
Literature Review Basics
- What is a Literature Review?
- Synthesizing Research
- Using Research & Synthesis Tables
- Additional Resources

About the Research and Synthesis Tables
Research Tables and Synthesis Tables are useful tools for organizing and analyzing your research as you assemble your literature review. They represent two different parts of the review process: assembling relevant information and synthesizing it. Use a Research table to compile the main info you need about the items you find in your research -- it's a great thing to have on hand as you take notes on what you read! Then, once you've assembled your research, use the Synthesis table to start charting the similarities/differences and major themes among your collected items.
We've included an Excel file with templates for you to use below; the examples pictured on this page are snapshots from that file.
- Research and Synthesis Table Templates This Excel workbook includes simple templates for creating research tables and synthesis tables. Feel free to download and use!
Using the Research Table

This is an example of a research table, in which you provide a basic description of the most important features of the studies, articles, and other items you discover in your research. The table identifies each item according to its author/date of publication, its purpose or thesis, what type of work it is (systematic review, clinical trial, etc.), the level of evidence it represents (which tells you a lot about its impact on the field of study), and its major findings. Your job, when you assemble this information, is to develop a snapshot of what the research shows about the topic of your research question and assess its value (both for the purpose of your work and for general knowledge in the field).
Think of your work on the research table as the foundational step for your analysis of the literature, in which you assemble the information you'll be analyzing and lay the groundwork for thinking about what it means and how it can be used.
Using the Synthesis Table

This is an example of a synthesis table or synthesis matrix , in which you organize and analyze your research by listing each source and indicating whether a given finding or result occurred in a particular study or article ( each row lists an individual source, and each finding has its own column, in which X = yes, blank = no). You can also add or alter the columns to look for shared study populations, sort by level of evidence or source type, etc. The key here is to use the table to provide a simple representation of what the research has found (or not found, as the case may be). Think of a synthesis table as a tool for making comparisons, identifying trends, and locating gaps in the literature.
How do I know which findings to use, or how many to include? Your research question tells you which findings are of interest in your research, so work from your research question to decide what needs to go in each Finding header, and how many findings are necessary. The number is up to you; again, you can alter this table by adding or deleting columns to match what you're actually looking for in your analysis. You should also, of course, be guided by what's actually present in the material your research turns up!
- << Previous: Synthesizing Research
- Next: Additional Resources >>
- Last Updated: Sep 26, 2023 12:06 PM
- URL: https://usi.libguides.com/literature-review-basics
- Research Guides
- Vanderbilt University Libraries
- Peabody Library
Literature Reviews
Literature review table.
- Steps to Success: The Literature Review Process
- Literature Reviews Webinar Recording
- Writing Like an Academic
- Publishing in Academic Journals
- Managing Citations This link opens in a new window
- Literature Review Table Template Peabody Librarians have created a sample literature review table to use to organize your research. Feel free to download this file and use or adapt as needed.
- << Previous: Literature Reviews Webinar Recording
- Next: Writing Like an Academic >>
- Last Updated: Feb 29, 2024 4:20 PM
- URL: https://researchguides.library.vanderbilt.edu/peabody/litreviews

MSU Libraries
- Need help? Ask a Librarian
Nursing Literature Reviews
- Literature and Other Types of Reviews
- Starting Your Search
- Developing a Research Question and the Literature Search Process
- Conducting a Literature Search
- Levels of Evidence
- Creating a PRISMA Table
- Literature Table and Synthesis
- Other Resources
About Literature Tables and Writing a Synthesis
A literature table is a way to organize the articles you've selected for inclusion in your publication. There are many different types of literature tables-the main thing is to determine the important pieces that help draw out the comparisons and contrasts between the articles included in your review. The first few columns should include the basic info about the article (title, authors, journal), publication year, and the purpose of the paper.
While the table is a step to help you organize the articles you've selected for your research, the literature synthesis can take many forms and can have multiple parts. This largely depends on what type of review you've undertaken. Look back at the examples under Literature and Other Types of Reviews to see examples of different types of reviews.
- Example of Literature Table
Examples of Literature Tables

Camak, D.J. (2015), Addressing the burden of stroke caregivers: a literature review. J Clin Nurs, 24: 2376-2382. doi: 10.1111/jocn.12884

Balcombe, L., Miller, C., & McGuiness, W. (2017). Approaches to the application and removal of compression therapy: A literature review. British Journal of Community Nursing , 22 , S6–S14. https://doi-org.proxy1.cl.msu.edu/10.12968/bjcn.2017.22.Sup10.S6
- << Previous: Creating a PRISMA Table
- Next: Other Resources >>
- Last Updated: May 10, 2024 9:36 AM
- URL: https://libguides.lib.msu.edu/nursinglitreview
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, automatically generate references for free.
- Knowledge Base
- Dissertation
- What is a Literature Review? | Guide, Template, & Examples
What is a Literature Review? | Guide, Template, & Examples
Published on 22 February 2022 by Shona McCombes . Revised on 7 June 2022.
What is a literature review? A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research.
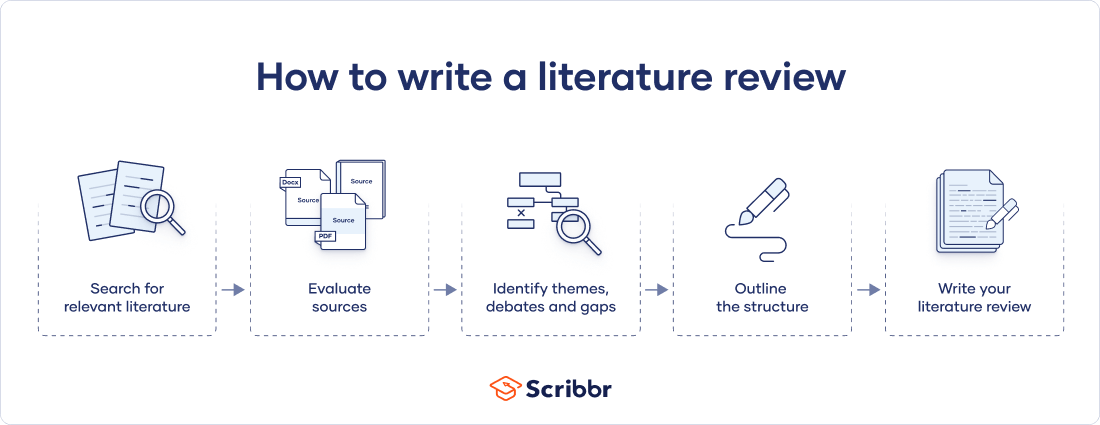
There are five key steps to writing a literature review:
- Search for relevant literature
- Evaluate sources
- Identify themes, debates and gaps
- Outline the structure
- Write your literature review
A good literature review doesn’t just summarise sources – it analyses, synthesises, and critically evaluates to give a clear picture of the state of knowledge on the subject.
Instantly correct all language mistakes in your text
Be assured that you'll submit flawless writing. Upload your document to correct all your mistakes.

Table of contents
Why write a literature review, examples of literature reviews, step 1: search for relevant literature, step 2: evaluate and select sources, step 3: identify themes, debates and gaps, step 4: outline your literature review’s structure, step 5: write your literature review, frequently asked questions about literature reviews, introduction.
- Quick Run-through
- Step 1 & 2
When you write a dissertation or thesis, you will have to conduct a literature review to situate your research within existing knowledge. The literature review gives you a chance to:
- Demonstrate your familiarity with the topic and scholarly context
- Develop a theoretical framework and methodology for your research
- Position yourself in relation to other researchers and theorists
- Show how your dissertation addresses a gap or contributes to a debate
You might also have to write a literature review as a stand-alone assignment. In this case, the purpose is to evaluate the current state of research and demonstrate your knowledge of scholarly debates around a topic.
The content will look slightly different in each case, but the process of conducting a literature review follows the same steps. We’ve written a step-by-step guide that you can follow below.
The only proofreading tool specialized in correcting academic writing
The academic proofreading tool has been trained on 1000s of academic texts and by native English editors. Making it the most accurate and reliable proofreading tool for students.
Correct my document today
Writing literature reviews can be quite challenging! A good starting point could be to look at some examples, depending on what kind of literature review you’d like to write.
- Example literature review #1: “Why Do People Migrate? A Review of the Theoretical Literature” ( Theoretical literature review about the development of economic migration theory from the 1950s to today.)
- Example literature review #2: “Literature review as a research methodology: An overview and guidelines” ( Methodological literature review about interdisciplinary knowledge acquisition and production.)
- Example literature review #3: “The Use of Technology in English Language Learning: A Literature Review” ( Thematic literature review about the effects of technology on language acquisition.)
- Example literature review #4: “Learners’ Listening Comprehension Difficulties in English Language Learning: A Literature Review” ( Chronological literature review about how the concept of listening skills has changed over time.)
You can also check out our templates with literature review examples and sample outlines at the links below.
Download Word doc Download Google doc
Before you begin searching for literature, you need a clearly defined topic .
If you are writing the literature review section of a dissertation or research paper, you will search for literature related to your research objectives and questions .
If you are writing a literature review as a stand-alone assignment, you will have to choose a focus and develop a central question to direct your search. Unlike a dissertation research question, this question has to be answerable without collecting original data. You should be able to answer it based only on a review of existing publications.
Make a list of keywords
Start by creating a list of keywords related to your research topic. Include each of the key concepts or variables you’re interested in, and list any synonyms and related terms. You can add to this list if you discover new keywords in the process of your literature search.
- Social media, Facebook, Instagram, Twitter, Snapchat, TikTok
- Body image, self-perception, self-esteem, mental health
- Generation Z, teenagers, adolescents, youth
Search for relevant sources
Use your keywords to begin searching for sources. Some databases to search for journals and articles include:
- Your university’s library catalogue
- Google Scholar
- Project Muse (humanities and social sciences)
- Medline (life sciences and biomedicine)
- EconLit (economics)
- Inspec (physics, engineering and computer science)
You can use boolean operators to help narrow down your search:
Read the abstract to find out whether an article is relevant to your question. When you find a useful book or article, you can check the bibliography to find other relevant sources.
To identify the most important publications on your topic, take note of recurring citations. If the same authors, books or articles keep appearing in your reading, make sure to seek them out.
You probably won’t be able to read absolutely everything that has been written on the topic – you’ll have to evaluate which sources are most relevant to your questions.
For each publication, ask yourself:
- What question or problem is the author addressing?
- What are the key concepts and how are they defined?
- What are the key theories, models and methods? Does the research use established frameworks or take an innovative approach?
- What are the results and conclusions of the study?
- How does the publication relate to other literature in the field? Does it confirm, add to, or challenge established knowledge?
- How does the publication contribute to your understanding of the topic? What are its key insights and arguments?
- What are the strengths and weaknesses of the research?
Make sure the sources you use are credible, and make sure you read any landmark studies and major theories in your field of research.
You can find out how many times an article has been cited on Google Scholar – a high citation count means the article has been influential in the field, and should certainly be included in your literature review.
The scope of your review will depend on your topic and discipline: in the sciences you usually only review recent literature, but in the humanities you might take a long historical perspective (for example, to trace how a concept has changed in meaning over time).
Remember that you can use our template to summarise and evaluate sources you’re thinking about using!
Take notes and cite your sources
As you read, you should also begin the writing process. Take notes that you can later incorporate into the text of your literature review.
It’s important to keep track of your sources with references to avoid plagiarism . It can be helpful to make an annotated bibliography, where you compile full reference information and write a paragraph of summary and analysis for each source. This helps you remember what you read and saves time later in the process.
You can use our free APA Reference Generator for quick, correct, consistent citations.
To begin organising your literature review’s argument and structure, you need to understand the connections and relationships between the sources you’ve read. Based on your reading and notes, you can look for:
- Trends and patterns (in theory, method or results): do certain approaches become more or less popular over time?
- Themes: what questions or concepts recur across the literature?
- Debates, conflicts and contradictions: where do sources disagree?
- Pivotal publications: are there any influential theories or studies that changed the direction of the field?
- Gaps: what is missing from the literature? Are there weaknesses that need to be addressed?
This step will help you work out the structure of your literature review and (if applicable) show how your own research will contribute to existing knowledge.
- Most research has focused on young women.
- There is an increasing interest in the visual aspects of social media.
- But there is still a lack of robust research on highly-visual platforms like Instagram and Snapchat – this is a gap that you could address in your own research.
There are various approaches to organising the body of a literature review. You should have a rough idea of your strategy before you start writing.
Depending on the length of your literature review, you can combine several of these strategies (for example, your overall structure might be thematic, but each theme is discussed chronologically).
Chronological
The simplest approach is to trace the development of the topic over time. However, if you choose this strategy, be careful to avoid simply listing and summarising sources in order.
Try to analyse patterns, turning points and key debates that have shaped the direction of the field. Give your interpretation of how and why certain developments occurred.
If you have found some recurring central themes, you can organise your literature review into subsections that address different aspects of the topic.
For example, if you are reviewing literature about inequalities in migrant health outcomes, key themes might include healthcare policy, language barriers, cultural attitudes, legal status, and economic access.
Methodological
If you draw your sources from different disciplines or fields that use a variety of research methods , you might want to compare the results and conclusions that emerge from different approaches. For example:
- Look at what results have emerged in qualitative versus quantitative research
- Discuss how the topic has been approached by empirical versus theoretical scholarship
- Divide the literature into sociological, historical, and cultural sources
Theoretical
A literature review is often the foundation for a theoretical framework . You can use it to discuss various theories, models, and definitions of key concepts.
You might argue for the relevance of a specific theoretical approach, or combine various theoretical concepts to create a framework for your research.
Like any other academic text, your literature review should have an introduction , a main body, and a conclusion . What you include in each depends on the objective of your literature review.
The introduction should clearly establish the focus and purpose of the literature review.
If you are writing the literature review as part of your dissertation or thesis, reiterate your central problem or research question and give a brief summary of the scholarly context. You can emphasise the timeliness of the topic (“many recent studies have focused on the problem of x”) or highlight a gap in the literature (“while there has been much research on x, few researchers have taken y into consideration”).
Depending on the length of your literature review, you might want to divide the body into subsections. You can use a subheading for each theme, time period, or methodological approach.
As you write, make sure to follow these tips:
- Summarise and synthesise: give an overview of the main points of each source and combine them into a coherent whole.
- Analyse and interpret: don’t just paraphrase other researchers – add your own interpretations, discussing the significance of findings in relation to the literature as a whole.
- Critically evaluate: mention the strengths and weaknesses of your sources.
- Write in well-structured paragraphs: use transitions and topic sentences to draw connections, comparisons and contrasts.
In the conclusion, you should summarise the key findings you have taken from the literature and emphasise their significance.
If the literature review is part of your dissertation or thesis, reiterate how your research addresses gaps and contributes new knowledge, or discuss how you have drawn on existing theories and methods to build a framework for your research. This can lead directly into your methodology section.
A literature review is a survey of scholarly sources (such as books, journal articles, and theses) related to a specific topic or research question .
It is often written as part of a dissertation , thesis, research paper , or proposal .
There are several reasons to conduct a literature review at the beginning of a research project:
- To familiarise yourself with the current state of knowledge on your topic
- To ensure that you’re not just repeating what others have already done
- To identify gaps in knowledge and unresolved problems that your research can address
- To develop your theoretical framework and methodology
- To provide an overview of the key findings and debates on the topic
Writing the literature review shows your reader how your work relates to existing research and what new insights it will contribute.
The literature review usually comes near the beginning of your dissertation . After the introduction , it grounds your research in a scholarly field and leads directly to your theoretical framework or methodology .
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the ‘Cite this Scribbr article’ button to automatically add the citation to our free Reference Generator.
McCombes, S. (2022, June 07). What is a Literature Review? | Guide, Template, & Examples. Scribbr. Retrieved 6 May 2024, from https://www.scribbr.co.uk/thesis-dissertation/literature-review/
Is this article helpful?
Shona McCombes
Other students also liked, how to write a dissertation proposal | a step-by-step guide, what is a theoretical framework | a step-by-step guide, what is a research methodology | steps & tips.
Get science-backed answers as you write with Paperpal's Research feature
What is a Literature Review? How to Write It (with Examples)

A literature review is a critical analysis and synthesis of existing research on a particular topic. It provides an overview of the current state of knowledge, identifies gaps, and highlights key findings in the literature. 1 The purpose of a literature review is to situate your own research within the context of existing scholarship, demonstrating your understanding of the topic and showing how your work contributes to the ongoing conversation in the field. Learning how to write a literature review is a critical tool for successful research. Your ability to summarize and synthesize prior research pertaining to a certain topic demonstrates your grasp on the topic of study, and assists in the learning process.
Table of Contents
- What is the purpose of literature review?
- a. Habitat Loss and Species Extinction:
- b. Range Shifts and Phenological Changes:
- c. Ocean Acidification and Coral Reefs:
- d. Adaptive Strategies and Conservation Efforts:
- How to write a good literature review
- Choose a Topic and Define the Research Question:
- Decide on the Scope of Your Review:
- Select Databases for Searches:
- Conduct Searches and Keep Track:
- Review the Literature:
- Organize and Write Your Literature Review:
- Frequently asked questions
What is a literature review?
A well-conducted literature review demonstrates the researcher’s familiarity with the existing literature, establishes the context for their own research, and contributes to scholarly conversations on the topic. One of the purposes of a literature review is also to help researchers avoid duplicating previous work and ensure that their research is informed by and builds upon the existing body of knowledge.

What is the purpose of literature review?
A literature review serves several important purposes within academic and research contexts. Here are some key objectives and functions of a literature review: 2
- Contextualizing the Research Problem: The literature review provides a background and context for the research problem under investigation. It helps to situate the study within the existing body of knowledge.
- Identifying Gaps in Knowledge: By identifying gaps, contradictions, or areas requiring further research, the researcher can shape the research question and justify the significance of the study. This is crucial for ensuring that the new research contributes something novel to the field.
- Understanding Theoretical and Conceptual Frameworks: Literature reviews help researchers gain an understanding of the theoretical and conceptual frameworks used in previous studies. This aids in the development of a theoretical framework for the current research.
- Providing Methodological Insights: Another purpose of literature reviews is that it allows researchers to learn about the methodologies employed in previous studies. This can help in choosing appropriate research methods for the current study and avoiding pitfalls that others may have encountered.
- Establishing Credibility: A well-conducted literature review demonstrates the researcher’s familiarity with existing scholarship, establishing their credibility and expertise in the field. It also helps in building a solid foundation for the new research.
- Informing Hypotheses or Research Questions: The literature review guides the formulation of hypotheses or research questions by highlighting relevant findings and areas of uncertainty in existing literature.
Literature review example
Let’s delve deeper with a literature review example: Let’s say your literature review is about the impact of climate change on biodiversity. You might format your literature review into sections such as the effects of climate change on habitat loss and species extinction, phenological changes, and marine biodiversity. Each section would then summarize and analyze relevant studies in those areas, highlighting key findings and identifying gaps in the research. The review would conclude by emphasizing the need for further research on specific aspects of the relationship between climate change and biodiversity. The following literature review template provides a glimpse into the recommended literature review structure and content, demonstrating how research findings are organized around specific themes within a broader topic.
Literature Review on Climate Change Impacts on Biodiversity:
Climate change is a global phenomenon with far-reaching consequences, including significant impacts on biodiversity. This literature review synthesizes key findings from various studies:
a. Habitat Loss and Species Extinction:
Climate change-induced alterations in temperature and precipitation patterns contribute to habitat loss, affecting numerous species (Thomas et al., 2004). The review discusses how these changes increase the risk of extinction, particularly for species with specific habitat requirements.
b. Range Shifts and Phenological Changes:
Observations of range shifts and changes in the timing of biological events (phenology) are documented in response to changing climatic conditions (Parmesan & Yohe, 2003). These shifts affect ecosystems and may lead to mismatches between species and their resources.
c. Ocean Acidification and Coral Reefs:
The review explores the impact of climate change on marine biodiversity, emphasizing ocean acidification’s threat to coral reefs (Hoegh-Guldberg et al., 2007). Changes in pH levels negatively affect coral calcification, disrupting the delicate balance of marine ecosystems.
d. Adaptive Strategies and Conservation Efforts:
Recognizing the urgency of the situation, the literature review discusses various adaptive strategies adopted by species and conservation efforts aimed at mitigating the impacts of climate change on biodiversity (Hannah et al., 2007). It emphasizes the importance of interdisciplinary approaches for effective conservation planning.

How to write a good literature review
Writing a literature review involves summarizing and synthesizing existing research on a particular topic. A good literature review format should include the following elements.
Introduction: The introduction sets the stage for your literature review, providing context and introducing the main focus of your review.
- Opening Statement: Begin with a general statement about the broader topic and its significance in the field.
- Scope and Purpose: Clearly define the scope of your literature review. Explain the specific research question or objective you aim to address.
- Organizational Framework: Briefly outline the structure of your literature review, indicating how you will categorize and discuss the existing research.
- Significance of the Study: Highlight why your literature review is important and how it contributes to the understanding of the chosen topic.
- Thesis Statement: Conclude the introduction with a concise thesis statement that outlines the main argument or perspective you will develop in the body of the literature review.
Body: The body of the literature review is where you provide a comprehensive analysis of existing literature, grouping studies based on themes, methodologies, or other relevant criteria.
- Organize by Theme or Concept: Group studies that share common themes, concepts, or methodologies. Discuss each theme or concept in detail, summarizing key findings and identifying gaps or areas of disagreement.
- Critical Analysis: Evaluate the strengths and weaknesses of each study. Discuss the methodologies used, the quality of evidence, and the overall contribution of each work to the understanding of the topic.
- Synthesis of Findings: Synthesize the information from different studies to highlight trends, patterns, or areas of consensus in the literature.
- Identification of Gaps: Discuss any gaps or limitations in the existing research and explain how your review contributes to filling these gaps.
- Transition between Sections: Provide smooth transitions between different themes or concepts to maintain the flow of your literature review.
Conclusion: The conclusion of your literature review should summarize the main findings, highlight the contributions of the review, and suggest avenues for future research.
- Summary of Key Findings: Recap the main findings from the literature and restate how they contribute to your research question or objective.
- Contributions to the Field: Discuss the overall contribution of your literature review to the existing knowledge in the field.
- Implications and Applications: Explore the practical implications of the findings and suggest how they might impact future research or practice.
- Recommendations for Future Research: Identify areas that require further investigation and propose potential directions for future research in the field.
- Final Thoughts: Conclude with a final reflection on the importance of your literature review and its relevance to the broader academic community.

Conducting a literature review
Conducting a literature review is an essential step in research that involves reviewing and analyzing existing literature on a specific topic. It’s important to know how to do a literature review effectively, so here are the steps to follow: 1
Choose a Topic and Define the Research Question:
- Select a topic that is relevant to your field of study.
- Clearly define your research question or objective. Determine what specific aspect of the topic do you want to explore?
Decide on the Scope of Your Review:
- Determine the timeframe for your literature review. Are you focusing on recent developments, or do you want a historical overview?
- Consider the geographical scope. Is your review global, or are you focusing on a specific region?
- Define the inclusion and exclusion criteria. What types of sources will you include? Are there specific types of studies or publications you will exclude?
Select Databases for Searches:
- Identify relevant databases for your field. Examples include PubMed, IEEE Xplore, Scopus, Web of Science, and Google Scholar.
- Consider searching in library catalogs, institutional repositories, and specialized databases related to your topic.
Conduct Searches and Keep Track:
- Develop a systematic search strategy using keywords, Boolean operators (AND, OR, NOT), and other search techniques.
- Record and document your search strategy for transparency and replicability.
- Keep track of the articles, including publication details, abstracts, and links. Use citation management tools like EndNote, Zotero, or Mendeley to organize your references.
Review the Literature:
- Evaluate the relevance and quality of each source. Consider the methodology, sample size, and results of studies.
- Organize the literature by themes or key concepts. Identify patterns, trends, and gaps in the existing research.
- Summarize key findings and arguments from each source. Compare and contrast different perspectives.
- Identify areas where there is a consensus in the literature and where there are conflicting opinions.
- Provide critical analysis and synthesis of the literature. What are the strengths and weaknesses of existing research?
Organize and Write Your Literature Review:
- Literature review outline should be based on themes, chronological order, or methodological approaches.
- Write a clear and coherent narrative that synthesizes the information gathered.
- Use proper citations for each source and ensure consistency in your citation style (APA, MLA, Chicago, etc.).
- Conclude your literature review by summarizing key findings, identifying gaps, and suggesting areas for future research.
The literature review sample and detailed advice on writing and conducting a review will help you produce a well-structured report. But remember that a literature review is an ongoing process, and it may be necessary to revisit and update it as your research progresses.
Frequently asked questions
A literature review is a critical and comprehensive analysis of existing literature (published and unpublished works) on a specific topic or research question and provides a synthesis of the current state of knowledge in a particular field. A well-conducted literature review is crucial for researchers to build upon existing knowledge, avoid duplication of efforts, and contribute to the advancement of their field. It also helps researchers situate their work within a broader context and facilitates the development of a sound theoretical and conceptual framework for their studies.
Literature review is a crucial component of research writing, providing a solid background for a research paper’s investigation. The aim is to keep professionals up to date by providing an understanding of ongoing developments within a specific field, including research methods, and experimental techniques used in that field, and present that knowledge in the form of a written report. Also, the depth and breadth of the literature review emphasizes the credibility of the scholar in his or her field.
Before writing a literature review, it’s essential to undertake several preparatory steps to ensure that your review is well-researched, organized, and focused. This includes choosing a topic of general interest to you and doing exploratory research on that topic, writing an annotated bibliography, and noting major points, especially those that relate to the position you have taken on the topic.
Literature reviews and academic research papers are essential components of scholarly work but serve different purposes within the academic realm. 3 A literature review aims to provide a foundation for understanding the current state of research on a particular topic, identify gaps or controversies, and lay the groundwork for future research. Therefore, it draws heavily from existing academic sources, including books, journal articles, and other scholarly publications. In contrast, an academic research paper aims to present new knowledge, contribute to the academic discourse, and advance the understanding of a specific research question. Therefore, it involves a mix of existing literature (in the introduction and literature review sections) and original data or findings obtained through research methods.
Literature reviews are essential components of academic and research papers, and various strategies can be employed to conduct them effectively. If you want to know how to write a literature review for a research paper, here are four common approaches that are often used by researchers. Chronological Review: This strategy involves organizing the literature based on the chronological order of publication. It helps to trace the development of a topic over time, showing how ideas, theories, and research have evolved. Thematic Review: Thematic reviews focus on identifying and analyzing themes or topics that cut across different studies. Instead of organizing the literature chronologically, it is grouped by key themes or concepts, allowing for a comprehensive exploration of various aspects of the topic. Methodological Review: This strategy involves organizing the literature based on the research methods employed in different studies. It helps to highlight the strengths and weaknesses of various methodologies and allows the reader to evaluate the reliability and validity of the research findings. Theoretical Review: A theoretical review examines the literature based on the theoretical frameworks used in different studies. This approach helps to identify the key theories that have been applied to the topic and assess their contributions to the understanding of the subject. It’s important to note that these strategies are not mutually exclusive, and a literature review may combine elements of more than one approach. The choice of strategy depends on the research question, the nature of the literature available, and the goals of the review. Additionally, other strategies, such as integrative reviews or systematic reviews, may be employed depending on the specific requirements of the research.
The literature review format can vary depending on the specific publication guidelines. However, there are some common elements and structures that are often followed. Here is a general guideline for the format of a literature review: Introduction: Provide an overview of the topic. Define the scope and purpose of the literature review. State the research question or objective. Body: Organize the literature by themes, concepts, or chronology. Critically analyze and evaluate each source. Discuss the strengths and weaknesses of the studies. Highlight any methodological limitations or biases. Identify patterns, connections, or contradictions in the existing research. Conclusion: Summarize the key points discussed in the literature review. Highlight the research gap. Address the research question or objective stated in the introduction. Highlight the contributions of the review and suggest directions for future research.
Both annotated bibliographies and literature reviews involve the examination of scholarly sources. While annotated bibliographies focus on individual sources with brief annotations, literature reviews provide a more in-depth, integrated, and comprehensive analysis of existing literature on a specific topic. The key differences are as follows:
References
- Denney, A. S., & Tewksbury, R. (2013). How to write a literature review. Journal of criminal justice education , 24 (2), 218-234.
- Pan, M. L. (2016). Preparing literature reviews: Qualitative and quantitative approaches . Taylor & Francis.
- Cantero, C. (2019). How to write a literature review. San José State University Writing Center .
Paperpal is an AI writing assistant that help academics write better, faster with real-time suggestions for in-depth language and grammar correction. Trained on millions of research manuscripts enhanced by professional academic editors, Paperpal delivers human precision at machine speed.
Try it for free or upgrade to Paperpal Prime , which unlocks unlimited access to premium features like academic translation, paraphrasing, contextual synonyms, consistency checks and more. It’s like always having a professional academic editor by your side! Go beyond limitations and experience the future of academic writing. Get Paperpal Prime now at just US$19 a month!
Related Reads:
- Empirical Research: A Comprehensive Guide for Academics
- How to Write a Scientific Paper in 10 Steps
- Life Sciences Papers: 9 Tips for Authors Writing in Biological Sciences
- What is an Argumentative Essay? How to Write It (With Examples)
6 Tips for Post-Doc Researchers to Take Their Career to the Next Level
Self-plagiarism in research: what it is and how to avoid it, you may also like, how paperpal’s research feature helps you develop and..., how paperpal is enhancing academic productivity and accelerating..., how to write a successful book chapter for..., academic editing: how to self-edit academic text with..., 4 ways paperpal encourages responsible writing with ai, what are scholarly sources and where can you..., how to write a hypothesis types and examples , measuring academic success: definition & strategies for excellence, what is academic writing: tips for students, why traditional editorial process needs an upgrade.

- Walden University
- Faculty Portal
Common Assignments: Literature Review Matrix
Literature review matrix.
As you read and evaluate your literature there are several different ways to organize your research. Courtesy of Dr. Gary Burkholder in the School of Psychology, these sample matrices are one option to help organize your articles. These documents allow you to compile details about your sources, such as the foundational theories, methodologies, and conclusions; begin to note similarities among the authors; and retrieve citation information for easy insertion within a document.
You can review the sample matrixes to see a completed form or download the blank matrix for your own use.
- Literature Review Matrix 1 This PDF file provides a sample literature review matrix.
- Literature Review Matrix 2 This PDF file provides a sample literature review matrix.
- Literature Review Matrix Template (Word)
- Literature Review Matrix Template (Excel)
Related Resources
Didn't find what you need? Email us at [email protected] .
- Previous Page: Commentary Versus Opinion
- Next Page: Professional Development Plans (PDPs)
- Office of Student Disability Services
Walden Resources
Departments.
- Academic Residencies
- Academic Skills
- Career Planning and Development
- Customer Care Team
- Field Experience
- Military Services
- Student Success Advising
- Writing Skills
Centers and Offices
- Center for Social Change
- Office of Academic Support and Instructional Services
- Office of Degree Acceleration
- Office of Research and Doctoral Services
- Office of Student Affairs
Student Resources
- Doctoral Writing Assessment
- Form & Style Review
- Quick Answers
- ScholarWorks
- SKIL Courses and Workshops
- Walden Bookstore
- Walden Catalog & Student Handbook
- Student Safety/Title IX
- Legal & Consumer Information
- Website Terms and Conditions
- Cookie Policy
- Accessibility
- Accreditation
- State Authorization
- Net Price Calculator
- Contact Walden
Walden University is a member of Adtalem Global Education, Inc. www.adtalem.com Walden University is certified to operate by SCHEV © 2024 Walden University LLC. All rights reserved.
The Sheridan Libraries
- Write a Literature Review
- Sheridan Libraries
- Find This link opens in a new window
- Evaluate This link opens in a new window
Get Organized
- Lit Review Prep Use this template to help you evaluate your sources, create article summaries for an annotated bibliography, and a synthesis matrix for your lit review outline.
Synthesize your Information
Synthesize: combine separate elements to form a whole.
Synthesis Matrix
A synthesis matrix helps you record the main points of each source and document how sources relate to each other.
After summarizing and evaluating your sources, arrange them in a matrix or use a citation manager to help you see how they relate to each other and apply to each of your themes or variables.
By arranging your sources by theme or variable, you can see how your sources relate to each other, and can start thinking about how you weave them together to create a narrative.
- Step-by-Step Approach
- Example Matrix from NSCU
- Matrix Template
- << Previous: Summarize
- Next: Integrate >>
- Last Updated: Sep 26, 2023 10:25 AM
- URL: https://guides.library.jhu.edu/lit-review
- UWF Libraries
Literature Review: Conducting & Writing
- Sample Literature Reviews
- Steps for Conducting a Lit Review
- Finding "The Literature"
- Organizing/Writing
- APA Style This link opens in a new window
- Chicago: Notes Bibliography This link opens in a new window
- MLA Style This link opens in a new window
Sample Lit Reviews from Communication Arts
Have an exemplary literature review.
- Literature Review Sample 1
- Literature Review Sample 2
- Literature Review Sample 3
Have you written a stellar literature review you care to share for teaching purposes?
Are you an instructor who has received an exemplary literature review and have permission from the student to post?
Please contact Britt McGowan at [email protected] for inclusion in this guide. All disciplines welcome and encouraged.
- << Previous: MLA Style
- Next: Get Help! >>
- Last Updated: Mar 22, 2024 9:37 AM
- URL: https://libguides.uwf.edu/litreview
Jump to navigation

Cochrane Training
Chapter 14: completing ‘summary of findings’ tables and grading the certainty of the evidence.
Holger J Schünemann, Julian PT Higgins, Gunn E Vist, Paul Glasziou, Elie A Akl, Nicole Skoetz, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group
Key Points:
- A ‘Summary of findings’ table for a given comparison of interventions provides key information concerning the magnitudes of relative and absolute effects of the interventions examined, the amount of available evidence and the certainty (or quality) of available evidence.
- ‘Summary of findings’ tables include a row for each important outcome (up to a maximum of seven). Accepted formats of ‘Summary of findings’ tables and interactive ‘Summary of findings’ tables can be produced using GRADE’s software GRADEpro GDT.
- Cochrane has adopted the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation) for assessing certainty (or quality) of a body of evidence.
- The GRADE approach specifies four levels of the certainty for a body of evidence for a given outcome: high, moderate, low and very low.
- GRADE assessments of certainty are determined through consideration of five domains: risk of bias, inconsistency, indirectness, imprecision and publication bias. For evidence from non-randomized studies and rarely randomized studies, assessments can then be upgraded through consideration of three further domains.
Cite this chapter as: Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from www.training.cochrane.org/handbook .
14.1 ‘Summary of findings’ tables
14.1.1 introduction to ‘summary of findings’ tables.
‘Summary of findings’ tables present the main findings of a review in a transparent, structured and simple tabular format. In particular, they provide key information concerning the certainty or quality of evidence (i.e. the confidence or certainty in the range of an effect estimate or an association), the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes. Cochrane Reviews should incorporate ‘Summary of findings’ tables during planning and publication, and should have at least one key ‘Summary of findings’ table representing the most important comparisons. Some reviews may include more than one ‘Summary of findings’ table, for example if the review addresses more than one major comparison, or includes substantially different populations that require separate tables (e.g. because the effects differ or it is important to show results separately). In the Cochrane Database of Systematic Reviews (CDSR), all ‘Summary of findings’ tables for a review appear at the beginning, before the Background section.
14.1.2 Selecting outcomes for ‘Summary of findings’ tables
Planning for the ‘Summary of findings’ table starts early in the systematic review, with the selection of the outcomes to be included in: (i) the review; and (ii) the ‘Summary of findings’ table. This is a crucial step, and one that review authors need to address carefully.
To ensure production of optimally useful information, Cochrane Reviews begin by developing a review question and by listing all main outcomes that are important to patients and other decision makers (see Chapter 2 and Chapter 3 ). The GRADE approach to assessing the certainty of the evidence (see Section 14.2 ) defines and operationalizes a rating process that helps separate outcomes into those that are critical, important or not important for decision making. Consultation and feedback on the review protocol, including from consumers and other decision makers, can enhance this process.
Critical outcomes are likely to include clearly important endpoints; typical examples include mortality and major morbidity (such as strokes and myocardial infarction). However, they may also represent frequent minor and rare major side effects, symptoms, quality of life, burdens associated with treatment, and resource issues (costs). Burdens represent the impact of healthcare workload on patient function and well-being, and include the demands of adhering to an intervention that patients or caregivers (e.g. family) may dislike, such as having to undergo more frequent tests, or the restrictions on lifestyle that certain interventions require (Spencer-Bonilla et al 2017).
Frequently, when formulating questions that include all patient-important outcomes for decision making, review authors will confront reports of studies that have not included all these outcomes. This is particularly true for adverse outcomes. For instance, randomized trials might contribute evidence on intended effects, and on frequent, relatively minor side effects, but not report on rare adverse outcomes such as suicide attempts. Chapter 19 discusses strategies for addressing adverse effects. To obtain data for all important outcomes it may be necessary to examine the results of non-randomized studies (see Chapter 24 ). Cochrane, in collaboration with others, has developed guidance for review authors to support their decision about when to look for and include non-randomized studies (Schünemann et al 2013).
If a review includes only randomized trials, these trials may not address all important outcomes and it may therefore not be possible to address these outcomes within the constraints of the review. Review authors should acknowledge these limitations and make them transparent to readers. Review authors are encouraged to include non-randomized studies to examine rare or long-term adverse effects that may not adequately be studied in randomized trials. This raises the possibility that harm outcomes may come from studies in which participants differ from those in studies used in the analysis of benefit. Review authors will then need to consider how much such differences are likely to impact on the findings, and this will influence the certainty of evidence because of concerns about indirectness related to the population (see Section 14.2.2 ).
Non-randomized studies can provide important information not only when randomized trials do not report on an outcome or randomized trials suffer from indirectness, but also when the evidence from randomized trials is rated as very low and non-randomized studies provide evidence of higher certainty. Further discussion of these issues appears also in Chapter 24 .
14.1.3 General template for ‘Summary of findings’ tables
Several alternative standard versions of ‘Summary of findings’ tables have been developed to ensure consistency and ease of use across reviews, inclusion of the most important information needed by decision makers, and optimal presentation (see examples at Figures 14.1.a and 14.1.b ). These formats are supported by research that focused on improved understanding of the information they intend to convey (Carrasco-Labra et al 2016, Langendam et al 2016, Santesso et al 2016). They are available through GRADE’s official software package developed to support the GRADE approach: GRADEpro GDT (www.gradepro.org).
Standard Cochrane ‘Summary of findings’ tables include the following elements using one of the accepted formats. Further guidance on each of these is provided in Section 14.1.6 .
- A brief description of the population and setting addressed by the available evidence (which may be slightly different to or narrower than those defined by the review question).
- A brief description of the comparison addressed in the ‘Summary of findings’ table, including both the experimental and comparison interventions.
- A list of the most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes.
- A measure of the typical burden of each outcomes (e.g. illustrative risk, or illustrative mean, on comparator intervention).
- The absolute and relative magnitude of effect measured for each (if both are appropriate).
- The numbers of participants and studies contributing to the analysis of each outcomes.
- A GRADE assessment of the overall certainty of the body of evidence for each outcome (which may vary by outcome).
- Space for comments.
- Explanations (formerly known as footnotes).
Ideally, ‘Summary of findings’ tables are supported by more detailed tables (known as ‘evidence profiles’) to which the review may be linked, which provide more detailed explanations. Evidence profiles include the same important health outcomes, and provide greater detail than ‘Summary of findings’ tables of both of the individual considerations feeding into the grading of certainty and of the results of the studies (Guyatt et al 2011a). They ensure that a structured approach is used to rating the certainty of evidence. Although they are rarely published in Cochrane Reviews, evidence profiles are often used, for example, by guideline developers in considering the certainty of the evidence to support guideline recommendations. Review authors will find it easier to develop the ‘Summary of findings’ table by completing the rating of the certainty of evidence in the evidence profile first in GRADEpro GDT. They can then automatically convert this to one of the ‘Summary of findings’ formats in GRADEpro GDT, including an interactive ‘Summary of findings’ for publication.
As a measure of the magnitude of effect for dichotomous outcomes, the ‘Summary of findings’ table should provide a relative measure of effect (e.g. risk ratio, odds ratio, hazard) and measures of absolute risk. For other types of data, an absolute measure alone (such as a difference in means for continuous data) might be sufficient. It is important that the magnitude of effect is presented in a meaningful way, which may require some transformation of the result of a meta-analysis (see also Chapter 15, Section 15.4 and Section 15.5 ). Reviews with more than one main comparison should include a separate ‘Summary of findings’ table for each comparison.
Figure 14.1.a provides an example of a ‘Summary of findings’ table. Figure 15.1.b provides an alternative format that may further facilitate users’ understanding and interpretation of the review’s findings. Evidence evaluating different formats suggests that the ‘Summary of findings’ table should include a risk difference as a measure of the absolute effect and authors should preferably use a format that includes a risk difference .
A detailed description of the contents of a ‘Summary of findings’ table appears in Section 14.1.6 .
Figure 14.1.a Example of a ‘Summary of findings’ table
Summary of findings (for interactive version click here )
a All the stockings in the nine studies included in this review were below-knee compression stockings. In four studies the compression strength was 20 mmHg to 30 mmHg at the ankle. It was 10 mmHg to 20 mmHg in the other four studies. Stockings come in different sizes. If a stocking is too tight around the knee it can prevent essential venous return causing the blood to pool around the knee. Compression stockings should be fitted properly. A stocking that is too tight could cut into the skin on a long flight and potentially cause ulceration and increased risk of DVT. Some stockings can be slightly thicker than normal leg covering and can be potentially restrictive with tight foot wear. It is a good idea to wear stockings around the house prior to travel to ensure a good, comfortable fit. Participants put their stockings on two to three hours before the flight in most of the studies. The availability and cost of stockings can vary.
b Two studies recruited high risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous two years, large varicose veins or, in one of the studies, participants taller than 190 cm and heavier than 90 kg. The incidence for the seven studies that excluded high risk participants was 1.45% and the incidence for the two studies that recruited high-risk participants (with at least one risk factor) was 2.43%. We have used 10 and 30 per 1000 to express different risk strata, respectively.
c The confidence interval crosses no difference and does not rule out a small increase.
d The measurement of oedema was not validated (indirectness of the outcome) or blinded to the intervention (risk of bias).
e If there are very few or no events and the number of participants is large, judgement about the certainty of evidence (particularly judgements about imprecision) may be based on the absolute effect. Here the certainty rating may be considered ‘high’ if the outcome was appropriately assessed and the event, in fact, did not occur in 2821 studied participants.
f None of the other studies reported adverse effects, apart from four cases of superficial vein thrombosis in varicose veins in the knee region that were compressed by the upper edge of the stocking in one study.
Figure 14.1.b Example of alternative ‘Summary of findings’ table
14.1.4 Producing ‘Summary of findings’ tables
The GRADE Working Group’s software, GRADEpro GDT ( www.gradepro.org ), including GRADE’s interactive handbook, is available to assist review authors in the preparation of ‘Summary of findings’ tables. GRADEpro can use data on the comparator group risk and the effect estimate (entered by the review authors or imported from files generated in RevMan) to produce the relative effects and absolute risks associated with experimental interventions. In addition, it leads the user through the process of a GRADE assessment, and produces a table that can be used as a standalone table in a review (including by direct import into software such as RevMan or integration with RevMan Web), or an interactive ‘Summary of findings’ table (see help resources in GRADEpro).
14.1.5 Statistical considerations in ‘Summary of findings’ tables
14.1.5.1 dichotomous outcomes.
‘Summary of findings’ tables should include both absolute and relative measures of effect for dichotomous outcomes. Risk ratios, odds ratios and risk differences are different ways of comparing two groups with dichotomous outcome data (see Chapter 6, Section 6.4.1 ). Furthermore, there are two distinct risk ratios, depending on which event (e.g. ‘yes’ or ‘no’) is the focus of the analysis (see Chapter 6, Section 6.4.1.5 ). In the presence of a non-zero intervention effect, any variation across studies in the comparator group risks (i.e. variation in the risk of the event occurring without the intervention of interest, for example in different populations) makes it impossible for more than one of these measures to be truly the same in every study.
It has long been assumed in epidemiology that relative measures of effect are more consistent than absolute measures of effect from one scenario to another. There is empirical evidence to support this assumption (Engels et al 2000, Deeks and Altman 2001, Furukawa et al 2002). For this reason, meta-analyses should generally use either a risk ratio or an odds ratio as a measure of effect (see Chapter 10, Section 10.4.3 ). Correspondingly, a single estimate of relative effect is likely to be a more appropriate summary than a single estimate of absolute effect. If a relative effect is indeed consistent across studies, then different comparator group risks will have different implications for absolute benefit. For instance, if the risk ratio is consistently 0.75, then the experimental intervention would reduce a comparator group risk of 80% to 60% in the intervention group (an absolute risk reduction of 20 percentage points), but would also reduce a comparator group risk of 20% to 15% in the intervention group (an absolute risk reduction of 5 percentage points).
‘Summary of findings’ tables are built around the assumption of a consistent relative effect. It is therefore important to consider the implications of this effect for different comparator group risks (these can be derived or estimated from a number of sources, see Section 14.1.6.3 ), which may require an assessment of the certainty of evidence for prognostic evidence (Spencer et al 2012, Iorio et al 2015). For any comparator group risk, it is possible to estimate a corresponding intervention group risk (i.e. the absolute risk with the intervention) from the meta-analytic risk ratio or odds ratio. Note that the numbers provided in the ‘Corresponding risk’ column are specific to the ‘risks’ in the adjacent column.
For the meta-analytic risk ratio (RR) and assumed comparator risk (ACR) the corresponding intervention risk is obtained as:
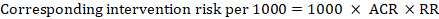
As an example, in Figure 14.1.a , the meta-analytic risk ratio for symptomless deep vein thrombosis (DVT) is RR = 0.10 (95% CI 0.04 to 0.26). Assuming a comparator risk of ACR = 10 per 1000 = 0.01, we obtain:
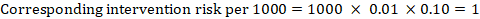
For the meta-analytic odds ratio (OR) and assumed comparator risk, ACR, the corresponding intervention risk is obtained as:
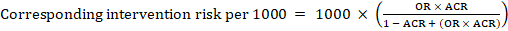
Upper and lower confidence limits for the corresponding intervention risk are obtained by replacing RR or OR by their upper and lower confidence limits, respectively (e.g. replacing 0.10 with 0.04, then with 0.26, in the example). Such confidence intervals do not incorporate uncertainty in the assumed comparator risks.
When dealing with risk ratios, it is critical that the same definition of ‘event’ is used as was used for the meta-analysis. For example, if the meta-analysis focused on ‘death’ (as opposed to survival) as the event, then corresponding risks in the ‘Summary of findings’ table must also refer to ‘death’.
In (rare) circumstances in which there is clear rationale to assume a consistent risk difference in the meta-analysis, in principle it is possible to present this for relevant ‘assumed risks’ and their corresponding risks, and to present the corresponding (different) relative effects for each assumed risk.
The risk difference expresses the difference between the ACR and the corresponding intervention risk (or the difference between the experimental and the comparator intervention).
For the meta-analytic risk ratio (RR) and assumed comparator risk (ACR) the corresponding risk difference is obtained as (note that risks can also be expressed using percentage or percentage points):
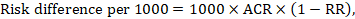
As an example, in Figure 14.1.b the meta-analytic risk ratio is 0.41 (95% CI 0.29 to 0.55) for diarrhoea in children less than 5 years of age. Assuming a comparator group risk of 22.3% we obtain:
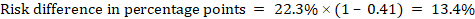
For the meta-analytic odds ratio (OR) and assumed comparator risk (ACR) the absolute risk difference is obtained as (percentage points):
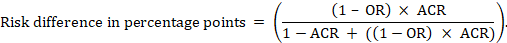
Upper and lower confidence limits for the absolute risk difference are obtained by re-running the calculation above while replacing RR or OR by their upper and lower confidence limits, respectively (e.g. replacing 0.41 with 0.28, then with 0.55, in the example). Such confidence intervals do not incorporate uncertainty in the assumed comparator risks.
14.1.5.2 Time-to-event outcomes
Time-to-event outcomes measure whether and when a particular event (e.g. death) occurs (van Dalen et al 2007). The impact of the experimental intervention relative to the comparison group on time-to-event outcomes is usually measured using a hazard ratio (HR) (see Chapter 6, Section 6.8.1 ).
A hazard ratio expresses a relative effect estimate. It may be used in various ways to obtain absolute risks and other interpretable quantities for a specific population. Here we describe how to re-express hazard ratios in terms of: (i) absolute risk of event-free survival within a particular period of time; (ii) absolute risk of an event within a particular period of time; and (iii) median time to the event. All methods are built on an assumption of consistent relative effects (i.e. that the hazard ratio does not vary over time).
(i) Absolute risk of event-free survival within a particular period of time Event-free survival (e.g. overall survival) is commonly reported by individual studies. To obtain absolute effects for time-to-event outcomes measured as event-free survival, the summary HR can be used in conjunction with an assumed proportion of patients who are event-free in the comparator group (Tierney et al 2007). This proportion of patients will be specific to a period of time of observation. However, it is not strictly necessary to specify this period of time. For instance, a proportion of 50% of event-free patients might apply to patients with a high event rate observed over 1 year, or to patients with a low event rate observed over 2 years.
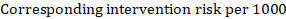
As an example, suppose the meta-analytic hazard ratio is 0.42 (95% CI 0.25 to 0.72). Assuming a comparator group risk of event-free survival (e.g. for overall survival people being alive) at 2 years of ACR = 900 per 1000 = 0.9 we obtain:
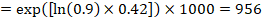
so that that 956 per 1000 people will be alive with the experimental intervention at 2 years. The derivation of the risk should be explained in a comment or footnote.
(ii) Absolute risk of an event within a particular period of time To obtain this absolute effect, again the summary HR can be used (Tierney et al 2007):
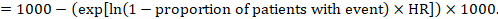
In the example, suppose we assume a comparator group risk of events (e.g. for mortality, people being dead) at 2 years of ACR = 100 per 1000 = 0.1. We obtain:
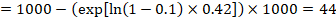
so that that 44 per 1000 people will be dead with the experimental intervention at 2 years.
(iii) Median time to the event Instead of absolute numbers, the time to the event in the intervention and comparison groups can be expressed as median survival time in months or years. To obtain median survival time the pooled HR can be applied to an assumed median survival time in the comparator group (Tierney et al 2007):
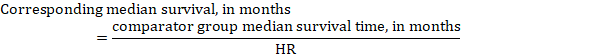
In the example, assuming a comparator group median survival time of 80 months, we obtain:
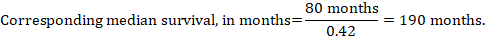
For all three of these options for re-expressing results of time-to-event analyses, upper and lower confidence limits for the corresponding intervention risk are obtained by replacing HR by its upper and lower confidence limits, respectively (e.g. replacing 0.42 with 0.25, then with 0.72, in the example). Again, as for dichotomous outcomes, such confidence intervals do not incorporate uncertainty in the assumed comparator group risks. This is of special concern for long-term survival with a low or moderate mortality rate and a corresponding high number of censored patients (i.e. a low number of patients under risk and a high censoring rate).
14.1.6 Detailed contents of a ‘Summary of findings’ table
14.1.6.1 table title and header.
The title of each ‘Summary of findings’ table should specify the healthcare question, framed in terms of the population and making it clear exactly what comparison of interventions are made. In Figure 14.1.a , the population is people taking long aeroplane flights, the intervention is compression stockings, and the control is no compression stockings.
The first rows of each ‘Summary of findings’ table should provide the following ‘header’ information:
Patients or population This further clarifies the population (and possibly the subpopulations) of interest and ideally the magnitude of risk of the most crucial adverse outcome at which an intervention is directed. For instance, people on a long-haul flight may be at different risks for DVT; those using selective serotonin reuptake inhibitors (SSRIs) might be at different risk for side effects; while those with atrial fibrillation may be at low (< 1%), moderate (1% to 4%) or high (> 4%) yearly risk of stroke.
Setting This should state any specific characteristics of the settings of the healthcare question that might limit the applicability of the summary of findings to other settings (e.g. primary care in Europe and North America).
Intervention The experimental intervention.
Comparison The comparator intervention (including no specific intervention).
14.1.6.2 Outcomes
The rows of a ‘Summary of findings’ table should include all desirable and undesirable health outcomes (listed in order of importance) that are essential for decision making, up to a maximum of seven outcomes. If there are more outcomes in the review, review authors will need to omit the less important outcomes from the table, and the decision selecting which outcomes are critical or important to the review should be made during protocol development (see Chapter 3 ). Review authors should provide time frames for the measurement of the outcomes (e.g. 90 days or 12 months) and the type of instrument scores (e.g. ranging from 0 to 100).
Note that review authors should include the pre-specified critical and important outcomes in the table whether data are available or not. However, they should be alert to the possibility that the importance of an outcome (e.g. a serious adverse effect) may only become known after the protocol was written or the analysis was carried out, and should take appropriate actions to include these in the ‘Summary of findings’ table.
The ‘Summary of findings’ table can include effects in subgroups of the population for different comparator risks and effect sizes separately. For instance, in Figure 14.1.b effects are presented for children younger and older than 5 years separately. Review authors may also opt to produce separate ‘Summary of findings’ tables for different populations.
Review authors should include serious adverse events, but it might be possible to combine minor adverse events as a single outcome, and describe this in an explanatory footnote (note that it is not appropriate to add events together unless they are independent, that is, a participant who has experienced one adverse event has an unaffected chance of experiencing the other adverse event).
Outcomes measured at multiple time points represent a particular problem. In general, to keep the table simple, review authors should present multiple time points only for outcomes critical to decision making, where either the result or the decision made are likely to vary over time. The remainder should be presented at a common time point where possible.
Review authors can present continuous outcome measures in the ‘Summary of findings’ table and should endeavour to make these interpretable to the target audience. This requires that the units are clear and readily interpretable, for example, days of pain, or frequency of headache, and the name and scale of any measurement tools used should be stated (e.g. a Visual Analogue Scale, ranging from 0 to 100). However, many measurement instruments are not readily interpretable by non-specialist clinicians or patients, for example, points on a Beck Depression Inventory or quality of life score. For these, a more interpretable presentation might involve converting a continuous to a dichotomous outcome, such as >50% improvement (see Chapter 15, Section 15.5 ).
14.1.6.3 Best estimate of risk with comparator intervention
Review authors should provide up to three typical risks for participants receiving the comparator intervention. For dichotomous outcomes, we recommend that these be presented in the form of the number of people experiencing the event per 100 or 1000 people (natural frequency) depending on the frequency of the outcome. For continuous outcomes, this would be stated as a mean or median value of the outcome measured.
Estimated or assumed comparator intervention risks could be based on assessments of typical risks in different patient groups derived from the review itself, individual representative studies in the review, or risks derived from a systematic review of prognosis studies or other sources of evidence which may in turn require an assessment of the certainty for the prognostic evidence (Spencer et al 2012, Iorio et al 2015). Ideally, risks would reflect groups that clinicians can easily identify on the basis of their presenting features.
An explanatory footnote should specify the source or rationale for each comparator group risk, including the time period to which it corresponds where appropriate. In Figure 14.1.a , clinicians can easily differentiate individuals with risk factors for deep venous thrombosis from those without. If there is known to be little variation in baseline risk then review authors may use the median comparator group risk across studies. If typical risks are not known, an option is to choose the risk from the included studies, providing the second highest for a high and the second lowest for a low risk population.
14.1.6.4 Risk with intervention
For dichotomous outcomes, review authors should provide a corresponding absolute risk for each comparator group risk, along with a confidence interval. This absolute risk with the (experimental) intervention will usually be derived from the meta-analysis result presented in the relative effect column (see Section 14.1.6.6 ). Formulae are provided in Section 14.1.5 . Review authors should present the absolute effect in the same format as the risks with comparator intervention (see Section 14.1.6.3 ), for example as the number of people experiencing the event per 1000 people.
For continuous outcomes, a difference in means or standardized difference in means should be presented with its confidence interval. These will typically be obtained directly from a meta-analysis. Explanatory text should be used to clarify the meaning, as in Figures 14.1.a and 14.1.b .
14.1.6.5 Risk difference
For dichotomous outcomes, the risk difference can be provided using one of the ‘Summary of findings’ table formats as an additional option (see Figure 14.1.b ). This risk difference expresses the difference between the experimental and comparator intervention and will usually be derived from the meta-analysis result presented in the relative effect column (see Section 14.1.6.6 ). Formulae are provided in Section 14.1.5 . Review authors should present the risk difference in the same format as assumed and corresponding risks with comparator intervention (see Section 14.1.6.3 ); for example, as the number of people experiencing the event per 1000 people or as percentage points if the assumed and corresponding risks are expressed in percentage.
For continuous outcomes, if the ‘Summary of findings’ table includes this option, the mean difference can be presented here and the ‘corresponding risk’ column left blank (see Figure 14.1.b ).
14.1.6.6 Relative effect (95% CI)
The relative effect will typically be a risk ratio or odds ratio (or occasionally a hazard ratio) with its accompanying 95% confidence interval, obtained from a meta-analysis performed on the basis of the same effect measure. Risk ratios and odds ratios are similar when the comparator intervention risks are low and effects are small, but may differ considerably when comparator group risks increase. The meta-analysis may involve an assumption of either fixed or random effects, depending on what the review authors consider appropriate, and implying that the relative effect is either an estimate of the effect of the intervention, or an estimate of the average effect of the intervention across studies, respectively.
14.1.6.7 Number of participants (studies)
This column should include the number of participants assessed in the included studies for each outcome and the corresponding number of studies that contributed these participants.
14.1.6.8 Certainty of the evidence (GRADE)
Review authors should comment on the certainty of the evidence (also known as quality of the body of evidence or confidence in the effect estimates). Review authors should use the specific evidence grading system developed by the GRADE Working Group (Atkins et al 2004, Guyatt et al 2008, Guyatt et al 2011a), which is described in detail in Section 14.2 . The GRADE approach categorizes the certainty in a body of evidence as ‘high’, ‘moderate’, ‘low’ or ‘very low’ by outcome. This is a result of judgement, but the judgement process operates within a transparent structure. As an example, the certainty would be ‘high’ if the summary were of several randomized trials with low risk of bias, but the rating of certainty becomes lower if there are concerns about risk of bias, inconsistency, indirectness, imprecision or publication bias. Judgements other than of ‘high’ certainty should be made transparent using explanatory footnotes or the ‘Comments’ column in the ‘Summary of findings’ table (see Section 14.1.6.10 ).
14.1.6.9 Comments
The aim of the ‘Comments’ field is to help interpret the information or data identified in the row. For example, this may be on the validity of the outcome measure or the presence of variables that are associated with the magnitude of effect. Important caveats about the results should be flagged here. Not all rows will need comments, and it is best to leave a blank if there is nothing warranting a comment.
14.1.6.10 Explanations
Detailed explanations should be included as footnotes to support the judgements in the ‘Summary of findings’ table, such as the overall GRADE assessment. The explanations should describe the rationale for important aspects of the content. Table 14.1.a lists guidance for useful explanations. Explanations should be concise, informative, relevant, easy to understand and accurate. If explanations cannot be sufficiently described in footnotes, review authors should provide further details of the issues in the Results and Discussion sections of the review.
Table 14.1.a Guidance for providing useful explanations in ‘Summary of findings’ (SoF) tables. Adapted from Santesso et al (2016)
14.2 Assessing the certainty or quality of a body of evidence
14.2.1 the grade approach.
The Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) has developed a system for grading the certainty of evidence (Schünemann et al 2003, Atkins et al 2004, Schünemann et al 2006, Guyatt et al 2008, Guyatt et al 2011a). Over 100 organizations including the World Health Organization (WHO), the American College of Physicians, the American Society of Hematology (ASH), the Canadian Agency for Drugs and Technology in Health (CADTH) and the National Institutes of Health and Clinical Excellence (NICE) in the UK have adopted the GRADE system ( www.gradeworkinggroup.org ).
Cochrane has also formally adopted this approach, and all Cochrane Reviews should use GRADE to evaluate the certainty of evidence for important outcomes (see MECIR Box 14.2.a ).
MECIR Box 14.2.a Relevant expectations for conduct of intervention reviews
For systematic reviews, the GRADE approach defines the certainty of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. Assessing the certainty of a body of evidence involves consideration of within- and across-study risk of bias (limitations in study design and execution or methodological quality), inconsistency (or heterogeneity), indirectness of evidence, imprecision of the effect estimates and risk of publication bias (see Section 14.2.2 ), as well as domains that may increase our confidence in the effect estimate (as described in Section 14.2.3 ). The GRADE system entails an assessment of the certainty of a body of evidence for each individual outcome. Judgements about the domains that determine the certainty of evidence should be described in the results or discussion section and as part of the ‘Summary of findings’ table.
The GRADE approach specifies four levels of certainty ( Figure 14.2.a ). For interventions, including diagnostic and other tests that are evaluated as interventions (Schünemann et al 2008b, Schünemann et al 2008a, Balshem et al 2011, Schünemann et al 2012), the starting point for rating the certainty of evidence is categorized into two types:
- randomized trials; and
- non-randomized studies of interventions (NRSI), including observational studies (including but not limited to cohort studies, and case-control studies, cross-sectional studies, case series and case reports, although not all of these designs are usually included in Cochrane Reviews).
There are many instances in which review authors rely on information from NRSI, in particular to evaluate potential harms (see Chapter 24 ). In addition, review authors can obtain relevant data from both randomized trials and NRSI, with each type of evidence complementing the other (Schünemann et al 2013).
In GRADE, a body of evidence from randomized trials begins with a high-certainty rating while a body of evidence from NRSI begins with a low-certainty rating. The lower rating with NRSI is the result of the potential bias induced by the lack of randomization (i.e. confounding and selection bias).
However, when using the new Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool (Sterne et al 2016), an assessment tool that covers the risk of bias due to lack of randomization, all studies may start as high certainty of the evidence (Schünemann et al 2018). The approach of starting all study designs (including NRSI) as high certainty does not conflict with the initial GRADE approach of starting the rating of NRSI as low certainty evidence. This is because a body of evidence from NRSI should generally be downgraded by two levels due to the inherent risk of bias associated with the lack of randomization, namely confounding and selection bias. Not downgrading NRSI from high to low certainty needs transparent and detailed justification for what mitigates concerns about confounding and selection bias (Schünemann et al 2018). Very few examples of where not rating down by two levels is appropriate currently exist.
The highest certainty rating is a body of evidence when there are no concerns in any of the GRADE factors listed in Figure 14.2.a . Review authors often downgrade evidence to moderate, low or even very low certainty evidence, depending on the presence of the five factors in Figure 14.2.a . Usually, certainty rating will fall by one level for each factor, up to a maximum of three levels for all factors. If there are very severe problems for any one domain (e.g. when assessing risk of bias, all studies were unconcealed, unblinded and lost over 50% of their patients to follow-up), evidence may fall by two levels due to that factor alone. It is not possible to rate lower than ‘very low certainty’ evidence.
Review authors will generally grade evidence from sound non-randomized studies as low certainty, even if ROBINS-I is used. If, however, such studies yield large effects and there is no obvious bias explaining those effects, review authors may rate the evidence as moderate or – if the effect is large enough – even as high certainty ( Figure 14.2.a ). The very low certainty level is appropriate for, but is not limited to, studies with critical problems and unsystematic clinical observations (e.g. case series or case reports).
Figure 14.2.a Levels of the certainty of a body of evidence in the GRADE approach. *Upgrading criteria are usually applicable to non-randomized studies only (but exceptions exist).
14.2.2 Domains that can lead to decreasing the certainty level of a body of evidence
We now describe in more detail the five reasons (or domains) for downgrading the certainty of a body of evidence for a specific outcome. In each case, if no reason is found for downgrading the evidence, it should be classified as 'no limitation or not serious' (not important enough to warrant downgrading). If a reason is found for downgrading the evidence, it should be classified as 'serious' (downgrading the certainty rating by one level) or 'very serious' (downgrading the certainty grade by two levels). For non-randomized studies assessed with ROBINS-I, rating down by three levels should be classified as 'extremely' serious.
(1) Risk of bias or limitations in the detailed design and implementation
Our confidence in an estimate of effect decreases if studies suffer from major limitations that are likely to result in a biased assessment of the intervention effect. For randomized trials, these methodological limitations include failure to generate a random sequence, lack of allocation sequence concealment, lack of blinding (particularly with subjective outcomes that are highly susceptible to biased assessment), a large loss to follow-up or selective reporting of outcomes. Chapter 8 provides a discussion of study-level assessments of risk of bias in the context of a Cochrane Review, and proposes an approach to assessing the risk of bias for an outcome across studies as ‘Low’ risk of bias, ‘Some concerns’ and ‘High’ risk of bias for randomized trials. Levels of ‘Low’. ‘Moderate’, ‘Serious’ and ‘Critical’ risk of bias arise for non-randomized studies assessed with ROBINS-I ( Chapter 25 ). These assessments should feed directly into this GRADE domain. In particular, ‘Low’ risk of bias would indicate ‘no limitation’; ‘Some concerns’ would indicate either ‘no limitation’ or ‘serious limitation’; and ‘High’ risk of bias would indicate either ‘serious limitation’ or ‘very serious limitation’. ‘Critical’ risk of bias on ROBINS-I would indicate extremely serious limitations in GRADE. Review authors should use their judgement to decide between alternative categories, depending on the likely magnitude of the potential biases.
Every study addressing a particular outcome will differ, to some degree, in the risk of bias. Review authors should make an overall judgement on whether the certainty of evidence for an outcome warrants downgrading on the basis of study limitations. The assessment of study limitations should apply to the studies contributing to the results in the ‘Summary of findings’ table, rather than to all studies that could potentially be included in the analysis. We have argued in Chapter 7, Section 7.6.2 , that the primary analysis should be restricted to studies at low (or low and unclear) risk of bias where possible.
Table 14.2.a presents the judgements that must be made in going from assessments of the risk of bias to judgements about study limitations for each outcome included in a ‘Summary of findings’ table. A rating of high certainty evidence can be achieved only when most evidence comes from studies that met the criteria for low risk of bias. For example, of the 22 studies addressing the impact of beta-blockers on mortality in patients with heart failure, most probably or certainly used concealed allocation of the sequence, all blinded at least some key groups and follow-up of randomized patients was almost complete (Brophy et al 2001). The certainty of evidence might be downgraded by one level when most of the evidence comes from individual studies either with a crucial limitation for one item, or with some limitations for multiple items. An example of very serious limitations, warranting downgrading by two levels, is provided by evidence on surgery versus conservative treatment in the management of patients with lumbar disc prolapse (Gibson and Waddell 2007). We are uncertain of the benefit of surgery in reducing symptoms after one year or longer, because the one study included in the analysis had inadequate concealment of the allocation sequence and the outcome was assessed using a crude rating by the surgeon without blinding.
(2) Unexplained heterogeneity or inconsistency of results
When studies yield widely differing estimates of effect (heterogeneity or variability in results), investigators should look for robust explanations for that heterogeneity. For instance, drugs may have larger relative effects in sicker populations or when given in larger doses. A detailed discussion of heterogeneity and its investigation is provided in Chapter 10, Section 10.10 and Section 10.11 . If an important modifier exists, with good evidence that important outcomes are different in different subgroups (which would ideally be pre-specified), then a separate ‘Summary of findings’ table may be considered for a separate population. For instance, a separate ‘Summary of findings’ table would be used for carotid endarterectomy in symptomatic patients with high grade stenosis (70% to 99%) in which the intervention is, in the hands of the right surgeons, beneficial, and another (if review authors considered it relevant) for asymptomatic patients with low grade stenosis (less than 30%) in which surgery appears harmful (Orrapin and Rerkasem 2017). When heterogeneity exists and affects the interpretation of results, but review authors are unable to identify a plausible explanation with the data available, the certainty of the evidence decreases.
(3) Indirectness of evidence
Two types of indirectness are relevant. First, a review comparing the effectiveness of alternative interventions (say A and B) may find that randomized trials are available, but they have compared A with placebo and B with placebo. Thus, the evidence is restricted to indirect comparisons between A and B. Where indirect comparisons are undertaken within a network meta-analysis context, GRADE for network meta-analysis should be used (see Chapter 11, Section 11.5 ).
Second, a review may find randomized trials that meet eligibility criteria but address a restricted version of the main review question in terms of population, intervention, comparator or outcomes. For example, suppose that in a review addressing an intervention for secondary prevention of coronary heart disease, most identified studies happened to be in people who also had diabetes. Then the evidence may be regarded as indirect in relation to the broader question of interest because the population is primarily related to people with diabetes. The opposite scenario can equally apply: a review addressing the effect of a preventive strategy for coronary heart disease in people with diabetes may consider studies in people without diabetes to provide relevant, albeit indirect, evidence. This would be particularly likely if investigators had conducted few if any randomized trials in the target population (e.g. people with diabetes). Other sources of indirectness may arise from interventions studied (e.g. if in all included studies a technical intervention was implemented by expert, highly trained specialists in specialist centres, then evidence on the effects of the intervention outside these centres may be indirect), comparators used (e.g. if the comparator groups received an intervention that is less effective than standard treatment in most settings) and outcomes assessed (e.g. indirectness due to surrogate outcomes when data on patient-important outcomes are not available, or when investigators seek data on quality of life but only symptoms are reported). Review authors should make judgements transparent when they believe downgrading is justified, based on differences in anticipated effects in the group of primary interest. Review authors may be aided and increase transparency of their judgements about indirectness if they use Table 14.2.b available in the GRADEpro GDT software (Schünemann et al 2013).
(4) Imprecision of results
When studies include few participants or few events, and thus have wide confidence intervals, review authors can lower their rating of the certainty of the evidence. The confidence intervals included in the ‘Summary of findings’ table will provide readers with information that allows them to make, to some extent, their own rating of precision. Review authors can use a calculation of the optimal information size (OIS) or review information size (RIS), similar to sample size calculations, to make judgements about imprecision (Guyatt et al 2011b, Schünemann 2016). The OIS or RIS is calculated on the basis of the number of participants required for an adequately powered individual study. If the 95% confidence interval excludes a risk ratio (RR) of 1.0, and the total number of events or patients exceeds the OIS criterion, precision is adequate. If the 95% CI includes appreciable benefit or harm (an RR of under 0.75 or over 1.25 is often suggested as a very rough guide) downgrading for imprecision may be appropriate even if OIS criteria are met (Guyatt et al 2011b, Schünemann 2016).
(5) High probability of publication bias
The certainty of evidence level may be downgraded if investigators fail to report studies on the basis of results (typically those that show no effect: publication bias) or outcomes (typically those that may be harmful or for which no effect was observed: selective outcome non-reporting bias). Selective reporting of outcomes from among multiple outcomes measured is assessed at the study level as part of the assessment of risk of bias (see Chapter 8, Section 8.7 ), so for the studies contributing to the outcome in the ‘Summary of findings’ table this is addressed by domain 1 above (limitations in the design and implementation). If a large number of studies included in the review do not contribute to an outcome, or if there is evidence of publication bias, the certainty of the evidence may be downgraded. Chapter 13 provides a detailed discussion of reporting biases, including publication bias, and how it may be tackled in a Cochrane Review. A prototypical situation that may elicit suspicion of publication bias is when published evidence includes a number of small studies, all of which are industry-funded (Bhandari et al 2004). For example, 14 studies of flavanoids in patients with haemorrhoids have shown apparent large benefits, but enrolled a total of only 1432 patients (i.e. each study enrolled relatively few patients) (Alonso-Coello et al 2006). The heavy involvement of sponsors in most of these studies raises questions of whether unpublished studies that suggest no benefit exist (publication bias).
A particular body of evidence can suffer from problems associated with more than one of the five factors listed here, and the greater the problems, the lower the certainty of evidence rating that should result. One could imagine a situation in which randomized trials were available, but all or virtually all of these limitations would be present, and in serious form. A very low certainty of evidence rating would result.
Table 14.2.a Further guidelines for domain 1 (of 5) in a GRADE assessment: going from assessments of risk of bias in studies to judgements about study limitations for main outcomes across studies
Table 14.2.b Judgements about indirectness by outcome (available in GRADEpro GDT)
Intervention:
Comparator:
Direct comparison:
Final judgement about indirectness across domains:
14.2.3 Domains that may lead to increasing the certainty level of a body of evidence
Although NRSI and downgraded randomized trials will generally yield a low rating for certainty of evidence, there will be unusual circumstances in which review authors could ‘upgrade’ such evidence to moderate or even high certainty ( Table 14.3.a ).
- Large effects On rare occasions when methodologically well-done observational studies yield large, consistent and precise estimates of the magnitude of an intervention effect, one may be particularly confident in the results. A large estimated effect (e.g. RR >2 or RR <0.5) in the absence of plausible confounders, or a very large effect (e.g. RR >5 or RR <0.2) in studies with no major threats to validity, might qualify for this. In these situations, while the NRSI may possibly have provided an over-estimate of the true effect, the weak study design may not explain all of the apparent observed benefit. Thus, despite reservations based on the observational study design, review authors are confident that the effect exists. The magnitude of the effect in these studies may move the assigned certainty of evidence from low to moderate (if the effect is large in the absence of other methodological limitations). For example, a meta-analysis of observational studies showed that bicycle helmets reduce the risk of head injuries in cyclists by a large margin (odds ratio (OR) 0.31, 95% CI 0.26 to 0.37) (Thompson et al 2000). This large effect, in the absence of obvious bias that could create the association, suggests a rating of moderate-certainty evidence. Note : GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. However, if the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0, then some hesitation would be appropriate in the decision to rate up for a large effect. Another situation allows inference of a strong association without a formal comparative study. Consider the question of the impact of routine colonoscopy versus no screening for colon cancer on the rate of perforation associated with colonoscopy. Here, a large series of representative patients undergoing colonoscopy may provide high certainty evidence about the risk of perforation associated with colonoscopy. When the risk of the event among patients receiving the relevant comparator is known to be near 0 (i.e. we are certain that the incidence of spontaneous colon perforation in patients not undergoing colonoscopy is extremely low), case series or cohort studies of representative patients can provide high certainty evidence of adverse effects associated with an intervention, thereby allowing us to infer a strong association from even a limited number of events.
- Dose-response The presence of a dose-response gradient may increase our confidence in the findings of observational studies and thereby enhance the assigned certainty of evidence. For example, our confidence in the result of observational studies that show an increased risk of bleeding in patients who have supratherapeutic anticoagulation levels is increased by the observation that there is a dose-response gradient between the length of time needed for blood to clot (as measured by the international normalized ratio (INR)) and an increased risk of bleeding (Levine et al 2004). A systematic review of NRSI investigating the effect of cyclooxygenase-2 inhibitors on cardiovascular events found that the summary estimate (RR) with rofecoxib was 1.33 (95% CI 1.00 to 1.79) with doses less than 25mg/d, and 2.19 (95% CI 1.64 to 2.91) with doses more than 25mg/d. Although residual confounding is likely to exist in the NRSI that address this issue, the existence of a dose-response gradient and the large apparent effect of higher doses of rofecoxib markedly increase our strength of inference that the association cannot be explained by residual confounding, and is therefore likely to be both causal and, at high levels of exposure, substantial. Note : GRADE guidance suggests the possibility of rating up one level for a large effect if the relative effect is greater than 2.0. Here, the fact that the point estimate of the relative effect is greater than 2.0, but the confidence interval is appreciably below 2.0 might make some hesitate in the decision to rate up for a large effect
- Plausible confounding On occasion, all plausible biases from randomized or non-randomized studies may be working to under-estimate an apparent intervention effect. For example, if only sicker patients receive an experimental intervention or exposure, yet they still fare better, it is likely that the actual intervention or exposure effect is larger than the data suggest. For instance, a rigorous systematic review of observational studies including a total of 38 million patients demonstrated higher death rates in private for-profit versus private not-for-profit hospitals (Devereaux et al 2002). One possible bias relates to different disease severity in patients in the two hospital types. It is likely, however, that patients in the not-for-profit hospitals were sicker than those in the for-profit hospitals. Thus, to the extent that residual confounding existed, it would bias results against the not-for-profit hospitals. The second likely bias was the possibility that higher numbers of patients with excellent private insurance coverage could lead to a hospital having more resources and a spill-over effect that would benefit those without such coverage. Since for-profit hospitals are likely to admit a larger proportion of such well-insured patients than not-for-profit hospitals, the bias is once again against the not-for-profit hospitals. Since the plausible biases would all diminish the demonstrated intervention effect, one might consider the evidence from these observational studies as moderate rather than low certainty. A parallel situation exists when observational studies have failed to demonstrate an association, but all plausible biases would have increased an intervention effect. This situation will usually arise in the exploration of apparent harmful effects. For example, because the hypoglycaemic drug phenformin causes lactic acidosis, the related agent metformin was under suspicion for the same toxicity. Nevertheless, very large observational studies have failed to demonstrate an association (Salpeter et al 2007). Given the likelihood that clinicians would be more alert to lactic acidosis in the presence of the agent and over-report its occurrence, one might consider this moderate, or even high certainty, evidence refuting a causal relationship between typical therapeutic doses of metformin and lactic acidosis.
14.3 Describing the assessment of the certainty of a body of evidence using the GRADE framework
Review authors should report the grading of the certainty of evidence in the Results section for each outcome for which this has been performed, providing the rationale for downgrading or upgrading the evidence, and referring to the ‘Summary of findings’ table where applicable.
Table 14.3.a provides a framework and examples for how review authors can justify their judgements about the certainty of evidence in each domain. These justifications should also be included in explanatory notes to the ‘Summary of Findings’ table (see Section 14.1.6.10 ).
Chapter 15, Section 15.6 , describes in more detail how the overall GRADE assessment across all domains can be used to draw conclusions about the effects of the intervention, as well as providing implications for future research.
Table 14.3.a Framework for describing the certainty of evidence and justifying downgrading or upgrading
14.4 Chapter information
Authors: Holger J Schünemann, Julian PT Higgins, Gunn E Vist, Paul Glasziou, Elie A Akl, Nicole Skoetz, Gordon H Guyatt; on behalf of the Cochrane GRADEing Methods Group (formerly Applicability and Recommendations Methods Group) and the Cochrane Statistical Methods Group
Acknowledgements: Andrew D Oxman contributed to earlier versions. Professor Penny Hawe contributed to the text on adverse effects in earlier versions. Jon Deeks provided helpful contributions on an earlier version of this chapter. For details of previous authors and editors of the Handbook , please refer to the Preface.
Funding: This work was in part supported by funding from the Michael G DeGroote Cochrane Canada Centre and the Ontario Ministry of Health.
14.5 References
Alonso-Coello P, Zhou Q, Martinez-Zapata MJ, Mills E, Heels-Ansdell D, Johanson JF, Guyatt G. Meta-analysis of flavonoids for the treatment of haemorrhoids. British Journal of Surgery 2006; 93 : 909-920.
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O'Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer TT, Varonen H, Vist GE, Williams JW, Jr., Zaza S. Grading quality of evidence and strength of recommendations. BMJ 2004; 328 : 1490.
Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011; 64 : 401-406.
Bhandari M, Busse JW, Jackowski D, Montori VM, Schünemann H, Sprague S, Mears D, Schemitsch EH, Heels-Ansdell D, Devereaux PJ. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. Canadian Medical Association Journal 2004; 170 : 477-480.
Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Annals of Internal Medicine 2001; 134 : 550-560.
Carrasco-Labra A, Brignardello-Petersen R, Santesso N, Neumann I, Mustafa RA, Mbuagbaw L, Etxeandia Ikobaltzeta I, De Stio C, McCullagh LJ, Alonso-Coello P, Meerpohl JJ, Vandvik PO, Brozek JL, Akl EA, Bossuyt P, Churchill R, Glenton C, Rosenbaum S, Tugwell P, Welch V, Garner P, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 1: a randomized trial shows improved understanding of content in summary of findings tables with a new format. Journal of Clinical Epidemiology 2016; 74 : 7-18.
Deeks JJ, Altman DG. Effect measures for meta-analysis of trials with binary outcomes. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care: Meta-analysis in Context . 2nd ed. London (UK): BMJ Publication Group; 2001. p. 313-335.
Devereaux PJ, Choi PT, Lacchetti C, Weaver B, Schünemann HJ, Haines T, Lavis JN, Grant BJ, Haslam DR, Bhandari M, Sullivan T, Cook DJ, Walter SD, Meade M, Khan H, Bhatnagar N, Guyatt GH. A systematic review and meta-analysis of studies comparing mortality rates of private for-profit and private not-for-profit hospitals. Canadian Medical Association Journal 2002; 166 : 1399-1406.
Engels EA, Schmid CH, Terrin N, Olkin I, Lau J. Heterogeneity and statistical significance in meta-analysis: an empirical study of 125 meta-analyses. Statistics in Medicine 2000; 19 : 1707-1728.
Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta-analyses. International Journal of Epidemiology 2002; 31 : 72-76.
Gibson JN, Waddell G. Surgical interventions for lumbar disc prolapse: updated Cochrane Review. Spine 2007; 32 : 1735-1747.
Guyatt G, Oxman A, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann H. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336 : 3.
Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer H, Jaeschke R, Rind D, Meerpohl J, Dahm P, Schünemann HJ. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011a; 64 : 383-394.
Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, Jaeschke R, Williams JW, Jr., Murad MH, Sinclair D, Falck-Ytter Y, Meerpohl J, Whittington C, Thorlund K, Andrews J, Schünemann HJ. GRADE guidelines 6. Rating the quality of evidence--imprecision. Journal of Clinical Epidemiology 2011b; 64 : 1283-1293.
Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, McGinn T, Hayden J, Williams K, Shea B, Wolff R, Kujpers T, Perel P, Vandvik PO, Glasziou P, Schünemann H, Guyatt G. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ 2015; 350 : h870.
Langendam M, Carrasco-Labra A, Santesso N, Mustafa RA, Brignardello-Petersen R, Ventresca M, Heus P, Lasserson T, Moustgaard R, Brozek J, Schünemann HJ. Improving GRADE evidence tables part 2: a systematic survey of explanatory notes shows more guidance is needed. Journal of Clinical Epidemiology 2016; 74 : 19-27.
Levine MN, Raskob G, Landefeld S, Kearon C, Schulman S. Hemorrhagic complications of anticoagulant treatment: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126 : 287S-310S.
Orrapin S, Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database of Systematic Reviews 2017; 6 : CD001081.
Salpeter S, Greyber E, Pasternak G, Salpeter E. Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2007; 4 : CD002967.
Santesso N, Carrasco-Labra A, Langendam M, Brignardello-Petersen R, Mustafa RA, Heus P, Lasserson T, Opiyo N, Kunnamo I, Sinclair D, Garner P, Treweek S, Tovey D, Akl EA, Tugwell P, Brozek JL, Guyatt G, Schünemann HJ. Improving GRADE evidence tables part 3: detailed guidance for explanatory footnotes supports creating and understanding GRADE certainty in the evidence judgments. Journal of Clinical Epidemiology 2016; 74 : 28-39.
Schünemann HJ, Best D, Vist G, Oxman AD, Group GW. Letters, numbers, symbols and words: how to communicate grades of evidence and recommendations. Canadian Medical Association Journal 2003; 169 : 677-680.
Schünemann HJ, Jaeschke R, Cook DJ, Bria WF, El-Solh AA, Ernst A, Fahy BF, Gould MK, Horan KL, Krishnan JA, Manthous CA, Maurer JR, McNicholas WT, Oxman AD, Rubenfeld G, Turino GM, Guyatt G. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006; 174 : 605-614.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, Williams JW, Jr., Kunz R, Craig J, Montori VM, Bossuyt P, Guyatt GH. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008a; 336 : 1106-1110.
Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Bossuyt P, Chang S, Muti P, Jaeschke R, Guyatt GH. GRADE: assessing the quality of evidence for diagnostic recommendations. ACP Journal Club 2008b; 149 : 2.
Schünemann HJ, Mustafa R, Brozek J. [Diagnostic accuracy and linked evidence--testing the chain]. Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen 2012; 106 : 153-160.
Schünemann HJ, Tugwell P, Reeves BC, Akl EA, Santesso N, Spencer FA, Shea B, Wells G, Helfand M. Non-randomized studies as a source of complementary, sequential or replacement evidence for randomized controlled trials in systematic reviews on the effects of interventions. Research Synthesis Methods 2013; 4 : 49-62.
Schünemann HJ. Interpreting GRADE's levels of certainty or quality of the evidence: GRADE for statisticians, considering review information size or less emphasis on imprecision? Journal of Clinical Epidemiology 2016; 75 : 6-15.
Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, Morgan RL, Gartlehner G, Kunz R, Katikireddi SV, Sterne J, Higgins JPT, Guyatt G, Group GW. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of Clinical Epidemiology 2018.
Spencer-Bonilla G, Quinones AR, Montori VM, International Minimally Disruptive Medicine W. Assessing the Burden of Treatment. Journal of General Internal Medicine 2017; 32 : 1141-1145.
Spencer FA, Iorio A, You J, Murad MH, Schünemann HJ, Vandvik PO, Crowther MA, Pottie K, Lang ES, Meerpohl JJ, Falck-Ytter Y, Alonso-Coello P, Guyatt GH. Uncertainties in baseline risk estimates and confidence in treatment effects. BMJ 2012; 345 : e7401.
Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JPT. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355 : i4919.
Thompson DC, Rivara FP, Thompson R. Helmets for preventing head and facial injuries in bicyclists. Cochrane Database of Systematic Reviews 2000; 2 : CD001855.
Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8 .
van Dalen EC, Tierney JF, Kremer LCM. Tips and tricks for understanding and using SR results. No. 7: time‐to‐event data. Evidence-Based Child Health 2007; 2 : 1089-1090.
For permission to re-use material from the Handbook (either academic or commercial), please see here for full details.

Redesigning Continuing Education in the Health Professions (2010)
Chapter: appendix a: literature review tables, appendix a literature review tables.
E vidence on the effectiveness of continuing education (CE) and CE methods was identified through a literature review. Although nonexhaustive, the review included a comprehensive search of the Research and Development Resource Base (RDRB), a bibliographic database of more than 18,000 articles from fields including CE, knowledge translation, interprofessional literature, and faculty development. Articles in the RDRB are culled from Medline, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Excerpta Medica Database (EMBASE), Education Resources Information Center (ERIC), Sociological Abstracts, PsychoInfo, Library Information and Science Abstracts (LISA), and business databases, as well as automatic retrieval of articles from journals dedicated to medical education (e.g., Journal of Continuing Education in the Health Professions , Medical Education , Studies in Continuing Education ).
The RDRB was searched using keywords, 1 and the results of the searches were culled by two independent reviewers using an iterative approach. Studies collected were from 1989 to April 2009.
Abstracts of search results were reviewed to eliminate articles that clearly did not pertain to CE methods, cost-effectiveness, or educational theory and to categorize the studies as informative, equivocal, or not informative of CE effectiveness. A wide range of designs were classified as informative, including randomized controlled trials, prospective cohort studies, observational studies, and studies with pre- and post-intervention assessment methodologies. Quantitative and qualitative approaches were included, and inclusion was not limited to studies with positive results. The most common reasons articles were classified as not informative were absence of a trial design, small sample size, and high likelihood of confounding factors in the design that could affect outcomes. The two reviewers independently classified abstracts and full texts of the articles and then compared their classification results. Interreviewer reliability was greater than 80 percent, and discrepancies were resolved by a consensus process. A third reviewer verified the results classified as informative or equivocal in a final round of detailed assessment of the study design, populations, intervention, type of outcome, and conclusions for each article. Systematic reviews and metaanalyses are included in Table A-1 ; studies and articles are included in Table A-2 .
Table A-1 begins on the next page.
TABLE A-1 Summary of Systematic Reviews on Effectiveness of CE Methods
TABLE A-2 Literature Review on the Effectiveness of CE Methods
This page intentionally left blank.
Today in the United States, the professional health workforce is not consistently prepared to provide high quality health care and assure patient safety, even as the nation spends more per capita on health care than any other country. The absence of a comprehensive and well-integrated system of continuing education (CE) in the health professions is an important contributing factor to knowledge and performance deficiencies at the individual and system levels.
To be most effective, health professionals at every stage of their careers must continue learning about advances in research and treatment in their fields (and related fields) in order to obtain and maintain up-to-date knowledge and skills in caring for their patients. Many health professionals regularly undertake a variety of efforts to stay up to date, but on a larger scale, the nation's approach to CE for health professionals fails to support the professions in their efforts to achieve and maintain proficiency.
Redesigning Continuing Education in the Health Professions illustrates a vision for a better system through a comprehensive approach of continuing professional development, and posits a framework upon which to develop a new, more effective system. The book also offers principles to guide the creation of a national continuing education institute.
READ FREE ONLINE
Welcome to OpenBook!
You're looking at OpenBook, NAP.edu's online reading room since 1999. Based on feedback from you, our users, we've made some improvements that make it easier than ever to read thousands of publications on our website.
Do you want to take a quick tour of the OpenBook's features?
Show this book's table of contents , where you can jump to any chapter by name.
...or use these buttons to go back to the previous chapter or skip to the next one.
Jump up to the previous page or down to the next one. Also, you can type in a page number and press Enter to go directly to that page in the book.
Switch between the Original Pages , where you can read the report as it appeared in print, and Text Pages for the web version, where you can highlight and search the text.
To search the entire text of this book, type in your search term here and press Enter .
Share a link to this book page on your preferred social network or via email.
View our suggested citation for this chapter.
Ready to take your reading offline? Click here to buy this book in print or download it as a free PDF, if available.
Get Email Updates
Do you enjoy reading reports from the Academies online for free ? Sign up for email notifications and we'll let you know about new publications in your areas of interest when they're released.
Purdue Online Writing Lab Purdue OWL® College of Liberal Arts
Welcome to the Purdue Online Writing Lab

Welcome to the Purdue OWL
This page is brought to you by the OWL at Purdue University. When printing this page, you must include the entire legal notice.
Copyright ©1995-2018 by The Writing Lab & The OWL at Purdue and Purdue University. All rights reserved. This material may not be published, reproduced, broadcast, rewritten, or redistributed without permission. Use of this site constitutes acceptance of our terms and conditions of fair use.
The Online Writing Lab at Purdue University houses writing resources and instructional material, and we provide these as a free service of the Writing Lab at Purdue. Students, members of the community, and users worldwide will find information to assist with many writing projects. Teachers and trainers may use this material for in-class and out-of-class instruction.
The Purdue On-Campus Writing Lab and Purdue Online Writing Lab assist clients in their development as writers—no matter what their skill level—with on-campus consultations, online participation, and community engagement. The Purdue Writing Lab serves the Purdue, West Lafayette, campus and coordinates with local literacy initiatives. The Purdue OWL offers global support through online reference materials and services.
A Message From the Assistant Director of Content Development
The Purdue OWL® is committed to supporting students, instructors, and writers by offering a wide range of resources that are developed and revised with them in mind. To do this, the OWL team is always exploring possibilties for a better design, allowing accessibility and user experience to guide our process. As the OWL undergoes some changes, we welcome your feedback and suggestions by email at any time.
Please don't hesitate to contact us via our contact page if you have any questions or comments.
All the best,
Social Media
Facebook twitter.
What’s Included: Literature Review Template
This template is structure is based on the tried and trusted best-practice format for formal academic research projects such as dissertations and theses. The literature review template includes the following sections:
- Before you start – essential groundwork to ensure you’re ready
- The introduction section
- The core/body section
- The conclusion /summary
- Extra free resources
Each section is explained in plain, straightforward language , followed by an overview of the key elements that you need to cover. We’ve also included practical examples and links to more free videos and guides to help you understand exactly what’s required in each section.
The cleanly-formatted Google Doc can be downloaded as a fully editable MS Word Document (DOCX format), so you can use it as-is or convert it to LaTeX.
PS – if you’d like a high-level template for the entire thesis, you can we’ve got that too .
FAQs: Literature Review Template
What format is the template (doc, pdf, ppt, etc.).
The literature review chapter template is provided as a Google Doc. You can download it in MS Word format or make a copy to your Google Drive. You’re also welcome to convert it to whatever format works best for you, such as LaTeX or PDF.
What types of literature reviews can this template be used for?
The template follows the standard format for academic literature reviews, which means it will be suitable for the vast majority of academic research projects (especially those within the sciences), whether they are qualitative or quantitative in terms of design.
Keep in mind that the exact requirements for the literature review chapter will vary between universities and degree programs. These are typically minor, but it’s always a good idea to double-check your university’s requirements before you finalize your structure.
Is this template for an undergrad, Master or PhD-level thesis?
This template can be used for a literature review at any level of study. Doctoral-level projects typically require the literature review to be more extensive/comprehensive, but the structure will typically remain the same.

Can I modify the template to suit my topic/area?
Absolutely. While the template provides a general structure, you should adapt it to fit the specific requirements and focus of your literature review.
What structural style does this literature review template use?
The template assumes a thematic structure (as opposed to a chronological or methodological structure), as this is the most common approach. However, this is only one dimension of the template, so it will still be useful if you are adopting a different structure.
Does this template include the Excel literature catalog?
No, that is a separate template, which you can download for free here . This template is for the write-up of the actual literature review chapter, whereas the catalog is for use during the literature sourcing and sorting phase.
How long should the literature review chapter be?
This depends on your university’s specific requirements, so it’s best to check with them. As a general ballpark, literature reviews for Masters-level projects are usually 2,000 – 3,000 words in length, while Doctoral-level projects can reach multiples of this.
Can I include literature that contradicts my hypothesis?
Yes, it’s important to acknowledge and discuss literature that presents different viewpoints or contradicts your hypothesis. So, don’t shy away from existing research that takes an opposing view to yours.
How do I avoid plagiarism in my literature review?
Always cite your sources correctly and paraphrase ideas in your own words while maintaining the original meaning. You can always check our plagiarism score before submitting your work to help ease your mind.
Do you have an example of a populated template?
We provide a walkthrough of the template and review an example of a high-quality literature research chapter here .
Can I share this literature review template with my friends/colleagues?
Yes, you’re welcome to share this template in its original format (no editing allowed). If you want to post about it on your blog or social media, all we ask is that you reference this page as your source.
Do you have templates for the other dissertation/thesis chapters?
Yes, we do. You can find our full collection of templates here .
Can Grad Coach help me with my literature review?
Yes, you’re welcome to get in touch with us to discuss our private coaching services , where we can help you work through the literature review chapter (and any other chapters).

- Open access
- Published: 17 August 2023
Data visualisation in scoping reviews and evidence maps on health topics: a cross-sectional analysis
- Emily South ORCID: orcid.org/0000-0003-2187-4762 1 &
- Mark Rodgers 1
Systematic Reviews volume 12 , Article number: 142 ( 2023 ) Cite this article
3614 Accesses
13 Altmetric
Metrics details
Scoping reviews and evidence maps are forms of evidence synthesis that aim to map the available literature on a topic and are well-suited to visual presentation of results. A range of data visualisation methods and interactive data visualisation tools exist that may make scoping reviews more useful to knowledge users. The aim of this study was to explore the use of data visualisation in a sample of recent scoping reviews and evidence maps on health topics, with a particular focus on interactive data visualisation.
Ovid MEDLINE ALL was searched for recent scoping reviews and evidence maps (June 2020-May 2021), and a sample of 300 papers that met basic selection criteria was taken. Data were extracted on the aim of each review and the use of data visualisation, including types of data visualisation used, variables presented and the use of interactivity. Descriptive data analysis was undertaken of the 238 reviews that aimed to map evidence.
Of the 238 scoping reviews or evidence maps in our analysis, around one-third (37.8%) included some form of data visualisation. Thirty-five different types of data visualisation were used across this sample, although most data visualisations identified were simple bar charts (standard, stacked or multi-set), pie charts or cross-tabulations (60.8%). Most data visualisations presented a single variable (64.4%) or two variables (26.1%). Almost a third of the reviews that used data visualisation did not use any colour (28.9%). Only two reviews presented interactive data visualisation, and few reported the software used to create visualisations.
Conclusions
Data visualisation is currently underused by scoping review authors. In particular, there is potential for much greater use of more innovative forms of data visualisation and interactive data visualisation. Where more innovative data visualisation is used, scoping reviews have made use of a wide range of different methods. Increased use of these more engaging visualisations may make scoping reviews more useful for a range of stakeholders.
Peer Review reports
Scoping reviews are “a type of evidence synthesis that aims to systematically identify and map the breadth of evidence available on a particular topic, field, concept, or issue” ([ 1 ], p. 950). While they include some of the same steps as a systematic review, such as systematic searches and the use of predetermined eligibility criteria, scoping reviews often address broader research questions and do not typically involve the quality appraisal of studies or synthesis of data [ 2 ]. Reasons for conducting a scoping review include the following: to map types of evidence available, to explore research design and conduct, to clarify concepts or definitions and to map characteristics or factors related to a concept [ 3 ]. Scoping reviews can also be undertaken to inform a future systematic review (e.g. to assure authors there will be adequate studies) or to identify knowledge gaps [ 3 ]. Other evidence synthesis approaches with similar aims have been described as evidence maps, mapping reviews or systematic maps [ 4 ]. While this terminology is used inconsistently, evidence maps can be used to identify evidence gaps and present them in a user-friendly (and often visual) way [ 5 ].
Scoping reviews are often targeted to an audience of healthcare professionals or policy-makers [ 6 ], suggesting that it is important to present results in a user-friendly and informative way. Until recently, there was little guidance on how to present the findings of scoping reviews. In recent literature, there has been some discussion of the importance of clearly presenting data for the intended audience of a scoping review, with creative and innovative use of visual methods if appropriate [ 7 , 8 , 9 ]. Lockwood et al. suggest that innovative visual presentation should be considered over dense sections of text or long tables in many cases [ 8 ]. Khalil et al. suggest that inspiration could be drawn from the field of data visualisation [ 7 ]. JBI guidance on scoping reviews recommends that reviewers carefully consider the best format for presenting data at the protocol development stage and provides a number of examples of possible methods [ 10 ].
Interactive resources are another option for presentation in scoping reviews [ 9 ]. Researchers without the relevant programming skills can now use several online platforms (such as Tableau [ 11 ] and Flourish [ 12 ]) to create interactive data visualisations. The benefits of using interactive visualisation in research include the ability to easily present more than two variables [ 13 ] and increased engagement of users [ 14 ]. Unlike static graphs, interactive visualisations can allow users to view hierarchical data at different levels, exploring both the “big picture” and looking in more detail ([ 15 ], p. 291). Interactive visualizations are often targeted at practitioners and decision-makers [ 13 ], and there is some evidence from qualitative research that they are valued by policy-makers [ 16 , 17 , 18 ].
Given their focus on mapping evidence, we believe that scoping reviews are particularly well-suited to visually presenting data and the use of interactive data visualisation tools. However, it is unknown how many recent scoping reviews visually map data or which types of data visualisation are used. The aim of this study was to explore the use of data visualisation methods in a large sample of recent scoping reviews and evidence maps on health topics. In particular, we were interested in the extent to which these forms of synthesis use any form of interactive data visualisation.
This study was a cross-sectional analysis of studies labelled as scoping reviews or evidence maps (or synonyms of these terms) in the title or abstract.
The search strategy was developed with help from an information specialist. Ovid MEDLINE® ALL was searched in June 2021 for studies added to the database in the previous 12 months. The search was limited to English language studies only.
The search strategy was as follows:
Ovid MEDLINE(R) ALL
(scoping review or evidence map or systematic map or mapping review or scoping study or scoping project or scoping exercise or literature mapping or evidence mapping or systematic mapping or literature scoping or evidence gap map).ab,ti.
limit 1 to english language
(202006* or 202007* or 202008* or 202009* or 202010* or 202011* or 202012* or 202101* or 202102* or 202103* or 202104* or 202105*).dt.
The search returned 3686 records. Records were de-duplicated in EndNote 20 software, leaving 3627 unique records.
A sample of these reviews was taken by screening the search results against basic selection criteria (Table 1 ). These criteria were piloted and refined after discussion between the two researchers. A single researcher (E.S.) screened the records in EPPI-Reviewer Web software using the machine-learning priority screening function. Where a second opinion was needed, decisions were checked by a second researcher (M.R.).
Our initial plan for sampling, informed by pilot searching, was to screen and data extract records in batches of 50 included reviews at a time. We planned to stop screening when a batch of 50 reviews had been extracted that included no new types of data visualisation or after screening time had reached 2 days. However, once data extraction was underway, we found the sample to be richer in terms of data visualisation than anticipated. After the inclusion of 300 reviews, we took the decision to end screening in order to ensure the study was manageable.
Data extraction
A data extraction form was developed in EPPI-Reviewer Web, piloted on 50 reviews and refined. Data were extracted by one researcher (E. S. or M. R.), with a second researcher (M. R. or E. S.) providing a second opinion when needed. The data items extracted were as follows: type of review (term used by authors), aim of review (mapping evidence vs. answering specific question vs. borderline), number of visualisations (if any), types of data visualisation used, variables/domains presented by each visualisation type, interactivity, use of colour and any software requirements.
When categorising review aims, we considered “mapping evidence” to incorporate all of the six purposes for conducting a scoping review proposed by Munn et al. [ 3 ]. Reviews were categorised as “answering a specific question” if they aimed to synthesise study findings to answer a particular question, for example on effectiveness of an intervention. We were inclusive with our definition of “mapping evidence” and included reviews with mixed aims in this category. However, some reviews were difficult to categorise (for example where aims were unclear or the stated aims did not match the actual focus of the paper) and were considered to be “borderline”. It became clear that a proportion of identified records that described themselves as “scoping” or “mapping” reviews were in fact pseudo-systematic reviews that failed to undertake key systematic review processes. Such reviews attempted to integrate the findings of included studies rather than map the evidence, and so reviews categorised as “answering a specific question” were excluded from the main analysis. Data visualisation methods for meta-analyses have been explored previously [ 19 ]. Figure 1 shows the flow of records from search results to final analysis sample.
Flow diagram of the sampling process
Data visualisation was defined as any graph or diagram that presented results data, including tables with a visual mapping element, such as cross-tabulations and heat maps. However, tables which displayed data at a study level (e.g. tables summarising key characteristics of each included study) were not included, even if they used symbols, shading or colour. Flow diagrams showing the study selection process were also excluded. Data visualisations in appendices or supplementary information were included, as well as any in publicly available dissemination products (e.g. visualisations hosted online) if mentioned in papers.
The typology used to categorise data visualisation methods was based on an existing online catalogue [ 20 ]. Specific types of data visualisation were categorised in five broad categories: graphs, diagrams, tables, maps/geographical and other. If a data visualisation appeared in our sample that did not feature in the original catalogue, we checked a second online catalogue [ 21 ] for an appropriate term, followed by wider Internet searches. These additional visualisation methods were added to the appropriate section of the typology. The final typology can be found in Additional file 1 .
We conducted descriptive data analysis in Microsoft Excel 2019 and present frequencies and percentages. Where appropriate, data are presented using graphs or other data visualisations created using Flourish. We also link to interactive versions of some of these visualisations.
Almost all of the 300 reviews in the total sample were labelled by review authors as “scoping reviews” ( n = 293, 97.7%). There were also four “mapping reviews”, one “scoping study”, one “evidence mapping” and one that was described as a “scoping review and evidence map”. Included reviews were all published in 2020 or 2021, with the exception of one review published in 2018. Just over one-third of these reviews ( n = 105, 35.0%) included some form of data visualisation. However, we excluded 62 reviews that did not focus on mapping evidence from the following analysis (see “ Methods ” section). Of the 238 remaining reviews (that either clearly aimed to map evidence or were judged to be “borderline”), 90 reviews (37.8%) included at least one data visualisation. The references for these reviews can be found in Additional file 2 .
Number of visualisations
Thirty-six (40.0%) of these 90 reviews included just one example of data visualisation (Fig. 2 ). Less than a third ( n = 28, 31.1%) included three or more visualisations. The greatest number of data visualisations in one review was 17 (all bar or pie charts). In total, 222 individual data visualisations were identified across the sample of 238 reviews.
Number of data visualisations per review
Categories of data visualisation
Graphs were the most frequently used category of data visualisation in the sample. Over half of the reviews with data visualisation included at least one graph ( n = 59, 65.6%). The least frequently used category was maps, with 15.6% ( n = 14) of these reviews including a map.
Of the total number of 222 individual data visualisations, 102 were graphs (45.9%), 34 were tables (15.3%), 23 were diagrams (10.4%), 15 were maps (6.8%) and 48 were classified as “other” in the typology (21.6%).
Types of data visualisation
All of the types of data visualisation identified in our sample are reported in Table 2 . In total, 35 different types were used across the sample of reviews.
The most frequently used data visualisation type was a bar chart. Of 222 total data visualisations, 78 (35.1%) were a variation on a bar chart (either standard bar chart, stacked bar chart or multi-set bar chart). There were also 33 pie charts (14.9% of data visualisations) and 24 cross-tabulations (10.8% of data visualisations). In total, these five types of data visualisation accounted for 60.8% ( n = 135) of all data visualisations. Figure 3 shows the frequency of each data visualisation category and type; an interactive online version of this treemap is also available ( https://public.flourish.studio/visualisation/9396133/ ). Figure 4 shows how users can further explore the data using the interactive treemap.
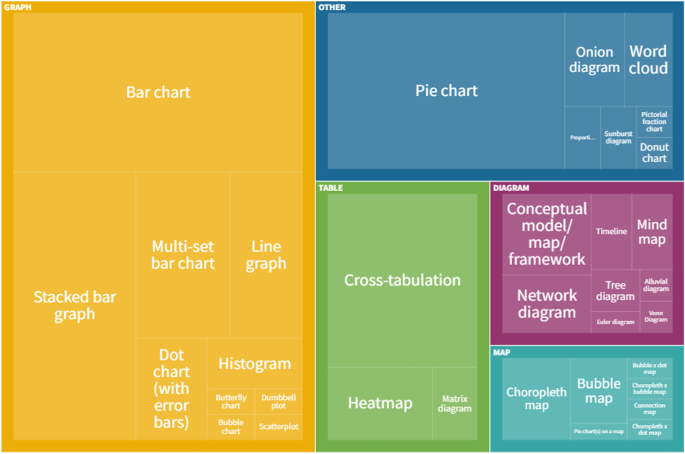
Data visualisation categories and types. An interactive version of this treemap is available online: https://public.flourish.studio/visualisation/9396133/ . Through the interactive version, users can further explore the data (see Fig. 4 ). The unit of this treemap is the individual data visualisation, so multiple data visualisations within the same scoping review are represented in this map. Created with flourish.studio ( https://flourish.studio )
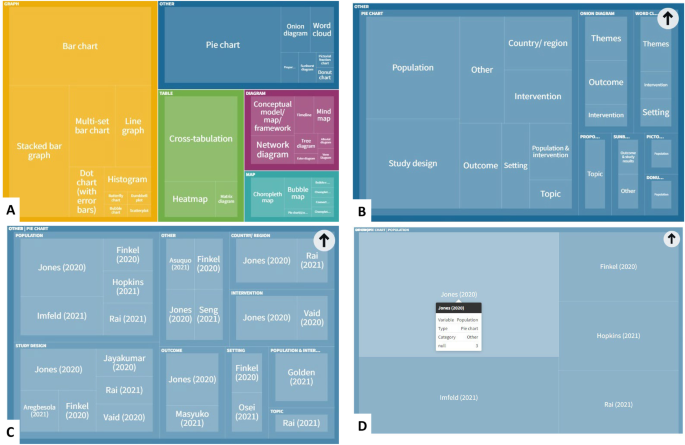
Screenshots showing how users of the interactive treemap can explore the data further. Users can explore each level of the hierarchical treemap ( A Visualisation category > B Visualisation subcategory > C Variables presented in visualisation > D Individual references reporting this category/subcategory/variable permutation). Created with flourish.studio ( https://flourish.studio )
Data presented
Around two-thirds of data visualisations in the sample presented a single variable ( n = 143, 64.4%). The most frequently presented single variables were themes ( n = 22, 9.9% of data visualisations), population ( n = 21, 9.5%), country or region ( n = 21, 9.5%) and year ( n = 20, 9.0%). There were 58 visualisations (26.1%) that presented two different variables. The remaining 21 data visualisations (9.5%) presented three or more variables. Figure 5 shows the variables presented by each different type of data visualisation (an interactive version of this figure is available online).
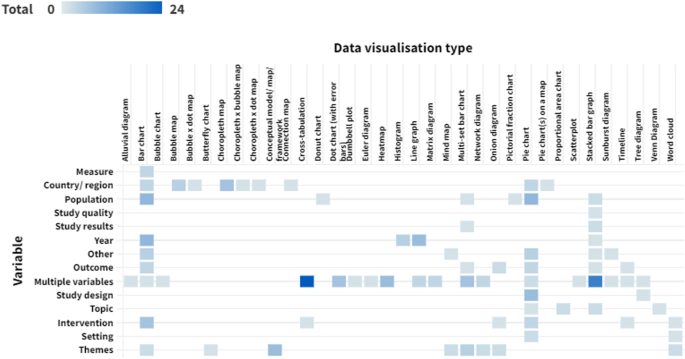
Variables presented by each data visualisation type. Darker cells indicate a larger number of reviews. An interactive version of this heat map is available online: https://public.flourish.studio/visualisation/10632665/ . Users can hover over each cell to see the number of data visualisations for that combination of data visualisation type and variable. The unit of this heat map is the individual data visualisation, so multiple data visualisations within a single scoping review are represented in this map. Created with flourish.studio ( https://flourish.studio )
Most reviews presented at least one data visualisation in colour ( n = 64, 71.1%). However, almost a third ( n = 26, 28.9%) used only black and white or greyscale.
Interactivity
Only two of the reviews included data visualisations with any level of interactivity. One scoping review on music and serious mental illness [ 22 ] linked to an interactive bubble chart hosted online on Tableau. Functionality included the ability to filter the studies displayed by various attributes.
The other review was an example of evidence mapping from the environmental health field [ 23 ]. All four of the data visualisations included in the paper were available in an interactive format hosted either by the review management software or on Tableau. The interactive versions linked to the relevant references so users could directly explore the evidence base. This was the only review that provided this feature.
Software requirements
Nine reviews clearly reported the software used to create data visualisations. Three reviews used Tableau (one of them also used review management software as discussed above) [ 22 , 23 , 24 ]. Two reviews generated maps using ArcGIS [ 25 ] or ArcMap [ 26 ]. One review used Leximancer for a lexical analysis [ 27 ]. One review undertook a bibliometric analysis using VOSviewer [ 28 ], and another explored citation patterns using CitNetExplorer [ 29 ]. Other reviews used Excel [ 30 ] or R [ 26 ].
To our knowledge, this is the first systematic and in-depth exploration of the use of data visualisation techniques in scoping reviews. Our findings suggest that the majority of scoping reviews do not use any data visualisation at all, and, in particular, more innovative examples of data visualisation are rare. Around 60% of data visualisations in our sample were simple bar charts, pie charts or cross-tabulations. There appears to be very limited use of interactive online visualisation, despite the potential this has for communicating results to a range of stakeholders. While it is not always appropriate to use data visualisation (or a simple bar chart may be the most user-friendly way of presenting the data), these findings suggest that data visualisation is being underused in scoping reviews. In a large minority of reviews, visualisations were not published in colour, potentially limiting how user-friendly and attractive papers are to decision-makers and other stakeholders. Also, very few reviews clearly reported the software used to create data visualisations. However, 35 different types of data visualisation were used across the sample, highlighting the wide range of methods that are potentially available to scoping review authors.
Our results build on the limited research that has previously been undertaken in this area. Two previous publications also found limited use of graphs in scoping reviews. Results were “mapped graphically” in 29% of scoping reviews in any field in one 2014 publication [ 31 ] and 17% of healthcare scoping reviews in a 2016 article [ 6 ]. Our results suggest that the use of data visualisation has increased somewhat since these reviews were conducted. Scoping review methods have also evolved in the last 10 years; formal guidance on scoping review conduct was published in 2014 [ 32 ], and an extension of the PRISMA checklist for scoping reviews was published in 2018 [ 33 ]. It is possible that an overall increase in use of data visualisation reflects increased quality of published scoping reviews. There is also some literature supporting our findings on the wide range of data visualisation methods that are used in evidence synthesis. An investigation of methods to identify, prioritise or display health research gaps (25/139 included studies were scoping reviews; 6/139 were evidence maps) identified 14 different methods used to display gaps or priorities, with half being “more advanced” (e.g. treemaps, radial bar plots) ([ 34 ], p. 107). A review of data visualisation methods used in papers reporting meta-analyses found over 200 different ways of displaying data [ 19 ].
Only two reviews in our sample used interactive data visualisation, and one of these was an example of systematic evidence mapping from the environmental health field rather than a scoping review (in environmental health, systematic evidence mapping explicitly involves producing a searchable database [ 35 ]). A scoping review of papers on the use of interactive data visualisation in population health or health services research found a range of examples but still limited use overall [ 13 ]. For example, the authors noted the currently underdeveloped potential for using interactive visualisation in research on health inequalities. It is possible that the use of interactive data visualisation in academic papers is restricted by academic publishing requirements; for example, it is currently difficult to incorporate an interactive figure into a journal article without linking to an external host or platform. However, we believe that there is a lot of potential to add value to future scoping reviews by using interactive data visualisation software. Few reviews in our sample presented three or more variables in a single visualisation, something which can easily be achieved using interactive data visualisation tools. We have previously used EPPI-Mapper [ 36 ] to present results of a scoping review of systematic reviews on behaviour change in disadvantaged groups, with links to the maps provided in the paper [ 37 ]. These interactive maps allowed policy-makers to explore the evidence on different behaviours and disadvantaged groups and access full publications of the included studies directly from the map.
We acknowledge there are barriers to use for some of the data visualisation software available. EPPI-Mapper and some of the software used by reviews in our sample incur a cost. Some software requires a certain level of knowledge and skill in its use. However numerous online free data visualisation tools and resources exist. We have used Flourish to present data for this review, a basic version of which is currently freely available and easy to use. Previous health research has been found to have used a range of different interactive data visualisation software, much of which does not required advanced knowledge or skills to use [ 13 ].
There are likely to be other barriers to the use of data visualisation in scoping reviews. Journal guidelines and policies may present barriers for using innovative data visualisation. For example, some journals charge a fee for publication of figures in colour. As previously mentioned, there are limited options for incorporating interactive data visualisation into journal articles. Authors may also be unaware of the data visualisation methods and tools that are available. Producing data visualisations can be time-consuming, particularly if authors lack experience and skills in this. It is possible that many authors prioritise speed of publication over spending time producing innovative data visualisations, particularly in a context where there is pressure to achieve publications.
Limitations
A limitation of this study was that we did not assess how appropriate the use of data visualisation was in our sample as this would have been highly subjective. Simple descriptive or tabular presentation of results may be the most appropriate approach for some scoping review objectives [ 7 , 8 , 10 ], and the scoping review literature cautions against “over-using” different visual presentation methods [ 7 , 8 ]. It cannot be assumed that all of the reviews that did not include data visualisation should have done so. Likewise, we do not know how many reviews used methods of data visualisation that were not well suited to their data.
We initially relied on authors’ own use of the term “scoping review” (or equivalent) to sample reviews but identified a relatively large number of papers labelled as scoping reviews that did not meet the basic definition, despite the availability of guidance and reporting guidelines [ 10 , 33 ]. It has previously been noted that scoping reviews may be undertaken inappropriately because they are seen as “easier” to conduct than a systematic review ([ 3 ], p.6), and that reviews are often labelled as “scoping reviews” while not appearing to follow any established framework or guidance [ 2 ]. We therefore took the decision to remove these reviews from our main analysis. However, decisions on how to classify review aims were subjective, and we did include some reviews that were of borderline relevance.
A further limitation is that this was a sample of published reviews, rather than a comprehensive systematic scoping review as have previously been undertaken [ 6 , 31 ]. The number of scoping reviews that are published has increased rapidly, and this would now be difficult to undertake. As this was a sample, not all relevant scoping reviews or evidence maps that would have met our criteria were included. We used machine learning to screen our search results for pragmatic reasons (to reduce screening time), but we do not see any reason that our sample would not be broadly reflective of the wider literature.
Data visualisation, and in particular more innovative examples of it, is currently underused in published scoping reviews on health topics. The examples that we have found highlight the wide range of methods that scoping review authors could draw upon to present their data in an engaging way. In particular, we believe that interactive data visualisation has significant potential for mapping the available literature on a topic. Appropriate use of data visualisation may increase the usefulness, and thus uptake, of scoping reviews as a way of identifying existing evidence or research gaps by decision-makers, researchers and commissioners of research. We recommend that scoping review authors explore the extensive free resources and online tools available for data visualisation. However, we also think that it would be useful for publishers to explore allowing easier integration of interactive tools into academic publishing, given the fact that papers are now predominantly accessed online. Future research may be helpful to explore which methods are particularly useful to scoping review users.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
Organisation formerly known as Joanna Briggs Institute
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Munn Z, Pollock D, Khalil H, Alexander L, McLnerney P, Godfrey CM, Peters M, Tricco AC. What are scoping reviews? Providing a formal definition of scoping reviews as a type of evidence synthesis. JBI Evid Synth. 2022;20:950–952.
Peters MDJ, Marnie C, Colquhoun H, Garritty CM, Hempel S, Horsley T, Langlois EV, Lillie E, O’Brien KK, Tunçalp Ӧ, et al. Scoping reviews: reinforcing and advancing the methodology and application. Syst Rev. 2021;10:263.
Article PubMed PubMed Central Google Scholar
Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18:143.
Sutton A, Clowes M, Preston L, Booth A. Meeting the review family: exploring review types and associated information retrieval requirements. Health Info Libr J. 2019;36:202–22.
Article PubMed Google Scholar
Miake-Lye IM, Hempel S, Shanman R, Shekelle PG. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev. 2016;5:28.
Tricco AC, Lillie E, Zarin W, O’Brien K, Colquhoun H, Kastner M, Levac D, Ng C, Sharpe JP, Wilson K, et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16:15.
Khalil H, Peters MDJ, Tricco AC, Pollock D, Alexander L, McInerney P, Godfrey CM, Munn Z. Conducting high quality scoping reviews-challenges and solutions. J Clin Epidemiol. 2021;130:156–60.
Lockwood C, dos Santos KB, Pap R. Practical guidance for knowledge synthesis: scoping review methods. Asian Nurs Res. 2019;13:287–94.
Article Google Scholar
Pollock D, Peters MDJ, Khalil H, McInerney P, Alexander L, Tricco AC, Evans C, de Moraes ÉB, Godfrey CM, Pieper D, et al. Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI Evidence Synthesis. 2022;10:11124.
Google Scholar
Peters MDJ GC, McInerney P, Munn Z, Tricco AC, Khalil, H. Chapter 11: Scoping reviews (2020 version). In: Aromataris E MZ, editor. JBI Manual for Evidence Synthesis. JBI; 2020. Available from https://synthesismanual.jbi.global . Accessed 1 Feb 2023.
Tableau Public. https://www.tableau.com/en-gb/products/public . Accessed 24 January 2023.
flourish.studio. https://flourish.studio/ . Accessed 24 January 2023.
Chishtie J, Bielska IA, Barrera A, Marchand J-S, Imran M, Tirmizi SFA, Turcotte LA, Munce S, Shepherd J, Senthinathan A, et al. Interactive visualization applications in population health and health services research: systematic scoping review. J Med Internet Res. 2022;24: e27534.
Isett KR, Hicks DM. Providing public servants what they need: revealing the “unseen” through data visualization. Public Adm Rev. 2018;78:479–85.
Carroll LN, Au AP, Detwiler LT, Fu T-c, Painter IS, Abernethy NF. Visualization and analytics tools for infectious disease epidemiology: a systematic review. J Biomed Inform. 2014;51:287–298.
Lundkvist A, El-Khatib Z, Kalra N, Pantoja T, Leach-Kemon K, Gapp C, Kuchenmüller T. Policy-makers’ views on translating burden of disease estimates in health policies: bridging the gap through data visualization. Arch Public Health. 2021;79:17.
Zakkar M, Sedig K. Interactive visualization of public health indicators to support policymaking: an exploratory study. Online J Public Health Inform. 2017;9:e190–e190.
Park S, Bekemeier B, Flaxman AD. Understanding data use and preference of data visualization for public health professionals: a qualitative study. Public Health Nurs. 2021;38:531–41.
Kossmeier M, Tran US, Voracek M. Charting the landscape of graphical displays for meta-analysis and systematic reviews: a comprehensive review, taxonomy, and feature analysis. BMC Med Res Methodol. 2020;20:26.
Ribecca, S. The Data Visualisation Catalogue. https://datavizcatalogue.com/index.html . Accessed 23 November 2021.
Ferdio. Data Viz Project. https://datavizproject.com/ . Accessed 23 November 2021.
Golden TL, Springs S, Kimmel HJ, Gupta S, Tiedemann A, Sandu CC, Magsamen S. The use of music in the treatment and management of serious mental illness: a global scoping review of the literature. Front Psychol. 2021;12: 649840.
Keshava C, Davis JA, Stanek J, Thayer KA, Galizia A, Keshava N, Gift J, Vulimiri SV, Woodall G, Gigot C, et al. Application of systematic evidence mapping to assess the impact of new research when updating health reference values: a case example using acrolein. Environ Int. 2020;143: 105956.
Article CAS PubMed PubMed Central Google Scholar
Jayakumar P, Lin E, Galea V, Mathew AJ, Panda N, Vetter I, Haynes AB. Digital phenotyping and patient-generated health data for outcome measurement in surgical care: a scoping review. J Pers Med. 2020;10:282.
Qu LG, Perera M, Lawrentschuk N, Umbas R, Klotz L. Scoping review: hotspots for COVID-19 urological research: what is being published and from where? World J Urol. 2021;39:3151–60.
Article CAS PubMed Google Scholar
Rossa-Roccor V, Acheson ES, Andrade-Rivas F, Coombe M, Ogura S, Super L, Hong A. Scoping review and bibliometric analysis of the term “planetary health” in the peer-reviewed literature. Front Public Health. 2020;8:343.
Hewitt L, Dahlen HG, Hartz DL, Dadich A. Leadership and management in midwifery-led continuity of care models: a thematic and lexical analysis of a scoping review. Midwifery. 2021;98: 102986.
Xia H, Tan S, Huang S, Gan P, Zhong C, Lu M, Peng Y, Zhou X, Tang X. Scoping review and bibliometric analysis of the most influential publications in achalasia research from 1995 to 2020. Biomed Res Int. 2021;2021:8836395.
Vigliotti V, Taggart T, Walker M, Kusmastuti S, Ransome Y. Religion, faith, and spirituality influences on HIV prevention activities: a scoping review. PLoS ONE. 2020;15: e0234720.
van Heemskerken P, Broekhuizen H, Gajewski J, Brugha R, Bijlmakers L. Barriers to surgery performed by non-physician clinicians in sub-Saharan Africa-a scoping review. Hum Resour Health. 2020;18:51.
Pham MT, Rajić A, Greig JD, Sargeant JM, Papadopoulos A, McEwen SA. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods. 2014;5:371–85.
Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, McInerney P, Godfrey CM, Khalil H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18:2119–26.
Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169:467–73.
Nyanchoka L, Tudur-Smith C, Thu VN, Iversen V, Tricco AC, Porcher R. A scoping review describes methods used to identify, prioritize and display gaps in health research. J Clin Epidemiol. 2019;109:99–110.
Wolffe TAM, Whaley P, Halsall C, Rooney AA, Walker VR. Systematic evidence maps as a novel tool to support evidence-based decision-making in chemicals policy and risk management. Environ Int. 2019;130:104871.
Digital Solution Foundry and EPPI-Centre. EPPI-Mapper, Version 2.0.1. EPPI-Centre, UCL Social Research Institute, University College London. 2020. https://eppi.ioe.ac.uk/cms/Default.aspx?tabid=3790 .
South E, Rodgers M, Wright K, Whitehead M, Sowden A. Reducing lifestyle risk behaviours in disadvantaged groups in high-income countries: a scoping review of systematic reviews. Prev Med. 2022;154: 106916.
Download references
Acknowledgements
We would like to thank Melissa Harden, Senior Information Specialist, Centre for Reviews and Dissemination, for advice on developing the search strategy.
This work received no external funding.
Author information
Authors and affiliations.
Centre for Reviews and Dissemination, University of York, York, YO10 5DD, UK
Emily South & Mark Rodgers
You can also search for this author in PubMed Google Scholar
Contributions
Both authors conceptualised and designed the study and contributed to screening, data extraction and the interpretation of results. ES undertook the literature searches, analysed data, produced the data visualisations and drafted the manuscript. MR contributed to revising the manuscript, and both authors read and approved the final version.
Corresponding author
Correspondence to Emily South .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1..
Typology of data visualisation methods.
Additional file 2.
References of scoping reviews included in main dataset.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
South, E., Rodgers, M. Data visualisation in scoping reviews and evidence maps on health topics: a cross-sectional analysis. Syst Rev 12 , 142 (2023). https://doi.org/10.1186/s13643-023-02309-y
Download citation
Received : 21 February 2023
Accepted : 07 August 2023
Published : 17 August 2023
DOI : https://doi.org/10.1186/s13643-023-02309-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Scoping review
- Evidence map
- Data visualisation
Systematic Reviews
ISSN: 2046-4053
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Open access
- Published: 01 May 2024
The effectiveness of virtual reality training on knowledge, skills and attitudes of health care professionals and students in assessing and treating mental health disorders: a systematic review
- Cathrine W. Steen 1 , 2 ,
- Kerstin Söderström 1 , 2 ,
- Bjørn Stensrud 3 ,
- Inger Beate Nylund 2 &
- Johan Siqveland 4 , 5
BMC Medical Education volume 24 , Article number: 480 ( 2024 ) Cite this article
416 Accesses
1 Altmetric
Metrics details
Virtual reality (VR) training can enhance health professionals’ learning. However, there are ambiguous findings on the effectiveness of VR as an educational tool in mental health. We therefore reviewed the existing literature on the effectiveness of VR training on health professionals’ knowledge, skills, and attitudes in assessing and treating patients with mental health disorders.
We searched MEDLINE, PsycINFO (via Ovid), the Cochrane Library, ERIC, CINAHL (on EBSCOhost), Web of Science Core Collection, and the Scopus database for studies published from January 1985 to July 2023. We included all studies evaluating the effect of VR training interventions on attitudes, knowledge, and skills pertinent to the assessment and treatment of mental health disorders and published in English or Scandinavian languages. The quality of the evidence in randomized controlled trials was assessed with the Cochrane Risk of Bias Tool 2.0. For non-randomized studies, we assessed the quality of the studies with the ROBINS-I tool.
Of 4170 unique records identified, eight studies were eligible. The four randomized controlled trials were assessed as having some concern or a high risk of overall bias. The four non-randomized studies were assessed as having a moderate to serious overall risk of bias. Of the eight included studies, four used a virtual standardized patient design to simulate training situations, two studies used interactive patient scenario training designs, while two studies used a virtual patient game design. The results suggest that VR training interventions can promote knowledge and skills acquisition.
Conclusions
The findings indicate that VR interventions can effectively train health care personnel to acquire knowledge and skills in the assessment and treatment of mental health disorders. However, study heterogeneity, prevalence of small sample sizes, and many studies with a high or serious risk of bias suggest an uncertain evidence base. Future research on the effectiveness of VR training should include assessment of immersive VR training designs and a focus on more robust studies with larger sample sizes.
Trial registration
This review was pre-registered in the Open Science Framework register with the ID-number Z8EDK.
Peer Review reports
A robustly trained health care workforce is pivotal to forging a resilient health care system [ 1 ], and there is an urgent need to develop innovative methods and emerging technologies for health care workforce education [ 2 ]. Virtual reality technology designs for clinical training have emerged as a promising avenue for increasing the competence of health care professionals, reflecting their potential to provide effective training [ 3 ].
Virtual reality (VR) is a dynamic and diverse field, and can be described as a computer-generated environment that simulates sensory experiences, where user interactions play a role in shaping the course of events within that environment [ 4 ]. When optimally designed, VR gives users the feeling that they are physically within this simulated space, unlocking its potential as a dynamic and immersive learning tool [ 5 ]. The cornerstone of the allure of VR is its capacity for creating artificial settings via sensory deceptions, encapsulated by the term ‘immersion’. Immersion conveys the sensation of being deeply engrossed or enveloped in an alternate world, akin to absorption in a video game. Some VR systems will be more immersive than others, based on the technology used to influence the senses. However, the degree of immersion does not necessarily determine the user’s level of engagement with the application [ 6 ].
A common approach to categorizing VR systems is based on the design of the technology used, allowing them to be classified into: 1) non-immersive desktop systems, where users experience virtual environments through a computer screen, 2) immersive CAVE systems with large projected images and motion trackers to adjust the image to the user, and 3) fully immersive head-mounted display systems that involve users wearing a headset that fully covers their eyes and ears, thus entirely immersing them in the virtual environment [ 7 ]. Advances in VR technology have enabled a wide range of VR experiences. The possibility for health care professionals to repeatedly practice clinical skills with virtual patients in a risk-free environment offers an invaluable learning platform for health care education.
The impact of VR training on health care professionals’ learning has predominantly been researched in terms of the enhancement of technical surgical abilities. This includes refining procedural planning, familiarizing oneself with medical instruments, and practicing psychomotor skills such as dexterity, accuracy, and speed [ 8 , 9 ]. In contrast, the exploration of VR training in fostering non-technical or ‘soft’ skills, such as communication and teamwork, appears to be less prevalent [ 10 ]. A recent systematic review evaluates the outcomes of VR training in non-technical skills across various medical specialties [ 11 ], focusing on vital cognitive abilities (e.g., situation awareness, decision-making) and interprofessional social competencies (e.g., teamwork, conflict resolution, leadership). These skills are pivotal in promoting collaboration among colleagues and ensuring a safe health care environment. At the same time, they are not sufficiently comprehensive for encounters with patients with mental health disorders.
For health care professionals providing care to patients with mental health disorders, acquiring specific skills, knowledge, and empathic attitudes is of utmost importance. Many individuals experiencing mental health challenges may find it difficult to communicate their thoughts and feelings, and it is therefore essential for health care providers to cultivate an environment where patients feel safe and encouraged to share feelings and thoughts. Beyond fostering trust, health care professionals must also possess in-depth knowledge about the nature and treatment of various mental health disorders. Moreover, they must actively practice and internalize the skills necessary to translate their knowledge into clinical practice. While the conventional approach to training mental health clinical skills has been through simulation or role-playing with peers under expert supervision and practicing with real patients, the emergence of VR applications presents a compelling alternative. This technology promises a potentially transformative way to train mental health professionals. Our review identifies specific outcomes in knowledge, skills, and attitudes, covering areas from theoretical understanding to practical application and patient interaction. By focusing on these measurable concepts, which are in line with current healthcare education guidelines [ 12 ], we aim to contribute to the knowledge base and provide a detailed analysis of the complexities in mental health care training. This approach is designed to highlight the VR training’s practical relevance alongside its contribution to academic discourse.
A recent systematic review evaluated the effects of virtual patient (VP) interventions on knowledge, skills, and attitudes in undergraduate psychiatry education [ 13 ]. This review’s scope is limited to assessing VP interventions and does not cover other types of VR training interventions. Furthermore, it adopts a classification of VP different from our review, rendering their findings and conclusions not directly comparable to ours.
To the best of our knowledge, no systematic review has assessed and summarized the effectiveness of VR training interventions for health professionals in the assessment and treatment of mental health disorders. This systematic review addresses the gap by exploring the effectiveness of virtual reality in the training of knowledge, skills, and attitudes health professionals need to master in the assessment and treatment of mental health disorders.
This systematic review follows the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis [ 14 ]. The protocol of the systematic review was registered in the Open Science Framework register with the registration ID Z8EDK.
We included randomized controlled trials, cohort studies, and pretest–posttest studies, which met the following criteria: a) a population of health care professionals or health care professional students, b) assessed the effectiveness of a VR application in assessing and treating mental health disorders, and c) reported changes in knowledge, skills, or attitudes. We excluded studies evaluating VR interventions not designed for training in assessing and treating mental health disorders (e.g., training of surgical skills), studies evaluating VR training from the first-person perspective, studies that used VR interventions for non-educational purposes and studies where VR interventions trained patients with mental health problems (e.g., social skills training). We also excluded studies not published in English or Scandinavian languages.
Search strategy
The literature search reporting was guided by relevant items in PRISMA-S [ 15 ]. In collaboration with a senior academic librarian (IBN), we developed the search strategy for the systematic review. Inspired by the ‘pearl harvesting’ information retrieval approach [ 16 ], we anticipated a broad spectrum of terms related to our interdisciplinary query. Recognizing that various terminologies could encapsulate our central ideas, we harvested an array of terms for each of the four elements ‘health care professionals and health care students’, ‘VR’, ‘training’, and ‘mental health’. The pearl harvesting framework [ 16 ] consists of four steps which we followed with some minor adaptions. Step 1: We searched for and sampled a set of relevant research articles, a book chapter, and literature reviews. Step 2: The librarian scrutinized titles, abstracts, and author keywords, as well as subject headings used in databases, and collected relevant terms. Step 3: The librarian refined the lists of terms. Step 4: The review group, in collaboration with a VR consultant from KildeGruppen AS (a Norwegian media company), validated the refined lists of terms to ensure they included all relevant VR search terms. This process for the element VR resulted in the inclusion of search terms such as ‘3D simulated environment’, ‘second life simulation’, ‘virtual patient’, and ‘virtual world’. We were given a peer review of the search strategy by an academic librarian at Inland Norway University of Applied Sciences.
In June and July 2021, we performed comprehensive searches for publications dating from January 1985 to the present. This period for the inclusion of studies was chosen since VR systems designed for training in health care first emerged in the early 1990s. The searches were carried out in seven databases: MEDLINE and PsycInfo (on Ovid), ERIC and CINAHL (on EBSCOhost), the Cochrane Library, Web of Science Core Collection, and Scopus. Detailed search strategies from each database are available for public access at DataverseNO [ 17 ]. On July 2, 2021, a search in CINAHL yielded 993 hits. However, when attempting to transfer these records to EndNote using the ‘Folder View’—a feature designed for organizing and managing selected records before export—only 982 records were successfully transferred. This discrepancy indicates that 11 records could not be transferred through Folder View, for reasons not specified. The process was repeated twice, consistently yielding the same discrepancy. The missing 11 records pose a risk of failing to capture relevant studies in the initial search. In July 2023, to make sure that we included the latest publications, we updated our initial searches, focusing on entries since January 1, 2021. This ensured that we did not miss any new references recently added to these databases. Due to a lack of access to the Cochrane Library in July 2023, we used EBMR (Evidence Based Medicine Reviews) on the Ovid platform instead, including the databases Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, and Cochrane Clinical Answers. All references were exported to Endnote and duplicates were removed. The number of records from each database can be observed in the PRISMA diagram [ 14 ], Fig. 1 .
PRISMA flow chart of the records and study selection process
Study selection and data collection
Two reviewers (JS, CWS) independently assessed the titles and abstracts of studies retrieved from the literature search based on the eligibility criteria. We employed the Rayyan website for the screening process [ 18 ]. The same reviewers (JS, CWS) assessed the full-text articles selected after the initial screening. Articles meeting the eligibility criteria were incorporated into the review. Any disagreements were resolved through discussion.
Data extracted from the studies by the first author (CWS) and cross-checked by another reviewer (JS) included: authors of the study, publication year, country, study design, participant details (education, setting), interventions (VR system, class label), comparison types, outcomes, and main findings. This data is summarized in Table 1 and Additional file 1 . In the process of reviewing the VR interventions utilized within the included studies, we sought expertise from advisers associated with VRINN, a Norwegian immersive learning cluster, and SIMInnlandet, a center dedicated to simulation in mental health care at Innlandet Hospital Trust. This collaboration ensured a thorough examination and accurate categorization of the VR technologies applied. Furthermore, the classification of the learning designs employed in the VP interventions was conducted under the guidance of an experienced VP scholar at Paracelcus Medical University in Salzburg.
Data analysis
We initially intended to perform a meta-analysis with knowledge, skills, and attitudes as primary outcomes, planning separate analyses for each. However, due to significant heterogeneity observed among the included studies, it was not feasible to carry out a meta-analysis. Consequently, we opted for a narrative synthesis based on these pre-determined outcomes of knowledge, skills, and attitudes. This approach allowed for an analysis of the relationships both within and between the studies. The effect sizes were calculated using a web-based effect size calculator [ 27 ]. We have interpreted effect sizes based on commonly used descriptions for Cohen’s d: small = 0.2, moderate = 0.5, and large = 0.8, and for Cramer’s V: small = 0.10, medium = 0.30, and large = 0.50.
Risk of bias assessment
JS and CWS independently evaluated the risk of bias for all studies using two distinct assessment tools. We used the Cochrane risk of bias tool RoB 2 [ 28 ] to assess the risk of bias in the RCTs. With the RoB 2 tool, the bias was assessed as high, some concerns or low for five domains: randomization process, deviations from the intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result [ 28 ].
We used the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) tool [ 29 ] to assess the risk of bias in the cohort and single-group studies. By using ROBINS-I for the non-randomized trials, the risk of bias was assessed using the categories low, moderate, serious, critical or no information for seven domains: confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of the reported result [ 29 ].
We included eight studies in the review (Fig. 1 ). An overview of the included studies is presented in detail in Table 1 .
Four studies were RCTs [ 19 , 20 , 21 , 22 ], two were single group pretest–posttest studies [ 23 , 26 ], one was a controlled before and after study [ 25 ], and one was a cohort study [ 24 ]. The studies included health professionals from diverse educational backgrounds, including some from mental health and medical services, as well as students in medicine, social work, and nursing. All studies, published from 2009 to 2021, utilized non-immersive VR desktop system interventions featuring various forms of VP designs. Based on an updated classification of VP interventions by Kononowicz et al. [ 30 ] developed from a model proposed by Talbot et al. [ 31 ], we have described the characteristics of the interventions in Table 1 . Four of the studies utilized a virtual standardized patient (VSP) intervention [ 20 , 21 , 22 , 23 ], a conversational agent that simulates clinical presentations for training purposes. Two studies employed an interactive patient scenario (IPS) design [ 25 , 26 ], an approach that primarily uses text-based multimedia, enhanced with images and case histories through text or voice narratives, to simulate clinical scenarios. Lastly, two studies used a virtual patient game (VP game) intervention [ 19 , 24 ]. These interventions feature training scenarios using 3D avatars, specifically designed to improve clinical reasoning and team training skills. It should be noted that the interventions classified as VSPs in this review, being a few years old, do not encompass artificial intelligence (AI) as we interpret it today. However, since the interventions include some kind of algorithm that provides answers to questions, we consider them as conversational agents, and therefore as VSPs. As the eight included studies varied significantly in terms of design, interventions, and outcome measures, we could not incorporate them into a meta-analysis.
The overall risk of bias for the four RCTs was high [ 19 , 20 , 22 ] or of some concern [ 21 ] (Fig. 2 ). They were all assessed as low or of some concern in the domains of randomization. Three studies were assessed with a high risk of bias in one [ 19 , 20 ] or two domains [ 22 ]; one study had a high risk of bias in the domain of selection of the reported result [ 19 ], one in the domain of measurement of outcome [ 20 ], and one in the domains of deviation from the intended interventions and missing outcome data [ 22 ]. One study was not assessed as having a high risk of bias in any domain [ 21 ].
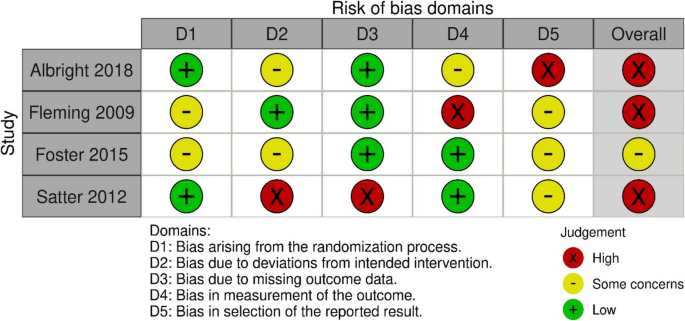
Risk of bias summary: review authors assessments of each risk of bias item in the included RCT studies
For the four non-randomized studies, the overall risk of bias was judged to be moderate [ 26 ] or serious [ 23 , 24 , 25 ] (Fig. 3 ). One study had a serious risk of bias in two domains: confounding and measurement of outcomes [ 23 ]. Two studies had a serious risk of bias in one domain, namely confounding [ 24 , 25 ], while one study was judged not to have a serious risk of bias in any domain [ 26 ].
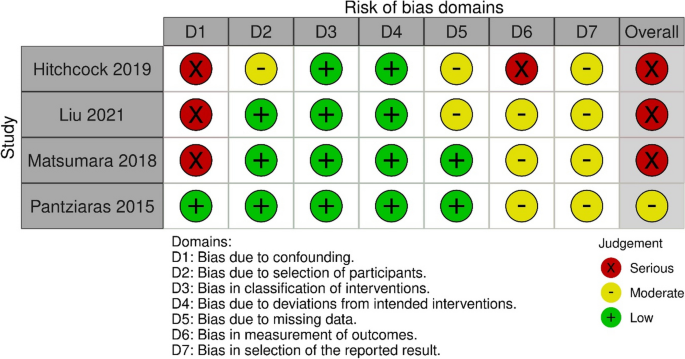
Risk of bias summary: review authors assessments of each risk of bias item in the included non-randomized studies
Three studies investigated the impact of virtual reality training on mental health knowledge [ 24 , 25 , 26 ]. One study with 32 resident psychiatrists in a single group pretest–posttest design assessed the effect of a VR training intervention on knowledge of posttraumatic stress disorder (PTSD) symptomatology, clinical management, and communication skills [ 26 ]. The intervention consisted of an IPS. The assessment of the outcome was conducted using a knowledge test with 11 multiple-choice questions and was administered before and after the intervention. This study reported a significant improvement on the knowledge test after the VR training intervention.
The second study examined the effect of a VR training intervention on knowledge of dementia [ 25 ], employing a controlled before and after design. Seventy-nine medical students in clinical training were divided into two groups, following a traditional learning program. The experimental group received an IPS intervention. The outcome was evaluated with a knowledge test administered before and after the intervention with significantly higher posttest scores in the experimental group than in the control group, with a moderate effects size observed between the groups.
A third study evaluated the effect of a VR training intervention on 299 undergraduate nursing students’ diagnostic recognition of depression and schizophrenia (classified as knowledge) [ 24 ]. In a prospective cohort design, the VR intervention was the only difference in the mental health related educational content provided to the two cohorts, and consisted of a VP game design, developed to simulate training situations with virtual patient case scenarios, including depression and schizophrenia. The outcome was assessed by determining the accuracy of diagnoses made after reviewing case vignettes of depression and schizophrenia. The study found no statistically significant effect of VR training on diagnostic accuracy between the simulation and the non-simulation cohort.
Summary: All three studies assessing the effect of a VR intervention on knowledge were non-randomized studies with different study designs using different outcome measures. Two studies used an IPS design, while one study used a VP game design. Two of the studies found a significant effect of VR training on knowledge. Of these, one study had a moderate overall risk of bias [ 26 ], while the other was assessed as having a serious overall risk of bias [ 25 ]. The third study, which did not find any effect of the virtual reality intervention on knowledge, was assessed to have a serious risk of bias [ 24 ].
Three RCTs assessed the effectiveness of VR training on skills [ 20 , 21 , 22 ]. One of them evaluated the effect of VR training on clinical skills in alcohol screening and intervention [ 20 ]. In this study, 102 health care professionals were randomly allocated to either a group receiving no training or a group receiving a VSP intervention. To evaluate the outcome, three standardized patients rated each participant using a checklist based on clinical criteria. The VSP intervention group demonstrated significantly improved posttest skills in alcohol screening and brief intervention compared to the control group, with moderate and small effect sizes, respectively.
Another RCT, including 67 medical college students, evaluated the effect of VR training on clinical skills by comparing the frequency of questions asked about suicide in a VSP intervention group and a video module group [ 21 ]. The assessment of the outcome was a psychiatric interview with a standardized patient. The primary outcome was the frequency with which the students asked the standardized patient five questions about suicide risk. Minimal to small effect sizes were noted in favor of the VSP intervention, though they did not achieve statistical significance for any outcomes.
One posttest only RCT evaluated the effect of three training programs on skills in detecting and diagnosing major depressive disorder and posttraumatic stress disorder (PTSD) [ 22 ]. The study included 30 family physicians, and featured interventions that consisted of two different VSPs designed to simulate training situations, and one text-based program. A diagnostic form filled in by the participants after the intervention was used to assess the outcome. The results revealed a significant effect on diagnostic accuracy for major depressive disorder for both groups receiving VR training, compared to the text-based program, with large effect sizes observed. For PTSD, the intervention using a fixed avatar significantly improved diagnostic accuracy with a large effect size, whereas the intervention with a choice avatar demonstrated a moderate to large effect size compared to the text-based program.
Summary: Three RCTs assessed the effectiveness of VR training on clinical skills [ 20 , 21 , 22 ], all of which used a VSP design. To evaluate the effect of training, two of the studies utilized standardized patients with checklists. The third study measured the effect on skills using a diagnostic form completed by the participants. Two of the studies found a significant effect on skills [ 20 , 22 ], both were assessed to have a high risk of bias. The third study, which did not find any effect of VR training on skills, had some concern for risk of bias [ 21 ].
Knowledge and skills
One RCT study with 227 health care professionals assessed knowledge and skills as a combined outcome compared to a waitlist control group, using a self-report survey before and after the VR training [ 19 ]. The training intervention was a VP game designed to practice knowledge and skills related to mental health and substance abuse disorders. To assess effect of the training, participants completed a self-report scale measuring perceived knowledge and skills. Changes between presimulation and postsimulation scores were reported only for the within treatment group ( n = 117), where the composite postsimulation score was significantly higher than the presimulation score, with a large effect size observed. The study was judged to have a high risk of bias in the domain of selection of the reported result.
One single group pretest–posttest study with 100 social work and nursing students assessed the effect of VSP training on attitudes towards individuals with substance abuse disorders [ 23 ]. To assess the effect of the training, participants completed an online pretest and posttest survey including questions from a substance abuse attitudes survey. This study found no significant effect of VR training on attitudes and was assessed as having a serious risk of bias.
Perceived competence
The same single group pretest–posttest study also assessed the effect of a VSP training intervention on perceived competence in screening, brief intervention, and referral to treatment in encounters with patients with substance abuse disorders [ 23 ]. A commonly accepted definition of competence is that it comprises integrated components of knowledge, skills, and attitudes that enable the successful execution of a professional task [ 32 ]. To assess the effect of the training, participants completed an online pretest and posttest survey including questions on perceived competence. The study findings demonstrated a significant increase in perceived competence following the VSP intervention. The risk of bias in this study was judged as serious.
This systematic review aimed to investigate the effectiveness of VR training on knowledge, skills, and attitudes that health professionals need to master in the assessment and treatment of mental health disorders. A narrative synthesis of eight included studies identified VR training interventions that varied in design and educational content. Although mixed results emerged, most studies reported improvements in knowledge and skills after VR training.
We found that all interventions utilized some type of VP design, predominantly VSP interventions. Although our review includes a limited number of studies, it is noteworthy that the distribution of interventions contrasts with a literature review on the use of ‘virtual patient’ in health care education from 2015 [ 30 ], which identified IPS as the most frequent intervention. This variation may stem from our review’s focus on the mental health field, suggesting a different intervention need and distribution than that observed in general medical education. A fundamental aspect of mental health education involves training skills needed for interpersonal communication, clinical interviews, and symptom assessment, which makes VSPs particularly appropriate. While VP games may be suitable for clinical reasoning in medical fields, offering the opportunity to perform technical medical procedures in a virtual environment, these designs may present some limitations for skills training in mental health education. Notably, avatars in a VP game do not comprehend natural language and are incapable of engaging in conversations. Therefore, the continued advancement of conversational agents like VSPs is particularly compelling and considered by scholars to hold the greatest potential for clinical skills training in mental health education [ 3 ]. VSPs, equipped with AI dialogue capabilities, are particularly valuable for repetitive practice in key skills such as interviewing and counseling [ 31 ], which are crucial in the assessment and treatment of mental health disorders. VSPs could also be a valuable tool for the implementation of training methods in mental health education, such as deliberate practice, a method that has gained attention in psychotherapy training in recent years [ 33 ] for its effectiveness in refining specific performance areas through consistent repetition [ 34 ]. Within this evolving landscape, AI system-based large language models (LLMs) like ChatGPT stand out as a promising innovation. Developed from extensive datasets that include billions of words from a variety of sources, these models possess the ability to generate and understand text in a manner akin to human interaction [ 35 ]. The integration of LLMs into educational contexts shows promise, yet careful consideration and thorough evaluation of their limitations are essential [ 36 ]. One concern regarding LLMs is the possibility of generating inaccurate information, which represents a challenge in healthcare education where precision is crucial [ 37 ]. Furthermore, the use of generative AI raises ethical questions, notably because of potential biases in the training datasets, including content from books and the internet that may not have been verified, thereby risking the perpetuation of these biases [ 38 ]. Developing strategies to mitigate these challenges is imperative, ensuring LLMs are utilized safely in healthcare education.
All interventions in our review were based on non-immersive desktop VR systems, which is somewhat surprising considering the growing body of literature highlighting the impact of immersive VR technology in education, as exemplified by reviews such as that of Radianti et al. [ 39 ]. Furthermore, given the recent accessibility of affordable, high-quality head-mounted displays, this observation is noteworthy. Research has indicated that immersive learning based on head-mounted displays generally yields better learning outcomes than non-immersive approaches [ 40 ], making it an interesting research area in mental health care training and education. Studies using immersive interventions were excluded in the present review because of methodological concerns, paralleling findings described in a systematic review on immersive VR in education [ 41 ], suggesting the potential early stage of research within this field. Moreover, the integration of immersive VR technology into mental health care education may encounter challenges associated with complex ethical and regulatory frameworks, including data privacy concerns exemplified by the Oculus VR headset-Facebook integration, which could restrict the implementation of this technology in healthcare setting. Prioritizing specific training methodologies for enhancing skills may also affect the utilization of immersive VR in mental health education. For example, integrating interactive VSPs into a fully immersive VR environment remains a costly endeavor, potentially limiting the widespread adoption of immersive VR in mental health care. Meanwhile, the use of 360-degree videos in immersive VR environments for training purposes [ 42 ] can be realized with a significantly lower budget. Immersive VR offers promising opportunities for innovative training, but realizing its full potential in mental health care education requires broader research validation and the resolution of existing obstacles.
This review bears some resemblance to the systematic review by Jensen et al. on virtual patients in undergraduate psychiatry education [ 13 ] from 2024, which found that virtual patients improved learning outcomes compared to traditional methods. However, these authors’ expansion of the commonly used definition of virtual patient makes their results difficult to compare with the findings in the present review. A recognized challenge in understanding VR application in health care training arises from the literature on VR training for health care personnel, where ‘virtual patient’ is a term broadly used to describe a diverse range of VR interventions, which vary significantly in technology and educational design [ 3 , 30 ]. For instance, reviews might group different interventions using various VR systems and designs under a single label (virtual patient), or primary studies may use misleading or inadequately defined classifications for the virtual patient interventions evaluated. Clarifying the similarities and differences among these interventions is vital to inform development and enhance communication and understanding in educational contexts [ 43 ].
Strengths and limitations
To the best of our knowledge, this is the first systematic review to evaluate the effectiveness of VR training on knowledge, skills, and attitudes in health care professionals and students in assessing and treating mental health disorders. This review therefore provides valuable insights into the use of VR technology in training and education for mental health care. Another strength of this review is the comprehensive search strategy developed by a senior academic librarian at Inland Norway University of Applied Sciences (HINN) and the authors in collaboration with an adviser from KildeGruppen AS (a Norwegian media company). The search strategy was peer-reviewed by an academic librarian at HINN. Advisers from VRINN (an immersive learning cluster in Norway) and SIMInnlandet (a center for simulation in mental health care at Innlandet Hospital Trust) provided assistance in reviewing the VR systems of the studies, while the classification of the learning designs was conducted under the guidance of a VP scholar. This systematic review relies on an established and recognized classification of VR interventions for training health care personnel and may enhance understanding of the effectiveness of VR interventions designed for the training of mental health care personnel.
This review has some limitations. As we aimed to measure the effect of the VR intervention alone and not the effect of a blended training design, the selection of included studies was limited. Studies not covered in this review might have offered different insights. Given the understanding that blended learning designs, where technology is combined with other forms of learning, have significant positive effects on learning outcomes [ 44 ], we were unable to evaluate interventions that may be more effective in clinical settings. Further, by limiting the outcomes to knowledge, skills, and attitudes, we might have missed insights into other outcomes that are pivotal to competence acquisition.
Limitations in many of the included studies necessitate cautious interpretation of the review’s findings. Small sample sizes and weak designs in several studies, coupled with the use of non-validated outcome measures in some studies, diminish the robustness of the findings. Furthermore, the risk of bias assessment in this review indicates a predominantly high or serious risk of bias across most of the studies, regardless of their design. In addition, the heterogeneity of the studies in terms of study design, interventions, and outcome measures prevented us from conducting a meta-analysis.
Further research
Future research on the effectiveness of VR training for specific learning outcomes in assessing and treating mental health disorders should encompass more rigorous experimental studies with larger sample sizes. These studies should include verifiable descriptions of the VR interventions and employ validated tools to measure outcomes. Moreover, considering that much professional learning involves interactive and reflective practice, research on VR training would probably be enhanced by developing more in-depth study designs that evaluate not only the immediate learning outcomes of VR training but also the broader learning processes associated with it. Future research should also concentrate on utilizing immersive VR training applications, while additionally exploring the integration of large language models to augment interactive learning in mental health care. Finally, this review underscores the necessity in health education research involving VR to communicate research findings using agreed terms and classifications, with the aim of providing a clearer and more comprehensive understanding of the research.
This systematic review investigated the effect of VR training interventions on knowledge, skills, and attitudes in the assessment and treatment of mental health disorders. The results suggest that VR training interventions can promote knowledge and skills acquisition. Further studies are needed to evaluate VR training interventions as a learning tool for mental health care providers. This review emphasizes the necessity to improve future study designs. Additionally, intervention studies of immersive VR applications are lacking in current research and should be a future area of focus.
Availability of data and materials
Detailed search strategies from each database is available in the DataverseNO repository, https://doi.org/10.18710/TI1E0O .
Abbreviations
Virtual Reality
Cave Automatic Virtual Environment
Randomized Controlled Trial
Non-Randomized study
Virtual Standardized Patient
Interactive Patient Scenario
Virtual Patient
Post Traumatic Stress Disorder
Standardized Patient
Artificial intelligence
Inland Norway University of Applied Sciences
Doctor of Philosophy
Frenk J, Chen L, Bhutta ZA, Cohen J, Crisp N, Evans T, et al. Health professionals for a new century: transforming education to strengthen health systems in an interdependent world. Lancet. 2010;376(9756):1923–58.
Article Google Scholar
World Health Organization. eLearning for undergraduate health professional education: a systematic review informing a radical transformation of health workforce development. Geneva: World Health Organization; 2015.
Google Scholar
Talbot T, Rizzo AS. Virtual human standardized patients for clinical training. In: Rizzo AS, Bouchard S, editors. Virtual reality for psychological and neurocognitive interventions. New York: Springer; 2019. p. 387–405.
Chapter Google Scholar
Merriam-Webster dictionary. Springfield: Merriam-Webster Incorporated; c2024. Virtual reality. Available from: https://www.merriam-webster.com/dictionary/virtual%20reality . [cited 2024 Mar 24].
Winn W. A conceptual basis for educational applications of virtual reality. Technical Publication R-93–9. Seattle: Human Interface Technology Laboratory, University of Washington; 1993.
Bouchard S, Rizzo AS. Applications of virtual reality in clinical psychology and clinical cognitive neuroscience–an introduction. In: Rizzo AS, Bouchard S, editors. Virtual reality for psychological and neurocognitive interventions. New York: Springer; 2019. p. 1–13.
Waller D, Hodgson E. Sensory contributions to spatial knowledge of real and virtual environments. In: Steinicke F, Visell Y, Campos J, Lécuyer A, editors. Human walking in virtual environments: perception, technology, and applications. New York: Springer New York; 2013. p. 3–26. https://doi.org/10.1007/978-1-4419-8432-6_1 .
Choudhury N, Gélinas-Phaneuf N, Delorme S, Del Maestro R. Fundamentals of neurosurgery: virtual reality tasks for training and evaluation of technical skills. World Neurosurg. 2013;80(5):e9–19. https://doi.org/10.1016/j.wneu.2012.08.022 .
Gallagher AG, Ritter EM, Champion H, Higgins G, Fried MP, Moses G, et al. Virtual reality simulation for the operating room: proficiency-based training as a paradigm shift in surgical skills training. Ann Surg. 2005;241(2):364–72. https://doi.org/10.1097/01.sla.0000151982.85062.80 .
Kyaw BM, Saxena N, Posadzki P, Vseteckova J, Nikolaou CK, George PP, et al. Virtual reality for health professions education: systematic review and meta-analysis by the Digital Health Education Collaboration. J Med Internet Res. 2019;21(1):e12959. https://doi.org/10.2196/12959 .
Bracq M-S, Michinov E, Jannin P. Virtual reality simulation in nontechnical skills training for healthcare professionals: a systematic review. Simul Healthc. 2019;14(3):188–94. https://doi.org/10.1097/sih.0000000000000347 .
World Health Organization. Transforming and scaling up health professionals’ education and training: World Health Organization guidelines 2013. Geneva: World Health Organization; 2013. Available from: https://www.who.int/publications/i/item/transforming-and-scaling-up-health-professionals%E2%80%99-education-and-training . Accessed 15 Jan 2024.
Jensen RAA, Musaeus P, Pedersen K. Virtual patients in undergraduate psychiatry education: a systematic review and synthesis. Adv Health Sci Educ. 2024;29(1):329–47. https://doi.org/10.1007/s10459-023-10247-6 .
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):89. https://doi.org/10.1186/s13643-021-01626-4 .
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10(1):39. https://doi.org/10.1186/s13643-020-01542-z .
Sandieson RW, Kirkpatrick LC, Sandieson RM, Zimmerman W. Harnessing the power of education research databases with the pearl-harvesting methodological framework for information retrieval. J Spec Educ. 2010;44(3):161–75. https://doi.org/10.1177/0022466909349144 .
Steen CW, Söderström K, Stensrud B, Nylund IB, Siqveland J. Replication data for: the effectiveness of virtual reality training on knowledge, skills and attitudes of health care professionals and students in assessing and treating mental health disorders: a systematic review. In: Inland Norway University of Applied S, editor. V1 ed: DataverseNO; 2024. https://doi.org/10.18710/TI1E0O .
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210. https://doi.org/10.1186/s13643-016-0384-4 .
Albright G, Bryan C, Adam C, McMillan J, Shockley K. Using virtual patient simulations to prepare primary health care professionals to conduct substance use and mental health screening and brief intervention. J Am Psych Nurses Assoc. 2018;24(3):247–59. https://doi.org/10.1177/1078390317719321 .
Fleming M, Olsen D, Stathes H, Boteler L, Grossberg P, Pfeifer J, et al. Virtual reality skills training for health care professionals in alcohol screening and brief intervention. J Am Board Fam Med. 2009;22(4):387–98. https://doi.org/10.3122/jabfm.2009.04.080208 .
Foster A, Chaudhary N, Murphy J, Lok B, Waller J, Buckley PF. The use of simulation to teach suicide risk assessment to health profession trainees—rationale, methodology, and a proof of concept demonstration with a virtual patient. Acad Psych. 2015;39:620–9. https://doi.org/10.1007/s40596-014-0185-9 .
Satter R. Diagnosing mental health disorders in primary care: evaluation of a new training tool [dissertation]. Tempe (AZ): Arizona State University; 2012.
Hitchcock LI, King DM, Johnson K, Cohen H, McPherson TL. Learning outcomes for adolescent SBIRT simulation training in social work and nursing education. J Soc Work Pract Addict. 2019;19(1/2):47–56. https://doi.org/10.1080/1533256X.2019.1591781 .
Liu W. Virtual simulation in undergraduate nursing education: effects on students’ correct recognition of and causative beliefs about mental disorders. Comput Inform Nurs. 2021;39(11):616–26. https://doi.org/10.1097/CIN.0000000000000745 .
Matsumura Y, Shinno H, Mori T, Nakamura Y. Simulating clinical psychiatry for medical students: a comprehensive clinic simulator with virtual patients and an electronic medical record system. Acad Psych. 2018;42(5):613–21. https://doi.org/10.1007/s40596-017-0860-8 .
Pantziaras I, Fors U, Ekblad S. Training with virtual patients in transcultural psychiatry: Do the learners actually learn? J Med Internet Res. 2015;17(2):e46. https://doi.org/10.2196/jmir.3497 .
Wilson DB. Practical meta-analysis effect size calculator [Online calculator]. n.d. https://campbellcollaboration.org/research-resources/effect-size-calculator.html . Accessed 08 March 2024.
Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. 2019;366:l4898.
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. 2016;355:i4919. https://doi.org/10.1136/bmj.i4919 .
Kononowicz AA, Zary N, Edelbring S, Corral J, Hege I. Virtual patients - what are we talking about? A framework to classify the meanings of the term in healthcare education. BMC Med Educ. 2015;15(1):11. https://doi.org/10.1186/s12909-015-0296-3 .
Talbot TB, Sagae K, John B, Rizzo AA. Sorting out the virtual patient: how to exploit artificial intelligence, game technology and sound educational practices to create engaging role-playing simulations. Int J Gaming Comput-Mediat Simul. 2012;4(3):1–19.
Baartman LKJ, de Bruijn E. Integrating knowledge, skills and attitudes: conceptualising learning processes towards vocational competence. Educ Res Rev. 2011;6(2):125–34. https://doi.org/10.1016/j.edurev.2011.03.001 .
Mahon D. A scoping review of deliberate practice in the acquisition of therapeutic skills and practices. Couns Psychother Res. 2023;23(4):965–81. https://doi.org/10.1002/capr.12601 .
Ericsson KA, Lehmann AC. Expert and exceptional performance: evidence of maximal adaptation to task constraints. Annu Rev Psychol. 1996;47(1):273–305.
Roumeliotis KI, Tselikas ND. ChatGPT and Open-AI models: a preliminary review. Future Internet. 2023;15(6):192. https://doi.org/10.3390/fi15060192 .
Kasneci E, Sessler K, Küchemann S, Bannert M, Dementieva D, Fischer F, et al. ChatGPT for good? On opportunities and challenges of large language models for education. Learn Individ Differ. 2023;103:102274. https://doi.org/10.1016/j.lindif.2023.102274 .
Thirunavukarasu AJ, Ting DSJ, Elangovan K, Gutierrez L, Tan TF, Ting DSW. Large language models in medicine. Nat Med. 2023;29(8):1930–40. https://doi.org/10.1038/s41591-023-02448-8 .
Touvron H, Lavril T, Gautier I, Martinet X, Marie-Anne L, Lacroix T, et al. LLaMA: open and efficient foundation language models. arXivorg. 2023;2302.13971. https://doi.org/10.48550/arxiv.2302.13971 .
Radianti J, Majchrzak TA, Fromm J, Wohlgenannt I. A systematic review of immersive virtual reality applications for higher education: design elements, lessons learned, and research agenda. Comput Educ. 2020;147:103778. https://doi.org/10.1016/j.compedu.2019.103778 .
Wu B, Yu X, Gu X. Effectiveness of immersive virtual reality using head-mounted displays on learning performance: a meta-analysis. Br J Educ Technol. 2020;51(6):1991–2005. https://doi.org/10.1111/bjet.13023 .
Di Natale AF, Repetto C, Riva G, Villani D. Immersive virtual reality in K-12 and higher education: a 10-year systematic review of empirical research. Br J Educ Technol. 2020;51(6):2006–33. https://doi.org/10.1111/bjet.13030 .
Haugan S, Kværnø E, Sandaker J, Hustad JL, Thordarson GO. Playful learning with VR-SIMI model: the use of 360-video as a learning tool for nursing students in a psychiatric simulation setting. In: Akselbo I, Aune I, editors. How can we use simulation to improve competencies in nursing? Cham: Springer International Publishing; 2023. p. 103–16. https://doi.org/10.1007/978-3-031-10399-5_9 .
Huwendiek S, De leng BA, Zary N, Fischer MR, Ruiz JG, Ellaway R. Towards a typology of virtual patients. Med Teach. 2009;31(8):743–8. https://doi.org/10.1080/01421590903124708 .
Ødegaard NB, Myrhaug HT, Dahl-Michelsen T, Røe Y. Digital learning designs in physiotherapy education: a systematic review and meta-analysis. BMC Med Educ. 2021;21(1):48. https://doi.org/10.1186/s12909-020-02483-w .
Download references
Acknowledgements
The authors thank Mole Meyer, adviser at SIMInnlandet, Innlandet Hospital Trust, and Keith Mellingen, manager at VRINN, for their assistance with the categorization and classification of VR interventions, and Associate Professor Inga Hege at the Paracelcus Medical University in Salzburg for valuable contributions to the final classification of the interventions. The authors would also like to thank Håvard Røste from the media company KildeGruppen AS, for assistance with the search strategy; Academic Librarian Elin Opheim at the Inland Norway University of Applied Sciences for valuable peer review of the search strategy; and the Library at the Inland Norway University of Applied Sciences for their support. Additionally, we acknowledge the assistance provided by OpenAI’s ChatGPT for support with translations and language refinement.
Open access funding provided by Inland Norway University Of Applied Sciences The study forms a part of a collaborative PhD project funded by South-Eastern Norway Regional Health Authority through Innlandet Hospital Trust and the Inland University of Applied Sciences.
Author information
Authors and affiliations.
Mental Health Department, Innlandet Hospital Trust, P.B 104, Brumunddal, 2381, Norway
Cathrine W. Steen & Kerstin Söderström
Inland Norway University of Applied Sciences, P.B. 400, Elverum, 2418, Norway
Cathrine W. Steen, Kerstin Söderström & Inger Beate Nylund
Norwegian National Advisory Unit On Concurrent Substance Abuse and Mental Health Disorders, Innlandet Hospital Trust, P.B 104, Brumunddal, 2381, Norway
Bjørn Stensrud
Akershus University Hospital, P.B 1000, Lørenskog, 1478, Norway
Johan Siqveland
National Centre for Suicide Research and Prevention, Oslo, 0372, Norway
You can also search for this author in PubMed Google Scholar
Contributions
CWS, KS, BS, and JS collaboratively designed the study. CWS and JS collected and analysed the data and were primarily responsible for writing the manuscript text. All authors contributed to the development of the search strategy. IBN conducted the literature searches and authored the chapter on the search strategy in the manuscript. All authors reviewed, gave feedback, and granted their final approval of the manuscript.
Corresponding author
Correspondence to Cathrine W. Steen .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Not applicable .
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: table 2..
Effects of VR training in the included studies: Randomized controlled trials (RCTs) and non-randomized studies (NRSs).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Steen, C.W., Söderström, K., Stensrud, B. et al. The effectiveness of virtual reality training on knowledge, skills and attitudes of health care professionals and students in assessing and treating mental health disorders: a systematic review. BMC Med Educ 24 , 480 (2024). https://doi.org/10.1186/s12909-024-05423-0
Download citation
Received : 19 January 2024
Accepted : 12 April 2024
Published : 01 May 2024
DOI : https://doi.org/10.1186/s12909-024-05423-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Health care professionals
- Health care students
- Virtual reality
- Mental health
- Clinical skills
- Systematic review
BMC Medical Education
ISSN: 1472-6920
- Submission enquiries: [email protected]
- General enquiries: [email protected]
- Systematic Review
- Open access
- Published: 10 May 2024
Network meta-analysis of the intervention effects of different exercise measures on Sarcopenia in cancer patients
- Rui Liu 1 na1 ,
- XY Gao 1 na1 &
- Li Wang 1
BMC Public Health volume 24 , Article number: 1281 ( 2024 ) Cite this article
20 Accesses
2 Altmetric
Metrics details
This study aims to investigate the impact of four exercise modes (aerobic exercise, resistance exercise, aerobic combined with resistance multimodal exercise, and stretching) on the physical performance of cancer patients.
Randomized controlled trials (RCTs) were exclusively collected from PubMed, EMBASE, Web of Science, and The Cochrane Library, with a search deadline of April 30, 2023. Different exercise interventions on the physical performance of cancer patients were studied, and the Cochrane risk of bias assessment tool was employed to evaluate the quality of the included literature. Data analysis was conducted using STATA 15.1 software.
This study included ten randomized controlled trials with a combined sample size of 503 participants. Network meta-analysis results revealed that aerobic combined with resistance multimodal exercise could reduce fat mass in cancer patients (SUCRA: 92.3%). Resistance exercise could improve lean mass in cancer patients (SUCRA: 95.7%). Furthermore, resistance exercise could enhance leg extension functionality in cancer patients with sarcopenia (SUCRA: 83.0%).
This study suggests that resistance exercise may be more beneficial for cancer-related sarcopenia.In clinical practice, exercise interventions should be tailored to the individual patients’ circumstances.
Registration number
This review was registered on INPLASY2023110025; DOI number is https://doi.org/10.37766/inplasy2023.11.0025 .
Peer Review reports
Sarcopenia is a systemic syndrome characterized primarily by the weakening or loss of muscle mass and function [ 1 ]. Its pathogenesis is associated with inflammatory responses, hormone levels, and insulin resistance within the body, subsequently leading to disruptions in protein synthesis and the onset of sarcopenia [ 2 , 3 ]. Based on differing mechanisms of occurrence, sarcopenia is classified into primary (i.e., degenerative) and secondary types, with the former being more prevalent among older people. At the same time, the latter is commonly observed in cases of chronic wasting. Tumor patients constitute a prominent demographic affected by secondary muscle atrophy, with an incidence rate of approximately 38.6%. This condition is closely linked to postoperative complications, chemotherapy toxicity reactions, and overall survival rates [ 4 ]. Statistics reveal that for tumor patients, a 25% reduction in body mass corresponds to a loss of 75% of skeletal muscle myocardium protein. Even more concerning, sarcopenia is responsible for approximately one-fifth of all tumor-related fatalities [ 5 , 6 , 7 ].
So far, the existing drug treatments have not been entirely satisfactory, and the accompanying side effects and high medical costs have restricted the clinical application of such therapies. Therefore, the search for a cost-effective, low-side-effect, non-pharmaceutical alternative has become increasingly important. Several studies suggest that exercise interventions can effectively delay the onset of sarcopenia in cancer patients, improve their quality of life, and extend their survival periods, making them the most efficient measure for treating sarcopenia [ 8 , 9 , 10 , 11 ]. However, due to the diverse nature and distinct characteristics of exercise intervention measures, there is currently no unanimous consensus on which exercise intervention is the most effective.
Network meta-analysis is an advanced, evidence-based technique that enables direct or indirect comparisons of the effects of multiple interventions on a particular disease and ranks their relative efficacy for improvement [ 12 ]. In this study, we aim to evaluate the impact of various exercise interventions on the physical performance of cancer patients with sarcopenia. By comparing these exercise interventions, we aim to provide valuable insights for healthcare professionals and patients.
Materials and methods
Search strategy.
Through a computer search encompassing four electronic databases, namely PubMed, EMBASE, Web of Science, and The Cochrane Library, covering the period from their inception to April 2023, the retrieval strategy was structured by the PICOS framework: (P) Population: cancer patients; (I) Intervention: exercise; (C) Control Group: a control group receiving standard care or stretching exercises exclusively; (O) Outcomes: lean mass, fat mass, and leg extension test (leg extension); (S) Study Type: randomized controlled trials. Taking PubMed as an example, the detailed search strategy is shown in Table 1 .
Inclusion criteria
(1) The experimental group and various exercise training methods as interventions for tumor patients. (2) The control group comprises patients receiving exercise interventions distinct from those in the experimental group or receiving routine care. (3) A clinical randomized controlled trial. (4) Outcomes encompass at least one of the following indicators: lean mass, fat mass, and leg extension test.
Exclusion criteria
(1) Literature lacking complete or accessible data; (2) non-randomized controlled trials, including quasi-randomized controlled trials and animal studies; (3) conference abstracts, case reports, and communications; (4) outcome measures that cannot be converted or aggregated; (5) literature not in the English language.
Study selection
Literature screening and exclusion were carried out using EndNote 20, a literature management software. Initially, two researchers independently conducted literature screening using the inclusion and exclusion criteria. Duplicate titles, non-randomized controlled trial studies, retrospective papers, conference papers, protocols, and correspondence were eliminated. Subsequently, the abstracts of the remaining literature were reviewed to determine their inclusion or exclusion. Any remaining literature was then subjected to a cross-check and comparison by both researchers. If the assessments were identical, the literature was included; in cases of disagreement, the third investigator facilitated discussion and resolution.
Data extraction
Seven predetermined data elements were chosen: (1) author’s name, (2) year of publication, (3) country, (4) study duration, (5) sample size, (6) mean age, and (7) outcome measures for exercise intervention.
Literature quality evaluation
The assessment of literature quality was conducted independently by two researchers, with a subsequent thorough review of the results. In cases of disagreement, a third party was consulted for evaluation. The evaluation of the risk of bias was carried out using the Cochrane 5.1.0 Risk Assessment Tool (ROB), considering seven key domains: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and experimenters; (4) investigator blinding; (5) completeness of outcome data; (6) selective reporting of results; and (7) other potential sources of bias. Each domain was categorized as having “high risk of bias,” “low risk of bias,” or “unclear.” Trials were then stratified into three levels of risk of bias based on the number of components with high ROB: high risk (5 or more), moderate risk (3 to 4), and low risk (2 or fewer) [ 13 ]. The results were ultimately presented in charts and tables.
Data analysis
Employing various exercise interventions, all outcome measures were treated as continuous variables, and the presentation and analysis included mean, standard deviation (SD) and mean-variance (MD, representing the absolute difference between the treatment and control groups and calculated using the same sample size) or standardized mean difference (SMD, indicating the mean of the groups divided by the standard deviation between subjects, suitable for data analysis in trials of varying sizes), along with 95% confidence interval (CI) [ 14 ]. Given the heterogeneity among studies, we opted for a random effects model for the analysis rather than a fixed effects model [ 15 ].
The Stata software, version 15.1, was employed for the NMA summary and analysis, utilizing the Bayesian Markov Chain Monte Carlo algorithm. To assess consistency, node splitting was applied, with a threshold of a p -value greater than 0.05 indicating the use of the consistency model; otherwise, the inconsistency model was employed [ 16 ]. Stata generated the network graph, where each node represents an independent intervention, and the connecting lines between nodes signify direct comparisons between interventions. The size of each node and the width of the lines are proportional to the number of trials conducted [ 17 ].
The greater the SUCRA value, the higher the likelihood of being the most effective intervention [ 17 ]. When determining the ranking of SUCRA, in addition to comparing the area under the cumulative ranking probability curve for different exercise interventions (surface under the cumulative ranking, SUCRA), it is essential to interpret the clinical significance of these interventions carefully. Furthermore, to address the possibility of publication bias in NMA, we constructed a network funnel plot and visually assessed its symmetry to detect the presence of small-sample effects [ 17 ].
Literature screening process
Following a thorough search across multiple databases, an initial screening identified 1,541 relevant articles. A manual search yielded nine more articles, and with the assistance of Endnote software, 339 duplicate entries were removed. Subsequent examinations of titles and abstracts resulted in excluding 1,106 articles deemed irrelevant. This process left 73 articles for full-text evaluation, eventually culminating in the inclusion of 10 pieces in the meta-analysis (Fig. 1 ).
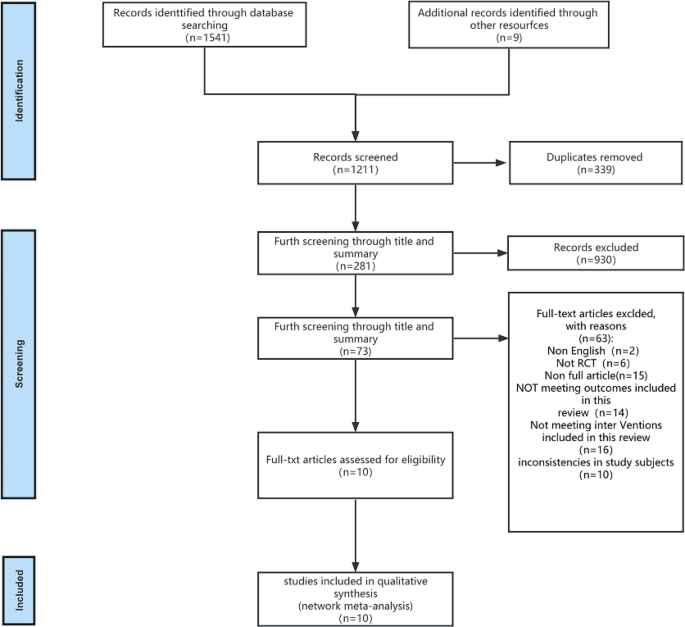
Flow chart of literature screening
Out of the ten studies included [ 11 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 ], 2 of them [ 11 , 22 ] were categorized as low risk, while 8 [ 11 , 20 , 22 , 25 ] were classified as medium risk. All the included studies referred to random allocation, with 4 [ 11 , 18 , 20 , 21 ] explicitly mentioning the use of computerized grouping, while the remaining literature indicated randomization. In three [ 11 , 21 , 22 ] of the studies, a specific concealed allocation scheme was proposed. Due to the nature of the exercise intervention, achieving blinding for both the subjects and assessors was challenging, as patients and their families needed to provide informed consent before participating in the experiments. All the studies described the rate and reasons for loss to follow-up, and the outcome measures were comprehensive. The baseline characteristics of the intervention groups were reasonably balanced, with no signs of selective reporting. For detailed information, please refer to Fig. 2 A and B.
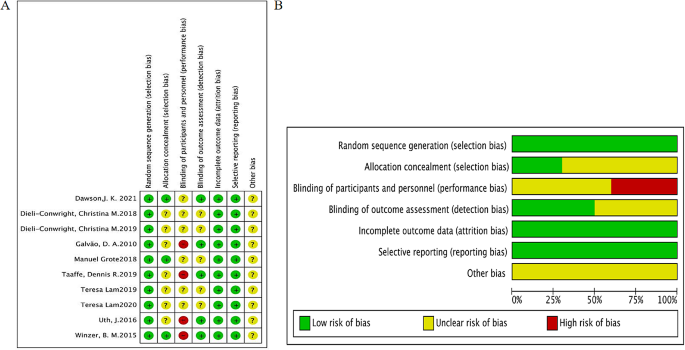
( A ) Risk of bias plot for literature quality assessment; ( B ) Scale plot of risk of bias for literature quality evaluation
Basic characteristics of the included literature
This study incorporated ten randomized controlled trials (RCTs), encompassing 503 patients diagnosed with malignancies, with 310 male and 193 female participants. The selected studies contained four distinct types of exercises models: 5 RCTs introduced resistance exercises in the experimental group [ 11 , 19 , 21 , 25 ], 5 RCTs implemented aerobic combined with resistance multimodal exercise [ 16 , 18 , 22 , 23 , 24 ], and 2 RCTs within the control group utilized stretching exercises [ 11 , 22 ], while the remaining control groups involved routine activities without any additional interventions. All ten of the included studies reported fat mass as an outcome measure [ 11 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 ], lean mass as an outcome measure [ 11 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 ], and six studies measured leg extension as an outcome indicator [ 11 , 18 , 19 , 20 , 21 , 22 ]. These studies were distributed across regions, with three originating from the Americas, two from Europe, and six from Oceania. Detailed characteristics of the included studies are presented in Table 2 .
Mesh Meta-analysis results
The full figures are detailed in Figs. 3 A and 4 A, and 5 A.
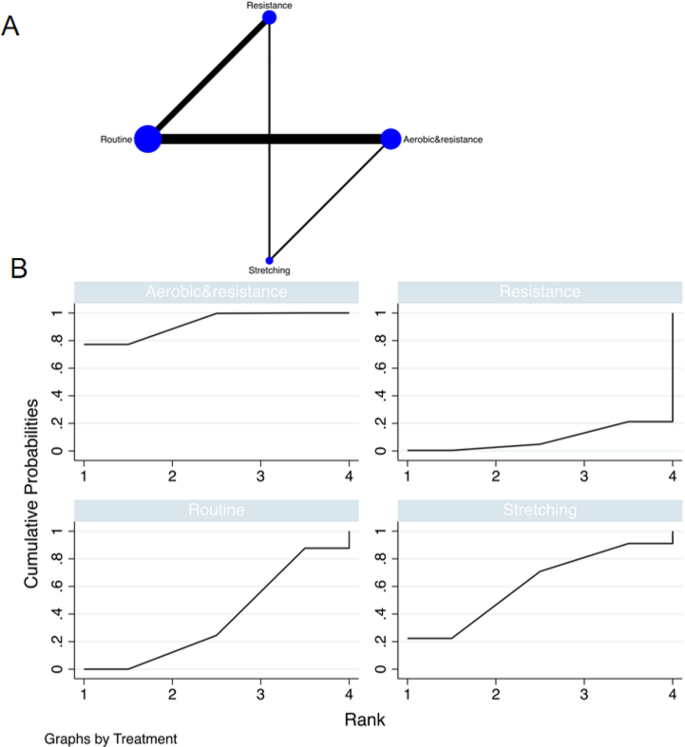
The NMA plot of fat mass; B Fat mass SUCRA Fig
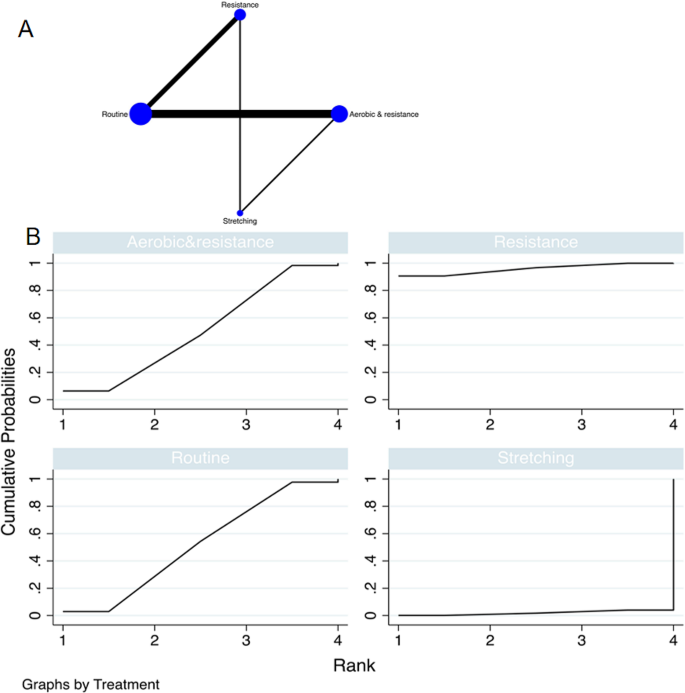
The NMA plot of lean mass; B Lean mass SUCRA Fig
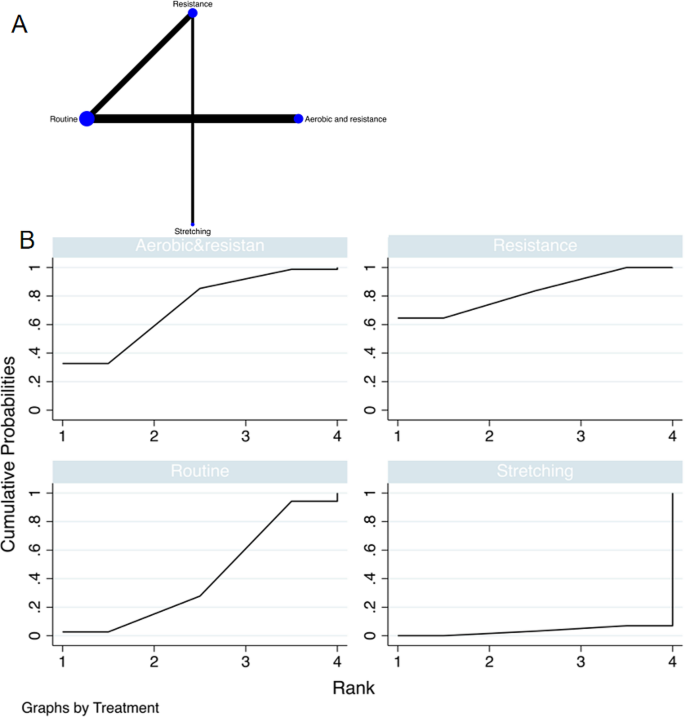
The NMA plot of leg extension; B Leg extension SUCRA Fig
A total of 10 randomized controlled trials (RCTs) was included, and multiple exercise interventions, primarily focusing on routine exercise, formed a complex network structure comprising two interconnected loops. The node-splitting test assessed the consistency between indirect and direct outcomes from all studies. The results of the network meta-analysis indicated that stretching [MD = -4.02, 95% CI = (10.42, 2.38)] and aerobic combined with resistance multi modal exercise [MD = -6.09, 95% CI = (-10.84, -1.34)] exhibited superior performance compared to conventional practice in comparison with the control group. The top-ranking intervention, based on the best-ranking results, was aerobic combined with resistance multimodal exercise, demonstrating the most effective reduction in tumor fat mass (SUCRA: 92.3%, as depicted in Fig. 3 B). Further comparisons between different exercise interventions are presented in Table 3 .
A total of 10 randomized controlled trials (RCTs) were included in the analysis. Multiple exercise interventions, predominantly centered around routine exercises, formed a complex network structure, resulting in two interconnected closed loops. Consistency between indirect and direct outcome indicators from all studies was evaluated. The network meta-analysis revealed that resistance exercise [MD = 14.00, 95% CI = (4.41, 23.60)] outperformed conventional exercise compared to the control group. The highest-ranking results demonstrated that resistance exercise was the most effective in increasing lean mass in tumor patients with sarcopenia (SUCRA: 95.7%, as depicted in Fig. 4 B). Detailed comparisons between the various exercise interventions can be found in Table 4 .
Leg extension
A total of 6 randomized controlled trials (RCTs) were incorporated into the study, and various exercise interventions were primarily based on routine exercises, resulting in a complex network structure with a closed loop. To assess the consistency between indirect and direct indicators from all studies, the node-splitting test was employed. The network meta-analysis indicated that resistance exercise [MD = 68.27, 95% CI = (26.25, 110.30)] and aerobic combined with resistance multimodal exercise [MD = 57.97, 95% CI = (5.23, 121.16)] outperformed the control group. The top-ranking results highlighted that resistance exercise was the most effective in enhancing the Leg extension function in cancer patients with sarcopenia (SUCRA: 83.0%, as displayed in Fig. 5 B). Detailed comparisons between the diverse exercise interventions can be found in Table 5 .
Publication bias trial
A publication bias funnel plot was generated for the intervention effect indicators of various exercise modalities on sarcopenia in cancer patients (Fig. 6 A, B and C), and no notable publication bias was observed upon visual inspection of the funnel plot.
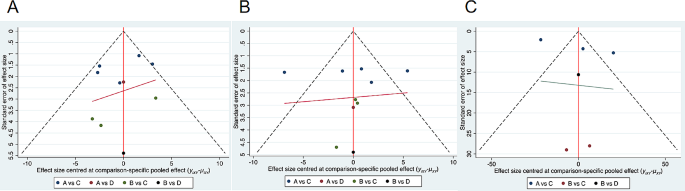
( A ) The funnel plot of fat mass bias; ( B ) The funnel plot of lean mass bias; ( C ) The funnel plot of leg extension bias
A total of 10 randomized controlled trials (RCTs) were included, comprising a combined total of 503 cancer patients. Through the comparative analysis of the effects of various exercise interventions, including aerobic exercise, resistance exercise, aerobic resistance combined with multimodal training, and stretching exercise in cancer patients, our study demonstrates that differences in exercise interventions highlight the variability in their capacity to enhance sarcopenia and function in patients with sarcopenia-related tumours. Cancer patients must select targeted exercise interventions carefully. Resistance exercise exhibits the most favourable impact on improving lean mass and leg extension, while aerobic combined with resistance multimodal exercise is most effective in reducing fat mass in tumor patients with sarcopenia. Upon a comprehensive assessment, we assert that resistance exercise is competitive in ameliorating sarcopenia in cancer patients.
While ‘sarcopenia’ is defined as the loss of muscle mass or function, it is increasingly acknowledged that sarcopenia can also coexist with obesity. Excessive fat can obscure the absence of skeletal muscle mass. Thus, the measurement of fat mass is an essential component in the diagnosis of sarcopenia. Early detection, diagnosis, and exercise intervention are pivotal for prognosis improvement and sarcopenia treatment [ 27 , 28 , 29 ]. For cancer patients, engaging in 150 min of moderate or 75 min of high-intensity exercise per week is deemed safe. It can reduce abnormal lipid accumulation in skeletal muscle, enhance glucose circulation metabolism, and decrease fat mass [ 18 , 30 , 31 , 32 ]. Regular fat mass measurements enable dynamic patient monitoring and adjustment of the regimen to achieve the optimal treatment outcome. The results demonstrate that combined resistance exercise is effective in reducing fat mass, which is statistically significant when compared to the control group. In contrast to resistance exercise, aerobic combined with resistance exercise offers more diversity, not only increasing exercise engagement but also boosting participants’ motivation, thus yielding more pronounced exercise effects.
Within the domain of studies about “sarcopenia,” assessments of lean mass (also known as lean weight or fat-free mass) are customarily incorporated, as they represent one of the paramount indicators of sarcopenia [ 31 ]. The reduction in muscle mass observed in sarcopenia often stems from a combination of muscle atrophy and cell death. At the molecular level, previous research has pointed to the association between sarcopenia and mitochondrial dysfunction, along with alterations in protein synthesis and degradation [ 32 , 33 , 34 ]. The measurement of lean mass allows us to furnish concrete evidence regarding the effectiveness of exercise interventions on skeletal muscle. Exercise interventions have the potential to stimulate the production of crucial regulatory components within skeletal muscle mitochondria, suppress ubiquitin-proteasome system (UPS) activity, enhance the expression of autophagy-related genes, improve mitochondrial oxidative capacity, and increase muscle blood flow [ 35 , 36 , 37 ]. Our findings indicate that resistance exercise yields significant intervention effects on lean mass in patients with tumor-related sarcopenia. This can be attributed to the stimulation of mitochondrial “biogenesis” through resistance exercise, which accelerates muscle cell signalling, increases mitochondrial count, enhances glucose transporter capacity and reduces the production of muscle growth inhibitors. Consequently, this process inhibits the proliferation and differentiation of myoblasts in developing muscles [ 38 , 39 , 40 ].
The relationship between lower limb strength and physical function is more closely intertwined than that of the upper limbs. Resistance exercise has found widespread use in the treatment of tumor-related sarcopenia patients. Impedance exercise, involving the application of external force to facilitate synergistic and antagonistic muscle training, can enhance lower limb muscle strength and endurance among patients with tumor-related sarcopenia, thus leading to improved leg extension function. Our results demonstrate a significant intervention effect of resistance exercise on leg extension function in tumor-related sarcopenia patients. This can be attributed to the requirements of muscle strength during resistance exercise, which consequently impacts the power of muscle motor units and the number and type of muscle fibers and fosters muscle growth and repair. Such improvements in muscle contraction afford patients greater control during leg extension, reducing muscle fatigue and pain [ 38 , 39 , 40 ]. Furthermore, the persistent mechanical strain on osteocytes induced by resistance training can promote their physical deformation, subsequently expediting bone remodeling and tissue regeneration and ultimately enhancing bone density [ 41 ]. Therefore, the study of tumor-related sarcopenia patients underscores that regular resistance exercise training represents a practical approach for restoring and enhancing leg extension function, ultimately improving the overall quality of life.
Advantage and limitations
Therapies aimed at addressing tumor-induced sarcopenia have garnered significant attention. Moderate exercise has demonstrated its potential to enhance overall bodily function and augment muscle mass in tumor patients. This study consolidates data from 10 eligible studies involving 503 patients to corroborate the efficacy of four exercise modes: resistance exercise, aerobic combined resistance multimodal exercise, and stretching exercise. In comparison to the meta-analysis of relevant literature, resistance exercise has shown a more pronounced impact on patients by bolstering both muscle strength and mass. This study provides valuable guidance for future research endeavors.
This study has its limitations. First, it relies on currently available English literature about tumor sarcopenia, which may introduce certain constraints to the study findings. Second, there exists some degree of heterogeneity and bias in the study results. Third, the research is influenced by variations in disease types, cultural backgrounds, and the healthcare systems of cancer patients. Therefore, it is imperative to tailor exercise interventions to individual needs based on the specific circumstances of each patient. Our study has not yet included aerobic capacity testing and leg press testing, and thus cannot assess physical performance, which is what we are working towards in the future.
The findings of this study underscore the superiority of resistance exercise over other exercise modalities in enhancing muscle mass and function. Consequently, the inclusion of resistance exercise in the rehabilitation regimen for cancer patients is of paramount importance. Tailoring the intervention to the individual circumstances of the patients and implementing it promptly is crucial for achieving optimal outcomes. Additionally, further investigation into the specific application methods and results of exercise in the rehabilitation of cancer patients is warranted to offer more scientifically informed guidance for clinical practice.
Data availability
The datasets used and/or analysis during the current study are available from the corresponding author on reasonable request.
Abbreviations
Randomized controlled trials
Risk Assessment Tool
Standard deviation
Confidence interval
Kim JW, Kim R, Choi H, Lee SJ, Bae GU. Understanding of Sarcopenia: from definition to therapeutic strategies. Arch Pharm Res. 2021;44:876–89.
Article CAS PubMed Google Scholar
Liguori I, Russo G, Aran L, Bulli G, Curcio F, Della-Morte D, et al. Sarcopenia: assessment of disease burden and strategies to improve outcomes. Clin Interv Aging. 2018;13:913–27.
Article CAS PubMed PubMed Central Google Scholar
Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90:1579–85.
Couderc AL, Liuu E, Boudou-Rouquette P, Poisson J, Frelaut M, Montégut C et al. Pre-therapeutic Sarcopenia among Cancer patients: an Up-to-date Meta-analysis of Prevalence and Predictive Value during Cancer Treatment. Nutrients. 2023;15.
Halvorsen TO, Valan CD, Slaaen M, Grønberg BH. Associations between muscle measures, survival, and toxicity in patients with limited stage small cell lung cancer. J Cachexia Sarcopenia Muscle. 2020;11:1283–90.
Article PubMed PubMed Central Google Scholar
Feliciano EMC, Kroenke CH, Meyerhardt JA, Prado CM, Bradshaw PT, Kwan ML, et al. Association of Systemic Inflammation and Sarcopenia with Survival in Nonmetastatic Colorectal Cancer: results from the C SCANS study. JAMA Oncol. 2017;3:e172319.
Article PubMed Google Scholar
Ryan AM, Prado CM, Sullivan ES, Power DG, Daly LE. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition. 2019;67–68:110539.
Yoshimura Y, Wakabayashi H, Yamada M, Kim H, Harada A, Arai H. Interventions for treating Sarcopenia: a systematic review and Meta-analysis of Randomized Controlled studies. J Am Med Dir Assoc. 2017;18:e5531–16.
Article Google Scholar
Koya S, Kawaguchi T, Hashida R, Hirota K, Bekki M, Goto E, et al. Effects of in-hospital exercise on Sarcopenia in hepatoma patients who underwent transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2019;34:580–8.
Mahalakshmi B, Maurya N, Lee SD, Bharath Kumar V. Possible neuroprotective mechanisms of Physical Exercise in Neurodegeneration. Int J Mol Sci. 2020;21.
Winzer BM, Paratz JD, Whitehead JP, Whiteman DC, Reeves MM. The feasibility of an exercise intervention in males at risk of oesophageal adenocarcinoma: a randomized controlled trial. PLoS ONE. 2015;10:e0117922.
Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12:103–11.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Yin Y, Wang J, Yu Z, Zhou L, Liu X, Cai H, et al. Does whole-body vibration training have a positive effect on balance and walking function in patients with stroke? A meta-analysis. Front Hum Neurosci. 2022;16:1076665.
Jackson D, Riley R, White IR. Multivariate meta-analysis: potential and promise. Stat Med. 2011;30:2481–98.
van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. 2016;7:80–93.
Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS ONE. 2013;8:e76654.
Lam T, McLean M, Hayden A, Poljak A, Cheema B, Gurney H, et al. A potent liver-mediated mechanism for loss of muscle mass during androgen deprivation therapy. Endocr Connect. 2019;8:605–15.
Dieli-Conwright CM, Courneya KS, Demark-Wahnefried W, Sami N, Lee K, Sweeney FC, et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: a randomized controlled trial. Breast Cancer Res. 2018;20:124.
Lam T, Cheema B, Hayden A, Lord SR, Gurney H, Gounden S, et al. Androgen deprivation in prostate cancer: benefits of home-based resistance training. Sports Med Open. 2020;6:59.
Galvão DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28:340–7.
Dawson JK, Dorff TB, Tuzon C, Rice JC, Schroeder ET, Lane CJ, et al. Effect of Periodized Resistance training on skeletal muscle during androgen deprivation therapy for prostate Cancer: a pilot randomized Trial. Integr Cancer Ther. 2021;20:15347354211035442.
Uth J, Hornstrup T, Christensen JF, Christensen KB, Jørgensen NR, Schmidt JF, et al. Efficacy of recreational football on bone health, body composition, and physical functioning in men with prostate cancer undergoing androgen deprivation therapy: 32-week follow-up of the FC prostate randomised controlled trial. Osteoporos Int. 2016;27:1507–18.
Dieli-Conwright CM, Sweeney FC, Courneya KS, Tripathy D, Sami N, Lee K, et al. Hispanic ethnicity as a moderator of the effects of aerobic and resistance exercise in survivors of breast cancer. Cancer. 2019;125:910–20.
Taaffe DR, Galvão DA, Spry N, Joseph D, Chambers SK, Gardiner RA, et al. Immediate versus delayed exercise in men initiating androgen deprivation: effects on bone density and soft tissue composition. BJU Int. 2019;123:261–9.
Grote M, Maihöfer C, Weigl M, Davies-Knorr P, Belka C. Progressive resistance training in cachectic head and neck cancer patients undergoing radiotherapy: a randomized controlled pilot feasibility trial. Radiat Oncol. 2018;13:215.
Dhillon RJ, Hasni S. Pathogenesis and management of Sarcopenia. Clin Geriatr Med. 2017;33:17–26.
Lam T, Birzniece V, McLean M, Gurney H, Hayden A, Cheema BS. The adverse effects of Androgen deprivation therapy in prostate Cancer and the benefits and potential anti-oncogenic mechanisms of Progressive Resistance Training. Sports Med Open. 2020;6:13.
Rock CL, Thomson CA, Sullivan KR, Howe CL, Kushi LH, Caan BJ, et al. American Cancer Society nutrition and physical activity guideline for cancer survivors. CA Cancer J Clin. 2022;72:230–62.
Colleluori G, Villareal DT. Aging, obesity, Sarcopenia and the effect of diet and exercise intervention. Exp Gerontol. 2021;155:111561.
Evans WJ, Hellerstein M, Orwoll E, Cummings S, Cawthon PM. D(3) -Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle. 2019;10:14–21.
Chen X, Ji Y, Liu R, Zhu X, Wang K, Yang X, et al. Mitochondrial dysfunction: roles in skeletal muscle atrophy. J Transl Med. 2023;21:503.
Kamel HK. Sarcopenia and Aging Nutrition Reviews. 2003;61:157– 67.
Dickinson JM, Volpi E, Rasmussen BB. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev. 2013;41:216–23.
Borges IBP, de Oliveira DS, Marie SKN, Lenario AM, Oba-Shinjo SM, Shinjo SK. Exercise Training attenuates ubiquitin-proteasome pathway and increases the genes related to Autophagy on the skeletal muscle of patients with inflammatory myopathies. J Clin Rheumatol. 2021;27:S224–31.
Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. Faseb j. 2002;16:1879–86.
Pascual-Fernández J, Fernández-Montero A, Córdova-Martínez A, Pastor D, Martínez-Rodríguez A, Roche E. Sarcopenia: Molecular pathways and potential targets for intervention. Int J Mol Sci. 2020;21.
Emerson NS, Stout JR, Fukuda DH, Robinson EH, Iv, Scanlon TC, et al. Resistance training improves capacity to delay neuromuscular fatigue in older adults. Arch Gerontol Geriatr. 2015;61:27–32.
Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res. 1981;219:45–55.
Bryanton MA, Carey JP, Kennedy MD, Chiu LZ. Quadriceps effort during squat exercise depends on hip extensor muscle strategy. Sports Biomech. 2015;14:122–38.
Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American College of Sports Medicine Position stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36:1985–96.
Download references
Acknowledgements
We would like to acknowledge the reviewers for their helpful comments on this paper.
This work was supported by the Nursing project of Beijing Hope Marathon Fund of China Cancer Foundation (Item number: LC2022C03).
Author information
Rui Liu and XaoYing Gao contributed equally to this work.
Authors and Affiliations
Department of Special Medical Care, Cancer Hospital, Chinese Academy of Medical Sciences, Chaoyang district, 100021, Beijing, China
Rui Liu, XY Gao & Li Wang
You can also search for this author in PubMed Google Scholar
Contributions
Rui Liu and XY Gao participated in protocol design, data extraction, quality assessment, statistical analyses and manuscript preparation. Li Wang participated in protocol design, quality assessment, and manuscript revision. All authors have read and approved the final manuscript.
Corresponding author
Correspondence to Li Wang .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Liu, R., Gao, X. & Wang, L. Network meta-analysis of the intervention effects of different exercise measures on Sarcopenia in cancer patients. BMC Public Health 24 , 1281 (2024). https://doi.org/10.1186/s12889-024-18493-y
Download citation
Received : 11 December 2023
Accepted : 31 March 2024
Published : 10 May 2024
DOI : https://doi.org/10.1186/s12889-024-18493-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Exercise intervention
- Network Meta-analysis
BMC Public Health
ISSN: 1471-2458
- Submission enquiries: [email protected]
- General enquiries: [email protected]

COMMENTS
Here, we offer five tips for authors of the review articles, relevant to all types of reviews, for creating useful and relevant literature summary tables. We also provide examples from our published reviews to illustrate how useful literature summary tables can be developed and what sort of information should be provided.
Examples of literature reviews. Step 1 - Search for relevant literature. Step 2 - Evaluate and select sources. Step 3 - Identify themes, debates, and gaps. Step 4 - Outline your literature review's structure. Step 5 - Write your literature review.
This is an example of a research table, in which you provide a basic description of the most important features of the studies, articles, and other items you discover in your research.The table identifies each item according to its author/date of publication, its purpose or thesis, what type of work it is (systematic review, clinical trial, etc.), the level of evidence it represents (which ...
Literature Review Table. Literature Review Table Template. Peabody Librarians have created a sample literature review table to use to organize your research. Feel free to download this file and use or adapt as needed. << Previous: Literature Reviews Webinar Recording; Next: Writing Like an Academic >>
in the summary tables (see figure 1) demonstrates to. the readers that a robust method of data extraction and. synthesis has been followed. Tip 5: create your person alised template for lit ...
While the table is a step to help you organize the articles you've selected for your research, the literature synthesis can take many forms and can have multiple parts. This largely depends on what type of review you've undertaken. Look back at the examples under Literature and Other Types of Reviews to see examples of different types of reviews.
A literature review is a survey of scholarly sources on a specific topic. It provides an overview of current knowledge, allowing you to identify relevant theories, methods, and gaps in the existing research. There are five key steps to writing a literature review: Search for relevant literature. Evaluate sources. Identify themes, debates and gaps.
As mentioned above, writing your literature review is a process, which I'll break down into three steps: Finding the most suitable literature. Understanding, distilling and organising the literature. Planning and writing up your literature review chapter. Importantly, you must complete steps one and two before you start writing up your chapter.
When writing a literature review it is important to start with a brief introduction, followed by the text broken up into subsections and conclude with a summary to bring everything together. A summary table including title, author, publication date and key findings is a useful feature to present in your review (see Table 1 for an example). This ...
tables making it a complex task specialty for the novice researchers or reviewers. Here, we offer five tips for authors of the review articles, relevant to all types of reviews, for creating useful and relevant literature summary tables. We also provide examples from our published reviews to illustrate how useful literature summary tables can ...
A literature review is a critical analysis and synthesis of existing research on a particular topic. It provides an overview of the current state of knowledge, identifies gaps, and highlights key findings in the literature. 1 The purpose of a literature review is to situate your own research within the context of existing scholarship ...
Literature Review Matrix. As you read and evaluate your literature there are several different ways to organize your research. Courtesy of Dr. Gary Burkholder in the School of Psychology, these sample matrices are one option to help organize your articles. These documents allow you to compile details about your sources, such as the foundational ...
If you're working on a dissertation or thesis and are looking for an example of a strong literature review chapter, you've come to the right place.. In this video, we walk you through an A-grade literature review from a dissertation that earned full distinction.We start off by discussing the five core sections of a literature review chapter by unpacking our free literature review template.
Steps to Completing a Literature Review. Find. Conduct searches for relevant information. Evaluate. Critically review your sources. Summarize. Determine the most important and relevant information from each source, theories, findings, etc. Synthesize. Create a synthesis matrix to find connections between resources, and ensure your sources ...
Demonstrate your knowledge of the research topic. Identify the gaps in the literature and show how your research links to these. Provide the foundation for your conceptual framework (if you have one) Inform your own methodology and research design. To achieve this, your literature review needs a well-thought-out structure.
for example, that there should be "some kind of structure to the chapter" (Oliver, 2004, p.109). One guide depressingly takes it for granted that writing a review ... Table 1: Aims of the literature review for thesis writers To show a thorough professional grasp of the area Identifies the relevant literature
A literature review is a document or section of a document that collects key sources on a topic and discusses those sources in conversation with each other (also called synthesis ). The lit review is an important genre in many disciplines, not just literature (i.e., the study of works of literature such as novels and plays).
- The presence of negative consequences cited in the literature indicates a need to further evaluate the risks associated with eHealth Harrington, L., D. Kennerly, and C. Johnson. 2011. Safety issues related to the electronic medi-cal record (EMR): Synthesis of the literature from the last decade, 2000-2009. Journal of Health Care Management
A synthesis matrix helps you record the main points of each source and document how sources relate to each other. After summarizing and evaluating your sources, arrange them in a matrix or use a citation manager to help you see how they relate to each other and apply to each of your themes or variables. By arranging your sources by theme or ...
Steps for Conducting a Lit Review; Finding "The Literature" Organizing/Writing; APA Style This link opens in a new window; Chicago: Notes Bibliography This link opens in a new window; MLA Style This link opens in a new window; Sample Literature Reviews. Sample Lit Reviews from Communication Arts; Have an exemplary literature review? Get Help!
Table 14.3.a provides a framework and examples for how review authors can justify their judgements about the certainty of evidence in each domain. These justifications should also be included in explanatory notes to the 'Summary of Findings' table (see Section 14.1.6.10 ).
Evidence on the effectiveness of continuing education (CE) and CE methods was identified through a literature review.Although nonexhaustive, the review included a comprehensive search of the Research and Development Resource Base (RDRB), a bibliographic database of more than 18,000 articles from fields including CE, knowledge translation, interprofessional literature, and faculty development.
The Online Writing Lab at Purdue University houses writing resources and instructional material, and we provide these as a free service of the Writing Lab at Purdue.
The literature review template includes the following sections: Each section is explained in plain, straightforward language, followed by an overview of the key elements that you need to cover. We've also included practical examples and links to more free videos and guides to help you understand exactly what's required in each section.
Scoping reviews and evidence maps are forms of evidence synthesis that aim to map the available literature on a topic and are well-suited to visual presentation of results. A range of data visualisation methods and interactive data visualisation tools exist that may make scoping reviews more useful to knowledge users. The aim of this study was to explore the use of data visualisation in a ...
Although our review includes a limited number of studies, it is noteworthy that the distribution of interventions contrasts with a literature review on the use of 'virtual patient' in health care education from 2015 , which identified IPS as the most frequent intervention. This variation may stem from our review's focus on the mental ...
Sarcopenia is a systemic syndrome characterized primarily by the weakening or loss of muscle mass and function [].Its pathogenesis is associated with inflammatory responses, hormone levels, and insulin resistance within the body, subsequently leading to disruptions in protein synthesis and the onset of sarcopenia [2, 3].Based on differing mechanisms of occurrence, sarcopenia is classified into ...