What is a contract research organization (CRO)?
Clinical trial services.

What does a CRO do?
CROs are hired by sponsors who want to run a clinical trial. This eliminates the need to hire full time staff to complete the project and provides an opportunity to work with the CRO on a project-by-project basis. The CRO is hired to plan, coordinate, execute, and manage the lifecycle of the clinical trial, safely and efficiently. Serving as the main contact between the sponsor and other stakeholders throughout the trial, the CRO communicates with ethics and compliance committees, regulatory personnel, vendors, physicians, and research coordinators.
What does a CRO mean in clinical trials?
CROs have the knowledge, capabilities, processes and procedures that are needed to develop and run a successful clinical trial, while ensuring trial quality and compliance with national and international standards. Working with a contract research organization can offer innovative tools that can increase efficiencies, leading to decreased timelines and cost. Choosing the right CRO to run your clinical trial is crucial to the trial success. In addition to consideration of their own project needs, requirements, and budget when selecting a CRO, Sponsors should evaluate the qualification, experience, and quality system processes of the CRO.
Our CRO services
MED Institute is a full service contract research organization with over 35 years of experience designing and executing clinical trials, ranging from early feasibility studies to multinational, controlled pivotal trials to post-market registries. Our clinical trial services include clinical trial planning, clinical data management, clinical project management, and clinical trial monitoring. Our MED team is able to support your clinical study from start to finish or is able to help with one specific aspect of the clinical study (e.g., monitoring).
Clinical trial planning and study start-up include the process of creating the clinical plan and clinical protocol and obtaining the necessary approvals from regulatory authorities and ethics committees. We can help you design a clinical study tailored to your product’s specific needs, to collect data efficiently while meeting all regulatory requirements. Our protocol development process involves working in collaboration with clinical experts and other study stakeholders to identify reasonable performance goals, to determine appropriate examinations and procedures, and to define the right patient population. It ensures the clinical study protocol will successfully implement the study plan and maintain compliance with all applicable regulatory requirements. A well-written protocol is essential to maximizing efficiency and minimizing risks in gathering the necessary clinical data for your product.
Clinical data management is critical to the clinical trial process. We are dedicated to providing high quality clinical data management services to support your product development needs, obtaining clinical trial data you can trust to save time and resources. We can create case report forms (CRFs) that ask the right questions to achieve your clinical trial’s goals in a simple and intuitive way. These CRFs are customized to suit the project type and individual requirements for streamlined data entry, avoiding the unnecessary delays and potentially inaccurate data that often result from poorly designed CRFs. We can generate paper or electronic CRFs based on the clinical trial and Sponsor requirements.
Drawing from our experiences working with hundreds of investigators worldwide, we are able to build efficient, intuitive electronic data capture (EDC) systems that are simple for investigators to learn and use. The benefits of a well-designed, well-built EDC system contribute to minimizing the time and cost to run a clinical trial. Learn more about designing and building an EDC system in our “Considerations for designing and building an electronic data capture (EDC) system” blog series- Part 1: with users in mind and Part 2: with sponsors/CRO in mind .
Clinical project management is important to ensure that your trial runs as smoothly as possible. Our clinical project management team has decades of experience managing trials ranging from first-in-human proof of concept to multinational, multicenter, controlled pivotal trials. We emphasize efficiency in conducting clinical trials, while maintaining exemplary quality and compliance. Our team can complete clinical site assessments, help you prepare documentation for IRB/Ethics Committees, complete the site initiation and training, and complete the necessary steps for trial closeout. Our experience with a multitude of trial sites around the world has taught us how to evaluate potential sites, and the right questions to ask to determine the best fit for each clinical trial. We also strive to see that candidate sites learn enough about the requirements of the clinical trial to determine whether it is a good fit for them. Smart site selection contributes greatly to completing a clinical trial on time and on budget.
Clinical trial monitoring helps ensure that the trial is conducted, recorded, and reported in accordance to the protocol, ethics committees, and Good Clinical Practice (GCP) . We create comprehensive, but efficient, monitoring plans to manage clinical trials and engender confidence in clinical trial integrity. Analytics are used to implement a risk-based approach to monitoring, which reduces costs without sacrificing assurance of data reliability, investigator compliance, or meeting regulatory reporting obligations. Active clinical monitoring can help predict potential clinical trial challenges or at least identify issues early so they may be addressed before they lead to significant delays leading to increased costs, or derail the trial completely.
How to choose a good contract research organization
Choosing a good CRO is very important and contributes to the success of the study and helps the trial to run as smoothly as possible. Some of the questions to ask when choosing a CRO are:
- Do they have an established quality system, with procedures to address the assigned clinical study activities?
- Are they responsive and willing to work with you and communicate throughout the project?
- Do they have stable project teams is there high turnover?
- Can they assist with data management?
- Will they help you recruit and manage safety boards or committees?
- Will they be able to conduct audits to help you prepare to pass BIMO FDA inspections ?
- Can they provide general site support and project management help in addition to clinical monitoring?
CROs are hired by Sponsors who want to run a clinical trial and become a fundamental part of the trial. They offer a wide variety of services associated with conducting the study from start to finish. It is important to choose a good CRO as the success of your trial is dependent on the management and capabilities of the CRO.
We can partner with you to successfully take your clinical trial from start-up to trial close out. Contact us today to discuss your clinical trial needs 855.463.1633 | [email protected] | medinstitute.com.

Get email about news, services, and events from MED Institute.
- First Name *
- Last Name *
- Please see our customer data privacy notice relating to our collection and use of your data. You always have the right to unsubscribe.
OUR COMMITTMENT
We are committed to consistently performing services with high quality, that deliver exceptional results, and add value to the client’s business. For client surveys sent in the last two quarters, we received ratings of 4.76/5 points (10).
News & Articles
What is a contract research organization.
In the rapidly evolving landscape of medical research and development, the role of a Contract Research Organization (CRO) is becoming increasingly crucial. These organizations offer a comprehensive spectrum of services that enable pharmaceutical, biotechnology, and medical device companies to accelerate their product development process.
Contract Research Organization: Essential Partner in Medical Research
A Contract Research Organization (CRO) is a specialized service provider that offers end-to-end support for clinical trials, ranging from early development to post-approval studies. They leverage their expertise, resources, and global reach to help sponsors navigate through the complex processes of drug and device development.
Defining the Role of a CRO
The fundamental responsibility of a CRO is to assist sponsors in conducting clinical trials in a manner that is efficient, safe, and compliant with regulatory standards. This involves a wide range of activities, including but not limited to, clinical trial planning and design, site selection, data management, regulatory affairs, and project management.
Understanding the Significance of a CRO in Clinical Trials
The involvement of a CRO in clinical trials is essential for ensuring trial quality and compliance with national and international standards. They bring to the table innovative tools and strategies that can enhance efficiencies, thereby reducing timelines and costs. The choice of a suitable CRO is instrumental in determining the success of a clinical trial.
Vision Lifesciences as Your Trusted CRO Partner
At Vision Lifesciences, we pride ourselves on being a full-service Contract Research Organization with experience in designing and implementing clinical trials.
Our Expertise in Clinical Trial Services
Our suite of clinical trial services is designed to support your clinical study from inception to completion. We can either manage the entire study or assist with specific components, depending on your requirements.
Clinical Trial Planning
Our clinical trial planning services encompass creating the clinical plan and protocol, and facilitating the necessary approvals from regulatory authorities and ethics committees. We collaborate with clinical experts and other stakeholders to tailor a clinical study that suits your product’s specific needs, ensuring data is collected efficiently while complying with all regulatory requirements.
Clinical Data Management
Robust clinical data management is integral to the clinical trial process. We take meticulous measures to deliver high-quality clinical data management services to support your product development needs. This includes the creation of case report forms (CRFs), the establishment of efficient electronic data capture (EDC) systems, and the management of data entry and validation processes.
Clinical Project Management
We understand the importance of smooth project management in conducting clinical trials. We ensure efficiency while maintaining superior quality and compliance. Our project management services include site assessments, documentation for IRB/Ethics Committees, site initiation and training, and trial closeout steps.
Clinical Trial Monitoring
Clinical trial monitoring is crucial in ensuring that the trial is conducted, recorded, and reported in accordance with the protocol, ethics committees, and Good Clinical Practice (GCP). Our comprehensive monitoring plans help manage clinical trials and foster confidence in clinical trial integrity.
Choosing the Right Contract Research Organization
Selecting the right CRO for your clinical trial is a critical decision. It involves an evaluation of the qualification, experience, and quality system processes of the CRO. Here are a few questions to consider when choosing a CRO:
- Do they have a well-established quality system?
- Are they responsive and communicative throughout the project?
- Do they have a stable project team with low turnover?
- Can they assist with data management?
- Will they help in recruiting and managing safety boards or committees?
- Can they conduct audits to help you prepare for BIMO FDA inspections?
- Can they provide general site support and project management help in addition to clinical monitoring?
Why Choose Vision Lifesciences as Your CRO Partner?
At Vision Lifesciences , we bring our extensive experience and expertise to the table to ensure the success of your clinical trial. We understand that every trial is unique, and we tailor our services to meet your specific needs.
Commitment to Quality
We are committed to consistently delivering services of the highest quality that yield exceptional results and add value to your business. Our track record speaks volumes about our commitment to quality, with consistently high ratings from our clients in the past two quarters.
Comprehensive Services
Our comprehensive suite of services ensures that you have the support you need at every step of your clinical trial. From medical device engineering and testing to regulatory services and clinical trial services, we have you covered.
Global Reach
With a global presence, we have the capability to conduct clinical trials across multiple geographical locations, allowing for diverse patient populations and improved recruitment rates.
In conclusion, a Contract Research Organization is an invaluable partner in the realm of clinical trials. Their specialized skills and services allow sponsors to conduct trials efficiently, safely, and in compliance with regulatory standards.
At Vision Lifesciences, we are committed to being your trusted partner in your clinical trial journey. Contact us today to discuss your clinical trial needs. Our team of experts is ready and eager to support you in bringing your product to market.
Further Reading
The top 11 contract research organizations (cros) shaping the future of pharma, biotech, and medtech in 2023, quick links.
- [email protected]
- Ulica Republike Austrije 23, Zagreb, Croatia
- World Trust Tower, 50 Stanley Street, Central, Hong Kong
Subscribe To Our Newsletter!
By submitting, you are agreeing to our terms and privacy policy
- Privacy Notice
- Terms and Conditions
Copyright © 2024 Vision Life Sciences. All Rights Reserved.
Clinical Research Organization (CRO): How they work?
- Author Company: Clariwell Global Services
- Author Name: Zain Malik
- Author Email: [email protected]
- Author Telephone: +917709551477
- Author Website: https://www.clariwell.in/best-clinical-research-courses-in-pune-with-100-percent-job-guarantee
A clinical research organization (CRO) is often called a contract research organization (CRO). CRO is a service organization that provides support to pharmaceutical and biotechnology industries in the form of outsourced clinical research courses and services for both medical devices and drugs.
The main functions required to conduct clinical researches, which are usually departments of the clinical research organization, are:
- The function of medical operations: People working in this area are given clinical research training from various clinical research institutes. This sector includes medically qualified people who are capable of constructing and designing clinical studies, clinical trials, and their protocols to provide medical-related input throughout the study. This includes roles like medical monitor, clinical research physician, advisors, etc.
- Clinical operations: This is the most organized and the largest team in any contract research organization. It consists of medical research associates, clinical trial assistants, managers, etc. This is the team of the CRO, which selects the clinical trial sites and locations, assists the studies, monitors the studies, etc.
- Data Management: This team helps in managing and designing various tools and databases. This is done in order to collect data from the trial. They help in ensuring that the data collected from the trials is accurate and is suitable for analysis. To keep track and organized, this team uses sophisticated software and modern technologies.
- Biostatistics: This team plays a major role in the outcome of the trial. They help in analyzing the study data and figuring out whether the study has yielded positive results or negative results while adhering strictly to all the protocols. They also generate statistical tables, figures, and graphs with their interpretations which are all then passed down to the medical writers to compile into reports.
- Writing team: these writers compile all the data into medical reports that can easily be understood by the public as well. They also manage the study protocols, study analysis reports, promotional material, etc.
- Quality assurance: The audits to ensure that the guidelines, standard operations, and regulation procedures are followed throughout are assessed by these trained professionals. This department is entirely dedicated to ensuring the quality of the product.
- Human Resources: Every clinical research organization has a dedicated human resource organization that is responsible for hiring trained professionals for various job roles and positions within the organization. They have to maintain a certain level of talent in the talent pool available.
- IT team: This is generally considered to be the support staff of the Clinical research organizations. They take care of all the IT-related needs like maintenance and purchases of laptops, desktops, telephones, software, etc.
- The finance team: All the monetary controls and the finance part, including the administration of the whole CRO, is managed by this department of the CRO.
- Training and Development: This is a dedicated department to training and developing in the CRO. It focuses on the professional development of all its employees and conducts routine training to make sure that their staffs remain up to date with all the developments in technology. It acts as a clinical research institute that provides the best clinical research courses and clinical research training .
You are leaving PharmiWeb.com

Disclaimer: You are now leaving PharmiWeb.com website and are going to a website that is not operated by us. We are not responsible for the content or availability of linked sites.
ABOUT THIRD PARTY LINKS ON OUR SITE
PharmiWeb.com offers links to other third party websites that may be of interest to our website visitors. The links provided in our website are provided solely for your convenience and may assist you in locating other useful information on the Internet. When you click on these links you will leave the PharmiWeb.com website and will be redirected to another site. These sites are not under the control of PharmiWeb.com.
PharmiWeb.com is not responsible for the content of linked third party websites. We are not an agent for these third parties nor do we endorse or guarantee their products. We make no representation or warranty regarding the accuracy of the information contained in the linked sites. We suggest that you always verify the information obtained from linked websites before acting upon this information.
Also, please be aware that the security and privacy policies on these sites may be different than PharmiWeb.com policies, so please read third party privacy and security policies closely.
If you have any questions or concerns about the products and services offered on linked third party websites, please contact the third party directly.
- Investigators
- Clinical Data Management
- Medical Device CRO
Selecting a CRO
WHAT IS A CRO?
- Request for Proposal

What is a Contract Research Organization?
A Contract Research Organization (CRO), sometimes known as a Clinical Research Organization, is an organization contracted by another company to take the lead in managing that company’s trials and complex medical testing responsibilities. Contract Research Organizations reduce the cost of research and development to help businesses and institutions meet the needs of the evolving medical device and pharma industry.
Topics covered below:
- What is Clinical Outsourcing?
- What Does a Contract Research Organization Do?
Benefits of Outsourcing to a CRO
- Working With a CRO
- Contract Research and Manufacturing Services (CRAMS)
What Is Clinical Outsourcing?
Clinical outsourcing occurs when a company hires another company made up of researchers and experts to conduct comprehensive and complex medical research so that the hiring company does not have to staff experts in-house, or maintain the required infrastructure and office space. This enables the hiring company to receive expert medical testing while also saving both time and money.
To reduce the cost of research and development, drug companies are increasingly outsourcing their medical testing responsibilities to alleviate the growing and ongoing cost of maintaining medical facilities and full-time staff year-round.
What Does A Contract Research Organization Do?
Contract Research Organizations (CROs) conduct clinical trials and research support services for biotechnology, medical device, and pharmaceutical industries, as well as universities, government organizations, and foundations.
CROs are able to provide a wide range of clinical research services to medical sponsors, including, but not limited to:
- Procedures and Logistics
- Resource Allocation Management
- Database Design
- Data Entry and Validation
- Database Maintenance and Archival
- Security Procedures
- Site Recruitment
- Patient Recruitment
- Study Monitoring
- Site Management
- Audits/FDA inspection support
- Clinical SOP Development
- GCP Seminars
- Medicine and Disease Coding
- Validation Programming
- Market Assessments
- Market Research
- Strategic Planning Consultation
- Medical Product Registration
- Product Launch
- Product Marketing
- Product Sales
- Safety and Efficacy Summaries
- Quality Reporting
- Statistical Methodology Consultation
- Statistical Analysis Plan Development
- Statistical Analysis Reports
- Final Study Reports
- Clinical Study Protocol Documents
- “Instructions for Use”
- Manuscripts
- PowerPoint Presentations
- White Papers
Outsourcing to a Contract Research Organization can bring multiple benefits to clinical professionals and institutions.
When choosing to outsource clinical work, consider:
Time Savings
Cost Savings
Advanced Technological Needs
Evolving and Complex Regulatory Requirements
Outsourcing to a CRO saves critical time in the trial and development phase. Working with a CRO to conduct a trial often significantly reduces the time it takes compared to completing the trial in-house. CROs are already set up with all of the necessary tools and resources needed as well as a team of in-house experts who are experienced in all areas of clinical testing, development, and compliance.
There are significant cost savings in hiring a CRO. A faster trial process alone offers medical institutions a reduction in costs. There are also financial savings in each long-term purchase an entity would incur in order to run sufficient research trials. Full-time staff and medical facilities are costly year-round, especially when they are not needed during all parts of the year.
Working with a CRO gives hiring companies access to the most advanced technology and systems for data management, product development, research analysis, and other clinical research services. Clinical research is a rapidly changing industry. It is essential that software and hardware IT capabilities, as well as Internet-based applications, are the best in the industry to facilitate the acceleration of clinical trials while maintaining comprehensive quality control.
The FDA and other relevant regulatory authorities require intricate and accurate data for approvals. CROs work within clinical compliance on a daily basis, which gives them intricate knowledge of regulatory requirements and audits such as Good Clinical Practice (GCP) audits or Good Laboratory Practice (GLP) audits. CROs work with hiring companies to optimize audit results through careful review of any previous issues, close inspection of infrastructure, and adherence to current protocols.
Working with a CRO
There is a wide variety in CROs. Some are large, publicly owned companies with global coverage and a range of comprehensive services, while other CROs are small, privately owned companies that specialize in a specific niche area. Comparing one CRO to another can be very difficult, as CRO budgets and services often vary significantly from one company to the next. When selecting a CRO, take into account the company’s previous experience, including the types of projects completed, the clients they have worked with, any niche services they provide, as well as their overall track record in the industry.
Before you decide, read our guide on how to efficiently and effectively evaluate Clinical Research Companies and download our CRO Evaluation Checklist .
Sponsor’s Responsibility
A business, organization, or institution may transfer any or all of their clinical trials and research responsibilities over to a Contract Research Organization, but the responsibility remains with the original company hiring the CRO. The quality and integrity of the clinical research data continue to reside with the entity sponsoring the work. CROs should be backed by a spotless track record of quality assurance and quality control.
Get It In Writing
When hiring a CRO, always ensure each delegated task is outlined in writing and signed by both parties. All agreements should be thoroughly documented by all of the involved parties to avoid any costly misunderstandings or complications. It should be very clear which organization is responsible for each aspect of the medical research, development, or other clinical services. Any services or components that are not specified in the agreement will remain the responsibility of the hiring entity.
What Is Contract Research And Manufacturing Services?
Contract Research and Manufacturing Services (CRAMS) is a broader clinical outsourcing term that includes CROs as well as CMOs, Contract Manufacturing Organizations.
Contract Manufacturing Organizations (CMOs) are similar to CROs, in that they are also hired by another pharmaceutical company on a contract basis. The purview of a CMO is in the development of a drug through to its manufacturing.
They all make up a rapidly expanding segment of the biotechnological and pharmaceutical industry. Outsourcing specialized clinical research and manufacturing work continues to develop as organizations aim to meet the needs of an evolving industry.
Promedica Intl. (PMI) delivers the quality and consistent results of a large Clinical Research Organization with the efficiencies and responsiveness of a small CRO. PMI can support your needs with a range of specialized services, including Project Management, Product Development Planning, Clinical Study Management, Product Commercialization, Medical Writing, Biostatistics, Data Management, and Research Compliance & Education.
Learn more about our wide range of clinical services , contact our team , or submit a Request for Proposal .
PMI’s quality control procedures for statistical output involve a combination of independent programming and internal review to verify accuracy and completeness of tables, figures, and listings.
CORPORATE INFORMATION
News & Events
Audits/FDA Inspections Data Management GCP Seminars
Clinical Information Technology
Employment Opportunities Contract Consulting
Request for Proposal Selecting a CRO Visiting PMI Study Opportunities Links & Resources

Site Map | Privacy Policy | Disclaimer
© 2019-2024 Promedica International, a California Corporation

Everything You Need to Know About CROs
The clinical research industry has a lot of acronyms. It can be very confusing to navigate through the field’s vocabulary, especially if you are just starting to read about it. Below, I will tell you everything you need to know about CROs and make your life just a little bit easier.
What is a CRO?
CRO, or a clinical research organization, is a company that assist drug manufacturers and pharmaceutical companies by outsourcing the necessary stages and clinical trial processes. CROs streamline clinical trial processes and help in research and development (R&D). To better understand the roles within a CRO, consider exploring the Clinical Research Coordinator course or the Clinical Trials Assistant Training .
What is the outlook for CROs?
The CROs are critical to the R&D process, and they create growing field. In 2017, the pharmaceutical outsourcing market was valued at $3.37 billion . By 2024, it is estimated to become valued at $7.26 billion .
Many large companies have their own clinical research organization or rely on an outside company to conduct drug development and testing. Outsourcing these services save pharmaceutical companies a lot of money. By hiring a CROs, companies no longer have to recruit or maintain their own clinical department. This helps a company cut down costs overtime.
The two major CROs are the LabCorp and IQVIA .
LabCorp, or Laboratory Corporation of America Holdings, generated $11.5 billion of revenue in 2019 . LabCorp is split into two: Covance Drug Development and LabCorp Diagnostics. One focuses on clinical research from the early stage research to post regulatory approval, while the other focuses on the diagnostic tests.
IQVIA was the result of a merger between IMS Health and Quintiles in 2016 . It had made $11.09 billion in revenue in 2019 .
Why are CROs Growing?
Before a drug or medical device can be sold to the public, they must be approved by the relevant bodies. For example, in the U.S., a new drug must be FDA approved before it is released on the market. However, most evaluating bodies have high requirements and need a lot of quality, positive data approving a product. When companies expect unbiased and high-quality results, they hire a CRO.
CROs are experts at what they do. They provide skilled personnel to generate high-quality data and assist with the necessary paperwork so that an approval can go through. One of the most compelling reasons to hire a CRO is that they are unbiased. CROs work closely with the companies they work for, but are separate from them. They do not share a conflicts of interest and can be trusted to report accurate and truthful data.
Want to work for a CRO?
CROs are a part of a growing field, and they need a lot of manpower. There are so many postings for positions within a CROs right here . Don’t have any experience? Don’t worry! You can take courses from CCRPS and learn how to become a clinical research professional. Explore specialized courses like Pharmacovigilance Certification , CRA Training , ICH-GCP Training , Advanced Clinical Research Project Manager Certification , or Advanced Principal Investigator Physician Certification to further your understanding and skills in the field.
Take courses from CCRPS and learn more on how to become a clinical research professional.
Discover more from Clinical Research Training | Certified Clinical Research Professionals Course

What is a Clinical Research Scientist
Clinical research positions.
- Phase I – III
- Phase IV & RWE
- Resourcing Solutions
- Functional Services (FSP)
- Dermatology, Immunology & Inflammatory Diseases
- Internal Medicine
- Neuroscience
- Oncology & Hematology
- Ophthalmology
- Rare Diseases & Orphan Drugs
- Global Presence
- Sustainability
- Investigators
- Intellect Hub
- Request a Proposal
What Does a CRO Actually Do?

A contract research organization, or CRO for short, is a specialized provider that is outsourced on a contractual basis by pharmaceutical, biotechnology, and medical device companies (sponsor) in the clinical research industry to manage various aspects of clinical trials . Typically, sponsors can transfer one or more of their own trial-related duties and functions to a CRO throughout the lifecycle of clinical trials, wherein the transferred duties are specified in writing .
For sponsors looking to conduct clinical trials efficiently and effectively, understanding the functions and responsibilities of a CRO is paramount. In this article, we will explore the key aspects of what a CRO actually does and why their expertise is vital for successful clinical trial management.
The Global Demand for Contract Research Organizations (CROs)
Estimates by Global Market Insights valued the CRO market size at US$56.7 billion in 2022 and projected a compound annual growth rate (CAGR) of 6.9% between 2023 and 2032 to US$108 billion in 2032 . Although the COVID-19 lockdown restrictions in 2020 initially slowed the market, it quickly rose again as R&D and manufacturing processes resumed and CRO partnerships in clinical research were being embraced by biopharma and biotech sponsors worldwide . The COVID-19 pandemic also accelerated the transition from traditional paper-based records to digital data solutions, creating a niche in which the advanced technologies typically offered by CROs could especially flourish . Overall, the projected market growth of this industry is driven primarily by increasing R&D expenditure worldwide, greater number of clinical trials emerging in different countries, and increasing capabilities of globalized clinical research as a result of rising technological advancements .
The Role of the Contract Research Organization
Nearly three out of every four clinical trials are estimated to be conducted by a CRO contracted by sponsors, underscoring the value of these companies to the drug development process today . Although they may have started out as a peripheral vendor to which clinical trial companies could outsource certain R&D activities, CROs are now considered an integral partner within the pharmaceutical, biotech, and medical device industries. The primary advantage of such a partnership is the flexibility and resources CROs provide to sponsors, freeing up their time to invest more in R&D innovation and allowing them to thrive in a competitive fast-paced marketplace .
Clinical Trials Services Provided by Contract Research Organizations
There is a significant variety in the types of services that a CRO can offer for sponsors, comprehensively covering multiple areas spanning the drug development pipeline, from initial discovery of a new molecule to post-marketing surveillance . Visit our article on the TFS Intellect Hub to discover the top 10 must-have services offered by leading CROs .
- Pre-clinical research: Providing facilities to support initial testing and analysis of a new drug or medical device.
- Clinical research: Providing trained teams of clinical trial professionals to oversee the administration and management of clinical trials, as well as navigate local regulatory guidelines to maintain strict compliance.
- Other services: Providing specialty services in bioanalysis, data management, biostatistics, central laboratory capabilities, and many more.
In terms of the services they provide, CROs are typically classified as either full-service, specialty, or some combination thereof . Whereas a full-service CRO can support clinical trials through a comprehensive array of services (e.g., project management, site selection, data management, etc.), specialty CROs offer a focused scope of services that is limited to a specific area (e.g., oncology, preclinical development, medical devices, etc.) . For example, TFS HealthScience is a full-service CRO with teams supporting numerous topics of expertise (see here ) and solutions (see here ).
Regardless of the type of CRO chosen, the vast diversity of duties they can perform for clinical research companies creates a strong support system that enables sponsors to streamline their operations and focus their resources on innovation and growth.
How Contract Research Organization Partnerships Impact Clinical Research
According to a 2023 report released by PPD, The Pulse: Global R&D Insights in Pharmaceuticals, many of the respondents noted a growing strain of talent shortages, of which 41% increased their reliance on CROs through functional service provider partnerships . By leveraging the specialization and expertise of a CRO, biopharma and biotech companies can accelerate their drug discovery and development processes, thereby bringing vital new treatments to market more quickly. Furthermore, as clinical trials become increasingly globalized, CROs act as an invaluable partner enabling sponsors to navigate numerous local regulatory restrictions and overcome such challenges to ensure their clinical research activities are fully compliant .
Conclusion
CROs play a pivotal role in the pharmaceutical, biotech, and medical device clinical research industries as a result of the broad and diverse array of services they provide in the pursuit of bringing new therapies to market. By outsourcing their research and development activities to a CRO, companies can maintain a focus on their core competencies, while also benefiting from the specialized expertise and substantial infrastructure of the CRO. As the landscape of these industries continues to evolve and grow, the role of CROs within this ecosystem is likely to become even more critical and indispensable in the future, evidenced by the expected growth of the CRO market over the next decade alone .
About TFS HealthScience CRO
TFS HealthScience is a global CRO that supports biotechnology and pharmaceutical companies throughout their entire clinical development journey. In partnership with customers, we build solution-driven teams working for a healthier future. As a trusted CRO partner throughout the entire clinical development journey, we understand the importance of providing essential and diverse services to streamline clinical trials for our clients.
Visit our website to learn more about the solutions TFS can offer for your next clinical trial or connect with a TFS representative today!
Learn more about our related services and resources:
Contact us:.
Contact us today to learn more.
Previous Post A Candid Look at the Employee Experiences within the Top CROs of 2024
Next post empowher spotlight: noelia ortega, business unit head of strategic resourcing solutions.
Comments are closed.
Our Expertise
Our solutions, our resources.
- Join Investigator Database
Connect with TFS
© 2024 TFS HealthScience | Contract Research Organization | Global Resourcing Provider | CRO. All rights reserved. Raise a Concern Privacy Policy and Cookies Terms and Conditions Cookie Settings

Thank you for Subscribe us
Thanks for your interest, we will get back to you shortly
Home » Digital Adoption » What Is A CRO? The Pharmaceutical, Biotechnology, and Healthcare Device Industries Explained
What Is A CRO? The Pharmaceutical, Biotechnology, and Healthcare Device Industries Explained

- Updated February 9, 2023

This article will explain what a CRO is, an acronym for Clinical Research Organization or Contract Research Organization. Anyone involved in healthcare-related industries will have some experience working with these outsourcing companies that deal in clinical trials.
After all, medical outsourcing is a huge industry. A 2022 Fortune report predicted that the industry will be US$90 Billion by 2026. Companies across the pharmaceutical industry seek to reduce costs and improve outcomes, making it more critical than ever to consider the most efficient way to conduct clinical trials.

The healthcare industry is on a mass digital adoption spree, and outsourcing clinical trials has become a viable option with the assistance of new technology such as blockchain, AI, and cloud storage. These new technologies have allowed companies to reduce costs and increase efficiency in their clinical trials by streamlining processes and creating a secure platform for data exchange.
However, choosing a CRO can be a complex process. This article will explain what a CRO is, how they can support major companies and the criteria for selecting the right CRO.
What Is A Contract Research Organization (CRO)?
A CRO is an organization that undertakes short-term contracts in research and development across the life sciences industries. CROs are especially valuable in medical sectors, including pharmaceutical companies, biotechnology, and medical technology.
They support efficiency by staying up-to-date in all aspects of R&D. The contracting company does not need to maintain their research experts or provide the infrastructure for them to work.
The acronym CRO usually refers to a “contract research organization,” but this is synonymous with “clinical research organization.”
Contract research organizations choose to specialize their services in different ways. Some will provide services to one particular niche or industry. Others may specialize in a specific kind of medical research.
How a Clinical Research Organization Can Support the Clinical Trial Process
Clinical trials are complicated, expensive, and time-consuming. Even the largest pharmaceutical company won’t always have the research and development capacity to complete successful trials internally.
A contract research organization can support companies by taking charge of all trial-related duties. In doing so, CROs ensure oversight of the quality assurance process in a cost-effective way.
A successful trial relies on a tight network of specialist medical staff, reliable participants, and regulatory knowledge and involves manufacturers, sponsors, and moral authorities. Moreover, valuable trial data depends on outstanding quality at every step of the process.
Companies dealing with drug development, medical devices, or biotechnology require extensive clinical trials to guarantee that their products are thoroughly tested and ready to market. CROs efficiently provide the peace of mind that only comes with thorough research and documentation.
In an increasingly digital world, communications between CROs and their sponsors often benefit from digital transformation. On the consumer side of healthcare, digital adoption has been shown to improve customer experiences. Digital adoption solutions can likewise integrate companies seamlessly.
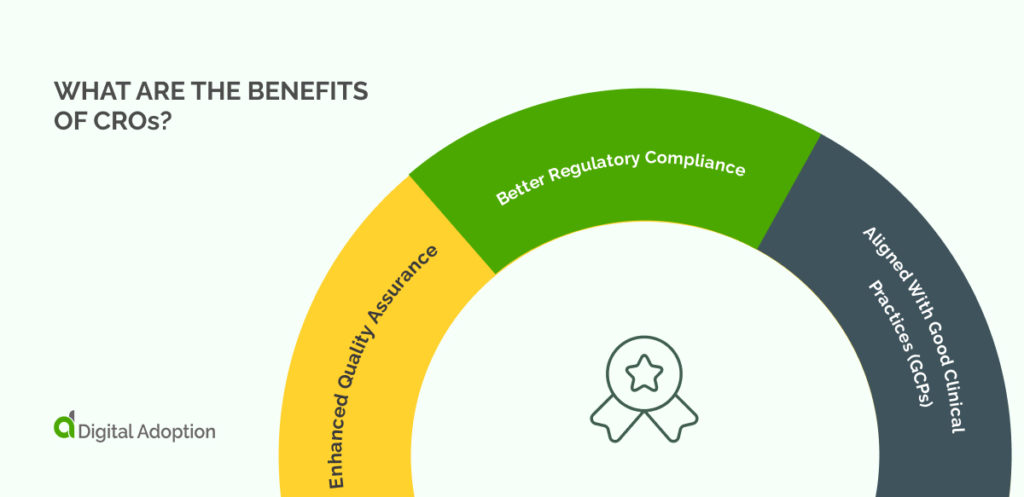
What Are The Benefits Of CROs?
Many benefits of business process outsourcing medical trials are similar to other kinds of outsourcing. A CRO helps a company to access specialized staff, manage financial constraints, and tailor solutions that match their needs.
However, the specific demands of trial logistics mean that there are some more specific benefits of contracting with a CRO:
Enhanced Quality Assurance
The CRO looks after a vast range of assurance responsibilities that lead to a consistently high trial quality.
A CRO has the right research staff, innovation knowledge, and physical capabilities to conduct an excellent trial from start to finish. From clinical monitoring and site selection to medical writing, the CRO does vital work in ensuring trial quality.
Better Regulatory Compliance
Regulatory authorities of all sizes govern clinical trials with non-negotiable policies and legally binding rules. Many organizations would struggle to keep up with the most relevant compliance issues. However, the specific regulatory requirements in an industry are a fundamental part of the CRO’s work.
Aligned With Good Clinical Practices (GCPs)
The UK government explains that Good Clinical Practice is “a set of internationally-recognized ethical and scientific quality requirements that must be followed when designing, conducting, recording and reporting clinical trials that involve people.”
These requirements place a necessary burden on trial sites to report problems and accept inspections. In other words, maintaining a trial center is much more than allocating the right office space for the job. CROs do a lot of work to keep their centers ready for inspections at all times.
What Clinical Trial Management Services Do CROs Offer?
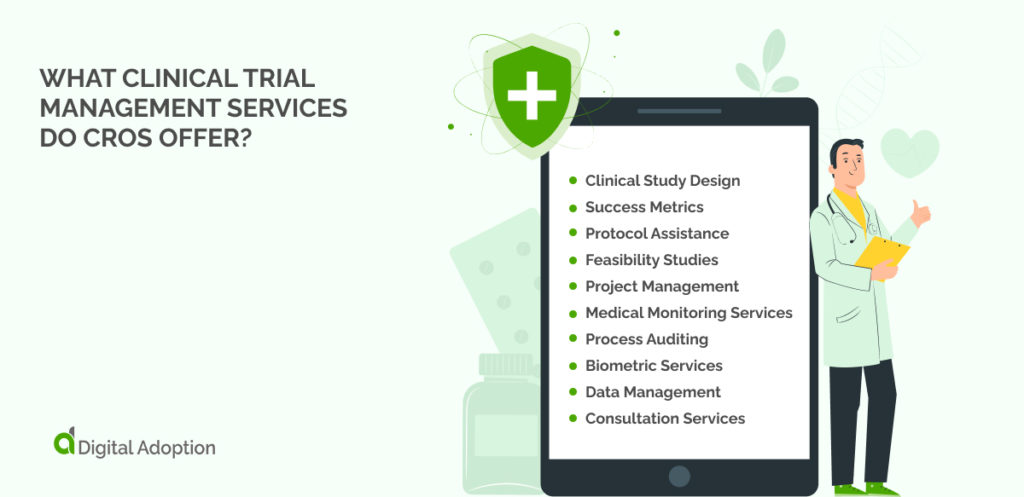
A CRO can support all stages of the planning, execution, and review of a clinical trial. A business leader can hand over all of the sponsor’s trial-related duties to the CRO if necessary.
However, many CROS can support sponsors with just one or more aspects of functions carried out in the trial process. Some of the typical CRO services that businesses can use include the following.
Clinical Study Design
The design stage is the moment for the CRO to find a solution that meets the sponsor’s needs. They will advise on the best type of approach, the type of participants, the budget, and the schedule.
Success Metrics
The planning team at a CRO will be able to advise on the most reliable measures of success. This is an excellent stage for dialogue between the sponsoring organization and the contract research organization itself.
Protocol Assistance
CROs deal with the details of trial protocols every day of the week. They are best placed to rigorously monitor documentation around ethics and regulatory affairs, ensuring that national and international standards are kept.

A wonderful team of Digital Adoption, Digital Transformation & Change Management Experts.
RELATED ARTICLES

Cloud Adoption: What Is It And Why Is It Important?

The Power of Cross-Functional Teams

16 Employee engagement survey questions
Most popular.

Conversational AI vs Generative AI

24 Best AI productivity tools for 2024

Contact Us: [email protected]
This blog is powered by WalkMe. By subscribing to our newsletter, you agree to the Terms and Conditions . For more information about the processing of your personal data please check our Privacy Policy .
POPULAR POSTS

POPULAR CATEGORY
- Chinese, Simplified
- Chinese, Traditional

- News & Resources
- Latest News
- Latest Articles
- Latest Reports
- Latest Webinars
- Upcoming Events
- Latest Case Studies
- Latest Whitepapers
- Latest FAQs
- Covid Notices
- Novotech Announces MOU with Experienced Psychedelics Research Center at the 4th Annual Psychedelic Therapeutics and Drug Development Conference in Boston
- Novotech Appoints Commercial and Clinical Life Sciences Specialist to Head Up Global Drug Development Consulting Team
- Novotech Publishes Research Report on Acute Myeloid Leukaemia Clinical Trial Landscape for Clinical Stage Biotechs
- View All News
Endometrial cancer - Global Clinical Trial Landscape (2024)
Cystic fibrosis: global clinical trial landscape (2024).
- Acute Myeloid Leukaemia: Global Clinical Trial Landscape (2024)
- View All Reports
Transitioning from Healthy Volunteers to Patients: Navigating the Clinical Development Journey
Clinical trials in china: unlocking the potential of advanced therapies.
- Endpoints at BIO 2023: Forging new biotech ties with China
- View All Webinars
Psychedelic Therapeutics and Drug Development Conference 2024
Asco annual meeting 2024.
- BIO International Convention 2024
- View All Events
Examining a Phase 1 COVID 19 Clinical Trial
- Muscular Dystrophy – Multi-National and Multi-Site Trials Case Study
- Expert Consulting and Multi-Regional Clinical Trial (MRCT) Strategy Rescues Oncology Program
- View All Case Studies
Unveiling the Potential of Antibody-Drug Conjugates
- Overview of Endometrial cancer: Global Clinical Trials Landscape
- Insights into the Clinical Trials Landscape of Cystic Fibrosis
- View All Faqs
Comprehensive Report on Antibody-Drug Conjugates Clinical Trials 2024
- Precision Oncology Clinical Trials & Statistics 2024
- Immune Checkpoint Inhibitors Global Clinical Trials Landscape (2023)
- View All Whitepapers
- Assessing the pros and cons of basing clinical trials in today’s European landscape
- Q&A with Judith Ng-Cashin, CRO Novotech's chief medical officer, on 2023 and beyond
- Navigating Global Innovation: Novotech's Insights on Accelerating China’s Biotech Development
- View All Article
Medical and Regulatory Consulting
Patient recruitment and site selection, early phase trials in australia, clinical operations and project management, site management organization (smo), biometrics and data management, virtual clinical trials, real world data, laboratory services, oncology cro services, pharmacometric services, drug development consulting, gmo solutions, liver disease cro services, infectious diseases and vaccines cro services, orphan and rare disease cro services, clinical and regulatory strategy, novotech announces mou with experienced psychedelics research center at the 4th annual psychedelic….
Boston, USA - Novotech, the global full-service cli...
Novotech Appoints Commercial and Clinical Life Sciences Specialist to Head Up Global Drug…
Boston, USA - Novotech,...
Novotech Publishes Research Report on Acute Myeloid Leukaemia Clinical Trial Landscape for Clinical…
Boston, USA - Novotech, the global full-service clinical Contract Resea...
Explore cutting-edge endometrial cancer research with Novotech CRO's disease report....
Explore cutting-edge cystic fibrosis research with Novotech CRO's disease report. Learn about new treatments, clinical trials, and future innovations in...
As you embark on Phase 2 after successfully launching your Phase 1 healthy volunteer study in Australia or New Zealand, it’s time…
In recent years, China has become an important player in the field of advanced therapies by increasing its focus on cutting-edge…
Background:Phase I clinical studies are critical in the development of new therapies especially in the rapidly changing biotech...
This FAQ section explores key trends in clinical trials, applications, and cutting-edge research shaping the future of these targeted therapies....
Download the 2024 clinical trial report on Antibody-Drug Conjugates, covering new drug developments and market insigh...
Covid-19 Notice important updates
Find content relevant to:.
Chicago, Illinois
Novotech’s Medical and Regulatory Consulting team offers full range of pre-clinical, regulatory affairs support, medical and pharmacovigilance consulting services....
Novotech relies on years of experience, in-country knowledge and real-life big data to identify and propose the best-performing sites for...
Australia is a preferred destination for early phase trials because of simple and fast regulatory stream and lucrative R&D cash...
Novotech’s streamlined and integrated clinical trial services are delivered by a dedicated team of professionals with deep industry and therapeutic...
Discover the power of clinical excellence with Acrostar's SMO Division, a dedicated entity operating as part of Novotech....
Delivering accurate, high-quality and timely biostatistics in clinical trials services, including statistical planning, analysis and reporting....
How virtual clinical trials can offer patient retention and cost benefits compared to traditional trials....
Accelerating patient recruitment and drug development with real world data (RWD)...
Our bioanalytical services assist our customers in every stage of their molecule development....
The global Oncology landscape for Biotechnology companies...
Our team can assist in all clinical study phases and in study designs ranging from first in human, single ascending...
Novotech Drug Development Consulting is a full-service global product development and strategic regulatory group, providing comprehensive “lab to launch” program...
GMO Solutions: Novotech initiated the first national, privately owned, commercial Institutional Biosafety Committee (IBC) in Australia....
The global Liver Disease landscape for Biotechnology companies...
The global Infectious diseases and Vaccines CRO services landscape for Biotechnology companies...
The global Orphan and rare disease CRO services landscape for Biotechnology companies...
Clinical and Regulatory Strategy: Many biotechnology companies come to Australia to conduct early phase clinical trials and take advantage of...
Our workplace culture reflects the passion of our people, and we will support you to develop and achieve at all...
Novotech is a global full-service contract research organization (cro) providing clinical development services across all clinical trial phases and therapeutic..., there are many reasons people love working at novotech, but when you join it will be our open, inclusive, and..., we are committed to providing ongoing professional development training, a competitive bonus structure, a supportive work environment, variety in their..., our mission is to create career development opportunity for everyone...., we are committed to hiring ambitious and ethical professionals genuinely excited to be a part of the dynamic life sciences industry and who relish a challenge., search novotech, how cro companies work - a basic overview.
It’s quite common for pharmaceutical and biotechnology companies introducing new drugs and treatment to outsource trials of new medication or procedures to CRO Companies.
What Is a Clinical Research Organization?
A Clinical Research Organization (CRO) is contracted by a pharma, biotech or related entity to manage and lead their clinical trials.
Organizations contract with CRO Companies so they can acquire specific expertise without having to invest in their own staff. Reducing the time it takes to conduct a trial compared to conducting the trial in-house results in substantial cost savings. It also eliminates the need for infrastructure and office space to run the trials themselves.
Some CRO Companies manage most aspects of a clinical trial, right from the site selection and patient enrolment to the final regulatory approval.
Although the sponsoring company transfers the trial functions to a CRO Clinical Research Company, the sponsor is responsible for the trial’s integrity and needs to ensure results are factual and scientifically backed.
Services Provided by Clinical Research Organization Companies
Clinical Research Organizations can be involved in a range of services from project management, bioanalytical services , database design, data entry and validation, data management, disease coding, reporting, statistical analysis, validation programming, safety & efficacy summaries and the final study report.
The main areas which CRO Companies are involved in include infectious disease, oncology, infectious disease, the central nervous system, cardiovascular disease and metabolic disorders.
Helping to Drive Down Treatment Costs
Pharmaceutical and medical devices companies are facing an increasing pressure to make drugs and treatments more affordable.
They are constantly looking for ways to lower the costs of prescription drugs without sacrificing profits. Outsourcing clinical trials to clinical trial organization companies is a way to significantly lower costs and make up for the money they have to forego because of lower medication prices.
Utilizing the services of CRO Companies is also a cost-effective way for companies to develop medications for rare conditions or conditions which only a few people suffer from.
Other relevant content
What is an investigator site file (isf), corrective and preventive action, reporting serious breaches in clinical trials, how long it take a cro company to complete a clinical trial, what is the driving force behind china’s biotech revolution, glossary of important terms related to clinical trials, placebos: why they play an important role in clinical trials, what makes a good clinical trial site, the importance of control groups in clinical trials, randomisation in clinical trials, industry survey identifies successful biotech clinical trial strategies.
A Comprehensive Guide to Clinical Research Organizations (CROs)

Clinical Research Organizations (CROs) play a crucial role in the pharmaceutical, biotechnology, and medical device industries. They provide support to companies in the form of research services outsourced on a contract basis. In this comprehensive guide, we will explore what CROs are, who their clients are, the stages of the research process they are typically involved in, and delve into the exciting career opportunities within the field of clinical research.
Table of Contents
Introduction to Clinical Research Organizations (CROs) The Role of CROs in the Research Process Services Offered by CROs Clients and Partners of CROs Careers in Clinical Research Clinical Research Associate (CRA) Roles and Responsibilities Educational and Professional Requirements for Clinical Research Careers Advancement Opportunities in Clinical Research Tips for Success in Clinical Research Careers Resources and Professional Organizations Conclusion
Introduction to Clinical Research Organizations (CROs)
Clinical Research Organizations (CROs) are companies that provide support to the pharmaceutical, biotechnology, and medical device industries in the form of research services outsourced on a contract basis. They offer a wide range of services , including biopharmaceutical development, clinical development, clinical trials management, and pharmacovigilance. CROs aim to simplify the entry into drug markets and streamline the drug development process by providing specialized expertise and resources.
The Role of CROs in the Research Process
CROs play a crucial role in the research process, from the early stages of drug discovery and development to the final stages of clinical trials and commercialization. They work closely with their clients to design and execute clinical trials, ensuring adherence to regulatory requirements and ethical standards. CROs also provide support in data management, statistical analysis, and the preparation of regulatory submissions.
Services Offered by CROs
CROs offer a wide range of services to their clients, including but not limited to:
Biopharmaceutical development : CROs assist in the development of new drugs, from preclinical studies to early-phase clinical trials. Clinical development: CROs design and manage clinical trials, ensuring compliance with regulatory requirements and ethical standards.
Clinical trials management: CROs oversee all aspects of clinical trials, including site selection, patient recruitment, data collection, and safety monitoring.
Pharmacovigilance : CROs monitor the safety of drugs and medical devices during clinical trials and after they are on the market.
Real-world evidence and outcomes research : CROs collect and analyze data from real-world sources, such as electronic health records, to generate evidence on the safety and effectiveness of drugs and medical devices. CROs conduct studies to evaluate the effectiveness and safety of drugs and medical devices in real-world settings.
Clients and Partners of CROs
CROs work with a diverse range of clients, including pharmaceutical companies, biotechnology firms, medical device manufacturers, research institutions, and government organizations . They collaborate closely with their clients to ensure that research studies are conducted efficiently, safely, and in compliance with regulatory requirements. CROs also partner with academic institutions and foundations to support their research initiatives.
Careers in Clinical Research
Clinical research offers exciting career opportunities for individuals interested in the scientific, regulatory, and operational aspects of drug development.
Careers in clinical research span a wide range of roles, including Clinical Research Associate (CRA), Clinical Project Manager, Data Manager, Biostatistician, and Medical Writer, among others. These roles require a combination of scientific knowledge, attention to detail, critical thinking, and strong communication skills.
Clinical Research Associate (CRA) Roles and Responsibilities
Clinical Research Associates (CRAs) play a crucial role in the execution and monitoring of clinical trials. Their responsibilities include site selection and initiation, monitoring study progress, ensuring compliance with protocols and regulatory requirements, and maintaining accurate and complete documentation. CRAs work closely with investigators, study coordinators, and other stakeholders to ensure that trials are conducted safely and efficiently.
Educational and Professional Requirements for Clinical Research Careers
Careers in clinical research typically require a strong educational background in life sciences or a related field. Many positions, such as CRAs, require a bachelor's or master's degree in a scientific discipline. Professional certifications, such as the Certified Clinical Research Associate (CCRA) certification, can enhance career prospects and demonstrate expertise in the field.
Advancement Opportunities in Clinical Research
Clinical research offers ample opportunities for career advancement and professional growth. Experienced professionals can progress to more senior roles, such as Clinical Project Manager or Clinical Operations Director, where they oversee the planning and execution of multiple clinical trials. Continuing education, networking, and staying updated with industry trends are essential for career advancement in clinical research.
Tips for success in seeking a Career in Clinical Research
To succeed in clinical research careers, professionals should continuously develop their scientific knowledge, stay updated with regulatory requirements, and enhance their communication and project management skills. Networking, building relationships with key stakeholders, and seeking mentorship can also contribute to career advancement in the field.
Resources and Professional Organizations for Clinical Research Professionals
Several resources and professional organizations cater to the needs of clinical research professionals. These include industry publications, online forums, conferences, and professional associations. Organizations such as the Association of Clinical Research Professionals (ACRP) and the Society of Clinical Research Associates (SoCRA) provide educational resources, networking opportunities, and professional certifications for clinical research professionals.
Clinical Research Organizations (CROs) play a vital role in the pharmaceutical, biotechnology, and medical device industries by providing research services on a contract basis. They offer a wide range of services to support the development and execution of clinical trials, ensuring compliance with regulatory requirements and ethical standards.
Careers in clinical research offer exciting opportunities for individuals interested in the scientific, regulatory, and operational aspects of drug development. By partnering with CROs and pursuing careers in clinical research, professionals can contribute to the advancement of medical science and the development of innovative therapies.
If you're interested in exploring careers opportunities in clinical research, view our current vacancies at ICON today.
Sign up for post alerts
Icon & you the potential of together..
Careers that improve the lives of patients, our clients and each other. Are you ready to make a difference?
Related jobs at ICON
United States
Regulatory, Drug Safety/ Quality Assurance & Other roles
Remote Working
Business Area
ICON Strategic Solutions
Job Categories
Description
As a Clinical Supplies Specialist you will be joining the world’s largest & most comprehensive clinical research organisation, powered by healthcare intelligence.
2024-110322
Expiry date

Romania, Bucharest
Hybrid: Office/Remote
ICON Full Service & Corporate Support
Study Start Up
At ICON, it’s our people that set us apart. Our diverse teams enable us to become a better partner to our customers and help us to fulfil our mission to advance and improve patients’ lives. Our ‘Own

Poland, Warsaw
Full Service - Development & Commercialisation Solutions
ICON plc is a world-leading healthcare intelligence and clinical research organisation. From molecule to medicine, we advance clinical research providing outsourced services to pharmaceutical, biotech
Biometrics Roles
Biometrics Portfolio
ICON plc is a world-leading healthcare intelligence and clinical research organization. We’re proud to foster an inclusive environment driving innovation and excellence, and we welcome you to join us
2024-107697
Related stories

Teaser label
Content type
Publish date
Deepak is a Clinical Data Management Project Manager at ICON Strategic Solutions, the largest global provider of Functional Service Provision (FSP). He works as a dedicated resource within one of o
Deepak shares his experience as Clinical Data Management Project Manager at ICON Strategic Solutions.
.png)
Overcoming Resume Gaps In an ideal world, resumes would neatly showcase an uninterrupted career progression. However, In today's dynamic job market, it's not uncommon for professionals to encoun
Periods of unemployment don't have to be a red flag. Learn proven strategies for addressing resume gaps.
.png)
The Art of Customisation: How to Tailor Your CV for Any Role or Industry In today's competitive job market, a one-size-fits-all CV often misses the mark. To truly stand out and position yourself a
Discover strategies to highlight your expertise, role-specific skills, and industry knowledge to help your CV stand out.
Recently viewed jobs
A better career. A better world. A better you.
Browse popular job categories below or search all jobs above
What is a CRO?
Patricio ledesma, contract research organization, 24 january, 2024.

A CRO (Contract Research Organization) is a company that provides clinical trial management services for the pharmaceutical, biotech, and medical device industries.
Although there are different types of CROs and diverse levels of specialization (distinct therapeutic areas for instance), typical CRO services include regulatory affairs, site selection and activation, recruitment support, clinical monitoring, data management, trial logistics, pharmacovigilance, biostatistics, medical writing, and project management, among others.
In a clinical trial, CROs are hired by sponsors to perform a set of tasks, taking various technical and administrative responsibilities on the sponsor’s behalf.
The main role of the CRO is to plan, coordinate, execute, and supervise the processes involved in the development of a clinical trial, being a central contact point between the sponsor and other trial actors (e.g. ethics committees, regulatory agencies, vendors, and hospitals).
CROs are key players in clinical research, since they have the knowledge and the capabilities needed for the proper development of a clinical study. They help sponsors by reducing their workload, while ensuring trial quality and compliance with national and international standards.
At the same time, many CROs supply innovative technological tools to increase efficiency in the study processes, which translates into cost reductions.
Without doubt, CROs play a crucial role in the success of a clinical trial. Sponsors should carefully assess the particular needs of their projects, and look for the CRO that best meets their technical requirements and budget.

Patricio Ledesma ( B.Eng Concordia University, Montreal, Canada and Master’s Degree in Clinical Trials, University of Seville, Spain) is the Head of Clinical Operations and Founder at Sofpromed CRO. Patricio is a professional consultant providing comprehensive, one-stop expert advice and guidance to biotechnology and pharmaceutical companies worldwide in the field of clinical trials and drug development. He is personally and enthusiastically devoted to helping biotech Chief Executive, Operations, Scientific, Medical, and Regulatory Officers in the planning and execution of phase I-IV clinical trials across North America, Europe, Asia-Pacific, Latin America, and Middle East regions. You can contact Patricio at: +34 607 939 266 [email protected]

To our articles and updates
- Follow Follow
You may also be interested…

What Are Community-Based Clinical Trials?
Community-Based Clinical Trials
If you are a Sponsor seeking to run a community-based clinical trial in underserved populations, please contact us at [email protected] Clinical trials are fundamental to advance medical knowledge and improve patient care. However, one significant challenge in...

Clinical Research Site Networks: Accelerating Clinical Trials
Site Networks
If you are a Sponsor seeking to run a clinical trial through a clinical research site network, please contact us at [email protected] Clinical research plays a central role in advancing medical treatments and improving healthcare outcomes. To ensure the smooth...

How Community-Based Clinical Trials Are Advancing Healthcare in Underserved Populations
If you are a Sponsor interested in running a community-based clinical trial in underserved populations, please contact us at [email protected] Clinical trials are instrumental in advancing healthcare by evaluating the safety and effectiveness of new treatments and...

Advantages of Clinical Research Site Networks in North Carolina
If you are a Sponsor interested in running a clinical trial through a clinical research site network in North Carolina, please contact us at [email protected] Clinical research plays a pivotal role in advancing medical knowledge, improving patient care, and driving...

Benefits of Clinical Research Site Networks in Illinois
If you are a Sponsor interested in running a clinical trial through a clinical research site network in Illinois, please contact us at [email protected] Clinical site networks play a central role in advancing medical research and improving patient care. In this...

Advantages of Clinical Research Site Networks in Pennsylvania
If you are a Sponsor interested in running a clinical trial through a clinical research site network, please contact us at [email protected] Pennsylvania is a hub for clinical research, with numerous reputable clinical site networks offering a wide range of trials to...

Benefits of Clinical Research Site Networks in New York
If you are a Sponsor interested in running a clinical trial through a clinical research site network in New York, please contact us at [email protected] New York, with its vibrant healthcare landscape, is home to several prominent clinical research site networks.In...

Enhancing Clinical Trial Diversity Through Community-Based Site Networks
If you are a Sponsor interested in running a clinical trial through a community-based clinical research site network, please contact us at [email protected] One significant challenge in clinical trials is the lack of diversity among participants, particularly from...
Patricio Ledesma ( B.Eng Concordia University, Montreal, Canada and Master’s Degree in Clinical Trials, University of Seville, Spain) is the Head of Clinical Operations and Founder at Sofpromed CRO. Patricio is a professional consultant providing comprehensive, one-stop expert advice and guidance to biotechnology and pharmaceutical companies worldwide in the field of clinical trials and drug development. He is personally and enthusiastically devoted to helping biotech Chief Executive, Operations, Scientific, Medical, and Regulatory Officers in the planning and execution of phase I-IV clinical trials across North America, Europe, Asia-Pacific, Latin America, and Middle East regions. You can contact Patricio at: +34 607 939 266 [email protected]
Your session is about to expire
What is a contract research organization plus, top 5 cros to check out in 2022.

What is a CRO?
A contract research organization – CRO – is a company that provides outsourced services related to drug development and clinical research. Pharma and biotech firms and other sponsors may rely on a CRO for support in one or more aspects of developing and bringing new drugs and medical devices to market.
Keep reading as we explore the role of CROs in drug development, what services they offer, and why many sponsors work with one. We’ve also included a list of the top 5 CROs, and questions to ask before signing a contract with a CRO.
What Do CROs Do?

The main role of CROs is to carry out activities that the sponsoring pharmaceutical company cannot or chooses not to do in-house.
Pharmaceutical companies often count on both internal and external resources, and sometimes they need assistance with tasks that fall outside of their core capabilities. Most modern CROs provide services spanning the drug development pipeline, from running entire clinical trials to individual services such as data management, patient recruitment, protocol development, drug manufacturing, and the list goes on. CROs themselves might partner with other companies to provide robust services across the full spectrum of clinical trial activities. For example, CROs might partner with Power as a part of a multi-layered recruitment strategy for a trial.
The goal of a CRO is to provide streamlined services to pharmaceutical companies in order to help them get their new drugs developed, tested, and approved. It’s often more resource efficient and quicker to outsource certain aspects of clinical development, allowing sponsors to focus on their core operations.
For example, let’s say a biotech company has developed a new drug that looks promising in preclinical laboratory tests, but they’ve never conducted a clinical trial. It would generally be much more straightforward and efficient to partner with a CRO to conduct clinical trials than to dive into the complex regulatory landscape and risk inefficiencies or failures in the clinical testing of their promising new drug. Although the biotech firm would still be the sponsor of the trial, retaining responsibility for regulatory compliance, the CRO would be responsible for recruiting patients, administering the study drug, monitoring patients and overseeing the trial, and reporting the results back to the company. Each of these aspects is, in and of itself, a whole world that requires specialized expertise. It makes much more sense to outsource these aspects to dedicated professionals.
See the image below for some example services offered by CROs:
Are contract research organizations the same as clinical research organizations?
Contract research organizations (CROs) may get involved in all aspects of the clinical development process, from initial drug discovery through to pre-clinical and clinical trials and post-marketing surveillance studies. A CROs might help design a study, recruit patients for the study, perform laboratory tests, monitor patients during the study, analyze study data, prepare study reports for the sponsor, and present reports or summaries to regulatory bodies.
Clinical research organization is a less-common term which might be used to refer to a specific type of CRO that specializes in conducting clinical trials for pharmaceutical companies and other sponsor organizations. Thus, where a CRO might offer comprehensive service across the clinical development spectrum, a clinical research organization would be focused primarily on conducting phase I through phase IV studies for new drugs and medical devices.
Why do sponsors work with CROs?
CROs play an important role in pharmaceutical and biotechnology product development. Here are just some of the reasons why sponsors may decide to work with CROs:
1. Specialized operations and cost control
Building and managing clinical trial operations completely in-house is complicated, expensive, and requires specialization in a multitude of areas. The rise of CROs meant that pharmaceutical companies no longer needed to own all of their own scientific and clinical research facilities. Full-service CROs offer sponsors a complete set of solutions, allowing them to delegate as much or as little of the overall clinical development operation as they desire so as to optimize resource use and development timelines.
2. Expanded access to technology
CROs offer various technological solutions to support sponsors in designing, conducting, and managing clinical trials. Many of these tools are specific to clinical trials, and would not be commonplace in a lab-focused pharmaceutical company, for example. Sponsors can choose to leverage these technologies or product suites depending on the needs of each unique study. Some examples of eClinical technologies that sponsors may use include: planning tools (protocol design, patient enrollment, etc.); site management tools (activation, payments, etc.); modern patient recruitment tools (e.g., Power ); clinical trial management systems ( CTMS ); clinical data management systems (CDMS); analytics platforms.
3. Ability to handle large amounts of data
There are a lot of moving pieces involved in successfully running a clinical trial. CROs with experience in clinical trials will have efficient data handling procedures in place, making them a reliable partner for this complex aspect of modern trials which often draw from various data sources such as wearable devices, EHRs, eCOA / ePRO interfaces, etc.
4. Streamlined regulatory affairs (FDA regulatory compliance)
CROs typically have experience working with regulatory agencies such as the Food and Drug Administration (FDA). The FDA regulates the entirety of clinical trials, ensuring that ethical research guidelines and patient privacy safety standards are upheld. Sponsors must adhere to a multitude of FDA and HHS guidelines when conducting any clinical trial. Regulatory affairs is a complex landscape requiring familiarity with various parallel and overlapping regulations, as we discuss in our explainer on regulatory compliance in clinical trials . An experienced CRO will be able to help pharmaceutical companies get their products to market faster by facilitating regulatory submissions and navigating the intricacies of guidelines laid out in Title 21 and 45 CFR, GCP, HIPAA, etc. Partnering with a CRO offers an attractive alternative to hiring and training internal experts specializing in regulatory affairs, particularly for sponsors who aren’t conducting trials frequently.
5. Multi-disciplinary expertise leads to quicker and more effective trials
CROs often consist of a team of professionals with diverse backgrounds and specializations, which means that they can offer the appropriate expertise needed to effectively manage various aspects of clinical trial operations.
What to ask when choosing a CRO partner for clinical research

With the massive offer of CROs currently available, it’s important to realize the weight of the decision when selecting partners for clinical research. As the sponsor, regulatory compliance falls entirely under your responsibility, so choosing a trustworthy partner with a proven record of compliance can help you be sure your trial is in the right hands. Partnering with an incompetent or inexperienced CRO may have the opposite effect of streamlining trial timelines and resource usage, causing delays and risking valuable resources or even patient health.
The following 3 points act as a guideline for choosing a potential CRO before signing a provider agreement with them for a clinical trial, regardless of the extent of their involvement.
1. Obtain trustworthy information. It's important to gather as much information as possible about a potential research partner, as there is significant weight placed on such agreements. The website of the CRO itself will not tell the whole story; it is a good idea to look for real reviews and feedback from other companies who have worked with the CRO.
Make sure that the CRO you are considering has an established track record of success with past and current clients. Check that the CRO has an active online presence and transparent contact information, along with active client support.
The CRO should also have a good reputation among its employees; complaints by employees might be a red flag, particularly if they’re related to protocol deviations, non-compliance, mismanaged client disputes, internal uncleanliness or disorganization, or other issues that could impact patient safety and regulatory compliance.
2. Check that the CRO is up-to-date with regulatory standards and accreditations. The regulatory landscape is updated frequently, so it’s important to verify that the CRO in question has been active recently and has up-to-date authorization and accreditation for the specific operations you are considering contracting them for. If you can’t find this information on the website, contact them and have an open discussion to get clear answers.
3. Verify that the CRO has real expertise in the specific task you wish to outsource. This may seem obvious, but just because a given CRO is world-renowned in conducting clinical trials does not mean they will be experts in analyzing your specific data, particularly if you’re working in rare diseases or other niche areas. Direct experience in a similar therapeutic field and trial type is important, as it translates into efficiency and tells you that they will be comfortable navigating the particular waters you’re entering together. After all, the point is for the partnership to make your operations smoother, and for that, you need a partner with relevant experience.
What Are The Top CROs in 2022?
The contract research organization (CRO) industry is rapidly growing, and it's easy to see why due to the unique roles they fulfill in pharmaceutical and biomedical research.
We’ve curated a list of the top 5 contract research organizations (CROs) in 2022:
IQVIA is a leading healthcare technology company providing integrated services spanning the entire healthcare and clinical development spectrum. IQVIA offers solutions for clinical trials and research/development, real-world evidence studies, safety and regulatory compliance, and commercialization, and features a vast suite of technological tools powering “smarter healthcare.”
2) Labcorp Drug Development
Labcorp Drug Development, formerly Covance, is a contract research organization that offers drug development, clinical trial, and laboratory services to the pharmaceutical industry. The company's mission is to help advance patient care by providing clients with high-quality services and solutions.
Labcorp was founded in 1996 and has since grown into one of the world's largest CROs, with over 40 offices worldwide.
PPD is part of Thermo Fisher Scientific, serving as the clinical research services arm of the research giant. PPD is known internationally, having conducted clinical trials in over 100 countries, and currently consisting of over 35,000 employees worldwide.
PPD offers a comprehensive range of clinical development and laboratory services, providing sponsors with customizable solutions backed with a proven track record of professionalism and success.
ICON plc offers a full range of consulting, clinical development, and commercialization services to pharma, biotech, and other healthcare sector actors. Founded in Dublin, Ireland in 1990 and now counting on offices in 46 countries worldwide, ICON has a global team of experts and extensive experience in a wide range of therapeutic areas.
Medpace provides full-service clinical development services aimed particularly at biotech firms, with services ranging from study start-up through to monitoring, pharmacovigilance, regulatory affairs, and even medical writing. Founded in 1992, Medpace now operates in 6 continents and their teams of medical, operational, and regulatory specialists support their “therapeutically-aligned” scientific expertise and the delivery of their full-service, solution-oriented trial execution model.
CROs have become ubiquitous in clinical research amidst the trend toward partnering with third-party providers and the rise of specialized end-to-end clinical development services. A well-equipped CRO will be a valuable ally in managing drug development and clinical trial operations, bringing expertise and experience as well as access to state-of-the-art technology and facilities. Often, partnering with a CRO means optimizing resource allocation and thus keeping costs down. If you’re considering partnering with a CRO to enhance your research, development, or clinical trial efforts, it’s important to make an informed decision to ensure your operations are in the right hands. If you’re looking for a partner specializing in modern patient recruitment, reach out to us – we will be happy to show you the incredible power that lies within our patient recruitment marketplace and advanced recruitment solutions.
Need a predictable recruitment solution?
Tap into the largest source of independent patients looking for trials., other trials to consider.
Part 1a - Dose Escalation
Telephone-based early childhood developmental care coordination, incmga00012 (pd-1 antibody), tcu opioid-treatment linkage model (o-tlm), popular categories.
Myasthenia Gravis Clinical Trials 2024
Aricept Clinical Trials
Clinical Trials in Knoxville, TN
Clinical Trials in Florida
Clinical Trials in Iowa
Paid Clinical Trials in Austin, TX
Paid Clinical Trials in Glendale, AZ
Sickle Cell Disease Clinical Trials 2023
Diabetic Nephropathy Clinical Trials 2023
Anal Fistula Clinical Trials 2023
Popular guides.

The Growing Role of CROs in Clinical Trials
In today’s environment, drug developers must decide whether to leverage a CRO partner even earlier in their development timelines.

It’s estimated that nearly three out of every four clinical trials are conducted by contract research organizations (CROs), highlighting just how much sponsors value — and rely on — the work that CROs perform.
As clinical trials become more complex, sponsors are seeking business partnerships with a congruency in the management and prioritization of pipeline.
In our conversations with biopharma and biotech organizations over the past year, it’s become increasingly evident that the rising number of clinical trials and economic climate mean that the industry is feeling the effects of resource constraints.
As a result, sponsors must decide whether to leverage a CRO partner even earlier in their drug development timelines to ensure proper planning for these constraints.
How a CRO Accelerates Clinical Development
Clinical trials are becoming increasingly complex as the industry evolves. Our recent survey on industry trends found that 51% of drug developers denote the increasing complexity of clinical trials as a top challenge, along with patient recruitment (55%) and regulatory hurdles (46%).
From oncology clinical research to vaccine development , successfully executing and overseeing the many moving parts of a clinical trial may become overwhelming for sponsors to handle alone.
Undoubtedly, a CRO’s ability to respond to increased urgency by assuring certainty and speed in project delivery is the gold standard. Surveys show that CROs improve trial efficiency and increase productivity.
As a trusted extension of a drug developer’s team, CROs have the capacity to complete the day-to-day research activities that are either not possible or too expensive to achieve in-house. That can range from designing the clinical trial itself to bioanalytical testing and regulatory consultation.
CROs in Action
In today’s competitive landscape, with early decision and engagement, CROs enable their customers to navigate and overcome challenges.
Here are some examples:
- A European biotech firm with a promising pipeline and large investments needed to ramp up across multiple functions and therapeutic areas . The organization sought to build a dedicated FTE model for a broad range of services, including regulatory, medical writing and data management. It tapped a CRO with a strong FSP program to consolidate and streamline resourcing, and in doing so reduced time-to-offer from more than 50 days to 36.
- A sponsor needed a reliable laboratory partner to deliver product characterization and stability registration data for two different inhaled drug-device combination products. While this may have been too expensive or impossible to complete in-house, partnering with a knowledgeable and experienced CRO in this area provided both the capacity and the harmonized, quality data needed for successful new drug application (NDA) submissions.
- Amid a pause in clinical studies during the pandemic, sponsors needed a way to ensure trial continuity while keeping patients safe . Forward-thinking CROs used their experience, operational capabilities and regulatory knowledge to quickly deploy innovative, digital strategies — such as a visual communication tool to facilitate investigator and patient interaction — to safely continue data collection.
Four Key Factors to Consider When Selecting a CRO Partner
PPD experts see greatest sponsor success when they carefully consider the following factors in their CRO partner.
- A partnership model. A partnership model includes early engagement and shared governance with the CRO to prioritize and co-manage the pipeline. This brain trust extends to creative problem-solving, the ability to lean on global capabilities together, and a consistent and engaged team — translating to consistency and better prioritization of a sponsor’s trials.
- A talent retention and retraining strategy. Seek out CROs that invest in their people. Research suggests that companies that prioritize education become talent magnets and report increased retention rates. Sponsors considering an outsourcing or functional service provider (FSP) model, in particular, should look for a CRO that prioritizes developing staff knowledge and skillsets. The full force of global training, upskilling and reskilling, and mastery of data, infrastructure and systems encourages lower turnover and, in turn, study stability.
- Agility and scalability. Ensure the CRO can adapt its services and speed to your specific needs and goals, both today and in the future. If breaking into new geographic markets is an objective next year, will a given CRO be stable and sizeable enough to enable that? It’s also smart to partner with a CRO that can extend beyond traditional services and stay on the cutting edge — whether that’s early involvement with mRNA vaccines or trial decentralization .
- Experience and capabilities. Clients will initially interact with the business development team. Don’t be afraid to go a step further by ensuring the potential project team and leaders have relevant indication or product experience. Has the company worked with projects or drug products similar to yours before, and does it have the integrated global capabilities to provide support for your study? CROs should also be able to supply a list of client references or customer testimonials to demonstrate its ability to accelerate development.
These factors are, of course, coupled with the non-negotiables in a CRO partner: dependability, diversity, a patient-centric approach and collaborative engagement.
A strong CRO partner is prepared to navigate the explosive R&D landscape alongside you.
CROs bring more than just reassurance to drug developers’ clinical programs — they also provide a wealth of expertise, drive time and cost efficiencies, and deliver outstanding project management.
By choosing a CRO that is committed to operational excellence, customers gain more than clinical trial capabilities — they secure a valued partner with a shared commitment to the success of their clinical program.
More than a third of Biotech respondents in our 2023 report, The Pulse: Global R&D Insights in Pharmaceuticals, are feeling the strain of talent shortages . As a result, 41% have increased their reliance on CROs though FSP partnerships.
Access the insights in our full 2023 report.

Top 17 Clinical Research Organizations (CRO) in 2023
In clinical research and treatment development, clinical research organizations (CROs) are frequently a sponsor’s most important partner and ally.
Depending on the nature of the clinical trial, and your existing capabilities as a sponsor to run the trial, the CRO company of your choice will typically be responsible for facilitating most of the micro and macro processes that go into designing and running a successful clinical trial.
When contracting a CRO to help you with your trial, you are transferring over a large portion of responsibility into the hands of your clinical research partner. The CRO of your choice will have the responsibility to control a variety of factors and processes of a clinical trial, and depending on their expertise, team structures, service offerings, internal resources and many other capabilities.
Your ability to find and contract a top CRO company that is the right fit for your unique trial will be a determinant of whether or not you will be able to operate a high-quality clinical trial that meets your expected timelines, budget and delivers a top-notch patient experience.
At ClaraHealth (a patient-centric recruitment acceleration platform) , we have put together an extensive list of the top CRO companies in the US and around the world.
This is not a cro rankings list, but rather a compiled list of some of the top clinical research organizations around the world. We have highlighted their strengths and core service offerings to make it easier for you to find the right fit clinical research partner.
In addition, we’ve put together a list of 9 fundamental questions to ask the prospective clinical research organization , which will help you to save time and ensure a right fit in picking the CRO.
Formerly known as Quintiles and IMS Health, IQVIA is one of the largest CROs in the world, with a large range of service offerings to help advance clinical research.
The company was founded in North Carolina in 1982, and has since grown to over 88,000 employees in more than 100 countries.
Some clinical trial solutions offered by IQVIA include:
- Assistance with protocol design
- Design of phase 1 clinical trials
- Assessment and improvement of phase 2 and 3 clinical trials
- Site identification & selection
- Patient recruitment
- Access to global laboratories via their wholly owned subsidiary Q2 Solutions
Parexel is a global clinical research organization that was founded in 1982, and specializes in conducting clinical studies on behalf of its pharmaceutical partners in order to accelerate and ensure the drug approval process of up-and-coming potential treatments. It currently operates in more than 50 countries, and is run by more than 18,000 employees around the world.
The company has a wide range of service offerings, covering nearly every type of clinical trial service to assist sponsors in running successful clinical studies.
Some clinical trial solutions offered by Parexel include:
- Clinical trial design and development for early phase, phase 2 & 3, and late phase clinical trials
- Clinical data management
- Decentralized clinical trials
- Clinical supply chain management
- Medical writing
- Regulatory affairs consulting
- Pharmacovigilance
3. PRA Health Sciences
PRA Health Sciences is one of the largest contract research organizations in the world. Founded in 1976 under the name “Anti-Inflammatory Drug Study Group”, the company was renamed to PRA in 1982. PRA Health Sciences employees more than 17,000 people, and provides coverage to more than 90 countries.
In 2021, PRA Health Sciences was acquired by the Ireland-headquartered global CRO leader ICON, which is also reviewed in this list.
Some clinical trial solutions offered by PRA Health Sciences include:
- Decentralized Clinical Trials Platform
- Protocol Consultation & Study Design
- Onsite Support services
- Customized Solutions for Biotech (such as asset valuation, regulatory strategy, engagement and support, drug development strategy and funding solutions)
- Clinical Diagnostics
- Site Commercial Solutions
- PRA’s Laboratories for Drug Development
Headquartered in Ireland, ICON was founded in 1990 in Dublin by co-founders John Climax and Ronan Lambre. The company has since grown to be one of the largest CROs in the world. As of September 2020, the company employs more than 15,000 people in 94 locations and across 40 countries.
ICON offers clinical research services which include consulting, clinical development and commercialization across a wide range of therapeutic areas.
In 2021, ICON acquired PRA Health Sciences, which is another CRO and global leader in clinical research services.
Some clinical trial solutions offered by ICON:
- Commercial Positioning
- Early Phase
- Functional Services Provision
- Laboratories
- Language Services
- Medical Imaging
- Real World Intelligence
- Site & Patient Solutions
- COVID-19 Clinical Operations
5. Syneos Health
Formerly known as InVentiv Health Incorporated and INC Research, Syneos Health is a publicly listed and global contract research organization. The company is based in Morrisville, North Carolina, and specializes in assisting companies with late-stage clinical trials. Syneos Health currently employs more than 25,000 people, and has offices across 91 locations.
In early 2018, INC Research was acquired inVentiv Health, and the merged company was named Syneos Health.
Some clinical trial solutions offered by Syneos Health include:
- Decentralized Clinical Trials Solutions
- Bioanalytical Solutions
- Phase II-III/Phase IIIb-IIIV
- Medical Device Diagnostics
- Clinical Data Management
- Clinical Project Management
- Clinical Monitoring
- Drug Safety & Pharmacovigilance
- Site and Patient Access
6. Labcorp Drug Development (Formerly Covance)
Formerly known as Covance and renamed to Labcorp Drug Development in early 2021, this CRO is one of the largest contract research organizations in the world. The company claims to provide the world’s largest central laboratory network, and has been rated as one of the best places to work for LGBTQ+ equality by the Human Rights Campaign organization in 2018 to 2021. Currently, Labcorp employs over 70,000 people and is able to support clinical research efforts in almost 100 countries around the world.
Some clinical trial solutions offered by Labcorp Drug Development include:
- Preclinical Services
- Clinical Trials
- Clinical Trial Laboratory Services
- Post-Marketing Solutions
- Medical Devices
- Data & Technology
Also known as Pharmaceutical Product Development, PPD is a large global contract research organization headquartered in Wilmington, North Carolina. Started as a one-person consulting firm in 1985, PPD has grown to over 27,000 employees worldwide, and provides a wide range of clinical research services to pharmaceutical and biotech companies.
Some clinical trial solutions offered by PPD include:
- Clinical Development
- Early Development
- Peri- and Post-Approval
- PPD Biotech
- PPD Laboratories
- Product Development and Consulting
- Site and Patient Centric Solutions
8. Fisher Clinical Services
Part of Thermo Fisher Scientific, Fisher Clinical Services is a global clinical research organization with headquarters in Center Valley, Philadelphia.
The company has been in the business of clinical supply chain management for over 20 years, and is focused exclusively on working with the packaging and distribution requirements of clinical trials across the globe.
Some clinical trial solutions offered by Fisher Clinical Services include:
- Biologistics Management
- Cell & Gene Therapy
- Clinical Ancillary Management
- Clinical Label Services
- Clinical Trial Packaging & Storage
- Clinical Supply Optimization Services
- Cold Chain Management & Expertise
- Direct-to-Patient
- Distribution & Logistics
- Strategic Comparator Sourcing
- Public Health Research
Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
KCR operates globally, and has offices in North America, Western Europe, Central Europe and Eartern Europe. The company currently employs more than 700 staff.
Some clinical trial solutions offered by KCR include:
- Trial Execution
10. Medpace
Founded in 1992 and based in Cincinnati, Ohio, Medpace is a midsize clinical contract research organization. The company has operations in over 45 countries, and employs over 2,800 people. Medpace provides support services for Phase I-IV clinical trials for pharmaceutical and biotechnology companies, which include central laboratory services and regulatory services.
Some clinical trial solutions offered by Medpace include:
- Biostatistics and Data Sciences
- Clinical Trial Management
- Drug Safety and Pharmacovigilance
- Medical Writing
- Quality Assurance
- Regulatory Affairs
- Risk-Based Monitoring
- Medpace Laboratories
11. Clintec
Now in business for over 22 years, Clintec is a medium-sized global contract research organization for pharmaceutical, biotech and medical device industries, with large expertise in oncology and rare diseases.
The company provides the flexibility and agility of a smaller-sized CRO, while also having a wide global coverage that large CRO companies are known for. Clintec is based in more than 50 countries, and was acquired by the leading global CRO IQVIA in late 2018.
Some clinical trial solutions offered by Clintec include:
- Project Management
- Data Management
- Biostatistics
- Global Feasibilities
- Patient Recruitment & Retention
12. Worldwide Clinical Trials
Bringing over 30 years of experience to the clinical research market, Worldwide Clinical Trials is a leading medium-sized global contract research organization. Founded by physicians with a dedication and commitment to advancing medical research, Worldwide Clinical Trials was the first customer-centric CRO.
Currently the company has coverage in more than 60 countries, and has extensive experience in a wide range of therapeutic areas, including central nervous system, metabolic, cardiovascular, oncology, rare diseases and general medicine.
Some clinical trial solutions offered by Worldwide Clinical Trials include:
- Bioanalytical Lab
- Early Phase Development
- Clinical Phase IIB-II Clinical Trials
- Phase IIIB-IV Clinical Trials
- Trial Management Technologies
Named #1 CRO in the world for operational excellence at the 2021 CRO Leadership Awards, CTI Clinical Trial And Consulting Services is a medium-sized global contract research organization that has been serving pharmaceutical companies since 1999.
Based in Covington, Kentucky, CTI has offices around the world in more than 60 countries, with coverage in North America, Europe, Latin America, Middle-East, Africa, and Asia-Pacific regions.
Some clinical trial solutions offered by CTI include:
- Feasibility
- Regulatory Affairs Study Start-Up
- Medical Monitoring
- Safety & Pharmacovigilance
- Clinical Services
14. Wuxi AppTec
Founded in 2000 as WuXi PharmaTech in the city of Wuxi, China, Wuxi AppTec has grown from a single laboratory into a leading global contract research organization with more than 28,000 employees, including 23,000 scientists and more than 30 research & development and manufacturing sites around the world.
With offices in Asia, U.S, Europe and the Middle East, the company is able to provide coverage to more than 30 countries around the world.
Some clinical trial solutions offered by Wuxi AppTec include:
- Small Molecule Drug R&D and Manufacturing
- Cell Therapy and Gene Therapy
- Drug R&D and Medical Device Testing
- Clinical Services (Phase I-IV)
15. Advanced Clinical
Founded in 1994 and based out of Deerfield, Illinois, Advanced Clinical is a midsize and full-service CRO that helps sponsors with running clinical trials. The company employs more than 700 staff, and offers a wide variety of services across many therapeutic areas. Advanced Clinical has global representation in over 50 countries around the world.
Some clinical trial solutions offered by Advanced Clinical include:
- eTMF & Document Management
- Global Medical Services
- Quality & Validation
16. Pharm-Olam
Pharm-Olam is a leading midsize CRO with global headquarters located in Houston, Texas and its European headquarters in Bracknell, United Kingdom. The company employs more than 800 staff, and has 25 offices around the world, with a global coverage in more than 60 countries.
The company has therapeutic expertise in 5 areas, including Rare & Orphan Disease, Infectious Disease & Vaccine, Oncology-Hematology, Allergy and Autoimmune.
Some clinical trial solutions offered by Pharm-Olam include:
- Study Feasibility
- Site Activation
- Patient Recruitment
- Medical Affairs
- Compliance & Training
- Clinical Monitoring & Operations
17. Clinipace
Founded in 2003 and based out of Morrisville, North Carolina, Clinipace is a global midsize full-service CRO with a focus on solution customization for clinical trials. The company has a large global coverage in more than 50 countries, and has offices in North America, South America, Europe and Asia-Pacific regions.
Clinipace’s therapeutic focus areas include Oncology, Nephrology and Urology, Rare Disease, Gastroenterology and Women’s Health. The company also has complete therapeutic expertise in Infectious Disease & Vaccines, Cardiology, CNS, Immunology, and Respiratory.
Some clinical trial solutions offered by Clinipace include:
- Clinical Analytics
- Clinical Technology and Ecosystem
- Functional Service Partnership (FSP)
- Regulatory & Strategic Product Development
9 Fundamental Questions To Ask A Top CRO Company Before Signing The Contract
1. which services does the cro provide.
CROs offload a lot of operational tasks from trial sponsors, which can touch any component of clinical trial operations. From formulating an overall study strategy and implementing technologies to support the operational processes of the trial, to picking and identifying sites, and supporting patients during the trial, the range of clinical services offered by a CRO tends to be vast and inclusive of all the typical services and support you will require for running a successful clinical trial.
However, not all CROs are the same in their service offerings, or are able to offer the same depth of capability within a seemingly same clinical trial support process. For this reason it is important to understand exactly which kind of clinical services and support you are looking to receive from the prospective CRO when running your clinical trial.
While services such as clinical monitoring and clinical trial management are offered by the majority of CROs, the specific needs of each trial are unique, and for this reason it is important to first identify what will be the unique services your trial requires. Completing this internal analysis first will help you to understand the extent to which a potential CRO partner will be able to provide all of these services.
Some CROs specialize in specific clinical trial functions which the company may label as a “core services”, in which case this is a sign the company will have more expertise, experience, and will be set up in a way to maximize their capabilities in providing support for these services compared to other services that the CRO offers.
For example, a CRO may include patient recruitment as part of its “core services”, which implies that they are highly skilled in and have the necessary infrastructure to design and implement a high-quality patient recruitment strategy.
Clara Health CRO Support Services: At Clara Health our specialty services include technology-augmented digital and patient advocacy recruitment, as well as patient support via our signature patient recruitment platform, which we use to upgrade clinical trials and deliver results sponsors look for in their recruitment and retention campaigns.
At Clara, we work alongside CROs to supplement and support clinical trials with modern and personalized capabilities that CROs do not typically have the bandwidth, corporate structure or infrastructure to support.
If you would like to learn more about exactly how our platform can upgrade your unique trial, feel free to book a Free 30 Minute Consultation Session Here with one of our in-house experts.
2. What Related Experience Does The CRO Have?
It is helpful to ask the prospective CRO company if they have any relevant experience in running clinical trials that would be an asset in designing and running your study. Previous experience in a related therapeutic area or in running a trial with a similar design allows CROs to have a deeper understanding into potential opportunities and challenges, increasing the likelihood of your clinical study being successful.
For example, if a sponsor is planning to run a trial in oncology, for the purpose of site identification and selection it would be valuable to partner with a CRO vendor that has expertise in this area, as they likely already have a good understanding of which sites will lead to optimal results.
However, it is also important to consider all factors when selecting a CRO vendor and not to rely on therapeutic experience as the sole qualifier for whether or not a potential CRO is a fit for your trial. While previous experience is beneficial, some sponsors close themselves off from working with vendors that have not worked in their therapeutic area, which significantly limits options when choosing a CRO partner that is truly a good fit for their clinical study.
This can impact the end result of your clinical study, as sponsors that are not successful in choosing a CRO vendor that is the right overall fit may face difficulties if the needs of their clinical study aren’t being properly met.
Clara Health: We have worked to provide support for clinical trials across a wide range of therapeutic areas and trial designs. Our specialty is filling in the gaps that CROs traditionally did not have to think about, which include digital patient recruitment, patient advocacy recruitment, and technology-augmented patient support.
Additionally, we are constantly building our proprietary data and running tests in a variety of therapeutic areas. These research efforts allow us to have a detailed understanding of the expected level of difficulty when recruiting particular patient populations, as well as allow us to predict with accuracy which segments of the targeted population will be likely to qualify in a particular study.
3. What Are The Communication Workflows & Expectations For Performing And Delivering Contracted Services?
It is important that you clarify what the expectations for communication will be between your prospective CRO vendor and your internal teams, as you will most likely be working with the CRO of your choice for the entire duration of your clinical trial.
There are a vast variety of factors and success determinants for a clinical trial, which are continuously undergoing change as the study unfolds. For this reason, it is recommended that you work with a CRO that is proactive in their communication, so that you are kept up to date with information about important changes as your clinical trial progresses.
A vendor that is proactive rather than reactive in their communication and approach to dealing with arising issues is one of the most important qualities in CRO. Challenging situations will naturally arise, and the promptness with which they are taken care of will significantly impact your clinical trial’s degree of success. Therefore, seeking a vendor that is able to match the standard of communication that you as a sponsor would like to experience throughout the duration of your partnership is one of the most critical steps in determining which CRO is the right fit for your clinical trial.
We’ve included a few additional questions pertaining to the communication structure and reporting expectations that you can ask a prospective CRO vendor to determine the degree of fit in this particular category:
Communication Expectations:
- If we were to move forward with you, which of your team members will be our main point of contact?
- How available will you be outside of the scheduled meetings to address any of our concerns or additional requests?
- What will be the frequency at which update meetings will be conducted, and who will be present at those meetings?
- Which clinical study processes will be reported on, and what will be the workflow for how we will receive this information?
- What will be the cadence at which we will receive progress reports?
- Would we be able to access metrics electronically via an interactive dashboard, or will you send us formal reports?
Clara Health: At Clara Health, we directly interact and actively work with several key stakeholders involved in running a clinical trial, which includes sponsors, CROs, sites, and patients. This unique position allows us to have a centralized perspective which helps us to see all the moving parts of a clinical trial at the same time, which helps to identify issues and relay this vital information and insight back to the sponsor (or other appropriate stakeholders) in the shortest time possible.
The ability to access this perspective allows us to gather the most accurate, complete, and up-to-date information about how the clinical trial is unfolding, and quickly becomes very valuable to sponsors for their clinical trial.
As an example, we may receive feedback from patients about having an unsatisfactory experience with a particular study site. We are able to aggregate and analyze this information, and relay our findings back to the sponsor and the study site to improve the experience for other patients.
4. What Is The CRO’s Client Satisfaction Record?
It is a good practice to request information or metrics from the prospective CRO vendor that can point to the degree of satisfaction of their past clients. Prior to signing the contract, vendors will naturally do their best to uplift their image and future value to you during their sales conversations with you and your team. It can be tricky to get an objective understanding of what the partnership experience will actually entail, especially when there are multiple vendors fighting for your commitment.
We recommend that you ask the prospective vendor to provide success metrics regarding areas of clinical trial operations that are going to be important for your trial.
For example, you may be interested in learning about the vendor’s relationship to finances, in which case it will be useful to ask them about situations in which they went over the planned budget, and investigate into the reasons behind that. Alternatively you may be concerned about potential delays in timelines, in which case it would be helpful to learn about metrics regarding the CRO’s ability to meet timeline expectations.
You may also request to talk to the prospective CRO’s past clients, which will help you to gain insight into what the relationship was like and give you the opportunity to examine if the way in which the particular CRO manages its relationships and performs its services meets the expectations that you would have for your potential relationship and for your clinical trial.
Clara Health: At Clara Health, our relationships with our partners and with our patients are most important to us. In the unique position where we fit in the clinical trial process, we have the opportunity to directly co-create the clinical trial patient experience with a variety of stakeholders, including sponsors, sites, CROs, and patients.
Our company’s values and culture have been directed and developed to be such that the client and patient experience is at the top of priority for all of our internal teams, and we work to provide the best quality of care to all stakeholders.
We have many testimonials from every type of partner we’ve worked with which we can happily share with you.
5. How Do You Adapt When Encountering Challenges With Running A Clinical Trial?
It is inevitable that challenges and unforeseen changes will arise throughout the operational clinical trial process, and for this reason it is important to work with a CRO vendor that can provide you with evidence of their flexibility and ability to adapt to sudden changes.
The ideal CRO partner is one that is highly consultative throughout the entire process, and has an ability and the initiative to deal with challenges at their seed stage, prior to them turning into major obstacles for the success of your trial.
CROs naturally have a large reach, and there are a lot of different clinical trial mechanisms and processes that are under their control. They are able to monitor and respond to what is going on in every key link in the chain of the clinical trial operation.
It is reasonable to expect this level of oversight from a CRO, and additional questions that can help you gain insight into this include:
- What are some examples where the CRO was effective at monitoring the health of clinical trials they’ve helped operate in the past?
- How quickly does the CRO respond to challenges or opportunities for improving the clinical trial experience?
- How well does the CRO gather & process information from study sites, study teams, patients & the sponsor, and what are their typical data analysis workflows?
It is also recommended to speak to the prospective CROs past clients to help you gain insight into how well they respond and adapt to the naturally arising challenges in clinical trials.
Clara Health: While CROs do have a large reach within the clinical trial, no CRO has complete visibility into every clinical process. They are not typically set up to support full visibility, which can manifest as a potential threat to your clinical trial as it unfolds. This is especially true for parts of the clinical trial processes that CROs naturally do not specialize and often subcontract, such as clinical trial recruitment.
At Clara, we are in a unique position in relation to other key partners involved in operating the clinical trial. We are in direct and frequent contact with patients, CROs, study sites, study teams, and the sponsor, and have a very deep understanding of the patient pipeline. This allows us the unique ability to go very deep into specific parts of the recruitment chain and investigate what is working and what is not working.
In addition, Clara functions as a resource for all partners in the clinical trial. For example, we work directly with site teams to ensure that they have access to a 3rd party that they can relay their needs to and receive fast support in case there is anything they require that can improve the patient recruitment process.
6. Which Parts Of Operating The Clinical Trial Will You Be Outsourcing?
Since there are so many processes and mechanisms that go into operating a clinical trial, CROs will always outsource some parts of running and managing the study. While you can expect that the prospective CRO will subcontract some of the work, it is important to find out which exact parts the clinical study will be outsourced.
There are certain basic and key clinical processes (such as site selection) that CROs almost always help with, and if you find that these parts of your trial are going to be subcontracted to another company, it is recommended to find out why the CROs operations are set up this way and how this would impact the service you will receive.
Ultimately what matters to you as a partner and client is that the quality of service and care that you will receive will be up to standard, and meet what was promised and what you are expecting. While this trust is important after you have signed the contract, it is recommended that prior to entering into such a significant commitment that you have evidence and the conviction that the CRO of your choice is truly the right fit and will deliver the quality of service that was being discussed.
Since it is impossible to predict exactly what the quality of this relationship and services performed will actually be like in practice, it is recommended that you understand the details of what will be done for your trial and how. Investigating how the CRO outsources and subcontracts services for a clinical trial will help you to gain necessary insight that you would need to make the correct vendor selection decision.
Clara Health: At Clara, we maximize the effectiveness of the digital component across the entire digital & recruitment spectrum, which is added on top of the existing capabilities of the CROs and other vendors involved in operating your clinical trial. In addition, we offer services that augment the CROs efforts, which has the potential to significantly improve the patient experience, operations flows, recruitment and retention performance, which is so important in ensuring the success of a clinical trial.
For example, if a CRO wants to have a great site relationship, we are able to come in as a third party on behalf of the sponsor and CRO and act as a resource and additional support for sites.
In another example, If a sponsor wants to have great relationships with the patient community, Clara is able to come in on behalf of the sponsor and develop these relationships while being perceived more neutrally by the patient community.
7. Do You Have Experience Running International Trials?
If you are planning on operating an international clinical trial, it is recommended to work with a CRO that has extensive experience in this area. While many CROs will offer near-global coverage, the level of experience with specific geographic locations can significantly vary from one vendor to another.
It is important to work with a CRO that has experience running clinical trials in the specific countries and regions you are planning to conduct your research in. Being compliant with the local rules and regulations for clinical testing is a very complex process that requires existing understanding and familiarity in order to ensure logistical smoothness and to mitigate legal risks. In operating a clinical trial, there are a multitude of clinical services and processes, which can greatly vary across the many regions in which you can conduct clinical testing.
A CRO that is lacking experience in operating international trials or operating in particular regions where you plan on conducting research may not be able to meet your desired quality and agility expectations, and therefore may not be the right fit for your international clinical trial.
Clara Health: In the past, we have provided international patient recruitment and digitally-augmented trial support services for clinical trials in the EU, Canada, UK, Australia and South America.
Clara Health is fully compliant to operate international studies everywhere in the world, with the exception of Russia and China.
8. What Is Your Relationship With Patients?
Patient-centric approach to designing and operating a clinical trial is becoming more and more crucial in the clinical research space. The ability of a sponsor and their CRO partner to understand the needs and characteristics of their target patient community is a significant determinant of whether or not the study will be a success.
A sponsor that has close and authentic relationships with the patient community tends to have a deeper understanding of how to create the best clinical trial experience that will attract patients and keep their interest throughout the clinical trial.
In addition, strong relationships with patients allow sponsors and CROs to forecast recruitment and patient retention pipeline with much higher accuracy. This ability is critical for ensuring the success of the trial and mitigating the risk of low enrollment. After an understanding of the patient population is acquired, sponsors gain the necessary insight to design a clinical trial that is not only favorable to their research results, but is also practical and will result in the enrollment numbers they are looking for.
While many CROs have already recognized the importance of patient-centricity and evolved the ways in which they design and operate clinical trials, other CROs have not yet made such a pivot in their values. It is important to understand the degree of importance the prospective CRO places on creating a favorable patient experience, and what kind of infrastructure the company has to support it.
At Clara, we recommend choosing a CRO partner that is adapting to the patient-centric model which is becoming more and more important for running a successful clinical trial.
Clara Health: Since early stages of our development, we’ve had a dedicated patient advocacy team that has been integral in shaping our company’s vision and operations. We have built our entire platform and recruitment infrastructure around creating the best experience for patients. Our teams, corporate values, service offerings and company infrastructure all work in the service of the patient.
In addition, over the many years of being in business we have heavily invested in building authentic patient community relationships that span across a variety of therapeutic areas. This has given us a unique ability to receive feedback directly from patients that is genuine and authentic around marketing materials, strategy for patient recruitment, and other services that we build for specific trials.
This ability to build partnerships with the patient community in an authentic way gives us a very unique ability to engage with the patient community on behalf of a pharmaceutical company, allowing our sponsor & CRO partners the opportunity to start conversations with patients through our in-house patient advocacy team.
If you would like to learn how Clara can help you to build a strong & authentic relationship with your target patient community, get in touch with us and we’d be happy to share our capabilities and previous results with you as they relate to your current or upcoming clinical trial.
9. How Is The CRO Going To Utilize Patient Input For Developing The Trial?
In the initial stages of clinical trial design, sponsors often determine the ideal patient profiles that would help them to drive the most favorable research outcomes for their study. While it is important for the success of your trial to determine who your ideal patients are, very often these projections do not match up with what is viable in practice.
At Clara, we often encounter study protocols that are not set up realistically for successful recruitment to be possible.
Common mistakes that are made when determining trial eligibility criteria and trial design include:
- Overestimating the interest in the clinical trial from the target patient population
- A lack of patient focus in the trial design
- A lack of convenience for patients in their participation
- Complicated and/or inefficient study experience flows
- Crafting the eligibility criteria around the patient population that is most likely to lead to favorable study outcomes, without conducting sufficient research to more accurately estimate the recruitment and retention difficulty of the group for a particular study
It is natural for there to be a “push & pull” between the research ideal and the real world practicality. It is important to determine the correct balance between these two sides for your trial, as going too far in either direction will decrease the chance of your clinical study’s success.
The nature of the industry as it is right now is such that there is excess research idealization and not enough emphasis on patient centricity. This distorted orientation has resulted in many clinical trials being unsuccessful, negatively impacting sponsors, patients and the entire clinical trials industry.
The ideal CRO partner should help you make sure that your protocol design sets your study up for success. The CRO should be able to help you determine the proper balance between the research ideal and the real world practicality, and back up their findings with sufficient research and patient data that can project your trial being a success.
Clara Health: When formulating a recruitment and retention plan for our clients, we begin with conducting thorough research into the target trial patient population. This allows us to get a clear understanding of which recruitment channels will yield the best results and what kind of marketing materials will resonate with the prospective study participants.
To ensure accuracy and real-world applicability of our research, we consult and collaborate with our internal patient advocacy and patient support teams, as well as with our clients and patients representing the target trial patient profiles. We then tie our findings back with any existing proprietary data that we have in connection with the therapeutic area or the prospective target patient group.
Our unique position within the clinical recruitment chain gives us the presence and deep-rooted access needed to effectively tap into any of the three patient traffic sources: digital recruitment, offline recruitment, or patient advocacy recruitment.
Once a recruitment campaign has gone live, we constantly monitor, analyze and optimize our performance to make sure that the processes we have in place are as efficient as possible and drive the greatest results. In addition, we have the capability to layer in any traditional advertising (such as billboard ads) if requested by the study sponsor.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Ger Med Sci

Language: English | German
Current practice and perspectives in CRO oversight based on a survey performed among members of the German Association of Research-Based Pharmaceutical Companies (vfa)
Aktuelle praxis und perspektiven beim cro oversight – umfrage unter den mitgliedern des verbandes forschender arzneimittelhersteller (vfa), michael hennig.
1 GlaxoSmithKline GmbH & Co. KG, Munich, Germany
Ferdinand Hundt
2 Doc-Dog-Consulting, Berlin, Germany
Susanne Busta
3 Bristol-Myers Squibb, Munich, Germany
Stefan Mikus
4 Pfizer Pharma GmbH, Berlin, Germany
Per-Holger Sanden
5 Merck KGaA, Darmstadt, Germany
Andrea Sörgel
6 MSD Sharp & Dohme GmbH, Munich, Germany
Thorsten Ruppert
7 vfa, Berlin, Germany
Associated Data
In recent years, the number and scope of outsourced activities in the pharmaceutical industry have increased heavily. In addition, also the type of outsourcing has changed significantly in that time.
This raises the question of whether and how sponsors retain the capability to select and to control the contract research organizations (CROs) involved and what expertise still has to be present in the development department as well as other relevant departments to ensure adequate oversight, also in line with the expectations of regulators and health authorities. In order to answer these questions, a survey was conducted among the German vfa member companies. The survey describes the latest developments and experiences in outsourcing by 18 German vfa member companies. It concentrates on measures how to implement Quality Assurance (QA) when performing outsourced clinical studies.
This study shows that the majority of companies apply a full-outsourcing, preferred-provider model of clinical trial services, with the clinical research department playing the major role in this process. A large amount of guiding documents, processes and tools are used to ensure an adequate oversight of the services performed by the CRO(s).
Finally the guiding principles for all oversight processes should be transparent communication, a clearly established expectation for quality, a precise definition of accountability and responsibility while avoiding silo mentality, and a comprehensive documentation of the oversight’s evidence. For globally acting and outsourcing sponsors, oversight processes need to be aligned with regards to local and global perspectives.
This survey shows that the current implementation of oversight processes in the participating companies covers all relevant areas to ensure highest quality and integrity of the data produced by the outsourced clinical trial.
Zusammenfassung
In den letzten Jahren haben sich sowohl die Anzahl als auch der Umfang der ausgelagerten Tätigkeiten in der pharmazeutischen Industrie stark erhöht. Darüber hinaus hat sich auch die Art des Outsourcings in dieser Zeit verändert.
Dies wirft die Frage auf, ob und wie die Sponsoren die Fähigkeit, geeignete CROs auszuwählen und zu überprüfen, beibehalten und welches Know-how noch in den Forschungs-, Entwicklungs- und anderen Abteilungen vorhanden sein muss, um eine angemessene Aufsicht zu gewährleisten, wie sie auch vom Gesetzgeber und den Überwachungsbehörden erwartet wird. Um diese Fragen zu beantworten, wurde eine Umfrage unter den deutschen vfa-Mitgliedsunternehmen durchgeführt. Die Studie beschreibt die neuesten Entwicklungen und Erfahrungen im Outsourcing von 18 deutschen Vfa-Mitgliedsunternehmen. Es werden Maßnahmen zur Umsetzung von Quality Assurance (QA) beschrieben, die bei der Auslagerung von klinischen Studien angewendet werden sollten.
Diese Studie zeigt, dass die Mehrheit der Unternehmen ein Full-Outsourcing-Modell mit bevorzugten Anbietern („Preferred Provider“) im Bereich der klinischen Studien anwendet; dabei spielt die Abteilung „Klinische Forschung“ die Hauptrolle in diesem Prozess. Eine große Menge von Guidelines, Prozessen und Werkzeugen werden verwendet, um eine angemessene Aufsicht über die Leistungen von den CROs zu gewährleisten.
Schließlich sollten die Leitprinzipien für alle Oversight-Prozesse folgende Punkte umfassen: transparente Kommunikation, klar festgelegte Erwartung hinsichtlich der Qualität, genaue Definition der Verantwortlichkeiten ohne Silodenken, sowie eine umfassende Dokumentation der Oversight-Tätigkeiten. Für global agierende Sponsoren sollten die Oversight-Prozesse in Bezug auf lokale und globale Perspektiven abgestimmt sein.
Diese Studie zeigt, dass die aktuelle Implementierung der Oversight-Prozesse in den beteiligten Unternehmen alle relevanten Bereiche abdeckt, um höchste Qualität und Integrität der durch die ausgelagerten klinischen Studien generierten Daten zu gewährleisten.
1 Introduction
In the past the majority of clinical study activities were performed largely in-house. Most activities, especially regarding Quality Management (QMS – Quality Management System and CAPA – Corrective Actions/Preventive Actions) were done by the sponsor itself, and only individual activities were awarded to specialized contract research organizations (CROs). Today the trend is increasingly towards completely outsourced studies with a full-service provider and a so-called strategic partnership between a sponsor and its main CRO (preferred provider). Major areas of previous sponsor tasks are assumed by CROs, including Quality Management – however, according to ICH E6, “ the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor ” [ 1 ] and in the recent update (R2) of the guideline the amended introduction describes the objective " to encourage implementation of improved and more efficient approaches to [...] oversight [...]" [ 2 ]. Already before, the health authorities made clear in their last year's inspections that it is the sponsor’s responsibility to actively ensure by oversight that trial conduct follows Good Clinical Practice (GCP). Consequently e.g. the FDA (U.S. Food and Drug Administration) issued more and more 483s (list of inspectional observations) and warning letters directly to the sponsor that in the past were mainly issued to investigators and CROs.
This trend towards outsourcing is illustrated by 375 industry professionals who responded to Contract Pharma’s Eleventh Annual Outsourcing Survey 2015 [ 3 ]. Forty-five percent of respondents were from pharmaceutical sponsor companies, and the remaining 55% represented service providers. When asked if there is an increasing demand for outsourcing, 80% of respondents answered yes. The number one reason for this, according to 41% of respondents, is to focus on core competencies. Pharmaceutical company sponsors say they are also outsourcing more because they are virtual (30%), while 14% say they lack the capabilities in-house. Companies were focusing their outsourcing efforts in 2015 on the following fields: analytical and testing services (37%); clinical trials, phases I-IV (34%); API (active pharmaceutical ingredient) manufacturing (31%); solid dosage manufacturing (28%); formulation development (20%); clinical trials materials (15%).
These figures demonstrate the high relevance of outsourcing in clinical trials run by the pharmaceutical industry.
As a result, the competent authorities like EMA (European Medicine Agency) and FDA increased their expectations of oversight of service providers by the sponsor and focus on this aspect during inspections. Important to note is that competent authorities do not limit the need for quality management to specific activities like monitoring but e.g. “ FDA considers monitoring to be just one component of a multi-factor approach to ensuring the quality of clinical investigations ” [ 4 ]. The EMA as well proposes a risk-based approach to quality management including oversight in their reflection paper [ 5 ]. In consequence, proof of a broader scope of oversight is demanded in inspections.
Furthermore, in the recent Addendum of the ICH-GCP a new sentence has been added in 5.2. regarding the involvement of a CRO stating that „ the sponsor should ensure oversight of any trial-related duties and functions carried out on its behalf. ” [ 2 ]. All this raises the question of whether and how sponsors retain the capability to select and to control the CRO(s) involved and what expertise still has to be present in the development department as well as other relevant departments to ensure adequate oversight. In order to answer these questions, a survey was conducted among the German Association of Research-Based Pharmaceutical Companies (vfa; Verband forschender Arzneimittelhersteller) member companies. The survey results first describe the latest developments and experiences in outsourcing by the German vfa member companies and second concentrates on measures how to implement Quality Assurance (QA) when performing outsourced clinical studies.
The vfa, the Association of Research-Based Pharmaceutical Companies, is the trade organization of research-based pharmaceutical companies in Germany which represents 2/3 of the pharmaceutical market in Germany. 44 leading research-based pharmaceutical companies are currently organized in the vfa. In a joint project of the sub-committee on clinical trials and quality assurance (UA KliFo/QS) and the working group Biostatistics within the vfa, a questionnaire (see Attachment 1 ) covering the major aspects on the current practice of CRO selection and oversight was developed – in these committees 25 vfa member companies are involved. This survey was based on a first version of a questionnaire covering mainly biostatistics and data management aspects of CRO oversight, developed in 2014 by the working group Biostatistics of the vfa.
The questionnaire referred to interventional clinical studies of phases II–IV, as studies of these phases are similar with regards to the outsourced services. It started with a section, in which the key elements were defined – to ensure a common understanding and interpretation of these elements, as shown below:
- The term “CRO oversight” is used for any measure to control the performance, the deliverables and the efficiency of contract research organizations (CROs) performing outsourced tasks on behalf of the pharmaceutical company or acting as the sponsor of a clinical study – not covered in this questionnaire: insourcing/temporary employment. Other terms typically used in this context include “CRO management”, “CRO supervision”.
- The term “preferred provider” is used for any outsourcing model, in which one or several CROs are selected as primary supplier by a pharmaceutical company in order to perform defined tasks for a series of clinical studies. Other terms typically used in this context include “strategic (alliance) partner/vendor/CRO”.
- The terms “local” and “global” refer to international companies with local subsidiaries in various countries. Here “global” refers to the CRO outsourcing on the international level within a company, whereas “local” refers to the German subsidiary and studies on the local German level – if applicable.
The questionnaire (see Attachment 1 ) consisted of three sections:
- In a general part questions about outsourcing models, the outsourced services, the selection and decision-making were asked. Here it was e.g. assessed whether the outsourcing is organized locally or globally as well as the reasons for outsourcing. The global and local perspectives were addressed separately as the vfa member companies are acting with a global and local focus.
- The second section dealt with the procedures ensuring CRO oversight and covered issues like CRO qualification, audits, SOPs, other oversight tools and escalation processes.
- The third part covered specific oversight topics for the outsourcing of data management and biostatistics services , e. g. requirements for data quality or coding. The results of this part will be published separately.
Finally the complete questionnaire covered 52 items. The survey was conducted from August till October 2015 and captured the companies’ outsourcing status quo applicable at this point in time. English language was selected for this questionnaire in order to ease the use within the companies. The questionnaire was sent out electronically by the vfa. The completed questionnaire was returned to the vfa and blinded afterwards by the vfa, ensuring that no identification of the companies was possible for the analysis team, lead by one of the authors (MH). Before analyzing the questionnaire descriptively, several quality control measures were performed in order to clean any data deficiencies and inconsistencies. In case of obvious data errors (e.g. an initial question was not answered, but the follow-up question was answered) the corresponding missing data was substituted. Some free text answers were clustered post-hoc by one of the authors (AS) to allow for a descriptive analysis of relevant categories.
In addition, relevant articles were identified in a systematic literature search in Embase, Medline, and other internet sources, resulting in a total of 257 publications of potential relevance. After screening of the abstracts and full-texts finally a total of 10 relevant articles were selected [ 6 ], [ 7 ], [ 8 ], [ 9 ], [ 10 ], [ 11 ], [ 12 ], [ 13 ], [ 14 ], [ 15 ].
There are, according to our research, only a few articles concentrating on the quality aspect of CRO oversight [ 14 ], [ 16 ] in the field of clinical trials, most papers – not from peer reviewed journals – concentrated on operational aspects [ 7 ], [ 10 ], [ 11 ], [ 17 ], [ 18 ], [ 19 ] or extrapolated experiences with contract manufacturing organisations (CMOs) [ 6 ].
Twenty-five companies within the vfa were contacted, from which 18 companies participated (72%). Three companies provided multiple feedbacks: One company provided two questionnaires – one covering the local (German) outsourcing perspective and one covering the global outsourcing perspective. One company provided three questionnaires: one covering the local perspective, one for the global perspective and one additional questionnaire covering the outsourcing of monitoring activities only. Finally one company divided their answers on two questionnaires: one for partly outsourcing activities, the other for full outsourcing activities.
In total the survey is based on 22 questionnaires from 18 different companies.
3.1 General questions
The first block of questions dealt with the general perspective of the outsourcing model.
Outsourcing models
All companies performed outsourcing of services to CROs. The majority of questionnaires (55%) referred to CRO outsourcing on an international/global level and to a full outsourcing model, in which all or the vast majority of services are outsourced (Table 1 (Tab. 1) ).

Full outsourcing
For those companies applying a full outsourcing model the vast majority (93%) used a preferred provider model. Within these models in 64% there was cooperation with more than one CRO acting as preferred provider, with an average of about 3 CROs per sponsor (Table 2 (Tab. 2) ).

Partly outsourcing
Companies applying a partly outsourcing model used CROs (90%) and freelancers (70%) as partners. For those companies performing a partly outsourcing model to CROs the selected CRO(s) typically acted as preferred provider (89%). In this model the majority of companies (63%) cooperated with one CRO as preferred provider (Table 3 (Tab. 3) ).

Monitoring services were outsourced by all companies performing a partly outsourcing model, followed by data management (60%) and medical writing services (50%) (Figure 1 (Fig. 1) ).

Decision for outsourcing
A large number of sponsor departments were involved in the decision on which outsourcing model to apply for a specific study: the clinical research department is involved in 62%, followed by R&D business operations (38%), medical management (33%) and biostatistics (19%). The three main criteria for outsourcing services were “Decision by global” (73%), “Costs” (67%) and “Availability of internal resources/Flexibility in headcount planning” (60%). The main criterion for selecting specific services for outsourcing was “Decision by global/Strategic decision” (84%) (Table 4 (Tab. 4) ).

Selection of preferred providers
When selecting a preferred provider the three key sponsor departments involved are: 1. Procurement, 2. Quality Management, and 3. Clinical Research Department (Table 5 (Tab. 5) ).

Sponsor department involvement: Comparison of preferred provider/non-preferred provider model
The involvement of sponsor departments into the process of outsourcing of services to a CRO was investigated for the two outsourcing models: a) preferred provider model and b) non-preferred provider model. In both models the clinical research department is the key department. The involvement of other departments like “Dedicated Outsourcing Unit”, “Study Team”, “Biostatistics”, “R&D Business Operations”, “Data Management”, “Medical Management” and “Monitoring Organization” was also similar for the two models. In the non-preferred provider model there was a more prominent involvement of the departments “Procurement”, “Quality Management”, “Legal Department” and “Pharmacovigilance” – compared to the preferred provider model (Figure 2 (Fig. 2) ).

3.2 CRO oversight
Guiding documents.
There is a SOP or any other quality/guiding document available in 81% of the responders. The following guiding documents are used: “Oversight plan”, “Partnership trainings“, „Guidance document on distribution of tasks”, “Job aides”. The vast majority (94%) of SOPs resp. guidance documents were declared as global documents (Table 6 (Tab. 6) ).

Check of the CRO’s qualification before procurement
The top-5-criteria checked during the CRO selection phase were: former experience with this CRO, costs, qualification of staff, and experience in indication, financial stability. These criteria were typically assessed by standardized documents like bid grids/templates (81%) or questionnaires (69%). A qualification audit prior to start of the study is performed in 69% of the responders (Table 7 (Tab. 7) ).

Extent and effort of CRO oversight during the study
A vendor audit is performed at least sometimes during the study in all responders; in 21% a vendor audit is a mandatory oversight tool. Those 79% of responders performing a vendor audit occasionally triggered an audit mainly by quality issues. The study duration was another trigger factor for an audit (39%).
Most of the responders (84%) used standardized tools for performing CRO oversight, like standardized metrics, meetings, oversight plans, action logs, monitoring visit cycle time reports, and regular CRO assessments.
A training program on how to perform CRO oversight is available in 76% of the responders. Also a risk-based CRO oversight based on the basis of previous experiences is conducted by 75%.
CRO oversight is typically conducted by the clinical research department (70%), quality management (65%) and the study team (60%).
A CRO oversight per CRO – across studies – (in the sense of an overall assessment) is performed by 85%.
A lessons learned process is implemented as mandatory requirement in 75% of the responders.
The documentation of CRO oversight measures is mostly done via meeting minutes (89%) and standardized documents (74%), like standardized oversight/surveillance plans, performance reports and metrics, action item logs and metrics/KPIs (key performance indicators).
For implementation and support of the CRO oversights the following main instruments were used: RACI matrix (RACI: R esponsible, A ccountable, C onsulted and I nformed – a responsibility assignment matrix) of responsibilities (95%), matrix of valid SOPs (sponsor’s/CRO’s/both – as tick box) (86%), communication plan (86%) (Table 8 (Tab. 8) ).

Size of CRO
There was experience with large CROs in 18 responders (82%), compared to less experience with small CROs (10 responders, corresponding to 45%). The sequence of the main criteria for a partnership with a large CRO (ranked by importance) was: 1. Quality, 2. Delivery in time, 3. Communication, 4. Costs – compared to the following sequence for small CROs: 1. Delivery in time, 2. Quality, 3. Costs, 4. Communication (Figure 3 (Fig. 3) ).

The majority of responders (61%) have specific outsourcing areas dependent on the size of the CRO. Large CROs are typically selected for services like monitoring (67%), study management (56%) and data management (44%); whereas small CROs are typically selected for “other services” (60%) (Table 9 (Tab. 9) ).

Multiple CROs
A minority of responders cooperate with several CROs for one study (29%). For them the main reason is the functional service provider strategy, and they use mainly portals and sharepoints for their exchange with the CROs (Table 10 (Tab. 10) ).

An established escalation plan is available in 90% of the responders. In case of QA findings all responders take action, with a timeframe depending on the issue (Table 11 (Tab. 11) ).

4 Discussion
This study describes the implementation of CRO oversight measures in 18 Germany based pharmaceutical companies organized within the vfa. It shows that the majority of companies apply a full outsourcing, preferred provider model of clinical trial services, with the clinical research department playing the major role in this process. A large amount of guiding documents, processes and tools are used to ensure an adequate oversight of the services performed by the CRO(s).
This survey represents a large proportion of the current practice in pharmaceutical companies located in Germany, but it has to be taken into account that the representativeness is limited by three factors: the selection process of this survey, in which only 25 pharmaceutical companies represented in the vfa were considered, the return rate (of 72%) and by the fact that 3 companies submitted multiple questionnaires.
Another limitation of this study is some degree of inconsistency in some answers, i.e. not all relevant questions were answered by all parties, leading to some missing values. This limitation however, is mainly caused by the diversity of the oversight process and also the oversight language within the participating companies.
The survey showed that the main criteria for selecting outsourcing services are “decisions by global” resp. “strategic decisions”, followed by “lack of internal resources”. This finding may be interpreted in the sense that the specific strategic reasons, typically considered on a global company level, are not always fully transparent to the company’s local affiliate executing the outsourcing measures. It seems that local experience and expertise with local CROs is only considered partly when deciding for an outsourcing strategy. Compared to a global perspective local affiliates may have more insight in CRO performance as they are actively overseeing the quality of CRO performance. It can be assumed that the remaining know-how within the sponsor is a critical issue for most of the companies, especially for small companies.
With regards to the size of a CRO, it turned out that small CROs are mostly preferred for services other than the “typical” services, as monitoring, study management, data management, etc. Although no further information on the kind of “other services” was collected, this may be interpreted as a niche for small CROs specialized on specific services like quality control visits, administrative services, recruitment services and laboratory services. It also seems that small CROs are preferred for services, where delivery in time is essential; whereas large CROs are preferred mainly because of the better communication.
Although the large majority of participating companies report the existence of a SOP or any other guiding document, there remain five feedbacks from companies without these measures. This feedback may come from companies with only limited outsourcing-activities or from small companies, which have just started to set up a corresponding SOP-system.
The word “oversight” can be interpreted twofold: in the sense of supervision but also in the sense of error. Throughout this manuscript CRO oversight is used in the context of supervision, control, and project progress and quality.
The term “oversight” is also strongly related to the term “risk-based quality management”, topic of publications from authorities like the FDA (US) and the EMA (EU) but also from organizations like the ICH – International Council of Harmonisation [ 4 ], [ 5 ], [ 20 ], [ 21 ]. The FDA states in her procedural paper in 2013: “ Although sponsors can transfer responsibilities for monitoring to a CRO(s), they retain responsibility for oversight of the work completed by the CRO(s) that assume this responsibility. Sponsors should evaluate CRO compliance with regulatory requirements and contractual obligations in an ongoing manner. For example, sponsor oversight of monitoring performed by a CRO may include the sponsor’s periodic review of monitoring reports and vendor performance or quality metrics and documented communication between the sponsor and CRO regarding monitoring progress and findings. ” [ 4 ]
This is in line with ICH E6, stating that “the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor ” [ 1 ].
Looking into the EU, the “Reflection paper on risk based quality management in clinical trials” [ 5 ] concentrates on the obligations of sponsors and/or CRO or vendors to whom the sponsor has delegated trial related duties, risk assessment, risk control (risk mitigation/risk acceptance), quality tolerance limits concerning trial data, trial protocol procedures and trial management and risk review and reporting quality. Checking further the recommendations for risk-based quality management like ICH Q9, unfortunately nothing is mentioned concerning the sponsor-CRO relation [ 20 ].
There are some publications concerning quality management while outsourcing clinical trials [ 14 ], [ 16 ] focusing on “precontract audits” and on a four-phase program (credit history, qualification audit, audit during the conduct of the study, evaluation during and after by the department concerned).
In consequence various SOPs have been implemented by sponsors outsourcing clinical trials partly or completely. Internationally acting pharmaceutical companies organized in the vfa accomplished this with global guiding documents on e.g. vendor identification, vendor management and vendor qualification. Other guiding documents describe the RACI system (RACI: R=responsible, A=accountable, C=consulted, I=informed) defining the collaboration between sponsor and vendor in detail: i.e. start up, protocol development, site selection, monitoring, safety, and also project oversight/management). In some companies the entire process of vendor engagement and outsourcing activities have been centralized by creating a single platform/outsourcing department coordinating all related outsourcing tasks. In this model a standardized oversight process covering the vendor selection, the execution of oversight measures and the close-out activities has been established.
With regards to the commonly used preferred provider model, some companies follow the approach to govern all interfaces between sponsor and preferred provider in one document, allowing both parties to act according to their SOPs. Other sponsors have established regular partnership meetings with the preferred CRO(s), in which a standardized regional review of all outsourced studies is performed. However, it seems that the oversight with non-preferred providers is less regulated by most sponsors, leaving more room for interpretation, which may result in lower quality.
There is definitely a very large number of tools available for performing and measuring the oversight – for all relevant levels, like: investigator level, study level, asset level, process level, enterprise level and relationship level. These tools cover aspects like on-site oversight visits, investigator site audits, study level performance metrics, study team meetings, quality standards, training events, balanced scorecards, etc. A single tool may be highly effective in one trial but only of limited value in another trial – this may also explain why none of the tools addressed in this survey reached a 100% consent. In order to establish an efficient CRO oversight it is rather essential to combine the relevant tools into a bundle. Here the key task for the sponsor is to identify and implement the adequate bundle of tools into an oversight plan for a specific study.
In addition there are typically further local guiding documents like manuals and/or SOPs, regulating the local specifics within a country. The impact of all guiding documents should be checked on a regular basis e.g. by implementation of a lessons learned process as a mandatory requirement. This important element is established in the vast majority of companies participating in this survey.
As a consequence this leads to a large amount of SOPs, guidance documents, forms, templates – a total number of more than 20 relevant documents can be available. In this context it is of importance to highlight the relevant publication by Schmidt et al. to avoid overregulation, creating too much – unnecessary – interfaces, just endangering the original aim: quality [ 22 ]. As almost all registration studies are multinational, SOPs need to be globally usable. Therefore, global SOPs should describe all globally defined processes to ensure harmonization and efficiency across the whole organization. However, regional or local amendments to global SOPs need to be possible but should only be introduced if required by regional/local law/regulations or organizational structures of affiliates. A SOP should describe the standard situation of a process. Therefore, special rules and exceptions should be avoided. SOPs do not need to consider all imaginable situations. SOPs and associated workflows should be kept as simple as possible [ 22 ]. However, in relation to the guidance documents by EU and US authorities containing information on CRO oversight it seems required for sponsors, who outsource parts of or complete clinical trials, to implement quality management plans. These plans should contain information on how continuous CRO oversight on a global and local level is organized, including risk assessment, risk control and risk review (cycle) [ 5 ], including defined escalation processes. In this context involved functions should not treat their part of a study as an isolated piece of work because an integrated cross departmental and risk-based sponsor oversight approach can help to further increase the quality [ 23 ].
Finally the guiding principles for all oversight processes should be transparent communication, a clearly established expectation for quality, a precise definition of accountability and responsibility and a comprehensive documentation of the oversight’s evidence. For globally acting and outsourcing sponsors oversight processes need to be aligned with regards to local and global perspectives. This survey shows that the current implementation of oversight processes in the participating companies covers all relevant areas to ensure highest quality and integrity of the data produced by the outsourced clinical trial.
5 Conclusion
This survey shows that the current implementation of oversight processes in the participating companies covers all relevant areas to ensure highest quality and integrity of the data produced by the outsourced clinical trial. It remains the ultimate responsibility of any sponsor to apply the implemented measures adequately.
Competing interests
The authors declare that they have no competing interests.
Supplementary Material

IMAGES
VIDEO
COMMENTS
Definition, regulatory aspects. The International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, a 2015 Swiss NGO of pharmaceutical companies and others, defined a contract research organization (CRO), specifically pertaining to clinical trials services as:: 10 "A person or an organization (commercial, academic, or other) contracted by the ...
Clinical or Contract Research Organization or CRO is an organization that offers clinical trial and related services for pharmaceutical drug development. ... According to the definition of the International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH), ...
The Essence of a CRO: Clinical Research Organizations, abbreviated as CROs, epitomize entities entrusted with the management of clinical trials on behalf of pharmaceutical and biotechnological giants. Their mandates encompass the selection of patients, procurement of informed consent, aggregation of data, and the persistent oversight of safety ...
A Contract Research Organization (CRO) is a company that provides clinical trial services for the pharmaceutical, biotechnology, and medical device industries. There are different types of CROs, but typical CRO services in the medical device industry include regulatory affairs, clinical trial planning, site selection and initiation, recruitment support, clinical monitoring, data management ...
A Contract Research Organization (CRO) is a specialized service provider that offers end-to-end support for clinical trials, ranging from early development to post-approval studies. They leverage their expertise, resources, and global reach to help sponsors navigate through the complex processes of drug and device development.
A clinical research organization (CRO) is often called a contract research organization (CRO). CRO is a service organization that provides support to pharmaceutical and biotechnology industries in the form of outsourced clinical research courses and services for both medical devices and drugs. The main functions required to conduct clinical ...
A contract research organization (CRO) is a specialized clinical research provider engaged on a contractual basis to manage various aspects of clinical trials. CROs can be traced back to the 1930s. They have evolved to be of various sizes, have different areas of expertise and geographical focus, and provide a range of services matching the ...
A CRO, or Clinical Research Organization, is an outsourced company that specializes in managing and overseeing clinical trials on behalf of sponsors. They act as a bridge between the sponsor and other stakeholders involved in the trial, ensuring its proper development and adherence to regulatory standards.
Discover the vital role of Contract Research Organizations (CROs) in the field of clinical research. This comprehensive guide explores their functions, services, and global impact. Learn how CROs accelerate drug development, ensure quality and compliance, and contribute to the advancement of patient care. Contract Research Organizations (CROs) are pivotal players in the field of clinical ...
A CRO is an organization contracted by another company to manage its clinical trials and complex medical testing responsibilities. CROs offer a range of services, such as project management, data management, biostatistics, medical writing, and regulatory compliance, to reduce the cost and time of research and development.
CRO, or a clinical research organization, is a company that assist drug manufacturers and pharmaceutical companies by outsourcing the necessary stages and clinical trial processes. CROs streamline clinical trial processes and help in research and development (R&D). To better understand the roles within a CRO, consider exploring the Clinical ...
A contract research organization, or CRO for short, is a specialized provider that is outsourced on a contractual basis by pharmaceutical, biotechnology, and medical device companies (sponsor) in the clinical research industry to manage various aspects of clinical trials.
A CRO is an organization that undertakes short-term contracts in research and development across the life sciences industries. CROs are especially valuable in medical sectors, including pharmaceutical companies, biotechnology, and medical technology. They support efficiency by staying up-to-date in all aspects of R&D.
A Clinical Research Organization (CRO) is contracted by a pharma, biotech or related entity to manage and lead their clinical trials. Organizations contract with CRO Companies so they can acquire specific expertise without having to invest in their own staff. Reducing the time it takes to conduct a trial compared to conducting the trial in ...
Clinical Research Organizations (CROs) play a crucial role in the pharmaceutical, biotechnology, and medical device industries. They provide support to companies in the form of research services outsourced on a contract basis. In this comprehensive guide, we will explore what CROs are, who their clients are, the stages of the research process ...
Learn how a CRO (contract research organization) supports clinical trials, from planning to execution, and the benefits of outsourcing research to a CRO. Vial is a leading CRO platform.
A CRO (Contract Research Organization) is a company that provides clinical trial management services for the pharmaceutical, biotech, and medical device industries. Although there are different types of CROs and diverse levels of specialization (distinct therapeutic areas for instance), typical CRO services include regulatory affairs, site ...
We've curated a list of the top 5 contract research organizations (CROs) in 2022: 1) IQVIA. IQVIA is a leading healthcare technology company providing integrated services spanning the entire healthcare and clinical development spectrum. IQVIA offers solutions for clinical trials and research/development, real-world evidence studies, safety ...
A CRO is a contract research organization that provides research services to the biotech, pharma, and medical device industries. Learn how CROs can help you accelerate your clinical trials and reduce your costs with Vial, a trusted partner in ophthalmology research.
CROs bring more than just reassurance to drug developers' clinical programs — they also provide a wealth of expertise, drive time and cost efficiencies, and deliver outstanding project management. By choosing a CRO that is committed to operational excellence, customers gain more than clinical trial capabilities — they secure a valued ...
9. KCR. Established in 1997 under the name Kiecana Clinical Research, KCR is a full-service contract research organization that provides a variety of services for clinical monitoring, safety & pharmacovigilance, clinical project management, quality assurance and regulatory affairs.
CROs play a major role in ensuring clinical trials (CTs) are executed in line with ethical and regulatory requirements, ultimately to support the development of new drugs that benefit patients worldwide. To meet the evolving needs of pharmaceutical and biotech companies, CROs have expanded their services they provide.
The term "CRO oversight" is used for any measure to control the performance, the deliverables and the efficiency of contract research organizations (CROs) performing outsourced tasks on behalf of the pharmaceutical company or acting as the sponsor of a clinical study - not covered in this questionnaire: insourcing/temporary employment.