
- Meetings Archive
- Browse By Category
- Browse By Keyword

A CASE OF PERNICIOUS ANEMIA PRESENTING WITH HYPERPIGMENTATION AND SEVERE HEMOLYSIS
Case Presentation: A 75 year old woman with past medical history of hypothyroidism presented with weakness and the sensation of “large folds of skin” on the bottom of her feet. Pertinent physical exam findings included conjunctival pallor, nonspecific diffuse abdominal tenderness, and hyperpigmented macules of the dorsal finger tips and soles, which were also new since the onset of weakness.Initial lab results were significant for pancytopenia: platelets 68, white blood cell count 2.7, and hemoglobin 5.2 with an MCV of 119. The patient was admitted for work up of anemia and was treated with 2 units of packed red blood cells. Subsequent labs revealed vitamin B12 <60 and normal folate. She had evidence of hemolysis (decreased haptoglobin, LDH 2558) and an increased absolute reticulocyte count, but her Coombs test was negative. Peripheral smear revealed macrocytic normochromic anemia with anisopoikylocytosis, leukopenia with hypersegmented neutrophils, thrombocytopenia without blasts (Figure 1). Given new onset of rash on palms and soles, an RPR was ordered which was negative. With the presumption of B12 deficiency, and given the initial severity of the deficiency, the patient was treated with a regiment of intramuscular B12 injections. Final labs after discharge revealed positive antibodies to intrinsic factor and parietal cell, thus confirming the diagnosis of pernicious anemia in our patient.
Discussion: Vitamin B12 deficiency is a common cause of megaloblastic anemia and may be accompanied by various neuropsychiatric symptoms. It is most commonly due to autoimmune gastritis, or pernicious anemia. Diagnosis can be made with serum B12 <350 pg/ml (90% sensitive), anti-intrinsic factor antibody (50% sensitive, 100% specific), and anti-parietal cell antibody (80% sensitive, 50-100% specific) (1). Although most B12-deficient cases have only mild findings, 10% of patients may develop symptomatic pancytopenia, severe anemia and less commonly hemolytic anemia (about 1.5% of cases) (2). In cases of severe pancytopenia and hemolysis in elderly patients, there may be suspicion for malignancy or leukemia, but bone marrow biopsy and aspiration are not necessary and may confuse the diagnosis.B12 deficiency may present with peripheral neuropathy, areflexia, and loss of proprioception and vibratory sense. Hyperpigmentation is rarely reported as the presenting manifestation of B12 deficiency, but the rash is characterized as hyperpigmented macules of the extremities with a predilection for the dorsum of the palms and soles, as well as accentuation over the interphalangeal joints, as seen in our patient (3, 4). When patients present with severely low levels of B12, intramuscular administration can be considered for rapid improvement of symptoms, although oral administration of high-dose vitamin B12 (1 to 2 mg daily) is just as effective (5).
Conclusions: This vignette describes an uncommon case of B12 deficiency presenting with severe hemolysis and new onset hyperpigmented rash of the palms and soles. Clinicians should maintain a wide differential when evaluating pancytopenia in an elderly patient, but characteristic peripheral smear and negative hemolysis work up should be reassuring for B12 deficiency.
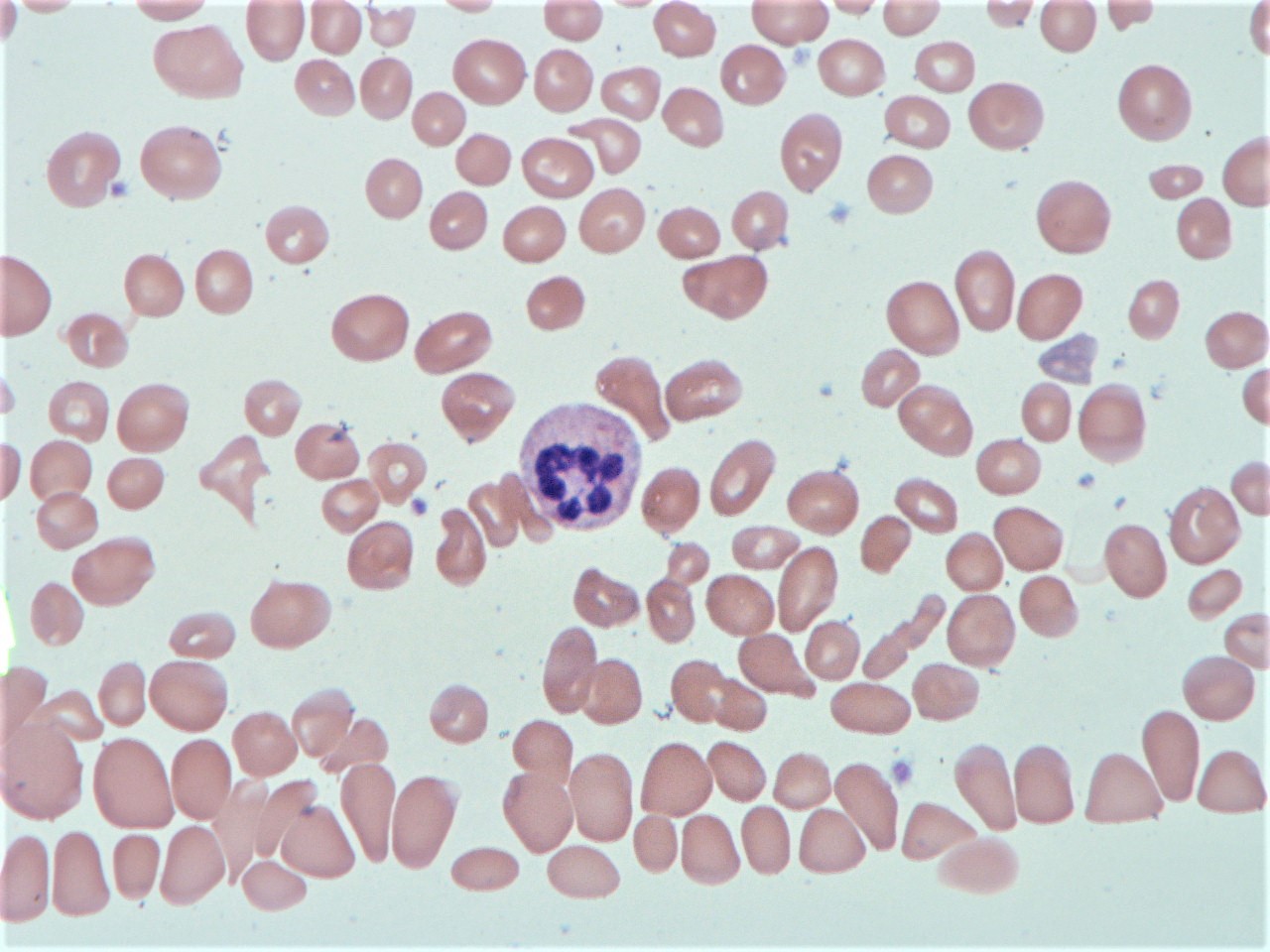
To cite this abstract:
Accetta, J; Ali, A; Lyons, J; Reynaud, P.
A CASE OF PERNICIOUS ANEMIA PRESENTING WITH HYPERPIGMENTATION AND SEVERE HEMOLYSIS.
Abstract published at Hospital Medicine 2020, Virtual Competition.
Journal of Hospital Medicine.
https://shmabstracts.org/abstract/a-case-of-pernicious-anemia-presenting-with-hyperpigmentation-and-severe-hemolysis/.
May 17th 2024.
<< Go back
Journals | Policy | Permission Journal of Medical Cases
INFORMATION
- For Authors
- For Reviewers
- For Editors
- For Readers
- Admin-login
(For the last 6-month published articles)
Web of Science (ESCI)

All content of World Journal of Oncology is permanently archived and preserved in Portico .

- Online-First
- Case Report
Volume 10, Number 3, March 2019, pages 81-83
Pernicious Anemia Presenting With Pancytopenia and Hemolysis: A Case Report
Eric Gladstone
Department of Medicine, Lenox Hill Hospital, New York, NY, USA
Manuscript submitted February 8, 2019, accepted February 25, 2019 Short title: Pernicious Anemia Presenting With Pancytopenia and Hemolysis doi: https://doi.org/10.14740/jmc3269
- Introduction
Vitamin B12 deficiency is a well-known cause of anemia. Pancytopenia and hemolysis are less frequently observed in vitamin B12 deficiency. Reported is the case of a 58-year-old man who presented with dyspnea on exertion, fatigue, and unintentional weight loss. He was found to have severe anemia (hemoglobin 3.3 g/dL) in association with pancytopenia and hemolysis. Extensive workup revealed vitamin B12 deficiency secondary to pernicious anemia. A literature review confirmed that this case demonstrates the rare occurrence of severe anemia in association with pancytopenia and hemolysis due to B12 deficiency.
Keywords: Pernicious; Anemia; Pancytopenia; B12; Hemolysis
Vitamin B12 deficiency is a common cause of anemia. Pernicious anemia leading to pancytopenia and hemolysis is rare. Providers should be cognizant of this phenomenon as the workup of pancytopenia and hemolysis can be invasive and costly.
A 58-year-old African American male presented to the emergency room (ER) with 6 weeks of progressively worsening dyspnea on exertion, fatigue and unintentional weight loss. He had a past medical history of iron deficiency anemia, gout, and osteoarthritis. He denied other respiratory symptoms. He never used tobacco. He reported frequent gum bleeding when brushing his teeth but denied nosebleeds, hemoptysis, hematochezia, melena or hematuria. He reported intermittent chills and a 15-pound weight loss which he attributed to poor appetite. Review of systems was otherwise unremarkable. He ate a balanced diet and was not a vegetarian or vegan. He recalled being diagnosed with iron deficiency anemia at an ER 5 year prior, requiring transfusion at the time of diagnosis. He did not recall any workup for the cause of his anemia. He did not have a primary care physician. Since then, he had been taking iron supplements twice a week. His only other medication was naproxen, which he took only sparingly on occasion. He sometimes smoked marijuana for recreation, but denied alcohol use. He was recently divorced and was sexually active with a few new female partners, using condoms consistently. His father developed prostate carcinoma at around the age of 70. There was no other known family history of malignancy or rheumatologic disorders.
At presentation, his vital signs were stable. Exam revealed subconjunctival pallor, two mildly enlarged submandibular lymph nodes that were mobile and nontender, hepatomegaly with right upper quadrant tenderness, scattered ecchymoses of the lower extremities and hyperpigmentation of the gums and interphalangeal joints. Labwork revealed hemoglobin of 3.3 g/dL, hematocrit 9.4%, mean corpuscular volume (MCV) 119 fL, red cell distribution width (RDW) 26%, white blood cell (WBC) 3,000/µL (55% lymphocytes, 42% neutrophils, 2% eosinophils, 1% basophils), platelets 41,000/µL, and reticulocyte percent 1.4 (reticulocyte index 0.13). Manual smear showed macrocytosis, hypersegmented neutrophils, large platelets, and many atypical lymphocytes as well as occasional schistocytes. Prothrombin time (PT) was 13.1 s, partial thromboplastin time (PTT) 28.4 s, fibrinogen 269 mg/dL, D-dimer 573 ng/mL, lactate dehydrogenase (LDH) > 2,500 U/L, and haptoglobin < 10 mg/dL. Comprehensive metabolic panel (CMP) was unremarkable. Urinalysis (UA) showed small bilirubin, urobilinogen > 8 EU/dL, trace leukocyte esterase and trace blood. Chest X-ray (CXR) was unremarkable. Computed tomography (CT) abdomen and pelvis with intravenous (IV) contrast showed hepatomegaly (right lobe spanning 19 cm) and several small hepatic hypodensities too small to be characterized, but no splenomegaly, lymphadenopathy or other evidence of malignancy. He was transfused four units of packed red blood cells and was admitted for further workup. Serum vitamin B12 level was low at < 150 pg/mL. Folate level was normal at 13.7 ng/mL. Iron studies showed elevated total serum iron, ferritin, and percentage of saturation with low total iron binding capacity (TIBC). Human immunodeficiency virus (HIV) and hepatitis C screens were negative. Epstein-Barr virus (EBV) serologies were consistent with prior exposure. Antinuclear antibody (ANA) titer was 1:160. Quantitative rheumatoid factor was 9 IU/mL (WNL). Transglutaminase antibodies were negative. Positron emission tomography (PET) CT showed no evidence of malignancy. A bone marrow biopsy was performed. The marrow showed myeloid and erythroid hyperplasia with megaloblastic changes of erythroid elements. Cytogenetic analysis showed no consistent abnormalities and no evidence of leukemia or lymphoma. Lab findings conclusive for pernicious anemia returned on hospital day 3; these included elevated levels of homocysteine (155 mol/L) and MMA (88,883 nmol/L) and the detection of intrinsic factor antibodies.
Vitamin B12, also termed cobalamin, is required for formation of hematopoietic cells. Cobalamin is a cofactor used in the synthesis of DNA as well as in metabolism of fatty acids and amino acids. Additionally, through an incompletely known mechanism, vitamin B12 is used in neuronal myelination.
Pancytopenia is a rare manifestation of vitamin B12 deficiency. More commonly, simple anemia is observed. The differential for pancytopenia is broad, including infection, autoimmune conditions, marrow failure, and marrow space-occupying lesions. Pancytopenia due to vitamin B12 deficiency is more common in developing countries [ 1 ]. Pancytopenia occurs in cases of severe vitamin B12 deficits that are sustained over time. The mechanism of the cytopenias is defective DNA synthesis [ 2 ]. While all hematopoietic cell lines are affected, the impact on erythrocytes is greatest. In one study of 201 patients with documented cobalamin deficiency, two-thirds of patients showed hematologic abnormalities [ 3 ]. Macrocytosis was the most commonly observed finding (54%), followed by anemia (37%), hypersegmented neutrophils (32%), leukopenia (13.9%), and thrombocytopenia (9.9%) [ 3 ]. Pancytopenia was seen in 5% of patients and hemolytic anemia in 1.5% [ 3 ]. Thrombocytopenia is usually due to ineffective platelet production. While some patients have marrow aplasia, megakaryocytic mass and platelet survival are usually normal [ 4 , 5 ]. There are cases in the literature of isolated thrombocytopenia; however this is rare [ 6 ]. Leukopoietic cells may reach large dimensions and have trouble crossing marrow sinuses to reach the peripheral blood, contributing to leukopenia [ 7 ]. Only rarely does the thrombocytopenia and leukopenia cause clinical problems.
Cobalamin deficiency can lead to both intramedullary and extramedullary hemolysis. Intramedullary hemolysis is more common and occurs due to macrophage destruction of megaloblastic cells in the bone marrow. Conversely, extramedullary hemolysis in cobalamin deficiency (“pseudothrombotic microangiopathy”) occurs due to fragmentation of erythrocytes in capillaries. This appears to be multifactorial. Cobalamin deficiency leads to decreased deformability of the red blood cell (RBC) membrane as well hyperhomocysteinemia promoting endothelial dysfunction [ 8 - 10 ]. In both intramedullary and extramedullary hemolysis, serum LDH levels will be high while haptoglobin levels will be low. In purely intramedullary hemolysis, schistocytes would not be expected on peripheral smear. When schistocytes are seen, other types of microangiopathic hemolytic anemia, most importantly thrombotic thrombocytopenic purpura (TTP), should be considered. TTP is a syndrome of thrombocytopenia, hemolytic anemia, kidney injury, fever and changes in mental status. Distinguishing TTP from pseudothrombotic microangiopathy based on routine labs is imperative as ADAMTS13 assay results are not rapidly available. In pseudothrombotic microangiopathy, the reticulocyte count should be low. Bilirubin levels will be lower than expected as destruction of immature erythroblasts releases less hemoglobin than with mature erythrocytes. Alternatively, LDH levels and platelets are generally lower in TTP.
Animal products are the main source of cobalamin. Strict vegetarians are at greater risk of deficiency. Other causes of deficiency include pernicious anemia, gastritis and impaired absorption. Impaired absorption is often secondary to inflammatory bowel disease (IBD), surgery, pancreatic insufficiency and celiac disease. Bacterial overgrowth and infection with fish tapeworm are also predisposing factors. Common medications such as metformin, proton pump inhibitors and histamine receptor antagonists can interfere with cobalamin absorption. Rarely, transcobalamin deficiency (an autosomal recessive genetic disorder) is the culprit. The most common cause of deficiency is pernicious anemia. Pernicious anemia is caused by autoantibodies targeting intrinsic factor or gastric parietal cells.
Stores of vitamin B12 are generally large enough to supply adequate levels for over 5 years. Aside from anemia, signs of deficiency include glossitis, neuropathies and cognitive dysfunction. Degeneration of the posterior and lateral columns of the spinal cord in cobalamin deficiency is termed subacute combined degeneration. Classically, the neuropathy manifests as symmetric paresthesias, numbness or problems with ambulation. The legs are affected before the arms.
Diagnosing deficiency usually begins with testing of the serum vitamin B12 level. The Schilling test is not routinely used anymore. Confirmation of deficiency should be performed by measuring levels of homocysteine and methylmalonic acid. Both will be elevated in vitamin B12 deficiency, as opposed to folate deficiency where MMA will be normal. The CBC may show an elevated mean corpuscular volume. On a peripheral smear one may observe hypersegmented neutrophils. Hypersegmented neutrophils possess ≥ five lobes. A bone marrow evaluation is likely to reveal a markedly hypercellular marrow with megaloblastic erythroid hyperplasia, giant metamyelocytes, and frequent mitoses.
In this case, the patient presented with many classical features of vitamin B12 deficiency. The best initial clue was his MCV of 119 fL. While vitamin B12 deficiency does not always present with an elevated MCV, an MCV of > 120 fL is 99% specific for vitamin B12 deficiency [ 11 ]. Other causes of macrocytosis such as heavy alcohol use, folate deficiency, and reticulocytosis were excluded. The finding of hypersegmented neutrophils in addition to MCV > 100 fL has a specificity of 97% for vitamin B12 deficiency [ 11 ]. Although vitamin B12 deficiency can account for pancytopenia, we decided to perform a bone marrow biopsy and PET-CT to look for evidence of malignancy. The patient’s history of unintentional weight loss was one driving factor. Interestingly, vitamin B12 deficiency has been associated with constitutional symptoms including fevers [ 12 ]. The patient’s elevated LDH and low haptoglobin likely reflected a primarily intramedullary hemolysis as few schistocytes were observed.
Acknowledgments
None to declare.
Financial Disclosure
Conflict of Interest
There is no financial support or relationships that may pose conflict of interest.
Informed Consent
Written consent was obtained from the patient.
Author Contributions
EG was involved in patient care and writing the manuscript.
- Premkumar M, Gupta N, Singh T, et al. Cobalamin and folic acid status in relation to the etiopathogenesis of pancytopenia in adults at a tertiary care centre in North India. Anemia. 2012;2012:707402. doi pubmed
- Wadood Khan ZA, Vidyasagar S, Bekur R, Belurkars S, et al. Subhyaloid haemorrhage in a patient with vitamin B12 deficiency: a unique presentation. J Clin Sci Res. 2013;2:161-164.
- Andres E, Affenberger S, Zimmer J, Vinzio S, Grosu D, Pistol G, Maloisel F, et al. Current hematological findings in cobalamin deficiency. A study of 201 consecutive patients with documented cobalamin deficiency. Clin Lab Haematol. 2006;28(1):50-56. doi pubmed
- Edited by McKrae. Thrombocytopenia. 2006.
- Ghosh K, Sarade R, Varma N, et al. Megakaryocytic thrombocytopenia of nutritional B12 deficiency. Trop Geogr Med. 1988;40(2):158-160. pubmed
- Okur M, Ozkan A, Gunes C, Kaya M, Kocabay K. Case of isolated thrombocytopenia due to cobalamin deficiency. Int J Hematol. 2011;94(5):488-490. doi pubmed
- Briani C, Dalla Torre C, Citton V, Manara R, Pompanin S, Binotto G, Adami F. Cobalamin deficiency: clinical picture and radiological findings. Nutrients. 2013;5(11):4521-4539. doi pubmed
- Malla M, Seetharam M. To treat or not to treat: a rare case of pseudo-thrombotic thrombocytopenia purpura in a Jehova's Witness. Transfusion. 2016;56(1):160-163. doi pubmed
- Tuten N, Bennett C, Babcock W. Thrombotic thrombocytopenic purpura or cobalamin deficiency? A case report and review. Clin Case Rep Rev. 2015;1(8):157-159. doi
- Acharya U, Gau JT, Horvath W, Ventura P, Hsueh CT, Carlsen W. Hemolysis and hyperhomocysteinemia caused by cobalamin deficiency: three case reports and review of the literature. J Hematol Oncol. 2008;1:26. doi pubmed
- Savage DG, Ogundipe A, Allen RH, Stabler SP, Lindenbaum J. Etiology and diagnostic evaluation of macrocytosis. Am J Med Sci. 2000;319(6):343-352. doi
- Manuel K, Padhi S, G'Boy Varghese R. Pyrexia in a patient with megaloblastic anemia: a case report and literature review. Iran J Med Sci. 2013;38(2 Suppl):198-201. pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. Journal of Medical Cases is published by Elmer Press Inc.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Case Rep Hematol
- v.2016; 2016

Pernicious Anemia with Autoimmune Hemolytic Anemia: A Case Report and Literature Review
Sri lakshmi hyndavi yeruva.
Division of Hematology and Oncology, Department of Internal Medicine, Howard University Hospital, Washington, DC 20060, USA
Raj Pal Manchandani
Patricia oneal.
Pernicious anemia is a common cause of vitamin B12 deficiency. Here, we discuss a case of a young woman who presented with severe anemia along with a history of iron deficiency anemia. After a review of her clinical presentation and laboratory data, we identified an autoimmune hemolytic anemia and a concomitant pernicious anemia. The concurrence of both these hematological diagnoses in a patient is rare.
1. Introduction
Vitamin B12 deficiency is common in the United States and pernicious anemia is one of the most common causes of vitamin B12 deficiency throughout the world [ 1 ]. Patients with vitamin B12 deficiency usually present with hematologic and neurologic findings. The hematologic manifestations can range from megaloblastic anemia to pancytopenia and can be life threatening in approximately 10% of patients [ 2 ]. In this paper, we present a young female with a history of iron deficiency anemia who came with severe anemia secondary to autoimmune hemolytic anemia (AIHA) and a vitamin B12 deficiency caused by a deficiency in intrinsic factor leading to pernicious anemia.
2. Case Report
A 22-year-old African American female with past medical history of hypertension and iron deficiency anemia presented with generalized fatigue and new onset dyspnea on exertion of three-week duration. She started having heavy menstrual bleed requiring blood transfusions in last six months and recent transfusion of four units of packed red blood cells (PRBCs) was one month ago at an outside facility. Her last menstrual cycle was also a month ago. She denied any history of autoimmune diseases or history of miscarriages. She is sexually active with no history of sexually transmitted diseases. Her only home medications included iron supplements and oral contraceptive pills.
On admission, her vitals and physical examination were normal except for a heart rate of 132 beats/minute, pale conjunctiva with an icteric sclera, and morbid obesity. She did not have any organomegaly and neurologic examination was normal. Further review of labs showed hemoglobin and hematocrit of 4.8 g/dL (normal range: 12.1–15.9) and 13.4% (normal range: 34.3–46.6), respectively, with platelets of 91,000 (normal range: 177000–406000), MCV 87.7 fL (normal range: 77.8–98), RDW 14.4%, absolute reticulocyte count of 97,000, and reticulocyte percent of 7.8. Additional labs included serum iron 273 mc/dL (normal range: 55–185), ferritin 500 ng/mL (normal range: 20–400), transferrin of 234 mg/dL (normal range: 192–382), haptoglobin 2 mg/dL (normal range: 36–195), and LDH 1868 IU/L (normal range: 0–250). Renal, coagulation, and hepatic function tests were normal except for elevated total bilirubin of 1.5 mg/dL (normal range: 0.2–1.2). The peripheral smear showed marked anisocytosis, hypochromasia with poikilocytosis, many macrocytes, microcytes, and few schistocytes. Given her recent blood transfusion, we tried to determine if the hemolytic process was secondary to AIHA versus delayed hemolytic transfusion reaction. Her results showed no alloantibodies; however, a direct antiglobin test (Coombs) with IgG was found to be positive which was suggestive of AIHA. Further anemia workup also revealed a vitamin B12 deficiency at 60 pg/mL (250–950), folate 7.26 ng/mL (5.9–24.8), and normal thyroid profile. Homocysteine and methylmalonic acid levels were elevated at 22.4 μ mol/L (<10.4) and 2300 nmol/L (87–813), respectively. These laboratory results confirmed a vitamin B12 deficiency. The etiology of the vitamin B12 deficiency was then ascertained with presence of intrinsic factor blocking and anti-parietal cell antibodies as lack of intrinsic factor and loss of parietal cells, resulting in pernicious anemia. Autoimmune workup with anti-centromere antibody, cyclic citrullinated peptide, ANA levels, rheumatoid factor along with serum immunoglobulins, and complements were negative. Her hepatitis panel showed immunity to hepatitis B and nonreactivity to hepatitis C and HIV.
After receiving two units of PRBCs for her symptomatic anemia, she was started on prednisone 100 mg (1 mg/kg of body weight) daily for the treatment of AIHA. Meanwhile, given her concomitant vitamin B12 deficiency, she was also initiated on daily parenteral vitamin B12 supplementation along with iron and folic acid supplementation. With these treatments, her hemoglobin and hematocrit levels improved to 7.1 g/dL and 20.9% with a good reticulocyte response of 345,000 over a course of five days of treatment. Steroids were then decreased to prednisone 40 mg and patient was discharged home.
Outpatient upper GI endoscopy was performed which showed patchy area of nonerosive gastritis in the antrum and atrophic mucosa in the body of the stomach. Biopsy of the atrophic mucosa revealed chronic active atrophic gastritis with intestinal metaplasia and immunostaining for Helicobacter pylori was negative. These findings were consistent with pernicious anemia.
On her subsequent clinic visits, we continued her on prednisone 40 mg daily and monthly subcutaneous vitamin B12 supplementation until her hemoglobin and hematocrit stabilized. After the direct Coombs test became negative at six months, with a stable hemoglobin more than 10 g/dL, normal LDH, and reticulocyte count < 100,000, the steroids were gradually tapered off over two months while she was continued on subcutaneous vitamin B12 supplements.
Her laboratory data from most recent follow-up in clinic, which was two years after her initial presentation, was hemoglobin and hematocrit of 12.4 g/dL and 38%, respectively, MCV 88 fL with a reticulocyte percent of 3.5.
3. Discussion
Vitamin B12 deficiency varies and increases with age. Prevalence of vitamin B12 deficiency is 3% in patients aged 20 to 39 years [ 3 , 4 ]. Pernicious anemia is an autoimmune disease caused by vitamin B12 deficiency due to atrophic gastritis or loss of parietal cells or lack of intrinsic factor. A diagnosis of pernicious anemia is made when patients have a low vitamin B12 level and positive intrinsic factor antibody or parietal cell antibody or low vitamin B12 level and presence of atrophic gastric mucosa, establishing the autoimmune nature of the disease and presence of vitamin B12 deficiency. Given the difficulty with accurate diagnosing of vitamin B12 deficiency on the currently available methods, patients with low normal vitamin B12 levels could still be deficient in it and in these instances, a diagnosis is made by elevated methylmalonic acid and homocysteine levels [ 5 ].
Vitamin B12 deficiency can present with a hemolytic picture in 1.5% of patients with elevated LDH, low haptoglobin, and elevated indirect bilirubin mostly due to ineffective erythropoiesis and intramedullary destruction [ 2 , 4 ]. Managing our patient was challenging as she presented with a severe normocytic anemia and hemolytic picture, none of which were suggesting vitamin B12 deficiency. Further evaluation for causes of anemia revealed a diagnosis of vitamin B12 deficiency due to deficiency of intrinsic factor and subsequently a diagnosis of pernicious anemia was made. As no alloantibodies were detected on further workup, a delayed hemolytic transfusion reaction as a cause for her hemolysis was ruled out.
As the management of anemia due to pernicious anemia with hemolysis is different from patients presenting with autoimmune hemolytic anemia (AIHA) with a positive direct Coombs test, differentiating these two conditions early on is important. Although it has been reported that patients with pernicious anemia can transiently have a positive Coombs test which becomes negative on treatment with vitamin B12 supplementation, patients with true AIHA would not respond to vitamin B12 supplementation, unless they are treated with steroids [ 6 ].
The autoimmune nature of the pernicious anemia is not fully understood but patients with this disease can have higher incidence of other autoimmune diseases, which might be due to a shared susceptibility in some individuals [ 5 ]. These two autoimmune mediated conditions (pernicious anemia and AIHA) can present concomitantly as in our patient or can be preceded by one another. The presence of autoimmune hemolytic anemia with a positive antiglobin test is not a common finding. In a study by Pirofsky and Vaughn, it was found that, among 103 patients with pernicious anemia, only 5 patients had positive antiglobin test [ 7 ]. In another study involving 865 patients with autoimmune hemolytic anemia, only three patients with pernicious anemia were positive for direct Coombs test [ 8 ].
A further literature search was done of published case reports and case series on autoimmune hemolytic anemia with a positive direct Coombs test in patients with pernicious anemia. As seen in Table 1 , this association was noted mostly in women who were middle aged with other autoimmune conditions.
Reported cases of pernicious anemia with autoimmune hemolytic anemia.
4. Conclusion
Though AIHA and pernicious anemia are not common, clinicians should have a high index of suspicion when patients present with hemolytic picture and severe megaloblastic anemia, as a careful and systematic approach to diagnose the etiology of anemia can prevent patients from undergoing unnecessary procedures and treatments.
Competing Interests
The authors declare that they have no competing interests.
- Open access
- Published: 13 May 2024
Causal inference between pernicious anemia and cancers: a bidirectional two-sample mendelian randomization analysis
- Bangwei Che 1 ,
- Shenglan Yuan 2 ,
- Hongyan Zhang 3 ,
- Jiancheng Zhai 1 ,
- Yang Zhang 2 ,
- Chuanchuan Wu 2 &
- Kaifa Tang 1
BMC Cancer volume 24 , Article number: 586 ( 2024 ) Cite this article
116 Accesses
Metrics details
Observational study investigated the association between pernicious anemia (PA) and cancers. However, with the exception of gastric cancer, the results are mostly contradictory. The purpose of this study was to investigate the potential causal relationship between PA and cancers through bidirectional two-sample Mendelian randomized (MR) analysis.
The European sample FinnGen project provided the genetic summary data for PA and 20 site-specific cancers. This bidirectional two-sample MR design mainly used the inverse variance weighting (IVW) method to evaluate the causal relationship between PA and cancer risk. Benjamini-Hochberg correction was performed to reduce the bias caused by multiple tests.
Our study shows that there was a causal relationship between PA and gastric cancer, prostate cancer, testicular cancer and malignant melanoma of skin, and there was a reverse causal relationship between prostate cancer or gastric cancer and PA ( P < 0.05). After Benjamini-Hochberg correction test, there was still a causal correlation between PA and gastric or prostate cancer ( P’ < 0.05), while there was only an implied causal association between PA and testicular cancer and malignant melanoma of skin ( P ’> 0.05). There was still a reverse causal relationship between gastric cancer and PA ( P ‘< 0.05), while prostate cancer shows an implied reverse causal relationship( P ’> 0.05). In addition, MR-Egger and MR-PRESSO tests showed no significant horizontal pleiotropy.
Conclusions
PA may be genetically associated with testicular cancer, prostate cancer, gastric cancer, and malignant melanoma of skin.
Peer Review reports
Introduction
Pernicious anemia (PA) is an autoimmune disease, mainly due to autoantibodies targeting and destroying gastric parietal cells, resulting in vitamin B12 deficiency caused by a lack of internal factors [ 1 ]. This condition is often mistaken for simple vitamin B12 deficiency, but it specifically refers to the vitamin B12 deficiency caused by gastric atrophy and/or the lack of intrinsic factor [ 2 ]. PA is the end stage of autoimmune gastritis, a disease characterized by immune-mediated damage to gastric parietal cells, accompanied by gastric body atrophy and the absence of intrinsic factor.
A critical component of PA’s pathophysiology involves the destruction of the oxyntic mucosa, resulting in the subsequent emergence of hypo- and achlorhydria, as well as elevated circulating gastrin levels. Hypergastrinemia, a defining characteristic of PA, plays a significant role in the development of gastric neuroendocrine tumors (NETs) [ 3 ]. Gastrin, a peptide hormone, stimulates the growth and function of gastric cells, particularly endocrine cells. In the setting of PA, persistent hypergastrinemia can promote the proliferation of gastrointestinal chromaffin cells, precursors to NETs. Type I gastric NETs, also known as gastrinomas, are a subtype strongly linked to hypergastrinemia. These tumors are typically numerous, small, and located in the gastric mucosa or submucosa. They are thought to arise from the hyperplasia of enterochromaffin-like cells in response to chronically elevated gastrin levels. Consequently, individuals with PA, who often exhibit persistently elevated gastrin levels, are at an increased risk of developing type I gastric NETs [ 4 ]. The clinical management of PA patients should therefore include regular surveillance for the early detection of gastric NETs. This longstanding understanding has also contributed to the belief that patients with PA face a higher risk of gastric cancer [ 5 , 6 ].
According to genetic epidemiological research, the genome of an individual plays a major role in determining their susceptibility to autoimmune diseases [ 7 ]. Therefore, it is generally believed that autoimmune diseases may lead to the development of cancer [ 8 , 9 ]. In addition to gastric cancer, observational studies have shown inconsistent or missing evidence for the association between PA and other cancers [ 10 , 11 ]. On the one hand, this may be due to the fact that PA is usually asymptomatic, which leads to an underestimation of the true prevalence of PA diagnosis and complications [ 12 ]. On the other hand, observational studies could be skewed by reverse causality and confounding variables [ 13 ]. This implies that in order to systematically evaluate the relationship between PA and cancer risk, genetic causal association analysis is required.
The Mendelian randomization (MR) study uses single nucleoside polymers (SNPs) as instrumental variables (IVs) to study the association between disease / exposure factors and disease, which can effectively solve the confounding and reverse causality of traditional observational studies, so it is regarded as a complementary strategy for randomized controlled trials [ 14 ]. Thus, in order to ascertain the causal relationship between PA and 20 site-specific cancers, this study employed a bidirectional two-way MR design.
Study design
PA and cancer were assessed as causally related using a bidirectional two-sample MR analysis. Three fundamental presumptions served as the foundation for the MR study: (I) Relevance hypothesis, genetic IVs must be strongly related to PA (cancers). (II) Independence hypothesis, the selected IVs cannot be associated with confounding factors. (III) Exclusive hypothesis, IVs can only affect cancer (PA) risk through PA (cancer). In this study, the direction of the causal relationship between PA and cancer was further determined by a two-way MR design, and the potential reverse causal relationship was determined (Fig. 1 ).
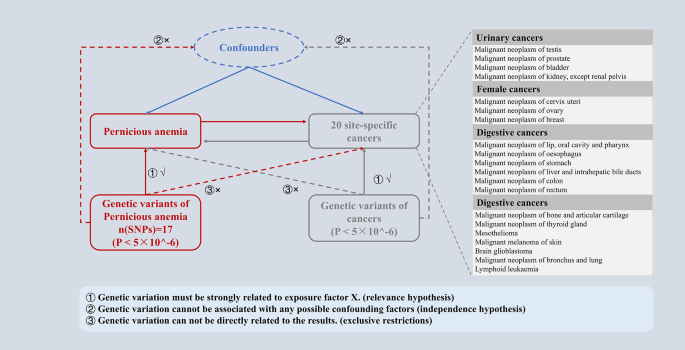
Overview of the study design in this bidirectional MR study
Exposure / outcome data source
PA and cancer data, including publicly summarized statistics of 21 genome-wide association studies (GWAS), were used in this study from the European sample FinnGen Project R5 ( https://www.finngen.fi/en ) (Supplementary Table 1 ). In order to minimize population stratification bias, the exposure and outcome cohorts were restricted to participants of European descent, and the cancer GWAS data excluded cases of other cancers.
This study does not require institutional review committee ethical approval because all FinnGen Project studies have been approved by the local institutional review committee and ethics committee.
Instrumental variable selection and statistical analysis
For univariate two-sample MR analyses, the reference/alternative alleles were first examined for coordination between exposure and outcome. SNPs were limited by linkage disequilibrium (LD, r2 equilibrium = 0.001, and window size = 1000 kb). SNPs associated with PA (cancer) that have suggestive genome-wide significance ( P < 5 × 10^-6) were employed as IVs for the disease because PA SNPs found by GWASs rarely reach the level of genome-wide significance ( P < 5 × 10^-8). IVs associated with confounding factors of outcome were removed by examining the secondary phenotype of each SNP on the PhenoScanner. For the statistically significant IVs, F statistics are further calculated to verify whether they were strong tools [ 15 , 16 ].
The Inverse variance weighted (IVW) were the main MR methods in this study. Benjamini-Hochberg correction was performed to reduce the bias caused by multiple tests. When IVW P < 0.05, but Benjamini-Hochberg corrected P > 0.05, the correlation was considered suggestive. On the contrary, if the Benjamini-Hochberg corrected P < 0.05, it was considered significant. MRlap was used to detect potential sample overlap and its impact on result bias [ 17 ]. MR-PRESSO was used to detect the existence of horizontal pleiotropy and to eliminate abnormal SNP (outliers) and estimate the corrected results (Supplementary Table 3 ). The MR-Egger intercept test was used to investigate the presence of directional pleiotropy in the effect estimates ( Pmr < 0.05 indicating its existence), and the MR-Egger adjusted effect estimates, which accounts for average directional pleiotropy, were also reported. Cochran’s Q was used to test heterogeneity (pval < 0.05 indicating heterogeneity). In addition, leave-one-out was used to determine whether the estimated value is driven by a single SNP. Through a search on the PhenoScanner platform to determine the pleiotropy between PA-related SNPs and cancer. As PA and cancer phenotypes were binary, the effect estimates (log odds ratio) were converted by multiplying 0.693 (ln2), which emphasizes causal correlation and weakens causal effect size. The results could be explained by the effect of doubling the risk of PA on the risk of cancer. In the opposite direction, it showed the effect of doubling the risk of cancer on the risk of PA [ 18 , 19 ].
All data processing, MR analysis and mapping are carried out in R software (version 4.3.1). The main software packages used include “TwoSampleMR”, “MRlap”, “dplyr”, “tidyverse”, “grid”, “forestploter”, “cmplot” and “MRPRESSO”. P < 0.05 was considered statistically significant.
Selection of SNPs: PA IVs
We finally identified 17 independent SNPs significantly associated with PA as tool variables. Each SNP’s F statistical value was more than 10, which rules out any possibility of weak instrument bias (Supplementary Table 2 ). Based on the PhenoScanner search results, except for rs28407950 (cervical cancer) and rs17656368 (skin cancer), none of the other 15 IVs has 20 cancer-related SNPs. Subsequently, after removing the SNPs with horizontal pleiotropy, the final IVs were obtained.
The Leave-one method did not detect high-impact SNPs in any of the tests (Supplementary Figs. 1 , 4 , 7 , and 10 ). MRlap method showed that the results of this study were not affected by sample overlap. There was no difference between MR-Egger intercept and zero, indicating that there was no directional pleiotropy. The heterogeneity between IVs was not detected by the Cochran Q test in this study ( P > 0.05).

Causal effects of PA on urinary cancers
In the MR analysis of PA and urinary tumors, the fixed effect / random effect IVW (IVW-FE / IVW-RE) model showed PA and testicular cancer (IVW-FE, beta = -0.075; 95%CI = -0.144, -0.003; P = 0.04; IVW-RE, beta = -0.075; 95%CI = -0.144, -0.003; P = 0.04) or prostate cancer (IVW-FE, beta = -0.022; 95% CI = -0.035, -0.006; P = 0.007; IVW-RE, beta = -0.022, 95%CI = -0.035, -0.006; P = 0.005) was a significant causal relationship between risks. The results of Benjamini-Hochberg correction test showed that there was still a significant causal relationship between prostate cancer and PA ( P’ <0.05). And the causal relationship between PA and testicular cancer was suggestive only ( P’ >0.05). No significant causal relationship was observed between PA and bladder cancer or renal cell carcinoma ( P > 0.05) (Fig. 2 , Supplementary Figs. 2 – 3 ).
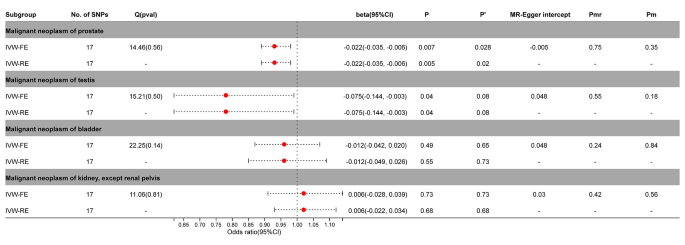
Association between genetic susceptibility to pernicious anemia and urinary cancers. IVW-FE; Inverse Variance Weighted Fixed Effects estimation method; IVW-RE; Inverse Variance Weighted Random Effects estimation method; beta, the average change (when exposed to the variable) in the outcome per 2-fold increase; SNP, single nucleotide polymorphism; 95%CI, 95% Confidence Interval; P, The IVW estimated P-value; P’, The IVW estimated P-value adjusted by the Benjamin-Hochberg method; Q(pval), Cochran Heterogeneity Test P-value; Pmr, MR–Egger P-value; Pm, MRlap P-value; -, Not applicable
Causal effects of PA on female cancers
After adjustment by MR-PRESSO, SNPs with horizontal pleiotropy in cervical cancer (rs9270535, rs140650994, rs79132259, rs75973258, rs7310615, rs151234, rs35056955, and rs73597298) were removed. Breast cancer or cervical cancer did not significantly correlate with PA ( P > 0.05). In ovarian cancer, the IVW-FE model (beta =-0.035; 95%CI = -0.075, 0.003, P = 0.06) showed no significant causal relationship, while the IVW-RE model (beta =-0.035; 95%CI = -0.071, 0.003, P = 0.04) showed a significant causal relationship. After Benjamini-Hochberg correction, the results indicated a possible causal relationship between ovarian cancer and PA (IVW-FE, P’ > 0.05) (Fig. 3 , Supplementary Figs. 5 – 6 ).
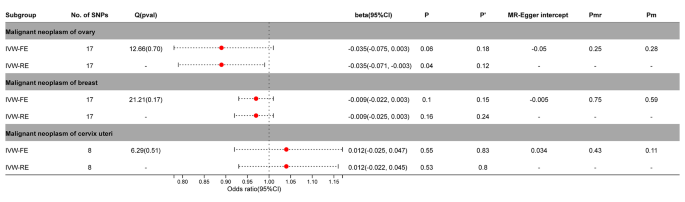
Association between genetic susceptibility to pernicious anemia and female cancers. IVW-FE; Inverse Variance Weighted Fixed Effects estimation method; IVW-RE; Inverse Variance Weighted Random Effects estimation method; beta, the average change (when exposed to the variable) in the outcome per 2-fold increase; SNP, single nucleotide polymorphism; 95%CI, 95% Confidence Interval; P, The IVW estimated P-value; P’, The IVW estimated P-value adjusted by the Benjamin-Hochberg method; Q(pval), Cochran Heterogeneity Test P-value; Pmr, MR–Egger P-value; Pm, MRlap P-value; -, Not applicable
Causal effects of PA on digestive cancers
The IVW-FE / IVW-RE model before and after correction by Benjamini-Hochberg showed a significant causal relationship between PA and gastric cancer (IVW-FE, beta = 0.067; 95%CI = 0.029, 0.110, P = 0.001, P’ <0.05; IVW-FE, beta = 0.067; 95%CI = 0.037, 0.101, P < 0.001, P’ <0.05) in the MR analysis of digestive tumors and PA (Fig. 4 , Supplementary Figs. 8 – 9 ).
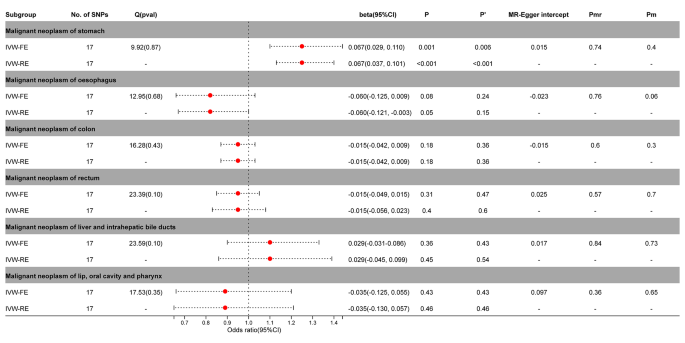
Association between genetic susceptibility to pernicious anemia and digestive cancers. IVW-FE; Inverse Variance Weighted Fixed Effects estimation method; IVW-RE; Inverse Variance Weighted Random Effects estimation method; beta, the average change (when exposed to the variable) in the outcome per 2-fold increase; SNP, single nucleotide polymorphism; 95%CI, 95% Confidence Interval; P, The IVW estimated P-value; P’, The IVW estimated P-value adjusted by the Benjamin-Hochberg method; Q(pval), Cochran Heterogeneity Test P-value; Pmr, MR–Egger P-value; Pm, MRlap P-value; -, Not applicable
Causal effects of PA on other cancers
The IVW-FE / IVW-RE model demonstrated a significant causal relationship between PA and malignant melanoma of skin (IVW-FE, beta = 0.134; 95%CI = 0.029, 0.237; P = 0.01; IVW-RE, beta = 0.134; 95%CI = 0.039, 0.226; P = 0.005)in the MR analysis of PA and other tumors. After correction by Benjamini-Hochberg, the random effects model still supported a significant causal relationship between PA and malignant melanoma of skin ( P < 0.05), while the fixed effects model only suggested an implied causal relationship ( P > 0.05) (Fig. 5 , Supplementary Figs. 11 – 12 ).
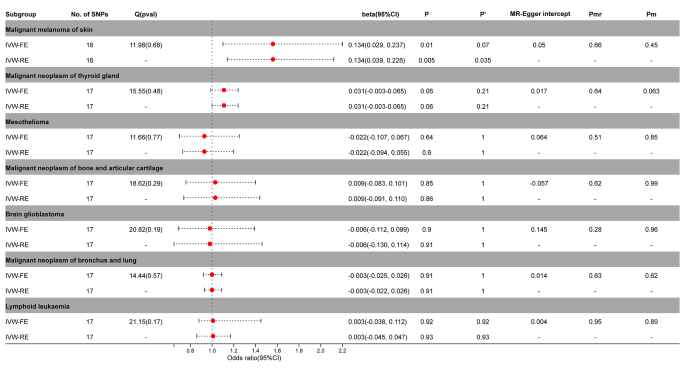
Association between genetic susceptibility to pernicious anemia and other cancers. IVW-FE; Inverse Variance Weighted Fixed Effects estimation method; IVW-RE; Inverse Variance Weighted Random Effects estimation method; beta, the average change (when exposed to the variable) in the outcome per 2-fold increase; SNP, single nucleotide polymorphism; 95%CI, 95% Confidence Interval; P, The IVW estimated P-value; P’, The IVW estimated P-value adjusted by the Benjamin-Hochberg method; Q(pval), Cochran Heterogeneity Test P-value; Pmr, MR–Egger P-value; Pm, MRlap P-value; -, Not applicable
Causal effects of cancers on PA
We also created a reverse MR to help further elucidate the causal link between PA and malignant melanoma of skin, prostate cancer, gastric cancer, and testicular cancer. The findings demonstrated a significant reverse causal relationship between PA and prostate cancer (IVW-FE, beta =-0.022; 95%CI = -0.042, -0.003; P = 0.02; IVW-RE, beta =-0.022; 95%CI = -0.045, -0.003; P = 0.03) or gastric cancer (IVW-FE, beta = 0.065; 95%CI = 0.042, 0.088; P < 0.001; IVW-RE, beta = 0.065; 95%CI = 0.047, 0.081; P < 0.001). After correction by Benjamini-Hochberg, there was still a significant causal relationship between PA and gastric cancer ( P < 0.05). For prostate cancer, fixed effects model supported a causal relationship with PA ( P < 0.05), while random effects model had only suggestive effects ( P > 0.05). Testicular cancer and malignant melanoma of the skin did not exhibit a reverse causal connection with PA ( P > 0.05) (Fig. 6 , Supplementary Figs. 13 – 15 ).
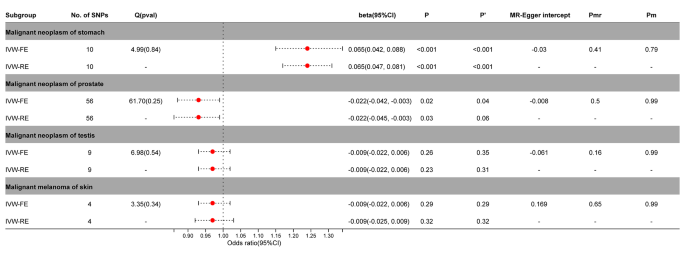
Association between genetic susceptibility to cancers and pernicious anemia. IVW-FE; Inverse Variance Weighted Fixed Effects estimation method; IVW-RE; Inverse Variance Weighted Random Effects estimation method; beta, the average change (when exposed to the variable) in the outcome per 2-fold increase; SNP, single nucleotide polymorphism; 95%CI, 95% Confidence Interval; P, The IVW estimated P-value; P’, The IVW estimated P-value adjusted by the Benjamin-Hochberg method; Q(pval), Cochran Heterogeneity Test P-value; Pmr, MR–Egger P-value; Pm, MRlap P-value; -, Not applicable
PA and subsequent cancer risk have been a focus of attention [ 6 , 10 , 11 , 20 ]. However, so far, the association between PA and cancer has been explored only in observational studies for a variety of reasons. Due to the complexity of PA diagnosis, the prevalence of PA is often underestimated in observational studies, so reliable data are not available to assess the subsequent risk of cancer in these patients [ 12 ]. It may even get the opposite result. While MR uses the random distribution of genetic variation, its advantage is that it is not disturbed by confounding factors and is not easily affected by reverse causality, which improves the validity of causal inference [ 14 ].
This was the first study to investigate the association between PA and 20 site-specific malignancies in the European population using a bidirectional two-sample MR investigation. The findings demonstrated a strong causative link between PA and testicular cancer, prostate cancer, gastric cancer, and malignant melanoma of skin. There was, however, no proof of a connection to the remaining sixteen site-specific malignancies. Reverse MR results showed that prostate cancer and gastric cancer also had causal effects on PA.
PA remains a neglected disease in many healthcare settings and is related to but distinct from autoimmune gastritis. PA occurs in the later stages of autoimmune atrophic gastritis, when gastric factor deficiency and consequent vitamin B12 deficiency may occur [ 2 ]. In addition to vitamin B12 deficiency, chronic autoimmune atrophic gastritis is often accompanied by other trace element deficiencies, including vitamin D, etc [ 21 , 22 ]. Observational studies have confirmed that autoimmune atrophic gastritis is associated with gastric cancer (including gastric neuroendocrine tumors), but the specific pathophysiological mechanism is still unclear. The available evidence supports the relationship between the prevalence of gastric cancer and vitamin D deficiency [ 23 , 24 ]. The biological function of vitamin D is usually activated by genomic regulation related to VDR in the nucleus or non-genomic action on the cell membrane [ 25 ]. After VDR is activated by its antagonist, in most cases, it translocates in the nucleus, regulates the expression of p21, p27 and other target genes, and plays the anticancer effect of vitamin D, including inhibiting cell proliferation and inducing cell apoptosis. In addition to disorders of nutrition and metabolism, it itself acts as an immune system disease [ 26 , 27 ]。This immune system abnormality may lead to a loss of immune tolerance [ 28 ], which may make the body more vulnerable to cancer. In addition, immune system abnormalities may also affect tumor immune surveillance and clearance functions, thereby increasing the risk of cancer. Therefore, the subsequent clinical manifestations of autoimmune atrophic gastritis may be the main reason for increasing the risk of gastric cancer or other cancer.
There is still much controversy about the association between vitamin B12 deficiency and gastric cancer [ 29 ]. Vitamin B12 deficiency is characterized by effects on the blood and nervous system. Therefore, the instability of observational studies on the relationship between vitamin B12 and gastric cancer may be related to its complex clinical manifestations, so it may need to be explored for a particular clinical manifestation, such as PA. This may be more helpful in helping people with autoimmune atrophic gastritis / vitamin B12 deficiency benefit from tumor prevention. It is worth noting that, despite the lack of research on PA and cancer, some clinical guidelines still regard PA as a precancerous lesion of gastric cancer and recommend the need for endoscopic monitoring [ 30 , 31 ]. According to a prior meta-analysis, those with PA had a roughly seven-fold increased relative risk of gastric cancer compared to those without PA [ 32 ]. Patients with PA had nearly tripled risk of gastric adenocarcinoma and an 11-fold increased risk of stomach carcinoid tumors, according to another large case-control research [ 6 ]. The mechanism underlying the link between PA and the risk of gastric cancer, as well as whether it indicates causation, remain poorly understood, despite the observational studies’ accumulated evidence supporting this association. This may be related to the changes in the environment of the stomach [ 33 ]. Due to the lack of gastric acidity caused by the destruction of parietal cells, the environment with high PH may be more conducive to the survival of conditional pathogenic bacteria [ 34 , 35 ]. Although the exact mechanism is unknown, the results of this study suggest that there is a significant two-way causal relationship between PA patients and gastric cancer, and this MR study can provide additional evidence of a genetic association between PA and gastric cancer.
In addition to digestive tract tumors, the findings provide genetic evidence for a link between PA and androgen-related urinary tumors (prostate and testicular cancer). We speculate that it may be related to the level of vitamin B12. Low levels of vitamin B12 in the body reduced the catalytic activity of methionine synthetase from homocysteine to methionine, resulting in the accumulation of homocysteine in plasma [ 36 ]. A number of studies have shown that homocysteine may be related to androgen levels [ 37 , 38 ]. Therefore, although there is not enough genetic evidence to show that vitamin B12 has a causal relationship with androgen-related urinary tumors [ 39 ]. However, previous studies have still observed that vitamin B12 is associated with the risk of prostate cancer [ 40 , 41 ]. Vitamin B12 supplementation may slow the growth of prostate cancer to some extent [ 42 ].
In addition, this study also shows that there was a causal relationship between PA and malignant melanoma of skin. And this is consistent with some previous studies. And a recent large meta-analysis also suggests that PA may increase the risk of malignant melanoma of skin [ 20 ]. Therefore, the role of PA in the development of cutaneous melanoma needs more research.
The main advantage of this study is the MR study, which reduces the potential impact of confounding factors compared to observational studies. Secondly, the European sample FinnGen project involved in this MR design has a large sample size to ensure sufficient statistical effectiveness. Finally, we evaluated the association between PA and a variety of cancers for the first time. Of course, there are some limitations. On the one hand, our analysis only covers people of European origin, which, although reducing the impact of race, also limits the universality of our results to other ethnic groups. On the other hand, this study may also be affected by inherent defects in MR analysis, such as the inability to obtain data at the individual level, which may affect the selection bias of IVs. Finally, it is important to emphasize that a binary exposure can lead to violation of the exclusion restriction assumption and limit the inferences drawn from an MR study [ 43 ]. Nevertheless, MR remains a robust method to test the causal hypothesis. Therefore, the main purpose of this study was to determine whether a causal relationship exists rather than to estimate the magnitude of the effect.
In conclusion, this study provides genetic evidence that PA may play a role in the development of several site-specific cancers. Future research is necessary to help us complement cancer prevention strategies.
Data availability
The datasets analyzed during the current study are available in the FinnGen repository, https://r5.finngen.fi/.
Bizzaro N, Antico A. Diagnosis and classification of pernicious anemia. Autoimmun Rev. 2014;13(4–5):565–8.
Article PubMed Google Scholar
Esposito G, Dottori L, Pivetta G, Ligato I, Dilaghi E, Lahner E. Pernicious Anemia: the hematological presentation of a multifaceted disorder caused by Cobalamin Deficiency. Nutrients. 2022; 14(8).
Massironi S, Gallo C, Elvevi A, Stegagnini M, Coltro LA, Invernizzi P. Incidence and prevalence of gastric neuroendocrine tumors in patients with chronic atrophic autoimmune gastritis. World J Gastrointest Oncol. 2023;15(8):1451–60.
Article PubMed PubMed Central Google Scholar
Vanoli A, La Rosa S, Luinetti O, Klersy C, Manca R, Alvisi C, Rossi S, Trespi E, Zangrandi A, Sessa F, et al. Histologic changes in type a chronic atrophic gastritis indicating increased risk of neuroendocrine tumor development: the predictive role of dysplastic and severely hyperplastic enterochromaffin-like cell lesions. Hum Pathol. 2013;44(9):1827–37.
Article CAS PubMed Google Scholar
Zadori N, Szako L, Vancsa S, Vorhendi N, Ostarijas E, Kiss S, Frim L, Hegyi P, Czimmer J. Six Autoimmune disorders are Associated with increased incidence of gastric Cancer: a systematic review and Meta-analysis of half a million patients. Front Immunol. 2021;12:750533.
Article CAS PubMed PubMed Central Google Scholar
Murphy G, Dawsey SM, Engels EA, Ricker W, Parsons R, Etemadi A, Lin SW, Abnet CC, Freedman ND. Cancer Risk after Pernicious Anemia in the US Elderly Population. Clin Gastroenterol Hepatol. 2015;13(13):2282–9. e1-4.
Zhernakova A, van Diemen CC, Wijmenga C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet. 2009;10(1):43–55.
Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev. 2017;16(10):1049–57.
Elkoshi Z. Cancer and Autoimmune diseases: a tale of two immunological opposites? Front Immunol. 2022;13:821598.
Mellemkjaer L, Gridley G, Moller H, Hsing AW, Linet MS, Brinton LA, Olsen JH. Pernicious anaemia and cancer risk in Denmark. Br J Cancer. 1996;73(8):998–1000.
Brinton LA, Gridley G, Hrubec Z, Hoover R, Fraumeni JJ. Cancer risk following pernicious anaemia. Br J Cancer. 1989;59(5):810–3.
Htut TW, Thein KZ, Oo TH. Pernicious anemia: pathophysiology and diagnostic difficulties. J Evid Based Med. 2021;14(2):161–9.
Meuli L, Dick F. Understanding confounding in Observational studies. Eur J Vasc Endovasc Surg. 2018;55(5):737.
Sekula P, Del GMF, Pattaro C, Kottgen A. Mendelian randomization as an Approach to assess causality using Observational Data. J Am Soc Nephrol. 2016;27(11):3253–65.
Burgess S, Thompson SG. Avoiding bias from weak instruments in mendelian randomization studies. Int J Epidemiol. 2011;40(3):755–64.
Flatby HM, Ravi A, Damas JK, Solligard E, Rogne T. Circulating levels of micronutrients and risk of infections: a mendelian randomization study. BMC Med. 2023;21(1):84.
Mounier N, Kutalik Z. Bias correction for inverse variance weighting mendelian randomization. Genet Epidemiol. 2023;47(4):314–31.
Burgess S, Labrecque JA. Mendelian randomization with a binary exposure variable: interpretation and presentation of causal estimates. Eur J Epidemiol. 2018;33(10):947–52.
Cheung CL, Ho SC, Krishnamoorthy S, Li GH. COVID-19 and platelet traits: a bidirectional mendelian randomization study. J Med Virol. 2022;94(10):4735–43.
Lahner E, Capasso M, Carabotti M, Annibale B. Incidence of cancer (other than gastric cancer) in pernicious anaemia: a systematic review with meta-analysis. Dig Liver Dis. 2018;50(8):780–6.
Massironi S, Cavalcoli F, Zilli A, Del GA, Ciafardini C, Bernasconi S, Felicetta I, Conte D, Peracchi M. Relevance of vitamin D deficiency in patients with chronic autoimmune atrophic gastritis: a prospective study. BMC Gastroenterol. 2018;18(1):172.
Zilli A, Cavalcoli F, Ciafardini C, Massironi S. Deficiency of micronutrients in patients affected by chronic atrophic autoimmune gastritis: a single-institution observational study. Dig Liver Dis. 2019;51(4):505–9.
Nguyen MT, Huynh N, Nguyen DD, Ta NH, Van Nguyen T, Dang HT, Le NT. Vitamin D intake and gastric cancer in Viet Nam: a case-control study. BMC Cancer. 2022;22(1):838.
Kwak JH, Paik JK. Vitamin D status and gastric Cancer: a cross-sectional study in koreans. Nutrients. 2020; 12(7).
Heikkinen S, Vaisanen S, Pehkonen P, Seuter S, Benes V, Carlberg C. Nuclear hormone 1alpha,25-dihydroxyvitamin D3 elicits a genome-wide shift in the locations of VDR chromatin occupancy. Nucleic Acids Res. 2011;39(21):9181–93.
Kajdaniuk D, Foltyn W, Morawiec-Szymonik E, Czuba Z, Szymonik E, Kos-Kudla B, Marek B. Th17 cytokines and factors modulating their activity in patients with pernicious anemia. Immunol Res. 2023;71(6):873–82.
Carmel R, Boone D, Parker JW. Lymphocyte surface phenotypes in pernicious anemia. Dig Dis Sci. 1987;32(8):846–50.
Vargas JA, Alvarez-Mon M, Manzano L, Albillos A, Fernandez-Corugedo A, Albarran F, Durantez A. Functional defect of T cells in autoimmune gastritis. Gut. 1995;36(2):171–5.
He J, Fu H, Li C, Deng Z, Chang H. Association between vitamin B(12) and risk of gastric Cancer: a systematic review and Meta-analysis of Epidemiological studies. Nutr Cancer. 2022;74(9):3263–73.
Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA clinical practice update on the diagnosis and management of Atrophic gastritis: Expert Review. Gastroenterology. 2021;161(4):1325–e13327.
Banks M, Graham D, Jansen M, Gotoda T, Coda S, di Pietro M, Uedo N, Bhandari P, Pritchard DM, Kuipers EJ, et al. British Society of Gastroenterology guidelines on the diagnosis and management of patients at risk of gastric adenocarcinoma. Gut. 2019;68(9):1545–75.
Vannella L, Lahner E, Osborn J, Annibale B. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther. 2013;37(4):375–82.
Tenca A, Massironi S, Pugliese D, Consonni D, Mauro A, Cavalcoli F, Franchina M, Spampatti M, Conte D, Penagini R. Gastro-esophageal reflux and antisecretory drugs use among patients with chronic autoimmune atrophic gastritis: a study with pH-impedance monitoring. Neurogastroenterol Motil. 2016;28(2):274–80.
Kuster GG, Remine WH, Dockerty MB. Gastric cancer in pernicious anemia and in patients with and without achlorhydria. Ann Surg. 1972;175(5):783–9.
Borriello SP, Reed PJ, Dolby JM, Barclay FE, Webster AD. Microbial and metabolic profile of achlorhydric stomach: comparison of pernicious anaemia and hypogammaglobulinaemia. J Clin Pathol. 1985;38(8):946–53.
Jakubowski H. Homocysteine Modification in protein Structure/Function and human disease. Physiol Rev. 2019;99(1):555–604.
Sung SH, Kim NH, Hong SP, Lee JK, Choi SJ. Associations of metabolic syndrome with total testosterone and homocysteine levels in male Korean workers. Endocrinol Metab (Seoul). 2019;34(2):158–68.
Prudova A, Albin M, Bauman Z, Lin A, Vitvitsky V, Banerjee R. Testosterone regulation of homocysteine metabolism modulates redox status in human prostate cancer cells. Antioxid Redox Signal. 2007;9(11):1875–81.
Kim JY, Song M, Kim MS, Natarajan P, Do R, Myung W, Won HH. An atlas of associations between 14 micronutrients and 22 cancer outcomes: mendelian randomization analyses. BMC Med. 2023;21(1):316.
Hultdin J, Van Guelpen B, Bergh A, Hallmans G, Stattin P. Plasma folate, vitamin B12, and homocysteine and prostate cancer risk: a prospective study. Int J Cancer. 2005;113(5):819–24.
Price AJ, Travis RC, Appleby PN, Albanes D, Barricarte GA, Bjorge T, Bueno-De-Mesquita HB, Chen C, Donovan J, Gislefoss R, et al. Circulating folate and Vitamin B(12) and risk of prostate Cancer: a Collaborative Analysis of Individual Participant Data from six cohorts including 6875 cases and 8104 controls. Eur Urol. 2016;70(6):941–51.
Tisman G, Kutik S, Rainville C. Coexistence of pernicious anemia and prostate cancer - ‘an experiment of nature’ involving vitamin B(12)modulation of prostate cancer growth and metabolism: a case report. J Med Case Rep. 2009;3:9295.
Carter AR, Fraser A, Howe LD, Harris S, Hughes A. Why caution should be applied when interpreting and promoting findings from mendelian randomisation studies. Gen Psychiatr. 2023;36(4):e101047.
Download references
Acknowledgements
We want to acknowledge the participants and investigators of the FinnGen study.
This study was supported by the High-level Talent Project of the First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine (GYZYYFY-BS-2023 (14)).
Author information
Authors and affiliations.
Department of Urology & Andrology, The First Affiliated of Guizhou University of Traditional Chinese Medicine, Guiyang, Guiyang, 550001, China
Bangwei Che, Jiancheng Zhai & Kaifa Tang
The First Clinical College, Guizhou University of Traditional Chinese Medicine, Guiyang, 550001, China
Shenglan Yuan, Yang Zhang & Chuanchuan Wu
Physical examination center, The First Affiliated of Guizhou University of Traditional Chinese Medicine, Guiyang, 550001, China
Hongyan Zhang
You can also search for this author in PubMed Google Scholar
Contributions
Study concept and design: KT. Collection and assembly of data: BC, SY, and HZ. Data analysis and interpretation: BC, JZ, YZ, and CW. Manuscript revised: BC and KT. Manuscript writing and review: BC and KT. All authors contributed to the article and approved the submitted version.
Corresponding author
Correspondence to Kaifa Tang .
Ethics declarations
Ethics approval and consent to participate.
This study does not require institutional review committee ethical approval because all FinnGen ( https://www.finngen.fi/en ) Project studies have been approved by the local institutional review committee and ethics committee.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, supplementary material 3, supplementary material 4, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Che, B., Yuan, S., Zhang, H. et al. Causal inference between pernicious anemia and cancers: a bidirectional two-sample mendelian randomization analysis. BMC Cancer 24 , 586 (2024). https://doi.org/10.1186/s12885-024-12354-y
Download citation
Received : 20 December 2023
Accepted : 07 May 2024
Published : 13 May 2024
DOI : https://doi.org/10.1186/s12885-024-12354-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Pernicious Anemia
- Vitamin B12 deficiency
- Mendelian randomization
- Cancer genetics
ISSN: 1471-2407
- Submission enquiries: [email protected]
- General enquiries: [email protected]

COMMENTS
Study with Quizlet and memorize flashcards containing terms like Introduction, The nurse receives shift report and proceeds to the client's room, bringing equipment to measure his vital signs. Which vital sign should concern the nurse the most? A. Blood pressure is 142/80 mmHg. B. Respiration rate of 24 breaths/minute. C. Heart rate of 98 beats/minute. D. Pulse oxygenation of 94%., The nurse ...
pernicious anemia case study. John Baker is a 45-year-old Caucasian male who was admitted to the medical-surgical unit 6 hours ago from the Emergency Department due to nausea, vomiting, shortness of breath, pallor, weakness, and severe fatigue. John is divorced with no children and has been practicing law for 15 years.
Blood pressure is 142/80 mmHg. This elevated blood pressure indicates an underlying issue that must be addressed. Mr. Baker says that he is feeling better and it is now time to give the scheduled medications. Because of the new medical diagnosis of pernicious anemia, the healthcare provider has prescribed vitamin B12 (cyanocobalamin) 100 mcg ...
Pernicious anemia (PA) is a megaloblastic anemia caused by the deficiency of vitamin B12 (cobalamin). Vitamin B12 (Vit.B12) is a crucial vitamin for DNA synthesis. Vit.B12 is absorbed in the terminal ileum as a complex with intrinsic factor, secreted by parietal cells of the stomach.
receives shift report and proceeds to the client's room, bringing equipment to measure his vital signs. Which vital sign should concern the nurse the most? A. Blood pressure is 142/80 mmHg. B. Respiration rate of 24 breaths/minute. C. Heart rate of 98 beats/minute. D. Pulse oxygenation of 94%. - A This elevated blood pressure could indicate an underlying issue that should be addressed. The ...
Pernicious anemia is a relatively rare autoimmune disorder that causes diminishment in dietary vitamin B12 (cobalamin) absorption, resulting in B12 deficiency and subsequent megaloblastic anemia. It affects people of all ages worldwide, particularly those over 60. Despite the advances in understanding, making the diagnosis can be challenging for clinicians due to its complexity, broad spectrum ...
Pernicious anemia (PA) is a macrocytic normochromic anemia. 1, 2, 3 Here, we reported a case of PA in a 64-year-old female patient who was treated with intramuscular injection of vitamin B12 and oral administration of folic acid and iron tablets and showed a good clinical outcome with the abnormal blood data returning to the normal values in ...
Popular books. Biology Mary Ann Clark, Jung Choi, Matthew Douglas. College Physics Raymond A. Serway, Chris Vuille. Essential Environment: The Science Behind the Stories Jay H. Withgott, Matthew Laposata. Everything's an Argument with 2016 MLA Update University Andrea A Lunsford, University John J Ruszkiewicz. Lewis's Medical-Surgical Nursing Diane Brown, Helen Edwards, Lesley Seaton, Thomas ...
PERNICIOUS ANEMIA: A STUDY OF FIFTY CASES. BY RICHARD C. CABOT, M.D. (Concludedfrom No. 5,p. 107.) Blood Examination — In one case only an exami- nation of the fresh blood is recorded, but ...
Pernicious anaemia (PA) is an autoimmune disease of multifactorial aetiology involving environmental and immunological factors. It is the most common cause of cobalamin deficiency anaemia worldwide. The disease is a macrocytic anaemia caused by a vitamin B12 deficiency, which, in turn, is the result of intrinsic factor deficiency, a protein ...
Pernicious anemia (PA) is the most common cause of vitamin B12 (cobalamin) defi-. ciency anemia in the world. It is an autoimmune disease, comprising of salient fea-. tures of autoimmune chronic atrophic gastritis (CAG) and cobalamin deficiency (CD). Although the anemia was first described as pernicious, it may well be controlled with.
Pernicious anemia is an autoimmune condition that happens when your immune system produces antibodies that attack cells in the mucosal lining of your stomach and nerve cells. Your immune system's response affects your body's ability to absorb vitamin B12. The antibodies also block a critical protein called intrinsic (in-TRIN-sic) factor.
Introduction. Pernicious anemia is an autoimmune disease characterized by vitamin B12 deficiency due to antibodies that target intrinsic factor (IF) or parietal cells, both directly involved in vitamin B12 absorption [].In the United States, vitamin B12 deficiency affects about 20% of individuals over 60 years old; however, the prevalence is significantly decreased in patients younger than 60 ...
Pernicious anemia is a common cause of vitamin B12 deficiency. Here, we discuss a case of a young woman who presented with severe anemia along with a history of iron deficiency anemia. After a review of her clinical presentation and laboratory data, we identified an autoimmune hemolytic anemia and a concomitant pernicious anemia. The concurrence of both these hematological diagnoses in a ...
Use your knowledge and apply key concepts to realistic patient care scenarios. HESI Case Studies provide real-world patient care scenarios accompanied by application-based questions and rationales that will help you learn how to manage complex patient conditions and make sound clinical judgments. Questions cover nursing care for patients with a wide variety physiological and psychosocial ...
Pernicious anemia (PA) is a chronic condition that stems from an inability to absorb and use vitamin B12 ().The condition is prevalent in 0.1% of the general population and is more frequently diagnosed in older adults (1.9%) ().Common symptoms of PA are fatigue, memory loss, and difficulty maintaining concentration ().Pernicious anemia is treated via regular intramuscular injections of ...
Final labs after discharge revealed positive antibodies to intrinsic factor and parietal cell, thus confirming the diagnosis of pernicious anemia in our patient. Discussion: Vitamin B12 deficiency is a common cause of megaloblastic anemia and may be accompanied by various neuropsychiatric symptoms. It is most commonly due to autoimmune ...
Background. Inadequate consumption of animal foods and pernicious anaemia (loss of intrinsic factor due to autoimmune atrophic gastritis) are the most common causes of severe vitamin B 12 deficiency worldwide in children and adults, respectively. 1 2. Vitamin B 12 deficiency may cause reversible megaloblastic anaemia and/or demyelinating central nervous system disease.
Study with Quizlet and memorize flashcards containing terms like The nurse receives shift report and proceeds to the client's room, bringing equipment to measure his vital signs. Which vital sign should concern the nurse the most? A. Blood pressure is 142/80 mmHg. B. Respiration rate of 24 breaths/minute. C. Heart rate of 98 beats/minute. D. Pulse oxygenation of 94%., The nurse asks the client ...
Reported is the case of a 58-year-old man who presented with dyspnea on exertion, fatigue, and unintentional weight loss. He was found to have severe anemia (hemoglobin 3.3 g/dL) in association with pancytopenia and hemolysis. Extensive workup revealed vitamin B12 deficiency secondary to pernicious anemia.
B - He has a history of chronic gastritis and his symptoms are nausea, vomiting, and weight loss, and he has developed pernicious anemia. C He is exhibiting the signs of pernicious anemia, such as weight loss, nausea, vomiting, shortness of breath, pallor, weakness, inflamed tongue, tremors, and severe fatigue.
Discussion. Vitamin B12 deficiency varies and increases with age. Prevalence of vitamin B12 deficiency is 3% in patients aged 20 to 39 years [ 3, 4 ]. Pernicious anemia is an autoimmune disease caused by vitamin B12 deficiency due to atrophic gastritis or loss of parietal cells or lack of intrinsic factor. A diagnosis of pernicious anemia is ...
Study with Quizlet and memorize flashcards containing terms like The nurse receives shift report and proceeds to the client's room, bringing equipment to measure his vital signs. Which vital sign should concern the nurse the most? a. blood pressure is 142/80 mmHG b. respiration rate of 24 breaths/min c. heart rate of 98 beats/min d. pulse ox of 94%, The nurse asks the client how he is feeling ...
Background Observational study investigated the association between pernicious anemia (PA) and cancers. However, with the exception of gastric cancer, the results are mostly contradictory. The purpose of this study was to investigate the potential causal relationship between PA and cancers through bidirectional two-sample Mendelian randomized (MR) analysis. Methods The European sample FinnGen ...