference genomes for 40 species (some of which are currently in their 1-year confidentiality period) with additional genomes in the pipeline to reach a currently planned total of 55 species (Table 1 ). These genomes include both de novo reference genomes generated or in progress by the IWGC (31 species; Table 1 ), and publicly available genome assemblies of 24 weedy or related species that were generated by independent research groups (Table 2 ). As of May 2024, WeedPedia has over 370 registered users from more than 27 countries spread across 6 continents.
The IWGC reference genomes are generated in partnership with the Corteva Agriscience Genome Center of Excellence (Johnston, Iowa) using a combination of single-molecule long-read sequencing, optical genome maps, and chromosome conformation mapping. This strategy has already yielded highly contiguous, phased, chromosome-level assemblies for 26 weed species, with additional assemblies currently in progress (Table 1 ). The IWGC assemblies have been completed as single or haplotype-resolved double-haplotype pseudomolecules in inbreeding and outbreeding species, respectively, with multiple genomes being near gapless. For example, the de novo assemblies of the allohexaploids Conyza sumatrensis and Chenopodium album have all chromosomes captured in single scaffolds and most chromosomes being gapless from telomere to telomere. Complementary full-length isoform (IsoSeq) sequencing of RNA collected from diverse tissue types and developmental stages assists in the development of gene models during annotation.
As with accessibility of data, a core objective of the IWGC is to facilitate open access to sequenced germplasm when possible for featured species. Historically, the weed science community has rarely shared or adopted standard germplasm (e.g., specific weed accessions). The IWGC has selected a specific accession of each species for reference genome assembly (typically susceptible to herbicides). In collaboration with a parallel effort by the Herbicide Resistant Plants committee of the Weed Science Society of America, seeds of the sequenced weed accessions will be deposited in the United States Department of Agriculture Germplasm Resources Information Network [ 186 ] for broad access by the scientific community and their accession numbers will be listed on the IWGC website. In some cases, it is not possible to generate enough seed to deposit into a public repository (e.g., plants that typically reproduce vegetatively, that are self-incompatible, or that produce very few seeds from a single individual). In these cases, the location of collection for sequenced accessions will at least inform the community where the sequenced individual came from and where they may expect to collect individuals with similar genotypes. The IWGC ensures that sequenced accessions are collected and documented to comply with the Nagoya Protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization under the Convention on Biological Diversity and related Access and Benefit Sharing Legislation [ 187 ]. As additional accessions of weed species are sequenced (e.g., pangenomes are obtained), the IWGC will facilitate germplasm sharing protocols to support collaboration. Further, to simplify the investigation of herbicide resistance, the IWGC will link WeedPedia with the International Herbicide-Resistant Weed Database [ 104 ], an already widely known and utilized database for weed scientists.
Training and collaboration in weed genomics
Beyond producing genomic tools and resources, a priority of the IWGC is to enable the utilization of these resources across a wide range of stakeholders. A holistic approach to training is required for weed science generally [ 188 ], and we would argue even more so for weed genomics. To accomplish our training goals, the IWGC is developing and delivering programs aimed at the full range of IWGC stakeholders and covering a breadth of relevant topics. We have taken care to ensure our approaches are diverse as to provide training to researchers with all levels of existing experience and differing reasons for engaging with these tools. Throughout, the focus is on ensuring that our training and outreach result in impacts that benefit a wide range of stakeholders.
Although recently developed tools are incredibly enabling and have great potential to replace antiquated methodology [ 189 ] and to solve pressing weed science problems [ 14 ], specialized computational skills are required to fully explore and unlock meaning from these highly complex datasets. Collaboration with, or training of, computational biologists equipped with these skills and resources developed by the IWGC will enable weed scientists to expand research programs and better understand the genetic underpinnings of weed evolution and herbicide resistance. To fill existing skill gaps, the IWGC is developing summer bootcamps and online modules directed specifically at weed scientists that will provide training on computational skills (Fig. 1 ). Because successful utilization of the IWGC resources requires more than general computational skills, we have created three targeted workshops that teach practical skills related to genomics databases, molecular biology, and population genomics (available at [ 190 ]). The IWGC has also hosted two official conference meetings, one in September of 2021 and one in January of 2023, with more conferences planned. These conferences have included invited speakers to present successful implementations of weed genomics, educational workshops to build computational skills, and networking opportunities for research to connect and collaborate.
Engagement opportunities during undergraduate degrees have been shown to improve academic outcomes [ 191 , 192 ]. As one activity to help achieve this goal, the IWGC has sponsored opportunities for US undergraduates to undertake a 10-week research experience, which includes an introduction to bioinformatics, a plant genomics research project that results in a presentation, and access to career building opportunities in diverse workplace environments. To increase equitable access to conferences and professional communities, we supported early career researchers to attend the first two IWGC conferences in the USA as well as workshops and bootcamps in Europe, South America, and Australia. These hybrid or in-person travel grants are intentionally designed to remove barriers and increase participation of individuals from backgrounds and experiences currently underrepresented within weed/plant science or genomics [ 193 ]. Recipients of these travel awards gave presentations and gained the measurable benefits that come from either virtual or in-person participation in conferences [ 194 ]. Moving forward, weed scientists must amass skills associated with genomic analyses and collaborate with other area experts to fully leverage resources developed by the IWGC.
The tools generated through the IWGC will enable many new research projects with diverse objectives like those listed above. In summary, contiguous genome assemblies and complete annotation information will allow weed scientists to join plant breeders in the use of genetic mapping for many traits including stress tolerance, plant architecture, and herbicide resistance (especially important for cases of NTSR). These assemblies will also allow for investigations of population structure, gene flow, and responses to evolutionary mechanisms like genetic bottlenecking and artificial selection. Understanding gene sequences across diverse weed species will be vital in modeling new herbicide target site proteins and designing novel effective herbicides with minimal off-target effects. The IWGC website will improve accessibility to weed genomics data by providing a single hub for reference genomes as well as phenotypic and genotypic information for accessions shared with the IWGC. Deposition of sequenced germplasm into public repositories will ensure that researchers are able to access and utilize these accessions in their own research to make the field more standardized and equitable. WeedPedia allows users of all backgrounds to quickly access information of interest such as herbicide target site gene sequence or subcellular localization of protein products for different genes. Users can also utilize server-based tools such as BLAST and genome browsing similar to other public genomic databases. Finally, the IWGC is committed to training and connecting weed genomicists through hosting trainings, workshops, and conferences.
Conclusions
Weeds are unique and fascinating plants, having significant impacts on agriculture and ecosystems; and yet, aspects of their biology, ecology, and genetics remain poorly understood. Weeds represent a unique area within plant biology, given their repeated rapid adaptation to sudden and severe shifts in the selective landscape of anthropogenic management practices. The production of a public genomics database with reference genomes and annotations for over 50 weed species represents a substantial step forward towards research goals that improve our understanding of the biology and evolution of weeds. Future work is needed to improve annotations, particularly for complex gene families involved in herbicide detoxification, structural variants, and mobile genetic elements. As reference genome assemblies become available; standard, affordable methods for gathering genotype information will allow for the identification of genetic variants underlying traits of interest. Further, methods for functional validation and hypothesis testing are needed in weeds to validate the effect of genetic variants detected through such experiments, including systems for transformation, gene editing, and transient gene silencing and expression. Future research should focus on utilizing weed genomes to investigate questions about evolutionary biology, ecology, genetics of weedy traits, and weed population dynamics. The IWGC plans to continue the public–private partnership model to host the WeedPedia database over time, integrate new datasets such as genome resequencing and transcriptomes, conduct trainings, and serve as a research coordination network to ensure that advances in weed science from around the world are shared across the research community (Fig. 1 ). Bridging basic plant genomics with translational applications in weeds is needed to deliver on the potential of weed genomics to improve weed management and crop breeding.
Availability of data and materials
All genome assemblies and related sequencing data produced by the IWGC will be available through NCBI as part of publications reporting the first genome-wide analysis for each species.
Gianessi LP, Nathan PR. The value of herbicides in U.S. crop production. Weed Technol. 2007;21(2):559–66.
Article Google Scholar
Pimentel D, Lach L, Zuniga R, Morrison D. Environmental and economic costs of nonindigenous species in the United States. Bioscience. 2000;50(1):53–65.
Barrett SH. Crop mimicry in weeds. Econ Bot. 1983;37(3):255–82.
Powles SB, Yu Q. Evolution in action: plants resistant to herbicides. Annu Rev Plant Biol. 2010;61:317–47.
Article CAS PubMed Google Scholar
Thurber CS, Reagon M, Gross BL, Olsen KM, Jia Y, Caicedo AL. Molecular evolution of shattering loci in U.S. weedy rice. Mol Ecol. 2010;19(16):3271–84.
Article PubMed PubMed Central Google Scholar
Comont D, Lowe C, Hull R, Crook L, Hicks HL, Onkokesung N, et al. Evolution of generalist resistance to herbicide mixtures reveals a trade-off in resistance management. Nat Commun. 2020;11(1):3086.
Article CAS PubMed PubMed Central Google Scholar
Ashworth MB, Walsh MJ, Flower KC, Vila-Aiub MM, Powles SB. Directional selection for flowering time leads to adaptive evolution in Raphanus raphanistrum (wild radish). Evol Appl. 2016;9(4):619–29.
Chan EK, Rowe HC, Kliebenstein DJ. Understanding the evolution of defense metabolites in Arabidopsis thaliana using genome-wide association mapping. Genetics. 2010;185(3):991–1007.
Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–94.
Harkess A, Zhou J, Xu C, Bowers JE, Van der Hulst R, Ayyampalayam S, et al. The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat Commun. 2017;8(1):1279.
Periyannan S, Moore J, Ayliffe M, Bansal U, Wang X, Huang L, et al. The gene Sr33 , an ortholog of barley Mla genes, encodes resistance to wheat stem rust race Ug99. Science. 2013;341(6147):786–8.
Ågren J, Oakley CG, McKay JK, Lovell JT, Schemske DW. Genetic mapping of adaptation reveals fitness tradeoffs in Arabidopsis thaliana . Proc Natl Acad Sci U S A. 2013;110(52):21077–82.
Article PubMed Central Google Scholar
Schartl M, Walter RB, Shen Y, Garcia T, Catchen J, Amores A, et al. The genome of the platyfish, Xiphophorus maculatus , provides insights into evolutionary adaptation and several complex traits. Nat Genet. 2013;45(5):567–72.
Ravet K, Patterson EL, Krähmer H, Hamouzová K, Fan L, Jasieniuk M, et al. The power and potential of genomics in weed biology and management. Pest Manag Sci. 2018;74(10):2216–25.
Hufford MB, Seetharam AS, Woodhouse MR, Chougule KM, Ou S, Liu J, et al. De novo assembly, annotation, and comparative analysis of 26 diverse maize genomes. Science. 2021;373(6555):655–62.
Liao W-W, Asri M, Ebler J, Doerr D, Haukness M, Hickey G, et al. A draft human pangenome reference. Nature. 2023;617(7960):312–24.
Huang Y, Wu D, Huang Z, Li X, Merotto A, Bai L, et al. Weed genomics: yielding insights into the genetics of weedy traits for crop improvement. aBIOTECH. 2023;4:20–30.
Chen K, Yang H, Wu D, Peng Y, Lian L, Bai L, et al. Weed biology and management in the multi-omics era: progress and perspectives. Plant Commun. 2024;5(4):100816.
De Wet JMJ, Harlan JR. Weeds and domesticates: evolution in the man-made habitat. Econ Bot. 1975;29(2):99–108.
Mahaut L, Cheptou PO, Fried G, Munoz F, Storkey J, Vasseur F, et al. Weeds: against the rules? Trends Plant Sci. 2020;25(11):1107–16.
Neve P, Vila-Aiub M, Roux F. Evolutionary-thinking in agricultural weed management. New Phytol. 2009;184(4):783–93.
Article PubMed Google Scholar
Sharma G, Barney JN, Westwood JH, Haak DC. Into the weeds: new insights in plant stress. Trends Plant Sci. 2021;26(10):1050–60.
Vigueira CC, Olsen KM, Caicedo AL. The red queen in the corn: agricultural weeds as models of rapid adaptive evolution. Heredity (Edinb). 2013;110(4):303–11.
Donohue K, Dorn L, Griffith C, Kim E, Aguilera A, Polisetty CR, et al. Niche construction through germination cueing: life-history responses to timing of germination in Arabidopsis thaliana . Evolution. 2005;59(4):771–85.
PubMed Google Scholar
Exposito-Alonso M. Seasonal timing adaptation across the geographic range of Arabidopsis thaliana . Proc Natl Acad Sci U S A. 2020;117(18):9665–7.
Fournier-Level A, Korte A, Cooper MD, Nordborg M, Schmitt J, Wilczek AM. A map of local adaptation in Arabidopsis thaliana . Science. 2011;334(6052):86–9.
Hancock AM, Brachi B, Faure N, Horton MW, Jarymowycz LB, Sperone FG, et al. Adaptation to climate across the Arabidopsis thaliana genome. Science. 2011;334(6052):83–6.
Initiative TAG. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana . Nature. 2000;408(6814):796–815.
Alonso-Blanco C, Andrade J, Becker C, Bemm F, Bergelson J, Borgwardt KM, et al. 1,135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana . Cell. 2016;166(2):481–91.
Durvasula A, Fulgione A, Gutaker RM, Alacakaptan SI, Flood PJ, Neto C, et al. African genomes illuminate the early history and transition to selfing in Arabidopsis thaliana . Proc Natl Acad Sci U S A. 2017;114(20):5213–8.
Frachon L, Mayjonade B, Bartoli C, Hautekèete N-C, Roux F. Adaptation to plant communities across the genome of Arabidopsis thaliana . Mol Biol Evol. 2019;36(7):1442–56.
Fulgione A, Koornneef M, Roux F, Hermisson J, Hancock AM. Madeiran Arabidopsis thaliana reveals ancient long-range colonization and clarifies demography in Eurasia. Mol Biol Evol. 2018;35(3):564–74.
Fulgione A, Neto C, Elfarargi AF, Tergemina E, Ansari S, Göktay M, et al. Parallel reduction in flowering time from de novo mutations enable evolutionary rescue in colonizing lineages. Nat Commun. 2022;13(1):1461.
Kasulin L, Rowan BA, León RJC, Schuenemann VJ, Weigel D, Botto JF. A single haplotype hyposensitive to light and requiring strong vernalization dominates Arabidopsis thaliana populations in Patagonia. Argentina Mol Ecol. 2017;26(13):3389–404.
Picó FX, Méndez-Vigo B, Martínez-Zapater JM, Alonso-Blanco C. Natural genetic variation of Arabidopsis thaliana is geographically structured in the Iberian peninsula. Genetics. 2008;180(2):1009–21.
Atwell S, Huang YS, Vilhjálmsson BJ, Willems G, Horton M, Li Y, et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature. 2010;465(7298):627–31.
Flood PJ, Kruijer W, Schnabel SK, van der Schoor R, Jalink H, Snel JFH, et al. Phenomics for photosynthesis, growth and reflectance in Arabidopsis thaliana reveals circadian and long-term fluctuations in heritability. Plant Methods. 2016;12(1):14.
Marchadier E, Hanemian M, Tisné S, Bach L, Bazakos C, Gilbault E, et al. The complex genetic architecture of shoot growth natural variation in Arabidopsis thaliana . PLoS Genet. 2019;15(4):e1007954.
Tisné S, Serrand Y, Bach L, Gilbault E, Ben Ameur R, Balasse H, et al. Phenoscope: an automated large-scale phenotyping platform offering high spatial homogeneity. Plant J. 2013;74(3):534–44.
Tschiersch H, Junker A, Meyer RC, Altmann T. Establishment of integrated protocols for automated high throughput kinetic chlorophyll fluorescence analyses. Plant Methods. 2017;13:54.
Chen X, MacGregor DR, Stefanato FL, Zhang N, Barros-Galvão T, Penfield S. A VEL3 histone deacetylase complex establishes a maternal epigenetic state controlling progeny seed dormancy. Nat Commun. 2023;14(1):2220.
Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, et al. Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci U S A. 2009;106(45):19096–101.
Davey JW, Blaxter ML. RADSeq: next-generation population genetics. Brief Funct Genomics. 2010;9(5–6):416–23.
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES, et al. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE. 2011;6(5):e19379.
MacGregor DR. What makes a weed a weed? How virus-mediated reverse genetics can help to explore the genetics of weediness. Outlooks Pest Manag. 2020;31(5):224–9.
Mellado-Sánchez M, McDiarmid F, Cardoso V, Kanyuka K, MacGregor DR. Virus-mediated transient expression techniques enable gene function studies in blackgrass. Plant Physiol. 2020;183(2):455–9.
Dimaano NG, Yamaguchi T, Fukunishi K, Tominaga T, Iwakami S. Functional characterization of Cytochrome P450 CYP81A subfamily to disclose the pattern of cross-resistance in Echinochloa phyllopogon . Plant Mol Biol. 2020;102(4–5):403–16.
de Figueiredo MRA, Küpper A, Malone JM, Petrovic T, de Figueiredo ABTB, Campagnola G, et al. An in-frame deletion mutation in the degron tail of auxin coreceptor IAA2 confers resistance to the herbicide 2,4-D in Sisymbrium orientale . Proc Natl Acad Sci U S A. 2022;119(9):e2105819119.
Patzoldt WL, Hager AG, McCormick JS, Tranel PJ. A codon deletion confers resistance to herbicides inhibiting protoporphyrinogen oxidase. Proc Natl Acad Sci U S A. 2006;103(33):12329–34.
Zabala-Pardo D, Gaines T, Lamego FP, Avila LA. RNAi as a tool for weed management: challenges and opportunities. Adv Weed Sci. 2022;40(spe1):e020220096.
Fattorini R, Glover BJ. Molecular mechanisms of pollination biology. Annu Rev Plant Biol. 2020;71:487–515.
Rollin O, Benelli G, Benvenuti S, Decourtye A, Wratten SD, Canale A, et al. Weed-insect pollinator networks as bio-indicators of ecological sustainability in agriculture. A review Agron Sustain Dev. 2016;36(1):8.
Irwin RE, Strauss SY. Flower color microevolution in wild radish: evolutionary response to pollinator-mediated selection. Am Nat. 2005;165(2):225–37.
Ma B, Wu J, Shi T-L, Yang Y-Y, Wang W-B, Zheng Y, et al. Lilac ( Syringa oblata ) genome provides insights into its evolution and molecular mechanism of petal color change. Commun Biol. 2022;5(1):686.
Xing A, Wang X, Nazir MF, Zhang X, Wang X, Yang R, et al. Transcriptomic and metabolomic profiling of flavonoid biosynthesis provides novel insights into petals coloration in Asian cotton ( Gossypium arboreum L.). BMC Plant Biol. 2022;22(1):416.
Zheng Y, Chen Y, Liu Z, Wu H, Jiao F, Xin H, et al. Important roles of key genes and transcription factors in flower color differences of Nicotiana alata . Genes (Basel). 2021;12(12):1976.
Krizek BA, Anderson JT. Control of flower size. J Exp Bot. 2013;64(6):1427–37.
Powell AE, Lenhard M. Control of organ size in plants. Curr Biol. 2012;22(9):R360–7.
Spencer V, Kim M. Re"CYC"ling molecular regulators in the evolution and development of flower symmetry. Semin Cell Dev Biol. 2018;79:16–26.
Amrad A, Moser M, Mandel T, de Vries M, Schuurink RC, Freitas L, et al. Gain and loss of floral scent production through changes in structural genes during pollinator-mediated speciation. Curr Biol. 2016;26(24):3303–12.
Delle-Vedove R, Schatz B, Dufay M. Understanding intraspecific variation of floral scent in light of evolutionary ecology. Ann Bot. 2017;120(1):1–20.
Pichersky E, Gershenzon J. The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol. 2002;5(3):237–43.
Ballerini ES, Kramer EM, Hodges SA. Comparative transcriptomics of early petal development across four diverse species of Aquilegia reveal few genes consistently associated with nectar spur development. BMC Genom. 2019;20(1):668.
Corbet SA, Willmer PG, Beament JWL, Unwin DM, Prys-Jones OE. Post-secretory determinants of sugar concentration in nectar. Plant Cell Environ. 1979;2(4):293–308.
Galliot C, Hoballah ME, Kuhlemeier C, Stuurman J. Genetics of flower size and nectar volume in Petunia pollination syndromes. Planta. 2006;225(1):203–12.
Vila-Aiub MM, Neve P, Powles SB. Fitness costs associated with evolved herbicide resistance alleles in plants. New Phytol. 2009;184(4):751–67.
Baucom RS. Evolutionary and ecological insights from herbicide-resistant weeds: what have we learned about plant adaptation, and what is left to uncover? New Phytol. 2019;223(1):68–82.
Bajwa AA, Latif S, Borger C, Iqbal N, Asaduzzaman M, Wu H, et al. The remarkable journey of a weed: biology and management of annual ryegrass ( Lolium rigidum ) in conservation cropping systems of Australia. Plants (Basel). 2021;10(8):1505.
Bitarafan Z, Andreasen C. Fecundity allocation in some european weed species competing with crops. Agronomy. 2022;12(5):1196.
Costea M, Weaver SE, Tardif FJ. The biology of Canadian weeds. 130. Amaranthus retroflexus L., A. powellii , A. powellii S. Watson, and A. hybridus L. Can J Plant Sci. 2004;84(2):631–68.
Dixon A, Comont D, Slavov GT, Neve P. Population genomics of selectively neutral genetic structure and herbicide resistance in UK populations of Alopecurus myosuroides . Pest Manag Sci. 2021;77(3):1520–9.
Kersten S, Chang J, Huber CD, Voichek Y, Lanz C, Hagmaier T, et al. Standing genetic variation fuels rapid evolution of herbicide resistance in blackgrass. Proc Natl Acad Sci U S A. 2023;120(16):e2206808120.
Qiu J, Zhou Y, Mao L, Ye C, Wang W, Zhang J, et al. Genomic variation associated with local adaptation of weedy rice during de-domestication. Nat Commun. 2017;8(1):15323.
Kreiner JM, Caballero A, Wright SI, Stinchcombe JR. Selective ancestral sorting and de novo evolution in the agricultural invasion of Amaranthus tuberculatus . Evolution. 2022;76(1):70–85.
Kreiner JM, Latorre SM, Burbano HA, Stinchcombe JR, Otto SP, Weigel D, et al. Rapid weed adaptation and range expansion in response to agriculture over the past two centuries. Science. 2022;378(6624):1079–85.
Wu D, Shen E, Jiang B, Feng Y, Tang W, Lao S, et al. Genomic insights into the evolution of Echinochloa species as weed and orphan crop. Nat Commun. 2022;13(1):689.
Yeaman S, Hodgins KA, Lotterhos KE, Suren H, Nadeau S, Degner JC, et al. Convergent local adaptation to climate in distantly related conifers. Science. 2016;353(6306):1431–3.
Haudry A, Platts AE, Vello E, Hoen DR, Leclercq M, Williamson RJ, et al. An atlas of over 90,000 conserved noncoding sequences provides insight into crucifer regulatory regions. Nat Genet. 2013;45(8):891–8.
Sackton TB, Grayson P, Cloutier A, Hu Z, Liu JS, Wheeler NE, et al. Convergent regulatory evolution and loss of flight in paleognathous birds. Science. 2019;364(6435):74–8.
Ye CY, Fan L. Orphan crops and their wild relatives in the genomic era. Mol Plant. 2021;14(1):27–39.
Clements DR, Jones VL. Ten ways that weed evolution defies human management efforts amidst a changing climate. Agronomy. 2021;11(2):284.
Article CAS Google Scholar
Weinig C. Rapid evolutionary responses to selection in heterogeneous environments among agricultural and nonagricultural weeds. Int J Plant Sci. 2005;166(4):641–7.
Cousens RD, Fournier-Level A. Herbicide resistance costs: what are we actually measuring and why? Pest Manag Sci. 2018;74(7):1539–46.
Lasky JR, Josephs EB, Morris GP. Genotype–environment associations to reveal the molecular basis of environmental adaptation. Plant Cell. 2023;35(1):125–38.
Lotterhos KE. The effect of neutral recombination variation on genome scans for selection. G3-Genes Genom Genet. 2019;9(6):1851–67.
Lovell JT, MacQueen AH, Mamidi S, Bonnette J, Jenkins J, Napier JD, et al. Genomic mechanisms of climate adaptation in polyploid bioenergy switchgrass. Nature. 2021;590(7846):438–44.
Todesco M, Owens GL, Bercovich N, Légaré J-S, Soudi S, Burge DO, et al. Massive haplotypes underlie ecotypic differentiation in sunflowers. Nature. 2020;584(7822):602–7.
Revolinski SR, Maughan PJ, Coleman CE, Burke IC. Preadapted to adapt: Underpinnings of adaptive plasticity revealed by the downy brome genome. Commun Biol. 2023;6(1):326.
Kuester A, Conner JK, Culley T, Baucom RS. How weeds emerge: a taxonomic and trait-based examination using United States data. New Phytol. 2014;202(3):1055–68.
Arnaud JF, Fénart S, Cordellier M, Cuguen J. Populations of weedy crop-wild hybrid beets show contrasting variation in mating system and population genetic structure. Evol Appl. 2010;3(3):305–18.
Ellstrand NC, Schierenbeck KA. Hybridization as a stimulus for the evolution of invasiveness in plants? Proc Natl Acad Sci U S A. 2000;97(13):7043–50.
Nakabayashi K, Leubner-Metzger G. Seed dormancy and weed emergence: from simulating environmental change to understanding trait plasticity, adaptive evolution, and population fitness. J Exp Bot. 2021;72(12):4181–5.
Busi R, Yu Q, Barrett-Lennard R, Powles S. Long distance pollen-mediated flow of herbicide resistance genes in Lolium rigidum . Theor Appl Genet. 2008;117(8):1281–90.
Délye C, Clément JAJ, Pernin F, Chauvel B, Le Corre V. High gene flow promotes the genetic homogeneity of arable weed populations at the landscape level. Basic Appl Ecol. 2010;11(6):504–12.
Roumet M, Noilhan C, Latreille M, David J, Muller MH. How to escape from crop-to-weed gene flow: phenological variation and isolation-by-time within weedy sunflower populations. New Phytol. 2013;197(2):642–54.
Moghadam SH, Alebrahim MT, Mohebodini M, MacGregor DR. Genetic variation of Amaranthus retroflexus L. and Chenopodium album L. (Amaranthaceae) suggests multiple independent introductions into Iran. Front Plant Sci. 2023;13:1024555.
Muller M-H, Latreille M, Tollon C. The origin and evolution of a recent agricultural weed: population genetic diversity of weedy populations of sunflower ( Helianthus annuus L.) in Spain and France. Evol Appl. 2011;4(3):499–514.
Wesse C, Welk E, Hurka H, Neuffer B. Geographical pattern of genetic diversity in Capsella bursa-pastoris (Brassicaceae) -A global perspective. Ecol Evol. 2021;11(1):199–213.
Fraimout A, Debat V, Fellous S, Hufbauer RA, Foucaud J, Pudlo P, et al. Deciphering the routes of invasion of Drosophila suzukii by means of ABC random forest. Mol Biol Evol. 2017;34(4):980–96.
CAS PubMed PubMed Central Google Scholar
Battlay P, Wilson J, Bieker VC, Lee C, Prapas D, Petersen B, et al. Large haploblocks underlie rapid adaptation in the invasive weed Ambrosia artemisiifolia . Nat Commun. 2023;14(1):1717.
van Boheemen LA, Hodgins KA. Rapid repeatable phenotypic and genomic adaptation following multiple introductions. Mol Ecol. 2020;29(21):4102–17.
Putra A, Hodgins K, Fournier-Level A. Assessing the invasive potential of different source populations of ragweed ( Ambrosia artemisiifolia L.) through genomically-informed species distribution modelling. Authorea. 2023;17(1):e13632.
Google Scholar
Bourguet D, Delmotte F, Franck P, Guillemaud T, Reboud X, Vacher C, et al. Heterogeneity of selection and the evolution of resistance. Trends Ecol Evol. 2013;28(2):110–8.
The International Herbicide-Resistant Weed Database. www.weedscience.org . Accessed 20 June 2023.
Powles S. Herbicide discovery through innovation and diversity. Adv Weed Sci. 2022;40(spe1):e020220074.
Murphy BP, Tranel PJ. Target-site mutations conferring herbicide resistance. Plants (Basel). 2019;8(10):382.
Gaines TA, Duke SO, Morran S, Rigon CAG, Tranel PJ, Küpper A, et al. Mechanisms of evolved herbicide resistance. J Biol Chem. 2020;295(30):10307–30.
Lonhienne T, Cheng Y, Garcia MD, Hu SH, Low YS, Schenk G, et al. Structural basis of resistance to herbicides that target acetohydroxyacid synthase. Nat Commun. 2022;13(1):3368.
Comont D, MacGregor DR, Crook L, Hull R, Nguyen L, Freckleton RP, et al. Dissecting weed adaptation: fitness and trait correlations in herbicide-resistant Alopecurus myosuroides . Pest Manag Sci. 2022;78(7):3039–50.
Neve P. Simulation modelling to understand the evolution and management of glyphosate resistance in weeds. Pest Manag Sci. 2008;64(4):392–401.
Torra J, Alcántara-de la Cruz R. Molecular mechanisms of herbicide resistance in weeds. Genes (Basel). 2022;13(11):2025.
Délye C, Gardin JAC, Boucansaud K, Chauvel B, Petit C. Non-target-site-based resistance should be the centre of attention for herbicide resistance research: Alopecurus myosuroides as an illustration. Weed Res. 2011;51(5):433–7.
Chandra S, Leon RG. Genome-wide evolutionary analysis of putative non-specific herbicide resistance genes and compilation of core promoters between monocots and dicots. Genes (Basel). 2022;13(7):1171.
Margaritopoulou T, Tani E, Chachalis D, Travlos I. Involvement of epigenetic mechanisms in herbicide resistance: the case of Conyza canadensis . Agriculture. 2018;8(1):17.
Pan L, Guo Q, Wang J, Shi L, Yang X, Zhou Y, et al. CYP81A68 confers metabolic resistance to ALS and ACCase-inhibiting herbicides and its epigenetic regulation in Echinochloa crus-galli . J Hazard Mater. 2022;428:128225.
Sen MK, Hamouzová K, Košnarová P, Roy A, Soukup J. Herbicide resistance in grass weeds: Epigenetic regulation matters too. Front Plant Sci. 2022;13:1040958.
Han H, Yu Q, Beffa R, González S, Maiwald F, Wang J, et al. Cytochrome P450 CYP81A10v7 in Lolium rigidum confers metabolic resistance to herbicides across at least five modes of action. Plant J. 2021;105(1):79–92.
Kubis GC, Marques RZ, Kitamura RS, Barroso AA, Juneau P, Gomes MP. Antioxidant enzyme and Cytochrome P450 activities are involved in horseweed ( Conyza sumatrensis ) resistance to glyphosate. Stress. 2023;3(1):47–57.
Qiao Y, Zhang N, Liu J, Yang H. Interpretation of ametryn biodegradation in rice based on joint analyses of transcriptome, metabolome and chemo-characterization. J Hazard Mater. 2023;445:130526.
Rouse CE, Roma-Burgos N, Barbosa Martins BA. Physiological assessment of non–target site restistance in multiple-resistant junglerice ( Echinochloa colona ). Weed Sci. 2019;67(6):622–32.
Abou-Khater L, Maalouf F, Jighly A, Alsamman AM, Rubiales D, Rispail N, et al. Genomic regions associated with herbicide tolerance in a worldwide faba bean ( Vicia faba L.) collection. Sci Rep. 2022;12(1):158.
Gupta S, Harkess A, Soble A, Van Etten M, Leebens-Mack J, Baucom RS. Interchromosomal linkage disequilibrium and linked fitness cost loci associated with selection for herbicide resistance. New Phytol. 2023;238(3):1263–77.
Kreiner JM, Tranel PJ, Weigel D, Stinchcombe JR, Wright SI. The genetic architecture and population genomic signatures of glyphosate resistance in Amaranthus tuberculatus . Mol Ecol. 2021;30(21):5373–89.
Parcharidou E, Dücker R, Zöllner P, Ries S, Orru R, Beffa R. Recombinant glutathione transferases from flufenacet-resistant black-grass ( Alopecurus myosuroides Huds.) form different flufenacet metabolites and differ in their interaction with pre- and post-emergence herbicides. Pest Manag Sci. 2023;79(9):3376–86.
Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21(3):181–200.
Acuner Ozbabacan SE, Engin HB, Gursoy A, Keskin O. Transient protein-protein interactions. Protein Eng Des Sel. 2011;24(9):635–48.
Lu H, Zhou Q, He J, Jiang Z, Peng C, Tong R, et al. Recent advances in the development of protein–protein interactions modulators: mechanisms and clinical trials. Signal Transduct Target Ther. 2020;5(1):213.
Benson CW, Sheltra MR, Maughan PJ, Jellen EN, Robbins MD, Bushman BS, et al. Homoeologous evolution of the allotetraploid genome of Poa annua L. BMC Genom. 2023;24(1):350.
Robbins MD, Bushman BS, Huff DR, Benson CW, Warnke SE, Maughan CA, et al. Chromosome-scale genome assembly and annotation of allotetraploid annual bluegrass ( Poa annua L.). Genome Biol Evol. 2022;15(1):evac180.
Montgomery JS, Giacomini D, Waithaka B, Lanz C, Murphy BP, Campe R, et al. Draft genomes of Amaranthus tuberculatus , Amaranthus hybridus and Amaranthus palmeri . Genome Biol Evol. 2020;12(11):1988–93.
Jeschke MR, Tranel PJ, Rayburn AL. DNA content analysis of smooth pigweed ( Amaranthus hybridus ) and tall waterhemp ( A. tuberculatus ): implications for hybrid detection. Weed Sci. 2003;51(1):1–3.
Rayburn AL, McCloskey R, Tatum TC, Bollero GA, Jeschke MR, Tranel PJ. Genome size analysis of weedy Amaranthus species. Crop Sci. 2005;45(6):2557–62.
Laforest M, Martin SL, Bisaillon K, Soufiane B, Meloche S, Tardif FJ, et al. The ancestral karyotype of the Heliantheae Alliance, herbicide resistance, and human allergens: Insights from the genomes of common and giant ragweed. Plant Genome . 2024;e20442. https://doi.org/10.1002/tpg2.20442 .
Mulligan GA. Chromosome numbers of Canadian weeds. I Canad J Bot. 1957;35(5):779–89.
Meyer L, Causse R, Pernin F, Scalone R, Bailly G, Chauvel B, et al. New gSSR and EST-SSR markers reveal high genetic diversity in the invasive plant Ambrosia artemisiifolia L. and can be transferred to other invasive Ambrosia species. PLoS One. 2017;12(5):e0176197.
Pustahija F, Brown SC, Bogunić F, Bašić N, Muratović E, Ollier S, et al. Small genomes dominate in plants growing on serpentine soils in West Balkans, an exhaustive study of 8 habitats covering 308 taxa. Plant Soil. 2013;373(1):427–53.
Kubešová M, Moravcova L, Suda J, Jarošík V, Pyšek P. Naturalized plants have smaller genomes than their non-invading relatives: a flow cytometric analysis of the Czech alien flora. Preslia. 2010;82(1):81–96.
Thébaud C, Abbott RJ. Characterization of invasive Conyza species (Asteraceae) in Europe: quantitative trait and isozyme analysis. Am J Bot. 1995;82(3):360–8.
Garcia S, Hidalgo O, Jakovljević I, Siljak-Yakovlev S, Vigo J, Garnatje T, et al. New data on genome size in 128 Asteraceae species and subspecies, with first assessments for 40 genera, 3 tribes and 2 subfamilies. Plant Biosyst. 2013;147(4):1219–27.
Zhao X, Yi L, Ren Y, Li J, Ren W, Hou Z, et al. Chromosome-scale genome assembly of the yellow nutsedge ( Cyperus esculentus ). Genome Biol Evol. 2023;15(3):evad027.
Bennett MD, Leitch IJ, Hanson L. DNA amounts in two samples of angiosperm weeds. Ann Bot. 1998;82:121–34.
Schulz-Schaeffer J, Gerhardt S. Cytotaxonomic analysis of the Euphorbia spp. (leafy spurge) complex. II: Comparative study of the chromosome morphology. Biol Zentralbl. 1989;108(1):69–76.
Schaeffer JR, Gerhardt S. The impact of introgressive hybridization on the weediness of leafy spurge. Leafy Spurge Symposium. 1989;1989:97–105.
Bai C, Alverson WS, Follansbee A, Waller DM. New reports of nuclear DNA content for 407 vascular plant taxa from the United States. Ann Bot. 2012;110(8):1623–9.
Aarestrup JR, Karam D, Fernandes GW. Chromosome number and cytogenetics of Euphorbia heterophylla L. Genet Mol Res. 2008;7(1):217–22.
Wang L, Sun X, Peng Y, Chen K, Wu S, Guo Y, et al. Genomic insights into the origin, adaptive evolution, and herbicide resistance of Leptochloa chinensis , a devastating tetraploid weedy grass in rice fields. Mol Plant. 2022;15(6):1045–58.
Paril J, Pandey G, Barnett EM, Rane RV, Court L, Walsh T, et al. Rounding up the annual ryegrass genome: high-quality reference genome of Lolium rigidum . Front Genet. 2022;13:1012694.
Weiss-Schneeweiss H, Greilhuber J, Schneeweiss GM. Genome size evolution in holoparasitic Orobanche (Orobanchaceae) and related genera. Am J Bot. 2006;93(1):148–56.
Towers G, Mitchell J, Rodriguez E, Bennett F, Subba Rao P. Biology & chemistry of Parthenium hysterophorus L., a problem weed in India. Biol Rev. 1977;48:65–74.
CAS Google Scholar
Moghe GD, Hufnagel DE, Tang H, Xiao Y, Dworkin I, Town CD, et al. Consequences of whole-genome triplication as revealed by comparative genomic analyses of the wild radish ( Raphanus raphanistrum ) and three other Brassicaceae species. Plant Cell. 2014;26(5):1925–37.
Zhang X, Liu T, Wang J, Wang P, Qiu Y, Zhao W, et al. Pan-genome of Raphanus highlights genetic variation and introgression among domesticated, wild, and weedy radishes. Mol Plant. 2021;14(12):2032–55.
Chytrý M, Danihelka J, Kaplan Z, Wild J, Holubová D, Novotný P, et al. Pladias database of the Czech flora and vegetation. Preslia. 2021;93(1):1–87.
Patterson EL, Pettinga DJ, Ravet K, Neve P, Gaines TA. Glyphosate resistance and EPSPS gene duplication: Convergent evolution in multiple plant species. J Hered. 2018;109(2):117–25.
Jugulam M, Niehues K, Godar AS, Koo DH, Danilova T, Friebe B, et al. Tandem amplification of a chromosomal segment harboring 5-enolpyruvylshikimate-3-phosphate synthase locus confers glyphosate resistance in Kochia scoparia . Plant Physiol. 2014;166(3):1200–7.
Patterson EL, Saski CA, Sloan DB, Tranel PJ, Westra P, Gaines TA. The draft genome of Kochia scoparia and the mechanism of glyphosate resistance via transposon-mediated EPSPS tandem gene duplication. Genome Biol Evol. 2019;11(10):2927–40.
Zhang C, Johnson N, Hall N, Tian X, Yu Q, Patterson E. Subtelomeric 5-enolpyruvylshikimate-3-phosphate synthase ( EPSPS ) copy number variation confers glyphosate resistance in Eleusine indica . Nat Commun. 2023;14:4865.
Koo D-H, Molin WT, Saski CA, Jiang J, Putta K, Jugulam M, et al. Extrachromosomal circular DNA-based amplification and transmission of herbicide resistance in crop weed Amaranthus palmeri . Proc Natl Acad Sci U S A. 2018;115(13):3332–7.
Molin WT, Yaguchi A, Blenner M, Saski CA. The eccDNA Replicon: A heritable, extranuclear vehicle that enables gene amplification and glyphosate resistance in Amaranthus palmeri . Plant Cell. 2020;32(7):2132–40.
Jugulam M. Can non-Mendelian inheritance of extrachromosomal circular DNA-mediated EPSPS gene amplification provide an opportunity to reverse resistance to glyphosate? Weed Res. 2021;61(2):100–5.
Kreiner JM, Giacomini DA, Bemm F, Waithaka B, Regalado J, Lanz C, et al. Multiple modes of convergent adaptation in the spread of glyphosate-resistant Amaranthus tuberculatus . Proc Natl Acad Sci U S A. 2019;116(42):21076–84.
Cai L, Comont D, MacGregor D, Lowe C, Beffa R, Neve P, et al. The blackgrass genome reveals patterns of non-parallel evolution of polygenic herbicide resistance. New Phytol. 2023;237(5):1891–907.
Chen K, Yang H, Peng Y, Liu D, Zhang J, Zhao Z, et al. Genomic analyses provide insights into the polyploidization-driven herbicide adaptation in Leptochloa weeds. Plant Biotechnol J. 2023;21(8):1642–58.
Ohadi S, Hodnett G, Rooney W, Bagavathiannan M. Gene flow and its consequences in Sorghum spp. Crit Rev Plant Sci. 2017;36(5–6):367–85.
Renzi JP, Coyne CJ, Berger J, von Wettberg E, Nelson M, Ureta S, et al. How could the use of crop wild relatives in breeding increase the adaptation of crops to marginal environments? Front Plant Sci. 2022;13:886162.
Ward SM, Cousens RD, Bagavathiannan MV, Barney JN, Beckie HJ, Busi R, et al. Agricultural weed research: a critique and two proposals. Weed Sci. 2014;62(4):672–8.
Evans JA, Tranel PJ, Hager AG, Schutte B, Wu C, Chatham LA, et al. Managing the evolution of herbicide resistance. Pest Manag Sci. 2016;72(1):74–80.
International Weed Genomics Consortium Website. https://www.weedgenomics.org . Accessed 20 June 2023.
WeedPedia Database. https://weedpedia.weedgenomics.org/ . Accessed 20 June 2023.
Hall N, Chen J, Matzrafi M, Saski CA, Westra P, Gaines TA, et al. FHY3/FAR1 transposable elements generate adaptive genetic variation in the Bassia scoparia genome. bioRxiv . 2023; DOI: https://doi.org/10.1101/2023.05.26.542497 .
Jarvis DE, Sproul JS, Navarro-Domínguez B, Krak K, Jaggi K, Huang Y-F, et al. Chromosome-scale genome assembly of the hexaploid Taiwanese goosefoot “Djulis” ( Chenopodium formosanum ). Genome Biol Evol. 2022;14(8):evac120.
Ferreira LAI, de Oliveira RS, Jr., Constantin J, Brunharo C. Evolution of ACCase-inhibitor resistance in Chloris virgata is conferred by a Trp2027Cys mutation in the herbicide target site. Pest Manag Sci. 2023;79(12):5220–9.
Laforest M, Martin SL, Bisaillon K, Soufiane B, Meloche S, Page E. A chromosome-scale draft sequence of the Canada fleabane genome. Pest Manag Sci. 2020;76(6):2158–69.
Guo L, Qiu J, Ye C, Jin G, Mao L, Zhang H, et al. Echinochloa crus-galli genome analysis provides insight into its adaptation and invasiveness as a weed. Nat Commun. 2017;8(1):1031.
Sato MP, Iwakami S, Fukunishi K, Sugiura K, Yasuda K, Isobe S, et al. Telomere-to-telomere genome assembly of an allotetraploid pernicious weed, Echinochloa phyllopogon . DNA Res. 2023;30(5):dsad023.
Stein JC, Yu Y, Copetti D, Zwickl DJ, Zhang L, Zhang C, et al. Genomes of 13 domesticated and wild rice relatives highlight genetic conservation, turnover and innovation across the genus Oryza . Nat Genet. 2018;50(2):285–96.
Wu D, Xie L, Sun Y, Huang Y, Jia L, Dong C, et al. A syntelog-based pan-genome provides insights into rice domestication and de-domestication. Genome Biol. 2023;24(1):179.
Wang Z, Huang S, Yang Z, Lai J, Gao X, Shi J. A high-quality, phased genome assembly of broomcorn millet reveals the features of its subgenome evolution and 3D chromatin organization. Plant Commun. 2023;4(3):100557.
Mao Q, Huff DR. The evolutionary origin of Poa annua L. Crop Sci. 2012;52(4):1910–22.
Benson CW, Sheltra MR, Maughan JP, Jellen EN, Robbins MD, Bushman BS, et al. Homoeologous evolution of the allotetraploid genome of Poa annua L. Res Sq. 2023. https://doi.org/10.21203/rs.3.rs-2729084/v1 .
Brunharo C, Benson CW, Huff DR, Lasky JR. Chromosome-scale genome assembly of Poa trivialis and population genomics reveal widespread gene flow in a cool-season grass seed production system. Plant Direct. 2024;8(3):e575.
Mo C, Wu Z, Shang X, Shi P, Wei M, Wang H, et al. Chromosome-level and graphic genomes provide insights into metabolism of bioactive metabolites and cold-adaption of Pueraria lobata var. montana . DNA Research. 2022;29(5):dsac030.
Thielen PM, Pendleton AL, Player RA, Bowden KV, Lawton TJ, Wisecaver JH. Reference genome for the highly transformable Setaria viridis ME034V. G3 (Bethesda, Md). 2020;10(10):3467–78.
Yoshida S, Kim S, Wafula EK, Tanskanen J, Kim Y-M, Honaas L, et al. Genome sequence of Striga asiatica provides insight into the evolution of plant parasitism. Curr Biol. 2019;29(18):3041–52.
Qiu S, Bradley JM, Zhang P, Chaudhuri R, Blaxter M, Butlin RK, et al. Genome-enabled discovery of candidate virulence loci in Striga hermonthica , a devastating parasite of African cereal crops. New Phytol. 2022;236(2):622–38.
Nunn A, Rodríguez-Arévalo I, Tandukar Z, Frels K, Contreras-Garrido A, Carbonell-Bejerano P, et al. Chromosome-level Thlaspi arvense genome provides new tools for translational research and for a newly domesticated cash cover crop of the cooler climates. Plant Biotechnol J. 2022;20(5):944–63.
USDA-ARS Germplasm Resources Information Network (GRIN). https://www.ars-grin.gov/ . Accessed 20 June 2023.
Buck M, Hamilton C. The Nagoya Protocol on access to genetic resources and the fair and equitable sharing of benefits arising from their utilization to the convention on biological diversity. RECIEL. 2011;20(1):47–61.
Chauhan BS, Matloob A, Mahajan G, Aslam F, Florentine SK, Jha P. Emerging challenges and opportunities for education and research in weed science. Front Plant Sci. 2017;8:1537.
Shah S, Lonhienne T, Murray CE, Chen Y, Dougan KE, Low YS, et al. Genome-guided analysis of seven weed species reveals conserved sequence and structural features of key gene targets for herbicide development. Front Plant Sci. 2022;13:909073.
International Weed Genomics Consortium Training Resources. https://www.weedgenomics.org/training-resources/ . Accessed 20 June 2023.
Blackford S. Harnessing the power of communities: career networking strategies for bioscience PhD students and postdoctoral researchers. FEMS Microbiol Lett. 2018;365(8):fny033.
Pender M, Marcotte DE, Sto Domingo MR, Maton KI. The STEM pipeline: The role of summer research experience in minority students’ Ph.D. aspirations. Educ Policy Anal Arch. 2010;18(30):1–36.
PubMed PubMed Central Google Scholar
Burke A, Okrent A, Hale K. The state of U.S. science and engineering 2022. Foundation NS. https://ncses.nsf.gov/pubs/nsb20221 . 2022.
Wu J-Y, Liao C-H, Cheng T, Nian M-W. Using data analytics to investigate attendees’ behaviors and psychological states in a virtual academic conference. Educ Technol Soc. 2021;24(1):75–91.
Download references
Peer review information
Wenjing She was the primary editor of this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
The International Weed Genomics Consortium is supported by BASF SE, Bayer AG, Syngenta Ltd, Corteva Agriscience, CropLife International (Global Herbicide Resistance Action Committee), the Foundation for Food and Agriculture Research (Award DSnew-0000000024), and two conference grants from USDA-NIFA (Award numbers 2021–67013-33570 and 2023-67013-38785).
Author information
Authors and affiliations.
Department of Agricultural Biology, Colorado State University, 1177 Campus Delivery, Fort Collins, CO, 80523, USA
Jacob Montgomery, Sarah Morran & Todd A. Gaines
Protecting Crops and the Environment, Rothamsted Research, Harpenden, Hertfordshire, UK
Dana R. MacGregor
Department of Crop, Soil, and Environmental Sciences, Auburn University, Auburn, AL, USA
J. Scott McElroy
Department of Plant and Environmental Sciences, University of Copenhagen, Taastrup, Denmark
Paul Neve & Célia Neto
IFEVA-Conicet-Department of Ecology, University of Buenos Aires, Buenos Aires, Argentina
Martin M. Vila-Aiub & Maria Victoria Sandoval
Department of Ecology, Faculty of Agronomy, University of Buenos Aires, Buenos Aires, Argentina
Analia I. Menéndez
Department of Botany, The University of British Columbia, Vancouver, BC, Canada
Julia M. Kreiner
Institute of Crop Sciences, Zhejiang University, Hangzhou, China
Longjiang Fan
Department of Biology, University of Massachusetts Amherst, Amherst, MA, USA
Ana L. Caicedo
Department of Plant and Wildlife Sciences, Brigham Young University, Provo, UT, USA
Peter J. Maughan
Bayer AG, Weed Control Research, Frankfurt, Germany
Bianca Assis Barbosa Martins, Jagoda Mika, Alberto Collavo & Bodo Peters
Department of Crop Sciences, Federal University of Rio Grande Do Sul, Porto Alegre, Rio Grande Do Sul, Brazil
Aldo Merotto Jr.
Department of Soil and Crop Sciences, Texas A&M University, College Station, TX, USA
Nithya K. Subramanian & Muthukumar V. Bagavathiannan
Department of Plant, Soil and Microbial Sciences, Michigan State University, East Lansing, MI, USA
Luan Cutti & Eric L. Patterson
Department of Agronomy, Kansas State University, Manhattan, KS, USA
Md. Mazharul Islam & Mithila Jugulam
Department of Plant Pathology, Kansas State University, Manhattan, KS, USA
Bikram S. Gill
Crop Protection Discovery and Development, Corteva Agriscience, Indianapolis, IN, USA
Robert Cicchillo, Roger Gast & Neeta Soni
Genome Center of Excellence, Corteva Agriscience, Johnston, IA, USA
Terry R. Wright, Gina Zastrow-Hayes, Gregory May, Kevin Fengler & Victor Llaca
School of Agriculture, Food and Wine, University of Adelaide, Glen Osmond, South Australia, Australia
Jenna M. Malone
Jealott’s Hill International Research Centre, Syngenta Ltd, Bracknell, Berkshire, UK
Deepmala Sehgal, Shiv Shankhar Kaundun & Richard P. Dale
Department of Plant and Soil Sciences, University of Pretoria, Pretoria, South Africa
Barend Juan Vorster
BASF SE, Ludwigshafen Am Rhein, Germany
Jens Lerchl
Department of Crop Sciences, University of Illinois, Urbana, IL, USA
Patrick J. Tranel
Senior Scientist Consultant, Herbicide Resistance Action Committee / CropLife International, Liederbach, Germany
Roland Beffa
School of BioSciences, University of Melbourne, Parkville, VIC, Australia
Alexandre Fournier-Level
You can also search for this author in PubMed Google Scholar
Contributions
JMo and TG conceived and outlined the article. TG, DM, EP, RB, JSM, PJT, MJ wrote grants to obtain funding. MMI, BSG, and MJ performed mitotic chromosome visualization. VL performed sequencing. VL and KF assembled the genomes. LC and ELP annotated the genomes. JMo, SM, DRM, JSM, PN, CN, MV, MVS, AIM, JMK, LF, ALC, PJM, BABM, JMi, AC, MVB, LC, AFL, and ELP wrote the first draft of the article. All authors edited the article and improved the final version.
Corresponding author
Correspondence to Todd A. Gaines .
Ethics declarations
Ethics approval and consent to participate.
Ethical approval is not applicable for this article.
Competing interests
Some authors work for commercial agricultural companies (BASF, Bayer, Corteva Agriscience, or Syngenta) that develop and sell weed control products.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13059_2024_3274_moesm1_esm.docx.
Additional file 1. List of completed and in-progress genome assemblies of weed species pollinated by insects (Table S1).
13059_2024_3274_MOESM2_ESM.docx
Additional file 2. Methods and results for visualizing and counting the metaphase chromosomes of hexaploid Avena fatua (Fig S1); diploid Lolium rigidum (Fig S2); tetraploid Phalaris minor (Fig S3); and tetraploid Salsola tragus (Fig S4).
Additional file 3. Review history.
Rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Montgomery, J., Morran, S., MacGregor, D.R. et al. Current status of community resources and priorities for weed genomics research. Genome Biol 25 , 139 (2024). https://doi.org/10.1186/s13059-024-03274-y
Download citation
Received : 11 July 2023
Accepted : 13 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s13059-024-03274-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Weed science
- Reference genomes
- Rapid adaptation
- Herbicide resistance
- Public resources
Genome Biology
ISSN: 1474-760X
- Submission enquiries: [email protected]
- General enquiries: [email protected]

IMAGES
VIDEO
COMMENTS
Biology definition: A null hypothesis is an assumption or proposition where an observed difference between two samples of a statistical population is purely accidental and not due to systematic causes. It is the hypothesis to be investigated through statistical hypothesis testing so that when refuted indicates that the alternative hypothesis is true. . Thus, a null hypothesis is a hypothesis ...
The null hypothesis states there is no relationship between the measured phenomenon (the dependent variable) and the independent variable, which is the variable an experimenter typically controls or changes.You do not need to believe that the null hypothesis is true to test it. On the contrary, you will likely suspect there is a relationship between a set of variables.
Null Hypothesis Examples. "Hyperactivity is unrelated to eating sugar " is an example of a null hypothesis. If the hypothesis is tested and found to be false, using statistics, then a connection between hyperactivity and sugar ingestion may be indicated. A significance test is the most common statistical test used to establish confidence in a ...
Whenever we perform a hypothesis test, we always write a null hypothesis and an alternative hypothesis, which take the following forms: H0 (Null Hypothesis): Population parameter =, ≤, ≥ some value. HA (Alternative Hypothesis): Population parameter <, >, ≠ some value. Note that the null hypothesis always contains the equal sign.
An example of the null hypothesis is that light color has no effect on plant growth. The null hypothesis (H 0) is the hypothesis that states there is no statistical difference between two sample sets. In other words, it assumes the independent variable does not have an effect on the dependent variable in a scientific experiment.
The null hypothesis is proposed by a scientist before completing an experiment, and it can be either supported by data or disproved in favor of an alternate hypothesis.
Basic definitions. The null hypothesis and the alternative hypothesis are types of conjectures used in statistical tests to make statistical inferences, which are formal methods of reaching conclusions and separating scientific claims from statistical noise.. The statement being tested in a test of statistical significance is called the null hypothesis. . The test of significance is designed ...
Revised on June 22, 2023. The null and alternative hypotheses are two competing claims that researchers weigh evidence for and against using a statistical test: Null hypothesis (H0): There's no effect in the population. Alternative hypothesis (Ha or H1): There's an effect in the population. The effect is usually the effect of the ...
null hypothesis. The statistical hypothesis that states that there will be no differences between observed and expected data.
The null and alternative hypotheses are two competing claims that researchers weigh evidence for and against using a statistical test: Null hypothesis (H0): There's no effect in the population. Alternative hypothesis (HA): There's an effect in the population. The effect is usually the effect of the independent variable on the dependent ...
A hypothesis is a prediction of the outcome of a test. It forms the basis for designing an experiment in the scientific method. A good hypothesis is testable, meaning it makes a prediction you can check with observation or experimentation. Here are different hypothesis examples. Null Hypothesis Examples
The actual test begins by considering two hypotheses.They are called the null hypothesis and the alternative hypothesis.These hypotheses contain opposing viewpoints. H 0, the —null hypothesis: a statement of no difference between sample means or proportions or no difference between a sample mean or proportion and a population mean or proportion. In other words, the difference equals 0.
The null hypothesis (H 0) is a hypothesis which the researcher tries to disprove, reject or nullify. The 'null' often refers to the common view of something, while the alternative hypothesis is what the researcher really thinks is the cause of a phenomenon. The simplistic definition of the null is as the opposite of the alternative hypothesis ...
The null hypothesis in statistics states that there is no difference between groups or no relationship between variables. It is one of two mutually exclusive hypotheses about a population in a hypothesis test. When your sample contains sufficient evidence, you can reject the null and conclude that the effect is statistically significant.
The null hypothesis is defined as any observable differences in treatments or variables is likely due to chance. In other words, the null hypothesis states that there is no significant difference ...
Therefore, DF = 4 - 1 = 3 and choosing p < 0.05 to be the threshold for significance (rejection of the null hypothesis), the X 2 must be greater than 7.82 in order to be significantly deviating from what is expected. With this dihybrid cross example, we expect a ratio of 9:3:3:1 in phenotypes where 1/16th of the population are recessive for ...
6. Write a null hypothesis. If your research involves statistical hypothesis testing, you will also have to write a null hypothesis. The null hypothesis is the default position that there is no association between the variables. The null hypothesis is written as H 0, while the alternative hypothesis is H 1 or H a.
Luke J. Harmon. University of Idaho. Standard hypothesis testing approaches focus almost entirely on rejecting null hypotheses. In the framework (usually referred to as the frequentist approach to statistics) one first defines a null hypothesis. This null hypothesis represents your expectation if some pattern, such as a difference among groups ...
It is the opposite of your research hypothesis. The alternative hypothesis--that is, the research hypothesis--is the idea, phenomenon, observation that you want to prove. If you suspect that girls take longer to get ready for school than boys, then: Alternative: girls time > boys time. Null: girls time <= boys time.
Overview of null hypothesis, examples of null and alternate hypotheses, and how to write a null hypothesis statement.
The null hypothesis is a general statement that states that there is no relationship between two phenomenons under consideration or that there is no association between two groups. An alternative hypothesis is a statement that describes that there is a relationship between two selected variables in a study. Symbol. It is denoted by H 0.
The null hypothesis is rejected using the P-value approach. If the P-value is less than or equal to the α, there should be a rejection of the null hypothesis in favour of the alternate hypothesis. In case, if P-value is greater than α, the null hypothesis is not rejected. Null Hypothesis and Alternative Hypothesis
a Example current recording from an outside ... sitting at the heart of this biology. Our hypothesis is that activation of these channels is a first or near-first event when force is applied at ...
Weeds are attractive models for basic and applied research due to their impacts on agricultural systems and capacity to swiftly adapt in response to anthropogenic selection pressures. Currently, a lack of genomic information precludes research to elucidate the genetic basis of rapid adaptation for important traits like herbicide resistance and stress tolerance and the effect of evolutionary ...