

At a glance: The STEP trials
A round-up of the STEP phase 3 clinical trials evaluating semaglutide for weight loss in people with overweight or obesity.
Springer Medicine
Breast cancer research and treatment, breast cancer research and treatment onlinefirst articles, be-wel trial (breast: evaluation of weight and exercise for lymphoedema) testing weight control and exercise programmes for women with breast cancer related lymphoedema: a feasibility trial, alteration of pd-l1 (sp142) status after neoadjuvant chemotherapy and its clinical significance in triple-negative breast cancer, combined transcriptome and proteome analysis reveals msn and arfip2 as biomarkers for trastuzumab resistance of breast cancer, racial differences in familiarity, interest, and use of integrative medicine among patients with breast cancer, an exploratory clinical trial of preoperative non-invasive localization before breast-conserving surgery using augmented reality technology, the emerging predictive and prognostic role of her2 in her2-negative early breast cancer: a retrospective study, measuring cfdna integrity as a biomarker for predicting neoadjuvant chemotherapy response in breast cancer patients: a pilot study, characterizing “collateral damage” in men and women with metastatic breast cancer (mbc) from diverse racial and ethnic backgrounds in new york city, predictive analysis of breast cancer response to neoadjuvant chemotherapy through plasma metabolomics, ductal carcinoma in situ and cause-specific mortality among younger and older postmenopausal women: the women’s health initiative, latest issues, breast cancer research and treatment 1/2024, breast cancer research and treatment 2/2024, breast cancer research and treatment 3/2024, breast cancer research and treatment 3/2023, breast cancer research and treatment 1/2023, breast cancer research and treatment 2/2023.
scroll for more
use your arrow keys for more
scroll or use arrow keys for more
About this journal
Breast Cancer Research and Treatment provides the surgeon, radiotherapist, medical oncologist, endocrinologist, epidemiologist, immunologist or cell biologist investigating problems in breast cancer a single forum for communication. The journal creates a "market place" for breast cancer topics which cuts across all the usual lines of disciplines, providing a site for presenting pertinent investigations, and for discussing critical questions relevant to the entire field. It seeks to develop a new focus and new perspectives for all those concerned with breast cancer. Oncology is undoubtedly the most rapidly growing subspecialty in the field of medicine, and breast cancer is one of the most serious problems of oncology. It is the leading cause of death of women in many countries, and is truly a multidisciplinary problem without geographic restrictions. Yet this very multidisciplinary aspect accounts for breast cancer literature appearing in any of the dozens of existing medical journals. None of these journals provides a focus on the unique problems of breast cancer. There has been no convenient arena for the discussion and resolution of ongoing controversies in breast cancer treatment, or for the consideration of thoughtful speculation and comments on current work. Breast Cancer Research and Treatment aims to fill this need. Each issue contains several articles dealing with original laboratory investigations and articles dealing with clinical studies. There are sections devoted to review articles, pro and con discussions of controversial subjects, meeting reports, and editorials. The panel discussions encourage experts to consider important topics.There is a section for letters to the editor, which provides for a lively exchange of opinions on previously published articles or other topics of interest. There is also an opportunity to publish the proceedings of special workshops, symposia, etc., devoted to breast cancer. All man uscripts are peer reviewed by a distinguished group of advisory editors from many countries covering all of the various disciplines of breast cancer.
Keynote webinar | Spotlight on antibody–drug conjugates in cancer
Antibody–drug conjugates (ADCs) are novel agents that have shown promise across multiple tumor types. Explore the current landscape of ADCs in breast and lung cancer with our experts, and gain insights into the mechanism of action, key clinical trials data, existing challenges, and future directions.
- Medical Journals
- Webcasts & Webinars
- CME & eLearning
- Newsletters
- ESMO Congress 2023
- 2023 ERS Congress
- ESC Congress 2023
- Advances in Alzheimer’s
- About Springer Medicine
- Diabetology
- Endocrinology
- Gastroenterology
- Geriatrics and Gerontology
- Gynecology and Obstetrics
- Infectious Disease
- Internal Medicine
- Respiratory Medicine
- Rheumatology
Breast Cancer Research and Treatment

Subject Area and Category
- Cancer Research
Springer New York
Publication type
01676806, 15737217
Information
How to publish in this journal
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scientific influence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scientific influence of the average article in a journal, it expresses how central to the global scientific discussion an average article of the journal is.
Evolution of the number of published documents. All types of documents are considered, including citable and non citable documents.
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year. The two years line is equivalent to journal impact factor ™ (Thomson Reuters) metric.
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years. Journal Self-citation is defined as the number of citation from a journal citing article to articles published by the same journal.
Evolution of the number of total citation per document and external citation per document (i.e. journal self-citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
Evolution of the percentage of female authors.
Evolution of the number of documents cited by public policy documents according to Overton database.
Evoution of the number of documents related to Sustainable Development Goals defined by United Nations. Available from 2018 onwards.
Leave a comment
Name * Required
Email (will not be published) * Required
* Required Cancel
The users of Scimago Journal & Country Rank have the possibility to dialogue through comments linked to a specific journal. The purpose is to have a forum in which general doubts about the processes of publication in the journal, experiences and other issues derived from the publication of papers are resolved. For topics on particular articles, maintain the dialogue through the usual channels with your editor.

Follow us on @ScimagoJR Scimago Lab , Copyright 2007-2024. Data Source: Scopus®

Cookie settings
Cookie Policy
Legal Notice
Privacy Policy
Racial differences in familiarity, interest, and use of integrative medicine among patients with breast cancer
- Open access
- Published: 15 May 2024
Cite this article
You have full access to this open access article

- Jincong Q. Freeman ORCID: orcid.org/0000-0002-1119-2146 1 , 2 , 3 na1 ,
- Jori B. Sheade ORCID: orcid.org/0000-0002-1283-8308 4 , 5 na1 ,
- Fangyuan Zhao ORCID: orcid.org/0000-0001-9776-3677 1 ,
- Olufunmilayo I. Olopade ORCID: orcid.org/0000-0002-9936-1599 4 , 6 ,
- Dezheng Huo ORCID: orcid.org/0000-0002-4041-1678 1 , 6 &
- Rita Nanda ORCID: orcid.org/0000-0001-5248-0876 4 , 6 , 7
Integrative medicine (IM) has received the American Society of Clinical Oncology’s endorsement for managing cancer treatment-related side effects. Little is known about racial differences in familiarity, interest, and use of IM among patients with breast cancer.
Patients with breast cancer enrolled in the Chicago Multiethnic Epidemiologic Breast Cancer Cohort were surveyed regarding familiarity, interest, and use of acupuncture, massage, meditation, music therapy, and yoga. Familiarity and interest, measured by a 5-point Likert scale, was modeled using proportional odds. Use was self-reported, and modeled using binary logistic regression.
Of 1,300 respondents (71.4% White and 21.9% Black), Black patients were less likely than White patients to be familiar with acupuncture (aOR 0.60, 95% CI 0.41–0.87); there were no racial differences in familiarity with massage, meditation, music therapy, and yoga. While there were no differences in interest in acupuncture between Black and White patients (aOR 1.12, 95% CI 0.76–1.65), Black patients were more interested in massage (aOR 1.86, 95% CI 1.25–2.77), meditation (aOR 2.03, 95% CI 1.37–3.00), music therapy (aOR 2.68, 95% CI 1.80–3.99), and yoga (aOR 2.10, 95% CI 1.41–3.12). Black patients were less likely than White patients to have used acupuncture (aOR 0.49, 95% CI 0.29–0.84); but there were no racial differences in use of massage, meditation, music therapy, and yoga.
Black patients expressed more interest in IM than their White counterparts; there were no racial differences in IM use, except lower acupuncture use among Black patients. A breast program focused on equity should provide access to these services for patients with breast cancer.
Similar content being viewed by others

Real-world experiences with acupuncture among breast cancer survivors: a cross-sectional survey study

Integrative Therapies in Cancer Pain

Electro-acupuncture versus battle field auricular acupuncture in breast cancer survivors with chronic musculoskeletal pain: subgroup analysis of a randomized clinical trial
Avoid common mistakes on your manuscript.
Introduction
In the United States (U.S.), breast cancer is the most common cancer type in women, accounting for approximately 30% of all cancers in women and with more than 3.6 million people alive with breast cancer in 2020 [ 1 ]. Breast cancer and its treatment can be associated with numerous side effects and symptoms, from cancer pain to lymphedema to hot flashes, [ 2 ] that can negatively impact patients’ treatment adherence and quality of life [ 3 ]. Reduction of side effects and management of symptoms typically consists of further medications, which carry their own adverse effect profiles. In 2017, the Society for Integrative Oncology (SIO) performed a systemic review focusing on randomized controlled trials from 1990 through 2015 of the use of integrative medicine (IM) modalities during and after breast cancer treatment [ 4 ]. This resulted in a set of evidence-based practice guidelines on the use of IM in breast cancer, which was subsequently endorsed by the American Society of Clinical Oncology (ASCO) in 2018 [ 5 ]. IM modalities, including acupuncture, therapeutic massage, meditation therapy, music therapy, and yoga therapy all have received ASCO’s endorsement for the treatment or management of various side effects and symptoms, particularly: hot flashes, nausea, anxiety or stress reduction, depression or mood disorders, and improving quality of life [ 5 ].
A 2021 study documented that more than 50.0% of cancer patients and their caregivers are familiar with acupuncture, yoga therapy, and meditation therapy [ 6 ]. Multiple previous studies have reported breast and gynecological cancer patients’ growing interest in and demand for IM, however, these studies included only White female patients [ 7 , 8 , 9 , 10 ]. Use of IM has increased in recent decades [ 11 , 12 , 13 ]. A 2005 survey conducted in breast cancer survivors found that more than 80.0% of the survivors had either used a complementary and alternative therapy or visited a therapist in the past [ 11 ]. According to a study using the 2002 National Health Interview Survey, Black race and lower socioeconomic status (SES) have historically been associated with lower prevalence of IM utilization among U.S. adults [ 12 ]. Therefore, IM services are generally marketed toward White populations and those with higher SES. However, little is known about familiarity, interest, and use of IM among Black or African American patients with breast cancer and survivors.
To date, the best study about racial differences in interest and use of IM comes from a 2017 study at the University of Texas MD Anderson Cancer Center, which surveyed 165 cancer patients, 43% of which were Black or African Americans, at an urban community hospital about interest and use of complementary and alternative therapies [ 14 ]. The study found that 90.6% of the patients were interested in therapeutic massage, followed by 72.7% in meditation therapy, 69.8% in yoga therapy, and 49.7% in acupuncture [ 14 ]. However, most were unprofessionally guided use, and both past and current IM use were low. In this study, 13.8% of Black patients had used yoga therapy as compared to 42.9% of Asian and 25.7% of White patients, with no significant differences among these racial groups based on a Pearson’s Chi-square test. Moreover, the study did not perform multivariable regression analyses due to the small sample size [ 14 ].
Prior studies are small and descriptive, with the majority of cancer patients and survivors being White. Additionally, there is paucity of data on racial differences in familiarity, interest, and use of IM specifically among patients with breast cancer. To fill these gaps in the literature, we sought to assess racial differences in familiarity, interest, and use of five ASCO-endorsed IM modalities for breast cancer symptom management: acupuncture, therapeutic massage, meditation therapy, music therapy, and yoga therapy in a large cohort of patients with breast cancer having been treated at the University of Chicago Medicine.
Study design and study population
We conducted a cross-sectional survey among patients with breast cancer who were enrolled in the Chicago Multiethnic Epidemiologic Breast Cancer Cohort (ChiMEC). Briefly, ChiMEC is a hospital-based study having been enrolling patients diagnosed with breast cancer since 1993. Detailed information of ChiMEC has been previously published [ 15 ]. Eligible participants were aged 18 years or older. From July to September 2021, a REDCap survey was sent to 2,788 ChiMEC participants who consented to be followed up for subsequent surveys. All patients provided their written informed consent prior to study participation. The University of Chicago Institutional Review Board reviewed and approved this study.
Measures of key variables
Familiarity was measured by asking participants how familiar they were with these types of IM, using a 5-point Likert scale including not familiar at all, not very familiar, neutral, familiar, and very familiar. Interest was measured by asking participants how interested they would be in these IM modalities if offered at the center, using a 5-point Likert scale including not interested at all, not very interested, neutral, interested, and very interested.
We also assessed cancer treatment-related symptoms as facilitators by asking participants how interested they would be in any type of IM if it treated hot flashes, chemotherapy-induced neuropathy, nausea, joint pain, back or other pain, depression or mood change, fatigue or tiredness, and anxiety or stress reduction. Other facilitating factors for interest included recommendation from a provider, cost not being a barrier, being covered by health insurance, and price willing to pay out-of-pocket for a session.
To measure use of IM, we asked patients to “Select all of the therapies that you have had or received in the past.” Response options for each item were yes and no. We also assessed major barriers to IM use, including cost/money, lack of access to services, lack of transportation to service-providing facilities, lack of time, lack of interest, unaware of benefits of these services, low confidence about the benefits on these services, and lack of trusted information on these services by asking what, in general, prevents participants from using any IM modality.
Demographic and behavioral characteristics, including age, race/ethnicity (Asian, Black, Hispanic, and White), highest level of education (High school/GED or less, post high school, trade/technical school, or some college, Associate’s degree, Bachelor’s degree, and graduate or professional degree), marital status, annual household income, type of health insurance (Medicare, Medicaid, private, and other), and history of tobacco and alcohol consumption (never, current, and past), were collected from the survey. Clinical characteristics such as duration from diagnosis to survey, American Joint Committee on Cancer (AJCC) stage group, hormone receptor (HR) status (HR-positive/ human epidermal growth factor receptor 2 [HER2]-negative, HER2-positive, and triple negative breast cancer [TNBC]), and Charlson comorbidity index (CCI, i.e., 0, 1, and ≥ 2) was obtained through clinical chart abstraction.
Statistical analysis
We calculated means and standard deviations (SD) for continuous data and tabulated frequencies and percentages (%) for categorical data. Demographic, behavioral, and clinical characteristics between racial/ethnic groups were compared using Student’s t tests or ANOVA for continuous variables and Pearson’s chi-square or Fisher’s exact tests for categorical variables. Of note, Asian and Hispanic patients were included in the descriptive analysis but were excluded in subsequent analyses due to small group sample size. Multivariable proportional odds were modeled for familiarity with and interest in different IM modalities. Multivariable binary logistic regression was modeled for self-report of having used these modalities in the past. All models were adjusted for age, highest level of education, marital status, annual household income, type of health insurance, CCI, HR status, and AJCC stage group. To assess racial differences in familiarity, interest, and use of IM modalities, we calculated adjusted odds ratios (aOR) and 95% confidence intervals (CI). The level of significance was set at 0.05. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Patient characteristics
We received 1,300 survey responses from ChiMEC participants. Of the total, 71.4% were White, followed by 21.9% Black, 3.3% Asian, and 3.3% Hispanic; 59.1% were aged 40–65 years; 39.3% obtained a graduate or professional degree; 69.6% were married; 74.8% had private insurance and 23.5% were on Medicaid/Medicare; 65.8% were HR-positive/HER2-negative; and 98.9% had stage 0-III disease (Table 1 ). Given low percentages of Asian and Hispanic respondents, they were excluded in the following analyses. The mean age of White respondents was 61.4 (SD = 11.3) years, whereas Black respondents’ mean age was 62.6 (SD = 12.3) years. Compared with White patients, Black patients had higher percentages of having obtained high school/GED or less education (16.7% vs. 8.1%), a lower annual household income of less than $50,000 (43.4% vs. 11.3%), been enrolled in Medicaid (16.3% vs. 1.5%) or Medicare (25.2% vs. 17.2%), and a CCI of ≥ 2 (10.8% vs. 5.7%). A higher percentage of Black patients had TNBC than White patients (28.7% vs. 14.4%) (Table 1 ).
Familiarity with integrative medicine
Overall, 59.8% of the patients were familiar or very familiar with therapeutic massage, followed by 47.7% acupuncture, 47.6% meditation therapy, 47.0% yoga therapy, and 35.4% music therapy. Compared with White patients, Black patients had a higher percentage of being familiar or very familiar with music therapy (44.2% vs. 32.7%). However, a higher percentage of White patients reported being familiar or very familiar with therapeutic massage (60.9% vs. 56.4%), acupuncture (49.9% vs. 40.5%), or yoga therapy (47.8% vs. 44.3%) than Black patients (Table 2 ). After adjusting for covariates, Black patients were less likely than their White counterparts to be familiar with acupuncture (aOR 0.60, 95% CI 0.41–0.87). We did not observe differences between Black and White patients in familiarity with music therapy, meditation therapy, therapeutic, or yoga therapy (Table 3 ).
Interest in integrative medicine and facilitators
Overall, 62.9% of the patients reported being interested or very interested in therapeutic massage, followed by 49.4% yoga therapy, 47.1% meditation therapy, 43.9% acupuncture, and 40.4% music therapy. By race, Black patients had higher percentages of being interested or very interested in therapeutic massage (74.0% vs. 59.5%), yoga therapy (62.6% vs. 45.5%), meditation therapy (60.7% vs. 43.1%), music therapy (56.2% vs. 35.8%), or acupuncture (49.2% vs. 42.3%) than did White patients (Table 4 ). In the adjusted proportional odds model, Black patients were significantly more interested in the use of music therapy (aOR 2.68, 95% CI 1.80–3.99), yoga therapy (aOR 2.10, 95% CI 1.41–3.12), meditation therapy (aOR 2.03, 95% CI 1.37–3.00), and therapeutic massage (aOR 1.86, 95% CI 1.25–2.77) than their White counterparts. There were no differences in interest in acupuncture between the racial groups (Table 3 ).
Participants reported being interested or very interested when asked for interest in any of the IM modalities to address specific symptoms: joint paints due to aromatase inhibitors (Black 71.6% vs. White 66.2%), back pain or other pain (Black 71.0% vs. White 65.9%), fatigue (Black 63.8% vs. White 64.0%), anxiety or stress reduction (Black 61.3% vs. White 63.25), depression or mood changes (Black 50.4% vs. White 52.5%), hot flashes (Black 49.2% vs. White 44.1%), chemotherapy-induced neuropathy (Black 43.2% vs. White 38.0%), and nausea (Black 32.0% vs. White 30.2%). However, there were no significant differences in symptoms as facilitators for interest in IM between the racial groups (Table 5 ). Generally, Black respondents expressed more interest in IM modalities if they were recommended by their doctors or nurses and were covered by health insurance. Black respondents typically were less willing to pay more than $0–$19 out of pocket for a session of any IM services (Table 6 ).
Use of integrative medicine and barriers
Overall, 41.6% of the patients had used therapeutic massage, followed by 26.1% acupuncture, 19.0% yoga therapy, 18.5% meditation therapy, and only 7.7% music therapy. Higher percentages of White patients reported having used therapeutic massage (42.9% vs. 37.5%), acupuncture (29.5% vs. 14.7%), yoga therapy (20.8% vs. 13.0%), and meditation therapy (18.8% vs. 17.5%) than Black patients. However, Black patients had a higher percentage of prior use of music therapy than did White patients (11.2% vs. 6.6%) (Table 7 ). In the adjusted logistic regression model, there were no differences between Black and White patients in self-reported use of the IM modalities surveyed, with the exception of acupuncture as Black patients were less likely than their White counterparts to have used acupuncture (aOR 0.49, 95% CI 0.29–0.84) (Table 3 ).
When participants were asked about barriers to use of any IM modality, higher percentages of Black patients reported cost (55.1% vs. 31.4%), lack of awareness of benefits of IM services (35.8% vs. 23.9%), lack of access to services (24.6% vs. 19.4%), and lack of transportation to service-providing facilities (13.0% vs. 9.3%) than White patients. White patients had a higher percentage of lack of time as a barrier to use than their Black counterparts (32.2% vs. 19.3%). Of note, confidence in the benefits of IM was high in both races (White 91% vs. Black 90.5%) (Table 8 ).
To our knowledge, this is of the first and the largest study to examine racial differences in familiarity, interest, and use of five ASCO-endorsed IM modalities and to assess specific symptom-related facilitating factors for interest in, and key barriers to use of, these modalities among U.S. patients with breast cancer. In this diverse cohort of patients with breast cancer, familiarity of IM modalities was prevalent among Black and White patients. However, higher proportions, ranging from 26.9% to 48.6%, of the patients across the racial groups were still not familiar with these modalities. Black patients were less likely to be familiar with acupuncture than their White counterparts, but there were no differences between them in familiarity with therapeutic massage, meditation therapy, music therapy, and yoga therapy. These findings are somewhat consistent with a recent study of familiarity and interest in IM among cancer patients and their caregivers that non-White patients are less familiar with therapeutic massage than White patients, while levels of familiarity with acupuncture, meditation therapy, music therapy, and yoga therapy are similar between White and non-White patients [ 6 ]. Our findings suggest that patient education on IM and its associated benefits may be needed among patients with breast cancer in order to increase patients’ knowledge and awareness of IM. Future research may be needed to explore reasons related to level of familiarity and how the findings could help inform and tailor IM education campaigns and programs specifically toward patients with breast cancer and survivors.
We found that most patients across the two racial groups were interested in the use of IM modalities, and the percentages of interest of IM use also increased when the patients were asked if any of these modalities were treated for a particular symptom such as joint pains, back pain, fatigue, anxiety or stress reduction, hot flashes, and chemotherapy-induced neuropathy. Our finding in increased interest of IM use aligns with a published study that patients who experience back, joint, or other pain are more likely to use acupuncture and therapeutic massage, though racial differences were not assessed [ 16 ]. Further, Black patients were twice as likely as their White counterparts to have expressed interest in music therapy, yoga therapy, meditation therapy, and therapeutic massage, which is contrary to prior findings in the general population that conclude Black patients are less interested in IM services than White patients [ 12 ]. Patients with breast cancer may have unique needs, as a population-based research has indicated that cancer patients and survivors are more likely than the general population to have discussed IM use with a provider and have used these modalities in the past 12 months [ 17 ]. Our result also contradicts a recent study finding that levels of interest in IM are similar between non-White and White patients [ 6 ]. However, this study sample was relatively small, probably lacking statistical power. In addition, it included patients with different cancer types and their caregivers, and thus, the findings may not be comparable to our cohort of patients with breast cancer [ 6 ]. It is worth noting that approximately 21.6%–40.6% of the patients across the racial groups reported being not very interested or not interested at all in using these IM modalities, even when they were asked if these modalities addressed specific common cancer treatment-associated symptoms. It is also important to note that most patients expressed greater interest in IM use if recommended by their providers and were willing to pay no more than $19 out of pocket for a session. Our findings indicate that Black patients may be in greater need for IM and that both provision and coverage of these modalities should be integrated as part of standard cancer care and services at comprehensive cancer centers in the U.S.
The percentages of past use of IM modalities were low among Black and White patients with breast cancer; the majority of the patients, between 57.1% and 93.4%, had not used these modalities in the past. These results are in line with a previous study that both past and current use of acupuncture, meditation therapy, yoga therapy, and therapeutic massage were low, ranging from only 5.6% to 46.3% [ 14 ]. Compared with White patients, a higher proportion of Black patients reported cost, lack of access to services, unaware of the benefits of these services as major barriers to IM use. Less than 10.0% of both Black and White patients reported low confidence in the benefits of the five surveyed IM modalities. After adjusting for key demographic and clinical characteristics, Black patients in our cohort were significantly less likely than their White counterparts to have used acupuncture, while there were no differences between the racial groups in past use of therapeutic massage, meditation therapy, music therapy, or yoga therapy. Our finding is consistent with the previous study partially that use of yoga therapy was similar between Black and White patients, however, multivariable regression modeling was not performed due to the small sample size [ 14 ]. It is important to point out that we did not ask whether the patients had used these IM modalities before, during, and/or after their cancer therapies, which is worth doing in future research to evaluate whether there are racial and ethnic differences in IM utilization over time and how these differences would impact patients’ treatment adherence and quality of life. Our findings also suggest that providers at cancer centers should be promoting these IM services as recommended by ASCO guidelines to all patients which, as shown, may be likely to increase interest in patients with breast cancer and survivors.
Furthermore, Black patients with breast cancer more frequently report nonadherence to endocrine therapy than their White counterparts, with side effect profile being one of the main causes of discontinuation [ 18 , 19 ]. Therefore, the use of IM modalities to reduce side effects from breast cancer treatment and to manage symptoms may lead to greater adherence to endocrine therapy in Black patients. As we have shown Black patients are just as interested, if not more interested, in IM as their White counterparts, and there are likely unmet needs of IM among patients with breast cancer, we should ensure equity to access these services for all our patients, regardless of race.
Several limitations of this study need to be noted. First, the data collected through the survey were self-reported, which were subject to recall error or social desirability bias. However, we expect such bias to be minimal since our research staff had little interaction with the patients. Therefore, their responses were unlikely influenced. Second, because we did not ask the participants whether they had use these services before their cancer diagnoses, during, and/or after their cancer treatment, with an approximately 47.0% response rate, the percentages of use of these IM modalities may have been either overestimated or underestimated. Third, we were not able to assess unmeasured characteristics, e.g., cultural background/influence, employment status, patient-provider discussion of IM, which might affect or help better explain the observed racial differences in familiarity, interest, and past use of IM. Thus, additional cultural and behavioral factors should be taken into consideration in future research. Lastly, participants in the ChiMEC may not be representative of all U.S. patients with breast cancer or other patient populations, and therefore, limiting the generalizability of our study findings.
Despite the above limitations, this study has several strengths. Our study is the largest to date examining racial differences in familiarity, interest, and use of the five ASCO-endorsed IM modalities among patients with breast cancer. Another strength of this study was the inclusion of a racially diverse cohort of patients with breast cancer and key clinical characteristics that previous research was not able to assess.
In conclusion, both Black and White patients with breast cancer were familiar with the five ASCO-endorsed IM modalities, but Black patients expressed greater interest in the use of these modalities. There were no racial differences in prior use of IM, except an increased use of acupuncture among White patients. However, Black patients reported more health care and services access-related barriers than did their White counterparts. Promoting benefits of IM among patients with breast cancer and facilitating patient-provider discussion of IM use may be needed. Furthermore, breast programs focused on health equity should provide access to these services for all patients.
Data availability
The data analyzed during the current study are not publicly available due to the ethics for patient information but can be made available from the corresponding author on reasonable request.
Cancer Stat Facts: Female Breast Cancer (2022) National Institute of Health. https://seer.cancer.gov/statfacts/html/breast.html . Accessed 3 Feb 2022
Side Effects of Cancer Treatment (2002) National Cancer Institute. https://www.cancer.gov/about-cancer/treatment/side-effects . Accessed 3 Feb 2022
Paraskevi T (2012) Quality of life outcomes in patients with breast cancer. Oncol Rev 6(1):e2. https://doi.org/10.4081/oncol.2012.e2
Article PubMed PubMed Central Google Scholar
Greenlee H, DuPont-Reyes MJ, Balneaves LG et al (2017) Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin 67(3):194–232. https://doi.org/10.3322/caac.21397
Lyman GH, Greenlee H, Bohlke K et al (2018) Integrative therapies during and after breast cancer treatment: ASCO endorsement of the SIO clinical practice guideline. J Clin Oncol 36(25):2647–2655. https://doi.org/10.1200/JCO.2018.79.2721
Article CAS PubMed Google Scholar
Larbi OM, Jiang C, McLane B et al (2021) Interest and willingness to pay for integrative therapies of patients with cancer and caregivers. JCO Oncol Pract 17(11):e1622–e1630. https://doi.org/10.1200/OP.20.00471
Article PubMed Google Scholar
Grimm D, Mathes S, Woelber L et al (2021) Demand for integrative medicine among women with breast and gynecological cancer: a multicenter cross-sectional study in Southern and Northern Germany. Arch Gynecol Obstet 303(5):1315–1330. https://doi.org/10.1007/s00404-020-05880-0
Schuerger N, Klein E, Hapfelmeier A, Kiechle M, Brambs C, Paepke D (2019) Evaluating the demand for integrative medicine practices in breast and gynecological cancer patients. Breast Care (Basel) 14(1):35–40. https://doi.org/10.1159/000492235
Hack CC, Fasching PA, Fehm T, de Waal J, Rezai M, Baier B et al (2017) Interest in integrative medicine among postmenopausal hormone receptor-positive breast cancer patients in the EvAluate-TM study. Integr Cancer Ther 16(2):165–175. https://doi.org/10.1177/1534735416668575
Viscuse PV, Price K, Millstine D, Bhagra A, Bauer B, Ruddy KJ (2017) Integrative medicine in cancer survivors. Curr Opin Oncol 29(4):235–242. https://doi.org/10.1097/CCO.0000000000000376
Boon HS, Olatunde F, Zick SM (2007) Trends in complementary/alternative medicine use by breast cancer survivors: comparing survey data from 1998 and 2005. BMC Womens Health 7:4. https://doi.org/10.1186/1472-6874-7-4
Tindle HA, Davis RB, Phillips RS, Eisenberg DM (2005) Trends in use of complementary and alternative medicine by US adults: 1997–2002. Altern Ther Health Med 11(1):42–49
PubMed Google Scholar
Cutshall SM, Cha SS, Ness SM, Stan DL, Christensen SA, Bhagra A et al (2015) Symptom burden and integrative medicine in cancer survivorship. Support Care Cancer 23(10):2989–2994. https://doi.org/10.1007/s00520-015-2666-0
Jones D, Cohen L, Rieber AG, Urbauer D, Fellman B, Fisch MJ, Nazario A (2018) Complementary and alternative medicine use in minority and medically underserved oncology patients: assessment and implications. Integr Cancer Ther 17(2):371–379. https://doi.org/10.1177/1534735417735892
Zhao F, Copley B, Niu Q, Liu F, Johnson JA, Sutton T et al (2021) Racial disparities in survival outcomes among breast cancer patients by molecular subtypes. Breast Cancer Res Treat 185(3):841–849. https://doi.org/10.1007/s10549-020-05984-w
Pang R, Wang S, Tian L, Lee MC, Do A, Cutshall SM et al (2015) Complementary and integrative medicine at Mayo Clinic. Am J Chin Med 43(8):1503–1513. https://doi.org/10.1142/S0192415X15500858
Mao JJ, Palmer CS, Healy KE, Desai K, Amsterdam J (2011) Complementary and alternative medicine use among cancer survivors: a population-based study. J Cancer Surviv 5(1):8–17. https://doi.org/10.1007/s11764-010-0153-7
Spencer JC, Reeve BB, Troester MA, Wheeler SB (2020) Factors associated with endocrine therapy non-adherence in breast cancer survivors. Psychooncology 29(4):647–654. https://doi.org/10.1002/pon.5289
Wheeler SB, Spencer J, Pinheiro LC, Murphy CC, Earp JA, Carey L, Olshan A, Tse CK, Bell ME, Weinberger M, Reeder-Hayes KE (2019) Endocrine therapy nonadherence and discontinuation in Black and White women. J Natl Cancer Inst 111(5):498–508. https://doi.org/10.1093/jnci/djy136
Download references
Acknowledgements
We are grateful to the participants who are enrolled in the Chicago Multiethnic Epidemiologic Breast Cancer Cohort. We thank the Iannessa family for their generous donation to support this study and our acupuncture program at the University of Chicago Medicine.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by a donation from the Iannessa family, the National Cancer Institute (P20CA233307), Susan G. Komen ® (TREND21675016), Breast Cancer Research Foundation (BCRF-23-071), and the National Institute on Aging (T32AG000243 and R24AG066588). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer and the National Institute on Aging.
Author information
Jincong Q. Freeman and Jori B. Sheade have contributed equally to this study.
Authors and Affiliations
Department of Public Health Sciences, The University of Chicago, Chicago, IL, USA
Jincong Q. Freeman, Fangyuan Zhao & Dezheng Huo
Cancer Prevention and Control Program, UChicago Medicine Comprehensive Cancer Center, Chicago, IL, USA
Jincong Q. Freeman
Center for Health and the Social Sciences, The University of Chicago, Chicago, IL, USA
Section of Hematology/Oncology, Department of Medicine, The University of Chicago, Chicago, IL, USA
Jori B. Sheade, Olufunmilayo I. Olopade & Rita Nanda
Department of Hematology and Medical Oncology, Lake Forest Hospital Cancer Center, Northwestern Medicine, Lake Forest, IL, USA
Jori B. Sheade
Center for Clinical Cancer Genetics & Global Health, The University of Chicago, Chicago, IL, USA
Olufunmilayo I. Olopade, Dezheng Huo & Rita Nanda
Department of Medicine, The University of Chicago, 5841 S Maryland Ave, Chicago, IL, 60637, USA
You can also search for this author in PubMed Google Scholar
Contributions
J.Q.F.: Conceptualization, data analysis, creating statistical tables, writing the initial manuscript, and formatting and preparation of manuscript submission. J.B.S.: Conceptualization, design, survey development, research implementation, writing the initial manuscript, and formatting and preparation of manuscript submission. F.Z.: Conceptualization, design, survey development, research implementation, data collection and cleaning. O.I.O.: Conceptualization, design, research implementation. D.H.: Conceptualization, design, survey development, research implementation, and overall supervision. R.N.: Conceptualization, design, survey development, research implementation, and overall supervision. All authors contributed to interpretations of the findings, writing, review, and editing of the manuscript, and approval of the final manuscript.
Corresponding author
Correspondence to Rita Nanda .
Ethics declarations
Competing interests.
J.Q.F., J.B.S., F.Z., and D.H. have no relevant financial or non-financial conflict of interests to disclose. R.N. disclosed advisory board involvement with and research funding from Arvinas, AstraZeneca, BeyondSpring, Celgene, FujiFilm, Genentech/Roche, Gilead, Infinity, iTeos, Merck, OBI Pharma, OncoSec, Pfizer, Relay Therapeutics, SeaGen, Sun Pharma, and Taiho. O.I.O. disclosed financial relationships with CancerIQ, 54gnene, HealthWell Solutions, Tempus; research funding from Ayala Pharmaceuticals, Cepheid, Color Genomics, Novartis, and Roche/Genentech.
Ethical approval
This study was conducted in line with the principles of the Declaration of Helsinki. Ethnics approval was granted by the University of Chicago Institutional Review Board.
Consent to participate
Written informed consent was obtained from all patients prior to study enrollment and participation.
Consent to publish
Not applicable.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Freeman, J.Q., Sheade, J.B., Zhao, F. et al. Racial differences in familiarity, interest, and use of integrative medicine among patients with breast cancer. Breast Cancer Res Treat (2024). https://doi.org/10.1007/s10549-024-07363-1
Download citation
Received : 29 January 2024
Accepted : 24 April 2024
Published : 15 May 2024
DOI : https://doi.org/10.1007/s10549-024-07363-1
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Integrative medicine
- Racial disparities
- Breast cancer
- Find a journal
- Publish with us
- Track your research

Die Highlights vom Kongress des American College of Cardiology 2024
Im April diskutierten die Herz-Kreislauf-Spezialisten aus der ganzen Welt auf dem Kongress des American College of Cardiology (ACC) u.a. neue Therapieansätze bei akutem Myokardinfarkt, koronarer Herzerkrankung, kardiogenem Schock oder Aortenklappenstenose. In unserem Kongress-Dossier haben wir für Sie Berichte über die „Late-Breaking Trials“ und andere wichtige Studien zusammengestellt.
Springer Medizin
Breast cancer research and treatment.
- Alle Ausgaben
Ausgabe 1/2023
Inhalt (15 artikel), no more disparities among regions in italy: recent approval of genomic test reimbursability for early breast cancer patients in the country.
Sara Bravaccini, Massimiliano Mazza, Roberta Maltoni
Identifying miRNA biomarkers for breast cancer and ovarian cancer: a text mining perspective
Xin Li, Andrea Dai, Richard Tran, Jie Wang
Advances of neuroimaging in chemotherapy related cognitive impairment (CRCI) of patients with breast cancer
Senbang Yao, Qianqian Zhang, Xinxin Yao, Xiuqing Zhang, Lulian Pang, Sheng Yu, Huaidong Cheng
Heterogeneous circulating tumor cells correlate with responses to neoadjuvant chemotherapy and prognosis in patients with locally advanced breast cancer
Ge Ma, Jingyi Wang, Jingyue Fu, Rui Chen, Mengdi Liang, Minghui Li, Tiansong Xia, Xiaoan Liu, Shui Wang
Modeling the novel SERD elacestrant in cultured fulvestrant-refractory HR-positive breast circulating tumor cells
Taronish D. Dubash, Aditya Bardia, Brian Chirn, Brittany A. Reeves, Joseph A. LiCausi, Risa Burr, Ben S. Wittner, Sumit Rai, Hitisha Patel, Teeru Bihani, Heike Arlt, Francois-Clement Bidard, Virginia G. Kaklamani, Philippe Aftimos, Javier Cortés, Simona Scartoni, Alessio Fiascarelli, Monica Binaschi, Nassir Habboubi, A. John Iafrate, Mehmet Toner, Daniel A. Haber, Shyamala Maheswaran
Conventional specimen radiography in breast-conserving therapy: a useful tool for intraoperative margin assessment after neoadjuvant therapy?
Benedikt Schäfgen, Annabelle Haller, Hans-Peter Sinn, Manuel Feisst, Christina Gomez, Anne Stieber, Juliane Nees, Riku Togawa, André Pfob, André Hennigs, Johanna Hederer, Fabian Riedel, Sarah Fastner, Jörg Heil, Michael Golatta
Radiofrequency localization of nonpalpable breast cancer in a multicentre prospective cohort study: feasibility, clinical acceptability, and safety
Anke Christenhusz, Bianca M. den Dekker, Thijs van Dalen, Lisa Jongen, Margreet C. van der Schaaf, Lejla Alic, Bennie ten Haken, Ruud M. Pijnappel, Anneriet E. Dassen
Impact of non-adherence to endocrine therapy on recurrence risk in older women with stage I breast cancer after breast-conserving surgery
Danielle Rodin, Rinku Sutradhar, Katarzyna J. Jerzak, Ezra Hahn, Lena Nguyen, Matthew Castelo, Omolara Fatiregun, Cindy Fong, Danilo Giffoni M. M. Mata, Sabina Trebinjac, Lawrence Paszat, Eileen Rakovitch
Survival benefits associated with being adherent and having longer persistence to adjuvant hormone therapy across up to five years among U.S. Medicare population with breast cancer
Dandan Zheng, Joseph Thomas III
Treatment patterns and outcomes associated with sequential and non-sequential use of CDK4 & 6 inhibitors in patients with HR+, HER2− MBC in the real world
Megan Kruse, Emily Nash Smyth, Lee Bowman, Santosh Gautam, Claudia M. Guimaraes, Alnecia R. Nisbett, Maxine D. Fisher, Zhanglin Lin Cui, Kristin M. Sheffield, Kevin Kalinsky
Development of cardiometabolic risk factors following endocrine therapy in women with breast cancer
Eileen Rillamas-Sun, Marilyn L. Kwan, Carlos Iribarren, Richard Cheng, Romain Neugebauer, Jamal S. Rana, Mai Nguyen-Huynh, Zaixing Shi, Cecile A. Laurent, Valerie S. Lee, Janise M. Roh, Yuhan Huang, Hanjie Shen, Dawn L. Hershman, Lawrence H. Kushi, Heather Greenlee
Surviving the COVID-19 pandemic: navigating the recovery of breast imaging services in a safety-net hospital
Jessica H. Porembka, Stephen J. Seiler, B. Bersu Ozcan, W. Phil Evans, Jasmin Tiro, Basak E. Dogan
Validating the PROMIS cognitive function short form in cancer survivors
Ashley M. Henneghan, Kathleen Van Dyk, Xingtao Zhou, Raeanne C. Moore, James C. Root, Tim A. Ahles, Zev M. Nakamura, Jeanne Mandeblatt, Patricia A. Ganz
Is there any association between declined creatinine clearence and higher endoxifen levels in older patients with non-metastatic breast cancer?
Kadri Altundag
It is the impact of racism, not race, that causes breast cancer inequities
Francois G. Rollin, Shub S. Agrawal, Mackenzie L. W. Garcia
Aktuelle Ausgaben
Breast cancer research and treatment 1/2024, breast cancer research and treatment 3/2024, breast cancer research and treatment 2/2024, breast cancer research and treatment 3/2023, breast cancer research and treatment 1/2023, breast cancer research and treatment 2/2023.
Scrollen für mehr
Benutzen Sie die Pfeiltasten für mehr
Scrollen oder Pfeiltasten für mehr
Neu im Fachgebiet Onkologie
Alphablocker schützt vor Miktionsproblemen nach der Biopsie
Nach einer Prostatabiopsie treten häufig Probleme beim Wasserlassen auf. Ob sich das durch den periinterventionellen Einsatz von Alphablockern verhindern lässt, haben australische Mediziner im Zuge einer Metaanalyse untersucht.
Antikörper-Wirkstoff-Konjugat hält solide Tumoren in Schach
Trastuzumab deruxtecan scheint auch jenseits von Lungenkrebs gut gegen solide Tumoren mit HER2-Mutationen zu wirken. Dafür sprechen die Daten einer offenen Pan-Tumor-Studie.
Mammakarzinom: Senken Statine das krebsbedingte Sterberisiko?
Frauen mit lokalem oder metastasiertem Brustkrebs, die Statine einnehmen, haben eine niedrigere krebsspezifische Mortalität als Patientinnen, die dies nicht tun, legen neue Daten aus den USA nahe.
Labor, CT-Anthropometrie zeigen Risiko für Pankreaskrebs
Gerade bei aggressiven Malignomen wie dem duktalen Adenokarzinom des Pankreas könnte Früherkennung die Therapiechancen verbessern. Noch jedoch klafft hier eine Lücke. Ein Studienteam hat einen Weg gesucht, sie zu schließen.
Update Onkologie
Bestellen Sie unseren Fach-Newsletter und bleiben Sie gut informiert.
- Facharzt-Training
- Zeitschriften
- Springer Medizin Podcast – der Talk für Gesundheitsprofis
- Info & Hilfe
- Anästhesiologie
- Allgemeinmedizin
- Arbeitsmedizin
- Augenheilkunde
- Dermatologie
- Gynäkologie und Geburtshilfe
- Innere Medizin
- Kardiologie
- Onkologie und Hämatologie
- Orthopädie und Unfallchirurgie
- Psychiatrie
- Rechtsmedizin
- Zahnmedizin
- Typ-2-Diabetes und Adipositas
- Klimawandel und Gesundheit
- Neues aus dem Markt
- Praxis und Beruf
- Seltene Erkrankungen
- GOÄ & EBM
- Kasuistiken
- Algorithmen & Infografiken
- Blickdiagnosen
- Arthropedia
- Kongressberichterstattung
- Medizin für Apothekerinnen und Apotheker
- Für Ärztinnen und Ärzte in Weiterbildung
- Für Medizinstudierende
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- PMC10020306

Estrogen therapy after breast cancer diagnosis and breast cancer mortality risk
1 Department of Oncology, Faculty of Medicine and Health, Örebro University Hospital, Örebro University, 70182 Örebro, Sweden
2 Sweden Regional Cancer Center, Uppsala University Hospital, Uppsala University, Uppsala, Sweden
Anne Andersson
3 Department of Radiation Sciences, Oncology, Umeå University, Umeå, Sweden
Sara Margolin
4 Department of Oncology, Södersjukhuset, Stockholm, Sweden
5 Department of Clinical Science and Education, Södersjukhuset, Karolinska Institutet, Stockholm, Sweden
Johan Ahlgren
Antonis valachis, associated data.
The data generated and analyzed during this study are described in the following data record: https://doi.org/10.6084/m9.figshare.1454142340 . The Breast Cancer DataBase Sweden (BCBaSe) cohort was used in this study. It is a population-based database that comprises all new cases of invasive breast cancer in women from 1992 to 2012 in three Swedish health care regions. The cohort was linked to a number of national population-based registries. Since BCBaSe contains sensitive health information, it cannot be published in open repositories. Those interested in data from BCBaSe should contact the corresponding author.
The safety of local estrogen therapy in patients on adjuvant endocrine treatment is questioned, but evidence on the issue is scarce. This nested case–control registry-based study aimed to investigate whether estrogen therapy affects breast cancer mortality risk in women on adjuvant endocrine treatment.
In a cohort of 15,198 women diagnosed with early hormone receptor (HR)-positive breast cancer and adjuvant endocrine treatment, 1262 women died due to breast cancer and were identified as cases. Each case was matched with 10 controls. Exposure to estrogen therapy with concurrent use of aromatase inhibitors (AIs), tamoxifen, or both sequentially, was compared between cases and controls.
No statistically significant difference in breast cancer mortality risk was seen in patients with exposure to estrogen therapy concurrent to endocrine treatment, neither in short-term or in long-term estrogen therapy use.
Conclusions
The study strengthens current evidence on local estrogen therapy use in breast cancer survivors, showing no increased risk for breast cancer mortality in patients on adjuvant AIs or tamoxifen.
Introduction
Adjuvant endocrine treatment has shown to improve survival in breast cancer patients with hormone-receptor (HR)-positive disease, with tamoxifen being the treatment of choice for premenopausal women whereas AIs are preferred options in postmenopausal women [ 1 , 2 ]. However, the adherence to endocrine treatment is considerably low, mainly due to the presence of side effects influencing patients’ quality of life [ 3 , 4 ]. Poor adherence to endocrine treatment impacts the prognosis [ 5 ].
A common side effect of endocrine treatment, with possible effects on adherence, is genitourinary symptoms due to vaginal atrophy [ 6 – 8 ]. The treatment strategy for women without prior breast cancer suffering from vaginal atrophy is based on the use of systemic and local estrogen therapy. In patients with prior breast cancer, use of systemic estrogen therapy is not recommended, based on three randomized controlled trials investigating estrogen replacement therapy in breast cancer survivors that stopped early due to increased risk for development of a new breast cancer or recurrence in two of them [ 9 – 11 ].
Local estrogen therapy can improve genitourinary symptoms [ 12 ]. However, the safety of local estrogen therapy in breast cancer patients has been questioned based on some small prospective studies which found increased blood hormone levels when local estrogen therapy was used in patients with adjuvant endocrine treatment [ 13 – 15 ]. Whether the increased blood hormone levels could be translated to a clinically relevant increase in risk for recurrence or mortality due to breast cancer is largely unknown. Two observational studies investigating the potential impact of local estrogen therapy on breast cancer recurrence have been published so far without indications on increased risk for patients using local therapy [ 16 , 17 ]. However, these studies are prone to bias due to small sample size [ 16 ] as well as lacking power to study AI users separately [ 17 ], thus, making the interpretation of results questionable.
The purpose of the present study was to investigate the impact of local estrogen therapy in HR-positive breast cancer patients with adjuvant endocrine treatment on breast cancer mortality and whether the duration of exposure to estrogen therapy or the type of adjuvant endocrine treatment could influence the potential association.
Data source
For this study, the research database Breast Cancer Database Sweden (BCBaSe) was used. BCBaSe is a linkage between breast cancer quality registers and several other population-based registries, with information on individuals diagnosed with breast cancer between 1992 and 2012 in three Swedish health care regions; Stockholm-Gotland, Uppsala-Orebro and the Northern health care regions, comprising approximately 50% of the Swedish population.
The breast cancer quality registers hold information on tumor characteristics, treatment, and follow-up [ 18 , 19 ]. Population-based registers with linkage in BCBaSe include the Cause of Death Register, with information on date of death and underlying cause of death [ 20 ], the Prescribed Drug Register, with information on all prescribed medications filled in Swedish pharmacies with information regarding defined daily doses (DDD) [ 21 , 22 ], Longitudinal Integration Database for Health Insurance and Labour Market Studies (LISA), with data on socioeconomic variables such as marital status, education level and income [ 23 ], as well as the National Patient Register with information on diagnoses in hospital care [ 24 , 25 ]. By combining relevant data from BCBaSe, the Charlson Comorbidity Index (CCI) as well as a Drug Comorbidity Index (DCI) for each patient was calculated. Both CCI and DCI have prognostic value in terms of overall survival in cancer patients [ 26 , 27 ]. BCBaSe is previously described in detail [ 28 ].
Study cohort
The study cohort included women from BCBaSe diagnosed with HR-positive breast cancer between July 1, 2006 and December 31, 2012, with at least six months of treatment with AIs or tamoxifen in total and with a filled prescription of AIs or tamoxifen within 1 year after breast cancer diagnosis. We excluded patients with distant metastases at diagnosis, patients who emigrated or died before six months of endocrine treatment, as well as patients who had filled prescription of AIs or tamoxifen more than 1 year prior to breast cancer diagnosis. Last day of follow-up was December 31, 2019. See flowchart in Fig. 1 .

Flowchart diagram of study cohort
Study design
The study was conducted with nested case–control design. Patients in the study cohort who died due to breast cancer before end of follow-up were selected as cases, with date of breast cancer-related death serving as index date. By incidence density sampling, each case was matched with 10 randomly selected controls from the study cohort, alive, and at risk at index date.
Exposure to estrogen therapy
Exposure to estrogen therapy was defined as at least one filled prescription of either estriol (Anatomical Therapeutic Chemical (ATC) code G03CA04) or estradiol (ATC code G03CA03), at least 1 year before index date. Exposure to estrogen therapy was compared regarding concurrent use of AI, tamoxifen, or both, as well as no concurrent use of endocrine treatment. Concurrent use was defined as a filled prescription of AI or tamoxifen within 90 days before or after a filled prescription of estrogen therapy. No exposure to estrogen therapy served as reference.
Short- and long-term estrogen exposure
To investigate the potential impact of estrogen therapy exposure on breast cancer mortality, the study cohort exposed to estrogen therapy was divided into two groups, based on DDD of estrogen therapy. Short -term exposure was defined as a DDD of estrogen therapy less than the 66th percentile of the total estrogen therapy exposed patient cohort, whereas long-term exposure was defined as a DDD of more than the 66th percentile. The data-driven cut-off value of DDD for defining short- or long-term exposure was 90 days.
Statistical analysis
Conditional logistic regression analyses were used for estimation of Odds Ratio (OR) and 95% Confidence Interval (CI) concerning exposure in uni- and multivariate analyses. The multivariate analysis was performed in two steps: first including potential confounders related to tumor characteristics and patients’ age; age at breast cancer diagnosis, time from breast cancer diagnosis to index date, tumor stage, nodal stage, pre- or postoperative chemotherapy. The second step also included covariates related to patient characteristics; family income, marital status, educational level, previous estradiol or estriol treatment more than 1 year before breast cancer diagnosis, DCI, CCI, and health care region.
All analyses were performed on R version R version 4.1.2 ("Bird Hippie" Copyright (C) 2021 The R Foundation for Statistical Computing). Statistical significance was considered when 95% CI did not include the value of 1.
During follow-up, 1262 women in the cohort died due to breast cancer and were identified as cases. All cases were matched with 10 controls each, resulting in 12,620 controls. Median time from breast cancer diagnosis to index date was 5.1 years (range: 0.4–13.3 years). The characteristics of cases and controls are presented in Table Table1. 1 . Cases comprised older patients, with higher T- and N-stage, more frequently treated with chemotherapy and AIs, higher DCI and CCI scores, as well as a lower socioeconomic status.
Characteristics of cases and controls
Estrogen therapy exposure and breast cancer mortality risk
Exposure to estrogen therapy with or without concurrent use of AI, tamoxifen, or both, was compared between cases and controls (Table (Table2). 2 ). No statistically significant difference in breast cancer mortality was seen in patients using estrogen therapy concurrent to endocrine treatment. Estrogen therapy use without concurrent endocrine treatment was associated with decreased risk for breast cancer mortality.
Breast cancer mortality risk based on exposure to estrogen therapy
OR odds ratio, CI confidence interval
*Adjusted for age, time from breast cancer diagnosis to index date, T stage, N stage, and chemotherapy
**Adjusted for variables in previous adjustment*, as well as educational level, family income, marital status, CCI, DCI, previous estrogen therapy exposure, region
To investigate whether estrogen therapy exposure period effects breast cancer mortality, the study cohort exposed to estrogen therapy were divided into two groups; short- and long-term exposure, based on the DDD of estrogen therapy (Table (Table3). 3 ). We found no statistically significant differences in breast cancer mortality risk either in short- or long-term estrogen exposure groups concurrent with AI, tamoxifen, or both AI and tamoxifen. Long-term exposure to estrogen therapy without concurrent endocrine treatment was associated with decreased risk for breast cancer mortality.
Short- and long-term exposure to estrogen therapy and breast cancer mortality risk
*Adjusted for age, time from breast cancer diagnosis to index date, T stage, N stage and chemotherapy
#More or less than 90 DDD
This population-based case–control study aimed to investigate potential risks with local estrogen therapy in breast cancer patients with adjuvant endocrine treatment in terms of breast cancer mortality. No statistically significant association between estrogen therapy and breast cancer mortality in patients treated with concurrent tamoxifen, AI, or AI and tamoxifen was observed. The lack of association remained consistent irrespective of the duration of exposure to estrogen therapy.
The study results are in line with the two previous observational studies that did not find an association between local estrogen therapy and breast cancer recurrence or mortality in patients with endocrine treatment [ 16 , 17 ]. Our study strengthens the current limited evidence with results from a larger cohort with longer follow-up and adds some new insights in this clinically relevant question by presenting separate analyses depending on the type of endocrine treatment and considering the potential impact of short- or long-term exposure to estrogen therapy on the outcome of interest. In fact, Le Ray et al. did not perform a separate analysis for AI users due to lack of adequate data, and they were not able to consider the duration of exposure to estrogen therapy in their analyses. Dew et al. analyzed data from a smaller unselected cohort (1472 women) that included a mixed population of both HR-positive with or without tamoxifen treatment and HR-negative breast cancer, hence, not being able to draw conclusions on possible risks with local estrogen in HR-positive breast cancer patients with concurrent endocrine treatment.
An interesting finding from our analyses was that breast cancer patients exposed to estrogen therapy without concurrent use of endocrine therapy seemed to have a decreased risk for breast cancer mortality. We hypothesize that this patient group might correspond to a low-risk breast cancer group treated with estrogen therapy after the end of adjuvant endocrine treatment and the exposure to estrogen therapy might serve as an indicator of well-being, an association previously described [ 27 ].
Our study has several limitations that should be discussed. First, we were not able to distinguish the pharmaceutical form of estrogen therapy. However, as systemic estrogen therapy after breast cancer diagnosis is contraindicated according to the Swedish National Guidelines for breast cancer [ 29 ] and this was the case during the time period for study cohort as well, it is reasonable to presume that the vast majority of estrogen therapy prescribed in our study cohort was local estrogen therapy. Further, an unmeasured risk for over-the-counter use of estrogen therapy do exist and should be considered when interpreting our findings. Another potential limitation is the choice of breast cancer mortality as study endpoint. Although breast cancer mortality is a robust and objective outcome, one could argue that the follow-up time is not long enough to investigate mortality as outcome. However, breast cancer recurrence is a less reliable measure than mortality in BCBaSe and choosing breast cancer mortality as endpoint increases the validity of study results. Finally, a causal relationship between exposure and outcome is difficult to be proved with this study design but the dose–response analysis based on exposure to estrogen therapy and the requirement of at least 1 year between exposure to estrogen therapy and mortality are efforts to increase the validity of study results in terms of causality.
In summary, our study did not find any associations between exposure to estrogen therapy and breast cancer mortality in patients treated with tamoxifen or AI, which reassures that local estrogen therapy seems to be safe and can be considered in breast cancer patients with genitourinary symptoms when non-hormonal products are ineffective. Further studies, with more detailed information on prescription patterns and pharmaceutical forms of estrogen therapy, as well as longer follow-up with recurrence as an added endpoint of interest, are warranted to further expand the current evidence.
Author contributions
MS: Methodology, formal analysis, investigation, writing (original draft). HG: Methodology, formal analysis, writing (review & editing). AA, SM: Writing (review & editing). JA: Writing (review & editing). AV: Conceptualization, methodology, writing (original draft), supervision.
Open access funding provided by Örebro University. This work was supported by Bröstcancerförbundet and ALF Funding Region Örebro County.
Data availability
Declarations.
The authors have no relevant financial or non-financial interests to disclose.
The study was approved by the Ethical Review Board in Stockholm (EPN Stockholm) dnr 2013/1272–31/4, with supplements approved dnr 2020–06312.
Considering the observational nature of this register-based study, informed consent was waived from the Ethical Review Board.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Breast Cancer-Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies-An Updated Review
Affiliations.
- 1 Department of Surgical Oncology, Center of Oncology of the Lublin Region St. Jana z Dukli, 20-091 Lublin, Poland.
- 2 Department of Forensic Medicine, Medical University of Lublin, 20-090 Lublin, Poland.
- 3 Department of Human Anatomy, Medical University of Lublin, 20-090 Lublin, Poland.
- 4 Department of Oncology, Chair of Oncology and Environmental Health, Medical University of Lublin, 20-081 Lublin, Poland.
- PMID: 34503097
- PMCID: PMC8428369
- DOI: 10.3390/cancers13174287
Breast cancer (BC) is the most frequently diagnosed cancer in women worldwide with more than 2 million new cases in 2020. Its incidence and death rates have increased over the last three decades due to the change in risk factor profiles, better cancer registration, and cancer detection. The number of risk factors of BC is significant and includes both the modifiable factors and non-modifiable factors. Currently, about 80% of patients with BC are individuals aged >50. Survival depends on both stage and molecular subtype. Invasive BCs comprise wide spectrum tumors that show a variation concerning their clinical presentation, behavior, and morphology. Based on mRNA gene expression levels, BC can be divided into molecular subtypes (Luminal A, Luminal B, HER2-enriched, and basal-like). The molecular subtypes provide insights into new treatment strategies and patient stratifications that impact the management of BC patients. The eighth edition of TNM classification outlines a new staging system for BC that, in addition to anatomical features, acknowledges biological factors. Treatment of breast cancer is complex and involves a combination of different modalities including surgery, radiotherapy, chemotherapy, hormonal therapy, or biological therapies delivered in diverse sequences.
Keywords: breast cancer; classification; diagnosis; epidemiology; marker; prognosis; risk factors; treatment.
Publication types
Breast Cancer Research

Breast Cancer Risk Factors
Breast Cancer Research is presenting our Retrospective Collection on "Breast Cancer Risk Factors." Celebrating 'Breast Cancer Awareness Month (1 October- 31 October)', with this Collection, we aim to gain valuable insights into the multifaceted aspects of breast cancer risk to promote awareness, prevention, and early detection.
NEW CROSS-JOURNAL COLLECTIONS Find out more by clicking the links below:
Artif icial Intelligence in Breast Imaging PDGFB in Br east Cancer Initiation,Progression, and Metastasis

Aims and scope
- Most accessed
Association of area- and volumetric-mammographic density and breast cancer risk in women of Asian descent: a case control study
Authors: Shivaani Mariapun, Weang-Kee Ho, Mikael Eriksson, Nur Aishah Mohd Taib, Cheng-Har Yip, Kartini Rahmat, Per Hall and Soo-Hwang Teo
Fusogenic vesicular stomatitis virus combined with natural killer T cell immunotherapy controls metastatic breast cancer
Authors: Adam Nelson, Nichole McMullen, Simon Gebremeskel, Roberto De Antueno, Duncan Mackenzie, Roy Duncan and Brent Johnston
Enhancing pathological complete response prediction in breast cancer: the role of dynamic characterization of DCE-MRI and its association with tumor heterogeneity
Authors: Xinyu Zhang, Xinzhi Teng, Jiang Zhang, Qingpei Lai and Jing Cai
Proteogenomic characterization of difficult-to-treat breast cancer with tumor cells enriched through laser microdissection
Authors: Praveen-Kumar Raj-Kumar, Xiaoying Lin, Tao Liu, Lori A. Sturtz, Marina A. Gritsenko, Vladislav A. Petyuk, Tyler J. Sagendorf, Brenda Deyarmin, Jianfang Liu, Anupama Praveen-Kumar, Guisong Wang, Jason E. McDermott, Anil K. Shukla, Ronald J. Moore, Matthew E. Monroe, Bobbie-Jo M. Webb-Robertson…
CD163 + macrophages in the triple-negative breast tumor microenvironment are associated with improved survival in the Women’s Circle of Health Study and the Women’s Circle of Health Follow-Up Study
Authors: Angela R. Omilian, Rikki Cannioto, Lucas Mendicino, Leighton Stein, Wiam Bshara, Bo Qin, Elisa V. Bandera, Nur Zeinomar, Scott I. Abrams, Chi-Chen Hong, Song Yao, Thaer Khoury and Christine B. Ambrosone
Most recent articles RSS
View all articles
Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib
Authors: Nusayba Bagegni, Shana Thomas, Ning Liu, Jingqin Luo, Jeremy Hoog, Donald W. Northfelt, Matthew P. Goetz, Andres Forero, Mattias Bergqvist, Jakob Karen, Magnus Neumüller, Edward M. Suh, Zhanfang Guo, Kiran Vij, Souzan Sanati, Matthew Ellis…
Choosing the right cell line for breast cancer research
Authors: Deborah L Holliday and Valerie Speirs
Breast asymmetry and predisposition to breast cancer
Authors: Diane Scutt, Gillian A Lancaster and John T Manning
Triple-negative breast cancer molecular subtyping and treatment progress
Authors: Li Yin, Jiang-Jie Duan, Xiu-Wu Bian and Shi-cang Yu
Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer
Authors: Suzanne A Eccles, Eric O Aboagye, Simak Ali, Annie S Anderson, Jo Armes, Fedor Berditchevski, Jeremy P Blaydes, Keith Brennan, Nicola J Brown, Helen E Bryant, Nigel J Bundred, Joy M Burchell, Anna M Campbell, Jason S Carroll, Robert B Clarke, Charlotte E Coles…
Most accessed articles RSS

Editor-in-Chief
Lewis Chodosh , University of Pennsylvania, USA

Trending in the Media
Click here to see the most popular articles published in Breast Cancer Research in the past three months.

BCR's 20th Anniversary
20 years ago Breast Cancer Research published its first articles with BMC. Well-respected in the field, the journal has continually placed in the first quartile of the ‘Oncology’ category of Journal Citation Reports. Over the past decade, Breast Cancer Research (BCR) has also become the highest ranked breast cancer focused title in the field.
Look back at the journal’s milestone achievements and article highlights .

Featured Review - Artificial intelligence in mammographic phenotyping of breast cancer risk: a narrative review
In this review, we provide a useful reference for AI researchers investigating image-based breast cancer risk assessment while indicating key priorities and challenges that, if properly addressed, could accelerate the implementation of AI-assisted risk stratification to future refine and individualize breast cancer screening strategies.
Springer Nature Oncology Portfolio
Discover the range of academic oncology titles at Springer Nature here .


Annual Journal Metrics
2022 Citation Impact 7.4 - 2-year Impact Factor 7.4 - 5-year Impact Factor 1.764 - SNIP (Source Normalized Impact per Paper) 2.408 - SJR (SCImago Journal Rank)
2023 Speed 20 days submission to first editorial decision for all manuscripts (Median) 129 days submission to accept (Median)
2023 Usage 2,432,781 downloads 1,561 Altmetric mentions
- More about our metrics
ISSN: 1465-542X
- Submission enquiries: [email protected]

Book series
Cancer Treatment and Research
About this book series.
- Steven T. Rosen
Book titles in this series
Gastrointestinal malignancies.
- David Bentrem
- Al B. Benson
- Copyright: 2024
Available Renditions

Nutrition and Dietary Interventions in Cancer
- Rida Fatima Saeed
- Sadr Shaheed

Breast Cancer Research and Treatment
Innovative Concepts
- Ouissam Al Jarroudi
- Khalid El Bairi
- Giuseppe Curigliano
- Copyright: 2023

Epigenetics in Oncology
- Jianjun Chen
- G. Greg Wang

Cancer Drug Safety and Public Health Policy
A Changing Landscape
- Charles Bennett
- Courtney Lubaczewski
- Bartlett Witherspoon
- Copyright: 2022

Publish with us
Abstracted and indexed in.
- Chemical Abstracts Service (CAS)
- INIS Atomindex
- Open access
- Published: 11 May 2024
Erianin inhibits the progression of triple-negative breast cancer by suppressing SRC-mediated cholesterol metabolism
- Ming Li 1 ,
- Shiyao Kang 1 ,
- Xuming Deng 1 ,
- Huimin Li 1 ,
- Yuan Zhao 2 ,
- Wenru Tang 1 &
- Miaomiao Sheng 1
Cancer Cell International volume 24 , Article number: 166 ( 2024 ) Cite this article
207 Accesses
8 Altmetric
Metrics details
Triple-negative breast cancer (TNBC) is highly malignant and lacks effective biotherapeutic targets. The development of efficient anticancer drugs with low toxicity and few side effects is a hotspot in TNBC treatment research. Although erianin is known to have potent antitumor activity, its regulatory mechanism and target in TNBC have not been fully elucidated, hampering further drug development. This study showed that erianin can significantly inhibit TNBC cell proliferation and migration, promote cell apoptosis, and inhibit the growth of transplanted tumors in mice. Mechanistically, through network pharmacology analysis, molecular docking and cellular thermal shift assays, we preliminarily identified SRC as the cellular target of erianin. Erianin potently inhibited the expression of SRC, which mediated the anticancer effect of erianin in TNBC. Moreover, erianin can downregulate the expression of genes related to cholesterol synthesis and uptake by targeting SRC, interfering with cholesterol levels in TNBC, thereby inhibiting the progression of TNBC in vivo and in vitro. Taken together, our results suggest that erianin may inhibit the progression of TNBC by suppressing SRC-mediated cholesterol metabolism, and erianin has the great potential to be an effective treatment for TNBC patients.
Introduction
Breast cancer (BC) is the most commonly diagnosed cancer among women and poses a significant threat to their health. According to the latest worldwide cancer statistics of 2020, BC accounted for the highest number of new cases (2.26 million) among cancers, surpassing lung cancer (2.2 million) [ 1 ]. In China, BC contributes 12.2% and 9.6% of the world’s total annual number of new cancer cases and related deaths, respectively [ 2 ]. TNBC is a subtype of BC that accounts for 10–20% of invasive BC cases [ 3 ]. Because TNBC patients do not express the estrogen receptor, progesterone receptor or human epidermal growth factor receptor 2 (Her-2) and lack clear therapeutic targets, they often cannot benefit from endocrine therapy or anti-Her-2-targeted therapy. In addition, TNBC is characterized by high metastasis and recurrence rates [ 4 , 5 , 6 ]. Currently, the combination of taxane and anthracycline is a popular option for treating TNBC clinically. However, anthracycline causes irreversible toxic damage to the heart, which is difficult for many patients to tolerate. Moreover, once patients develop chemotherapy drug resistance, tumor relapse occurs, and the tumor metastasizes rapidly [ 7 , 8 ]. Therefore, the research and development of anticancer drugs with high efficacy and low toxicity has become an interesting topic and a difficult goal in the treatment of TNBC.
Reprogramming of lipid metabolism is a hallmark of cancer [ 9 , 10 ]. Cholesterol, an important component of blood lipids, is thought to be fundamental to cancer cell proliferation and survival [ 11 ]. In addition to being a constituent of the cell membrane, cholesterol is also a precursor to bile acids, steroids and vitamin D and plays a crucial role in cell growth and differentiation. Moreover, cholesterol is distributed to a large extent in lipid rafts, which are small regions in the cell membrane that act as cellular signal transducers [ 12 ]. Cholesterol homeostasis in mammalian cells is maintained by the regulation of de novo synthesis, uptake, efflux, and storage processes [ 13 ]. Multiple cancers have been found to have enhanced cholesterol biosynthesis, which promotes tumor growth, metastasis, stem cell maintenance, and resistance to treatments [ 11 , 14 ]. Cholesterol and its metabolites were discovered to preclinically and clinically enhance tumor progression in patients with BC. In comparison to other subtypes, TNBC demonstrates increased cholesterol biosynthesis, which may have profound biological effects and provide ideas for potential therapeutic approaches [ 13 ].
Increasing amounts of attention are being given to researching the study and application of substrates derived from natural plants. Erianin (2-methoxy-5-[2-(3,4,5-trimethoxy phenyl) ethyl]-phenol), a bibenzyl compound, is an active compound extracted from Dendrobium. It has numerous biological functions, including programmed cell death induction, angiogenesis inhibition, and antioxidant and antitumor effects. Previous studies have shown that erianin can inhibit the growth of tumor cells by inducing apoptosis [ 15 , 16 ], autophagy [ 17 , 18 ], ferroptosis [ 19 ] and other pathways. Sheng et al. [ 20 ] found that erianin could exert its anti-liver cancer effect by inhibiting the activity of pyruvate carboxylase. Chen P et al. [ 19 ] found that erianin could inhibit the proliferation and migration of lung cancer cells via calcium/calmodulin-dependent ferroptosis. At present, little is known about the antitumor effects of erianin on human BC cells in the BC setting, and it has been reported that erianin can inhibit the proliferation and migration of T47D cells and can also induce cell apoptosis [ 21 ]. Erianin and its derivative (Ecust004) can suppress BC cell growth, invasion, and migration via EMT regulation [ 22 ]. Erianin induces apoptosis in TNBC cells by inhibiting the PI3K/Akt pathway [ 23 ]. However, the molecular mechanism of action and the drug targets of erianin in TNBC are unclear, which limits the further development of this natural anticancer product.
Thus, in the present study, we explored the effects of erianin on TNBC and its underlying mechanisms. Erianin has been shown to significantly inhibit the proliferation and migration of TNBC cells and the growth of transplanted tumors. Mechanistically, our study revealed that erianin may inhibit the progression of TNBC by downregulating the expression of SRC and interfering with cholesterol metabolism. Overall, we showed that erianin exhibited potent anticancer efficacy both in vitro and in vivo and has great potential to be developed as an effective therapeutic agent for TNBC patients.
Materials and methods
Purified erianin (HPLC, ≥ 98%) was purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China, B20844). A stock solution of 800 µM was made in dimethyl sulfoxide (DMSO, D1435, Sigma, USA) and stored in the dark at -80 °C. A CCK-8 detection kit was purchased from RiboBio (C-005), goat serum was purchased from Jackson (005-000-121), and an immunohistochemical secondary antibody and DAB developer were purchased from Dako (K5007). SRC (2109 S), as well as secondary antibodies, were purchased from Cell Signaling Technology (Beverly, California, USA). GAPDH (AC001) was purchased from ABclonal (Massachusetts, USA). Vinculin (bsm-54148R) was purchased from Bioss(BeiJing, China), PP2(HY-13,805) was purchased from MCE (New Jersey, USA), 7.5% and 10% PAGE Gel Fast Preparation Kit (PG111 and PG112) were purchased from Shanghai Epizyme Biomedical Technology Co., Ltd. (Shang Hai, China), alanine aminotransferase assay kit (C009-3-1), aspartate aminotransferase assay kit (C010-1-1) and Total cholesterol assay kit (A111-1-1) were purchased from Nanjing Jiancheng Bioengineering Institute (Nan Jing, China), High-sig ECL Western Blotting Substrate (80-5001) was purchased from Tanon (Shang Hai, China), modified sodium citrate antigen repair solution (P0083), Cholesterol (≥ 99%, ST1155), RIPA Lysis Buffer (P0013B) and PMSF (ST505) were purchased from Beyotime Biotechnology (Shanghai, China), EndoFree Maxi Plasmid Kit (DP117) and BCA Protein Assay Kit (X0927) were purchased from TIANGEN (Bei Jing, China), bovine serum albumin (BSA) was purchased from Sigma‒Aldrich(9048-46-8), Annexin-V-FLUOS Statining Kit was purchased from Roche (Germany), TransIntro EL transfection reagent (FT201) was purchased from TransGen Biotech(Beijing, China). FBS (10,099,141), RPMI-1640 (C11875500BT), Opti-MEM™ (31,985,070) and DMEM (C11995500BT) were purchased from Thermo Fisher Scientific.
Cell culture
The MDA-MB-231 and 4T1 cell lines were acquired from the Cell Culture Center of Peking Union Medical College, Beijing. The cell lines were incubated in humidified incubators at 37 °C and 5% CO2. 4T1 cells were cultured in RPMI-1640 medium supplemented with 10% FBS. MDA-MB-231 cells (triple-negative/basal-like BC cells) were cultured in DMEM supplemented with 10% FBS.
Detection of cell proliferation
A CCK-8 assay was used to evaluate the effect of drug treatment on the proliferation and viability of MDA-MB-231 and 4T1 cells. The cells were inoculated into a 48-well plate at a concentration of 2 × 10 4 cells per well. When the cells were 70–80% confluent, they were treated with different drugs. After the treatment was completed, 20 µl of CCK-8 solution was added to each well at different times, and an enzyme labeling instrument was used to determine the absorbance at 450 nm. Cell survival rate (%) = (D experimental group-D blank group)/(D negative control group-D blank group)×100%.
Apoptosis detection
A propidium iodide (PI)/annexin V-FITC kit was used to detect apoptosis in two TNBC cell lines. MDA-MB-231 and 4T1 cells were plated in 6-well cell culture plates at 1 × 10 5 cells/well and incubated to achieve adherence. The cells were treated with DMSO (control) or erianin for 24 h, digested with 0.25% trypsin without EDTA, washed three times with 1× phosphate buffer saline (PBS) and finally resuspended in 1× combined buffer. Then, Annexin V-FITC/PI double staining and an Annexin V-FITC/PI apoptosis detection kit was used for staining at room temperature for 10 min. Then, the cells were analyzed by a C6 flow cytometer (USA, BD). Approximately 100,000 counts were made for each sample, and the percentage of apoptotic cells was calculated.
Cell transfection
The pcDNA3.1-SRC vector (General Biosystems, Anhui, China) was transfected into MDA-MB-231 cells using the TransIntro EL transfection reagent to determine the role of SRC. When the cell confluence reached 70%, the cell culture medium was removed, and Opti-MEM was used to mix the plasmid with TransIntro EL transfection reagent (with pcDNA3.1 empty plasmid in the control group and with pcDNA3.1-SRC plasmid in the experimental group); the mixture was incubated for 15–20 min at room temperature without light and then added to the cell petri dish. The cells were subsequently transferred to the cell incubator. The medium was replenished with complete medium after 4–6 h, and the cells were collected for follow-up experiments after 24 h.
Transwell assays
After TNBC cells were treated with 40 nM erianin for 24 h, the cells were digested and counted, and the cells were mixed with medium containing 1% FBS. Subsequently, a 24-well plate was prepared, and complete medium (500 µL) was added to the lower chamber. Additionally, 1% FBS medium (200 µL) was added to each well of the upper chamber, containing approximately 1 × 10 4 cells per well. The 24-well plate was removed after 16 h, and the liquid in the upper chamber was removed, followed by the addition of PBS and two rounds of washing. The nonmigratory cells were removed using a cotton swab. The remaining cells were fixed in 4% paraformaldehyde and stained with 1% hematoxylin for cells that had migrated to the lower surface. Observations of cell migration were made and recorded under a microscope after staining.
Animal experiment
BALB/c mice and BALB/c nude mice were purchased from Yunnan University Experimental Animal Center, Kunming University of Science and Technology. We chose 6- to 8-week-old female mice for the experiment. The mice were housed under a specific pathogen-free (SPF) grade experimental system. 4T1 cells were injected into each BALB/c mouse at 3 × 10 5 , and MDA-MB-231 cells were injected into each BALB/c nude mouse at 3 × 10 5 on the second left mammary fat pad. When the tumor size reached approximately 15 mm 3 , the mice were randomly divided into a control group and an experimental group, and the body weights of the mice were recorded. The mice in the experimental group were injected intraperitoneally with erianin at 4 mg/kg or PP2 at 8 mg/kg. The control group mice received an injection of the same volume of solvent (1% DMSO). When the mice were treated with cholesterol, 8 mg/kg cholesterol was given to the mice, and the control group was given the same volume of solvent (1% methanol). The treatments mentioned above were administered every two days, whereas the longest and shortest tumor diameters were measured using Vernier calipers, and the analysis was performed every two days. The tumor volume was calculated as follows: tumor volume = long diameter × short diameter 2 /2. After the completion of the experiment (BALB/c nude mice were treated for 21 days and BALB/c mice for 17 days), the mice were deeply anesthetized with 3% pentobarbital sodium and weighed. The mice were killed by cervical dislocation after blood was drawn from their eyeballs, after which the tumors were dissected, photographed, and weighed. All protocols were approved by the Animal Ethics Committee of Kunming University of Science and Technology (PZWH K2019-0005).
Hematoxylin and eosin (HE) assays
The liver tissue taken from the mice was placed in a 10% neutral formalin solution for 24 h, fixed for 24 h, and washed with running water for 24 h. Then, the tissue was subjected to alcohol gradient dehydration, xylene transparency, wax soaking, and embedding. The embedded wax blocks were fixed on a slicer to a thickness of 5 μm. The slices were dewaxed and rehydrated for 5 min after hematoxylin staining. After the excess hematoxylin was washed with tap water, hydrochloric acid and alcohol differentiation were carried out for 1 s, after which the ammonia was returned to blue. Three minutes later, the sections were stained with eosin, rinsed with tap water and sealed via rehydration.
Detection of glutamic oxaloacetic transaminase (GOT) and glutamic pyruvic transaminase (GPT)
GOT and GPT in blood can be used as indicators for evaluatingliver damage, and we evaluated the hepatotoxicity of erianin in mice using GOT and GPT assays. After blood was taken from the eyeballs of the mice, the blood was incubated overnight at 4 °C and centrifuged at 4 °C (2000 rpm, 15 min). The upper serum was taken for the detection of GOT and GPT. Serum (5 µl) was added to the 96-well plate, 20 µl of preheated matrix solution was added at 37 °C, and the plate was incubated at 37 °C for 30 min. Then, 2,4-dinitrophenylhydrazine solution (20 µl) was added, and the mixture was reacted at 37 °C for 20 min. Finally, 0.4 mol/L sodium hydroxide (200 µl) was added, the 96-well plate was gently shaken at room temperature for 15 min at a wavelength of 510 nm, and the optical density (OD) of each well was determined according to the absolute OD value (determined OD value - control OD value). The corresponding activity units of GOT and GPT were obtained by checking the standard curve.
Predicting the target genes of erianin in TNBC
The PubChem database ( https://pubchem.ncbi.nlm.nih.gov/ ) contains biological activity data for small organic molecules, and the molecular structure of erianin was obtained from PubChem. The PharmMapper ( http://www.lilab-ecust.cn/pharmmapper/ ) server was designed to identify potential target candidates for a given small molecule. The Comparative Toxicogenomics Database (CTD; http://ctdbase.org/ ) provides key information about gene‒protein interactions and chemical–disease and gene–disease relationships. The genes related to erianin were screened in the PharmMapper and CTD databases with “erianin” as the keyword. The GeneCards ( https://www.genecards.org/ ) and OMIM ( http://www.omim.org/ ) databases were searched using the keyword “TNBC” and the species “ Homo sapiens ”, respectively. These two sets were subsequently combined, and duplicates were removed using the UniProt ( https://www.uniprot.org/ ) database. Furthermore, Venn diagrams were generated to display the common targets.
Construction of protein‒protein interactions and hub gene networks
The online network analysis platform STRING 11.5 [ 24 ] ( http://string-db.org/ ) was used to analyze the network topology. The common targets were uploaded to the STRING 11.5 database to construct PPIs with a confidence score > 0.9, and the species “ Homo sapiens ” was selected. The PPI network was then visualized and analyzed using Cytoscape 3.8.0. NetworkAnalyzer and CytoNCA were subsequently used to screen the hub genes based on degree centrality in the PPI network.
Gene expression and survival analysis
The breast cancer gene expression dataset was downloaded from The Cancer Genome Atlas (TCGA) database ( https://www.cancer.gov/ ) after filtering patients according to the following criteria: breast, TCGA, TCGA-BRCA, ductal and lobular neoplasm, and female. Files: transcriptome profiling, gene expression quantification, and HTSeq-FPKM. The dataset comprises samples of tumor tissue and paraneoplastic normal tumors. The female participants were analyzed through transcriptome profiling and gene expression quantification using the HTSeq-FPKM dataset. The samples consisted of both tumor tissues and paraneoplastic normal samples, and patient-related clinical data were also downloaded. TNBC patients were selected based on clinical information screening for PR-negative, ER-negative, and Her-2-negative conditions through either immunohistochemistry or FISH. The expression matrices of both the normal and tumor groups were analyzed using R software. In addition, survival analysis of potential target genes of erianin action in TNBC was performed online using Kaplan‒Meier Plotter ( https://kmplot.com/analysis/ ). The best nodes were selected for grouping, and the ER, PR and Her-2 statuses were set to negative.
Molecular docking analysis
Molecular docking can be used to predict the binding abilities of small molecular compounds to target genes effectively. The 2D structure of erianin was downloaded from the PubChem database. Chem3D software was subsequently used to convert the 2D structure into a 3D structure. The 3D crystal structures of the 8 hub genes were retrieved from the PDB database. AutoDockTools 1.5.6 software was used to remove all ligands from the protein receptors and add hydrogens and charges before molecular docking simulation. Ligands and protein receptors were recorded in PDBQT format. The binding sites of erianin to the target gene receptor protein were subsequently examined using PyMOL 2.4.1. The binding energy was determined from the affinity. The higher the absolute affinity value is, the stronger the binding affinity of the erianin protein.
MDA-MB-231 cells (5 × 10 4 ) were seeded in 48-well plates. After 24 h, 100 nM si-SRC and the si-native control (Guangzhou RiboBio Biotechnology Co., Ltd.) were introduced into the cells using TransIntro® EL Transfection Reagent (FT201-01, trans, China) according to the manufacturer’s instructions. After 6 h, the cells were switched to fresh DMEM containing 10% FBS and cultured for 18 h.
Cellular thermal shift assay (CETSA)
CETSA is an assay that detects the efficiency of intracellular drug binding to target proteins based on the principle that target proteins usually become stable when bound to drug molecules. That is, as the temperature increases, the protein degrades; when the protein binds to the drug, the amount of undegraded protein increases at the same temperature. The CETSA can be used to detect the binding of small molecules to target proteins. MDA-MB-231 cells were harvested and resuspended in PBS containing 0.5% PMSF. Subsequently, the cells were lysed by three repeated freeze‒thaw cycles using liquid nitrogen and a 37 °C water bath. The cell suspension was centrifuged (12,000 rpm, 4 °C, 20 min), after which the supernatant was collected and divided into two groups. One group was treated with erianin, and the other group was treated with the same amount of DMSO for 30 min at 37 °C. Then, the samples were incubated at various temperatures (50 °C, 55 °C, 60 °C, 65 °C) for 3 min, cooled to room temperature for an additional 3 min, and finally centrifuged (12,000 rpm, 4 °C, 20 min). The protein content in the supernatant was collected and analyzed via WB.
RNA sequencing
Total RNA was extracted using TRIzol Reagent (Novogene Technology Co., Ltd.). High-throughput sequencing and analyses were subsequently performed on the mRNAs. The integrity of the RNA was tested on an Agilent 2100 bioanalyzer according to the manufacturer’s instructions, and the library was constructed with the NEBNext® Ultra™ Directional RNA Library Prep Kit for Illumina®. After the library was constructed, the library was initially quantified using a Qubit2.0 fluorometer, and the library was diluted to 1.5 ng/µl. The library’s insert size and effective concentration were subsequently detected by an Agilent 2100 bioanalyzer and qRT‒PCR. Finally, the total RNA from the indicated cells was subjected to mRNA sequencing on an Illumina NovaSeq 6000 platform. DESeq2 was used to identify the differentially expressed genes (DEGs). ClusterProfiler software was used to carry out a GO functional enrichment analysis and a KEGG pathway enrichment analysis of the differential gene sets.
Determination of cholesterol levels
A total cholesterol assay kit was used to detect the effects of different drug treatments on cholesterol levels in TNBC cells or tumor tissues. Cell samples: The prepared cell suspension was centrifuged (1500 rpm, 4 °C, 5 min), the supernatant was discarded, and the cell residue was left. RIPA lysis buffer was added for homogenization, and the cell sample was lysed by ultrasonication in an ice water bath. For tissue samples, RIPA lysis buffer and steel balls were added to the tissue block, which was subsequently ground in a tissue grinder. The homogenate prepared from the two samples was not centrifuged. During cholesterol detection, 200 µl of the working solution was added to a 96-well plate, after which 5 µl of the homogenate or standard solution was added. Following mixing, the homogenate was incubated at 37 °C for 10 min. The absorbance of each sample was measured at a wavelength of 510 nm. In addition, 100 µl of the homogenate was centrifuged (12,000 rpm, 4 °C, 30 min), and BCA was used to determine the protein concentration. Cholesterol levels were calculated as [(A sample-A blank)/(A standard-A blank)×C standard]/Cpc (C standard: standard concentration, Cpr: homogenate protein concentration).
Western blotting (WB)
TNBC cells were cultured at a density of 1 × 10 6 per well in 6-well plates. After treatment with 40 nM erianin for 24 h, the cells were lysed in precooled RIPA buffer. RIPA lysis buffer and steel balls were added to the tumor tissue blocks, which were ground in a tissue grinder. A BCA protein assay kit was used to determine the protein concentrations. Equal amounts of protein were separated by SDS‒PAGE and transferred to 0.45 μm polyvinylidene fluoride membranes (Millipore, IPVH00010)1. After blocking with 2% BSA for 2 h at room temperature, the membranes were incubated with primary antibodies at 4 °C overnight. Then, the membranes were washed with TBST buffer 3 times and incubated with an HRP-conjugated secondary antibody. The results were developed and recorded by a chemiluminescent analysis system (Tanon-5200, Tanon Science and Technology). ImageJ software was used for data analysis.
Immunohistochemistry (IHC)
The tumor tissues were fixed in 4% paraformaldehyde for 24 h, washed in running water, and embedded in paraffin. Then, the paraffin-embedded tissue was cut into 5 μm thick sections. Sodium citrate buffer was used to boil the slides for 10 min to retrieve the antigens. After blocking with goat serum solution for 30 min, the sections were incubated overnight at 4 °C with primary antibodies. The next day, a secondary antibody was introduced, followed by the addition of DAB chromogenic reagent and counterstaining with hematoxylin. The tissue sections were scored based on the intensity of the immunohistochemical signal and the number of areas that were positively stained. Finally, Image-Pro Plus 6.0 was used for quantitative analysis of the images.
Real-time PCR
TRIzol reagent (Invitrogen) was used to extract total RNA from frozen cell samples. The RNA quality and concentration were determined via agarose gel electrophoresis and spectrophotometry. Single-stranded complementary DNA was obtained by reverse transcription of 1 µg of RNA using an RT‒PCR kit (BD Biosciences, Franklin, NJ, USA). The mRNA levels of the genes detected were quantified using an Applied Biosystems 7300 Real-Time PCR System (USA) with SYBR Green Master Mix (Takara Bio, Osaka Prefecture, Osaka City, Japan). Gene expression was analyzed using the 2 −ΔΔCt method. The primers used for the real-time PCR analysis of genes related to cholesterol synthesis and uptake are shown in Table 1 . Each experiment had three independent replicates.
Statistical analysis
All the values are expressed as the means ± SDs. All the statistical analyses and graphs were generated using GraphPad Prism software. Significant differences between mean values were determined by ANOVA, followed by Fisher’s protected least significance difference test.
Erianin exerts anti-TNBC effects in vitro
To evaluate the effect of erianin on the proliferation of TNBC cells, MDA-MB-231 and 4T1 cells were treated with DMSO or different concentrations (0, 20 and 40 nM) of erianin for different times (12, 24 and 48 h). Then, a CCK-8 assay was used to detect cell proliferation. The results showed that there was a time- and dose-dependent inhibition of erianin on TNBC cell proliferation (Fig. 1 A, B). Moreover, after treatment with erianin for 24 h, erianin induced morphological changes, including cell shrinkage, an increase in the number of floating cells and increased cell debris in MDA-MB-231 and 4T1 cells (Fig. 1 C). Next, we explored the roles of erianin in cell apoptosis and migration. According to the flow cytometry analysis, erianin considerably induced TNBC cell apoptosis (Fig. 1 D, E). Transwell assays also showed that erianin significantly inhibited TNBC cell migration (Fig. 1 F). Taken together, these results indicated that erianin can inhibit the development of TNBC cells in vitro.
Erianin inhibits the growth of transplanted tumors in vivo
To investigate the antitumor effect of erianin in vivo, MDA-MB-231 cells and 4T1 cells were injected into BALB/c nude mice and BALB/c normal mice in situ. When the tumor size reached approximately 15 mm 3 , the mice were randomized into control (DMSO) or erianin (4 mg/kg) groups. Consistent with the in vitro results, erianin treatment obviously suppressed the growth of tumors (Fig. 2 A, B and D, E). While, after the administration of erianin, the weight of the mice did not decrease (Fig. 2 C and F). Further HE staining revealed no significant difference in liver morphology between the erianin group and the control group (Fig. 2 G). Moreover, erianin did not cause an increase in the blood concentration of GOT or GPT (Fig. 2 H). Collectively, these results demonstrated that erianin obviously inhibited tumor growth and had no liver toxicity in vivo.
SRC is a potential target of erianin in TNBC
Based on network pharmacology, 381 and 131 genes related to erianin were obtained from the PharmMapper and CTD databases, respectively. A total of 178 TNBC-related genes were identified by searching the OMIM database, and 897 TNBC-related genes were identified in the GeneCards database. After removing duplicate genes, 984 genes were selected for further analysis. Thereafter, both erianin targets and TNBC related genes were analyzed via a Venn diagram, and 120 common targets were identified (Fig. 3 A, B).
To further explore the targets of erianin, the above 120 common targets were uploaded to the STRING database to construct the PPI network. Cytoscape software was used to visualize the PPI network, and 120 nodes and 600 edges were obtained (Fig. 3 C). To identify the core target genes, node degree was utilized as a screening criterion, and the top 8 core target genes were selected: SRC, STAT3, AKT1, GRB2, HRAS, MAPK1, PIK3R1 and PTPN11. Subsequently, we obtained the 3D structure of erianin to predict its reliable target (Fig. 3 D). AutoDock was used to calculate the binding active pockets and binding energies between erianin and the 8 hub genes. The smaller the binding energy is, the stronger the binding force and the more stable the structure. As shown in Fig. 3 E, erianin had the strongest binding with SRC, and its potential interaction is shown in Fig. 3 F. These data suggested that erianin may affect the progression of TNBC by interacting with SRC.
To further verify the interaction of erianin with SRC, we conducted a CETSA to determine the binding capacity of erianin for SRC. MDA-MB-231 cells were exposed to 10 µM erianin at different temperatures. The results showed that the stability of SRC was significantly enhanced by binding with erianin, indicating that SRC could be a potential protein target of erianin (Fig. 3 G, H). Next, we examined the effect of erianin on SRC expression. MDA-MB-231 and 4T1 cells were treated with erianin for 24 h. The results showed that the expression of the SRC protein was significantly inhibited by erianin (Fig. 3 I, J). However, Erianin did not have a significant effect on SRC gene expression (Fig. 3 K). Moreover, in two xenograft transplantation models, the expression of SRC was also decreased in the erianin group (Fig. 3 L, M), which suggested that erianin can inhibit the expression of SRC in TNBC.
SRC can affect TNBC cell proliferation in vitro and in vivo
The above results suggested that the anticancer effect of erianin may be influenced by SRC in TNBC. Subsequently, we obtained a total of 101 TNBC samples and 113 normal tissue samples by screening TCGA database analysis, and the expression of SRC in TNBC tissues was significantly greater than that in normal tissue samples (Fig. 4 A). Survival analysis via Kaplan‒Meier Plotter showed that TNBC patients with high SRC expression had a relatively poor prognosis, although the results were not significantly different ( p > 0.05) (Fig. 4 B). To investigate the role of SRC in TNBC cell proliferation, we overexpressed the SRC gene in MDA-MB-231 cells (Fig. 5 A, B), and CCK-8 analysis showed that the SRC-overexpressing cells exhibited greater proliferation than the control cells did (Fig. 5 C). Moreover, we found that SRC overexpression attenuated the inhibitory effect of erianin on the proliferation of MDA-MB-231 cells (Fig. 5 D). Then, we further treated MDA-MB-231 and 4T1 cells with PP2, a specific inhibitor of SRC. The results showed that PP2 significantly reduced the expression of the SRC protein in cells (Fig. 6 A, B), and the proliferation of cells with low SRC expression was significantly inhibited (Fig. 6 C). These results suggested that erianin may affect TNBC proliferation by downregulating SRC. To further confirm these findings, we established two xenograft transplantation models, and the results showed that, compared with those in the control group, the tumor volume and weight were significantly lower in the PP2 treatment group (Fig. 6 D-I), as was the level of SRC expression in tumor tissues (Fig. 6 J, K). These results were consistent with the in vitro findings, indicating that SRC plays a vital role in the antitumor effects of erianin.
Erianin inhibits the proliferation of TNBC by reducing cholesterol levels
To gain insight into the signaling pathway underlying the erianin-mediated inhibition of cell proliferation, transcriptome analysis was performed. KEGG pathway analysis indicated that the steroid biosynthesis pathway was the most downregulated pathway in the erianin group (Fig. 7 A; Table 2 ), and cholesterol was the most common class of steroids. Thus, we confirmed these findings by measuring cellular cholesterol levels and found that erianin significantly decreased the cholesterol levels in MDA-MB-231 cells and 4T1 cells (Fig. 7 B). The same results were observed in transplanted tumors in mice (Fig. 7 C). Further analysis revealed that the expression of four molecules involved in cholesterol synthesis and absorption, HMGCR, SERBP2, DHCR24 and LDLR, was significantly inhibited upon erianin treatment (Fig. 7 D). The above results indicated that erianin can reduce cholesterol levels in TNBC patients. Studies have shown that cholesterol can promote the proliferation of tumor cells. To investigate the effect of cholesterol on the proliferation of TNBC cells, we treated MDA-MB-231 and 4T1 cells with cholesterol and lovastatin (an inhibitor of cholesterol synthesis), respectively. The results showed that cholesterol increased the cholesterol level in the two kinds of TNBC cells (Fig. 7 E) and significantly promoted cell proliferation (Fig. 7 F). In contrast, lovastatin reduced the cholesterol level in both TNBC cell lines (Fig. 7 G) and significantly suppressed cell proliferation (Fig. 7 H). Two mouse orthotopic transplanted tumor models were constructed to investigate the impact of cholesterol on tumor growth in vivo. The results showed that the tumor growth rate, tumor volume and weight in the cholesterol group were significantly greater than those in the control group (Fig. 7 I-M). Moreover, the cholesterol level in the tumors of the cholesterol group was significantly increased (Fig. 7 N).
Subsequently, we treated MDA-MB-231 cells with both 40 nM erianin and 20 µM cholesterol for 24 h and found that the decreases in cell proliferation and cholesterol levels induced by erianin were partially rescued by exogenous cholesterol treatment (Fig. 8 A, B). These results were further confirmed in vivo. Cholesterol treatment interfered with the inhibitory effect of erianin on tumor growth and restored cholesterol levels (Fig. 8 C-F). Taken together, these results indicated that erianin inhibits TNBC cells proliferation by reducing cholesterol levels in vitro and in vivo.
Erianin inhibits cholesterol metabolism by downregulating SRC and affects the proliferation of TNBC cells
Our study suggested that erianin may regulate TNBC cell proliferation by targeting the SRC molecule and that it can also regulate the cholesterol metabolism pathway. We then investigated whether erianin decreases cholesterol levels via SRC. To determine the relationship between SRC and cholesterol levels in TNBC, we first overexpressed SRC in MDA-MB-231 cells and showed that the overexpression of SRC significantly increased cholesterol levels in the cells (Fig. 9 A). Moreover, SRC overexpression rescued the inhibitory effect of erianin on cholesterol (Fig. 9 B). We then treated TNBC cells with 200 nM PP2. After 24 h, compared with those in the control group, the cholesterol levels in the MDA-MB-231 and 4T1 cells were significantly lower (Fig. 9 C), and the expression of genes related to cholesterol synthesis and absorption was significantly lower (Fig. 9 D). Moreover, PP2 treatment significantly reduced the cholesterol level in vivo (Fig. 9 E). These results demonstrated that erianin inhibits the progression of TNBC by downregulating SRC-mediated cholesterol metabolism (Fig. 9 F).
Erianin is an active compound found in Dendrobium that has various effects, including antioxidant, antiangiogenic, and antitumor effects. Earlier investigations have suggested that erianin may regulate the onset and progression of several cancer types, including lung and liver cancer [ 16 , 25 ], by suppressing cell proliferation, inducing cell cycle arrest, and enhancing apoptosis. Limited research has revealed that erianin can inhibit the proliferation and migration of the BC cell line T47D and induce cell apoptosis [ 21 ]. However, further in vivo experiments have not been conducted, and the drug targets and specific molecular mechanisms of erianin in TNBC have not been elucidated.
This study aimed to elucidate the regulatory role of erianin in TNBC progression and identify its drug target and molecular mechanism. We found that erianin obviously inhibit the proliferation and migration of TNBC cells and the growth of transplanted tumors, meanwhile it had no liver toxicity in vivo. To investigate the mechanism of erianin in TNBC, we performed network pharmacology analysis. The analysis revealed 120 potential target genes shared by erianin and TNBC. To determine the crucial targets of erianin in the anti-TNBC process, we conducted a PPI analysis, the results revealed that SRC, STAT3, AKT1, GRB2, HTRAS, MAPK1, PIK3R1, and PTPN11 were eight significant hub proteins.
Notably, these eight genes are commonly associated with the incidence and progression of TNBC in the literature. For instance, various studies had demonstrated that the SRC pathway was a crucial pathway that drives TNBC [ 26 ]. SRC-mediated cellular signaling can affect the proliferation, survival, migration, and invasion of TNBC cells [ 27 ]. In TNBC, suppression of STAT3 expression inhibited tumor proliferation and migration [ 28 ]. Additionally, HRAS [ 29 ] and MAPK1 [ 30 ] levels were significantly increased in TNBC patients. Similarly, PIK3R1 [ 31 ] and AKT1 [ 32 ] played crucial roles in the development of TNBC. PTPN11 downregulation may inhibite the proliferation, migration, and invasion of TNBC cells and played a pivotal role in TNBC progression [ 33 ].
To further clarify the specific targets of erianin in anti-TNBC, we performed molecular docking experiments. The results indicated that there was a strong binding interaction between erianin and SRC. We then verified the binding efficiency of erianin to SRC proteins by CETSA. SRC is a nonreceptor tyrosine kinase and the first confirmed oncogene. It was also the first protein tyrosine kinase to be described. The SRC functions as a signal transduction center and allows for coordinated cell responses to extracellular stimuli. SRC-mediated abnormal activation of SRC kinase could promote tumor cell movement, proliferation, invasion, and metastasis [ 34 ]. So, targeting SRC kinase is an attractive strategy for treating cancer. To investigate the effect of SRC on TNBC proliferation, we inhibited SRC expression with the SRC inhibitor PP2 in two TNBC cell lines. Consequently, SRC expression and cell proliferation ability were significantly reduced. In addition, PP2 also substantially inhibited the growth of transplanted tumors in mice.
To gain a better understanding of the signaling pathways through which erianin inhibited the progression of TNBC, we performed transcriptome sequencing on tumor tissues from mice treated with erianin. The results indicated that erianin strongly inhibited the biosynthesis of steroids, with cholesterol being the largest class of steroids. Cholesterol metabolism is abnormally active in tumor cells, accompanied by elevated levels of intracellular cholesterol and its metabolic products, leading to enhanced proliferation and invasion [ 35 ] and tumor drug resistance [ 36 , 37 ], affecting cell stemness [ 38 ], patient survival [ 39 ] and recurrence [ 40 ]. A large number of retrospective studies have shown that high plasma cholesterol levels are associated with a high risk of BC and are an independent risk factor for BC onset and recurrence [ 41 ]. For example, after menopause in women, a high-cholesterol diet increased the risk of BC [ 42 ]; compared with mice on a normal diet, mice on a high-fat diet had significantly elevated blood cholesterol levels and accelerated tumor growth [ 43 ]. In addition, Qiu T and others observed increased cholesterol biosynthesis in tumor stem cells derived from BC patients, and cholesterol promoted the occurrence of BC by enhancing Hedgehog signaling and tumor stem cell-like cell populations [ 44 ]. These studies indicated that cholesterol played a crucial role in the progression of BC. We treated MDA-MB-231 and 4T1 cells with cholesterol and cholesterol synthesis inhibitors (lovastatin). The results indicated that the addition of cholesterol promoted the proliferation of MDA-MB-231 and 4T1 cells. After cholesterol levels were reduced, the proliferation of TNBC cells was inhibited. Moreover, in the mouse-transplanted tumors, cholesterol significantly promoted tumor growth. In addition, we found that erianin significantly reduced cholesterol levels in both TNBC cells and xenografts. RT‒PCR experiments showed that by inhibiting the expression of genes involved in cholesterol synthesis and absorption, erianin can reduce cholesterol levels in TNBC cells. These findings indicated that erianin could inhibit the development of TNBC by reducing cholesterol levels.
Studies have shown that SRC may regulate intracellular cholesterol accumulation through multiple pathways [ 45 , 46 , 47 ]. For example, in glioblastoma, SRC could promote cholesterol imbalance through the ERK pathway [ 48 ]. Doxorubicin can reduce the expression of the key enzyme HMGCR in cholesterol synthesis by inhibiting the EGFR/SRC pathway [ 49 ]. To explore the effect of SRC on cholesterol in TNBC, we overexpressed SRC in MDA-MB-231 cells and found that the intracellular cholesterol levels significantly increased. Treatment of TNBC with PP2 resulted in a significant decrease in intracellular cholesterol levels. Moreover, RT‒PCR showed that PP2 could reduce cholesterol levels in TNBC cells by inhibiting the expression of genes related to cholesterol synthesis and absorption. In addition, we conducted a rescue experiment by treating MDA-MB-231 cells with erianin and overexpressing the SRC gene. The results showed that overexpression of the SRC gene could rescue the inhibitory effect of erianin on MDA-MB-231 cell proliferation and cholesterol. Furthermore, MDA-MB-231 cells were simultaneously treated with erianin and cholesterol. The results showed that high cholesterol levels in MDA-MB-231 cells could also rescue the inhibitory effect of erianin on cell proliferation.
However, this study still has several limitations. For instance, we only used molecular docking and thermal drift experiments to identify erianin targets, and we should further validate the above results using the SM pull-down technique. Moreover, the specific mechanism by which SRC regulates cholesterol metabolism in TNBC needs to be elucidated. Additionally, in future studies, we should investigate SRC expression and cholesterol levels and their correlation with various pathological indicators as well as TNBC patient outcomes in clinical samples.
In conclusion, our study clarified the regulatory role of erianin in the progression of TNBC and identified the drug targets and molecular mechanisms of erianin in the anti-TNBC process. The results showed that erianin significantly reduced TNBC cell proliferation, migration and inhibited the growth of transplanted tumors. Mechanistically, erianin may inhibit the progression of TNBC by downregulating the expression of SRC and interfering with cholesterol metabolism, indicating that erianin has the potential to be an effective treatment for TNBC. Moreover, this study provides a new perspective on the role of natural small molecules in treating malignant tumors.
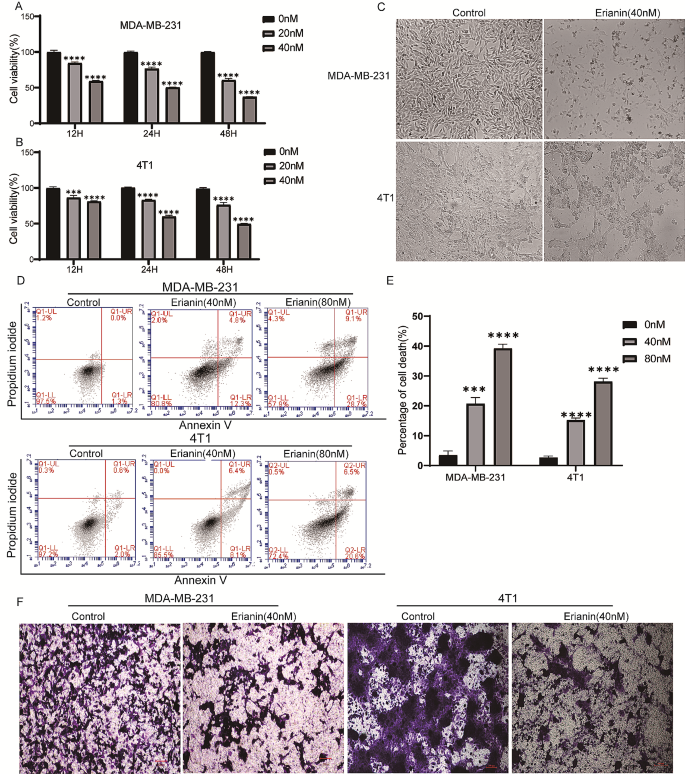
Erianin inhibits the progression of TNBC cells in vitro. MDA-MB-21 ( A ) and 4T1 ( B ) cells were treated with different concentrations (0, 20 and 40 nM) of erianin for different times (12, 24 and 48 h), after which cell viability was determined via CCK-8 assay. C . Changes in the morphology of TNBC cells were examined before and after exposure to 40 nM erianin. D . Flow cytometry results showing the distribution of MDA-MB-231 and 4T1 cell apoptosis after treatment with erianin for 24 h. E . Statistical analysis of apoptosis. F . Transwell assays were used to evaluate the effect of erianin treatment on the migration of TNBC cells. The data are presented as the means ± SDs ( n = 3); *** p < 0.001, **** p < 0.0001 compared to the control groups
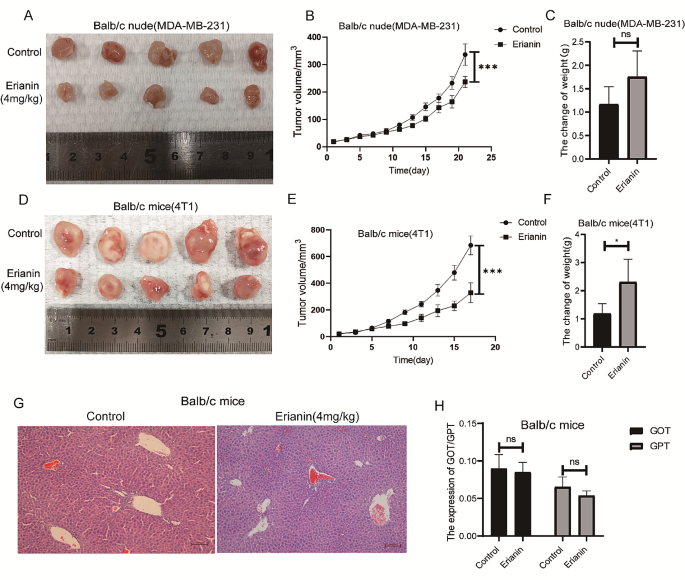
Erianin inhibits the proliferation of TNBC cells in vivo. A . A representative image of the xenograft tumors at the end of the experiment. B . Tumor volumes in the different groups. C . Changes in the body weight of BALB/c nude mice in each group. D . A representative image of the xenograft tumors at the end of the experiment. E . Tumor volumes in the different groups. F . Changes in the body weight of BALB/c mice in each group. G . Effect of erianin on liver injury in BALB/c mice. H . Effect of erianin on the blood content of GOT and GPT. The data are presented as the means ± SDs ( n = 3); * p < 0.05, *** p < 0.001, **** p < 0.0001 compared to the control group
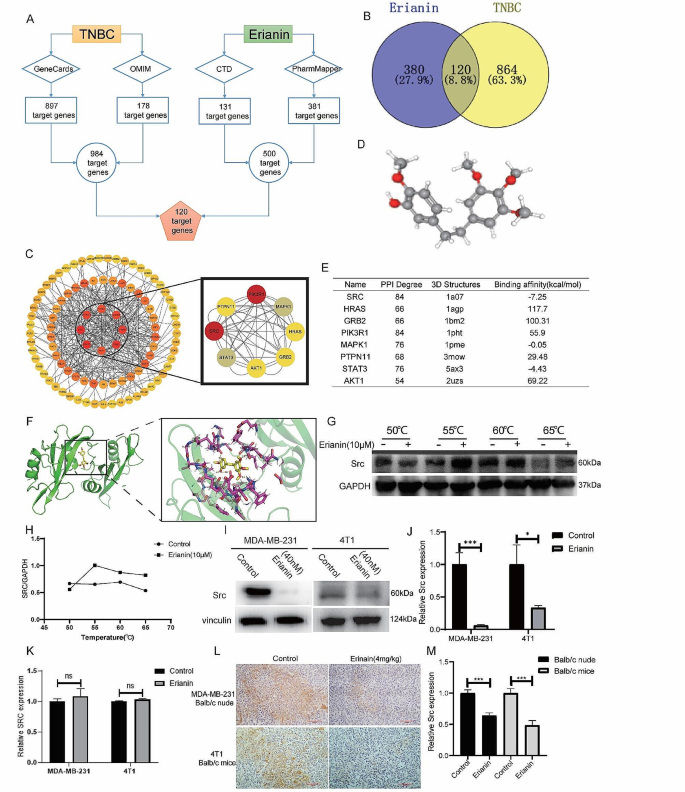
Erianin inhibits the expression of SRC in TNBC. A . Schematic diagram of 120 target genes. B . Venn diagram of common target genes between erianin and TNBC. C . PPI network of the hub genes. D . The 3D structure of erianin. E . Molecular docking results of erianin with target proteins. F . Molecular docking visualization results showing that erianin binds to SRC. G . The binding capacity of erianin with SRC was measured through CETSA. H . Quantitative analysis of the relative expression levels of SRC in CETSA. I . After 24 h of treatment with erianin, the expression of SRC was analyzed via WB. J . Quantitative analysis of the relative expression levels of SRC by Western blotting. K . After 24 h of treatment with erianin, the expression of SRC was analyzed via RT-qPCR. L . Representative images of IHC staining for SRC in xenograft tumors after treatment with erianin. M . Quantitative analysis of the relative expression levels of SRC by IHC. The data are representative of 3 independent experiments and are presented as the mean ± standard deviation. * p < 0.05, ** p < 0.01, *** p < 0.001
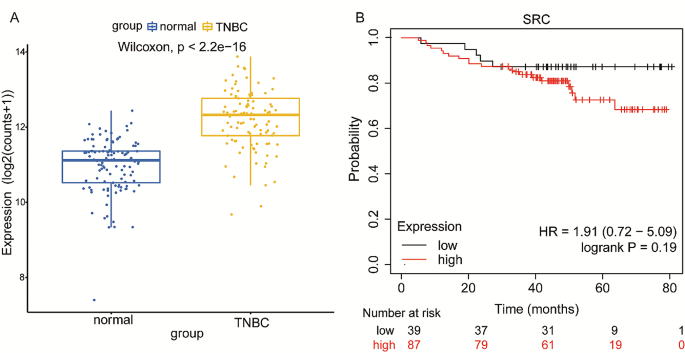
Expression of SRC in TNBC patients and its effect on survival. A . The expression of SRC in TNBC tissues was significantly greater than that in normal tissues. B . Effect of SRC expression on survival in TNBC patients
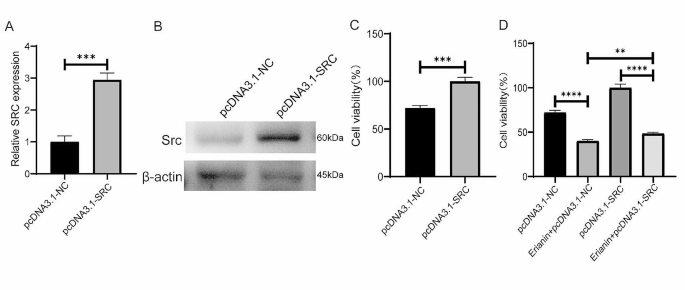
Overexpression of SRC in MDA-MB-231 cells promoted cell proliferation and attenuated the inhibitory effect of erianin on cell proliferation. A . The expression of SRC gene in MDA-MB-231 cells after the overexpression of SRC. B . The expression of Src protein in MDA-MB-231 cells after the overexpression of SRC. C . Effect of SRC overexpression on the proliferation of MDA-MB-231 cells. D . SRC overexpression attenuated the inhibitory effect of erianin on the proliferation of MDA-MB-231 cells. The data are presented as the means ± SDs ( n = 3); ** p < 0.01, *** p < 0.001, **** p < 0.0001 compared to the control group
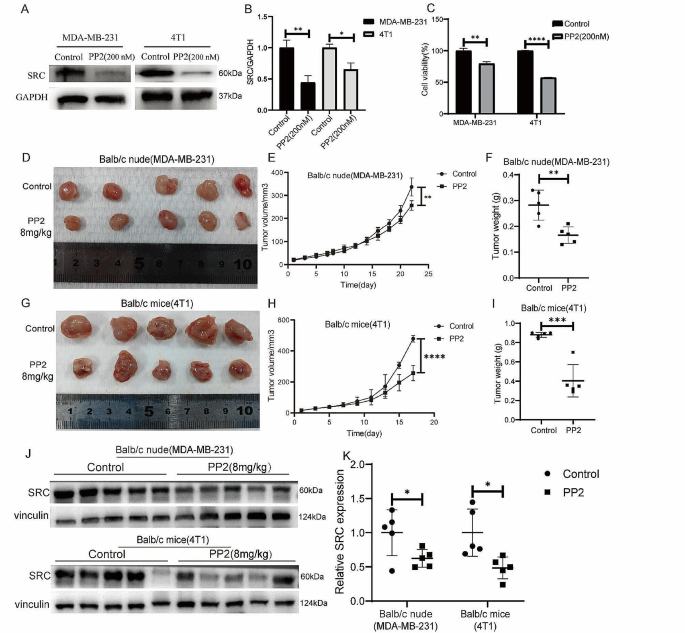
Low SRC expression inhibits the proliferation of TNBC cells in vitro and in vivo. A . Changes in Src protein levels in MDA-MB-231 and 4T1 cells treated with PP2. B . Statistical analysis of the changes in SRC protein levels in two TNBC cell lines treated with PP2. C . CCK-8 assay of MDA-MB-231 and 4T1 cells treated with PP2. D . Images of BALB/c nude mouse xenografts after PP2 treatment. E . Tumor volume in each group ( n = 5). F . Tumor weight in each group ( n = 5). G . Images of BALB/c mouse xenografts after PP2 treatment. H . Tumor volume in each group ( n = 5). I . Tumor weight in each group ( n = 5). J . The expression of SRC in two kinds of transplanted tumors treated with PP2. K . Quantitative analysis of protein expression in J. The data are presented as the means ± SDs ( n = 3); * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 compared to the control groups
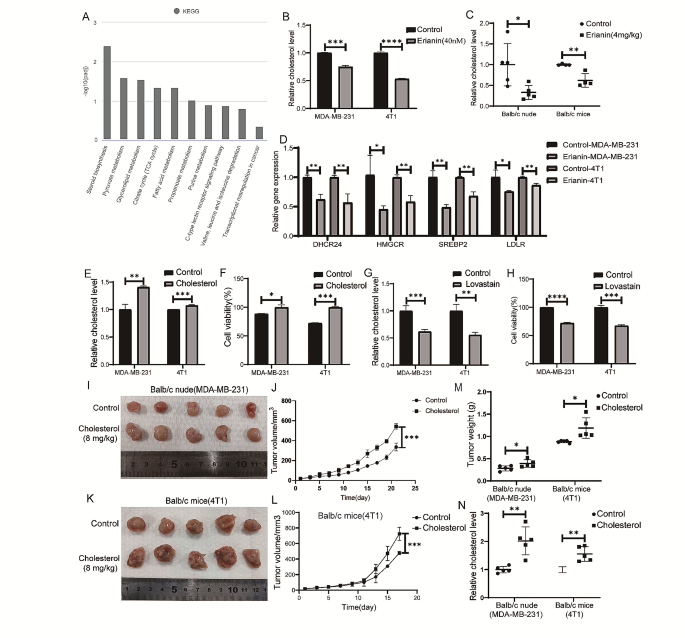
Erianin inhibits TNBC cell proliferation in vitro and in vivo by reducing cholesterol levels. A . KEGG pathway analysis. B . Changes in cholesterol levels in TNBC cells after treatment with erianin. C . Changes in cholesterol levels in transplanted tumors from mice after treatment with erianin. D . RT-PCR analysis of HMGCR, SERBP2, DHCR24, and LDLR in erianin-treated cells. E . Changes in intracellular cholesterol levels in TNBC cells treated with cholesterol. F . CCK-8 assay of TNBC cells treated with cholesterol for 24 h. G . Changes in intracellular cholesterol levels in TNBC cells treated with lovastatin. H . CCK-8 assay of TNBC cells treated with lovastatin. I . MDA-MB-231 cells were injected into BALB/c nude mice in situ, and images of xenograft tumors after cholesterol treatment were shown. J . Tumor volume in each group ( n = 5). K . 4T1 cells were injected into BALB/c mice in situ, and images of xenograft tumors after cholesterol treatment were shown. L . Tumor volume in each group ( n = 5). M . Tumor weight in each group ( n = 5). N . Cholesterol levels in tumors from two kinds of transplanted tumors. The data are presented as the means ± SDs ( n = 3); * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 compared to the control group
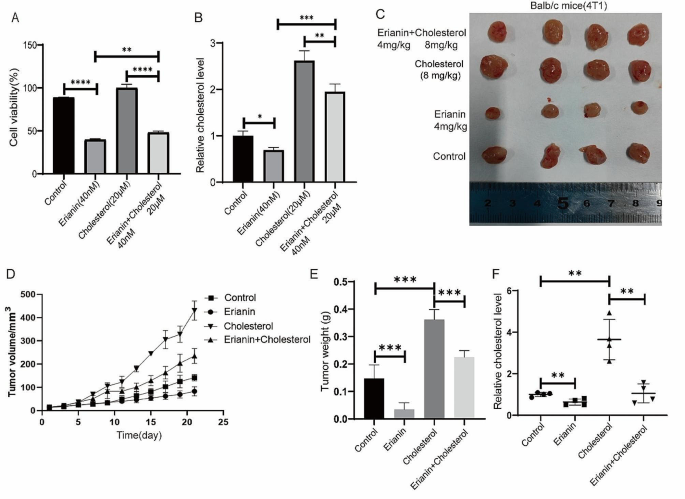
High cholesterol rescues the inhibitory effect of erianin on TNBC proliferation. A . Cell proliferation detection after MDA-MB-231 cells were treated with erianin and cholesterol. B . Changes in cholesterol levels after treatment with erianin and cholesterol in MDA-MB-231 cells. C . Representative image of xenograft tumors at the endpoint of the experiment ( n = 4). D . The tumor volume in the different groups ( n = 4). E . Tumor weight in each group ( n = 4). F . Cholesterol levels in transplanted tumors. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 compared to the control groups
Erianin inhibits the progression of TNBC by suppressing SRC-mediated cholesterol metabolism. A . Changes in the intracellular cholesterol concentration in MDA-MB-231 cells after the overexpression of SRC. B . Changes in cholesterol content after the overexpression of SRC in MDA-MB-231 cells treated with erianin. C . PP2 decreased the cholesterol levels in TNBC cells. D . RT-PCR analysis of HMGCR, SERBP2, DHCR24, and LDLR in PP2-treated cells. E . PP2 reduced cholesterol levels in mouse xenograft tumors. F . Molecular mechanism by which erianin inhibits TNBC progression. The data are presented as the means ± SDs ( n = 3); * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001 compared to the control group
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
triple-negative breast cancer
human epidermal growth factor receptor 2
breast cancer
cellular thermal shift assay
differentially expressed genes
Western blotting
immunohistochemistry
fetal bovine serum
phosphate buffer saline
optical density
bovine serum albumin
hematoxylin and eosin
dimethyl sulfoxide
The Cancer Genome Atlas
glutamic oxaloacetic transaminase
glutamic pyruvic transaminase
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Article PubMed Google Scholar
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Kumar P, Aggarwal R. An overview of triple-negative breast cancer. Arch Gynecol Obstet. 2016;293(2):247–69.
Article CAS PubMed Google Scholar
Lee KL, Kuo YC, Ho YS, Huang YH. Triple-negative breast Cancer: current understanding and future therapeutic breakthrough Targeting Cancer Stemness. Cancers (Basel) 2019, 11(9).
Geiger S, Cnossen JA, Horster S, DiGioia D, Heinemann V, Stemmler HJ. Long-term follow-up of patients with metastatic breast cancer: results of a retrospective, single-center analysis from 2000 to 2005. Anticancer Drugs. 2011;22(9):933–9.
Garrido-Castro AC, Lin NU, Polyak K. Insights into Molecular classifications of Triple-negative breast Cancer: improving patient selection for treatment. Cancer Discov. 2019;9(2):176–98.
Article CAS PubMed PubMed Central Google Scholar
Hurley J, Reis IM, Rodgers SE, Gomez-Fernandez C, Wright J, Leone JP, Larrieu R, Pegram MD. The use of neoadjuvant platinum-based chemotherapy in locally advanced breast cancer that is triple negative: retrospective analysis of 144 patients. Breast Cancer Res Treat. 2013;138(3):783–94.
Goto W, Kashiwagi S, Takada K, Asano Y, Takahashi K, Fujita H, Takashima T, Tomita S, Hirakawa K, Ohira M. Significance of intrinsic breast cancer subtypes on the long-term prognosis after neoadjuvant chemotherapy. J Transl Med. 2018;16(1):307.
Hirsch HA, Iliopoulos D, Joshi A, Zhang Y, Jaeger SA, Bulyk M, Tsichlis PN, Shirley Liu X, Struhl K. A transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell. 2010;17(4):348–61.
Cheng C, Geng F, Cheng X, Guo D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun (Lond). 2018;38(1):27.
PubMed Google Scholar
Kuzu OF, Noory MA, Robertson GP. The role of cholesterol in Cancer. Cancer Res. 2016;76(8):2063–70.
Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327(5961):46–50.
Giacomini I, Gianfanti F, Desbats MA, Orso G, Berretta M, Prayer-Galetti T, Ragazzi E, Cocetta V. Cholesterol metabolic reprogramming in Cancer and its pharmacological modulation as therapeutic strategy. Front Oncol. 2021;11:682911.
Pelton K, Freeman MR, Solomon KR. Cholesterol and prostate cancer. Curr Opin Pharmacol. 2012;12(6):751–9.
Zhu Q, Sheng Y, Li W, Wang J, Ma Y, Du B, Tang Y. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits bladder cancer cell growth via the mitochondrial apoptosis and JNK pathways. Toxicol Appl Pharmacol. 2019;371:41–54.
Yang L, Hu Y, Zhou G, Chen Q, Song Z. Erianin suppresses hepatocellular carcinoma cells through down-regulation of PI3K/AKT, p38 and ERK MAPK signaling pathways. Biosci Rep 2020, 40(7).
Chen YT, Hsieh MJ, Chen PN, Weng CJ, Yang SF, Lin CW. Erianin induces apoptosis and autophagy in oral squamous cell carcinoma cells. Am J Chin Med. 2020;48(1):183–200.
Wang H, Zhang T, Sun W, Wang Z, Zuo D, Zhou Z, Li S, Xu J, Yin F, Hua Y, et al. Erianin induces G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2016;7(6):e2247.
Chen P, Wu Q, Feng J, Yan L, Sun Y, Liu S, Xiang Y, Zhang M, Pan T, Chen X, et al. Erianin, a novel dibenzyl compound in Dendrobium extract, inhibits lung cancer cell growth and migration via calcium/calmodulin-dependent ferroptosis. Signal Transduct Target Ther. 2020;5(1):51.
Sheng Y, Chen Y, Zeng Z, Wu W, Wang J, Ma Y, Lin Y, Zhang J, Huang Y, Li W, et al. Identification of Pyruvate Carboxylase as the Cellular Target of Natural bibenzyls with potent anticancer activity against Hepatocellular Carcinoma via metabolic reprogramming. J Med Chem. 2022;65(1):460–84.
Sun J, Fu X, Wang Y, Liu Y, Zhang Y, Hao T, Hu X. Erianin inhibits the proliferation of T47D cells by inhibiting cell cycles, inducing apoptosis and suppressing migration. Am J Transl Res. 2016;8(7):3077–86.
CAS PubMed PubMed Central Google Scholar
Liu Z, Huang L, Sun L, Nie H, Liang Y, Huang J, Wu F, Hu X. Ecust004 suppresses breast Cancer Cell Growth, Invasion, and Migration via EMT regulation. Drug Des Devel Ther. 2021;15:3451–61.
Article PubMed PubMed Central Google Scholar
Xu Y, Fang R, Shao J, Cai Z. Erianin induces triple-negative breast cancer cells apoptosis by activating PI3K/Akt pathway. Biosci Rep 2021, 41(6).
Fang T, Liu L, Liu W. Network pharmacology-based strategy for predicting therapy targets of Tripterygium Wilfordii on acute myeloid leukemia. Med (Baltim). 2020;99(50):e23546.
Article CAS Google Scholar
Zhang HQ, Xie XF, Li GM, Chen JR, Li MT, Xu X, Xiong QY, Chen GR, Yin YP, Peng F et al. Erianin inhibits human lung cancer cell growth via PI3K/Akt/mTOR pathway in vitro and in vivo. Phytother Res 2021.
Canonici A, Browne AL, Ibrahim MFK, Fanning KP, Roche S, Conlon NT, O’Neill F, Meiller J, Cremona M, Morgan C, et al. Combined targeting EGFR and SRC as a potential novel therapeutic approach for the treatment of triple negative breast cancer. Ther Adv Med Oncol. 2020;12:1758835919897546.
Hamurcu Z, Delibasi N, Gecene S, Sener EF, Donmez-Altuntas H, Ozkul Y, Canatan H, Ozpolat B. Targeting LC3 and Beclin-1 autophagy genes suppresses proliferation, survival, migration and invasion by inhibition of Cyclin-D1 and uPAR/Integrin beta1/ src signaling in triple negative breast cancer cells. J Cancer Res Clin Oncol. 2018;144(3):415–30.
Zhu Z, Yuan J, Xu X, Wei Y, Yang B, Zhao H. Eucannabinolide, a novel sesquiterpene lactone, suppresses the growth, metastasis and BCSCS-like traits of TNBC via inactivation of STAT3. Neoplasia. 2021;23(1):36–48.
Peng C, Ma W, Xia W, Zheng W. Integrated analysis of differentially expressed genes and pathways in triplenegative breast cancer. Mol Med Rep. 2017;15(3):1087–94.
Huang S, Huang P, Wu H, Wang S, Liu G. Soyasaponin Ag inhibits triple-negative breast cancer progression via targeting the DUSP6/MAPK signaling. Folia Histochem Cytobiol. 2021;59(4):291–301.
Kim S, Lee E, Jung J, Lee JW, Kim HJ, Kim J, Yoo HJ, Lee HJ, Chae SY, Jeon SM, et al. microRNA-155 positively regulates glucose metabolism via PIK3R1-FOXO3a-cMYC axis in breast cancer. Oncogene. 2018;37(22):2982–91.
Yang SJ, Wang DD, Zhong SL, Chen WQ, Wang FL, Zhang J, Xu WX, Xu D, Zhang Q, Li J, et al. Tumor-derived exosomal circPSMA1 facilitates the tumorigenesis, metastasis, and migration in triple-negative breast cancer (TNBC) through miR-637/Akt1/beta-catenin (cyclin D1) axis. Cell Death Dis. 2021;12(5):420.
Liu Z, Tian Y, Chen Q, Zhang G, Li C, Luo DQ. Transcriptome Analysis of MDA-MB-231 Cells Treated with Fumosorinone isolated from insect pathogenic Fungi. Anticancer Agents Med Chem. 2020;20(4):417–28.
Sivaganesh V, Sivaganesh V, Scanlon C, Iskander A, Maher S, Le T, Peethambaran B. Protein tyrosine phosphatases: mechanisms in Cancer. Int J Mol Sci 2021, 22(23).
Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metabolism. 2020;2(2):132–41.
Article Google Scholar
Ghanbari F, Mader S, Philip A. Cholesterol as an endogenous ligand of ERRalpha promotes ERRalpha-Mediated Cellular Proliferation and Metabolic Target Gene expression in breast Cancer cells. Cells 2020, 9(8).
Ghanbari F, Fortier AM, Park M, Philip A. Cholesterol-Induced metabolic reprogramming in breast Cancer cells is mediated via the ERRalpha Pathway. Cancers (Basel) 2021, 13(11).
Qin Y, Hou Y, Liu S, Zhu P, Wan X, Zhao M, Peng M, Zeng H, Li Q, Jin T, et al. A Novel Long non-coding RNA lnc030 maintains breast Cancer stem cell stemness by stabilizing SQLE mRNA and increasing cholesterol synthesis. Adv Sci (Weinh). 2021;8(2):2002232.
Moksud N, Loo LWM, Yang J, Huang CY, Haiman CA, Le Marchand L, Wilkens LR, Cheng I. Cholesterol lowering drug use and breast cancer survival: the multiethnic cohort study. Breast Cancer Res Treat. 2021;190(1):165–73.
Mansourian M, Haghjooy-Javanmard S, Eshraghi A, Vaseghi G, Hayatshahi A, Thomas J. Statins use and risk of breast Cancer recurrence and death: a systematic review and Meta-analysis of Observational studies. J Pharm Pharm Sci. 2016;19(1):72–81.
Kitahara CM, Berrington de Gonzalez A, Freedman ND, Huxley R, Mok Y, Jee SH, Samet JM. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncology: Official J Am Soc Clin Oncol. 2011;29(12):1592–8.
Danilo C, Frank PG. Cholesterol and breast cancer development. Curr Opin Pharmacol. 2012;12(6):677–82.
Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, et al. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342(6162):1094–8.
Qiu T, Cao J, Chen W, Wang J, Wang Y, Zhao L, Liu M, He L, Wu G, Li H, et al. 24-Dehydrocholesterol reductase promotes the growth of breast cancer stem-like cells through the hedgehog pathway. Cancer Sci. 2020;111(10):3653–64.
Kasahara K, Nakayama Y, Sato I, Ikeda K, Hoshino M, Endo T, Yamaguchi N. Role of src-family kinases in formation and trafficking of macropinosomes. J Cell Physiol. 2007;211(1):220–32.
Hennuyer N, Duplan I, Paquet C, Vanhoutte J, Woitrain E, Touche V, Colin S, Vallez E, Lestavel S, Lefebvre P, et al. The novel selective PPARalpha modulator (SPPARMalpha) pemafibrate improves dyslipidemia, enhances reverse cholesterol transport and decreases inflammation and atherosclerosis. Atherosclerosis. 2016;249:200–8.
McLaren JE, Michael DR, Salter RC, Ashlin TG, Calder CJ, Miller AM, Liew FY, Ramji DP. IL-33 reduces macrophage foam cell formation. J Immunol. 2010;185(2):1222–9.
Fang R, Chen X, Zhang S, Shi H, Ye Y, Shi H, Zou Z, Li P, Guo Q, Ma L, et al. EGFR/SRC/ERK-stabilized YTHDF2 promotes cholesterol dysregulation and invasive growth of glioblastoma. Nat Commun. 2021;12(1):177.
Yun UJ, Lee JH, Shim J, Yoon K, Goh SH, Yi EH, Ye SK, Lee JS, Lee H, Park J, et al. Anti-cancer effect of doxorubicin is mediated by downregulation of HMG-Co A reductase via inhibition of EGFR/Src pathway. Lab Invest. 2019;99(8):1157–72.
Download references
This work was supported by the National Natural Science Foundation of China (grant number 82360796), the Yunnan High-level Personnel Training Support Program (grant number YNWR-QNBJ-2020-243), the Yunnan Province Science and Technology Program (grant number 202101AT070071) and Pu er city Science and Technology Program project (grant number PRKJ2023003Z).
Author information
Authors and affiliations.
Laboratory of Molecular Genetics of Aging & Tumor, Medical School, Kunming University of Science and Technology, Kunming, Yunnan, 650500, China
Ming Li, Shiyao Kang, Xuming Deng, Huimin Li, Wenru Tang & Miaomiao Sheng
Kunming University of Science and Technology Affiliated Puer City People’s Hospital, Puer, Yunnan, 665000, China
You can also search for this author in PubMed Google Scholar
Contributions
Ming Li: Writing – review and editing, writing – original draft, conceptualization; Shiyao Kang: Methodology, Data curation and Conceptualization, Supplemental article experiments; Xuming Deng: Methodology, Data curation, Conceptualization, Supplemental article experiments; Huiming Li: Methodology, Data curation, Conceptualization; Yuan Zhao: Writing – review & editing. Wenru Tang: Methodology; Miaomiao Sheng: Methodology, Data curation, Writing – original draft.
Corresponding author
Correspondence to Miaomiao Sheng .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval and consent to participate
The animal experiments were approved by the Animal Ethics Committee of Kunming University of Science and Technology.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Li, M., Kang, S., Deng, X. et al. Erianin inhibits the progression of triple-negative breast cancer by suppressing SRC-mediated cholesterol metabolism. Cancer Cell Int 24 , 166 (2024). https://doi.org/10.1186/s12935-024-03332-2
Download citation
Received : 12 December 2023
Accepted : 18 April 2024
Published : 11 May 2024
DOI : https://doi.org/10.1186/s12935-024-03332-2
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cholesterol levels
- Molecular mechanism
Cancer Cell International
ISSN: 1475-2867
- General enquiries: [email protected]
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- 16 May 2024
Gut microbes linked to fatty diet drive tumour growth
- Gillian Dohrn
You can also search for this author in PubMed Google Scholar
A Desulfovibrio bacterium. This group of gut bacteria has been linked to a suppressed immune system, which can allow breast-cancer tumours to flourish. Credit: PNWL/Alamy
Researchers have found a link between diet, a type of gut bacterium and breast cancer. The study, published on 6 May in the Proceedings of the National Academy of Science 1 , found that a high-fat diet increased the number of Desulfovibrio bacteria in the guts of mice, suppressing their immune systems and accelerating tumour growth.
Researchers say the finding could spark new ideas for therapies for breast cancer, the most common malignancy affecting women worldwide.
Erwei Song, a breast-cancer surgeon at the SunYat-Sen Memorial Hospital in Guangzhou, China, and colleagues investigated the gut bacteria of people with breast cancer after collecting data showing that those with a high body-mass index had reduced chances of survival.
“A high-fat diet might promote progression of tumours or induce recurrence of tumours,” notes Song.
The researchers took tissue and fecal samples from 61 people with breast cancer at the SunYat-Sen Memorial Hospital, before the participants started treatment.
Women whose BMI exceeded 24 — the authors’ cut-off for obesity — had higher levels of bacteria in the Desulfovibrio genus than did those whose BMI was lower than 24.
The researchers then turned to mice to further explore this link. Mice that are fed a high-fat diet often serve as a proxy for human obesity in animal studies. The team found that mice consuming a high-fat diet had more Desulfovibrio bacteria and had elevated levels of a type of cell that suppresses the immune system, myeloid-derived suppressor cells (MDSCs), which originate in the bone marrow. This suggested to the researchers that higher numbers of Desulfovibrio bacteria and a suppressed immune system were linked; they just needed to find out how.
High-fat-diet mice also had higher levels of the amino acid leucine circulating in their blood than did mice fed a normal diet. Knowing that leucine can be made by some kinds of gut bacteria, the team treated the mice with antibiotics that killed Desulfovibrio . This caused both MDSC and leucine levels to return to normal.
Of mice and people
Armed with this information, the researchers went back to the blood samples that they had taken from the people with breast cancer. As anticipated, those with a BMI of more than 24 had higher levels of leucine, more immunosuppressive MDSCs and survived fewer years post-treatment than those with a lower BMI.
In other words, Desulfovibrio bacteria, benefiting from a high-fat diet, made excess leucine. This caused a spike in the numbers of MDSCs, which suppress the immune system and allow tumours to grow.
“It’s a provocative finding that will open up new avenues that we should be thinking about,” says Stephen Hursting, a nutritional biologist at the University of North Carolina at Chapel Hill.
Katherine Loree Cook, a cancer biologist focused on microbiome research at Wake Forest University School of Medicine in Winston-Salem, North Carolina, agrees, saying the study provides “strong evidence” of a new signalling mechanism involving gut bacteria.
“The influence of the microbiome on cancer development, disease progression and response to therapy is currently a critical area of research,” says Cook.

How gut microbes are joining the fight against cancer
But, she says, the composition of the gut microbiome can vary with geography and diet, so the findings might not translate to other populations. “Gut-microbiome studies across the world often report different populations associated with different outcomes,” she says.
But both Cook and Hursting see how the findings could lead to new treatment options. “If bacterial-derived leucine seems to be driving some of the cancer risk of a high-fat diet, how do we maybe reduce that, and encourage non-leucine-producing bacteria?” asks Hursting.
Hursting has studied leucine in the context of cancer cachexia, a wasting syndrome seen in people with certain cancers that causes severe weight loss, but hadn’t considered that high levels of it could be produced by gut microbes.
“This opened up a whole new avenue to me,” Hursting says.
doi: https://doi.org/10.1038/d41586-024-01443-4
Chen, J. et al. Proc. Natl Acad. Sci. USA 121 , e2306776121 (2024).
Article PubMed Google Scholar
Download references
Reprints and permissions
Related Articles

- Medical research
Dual-action obesity drug rewires brain circuits for appetite
News & Views 15 MAY 24

Experimental obesity drug packs double punch to reduce weight
News 15 MAY 24

GLP-1-directed NMDA receptor antagonism for obesity treatment
Article 15 MAY 24
Dad’s gut microbes matter for pregnancy health and baby’s growth
News & Views Forum 01 MAY 24

Exploring the lung microbiome’s role in disease
Outlook 17 APR 24

Gut bacteria break down cholesterol — hinting at probiotic treatments
News 02 APR 24
Pig-organ transplants: what three human recipients have taught scientists
News 17 MAY 24

Neglecting sex and gender in research is a public-health risk
Comment 15 MAY 24
Brain-reading device is best yet at decoding ‘internal speech’
News 13 MAY 24
Faculty Positions& Postdoctoral Research Fellow, School of Optical and Electronic Information, HUST
Job Opportunities: Leading talents, young talents, overseas outstanding young scholars, postdoctoral researchers.
Wuhan, Hubei, China
School of Optical and Electronic Information, Huazhong University of Science and Technology
Postdoc in CRISPR Meta-Analytics and AI for Therapeutic Target Discovery and Priotisation (OT Grant)
APPLICATION CLOSING DATE: 14/06/2024 Human Technopole (HT) is a new interdisciplinary life science research institute created and supported by the...
Human Technopole
Research Associate - Metabolism
Houston, Texas (US)
Baylor College of Medicine (BCM)
Postdoc Fellowships
Train with world-renowned cancer researchers at NIH? Consider joining the Center for Cancer Research (CCR) at the National Cancer Institute
Bethesda, Maryland
NIH National Cancer Institute (NCI)
Faculty Recruitment, Westlake University School of Medicine
Faculty positions are open at four distinct ranks: Assistant Professor, Associate Professor, Full Professor, and Chair Professor.
Hangzhou, Zhejiang, China
Westlake University
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Risk Factors
- Breast Cancer Resources to Share
- What CDC Is Doing About Breast Cancer
- Advisory Committee on Breast Cancer in Young Women
- MMWR Appendix
- National Breast and Cervical Cancer Early Detection Program
- Bring Your Brave Campaign
Treatment of Breast Cancer
- Breast cancer can be treated in several ways. It depends on the type of breast cancer and how far it has spread.
- People with breast cancer often get more than one kind of treatment.
Treatment options

- Surgery: Is an operation in which doctors cut out the cancer.
- Chemotherapy: Uses special medicines to shrink or kill the cancer. The drugs can be pills you take or medicines given in your veins, or sometimes both.
- Hormonal therapy: Blocks cancer cells from getting the hormones they need to grow.
- Biological therapy: Works with your body's immune system to help it fight cancer cells or to control side effects from other cancer treatments.
- Radiation therapy: Uses high-energy rays (similar to x-rays) to kill the cancer.
For more information, visit the National Cancer Institute's Breast Cancer Treatment Option Overview. This site can also help you find health care services.
Which treatment is right for me?
Talk to your cancer doctor about the treatment options available for your type and stage of cancer. Your doctor can explain the risks and benefits of each treatment and their side effects. Side effects are how your body reacts to drugs or other treatments.
Sometimes people get an opinion from more than one cancer doctor. This is called a "second opinion." Getting a second opinion may help you choose the treatment that is right for you.
Clinical trials
Clinical trials use new treatment options to see if they are safe and effective. If you have cancer, you may want to take part. Visit the sites listed below for more information.
- NIH Clinical Research Trials and You (National Institutes of Health)
- Learn About Clinical Trials (National Cancer Institute)
- Search for Clinical Trials (National Cancer Institute)
- ClinicalTrials.gov (National Institutes of Health)
Complementary and alternative medicine
Complementary and alternative medicine are medicines and health practices that are not standard cancer treatments. Complementary medicine is used in addition to standard treatments. Alternative medicine is used instead of standard treatments. Acupuncture and supplements like vitamins and herbs are some examples.
Many kinds of complementary and alternative medicine have not been tested scientifically and may not be safe. Talk to your doctor about the risks and benefits before you start any kind of complementary or alternative medicine.
- Types of Treatment for Breast Cancer (National Cancer Institute)
- Complementary and Alternative Medicine for Patients (National Cancer Institute)
Breast Cancer
Talk to your doctor about when to start and how often to get a mammogram.
For Everyone
Public health.
- Open access
- Published: 16 May 2024
Cannabis and cancer: unveiling the potential of a green ally in breast, colorectal, and prostate cancer
- Husam A. ALSalamat 1 , 2 , 3 ,
- Sara Feras Abuarab 4 ,
- Hazem Mohamed Salamah 3 , 5 ,
- Anas Hasan Ishqair 3 , 6 ,
- Mohammad Fuad Dwikat 3 , 7 ,
- Anas Zakarya Nourelden 3 , 8 ,
- Aseel N. Qandil 1 ,
- Yasmeen Barakat 4 &
- Muna Barakat ORCID: orcid.org/0000-0002-7966-1172 4
Journal of Cannabis Research volume 6 , Article number: 24 ( 2024 ) Cite this article
4 Altmetric
Metrics details
Cancer comes in second place on the list of causes of death worldwide. In 2018, the 5-year prevalence of breast cancer (BC), prostate cancer (PC), and colorectal cancer (CRC) were 30%, 12.3%, and 10.9%, respectively. Cannabinoids are chemicals derived from the Cannabis sativa plant; the most investigated cannabinoids are cannabinol, delta 9-tetrahydrocannabinol (Δ 9 -THC), and cannabidiol. In humans, the endogenous endocannabinoid system consists of endocannabinoids, cannabinoids receptors (CBs), and enzymes that degrade the endocannabinoids. In this review, we will review the most recent literature for evidence that discusses the role of cannabis in the treatment of the three types of neoplasms mentioned. Studies have proved that BC cells express CB receptors; many in- vivo studies showed that cannabinoids cause apoptosis and inhibit proliferation and migration. Also, researchers found that treating BC mice with THC and JWH-133 (CB2 receptor agonist) slowed the tumor growth. Regarding CRC, cannabidiol was found to decrease the viability of chemotherapy-resistant CRC cells and inhibit metastasis by antagonizing the G-protein-coupled receptor 55 (GPR55; a novel cannabinoid receptor) necessary for metastasis. Moreover, cannabidiol had anti-angiogenetic effects by reducing the expression of vascular endothelial growth factor (VEGF) in addition to anti-inflammatory effects. Finally, studies demonstrated that PC cells highly express CB1 and CB2 receptors and that cannabinoids are capable of inhibiting the release of exosomes and microvesicles related to cancer progression. Cannabinoids also have antiproliferative, anti-invasive, anti-fibroblastic, cell cycle arrest, and proapoptotic effects on PC cells.
Introduction
Cancer is the second most common cause of death worldwide. In 2018, 9.6 million deaths were attributed to cancer while 18 million new cancer cases were diagnosed. By 2030, deaths and new cases are expected to increase by 37.9% and 36.3%, respectively. Breast (30%) and prostate (17.7%) cancers (BC and PC) are the most prevalent in females and males, respectively, while colorectal cancer (CRC) is the second most prevalent cancer in both sexes (Ferlay et al. 2019 ).
In 2018, over 600,000 women and 350,000 men died due to BC (number one cancer killer in females) and PC (number five cancer killer in males), respectively. In the same year, BC was the most occurring among women with an incidence of over two million women diagnosed, and PC was the second most occurring in men with an incidence of 1.2 million new cases. In the same year, the 5-year prevalence of BC was approximately 30% of all female cancer patients and the 5-year prevalence of PC was 12.3% among males. CRC was the fourth leading cause of cancer-related deaths in males, the 3 rd in females, and the 2 nd in both sexes. Also, CRC had an incidence of 1.8 million new cases making it the 3 rd most occurring in both sexes with a 5-year prevalence of 10.9% (Ferlay et al. 2019 ).
The most evident risk factors of BC include obesity, using combined estrogen and progestin hormones after menopause, alcohol consumption, early menarche, late menopause, family history of BC, and genetic predisposition especially BRCA1 and BRCA2 mutations (Friedman et al. 2006 , Smith-Warner et al. 1998 , Tamimi et al. 2016 ). The most clinically evident complication of BC is metastasis and the most common site is the bone (Kennecke et al. 2010 , Xiao et al. 2018 ). For PC, the most evident and researched risk factors are age which has a direct relationship with the risk of developing PC, ethnicity (making the highest risk among African Americans), in addition to family history (Cuzick et al. 2014 , Markozannes et al. 2016 ). PC has a very high chance of metastasis compared with other neoplasms, especially to the bones (Bubendorf et al. 2000 , Harada et al. 1992 ). CRC has many risk factors; the most important and well-evident ones are gender (males have an increased risk), age (direct relationship with the risk of developing CRC), family history, which increases the risk of developing inherited CRC syndromes such as hereditary nonpolyposis colorectal cancer (HNPCC) (that also referred to as Lynch syndrome) and familial adenomatous polyposis (FAP). Besides, patients having Inflammatory Bowel Disease (IBD) or previously diagnosed with CRC also have a higher risk of developing CRC (Cottet et al. 2012 , Czene et al. 2002 , Jess et al. 2012 , Schoen et al. 2015 ). Approximately 20% of CRC patients already have metastasis at the time of diagnosis with the liver being the most common organ (Cejas et al. 2009 , Sun et al. 2014 , Pool et al. 2012 ).
Among the oldest chemicals used in medicine throughout history are the cannabinoids. These are chemicals derived from the Cannabis sativa plant and have been used for thousands of years for their medicinal purposes and their well-known strong psychotropic effects. The oldest reports that mention the medical use of cannabis go back to 2700 B.C in China and are mentioned in the Pen Ts'ao Ching (The Classic of Herbal Medicine) for the treatment of migraine, constipation, asthma, and malaria (Touw 1981 ). Besides, people in India grew cannabis and used it as preparations (Bhang) to reduce phlegm (Grierson 1894 ). The use of cannabis has been very controversial worldwide. It has been introduced into the United States Pharmacopoeia (USP) in 1851. It gained its popularity because other hypnotics and analgesics were not yet discovered, e.g., chloral hydrate was discovered in 1869, and paraldehyde and barbitals were identified in the following 30 years (Todd 1939 ). In 1942, however, cannabis was removed from the Pharmacopoeia due to its abuse potential, variation in its quality, fear of its unidentified active compounds, and because other conventional medications that were known for their efficacy were used as alternatives (Zuardi 2006 ). Many countries around the world allowed the use of cannabis for medicinal purposes and some countries made it legal partially or under certain conditions (Hammond et al. 2020 ).
Researchers found more than 500 compounds in cannabis, of which, 60 are considered phytocannabinoids (Hanuš et al. 2016 ). The most common and extensively researched cannabinoids (CBD), which differ in their structures are cannabinol (Wood et al. 1899 ), delta 9-tetrahydrocannabinol (Δ 9 -THC) (Gaoni and Mechoulam 1964 ), and cannabidiol (Mechoulam and Shvo 1963 ). CBD is a non-psychoactive chemical present in the cannabis plant (Ahmed 2022 ). It has been demonstrated that it possesses anti-inflammatory, analgesic, and anti-tumor activities (Ahmed 2022 ). CBD may be useful in combating chemotherapy resistance in cancer cells (Ahmed 2022 ). A study discovered that CBD may be able to sensitize canine cancer cells to chemotherapy treatments (Ahmed 2022 ). THC is the major cannabinoid causing the psychoactive effects of cannabis. On the other hand, the Endocannabinoid System ECS (Gorzo et al. 2022 ) endogenously produced by our body consists of endocannabinoids, cannabinoid receptors (CBs), and the enzymes that break down endocannabinoids after executing their functions. So far, researchers have discovered two endocannabinoids, N-arachidonoylethanolamine (AEA; also referred to as anandamide) in addition to 2-arachidonylglycerol (2-AG) (Kwee et al. 2023 ). Cannabinoid receptors namely cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2) were discovered a few years later (Han et al. 2023 ). Recently, G protein-coupled receptors 55 and 119 (GPR55 and GPR119) were identified as cannabinoid receptors (dos Santos Sampaio MdF, de Paiva YB, Sampaio TB, Pereira MG, Coimbra NC. 2023 ) along with the transient receptor potential (TRP) channels which are a group of ion gated channels mostly located on the plasma membrane of several animal cell types (specifically TRPA1, TRPM8, TRPV1, and TRPV2) (Fernanda et al. 2023 ). CB1 receptors are heavily distributed in the Central Nervous System (CNS); predominantly occupying the basal ganglia and hippocampus (Yanar a, Karolin, Yazıcı Z. Cannabis 2023 ). In fact, CB1 receptors are considered the most copious type of receptors in the CNS (Araújo et al. 2023 ). While, CB2 receptors are expressed extensively in immune and hematopoietic cells, tonsils, and spleen, in addition to their presence in low quantities in the CNS (Brust et al. 2023 ). The major enzymes in the ECS responsible for degrading endocannabinoids are Fatty acid amide hydrolase (FAAH) that mainly works on AEA (Ramsay et al. 2023 ) and Monoacylglycerol Lipase (Han et al. 2023 , Vatrella et al. 2020 ) that mainly degrades 2-AG (Simard et al. 2022 ).
Recently, many studies have proven the expression of the different components of ECS in breast, colorectal, and prostate tumors. These studies demonstrate that medications targeting these components could be advantageous as antineoplastic agents. In this review, we will discuss this evidence in-depth and highlight any potential clinical values for cannabinoids in the three cancer types. Figure 1 summarizes the main effects of cannabinoids on breast, colorectal, and prostate cancer.
Summary of Cannabis effects on breast, colorectal, and prostate cancer
Cannabis in breast cancer
BC is highly prevalent in women compared to men, as 10% of women develop BC at any point in their lives (Ammar-Shehada et al. 2023 ). BC has different pathological and molecular subtypes, and each of these subtypes has different treatments with each showing distinct outcomes (Furtney et al. 2023 ). Although some therapies showed great success in treating BC some subtypes of BC did not respond adequately to these therapies, and some of them relapse. Thus, the need for new therapies are emerging, and the real challenge is to find specific therapy that targets a specific subtype of BC (Bimonte et al. 2023 ).
Many risk factors could enhance the opportunity to develop BC, such as genetic factors, nulliparity, increase hormone levels, and a decrease in both iodine level and breastfeeding (Bimonte et al. 2023 ). As, the most common breast tissues from which cancer originates are the milk ducts (ductal carcinomas) and the lobules (lobular carcinomas), which secrete milk into ducts (Kadys et al. 2023 ). Preclinical evidence has arisen in the last recent years about the effectiveness of cannabinoids in treating cancer. This evidence has been tested both in-vivo and in-vitro on both cell cultures and mice (Voicu et al. 2023 ).
Cannabinoid receptors in breast cancer
Different breast cancers have shown the expression of cannabinoid receptors (CB). In human breast carcinoma, the expression of CB1 immunoreactivity was 28% while for CB2 it was 35% (Prateeksha et al. 2023 ). A study found that CB1 receptor was expressed in 14% of the tumors and CB2 was expressed in 72% of the tumors, and in 91% of ErbB2-positive breast tumors, CB2 was expressed (Oliveira et al. 2023 ). This suggests that there is a link between CB2 and ErbB2- expression, but not between CB1 and ErbB2- expression (Selvaraj et al. 2023 ). ErbB2 (HER2) is a gene that encodes a protein involved in cell growth and division. In some subtypes of BC, ErbB2 is overexpressed or amplified, making it more aggressive and less susceptible to hormone therapy (Selvaraj et al. 2023 ). One of the BC subtypes that may be detected by measuring the expression of ErbB2 is ErbB2-positive BC (Selvaraj et al. 2023 ). ErbB2's oncogenic effect is mediated by a complex signaling network that closely regulates malignant cell motility and invasion, and therefore metastatic potential (Selvaraj et al. 2023 ). Recent attempts have been undertaken to discover gene expression profiles of ErbB2-positive invasive breast tumors, which may be key mediators of ErbB2-induced tumorigenesis and metastatic development (Selvaraj et al. 2023 ).
GPR55 is a new cannabinoid receptor, and a study found that its expression is extensive in the highly metastatic MDA-MB-231 cell lines and low by 30 folds in the low metastatic MCF-7 cell line (Morales et al. 2023 ). This study revealed that stimulating GPR55 by its endogenous ligand L-α lysophosphatidylinositol (LPI) stimulates cell migration and invasion in an MDA-MB-231 cell line. When the MCF-7 cell line transfected with pcDNA3.1 plasmid encoding human HA-GPR55 to increase the expression of GPR55, LPI enhanced the migration of the MCF-7 cell line. When the authors pretreated the MDA-MB-231 cell line with cannabidiol (which acts as a GPR55 antagonist, the effect of LPI on migration significantly decreased (Morales et al. 2023 ). On the other hand, another study reported that LPI enhances cell proliferation and Cannabidiol (CBD) blocked this effect (Martínez-Aguilar et al. 2023 ).
Anticancer effects of cannabinoids against breast cancer
The effect of cannabinoids on different breast carcinoma cell lines have been examined in- vitro, and confirmed their capability to enhance apoptosis and inhibit both proliferation and migration (Lin et al. 2023 ). A study found that ∆ 9 -THC induces apoptosis and inhibits BC cell proliferation through the activation of CB2 receptor (Zhong et al. 2023 ). A metabolite of ∆ 9 -THC called Cannabinol (CBN), which acts as an agonist on CB1 and CB2 receptors found to inhibit cell proliferation (Bimonte et al. 2023 ). As well, it was stated that endogenous CB1 agonists as Anandamide, oleamide, and 2-Arachidonoylglycerol (works as CB1 and CB2 agonist) have an antiproliferative effect (Coelho et al. 2023 ). Moreover, arvanil (which is a synthetic CB1 agonist) also inhibits cell proliferation (Tundidor et al. 2023 ). Phytocannabinoids compounds were also tested in a study to investigate their abilities in inhibiting BC growth. The study found that CBD was the most potent, followed by Cannabigerol, then cannabichromene, while cannabidiol acid was the weakest compound (Younes 2023 ).
Moreover, a research has shown an inhibitory effect for exosome and microvesicle (EMV), which play a crucial role in tumor metastasis and released by several cancer types including BC (Tomko et al. 2022 ). Exosomes are defined as the smallest vesicles (30–100 nm) fused to the plasma membrane of the cell and releasing multivesicular bodies to the out-side milieu (Tomko et al. 2022 ). Microvesicles are vesicles (0.1–1.0 μm) that are produced from cells and shed by outward blebbing of the cell membrane (Tomko et al. 2022 ). CBD enhanced cisplatin apoptotic effect against MDA-MB-231 cancer cells, in addition to significantly reducing cell viability when cells were pretreated with CBD compared to the use of cisplatin alone (Kosgodage et al. 2018 ). Cisplatin is an alkylating agent, which is a kind of chemotherapy medication (Dasari et al. 2022 ). Platinum is present in it. It causes irreversible harm to the DNA of dividing cells (Dasari et al. 2022 ). This causes cancer cells and other quickly dividing cells to die by stopping or slowing their proliferation (Dasari et al. 2022 ). Cisplatin is licensed to treat bladder cancer, ovarian cancer that has spread to other parts of the body, and testicular cancer that has spread to other parts of the body alone or in combination with other medications. It is also being researched as a therapy for other forms of cancer (Dasari et al. 2022 ). CBD is a non-psychoactive chemical present in the cannabis plant (Ahmed 2022 ). It has been demonstrated that it possesses anti-inflammatory, analgesic, and anti-tumor activities (Ahmed 2022 ). CBD may be useful in combating chemotherapy resistance in cancer cells (Ahmed 2022 ). A study discovered that CBD may be able to sensitize canine cancer cells to chemotherapy treatments (Ahmed 2022 ).
Anandamide was found to decrease the synthesis of prolactin receptors, which reduces the effect of prolactin on breast cells. This leads to decrease the synthesis of BC susceptibility gene product (BRCA1), which is a marker for the proliferation of human mammary epithelial cells (Custódio et al. 2022 ). However, ∆ 9 -THC decreases the proliferation of BC cells by arresting the cell cycle at G2-M, which leads to the induction of apoptosis. This effect was explained through activating CB2 receptors and subsequently reducing cyclin-dependent kinase-2 (Cdc2) levels, which makes the cell enter mitosis (Feng et al. 2022 ). This leads to cell cycle arrest and subsequent apoptosis (Zhong et al. 2023 ). Study found that cannabinoid receptor agonists, JWH-133 and WIN-55,212-2, decreased cell viability and migration in BC cell lines, MDA-MB-231, and MDA-MB-468 cell lines (Khunluck et al. 2022 ). It also found that JWH-133 and WIN-55,212-2 decreased tumor volume and angiogenesis in a group of mice that were subcutaneously injected with BC cell line, MDA-MB-231 cells. It also found that both JWH-133 and WIN-55,212 reduced lung metastasis (Khunluck et al. 2022 ).
During another in-vivo study, a group of MMTV (mouse mammary tumor virus)-neu mice (mice with BC) was divided into three experimental groups: control group ( n =15), 6 received THC treatment, and 8 received JWH-133, which is a synthetic CB2 receptor-selective agonist (Caffarel et al. 2010 ). At the end of treatment, the THC and JWH-133 groups showed slower tumor growth, and the lesion was smaller than the control group. Also, the number of tumors developed in the cannabinoid-treated mice was less than three tumors, while 41% of the control group showed four or more than four tumors. The researchers discovered that THC and JWH-133 caused apoptosis (shown by an increase in the number of active caspase 3-positive cells), inhibited tumor cell proliferation (as demonstrated by a decrease in the number of Ki67 positive cells), and had an anti-angiogenic effect by reducing the number of blood vessels and vascularization (as shown by CD 31 staining). Additionally, they discovered that THC reduced the proportion of animals with lung metastasis in comparison to the control group, which displayed 67% of the mice to have lung metastasis. The JWH-133 group did not exhibit a decrease in this proportion, but 50% of the lesions were smaller and could only be seen under a microscope (Caffarel et al. 2010 ).
Furthermore, the phytocannabinoid cannabidiol also reduces tumor growth and decreases the number of lung metastases in mice injected with 4T1 BC cell lines (Suttithumsatid et al. 2023 ). Moreover, the Anandamide analogue, Methanandamide, also reduces the number and size of lung tumor nodules in mice injected with TSA-1 mammary carcinoma cell line through a CB1 receptor mechanism . Strikingly, when the CB1 antagonist SR141716A administered alone, has also been reported to decrease tumor size in mice injected with MDA-MB-231 cancer cells (Li et al. 2022 ).
Effects of cannabinoids on COX-2 and prostaglandins in breast cancer
In about 40% of breast cancers, there is overexpression of Cyclooxygenase-2 (COX-2). COX-2 produces prostaglandin-E2 (PGE 2 ), which promotes angiogenesis and tumor growth (Sahu et al. 2023 ). Treating MDA-MB-231 cells with JWH-133 or WIN-55 resulted in decreasing the levels of PGE 2 in the supernatants of MDA-MB-231 cells compared to the control group. There was also a decrease in (COX-2) expression in JWH-133– and WIN-55,212-2–treated MDA-MB-231 cells (Shah et al. 2021 ). As shown in Fig. 2 , reducing PGE2 and COX-2 levels prevent cancer cell migration and metastasis.
Cannabinoid effect on BC. This figure has been redrawn form (Caffarel et al. 2012 )
Cannabis in colorectal cancer
CRC is one of the most worldwide spread of cancers. In 2018, the world health organization declared that CRC is the third most common cancer diagnosed in the world by 1.8 million cases and the second cause of death from cancer worldwide by 862,000 deaths (Wang et al. 2023 ). It Is the third common cancer in men after lung and prostate cancer and the second common cancer in women after BC.
CRC arises more common in the sigmoid part of the large intestine and the rectum (Ingleby et al. 2022 ). The causes and risk factors of CRC are multiple. Some of these risk factors are Inflammatory bowel disease, family history, obesity, diet, lifestyle, age, smoking, and genes (Hossain et al. 2022 ). Most colorectal cancers are preceded by adenomas and polyps (i.e. precancerous lesions) (Garber and Chung 2022 ).
The current treatment of CRC is the surgical removal of the tumor followed by chemotherapy such as Oxaliplatin, Fluorouracil, and Leucovorin (Garber and Chung 2022 ). Unfortunately, studies have shown that patients develop resistance against some chemotherapies such as Oxaliplatin and Fluorouracil (Hsieh et al. 2022 ). Accordingly, there is a serious need for new therapies to treat CRC and to overcome this resistance.
Cannabis has shown good evidence to be effective in CRC at both levels in-vivo and in-vitro . It shows anticancer effects either alone or in combination with other chemotherapies (Perera and Diddeniya 2022 ). However, most of the in- vivo trials were conducted on animal models. Therefore, further clinical trials on humans are required to confirm its clinical effectiveness and safety.
Cannabidiol in chemotherapy-resistant colorectal cancer
Oxaliplatin is a chemotherapeutic medication that is used to treat cancer. It is a platinum medication with alkylating properties (O'Dowd et al. 2023 ). Oxaliplatin, like other alkylating drugs, operates by interfering with the development of DNA in a cell. It kills cells by preventing them from growing and replicating (O'Dowd et al. 2023 ). This aids in the treatment of cancer, which is characterized by uncontrollable cell growth and division (O'Dowd et al. 2023 ). Exploring novel techniques to improve the efficacy of CRC treatment by identifying molecules and mechanisms linked with oxaliplatin resistance is necessary (Jeong et al. 2019 ). CBDhas the potential to assist human CRC cells overcome Oxaliplatin resistance. Jeong et al. conducted a study to demonstrate the effect of CBD on inducing autophagy in Oxaliplatin resistance colorectal cancer cell (CRC), they generated oxaliplatin-resistant cell lines, which didn’t respond to oxaliplatin treatment (Jeong et al. 2019 ). When the cell lines were treated with a combination of CBD and oxaliplatin, the death of oxaliplatin-resistant CRC was considerably raised (Jeong et al. 2019 ). The authors also performed an in-vivo study on mice. They injected a group of mice with oxaliplatin-resistant cell lines subcutaneously, then they measured the tumor size and weight every 2 days. They found that both size and weight of tumor were lower in mice that were treated with both oxaliplatin and CBD than in the non-treated control group and mice that were treated with either drug. The mechanism behind this is that CBDdecreases NOS 3 phosphorylation-which is essential for Oxaliplatin resistance development- and superoxide dismutase-2 (which is an intracellular antioxidant) increasing Reactive Oxygen Species (ROS) through mitochondrial dysfunction leading to induce autophagy (Jeong et al. 2019 ).
Autophagy (macroautophagy) is a lysosomal breakdown of cytosolic proteins, damaged organelles, and invasive microorganisms in autophagosomes, which are double-membrane vesicles formed by phagophores extending (Jeong et al. 2019 ). Chemotherapy causes stress in cells, increasing apoptosis inhibition, autophagy, and EMT-competent phenotypes via Beclin-1, Bcl-2, mammalian target of rapamycin (mTOR), adenisine monophosphate (AMP)-activated protein kinase (AMPK), and select microRNAs (Jeong et al. 2019 ). CBD promotes oxaliplatin-mediated autophagy via NOS 3 -mediated mitochondrial dysfunction, implying that NOS 3 is a viable therapeutic target for overcoming oxaliplatin resistance and that CBD could be a novel treatment option for CRC (Jeong et al. 2019 ).
Anticancer effects of CBD against colorectal cancer
Effect on apoptosis.
A recent study was conducted both in vivo and in vitro to demonstrate the mechanism of CBD in inducing apoptosis in colorectal cancer cells. CBD reduced the viability of colorectal cancer cells by causing apoptosis, as evidenced by increased production of apoptotic markers. The authors discovered that CBD activated Noxa (a protein associated with apoptosis (Jeong et al. 2019 ) and that Noxa activation increased ROS generation, resulting in DNA damage and apoptosis (Jeong et al. 2019 ).
CBD triggered apoptosis via regulating numerous pro- and anti-apoptotic proteins, of which Noxa showed significantly higher expression (Jeong et al. 2019 ). To further understand the link between Noxa and CBD-induced apoptosis, Noxa levels were reduced using siRNA, and the expression of apoptotic markers was reduced (Jeong et al. 2019 ). After ROS production was inhibited, the level of Noxa fell, suggesting that ROS is involved in the control of Noxa, which is a well-known pro-apoptotic signaling agent along with ROS (Jeong et al. 2019 ). As a consequence, in a Noxa- and ROS-dependent way, CBD promoted apoptosis (Jeong et al. 2019 ).
Another mechanism by which CBD induces apoptosis is increasing the expression of death receptor-5 (DR5), to which TNF-Related Apoptosis-Inducing Ligand (TRAIL) binds and stimulates apoptosis in colorectal cancer cells, which in return increases the sensitivity to TRAIL leading to increased apoptosis in CRC, but it didn’t affect the normal colorectal cells .
Effect on metastasis
Metastasis, especially liver metastasis, is the reason behind the poor diagnosis of most CRC (Dillekås et al. 2019 ). It is the most common cause of death in CRC patients. Early detection and treatment of CRC have a 90% five-year survival rate, but once metastasis occurs this rate decreases to 10 to 15% (Cherkasova et al. 2021 ). A population-based study on CRC liver metastases found that 25-30% of patients with CRC have liver metastases, which is a primary cause of cancer-related fatalities (Dillekås et al. 2019 ). The incidence rate of metastasis that results in mortality in CRC patients, on the other hand, is not expressly reported (Dillekås et al. 2019 ).
To treat CRC metastasis, CBD can be used. CBD acts as an antagonist to GPR55 (Pulgar et al. 2022 ). GPR55 activation has been demonstrated to promote cancer metastasis via the G12/13 proteins (Rasheed et al. 2022 ). A study discovered that after in-vitro treatment of colorectal cancer cells with CBD, CBD inhibited the GPR55 receptor, lowering colorectal cancer cell adhesion, invasiveness, and migration (Wang et al. 2023 ).
Effect on proliferation
Another mechanism by which CBD fights CRC is through the suppression of cell proliferation. Aviello et al. tested the ability of CBD to decrease colorectal cancer cells’ proliferations. CBD was demonstrated to have a substantial antiproliferative affect (Aviello et al. 2012 ). They also conducted an in-vivo trial on mice to examine cannabidiol's ability as chemo-preventive therapy (Aviello et al. 2012 ). CBD was investigated for its ability to inhibit the production of aberrant crypt foci (ACF), polyps (both of which are precancerous lesions) (Reddy et al. 2023 ), and tumors in mice treated with azoxymethane (AOM) (carcinogenic compound effective for the induction of a colon carcinoma) (Aviello et al. 2012 ). They discovered that when compared to the control group, CBD dramatically reduced the formation of ACF, polyps, and tumors in cannabidiol-pretreated mice (Aviello et al. 2012 ).
Effect on angiogenesis
Angiogenesis is very important for cancer to progress and metastasize which makes this process an effective target for cancer treatment (Praphasawat et al. 2023 ). Honarmand et al. found that CBD has an anti-angiogenesis effect subsequently, has an anticancer and antimetastatic effect on a group of mice with colon cancer by reducing the expression of VEGF (vascular endothelial growth factor) in cannabidiol-treated mice more than the non-cannabidiol-treated control group (Honarmand et al. 2019 ). The same study also reported a decrease in the tumor size in a cannabidiol-treated group compared with the non-cannabidiol-treated group (Honarmand et al. 2019 )).
Anti-inflammatory effect of cannabinoids in colorectal cancer
Inflammation can cause cancer and vice versa (Taffoni et al. 2023 ). Cannabinoids showed promising results in treating both inflammations (which could cause cancer, such as ulcerative colitis and other inflammatory bowel diseases) and cancer-induced inflammation (Bereketoğlu 2020 ). Study found that CB1 and CB2 agonists administration reduced the mice-colon inflammation, cellular infiltration subsided and the epithelium returned to the normal appearance (Wardill et al. 2023 ). The effect of endocannabinoid system agonists on inflammatory bowel disease was reviewed deeply for 51 publications (Nduma et al. 2023 ). Scientists found that cannabinoids significantly reduced macroscopic colitis severity, expressed by disease activity index (DAI) (Nduma et al. 2023 ).
Besides the anti-inflammatory effect prior to cancer, cannabinoids also decrease cancer-induced inflammation (Lyons 2023 ). Honarmand et al. found that inflammatory cytokines, IL-6 and IL-8, in mice with colon cancer were elevated significantly in the serum compared to normal mice; indicating the presence of cancer-induced inflammation. Moreover, in cannabidiol-treated mice, the IL-6 and IL-8 serum levels were significantly lower than the untreated mice with colon cancer (Honarmand et al. 2019 ), indicating that CBD decreases the cancer-induced inflammation (Honarmand et al. 2019 ).
Controversy
Although all previous evidence about the effectiveness of cannabis in treating CRC, there is a controversy about its anti-cancer effect. Some studies have shown that CB2 receptor activation with small-dose of exogenous agonist, induces cell proliferation leading to CRC (Lee et al. 2022 ). This study suggests that a low-dose agonist similar to the dose of endogenous cannabinoids promotes cancer progression. On the contrary, large doses of this agonist inhibit cancer development. Accordingly, physiological doses of CB2-agonists could promote cancer development, but using pharmacological doses (high) will inhibit cancer growth. Further studies must be conducted to clarify the effect of each receptor on CRC and to test the dose-dependent effect of cannabinoids on CB2 receptor.
Cannabis in prostate cancer
PC has been reported as the fourth most common cause of cancer globally. World health organization (WHO) estimated the prevalence of PC in 2018 as 1.3 million cases worldwide (Pak et al. 2022 ). PC comes in second place as the most common cancer in men (13.5% of total cancer cases in men) after lung cancer (14.5%) (Maldonado Ortiz 2022 ).
Current treatments of local non-metastatic PC are active surveillance, radical radiotherapy, or radical proctectomy (Reddy et al. 2023 ). In metastatic PC, androgen-deprivation therapy (ADT) is used, and the castration is accomplished either by surgical, chemical with anti-androgens, luteinizing hormone-releasing hormone (LHRH) agonists, or antagonists (Zhang et al. 2023 ). Unfortunately, after 2 to 3 years, resistance starts to develop in PC against ADT, which is then become castration-resistant prostate cancer (CRPC) (Zhang et al. 2023 ). Several drugs have been used to overcome this resistance such as docetaxel, abiraterone acetate, cabazitaxel, enzalutamide, taxanes, radium-223, and sipuleucel-T (Dell’Atti and Aguiari 2023 ). All the previous drugs have demonstrated relatively small survival benefits and resistance developed eventually (Zhang et al. 2023 ). So, still more new treatments are required.
Noteworthy, it has been demonstrated that prostatic cancer highly expresses cannabinoid receptors, CB1, and CB2. This expression is also associated with the severity of cancer; higher in the more aggressive cancers (Mahmoud et al. 2023 ). Cannabinoids showed good evidence for possible anticancer effects, through multiple mechanisms in inhibiting the growth and progression of PC.
The study found that treating prostate cancer cell lines with endocannabinoids resulted in a substantial drop in cell viability and an increase in the frequency of apoptotic cells. These findings were linked to an increase in the active form of caspase-3 and a reduction in Bcl-2, indicating apoptotic pathway activation. They also boosted the amount of Erk while decreasing the level of Akt. All of the above processes explain the decrease in cell viability and suggest that endocannabinoids may be beneficial in treating prostate tumors that do not respond to standard therapies (Singh et al. 2021 ). Caspase-3 belongs to the caspase family, which consists of 13 aspartate-specific cysteine proteases that play an important role in the execution of the apoptotic program (Asadi et al. 2022 ). It is mainly responsible for the cleavage of PARP during cell death (Asadi et al. 2022 ). Caspase-3 reduces ROS generation after activation by caspase-9 and is needed for the successful execution of apoptosis (Asadi et al. 2022 ). Activated caspase-3 cleaves a wide range of downstream substrates during apoptosis, resulting in the usual morphological alterations seen in apoptotic cells (Asadi et al. 2022 ). Bcl-2 is a member of a family of proteins that work together to decide a cell's destiny (Zupo et al. 2009 ). By maintaining the integrity of the mitochondrial membrane and inhibiting the release of apoptogenic chemicals, Bcl-2 suppresses apoptosis (Zupo et al. 2009 ). One important signaling cascade that controls a number of biological functions, including as cell division, proliferation, motility, and survival, is the extracellular signal-regulated kinase ERK pathway (Hirashima et al. 2023 ). Tumor cells that are exposed to different anticancer treatments may undergo apoptosis due to ERK activation (Hirashima et al. 2023 ).
A recent study was conducted both in-vitro and in-vivo experiments to investigate the effect of a synthetic cannabinoid, WIN-55, on PC. The authors conducted the in-vitro experiment using prostate cancer cell lines (PC3, LNCaP, and DU145). WIN-55 showed a dose-dependent antiproliferative effect on all three cell lines. The effect ranges from 46% to 69% reduction in cell proliferation according to the dose of WIN-55, and the cell line. Also, WIN showed a significant increase in the apoptotic cells’ viability in PC3 and DU145 cells in a dose-dependent manner. WIN also showed a significant increase in the percentage of cells in the G1 phase and a decrease in the percentage of cells in the S phase suggesting that WIN cause cell cycle arrest in prostate cancer cells, although a previous study reported that there was no significant difference in the distribution of PC3 cells treated with endocannabinoids across the cell cycle phases compared with the control non-treated group of PC3 cells (Singh et al. 2021 ). Also, they found that WIN significantly reduces the migration and invasion of prostate cancer cells. They did the in vivo experiment by injecting PC3 cells subcutaneously in mice. Then, they divided the mice into the control group and WIN-treated group. The WIN-treated group showed significant reductions in the tumor size compared to the control group (Pennant and Hinton 2023 ).
Effect of cannabinoids on exosomes and microvesicles released in prostate cancer
Microvesicles may carry caspase-3 away from cells as a protective mechanism against apoptosis. Exosomes and microvesicles (EMV) are associated with tumor spread and chemotherapy resistance, through expelling drugs outside the cancer cells (Liu and Wang 2023 ). Their inhibition will enhance the accumulation of chemotherapy drugs inside the cancer cells and produce the same efficacy with the lower dose, accordingly potentiate the apoptosis in the cancer cells (Liguori and Kralj-Iglič 2023 ).
In general, cannabinoids have shown a significant inhibitory effect on EMV released by different types of cancer. A study revealed that PC3 cells produced much more EMVs than BC cells or hepatocellular cancer cells, and treatment of PC3 cells with CBD (using 1 and 5 μM) reduced the EMVs by 44.5% 98.1%, respectively (Kosgodage et al. 2018 ). The reduction in EMVs with 5 μM CBD was significantly greater than the reduction with Cl-amidine, which is an effective EMVs inhibitor used as a comparator intervention. The proposed mechanism for this substantial activity is a reduction in ATP production and proton leakage, in addition to the suppression of mitochondrial respiration resulting in absence of pseudo-apoptotic responses in the PC cancer cells (Kosgodage et al. 2018 ).
In parallel, CBD also significantly decreased EMVs release from the other tumor cell lines, human hepatocellular carcinoma (HEPG2 and ECACC) and human breast adenocarcinoma (MDA-MB-231) compared to their control cells (Kosgodage et al. 2018 ). Also, CBD enhanced the apoptotic effect of cisplatin on MDA-MB-231 and HEPG2 cancer cells (Kosgodage et al. 2018 ). Prior treatment with CBD then treatment with cisplatin significantly decreased cell viability more than cisplatin-treated cells without prior treatment with CBD (Kosgodage et al. 2018 ). This shows that CBD increases the sensitivity of cancer cells to chemotherapy drugs.
Effect of Cannabinoids on cancer-associated fibroblasts in prostate cancer
Fibroblasts are the major components of the stroma of PC (Pederzoli et al. 2023 ). Fibroblasts are necessary for cancer progression, cancer metastasis, and being androgen-independent (Lasorsa et al. 2023 ). A recent study was conducted on three prostate cancer cell lines (PC-3 and DU-145, and LNCaP) and healthy prostate cells as control (PNT-1) (Pietrovito et al. 2020 ). Without harming healthy tissues, they discovered that WIN-55 can specifically reduce the cell viability of prostate cancer cell lines (Pietrovito et al. 2020 ). They found that there is a significant increase in the expression of CB1 and CB2 receptors in cancer-associated fibroblasts (CAFs) compared to the normal fibroblasts (HFPs), although treating the HFPs and CAFs with CBD showed more decrease in the viability than WIN-55, this indicates that CBD acts through different receptors, such as peroxisome proliferator-activated receptor (PPAR)-γ (Pietrovito et al. 2020 ). CAFs were associated with a significant decrease in the expression of α-smooth muscle actin, matrix metalloproteinase, and invasion abilities with WIN-55 treatment (Pietrovito et al. 2020 ).When PC-3 cells were incubated with CAFs treated with/out cannabinoids, WIN was able to significantly reduce the CAFs-induced invasion of PC-3 cells (Pietrovito et al. 2020 ).
In contrast, the same study (Pietrovito et al. 2020 ) also reported that endocannabinoid antagonists could inhibit both PC-3 cell migration and CAFs activity, which indicates that endocannabinoids can promote cancer progression (Pietrovito et al. 2020 ).
Effect of cannabinoids on ADT-treated patients
One of the reasons behind PC recurrence and progression in the ADT-treated patients is the differentiation of the prostate cancer cells into the neuroendocrine (NE)-like cells which correlates with tumor progression and poor prognosis (Bennett et al. 2023 ). This occurs in a hormone-deficient medium, which occurs in patients receiving ADT (Bennett et al. 2023 ). Using this principle, Morell et al. experimented to see the inhibitory effect of WIN on the differentiation of the prostate cancer cells into neuroendocrine-like cells (Morell et al. 2016 ). They found that WIN decreased the cell viability in both LNCaP and the NE differentiated cells, and when they incubated the LNCaP cell line with WIN, it showed a significant decrease in the NE markers in the resulting NE cells (106). The way that WIN inhibits PI3K/Akt causes AMPK to be activated, which in turn reduces NE differentiation (Morell et al. 2016 ). This is the mechanism underlying the inhibitory action. This study also found that cannabinoid receptors show a decrease during NE differentiation, and cannabinoid receptors have a tonic inhibitory effect on NE differentiation (Morell et al. 2016 ).
Treatment for ADT adverse effects may potentially benefit from cannabinoids (Mousa et al. 2020 ). According to a study, the majority of patients with advanced PC who had androgen-deprivation treatment reported feeling somewhat relieved from its side effects (Mousa et al. 2020 ). For example, pain, fatigue, sleeplessness, hot flashes, irritability, depression, headache, nausea, and vomiting are common adverse effects of androgen deprivation therapy (ADT) for individuals with PC (Mousa et al. 2020 ). Numerous research studies have demonstrated the potential effectiveness of cannabis in treating neuropathic pain, nausea, and vomiting (Mousa et al. 2020 ).
Palliative actions of cannabinoids in cancer
Night sweats.
Dronabinol (a synthetic form of delta-9-tetrahydrocannabinol) was found to be an effective therapy for paraneoplastic night sweats in cancer patients (Carr et al. 2019 ). Five patients were considered if they had a cancer diagnosis and complained of night sweats that interfered with their quality of life. All decided to try oral dronabinol to alleviate their night sweats (Carr et al. 2019 ). There were two female patients and three male patients. Two had Acute Myeloblastic Leukemia, while the others had colon cancer, rectal cancer, and BC. Patients were at various phases of disease-modifying treatments. Three patients were given 5 mg at bedtime, while one was started on 5 mg three times a day (TID) and gradually raised to 10 mg TID due to worsening symptoms (Carr et al. 2019 ). One older patient was given 2.5 mg twice a day (BID) to begin with. Patients were examined one to four weeks after starting treatment at their next planned appointment. One patient noticed a decrease in the intensity of night sweats three days after starting treatment. Two patients experienced total relief from nocturnal sweats (Carr et al. 2019 ). The other three patients indicated that the severity of their night sweats had decreased, requiring them to change clothes just once or not at all during the night. Due to sedation, one patient ceased the dronabinol and noted that his night sweats reappeared (Carr et al. 2019 ). Once the patients' night sweats were resolved, other symptoms such as anxiety and fatigue improved, leading to an improvement in their overall quality of life. All five patients' symptoms resolved after one week of starting dronabinol (Carr et al. 2019 ).
The cannabinoid receptors are highly abundant in the brain (Patthy et al. 2023 ). Normally, Pain begins when tissue is damaged, as damaged tissues release nerve growth factor, which activates mast cells. Mast cells degranulate and produce bradykinin, which activates nociceptors. The impulse is then carried through peripheral nerve fibers to the dorsal root ganglia, which merges with the spinal cord and travels to the brain (Boissoneault et al. 2023 ). The brain responds by releasing gamma-Aminobutyric acid (GABA) and other substances to inhibit pain and excitatory impulses (Boissoneault et al. 2023 ). Interestingly, CB2 receptor is found on mast cells and upon its activation by CB2 ligands and nonselective CB1/CB2 agonists, it inhibits cell degranulation . This leads to decreasing bradykinin release and nociceptor stimulation. Moreover, CB1 receptors were also found to have an analgesic effect by reducing nerve-C-fiber-driven post-discharge responses .
In reality, opioids are the most common drugs used to relieve cancer-associated pain (Dupoiron et al. 2022 ). Therefore, more than one-third of the patients receiving opioids, suffer from opioid-refractory cancer pain, in addition to opioids’ adverse effects (Diernberger et al. 2023 ). This spotlight the need to find new drugs to be used alone or as add-on therapy with opioids. Remarkably, cannabinoids showed promising results in treatment-refractory patients (Sá et al. 2023 ). A randomized, placebo-controlled trial tested the effect of Nabiximols (which is a new cannabinoid drug) as add-on therapy on opioid-refractory cancer patients. It showed that Nabiximols-treated patients reported significant analgesic effect and a decrease in pain score more than the placebo group (Sá et al. 2023 ).
Furthermore, a recent retrospective study collected 232 cancer patients (53 patients with gastrointestinal cancer) and divided them into 137 patients with THC+ and 95 patients without THC-. It found that the THC+ group showed a decrease in opioid use by 33% and those in THC- showed an increase in opioid use by 23%. Moreover, in THC- a group it is required to increase the opioid daily use by 63% to achieve the same pain relief as the THC+ group (Pawasarat et al. 2020 ). Also, an observational study included 2970 cancer patients of which 236 patients had CRC, more than 50% of patients reported high pain score (8-10 out of 10) before starting treatment, but after 6 months of using medical cannabis, less than 5% reported such high score (Bar-Lev Schleider et al. 2022 ). The same study also reported an improvement in nausea and vomiting associated with cancer (Bar-Lev Schleider et al. 2022 ).
Cannabinoids are chemicals derived from the Cannabis sativa plant and have been used for their medicinal purposes, especially for their well-known strong psychotropic effects. There is growing evidence supporting the role of Cannabinoids in numerous pathological conditions, including their role in several cancer types such as breast, colorectal, and prostate cancer. Accordingly, cannabinoids could have a promising potential as adjunctive therapy for the treatment of these types of cancers.
Availability of data and materials
Not applicable.
Abbreviations
Aberrant crypt foci
Androgen-deprivation therapy
N-arachidonoylethanolamine; anandamide
Arachidonylglycerol
Adenosine monophosphate-activated protein kinase
Azoxymethane
Breast cancer
Twice a day
Breast cancer susceptibility protein
Cancer-associated fibroblasts
Cannabinoids receptor
Cannabidiol
Crohn's Disease Activity Index
Cyclin-dependent kinase
Central nervous system
Cyclooxygenase
Colorectal cancer
Castration-resistant prostate cancer
Disease activity index
Deoxyribonucleic acid
Death receptor
Endocannabinoid system
Exosome and macrovesicle
Fatty acid amide hydrolase
Familial adenomatous polyposis
Gamma-aminobutyric acid
G-protein-coupled receptor
Hereditary nonpolyposis colorectal cancer
Inflammatory Bowel Disease
Interleukin
Luteinizing hormone-releasing hormone
Lysophosphatidylinositol
Monoacylglycerol Lipase
Medical marijuana
Mouse mammary tumor virus
Macrovesicle
Neuroendocrine
Prostate cancer
Prostaglandin
Peroxisome proliferator-activated receptor
Transforming growth factor
Tetrahydrocannabinol
Three times a day
TNF-Related Apoptosis-Inducing Ligand
Transient receptor potential
United States Pharmacopoeia
Vascular endothelial growth factor
World health organization
Ahmed L. Cytotoxicity of Non-Psychoactive Cannabinoids with an Emphasis on Cannabidiol and Ovarian Cancer. Canada: University of Toronto; 2022.
Google Scholar
Ammar-Shehada W, Abusaman K, Bracke P. Perceived support, social and marital challenges in the lives of breast cancer survivors after illness: a self-administered cross-sectional survey. Front Sociol. 2023;8:1227529.
Article PubMed PubMed Central Google Scholar
Araújo M, Almeida MB, Araújo LLN. The cannabinoids mechanism of action: an overview. BrJP. 2023.
Asadi M, Taghizadeh S, Kaviani E, Vakili O, Taheri-Anganeh M, Tahamtan M, et al. Caspase-3: structure, function, and biotechnological aspects. Biotechnol Appl Biochem. 2022;69(4):1633–45.
Article CAS PubMed Google Scholar
Aviello G, Romano B, Borrelli F, Capasso R, Gallo L, Piscitelli F, et al. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J Mol Med. 2012;90:925–34.
Bar-Lev Schleider L, Mechoulam R, Sikorin I, Naftali T, Novack V. Adherence, safety, and effectiveness of medical cannabis and epidemiological characteristics of the patient population: a prospective study. Front Med. 2022;9:827849.
Article Google Scholar
Bennett JL, Jackson BN, Miller RJ, Tsui H, Martin-Caraballo M. IL-6 evoked biochemical changes in prostate cancer cells. Cytokine. 2023;161:156079.
Bereketoğlu C. Delivery of medicinal cannabis. 2020.
Bimonte S, Palma G, Cascella M, Cuomo A. Phytocannabinoids in Triple Negative Breast Cancer Treatment: Current Knowledge and Future Insights. Anticancer Research. 2023;43(3):993–1000.
Boissoneault J, Stennett-Blackmon B, Gilmour C, Blaes S. Neural and psychosocial mechanisms underlying alcohol use and pain interactions: overview of current evidence and future directions. Curr Addict Rep. 2023;10:1–13.
Brust CA, Swanson MA, Bohn LM. Structural and functional insights into the G protein-coupled receptors: CB1 and CB2. Biochem Soc Trans. 2023;51(4):1533–43.
Article CAS PubMed PubMed Central Google Scholar
Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–83.
Caffarel MM, Andradas C, Mira E, Pérez-Gómez E, Cerutti C, Moreno-Bueno G, et al. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol Cancer. 2010;9(1):1–11.
Caffarel MM, Andradas C, Pérez-Gómez E, Guzmán M, Sánchez C. Cannabinoids: a new hope for breast cancer therapy? Cancer Treat Rev. 2012;38(7):911–8.
Carr C, Vertelney H, Fronk J, Trieu S. Dronabinol for the treatment of paraneoplastic night sweats in cancer patients: a report of five cases. J Palliat Med. 2019;22(10):1221–3.
Article PubMed Google Scholar
Cejas P, Lopez-Gomez M, Aguayo C, Madero R, de Castro Carpeno J, Belda-Iniesta C, et al. KRAS mutations in primary colorectal cancer tumors and related metastases: a potential role in prediction of lung metastasis. PloS one. 2009;4(12):e8199.
Cherkasova V, Kovalchuk O, Kovalchuk I. Cannabinoids and endocannabinoid system changes in intestinal inflammation and colorectal cancer. Cancers. 2021;13(17):4353.
Coelho MP, Duarte P, Calado M, Almeida AJ, Reis CP, Gaspar MM. The current role of cannabis and cannabinoids in health: A comprehensive review of their therapeutic potential. Life Sci. 2023;329:121838.
Cottet V, Jooste V, Fournel I, Bouvier A-M, Faivre J, Bonithon-Kopp C. Long-term risk of colorectal cancer after adenoma removal: a population-based cohort study. Gut. 2012;61(8):1180–6.
Custódio N, Savisaar R, Carvalho C, Bak-Gordon P, Ribeiro MI, Tavares J, et al. Expression Profiling in Ovarian Cancer Reveals Coordinated Regulation of BRCA1/2 and Homologous Recombination Genes. Biomed. 2022;10(2):199.
Cuzick J, Thorat MA, Andriole G, Brawley OW, Brown PH, Culig Z, et al. Prevention and early detection of prostate cancer. Lancet Oncol. 2014;15(11):e484–92.
Czene K, Lichtenstein P, Hemminki K. Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish family‐cancer database. Int J Cancer. 2002;99(2):260–6.
Dasari S, Njiki S, Mbemi A, Yedjou CG, Tchounwou PB. Pharmacological effects of cisplatin combination with natural products in cancer chemotherapy. Int J Mol Sci. 2022;23(3):1532.
Dell’Atti L, Aguiari G. The role of genetic polymorphisms in the diagnosis and management of prostate cancer: an update. Anticancer Res. 2023;43(1):317–22.
Diernberger K, Clausen E, Murray G, Wee B, Kaasa S, Hall P, et al. Cancer pain assessment and management: does an institutional approach individualise and reduce cost of care? BMJ Support Palliat Care. 2023;13:e1258–64.
Article PubMed Central Google Scholar
Dillekås H, Rogers MS, Straume O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019;8(12):5574–6.
de Fátima Dos Santos Sampaio M, de Paiva YB, Sampaio TB, Pereira MG, Coimbra NC. Therapeutic applicability of cannabidiol and other phytocannabinoids in epilepsy, multiple sclerosis and Parkinson's disease and in comorbidity with psychiatric disorders. Basic Clin Pharmacol Toxicol. 2024;134(5):574–601.
Dupoiron D, Duarte R, Carvajal G, Aubrun F, Eldabe S. Rationale and recent advances in targeted drug delivery for cancer pain: is it time to change the paradigm? Pain physician. 2022;25(3):E414–25.
PubMed Google Scholar
Feng H, Lane KA, Roumeliotis TI, Jeggo PA, Somaiah N, Choudhary JS, et al. PBAF loss leads to DNA damage-induced inflammatory signaling through defective G2/M checkpoint maintenance. Genes Devel. 2022;36(13–14):790–806.
Ferlay J, Colombet M, Soerjomataram I, Mathers C, Parkin DM, Piñeros M, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–53.
Fernanda S, Valentina D, Caterina C, Andrea C. Functional TRPA1 Channels Regulate CD56dimCD16+ NK Cell Cytotoxicity against Tumor Cells. Int J Mol Sci. 2023;24(19):14736.
Friedman E, Kotsopoulos J, Lubinski J, Lynch HT, Ghadirian P, Neuhausen SL, et al. Spontaneous and therapeutic abortions and the risk of breast cancer among BRCA mutation carriers. Breast Cancer Res. 2006;8:1–7.
Furtney I, Bradley R, Kabuka MR. Patient Graph Deep Learning to Predict Breast Cancer Molecular Subtype. IEEE/ACM Trans Comput Biol Bioinform. 2023;20(5):3117–27.
Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86(8):1646–7.
Article CAS Google Scholar
Garber JJ, Chung DC. Polyps of the Colon and Rectum. Yamada's Textbook of Gastroenterology. 2022:1447-63.
Gorzo A, Havași A, Spînu Ș, Oprea A, Burz C, Sur D. Practical Considerations for the Use of Cannabis in Cancer Pain Management—What a Medical Oncologist Should Know. Journal of Clinical Medicine. 2022;11(17):5036.
Grierson GA. The hemp plant in Sanskrit and Hindi literature. Indian Antiquary. 1894;23:260–2.
Hammond D, Goodman S, Wadsworth E, Rynard V, Boudreau C, Hall W. Evaluating the impacts of cannabis legalization: the International Cannabis Policy Study. Int J Drug Policy. 2020;77:102698.
Han Y, Dong Q, Peng J, Li B, Ma C. Laminar Distribution of Cannabinoid Receptor 1 in the Prefrontal Cortex of Nonhuman Primates. Mol Neurobiol. 2023.
Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Product Rep. 2016;33(12):1357–92.
Harada M, Iida M-i, Yamaguchi M, Shida K Analysis of bone metastasis of prostatic adenocarcinoma in 137 autopsy cases. Prostate Cancer and Bone Metastasis. 1992:173-82.
Hirashima T, Hino N, Aoki K, Matsuda M. Stretching the limits of extracellular signal-related kinase (ERK) signaling—Cell mechanosensing to ERK activation. Curr Opin Cell Biol. 2023;84:102217.
Honarmand M, Namazi F, Mohammadi A, Nazifi S. Can cannabidiol inhibit angiogenesis in colon cancer? Comparative Clinical Pathology. 2019;28:165–72.
Hossain MS, Karuniawati H, Jairoun AA, Urbi Z, Ooi DJ, John A, et al. Colorectal cancer: a review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers. 2022;14(7):1732.
Hsieh C-H, Jian C-Z, Lin L-I, Low G-S, Ou P-Y, Hsu C, et al. Potential role of CXCL13/CXCR5 signaling in immune checkpoint inhibitor treatment in cancer. Cancers. 2022;14(2):294.
Ingleby FC, Woods LM, Atherton IM, Baker M, Elliss-Brookes L, Belot A. An investigation of cancer survival inequalities associated with individual-level socio-economic status, area-level deprivation, and contextual effects, in a cancer patient cohort in England and Wales. BMC Public Health. 2022;22:1–12.
Jeong S, Kim BG, Kim DY, Kim BR, Kim JL, Park SH, et al. Cannabidiol overcomes oxaliplatin resistance by enhancing NOS3-and SOD2-induced autophagy in human colorectal cancer cells. Cancers. 2019;11(6):781.
Jeong S, Yun HK, Jeong YA, Jo MJ, Kang SH, Kim JL, et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer letters. 2019;447:12–23.
Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10(6):639–45.
Kadys A, Gremke N, Schnetter L, Kostev K, Kalder M. Intercontinental comparison of women with breast cancer treated by oncologists in Europe, Asia, and Latin America: a retrospective study of 99,571 patients. J Cancer Res Clin Oncol. 2023;149:1–8.
Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28(20):3271–7.
Khunluck T, Lertsuwan K, Chutoe C, Sooksawanwit S, Inson I, Teerapornpuntakit J, et al. Activation of cannabinoid receptors in breast cancer cells improves osteoblast viability in cancer-bone interaction model while reducing breast cancer cell survival and migration. Sci Rep. 2022;12(1):7398.
Kosgodage US, Mould R, Henley AB, Nunn AV, Guy GW, Thomas EL, et al. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front Pharmacol. 2018;9:889.
Kwee CM, Leen NA, Van der Kamp RC, Van Lissa CJ, Cath DC, Groenink L, et al. Anxiolytic effects of endocannabinoid enhancing compounds: A systematic review and meta-analysis. Eur Neuropsychopharmacol. 2023;72:79–94.
Lasorsa F, di Meo NA, Rutigliano M, Ferro M, Terracciano D, Tataru OS, et al. Emerging Hallmarks of Metabolic Reprogramming in Prostate Cancer. Int J Mol Sci. 2023;24(2):910.
Lee H-S, Tamia G, Song H-J, Amarakoon D, Wei C-I, Lee S-H. Cannabidiol exerts anti-proliferative activity via a cannabinoid receptor 2-dependent mechanism in human colorectal cancer cells. Int Immunopharmacol. 2022;108:108865.
Li P, Lin Q, Sun S, Yang N, Xia Y, Cao S, et al. Inhibition of cannabinoid receptor type 1 sensitizes triple-negative breast cancer cells to ferroptosis via regulating fatty acid metabolism. Cell Death Dis. 2022;13(9):808.
Liguori GL, Kralj-Iglič V. Pathological and Therapeutic Significance of Tumor-Derived Extracellular Vesicles in Cancer Cell Migration and Metastasis. Cancers. 2023;15(18):4425.
Lin Y-S, Huang W-H, Hsu K-F, Tang M-J, Chiu W-T. Reversion of chemoresistance by endocannabinoid-induced ER stress and autophagy activation in ovarian cancer. Am J Cancer Res. 2023;13(9):4163.
CAS PubMed PubMed Central Google Scholar
Liu Y-J, Wang C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Communication and Signaling. 2023;21(1):1–12.
Lyons D. Regulators of Mast Cell Activation: University of Northern Colorado; Dissertations [Internet]. 2023:960. https://digscholarship.unco.edu/dissertations/960 .
Mahmoud AM, Kostrzewa M, Marolda V, Cerasuolo M, Maccarinelli F, Coltrini D, et al. Cannabidiol alters mitochondrial bioenergetics via VDAC1 and triggers cell death in hormone-refractory prostate cancer. Pharmacol Res. 2023;189:106683.
Maldonado Ortiz YI. Extreme environments promote cancerous behavior in healthy cells. 2022.
Markozannes G, Tzoulaki I, Karli D, Evangelou E, Ntzani E, Gunter MJ, et al. Diet, body size, physical activity and risk of prostate cancer: An umbrella review of the evidence. Eur J Cancer. 2016;69:61–9.
Martínez-Aguilar LM, Ibarra-Sánchez A, Guerrero-Morán DJ, Macías-Silva M, Muñoz-Bello JO, Padilla A, et al. Lysophosphatidylinositol Promotes Chemotaxis and Cytokine Synthesis in Mast Cells with Differential Participation of GPR55 and CB2 Receptors. Int J Mol Sci. 2023;24(7):6316.
Mechoulam R, Shvo Y. Hashish—I: the structure of cannabidiol. Tetrahedron. 1963;19(12):2073–8.
Morales P, Guerrero-Alba R, Marichal-Cancino BA. Linking the G-protein-coupled receptor 55 (GPR55) to the cannabinoid receptors (CB1 and CB2): A new narrative. Cannabis Use, Neurobiology, Psychology, and Treatment: Elsevier; 2023:395-406.
Morell C, Bort A, Vara D, Ramos-Torres A, Rodríguez-Henche N, Díaz-Laviada I. The cannabinoid WIN 55,212–2 prevents neuroendocrine differentiation of LNCaP prostate cancer cells. Prostate Cancer Prostatic Dis. 2016;19(3):248–57.
Mousa A, Petrovic M, Fleshner NE. Prevalence and predictors of cannabis use among men receiving androgen-deprivation therapy for advanced prostate cancer. Can Urological Assoc J. 2020;14(1):E20.
Nduma BN, Mofor KA, Tatang J, Ekhator C, Ambe S, Fonkem E, et al. The Use of Cannabinoids in the Treatment of Inflammatory Bowel Disease (IBD): A Review of the Literature. Cureus. 2023;15(3):e36148.
PubMed PubMed Central Google Scholar
O’Dowd PD, Sutcliffe DF, Griffith DM. Oxaliplatin and its derivatives–An overview. Coord Chem Rev. 2023;497:215439.
Oliveira HA, Somvanshi RK, Kumar U. Comparative changes in breast cancer cell proliferation and signalling following somatostatin and cannabidiol treatment. Biochem Biophys Res Commun. 2023;643:30–8.
Pak S, Jung K-W, Park E-H, Ko YH, Won Y-J, Joung JY. Incidence and mortality projections for major cancers among Korean men until 2034, with a focus on prostate cancer. Invest Clin Urology. 2022;63(2):175.
Patthy Á, Hanics J, Zachar G, Kovács GG, Harkany T, Alpár A. Regional redistribution of CB1 cannabinoid receptors in human foetal brains with Down’s syndrome and their functional modifications in Ts65Dn+/+ mice. Neuropathol Appl Neurobiol. 2023;49(1):e12887.
Pawasarat IM, Schultz EM, Frisby JC, Mehta S, Angelo MA, Hardy SS, et al. The efficacy of medical marijuana in the treatment of cancer-related pain. Journal of palliative medicine. 2020;23(6):809–16.
Pederzoli F, Raffo M, Pakula H, Ravera F, Nuzzo PV, Loda M. Stromal cells in prostate cancer pathobiology: friends or foes? Br J Cancer. 2023;128(6):930–9.
Pennant NM, Hinton CV. The evolution of cannabinoid receptors in cancer. WIREs Mech Dis. 2023;15:e1602.
Perera PK, Diddeniya JID, editors. In-vitro and in-vivo supportive research on medicinal properties of Cannabis sativa: a comprehensive review. 2022.
Pietrovito L, Iozzo M, Bacci M, Giannoni E, Chiarugi P. Treatment with cannabinoids as a promising approach for impairing fibroblast activation and prostate cancer progression. Int J Mol Sci. 2020;21(3):787.
Praphasawat R, Klajing W, Palipoch S, Wimuttiyanon J, Wutti J, Saypeark N, et al. Cancer Signaling Pathway and Anti-Cancer Mechanism of Cannabidiol. J Med Assoc Thai. 2023;106(2):219–27.
Prateeksha P, Sharma VK, Singh SM, Sharma M, Diwan D, Hesham AE-L, et al. Tetrahydrocannabinols: potential cannabimimetic agents for cancer therapy. Cancer Metastasis Rev. 2023:1-23.
Pulgar VM, Howlett AC, Eldeeb K. WIN55212-2 Modulates Intracellular Calcium via CB1 Receptor-Dependent and Independent Mechanisms in Neuroblastoma Cells. Cells. 2022;11(19):2947.
Ramsay S, Spencer NJ, Zagorodnyuk V. Endocannabinoids, anandamide and 2-AG, regulate mechanosensitivity of mucosal afferents in the Guinea pig bladder. Eur J Pharmacol. 2023;945:175624.
Rasheed SAK, Subramanyan LV, Lim WK, Udayappan UK, Wang M, Casey PJ. The emerging roles of Gα12/13 proteins on the hallmarks of cancer in solid tumors. Oncogene. 2022;41(2):147–58.
Reddy D, van Son M, Peters M, Bertoncelli Tanaka M, Dudderidge T, Cullen E, et al. Focal therapy versus radical prostatectomy and external beam radiotherapy as primary treatment options for non-metastatic prostate cancer: results of a cost-effectiveness analysis. J Med Econ. 2023;26(1):1099–107.
Sá SS, Melo-Alvim C, Reis-Pina P. Effects of cannabinoids on pain control, quality of life and opioid-sparing in cancer patients: systematic review. BrJP. 2023;6:320–9.
Sahu A, Raza K, Pradhan D, Jain AK, Verma S. Cyclooxygenase-2 as a therapeutic target against human breast cancer: A comprehensive review. WIREs Mech Dis. 2023;15(3):e1596.
Schoen RE, Razzak A, Kelly JY, Berndt SI, Firl K, Riley TL, et al. Incidence and mortality of colorectal cancer in individuals with a family history of colorectal cancer. Gastroenterol. 2015;149(6):1438–45 e1.
Selvaraj NB, Swaroop AK, Mariappan E, Natarajan J, Thangavelu P, Selvaraj J. Effect of Calcitriol in Inhibiting the Cancer Cell Growth and Promoting Apoptosis in ErbB2-positive Breast Cancer Cells. Anticancer Agents Med Chem. 2023;23(18):2056–71.
Shah SA, Gupta AS, Kumar P. Emerging role of cannabinoids and synthetic cannabinoid receptor 1/cannabinoid receptor 2 receptor agonists in cancer treatment and chemotherapy-associated cancer management. J Cancer Res Ther. 2021;17(1):1–9.
Simard M, Archambault A-S, Lavoie J-PC, Dumais É, Di Marzo V, Flamand N. Biosynthesis and metabolism of endocannabinoids and their congeners from the monoacylglycerol and N-acyl-ethanolamine families. Biochemical Pharmacol. 2022;205:115261.
Singh K, Nassar N, Bachari A, Schanknecht E, Telukutla S, Zomer R, et al. The pathophysiology and the therapeutic potential of cannabinoids in prostate cancer. Cancers. 2021;13(16):4107.
Smith-Warner SA, Spiegelman D, Yaun S-S, Van Den Brandt PA, Folsom AR, Goldbohm RA, et al. Alcohol and breast cancer in women: a pooled analysis of cohort studies. Jama. 1998;279(7):535–40.
Sun V, Grant M, McMullen CK, Altschuler A, Mohler MJ, Hornbrook MC, et al. From diagnosis through survivorship: health-care experiences of colorectal cancer survivors with ostomies. Support Care Cancer. 2014;22:1563–70.
Suttithumsatid W, Sukketsiri W, Panichayupakaranant P. Cannabinoids and standardized cannabis extracts inhibit migration, invasion, and induce apoptosis in MCF-7 cells through FAK/MAPK/Akt/NF-κB signaling. Toxicol Vitro. 2023;93:105667.
Taffoni C, Marines J, Chamma H, Guha S, Saccas M, Bouzid A, et al. DNA damage repair kinase DNA-PK and cGAS synergize to induce cancer-related inflammation in glioblastoma. The EMBO Journal. 2023;42(7):e111961.
Tamimi RM, Spiegelman D, Smith-Warner SA, Wang M, Pazaris M, Willett WC, et al. Population attributable risk of modifiable and nonmodifiable breast cancer risk factors in postmenopausal breast cancer. Am J Epidemiol. 2016;184(12):884–93.
Todd A. Marihuana. America’s New Drug Problem: Nature Publishing Group UK London; 1939.
Tomko AM, Whynot EG, O’Leary LF, Dupré DJ. Anti-cancer potential of cannabis terpenes in a taxol-resistant model of breast cancer. Can J Physiol Pharmacol. 2022;100(8):806–17.
Touw M. The religious and medicinal uses of Cannabis in China, India and Tibet. J Psychoactive Drugs. 1981;13(1):23–34.
Tundidor I, Seijo-Vila M, Blasco-Benito S, Rubert-Hernández M, Adámez S, Andradas C, et al. Identification of fatty acid amide hydrolase as a metastasis suppressor in breast cancer. Nat Commun. 2023;14(1):3130.
Van der Pool A, Damhuis R, Ijzermans J, de Wilt J, Eggermont A, Kranse R, et al. Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Colorectal Dis. 2012;14(1):56–61.
Vatrella A, Maglio A, Pelaia C, Pelaia G, Vitale C. Pharmacotherapeutic strategies for critical asthma syndrome: a look at the state of the art. Expert Opin Pharmacother. 2020;21(12):1505–15.
Voicu V, Brehar F-M, Toader C, Covache-Busuioc R-A, Corlatescu AD, Bordeianu A, et al. Cannabinoids in Medicine: A Multifaceted Exploration of Types, Therapeutic Applications, and Emerging Opportunities in Neurodegenerative Diseases and Cancer Therapy. Biomolecules. 2023;13(9):1388.
Wang T, Xia K, Qiu T, Han S, Chen Z, Ma X, et al. A comprehensive survival and prognosis analysis of GPR55 expression in hepatocellular carcinoma. Aging (Albany NY). 2023;15(17):8930.
Wang X, Sun J, Yin X, Zou C, Li H. Effects of behavioral change techniques on diet and physical activity in colorectal cancer patients: a systematic review and meta-analysis. Support Care Cancer. 2023;31(1):29.
Wardill HR, Wooley LT, Bellas OM, Cao K, Cross CB, van Dyk M, et al. Supporting gut health with medicinal cannabis in people with advanced cancer: potential benefits and challenges. Br J Cancer. 2023;130:1–12.
Wood TB, Spivey WN, Easterfield TH III. Cannabinol. Part I J Chem Soc Trans. 1899;75:20–36.
Xiao W, Zheng S, Yang A, Zhang X, Zou Y, Tang H, et al. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: a population-based study. Cancer Manag Res. 2018;10:5329–38.
Yanar a, Karolin, Yazıcı Z. Cannabis: Action Mechanisms and Potential Roles in the Management of Type 2 Diabetes Mellitus. Natural Products and their Bioactives in Antidiabetic Drug Discovery. 2023:232-43.
Younes M. The Combination of CBD and THC Isolated from the Lebanese Cannabis sativa Exerts Anti-cancer Effects in Breast Cancer Cells: Lebanese American University. 2023.
Zhang W, Lee AM, Jena S, Huang Y, Ho Y, Tietz KT, et al. Computational drug discovery for castration-resistant prostate cancers through in vitro drug response modeling. Proc Natl Acad Sci. 2023;120(17):e2218522120.
Zhong N, Li D, Wang B, Kovalchuk O, Kovalchuk I. Cannabinol inhibits cell growth and triggers cell cycle arrest and apoptosis in cancer cells. Biocatalysis Agric Biotechnol. 2023;48:102627.
Zuardi AW. História da cannabis como medicamento: uma revisão. Braz J Psychiatry. 2006;28:153–7.
Zupo V, Costantini M, Aflalo ED, Levy T, Chalifa-Caspi V, Obayomi O, et al. Ferroptosis precedes apoptosis to facilitate specific death signalling by fatty acids. Proc R Soc B. 2009;2023(290):20231327.
Download references
Acknowledgements
Authors would like to thank the International Medical Students Association (IMedRA) for their technical facilitation and networking.
Authors declare that this research has received no financial support.
Author information
Authors and affiliations.
Department of Basic Medical Sciences, Faculty of Medicine, Al-Balqa Applied University, Al-Salt, 19117, Jordan
Husam A. ALSalamat & Aseel N. Qandil
Department of Biopharmaceutics and Clinical Pharmacy, School of Pharmacy,, University of Jordan, Amman, 19328, Jordan
Husam A. ALSalamat
International Medical Research Association (IMedRA), Cairo, Egypt
Husam A. ALSalamat, Hazem Mohamed Salamah, Anas Hasan Ishqair, Mohammad Fuad Dwikat & Anas Zakarya Nourelden
Department of Clinical Pharmacy and Therapeutics, School of Pharmacy, Applied Science Private University, Amman, 541350, Jordan
Sara Feras Abuarab, Yasmeen Barakat & Muna Barakat
School of Medicine, Zagazig University, Zagazig, 44519, Egypt
Hazem Mohamed Salamah
Faculty of Medicine, The Hashemite University, Zarqa, 13133, Jordan
Anas Hasan Ishqair
Faculty of Medicine, An-Najah National University, Nablus, Palestine
Mohammad Fuad Dwikat
Faculty of Medicine, Al-Azhar University, Cairo, Egypt
Anas Zakarya Nourelden
You can also search for this author in PubMed Google Scholar
Contributions
Concept – H.A.A.; Design – H.A.A., H.M.S., A.H.I., M.F.D., A.Z.N., M.B.; Supervision – H.A.A.; Facilitation – H.A.A., A.Z.N.; Data Collection and/or Processing – H.A.A., H.M.S., A.H.I., M.F.D., M.B.; Analysis and/or interpretation – H.A.A., H.M.S., A.H.I., M.F.D., M.B.; Literature search – H.A.A., S.F.A., H.M.S., A.H.I., M.F.D., M.B.; Writing the manuscript – H.A.A., S.F.A., H.M.S., A.H.I., M.F.D., A.Q, Y.B, M.B.; Manuscript review – H.A.A., S.F.A., H.M.S., A.H.I., M.F.D., A.Z.N., A.Q, Y.B, M.B.; Figures and/or illustrations – M.B.; Critical review – H.A.A., M.B.
Corresponding author
Correspondence to Muna Barakat .
Ethics declarations
Ethics approval and consent to participate, consent for publication, competing interests.
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
ALSalamat, H.A., Abuarab, S.F., Salamah, H.M. et al. Cannabis and cancer: unveiling the potential of a green ally in breast, colorectal, and prostate cancer. J Cannabis Res 6 , 24 (2024). https://doi.org/10.1186/s42238-024-00233-z
Download citation
Received : 18 November 2023
Accepted : 14 April 2024
Published : 16 May 2024
DOI : https://doi.org/10.1186/s42238-024-00233-z
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cannabinoids
- Anti-cancer
Journal of Cannabis Research
ISSN: 2522-5782
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]

COMMENTS
Breast Cancer Research and Treatment is a comprehensive forum dedicated to all aspects of breast cancer research. The journal's focus spans across various disciplines including surgery, radiotherapy, medical oncology, endocrinology, epidemiology, immunology and cell biology. Provides an international platform for the discussion and resolution ...
Breast Cancer Research and Treatment provides the surgeon, radiotherapist, medical oncologist, endocrinologist, epidemiologist, immunologist or cell biologist investigating problems in breast cancer a single forum for communication. The journal creates a "market place" for breast cancer topics which cuts across all the usual lines of disciplines, providing a site for presenting pertinent ...
beginning to develop drugs for treatment of breast cancer, all of which were non-specific and empirically based. We started with nitrogen mustard, then anthra-cyclines, fluorouracil, methotrexate and a few others. Breast cancer had already been noted to have one target, the estrogen receptor.
Focuses on treatment options for breast cancer, such as radiotherapy, systemic therapy and immunotherapy. Addresses ongoing research in screening, diagnosis and management for all subtypes of breast cancer. Edited and authored by leading experts in the field. Part of the book series: Cancer Treatment and Research (CTAR, volume 188) 5282 Accesses.
1. Introduction. Breast cancer has a very long history as it was first reported by the ancient Egyptians more than 3500 years ago in about 1500 B.C [].Today, breast cancer is the second most prevalent type of cancer and is a leading cause of most cancer-related deaths in women in the United States [].Around 281,550 women are projected to be diagnosed with breast cancer in 2021, and 43,600 ...
4.872 (2020) ISO 4. 0167-6806. Breast Cancer Research and Treatment is a scientific journal focused on the treatment of and investigations in breast cancer. It is targeted towards a wide audience of clinical researchers, epidemiologists, immunologists, or cell biologists interested in breast cancer. The types of articles in this journal include ...
Introduction. Breast cancer is the most commonly diagnosed cancer among female patients and is the leading cause of cancer-related death. 1 There were 300,590 new cases and 43,700 deaths of invasive breast cancer in the United States based on the 2023 prediction, accounting for approximately 30% of female cancers. 1 The treatments of breast cancer include surgery, chemotherapy, radiotherapy ...
Abstract. Breast cancer is categorized at the molecular level according to the status of certain hormone and growth factor receptors, and this classification forms the basis of current diagnosis and treatment. The development of resistance to treatment and recurrence of the disease have led researchers to develop new therapies.
FDG-PET/CT in high-risk primary breast cancer—a prospective study of stage migration and clinical impact. Marianne Vogsen, Jeanette Dupont Jensen, Ivar Yannick Christensen, Oke Gerke, Anne Marie Bak Jylling, Lisbet Brønsro Larsen, Poul-Erik Braad, Katrine Lydolph Søe, Camilla Bille, Marianne Ewertz, Malene Grubbe Hildebrandt. Clinical trial.
Scope. Breast Cancer Research and Treatment provides the surgeon, radiotherapist, medical oncologist, endocrinologist, epidemiologist, immunologist or cell biologist investigating problems in breast cancer a single forum for communication. The journal creates a "market place" for breast cancer topics which cuts across all the usual lines of ...
Purpose Integrative medicine (IM) has received the American Society of Clinical Oncology's endorsement for managing cancer treatment-related side effects. Little is known about racial differences in familiarity, interest, and use of IM among patients with breast cancer. Methods Patients with breast cancer enrolled in the Chicago Multiethnic Epidemiologic Breast Cancer Cohort were surveyed ...
Rational development of targeted therapies has revolutionized metastatic breast cancer outcomes, although resistance to treatment remains a major challenge. Advances in molecular profiling and imaging technologies have provided evidence for the impact of clonal diversity in cancer treatment resistance, through the outgrowth of resistant clones.
Impact of non-adherence to endocrine therapy on recurrence risk in older women with stage I breast cancer after breast-conserving surgery. Danielle Rodin, Rinku Sutradhar, Katarzyna J. Jerzak, Ezra Hahn, Lena Nguyen, Matthew Castelo, Omolara Fatiregun, Cindy Fong, Danilo Giffoni M. M. Mata, Sabina Trebinjac, Lawrence Paszat, Eileen Rakovitch
Breast Cancer Research and Treatment. Breast Cancer Research and Treatment More about the journal. ... Springer Nature makes no representations, warranties or guarantees, whether express or implied, that the content on this community is accurate, complete or up to date, and to the fullest extent permitted by law all liability is excluded. ...
Subgroup analyses from the phase 3 ASCENT study of sacituzumab govitecan in metastatic triple-negative breast cancer. Sara A. Hurvitz. Aditya Bardia. Sara M. Tolaney. Article Open Access 25 Apr 2024.
Data source. For this study, the research database Breast Cancer Database Sweden (BCBaSe) was used. BCBaSe is a linkage between breast cancer quality registers and several other population-based registries, with information on individuals diagnosed with breast cancer between 1992 and 2012 in three Swedish health care regions; Stockholm-Gotland, Uppsala-Orebro and the Northern health care ...
Breast cancer (BC) is the most frequently diagnosed cancer in women worldwide with more than 2 million new cases in 2020. ... Treatment of breast cancer is complex and involves a combination of different modalities including surgery, radiotherapy, chemotherapy, hormonal therapy, or biological therapies delivered in diverse sequences.
Aims and scope. Breast Cancer Research is an international, peer-reviewed online journal, publishing original research, reviews, editorials and reports. Open access research articles of exceptional interest are published in all areas of biology and medicine relevant to breast cancer, including normal mammary gland biology, with special emphasis ...
About this book series. This book series provides detailed updates on the state of the art in the treatment of different forms of cancer and also covers a wide spectrum of topics of current research interest. Clinicians will benefit from expert analysis of both standard treatment options and the latest therapeutic innovations and from provision ...
Triple-negative breast cancer (TNBC) is highly malignant and lacks effective biotherapeutic targets. The development of efficient anticancer drugs with low toxicity and few side effects is a hotspot in TNBC treatment research. Although erianin is known to have potent antitumor activity, its regulatory mechanism and target in TNBC have not been fully elucidated, hampering further drug development.
Researchers have found a link between diet, a type of gut bacterium and breast cancer. The study, published on 6 May in the Proceedings of the National Academy of Science 1, found that a high-fat ...
Treatment options. Breast cancer is treated in several ways. It depends on the kind of breast cancer and how far it has spread. Surgery: Is an operation in which doctors cut out the cancer. Chemotherapy: Uses special medicines to shrink or kill the cancer. The drugs can be pills you take or medicines given in your veins, or sometimes both.
Cancer comes in second place on the list of causes of death worldwide. In 2018, the 5-year prevalence of breast cancer (BC), prostate cancer (PC), and colorectal cancer (CRC) were 30%, 12.3%, and 10.9%, respectively. Cannabinoids are chemicals derived from the Cannabis sativa plant; the most investigated cannabinoids are cannabinol, delta 9-tetrahydrocannabinol (Δ9-THC), and cannabidiol. In ...