Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article

Research ethics review during the COVID-19 pandemic: An international study
Roles Conceptualization, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft
Current address: Dalla Lana School of Public Health, University of Toronto, Toronto, Canada
Affiliation Lunenfeld-Tanenbaum Research Institute, Bridgepoint Collaboratory for Research and Innovation, Sinai Health, Toronto, Canada
Roles Conceptualization, Writing – review & editing
Affiliation Institute for the History and Philosophy of Science and Technology, University of Toronto, Toronto, Canada
Roles Conceptualization, Funding acquisition, Methodology, Validation, Writing – review & editing
Affiliation School of Public Health, The University of Sydney, Sydney, Australia
Roles Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing – review & editing
Affiliations Lunenfeld-Tanenbaum Research Institute, Bridgepoint Collaboratory for Research and Innovation, Sinai Health, Toronto, Canada, Dalla Lana School of Public Health, University of Toronto, Toronto, Canada
Roles Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing
* E-mail: [email protected]
Affiliation Faculty of Health Sciences, Western University, London, Canada
- Fabio Salamanca-Buentello,
- Rachel Katz,
- Diego S. Silva,
- Ross E. G. Upshur,
- Maxwell J. Smith

- Published: April 16, 2024
- https://doi.org/10.1371/journal.pone.0292512
- Reader Comments
Research ethics review committees (ERCs) worldwide faced daunting challenges during the COVID-19 pandemic. There was a need to balance rapid turnaround with rigorous evaluation of high-risk research protocols in the context of considerable uncertainty. This study explored the experiences and performance of ERCs during the pandemic. We conducted an anonymous, cross-sectional, global online survey of chairs (or their delegates) of ERCs who were involved in the review of COVID-19-related research protocols after March 2020. The survey ran from October 2022 to February 2023 and consisted of 50 items, with opportunities for descriptive responses to open-ended questions. Two hundred and three participants [130 from high-income countries (HICs) and 73 from low- and middle-income countries (LMICs)] completed our survey. Respondents came from diverse entities and organizations from 48 countries (19 HICs and 29 LMICs) in all World Health Organization regions. Responses show little of the increased global funding for COVID-19 research was allotted to the operation of ERCs. Few ERCs had pre-existing internal policies to address operation during public health emergencies, but almost half used existing guidelines. Most ERCs modified existing procedures or designed and implemented new ones but had not evaluated the success of these changes. Participants overwhelmingly endorsed permanently implementing several of them. Few ERCs added new members but non-member experts were consulted; quorum was generally achieved. Collaboration among ERCs was infrequent, but reviews conducted by external ERCs were recognized and validated. Review volume increased during the pandemic, with COVID-19-related studies being prioritized. Most protocol reviews were reported as taking less than three weeks. One-third of respondents reported external pressure on their ERCs from different stakeholders to approve or reject specific COVID-19-related protocols. ERC members faced significant challenges to keep their committees functioning during the pandemic. Our findings can inform ERC approaches towards future public health emergencies. To our knowledge, this is the first international, COVID-19-related study of its kind.
Citation: Salamanca-Buentello F, Katz R, Silva DS, Upshur REG, Smith MJ (2024) Research ethics review during the COVID-19 pandemic: An international study. PLoS ONE 19(4): e0292512. https://doi.org/10.1371/journal.pone.0292512
Editor: Collins Atta Poku, Kwame Nkrumah University of Science and Technology, GHANA
Received: September 5, 2023; Accepted: March 23, 2024; Published: April 16, 2024
Copyright: © 2024 Salamanca-Buentello et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data for this study are within the paper and its Supporting Information files. Additionally, the raw survey data are available from the figshare database ( https://doi.org/10.6084/m9.figshare.24076704 ).
Funding: This study was funded by Canadian Institutes of Health Research grant #C150-2019-11 ( https://cihr-irsc.gc.ca/e/193.html ) awarded to MJS. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The ethical review of research protocols during public health emergencies (PHEs) such as the COVID-19 pandemic is a daunting endeavour. Committees tasked with assessing the ethical acceptability of research projects, which we refer to as ethics review committees (ERCs) but are also variably called research ethics boards, research ethics committees, ethics review boards, and institutional review boards, face challenges to reviewing research protocols swiftly while maintaining a high degree of rigour, all under suboptimal conditions and uncertainty. ERCs must balance the urge for rapid turnaround and flexibility with the requirement for intense scrutiny given that new projects often propose innovative but high-risk diagnostic, therapeutic, or preventive approaches to address the PHE. This is especially challenging in the case of countries with fragile health systems, poor infrastructure, and little experience conducting medical research, and also of countries experiencing protracted emergencies [ 1 – 5 ].
Failure to ensure rigour and depth during rapid ethics reviews in public health emergencies may place research participants at risk [ 6 ]. In such challenging circumstances, ERCs must consider how interventions, study design, eligibility criteria, community engagement, and approaches to vulnerable populations impact scientific validity, participant autonomy, respect for persons, welfare, justice, and social value [ 2 , 7 – 9 ]. Additional demands on ERCs may include the ability to incorporate and respond swiftly to newly available knowledge, to provide monitoring and oversight of research, and to grapple with the impact of the PHE on those involved in the research process, such as research participants, investigators, and ERC members and staff [ 7 ].
Public health emergencies force ERCs to make reasonable adjustments and design innovative strategies to address the various components of research ethics review while still adhering to ethical principles [ 3 , 6 , 7 , 10 ]. Moreover, after a PHE, changes implemented to secure continued operations of ERCs must be evaluated to determine their success and whether they should be permanently put in place to improve the everyday functioning of the committees.
Given the challenges that ERCs worldwide faced during the COVID-19 pandemic, we aimed in this exploratory study to identify their experiences in the attempt to adapt to this PHE. We were particularly interested in the availability of pandemic-specific support, the promptness of protocol review, the volume of protocols received, the modifications to and innovations in operational procedures and policies and the evaluation of their outcomes, the anticipated permanence of such changes beyond the pandemic, the presence of pressure from different stakeholders on ERCs, the efforts to ensure quorum, the changes to the composition of ERCs, and the approaches to strengthen inter-ERC collaboration. To our knowledge, this is the first international, COVID-19-related study of its kind.
This international, cross-sectional, exploratory online survey was conducted by researchers from Western University, the University of Toronto, and the Lunenfeld–Tanenbaum Research Institute in Canada, and the University of Sydney in Australia, in collaboration with the World Health Organization’s COVID-19 Ethics and Governance Working Group.
Inclusion criteria
We used targeted purposive and criterion sampling to invite Chairs and members of ERCs who were actively involved in the ethics review of COVID-19 research protocols to participate in this study. To ensure eligibility of participants, the first question of the survey asked respondents to confirm whether they had reviewed COVID-19-related research protocols during the pandemic. Responding to our survey was entirely voluntary. For the purposes of this study, we considered March 2020 as the beginning of this PHE. We specifically targeted individuals from all WHO regions. Participants were assigned to either of two categories: high-income countries (HICs) or low- and middle-income countries (LMICs), according to their reported country of residence. To do this, we used the World Bank classification of countries ( https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups ), which is based on gross national income per capita. We adopted this widely used categorization notwithstanding its limitations in terms of hiding power imbalances and reducing important differences among countries to questions of economics [ 11 ].
Survey questionnaire
The complete questionnaire is available as S1 Appendix . The overall structure and flow of the survey questionnaire, which consisted of a main “trunk” of 37 items organized into 11 thematic categories, is shown in Fig 1 . As can be seen in this figure, eight of these items branched into different survey flow elements based on respondents’ answers; seven of these eight items branched into elements with questions (six of them contained two questions). Thus, in total, the questionnaire, written in English, included 50 questions. We privileged close-ended over open-ended questions, but we allowed respondents the opportunity to provide additional comments for some items. We pilot-tested the online questionnaire with a small group of experts who fulfilled the inclusion criteria. This helped polish the wording of the questions and also assess and improve the logistics of the administration of the survey.
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0292512.g001
Data collection
The invitation to participate in the survey explained the nature and purpose of our study, the inclusion criteria used to select participants, a summary of the procedures involved, and the URL link to the survey. These invitations were initially distributed by email by the WHO’s COVID-19 Ethics and Governance Working Group through the email listserv of the 13th Global Summit of National Ethics Committees (an event that took place in September 2022). The Working Group identified additional potential participants among its extensive contact networks. We also circulated the invitation to experts identified by the research team. Invitations could also be forwarded to individuals designated by ERCs.
Our survey was active from October 11, 2022, to February 28, 2023. We used the Qualtrics Experience Management (XM) online platform to administer the questionnaire, which was open only to individuals who received the invitation with the link to the survey.
Data analysis
The analysis of the findings of this exploratory study employed descriptive statistics and stratified the comparison between responses of participants from HICs with those of participants from LMICs. To facilitate the examination of the results, tables were prepared showing the number and percentage of respondents from HICs and LMICs who answered each question in the survey. Qualitative data (descriptive responses to open-ended questions) were evaluated using thematic analysis and the constant comparative method.
Research ethics approval
Our study received approval from Western University’s Non-Medical Research Ethics Board (Protocol ID 120455). Additionally, it was evaluated by the World Health Organization Research Ethics Review Committee (Protocol ID CERC.0181) and was exempted from further review. The use of the Qualtrics platform facilitated data collection and management while respecting the privacy and confidentiality of participants. Respondents indicated their consent to participate in our survey by selecting a button labelled “I consent” at the end of the letter of information and consent, which appeared on the first page of the questionnaire. Responses were anonymous to protect participants’ privacy and confidentiality and encourage the open sharing of experiences.
Reporting survey results
While no universally agreed-upon reporting standards for surveys exist like there are for clinical trials and meta-analyses, the EQUATOR (Enhancing the QUAlity and Transparency Of health Research) Network has recently (2021) proposed a checklist for reporting of survey studies [ 12 ]. EQUATOR has been responsible for the development of several of these standards, including the Consolidated Standards of Reporting Trials (CONSORT) for randomized control trials; Strengthening the Reporting of Observational studies in Epidemiology (STROBE) for observational studies; and Preferred Reporting Items for Systemic Reviews and Meta-analyses (PRISMA) for systematic reviews and meta-analyses. Even though this is not a globally recognized official standard, it is quite useful, and we have ensured that our manuscript fulfills all the reporting requirements included in this checklist.
Characterization of survey respondents
Two hundred and eighty-one individuals opened our survey. Of these, 250 answered the first question, which confirmed whether respondents fulfilled our inclusion criteria, and with which we could confirm their eligibility. Forty-three individuals explicitly indicated that they did not meet our criteria. Thus, the initial number of suitable respondents was 207. As expected in surveys such as ours in which participants are allowed to skip questions, the number of respondents per question varied slightly, from a maximum of 207 to a minimum of 147.
Of the 204 participants who indicated their sex / gender, 120 (58.8%) were female, 82 (40.2%) were male, one (0.5%) preferred to self-describe, and one (0.5%) preferred not to disclose this information ( Box 1 , Table a ). The proportion of females was higher in HICs (64.9%) than in LMICs (47.9%); thus, the distribution of respondents by sex / gender was more balanced in LMICs than in HICs. As shown in Box 1 , Table b , more than three quarters of respondents (77.9%) were 45 years old or older. This was true for both HICs and LMICs. Most respondents provided ethics review for national bodies such as national ethics committees or national public health organizations; more than a quarter participated in ERCs linked to academic or research institutions ( Box 1 , Table c ). However, while almost half of respondents from HICs were members of ERCs affiliated with national bodies, only one quarter of participants from LMICs provided ethics review for such organizations. In contrast, in LMICs, 40% of respondents were members of ERCs associated with academic or research institutions. Furthermore, only 20% of participants from LMICs and 13.9% of participants from HICs provided ethics review for health care facilities.
Box 1. Characterization of survey participants
https://doi.org/10.1371/journal.pone.0292512.t001
In terms of the WHO region for which ethics review was provided, all regions were represented in our survey ( S1 Table in S2 Appendix ). More than one third of respondents reviewed research protocols from Europe, almost one fifth from the Americas, one tenth from Africa, and less than one tenth each from the other WHO regions.
Table 1 shows the number of respondents by country of residence. Participants from 48 countries (19 HICs and 29 LMICs) responded to our survey. Of the 203 individuals who indicated their country of residence, 130 (64%) were from HICs and 73 (36%) from LMICs. There was a large contingent of respondents from the UK (93).
https://doi.org/10.1371/journal.pone.0292512.t002
Two thirds of respondents had six or more years of experience as ERC members. This is true for participants from both HICs and LMICs ( S2 Table in S2 Appendix ).
As shown in S3 Table in S2 Appendix , about one half of respondents (52%) were involved in only one ERC. This pattern was common for participants from HICs and LMICs. However, more than one third of respondents from HICs participated in three or more ERCs during the COVID-19 pandemic. Of those who indicated involvement with multiple ERCs, close to one half specified that such participation was simultaneous ( S4 Table in S2 Appendix ).
Support for the operation of ERCs during the pandemic
As shown in S5 Table in S2 Appendix , an overwhelming majority (78.4%) of respondents indicated that their ERCs received no additional support for the operation of their committees during the pandemic. This lack of support was more pronounced in the case of ERCs in LMICs. For the minority of ERCs that did receive support, this consisted mainly of administrative and human resources, with one quarter of respondents from LMICs stating that their ERCs also received financial support, in contrast to only 12.5% of those from HICs ( S6 Table in S2 Appendix ). In terms of specific areas supported, participants from both HICs and LMICs mentioned teleconferencing and virtual meeting capabilities, information technology, support staff, assistance for ERC reviewers, and training of ERC members ( Table 2 ). Interestingly, while 20% of respondents from HICs chose ERC support staff as one of the areas that received assistance, only 7.5% of those from LMICs did.
https://doi.org/10.1371/journal.pone.0292512.t003
In their descriptive responses, participants alluded to support for covering the costs of using online platforms for meetings and protocol review, and for acquiring or upgrading hardware such as laptops and webcams. In one ERC, members were able to claim costs of setting up teleconferencing and of telephone calls if dialling into a meeting. In other ERCs, information technology training was offered, along with technical support for the use of online platforms. It is important to note that almost half of respondents from HICs, but close to the totality (91.4%) of those from LMICs, indicated that their ERCs lacked any pre-pandemic financial planning that included provisions for the support of the committees during a public health emergency ( S7 Table in S2 Appendix ).
Modification of existing procedures or policies
Respondents from both HICs and LMICs overwhelmingly (more than 75% of participants in both cases) reported that their ERCs modified existing procedures or policies to operate during the pandemic ( S8 Table in S2 Appendix ). The most frequently modified domain was meeting logistics, followed by meeting frequency and procedures for protocol review and approval ( S9 Table in S2 Appendix ).
In terms of modifications to review procedures, several participants pointed out in their descriptive responses that their ERCs fast-tracked the review of pandemic-related studies, shortening the timeline to review and approve protocols. ERC members were expected to complete the review of these protocols within a few days and, in some cases, 24 hours. To facilitate such a quick turnaround, some ERCs created special sub-committees that would conduct very fast protocol reviews. Moreover, participants emphasized the importance of simplifying and increasing the flexibility of administrative processes. For example, several respondents indicated that their ERCs switched entirely to the use of online platforms for protocol review, eliminating the need for paper documents.
Numerous participants stated that all ERC meetings were conducted virtually (as opposed to face-to-face) during the pandemic, which, in their view, enabled ERC members and researchers to participate regardless of geographical location, prevented contagion, and allowed rapid turnaround of reviews. Even in the case of virtual sessions, all other full meeting requirements such as quorum had to be met. Some ERCs modified their meetings to open a permanent slot in their agendas for COVID-19-related research or added urgent full meetings to discuss top-priority pandemic-related trial protocols. In other cases, members were permanently available to review COVID-19-related protocols, with those pertaining to other topics addressed less frequently.
While most respondents acknowledged the advantages of using online platforms during the pandemic to organize ERC meetings and to review research protocols, several participants highlighted the challenges that the use of such technologies entailed, particularly for new and more senior members of the ERCs who felt uncomfortable using these platforms. Some individuals deplored the loss of quality in the dynamics among ERC members (stilted conversations, fewer informal interactions) compared against the benefits of face-to-face meetings. Resistance to working online for some was compounded by difficulties accessing the internet and the lack of adequate electronic devices to do so.
Regarding the modification of protocol requirements, respondents mentioned adding safety procedures for study participants and members of the research teams, facilitating remote documentation of consent, and changing the policies regarding the use of non-anonymized data from health service and public health records for the duration of the pandemic to allow more unrestrained use of data. Some ERCs transitioned from requiring the physical signature of conflict-of-interest declaration forms to an email declaration.
As shown in Table 3 , only a minority of respondents indicated that their ERCs conducted a formal evaluation of the success or failure of modifying existing procedures or policies (28% of participants from HICs and 17% of those from LMICs). More than one quarter of respondents did not know whether such modifications had been assessed.
https://doi.org/10.1371/journal.pone.0292512.t004
Design and implementation of new procedures and policies
Almost two-thirds of respondents from both HICs and LMICs reported that their ERCs had designed and implemented new procedures and policies to address the challenges brought about by the pandemic ( S10 Table in S2 Appendix ). As in the case of modifications to ERC processes, innovations occurred mainly in the areas of meeting logistics and frequency, and of procedures for protocol review and approval ( S11 Table in S2 Appendix ). This was the case for ERCs in both HICs and LMICs.
In their descriptive responses, participants mentioned the development and implementation of new standard operating procedures (SOPs) and the integration of ad hoc committees, some including specialists, for urgent, accelerated protocol review. Such fast-track ERCs could review studies in one or two days, considerably shortening the time to complete reviews. One respondent considered the most successful innovation to be the formation of a “pool” of committee members ready to be convened at very short notice to quickly review COVID-19-related protocols. Such an ad hoc committee enabled applications to be reviewed and turned around very quickly. Of note, survey participants did not explicitly specify in their descriptive responses whether these ad hoc committees were integrated exclusively by existing ERC members, or if external experts and specialists were invited to take part in them. Similarly, respondents did not comment on whether existing SOPs contemplated the creation of ad hoc committees, on the way these entities were governed, or on the modifications made to SOPs to allow the integration of such committees.
The proportion of ERCs that formally evaluated the success or failure of new procedures and policies was analogous to that described for modifications to SOPs. Table 4 shows that just 37% of respondents from HICs and 21% of those from LMICs reported that their ERCs conducted such an evaluation.
https://doi.org/10.1371/journal.pone.0292512.t005
Permanently putting into effect modifications and innovations
A substantial majority of respondents (almost three quarters of those from HICs and more than four-fifths of those from LMICs) stated that many of the modifications and innovations to operating procedures implemented during the pandemic should be permanently put into effect ( S12 Table in S2 Appendix ), particularly in the areas of meeting logistics and frequency, procedures for protocol review and approval, and training of ethics review committee members in new or modified procedures ( S13 Table in S2 Appendix ). Several participants argued in their descriptive responses that virtual online meetings should be a permanent feature of ERC operations, as they increase efficiency and preclude many of the disadvantages of face-to-face meetings. Another recommendation was to enable the integration of ad hoc committees during times of increased demand. Similarly, respondents emphasized the relevance of facilitating the incorporation of new expert members to the ERCs as required. However, 20% of participants from HICs and 50% of those from LMICs indicated that their ERCs had no support to permanently implement modifications or innovations established during the COVID-19 pandemic ( S14 Table in S2 Appendix ).
Policies, procedures, and guidelines for public health emergencies
It is noteworthy that almost half of respondents from HICs and three-quarters of participants from LMICs indicated that their ERCs did not have internal policies, procedures, or guidelines before the pandemic that could orient members regarding the functioning of the committees during PHEs ( S15 Table in S2 Appendix ). Regarding the use of internal guidelines, some ERCs adapted existing documents, while others developed entirely new procedures. In the absence of specific internal guidelines, some SOPs explicitly privileged expedited review during health crises.
In contrast to the widespread absence of internal guidelines, the ERCs of one quarter of respondents from HICs and of almost half of those from LMICs used external guidelines not developed by their committees to govern their operation during the pandemic ( S16 Table in S2 Appendix ). Members of several committees referred to publicly available national and international guidelines. A selection of the most consulted documents appears in Box 2 .
Box 2. National and international external guidelines* that survey respondents reported were used by their ERCs to manage operations during the COVID-19 pandemic
International health organizations.
• Council for International Organizations of Medical Sciences, & World Health Organization (2016). International Ethical Guidelines for Health-related Research Involving Humans (Fourth Ed.). Council for International Organizations of Medical Sciences. https://doi.org/10.56759/rgxl7405
• Pan-American Health Organization (2020). Guidance for ethics oversight of COVID-19 research in response to emerging evidence. https://iris.paho.org/handle/10665.2/53021
• Pan-American Health Organization (2020). Guidance and strategies to streamline ethics review and oversight of COVID-19-related research. https://iris.paho.org/handle/10665.2/52089
• Pan-American Health Organization (2020). Template and operational guidance for the ethics review and oversight of COVID-19-related research. https://iris.paho.org/handle/10665.2/52086
• Pan-American Health Organization (2022). Catalyzing ethical research in emergencies. Ethics guidance, lessons learned from the COVID-19 pandemic, and pending agenda. https://iris.paho.org/handle/10665.2/56139
• Red de América Latina y el Caribe de Comités Nacionales de Bioética—United Nations Educational, Scientific and Cultural Organization (UNESCO) (2020). Ante las investigaciones biomédicas por la pandemia de enfermedad infecciosa por coronavirus Covid-19. https://redbioetica.com.ar/wp-content/uploads/2020/03/Declaracion-RED-ALAC-CNBS-Investigaciones-Covid-19.pdf
• World Health Organization (2016). Guidance for managing ethical issues in infectious disease outbreaks. World Health Organization. https://apps.who.int/iris/handle/10665/250580
• World Health Organization (2020). Key criteria for the ethical acceptability of COVID-19 human challenge studies. https://apps.who.int/iris/handle/10665/331976
• World Health Organization (2020). Guidance for research ethics committees for rapid review of research during public health emergencies. https://apps.who.int/iris/handle/10665/332206
• World Health Organization (2020). Ethical standards for research during public health emergencies: distilling existing guidance to support COVID-19 R&D. https://apps.who.int/iris/handle/10665/331507
Bioethics centres
• Nuffield Council of Bioethics (2020). Ethical considerations in responding to the COVID-19 pandemic. https://www.nuffieldbioethics.org/assets/pdfs/Ethical-considerations-in-responding-to-the-COVID-19-pandemic.pdf
The Hastings Center: Berlinger N et al . (2020). Ethical Framework for Health Care Institutions Responding to Novel Coronavirus SARS-CoV-2 (COVID-19). Guidelines for Institutional Ethics Services Responding to COVID-19. https://www.thehastingscenter.org/ethicalframeworkcovid19/
Scientific publications mentioned by respondents
• Saxena et al. (2019). Ethics preparedness: facilitating ethics review during outbreaks—recommendations from an expert panel. https://bmcmedethics.biomedcentral.com/articles/10.1186/s12910-019-0366-x
National guidelines
Resolución 908/2020. Ministerio de Salud de Argentina: https://www.argentina.gob.ar/normativa/nacional/resoluci%C3%B3n-908-2020-337359/texto
• Normativas da Comissão Nacional de Ética em Pesquisa: http://conselho.saude.gov.br/normativas-conep?view=default
• Consejo Nacional de Investigación en Salud de Costa Rica (CONIS) (2020). COMUNICADO 2: Recomendaciones para realizar investigación biomédica durante el periodo de la emergencia sanitaria por COVID-19 en Costa Rica. https://www.ministeriodesalud.go.cr/gestores_en_salud/conis/circulares/comunicado_cec_oac_oic_20082020.pdf
El Salvador
• Comité Nacional de Ética de la Investigación en Salud de El Salvador (2015). Manual de procedimientos operativos estándar para comités de ética de la investigación en salud. https://www.cneis.org.sv/wp-content/uploads/2018/07/MANUAL-CNEIS.pdf
• Indian Council of Medical Research (2017). National ethical guidelines for biomedical and health research involving human participants. https://ethics.ncdirindia.org/asset/pdf/ICMR_National_Ethical_Guidelines.pdf
• Indian Council of Medical Research (2020).National guidelines for ethics committees reviewing biomedical & health research during COVID-19 pandemic. https://main.icmr.nic.in/sites/default/files/guidelines/EC_Guidance_COVID19_06_05_2020.pdf
• Kenya Medical Research Institute Scientific and Ethics Review Unit (2019). KEMRI SERU guidelines for the conduct of research during the covid-19 pandemic in Kenya. https://www.kemri.go.ke/wp-content/uploads/2019/11/KEMRI-SERU_GUIDELINES-FOR-THE-CONDUCT-OF-RESEARCH-DURING-THE-COVID_8-June-2020_Final.pdf
Garis Panduan Pengurusan COVID-19 di Malaysia No.5 [COVID-19 Management Guidelines in Malaysia No.5] (2020). Ministry of Health of Malaysia. https://covid-19.moh.gov.my/garis-panduan/garis-panduan-kkm
• Government of Pakistan National COVID Command and Operation Center (NCOC) Guidelines (2020). [No longer available, as NCOC ceased operations on April 1, 2022)]
South Africa
• Department of Health, Republic of South Africa (2015). Ethics in Health Research: Principles, Processes and Structures (2d Ed). https://www.sun.ac.za/english/research-innovation/Research-Development/Documents/Integrity%20and%20Ethics/DoH%202015%20Ethics%20in%20Health%20Research%20-%20Principles,%20Processes%20and%20Structures%202nd%20Ed.pdf
South Korea
• Government of the Republic of Korea (2014). Bioethics and Safety Act (Act No. 12844). https://elaw.klri.re.kr/eng_mobile/viewer.do?hseq=33442&type=part&key=36
United Kingdom
• United Kingdom Health Departments / Research Ethics Service (2022). Standard Operating Procedures for Research Ethics Committees (Version 7.6). https://www.hra.nhs.uk/documents/3090/RES_Standard_Operating_Procedures_Version_7.6_September_2022_Final.pdf . [In particular, several respondents from the UK mentioned Section 9 of this document, which addresses expedited review in situations such as public health emergencies.]
• Health Research Authority (2020). https://www.hra.nhs.uk/approvals-amendments/
• Health Research Authority (2020). https://www.hra.nhs.uk/covid-19-research/covid-19-guidance-sponsors-sites-and-researchers/
• Department of Health and Social Care (2020). Coronavirus (COVID-19): notification to organisations to share information. https://www.gov.uk/government/publications/coronavirus-covid-19-notification-of-data-controllers-to-share-information
* We defined “external guidelines” as those not developed internally by participants’ ERCs
Changes in workload
Respondents stated that the workload of ERC members increased considerably during the pandemic because of the increase in the number of protocols reviewed and also due to the urgency that the approval of COVID-19-related studies demanded. More than half of participants indicated that the volume of protocols received for review increased, both for studies assigned to delegated / expedited review, and for protocols that underwent full review ( S17 Table in S2 Appendix ). The increase in the volume of protocols had unexpected consequences. For example, in one HIC, the number of applicants who were summoned to discuss their protocols with ERCs in online meetings increased proportionally to the escalation in the volume of protocols submitted. In another case, ERC members were burdened with additional tasks such as working closely with the investigators of rejected COVID-19 protocols to improve their applications until these could be approved.
In terms of the time it took ERCs to process and approve protocols during the pandemic, participants confirmed in their descriptive responses that the turnaround time for ERC review was markedly shortened, from weeks or even months to just a few days. In general, more than half of survey participants indicated that, before the pandemic, the duration of the review process, from the time of initial submission to full approval, was between three and eight weeks ( S18 Table in S2 Appendix ). In contrast, during the pandemic, this process was substantially reduced to less than two weeks for both delegated / expedited review and full review. However, this decrease was more pronounced in HICs than in LMICs ( S19 Table in S2 Appendix ). Unsurprisingly, the approval of COVID-19-related research protocols was faster than that of non-COVID-19 studies. More than two-thirds of respondents indicated that delegated / expedited review of COVID-19-related protocols took less than five weeks; this was the case for more than half of full reviews. The process was longer in LMICs, though ( S20 Table in S2 Appendix ). Conversely, protocol review was slightly longer for non-COVID-19 studies, except in the case of full reviews in LMICs, which participants reported took between three and more than 12 weeks ( S21 Table in S2 Appendix ).
Presence of external pressure on ERCs
While only 14% of respondents from HICs reported that their ERCs were subjected to different types of external pressure to both approve and reject research protocols, one third of participants from LMICs (34%) faced such a challenge ( S22 Table in S2 Appendix ). The perceived demand mentioned most frequently involved pressures to rush studies through the review process at the expense of proper examination and ethical oversight. This was especially evident in the case of COVID-19 vaccine clinical trials. Some participants highlighted their defense of the autonomy of their ERCs in the face of external influences by using, for example, research policies developed and implemented specifically for the pandemic as a tool for transparent decision-making and as a safeguard against external pressures. One ERC successfully resisted government pressure to approve a research protocol related to a domestic PCR test, human trials of locally developed ventilators, and a placebo-controlled vaccine trial proposed despite the existence of six emergency-authorized vaccines and ongoing mass vaccination.
While some respondents acknowledged that entities such as national governments were understandably impatient for preventive, diagnostic, and therapeutic measures to combat the pandemic, they still emphasized the need for proper and thorough review of research protocols. One respondent stated that institutional authorities that favoured or sponsored certain studies sought their immediate approval and considered ERCs as inconvenient hindrances to achieve this goal. Several participants described instances in which ERCs, particularly in LMICs, received pressure to approve alternative medicine clinical trials.
Types of COVID-19 protocols reviewed by ERCs
Given the range of challenges brought about by the COVID-19 pandemic, it was interesting to determine the proportion of protocols received by ERCs according to the research area in which they could be classified, namely, diagnostics, therapeutics, vaccines, pharmacovigilance, or other topics such as behavioural research. Our results suggest that between one-half and two-thirds of ERCs received from one to 10 studies in each area ( Table 5 ). In other words, all areas of COVID-19 research were covered in these protocols submitted to ERCs of both HICs and LMICs. However, it must be noted that between one-third and one-half of respondents could not classify the protocols received by their ERCs (perhaps due to not tracking such information).
https://doi.org/10.1371/journal.pone.0292512.t006
Prioritization of protocols for ethics review
Overwhelmingly, and as expected, participants reported that their ERCs considered COVID-19-related protocols urgent and thus prioritized their review and approval over that of others, particularly in terms of expediting the review of these studies and privileging their discussion during committee meetings. More than three quarters of respondents from HICs and almost two-thirds of those from LMICs indicated that their ERCs gave priority to COVID-19-related studies ( S23 Table in S2 Appendix ). In fact, in one case, an ERC stopped reviewing non-COVID-19-related protocols altogether. Some ERCs gave precedence to the review of COVID-19-related studies according to the priorities determined by their national governments. Others were assigned studies by an ad hoc national entity that triaged the research protocols. Interestingly, however, as shown in S23 Table in S2 Appendix , 15% of respondents from HICs and 27% of those from LMICs stated that their ERCs did not give priority to pandemic-related studies.
Furthermore, our results show that almost one-third of respondents from HICs and almost half of those from LMICs indicated that, for their ERCs, the review of some types of COVID-19-related studies took precedence over that of others ( S24 Table in S2 Appendix ). In their descriptive responses, participants explicitly mentioned prioritizing clinical trials, particularly those focused on COVID-19 vaccine development and safety monitoring; studies related to therapeutic agents for the treatment of COVID-19; protocols about diagnostics and prognostic factors; epidemiological studies, including those related to the natural history of COVID-19 and serosurveillance; and research affecting public health policy. In the case of one ERC in a HIC with very low infection rates resulting from successful public health measures, priority was given to vaccine trials and observational research on vaccine monitoring and community incidence.
Membership of ERCs during the pandemic
One of the main challenges that ERCs worldwide faced during the pandemic was making certain that the number and expertise of their members enabled the efficient operation of the committees under such demanding circumstances. Most survey respondents indicated that their ERCs were able to ensure quorum (80% of participants from HICs, but only 60% of those from LMICs); however, one-third of respondents from LMICs stated that quorum in their ERCs was infrequently met ( S25 Table in S2 Appendix ). Two-thirds of participants from HICs and three quarters of those from LMICs reported that their committees had taken measures to ensure continuity of adequate review of research protocols in case existing members became unavailable due to the pandemic ( S26 Table in S2 Appendix ).
ERCs in both HICs and LMICs did invite new members or appointed alternate ones to ensure quorum and inclusion of individuals with appropriate expertise. Yet, consulting expert non-members seems to have been preferred to incorporating individuals to the committee. Only 13% of respondents from HICs and 24% of those from LMICs indicated that their ERCs had added new members to accelerate protocol review during the pandemic ( S27 Table in S2 Appendix ). Similarly, 11% of participants from HICs and 37% of those from LMICs added new members with specific expertise ( S28 Table in S2 Appendix ). In contrast, almost one-third of individuals from HICs, but close to two-thirds of those from LMICs, stated that their committees had consulted expert non-members to address novel areas of research or to provide enhanced scrutiny of research protocols ( S29 Table in S2 Appendix ). In their descriptive responses, participants expressed that, in some cases, ERCs incorporated new members who were available at quick notice and comfortable with the use of online platforms for meetings and protocol review. A similar approach consisted of integrating virtual ad hoc committees solely to review COVID-19-related-protocols. For some ERCs, national legislation complicated getting additional support or adding new members. Another factor complicating the integration of ERCs was that clinical responsibilities of individuals directly in the care of COVID-19 patients soared, hindering their participation in committee meetings. One participant reported that some ERC members could not fulfill their duties in their respective ERCs because they had become highly sought-after “media celebrity” experts.
Survey respondents suggested that it would be worthwhile to assess the psychological and emotional challenges that ERC members faced when having to evaluate protocols using new, unfamiliar procedures under extreme pressure. Also, it is worth reiterating that, according to several participants, many ERC members, particularly older ones, deplored the loss of features common to face-to-face meetings, such as a warmer, more informal and welcoming environment that favoured interpersonal interactions. Other respondents expressed their desire for constructive and supportive feedback and for more appreciative and generous gestures of gratitude for the extraordinary efforts of ERCs. However, a few participants considered that being able to respond in a useful way to a public health crisis as ERC members was in itself very gratifying and validating.
National and international collaboration
While 38.5% of respondents from HICs and 40% of those from LMICs reported the presence of national and international collaboration among ERCs to standardize emergency operations and procedures during the pandemic, almost one-third of participants from HICs were unsure about the existence of such collaboration ( S30 Table in S2 Appendix ). Almost half of respondents from HICs, but more than two-thirds of those from LMICs, indicated that their ERCs did not have strategies to harmonize multiple review processes ( S31 Table in S2 Appendix ). Most participants (55% of those from HICs and 63% of those from LMICs) reported that their ERCs relied on established procedures to recognize and validate research protocol reviews conducted by other committees ( S32 Table in S2 Appendix ). About one half of respondents from HICs, but almost two-thirds of those from LMICs, affirmed that their ERCs collaborated with scientific committees that pre-reviewed or prioritized pandemic-related research protocols ( S33 Table in S2 Appendix ).
Almost 50% of participants from HICs, but little more than a third of those from LMICs, reported the presence of centralized ethics review of research protocols for multicentre studies related to COVID-19 ( S34 Table in S2 Appendix ). Conversely, one-third of respondents from HICs, but more than two-thirds of those from LMICs, stated that their ERCs did not consider the formation of Joint Scientific Advisory Committees, Data Safety Review Committees, Data Access Committees, or a Joint Ethics Review Committee integrated with representatives of ethics committees of all institutions and countries involved in COVID-19-related research ( S35 Table in S2 Appendix ).
In their descriptive responses, participants noted the need for better inter-ERC collaboration and communication at the national and international levels to share successful strategies and avoid effort duplication. A case of very successful national inter-ERC collaboration is worth mentioning. Respondents from one particular LMIC stated that, given the critical and unforeseen absence of the national entity responsible for health research ethics during the pandemic, ERCs throughout the country joined forces to create an ad hoc spontaneous informal national network of all ERC chairs and co-chairs (it also included members of the national drug regulator) to strengthen mutual support, enhance communication among ERCs, identify best practices, and share academic and ethics resources.
ERCs faced considerable challenges during the COVID-19 pandemic. Demands were placed on them to urgently review an increased volume of protocols while maintaining rigour, all under suboptimal conditions and uncertainty. Yet, our findings suggest ERCs reviewed a greater volume of protocols and did so faster than before the pandemic. Against this backdrop, our results also reveal that, despite billions of dollars having been invested into the research and development (R&D) ecosystem to support the COVID-19 research response, little to no additional resources were directed to ERCs to support or expedite their functions. This should be particularly sobering for those who raise complaints about ERCs being an “obstacle” to research [ 13 – 16 ]. It may also help to explain other challenges experienced by ERCs during the pandemic, such as the absence of internal policies or guidelines for adapting to a PHE, the collateral damage sustained from deprioritizing non-COVID-19 protocols, and the pressures felt to rush protocols through review.
Our finding that ERCs wish to sustain many of the modifications made to their operations during the COVID-19 pandemic should be interpreted in light of the fact that ERCs also report having received little or no support during the COVID-19 pandemic as well as exiguous support for the maintenance of any modifications they would like to make permanent into the future. While it is expected that the research ethics ecosystem learn from this experience and enhance operations for future threats, it is difficult to see how this will be possible without significant investment. While no one seems to disagree that the research ethics ecosystem should strive for greater efficiency and collaboration, especially during PHEs, investments are required to achieve these aims. Simply put, the experience of ERCs during the COVID-19 pandemic, while herculean in many respects, was a function of necessity and is unlikely to be sustainable.
Extant literature reporting the challenges faced by ERCs during the COVID-19 pandemic is scant and tends to be limited to the early phases of this PHE. Most studies published on this topic are confined to single countries or geographical regions, with only one study including 14 countries in Africa, Asia, Australia, and Europe[ 17 ]. Several of these contributions focus exclusively on one ERC, usually associated with an academic or health care institution. The literature includes descriptions of ERC operations during the pandemic in Central America and the Dominican Republic [ 18 ], China [ 19 ], Ecuador [ 20 ], Egypt [ 21 ], Germany [ 22 ], India [ 23 – 25 ], Iran [ 10 ], Ireland [ 26 ], Kenya [ 27 ], Kyrgyzstan [ 28 ], Latin America [ 29 ], the Netherlands [ 30 ], Pakistan [ 31 ], South Africa [ 32 , 33 ], Turkey [ 34 ], and the United States [ 35 – 37 ]. Most of these studies reported results from surveys, interviews, focus groups, and documentary analysis, including review of research protocols, ERC meeting minutes, and existing SOPs. Participants usually consisted of ERC chairpersons and members, clinical and biomedical researchers, institutional representatives, and laypeople. Most studies based on surveys and interviews included fewer than 30 respondents, with only some having more than 100 participants.
Our findings agree with this literature. Given that our study is truly global in scope, it considerably broadens what is known about the operation of ERCs during the COVID-19 pandemic and clears a path towards greater consensus on strategies to prepare for and respond during future PHEs.
In this literature, several studies emphasize the lack of support and resources to operate during the pandemic. The vast majority of ERCs made numerous modifications to their SOPs. In particular, the use of online platforms for ERC meetings and for protocol review was ubiquitous. However, ERC members across studies pointed out several disadvantages of such platforms, including lack of familiarity and technical know-how, particularly in the case of more senior members of the committees. Only a few institutions provided training, equipment, and technical support for the use of these online platforms. Consistent with our findings, almost no ERCs in these studies reported having internal policies, procedures, or guidelines to operate during a PHE. National regulations on this topic, where available, were often unclear, contradictory, rapidly changing, vague, or difficult to interpret. Conversely, several ERCs availed themselves of international guidelines ( Box 2 ), in particular those prepared by WHO [ 38 – 41 ] and PAHO [ 42 – 45 ].
In terms of changes in workload, all ERCs in the studies mentioned earlier experienced a dramatic increase in the number of COVID-19-related protocols received, which had to be reviewed very quickly in the face of pressure for expedited approvals from researchers, institutions, governments, and the media. The surge in the volume of protocols, along with shortened timelines for turnaround, severely strained ERC members’ ability to conduct rigorous, thorough, high-quality assessments. Despite feeling overwhelmed, ERC members participating in these studies managed to fulfill their responsibilities, sometimes at great personal cost.
Given the urgency to examine and approve an ever-increasing number of COVID-19 research protocols, previous studies report several strategies implemented by ERCs worldwide to prioritize their review. This frequently meant that the assessment of non-COVID-19-related studies was postponed or even abandoned. Similarly, non-interventional COVID-19 protocols were given secondary importance. Prioritization of COVID-19 protocols by type of study was rare.
Despite numerous staffing challenges, most ERCs in the studies examined were able to ensure quorum. In some cases, their institutions provided training sessions to update committee members on the rapidly changing landscape of basic and clinical knowledge about COVID-19. A less frequently used approach was to incorporate new members with relevant expertise into the ERCs. One common strategy across different countries was the integration of ad hoc committees focused exclusively on the review of COVID-19 -related protocols.
The topic of centralized review of pandemic-related research is rather contentious in this literature. While some studies report ERC members favouring such an approach, others consider that a single national ERC in charge of PHE-specific ethics review is bound to be unsuccessful due to the importance of local context in responding to PHEs. In Ecuador, forcing researchers to submit their protocols to a seven-member centralized ad hoc ERC caused considerable delays in the approval process and instead severely impeded the execution of COVID-19-related studies [ 20 ].
As shown in our results, in some countries ERCs strengthened collaboration networks during the pandemic. A notable case was the creation of a spontaneous, informal, ad hoc group in South Africa—the Research Ethics Support in COVID-19 Pandemic (RESCOP)—by ERC chairpersons and members as a response to the lack of national ethics guidance and the unexpected critical absence of the National Health Research Ethics Council at the most crucial moment in the pandemic [ 33 ]. This example highlights the clear need for national governance and oversight for research ethics to ensure accountability and responsiveness of ERCs [ 46 ].
A common topic of concern across ERCs in several countries was the set of unique challenges to obtaining informed consent during the pandemic, especially in the case of patients unable to give consent, such as those who were severely ill, isolated, or in the intensive care unit. Thus, it was necessary to find innovative alternative strategies to obtain consent.
Recommendations
The recommendations presented in Box 3 aim to strengthen the resiliency of ERCs during future public health emergencies. They are based upon our careful analysis of survey responses, and thus articulate, in a way, the concerns and expectations of the participants in our study. These recommendations closely align with the national and international guidelines listed in Box 2 , particularly with those published by WHO.
Box 3. Recommendations to strengthen the resiliency of ERCs during future public health emergencies
• Increase and assign an adequate proportion of the budgets of ethics review committees (ERCs) for:
○ their continued operation during public health emergencies (PHEs), especially in terms of online teleconferencing and review platforms
○ sustaining select modifications and innovations designed and implemented during PHEs
• Increase awareness of the value of ERCs in the research and development (R&D) ecosystem as a means of protecting research participants, ensuring social value, and promoting public trust in research outputs, rather than as a bureaucratic nuisance
• Evaluate the success or failure of modifications and innovations designed and implemented during PHEs
• Develop a “first aid kit” for each ERC that includes:
○ Existing external guidelines for committee operation during a PHE
○ Internal contingency plans designed by the ERC or its home institution that adapt existing external guidelines to local contexts
○ A directory of expert non-members available for consultation
○ Easy-to-follow checklists that incorporate the essential elements needed to function during a PHE
• Familiarize ERC members with the “first aid kit” through periodic capacity building activities
• Consider the psychological and emotional challenges that ERC members face during PHEs
• Devise strategies to defend and safeguard ERCs’ autonomy against external pressures
• Promote national and regional collaboration networks of ERCs that strengthen their resiliency during PHEs
• Facilitate collaboration between ERCs and scientific committees
Limitations and strengths
In terms of the limitations of our study, it would have been desirable to include participants from more countries, and a larger number of respondents from each country. It was probably difficult to reach a higher response rate due to “pandemic fatigue”. Non-native English speakers, especially in LMICs, may have excluded themselves from our survey. Absent or unreliable internet access could have limited the participation of some participants, particularly in LMICs. The number of ERC members that provided ethics review for health care facilities was relatively low. Despite the anonymity of their answers, respondents may have been reluctant to share specific instances of external pressures impinging upon their ERCs. The large number of participants from the UK (93 out of 281) likely skewed the results from HICs, and from experiences in the UK in particular.
We chose to present our results descriptively and did not perform any analytic tests for statistically significant differences in responses. This was because we were unable to determine a denominator, so we could not meet the requirements for many significance tests. Non-parametric tests could have been used, but we think reporting statistical significance in this context would not be informative. Non-response bias could also influence our results. This could be non-differential in its effects as our results cohere with the literature thus far reported.
To our knowledge, this is the first examination at a global level of the challenges faced by ERCs during the COVID-19 pandemic, and the strategies used to address them. Also, our study compares for the first time several dimensions of the operation of ERCs during the pandemic between committees in HICs and those in LMICs. All WHO regions were represented in our study, as participants from 48 countries (19 HICs and 29 LMICs) responded to our survey. There was an adequate balance in terms of the sex / gender of respondents. Furthermore, the ample experience of the study participants as ERC members (two thirds of respondents had six or more years of experience in this role) strengthens the generalizability of our findings. The recommendations suggested by the study participants are quite relevant to combating future public health emergencies. In general, all these strengths give credence to the validity, reliability, and accuracy of our results.
Supporting information
S1 appendix. qualtrics questionnaire..
https://doi.org/10.1371/journal.pone.0292512.s001
S2 Appendix. Supplementary tables.
https://doi.org/10.1371/journal.pone.0292512.s002
Acknowledgments
We are very grateful to all the members of ethics review committees from around the world who participated in our survey. We also want to express our gratitude to Andreas Reis and Katherine Littler of the Global Health Ethics and Governance Unit, World Health Organization, and to the following members of the World Health Organization COVID-19 Ethics and Governance Working Group, for their inputs on the survey instrument and manuscript: Aasim Ahmad, Thalia Arawi, Caesar Atuire, Oumou Bah-Sow, Anant Bhan, Ingrid Callies, Angus Dawson, Jean-François Delfraissy, Ezekiel Emanuel, Ruth Faden, Tina Garanis-Papadatos, Prakash Ghimire, Dirceu Greco, Calvin Ho, Patrik Hummel, Zubairu Iliyasu, Mohga Kamal-Yanni, Sharon Kaur, So Yoon Kim, Sonali Kochhar, Ruipeng Lei, Ahmed Mandil, Julian März, Ignacio Mastroleo, Roli Mathur, Signe Mežinska, Ryoko Miyazaki-Krause, Keymanthri Moodley, Suerie Moon, Michael Parker, Carla Saenz, G. Owen Schaefer, Ehsan Shamsi-Gooshki, Jerome Singh, Beatriz Thomé, Teck Chuan Voo, Jonathan Wolff, and Xiaomei Zhai.
- View Article
- PubMed/NCBI
- Google Scholar
- 3. Council for International Organizations of Medical Sciences, World Health Organization. International Ethical Guidelines for Health-related Research Involving Humans. Fourth Ed. Geneva: Council for International Organizations of Medical Sciences; 2016.
- 13. Schneider CE. The Censor’s Hand: The Misregulation of Human-Subject Research. 1st edition. Cambridge, Massachusetts: The MIT Press; 2015.
- 38. World Health Organization. Guidance for managing ethical issues in infectious disease outbreaks. Geneva; 2016. Available: https://apps.who.int/iris/handle/10665/250580 .
- 46. World Health Organization. WHO tool for benchmarking ethics oversight of health-related research with human participants (Draft). Geneva; 2022. Available: https://www.who.int/publications/m/item/who-tool-for-benchmarking-ethics-oversight-of-health-related-research-with-human-participants .
- Open access
- Published: 30 April 2021
Ethics review of big data research: What should stay and what should be reformed?
- Agata Ferretti ORCID: orcid.org/0000-0001-6716-5713 1 ,
- Marcello Ienca 1 ,
- Mark Sheehan 2 ,
- Alessandro Blasimme 1 ,
- Edward S. Dove 3 ,
- Bobbie Farsides 4 ,
- Phoebe Friesen 5 ,
- Jeff Kahn 6 ,
- Walter Karlen 7 ,
- Peter Kleist 8 ,
- S. Matthew Liao 9 ,
- Camille Nebeker 10 ,
- Gabrielle Samuel 11 ,
- Mahsa Shabani 12 ,
- Minerva Rivas Velarde 13 &
- Effy Vayena 1
BMC Medical Ethics volume 22 , Article number: 51 ( 2021 ) Cite this article
15k Accesses
39 Citations
19 Altmetric
Metrics details
Ethics review is the process of assessing the ethics of research involving humans. The Ethics Review Committee (ERC) is the key oversight mechanism designated to ensure ethics review. Whether or not this governance mechanism is still fit for purpose in the data-driven research context remains a debated issue among research ethics experts.
In this article, we seek to address this issue in a twofold manner. First, we review the strengths and weaknesses of ERCs in ensuring ethical oversight. Second, we map these strengths and weaknesses onto specific challenges raised by big data research. We distinguish two categories of potential weakness. The first category concerns persistent weaknesses, i.e., those which are not specific to big data research, but may be exacerbated by it. The second category concerns novel weaknesses, i.e., those which are created by and inherent to big data projects. Within this second category, we further distinguish between purview weaknesses related to the ERC’s scope (e.g., how big data projects may evade ERC review) and functional weaknesses, related to the ERC’s way of operating. Based on this analysis, we propose reforms aimed at improving the oversight capacity of ERCs in the era of big data science.
Conclusions
We believe the oversight mechanism could benefit from these reforms because they will help to overcome data-intensive research challenges and consequently benefit research at large.
Peer Review reports
The debate about the adequacy of the Ethics Review Committee (ERC) as the chief oversight body for big data studies is partly rooted in the historical evolution of the ERC. Particularly relevant is the ERC’s changing response to new methods and technologies in scientific research. ERCs—also known as Institutional Review Boards (IRBs) or Research Ethics Committees (RECs)—came to existence in the 1950s and 1960s [ 1 ]. Their original mission was to protect the interests of human research participants, particularly through an assessment of potential harms to them (e.g., physical pain or psychological distress) and benefits that might accrue from the proposed research. ERCs expanded in scope during the 1970s, from participant protection towards ensuring valuable and ethical human subject research (e.g., having researchers implement an informed consent process), as well as supporting researchers in exploring their queries [ 2 ].
Fast forward fifty years, and a lot has changed. Today, biomedical projects leverage unconventional data sources (e.g., social media), partially inscrutable data analytics tools (e.g., machine learning), and unprecedented volumes of data [ 3 , 4 , 5 ]. Moreover, the evolution of research practices and new methodologies such as post-hoc data mining have blurred the concept of ‘ human subject’ and elicited a shift towards the concept of data subject —as attested in data protection regulations. [ 6 , 7 ]. With data protection and privacy concerns being in the spotlight of big data research review, language from data protection laws has worked its way into the vocabulary of research ethics. This terminological shift further reveals that big data, together with modern analytic methods used to interpret the data, creates novel dynamics between researchers and participants [ 8 ]. Research data repositories about individuals and aggregates of individuals are considerably expanding in size. Researchers can remotely access and use large volumes of potentially sensitive data without communicating or actively engaging with study participants. Consequently, participants become more vulnerable and subjected to the research itself [ 9 ]. As such, the nature of risk involved in this new form of research changes too. In particular, it moves from the risk of physical or psychological harm towards the risk of informational harm, such as privacy breaches or algorithmic discrimination [ 10 ]. This is the case, for instance, with projects using data collected through web search engines, mobile and smart devices, entertainment websites, and social media platforms. The fact that health-related research is leaving hospital labs and spreading into online space creates novel opportunities for research, but also raises novel challenges for ERCs. For this reason, it is important to re-examine the fit between new data-driven forms of research and existing oversight mechanisms [ 11 ].
The suitability of ERCs in the context of big data research is not merely a theoretical puzzle but also a practical concern resulting from recent developments in data science. In 2014, for example, the so-called ‘emotional contagion study’ received severe criticism for avoiding ethical oversight by an ERC, failing to obtain research consent, violating privacy, inflicting emotional harm, discriminating against data subjects, and placing vulnerable participants (e.g., children and adolescents) at risk [ 12 , 13 ]. In both public and expert opinion [ 14 ], a responsible ERC would have rejected this study because it contravened the research ethics principles of preventing harm (in this case, emotional distress) and adequately informing data subjects. However, the protocol adopted by the researchers was not required to undergo ethics review under US law [ 15 ] for two reasons. First, the data analyzed were considered non-identifiable, and researchers did not engage directly with subjects, exempting the study from ethics review. Second, the study team included both scientists affiliated with a public university (Cornell) and Facebook employees. The affiliation of the researchers is relevant because—in the US and some other countries—privately funded studies are not subject to the same research protections and ethical regulations as publicly funded research [ 16 ]. An additional example is the 2015 case in which the United Kingdom (UK) National Health Service (NHS) shared 1.6 million pieces of identifiable and sensitive data with Google DeepMind. This data transfer from the public to the private party took place legally, without the need for patient consent or ethics review oversight [ 17 ]. These cases demonstrate how researchers can pursue potentially risky big data studies without falling under the ERC’s purview. The limitations of the regulatory framework for research oversight are evident, in both private and public contexts.
The gaps in the ERC’s regulatory process, together with the increased sophistication of research contexts—which now include a variety of actors such as universities, corporations, funding agencies, public institutes, and citizens associations—has led to an increase in the range of oversight bodies. For instance, besides traditional university ethics committees and national oversight committees, funding agencies and national research initiatives have increasingly created internal ethics review boards [ 18 , 19 ]. New participatory models of governance have emerged, largely due to an increase in subjects’ requests to control their own data [ 20 ]. Corporations are creating research ethics committees as well, modelled after the institutional ERC [ 21 ]. In May 2020, for example, Facebook welcomed the first members of its Oversight Board, whose aim is to review the company’s decisions about content moderation [ 22 ]. Whether this increase in oversight models is motivated by the urge to fill the existing regulatory gaps, or whether it is just ‘ethics washing’, is still an open question. However, other types of specialized committees have already found their place alongside ERCs, when research involves international collaboration and data sharing [ 23 ]. Among others, data safety monitoring boards, data access committees, and responsible research and innovation panels serve the purpose of covering research areas left largely unregulated by current oversight [ 24 ].
The data-driven digital transformation challenges the purview and efficacy of ERCs. It also raises fundamental questions concerning the role and scope of ERCs as the oversight body for ethical and methodological soundness in scientific research. Footnote 1 Among these questions, this article will explore whether ERCs are still capable of their intended purpose, given the range of novel (maybe not categorically new, but at least different in practice) issues that have emerged in this type of research. To answer this question, we explore some of the challenges that the ERC oversight approach faces in the context of big data research and review the main strengths and weaknesses of this oversight mechanism. Based on this analysis, we will outline possible solutions to address current weaknesses and improve ethics review in the era of big data science.
Strengths of the ethics review via ERC
Historically, ERCs have enabled cross disciplinary exchange and assessment [ 27 ]. ERC members typically come from different backgrounds and bring their perspectives to the debate; when multi-disciplinarity is achieved, the mixture of expertise provides the conditions for a solid assessment of advantages and risks associated with new research. Committees which include members from a variety of backgrounds are also suited to promote projects from a range of fields, and research that cuts across disciplines [ 28 ]. Within these committees, the reviewers’ expertise can be paired with a specific type of content to be reviewed. This one-to-one match can bring timely and, ideally, useful feedback [ 29 ]. In many countries (e.g., European countries, the United States (US), Canada, Australia), ERCs are explicitly mandated by law to review many forms of research involving human participants; moreover, these laws also describe how such a body should be structured and the purview of its review [ 30 , 31 ]. In principle, ERCs also aim to be representative of society and the research enterprise, including members of the public and minorities, as well as researchers and experts [ 32 ]. And in performing a gatekeeping function to the research enterprise, ERCs play an important role: they recognize that both experts and lay people should have a say, with different views to contribute [ 33 ].
Furthermore, the ERC model strives to ensure independent assessment. The fact that ERCs assess projects “from the outside” and maintain a certain degree of objectivity towards what they are reviewing, reduces the risk of overlooking research issues and decreases the risk for conflicts of interest. Moreover, being institutionally distinct—for example, being established by an organization that is distinct from the researcher or the research sponsor—brings added value to the research itself as this lessens the risk for conflict of interest. Conflict of interest is a serious issue in research ethics because it can compromise the judgment of reviewers. Institutionalized review committees might particularly suffer from political interference. This is the case, for example, for universities and health care systems (like the NHS), which tend to engage “in house” experts as ethics boards members. However, ERCs that can prove themselves independent are considered more trustworthy by the general public and data subjects; it is reassuring to know that an independent committee is overseeing research projects [ 34 ].
The ex-ante (or pre-emptive) ethical evaluation of research studies is by many considered the standard procedural approach of ERCs [ 35 ]. Though the literature is divided on the usefulness and added value provided by this form of review [ 36 , 37 ], ex-ante review is commonly used as a mechanism to ensure the ethical validity of a study design before the research is conducted [ 38 , 39 ]. Early research scrutiny aims at risk-mitigation: the ERC evaluates potential research risks and benefits, in order to protect participants’ physical and psychological well-being, dignity, and data privacy. This practice saves researchers’ resources and valuable time by preventing the pursuit of unethical or illegal paths [ 40 ]. Finally, the ex-ante ethical assessment gives researchers an opportunity to receive feedback from ERCs, whose competence and experience may improve the research quality and increase public trust in the research [ 41 ].
All strengths mentioned in this section are strengths of the ERC model in principle. In practice, there are many ERCs that are not appropriately interdisciplinary or representative of the population and minorities, that lack independence from the research being reviewed, and that fail to improve research quality, and may in fact hinder it. We now turn to consider some of these weaknesses in more detail.
Weaknesses of the ethics review via ERC
In order to assess whether ERCs are adequately equipped to oversee big data research, we must consider the weaknesses of this model. We identify two categories of weaknesses which are described in the following section and summarized in Fig. 1 :
Persistent weaknesses : those existing in the current oversight system, which could be exacerbated by big data research
Novel weaknesses : those brought about by and specific to the nature of big data projects
Within this second category of novel weaknesses, we further differentiate between:
Purview weaknesses : reasons why some big data projects may bypass the ERCs’ purview
Functional weaknesses : reasons why some ERCs may be inadequate to assess big data projects specifically
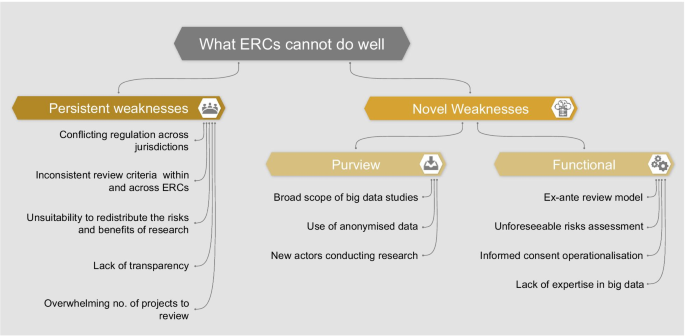
Weaknesses of the ERCs
We base the conceptual distinction between persistent and novel weaknesses on the fact that big data research diverges from traditional biomedical research in many respects. As previously mentioned, big data projects are often broad in scope, involve new actors, use unprecedented methodologies to analyze data, and require specific expertise. Furthermore, the peculiarities of big data itself (e.g., being large in volume and from a variety of sources) make data-driven research different in practice from traditional research. However, we should not consider the category of “novel weaknesses” a closed category. We do not argue that weaknesses mentioned here do not, at least partially, overlap with others which already exist. In fact, in almost all cases of ‘novelty’, (i) there is some link back to a concept from traditional research ethics, and (ii) some thought has been given to the issue outside of a big data or biomedical context (e.g., the problem of ERCs’ expertise has arisen in other fields [ 42 ]). We believe that by creating conceptual clarity about novel oversight challenges presented by big data research, we can begin to identify tailored reforms.
Persistent weaknesses
As regulation for research oversight varies between countries, ERCs often suffer from a lack of harmonization. This weakness in the current oversight mechanism is compounded by big data research, which often relies on multi-center international consortia. These consortia in turn depend on approval by multiple oversight bodies demanding different types of scrutiny [ 43 ]. Furthermore, big data research may give rise to collaborations between public bodies, universities, corporations, foundations, and citizen science cooperatives. In this network, each stakeholder has different priorities and depends upon its own rules for regulation of the research process [ 44 , 45 , 46 ]. Indeed, this expansion of regulatory bodies and aims does not come with a coordinated effort towards agreed-upon review protocols [ 47 ]. The lack of harmonization is perpetuated by academic journals and funding bodies with diverging views on the ethics of big data. If the review bodies which constitute the “ethics ecosystem” [ 19 ] do not agree to the same ethics review requirements, a big data project deemed acceptable by an ERC in one country may be rejected by another ERC, within or beyond the national borders.
In addition, there is inconsistency in the assessment criteria used within and across committees. Researchers report subjective bias in the evaluation methodology of ERCs, as well as variations in ERC judgements which are not based on morally relevant contextual considerations [ 48 , 49 ]. Some authors have argued that the probability of research acceptance among experts increases if some research peer or same-field expert sits on the evaluation committee [ 50 , 51 ]. The judgement of an ERC can also be influenced by the boundaries of the scientific knowledge of its members. These boundaries can impact the ERC’s approach towards risk taking in unexplored fields of research [ 52 ]. Big data research might worsen this problem since the field is relatively new, with no standardized metric to assess risk within and across countries [ 53 ]. The committees do not necessarily communicate with each other to clarify their specific role in the review process, or try to streamline their approach to the assessment. This results in unclear oversight mandates and inconsistent ethical evaluations [ 27 , 54 ].
Additionally, ERCs may fall short in their efforts to justly redistribute the risks and benefits of research. The current review system is still primarily tilted toward protecting the interests of individual research participants. ERCs do not consistently assess societal benefit, or risks and benefits in light of the overall conduct of research (balancing risks for the individual with collective benefits). Although demands on ERCs vary from country to country [ 55 ], the ERC approach is still generally tailored towards traditional forms of biomedical research, such as clinical trials and longitudinal cohort studies with hospital patients. These studies are usually narrow in scope and carry specific risks only for the participants involved. In contrast, big data projects can impact society more broadly. As an example, computational technologies have shown potential to determine individuals’ sexual orientation by screening facial images [ 56 ]. An inadequate assessment of the common good resulting from this type of study can be socially detrimental [ 57 ]. In this sense, big data projects resemble public health research studies, with an ethical focus on the common good over individual autonomy [ 58 ]. Within this context, ERCs have an even greater responsibility to ensure the just distribution of research benefits across the population. Accurately determining the social value of big data research is challenging, as negative consequences may be difficult to detect before research begins. Nevertheless, this task remains a crucial objective of research oversight.
The literature reports examples of the failure of ERCs to be accountable and transparent [ 59 ]. This might be the result of an already unclear role of ERCs. Indeed, the ERCs practices are an outcome of different levels of legal, ethical, and professional regulations, which largely vary across jurisdictions. Therefore, some ERCs might function as peer counselors, others as independent advisors, and still others as legal controllers. What seems to be common across countries, though, is that ERCs rarely disclose their procedures, policies, and decision-making process. The ERCs’ “secrecy” can result in an absence of trust in the ethical oversight model [ 60 ].This is problematic because ERCs rely on public acceptance as accountable and trustworthy entities [ 61 ]. In big data research, as the number of data subjects is exponentially greater, a lack of accountability and an opaque deliberative process on the part of ERCs might bring even more significant public backlash. Ensuring truthfulness of the stated benefits and risks of research is a major determinant of trust in both science and research oversight. Researchers are another category of stakeholders negatively impacted by poor communication and publicity on the part of the ERC. Commentators have shown that ERCs often do not clearly provide guidance about the ethical standards applied in the research review [ 62 ]. For instance, if researchers provide unrealistic expectations of privacy and security to data subjects, ERCs have an institutional responsibility to flag those promises (e.g., about data security and the secondary-uses of subject data), especially when the research involves personal and high sensitivity data [ 63 ]. For their part, however, ERCs should make their expectations and decision-making processes clear.
Finally, ERCs face the increasing issue of being overwhelmed by the number of studies to review [ 64 , 65 ]. Whereas ERCs originally reviewed only human subjects research happening in natural sciences and medicine, over time they also became the ethical body of reference for those conducting human research in the social sciences (e.g., in behavioral psychology, educational sciences, etc.). This increase in demand creates pressure on ERC members, who often review research pro bono and on a voluntary basis. The wide range of big data research could exacerbate this existing issue. Having more research to assess and less time to accomplish the task may negatively impact the quality of the ERC’s output, as well as increase the time needed for review [ 66 ]. Consequently, researchers might carry out potentially risky studies because the relevant ethical issues of those studies were overlooked. Furthermore, research itself could be significantly delayed, until it loses its timely scientific value.
Novel weaknesses: purview weaknesses
To determine whether the ERC is still the most fit-for-purpose entity to oversee big data research, it is important to establish under which conditions big data projects fall under the purview of ERCs.
Historically, research oversight has primarily focused on human subject research in the biomedical field, using public funding. In the US for instance, each review board is responsible for a subtype of research based on content or methodology (for example there are IRBs dedicated to validating clinical trial protocols, assessing cancer treatments, examining pediatric research, and reviewing qualitative research). This traditional ethics review structure cannot accommodate big data research [ 2 ]. Big data projects often reach beyond a single institution, cut across disciplines, involve data collected from a variety of sources, re-use data not originally collected for research purposes, combine diverse methodologies, orient towards population-level research, rely on large data aggregates, and emerge from collaboration with the private sector. Given this scenario, big data projects may likely fall beyond the purview of ERCs.
Another case in which big data research does not fall under ERC purview is when it relies on anonymized data. If researchers use data that cannot be traced back to subjects (anonymized or non-personal data), then according to both the US Common Rule and HIPAA regulations, the project is considered safe enough to be granted an ethics review waiver. If instead researchers use pseudonymized (or de-identified) data, they must apply for research ethics review, as in principle the key that links the de-identified data with subjects could be revealed or hacked, causing harm to subjects. In the European Union, it would be left to each Member State (and national laws or policies at local institutions) to define whether research using anonymized data should seek ethical review. This case shows once more that current research ethics regulation is relatively loose and disjointed across jurisdictions, and may leave areas where big data research is unregulated. In particular, the special treatment given anonymized data comes from an emphasis on risk at the individual level. So far in the big data discourse, the concept of harm has been mainly linked to vulnerability in data protection. Therefore if privacy laws are respected, and protection is built into the data system, researchers can prevent harmful outcomes [ 40 ]. However, this view is myopic as it does not include other misuses of data aggregates, such as group discrimination and dignitary harm. These types of harm are already emerging in the big data ecosystem, where anonymized data reveal health patterns of a certain sub-group, or computational technologies include strong racial biases [ 67 , 68 ]. Furthermore, studies using anonymized data should not be deemed oversight-free by default, as it is increasingly hard to anonymize data. Technological advancements might soon make it possible to re-identify individuals from aggregate data sets [ 69 ].
The risks associated with big data projects also increase due to the variety of actors involved in research alongside university researchers (e.g., private companies, citizen science associations, bio-citizen groups, community workers cooperatives, foundations, and non-profit organizations) [ 70 , 71 ]. The novel aspect of health-related big data research compared with traditional research is that anyone who can access large amounts of data about individuals and build predictive models based on that data, can now determine and infer the health status of a person without directly engaging with that person in a research program [ 72 ]. Facebook, for example, is carrying out a suicide prediction and prevention project, which relies exclusively on the information that users post on the social network [ 18 ]. Because this type of research is now possible, and the available ethics review model exempts many big data projects from ERC appraisal, gaps in oversight are growing [ 17 , 73 ]. Just as corporations can re-use publicly available datasets (such as social media data) to determine life insurance premiums [ 74 ], citizen science projects can be conducted without seeking research oversight [ 75 ]. Indeed, participant-led big data research (despite being increasingly common) is another area where the traditional overview model is not effective [ 76 ]. In addition, ERCs might consider research conducted outside academia or publicly funded institutions to be not serious. Thus ERCs may disregard review requests from actors outside the academic environment (e.g., by the citizen science or health tech start up) [ 77 ].
Novel weaknesses: functional weaknesses
Functional weaknesses are those related to the skills, composition, and operational activities of ERCs in relation to big data research.
From this functional perspective, we argue that the ex-ante review model might not be appropriate for big data research. Project assessment at the project design phase or at the data collection level is insufficient to address emerging challenges that characterize big data projects – especially as data, over time, could become useful for other purposes, and therefore be re-used or shared [ 53 ]. Limitations of the ex-ante review model have already become apparent in the field of genetic research [ 78 ]. In this context, biobanks must often undergo a second ethics assessment to authorize the specific research use on exome sequencing of their primary data samples [ 79 ]. Similarly, in a case in which an ERC approved the original collection of sensitive personal data, a data access committee would ensure that the secondary uses are in line with original consent and ethics approval. However, if researchers collect data from publicly accessible platforms, they can potentially use and re-use data for research lawfully, without seeking data subject consent or ERC review. This is often the case in social media research. Social media data, which are collected by researchers or private companies using a form of broad consent, can be re-used by researchers to conduct additional analysis without ERC approval. It is not only the re-use of data that poses unforeseeable risks. The ex-ante approach might not be suitable to assess other stages of the data lifecycle [ 80 ], such as deployment machine learning algorithms.
Rather than re-using data, some big data studies build models on existing data (using data mining and machine learning methods), creating new data, which is then used to further feed the algorithms [ 81 ]. Sometimes it is not possible to anticipate which analytic models or tools (e.g., artificial intelligence) will be leveraged in the research. And even then, the nature of computational technologies which extract meaning from big data make it difficult to anticipate all the correlations that will emerge from the analysis [ 37 ]. This is an additional reason that big data research often has a tentative approach to a research question, instead of growing from a specific research hypothesis [ 82 ].The difficulty of clearly framing the big data research itself makes it even harder for ERCs to anticipate unforeseeable risks and potential societal consequences. Given the existing regulations and the intrinsic exploratory nature of big data projects, the mandate of ERCs does not appear well placed to guarantee research oversight. It seems even less so if we consider problems that might arise after the publication of big data studies, such as repurposing or dual-use issues [ 83 ].
ERCs also face the challenge of assessing the value of informed consent for big data projects. To re-obtain consent from research subjects is impractical, particularly when using consumer generated data (e.g., social media data) for research purposes. In these cases, researchers often rely on broad consent and consent waivers. This leaves the data subjects unaware of their participation in specific studies, and therefore makes them incapable of engaging with the research progress. Therefore, the data subjects and the communities they represent become vulnerable towards potential negative research outcomes. The tool of consent has limitations in big data research—it cannot disclose all possible future uses of data, in part because these uses may be unknown at the time of data generation. Moreover, researchers can access existing datasets multiple times and reuse the same data with alternative purposes [ 84 ]. What should be the ERCs’ strategy, given the current model of informed consent leaves an ethical gap in big data projects? ERCs may be tempted to focus on the consent challenge, neglecting other pressing big data issues [ 53 ]. However, the literature reports an increasing number of authors who are against the idea of a new consent form for big data studies [ 5 ].
A final widely discussed concern is the ERC’s inadequate expertise in the area of big data research [ 85 , 86 ]. In the past, there have been questions about the technical and statistical expertise of ERC members. For example, ERCs have attempted to conform social science research to the clinical trial model, using the same knowledge and approach to review both types of research [ 87 ]. However, big data research poses further challenges to ERCs’ expertise. First, the distinct methodology of big data studies (based on data aggregation and mining) requires a specialized technical expertise (e.g., information systems, self-learning algorithms, and anonymization protocols). Indeed, big data projects have a strong technical component, due to data volume and sources, which brings specific challenges (e.g., collecting data outside traditional protocols on social media) [ 88 , 89 ]. Second, ERCs may be unfamiliar with new actors involved in big data research, such as citizen science actors or private corporations. Because of this lack of relevant expertise, ERCs may require unjustified amendments to research studies, or even reject big data projects tout-court [ 36 ]. Finally, ERCs may lose credibility as an oversight body capable of assessing ethical violations and research misconduct. In the past, ERCs solved this challenge by consulting independent experts in a relevant field when reviewing a protocol in that domain. However, this solution is not always practical as it depends upon the availability of an expert. Furthermore, experts may be researchers working and publishing in the field themselves. This scenario would be problematic because researchers would have to define the rules experts must abide by, compromising the concept of independent review [ 19 ]. Nonetheless, this problem does not disqualify the idea of expertise but requires high transparency standards regarding rule development and compliance. Other options include ad-hoc expert committees or provision of relevant training for existing committee members [ 47 , 90 , 91 ]. Given these options, which one is best to address ERCs’ lack of expertise in big data research?
Reforming the ERC
Our analysis shows that ERCs play a critical role in ensuring ethical oversight and risk–benefit evaluation [ 92 ], assessing the scientific validity of a project in its early stages, and offering an independent, critical, and interdisciplinary approach to the review. These strengths demonstrate why the ERC is an oversight model worth holding on to. Nevertheless, ERCs carry persistent big data-specific weaknesses, reducing their effectiveness and appropriateness as oversight bodies for data-driven research. To answer our initial research question, we propose that the current oversight mechanism is not as fit for purpose to assess the ethics of big data research as it could be in principle. ERCs should be improved at several levels to be able to adequately address and overcome these challenges. Changes could be introduced at the level of the regulatory framework as well as procedures. Additionally, reforming the ERC model might mean introducing complementary forms of oversight. In this section we explore these possibilities. Figure 2 offers an overview of the reforms that could aid ERCs in improving their process.
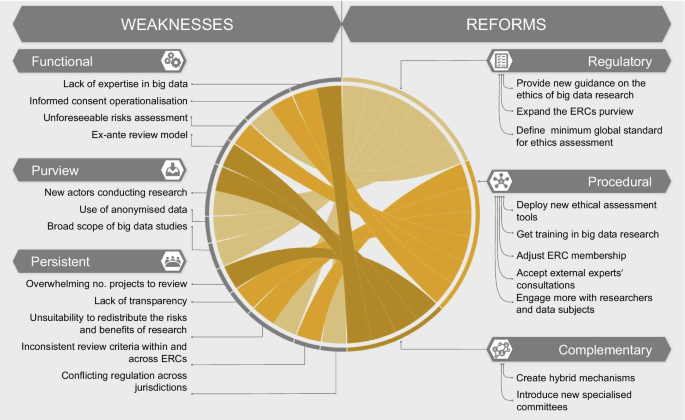
Reforms overview for the research oversight mechanism
Regulatory reforms
The regulatory design of research oversight is the first aspect which needs reform. ERCs could benefit from new guidance (e.g., in the form of a flowchart) on the ethics of big data research. This guidance could build upon a deep rethinking of the importance of data for the functioning of societies, the way we use data in society, and our justifications for this use. In the UK, for instance, individuals can generally opt out of having their data (e.g., hospital visit data, health records, prescription drugs) stored by physicians’ offices or by NHS digital services. However, exceptions to this opt-out policy apply when uses of the data are vital to the functioning of society (for example, in the case of official national statistics or overriding public interest, such as the COVID-19 pandemic) [ 93 ].
We imagine this new guidance also re-defining the scope of ERC review, from protection of individual interest to a broader research impact assessment. In other words, it will allow the ERC’s scope to expand and to address purview issues which were previously discussed. For example, less research will be oversight-free because more factors would trigger ERC purview in the first place. The new governance would impose ERC review for research involving anonymized data, or big data research within public–private partnerships. Furthermore, ERC purview could be extended beyond the initial phase of the study to other points in the data lifecycle [ 94 ]. A possible option is to assess a study after its conclusion (as is the case in the pharmaceutical industry): ERCs could then decide if research findings and results should be released and further used by the scientific community. This new ethical guidance would serve ERCs not only in deciding whether a project requires review, but also in learning from past examples and best practices how to best proceed in the assessment. Hence, this guidance could come in handy to increase transparency surrounding assessment criteria used across ERCs. Transparency could be achieved by defining a minimum global standard for ethics assessment that allows international collaboration based on open data and a homogenous evaluation model. Acceptance of a global standard would also mean that the same oversight procedures will apply to research projects with similar risks and research paths, regardless of whether they are carried on by public or private entities. Increased clarification and transparency might also streamline the review process within and across committees, rendering the entire system more efficient.
Procedural reforms
Procedural reforms might target specific aspects of the ERC model to make it more suitable for the review of big data research. To begin with, ERCs should develop new operational tools to mitigate emerging big data challenges. For example, the AI Now algorithmic impact assessment tool, which appraises the ethics of automated decision systems, and informs decisions about whether or not to deploy the systems in society, could be used [ 95 ]. Forms of broad consent [ 96 ] and dynamic consent [ 20 ] can also address some of the issues raised, by using, re-using, and sharing big data (publicly available or not). Nonetheless, informed consent should not be considered a panacea for all ethical issues in big data research—especially in the case of publicly available social media data [ 97 ]. If the ethical implications of big data studies affect the society and its vulnerable sub-groups, individual consent cannot be relied upon as an effective safeguard. For this reason, ERCs should move towards a more democratic process of review. Possible strategies include engaging research subjects and communities in the decision-making process or promoting a co-governance system. The recent Montreal Declaration for Responsible AI is an example of an ethical oversight process developed out of public involvement [ 98 ]. Furthermore, this inclusive approach could increase the trustworthiness of the ethics review mechanism itself [ 99 ]. In practice, the more that ERCs involve potential data subjects in a transparent conversation about the risks of big data research, the more socially accountable the oversight mechanism will become.
ERCs must also address their lack of big data and general computing expertise. There are several potential ways to bridge this gap. First, ERCs could build capacity with formal training on big data. ERCs are willing to learn from researchers about social media data and computational methodologies used for data mining and analysis [ 85 ]. Second, ERCs could adjust membership to include specific experts from needed fields (e.g., computer scientists, biotechnologists, bioinformaticians, data protection experts). Third, ERCs could engage with external experts for specific consultations. Despite some resistance to accepting help, recent empirical research has shown that ERCs may be inclined to rely upon external experts in case of need [ 86 ].
In the data-driven research context, ERCs must embrace their role as regulatory stewards, and walk researchers through the process of ethics review [ 40 ]. ERCs should establish an open communication channel with researchers to communicate the value of research ethics while clarifying the criteria used to assess research. If ERCs and researchers agree to mutually increase transparency, they create an opportunity to learn from past mistakes and prevent future ones [ 100 ]. Universities might seek to educate researchers on ethical issues that can arise when conducting data-driven research. In general, researchers would benefit from training on identifying issues of ethics or completing ethics self-assessment forms, particularly if they are responsible for submitting projects for review [ 101 ]. As biomedical research is trending away from hospitals and clinical trials, and towards people’s homes and private corporations, researchers should strive towards greater clarity, transparency, and responsibility. Researchers should disclose both envisioned risks and benefits, as well as the anticipated impact at the individual and population level [ 54 ]. ERCs can then more effectively assess the impact of big data research and determine whether the common good is guaranteed. Furthermore, they might examine how research benefits are distributed throughout society. Localized decision making can play a role here [ 55 ]. ERCs may take into account characteristics specific to the social context, to evaluate whether or not the research respects societal values.
Complementary reforms
An additional measure to tackle the novelty of big data research might consist in reforming the current research ethics system through regulatory and procedural tools. However, this strategy may not be sufficient: the current system might require additional support from other forms of oversight to complement its work.
One possibility is the creation of hybrid review mechanisms and norms, merging valuable aspects of the traditional ERC review model with more innovative models, which have been adopted by various partners involved in the research (e.g., corporations, participants, communities) [ 102 ]. This integrated mechanism of oversight would cover all stages of big data research and involve all relevant stakeholders [ 103 ]. Journals and the publishing industry could play a role within this hybrid ecosystem in limiting potential dual use concerns. For instance, in the research publication phase, resources could be assigned to editors so as to assess research integrity standards and promote only those projects which are ethically aligned. However, these implementations can have an impact only when there is a shared understanding of best practice within the oversight ecosystem [ 19 ].
A further option is to include specialized and distinct ethical committees alongside ERCs, whose purpose is to assess big data research and provide sectorial accreditation to researchers. In this model, ERCs would not be overwhelmed by the numbers of study proposals to review and could outsource evaluations requiring specialist knowledge in the field of big data. It is true that specialized committees (data safety monitoring boards, data access committees, and responsible research and innovation panels) already exist and support big data researchers in ensuring data protection (e.g., system security, data storage, data transfer). However, something like a “data review board” could assess research implications both for the individual and society, while reviewing a project’s technical features. Peer review could play a critical role in this model: the research community retains the expertise needed to conduct ethical research and to support each other when the path is unclear [ 101 ].
Despite their promise, these scenarios all suffer from at least one primary limitation. The former might face a backlash when attempting to bring together the priorities and ethical values of various stakeholders, within common research norms. Furthermore, while decentralized oversight approaches might bring creativity over how to tackle hard problems, they may also be very dispersive and inefficient. The latter could suffer from overlapping scope across committees, resulting in confusing procedures, and multiplying efforts while diluting liability. For example, research oversight committees have multiplied within the United States, leading to redundancy and disharmony across committees [ 47 ]. Moreover, specialized big data ethics committees working in parallel with current ERCs could lead to questions over the role of the traditional ERC, when an increasing number of studies will be big data studies.
ERCs face several challenges in the context of big data research. In this article, we sought to bring clarity regarding those which might affect the ERC’s practice, distinguishing between novel and persistent weaknesses which are compounded by big data research. While these flaws are profound and inherent in the current sociotechnical transformation, we argue that the current oversight model is still partially capable of guaranteeing the ethical assessment of research. However, we also advance the notion that introducing reform at several levels of the oversight mechanism could benefit and improve the ERC system itself. Among these reforms, we identify the urgency for new ethical guidelines and new ethical assessment tools to safeguard society from novel risks brought by big data research. Moreover, we recommend that ERCs adapt their membership to include necessary expertise for addressing the research needs of the future. Additionally, ERCs should accept external experts’ consultations and consider training in big data technical features as well as big data ethics. A further reform concerns the need for transparent engagement among stakeholders. Therefore, we recommend that ERCs involve both researchers and data subjects in the assessment of big data research. Finally, we acknowledge the existing space for a coordinated and complementary support action from other forms of oversight. However, the actors involved must share a common understanding of best practice and assessment criteria in order to efficiently complement the existing oversight mechanism. We believe that these adaptive suggestions could render the ERC mechanism sufficiently agile and well-equipped to overcome data-intensive research challenges and benefit research at large.
Availability of data and materials
Not applicable.
There is an unsettled discussion about whether ERCs ought to play a role in evaluating both scientific and ethical aspects of research, or whether these can even come apart—but we will not go into detail here. 25.Dawson AJ, Yentis SM. Contesting the science/ethics distinction in the review of clinical research. Journal of Medical Ethics. 2007;33(3):165–7, 26.Angell EL, Bryman A, Ashcroft RE, Dixon-Woods M. An analysis of decision letters by research ethics committees: the ethics/scientific quality boundary examined. BMJ Quality & Safety. 2008;17(2):131–6.
Abbreviations
Ethics Review Committee(s)
Health Insurance Portability and Accountability Act
Institutional Review Board(s)
National Health Service
Research Ethics Committee(s)
United Kingdom
United States
Moon MR. The history and role of institutional review boards: A useful tension. AMA J Ethics. 2009;11(4):311–6.
Article Google Scholar
Friesen P, Kearns L, Redman B, Caplan AL. Rethinking the Belmont report? Am J Bioeth. 2017;17(7):15–21.
Nebeker C, Torous J, Ellis RJB. Building the case for actionable ethics in digital health research supported by artificial intelligence. BMC Med. 2019;17(1):137.
Ienca M, Ferretti A, Hurst S, Puhan M, Lovis C, Vayena E. Considerations for ethics review of big data health research: A scoping review. PloS one. 2018;13(10).
Hibbin RA, Samuel G, Derrick GE. From “a fair game” to “a form of covert research”: Research ethics committee members’ differing notions of consent and potential risk to participants within social media research. J Empir Res Hum Res Ethics. 2018;13(2):149–59.
Maldoff G. How GDPR changes the rules for research: International Association of Privacy Protection; 2020 [Available from: https://iapp.org/news/a/how-gdpr-changes-the-rules-for-research/ .
Samuel G, Buchanan E. Guest Editorial: Ethical Issues in Social Media Research. SAGE Publications Sage CA: Los Angeles, CA; 2020. p. 3–11.
Shmueli G. Research Dilemmas with Behavioral Big Data. Big Data. 2017;5(2).
Sula CA. Research ethics in an age of big data. Bull Assoc Inf Sci Technol. 2016;42(2):17–21.
Metcalf J, Crawford K. Where are human subjects in Big Data research? The emerging ethics divide. Big Data Soc. 2016;3(1):2053951716650211.
Vayena E, Gasser U, Wood AB, O'Brien D, Altman M. Elements of a new ethical framework for big data research. Washington and Lee Law Review Online. 2016;72(3).
Goel V. As Data Overflows Online, Researchers Grapple With Ethics: The New York Times; 2014 [Available from: https://www.nytimes.com/2014/08/13/technology/the-boon-of-online-data-puts-social-science-in-a-quandary.html .
Vitak J, Shilton K, Ashktorab Z, editors. Beyond the Belmont principles: Ethical challenges, practices, and beliefs in the online data research community. Proceedings of the 19th ACM Conference on Computer-Supported Cooperative Work & Social Computing; 2016.
BBC World. Facebook emotion experiment sparks criticism 2014 [Available from: https://www.bbc.com/news/technology-28051930 .
Fiske ST, Hauser RM. Protecting human research participants in the age of big data. Proc Natl Acad Sci USA. 2014;111(38):13675.
Klitzman R, Appelbaum PS. Facebook’s emotion experiment: Implications for research ethics: The Hastings Center; 2014 [Available from: https://www.thehastingscenter.org/facebooks-emotion-experiment-implications-for-research-ethics/ .
Ballantyne A, Stewart C. Big Data and Public-Private Partnerships in Healthcare and Research. Asian Bioethics Review. 2019;11(3):315–26.
Barnett I, Torous J. Ethics, transparency, and public health at the intersection of innovation and Facebook's suicide prevention efforts. American College of Physicians; 2019.
Samuel G, Derrick GE, van Leeuwen T. The ethics ecosystem: Personal ethics, network governance and regulating actors governing the use of social media research data. Minerva. 2019;57(3):317–43.
Vayena E, Blasimme A. Biomedical big data: new models of control over access, use and governance. Journal of bioethical inquiry. 2017;14(4):501–13.
BBC World. Google announces AI ethics panel: BBC World; 2019 [Available from: https://www.bbc.com/news/technology-47714921 .
Clegg N. Welcoming the Oversight Board - About Facebook: FACEBOOK; 2020 [updated 2020–05–06. Available from: https://about.fb.com/news/2020/05/welcoming-the-oversight-board/ .
Shabani M, Dove ES, Murtagh M, Knoppers BM, Borry P. Oversight of genomic data sharing: what roles for ethics and data access committees? Biopreservation and biobanking. 2017;15(5):469–74.
Joly Y, Dove ES, Knoppers BM, Bobrow M, Chalmers D. Data sharing in the post-genomic world: the experience of the International Cancer Genome Consortium (ICGC) Data Access Compliance Office (DACO). PLoS Comput Biol. 2012;8(7):e1002549.
Dawson AJ, Yentis SM. Contesting the science/ethics distinction in the review of clinical research. J Med Ethics. 2007;33(3):165–7.
Angell EL, Bryman A, Ashcroft RE, Dixon-Woods M. An analysis of decision letters by research ethics committees: the ethics/scientific quality boundary examined. BMJ Qual Saf. 2008;17(2):131–6.
Nichols AS. Research ethics committees (RECS)/institutional review boards (IRBS) and the globalization of clinical research: Can ethical oversight of human subjects research be standardized. Wash U Global Stud L Rev. 2016;15:351.
Google Scholar
Garrard E, Dawson A. What is the role of the research ethics committee? Paternalism, inducements, and harm in research ethics. J Med Ethics. 2005;31(7):419–23.
Page SA, Nyeboer J. Improving the process of research ethics review. Research Integrity and Peer Review. 2017;2(1):14.
Bowen AJ. Models of institutional review board function. 2008.
McGuinness S. Research ethics committees: the role of ethics in a regulatory authority. J Med Ethics. 2008;34(9):695–700.
Kane C, Takechi K, Chuma M, Nokihara H, Takagai T, Yanagawa H. Perspectives of non-specialists on the potential to serve as ethics committee members. J Int Med Res. 2019;47(5):1868–76.
Kirkbride J, George A. Lay REC members: patient and public. J Med Ethics. 2020;39(12):780–2.
Resnik DB. Trust as a Foundation for Research with Human Subjects. The Ethics of Research with Human Subjects: Protecting People, Advancing Science, Promoting Trust. Cham: Springer International Publishing; 2018. p. 87–111.
Kritikos M. Research Ethics Governance: The European Situation. Handbook of Research Ethics and Scientific Integrity. 2020:33–50.
Molina JL, Borgatti SP. Moral bureaucracies and social network research. Social Networks [Internet]. 2019;16(11):2020.
Sheehan M, Dunn M, Sahan K. Reasonable disagreement and the justification of pre-emptive ethics governance in social research: a response to Hammersley. J Med Ethics. 2018;44:719–20.
Mustajoki H. Pre-emptive research ethics: Finnish NationalBoard on Research Integrity Tenk; 2018 [Available from: https://vastuullinentiede.fi/en/doing-research/pre-emptive-research-ethics .
Biagetti M, Gedutis A. Towards Ethical Principles of Research Evaluation in SSH. The Third Research Evaluation in SSH Conference, Valencia, 19–20 September 20192019. p. 19–20.
Dove ES. Regulatory Stewardship of Health Research: Edward Elgar Publishing; 2020.
Tene O, Polonetsky J. Beyond IRBs: Ethical guidelines for data research. Washington and Lee Law Review Online. 2016;72(3):458.
Bloss C, Nebeker C, Bietz M, Bae D, Bigby B, Devereaux M, et al. Reimagining human research protections for 21st century science. J Med Internet Res. 2016;18(12):e329.
Dove ES, Garattini C. Expert perspectives on ethics review of international data-intensive research: Working towards mutual recognition. Research Ethics. 2018;14(1):1–25.
van den Broek T, van Veenstra AF. Governance of big data collaborations: How to balance regulatory compliance and disruptive innovation. Technol Forecast Soc Chang. 2018;129:330–8.
Jackman M, Kanerva L. Evolving the IRB: building robust review for industry research. Washington and Lee Law Review Online. 2016;72(3):442.
Someh I, Davern M, Breidbach CF, Shanks G. Ethical issues in big data analytics: A stakeholder perspective. Commun Assoc Inf Syst. 2019;44(1):34.
Friesen P, Redman B, Caplan A. Of Straws, Camels, Research Regulation, and IRBs. Therapeutic innovation & regulatory science. 2019;53(4):526–34.
Kohn T, Shore C. The ethics of university ethics committees. Risk management and the research imagination, in Death of the public university. 2017:229–49.
Friesen P, Yusof ANM, Sheehan M. Should the Decisions of Institutional Review Boards Be Consistent? Ethics & human research. 2019;41(4):2–14.
Binik A, Hey SP. A framework for assessing scientific merit in ethical review of clinical research. Ethics & human research. 2019;41(2):2–13.
Derrick GE, Haynes A, Chapman S, Hall WD. The association between four citation metrics and peer rankings of research influence of Australian researchers in six fields of public health. PLoS ONE. 2011;6(4):e18521.
Luukkonen T. Conservatism and risk-taking in peer review: Emerging ERC practices. Research Evaluation. 2012;21(1):48–60.
Dove ES, Townend D, Meslin EM, Bobrow M, Littler K, Nicol D, et al. Ethics review for international data-intensive research. Science. 2016;351(6280):1399–400.
Abbott L, Grady C. A systematic review of the empirical literature evaluating IRBs: What we know and what we still need to learn. J Empir Res Hum Res Ethics. 2011;6(1):3–19.
Shaw DM, Elger BS. The relevance of relevance in research. Swiss Medical Weekly. 2013;143(1920).
Kosinski Y, Wang M. Deep neural networks are more accurate than humans at detecting sexual orientation from facial images. J Pers Soc Psychol. 2018;114(2):246–57.
Levin S. LGBT groups denounce 'dangerous' AI that uses your face to guess sexuality: The Guardian; 2017 [updated 2017–09–09. Available from: http://www.theguardian.com/world/2017/sep/08/ai-gay-gaydar-algorithm-facial-recognition-criticism-stanford .
Tan S, Zhao Y, Huang W. Neighborhood Social Disadvantage and Bicycling Behavior: A Big Data-Spatial Approach Based on Social Indicators. Soc Indic Res. 2019;145(3):985–99.
Lynch HF. Opening closed doors: Promoting IRB transparency. J Law Med Ethics. 2018;46(1):145–58.
Samuel GN, Farsides B. Public trust and ‘ethics review’as a commodity: the case of Genomics England Limited and the UK’s 100,000 genomes project. Med Health Care Philos. 2018;21(2):159–68.
Nebeker C, Lagare T, Takemoto M, Lewars B, Crist K, Bloss CS, et al. Engaging research participants to inform the ethical conduct of mobile imaging, pervasive sensing, and location tracking research. Translational behavioral medicine. 2016;6(4):577–86.
Clapp JT, Gleason KA, Joffe S. Justification and authority in institutional review board decision letters. Soc Sci Med. 2017;194:25–33.
Sheehan M, Friesen P, Balmer A, Cheeks C, Davidson S, Devereux J, et al. Trust, trustworthiness and sharing patient data for research. Journal of Medical Ethics [Internet]. 2020.
Klitzman R. The ethics police?: The struggle to make human research safe: Oxford University Press; 2015.
Cantonal Ethics Committee Zurich. Annual Report 2019. 2019 [Available from: https://www.zh.ch/content/dam/zhweb/bilder-dokumente/organisation/gesundheitsdirektion/ethikkommission-/jahresberichte-kek/Jahresbericht_KEK%20ZH%202019_09-03-2020_PKL.pdf .
Lynch HF, Abdirisak M, Bogia M, Clapp J. Evaluating the quality of research ethics review and oversight: a systematic analysis of quality assessment instruments. AJOB Empirical Bioethics. 2020:1–15.
Hoffman S. What genetic testing teaches about long-term predictive health analytics regulation. 2019.
Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447–53.
Yoshiura H. Re-identifying people from anonymous histories of their activities. 2019 IEEE 10th International Conference on Awareness Science and Technology (iCAST); 23–25 Oct. 20192019. p. 1–5.
Holm S, Ploug T. Big Data and Health Research—The Governance Challenges in a Mixed Data Economy. Journal of Bioethical Inquiry. 2017;14(4):515–25.
Nebeker C. mHealth Research Applied to Regulated and Unregulated Behavioral Health Sciences. The Journal of Law, Medicine & Ethics. 2020;48(1_suppl):49–59.
Marks M. Emergent Medical Data: Health Information Inferred by Artificial Intelligence. UC Irvine Law Review (2021, Forthcoming). 2020.
Friesen P, Douglas Jones R, Marks M, Pierce R, Fletcher K, Mishra A, et al. Governing AI-driven health research: are IRBs up to the task? Ethics & Human Research. 2020 Forthcoming
Baron J. Life Insurers Can Use Social Media Posts To Determine Premiums, As Long As They Don't Discriminate: Forbes; 2019 [Available from: https://www.forbes.com/sites/jessicabaron/2019/02/04/life-insurers-can-use-social-media-posts-to-determine-premiums/ .
Wiggins A, Wilbanks J. The rise of citizen science in health and biomedical research. Am J Bioeth. 2019;19(8):3–14.
Ienca M, Vayena E. “Hunting Down My Son’s Killer”: New Roles of Patients in Treatment Discovery and Ethical Uncertainty. Journal of Bioethical Inquiry. 2020:1–11.
Grant AD, Wolf GI, Nebeker C. Approaches to governance of participant-led research: a qualitative case study. BMJ Open. 2019;9(4):e025633.
Mascalzoni D, Hicks A, Pramstaller P, Wjst M. Informed consent in the genomics era. PLoS Med. 2008;5(9):e192.
McGuire AL, Beskow LM. Informed consent in genomics and genetic research. Annu Rev Genomics Hum Genet. 2010;11:361–81.
Roth S, Luczak-Roesch M. Deconstructing the data life-cycle in digital humanitarianism. Inf Commun Soc. 2020;23(4):555–71.
Gal A, Senderovich A. Process Minding: Closing the Big Data Gap. International Conference on Business Process Management: Springer; 2020. p. 3–16.
Ferretti A, Ienca M, Hurst S, Vayena E. Big Data, Biomedical Research, and Ethics Review: New Challenges for IRBs. Ethics & human research. 2020;42(5):17–28.
Ienca M, Vayena E. Dual use in the 21st century: emerging risks and global governance. Swiss Med Wkly. 2018;148:w14688.
Shabani M, Borry P. Rules for processing genetic data for research purposes in view of the new EU General Data Protection Regulation. Eur J Hum Genet. 2018;26(2):149–56.
Nebeker C, Harlow J, Espinoza Giacinto R, Orozco-Linares R, Bloss CS, Weibel N. Ethical and regulatory challenges of research using pervasive sensing and other emerging technologies: IRB perspectives. AJOB empirical bioethics. 2017;8(4):266–76.
Sellers C, Samuel G, Derrick G. Reasoning, “uncharted territory”: notions of expertise within ethics review panels assessing research use of social media. J Empir Res Hum Res Ethics. 2020;15(1–2):28–39.
Schrag ZM. The case against ethics review in the social sciences. Research Ethics. 2011;7(4):120–31.
Beskow LM, Hammack-Aviran CM, Brelsford KM, O'Rourke PP. Expert Perspectives on Oversight for Unregulated mHealth Research: Empirical Data and Commentary. The Journal of Law, Medicine & Ethics. 2020;48(1_suppl):138–46.
Huh-Yoo J, Rader E. It’s the Wild, Wild West: Lessons Learned From IRB Members’ Risk Perceptions Toward Digital Research Data. Proceedings of the ACM on Human-Computer Interaction. 2020;4(CSCW1):1–22.
Research; NHA. Gene Therapy Advisory Committee 2020 [Available from: https://www.hra.nhs.uk/about-us/committees-and-services/res-and-recs/gene-therapy-advisory-committee/ .
Research; NHA. The Social Care Research Ethics Committee (REC) 2020 [Available from: https://www.hra.nhs.uk/planning-and-improving-research/policies-standards-legislation/social-care-research/ .
Sheehan M, Dunn M, Sahan K. In defence of governance: ethics review and social research. J Med Ethics. 2017;44(10):710–6.
NHS UK. When your choice does not apply. 2019 [Available from: https://www.nhs.uk/your-nhs-data-matters/where-your-choice-does-not-apply/ .
Master Z, Martinson BC, Resnik DB. Expanding the scope of research ethics consultation services in safeguarding research integrity: Moving beyond the ethics of human subjects research. Am J Bioeth. 2018;18(1):55–7.
Reisman D, Schultz J, Crawford K. Whittaker M. Algorithmic impact assessments: A practical framework for public agency accountability. AI Now Institute; 2018. p. 1–22.
Sheehan M. Broad consent is informed consent Bmj. 2011;343:d6900.
Sheehan M, Thompson R, Fistein J, Davies J, Dunn M, Parker M, et al. Authority and the Future of Consent in Population-Level Biomedical Research. Public Health Ethics. 2019;12(3):225–36.
Montréal; Ud. Montréal Declaration for a Responsible Development of Artificial Intelligence 2019 [Available from: https://www.montrealdeclaration-responsibleai.com .
McCoy MS, Jongsma KR, Friesen P, Dunn M, Neuhaus CP, Rand L, et al. National Standards for Public Involvement in Research: missing the forest for the trees. J Med Ethics. 2018;44(12):801–4.
Brown C, Spiro J, Quinton S. The role of research ethics committees: Friend or foe in educational research? An exploratory study. Br Edu Res J. 2020;46(4):747–69.
Pagoto S, Nebeker C. How scientists can take the lead in establishing ethical practices for social media research. J Am Med Inform Assoc. 2019;26(4):311–3.
Harlow J, Weibel N, Al Kotob R, Chan V, Bloss C, Linares-Orozco R, et al. Using participatory design to inform the Connected and Open Research Ethics (CORE) commons. Sci Eng Ethics. 2020;26(1):183–203.
Vayena E, Blasimme A. Health research with big data: Time for systemic oversight. J Law Med Ethics. 2018;46(1):119–29.
Download references
Acknowledgements
This article reports the ideas and the conclusions emerged during a collaborative and participatory online workshop. All authors participated in the “Big Data Challenges for Ethics Review Committees” workshop, held online the 23-24 April 2020 and organized by the Health Ethics and Policy Lab, ETH Zurich.
This research is supported by the Swiss National Science Foundation under award 407540_167223 (NRP 75 Big Data). MS1 is grateful for funding from the National Institute for Health Research (NIHR) Oxford Biomedical Research Centre (BRC). The funding bodies did not take part in designing this research and writing the manuscript.
Author information
Authors and affiliations.
Health Ethics and Policy Lab, Department of Health Sciences and Technology, ETH Zürich, Hottingerstrasse 10 (HOA), 8092, Zürich, Switzerland
Agata Ferretti, Marcello Ienca, Alessandro Blasimme & Effy Vayena
The Ethox Centre, Department of Population Health, University of Oxford, Oxford, UK
Mark Sheehan
School of Law, University of Edinburgh, Edinburgh, UK
Edward S. Dove
Brighton and Sussex Medical School, Brighton, UK
Bobbie Farsides
Biomedical Ethics Unit, Department of Social Studies of Medicine, McGill University, Montreal, Canada
Phoebe Friesen
Johns Hopkins Berman Institute of Bioethics, Baltimore, USA
Mobile Health Systems Lab, Department of Health Sciences and Technology, ETH Zürich, Zürich, Switzerland
Walter Karlen
Cantonal Ethics Committee Zürich, Zürich, Switzerland
Peter Kleist
Center for Bioethics, Department of Philosophy, New York University, New York, USA
S. Matthew Liao
Research Center for Optimal Digital Ethics in Health (ReCODE Health), Herbert Wertheim School of Public Health and Longevity Science, University of California, San Diego, USA
Camille Nebeker
Department of Global Health and Social Medicine, King’s College London, London, UK
Gabrielle Samuel
Faculty of Law and Criminology, Ghent University, Ghent, Belgium
Mahsa Shabani
Department of Radiology and Medical Informatics, Faculty of Medicine, University of Geneva, Geneva, Switzerland
Minerva Rivas Velarde
You can also search for this author in PubMed Google Scholar
Contributions
AF drafted the manuscript, MI, MS1 and EV contributed substantially to the writing. EV is the senior lead on the project from which this article derives. All the authors (AF, MI, MS1, AB, ESD, BF, PF, JK, WK, PK, SML, CN, GS, MS2, MRV, EV) contributed greatly to the intellectual content of this article, edited it, and approved the final version. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Agata Ferretti .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Ferretti, A., Ienca, M., Sheehan, M. et al. Ethics review of big data research: What should stay and what should be reformed?. BMC Med Ethics 22 , 51 (2021). https://doi.org/10.1186/s12910-021-00616-4
Download citation
Received : 23 November 2020
Accepted : 15 April 2021
Published : 30 April 2021
DOI : https://doi.org/10.1186/s12910-021-00616-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Research ethics
- Ethics review
- Biomedical research
BMC Medical Ethics
ISSN: 1472-6939
- General enquiries: [email protected]

Research ethics guidance - ESRC
Our framework for research ethics helps you to consider ethics issues during the complete lifecycle of a project and includes information and guidelines on good research conduct and governance.
Our policy and guidelines for good research conduct
Find out about your responsibilities to carry out research to the highest scientific and ethical standards
Framework for research ethics
Find out about our principles, commitment, terms and conditions and when to contact us
Researchers and research teams
Our principles and expectations for research collaboration
What to expect as a research participant
Find out how researchers should treat you if you are participating in their research
Risk and benefit
Answers to commonly asked questions about risk and benefit
Answers to commonly asked questions about consent
Internet mediated research
Learn about the specific ethical considerations when using the internet and social media for research
Research with children and young people
Considerations when using children in research
Research with potentially vulnerable people
Considerations when using vulnerable people in research
International research
What should you consider if your research has an international dimension
Data requirements
Your responsibilities when gathering and sharing data
Research organisations and research ethics committees
Build and maintain appropriate structures and support for good practice
Ethics reviews
Ensure your ethical approach is properly reviewed
Ethics statement examples
Read ethics statements from successful applications
Useful resources
Useful links to ethical codes, forms and templates and guidance
This is the website for UKRI: our seven research councils, Research England and Innovate UK. Let us know if you have feedback or would like to help improve our online products and services .
Ethical Considerations of Conducting Systematic Reviews in Educational Research
- Open Access
- First Online: 22 November 2019
Cite this chapter
You have full access to this open access chapter

- Harsh Suri 6
136k Accesses
41 Citations
1 Altmetric
Ethical considerations of conducting systematic reviews in educational research are not typically discussed explicitly. However, systematic reviews are frequently read and cited in documents that influence educational policy and practice. Hence, ethical issues associated with what and how systematic reviews are produced and used have serious implications. It becomes imperative for systematic reviewers to reflexively engage with a variety of ethical issues associated with potential conflicts of interest and issues of voice and representation. This chapter discusses how systematic reviewers can draw upon the philosophical traditions of consequentialism, deontology or virtue ethics to situate their ethical decision-making.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others

Quality Criteria in Educational Research: Is Beauty More Important Than Popularity?
Questions of legitimacy and quality in educational research.

Methodological Approaches to Literature Review
Ethical considerations of conducting systematic reviews in educational research are not typically discussed explicitly. As an illustration, ‘ethics’ is not listed as a term in the index of the second edition of ‘An Introduction to Systematic Reviews’ (Gough et al. 2017 ). This chapter draws from my earlier in-depth discussion of this topic in the Qualitative Research Journal (Suri 2008 ) along with more recent publications by colleagues in the field of research ethics and methods of research synthesis.
Unlike primary researchers, systematic reviewers do not collect deeply personal, sensitive or confidential information from participants. Systematic reviewers use publicly accessible documents as evidence and are seldom required to seek an institutional ethics approval before commencing a systematic review. Institutional Review Boards for ethical conduct of research do not typically include guidelines for systematic reviews. Nonetheless, in the past four decades systematic reviews have evolved to become more methodologically inclusive and play a powerful role in influencing policy, practice, further research and public perception. Hence, ethical considerations of how interests of different stakeholders are represented in a research review have become critical (Franklin 1999 ; Hammersley 2003 ; Harlen and Crick 2004 ; Popkewitz 1999 ).
Educational researchers often draw upon the philosophical traditions of consequentialism, deontology or virtue ethics to situate their ethical decision-making. Consequentialism or utilitarianism focuses on maximising benefit and minimising harm by undertaking a cost-benefit analysis of potential positive and negative impacts of research on all stakeholders. Deontology or universalism stems from Immanuel Kant’s logic that certain actions are inherently right or wrong and hence ends cannot justify the means. A deontological viewpoint is underpinned by rights-based theories that emphasise universal adherence to the principles of beneficence (do good), non-maleficence (prevent harm), justice, honesty and gratitude. While both consequentialism and deontology focus on actions and behaviour, virtue ethics focuses on being virtuous, especially in relationships with various stakeholders. There are several overlaps, as well as tensions, between and across these philosophical traditions (Brooks et al. 2014 ; Cohen et al. 2018 ).
Recognising the inherently situated nature of ethical decision-making, I am selectively eclectic in drawing from each of these traditions. I discuss a variety of ethical considerations of conducting systematic reviews informed by rights-based theories, ethics of care and Foucauldian ethics. Rights-based theories underpin deontology and consequentialism. Most regulatory research ethics guidelines, such as those offered by British Educational Research Association (BERA 2018 ) and American Educational Research Association are premised on rights-based theories that emphasises basic human rights, such as liberty, equality and dignity. Ethics of care prioritises attentiveness, responsibility, competence and responsiveness (Tronto 2005 ). Foucauldian ethics highlights the relationship of power and knowledge (Ball 2013 ).
In my earlier publications, I have identified the following three guiding principles for a quality research synthesis (Suri 2018 ; Suri and Clarke 2009 ):
Informed subjectivity and reflexivity
Purposefully informed selective inclusivity
Audience-appropriate transparency
In the rest of this chapter, I will discuss how these guiding principles can support ethical decision making in systematic reviews in each of the following six phases of systematic reviews as identified in my earlier publications (Suri 2014 ):
identifying an appropriate epistemological orientation
identifying an appropriate purpose
searching for relevant literature
evaluating, interpreting and distilling evidence from selected reports
constructing connected understandings
communicating with an audience
To promote ethical production and use of systematic reviews through this chapter, I have used questioning as a strategic tool with the purpose of raising awareness about a variety of ethical considerations among systematic reviewers and their audience
1 Identifying an Appropriate Epistemological Orientation
What philosophical traditions are amenable for guiding ethical decision - making in systematic reviews positioned along distinct epistemologies?
Practising informed subjectivity and reflexivity, all systematic reviewers must identify an appropriate epistemological orientation, such as post-positivist, interpretive, participatory and/or critical, that is aligned with their review purpose and research competence (Suri 2013 , 2018 ).
Deontological ethics is more relevant to post-positivist reviewers who focus on explaining, predicting or describing educational phenomena as generalisable laws expressed through relationships between measurable constructs and variables. The ethical focus of post-positivist systematic reviews tends to be on minimising threats to internal validity, external validity, internal reliability and external reliability of review findings. This is typically achieve by using a priori synthesis protocols, defining all key constructs conceptually and operationally in behavioural terms, employing exhaustive sampling strategies and employing variable oriented statistical analyses (Matt and Cook 2009 ; Petticrew and Roberts 2006 ).
Teleological ethics is more relevant to interpretive systematic reviews aiming to construct a holistic understanding of the educational phenomena that takes into account subjective experiences of diverse groups in varied contexts. Ethical decision making in interpretive systematic reviews lays an emphasis on authentically representing experiences and perceptions of diverse groups, especially those whose viewpoints tend to be less represented in the literature, to the extent that is permissible from the published literature. Maintaining a questioning gaze and a genuine engagement with diverse viewpoints, interpretive systematic reviewers focus on how individual accounts of a phenomenon reinforce, refute or augment each other (Eisenhart 1998 ; Noblit and Hare 1988 ).
Ethics of care is amenable to participatory systematic reviews that are designed to improve participant reviewers’ local world experientially through critical engagement with the relevant research. Ethical decision making in participatory systematic reviews promotes building teams of practitioners with the purpose of co-reviewing research that can transform their own practices and representations of their lived experiences. Participant co-reviewers exercise greater control throughout the review process to ensure that the review remains relevant to generating actionable knowledge for transforming their practice (Bassett and McGibbon 2013 ).
Foucauldian ethics is aligned with critical systematic reviews that contest dominant discourse by problematizing the prevalent metanarratives. Ethical decision making in critical systematic reviews focuses on problematizing ‘what we might take for granted’ (Schwandt 1998 , p. 410) in a field of research by raising ‘important questions about how narratives get constructed, what they mean, how they regulate particular forms of moral and social experiences, and how they presuppose and embody particular epistemological and political views of the world’ (Aronowitz and Giroux 1991 , pp. 80–81).
2 Identifying an Appropriate Purpose
What are key ethical considerations associated with identifying an appropriate purpose for a systematic review?
In this age of information explosion, systematic reviews require substantial resources. Guided by teleological ethics, systematic reviewers must conduct a cost-benefit analysis with a critical consideration of the purpose and scope of the review and its potential benefits to various groups of stakeholders.
If we consider the number of views or downloads as a proxy measure of impact, then we can gain useful insights by examining the teleological underpinnings of some of the highly read systematic reviews. Review of Educational Research (RER) tends to be regarded as the premiere educational research review journal internationally. Let us examine the scope and purpose of the three ‘most read’ articles in RER, as listed on 26 September 2018. Given the finite amount of resources available, an important question for educators is ‘what interventions are likely to be most effective, and under what circumstances?’. The power of feedback (Hattie and Timperley 2007 ), with 11463 views and downloads, is a conceptual analysis primarily drawing from the findings of published systematic reviews (largely meta-analyses) conducted to address this important question. In addition to effectively teaching what is deemed important, educators also have an important role of critiquing what is deemed important and why. The theory and practice of culturally relevant education: A synthesis of research across content areas (Aronson and Laughter 2016 ), with 8958 views and downloads, is an example of such a systematic review. After highlighting the positive outcomes of culturally relevant education, the authors problematise the validity of standardised testing as an unbiased form of a desirable educational outcome for all. As education is essentially a social phenomenon, understanding how different stakeholders perceive various configurations of an educational intervention is critical. Making sense of assessment feedback in higher education (Evans 2013 ), with 5372 views and downloads, is an example of a systematic review that follows such a pursuit. Even though each of these reviews required significant resources and expertise, the cost is justified by the benefits evident from the high number of views and downloads of these articles. Each of these three reviews makes clear recommendations for practitioners and researchers by providing an overview, as well as interrogating, current practices.
All educational researchers are expected to prevent, or disclose and manage, ethical dilemmas arising from any real or perceived conflicts of interest (AERA 2011 ; BERA 2018 ). Systematic reviewers should also carefully scrutinise how their personal, professional or financial interests may influence the review findings in a specific direction. As systematic reviews require significant effort and resources, it is logical for systematic reviewers to bid for funding. Recognising the influence of systematic reviews in shaping perceptions of the wider community, many profit and not profit organisations have become open to funding systematic reviews. Before accepting funding for conducting a systematic review, educational researchers must carefully reflect on the following questions:
How does the agenda of the funding source intersect with the purpose of the review?
How might this potentially influence the review process and findings? How will this be managed ethically to ensure integrity of the systematic review findings?
In case of sponsored systematic reviews, it is important to consider at the outset how potential ethical issues will be managed if the interest of the funding agency conflicts with the interests of relatively less influential or less represented groups. Systematic reviews funded by a single agency with a vested interest in the findings are particularly vulnerable to ethical dilemmas arising from a conflict of interest (The Methods Coordinating Group of the Campbell Collaboration 2017 ). One approach could be to seek funding from a combination of agencies representing interests of different stakeholder groups. Exploring the option of crowdfunding is another option that systematic reviewers could use to represent the interests of marginalised groups whose interests are typically overlooked in the agenda of powerful funding agencies. In participatory synthesis, it is critical that the purpose of the systematic review evolves organically in response to the emerging needs of the practitioner participant reviewers.
3 Searching for Relevant Literature
What are key ethical considerations associated with developing an appropriate strategy for sampling and searching relevant primary research reports to include in a systematic review?
A number of researchers in education and health sciences have found that studies with certain methodological orientations or types of findings are more likely to be funded, published, cited and retrieved through common search channels (Petticrew and Roberts 2006 ). Serious ethical implications arise when systematic reviews of biased research are drawn upon to make policy decisions with an assumption that review findings are representative of the larger population. In designing an appropriate sampling and search strategy, systematic reviewers should carefully consider the impact of potential publication biases and search biases.
Funding bias, methodological bias, outcome bias and confirmatory bias are common forms of publication bias in educational research. For instance, studies with large sample-sizes are more likely to attract research funding, being submitted for publishing and getting published in reputable journals (Finfgeld-Connett and Johnson 2012 ). Research that reports significantly positive effects of an innovative intervention is more likely to be submitted for publishing by primary researchers and being accepted for publishing by journal editors (Dixon-Woods 2011 ; Rothstein et al. 2004 ). Rather than reporting on all the comparisons made in a study, often authors report on only those comparisons that are significant (Sutton 2009 ). As a result, the effectiveness of innovative educational interventions gets spuriously inflated in published literature. Often, when an educational intervention is piloted, additional resources are allocated for staff capacity building. However, in real life when the same intervention is rolled out at scale, the same degree of support is not provided to teachers whose practice is impacted by the intervention (Schoenfeld 2006 ).
Even after getting published, certain types of studies are more likely to be cited and retrieved through common search channels, such as key databases and professional networks (Petticrew and Roberts 2006 ). Systematic reviewers must carefully consider common forms of search biases, such as database bias, citation bias, availability bias, language bias, country bias, familiarity bias and multiple publication bias. The term ‘grey literature’ is sometimes used to refer to published and unpublished reports, such as government reports, that are not typically included in common research indexes and databases (Rothstein and Hopewell 2009 ). Several scholars recommend inclusion of grey literature to minimise potential impact of publication bias and search bias (Glass 2000 ) and to be inclusive of key policy documents and government reports (Godin et al. 2015 ). On the other hand, several other scholars argue that systematic reviewers should include only published research that has undergone the peer-review process of academic community to include only high-quality research and to minimise the potential impact of multiple publications based on the same dataset (La Paro and Pianta 2000 ).
With the ease of internet publishing and searching, the distinction between published and unpublished research has become blurred and the term grey literature has varied connotations. While most systematic reviews employ exhaustive sampling, in recent years there has been an increasing uptake of purposeful sampling in systematic reviews as evident from more than 1055 Google Scholar citations of a publication on this topic: Purposeful sampling in qualitative research synthesis (Suri 2011 ).
Aligned with the review’s epistemological and teleological positioning, all systematic reviewers must prudently design a sampling strategy and search plan, with complementary sources, that will give them access to most relevant primary research from a variety of high-quality sources that is inclusive of diverse viewpoints. They must ethically consider positioning of the research studies included in their sample in relation to the diverse contextual configurations and viewpoints commonly observed in practical settings.
4 Evaluating, Interpreting and Distilling Evidence from the Selected Research Reports
What are key ethical considerations associated with evaluating, interpreting and distilling evidence from the selected research reports in a systematic review?
Systematic reviewers typically do not have direct access to participants of primary research studies included in their review. The information they analyse is inevitably refracted through the subjective lens of authors of individual studies. It is important for systematic reviewers to critically reflect upon contextual position of the authors of primary research studies included in the review, their methodological and pedagogical orientations, assumptions they are making, and how they might have influenced the findings of the original studies. This becomes particularly important with global access to information where critical contextual information, that is common practice in a particular context but not necessarily in other contexts, may be taken-for-granted by the authors of the primary research report and hence may not get explicitly mentioned.
Systematic reviewers must ethically consider the quality and relevance of evidence reported in primary research reports with respect to the review purpose (Major and Savin-Baden 2010 ). In evaluating quality of evidence in individual reports, it is important to use the evaluation criteria that are commensurate with the epistemological positioning of the author of the study. Cook and Campbell’s ( 1979 ) constructs of internal validity, construct validity, external validity and statistical conclusion are amenable for evaluating postpositivist research. Valentine ( 2009 ) provides a comprehensive discussion of criteria suitable for evaluating research employing a wide range of postpositivist methods. Lincoln and Guba’s ( 1985 ) constructs of credibility, transferability, dependability and confirmability are suitable for evaluating interpretive research. The Centre for Reviews and Dissemination (CRD 2009 ) provides a useful comparison of common qualitative research appraisal tools in Chap. 6 of its open access guidelines for systematic reviews. Herons and Reason’s ( 1997 ) constructs of critical subjectivity, epistemic participation and political participation emphasising a congruence of experiential, presentational, propositional, and practical knowings are appropriate for evaluating participatory research studies. Validity of transgression, rather than correspondence, is suitable for evaluating critically oriented research reports using Lather’s constructs of ironic validity, paralogical validity, rhizomatic validity and voluptuous validity (Lather 1993 ). Rather than seeking perfect studies, systematic reviewers must ethically evaluate the extent to which findings reported in individual studies are grounded in the reported evidence.
While interpreting evidence from individual research reports, systematic reviewers should be cognisant of the quality criteria that are commensurate with the epistemological positioning of the original study. It is important to ethically reflect on plausible reasons for critical information that may be missing from individual reports and how might that influence the report findings (Dunkin 1996 ). Through purposefully informed selective inclusivity, systematic reviewers must distil information that is most relevant for addressing the synthesis purpose.
Often a two-stage approach is appropriate for evaluating, interpreting and distilling evidence from individual studies. For example, in their review that won the American Educational Research Association’s Review of the Year Award , Wideen et al. ( 1998 ) first evaluated individual studies using the criteria aligned with the methodological orientation of individual studies. Then, they distilled information that was most relevant for addressing their review purpose. In this phase, systematic reviewers must ethically pay particular attention to the quality criteria that are aligned with the overarching methodological orientation of their review, including some of the following criteria: reducing any potential biases, honouring representations of the participants of primary research studies, enriching praxis of participant reviewers or constructing a critically reflexive account of how certain discourses of an educational phenomenon have become more powerful than others. The overarching orientation and purpose of the systematic review should influence the extent to which evidence from individual primary research studies is drawn upon in a systematic review to shape the review findings (Major and Savin-Baden 2010 ; Suri 2018 ).
5 Constructing Connected Understandings
What are key ethical considerations associated with constructing connected understandings in a systematic review?
Through informed subjectivity and reflexivity, systematic reviewers must ethically consider how their own contextual positioning is influencing the connected understandings they are constructing from the distilled evidence. A variety of systematic techniques can be used to minimise unacknowledged biases, such as content analysis, statistical techniques, historical methods, visual displays, narrative methods, critical sensibilities and computer-based techniques. Common strategies for enhancing quality of all systematic reviews include ‘reflexivity; collaborative sense-making; eliciting feedback from key stakeholders; identifying disconfirming cases and exploring rival connections; sensitivity analyses and using multiple lenses’ (Suri 2014 , p. 144).
In addition, systematic reviewers must pay specific attention to ethical considerations particularly relevant to their review’s epistemological orientation. For instance, all post-positivist systematic reviewers should be wary of the following types of common errors: unexplained selectivity, not discriminating between evidence of varying quality, inaccurate coding of contextual factors, overstating claims made in the review beyond what can be justified by the evidence reported in primary studies and not paying adequate attention to the findings that are at odds with the generalisations made in the review (Dunkin 1996 ). Interpretive systematic reviews should focus on ensuring authentic representation of the viewpoints of the participants of the original studies as expressed through the interpretive lens of the authors of those studies. Rather than aiming for generalisability of the findings, they should aim at transferability by focusing on how the findings of individual studies intersect with their methodological and contextual configurations. Ethical considerations in participatory systematic reviews should pay attention to the extent to which practitioner co-reviewers feel empowered to drive the agenda of the review to address their own questions, change their own practices through the learning afforded by participating in the experience of the synthesis and have practitioner voices heard through the review (Suri 2014 ). Critically oriented systematic reviews should highlight how certain representations silence or privilege some discourses over the others and how they intersect with the interests of various stakeholder groups (Baker 1999 ; Lather 1999 ; Livingston 1999 ).
6 Communicating with an Audience
What are key ethical considerations associated with communicating findings of a systematic review to diverse audiences?
All educational researchers are expected to adhere to the highest standards of quality and rigour (AERA 2011 ; BERA 2018 ). The PRISMA-P group have identified a list of ‘Preferred reporting items for systematic review and meta-analysis protocols’ (Moher et al. 2015 ) which are useful guidelines to improve the transparency of the process in systematic reviews. Like all educational researchers, systematic reviewers also have an obligation to disclose any sources of funding and potential conflicts of interest that could have influenced their findings.
All researchers should reflexively engage with issues that may impact on individuals participating in the research as well as the wider groups whose interests are intended to be addressed through their research (Greenwood 2016 ; Pullman and Wang 2001 ; Tolich and Fitzgerald 2006 ). Systematic reviewers should also critically consider the potential impact of the review findings on the participants of original studies and the wider groups whose practices or experiences are likely to be impacted by the review findings. They should carefully articulate the domain of applicability of a review to deter the extrapolation of the review findings beyond their intended use. Contextual configurations of typical primary research studies included in the review must be comprehensively and succinctly described in a way that contextual configurations missing from their sample of studies become visible.
Like primary researchers, systematic reviewers should reflexively engage with a variety of ethical issues associated that potential conflicts of interest and issues of voice and representation. Systematic reviews are frequently read and cited in documents that influence educational policy and practice. Hence, ethical issues associated with what and how systematic reviews are produced and used have serious implications. Systematic reviewers must pay careful attention to how perspectives of authors and research participants of original studies are represented in a way that makes the missing perspectives visible. Domain of applicability of systematic reviews should be scrutinised to deter unintended extrapolation of review findings to contexts where they are not applicable. This necessitates that they systematically reflect upon how various publication biases and search biases may influence the synthesis findings. Throughout the review process, they must remain reflexive about how their own subjective positioning is influencing, and being influenced, by the review findings. Purposefully informed selective inclusivity should guide critical decisions in the review process. In communicating the insights gained through the review, they must ensure audience-appropriate transparency to maximise an ethical impact of the review findings.
AERA. (2011). Code of ethics (Approved by Amercian Educational Research Association Council February 2011). Educational Researcher, 40 (3), 145–156.
Article Google Scholar
Aronowitz, S., & Giroux, H. A. (1991). Postmodern education: Politics, culture, and social criticism . Minneapolis: University of Minnesota Press.
Google Scholar
Aronson, B., & Laughter, J. (2016). The theory and practice of culturally relevant education: A synthesis of research across content areas. Review of Educational Research, 86 (1), 163–206.
Baker, B. (1999). What is voice? Issues of identity and representation in the framing of reviews. Review of Educational Research, 69 (4), 365–383.
Ball, S. (2013). Foucault, power and education . NY: Routledge.
Book Google Scholar
Bassett, R., & McGibbon, E. (2013). A critical participatory and collaborative method for scoping the literature. Quality and quantity, 47 (6), 3249–3259.
BERA. (2018). British Educational Research Associations’ ethical guidelines for educational research . Retrieved 4 April 2018 from https://www.bera.ac.uk/wp-content/uploads/2018/06/BERA-Ethical-Guidelines-for-Educational-Research_4thEdn_2018.pdf?noredirect=1 .
Brooks, R., Kitty, t. R., & Maguire, M. (2014). Ethics and Education Research . London: Sage.
Cohen, L., Manion, L., & Morrison, K. (2018). Research Methods in Education (8th ed.). Abingdon: Routledge.
Cook, T. D., & Campbell, D. T. (1979). Quasi-experimentation: Design & analysis issues for field settings . Chicago: Rand McNally.
CRD. (2009). Systematic reviews: CRD’s guidance for undertaking reviews in health care. Retrieved April 10, 2019 from https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf .
Dixon-woods, M. (2011). Using framework-based synthesis for conducting reviews of qualitative studies. BMC Medicine, 9 (3), 39–40.
Dunkin, M. J. (1996). Types of errors in synthesizing research in education. Review of Educational Research, 66 (2), 87–97.
Eisenhart, M. (1998). On the subject of interpretive reviews. Review of Educational Research, 68 (4), 391–399.
Evans, C. (2013). Making sense of assessment feedback in higher education. Review of Educational Research, 83 (1), 70–120. https://doi.org/10.3102/0034654312474350 .
Finfgeld-Connett, D., & Johnson, E. D. (2012). Literature search strategies for conducting knowledge-building and theory-generating qualitative systematic reviews. Journal of Advanced Nursing, 69 (1), 194–203. https://doi.org/10.1111/j.1365-2648.2012.06037.x .
Franklin, B. M. (1999). Discourse, rationality and educational research: A historical perspective of RER. Review of Educational Research, 69 (4), 347–363.
Glass, G. V. (2000, January). Meta-analysis at 25. Retrieved April 10, 2019, from http://www.gvglass.info/papers/meta25.html .
Godin, K., Stapleton, J., Kirkpatrick, S. I., Hanning, R. M., & Leatherdale, S. T. (2015). Applying systematic review search methods to the grey literature: A case study examining guidelines for school-based breakfast programs in Canada. Systematic Reviews, 4 (1), 138. https://doi.org/10.1186/s13643-015-0125 .
Gough, D., Oliver, S., & Thomas, J. (Eds.). (2017). An introduction to systematic reviews (2nd ed.). London: Sage.
Greenwood, M. (2016). Approving or improving research ethics in management journals. Journal of Business Ethics, 137 , 507–520.
Hammersley, M. (2003). Systematic or unsystematic, is that the question? Some reflections on the science, art, and politics of reviewing research. Paper presented at the Department of Epidemiology and Public Health, University of Leicester.
Harlen, W., & Crick, R. D. (2004). Opportunities and challenges of using systematic reviews of research for evidence-based policy in education. Evaluation and Research in Education, 18 (1–2), 54–71.
Hattie, J., & Timperley, H. (2007). The power of feedback. Review of Educational Research, 77 (1), 81–113.
Heron, J., & Reason, P. (1997). A participatory inquiry paradigm. Qualitative Inquiry, 3 (3), 274–294.
La Paro, K., & Pianta, R. (2000). Predicting children’s competence in the early school years: A meta-analytic review. Review of Educational Research, 70 (4), 443–484.
Lather, P. (1993). Fertile obsession: Validity after poststructuralism. The Sociological Quaterly, 34 (4), 673–693.
Lather, P. (1999). To be of use: The work of reviewing. Review of Educational Research, 69 (1), 2–7.
Lincoln, Y. S., & Guba, E. G. (1985). Naturalistic inquiry . Beverly Hills, CA: Sage.
Livingston, G. (1999). Beyond watching over established ways: A review as recasting the literature, recasting the lived. Review of Educational Research, 69 (1), 9–19.
Major, C. H., & Savin-Baden, M. (2010). An introduction to qualitative research synthesis: Managing the information explosion in social science research . London: Routledge.
Matt, G. E., & Cook, T. D. (2009). Threats to the validity of generalized inferences. In H. M. Cooper, L. V. Hedges & J. C. Valentine (Eds.), The handbook of research synthesis and meta-analysis (2nd ed., pp. 537–560). New York: Sage.
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M.,... Stewart, L. (2015). Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews, 4 (1–9).
Noblit, G. W., & Hare, R. D. (1988). Meta-ethnography: Synthesizing qualitative studies . Newbury Park: Sage.
Petticrew, M., & Roberts, H. (2006). Systematic reviews in the social sciences: A practical guide . Malden, MA: Blackwell.
Popkewitz, T. S. (1999). Reviewing reviews: RER , research and the politics of educational knowledge. Review of Educational Research, 69 (4), 397–404.
Pullman, D., & Wang, A. T. (2001). Adaptive designs, informed consent, and the ethics of research. Controlled Clinical Trials, 22 (3), 203–210.
Rothstein, H. R., & Hopewell, S. (2009). Grey literature. In H. M. Cooper, L. V. Hedges & J. C. Valentine (Eds.), The handbook of research synthesis and meta-analysis (2nd ed., pp. 103–125). New York: Sage.
Rothstein, H. R., College, B., Turner, H. M., & Lavenberg, J. G. (2004). The Campbell Collaboration information retrieval policy brief . Retrieved 2006, June 05, from http://www.campbellcollaboration.org/MG/IRMGPolicyBriefRevised.pdf .
Schoenfeld, A. H. (2006). What doesn’t work: The challenge and failure of the What Works Clearinghouse to conduct meaningful reviews of studies of mathematics curricula. Educational Researcher, 35 (2), 13–21.
Schwandt, T. A. (1998). The interpretive review of educational matters: Is there any other kind? Review of Educational Research, 68 (4), 409–412.
Suri, H. (2008). Ethical considerations in synthesising research: Whose representations? Qualitative Research Journal, 8 (1), 62–73.
Suri, H. (2011). Purposeful sampling in qualitative research synthesis. Qualitative Research Journal, 11 (2), 63–75.
Suri, H. (2013). Epistemological pluralism in qualitative research synthesis. International Journal of Qualitative Studies in Education 26 (7), 889–911.
Suri, H. (2014). Towards methodologically inclusive research synthesis . UK: Routledge.
Suri, H. (2018). ‘Meta-analysis, systematic reviews and research syntheses’ In L. Cohen, L. Manion & K. R. B. Morrison Research Methods in Education (8th ed., pp. 427–439). Abingdon: Routledge.
Suri, H., & Clarke, D. J. (2009). Advancements in research synthesis methods: From a methodologically inclusive perspective. Review of Educational Research, 79 (1), 395–430.
Sutton, A. J. (2009). Publication bias. In H. M. Cooper, L. V. Hedges & J. C. Valentine (Eds.), The handbook of research synthesis and meta-analysis (2nd ed., pp. 435–452). New York: Sage.
The Methods Coordinating Group of the Campbell Collaboration. (2017). Methodological expectations of Campbell Collaboration intervention reviews: Reporting standards. Retrieved April 21, 2019, from https://www.campbellcollaboration.org/library/campbell-methods-reporting-standards.html .
Tolich, M., & Fitzgerald, M. (2006). If ethics committees were designed for ethnography. Journal of Empirical Research on Human Research Ethics. Journal of Empirical Research on Human Research Ethics, 12 (2), 71–78.
Tronto, J. C. (2005). An ethic of care. In A. E. Cudd & R. O. Andreasen (Eds.), Feminist theory: A philosophical anthology (pp. 251–263). Oxford, UK Malden, Massachusetts: Blackwell.
Valentine, J. C. (2009). Judging the quality of primary research. In H. M. Cooper, L. V. Hedges & J. C. Valentine (Eds.), The handbook of research synthesis and meta-analysis (2nd ed., pp. 129–146). New York: Sage.
Wideen, M., Mayer-Smith, J., & Moon, B. (1998). A critical analysis of the research on learning to teach: Making the case for an ecological perspective on inquiry. Review of Educational Research, 68 (2), 130–178.
Download references
Author information
Authors and affiliations.
Deakin University, Melbourne, Australia
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Harsh Suri .
Editor information
Editors and affiliations.
Oldenburg, Germany
Olaf Zawacki-Richter
Essen, Germany
Michael Kerres
Svenja Bedenlier
Melissa Bond
Katja Buntins
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions
Copyright information
© 2020 The Author(s)

About this chapter
Suri, H. (2020). Ethical Considerations of Conducting Systematic Reviews in Educational Research. In: Zawacki-Richter, O., Kerres, M., Bedenlier, S., Bond, M., Buntins, K. (eds) Systematic Reviews in Educational Research. Springer VS, Wiesbaden. https://doi.org/10.1007/978-3-658-27602-7_3
Download citation
DOI : https://doi.org/10.1007/978-3-658-27602-7_3
Published : 22 November 2019
Publisher Name : Springer VS, Wiesbaden
Print ISBN : 978-3-658-27601-0
Online ISBN : 978-3-658-27602-7
eBook Packages : Education Education (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
Reflections on the NeurIPS 2023 Ethics Review Process
Communications Chairs 2024 2023 Conference
By the NeurIPS 2023 Ethics Review Co-Chairs
The NeurIPS 2023 Ethic Review process began with the publication of the Code of Ethics . This step formally codified a foundation for ethics within the conference framework.
The Ethics Guidelines for Reviewers were also updated to reflect incorporation of the Code of Ethics .
This year, 502 papers (3.77% of all submissions) were flagged for ethics review. This represents an increase in the number of papers flagged last year (474) but a decrease in the overall rate of papers being flagged (from 4.37% in 2022).
In particular, we are heartened that fewer submissions in the Datasets and Benchmarks track were flagged for ethics review, despite the number of submissions more than doubling since last year. We believe that these trends mean that the ethics of ML research is being taken more seriously by the research community.
This year, reviewers in the main track were required to flag at least one specific area for ethics review. Note that papers can be flagged for multiple areas, so the percentages below do not add up to 100%.
Notably, discrimination, bias and fairness concerns have been flagged at nearly triple the rate this year compared to last year; the rate of papers flagged for legal compliance or responsible research practice also doubled.
This year, we invited everyone contacted for ethics reviews from last year to serve again as ethics reviewers, and also issued an open call for new reviewers . A total of 396 ethics reviewers answered the call.
In addition, we also asked reviewers if they were available as emergency ethics reviewers , to provide missing reviews and reviews for papers flagged for review on short notice. 76 people volunteered as emergency ethics reviewers.
We are immensely grateful to everyone who answered the call to service, which allowed us to handle the increased workload for ethics reviews, while broadening the community and reducing the average number of reviews per reviewer.
Thank you all for a wonderful and successful ethical review process.
Yours truly,
Jiahao Chen, Lester Mackey, and Cherie Poland NeurIPS 2023 Ethics Review Co-Chairs
Related Posts
2023 Conference
Announcing the NeurIPS 2023 Paper Awards
Announcing neurips 2023 invited talks, neurips newsletter – november 2023.
Advarra Clinical Research Network
Onsemble Community
Diversity, Equity, & Inclusion
Participants & Advocacy
Advarra Partner Network
Community Advocacy

Institutional Review Board (IRB)
Institutional Biosafety Committee (IBC)
Data Monitoring Committee (DMC)
Endpoint Adjudication (EAC)
GxP Services
Research Compliance & Site Operations
Professional Services
Coverage Analysis
Budget Negotiation
Calendar Build
Technology Staffing
Clinical Research Site Training
Research-Ready Training
Custom eLearning
Services for
Sponsors & CROs
Sites & Institutions
BioPharma & Medical Tech
Oncology Research
Decentralized Clinical Trials
Early Phase Research
Research Staffing
Cell and Gene Therapy
Ready to Increase Your Research Productivity?
Solutions for need.
Clinical Trial Management
Clinical Data Management
Research Administration
Study Startup
Site Support and Engagement
Secure Document Exchange
Research Site Cloud
Solutions for Sites
Enterprise Institution CTMS
Health System/Network/Site CTMS
Electronic Consenting System
eSource and Electronic Data Capture
eRegulatory Management System
Research ROI Reporting
Automated Participant Payments
Life Sciences Cloud
Solutions for Sponsors/CROs
Clinical Research Experience Technology
Center for IRB Intelligence
Insights for Feasibility
Not Sure Where To Start?
Resource library.
White Papers
Case Studies
Ask the Experts
Frequently Asked Questions
COVID-19 Support

About Advarra
Consulting Opportunities
Leadership Team
Our Experts
Accreditation & Compliance
Join Advarra
Learn more about our company team, careers, and values. Join Advarra’s Talented team to take on engaging work in a dynamic environment.
Navigating Local Considerations When Developing sIRB Reliance Policies

In recent years, the use of single institutional review board (sIRB) review has gained significant traction in the world of research ethics and compliance. sIRBs are intended to streamline the ethical review process for multisite research studies, ensuring research is conducted safely and efficiently.
However, implementing an sIRB reliance model involves important local considerations researchers and institutions must carefully address. In this blog, we explore these local considerations to help research teams navigate the complexities of sIRB implementation successfully.
What is an sIRB?
Before delving into the local considerations, it’s essential to understand what an sIRB is and why it’s used in research. An sIRB is a single ethics review board assuming responsibility for reviewing research protocols, and ensuring ongoing IRB oversight, across multiple institutions or sites engaged in human subjects research.
The primary goals of utilizing sIRBs are to simplify the ethical review process, reduce redundancy and administrative burden, and ensure the same ethical standards are applied consistently across all participating sites.
Local Considerations in sIRB Implementation
Local context, expertise, compliance, and collaboration are all critical factors to sIRB success. By carefully addressing these considerations, research institutions can effectively implement sIRB policies while respecting their local research environments’ unique characteristics and ensuring the highest ethical standards in their studies.
“Local context” is a concept mentioned in regulatory guidance but lacking an official definition. However, the research community generally agrees local context covers key site-level requirements and other local considerations an sIRB must include as part of its review.
Communication and Coordination
Effective communication and coordination are critical when implementing an sIRB policy. Additionally, the National Institutes of Health (NIH) requires grant applicants to provide a communication plan for sIRB reliance.
To ensure the review process runs smoothly, sites should work with the sIRB to establish clear lines of communication. This may involve establishing key contacts at both organizations, regular meetings, updates on protocol changes, and a transparent process for addressing questions or concerns. At the site, local communications should be organized to include the HRPP as well as the research team.
Consent Form Local Requirements
Many institutions have unique consent requirements related to the community they serve as well as state, local, or other organizational needs. This may include birth control language for institutions with religious affiliations or compensation for injury verbiage.
When developing your institution’s consent language for sIRB use, consider what is truly necessary and limit your requirements to just those protecting participants or meeting other local requirements. Establishing clear and reasonable local consent requirements helps ensure the sIRB appropriately addresses local needs while preserving sIRB efficiencies. Avoid the temptation to rewrite the entire informed consent or reword portions for editorial purposes, as this detracts from the purpose of sIRB and delays review timelines.
Local IRB Engagement/Transition Plan
Engage your local human research protections program (HRPP) in evolving the sIRB process. Even though the sIRB is responsible for IRB oversight of the overall protocol, the local HRPP staff will likely still have responsibilities, such as providing local informed consent requirements, reviewing sIRB applications, and ensuring local ancillary reviews (e.g., conflict of interest, feasibility, pharmacy, etc.) are completed as required.
Local HRPPs can provide valuable input in ensuring the sIRB understands local risk factors and respects community values and norms. The ceding institution (i.e., the institutional site giving up local IRB oversight and relying on an sIRB’s review) should be prepared to provide any relevant local context information to the reviewing IRB; for example, some communities may have long standing mistrust of researchers related to historical context not known to the reviewing IRB.
This essential information may inform the IRB review of the study and should be communicated as a part of the local context information. Institutions should consider developing a dedicated sIRB local context resource, perhaps a single document outlining the unique specifics that is shared with sIRBs periodically and whenever there is a revision.
Researcher Training
In developing institutional policies, ensure you include a process for training investigators and study teams on local requirements for ceding IRB review. Researchers and other stakeholders should be trained on the sIRB process, local considerations, and institutional expectations for sIRB research. This should include both initial requirements to cede review, and any requirements for ongoing updates and reporting to the local HRPP.
Researchers should also be aware of the unique ethical, cultural, and regulatory aspects of conducting research in their region so they can appropriately include such details in the sIRB submission process. They should also be familiar with the technology required for sIRB submission, including the sIRB’s platform and any local tools involved in the process.
Equip stakeholders with any special institutional sIRB submission requirements, process details for relying on an sIRB, and any other local elements potentially impacting sIRB review. For example, some institutions may require investigators to complete a reliance form prior to ceding review to an sIRB.
Finally, ensure stakeholders are made aware of and can easily find information regarding ongoing local requirements; for example, do researchers need local approval for a principal investigator (PI) change, and how is local approval obtained? Are study teams required to submit annual reports, or does the local HRPP only need updates if certain changes are made to the research? Are there local reporting requirements for unanticipated events that are in addition to and/or differ from sIRB requirements?
Institutional Culture and Policies
Each research institution has its own unique culture, policies, standards of care, and community expectations affecting how they approach research ethics. When relying on an sIRB, it is crucial to consider how the sIRB process aligns with the institution’s local culture and policies.
Consider what local processes and reviews (e.g., conflict of interest review, radiation safety committee review, etc.) must take place in conjunction with the sIRB review process. From there, think about how these local activities may impact sIRB review timelines. It may be valuable to assess which local processes have minimal impact on sIRB review. This proactive assessment then, can be done in parallel with the sIRB review, instead of waiting to submit until all ancillary reviews are completed.
Engaging an independent third party’s perspective could prove indispensable in assessing current processes and developing new approaches that capitalize on the efficiencies inherent to sIRB review.
Regulatory Compliance
Different communities and regions may have distinct regulatory requirements governing research ethics. Researchers and institutions must ensure the sIRB is aware of local requirements, state laws and statutes, and other regulations governing regulatory compliance.
This includes addressing any differences in informed consent procedures, reporting requirements, and other ethical considerations varying by location (e.g., reporting for-cause FDA or OHRP audits to the sIRB). For example, consent requirements for minors and the age of majority may vary by state. Additionally, it’s critical to notify the sIRB whether the institution has “checked the box” on its Federalwide Assurance (FWA) .
Local Expertise
In some cases, local expertise may be required to provide insights into specific cultural or contextual nuances potentially impacting research ethics. Institutions should alert the sIRB regarding local researchers, ethicists, or community representatives whose perspectives may be necessary to the review process.
Data Privacy and Security
Local data privacy and security regulations may vary, and the institution should inform the sIRB of data handling compliance. This may involve encrypting data, obtaining specific permissions, or adhering to local data storage requirements.
Budget and Resource Allocation
Implementing an sIRB process may require additional resources, including financial support, administrative staff, and training. Institutions should carefully consider the budgetary and resource implications of sIRB implementation to ensure the process is sustainable.
Some institutional IRBs may decide to act as an sIRB in certain situations. While this might be easier for the local investigator, many operational considerations must also be made (e.g., local resources, technology, etc.). To do this sIRB work, institutional IRBs charge fees for their services, but many do so arbitrarily, without a clear understanding of their actual operating costs. This lack of cost clarity is a common shortcoming among many HRPPs . It may be valuable to assess this cost to help inform budget considerations for grant applications, to advocate for increased staffing, and other metrics.
Successfully Implementing an sIRB Process
While implementing an sIRB process can significantly streamline the ethical review process for multisite research studies, it’s essential to navigate the local considerations thoughtfully.
By acknowledging and addressing the unique cultural, regulatory, and logistical aspects of each participating institution, and establishing a consistent process for ceding IRB oversight, researchers and institutions can ensure the sIRB process is both efficient and ethically sound.
Effective communication, collaboration, and flexibility are key to successfully implementing an sIRB model designed to benefit all stakeholders involved in research.
Tagged in: FDA Mandate , IRB , research compliance , Research Institutions , Reviews , Single IRB (sIRB)
Back to Resources
For a deeper dive into sIRB topics, download our white paper.
Subscribe to our monthly email.
Receive updates monthly about webinars for CEUs, white papers, podcasts, and more.
Advancing Clinical Research: Safer, Smarter, Faster ™
Copyright © 2024 Advarra. All Rights Reserved.
- Privacy Policy
- Terms of Use
- Cookie Policy
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 25 May 2024
Neither right nor wrong? Ethics of collaboration in transformative research for sustainable futures
- Julia M. Wittmayer ORCID: orcid.org/0000-0002-4738-6276 1 , 2 ,
- Ying-Syuan (Elaine) Huang 3 ,
- Kristina Bogner ORCID: orcid.org/0000-0002-1871-9828 4 ,
- Evan Boyle 5 ,
- Katharina Hölscher 6 ,
- Timo von Wirth 2 , 7 ,
- Tessa Boumans 2 ,
- Jilde Garst 8 ,
- Yogi Hale Hendlin 9 ,
- Mariangela Lavanga ORCID: orcid.org/0000-0001-5925-9509 10 ,
- Derk Loorbach 1 ,
- Neha Mungekar ORCID: orcid.org/0000-0002-4663-0716 1 , 2 ,
- Mapula Tshangela 11 ,
- Pieter Vandekerckhove 12 &
- Ana Vasques 13
Humanities and Social Sciences Communications volume 11 , Article number: 677 ( 2024 ) Cite this article
541 Accesses
Metrics details
- Environmental studies
- Science, technology and society
Transformative research is a broad and loosely connected family of research disciplines and approaches, with the explicit normative ambition to fundamentally question the status quo, change the dominant structures, and support just sustainability transitions by working collaboratively with society. When engaging in such science-practice collaborations for transformative change in society, researchers experience ethical dilemmas. Amongst others, they must decide, what is worthwhile to be researched, whose reality is privileged, and whose knowledge is included. Yet, current institutionalised ethical standards, which largely follow the tradition of medical ethics, are insufficient to guide transformative researchers in navigating such dilemmas. In addressing this vacuum, the research community has started to develop peer guidance on what constitutes morally good behaviour. These formal and informal guidelines offer a repertoire to explain and justify positions and decisions. However, they are only helpful when they have become a part of researchers’ practical knowledge ‘in situ’. By focusing on situated research practices, the article addresses the need to develop an attitude of leaning into the uncertainty around what morally good behaviour constitutes. It also highlights the significance of combining this attitude with a critical reflexive practice both individually and collaboratively for answering questions around ‘how to’ as well as ‘what is the right thing to do’. Using a collaborative autoethnographic approach, the authors of this paper share their own ethical dilemmas in doing transformative research, discuss those, and relate them to a practical heuristic encompassing axiological, ontological, and epistemological considerations. The aim is to support building practical wisdom for the broader research community about how to navigate ethical questions arising in transformative research practice.
Similar content being viewed by others
A tool for reflecting on research stances to support sustainability transitions
Negotiating the ethical-political dimensions of research methods: a key competency in mixed methods, inter- and transdisciplinary, and co-production research
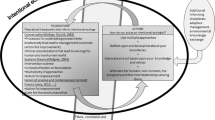
Intentional Ecology: Integrating environmental expertise through a focus on values, care and advocacy
Introduction.
There is a growing recognition that current research has failed to adequately address persistent societal challenges, which are complex, uncertain, and evaluative in nature (Ferraro et al., 2015 ; Loorbach et al., 2017 ; Saltelli et al., 2016 ). Along with this recognition come calls for science to help address these increasingly urgent and complex challenges faced at a global and local level, such as biodiversity loss, climate change, or social inequalities (Future Earth, 2014 ; Parks et al., 2019 ; WBGU, 2011 ). This call is echoed from within academia (Bradbury et al., 2019 ; Fazey et al., 2018 ; Norström et al., 2020 ) and has also translated into corresponding research funding (Arnott et al., 2020 ; Gerber et al., 2020 ; Vermeer et al., 2020 ). The fundamental premise is that addressing complex societal challenges requires more than disciplinary knowledge alone and extends beyond the confines of academia (Gibbons et al., 1994 ; Hirsch Hadorn et al., 2008 ; Lang et al., 2012 ). That is, addressing them necessitates interactive knowledge co-production and social learning with societal actors to produce actionable and contextually embedded knowledge for societal transformations (Chambers et al., 2021 ; Hessels et al., 2009 ; Schäpke et al., 2018 ). This trend has prompted a (re)surge of socially engaged approaches to research, including transdisciplinary research, phronetic social sciences, participatory research, action- and impact-oriented research, and transformative research. These approaches involve collaboration between academics and various societal stakeholders, such as policymakers, communities, enterprises, and civil society organisations.
However, often, such socially engaged research approaches are at odds with the institutional traditions designed for monodisciplinary knowledge production. Transformative research, for instance, does not claim an objective observer position; instead, it explicitly embraces a normative orientation. Its goal, as many have argued, is to facilitate transformative societal change towards justice and sustainability by recognising and addressing the deep and persistent socio-ecological challenges inherent in our current society (Mertens, 2007 ; Wittmayer et al., 2021 ). This motive to transform existing systems through collaborative research, in our view, obliges researchers to be more critical and vigilant in their decisions (Fazey et al., 2018 ). As we will present later in this paper, many of these decisions constitute ethical dilemmas, such as who decides what ‘good’ research is, whose knowledge to prioritise, or who should engage and under which circumstances. These ethical dilemmas are only poorly addressed by the ethical review processes in place at most universities, which remain dominated by linear and positivist framings of knowledge production and research design (Wood and Kahts-Kramer, 2023 ). Consequently, transformative researchers are often left struggling to choose “ between doing good (being ethically responsive to the people being researched) and doing good research (maintaining pre-approved protocols) ” (Macleod et al., 2018 , p. 10). The translation of the values and principles of transformative research into formal and informal ethical guidelines is only starting (Caniglia et al., 2023 ; Fazey et al., 2018 ; West and Schill, 2022 ).
Confronting these ethical dilemmas calls for greater reflexivity and dialogue with ourselves, among researchers, between researchers and their collaborators (including funders and professionals), and between researchers and the institutions within which they operate (Finlay, 2002 ; Horcea-Milcu et al., 2022 ; Pearce et al., 2022 ). Attesting to this call, the authors of this paper engaged in a ‘collaborative autoethnography’ (Lapadat, 2017 ; Miyahara & Fukao, 2022 ; Phillips et al., 2022 ) to explore the following research question: Which ethical dilemmas do researchers face in research collaborations that seek to catalyse transformations? And how do they navigate these in their collaborative practice? Thus, as an interdisciplinary group of researchers affiliated with academic research institutes, we shared, compared, and discussed our experiences concerning ethical dilemmas in our transformative research endeavours. In these discussions, we considered our interactions, engagements, and relationships with collaborators along with how institutional rules and norms influence or constrain our practices and relations.
This paper begins with an overview of transformative research and the challenges that arise when working collaboratively. It also testifies to the formal and informal attempts to support researchers in navigating those challenges (“Ethics in transformative research”). From there, we develop the argument that formal or informal guidelines are most meaningful when they have become a part of the practical wisdom of researchers. When they are, they support researchers in leaning into the uncertainty of what constitutes morally good behaviour and in navigating collaboration ‘in situ’. Inspired by Mertens ( 2017 ), we relate our own dilemmas to the three philosophical commitments that comprise a research paradigm: axiology, ontology, and epistemology (“Transformative research practice investigated through collaborative autoethnography”, also for an elaboration of the terms). We share concrete dilemmas while embedding and relating them to a broader body of knowledge around similar dilemmas and questions (“Collaboration in transformative research practice”). We close the paper by pointing to the importance of bottom-up ethics and the need to embed those into revalued and redesigned ethical standards, processes, and assessments that can provide external guidance and accountability (“Concluding thoughts”).
Ethics in transformative research
In this section, we first introduce transformative research (TR) in terms of its underlying values and its ontological and epistemological premises (Mertens, 2007 , 2017 ) (“Introducing transformative research”). We then connect it to its institutional context, where ethical standards and procedures fit the linear production of knowledge, leading to tensions with TR practices (“Institutional context: Formal ethical standards and processes”). Finally, we outline how the research community tries to address this misfit and the felt need for understanding what constitutes morally ‘right’ behaviour by providing peer guidance on the ethical conduct of TR (“Peer context: Informal heuristics for transformative research”).
Introducing transformative research
TR refers to a broad and loosely connected family of research disciplines and approaches, with the explicit normative ambition to fundamentally question the status quo, change the dominant structures, and support just sustainability transitions (Hölscher et al., 2021 ; Jaeger-Erben et al., 2018 ; Mertens, 2021 ; Schneidewind et al., 2016 ; Wittmayer et al., 2021 ). Transformative researchers thus start from the basic premise that “ all researchers are essentially interveners ” (Fazey et al., 2018 , p. 63). Consequently, they are explicit about the kind of normative orientation of their interventions to further a social justice and environmental sustainability agenda. There is no denying the fact that such research approaches can also be used with a different normative mindset and value orientation, which will have other ethical consequences.
TR builds on methodological and theoretical pluralism that knits together kindred, or even conflicting, perspectives to complement disciplinary specialism (Hoffmann et al., 2017 ; Horcea-Milcu et al., 2022 ; Midgley, 2011 ). As such, it also comes as a diverse phenomenon, and where such diversity is “ not haphazard […] we must be cautious about developing all-embracing standards to differentiate the ‘good’ from the ‘bad ’” (Cassell and Johnson, 2006 , p. 783). Such an ontological stance involves letting go of the idea of absolute truth and the need to tightly control the research process and outcomes (van Breda and Swilling, 2019 ). Instead, TR encourages continuous societal learning to generate actionable knowledge and transformative action that manifests in real-world changes in behaviours, values, institutions, etc. (Bartels and Wittmayer, 2018 ; Hölscher et al., 2021 ). In doing so, TR is often based upon pragmatist assumptions about the ways knowledge and action inform one another, generating contingent knowledge in a process of action and experimentation (Harney et al., 2016 ; Popa et al., 2015 ). The research process serves as a means to assess ideas in practical application, blending a critical realist stance on socially constructed reality with acknowledging subjectivism and the existence of multiple realities (Cassell and Johnson, 2006 ).
TR also represents an epistemological shift from the notion of the distanced, presumably unbiased, and all-knowing researcher and recognises individuals as sense-makers, agency holders, and change agents (Horcea-Milcu et al., 2022 ; Hurtado, 2022 ). Collaboration enables the elicitation of different kinds of knowledge, including scientific knowledge across disciplines as well as phronetic and tacit knowledge from practice. It aims at capturing the plurality of knowing and doing that is relevant to specific contexts and actors (Frantzeskaki and Kabisch, 2016 ; Nugroho et al., 2018 ; Pohl, 2008 ). This sort of mutual social learning supports joint sense-making and experimental processes. These then invite us to rethink existing situations, (re)define desired futures, and (re)position short-term action (Fazey et al., 2018 ; Lotz-Sisitka et al., 2016 ; Schneider et al., 2019 ). The co-creation of knowledge and action can increase ownership, legitimacy, and accountability and can help facilitate trust-building among diverse societal groups (Hessels et al., 2009 ; Lang et al., 2012 ). The latter is an essential ingredient for tackling complex societal problems during times of discrediting science and the rise of populist, antidemocratic movements (Saltelli et al., 2016 ).
Institutional context: formal ethical standards and processes
The institutional environment is challenging for researchers engaging in TR for multiple reasons; one challenge is the formal ethical standards and processes. Current approaches to ethical assessment in social science emerged from several international conventions in the field of medical ethics (BMJ, 1996 ; General Assembly of the World Medical Association, 2014 ; National Commission for the Protection of Human Subjects of Biomedical, & Behavioural Research, 1979 ). Most formal research ethics reviews adopt the four principles of Beauchamp and Childress ( 2001 ), which include: (1) non-maleficence by attempting to not harm others; (2) respect for autonomy by attempting to provide information about the research that allows decisions to be taken; (3) beneficence by attempting to achieve useful outcomes outweighing the risks of participation; and (4) justice by attempting fairness in participation and distribution of benefits. These principles have found their way into formal ethical reviews, often practicing value-neutral and utilitarian ethics. This approach is debatable for TR approaches (Detardo-Bora, 2004 ) and seems more effective at protecting research institutions (foregrounding bureaucratically controllable compliance) than research participants (Christians, 2005 ). Indeed, many engaged in TR have raised concerns that neither these principles nor their formal translation account for the particularity, situatedness, epistemic responsibilities, and relationality that are key to the conduct and ethics of TR (Cockburn and Cundill, 2018 ; Lincoln, 2001 ; Parsell et al., 2014 ; Wijsman and Feagan, 2019 ). In the following paragraphs, we highlight several tensions between the understanding of research, as it informs many ethical standards in place, and an understanding of TR.
First, a pre-defined versus an emerging research design. Due to its real-world orientation, TR needs to be able to deal flexibly with changing contexts and windows of opportunity that might arise (Hurtado, 2022 ). Due to the relationality of TR, it requires ongoing interaction and negotiation between researchers and their collaborators (Bartels and Wittmayer, 2018 ; Bournot-Trites and Belanger, 2005 ; Williamson and Prosser, 2002 ). One-off general consent at the start (e.g., through informed consent forms), as is common for ethical review processes, is thus at odds with the emergent design of TR and is also argued to be insufficient in maintaining participants’ autonomy (Smith, 2008 ). As an alternative, Locke et al. ( 2013 ) posit that informed consent should be seen as a collective, negotiated, continuous process, especially in collaborative action research.
Second, assumed neutrality versus dynamic aspects of researchers’ positionalities. Ethical review protocols are geared towards upholding the objective position of researchers as outsiders in the investigated context, ensuring that they will not influence this research context in any way. However, TR explicates its ambition to influence real-world problems through engagement, acknowledging that research needs to confront existing hegemonic orders and emancipate those involved through a democratic process (Cassell and Johnson, 2006 ). Furthermore, researchers co-design, facilitate, and participate in the process of knowledge co-production, making them also participants and subjects of their own research (Janes, 2016 ). To enhance the validity and integrity of the research, Wood, and Kahts-Kramer ( 2023 ), among others, suggest that transformative researchers explicitly state their positionality. This involves reflecting on their assumptions, values, and worldviews.
Third, the primacy of knowledge generation versus the importance of action. Ethical review protocols, given their historical roots in medical practice, assume that the act of falsifying, generating, or improving theories alone would benefit participants, collaborators, and the public at large. Yet, researchers engaged in TR take a step further, seeking to develop both scientific and actionable knowledge in a way that addresses persistent societal problems and stimulates social change (Bartels and Wittmayer, 2018 ; Caniglia et al., 2021 ; Greenwood and Levin, 2007 ). As put by Wood and Kahts-Kramer ( 2023 , p. 7), “ the ethical imperative of participatory research is to bring about positive change and generate theory from reflection on the purposeful action ”. This approach strengthens the responsiveness of research to societal and political needs (Stilgoe et al., 2013 ).
Transformative researchers thus perceive a lack of utility and guidance from ethical standards and processes in place that have institutionalised a certain understanding of research and related sets of principles. Following Clouser and Gert ( 1990 ), one might question whether such institutionalisation of a moral consciousness is possible in the first place. They argue that so-called ‘principlism,’ “ the practice of using ‘principles’ to replace both moral theory and particular moral rules and ideals in dealing with the moral problems that arise in medical practice ” (Clouser and Gert, 1990 , p. 219), has reduced the much-needed debates on morality vis-à-vis research and results in inconsistent and ambiguous directives for morally ‘right’ action in practice. In response to the vacuum left by institutionalised ethics standards and processes and the perceived necessity of defining morally ‘right’ behaviour, the research community is turning inward to develop peer guidance on ethical conduct in TR. The subsequent section highlights several contributions to this endeavour.
Peer context: Informal heuristics for transformative research
Transformative researchers have started offering general principles or frameworks as informal heuristics for what constitutes ‘ethical’ TR. Caniglia et al. ( 2023 ), for example, argue that practical wisdom can serve as a moral compass in complex knowledge co-production contexts, and propose four central ‘wills’ for researchers to follow: committing to justice, embracing care, fostering humility, and developing courage. Under the framing of post-normal or Mode-2 science (Funtowicz and Ravetz, 1994 ; Gibbons et al., 1994 ; Nowotny et al., 2003 ), Fazey et al. ( 2018 ) present ten ‘essentials’ of action-oriented research on transforming energy systems and climate change research Footnote 1 . One of these essentials highlights that, as researchers, we intervene, and that failing to acknowledge and engage with this reality opens the doors to sustaining unjust power relations or positioning science as apolitical. To address this, they echo Lacey et al.’s ( 2015 , p. 201) assertion that such acknowledgment means “ be[ing] transparent and accountable about the choices made about what science is undertaken, and how it is funded and communicated ”.
Looking beyond sustainability scholarship, other researchers have also developed practical actions or strategies for enhancing their ethical behaviours in the research collaboration. Taking the unique attributes of community-based participatory research, Kwan and Walsh ( 2018 , p. 382) emphasise a “ focus on equity rather than equality ” and on practicing a constructive or generative use of power “ rather than adopting a power neutral or averse position ”. Others provide guiding questions to think about the forms and quality of relationships between researchers and participants (Rowan, 2000 ) and to support the navigation of the relationship between action research and other participants (Williamson and Prosser, 2002 ). Such questions should cover not only process-focused questions but also the risks and benefits of the intended outcomes, as well as questions around purpose, motivation, and directionalities (Stilgoe et al., 2013 ). Others also propose broader guidelines in which they pay attention to non-Western and non-human-centred virtue ethics, such as ‘Ubuntu’ (I am because we are) (Chilisa, 2020 ). In forwarding climate change as a product of colonisation, Gram-Hanssen et al. ( 2022 ) join Donald’s ( 2012 ) call for an ethical relationality and reiterate the need to ground all transformation efforts on a continuous process of embodying ‘right relations’ (see also Chilisa, 2020 ; Wilson, 2020 ).
Yet, as argued before, ethics in collaboration cannot be approached through developing principles and strategies alone. Not only might they not be at hand or on top of one’s mind when being immersed in a collaborative practice, which often requires a certain reaction on the spot. They also cannot or should not replace the quest for what morality means within that collaboration (cf. Clouser and Gert, 1990 ). Further questions have been prompted about the necessary skillsets for realising ethical principles in practice (Jaeger-Erben et al., 2018 ; Pearce et al., 2022 ; West and Schill, 2022 ). Caniglia et al. ( 2023 ), for example, propose that researchers need skills such as dealing with plural values with agility and traversing principles and situations with discernment. Others focus on competency building among research participants (Menon and Hartz-Karp, 2023 ). The subsequent section turns to the point of supporting researchers in navigating collaboration ‘in situ’ and in leaning into the uncertainty around what morally good behaviour constitutes—in concrete TR contexts that are plural and uncertain.
Transformative research practice investigated through collaborative autoethnography
Transformative research as a situated practice.
The aforementioned institutionalised ethical standards and procedures, as well as the informal peer heuristics, are two vantage points for guidance on what constitutes morally good behaviour for transformative researchers. These existing vantage points are either developed based on theoretical and philosophical framings or based on researchers’ actual experiences of doing TR. They do offer a repertoire to explain and justify positions and decisions in ethical dilemmas during research collaborations. However, it is not until such heuristics or principles have become part of the practical knowledge of researchers that they are useful for actual TR in situ.
Considering research more as a practice situates it as a social activity in a ‘real-world context’. In such a practice, researchers often make decisions on the spot. Moreover, due to the constraints posed by available time and resources, researchers often engage in what Greenwood and Levin ( 2007 , p. 130) term “ skilful improvisation ” or “ pragmatic concessions ” (Greenwood and Levin, 2007 , p. 85). This “ improvisational quality ” (Yanow, 2006 , p. 70) of the research process does not mean it is not carried out systematically. Such systematicity is based on “ action repertoires ” (Yanow, 2006 , p. 71) that researchers creatively use and remake (Malkki, 2007 ). This improvisation is thus neither spontaneous nor random; rather, it builds on and is based on the practical knowledge of researchers (formed through their experiences and their situatedness) guiding their behaviours in normatively complex situations. Using ‘organic design’ (Haapala et al., 2016 ), the researchers blend real-world settings into formal spaces, fostering bricolage and driving sustainable institutional evolution over time. Such practical knowledge includes “ both ‘know how’ knowledge (techne), […] and ethical and political-practical knowledge (phronesis)” (Fazey et al., 2018 , p. 61). Research can thus be considered a craft (Wittmayer, 2016 ): the skilful mastery of which develops over time through learning based on experience and reflection (Kolb, 1984 ).
Such experiential learning should go beyond reflecting on what lies in view to include seeing how attributes of the viewer shape what is being viewed (cf. Stirling, 2006 ). Engaging in TR includes being one’s own research instrument, which puts a researcher’s positionality, i.e., their social, cultural, and political locations, centre stage. It reminds us that researchers are “ located within networks of power and participate in the (re)configuration of power relations ” (Wijsman and Feagan, 2019 , p. 74). This positionality, the sum of what makes a person and how this informs their actions (Haraway, 1988 ; Kwan and Walsh, 2018 ; Marguin et al., 2021 ), is increasingly being acknowledged in academia. It has a long history in feminist theories, participatory action research, and the critical pedagogy of decolonisation. Positionality refers to the “ researcher’s self-understanding and social vision ” (Coghlan and Shani, 2005 , p. 539) as well as their motivation to ‘better society’ (Boyle et al., 2023 ; Kump et al., 2023 ) and how these affect how researchers interpret ethical guidelines, conduct research, interpret data, and present findings. Consequently, one’s positionality can make certain research choices seem unethical. Mertens ( 2021 , p. 2), for example, considers “ continuing to do research in a business-as-usual manner” unethical as it makes the researcher “ complicit in sustaining oppression ”.
Acknowledging one’s positionality and normative role is part of a broader reflexive practice of critically questioning, reflecting on, and being transparent about values, as well as taking responsibility and accountability for research processes and outcomes (Fazey et al., 2018 ; Pearce et al., 2022 ; Wijsman and Feagan, 2019 ). Such a reflexive practice can support individual researchers to act ethically, but more so, to improve our collective ways of being and doing (i.e., an ethically informed research community) by constantly connecting what should be (i.e., the guidelines) and how it has been done (i.e., the practices) through critical reflexive practices. This improvement at the collective level includes a re-valuation and redesign of existing processes and guidelines for morally good research.
A collaborative autoethnography
Responding to this need for critical reflexivity, we engaged with our storied experience in navigating concrete and immediate ethical dilemmas that we have encountered when collaborating with others for TR in practice. We did so through collaborative autoethnography, a multivocal approach in which two or more researchers work together to share personal stories and interpret the pooled autoethnographic data (Chang et al., 2016 ; Lapadat, 2017 ; Miyahara and Fukao, 2022 ). Collaborative autoethnography is appropriate for our inquiry as it broadens the gaze from the dilemmas of the self to locate them within categories of experience shared by many. Interrogating our personal narratives and understanding the shared experiences through multiple lenses not only facilitates a more rigorous, polyvocal analysis but also reveals possibilities for practical action or intervention (Lapadat, 2017 ). Collaborative auto-ethnography can thus be considered an approach that moves “ beyond the clichés and usual explanations to the point where the written memories come as close as they can make them to ‘an embodied sense of what happened’ ” (Davies and Gannon, 2006 , p. 3). It also supports developing researcher reflexivity (Miyahara and Fukao, 2022 ).
Overall, we engaged in two types of collaborative activities over the course of a period of 18 months: writing and discussing. In hindsight, this period can be divided into three phases: starting up, exploring, and co-working. The first phase was kicked off by an online dialogue session with about 30 participants convened by the Design Impact Transition Platform of the Erasmus University Rotterdam in April 2022. The session was meant to explore and share experiences with a wide range of ethical dilemmas arising from TR collaboration in practice. Following this session, some participants continued deliberating on the questions and dilemmas raised in differing constellations and developed the idea of codifying and sharing our experiences and insights via a publication. In a second phase, we started writing down individual ethical dilemmas, both those we had discussed during the seminar and additional ones. These writings were brought together in an online shared file, where we continued our discussions. This was accompanied by meetings in differing constellations and of differing intensity for the researchers involved.
A third phase of intense co-work was framed by two broader online sessions. During a session in May 2023, we shared and discussed a first attempt at an analysis and sense-making of our individual dilemmas. During this session, we discerned the heuristic by Mertens et al. (2017) and discussed how it could be helpful in structuring our different experiences. Inspired by Mertens et al. (2017), we re-engaged with the three critical dimensions of any research paradigm to scrutinise our philosophical commitments to doing TR. A re-engagement with issues of axiology (the nature of ethics and values), ontology (the nature of reality), and epistemology (the nature of knowledge), as illustrated in Table 1 , allowed us to reconcile our ethical dilemmas and opened a space for a more nuanced understanding and bottom-up approach to the ethics of collaboration in TR. In moving forward, the heuristic also helped to guide the elicitation of additional dilemmas. This session kicked off a period of focused co-writing leading up to a second session in December 2023, where we discussed writing progress and specifically made sense of and related the ethical dilemmas to existing literature and insights.
Especially in this last phase, as we interacted dialogically to analyse and interpret the collection of storied experiences of ethical dilemmas, our thinking about the ethics of collaboration has evolved. It went beyond considering the inadequacy of institutional rules and how we navigated those, towards acknowledging their interplay with individual positionality and a researcher’s situated practice. Closer attention to the contexts within which the ethical dilemmas have arisen has led us to return to our philosophical commitments as transformative researchers and reflect on our assumptions about collaboration and research from a transformative standpoint.
The author team thus comprises a high proportion of those participating in the initial session, as well as others who joined the ensuing collective interpretation and analysis resulting in this paper. An important characteristic of the authors is that we are all affiliated with academic research institutions and that all but one of these institutions are based in high-income countries. It is in this context that we have shared our experiences, which is also limited by it. As such, this paper will mainly speak to other researchers affiliated with academic institutions in comparable settings. Acknowledging these limitations, we are from different (inter)disciplinary backgrounds Footnote 2 , nationalities, and work in different national settings and urban and rural locations. This diversity of contexts impacts the constellation of ethical dilemmas that we were faced with. We thus synthesise lessons from disparate yet still limited contexts, whilst remaining cognisant of the ungeneralisable nature of such a study.
Collaboration in transformative research practice
At the heart of our collaborative autoethnographic experience was the sharing and sensemaking of ethical dilemmas. In this section, we share those dilemmas (see Tables 2 – 4 ) clustered along the three philosophical commitments that served to deepen the analysis and interpretation of our storied experience. We embed our dilemmas with the broader body of knowledge around similar issues to discuss ways forward for practical knowledge around ‘what is good’ TR practice and ‘how to’ navigate ethical dilemmas.
Axiological dimension
Axiology is the study of value, which concerns what is considered ‘good’, what is valued, and most importantly, what ‘ought to be’. The axiological standpoint of TR is to address persistent societal problems and to contribute to transitions towards more just and sustainable societies. The commitment to knowledge development and transformative actions is also shaped by different personal judgements, disciplinary traditions, and institutional contexts. Together, these raise ethical concerns around the shape and form of research collaborations, the research lines being pursued, and where and for whom the benefits of the research accrue. Table 2 provides the details of the ethical dilemmas (described as encounters) that we discuss in the following.
Taking up a transformative stance goes hand in hand with individual researchers holding different roles at the same time (Hoffmann et al., 2022 ; Horlings et al., 2020 ; Jhagroe, 2018 ; Schut et al., 2014 ). Often resulting from this, they also perceive a wide range of responsibilities towards diverse groups (stakeholders, peers, the academic community, etc.). This is why transformative researchers face questions of who is responsible for what and whom in front of whom, and these questions influence and are influenced by what they consider the ‘right’ thing to do in relation to others in a collaborative setting. As a result, their axiological position is constructed intersubjectively in and through interactions unfolding in the communities of important others. It is thus relational and may differ depending on ‘the other’ in the research collaboration (Arrona & Larrea, 2018 ; Bartels and Wittmayer, 2018 ). Encounter 1 illustrates this through a constellation of the research collaboration that holds the potential to become a conflict of interest.
Such conflicts of interest can also occur in the very choice of which ‘community’ is being considered as the main beneficiary of the collaboration. The emphasis on action in TR, especially with regards to the principles of beneficence and justice that we mentioned in “Ethics in transformative research”, can increase this dilemma. Researchers are to continuously evaluate their (perceived) obligations. This includes, for example, obligations towards the scientific community (contributions to the academic discourse via publications) vs. obligations towards stakeholders (being a provider of free practical advice or consultant) vs. scientific requirements (academic rigour and independence) vs. stakeholder requests (answering practical questions). Researchers have to position themselves in this contested field of what ‘good research’ and ‘useful outcomes’ mean and sometimes question or challenge their peers or the academic system at large (see also Kump et al., 2023 ). This is the very question raised by Encounter 2 , where researchers are forced to decide which stakeholders’ values and needs should be prioritised in transforming clinical practice and improving the lives of patients.
Moreover, a similar prioritisation between the interests of different groups needs to be made between whether to create knowledge according to traditional scientific standards of systematicity and rigour or supporting collaborators in developing usable knowledge. This is surely a dilemma that arises from being embedded in an institutional context that judges according to different standards, but it also arises from the double commitment of TR to knowledge development and transformative action (Bartels et al., 2020 ). Huang et al. ( 2024 ) for example show how axiological assumptions serve as the base from which different notions of research excellence (e.g., scientific rigour, ‘impactful’ scholarship) are operationalised and supported institutionally. Encounter 3 reflects a similar dilemma as the lecturer juggles conflicting priorities that are inherent to the axiological concerns of TR. That is, can the goals of knowledge development in the traditional academic sense and transformative action be achieved simultaneously? The answer provided by Encounter 3 seems to suggest a redefinition of what ‘good’ scientific knowledge is, for immediate action to be possible.
Yet, perceived responsibilities—towards human and non-human actors, but also towards the own university, the institutional arrangements in which we partake, and what we understand as ethical behaviours—exist in a close, interdependent relationship with our inner ethical standards. Creed et al. ( 2022 , p. 358) capture this “ collection of sedimented evaluations of experiences, attachments, and commitments ” as an ‘embodied world of concern’. This can illustrate the complexity of how an individual researcher’s values, emotions, or sentiments tend to intertwine, and can sometimes clash, with the concerns of their communities and the social-political situation where they operate. Given that one’s embodied world of concern is not fixed but characterised by emerging pluralism, as Encounter 4 illustrates, the consequence of an ethical decision tends to fall more heavily on those with less axiological privilege, such as early career researchers or those located in regions where the opportunity for scientific publishing is limited (Kruijf et al., 2022 ).
As transformative researchers seek systemic change, their values cannot help but influence their research collaboration, including the choice of whom they work with and which methods to use. However, the intention of strengthening the responsiveness of research to societal and political needs through TR collaborations risks being co-opted by the interests of those funding research activities (Bauwens et al., 2023 ; Strydom et al., 2010 ). As illustrated in Encounter 5 , this might cause dilemmas when being approached by stakeholders (e.g., oil and gas companies) to do research, which may not sit well with the subjective judgements of the researcher or with an overall need for transformative change. Researchers can be caught in an odd position and left to wonder whether a compromise of values is worth the risks and end gain, depending on whether a positive contribution can still be achieved. Negotiating our axiological stances with collaborators thus allows researchers to be seen as social beings embedded in patterns of social interdependence, who are not only “ capable and can flourish ” but also “ vulnerable and susceptible to various kinds of loss or harm [and] can suffer ” (Sayer, 2011 , p. 1).
Ontological dimension
Ontology is the philosophical study of being, which concerns the nature of reality and what really exists. TR can start from diverse ontological stances, including critical realist, pragmatist, or subjectivist perspectives. This includes a strong acknowledgement that “ there are multiple versions of what is believed to be real ” (Mertens, 2017 , p. 21). Yet, such a pluralist stance remains a theoretical exercise up until the point that researchers ought to define what are ‘the things’ that need to be transformed and into what. In this situation, at least two debates arise: Do ‘the things’ exist based on a specific ontological commitment, such as the divide between measurable constructs and socially constructed understandings of risks and inequities. And is the existence of ‘the things’ universal or merely a construct of a specific time, space, or social group? As the researcher illustrated in Encounter 6 (see Table 3 for the detailed encounters), if maths anxiety and eco-anxiety are recognised as ‘real’ because of growing clinical research, why can’t the research team accept the construct of ‘science anxiety’ that their teacher collaborators have perceived in their classrooms? Collaboration thus remains especially challenging when researchers strive for academic rigour from an empiricist standpoint while having to cross paths or work with individuals from different ontological positions (Midgley, 2011 ).
Commitments to working collaboratively with members of ‘marginalised’ and ‘vulnerable’ communities add to this dilemma, as researchers are bound to encounter the ethical dilemmas of whose reality is privileged, whose reality can or should be legitimised and considered ‘true’ in a TR process (Kwan and Walsh, 2018 ). In Encounter 7 , for instance, research participants do not recognise themselves as ‘climate displaced persons’ or ‘climate migrants’ because they have a long history of migration for a plethora of reasons. Now, should researchers continue using this term with a view to gain political attention to the issues of climate change, or should they abstain from doing so? How does this relate to their commitment to transformative action, including shaping political agendas? The intention to target system-level change in TR (Burns, 2014 ; Kemmis, 2008 ) also means that researchers ought to interrogate the mechanisms that inflict certain perceived realities on the powerless in the name of good causes (Edelman, 2018 ; Feltham-King et al., 2018 ), the ways in which these narratives are deployed by powerful stakeholders (Thomas and Warner, 2019 ) and how these are translated into (research) action.
Moreover, research and action on ‘scientific’ problems can deflect attention from other problems that local communities most care about or lead to unexpected, even negative, implications for some stakeholders. With increasing pressure on the societal impact of research and funding tied to certain policy goals, the issues of labelling and appropriation might only perpetuate a deficit perspective on specific groups (Eriksen et al., 2021 ; Escobar, 2011 ; van Steenbergen, 2020 ). Encounter 8 highlights that, without caution, well-intended efforts risk perpetuating harm and injustice —upholding a certain deficit perspective of the community in question. Communities accustomed to ‘helicopter’ research, where academics ‘fly-in, fly-out’ to further their careers at the expense of the communities, may be reluctant to collaborate. This necessitates transparency, active listening, deliberative involvement, and trust building (Adame, 2021 ; Haelewaters et al., 2021 ). It also reminds us of the ‘seagull syndrome’,’ which attests to the frustration felt by community members towards outsider ‘experts’ making generalisations and false diagnoses based on what is usually a superficial or snapshot understanding of local community dynamics (Porter, 2016 ). In some incidents, transformative researchers may need to redesign collaboration processes in TR that centre on the realities of people in the study (Hickey et al., 2018 ).
Epistemological dimension
Epistemology is the philosophical study of knowledge, and its primary concern is the relationship between the knower and what can be known. Transformative researchers usually work at the interface of disciplines, each with their own ideas on what constitutes ‘scientifically sound’ but also ‘socially robust’ or ‘actionable’ knowledge (Mach et al., 2020 ; Nowotny et al., 2003 ). Many thus hold the epistemological assumption that knowledge is created through multiple ways of knowing, and the processes of knowledge generation need to recognise how power inequities may shape the normative definition of legitimate knowledge. This stance raises ethical concerns about whose knowledge systems and ways of knowing are included, privileged, and/or legitimised in TR practice. Moreover, it raises concerns about ways of ensuring a plurality of knowledge spaces (Savransky, 2017 ).
Using an epistemological lens to interrogate collaborative practice in TR can illuminate a wide range of ethical dilemmas associated with longstanding critiques of Western norms and ‘scientific superiority’ (Dotson, 2011 ; Dutta et al., 2022 ; Wijsman and Feagan, 2019 ). It also brings to the fore the power dynamics inherent within collaborative processes of TR for sustainability (de Geus et al., 2023 ; Frantzeskaki and Rok, 2018 ; Kanemasu and Molnar, 2020 ; Kok et al., 2021 ; Strumińska-Kutra and Scholl, 2022 ). A particular ethical challenge is related to the fact that it is typically researchers from the Global North who design and lead research collaborations, even when these take place in the Global South. This immediately creates “ an inequality that is not conducive to effective co-production ” and requires “ dedicated commitment to identify and confront the embodied power relations [and] hegemonic knowledge systems among the participants in the process ” (Vincent, 2022 , p. 890). See Table 4 for details on the ethical dilemmas that we discuss in the following.
Concerns about epistemic justice (Ackerly et al., 2020 ; Harvey et al., 2022 ; Temper and Del Bene, 2016 ) and interpretation of voices (Komulainen, 2007 ) are largely rooted in the deficit narratives about the capacity of certain groups for producing knowledge or for being knowers. Encounter 9 shows how easily certain voices can be muted as not being considered to speak from a position of knowledge. Research processes can usefully be expanded to include disinterested or disengaged citizens (Boyle et al., 2022 ), or those opposing a project or initiative so as to lay bare the associated tensions of knowledge integration and co-production (Cockburn, 2022 ). Encounter 10 illustrates that such silencing also relates to the question of who holds legitimate knowledge. This research has three parties that may hold legitimate knowledge: the researcher, the corporation, and the local community. However, the extent to which the researchers’ knowledge is heard remains unclear since the corporation does not consider it in its actions. It also illustrates common insecurities about what one can attain using certain research methods. The reliance of political institutions and citizens on expert advice, particularly when dealing with acute crises (e.g., Covid-19 pandemic), also tends to exacerbate the depoliticisation of decisions (Rovelli, 2021 ).
Moreover, TR practice nearly inevitably results in privileging certain ways of knowing and knowledges. Researchers make space for shared action or dialogue around a certain issue, inviting certain groups but not others, and choosing certain methods and not others. Encounter 11 illustrates the issue of favouritism in research collaboration. It elaborates on how thoughtful facilitation can intervene to level the playing field and provide a way out of the dilemma going beyond the question of whose benefit it serves. This facilitation enables meaningful collaboration among all parties involved. Particularly in policy sectors dominated by political and economic considerations, which exhibit strong vested interests, there is a need to foster meaningful and safe participation (Nastar et al., 2018 ). Skilled facilitation is crucial for uniting marginalised groups, preparing them to deal with the intricacies of scientific jargon and technological hegemony (Djenontin and Meadow, 2018 ; Reed and Abernethy, 2018 ). The contextual dimensions of collaborators, their associated worldviews, and the social networks in which they are situated are important epistemological foundations. Yet, these are not static and can shift over time throughout collaborative partnerships.
As explicated in “Introducing transformative research”, TR represents an epistemological shift to recognise researchers as sense-makers, agency holders, and change agents. This philosophical commitment can create dilemmas for ‘embedded researchers’ seeking to strengthen the science-policy interface. Encounter 12 illustrates how occupying a dual role — to dive into action and to publish scientifically — can be at odds. This encounter alludes to the fact that transformative researchers often navigate different roles, which come with different, at times conflicting, epistemological priorities and ways of knowing (e.g., roles as a change agent and a reflective scientist, the approach of ‘Two-Eyed Seeing’ by Indigenous scholars) (Bulten et al., 2021 ; Temper et al., 2019 ; Wittmayer and Schäpke, 2014 ). Importantly, such roles change over time in a TR practice and over the course of a researcher’s career (McGowan et al., 2014 ; Pohl et al., 2017 ).
Involving diverse stakeholders in knowledge co-production also inevitably leads to ethical questions concerning how to integrate diverse knowledge systems, especially those using multi-method research designs or models to aid decision-making (Hoffmann et al., 2017 ). Models can be useful in providing scenarios, however, they are constructed by people based on certain assumptions. These assumptions serve as the fundamental lenses through which complex real-world systems are simplified, analysed, and interpreted within the model framework. Despite the well-intention of researchers, the practice of establishing a shared understanding and reaching consensus about key constructs in a model is often unattainable. As Encounter 13 illustrates, participatory model building requires the capacity and willingness of all involved to knit together kindred, or even conflicting, perspectives to complement disciplinary specialism.
We explored the dilemmas of researchers pertaining to knowing ‘how to’ act in a certain situation and considering ‘what is doing good’ in that situation. Transformative researchers (re)build their practical knowledge of what doing research means through cultivating a reflexive practice that puts experiences in context and allows to learn from them. From a meta-perspective, doing TR is a form of experiential learning (Kolb, 1984 ) and doing TR involves traversing an action research cycle: experiencing and observing one’s action research practice, abstracting from it, building knowledge, and experimenting with it again to cultivate what has been referred to as first person inquiry (Reason and Torbert, 2001 ).
Concluding thoughts
In this article, we set out to explore which ethical dilemmas researchers face in TR and how they navigate those in practice. We highlighted that researchers engaging in TR face a context of uncertainty and plurality around what counts as ethically acceptable collaboration. With TR emphasising collaboration, it becomes important to discern the notion of ‘right relations’ with others (Gram-Hanssen et al., 2022 ), to attend to the positionality of the researcher, and to reconfigure power relations. Importantly, with TR emphasising the need for structural and systematic changes, researchers need to be aware of how research itself is characterised by structural injustices.
Using a collaborative autoethnography, we shared ethical dilemmas to uncover the messiness of collaborative TR practice. We established how guidance from institutionalised reference systems (i.e., ethical review boards and procedures) currently falls short in recognising the particularities of TR. We described how the research community generates informal principles, or heuristics to address this gap. However, we also appreciated that in actual collaboration, researchers are often ‘put on the spot’ to react ‘ethically’ in situ, with limited time and space to withdraw and consult guidelines on ‘how to behave’. Such informal heuristics are thus but a start and a helpful direction for developing the practical knowledge of researchers on how to navigate a plural and uncertain context.
This practical knowledge is based on an awareness of the uncertainty around what constitutes morally good behaviour and builds through experience and a critical reflexive practice. Our aim is not to share another set of principles, but rather to highlight the situatedness of TR and the craftsmanship necessary to navigate it and, in doing so, build practical knowledge through experiential learning and insight discovery (Kolb, 1984 ; Pearce et al., 2022 ). Such a bottom-up approach to research ethics builds on the experiences of researchers engaging in TR as a situated practice vis-à-vis their personal motivations and normative ambitions and the institutional contexts they are embedded in. This approach nurtures the critical reflexivity of researchers about how they relate to ethical principles and how they translate this into their normative assumptions, practical hypotheses, and methodological strategy.
Next to continuous learning, this critical reflexivity on TR as craftmanship can enhance practical wisdom not only for the individual but also for the broader community of researchers. We envision such wisdom not as a set of closed-ended guidelines or principles, but rather as a growing collection of ethical questions enabling the TR community to continuously deepen the interrogation of their axiological, ontological, and epistemological commitments (see Table 5 ). Only through this ongoing process of reacting, reflecting, and questioning—or as referred to by Pearce et al. ( 2022 , p. 4) as “an insight discovery process”—can we collectively learn from the past to improve our future actions.
However, such a bottom-up approach to ethics can only form one part of the answer, set in times of an evolving research ethics landscape. Researchers engaging in transformative academic work cannot and should not be left alone. Additionally, researchers’ ethical judgements cannot be left to their goodwill and virtuous values alone. Therefore, another important part of the answer is the carving out of appropriate institutions that can provide external guidance and accountability. This will require nothing less than structural and cultural changes in established universities and research environments. Rather than having researchers decide between doing good and doing ‘good’ research, such environments should help to align those goals.
From this work, questions arise on how institutional environments can be reformed or transformed to be more conducive to the particularities of TR, and to help nurture critical reflexivity. We highlight the critical role that ethic review boards can play in starting to rethink their roles, structures, and underlying values. Practical ideas include employing mentors for transformative research ethics, having ethical review as a process rather than as a one-off at the start of the project, or continuously investing in moral education. Thus, we underscore the importance of individual reflexivity and learning. However, we would like to set this in the broader context of organisational learning, and even unlearning, among academic institutions to overhaul our academic systems in response to the urgent imperative of tackling socio-ecological challenges globally. In this transformative endeavour, careful consideration of how the ethics of research and collaboration shape academics’ socially engaged work is indispensable.
The full set of essentials is the following: (1) Focus on transformations to low-carbon, resilient living; (2) Focus on solution processes; (3) Focus on ‘how to’ practical knowledge; (4) Approach research as occurring from within the system being intervened; (5) Work with normative aspects; (6) Seek to transcend current thinking; (7) Take a multi-faceted approach to understand and shape change; (8) Acknowledge the value of alternative roles of researchers; (9) Encourage second-order experimentation; and (10) Be reflexive. Joint application of the essentials would create highly adaptive, reflexive, collaborative, and impact-oriented research able to enhance capacity to respond to the climate challenge.
Disciplines include amongst others anthropology, business administration, climate change adaptation, cultural economics, economics, economic geography, education, health sciences, human geography, international development studies, philosophy, political science, sociology, urban planning.
Ackerly BA, Friedman EJ, Menon K, Zalewski M (2020) Research ethics and epistemic oppression. Int. Feminist J Politics 22(3):309–311. https://doi.org/10.1080/14616742.2020.1771006
Article Google Scholar
Adame F (2021) Meaningful collaborations can end ‘helicopter research’. Nature d41586-021-01795–1. https://doi.org/10.1038/d41586-021-01795-1
Arnott JC, Neuenfeldt RJ, Lemos MC (2020) Co-producing science for sustainability: can funding change knowledge use? Glob Environ Change 60:101979. https://doi.org/10.1016/j.gloenvcha.2019.101979
Arrona A, Larrea M (2018) Soft resistance: balancing relationality and criticality to institutionalise action research for territorial development. In: Bartels KPR, Wittmayer JM (eds.) Action research in policy analysis. critical and relational approaches to sustainability transitions. Routledge Taylor & Francis Group, London and New York, pp 134–152
Bartels KPR, Greenwood DJ, Wittmayer JM (2020) How action research can make deliberative policy analysis more transformative. Policy Stud 41(4):392–410. https://doi.org/10.1080/01442872.2020.1724927
Bartels KPR, Wittmayer JM (eds) (2018) Action research in policy analysis. critical and relational approaches to sustainability transitions. Routledge Taylor & Francis Group, London and New York
Bauwens T, Reike D, Calisto-Friant M (2023) Science for sale? Why academic marketization is a problem and what sustainability research can do about it. Environ Innov Soci Transit 48:100749. https://doi.org/10.1016/j.eist.2023.100749
Beauchamp T, Childress J (2001) Principles Biomedical Ethics (5th ed.). Oxford University Press
BMJ. (1996) The Nuremberg Code (1947). 313(7070), 1448. https://doi.org/10.1136/bmj.313.7070.1448
Bournot-Trites M, Belanger J (2005) Ethical dilemmas facing action researchers. J Educ Thought (JET)/Rev de La Pensée Éducative 39(2):197–215
Google Scholar
Boyle E, Galvin M, Revez A, Deane A, Ó Gallachóir B, Mullally G (2022) Flexibility & structure: community engagement on climate action & large infrastructure delivery. Energy Policy 167:113050. https://doi.org/10.1016/j.enpol.2022.113050
Boyle E, McGookin C, O’Mahony C, Bolger P, Byrne E, Gallachóir BÓ, Mullally G (2023) Understanding how institutions may support the development of transdisciplinary approaches to sustainability research. Research for All, 7 (1). https://doi.org/10.14324/RFA.07.1.07
Bradbury H, Waddell S, O’Brien K, Apgar M, Teehankee B, Fazey I (2019) A call to action research for transformations: the times demand it. Action Res 17(1):3–10. https://doi.org/10.1177/1476750319829633
Bulten E, Hessels LK, Hordijk M, Segrave AJ (2021) Conflicting roles of researchers in sustainability transitions: balancing action and reflection. Sustain Sci 16(4):1269–1283. https://doi.org/10.1007/s11625-021-00938-7
Burns D (2014) Systemic action research: changing system dynamics to support sustainable change. Action Res 12(1):3–18. https://doi.org/10.1177/1476750313513910
Caniglia G, Freeth R, Luederitz C, Leventon J, West SP, John B, Peukert D, Lang DJ, von Wehrden H, Martín-López B, Fazey I, Russo F, von Wirth T, Schlüter M, Vogel C (2023) Practical wisdom and virtue ethics for knowledge co-production in sustainability science. Nat Sustain 6(5):493–501. https://doi.org/10.1038/s41893-022-01040-1
Caniglia G, Luederitz C, von Wirth T, Fazey I, Martín-López B, Hondrila K, König A, von Wehrden H, Schäpke NA, Laubichler MD, Lang DJ (2021) A pluralistic and integrated approach to action-oriented knowledge for sustainability. Nat Sustain 4(2):93–100. https://doi.org/10.1038/s41893-020-00616-z
Cassell C, Johnson P (2006) Action research: explaining the diversity. Hum Relat 59(6):783–814. https://doi.org/10.1177/0018726706067080
Chambers JM, Wyborn C, Ryan ME, Reid RS, Riechers M, Serban A, Bennett NJ, Cvitanovic C, Fernández-Giménez ME, Galvin KA, Goldstein BE, Klenk NL, Tengö M, Brennan R, Cockburn JJ, Hill R, Munera C, Nel JL, Österblom H, Pickering T (2021) Six modes of co-production for sustainability. Nat. Sustainability 4(11):983–996. https://doi.org/10.1038/s41893-021-00755-x
Chang H, Ngunjiri F, Hernandez K (2016) Collaborative autoethnography (Vol. 8). Routledge. https://www.taylorfrancis.com/books/mono/10.4324/9781315432137/collaborative-autoethnography-kathy-ann-hernandez-heewon-chang-faith-ngunjiri
Chilisa B (2020) Indigenous research methodologies. SAGE Publications, Thousand Oaks
Christians C (2005) Ethics and politics in qualitative research. In: Y Lincoln (ed) The Sage Handbook of Qualitative Research. SAGE Publications, Thousand Oaks, pp 139–164
Clouser K, Gert B (1990) A critique of principlism. J Med Philos 152(2):219–236. https://doi.org/10.1093/jmp/15.2.219
Cockburn J (2022) Knowledge integration in transdisciplinary sustainability science: Tools from applied critical realism. Sustain Dev 30(2):358–374
Article MathSciNet Google Scholar
Cockburn J, Cundill G (2018) Ethics in transdisciplinary research: Reflections on the implications of ‘Science with Society’. In: Macleod CI, Marx J, Mnyaka P, Treharne GJ (eds) The Palgrave Handbook of Ethics in Critical Research. Springer, Cham, pp 81–97
Coghlan D, Shani ABR (2005) Roles, Politics, and Ethics in Action Research Design. Syst Pract Action Res 18(6):533–546. https://doi.org/10.1007/s11213-005-9465-3
Creed WED, Hudson BA, Okhuysen GA, Smith-Crowe K (2022) A place in the world: vulnerability, well-Being, and the Ubiquitous evaluation that animates participation in institutional processes. Acad Manag Rev 47(3):358–381. https://doi.org/10.5465/amr.2018.0367
Davies B, Gannon S (2006) The practices of collective biography. In: Davies B, Gannon S (eds) Doing collective biography. Investigating the production of subjectivity, Open University Press, Maidenhead, pp 1–15
de Geus T, Avelino F, Strumińska-Kutra M, Pitzer M, Wittmayer JM, Hendrikx L, Joshi V, Schrandt N, Widdel L, Fraaije M, Iskandarova M, Hielscher S, Rogge K (2023) Making sense of power through transdisciplinary sustainability research: insights from a transformative power lab. Sustain Sci. 18(3):1311–1327. https://doi.org/10.1007/s11625-023-01294-4
Detardo-Bora K (2004) Action research in a world of positivist-oriented review boards. Action Res 2(3):237–253
Djenontin INS, Meadow AM (2018) The art of co-production of knowledge in environmental sciences and management: lessons from international practice. Environ Manag 61(6):885–903. https://doi.org/10.1007/s00267-018-1028-3
Article ADS Google Scholar
Donald D (2012) Indigenous Métissage: a decolonizing research sensibility. Int J Qual Stud Educ 25(5):533–555. https://doi.org/10.1080/09518398.2011.554449
Dotson K (2011) Tracking epistemic violence, tracking practices of silencing. Hypatia 26(2):236–257. https://doi.org/10.1111/j.1527-2001.2011.01177.x
Dutta U, Azad AK, Hussain SM (2022) Counterstorytelling as epistemic justice: decolonial community‐based praxis from the global south. Am J Commun Psychol 69(1–2):59–70. https://doi.org/10.1002/ajcp.12545
Edelman NL (2018) Researching sexual healthcare for women with problematic drug use: returning to ethical principles in study processes. In: In: Macleod CI, Marx J, Mnyaka P, Treharne GJ (eds) The Palgrave Handbook of Ethics in Critical Research, Springer, Cham, pp 47–62
Eriksen S, Schipper ELF, Scoville-Simonds M, Vincent K, Adam HN, Brooks N, Harding B, Khatri D, Lenaerts L, Liverman D, Mills-Novoa M, Mosberg M, Movik S, Muok B, Nightingale A, Ojha H, Sygna L, Taylor M, Vogel C, West JJ (2021) Adaptation interventions and their effect on vulnerability in developing countries: Help, hindrance or irrelevance? World Dev 141:105383. https://doi.org/10.1016/j.worlddev.2020.105383
Escobar A (2011) Encountering development: the making and unmaking of the Third World, vol 1. Princeton University Press, Princeton
Fazey I, Schäpke N, Caniglia G, Patterson J, Hultman J, van Mierlo B, Säwe F, Wiek A, Wittmayer J, Aldunce P, Woods M, Wyborn C (2018) Ten essentials for action-oriented and second order energy transitions, transformations and climate change research. Energy Res Soc Sci 40:54–70. https://doi.org/10.1016/j.erss.2017.11.026
Feltham-King T, Bomela Y, Macleod CI (2018) Contesting the nature of young pregnant and mothering women: critical healthcare nexus research, ethics committees, and healthcare institutions. In: Macleod CI, Marx J, Mnyaka P, Treharne GJ (eds) The Palgrave Handbook of Ethics in Critical Research, Springer, Cham, pp 63–79
Ferraro F, Etzion D, Gehman J (2015) Tackling grand challenges pragmatically: robust action revisited. Organ Stud 36(3):363–390. https://doi.org/10.1177/0170840614563742
Finlay L (2002) Negotiating the swamp: the opportunity and challenge of reflexivity in research practice. Qua Res 2:209–230. https://doi.org/10.1177/146879410200200205
Frantzeskaki N, Kabisch N (2016) Designing a knowledge co-production operating space for urban environmental governance—Lessons from Rotterdam, Netherlands and Berlin, Germany. Environ Sci Policy 62:90–98. https://doi.org/10.1016/j.envsci.2016.01.010
Frantzeskaki N, Rok A (2018) Co-producing urban sustainability transitions knowledge with community, policy and science. Environ Innov Soc Transit 29(August):47–51. https://doi.org/10.1016/j.eist.2018.08.001
Funtowicz SO, Ravetz JR (1994) The worth of a songbird: ecological economics as a post-normal science. Ecol Econ 10(3):197–207. https://doi.org/10.1016/0921-8009(94)90108-2
Future Earth (2014) Future Earth 2025 Vision. International Council for Science (ICSU), Paris. Online at: https://futureearth.org/wp-content/uploads/2019/03/future-earth_10-year-vision_web.pdf
General Assembly of the World Medical Association (2014) World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J Am Coll Dent 81(3):14–18
Gerber A, Forsberg E-M, Shelley-Egan C, Arias R, Daimer S, Dalton G, Cristóbal AB, Dreyer M, Griessler E, Lindner R, Revuelta G, Riccio A, Steinhaus N (2020) Joint declaration on mainstreaming RRI across Horizon Europe. J Responsible Innov 7(3):708–711. https://doi.org/10.1080/23299460.2020.1764837
Gibbons M, Limoges C, Nowotny H, Schwartzmann S, Scott P, Trow M (1994) The new production of knowledge. The dynamics of science and research in contemporary societies. Sage Publications, London
Gram-Hanssen I, Schafenacker N, Bentz J (2022) Decolonizing transformations through ‘right relations. Sustain Sci 17(2):673–685. https://doi.org/10.1007/s11625-021-00960-9
Greenwood DJ, Levin M (2007) Introduction to Action Research. Social Research for Social Change. Sage, Thousand Oaks
Haapala J, Rautanen S-L, White P, Keskinen M, Varis O (2016) Facilitating bricolage through more organic institutional designs? The case of water users’ associations in rural Nepal. Int J Commons 10(2):1172. https://doi.org/10.18352/ijc.688
Haelewaters D, Hofmann TA, Romero-Olivares AL (2021) Ten simple rules for Global North researchers to stop perpetuating helicopter research in the Global South. PLOS Comput Biol 17(8):e1009277. https://doi.org/10.1371/journal.pcbi.1009277
Article ADS CAS PubMed PubMed Central Google Scholar
Haraway D (1988) Situated knowledges: the science question in feminism and the privilege of partial perspective. Feminist Stud 14(3):575. https://doi.org/10.2307/3178066
Harney L, Mccurry J, Wills J (2016) Developing ‘ process pragmatism ’ to underpin engaged research in human geography. Prog Hum Geogr 40(3):316–333. https://doi.org/10.1177/0309132515623367
Harvey B, Huang Y-S, Araujo J, Vincent K, Sabiiti G (2022) Breaking vicious cycles? A systems perspective on Southern leadership in climate and development research programmes. Clim Dev 14(10):884–895. https://doi.org/10.1080/17565529.2021.2020614
Hessels LK, van Lente H, Smits R (2009) In search of relevance: the changing contract between science and society. Sci Public Policy 36(5):387–401. https://doi.org/10.3152/030234209X442034
Hickey G, Richards T, Sheehy J (2018) Co-production from proposal to paper Three examples show how public participation in research can be extended at every step of the process to generate useful knowledge. Nature 562:29–31. https://doi.org/10.1038/d41586-018-06861-9
Article ADS CAS PubMed Google Scholar
Hirsch Hadorn G, Hoffmann-Riem H, Biber-Klemm S, Grossenbacher-Mansuy W, Joye, D, Pohl, C, Wiesmann U, Zemp E (2008) Handbook of transdisciplinary research. Handbook of Transdisciplinary Research. Springer Science + Business Media B.V., Cham https://doi.org/10.1007/978-1-4020-6699-3
Hoffmann S, Deutsch L, Klein JT, O’Rourke M (2022) Integrate the integrators! A call for establishing academic careers for integration experts. Hum Soc Sci Commun 9(1):147. https://doi.org/10.1057/S41599-022-01138-Z
Hoffmann S, Pohl C, Hering JG (2017) Exploring transdisciplinary integration within a large research program: empirical lessons from four thematic synthesis processes. Res Policy 46(3):678–692. https://doi.org/10.1016/j.respol.2017.01.004
Hölscher K, Wittmayer JM, Hirschnitz-garbers M, Olfert A, Schiller G, Brunnow B (2021) Transforming science and society? Methodological lessons from and for transformation research. Res Eval 1–17. https://doi.org/10.1093/reseval/rvaa034
Horcea-Milcu A-I, Leventon J, Lang DJ (2022) Making transdisciplinarity happen: Phase 0, or before the beginning. Environ Sci Policy 136:187–197. https://doi.org/10.1016/j.envsci.2022.05.019
Horlings LG, Nieto-Romero M, Pisters S, Soini K (2020) Operationalising transformative sustainability science through place-based research: the role of researchers. Sustain Sci. 15(2):467–484. https://doi.org/10.1007/s11625-019-00757-x
Huang YS, Harvey B, Vincent K (2024) Large-scale sustainability programming is reshaping research excellence: Insights from a meta-ethnographic study of 12 global initiatives Environ Sci Policy 155:103725. https://doi.org/10.1016/j.envsci.2024.103725
Hurtado S (2022) The transformative paradigm. In: Pasque PA (ed) Advancing culturally responsive research and researchers: Qualitative, quantitative, and mixed methods, Routledge, London, pp 15–17
Jaeger-Erben M, Nagy E, Schäfer M, Süßbauer E, Zscheischler J (2018) Von der Programmatik zur Praxis: Plädoyer für eine Grounded Theory transformationsorientierter Forschung. GAIA - Ecol Perspect Sci Soc 27(1):117–121. https://doi.org/10.14512/gaia.27.1.5
Janes J (2016) Democratic encounters? Epistemic privilege, power, and community-based participatory action research. Action Res 14(1):72–87
Jhagroe S (2018) Transition scientivism: on activist gardening and co-producing transition knowledge ‘from below’. In: Bartels KPR, Wittmayer JM (eds) Action Research in Policy Analysis. Critical and Relational Appoaches to Sustainability Transitions, Routledge Taylor & Francis Group, London and New York, pp 64–85
Kanemasu Y, Molnar G (2020) Representing’ the voices of Fijian women rugby players: Working with power differentials in transformative research. Int Rev Sociol Sport 55(4):399–415. https://doi.org/10.1177/1012690218818991
Kemmis S (2008) Critical theory and participatory action research. In: Reason P, Bradbury H (eds) The Sage Handbook of Action Research. Participative inquiry and practice, 2nd edn. SAGE Publications, London, pp 695–707
Kok KPW, Gjefsen MD, Regeer BJ, Broerse JEW (2021) Unraveling the politics of ‘doing inclusion’ in transdisciplinarity for sustainable transformation. Sustain Sci 16(6):1811–1826. https://doi.org/10.1007/s11625-021-01033-7
Article PubMed PubMed Central Google Scholar
Kolb, D (1984) The Process of Experiential Learning. In: Experiential Learning. Experience as The Source of Learning and Development. Prentice Hall, pp 20–38. https://doi.org/10.1016/B978-0-7506-7223-8.50017-4
Komulainen S (2007) The Ambiguity of the Child’s ‘Voice’ in Social Research. Childhood 14(1):11–28. https://doi.org/10.1177/0907568207068561
Kruijf JV, Verbrugge L, Schröter B, den Haan R, Cortes Arevalo J, Fliervoet J, Henze J, Albert C (2022) Knowledge co‐production and researcher roles in transdisciplinary environmental management projects. Sustain Dev 30(2):393–405. https://doi.org/10.1002/sd.2281
Kump B, Wittmayer J, Bogner K, Beekman M (2023) Navigating force conflicts: a case study on strategies of transformative research in the current academic system. J Clean Prod 412:137374. https://doi.org/10.1016/j.jclepro.2023.137374
Kwan C, Walsh C (2018) Ethical issues in conducting community-based participatory research: a narrative review of the literature. Qual Rep. https://doi.org/10.46743/2160-3715/2018.3331
Lacey J, Howden SM, Cvitanovic C, Dowd A-M (2015) Informed adaptation: ethical considerations for adaptation researchers and decision-makers. Glob Environ Change 32:200–210. https://doi.org/10.1016/j.gloenvcha.2015.03.011
Lang DJ, Wiek A, Bergmann M, Moll P, Swilling M, Thomas CJ (2012) Transdisciplinary research in sustainability science: practice, principles, and challenges. Sustainability Sci. 7:25–43. https://doi.org/10.1007/s11625-011-0149-x
Lapadat JC (2017) Ethics in autoethnography and collaborative autoethnography. Qual Inq 23(8):589–603. https://doi.org/10.1177/1077800417704462
Lincoln Y (2001) Engaging sympathies: Relationships between action research and social constructivism. In: Reason P, Bradbury H (eds) The Sage Handbook of Action Research. Participative inquiry and practice, SAGE Publications, London, pp 124–132
Locke T, Alcorn N, O’Neill J (2013) Ethical issues in collaborative action research. Educ Action Res 21(1):107–123. https://doi.org/10.1080/09650792.2013.763448
Loorbach D, Frantzeskaki N, Avelino F (2017) Sustainability transitions research: transforming science and practice for societal change. Annu Rev Environ Resour 42(1):599–626. https://doi.org/10.1146/annurev-environ-102014-021340
Lotz-Sisitka H, Ali MB, Mphepo G, Chaves M, Macintyre T, Pesanayi T, Wals A, Mukute M, Kronlid D, Tran DT, Joon D, McGarry D (2016) Co-designing research on transgressive learning in times of climate change. Curr Opin Environ Sustain 20:50–55. https://doi.org/10.1016/j.cosust.2016.04.004
Mach KJ, Lemos MC, Meadow AM, Wyborn C, Klenk N, Arnott JC, Ardoin NM, Fieseler C, Moss RH, Nichols L, Stults M, Vaughan C, Wong-Parodi G (2020) Actionable knowledge and the art of engagement. Curr Opin Environ Sustain 42:30–37. https://doi.org/10.1016/j.cosust.2020.01.002
Macleod CI, Marx J, Mnyaka P, Treharne GJ (eds) (2018) The Palgrave Handbook of Ethics in Critical Research. Springer, Cham
Malkki LH (2007) Tradition and Improvisation in Ethnographic Field Research. In: Cerwonka A, Malkki LH, Improvising Theory. Process and Temporality in Ethnographic Fieldwork, The University of Chicago Press, pp 162–187
Marguin S, Haus J, Heinrich AJ, Kahl A, Schendzielorz C, Singh A (2021) Positionality Reloaded: Über die Dimensionen der Reflexivität im Verhältnis von Wissenschaft und Gesellschaft: Ein EditorialPositionality Reloaded: Debating the Dimensions of Reflexivity in the Relationship Between Science and Society: An Editorial. Historical Soc Res 46:734. https://doi.org/10.12759/HSR.46.2021.2.7-34
McGowan KA, Westley F, Fraser EDG, Loring PA, Weathers KC, Avelino F, Sendzimir J, Roy Chowdhury R, Moore M-L (2014) The research journey: travels across the idiomatic and axiomatic toward a better understanding of complexity. Ecol Soc 19(3):art37. https://doi.org/10.5751/ES-06518-190337
Menon S, Hartz-Karp J (2023) Applying mixed methods action research to explore how public participation in an Indian City could better resolve urban sustainability problems. Action Res 21(2):230–253. https://doi.org/10.1177/1476750320943662
Mertens DM (2007) Transformative paradigm: mixed methods and social justice. J Mixed Methods Res 1(3):212–225. https://doi.org/10.1177/1558689807302811
Mertens DM (2017) Transformative research: Personal and societal. Int J Transform Res 4(1):18–24. https://doi.org/10.1515/ijtr-2017-0001
Mertens DM (2021) Transformative research methods to increase social impact for vulnerable groups and cultural minorities. Int J Qua Methods 20:160940692110515. https://doi.org/10.1177/16094069211051563
Midgley G (2011) Theoretical pluralism in systemic action research. Syst Pract Action Res 24:1–15. https://doi.org/10.1007/s11213-010-9176-2
Miyahara M, Fukao A (2022) Exploring the use of collaborative autoethnography as a tool for facilitating the development of researcher reflexivity. System 105:102751. https://doi.org/10.1016/j.system.2022.102751
Nastar M, Abbas S, Aponte Rivero C, Jenkins S, Kooy M (2018) The emancipatory promise of participatory water governance for the urban poor: Reflections on the transition management approach in the cities of Dodowa, Ghana and Arusha, Tanzania. Afr Stud 77(4):504–525. https://doi.org/10.1080/00020184.2018.1459287
National Commission for the Protection of Human Subjects of Biomedical, & Behavioral Research. (1979) The Belmont report: Ethical principles and guidelines for the protection of human subjects of research ( Vol. 1 ) . United States Department of Health, Education, and Welfare. Online available here: https://www.hhs.gov/ohrp/regulations-and-policy/belmont-report/read-the-belmont-report/index.html
Norström AV, Cvitanovic C, Löf MF, West S, Wyborn C, Balvanera P, Bednarek AT, Bennett EM, Biggs R, de Bremond A, Campbell BM, Canadell JG, Carpenter SR, Folke C, Fulton EA, Gaffney O, Gelcich S, Jouffray JB, Leach M, Österblom H (2020) Principles for knowledge co-production in sustainability research. Nat. Sustainability 3(3):182–190. https://doi.org/10.1038/s41893-019-0448-2
Nowotny H, Scott P, Gibbons M (2003) Mode 2’ Revisited: The New Production of Knowledge. Minerva 41(3):179–194
Nugroho K, Carden F, Antlov H (2018) Local knowledge matters: Power, context and policy making in Indonesia. Policy Press, Bristol
Parks S, Rincon D, Parkinson S, Manville C (2019) The changing research landscape and reflections on national research assessment in the future . RAND. https://www.rand.org/pubs/research_reports/RR3200.html
Parsell M, Ambler T, Jacenyik-Trawoger C (2014) Ethics in higher education research. Stud High Educ 39(1):166–179. https://doi.org/10.1080/03075079.2011.647766
Pearce BJ, Deutsch L, Fry P, Marafatto FF, Lieu J (2022) Going beyond the AHA! moment: insight discovery for transdisciplinary research and learning. Hum Soc Sci Commun 9(1):123. https://doi.org/10.1057/s41599-022-01129-0
Phillips L, Christensen-Strynø MB, Frølunde L (2022) Thinking with autoethnography in collaborative research: a critical, reflexive approach to relational ethics. Qual Res 22(5):761–776. https://doi.org/10.1177/14687941211033446
Pohl C (2008) From science to policy through transdisciplinary research. Environ Sci Policy 11(1):46–53. https://doi.org/10.1016/j.envsci.2007.06.001
Pohl C, Krütli P, Stauffacher M (2017) Ten reflective steps for rendering research societally relevant. GAIA-Ecol Perspect Sci Soc 26(1):43–51. https://doi.org/10.14512/gaia.26.1.10
Popa F, Guillermin M, Dedeurwaerdere T (2015) A pragmatist approach to transdisciplinarity in sustainability research: from complex systems theory to reflexive science. Futures 65:45–56. https://doi.org/10.1016/j.futures.2014.02.002
Porter A (2016) Decolonizing policing: indigenous patrols, counter-policing and safety. Theor Criminol 20(4):548–565
Reason P, Torbert W (2001) The action turn: toward a transformational social science. Concepts Transform 6(1):1–37. https://doi.org/10.1075/cat.6.1.02rea
Reed MG, Abernethy P (2018) Facilitating Co-production of transdisciplinary knowledge for sustainability: working with canadian biosphere reserve practitioners. Soc Nat Resour 31(1):39–56. https://doi.org/10.1080/08941920.2017.1383545
Rovelli C (2021) Politics should listen to science, not hide behind it. Nat Mater 20(2):272–272. https://doi.org/10.1038/s41563-020-00891-3
Rowan J (2000) Research ethics. Int J Psychother 5(2):103–110
Saltelli A, Ravetz JR, Funtowicz S (2016) Who will solve the crisis in science? In: Benessia A, Funtowicz S, Giampietro M, Guimaraes Pereira A, Ravetz J, Saltelli A, Strand R, van der Sluijs JP (eds) The rightful place of science: Science on the verge. Consortium for Science, Policy & Outcomes
Savransky M (2017) A decolonial imagination: sociology, anthropology and the politics of reality. Sociology 51(1):11–26
Sayer A (2011) Why things matter to people: Social science, values and ethical life. Cambridge University Press, Cambridge
Schäpke N, Bergmann M, Stelzer F, Lang DJ (2018) Labs in the real world: advancing transdisciplinary research and sustainability transformation: mapping the field and emerging lines of inquiry. Gaia 27:8–11. https://doi.org/10.14512/gaia.27.S1.4
Schneider F, Giger M, Harari N, Moser S, Oberlack C, Providoli I, Schmid L, Tribaldos T, Zimmermann A (2019) Transdisciplinary co-production of knowledge and sustainability transformations: three generic mechanisms of impact generation. Environ Sci Policy 102(October):26–35. https://doi.org/10.1016/j.envsci.2019.08.017
Schneidewind U, Singer-Brodowski M, Augenstein K (2016) Transformative science for sustainability transitions. In: Handbook on sustainability transition and sustainable peace, Springer, pp 123–136
Schut M, Paassen AV, Leeuwis C, Klerkx L (2014) Towards dynamic research configurations: A framework for reflection on the contribution of research to policy and innovation processes. 41(August 2013), 207–218. https://doi.org/10.1093/scipol/sct048
Smith L (2008) Ethical principles in practice. Evidence from participatory action research. Kairaranga 9(3):16–21. https://doi.org/10.54322/kairaranga.v9i3.135
Stilgoe J, Owen R, Macnaghten P (2013) Developing a framework for responsible innovation. Res Policy 42(9):1568–1580. https://doi.org/10.1016/j.respol.2013.05.008
Stirling A (2006) Precaution, foresight and sustainability: Reflection and reflexivity in the governance of science and technology. In: Voß JP, Bauknecht D, Kemp R (eds) Reflexive governance for sustainable development, Edward Elgar Publishing, Cheltenham, pp 225–272
Strumińska-Kutra M, Scholl C (2022) Taking power seriously: towards a power-sensitive approach for transdisciplinary action research. Futures 135(December 2021):1–9. https://doi.org/10.1016/j.futures.2021.102881
Strydom WF, Funke N, Nienaber S, Nortje K, Steyn M (2010) Evidence-based policymaking: a review. South Afr J Sci 106(5/6):8 pages. https://doi.org/10.4102/sajs.v106i5/6.249
Temper L, Del Bene D (2016) Transforming knowledge creation for environmental and epistemic justice. Curr Opin Environ Sustain 20:41–49. https://doi.org/10.1016/j.cosust.2016.05.004
Temper L, McGarry D, Weber L (2019) From academic to political rigour: insights from the ‘Tarot’ of transgressive research. Ecol Econ 164:106379. https://doi.org/10.1016/j.ecolecon.2019.106379
Thomas KA, Warner BP (2019) Weaponizing vulnerability to climate change. Glob Environ Change 57:101928. https://doi.org/10.1016/j.gloenvcha.2019.101928
van Breda J, Swilling M (2019) The guiding logics and principles for designing emergent transdisciplinary research processes: learning experiences and reflections from a transdisciplinary urban case study in Enkanini informal settlement, South Africa. Sustain Sci 14(3):823–841. https://doi.org/10.1007/s11625-018-0606-x
van Steenbergen F (2020) Zonder marge geen centrum. Een pleidooi voor een rechtvaardige transitie. [PhD-Thesis]. Erasmus University Rotterdam
Vermeer J, Pinheiro H, Petrosova L, Eaton D (2020) Evaluation of the Belmont Forum: Final report. Technolopolis Group. https://www.belmontforum.org/wp-content/uploads/2023/09/Belmont-Forum-External-Evaluation.pdf
Vincent K (2022) Development geography I: Co-production. Prog Hum Geogr 46(3):890–897. https://doi.org/10.1177/0309132522107905
WBGU (2011) Flagship report: world in transition—a social contract for sustainability. In: Berlin: German Advisory
West S, Schill C (2022) Negotiating the ethical-political dimensions of research methods: a key competency in mixed methods, inter- and transdisciplinary, and co-production research. Hum Soc Sci Commun 9(1):294. https://doi.org/10.1057/s41599-022-01297-z
Wijsman K, Feagan M (2019) Rethinking knowledge systems for urban resilience: Feminist and decolonial contributions to just transformations. Environ Sci Policy 98:70–76. https://doi.org/10.1016/j.envsci.2019.04.017
Williamson GR, Prosser S (2002) Action research: politics, ethics and participation. J Adv Nurs 40(5):587–593. https://doi.org/10.1046/j.1365-2648.2002.02416.x
Article PubMed Google Scholar
Wilson S (2020) Research is ceremony: Indigenous research methods. Fernwood Publishing. https://fernwoodpublishing.ca/book/research-is-ceremony-shawn-wilson
Wittmayer JM (2016) Transition Management, Action Research and Actor Roles: Understanding local sustainability transitions. Erasmus University Rotterdam
Wittmayer JM, Loorbach D, Bogner K, Hendlin Y, Hölscher K, Lavanga M, Vasques A, von Wirth T, de Wal M (2021) Transformative Research: Knowledge and action for just sustainability transitions (Design Impact Transition Platform Working Paper Series). Design Impact Transition Platform Erasmus University Rotterdam. https://pure.eur.nl/en/publications/transformative-research-knowledge-and-action-for-just-sustainabil
Wittmayer JM, Schäpke N (2014) Action, research and participation: roles of researchers in sustainability transitions. Sustain Sci 9(4):483–496. https://doi.org/10.1007/s11625-014-0258-4
Wood L, Kahts-Kramer S (2023) But how will you ensure the objectivity of the researcher?’ Guidelines to address possible misconceptions about the ethical imperatives of community-based research. Res Ethics 19(1):1–17. https://doi.org/10.1177/17470161221135882
Yanow D (2006) Neither rigorous nor objective? Interrogating criteria for knowledge claims in interpretive science. In: Yanow D, Schwartz-Shea P, Interpretation and Method. Empirical Research Methods and the Interpretive Turn, M.E. Sharpe, pp 67–88
Download references
Author information
Authors and affiliations.
DRIFT, Erasmus University Rotterdam, Rotterdam, The Netherlands
Julia M. Wittmayer, Derk Loorbach & Neha Mungekar
Erasmus School of Social and Behavioural Sciences, Erasmus University Rotterdam, Rotterdam, The Netherlands
Julia M. Wittmayer, Timo von Wirth, Tessa Boumans & Neha Mungekar
Faculty of Education, McGill University, Montreal, Canada
Ying-Syuan (Elaine) Huang
Copernicus Institute of Sustainable Development, Faculty of Geosciences, University of Utrecht, Utrecht, The Netherlands
Kristina Bogner
MaREI Centre for Energy Climate and Marine, University College Cork, Cork, Ireland
Department of Human Geography and Spatial Planning, Faculty of Geosciences, University of Utrecht, Utrecht, The Netherlands
Katharina Hölscher
Research Lab for Urban Transport (ReLUT), Frankfurt University of Applied Sciences, Frankfurt am Main, Germany
Timo von Wirth
Business Management & Organisation Group, Wageningen University, Wageningen, The Netherlands
Jilde Garst
Erasmus School of Philosophy, Erasmus University Rotterdam, Rotterdam, The Netherlands
Yogi Hale Hendlin
Erasmus School of History, Culture and Communication, Erasmus University Rotterdam, Rotterdam, The Netherlands
Mariangela Lavanga
Stellenbosch University, Stellenbosch, South Africa
Mapula Tshangela
Delft Centre for Entrepreneurship, Delft University of Technology, Delft, The Netherlands
Pieter Vandekerckhove
Erasmus University College Erasmus University Rotterdam, Rotterdam, The Netherlands
Ana Vasques
You can also search for this author in PubMed Google Scholar
Contributions
Julia M. Wittmayer and Ying-Syuan Huang drafted the work for important intellectual content, substantially contributed to the concept and design of the work, and contributed to the analysis and interpretation of data for the work. Kristina Bogner, Evan Boyle, Katharina Hölscher, and Timo von Wirth substantially contributed to the concept or design of the work and contributed to the analysis or interpretation of data for the work. Tessa Boumans, Jilde Garst, Yogi Hendlin, Mariangela Lavanga, Derk Loorbach, Neha Mungekar, Mapula Tshangela, Pieter Vandekerckhove, and Ana Vasues contributed to the analysis or interpretation of data for the work.
Corresponding author
Correspondence to Julia M. Wittmayer .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethical approval and Informed consent
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Wittmayer, J.M., Huang, YS.(., Bogner, K. et al. Neither right nor wrong? Ethics of collaboration in transformative research for sustainable futures. Humanit Soc Sci Commun 11 , 677 (2024). https://doi.org/10.1057/s41599-024-03178-z
Download citation
Received : 21 December 2023
Accepted : 13 May 2024
Published : 25 May 2024
DOI : https://doi.org/10.1057/s41599-024-03178-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Log in using your username and password
- Search More Search for this keyword Advanced search
- Latest content
- For authors
- Browse by collection
- BMJ Journals More You are viewing from: Google Indexer
You are here
- Volume 14, Issue 5
- Use of social network analysis in health research: a scoping review protocol
- Article Text
- Article info
- Citation Tools
- Rapid Responses
- Article metrics
- Eshleen Grewal 1 ,
- Jenny Godley 2 , 3 , 4 ,
- Justine Wheeler 5 ,
- http://orcid.org/0000-0001-9008-2289 Karen L Tang 1 , 3 , 4
- 1 Department of Medicine , University of Calgary , Calgary , Alberta , Canada
- 2 Department of Sociology , University of Calgary , Calgary , Alberta , Canada
- 3 Department of Community Health Sciences , University of Calgary , Calgary , Alberta , Canada
- 4 O’Brien Institute for Public Health , University of Calgary , Calgary , Alberta , Canada
- 5 Libraries and Cultural Resources , University of Calgary , Calgary , Alberta , Canada
- Correspondence to Dr Karen L Tang; klktang{at}ucalgary.ca
Introduction Social networks can affect health beliefs, behaviours and outcomes through various mechanisms, including social support, social influence and information diffusion. Social network analysis (SNA), an approach which emerged from the relational perspective in social theory, has been increasingly used in health research. This paper outlines the protocol for a scoping review of literature that uses social network analytical tools to examine the effects of social connections on individual non-communicable disease and health outcomes.
Methods and analysis This scoping review will be guided by Arksey and O’Malley’s framework for conducting scoping reviews. A search of the electronic databases, Ovid Medline, PsycINFO, EMBASE and CINAHL, will be conducted in April 2024 using terms related to SNA. Two reviewers will independently assess the titles and abstracts, then the full text, of identified studies to determine whether they meet inclusion criteria. Studies that use SNA as a tool to examine the effects of social networks on individual physical health, mental health, well-being, health behaviours, healthcare utilisation, or health-related engagement, knowledge, or trust will be included. Studies examining communicable disease prevention, transmission or outcomes will be excluded. Two reviewers will extract data from the included studies. Data will be presented in tables and figures, along with a narrative synthesis.
Ethics and dissemination This scoping review will synthesise data from articles published in peer-reviewed journals. The results of this review will map the ways in which SNA has been used in non-communicable disease health research. It will identify areas of health research where SNA has been heavily used and where future systematic reviews may be needed, as well as areas of opportunity where SNA remains a lesser-used method in exploring the relationship between social connections and health outcomes.
- Protocols & guidelines
- Social Interaction
- Social Support
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ .
https://doi.org/10.1136/bmjopen-2023-078872
Statistics from Altmetric.com
Request permissions.
If you wish to reuse any or all of this article please use the link below which will take you to the Copyright Clearance Center’s RightsLink service. You will be able to get a quick price and instant permission to reuse the content in many different ways.
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is a novel scoping review that fills an important gap—how and where social network analysis (SNA) (as a data collection and analytical tool) has been used in health research has not been systematically documented despite its increasing use in the discipline.
The breadth of the scoping review allows for a comprehensive mapping of the use of SNA to examine social connections and non-communicable disease and health outcomes, without limiting to any one population group or setting.
The use of the Arksey and O’Malley framework as well as the Levac et al recommendations to guide our scoping review will ensure that a rigorous and transparent process is undertaken.
Due to the scope of the review and the large volume of anticipated studies, only published articles in the English language will be included.
Introduction
Social connections are known to influence health. 1 People with many supportive social connections tend to be healthier and live longer than people who have fewer supportive social connections, while social isolation, or the absence of supportive social connections, is associated with the deterioration of physical and psychological health, and even death. 2–5 These associations hold even when accounting for socioeconomic status and health practices. 6 Additionally, having a low quantity of supportive social connections is associated with the development or worsening of medical conditions, such as atherosclerosis, hypertension, cardiovascular disease and cancer, potentially through chronic inflammation and changes to autonomic regulation and immune responses. 7–13 Unsupportive social connections can also have adverse effects on health due to emotional stress, which can then lead to poor health habits, psychological distress and negative physiological responses (eg, increased heart rate and blood pressure), all of which are detrimental to health over time. 14 The health of individuals is therefore connected to the people around them. 15
Social networks can influence health via five pathways. 15 16 First, networks can provide social support, to meet the needs of the individual. Dyadic relationships can provide informational, instrumental (ie, aid and assistance with tangible needs), appraisal (ie, help with decision-making) and/or emotional support; this support can be enhanced or hindered by the overall network structure. 17 In addition to the tangible aid and resources that are provided, social support—either perceived or actual—also has direct effects on mental health, well-being and feelings of self-efficacy. 18–20 Social support may also act as a buffer to stress. 16 19 The second pathway by which social networks influence health, and in particular health behaviours such as alcohol and cigarette use, physical activity, food intake patterns and healthcare utilisation, is through social influence. 16 21 That is, the attitudes and behaviour of individuals are guided and altered in response to other network members. 22 23 Social influence is difficult to disentangle from social selection from an empirical standpoint. That is, similarities in behaviour may be due to influences within a network, or alternatively, they may reflect the known phenomenon where individuals tend to form close connections with others who are like them. 22 24 The third pathway is through the promotion of social engagement and participation. Individuals derive a sense of identity, value and meaning through the roles they play (eg, parental roles, community roles, professional roles, etc) in their networks, and the opportunities for participation in social contexts. 16 The fourth pathway by which networks affect health is through transmission of communicable diseases through person-to-person contact. Finally, social networks overlap, resulting in differential access to resources and opportunities (eg, finances, information and jobs). 15 16 An individual’s structural position can result in differential health outcomes, similar to the inequities that stem from differences in social status. 16
There has been an explosion of literature in the area of social networks and health. In their bibliometric analysis, Chapman et al found that the number of studies that examine social networks and health has sextupled since 2000. 25 Similarly, the value of grants and contracts in this topic area, as awarded by the National Science Foundation and the National Institutes of Health, has increased 10-fold. 25 A turning point in the field was the HIV epidemic, where there was an urgent need to better understand its spread. 25 The exponential rise in the number of studies since then that examine social networks and health appears to reflect a widespread understanding that an individual’s health cannot be isolated from his or her social networks and context. There is, however, significant heterogeneity in what aspect of, and how, social networks are being studied. For example, many health research studies use proxies for social connectedness such as marital status or living alone status (as these variables tend to be commonly included in health surveys), without considering the quality of those social connections, and without further exploring the broader social network and their characteristics. 16 26 These proxy measures do little to describe the structure, quantity, quality or characteristics of social connections within which individuals are embedded. Another common approach in health research is to focus on social support measures and their effects on health. Individuals are asked about perceived, or received, social support (for example, through questions that ask about the availability of people who provide emotional support, informational support and/or assistance with daily tasks, with either binary or a Likert scale of responses). 27 28 While important, social support measures do not assess the structure of social networks and represent only one of many different mechanisms by which social networks influence health. 17 23
Social network analysis
Social network analysis (SNA) is a methodological tool, developed in the 1930s by social psychologists, used to study the structure and characteristics of the social networks within which individuals are embedded. 16 29 It has evolved over the past 100 years and has been used by researchers in many social science disciplines to analyse how structures of relationships impact social life. 29 30 SNA has the following key properties 3 30 31 : (1) it relies on empirical relational data (ie, data on actors (nodes) and the connections (ties) between them); (2) it uses mathematical models and graph theory to examine the structure of relationships within which individual actors are embedded; and (3) it models social action at both the group and the individual level arising from the opportunities and constraints determined by the system of relationships. The premise of SNA is that social ties are both drivers and consequences of human behaviour, and are therefore the object of study. 15 16 23 32 Social networks are comprised of nodes, representing the members within a network, connected by ties, representing relations among those individuals. 33 There are two types of SNA: egocentric network analysis and whole network analysis. Egocentric network analysis describes the characteristics of an individual’s (ie, the ‘egos’) personal network, while whole network analysis examines the structure of relationships among all the individuals in a bounded group, such as a school or classroom. 3
In egocentric network analysis, a list of ‘alters’ (ie, nodes) to whom the ego is connected, is obtained through a name generator. Name generator questions ask for a list of alters based on role relations (eg, friends or family), affect (eg, people to whom the ego feels close), interaction (eg, people with whom the ego has been in contact) or exchange (eg, people who provide social and/or financial support). 34 These are followed by name interpreters, where the ego is asked questions about the characteristics of each named alter. 35 Analyses of these data involve constructing measures that describe these egocentric networks. Such measures include network size, network density (ie, how tightly knit the network is), the strength of relationships (ie, the intensity and duration of relationships between ego and alter), network function (ie, the resources and/or support provided through the network) and the diversity of relations within the network (‘heterogeneity’). 23 36 In whole network analysis, the network boundary is determined a priori and network members are known, for example, through membership lists or rosters. 37 Each network member is surveyed, to identify the other network members with whom they are connected and/or affiliated; attributes of each member are obtained through surveying the network members themselves. Variables are constructed at the individual and network levels. Individual-level measures include the number of ties to other network members (‘degree’), types of relationships, and the strength and diversity of relationships. Network-level measures include but are not limited to: density (representing how tightly knit or ‘glued’ together the network is), reciprocity (ie, the proportion of network ties that are reciprocated), isolates (ie, nodes with no ties to other network members), centralisation (or the extent to which the network ties are focused on one node or a set of nodes), cliques and equivalence (ie, sets of nodes that have the same pattern of ties and therefore occupy the same position in the network). 33 38 The constructed measures can then be included in statistical models to explore associations between individual and/or network-level measures, and outcomes. 33 39
Study rationale
In medicine and health research, there has traditionally been a dichotomy between the individual and the context in which the individual is situated—such as in their relationships with others. 40 As such, epidemiology of diseases has historically focused on individual-level traditional risk and protective factors—such as biological markers, genetics, lifestyle and health behaviours, and psychological conditions. 41 While criticisms of this individualistic focus abound, attempts to develop and use different approaches in medicine and research have lagged behind. 42 The use and adoption of methods, like SNA, that frame issues of health and wellness differently, has the potential to offer new insights and solutions to clinical and healthcare delivery problems, 42 by more holistically considering ‘different levels of change’ beyond the individual. 41 We seek to examine the extent to which SNA has transcended the boundaries of its disciplines of origin in the social sciences, into health research. For example, while Chapman et al have clearly shown an explosion of publications at this intersection, 25 it remains unclear whether these studies use SNA tools (which were developed specifically to interrogate the nature and characteristics of social networks), or whether they suffer from the known problem of conflation of constructs like social support, social capital and social integration. 15 43 Many studies that report the impact of ‘social networks’ on health outcomes do not use SNA methods but rather use self-reported network size (without probing the network and its structure), 44 45 social support, 46 marital status 47 48 and/or household members 47 as proxies.
We will therefore undertake a scoping review to map the use of SNA as a data collection and analytical method in health research. More specifically, the scoping review will examine how SNA has been used to study associations across social networks and individual health and well-being (including both physical and psychological health), health knowledge, health engagement, health service use and health behaviours. Scoping reviews are a knowledge synthesis approach that aims to uncover the volume, range, reach and coverage of a body of literature on a specific topic. 49 They differ from systematic reviews, another type of knowledge synthesis, in their objectives. Systematic reviews seek to answer clinical or epidemiological questions and are conducted to fill gaps in knowledge. 50 Systematic reviews are used to establish the effectiveness of an intervention or associations between specific exposures and outcomes. On the other hand, scoping reviews do not seek to provide an answer to a question, but rather, aim to create a map of the existing literature. 49 They are used to provide clarity to the concepts and definitions used in literature, examine the way in which research is conducted in a specific field or on a specific topic, and uncover knowledge gaps. 49 A scoping review, therefore, is well suited as a research method to address our research question, of mapping the ways in which SNA has been used in health research. This scoping review can identify areas (eg, specific populations and specific health outcomes) where there has been a plethora of SNA research warranting future systematic reviews. It can also identify areas within health research where the use of SNA is scarce, highlighting topics, populations or outcomes for future study.
This scoping review will be limited to studies that use SNA in exploring network components and their associations with non-communicable diseases and health and well-being outcomes, for three reasons. The first is feasibility, given the large volume of studies anticipated, based on Chapman et al ’s bibliometric study on this topic. 25 Second, the use of SNA in understanding disease transmission of communicable diseases (such as sexually transmitted infections) is well established; its application to HIV was in fact one of the catalysts, as previously mentioned, to its broader uptake in health research. 25 Third, SNA in health research has shifted from focusing on communicable diseases to focusing on non-communicable diseases and their risk factors; SNA is now being applied much more frequently to the latter conditions than the former ones. 51
Methods and analysis
The scoping review will be informed by the framework developed by Arksey and O’Malley 52 for conducting scoping reviews, as well as the additional recommendations made by Levac et al . 53 Arksey and O’Malley’s framework recommends that the review process be organised into the following five steps: identifying the research question; identifying relevant studies; study selection; charting the data; and collating, summarising and reporting the results. 52 The reporting of this review will adhere to the Preferred Reporting Items for Systematic reviews and Meta-Analyses extension for Scoping Reviews. 54
Patient and public involvement
No patients will be involved.
Step 1: identifying the research question
A preliminary search of the literature identified a gap related to SNA and how it has been used to study the relationship between social networks and individual well-being and health outcomes. This led to the development of the research question that will guide this scoping review: how have social network analytical tools been used to study the associations between social networks and individual patient health? In this case, SNA is defined as a data analysis technique that uses either an egocentric or whole network analysis approach. For egocentric network analysis, we will include studies that involve peer nomination (ie, use of a name generator) and the collection of one or more characteristics of alters (ie, use of name interpreter(s)).
Step 2: identifying relevant studies
A search strategy will be constructed through consultation with an academic librarian (JW). The main concepts from the research question will be used for a preliminary search in Google Scholar. Additionally, the lead authors will provide the librarian with key studies that will be text-mined for relevant terms. These key studies will include a variety of populations (across different countries and age groups) and health outcomes. 55–58 Key studies will be searched in Ovid MEDLINE for appropriate subject headings. In consultation with team members, the librarian (JW) will construct a pilot search strategy. A title/abstract/keyword search will be conducted in Ovid MEDLINE against the known seed/key studies. Table 1 lists example keywords and terms relating to social networks that will be used, with the full search strategy detailed in online supplemental appendix A .
Supplemental material
- View inline
Search terms relating to social network analysis
Due to a significant number of irrelevant articles surrounding communicable diseases using this search strategy, we will exclude records with these terms in either the title or keyword fields. Table 2 lists the terms related to communicable diseases.
Search terms relating to communicable diseases
Of note, the search strategy will not include terms that relate to health-related outcomes of interest (outside of excluding communicable diseases). Prior literature has shown that the inclusion of outcome concepts in a search strategy reduces the recall and sensitivity of a search strategy. 59 60 This problem is further exacerbated when only generic health terms (for example, ‘morbidity’ or ‘health status’) or specific health terms (eg, specific diseases or conditions such as ‘diabetes mellitus’) are used. 61 Because the objective of this scoping review is to examine and map the use of SNA in health research, the outcomes of interest are very broad, including: physical health and well-being, psychological health and well-being, healthcare engagement, health knowledge, health behaviours, healthcare access and use, disease prevalence and outcomes (spanning every organ system), and mortality. It will be impossible for a search strategy to be sufficiently comprehensive, to capture all possible generic and specific terms relating to this broad range of outcomes. In keeping with recommendations to minimise the number of elements in a search strategy 62 —and in particular outcome elements 63 —our search strategy will entail searching for SNA terms in health databases without specifying health outcomes.
The search strategy will first be created in Medline (Ovid), then translated and adapted for the databases: (1) EMBASE (Ovid), (2) APA PsycInfo (Ovid) and (3) CINAHL (EBSCO). A search will be completed in April 2024. No date filters will be applied to the search. However, animal-only studies will be excluded. The current version of the search strategy including limits and filters, for all databases, is included in online supplemental appendix A .
Step 3: study selection
The criteria that will be used to determine which studies to include are as follows:
Studies that employ SNA as a data collection and/or analysis technique, as defined above. Of note, studies that elicit only the number of friends or other social contacts, without collection of any information about these social contacts, are not considered to be SNA and are therefore not included in the scoping review.
Studies that explore the social networks of individuals in whom the health outcome is measured.
Studies must include the exploration of non-communicable health outcomes. Examples include self-rated health or other global measures of health (including measures of physical health, mental health and well-being), health practices (eg, physical activity, dietary patterns, smoking, alcohol use, substance use), sexual and reproductive health, healthcare-seeking behaviours (eg, medication adherence, acute care use, attachment to a primary care provider), health knowledge, health beliefs, healthcare engagement, non-communicable disease prevalence and mortality.
The criteria that will be used to exclude studies are as follows:
Studies that explore the social networks of organisations or healthcare providers, rather than the social networks of the individual about whom the health outcome is measured or reported.
Studies that describe or use data analysis techniques other than SNA (eg, using proxies for social networks/social support that do not include peer nomination (such as marital status or living alone status), or studies where study participants report the number of social contacts but where no other information about each social contact is collected).
Studies that focus exclusively on online social networks (eg, social media, online forums, online support groups).
Studies related to prevention, transmission or outcomes of communicable diseases.
Non-English studies, for feasibility purposes.
We will not limit studies based on the study population or country in which the SNA is conducted. Studies in paediatric and adult populations will be included. The reasons for excluding SNA studies that focus solely on social media and online networks are twofold. First, we anticipate a very large number of articles, given the broad populations and outcomes of interest, and for feasibility purposes, we have needed to narrow the research objective to in-person and/or offline social networks only. Second, there are likely inherent differences in online and offline social networks. Individuals use health-related social networking sites and online networks primarily for information seeking, connection with others who share a similar lived experience while being able to maintain some emotional distance and interacting with health professionals 64 ; this differs from in-person networks, which individuals go to more for emotional and tangible or instrumental support. Friends met on online networks vary from friends met in person in other important ways. They tend to have less similarity in terms of age, gender and place of residence, 65 and the network ties more commonly arise spontaneously—that is, without common acquaintances or affiliations. 66 The social patterns and interactions among individuals and their online network contacts are also different—with entire relationships built on text-based interactions. 66 Therefore, while online social networks are an important area of study, they appear to be inherently different from the study of offline social networks, and are therefore excluded from this scoping review.
For the first step of the screening process, after removing duplicate articles, two reviewers will independently assess the titles and abstracts of the studies to determine whether they meet the inclusion criteria. Any studies that do not meet the inclusion criteria will be excluded from the review. Studies that either one of the two reviewers feels are potentially relevant will be included in the full-text review, to ensure that no article is prematurely excluded at this stage. During the second step of the screening process, two reviewers will independently review the full texts of the studies to ensure they meet the inclusion criteria. Conflicts will be resolved by third and fourth reviewers with expertise in SNA (JG) and health outcomes (KLT). The number of studies included in each step of the screening process will be reported using the Preferred Reporting Items for Systematic reviews and Meta-Analyses diagram. 67
Step 4: charting the data
A data charting document ( online supplemental appendix B ) will be created to extract data from the studies in the review. This document will include information about the authors, year of publication, study location, study population characteristics, outcomes of interest to this scoping review, and the scales and measures used for each outcome. Data about the social network analytical method will also be extracted, including whether studies used egocentric versus whole networks, the name generator used (in egocentric network studies) or the relationship being explored, the maximum number of peer nominations allowed, the lookback period used, whether (and which) alter attributes were collected, and whether alter-to-alter tie data were collected. Data extraction will be performed by at least one reviewer, with a second reviewer separately checking and confirming the inputted data. Disagreements in data extraction will be resolved through a consensus, and through the input of reviewers with content and methods expertise (KLT, JG).
Step 5: collating, summarising and reporting results
The results of the review will be presented in the form of figures and tables and will include descriptive numerical summaries. The numerical summary will include information about the number of studies included in the review, where the studies were conducted, when they were published and characteristics of the populations, such as the sample sizes and mean age. It will also include characteristics of the SNA conducted in these studies, including the number that are whole network studies versus egocentric network studies, the data sources used and the attributes of the social connections that are collected and analysed. Results will be synthesised in text, as well as through tables and figures.
Ethics and dissemination
This review does not require ethics approval. Data will be extracted from published material. Once the scoping review is complete, an article will be written to convey the findings of this review, and it will be submitted for publication in a peer-reviewed journal. We anticipate the results of this review will map out the ways in which SNA has been used in health research. Specifically, this scoping review will identify areas of potential saturation where SNA has been heavily used, opportunities for future systematic reviews (where there is a large body of primary research studies requiring synthesis) and health research gaps (eg, the health outcomes where SNA has been minimally used). The scoping review will also shed light on characteristics of SNA that have been used (eg, whether egocentric networks vs whole networks are used and in what settings, and whether a broad range of social network characteristics are captured and analysed), which will serve to inform the conduct of future SNA studies in health research.
Ethics statements
Patient consent for publication.
Not applicable.
- Schaefer DR
- Christiansen J ,
- Qualter P ,
- Friis K , et al
- Leong D , et al
- Umberson D ,
- Glymour MM ,
- Everson-Rose SA ,
- Robles TF ,
- Kiecolt-Glaser JK
- Kiecolt-Glaser JK ,
- McGuire L ,
- Robles TF , et al
- O’Brien E , et al
- Shattuck EC
- Christakis NA
- Berkman LF ,
- Brissette I , et al
- McFarlane AH ,
- Bellissimo A ,
- Rueger SY ,
- Malecki CK ,
- Pyun Y , et al
- Siciliano MD
- de la Haye K ,
- Barnett LM , et al
- Murray JM ,
- Sánchez-Franco SC ,
- Sarmiento OL , et al
- Pescosolido BA ,
- Borgatti SP
- Chapman A ,
- Verdery AM ,
- Holt-Lunstad J ,
- Gjesfjeld CD ,
- Greeno CG ,
- Poston WS , et al
- Fredericks KA ,
- Carrington P
- Crossley N ,
- Bellotti E ,
- Edwards G , et al
- Wasserman S ,
- Burgette JM ,
- Rankine J ,
- Culyba AJ , et al
- Kirkengen AL ,
- Ekeland T-J ,
- Getz L , et al
- Pescosolido BA
- Lucivero F , et al
- Vettore MV ,
- Ahmad SFH ,
- Machuca C , et al
- De Gagne JC
- Palmer Kelly E ,
- García EL ,
- Banegas JR ,
- Pérez-Regadera AG , et al
- Hempler NF ,
- Joensen LE ,
- Peters MDJ ,
- Stern C , et al
- Higgins JPT ,
- Chandler J , et al.
- Valente TW ,
- Colquhoun H ,
- Tricco AC ,
- Zarin W , et al
- Christakis NA ,
- Mohr P , et al
- O’Malley AJ ,
- Arbesman S ,
- Steiger DM , et al
- Watkins SC ,
- Jato MN , et al
- Frandsen TF ,
- Nielsen MFB ,
- Bruun Nielsen MF ,
- Lindhardt CL , et al
- Maclean A ,
- Sweeting H , et al
- Bramer WM ,
- de Jonge GB ,
- Rethlefsen ML , et al
- Lefebvre C ,
- Glanville J ,
- Briscoe S , et al
- Colineau N ,
- Doerfel ML ,
- Shamseer L ,
- Clarke M , et al
Supplementary materials
Supplementary data.
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
- Data supplement 1
- Data supplement 2
Contributors KLT and JG conceived of the study protocol. KLT, JG, EG and JW developed and revised the study protocol, the search strategy and the inclusion/exclusion criteria. EG and KLT drafted the protocol manuscript, and all authors provided critical revisions.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Read the full text or download the PDF:
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Research ethics.
Jennifer M. Barrow ; Grace D. Brannan ; Paras B. Khandhar .
Affiliations
Last Update: September 18, 2022 .
- Introduction
Multiple examples of unethical research studies conducted in the past throughout the world have cast a significant historical shadow on research involving human subjects. Examples include the Tuskegee Syphilis Study from 1932 to 1972, Nazi medical experimentation in the 1930s and 1940s, and research conducted at the Willowbrook State School in the 1950s and 1960s. [1] As the aftermath of these practices, wherein uninformed and unaware patients were exposed to disease or subject to other unproven treatments, became known, the need for rules governing the design and implementation of human-subject research protocols became very evident.
The first such ethical code for research was the Nuremberg Code, arising in the aftermath of Nazi research atrocities brought to light in the post-World War II Nuremberg Trials. [1] This set of international research standards sought to prevent gross research misconduct and abuse of vulnerable and unwitting research subjects by establishing specific human subject protective factors. A direct descendant of this code was drafted in 1978 in the United States, known as the Belmont Report, and this legislation forms the backbone of regulation of clinical research in the USA since its adoption. [2] The Belmont Report contains 3 basic ethical principles:
- Respect for persons
- Beneficence
Additionally, the Belmont Report details research-based protective applications for informed consent, risk/benefit assessment, and participant selection. [3]
- Issues of Concern
The first protective principle stemming from the 1978 Belmont Report is the principle of Respect for Persons, also known as human dignity. [2] This dictates researchers must work to protect research participants' autonomy while also ensuring full disclosure of factors surrounding the study, including potential harms and benefits. According to the Belmont Report, "an autonomous person is an individual capable of deliberation about personal goals and acting under the direction of such deliberation." [1]
To ensure participants have the autonomous right to self-determination, researchers must ensure that potential participants understand that they have the right to decide whether or not to participate in research studies voluntarily and that declining to participate in any research does not affect in any way their access to current or subsequent care. Also, self-determined participants must be able to ask the researcher questions and comprehend the questions asked by the researcher. Researchers must also inform participants that they may stop participating in the study without fear of penalty. [4] As noted in the Belmont Report definition above, not all individuals can be autonomous concerning research participation. Whether because of the individual's developmental level or because of various illnesses or disabilities, some individuals require special research protections that may involve exclusion from research activities that can cause potential harm or appointing a third-party guardian to oversee the participation of such vulnerable persons. [5]
Researchers must also ensure they do not coerce potential participants into agreeing to participate in studies. Coercion refers to threats of penalty, whether implied or explicit, if participants decline to participate or opt out of a study. Additionally, giving potential participants extreme rewards for agreeing to participate can be a form of coercion. The rewards may provide an enticing enough incentive that the participant feels they need to participate. In contrast, they would otherwise have declined if such a reward were not offered. While researchers often use various rewards and incentives in studies, they must carefully review this possibility of coercion. Some incentives may pressure potential participants into joining a study, thereby stripping participants of complete self-determination. [3]
An additional aspect of respecting potential participants' self-determination is to ensure that researchers have fully disclosed information about the study and explained the voluntary nature of participation (including the right to refuse without repercussion) and possible benefits and risks related to study participation. A potential participant cannot make a truly informed decision without complete information. This aspect of the Belmont Report can be troublesome for some researchers based on their study designs and research questions. Noted biases related to reactivity may occur when study participants know the exact guiding research questions and purposes. Some researchers may avoid reactivity biases using covert data collection methods or masking critical study information. Masking frequently occurs in pharmaceutical trials with placebos because knowledge of placebo receipt can affect study outcomes. However, masking and concealed data collection methods may not fully respect participants' rights to autonomy and the associated informed consent process. Any researcher considering hidden data collection or masking of some research information from participants must present their plans to an Institutional Review Board (IRB) for oversight, as well as explain the potential masking to prospective patients in the consent process (ie, explaining to potential participants in a medication trial that they are randomly assigned either the medication or a placebo). The IRB determines if studies warrant concealed data collection or masking methods in light of the research design, methods, and study-specific protections. [6]
The second Belmont Report principle is the principle of beneficence. Beneficence refers to acting in such a way to benefit others while promoting their welfare and safety. [7] Although not explicitly mentioned by name, the biomedical ethical principle of nonmaleficence (not harm) also appears within the Belmont Report's section on beneficence. The beneficence principle includes 2 specific research aspects:
- Participants' right to freedom from harm and discomfort
- Participants' rights to protection from exploitation [8]
Before seeking IRB approval and conducting a study, researchers must analyze potential risks and benefits to research participants. Examples of possible participant risks include physical harm, loss of privacy, unforeseen side effects, emotional distress or embarrassment, monetary costs, physical discomfort, and loss of time. Possible benefits include access to a potentially valuable intervention, increased understanding of a medical condition, and satisfaction with helping others with similar issues. [8] These potential risks and benefits should explicitly appear in the written informed consent document used in the study. Researchers must implement specific protections to minimize discomfort and harm to align with the principle of beneficence. Under the principle of beneficence, researchers must also protect participants from exploitation. Any information provided by participants through their study involvement must be protected.
The final principle contained in the Belmont Report is the principle of justice, which pertains to participants' right to fair treatment and right to privacy. The selection of the types of participants desired for a research study should be guided by research questions and requirements not to exclude any group and to be as representative of the overall target population as possible. Researchers and IRBs must scrutinize the selection of research participants to determine whether researchers are systematically selecting some groups (eg, participants receiving public financial assistance, specific ethnic and racial minorities, or institutionalized) because of their vulnerability or ease of access. The right to fair treatment also relates to researchers treating those who refuse to participate in a study fairly without prejudice. [3]
The right to privacy also falls under the Belmont Report's principle of justice. Researchers must keep any shared information in their strictest confidence. Upholding the right to privacy often involves procedures for anonymity or confidentiality. For participants' data to be completely anonymous, the researcher cannot have the ability to connect the participants to their data. The study is no longer anonymous if researchers can make participant-data connections, even if they use codes or pseudonyms instead of personal identifiers. Instead, researchers are providing participant confidentiality. Various methods can help researchers assure confidentiality, including locking any participant identifying data and substituting code numbers instead of names, with a correlation key available only to a safety or oversight functionary in an emergency but not readily available to researchers. [3]
- Clinical Significance
One of the most common safeguards for the ethical conduct of research involves using external reviewers, such as an Institutional Review Board (IRB). Researchers seeking to begin a study must submit a full research proposal to the IRB, which includes specific data collection instruments, research advertisements, and informed consent documentation. The IRB may perform a complete or expedited review depending on the nature of the study and the risks involved. Researchers cannot contact potential participants or start collecting data until they obtain full IRB approval. Sometimes, multi-site studies require approvals from several IRBs, which may have different forms and review processes. [3]
A significant study aspect of interest to IRB members is using participants from vulnerable groups. Vulnerable groups may include individuals who cannot give fully informed consent or those individuals who may be at elevated risk of unplanned side effects. Examples of vulnerable participants include pregnant women, children younger than the age of consent, terminally ill individuals, institutionalized individuals, and those with mental or emotional disabilities. In the case of minors, assent is also an element that must be addressed per Subpart D of the Code of Federal Regulations, 45 CFR 46.402, which defines consent as "a child's affirmative agreement to participate in research; mere failure to object should not, absent affirmative agreement, be construed as assent." [9] There is a lack in the literature on when minors can understand research, although current research suggests that the age by which a minor could assent is around 14. [10] Anytime researchers include vulnerable groups in their studies, they must have extra safeguards to uphold the Belmont Report's ethical principles, especially beneficence. [3]
- Enhancing Healthcare Team Outcomes
Research ethics is a foundational principle of modern medical research across all disciplines. The overarching body, the IRB, is intentionally comprised of experts across various disciplines, including ethicists, social workers, physicians, nurses, other scientific researchers, counselors, mental health professionals, and advocates for vulnerable subjects. There is also often a legal expert on the panel or available to discuss any questions regarding the legality or ramifications of studies.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Disclosure: Jennifer Barrow declares no relevant financial relationships with ineligible companies.
Disclosure: Grace Brannan declares no relevant financial relationships with ineligible companies.
Disclosure: Paras Khandhar declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Barrow JM, Brannan GD, Khandhar PB. Research Ethics. [Updated 2022 Sep 18]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- The historical, ethical, and legal background of human-subjects research. [Respir Care. 2008] The historical, ethical, and legal background of human-subjects research. Rice TW. Respir Care. 2008 Oct; 53(10):1325-9.
- The Belmont Report at 40: Reckoning With Time. [Am J Public Health. 2018] The Belmont Report at 40: Reckoning With Time. Adashi EY, Walters LB, Menikoff JA. Am J Public Health. 2018 Oct; 108(10):1345-1348. Epub 2018 Aug 23.
- Informed consent in human experimentation before the Nuremberg code. [BMJ. 1996] Informed consent in human experimentation before the Nuremberg code. Vollmann J, Winau R. BMJ. 1996 Dec 7; 313(7070):1445-9.
- Review The History of Human Subjects Research and Rationale for Institutional Review Board Oversight. [Nutr Clin Pract. 2021] Review The History of Human Subjects Research and Rationale for Institutional Review Board Oversight. Spellecy R, Busse K. Nutr Clin Pract. 2021 Jun; 36(3):560-567. Epub 2021 Jan 13.
- Review Ethical issues in research. [Best Pract Res Clin Obstet Gyn...] Review Ethical issues in research. Artal R, Rubenfeld S. Best Pract Res Clin Obstet Gynaecol. 2017 Aug; 43:107-114. Epub 2017 Jan 23.
Recent Activity
- Research Ethics - StatPearls Research Ethics - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers
UC researchers continue to blaze new trails in science, medicine, business, education, engineering and the arts — literally transforming the way we live, work and learn.
Findings is the Office of Research’s newsletter, which began in October 2017. It delivers monthly updates to faculty, staff, students, and community partners about impactful research, initiatives, partnerships, events, and opportunities at the University of Cincinnati. To view current and previous issues of the newsletter, go to the Findings page .

A Year in Review: Society & Culture
This past academic year has been a groundbreaking period for our Society & Culture (S&C) vertical, as we strive to expand our understanding of the human experience through the non-STEM disciplines. Focusing on the Arts, Humanities, and Social Sciences (AHSS) has led to several exciting developments that highlight our commitment to advancing research and fostering collaboration.
Next Lives Here Urban Futures Pathway: Society & Culture Initiative
The Society & Culture (S&C) initiative is dedicated to expanding our understanding of the human experience through the non-STEM disciplines. Focusing on the Arts, Humanities, and Social Sciences (AHSS), the S&C seeks to advance UC’s research reputation globally while also ensuring our institution is making a difference in the Greater Cincinnati region.
Pioneering Partnerships and Research Initiatives

he past academic year has been marked by several important additions to the S&C vertical, including the introduction of the Urban Futures Pathway Fellowship. The inaugural fellow, Dr. Todd Foley, has embarked on a 12-month research project in partnership with Digital Futures’ in-house nonprofit partner Adopt A Class (AAC). Dr. Foley’s work involves analyzing almost 20 years of AAC data to provide actionable insights, with the goal of scaling up AAC’s efforts through the ‘ResultsOHIO’ funding mechanism. This innovative project not only aims to enhance the impact of AAC, but also works as a proof of concept for expansion to other nonprofits.
Dr. Foley’s enthusiasm for the fellowship underscores the potential for broader applications: “If this project works out as we believe it will, we can then think about how we can apply our approach and partnership model to other nonprofits, starting with the backbone organizations co-located in the Digital Futures building.”
Under Dr. Foley’s guidance, UC research students have also begun a multi-year research study for AAC, aiming to optimize AAC’s use of Salesforce, implementing new strategies to improve efficiencies and elevate the program to new heights. This collaborative effort exemplifies the hands-on, impactful research opportunities available within the S&C vertical.
At our end of year event, Research + Innovation Week 2024, we held a panel discussion about AAC and Dr. Foley’s partnership. In it, expert panelists discuss how AAC effects the wider community and how Digital Futures has had a hand in that. Check out the recorded discussion here !
Launching the Society & Culture Research Fellowship Program
Capping off the year, Vice President for Research Pat Limbach announced the Society & Culture Research Fellowship program at the annual State of Research during Research + Innovation Week. This initiative matches external funding received by faculty in AHSS fields from the National Endowments for the Arts and National Endowment for the Humanities (NEA and NEH) . These matching funds allow our faculty to expand upon their federally sponsored activities while also providing opportunities to receive important course relief to dedicate themselves more fully towards the goals of their awards.
Anne Steinert

College of Arts & Sciences
Assistant Professor
NEH Award: Avondale Neighborhood History Initiative
Matching Funds: $30,000
It’s an opportunity to discover and celebrate the history of this community. The grant covers creation of a team—a corps of local history researchers who will be recruited from the Avondale community.
Hyesun Jeong

College of Design, Architecture, Art, and Planning
School of Planning
NEA Award: The Socio-Economic Impacts of Murals on Pedestrian Activities and Local Businesses: A Comparative Study of US Metropolitan Areas
Matching Funds: $20,000
This NEA award provides an opportunity for not only studying the socioeconomic impact of the arts but also creating a meaningful platform where I can work with the arts organizations, architects, planners and policymakers to draw local and national impacts through joining research and design.
Announcing the Society & Culture Research Advancement Awardees
The Office of Research uses the Society & Culture Research Advancement Award to incentivize novel research, exceptional scholarship, and the production of creative and performing art works that creatively address issues of increasing societal significance. The program follows a two-stage, LOI and finalist round process and is open to UC faculty whose proposed activities fall within the areas of the arts, humanities, and social sciences. Congratulations to the following awardees!

School of Architecture and Interior Design
Master Plan: Soft Power and the Rise of Architectural Expertise
The book project leverages archival materials to contextualize master plans not only within the oeuvres of their respective architects, but also within the evolution of their commissioning institutions or overseeing agencies—groups that have had an outsized impact on society through civic engagement and infrastructural transformation. In so doing, the book project tracks the production process and deduces the guiding principles of these master plans during their creative inception and, in some cases, their ultimate execution. While acting in a professional capacity, architects may have performed as unwitting agents in the Cold War era’s political and economic machinations, even if their personal beliefs misaligned with the prevailing ideology. We hope the readers of the book will be able to consider anew how the ambitions of master plans intersect with larger issues of race, equity, and inclusion at times of political and economic instability and uncertainty, in both the past and present.
Eunjee Kwon

Lindner College of Business
Department of Finance, Real Estate, and Insurance and Risk Management
Effectiveness of Housing Voucher Program: Evidence from Small Area Fair Market Rent
This research on the Housing Choice Voucher (HCV) Program and its implementation of Small Area Fair Market Rents (SAFMRs) addresses critical issues under the "Sustainable Cities and Communities" goal of the United Nations Sustainable Development Goals. By investigating how SAFMR policy influences housing choices and poverty exposure among low-income families, our project aims to foster more inclusive and equitable urban communities. We anticipate that this research will provide actionable insights to refine housing policies, ultimately promoting safer, more resilient, and sustainable urban environments where diverse populations can thrive.
John Leverso

College of Education, Criminal Justice, and Human Services
School of Criminal Justice
The Cincinnati Youth Survey
This research can help us understand the impact of social media on youth. Recently, scholars have called social media a catalyst for youth violence, while the U.S. Surgeon General has expressed concerns about the effects of social media on youth mental health. This project will allow for a more dynamic understanding of the associations between mental health, youth violence, and social media use among youth.
Gary Painter

Preventing homelessness and eviction
While we understand that low income, vulnerable populations are most likely to experience adverse housing events like eviction or homelessness, less is know about how to target scarce resources to prevent adverse housing events. This study will develop a methodology to identify the highest risk households and to co-develop and test an intervention to prevent adverse housing events.
Announcing the UC Coalition for Change Awardees
The UC Coalition for Change (C3) grant program (formerly the UC Community Change Collaborative Equitable Cities opportunity) is a funding opportunity from the Office of Research that builds upon previous Next Lives Here investments in the Urban Futures Pathway. Through this opportunity, the Office of Research seeks to incentivize novel, impactful research, exceptional scholarship, and the production of dynamic creative and performing artworks that address issues of increasing societal significance.
This grant program was open to all UC faculty whose proposed research and activities sought to address the most pressing issues faced by the people in and around Cincinnati and those in communities beyond our region. Applicants submitted a letter of interest that detailed the problem they aim to solve, their approach, and their impact. Then, finalists were invited to submit full proposals and pitch their ideas at a pitch event in competition for up to four $25,000 grants.
Congratulations to the 2023 UC Coalition for Change grantee!
Priyanka Gudsoorkar

College of Medicine
Department of Environmental & Public Health Sciences
Integrating Tradition and Innovation: Transforming Health in Rural Tanzania
This work will foster profound societal impacts by elevating rural Tanzanian communities’ oral and overall health, targeting vulnerable communities, and focusing on pediatric and maternal populations. Our comprehensive approach is designed to alleviate dental and periodontal diseases, advance women’s health, and address systemic health issues like hypertension and diabetes, also acknowledged as sustainable development goals (SDGs) health targets. This holistic health improvement will enhance the quality of life, create cultural shifts in health perception, diminish social stigma, and augment disease management, leading to robust community health and cultural shifts towards preventive health practices aligning with SDG3 for health and well-being.
Ayane Kozasa

College-Conservatory of Music
Creative Chamber Music Album & Tour with Owls
Owls' debut album includes works by a diverse group of composers (many of them living) who all have a special voice and have made a significant impact on music composition. Owls itself is a creative collection of musicians who are expanding the boundaries of what a classical chamber ensemble can produce by way of arranging, programming, and performing. Owls' mission is to show audiences and young musicians/aspiring artists the limitless possibilities of chamber music, and our debut album will bring these fresh ideas to a wider community.
Vikram Ravindra

College of Engineering and Applied Sciences
Department of Computer Science
An Investigation into NPO Leadership
This project works with Nonprofit Organizations (NPOs) that focus on broadening participation in STEM-degree programs. I will actively collaborate with underrepresented communities to ensure equitable access and opportunity in STEM education and career pathways. This project includes a 6-week nonprofit board leadership training program with Leadership Council to be an effective leader in NPOs.
- < Back to News Back to News
Want to stay up to date with the latest research news?
Subscribe to the Office of Research Newsletter

IMAGES
VIDEO
COMMENTS
Where research ethics review takes place under the auspices of an academic institution, the institutions must typically take responsibility to adequately support the functioning of their Boards and promote a positive culture of research ethics [3, 5]. Supporting the financial and human resource costs of participating in ongoing education (e.g ...
From the academic hallways to the literature, characterizations of REBs and the research ethics review process are seldom complimentary. While numerous criticisms have been levelled, it is the time to decision that is most consistently maligned [6,7,8,9,10,11].Factors associated with lengthy review time include incomplete or poorly completed applications [7, 12, 13], lack of administrative ...
Research Ethics is aimed at all readers and authors interested in ethical issues in the conduct of research, the regulation of research, the procedures and process of ethical review as well as broader ethical issues related to research such as scientific … | View full journal description. This journal is a member of the Committee on ...
The aim of this integrative review was to analyze and synthetize ethical dilemmas that occur during the progress of qualitative investigation and the strategies proposed to face them. The search for studies used LILACS and MEDLINE databases with descriptors "research ethics" and "qualitative research", originating 108 titles. Upon ...
The new WHO publication "Standards and operational guidance for ethics review of health-related research with human participants", is a compilation of 10 standards that are applicable to the ethics review of health related research with human participants. This document is intended to provide guidance on the research ethics review process, not to take a substantive position on how ...
Research ethics review thus remains an area ripe for investigation. 18.5 Conclusion . In this chapter, I have argued that RECs have become regulatory entities in their own right, governed by - depending on the jurisdiction - institutions, central regulatory agencies, administrative staff and offices, standardised forms and communications ...
It is important to adhere to ethical principles in order to protect the dignity, rights and welfare of research participants. As such, all research involving human beings should be reviewed by an ethics committee to ensure that the appropriate ethical standards are being upheld. Discussion of the ethical principles of beneficence, justice and ...
The UK Health Research Authority has established partnerships with others involved in research review. 20 For example, research ethics committees often (re)-review the study design and statistical issues. 21 Such review should be done once, by those with training and experience in statistics and design. The Research Ethics Committee should ask ...
The implementation of ethics review processes is an important first step for anticipating and mitigating the potential harms of AI research. Its long-term success, however, requires a coordinated ...
Research ethics review committees (ERCs) worldwide faced daunting challenges during the COVID-19 pandemic. There was a need to balance rapid turnaround with rigorous evaluation of high-risk research protocols in the context of considerable uncertainty. This study explored the experiences and performance of ERCs during the pandemic. We conducted an anonymous, cross-sectional, global online ...
Ethics review is the process of assessing the ethics of research involving humans. The Ethics Review Committee (ERC) is the key oversight mechanism designated to ensure ethics review. Whether or not this governance mechanism is still fit for purpose in the data-driven research context remains a debated issue among research ethics experts. In this article, we seek to address this issue in a ...
The Research Ethics Review Committee (ERC) is a 27-member committee established and appointed by the Director-General. Its mandate is to ensure WHO only supports research of the highest ethical standards. The ERC reviews all research projects, involving human participants supported either financially or technically by WHO.
Elisa Yule. There is a growing body of literature on the research ethics review process, a process that can have important effects on the nature of research in contemporary times. Yet, many people know little about what the actual process entails once an application has been submitted for review. This lack of knowledge can affect researchers ...
Research ethics are a set of principles that guide your research designs and practices in both quantitative and qualitative research. In this article, you will learn about the types and examples of ethical considerations in research, such as informed consent, confidentiality, and avoiding plagiarism. You will also find out how to apply ethical principles to your own research projects with ...
It is time for institutional review boards and research ethics committees to address the ethics of inclusion. The disproportionate impact of COVID-19 on certain populations, such as Black, Latinx, and Indigenous populations in the United States, has focused attention on inequalities in health and on the need to increase enrollment of racial and ...
Colnerud G. Ethical dilemmas in research in relation to ethical review: An empirical study. Research Ethics. 2015; 10 (4):238-253. doi: 10.1177/1747016114552339. [Google Scholar] Davison J. Dilemmas in Research: Issues of Vulnerability and Disempowerment for the Social Workers/Researcher. Journal of Social Work Practice. ...
Our framework for research ethics helps you to consider ethics issues during the complete lifecycle of a project and includes information and guidelines on good research conduct and governance. ... Ethics review application forms and protocols; This is the website for UKRI: our seven research councils, Research England and Innovate UK. ...
Ethical considerations of conducting systematic reviews in educational research are not typically discussed explicitly. As an illustration, 'ethics' is not listed as a term in the index of the second edition of 'An Introduction to Systematic Reviews' (Gough et al. 2017).This chapter draws from my earlier in-depth discussion of this topic in the Qualitative Research Journal (Suri 2008 ...
When most people think of ethics (or morals), they think of rules for distinguishing between right and wrong, such as the Golden Rule ("Do unto others as you would have them do unto you"), a code of professional conduct like the Hippocratic Oath ("First of all, do no harm"), a religious creed like the Ten Commandments ("Thou Shalt not kill..."), or a wise aphorisms like the sayings of Confucius.
Committee members. Chair Peter Olumese. Co-Chair Antonella Lavelanet. Co-Chair Melba Gomes. Co-Chair Leslie Olson. Its mandate is to ensure WHO only supports research of the highest ethical standards. The ERC reviews all research projects, involving human participants supported either financially or technically by WHO.
The NeurIPS 2023 Ethic Review process began with the publication of the Code of Ethics. This step formally codified a foundation for ethics within the conference framework. The Ethics Guidelines for Reviewers were also updated to reflect incorporation of the Code of Ethics . This year, 502 papers (3.77% of all submissions) were flagged for ...
The role of ethical review is to ensure that ethical standards in research are met. In Australia this process is governed by the National Statement on the Ethical Conduct of Research Involving Humans (National Health and Medical Research Council, 2007 (revised 2015)).The National Statement (as it is called) provides both guidelines on ethical research conduct for those designing and conducting ...
This paper presents a model based on stakeholder responsibilities in the process of research ethics review and illustrates how each makes contributions to the time an application spends in this process. This model focusses on REBs operating under the auspices of academic institutions, typical in Canada and the USA.
Before delving into the local considerations, it's essential to understand what an sIRB is and why it's used in research. An sIRB is a single ethics review board assuming responsibility for reviewing research protocols, and ensuring ongoing IRB oversight, across multiple institutions or sites engaged in human subjects research.
Most formal research ethics reviews adopt the four principles of Beauchamp and Childress , which include: (1) non-maleficence by attempting to not harm others; (2) respect for autonomy by ...
The Code of Ethics aims to guide the NeurIPS community towards higher standards of ethical conduct as it pertains to elements of research ethics and the broader societal and environmental impact of research submitted to NeurIPS. It outlines conference expectations about the ethical practices that must be adopted by the submitting authors ...
Ethics and dissemination This scoping review will synthesise data from articles published in peer-reviewed journals. The results of this review will map the ways in which SNA has been used in non-communicable disease health research. It will identify areas of health research where SNA has been heavily used and where future systematic reviews may be needed, as well as areas of opportunity where ...
Public Administration Review is the premier journal for public administration research, theory, and practice, publishing articles and book reviews on a wide range of topics American Society for Public Administration Code of Ethics - 2024 - Public Administration Review - Wiley Online Library
Multiple examples of unethical research studies conducted in the past throughout the world have cast a significant historical shadow on research involving human subjects. Examples include the Tuskegee Syphilis Study from 1932 to 1972, Nazi medical experimentation in the 1930s and 1940s, and research conducted at the Willowbrook State School in the 1950s and 1960s.[1] As the aftermath of these ...
A Year in Review: Society & Culture. Findings is the Office of Research's newsletter, which began in October 2017. It delivers monthly updates to faculty, staff, students, and community partners about impactful research, initiatives, partnerships, events, and opportunities at the University of Cincinnati. To view current and previous issues ...