An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Int J Health Sci (Qassim)
- v.6(1); 2012 Jan

Surgical site infections: A one year prospective study in a tertiary care center
Dr. varsha shahane.
* Dept. of Microbiology, Padmashree Dr DY Patil Medical College, Hospital and Research Center, Near Sant Tukaram nagar, Pimpri, Pune. Cell no- 0567076587. moc.liamg@9ahsravelel
Dr. Saikat Bhawal
** Dept. of Microbiology, Padmashree Dr DY Patil Medical College, Hospital and Research Center, Near Sant Tukaram nagar, Pimpri, Pune. [email protected]
Mr. Upendra Lele
** Dept. of Statistics, International School of Business and Media, Nande, Pune. moc.liamtoh@elelu
Surgical Site Infections (SSI) is the third most commonly reported nosocomial infection which has an adverse impact on the hospital as well as on the patient. A continuous surveillance is called for, to keep a check on the occurrence of SSI.
- to study the incidence of SSI in our hospital.
- to study the risk factors of SSI in our hospital.
- to identify the areas in our hospital, which need to be strengthened and dealt with proactive measures to curb the incidence of SSI, which is an indicator of health care system in a given set up.
Methodology
We conducted a one year study of SSI in our hospital. 300 cases of Surgery and Obstetrics and Gynaecology were included in the study. A few host factors, wound factors and surgery related factors that cause SSI were studied. Swabs were collected from the infected surgical wounds and processed by the conventional microbiological methods. Antimicrobial susceptibility was done by Kirby-Bauer disc diffusion method.
Prolonged surgery (>2hours) and insertion of drain were found to be significantly associated with occurrence of SSI and the clean surgeries showed minimum risk of infection. Escherichia coli (31.25%) was the commonest pathogen, followed by Pseudomonas aeruginosa (25 %) and Staphylococcus aureus 22%. The incidence of SSI in our set up is 6%.
Conclusions
The outcome of the SSI surveillance in our hospital revealed that in order to decrease the incidence of SSI we would have to: a) decrease the duration of the surgeries performed b) focus on regular and intensive drain care c) identify poor risk patients and ensure their proper management d) conduct periodic surveillance to keep a check on SSI.
Introduction
Surgical site infections (SSI) are the third most commonly reported nosocomial infection and they account for approximately a quarter of all nosocomial infections. It has an adverse impact on the hospital as well as on the patient. It is responsible for increasing length of stay of patient which results in social and economic loss to the patients and family. Host factors, wound factors and surgery related factors are implicated in the causation of SSI. ( 1 )
The present study was aimed at obtaining the incidence of SSI in our set up and to evaluate the risk factors as well as to formulate an antibiotic policy for patients posted for any surgery in our hospital.
SSI is the index of the health care system of any hospital. With the increase in incidence of nosocomial infections and multi drug resistance, a meticulous and periodic surveillance of various hospital acquired infections is called for.
With an active Infection Control team operating in the hospital, SSI is naturally one of the topmost priorities on the agenda. Hence the following study was undertaken.
Materials and Methods
The present study was conducted in the Dept. of Microbiology, Padm. Dr D Y Patil Medical College, Pune. 300 operated cases in Surgery and Obstetrics and Gynaecology were included in this study. Certain risk factors like – type of surgical wound, elective or emergency surgery, antibiotic prophylaxis, duration of surgery, presence or absence of drain and any underlying or predisposing conditions were noted. Swabs were obtained from the post operative infected wounds and processed by the conventional microbiological methods. ( 2 ) Antimicrobial susceptibility testing was done by Kirby-Bauer disc diffusion method ( 3 ) and interpretation was done according to CLSI guidelines. ( 4 )
CDC criteria were used to define the type of surgical wound i.e. Class I- Clean, Class II- Clean contaminated, Class III- Contaminated, Class IV- Dirty. ( 5 ) The statistical significance of the relative importance of various parameters affecting SSI has been tested using ‘p’ test at 95% confidence level (p< 0.05).
300 surgical wounds (from General Surgery wards and Obstetrics and Gynaecology wards) were studied over a period of one year.
The overall infection rate was 6%.
The incidence of SSI in our set up was 6%. ( Fig 1 ) This is in agreement with the SSI incidences in other studies. ( 6 , 7 ) However, infection rates varying from 20% to as high as 76.9% have also been reported. ( 8 – 12 )

Incidence of SSI at DYPMC and Hospital
Prolonged duration of operation results in increased exposure of operation site to air, prolonged trauma, stress of prolonged anaesthesia and sometimes blood loss. ( 13 ) Our study reveals a clear cut increased number of SSI cases i.e.13.1% cases, where surgery has been prolonged ≥ 2 hours. ( Fig 2 ). Studies conducted on SSI in Aurangabad, ( 6 ) Mumbai, ( 7 ) Hyderabad, ( 9 ) and Orissa ( 10 ) have reported a similar observation. In fact, this correlation has been established since 1964 by the Public health laboratory services (PHLS) in England and Wales. ( 13 )

Duration of Surgery of SSI cases
The use of drains has contributed significantly as a risk factor in causing SSI in this study i.e. 13.6% ( Fig 3 ). This could be due to the fact that they are more likely to be used in contaminated or dirty wounds and in emergency and prolonged operations which increases the probability of the wound getting infected. ( 13 ) Mumbai ( 7 ) has also observed 22.4% cases of drained and 3% undrained wounds getting infected while Aligarh ( 8 ) reported 30% and 11.6% respectively.

Insertion of Drain in SSI cases
The rate of infection was highest in contaminated type of wounds-12.3%, followed by clean contaminated wounds-8.0% and least in clean wounds-4.6% ( Fig 4 ). This is an expected observation. In Aurangabad, ( 6 ) similar rates were noted i.e. percentage of infection rate was 10.6 % and 4% in clean contaminated and clean cases respectively, in Mumbai ( 7 ) 22.4% and 3.0% respectively and in England ( 13 ) 10.8% and 3.0% respectively. However, in Orissa, ( 10 ) the difference between the rate of infection in clean contaminated (25%) and in clean surgeries (30%) was not statistically significant.

Type of Surgery in SSI cases
Certain underlying conditions like anaemia, diabetes, and smoking may alter or decrease the immune status thus significantly increasing the risk of SSI. They also are an important cause of increasing the pre operative stay of the patient which steeply increases the risk of SSI in such patients. ( 13 ) In our study, 7.6% of patients with SSI had some underlying conditions, anaemia and diabetes mellitus being the commonest ( Fig. 5 ). Each day of extra hospitalization adds to the risk of acquiring SSI and this has been confirmed by studies in Aurangabad, ( 6 ) Mumbai, ( 7 ) Hyderabad ( 9 ) and in Orissa. ( 10 ) In Aligarh ( 8 ) however, it was noted that diabetes mellitus and dehydration did not contribute to the occurrence of SSI.

Predisposing Condition/s in SSI cases
SSI have occurred more in elective surgeries-7.9%, than in emergency surgeries-2.7% ( Fig 6 ). This observation may seem very surprising, as emergency cases have known to land up in SSI more than the elective ones. ( 10 , 13 – 16 ) However, in our study, there have been more number of elective cases (63%) over the year than emergency cases (37%).

Category of Surgery in SSI cases
Also, the presence of underlying conditions like anaemia, diabetes mellitus etc was more in patients who had undergone elective surgery (38%) than in those who had emergency operations done (33%). These two factors could have been responsible for this unexpected outcome. In Aurangabad, ( 6 ) the infection rate in emergency surgeries was not statistically higher than in elective surgeries.
Pre operative antibiotics are known to decrease incidence of SSI cases. ( 1 , 6 , 13 ) However, prophylactic antimicrobials are more frequently given to patients who are poor risks from the stand point of susceptibility to infection. ( 13 ) This could explain our finding of a marginal increase of SSI in patients who have received prophylaxis (7.2%) than those who had not (5.6%) ( Fig 7 ).

Preoperative Antibiotics in SSI cases
Also, there is a marginal preponderance of male patients developing SSI (7.4%) over female patients with SSI (5.1%) which is not statistically significant ( Fig 8 ). In Aligarh, ( 8 ) females (27%) showed preponderance of SSI than males (18%). However, it has been known that sex is not a pre determinant of the risk of SSI. ( 13 )

Genderwise SSI
Gram negative bacilli, namely members of enterobacteriaceae, are the predominant pathogens in our setup according to the data provided by the Infection Control Committee of our hospital and our SSI bacteriological profile matched this observation. Of the 18 SSI cases, 4 patients’ swabs yielded no growth (22%) aerobically and anaerobically and showed no organisms on direct smear as well. Mumbai ( 7 ) study has also reported 17.6% culture negative SSI cases. The remaining 14 patients’ swabs yielded a total of 16 isolates in our laboratory of which Escherichia coli was 31.25%, Pseudomonas aeruginosa 25% and Staphylococcus aureus was 22%. This trend of gram negative bacilli i.e. enterobacteriaceae dominating the gram positive cocci has been observed in Aurangabad, ( 6 ) Orissa ( 10 ) and Navi Mumbai ( 11 ) also.
Conversely, in some studies ( 7 , 8 , 9 , 12 ) Staphylococcus aureus has dominated the scene. Multi drug resistance is a dreaded problem in nosocomial infections. Our study reveals enterobacteriaceae showing highest sensitivity to amikacin (78%) followed by gentamicin (71%). In comparison, very low sensitivity is noted with the cephalosporins and fluoroquinolones (10% and 58% respectively). This could be due to the overuse of these drugs and the high prevalence of extended spectrum beta lactamases (ESBLs) among these organisms. Surprisingly, our Pseudomonas isolates showed good sensitivity to piperacilin-tazobactam, ceftazidime and imipenem (83.5%, 83% and 100% respectively).
Staphylococcus aureus showed maximum sensitivity to linezolid (87.3%), gentamicin (100%), clindamycin (100%) vancomycin (100%).
In other studies, penicillins have shown very poor performance while gentamicin has showed maximum sensitivity (> 90%) against both gram positive cocci as well as gram negative bacilli. ( 6 , 7 , 9 , 10 , 12 ) Unlike our study, Hyderabad study ( 9 ) has shown first generation cephalosporins to be very effective for both.
Similar to our observation, vancomycin and linezolid have shown promise for Staphylococcus aureus in Navi Mumbai ( 11 ) too.
To conclude, we have observed that increased duration of surgery (> 2 hours), use of drains, and compromised immunity are responsible for SSI in our set up. Enterobacteriaceae (especially E.coli ) and S.aureus are the predominant pathogens showing maximum sensitivity to amikacin and gentamicin.
We can further bring down our SSI rate by implementing the following measures:
- ○ Efforts to decrease the duration of surgery without compromising the patient’s safety and the beneficial outcome.
- ○ Regular and intensive drain care
- ○ A thorough examination and investigations of poor risk patients should be done and accordingly all appropriate care should be taken to enable them to withstand the stress of surgery. Also utmost post operative care and efforts to boost their immunity would help in decreasing further the occurrence of SSI in this group of patients.
- ○ Periodic surveillance of SSI will guide the Infection Control Committee in laying down strict guidelines to further decrease the SSI incidence in our setup, which is an indicator of health care in a given system.
Undertaking
I, the undersigned, give an undertaking to the following effect with regard to our article entitled “Surgical Site Infections: A one year prospective study in a tertiary care center ” submitted for publication in International Journal of Health Sciences.
- The article mentioned above is original and has not been published or submitted to or accepted for publication in any form, in any other journal and does not infringe any existing copyright or any other third party rights.
- We also vouchsafe that the authorship of this article will not be contested by anyone whose name(s) is/are not listed by us here. Conflict of interests is none.
- I am authorized by my co-authors to enter into these arrangements.
- The article contains nothing that is unlawful, libelous, or which would, if published, constitute a breach of contract or of confidence or of commitment given to secrecy;
- Due care to ensure the integrity of the article has been taken.
Dr. Varsha D.Shahane (Corresponding and main author)
- Download PDF
- CME & MOC
- Share X Facebook Email LinkedIn
- Permissions
Surgical Site Infection Prevention : A Review
- 1 Duke Center for Antimicrobial Stewardship and Infection Prevention, Duke University School of Medicine, Durham, North Carolina
- 2 Department of Surgery, Duke University School of Medicine, Durham, North Carolina
- Original Investigation Effect of Incisional Negative Pressure Wound Therapy vs Standard Wound Dressing on Deep SSI Matthew L. Costa, PhD; Juul Achten, PhD; Ruth Knight, PhD; Julie Bruce, PhD; Susan J. Dutton, MSc; Jason Madan, PhD; Melina Dritsaki, PhD; Nick Parsons, PhD; Miguel Fernandez, PhD; Richard Grant; Jagdeep Nanchahal, PhD; WHIST Trial Collaborators; Peter Hull; Simon Scott; David Melling; Javed Salim; Hemant Sharma; William Eardley; Peter V Giannoudis; Jitendra Mangwani; Andrew Riddick; Paul Harnett; Edward Mills; Mike (R) Reed; Ben J Ollivere; Xavier L Griffin; Mark D Brinsden; Ravichandran Karthikeyan; Benedict A Rogers; Peter Bates; Haroon Majeed; Damian McClelland; Sharad Bhatnagar; Caroline B Hing; Rajarshi Bhattacharya; Usman Butt; George Cox; Khitish Mohanty; Mateen Arastu; Paul Harwood; Alex L Sims; Brett Rocos; Ian Baxter; Tanvir Khan; Paul M Guyver; Siddhant Kapoor; Michalis Kaminaris; Edward Massa; Richard Unsworth; Robert Jordan; Tarek Boutefnouchet; Laura Beddard; Graham Lawton JAMA
- JAMA Insights Preventing Surgical Site Infections—Looking Beyond the Current Guidelines Adam C. Fields, MD; Jason C. Pradarelli, MD, MS; Kamal M. F. Itani, MD JAMA
Importance Approximately 0.5% to 3% of patients undergoing surgery will experience infection at or adjacent to the surgical incision site. Compared with patients undergoing surgery who do not have a surgical site infection, those with a surgical site infection are hospitalized approximately 7 to 11 days longer.
Observations Most surgical site infections can be prevented if appropriate strategies are implemented. These infections are typically caused when bacteria from the patient’s endogenous flora are inoculated into the surgical site at the time of surgery. Development of an infection depends on various factors such as the health of the patient’s immune system, presence of foreign material, degree of bacterial wound contamination, and use of antibiotic prophylaxis. Although numerous strategies are recommended by international organizations to decrease surgical site infection, only 6 general strategies are supported by randomized trials. Interventions that are associated with lower rates of infection include avoiding razors for hair removal (4.4% with razors vs 2.5% with clippers); decolonization with intranasal antistaphylococcal agents and antistaphylococcal skin antiseptics for high-risk procedures (0.8% with decolonization vs 2% without); use of chlorhexidine gluconate and alcohol-based skin preparation (4.0% with chlorhexidine gluconate plus alcohol vs 6.5% with povidone iodine plus alcohol); maintaining normothermia with active warming such as warmed intravenous fluids, skin warming, and warm forced air to keep the body temperature warmer than 36 °C (4.7% with active warming vs 13% without); perioperative glycemic control (9.4% with glucose <150 mg/dL vs 16% with glucose >150 mg/dL); and use of negative pressure wound therapy (9.7% with vs 15% without). Guidelines recommend appropriate dosing, timing, and choice of preoperative parenteral antimicrobial prophylaxis.
Conclusions and Relevance Surgical site infections affect approximately 0.5% to 3% of patients undergoing surgery and are associated with longer hospital stays than patients with no surgical site infections. Avoiding razors for hair removal, maintaining normothermia, use of chlorhexidine gluconate plus alcohol–based skin preparation agents, decolonization with intranasal antistaphylococcal agents and antistaphylococcal skin antiseptics for high-risk procedures, controlling for perioperative glucose concentrations, and using negative pressure wound therapy can reduce the rate of surgical site infections.
Read More About
Seidelman JL , Mantyh CR , Anderson DJ. Surgical Site Infection Prevention : A Review . JAMA. 2023;329(3):244–252. doi:10.1001/jama.2022.24075
Manage citations:
© 2024
Artificial Intelligence Resource Center
Cardiology in JAMA : Read the Latest
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts
- Open access
- Published: 10 February 2020
Intraoperative surgical site infection control and prevention: a position paper and future addendum to WSES intra-abdominal infections guidelines
- Belinda De Simone 1 ,
- Massimo Sartelli 2 ,
- Federico Coccolini 3 ,
- Chad G. Ball 4 ,
- Pietro Brambillasca 5 ,
- Massimo Chiarugi 6 ,
- Fabio Cesare Campanile 7 ,
- Gabriela Nita 8 ,
- Davide Corbella 9 ,
- Ari Leppaniemi 10 ,
- Elena Boschini 11 ,
- Ernest E. Moore 12 ,
- Walter Biffl 13 ,
- Andrew Peitzmann 14 ,
- Yoram Kluger 15 ,
- Michael Sugrue 16 ,
- Gustavo Fraga 17 ,
- Salomone Di Saverio 18 ,
- Dieter Weber 19 ,
- Boris Sakakushev 20 ,
- Osvaldo Chiara 21 ,
- Fikri M. Abu-Zidan 22 ,
- Richard ten Broek 23 ,
- Andrew W. Kirkpatrick 24 ,
- Imtiaz Wani 25 ,
- Raul Coimbra 26 ,
- Gian Luca Baiocchi 27 ,
- Micheal D. Kelly 28 ,
- Luca Ansaloni 29 &
- Fausto Catena 30
World Journal of Emergency Surgery volume 15 , Article number: 10 ( 2020 ) Cite this article
46 Citations
1 Altmetric
Metrics details
A Correction to this article was published on 14 April 2021
This article has been updated
Surgical site infections (SSI) represent a considerable burden for healthcare systems. They are largely preventable and multiple interventions have been proposed over past years in an attempt to prevent SSI.
We aim to provide a position paper on Operative Room (OR) prevention of SSI in patients presenting with intra-abdominal infection to be considered a future addendum to the well-known World Society of Emergency Surgery (WSES) Guidelines on the management of intra-abdominal infections.
The literature was searched for focused publications on SSI until March 2019. Critical analysis and grading of the literature has been performed by a working group of experts; the literature review and the statements were evaluated by a Steering Committee of the WSES.
Wound protectors and antibacterial sutures seem to have effective roles to prevent SSI in intra-abdominal infections. The application of negative-pressure wound therapy in preventing SSI can be useful in reducing postoperative wound complications.
It is important to pursue normothermia with the available resources in the intraoperative period to decrease SSI rate.
The optimal knowledge of the pharmacokinetic/pharmacodynamic characteristics of antibiotics helps to decide when additional intraoperative antibiotic doses should be administered in patients with intra-abdominal infections undergoing emergency surgery to prevent SSI.
Conclusions
The current position paper offers an extensive overview of the available evidence regarding surgical site infection control and prevention in patients having intra-abdominal infections.
Surgical site infections (SSI) are a common type of healthcare-associated infections and frequent complication of hospitalization, responsible for prolonged hospital stay, increased intensive care unit admissions, hospital readmissions after surgery, significantly increased costs (1300–5000 USD per SSI), and delays to adjuvant systemic therapy; they occur in 2 to 5% of patients undergoing surgery in the USA [ 1 , 2 , 3 ].
Approximately 160,000 to 300,000 SSI are diagnosed and treated every year and represent a considerable burden for healthcare systems in terms of re-operation, increased post-surgical pain, poor wound healing, prolonged hospital stay, cosmetic appearance, and decreased quality of life [ 4 , 5 , 6 , 7 ].
SSI has also been shown to be an independent risk factor in the development of incisional hernia [ 8 ].
The incidence of all types of SSI following abdominal surgery can reach 14% of all hospital-acquired infections and the most common form is the incisional superficial SSI, which is often the first to appear and is easy to diagnose [ 9 ].
While more data are available from Western healthcare settings, SSI was the leading cause of hospital-acquired infection in a systematic review of studies in low- and middle-income countries [ 10 ].
They also a result in deleterious softer endpoints such as patient psychosocial distress, loss of income, and decreased productivity [ 1 , 2 , 3 ].
Multiple interventions have been proposed and employed over the past decades in an attempt to prevent SSI. These include skin cleansing protocols, hair removal, the maintenance of intraoperative normothermia, the preoperative antimicrobial prophylaxis administration, the use of plastic adhesive skin barriers, the high flow oxygen supplementation, the wound protection, the sterility of instruments, the bowel preparation, the length of the incision, and the delayed primary incision closure [ 11 , 12 , 13 , 14 , 15 ].
The development of SSI is multifactorial, and it may be related to patient’s risk factors such as age, comorbidities, smoking habit, obesity, malnutrition, immunosuppression, malignancies, and the class of contamination of the wound [ 9 , 16 ].
Emergency surgery is a risk factor for SSI because many strong risk factors for SSI such as contaminated and dirty wounds, prolonged duration of the operation, patient comorbidities, and high American Society of Anesthesiologists (ASA) score are commonly present in this type of surgery. For these reasons, the World Society of Emergency Surgery (WSES) developed a position paper for the prevention of SSI in the operative room (OR).
A panel of international experts discussed statements based on predetermined research questions and the results of related systematic literature reviews.
The literature search found few articles focused on SSI and emergency surgery; consequently, most of the reviewed studies considered the incidence of SSI in elective surgery because of the lack of valid data from an emergency setting. This is a consequence of the difficulty to conduct a good-quality study in an emergency environment: the workload is often intermittent and unpredictable, patient case-mix is heterogeneous with a wide variety of concomitant problems and severity of initial diagnosis; moreover, the emergency environment poses many barriers and obstacles to patient recruitment and data collection, and this has implications particularly for the staffing of prospective trials.
Considering all these limitations, we cannot ignore the potential benefit from using some devices and equipment or adopting some simple strategies in emergency surgery to decrease the incidence of SSI.
This position paper aims to provide recommendations on OR prevention of SSI in patients with intra-abdominal infections to be an addendum to the WSES Guidelines on the management of intra-abdominal infections.
Materials and methods
In July 2018, the Scientific Board of the WSES, the President of the Society and the President of the 5th World Congress of the WSES decided to prepare a position paper on OR prevention of SSI in patients with intra-abdominal infections in the emergency setting.
The Presidents and ten members of the Scientific Secretariat (SS) agreed on 11 key topics to develop in the position paper (Table 1 ); nine international experts, members of the WSES Board, were chosen as Steering Committee (SC).
Each topic was developed by members of the SS: the SC and the Presidents supervised every step of literature search, selection, and the final work.
The SS provided the electronic search in PubMed and EMBASE databases, according to specific keywords for each question as you can see in the Appendix 1 without time or language restrictions.
Each expert followed the PRISMA methodology in the selection of papers to consider for review: meta-analyses of randomized controlled trials, randomized control trials, prospective studies, observational studies, large case series, and systematic reviews were included in this study.
Each SS member developed a focused draft and a variable number of statements. Each statement has been evaluated according to the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) [ 17 ] summarized in Table 2 .
The provisional statements and the supporting literature were reviewed by all SS members and the Presidents, discussed with the SC members by email/call conferences and modified if necessary.
The designated member of SS presented the statements to SC along with the grade of recommendation (GoR) and the literature supporting each statement.
Clinicians and surgeons must be aware that the present position paper should be considered as an adjunctive tool for decision and management, but they do not substitute for the clinical judgment for individual patients.
How to close a surgical incision?
Statement 1.1: there is no significant difference in terms of ssi incidence and length of hospital stay between patients in which the skin is sutured by continuous versus interrupted stitches (gor 1b)., statement 1.2: superficial wound dehiscence is lower in subcuticular continuous suture versus interrupted stitches (gor 1b)., statement 1.3: the use of steri-strips or tissue adhesives doesn't reduce the incidence of ssi (gor 1b)..
The method of skin closure may have a role in preventing the development of SSI. Compared with interrupted sutures, continuous sutures can provide a better seal preventing the exogenous bacterial invasion of the surgical wound [ 16 ].
However, a continuous tightly pulled suture can strangulate the wound edges [ 18 , 19 ].
Many published trials have demonstrated the benefit of skin closure by subcuticular interrupted sutures compared with conventional skin stapling in different surgical scenarios [ 9 , 16 , 17 ].
On the other hand, very few papers have been designed to investigate differences in the outcome when the skin is closed by continuous or by interrupted sutures.
In a Cochrane meta-analysis [ 19 ] published in 2014 and focused on the impact that different methods of skin closure could have on superficial SSI, superficial wound dehiscence, and length of hospital stay, only five RCTs comparing continuous versus interrupted sutures were identified. The five RCTs included a total of 827 participants undergoing abdominal or groin operations (non-obstetric surgery) [ 19 , 20 , 21 , 22 , 23 ]. Most of the enrolled patients were children or adolescents, and appendectomy was the most performed surgery.
Comparisons were made irrespectively of the material of the sutures. From this meta-analysis, no statistically significant differences were found between the two methods of suture regarding the prevalence of superficial SSI (RR 0.73; 95% CI 0.40 to 1.33) and length of hospital stay. However, a lower rate of superficial wound dehiscence was recorded in the continuous suture group (RR 0.08; 95%, CI 0.02 to 0.35).
It should be noted that in these trials the continuous skin suture groups received absorbable subcuticular sutures, while the interrupted skin suture groups received non-absorbable transcutaneous sutures. The non-absorbable sutures were removed 7 to 9 days after surgery, which is generally considered to be a suitable time for removal of sutures. The removal of sutures was not necessary for the absorbable subcuticular continuous suture group. The suture material used in the continuous suture groups was 4-0 poliglecaprone and 4-0 polyglactin [ 22 , 23 ].
This kind of sutures retains approximately 50 to 75% of their original tensile strength after 1 week in situ. This extra support for the wound after 1 week may be the main reason for the difference between the continuous suture group and the interrupted suture group regarding the development of superficial wound dehiscence [ 19 ].
Conclusions of the meta-analysis were that superficial wound dehiscence may be reduced by using continuous subcuticular sutures and that continuous or interrupted skin closure does not have any impact on the development of superficial SSI and on the length of hospital stay. Due to the quality of the evidence, a high grade of uncertainty remains.
In addition to the abovementioned meta-analysis, only one study compared continuous versus interrupted skin suture for abdominal surgery in a non-intra-abdominal infection setting [ 24 ].
This review included 586 patients from a single Japanese institution to compare the incidence of incisional SSI after elective hepato-pancreatobiliary surgery (HPB) by different methods of skin closure. The study showed statistically significant efficacy of the subcuticular continuous sutures to prevent incisional SSI in patients undergoing HPB surgery (1.8% in the subcuticular continuous suture group and 10.0% in the stapling group, P < 0.01). However, the retrospective and single-institution design substantially affect the evidence of the results.
Many papers showing the benefits of subcuticular sutures versus stapling in terms of reduction of SSI and wound dehiscence are available from the literature, but unfortunately they were designed to compare interrupted rather than continuous subcuticular sutures versus stapling, or they merge continuous and interrupted techniques in a single group [ 9 , 16 , 25 ].
For these reasons, further well-designed RCTs with a low risk of bias should be conceived to establish which type of skin suturing provides better results.
A common practice in OR is to cover the closed wound with adhesive steri-strips.
Custis et al. [ 26 ] carried out a prospective study to assess whether the addition of adhesive strips to a wound closed with buried interrupted subcuticular sutures improves outcomes following wound closure. The study enrolled 45 patients and showed that there was no significant difference in the total patient assessment scale score between the combination closure (14.0 [7.6]) and sutures only (14.7 [7.6]) sides at 3 months ( P = .39). There was also no significant difference between the two closure methods in terms of mean (SD) scar width (both methods, 1.1 [0.8] mm, P = .89) at follow-up. There was one case of wound dehiscence at a site that used adhesive strips and two cases at sites without adhesive strips. Three suture abscesses were documented at sites with adhesive strips and six at sites without adhesive strips. One patient had a spitting suture, which was not classified as an abscess; this event occurred at a site without adhesive strips. There were no documented infections, hematomas, or seromas. None of the adverse effects were statistically significant between study arms. The authors concluded that similar outcomes were observed whether or not adhesive strips were applied in addition to buried dermal sutures when performing cutaneous surgical procedures and that the use of adhesive strips cannot be recommended to improve cosmetic outcomes or reduce scar width.
An updated Cochrane review [ 27 ] was carried out to determine the effects of various tissue adhesives compared with conventional skin closure techniques for the closure of surgical wounds included 33 studies with a total of 2793 participants and demonstrated that there was low-quality evidence that sutures were significantly better than tissue adhesives for reducing the risk of wound breakdown (dehiscence; RR 3.35; 95% CI 1.53 to 7.33; 10 trials, 736 participants that contributed data to the meta-analysis). The number needed to treat for an additional harmful outcome was calculated as 43. For all other outcomes—infection, patient and operator satisfaction and cost—there was no evidence of a difference for either sutures or tissue adhesives. No evidence of differences was found between tissue adhesives and tapes for minimizing dehiscence, infection, patients’ assessment of cosmetic appearance, patient satisfaction, or surgeon satisfaction. The authors concluded that sutures are significantly better than tissue adhesives for minimizing dehiscence. In some cases, tissue adhesives may be quicker to apply than sutures.
Coated sutures: are they useful?
Statement 2.: triclosan-coated suture significantly reduces ssi prevalence compared with the non-coated sutures (gor 1b)..
Sutures with antimicrobial properties were developed to prevent microbial colonization of the suture material in operative incisions. Early studies showed a reduction of the number of bacteria in vitro and wound infections in animals using triclosan-coated sutures, and this effect was subsequently confirmed in clinical studies [ 28 , 29 ]. Several novel antimicrobial coatings are now available, but still, no clinical studies have been done that compare the efficacy with non-coated sutures [ 30 ].
Wu et al. performed a systematic review to assess whether the use of antimicrobial-coated sutures is more effective in reducing the risk of SSI than the use of non-coated sutures.
Eighteen studies comparing triclosan-coated sutures vs non-coated sutures (13 randomized controlled studies and 5 observational studies) were included in the meta-analysis for a total of 7458 patients; all studies investigated triclosan-coated sutures and focused on adult patients, apart from one done in a pediatric population [ 31 ]. The meta-analysis of the data demonstrated that antimicrobial sutures significantly reduced SSI risk (for RCTs: OR 0.72, 95% CI 0.59–0.88, P = 0.001, I2 = 14%; for observational studies: OR 0.58, 95% CI 0.40–0.83, P = 0.003, I2 = 22%). Only Vicryl Plus vs Vicryl revealed consistent results in favor of antimicrobial sutures (for 7 RCTs: OR 0.62, 95% CI 0.44–0.88, P = 0.007, I2 = 3%; for 4 observational studies: OR 0.58, 95% CI 0.37–0.92, P = 0.02, I2 = 41%). Besides, the effect of antimicrobial coating was similar between different suture, wound (clean, clean-contaminated, and mixed), and procedure types (colorectal, cardio-vascular, head and neck, breast surgical procedures). Quality of RCT evidence was judged moderate, and observational studies’ evidence was judged of very low quality and many studies had conflicts of interest. The authors concluded that triclosan-coated sutures may reduce SSI risk.
Uchino et al. [ 32 ] have recently analyzed the efficacy of antimicrobial-coated sutures in preventing SSIs in digestive surgery. A total of 5188 patients in 15 studies were included, with 10 randomized controlled trials (RCT) and 5 observational studies (OBS). One study enrolled pediatric patients. The sutured surgical sites in the included studies were the abdominal fascia in 12 studies, the subcutaneous alone in 1 study, and unknown in 2 studies.
Regarding the types of surgeries represented, there were 9 colorectal surgeries, 4 mixed digestive surgeries, 1 gastric surgery, and 1 pancreaticoduodenectomy. The RCTs included 6 studies that performed surgeries limited to class 2 wounds or described the incidence distinct from the wound class. Only one study was performed during emergent surgeries and was limited to the dirty/infected wound classes. The remaining 3 studies were analyses conducted together with mixed wound classes. Regarding the suture materials in the RCTs, monofilament sutures were used in 4 RCTs, and poly-filament sutures were used in 4 RCTs. Two RCTs used mixed suture materials. In OBSs, nearly half of the participants had upper gastrointestinal surgery. The meta-analysis showed that in the 10 RCTs, the incidence rates of incisional SSIs were 160/1798 (8.9%) with coated sutures and 205/1690 (12.1%) with non-coated sutures. Overall, antimicrobial-coated sutures were superior for reducing the incidence of incisional SSI (RR 0.67, 95% CI 0.48–0.94, P = 0.02) in RCTs for digestive surgery with the mixed wound class and surgeries limited to a clean-contaminated wound (RR 0.66, 95% CI 0.44–0.98, P = 0.04). A superior effect of antimicrobial-coated sutures was found in 9 RCTs that involved only colorectal surgeries (RR 0.69, 95% CI 0.49–0.98, P = 0.04). The superior effect of antimicrobial-coated sutures was also found in OBSs (OR 0.4, 95% CI 0.3 to 0.54, P < 0.001). The mean hospital stay length was similar to coated or uncoated sutures in 5 RCTs involving colorectal surgery (mean difference (MD) − 5.00, 95% CI 16.68-6.69, P = 0.4) [ 32 ].
Guo et al. demonstrated that triclosan-coated sutures were associated with a lower risk of SSI than uncoated sutures across all surgeries (risk ratio [RR] 0.76, 95% confidence interval [CI] 0.65–0.88, P < 0.001). Similar proportions of patients experienced wound dehiscence with either type of suture (RR 0.97, 95% CI 0.49–1.89, P = 0.92). Subgroup analysis showed lower risk of SSI with triclosan-coated sutures in abdominal surgeries (RR 0.70, 95% CI 0.50–0.99, P = 0.04) and group with prophylactic antibiotic (RR 0.79, 95% CI 0.63–0.99, P = 0.04). However, such risk reduction was not observed in cardiac surgeries, breast surgeries, or the group without prophylactic antibiotics [ 33 ].
Henriksen et al. [ 34 ] in an overall comparison including both triclosan-coated Vicryl and PDS sutures for fascial closure, reported that triclosan-coated sutures were superior in reducing the rate of SSI (OR 0.67; CI 0.46–0.98). The majority of the studies included only elective surgery procedures. Four of these included only colorectal procedures, whereas Diener et al. [ 35 ] included all types of elective procedures through a midline laparotomy. Justinger et al. [ 36 ] included both elective and emergency laparotomies, whereas Ruiz-Tovar et al. [ 37 ] included only cases with fecal peritonitis and Mingmalairak et al. [ 38 ] studied patients undergoing open appendectomies. When evaluating PDS sutures separately, there was no effect of triclosan coating on the rate of SSI (OR 0.85; CI 0.61–1.17). After trial sequential analysis, authors concluded that triclosan-coated Vicryl sutures for abdominal fascial closure significantly decrease the risk of SSI and performing further RCTs will not change this outcome, but there was no effect on SSI rate with the use of triclosan-coated PDS sutures for abdominal fascial closure [ 34 ]. That means that PDS commonly used in abdominal surgery was not different.
Konstantelias et al. [ 39 ] analyzed 30 studies (19 randomized, 11 non-randomized; 15,385 procedures) giving evidence that triclosan-coated sutures were associated with a lower risk of SSIs (risk ratio [RR] = 0.68; 95% confidence interval [CI] 0.57–0.81). Triclosan-coated sutures were associated with a lower risk for SSIs in high-quality randomized studies (Jadad score 4 or 5). A lower risk for the development of SSIs based on wound classification was observed in clean, clean-contaminated, and contaminated but not for dirty procedures. No benefit was observed in specific types of surgery: colorectal, cardiac, lower limb vascular, or breast surgery.
A specific study on emergency surgery was also carried out confirming these findings [ 40 ].
What is the role of intraoperative intraperitoneal irrigation vs topic wound lavage with antibiotic solutions to prevent surgical site infections?
Statement 3: there are insufficient data to support the role of intraperitoneal or topic wound irrigation with antibiotics in preventing ssi (gor 2b)..
Although intraoperative irrigation with antibiotic solutions has been suggested to be beneficial in the prevention of infections, no evidence-based results have been made available. The effectiveness of intra-abdominal lavage with antibiotic solutions on the prevention of postoperative SSI is controversial. Furthermore, issues about its safety need to be examined as well as local adverse effects (increased adhesion formation, postoperative pain), selection of resistant bacteria, and tissue toxicity.
The safety of the intraperitoneal administration of antibacterial agents during or after surgery as prophylaxis or treatment of infection has been investigated in a systematic review that included 29 RCTs and 50 observational studies [ 41 ].
The objective of this systematic review was to analyze perioperative intraperitoneal administration of antibacterial agents, to characterize the drugs used, and their safety profile. Administration of topical intraperitoneal antibiotics both during and after surgery was studied. Aminoglycosides, first- and second-generation cephalosporins, tetracyclines, and penicillins were most commonly administered intraperitoneally during or after surgery. The antibacterial agent was usually administered intraperitoneally as monotherapy. However, some studies administered combination regimens with heparin or with another antibacterial agent. The most frequent combination was aminoglycosides and lincosamides. Only a few and mild adverse events were reported and the authors concluded that antibacterial agents can safely be administered intraperitoneally. However, they acknowledged that in 43% of the included articles the adverse events were not reported while 41% of the studies specified that there were no adverse events related to the intraperitoneal administration of drugs. The most frequently reported adverse event was discomfort or pain during administration, especially with the use of oxytetracycline [ 41 ].
Animal data about the relationship between intraperitoneal antibiotics and adhesion development are conflicting [ 42 , 43 , 44 , 45 , 46 ].
In the experimental study conducted by Sortini et al. [ 43 ], the peritoneal lavage solution showing low adhesion formation and high survival rates was saline solution at 37 °C. In this study, lavage with antiseptics was associated with higher mortality (55–80% versus 0% for chlorhexidine–iodine solutions and saline solution, respectively, P < 0.001) but less adhesion formation ( P < 0.001) as compared to saline solution. The use of antibiotic solutions was associated with 3% mortality in the treatment of peritonitis but with higher Zühlke scores and adhesion formation as compared to saline solution ( P < 0.001).
According to these data, antiseptic solutions should not be recommended for peritoneal lavage.
Another experimental study was carried out to test the effectiveness of the intraperitoneal application of alternate antibiotics (Imipenem, ceftriaxone, and cefazolin) in an abdominal sepsis model. These data suggest that cephalosporins may be effective in preventing adhesion formation in septic abdomens compared to metronidazole [ 46 ].
Tetikcok et al. [ 47 ] have recently demonstrated that in rats, peritoneal lavage with prednisolone improved survival rates with increasing doses in abdominal sepsis. Abdominal lavage in rats was made using saline in group 1, equal volumes of cefazolin sodium in group 2, low-dose methylprednisolone (1 mg/kg) in group 3, and high-dose methylprednisolone (2 mg/kg) in group 4. The study showed that the mortality rate of the rats in group 2 was significantly higher than that in group 4, which had no mortality ( P = 0.032). Although insignificant, the lowest mean value of IL-1β, IL-2, and TNF-α was in group 1, and the highest was in group 2. The lowest IL-4 level was in group 3, and the highest level was in group 2 ( P = 0.41). Interleukin-10 levels were significantly lower in group 4 and higher in group 2 ( P = 0.014). The administration of prednisolone in this abdominal sepsis model does not reflect a real-world situation; however, the administration of prednisolone alone helped to understand the effect of corticosteroids without masking the effects with antibiotics.
A 2017 Cochrane review included 36 studies (6163 participants) comparing the use of antibacterial irrigation with non-antibacterial irrigation [ 48 ]; authors reported a lower incidence of SSI in patients treated with antibacterial irrigation compared with non-antibacterial irrigation (RR 0.57, 95% CI 0.44 to 0.75; I2 = 53%; 30 studies, 5141 participants). This was low-certainty evidence downgraded once because 54% of the analysis weight was contributed by studies at high risk of bias in one or more domains, and once because publication bias was considered likely to have affected the result. Besides, the review pools together studies about intra-cavitary and wound irrigation, antibiotics, and antiseptics as antibacterial agents.
The possible benefit was present in each of the surgical contamination subgroups (clean versus clean-contaminated versus contaminated or dirty). The difference in adverse events, mortality, and abscess formation did not reach statistical significance. The hospital stay was reduced in the antibacterial irrigation group.
Concerning intraoperative wound irrigation, Mueller et al. in a meta-analysis of RCTs investigating the incidence of postoperative SSI after intraoperative irrigation of the surgical incision (after the closure of the fascia or peritoneum and before skin closure) performed a subgroup analysis comparing intraoperative wound irrigation with topical antibiotics vs saline solution irrigation. The study showed a significant reduction of postoperative SSI when antibiotic solution irrigation was used compared to both saline solution only irrigation and no irrigation.
The reported length of follow-up in the included trials was 30 days or more in only 21 out of 41 trials. The remaining trials reported follow-up times of as short as 5–10 days or did not specify the follow-up time at all. Besides, the number and frequency of follow-up visits varied largely, as did the type and blinding status of the primary outcome assessor [ 49 ].
However, the considerable risk for bias of all the included trials, their large heterogeneity, and the need to balance those findings against the risk of impaired wound healing and the potential increase of the bacterial resistance suggest caution in the clinical application of these results.
Could wound irrigation with saline and/or povidone iodine solution be useful to prevent surgical site infection?
Statement 4.: there are insufficient data to determine the role of saline or povidone irrigation of incisional wounds before closure to prevent ssi (gor 2b).
Intraoperative wound irrigation refers to the flow of a solution across the surface of an open wound. It is a widely practiced procedure and considered to help prevent SSI.
Among other benefits, wound irrigation is intended to physically remove foreign material, cellular debris, surface bacteria, and body fluids, to dilute possible contamination and to function as a local antibacterial agent when an antiseptic or antibiotic agent is used.
Wound irrigation must be vigorous enough to perform the above goals but gentle enough to avoid further tissue trauma or passage of bacteria and foreign material deeper into the wound. Practices vary depending on the patient population, the surface of the application, and the solution used.
On the other hand, vigorous irrigation may remove protective immunologic cells that are enable healing to progress through a natural series of processes, including inflammation and granulation, to final re-epithelialization and remodeling. Exposed subcutaneous tissue provides a favorable substratum for a wide variety of microorganisms to contaminate and colonize, and if the involved tissue is devitalized (e.g., ischemic, hypoxic, or necrotic) and the host immune response is compromised, the conditions become optimal for microbial growth [ 50 ]. A systematic review was carried out to investigate whether intraoperative wound irrigation (with or without active agents or pressured application) affects the incidence of SSI. Studies investigating the topical application of antibiotics or antiseptics (e.g., powder, gels, sponges) were not included.
Twenty-one RCTs were identified comparing wound irrigation with no wound irrigation in patients undergoing various surgical procedures, and the results were substantially heterogeneous [ 51 ]
Saline irrigation was not effective in reducing SSIs [ 52 ]. However, when the saline was applied with a syringe to generate some pressure [ 53 ], a reduction in the risk of SSI compared with no irrigation was shown in one study (OR 0.35; 95% CI 0.19–0.65; P = 0.0009). This benefit also was demonstrated when pulse pressure irrigation with saline was compared with normal saline irrigation in a meta-analysis of two RCTs [ 54 , 55 ] (OR 0.30; 95% CI 0.08–0.86; P = 0.0003).
In the same meta-analysis, a low quality of evidence demonstrated a statistically significant benefit for incisional wound irrigation with an aqueous povidone iodine solution in clean and clean-contaminated wounds (OR 0.31; 95% CI 0.13–0.73; P = 0.007); 50 fewer SSI per 1000 procedures (from 19 fewer to 64 fewer) [ 51 ].
The 2017 Cochrane review comparing antibacterial irrigation with non-antibacterial irrigation (36 studies, 6163 participants), the largest meta-analysis published, reported a lower incidence of SSI in participants treated with antibacterial irrigation compared with non-antibacterial irrigation (RR 0.57, 95% CI 0.44 to 0.75; I2 = 53%; 30 studies, 5141 participants) but evidence are of low certainty [ 48 ].
Therefore, where a possible difference in the incidence of SSI was identified (in comparisons of antibacterial and non-antibacterial interventions, and pulsatile versus standard methods), these should be considered in the context of uncertainty, particularly given the possibility of publication bias for the comparison of antibacterial and non-antibacterial interventions.
Clinicians should also consider whether the evidence is relevant to the surgical populations (wound classification and setting) under consideration.
Are wound protector devices useful? (Table 3 )
Statement 5.1: the use of wound protectors has protective effects in reducing incisional ssi (gor 1a);, statement 5.2: the use of dual-ring constructed wound protectors appears to be superior to single-ring devices in preventing ssi (gor 1b)..
Wound protector devices (alternatively called “wound guards” or “wound retractors”) have been increasingly used in the effort to reduce SSI rates. These devices form a physical barrier between the wound edges and the contaminated surgical field. More specifically, the impervious plastic barrier prevents both endogenous and exogenous pathogens from imbedding themselves within the wound (skin, fat, fascia, peritoneum). This mechanism, in conjunction with maintaining wound humidity and reducing direct physical trauma from fixed retractors, is believed to reduce the risk of incisional SSI. It must be noted however that some bacterial invasion could occur immediately before the insertion, or more likely after the removal of the wound protector itself. There are two widely available forms: a single ring that lies within the abdominal cavity connected to a protective drape that extends outward, or two rings that are connected cylindrically by impenetrable plastic with one ring inside the wound and the other secured on the outside [ 64 ].
The ROSSINI trial [ 56 ] is a multicenter observer-blinded RCT carried up to determine the clinical effectiveness of wound edge protection device (the device used was the 3 M Steri-Drape Wound Edge Protector) in reducing surgical site infection after abdominal surgery, enrolling 760 patients with 382 patients assigned to the device group and 378 to the control group, reported that a total of 184 patients experienced surgical site infection within 30 days of surgery, 91/369 (24.7%) in the device group and 93/366 (25.4%) in the control group (odds ratio 0.97, 95% confidence interval 0.69 to 1.36; P = 0.85). In the secondary analyses, no subgroup could be identified in which there was evidence of clinical benefit associated with the use of the device. The authors concluded that wound edge protection devices cannot be recommended to reduce the rate of SSI in patients undergoing laparotomy.
Gheorghe et al. cost-effectiveness analysis suggests that the use of wound protector devices for SSI reduction cannot be justified and should be discontinued [ 64 ].
Previously, in 2012, Gheorghe et al. [ 57 ] reviewed 12 studies (2 prospective controlled studies +10 RCTs) reporting primary data from 1933 patients. The quality assessment found all of them to be at considerable risk of bias. An exploratory meta-analysis was performed to provide a quantitative indication of the wound edge protector device effect. The pooled risk ratio under a random-effects model was 0.60 (95% confidence interval, 0.41–0.86), indicating a potentially significant benefit from the use of the dispositive. No indications of significant between-study heterogeneity or publication bias, respectively, were identified.
In 2012, Edwards et al. [ 58 ] analyzed 6 RCTs for a total of 1008 patients were included. They reported that the use of a wound protector was associated with a significant decrease in SSI (RR = 0.55, 95% CI 0.31–0.98, P = 0.04). Data showed also a nonsignificant trend toward greater protective effect in studies using a dual-ring protector (RR = 0.31, 95% CI 0.14–0.67, P = 0.003), rather than a single-ring protector (RR = 0.83, 95% CI 0.38–1.83, P = 0.64).
To assess these controversial results, several meta-analyses have been published looking at the effectiveness of wound protectors in preventing SSIs in abdominal surgeries.
In 2015, Mihaljevic et al. [ 59 ] analyzed 16 RCTs including 3695 patients investigating wound edge protectors published between 1972 and 2014. Data reported that wound edge protectors significantly reduced the rate of surgical site infections (risk ratio 0.65; 95%CI, 0.51–0.83; P = 0.0007; I 2 2 = 52%). A similar effect size was found in the subgroup of patients undergoing colorectal surgery (risk ratio 0.65; 95%CI, 0.44–0.97; P = 0.04; I 2 2 = 56%). Of the two common types of wound protectors, double-ring devices were found to exhibit a greater protective effect (risk ratio 0.29; 95%CI, 0.15–0.55) than single-ring devices (risk ratio 0.71; 95%CI, 0.54–0.92), but this might largely be due to the lower quality of available data for double-ring devices. Exploratory subgroup analyses for the degree of contamination showed a larger protective effect in contaminated cases (0.44; 95%CI, 0.28–0.67; P = 0.0002, I 2 2 = 23%) than in clean-contaminated surgeries (0.72, 95%CI, 0.57–0.91; P = 0.005; I 2 2 = 46%) and a strong effect on the reduction of superficial surgical site infections (risk ratio 0.45; 95%CI, 0.24–0.82; P = 0.001; I 2 2 = 72%) [ 59 ].
Zhang et al. reviewed 11 RCTs including 2344 patients. In particular, 6 trials (1589 patients) testing the single-ring design wound edge protector did not show a statistically significant reduction in SSI of laparotomy (RR 0.76, 95% CI 0.51–1.12). Pooled analysis of the five trials (755 patients) that tested the effect of dual-ring wound protector on SSI showed a significant reduction (RR 0.29, 95% CI 0.15–0.55). The combined data of the 11 trials favored the wound edge protector effect (RR 0.58, 95% CI 0.39–0.87). Analysis adjusted by the degrees of contamination revealed that wound protector device is effective in reducing the incidence of SSI after laparotomy incision contamination (RR 0.43, 0.26–0.72) but failed to demonstrate such effect in clean/contaminated and dirty incisions (RR 0.72, 95% CI 0.43–1.21; RR 0.82, 95% CI 0.43–1.55, respectively) [ 60 ]
More specifically, two extremely recent systematic reviews that evaluated 2684-patient and 3808-patient RCTs respectively once again confirm this observation.
The first from Kang et al. [ 61 ] identified and analyzed 14 randomized controlled trials with a total of 2684 patients. The pooled risk ratio under a random-effects model was 0.70 (95% confidence interval, 0.51-0.96; I2, 56.8%), indicating a potentially significant benefit from impervious plastic wound protector use. There was a significant trend toward greater protective effect in studies using a dual-ring protector (relative risk = 0.31; 95% confidence interval, 0.15–0.58), rather than a single-ring protector (relative risk = 0.84; 95% confidence interval, 0.71–1.00). There was no significant between-study heterogeneity or publication bias.
The second from Said et al. [ 62 ] analyzed 18 RCTs and demonstrated that wound edge protector is associated with the reduced incidence of overall SSI (OR 0.59; 95% CI 0.43–0.81; z = 3.30; P < 0.001) and superficial SSI (OR 0.42; 95% CI 0.18–0.95; z = 2.09; P < 0.04). In addition, it also successfully reduced the risk of SSI in clean-contaminated wounds (OR 0.67; 95% CI 0.46–0.98; z = 2.06; P < 0.04) as well as in contaminated wounds (OR 0.24; 95% CI 0.12–0.49; z = 3.96; P < 0.0001). The reported overall reduction in SSI was substantial in both reviews (OR = 0.70 and 0.59 respectively).
When superficial (wound) SSI is the focus of the analysis, there is a further reduction in the postoperative rate (OR = 0.42). Furthermore, these trends appear to extend to both clean-contaminated and contaminated wounds (OR = 0.67 and 0.24 respectively). While these comprehensive reviews and statistical analyses are compelling, they omit a single large recent RCT that evaluated the role of wound protectors in high-risk non-colorectal scenarios (i.e., pancreaticoduodenectomies (PD) following preoperative insertion of biliary stents for obstruction). This study including a total of 107 patients reported a significant reduction in the incidence of incisional SSI in the wound protector group (21.1% vs 44.0%; relative risk reduction 52%; P = 0.010). Patients with completed PD had a decrease in incisional SSI with the use of the wound protector compared with those undergoing palliative operations (27.3% vs 48.7%; P = 0.04). Multivariate analysis did not identify any significant modifying factor relationships (estimated blood loss, duration of surgery, hospital site, etc.) ( P > 0.05) [ 63 ].
While the utility of wound protectors is clear, the superior mechanical configuration of these devices remains debated. More specifically, both single-ring (with or without large adhesive drape components) and dual-ring modalities (internal and external ring connected by impervious plastic) are currently available. Two high-quality analyses [ 61 , 62 ] have both noted a strong trend toward a greater protective effect with dual-ring variants when compared to devices constructed with a single external ring and associated semi-adhesive drape. It is also interesting to note that among this level 1 RCT data, there is a clear modifying effect of the publication year. In other words, as time has progressed in the study of wound protectors (and therefore the evaluation of more diverse surgical subgroups), their protective effect has become increasingly evident.
In clinical practice, the only possible barrier to the routine use of these types of devices is cost and availability. A possible solution to decrease cost is to reserve wound protectors for high-risk patients or dirty surgical incisions to reduce SSI and equate costs related to wound protectors and hospitalization(s).
Are adhesive sterile surgical incise drapes useful?
Statement 6.1: there is no evidence that plastic adhesive drapes with or without antimicrobial properties are useful to decrease ssi (gor 2c)..
Adhesive plastic incise drapes are used on a patient’s skin after surgical site preparation, with or without antimicrobial impregnation, and the surgeon performs the incision of the drape and the skin simultaneously. There are conflicting recommendations on the use of plastic adhesive drapes, mainly discouraging their use.
In 2015, the fourth update of the Cochrane review carried out to investigate the advantages about using plastic adhesive drapes to protect the wound from organisms that may be present on the surrounding skin during surgery, analyzed 5 studies with a total 3082 participants comparing plastic adhesive drapes with no drapes and 2 studies involving 1113 participants comparing iodine-impregnated adhesive drapes with no drapes. A significantly higher proportion of patients in the adhesive drape group developed a surgical site infection when compared with no drapes (risk ratio (RR) 1.23, 95% confidence interval (CI) 1.02 to 1.48, P = 0.03). Iodine-impregnated adhesive drapes did not affect the surgical site infection rate (RR 1.03, 95% CI 0.06 to 1.66, P = 0.89). The length of hospital stay was similar in the adhesive drape and non-adhesive drape groups. There was no evidence from the 7 trials that plastic adhesive drapes reduce surgical site infection rates and some evidence that they increase infection rates [ 65 ].
In 2016, Allegranzi et al. analyzed 4 studies (one RCT, one quasi-RCT, and two observational studies) comparing adhesive iodine-impregnated incise drapes with no drapes and showed no difference in the SSI risk (RCTs: OR 2·62; 0·68–10·04; observational studies: OR 0·49; 0·16–1·49). Similarly, a meta-analysis of two RCTs comparing non-impregnated adhesive incise drapes to no drapes showed no difference in the SSI risk (OR 1·10; 0·68–1·78) [ 66 ].
Recently, Rezapoor et al. carried out a prospective, randomized clinical trial to evaluate the efficacy of iodophor-impregnated adhesive drapes for reducing bacterial contamination and counts at the incision site during hip surgery. The study enrolled 101 patients undergoing open joint preservation procedure of the hip. Half the patients had the adhesive drape applied to the skin before incision, while the remainder underwent the same surgery without a drape. Culture swabs were taken from the surgical site at 5 points (pre skin preparation, after skin preparation, post-incision, before subcutaneous closure, before dressing application) and sent for culture and colony counts. After surgery, 12.0% of incisions with adhesive drapes and 27.4% without adhesive drapes were positive for bacterial colonization. It appears that the iodophor-impregnated adhesive draping significantly reduces bacterial colonization of the incision [ 67 ].
Recently, Zarei et al. have conducted a quasi-experimental study with non-equivalent control group design enrolling 88 patients who were the candidate for lumbar spine surgery in the elective operating room to investigate the effect of the incise drape on the rate of bacterial contamination of surgical wound, and they concluded that the use of ID is unable to reduce surgical wound bacterial contamination in clean lumbar spine surgery [ 68 ].
To drain or not to drain in closing surgical incision?
Statement 7.1: there are insufficient data to determine the role of subcutaneous drainage of incisional wounds before closure to prevent ssi in high-risk patients (gor 2b)..
Evidence regarding the utility of subcutaneous drains in preventing incisional SSI are controversial.
The presence of fluid collection between the skin sutures and underlying fascia is thought to increase the risk for SSIs, as it can provide a medium for bacterial growth. The concept of subcutaneous drainage is to remove these fluids before they become infected, resulting in a reduction of SSI.
Recently, several studies have examined suctioning/active drainage systems as a means to prevent SSI in digestive surgery, but the utility of these systems is still controversial [ 69 , 70 ].
Fuji et al. assessed the efficiency of subcutaneous drains for high-risk patients undergoing colorectal surgery, including patients with thick subcutaneous fat tissue and those undergoing emergency operations. They enrolled in their 79 high-risk patients for SSI. The overall incidence of incisional SSI was 27.8%. The incidences of incisional SSI in these cases with or without a subcutaneous drain were 14.3% and 38.6%, respectively. The authors concluded that subcutaneous drains are effective for preventing incisional SSI in patients with thick subcutaneous fat in colorectal surgery [ 71 ].
In 2013, Kosins et al. [ 72 ] reviewed and analyzed 52 randomized controlled trials with a total of 6930 operations aimed to determine the evidenced-based value of prophylactic drainage of subcutaneous wounds in surgery. Subgroups were determined by specific surgical procedures or characteristics (cesarean delivery, abdominal wound, breast reduction, breast biopsy, femoral wound, axillary lymph node dissection, hip and knee arthroplasty, obesity, and clean-contaminated wound). There were 3495 operations in the drain group and 3435 in the no-drain group. Prophylactic subcutaneous drainage offered a statistically significant advantage only for the prevention of hematomas in breast biopsy procedures and the prevention of seromas in axillary node dissections. In all other procedures studied, drainage did not offer an advantage.
The authors concluded that drain placement following a surgical procedure is the surgeon’s choice and can be based on multiple factors beyond the type of procedure being performed or the patient’s body habitus [ 72 ].
All the previous studies assessed the usefulness of active-suctioning subcutaneous drain in a closed surgical wound. Numata et al. [ 73 ] decided to evaluate the efficacy of a passive drainage system for preventing surgical site infections during major colorectal surgery, enrolling 246 (124 underwent passive drainage, and 122 underwent no drainage) patients who underwent major colorectal surgery. Patients were randomly assigned to receive subcutaneous passive drainage or no drainage. The primary outcome measured was the incidence of superficial SSI. The secondary outcomes measured were the development of hematomas, seromas, and wound dehiscence.
They reported a significant difference in the incidence of superficial SSIs between patients assigned to the passive drainage and no drainage groups (3.2% vs 9.8%, respectively, P = 0.041). There were no cases that developed a hematoma, seroma, or wound dehiscence in either group. The authors concluded that subcutaneous passive drainage provides benefits over no drainage in patients undergoing major colorectal surgery.
The benefit of subcutaneous drainage was studied also in ileostomy closure that is in a dirty surgical field; after having conducted an RCT, Lauscher et al. [ 74 ] were able to affirm that the omission of subcutaneous suction drains is not inferior to the use of subcutaneous suction drains after ileostomy reversal in terms of length of hospital stay, surgical site infections, and hematomas/seromas.
In another RCT, the rate of SSI appeared to be reduced with subcutaneous suction drains in open abdominal surgery, but the authors concluded that prospective randomized larger-scale studies should be performed to confirm data [ 75 ].
Recently, Watanabe et al. [ 76 ] decided to evaluate the effects of subcutaneous closed-suction Blake drain for preventing SSIs after colorectal surgery performing an RCT, enrolling 240 patients. The incidence of incisional SSI was 8.7% in the overall patients. The incidence of incisional SSI was 12.8% in the control arm and 4.5% in the subcutaneous drainage arm. They reported a significant reduction of the incidence of SSI in the subcutaneous drainage arm than in the control arm ( P = 0.025). Logistic regression analysis demonstrated that thickness of subcutaneous fat > 3.0 cm, forced expiratory volume in 1 s as percent of forced vital capacity (FEV1.0%) > 70%, and subcutaneous drain were independent predictors of postoperative incisional SSIs ( P = 0.008, P = 0.004, and P = 0.017, respectively). The authors affirmed that a subcutaneous Blake drain is beneficial for preventing incisional SSIs in patients undergoing colorectal surgery [ 76 ].
Manzoor et al. [ 77 ] after reviewing the literature to assess the evidence on the efficacy of subcutaneous wound drainage in reducing SSI concluded that not all patients will benefit from subcutaneous drainage. Subcutaneous wound drainage seems to be useful in patients with high risk to develop an SSI including patients who are obese and/or have contaminated wounds but in clean and clean-contaminated surgical wounds, it remains a surgeon’s choice [ 77 ].
When is double gloving recommended? When is changing gloves recommended during an operation?
Statement 8.1: there are insufficient data to determine the role of double gloving to prevent ssi (gor 2b)., statement 8.2: the mechanical resistance of latex gloves depends on the duration of wear. it may be beneficial for surgical team members and their protection to change gloves at certain intervals during surgery [gor 2c]..
Surgical gloves are an important physical barrier between the surgical staff and the patient. They enable the prevention of transmission of microorganisms in both directions, from the surgeons’ hands to the patient.
The integrity of gloves depends on the duration of wearing, the role within the surgical team, and the type of surgery performed.
Their use since the beginning was a barrier against infections. With the recognition of HIV infection and the associated concerns about transmission of HBV and hepatitis C virus in the operating room during the 1980s and early 1990s, considerable interest emerged in the provision of better protection of the hands for surgical personnel [ 78 ].
The intact surgical glove is the most important barrier to the bi-directional migration of microorganisms between the hands of the members of a surgical team and the patient. Several studies have shown that undetected perforations of surgical gloves are common and that the frequency of such defects increases with the duration of glove wear. The risk of glove defects is related to the type of surgery being done, ranging from 7% in urologic surgery to 65% in cardiothoracic surgery [ 78 , 79 ].
Various measures have been developed to reduce the risk of surgical site contamination with microorganisms originating from the surgeon’s hands.
Standard practice for decreasing the microbial bio-burden on the hands of surgeons and other surgical team members is preoperative surgical hand disinfection with an antimicrobial soap (surgical scrub) or an alcohol-based hand disinfectant (surgical rub). Preoperative surgical hand disinfection can reduce, but not eradicate, the resident flora on the surgeon’s hands. Because of the re-growth of skin flora during a surgical procedure, original levels of skin flora on a surgeon’s hands can be re-established within 3–6 h, depending on the formulation of the product used to disinfect the hands [ 78 ].
A novel sterile antimicrobial surgical glove, featuring a proprietary complex coating with 14 ingredients and chlorhexidine as an active antimicrobial ingredient on its inner surface, has been developed to reduce the risk of contamination of the surgical site in the event of a glove breach. Further clinical studies are needed to confirm this concept [ 79 ].
Double gloving has been demonstrated to reduce blood contact with the hands of the operating team. Quebbeman and colleagues noted a nearly 90% reduction in hand exposure to blood with double gloving in a prospective, randomized trial [ 80 ]. Wearing two pairs of latex gloves significantly reduces the number of perforations to the innermost glove. This evidence comes from trials undertaken in “low-risk” surgical specialties. Wearing two pairs of latex gloves does not cause the glove wearer to sustain more perforations to their outermost glove. Wearing double latex indicator gloves enables the glove wearer to detect perforations to the outermost glove more easily than when wearing double latex gloves. However wearing a double latex indicator system will not assist with the detection of perforations to the innermost glove, nor reduce the number of perforations to either the outermost or the innermost glove. There is no direct evidence that additional glove protection worn by the surgical team reduces surgical site infections in patients; however, the most important published review has insufficient power for this outcome [ 81 ]..
The adequate protection, however, requires that the glove material remain intact. The electrical conductivity, insulation, and mechanical resistance of glove latex depend on the duration of wear. Latex is subject to hydration; 30 min of surgical use was associated with measurable hydration of glove latex and a statistically significant loss of electrical and mechanical resistance, with rupture load decreasing by 24% [ 82 ].
Parteke et al. prospectively collected 898 consecutive pairs of used surgical gloves over 9 months in a single institution and reported that wearing gloves for 90 min or less resulted in microperforations in 46 (15.4%) of 299 pairs of gloves, whereas wearing gloves for 91–150 min resulted in perforation of 54 (18.1%) of 299 pairs, and 71 of (23.7%) of 300 pairs were perforated when the duration of wear was longer than 150 min ( P = .05). Because of the increase in the rate of microperforation over time, authors recommended that surgeons, first assistants, and surgical nurses directly assisting in the operating field change gloves after 90 min of surgery [ 83 ].
Several studies demonstrated that the occurrence of microperforations in surgical gloves increases over time.
Even in orthopedic surgery, surgical gloves should be changed when they are excessively contaminated with surgical fluids and the surgeon and first assistant should also change their outer gloves at an average of every 90 min [ 84 ].
Glove perforation rates are high in open abdominal surgery; considering data available, it may be beneficial for surgical team members to change gloves at certain intervals during surgery or use indicator glove systems [ 84 ].
Is negative-pressure wound dressing useful to prevent surgical site infections? (Table 4 )
Statement 9: the application of negative-pressure wound therapy in preventing ssi may be effective in reducing postoperative wound complications and it may be an option, especially in patients with a high risk of ssi. (gor 2c)..
Gomoll et al. [ 93 ] first reported the application of negative-pressure wound therapy in closed incisions (cINPT), and their outcomes showed that its use for treating closed incisions in orthopedic surgery can reduce the incidence of SSI.
A subsequent series of reports [ 85 , 86 , 87 ] confirmed the effectiveness of cINPT in reducing SSI.
In 2015, Sandy-Hodgetts et al. [ 88 ] decided to conduct a systematic review and meta-analysis of all papers available from 1990 to 2013 evaluating the effectiveness of cINPT in preventing postoperative surgical wound complications. Eight studies were included in the review. Meta-analyses revealed a statistically significant difference in favor of the use of cINPT as compared with standard surgical dressings in managing SSI, but conflicting results were found for wound dehiscence and seroma. Considering the small number of studies included and that most of them were retrospective comparative cohort in design, authors could not recommend cINPT to prevent SSI even if the study demonstrated an association between the use of cINPT and reduction of SSI.
A more recent meta-analysis by Strugala et al. [ 89 ] investigated the effectiveness of prophylactic use of a specific design of cINPT device on surgical site complications. The authors considered all articles comparing the specific single-use cINPT device (PICO) with standard care for SSI in closed surgical wounds. Ten randomized and 6 observational studies were selected with a total of 1863 patients (2202 incisions) included. The randomized studies reported a significant reduction in SSI rate of 51% from 9.7 to 4.8% with cINPT intervention (RR 0.49 [95% CI 0.34–0.69] P < 0.0001). The observational studies assessed a reduction in SSI rate of 67% from 22.5 to 7.4% with cINPT (RR 0.32 [95% CI 0.18–0.55] P < 0.0001). Pooling all the data, there was a significant reduction in SSI of 58% from 12.5 to 5.2% with cINPT (RR 0.43 [95% CI 0.32–0.57] P < 0.0001) regardless of the type of surgery (orthopedic, abdominal, colorectal, or cesarean section), although the numbers needed to treat were lower in operations with higher frequencies of complications. Furthermore, meta-analysis showed a significant reduction in dehiscence from 17.4 to 12.8% with cINPT (RR 0.71 [95% CI 0.54–0.92] P < 0.01) and in-hospital length of stay by cINPT (− 0.47 days [95% CI − 0.71 to − 0.23] P < 0.0001).
Another meta-analysis carried out by Sahebally et al. [ 90 ] in 2018 evaluated the association of prophylactic cINPT with SSI rates in general and colorectal surgery in elective and emergency settings.
Three randomized trials and 2 prospective and 4 retrospective studies were selected for the meta-analysis, involving 1187 patients with 1189 incisions. The authors found significant clinical and methodologic heterogeneity among the studies. On random-effects analysis, cINPT was associated with a significantly lower rate of SSI compared with standard dressings (pooled odds ratio [OR], 0.25; 95% CI, 0.12–0.52; P < .001) but no difference in rates of seroma (pooled OR, 0.38; 95% CI, 0.12–1.23; P = .11) or wound dehiscence (pooled OR, 2.03; 95% CI, 0.61–6.78; P = 0.25). On sensitivity analysis, focusing solely on colorectal procedures, cINPT significantly reduced SSI rates (pooled OR, 0.16; 95% CI, 0.07–0.36; P < .001). Thus, this study demonstrated that the application of cINPT on closed laparotomy wounds in general and in colorectal surgery is associated with reduced SSI rates but no different significant rates of seroma and wound dehiscence compared with traditional dressings.
Readership expressed some criticisms about the clinical value of these outcomes considering the high level of statistical heterogeneity associated with the included studies in the discussion and the necessity for randomized controlled trials before recommending the application of cINPT in clinical practice.
Uncertainty in the indications for the use of cINPT had been reported in 2012 [ 91 ] and then confirmed in 2014 [ 92 ] and the updated 2019 [ 94 ] version of the Cochrane systematic review. In the last systematic review, despite the addition of 25 trials, the authors judged the evidence to be low or very low certainty for all outcomes.
The study involved 2957 participants (30 intervention trials and two economic studies nested in trials). Surgeries included abdominal and colorectal ( n = 5); cesarean sections ( n = 5); knee or hip arthroplasties ( n = 5); groin surgery ( n = 5); fractures ( n = 5); laparotomy ( n = 1); vascular surgery ( n = 1); sternotomy ( n = 1); breast reduction mammoplasty ( n = 1); and mixed ( n = 1). Webster et al. showed uncertainty about whether cINPT compared with a standard dressing reduces or increases the incidence of important outcomes such as mortality, dehiscence, and seroma or if it increases costs. Given the cost and widespread use of cINPT for SSI prophylaxis, authors claimed an urgent need for larger, well-designed and well-conducted trials to evaluate the effects of newer cINPT products designed for use on clean, closed surgical incisions.
Several studies investigated the role of cINPT in contaminated and dirty surgical wounds.
Danno et al. [ 95 ] prospectively included in their study 28 patients undergoing abdominal surgery for peritonitis caused by a lower-gastrointestinal perforation. They compared data from this group with a 19 patients historical control group who had undergone primary suturing for managing peritonitis incisions for a lower-gastrointestinal perforation. Authors reported a significant association between the SSI incidence and the type of incision management (10.7% with cINPT and delayed closure vs. 63.2% with primary suturing; P < 0.001); no significant difference between the groups in the length of the hospital stay (22 days for cINPT and delayed closure vs. 27 days for primary suturing; P = 0.45) was found.
Therefore, the association of cINPT and delayed closure of the abdominal wall is an effective method to prevent SSI.
A Spanish group [ 96 ] decided to compare outcomes about three techniques used for wound management after laparotomy in contaminated and dirty/infected wounds: the primary, delayed primary, and vacuum-assisted closures in terms of SSI. Eighty-one patients undergone laparotomy with Class III or IV surgical wounds were enrolled in a three-arm randomized prospective study. Twenty-seven patients received primary closure, 29 delayed primary closure, and 25 vacuum-assisted closure, with no exclusions for analysis. Surgical site infection was present in 10 (37%) patients treated with primary closure, 5 (17%) with primary delayed closure, and 0 (0%) patients receiving vacuum-assisted closure. Statistical significance was found between infection rates of the vacuum-assisted group and the other two groups. No significant difference was found between the primary and primary delayed closure groups. The infection rate in contaminated/dirty-infected laparotomy wounds decreases from 37 and 17% with primary and delayed closures, respectively, to 0% with vacuum-assisted systems [ 96 ]. We have to consider that in this study the number of patients is very small for each group.
Several studies evaluated the cost-utility of cINPT in preventing SSIs compared to standard dressings and demonstrated that the use of closed-incision negative-pressure therapy is cost-saving following the closure of abdominal incisions in high-risk patients [ 97 , 98 , 99 ].
Furthermore, to obviate the high costs related to current equipment for cINPT, more cost-effective alternatives were developed using standard gauze sealed with an occlusive dressing and wall suction. Several studies comparing both methods of treatment appear to be similarly effective for reducing wound surface area and volume [ 94 , 100 , 101 ].
Is intraoperative normothermia useful to prevent surgical site infections?
Statement 10.1: intraoperative normothermia decreases the rate of ssi (gor 1a)., statement 10.2: the use of active warming devices in operating room is useful to keep normothermia and reduce ssi (gor 1b)..
Core body temperature is kept in a narrow range by several mechanisms, namely heat genesis and thermal insulation (mainly vasoconstriction or dilatation). This balance is greatly challenged during major surgery. On the one hand, surgery may imply exposure of large surface areas with consequent loss of heat and fluids. On the other hand, anesthesia disrupts the temperature setpoint (i.e., a lower than usual temperature triggers an adaptive reflex as shivering or metabolic thermogenesis) and can increase heat loss by vasodilatation [ 102 ]. Animal studies have shown that hypothermia increases complications such as infection, myocardial infarction, and coagulation derangements. Perioperative hypothermia can increase SSI due to its reflex vasoconstriction and mediated local immunosuppression. Vasoconstriction reduces partial oxygen pressure which lowers resistance to infections in animal models [ 103 ].
Perioperative normothermia has been addressed by several studies, papers, and meta-analysis. Considering only RCTs, the subsequent comparisons, but not limited to them, have been evaluated: head-to-head RCTs of one active warming device vs another, different extension of the active warming period through the perioperative one, active warming device vs no warming, warming of fluids and or insufflation gases during laparoscopic vs no active warming. We decided to focus on RCTs comparing interventions aimed at preventing hypothermia vs a control group where no such an intervention was implemented (a placebo group), the outcome was the incidence of SSI. Four relevant papers were analyzed [ 104 , 105 ]. All of them dealt with an active body warming device against the placebo.
Kurz et al. [ 105 ] in 1996 randomized 200 patients scheduled for major abdominal contaminated surgery to receive active body surface warming by a forced-air warmer device. The incidence of SSI was 6/104 in the intervention group and 18/96 in the control one ( P = 0.009).
Melling et al. [ 106 ] in 2001 randomized 421 patients scheduled for clean surgery into three arms placebo, local warming (non-contact, radiant heat dressing), and systemic warming (forced-air warming device). Pooling the data of the two intervention groups, the incidence of SSI was 19/139 in the placebo group vs 13/277 in the intervention group ( P = 0.001).
Pu et al. [ 107 ] in 2014 randomized 110 patients scheduled for laparoscopic gastrointestinal procedure into placebo group vs systemic warming (disposable underbody warming blanket with reusable forced-air warming system). The incidence of SSI was 0 in both the intervention and control groups.
Yi et al. [ 104 ] in 2018 randomized, in an open-label, pilot study 62 patients scheduled for open thoracic or hip replacement surgery to systemic warming (forced-air warming device) vs control (quilt). The incidence of SSI was 0/32 in the control group and 3/30 in the warming group ( P = 0.238).
The effectiveness of temperature measurement in preventing SSIs has been assessed in a large cohort 2013 study in the colonic surgery population [ 108 ]. Several meta-analyses have been published on the topic. A recent Cochrane review from Madrid et al. [ 106 ] reviewed the literature and found a significant decrease in SSI after the implementation of an active warming intervention (risk ratio (RR) 0.36, 95% confidence interval (CI) 0.20 to 0.66; P = 0.0008; I 2 = 0%); the studies were rated of fair quality. Another meta-analysis reached the same conclusions [ 106 ]. There exists little debate around the effectiveness of reducing SSI by keeping the patients normothermic throughout the perioperative period. Four RCTs [ 100 , 101 , 102 , 103 ] and at least two meta-analyses [ 109 , 110 ] confirm this risk reduction. It seems unlikely that other RCTs comparing a device to keep normothermia will be compared with a placebo group as this recommendation has been implemented in several national and international guidelines [ 111 , 112 , 113 , 114 ]. The last two RCTs [ 104 , 107 ] with a real placebo group have been carried out in a nation where it is not common practice to warm patients during surgery. Those studies [ 100 , 103 ] were meant to be pilot studies to assess the feasibility of forced-air warming in that context.
The two open questions are which device and/or strategy should be used and when (only intraoperative or intraoperative and pre- and/or postoperative?). There are three main devices to warm up the patients: forced-air warming (so far the most studied and used worldwide), resistive polymer fabric warming, and circulatory warming systems using a closed fluid circuit. The use of radiant heating systems is considered feasible only during pediatric procedures. On the other side, other strategies have been implemented to reduce heat loss and prevent hypothermia (e.g., warm iv infusion, warm irrigation fluids or gases for pneumoperitoneum during laparoscopic, preoperative infusion of nutrients to increase metabolic rate and protein turn-over, reflective blankets). A thorough evaluation of those questions is outside the statement. The majority of those studies has as main outcome the achievement of normothermia and were not powered enough to detect a difference in SSI. To date, Madrid et al. [ 109 ] evaluated in their meta-analysis the studies comparing head-to-head the different modality to warm up the patients and found no differences in SSI incidence. The main concern is the use of forced-air warming devices in surgery where air-borne pathogens are a major threat to orthopedic prosthesis surgery. In this particular scenario, the surgery takes place under the condition of ultra clean ventilation, at least in affluent countries, and it is known that forced-air disrupt the laminar flow and increases a load of bacteria at the operation site (in lab models). The bacterial load is the main risk factor for prosthesis colonization [ 115 ]. A systematic review is available but results are inconclusive [ 116 ]. Anyway, this hypothesis has not been formally tested in an adequately powered RCT.
The timing of warming has been evaluated in several papers. Pre-emptive warming plus intraoperative warming has shown better results in providing normothermia than intraoperative warming alone in small RCTs [ 117 , 118 , 119 ] and in a systematic meta-analysis [ 120 ]. Heterogeneity between the studies is high as well as the results from the single trials and the meta-analysis was not conclusive.
Several guidelines from national and international institutions stated in favor of achieving normothermia in the perioperative period to reduce the incidence of SSI [ 111 , 112 , 113 , 114 ].
Is perioperative supplemental oxygen effective to reduce SSI?
Statement 11: perioperative hyperoxygenation does not reduce ssi (gor 2b)..
The most important defense against SSI is oxidative killing by neutrophils, and molecular oxygen is the substrate of the process. The easiest way to increase tissue oxygenation is to increase inspired oxygen. For example, intraoperative tissue oxygen partial pressure is typically about 6.6 kPa in patients given 30% inspired oxygen and about13.3 kPa in those given 80% inspired oxygen [ 121 ].
Despite some early evidence [ 121 ], there have since been conflicting results from numerous randomized clinical trials.
Two well-conducted randomized trials ( n = 500 and n = 300) [ 121 , 122 ], a smaller trial [ 123 ] and a registry analysis [ 124 ], suggested that supplemental oxygen (80% vs 30%) halved infection risk, supporting the role of supplemental oxygen in reducing the risk of SSI. However, other studies have not been able to confirm this.
The PROXI trial [ 125 ], that is a large, multicenter, randomized trial involving 1400 patients undergoing abdominal surgery, found no evidence of any beneficial effect of supplemental oxygen; in fact, SSI occurred in 131 of 685 patients (19%) receiving 80% oxygen and in 141 of 701 (20%) receiving 30% oxygen [odds ratio 0.94 (95% confidence interval 0.72–1.22), P = 0.64]. Indeed, a long-term follow-up study (median 2.3 years after surgery) found poorer survival in the supplemental oxygen group [ 126 ].
Another recently published randomized, blinded trial including 400 patients [ 127 ] tested the hypothesis that extending intraoperative supplemental oxygen 12 to 16 h into the postoperative period reduces the risk of SSI and healing-related complications in the morbidly obese patients and reported no benefit of supplemental oxygen.
In 2018, Cohen et al. [ 128 ] published a meta-analysis including 26 trials with a total of 14,710 patients, to investigate the effect. The RR [95%CI] for wound infection was 0.81 [0.70, 0.94] in the high vs. low inspired oxygen groups. The effect remained significant in colorectal patients (10,469 patients), 0.79 [0.66, 0.96], but not in other patients (4,241 patients), 0.86 [0.69, 1.09]. When restricting the analysis to studies with low risk of bias, either by strict inclusion criteria (5047 patients) or by researchers’ judgment (12,547 patients), no significant benefit remained: 0.84 [0.67, 1.06] and 0.89 [0.76, 1.05], respectively. The authors concluded that meta-analysis of the most reliable studies does not suggest that supplemental oxygen substantively reduces wound infection risk when considering all available data, but more research is needed to fully answer this question.
Whether supplemental oxygen, which is inexpensive and easy to provide, reduces infection risk, thus remains in dispute.
Leaving the skin open for delayed primary closure can reduce SSI?
Statement 12.1: delayed primary skin closure may reduce the incidence of ssi (gor2c)., statement 12.2: delayed primary closure of a surgical incision is an option to take into consideration in contaminated abdominal surgeries in high-risk patients (gor 2c)..
Delayed primary closure of dirty wounds has been widely practiced in war surgery; it is a procedure which aims to reduce the rate of SSI by suturing a wound later after proper dressing, considering the fundamental principles of decreasing bacterial inoculums and potentiating local wound resistance from increasing wound oxygenation and blood supply from developing granulation tissue. It was first applied to traumatic wounds and later was more widely applied to various types of operations with the demonstration of good efficacy [ 129 , 130 , 131 ].
These results were mainly from observational studies that may be prone to selection and confounding biases.
Besides, the delayed primary closure also has its disadvantages including pain from routine dressing, the necessity for later wound suturing, and increase the cost of treatments [ 129 , 130 , 131 , 132 ].
In 2013, Bhangu et al. [ 132 ] decided to determine using meta-analysis whether delayed primary skin closure of contaminated and dirty abdominal incisions reduces the rate of SSI compared with primary skin closure.
The authors included in the final analysis 8 studies randomizing 623 patients with contaminated or dirty abdominal wounds to either delayed primary skin closure or primary closure. The most common diagnosis was appendicitis (77.4%), followed by perforated abdominal viscus (11.5%), ileostomy closure (6.5%), trauma (2.7%), and intra-abdominal abscess/other peritonitis (1.9%). The time to the first review for delayed primary skin closure was provided at between 2 and 5 days postoperatively. All studies were found to be at high risk of bias, with marked deficiencies in study design and outcome assessment. When SSI was assessed across all studies using a fixed-effect model, delayed primary skin closure significantly reduced the chance of SSI (odds ratio, 0.65; 95% CI, 0.40–0.93; P = .02). However, heterogeneity was high (72%), and using a random-effects model, the effect was no longer significant (odds ratio, 0.65; 95% CI, 0.25–1.64; P = .36).
The authors concluded that delayed primary skin closure may reduce the rate of SSI, but current trials fail to provide definitive evidence.
In 2014, Siribumrungwong et al. [ 133 ] decided to investigate the same topic carrying out a systematic review and meta-analysis to compare SSI between delayed primary and primary wound closure in complicated appendicitis and other contaminated abdominal wounds. Eight studies were considered for meta-analysis: 5 studies were done in complicated appendicitis, 2 with mixed complicated appendicitis and other types of abdominal operation, and 1 with ileostomy closure. Most studies (75%) had a high risk of bias in sequence generation and allocation concealment. Among 6 RCTs of complicated appendicitis that underwent open appendectomy, the SSI between primary closure and delayed primary closure were not significantly different with a risk ratio of 0.89 (95% CI, 0.46, 1.73). Delayed primary closure had significantly 1.6 days (95% CI: 1.41, 1.79) longer length of stay than primary closure.
Based on a small number of studies with low-quality, a meta-analysis suggested there might be no advantage of delayed primary closure over primary closure in reducing SSI in complicated appendicitis.
After this meta-analysis, Siribumrungwong et al. [ 134 ] carried out a multicenter randomized controlled trial to compare superficial SSI rates between delayed primary wound closure and primary wound closure for complicated appendicitis.
The study enrolled and randomized 300 and 298 patients with gangrenous and ruptured appendicitis to primary closure and delayed primary closure (at postoperative days 3–5) groups.
The superficial SSI rate was lower in the primary closure than in delayed primary closure groups [i.e., 7.3% (95% confidence interval 4.4, 10.3) vs 10% (95% CI 6.6, 13.3)] with a risk difference (RD) of − 2.7% (− 7.1%, 1.9%), but this RD was not significant. Postoperative pain, length of stay, recovery times, and quality of life were nonsignificantly different with corresponding RDs of 0.3 (− 2.5, 3.0), − 0.1 (− 0.5, 0.3), − 0.2 (− 0.8, 0.4), and 0.02 (− 0.01, 0.04), respectively. However, costs for primary closure were 2083 (1410, 2756) cheaper than DPC ($60 USD).
The authors showed that superficial SSI rates for the primary closure group were slightly lower than the delayed group, even if there is no statistical significance. Costs were significantly lower for the primary closure group.
Recently, Tang et al. [ 135 ] published a meta-analysis about the benefits of a delayed primary closure over primary closure of a surgical incision in contaminated abdominal surgery.
Of the 12 studies included in the analysis, 5 were from third world countries (i.e., India and Pakistan), and all of these demonstrated an improvement in the SSI rate with delayed primary closure. When the fixed-effect model was used, compared with primary closure, SSI was significantly reduced in delayed primary closure with a risk ratio of 0.64 (0.51–0.79) ( P < 0.0001), and a significant difference in LOS between delayed primary closure and primary closure was also identified with a mean difference of 0.39 (0.17–0.60) ( P = 0.0004). Although the random-effect model was used, no significant difference in SSI between delayed and primary closure was observed with a risk ratio of 0.65 (0.38–1.12) ( P = 0.12), and no significant difference in LOS with a mean difference of 1.19 (− 1.03 to 3.41) ( P = 0.29).
The authors suggested that delayed primary closure may be the preferable choice in contaminated abdominal surgeries, especially in patients with a high risk of infection, and particularly in resource-constrained environments, even if more high-quality studies are needed to provide clear evidence.
When should additional antibiotic doses be administered intraoperatively?
Statement 13: optimal knowledge and use of the pharmacokinetic/pharmacodynamic characteristics of antibiotics are important to evaluate when additional antibiotic doses should be administered intraoperatively in patients with intra-abdominal infections undergoing emergency surgery (gor 1c)..
Optimal use of the pharmacokinetic/pharmacodynamic characteristics of antibiotics is helpful to evaluate when additional antibiotic doses should be administered intraoperatively in patients with intra-abdominal infections undergoing emergency surgery.
Antibiotics should be used after a treatable intra-abdominal infection (IAI) has been recognized or there is a high degree of suspicion of infection. Initial antimicrobial therapy for patients with IAI should be prompt because especially critically ill patients need immediate treatment. It may be interesting to evaluate when additional antibiotic doses should be administered intraoperatively in patients with intra-abdominal infections undergoing emergency surgery.
To define how to administrate antibiotics in patients with IAIs, it is necessary to know the pharmacokinetic/pharmacodynamic relationship of antibiotics. Knowledge of the pharmacokinetic and pharmacodynamic antibiotic properties may provide a more rational determination of optimal dosing regimens in terms of the dose and the dosing interval [ 136 ].
Antibiotic pharmacodynamics integrates the complex relationship between organism susceptibility and patient pharmacokinetics. Pharmacokinetics describes the fundamental processes of absorption, distribution, metabolism, and elimination and the resulting concentration-versus-time profile of an agent administered in vivo. The achievement of appropriate target site concentrations of antibiotics is essential to eradicate the pathogens [ 136 ]. Suboptimal target site concentrations may have important clinical implications and may explain therapeutic failures, in particular, for bacteria for which in vitro MICs are high. During the operation, target site concentrations should remain steadily optimal.
Dosing frequency is related to the concept of time-dependent versus concentration-dependent killing. Beta-lactam agents exhibit time-dependent activity and exert optimal bactericidal activity when drug concentrations are maintained above the MIC [ 137 ]. Therefore, the serum concentration must exceed the MIC for the appropriate duration of the dosing interval. Higher-frequency dosing, prolonged infusions, and continuous infusions have been utilized to achieve this effect. It is well known that for beta-lactams, prolonged or continuous infusions have been advocated to maximize the time that the drug concentration exceeds the MIC, whereas high peak concentrations are not beneficial. This concept should be extended also to patients undergoing an emergency operation and higher-frequency dosing, prolonged infusions, and continuous infusions should be suggested also in the operatory room.
In contrast, antibiotics such as aminoglycosides exhibit concentration-dependent activity and should be administered in a once-daily manner (or with the least possible number of daily administrations) to achieve high peak plasma concentrations [ 137 ].
With these agents, the peak serum concentration, and not the time the concentration remains above the MIC, is more closely associated with efficacy. In these patients, additional doses are not necessary during operation.
We conceived this position paper to offer an extensive overview of available evidence regarding OR prevention of surgical site infection in emergency surgery as a potential addendum to WSES guidelines on the management of intra-abdominal infections.
The use of triclosan-coated suture significantly reduces SSI prevalence compared with the non-coated sutures.
The use of wound protectors has protective effects in reducing incisional SSI, in particular, the use of dual-ring constructed wound protectors appears to be superior to single-ring devices in preventing SSI.
The application of negative-pressure wound therapy in preventing SSI may be effective in reducing postoperative wound complications and it may be an option to take into consideration especially in patients with a high risk of infection.
Intraoperative normothermia decreases the rate of SSI, and the use of active warming devices in the operating room is useful to keep normothermia.
Perioperative supplemental oxygenation does not reduce SSI.
There is no strong evidence that delayed primary skin closure may reduce the incidence of SSI but it may be a valid option to primary skin closure in highly contaminated or “dirty” abdominal operations, especially in patients at high risk of infection.
The optimal knowledge and use of the pharmacokinetic/pharmacodynamic characteristics of antibiotics are important to evaluate when additional antibiotic doses should be administered intraoperatively in patients with intra-abdominal infections undergoing emergency surgery.
Availability of data and materials
Not applicable.
Change history
14 april 2021.
A Correction to this paper has been published: https://doi.org/10.1186/s13017-021-00361-4
Abbreviations
Closed-incision negative-pressure therapy
Negative-pressure wound therapy
Observational trial(s)
- Operating room
Randomized controlled trial(s)
Steering committee
Scientific secretary
Surgical site infection(s)
Al-Dabbagh MA, Dobson S. The evidence behind prophylaxis and treatment of wound infection after surgery. Adv Exp Med Biol. 2013;764:141-150. Review.
Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;20(11):725–30.
CAS PubMed Google Scholar
de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB.Surgical site infection: incidence and impact on hospital utilization and treatment costs.Am J Infect Control 2009;37(5):387-397. doi: https://doi.org/10.1016/j.ajic.2008.12.010 . Epub 2009 Apr 23.
Anderson DJ, Podgorny K, Berrios-Torres SI, et al. Strategies to prevent surgical site infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:605e627.
Google Scholar
Bratzler DW, Dellinger EP, Olsen KM, et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195e283.
Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999;20:247e278.
Magill SS, Edwards JR, Bamberg W, et al. Multistate point prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198e1208.
Murray BW, et al. The impact of surgical site infection on the development of incisional hernia and small bowel obstruction in colorectal surgery. Am J Surg. 2011;202(5):558–60.
PubMed Google Scholar
Kazuhiro Imamura, Kensuke Adachi, Ritsuko Sasaki, Satoko Monma, Sadaaki Shioiri, Yasuji Seyama, Masaru Miura, Yoshihiko Morikawa, Tetsuji Kaneko. 2016. Randomized comparison of subcuticular sutures versus staples for skin closure after open abdominal surgery: a multicenter open-label randomized controlled trial. J Gastrointest Surg 2016; 20:2083208330.
Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, Pittet D. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228–41. https://doi.org/10.1016/S0140-6736(10)61458-4 .
Article PubMed Google Scholar
Horan TC, Culver DH, Gaynes RP, et al. Nosocomial infections in surgical patients in the United States. January 1986-June 1992. National Nosocomial Infections Surveillance (NNIS) System. Infect Control Hosp Epidemiol. 1993;14:73993.
Le Huu NR, Mege D, Quaissi M, et al. Incidence and prevention of incisional hernia. J Visc Surg. 2012;149:e39–n.
Alfonso JL, Pereperez SB, Canoves JM, et al. Are we really seeing the total costs of surgical site infections? A Spanish study. Wound Repair Regen. 2007;15:4745–474.
Perencevich EN, Sands KE, Cosgrove SE, et al. Health and economic impact of surgical site infections diagnosed after hospital discharge. Emerg Infect Dis. 2003;9:1969–196.
Cruse PJE, Foord R. A five-year prospective study of 23, 649 surgical wounds. Arch Surg. 1973;107:2067–206.
Tsujinaka T, Yamamoto K, Fujita J, Endo S, Kawada J, Nakahira S, Shimokawa T, Kobayashi S. Subcuticular sutures versus staples for skin closure after open gastrointestinal surgery: a phase 3, multicentre, open-label, randomised controlled trial. Lancet. 2013;382:1105–382.
Brozek JL, Akl EA, Compalati E, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 3 of 3. The GRADE approach to developing recommendations. Allergy. 2011;64(8):1109109reco doi: https://doi.org/10.1111/j1398-9995.2009.02083.x . [PubMed] [CrossRef].
McLean NR, Fyfe AH, Flint EF, Irvine BH, Calvert MH. Comparison of skin closure using continuous and interrupted nylon sutures. Br J Surg. 1980;67:633–5.
Gurusamy KS, Toon CD, Allen VB, Davidson BR. Continuous versus interrupted skin sutures for non-obstetric surgery. Cochrane Database Syst Rev 2014;(2):CD010365. doi: https://doi.org/10.1002/14651858.CD010365.pub2 . Review.
Hopkinson GB, Bullen BR. Removable subcuticular skin suture in acute appendicitis: a prospective comparative clinical trial. Br Med J (Clin Res Ed). 1982;284:869.
CAS Google Scholar
Anatol TI, Roopchand R, Holder Y, Shing HG. A comparison of the use of plain catgut, skin tapes and plygalactin stures for skin closure: a prospective clinical trial. J R Coll Surg Edinb. 1997;42:124–7.
Pauniaho SL, Lahdes-Vasama T, Helminen MT, Iber T, Makela E, Pajulo O. Non-absorbable interrupted versus absorbable continuous skin closure in pediatric appendectomies. Scand J Surg. 2010;99:142–6.
Kataluoto S, Pauniaho SL, Helminen M, Kuokkanen H, Rantanen T. Wound healing after open appendectomies in adult patients: a prospective, randomised trial comparing two methods of wound closure. World J Surg. 2012;36:2305–10.
Okubo S, Gotohda N, Sugimoto M, Nomura S, Kobayashi S, Takahashi S, Hayashi R, Konishi M. Abdominal skin closure using subcuticular sutures prevents incisional surgical site infection in hepatopancreatobiliary surgery. Surgery. 2018;164(2):251–6.
Kobayashi S, Ito M, Yamamoto S, Kinugasa Y, Kotake M, Saida Y, Kobatake T, Yamanaka T, Saito N, Moriya Y. Randomized clinical trial of skin closure by subcuticular suture or skin stapling after elective colorectal cancer surgery. Br J Surg. 2015;102:495–500.
Matl FD, Zlotnyk J, Obermeier A, Friess W, Vogt S. Bcular suture or skin STTI-infective coatings of surgical sutures based on a combination of antiseptics and fatty acids. J Biomater Sci Polym Ed. 2009;20(10):1439–49. https://doi.org/10.1163/092050609X12457418973107 .
Article CAS PubMed Google Scholar
Custis T, Armstrong AW, King TH, Sharon VR, Eisen DB. Effect of adhesive strips and dermal sutures vs dermal sutures only on wound closure: a randomized clinical trial. JAMA Dermatol. 2015;151(8):862–7. https://doi.org/10.1001/jamadermatol.2015.0174 .
Article PubMed PubMed Central Google Scholar
Dumville JC, Coulthard P, Worthington HV, Riley P, Patel N, Darcey J, Esposito M, van der Elst M, van Waes OJF. Tissue adhesives for closure of surgical incisions. Cochrane Database Syst Rev. 2014;11:CD004287. https://doi.org/10.1002/14651858.CD004287.pub4 .
Article Google Scholar
Obermeier A, Schneider J, Wehner S, Matl FD, Schieker M, von Eisenhart-Rothe R, et al. Novel high efficient coatings for anti-microbial surgical sutures using chlorhexidine in fatty acid slow-release carrier systems. PLoS One. 2014;9(7):e101426. https://doi.org/10.1371/journal.pone.0101426 .
Article CAS PubMed PubMed Central Google Scholar
Park JH, Kim JK, et al. Comparison of intraoperative handling and wound healing between (NEOSORBow-release ccoated polyglactin 910 suture NEOSORB®): a prospective, single-blind, randomized controlled trial. BMC Surg. 2018;18(1):45. https://doi.org/10.1186/s12893-018-0377-4 .
Wu X, Kubilay NZ, Ren J, Allegranzi B, Bischoff P, Zayed B, Pittet D, Li J. Antimicrobial-coated sutures to decrease surgical site infections: a systematic review and meta-analysis. Eur J Clin Microbiol Infect Dis. 2017;36(1):19–32. https://doi.org/10.1007/s10096-016-2765-y Epub 2016 Sep 2. Review. Erratum in: Eur J Clin Microbiol Infect Dis. 2018 Oct;37(10):2031-2034.
Uchino M, Mizuguchi T, Ohge H, et al. The efficacy of antimicrobial-coated sutures for preventing incisional surgical site infections in digestive surgery: a systematic review and meta-analysis. J Gastrointest Surg. 2018;22(10):1832–41. https://doi.org/10.1007/s11605-018-3832-8 Epub 2018 Jun 20.
Guo J, Pan LH, Li YX, Yang XD, Li LQ, Zhang CY, Zhong JH. Efficacy of triclosan-coated sutures for reducing risk of surgical site infection in adults: a meta-analysis of randomized clinical trials. J Surg Res. 2016;201(1):105–17. https://doi.org/10.1016/j.jss.2015.10.015 Epub 2015 Oct 23.
Henriksen NA, Deerenberg EB, Venclauskas L, Fortelny RH, Garcia-Alamino JM, Miserez M, Muysoms FE. Triclosan-coated sutures and surgical site infection in abdominal surgery: the TRISTAN review, meta-analysis and trial sequential analysis. Hernia. 2017;21(6):833–41. https://doi.org/10.1007/s10029-017-1681-0 Epub 2017 Oct 17. Review.
Diener MK, Knebel P, Kieser M, Schuler P, Schiergens TS, Atanassov V, Neudecker J, Stein E, Thielemann H, Kunz R, von Frankenberg M, Schernikau U, Bunse J, Jansen-Winkeln B, Partecke LI, Prechtl G, Pochhammer J, Bouchard R, Hodina R, Beckurts KT, Leissner L, Lemmens HP, Kallinowski F, Thomusch O, Seehofer D, Simon T, Hyhlik-Durr A, Seiler CM, Hackert T, Reissfelder C, Hennig R, Doerr-Harim C, Klose C, Ulrich A, Buchler MW (2014) Effectiveness of triclosan-coated PDS Plus versus uncoated PDS II sutures for prevention of surgical site infection after abdominal wall closure: the randomised controlled PROUD trial. Lancet 384(9938):142(9938):142 abdominal wall c16/S0140-6736(14)60238-5.
Justinger C, Slotta JE, Ningel S, Graber S, Kollmar O, Schilling MK. Surgical-site infection after abdominal wall closure with triclosan-impregnated polydioxanone sutures: results of a randomized clinical pathway facilitated trial (NCT00998907). Surgery. 2013;154(3):589ry–154 https://doi.org/10.1016/j.surg.2013.04.01 .
Ruiz-Tovar J, Alonso N, Morales V, Llavero C (2015) Association between triclosan-coated sutures for abdominal wall closure and incisional surgical site infection after open surgery in patients presenting with fecal peritonitis: a randomized clinical trial. Surg Infect 16(5):588(5):58816(5):588:588riclosan-coated sutures.
Mingmalairak CUP, Paocharoen V (2009) Efficacy of antimicrobial coating suture coated polyglactin 910 with triclosan (Vicryl Plus) compared with polyglactin 910 (Vicryl) in reduced surgical site infection of appendicitis, double blind randomized control trial, preliminary safety report. J Med Assoc Thail 92(6):770):77.
Konstantelias AA, Andriakopoulou CS, Mourgela S. Triclosan-coated sutures for the prevention of surgical-site infections: a meta-analysis . Acta Chir Belg. 2017;117(3):137-148. doi: https://doi.org/10.1080/00015458.2017.1287396 . Epub 2017 Feb 9. Review.
Darvin VV, Lobanov DS, Krasnov EA, Gvozdetsky AN. Assessment of the effectiveness of the suture with triclosan coated in emergency surgery. Khirurgiia (Mosk). 2017;3:70–5. https://doi.org/10.17116/hirurgia2017370-75 .
Fonnes S, Holzknecht B, Arpi M, Rosenberg J. Characterisation and safety of intraperitoneal perioperative administration of antibacterial agents: a systematic review. Drug Research. 2017;67(12):688l per.
Tolhurst Cleaver CL, Hopkins AD, Kee Kwong KC, Raftery AT. The effect of postoperative peritoneal lavage on survival, peritoneal wound healing and adhesion formation following fecal peritonitis: an experimental study in the rat. Br J Surg. 1974;61(8):601–4.
Sortini D, Feo CV, Maravegias K, Carcoforo P, Pozza E, Liboni A, Sortini A. Role of peritoneal lavage in adhesion formation and survival rate in rats: an experimental study. J Investig Surg. 2006;19(5):291–7.
Jallouli M, Hakim A, Znazen A, Sahnoun Z, Kallel H, Zghal K, Hammami A, Mhiri R. Rifamycin lavage in the treatment of experimental intra-abdominal infection. J Surg Res. 2009;155(2):191–4.
Wang XC, Gui CQ, Zheng QS. Combined therapy of allantoin, metronidazole, dexamethasone on the prevention of intra-abdominal adhesion in dogs and its quantitative analysis. World J Gastroenterol. 2003;9(3):56868):5.
Kayaoglu HA, Ozkan N, Yenidogan E, Koseoglu RD. Effect of antibiotic lavage in adhesion prevention in bacterial peritonitis. Ulus Travma Acil Cerrahi Derg. 2013;19(3):189189A.
Tetikcok R, Kayaoglu HA, Ozsoy Z, Yenidogan E, Ozkan N, Celik A, Sahin S, Ersoy OF. The effect of peritoneal prednisolone lavage in bacterial peritonitis: an experimental study. Wounds. 2016;28(10):354–9.
Norman G, Atkinson RA, Smith TA, Rowlands C, Rithalia AD, Crosbie EJ, Dumville JC. Intracavity lavage and wound irrigation for prevention of surgical site infection. Cochrane Database Syst Rev. 2017;10:CD012234.
Mueller TC, Loos M, Haller B, Mihaljevic AL, Nitsche U, Wilhelm D, Friess H, Kleeff J, Bader FG. Intra-operative wound irrigation to reduce surgical site infections after abdominal surgery: a systematic review and meta-analysis. Langenbeck F Archives of Surgery. 2015;400(2):167erat.
Bowler PG, Duerden BI, Armstrong DG, et al. Clin Microbiol Rev. 2001;14(2):244–69. https://doi.org/10.1128/CMR.14.2.244-269.2001 .
de Jonge SW, Boldingh QJJ, Solomkin JS, Allegranzi B, Egger M, Dellinger EP, Boermeester MA. Systematic Review and Meta-Analysis of Randomized Controlled Trials Evaluating Prophylactic Intra-Operative Wound Irrigation for the Prevention of Surgical Site Infections. Surg Infect (Larchmt). 2017 May/Jun;18(4):508-519. doi: https://doi.org/10.1089/sur.2016.272 . Epub 2017 Apr 27.
Al-Ramahi M, Bata M, Sumreen I, et al. Saline irrigation and wound infection in abdominal gynecologic surgery. Int J Gynaecol Obstet 2006;94:33;94:.
Cervantes-Sanchez CR, Gutierrez-Vega R, Vazquez-Carpizo JA, et al. Syringe pressure irrigation of subdermic tissue after appendectomy to decrease the incidence of postoperative wound infection. World J Surg 2000;24: 38: 38.
Hargrove R, Ridgeway S, Russell R, et al. Does pulse lavage reduce hip hemiarthroplasty infection rates? J Hosp Infect. 2006;62:446.
Nikfarjam M, Weinberg L, Fink MA, et al. Pressurized pulse irrigation with saline reduces surgical-site infections following major hepatobiliary and pancreatic surgery: randomized controlled trial. World J Surg 2014;38:447:447l.
Pinkney TD, Calvert M, Bartlett DC, et al. Impact of wound edge protection devices on surgical site infection after laparotomy: multicentre randomised controlled trial (ROSSINI Trial). BMJ. 2013;347:f4305. Published 2013 Jul 31. doi:10.1136/bmj.f4305.
Gheorghe A, Calvert M, Pinkney TD, Fletcher BR, Bartlett DC, Hawkins WJ, Mak T, Youssef H, Wilson S, West Mid- lands Research Collaborative, ROSSINI Trial Management Group (2012) Systematic review of the clinical effectiveness of wound-edge protection devices in reducing surgical site infection in patients undergoing open abdominal surgery. Ann Surg 255(6):10171017 un.
Edwards JP, Ho AL, Tee MC, Dixon E, Ball CG (2012) Wound protectors reduce surgical site infection: a meta-analysis of randomized controlled trials. Ann Surg 256(1):53):5.
Mihaljevic AL, ML, Tee MC, Kehl V, Friess H, Kleeff J. Wound edge protectors in open abdominal surgery to reduce surgical site infections: a systematic review and meta-analysis. PLoS One. 2015;10(3):e0121187.
PubMed PubMed Central Google Scholar
Zhang MX, Sun YH, Xu Z, Zhou P, Wang HX, Wu YY (2015) Wound edge protector for prevention of surgical site infection in laparotomy: an updated systematic review and meta-analysis. ANZ J Surg 85(5):308:308n.
Kang SI, Oh HK, Kim MH, Kim MJ, Kim DW, Kim HJ, Kang SB. Systematic review and meta-analysis of randomized controlled trials of the clinical effectiveness of impervious plastic wound protectors in reducing surgical site infections in patients undergoing abdominal surgery. Surgery. 2018 Nov;164(5):939-945. doi: 10.1016/j.surg.2018.05.024. Epub 2018 Aug 9.
Sajid MS, Rathore MA, Sains P, Singh KK. A systematic review of clinical effectiveness of wound edge protector devices in reducing surgical site infections in patients undergoing abdominal surgery. Updates Surg. 2017 Mar;69(1):21-28. doi: 10.1007/s13304-017-0415-2. Epub 2017 Jan 25.
Bressan AK, Aubin JM, Martel G, Dixon E, Bathe OF, Sutherland FR, Balaa F, Mimeault R, Edwards JP, Grondin SC, Isherwood S, Lillemoe KD, Saeed S, Ball CG. Efficacy of a dual-ring wound protector for prevention of surgical site infections after pancreaticoduodenectomy in patients with intrabiliary stents: a randomized clinical trial. Ann Surg. 2018 Jul;268(1):35-40. doi: 10.1097/SLA.0000000000002614.
Gheorghe A, Roberts TE, Pinkney TD, Bartlett DC, Morton D, Calvert M, West Midlands Research Collaborative, ROSSINI Trial Investigators. The cost-effectiveness of wound-edge protection devices compared to standard care in reducing surgical site infection after laparotomy: an economic evaluation alongside the ROSSINI trial. PLoS One. 2014;9(4):e95595 Vol.:(01233456789).
Webster J, Alghamdi A. Use of plastic adhesive drapes during surgery for preventing surgical site infection. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No.: CD006353. DOI: 10.1002/14651858.CD006353.pub4.
Allegranzi B, Zayed B, Bischoff P, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16:e288bdomi.
Rezapoor, Maryam et al. Incise draping reduces the rate of contamination of the surgical site during hip surgery: a prospective, randomized trial 2018;The Journal of Arthroplasty , Volume 33 , Issue 6 , 1891 - 1895.
Zarei M, Tabesh H, Fazeli H, Aarabi A. Effect of incise drape on contamination rate of surgical wound during surgical procedures of lumbar spine. Adv Biomed Res . 2019;8:8. Published 2019 Jan 31. doi:10.4103/abr.abr_226_18.
Baier PK, Gluck NC, Baumgartner U, Adam U, Fischer A, Hopt UT (2010) Subcutaneous Redon drains do not reduce the incidence of surgical site infections after laparotomy. A randomized controlled trial on 200 patients. Int J Color Dis 25:639 tri.
Kaya E, Paksoy E, Ozturk E, Sigirli D, Bilgel H (2010) Subcutaneous closed-suction drainage does not affect surgical site infection rate following elective abdominal operations: a prospective randomized clinical trial. Acta Chir Belg 110:457:457.
Fujii, T., Tabe, Y., Yajima, R., Yamaguchi, S., Tsutsumi, S., Asao, T., & Kuwano, H. (2011). Effects of subcutaneous drain for the prevention of incisional SSI in high-risk patients undergoing colorectal surgery. Int J Color Dis, 26(9), 1151ional Sdoi:10.1007/s00384-011-1228-2.
Kosins AM, Scholz T, Cetinkaya M, Evans GR. Evidence-based value of subcutaneous surgical wound drainage: the largest systematic review and meta-analysis. Plast Reconstr Surg. 2013;132(2):443–50. https://doi.org/10.1097/PRS.0b013e3182958945 .
Numata, M., Godai, T., Shirai, J., Watanabe, K., Inagaki, D., Hasegawa, S., a Masuda, M. (2014). A prospective randomized controlled trial of subcutaneous passive drainage for the prevention of superficial surgical site infections in open and laparoscopic colorectal surgery. International Journal of Colorectal Disease, 29(3), 353s passdoi:10.1007/s00384-013-1810-x.
Lauscher JC, Schneider V, Lee LD, Stroux A, Buhr HJ, Kreis ME, Ritz JP. Necessity of subcutaneous suction drains in ileostomy reversal (DRASTAR) randomized, controlled bi-centered trial. Arch Surg. 2016;401(4):409–18.
Arer IM, Yabanoglu H, Aytac HO, Ezer A. The effect of subcutaneous suction drains on surgical site infection in open abdominal surgery A prospective randomized study. Ann Ital Chir. 2016;87:49–55.
Watanabe J, Ota M, Kawamoto M, Akikazu Y, Suwa Y, Suwa H, Momiyama M, Ishibe A, Watanabe K, Masui H, Nagahori K. A randomized controlled trial of subcutaneous closed-suction Blake drains for the prevention of incisional surgical site infection after colorectal surgery. Int J Colorectal Dis. 2017 Mar;32(3):391-398. doi: 10.1007/s00384-016-2687-2. Epub 2016 Oct 25.
Manzoor B, Heywood N, Sharma A. Review of subcutaneous wound drainage in reducing surgical site infections after laparotomy. Surg Res Pract. 2015;2015:715803. doi: 10.1155/2015/715803. Epub 2015 Dec 13.
Fry DE, Harris WE, Kohnke EN, Twomey CL. Influence of double-gloving on manual dexterity and tactile sensation of surgeons. J Am Coll Surg. 2010 Mar;210(3):325–30.
Assadian O, Kramer A, Ouriel K, et al. Suppression of surgeons' bacterial hand flora during surgical procedures with a new antimicrobial surgical glove. Surg Infect. 2014;15(1):43–9. https://doi.org/10.1089/sur.2012.230 .
Quebbeman EJ, Telford GL,Wadsworth K, et al. Double gloving: protecting surgeons from blood contamination in the operating room. Arch Surg 1992;127:213:213 g.
Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No.: CD003087. DOI: 10.1002/14651858.CD003087.
Hentz RV, Traina GC, Cadossi R, Zucchini P, Muglia MA, Giordani M. The protective efficacy of surgical latex gloves against the risk of skin contamination: how well are the operators protected? J Mater Sci Mater Med. 2000 Dec;11(12):825–3.
Partecke LI, Goerdt AM, Langner I, Jaeger B, Assadian O, Heidecke CD, Kramer A, Huebner NO.] Incidence of microperforation for surgical gloves depends on duration of wear. Infect Control Hosp Epidemiol. 2009 May;30(5):409-414. doi: 10.1086/597062.
Kaya I, Uğraş A, Sungur I, Yilmaz M, Korkmaz M, Cetinus E. Glove perforation time and frequency in total hip arthroplasty procedures. Acta Orthop Traumatol Turc. 2012;46(1):57–60.
Stannard JP, Volgas DA, McGwin GR et al: Incisional negative pressure wound therapy after high-risk lower extremity fractures. J Orthop Trauma 2012;26(1):37:37JP.
Grauhan O, Navasardyan A, Hofmann M et al: Prevention of poststernotomy wound infections in obese patients by negative pressure wound therapy. J Thorac Cardiovasc Surg 2013;145(5):13871387avasa.
Blackham AU, Farrah JP, McCoy TP et al: Prevention of surgical site infections in high-risk patients with laparotomy incisions using negative-pressure therapy. Am J Surg 2013;205(6): 647 647 Far.
Sandy-Hodgetts K, Watts R. Effectiveness of negative pressure wound therapy/closed incision management in the prevention of post-surgical wound complications: a systematic review and meta-analysis. JBI Database System Rev Implement Rep. 2015 Jan;13(1):253–303. https://doi.org/10.11124/jbisrir-2015-1687 .
Strugala V, Martin R. Meta-Analysis of comparative trials evaluating a prophylactic single-use negative pressure wound therapy system for the prevention of surgical site complications. Surg Infect. 2017;18(7):810–9.
Sahebally SM, McKevitt K, Stephens I, et al. Negative pressure wound therapy for closed laparotomy incisions in general and colorectal surgery: a systematic review and meta-analysis. JAMA Surg. 2018;153(11):e183467. https://doi.org/10.1001/jamasurg.2018.3467 .
Webster J, Scuffham P, Sherriff KL, Stankiewicz M, Chubbier WP. Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. Cochrane Database of Systematic Reviews 2012, Issue 4. Art. No.: CD009261. DOI: 10.1002/14651858.CD009261.pub2.
Webster J, Scuffham P, Stankiewicz M, Chaboyer WP. Negative pressure wound therapy for skin grafts and surgical wounds healing by primary intention. Cochrane Database of Systematic Reviews 2014, Issue 10. Art. No.: CD009261. DOI: 10.1002/14651858.CD009261.pub3.
Gomoll AH, Lin A, Harris MB: Incisional vacuum-assisted closure therapy. J Orthop Trauma 2006;20(10):705705Li).
Webster J, Liu Z, Norman G, Dumville JC, Chiverton L, Scuffham P, Stankiewicz M, Chaboyer WP. Negative pressure wound therapy for surgical wounds healing by primary closure. Cochrane Database of Systematic Reviews 2019, Issue 3. Art. No.: CD009261. DOI: 10.1002/14651858.CD009261.pub4.
Danno K, Matsuda C, Miyazaki S, Komori T, Nakanishi M, Motoori M, Kashiwazaki M, Fujitani K. Efficacy of negative-pressure wound therapy for preventing surgical site infections after surgery for peritonitis attributable to lower-gastrointestinal perforation: a single-institution experience. Surg Infect. 2018;19(7):711–6.
Lozano-Balderas G, Ruiz-Velasco-Santacruz A, Dr Preventing surgical site infections after surgery for peritonitis attributable to lower-gastrointestinal perforation: a single-institution experience. ematic Reviews 2019, Issue 3. Art. No.: CD0;83(5):512-514.
Tuffaha HW, Gillespie BM, Chaboyer W, Gordon LG, Scuffham PA. Cost-utility analysis of negative pressure wound therapy in high-risk cesarean section wounds. J Surg Res. 2015;195(2):612–22. https://doi.org/10.1016/j.jss.2015.02.008 . Epub 2015 Feb 13.
Chopra K, Gowda AU, Morrow C, Holton L 3rd, Singh DP. The economic impact of closed-incision negative-pressure therapy in high-risk abdominal incisions: a cost-utility analysis. Plast Reconstr Surg. 2016 Apr;137(4):1284-1289. https://doi.org/10.1097/PRS.0000000000002024 .
Hyldig N, Joergensen JS, Wu C, Bille C, Vinter CA, Sorensen JA, Mogensen O, Lamont RF, Möller S, Kruse M. Cost-effectiveness of incisional negative pressure wound therapy compared with standard care after caesarean section in obese women: a trial-based economic evaluation. BJOG. 2019 Apr;126(5):619-627. doi: 10.1111/1471-0528.15573. Epub 2018 Dec 29.
Nguyen TQ, Franczyk M, Lee JC, Greives MR, O'Connor A, Gottlieb LJ. Prospective randomized controlled trial comparing two methods of securing skin grafts using negative pressure wound therapy: vacuum-assisted closure and gauze suction. J Burn Care Res. 2015 Mar-Apr;36(2):324-328. doi: 10.1097/BCR.0000000000000089.
Dorafshar AH, Franczyk M, Karian L, Teven C, Wroblewski K, Gottlieb LJ, Lohman RF. A prospective randomized trial comparing subatmospheric wound therapy with a sealed gauze dressing and the standard vacuum-assisted closure device: a supplementary subgroup analysis of infected wounds. Wounds. 2013 May;25(5):121–30.
Sessler, D. I. Perioperative thermoregulation and heat balance. Lancet 2016; 387, 2655perative ther.
Jonsson, K., Hunt, T. K. & Mathes, S. J. Oxygen as an isolated variable influences resistance to infection. Ann Surg. 1988; 208, 7833 & Mathes,.
Yi J, Liang H, Song R, Xia H, Huang Y. Maintaining intraoperative normothermia reduces blood loss in patients undergoing major operations: a pilot randomized controlled clinical trial. BMC Anesthesiol. 2018;18.
Melling, A. C., Ali, B., Scott, E. M. & Leaper, D. J. Effects of preoperative warming on the incidence of wound infection after clean surgery: a randomised controlled trial. Lancet Lond Engl. 2001; 358, 8766gl. 358, 87.
Kurz, A., Sessler, D. I. & Lenhardt, R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of Wound Infection and Temperature Group. N Engl J Med. 1996; 334, 1209nd Temperatur.
Pu, Y. et al. Warming with an underbody warming system reduces intraoperative hypothermia in patients undergoing laparoscopic gastrointestinal surgery: a randomized controlled study. Int J Nurs Stud. 51, 181et al. 2014).
Melton, G. B. et al. Continuous intraoperative temperature measurement and surgical site infection risk: analysis of anesthesia information system data in 1008 colorectal procedures. Ann Surg. 2013; 258, 606. Continuous intraoperative temp.
Madrid E, et al. Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev. 2016. https://doi.org/10.1002/14651858.CD009016.pub2 .
Sajid, M. S., Shakir, A. J., Khatri, K. & Baig, M. K. The role of perioperative warming in surgery: a systematic review. Sao Paulo Med J Rev Paul Med. 2009; 127, 231perioperativ.
BerríerrTorres, S. I. et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 152, 7842, 784ul. D.
Hypothermia: prevention and management in adults having surgery | Guidance and guidelines | NICE. Available at: https://www.nice.org.uk/guidance/cg65 . (Accessed: 30 Nov 2018).
Global Guidelines for the Prevention of Surgical Site Infection. (World Health Organization, 2016).
Forbes SS, et al. Evidence-based guidelines for prevention of perioperative hypothermia. J Am Coll Surg. 2009;209:492–503.e1.
Wood, A. M., Moss, C., Keenan, A., Reed, M. R. & Leaper, D. J. Infection control hazards associated with the use of forced-air warming in operating theatres. J Hosp Infect. 2014; 88, 132. M., Moss,.
Haeberle, H. S. et al. No evidence of increased infection risk with forced-air warming devices: a systematic review. Surg Technol Int. 2017; 31, 295on risk with.
Adriani, M. B. & Moriber, N. Preoperative forced-air warming combined with intraoperative warming versus intraoperative warming alone in the prevention of hypothermia during gynecologic surgery. AANA J. 2013; 81, 446& Moriber, N.
Fettes, S., Mulvaine, M. & Van Doren, E. Effect of preoperative forced-air warming on postoperative temperature and postanesthesia care unit length of stay. AORN J. 2013; 97, 323vaine, M. &.
Lau A, et al. Effect of preoperative warming on intraoperative hypothermia: a randomized-controlled trial. Can J Anaesth J Can Anesth. 2018. https://doi.org/10.1007/s12630-018-1161-8 .
de Brito Poveda, V., Clark, A. M. & Galvlv630-018-. A systematic review on the effectiveness of prewarming to prevent perioperative hypothermia. J Clin Nurs. 22, 9066, 906review.
Greif R, Akca O, Horn E, Kurz A, Sessler D. Supplemental perioperative oxygen to reduce the incidence of surgical wound infection. N Engl J Med 2000;342:161–1.
Belda FJ, Aguilera L, Garcia dela Asuncion J, et al. Supplemental perioperative oxygen and the risk of surgical wound infection: a randomized controlled trial. JAMA 2005; 294: 2035 20.
Turtiainen J, Saimanen EI, Partio TJ, et al. Supplemental postoperative oxygen in the prevention of surgical wound infection after lower limb vascular surgery: a randomized controlled trial. World J Surg. 2011;35:1387138.
Maragakis LL, Cosgrove SE, Martinez EA, Tucker MG, Cohen DB, Perl TM. Intraoperative fraction of inspired oxygen is a modifiable risk factor for surgical site infection after spinal surgery. Anesthesiology. 2009;110:556–62.
Meyhoff CS, Wetterslev J, Jorgensen LN, et al. Effect of high perioperative oxygen fraction on surgical site infection and pulmonary complications after abdominal surgery: the PROXI randomized clinical trial. JAMA. 2009;302:1543–50. https://doi.org/10.1001/jama.2009.1452 .
Meyhoff CS, Jorgensen LN, Wetterslev J, Christensen KB, Rasmussen LS. Increased long-term mortality after a high perioperative inspiratory oxygen fraction during abdominal surgery: follow-up of a randomized clinical trial. Anesth Analg 2012; 115: 849: 8.
Wadhwa A, Kabon B, Fleischmann E, Kurz A, Sessler DI. Supplemental postoperative oxygen does not reduce surgical site infection and major healing-related complications from bariatric surgery in morbidly obese patients: a randomized, blinded trial. Anesth Analg 2014; 119: 357: 3.
Cohen, B. et al. Effect of intraoperative hyperoxia on the incidence of surgical site infections: a meta-analysis. Br J Anaesth, 2018; Volume 120, Issue 6, 1176 ue 6,.
Hepburn HH. Delayed primary suture of wounds. Br Med J. 1919;1:181tish Journal of Anaesthesia, Volum.
Fogdestam I, Niinikoski J. Delayed primary closure. Tissue gas tensions in healing rat skin incisions. Scand J Plast Reconstr Surg. 1981;15:9981;15:91981;15:9;15:9s tensions in.
Fogdestam I, Jensen FT, Nilsson SK. Delayed primary closure. Blood-flow in healing rat skin incisions. Scand J Plast Reconstr Surg. 1981;15:8181;15:81981;15:8115:81ary closure.
Bhangu A, Singh P, Lundy J, Bowley DM. Systemic Review and Meta-analysis of Randomized Clinical Trials Comparing Primary vs Delayed Primary Skin Closure in Contaminated and Dirty Abdominal Incisions. JAMA Surg. 2013;148(8):779:779 2013;148(8):779 Bowley DM. Syst.
Siribumrungwong B, Noorit P, Wilasrusmee C, Thakkinstian A. A systematic review and meta-analysis of randomised controlled trials of delayed primary wound closure in contaminated abdominal wounds. World J Emerg Surg. 2014;9(1):49. Published 2014 Sep 6. doi:10.1186/1749-7922-9-49.
Siribumrungwong B, Chantip A, Noorit P, et al. Comparison of superficial surgical site infection between delayed primary versus primary wound closure in complicated appendicitis: a randomized controlled trial. Ann Surg. 2018; 267(4):631:6314):631Versus Primary Wo00000002464.
Tang S, Hu W, Hu L, Zhou J. Primary versus delayed primary incision closure in contaminated abdominal surgery: a meta-analysis. J Surg Res. 2019 Jul;239:22-30. doi: 10.1016/j.jss.2019.01.047. Epub 2019 Feb 19.
Sartelli M, Weber DG, Ruppe E, et al. Antimicrobials: a global alliance for optimizing their rational use in intra-abdominal infections (AGORA). World J Emerg Surg. 2016;11:33.
Pea F, Viale P. Bench-to-bedside review: appropriate antibiotic therapy in severe sepsis and septic shock-does the dose matter? Crit Care. 2009;13:214.
Download references
Acknowledgements
No funding received for this article.
Author information
Authors and affiliations.
Department of General Surgery, Azienda USL-IRCSS di Reggio Emilia, Guastalla Hospital, Via Donatori di sangue 1, 42016, Guastalla, RE, Italy
Belinda De Simone
Department of General Surgery, Macerata Hospital, 62100, Macerata, Italy
Massimo Sartelli
General, Emergency and Trauma Surgery, Pisa University Hospital, 56124, Pisa, Italy
Federico Coccolini
Department of Surgery and Oncology, Hepatobiliary and Pancreatic Surgery, Trauma and Acute Care Surgery, University of Calgary Foothills Medical Center, Calgary, Alberta, T2N 2T9, Canada
Chad G. Ball
Anesthesia and Critical Care Department, Papa Giovanni XXIII Hospital, P.zza OMS 1, 24128, Bergamo, Italy
Pietro Brambillasca
Emergency Surgery Unit and Trauma Center, Cisanello Hospital, Pisa, Italy
Massimo Chiarugi
Fabio Cesare Campanile, Ospedale Andosilla, Civita Castellana, Italy
Fabio Cesare Campanile
Unit of General Surgery, Castelnuovo ne’Monti Hospital, AUSL, Reggio Emilia, Italy
Gabriela Nita
Davide Corbella
Abdominal Center, Helsinki University Hospital Meilahti, Helsinki, Finland
Ari Leppaniemi
Medical Library, Papa Giovanni XXIII Hospital, P.zza OMS 1, 24128, Bergamo, Italy
Elena Boschini
Ernest E Moore Shock Trauma Center at Denver Health and University of Colorado, Denver, USA
Ernest E. Moore
Trauma and Acute Care Surgery, Scripps memorial Hospital, La Jolla, CA, USA
Walter Biffl
Department of Surgery, University of Pittsburgh School of Medicine, Pittsburgh, USA
Andrew Peitzmann
Division of General Surgery, Rambam Health Care Campus, Haifa, Israel
Yoram Kluger
Department of Surgery, Letterkenny University Hospital and Donegal Clinical Research Academy, Letterkenny, Ireland
Michael Sugrue
Division of Trauma Surgery, School of Medical Sciences, University of Campinas, Campinas, SP, Brazil
Gustavo Fraga
Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK
Salomone Di Saverio
Trauma and General Surgery, Royal Perth Hospital, Perth, Australia
Dieter Weber
University Hospital St George First, Clinic of General Surgery, Plovdiv, Bulgaria
Boris Sakakushev
State University of Milan, Acute Care Surgery Niguarda Hospital, Milan, Italy
Osvaldo Chiara
Department of Surgery, College of Medicine and Health Sciences, UAE University, Al-Ain, United Arab Emirates
Fikri M. Abu-Zidan
Radboud University Nijmegen, Nijmegen, The Netherlands
Richard ten Broek
Foothills Medical Centre and the University of Calgary, Calgary, AB, Canada
Andrew W. Kirkpatrick
Department of Minimal Access and General Surgery, Government Gousia Hospital, Sringar, Kashmir, India
Imtiaz Wani
Department of Surgery, UC San Diego Medical Center, San Diego, USA
Raul Coimbra
Department of Surgery, University of Brescia, Brescia, Italy
Gian Luca Baiocchi
Department of General Surgery, Albury Hospital, Albury, NSW, 2640, Australia
Micheal D. Kelly
Department of Emergency and Trauma Surgery, Bufalini Hospital, 47521, Cesena, Italy
Luca Ansaloni
Department of Emergency and Trauma Surgery, University Hospital of Parma, 43100, Parma, Italy
Fausto Catena
You can also search for this author in PubMed Google Scholar
Contributions
FC and MS conceived the study; all the experts nominated contributed writing a summary of evidence; BDS collected summaries, updated the literature, and wrote the manuscript; all the authors read and revised the manuscript; BDS revised the final manuscript; MK checked the English language. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Belinda De Simone .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key words’ list for literature searching:
“surgical incision” and “closure”“suture” and “surgical site infection”
“irrigation” and “incisional wound”;
“wound protector” and “surgical site infection”;
“dual ring” and “wound protector” and “wound infection”;
“incisional drape” and “wound infection”;
“drainage” and “subcutaneous” and “surgical incision”;
“gloves” and “surgical site infection”;
“negative pressure wound therapy”and wound infection” and surgical incision”;
“normothermia” and “surgical site infection” and warming device”;
“antibiotics” and “surgical wound infection” and “prevention”;
“hyperoxia/hyperoxigenation”and “surgical site infection”;
“timing skin closure” and “early” and “delayed” and “wound infection” and “dirty surgical incision”.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated.
Reprints and permissions
About this article
Cite this article.
De Simone, B., Sartelli, M., Coccolini, F. et al. Intraoperative surgical site infection control and prevention: a position paper and future addendum to WSES intra-abdominal infections guidelines. World J Emerg Surg 15 , 10 (2020). https://doi.org/10.1186/s13017-020-0288-4
Download citation
Received : 14 August 2019
Accepted : 01 January 2020
Published : 10 February 2020
DOI : https://doi.org/10.1186/s13017-020-0288-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Surgical site infection
- Intra-abdominal infection
World Journal of Emergency Surgery
ISSN: 1749-7922
- Submission enquiries: [email protected]
- Open access
- Published: 07 November 2023
Bacterial profile of surgical site infection and antimicrobial resistance patterns in Ethiopia: a multicentre prospective cross-sectional study
- Seble Worku ORCID: orcid.org/0000-0002-6621-0399 1 , 2 , 3 ,
- Tamrat Abebe 3 ,
- Ashenafi Alemu 2 ,
- Berhanu Seyoum 2 ,
- Göte Swedberg 4 ,
- Alemseged Abdissa 2 ,
- Adane Mihret 2 , 3 &
- Getachew Tesfaye Beyene 2
Annals of Clinical Microbiology and Antimicrobials volume 22 , Article number: 96 ( 2023 ) Cite this article
3 Citations
1 Altmetric
Metrics details
Globally, surgical site infections (SSI) are the most commonly reported healthcare-associated infections.
A multicentre study was conducted among patients who underwent surgical procedures at four hospitals located in Northern (Debre Tabor), Southern (Hawassa), Southwest (Jimma), and Central (Tikur Anbessa) parts of Ethiopia. A total of 752 patients clinically studied for surgical site infection were enrolled. The number of patients from Debre Tabor, Hawassa, Jimma, and Tikur Anbessa, hospitals was 172, 184, 193, and 203, respectively. At each study site, SSI discharge culture was performed from all patients, and positive cultures were characterized by colony characteristics, Gram stain, and conventional biochemical tests. Each bacterial species was confirmed using Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI TOF). An antimicrobial susceptibility test (AST) was done on Mueller–Hinton agar using the disk diffusion method. Logistic regression analysis was used to assess associations of dependent and independent variables. A p-value < 0.05 was considered statistically significant. Data were analysed using STATA 16 software.
Among 752 wound discharge cultures performed, 65.5% yielded growth. Among these, 57.9% and 42.1% were Gram-negative and Gram-positive isolates, respectively. In this study, a total of 494 bacteria were isolated; Staphylococcus aureus (31%), Escherichia coli (20.7%), and Klebsiella pneumoniae (9.8%) were the most common. Rare isolates (0.8% each) included Raoultella ornithinolytica, Stenotrophomonas maltophilia , Alcalignes faecalis, Pantoea ecurina, Bacillus flexus, and Paenibacillus tylopili . Enterobacteriaceae showed high levels of resistance to most of the tested antibiotics but lower levels of ertapenem (32.9%), amikacin (24.3%), imipenem (20.3%), and meropenem (17.6%) resistance. Multidrug-resistant (MDR) frequency of Enterobacteriaceae at Debre Tabor, Hawassa, Jimma, and Tikur Anbessa hospitals was 84.5%, 96.5%, 97.3%, and 94%, respectively. Ages ≥ 61 years (AOR = 2.83, 95% CI: 1.02–7.99; P 0.046), prolonged duration of hospital stay (AOR = 4.15, 95% CI: 2.87–6.01; P 0.000), history of previous antibiotics use (AOR = 2.83, 95% CI: 1.06–2.80; P 0.028), history of smoking (AOR = 2.35, 95% CI: 1.44–3.83; P 0.001), emergency surgery (AOR = 2.65, 95% CI: 1.92–3.66; P 0.000), and duration of operation (AOR = 0.27, 95% CI: 0.181–0.392; P 0.000) were significant risk factors.
The most prevalent isolates from Gram-positive and Gram-negative bacteria across all hospitals were S. aureus and E. coli, respectively . Many newly emerging Gram-negative and Gram-positive bacteria were identified. Variation between hospitals was found for both SSI etiology type and MDR frequencies. Hence, to prevent the emergence and spread of MDR bacteria, standard bacteriological tests and their AST are indispensable for effective antimicrobial stewardship.
Introduction
Surgical site infection (SSI) is the major costliest healthcare-associated infection and a substantial cause of morbidity and mortality throughout the world [ 1 , 2 ]. It occurs near or at the incision site and/or deeper underlying tissue spaces and organs within 30 days of a surgical procedure performed (or up to 90 days for implanted prosthetics) [ 3 ]. In low and middle-income countries SSI ranked the most frequently reported case of nosocomial infections [ 4 ], and in some settings, up to one-third of patients who are operated on [ 5 ] can catch SSI, despite standard protocols of preoperative preparation and antibiotic prophylaxis are practiced [ 6 ]. The SSI rate in Ethiopia has been reported to be between 14.8 and 20% [ 5 , 7 , 8 , 9 ], and surgical patients account for 38% of general surgical wards at various teaching hospitals [ 10 ]. It results from mostly bacterial contamination during or after the surgical procedure but only a small portion progresses to clinical infection due to innate host defences removing contaminants. The contamination that will lead to surgical site infection depends on the dose of bacterial contamination, the virulence, and drug resistance of the bacteria [ 11 ]. Most SSI infections are preventable [ 11 ], however probable development of an infection depends on the age, immunocompromising conditions of the host, or the antimicrobial-resistance (AMR) nature of the infecting microorganisms [ 12 ]. The frequency varies from one hospital to the other and is related to complications [ 13 ]. Patients with SSI are twice as likely to die, 60% more likely to spend time in an intensive care unit (ICU), and more than five times more likely to be readmitted to the hospital after discharge [ 14 ]. The most common pathogens associated with surgical wound infections are Staphylococcus aureus, Escherichia coli, Klebsiella spp., Proteus spp ., Citrobacter spp., Acinetobacter spp., Coagulase negative Staphylococcus aureus and pseudomonas aeruginosa [ 7 , 15 ]. Beta-lactam antibiotics are the most widely used antibiotics for SSI prophylaxis and therapy; however, 30% to 90% of antibiotics are misused or overused [ 16 , 17 ]. This inappropriate overuse increases selection pressure, favouring the emergence of drug-resistant bacteria, making the choice of empirical therapy more difficult and expensive, and poses a serious threat to public health, thus increasing the global risk of SSI [ 18 , 19 ]. The condition is more serious due to irrational antimicrobial prescriptions and un-updated empirical therapy. Hence, the use of data from clinical laboratories' antibiotics susceptibility testing (AST) or solid epidemiological data from ongoing nosocomial infection surveillance is needed to minimize the problem [ 20 ]. In developing countries, including Ethiopia, published reports on bacterial pathogens and their antibiotics resistance patterns of frequently causing SSIs are relatively scarce [ 21 ] compared to the developed parts of the world. Besides, virtually all earlier reports depend on phenotypic laboratory methods to characterize pathogenic bacteria and studies were done at single sites with small sample sizes [ 9 , 22 , 23 ]. A recent systematic review and meta-analyses study by Birhanu Y et al. [ 24 ] focused on the pooled prevalence of SSI and its aetiology in Ethiopia. Also, the study had limitations, the papers included used only phenotypic laboratory methods and the result did not display AMR data. Thus, there have always been ambiguities in the interpretation of the findings when using phenotypic bacterial identification methods. In this study, to avoid the ambiguity in the interpretation of strain identification, we employed Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI TOF) technique for the confirmation of bacterial isolates. Furthermore, this is comprehensive study with a large sample size conducted to determine the bacterial profile of surgical site infection and antimicrobial resistance patterns at four major hospitals in Ethiopia.
Study site and design
A multicentre cross-sectional study was conducted between July 2020 and August 2021 at four selected hospitals in Northern, Central, Southern and Southwest Ethiopia. The study was conducted in purposively selected University Teaching Hospitals in Ethiopia, namely, Debre Tabor Comprehensive Specialized Hospital (DTCSH), Hawassa University Teaching Hospital (HUTH), Jimma University Teaching Hospital (JUTH), and Tikur Anbessa Specialized Hospital (TASH) (Fig. 1 ).
The map of the geographic locations of the four referral hospitals selected for this study
DTCSH comprehensive specialized hospital provides health service to over 5 million people located in Debre Tabor town of South Gondar Administrative Zone, Amhara Regional State. Debre Tabor town is about 98 km away to the East of Bahir-Dar (the capital of Amhara regional state) and about 666 km North of Addis Ababa (the capital of Ethiopia). It is the only specialized hospital in the south Gondar Zone having over 400 beds.
The hospital provides surgery, pediatrics, emergency, maternity, gynecologic/obstetric, and psychiatric, including other departments. In addition, the hospital serves as a teaching centre for the region.
HUCSH is located in Hawassa city in Southern Ethiopia, 280 km from Addis Ababa. HUCSH is one of the largest health facilities in the Southern part of the country and provides teaching, public health services and research activities. It serves more than 20 million people locally and in the neighbouring regions. Currently, the hospital has over 400 beds and provides patient care to 90,200 outpatients, 18,100 hospitalized patients and 1100 emergency cases annually.
TASH is the teaching hospital of Addis Ababa University located in Addis Ababa, the capital of Ethiopia and the largest specialized hospital in Ethiopia, with over 700 beds. It is also an institution where specialized clinical services that are not available in other public or private institutions are rendered to the whole nation.
The TASH has 200 doctors, 379 nurses and 115 other health professionals dedicated to providing health care services. The various departments, faculties and residents under specialty training in the School of Medicine provide patient care in the hospital.
In their outpatient and inpatient units, the hospital offers a variety of services. They also have microbiology laboratories that perform culture and antimicrobial sensitivity testing.
While three hospitals had established microbiology laboratories the DTCSH had started performing bacteriological culture and antimicrobial susceptibility testing at the time of this study. Therefore, with the help of Armauer Hansen Research Institute (AHRI), DTCSH and my home institutions Debre Tabor University we established a bacteriology laboratory, which was used for wound culture processing and antimicrobial susceptibility testing.
Patient recruitment and sample size calculation
The source population the study participants drawn were all patients with suspected cases of SSI who were admitted for elective and emergency surgery. Those who developed signs and symptoms of SSI within 30 or 90 (received implant) days and gave consent and/or assent to participate in the study were enrolled and decision to identify eligible patients as SSI cases were done by attending physicians. All age groups were included, but patients who had been on antibiotic treatment within the preceding ten days, SSI later than 30 days after the operation, or refused to give assent or consent (participate in the study) as well as patients with infected burn wounds, were excluded from the study. A total of 752 clinically diagnosed cases of SSI from different wards were enrolled in the study. The sample size was calculated based on a single proportion sample size estimation formula (n = Z 2 P (1—P) /d 2 ) using a proportion of 20% [ 25 ]. As this was a multicenter study, to increasing the sample size a precision (d) of 0.03 was used, where Z stands for Z statistic with a level of confidence of 95%, and the Z value of 1.96. With a 10% non-response rate, the total sample size came to 752. A convenient sampling technique was used to recruit study participants until the required sample size was achieved, and proportional allocation was made among different hospitals based on the patient flow across the four study sites.
Operational definitions
Surgical site infection occurs near or at the incision site and/or deeper underlying tissue spaces and organs within 30 days of a surgical procedure performed (or up to 90 days for implanted prosthetics) [ 3 ].
Clean wound where no inflammation is encountered and the respiratory, alimentary or genitourinary tracts were not entered.
Clean contaminated wound is where the respiratory, alimentary or genitourinary tracts were entered but without significant spillage.
Contaminated when acute inflammation is encountered, or there is visible contamination of the wound.
Dirty wound wound in the presence of pus, where there is a previously perforated hollow viscous or compound/open injury more than four hours old [ 26 ].
Antibiotic a drug, which is products of fungi or bacteria that kills bacteria or inhibits their growth. Antibiotics are not effective against viruses (also referred to as an antimicrobial).
Multidrug resistance (MDR) refers to resistance at least one antimicrobial agent in three or more antimicrobial classes.
Data collection
Professional nurses who had experience of wound swabs sample collection and microbiologists who were working in the bacteriology laboratory were recruited as data collectors. Training on socio-demographic and clinical data collection using structured questionnaires, wound swab sample collection and sample transportation to bacteriology laboratories and culture were given to all data collectors. Wound swab cultures, bacterial identification and drug susceptibility testing were performed in accordance with a standardized laboratory protocol that was uniformly applied in all study sites (Fig. 2 ). The findings of each culture were communicated to attending physicians for patient management. All bacterial strains were stored at − 80 °C and transported to the Armauer Hansen Research Institute (AHRI) and Sweden for further characterization. The isolates were transported to Sweden utilizing a triple packaging method with dry ice, specifically engineered for the safe carriage of Category A and B for Infectious and non-infectious substances, in accordance with UN regulation UN3373 for DNA samples and UN2814 for bacterial isolates.
Laboratory workflow illustrating through patient recruitment to confirmation of the species of bacterial isolates
Sample collection and transportation
Trained personnel collected SSI discharge aseptically (pus, pus aspirates, and wound swabs) with sterile syringe with needle pus aspirate or sterile cotton-tipped swabs from inside to outside. After cleaning the infected area with 10% povidone-iodine, a sterile cotton-tipped swab was placed in the center, and the rolling technique was used. All wound swabs were dipped in modified Stuart's Transport Medium and immediately transported to the bacteriology laboratory for culture and drug susceptibility testing within 1 h. Following that, all collected specimens were processed for the identification of bacteria implicated in SSIs.
Biochemical tests
For the final identification of the isolates, the following biochemical tests were performed using colonies from pure cultures. For Gram-negative rods, gas production, sugar fermentation, H2S production, indole production, citrate utilization, lysine decarboxylase production, urea hydrolysis, and motility tests were used. Gram-positive cocci were identified using the results of the Gram reaction, catalase, coagulase, bacitracin, and optochin tests [ 27 ].
Bacterial strain confirmation using matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS)
The isolates were transported to Sweden utilizing a triple packaging method with dry ice, specifically engineered for the safe carriage of Category A and B Infectious and non-infectious substances. All bacteria were re-identified and confirmed using MALDI-TOF MS at the Clinical Microbiology Department of Uppsala University Hospital Uppsala, Sweden. From fresh cultures, a single colony of bacteria was smeared onto a MALDI-TOF plate, and the sample was air-dried. Next, 1 µl of formic acid was added to each cell and air-dried, and then 1 µl of MALDI matrix solution was applied to the cells and air-dried before reading. MALDI-TOF identification was automatically scored by the system software between 1 and 3 points. All isolates with scores two and above were accepted, and all results below 1.7 and flagged red were rejected. Samples with scores 1.7–2 and flagged yellow were re-analysed.
Antimicrobial susceptibility testing (AST)
The antibiotics susceptibility tests were performed on Muller-Hinton agar (Oxoid) by using the Kirby-Bauer disk diffusion technique. Using a sterile wire loop, 3–5 pure colonies were transferred to a tube containing 5 mL of sterile normal saline (0.85% NaCl) and gently mixed until a uniform suspension formed. Standard inoculum density was adjusted to 0.5 McFarland units. The excess broth suspension was removed by tapping against the tube wall. The bacterial suspension was swabbed on MHA surface by using sterile swab then a set of antibiotic discs placed with sterile forceps at least 24 mm apart from one another [ 28 ]. All antibiotics discs were OXOID products (Oxoid Ltd, UK), and susceptibility of Gram-negative isolates was tested against: ampicillin (10 µg), gentamicin (10 µg), amikacin (30 µg), ciprofloxacin (5 µg), chloramphenicol (30 µg), ceftazidime (30 µg), cefotaxime (30 µg), ceftriaxone (30 μg), cefuroxime (30 µg), cefepime (30 µg), tetracycline (30 µg), amoxycillin + Clavulanate (20/10 μg), Trimethoprim-sulfamethoxazole (1.25/23.75 µg), ampicillin-sulbactam (10/10 µg), aztreonam (30 µg), meropenem (10 µg), Imipenem (10 µg), ertapenem (30 µg). Gram-positive isolates were tested against penicillin (10units), ampicillin (10 µg), vancomycin (30 µg), erythromycin (15 µg), ciprofloxacin (5 µg), cefoxitin (30 µg), clindamycin (30 µg), erythromycin (15 µg), doxycycline (30 µg), chloramphenicol (30 µg), gentamicin (10 µg), and oxacillin (5 µg), tetracycline (30 µg), [ 28 ]. Following that, the plates were incubated at 37 °C for 18–24 h. Each zone of inhibition was measured to the nearest millimeter, and classified as sensitive, intermediate, or resistant using the standard technique [ 28 ]. MDR was a bacterium that was simultaneously resistant at least one drug in three or more categories.
Quality control
All specimens were collected according to the standard operating procedure (SOP) [ 21 ]. A double data entry method was used to ensure the accuracy of the data. The performance of all prepared media was checked by inoculating control strains, E. coli (ATCC 25922) and S. aureus ( ATCC 25923), for each new batch of agar plates [ 22 ]. In addition, the sterility of culture media was checked by incubating 5% of the prepared media at 37 °C for 24–48 h. In addition, reagents for Gram-stain and biochemical tests were checked against control strains of S. aureus and E. coli. The 0.5 McFarland standard was used to standardize the inoculum density of the bacterial suspension for the susceptibility test. Each MALDI-TOF run also included quality control strains using E. coli (ATCC 25922) and S. aureus ( ATCC 25923).
Data analysis
The data were checked for completeness, missing values, and coding of questionnaires entered into Research Electronic Data Capture (RED-Cap) and exported to STATA version 16.0. Frequencies and cross-tabulations were used to summarize descriptive statistics (median, percentages or frequency). Associations of possible risk factors with SSIs was assessed using bivariate and multivariate logistic regression to study the effect of independent variables on the dependent variables . P-value less than 0.05 were considered statistically significant.
Ethical considerations
The Department of Medical Microbiology, Immunology, and Parasitology (DMIP) and the AHRI/ALERT Research Ethics Committee (AAREC) reviewed and approved the study, and institutional review board (IRB) approval was obtained from Addis Ababa University's College of Health Sciences and AAREC, AAUMF03-008/2020. Written permission letter was obtained from each study site before starting the data collection. The purpose and procedures of the study was explained to the study participants, participants’ parents or guardians before recruitment to the study. Those study participants who gave written informed consent and those children whose parents or guardians gave informed assent were selected and enrolled in this study. Results obtained from all patients were communicated to attending physicians and all patient’s information was kept confidentially.
In the present study, a total of 752 patients from four different hospitals were investigated for SSIs. The number of patients from DTCSH was172, and the numbers from HUCSH, JUSTH, and TASH, were184, 193, and 203, respectively (Table 1 ). Of the 752 study participants whose SSI discharge was inoculated onto growth media, 65.5% (493 /752 ) showed bacterial growth (Table 1 ). DTCSH had the highest percentage of positive cultures (78.5%), followed by JUTSH (65.3%), and HUCSH and TASH, respectively, had 65.2% and 55.7% of SSI bacterial growth (Table 1 ). The study participants age ranged from 3 days to 85 years with median of 28 years and 418 (55.6%) were males. Approximately 487 (64.8%) of patients had deep SSI, 454 (60.4%) preoperative hospital stay > 7 days, 619 (82.4%) history of hospital admission, 388 (52.9%) had previous use of antibiotics, 448 (59.6%) had smoking history, 506 (67.2%) of surgical procedures were emergency surgery, 724 (96.2%) of patients with clean or clean contaminated wounds dominated the wound class, 548 (72.8%) required antimicrobial prophylaxis before the procedure, and 55.3% underwent surgeries lasting greater than an hour (Table 1 ).
Bivariate and multivariable logistic regression analyses were used to see the relationship between the independent variables over the dependent variable. On bivariate regression analysis, male sex, age ≥ 61, SSI type, preoperative hospital stays, history of hospital admission, previous use of antibiotics, smoking, emergency surgery, and duration of operation ≥ 1 h had a statistically significant association with the occurrence of SSI. The type of surgery (wound), alcohol history and the timing of prophylactic antibiotics ≥ 1 h had no statistically significant association (Table 2 ). The result of the multivariate regression showed that ages ≥ 61 years (AOR = 2.83, 95% CI: 1.02–7.99; P 0.046), prolonged duration of hospital stay (AOR = 4.15, 95% CI: 2.87–6.01; P 0.000), history of previous antibiotics use (AOR = 2.83, 95% CI: 1.06–2.80; P 0.028), history of smoking (AOR = 2.35, 95% CI:1.44–3.83; P 0.001), emergency surgery (AOR = 3.24, 95% CI: 2.29–4.77; P 0.000), and duration of operation (AOR = 0.27, 95% CI: 0.181–0.39; P 0.000) were significant risk factors (Table 2 ).
Frequency and distribution of identified bacterial isolates
The total number of pathogenic bacterial isolates were 65.7% (494/752 ) from all SSI culture (Figs. 3 , 4 A). Gram-negative were 57.9% (286/752 ) , and Gram positive 42.1% (208/752) according to Fig. 2 B, C). Of these, 2.6% (13/493 ) of cultures were a mixture of two colony types, while 2.4% (12/493 ) wer e commensals or contaminants and 97.4% showed single bacterial growth. Species of the mixed cultures were Raoultella ornithinolytica, Paenibacillus tylopili , S. aureus and coagulase negative staphylococci. Among the identified types of bacteria, Staphylococcus aureus was the predominant one (31%), followed by E. coli (20.7%) and Klebsiella pneumonia (9.8%) among SSIs (Fig. 1 ). Other less frequently detected species were Acinetobacter baumannii (7.6%) , Enterobacter cloacae (5.1%) , Pseudomonas aeruginosa (3.7%) , Klebsiella variicola, and Enterobacter hormaeche (1% each). Diverse species of Acinetobacter, Enterobacter, Enterococcus, Staphylococcus, Aerococcus, Bacillus, Citrobacter, and Pseudomonas were identified. While Gram-positives was found at all four hospitals (42.1%), it was mainly detected at DTCSH (40.4%), with 21.6%, 21.6%, and 16.3% isolated at TASH, HUCSH, and JUTSH, respectively (Fig. 2 B). In addition, Raoultella ornithinolytica, Stenotrophomonas maltophilia, Pantoea ecurina, Providencia rettgeri, Alcalignes faecalis, and Morganella morganii were detected as rare bacterial pathogens. Figure 2 A shows the frequency and distribution of Gram-negative bacterial isolates at the four hospitals.
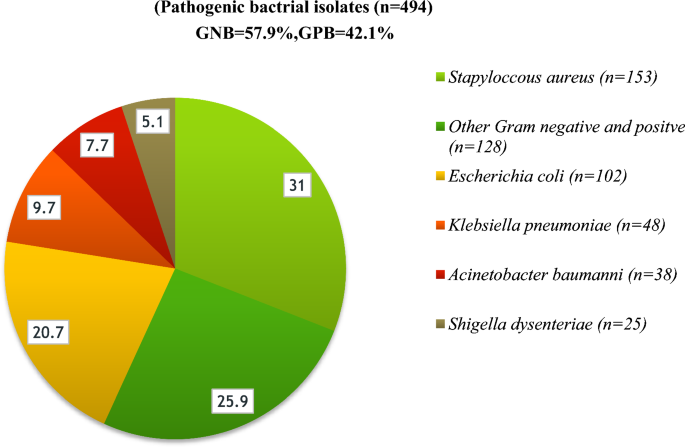
Frequency and distribution of bacteria isolated from patients investigated for surgical site infection at four different hospitals in Ethiopia. GNB Gram-negative bacteria, GPB Gram-positive bacteria, Other GN and GP (n = 128): Raoultella ornithinolytica (n = 1), Stenotrophomonas maltophilia (n = 1), Acinetobacter soli (n = 2), Acinetobacter pitti (n = 2), Acinetobacter lactucae (n = 1), Pseudomonas plecoglossicida (n = 1), Pantoea ecurina (n = 1), Citrobacter freundii (n = 1), Citrobacter sedlakii (n = 2), Providencia rettgeri (n = 2), Alcalignes faecalis (n = 2), Proteus mirabilis (n = 4), Morganella morganii (n = 1), Aerococcus viridans (n = 3), Bacillus flexus(n = 1) Paenibacillus tylopili (n = 1), Enterobacter cloacae (n = 19), Enterobacter asburiae (n = 1), Enterobacter bugandensis (n = 4), Enterobacter hormaeche (n = 5), Enterococcus faecium (n = 8): Enterococcus gallinarum (n = 3), Enterococcus hirae (n = 2) , Enterococcus durans (n = 2), Staphylococcus hominis (n = 4), Staphylococcus haemolyticus (n = 3), Staphylococcus warneri (n = 2), Staphylococcus sciuri (n = 6), S. epidermidis (n = 8)
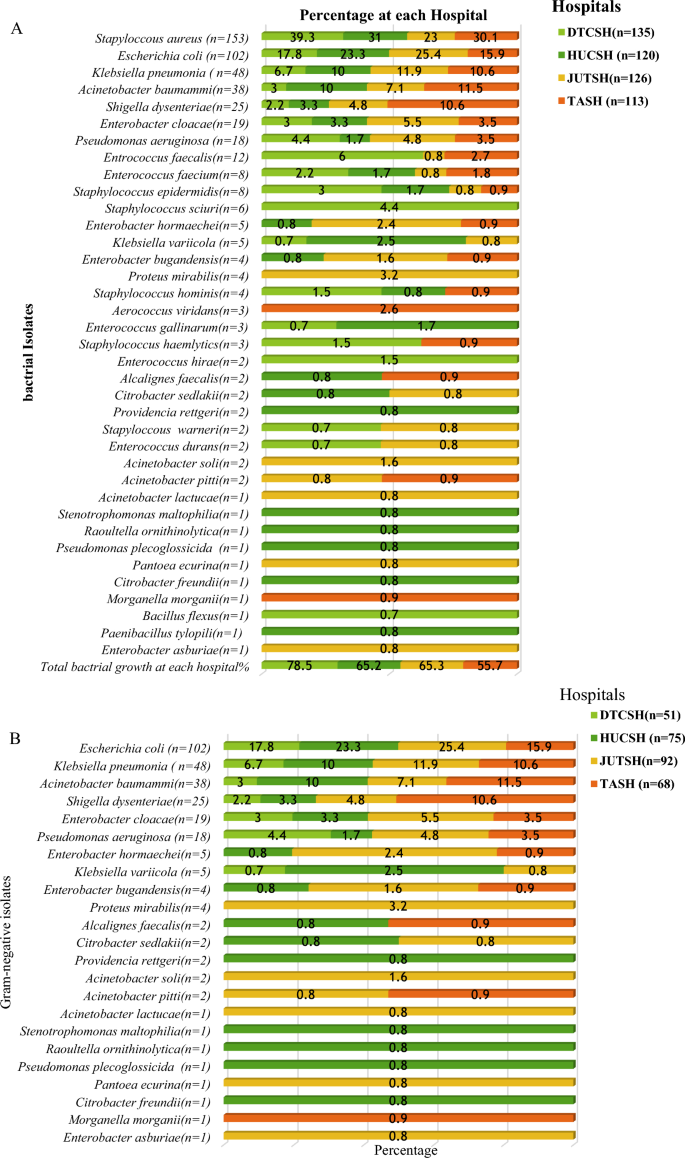
Frequency and distribution of bacterial isolates from the total number of bacteria isolated at each hospital A total identified bacteria at each site, B Gram-negative isolates and C Gram-positive isolates. DTCSH Debre Tabor Comprehensive Specialized Hospital, HUCSH Hawassa University Comprehensive Specialized Hospital, JUTSH Jimma University Teaching Specialized Hospital, TASH Tikur Anbessa Specialized Hospital, n number of bacterial isolates
Antibiotic resistance pattern of SSI bacterial isolates
The predominant isolate from Gram-positives, S. aureus , revealed a high level of resistance toward penicillin 90.1%, and ampicillin 76.5%, while 7.8%, 10.6%, and 12.4% of the isolates were resistant to clindamycin, chloramphenicol, and gentamicin respectively but 100% of S. aureus were sensitive to vancomycin (Table 3 ). All isolates of S. aureus showed multiple drug resistance (resistance to two or more drugs).
Cefoxitin, which is a surrogate marker of methicillin, showed 22.7% resistance against S. aureus . Enterococcus species showed 70.4% resistance to ampicillin and 66.7% to erythromycin. Table 3 shows the AMR pattern of Gram-positive bacteria.
The Enterobacteriaceae showed high resistance toward ampicillin (93.2%), ceftriaxone (90.5%), cefuroxime (88.7%), aztreonam (82.9%), ceftazidime (80.6%), cefepime (77%), ampicillin-sulbactam (76.1%), trimethoprim-sulfamethoxazole (77.5%), tetracycline (72.5%), amoxicillin-clavulanic acid (71.2%) (Table 4 ).
Low resistance frequency of Enterobacteriaceae was detected for amikacin (24.3%), imipenem (20.3%), meropenem (17.6%), and ertapenem (32.9%) (Table 4 ).
The resistance of Enterobacteriaceae to meropenem and imipenem was (11.6%,18.6%), (18%, 22.9%), (12.2%,13.5%) and (26.9%,25%) at DTCSH, HUCSH, JUTSH, and TASH, respectively (Fig. 5 A).
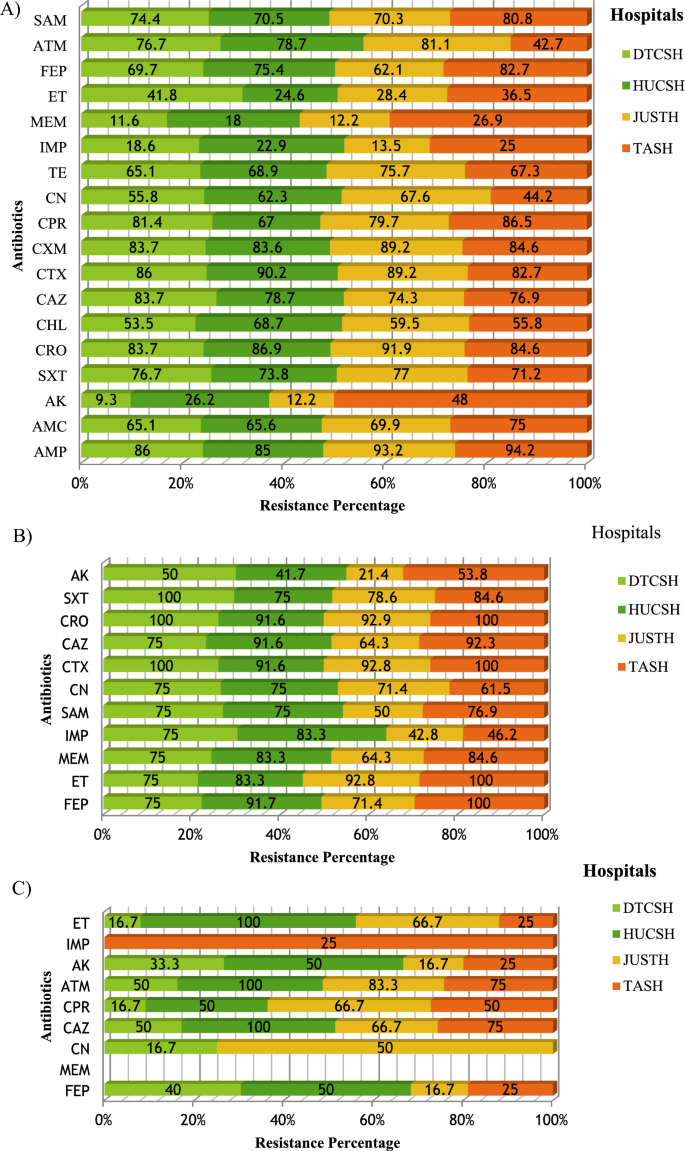
Frequency of antibiotic resistance at four hospitals; A Enterobacteriaceae B Acinetobacter species C Pseudomonas species. The percentage represents the numbers of resistant isolates, out of the total number of isolates at all hospitals. AMP ampicillin, AMC amoxicillin/clavulanate, AK amikacin, SXT trimethoprim-sulfamethoxazole, C chloramphenicol, CAZ ceftazidime, CTX cefotaxime, CRO ceftriaxone, CXM cefuroxime, CIP ciprofloxacin, CN gentamicin, TE tetracycline, ATM aztreonam, SAM ampicillin-sulbactam, FEP cefepime, IMP Impemene, MEM meropeneme, ET Ertapeneme, DTCSH Debre Tabor Comprehensive Specialized Hospital, HUCSH Hawassa University Comprehensive Specialized Hospital, JUTSH Jimma University Teaching Specialized Hospital, TASH Tikur Anbessa Specialized Hospital
The predominant isolate, E. coli (n = 102) revealed a high level of resistance to ampicillin (94.6%), ceftriaxone (99%), cefotaxime (93.8), ceftazidime (79.4%), cefepime (77%), cefuroxime (73.5%), ampicillin-sulbactam (72%), trimethoprim-sulfamethoxazole (71.5%), tetracycline (70.6%), and low-level resistance to gentamicin (57.8%), chloramphenicol (41.2%), ertapenem (24.5), imipenem (11.6%), amikacin (10.8%), meropenem (9.8).
K. pneumoniae (n = 48) were resistant to ampicillin (100%), ceftriaxone (100%), cefotaxime (93.8%), amoxicillin-clavulanic acid (91.7%), ceftazidime (88.5%), cefepime (81.2%), cefuroxime (77.1%), tetracycline (66.7%), ertapenem (43.8%), meropenem (41.7%), amikacin (33.3%), imipenem (29.2%). Amikacin and meropenem were 100% effective against all of the isolates of Klebsiella variicola and Proteus mirabilis. In the non-fermenter group, A. baumannii showed the highest resistance to cefotaxime (95.3%), ertapenem (92.1%), ceftazidime (89.5%), gentamicin (86.8%), cefepime (84.2%), meropenem (84.2%), and SXT (81.4%). In addition, A. baumannii has lower-level resistance to imipenem (65.9%) and ampicillin-sulbactam (63.1%) (Table 4 ). The resistance frequency of Acinetobacter species to meropenem at DRH, HUCSH, JUSTH, and TASH was 75%, 83.3%, 42.8%, and 46.2%, respectively (Fig. 3 B). P. aeruginosa showed minimal resistance to ceftazidime (66.7%), cefepime (55.5%), gentamicin (47.8%), ciprofloxacin (22.2%), and amikacin (10.5%) (Table 4 ). In addition, 100% and 94.5% of Pseudomonas species were sensitive to meropenem and imipenem, respectively (Table 4 , Fig. 5 C).
- Multidrug resistance
The overall Multidrug resistance (MDR) to three or more antibiotics was observed in 100% of S. aureus (Table 3 ) and 93.3% Enterobacteriaceae (Table 4 ). Enterobacteriaceae that showed MDR to eight (R-9), nine (R-10) and ten (R- ≥ 11) antibiotics from different groups had a frequency of 6.3%, 6.7%, and 64.9%, respectively. Only 0.5% Enterobacteriaceae showed zero resistance (R-0) to all antibiotic classes tested, whereas 3.1% Enterobacteriaceae showed resistance to one antibiotic (R-1) class. For Enterobacteriaceae , the MDR frequency at DTCSH, HUCSH, JUSTH, and TASH was 84.5%, 96.5%, 97.3%, and 94%, respectively (Fig. 6 A). E. coli, K. pneumoniae, E. cloacae, S. dysenteriae, K. variicola, and P. mirabilis showed an overall MDR frequency of 96.1%, 95.9%, 79.3%, 82%, 100%, and 100%, respectively. The overall MDR frequency of A. baumannii and P. aeruginosa was 95% and 77.8%, respectively. The MDR frequency for Acinetobacter species was 73% at DTCSH, 83.3% at HUCSH, 100% at JUTSH, and 100% at TASH (Fig. 4 B). On the other hand, MDR frequency for Pseudomonas species was 66.7%, 66.7%, 83.3% and 50% at DTCSH, HUCSH, and JUSTH and TASH, respectively (Fig. 6 C).
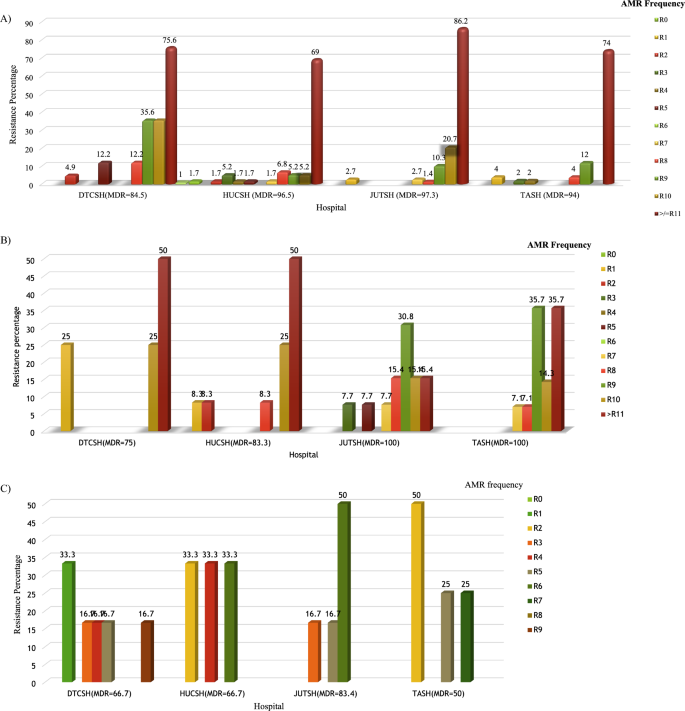
Frequency of multidrug resistance at four hospitals. A Enterobacteriaceae B Acinetobacter species C Pseudomonas species. Percentages represent the number of resistant isolates out of the total number of isolates at each hospital. Debre Tabor Comprehensive Specialized Hospital (DTCSH), HUCSH Hawassa University Comprehensive Specialized Hospital, and Jimma University Teaching Specialized Hospital (JUTH) and Tikur Anbessa Specialized Hospital (TASH), MDR multidrug resistance
In surgically treated patients, post-operative SSI is still one of the leading causes of morbidity and mortality, and it increases the cost of health care due to repeated readmission. In order to effectively manage bacterial infection, it is crucial to identify the bacterial pathogens and choose an antibiotic that is efficient against the organism [ 1 , 2 ].
As far as risk factors associated with the occurrence of SSI are concerned, in the present study, the likelihood of SSI occurrences among patients aged ≥ 61 years increased by a factor of 2.8. Similar findings have been conducted in Ethiopia [ 15 , 16 , 29 ] and elsewhere [ 30 ]. This might be due to a weakened immune response to infectious agents and poor nutritional status [ 31 ].
Patients who had a longer duration of hospital stay developed SSIs 4.1 times more frequently (P = 0.000) than those who had shorter time. This finding was in agreement with many studies in Ethiopia [ 29 , 32 , 33 ] and elsewhere [ 30 ]. This is a notable finding because it is associated with additional costs in a country with a staggering economy and healthcare system [ 34 ].
Similarly, the present study demonstrated that with previous use of antibiotics, patients had a 2.8 times higher chance of developing SSI than with non-previous use of antibiotics [ 35 ]. This could be because broad-spectrum antibiotics have a high risk of causing superinfection of resistant strains due to selective pressure [ 18 , 19 ].
The type of surgery was also statistically associated with SSI in the present study. Undergoing emergency surgery showed approximately 3.24 times higher chances of acquiring SSIs when compared to elective surgery (P = 0.000), which complies with related studies [ 35 ].
The risk of developing SSI with smoking histories was found to be 2.35 times more than in those who did not have smoking history. There was a significant association between smoking patients’ history and SSI (P = 0.001). This finding was in agreement with smoking history were independent predictors of SSIs in multivariate logistic regression analysis [ 36 ]. Smoking weakens immunity and increases the risk of SSI [ 37 ].
The overall culture positivity rate from patients with SSI in the current study was 65.5%. Similar results reported from India (68%) [ 38 ]. Which was slightly smaller than results previously reported from Jimma (71.7%) [ 9 ]. In contrast, the current study was lower than reports from Tikur Anbessa (75.6%) [ 39 ], Gondar (83.9%) [ 40 ], and elsewhere (82%) [ 41 ]. Lower rates of positive culture were reported from India Bangladesh (61.8%) [ 42 ].
In this study, the Gram-negative bacterial isolation rate was greater (57.9%) than the Gram-positive isolates (42.1%), which is comparable with the study done in Addis Ababa [ 43 ]. On the other hand, the current study was lower in Gram- negative isolates than reports from Mizan-Tepi (73.2%) and higher in Gram-positive isolates (24.8%) [ 22 ]. This could be due to a difference in the study population.
In the current study, the profiles of bacterial isolates highly associated with SSI were S. aureus (31%), followed by E. coli (20.7%), and K. pneumoniae (8.9%). Studies from Gondar [ 44 ], Addis Ababa [ 45 ], and India [ 40 ] reported similar results. The high prevalence of S. aureus infection could be due to an endogenous source as well as environmental contamination.
In contrast to previous reports, a study from Addis Ababa found E. coli 23.1%, followed by multidrug-resistant Acinetobacter species 22.1% [ 34 ]. Similarly, in a study done by Jimma, E. coli was frequently identified and followed by Klebsiella spp. [ 41 ]. This variation in the distribution pattern of bacterial isolates may be due to the diversity of the study population, setting, and local antimicrobial usage pattern, which leads to the introduction of pathogens that may be resistant to currently used antibiotics.
Rare surgical site infections isolate including Raoultella ornithinolytica , Stenotrophomonas maltophilia, Alcalignes faecalis, and Paenibacillus tylopili, were identified. Such newly emerging bacteria-causing infections in SSI patients may result in future challenges [ 46 , 47 , 48 ]. These species, along with K. variicola and Pantoea ecurina , have never before been identified in patients in Ethiopia who were being evaluated for SSI. The isolation of these new SSI etiologies emphasizes the necessity of institution-based diagnostic and intervention practices.
In our finding, S. aureus revealed a high level of resistance to penicillin (88.6%) and ampicillin (77.3%), which was comparable to study in Turkey [ 46 ]. On the other hand, all isolated S. aureus were susceptible to vancomycin, and our finding was the same as earlier studies in Jimma [ 36 ] and Turkey [ 46 ].
Gram-negative bacteria showed higher resistance to ampicillin (93.2%), ceftriaxone (90.5%), cefuroxime (88.7%), aztreonam (82.9%), ceftazidime (80.6%), cefepime (77%), ampicillin-sulbactam (76.1%), trimethoprim-sulfamethoxazole (77.5%), tetracycline (72.5%), and amoxicillin-clavulanic acid (71.2%). Since β-lactam antibiotics are the most widely used antibiotics, many studies in Ethiopia [ 11 , 34 ] and around the world have found similar resistant patterns [ 40 ]. This shows antibiotics require a periodic evaluation and the establishment of antibiotic policies for prophylaxis and treatment guidelines in the Ethiopian setting.
In this study, multidrug resistance (MDR) was observed (100%) in S. aureus and (93.4%) in Gram-negative bacteria, similar to findings from Bahir Dar [ 18 ].
In the current study, A. baumannii showed the highest to resistance cefotaxime (95.3%), ceftazidime (89.5%), gentamicin (86.8%), cefepime (84.2%), and trimethoprim-sulfamethoxazole (81.4%). Many studies have found that these organisms have a high resistance to the most commonly used antibiotics [ 34 , 47 , 48 ]. In addition, A. baumannii also showed remarkably high resistance to ertapenem (92.1%), meropenem (84.2%), and imipenem (65.9%).
Amikacin and meropenem were 100% effective against all of the isolates of P. mirabilis and K. variicola in our study. However, studies done in Mekelle [ 37 ], and elsewhere [ 29 ] showed that ciprofloxacin were effective against Proteus and Pseudomonas isolates. The differences maybe the rational use of antibiotics and the fact that the cost of the drugs may be higher relative to others, so people do not take these drugs for self-medication in the study area [ 49 ].
Carbapenem resistance among Enterobacteriaceae was 17.6%, 20.3%, and 32.9% to meropenem, imipenem, and ertapenem, respectively. Effective treatment options for Enterobacteriaceae were limited to amikacin, meropenem, and imipenem. The most frequent isolate is E. coli, which showed the highest resistance to ampicillin (ceftriaxone, cefotaxime, ceftazidime, cefepime, cefuroxime, ampicillin-sulbactam, trimethoprim-sulfamethoxazole, and tetracycline. Similar studies were conducted in Ethiopia [ 33 ] and Iraq [ 50 ]. This might be due to the indiscriminate use of antibiotics in both hospitals [ 18 , 19 ].
In our study, an alarming level of carbapenem-resistant E. coli to ertapenem (24.5%), imipenem (11.8%) , and meropenem (9.8%) was detected; however, it was lower than in another study [ 51 ]. The cause of the higher rates compared to other settings may be irrational use or misuses of antibiotics. The discrepancy of antibiotics resistance across sites includes differences in the rational use of antibiotics [ 52 ].
Strengths and limitation
The strength of this study was multicentred, enrollment of all age groups, a reasonably large sample size and re-characterizing bacteria using MALDI TOF–MS an advanced bacterial identification method, and the limitation were unable to investigate anaerobic bacterial and fungal agents due to limited laboratory resources at the hospitals.
Conclusions
This multicenter study identified frequent and diverse Gram-negative and Gram-positive SSI etiologies without significant variation in primary etiologies between hospitals. Isolation of various newly emerging bacterial strains in all sites showed the growing epidemiology and diversity of SSI etiologies. E. coli and S. aureus were the leading Gram-negative and Gram-positive isolates, respectively. High antimicrobial resistance was detected with varying frequency between hospitals. Gram-positive isolates revealed maximum sensitivity to vancomycin and clindamycin, whereas, among Gram-negative isolates, amikacin, imipenem, and meropenem were the most effective antibiotics Furthermore, the overall ceftriaxone resistance is about 90.5%. Among the study participants, 72.1% took prophylaxis and developed SSIs. The finding of high levels of carbapenem resistance, especially towards ertapenem, is alarming.
Hence, to prevent the emergence and spread of MDR SSI, we recommend effective antimicrobial stewardship and antibiotic treatment to based on AST of the pathogens. At the national level, regular surveillance and monitoring of antimicrobial resistance patterns are indispensable. This includes the careful monitoring of the antibiotics used as prophylaxis and empiric treatment by the concerned authorities.
Availability of data and materials
The data sets generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
Abbreviations
AHRI/ALERT Ethics Review Committee
Armauer Hansen Research Institute
Antimicrobial resistance
Antimicrobial susceptibility testing
American Type of Culture Collection
Center for Disease Control and Prevention
Clinical Laboratory Standards Institute
Department of Microbiology Immunology and Parasitology
Debre Tabor Teaching, and Referral Hospital
Hawassa University Teaching Hospital
Jimma University Teaching Hospital
Matrix-assisted laser desorption ionization-time of flight mass spectrometry
Multi drug resistance
Mueller Hinton Agar
Standard operation procedure
Surgical site infections
South Texas Art Therapy Association
Tikur Anbessa Specialized Hospital
Extended-spectrum beta lactamases
Forrester JD, Maggio PM, Tennakoon L. Cost of health care-associated infections in the United States. J Patient Saf. 2022;18(2):e477–9.
Article PubMed Google Scholar
Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg. 2017;152(8):784–91.
Borchardt RA, Tzizik D. Update on surgical site infections: the new CDC guidelines. Jaapa. 2018;31(4):52–4.
Lewis SS, Moehring RW, Chen LF, Sexton DJ, Anderson DJ. Assessing the relative burden of hospital-acquired infections in a network of community hospitals. Infect Control Hosp Epidemiol. 2013;34(11):1229–30.
Article PubMed PubMed Central Google Scholar
Mengesha RE, Kasa BG-S, Saravanan M, Berhe DF, Wasihun AG. Aerobic bacteria in post surgical wound infections and pattern of their antimicrobial susceptibility in Ayder Teaching and Referral Hospital, Mekelle, Ethiopia. BMC Res Notes. 2014;7(1):575.
Raza MS, Chander A, Ranabhat A. Antimicrobial susceptibility patterns of the bacterial isolates in post-operative wound infections in a tertiary care hospital, Kathmandu, Nepal. Open J Med Microbiol. 2013;3(3):159.
Article Google Scholar
Godebo G, Kibru G, Tassew H. Multidrug-resistant bacterial isolates in infected wounds at Jimma University Specialized Hospital, Ethiopia. Ann Clin Microbiol Antimicrob. 2013;12:17.
Halawi E, Assefa T, Hussen S. Pattern of antibiotics use, incidence and predictors of surgical site infections in a Tertiary Care Teaching Hospital. BMC Res Notes. 2018;11(1):538.
Misha G, Chelkeba L, Melaku T. Bacterial profile and antimicrobial susceptibility patterns of isolates among patients diagnosed with surgical site infection at a tertiary teaching hospital in Ethiopia: a prospective cohort study. Ann Clin Microbiol Antimicrob. 2021;20(1):33.
Article CAS PubMed PubMed Central Google Scholar
Spagnolo A, Ottria G, Amicizia D, Perdelli F, Cristina ML. Operating theatre quality and prevention of surgical site infections. J Prev Med Hyg. 2013;54(3):131.
CAS PubMed PubMed Central Google Scholar
National Institute for Health and Care Excellence: Clinical Guidelines. Surgical site infections: prevention and treatment. London: National Institute for Health and Care Excellence (NICE). Copyright © NICE 2020. 2020.
Moradali MF, Ghods S, Rehm BHA. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
Isibor JO, Oseni A, Eyaufe A, Turay A. Incidence of aerobic bacteria and Candida albicans in post-operative wound infections. Afr J Microbiol Res. 2008;2(11):288–91.
Google Scholar
Organization WH. Global guidelines for the prevention of surgical site infection. Geneva: WHO; 2016.
Biadglegne F, Abera B, Alem A, Anagaw B. Bacterial isolates from wound infection and their antimicrobial susceptibility pattern in Felege Hiwot referral Hospital North West Ethiopia. Ethiop J Health Sci. 2009;19(3).
Mulu W, Kibru G, Beyene G, Damtie M. Postoperative nosocomial infections and antimicrobial resistance pattern of bacteria isolates among patients admitted at Felege Hiwot Referral Hospital, Bahirdar, Ethiopia. Ethiop J Health Sci. 2012;22(1):7–18.
PubMed PubMed Central Google Scholar
Munckhof W. Antibiotics for surgical prophylaxis. Aust Prescr. 2005;28(2).
Ranjan KP, Ranjan N, Bansal SK, Arora D. Prevalence of Pseudomonas aeruginosa in post-operative wound infection in a referral hospital in Haryana, India. J Lab Phys. 2011;2(2):129–32.
Li B, Webster TJ. Bacteria antibiotic resistance: new challenges and opportunities for implant-associated orthopedic infections. J Orthop Res. 2018;36(1):22–32.
Fadeyi A, Adigun I, Rahman G. Bacteriological pattern of wound swab isolates in patients with chronic leg ulcer. Int J Health Res. 2008. https://doi.org/10.4314/ijhr.v1i4.55375 .
Iskandar K, Sartelli M, Tabbal M, Ansaloni L, Baiocchi GL, Catena F, et al. Highlighting the gaps in quantifying the economic burden of surgical site infections associated with antimicrobial-resistant bacteria. World J Emerg Surg. 2019;14(1):50.
Abayneh M, Asnake M, Muleta D, Simieneh A. Assessment of bacterial profiles and antimicrobial susceptibility pattern of isolates among patients diagnosed with surgical site infections at Mizan-Tepi University Teaching Hospital, Southwest Ethiopia: a prospective observational cohort study. Infect Drug Resist. 2022;15:1807–19.
Bitew Kifilie A, Dagnew M, Tegenie B, Yeshitela B, Howe R, Abate E. Bacterial profile, antibacterial resistance pattern, and associated factors from women attending postnatal health service at University of Gondar Teaching Hospital, Northwest Ethiopia. Int J Microbiol. 2018;2018:3165391.
Birhanu Y, Endalamaw A. Surgical site infection and pathogens in Ethiopia: a systematic review and meta-analysis. Patient Saf Surg. 2020;14:7.
Mengesha RE, Kasa BG, Saravanan M, Berhe DF, Wasihun AG. Aerobic bacteria in post surgical wound infections and pattern of their antimicrobial susceptibility in Ayder Teaching and Referral Hospital, Mekelle, Ethiopia. BMC Res Notes. 2014;7:575.
Garner JS. Hospital Infection Control Practice Advisory Committee. Guideline for isolation precaution in hospitals. Infection control and hospital epidemiology. Infect Control Hosp Epidemiol. 1996;17:53–80.
Article CAS PubMed Google Scholar
Cheesbrough M. District laboratory practice in tropical countries part II. 2nd ed. New York: Cambridge University Press; 2006. p. 45–58.
Book Google Scholar
Clinical Laboratory Standard Institute. Performance standards for antimicrobial susceptibility testing; 30th ed. CLSI document M100. CLSI. 2021;M100.
Awoke N, Arba A, Girma A. Magnitude of surgical site infection and its associated factors among patients who underwent a surgical procedure at Wolaita Sodo University Teaching and Referral Hospital, South Ethiopia. PLoS ONE. 2019;14(12): e0226140.
Narula H, Chikara G, Gupta P. A prospective study on bacteriological profile and antibiogram of postoperative wound infections in a tertiary care hospital in Western Rajasthan. J Fam Med Prim Care. 2020;9(4):1927–34.
Ayala D, Tolossa T, Markos J, Yilma MT. Magnitude and factors associated with surgical site infection among mothers underwent cesarean delivery in Nekemte town public hospitals, western Ethiopia. PLoS ONE. 2021;16(4): e0250736.
Lubega A, Joel B, Justina LN. Incidence and etiology of surgical site infections among emergency postoperative patients in Mbarara regional referral hospital, South Western Uganda. Surg Res Pract. 2017. https://doi.org/10.1155/2017/6365172 .
Shiferaw WS, Aynalem YA, Akalu TY, Petrucka PM. Surgical site infection and its associated factors in Ethiopia: a systematic review and meta-analysis. BMC Surg. 2020;20(1):1–15.
Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect. 2017;96(1):1–15.
Misha G, Chelkeba L, Melaku T. Incidence, risk factors and outcomes of surgical site infections among patients admitted to Jimma Medical Center, South West Ethiopia: prospective cohort study. Ann Med Surg (Lond). 2021;65: 102247.
PubMed Google Scholar
Costa T, Medeiros P, Salles M. Smoking increases the risk of surgical site infection after hydrocelectomy in adults: a retrospective cohort study in Brazil. J Infect Dev Ctries. 2018;11:950.
Jiang C, Chen Q, Xie M. Smoking increases the risk of infectious diseases: a narrative review. Tob Induc Dis. 2020;18:60.
Vasundhara Devi P, Sreenivasulu Reddy P, Shabnum M. Microbial profile and antibiotic susceptibility pattern of orthopedic infections in a tertiary care hospital: a study from South India. Int J Med Sci Public Health. 2017;6(5):838–41.
Asres GS, Legese MH, Woldearegay GM. Prevalence of multidrug resistant bacteria in postoperative wound infections at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. Arch Med. 2017;9 (4):0.
Asres GS, Legese MH, Woldearegay GM. Prevalence of multidrug resistant Bacteria in postoperative wound infections at Tikur Anbessa specialized hospital, Addis Ababa, Ethiopia. Archives of Medicine. 2017;9(4).
Mohammed A, Adeshina GO, Ibrahim YK. Incidence and antibiotic susceptibility pattern of bacterial isolates from wound infections in a tertiary hospital in Nigeria. Trop J Pharm Res. 2013;12(4):617–21.
Khanam RA, Islam MR, Sharif A, Parveen R, Sharmin I, Yusuf MA. Bacteriological profiles of pus with antimicrobial sensitivity pattern at a teaching hospital in Dhaka City. Bangladesh J. 2018. https://doi.org/10.3329/bjid.v5i1.37710 .
Dessie W, Mulugeta G, Fentaw S, Mihret A, Hassen M, Abebe E. Pattern of bacterial pathogens and their susceptibility isolated from surgical site infections at selected referral hospitals, Addis Ababa, Ethiopia. Int J Microbiol. 2016. https://doi.org/10.1155/2016/2418902 .
Bitew Kifilie A, Dagnew M, Tegenie B, Yeshitela B, Howe R, Abate E. Bacterial profile, antibacterial resistance pattern, and associated factors from women attending postnatal health service at University of Gondar Teaching Hospital, Northwest Ethiopia. Int J Microbiol. 2018. https://doi.org/10.1155/2018/3165391 .
Kalayu AA, Diriba K, Girma C, Abdella E. Incidence and bacterial etiologies of surgical site infections in a Public Hospital, Addis Ababa, Ethiopia. Open Microbiol J. 2019. https://doi.org/10.2174/1874285801913010301 .
Szaniawski MA, Spivak AM. Recurrent Paenibacillus infection. Oxf Med Case Rep. 2019;2019(5):omz034.
Tena D, Fernández C, Lago MR. Alcaligenes faecalis: an unusual cause of skin and soft tissue infection. Jpn J Infect Dis. 2015;68(2):128–30.
Patterson SB, Mende K, Li P, Lu D, Carson ML, Murray CK, et al. Stenotrophomonas maltophilia infections: clinical characteristics in a military trauma population. Diagn Microbiol Infect Dis. 2020;96(2): 114953.
de With K, Allerberger F, Amann S, Apfalter P, Brodt HR, Eckmanns T, et al. Strategies to enhance rational use of antibiotics in hospital: a guideline by the German Society for Infectious Diseases. Infection. 2016;44(3):395–439.
Ali KM, Al-Jaff BM. Source and antibiotic susceptibility of Gram-negative bacteria causing superficial incisional surgical site infections. Int J Surg Open. 2021;30:100318.
Mora-Guzmán I, Rubio-Perez I, Maqueda González R, Domingo Garcia D, Martín-Pérez E. Surgical site infection by carbapenemase-producing Enterobacteriaceae. A challenge for today's surgeons. Cir Esp (Engl Ed). 2020;98(6):342–9.
Ma F, Xu S, Tang Z, Li Z, Zhang L. Use of antimicrobials in food animals and impact of transmission of antimicrobial resistance on humans. Biosaf Health. 2021;3(1):32–8.
Download references
Acknowledgements
The authors would like to thank Addis Ababa University, Armauer Hansen Research Institute, Debre Tabor University, Uppsala University, and The Centre for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa) for supporting this study. We would like to extend our gratitude to the Debre Tabor compressive specialized hospital , Tikur Anbessa Specialized Hospital, Hawassa University Teaching Hospital, and Jimma University Teaching Specialized Hospital for allowing us to conduct the study. We are thankful to the physicians, nurses, and microbiologists from all the study sites who helped us undertake this study. Lastly, our gratitude goes to all the study participants.
This study was sponsored by Addis Ababa University, Armauer Hansen Research Institute, Uppsala University, SIDA/SAREC bilateral research cooperation with Ethiopia and The Centre for Innovative Drug Development and Therapeutic Trials for Africa.
Author information
Authors and affiliations.
Department of Medical Laboratory Science, College of Medicine and Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
Seble Worku
Bacterial and Viral Diseases Research Directorate, Armauer Hansen Research Institute, Addis Ababa, Ethiopia
Seble Worku, Ashenafi Alemu, Berhanu Seyoum, Alemseged Abdissa, Adane Mihret & Getachew Tesfaye Beyene
Department of Microbiology, Immunology and Parasitology, College of Health Sciences, School of Medicine, Addis Ababa University, Addis Ababa, Ethiopia
Seble Worku, Tamrat Abebe & Adane Mihret
Department of Medical Biochemistry and Microbiology, Uppsala University, Uppsala, Sweden
Göte Swedberg
You can also search for this author in PubMed Google Scholar

Contributions
SW: was the primary researcher, conceived the study, data collection, analysis, interpretation of the findings, drafting the manuscript, and write-up. AAl substantially participated in laboratory work. TA, BS, GS, AAb, AM and GTB: substantially participated in the design of the study, reviewed the manuscript, and provided critical intellectual content. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Seble Worku .
Ethics declarations
Ethics approval and consent to participate.
Ethical clearance and approval were obtained from Addis Ababa University's College of Health Sciences and AAREC, AAUMF03-008/2020. Above all the data were collected after full informed and written consent or assent was obtained from each participant.
Consent for publication
Not applicable.
Competing interests
We authors declare that no competing interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Worku, S., Abebe, T., Alemu, A. et al. Bacterial profile of surgical site infection and antimicrobial resistance patterns in Ethiopia: a multicentre prospective cross-sectional study. Ann Clin Microbiol Antimicrob 22 , 96 (2023). https://doi.org/10.1186/s12941-023-00643-6
Download citation
Received : 04 July 2023
Accepted : 10 October 2023
Published : 07 November 2023
DOI : https://doi.org/10.1186/s12941-023-00643-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Surgical site infections etiologies
- Emerging pathogens
- Risk factors
Annals of Clinical Microbiology and Antimicrobials
ISSN: 1476-0711
- Submission enquiries: [email protected]
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Magnitude of surgical site infection and its associated factors among patients who underwent a surgical procedure at Wolaita Sodo University Teaching and Referral Hospital, South Ethiopia
Roles Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliation Department of Nursing, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia
Roles Conceptualization, Data curation, Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing
Roles Conceptualization, Investigation, Software, Writing – original draft
Affiliation Wolaita Sodo University Teaching and Referral Hospital, Wolaita Sodo University, Wolaita Sodo, Ethiopia
- Nefsu Awoke,
- Aseb Arba,

- Published: December 5, 2019
- https://doi.org/10.1371/journal.pone.0226140
- Reader Comments
Introduction
Surgical site infections are infections that take place within 30 days of an operative procedure. Worldwide, 23% of patients develop surgical site infections among all surgeries annually with the worst complications causing prolonged hospital stays, increased resistance of microorganisms to antimicrobials, higher health system costs, emotional stress for patients and their families, and substantial economic burdens on hospitals. Therefore, this study was created to assess the magnitude and associated factors of surgical site infection at Wolaita Sodo University Teaching and Referral Hospital.
We conducted a hospital-based cross-sectional study on patients who underwent a surgical procedure in 2018 at Wolaita Sodo University Teaching and Referral Hospital. We applied a systematic random sampling technique to obtain 261 patient records from all records of surgical patients from January 1, 2018, to December 30, 2018. We collected data using a pretested checklist. We used bivariate and multivariate logistic regression analysis to identify factors associated with surgical site infection. We considered a P-value < 0.05 as statistically significant. Summary measures, texts, tables, and figures present the results of the analysis.
Among the 261 patients, 34 or 13% (95% CI = 9.2%, 17.2%) developed surgical site infection. Patients younger than 40 years old [AOR 6.45; 95% CI (1.56, 26.67)], illiterate [AOR 4.25; 95% CI (1.52, 11.84)], with a history of previous hospitalization [AOR 4.50; 95% CI (1.44, 14.08)], with a prolonged preoperative hospital stay (≥ 7 days) [AOR 3.88; 95% CI (1.46, 10.29)], and admitted to the public wing of the ward [AOR 0.24; 95% CI (0.07, 0.79)] possessed factors associated with surgical site infection.
The magnitude of surgical site infection in this study was high. Shortening preoperative hospital stays, delivering intravenous antimicrobial prophylaxis before surgery, and giving wound care as ordered would significantly reduce the incidence of surgical site infection.
Citation: Awoke N, Arba A, Girma A (2019) Magnitude of surgical site infection and its associated factors among patients who underwent a surgical procedure at Wolaita Sodo University Teaching and Referral Hospital, South Ethiopia. PLoS ONE 14(12): e0226140. https://doi.org/10.1371/journal.pone.0226140
Editor: Lars-Peter Kamolz, Medical University Graz, AUSTRIA
Received: June 11, 2019; Accepted: November 20, 2019; Published: December 5, 2019
Copyright: © 2019 Awoke et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript.
Funding: The source of funding for this research was Wolaita Sodo University, College of Health Science and Medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Surgical site infection (SSI) refers to infections that take place within 30 days of an operative procedure and may extend to more than 30 days according to the surgical procedure [ 1 ]. One of the common problems in a hospital setting, reports from the World Health Organization in 2009, 23% of surgical patients worldwide developed SSIs [ 2 ]. In the US, 500,000 SSIs occur every year and are the second most common health care institution infection [ 3 ]. In 2012, a São Paulo, Brazil, study revealed that 22% of 195 patients admitted to an intensive care unit developed a hospital acquired infection [ 4 ]. According to a 2012 study conducted in Nigerian pediatric hospital, 30.9% of all operation sites were infected [ 5 ].
The impact of healthcare-associated infection is multifactorial, including prolonged hospital stays, long-term disabilities, increased resistance of microorganisms to antimicrobials, high health system costs, emotional stress for patients and their families, and substantial economic burdens for hospitals. SSIs and hospital stays can lead to pressure ulcers, hypoglycemia, additional economic burden, and death [ 6 , 7 ]. Different studies have shown that the most common causes of SSIs relate to inadequate supplies of personal protective equipment, a lack of training on infection control measures, an absence of hospital policy on infection control, and inadequate hand washing practices [ 8 , 9 ]. Infections might also be related to direct contact between a patient and an inanimate object without proper hand washing or using appropriate antisepsis [ 2 , 6 ]. Excessive nursing workload is an additional factor of SSIs [ 4 ].
Most SSIs are preventable through basic and advanced nursing procedures of wound care. To provide effective infection prevention care, health care professionals should stay updated with the knowledge and skills to provide the best possible practice [ 5 , 10 ]. In Sub-Saharan Africa (including Ethiopia and especially the southern part of the country), there are few evidential studies regarding the magnitude of SSI and its associated factors. Therefore, this study was created to assess the magnitude and associated factors of SSI at Wolaita Sodo University Teaching and Referral Hospital (WSUTRH).
Method and materials
Study setting.
We conducted the study in WSUTRH. The total number of beds in the hospital is 268, covering medical, pediatrics, surgical, gynecology, and obstetrics wards. The hospital gives service to approximately 3.5–5 million patients annually. We conducted a hospital-based cross-sectional study design using a retrospective chart review. The source populations were charts of patients who underwent surgery at WSUTRH from January 1, 2018, to December 30, 2018.
Inclusion criteria
- We included all patients who underwent surgery during the study period.
Exclusion criteria
- We excluded patients with incomplete charts.
- We excluded patients who had undergone an operation with another institution before coming to WSUTRH for a follow-up.
Sample size determination
We determined the sample size using a single population proportion formula and the following assumptions: p being the prevalence of 19.1% from a study conducted in Hawassa [ 11 ], d being the expected margin of error (5%), Z being the standard score corresponding to a 95% confidence interval, and α being the risk of rejecting the null hypothesis (0.05). The required sample size was determined to be 261.
Sampling technique
A total of 3,715 patients underwent a surgical procedure at WSUTRH from January 1, 2018, to December 30, 2018. Using a systematic random sampling technique, we selected 261 patient charts at every fourteenth interval. The sampling interval was determined by dividing the total study population who underwent a surgical procedure in the last one year at WSUTRH by the sample size, and then the starting point was randomly selected by lottery method.
Data collection tool and technique
We collected data using a pretested checklist, which we developed by reviewing different literature. Review of microbiology reports and patient medical records used indirect measurement of the surgical site infection method. The indirect method of SSI surveillance is both reliable (sensitivity, 84%–89%) and specific (specificity, 99.8%) [ 8 , 12 ]. We involved two data collectors who have a BSc degree in nursing in the data collection process. Using a card number of patients, data collectors traced and collected data from randomly identified charts of a patient using a checklist.
Data processing and analysis
We entered the collected data and analyzed the data using SPSS version 22. We assessed the statistical significance with the dependent variable at a p-value of less than 0.05. We used descriptive statistics including tables to describe the data. We performed bivariate and multivariable logistic regression analysis to see the association between dependent and independent variables. Variables that found to be statistically significant in the bivariate analysis at a p-value of less than 0.25 entered the multivariable logistic regression model. A p-value of less than 0.05 considered statistically significant in a multivariable logistic regression analysis and Odds ratio along with its 95% CI used to assess the association between dependent and independent variables. Finally, the level of statistical significance declared at a p-value less than 0.05.
Data quality control
We did the pretest of the checklist on 5% of the sample size out of the study area to ensure its validity. Two-day training (one day theoretical and one day practical) given on the data collection tool and how to conduct data collection. The principal investigator supervised the activities of the data collector. The principal investigator checked completeness and consistency of data on a daily basis. We did double data entry by two data clerks and consistencies of the entered data were cross-checked by comparing the two separately entered data on SPSS.
Ethical considerations
Ethical approval was first got from the Ethical Clearance Committee of Wolaita Sodo University. Then a letter of cooperation written to Wolaita Sodo University Teaching and Referral Hospital (WSUTRH) administration. Ethical Clearance Committee waived the requirement for informed consent to have data from the patient medical records. Participants’ confidentiality of information assured by excluding names and identifiers in the checklist.
Socio-demographic characteristics
A total, 261 patients were included in the analysis. Forty-six percent of the respondents aged>40 years. Males account for a majority of 62.8% among the participants. Literate was 59.4% and 18.8% were government workers. About half of the participants were from urban in residence. The majority had a previous history of hospitalization, and 77.4% of the participants admitted in the public ward of the hospital. Sixty-seven percent of the participants have stayed in the hospital for over seven days ( Table 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pone.0226140.t001
Surgery related factors
Informed consent was obtained from all the participants. The Majority, 62.8% of the participant underwent elective surgery. Sixty-three percent of the participant’s hand no previous history of surgery. Abdominal surgery was conducted among 42.9% of the participants. The total duration of surgery lasted from 1-2hrs among 54.8% of the participants. About half 50.6% of the respondent lost 500-1500ml of blood during the surgery. Only 9.6% of the participants had an implant inserted at the site of operation ( Table 2 ).
https://doi.org/10.1371/journal.pone.0226140.t002
Comorbidities and wound related factors
Among the participants, 20 (7.7%) had a comorbid medical condition and among them, 8 (3.1%) were diabetes mellitus patients. The remaining others had hypertension 6(2.3%), HIV/ADIS 3(1.1%) and Malignancy 3(1.1%). Majority 248(95%) of the participants received wound care as ordered. Among them, 62.8% received twice daily ( Table 3 ).
https://doi.org/10.1371/journal.pone.0226140.t003
Anesthesia and medication related factor.
Majority 66.7% of the study participant received general anesthesia and about half 50.2% of the study subject received the anesthesia for the duration of 30–60 min. Antibiotic prophylaxis was given for 86.6% of the study participants. Ninety-three percent of the participants received medication as ordered ( Table 4 ).
https://doi.org/10.1371/journal.pone.0226140.t004
Magnitude of surgical site infection.
The magnitude of Surgical Site infection in this study was found to be 13% (95% CI = 9.2%, 17.2%) ( Fig 1 ).
https://doi.org/10.1371/journal.pone.0226140.g001
Factors associated with surgical site infection.
There were 15 variables in binary logistic regression that had a p-value of ≤ 0.25 and became a candidate for multiple logistic regressions. In multiple logistic regressions, only five were significantly associated with surgical site infection, with P value ≤ 0.05. Patients whose age is between >40 years were 6.45 times more likely to develop surgical infection compared to the age group of 1–18 years [AOR 6.45; 95%Cl (1.56, 26.67)]. Illiterates were 4.25 times more likely to develop surgical site infection compared to literate [AOR 4.25; 95%Cl (1.52, 11.84)]. History of the previous hospitalization was significantly associated with surgical site infection; patients who had hospitalization history were 4.5 times more likely to develop a surgical infection than those who had no history [AOR 4.50; 95%Cl (1.44, 14.08)]. Patients who had prolonged preoperative hospital stay (≥7 days) were 3.88 times more likely to develop surgical site infection compared to those who had < 7 days of the stay [AOR 3.88; 95%Cl (1.46, 10.29)]. Patients who admitted on the public wing of the ward were less likely to develop surgical site infection compared to patients admitted on a private wing [AOR 0.24; 95%Cl (0.07, 0.79)] ( Table 5 ).
https://doi.org/10.1371/journal.pone.0226140.t005
The Magnitude of Surgical Site Infection in this study was found to be 13% (95% CI = 9.2%, 17.2%). Age, Educational status, Previous history of hospitalization, ward condition, Duration of preoperative hospital stay were factors associated with surgical site infection.
The magnitude of surgical site infection in this study was comparable to study conducted in Ethiopia with the magnitudes of 10.9% in Bahir Dar, North West Ethiopia [ 13 ] and 11.1% in Suhul Hospital, Northern Ethiopia [ 14 ]. Also study from Saudi Arabia had consistent finding with this study with magnitude 11.4% [ 15 ]. But our study was lower than the study conducted in Hawassa with magnitude 19.1%[ 11 ] and studies conducted in different parties of Africa with magnitude ranging from (20.6%– 27.56%) [ 16 – 18 ]. But our study was higher than studies conducted in Algeria (5.4%) [ 14 ] and Tunisia (8.6%) [ 19 ]. This might be attributed to the difference in study design, study period and sample size.
In this study Patients whose age is between >40 years were 6.45 times more likely to develop surgical infection compared to the age group of 1–18 years [AOR 6.45; 95%Cl (1.56, 26.67)] which is consistent with studies conducted in Bahir Dar, North West Ethiopia[ 13 ], Hawassa [ 11 ], Algeria[ 14 ] and Cameroon [ 18 ]. This is in fact that as age advances there was an increased incidence of the surgical site. This was also described by different studies in that age is one of non-modifiable risk factor that influence wound healing process and increases the likelihood of a positive surgical outcome [ 20 ]. Also in comparison to the younger population, these patients are usually characterized by an impaired immune response to infectious agents, inferior nutritional status, and possibly more comorbidities [ 21 ].
The Educational level had a positive effect on surgical site infection. This was also indicated on this study that Illiterates were 4.25 times more likely to develop surgical site infection compared to literate [AOR 4.25; 95%Cl (1.52, 11.84)] this was consistent with the study conducted in Saudi Arabia 15 . In fact that the levels of educations are important for minimizing perioperative SSI risk through the implementation of recommended process measures [ 12 ].
The Previous history of hospitalization was significantly associated with surgical site infection. Indicated in this study patients with the previous history of hospitalization were 4.5 times more likely to develop infection compared to those who had no history [AOR 4.50; 95%Cl (1.44, 14.08)] this was in agreement with a study conducted in India [ 22 ]. This might be due to that prior exposure to resistant microorganisms increase the likelihood of the rate of infection [ 23 , 24 ].
In this study patients who had ≥ 7 days of Preoperative Hospital Stay were 3.88 times more likely to develop surgical site infection compared to those who had less stay [AOR 3.88; 95%Cl (1.46, 10.29)] this is matched with study conducted in India [ 22 ], Tunisia [ 19 ] and Hawassa [ 11 ] this might be due to that global spread of multi-drug resistant infections in health care set-ups and its ubiquitous diagnostic procedures, therapies and microflora have been shown to increase the rate of surgical site infection [ 23 , 24 ].
The Magnitude of Surgical Site Infection in this study was high. Age, Educational status, Previous history of hospitalization, ward condition, Duration of preoperative hospital stay were factors associated with surgical site infection. Shortening the preoperative hospital stay, delivery of intravenous antimicrobial prophylaxis before surgery, giving wound care and medication as ordered were important measures to reduce the incidence of surgical site infection.
Declarations
Ethics approval.
Ethical approval was first got from the Ethical Clearance Committee of Wolaita Sodo University. Then a letter of cooperation written to Wolaita Sodo University Teaching and Referral Hospital (WSUTRH) administration. Participants’ confidentiality of information assured by excluding names and identifiers in the checklist.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
The source of funding for this research was Wolaita Sodo University, College of Health science and medicine. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
The authors like to express their gratefulness for all study participants for their genuine participation and staffs of Wolaita Sodo University Teaching and Referral Hospitals for their cooperation.
- 1. Heather L. O., David H. K., Louise F. L., Janet L. K., Deirdre O.D., Susie J., et al. Foundations of Best Practice for Skin and Wound Management: Best Practice Recommendations for The Prevention and Management of Wounds. 2018.
- 2. World Health Organization (WHO). First Global Patient Safety Challenge Clean Care is Safer Care. 2009. whqlibdoc.who.int/publications/2009/9789241597906_eng.pdf
- View Article
- Google Scholar
- 6. Suzanne M. Pear. Patient Risk Factors and Best Practices for Surgical Site Infection Prevention. 2007. https://www.halyardhealth.com/media/1515/patient_risk_factors_best_practices_ssi.pdf
- 10. World Health Organization (WHO). Global guidelines for the prevention of surgical site infection. 2016. https://www.who.int/gpsc/global-guidelines-web.pdf?ua=1
- PubMed/NCBI
- 22. Atif M.L., Azouaou A., Bouadda N., Bezzaoucha A., Si-Ahmed M., Bellouni R. Incidence and predictors of surgical site infection in a general surgery department in Algeria. Elsevier Masson SAS. 2015.
Risk of surgical site infection after hip hemiarthroplasty of femoral neck fractures: a systematic review and meta-analysis
- Hip Arthroplasty
- Open access
- Published: 28 May 2024
Cite this article
You have full access to this open access article

- Ubong Silas ORCID: orcid.org/0000-0002-6922-6599 1 ,
- Christof Berberich ORCID: orcid.org/0000-0002-5220-1889 2 ,
- Priscilla Anyimiah ORCID: orcid.org/0009-0006-3760-8337 3 ,
- Dominik Szymski ORCID: orcid.org/0000-0002-1739-8524 4 &
- Markus Rupp ORCID: orcid.org/0000-0001-7221-3783 4
Introduction
Surgical site infection (SSI) is a major complication following hemiarthroplasty surgery for displaced neck of femur fractures. Our aim is to systematically analyse relevant peer-reviewed studies for recent clinical information on the quantitative risk of surgical site infection (SSI) after hemiarthroplasty (HA) of hip fracture patients and on the factors which influence the SSI rates.
A comprehensive search of electronic databases (PubMed, Cochrane) was performed for clinical articles published between 2005 and 2023 and systematically reviewed with a defined list of inclusion and exclusion criteria. The methodology was undertaken and reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement checklist, while the detailed search strings and study protocol were published in PROSPERO (CRD42023458150). The pooled risks of SSIs were calculated in both primary and subgroup analyses.
The primary analysis showed a pooled superficial SSI rate after hemiarthroplasty of 1.3% (95% confidence interval (CI) 0.71; 2.04) from 17 studies with 29,288 patients and a deep SSI rate of 2.14% (1.87; 2.42) from 29 studies with 192,392 patients. Higher infection rates were observed with longer follow-up periods for deep SSI: pooled rates increased from 1.24% (0.73; 1.87) at 1 month to 2.64% (2.03; 3.31) at 12 months. Additionally, studies using defined criteria for infection diagnosis reported higher rates compared to undefined criteria: pooled deep SSI rates were 2.91% (1.40; 4.92) vs. 0.62% (0.34; 0.96) for defined vs. undefined criteria respectively, and 3.18% (2.23; 4.29) vs. 1.7% (1.44; 1.99) for superficial SSI.
Conclusions
The results of this study demonstrate a substantial SSI risk and a high variability of the infection rates following hemiarthroplasty for hip fracture patients. A standardization of infection criteria and an extended follow-up period are advisable and should be considered in guidelines aimed at improving the standard of care for these patients.
Avoid common mistakes on your manuscript.
Population ageing is an increasing demographic trend which affects many countries across the globe. Associated with ageing is a rise in severe low energy injuries such as falls from a standing position, affecting above all the frail geriatric patient. Such traumas often lead to hip fractures (HF) as a consequence of poor bone quality and severe osteoporosis. The global number of HF is expected to increase from 1.26 million in 1990 to 4.5 million by the year 2050 [ 1 ]. In the UK, the health community and orthopaedic surgeons have been prepared for an increasing volume of osteoporotic fractures which may in future overwhelm the medical services and lead to estimated costs between £2–3 billion by 2030 [ 2 ]. These calculations do not even include the enormous social costs due to the high mortality and morbidity post-discharge.
Usually, HF treatment requires surgery to repair or replace the fractured hip. Displaced neck of femur fractures are particularly problematic as a consequence of the blood supply disruption to the head of the femur and are therefore typically treated with urgent hemi- or total hip arthroplasty, especially in the elderly population [ 3 ]. Given the high level of frailty such patients are even more susceptible to developing some of the hip replacement associated complications including periprosthetic fractures, loosening, dislocations or infections [ 4 ]. However, the incidence of these complications in HF populations have been largely reported on basis of single centre experiences or on larger national cohorts in the past and may not mirror the now more stringent contemporary femur fracture treatment recommendations issued by some recent national guidelines [ 5 , 6 , 7 ]. In addition, substantial cross-country variations in care for patients presenting with HFs seem to exist [ 8 ].
Surgical site infections (SSI) have been recognised as one of the most debilitating complications in HF patients. They are associated with higher morbidity and mortality rates, increased length of hospital stays, extensive use of antibiotics, enhanced follow-up, and revision surgeries [ 9 , 10 , 11 ]. A particularly severe situation occurs if the infection also affects the deeper joint space and the prosthesis. The burden of SSI on the already high cost of HF has led to the introduction of new surgical methods, bundled payment system, innovative products as well as research with the aim of reducing the rate of SSI in patients after arthroplasty. This rate also remains one of the significant outcomes in clinical and economic studies related to the management of HF.
Given the high degree of variations in care on the one hand and the high impact of SSI in these patients on the other, it is important to gain a comprehensive picture on the overall burden of infections after hemiarthroplasty. Therefore, a qualitative and quantitative systematic review was performed with the objectives of (1) summarizing the available information on SSI rate after hemiarthroplasty in HF patients based on literature from 2005 to July 2023 and (2) identifying factors (e.g. types of SSI, criteria for infection definition, study design, observation periods) that can help explain the variations in these rates.
A systematic review of published studies was conducted. The methodology was undertaken and reported according to the PRISMA statement checklist (see Supplementary material) [ 12 ]. The study protocol was registered prior to conducting the review with PROSPERO registration number: CRD42023458150.
Eligibility criteria
Randomized control trials and observational studies that reported rates of SSI after HA following a femoral neck fracture were analysed. The differentiation of superficial SSI and deep SSI was based on the infection criteria provided by the Centers for Disease Control and Prevention’s guidelines which are summarized in the draft guideline [ 13 ]. Studies were included and excluded as described in Table 1 .
Search strategy
Searches were carried out on PubMed (including subsets such as MEDLINE, PMC, etc.) and Cochrane Library. The literature search of PubMed and Cochrane® library was performed on July 13th, 2023. With the aim of identifying recently published literature, the search string was limited to studies published from 2005 onwards. The key search terms were “hip fracture”, “fractured neck of femur”, “hemiarthroplasty”, “PJI”, “SSI”. Detailed search strings were published in the PROSPERO protocol. The search was restricted to human randomized control trials and observational studies published in English.
The abstracts and full tests of the identified records were screened by two individuals using PICO Portal (New York, United States). Studies that passed the screening were managed in the Citavi reference software (Swiss Academic Software, Wädenswil, Switzerland).
Study selection
The studies were included based on the inclusion criteria outlined in Table S1 (see Supplementary material). Titles and abstracts of studies were initially screened to determine study’s eligibility, then full text of studies reviewed. The studies that fully met the inclusion criteria were included for data extraction.
All records identified by the literature search were retrieved and screened independently by two researchers. The researchers were blinded to each other’s decisions and disagreements between the two researchers were settled by a third reviewer. Detailed methodology of data extract and risk assessment can be found in the Supplementary material.
Quantitative synthesis
Primary analysis.
Dichotomous outcomes used in the pooled analysis were reported as number of events per group. In included studies where this is not the case, the number of events was estimated by multiplying the reported risk of events by the total number of patients per group.
Meta-analysis of proportion was carried out to estimate the pooled rate of dichotomous outcomes. It involved the synthesis of a one-dimensional binomial measure known as (weighted) average proportions, estimated by pooling the results (proportions) from various studies and weighted by the inverse of their sampling variances [ 14 ]. The heterogeneity of each analysis was assessed using the I2 statistic. The meta-analysis was performed in R using the “metafor” and “meta” packages and the random effects model [ 14 ].
Subgroup analyses
Due to the differences in the follow-up periods reported by the different studies, subgroup analyses were performed for the primary outcomes using follow-up periods of 1-month, 3-months, and 12-months. If the reported rates were outside of these follow-up periods, a rate conversion was performed to the closest follow-up period before the meta-analysis.
A subgroup analysis comparing pooled SSI rates between defined versus undefined SSI was carried out to minimize the effect of missing definition in several papers.
Sensitivity analyses
Sensitivity analyses was conducted to evaluate the reliability of the generated findings. Studies deemed to have a high risk of bias, those with an unusually large sample size (characterized as studies exceeding 100% more participants than others in each group), and studies reporting exceptionally extreme outcomes were excluded. The subsequent findings were then compared with the initial analysis to assess the influence of these studies on the overall results.
The screening process and the steps involved in the selection of included studies are summarized in the PRISMA protocol flow diagram (see Fig. 1 ). A total of 270 and 141 abstracts were retrieved from the literature search. In total, 122 full text articles were screened, and 38 studies selected for data extraction after application of the eligibility criteria.
Study selection outlined in a PRISMA flow diagram
Study characteristics
A summary of the study characteristics including study quality is provided in Table S2 (Supplementary material). The majority of the included studies (71%, 27 studies) were published between 2019 and 2023. The remaining 29% (11 studies) were published between 2005 and 2018. Observational studies (31) reporting retrospective data from databases were most frequent. Two and three of the studies were randomized control trials and clinical studies, respectively. The studies were conducted in a total of 14 countries: China, Finland, France, Germany, Israel, Japan, Netherlands, Norway, Singapore, South Korea, Spain, Turkey, United Kingdom and the United States of America. Most studies (26%,10 studies) were conducted in the United Kingdom, while six and five were reported from the United States and Netherlands, respectively.
In 50% of the studies a definition of SSI was used. Most studies (74%), which reported a definition of infection used the Centres for Disease control and prevention (CDC) definition [ 13 ]. Two studies each applied the definition issued by the Infectious Disease Society of America [ 15 ], and the UK Health Security Agency [ 16 ], while one study used the International Consensus Meeting on Prosthetic Joint Infections (ICMPJI) definition [ 17 , 18 ].
Included studies reported on superficial SSI, deep SSI, both superficial and deep SSI or combined SSI. Follow-up periods reported by the various studies ranged from 1 month to 10 years. Six of the studies did not report the follow-up period.
Risk of bias
An overview of the individual study quality per the Newcastle-Ottawa scale can be found in Table S2 (Supplementary material). Eighteen of the included studies had quality ratings of “good” with another eighteen studies rated as “fair”. The remaining two studies were rated as “poor”.
Superficial surgical site infection
A total of 17 studies with a total of 22,679 patients reported on the rate of superficial SSI post hemiarthroplasty with a pooled rate estimated as 1.44% (95% CI 0.77; 2.28) and heterogeneity of 95% (see Fig. 2 ).
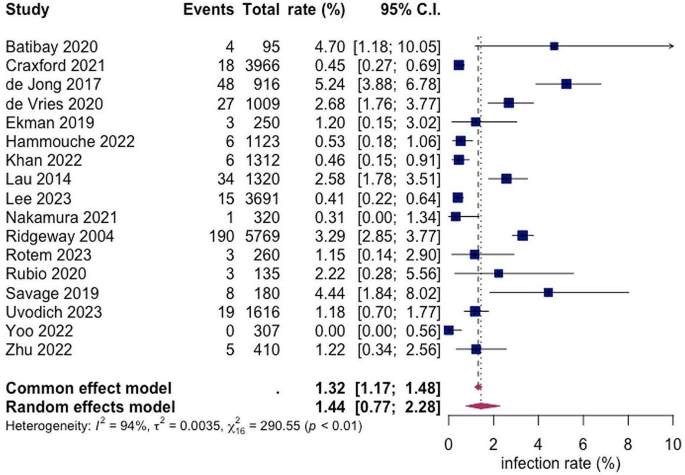
Forest Plot showing the risk of superficial SSI after hemiarthroplasty of hip fracture patients. Overall pooled SSI rate was calculated on basis of 17 studies including 22,679 patients with a value of 1.44% (95% CI 0.77; 2.28). Random effects model was used
Deep surgical site infection
A total of 29 studies with a total of 197,092 patients reported on the rate of deep SSI post hemiarthroplasty with a pooled rate estimate of 2.14% (1.87; 2.42) and heterogeneity of 95% (see Fig. 3 ). Within the included studies, the reported rates of deep SSI varied with the lowest being 0.00% and 14.94% as the highest (see Fig. 3 ).
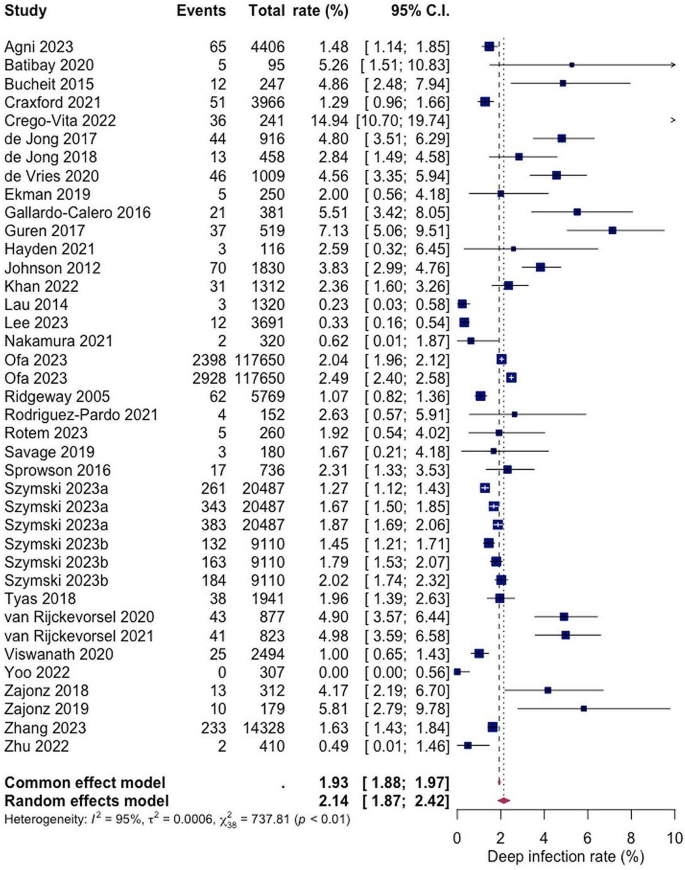
Forest Plot showing the risk of deep SSI after hemiarthroplasty of hip fracture patients. Overall pooled deep SSI rate was calculated on basis 29 studies including 197,092 patients with a value of 2.14% (95% CI 1.87; 2.42). Random effects model was used
In the subgroup analyses, rates of superficial SSI following HA in hip fracture patients varied across different follow-up periods. For a 1-month follow-up the pooled rate was estimated at 1.78% (0.49%; 3.77%), while for 3-month follow-up an infection rate of 2.46% (1.26%; 4.02%) was calculated. The pooled 12-month rate at 0.63% (0.01; 1.95) was notably lower than the others, this could be because superficial SSI appear within the first week post as they rather involve more virulent bacteria, such as Staphylococcus aureus [ 19 ]. Heterogeneity was observed, measuring at 92%, 90% and 91%, for the 1-month, 3-month and 12-month follow-ups, respectively (see Fig. 4 ).

Forest plots showing pooled rates of superficial SSI at different follow-up period; 1-month, 3-month, and 12-month. Random effects model was used
Similarly, for deep SSIs, the pooled rates following HA in hip fracture patients were analysed for different follow-up periods. After 1-month 1.24% (0.73%; 1.87%), after 3-months 2.26% (1.90%; 2.66%) and after 12 months 2.64% (2.03%; 3.31%) were identified with a deep SSI. Heterogeneity percentages were observed, measuring at 90%, 93%, and 95% for the 1-month, 3-month, and 12-month follow-ups, respectively (see Fig. 5 ).
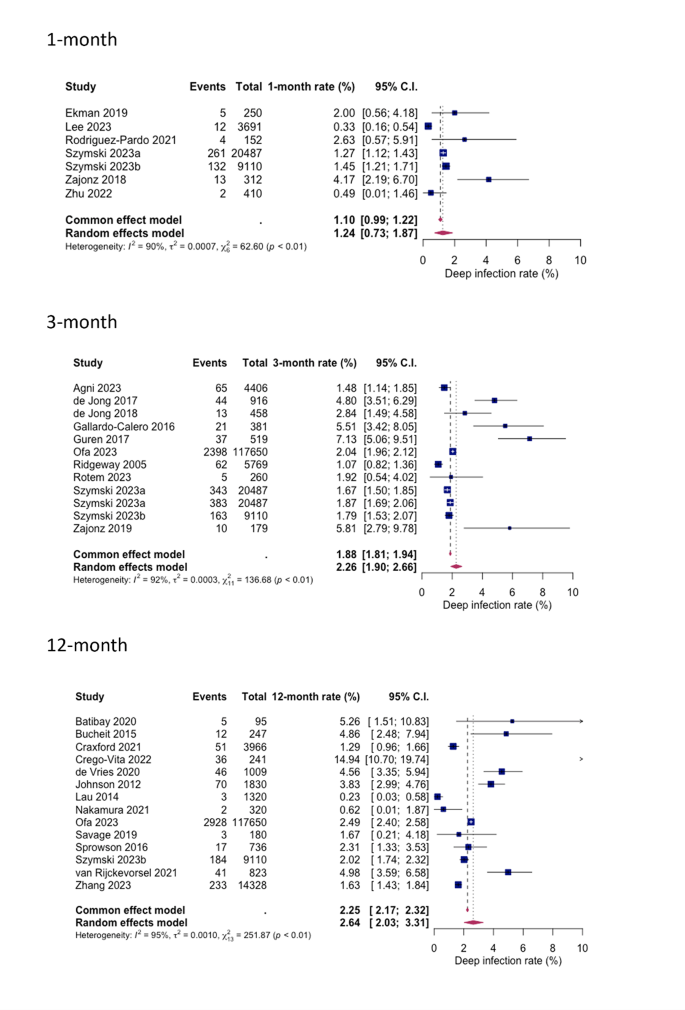
Forest plots showing pooled rates of deep SSI at different follow-up period; 1-month, 3-month, and 12-month. Random effects model was used
Defined versus undefined SSI criteria
For both superficial and deep infections, a statistically significant difference in SSI rates was shown between studies with explicitly defined criteria and those with undefined criteria (see Figs. 6 and 7 ). The pooled superficial SSI rate for defined and undefined criteria were 2.91% (1.40%; 4.92%) and 0.62% (0.34%; 0.96%) respectively, while the pooled deep SSI rates were 3.15% (2.23%; 4.29%) and 1.70% (1.44%; 1.99%) respectively.
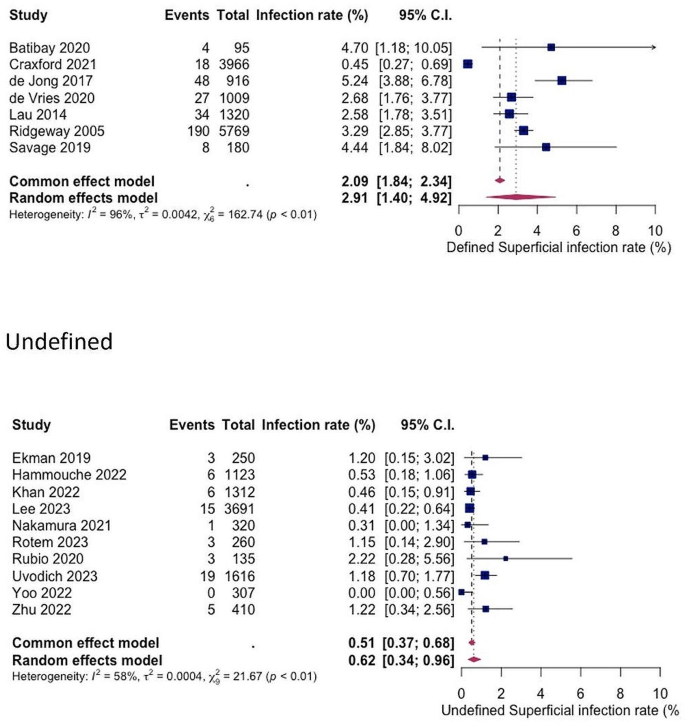
Forest plots showing pooled rates of superficial SSI between studies that used defined and undefined infection criteria. Random effects model was used
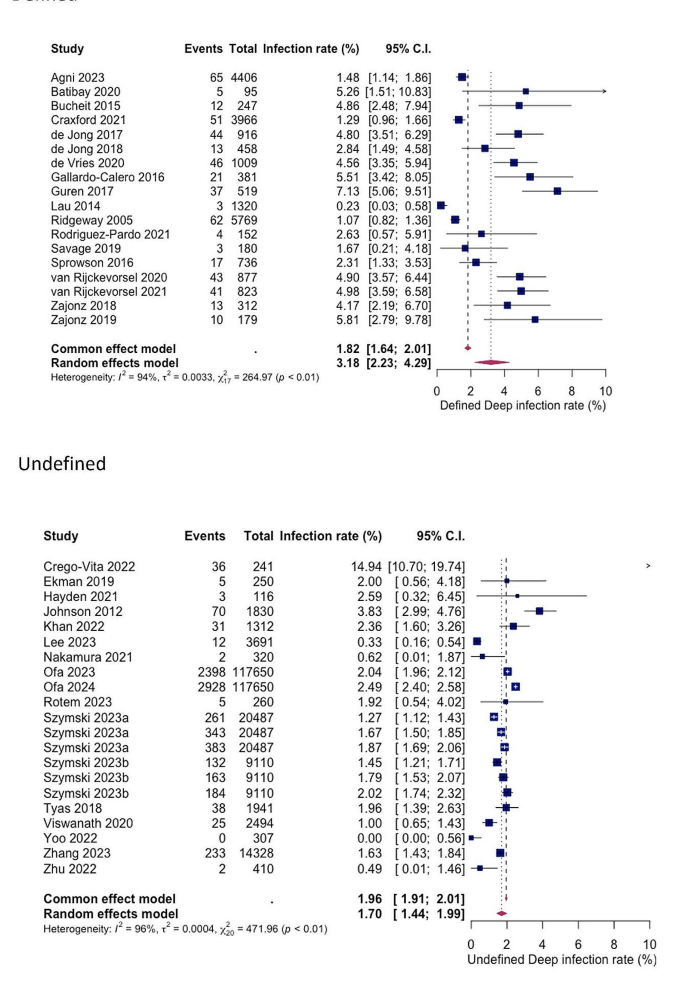
Forest plots showing pooled rates of deep SSI between studies that used defined and undefined infection criteria. Random effects model was used
The impact of outliers, study quality, and sample size on the results of the primary analysis is shown in Figure S1 (see Supplementary material). Overall, the sensitivity analyses showed that the results were mostly robust and consistent.
Our first aim was to assess the overall burden of SSI following HA in hip fracture patients. The results are consistent with previous observations showing that infections are among the most frequent complications in these patients. With a pooled overall rate of 1.3% for superficial SSI and a pooled overall rate of 2.14% for deep SSI with even higher values for extended follow-up periods and defined infection criteria the incidence of infections was higher than usually reported for elective hip arthroplasty procedures [ 20 , 21 ]. Data from the American Surgical Quality Improvement Program [ 22 ] and from the European ECDC Surveillance Atlas for infections [ 23 ] report SSI rates following elective primary hip replacement of around 1%. There was also a trend observed towards lower infection rates over the last years which may reflect the implementation of surgical quality improvement programmes in several countries with focus on pre-operative patient optimization, better sterile protocols and surgical techniques or minimizing wound drainage. While these approaches have been effective in primary THA, their impact on hemiarthroplasty is less clear. This is also partially to be expected given the high index of frailty among hip fracture patients and the difficulty to optimize patient risk factors prior to emergency surgery [ 24 ]. In fact, a recent population-based cohort study on 74,771 hip fracture patients ≥ 65 years old in Denmark revealed a significantly higher 30-days mortality for those patients who experienced a hospital-treated infection after hip fracture surgery compared to those without infection (hazard ratio = 2.7) [ 25 ]. In particular, the risk of developing a subsequent episode of pneumonia or even systemic sepsis was increased several-fold in the infection cohort [ 25 ].
Our second aim was to analyse factors influencing the SSI rate. Significant variations in infection rates were observed among the studies considered, attributable to differences in (1) infection follow-up periods and (2) infection definition criteria. Notably, extended observation periods beyond the acute phase of one month showed a clear tendency towards higher infection rates. This trend was particularly evident in cases of deep, prosthesis-associated infections, with rates increasing from 1.24% at one month to 2.26% at three months and 2.64% at one year. Given the chronic nature of many implant-related infections, longer follow-up times of at least one year may be necessary to accurately capture delayed infections and ascertain the true incidence of periprosthetic joint infections (PJI). This concern was also highlighted in a recent commentary published in The Lancet, which discussed the observation of a lower-than-assumed overall infection rate during the three-month observation period in a large randomized clinical study involving hemiarthroplasty patients [ 26 ].
Our observations on the second point reveal significant variations in infection rates depending on the clinical criteria employed for diagnosis. When restricting our analysis to studies utilizing defined and widely accepted infection criteria (such as those outlined by the CDC or UK Health Security Agency) [ 13 , 15 ], we noted substantially higher infection rates for both types of SSI compared to studies lacking explicit criteria (superficial SSI: defined = 2.91%, undefined = 0.62%; deep SSI: defined = 3.18%, undefined = 1.70%). The CDC definition of SSI, being the earliest and most recognised, classifies infections into superficial incisional, deep incisional, and organ/space categories. Superficial SSI affects only the skin and subcutaneous tissue at the incision site within 30 days post-surgery, while deep SSI involves the fascial and muscle layers within 90 days post-surgery. Organ/space SSI extends beyond these layers to involve bone or joint structures. Despite the lack of a universally accepted definition for PJI, recent evidence suggests that the newly proposed European Bone and Joint Infection Society (EBJIS) classification may offer the highest sensitivity and reliability for diagnosing PJI [ 27 , 28 ]. Our findings support the notion that unclear infection criteria in clinical practice pose a significant risk of overlooking cases requiring treatment.
Our study has several limitations. The review included observational retrospective studies, which are considered lower in the hierarchy of study qualities. For the prospective studies included, either the average risk of SSI of both control and intervention or the risk reported for only the control was used in the statistical analysis, leading to a potential bias. The review was limited to English language literature. Furthermore, the incidence data were mostly reported only as proportions with no standard deviation around the SSI rates but were only accounted for by meta-analyses of proportion approach. The variation in the duration of follow-up according to the individual objective of the included studies might be responsible for a potential attrition bias used in the pooled estimate. However, this systematic review also has several strengths including a comprehensive search strategy, screening, risk of bias quality assessment, and methodical implementation of the PRISMA.
The infection rates for both, superficial and deep SSI following HA in hip fracture patients demonstrate a high level of variation within the literature. It was reported that the overall reported infection rates are higher if the follow-up period is extended to up to one year and if established diagnostic criteria for infection definition are used. To enable meaningful analysis and interpretation of studies in the future, the standardized application of established infection criteria for clinical trials is advisable and should be demanded by specialized journals.
Veronese N, Maggi S (2018) Epidemiology and social costs of hip fracture. Injury 49:1458–1460. https://doi.org/10.1016/j.injury.2018.04.015
Article PubMed Google Scholar
Kelly M (2023) Implementing findings from (hip) fracture registries. Injury 54 Suppl. https://doi.org/10.1016/j.injury.2023.110961 . 5:110961
Article Google Scholar
Lewis SR, Macey R, Stokes J et al (2022) Surgical interventions for treating intracapsular hip fractures in older adults: a network meta-analysis. Cochrane Database of Systematic Reviews 2022. https://doi.org/10.1002/14651858.CD013404.pub2
de Haan E, Roukema GR, van Rijckevorsel VAJIM et al (2024) Risk factors for prosthetic joint infections after hemiarthroplasty of the hip following a femoral neck fracture. Injury 55:111195. https://doi.org/10.1016/j.injury.2023.111195
de Haan E, Roukema GR, van Rijckevorsel VAJIM et al (2011) NICE Hip fracture: management. Clinical guideline. https://www.boa.ac.uk/standards-guidance/nice-guidelines.html . Accessed 04 Feb 2024
Australian and New Zealand Guideline for Hip Fracture Care Improving Outcomes in Hip Fracture Management of Adults https://anzhfr.org/wp-content/uploads/sites/1164/2021/12/ANZ-Guideline-for-Hip-Fracture-Care.pdf . Accessed 04 Feb 2024
Hip Fractures in the Elderly - Clinical Practice Guideline | American Academy of Orthopaedic Surgeons https://www.aaos.org/quality/quality-programs/lower-extremity-programs/hip-fractures-in-the-elderly/ . Accessed 04 Feb 2024
Burrack N, Hatfield LA, Bakx P et al (2023) Variation in care for patients presenting with hip fracture in six high-income countries: a cross-sectional cohort study. J Am Geriatr Soc 71:3780–3791. https://doi.org/10.1111/jgs.18530
Guren E, Figved W, Frihagen F et al (2017) Prosthetic joint infection-a devastating complication of hemiarthroplasty for hip fracture. Acta Orthop 88:383–389. https://doi.org/10.1080/17453674.2017.1301009
Article PubMed PubMed Central Google Scholar
Noailles T, Brulefert K, Chalopin A et al (2016) What are the risk factors for post-operative infection after hip hemiarthroplasty? Systematic review of literature. Int Orthop 40:1843–1848. https://doi.org/10.1007/s00264-015-3033-y
Pollmann CT, Dahl FA, Røtterud JHM et al (2020) Surgical site infection after hip fracture - mortality and risk factors: an observational cohort study of 1,709 patients. Acta Orthop 91:347–352. https://doi.org/10.1080/17453674.2020.1717841
Shamseer L, Moher D, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 350:g7647. https://doi.org/10.1136/bmj.g7647
Shamseer L, Moher D, Clarke M et al (1998) Draft guideline for the prevention of surgical site infection, 1998–CDC. Notice Fed Regist 63:33168–33192
Google Scholar
Wang N (2023) Conducting Meta-analyses of proportions in R. JBDS 3. https://doi.org/10.35566/jbds/v3n2/wang
Stevens DL et al (2015) (Clin Infect Dis 2014; 59:147 – 59). Clin Infect Dis 60:1448. https://doi.org/10.1093/cid/civ114
UKHSA (2013) Protocol for the Surveillance of Surgical Site Infection version 6. https://assets.publishing.service.gov.uk/media/61e989028fa8f505985ef463/Protocol_for_the_Surveillance_of_Surgical_Site_Infection.pdf . Accessed 09 Feb 2024
Parvizi J, Gehrke T, Chen AF (2013) Proceedings of the International Consensus on Periprosthetic Joint Infection. Bone Joint J 95-B:1450–1452. https://doi.org/10.1302/0301-620X.95B11.33135
Parvizi J, Tan TL, Goswami K et al (2018) The 2018 definition of Periprosthetic hip and knee infection: an evidence-based and validated Criteria. J Arthroplasty 33:1309–1314e2. https://doi.org/10.1016/j.arth.2018.02.078
Ridgeway S, Wilson J, Charlet A et al (2005) Infection of the surgical site after arthroplasty of the hip. J Bone Joint Surg Br 87:844–850. https://doi.org/10.1302/0301-620X.87B6.15121
Article CAS PubMed Google Scholar
Beam E, Osmon D (2018) Prosthetic joint infection update. Infect Dis Clin North Am 32:843–859. https://doi.org/10.1016/j.idc.2018.06.005
Qvistgaard M, Nåtman J, Lovebo J et al (2022) Risk factors for reoperation due to periprosthetic joint infection after elective total hip arthroplasty: a study of 35,056 patients using linked data of the Swedish hip Arthroplasty Registry (SHAR) and Swedish Perioperative Registry (SPOR). BMC Musculoskelet Disord 23:275. https://doi.org/10.1186/s12891-022-05209-9
Article CAS PubMed PubMed Central Google Scholar
Sodhi N, Anis HK, Garbarino LJ et al (2019) Have we actually reduced our 30-Day short-term Surgical Site infection rates in primary total hip arthroplasty in the United States? J Arthroplasty 34:2102–2106. https://doi.org/10.1016/j.arth.2019.04.045
ECDC Surveillance Atlas of Infectious Diseases (2024) https://atlas.ecdc.europa.eu/public/index.aspx
Antonelli B, Chen AF (2019) Reducing the risk of infection after total joint arthroplasty: preoperative optimization. Arthroplasty 1:4. https://doi.org/10.1186/s42836-019-0003-7
Kjørholt KE, Kristensen NR, Prieto-Alhambra D et al (2019) Increased risk of mortality after postoperative infection in hip fracture patients. Bone 127:563–570. https://doi.org/10.1016/j.bone.2019.07.023
Tarabichi S, Parvizi J (2023) High-dose dual-antibiotic loaded bone cement in patients undergoing hemiarthroplasty. Lancet 402:162–163. https://doi.org/10.1016/S0140-6736(23)01089-9
McNally M, Sousa R, Wouthuyzen-Bakker M et al (2021) The EBJIS definition of periprosthetic joint infection. Bone Joint J 103–B:18–25. https://doi.org/10.1302/0301-620X.103B1.BJJ-2020-1381.R1
Sousa R, Ribau A, Alfaro P et al (2023) The European bone and Joint Infection Society definition of periprosthetic joint infection is meaningful in clinical practice: a multicentric validation study with comparison with previous definitions. Acta Orthop 94:8–18. https://doi.org/10.2340/17453674.2023.5670
Download references
Author information
Authors and affiliations.
Coreva Scientific GmbH & Co. KG, Koenigswinter, Germany
Ubong Silas
Heraeus Medical GmbH, Wehrheim, Germany
Christof Berberich
Hochschule Fresenius, Wiesbaden, Germany
Priscilla Anyimiah
Department of Trauma Surgery, University Hospital Regensburg, Regensburg, Germany
Dominik Szymski & Markus Rupp
You can also search for this author in PubMed Google Scholar
Contributions
US – conceptualization, formal analysis, data curation, methodology, project administration, writing – original draft. CB – methodology, supervision, validation, writing – original draft. PA – data curation, visualization, writing – original draft. DS – supervision, validation, writing – review and editing. MR – methodology, supervision, validation, writing – review and editing.
Corresponding author
Correspondence to Ubong Silas .
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Material 1
Supplementary material 2, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Silas, U., Berberich, C., Anyimiah, P. et al. Risk of surgical site infection after hip hemiarthroplasty of femoral neck fractures: a systematic review and meta-analysis. Arch Orthop Trauma Surg (2024). https://doi.org/10.1007/s00402-024-05384-5
Download citation
Received : 08 April 2024
Accepted : 15 May 2024
Published : 28 May 2024
DOI : https://doi.org/10.1007/s00402-024-05384-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Hip fracture
- Surgical site infection
- Hemiarthroplasty
- Find a journal
- Publish with us
- Track your research
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Guidelines and Guidance Library
- Core Practices
- Isolation Precautions Guideline
- Disinfection and Sterilization Guideline
- Environmental Infection Control Guidelines
- Hand Hygiene Guidelines
- Multidrug-resistant Organisms (MDRO) Management Guidelines
- Catheter-Associated Urinary Tract Infections (CAUTI) Prevention Guideline
- Tools and resources
- Evaluating Environmental Cleaning
Infection Control Basics
- Infection control prevents or stops the spread of infections in healthcare settings.
- Healthcare workers can reduce the risk of healthcare-associated infections and protect themselves, patients and visitors by following CDC guidelines.
Germs are a part of everyday life. Germs live in our air, soil, water and in and on our bodies. Some germs are helpful, others are harmful.
An infection occurs when germs enter the body, increase in number and the body reacts. Only a small portion of germs can cause infection.
Terms to know
- Sources : places where infectious agents (germs) live (e.g., sinks, surfaces, human skin). Sources are also called reservoirs.
- Susceptible person: someone who is not vaccinated or otherwise immune. For example, a person with a weakened immune system who has a way for the germs to enter the body.
- Transmission: a way germs move to the susceptible person. Germs depend on people, the environment and/or medical equipment to move in healthcare settings. Transmission is also called a pathway.
- Colonization: when someone has germs on or in their body but does not have symptoms of an infection. Colonized people can still transmit the germs they carry.
For an infection to occur, germs must transmit to a person from a source, enter their body, invade tissues, multiply and cause a reaction.
How it works in healthcare settings
Sources can be:.
- People such as patients, healthcare workers and visitors.
- Dry surfaces in patient care areas such as bed rails, medical equipment, countertops and tables).
- Wet surfaces, moist environments and biofilms (collections of microorganisms that stick to each other and surfaces in moist environments, like the insides of pipes).
- Cooling towers, faucets and sinks, and equipment such as ventilators.
- Indwelling medical devices such as catheters and IV lines.
- Dust or decaying debris such as construction dust or wet materials from water leaks.
Transmission can happen through activities such as:
- Physical contact, like when a healthcare provider touches medical equipment that has germs on it and then touches a patient before cleaning their hands.
- Sprays and splashes when an infected person coughs or sneezes. This creates droplets containing the germs, and the droplets land on a person's eyes, nose or mouth.
- Inhalation when infected patients cough or talk, or construction zones kick up dirt and dust containing germs, which another person breathes in.
- Sharps injuries such as when someone is accidentally stuck with a used needle.
A person can become more susceptible to infection when:
- They have underlying medical conditions such as diabetes, cancer or organ transplantation. These can decrease the immune system's ability to fight infection.
- They take medications such as antibiotics, steroids and certain cancer fighting medications. These can decrease the body's ability to fight infection.
- They receive treatments or procedures such as urinary catheters, tubes and surgery, which can provide additional ways for germs to enter the body.
Recommendations
Healthcare providers.
Healthcare providers can perform basic infection prevention measures to prevent infection.
There are 2 tiers of recommended precautions to prevent the spread of infections in healthcare settings:
- Standard Precautions , used for all patient care.
- Transmission-based Precautions , used for patients who may be infected or colonized with certain germs.
There are also transmission- and germ-specific guidelines providers can follow to prevent transmission and healthcare-associated infections from happening.
Learn more about how to protect yourself from infections in healthcare settings.
For healthcare providers and settings
- Project Firstline : infection control education for all frontline healthcare workers.
- Infection prevention, control and response resources for outbreak investigations, the infection control assessment and response (ICAR) tool and more.
- Infection control specifically for surfaces and water management programs in healthcare settings.
- Preventing multi-drug resistant organisms (MDROs).
Infection Control
CDC provides information on infection control and clinical safety to help reduce the risk of infections among healthcare workers, patients, and visitors.
For Everyone
Health care providers, public health.

IMAGES
VIDEO
COMMENTS
Methodology. We conducted a one year study of SSI in our hospital. 300 cases of Surgery and Obstetrics and Gynaecology were included in the study. A few host factors, wound factors and surgery related factors that cause SSI were studied. Swabs were collected from the infected surgical wounds and processed by the conventional microbiological ...
Surgical site infections have a wide range of consequences for both patients and healthcare systems, including discomfort, extended hospital stays, and missed work. 12,13 For example, SSIs approximately increase the length of hospital stays by 10 days. 13 Similarly, it increased the cost of therapy and the cost of an operation by 300% to 400% 12,13 and increased the rate of hospital ...
A Review. Jessica L. Seidelman, MD, MPH; Christopher R. Mantyh, MD; Deverick J. Anderson, MD, MPH. A surgical site infection is defined as infection following an. operation at an incision site or ...
Appendix 23: Antimicrobial-coated sutures to decrease surgical site infections: a systematic review and meta-analysis Appendix 24: Effect of laminar airflow ventilation on surgical site infections: a systematic review and meta-analysis Appendix 25: Summary of the systematic review on surgical antibiotic prophylaxis prolongation
Background. Surgical site infections (SSIs) are post-operative infections of incisions, organs, and/or spaces involved in a surgical procedure and result in increased patient morbidity and mortality [1, 2].Many patient and facility level factors can increase SSI risk, but it is estimated that approximately half of SSIs may be eliminated or mitigated with the use of emergent, evidence-based ...
The final article, "Preventing surgical site infections: implementing strategies throughout the perioperative continuum," 10 provides an overview of protocols for SSI prevention based on four seminal infection prevention guidelines. This article highlights the most effective infection prevention strategies for the perioperative nurse to ...
Introduction. Surgical site infection (SSI) previously termed postoperative wound infection is defined as that infection presenting up to 30 days after a surgical procedure if no prosthetic is placed and up to 1 year if a prosthetic is implanted in the patient 1.In the United States, SSI is found to be a serious complication with an incidence of 2% to 5% in patients undergoing surgery 2.
We read with great interest the Personal View by John Alverdy and colleagues on surgical site infections (SSIs) following elective surgery in the current era of asepsis. We fully agree with the conclusion that further research, including microbiome research and the use of whole-genome sequencing, is required in the field of SSI prevention ...
Conclusions and RelevanceSurgical site infections affect approximately 0.5% to 3% of patients undergoing surgery and are associated with longer hospital stays than patients with no surgical site infections. Avoiding razors for hair removal, maintaining normothermia, use of chlorhexidine gluconate plus alcohol-based skin preparation agents ...
In some cases, robotic procedures have been reported to increase costs and complications, including infection. 10. SSI PREVENTION EFFORTS. The CDC estimates that 50% of all SSIs are preventable. 11 Surgical site infection prevention is the responsibility of both the patient and the health care providers. For the patient, smoking cessation ...
Surgical site infections (SSI) represent a considerable burden for healthcare systems. They are largely preventable and multiple interventions have been proposed over past years in an attempt to prevent SSI. We aim to provide a position paper on Operative Room (OR) prevention of SSI in patients presenting with intra-abdominal infection to be considered a future addendum to the well-known World ...
Surgical site infection (SSI) is the major costliest healthcare-associated infection and a substantial cause of morbidity and mortality throughout the world [1, 2].It occurs near or at the incision site and/or deeper underlying tissue spaces and organs within 30 days of a surgical procedure performed (or up to 90 days for implanted prosthetics) [].
Introduction Surgical site infections (SSIs) remain a significant cause of morbidity for surgical patients worldwide and with growing rates of antibiotic resistance, the development of new nonantimicrobial techniques to target SSI reduction is crucial. This review aimed to explore available nonantibiotic intraoperative interventions to reduce the risk of SSI. Methods A literature search was ...
Introduction Surgical site infections are infections that take place within 30 days of an operative procedure. Worldwide, 23% of patients develop surgical site infections among all surgeries annually with the worst complications causing prolonged hospital stays, increased resistance of microorganisms to antimicrobials, higher health system costs, emotional stress for patients and their ...
Research Paper. Incidence and outcomes of surgical site infection following emergency laparotomy during the COVID-19 pandemic in a low resource setting: A retrospective cohort ... Presence of surgical site infection increased the risk for re-operation (Adjusted risk ratio (ARR) = 1.532, P < 0.001) and prolonged hospital stay (ARR = 3.022, ...
Introduction. Surgical site infection (SSI) is a major complication following hemiarthroplasty surgery for displaced neck of femur fractures. Our aim is to systematically analyse relevant peer-reviewed studies for recent clinical information on the quantitative risk of surgical site infection (SSI) after hemiarthroplasty (HA) of hip fracture patients and on the factors which influence the SSI ...
Surgical masks are primarily intended for interception of liquids and ballistic droplets as either source or exposure control. Certified surgical or medical masks require objective testing, including against fluid penetration and skin irritation, and for bacterial filtering efficiency under standards such as ASTM F2100-21. Standards for such ...
Research Preventing MDROs Laboratory Resources All Related Topics. Antibiotic Prescribing and Use ... Surgical Site Infection Basics. A surgical site infection occurs in the part of the body where a surgery took place. Jan. 17, 2024. Ventilator-associated Pneumonia Basics.
Appendix A: Type and Duration of Precautions Recommended for Selected Infections and Conditions. Appendix A: Table 1. History of Guidelines for Isolation Precautions in Hospitals. Appendix A: Table 2. Clinical Syndromes or Conditions Warranting Empiric Transmission-Based Precautions in Addition to Standard Precautions. Appendix A. Table 3.
Semantic Scholar extracted view of "Hypocholesterolaemia: An Overlooked Risk Factor for Surgical Site Infection" by J. A Jayalal S
Wet your hands with warm water. Use liquid soap if possible. Apply a nickel- or quarter-sized amount of soap to your hands. Rub your hands together until the soap forms a lather and then rub all over the top of your hands, in between your fingers and the area around and under the fingernails. Continue rubbing your hands for at least 15 seconds.
Infection prevention, control and response resources for outbreak investigations, the infection control assessment and response (ICAR) tool and more. Infection control specifically for surfaces and water management programs in healthcare settings. Preventing multi-drug resistant organisms (MDROs). Sources. Infection control prevents or stops ...