Report Description
Table of content, methodology.
- Medical Devices & Supplies
- Medical Device Contract Research Organization Market Share & Growth [2031]

Medical Device Contract Research Organization Market
Segments - by Phase (Preclinical and Clinical), Device Type (Diagnostics Devices, MedTech Devices, Handheld Devices, and Others), Service (Laboratory, Project Management/Clinical Supply Management, Clinical Monitoring, Data Management, Regulatory/Medical Affairs, Quality Management/Assurance, Medical Writing, Patient & Site Recruitment, Bio-statistics, Technology, Investigator Payments, and Others), and Region (Asia Pacific, North America, Latin America, Europe, and Middle East & Africa) - Global Industry Analysis, Growth, Share, Size, Trends, and Forecast 2023 – 2031
Raksha Sharma
Fact-checked by :
Vineet Pandey
Vishal Golekar
Medical Device Contract Research Organization Market Outlook 2031
The global medical device contract research organization market size was USD 7.15 Billion in 2022 and is likely to reach USD 15.40 Billion by 2031 , expanding at a CAGR of 8.9% during 2023–2031 . The market growth is attributed to the speedy development and cost-saving benefits facilitated by contract research organizations. Advanced medical devices are becoming key components in the healthcare industry, which drives their demand across diagnostics and treatments. Healthcare device manufacturers, across the globe are working with/collaborating with/partnering with with contract research organizations (CROs) specializing in medical devices, to speed up the development and trials of innovative devices. Growing partnerships/collaborations with CROs are allowing the commercialization ofnew medical devices at a fast pace while saving costs on various procedures. These factors are projected to propel the market during the forecast period.
In June 2023, Vial, a major global tech-focused CRO offering next-generation healthcare trial management services, announced a strategic partnership with Royale International Group , one of the leading independent logistics providers in the Asia Pacific. Royale has agreed to provide temperature-controlled logistics solutions to Vial for clinical trials.
Development of medical devices is increasing with advancements in technology. New companies are venturing into the healthcare manufacturing space with growing opportunities and demand. Along with this, the existing healthcare manufacturers are coming up with different innovative ideas from demand analysis, to improve the convenience of patients and healthcare professionals during healthcare procedures. Medical device CROs are likely to play a crucial role in various stages of development of these solutions, which increases opportunities in the market. The research report finds that the COVID-19 pandemic propelled the medical device CRO market. The tremendous growth in patient load due to infection and other subsequent healthcare complications gave rise to various medical device demands. The need for innovative devices for checking infection, and measuring temperature, and oxygen levels grew significantly. As the CROs have the necessary equipment and mechanisms to streamline the development and trial of new medical solutions, their demand rose high during the period.
Impact of Artificial Intelligence (AI) on Medical Device Contract Research Organization Market
AI is projected to fuel the growth of the market during the assessment period. Advances in this technology are supporting the optimization of various stages in medical device development. AI is expected to enhance the clinical trial efficacy for CROs. Leveraging AI analysis of vast data sets allows the elimination of errors in the early stages of medical trials. AI implementation at CRO is benefitting researchers and biostatisticians in saving time and speeding up trial design procedures. In addition, AI-powered solutions are likely to aid in screening, recruiting, and retaining patients for medical trials. Such diverse and evolving applications of AI in healthcare CROs are expected to benefit the market.
In September 2023, Belong.life, a patient engagement and communities & care healthtech platform, launched its new AI trial matching assistant Tara for cancer patients. It is offered as a Software as a Service (SaaS) for health systems, hospitals, and CROs. This platform uses machine learning (ML) and natural language processing (NLP) to connect patients to potentially life-saving treatments.
Medical Device Contract Research Organization Market Dynamics
Major drivers.
Growing demand for advanced medical devices is one of the key drivers of the market. These devices are used in diverse healthcare procedures from band aging of injured ankle to the diagnosis of various diseases. A medical device CRO specializes in providing contract-based services of clinical trial, data management, and development. Hiring of these services enables the medical device industry to launch their products for commercial use speedily, without compromising on quality standards and compliance.
According to the latest information published by the World Health Organization (WHO), it is estimated that worldwide there are two million medical devices for different purposes. These devices are categorized in the 7000 plus generic devices group. As medical devices are advancing the quality of healthcare, their demand is expected to grow all over the world in the coming years.
Time & cost-saving advantages facilitated by medical device CROs are projected to propel the market. New devices have to go through complex trials, regulatory procedures, and audits, which keep on consuming time and other resources until they meet expectations.Having these tasks carried out by a CRO possessing detailed expertise, aids in eliminating redundant procedures, allowing to save time and costs.
Existing Restraints
Lack of skilled professionals and challenges in patient recruiting & retention for clinical trials are likely to restrain the medical device contract research organization market. Since medical trials need to meet the regulatory standards setin different nations, the requirement for experienced clinical research associates is consistent in this sector. However, the availability of such skilled personnel does not meet the demand, which creates complications in understanding therapeutic areas and other complex concepts. Finding and retaining patients suitable for medical device trials is another major challenge, owing to protocol complexities and a lack of willingness from individuals to continue for a longer period.
Emerging Opportunities
Growing investment in healthcare research and development activities from government and private players is expected to create significant opportunities in the market. The rise in the application of medical devices in hospital and home care settings has boosted their demands all over the world. There is a surging use of disposable medical devices and supplies in hospitals and clinics, as they enable the prevention of hospital-acquired infections. CROs are integrating new-age technologies such as real world evidence (RWE), cloud computing , and advanced data analytics & risk monitoring tools, which is further fueling the growth opportunities for the players in the market.
Scope of the Medical Device Contract Research Organization Market Report
The global research report includes an assessment of the market trends, market segments, and regional markets. Overview and dynamics have also been included in the report.
Market Segment Insights
Phase analysis.
Based on phase , the medical device contract research organization market is bifurcated into preclinical and clinical.The clinical segment held the major share in 2022 and is expected to expand at a robust growth rate during the projection period,owing to the growing development of advanced medical devices. The rise in innovations of new devices with advancing technologies such as AI, is leading to a high number of medical devices entering in clinical phase.The focus by manufacturers in healthcare on cutting expenses associated with research and development stages further supports the growth of the segment.
In August 2023 , Venus Medtech (Hangzhou) Inc. , a major provider in China of integrated solutions for transcatheter structural heart valvular therapies, announced the Investigational Device Exemption full approval for its in-house developed VenusP-Valve from the Food and Drug Administration (FDA) in the US. This device has become the first China-made heart valvular system to be approved by the FDA for clinical trials, which is significant for the expansion of the global footprint of Chinese valves.
The preclinical segment is projected to grow at a rapid pace in the coming years, owing to complexities and varying government regulations involved in approval processes across the world. Growing innovations in the medical field are further amplifying the number of preclinical trials. The support of CROs allows the conversion of concepts into actual products speedily. This enables an opportunity to commercialize innovative medical devices faster than the conventional approaches.
Device Type Analysis
On the basis of device type , the global market is segregated into diagnostics devices, MedTech devices, hand held devices, and others.The diagnostics devices segment is projected to witness the fastest growth during the forecast period, owing to the increasing patient load on the global healthcare system. The medical devices purposed for diagnosis aid in speedy diagnosis, and are simple & safe to use.
Diagnostics devices help in measuring and observing different aspects of a patient’s health, this, in turn, allows quick diagnosis and decision-making. The prevalence of chronic diseases such as diabetes, cancer, and Asthma coupled with rising concerns about infectious diseases including COVID-19 has propelled the need for innovative diagnostics devices. CROs are providing crucial resources and support mechanisms to medical manufacturers, in stream lining device development, trials, and approval procedures for diagnostic medical devices.
Service Analysis
On the basis of service , the medical device contract research organization market is classified as laboratory, project management/clinical supply management, clinical monitoring, data management, regulatory/medical affairs, quality management/assurance, medical writing, patient & site recruitment, bio-statistics, technology, investigator payments, and others. The clinical monitoring segment is expected to hold a large revenue share in the market during the forecast period, due to the rise in demand for monitoring with increasing clinical trials. The integration of smart AI analytics and real world evidence is projected to enhance clinical trial monitoring procedures, providing more precise results. The expert team available with medical device CROs further improves the outcomes of the monitoring procedures.
Regional Outlook
In terms of region, the global medical device contract research organization market is classified as Asia Pacific, North America, Latin America, Europe, and Middle East & Africa.Asia Pacific is anticipated to dominate the market during the projection period,due to rising opportunities of growth in medical innovation space in this region.Emerging economies in the region including India, China, and Japan are becoming innovation hubs, owing to cost-saving advantages, enhanced regulatory standards, and higher availability of skilled professionals.The rising number of medical device CROs further supports the growth of the market in this region.
According to the recently published report, Asia is a hot spot for manufacturers of medical devices in terms of new opportunities, investments, sales, and expansion. The Medical Device industry in the region is anticipated to surpass USD 112 Billion in 2023 . The growth of the industry is at a significantly high CAGR in comparison to other regions, owing to the presence of a high population, rising healthcare spending, and supportive government policies.
The market in North America is expected to grow at a rapid pace, owing to an increasing number of medical innovations in the region, leading to a rise in the requirement for clinical trials. The increase in investment by government and private players along with the presence of well-established medical device CROs further boost the market in this region.
The global Medical Device Contract Research Organization Market has been segmented on the basis of
- Preclinical
- Diagnostics Devices
- MedTech Devices
- hand held Devices
- Project Management/Clinical Supply Management
- Clinical Monitoring
- Data Management
- Regulatory/Medical Affairs
- Quality Management/Assurance
- Medical Writing
- Patient & Site Recruitment
- Bio-statistics
- Investigator Payments
- Asia Pacific
- North America
- Latin America
- Middle East & Africa
Key Players
- IQVIA, Inc.
- Charles River Laboratories
- Syneos Health
- Laboratory Corporation of America Holdings
- Eurofins Scientific
- Promedica International
Competitive Landscape
Key players competing in the medical device contract research organization market are IQVIA, Inc.; Qserve; ICON, plc; Charles River Laboratories; Syneos Health; WuXiAppTec; Laboratory Corporation of America Holdings; Eurofins Scientific; Medpace; Promedica International; and Others. These companies adopted development strategies including mergers, acquisitions, partnerships, collaboration, product launches, and production expansion to expand their consumer base worldwide. The competitive landscape covers key insights into growth strategies adopted by major market players.
- In September 2023 , Syneos Health , a leading player in the industry and provider of integrated biopharmaceutical products, announced a strategic collaboration that expands its relationship with Oracle. The company is to use Oracle’s Cerner Learning Health Network and Oracle Suite of study startups to reduce the time taken for patient recruiting in clinical trials. This solution is also aimed at improving the participation of patients from diverse backgrounds in medical research.
- In January 2023 , Charles River Laboratories announced a partnership with Rznomics, a Biopharma firm based in South Korea, for the development and manufacturing of gene therapy viral vectors for the treatment of liver cancer. This deal centers on Rznomics’ RZ-001, the first ribozyme-based RNA reprogramming method that received FDA authorization for evaluation in patients.
Purchase Premium Report
- Single User $4200
- Multi User $5200
- Corporate User $6600
- Online License $2999
- Excel Data Pack $2599
Customize This Report
- Ask for Research To Be Focused On Specific Regions or Segments
- Receive Data As Per Your Format and Definition
- Companies Profiled based on Your Requirements
- Breaking Down Competitive Landscape as per Your Requirements
- Any Level of Customization
Our Clients

We needed a highly accurate and precise report, which Growth Market Reports delivered promptly. The company compiled information from a wide array of reliable agencies and sourcess.It is extremely satisfactory to be working with you. Strategy Head of Major Tech Company
We were very pleased to contact Growth Market Reports as they tailored reports precisely as per our requirements. As we are dealing with the aerospace and defense industry, we need reports of high accuracy and substantial quality. Major Player in Defense Industry
Extremely delighted to have a well-crafted report on “Global Packaging Solutions Market Research Report” from your team. Thank you for providing me with all our requirements and for incorporating our suggestions. CMO of Leading Packaging Company from USA
I had a good experience working with Growth Market Reports as they were very open to all constructive changes in the report. I found that the report had its charm embedded with ample of data. Founder and Managing Partner of Major Korean Company
Our company has been working with Growth Market Reports for some years now and we are very happy with the quality of the reports provided by the company.I, on behalf of my organization, would like to thank you for offering professional reports. Global Consulting Firm
Quick Contact
+1 909 414 1393
Growth Market Report
Certified By
Related Reports
Some other reports from this category!
Vaccines Storage Equipment Market Size, Share, Analysis 2030
Nitinol based medical device market size, share & trend 2031, orthopedic screws market size, opportunities, industry 2031, scoliosis braces market size, industry trends report | 2031, color contact lenses market size, share & growth report 2031, eecea mea healthcare personal protective equipment market.

Laryngeal Mask Airway Market Size, Share & Trend Report 2030
Healthcare personal protective equipment (ppe) market | 2031, lens monomer market size, share, trends & growth report 2030, leukocyte reduction filter market size, share, analysis 2031.
- Request For Sample

Medical Device Contract Research Organization Market (By Phase: Preclinical, Clinical; By Service: Project Management/Clinical Supply Management, Data Management, Regulatory/Medical Affairs, Medical Writing, Clinical Monitoring, Quality Management/Assurance, Bio-statistics, Investigator Payments, Laboratory, Patient & Site Recruitment, Technology) - Global Industry Analysis, Size, Share, Growth, Trends, Revenue, Regional Outlook 20212030
- Report Description
- Table of Content
- Request Customization
- Research Methodology
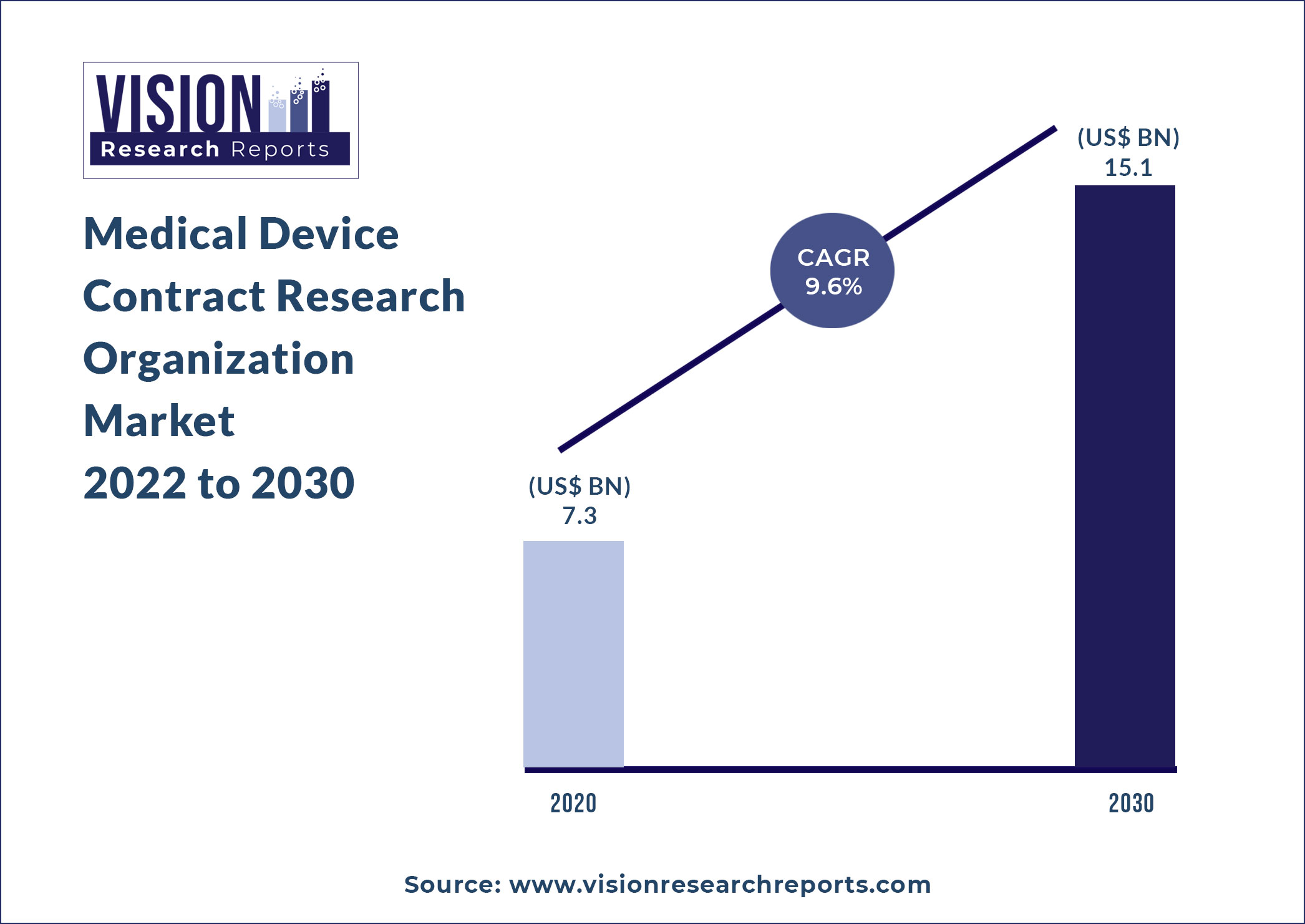
The global Medical Device Contract Research Organization market size is expected to be worth around US$ 15.1 billion by 2030, according to a new report by Vision Research Reports.
The global Medical Device Contract Research Organization market size was valued at US$ 7.3 billion in 2020 and is anticipated to grow at a CAGR of 9.6% during forecast period 2021 to 2030.
Report Coverage
Growth Factors
During the second half of the COVID-19 pandemic in 2020, the market witnessed a surge in demand for CRO services as compared to the first half, where the growth was stagnant. The main driver of the market includes time-saving, cost efficiency, and expertise in the area, which accelerates the process of devices reaching the market. In addition, outsourcing to a CRO with precise expertise in a medical device helps in meeting the complex regulatory requirements and audits as they work on it on a daily basis.
They also have access to the most advanced technological resources, such as all the latest and most advanced hardware, software, and internet-based applications, to make the process fast and maintain quality.
By Service Analysis
The clinical monitoring segment led the global market in 2020 accounting for a revenue share of 21.1% in 2020. The segment is anticipated to maintain its position over the forecast period owing to a large number of clinical trials performed and, to study them, increasing demand for clinical trial monitoring.
On the basis of services, the market is segmented into project management/clinical supply management, data management, regulatory/medical affairs, medical writing, clinical monitoring, quality management/assurance, bio-statistics, investigator payments, laboratory, patient & site recruitment, technology, and others.
By Phase Analysis
On the basis of phase, the market has been bifurcated into clinical and preclinical. The clinical segment dominated the market and accounted for the largest revenue share of more than 88% in 2020.
The preclinical segment is projected to register the fastest growth rate of 10.0% during the forecast period. An increase in the number of preclinical trials involving large molecules and the growing need to curb R&D expense is expected to contribute to the segment growth.
By Regional Analysis
Asia Pacific dominated the market accounting for the largest revenue share of 41.1% in 2020. This growth is due to several medical research organizations’ investments in developing the capability to analyze medical devices and technology.
In North America, the market for healthcare CROs held a substantial share in 2020 owing to the highest number of trials undertaken and outsourced in the region. This large number can be attributed to the presence of well-established CROs in the region and high investments by pharmaceutical companies.
Key Players
Charles River Laboratories
Syneos Health
Promedica International
Wuxi AppTec
Qserve Group
Market Segmentation
Preclinical
Project Management/Clinical Supply Management
Data Management
Regulatory/Medical Affairs
Medical Writing
Clinical Monitoring
Quality Management/Assurance
Bio-statistics
Investigator Payments
Patient & Site Recruitment
North America
Asia Pacific
South Korea
Latin America
Middle East & Africa
South Africa
Saudi Arabia
The Medical Device Contract Research Organization market research report covers definition, classification, product classification, product application, development trend, product technology, competitive landscape, industrial chain structure, industry overview, national policy and planning analysis of the industry, the latest dynamic analysis, etc., and also includes major. The study includes drivers and restraints of the global market. It covers the impact of these drivers and restraints on the demand during the forecast period. The report also highlights opportunities in the market at the global level.
The report provides size (in terms of volume and value) of Medical Device Contract Research Organization market for the base year 2020 and the forecast between 2021 and 2030. Market numbers have been estimated based on form and application. Market size and forecast for each application segment have been provided for the global and regional market.
This report focuses on the global Medical Device Contract Research Organization market status, future forecast, growth opportunity, key market and key players. The study objectives are to present the Medical Device Contract Research Organization market development in United States, Europe and China.
It is pertinent to consider that in a volatile global economy, we haven’t just conducted Medical Device Contract Research Organization market forecasts in terms of CAGR, but also studied the market based on key parameters, including Year-on-Year (Y-o-Y) growth, to comprehend the certainty of the market and to find and present the lucrative opportunities in market.
In terms of production side, this report researches the Medical Device Contract Research Organization capacity, production, value, ex-factory price, growth rate, market share for major manufacturers, regions (or countries) and type.
In terms of consumption side, this report focuses on the consumption of Medical Device Contract Research Organization by regions (countries) and application.
Buyers of the report will have access to verified market figures, including global market size in terms of revenue and volume. As part of production analysis, the authors of the report have provided reliable estimations and calculations for global revenue and volume by Type segment of the global Medical Device Contract Research Organization market. These figures have been provided in terms of both revenue and volume for the period 2017 to 2030. Additionally, the report provides accurate figures for production by region in terms of revenue as well as volume for the same period. The report also includes production capacity statistics for the same period.
With regard to production bases and technologies, the research in this report covers the production time, base distribution, technical parameters, research and development trends, technology sources, and sources of raw materials of major Medical Device Contract Research Organization market companies.
Regarding the analysis of the industry chain, the research of this report covers the raw materials and equipment of Medical Device Contract Research Organization market upstream, downstream customers, marketing channels, industry development trends and investment strategy recommendations. The more specific analysis also includes the main application areas of market and consumption, major regions and Consumption, major Chinese producers, distributors, raw material suppliers, equipment providers and their contact information, industry chain relationship analysis.
The research in this report also includes product parameters, production process, cost structure, and data information classified by region, technology and application. Finally, the paper model new project SWOT analysis and investment feasibility study of the case model.
Overall, this is an in-depth research report specifically for the Medical Device Contract Research Organization industry. The research center uses an objective and fair way to conduct an in-depth analysis of the development trend of the industry, providing support and evidence for customer competition analysis, development planning, and investment decision-making. In the course of operation, the project has received support and assistance from technicians and marketing personnel in various links of the industry chain.
Medical Device Contract Research Organization market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies’ focus related to Medical Device Contract Research Organization market.
Prominent players in the market are predicted to face tough competition from the new entrants. However, some of the key players are targeting to acquire the startup companies in order to maintain their dominance in the global market. For a detailed analysis of key companies, their strengths, weaknesses, threats, and opportunities are measured in the report by using industry-standard tools such as the SWOT analysis. Regional coverage of key companies is covered in the report to measure their dominance. Key manufacturers of Medical Device Contract Research Organization market are focusing on introducing new products to meet the needs of the patrons. The feasibility of new products is also measured by using industry-standard tools.
Key companies are increasing their investments in research and development activities for the discovery of new products. There has also been a rise in the government funding for the introduction of new Medical Device Contract Research Organization market. These factors have benefited the growth of the global market for Medical Device Contract Research Organization. Going forward, key companies are predicted to benefit from the new product launches and the adoption of technological advancements. Technical advancements have benefited many industries and the global industry is not an exception.
New product launches and the expansion of already existing business are predicted to benefit the key players in maintaining their dominance in the global market for Medical Device Contract Research Organization. The global market is segmented on the basis of region, application, en-users and product type. Based on region, the market is divided into North America, Europe, Asia-Pacific, Latin America and Middle East and Africa (MEA).
In this study, the years considered to estimate the market size of Medical Device Contract Research Organization are as follows:
- History Year: 2017-2020
- Base Year: 2021
- Forecast Year 2021 to 2030
Reasons to Purchase this Report:
- Market segmentation analysis including qualitative and quantitative research incorporating the impact of economic and policy aspects - Regional and country level analysis integrating the demand and supply forces that are influencing the growth of the market. - Market value USD Million and volume Units Million data for each segment and sub-segment - Competitive landscape involving the market share of major players, along with the new projects and strategies adopted by players in the past five years - Comprehensive company profiles covering the product offerings, key financial information, recent developments, SWOT analysis, and strategies employed by the major market players
Research Methodology:
In-depth interviews and discussions were conducted with several key market participants and opinion leaders to compile the research report.
This research study involved the extensive usage of both primary and secondary data sources. The research process involved the study of various factors affecting the industry, including the government policy, market environment, competitive landscape, historical data, present trends in the market, technological innovation, upcoming technologies and the technical progress in related industry, and market risks, opportunities, market barriers and challenges. The following illustrative figure shows the market research methodology applied in this report.
The study objectives of this report are:
- To analyze and study the global market capacity, production, value, consumption, status (2017-2020) and forecast (2021-2030);
- Focuses on the key manufacturers, to study the capacity, production, value, market share and development plans in future.
- Comprehensive company profiles covering the product offerings, key financial information, recent developments, SWOT analysis, and strategies employed by the major market players
- To define, describe and forecast the market by type, application and region.
- To analyze the global and key regions market potential and advantage, opportunity and challenge, restraints and risks.
- To identify significant trends and factors driving or inhibiting the market growth.
- To analyze the opportunities in the market for stakeholders by identifying the high growth segments.
- To strategically analyze each submarket with respect to individual growth trend and their contribution to the market
- To analyze competitive developments such as expansions, agreements, new product launches, and acquisitions in the market
- To strategically profile the key players and comprehensively analyze their growth strategies.
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Medical Device Contract Research Organization Market, By Phase
7.1. Medical Device Contract Research Organization Market, by Phase, 2021-2030
7.1.1. Preclinical
7.1.1.1. Market Revenue and Forecast (2017-2030)
7.1.2. Clinical
7.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 8. Global Medical Device Contract Research Organization Market, By Service
8.1. Medical Device Contract Research Organization Market, by Service, 2021-2030
8.1.1. Project Management/Clinical Supply Management
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Data Management
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Regulatory/Medical Affairs
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Medical Writing
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Clinical Monitoring
8.1.5.1. Market Revenue and Forecast (2017-2030)
8.1.6. Quality Management/Assurance
8.1.6.1. Market Revenue and Forecast (2017-2030)
8.1.7. Bio-statistics
8.1.7.1. Market Revenue and Forecast (2017-2030)
8.1.8. Investigator Payments
8.1.8.1. Market Revenue and Forecast (2017-2030)
8.1.9. Laboratory
8.1.9.1. Market Revenue and Forecast (2017-2030)
8.1.10. Patient & Site Recruitment
8.1.10.1. Market Revenue and Forecast (2017-2030)
8.1.11. Technology
8.1.11.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Medical Device Contract Research Organization Market , Regional Estimates and Trend Forecast
9.1. North America
9.1.1. Market Revenue and Forecast, by Phase (2017-2030)
9.1.2. Market Revenue and Forecast, by Service (2017-2030)
9.1.3. U.S.
9.1.3.1. Market Revenue and Forecast, by Phase (2017-2030)
9.1.3.2. Market Revenue and Forecast, by Service (2017-2030)
9.1.4. Rest of North America
9.1.4.1. Market Revenue and Forecast, by Phase (2017-2030)
9.1.4.2. Market Revenue and Forecast, by Service (2017-2030)
9.2. Europe
9.2.1. Market Revenue and Forecast, by Phase (2017-2030)
9.2.2. Market Revenue and Forecast, by Service (2017-2030)
9.2.3. UK
9.2.3.1. Market Revenue and Forecast, by Phase (2017-2030)
9.2.3.2. Market Revenue and Forecast, by Service (2017-2030)
9.2.4. Germany
9.2.4.1. Market Revenue and Forecast, by Phase (2017-2030)
9.2.4.2. Market Revenue and Forecast, by Service (2017-2030)
9.2.5. France
9.2.5.1. Market Revenue and Forecast, by Phase (2017-2030)
9.2.5.2. Market Revenue and Forecast, by Service (2017-2030)
9.2.6. Rest of Europe
9.2.6.1. Market Revenue and Forecast, by Phase (2017-2030)
9.2.6.2. Market Revenue and Forecast, by Service (2017-2030)
9.3. APAC
9.3.1. Market Revenue and Forecast, by Phase (2017-2030)
9.3.2. Market Revenue and Forecast, by Service (2017-2030)
9.3.3. India
9.3.3.1. Market Revenue and Forecast, by Phase (2017-2030)
9.3.3.2. Market Revenue and Forecast, by Service (2017-2030)
9.3.4. China
9.3.4.1. Market Revenue and Forecast, by Phase (2017-2030)
9.3.4.2. Market Revenue and Forecast, by Service (2017-2030)
9.3.5. Japan
9.3.5.1. Market Revenue and Forecast, by Phase (2017-2030)
9.3.5.2. Market Revenue and Forecast, by Service (2017-2030)
9.3.6. Rest of APAC
9.3.6.1. Market Revenue and Forecast, by Phase (2017-2030)
9.3.6.2. Market Revenue and Forecast, by Service (2017-2030)
9.4. MEA
9.4.1. Market Revenue and Forecast, by Phase (2017-2030)
9.4.2. Market Revenue and Forecast, by Service (2017-2030)
9.4.3. GCC
9.4.3.1. Market Revenue and Forecast, by Phase (2017-2030)
9.4.3.2. Market Revenue and Forecast, by Service (2017-2030)
9.4.4. North Africa
9.4.4.1. Market Revenue and Forecast, by Phase (2017-2030)
9.4.4.2. Market Revenue and Forecast, by Service (2017-2030)
9.4.5. South Africa
9.4.5.1. Market Revenue and Forecast, by Phase (2017-2030)
9.4.5.2. Market Revenue and Forecast, by Service (2017-2030)
9.4.6. Rest of MEA
9.4.6.1. Market Revenue and Forecast, by Phase (2017-2030)
9.4.6.2. Market Revenue and Forecast, by Service (2017-2030)
9.5. Latin America
9.5.1. Market Revenue and Forecast, by Phase (2017-2030)
9.5.2. Market Revenue and Forecast, by Service (2017-2030)
9.5.3. Brazil
9.5.3.1. Market Revenue and Forecast, by Phase (2017-2030)
9.5.3.2. Market Revenue and Forecast, by Service (2017-2030)
9.5.4. Rest of LATAM
9.5.4.1. Market Revenue and Forecast, by Phase (2017-2030)
9.5.4.2. Market Revenue and Forecast, by Service (2017-2030)
Chapter 10. Company Profiles
10.1. Icon Plc
10.1.1. Company Overview
10.1.2. Phase Offerings
10.1.3. Financial Performance
10.1.4. Recent Initiatives
10.2. IQVIA
10.2.1. Company Overview
10.2.2. Phase Offerings
10.2.3. Financial Performance
10.2.4. Recent Initiatives
10.3. Covance
10.3.1. Company Overview
10.3.2. Phase Offerings
10.3.3. Financial Performance
10.3.4. Recent Initiatives
10.4. Charles River Laboratories
10.4.1. Company Overview
10.4.2. Phase Offerings
10.4.3. Financial Performance
10.4.4. Recent Initiatives
10.5. Syneos Health
10.5.1. Company Overview
10.5.2. Phase Offerings
10.5.3. Financial Performance
10.5.4. Recent Initiatives
10.6. MedPace
10.6.1. Company Overview
10.6.2. Phase Offerings
10.6.3. Financial Performance
10.6.4. Recent Initiatives
10.7. Promedica International
10.7.1. Company Overview
10.7.2. Phase Offerings
10.7.3. Financial Performance
10.7.4. Recent Initiatives
10.8. Wuxi AppTec
10.8.1. Company Overview
10.8.2. Phase Offerings
10.8.3. Financial Performance
10.8.4. Recent Initiatives
10.9. Eurofins
10.9.1. Company Overview
10.9.2. Phase Offerings
10.9.3. Financial Performance
10.9.4. Recent Initiatives
10.10. Qserve Group
10.10.1. Company Overview
10.10.2. Phase Offerings
10.10.3. Financial Performance
10.10.4. Recent Initiatives
Chapter 11. Research Methodology
11.1. Primary Research
11.2. Secondary Research
11.3. Assumptions
Chapter 12. Appendix
12.1. About Us
12.2. Glossary of Terms
Report Details
- Report Code: 39074
- Category: Healthcare
- No. of Pages: 150+
- Format: PDF/PPT/Excel
- Published: January 2022
- Historical Year: 2021-2022
- Base Year: 2023
- Estimated Year: 2024-2033
Proceed To Buy
Customization offered.
Skip to main content
- Skip to main menu
- Skip to user menu

Filter News
- All (810,142)
- Topic (768,051)
- Hotbed/Location (738,027)
- Career Advice (3,903)
- Insights (181)
- Webinars (8)
- Podcasts (42)
Medical Device Contract Research Organization Market Size to Surpass US$ 15.1 BN by 2030
Published: Apr 26, 2022
The Medical Device Contract Research Organization Market size is expected to surpass around US$ 15.1 billion by 2030 from valued at US$ 7.3 billion in 2020 with a CAGR of 9.6% from 2021 to 2030.
Growth Factors
During the second half of the COVID-19 pandemic in 2020, the market witnessed a surge in demand for CRO services as compared to the first half, where the growth was stagnant. The main driver of the market includes time-saving, cost efficiency, and expertise in the area, which accelerates the process of devices reaching the market. In addition, outsourcing to a CRO with precise expertise in a medical device helps in meeting the complex regulatory requirements and audits as they work on it on a daily basis.
Get the sample copy of report@ https://www.visionresearchreports.com/report/sample/39074
They also have access to the most advanced technological resources, such as all the latest and most advanced hardware, software, and internet-based applications, to make the process fast and maintain quality.
By Service Analysis
The clinical monitoring segment led the global market in 2020 accounting for a revenue share of 21.1% in 2020. The segment is anticipated to maintain its position over the forecast period owing to a large number of clinical trials performed and, to study them, increasing demand for clinical trial monitoring.
On the basis of services, the market is segmented into project management/clinical supply management, data management, regulatory/medical affairs, medical writing, clinical monitoring, quality management/assurance, bio-statistics, investigator payments, laboratory, patient & site recruitment, technology, and others.
Report Coverage
By Phase Analysis
On the basis of phase, the market has been bifurcated into clinical and preclinical. The clinical segment dominated the market and accounted for the largest revenue share of more than 88% in 2020.
The preclinical segment is projected to register the fastest growth rate of 10.0% during the forecast period. An increase in the number of preclinical trials involving large molecules and the growing need to curb R&D expense is expected to contribute to the segment growth.
By Regional Analysis
Asia Pacific dominated the market accounting for the largest revenue share of 41.1% in 2020. This growth is due to several medical research organizations’ investments in developing the capability to analyze medical devices and technology.
In North America, the market for healthcare CROs held a substantial share in 2020 owing to the highest number of trials undertaken and outsourced in the region. This large number can be attributed to the presence of well-established CROs in the region and high investments by pharmaceutical companies.
Key Players
- Charles River Laboratories
- Syneos Health
- Promedica International
- Wuxi AppTec
- Qserve Group
Market Segmentatio n
- Preclinical
- Project Management/Clinical Supply Management
- Data Management
- Regulatory/Medical Affairs
- Medical Writing
- Clinical Monitoring
- Quality Management/Assurance
- Bio-statistics
- Investigator Payments
- Patient & Site Recruitment
- North America
- Asia Pacific
- Latin America
- Middle East & Africa
Click Here to View Full Report Table of Contents
Buy this Premium Research Report@ https://www.visionresearchreports.com/report/cart/39074
You can place an order or ask any questions, please feel free to contact at [email protected] | +1 9197 992 333
Market Segmentation
Back to news

- Healthcare /
- Biotechnology /
- Contract Research Organization

Medical Device and Diagnostics Contract Research Organization Market Forecast to 2028 - COVID-19 Impact and Global Analysis by Type and Services and Geography
- Region: Global
- The Insight Partners
- ID: 5394000
- Description
Table of Contents
- Companies Mentioned
Related Topics
Related reports.
- Purchase Options
- Ask a Question
- Recently Viewed Products
According to the publisher's new market research study on “Medical Device and Diagnostics Contract Research Organization Market Forecast to 2028 - COVID-19 Impact and Global Analysis - by Supplements, Application, Distribution Channel, and Geography,” the market is expected to reach US$ 20,336.08 million in 2028 from US$ 12,314.65 million in 2021. The market is estimated to grow with a CAGR of 7.4% from 2021 to 2028. Key factors driving the market such as increasing number of clinical trials worldwide and the rise in the adoption of outsourcing activities coupled with increasing R&D expenditures. However, the extensive competition in the CRO services market is a major factor hindering the market growth.
Based on services, the medical device and diagnostics contract research organization market is segmented into clinical data management, monitoring, clinical project management, medical writing, clinical auditing, digital health, clinical strategy, and others. The clinical data management segment held the largest share of the market in 2021. However, the digital health segment is estimated to register the highest CAGR in the market during the forecast period.
The CRO services industry is highly fragmented, with several hundred small and medium-sized limited-service providers, and a small number of large and full-service global CROs. There are a few barriers for smaller CROs to enter the global market. However, the full-service global CROs require efficient infrastructure with an ability to simultaneously manage multiple complex testing services across numerous geographies, establish the requisite relationships with strategic partners, and develop relevant therapeutic and expertise to meet the needs of end users. Over the past few years, the consolidation across the industry is an emerging trend followed by most of the prime players to strengthen their service offerings and garner the major market share in the global medical device & diagnostics contract research organization market. For instance, PAREXEL International Corp. acquired The Medical Affairs Company in 2017.
COVID-19 outbreak was first observed in December 2019 in Wuhan (China), and it has spread to more than 100 countries across the world, with the World Health Organization (WHO) stating it as a public health emergency. The contract-based research and manufacturing activities for all the non-essential medical devices were hampered due to strict imposition of company shutdowns in the region. Industries, however, need to adopt long-term, permanent solutions to challenges that arose due to COVID. Overall, the COVID-19 created a negative impact on the growth of medical devices and diagnostics contract research organization market.
PAREXEL International Corporation; ICON PLC; WUXI APPTEC; Charles River Laboratories; Laboratory Corporation of America Holdings; North American Science Associates, Inc.; Qserve Group B.V.; IQVIA; Proxima Clinical Research, Inc.; and Activa CRO are among the leading companies operating in the medical device and diagnostics contract research organization market.
The report segments global medical device and diagnostics contract research organization market as follows:
- Medical Devices
- Diagnostics
- Cardiac Biomarkers
- Diabetes Management
- Infectious Diseases
- Chemistry and Immunoassays
- Molecular Diagnostics
By Services
- Clinical Data Management
- Clinical Project Management
- Medical Writing
- Clinical Auditing
- Digital Health
- Clinical Strategy
By Geography
- North America
- Asia Pacific (APAC)
- South Korea
- Middle East and Africa (MEA)
- Saudi Arabia
- South Africa
- South and Central America (SCAM)
Reasons to Buy
- Save and reduce time carrying out entry-level research by identifying the growth, size, leading players and segments in the medical devices and diagnostics contract research organization market.
- Highlights key business priorities in order to assist companies to realign their business strategies.
- The key findings and recommendations highlight crucial progressive industry trends in the global medical devices and diagnostics contract research organization market, thereby allowing players across the value chain to develop effective long-term strategies.
- Develop/modify business expansion plans by using substantial growth offering developed and emerging markets.
- Scrutinize in-depth global market trends and outlook coupled with the factors driving the market, as well as those hindering it.
- Enhance the decision-making process by understanding the strategies that underpin security interest with respect to client products, segmentation, pricing and distribution.
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- PAREXEL International Corporation
- WUXI APPTEC
- Charles River Laboratories
- Laboratory Corporation of America Holdings
- North American Science Associates, Inc.
- Qserve Group B.V.
- Proxima Clinical Research, Inc.
Table Information
- Biotechnology
- Contract Research
- Drug Discovery

Contract Research Organization Services Market Forecasts from 2023 to 2028
- Report
- November 2023

Preclinical Contract Research Organizations - Global Strategic Business Report

Contract Research Organization (CRO) Services Market: Trends, Opportunities and Competitive Analysis 2023-2028

Global Contract Research Organization Services Market Analysis & Forecast to 2023-2033: Market By Service; By Application; By End-user; and By Region
- August 2023
ASK A QUESTION
We request your telephone number so we can contact you in the event we have difficulty reaching you via email. We aim to respond to all questions on the same business day.
Request a Quote
YOUR ADDRESS
YOUR DETAILS
PRODUCT FORMAT
DOWNLOAD SAMPLE
Please fill in the information below to download the requested sample.

The global medical device contract research organization market size is expected to reach USD 12.1 billion by 2028, registering a CAGR of 8.6%
During the second half of the COVID-19 pandemic in 2020, the market witnessed a surge in demand for CRO services as compared to the first half, where the growth was stagnant.
The main driver of the market includes time-saving, cost efficiency, and expertise in the area, which accelerates the process of devices reaching the market. In addition, outsourcing to a CRO with precise expertise in a medical device helps in meeting the complex regulatory requirements and audits as they work on it on a daily basis.
They also have access to the most advanced technological resources, such as all the latest and most advanced hardware, software, and internet-based applications, to make the process fast and maintain quality. The presence of well-established players, mid-size firms, and a number of new start-ups offering great opportunities are also contributing to the medical device CRO market growth.
Moreover, the focus of the regulatory authority towards the treatment of the disease and funding in the medical device market has increased for the rapid testing, production, and manufacturing of medical devices required for the treatment of COVID-19 infected patients.
Medical Device Contract Research Organization Market Report Highlights
- The clinical segment dominated the market in 2020 and is expected to grow at a significant CAGR over the forecasted period
- The clinical monitoring segment accounted for the largest revenue share in 2020 due to an increasing number of clinical trials, which require a large amount of monitoring
- Asia Pacific is expected to be the fastest-growing regional market over the forecast period
- North America was the second-largest regional market in 2020 and is estimated to maintain the leading position over the forecast period
- The government support for R&D activities through grants & funds to research institutes & companies has driven the market growth in this region
Key Topics Covered:
Chapter 1. Methodology and Scope
Chapter 2. Executive Summary
Chapter 3. Medical Device CRO Market Variables, Trends & Scope
3.1. Market Lineage outlook
3.1.1. Parent market outlook
3.1.2. Related/ancillary market outlook
3.2. Penetration & Growth Prospect Mapping
3.3. COVID-19 pandemic impact on medical device CRO activity
3.4. Market Dynamics
3.4.1. Market Driver Analysis
3.4.1.1. Rising demand for advanced products
3.4.1.2. Increasing complexity with respect to product design and engineering
3.4.1.3. Increasing outsourcing to emerging countries due to cost benefit
3.4.2. Market Restraint Analysis
3.4.2.1. Compliance issues while outsourcing
3.4.3. Industry Challenge
3.5. Medical Device CRO: Market Analysis Tools
3.5.1. Industry Analysis - Porter's
3.5.2. PESTEL Analysis
Chapter 4. Medical Device CRO Market: Segment Analysis, By Phase, 2016 - 2028 (USD Million)
4.1. Segment Dashboard
4.2. Phase Market Share Analysis, 2020 & 2028
4.3. Medical Device CRO Market, By Phase, 2016 to 2028
4.4. Market Size & Forecasts and Trend Analyses, 2016 to 2028 for the following
4.4.1. Preclinical
4.4.2. Clinical
Chapter 5. Medical Device CRO Market: Segment Analysis, By Service, 2016 - 2028 (USD Million)
5.1. Segment Dashboard
5.2. Service Market Share Analysis, 2020 & 2028
5.3. Medical Device CRO Market, By Service, 2016 to 2028
5.4. Market Size & Forecasts and Trend Analyses, 2016 to 2028 for the following
5.4.1. Project Management/Clinical Supply Management
5.4.2. Data Management
5.4.3. Regulatory/Medical Affairs
5.4.4. Medical Writing
5.4.5. Clinical Monitoring
5.4.6. Quality Management/Assurance
5.4.7. Bio-statistics
5.4.8. Investigator Payments
5.4.9. Laboratory
5.4.10. Patient & Site Recruitment
5.4.11. Technology
5.4.12. Others
Chapter 6. Medical Device CRO Market: Regional Market Analysis, 2016 - 2028 (USD Million)
6.1. Regional Market Dashboard
6.2. Regional Market Share Analysis, 2020 & 2028
6.3. Market Size, & Forecasts, and Trend Analysis, 2016 to 2028
Chapter 7. Medical Device CRO Market- Competitive Analysis
- Charles River Laboratories
- Syneos Health
- Promedica International
- Wuxi AppTec
- Qserve Group
For more information about this report visit https://www.researchandmarkets.com/r/90m9hb
ResearchAndMarkets.com Laura Wood, Senior Press Manager [email protected] For E.S.T Office Hours Call 1-917-300-0470 For U.S./CAN Toll Free Call 1-800-526-8630 For GMT Office Hours Call +353-1-416-8900

You are using an outdated browser. Please upgrade your browser to improve your experience.
Medical Device Contract Research Organization Market Size, Share & Trends Analysis Report By Phase (Preclinical, Clinical), By Service (Regulatory/Medical Affairs, Clinical Monitoring), By Type, By Region, And Segment Forecasts, 2023 - 2030
- Table of Contents
- Description
- Related Reports
- The clinical phase segment dominated the industry in 2022 owing to the high number of medical devices in the clinical stage
- The clinical monitoring services segment led the industry in 2022 with the largest revenue share
- This is due to an increasing number of clinical trials in medical devices, which require proper clinical monitoring
- The diagnostic devices segment held the largest revenue share in 2022 owing to the high prevalence of chronic and infectious diseases, which boosted the demand for advanced diagnostic medical devices
- North America dominated the industry, in terms of revenue share, in 2022 owing to the government support for R&D activities through grants and funds to research institutes and companies
Chapter 1 Methodology and Scope 1.1 Market Segmentation & Scope 1.1.1 Phase 1.1.2 Service 1.1.3 Device Type 1.1.4 Regional Scope 1.1.5 Estimates And Forecast Timeline 1.2 Research Methodology 1.3 Information Procurement 1.3.1 Purchased Database 1.3.2 GVR’S Internal Database 1.3.3 Secondary Sources 1.3.4 Primary Research 1.3.5 Details Of Primary Research 1.4 Information or Data Analysis 1.4.1 Data Analysis Models 1.5 Market Formulation & Validation 1.6 Model Details 1.6.1 Commodity Flow Analysis (Model 1) 1.6.2 Volume Price Analysis (Model 2) 1.7 List of Secondary Sources 1.8 List of Primary Sources 1.9 List of Abbreviations 1.10 Objectives 1.10.1 Objective - 1: 1.10.2 Objective - 2: 1.10.3 Objective - 3: 1.10.4 Objective - 4: Chapter 2 Executive Summary 2.1 Market Snapshot 2.2 Segment Snapshot 2.3 Segment Snapshot 2.4 Competitive Landscape Snapshot 2.5 Segment Outlook Chapter 3 Medical Device Contract Research Organization Market: Variables, Trends, & Scope 3.1 Market Lineage Outlook 3.1.1 Parent market outlook 3.1.2 Ancillary market outlook 3.2 COVID-19 Impact on the Clinical Trial Services Market 3.2.1 COVID-19 Impact on Medical Devices Clinical Trial 3.2.2 COVID-19 Impact on medical device approvals 3.2.3 COVID-19 Impact on Medical Device Supply Chain 3.2.4 COVID-19 Impact on the Revenue of Key Industry Players 3.2.5 Post-COVID-19 Impact on the Market 3.3 Market Dynamics 3.3.1 Market Driver Analysis 3.3.1.1 Rising Demand for Advanced Products 3.3.1.2 Increasing Complexity in Product Design and Engineering 3.3.1.3 Increasing Outsourcing to Emerging Countries due to Cost Benefit 3.3.2 Market Restraint Analysis 3.3.2.1 Compliance Issues While Outsourcing 3.4 Medical Device Contract Research Organization Market Analysis Tools 3.4.1 Porter’s Five Forces Analysis 3.4.2 PESTEL Analysis 3.5 Major Deals & Strategic Alliances Analysis 3.5.1 Mergers and acquisitions 3.5.2 Collaboration 3.5.3 Product Launch 3.5.4 Expansions Chapter 4 Medical Device Contract Research Organization Market: Phase Segment Analysis 4.1 Definitions & Scope 4.2 By Phase Market Share Analysis, 2022 & 2030 4.2.1 Preclinical 4.2.1.1 Preclinical market, 2018-2030 (USD Million) 4.2.2 Clinical 4.2.2.1 Clinical market, 2018-2030 (USD Million) Chapter 5 Medical Device Contract Research Organization Market: Service Segment Analysis 5.1 Definitions & Scope 5.2 By Service Market Share Analysis, 2022 & 2030 5.2.1 Project Management/Clinical Supply Management 5.2.1.1 Project Management/Clinical Supply Management market, 2018-2030 (USD Million) 5.2.2 Data Management 5.2.2.1 Data Management market, 2018-2030 (USD Million) 5.2.3 Regulatory/Medical Affairs 5.2.3.1 Regulatory/Medical Affairs market, 2018-2030 (USD Million) 5.2.4 Medical Writing 5.2.4.1 Medical Writing market, 2018-2030 (USD Million) 5.2.5 Clinical Monitoring 5.2.5.1 Clinical Monitoring market, 2018-2030 (USD Million) 5.2.6 Quality Management/Assurance 5.2.6.1 Quality Management/ Assurance market, 2018-2030 (USD Million) 5.2.7 Bio-statistics 5.2.7.1 Bio-statistics market, 2018-2030 (USD Million) 5.2.8 Investigator Payments 5.2.8.1 Investigator Payments market, 2018-2030 (USD Million) 5.2.9 Laboratory 5.2.9.1 Laboratory market, 2018-2030 (USD Million) 5.2.10 Patient and Site Recruitment 5.2.10.1 Patient and Site Recruitment market, 2018-2030 (USD Million) 5.2.11 Technology 5.2.11.1 Technology market, 2018-2030 (USD Million) 5.2.12 Others 5.2.12.1 Others market, 2018-2030 (USD Million) Chapter 6 Medical Device Contract Research Organization Market: Device Type Segment Analysis 6.1 Definitions & Scope 6.2 By Service Market Share Analysis, 2022 & 2030 6.2.1 MedTech devices 6.2.1.1 Medtech Devices market, 2018-2030 (USD Million) 6.2.2 Diagnostic Devices 6.2.2.1 Diagnostic Devices market, 2018-2030 (USD Million) 6.2.3 Handheld Devices 6.2.3.1 Handled Devices market, 2018-2030 (USD Million) 6.2.4 Others 6.2.4.1 Others market, 2018-2030 (USD Million) Chapter 7 Medical Device Contract Research Organization Market Regional Estimates and Trend Analysis 7.1 Medical Device Contract Research Organization Market Share by Region, 2022 & 2030 7.2 North America 7.2.1 North America Medical Device Contract Research Organization Market Estimates And Forecasts, 2018 - 2030 (USD Million) 7.2.2 U.S. 7.2.2.1 U.S. Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.2.3 Canada 7.2.3.1 Canada Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.3 Europe 7.3.1 Europe Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.3.2 U.K. 7.3.2.1 U.K. Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.3.3 Germany 7.3.3.1 Germany Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.3.4 France 7.3.4.1 France Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.3.5 Italy 7.3.5.1 Italy Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.3.6 Spain 7.3.6.1 Spain Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.3.7 Denmark 7.3.7.1 Denmark Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.3.8 Sweden 7.3.8.1 Sweden Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.3.9 Norway 7.3.9.1 Norway Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.4 Asia Pacific 7.4.1 Asia Pacific Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.4.2 India 7.4.2.1 India Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.4.3 Japan 7.4.3.1 Japan Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.4.4 China 7.4.4.1 China Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.4.5 Australia 7.4.5.1 Australia Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.4.6 South Korea 7.4.6.1 South Korea Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.4.7 Thailand 7.4.7.1 Thailand Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.5 Latin America 7.5.1 Latin America Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.5.2 Brazil 7.5.2.1 Brazil Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.5.3 Mexico 7.5.3.1 Mexico Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.5.4 Argentina 7.5.4.1 Argentina Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.6 Middle East & Africa 7.6.1 MEA Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.6.2 South Africa 7.6.2.1 South Africa Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.6.3 Saudi Arabia 7.6.3.1 Saudi Arabia Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.6.4 UAE 7.6.4.1 UAE Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) 7.6.5 Kuwait 7.6.5.1 Kuwait Medical Device Contract Research Organization Market, 2018 - 2030 (USD Million) Chapter 8 Competitive Analysis 8.1 Company Market Share Analysis In The Medical Device Contract Research Organization Market Chapter 9 Competitive Landscape 9.1 Participant’s Overview 9.2 Financial Performance 9.3 Participant Categorization 9.3.1 STRATEGY MAPPING 9.3.1.1 Expansions 9.3.1.2 Collaborations, Partnerships, Agreements 9.3.1.3 Product launch 9.3.1.4 Acquisition 9.3.1.4.1 IQVIA, Inc. 9.3.1.4.2 Charles River Laboratories 9.3.1.4.3 ICON, plc 9.3.1.4.4 Syneos Health 9.3.1.4.5 Laboratory Corporation of America Holdings 9.3.1.4.6 WuXi AppTec 9.3.1.4.7 Medpace 9.3.1.4.8 Eurofins Scientific SE 9.3.1.4.9 Promedica International 9.3.1.4.10 Qserve
Medical Device Contract Research Organization Market Size, Share & Trends Analysis Report By Phase (Preclinical, Clinical), By Service (Regulatory/Medical Affairs, Clinical Monitoring), By Region (APAC, North America), And Segment Forecasts, 2021 - 2028
Medical device contract research organization market size, trends, analysis, and outlook by phase (preclinical, clinical), by service (project management/clinical supply management, data management, regulatory/medical affairs, medical writing, clinical monitoring, quality management/assurance, bio-statistics, investigator payments, laboratory, patient & site recruitment, technology, others), by device type (medtech devices, diagnostic devices, handheld devices, others), by region, country, segment, and companies, 2024-2030, global medical device contract research organization market size study, by phase outlook (preclinical, clinical) by service outlook (project management/clinical supply management, data management, regulatory/medical affairs, medical writing, clinical monitoring, quality management/assurance, bio-statistics, investigator payments, laboratory, patient & site recruitment, technology, others) and regional forecasts 2022-2028, healthcare contract research organization market size, share & trends analysis report by type (drug discovery, pre-clinical, clinical), by service (clinical monitoring, regulatory/medical affairs), and segment forecasts, 2021 - 2028, global healthcare contract research organization market size study, by type (drug discovery, pre-clinical, clinical), by service (project management/clinical supply management, data management, regulatory/medical affairs, medical writing, clinical monitoring, others) and regional forecasts 2021-2027, preclinical contract research organizations, research assistance.

Learn how to effectively navigate the market research process to help guide your organization on the journey to success.
Other tasks
- Printer format
- Order by fax
- Currency converter
Request a Free Sample
Samples provide examples of tables, charts, and topics included in this report. Your sample will be emailed to the address provided.
MarketResearch.com, Inc. is committed to protecting and respecting your privacy, and we’ll only use your personal information to administer your account and to provide the products and services you requested from us. From time to time, we would like to contact you about our products and services, as well as other content that may be of interest to you. If you consent to us contacting you for this purpose, please tick below to say how you would like us to contact you:
You can unsubscribe from these communications at any time. For more information on how to unsubscribe, our privacy practices, and how we are committed to protecting and respecting your privacy, please review our Privacy Policy.
By clicking submit below, you consent to allow MarketResearch.com, Inc. to store and process the personal information submitted above to provide you the content requested.
Medical Device Contract Research Organization Global Market Report 2023: Featuring IQVIA, Charles River Laboratories, ICON, Syneos Health & More
May 18, 2023 08:18 ET | Source: Research and Markets Research and Markets
Dublin, May 18, 2023 (GLOBE NEWSWIRE) -- The "Medical Device Contract Research Organization Market Size, Share & Trends Analysis Report By Phase (Preclinical, Clinical), By Service (Regulatory/Medical Affairs, Clinical Monitoring), By Type, By Region, And Segment Forecasts, 2023 - 2030" report has been added to ResearchAndMarkets.com's offering. The global medical device contract research organization market size is expected to reach USD 14.01 billion by 2030, registering a CAGR of 8.8% from 2023 to 2030.
Key drivers of the industry includes benefits, such as time-saving, cost efficiency, and expertise in the area, which accelerates the process of devices reaching the market. In addition, outsourcing to a Contract Research Organization (CRO) with detailed expertise in a medical device helps in meeting the complex regulatory requirements and audits as they work on it on a daily basis. This further supports the demand for medical device CRO services. Contract research organizations also have access to the most advanced technological resources, such as all the latest and most advanced hardware, software, and internet-based applications, to maintain quality and make the process fast.
A significant number of CROs, such as Iqvia, Inc., ICON Plc, and Charles River, are expected to improve medical device-related research collaborations in the coming years and, thus, enhance industry growth. Nowadays, medical procedures are moving toward more laparoscopic and catheter-based technology. There has been a growing demand for minimally invasive procedures worldwide, as they are less risky compared to other surgical procedures. This is expected to boost the demand for advanced devices for screening and monitoring surgical procedures. Minimally invasive procedures are also enhancing the demand for new miniature technology. This is expected to improve the number of research in advanced less invasive medical devices and, thus, promote overall industry growth.
Public organizations worldwide are providing funding to improve healthcare R&D. For instance, in March 2022, over USD 188.0 million was funded by the U.K. government to support NHS-led health research pertaining to diagnostics and treatments. Such investments are expected to drive the industry growth post-pandemic. Medical Device Contract Research Organization Market Report Highlights
- The clinical phase segment dominated the industry in 2022 owing to the high number of medical devices in the clinical stage
- The clinical monitoring services segment led the industry in 2022 with the largest revenue share
- This is due to an increasing number of clinical trials in medical devices, which require proper clinical monitoring
- The diagnostic devices segment held the largest revenue share in 2022 owing to the high prevalence of chronic and infectious diseases, which boosted the demand for advanced diagnostic medical devices
- North America dominated the industry, in terms of revenue share, in 2022 owing to the government support for R&D activities through grants and funds to research institutes and companies
Key Attributes:
Key Topics Covered: Chapter 1 Methodology and Scope Chapter 2 Executive Summary
Chapter 3 Medical Device Contract Research Organization Market: Variables, Trends, & Scope 3.1 Market Lineage Outlook 3.1.1 Parent market outlook 3.1.2 Ancillary market outlook 3.2 COVID-19 Impact on the Clinical Trial Services Market 3.2.1 COVID-19 Impact on Medical Devices Clinical Trial 3.2.2 COVID-19 Impact on medical device approvals 3.2.3 COVID-19 Impact on Medical Device Supply Chain 3.2.4 COVID-19 Impact on the Revenue of Key Industry Players 3.2.5 Post-COVID-19 Impact on the Market 3.3 Market Dynamics 3.3.1 Market Driver Analysis 3.3.1.1 Rising Demand for Advanced Products 3.3.1.2 Increasing Complexity in Product Design and Engineering 3.3.1.3 Increasing Outsourcing to Emerging Countries due to Cost Benefit 3.3.2 Market Restraint Analysis 3.3.2.1 Compliance Issues While Outsourcing Chapter 4 Medical Device Contract Research Organization Market: Phase Segment Analysis 4.1 Definitions & Scope 4.2.1 Preclinical 4.2.2 Clinical Chapter 5 Medical Device Contract Research Organization Market: Service Segment Analysis 5.1 Definitions & Scope 5.2 By Service Market Share Analysis, 2022 & 2030 5.2.1 Project Management/Clinical Supply Management 5.2.2 Data Management 5.2.3 Regulatory/Medical Affairs 5.2.4 Medical Writing 5.2.5 Clinical Monitoring 5.2.6 Quality Management/Assurance 5.2.7 Bio-statistics 5.2.8 Investigator Payments 5.2.9 Laboratory 5.2.10 Patient and Site Recruitment 5.2.11 Technology 5.2.12 Others Chapter 6 Medical Device Contract Research Organization Market: Device Type Segment Analysis 6.1 Definitions & Scope 6.2 By Service Market Share Analysis, 2022 & 2030 6.2.1 MedTech devices 6.2.2 Diagnostic Devices 6.2.3 Handheld Devices 6.2.4 Others Chapter 7 Medical Device Contract Research Organization Market Regional Estimates and Trend Analysis Chapter 8 Competitive Analysis Chapter 9 Competitive Landscape Companies Mentioned
- IQVIA, Inc.
- Charles River Laboratories
- Syneos Health
- Laboratory Corporation of America Holdings
- WuXi AppTec
- Eurofins Scientific SE
- Promedica International
For more information about this report visit https://www.researchandmarkets.com/r/7w1bu9
About ResearchAndMarkets.com ResearchAndMarkets.com is the world's leading source for international market research reports and market data. We provide you with the latest data on international and regional markets, key industries, the top companies, new products and the latest trends.
- Global Medical Device Contract Research Organization Market

Contact Data
- Healthcare IT
- Contract Research Organization (CRO) Services Market
"Designing Growth Strategies is in our DNA"
Contract Research Organization (CRO) Services Market Size, Share & COVID-19 Impact Analysis, By Service Type (Early Phase Development Services, Clinical, Laboratory Service and Others), By Application (Oncology, CNS Disorder, Cardiology, Infectious Disease, Metabolic Disorder, and Others), By End-User (Pharmaceutical and Biotech Companies, Medical Device Companies, Academic and Research Institutes and Others), and Regional Forecast, 2023-2030
Last Updated: April 15, 2024 | Format: PDF | Report ID: FBI100864
- Segmentation
- Methodology
- Infographics
- Request Sample PDF
KEY MARKET INSIGHTS
The contract research organization (CRO) services market size was valued at USD 82.60 billion in 2023 and is projected to grow USD 188.52 billion by 2030, exhibiting a CAGR of 12.5% during 2023-2030. Rising chronic disease burden and pressure for new treatments drive the CRO market.
Contract research organization (CRO) services provides clinical management services to pharmaceutical, biotechnology, and medical device manufacturers. Clinical trial management gets complicated due to many factors, such as sponsors, manufacturers, ethical committees, competent authorities, foundations, researchers, centers, and legal departments. Moreover, it is crucial to work under the guidelines of good clinical practices and harmonization guides, ensuring the unaffected quality of the study.
CRO services sources of revenue are generally from the R&D budgets of pharmaceutical, biotechnology, medical device companies, governments, and other medical research entities. Similarly, the future revenue growth for the CROs can be attributed to overall R&D spending and growth in pharmaceutical R&D outsourcing. The increasing need for time-efficient and cost-effective drug development with high therapeutic expertise has increased the demand for CRO services. In many cases, outsourcing R&D functions provide the opportunity to optimize internal cost structures by exploiting CROs' capacity, scale, and expertise. Therefore, increased outsourced penetration is anticipated in the clinical development and post-approval stages. Certain early-stage functions like safety assessment and regulatory-related services also boost the outsourcing trends, thus propelling the market's growth. The organizations collaborate with other companies to develop innovative products that help in their trial services.
For instance, in April 2021, Pharmaceutical Product Development, LLC. (PPD) and Science 37 entered into a collaboration in which PPD would use Science 37’s Decentralized clinical trial Software as a Service (SaaS) -based technology platform to design, build, test, implement, and execute digital trials. This factor would eventually help the organization in expanding its market share.
COVID-19 IMPACT
Accelerated Drug Development Efforts Augmented Market Growth During Pandemic
COVID-19 has had both positive and negative impacts on the market. During 2020 the CRO services market growth was slowed due to lockdown restrictions. But soon, the market observed a spike in its growth. During COVID-19, many pharmaceutical and biopharmaceutical companies boosted their R&D and manufacturing processes to develop and distribute test kits, vaccines, and drugs against the SARS-CoV-2 virus. To boost R&D, many pharma and biotech companies have collaborated with CROs through long-term agreements, partnerships, and collaborations worldwide. For instance, in January 2021, ICON partnered with Pfizer and BioNTech to develop an experimental program for COVID-19 vaccines.
Moreover, for COVID-19 research, Contract research organization services provide dedicated research. After the COVID-19 outbreak, many new services, partnerships, and collaborations worldwide were recorded with pharma companies and academic institutes for COVID-19 vaccine research and development.
Amid the COVID-19 pandemic, the global market observed a significant increase in its market value due to the high demand for these services and increased revenue generated by the market players. For instance, IQVIA generated an estimated revenue of USD 13.65 billion in 2021, increasing 20.2% from the prior year.
LATEST TRENDS
Request a Free sample to learn more about this report.
Growing Investment by Pharma, Biopharma, and Medical Devices Makers to Boost Market Growth
Most biopharmaceutical, pharmaceutical, and medical devices businesses continue investing significant resources into developing new medications and technologies. Sponsors generally outsource therapeutic and other product development functions to independent services providers, which leads them to use a more flexible cost structure and avoid maintaining redundant development capabilities worldwide.
Increasing investments by many key businesses in clinical and non-clinical research activities and outsourcing to many contract research organizations services that help provide cost-effective development options fuel the global market growth. The trend shows that the key pharma companies are enhancing their R&D efficiencies and collaborating on added R&D. The pharma R&D sector is changing as companies are looking to allow more people to work remotely and focus on making trial endpoints more patient-centric and responsive. These factors will augment the market growth.
DRIVING FACTORS
Rise in Prevalence of Chronic Diseases is Fueling Contract Research Organization Services Market Growth
The global burden of chronic diseases is swiftly rising. For instance, according to the UN's Food and Agriculture Organization, chronic diseases accounted for approximately 60% of the world's 56.5 million documented deaths and 46% of the global disease burden. Based on an article from BMC Public Health in June 2020, WHO estimated that by 2020, non-communicable diseases (NCDs) would account for 80% of the global disease burden. Cardiovascular diseases account for more than half of all chronic disease deaths, while obesity and diabetes rise due to their widespread prevalence and earlier onset.
Chronic disease is a problem that affects people worldwide, not only in developed countries. Despite common belief, emerging countries are increasingly facing chronic disease-related public health problems. According to WHO, in five of the WHO's six regions, chronic diseases such as cancer, neurological disorders, immunological disorders, and others account for most mortality. Infectious diseases like HIV/AIDS, TB, malaria, and other similar disorders are still prevalent in Sub-Saharan Africa, and this trend is predicted to continue.
Although worldwide healthy life expectancy has climbed gradually (by over 6.5 years) between 1990 and 2019, it has not increased as much as overall life expectancy in 198 of the 204 nations studied, indicating that mortality has decreased even with the increasing prevalence of chronic diseases. Based on a WHO article published in April 2021, around 77% of NCD deaths happen in low and middle-income countries. Cardiovascular diseases accounted for most NCD deaths, approximately 17.9 million annually. Number of deaths due to cancer, respiratory diseases and diabetes were 9.3 million, 4.1 million and 1.5 million, respectively. The increasing prevalence of chronic diseases and rising awareness against these diseases have increased the pressure on the market players to develop efficient medications and is expected to boost the market significantly. These factors, in turn, drive the market.
Increasing Number of Clinical Trials Resulting in Increased Outsourcing to CROs
Clinical trials are essential for medication development processes all over the world. It helps discover novel treatments for diseases and new techniques for detecting, diagnosing, and preventing disease development. Clinical trials offer the scientific foundation for guiding and treating patients and evaluating novel medications and equipment. Clinical trial results help direct the right direction even though researchers may not get the expected results. Clinical trials in the United States are comparatively less than those conducted in other nations worldwide. Many clinical trials are conducted outside the United States and the European Union since it is often easier and less expensive.
Clinical trial success rates are highly dependent on the study's stage and the treatments or items being developed. Based on the article from Biotechnology Innovation Organization, only about 9% of medications make it through phase I to approval. Recently, the number of clinical trials that have been filed has risen significantly. Clinical trials have become increasingly sophisticated, yet they are still necessary to study and develop novel medications and technologies. This increase in the number of clinical trials is anticipated to boost the development of new drugs and is expected to drive the market's growth in the forecast period.
RESTRAINING FACTORS
Lack of Skilled Professionals to Restrain Growth of the Market
The surge in globalization is boosting the rate of innovation and technology. New opportunities are constantly being added in terms of occupation. Furthermore, increasing industrialization and new services have increased the demand for new skills. This trend has also increased competency in job opportunities. CROs face issues attracting and maintaining highly competent experts as they compete for qualified and experienced scientists with biotechnology, pharmaceutical, medical devices businesses, and academic and research institutions. Companies must offer better compensation and incentives to better compete, affecting the finances and outcomes of players, mainly small-scale analytical testing providers.
This scarcity of qualified specialists is a barrier to adopting novel procedures and technologies, restricting market growth. For instance, as per Labor Statistics, the U.S. market experienced a lack of experienced workforce in 2019. Skill development has not yet been successfully delivered by many countries worldwide. There are gaps between the working-age population and basic literacy. For instance, according to World Bank, around 750 million people aged 15 and above globally are not reading or writing, making it 18% of the global population.
SEGMENTATION
By service type analysis.
To know how our report can help streamline your business, Speak to Analyst
Clinical Segment Dominated Global CRO Services Market Due to Rising Number of Clinical Trials for Chronic Diseases
By service type, the market is divided into early phase development services, laboratory services, clinical, and others.
The clinical segment held a significant portion of the global contract research organization services market share in 2022. This is attributed to the high prevalence and rise in the number of chronic disease cases. Also, the growth in outsourcing R&D activities in the biotechnology & pharmaceutical sectors propels this market.
The early phase development services segment is estimated to expand at a comparatively higher CAGR during the forecast period. This is because of the increasing investment in the development of life-saving drugs.
By Application Analysis
Oncology Segment to Dominate Led by Continuous Development of Cancer Treatment Solutions
Based on application, the market can be segmented into CNS disorder, oncology, cardiology, metabolic disorder, infectious disease, and others.
Oncology has the highest market share as there is advancement in research and technology, and there is more focus on addressing broader global disease threats such as oncology. The stringent regulatory protocols and burdensome penalties for non-compliance have also increased the duration, cost, and complexity of clinical trials. It has been observed that the most active and fastest-growing therapeutic area for the CRO is oncology, representing the single most considerable focus in clinical development at present.
CNS Disorder has the second-highest CAGR due to the growing prevalence of Alzheimer's, Dementia, and Parkinson's disease.
By End User Analysis
Pharmaceutical Biotech Companies to Hold Dominant Share Backed by Increasing R&D
Based on end-users, the market can be segmented into medical device companies, academic and research institutes, pharmaceutical and biotech companies, and others.
Pharmaceutical and biotech companies have the highest market share as pharmaceutical sponsors are investing more in R&D and outsourcing a larger portion of R&D to independent service providers. According to Credit Suisse, only 41% of the clinical development was outsourced in 2016. It was anticipated to increase to 50% in 2020 because the cost and complexity of the development increase in onerous reimbursement and regulatory environment as using CRO partners, these pharmaceutical organizations seek more efficient solutions.
CROs for medical device is also growing at a faster pace. Management of medical technologies and new developments in healthcare by the CRO service providers has increased the market size share for medical devices.
REGIONAL INSIGHTS
North America Contract Research Organization (CRO) Services Market Size, 2022 (USD Billion)
To get more information on the regional analysis of this market, Request a Free sample
In terms of geography, the global market can be categorized into North America, Europe, Asia Pacific, and the Rest of the World.
The North America CRO services market was valued at USD 36.97 billion in 2022 and is expected to hold a major share of the global market in the forecast period. Factors responsible for the region’s dominance include the important pharmaceutical companies located in the region and their overall drug development activity and good healthcare infrastructure. Pharmaceutical organizations have increased their focus on outsourcing clinical trials to treat different disease conditions. Also, these organizations are investing more in R&D activities.
Europe was the second largest region in the market globally in 2022 and is estimated to hold this position during the forecast period. This is due to the surge in the incidence of diseases. Based on the article from Strategic Policy Group, the number of older people aged 65 years or more in the region would increase significantly, rising from around 90.5 million in 2019 to 129.8 million by 2050. Moreover, healthcare expenditure is surging, and pharma companies depend more on contract research organizations to offer more efficiency and productivity.
The Asia Pacific market is projected to grow at the fastest CAGR.The high growth is mainly attributed to the surge in R&D activity and growing shift towards outsourcing. Furthermore, low cost resources in the Asia Pacific region is one of the major factors driving the market growth in the region.
For instance, as per IBEF 2019 report, India’s cost of production is approximately 33.0% lower than that of the U.S.
The market in Rest of the World is expected to hold limited share in the market during the forecast period. However, increasing healthcare expenditure in the countries in rest of the world is expected to fuel market growth.
KEY INDUSTRY PLAYERS
Labcorp Adopts Merger & Acquisition Strategies to Bolster Portfolio and Strengthen Market Foothold
Labcorp Drug Development accounted for the major shares of the market 2022. The increased growth of the market was majorly due to the company’s strong focus on mergers and acquisition to upskill their services. For instance, in July 2021, Labcorp announced the expansion of its oncology portfolio by acquiring Omniseq. This acquisition upskilled the company’s contract research organization services portfolio.
Other key players, such as IQVIA, Pharmaceutical Product Development, LLC, and ICON pIc, held significant shares in the market. This was due to its strong focus on R&D to expand its product offerings and strengthen its global presence. Furthermore, companies are also focusing on incorporating new technologies, such as artificial intelligence , to increase the efficacy of their service offerings.
LIST OF KEY COMPANIES PROFILED:
- Pharmaceutical Product Development, LLC (U.S.)
- Medpace Holdings, Inc. (U.S.)
- Iqvia (U.S.)
- ICON plc (Ireland)
- KCR SA (U.S.)
- PSI (Switzerland)
- Parexel International Corporation (U.S.)
- Labcorp Drug Development (U.S.)
KEY INDUSTRY DEVELOPMENTS:
- December 2021 –Thermo Fisher Scientific Inc. completed the acquisition of Pharmaceutical Product Development, LLC. This acquisition enhanced the key services and offerings of the company.
- December 2021 – Laboratory Corporation of America Holdings completed the acquisition of Toxikon Corporation. This acquisition bolstered the company’s strong non-clinical development portfolio.
- November 2021 – Icon plc announced that its Accellacare Site Network has expanded in reach and capabilities through new partnerships with six research sites across four countries.
- November 2021 – Iqvia announced data aggregation strategy as the foundation to optimize the market insights. It helps to harness the right data and services to optimize the patient. This increased the efficiency of the company’s services.
- October 2021 – Parexel International Corporation and Kyoto University Hospital formed a strategic partnership to expand clinical research opportunities.
REPORT COVERAGE
An Infographic Representation of Contract Research Organization (CRO) Services Market

To get information on various segments, share your queries with us
The global market report provides a detailed market analysis. It focuses on key aspects such as an overview of the market, penetration of outsourcing in R&D and key countries, pricing analysis. Additionally, it includes key industry developments such as mergers, partnerships, & acquisitions, the impact of COVID-19 on the market, and brand analysis. Besides these, the report offers insights into the market trends and highlights key industry developments. In addition to the aforementioned factors, the report encompasses several factors that have contributed to the growth of the market over recent years. The report also covers a regional analysis of different segments.
Report Scope & Segmentation
Frequently asked questions.
Fortune Business Insights says that the global market size was USD 73.38 billion in 2022 and is projected to reach USD 188.52 billion by 2030.
In 2022, North America stood at USD 36.97 billion.
Growing at a CAGR of 12.5%, the market will exhibit steady growth in the forecast period (2023-2030).
The clinical segment is expected to be the leading segment in this market during the forecast period.
The increasing number of clinical trials and surge in the cases of chronic disease are some of the major factors driving the markets growth.
Labcorp and IQVIA are some of the major players in the global market.
North America dominated the market in 2022.
The surge in the outsourcing for R&D by the pharmaceutical organizations more efficiency and productivity are factors that drive the adoption of the service.
Seeking Comprehensive Intelligence on Different Markets? Get in Touch with Our Experts
- STUDY PERIOD: 2019-2030
- BASE YEAR: 2022
- HISTORICAL DATA: 2019-2021
- NO OF PAGES: 121
Personalize this Research
- Granular Research on Specified Regions or Segments
- Companies Profiled based on User Requirement
- Broader Insights Pertaining to a Specific Segment or Region
- Breaking Down Competitive Landscape as per Your Requirement
- Other Specific Requirement on Customization

Healthcare Clients

Related Reports
- Contract Development and Manufacturing Organization (CDMO) Outsourcing Market
- ASEAN Contract Development and Manufacturing Organization (CDMO) Market
- Contract Manufacturing Organization (CMO) Market
- Europe CRO Services Market
- Clinical Trials Market
Client Testimonials
“We are quite happy with the methodology you outlined. We really appreciate the time your team has spent on this project, and the efforts of your team to answer our questions.”
“Thanks a million. The report looks great!”
“Thanks for the excellent report and the insights regarding the lactose market.”
“I liked the report; would it be possible to send me the PPT version as I want to use a few slides in an internal presentation that I am preparing.”
“This report is really well done and we really appreciate it! Again, I may have questions as we dig in deeper. Thanks again for some really good work.”
“Kudos to your team. Thank you very much for your support and agility to answer our questions.”
“We appreciate you and your team taking out time to share the report and data file with us, and we are grateful for the flexibility provided to modify the document as per request. This does help us in our business decision making. We would be pleased to work with you again, and hope to continue our business relationship long into the future.”
“I want to first congratulate you on the great work done on the Medical Platforms project. Thank you so much for all your efforts.”
“Thank you very much. I really appreciate the work your team has done. I feel very comfortable recommending your services to some of the other startups that I’m working with, and will likely establish a good long partnership with you.”
“We received the below report on the U.S. market from you. We were very satisfied with the report.”
“I just finished my first pass-through of the report. Great work! Thank you!”
“Thanks again for the great work on our last partnership. We are ramping up a new project to understand the imaging and imaging service and distribution market in the U.S.”
“We feel positive about the results. Based on the presented results, we will do strategic review of this new information and might commission a detailed study on some of the modules included in the report after end of the year. Overall we are very satisfied and please pass on the praise to the team. Thank you for the co-operation!”
“Thank you very much for the very good report. I have another requirement on cutting tools, paper crafts and decorative items.”
“We are happy with the professionalism of your in-house research team as well as the quality of your research reports. Looking forward to work together on similar projects”
“We appreciate the teamwork and efficiency for such an exhaustive and comprehensive report. The data offered to us was exactly what we were looking for. Thank you!”
“I recommend Fortune Business Insights for their honesty and flexibility. Not only that they were very responsive and dealt with all my questions very quickly but they also responded honestly and flexibly to the detailed requests from us in preparing the research report. We value them as a research company worthy of building long-term relationships.”
“Well done Fortune Business Insights! The report covered all the points and was very detailed. Looking forward to work together in the future”
“It has been a delightful experience working with you guys. Thank you Fortune Business Insights for your efforts and prompt response”
“I had a great experience working with Fortune Business Insights. The report was very accurate and as per my requirements. Very satisfied with the overall report as it has helped me to build strategies for my business”
“This is regarding the recent report I bought from Fortune Business insights. Remarkable job and great efforts by your research team. I would also like to thank the back end team for offering a continuous support and stitching together a report that is so comprehensive and exhaustive”
“Please pass on our sincere thanks to the whole team at Fortune Business Insights. This is a very good piece of work and will be very helpful to us going forward. We know where we will be getting business intelligence from in the future.”
“Thank you for sending the market report and data. It looks quite comprehensive and the data is exactly what I was looking for. I appreciate the timeliness and responsiveness of you and your team.”
Get in Touch with Us
+1 424 253 0390 (US)
+44 2071 939123 (UK)
+91 744 740 1245 (APAC)
[email protected]
- Request Sample
Sharing this report over the email

The global contract research organization (CRO) services market is projected to grow from $82.60 billion in 2023 to $188.52 billion by 2030
Read More at:-

+1-866-353-3335
- Custom Research
- Research Partners
- Enterprise Solution
PUBLISHER: Grand View Research | PRODUCT CODE: 1233155

Medical Device Contract Research Organization Market Size, Share & Trends Analysis Report By Phase (Preclinical, Clinical), By Service (Regulatory/Medical Affairs, Clinical Monitoring), By Type, By Region, And Segment Forecasts, 2023 - 2030
Add to Cart
Description
Table of contents, list of tables, medical device contract research organization market growth & trends:.
The global medical device contract research organization market size is expected to reach USD 14.01 billion by 2030, registering a CAGR of 8.8% from 2023 to 2030, according to a new report by Grand View Research, Inc . Key drivers of the industry includes benefits, such as time-saving, cost efficiency, and expertise in the area, which accelerates the process of devices reaching the market. In addition, outsourcing to a Contract Research Organization (CRO) with detailed expertise in a medical device helps in meeting the complex regulatory requirements and audits as they work on it on a daily basis. This further supports the demand for medical device CRO services.
Contract research organizations also have access to the most advanced technological resources, such as all the latest and most advanced hardware, software, and internet-based applications, to maintain quality and make the process fast. A significant number of CROs, such as Iqvia, Inc., ICON Plc, and Charles River, are expected to improve medical device-related research collaborations in the coming years and, thus, enhance industry growth. Nowadays, medical procedures are moving toward more laparoscopic and catheter-based technology. There has been a growing demand for minimally invasive procedures worldwide, as they are less risky compared to other surgical procedures.
This is expected to boost the demand for advanced devices for screening and monitoring surgical procedures. Minimally invasive procedures are also enhancing the demand for new miniature technology. This is expected to improve the number of research in advanced less invasive medical devices and, thus, promote overall industry growth. Public organizations worldwide are providing funding to improve healthcare R&D. For instance, in March 2022, over USD 188.0 million was funded by the U.K. government to support NHS-led health research pertaining to diagnostics and treatments. Such investments are expected to drive the industry growth post-pandemic.
Medical Device Contract Research Organization Market Report Highlights:
Table of content
Chapter 1 Methodology and Scope
Chapter 2 executive summary, chapter 3 medical device contract research organization market: variables, trends, & scope, chapter 4 medical device contract research organization market: phase segment analysis, chapter 5 medical device contract research organization market: service segment analysis, chapter 6 medical device contract research organization market: device type segment analysis, chapter 7 medical device contract research organization market regional estimates and trend analysis, chapter 8 competitive analysis, chapter 9 competitive landscape.
- 1. List of secondary sources
- 2. List of abbreviations
- 3. Impact of COVID-19 on new patients entering trials
- 4. Impact of COVID-19 on other therapeutic areas
- 5. Revenue of market players in 2020 and 2021
- 6. Mergers & Acquisitions
- 7. Collaboration
- 8. Product launch
- 9. Expansions
- 10. Saudi Arabia regulatory approval process for medical devices
- 11. Key companies undertaking expansions.
- 12. Key companies undergoing collaborations, partnerships, and agreements
- 13. Key companies launching new products.
- 14. Key companies undergoing acquisitions.
List of Figures
- 1. Market research process
- 2. Information procurement
- 3. Primary research pattern
- 4. Market research approaches
- 5. QFD modeling for market share assessment
- 6. Market formulation & validation
- 7. Commodity flow analysis
- 8. Volume price analysis
- 9. Medical device contract research organization market segmentation
- 10. Parent market outlook (2022)
- 11. Related/ancillary market outlook (2022)
- 12. Medical device clinical trial scenario
- 13. Clinical trial scenario (As of January 2022)
- 14. Market driver relevance analysis (Current & future impact)
- 15. Market restraint relevance analysis (Current & future impact)
- 16. Porter's five forces analysis
- 17. SWOT analysis, by factor (political & legal, economic and technological)
- 18. Medical Device Contract Research Organization Market phase outlook: Segment dashboard
- 19. Medical Device Contract Research Organization market: Phase movement analysis
- 20. Class of medical device
- 21. Preclinical market, 2018 - 2030 (USD Million)
- 22. Clinical market, 2018 - 2030 (USD Million)
- 23. Medical Device Contract Research Organization market service outlook: Segment dashboard
- 24. Medical Device Contract Research Organization market: Service movement analysis
- 25. Parameters for measuring success of provider solution
- 26. Project Management/Clinical Supply Management market, 2018 - 2030 (USD Million)
- 27. Clinical data management flow
- 28. Data Management market, 2018 - 2030 (USD Million)
- 29. Class of medical device
- 30. Regulatory/Medical Affairs market, 2018 - 2030 (USD Million)
- 31. Medical Writing market, 2018 - 2030 (USD Million)
- 32. Clinical Monitoring market, 2018 - 2030 (USD Million)
- 33. The PDCA lifecycle of the medical device manufacturing
- 34. Quality Management/ Assurance market, 2018 - 2030 (USD Million)
- 35. Bio-statistics market, 2018 - 2030 (USD Million)
- 36. Investigator Payments market, 2018 - 2030 (USD Million)
- 37. Laboratory market, 2018 - 2030 (USD Million)
- 38. Strategic initiatives with positive impact on patient recruitment
- 39. Patient and Site Recruitment market, 2018 - 2030 (USD Million)
- 40. Technology market, 2018 - 2030 (USD Million)
- 41. Others market, 2018 - 2030 (USD Million)
- 42. Medical Device Contract Research Organization market device type outlook: Segment dashboard
- 43. Medical Device Contract Research Organization market: Device type movement analysis
- 44. MedTech devices market, 2018 - 2030 (USD Million)
- 45. Diagnostic devices market, 2018 - 2030 (USD Million)
- 46. Handled devices market, 2018 - 2030 (USD Million)
- 47. Others market, 2018 - 2030 (USD Million)
- 48. Regional marketplace: Key takeaways
- 49. Regional outlook, 2022 & 2030
- 50. North America clinical trials for medical devices (As of September 2022)
- 51. North America market, 2018 - 2030 (USD Million)
- 52. U.S. medical device approval
- 53. U.S. market, 2018 - 2030 (USD Million)
- 54. Canada market, 2018 - 2030 (USD Million)
- 55. Europe clinical trials for medical devices (As of September 2022)
- 56. Europe market, 2018 - 2030 (USD Million)
- 57. U.K. market, 2018 - 2030 (USD Million)
- 58. Germany market, 2018 - 2030 (USD Million)
- 59. France market, 2018 - 2030 (USD Million)
- 60. Italy market, 2018 - 2030 (USD Million)
- 61. Spain market, 2018 - 2030 (USD Million)
- 62. Denmark market, 2018 - 2030 (USD Million)
- 63. Sweden market, 2018 - 2030 (USD Million)
- 64. Norway market, 2018 - 2030 (USD Million)
- 65. Asia Pacific Clinical Trials for medical devices (As of September 2022)
- 66. Asia Pacific market, 2018 - 2030 (USD Million)
- 67. India market, 2018 - 2030 (USD Million)
- 68. Japan market, 2018 - 2030 (USD Million)
- 69. China market, 2018 - 2030 (USD Million)
- 70. Australia market, 2018 - 2030 (USD Million)
- 71. South Korea market, 2018 - 2030 (USD Million)
- 72. Thailand market, 2018 - 2030 (USD Million)
- 73. Latin America medical device clinical trials (As of September)
- 74. Latin America market, 2018 - 2030 (USD Million)
- 75. Brazil market, 2018 - 2030 (USD Million)
- 76. Mexico market, 2018 - 2030 (USD Million)
- 77. Argentina market, 2018 - 2030 (USD Million)
- 78. MEA clinical trials for medical devices (As of September 2022)
- 79. MEA market, 2018 - 2030 (USD Million)
- 80. South Africa market, 2018 - 2030 (USD Million)
- 81. Saudi Arabia market, 2018 - 2030 (USD Million)
- 82. UAE market, 2018 - 2030 (USD Million)
- 83. Kuwait market, 2018 - 2030 (USD Million)
- 84. Company Market Share Analysis in the Medical Device CRO Market (2022)

Jeroen Van Heghe
Manager - EMEA
+32-2-535-7543

Christine Sirois
Manager - Americas
+1-860-674-8796
瀼㰾散瑮牥㰾浩瑳汹㵥洢硡眭摩桴ㄺ〰∥爠晥牥敲灲汯捩㵹渢ⵯ敲敦牲牥•潬摡湩㵧氢穡≹搠捥摯湩㵧愢祳据•牳㵣栢瑴獰⼺眯睷献祫畱獥瑴挮浯愯獳瑥⽳牦湯整摮椯慭敧⽳灵潣業杮牟灥牯獴损癯牥楟慭敧樮杰•楷瑤㵨㈢〰•敨杩瑨∽㐲∰愠瑬∽敍楤慣敄楶散䌠湯牴捡⁴敒敳牡档传杲湡穩瑡潩慍歲瑥•楴汴㵥∢㰾振湥整㹲⼼㹰慍歲瑥传敶癲敩㩷.
瀼㰾散瑮牥㰾浩瑳汹㵥洢硡眭摩桴ㄺ〰∥爠晥牥敲灲汯捩㵹渢ⵯ敲敦牲牥•潬摡湩㵧氢穡≹搠捥摯湩㵧愢祳据•牳㵣栢瑴獰⼺眯睷献祫畱獥瑴挮浯愯獳瑥⽳牦湯整摮椯慭敧⽳牁扴慯摲ㅟ㉟瀮杮•楷瑤㵨㠢〰•敨杩瑨∽㔴∰愠瑬∽潆敲慣瑳片睯桴慒整•楴汴㵥∢㰾振湥整㹲⼼㹰桔慭歲瑥猠穩慷敤整浲湩摥戠⁹獥楴慭楴杮琠敨洠牡敫⁴桴潲杵潴⵰潤湷愠摮戠瑯潴灵愠灰潲捡ⱨ眠楨档眠獡映牵桴牥瘠污摩瑡摥眠瑩湩畤瑳祲椠瑮牥楶睥䌠湯楳敤楲杮琠敨渠瑡牵景琠敨洠牡敫⁴敷搠牥癩摥琠敨䰠晩捓敩据獥吠潯獬☠匠牥楶散祢猠来敭瑮愠杧敲慧楴湯桴潣瑮楲畢楴湯漠桴楌敦匠楣湥散潔汯…敓癲捩獥椠桐牡慭散瑵捩污ⱳ䈠潩整档潮潬祧☠䰠晩捓敩据獥愠摮瘠湥潤桳牡
敓浧湥慴楴湯䄠慮祬楳㩳
桔敍楤慣敄楶散䌠湯牴捡⁴敒敳牡档传杲湡穩瑡潩慍歲瑥椠敳浧湥整祢倠慨敳敓癲捩ⱥ䐠癥捩祔数敒楧湯污敗愠敲愠慮祬楺杮琠敨洠牡敫⁴景琠敨敳猠来敭瑮潴椠敤瑮晩⁹桷捩敳浧湥⁴獩琠敨氠牡敧瑳渠睯愠摮椠桴畦畴敲桷捩敳浧湥⁴慨桴楨桧獥⁴牧睯桴爠瑡ⱥ愠摮琠敨猠来敭瑮眠楨档漠晦牥桴灯潰瑲湵瑩⁹湩琠敨映瑵牵
- 慂敳湯倠慨敳琠敨洠牡敫⁴獩猠来敭瑮摥愠ⱳ倠敲汣湩捩污汃湩捩污
- 慂敳湯匠牥楶散琠敨洠牡敫⁴獩猠来敭瑮摥愠ⱳ倠潲敪瑣䴠湡条浥湥⽴汃湩捩污匠灵汰⁹慍慮敧敭瑮慄慴䴠湡条浥湥ⱴ删来汵瑡牯⽹敍楤慣晁慦物ⱳ䴠摥捩污圠楲楴杮汃湩捩污䴠湯瑩牯湩Ⱨ儠慵楬祴䴠湡条浥湥⽴獁畳慲据ⱥ䈠潩猭慴楴瑳捩ⱳ䤠癮獥楴慧潴慐浹湥獴慌潢慲潴祲慐楴湥⁴…楓整删捥畲瑩敭瑮敔档潮潬祧瑏敨獲
- 慂敳湯䐠癥捩祔数琠敨洠牡敫⁴獩猠来敭瑮摥愠ⱳ䴠摥敔档䐠癥捩獥楄条潮瑳捩䐠癥捩獥慈摮敨摬䐠癥捩獥瑏敨獲
- 慂敳湯删来潩慮桴慭歲瑥椠敳浧湥整獡潎瑲流牥捩ⱡ䔠牵灯ⱥ䄠楳慐楣楦Ᵽ䰠瑡湩䄠敭楲慣
敒楧湯污䄠慮祬楳㩳
瀼㰾散瑮牥㰾浩瑳汹㵥洢硡眭摩桴ㄺ〰∥爠晥牥敲灲汯捩㵹渢ⵯ敲敦牲牥•潬摡湩㵧氢穡≹搠捥摯湩㵧愢祳据•牳㵣栢瑴獰⼺眯睷献祫畱獥瑴挮浯愯獳瑥⽳牦湯整摮椯慭敧⽳牁扴慯摲ㅟ㕟瀮杮•楷瑤㵨㠢〰•敨杩瑨∽㔴∰愠瑬∽瑁牴捡楴敶敮獳湁污獹獩•楴汴㵥∢㰾振湥整㹲⼼㹰敍楤慣敄楶散䌠湯牴捡⁴敒敳牡档传杲湡穩瑡潩慍歲瑥㨠删獩湁污獹獩
歓兹敵瑳猧攠灸牥⁴湡污獹獴栠癡潣摮捵整楲歳愠慮祬楳潴甠摮牥瑳湡桴浩慰瑣漠硥整湲污攠瑸敲業楴獥漠敍楤慣敄楶散䌠湯牴捡⁴敒敳牡档传杲湡穩瑡潩慍歲瑥敗愠慮祬敺潨⁷敧灯汯瑩捩污椠普畬湥散慮畴慲楤慳瑳牥ⱳ挠楬慭整挠慨杮ⱥ氠来污猠散慮楲Ɐ攠潣潮業浩慰瑣牴摡…捥湯浯捩瀠汯捩敩ⱳ猠捯慩…瑥湨捩挠湯散湲ⱳ愠摮搠浥杯慲桰捩挠慨杮獥洠杩瑨愠晦捥⁴敍楤慣敄楶散䌠湯牴捡⁴敒敳牡档传杲湡穩瑡潩慍歲瑥猧猠灵汰⁹档楡Ɱ搠獩牴扩瑵潩Ɱ愠摮琠瑯污爠癥湥敵朠潲瑷
潃灭瑥瑩癩慬摮捳灡湩㩧
潔甠摮牥瑳湡桴潣灭瑥瑩癩慬摮捳灡ⱥ眠牡湡污穹湩敫⁹敍楤慣敄楶散䌠湯牴捡⁴敒敳牡档传杲湡穩瑡潩慍歲瑥瘠湥潤獲椠桴慭歲瑥潔甠摮牥瑳湡桴潣灭瑥瑩癩楲慶牬ⱹ眠牡潣灭牡湩桴敲敶畮ⱥ攠灸湥敳ⱳ爠獥畯捲獥牰摯捵⁴潰瑲潦楬Ɐ爠来潩潣敶慲敧慭歲瑥猠慨敲敫⁹湩瑩慩楴敶ⱳ瀠潲畤瑣氠畡据敨ⱳ愠摮愠祮渠睥敲慬整潴琠敨䴠摥捩污䐠癥捩潃瑮慲瑣删獥慥捲牏慧楮慺楴湯䴠牡敫
敋⁹汐祡牥潃敶敲湩琠敨删灥牯㩴
- 桃牡敬楒敶慌潢慲潴楲獥
- 慌潢慲潴祲䌠牯潰慲楴湯漠流牥捩潈摬湩獧
- 畅潲楦獮匠楣湥楴楦䕓
桔敍楤慣敄楶散䌠湯牴捡⁴敒敳牡档传杲湡穩瑡潩慍歲瑥椠敢湩湡污穹摥戠⁹歓兹敵瑳猧愠慮祬瑳楷桴琠敨栠汥⁰景㈠⬰猠档摥汵摥倠楲慭祲椠瑮牥楶睥牦浯戠瑯桴敤慭摮愠摮猠灵汰⁹楳敤圠慨敶愠牬慥祤椠癮獥整潭敲琠慨㔲‰潨牵湯琠楨敲潰瑲愠摮愠敲猠楴汬爠晥湩湩畯慤整琠牰癯摩畡桴湥楴慣整慤慴琠潹牵爠慥敤獲愠摮挠楬湥獴硅慨獵楴敶瀠楲慭祲愠摮猠捥湯慤祲爠獥慥捲獩挠湯畤瑣摥琠潣汬捥⁴湩潦浲瑡潩湯琠敨洠牡敫ⱴ瀠敥慭歲瑥湡慰敲瑮洠牡敫
畏牣獯湩畤瑳祲攠灸牥獴愠摮爠癥湥敵椭灭捡⁴潣獮汵慴瑮瑡匠祫畑獥⁴湥扡敬漠牵挠楬湥獴琠潣癮牥⁴慭歲瑥椠瑮汥楬敧据湩潴愠瑣潩慮汢ⱥ焠慵瑮晩慩汢敲畳瑬桴潲杵数獲湯污穩摥攠杮条浥湥
.
- 潔瀠潲楶敤愠搠瑥楡敬癯牥楶睥漠桴慶畬档楡湡湡污穹慭歲瑥琠敲摮楷桴琠敨倠牯整❲楦敶映牯散湡污獹獩
- 潔椠敤瑮晩⁹桴敫⁹汰祡牥湡潣灭敲敨獮癩汥⁹湡污穹桴楥慭歲瑥瀠獯瑩潩湩琠牥獭漠慲歮湩湡潣敲挠浯数整据敩ⱳ愠潬杮眠瑩敤慴汩湩桴潣灭瑥瑩癩慬摮捳灡潦桴慭歲瑥氠慥敤獲
桗瑡搠敯桴獩删灥牯⁴敄楬敶㽲
- 楈瑳牯捩污搠瑡潣敶慲敧›〲㘱琠〲㈲
- 片睯桴瀠潲敪瑣潩獮›〲㈲琠〲㠲
- 畃瑳浯穩瑡潩湯匠来敭瑮ⱳ删来潩獮湡潃灭湡⁹牐景汩獥
- 汇扯污愠摮䌠畯瑮祲䴠牡敫⁴牔湥獤
- 潃灭敲敨獮癩慍灰湩景䤠摮獵牴⁹慐慲敭整獲
- 敒畧慬潴祲猠散慮楲Ɐ爠来潩慮祤慮業獣湡湩楳桧獴漠敬摡湩潣湵牴敩湩攠捡敲楧湯
- 敓浧湥⁴牴湥獤愠慮祬楳ⱳ漠灰牯畴楮祴湡牧睯桴
- 灏潰瑲湵瑩⁹湡污獹獩戠⁹敲楧湯愠摮挠畯瑮祲
- 牐捩湩湡污獹獩
- 慐敲瑮洠牡敫⁴湡污獹獩
- 牐摯捵⁴潰瑲潦楬敢据浨牡楫杮
慔汢晏䌠湯整瑮
- 硅楨楢㩴䔠數畣楴敶匠浵慭祲鎀䐠瑡慔汢湯䴠牡敫⁴癏牥楶睥
- 硅楨楢㩴䔠數畣楴敶匠浵慭祲鎀䌠慨瑲漠敍楤慣敄楶散䌠湯牴捡⁴敒敳牡档传杲湡穩瑡潩慍歲瑥䌠慨慲瑣牥獩楴獣
- 硅楨楢㩴䔠數畣楴敶匠浵慭祲鎀䌠慨瑲漠湉牣浥湥慴片睯桴
慐敲瑮䴠牡敫⁴湁污獹獩
- 䔨桸扩瑩›敄慴汩摥嘠污敵䌠慨湩倠敲敳瑮瑡潩⥮
- 硅楨楢㩴倠牡湥⁴慍歲瑥䔠潣祳瑳浥䴠牡敫⁴湁污獹獩
- 硅楨楢㩴䴠牡敫⁴桃牡捡整楲瑳捩景倠牡湥⁴慍歲瑥
- 䔨桸扩瑩›慄慴吠扡敬›浅牥楧杮猠慴瑲灵敤慴汩⥳
- 䔨桸扩瑩›慄慴吠扡敬›慎敭漠潣灭湡敩湡楰数楬敮瀠潲畤瑣ⱳ爠来潩慮慭灰湩⥧
- 慍牣敯潣潮業湉楤慣潴獲
佃䥖⁄䵉䅐呃
- 湉牴摯捵楴湯
- 硅楨楢㩴䐠瑡湯䜠偄ⴠ夠慥癯牥礭慥牧睯桴㈠ⴶ〲㈲⠠⤥
- 硅楨楢㩴䄠慮祬楳湯欠祥猠牴瑡来敩摡灯整祢挠浯慰楮獥
- 硅楨楢㩴䌠浯数楴楴敶爠癩污祲䤠灭捡⁴景欠祥映捡潴獲〲ㄲ
- 硅楨楢㩴吠牨慥⁴景匠扵瑳瑩瑵牐摯捵獴䤠灭捡⁴景欠祥映捡潴獲〲ㄲ
- 硅楨楢㩴戠祵牥慢杲楡楮杮瀠睯牥䤠灭捡⁴景欠祥映捡潴獲〲ㄲ
- 潐楬楴慣浉慰瑣
- 敔档楮慣浉慰瑣
- 湅楶潲浮湥慴浉慰瑣
- 敌慧浉慰瑣
- 桃牡⁴湯䴠牡敫⁴桳牡祢朠潥牧灡票㈠㈰ⴱ〲㜲⠠⤥
- 慄慴吠扡敬漠慍歲瑥猠慨敲戠⁹敧杯慲桰⁹〲ㄲ㈭㈰⠷⤥
- 桃牡⁴湯䴠牡敫⁴桳牡祢挠畯瑮祲㈠㈰ⴱ〲㜲⠠⤥
- 慄慴吠扡敬漠慍歲瑥猠慨敲戠⁹潣湵牴⁹〲ㄲ㈭㈰⠷⤥
- 硅楨楢㩴䌠慨瑲漠慍歲瑥猠慨敲㈠㈰ⴱ〲㜲⠠⤥
䕋⁙佃偍乁⁙剐䙏䱉卅
- 硅楨楢㩴挠浯慰楮獥挠癯牥摥椠桴敲潰瑲〲ㄲ
- 硅楨楢㩴倠敩挠慨瑲愠慮祬楳湯挠浯慰祮洠牡敫⁴桳牡ⱥ㈠㈰⠱⤥
- 硅楨楢⁴潃灭湡⁹癏牥楶睥
- 硅楨楢⁴敋⁹敄敶潬浰湥獴

⸱䤠普牯慭楴湯倠潲畣敲敭瑮›桔獩猠慴敧椠癮汯敶桴牰捯牵浥湥⁴景䴠牡敫⁴慤慴漠敲慬整湩潦浲瑡潩楶牰浩牡⁹湡敳潣摮牡⁹潳牵散吠敨瘠牡潩獵猠捥湯慤祲猠畯捲獥甠敳湩汣摵摥瘠牡潩獵挠浯慰祮眠扥楳整ⱳ愠湮慵敲潰瑲ⱳ琠慲敤搠瑡扡獡獥湡慰摩搠瑡扡獡獥猠捵獡䠠潯敶❲ⱳ䈠潬浯敢杲䈠獵湩獥ⱳ䘠捡楴慶湡癁湥楴湯畏整浡搠摩㐠‵牰浩牡⁹湩整慲瑣潩獮䜠潬慢汬⁹桷捩湩汣摵摥猠癥牥污猠慴敫潨摬牥畳档愠慭畮慦瑣牵牥ⱳ挠獵潴敭獲敫⁹灯湩潩敬摡牥ⱳ攠捴癏牥污ⱬ椠普牯慭楴湯瀠潲畣敲敭瑮眠獡漠敮漠桴潭瑳攠瑸湥楳敶猠慴敧湩漠牵爠獥慥捲牰捯獥
⸲䤠普牯慭楴湯䄠慮祬楳㩳吠楨瑳灥椠癮汯敶牴慩杮汵瑡潩景搠瑡桴潲杵潢瑴浯甭⁰湡潴⵰潤湷愠灰潲捡敨潴攠瑳浩瑡湡慶楬慤整琠敨琠瑯污猠穩湡畦畴敲攠瑳浩瑡景琠敨䴠摥捩污䐠癥捩潃瑮慲瑣删獥慥捲牏慧楮慺楴湯䴠牡敫
⸳删灥牯⁴潆浲汵瑡潩㩮吠敨映湩污猠整⁰湥慴汩摥琠敨瀠慬散敭瑮漠慤慴瀠楯瑮湩愠灰潲牰慩整䴠牡敫⁴灳捡獥椠湡愠瑴浥瑰琠敤畤散瘠慩汢潣据畬楳湯
楗桴琠敨朠癩湥洠牡敫⁴慤慴畯敤楤慣整整浡漠湡污獹獴挠湡漠晦牥礠畯琠敨映汯潬楷杮挠獵潴業慺楴湯漠瑰潩獮愠敲愠慶汩扡敬映牯琠敨䴠摥捩污䐠癥捩潃瑮慲瑣删獥慥捲牏慧楮慺楴湯䴠牡敫㩴
潃灭瑥瑩癩湁污獹獩›敄慴汩摥愠慮祬楳湡牰景汩湩景愠摤瑩潩慮慍歲瑥瀠慬敹獲☠挠浯慰慲楴敶愠慮祬楳景挠浯数楴楴敶瀠潲畤瑣
潇琠慍歲瑥匠牴瑡来㩹䘠湩桴楨桧札潲瑷档湡敮獬琠湩敶瑳礠畯慭歲瑥湩晥潦瑲湡湩牣慥敳礠畯畣瑳浯牥戠獡
慃整潧祲䤠瑮汥楬敧据㩥䌠獵潴業敺湩整汬杩湥散琠慨⁴獩爠汥癥湡⁴潴琠敨物猠灵汰⁹慍歲瑥楷汬攠慮汢桴浥琠慭敫猠慭瑲牥猠畯捲湩敤楣楳湯湡浩牰癯桴楥慣整潧祲洠湡条浥湥
慗瑮琠畣瑳浯穩桴獩爠灥牯㽴
, , , , 敒畱獥⁴牆敥䌠獵潴業慺楴湯.
圢慨敶瀠牵档獡摥爠捥湥汴⁹敲潰瑲映潲歓兹敵瑳吠捥湨汯杯ⱹ愠摮眠牡慨灰⁹潴椠普牯潹⁵桴瑡琠楨敲潰瑲眠獡猠獵晥汵愠摮瀠慲瑣捩污映牯漠牵琠慥匠祫畱獥⁴敔浡眠獡瘠牥⁹捡楴敶愠摮漠牵焠敵楲獥眠牥潦汬睯摥甠⁰潣灭敬整祬䤮⁴慷浡穡湩∠
牍汁慚楬潃浭牥楣污䐠物捥潴Ⱳ䤠䥃䍉䤠慲
瀼㰾散瑮牥㰾浩瑳汹㵥洢硡眭摩桴ㄺ〰∥爠晥牥敲灲汯捩㵹渢ⵯ敲敦牲牥•潬摡湩㵧氢穡≹搠捥摯湩㵧愢祳据•牳㵣栢瑴獰⼺眯睷献祫畱獥瑴挮浯愯獳瑥⽳牦湯整摮椯慭敧⽳灵潣業杮牟灥牯獴损癯牥楟慭敧樮杰•楷瑤㵨㔢∰栠楥桧㵴㘢∰愠瑬∽敍楤慣敄楶散䌠湯牴捡⁴敒敳牡档传杲湡穩瑡潩慍歲瑥•楴汴㵥∢㰾振湥整㹲⼼㹰牐摯捵⁴䑉›䍕䥍㍇䨵〲㠸
, .
湅整潹牵攠慭汩愠摤敲獳礠畯胢犙獵湩潦潹牵愠捣畯瑮戠汥睯
敒敳⁴慰獳潷摲
Medical Device and Diagnostics Contract Research Organization Market to Grow at a CAGR of 7.4% to reach US$ 20,336.08 million from 2021 to 2028
Medical Device and Diagnostics Contract Research Organization Market Forecast to 2028 - COVID-19 Impact and Global Analysis By Type (Medical Devices and Diagnostics) and Services (Clinical Data Management, Monitoring, Clinical Project Management, Medical Writing, Clinical Auditing, Digital Health, Clinical Strategy, and Others) and Geography
Publication Month : Jun 2021
- Report Code : TIPHE100000958
- Category : Medical Device
- Status : Published
- No. of Pages : 188
- Description
- Table of content
- Research Methodology
- Free Download Sample
The medical device and diagnostics contract research organization market is expected to reach US$ 20,336.08 million in 2028 from US$ 12,314.65 million in 2021. The market is estimated to grow with a CAGR of 7.4% from 2021 to 2028.
Key factors driving the market such as increasing number of clinical trials worldwide and rise in adoption of outsourcing activities coupled with increasing R&D expenditures. However, the extensive competition in the CRO services market is a major factor hindering the market growth.
Lucrative Regions for Medical Device and Diagnostics Contract Research Organization Market
- Sample PDF showcases the content structure and the nature of the information with qualitative and quantitative analysis.
Market Insights
Increasing number of clinical trials worldwide.
Clinical trial is a crucial and significant step to evaluate the safety and effectiveness of a medical strategy, treatment, or device for commercial usage. These studies also help understand and determine the best medical approaches for a particular therapeutic area. Clinical trials are conducted primarily to collect data regarding the safety and efficacy of new drug and device development. Before the approval of drug molecules or medical devices by the regulatory authorities, a series of clinical studies are conducted. The increasing prevalence of various communicable and noncommunicable diseases is creating the need for the development of new drugs or medical devices, which is, in turn, propelling the demand for the medical device & diagnostics contract research organization.
Number of Registered Studies for Clinical Trials
Hence, the increasing number of clinical trials accelerates the demand for medical device & diagnostics contract research organization, which drives the growth of the medical device & diagnostics contract research organization market.
Type-Based Insights
In 2021, the medical devices segment accounted for the largest share of the global medical device and diagnostics contract research organization market, by type. The growth of the medical devices segment is attributed to the increasing number of clinical trials for medical devices, rising spending for research and development, and growing prevalence of chronic diseases. The market for diagnostics segment is estimated to grow at the highest CAGR during the forecast period owing to rapid product approvals and launches. For instance, in May 2019, Roche Diagnostics launched Cobas MTB-RIF/INH test that detects resistance to antibiotics in tuberculosis DNA. Such technological advancements are likely to favor the growth of the segment.
Medical Device and Diagnostics Contract Research Organization Market, by Type – 2021 and 2028
Service-Based Insights
Based on services, the medical device and diagnostics contract research organization market is segmented into clinical data management, monitoring, clinical project management, medical writing, clinical auditing, digital health, clinical strategy, and others. The clinical data management segment held the largest share of the market in 2021, whereas the digital health segment is estimated to register the highest CAGR in the market during the forecast period.
We can optimize and tailor the analysis and scope which is unmet through our standard offerings. This flexibility will help you gain the exact information needed for your business planning and decision making.
Medical Device and Diagnostics Contract Research Organization Market: Strategic Insights

Have a question?
Akshay will walk you through a 15-minute call to present the report’s content and answer all queries if you have any.
The key players such as PAREXEL International Corporation; ICON PLC; WUXI APPTEC; Charles River Laboratories; Laboratory Corporation of America Holdings; North American Science Associates, Inc.; Qserve Group B.V.; IQVIA; Proxima Clinical Research, Inc.; and Activa CRO adopt several organic and inorganic strategies to enhance their revenue and market standings. For instance, in March 2021, NAMSA acquired Minneapolis-based American Preclinical Services (APS), a full-service preclinical CRO. This purchase follows NAMSA’s acquisition of New York-based Syntactx, a leading clinical research services CRO, in January 2021. With the addition of APS’s portfolio of services, NAMSA will further enhance its already robust preclinical solutions by providing sponsors with a broader range of laboratory models and analysis tools, including innovative surgical instrumentation and several state-of-the-art catheterization labs, imaging capabilities, and surgical suites.
Medical Device and Diagnostics Contract Research Organization Market – by Type
- Medical Devices
- Cardiac Biomarkers
- Diabetes Management
- Infectious Diseases
- Chemistry and Immunoassays
- Molecular Diagnostics
Medical Device and Diagnostics Contract Research Organization Market – by Services
- Clinical Data Management
- Clinical Project Management
- Medical Writing
- Clinical Auditing
- Digital Health
- Clinical Strategy
Medical Device and Diagnostics Contract Research Organization Market – by Geography
North america.
- Rest of Europe
Asia Pacific (APAC)
- South Korea
- Rest of Asia Pacific
Middle East and Africa (MEA)
- South Africa
- Saudi Arabia
- Rest of Middle East &Africa
South and Central America (SCAM)
- Rest of South and Central America
Company Profiles
- PAREXEL International Corporation
- WUXI APPTEC
- Charles River Laboratories
- Laboratory Corporation of America Holdings
- North American Science Associates, Inc.
- Qserve Group B.V.
- Proxima Clinical Research, Inc.

Report Coverage
Revenue forecast, Company Analysis, Industry landscape, Growth factors, and Trends

Segment Covered
Type and Services and Geography

Regional Scope
North America, Europe, Asia Pacific, Middle East & Africa, South & Central America

Country Scope
Argentina, Australia, Brazil, Canada, China, France, Germany, India, Italy, Japan, Mexico, Saudi Arabia, South Africa, South Korea, Spain, United Arab Emirates, United Kingdom, United States
Get the Latest COVID-19 Analysis on this market Get them now
The List of Companies - Medical Device and Diagnostics Contract Research Organization Market
The Insight Partners performs research in 4 major stages: Data Collection & Secondary Research, Primary Research, Data Analysis and Data Triangulation & Final Review.
- Data Collection and Secondary Research:
As a market research and consulting firm operating from a decade, we have published many reports and advised several clients across the globe. First step for any study will start with an assessment of currently available data and insights from existing reports. Further, historical and current market information is collected from Investor Presentations, Annual Reports, SEC Filings, etc., and other information related to company’s performance and market positioning are gathered from Paid Databases (Factiva, Hoovers, and Reuters) and various other publications available in public domain.
Several associations trade associates, technical forums, institutes, societies and organizations are accessed to gain technical as well as market related insights through their publications such as research papers, blogs and press releases related to the studies are referred to get cues about the market. Further, white papers, journals, magazines, and other news articles published in the last 3 years are scrutinized and analyzed to understand the current market trends.
- Primary Research:
The primarily interview analysis comprise of data obtained from industry participants interview and answers to survey questions gathered by in-house primary team.
For primary research, interviews are conducted with industry experts/CEOs/Marketing Managers/Sales Managers/VPs/Subject Matter Experts from both demand and supply side to get a 360-degree view of the market. The primary team conducts several interviews based on the complexity of the markets to understand the various market trends and dynamics which makes research more credible and precise.
A typical research interview fulfils the following functions:
- Provides first-hand information on the market size, market trends, growth trends, competitive landscape, and outlook
- Validates and strengthens in-house secondary research findings
- Develops the analysis team’s expertise and market understanding
Primary research involves email interactions and telephone interviews for each market, category, segment, and sub-segment across geographies. The participants who typically take part in such a process include, but are not limited to:
- Industry participants: VPs, business development managers, market intelligence managers and national sales managers
- Outside experts: Valuation experts, research analysts and key opinion leaders specializing in the electronics and semiconductor industry.
Below is the breakup of our primary respondents by company, designation, and region:

Once we receive the confirmation from primary research sources or primary respondents, we finalize the base year market estimation and forecast the data as per the macroeconomic and microeconomic factors assessed during data collection.
- Data Analysis:
Once data is validated through both secondary as well as primary respondents, we finalize the market estimations by hypothesis formulation and factor analysis at regional and country level.
- 3.1 Macro-Economic Factor Analysis:
We analyse macroeconomic indicators such the gross domestic product (GDP), increase in the demand for goods and services across industries, technological advancement, regional economic growth, governmental policies, the influence of COVID-19, PEST analysis, and other aspects. This analysis aids in setting benchmarks for various nations/regions and approximating market splits. Additionally, the general trend of the aforementioned components aid in determining the market's development possibilities.
- 3.2 Country Level Data:
Various factors that are especially aligned to the country are taken into account to determine the market size for a certain area and country, including the presence of vendors, such as headquarters and offices, the country's GDP, demand patterns, and industry growth. To comprehend the market dynamics for the nation, a number of growth variables, inhibitors, application areas, and current market trends are researched. The aforementioned elements aid in determining the country's overall market's growth potential.
- 3.3 Company Profile:
The “Table of Contents” is formulated by listing and analyzing more than 25 - 30 companies operating in the market ecosystem across geographies. However, we profile only 10 companies as a standard practice in our syndicate reports. These 10 companies comprise leading, emerging, and regional players. Nonetheless, our analysis is not restricted to the 10 listed companies, we also analyze other companies present in the market to develop a holistic view and understand the prevailing trends. The “Company Profiles” section in the report covers key facts, business description, products & services, financial information, SWOT analysis, and key developments. The financial information presented is extracted from the annual reports and official documents of the publicly listed companies. Upon collecting the information for the sections of respective companies, we verify them via various primary sources and then compile the data in respective company profiles. The company level information helps us in deriving the base number as well as in forecasting the market size.
- 3.4 Developing Base Number:
Aggregation of sales statistics (2020-2022) and macro-economic factor, and other secondary and primary research insights are utilized to arrive at base number and related market shares for 2022. The data gaps are identified in this step and relevant market data is analyzed, collected from paid primary interviews or databases. On finalizing the base year market size, forecasts are developed on the basis of macro-economic, industry and market growth factors and company level analysis.
- Data Triangulation and Final Review:
The market findings and base year market size calculations are validated from supply as well as demand side. Demand side validations are based on macro-economic factor analysis and benchmarks for respective regions and countries. In case of supply side validations, revenues of major companies are estimated (in case not available) based on industry benchmark, approximate number of employees, product portfolio, and primary interviews revenues are gathered. Further revenue from target product/service segment is assessed to avoid overshooting of market statistics. In case of heavy deviations between supply and demand side values, all thes steps are repeated to achieve synchronization.
We follow an iterative model, wherein we share our research findings with Subject Matter Experts (SME’s) and Key Opinion Leaders (KOLs) until consensus view of the market is not formulated – this model negates any drastic deviation in the opinions of experts. Only validated and universally acceptable research findings are quoted in our reports.
We have important check points that we use to validate our research findings – which we call – data triangulation, where we validate the information, we generate from secondary sources with primary interviews and then we re-validate with our internal data bases and Subject matter experts. This comprehensive model enables us to deliver high quality, reliable data in shortest possible time.
Trends and growth analysis reports related to Medical Device and Diagnostics Contract Research Organization Market

Colonoscopes Market
Forecast to 2028 - COVID-19 Impact and Global Analysis By Product Type (Fiber Optic Colonoscopy Devices, Video Colonoscopy Devices); Application (Colorectal Cancer, Lynch Syndrome, Ulcerative Colitis, Crohn's Disease, Polyp); End User (Hospitals, Ambulatory Surgery Center, Others), and Geography
Noninvasive Fat Reduction Market
Size and Forecast (2021 - 2031), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Technology (Cryolipolysis, Laser Lipolysis, Ultrasound, and Others), End User (Hospitals, Dermatology Clinics & Cosmetic Clinics, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)
Medical Ultrasound Flow Meter Market
Size and Forecast (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Implementation Type (Clamp-On, Inline, and Others), Technology (Doppler, Transit Time, and Hybrid), Application (Heart and Lung Machines, Extracorporeal Membrane Oxygenation, Perfusion, Organ Transportation Systems, and Others), End User (Hospitals and Clinics, Ambulatory Surgical Centers, Research Laboratories, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)
Rapid Test Kits Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Type (Rapid Antigen Testing, Rapid Antibody Testing, and Others), Product (Over-the-Counter Rapid Testing Kit and Professional Rapid Testing Kit), Technology (Lateral Flow Assay, Solid Phase, Agglutination, Immunospot Assay, and Cellular Component-Based), Application (Blood Glucose Testing, Infectious Disease Testing, Pregnancy and Fertility, Cardiometabolic Testing, and Others), End User (Hospital and Clinics, Home Care, Diagnostics Centers, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)
Osteoarthritis Therapy Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trend, and Growth Opportunity Analysis Report Coverage: By Therapy Type [Transcutaneous Electrical Nerve Stimulation (TENS), Occupational Therapy, Physical Therapy, Platelet-Rich Plasma Therapy and Stromal Vascular Fraction, Prolotherapy, and Others], Disease Indication (Knee Osteoarthritis, Spine Osteoarthritis, Foot and Ankle Osteoarthritis, Shoulder Osteoarthritis, Hand Osteoarthritis, and Others), End User (Hospitals & Clinics, Specialty Clinics, Ambulatory Surgical Centers, Homecare, and Others), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)
Bariatric Surgeries Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Type [Adjustable Gastric Bands (AGB), Sleeve Gastrectomy, Gastric Bypass, Biliopancreatic Diversion with Duodenal Switch (BPD-DS), and Others], End User (Hospitals and Ambulatory Surgical Centers), and Geography (North America, Europe, Asia Pacific, Middle East & Africa, and South & Central America)
Post-Acute Care Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Service Type (Skilled Nursing Facilities, Inpatient Rehabilitation Facilities, Long-Term Care Hospitals, Home Health Agency, and Others), Age (Elderly, Adult, and Others), Disease Conditions (Amputations, Wound Management, Brain Injury and Spinal Cord Injury, Neurological Disorders, and Others), and Geography
Lung Cancer Therapy Market
Size and Forecasts (2020 - 2030), Global and Regional Share, Trends, and Growth Opportunity Analysis Report Coverage: By Therapy Type (Noninvasive and Minimally Invasive), Indication (Non-Small Cell Lung Cancer and Small Cell Lung Cancer), End User (Hospitals, Oncology Clinics, Research Centers, and Others), and Geography (North America, Europe, Asia Pacific, South & Central America, and Middle East & Africa)
Get Free Sample PDF
What's included in full report .
- Market Dynamics
- Competitive Analysis and Assessment
- Define Business Strategies
- Market Outlook and Trends
- Market Size and Share Analysis
- Growth Driving Factors
- Future Commercial Potential
- Identify Regional Growth Engines

More than a Quality Management System: Tools for the entire MedTech Lifecycle.
Featured Capabilities:

Experience the #1 QMS software for medical device companies first-hand. Click through an interactive demo.
Accelerate development with integrated design control and risk software.

Schedule a custom demo of Greenlight Guru Product now.
Data collection and management designed for MedTech clinical trials.

Get a personalized demo of Greenlight Guru Clinical today.
- By Initiative
- Migrating From Paper
- Managing and Assessing Risk
- Preparing for Regulatory Submissions
- Becoming Audit Ready
- Managing Postmarket Quality
- Small Business
- By Function
- Product / R&D
- ROI Calculator
- Customer Success
- Case Studies
- Checklists & Templates
- eBooks & Guides
- Content Hub
- Live & Virtual Events
- True Quality Roadshow
- Quality Pricing
- Clinical Pricing

Top 40+ Medical Device Contract Research Organizations (CROs)
-1.png)
In the rapidly evolving MedTech industry, companies encounter numerous challenges while striving to bring devices to market. Among these, conducting effective clinical trials stands out as a critical step, ensuring both compliance and go-to-market success. This means establishing a plan, selecting sites, collecting high-quality clinical data, meeting milestones, conducting analysis, and executing projects in compliance with regulations.
Developing a new medical device intended to improve the quality of lives requires the highest quality processes possible. For clinical studies, this means the resources involved in planning and executing these projects must be qualified and capable of achieving this outcome.
For many MedTech companies, a contract research organization (CRO) is an essential partner in this process, offering industry expertise, project management, risk mitigation, and trial conduct oversight.
At Greenlight Guru, we recognize how important it is to select the right CRO for your business. That’s why we partner with top CROs across the globe. Our Clinical Electronic Data Capture (EDC) solution is a key ingredient to the overall clinical trial process. It provides a comprehensive package specifically designed for the unique requirements of medical device clinical trials. If you’re a CRO interested in partnering with Greenlight Guru, learn more here .
If you’re a MedTech company looking to simplify your search for the right CRO, we’ve compiled a detailed list below. This list, while not a ranking, is a compilation of providers that highlights each of their strengths and core services to aid in your search.
FREE DOWNLOAD: Click here to download a free PDF version of the Top 40+ Contract Research Organizations in MedTech.
Website: https://www.avaniaclinical.com
Location: Marlborough, MA
Avania brings knowledgeable experts together to form a unique CRO that advances the research of medical devices, novel technology, and combination products across a wide range of therapeutic specialties. Their motivated team takes you from feasibility all the way through post-market trials. Avania’s experts in data management and analytics, clinical trial design & execution, strategic consulting, regulatory, reimbursement, and more will provide you with customized, scalable solutions that optimize efficiencies and streamline the advancement of your medical technology.
Website: http://mcra.com/
Location: Washington, DC
MCRA is the leading global full-service medical device, diagnostics, and biologics CRO and consulting advisory firm. MCRA delivers to its client’s industry experience, integrating its six business value creators: regulatory, clinical research, reimbursement, healthcare compliance, quality assurance, and distribution logistics to provide a dynamic, market-leading effort from innovation conception to commercialization. MCRA’s integrated application of these key value-creating initiatives provides unparalleled value for its clients. MCRA has offices in Washington, DC, Hartford, CT, New York, NY, and Tokyo, Japan and serves nearly clients globally.
Premier Research
Website: https://premier-research.com
Location: Morrisville , North Carolina
Premier Research, the clinical research company that’s Built for Biotech, is dedicated to helping biotech, specialty pharma, and device innovators transform life-changing ideas and breakthrough science into new medical treatments. More than just clinical services, they offer unique perspectives, intelligent study designs, and relentless focus on compliance and providing conclusive data. Ongoing investments into innovative technologies for smart study design and trial management to deliver clean, conclusive data to sponsors.
Website: https://www.fortrea.com
Location: Durham , North Carolina
As a leading global contract research organization (CRO) with a passion for scientific rigor and decades of clinical development experience, Fortrea provides pharmaceutical, biotechnology, and medical device customers a wide range of clinical development, patient access, and technology solutions across more than 20 therapeutic areas. With over 19,000 staff conducting operations in more than 90 countries, Fortrea is transforming drug and device development for partners and patients across the globe.
Website: https://www.iqvia.com
Location: Durham, North Carolina
I QVIA (NYSE:IQV) is a leading global provider of advanced analytics, technology solutions, and clinical research services to the life sciences industry. IQVIA creates intelligent connections across all aspects of healthcare through its analytics, transformative technology, big data resources and extensive domain expertise. IQVIA Connected Intelligence™ delivers powerful insights with speed and agility — enabling customers to accelerate the clinical development and commercialization of innovative medical treatments that improve healthcare outcomes for patients.
Website: https://namsa.com
Location: Atlanta, Georgia
NAMSA proudly serves the MedTech industry’s premier CRO partner for cost-efficient and efficacious outcomes that lead to accelerated development and marketplace success. NAMSA provides unparalleled medical device testing capabilities, strategic guidance, and tactical support to fast-track market introduction. They are committed to helping clients overcome development hurdles, mitigate concerns, and streamline commercialization efforts.
Clinius Ltd
Website: http://clinius.fi/
Location: Helsinki, Finland
Clinical device trial and Medical Regulations specialist Clinius is a device CRO with main focus on clinical trials and regulatory consulting. We support companies in the healthcare and well-being industry in understanding the needs of customers as well as meeting the regulatory requirements (MDR/IVDR). We have more than 20 years experience with supporting device development. Our key services- MDD to MDR transition - Documentation for Medical CE-marking - ISO 13485 quality management systems - Pre-audits - Clinical device trials - Clinical evaluations - Training
Website: 1med.ch
Location: Agno, Switzerland
1MED, is the leading European MedTech specialized solutions provider, offering end-to-end development expertise within the medical device, novel technology and combination products fields. Founded in 2014, with headquarter in Agno, Switzerland, and employing 100+ professionals across the Europe, 1MED's mission is to support all the MedTech organizations in facing the complexity associated with the development of innovative medical devices in compliance with MDR. Such support may include providing preclinical support, regulatory advice, clinical study management, post-marketing support and quality management systems across the clinical research continuum with a cross-functional approach which led to win the certification and certification maintenance of more than 500 different medical devices in the last 2 years
Website: https://www.iss-ag.ch/en
Location: Biel/Bienne, Switzerland
ISS AG, Integrated Scientific Services provides services to medtech companies for the development of medical devices, their introduction into the market and their maintenance. Typical services include medical software development, regulatory affairs and clinical evaluation report writing, clinical research, quality management, qualification and validation. The combination of these services with scientific work methods and project management puts ISS AG into a unique position as a service provider for the medtech industry.
Website: https://qservecro.com/
Location: Arnhem , The Netherlands
Qserve CRO is dedicated to driving the advancement of medical technologies and therapies through meticulous and impactful clinical investigations. As a leading Contract Research Organization, they specialize in providing comprehensive services that support the successful design, development, execution, and management of clinical trials. Their team of seasoned experts brings together a wealth of knowledge and experience, ensuring that your clinical trials are conducted with precision, compliance, and a commitment to delivering results that make a difference.
Website: https://veranex.com/
Location: Raleigh, North Carolina
Veranex offers best-in-class clinical and regulatory expertise, helping move your MedTech innovation to market. Veranex can help if you are in the process of moving your device to the market, looking to expand your claims, or working to comply with emerging post-market data collection requirements. Veranex’s in-depth regulatory expertise and clinical trial execution capabilities will ensure you’re checking the right boxes .
Website: http://clinchoice.com/
Location: Fort Washington, Pennsylvania
ClinChoice is a leading full-service clinical CRO offering high-quality solutions to pharmaceutical, biotechnology, medical device and consumer products clients. We contribute to a safer and better world by helping our sponsor clients accelerate drug and device approvals to market. We do this by combining our 25 years of proven quality and results with expertise in 30+ therapeutic areas, a flexible approach, and dedicated teams who enable rapid startups and fast timelines.
Website: https://www.evnia.dk/evnia-group/
Location: Copenhagen; Denmark
Evnia provides the Medical Device, Pharma and Biotech industries with specialised consulting services in the areas of Regulatory Affairs/Compliance, R&D, and Management by transforming data and regulations into measurable actions and tailored solutions.
They promote patient care and quality of life by striving, seeking, and finding solutions that support compliance and R&D decisions in investments, innovation and medical/regulatory communications.
Website: https://www.proximacro.com/
Location: Houston, Texas
Proxima CRO works with emerging biotech and medical device companies across all phases of development. As a contract research organization, Proxima organizes their team in two distinct areas: early-stage advisory consulting and all-inclusive clinical trial services.
Proxima CRO helps innovative companies overcome regulatory and clinical obstacles to get their medical products approved.
Website: http://hemex.ch/
Location: Liestal, Switzerland
HEMEX is a global provider of clinical research services to small and medium-sized life sciences companies in diverse therapeutic areas. By combining extensive clinical expertise, commitment to speed, and dedication to patient-centric approaches, HEMEX supports its clients to complete their clinical trials faster. Advancing healthcare and improving patient outcomes, HEMEX’s priority is ensuring patients can have access to lifesaving therapies within a short clinical journey. Through its subsidiaries, Hemex Germany GmbH and Hemex Benelux BV, the company can also secure project funding through different EU funds.
Website: https://qmed-consulting.com/
Location: Copenhagen, Denmark
Qmed is a full service Clinical Research Organisation(CRO) specializing in medical devices managing clinical trials and conducting strategic consultancy within regulatory affairs, quality assurance market access and reimbursement. Qmed provides strategic consulting services in connection with medical device approval as well as best-in-class expertise within clinical trial management, regulatory affairs, quality management and reimbursement and market access.
Clinical Accelerator
Website: https://clinicalaccelerator.com/
Location: Great Britain, United Kingdom
Clinical Accelerator is a full-service clinical CRO and a patient enrolment organization, with particular expertise in Central & Eastern Europe where they have thorough experience in all services relevant to clinical trials and research. In collaboration with their dedicated partners, they also operate in other countries of the world and are happy to discuss global requirements.
Website: https://www.confinis.com/
Location: Düdingen, Switzerland
Confinis is a global consulting firm in the field of medical devices, in vitro diagnostics and combination products based in Switzerland and the USA. Confinis services include regulatory affairs, quality management, design control, risk management, usability engineering/human factors, clinical evaluation, labeling, manufacturing, process validation, supply chain, post-market monitoring, auditing, and training. Their experienced and highly motivated team consists of electrical, mechanical, software, and biomedical engineers, as well as physicists, pharmacists, medical doctors, chemists, biochemists, biologists, and biotechnologists.
Website: http://bioaccessla.com/
Location: Orlando, Florida
bioaccess™ is a US-based CRO that bridges the gap between innovative Medtech companies and the untapped potential of conducting clinical research studies in Latin America. They are experts in delivering cost-effective and high-quality CRO services in Latin America. Bioaccess focuses on forward-looking Medtech companies seeking accelerated pilot, first-in-human (FIH), early-feasibility (EFS), pivotal, or post-market clinical study results.
Serena Group
Website: https://serenagroupinc.com/
Location: Cambridge, Massachusetts
SerenaGroup® Research (SGR), under the direction of Thomas Serena MD FACS, conducts clinical trials in a range of diseases related to wound healing, surgical site infections and diagnostics. SGR has set the standard in clinical trial design for chronic wounds, such as diabetic foot ulcers. To promote clinical research in the burgeoning field of wound care, SerenaGroup® established a site management organization (SMO) that assists hospitals and clinics in establishing compliant research programs. SGR has conducted more than 100 clinical trials at all phases.
novineon CRO
Website: http://novineon.com/
Location: Tubingen, Germany
Novineon CRO GmbH is a consulting and research company in the field of healthcare technology. As a professional service provider, they support international manufacturers of medical devices and in-vitro diagnostics. In close relationships with their clients, they actively manage all aspects of preclinical and clinical research, clinical evaluation, PMS (post-market surveillance) and PMCF (post-market clinical follow-up) strategies as well as performance evaluation. Furthermore, they offer regulatory services in conformity assessment procedures, also in non-European markets.
Hopkins Medtech Compliance
Website: http://www.hopkinsmedtech.com/
Location: Basking Ridge, New Jersey
Hopkins MedTech Compliance is a CRO dedicated for IVD Clinical Performance Studies for FDA & EU and regulatory filing. The organization helps medical device companies rapidly access the industry's best consultants, contractors, and candidates. Their resources assist in every stage of the product lifecycle, from clinical development to commercialization, with a focus in Quality Assurance, Regulatory Affairs, and Clinical Operations.
Website: http://excelya.com/
Location: Boulogne-Billancourt, France
Excelya is an independent partner that serves Biotech, Pharma, and Medical Device organizations with a comprehensive range of Full-service CRO, FSP, and Resourcing solutions guided by compassion, care, and excellence. Founded in 2014 and headquartered in Paris, and operating in 28 countries across Europe, USA and India, Excelya has a global team of 900+ experts who understand the critical needs of clients to provide bespoke and robust trial solutions across a broad spectrum of therapeutic areas, with specialty focus on Oncology & Hematology, Rare Disease & Pediatrics, Infectious Disease, Inflammation & Immunotherapy, Pain Treatment & CNS, and Late Phase & RWE.
Centaur Clinical Cro
Website: http://centaurclinical.com/
Location: Gardanne, France
Centaur Clinical is a CRO that runs clinical operations for Medical devices from pre-clinical trials to post-market up in Europe. The organisation runs your clinical studies with full respect of the regulatory framework for CE marking. Centaur Clinical has also set up partnerships all across the globe to provide the sponsor with an extensive service.
Website: http://oroxcell.com/
Location: Romainville, France
Oroxcell is an independent research organization (CRO) offering research services assessing the safety, toxicity and efficacy of active ingredients and finished formulations. Specialized in in-vitro testing, particularly in 3D Human Models, Oroxcell is developing its business on two fronts: safety & efficacy testing.
MDC Associates
Website: https://www.mdcassoc.com/
Location: Beverly, Massachusetts
For more than 30 years MDC has helped innovators and entrepreneurs bring In Vitro Diagnostics (IVD) and Medical Devices to market that impact the lives of millions of patients around the world. MDC’s mission is to continue to support diagnostic makers and innovators by applying our expertise in Regulatory and Clinical Affairs, Quality Systems, and Data Management to help launch new advances that promote and protect the health of our global and local communities.
Everest Clinical Research
Website: http://ecrscorp.com/
Location: Toronto, Canada
Everest Clinical Research ("Everest") is a full-service contract research organization (CRO) providing a broad range of expertise-based clinical research services to worldwide pharmaceutical, biotechnology, and medical device industries. They serve some of the best-known companies and work with many of the most advanced drugs, biologics, and medical devices in development today. Everest has been an independent CRO since 2004 with a strong foundation as a statistical and data management center of excellence. Building on this foundation, Everest has successfully developed and established itself as a full-service CRO. Everest is known in the industry for its high-quality deliverables, superior customer service, and flexibility in meeting client needs.
Clinical Research Strategies
Website: https://clinicalresearchstrategies.com/
Location: Wexford, Pennsylvania
Clinical Research Strategies assists new life sciences companies and healthcare IT entrepreneurs with their corporate start-ups. They distill the complexities of regulatory and research disciplines into accessible terms for life science executives, entrepreneurs, and investors alike who play a pivotal role in the development and investment of a new product. Clinical Research Strategies' goal is to improve your chances of clearing performance hurdles toward successful commercialization or exit.
Mediri Gmbh
Website: http://mediri.com/
Location: Heidelberg, Germany
Mediri works to make your image-based clinical trials more efficient, quick and safe. This is especially important when it comes to multicentre studies that place high demands on clinical data management.
Mediri provides the reinforcement you need with interdisciplinary services all from one single source. Their capability spans from imaging protocol and central data management to image data analysis with a clear evaluation of results. Mediri’s success is through the ability to put tremendous importance on creating shorter communication channels and providing highly qualified support for customers.
Website: http://profil.com/
Location: Neuss, Germany
Profil GmbH is a full service CRO based in Germany and certified according to ISO 9001 and 13485. Profil was founded as a spin-off of an academic study group at the Department of Metabolic Diseases and Nutrition at the University of Dusseldorf. Unlike other CROs, they focus their clinical research on diabetes and related diseases.
Requalite Gmbh
Website: http://requalite.com/
Location: Grafelfing, Germany
Requalite provides regulatory, quality, and clinical study services for in vitro diagnostic and medical device compliance in the EU. Requalite is located in the heart of Europe, in Munich-Germany. They provide full CRO services for clinical studies as per IVDR/MDR. In addition, they provide Technical and medical writing services to manufacturers of Medical Devices and In-vitro Diagnostic devices to obtain CE-marking as per EU regulations of MDR (2017/745) and IVDR (2017/746) for entering the European market.
Website: http://alvamed.com/
Location: Needham, Massachusetts
AlvaMed is a medical device consulting and outsourcing organization. They provide the full breadth of compliance services to their clients: quality assurance, quality system development, regulatory approval and compliance, and CRO/clinical management services. AlvaMed is an industry leader in medical device consulting and is comprised of technically savvy quality, clinical, and regulatory professionals with a broad range of complementary skills. The flexibility within their team allows them to quickly adapt their level of support to your changing needs or shift focus as you hire your own internal resources.
Archer Research
Website: archerresearch.eu
Location: Diepenbeek, Belgium
Archer Research is a full-service Contract Research Organization specializing in clinical studies with Medical Devices, located in the heart of Europe with a fast connection between Belgium, France, The Netherlands, The UK and Germany. They are experts in setting up and managing pre-CE as well as post-market medical device clinical studies from A to Z. Whether you are an investigator, an early-stage start-up, or a large organization, Archer Research can help you achieve your strategic goals through sound clinical and regulatory planning and execution.
Website: https://www.meditrial.net/clinical-trials/
Location: New York, New York
Meditrial is a leading provider of clinical trial services and digital cloud-based data management solutions for the medical device industry. As a Clinical Research Organization (CRO), Meditrial is renowned as the go-to partner for companies developing breakthrough devices that require a clearly defined path to first-in-man and pivotal clinical studies. Established in 2008, today Meditrial is a trusted European representative for large corporations as well as start-ups requiring support and resources to execute trials in Europe.
Aginko Research Ag
Website: aginko.com
Location: Marly, Switzerland
AGINKO Research is the premiere CRO for preclinical work in osteoarticular pathologies. They offer a wide range of contract research services to support Drug and established Medical Device companies as well as start-ups and academia. From industry-standard protocols to customized research design, their animal models and assays, both on an in vivo or in vitro basis, provide key tools for compound discovery and product development. Given their expertise, know-how, and network in the field of osteoarticular pathologies, AGINKO today also offers a full range of histology services for your preclinical and clinical trials as well as general consulting services (e.g, regulatory, strategy, financing) devoted to osteoarticular pathologies.
Clintrial Solutions
Website: https://www.clintrialsolutions.org/
Location: Roswell, Georgia
From protocol inception to trial execution and site management, all the way through to regulatory submissions, ClinTrialSolutions can help you with your clinical trial needs. With more than 30 years of experience in all aspects of clinical trials, including Investigator Initiated Trials (ISS) and Patient Reported Outcomes (PRO) Trials, ClinTrial Solutions is here to help you meet your clinical trial objectives on time and within budget.
Osmunda Medical Technology Service Group
Website: http://en.osmundacn.com/
Location: Berlin, Germany
OSMUNDA Medical Device Service Group, established in 2004, is a third-party service provider for medical devices and in-vitro diagnostic (IVDs), offering the medical device industry with global registrations, regulatory support, clinical trials (CRO), contract manufacturing (CDMO), as well as platforms for medical device management cloud (MDAC), medical-engineering cooperation & incubation, and industrial investment services. OSMUNDA Group has 12 wholly-owned branches with nearly 300 medical device experts worldwide (China, Germany, US, etc).
Appletree Ci Group Ag
Website: http://appletree-cig.com/
Location: Winterthur, Switzerland
Appletree CI Group is an expert niche CRO and global regulatory affairs service provider with track records in ophthalmology and medical device investigations. They are present in 11 European countries and have over 30 employees. By having an in-depth understanding of local cultures and customs, as well as experience with national regulations, they are able to facilitate your clinical development and regulatory projects.
Website: http://medqia.com/
Location: Los Angeles, California
MedQIA is a full-service global contract research organization specializing in Quantitative Image Analysis for clinical trials. It is their mission to deliver high-quality, innovative, computer-aided image interpretation and analysis for both clinical trials and radiological decision support. Biomarker Science MedQIA is a leader in bringing imaging biomarker science to clinical trials. The biomarker R&D program is a multidisciplinary, collaborative effort encompassing imaging physics, computer vision, pattern classification, biostatistics, and radiological sciences. Medqioa specializes in delivering cutting-edge quantitative imaging biomarkers in Phase I through IV studies for drugs and in medical devices.
Website: aicros.com
Location: Bremen, Germany
AICROS, the Association of International CROs, is an official operative network of small to midsize CROs which provide clinical research services for the medical device, pharmaceutical, and biotech industries. They understand the challenges sponsors can face in every step of their clinical development process. They overcome these constraints by quickly adapting to changing environments, adhering to specific client requirements, and applying proactive problem-solving approaches throughout the whole project. AICROS combines the advantages of large and small to midsize CROs, while offering the reliability of a global CRO.
Axonal-Biostatem
Website: http://axonalbiostatem.com/
Location: Nanterre, France
Axonal-Biostatem is a full-service European CRO specializing in Real World Evidence, providing solutions and expertise in conducting post-approval studies with high knowledge of regulatory particularities across Europe. Axonal-Biostatem provides project support to pharmaceutical, biotech companies and medical device manufacturers in the management of Phase II to IV clinical trials, pharmacoepidemiological studies, surveys, and observational studies. Throughout the years, Axonal-Biostatem has acquired an elite team of experienced scientists capable of conducting international studies and delivering accurate results on time.
While our list of top 40 CROs that service medical devices ends here – the possibilities for you and your device are just beginning. We hope this list of the top contract research organizations will help you choose a partner to develop a safe and reliable medical device that achieves regulatory compliance, surpasses your company goals, and ultimately improves the lives of the patients who use it.
When companies combine the expert services of a Contract Research Organizations (CROs) with Greenlight Guru Clinical , the leading Electronic Data Capture (EDC) platform purpose-built for MedTech, it’s a winning combination for enabling product to market.
So if you’re looking for a partner who will be with you every step of the way, then get your free demo of Greenlight Guru Clinical and learn how we can help you make the switch from your old EDC or paper to a purpose-built solution as smooth and efficient as possible.
Páll Jóhannesson
Páll Jóhannesson, M.Sc. in Medical Market Access, is the founder and Managing Director of Greenlight Guru Clinical (formerly SMART-TRIAL). Páll was previously the CEO of Greenlight Guru Clinical where he led the team to create the only EDC specifically made for medical devices.
Related Posts
Top 50 medical device product design and development companies, greenlight guru announces the 2024 true quality roadshow, greenlight guru receives strategic growth investment from jmi equity, subscribe to our blog.
Join 200,000+ other medical device professionals outperforming their peers.

Get your free PDF
- Checklists/Templates
- Request a Demo
- Content Title Description

- Product Development Strategy
- Medical Device Testing
- Biological Safety Consulting
- Clinical Research
- Preclinical Research
- Medical Device Regulatory & Quality
- Medical Writing Solutions
- Medical Device Reimbursement
- NAMSA APEX Program™
- MedTech Market Research Consulting
- Subject Matter Experts
- Therapeutic Expertise
- Resource Library
- MedTech Podcast and Video Series
- NAMSA Medical Device Test Navigators
- EU MDR & IVDR Planning Resources
- COVID-19 IVD Resources
- Monkeypox IVD Resources
- Accreditations & Certifications
- Client Testimonials
- Go to Client Portal
NAMSA IS THE WORLD’S LEADING MEDTECH CRO OFFERING GLOBAL DEVELOPMENT SERVICES
Contact Us Today
DRIVEN BY ITS GLOBAL REGULATORY EXPERTISE AND IN-DEPTH THERAPEUTIC KNOWLEDGE
From concept to post-market, delivering significant time savings vs. industry averages, helping medical device sponsors improve healthcare since 1967.
Our Services
Driven by our global regulatory expertise and in-depth therapeutic knowledge, NAMSA is a medical CRO offering only the most proven solutions to move your medical device through the development lifecycle as efficiently and cost-effectively as possible.
As a medical contract research organization, we are dedicated to helping you achieve accelerated, successful development and commercialization outcomes. NAMSA’s offerings include medical device testing; regulatory, reimbursement and quality consulting; and clinical research services.
Learn from the world’s leading medical CRO with complimentary access to our latest white papers, webinars, and other resources to learn proven strategies and best practices for successful medical device development and commercialization outcomes.
Always on the pulse of industry news, we interpret the latest regulatory guidelines and other medical device updates so you don’t have to. Learn from the world’s most trusted medical device CRO to stay up-to-date on recent news and what it means for your medical device development plans and commercial operations.
Navigating the New MDCG Guidance Documents on CIP and IB
Discover key insights on the latest MDCG guidance for EU medical device clinical investigations, aiding in compliant CIP and IB development.
Understanding the ISO 18562:2024 Standards Update for Medical Devices
The updated ISO 18562 standards, released in March 2024, now cover medical respiratory PPE like surgical masks and introduce new terms that could change testing requirements for medical devices. They emphasize the importance of considering a device's aging and life cycle, as well as providing detailed breathing volumes for expanded patient populations. These changes aim to enhance safety evaluations for devices throughout their use, presenting manufacturers with new challenges.
Collection of Race and Ethnicity Data in Clinical Studies – Standardizing the Sociocultural Construct of Our Society
The FDA's updated guidance on race and ethnicity data collection in clinical trials calls for using OMB-standardized categories to ensure accurate demographic representation. Sponsors must create a Diversity Action Plan, with mandatory implementation for new studies within 180 days after final guidance. Participants will self-report race and ethnicity, with the option to identify as multiracial and confirm their information against medical records.
Medical Device Software: Considerations for Device and Risk Characterization
On February 2, 2024, the IMDRF released a draft guidance for public comment on Software as a Medical Device (SaMD). This document aims to unify global regulations by detailing device characterization and risk assessment for medical software. It seeks to create a shared understanding that can navigate the varied regulatory landscapes, promoting safer digital health solutions. Public feedback is encouraged to refine these critical guidelines.
Revolutionizing Healthcare: The Rise of Medical Device Software and EU Regulatory Compliance
In the rapidly evolving field of healthcare technology, NAMSA provides expert guidance on navigating the complex regulatory landscape for Medical Device Software (MDSW) in Europe. The blog discusses the importance of clinical and performance evaluations according to EU regulations, highlighting the challenges manufacturers face in demonstrating safety and effectiveness. It emphasizes the need for thorough data analysis and risk assessment, particularly in areas like software safety, risk classification, and the lifespan of MDSW. NAMSA's specialized medical writing teams offer their extensive expertise to assist MDSW manufacturers in achieving compliance and fostering innovation within the digital health space.
HAVE QUESTIONS?
Let NAMSA’s medical device development experts guide you in the right direction.
Subscribe to NAMSA Events Newsletter
By submitting this form, you are providing NAMSA consent to contact you directly regarding NAMSA’s services. The information you are providing will be processed and stored by NAMSA to better understand your product needs and interests.
At any time, you can submit a request to withdraw your consent for the use of information provided by you by emailing [email protected] . For additional information, please visit our Privacy Policy or contact us at [email protected] .
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Join the NAMSA Network
Update your profile, post comments on the blog; sign-up for events, webinars and classes; receive email alerts and more.
Already have an account? Please Login Here
NAMSA Client Portal
Login to the NAMSA Client Portal for sample submissions, testing reports, project tracking, and more.
WELCOME TO THE NAMSA NETWORK!
Username or Email Address
Remember Me
Looking for Client Portal?
Visit NAMSA Client Portal for sample submissions, testing reports, project tracking, and more.
Not a member yet? Please register here .
Reset Password
Username or email
Get New Password
The Federal Register
The daily journal of the united states government, request access.
Due to aggressive automated scraping of FederalRegister.gov and eCFR.gov, programmatic access to these sites is limited to access to our extensive developer APIs.
If you are human user receiving this message, we can add your IP address to a set of IPs that can access FederalRegister.gov & eCFR.gov; complete the CAPTCHA (bot test) below and click "Request Access". This process will be necessary for each IP address you wish to access the site from, requests are valid for approximately one quarter (three months) after which the process may need to be repeated.
An official website of the United States government.
If you want to request a wider IP range, first request access for your current IP, and then use the "Site Feedback" button found in the lower left-hand side to make the request.

IMAGES
VIDEO
COMMENTS
The global medical device contract research organization market size was valued at USD 7.21 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 8.8% from 2023 to 2030. The market growth can be attributed to an increase in the number of medical device-specific clinical trials, the growing demand for advanced ...
The global medical device contract research organization market size was USD 7.15 Billion in 2022 and is likely to reach USD 15.40 Billion by 2031, expanding at a CAGR of 8.9% during 2023-2031. The market growth is attributed to the speedy development and cost-saving benefits facilitated by contract research organizations.
The Medical Device Contract Research Organization (CRO) market is a specialized segment of the medical device industry. It provides services to medical device companies, such as clinical trials, regulatory affairs, and product development.
The global Medical Device Contract Research Organization market size is expected to be worth around US$ 15.1 billion by 2030, according to a new report by Vision Research Reports.. The global Medical Device Contract Research Organization market size was valued at US$ 7.3 billion in 2020 and is anticipated to grow at a CAGR of 9.6% during forecast period 2021 to 2030.
The Medical Device Contract Research Organization Market size is expected to surpass around US$ 15.1 billion by 2030 from valued at US$ 7.3 billion in 2020 with a CAGR of 9.6% from 2021 to 2030.. Growth Factors. During the second half of the COVID-19 pandemic in 2020, the market witnessed a surge in demand for CRO services as compared to the first half, where the growth was stagnant.
DUBLIN--(BUSINESS WIRE)--The "Medical Device Contract Research Organization Market Size, Share & Trends Analysis Report By Phase (Preclinical, Clinical), By Service (Regulatory/Medical Affairs ...
One of the key objectives of the report was to estimate the existing market size and the future growth potential within the medical devices contract research market over the coming years. We have provided an informed estimate of the likely evolution of the market in the short to mid-term and mid to long term, for the period 2022-2035.
Medical Device and Diagnostics Contract Research Organization Market Forecast to 2028 - COVID-19 Impact and Global Analysis by Type and Services and Geography
The global medical device contract research organization market size is expected to reach USD 12.1 billion by 2028, registering a CAGR of 8.6% ... Medical Device Contract Research Organization ...
Medical Device Contract Research Organization Market Growth & Trends The global medical device contract research organization market size is expected to reach USD 12.1 billion by 2028, registering a CAGR of 8.6% over the forecast period, according to a new report by Grand View Research, Inc. During the second half of the COVID-19 pandemic in ...
The global medical device contract research organization market size is expected to reach USD 14.01 billion by 2030, registering a CAGR of 8.8% from 2023 to 2030, according to a new report by Grand View Research, Inc. Key drivers of the industry includes benefits, such as time-saving, cost efficiency, and expertise in the area, which ...
The Medical Device Contract Research Organization market can be segmented into various regions, including North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa. North America holds a significant market share, driven by the presence of major medical device manufacturers and a well-established healthcare infrastructure.
The global medical device contract research organization market size is expected to reach USD 14.01 billion by 2030, registering a CAGR of 8.8% from 2023 to 2030. Key drivers of the industry ...
KEY MARKET INSIGHTS. Listen to Audio Version. The contract research organization (CRO) services market size was valued at USD 82.60 billion in 2023 and is projected to grow USD 188.52 billion by 2030, exhibiting a CAGR of 12.5% during 2023-2030. Rising chronic disease burden and pressure for new treatments drive the CRO market.
Contract Research Organization Market size accounted for USD 56.7 billion in 2022 and is estimated to grow at 6.9% CAGR between 2023 and 2032. Rising adoption of advanced technologies for efficient R&D outcomes and the increasing outsourcing trends in the clinical trials industry has largely influenced the global market growth.
SAN FRANCISCO, Jan. 12, 2022 /PRNewswire/ -- The global medical device contract research organization market size is expected to reach USD 12.1 billion by 2028, registering a CAGR of 8.6% from ...
Medical Device Contract Research Organization Market Growth & Trends: The global medical device contract research organization market size is expected to reach USD 14.01 billion by 2030, registering a CAGR of 8.8% from 2023 to 2030, according to a new report by Grand View Research, Inc.Key drivers of the industry includes benefits, such as time-saving, cost efficiency, and expertise in the ...
Medical Device Contract Research Organization Market Size, Share, Growth Analysis, By Phase(Preclinical, Clinical), By Service(Project Management/Clinical Supply Management, Data Management), By Device Type(MedTech Devices, Diagnostic Devices), By Regional(North America, Europe) - Industry Forecast 2023-2030
Medical Device and Diagnostics Contract Research Organization Market was valued at US$ 12,314.65 million in 2021 and is projected to reach US$ 20,336.08 million by 2028 with a CAGR of 7.4% during 2021-2028 segmented into Type, Services and Geography.
Archer Research. Website: archerresearch.eu. Location: Diepenbeek, Belgium. Archer Research is a full-service Contract Research Organization specializing in clinical studies with Medical Devices, located in the heart of Europe with a fast connection between Belgium, France, The Netherlands, The UK and Germany.
Our Services. Driven by our global regulatory expertise and in-depth therapeutic knowledge, NAMSA is a medical CRO offering only the most proven solutions to move your medical device through the development lifecycle as efficiently and cost-effectively as possible. As a medical contract research organization, we are dedicated to helping you ...
Mobile apps are software applications that are downloaded and designed to run on mobile devices, such as smartphones and tablets. Many public entities use Start Printed Page 31327 mobile apps to provide services and reach the public in various ways, including the purposes for which public entities use websites, in addition to others.