

Monday, May 6, 2024
Maintenance alert for may 18-21, 2024.
Production System will go Offline Saturday, May 18, 2024 at 12:01 AM ET.
Production System will go Online Tuesday, May 21, 2024 at 6:00 AM ET.
For more information, please visit the System Enhancements & Server Maintenance Outages Calendar .
We appreciate your patience and cooperation as we continue to improve the Grants.gov user experience.
- The Grants.gov Program Management Office
Tuesday, April 23, 2024
Update: grants.gov training and production systems are back online.
Grants.gov Production and Training Systems are back Online .
For more information, please visit the System Enhancements & Server Maintenance Outages Calendar .
- The Grants.gov Program Management Office.
Monday, April 8, 2024
Maintenance alert for april 20-23, 2024.
Dear Grants.gov User,
The Grants.gov Production System and Grants.gov Training System will be offline for planned maintenance during the following period:
Production System will go Offline Saturday, April 20, 2024 at 12:01 AM ET.
Production System will go Online Sunday, April 21, 2024 at 6:00 AM ET.
Training System will go Offline Saturday, April 20, 2024 at 12:01 AM ET.
Training System will go Online Tuesday, April 23, 2024 at 6:00 AM ET.
Monday, February 5, 2024
Update: grants.gov technical difficulties with opportunity search.
Grants.gov has returned to normal operation.
If you have any questions, please contact the Grants.gov Applicant Support Center at [email protected] . -The Grants.gov Program Management Office
Friday, February 2, 2024
Grants.gov technical difficulties with opportunity search.
Grants.gov is currently experiencing technical difficulties with the apply button on the Opportunity Details page for some NOFOs listed in our system.
There are certain scenarios in which the apply button is greyed out on the Opportunity Details page at this time. However, this issue does not prevent the user from creating, completing, and submitting their application packages before submission deadlines.
Users are still able to create workspaces after logging into the Grants.gov system through the following steps:
1) Select login button at the top right corner.

3) On the left navigation, click on the Apply Now link to start a new application, or Manage Workspaces to continue an existing one.

4) For new applications, enter either Funding Opportunity Number or Opportunity Package Id and Application Filing Name, click Create Workspace button.

5) Once the workspace is created, follow the agency guidelines to fill in the forms and submit the application.
We are working to resolve the issue as quickly as possible.
If this presents an immediate concern, please contact the Grants.gov Applicant Support Center at [email protected] to create a ticket.
We apologize for any inconvenience.
Monday, December 18, 2023
Monday, december 4, 2023, maintenance alert for december 16-18, 2023.
Dear Grants.gov User ,
The Grants.gov Production System and Grants.gov Training System will be offline for planned maintenance during the following period:
- Production and Training Systems will go Offline Saturday, December 16, 2023 at 12:01 AM ET.
- Production and Training Systems will go Online Monday, December 18, 2023 at 6:00 AM ET.
Monday, November 20, 2023
Monday, november 6, 2023, maintenance alert for november 18-20, 2023.
- Production and Training System will go Offline Saturday, November 18, 2023 at 12:01 AM ET.
- Production and Training System will go Online Monday, November 20, 2023 at 6:00 AM ET.
Monday, October 30, 2023
Grants.gov training and production system are back online.
The planned maintenance has been completed and Grants.gov Training and Production System are back Online .
Action Required: Check Your Bookmarks
Please take a moment on or after October 31, 2023, to check the URLs of any bookmarks you may have for Grants.gov. Some page URLs may change, so updating your bookmarks will ensure uninterrupted access to Grants.gov resources.
Options for Support
We understand that during this period, you may encounter situations where you require assistance or have questions about the changes. We want to assure you that support is readily available.
You can learn more by checking out our latest blog post . If you have any questions or need assistance, please do not hesitate to contact our dedicated support team .
Your satisfaction and success using Grants.gov remain our top priorities. We encourage you to actively engage with us by filling out our feedback form . Thank you for being a valued member of the Grants.gov community.
Wednesday, October 4, 2023
Maintenance alert for october 25-31, 2023.
The Grants.gov Production System and Grants.gov Training System will be offline during the following period:
- Training System will go Offline Wednesday, October 25, 2023 at 12:01 AM ET.
- Training System will go Online Friday, October 27, 2023 at 6:00 AM ET.
- Production System will go Offline Saturday, October 28, 2023 at 12:01 AM ET.
- Production System will go Online Tuesday, October 31, 2023 at 11:59 PM ET.
Monday, July 17, 2023
Update: grants.gov production and training system are back online.
Grants.gov Production and Training System are back Online .
Monday, July 10, 2023
Maintenance alert for july 15-17, 2023.
- Production and Training System will go Offline Saturday, July 15, 2023 at 12:01 AM ET.
- Production and Training System will go Online Monday, July 17, 2023 at 6:00 AM ET.
Monday, June 19, 2023
Monday, june 5, 2023, maintenance alert for june 17-19, 2023.
The Grants.gov Production System and Grants.gov Training System will be offline for planned maintenance during the following period:
- Production and Training System will go Offline Saturday, June 17, 2023 at 12:01 AM ET.
- Production and Training System will go Online Monday, June 19, 2023 at 6:00 AM ET.
Description
Research grants to prevent firearm-related violence and injuries (r01).

Eligibility
Award sizing, visible records, all records, posted documents for rfa-ce-22-004, grant awards, grants awarded through rfa-ce-22-004, incumbent or similar grants, similar active opportunities, open grant opportunities similar to rfa-ce-22-004.

An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.

- Research Initiatives
- Meet Our Researchers
- Meet Our Program Officers
- RESEARCH LENSES
- Health Equity
- Social Determinants of Health
- Population and Community Health
- Prevention and Health Promotion
- Systems and Models of Care
- Funding Opportunities
- Small Business Funding
- Grant Applicant Resources
- Training Grants
- Featured Research
- Strategic Plan
- Budget and Legislation
- Connect With Us
- Jobs at NINR

Technical Assistance Webinar for RFA-NR-24-004: Transformative Research to Address Health Disparities and Advance Health Equity
February 13, 2024 | 2:00 pm - 3:30 pm (ET) | Virtual
On February 13, 2024 at 2:00 p.m. (ET), NINR hosted a technical assistance webinar for funding opportunity RFA-NR-24-004 : Transformative Research to Address Health Disparities and Advance Health Equity (U01 Clinical Trial Optional) .
NIH staff provided an overview of the funding opportunity and answered questions from potential applicants. Questions were submitted prior to the webinar through the registration form.
Funding Opportunity Purpose
The Transformative Research to Address Health Disparities and Advance Health Equity Initiative is soliciting applications to support unusually innovative intervention research addressing social determinants of health (SDOH) which, if successful, would have a major impact on preventing, reducing, or eliminating health disparities and advancing health equity. Projects should clearly demonstrate, based on the strength of the logic, a compelling potential to produce a major impact on advancing NIH’s commitment to addressing SDOH to accelerate progress in improving health for all. Preliminary data are not required for this initiative.
Health Promotion and Disease Prevention Research Centers: 2024 Special Interest Project Competitive Supplements (SIPS)
This Notice of Funding Opportunity (NOFO) will provide supplemental funding to the CDC Health Promotion and Disease Prevention Research Centers (RFA-DP-24-004) to conduct Special Interest Research Projects (SIP) to inform public health practice. Recipients will conduct high-quality applied health promotion and disease prevention research in real-world settings to identify, design, test, assess, evaluate, disseminate, and translate interventions (i.e., programs, practices, policies, or strategies) to prevent and reduce risk for the leading causes of illness, disability, and death in the United States.
Number of Applications. Applicants may apply to more than one SIP listed in Section VIII: Other Information - Special Interests Project Descriptions; however, a separate application is required for each SIP. Only one application per SIP per institution is allowed (e.g., multiple applications for the same SIP (listed in Section VIII) from the same institution are NOT permitted). Interested applicants from within Duke should contact [email protected] as early as possible.
Deadline: March 4, 2024
Below are the HP 2030 health topics and the associated SIPs:
Arthritis: • Arthritis Management and Well-being Research Network: (A-01, A-02, A-03, A-04)
People with Disabilities (includes Epilepsy) • Managing Epilepsy Well Network (MEW) (MHMD, HC)
Cancer: •Understanding the needs of Ovarian Cancer Survivors (C-11, C-R01) •Survivorship care plans and mortality among adult cancer survivors (C-11, C-R01) •Cancer Prevention and Control Research Network (C1-C11)) •Gauging men’s reaction to relabeling of GG1 prostate cancer and understanding of pathology reports (C-08, C-R01) •Understanding the impact of reducing social isolation and loneliness on health indices among cancer survivors (C-R01)
Health Communications:
• Mental Health and Chronic Disease Prevention Network (HIT-04)
Nutrition: • Nutrition Obesity Policy Research and Evaluation Network (NWS-01, NWS-04, NWS-06, NWS-08, NWS-09, NWS-10, MICH15-16)
Physical Activity: •Physical Activity Policy and Evaluation Research Network (PA01, PA-02, PA-06, PA-10, PA-11, EH02 and EVP06)
Immunizations: •Advancing Research in Immunization Services Network (IID-02-11, IID-D01-IID-D03)
Vaccinations: •Assessment of perceptions and effectiveness of interventions to increase MMR vaccination among children in close-knit communities with longstanding vaccine hesitancy (IID-02, IID-03, IID-04) •Understanding the potential of schools in promoting non-mandated childhood vaccinations (IID-07, IID-08, IID-09)
Applicants must be selected for funding under CDC Notice of Funding Opportunity RFA-DP-24-004 Health Promotion and Disease Prevention Research Centers in order to apply for SIP supplemental funding under this announcement. The institution name, EIN, and UEI of the SIP applicant must match the information of the institution selected for funding under RFA-DP-24-004 as listed in the Notice of Award. Special eligibly may apply to each SIP that will be listed in the NOFO.
Call or Text the Maternal Mental Health Hotline
Parents: don’t struggle alone
The National Maternal Mental Health Hotline provides free, confidential mental health support. Pregnant people, moms, and new parents can call or text any time, every day.
Start a call: 1-833-TLC-MAMA (1-833-852-6262)
Text now: 1-833-TLC-MAMA (1-833-852-6262)
Use TTY: Use your preferred relay service or dial 711 , then 1-833-852-6262 .
Learn more about the Hotline
- Find Grant Funding
Rural Health Research Center Program
About the program, who can apply.
Back to Listing

Grants toolbox
Apply for a grant.
- Determine Eligibility
- Complete Mandatory Registrations
- Prepare Your Application
- Track Your Application
- Frequently Asked Questions
Contact HRSA 1-877-464-4772 (TTY: 877-897-9910) M - F | 8 a.m. - 8 p.m. (except holidays)
Contact grants.gov Call 800-518-4726 Email grants.gov support
Report fraud Submit a Complaint Call: 1-800-HHS TIPS (1-800-447-8477) (TTY: 1-800-377-4950)
Become a grant reviewer
- Register as a HRSA grant reviewer
- Learn about the grant review process
Part 1. Overview Information
National Institutes of Health ( NIH )
This Notice Of Funding Opportunity (NOFO) is developed as a Common Fund initiative through the Office of the NIH Director, Office of Strategic Coordination. All NIH Institutes and Centers participate in Common Fund initiatives.
R01 Research Project Grant
- August 31, 2022 - Implementation Changes for Genomic Data Sharing Plans Included with Applications Due on or after January 25, 2023. See Notice NOT-OD-22-198 .
- August 5, 2022 - Implementation Details for the NIH Data Management and Sharing Policy. See Notice NOT-OD-22-189 .
See Section III. 3. Additional Information on Eligibility .
The NIH Director’s Transformative Research Award supports individual scientists or groups of scientists proposing bold, groundbreaking, exceptionally innovative, original, and/or unconventional research with the potential to create new scientific paradigms, establish entirely new and improved clinical approaches, or develop transformative technologies. To support innovative and novel research across the vast NIH mission, individuals from diverse backgrounds (including those from underrepresented groups; see Notice of NIH’s Interest in Diversity ) and from the full spectrum of eligible institutions in all geographic locations are encouraged to apply to this Notice of Funding Opportunity. Applications in all topics relevant to the broad mission of NIH are welcome in all topic areas relevant to the broad mission of NIH, including, but not limited to, behavioral, social, biomedical, applied, and formal sciences and topics that may involve basic, translational, or clinical research. No preliminary data are required. Projects must clearly demonstrate, based on the strength of the logic, a compelling potential to produce a major impact in a broad area of relevance to the NIH. The NIH Director’s Transformative Research Award is a component of the High-Risk, High-Reward Research (HRHR) Program of the NIH Common Fund .
Towards the objective of funding the best possible science, the Office of Strategic Coordination and the Center for Scientific Review are piloting a process for initial peer review of applications received in response to this NOFO in which the identity of the investigators and institutions are withheld until the last phase of review. Instructions for anonymizing components of the application are given in Section IV and must be carefully followed. A description of the review process is given in Section V.
Not Applicable
All applications are due by 5:00 PM local time of applicant organization.
Applicants are encouraged to apply early to allow adequate time to make any corrections to errors found in the application during the submission process by the due date.
No late applications will be accepted for this Notice of Funding Opportunity (NOFO).
It is critical that applicants follow the instructions in the Research (R) Instructions in the How to Apply - Application Guide , except where instructed to do otherwise (in this NOFO or in a Notice from NIH Guide for Grants and Contracts ).
Conformance to all requirements (both in the How to Apply - Application Guide and the NOFO) is required and strictly enforced. Applicants must read and follow all application instructions in the How to Apply - Application Guide as well as any program-specific instructions noted in Section IV. When the program-specific instructions deviate from those in the How to Apply - Application Guide , follow the program-specific instructions.
Applications that do not comply with these instructions may be delayed or not accepted for review.
There are several options available to submit your application through Grants.gov to NIH and Department of Health and Human Services partners. You must use one of these submission options to access the application forms for this opportunity.
- Use the NIH ASSIST system to prepare, submit and track your application online.
- Use an institutional system-to-system (S2S) solution to prepare and submit your application to Grants.gov and eRA Commons to track your application. Check with your institutional officials regarding availability.
- Use Grants.gov Workspace to prepare and submit your application and eRA Commons to track your application.
Part 2. Full Text of Announcement
Section i. notice of funding opportunity description.
The NIH Director's Transformative Research Award supports collaborative investigative teams or individual scientists who propose unusually bold and innovative research projects, which, if successful, would have a major impact in a broad area of relevance to the NIH. To be considered transformative, projects must have the potential to create or overturn fundamental scientific paradigms through novel concepts or perspectives, transform the way research is conducted through the development of novel tools or technologies, or lead to major improvements in health through the development of highly innovative diagnostic, therapeutic, or preventive strategies. Several key features of this NOFO are designed to emphasize to applicants and peer reviewers that Transformative Research applications are very different from conventional, investigator-initiated research applications. The Transformative Research application focuses on the importance of the problem, the novelty of the hypothesis and/or the proposed methodology, and the magnitude of the potential impact rather than on preliminary data or experimental details. Reviewers will be instructed to emphasize the significance and innovation of the application in their evaluations. Applicants and reviewers should keep the goal of the Transformative Research Award initiative in mind throughout the process– to solicit and fund unusually innovative and potentially transformative research. To support innovative and novel research across the NIH mission, the NIH recognizes the need to foster a diverse research workforce across the nation. Talented researchers from diverse backgrounds (including individuals from underrepresented racial and ethnic groups, individuals with disabilities, individuals from disadvantaged backgrounds, and women; see Notice of NIH's Interest in Diversity ), are strongly encouraged to work with their institutions to develop applications for this funding opportunity. As outstanding research is conducted at a broad spectrum of institutions, applications from the full range of eligible institutions (institutions that provide services to underrepresented or underserved communities, those that may be less research-intensive, and from all domestic geographic locations) are encouraged to apply. Applications are welcome in all research areas broadly relevant to the mission of NIH. These areas include, but are not limited to, the behavioral, medical, natural, social, applied, and formal sciences. Research may be basic, translational, or clinical. The primary requirements are that the research be highly innovative and have the potential for unusually broad impact.
Towards the objective of funding the best possible science, the Office of Strategic Coordination and the Center for Scientific Review are piloting a process for initial peer review of applications received in response to this NOFO in which the identity of the investigators and institutions are withheld until the last phase of review. Instructions for anonymizing components of the application are given in Section IV and must be carefully followed. If anonymization instructions are not followed, the application will be administratively withdrawn. A description of the review process is given in Section V.
Prospective applicants are invited to a pre-application webinar on June 26, 2024, from 3:30-5:00 PM EDT. NIH staff will discuss the initiative and answer questions about the application and review process. Questions for the webinar should be submitted ahead of time to [email protected] by 11:59 PM local time on June 21, 2024. Additional questions may be taken during the webinar if time allows. Register for the webinar and join on Webex . The webinar will be recorded and posted on the Transformative Research Award website.
The NIH Director's Transformative Research Award is part of the High-Risk, High-Reward Research program funded through the NIH Common Fund , which supports cross-cutting programs that are expected to have exceptionally high impact. All Common Fund initiatives invite investigators to develop bold, innovative, and often risky approaches to address problems that may seem intractable or to seize new opportunities that offer the potential for rapid progress.
See Section VIII. Other Information for award authorities and regulations.
Investigators proposing NIH-defined clinical trials may refer to the Research Methods Resources website for information about developing statistical methods and study designs.
Section II. Award Information
Grant: A financial assistance mechanism providing money, property, or both to an eligible entity to carry out an approved project or activity.
The OER Glossary and the SF424 (R&R) Application Guide provide details on these application types. Only those application types listed here are allowed for this NOFO.
The OER Glossary and the How to Apply - Application Guide provides details on these application types. Only those application types listed here are allowed for this NOFO.
Optional: Accepting applications that either propose or do not propose clinical trial(s).
Need help determining whether you are doing a clinical trial?
NIH intends to commit approximately $8 million in FY 2025 and make approximately 7 awards, depending on the size and scope of the most meritorious awards. The number of awards is contingent upon NIH appropriations and the submission of a sufficient number of meritorious applications.
The maximum project period is five years.
NIH grants policies as described in the NIH Grants Policy Statement will apply to the applications submitted and awards made from this NOFO.
Section III. Eligibility Information
1. Eligible Applicants
All organizations administering an eligible parent award may apply for a supplement under this NOFO.
Higher Education Institutions
- Public/State Controlled Institutions of Higher Education
- Private Institutions of Higher Education
The following types of Higher Education Institutions are always encouraged to apply for NIH support as Public or Private Institutions of Higher Education:
- Hispanic-serving Institutions
- Historically Black Colleges and Universities (HBCUs)
- Tribally Controlled Colleges and Universities (TCCUs)
- Alaska Native and Native Hawaiian Serving Institutions
- Asian American Native American Pacific Islander Serving Institutions (AANAPISIs)
Nonprofits Other Than Institutions of Higher Education
- Nonprofits with 501(c)(3) IRS Status (Other than Institutions of Higher Education)
- Nonprofits without 501(c)(3) IRS Status (Other than Institutions of Higher Education)
For-Profit Organizations
- Small Businesses
- For-Profit Organizations (Other than Small Businesses)
Local Governments
- State Governments
- County Governments
- City or Township Governments
- Special District Governments
- Indian/Native American Tribal Governments (Federally Recognized)
- Indian/Native American Tribal Governments (Other than Federally Recognized)
Federal Government
- Eligible Agencies of the Federal Government, which excludes NIH Intramural Investigators
- U.S. Territory or Possession
- Independent School Districts
- Public Housing Authorities/Indian Housing Authorities
- Native American Tribal Organizations (other than Federally recognized tribal governments)
- Faith-based or Community-based Organizations
- Regional Organizations
Non-domestic (non-U.S.) Entities (Foreign Organizations) are not eligible to apply.
Non-domestic (non-U.S.) components of U.S. Organizations are not eligible to apply.
Foreign components, as defined in the NIH Grants Policy Statement , are allowed.
Applicant Organizations
Applicant organizations must complete and maintain the following registrations as described in the How to Apply - Application Guide to be eligible to apply for or receive an award. All registrations must be completed prior to the application being submitted. Registration can take 6 weeks or more, so applicants should begin the registration process as soon as possible. Failure to complete registrations in advance of a due date is not a valid reason for a late submission, please reference NIH Grants Policy Statement Section 2.3.9.2 Electronically Submitted Applications for additional information.
- NATO Commercial and Government Entity (NCAGE) Code – Foreign organizations must obtain an NCAGE code (in lieu of a CAGE code) in order to register in SAM.
- Unique Entity Identifier (UEI) - A UEI is issued as part of the SAM.gov registration process. The same UEI must be used for all registrations, as well as on the grant application.
- eRA Commons - Once the unique organization identifier is established, organizations can register with eRA Commons in tandem with completing their Grants.gov registration; all registrations must be in place by time of submission. eRA Commons requires organizations to identify at least one Signing Official (SO) and at least one Program Director/Principal Investigator (PD/PI) account in order to submit an application.
- Grants.gov – Applicants must have an active SAM registration in order to complete the Grants.gov registration.
Program Directors/Principal Investigators (PD(s)/PI(s))
All PD(s)/PI(s) must have an eRA Commons account. PD(s)/PI(s) should work with their organizational officials to either create a new account or to affiliate their existing account with the applicant organization in eRA Commons. If the PD/PI is also the organizational Signing Official, they must have two distinct eRA Commons accounts, one for each role. Obtaining an eRA Commons account can take up to 2 weeks.
Any individual(s) with the skills, knowledge, and resources necessary to carry out the proposed research as the Program Director(s)/Principal Investigator(s) (PD(s)/PI(s)) is invited to work with their organization to develop an application for support. Individuals from diverse backgrounds, including individuals from underrepresented racial and ethnic groups, individuals with disabilities, and women are always encouraged to apply for NIH support. See, Reminder: Notice of NIH's Encouragement of Applications Supporting Individuals from Underrepresented Ethnic and Racial Groups as well as Individuals with Disabilities, NOT-OD-22-019 .
For institutions/organizations proposing multiple PDs/PIs, visit the Multiple Program Director/Principal Investigator Policy and submission details in the Senior/Key Person Profile (Expanded) Component of the How to Apply - Application Guide .
2. Cost Sharing
This NOFO does not require cost sharing as defined in the NIH Grants Policy Statement Section 1.2 Definition of Terms .
3. Additional Information on Eligibility
Number of Applications
Applicant organizations may submit more than one application, provided that each application is scientifically distinct.
The NIH will not accept duplicate or highly overlapping applications under review at the same time, per NIH Grants Policy Statement Section 2.3.7.4 Submission of Resubmission Application . This means that the NIH will not accept:
- A new (A0) application that is submitted before issuance of the summary statement from the review of an overlapping new (A0) or resubmission (A1) application.
- A resubmission (A1) application that is submitted before issuance of the summary statement from the review of the previous new (A0) application.
- An application that has substantial overlap with another application pending appeal of initial peer review (see NIH Grants Policy Statement 2.3.9.4 Similar, Essentially Identical, or Identical Applications ).
Section IV. Application and Submission Information
1. Requesting an Application Package
The application forms package specific to this opportunity must be accessed through ASSIST, Grants.gov Workspace or an institutional system-to-system solution. Links to apply using ASSIST or Grants.gov Workspace are available in Part 1 of this NOFO. See your administrative office for instructions if you plan to use an institutional system-to-system solution.
2. Content and Form of Application Submission
It is critical that applicants follow the instructions in the Research (R) Instructions in the How to Apply - Application Guide except where instructed in this notice of funding opportunity to do otherwise. Conformance to the requirements in the How to Apply - Application Guide is required and strictly enforced. Applications that are out of compliance with these instructions may be delayed or not accepted for review.
All page limitations described in the How to Apply – Application Guide and the Table of Page Limits must be followed.
The following section supplements the instructions found in the How to Apply – Application Guide and should be used for preparing an application to this NOFO.
All instructions in the How to Apply - Application Guide must be followed.
R&R or Modular Budget
R&R Subaward Budget
All instructions in the How to Apply - Application Guide must be followed.
All instructions in the How to Apply - Application Guide must be followed, with the following additional instructions:
Applications that do not comply with these instructions may be delayed or not accepted for review. Specific Aims: Do not list specific objectives of the proposed research. Instead, describe the overall project and why it is well aligned with the objectives of the Transformative Research Award initiative. Use two sections entitled "Significance, Innovation, and Impact" and "Insight and Rationale." The content of these two sections is described below. The text should be clear and cogent even to those who are not in the immediate field of the proposed research.
Significance, Innovation, and Impact : What challenge or opportunity is the focus of your proposed research? Why is this broadly significant? What is the overall approach you propose? What are the most innovative aspects of the proposed research? If successful, what would the impact be on scientific understanding and (ultimately) human health?
Insight and Rationale : What fundamental new insight motivates the proposed research? What is the underlying logic or rationale that supports pursuit of this insight despite little or no preliminary data?
Importantly, the text in the Specific Aims page must not contain any information that would allow reviewers to identify the PD(s)/PI(s), other participating individuals, or institutional affiliations. If such information is identified, the application will be deemed non-compliant and withdrawn.
For example, the Specific Aims page (including Figure Legends) must not include:
- Names of any individuals, whether participating or not in the proposed research (such as “Doe and colleagues showed” or “we will use the methodology reported by Doe”)
- Names of any institutions, whether participating or not in the proposed research (such as “the samples will be analyzed using core facilities at Doe University” or “under an IRB from U of Doe”), unless the named institution explicitly is in reference to a publicly available resource, such as a database or biobank
- Mention of any honors or awards, or other specific attributes of any participating investigator
- URLs and hyperlinks, unless the URL or hyperlink is to a publicly available resource, such as a database or biobank
- Reference to any investigator accomplishments in the public domain including in publications or available in preprint servers, (such as “our well-known method, named ‘Doe-seq'” “we developed this technology,” “the PIs demonstrated that,” “our team was the first to,” or “our collaborators are pioneers in”)
- Citations that provide specific information about the source (such as “Doe et al.” or “ Proceedings (May 2021)”). Use numeric citations only, which refer to the corresponding source in the “Bibliography & References Cited” component of the application. This is important to follow even if the references are not from the applicant’s laboratory. This applies to both text and figure legends.
- Any other text from which the identity of any participating individual or institution can be reasonably inferred (such as “we will recruit subjects through a large medical center located in the city of X”).
Inclusion of any text such as described above will result in the application being administratively withdrawn due to noncompliance.
Investigator suitability for the proposed research should be described in the biosketch, which will be available to reviewers in the last phase of review when applications will no longer be subject to anonymization.
Research Strategy: Organize the Research Strategy as a single document in the specified order using the instructions provided below. Start each section with the appropriate section heading as indicated. The presentation must be clear and compelling to both experts and non-experts in the field. Given the anonymized review process being used, the Research Strategy section must be as self-contained as possible.
Overview of research project: Describe briefly what is being proposed and why it is important. Describe briefly what is the state-of-the-art or a major challenge in the broad field of the application and how what is being proposed will advance well beyond the current status to transform the broad field. State the fundamental new insight driving this project.
Approach : Describe the overall approach. No detailed experimental plan or substantial preliminary data should be provided. Though preliminary data are not required, if limited preliminary data are provided, they will be evaluated. Provision of substantial preliminary data is not aligned with the intention of the Transformative Research Award. It is recommended to prominently state that, per the NOFO instructions, a detailed experimental plan and substantial preliminary data are not being provided. In lieu of preliminary data, provide the underlying logic or rationale for pursuing this project in the manner proposed. Summarize what you believe to be the major challenges or risks in the project and alternate approaches that may need to be pursued. Despite the lack of detailed plans and data, the reviewers still must have a clear sense of what is being proposed and why it is important. Reviewers must be convinced that the applicants have thought very deeply about the project, and that the research will be conducted in a robust, rigorous, and reproducible manner. Applicants proposing clinical trials may reference but should not repeat information submitted on the PHS Human Subjects Clinical Trial Information form.
Innovation: Describe the elements of exceptional innovation in your proposed research and why they should be considered exceptionally innovative compared to current approaches, paradigms, practices, or perspectives.
Appropriateness for the Transformative Research Award : Why is the proposed research well suited to the goals of the Transformative Research Award rather than a more traditional research grant program?
Timeline : The Transformative Research Award project must produce deliverables by the end of the project period with the potential for transformative impact. The project should not be framed as initiating a line of research that will have transformative impact only after subsequent periods of support. Provide a timeline within the project period for the proposed research indicating points where preliminary, intermediate, and long-range objectives will be assessed, the measurable outcomes that will be used to monitor progress, and the timing and process for reaching decisions regarding the course and direction of the continuing research effort. Given the high degree of risk involved in applications submitted under the Transformative Research Award, it is anticipated that investigators will continually reassess approaches based on experimental outcomes and potentially alter course to meet project goals. Possible alternative paths that may be followed at critical junctures in the project plan should be indicated on the timeline.
Importantly, the text in the Research Strategy component must not contain any information that would allow reviewers to identify the PD(s)/PI(s), other participating individuals, or institutional affiliations. If such information is identified the application will be deemed non-compliant and administratively withdrawn.
Thus, the Research Strategy component (including Figure Legends) must not include:
- Names of any institutions, whether participating or not in the proposed research (such as “the samples will be analyzed using core facilities at Doe University” or “under an IRB from U of Doe”), unless the named institution is in reference to a publicly available resource, such as a database or biobank
- Mention of any honors, awards, or other specific attributes of any participating investigator
- URLs or hyperlinks, unless the URL or hyperlink is to a publicly available resource, such as a database or biobank
- Reference to any investigator accomplishments in the public domain including in publications or available in preprint servers, (such as “our well-known method, named ‘Doe-seq’” “we developed this technology,” “the PIs demonstrated that,” “our team was the first to,” or “our collaborators are pioneers in”)
- Citations that provide specific information about the source (such as “Doe et al.” or “ Proceedings (May 2021)”). Use numeric citations only, which refer to the corresponding source in the “Bibliography & References Cited” component of the application. This is important to follow even if the references are not from the applicant’s laboratory. This applies to both text and figure legends.
Investigator suitability for the proposed research should be described in the biosketch, which will be available to reviewers in the last phase of review when the applications will no longer be subject to anonymization.
To help prospective applicants identify potential non-complaint text, an optional online tool is available called the TRA Anonymization Check (TRAAC) Tool . TRAAC scans documents for items that could violate anonymization requirements and highlights potential violations. Given the complexity of language and context, the tool does not provide a definitive determination on compliance. The applicant bears the sole responsibility to determine the text to include in the application. All applications will be checked for compliance by NIH and will be administratively withdrawn if found to be non-compliant by a team of NIH Review and Program staff.
Applicants considering clinical trial research are strongly recommended to discuss the proposed research with the most relevant NIH Institutes or Centers (ICs) to ensure that such scientific research conforms to the clinical trial research policies of those ICs. Funding of applications involving clinical trials is contingent upon conformity with the policies of the IC administering the award. For a list of IC staff contacts, see https://commonfund.nih.gov/highrisk/clinical.
Resource Sharing Plan:
Individuals are required to comply with the instructions for the Resource Sharing Plans as provided in the How to Apply - Application Guide .
Other Plan(s):
Note: Effective for due dates on or after January 25, 2023, the Data Management and Sharing Plan will be attached in the Other Plan(s) attachment in FORMS-H application forms packages.
All instructions in the How to Apply - Application Guide must be followed, with the following additional instructions:
- All applicants planning research (funded or conducted in whole or in part by NIH) that results in the generation of scientific data are required to comply with the instructions for the Data Management and Sharing Plan. All applications, regardless of the amount of Direct Costs requested for any one year, must address a Data Management and Sharing Plan.
Only limited Appendix materials are allowed. Follow all instructions for the Appendix as described in the How to Apply - Application Guide .
When involving human subjects research, clinical research, and/or NIH-defined clinical trials (and when applicable, clinical trials research experience) follow all instructions for the PHS Human Subjects and Clinical Trials Information form in the How to Apply - Application Guide , with the following additional instructions:
If you answered “Yes” to the question “Are Human Subjects Involved?” on the R&R Other Project Information form, you must include at least one human subjects study record using the Study Record: PHS Human Subjects and Clinical Trials Information form or Delayed Onset Study record.
Study Record: PHS Human Subjects and Clinical Trials Information
Delayed Onset Study
Note: Delayed onset does NOT apply to a study that can be described but will not start immediately (i.e., delayed start). All instructions in the How to Apply - Application Guide must be followed.
3. Unique Entity Identifier and System for Award Management (SAM)
See Part 2. Section III.1 for information regarding the requirement for obtaining a unique entity identifier and for completing and maintaining active registrations in System for Award Management (SAM), NATO Commercial and Government Entity (NCAGE) Code (if applicable), eRA Commons, and Grants.gov
Part I. contains information about Key Dates and times. Applicants are encouraged to submit applications before the due date to ensure they have time to make any application corrections that might be necessary for successful submission. When a submission date falls on a weekend or Federal holiday , the application deadline is automatically extended to the next business day.
Organizations must submit applications to Grants.gov (the online portal to find and apply for grants across all Federal agencies). Applicants must then complete the submission process by tracking the status of the application in the eRA Commons , NIH’s electronic system for grants administration. NIH and Grants.gov systems check the application against many of the application instructions upon submission. Errors must be corrected and a changed/corrected application must be submitted to Grants.gov on or before the application due date and time. If a Changed/Corrected application is submitted after the deadline, the application will be considered late. Applications that miss the due date and time are subjected to the NIH Grants Policy Statement Section 2.3.9.2 Electronically Submitted Applications .
Applicants are responsible for viewing their application before the due date in the eRA Commons to ensure accurate and successful submission.
Information on the submission process and a definition of on-time submission are provided in the How to Apply – Application Guide .
5. Intergovernmental Review (E.O. 12372)
This initiative is not subject to intergovernmental review.
All NIH awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
Pre-award costs are allowable only as described in the NIH Grants Policy Statement Section 7.9.1 Selected Items of Cost .
Applications must be submitted electronically following the instructions described in the How to Apply - Application Guide . Paper applications will not be accepted.
Applicants must complete all required registrations before the application due date. Section III. Eligibility Information contains information about registration.
For assistance with your electronic application or for more information on the electronic submission process, visit How to Apply – Application Guide . If you encounter a system issue beyond your control that threatens your ability to complete the submission process on-time, you must follow the Dealing with System Issues guidance. For assistance with application submission, contact the Application Submission Contacts in Section VII .
Important reminders:
All PD(s)/PI(s) must include their eRA Commons ID in the Credential field of the Senior/Key Person Profile form . Failure to register in the Commons and to include a valid PD/PI Commons ID in the credential field will prevent the successful submission of an electronic application to NIH. See Section III of this NOFO for information on registration requirements.
The applicant organization must ensure that the unique entity identifier provided on the application is the same identifier used in the organization’s profile in the eRA Commons and for the System for Award Management. Additional information may be found in the How to Apply - Application Guide .
See more tips for avoiding common errors.
Upon receipt applications will be evaluated for completeness and compliance with application instructions by the Center for Scientific Review. In parallel, compliance with anonymization instructions and responsiveness will be evaluated by the Office of Strategic Coordination (OSC). Applications that are incomplete, non-compliant with anonymization instructions, and/or non-responsive will not be reviewed.
Applicants are required to follow the instructions for post-submission materials, as described in the policy
Section V. Application Review Information
1. Criteria
Only the review criteria described below will be considered in the review process. Applications submitted to the NIH in support of the NIH mission are evaluated for scientific and technical merit through the NIH peer review system.
For this particular announcement, note the following: The NIH Director's Transformative Research Award is designed to support exceptionally innovative research projects with the potential to have a profound effect on an area of research relevant to the broad mission of NIH. The innovation may be technical, conceptual, or (often) a combination of both. Given the high level of innovation expected, conventionally detailed experimental plans and extensive preliminary data are not required. Accordingly, reviewers will emphasize the strength of the conceptual framework, the level of innovation, and the potential to significantly advance our understanding or capability in a field relevant to NIH.
For this review, an anonymized review process will be used in which the identity of the investigators and institutions is withheld until the last stage of review. In the first stage, the peer review panel will use the anonymized Specific Aims pages to identify a subset of applications with the most transformative potential. In the second stage, this subset of applications will be evaluated by "Mail Reviewers," who will only use the anonymized Specific Aims pages and anonymized Research Strategy components to provide feedback on the significance, innovation, and approach. The Mail Reviewers will submit written comments but will not meet to discuss the applications. In the third stage, the peer review panel, informed by the Mail Reviews, will prioritize the applications to be discussed. After this prioritization, the panel will be given access to the complete applications and identity of the investigators and institutions. They will finalize their selection of applications to discuss and will discuss and score them in a panel meeting. All criteria will be assessed in the discussion by the panel. All other applications will be considered as "Not Discussed."
A proposed Clinical Trial application may include study design, methods, and intervention that are not by themselves innovative but address important questions or unmet needs. Additionally, the results of the clinical trial may indicate that further clinical development of the intervention is unwarranted or lead to new avenues of scientific investigation.
Overall Impact
Reviewers will provide an overall impact score to reflect their assessment of the likelihood for the project to exert a sustained, powerful influence on the research field(s) involved, in consideration of the following review criteria and additional review criteria (as applicable for the project proposed).
Scored Review Criteria
Reviewers will consider each of the review criteria below in the determination of scientific merit and give a separate score for each. An application does not need to be strong in all categories to be judged likely to have major scientific impact. For example, a project that by its nature is not innovative may be essential to advance a field.
Significance
Does the project address an important problem or a critical barrier to progress in the field? Is the prior research that serves as the key support for the proposed project rigorous? If the aims of the project are achieved, how will scientific knowledge, technical capability, and/or clinical practice be improved? How will successful completion of the aims change the concepts, methods, technologies, treatments, services, or preventative interventions that drive this field?
In addition, for applications involving clinical trials
Are the scientific rationale and need for a clinical trial to test the proposed hypothesis or intervention well supported by preliminary data, clinical and/or preclinical studies, or information in the literature or knowledge of biological mechanisms? For trials focusing on clinical or public health endpoints, is this clinical trial necessary for testing the safety, efficacy or effectiveness of an intervention that could lead to a change in clinical practice, community behaviors or health care policy? For trials focusing on mechanistic, behavioral, physiological, biochemical, or other biomedical endpoints, is this trial needed to advance scientific understanding?
Specific to this NOFO :
How clear and convincing is the transformative potential of the proposed study? What are the impacts and consequences of the proposed research on the field? How fundamental is the proposed or challenged paradigm to the field?
Investigator(s)
Are the PD(s)/PI(s), collaborators, and other researchers well suited to the project? If Early Stage Investigators or those in the early stages of independent careers, do they have appropriate experience and training? If established, have they demonstrated an ongoing record of accomplishments that have advanced their field(s)? If the project is collaborative or multi-PD/PI, do the investigators have complementary and integrated expertise; are their leadership approach, governance, and organizational structure appropriate for the project?
With regard to the proposed leadership for the project, do the PD/PI(s) and key personnel have the expertise, experience, and ability to organize, manage and implement the proposed clinical trial and meet milestones and timelines? Do they have appropriate expertise in study coordination, data management and statistics? For a multicenter trial, is the organizational structure appropriate and does the application identify a core of potential center investigators and staffing for a coordinating center?
How will the PD/PI level of effort ensure the proposed research is prioritized?
Does the application challenge and seek to shift current research or clinical practice paradigms by utilizing novel theoretical concepts, approaches or methodologies, instrumentation, or interventions? Are the concepts, approaches or methodologies, instrumentation, or interventions novel to one field of research or novel in a broad sense? Is a refinement, improvement, or new application of theoretical concepts, approaches or methodologies, instrumentation, or interventions proposed?
Does the design/research plan include innovative elements, as appropriate, that enhance its sensitivity, potential for information or potential to advance scientific knowledge or clinical practice?
Are the overall strategy, methodology, and analyses well-reasoned and appropriate to accomplish the specific aims of the project? Have the investigators included plans to address weaknesses in the rigor of prior research that serves as the key support for the proposed project? Have the investigators presented strategies to ensure a robust and unbiased approach, as appropriate for the work proposed? Are potential problems, alternative strategies, and benchmarks for success presented? If the project is in the early stages of development, will the strategy establish feasibility and will particularly risky aspects be managed? Have the investigators presented adequate plans to address relevant biological variables, such as sex, for studies in vertebrate animals or human subjects?
If the project involves human subjects and/or NIH-defined clinical research, are the plans to address 1) the protection of human subjects from research risks, and 2) inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion or exclusion of individuals of all ages (including children and older adults), justified in terms of the scientific goals and research strategy proposed?
Does the application adequately address the following, if applicable
Study Design
Is the study design justified and appropriate to address primary and secondary outcome variable(s)/endpoints that will be clear, informative and relevant to the hypothesis being tested? Is the scientific rationale/premise of the study based on previously well-designed preclinical and/or clinical research? Given the methods used to assign participants and deliver interventions, is the study design adequately powered to answer the research question(s), test the proposed hypothesis/hypotheses, and provide interpretable results? Is the trial appropriately designed to conduct the research efficiently? Are the study populations (size, gender, age, demographic group), proposed intervention arms/dose, and duration of the trial, appropriate and well justified?
Are potential ethical issues adequately addressed? Is the process for obtaining informed consent or assent appropriate? Is the eligible population available? Are the plans for recruitment outreach, enrollment, retention, handling dropouts, missed visits, and losses to follow-up appropriate to ensure robust data collection? Are the planned recruitment timelines feasible and is the plan to monitor accrual adequate? Has the need for randomization (or not), masking (if appropriate), controls, and inclusion/exclusion criteria been addressed? Are differences addressed, if applicable, in the intervention effect due to sex/gender and race/ethnicity?
Are the plans to standardize, assure quality of, and monitor adherence to, the trial protocol and data collection or distribution guidelines appropriate? Is there a plan to obtain required study agent(s)? Does the application propose to use existing available resources, as applicable?
Data Management and Statistical Analysis
Are planned analyses and statistical approach appropriate for the proposed study design and methods used to assign participants and deliver interventions? Are the procedures for data management and quality control of data adequate at clinical site(s) or at center laboratories, as applicable? Have the methods for standardization of procedures for data management to assess the effect of the intervention and quality control been addressed? Is there a plan to complete data analysis within the proposed period of the award?
Despite the lack of experimental details and substantial preliminary data, how compelling is the logic and evidence of the approach? How strong is the evidence that the investigators will pursue the project (despite its inherent risks) in a robust, reproducible, and rigorous manner? How does the information in the timeline inspire confidence that the PD(s)/PI(s) will be able to assess progress each year of the award and either complete the project or demonstrate conclusively that it cannot be completed (despite good-faith efforts) during the term of the award?
Environment
Will the scientific environment in which the work will be done contribute to the probability of success? Are the institutional support, equipment, and other physical resources available to the investigators adequate for the project proposed? Will the project benefit from unique features of the scientific environment, subject populations, or collaborative arrangements?
If proposed, are the administrative, data coordinating, enrollment and laboratory/testing centers, appropriate for the trial proposed?
Does the application adequately address the capability and ability to conduct the trial at the proposed site(s) or centers? Are the plans to add or drop enrollment centers, as needed, appropriate?
If international site(s) is/are proposed, does the application adequately address the complexity of executing the clinical trial?
If multi-sites/centers, is there evidence of the ability of the individual site or center to: (1) enroll the proposed numbers; (2) adhere to the protocol; (3) collect and transmit data in an accurate and timely fashion; and, (4) operate within the proposed organizational structure?
Additional Review Criteria
As applicable for the project proposed, reviewers will evaluate the following additional items while determining scientific and technical merit, and in providing an overall impact score, but will not give separate scores for these items.
Study Timeline
Specific to applications involving clinical trials
Is the study timeline described in detail, taking into account start-up activities, the anticipated rate of enrollment, and planned follow-up assessment? Is the projected timeline feasible and well justified? Does the project incorporate efficiencies and utilize existing resources (e.g., CTSAs, practice-based research networks, electronic medical records, administrative database, or patient registries) to increase the efficiency of participant enrollment and data collection, as appropriate?
Are potential challenges and corresponding solutions discussed (e.g., strategies that can be implemented in the event of enrollment shortfalls)?
Protections for Human Subjects
For research that involves human subjects but does not involve one of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate the justification for involvement of human subjects and the proposed protections from research risk relating to their participation according to the following five review criteria: 1) risk to subjects, 2) adequacy of protection against risks, 3) potential benefits to the subjects and others, 4) importance of the knowledge to be gained, and 5) data and safety monitoring for clinical trials.
For research that involves human subjects and meets the criteria for one or more of the categories of research that are exempt under 45 CFR Part 46, the committee will evaluate: 1) the justification for the exemption, 2) human subjects involvement and characteristics, and 3) sources of materials. For additional information on review of the Human Subjects section, please refer to the Guidelines for the Review of Human Subjects .
Inclusion of Women, Minorities, and Individuals Across the Lifespan
When the proposed project involves human subjects and/or NIH-defined clinical research, the committee will evaluate the proposed plans for the inclusion (or exclusion) of individuals on the basis of sex/gender, race, and ethnicity, as well as the inclusion (or exclusion) of individuals of all ages (including children and older adults) to determine if it is justified in terms of the scientific goals and research strategy proposed. For additional information on review of the Inclusion section, please refer to the Guidelines for the Review of Inclusion in Clinical Research .
Vertebrate Animals
The committee will evaluate the involvement of live vertebrate animals as part of the scientific assessment according to the following three points: (1) a complete description of all proposed procedures including the species, strains, ages, sex, and total numbers of animals to be used; (2) justifications that the species is appropriate for the proposed research and why the research goals cannot be accomplished using an alternative non-animal model; and (3) interventions including analgesia, anesthesia, sedation, palliative care, and humane endpoints that will be used to limit any unavoidable discomfort, distress, pain and injury in the conduct of scientifically valuable research. Methods of euthanasia and justification for selected methods, if NOT consistent with the AVMA Guidelines for the Euthanasia of Animals, is also required but is found in a separate section of the application. For additional information on review of the Vertebrate Animals Section, please refer to the Worksheet for Review of the Vertebrate Animals Section.
Reviewers will assess whether materials or procedures proposed are potentially hazardous to research personnel and/or the environment, and if needed, determine whether adequate protection is proposed.
Resubmissions
Not Applicable.
As applicable for the project proposed, reviewers will consider each of the following items, but will not give scores for these items, and should not consider them in providing an overall impact score.
Applications from Foreign Organizations
Select Agent Research
Reviewers will assess the information provided in this section of the application, including 1) the Select Agent(s) to be used in the proposed research, 2) the registration status of all entities where Select Agent(s) will be used, 3) the procedures that will be used to monitor possession use and transfer of Select Agent(s), and 4) plans for appropriate biosafety, biocontainment, and security of the Select Agent(s).
Resource Sharing Plans
Reviewers will comment on whether the Resource Sharing Plan(s) (i.e., Sharing Model Organisms ) or the rationale for not sharing the resources, is reasonable.
Authentication of Key Biological and/or Chemical Resources:
For projects involving key biological and/or chemical resources, reviewers will comment on the brief plans proposed for identifying and ensuring the validity of those resources.
Budget and Period of Support
Reviewers will consider whether the budget and the requested period of support are fully justified and reasonable in relation to the proposed research.
2. Review and Selection Process
Applications will be evaluated for scientific and technical merit by (an) appropriate Scientific Review Group(s) convened by the Center for Scientific Review, in accordance with NIH peer review policy and procedures , with the modifications described below using the stated review criteria. Assignment to a Scientific Review Group will be shown in the eRA Commons.
As part of the scientific peer review, all applications will receive a written critique.
Applications may undergo a selection in which only those applications deemed to have the highest scientific and technical merit will be discussed and assigned an overall impact score.
For "Discussed" applications: the summary statement will contain a resume and summary of discussion and the Mail Reviewer comments. For “Not Discussed” applications: if chosen for stage 2, the summary statement will contain the Mail Reviewer comments; if not, it will contain a summary of the review process without evaluative comments.
Appeals of initial peer review will not be accepted for applications submitted in response to this NOFO.
Applications will be assigned to the appropriate NIH Institute or Center. Applications will compete for available funds with all other recommended applications submitted in response to this NOFO. Following initial peer review, recommended applications will receive a second level of review by the Council of Councils. The following will be considered in making funding decisions:
- Scientific and technical merit of the proposed project as determined by scientific peer review.
- Availability of funds
- Conformance to the clinical trial research policies of the administering Institute or Center.
- Despite inherent scientific and technical risks, the potential for the research to result in scientific breakthroughs of broad impact.
- Unusually cross-cutting science.
- Scientific balance in the portfolio of Transformative Research Award-supported research.
- Potential to invigorate exceptionally innovative and impactful science broadly across the nation.
3. Anticipated Announcement and Award Dates
After the peer review of the application is completed, the PD/PI will be able to access his or her Summary Statement (written critique) via the eRA Commons . Refer to Part 1 for dates for peer review, advisory council review, and earliest start date.
Information regarding the disposition of applications is available in the NIH Grants Policy Statement Section 2.4.4 Disposition of Applications .
Section VI. Award Administration Information
1. Award Notices
If the application is under consideration for funding, NIH will request "just-in-time" information from the applicant as described in the NIH Grants Policy Statement . This request is not a Notice of Award nor should it be construed to be an indicator of possible funding.
A formal notification in the form of a Notice of Award (NoA) will be provided to the applicant organization for successful applications. The NoA signed by the grants management officer is the authorizing document and will be sent via email to the recipient's business official.
Recipients must comply with any funding restrictions described in Section IV.6. Funding Restrictions. Selection of an application for award is not an authorization to begin performance. Any costs incurred before receipt of the NoA are at the recipient's risk. These costs may be reimbursed only to the extent considered allowable pre-award costs.
Any application awarded in response to this NOFO will be subject to terms and conditions found on the Award Conditions and Information for NIH Grants website. This includes any recent legislation and policy applicable to awards that is highlighted on this website.
Individual awards are based on the application submitted to, and as approved by, the NIH and are subject to the IC-specific terms and conditions identified in the NoA.
ClinicalTrials.gov: If an award provides for one or more clinical trials. By law (Title VIII, Section 801 of Public Law 110-85), the "responsible party" must register and submit results information for certain “applicable clinical trials” on the ClinicalTrials.gov Protocol Registration and Results System Information Website ( https://register.clinicaltrials.gov ). NIH expects registration and results reporting of all trials whether required under the law or not. For more information, see https://grants.nih.gov/policy/clinical-trials/reporting/index.htm
Institutional Review Board or Independent Ethics Committee Approval: Recipient institutions must ensure that all protocols are reviewed by their IRB or IEC. To help ensure the safety of participants enrolled in NIH-funded studies, the recipient must provide NIH copies of documents related to all major changes in the status of ongoing protocols.
Data and Safety Monitoring Requirements: The NIH policy for data and safety monitoring requires oversight and monitoring of all NIH-conducted or -supported human biomedical and behavioral intervention studies (clinical trials) to ensure the safety of participants and the validity and integrity of the data. Further information concerning these requirements is found at http://grants.nih.gov/grants/policy/hs/data_safety.htm and in the application instructions (SF424 (R&R) and PHS 398).
Investigational New Drug or Investigational Device Exemption Requirements: Consistent with federal regulations, clinical research projects involving the use of investigational therapeutics, vaccines, or other medical interventions (including licensed products and devices for a purpose other than that for which they were licensed) in humans under a research protocol must be performed under a Food and Drug Administration (FDA) investigational new drug (IND) or investigational device exemption (IDE).
2. Administrative and National Policy Requirements
All NIH grant and cooperative agreement awards include the NIH Grants Policy Statement as part of the NoA. For these terms of award, see the NIH Grants Policy Statement Part II: Terms and Conditions of NIH Grant Awards, Subpart A: General and Part II: Terms and Conditions of NIH Grant Awards, Subpart B: Terms and Conditions for Specific Types of Grants, Recipients, and Activities , including of note, but not limited to:
- Federal-wide Standard Terms and Conditions for Research Grants
- Prohibition on Certain Telecommunications and Video Surveillance Services or Equipment
- Acknowledgment of Federal Funding
If a recipient is successful and receives a Notice of Award, in accepting the award, the recipient agrees that any activities under the award are subject to all provisions currently in effect or implemented during the period of the award, other Department regulations and policies in effect at the time of the award, and applicable statutory provisions.
If a recipient receives an award, the recipient must follow all applicable nondiscrimination laws. The recipient agrees to this when registering in SAM.gov. The recipient must also submit an Assurance of Compliance ( HHS-690 ). To learn more, see the Laws and Regulations Enforced by the HHS Office for Civil Rights website .
HHS recognizes that NIH research projects are often limited in scope for many reasons that are nondiscriminatory, such as the principal investigator’s scientific interest, funding limitations, recruitment requirements, and other considerations. Thus, criteria in research protocols that target or exclude certain populations are warranted where nondiscriminatory justifications establish that such criteria are appropriate with respect to the health or safety of the subjects, the scientific study design, or the purpose of the research. For additional guidance regarding how the provisions apply to NIH grant programs, please contact the Scientific/Research Contact that is identified in Section VII under Agency Contacts of this NOFO.
In accordance with the statutory provisions contained in Section 872 of the Duncan Hunter National Defense Authorization Act of Fiscal Year 2009 (Public Law 110-417), NIH awards will be subject to System for Award Management (SAM.gov) requirements. SAM.gov requires Federal agencies to review and consider information about an applicant in the designated integrity and performance system (currently SAM.gov) prior to making an award. An applicant can review and comment on any information in the responsibility/qualification records available in SAM.gov. NIH will consider any comments by the applicant, in addition to the information available in the responsibility/qualification records in SAM.gov, in making a judgement about the applicant’s integrity, business ethics, and record of performance under Federal awards when completing the review of risk posed by applicants as described in 2 CFR Part 200.206 “Federal awarding agency review of risk posed by applicants.” This provision will apply to all NIH grants and cooperative agreements except fellowships.
3. Data Management and Sharing
Consistent with the 2023 NIH Policy for Data Management and Sharing, when data management and sharing is applicable to the award, recipients will be required to adhere to the Data Management and Sharing requirements as outlined in the NIH Grants Policy Statement . Upon the approval of a Data Management and Sharing Plan, it is required for recipients to implement the plan as described.
4. Reporting
When multiple years are involved, recipients will be required to submit the Research Performance Progress Report (RPPR) annually and financial statements as required in the NIH Grants Policy Statement .
A final RPPR, invention statement, and the expenditure data portion of the Federal Financial Report are required for closeout of an award, as described in the NIH Grants Policy Statement . NIH NOFOs outline intended research goals and objectives. Post award, NIH will review and measure performance based on the details and outcomes that are shared within the RPPR, as described at 2 CFR Part 200.301.
The Federal Funding Accountability and Transparency Act of 2006 as amended (FFATA), includes a requirement for recipients of Federal grants to report information about first-tier subawards and executive compensation under Federal assistance awards issued in FY2011 or later. All recipients of applicable NIH grants and cooperative agreements are required to report to the Federal Subaward Reporting System (FSRS) available at www.fsrs.gov on all subawards over $25,000. See the NIH Grants Policy Statement for additional information on this reporting requirement.
In accordance with the regulatory requirements provided at 2 CFR Part 200.113 and Appendix XII to 2 CFR Part 200, recipients that have currently active Federal grants, cooperative agreements, and procurement contracts from all Federal awarding agencies with a cumulative total value greater than $10,000,000 for any period of time during the period of performance of a Federal award, must report and maintain the currency of information reported in the System for Award Management (SAM) about civil, criminal, and administrative proceedings in connection with the award or performance of a Federal award that reached final disposition within the most recent five-year period. The recipient must also make semiannual disclosures regarding such proceedings. Proceedings information will be made publicly available in the designated integrity and performance system (Responsibility/Qualification in SAM.gov, formerly FAPIIS). This is a statutory requirement under section 872 of Public Law 110-417, as amended (41 U.S.C. 2313). As required by section 3010 of Public Law 111-212, all information posted in the designated integrity and performance system on or after April 15, 2011, except past performance reviews required for Federal procurement contracts, will be publicly available. Full reporting requirements and procedures are found in Appendix XII to 2 CFR Part 200 – Award Term and Conditions for Recipient Integrity and Performance Matters.
Section VII. Agency Contacts
We encourage inquiries concerning this funding opportunity and welcome the opportunity to answer questions from potential applicants.
eRA Service Desk (Questions regarding ASSIST, eRA Commons, application errors and warnings, documenting system problems that threaten submission by the due date, and post-submission issues)
Finding Help Online: https://www.era.nih.gov/need-help (preferred method of contact) Telephone: 301-402-7469 or 866-504-9552 (Toll Free)
General Grants Information (Questions regarding application instructions, application processes, and NIH grant resources) Email: [email protected] (preferred method of contact) Telephone: 301-480-7075
Grants.gov Customer Support (Questions regarding Grants.gov registration and Workspace) Contact Center Telephone: 800-518-4726 Email: [email protected]
Patricia Labosky, Ph.D. Office of the Director (OD) Email: [email protected]
Center for Scientific Review (CSR) Email: [email protected]
Michael Morse Office of the Director (OD) Telephone: 301-435-5446 Email: [email protected]
Section VIII. Other Information
Recently issued trans-NIH policy notices may affect your application submission. A full list of policy notices published by NIH is provided in the NIH Guide for Grants and Contracts . All awards are subject to the terms and conditions, cost principles, and other considerations described in the NIH Grants Policy Statement .
Awards are made under the authorization of Sections 301 and 405 of the Public Health Service Act as amended (42 USC 241 and 284) and under Federal Regulations 42 CFR Part 52 and 2 CFR Part 200.

Note: For help accessing PDF, RTF, MS Word, Excel, PowerPoint, Audio or Video files, see Help Downloading Files .
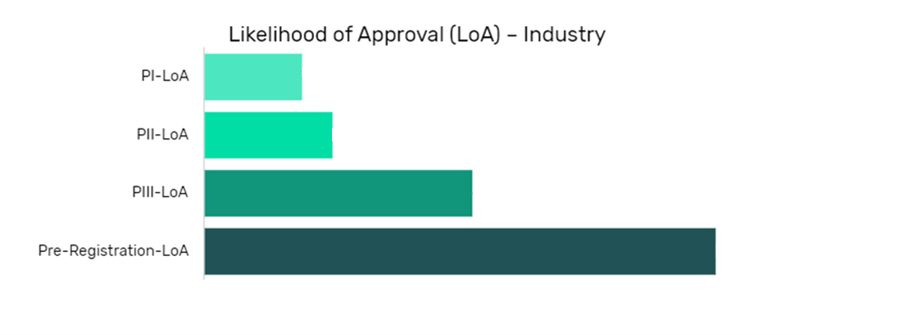
Likelihood of Approval and Phase Transition Success Rate Model – CYB-004 in Generalized Anxiety Disorder (GAD)
Unlock hidden opportunities in the LoA industry

Published: April 22, 2024 Report Code: GDHCDR28579LOA-MP-L5
- Share on Twitter
- Share on LinkedIn
- Share on Facebook
- Share on Threads
- Share via Email
- Report Overview
- Key Players
Empower your strategies with our Likelihood of Approval and Phase Transition Success Rate Model – CYB-004 in Generalized Anxiety Disorder (GAD) report and make more profitable business decisions.
This report provides you with the data that allows you to track and predict the specific likelihood of approval (LOA) and phase transition success rate (PTSR) of a drug using GlobalData’s proprietary machine learning algorithms developed using over 10 years of historical data.
CYB-004 in Generalized Anxiety Disorder (GAD) Drug Details:
CYB-004 is under development for the treatment of social anxiety disorder and generalized anxiety disorder. It is administered through inhalation route, intravenous bolus and intravenous route. The drug candidate is a deuterated forms of DMT and 5-MeO-DMT. It is a deuterated tryptamine and acts by targeting 5-hydroxytryptamine receptor 2A.
Report Coverage
The data is segmented by drug name per indication and shows the current likelihood of approval for the drug compared to the indication benchmark and the industry benchmark.
The Likelihood of Approval data is updated regularly based on events that take place which impact the clinical development process and regulatory considerations. GlobalData’s proprietary machine learning models consider these events in real time, to produce quantitative changes to the LOA and PTSR along with qualitative reasoning why the likelihood of approval has changed.
Reasons to Buy
- Precise Likelihood of Approval and Phase Transition Success Rates: Our machine learning and proprietary models provide accurate predictions, helping you gauge the potential success of a drug in the regulatory process.
- Competitive Strategy Planning: Access information on LOA and PTSR for competitors’ drugs, allowing you to plan your clinical development, commercialisation and marketing strategies
- Event-driven Updates: Track event-driven changes in LOA and PTSR benchmarked against indication LOA/PTSR. Get the latest insights to adapt your strategies promptly!
- Well-informed Investment Decisions: This data helps you navigate the dynamic landscape of drug development and regulatory considerations.
- Drug Details: Drug name, Drug type, Intervention type
- Administration Pathway
- Therapeutic Areas
- Key Manufacturers
- Drug Development Status
This is an on-demand report that will be delivered upon request. The report will be delivered within 2 business days of the purchase, excluding weekends and holidays. Certain sections of the report may be removed or altered based on data availability and relevance.
Frequently asked questions
Frequently asked questions, what is likelihood of approval (loa).
The probability of a drug ultimately receiving market authorization
What is Phase Transition Success Rate (PTSR)?
The probability of a drug’s advancement to the next stage of clinical development
Why is GlobalData’s product so powerful?
GlobalData’s Drug-Specific Likelihood of Approval (LoA) calculates the Phase Transition Success Rate (PTSR) and Likelihood of Approval (LoA) customized to individual drug. The model uses a combination of Machine Learning (ML) and a GlobalData proprietary algorithm to process data points from the Drugs, Clinical Trials, Regulatory Milestones, Company, and Financial databases.
What is the data scope (inclusions and exclusions)?
Data Scope:
- Drugs which have been approved in the past 10 years
- Drugs which have failed during clinical development in the past 18 years
- Drugs which are currently in development
Drug Phase Scope:
- Phase I, Phase II, Phase III, and Pre-Registration development stage
Drug Geography Scope:
- Drugs must meet one of the following criteria to be included in the model:
- The developer has specified the US as an intended market for approval.
- The developer has not specified any country as an intended market for approval, i.e. the “Drug Geography” is listed as “Global”
Drug Type Scope:
- Innovator drugs and biosimilars
Entity Type Scope:
Only drugs in development by companies are included in the model.
- Diagnostics, Imaging Agents, Biomarkers, stents and other drug delivery devices (covered in GlobalData’s Medical Intelligence Center).
- Nutraceuticals, dietary supplements, alternative medicines, imaging agents, radio emitter, transplants, transfusions, fillers, cosmetics, probiotics, antiseptics, antacids, mobilizing agents, veterinary drugs and drugs not seeking approval.
- Generic drugs
- Innovative drugs in Preclinical or Discovery Stage.
- Pipeline drugs sponsored by a Government or Institution.
- Drugs with a specific Drug Geography not the United States.
- Currency Conversion is for Indicative purpose only. All orders are processed in US Dollars only.
- USD - US Dollar
- AUD — Australian Dollar
- BRL — Brazilian Real
- CNY — Yuan Renminbi
- GBP — Pound Sterling
- INR — Indian Rupee
- JPY — Japanese Yen
- ZAR — South African Rand
- USD — US Dollar
- RUB — Russian Ruble
Can be used by individual purchaser only
Can be shared by unlimited users within one corporate location e.g. a regional office
Can be shared globally by unlimited users within the purchasing corporation e.g. all employees of a single company
Undecided about purchasing this report?
Get in touch to find out about multi-purchase discounts.
[email protected] Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.

“The GlobalData platform is our go-to tool for intelligence services. GlobalData provides an easy way to access comprehensive intelligence data around multiple sectors, which essentially makes it a one-for-all intelligence platform, for tendering and approaching customers.
GlobalData is very customer orientated, with a high degree of personalised services, which benefits everyday use. The highly detailed project intelligence and forecast reports can be utilised across multiple departments and workflow scopes, from operational to strategic level, and often support strategic decisions. GlobalData Analytics and visualisation solutions has contributed positively when preparing management presentations and strategic papers.”
“COVID-19 has caused significant interference to our business and the COVID-19 intelligence from GlobalData has helped us reach better decisions around strategy. These two highlights have helped enormously to understand the projections into the future concerning our business units, we also utilise the project database to source new projects for Liebherr-Werk to use as an additional source to pitch for new business.”
Your daily news has saved me a lot of time and keeps me up-to-date with what is happening in the market, I like that you almost always have a link to the source origin. We also use your market data in our Strategic Business Process to support our business decisions. By having everything in one place on the Intelligence Center it has saved me a lot of time versus looking on different sources, the alert function also helps with this.
Having used several other market research companies, I find that GlobalData manages to provide that ‘difficult-to-get’ market data that others can’t, as well as very diverse and complete consumer surveys.
Our experience with GlobalData has been very good, from the platform itself to the people. I find that the analysts and the account team have a high level of customer focus and responsiveness and therefore I can always rely on. The platform is more holistic than other providers. It is convenient and almost like a one stop shop. The pricing suite is highly competitive and value for our organisation.
I like reports that inform new segments such as the analysis on generation Z, millennials, the impact of COVID 19 to our banking customers and their new channel habits. Secondly the specialist insight on affluent sector significantly increases our understanding about this group of customers. The combination of those give us depth and breadth of the evolving market.
I’m in the business of answering and helping people make decisions so with the intelligence center I can do that, effectively and efficiently. I can share quickly key insights that answer and satisfy our country stakeholders by giving them many quality studies and primary research about competitive landscape beyond the outlook of our bank. It helps me be seen as an advisory partner and that makes a big difference. A big benefit of our subscription is that no one holds the whole data and because it allows so many people, so many different parts of our organisation have access, it enables all teams to have the same level of knowledge and decision support.
“I know that I can always rely on Globaldata’s work when I’m searching for the right consumer and market insights. I use Globaldata insights to understand the changing market & consumer landscape and help create better taste & wellbeing solutions for our customers in food, beverage and healthcare industries.
Globaldata has the right data and the reports are of very high quality compared to your competitors. Globaldata not only has overall market sizes & consumer insights on food & beverages but also provides insights at the ingredient & flavour level. That is key for B2B companies like Givaudan. This way we understand our customers’ business and also gain insight to our unique industry”
GlobalData provides a great range of information and reports on various sectors that is highly relevant, timely, easy to access and utilise. The reports and data dashboards help engagement with clients; they provide valuable industry and market insights that can enrich client conversations and can help in the shaping of value propositions. Moreover, using GlobalData products has helped increase my knowledge of the finance sector, the players within it, and the general threats and opportunities.
I find the consumer surveys that are carried out to be extremely beneficial and not something I have seen anywhere else. They provided an insightful view of why and which consumers take (or don’t) particular financial products. This can help shape conversations with clients to ensure they make the right strategic decisions for their business.
One of the challenges I have found is that data in the payments space is often piecemeal. With GD all of the data I need is in one place, but it also comes with additional market reports that provide useful extra context and information. Having the ability to set-up alerts on relevant movements in the industry, be it competitors or customers, and have them emailed directly to me, ensures I get early sight of industry activity and don’t have to search for news.
Related reports

Every Company Report we produce is powered by the GlobalData Intelligence Center.
Subscribing to our intelligence platform means you can monitor developments at Likelihood of Approval and Phase Transition Success Rate Model – CYB-004 in Generalized Anxiety Disorder (GAD) in real time.
- Access a live Likelihood of Approval and Phase Transition Success Rate Model – CYB-004 in Generalized Anxiety Disorder (GAD) dashboard for 12 months, with up-to-the-minute insights.
- Fuel your decision making with real-time deal coverage and media activity.
- Turn insights on financials, deals, products and pipelines into powerful agents of commercial advantage.

IMAGES
VIDEO
COMMENTS
January 30, 2024 - Pre-Application Webinar for RFA-NR-24-004: Transformative Research to Address Health Disparities and Advance Health Equity (U01 Clinical Trial Optional). See Notice NOT-NR-24-006; August 31, 2022- Implementation Changes for Genomic Data Sharing Plans Included with Applications Due on or after January 25, 2023.See Notice NOT-OD-22-198.
RFA-OH-24-004 World Trade Center Health Program Mentored Research Scientist Career Development Award (K01) Application Deadline: Tuesday, October 29, 2024, 5PM Eastern Time ... Note: World Trade Center Health Research related to WTC Survivors (U01-No Applications with Responders Accepted) RFA-OH-22-004 was reissued as R21 for FY24.
RFA-CE-22-004: Research Grants to Prevent Firearm-Related Violence and Injuries (R01) The National Center for Injury Prevention and Control, Division for Violence Prevention (DVP) is committing $2,539,786 to fund four research awards under RFA-CE-22-004: Research Grants to Prevent Firearm-Related Violence and Injuries.
Health Promotion and Disease Prevention Research Centers: RFA-DP-24-004 Activity Code U48 AMENDMENT 2: May 5, 2023 (1) In Section IV. "Application and Submission Information, 7. Page Limitations," the maximum pages for all appendices was increased to 50 pages. (2) In Section V. "Application Review Information, 1.
Deadline for Applications: June 23, 2023 by 11:59 p.m. Eastern time. CDC announces the availability of funding for the next cycle (2024-2029) of Health Promotion and Disease Prevention Research Centers (CDC-RFA-DP-24-004), also known as Prevention Research Centers (PRCs). This Notice of Funding Opportunity (NOFO) will provide funding to PRCs ...
On 11/8/21 Centers for Disease Control and Prevention posted grant opportunity RFA-CE-22-004 for Research Grants to Prevent Firearm-Related Violence and Injuries (R01) with funding of $33.0 million. The grant will be issued under grant program 93.136 Injury Prevention and Control Research and State and Community Based Programs.
On February 13, 2024 at 2:00 p.m. (ET), NINR hosted a technical assistance webinar for funding opportunity RFA-NR-24-004: Transformative Research to Address Health Disparities and Advance Health Equity (U01 Clinical Trial Optional).. NIH staff provided an overview of the funding opportunity and answered questions from potential applicants.
This Notice of Funding Opportunity (NOFO) will provide supplemental funding to the CDC Health Promotion and Disease Prevention Research Centers (RFA-DP-24-004) to conduct Special Interest Research Projects (SIP) to inform public health practice.
The purpose of this program is to provide opportunities for up to six consortia to develop or expand global health research training programs that meet the following objectives: (1) provide one-year mentored research training for pre-doctoral students from the U.S. and recent post-doctoral and post-professional degree graduates (collectively referred to as trainees) from the U.S. and low- and ...
The National Center for Medical Rehabilitation Research within NICHD seeks applications for intervention research on individuals with physical disabilities. Areas of interest include but are not limited to referrals for and access to health care and inpatient and outpatient rehabilitation services for both primary and secondary conditions, and ...
Funding Opportunity Number: HRSA-24-004. Dates to Apply: 03/22/2024 to 05/23/2024. Bureau/Office: Federal Office of Rural Health Policy. Status: Open. Estimated Award Date: 09/01/2024. Apply. Track Your Application. Email Us. This notice announces the opportunity to apply for funding under the Rural Health Research Center (RHRC) Program.
CLC 004 Market Research (Last modified:1/11/2023) No Schedule Available Download Course Objectives. ... This module provides a foundational understanding of the benefits of effective market research to reduce acquisition costs and cycle times, and provide greater access to advanced technologies. This module covers the differences between ...
RES-004: Research Misconduct. Date: 10/23/2005 Status: Final . Last Revised: 10/30/2019. Policy Type: University. ... The discovery of new knowledge through research is a fundamental part of the life of a faculty member at the University of Virginia. The formulation of testable hypotheses, the organization of research design, the mobilization ...
A. Scope. This Policy sets forth the implementing procedures to Regulation 1.0101 Research Integrity. These procedures apply to allegations of research misconduct and other research integrity deviations when the respondent is an individual employed by, affiliated with, or acting on behalf of the University during the time the alleged misconduct ...
Course Detail Page. CLC 004 Market Research. Description. THIS COURSE RETIRED AND REPLACED BY CON 0040 ON 10 JANUARY 2023. The requirement to conduct market research is not policy; it's the law. This module provides a foundational understanding of the benefits of effective market research to reduce acquisition costs and cycle times, and ...
The CDC announces the availability of funding for the next cycle of Health Promotion and Disease Prevention Research Centers (CDC-RFA-DP24-004), or Prevention Research Centers (PRCs). This Notice of Funding Opportunity (NOFO) will provide funding to PRCs through 5-year awards to:
MARKETING RESEARCH (004) EXAM 1. What is the purpose of marketing research? Click the card to flip 👆. DEFINITION: The function that links an organization to its market through the gathering of information. We do market research because often our beliefs and intuitions are wrong. Click the card to flip 👆. 1 / 26.
93.310. Funding Opportunity Purpose. The NIH Director's Transformative Research Award supports individual scientists or groups of scientists proposing bold, groundbreaking, exceptionally innovative, original, and/or unconventional research with the potential to create new scientific paradigms, establish entirely new and improved clinical ...
Fundamental Research is an open access, peer-reviewed, multidisciplinary journal, which is supervised by the National Natural Science Foundation of China (NSFC). Published bimonthly, it features high-calibre research covering all areas of the natural sciences and high-tech fields, including: Mathem…. View full aims & scope.
View Test prep - CLC004 - Market Research Exam.pdf from CLC 004 at Defense Acquisition University. DAU Page 1 of 3 Market Research Exam Here is your test result.The dots represent the choices you
CLC004 Ma rket Research Lesson 0 - Module Introduction TOC Introduction This module is designed to assist members of the DoD community who are involved in any stage of the acquisition process to better understand market research and its importance in ac. CLC 004. Defense Acquisition University. TEST PREP 186 views.
Prevention Research Centers (PRC) - ... NOFO RFA DP-24-004 CORRECTION • During the April 26. th. PRC NOFO Informational Webinar CDC incorrectly stated that the evaluation plan is to be developed and submitted as part of the application. • CORRECTION • An evaluation plan is not required as part of the application. The application should
In countries where both the public and private sectors participated in maize research, private sector research investment far exceeded that of the public sector. With more aggressive marketing programmes, the private sector captured 89% of the Asian maize seed market in the late 1990s. National public seed research agencies (including ...
CYB-004 in Generalized Anxiety Disorder (GAD) Drug Details: CYB-004 is under development for the treatment of social anxiety disorder and generalized anxiety disorder. It is administered through inhalation route, intravenous bolus and intravenous route. The drug candidate is a deuterated forms of DMT and 5-MeO-DMT.