Europe PMC requires Javascript to function effectively.
Either your web browser doesn't support Javascript or it is currently turned off. In the latter case, please turn on Javascript support in your web browser and reload this page.

Search life-sciences literature (44,131,415 articles, preprints and more)
- Full text links
- Citations & impact
- Similar Articles
A case study of overfeeding 3 different diets.
Author information, affiliations.
- Feltham S 1
- Westman EC 2
Current Opinion in Endocrinology, Diabetes, and Obesity , 01 Oct 2021 , 28(5): 446-452 https://doi.org/10.1097/med.0000000000000668 PMID: 34352821
Abstract
Purpose of review, recent findings, full text links .
Read article at publisher's site: https://doi.org/10.1097/med.0000000000000668
Citations & impact
Impact metrics, alternative metrics.

Smart citations by scite.ai Smart citations by scite.ai include citation statements extracted from the full text of the citing article. The number of the statements may be higher than the number of citations provided by EuropePMC if one paper cites another multiple times or lower if scite has not yet processed some of the citing articles. Explore citation contexts and check if this article has been supported or disputed. https://scite.ai/reports/10.1097/med.0000000000000668
Article citations, nutrition-related n-of-1 studies warrant further research to provide evidence for dietitians to practice personalized (precision) medical nutrition therapy: a systematic review..
Allman-Farinelli M , Boljevac B , Vuong T , Hekler E
Nutrients , 15(7):1756, 04 Apr 2023
Cited by: 1 article | PMID: 37049595 | PMCID: PMC10097352
The Niche of n-of-1 Trials in Precision Medicine for Weight Loss and Obesity Treatment: Back to the Future.
Grammatikopoulou MG , Gkouskou KK , Gkiouras K , Bogdanos DP , Eliopoulos AG , Goulis DG
Curr Nutr Rep , 11(2):133-145, 16 Feb 2022
Cited by: 4 articles | PMID: 35174475
Similar Articles
To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
A highly saturated fat-rich diet is more obesogenic than diets with lower saturated fat content.
Hariri N , Gougeon R , Thibault L
Nutr Res , 30(9):632-643, 01 Sep 2010
Cited by: 47 articles | PMID: 20934605
Effects of Short-Term Fasting and Different Overfeeding Diets on Thyroid Hormones in Healthy Humans.
Basolo A , Begaye B , Hollstein T , Vinales KL , Walter M , Santini F , Krakoff J , Piaggi P
Thyroid , 29(9):1209-1219, 09 Aug 2019
Cited by: 12 articles | PMID: 31298652 | PMCID: PMC6864752
Free full text in Europe PMC
The effects of varying dietary fat on the nutrient intake in male and female runners.
Horvath PJ , Eagen CK , Ryer-Calvin SD , Pendergast DR
J Am Coll Nutr , 19(1):42-51, 01 Feb 2000
Cited by: 16 articles | PMID: 10682875
Dietary fat consumption and health.
Lichtenstein AH , Kennedy E , Barrier P , Danford D , Ernst ND , Grundy SM , Leveille GA , Van Horn L , Williams CL , Booth SL
Nutr Rev , 56(5 pt 2):S3-19; discussion S19-28, 01 May 1998
Cited by: 108 articles | PMID: 9624878
The Effects of Overfeeding on Body Composition: The Role of Macronutrient Composition - A Narrative Review.
Leaf A , Antonio J
Int J Exerc Sci , 10(8):1275-1296, 01 Dec 2017
Cited by: 14 articles | PMID: 29399253 | PMCID: PMC5786199
Europe PMC is part of the ELIXIR infrastructure
A case study of overfeeding 3 different diets
Publisher URL: https://journals.lww.com/co-endocrinology/Fulltext/2021/10000/A_case_study_of_overfeeding_3_different_diets.5.aspx
DOI: 10.1097/med.0000000000000668
Keeping up-to-date with research can feel impossible, with papers being published faster than you'll ever be able to read them. That's where Researcher comes in: we're simplifying discovery and making important discussions happen. With over 19,000 sources, including peer-reviewed journals, preprints, blogs, universities, podcasts and Live events across 10 research areas, you'll never miss what's important to you. It's like social media, but better. Oh, and we should mention - it's free.

Researcher displays publicly available abstracts and doesn’t host any full article content. If the content is open access, we will direct clicks from the abstracts to the publisher website and display the PDF copy on our platform. Clicks to view the full text will be directed to the publisher website, where only users with subscriptions or access through their institution are able to view the full article.

Eric Charles Westman
Selected publications, ketogenic diet intervention on metabolic and psychiatric health in bipolar and schizophrenia: a pilot trial., correction: treating binge eating and food addiction symptoms with low-carbohydrate ketogenic diets: a case series., nutritional aspects, re: effect of a ketogenic diet versus mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: the interventional keto-med randomized crossover trial., a review of decision aids to assess cardiovascular risk., editorial introductions, competing paradigms of obesity pathogenesis: energy balance versus carbohydrate-insulin models., reply to a drewnowski et al, o devinsky, d a booth and e l gibson, and d j millward., the ketogenic diet for refractory mental illness: a retrospective analysis of 31 inpatients., the carbohydrate-insulin model: a physiological perspective on the obesity pandemic., editorial: carbohydrate restriction: from the 'bedside' to the 'bench'., a case study of overfeeding 3 different diets., carbohydrate-restricted diets and type 1 diabetes mellitus: research considerations., type 2 diabetes mellitus: a pathophysiologic perspective., editorial: carbohydrate-restricted nutrition and diabetes mellitus., application of nutrient essentiality criteria to dietary carbohydrates., using a low-carbohydrate diet to treat obesity and type 2 diabetes mellitus., editorial: exploring the untapped potential of low-carbohydrate diets., low-cost measurement of face mask efficacy for filtering expelled droplets during speech., unprocessed red meat and processed meat consumption., treating binge eating and food addiction symptoms with low-carbohydrate ketogenic diets: a case series., ketogenic diet for obesity and diabetes., a lifestyle intervention of weight loss via a low-carbohydrate diet plus walking to reduce metabolic disturbances caused by androgen deprivation therapy among prostate cancer patients: carbohydrate and prostate study 1 (caps1) randomized controlled trial., corrigendum to "dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base" [nutrition 31 (2015) 1-13]., the ketogenic diet and remission of psychotic symptoms in schizophrenia: two case studies., implementing a low-carbohydrate, ketogenic diet to manage type 2 diabetes mellitus., management of type 1 diabetes with a very low-carbohydrate diet., the effects of nicotine and non-nicotine smoking factors on working memory and associated brain function., weight loss: a patient and physician's perspective., residential lifestyle modification programs for the treatment of obesity, obesity: evaluation and treatment essentials, dietary treatment of the obese individual, medical treatment of pediatric obesity, lipids and bariatric procedures part 1 of 2: scientific statement from the national lipid association, american society for metabolic and bariatric surgery, and obesity medicine association: full report., lipids and bariatric procedures part 1 of 2: scientific statement from the national lipid association, american society for metabolic and bariatric surgery, and obesity medicine association: executive summary., lipids and bariatric procedures part 2 of 2: scientific statement from the american society for metabolic and bariatric surgery (asmbs), the national lipid association (nla), and obesity medicine association (oma)., dietary carbohydrate restriction as the first approach in diabetes management: critical review and evidence base., nicotine and non-nicotine smoking factors differentially modulate craving, withdrawal and cerebral blood flow as measured with arterial spin labeling., acp journal club. in overweight or obese patients with diabetes, a lifestyle intervention increased weight loss at 8 years., meclizine enhancement of sensorimotor gating in healthy male subjects with high startle responses and low prepulse inhibition., erratum: clinical management of individuals with obesity (journal of clinical lipidology (2014) 8:3 (237-248)), blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity., comparison of a reduced carbohydrate and reduced fat diet for ldl, hdl, and vldl subclasses during 9-months of weight maintenance subsequent to weight loss., reinforcing effects of nicotine and non-nicotine components of cigarette smoke., a randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss., low glycemic diet for weight loss in hypertriglyceridemic patients attending a lipid clinic., carbohydrate-restricted diets for obesity and related diseases: an update., precessation treatment with nicotine patch significantly increases abstinence rates relative to conventional treatment., a very low-carbohydrate diet improves symptoms and quality of life in diarrhea-predominant irritable bowel syndrome., the effects of varying dietary carbohydrate and fat content on survival in a murine lncap prostate cancer xenograft model., comparison of weight-loss diets., rethinking dietary saturated fats, effects of two weight-loss diets on health-related quality of life., schizophrenia, gluten, and low-carbohydrate, ketogenic diets: a case report and review of the literature., rethinking dietary saturated fat, the effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus., clinical experience using appetite suppressants and ssris., protein, weight management, and satiety., has carbohydrate-restriction been forgotten as a treatment for diabetes mellitus a perspective on the accord study design., dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: time for a critical appraisal., acid-base analysis of individuals following two weight loss diets., nonfasting triglycerides and cardiovascular risk., comparison of a low carbohydrate and low fat diet for weight maintenance in overweight or obese adults enrolled in a clinical weight management program., low-carbohydrate nutrition and metabolism., the effects of foods, beverages, and other factors on cigarette palatability., the effect of a low-carbohydrate, ketogenic diet on nonalcoholic fatty liver disease: a pilot study., the effects of a low-carbohydrate ketogenic diet and a low-fat diet on mood, hunger, and other self-reported symptoms., carbohydrate restriction is effective in improving atherogenic dyslipidemia even in the absence of weight loss., transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial., low carbohydrate diets in family practice: what can we learn from an internet-based support group., a very low-carbohydrate diet improves gastroesophageal reflux and its symptoms., effect of a low-carbohydrate, ketogenic diet program compared to a low-fat diet on fasting lipoprotein subclasses., precessation treatment with nicotine skin patch facilitates smoking cessation., dietary treatment of diabetes mellitus in the pre-insulin era (1914-1922)., the effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study., a low-carbohydrate, ketogenic diet to treat type 2 diabetes., is a low-carb, low-fat diet optimal, outcomes from an outpatient smoking-cessation clinic., comment: decreased warfarin effect after initiation of high-protein, low-carbohydrate diets., insulin resistance from a low carbohydrate, high fat diet perspective., prenatal and postpartum smoking abstinence a partner-assisted approach., update on smoking cessation, diet therapy for narcolepsy., the effects of controlled deep breathing on smoking withdrawal symptoms in dependent smokers., does binge eating disorder impact weight-related quality of life, a low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial., binge eating, quality of life and physical activity improve after roux-en-y gastric bypass for morbid obesity., postprandial triglycerides in response to high fat: role of dietary carbohydrate., clinical experience of a carbohydrate-restricted diet for the metabolic syndrome., trial finds low carb diets reduce weight more than low fat diets in the short term, but have more minor adverse effects, the effects of a low-carbohydrate regimen on glycemic control and serum lipids in diabetes mellitus, a review of low-carbohydrate ketogenic diets., pharmacologic and sensorimotor components of satiation in cigarette smoking., mecamylamine acutely increases human intravenous nicotine self-administration., clinical use of a carbohydrate-restricted diet to treat the dyslipidemia of the metabolic syndrome., clinical experience of a carbohydrate-restricted diet: effect on diabetes mellitus., a pilot trial of a low-carbohydrate, ketogenic diet in patients with type 2 diabetes., residential smoking therapy., pet studies of the influences of nicotine on neural systems in cigarette smokers., diets and clinical coronary events: the truth is out there., very-low-carbohydrate weight-loss diets revisited., relationship between obesity and health-related quality of life in men., effect of 6-month adherence to a very low carbohydrate diet program., asthma exacerbation after administration of nicotine nasal spray for smoking cessation., is dietary carbohydrate essential for human nutrition, is dietary carbohydrate essential for human nutrition [4] (multiple letters), oral nicotine solution for smoking cessation: a pilot tolerability study., use of bupropion sr in a pharmacist-managed outpatient smoking-cessation program., individual differences in smoking reward from de-nicotinized cigarettes., acute effects of nicotine and mecamylamine on tobacco withdrawal symptoms, cigarette reward and ad lib smoking., review: nicotine replacement treatments achieve smoking abstinence at 6-12 months: commentary, improvement of gastroesophageal reflux disease after initiation of a low-carbohydrate diet: five brief case reports., dissociating nicotine and nonnicotine components of cigarette smoking., cognitive behavioural therapy (cbt) with exercise led to higher levels of continuous smoking cessation in women than did cbt without exercise: commentary, combining the nicotine inhaler and nicotine patch for smoking cessation, arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: implications for addiction., factors influencing morning report case presentations., review: self help interventions alone minimally increase smoking cessation: commentary, naltrexone blockade of nicotine effects in cigarette smokers., blockade of smoking satisfaction using the peripheral nicotinic antagonist trimethaphan., integrating transdermal nicotine therapy into nicotine fading treatments: effects on salivary cotinine levels, safety of zyban in patients with co-existing medical and/or psychiatric conditions, implementing a patient's bill of rights with the personal health organizer, you're my father, not my patient, nicotine-mecamylamine treatment for smoking cessation: the role of pre-cessation therapy., helping patients take control of their medical records. a case study of a personal health organizer., smoking behavior on the first day of a quit attempt predicts long-term abstinence., should managed care organizations pay for nicotine replacement therapy, the safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease., airway sensory replacement as a treatment for smoking cessation, do heavy smokers need a higher replacement dose of nicotine to quit, dissociating the nicotine and airway sensory effects of smoking., nicotine/mecamylamine combination treatment for smoking cessation, curriculum survey of substance abuse teaching, accuracy and reliability of apical s3 gallop detection., does smokeless tobacco cause hypertension, combined administration of agonist-antagonist as a method of regulating receptor activation., airway sensory replacement combined with nicotine replacement for smoking cessation. a randomized, placebo-controlled trial using a citric acid inhaler., nicotine as a therapeutic drug., should physicians screen for oral disease a physical examination study of the oral cavity., mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone., nicotine skin patch treatment increases abstinence, decreases withdrawal symptoms, and attenuates rewarding effects of smoking., combined effects of nicotine and mecamylamine in attenuating smoking satisfaction, resident recognition of open-angle glaucoma: effects of an educational intervention., the nicotine patch in smoking cessation. a randomized trial with telephone counseling., treatment of acute myocardial infarction., smokeless tobacco use in an outpatient veteran population., the effect of sleeved arms on oscillometric blood pressure measurement., pharmacokinetics of a transdermal nicotine patch compared to nicotine gum, licorice, tobacco chewing, and hypertension..
The Effects of Overfeeding on Body Composition: The Role of Macronutrient Composition - A Narrative Review
Affiliations.
- 1 Human Nutrition and Functional Medicine, University of Western States, Portland, OR, USA.
- 2 Department of Health and Human Performance, Nova Southeastern University, Davie, FL, USA.
- PMID: 29399253
- PMCID: PMC5786199
Compared to investigations on hypocaloric diets, the effects of chronic overfeeding have been less studied. It has been posited that consuming calories in excess of daily caloric requirements will result in a gain in body weight and in particular fat mass regardless of which macronutrient(s) are consumed. However, recent evidence suggests that there is a quantitative difference in protein versus carbohydrate and/or fat overfeeding as it relates to body composition. Protein overfeeding or the consumption of a high protein diet may not result in a gain in body weight or fat mass despite consuming calories that exceed one's normal or habitual intake. Therefore, this review will provide an up-to-date narrative on the current scientific literature on various combinations of macronutrient overfeeding and its effects on body composition.
Keywords: Diet; body composition; bulking; energy surplus; fat gain; protein.
Publication types
Advertisement
Nutrition in the intensive care unit: from the acute phase to beyond
- Narrative Review
- Open access
- Published: 21 May 2024
Cite this article
You have full access to this open access article

- Angelique M. E. de Man ORCID: orcid.org/0000-0001-8738-8295 1 , 2 na1 ,
- Jan Gunst ORCID: orcid.org/0000-0003-2470-6393 3 , 4 &
- Annika Reintam Blaser ORCID: orcid.org/0000-0003-1211-7372 5 , 6 na1
3860 Accesses
125 Altmetric
Explore all metrics
Recent randomized controlled trials (RCTs) have shown no benefit but dose-dependent harm by early full nutritional support in critically ill patients. Lack of benefit may be explained by anabolic resistance, suppression of cellular repair processes, and aggravation of hyperglycemia and insulin needs. Also early high amino acid doses did not provide benefit, but instead associated with harm in patients with organ dysfunctions. However, most studies focused on nutritional interventions initiated during the first days after intensive care unit admission. Although the intervention window of some RCTs extended into the post-acute phase of critical illness, no large RCTs studied nutritional interventions initiated beyond the first week. Hence, clear evidence-based guidance on when and how to initiate and advance nutrition is lacking. Prolonged underfeeding will come at a price as there is no validated metabolic monitor that indicates readiness for medical nutrition therapy, and an adequate response to nutrition, which likely varies between patients. Also micronutrient status cannot be assessed reliably, as inflammation can cause redistribution, so that plasma micronutrient concentrations are not necessarily reflective of total body stores. Moreover, high doses of individual micronutrients have not proven beneficial. Accordingly, current evidence provides clear guidance on which nutritional strategies to avoid, but the ideal nutritional regimen for individual patients remains unclear. In this narrative review, we summarize the findings of recent studies, discuss possible mechanisms explaining the results, point out pitfalls in interpretation of RCTs and their effect on clinical practice, and formulate suggestions for future research.
Similar content being viewed by others
Toward nutrition improving outcome of critically ill patients: How to interpret recent feeding RCTs?
How to avoid harm with feeding critically ill patients: a synthesis of viewpoints of a basic scientist, dietitian and intensivist
The intensive care medicine research agenda in nutrition and metabolism.
Avoid common mistakes on your manuscript.
Introduction
In acute critical illness, catabolism is stimulated resulting in muscle wasting, weakness and failure to wean [ 1 ]. A low protein, energy, and micronutrient intake of these patients may aggravate catabolism and is associated with infections, delayed recovery, and increased mortality [ 2 , 3 , 4 ]. However, the traditional assumption that nutrients may counteract catabolism and thereby improve clinical outcome in critically ill patients is challenged by cumulative evidence from large randomized controlled trials (RCTs) revealing harm by providing full nutrition in the acute phase [ 5 , 6 , 7 , 8 ]. Even though a personalized approach has been suggested [ 9 , 10 ], monitoring tools that accurately quantify the actual energy, protein and micronutrient need for the individual patient are currently not available [ 4 ]. In this narrative review, we provide a condensed interpretation of recent RCT evidence, discuss the impact of evidence on clinical practice, and formulate some suggestions for future research.
Medical nutrition therapy in the ICU: evidence from RCTs
Observational studies have associated a cumulative protein and energy deficit with impaired outcome of critical illness [ 2 , 3 , 11 ]. However, the results of these studies might also be explained by feeding intolerance as a marker of severity of illness [ 2 , 3 , 12 ]. In the last decade, several large RCTs have addressed the timing, route and dosage of medical nutrition therapy in critically ill patients. The first RCT that challenged the assumption that early full nutrition would be beneficial was the EPaNIC RCT, published in 2011 [ 5 ]. In 4640 adult critically ill patients, initiation of parenteral nutrition to supplement insufficient enteral nutrition prolonged dependency on intensive care as compared to delaying supplemental parenteral nutrition until 1 week after intensive care unit (ICU) admission. Patients receiving early supplemental parenteral nutrition had a prolonged duration of vital organ support, more infections, a higher incidence of ICU-acquired weakness, and impaired recovery herefrom [ 5 , 13 ]. Supplemental parenteral nutrition also did not improve functional status at hospital discharge, as assessed by the 6-min walking distance and activities of daily living [ 5 ]. These results were subsequently confirmed in critically ill children (PEPaNIC RCT, N = 1440), in whom supplemental parenteral nutrition also adversely affected 2- and 4-year neurodevelopmental outcomes [ 8 , 14 ]. In both RCTs, mortality was unaffected, whereas harm by early parenteral nutrition was present in all studied subgroups, including patients with a high nutritional risk score (NRS score ≥ 5), patients with body mass index < 25 or ≥ 40, patients with sepsis, patients with a contraindication to enteral nutrition, and critically ill neonates [ 5 , 8 ]. Theoretically, harm in these RCTs could be explained by the higher feeding dose—early overfeeding—or by harm that is specific to the parenteral feeding route. Secondary analyses of these RCTs suggested dose-dependent harm rather than harm by the intravenous feeding route [ 15 , 16 ], which was corroborated by subsequent RCTs. Indeed, two large RCTs—the CALORIES ( N = 2400) [ 17 ] and NUTRIREA-2 RCT ( N = 2410) [ 6 ]—found no difference between the enteral and parenteral nutrition route with a similar energy dose in both groups. In the Nutrirea-2 RCT including ventilated patients with shock, early high-dose enteral nutrition was even more harmful as compared with early high-dose parenteral nutrition, by inducing potentially lethal gastrointestinal complications [ 6 ]. In both RCTs, patients were randomized shortly after ICU admission, and the intervention window was 5–7 days. Also in three RCTs that randomized critically ill patients to a lower or a higher dose of enteral nutrition initiated in the acute phase and continued for 6 (EDEN RCT, N = 1000), 14 (PermiT RCT, N = 894) or 28 days (TARGET RCT, N = 3957), a higher dose of enteral nutrition initiated in the acute phase did not provide benefit, and secondary outcomes suggested potential harm [ 18 , 19 , 20 ]. Also long-term functional outcome was not improved by higher feeding doses in the EDEN and TARGET RCTs [ 21 , 22 , 23 ]. The absence of benefit in the latter RCTs was consistent in all studied subgroups, including patients with high predicted risk of death [ 20 ], patients with body mass index (BMI) ≤ 18 [ 20 ], and patients with sepsis [ 19 , 20 ]. In a detailed post hoc analysis of the PermiT RCT, none of the studied baseline nutritional risk markers could identify patients who would benefit from early enhanced enteral nutrition, including the modified Nutrition Risk in Critically Ill (NUTRIC) score, BMI, transferrin, phosphate, urinary urea nitrogen, and nitrogen balance [ 24 ]. A low baseline prealbumin level, presumed to indicate high nutritional risk, even associated with significant mortality harm when receiving higher-dose enteral nutrition [ 24 ]. Likewise, the recent NUTRIREA-3 RCT, which randomized 3044 ventilated patients with shock to early high-dose nutrition (25 kcal/kg/day and 1.0–1.3 g protein per kg per day) versus low-dose nutrition in the first week (6 kcal/kg/day and 0.2–0.4 g protein/kg/day), found harm by early high-dose nutrition, with prolonged ICU dependency and increased complications in this group [ 7 ]. Importantly, in the NUTRIREA-3 RCT, the nutritional target was defined by randomization; medical nutrition therapy could be provided through either the enteral or parenteral route to reach that target [ 7 ]. A recent meta-analysis confirmed harm by early full feeding in critically ill patients as compared with permissive underfeeding. Although this meta-analysis did not include the EPaNIC RCT, it likely does not affect the conclusion, as EPaNIC patients were equally harmed by early full feeding [ 25 ]. A recent meta-analysis as part of the updated American feeding guidelines did not show benefit by supplemental parenteral nutrition as compared with enteral nutrition alone. Yet, the different design of included RCTs, with different timing of supplemental parenteral nutrition and different co-interventions and control groups, may complicate interpretation of the results [ 26 ]. Altogether, recent RCTs have shown dose-dependent harm by early medical nutrition therapy in critically ill patients, independent of the route of feeding and perceived nutritional risk. Table 1 summarizes the RCTs comparing a higher vs. lower amount of energy through the same or a different route, with the intervention initiated early after ICU admission. We report the energy intake on day 3 in these studies, as the higher energy target was reached in the respective high-dose group at that early time point. This time point also approximates the estimated end of the early period of the acute phase, as suggested by the European feeding guidelines [ 27 ]. Yet, the duration of the acute phase is debatable and likely variable between patients. American guidelines suggest a duration of 7–10 days for the acute phase, without any distinction between early and late periods [ 26 ].
The above-mentioned RCTs have been criticized for administering relatively low doses of proteins and for calculating the energy target by a fixed formula [ 35 , 36 ]. Recent trials do not support these critiques, however. Indeed, apart from increased energy intake, also high protein supply initiated early in critical illness did not provide benefit. The largest RCT on protein supplements, the EFFORT Protein RCT, which randomized 1329 critically ill patients to receive a high (≥ 2.2 g/kg/day) or a usual (≤ 1.2 g/kg/day) protein dose, initiated within 96 h after ICU admission and continued for up to 28 days did not show benefit by high protein doses [ 37 ]. On the contrary, an early high protein dose associated with prolonged ICU dependency and increased mortality in the most severely ill patients and in patients with acute kidney injury [ 37 ]. Also the Nephro-Protective RCT ( N = 474) did not find benefit by intravenous amino acid supplementation started on day 1 or 2 and continued until ICU discharge [ 38 ], and secondary analyses of the EPaNIC and PEPaNIC RCTs attributed harm by early parenteral nutrition specifically to the higher protein doses administered [ 15 , 16 ]. A recent meta-analysis on the effect of higher versus lower protein doses in critically ill patients confirmed no benefit of higher-dose protein. The meta-analysis suggested potential heterogeneity of treatment, however, with significant mortality harm restricted to patients with acute kidney injury, which requires further investigation [ 39 ].
A second reason why recent nutritional RCTs could have failed to show any benefit of early enhanced medical nutrition therapy has been suggested to be the absence of indirect calorimetry. Indeed, exact quantification of energy expenditure requires indirect calorimetry, as predictive equations do not accurately estimate energy expenditure in all patients [ 40 ]. However, there is no solid evidence that early indirect calorimetry-based feeding would be superior to nutrition based on a calculated energy target. Although a meta-analysis suggested a potential reduction in 28-day mortality in critically ill adults, there was no impact on 90-day mortality, and also morbidity outcomes were similar [ 41 ]. Such transient mortality difference could be explained by confounding in unblinded RCTs. Moreover, the perceived mortality difference at 28 days was borderline significant, as the statistical significance would have been lost if one patient would have had a different 28-day mortality outcome (fragility index of 1). In addition, the meta-analysis included RCTs with different design and co-interventions, which further complicates interpretation of these results. The largest RCT comparing calculated versus measured energy target feeding, the TICACOS-International RCT ( N = 580), found no benefit of indirect calorimetry-based feeding initiated early after ICU admission [ 42 ]. Moreover, the study was stopped prematurely because of slow recruitment, which may question the feasibility of routine use of indirect calorimetry. Evidently, these findings cannot necessarily be extrapolated to patients with prolonged critical illness.
Impact of evidence on clinical practice and guidelines
Published studies on the route, timing, and dose of nutrition may have changed practice in Europe remarkably as suggested by Veraar et al. [ 43 ]. The reported time point to start enteral and/or parenteral nutrition moved to a later time point soon after the EPaNIC study was published (Fig. 1 ). Nevertheless, a trend back to an earlier start slowly occurred over several years thereafter, with somewhat larger variation between respondents. The CALORIES RCT showing no difference between isocaloric enteral and parenteral nutrition may have contributed to this apparent change. Obviously, the trend only takes into account the timing and not the dose, which may have decreased during the time period presented in Fig. 1 . If not, one might expect that at least the dose of nutrition in the acute phase would decrease in future years, since the Nutrirea-3 RCT recently showed significant harm by early high-dose nutrition, regardless of the route [ 7 ]. Studies addressing dosage and route by interventions initiated beyond the first days in ICU are scarce. One RCT ( N = 305) starting supplemental parenteral nutrition in patients not tolerating 60% or more of measured energy needs via enteral route by day 4 after ICU admission did not show harm as compared with withholding supplemental parenteral nutrition until day 9 [ 44 ]. In this RCT, supplemental parenteral nutrition led to significantly less infections between day 9 and day 28, although this potential late protective effect was counteracted by more infections between day 4 and day 9 [ 45 ].

Evolution in nutritional practice in relation to large-scale randomized controlled trials. This graph presents multivariable regression of the start day of EN alone, PN alone, and EN in combination with PN from 2007 to 2018 in Europe, with 2007 as the reference year. Studies potentially influencing the actual dynamics of feeding practices are shown in the left. Data based on NutritionDay (16,032 patients admitted to 1389 intensive care units ([ 43 ], reproduced with permission). The reference (zero days) is set at the intercept of a multivariate model for year 2007 and does not represent real days since admission. EN enteral nutrition, PN parenteral nutrition, x axis days, y axis years
In response to RCTs performed in the last decade, international nutritional guidelines have changed. European guidelines have shifted toward recommending less aggressive nutrition in the acute phase (Table 2 ) [ 40 ]. Whereas the 2006/2009 guidelines recommended to start enteral nutrition within 24 h and to reach the caloric target (25 kcal/kg/day) within 2–3 days [ 46 , 47 ], the 2019 and partially updated 2023 guidelines recommend to initiate low-dose enteral nutrition within 48 h after ICU admission, unless contraindicated, and to advance toward energy target within 3–7 days [ 40 ]. If enteral nutrition is insufficient, parenteral nutrition is suggested to be initiated between days 4 and 7 instead of within the first 2 days [ 40 , 47 ] With regard to protein doses, a grade B recommendation to infuse protein at 1.3–1.5 g/kg/day was reformulated into a grade 0 recommendation stating that 1.3 g/kg/day can be delivered progressively [ 40 , 47 ]
Also the American nutritional guidelines changed over time, yet to a different extent. Whereas the 2009 guidelines recommended early initiation of enteral nutrition, and no medical nutrition therapy if enteral nutrition is not feasible [ 26 ], the 2022 guidelines recommend either enteral or parenteral nutrition as primary feeding modality in the first 7–10 days [ 48 ]. Despite a lower suggested feeding target than in the 2009 guidelines (12–25 kcal/kg/day in the first 7–10 days instead of 25–30 kcal/kg/day), the wider range still includes the full target immediately after ICU admission [ 26 , 48 ]. However, this recommendation was made before Nutrirea-3 RCT results were available, which showed harm by such high energy dose started early after ICU admission when compared to a dose twice lower than the lowest dose recommended by the American guidelines. If enteral nutrition is insufficient, the latest guidelines still recommend no supplemental parenteral nutrition prior to day 7 [ 48 ]. Regarding the protein target, the American guidelines contain a weak recommendation to administer 1.2–2 g protein/kg/day. However, this suggestion was made before the EFFORT Protein RCT was published [ 37 ], and one could anticipate that an updated version of the guidelines will no longer suggest such high doses.
The differences between the European and American guidelines reflect the persistent uncertainty regarding the optimal nutritional strategy in critically ill patients. Indeed, since recent large-scale RCTs were mainly negative, it has become clear which feeding strategies should be avoided, but not what one should do. We suggest that the varying level of evidence should be better reflected in the guidelines. In this regard, we suggest that guidelines contain strong recommendations what to avoid, and that suggestions what to do could be more imprecise. It has become clear that high doses of all macronutrients should be avoided in the acute phase. However, the duration of the acute phase remains unclear. This is illustrated by differences between the European and American guidelines, which allow below-target feeding for 3–7 days, or for maximum 7–10 days, respectively [ 40 , 48 ]. Differences between international guidelines may reflect different appreciations of the potential harm by a long period of relative starvation, versus the potential harm by starting full nutritional support too early. Also within guidelines, some recommendations may be confusing. Whereas the American guidelines allow early feeding doses up to 25 kcal/kg/day—which may need to be updated after Nutrirea-3—and suggest either enteral or parenteral nutrition as primary feeding modality, there is a strong recommendation against supplemental parenteral nutrition during the first 7 days. As of today, the optimal nutritional dose and feeding regimen in the acute phase and beyond remain unclear, as well as the ideal time point to start medical nutrition therapy. No RCT has evaluated whether low-dose feeding in the acute phase is superior as compared with progressive medical nutrition therapy, intermediate-dose feeding, or even no nutrition.
In addition, no large RCT has studied whether early enteral nutrition is actually superior to delayed enteral nutrition [ 49 ]. When medical nutrition therapy is initiated, however, we suggest that enteral nutrition is the primary feeding modality, unless contraindications. Although RCTs have not shown superiority of the enteral feeding route as compared with the parenteral route, the intervention window was short. When administered for a prolonged period of time, parenteral nutrition by itself can induce morbidity, as observed in patients with short bowel [ 50 ]. Moreover, costs of enteral nutrition are lower. A potential pragmatic feeding strategy is suggested in Fig. 2 .

Suggested pragmatic feeding strategy for ICU patients. In the hyperacute phase of critical illness, no nutrition is necessary. In patients developing spontaneous hypoglycemia, intravenous glucose is initiated to treat hypoglycemia. After initial stabilization, EN is started, when possible, considered “early” if started within 48 h. If EN is not possible and no non-nutritional energy is provided, low-dose glucose may need to be considered. If tolerated, EN is progressively increased toward target over several days. If EN is not possible or insufficient, PN should likely be initiated between days 4 and 8, and progressively increased toward target. The exact duration of the different phases shown in the figure is not known and likely varies individually. As full feeding should be avoided in the first days after ICU admission, it seems prudent to ensure sufficient micronutrient intake by maintenance doses of micronutrients, provided as long as the patient does not receive sufficient macronutrient intake via oral or enteral nutrition (unlike PN, standard commercial EN formulations contain micronutrients). If a patient develops hypophosphatemia upon initiation or increase of feeding, temporarily reducing macronutrient intake while correcting electrolyte abnormalities (in particular potassium and phosphate) is advised. Similarly, in patients developing a new severe insult (e.g., a new septic shock) during ICU stay while receiving full feeding, temporarily reducing or stopping macronutrient intake seems prudent. 1 Reasons to delay EN [ 40 ]. 2 No nutrition if high non-nutritional intake (e.g., propofol, citrate, glucose-containing solutions). 3 Consider non-nutritional intake. 4 A drop in phosphate by at least 0.16 mmol/l to below 0.65 mmol/l after initiating medical nutrition therapy. 5 Coverage of increased basal needs according to ESPEN micronutrient guidelines [ 51 ]. EN enteral nutrition, PN parenteral nutrition, ICU intensive care unit
Micronutrient administration in critical illness
Without external replenishment, most micronutrient stores become depleted within weeks to months in healthy persons [ 51 ]. In critically ill patients, micronutrient stores could decrease much faster. Additionally, patients may be malnourished upon ICU admission, and have increased micronutrient utilization and increased losses bodily fluids and continuous renal replacement therapy [ 52 , 53 ]. Moreover, the recent shift in feeding guidelines toward more restrictive feeding in the acute phase may contribute to development of micronutrient deficiencies. Micronutrients are crucial for vital functions such as ATP production, antioxidant and immune defenses, gene transcription and as cofactors for numerous enzymes [ 54 ]. Symptoms of micronutrient deficiencies are unspecific and resemble those of critical illness [ 55 ]. They may only become unmasked when medical nutrition therapy is started, manifesting as refeeding syndrome. Indeed, refeeding increases the need and intracellular uptake of several micronutrients (especially thiamine, but also other B-vitamins and trace elements) and electrolytes (predominantly phosphate and potassium). In case of low total body stores, this may lead to life-threatening arrhythmias, cardiac depression, lactic acidosis and severe muscle weakness. There are no validated standard criteria for diagnosing refeeding syndrome. A drop in phosphate levels by at least 0.16 mmol/l to below 0.65 mmol/l has been used as possible surrogate [ 56 , 57 ]. The risk of refeeding is increased when transitioning from prolonged fasting or prolonged low-dose feeding to full feeding. In the Refeeding RCT ( N = 339), temporarily restricting macronutrient intake in patients developing refeeding hypophosphatemia associated with decreased mortality as compared with continuing and increasing nutritional intake, while electrolytes were corrected with similar efficiency in both groups [ 57 ]. These data support cautious build-up of medical nutrition therapy in patients at risk of refeeding syndrome. Whether the risk of refeeding could be reduced with progressive medical nutrition therapy and/or by administering sufficient amounts of micronutrients and electrolytes in the acute phase to prevent micronutrient and electrolyte deficiencies has not been confirmed in studies. Nevertheless, administration of maintenance doses of micronutrients appears prudent. Ideal doses remain unclear, however. Plasma micronutrient concentrations cannot guide therapy, since they are also affected by redistribution due to inflammation. Hence, plasma micronutrient concentrations not necessarily reflect body stores and no accurate correction formulas for inflammation are available [ 58 ]. When the patient receives 1500 kcal via commercial enteral nutrition formula, this contains enough micronutrients to cover DRI (Dietary Reference Intake) for healthy people [ 51 ]. However, critically ill patients may have higher requirements to cover basal needs. Recommendations for presumed optimal intakes can be found in the ESPEN micronutrient guideline published in 2022 [ 51 ]. The suggested maintenance doses for critically ill patients are generally based on low-level evidence. Apart from maintenance doses, also much higher pharmacological doses have been suggested for individual micronutrients including vitamin C, vitamin D and selenium. Such practice should probably be separated from nutritional interventions and considered as pharmacological intervention aiming for a therapeutic effect of a drug. However, administration of such high pharmacological dose of any micronutrient has not been shown beneficial and is not recommended [ 51 , 59 ].
Mechanisms explaining lack of benefit of early high-dose nutrition
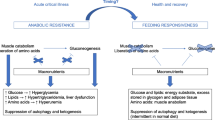
Several mechanisms may explain the lack of benefit of early high-dose nutrition, including anabolic resistance (inability to use nutrients for anabolism), suppression of cellular repair processes including autophagy and ketogenesis, and aggravation of stress hyperglycemia and insulin need (see Fig. 3 ) [ 4 ]. It appears that in acute critical illness, macronutrients cannot be used as in health [ 4 ]. Although medical nutrition therapy has been promoted to limit endogenous catabolism, recent evidence supports the opposite. Indeed, increased feeding intake did not prevent muscle loss in the EPaNIC RCT [ 13 , 60 ]. Moreover, several RCTs showed increased ureagenesis by increased protein or amino acid intake, suggesting futile catabolism of the extra provided amino acids [ 16 , 34 , 37 , 38 , 61 , 62 , 63 ].

Potential mechanisms for the lack of benefit by early full feeding in critical illness. This figure is a reproduction and adaptation from [ 4 ], under the Creative Commons Attribution 4.0 International License, ( http://creativecommons.org/licenses/by/4.0/ )
Medical nutrition therapy also powerfully suppresses autophagy and ketogenesis, which may be detrimental [ 4 ]. Indeed, autophagy is an important cellular recovery process that is able to clear macromolecular damage including damaged cell organelles, protein aggregates and intracellular microorganisms [ 64 , 65 ]. Increasing evidence has implicated activated autophagy as crucial pathway for recovery from critical illness-induced organ failure [ 64 ]. In normal physiology, autophagy is powerfully suppressed by feeding and insulin, and experimental studies confirm autophagy suppression by enhanced nutrition in critical illness [ 13 , 64 , 66 ]. In a subset of patients included in the EPaNIC RCT, muscular autophagy suppression by early parenteral nutrition associated with increased weakness, confirming the functional relevance of these findings [ 13 ]. Also suppression of ketogenesis has been implicated as potential mediator of harm by early high-dose nutrition. Apart from being an efficient energy source, ketones may stimulate autophagy and muscle regeneration, and have anti-inflammatory effects [ 67 ]. Experimental studies have shown that exogenous ketone administration protected septic mice against muscle weakness [ 68 , 69 ]. In a secondary analysis of the EPaNIC and PEPaNIC RCTs, withholding early parenteral nutrition activated ketogenesis, especially in critically ill children, in whom the effect on ketogenesis statistically mediated part of the outcome benefit of the intervention [ 70 , 71 ]. Hence, anorexia and enteral feeding intolerance could be adaptive in acute critical illness by activating cellular repair processes induced by relative fasting.
Early medical nutrition therapy also increases the degree of (stress) hyperglycemia, which is associated with poor outcome, although the ideal blood glucose target remains debated [ 72 ]. In contrast to the initial Leuven RCTs that showed morbidity and mortality benefit by tight glucose control in patients receiving early parenteral nutrition ( N = 3448) [ 73 , 74 , 75 ], the recent TGC-fast RCT ( N = 9230) in patients not receiving early parenteral nutrition did not show a benefit on mortality, although secondary endpoints suggested a potential benefit on morbidity [ 76 ]. A striking difference between the initial Leuven RCTs and the TGC-fast RCT is the much lower severity of hyperglycemia in the TGC-fast RCT, explained by considerably lower energy provision due to the omission of early parenteral nutrition [ 76 ]. Hence, excess mortality in the original Leuven RCTs seems explained by more severe hyperglycemia evoked by early parenteral nutrition that led to overfeeding in the first week [ 76 , 77 ]. Mechanistic studies have attributed harm by severe iatrogenic hyperglycemia to glucose overload in vital organs, and not to lack of insulin effect [ 78 ]. Indeed, insulin by itself may have unwanted side effects on organ recovery, as insulin is a powerful suppressor of autophagy and ketogenesis [ 79 , 80 ]. This may explain why lower glucose levels independently associated with improved outcome in RCTs, whereas increased insulin doses independently associated with harm [ 81 ]. Hence, avoiding early full feeding may be beneficial by avoiding iatrogenic severe hyperglycemia and by lowering the insulin need.
At present, no validated metabolic monitor can identify the time point when anabolic resistance switches into feeding responsiveness. Potential signs of overfeeding, including hyperglycemia and increased insulin need, hyperuremia, increased urea-over-creatinine ratio, and hypertriglyceridemia, are non-specific [ 82 ]. Moreover, also underfeeding could lead to hyperuremia and an increased urea-over-creatinine ratio [ 83 ]. Indirect calorimetry does not determine the amount of endogenous energy production that is not suppressible by nutrition [ 4 ]. Measurements of resting energy expenditure (REE) using indirect calorimetry after the acute phase could be helpful to guide the dose of nutrition; however, validation is needed, as well as certain expertise to interpret the results beyond one value of REE only [ 84 ]. It must be borne in mind that overfeeding leads to increased REE (not reflecting the actual needs) due to increased diet-induced thermogenesis which could erroneously prompt physicians to increase the nutritional dose, whereas underfeeding does not lead to decreased REE. Likely, the duration of anabolic resistance and of undesirable effects of nutritional support differ between patients, as the untoward response to feeding likely accompanies the acute stress response and its associated inflammatory and endocrine changes [ 4 ]. In the absence of a metabolic monitor and in view of the above insights, it may be prudent to temporarily restrict macronutrients again in patients confronted with a new severe insult in ICU, including de novo septic shock occurring later in ICU stay.
Future perspectives
Although recent evidence has shown benefits associated with relative fasting in acute critical illness, prolonged fasting will likely come at a price. Large nutritional RCTs have focused on initiation of medical nutrition therapy in the first days, and the intervention window was often restricted to the first week in ICU. In the RCTs with a longer intervention window, any benefit of a higher nutritional dose in a post-acute phase may have been counteracted by harm in the acute phase. Future RCTs should focus on interventions initiated beyond the acute phase, and interventions that extend into the recovery period [ 4 ]. In this regard, a RCT in hospitalized, non-critically ill patients ( N = 2088) found mortality benefit by protocol-guided individualized nutritional support as compared with standard feeding, suggesting that optimized nutrition may improve hard clinical endpoints when provided at the right dose to the right patient [ 85 ]. Yet, feeding optimization in this RCT was mainly achieved by optimizing oral intake, which is hardly possible in most ICU patients. Moreover, the protocol also ensured sufficient micronutrient intake in the intervention group. Hence, it remains unclear whether mortality benefit is explained by optimizing macronutrient intake, avoiding micronutrient deficiencies, or both. As consequences of over- and underfeeding are not readily visible, future RCTs should include outcomes beyond ICU discharge. Also, there may be an interaction between optimizing nutritional intake in the post-acute phase and exercise, which needs further study [ 86 , 87 ]. Future studies should explore whether indirect calorimetry may be helpful to guide energy dosing after the acute phase of critical illness, and evaluate the respiratory quotient as a variable to differentiate between over- and underfeeding, as suggested by some experts [ 84 ]. Also macronutrient composition has been scarcely studied, yet likely important based on physiology and indirect evidence. A metabolic monitor allowing monitoring of utilization of nutrients during its provision would be warranted to develop and validate nutritional interventions based on metabolic responses to feeding [ 4 ]. Scores monitoring gastrointestinal dysfunction should also be validated in future studies, as these may be useful in exploring this aspect in a complex response to enteral nutrition (EN).
As mechanistic studies suggest benefit by activating fasting responses in critical illness [ 4 ], future research should investigate whether these fasting-associated benefits could be exploited in novel feeding strategies. In this regard, intermittent fasting/feeding strategies, ketogenic diets and ketone supplementation have been suggested as alternative strategies [ 67 ]. Alternating feeding periods with fasting intervals could intermittently activate a fasting response with increased autophagy and ketogenesis, while avoiding prolonged starvation [ 67 ]. Intermittent fasting also improved insulin sensitivity in non-critically ill humans and animals [ 88 ]. Moreover, intermittent amino acid provision has been suggested to be more anabolic than continuous amino acid provision by avoiding the so-called muscle-full effect [ 89 , 90 , 91 ]. However, large-scale RCT evidence confirming the efficacy and safety of intermittent fasting/feeding, ketone supplementation and ketogenic diets is lacking. RCTs that studied intermittent versus continuous medical nutrition therapy have not shown consistent benefit [ 92 , 93 ]. However, apart from a lack of power to detect or exclude a benefit, the fasting interval was relatively short in these RCTs (in general only 4–6 h), which may have been too short to activate a full fasting response [ 94 ]. Although ketogenic diets have been successfully used in refractory status epilepticus, RCT evidence that would support widespread use in critically ill patients is not available [ 67 , 95 ]. Moreover, achieving ketosis can be cumbersome, as a considerable number of medication contains carbohydrate compounds [ 96 ].
With regard to the micronutrients, future research could aim at unraveling the individual basal needs. Most recommendations regarding coverage of basal needs are Good Clinical Practice Points [ 51 ]. Improving estimation of real losses and micronutrient status could help to optimize maintenance dosing.
Conclusions
Large-scale RCT evidence has shown harm by high macronutrient doses via any route during the acute phase of the critical illness, which may be explained by anabolic resistance, suppression of autophagy and ketogenesis, and overfeeding with more severe hyperglycemia and insulin need. The time point when anabolic resistance switches into feeding responsiveness remains unclear. Therefore, personalized medical nutrition therapy, even though desirable, is currently not feasible. Validated tools that monitor actual energy, protein and micronutrient needs and potentially allow minimization of both over- and underfeeding, are warranted. As postponing full nutrition may increase the risk of micronutrient deficiencies, and subsequent refeeding syndrome, it seems prudent to cover basal micronutrient needs in all critically ill patients.
Vanhorebeek I, Latronico N, Van den Berghe G (2020) ICU-acquired weakness. Intensive Care Med 46(4):637–653. https://doi.org/10.1007/s00134-020-05944-4
Article PubMed PubMed Central Google Scholar
Villet S, Chiolero RL, Bollmann MD, Revelly JP, Cayeux RNM, Delarue J, Berger MM (2005) Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr 24(4):502–509. https://doi.org/10.1016/j.clnu.2005.03.006
Article PubMed Google Scholar
Lew CCH, Wong GJY, Cheung KP, Chua AP, Chong MFF, Miller M (2017) Association between malnutrition and 28-day mortality and intensive care length-of-stay in the critically ill: a prospective cohort study. Nutrients. https://doi.org/10.3390/nu10010010
Gunst J, Casaer MP, Preiser JC, Reignier J, Van den Berghe G (2023) Toward nutrition improving outcome of critically ill patients: how to interpret recent feeding RCTs? Crit Care 27(1):43. https://doi.org/10.1186/s13054-023-04317-9
Casaer MP, Mesotten D, Hermans G, Wouters PJ, Schetz M, Meyfroidt G, Van Cromphaut S, Ingels C, Meersseman P, Muller J, Vlasselaers D, Debaveye Y, Desmet L, Dubois J, Van Assche A, Vanderheyden S, Wilmer A, Van den Berghe G (2011) Early versus late parenteral nutrition in critically ill adults. N Engl J Med 365(6):506–517. https://doi.org/10.1056/NEJMoa1102662
Article CAS PubMed Google Scholar
Reignier J, Boisrame-Helms J, Brisard L, Lascarrou JB, Ait Hssain A, Anguel N, Argaud L, Asehnoune K, Asfar P, Bellec F, Botoc V, Bretagnol A, Bui HN, Canet E, Da Silva D, Darmon M, Das V, Devaquet J, Djibre M, Ganster F, Garrouste-Orgeas M, Gaudry S, Gontier O, Guerin C, Guidet B, Guitton C, Herbrecht JE, Lacherade JC, Letocart P, Martino F, Maxime V, Mercier E, Mira JP, Nseir S, Piton G, Quenot JP, Richecoeur J, Rigaud JP, Robert R, Rolin N, Schwebel C, Sirodot M, Tinturier F, Thevenin D, Giraudeau B, Le Gouge A, Investigators N-T, Clinical Research in Intensive C, Sepsis g (2018) Enteral versus parenteral early nutrition in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group study (NUTRIREA-2). Lancet 391(10116):133–143. https://doi.org/10.1016/S0140-6736(17)32146-3
Reignier J, Plantefeve G, Mira JP, Argaud L, Asfar P, Aissaoui N, Badie J, Botoc NV, Brisard L, Bui HN, Chatellier D, Chauvelot L, Combes A, Cracco C, Darmon M, Das V, Debarre M, Delbove A, Devaquet J, Dumont LM, Gontier O, Groyer S, Guerin L, Guidet B, Hourmant Y, Jaber S, Lambiotte F, Leroy C, Letocart P, Madeux B, Maizel J, Martinet O, Martino F, Maxime V, Mercier E, Nay MA, Nseir S, Oziel J, Picard W, Piton G, Quenot JP, Reizine F, Renault A, Richecoeur J, Rigaud JP, Schneider F, Silva D, Sirodot M, Souweine B, Tamion F, Terzi N, Thevenin D, Thiery G, Thieulot-Rolin N, Timsit JF, Tinturier F, Tirot P, Vanderlinden T, Vinatier I, Vinsonneau C, Voicu S, Lascarrou JB, Le Gouge A, Investigators N-T, Clinical Research in Intensive C, Sepsis G (2023) Low versus standard calorie and protein feeding in ventilated adults with shock: a randomised, controlled, multicentre, open-label, parallel-group trial (NUTRIREA-3). Lancet Respir Med 11(7):602–612. https://doi.org/10.1016/S2213-2600(23)00092-9
Fivez T, Kerklaan D, Mesotten D, Verbruggen S, Wouters PJ, Vanhorebeek I, Debaveye Y, Vlasselaers D, Desmet L, Casaer MP, Garcia Guerra G, Hanot J, Joffe A, Tibboel D, Joosten K, Van den Berghe G (2016) Early versus late parenteral nutrition in critically ill children. N Engl J Med 374(12):1111–1122. https://doi.org/10.1056/NEJMoa1514762
Wischmeyer PE, Bear DE, Berger MM, De Waele E, Gunst J, McClave SA, Prado CM, Puthucheary Z, Ridley EJ, Van den Berghe G, van Zanten ARH (2023) Personalized nutrition therapy in critical care: 10 expert recommendations. Crit Care 27(1):261. https://doi.org/10.1186/s13054-023-04539-x
Reintam Blaser A, Rooyackers O, Bear DE (2023) How to avoid harm with feeding critically ill patients: a synthesis of viewpoints of a basic scientist, dietitian and intensivist. Crit Care 27(1):258. https://doi.org/10.1186/s13054-023-04543-1
Alberda C, Gramlich L, Jones N, Jeejeebhoy K, Day AG, Dhaliwal R, Heyland DK (2009) The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med 35(10):1728–1737. https://doi.org/10.1007/s00134-009-1567-4
Lv C, Jiang X, Long Y, Liu Z, Lin J, Wu C, Ye X, Ye R, Liu Y, Liu M, Liu Y, Chen W, Gao L, Tong Z, Ke L, Jiang Z, Li W (2022) Association between caloric adequacy and short-term clinical outcomes in critically ill patients using a weight-based equation: secondary analysis of a cluster-randomized controlled trial. Front Nutr 9:902986. https://doi.org/10.3389/fnut.2022.902986
Hermans G, Casaer MP, Clerckx B, Guiza F, Vanhullebusch T, Derde S, Meersseman P, Derese I, Mesotten D, Wouters PJ, Van Cromphaut S, Debaveye Y, Gosselink R, Gunst J, Wilmer A, Van den Berghe G, Vanhorebeek I (2013) Effect of tolerating macronutrient deficit on the development of intensive-care unit acquired weakness: a subanalysis of the EPaNIC trial. Lancet Respir Med 1(8):621–629. https://doi.org/10.1016/S2213-2600(13)70183-8
Jacobs A, Dulfer K, Eveleens RD, Hordijk J, Van Cleemput H, Verlinden I, Wouters PJ, Mebis L, Guerra GG, Joosten K, Verbruggen SC, Guiza F, Vanhorebeek I, Van den Berghe G (2020) Long-term developmental effect of withholding parenteral nutrition in paediatric intensive care units: a 4-year follow-up of the PEPaNIC randomised controlled trial. Lancet Child Adolesc Health 4(7):503–514. https://doi.org/10.1016/S2352-4642(20)30104-8
Casaer MP, Wilmer A, Hermans G, Wouters PJ, Mesotten D, Van den Berghe G (2013) Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. Am J Respir Crit Care Med 187(3):247–255. https://doi.org/10.1164/rccm.201206-0999OC
Vanhorebeek I, Verbruggen S, Casaer MP, Gunst J, Wouters PJ, Hanot J, Guerra GG, Vlasselaers D, Joosten K, Van den Berghe G (2017) Effect of early supplemental parenteral nutrition in the paediatric ICU: a preplanned observational study of post-randomisation treatments in the PEPaNIC trial. Lancet Respir Med 5(6):475–483. https://doi.org/10.1016/S2213-2600(17)30186-8
Harvey SE, Parrott F, Harrison DA, Bear DE, Segaran E, Beale R, Bellingan G, Leonard R, Mythen MG, Rowan KM, Investigators CT (2014) Trial of the route of early nutritional support in critically ill adults. N Engl J Med 371(18):1673–1684. https://doi.org/10.1056/NEJMoa1409860
National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N, Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N, Rock P (2012) Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 307(8):795–803. https://doi.org/10.1001/jama.2012.137
Article CAS Google Scholar
Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, Mehta S, McIntyre L, Solaiman O, Sakkijha MH, Sadat M, Afesh L, Permi TTG (2015) Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults. N Engl J Med 372(25):2398–2408. https://doi.org/10.1056/NEJMoa1502826
Target Investigators ftACTG, Chapman M, Peake SL, Bellomo R, Davies A, Deane A, Horowitz M, Hurford S, Lange K, Little L, Mackle D, O’Connor S, Presneill J, Ridley E, Williams P, Young P (2018) Energy-dense versus routine enteral nutrition in the critically ill. N Engl J Med 379(19):1823–1834. https://doi.org/10.1056/NEJMoa1811687
Article Google Scholar
Needham DM, Dinglas VD, Morris PE, Jackson JC, Hough CL, Mendez-Tellez PA, Wozniak AW, Colantuoni E, Ely EW, Rice TW, Hopkins RO, Network NNA (2013) Physical and cognitive performance of patients with acute lung injury 1 year after initial trophic versus full enteral feeding. EDEN trial follow-up. Am J Respir Crit Care Med 188(5):567–576. https://doi.org/10.1164/rccm.201304-0651OC
Needham DM, Dinglas VD, Bienvenu OJ, Colantuoni E, Wozniak AW, Rice TW, Hopkins RO, Network NNA (2013) One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial. BMJ 346:f1532. https://doi.org/10.1136/bmj.f1532
Deane AM, Little L, Bellomo R, Chapman MJ, Davies AR, Ferrie S, Horowitz M, Hurford S, Lange K, Litton E, Mackle D, O’Connor S, Parker J, Peake SL, Presneill JJ, Ridley EJ, Singh V, van Haren F, Williams P, Young P, Iwashyna TJ (2020) Outcomes six months after delivering 100% or 70% of enteral calorie requirements during critical illness (TARGET). A randomized controlled trial. Am J Respir Crit Care Med 201(7):814–822. https://doi.org/10.1164/rccm.201909-1810OC
Arabi YM, Aldawood AS, Al-Dorzi HM, Tamim HM, Haddad SH, Jones G, McIntyre L, Solaiman O, Sakkijha MH, Sadat M, Mundekkadan S, Kumar A, Bagshaw SM, Mehta S, Permi T (2017) Permissive underfeeding or standard enteral feeding in high- and low-nutritional-risk critically ill adults. Post hoc analysis of the PermiT Trial. Am J Respir Crit Care Med 195(5):652–662. https://doi.org/10.1164/rccm.201605-1012OC
Yue HY, Peng W, Zeng J, Zhang Y, Wang Y, Jiang H (2024) Efficacy of permissive underfeeding for critically ill patients: an updated systematic review and trial sequential meta-analysis. J Intensive Care 12(1):4. https://doi.org/10.1186/s40560-024-00717-3
McClave SA, Martindale RG, Vanek VW, McCarthy M, Roberts P, Taylor B, Ochoa JB, Napolitano L, Cresci G, Directors ASPENBo, American College of Critical Care M, Society of Critical Care M (2009) Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JPEN J Parenter Enteral Nutr 33(3):277–316. https://doi.org/10.1177/0148607109335234
Singer P, Blaser AR, Berger MM, Alhazzani W, Calder PC, Casaer MP, Hiesmayr M, Mayer K, Montejo JC, Pichard C, Preiser JC, van Zanten ARH, Oczkowski S, Szczeklik W, Bischoff SC (2019) ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr 38(1):48–79. https://doi.org/10.1016/j.clnu.2018.08.037
Arabi YM, Tamim HM, Dhar GS, Al-Dawood A, Al-Sultan M, Sakkijha MH, Kahoul SH, Brits R (2011) Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. Am J Clin Nutr 93(3):569–577. https://doi.org/10.3945/ajcn.110.005074
Rice TW, Mogan S, Hays MA, Bernard GR, Jensen GL, Wheeler AP (2011) Randomized trial of initial trophic versus full-energy enteral nutrition in mechanically ventilated patients with acute respiratory failure. Crit Care Med 39(5):967–974. https://doi.org/10.1097/CCM.0b013e31820a905a
Charles EJ, Petroze RT, Metzger R, Hranjec T, Rosenberger LH, Riccio LM, McLeod MD, Guidry CA, Stukenborg GJ, Swenson BR, Willcutts KF, O’Donnell KB, Sawyer RG (2014) Hypocaloric compared with eucaloric nutritional support and its effect on infection rates in a surgical intensive care unit: a randomized controlled trial. Am J Clin Nutr 100(5):1337–1343. https://doi.org/10.3945/ajcn.114.088609
Article CAS PubMed PubMed Central Google Scholar
Petros S, Horbach M, Seidel F, Weidhase L (2016) Hypocaloric vs normocaloric nutrition in critically ill patients: a prospective randomized pilot trial. JPEN J Parenter Enteral Nutr 40(2):242–249. https://doi.org/10.1177/0148607114528980
Braunschweig CA, Sheean PM, Peterson SJ, Gomez Perez S, Freels S, Lateef O, Gurka D, Fantuzzi G (2015) Intensive nutrition in acute lung injury: a clinical trial (INTACT). JPEN J Parenter Enteral Nutr 39(1):13–20. https://doi.org/10.1177/0148607114528541
Rugeles S, Villarraga-Angulo LG, Ariza-Gutierrez A, Chaverra-Kornerup S, Lasalvia P, Rosselli D (2016) High-protein hypocaloric vs normocaloric enteral nutrition in critically ill patients: a randomized clinical trial. J Crit Care 35:110–114. https://doi.org/10.1016/j.jcrc.2016.05.004
Allingstrup MJ, Kondrup J, Wiis J, Claudius C, Pedersen UG, Hein-Rasmussen R, Bjerregaard MR, Steensen M, Jensen TH, Lange T, Madsen MB, Moller MH, Perner A (2017) Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med 43(11):1637–1647. https://doi.org/10.1007/s00134-017-4880-3
Hoffer LJ, Bistrian BR (2016) Nutrition in critical illness: a current conundrum. F1000Res 5:2531. https://doi.org/10.12688/f1000research.9278.1
Hartl WH, Elke G (2023) Nutrition during the acute phase of critical illness: discussions on NUTRIREA-3. Lancet Respir Med 11(7):e61. https://doi.org/10.1016/S2213-2600(23)00212-6
Heyland DK, Patel J, Compher C, Rice TW, Bear DE, Lee ZY, Gonzalez VC, O’Reilly K, Regala R, Wedemire C, Ibarra-Estrada M, Stoppe C, Ortiz-Reyes L, Jiang X, Day AG, team EPT (2023) The effect of higher protein dosing in critically ill patients with high nutritional risk (EFFORT Protein): an international, multicentre, pragmatic, registry-based randomised trial. Lancet 401(10376):568–576. https://doi.org/10.1016/S0140-6736(22)02469-2
Doig GS, Simpson F, Bellomo R, Heighes PT, Sweetman EA, Chesher D, Pollock C, Davies A, Botha J, Harrigan P, Reade MC (2015) Intravenous amino acid therapy for kidney function in critically ill patients: a randomized controlled trial. Intensive Care Med 41(7):1197–1208. https://doi.org/10.1007/s00134-015-3827-9
Lee ZY, Dresen E, Lew CCH, Bels J, Hill A, Hasan MS, Ke L, van Zanten A, van de Poll MCG, Heyland DK, Stoppe C (2024) The effects of higher versus lower protein delivery in critically ill patients: an updated systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Crit Care 28(1):15. https://doi.org/10.1186/s13054-023-04783-1
Singer P, Blaser AR, Berger MM, Calder PC, Casaer M, Hiesmayr M, Mayer K, Montejo-Gonzalez JC, Pichard C, Preiser JC, Szczeklik W, van Zanten ARH, Bischoff SC (2023) ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin Nutr 42(9):1671–1689. https://doi.org/10.1016/j.clnu.2023.07.011
Pertzov B, Bar-Yoseph H, Menndel Y, Bendavid I, Kagan I, Glass YD, Singer P (2022) The effect of indirect calorimetry guided isocaloric nutrition on mortality in critically ill patients-a systematic review and meta-analysis. Eur J Clin Nutr 76(1):5–15. https://doi.org/10.1038/s41430-021-00919-0
Singer P, De Waele E, Sanchez C, Ruiz Santana S, Montejo JC, Laterre PF, Soroksky A, Moscovici E, Kagan I (2021) TICACOS international: A multi-center, randomized, prospective controlled study comparing tight calorie control versus Liberal calorie administration study. Clin Nutr 40(2):380–387. https://doi.org/10.1016/j.clnu.2020.05.024
Veraar C, Geilen J, Fischer A, Sulz I, Tarantino S, Mouhieddine M, Mora B, Schuh C, Singer P, Hiesmayr MJ (2021) Timing of parenteral nutrition in ICU patients: a transatlantic controversy. Clin Nutr ESPEN. 46:532–538. https://doi.org/10.1016/j.clnesp.2021.08.007
Heidegger CP, Berger MM, Graf S, Zingg W, Darmon P, Costanza MC, Thibault R, Pichard C (2013) Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet 381(9864):385–393. https://doi.org/10.1016/S0140-6736(12)61351-8
Heidegger CP, Berger MM, Thibault R, Zingg W, Pichard C (2013) Supplemental parenteral nutrition in critically ill patients–authors’ reply. Lancet 381(9879):1716–1717. https://doi.org/10.1016/S0140-6736(13)61072-7
Kreymann KG, Berger MM, Deutz NE, Hiesmayr M, Jolliet P, Kazandjiev G, Nitenberg G, van den Berghe G, Wernerman J, Dgem EC, Hartl W, Heymann C, Spies C, Espen (2006) ESPEN guidelines on enteral nutrition: intensive care. Clin Nutr 25(2):210–223. https://doi.org/10.1016/j.clnu.2006.01.021
Singer P, Berger MM, Van den Berghe G, Biolo G, Calder P, Forbes A, Griffiths R, Kreyman G, Leverve X, Pichard C, Espen (2009) ESPEN Guidelines on parenteral nutrition: intensive care. Clin Nutr 28(4):387–400. https://doi.org/10.1016/j.clnu.2009.04.024
Compher C, Bingham AL, McCall M, Patel J, Rice TW, Braunschweig C, McKeever L (2022) Guidelines for the provision of nutrition support therapy in the adult critically ill patient: the American Society for Parenteral and Enteral Nutrition. JPEN J Parenter Enteral Nutr 46(1):12–41. https://doi.org/10.1002/jpen.2267
Fuentes Padilla P, Martinez G, Vernooij RW, Urrutia G, Roque IFM, Bonfill Cosp X (2019) Early enteral nutrition (within 48 hours) versus delayed enteral nutrition (after 48 hours) with or without supplemental parenteral nutrition in critically ill adults. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD012340.pub2
Fuglsang KA, Brandt CF, Jeppesen PB (2022) Survival in patients initiating home parenteral support due to nonmalignant short bowel syndrome compared with background population. Clin Nutr ESPEN 50:170–177. https://doi.org/10.1016/j.clnesp.2022.05.023
Berger MM, Shenkin A, Schweinlin A, Amrein K, Augsburger M, Biesalski HK, Bischoff SC, Casaer MP, Gundogan K, Lepp HL, de Man AME, Muscogiuri G, Pietka M, Pironi L, Rezzi S, Cuerda C (2022) ESPEN micronutrient guideline. Clin Nutr 41(6):1357–1424. https://doi.org/10.1016/j.clnu.2022.02.015
Koekkoek WA, van Zanten AR (2016) Antioxidant vitamins and trace elements in critical illness. Nutr Clin Pract 31(4):457–474. https://doi.org/10.1177/0884533616653832
Berger MM, Broman M, Forni L, Ostermann M, De Waele E, Wischmeyer PE (2021) Nutrients and micronutrients at risk during renal replacement therapy: a scoping review. Curr Opin Crit Care 27(4):367–377. https://doi.org/10.1097/MCC.0000000000000851
Koekkoek KWA, Berger MM (2023) An update on essential micronutrients in critical illness. Curr Opin Crit Care 29(4):315–329. https://doi.org/10.1097/MCC.0000000000001062
Casaer MP, Bellomo R (2019) Micronutrient deficiency in critical illness: an invisible foe? Intensive Care Med 45(8):1136–1139. https://doi.org/10.1007/s00134-019-05678-y
Doig GS, Simpson F, Heighes PT, Bellomo R, Chesher D, Caterson ID, Reade MC, Harrigan PW, Refeeding Syndrome Trial Investigators G (2015) Restricted versus continued standard caloric intake during the management of refeeding syndrome in critically ill adults: a randomised, parallel-group, multicentre, single-blind controlled trial. Lancet Respir Med 3(12):943–952. https://doi.org/10.1016/S2213-2600(15)00418-X
Olthof LE, Koekkoek W, van Setten C, Kars JCN, van Blokland D, van Zanten ARH (2018) Impact of caloric intake in critically ill patients with, and without, refeeding syndrome: a retrospective study. Clin Nutr 37(5):1609–1617. https://doi.org/10.1016/j.clnu.2017.08.001
Berger MM, Talwar D, Shenkin A (2022) Pitfalls in the interpretation of blood tests used to assess and monitor micronutrient nutrition status. Nutr Clin Pract. https://doi.org/10.1002/ncp.10924
Reintam Blaser A, Alhazzani W, Belley-Cote E, Moller MH, Adhikari NKJ, Burry L, Coopersmith CM, Al Duhailib Z, Fujii T, Granholm A, Gunst J, Hammond N, Ke L, Lamontagne F, Loudet C, Morgan M, Ostermann M, Reinikainen M, Rosenfeld R, Spies C, Oczkowski S (2023) Intravenous vitamin C therapy in adult patients with sepsis: a rapid practice guideline. Acta Anaesthesiol Scand. https://doi.org/10.1111/aas.14311
Casaer MP, Langouche L, Coudyzer W, Vanbeckevoort D, De Dobbelaer B, Guiza FG, Wouters PJ, Mesotten D, Van den Berghe G (2013) Impact of early parenteral nutrition on muscle and adipose tissue compartments during critical illness. Crit Care Med 41(10):2298–2309. https://doi.org/10.1097/CCM.0b013e31828cef02
Gunst J, Vanhorebeek I, Casaer MP, Hermans G, Wouters PJ, Dubois J, Claes K, Schetz M, Van den Berghe G (2013) Impact of early parenteral nutrition on metabolism and kidney injury. J Am Soc Nephrol 24(6):995–1005. https://doi.org/10.1681/ASN.2012070732
Heyland DK, Wibbenmeyer L, Pollack JA, Friedman B, Turgeon AF, Eshraghi N, Jeschke MG, Belisle S, Grau D, Mandell S, Velamuri SR, Hundeshagen G, Moiemen N, Shokrollahi K, Foster K, Huss F, Collins D, Savetamal A, Gurney JM, Depetris N, Stoppe C, Ortiz-Reyes L, Garrel D, Day AG, Team R-ET (2022) A randomized trial of enteral glutamine for treatment of burn injuries. N Engl J Med 387(11):1001–1010. https://doi.org/10.1056/NEJMoa2203364
Heyland D, Muscedere J, Wischmeyer PE, Cook D, Jones G, Albert M, Elke G, Berger MM, Day AG, Canadian Critical Care Trials G (2013) A randomized trial of glutamine and antioxidants in critically ill patients. N Engl J Med 368(16):1489–1497. https://doi.org/10.1056/NEJMoa1212722
Vanhorebeek I, Casaer M, Gunst J (2023) Nutrition and autophagy deficiency in critical illness. Curr Opin Crit Care 29(4):306–314. https://doi.org/10.1097/MCC.0000000000001056
Mizushima N, Levine B (2020) Autophagy in human diseases. N Engl J Med 383(16):1564–1576. https://doi.org/10.1056/NEJMra2022774
Derde S, Vanhorebeek I, Guiza F, Derese I, Gunst J, Fahrenkrog B, Martinet W, Vervenne H, Ververs EJ, Larsson L, Van den Berghe G (2012) Early parenteral nutrition evokes a phenotype of autophagy deficiency in liver and skeletal muscle of critically ill rabbits. Endocrinology 153(5):2267–2276. https://doi.org/10.1210/en.2011-2068
Gunst J, Casaer MP, Langouche L, Van den Berghe G (2021) Role of ketones, ketogenic diets and intermittent fasting in ICU. Curr Opin Crit Care 27(4):385–389. https://doi.org/10.1097/MCC.0000000000000841
Weckx R, Goossens C, Derde S, Pauwels L, Vander Perre S, Van den Berghe G, Langouche L (2022) Efficacy and safety of ketone ester infusion to prevent muscle weakness in a mouse model of sepsis-induced critical illness. Sci Rep 12(1):10591. https://doi.org/10.1038/s41598-022-14961-w
Goossens C, Weckx R, Derde S, Dufour T, Vander Perre S, Pauwels L, Thiessen SE, Van Veldhoven PP, Van den Berghe G, Langouche L (2019) Adipose tissue protects against sepsis-induced muscle weakness in mice: from lipolysis to ketones. Crit Care 23(1):236. https://doi.org/10.1186/s13054-019-2506-6
De Bruyn A, Langouche L, Vander Perre S, Gunst J, Van den Berghe G (2021) Impact of withholding early parenteral nutrition in adult critically ill patients on ketogenesis in relation to outcome. Crit Care 25(1):102. https://doi.org/10.1186/s13054-021-03519-3
De Bruyn A, Gunst J, Goossens C, Vander Perre S, Guerra GG, Verbruggen S, Joosten K, Langouche L, Van den Berghe G (2020) Effect of withholding early parenteral nutrition in PICU on ketogenesis as potential mediator of its outcome benefit. Crit Care 24(1):536. https://doi.org/10.1186/s13054-020-03256-z
Gunst J, Verbruggen SC (2023) Insulin resistance in critical illness: consequences for nutrition therapy and glucose management. Curr Opin Crit Care 29(4):286–292. https://doi.org/10.1097/MCC.0000000000001055
van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R (2001) Intensive insulin therapy in critically ill patients. N Engl J Med 345(19):1359–1367. https://doi.org/10.1056/NEJMoa011300
Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R (2006) Intensive insulin therapy in the medical ICU. N Engl J Med 354(5):449–461. https://doi.org/10.1056/NEJMoa052521
Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, Mesotten D, Casaer MP, Meyfroidt G, Ingels C, Muller J, Van Cromphaut S, Schetz M, Van den Berghe G (2009) Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet 373(9663):547–556. https://doi.org/10.1016/S0140-6736(09)60044-1
Gunst J, Debaveye Y, Guiza F, Dubois J, De Bruyn A, Dauwe D, De Troy E, Casaer MP, De Vlieger G, Haghedooren R, Jacobs B, Meyfroidt G, Ingels C, Muller J, Vlasselaers D, Desmet L, Mebis L, Wouters PJ, Stessel B, Geebelen L, Vandenbrande J, Brands M, Gruyters I, Geerts E, De Pauw I, Vermassen J, Peperstraete H, Hoste E, De Waele JJ, Herck I, Depuydt P, Wilmer A, Hermans G, Benoit DD, Van den Berghe G, Collaborators TG-F (2023) Tight blood-glucose control without early parenteral nutrition in the ICU. N Engl J Med 389(13):1180–1190. https://doi.org/10.1056/NEJMoa2304855
Umpierrez GE (2023) Glucose control in the ICU. N Engl J Med 389(13):1234–1237. https://doi.org/10.1056/NEJMe2309442
Gunst J, Van den Berghe G (2016) Blood glucose control in the ICU: don’t throw out the baby with the bathwater! Intensive Care Med 42(9):1478–1481. https://doi.org/10.1007/s00134-016-4350-3
Gunst J (2017) Recovery from critical illness-induced organ failure: the role of autophagy. Crit Care 21(1):209. https://doi.org/10.1186/s13054-017-1786-y
Gunst J, De Bruyn A, Casaer MP, Vander Perre S, Langouche L, Van den Berghe G (2021) Impact of tight glucose control on circulating 3-hydroxybutyrate in critically ill patients. Crit Care 25(1):373. https://doi.org/10.1186/s13054-021-03772-6
Van den Berghe G, Wouters PJ, Bouillon R, Weekers F, Verwaest C, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P (2003) Outcome benefit of intensive insulin therapy in the critically ill: Insulin dose versus glycemic control. Crit Care Med 31(2):359–366. https://doi.org/10.1097/01.CCM.0000045568.12881.10
Berger MM, Reintam-Blaser A, Calder PC, Casaer M, Hiesmayr MJ, Mayer K, Montejo JC, Pichard C, Preiser JC, van Zanten ARH, Bischoff SC, Singer P (2019) Monitoring nutrition in the ICU. Clin Nutr 38(2):584–593. https://doi.org/10.1016/j.clnu.2018.07.009
Gunst J, Kashani KB, Hermans G (2019) The urea-creatinine ratio as a novel biomarker of critical illness-associated catabolism. Intensive Care Med 45(12):1813–1815. https://doi.org/10.1007/s00134-019-05810-y
Achamrah N, Delsoglio M, De Waele E, Berger MM, Pichard C (2021) Indirect calorimetry: The 6 main issues. Clin Nutr 40:4–14
Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, Kutz A, Tribolet P, Bregenzer T, Braun N, Hoess C, Pavlicek V, Schmid S, Bilz S, Sigrist S, Brandle M, Benz C, Henzen C, Mattmann S, Thomann R, Brand C, Rutishauser J, Aujesky D, Rodondi N, Donze J, Stanga Z, Mueller B (2019) Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet 393(10188):2312–2321. https://doi.org/10.1016/S0140-6736(18)32776-4
Kagan I, Cohen J, Bendavid I, Kramer S, Mesilati-Stahy R, Glass Y, Theilla M, Singer P (2022) Effect of combined protein-enriched enteral nutrition and early cycle ergometry in mechanically ventilated critically ill patients—a pilot study. Nutrients. https://doi.org/10.3390/nu14081589
Kim HK, Suzuki T, Saito K, Yoshida H, Kobayashi H, Kato H, Katayama M (2012) Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc 60(1):16–23. https://doi.org/10.1111/j.1532-5415.2011.03776.x
de Cabo R, Mattson MP (2019) Effects of intermittent fasting on health, aging, and disease. N Engl J Med 381(26):2541–2551. https://doi.org/10.1056/NEJMra1905136
Bohe J, Low JF, Wolfe RR, Rennie MJ (2001) Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 532(Pt 2):575–579. https://doi.org/10.1111/j.1469-7793.2001.0575f.x
Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ (2010) Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92(5):1080–1088. https://doi.org/10.3945/ajcn.2010.29819
Flower L, Haines RW, McNelly A, Bear DE, Koelfat K, Damink SO, Hart N, Montgomery H, Prowle JR, Puthucheary Z (2022) Effect of intermittent or continuous feeding and amino acid concentration on urea-to-creatinine ratio in critical illness. JPEN J Parenter Enteral Nutr 46(4):789–797. https://doi.org/10.1002/jpen.2258
Van Dyck L, Casaer MP (2019) Intermittent or continuous feeding: any difference during the first week? Curr Opin Crit Care 25(4):356–362. https://doi.org/10.1097/MCC.0000000000000617
McNelly AS, Bear DE, Connolly BA, Arbane G, Allum L, Tarbhai A, Cooper JA, Hopkins PA, Wise MP, Brealey D, Rooney K, Cupitt J, Carr B, Koelfat K, Damink SO, Atherton PJ, Hart N, Montgomery HE, Puthucheary ZA (2020) Effect of intermittent or continuous feed on muscle wasting in critical illness: a phase 2 clinical trial. Chest 158(1):183–194. https://doi.org/10.1016/j.chest.2020.03.045
Van Dyck L, Vanhorebeek I, Wilmer A, Schrijvers A, Derese I, Mebis L, Wouters PJ, Van den Berghe G, Gunst J, Casaer MP (2020) Towards a fasting-mimicking diet for critically ill patients: the pilot randomized crossover ICU-FM-1 study. Crit Care 24(1):249. https://doi.org/10.1186/s13054-020-02987-3
White H, Venkatesh B (2011) Clinical review: ketones and brain injury. Crit Care 15(2):219. https://doi.org/10.1186/cc10020
Katz JB, Owusu K, Nussbaum I, Beekman R, DeFilippo NA, Gilmore EJ, Hirsch LJ, Cervenka MC, Maciel CB (2021) Pearls and pitfalls of introducing ketogenic diet in adult status epilepticus: a practical guide for the intensivist. J Clin Med. https://doi.org/10.3390/jcm10040881
Download references
Acknowledgements
ARB holds a grant from Estonian Research Council (PRG1255). JG is granted a senior clinical investigator fellowship by Research Foundation—Flanders (1842724N), and has obtained research funding by grants from KU Leuven (STG/23/032) and the European Society of Intensive Care Medicine (Fundamental Research Award 2022).
Author information
Angelique M. E. de Man, Jan Gunst, and Annika Reintam Blaser have contributed equally to the manuscript.
Authors and Affiliations
Department of Intensive Care, Amsterdam UMC, Location Vrije Universiteit, Amsterdam, The Netherlands
Angelique M. E. de Man
Amsterdam Cardiovascular Sciences, Amsterdam, The Netherlands
Laboratory of Intensive Care Medicine, Department of Cellular and Molecular Medicine, KU Leuven, Leuven, Belgium
Department of Intensive Care Medicine, University Hospitals Leuven, Leuven, Belgium
Department of Anaesthesiology and Intensive Care, University of Tartu, Tartu, Estonia
Annika Reintam Blaser
Department of Intensive Care Medicine, Lucerne Cantonal Hospital, Spitalstrasse, 6000, Lucerne, Switzerland
You can also search for this author in PubMed Google Scholar
Contributions
All three authors equally contributed and approved all versions.
Corresponding author
Correspondence to Angelique M. E. de Man .
Ethics declarations
Conflicts of interest.
ARB has received speaker or consultancy fees from Nestle, Fresenius Kabi, Nutricia and VIPUN Medical. AdM has received a grant from the Netherlands Organisation for Health Research and Development to perform an RCT investigating high-dose vitamin C in patient post cardiac arrest. In addition, she received several reimbursements from congress organizations for travel and hotel expenses as a speaker, JG does not have conflicts of interest related to this manuscript.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/ .
Reprints and permissions
About this article
de Man, A.M.E., Gunst, J. & Reintam Blaser, A. Nutrition in the intensive care unit: from the acute phase to beyond. Intensive Care Med (2024). https://doi.org/10.1007/s00134-024-07458-9
Download citation
Received : 27 December 2023
Accepted : 21 April 2024
Published : 21 May 2024
DOI : https://doi.org/10.1007/s00134-024-07458-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Macronutrients
- Micronutrients
- Critical illness
- Intensive care
- Find a journal
- Publish with us
- Track your research
Antioxidants

Often used as a marketing buzzword, learn about the role of antioxidants beyond the hype, and some of the research on health and disease prevention.
Jump to: – What are antioxidants? – Health benefits of antioxidants: what’s the buzz? – Studies of antioxidant supplements and disease prevention – Antioxidants in food – Bottom line on antioxidants and disease prevention
What are antioxidants?
The body’s trillion or so cells face formidable threats, from lack of food to infection with a virus. Another constant threat comes from chemicals called free radicals. In very high levels, they are capable of damaging cells and genetic material. The body generates free radicals as the inevitable byproducts of turning food into energy. Free radicals are also formed after exercising or exposure to cigarette smoke, air pollution, and sunlight. [1]
Free radicals come in many shapes, sizes, and chemical configurations. What they all share is a voracious appetite for electrons, stealing them from any nearby substances that will yield them. This electron theft can radically alter the “loser’s” structure or function. Free radical damage can change the instructions coded in a strand of DNA. It can make a circulating low-density lipoprotein (LDL, sometimes called bad cholesterol) molecule more likely to get trapped in an artery wall. Or it can alter a cell’s membrane, changing the flow of what enters the cell and what leaves it. An excessive chronic amount of free radicals in the body causes a condition called oxidative stress, which may damage cells and lead to chronic diseases. [2]
We aren’t defenseless against free radicals. The body, long used to this relentless attack, makes many molecules that quench free radicals as surely as water douses fire. We also extract free-radical fighters from food. These defenders are labeled “antioxidants.” They work by generously giving electrons to free radicals without turning into electron-scavenging substances themselves. They are also involved in mechanisms that repair DNA and maintain the health of cells.
There are hundreds, probably thousands, of different substances that can act as antioxidants. The most familiar ones are vitamin C , vitamin E , beta-carotene , and other related carotenoids, along with the minerals selenium and manganese. They’re joined by glutathione, coenzyme Q10, lipoic acid, flavonoids, phenols, polyphenols, phytoestrogens, and many more. Most are naturally occurring, and their presence in food is likely to prevent oxidation or to serve as a natural defense against the local environment.
But using the term “antioxidant” to refer to substances is misleading. It is really a chemical property, namely, the ability to act as an electron donor. Some substances that act as antioxidants in one situation may be pro-oxidants—electron grabbers—in a different situation. Another big misconception is that antioxidants are interchangeable. They aren’t. Each one has unique chemical behaviors and biological properties. They almost certainly evolved as parts of elaborate networks, with each different substance (or family of substances) playing slightly different roles. This means that no single substance can do the work of the whole crowd.
Health benefits of antioxidants: what’s the buzz?
Antioxidants came to public attention in the 1990s, when scientists began to understand that free radical damage was involved in the early stages of artery-clogging atherosclerosis. It was also linked to cancer , vision loss, and a host of other chronic conditions. Some studies showed that people with low intakes of antioxidant-rich fruits and vegetables were at greater risk for developing these chronic conditions than were people who ate plenty of those foods. Clinical trials began testing the impact of single substances in supplement form, especially beta-carotene and vitamin E, as weapons against chronic diseases.
Even before the results of these trials were in, the media and the supplement and food industries began to hype the benefits of “antioxidants.” Frozen berries, green tea, and other foods labeled as being rich in antioxidants began popping up in stores. Supplement makers touted the disease-fighting properties of all sorts of antioxidants.
The research results were mixed, but most did not find the hoped-for benefits. Most research teams reported that vitamin E and other antioxidant supplements didn’t protect against heart disease or cancer. [3] One study even showed that taking beta-carotene supplements actually increased the chances of developing lung cancer in smokers. On the other hand, some trials reported benefits; for example, after 18 years of follow-up, the Physicians’ Health Study found that taking beta-carotene supplements was associated with a modest reduction in the rate of cognitive decline. [4]
These mostly disappointing results haven’t stopped food companies and supplement sellers from banking on antioxidants. Antioxidants are still added to breakfast cereals, sports bars, energy drinks, and other processed foods , and they are promoted as additives that can prevent heart disease, cancer, cataracts, memory loss, and other conditions.
Often the claims have stretched and distorted the data: While it’s true that the package of antioxidants, minerals , fiber , and other substances found naturally in fruits , vegetables , and whole grains helps prevent a variety of chronic diseases , it is unlikely that high doses of antioxidant supplements can accomplish the same feat.

Antioxidant foods hyped to super-status
Antioxidant supplements and disease prevention: little supportive evidence.
Randomized placebo-controlled trials, which can provide the strongest evidence, offer little support that taking vitamin C, vitamin E, beta-carotene, or other single antioxidants provides substantial protection against heart disease, cancer, or other chronic conditions. The results of the largest trials have been mostly negative.
Vitamin E, beta-carotene, and other antioxidants in supplement form aren’t the silver bullet against heart disease and stroke that researchers were hoping for. A modest effect of vitamin E has been found in some studies but more research is needed.
- In the Women’s Health Study, 39,876 initially healthy women took 600 IU of natural source vitamin E or a placebo every other day for 10 years. At the study’s end, the rates of major cardiovascular events and cancer were no lower among those taking vitamin E than those taking the placebo. However, the trial did observe a significant 24% reduction in total cardiovascular mortality. Although this was not a primary endpoint for the trial, it nevertheless represents an important outcome. [6]
- Earlier large vitamin E trials, conducted among individuals with previously diagnosed coronary disease or at high risk for it, generally showed no benefit. In the Heart Outcomes Prevention Evaluation (HOPE) trial, the rates of major cardiovascular events were essentially the same in the vitamin E (21.5%) and placebo (20.6%) groups, although participants taking vitamin E had higher risks of heart failure and hospitalization for heart failure. [7] In the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI) trial, the results were mixed but mostly showed no preventive effects after more than three years of treatment with vitamin E among 11,000 heart attack survivors. [8] However, some studies suggest potential benefits among certain subgroups. A recent trial of vitamin E in Israel, for example, showed a marked reduction in coronary heart disease among people with type 2 diabetes who have a common genetic predisposition for greater oxidative stress. [9]
- Beta-carotene, meanwhile, did not provide any protection against heart disease or stroke, as demonstrated by the Physicians’ Health Study. [10]
- What about combinations? In the Supplementation en Vitamines et Mineraux Antioxydants (SU.VI.MAX) study, 13,017 French men and women took a single daily capsule that contained 120 mg vitamin C, 30 mg vitamin E, 6 mg beta-carotene, 100 mcg selenium, and 20 mg zinc, or a placebo, for seven and a half years. The vitamins had no effect on overall rates of cardiovascular disease. [11]
- In the Women’s Antioxidant Cardiovascular Study, vitamin E, vitamin C, and beta-carotene had similar effects as a placebo on myocardial infarction, stroke, coronary revascularization, and cardiovascular death, although there was a modest and significant benefit for vitamin E among women with existing cardiovascular disease. [12]
A 2014 study from the Journal of Respiratory Research found that different isoforms of vitamin E (called tocopherols) had opposing effects on lung function. [13] The study analyzed data from the Coronary Artery Risk Development in Young Adults (CARDIA) cohort and measured serum levels of alpha- and gamma-tocopherol in 4,526 adults. Lung function was tested using spirometric parameters: higher parameters are indicative of increased lung function, while lower parameters are indicative of decreased lung function. The study found that higher serum levels of alpha-tocopherol were associated with higher spirometric parameters and that high serum levels of gamma-tocopherol were associated with lower spirometric parameters. Though the study was observational in nature, it confirmed the mechanistic pathway of alpha- and gamma-tocopherol in mice studies. [14]
When it comes to cancer prevention, the picture remains inconclusive for antioxidant supplements. Few trials have gone on long enough to provide an adequate test for cancer.
- In the long-term Physicians’ Health Study, cancer rates were similar among men taking beta-carotene and among those taking a placebo. [10] Other trials have also largely shown no effect, including HOPE. [7]
- The SU.VI.MAX randomized placebo-controlled trial showed a reduction in cancer risk and all-cause mortality among men taking an antioxidant cocktail (low doses of vitamins C and E, beta-carotene, selenium, and zinc) but no apparent effect in women, possibly because men tended to have low blood levels of beta-carotene and other vitamins at the beginning of the study. [11]
- A randomized trial of selenium in people with skin cancer demonstrated significant reductions in cancer and cancer mortality at various sites, including colon, lung, and prostate. [15] The effects were strongest among those with low selenium levels at baseline.
- A six-year trial, the Age-Related Eye Disease Study (AREDS), found that a combination of vitamin C, vitamin E, beta-carotene, and zinc offered some protection against the development of advanced age-related macular degeneration, but not cataracts, in people who were at high risk of the disease. [16,17]
- Lutein, a naturally occurring carotenoid found in green, leafy vegetables such as spinach and kale, may protect vision. However, relatively short trials of lutein supplementation for age-related macular degeneration have yielded conflicting findings. [18,19] A follow-up trial to the AREDS, the AREDS2, examined lutein/zeaxanthin supplementation on late age-related macular degeneration (AMD) in men and women for up to five years. [20] It found a favorable but not significant effect of the supplements on AMD.
- A Cochrane review of 19 randomized controlled trials compared antioxidant vitamin/mineral supplements (multivitamin, vitamin E, lutein, zeaxanthin, zinc) with placebo or no intervention in people with AMD. [21] The participants were generally well-nourished. The study found that people taking the vitamins were less likely to progress to late-stage AMD and vision loss. However, the study authors noted that taking lutein and zeaxanthin alone or vitamin E alone did not have a beneficial effect on these eye conditions.
- The Selenium and Vitamin E Cancer Prevention Trial (SELECT) Eye Endpoints Study, which followed 11,267 men for a mean of five years, did not find that vitamin E and selenium supplements, in combination or alone, protected from age-related cataracts. [22]
- The Physicians’ Health Study II, a randomized trial giving 50 mg beta-carotene supplements or a placebo to 5,956 men older than 65 years, found that longer-term supplementation for at least 15 years provided cognitive benefits. [4]
- The Prevention of Alzheimer’s Disease by Vitamin E and Selenium (PREADViSE) trial followed more than 3,700 men ages 60 and older for six years. It did not find that antioxidant supplements of vitamin E or selenium, alone or in combination, protected against dementia compared with a placebo. [23]
- A meta-analysis of 68 antioxidant supplement trials found that taking beta-carotene and vitamin A and E supplements increased the risk of dying. [24] Although healthy participants were included in 21 of the trials, most of the studies included people who already had some type of serious illness. It was also difficult to compare interventions because the types of supplements, the dosages taken, and the length of time they were taken varied widely.
- The same authors conducted another systematic review of 78 randomized clinical trials on antioxidant supplements including beta-carotene, vitamin A, vitamin C, vitamin E, and selenium (alone or in combination). [25] Again, the majority of trials included people with various established diseases. The study found that both people who were healthy and those with diseases taking beta-carotene and vitamin E supplements had a higher rate of death. The duration of the studies varied widely from one month to 12 years, with varying dosages.
If antioxidants were harmless, it wouldn’t much matter if you took them “just in case.” A few studies, though, have raised the possibility that taking antioxidant supplements, either single agents or combinations, could interfere with health.
- The first inkling came in a large trial of beta-carotene conducted among men in Finland who were heavy smokers, and therefore at high risk for developing lung cancer. The trial was stopped early when researchers saw a significant increase in lung cancer among those taking the supplement compared to those taking the placebo. [26]
- In another trial among heavy smokers and people exposed to asbestos, beta-carotene was combined with vitamin A. Again, an increase in lung cancer was seen in the supplement group. [27] Not all trials of beta-carotene show this harmful effect, however. In the Physicians’ Health Study, which included few active smokers, no increase in lung cancer or any other adverse affect was seen even after 18 years of follow-up. [10]
- In the SU.VI.MAX trial, rates of skin cancer were higher in women who were assigned to take vitamin C, vitamin E, beta-carotene, selenium, and zinc. [28]
- Vitamin E supplements were found to significantly increase the risk of prostate cancer by 17% in healthy men compared with those who took a placebo. These results came from the Selenium and Vitamin E Cancer Prevention Trial (SELECT) that followed 35,533 men for up to 12 years. [29]
High-dose antioxidant supplements can also interfere with medicines. Vitamin E supplements can have a blood-thinning effect and increase the risk of bleeding in people who are already taking blood-thinning medicines. Some studies have suggested that taking antioxidant supplements during cancer treatment might interfere with the effectiveness of the treatment. Inform your doctor if starting supplements of any kind. [1]
Antioxidants in food
One possible reason why many studies on antioxidant supplements do not show a health benefit is because antioxidants tend to work best in combination with other nutrients, plant chemicals, and even other antioxidants.
For example, a cup of fresh strawberries contains about 80 mg of vitamin C, a nutrient classified as having high antioxidant activity. But a supplement containing 500 mg of vitamin C (667% of the RDA) does not contain the plant chemicals (polyphenols) naturally found in strawberries like proanthocyanins and flavonoids, which also possess antioxidant activity and may team up with vitamin C to fight disease. Polyphenols also have many other chemical properties besides their ability to serve as antioxidants. There is a question if a nutrient with antioxidant activity can cause the opposite effect with pro-oxidant activity if too much is taken. This is why using an antioxidant supplement with a single isolated substance may not be an effective strategy for everyone.
Differences in the amount and type of antioxidants in foods versus those in supplements might also influence their effects. For example, there are eight chemical forms of vitamin E present in foods. However, vitamin E supplements typically only include one form, alpha-tocopherol. [1]
Epidemiological prospective studies show that higher intakes of antioxidant-rich fruits, vegetables, and legumes are associated with a lower risk of chronic oxidative stress-related diseases like cardiovascular diseases , cancer, and deaths from all causes. [30-33] A plant-based diet is believed to protect against chronic oxidative stress-related diseases. [2] It is not clear if this protective effect is due to the antioxidants, other substances in the foods, or a combination of both. The following are nutrients with antioxidant activity and the foods in which they are found:
- Vitamin C : Broccoli, Brussels sprouts , cantaloupe, cauliflower, grapefruit, leafy greens (turnip, mustard, beet, collards), honeydew, kale , kiwi, lemon, orange, papaya, snow peas, strawberries, sweet potato , tomatoes, and bell peppers (all colors)
- Vitamin E : Almonds , avocado, Swiss chard, leafy greens (beet, mustard, turnip), peanuts, red peppers, spinach (boiled), and sunflower seeds
- Carotenoids including beta-carotene and lycopene: Apricots, asparagus, beets, broccoli, cantaloupe, carrots, bell peppers, kale , mangos, turnip and collard greens, oranges, peaches, pink grapefruit, pumpkin, winter squash , spinach, sweet potato , tangerines, tomatoes, and watermelon
- Selenium: Brazil nuts, fish, shellfish, beef, poultry, barley, brown rice
- Zinc : Beef, poultry, oysters, shrimp, sesame seeds, pumpkin seeds, chickpeas , lentils , cashews, fortified cereals
- Phenolic compounds: Quercetin ( apples , red wine, onions), catechins ( tea , cocoa , berries), resveratrol ( red and white wine , grapes, peanuts, berries), coumaric acid (spices, berries), anthocyanins (blueberries, strawberries)
Bottom line on antioxidants and disease prevention
Excessive free radicals contribute to chronic diseases including cancer, heart disease, cognitive decline, and vision loss. This doesn’t automatically mean that substances with antioxidant properties will fix the problem, especially if they are taken out of their natural context. The studies so far are inconclusive but generally don’t provide strong evidence that antioxidant supplements have a substantial impact on disease. Keep in mind that most of the trials conducted have had fundamental limitations due to their relatively short duration and inclusion of people with existing disease. At the same time, abundant evidence suggests that eating whole in fruits , vegetables , and whole grains —all rich in networks of naturally occurring antioxidants and their helper molecules—provides protection against many scourges of aging.
- National Center for Complementary and Integrative Health (NCCIH). Antioxidants: In Depth. https://nccih.nih.gov/health/antioxidants/introduction.htm Accessed 7/1/19.
- Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L, Willey C, Senoo H, Umezono Y, Sanada C, Barikmo I. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutrition journal . 2010 Dec;9(1):3.
- Semba RD, Ferrucci L, Bartali B, Urpí-Sarda M, Zamora-Ros R, Sun K, Cherubini A, Bandinelli S, Andres-Lacueva C. Resveratrol levels and all-cause mortality in older community-dwelling adults. JAMA internal medicine . 2014 Jul 1;174(7):1077-84.
- Grodstein F, Kang JH, Glynn RJ, Cook NR, Gaziano JM. A randomized trial of beta carotene supplementation and cognitive function in men: the Physicians’ Health Study II. Archives of internal medicine . 2007 Nov 12;167(20):2184-90.
- USDA Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2 (2010). http://www.orac-info-portal.de/download/ORAC_R2.pdf Accessed 7/1/2019.
- Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA . 2005 Jul 6;294(1):56-65.
- Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA . 2005 Mar;293(11):1338-47.
- GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. The Lancet . 1999 Aug 7;354(9177):447-55.
- Milman U, Blum S, Shapira C, Aronson D, Miller-Lotan R, Anbinder Y, Alshiek J, Bennett L, Kostenko M, Landau M, Keidar S. Vitamin E supplementation reduces cardiovascular events in a subgroup of middle-aged individuals with both type 2 diabetes mellitus and the haptoglobin 2-2 genotype: a prospective double-blinded clinical trial. Arteriosclerosis, thrombosis, and vascular biology . 2008 Feb 1;28(2):341-7.
- Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, Belanger C, LaMotte F, Gaziano JM, Ridker PM, Willett W. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. New England Journal of Medicine . 1996 May 2;334(18):1145-9.
- Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, Roussel AM, Favier A, Briançon S. The SU. VI. MAX Study: a randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Archives of internal medicine . 2004 Nov 22;164(21):2335-42.
- Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Archives of internal medicine . 2007 Aug 13;167(15):1610-8.
- Marchese ME, Kumar R, Colangelo LA, Avila PC, Jacobs DR, Gross M, Sood A, Liu K, Cook-Mills JM. The vitamin E isoforms α-tocopherol and γ-tocopherol have opposite associations with spirometric parameters: the CARDIA study. Respiratory research . 2014 Dec;15(1):31.
- Berdnikovs S, Abdala-Valencia H, McCary C, Somand M, Cole R, Garcia A, Bryce P, Cook-Mills JM. Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment. The Journal of Immunology . 2009 Apr 1;182(7):4395-405.
- Duffield-Lillico AJ, Reid ME, Turnbull BW, Combs GF, Slate EH, Fischbach LA, Marshall JR, Clark LC. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiology and Prevention Biomarkers . 2002 Jul 1;11(7):630-9.
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Archives of ophthalmology . 2001 Oct;119(10):1417.
- Age-Related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Archives of Ophthalmology . 2001 Oct;119(10):1439.
- Richer S, Stiles W, Statkute L, Pulido J, Frankowski J, Rudy D, Pei K, Tsipursky M, Nyland J. Double-masked, placebo-controlled, randomized trial of lutein and antioxidant supplementation in the intervention of atrophic age-related macular degeneration: the Veterans LAST study (Lutein Antioxidant Supplementation Trial). Optometry-Journal of the American Optometric Association . 2004 Apr 1;75(4):216-29.
- Bartlett HE, Eperjesi F. Effect of lutein and antioxidant dietary supplementation on contrast sensitivity in age-related macular disease: a randomized controlled trial. European journal of clinical nutrition . 2007 Sep;61(9):1121.
- Chew EY, Clemons TE, SanGiovanni JP, Danis RP, Ferris FL, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA ophthalmology . 2014 Feb 1;132(2):142-9.
- Evans JR, Lawrenson JG. Antioxidant vitamin and mineral supplements for slowing the progression of age‐related macular degeneration. Cochrane Database of Systematic Reviews . 2017(7).
- Christen WG, Glynn RJ, Gaziano JM, Darke AK, Crowley JJ, Goodman PJ, Lippman SM, Lad TE, Bearden JD, Goodman GE, Minasian LM. Age-related cataract in men in the selenium and vitamin e cancer prevention trial eye endpoints study: a randomized clinical trial. JAMA ophthalmology . 2015 Jan 1;133(1):17-24.
- Kryscio RJ, Abner EL, Caban-Holt A, Lovell M, Goodman P, Darke AK, Yee M, Crowley J, Schmitt FA. Association of antioxidant supplement use and dementia in the prevention of Alzheimer’s disease by vitamin E and selenium trial (PREADViSE). JAMA neurology . 2017 May 1;74(5):567-73.
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA . 2007 Feb 28;297(8):842-57.
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane database of systematic reviews . 2012(3).
- Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ. α-Tocopherol and β-carotene supplements and lung cancer incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: effects of base-line characteristics and study compliance. JNCI: Journal of the National Cancer Institute . 1996 Nov 6;88(21):1560-70.
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens Jr FL, Valanis B, Williams Jr JH, Barnhart S. Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. New England journal of medicine . 1996 May 2;334(18):1150-5.
- Hercberg S, Ezzedine K, Guinot C, Preziosi P, Galan P, Bertrais S, Estaquio C, Briançon S, Favier A, Latreille J, Malvy D. Antioxidant supplementation increases the risk of skin cancers in women but not in men. The Journal of nutrition . 2007 Sep 1;137(9):2098-105.
- Klein EA, Thompson IM, Tangen CM, Crowley JJ, Lucia MS, Goodman PJ, Minasian LM, Ford LG, Parnes HL, Gaziano JM, Karp DD. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA . 2011 Oct 12;306(14):1549-56.
- Joshipura KJ, Hu FB, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Colditz G, Ascherio A, Rosner B, Spiegelman D, Willett WC. The effect of fruit and vegetable intake on risk for coronary heart disease. Annals of internal medicine . 2001 Jun 19;134(12):1106-14.
- Bhupathiraju SN, Wedick NM, Pan A, Manson JE, Rexrode KM, Willett WC, Rimm EB, Hu FB. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. The American journal of clinical nutrition . 2013 Oct 2;98(6):1514-23.
- Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. International journal of epidemiology . 2017 Jun 1;46(3):1029-56.
- Miller V, Mente A, Dehghan M, Rangarajan S, Zhang X, Swaminathan S, Dagenais G, Gupta R, Mohan V, Lear S, Bangdiwala SI. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. The Lancet . 2017 Nov 4;390(10107):2037-49.
Terms of Use
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website. The Nutrition Source does not recommend or endorse any products.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Antioxidants (Basel)
- PMC11117941

Effects of Berberine on Lipid Metabolism, Antioxidant Status, and Immune Response in Liver of Tilapia ( Oreochromis niloticus ) under a High-Fat Diet Feeding
1 Key Laboratory of Integrated Rice-Fish Farming Ecology, Ministry of Agriculture and Rural Affairs, Freshwater Fisheries Research Center, Chinese Academy of Fishery Sciences, Wuxi 214081, China; nc.crff@raij (R.J.); nc.crff@ryuoh (Y.H.); nc.crff@gnaiqilgnahz (L.Z.)
2 Wuxi Fisheries College, Nanjing Agricultural University, Wuxi 214081, China
Liqiang Zhang
Associated data.
All data are contained within the main manuscript.
Berberine, a natural alkaloid found abundantly in various medicinal plants, exhibits antioxidative, anti-inflammatory, and lipid metabolism-regulatory properties. Nonetheless, its protective effects and the molecular mechanisms underlying liver injury in fish have not been fully elucidated. The aims of this study were to investigate the antioxidative, anti-inflammatory, and lipid metabolism-regulating effects of berberine against high-fat diet (HFD)-induced liver damage and to clarify the underlying molecular mechanisms. Tilapia were fed diets containing two doses of berberine (50 and 100 mg/kg diet) alongside high fat for 60 days. The results showed that berberine treatments (50 and/or 100 mg/kg) significantly reduced elevated aminotransferases, triglycerides (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-c) in the plasma. In the liver, berberine treatments significantly increased the expression of peroxisome proliferator-activated receptor α ( pparα ) and carnitine palmitoyltransferase 1 ( cpt-1 ) genes, leading to a reduction in lipid accumulation. Meanwhile, berberine treatment suppressed lipid peroxidation formation and enhanced antioxidant capacity. Berberine upregulated the mRNA levels of erythroid 2-related factor 2 ( nrf2 ) and its downstream genes including heme oxygenase 1 ( ho-1 ) and glutathione-S-transferase ( gstα ). Additionally, berberine attenuated the inflammation by inhibiting the expression of toll-like receptor 2 ( tlr2 ), myeloid differential protein-88 ( myd88 ), relb , and inflammatory cytokines such as interleukin-1β ( il-1β ), tumor necrosis factor-α ( tnf-α ), and il-8 . In summary, this study suggested that berberine offers protection against HFD-induced liver damage in tilapia via regulating lipid metabolism, antioxidant status, and immune response. This protective effect may be attributed to the modulation of the Nrf2, TLR2/MyD88/NF-κB, and PPARα signaling pathways.
1. Introduction
The liver is crucial in fish, serving as the central organ for metabolism of substances and energy and providing vital barrier functions through detoxification and phagocytosis. It is susceptible to damage due to a variety of factors, including exposure to heavy metals, misuse of chemical medications or antibiotics, and changes in environments [ 1 , 2 ]. Abnormal liver function can suppress growth, disrupt normal metabolism, reduce immunity and stress tolerance, and may even lead to death. In aquaculture, fatty liver is a common metabolic dysfunction disease of fish liver [ 3 ]. There are numerous inducing factors, such as nutritional imbalances, environmental stress, and abnormalities in physiological functions, all of which can lead to excessive lipid accumulation or lipid metabolic disorders in the liver of fish, thereby causing liver lesions [ 4 ]. The mechanism underlying fatty liver injury has been extensively reported in fish, implicating lipid accumulation, oxidative stress, and inflammatory responses [ 5 , 6 ]. Diets rich in fat have been found to disrupt lipid metabolism, exacerbate lipid peroxidation, and impair immune function in the liver of tilapia ( Oreochromis niloticus ) [ 7 , 8 ]. Similarly, a high-fat diet (HFD) has been observed to cause lipid deposition, oxidative stress, and chronic inflammation in the liver of blunt snout bream ( Megalobrama amblycephala ) [ 9 , 10 ].
Given the adverse impact of fatty liver on aquaculture fish, researchers have been exploring preventive and therapeutic measures. Du et al., (2013) have recommended employing nutritionally balanced diets, preventing feed deterioration, enhancing aquaculture technology, and mitigating stress in fish as viable strategies [ 11 ]. Alongside, the exploration of pharmacological agents, particularly herbal extracts with hepatoprotective and antioxidant properties, has opened new avenues for treating fatty liver disease in fish. Notably, dietary Eucommia ulmoides leaf extract alleviated liver steatosis and improved liver function in Ictalurus punctatus [ 12 ]. Similarly, resveratrol has been found to regulate lipid synthesis and metabolism in the liver of O. niloticus , thus reducing liver damage [ 13 ]. Saikosaponin d, by acting on the AMPK/PPARα pathway, has been effective in countering hepatic steatosis induced by an HFD in hybrid grouper ( Epinephelus lanceolatusd ♂ × Epinephelus fuscoguttatus ♀) [ 14 ]. Additionally, dietary betaine has been shown to effectively mitigate hepatic inflammation induced by an HFD in Acanthopagrus schlegelii [ 15 ]. These findings clearly demonstrate the substantial potential of herbal extracts in improving liver health and combating lipid accumulation in fish. Therefore, integrating these natural compounds into aquaculture practices could serve as an effective strategy for mitigating fatty liver disease, ultimately enhancing the welfare and productivity of cultured fish.
Berberine, a natural alkaloid, is prevalent in numerous medicinal plants, especially in traditional Chinese medicinal species like Coptis chinensis , Berberis vulgaris , and Hydrastis Canadensis [ 16 ]. Recent studies highlighted berberine’s extensive potential in medicine, especially as a treatment option for diabetes, cardiovascular disease, fatty liver, and specific chronic inflammatory disorders [ 17 ]. Its capacity to regulate metabolic pathways, decrease blood glucose and cholesterol levels, alongside its antioxidant capabilities, further validate berberine’s therapeutic promise [ 18 ]. In aquaculture, berberine has been investigated as a dietary supplement, demonstrating beneficial effects in various fish species [ 19 ]. Dietary berberine was reported to promote growth and decrease the mortality of M. amblycephala induced by Aeromonas hydrophila exposure [ 20 ]. Yu et al. (2020) found that berberine improved intestinal barrier function by modulating the intestinal microbiota in M. amblycephala [ 21 ]. Furthermore, it has been demonstrated that berberine could promote lipid metabolism and enhance antioxidant capacity in Mylopharyngodon piceus [ 22 ]. In studies focusing on liver health, berberine has shown its effectiveness by reducing hepatocyte apoptosis induced by an HFD in M. amblycephala [ 23 ] and by alleviating chronic liver injury induced by copper exposure in Acrossocheilus fasciatus [ 24 ]. These findings highlight the necessity for further investigations into the impact of berberine on liver functions across a broader spectrum of fish species. Conducting such extensive research is crucial to deepen our understanding of berberine’s therapeutic potential and its possible application in aquaculture.
Tilapia ( O. niloticus ) is extensively cultured in regions including China, Asia, and Africa. In 2022, the production of tilapia in China reached 1,738,947 tons. In intensive tilapia aquaculture, the common practice of overfeeding or providing diets high in fats and sugars to accelerate growth frequently leads to the emergence of fatty liver disease. HFD has been confirmed to induce oxidative stress, disrupt lipid metabolism, reduce immune capacity, and damage liver tissue in tilapia [ 25 , 26 , 27 ]. Several extracts from traditional Chinese herbs, such as resveratrol [ 13 ], total flavanones from Sedum sarmentosum Bunge [ 28 ], and total flavones from Glycyrrhiza [ 29 ], have been found to regulate lipid metabolism, suppress oxidative stress, alleviate inflammation, and consequently ameliorate HFD-induced liver damage in tilapia. However, there is a notable absence of research on the protective effects and the underlying molecular mechanisms of berberine against fatty liver damage in tilapia. Therefore, it is interesting to investigate the protective effects of berberine using HFD-induced liver damage model in tilapia, focusing on its impacts on lipid metabolism, oxidative stress, and immune responses.
2. Materials and Methods
2.1. tilapia, experimental design, and sampling.
Juvenile tilapia, weighing 52 ± 2.2 g, were obtained from the Freshwater Fish Research Center of the Chinese Academy of Fishery Sciences (Wuxi, China) and underwent a two-week acclimatization to laboratory conditions in a recirculation system, which maintained a temperature of 29 ± 2 °C, dissolved oxygen levels above 6 mg/L, and a pH range of 7.4–7.9. Prior to initiating the experiment, these fish were fed a control diet twice daily.
Post-acclimatization, the tilapia were weighed and systematically allocated into four distinct groups: a normal control group (NC), a high-fat diet group (HFD), and two groups receiving 50 mg/kg and 100 mg/kg of berberine, respectively. The NC group received a control diet consisting of 6% fat, while the HFD group was fed a high-fat diet containing 21% fat. The berberine-supplemented groups were fed diets containing either 50 mg/kg or 100 mg/kg of berberine, complemented by 21% fat. The formulation of the high-fat diet was based on methodologies validated in previous studies [ 30 , 31 ]. The inclusion rate of berberine in the diets was selected according to previous studies [ 32 , 33 ]. Each group consisted of 60 fish, tested across three replicates. The fish were fed approximately 4% of their body weight twice daily (09:00 and 16:00), over a period of 60 days.
After 60 days of feeding, the tilapia were weighed, and nine fish from each group were randomly selected for the collection of liver and blood tissues, conducted under anesthesia using 100 mg/L MS-222 (Sigma-Aldrich, Shanghai, China). The plasma was isolated from the blood via centrifugation at 5000 rpm, 4 °C, for 10 min, facilitating the analysis of blood biochemical parameters. Liver samples were immediately flash-frozen in liquid nitrogen to preserve them for subsequent assessments of enzymatic activity and gene expression.
2.2. Biochemical Parameter Analysis
Plasma biochemical parameters, including total triacylglycerol (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), glutamate pyruvate transaminase (GPT), glutamate oxaloacetate transaminase (GOT), total protein (TP), albumin (Alb), alkaline phosphatase (AKP), and acid phosphatase (ACP), were quantified using commercial assay kits. These measurements followed the protocols provided by the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Antioxidative parameters, including superoxide dismutase (SOD), total antioxidant capacity (T-AOC), glutathione (GSH), and malondialdehyde (MDA), were measured in liver samples utilizing commercial assay kits, following the protocols specified by the manufacturer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.3. Measurement of Target Gene Expression
RNA was extracted from 100 mg of tilapia liver tissue employing RNAiso Plus (Takara, Beijing, China), chloroform, isopropanol, and ethanol. Spectrophotometric analysis measured the OD 260/280 values to assess RNA quality and concentration. The PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara) was utilized to convert RNA into first-strand cDNA via a two-step reverse transcription process. This cDNA was then used as a template for quantitative real-time PCR (qPCR). The qPCR reactions utilized TB Green TM Premix EX Taq TM II (Takara) in a total volume of 25 μL, comprising 12.5 μL TB Green TM Premix EX Taq TM II, 1 μL each of forward and reverse primers, 8.5 μL of ddH2O, and 2 μL of cDNA. For normalization of gene expression, the ubiquitin-conjugating enzyme ( ucbe ) was utilized as a reference gene. The relative mRNA levels were determined using the 2 −ΔΔCq method [ 34 ]. The specific primers used for qPCR in tilapia are detailed in Table 1 .
The primer sequences used in the present study.
2.4. Statistical Analysis
In this study, data were processed using SPSS 24.0 for analysis and GraphPad Prism 5 software for visualization. Analyses for normal distribution and homogeneity of variance were performed on all data. Differences between groups were determined using one-way analysis of variance (ANOVA), followed by the LSD test for instances of equal variances and Tamhane’s T2 test for cases of unequal variances, with significance established at p < 0.05. Results are expressed as mean ± standard error of the mean (mean ± SEM).
3.1. Changes in Hepatic Damage Parameters in Plasma
In the plasma, HFD feeding alone significantly elevated the levels of GPT and GOT after 60 days. However, these alterations were significantly mitigated by treatment with berberine at the dose of 50 mg/kg ( Figure 1 A,B). Similarly, the increased GOT was markedly decreased in the group treated with 100 mg/kg berberine ( Figure 1 B). Moreover, the AKP level was elevated in the HFD group, whereas berberine treatment failed to mitigate this increase ( Figure 1 E). Additionally, the levels of TP, Alb, and ACP were unchanged by either the HFD or berberine treatment ( Figure 1 C,D,F).

Changes in plasma hepatic damage parameters in tilapia fed berberine-inclusive high-fat diet. Different lowercase letters indicate significant differences between groups. ( A ) Glutamate pyruvate transaminase (GPT). ( B ) Glutamate oxaloacetate transaminase (GOT). ( C ) Total protein (TP) ( D ) Albumin (Alb). ( E ) Alkaline phosphatase (AKP). ( F ) Acid phosphatase (ACP).
3.2. Change in Lipid Metabolism in Plasma
As shown in Figure 2 , the plasma parameters’ analysis displayed that an HFD-alone treatment significantly raised the levels of TG, TC, LDL-c, and HDL-c after 60 days. Notably, the elevation in TG and TC levels was significantly ameliorated in the group receiving 50 mg/kg of berberine. Furthermore, berberine treatment at dosages of both 50 and 100 mg/kg markedly reduced the increases in LDL-c ( p < 0.05).

Changes in plasma lipid metabolism parameters in tilapia fed berberine-inclusive high-fat diet. Different lowercase letters indicate significant differences between groups. ( A ) Total triacylglycerol (TG). ( B ) Total cholesterol (TC). ( C ) Low-density lipoprotein cholesterol (LDL-c). ( D ) High-density lipoprotein cholesterol (HDL-c).
3.3. Changes in the Expression of Genes Related to Metabolism Function
To explore berberine’s role in regulating lipid metabolism, we examined the mRNA levels of fatty acid β-oxidation-related genes, including pparα , acox1 , and cpt-1 in the liver ( Figure 3 ). The results highlighted a significant decrease in the expression of pparα in the group treated with an HFD in comparison to that in the NC group ( p < 0.05; Figure 3 A). However, this decrease was significantly reversed by berberine treatment with 50 and 100 mg/kg when compared to the HFD-only treatment ( p < 0.05). The cpt-1 mRNA level was significantly reduced in the HFD group; however, this downregulation was mitigated by treatments with 100 mg/kg of berberine ( p < 0.05; Figure 3 C). In addition, acox1 mRNA level was notably lower in the HFD group, but the downregulation was not significantly counteracted by berberine treatments ( Figure 3 B).

Relative expression of genes related to metabolism function in liver of tilapia fed berberine-inclusive high-fat diet. Different lowercase letters indicate significant differences between groups. ( A ) Peroxisome proliferator activated receptor alpha ( pparα ). ( B ) Acyl-CoA oxidase 1 ( acox1 ). ( C ) Carnitine O-palmitoyltransferase 1 ( cpt-1 ). ( D ) Glutamine synthase a ( gs ). ( E ) UDP-glucuronosyltransferase 2A2 ( ugt2a2 ). ( F ) NADH-cytochrome b5 reductase 2 ( cbr2 ).
To investigate the relationship between other metabolism function and the hepatoprotective effects of berberine against HFD-induced liver damage, we measured the mRNA levels of gs , ugt2a2 , and cbr2 . Compared with the NC group, HFD treatment led to an increase in the mRNA levels of ugt2a2 and cbr2 , while causing a decrease in gs mRNA level ( p < 0.05; Figure 3 D–F). The mRNA levels of ugt2a2 and cbr2 were significantly reduced following berberine treatment with 100 mg/kg compared with the HFD group ( p < 0.05; Figure 3 D,E). However, berberine treatment did not change the expression of gs compared with the HFD group ( p > 0.05; Figure 3 F).
3.4. Changes in Antioxidation Status in Liver
In the liver, there was a marked reduction in the levels of SOD, GSH, and T-AOC, accompanied by an increase in the MDA level, in tilapia treated with an HFD alone, compared with those in the NC group ( p < 0.05; Figure 4 ). The decrease in SOD level was markedly improved by berberine treatment at both 50 and 100 mg/kg ( p < 0.05). Similarly, the reduction in GSH level was significantly ameliorated with berberine treatment at a dose of 100 mg/kg ( p < 0.05). Furthermore, the elevation in the MDA level was substantially reduced under berberine treatment at both 50 and 100 mg/kg doses ( p < 0.05).

Changes in antioxidant status in liver of tilapia fed berberine-inclusive high-fat diet. Different lowercase letters indicate significant differences between groups. ( A ) Superoxide dismutase (SOD). ( B ) Glutathione (GSH). ( C ) Total antioxidant capacity (T-AOC). ( D ) Malondialdehyde (MDA).
3.5. Changes in the Expression of Genes Related to Antioxidant Status
After HFD feeding, the mRNA levels of nrf2 and gstα were notably downregulated when compared with those in the NC group ( p < 0.05; Figure 5 A,C), while this downregulation was significantly alleviated in the groups treated with berberine at doses of 50 and 100 mg/kg ( p < 0.05; Figure 5 A,C). Similarly, the mRNA level of ho-1 was markedly reduced after 60 days of HFD feeding, whereas this reduction was prevented by treatment with 100 mg/kg of berberine ( p < 0.05; Figure 5 B). In addition, the expression of nqo1 was significantly reduced after HFD feeding, whereas berberine treatments did not influence the nqo1 expression ( p > 0.05; Figure 5 D).

Relative expression of genes related to antioxidant status in liver of tilapia fed berberine-inclusive high-fat diet. Different lowercase letters indicate significant differences between groups. ( A ) Nuclear factor erythroid 2-related factor 2 ( nrf2 ) ( B ) Heme oxygenase ( ho-1 ). ( C ) Glutathione S-transferase ( gsta ). ( D ) NAD(P)H dehydrogenase 1 ( nqo1 ).
3.6. Changes in the Expression of Genes Related to Inflammatory Response
The expression of genes associated with the inflammatory response in the liver of tilapia are depicted in Figure 6 . The mRNA levels of tlr2 , myd88 , relb , tnf-α , il-1β , and il-8 were significantly elevated in the HFD group compared to those in the NC group ( p < 0.05). These genes exhibited a decreasing trend following treatment with berberine, showing significant differences at a dosage of 100 mg/kg of berberine ( p < 0.05). Additionally, tnf-α expression was notably suppressed in the group treated with 50 mg/kg of berberine compared with that in the HFD group ( p < 0.05).

Relative expression of genes related to inflammatory response in liver of tilapia fed berberine-inclusive high-fat diet. Different lowercase letters indicate significant differences between groups. ( A ) Toll-like receptor 2 ( tlr2 ). ( B ) Myloid differentiation factor 88 ( myd88 ). ( C ) NF-kB subunit ( relb ). ( D ) Tumor necrosis factor-alpha ( tnf-α ). ( E ) Interleukin-1 beta ( il-1β ). ( F ) Interleukin-8 ( il-8 ).
3.7. Changes in the Expression of Genes Related to Immune Function
The qPCR analysis revealed that in the liver of tilapia subjected solely to an HFD, there was a significant reduction in the transcript levels of c3 , lzm , igm , and hep . However, this reduction was ameliorated under treatments with berberine at concentrations of 50 and 100 mg/kg ( p < 0.05; Figure 7 ).

Relative expression of genes related to immune function in liver of tilapia fed berberine-inclusive high-fat diet. Different lowercase letters indicate significant differences between groups. ( A ) Complement C3 ( c3 ). ( B ) Lysozyme C ( lzm ). ( C ) Immunoglobulin ( igm ). ( D ) Hepcidin ( hep ).
4. Discussion
Berberine has been identified as a therapeutic agent for liver diseases, including both chronic and acute hepatic damage [ 16 ]. Earlier studies have highlighted its hepatoprotective properties, demonstrating that berberine mitigates CCl 4 -induced acute hepatotoxicity in rats [ 38 ]. An in vitro study has further confirmed berberine’s capability to protect hepatocytes from hypoxia/reoxygenation (H/R)-induced damage [ 39 ]. In fish, berberine has been shown to protect against chronic copper-induced liver injury in A. fasciatus , significantly diminishing the serum levels of GPT and GOT [ 24 ]. Consistent with these findings, our study revealed a marked elevation in the activities of GPT and GOT in tilapia subjected to an HFD alone, indicative of severe liver injury. Conversely, this injurious trend was notably reversed following berberine treatment with 50 and/or 100 mg/kg, where the levels of these markers were nearly normalized, indicating berberine’s efficacy in counteracting HFD-induced liver damage in fish.
4.1. Effects of Berberine on the Metabolism Function
TC and TG are principal components of fish blood lipids, serving as critical markers for pathological diagnosis. They reflect the lipid metabolism of the liver and the overall health status of the fish. The elevations in plasma TC, TG, LDL-c, and HDL-c following an HFD indicate a significant disruption in lipid metabolism [ 40 ]. Specifically, the increase in LDL-c and TC are concerning, pointing to an elevated risk of fatty liver disease development, which can further compromise fish health through the enhancement of oxidative stress and inflammatory responses [ 41 ]. Although the increase in HDL-c is often deemed positive in health due to its role in facilitating the reverse transport of cholesterol, its simultaneous elevation with LDL-c and TG after an HFD feeding may suggest an incapacity in the lipid regulatory system in fish [ 42 ]. In this study, the ability of berberine (50 and/or 100 mg/kg) to significantly reduce LDL-c, TC, and TG levels in tilapia highlighted its comprehensive effectiveness in ameliorating disruptions in lipid metabolism. Similar results were also reported in other fish species. For instance, dietary berberine decreased serum levels of LDL-c, TC, and TG in Pelteobagrus fulvidraco [ 43 ]; similarly, it resulted in reductions in serum levels of LDL-c, HDL-c, TC, and TG in Ctenopharyngodon idella [ 44 ]. These findings suggest berberine’s potential beneficial impact in ameliorating lipid imbalances, highlighting its versatile role in aquaculture nutrition. Nonetheless, in M. amblycephala , dietary berberine failed to suppress the elevation of TG and TC induced by an HFD [ 23 ]. The observed variability in response across different species underscores the imperative for additional research to elucidate the underlying mechanisms of berberine’s effects.
In the liver, lipid accumulation is closely associated with fatty acid β-oxidation. The activity of rate-limiting enzymes for β-oxidation, such as CPT-1 and AOX-1, plays a significant role in this process. PPARα, in particular, is recognized as a central regulator of lipid metabolism. It activates specific target genes (e.g., cpt-1 and acox-1 ) to facilitate fatty acid transport, oxidation, and lipogenesis [ 45 ]. Activation of PPARα not only ameliorates the metabolic syndrome but also exhibits anti-inflammatory effects [ 46 ]. In HFD-fed fish, a significant consequence was the downregulation of cpt-1 , acox-1 , and pparα , leading to reduced β-oxidation [ 47 ]. This diminished β-oxidation signifies a dysregulation in hepatic fatty acids, which in turn accelerates lipid accumulation and induces liver injury. It has been reported that berberine can enhance fatty acid β-oxidation, leading to a reduction in lipid accumulation. For instance, in M. piceus , the inclusion of dietary berberine mitigated the suppression of cpt-1 , acox-1 , and pparα genes provoked by an HFD feeding [ 32 ]. Likewise, in M. amblycephala , there was a notable reduction in the expression of cpt-1 , pparα, and acox genes after an HFD feeding; however, supplementation with berberine effectively reversed this downregulation [ 32 , 48 ]. In line with these findings, our study revealed that the expression levels of cpt-1 and pparα genes were significantly lower in fish fed an HFD, but this downregulation was counteracted by 100 mg/kg of berberine supplementation. Additionally, the lower pparα expression was also ameliorated with 50 mg/kg of berberine. This suggests that berberine may alleviate liver lipid accumulation in tilapia by modulating β-oxidation through the PPARα pathway.
Fatty liver disease is associated not only with lipid metabolism but also with other metabolic functions. Glutamine synthase (GS) is pivotal in nitrogen metabolism, regulates the homeostasis of blood ammonium ions and glutamine, and contributes to the modulation of liver functions [ 49 ]. In the model of CCl 4 -induced liver damage, GS activity was observed to decrease [ 50 ]; similarly, in the case of HFD-induced metabolic disorders, the expression of hepatic GS was found to be downregulated [ 51 ]. This study also noted a reduction in gs expression following HFD feeding, yet this downregulation was not significantly reversed by berberine treatment, indicating that berberine may not effectively mitigate the abnormalities in glutamine metabolism caused by an HFD feeding.
UDP-glucuronosyltransferases (UGTs) are essential phase II drug-metabolizing enzymes that facilitate the detoxification of various substances, whose dysregulation can lead to metabolic disorders and improper management of xenobiotics. In rats subjected to chronic CCl 4 -induced liver fibrosis, an alteration in the mRNA level of ugt isoforms was observed, with ugt1a1, 1a6, 2b1, and 2b2 mRNA levels being elevated, while ugt2b3 , 2b6 , and 2b12 mRNA levels were found to be reduced [ 52 ]. Similarly, the progression of human non-alcoholic fatty liver disease (NAFLD) is accompanied by a selective upregulation of certain UGT isoforms, notably UGT1A1, UGT1A3, UGT2B10, and UGT1A9 [ 53 ]. In our study, the mRNA level of ugt2a2 was upregulated in the HFD group; however, treatment with 100 mg/kg of berberine mitigated this upregulation. This finding suggests that berberine possesses the potential to ameliorate UGT dysregulation and consequently alleviate metabolic disturbances.
NADH-cytochrome b5 reductase (CBR), an integral membrane enzyme, plays a pivotal role in several biochemical processes linked to liver health and disease, including fatty acid metabolism, drug processing, and antioxidant function. It has reported that the activity of CBR showed a significant increase in rats with liver injury induced by CCl 4 and HFD, while this augmentation is mitigated by hepatoprotective agents such as d-limonene, silymarin, and trans-anethole [ 54 , 55 ]. Consistent with these findings, an increase in the mRNA level of the cbr2 was observed in tilapia with HFD-induced liver injury, which was, however, alleviated by 50 and 100 mg/kg of berberine treatments. This suggests that berberine may have a regulatory effect on detoxification processes, contributing to the mitigation of liver damage.
4.2. Effect of Berberine on Antioxidant Status
Berberine is known for its antioxidant activity and its capacity to scavenge free radicals. It mitigates oxidative stress, as demonstrated by the modulation of antioxidant enzyme activities and the levels of oxidative stress indicators, including GSH and MDA [ 56 ]. Berberine can enhance the levels of T-AOC, SOD, and catalase (CAT), while suppressing the formation of MDA in the liver of Micropterus salmoides [ 57 ]. Berberine also prevents the reduction of antioxidant activity and formation of lipid peroxidation induced by acetaminophen in the liver of Cyprinus carpio [ 58 ]. Similarly, our findings demonstrated the remarkable antioxidative properties of berberine (50 and/or 100 mg/kg); it effectively mitigated oxidative damage induced by HFD feeding, maintained the normal levels of SOD (50 and 100 mg/kg) and GSH (100 mg/kg), and inhibited the formation of MDA (50 and 100 mg/kg) in liver tissues. This aligns with previously published findings that demonstrated the concomitant administration of berberine in HFD-fed M. amblycephala , I. punctatus , and rats significantly mitigated the reduction of antioxidant components (e.g., SOD and GSH) and the elevation of lipid peroxidation [ 23 , 59 , 60 ].
In liver injury induced by oxidative stress, a variety of critical molecules and pathways have been identified. Among these, the Nrf2 pathway is particularly notable for its extensive research focus related to oxidative stress. Nrf2, a transcription factor, plays a crucial role in protecting against oxidative damage via enhancing the expression of various cellular antioxidant defense proteins, including HO-1, GSTα, and NQO1 [ 61 ]. Studies have consistently demonstrated the therapeutic and biological effects of berberine through the activation of the Nrf2 pathway [ 62 ]. Specifically, berberine has been shown to mitigate liver injury induced by methotrexate, HFD, and CCl 4 through the activation of the Nrf2 pathway in rats [ 60 , 63 , 64 ]. In fish, it was also found that dietary berberine enhanced the antioxidant capacity by upregulating nrf2 expression [ 57 , 65 ]. In this study, the nrf2 expression was markedly downregulated in HFD-induced liver injury, but this downregulation was reversed to normal levels following treatments with 50 and 100 mg/kg of berberine. Correspondingly, the mRNA levels of its downstream antioxidant genes ho-1 and gstα were significantly downregulated in the HFD-treated group. However, berberine treatment at doses of 50 and 100 mg/kg notably increased gstα expression, and the 50 mg/kg of berberine markedly elevated ho-1 expression. These findings suggest that berberine’s antioxidative effects may be attributed not only to its ROS scavenging activity but also to its enhancement of detoxifying/antioxidant enzyme expression through the activation of the Nrf2 signaling pathway in the liver of tilapia.
4.3. Effect of Berberine on Inflammatory and Immune Response
TLR2, a pattern recognition receptor of the innate immune system, is essential in connecting inflammation and liver injury, especially through its regulation of inflammatory responses under HFD-induced liver damage [ 66 ]. It has been reported that TLR2 activation triggers MyD88, subsequently initiating the NF-κB pathway to modulate the inflammatory response [ 67 ]. Mice lacking TLR2 gene exhibited notable reductions in inflammation, steatosis, and the development of non-alcoholic steatohepatitis [ 68 , 69 ]. Meanwhile, TLR2/NF-κB pathway activation was observed in mice subjected to an HFD, contributing to vascular inflammation [ 70 ]. Our study also found similar results, as we noted significant upregulation of tlr2 , myd88 , and NF-κB ( relb ) mRNA levels in HFD-induced liver injury. Importantly, treatment with 100 mg/kg berberine significantly attenuated these upregulations, suggesting that berberine may mitigate inflammation through the modulation of the TLR2/Myd88/NF-kB pathway. This finding corroborates previous research results, demonstrating that the anti-inflammatory effects of berberine are associated with the TLR (including TLR2) pathway [ 71 ].
Activation of the TLR2/MyD88/NF-κB signaling pathway initiates a cascade of genes associated with inflammatory cytokines, such as IL-1β, IL-8, and TNF-α, thereby exacerbating hepatic injury [ 72 ]. Indeed, after HFD feeding, upregulation of pro-inflammatory cytokines in the livers of both mice and fish was observed [ 59 , 73 ]. Similarly, in this study, il-1β , il-8 , and tnf-α exhibited high expression levels in the liver of tilapia subjected to HFD treatment. In contrast, these elevated expressions of il-1β , il-8 , and tnf-α were significantly reduced following treatment with 100 mg/kg of berberine. These findings suggest that the protective effect of berberine on the liver may be attributed to its anti-inflammatory properties. Supporting this notion, Wang et al., (2022) found that berberine mitigated the highly expressed il-1β , tnf-α, and nf-κB in A. schlegelii treated with an HFD [ 33 ]. Moreover, berberine diminished inflammation and lowered the levels of TNF-α, IL-6, and IL-1β in rats with non-alcoholic fatty liver disease by regulating the TLR4/MyD88/NF-κB signaling pathway [ 74 ].
In addition to inflammation, a suppression of immunity emerged as another consequence of liver injury induced by an HFD [ 59 ]. In fish, several innate immune parameters, such as LZM, HEP, and C3, play a key role in protecting against pathogens and contributing to the overall defense mechanisms [ 75 , 76 ]. The reduced LZM activity was observed in the plasma of C. carpio fed an HFD [ 77 ]. Similarly, reductions in LZM and C3 were noted in the plasma of blunt snout bream fed an HFD [ 78 ]. Furthermore, an HFD led to the downregulation of these proteins in the liver of mice [ 79 , 80 ]. In addition, IgM, a key player in the primary immune response, was also found to be decreased in the plasma of C. carpio and O. niloticus after HFD feeding [ 77 , 81 ]. Our data aligned with those in these studies, demonstrating that HFD treatment led to the suppression of mRNA levels of lzm , hep , c3 , and igm in the liver of tilapia. The immunostimulatory effects of berberine have been well-documented, evidenced by a significant increase in LZM activity in the plasma of O. niloticus [ 82 ], alongside elevated levels of IgM and C3 in the intestine of A. schlegelii [ 65 ]. In line with these findings, our data demonstrated that berberine treatments (50 and 100 mg/kg) markedly reversed the HFD-induced downregulation of lzm, hep, c3 , and igm , suggesting that berberine exerted a positive effect on the immune function in the liver of tilapia.
5. Conclusions
In summary, our findings indicated that berberine conferred hepatoprotective effects in tilapia by enhancing antioxidative and immune capacity, reducing inflammation, and improving lipid metabolism ( Figure 8 ). The beneficial effects of berberine may be attributed to the enhancement of Nrf2 and PPARα signaling pathways, along with the inhibition of the TLR2/MyD88/NF-κB signaling pathway. Specifically, the upregulation of the Nrf2 pathway triggers the production of phase II detoxifying/antioxidant enzymes, such as HO-1 and GSTa, while the downregulation of the TLR2/MyD88/NF-κB pathway results in a decreased production of pro-inflammatory cytokines, including IL-1β, IL-8, and TNF-α. Additionally, increased PPARα is believed to enhance fatty acid β-oxidation, which alleviates liver lipid deposition.

Possible mechanisms of berberine in ameliorating liver injury induced by HFD in tilapia. Red arrows indicate stimulatory modification, green arrows indicate inhibitory modification.
Funding Statement
This research was funded by Wuxi modern industry development fund project (K20221053), Central Public-Interest Scientific Institution Basal Research Fund, CAFS (2023TD64), Young Science-Technology Talents Support Project of Jiangsu Association Science and Technology (TJ-2021-076), the National Key R&D Program of China (2019YFD0900305).
Author Contributions
Conceptualization, R.J. and J.Z.; methodology, Y.H.; software, R.J.; validation, R.J., Y.H., and L.Z.; formal analysis, B.L.; investigation, R.J.; resources, B.L.; data curation, L.Z.; writing—original draft preparation, R.J.; writing—review and editing, J.Z.; visualization, Y.H.; supervision, J.Z.; project administration, B.L.; funding acquisition, B.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All animals in this study were approved by Freshwater Fisheries Research Center (20 April 2023), and all procedures were performed according to Jiangsu Laboratory’s Animal Management Guidelines (014000319/2008-00079).
Informed Consent Statement
Not applicable.
Data Availability Statement
Conflicts of interest.
The authors declare no conflicts of interest.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 24 May 2024
Exploring the effect of prolonged fasting on kynurenine pathway metabolites and stress markers in healthy male individuals
- Varvara Louvrou 1 , 2 ,
- Rima Solianik ORCID: orcid.org/0000-0001-6822-4519 1 ,
- Marius Brazaitis ORCID: orcid.org/0000-0003-1369-7524 1 &
- Sophie Erhardt ORCID: orcid.org/0000-0001-7359-5250 1 , 2
European Journal of Clinical Nutrition ( 2024 ) Cite this article
370 Accesses
5 Altmetric
Metrics details
Background/objectives
Prolonged fasting triggers a stress response within the human body. Our objective was to investigate the impact of prolonged fasting, in conjunction with stress, on kynurenine pathway metabolites.

Subjects/methods
Healthy males were divided into fasting group (zero-calorie-restriction) for 6 days (FAST, n = 14), and control group (CON, n = 10). Blood and saliva samples were collected at baseline, Day 2, Day 4, Day 6 during fasting period, and 1 week after resuming regular diet. Plasma levels of kynurenine pathway metabolites were measured using ultra-performance liquid chromatography-mass spectrometry (UPLC-MS/MS). Plasma and salivary samples were analyzed for stress markers.
A pronounced activation of the kynurenine pathway in individuals on FAST trial was revealed. Concentrations of picolinic acid (PIC), kynurenic acid (KYNA) and 3-hydroxykynurenine (3-HK) were significantly increased, with peak levels observed on Day 6 ( P < 0.0001). Conversely, concentrations of tryptophan (TRP) and quinolinic acid (QUIN) decreased ( P < 0.0001), while kynurenine (KYN) and nicotinamide (NAM) levels remained stable. Cortisol and noradrenaline concentrations remained unchanged. However, adrenaline levels significantly increased on Day 4 within FAST compared to CON ( P = 0.005). Notably, all deviations in kynurenine pathway metabolite levels returned to baseline values upon resuming regular diet following the 6-day fasting regimen, even when weight and BMI parameters were not restored.
Conclusions
Extended fasting over 6 days induces the kynurenine pathway and has minimal effects on stress markers. Restoration of metabolite concentrations upon regular feeding implies rapid adaptation of the kynurenine pathway synthetic enzymes to maintain homeostasis when faced with perturbations.
Similar content being viewed by others

Associations of dietary patterns with brain health from behavioral, neuroimaging, biochemical and genetic analyses
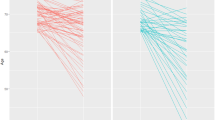
Investigating nutrient biomarkers of healthy brain aging: a multimodal brain imaging study

Temporal dynamics of the multi-omic response to endurance exercise training
Introduction.
Fasting is a practice that involves the intentional restriction or complete avoidance of food and caloric beverages for varying durations, spanning from hours to weeks. It is established that fasting induces ketogenesis, leading to significant alterations in metabolic pathways and cellular processes, including stress resistance, lipolysis, and autophagy. Fasting has been consistently highlighted for its dual role in enhancing overall health and serving as a potential disease modifier (see review: [ 1 ]). Moreover, it can complement pharmacological interventions, especially in specific conditions like epilepsy and diabetes [ 2 , 3 ]. Apart from effects on lipid, protein and glucose metabolism, fasting also elicits effects on psychological health, particularly in relation to mood regulation [ 4 , 5 ]. During fasting, hepatic gluconeogenesis stands out as a pivotal process [ 4 ], leading to the production of ketones, serving as vital energy source for the brain. Emerging evidence strongly suggests that the induction of peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) expression in the liver plays a crucial role in regulating energy utilization by boosting ATP production. This is particularly significant during fasting, where severe caloric restriction is imposed [ 6 ]. Fasting strongly induces liver expression of PGC-1α, which in turn induces the expression of kynurenine aminotransferase (KAT) enzymes and 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase (ACMSD) [ 7 , 8 ]. Interestingly, KAT and ACMSD are enzymes in the kynurenine pathway of TRP degradation through which several neuroactive metabolites are produced. Furthermore, it is shown that the kynurenine pathway, via KYN and KYNA agonist activity on the aryl hydrocarbon receptor, plays a role in T cell differentiation [ 9 ], consequently impacting immune system activity regarding tolerance and inflammation [ 10 ]. Interestingly, a recent study from our group showed that 2 days of fasting decreased TRP and increased plasma levels of the kynurenine pathway metabolites KYNA, 3-HK, PIC and the PIC/QUIN ratio in women [ 11 ].
Extended fasting induces a stress response in the human body. The physiology of stress response has two components: activation of the sympathetic-adreno-medullar axis, which acts fast, and the activation of the hypothalamic-pituitary-adrenal axis that acts slowly [ 12 ]. The former provides rapid physiological adaptation via secretion of catecholamines, noradrenaline and adrenaline [ 12 ]. The latter regulates feedback inhibition loops that involve the pituitary and adrenal glands, which control glucocorticoid production and cortisol release respectively. Cortisol exerts widespread effects due to the extensive distribution of its respective receptors in the body [ 12 ]. Importantly, many studies have shown that the kynurenine pathway can be influenced by different stressors, and induced by cortisol [ 13 ]. Our hypothesis posits that extended fasting will initiate a stress response, concurrently impacting kynurenine pathway metabolites levels. In this study, we aim to explore the effects of a 6-day fasting regimen on peripheral stress marker levels and plasma concentrations of kynurenine pathway metabolites in a male cohort.
Materials and methods
Participants.
Twenty-nine subjects were assessed for eligibility. The inclusion criteria were (i) male; (ii) aged between 18 and 44 years; (iii) with body mass index (BMI) from 19.5–29.9 kg/m 2 ; (iv) no participation in excessive regular moderate or vigorous physical activity (i.e. 3 times per week) and ≤ 150 min of moderate intensity or ≥ 75 min of vigorous-intensity activity per week; (v) no history of any eating, metabolic, skeletal, cardiovascular, oncological, neuromuscular, mental disabilities or conditions that could be negatively affected by fasting and affect experimental variables; (vi) no history of alcohol dependence or psychotropic drugs dependence and (vii) no blood/needle phobia. Participants were excluded if they were smokers, on any medications or participated in weight reduction programs and/or low-carbohydrate diets. Altogether, twenty-four males met the criteria and agreed to participate. Experiments were performed at the Institute of Sports Science and Innovations, Lithuanian Sports University, from February 2021 to September 2022. Data on 13 out of 14 FAST participants’ glucose, ketone, adrenaline and noradrenaline concentrations for timepoints ‘Baseline’ and ‘Day 6’ have been previously published.
Experimental protocol and study design
Baseline assessment commenced at 08.00–09.00 h when the participants arrived at the laboratory following an 8–13 h overnight fast. Participants were required to refrain from fatigue-related activities and abstain from ingesting caffeine, medication, and alcoholic beverages for at least 72 h before each experiment assessment. Anthropometric measurements were performed, and the participant rested in a semi-recumbent position for 20 min in a quiet room (temperature 24 °C, 60% humidity). Baseline values of capillary ketone and glucose concentrations were measured, and saliva and venous blood samples were collected (Fig. 1 ). Participants rested for a day before starting on one of the randomly prescribed trials: 6-day FAST trial ( n = 14) or CON trial ( n = 10) (Fig. 1 ). IBM Statistical Package for the Social Sciences (SPSS) for Windows version 22.0 (IBM Corp., Armonk, NY, USA) was used for randomization. FAST participants were instructed to follow a prescribed zero-calorie diet with water ad libitum over 6 days. CON participants were instructed to maintain their previous eating habits. On the 2nd, 4th, and 6th day of each trial, capillary ketone and glucose concentrations were measured, and saliva and venous blood samples were obtained (Fig. 1 ). Following a usual-diet period of 7 days (Day 13) after FAST and CON, capillary ketone and glucose concentrations were measured, saliva and venous blood samples were collected.
Blood and saliva samples taken at baseline, Day 2, Day 4, Day 6 and Day 13. FAST; fasting (0 kcal/day), CON; usual diet. Created with BioRender.com.
Capillary blood ketone and glucose concentrations
The capillary blood ketone and glucose concentrations were assessed via finger-prick capillary test using the Abbott FreeStyle Optium Neo H blood glucose and ketone monitoring system (Doncaster, Australia).
Blood sample preparation
Venous blood samples from the median antecubital vein were collected into 3 ml vacutainer tubes using EDTA with tri-potassium as an anticoagulant (K3EDTA tube; Fisher Scientific, Waltham, Massachusetts, USA), inverted 8–10 times and kept at 2–8 °C until centrifugation. Plasma was separated by centrifugation at 1200 × g for 15 min at 4 °C within 30 min of blood collection and stored in 0.5 ml aliquots at –80 °C until analysis.
Plasma preparation for UPLC-MS/MS
Thirty μl of human plasma sample, quality control or standard mix, was mixed with 30 μl of internal standard 0.5 μM in 10% ammonia for 15 s. Next, 60 μl of 200 nM ZnSO4 (5 °C) was added and mixed for 15 s. Then, 30 μl of methanol was added (5 °C) and mixed for 15 s. Additionally, the mixture was centrifuged for 10 min at 2841 × g at room temperature. 30 μl of the supernatant was mixed with 30 μl of 5% formic acid in LC-MS Certified Clear Glass 12 × 32-mm vials (product no. 186005662CV, Waters, Milford, Massachusetts, USA). Samples were transferred to an autosampler (5 °C). The volume injected into the system was 1.5 μl.
Detection of TRP and KYN metabolites in plasma
Blinded assessors used an UPLC–MS/MS system to measure plasma levels of TRP, KYN, KYNA, 3-HK, PIC, QUIN and NAM. The UPLC–MS/MS system used a Xevo TQ–XS triple quadrupole mass spectrometer (Waters) with a Z-spray electrospray interface and was operated in electrospray positive multiple reaction monitoring mode. UPLC conditions were as follows: separation was carried out using an Acquity UPLC® HSS T3 column (1.8 m, 2.1150 mm, Waters, part number: 186003540) at 50 °C with a guard column (Waters, Vanguard HSS T3 1.8 m, 2.150 mm column, part number: 186003976) to retain impurities from the mobile phase. The mobile phase consisted of: 0.6% formic acid in water (UPLC grade) and 0.6% formic acid in methanol (UPLC grade). The flow rate was 0.3 ml/min. While the MS was operating at a source temperature of 150 °C, the capillary voltage was set to +3.0 kV. The cone gas flow was 150 l/h and the desolvation gas flow rate was 1000 l/h, while the desolvation temperature was 650 °C. The autosampler was set at 5 °C and each sample took 13.0 min to run. All metabolites measured were detected in higher concentrations than the lowest level of quantification (TRP, 10 nM; KYN, 10 nM; KYNA, 10 nM; 3-HK, 10 nM; PIC, 10 nM; QUIN, 50 nM; and NAM, 10 nM). The coefficient of variation (CV) for quality controls (intra-assay, during 15 h) was less than 6% for all metabolites measured. Data processing and acquisition were performed using the software package MassLynx v 4.1 SCN943 SCN979 (© 2016 Waters Inc.). The detailed description of the method has been previously published [ 14 ].
Plasma stress hormones and salivary free cortisol concentrations
The researchers who analyzed saliva and venous blood samples were blinded to the experimental conditions. A minimum of 1.0 ml of saliva samples were collected in microtubes (1.5 ml; FLmedical, Italy) and stored at – 20 °C for later analysis. Cortisol and catecholamines concentrations were measured in duplicates using enzyme-linked immunosorbent assay kits (plasma cortisol: Cat. No. RE52061, catecholamines: Cat. No. RE59242, salivary free cortisol: Cat. No. RE52611, IBL International GmbH, Hamburg, Germany) and a Spark multimode microplate reader (Tecan, Grödig, Austria). The intra-assay CVs were 1.76%, 6.01% and 4.78%, and the inter-assay CVs were 2.42%, 7.32% and 4.94% for plasma cortisol, adrenaline, and noradrenaline, respectively. The intra-assay CV for salivary free cortisol was 3.10% and the inter-assay CV was 9.42%.
Statistical analysis
An a-priori power analysis was conducted using G*Power version 3.1.9.7 (Düsseldorf, Germany), using data from three participants in each group who completed the study. With a significance criterion of α = 0.05 and power = 0.80, the minimum required sample size was 8 participants per group to detect a main moderate effect of 0.6 for essential amino acid TRP. Furthermore, eight participants (6 in experimental group and 2 in control group) were added after considering the attrition rate and missing data.
Statistical analyses were performed using R programming language (RStudio version 2022.12.0 + 353, RStudio Team, Boston, MA, USA). Unpaired, two-tailed Welch t -tests were performed for comparisons between groups using the ggpubr R package. Homogeneity of variance was assessed using the Levene’s test, and all kynurenine pathway metabolite data were log10 transformed before fitting the model. In the case of ketones, the Levene’s test was not passed so robust standard errors were employed before fitting the model. A random linear mixed model with Bonferroni correction test was performed using the lme4 and emmeans R package. Age effect was adjusted as a co-variate factor. Subject was added as a random effect in the model. Pairwise comparisons were used to examine the effect of time within the group and to assess the effect of fasting between groups (FAST vs. CON). Normal distribution of the model residuals was assessed visually with the aid of QQ plots and histograms. All graphs were created using GraphPad Prism 9 (version 9.5.0 (525) GraphPad Software, San Diego, California, USA). Statistical significance was set at P < 0.05. Data are presented as the mean and standard error of the mean (SEM).
At baseline, the groups differed significantly in mean age (FAST 32.1 ± 1.9 vs. CON 23.4 ± 1.0, P = 0.0004) and BMI (kg/m 2 ) (FAST 25.7 ± 0.7 vs. CON 23.6 ± 0.7, P = 0.02), but not in weight (kg) (FAST 88.3 ± 3.9 vs. CON 78.9 ± 4.7, P = 0.15). Age is regarded as a co-variate, therefore the data have been adjusted accordingly (Supplementary Table 1 ).
Weight experienced a significant decrease during the fasting period ( P < 0.0001) and did not rebound to baseline values one week after resuming the regular diet ( P < 0.0001) (Fig. 2 ). Compliance with fasting regime was supported by capillary glucose and ketone changes. In the FAST trial, glucose concentrations were decreasing steadily ( P < 0.0001) while ketone concentrations showed a marked increase ( P < 0.0001). Both analytes returned to baseline values after recovery (Supplementary Fig. 1 ).
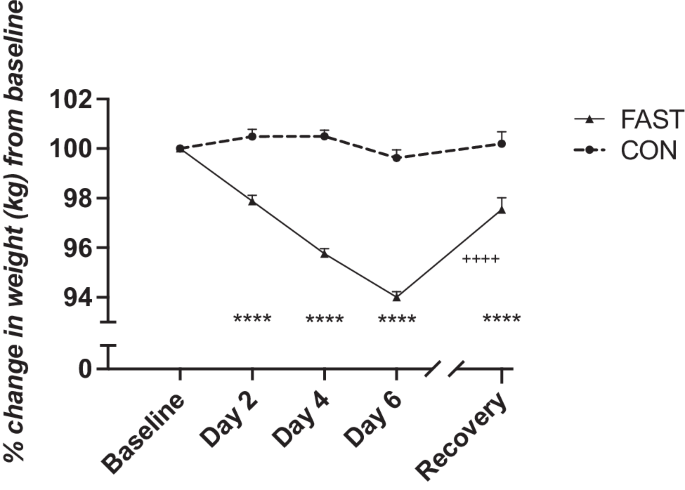
Weight was recorded every day during fasting and subsequently 1 week of recovery. Data are shown in mean ± SEM. **** P < 0.0001, compared with FAST group baseline values, ++++ P < 0.0001, for Day 6 -Recovery comparisons within FAST group. FAST; fasting (0 kcal/day), CON usual diet.
The effect of 6-day fasting on the kynurenine pathway was examined by measuring plasma concentrations of the metabolites (TRP, KYN, KYNA, QUIN, 3-HK, PIC, and NAM), as summarized in Fig. 3 . In the CON group, no significant time effects were observed for any of the metabolites (all P > 0.05). In contrast, in the FAST group, significant time effects were observed for plasma levels of all metabolites except KYN and NAM. Significant differences were revealed between FAST and CON groups regarding the concentrations of TRP ( P < 0.0001), KYNA ( P < 0.0001), 3-HK ( P < 0.05) and PIC ( P < 0.0001) during the 6 days of fasting. While concentrations of TRP significantly decreased ( P < 0.001), concentrations of KYNA, 3-HK and PIC increased significantly, peaking at 6 days of fasting. Although QUIN concentrations decreased significantly in the FAST group ( P < 0.0001), there were no significant differences between the two groups. All baseline metabolite concentrations were not significantly different from after-recovery metabolite concentrations ( P > 0.05), except KYNA ( P = 0.006). For 3-HK, KYNA, and PIC a trend of continuously increasing metabolite concentrations persisted until the sixth day of fasting, followed by a subsequent return to baseline metabolite concentrations after resuming to regular diet.
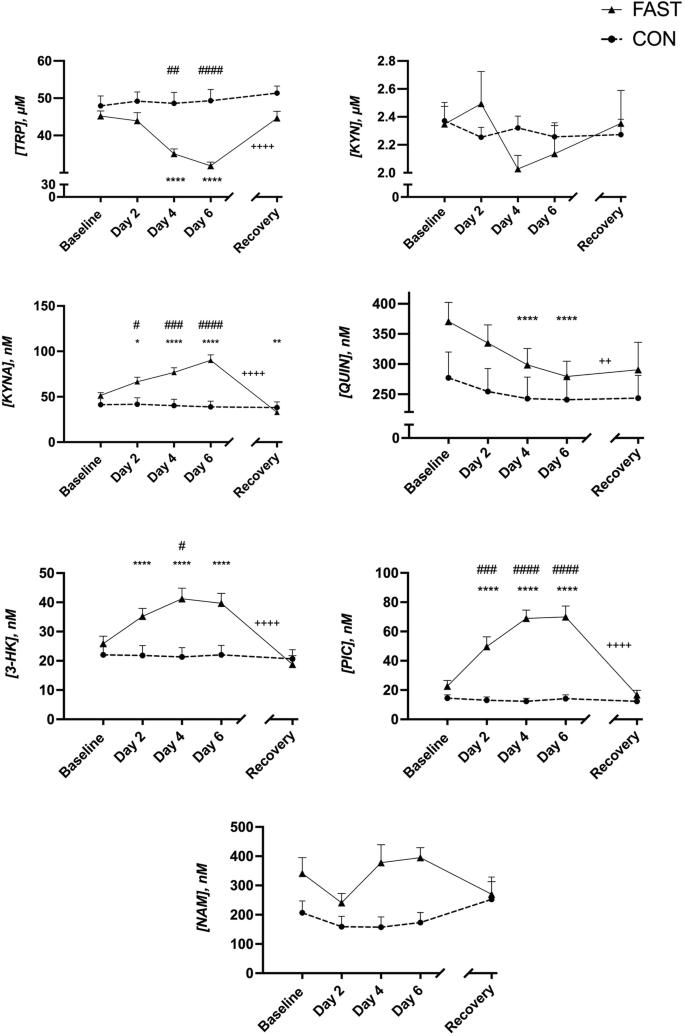
Data are shown in mean ± SEM. * P < 0.05, ** P < 0.01, **** P < 0.0001, compared with FAST baseline values. # P < 0.05, ## P < 0.01, ### P < 0.001, #### P < 0.0001 for between group comparisons, ++ P < 0.01, ++++ P < 0.0001, for Day 6 - Recovery comparisons within FAST group. FAST; fasting (0 kcal/day), CON usual diet.
Salivary and plasma cortisol concentrations showed no significant time or group effects (Fig. 4B, D ). Although noradrenaline concentrations appear more variable, no significant effects were observed (Fig. 4A ). Adrenaline levels showed no significant time effects within CON group but peaked at day 4 for the individuals in FAST group, resulting in a significant increase from baseline FAST value (Fig. 4C , P = 0.005).
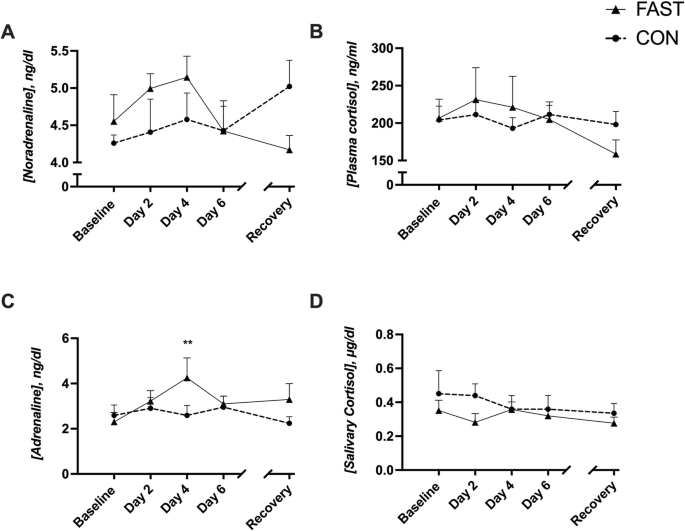
Data are shown in mean ± SEM. ** P < 0.01, compared with FAST baseline values. FAST fasting (0 kcal/day), CON usual diet.
The kynurenine pathway is the main route for TRP degradation, generating metabolites with widespread functions. The initial step involves conversion of TRP to KYN, catalyzed by enzymes tryptophan 2,3-dioxygenase (TDO) and enzyme indoleamine 2,3-dioxygenase (IDO) [ 15 , 16 ]. This pathway is implicated in numerous diseases related to immune response and excitatory neurotransmission, inevitably connecting it to neurological and psychiatric disorders [ 17 , 18 , 19 ]. It is also associated with malaria, diabetes, gastrointestinal disorders, and cancer [ 20 , 21 , 22 ]. KYN, KYNA and QUIN are proposed biomarkers for disease progression and severity [ 23 , 24 ], emphasizing the necessity of studies on pathway regulation in healthy individuals.
Accumulating evidence from human and animal studies underscores the preventive benefits of fasting against metabolic and inflammatory disorders [ 25 ]. Lifestyle changes, like physical activity and dietary restriction, rather than pharmaceuticals, have historically been the primary approach toward therapy.
The present study explores the impact of prolonged fasting, (over 6 days) on stress markers and plasma concentrations of kynurenine pathway metabolites in healthy males. The results reveal activation of the pathway during fasting, where some metabolites show significant increases, while others remain relatively stable. TRP and QUIN plasma levels decreased. Importantly, all metabolite concentrations reverted to their baseline upon resuming a regular diet, except QUIN, which remained below its baseline for unknown reasons. Notably, the metabolites’ return to baseline was not coupled with full recovery of weight and BMI parameters.
TRP concentration was expectedly decreased as it is an essential amino acid obtained from diet. TRP is vital for energy metabolism, contributing to NAD+ and acetyl-CoA formation, used for ATP generation [ 26 ]. Principally, TDO enzyme in the liver controls TRP to KYN conversion [ 16 ]. TDO activity has been described to be largely regulated by TRP availability but is also influenced by hormones, such as cortisol and insulin [ 27 ]. Notably, glucose inhibits TDO activity in rats [ 28 ], and our present study reveals a considerable decrease in glucose concentrations during fasting. KYN is the substrate for all subsequent metabolites in the pathway and may be tightly regulated, thus remained constant during fasting. Hence, the heightened activity of TDO may contribute to the elevation of downstream metabolites (KYNA, 3-HK, PIC).
Insulin suppresses the aminocarboxymuconate-semialdehyde decarboxylase (ACSMD) enzyme, inhibiting PIC production [ 29 ]. Reduced glucose and insulin levels attributed to fasting [ 30 ], alleviate the inhibition of TDO and ACMSD enzymes, aligning with the study’s findings.
Cortisol induces TDO activity, enhancing breakdown of TRP to KYN [ 31 ]. Moreover, cortisol concentration significantly increases after 8 or 10 days of water-only fasting in males and females [ 32 , 33 ].
Chronic stress activates the kynurenine pathway [ 34 , 35 ], an effect reversed by IDO inhibition [ 36 ]. Aerobic exercise may be protective against stress-induced depression via upregulated expression of KAT enzymes in muscle, consequently increasing KYN to KYNA conversion peripherally, which prevents KYN accumulation in the brain [ 37 ]. Stressful events also correlate with increased serum 3-HK levels [ 38 ].
Contrary to expectations, stress markers, like cortisol and noradrenaline did not significantly differ throughout fasting. Adrenaline levels were significantly increased after 4 days of fasting as compared to baseline, which may reflect sympathetic adreno-medullar axis activation. Although noradrenaline levels followed the same trend and peaked after 4 days of fasting, the change from baseline was insignificant. Due to cortisol circadian rhythm [ 39 ], one may speculate that the once a-day measurement of cortisol in the present study was insufficient to reveal a difference between FAST and CON groups cortisol levels throughout the day. In line with this, a study in primates [ 40 ] that employed a continuous 9-h cortisol measurement shows that change in cortisol levels after stress is more noticeable later in the day.
Fasting increased plasma concentrations of KYNA, 3-HK and PIC, whereas QUIN decreased significantly as compared to CON. Remarkably, the same shift was described in a previous 2-day fasting study [ 11 ], further supporting present findings. Increased PIC concentration might be attributed to PGC-1α involvement, a transcription coactivator that induces ACMSD expression. Indeed, fasting induces ACMSD via glucagon and glucocorticoid signaling [ 4 , 7 , 29 ]. ACMSD can be conceptualized as a regulator influencing the QUIN/PIC balance, favoring an increased PIC concentration and a decreased QUIN concentration in this context. The heightened PIC concentration also signifies an enhanced acetyl CoA synthesis, which enters the tricarboxylic acid cycle generating energy. Mice studies show the energy modulator PGC-1α increasing KAT enzyme expression in skeletal muscle, thus facilitating KYN to KYNA conversion, thereby enhancing energy efficiency and preventing fatigue [ 8 ]. Skeletal muscle mitochondria are essential in energy turnover suggesting PGC-1α expression as a critical factor in calorie restriction, energy storage mobilization, and energy propagation [ 41 ].
Increased plasma 3-HK concentration may be the product of increased kynurenine 3-monooxygenase enzyme activity. The enzyme can be influenced by anti-inflammatory cytokines such as IL-4 and IL-10, reflecting an anti-inflammatory function of fasting [ 42 ]. This aligns with previously published evidence that suggests the anti-inflammatory actions of PGC-1α on PPAR-α/PPAR-γ, as well as B-hydroxybutyrate on NLRP3 inflammasome [ 43 , 44 ].
In the present study, but also in a recent study [ 45 ], we uncovered a reduction in glucose levels and a significant increase in ketone levels after a 6-day fasting period in males. These effects may be attributed to depletion of liver glycogen stores, leading to fatty acid generation, which are subsequently converted into ketones, including acetoacetate and β-hydroxybutyrate. Ketones serve as primary alternative energy source in the brain. Notably, β-hydroxybutyrate has been shown to elevate KYNA levels in brain cortical slices, primary glial cultures, and in rat brain tissues. [ 46 , 47 ].
Further, a metabolomics study has demonstrated that fasting for 58 h increases ketone, leucine, and nicotinamide levels [ 48 ]. Excess leucine inhibits the quinolinate phosphoribosyltransferase enzyme and induces ACMSD, resulting in increased PIC levels [ 49 ], which supports this study’s findings.
Finally, it is crucial to evaluate the results’ reproducibility. By comparing the current data at the 2-day fasting timepoint with findings from another study from our lab involving a 2-day fasting regimen in women, we observe highly comparable levels of all metabolites. The persistent consistency in patterns and concentrations across studies reinforces our results [ 11 ].
Limitations
Considering the known sex differences in kynurenine pathway metabolite levels, even under baseline conditions [ 50 ], and recognizing that menstrual cycle transitions could significantly influence sex hormone levels, the present study only included males. As a future perspective, a separate study in females, accounting for all three menstrual cycle phases would provide more comprehensive understanding of how metabolic processes vary in females’ hormonal states.
Notably, the FAST and CON group had significantly different mean BMI at baseline. BMI can be considered as a covariate but given the study’s nature, BMI was not adjusted for. Collecting additional blood or saliva samples, especially considering cortisol’s circadian rhythm, would have provided valuable insights. Furthermore, this study did not account for protein binding of the metabolites. Measurements of free metabolites and albumin levels would offer deeper insight into tryptophan metabolism and should be prioritized for future investigations [ 51 , 52 ].
To conclude, this study demonstrates the effects of prolonged fasting on kynurenine pathway metabolites in healthy, young males. Recovery of metabolite concentrations to baseline after resuming regular diet, alongside non-restored weight and BMI parameters, suggests a rapid adaptation of kynurenine pathway synthetic enzymes to homeostatic perturbations. Further studies are needed to interpret the broader relevance of the observed fasting metabolic profile to diseases associated with the kynurenine pathway.
Data availability
The data analyzed in the current study are not publicly available due to data confidentiality reasons. Data analyzed in this study are available from the corresponding author upon reasonable request.
Code availability
We have followed best practices in open science by making all RStudio analysis scripts used in the study openly available on Github ( https://github.com/varlouv/6-day-fasting-study.git ).
Phillips MCL. Fasting as a therapy in neurological disease. Nutrients. 2019;11:2501.
Article CAS PubMed PubMed Central Google Scholar
Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19:181–92.
Wells J, Swaminathan A, Paseka J, Hanson C. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy-a review. Nutrients. 2020;12:1809.
Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, et al. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–8.
Article CAS PubMed Google Scholar
Wang Y, Wu R. The effect of fasting on human metabolism and psychological health. Dis Markers. 2022;2022:5653739.
PubMed PubMed Central Google Scholar
Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–22.
Koshiguchi M, Hirai S, Egashira Y. PGC1α regulates ACMSD expression through cooperation with HNF4α. Amino Acids. 2018;50:1769–73.
Agudelo LZ, Ferreira DMS, Dadvar S, Cervenka I, Ketscher L, Izadi M, et al. Skeletal muscle PGC-1α1 reroutes kynurenine metabolism to increase energy efficiency and fatigue-resistance. Nat Commun. 2019;10:2767.
Article PubMed PubMed Central Google Scholar
Stone TW, Williams RO. Modulation of T cells by tryptophan metabolites in the kynurenine pathway. Trends Pharm Sci. 2023;44:442–56.
Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature. 2014;511:184–90.
Solianik R, Schwieler L, Trepci A, Erhardt S, Brazaitis M. Two-day fasting affects kynurenine pathway with additional modulation of short-term whole-body cooling: a quasi-randomised crossover trial. British J Nutr. 2023;129:992–9.
Article CAS Google Scholar
Godoy LD, Rossignoli MT, Delfino-Pereira P, Garcia-Cairasco N, de Lima Umeoka EH. A comprehensive overview on stress neurobiology: basic concepts and clinical implications. Front Behav Neurosci. 2018;12:127.
Jovanovic F, Jovanovic V, Knezevic NN. Glucocorticoid hormones as modulators of the kynurenine pathway in chronic pain conditions. Cells. 2023;12:1178.
Trepci A, Imbeault S, Wyckelsma VL, Westerblad H, Hermansson S, Andersson DC, et al. Quantification of plasma kynurenine metabolites following one bout of sprint interval exercise. Int J Tryptophan Res IJTR. 2020;13:1178646920978241.
PubMed Google Scholar
Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49:15–23.
Article PubMed Google Scholar
Ball HJ, Jusof FF, Bakmiwewa SM, Hunt NH, Yuasa HJ. Tryptophan-catabolizing enzymes - party of three. Front Immunol. 2014;5:485.
Vécsei L, Szalárdy L, Fülöp F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. 2013;12:64–82.
Schwieler L, Samuelsson M, Frye MA, Bhat M, Schuppe-Koistinen I, Jungholm O, et al. Electroconvulsive therapy suppresses the neurotoxic branch of the kynurenine pathway in treatment-resistant depressed patients. J Neuroinflammation. 2016;13:51.
Erhardt S, Schwieler L, Imbeault S, Engberg G. The kynurenine pathway in schizophrenia and bipolar disorder. Neuropharmacology. 2017;112:297–306.
Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy States. Int J Tryptophan Res IJTR. 2009;2:1–19.
Holmberg D, Franzén-Röhl E, Idro R, Opoka RO, Bangirana P, Sellgren CM, et al. Cerebrospinal fluid kynurenine and kynurenic acid concentrations are associated with coma duration and long-term neurocognitive impairment in Ugandan children with cerebral malaria. Malar J. 2017;16:303.
Abd El-Fattah EE. IDO/kynurenine pathway in cancer: possible therapeutic approaches. J Transl Med. 2022;20:347.
Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Träskman-Bendz L, Guillemin GJ, et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-D-aspartate receptor in depression and suicidality. Brain Behav Immun. 2015;43:110–7.
Dschietzig TB, Kellner KH, Sasse K, Boschann F, Klüsener R, Ruppert J, et al. Plasma kynurenine predicts severity and complications of heart failure and associates with established biochemical and clinical markers of disease. Kidney Blood Press Res. 2019;44:765–76.
Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13.
Badawy AAB. Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int J Tryptophan Res IJTR. 2017;10. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5398323/
Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–77.
Badawy AA, Evans M. The regulation of rat liver tryptophan pyrrolase activity by reduced nicotinamide-adenine dinucleotide (phosphate). Experiments with glucose and nicotinamide. Biochem J. 1976;156:381–90.
Tanabe A, Egashira Y, Fukuoka SI, Shibata K, Sanada H. Expression of rat hepatic 2-amino-3-carboxymuconate-6-semialdehyde decarboxylase is affected by a high protein diet and by streptozotocin-induced diabetes. J Nutr. 2002;132:1153–9.
Jørgensen SW, Hjort L, Gillberg L, Justesen L, Madsbad S, Brøns C, et al. Impact of prolonged fasting on insulin secretion, insulin action, and hepatic versus whole body insulin secretion disposition indices in healthy young males. Am J Physiol-Endocrinol Metab. 2021;320:E281–90.
Messaoud A, Mensi R, Douki W, Neffati F, Najjar MF, Gobbi G, et al. Reduced peripheral availability of tryptophan and increased activation of the kynurenine pathway and cortisol correlate with major depression and suicide. World J Biol Psychiatry. 2019;20:703–11.
Colling C, Bredella MA, Fazeli PK, Pachón-Peña G, Singh RJ, Rosen CJ, et al. Changes in serum cortisol levels after 10 days of overfeeding and fasting. Am J Physiol-Endocrinol Metab. 2023;324:E506–13.
Stec K, Pilis K, Pilis W, Dolibog P, Letkiewicz S, Głębocka A. Effects of fasting on the physiological and psychological responses in middle-aged men. Nutrients. 2023;15:3444.
Chiappelli J, Pocivavsek A, Nugent KL, Notarangelo FM, Kochunov P, Rowland LM, et al. Stress-induced increase in kynurenic acid as a potential biomarker for patients with schizophrenia and distress intolerance. JAMA Psychiatry. 2014;71:761–8.
Chiappelli J, Rowland LM, Notarangelo FM, Wijtenburg SA, Thomas MAR, Pocivavsek A, et al. Salivary kynurenic acid response to psychological stress: inverse relationship to cortical glutamate in schizophrenia. Neuropsychopharmacology. 2018;43:1706–11.
Fuertig R, Azzinnari D, Bergamini G, Cathomas F, Sigrist H, Seifritz E, et al. Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: Both effects are reversed by inhibition of indoleamine 2,3-dioxygenase. Brain Behav Immun. 2016;54:59–72.
Agudelo LZ, Femenía T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45.
Küster OC, Laptinskaya D, Fissler P, Schnack C, Zügel M, Nold V. et al. Novel blood-based biomarkers of cognition, stress, and physical or cognitive training in older adults at risk of dementia: preliminary evidence for a role of BDNF, irisin, and the kynurenine pathway. J Alzheimers Dis. 2017;59:1097–111.
Nicolaides NC, Charmandari E, Kino T, Chrousos GP. Stress-related and circadian secretion and target tissue actions of glucocorticoids: impact on health. Front Endocrinol. 2017;8:70.
Article Google Scholar
Verspeek J, Behringer V, Laméris DW, Murtagh R, Salas M, Staes N, et al. Time-lag of urinary and salivary cortisol response after a psychological stressor in bonobos (Pan paniscus). Sci Rep. 2021;11:7905.
Halling JF, Pilegaard H. PGC-1α-mediated regulation of mitochondrial function and physiological implications. Appl Physiol Nutr Metab. 2020;45:927–36.
Kökten T, Hansmannel F, Ndiaye NC, Heba AC, Quilliot D, Dreumont N, et al. Calorie restriction as a new treatment of inflammatory diseases. Adv Nutr. 2021;12:1558–70.
Mandard S, Patsouris D. Nuclear control of the inflammatory response in mammals by peroxisome proliferator-activated receptors. PPAR Res. 2013;2013:613864.
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–9.
Solianik R, Židonienė K, Eimantas N, Brazaitis M. Prolonged fasting outperforms short-term fasting in terms of glucose tolerance and insulin release: a randomised controlled trial. British J Nutr. 2023;130:1500–9.
Chmiel-Perzyńska I, Kloc R, Perzyński A, Rudzki S, Urbańska EM. Novel aspect of ketone action: β-hydroxybutyrate increases brain synthesis of kynurenic acid in vitro. Neurotox Res. 2011;20:40–50.
Żarnowski T, Chorągiewicz T, Tulidowicz-Bielak M, Thaler S, Rejdak R, Żarnowski I, et al. Ketogenic diet increases concentrations of kynurenic acid in discrete brain structures of young and adult rats. J Neural Transm Vienna Austria 1996. 2012;119:679–84.
Google Scholar
Teruya T, Chaleckis R, Takada J, Yanagida M, Kondoh H. Diverse metabolic reactions activated during 58-hr fasting are revealed by non-targeted metabolomic analysis of human blood. Sci Rep. 2019;9:854.
Ghafoorunissa, Rao BSN. Effect of leucine on enzymes of the tryptophan–niacin metabolic pathway in rat liver and kidney. Biochem J. 1973;134:425–30.
Meier TB, Drevets WC, Teague TK, Wurfel BE, Mueller SC, Bodurka J, et al. Kynurenic acid is reduced in females and oral contraceptive users: Implications for depression. Brain Behav Immun. 2018;67:59–64.
Badawy AB. Plasma free tryptophan revisited: what you need to know and do before measuring it. J Psychopharmacol. 2010;24:809–15.
Coppens V, Verkerk R, Morrens M. Tracking TRYCAT: a critical appraisal of kynurenine pathway quantifications in blood. Front Pharm. 2022;13:825948.
Download references
Acknowledgements
We thank the participants of the study and Daiva Dainiene who collected the blood samples.
This work was supported by the Research Council of Lithuania (grant number S-MIP-23-84). Research Council of Lithuania had no role in the design, analysis or writing of this article. This work was supported by grants from the Swedish Research Council (2021-02251), and Åhlén-stiftelsen (SE). Open access funding provided by Karolinska Institute.
Author information
Authors and affiliations.
Institute of Sport Science and Innovations, Lithuanian Sports University, Kaunas, Lithuania
Varvara Louvrou, Rima Solianik, Marius Brazaitis & Sophie Erhardt
Department of Physiology and Pharmacology, Karolinska Institutet, Stockholm, Sweden
Varvara Louvrou & Sophie Erhardt
You can also search for this author in PubMed Google Scholar
Contributions
The research was conceived by SE and MB, designed, and conducted by all authors. The data were analyzed by VL and RS and the paper was written by VL. All authors provided constructive feedback and approved the final version of the manuscript.
Corresponding author
Correspondence to Sophie Erhardt .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Ethical approval
This study was approved by the Lithuanian Sports University Bioethics Committee (No. MNL-SFZ(M)-2021-339) and conducted according to the Declaration of Helsinki guidelines. A written informed consent was obtained from all subjects during the initial visit. The trial was registered at ClinicalTrials.gov (No. NCT05545943).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary material, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Louvrou, V., Solianik, R., Brazaitis, M. et al. Exploring the effect of prolonged fasting on kynurenine pathway metabolites and stress markers in healthy male individuals. Eur J Clin Nutr (2024). https://doi.org/10.1038/s41430-024-01451-7
Download citation
Received : 07 December 2023
Accepted : 08 May 2024
Published : 24 May 2024
DOI : https://doi.org/10.1038/s41430-024-01451-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
- Open access
- Published: 27 May 2024
A secondary analysis of indices of hepatic and beta cell function following 12 weeks of carbohydrate and energy restriction vs. free-living control in adults with type 2 diabetes
- Cody Durrer 1 ,
- Hashim Islam 1 ,
- Haoning Howard Cen 2 ,
- Maria Dolores Moya Garzon 3 , 4 ,
- Xuchao Lyu 3 , 4 ,
- Sean McKelvey 5 ,
- Joel Singer 4 ,
- Alan M. Batterham 6 ,
- Jonathan Z. Long 3 , 4 ,
- James D. Johnson 2 , 5 &
- Jonathan P. Little 1 , 5
Nutrition & Metabolism volume 21 , Article number: 29 ( 2024 ) Cite this article
112 Accesses
1 Altmetric
Metrics details
Substantial weight loss in people living with type 2 diabetes (T2D) can reduce the need for glucose-lowering medications while concurrently lowering glycemia below the diagnostic threshold for the disease. Furthermore, weight-loss interventions have also been demonstrated to improve aspects of underlying T2D pathophysiology related to ectopic fat in the liver and pancreatic beta-cell function. As such, the purpose of this secondary analysis was to explore the extent to which a low-carbohydrate and energy-restricted (LCER) diet intervention improves markers of beta-cell stress/function, liver fat accumulation, and metabolic related liver function in people with type 2 diabetes.
We conducted secondary analyses of blood samples from a larger pragmatic community-based parallel-group randomized controlled trial involving a 12-week pharmacist implemented LCER diet (Pharm-TCR: <50 g carbohydrates; ~850–1100 kcal/day; n = 20) versus treatment-as-usual (TAU; n = 16). Participants were people with T2D, using ≥ 1 glucose-lowering medication, and a body mass index of ≥ 30 kg/m 2 . Main outcomes were C-peptide to proinsulin ratio, circulating microRNA 375 (miR375), homeostatic model assessment (HOMA) beta-cell function (B), fatty liver index (FLI), hepatic steatosis index (HSI), HOMA insulin resistance (IR), and circulating fetuin-A and fibroblast growth factor 21 (FGF21). Data were analysed using linear regression with baseline as a covariate.
There was no observed change in miR375 ( p = 0.42), C-peptide to proinsulin ratio ( p = 0.17) or HOMA B ( p = 0.15). FLI and HSI were reduced by -25.1 ( p < 0.0001) and − 4.9 ( p < 0.0001), respectively. HOMA IR was reduced by -46.5% ( p = 0.011). FGF21 was reduced by -161.2pg/mL ( p = 0.035) with a similar tendency found for fetuin-A (mean difference: -16.7ng/mL; p = 0.11). These improvements in markers of hepatic function were accompanied by reductions in circulating metabolites linked to hepatic insulin resistance (e.g., diacylglycerols, ceramides) in the Pharm TCR group.
Conclusions
The Pharm-TCR intervention did not improve fasting indices of beta-cell stress; however, markers of liver fat accumulation and and liver function were improved, suggesting that a LCER diet can improve some aspects of the underlying pathophysiology of T2D.
Trial registration
Clinicaltrials.gov (NCT03181165).
Introduction
Type 2 diabetes (T2D) – one of the most prevalent lifestyle diseases in the world – puts a massive economic burden on healthcare systems worldwide with an estimated annual cost of ~$760 billion USD [ 1 ]. Despite availability of glucose-lowering medications, the prevalence of T2D is on the rise and is projected to continue increasing [ 1 ], suggesting that the current glucocentric approach to T2D care is insufficient to stem the rising prevalence of the disease. Nutritional interventions that employ low-carbohydrate [ 2 ] or energy restricted [ 3 ] strategies leading to weight loss improve glycemia, thereby reducing the need for glucose-lowering medications. There is also evidence that these approaches improve aspects of the underlying T2D pathophysiology (i.e., beta-cell function and insulin sensitivity) [ 4 , 5 , 6 , 7 ]. Reversal and remission of T2D via nutritional interventions are now recognized as realistic outcomes for managing glycemia in people with T2D [ 8 , 9 ]; however, for meaningful reversal/remission of the disease to occur, evidence of improvement in the underlying pathophysiology of T2D is needed.
One of the consequences of hyperglycemia and the associated heightened demand for insulin production and secretion in T2D is thought to be the development of endoplasmic reticulum (ER) stress in pancreatic beta-cells [ 10 , 11 ]. This can be reflected by an increased amount of proinsulin in the circulation for a given amount of C-peptide. In addition to beta-cell related impairments, hepatic steatosis is also highly prevalent among those living with T2D [ 12 ]; this is associated with elevated insulin resistance in the liver and aberrant secretion of hepatokines [ 13 , 14 ]. The presence of hepatic steatosis and T2D is associated with increased secretion of hepatokines that cause inflammation, insulin resistance, and glucose intolerance and decreased secretion/action of hepatokines that are associated with improved insulin sensitivity, improved liver steatosis, and lower adiposity [ 13 , 14 ]. Low-carbohydrate diets often result in lower postprandial glucose spikes and better glucose variability throughout the day [ 15 , 16 ]; this relief may allow for beta-cell rest and subsequent alleviation of beta-cell ER stress. When combined with an energy-restricted approach - which rapidly improves liver adiposity, liver insulin sensitivity, and fasting glucose [ 4 ] - the resultant reductions in hyperglycemia and liver adiposity could lead to both an alleviation of beta-cell stress and a healthier pattern of hepatokine secretion from the liver.
As such, the purpose of this secondary analysis was to explore the extent to which fasting markers of beta-cell stress/function, liver adiposity, insulin resistance, and hepatokine secretion are improved following the Pharmacist-led Therapeutic Carbohydrate Restriction (Pharm-TCR) intervention.
Study design and participants
Between July 7th 2017 and April 1st 2019 a total of 188 participants were enrolled in a pragmatic community-based randomized controlled trial following a parallel-group design across 12 pharmacies in British Columbia, Canada. Of the 188 participants, 36 are included in the subsample for this secondary exploratory analysis (Pharmacist-led therapeutic carbohydrate- and energy-restricted diet [Pharm-TCR] group: n = 20; Treatment-as usual [TAU] control group: n = 16). This n = 36 subsample includes participants from whom the study team was able to obtain fasting blood samples at baseline and after the 12-week trial; these participants were from five of the twelve original study sites. Fasting blood samples were not able to be obtained from all participants due to pragmatic reasons related to geographical location of the distributed pharmacy sites. The main paper for the study [ 17 ] and the trial protocol [ 18 ] are published elsewhere and provide details of the primary outcome results, participant recruitment and randomization, and study conduct. Ethical approval for the study was granted by the UBC Clinical Research Ethics Board (H16-01539) and written informed consent was obtained from all participants prior to enrollment in the study. The trial was registered on ClinicalTrials.gov (NCT03181165) on June 8th, 2017.
Participants were eligible for the study if they were able to provide written informed consent, were between the ages of 30–75 years, had been diagnosed with T2D by a physician, were using at least one glucose-lowering medication, and had a body mass index (BMI) of ≥ 30 kg/m 2 . Potential participants were excluded if they had had a heart attack within the previous two years, had any current unstable cardiovascular disorder, had a history of liver disease, kidney disease, or impaired renal function, were currently pregnant, lactating, or planning to become pregnant within the next 12 months, had a diagnosed neurological disorder, a history of bariatric surgery, a history of cancer within the previous five years, or any dietary restrictions that would inhibit adherence to the Pharm-TCR intervention diet.
Study procedures
Prior to randomization, baseline assessments of height, weight, waist circumference, and blood pressure were performed. Participants then visited a local clinical laboratory where they were assessed for HbA1c, fasting glucose, triglycerides, gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), and alanine aminotransferase (ALT). The waist circumference, triglycerides, GGT, AST, and ALT results are reported in the main paper [ 17 ] but were used in this study to calculate the hepatic steatosis index (HSI) and fatty liver index (FLI). A fasting blood sample was also collected into an EDTA containing vacutainer and processed by the local laboratories. Frozen plasma was sent to our university laboratory and stored at -80 °C for later batch analysis of insulin, proinsulin, C-peptide, microRNA (miR) 375, fetuin-A, and fibroblast growth factor 21 (FGF21).
Participants in the Pharm-TCR group followed a commercial weight loss plan (Ideal Protein) supplemented with whole foods comprising a daily macronutrient content of < 50 g carbohydrates, ~ 35–45 g fat, and ~ 110–120 g protein for a total of ~ 850–1100 kcal. Participants in this group had weekly visits to the pharmacy that involved meeting with a lifestyle coach and pharmacist to monitor progress and medication usage as well as collect intervention foods. The medication deprescription plan followed by the pharmacists is outlined in the supplementary information of the published protocol paper [ 18 ]. On the final visit, height, weight, waist circumference, and blood pressure were assessed and participants were sent to a local clinical laboratory for assessment of the same blood markers that were collected at baseline. A fasting blood sample was again collected, processed using the same procedures at the local laboratories, and frozen plasma sent to our university laboratory for batch analyses with the corresponding baseline samples after storage at -80 °C.
TAU control
Participants allocated to this group were given standard medication advice by their pharmacist as well as information pamphlets on diet and lifestyle conforming with 2013 Diabetes Canada (formerly the Canadian Diabetes Association) Clinical Practice Guidelines. The TAU group did not attend weekly meetings at the pharmacies during the 12-week period. Upon completion of the 12-weeks, participants returned to the pharmacy and were assessed for height, weight, waist circumference, and blood pressure. Participants were then sent to a local clinical laboratory for assessment of the same blood markers that were collected as baseline following the same procedures as the Pharm-TCR group.
Outcome measures
Insulin, proinsulin and c-peptide.
Fasting insulin was assessed in plasma samples with the U-PLEX Human Insulin Assay (intra assay CV: 4.8%; Meso Scale Discovery, Maryland, USA). Fasting proinsulin was measured in plasma samples using via either proinsulin chemiluminescence ELISA (intra assay CV: 3.2%; Alpco, Salem, NH, USA) or the U-PLEX Human Proinsulin Assay (intra assay CV: 2.7%; Meso Scale Discovery, Maryland, USA). Fasting C-peptide was measured in plasma samples via either C-peptide chemiluminescence ELISA (intra assay CV: 7.5%; Alpco, Salem, NH, USA) or the U-PLEX Human C-peptide Assay (intra assay CV: 3.2%; Meso Scale Discovery, Maryland, USA). Baseline and post-study samples from the same participant were assessed using the same assay.
Homeostatic model assessment
Insulin resistance was assessed using HOMA IR (HOMA2 Calculator Version 2.2.3). As this is a fasting assessment of insulin resistance, it primarily reflects the liver [ 19 ]. Beta-cell function was assessed via the homeostasis model assessment (HOMA) %B (HOMA2 Calculator Version 2.2.3). As recommended by Wallace et al. [ 20 ] C-peptide was used in the calculation for HOMA B rather than insulin.
Hepatokines and liver adiposity
The hepatokines fetuin-A and FGF21 were assessed in plasma samples collected at baseline and after 12-weeks via ELISA (both Quantikine, R&D Systems Inc, USA). The average intra assay CVs for fetuin-A and FGF21 were 6.3% and 9.7%, respectively. The fasting insulin to C-peptide ratio was calculated as a measure of insulin clearance by the liver. The degree of liver fat accumulation was assessed using the HSI and FLI at baseline and after 12-weeks. Fatty liver index (FLI) = (e 0.953*log e (triglycerides) +0.139*BMI+0.718*log e (GGT) +0.053*waist circumference−15.745 ) / (1 + e 0.953*log e (triglycerides) +0.139*BMI+0.718*log e (GGT) +0.053*waist circumference−15.745 ) * 100 [ 21 ]. Hepatic steatosis index (HSI) = 8 * (ALT/AST) + BMI(+ 2 if T2D, + 2 if female) [ 22 ].
MicroRNA 375
RNA was extracted from 100 µL plasma using the MagMax mir Vana Total RNA Isolation Kit (Applied Biosystems, Foster City, California, USA). 10 ng of total RNA were reverse transcribed into cDNA using TaqMan™ MicroRNA Reverse Transcription Kit (4,366,596, Applied Biosystems). MiR375 levels were determined on the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, California, USA) in duplicate qPCR reactions (3.35 ng cDNA/reaction) according to manufacturer’s instructions using the TaqMan™ MicroRNA Assay (#4,427,975, Assay ID 000564, Applied Biosystems) and the TaqMan™ Fast Advanced Master Mix (4,444,556, Applied Biosystems). Synthetic miR375 oligonucleotide (IDT, Iowa, USA) was used to determine cDNA synthesis efficiency and construct standard curves for absolute quantification during qPCR.
Anthropometrics and clinical blood markers
Body weight and body fat percentage were assessed using the Tanita model DF-430 U (IL, USA) scale, blood pressure was assessed using the PharmaSmart Model PS-2000 C (BC, Canada), height was measured using the Seca model 700 stadiometer (Germany), and waist circumference was assessed by measuring the distance around the waist at the top of the iliac crest with a tape measure. HbA1c and fasting plasma glucose, along with triglycerides, and liver function tests (GGT, ALT, and AST) were analyzed by provincially-accredited laboratories per standard clinical practice.
Untargeted plasma metabolomics
Preparation of plasma samples for lc-ms analysis.
Polar metabolites were extracted from plasma for LC-MS analysis by adding 150 uL of a 2:1 mixture of acetonitrile/methanol to 50 ul of plasma. This mixture was then centrifuged at 4 °C for 10 min at 15,000 rpm and the supernatant was transferred to a LC-MS vial.
Untargeted measurements of metabolites by LC-MS
Untargeted metabolomics measurements were performed on an Agilent 6520 Quadrupole time-of-flight (Q-TOF) LC/MS using electrospray ionization (ESI) in negative mode. The dual ESI source parameters were set as follows, the gas temperature was set at 250 °C with a drying gas flow of 12 L/min and the nebulizer pressure at 20 psi. The capillary voltage was set to 3500 V and the fragmentor voltage set to 100 V. Separation of polar metabolites was conducted using a Luna 5 μm NH2 100 Å LC column (Phenomenex 00B-4378-E0) with normal phase chromatography. Mobile phases were as follows: buffer A, 95:5 water: acetonitrile with 0.2% ammonium hydroxide and 10 mM ammonium acetate; buffer B, acetonitrile. The LC gradient started at 100% B with a flow rate of 0.2 mL min –1 from 0 to 2 min. The gradient was then linearly increased to 50% A/50% B at a flow rate of 0.7 mL min –1 from 2 to 20 min. From 20 to 25 min, the gradient was maintained at 50% A/50% B at a flow rate of 0.7 mL min –1 . Differential peak identification was performed with XCMS software.
XCMS analysis
LC-MS data were uploaded to Scripps XCMS Online to identify significantly changed metabolites by comparison of the pre ( N = 19) vs. post samples ( N = 19). Negative polarity was selected, mass error was set to 20 ppm, mz diff was set to 0.05, minimum peak width was set to 10 and maximum peak width was set to 60. Statistically significant peaks were searched in HMDB (Human Metabolome Database) selecting negative polarity and setting mass error to 20 ppm.
Statistical analysis
All analyses were performed using R version 4.3.0 [ 23 ]. Data were analyzed using linear models with the 12-week outcome value as the dependent variable and fixed effects for treatment (Pharm-TCR vs. TAU), sex, and the baseline outcome values. Model specification was assessed visually using normal probability plots and residuals vs. fitted values plots. When the behaviour of the model residuals warranted a log transformation, effect estimates and 95% confidence intervals were back-transformed to ratio (presented as percentage) differences using the emmeans package version 1.10.1 [ 24 ]. Significance was accepted at p < 0.05.
For untargeted plasma metabolics analyses, the row z-scores of the altered compounds ( p < 0.05) detected in untargeted metabolomics were plotted in the heatmap using ComplexHeatmap R package [ 25 ]. Because a few outliers (mean +/- 3 SD cutoffs) could drastically skew the z-score, they were excluded from the z-score calculation and are shown in grey color in the heatmap. The log2 fold changes of post- to pre-treatment measurements are calculated for each participant. The median log2 fold changes of each detected compound were plotted in the volcano plot. Pathways from all detected metabolites were predicted using the “Functional Analysis” module in MetaboAnalyst 5.0 online tool [ 26 ], following the official tutorial on the website. Briefly, a peak list of all detected compounds is uploaded, containing retention time in minutes and ranked by T scores. Negative ion mode, 20 ppm mass tolerance and “Enforce Primary Ions” are selected in the settings. Pathways are enriched from the human KEGG pathway library, and only pathways with > 3 entries are included. Both Mummichog 2.0 ( p value cutoff 0.05) and GSEA algorithms (ranked by T scores) are used to predict significant pathways, and their integrated p values are plotted. The pathway results are exported from MetaboAnalyst and plotted in R. The R code for metabolomics analysis is available at GitHub repository https://github.com/hcen/pharmTCR_metabolomics .
Baseline characteristics of study participants
Thirty-six participants, who had fasting blood samples taken at baseline and following 12-weeks, were included in this analysis. Baseline characteristics are reported in Table 1 . Only baseline levels of fetuin-A were different between groups.
Changes following the intervention period
Body mass and bmi.
There was a significant reduction in body mass and BMI (both p < 0.001) in the Pharm-TCR group in this subgroup analysis (Table 2 ). The magnitude of the mean differences is comparable to that in the primary analysis [ 17 ]. Changes in diabetes medication use are displayed in Table 3 .
Plasma analytes
There were significant reductions in HbA1c ( p = 0.007), fasting glucose ( p = 0.0017), insulin ( p = 0.045), and proinsulin ( p = 0.042) following the Pharm-TCR intervention. The hepatokine FGF21 was significantly reduced ( p = 0.035) in the Pharm-TCR group whereas a similar tendency was found for fetuin-A but this did not reach statistical significance ( p = 0.11) (Table 2 ).
Indices of beta-cell function/stress, liver adiposity and insulin resistance
There was no observed change in the proinsulin to C-peptide ratio ( p = 0.17), circulating miR375 ( p = 0.42), or HOMA B ( p = 0.15; Table 2 ). There was a significant reduction in both indices of liver fat accumulation, HSI and FLI, in the Pharm-TCR intervention (both p < 0.001; Table 2 ). HOMA2 IR was also significantly reduced following the Pharm-TCR intervention ( p = 0.023; Table 2 ). As TZDs and SGLT2 inhibitors have been reported to improve hepatic steatosis, sensitivity analyses excluding the participant taking a TZD at baseline and excluding the six participants taking SGLT2 inhibitors at baseline are included in Table S 1 and Table S2, respectively (Additional file 1). Furthermore, although HOMA2 IR can be calculated in patients taking exogenous insulin provided blood samples are taken when glucose and insulin concentrations are at a steady-state prior to medication use [ 20 ], and HOMA2 B can be calculated in these instances using C-peptide rather than insulin [ 20 ], an analysis of the data excluding participants taking exogenous insulin is provided in Table S3 (Additional file 1). In both of these sensitivity analyses, the findings were unchanged.
Plasma metabolites
A total of 114 metabolites were altered from pre- to post-intervention in the Pharm-TCR intervention with 21 metabolites upregulated and 93 metabolites downregulated (Fig. 1 A, p < 0.05). The 42 metabolites identified based on our mass error threshold of 20 ppm are labelled in Fig. 1 A. The most significantly altered metabolites are shown in Fig. 1 B. Notable downregulated metabolites linked to T2D pathophysiology included diacylglycerol, triacylglycerol, ceramide, phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol species (Fig. 1 A, C). Two pathways (ascorbate and alderate metabolism, metabolism of xenobiotics by cytochrome p50) showed negative normalized enrichment score (NES) while other pathways had undetermined directions (Fig. 1 D).
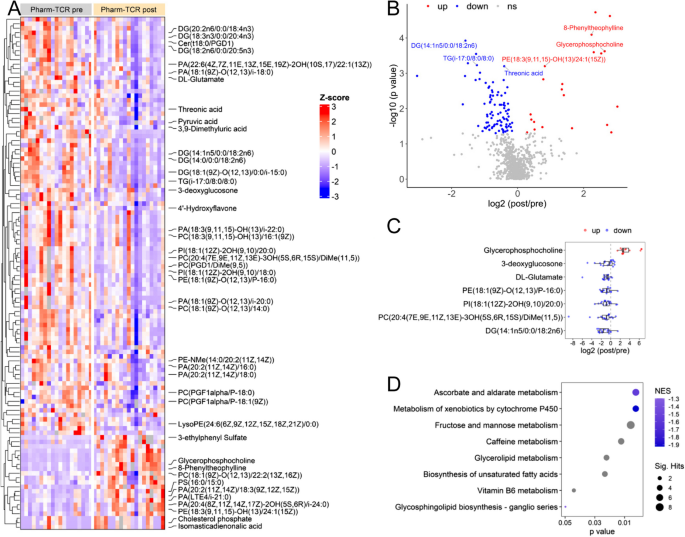
Untargeted metabolomics of the plasma sample at post vs. pre pharm-TCR treatment. A The heatmap of all the altered metabolites ( p < 0.05), among which the identified metabolites are labelled. B The volcano plot showing the log2 fold change (post/pre treatment) and –log10 (p value) of all detected metabolites. The metabolites with the most significant changes (FDR < 0.05) are labeled. C The log2 fold change (post/pre treatment) of selected metabolites. Each dot represents one participant. D Predicted pathways enriched using integrated Mummichog and GSEA methods. From GSEA, two pathways showed negative NES (normalized enrichment score), suggesting the metabolites in these pathways are mostly downregulated, while other pathways had undetermined directions
The present secondary analysis of a subgroup of participants in the Pharm-TCR trial provides evidence that a LCER diet in people living with type 2 diabetes may also improve some of the underlying pathophysiological defects in T2D. Namely, we observed improvements in markers of liver fat accumulation, insulin sensitivity, and hepatokine secretion.
The ratio of proinsulin to C-peptide is considered a marker of beta-cell ER stress, as the relative amount of improperly processed proinsulin released into the circulation increases as ER stress increases [ 10 , 11 ]. C-peptide, rather than insulin, is preferable to compare to proinsulin as this method removes any influence of insulin clearance by the liver. In this study, we did not observe a reduction in the proinsulin to C-peptide ratio despite the fact that glycemia (i.e., HbA1c and fasting glucose) insulin, proinsulin, and C-peptide were reduced in the Pharm-TCR group; however, it is possible that a more dynamic assessment of beta-cell function than what can be observed from fasting blood samples (e.g., oral glucose tolerance test) is required to observe a reduction in the proinsulin to C-peptide ratio. In support of this, a recent study by Mezza et al., assessed the similar proinsulin to insulin ratio as a measure of beta-cell stress during a mixed-meal tolerance test before and after a 50% pancreatectomy [ 27 ]. Although the loss of 50% of one’s beta-cells should be enough to induce stress during a meal challenge in the remaining beta-cells, they only observed a significant increase in the proinsulin to insulin ratio 180 min into the test [ 27 ]. In contrast, Skytte et al. observed an improvement in fasting proinsulin to insulin ratio after six weeks of dietary carbohydrate restriction [ 7 ]; however, people with T2D who were taking injectable diabetes medications (e.g., insulin) were excluded from that study, which likely led to a more recently diagnosed and less severe T2D disease status.
In addition to the absence of an improvement in the proinsulin to C-peptide ratio, we also did not observe an improvement in circulating levels of miR375 – a proposed marker of beta-cell stress that has been described in both type 1 diabetes (T1D) and T2D [ 28 , 29 ]. MicroRNA 375 is typically elevated in recent onset T1D, as well as in people with prediabetes and T2D (reviewed in 19). It is possible that absence of an effect on miR375 could be due to the fact that the rate of beta-cell death was not high enough to be impacted (i.e., a floor effect); however, it is also possible that the intervention simply did not impact beta-cell death. Finally, we also did not observe a change in HOMA2 B, but the fasting nature of the outcome is a limitation and a dynamic assessment of beta-cell function would have provided a broader perspective. Taken together, we did not observe any evidence of improvements in beta-cell function or stress outcomes. The advanced duration of T2D in the Pharm TCR group and use of sulfonylureas – both of which may adversely impact the capacity of beta cells to recover [ 5 , 30 ] – may have contributed to the lack of improvements in indices of beta cell function in our intervention.
As expected with any intervention that induces substantial weight loss, we observed improvements in both fatty liver disease indices (i.e., HSI and FLI) following the Pharm-TCR intervention. We also observed a significant reduction in the hepatokine FGF21 and a potential reduction in fetuin-A. Although considered a “good” hepatokine due to favourable effects on insulin sensitivity [ 31 ], FGF21 is chronically elevated in T2D [ 32 ] and the secretion of this hepatokine can be regulated by the circulating glucagon to insulin ratio [ 33 ]. As such, insulin resistance by the liver is speculated to play a role in mediating the higher circulating levels of FGF21 seen in T2D [ 33 ]. Fetuin-A is also increased in circulation in T2D and, unlike FGF21, causes insulin resistance and proinflammatory cytokine production from adipocytes and macrophages [ 34 ]. As such, lower circulating levels of these hepatokines following the Pharm-TCR intervention indicates a transition towards a healthy hepatokine secretion pattern. In addition to improvements in indices of liver fat accumulation and hepatokine secretion, the Pharm-TCR intervention also reduced insulin resistance as assessed by HOMA2 IR. Since HOMA is based off fasting measures of insulin and blood glucose, it primarily reflects liver insulin resistance [ 19 ]. A reduction in hepatokine secretion combined with improvements in HSI, FLI, and HOMA2 IR indicate that the Pharm-TCR intervention likely reduced liver fat accumulation and improved metabolic related liver function. This suggests that the intervention had a positive impact on liver pathophysiology in addition to improving measures of glycemia.
To further investigate the impact of the Pharm-TCR intervention on biomarkers linked to T2D pathophysiology, we performed exploratory untargeted metabolics analysis of plasma samples from the treatment group. We found significant reductions in various glycerolipid (di- and triacylglycerol) and ceramide species known to contribute to hepatic insulin resistance [ 35 ], thereby supporting the observed improvements in markers of liver fat and metabolic function in the Pharm TCR group. Moreover, several metabolites recently identified as markers of T2D risk (e.g., phosphatidylcholines, phosphatidylethanolamines, phosphatidylinositols) [ 36 ] were lowered after the intervention in the Pharm TCR group. Althought most of the metabolites identified were downregulated, glycerophosphocoline – an inverse independent predictor of T2D risk that is reduced in T2D [ 37 ] - was among the most robustly increased metabolites following the intervention. Collectively, these data support an improvement in certain aspects of the underlying T2D pathophysiology in the Pharm TCR group.
While the results from this study are positive, there are several limitations that must be addressed in this exploratory secondary analysis. First, although the main trial was a randomized controlled trial, the participants included in this secondary analysis are based off availability of fasting blood samples at baseline and following the 12-week intervention. Although there were no baseline imbalances in most outcomes (with the exception of FGF21), the use of a subsample allows the possibility that the randomization has been broken. In line with this, while there was no difference in baseline medication effect score (an index of the overall intensity of a pharmacological glucose-lowering regimen), there appears to be group imbalances in the use of individual medications in this subgroup. Regardless, mean reductions in HbA1c and body mass were consistent with those in the main paper, suggesting a representative subsample in those outcomes. In any case, the results of this secondary analysis need to be interpreted with caution. We chose not to adjust the criterion p-values for multiple testing (apart from the metabolomics analysis) given the exploratory nature of this study. The indirect nature of the outcome assessments in this study represents another limitation. Although it was not feasible, nor the purpose, of the main trial to assess these outcomes, the consistency in the results of this study (in particular, those regarding the liver) give us confidence that the findings are robust. It is important to note that the results of this study occurred with simultaneous reductions in diabetes medication use and the findings should be interpreted as such. Finally, the beta-cell function markers in this study were limited to fasting blood samples and therefore may not reflect the dynamic responses seen during a glucose challenge or meal.
In conclusion, the Pharm-TCR intervention resulted in no change in beta-cell outcomes, but did improve indices of liver fat accumulation, hepatokine secretion, and insulin sensitivity. Taken together, these findings suggest that a low-carbohydrate energy restricted diet represents a viable strategy for not only reducing hyperglycemia but also improving some of the underlying pathophysiological drivers of T2D in individuals with the disease.
Availability of data and materials
Data are available upon resonable request made to the corresponding author (JPL).
International Diabetes Federation. IDF Diabetes Atlas, 9th edn [Internet]. Brussels, Belgium. 2019. https://www.diabetesatlas.org .
Athinarayanan SJ, Adams RN, Hallberg SJ, McKenzie AL, Bhanpuri NH, Campbell WW, et al. Long-Term Effects of a Novel Continuous Remote Care Intervention Including Nutritional Ketosis for the Management of Type 2 Diabetes: A 2-Year Non-randomized Clinical Trial. Front Endocrinol. 2019;10:348. http://www.frontiersin.org/Endocrinology .
Article Google Scholar
Lean MEJ, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7(5):344–55. https://doi.org/10.1016/S2213-8587(19)30068-3 .
Article PubMed Google Scholar
Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia [Internet]. 2011;54(10):2506–14. Available from: https://doi.org/10.1007/s00125-011-2204-7 .
Article CAS PubMed Google Scholar
Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, Peters C, Barnes AC, Aribisala BS, et al. Remission of Human Type 2 Diabetes Requires Decrease in Liver and Pancreas Fat Content but Is Dependent upon Capacity for β Cell Recovery. Cell Metab. 2018;28(4):547-556.e3. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med15&NEWS=N&AN=30078554 .
Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP. Effect of a Low-Carbohydrate Diet on Appetite, Blood Glucose Levels, and Insulin Resistance in Obese Patients with Type 2 Diabetes. Ann Intern Med. 2005;142(6):403. http://annals.org/aim/fullarticle/718265 .
Skytte MJ, Samkani A, Astrup A, Frystyk J, Rehfeld JF, Holst JJ, et al. Effects of carbohydrate restriction on postprandial glucose metabolism, β -cell function, gut hormone secretion, and satiety in patients with Type 2 diabetes. Am J Physiol Metab. 2021;320(1):E7-18. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medl&NEWS=N&AN=33103448 .
Google Scholar
Buse JB, Caprio S, Cefalu WT, Ceriello A, Prato Del S, Inzucchi SE, et al. How Do We Define Cure of Diabetes? Diabetes Care. 2009;32(11):2133–5. http://care.diabetesjournals.org/content/diacare/32/11/2133.full.pdf .
Article PubMed PubMed Central Google Scholar
Dixon JB, Zimmet P, Alberti KG, Rubino F. Bariatric surgery: an IDF statement for obese Type 2 diabetes. Diabet Med. 2011;28(6):628–42. http://ubc.summon.serialssolutions.com/2.0.0/link/0/eLvHCXMwtV1LS8NAEF6qRRFEtCq-2ZOXkJIm220qeKit9QEiaL30EprsBgptKraF_nxnH8kmRdQKXkLZ0mmS-fh2ZvebWYQ8t-rYS5zgelHIQ4dEjZhRP3ToIKqHJIwo8xqRF5OiVKeUnpZmxv7V8TAGrheFtCs4PzMKA_AZIABXAAFcfwWDG8iFZRd-a6oKoGV1c2I9dLq .
Article CAS PubMed PubMed Central Google Scholar
Eizirik DL, Miani M, Cardozo AK. Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia. 2013;56(2):234–41. http://link.springer.com/10.1007/s00125-012-2762-3 .
Kulkarni A, Muralidharan C, May SC, Tersey SA, Mirmira RG. Inside the β Cell: Molecular Stress Response Pathways in Diabetes Pathogenesis. Endocrinology. 2022;164(1):bqac184. https://academic.oup.com/endo/article/doi/10.1210/endocr/bqac184/6783239 .
Younossi ZM, Golabi P, Avila de L, Paik JM, Srishord M, Fukui N, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. https://doi.org/10.1016/j.jhep.2019.06.021 .
Meex RCR, Watt MJ. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017;13(9):509–20. https://doi.org/10.1038/nrendo.2017.56 .
Ou H, Yang Y, Wu H, Wu J, Lu F, Chang C. Serum fetuin-A concentrations are elevated in subjects with impaired glucose tolerance and newly diagnosed type 2 diabetes. Clin Endocrinol (Oxf). 2011;75(4):450–5. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2265.2011.04070.x .
Chang CR, Francois ME, Little JP. Restricting carbohydrates at breakfast is sufficient to reduce 24-hour exposure to postprandial hyperglycemia and improve glycemic variability. Am J Clin Nutr. 2019;109(5):1302–9. https://academic.oup.com/ajcn/article/109/5/1302/5435774 .
Francois ME, Myette-Cote E, Bammert TD, Durrer C, Neudorf H, DeSouza CA, et al. Carbohydrate restriction with postmeal walking effectively mitigates postprandial hyperglycemia and improves endothelial function in type 2 diabetes. Am J Physiol Circ Physiol. 2018;314(1):H105–13.
Durrer C, McKelvey S, Singer J, Batterham AM, Johnson JD, Gudmundson K, et al. A randomized controlled trial of pharmacist-led therapeutic carbohydrate and energy restriction in type 2 diabetes. Nat Commun. 2021;12(1):5367. https://doi.org/10.1038/s41467-021-25667-4 .
Durrer C, McKelvey S, Singer J, Batterham AM, Johnson JD, Wortman J, et al. Pharmacist-led therapeutic carbohydrate restriction as a treatment strategy for type 2 diabetes: the Pharm-TCR randomized controlled trial protocol. Trials. 2019;20(1):781. https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-019-3873-7 .
Gutch M, Kumar S, Razi SM, Gupta KK, Gupta A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab. 2015;19(1):160.
Wallace TM, Levy JC, Matthews DR. Use and Abuse of HOMA Modeling. Diabetes Care. 2004;27(6):1487–95. http://care.diabetesjournals.org/content/27/6/1487.abstract .
Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6(1):33. https://doi.org/10.1186/1471-230X-6-33 .
Lee J-H, Kim D, Kim HJ, Lee C-H, Yang JI, Kim W, et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503–8 https://www.sciencedirect.com/science/article/pii/S1590865809003363 .
R Core Team. R: A Language and Environment for Statistical Computing [Internet]. Vienna, Austria. 2021. https://www.r-project.org/ .
Lenth Remmeans. Estimated Marginal Means, aka Least-Squares Means [Internet]. 2020. https://cran.r-project.org/package=emmeans .
Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32(18):2847–9. https://academic.oup.com/bioinformatics/article/32/18/2847/1743594 .
Chong J, Wishart DS, Xia J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr Protoc Bioinforma. 2019;68(1):e86.
Mezza T, Ferraro PM, Sun VA, Moffa S, Cefalo CMA, Quero G, et al. Increased β-Cell Workload Modulates Proinsulin-to-Insulin Ratio in Humans. Diabetes. 2018;67(11):2389–96. http://diabetes.diabetesjournals.org/lookup/doi/10.2337/db18-0279 .
Vasu S, Kumano K, Darden CM, Rahman I, Lawrence MC, Naziruddin B. MicroRNA Signatures as Future Biomarkers for Diagnosis of Diabetes States. Cells. 2019;8(12):1533. https://www.mdpi.com/2073-4409/8/12/1533 .
Mirmira RG, Sims EK, Syed F, Evans-Molina C. Biomarkers of β-Cell Stress and Death in Type 1 Diabetes. Curr Diab Rep. 2016;16(10):95. https://pubmed.ncbi.nlm.nih.gov/27541297 .
Maedler K, Carr RD, BoscO D, Zuellig RA, BerneY T, DonatH MY. Donath MY. Sulfonylurea induced β-cell apoptosis in cultured human islets. J Clin Endocrinol Metab [Internet]. 2005;90(1):501–6. https://doi.org/10.1210/jc.2004-0699 .
Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, et al. Circulating FGF21 Is Liver Derived and Enhances Glucose Uptake During Refeeding and Overfeeding. Diabetes. 2014;63(12):4057–63. http://diabetes.diabetesjournals.org/cgi/doi/10.2337/db14-0595 .
Chavez AO, Molina-Carrion M, Abdul-Ghani MA, Folli F, DeFronzo RA, Tripathy D. Circulating Fibroblast Growth Factor-21 Is Elevated in Impaired Glucose Tolerance and Type 2 Diabetes and Correlates With Muscle and Hepatic Insulin Resistance. Diabetes Care. 2009;32(8):1542–6. http://care.diabetesjournals.org/cgi/doi/10.2337/dc09-0684 .
Hansen JS, Pedersen BK, Xu G, Lehmann R, Weigert C, Plomgaard P. Exercise-Induced Secretion of FGF21 and Follistatin Are Blocked by Pancreatic Clamp and Impaired in Type 2 Diabetes. J Clin Endocrinol Metab. 2016;101(7):2816–25. https://www.semanticscholar.org/paper/3417f8ab018605064db4d13be11008ba3d3b3fe1 .
Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18(8):1279–85. https://www-nature-com.ep.fjernadgang.kb.dk/articles/nm.2851 .
Petersen MC, Shulman GI. Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol Sci. 2017;38(7):649–65. https://linkinghub.elsevier.com/retrieve/pii/S0165614717300962 .
Morze J, Wittenbecher C, Schwingshackl L, Danielewicz A, Rynkiewicz A, Hu FB, et al. Metabolomics and Type 2 Diabetes Risk: An Updated Systematic Review and Meta-analysis of Prospective Cohort Studies. Diabetes Care. 2022;45(4):1013–24. https://diabetesjournals.org/care/article/45/4/1013/144892/Metabolomics-and-Type-2-Diabetes-Risk-An-Updated .
Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam K-P, et al. Early Metabolic Markers of the Development of Dysglycemia and Type 2 Diabetes and Their Physiological Significance. Diabetes. 2013;62(5):1730–7. https://diabetesjournals.org/diabetes/article/62/5/1730/42895/Early-Metabolic-Markers-of-the-Development-of .
Download references
Peer-reviewed funding was obtained from the Mitacs Accelerate program (Grant No. IT08605). Matching funds for the Mitacs Accelerate fellowship to C.D. were provided by industry partner Pharmasave Drugs (Pacific) Ltd. J.P.L is supported by a Canadian Institutes for Health Research (MSH-141980) Project Grant and a Michael Smith Foundation for Health Research (MSFHR) Scholar Award (16890).
Author information
Authors and affiliations.
School of Health and Exercise Sciences, University of British Columbia, BC, Kelowna, V1V 1V7, Canada
Cody Durrer, Hashim Islam & Jonathan P. Little
Department of Cellular and Physiological Sciences, Diabetes Research Group, Life Sciences Institute, University of British Columbia, Vancouver, BC, Canada
Haoning Howard Cen & James D. Johnson
Department of Pathology, Stanford University School of Medicine, Stanford, CA, USA
Maria Dolores Moya Garzon, Xuchao Lyu & Jonathan Z. Long
School of Population and Public Health, University of British Columbia, Vancouver, BC, Canada
Maria Dolores Moya Garzon, Xuchao Lyu, Joel Singer & Jonathan Z. Long
Institute for Personalized Therapeutic Nutrition, Vancouver, BC, Canada
Sean McKelvey, James D. Johnson & Jonathan P. Little
Centre for Rehabilitation, School of Health and Life Sciences, Teesside University, Middlesbrough, United Kingdom
Alan M. Batterham
You can also search for this author in PubMed Google Scholar
Contributions
J.P.L. and S.M. conceived the study and designed the intervention. J.P.L., S.M., and C.D. performed the literature search prior to the study design. J.P.L., S.M., J.S., A.M.B., and C.D. contributed to the study design. S.M., C.D., J.D.J. and J.W. were involved in data collection, C.D. oversaw day-today conduct of the study with support from J.P.L. and S.M. A.M.B., J.S., C.D., H.I., H.H.C., M.D.M.G., and X.L. performed the data analysis and statistical analysis. The underlying data were verified by C.D. and J.P.L. Figures and tables were created by C.D., H.I., and H.H.C. All authors were involved in the interpretation of the data. The first draft of the manuscript was written by C.D. with guidance from J.P.L. All authors reviewed the manuscript, revised it critically for important intellectual content, and approved the final version.
Corresponding author
Correspondence to Jonathan P. Little .
Ethics declarations
Competing interests.
J.P.L. holds founder shares and advises for Metabolic Insights Inc., and is volunteer Chief Scientific Officer for the not-for-profit Institute for Personalized Therapeutic Nutrition. S.M. is employed as Chief Executive Officer for the not-for-profit Institute for Personalized Therapeutic Nutrition. J.D.J. is Chair of the Board for the Institute for Personalized Therapeutic Nutrition and receives no compensation.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Durrer, C., Islam, H., Cen, H.H. et al. A secondary analysis of indices of hepatic and beta cell function following 12 weeks of carbohydrate and energy restriction vs. free-living control in adults with type 2 diabetes. Nutr Metab (Lond) 21 , 29 (2024). https://doi.org/10.1186/s12986-024-00807-x
Download citation
Received : 14 February 2024
Accepted : 17 May 2024
Published : 27 May 2024
DOI : https://doi.org/10.1186/s12986-024-00807-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Nutrition & Metabolism
ISSN: 1743-7075
- General enquiries: [email protected]

- Previous Article
- Next Article
Nutritional management of pancreatitis and concurrent disease in dogs and cats
Click on author name to view affiliation information
- Download PDF
Nutrition is considered a key part of the management of pancreatitis in dogs and cats. While limited prospective research exists, experimental studies, retrospective studies, and anecdote allow for formulation of nutritional guidelines. Historically, fat has been considered the key nutrient of interest in pancreatitis; however, other nutrients and dietary factors, including energy density, digestibility, protein, carbohydrates, and fiber, are all of importance in these patients. Indeed protein particle size may be of greater significance than dietary fat in the management of pancreatitis in cats. Low-fat gastrointestinal diets are frequently recommended in the initial management of pancreatitis in dogs, while hydrolyzed diets are often considered first-line diets in cats with pancreatitis. The presence or absence of comorbid disease may also alter nutritional recommendations. When diseases occur concurrently, the dietary strategies for the most life-threatening illness, or the illness with the greatest impact on quality of life, is recommended to be prioritized. Many dogs and cats with pancreatitis can be transitioned back to their prediagnosis diet or another commercial maintenance diet, provided that significant comorbid disease is absent. Use of a low-fat diet in the long term may be prioritized in dogs with recurrent episodes of pancreatitis.
Introduction
The role of nutrition in pancreatitis is increasingly recognized in both human and veterinary medicine. Historically, “resting the gut” was proposed to prevent stimulation of pancreatic secretions and thus minimize autodigestion and inflammation. It has now been shown that pancreatic secretions are decreased during pancreatitis, and it is proposed that injured acinar cells are unable to fully respond to physiologic stimuli. 1 – 3 Thus the principles that underpinned the “rest the gut” theory were based in physiology and are thought to be of reduced significance in clinical disease. Fasting may also have detrimental effects, including intestinal mucosal atrophy, enterocyte apoptosis, gut barrier dysfunction, and bacterial translocation. 4 – 6 Many of these effects are mitigated via provision of enteral nutrition (EN). 7 – 10 Nutrition, either enterally or parenterally, has also been shown to reduce risk of death in humans with pancreatitis. 11 It has also been shown to reduce abdominal pain, opioid requirements, and risk of food intolerance as compared to nil per os. 12 When considering route of nutrition, EN is preferred. 13 , 14 This is likely also true for veterinary patients, leading to a new dogma of “feed the gut.”
While many articles have provided information on the medical management of pancreatitis, few have focused on the nutritional management of the disorder, in addition to how this may vary when comorbid disease is present. In this review we aim to describe best practices in the nutritional management of pancreatitis based on data from experimental models, clinical studies, and, where necessary, anecdotal recommendations based on our clinical experience.
Nutritional Assessment and Detailed Feeding Instructions
To provide nutritional recommendations, a veterinarian must understand each patient’s dietary history, its body composition (body condition score [BCS] and muscle condition score), 15 and the presence or absence of comorbid disease.
Management Principles in Dogs and Cats
Pancreatitis—no comorbid disease.
Clinical studies on nutritional management of pancreatitis are limited. We are reliant on low-quality evidence to support our recommendations. These recommendations are based on theory, retrospective studies, and clinical experience. Low-fat diets are frequently used in the initial management of dogs with pancreatitis, while hydrolyzed protein diets are often used in the initial management of cats with pancreatitis. Low- to moderate-fat hydrolyzed diets may also be used in dogs. Our definitions for low, moderate, and high concentrations of each nutrient are provided in Supplementary Material S1 .
Energy —It is important to meet each patient’s daily energy requirement to avoid negative energy balance, particularly in the long term, as it can have deleterious effects on the pancreas due to increased protein turnover. This is of increased importance in cats due to their susceptibility to hepatic lipidosis, which worsens prognosis. 16 In the short term, provision of some nutrition if full energy needs cannot be met should be prioritized over no nutritional intake.
Fat —Noncommercial high-fat diets have been shown to induce and worsen experimentally induced pancreatitis in dogs. 17 , 18 In contrast, the effect of high dietary fat in naturally occurring disease is less clear, and it is the authors’ opinion that fat is overemphasized in the management of pancreatitis.
Previous studies 19 , 20 assessing ketogenic diets and struvite dissolution diets, some of which are high in fat, demonstrated that 3 of 9 and 2 of 50 dogs developed pancreatitis, respectively. Therefore, it seems prudent to avoid high fat-diets and potentially reduce dietary fat intake, at least initially, in dogs with pancreatitis. Decreasing dietary fat also has the potential advantage of decreasing delayed gastric emptying. This is important because motility support may be a prerequisite to return of normal appetite. Particle size and food volume play major roles in gastric emptying, and focusing on low fat alone may not be sufficient to affect gastric emptying. Some nutritionists recommend reducing dietary fat by 50% of what the dog was originally eating prior to diagnosis of pancreatitis. There is no scientific basis behind this recommendation, but anecdote suggests this approach is effective in many cases. Interestingly, 1 study in healthy dogs did not show a significant effect of dietary fat on serum biomarkers of pancreatic injury (eg, canine pancreatic lipase immunoreactivity), questioning the focus on dietary fat in diets for dogs with pancreatitis. 21 However, it is unknown whether similar results would be found in dogs with pancreatitis. The role of dietary fat is even less certain in cats. 22 Dietary fat should be considered on a case-by-case basis in cats.
Protein —Dietary protein and amino acids are a stimulus for pancreatic secretion in cats, whereas products of protein digestion have been shown to stimulate pancreatic secretions in dogs. 23 , 24 Therefore, excess dietary protein should be avoided, while the diet should still provide sufficient protein for tissue repair and recovery. In cats, intact protein is more of a pancreatic stimulant than free amino acids. 25 Therefore, hydrolyzed protein diets are often recommended for cats, as they may result in less pancreatic secretion. This may be due to the fact that intact proteins are able to serve as substrates for proteases that break down cholecystokinin-releasing factors. 26 Another advantage of hydrolyzed protein diets in cats is their increased digestibility and reduced antigenicity, the latter of which may be beneficial if chronic enteropathy (CE) is present. It is also important to note that there are limited feline therapeutic hydrolyzed protein diets on the market at this time. Thus, if a cat does not accept this category of diet, some nutrition should be prioritized over strict adherence to the hydrolyzed protein diet.
Carbohydrate —Carbohydrates are less of a pancreatic stimulant when compared to fat and protein. 23 , 27 Some reduction may be necessary if glucose resistance or overt diabetes mellitus (DM) is present.
Fiber —Diets high in viscous fiber should be avoided in vomiting or regurgitating cases due to their effects on slowing gastric emptying. Prebiotic fibers could be considered to help restore intestinal dysbiosis. Further information on dietary fiber, in general, is available in a recent review article. 28
Considerations for chronic disease —Chronic pancreatitis (CP) may be differentiated from acute disease in that it is considered a progressive and irreversible process. Nutritional management of CP does not differ significantly from that of acute pancreatitis. It is the authors’ opinion that many dogs with acute disease do not require a long-term low-fat diet; however, dogs that are prone to repeated acute bouts of pancreatitis, or CP, may benefit from a long-term low-fat diet. It is also possible, albeit uncommon, to develop DM or exocrine pancreatic insufficiency (EPI) secondary to CP. Further management of these disorders can be found below.
Pancreatitis with comorbid disease
The nutritional management of pancreatitis and concurrent disease may differ from those with pancreatitis alone. A summary of nutritional strategies for the patient with comorbid disease is provided in Supplementary Material S2 .
Hypertriglyceridemia —Hypertriglyceridemia is an indication to reduce dietary fat. Pancreatic lipases hydrolyze triglycerides into free fatty acids, which when produced in excess can be toxic to pancreatic acinar cells. 29 The addition of triglycerides to a perfused canine pancreas has led to structural changes, suggestive of pancreatic injury. 30 While mild hypertriglyceridemia has been proposed to occur in dogs with pancreatitis, moderate to severe increases in serum triglyceride concentrations do not occur secondary to pancreatitis alone. 31 Dogs with a prior history of pancreatitis are more likely to have increased serum triglyceride concentrations (at pancreatic quiescence) than those without a history of pancreatitis, suggesting that moderate to severe hypertriglyceridemia is a cause rather than consequence of pancreatitis. 32 Therefore, recognizing and instigating dietary management of hypertriglyceridemia by decreasing dietary fat is paramount. The authors recommend measurement of serum triglycerides in dogs and believe that serum triglyceride concentrations > 600 mg/dL warrant therapeutic management. One study 33 showed that a commercial low-fat diet was effective in reducing serum triglyceride concentrations and correcting lipoprotein profiles in hypertriglyceridemic Miniature Schnauzers. Feeding a therapeutic low-fat diet or selecting a diet with 50% reduction in dietary fat is a reasonable first step. If fasting triglycerides do not return to within normal range, then further decreasing dietary fat is indicated; this may entail feeding an ultralow-fat home-prepared diet formulated by a board-certified veterinary nutritionist. Alternatively, fenofibrate may be added to the treatment protocol. 34
Studies 35 , 36 in humans and mice with hypertriglyceridemia have described benefits of using omega-3 fatty acids. One clinical study 37 aimed to evaluate the efficacy of omega-3 fatty acids in Schnauzers with primary hyperlipidemia by feeding one group a low-fat diet and the other a moderate-fat diet, with both groups receiving omega-3 fatty acids for 90 days. Both groups demonstrated decreased plasma cholesterol and triglyceride concentrations; however, the effect was greater in dogs fed the low-fat diet with omega-3 fatty acids. Unfortunately, it was not possible to tease out the individual effects of omega-3 fatty acids. The recommended 38 dose for the treatment of dogs with hyperlipidemia is 120 mg of eicosapentaenoic acid and docosahexaenoic acid/kg 0.75 . In healthy dogs, using medium-chain triglycerides, compared to long-chain saturated or unsaturated fatty acids, decreased postprandial triglycerides. 39
Obesity —Obesity has been associated with canine pancreatitis and is likely due to the inflammatory effects of adipose tissue. 40 The effect of obesity on feline pancreatitis is unknown. There is a wide range of dietary fat concentrations among therapeutic weight loss diets. Therefore, selecting a lower-fat diet may be considered, especially if dietary fat was suspected to be a predisposing factor in the pathogenesis of acute pancreatitis or if the animal has concurrent hypertriglyceridemia.
Chronic enteropathy —Chronic enteropathy in dogs and cats is subclassified based on treatment response, with food-responsive enteropathy comprising approximately two-thirds of cases. 41 Dietary management of these cases involves trial and error to determine the most effective strategy. 42 The specific nutrient profiles of the different therapeutic diets available for CE vary, and attention to the profiles may help guide specific diet choices in an individual patient.
Beyond ingredients, fat and fiber concentrations may be prioritized in certain cases. Several studies 43 – 47 have demonstrated the effectiveness of fiber-enriched diets in animals with large intestinal signs, and anecdote suggests similar use in small intestinal disease. Low-fat diets may be beneficial for some cases of CE, for example lymphangiectasia or in cases where there is reduced fat digestion and absorption in the small intestine, resulting in increased passage of undigested fat into the colon and thus secretory diarrhea. 48 As reduced dietary fat is an overlapping nutritional strategy for acute pancreatitis and some CE cases, managing a dog with these concurrent conditions involves prioritizing dietary fat when selecting the specific diet used for CE. Dietary fat does not seem to affect the outcome of cats with chronic diarrhea, and therefore dietary fat may be less of a concern in cats with CE; however, it is important to note that a final diagnosis was not reached in these cats. 49
Feline triaditis —Feline triaditis describes concurrent pancreatitis, cholangitis, and CE. The reported prevalence in ill referral cats is 17% to 39%. 50 – 52 While the underlying pathology of triaditis is poorly understood, overall, review articles suggest that the presence of inflammatory disease in the small intestine may be a common precipitating factor. 53 Cats with triaditis can present with vague nonspecific clinical signs, with 63% to 97% presenting with anorexia. 51 , 54 , 55 If anorexia is < 3 to 5 days, then nursing management, appetite stimulants, and addressing nausea and pain may help to increase appetite. For those cases where these interventions do not help or anorexia is prolonged, assisted EN should be started. For in-hospital feeding, the authors prefer to feed commercial gastrointestinal diets due to their higher digestibility; then, once the cat is home, a slow transition is made to a commercial hydrolyzed protein diet to address the CE. 53 The delayed introduction of the hydrolyzed protein diet is to prevent food aversion. If a cat does not accept the hydrolyzed protein diet, a slower transition over 2 to 3 weeks may help to improve acceptance, or this strategy can be abandoned to prioritize nutritional intake. Serum cobalamin should be assessed and supplemented if low, and serum potassium concentrations should also be maintained within normal concentrations to help with maintaining or improving appetite.
Feline EPI —Chronic pancreatitis is assumed to be a common cause of feline EPI, although pancreatic acinar atrophy is also reported. 56 Exocrine pancreatic insufficiency leads to a failure of intraluminal digestion and severe nutrient malassimilation. In addition, the lack of other pancreatic secretory products, such as gastrointestinal trophic factors, bicarbonate, intrinsic factor to help with cobalamin absorption, and antimicrobial factors, can result in impaired intestinal function and further nutrient malassimilation. The decrease or absence of gastrointestinal trophic factors and concurrent small intestinal bacterial overgrowth may also result in impaired mucosal enzyme activity, leading to decreased absorption of amino acids, fatty acids, and sugars. 57 Even though oral pancreatic enzyme supplementation is the cornerstone of treatment, EN also appears to be important in the management of EPI.
The most common clinical sign in feline EPI is weight loss. 58 Daily caloric intake should be adjusted to help achieve ideal BCS. Diets with high digestibility are prioritized to help with nutrient digestion and absorption, especially as studies 59 , 60 have demonstrated the benefit of such diets in dogs. Caloric density should be maintained, typically by avoiding excessive fat restriction. Higher-fiber diets are avoided, as dietary fiber has been shown to hinder pancreatic enzyme activity in vitro. 61 That said, if soft stool persists, even with enzyme supplementation, increasing dietary fiber may be considered to improve stool quality. The effect of dietary fat in feline EPI is unknown, and cats present less commonly with diarrhea compared to dogs. 58 When possible, cats with EPI should be fed multiple small meals per day in an attempt to maximize digestion and prevent intestinal overload and resultant osmotic diarrhea. 57
Cobalamin deficiency has been associated with EPI in 77% of cases. 58 Cobalamin supplementation also improves response to treatment. 58 Therefore, supplementation should be initiated as soon as possible in these cases. Supplementation with folate should be considered if low concentrations are documented. While hypofolatemia occurs uncommonly (5% of cases), supplementation should be considered in these cases, as folate deficiency has been shown to inhibit pancreatic exocrine function in rats. 58 , 62
Cats with EPI may be deficient in the fat-soluble vitamins A, D, E, and K due to fat malassimilation. 63 If a coagulopathy is documented in a cat with EPI, subcutaneous vitamin K therapy should be initiated. Empirical supplementation of vitamin A and D should be avoided, as excess levels may be harmful. 57 Vitamin E can be dosed orally at 10 IU/kg once a day. 57
Chronic kidney disease —Optimal nutritional management of chronic kidney disease (CKD) has been shown to slow disease progression and improve survival times. 64 , 65 Nutritional management is reviewed elsewhere. 66 It can be challenging to manage dogs with CP and CKD since many renal diets are high in fat. In these instances, it may be necessary to feed lower-fat diets that are still appropriately reduced in phosphorus (for CKD) and protein (for proteinuria). This can be achieved by selecting a low- to moderate-fat renal diet or by choosing a diet that may not be marketed for kidney disease but otherwise meets the desired nutrient profile. Selected diets are listed in Supplementary Material S3 . Similarly, for cats with concurrent CKD and pancreatitis, feeding a lower-phosphorus hydrolyzed protein diet should be prioritized whenever possible.
Diabetes mellitus —A subset of dogs and cats with CP develop DM. A review of nutritional management of DM in cats and dogs can be found elsewhere. 67 In dogs and cats with concurrent pancreatitis and DM, overall feeding strategies will greatly depend on BCS to choose a diet with an appropriate caloric density. For dogs, the presence or absence of moderate to severe hypertriglyceridemia is the next major factor to consider when selecting a diet. For cats with concurrent pancreatitis and DM, feeding a lower-carbohydrate, limited-ingredient diet should be considered. If the clinical signs of pancreatitis are minimal, there may be less priority given to a limited-ingredient diet. Selected diets are listed in Supplementary Material S3 .
Management of the Hospitalized Dog or Cat
Overview and oral food intake.
The authors support the use of early EN in acute presentations of pancreatitis due to high tolerance in both dogs and cats and a faster return to voluntary food intake and reduced rates of gastrointestinal upset in dogs with pancreatitis. 68 – 70 The duration of inappetence prior to hospitalization should be considered when determining nutrition requirements.
To promote oral food intake, it is important to recognize and treat nausea. Consideration of factors related to the diet, nursing, and environment may help to improve oral food intake. For example, the use of preferred ingredients, textures, and flavors of food; warming the food to increase acceptance; handfeeding; moistening food; and ensuring a stress-free environment should be considered to help increase intake. The authors do not recommend assisted oral feeding due to difficulty meeting daily caloric requirements as well as the risk of aspiration and food aversion.
If animals are not meeting their energy needs within 3 to 5 days, assisted EN should be initiated. On day 1, feeding is often reinstated at 25% of resting energy requirement (RER; 70 X body weight kg 0.75) and slowly titrated up to full RER over a few days based on tolerance. In the hospital, feeding should not exceed 100% of RER in order to prevent complications associated with overfeeding. 71 For EN, feedings should be spaced out and initially provide no more than 5 to 10 mL/kg of body weight. 72 If pain persists despite analgesia, reducing the rate of feeding, adjusting frequency, reducing fat content, or potentially using supplemental parenteral nutrition (PN) should be considered.
Methods of assisted EN
Nasogastric (NG) or nasoesophageal (NE) tubes are frequently utilized in the initial management of pancreatitis, as they can be placed with sedation and do not require general anesthesia. Complication rates are similar between NG and NE tubes. Nasogastric tubes allow for quantification of gastric residual volume, which may be of benefit in animals with pancreatitis, although this remains controversial and is affected by several factors. A study 69 involving 55 cats with suspected acute pancreatitis fed via an NG tube showed that this was well tolerated, with an overall survival rate of 91%. In that study, vomiting was uncommon (13%), and all cats that vomited had a history of vomiting prior to initiation of feeding. The study also documented a relatively low mechanical complication rate (13%), and low rate (9%) of hypersalivation following tube placement or feeding. One disadvantage of NE and NG tubes is their smaller lumen, which often precludes the use of slurried diets. A range of suitable commercial veterinary liquid diets are available ( Supplementary Material S4 ) . Caution is advised when using liquid diets intended for human consumption, as they have a lower amino acid content and adult formulas may be devoid of essential amino acids such as arginine, which may be detrimental to cats. 73
An esophagostomy tube can be placed if assisted feeding is anticipated to be needed for longer than 7 days and the animal is stable for general anesthesia. Additionally, an esophagostomy tube may allow for feeding of blenderized dry diets, which may be more energy dense and less costly than commercial liquid diets. Early EN with a commercial low-fat diet delivered proximal to the pylorus via esophagostomy tube was shown to be well tolerated in dogs with severe acute pancreatitis and resulted in fewer complications compared to PN. 68 For those dogs and cats with esophageal disease, percutaneous or surgically placed gastrostomy tubes can be considered. The placement of a jejunostomy tube allows avoidance of the stomach, duodenum, and pancreas, and a study 74 in experimental models of pancreatitis in dogs showed this does not stimulate pancreatic secretions. Two retrospective studies 75 , 76 in naturally occurring acute pancreatitis, 1 in dogs and 1 in cats, have described the application of these tubes in cases undergoing surgical management. In the canine study, 75 of the 30 dogs that had a jejunostomy tube placed, cellulitis associated with the jejunostomy site was noted in 6, and 1 suffered a major complication of septic peritonitis that was directly related to the tube. Although minimally invasive techniques for placement of nasojejunal tubes using fluoroscopy or endoscopy have been described in dogs, these are not widely used. Interestingly, even though delivering nutrients distal to the duodenum may allow for prevention of pancreatic enzyme secretion and reduction of inflammation, a meta-analysis 77 in humans showed no difference in incidence of mortality, infectious or digestive complications; achievement of energy balance; or length of hospital stay between NG and nasojejunal tube feeding. As NG and esophagostomy feeding tubes have been shown to be well tolerated in cats and dogs with acute pancreatitis, respectively, jejunostomy or nasojejunal tube feedings are not commonly used. 68 , 69
Parenteral nutrition
For those animals with intractable vomiting, dull mentation, or coagulopathy, an enteral feeding tube may not be possible, prompting consideration of PN. One study, 78 which evaluated the risk of PN in pancreatitis, among other diseases, reported a mortality rate of 31% for dogs and 19% for cats.
Transitioning From the In-Hospital to At-Home Diet
The amount of RER fed via assisted EN should be slowly decreased as the patient’s oral food intake increases. Once an animal is receiving > 50% RER orally and the animal is stable on oral medications, it is a candidate for discharge. To be able to accurately determine food intake, the amount of food should be weighed prior to feeding a patient, and the leftover food should be reweighed after the patient has finished eating. The difference between these values represents the weight of food ingested by the animal and can be used to accurately calculate the percentage of RER ingested. After hospital discharge, owners should be counseled to avoid the risks associated with pancreatitis or its recurrence, including abrupt food change, ingestion of unusual food items, trash, and table scraps, and should address obesity. 40
Conclusions and Future Outlook
This review article proposes the use of low-fat gastrointestinal diets and hydrolyzed protein diets during the initial management of pancreatitis in dogs and cats, respectively. If a cat does not readily accept the recommended diet, then it may need to be abandoned to prioritize nutritional intake. These recommendations may need to be edited on a case-by-case basis, particularly if comorbid disease is present. While these recommendations are based on the strongest available evidence, there is a lack of available research regarding the nutritional management of naturally occurring pancreatitis in both dogs and cats. The authors hope that this review article will stimulate future prospective studies. These guidelines should continue to be adapted over time as new data become available to clinicians and new dietary formulations come into existence.
Supplementary Materials
Supplementary materials are posted online at the journal website: avmajournals.avma.org .
Acknowledgments
The authors of this review thank Andrea Kepsel of the Michigan State University library service, who performed a comprehensive literature search to assist the authors in manuscript preparation.
Disclosures
Harry Cridge has nothing to disclose. Aarti Kathrani has received or is receiving funding from Purina and Royal Canin. Valerie J. Parker has received research funding and speaking honoraria from Purina, Hill’s, Royal Canin, and Boehringer Ingelheim.
No AI-assisted technologies were used in the generation of this manuscript.
The authors have nothing to disclose.
Niederau C , Niederau M , Lüthen R , Strohmeyer G , Ferrell LD , Grendell JH . Pancreatic exocrine secretion in acute experimental pancreatitis . Gastroenterology . 1990 ; 99 ( 4 ): 1120 - 1127 . doi: 10.1016/0016-5085(90)90633-C
- Search Google Scholar
- Export Citation
Boreham B , Ammori BJ . A prospective evaluation of pancreatic exocrine function in patients with acute pancreatitis: correlation with extent of necrosis and pancreatic endocrine insufficiency . Pancreatology . 2003 ; 3 ( 4 ): 303 - 308 . doi: 10.1159/000071768
O’Keefe SJ , Lee RB , Li J , Stevens S , Abou-Assi S , Zhou W . Trypsin secretion and turnover in patients with acute pancreatitis . Am J Physiol Gastrointest Liver Physiol . 2005 ; 289 ( 2 ): G181 - G187 . doi: 10.1152/ajpgi.00297.2004
Hernandez G , Velasco N , Wainstein C , et al. Gut mucosal atrophy after a short enteral fasting period in critically ill patients . J Crit Care . 1999 ; 14 ( 2 ): 73 - 77 . doi: 10.1016/S0883-9441(99)90017-5
Deitch EA , Winterton J , Li M , Berg R . The gut as a portal of entry for bacteremia: role of protein malnutrition . Ann Surg . 1987 ; 205 ( 6 ): 681 - 692 . doi: 10.1097/00000658-198706000-00010
Fukuyama K , Iwakiri R , Noda T , et al. Apoptosis induced by ischemia-reperfusion and fasting in gastric mucosa compared to small intestinal mucosa in rats . Dig Dis Sci . 2001 ; 46 ( 3 ): 545 - 549 . doi: 10.1023/A:1005695031233
Kotani J , Usami M , Nomura H , et al. Enteral nutrition prevents bacterial translocation but does not improve survival during acute pancreatitis . Arch Surg . 1999 ; 134 ( 3 ): 287 - 292 . doi: 10.1001/archsurg.134.3.287
Qin HL , Su ZD , Gao Q , Lin QT . Early intrajejunal nutrition: bacterial translocation and gut barrier function of severe acute pancreatitis in dogs . Hepatobiliary Pancreat Dis Int . 2002 ; 1 ( 1 ): 150 - 154 .
Qin HL , Su ZD , Hu LG , Ding ZX , Lin QT . Effect of early intrajejunal nutrition on pancreatic pathological features and gut barrier function in dogs with acute pancreatitis . Clin Nutr . 2002 ; 21 ( 6 ): 469 - 473 . doi: 10.1054/clnu.2002.0574
Mohr AJ , Leisewitz AL , Jacobson LS , Steiner JM , Ruaux CG , Williams DA . Effect of early enteral nutrition on intestinal permeability, intestinal protein loss, and outcome in dogs with severe parvoviral enteritis . J Vet Intern Med . 2003 ; 17 ( 6 ): 791 - 798 . doi: 10.1111/j.1939-1676.2003.tb02516.x
Petrov MS , Pylypchuk RD , Emelyanov NV . Systematic review: nutritional support in acute pancreatitis . Aliment Pharmacol Ther . 2008 ; 28 ( 6 ): 704 - 712 . doi: 10.1111/j.1365-2036.2008.03786.x
Petrov MS , McIlroy K , Grayson L , Phillips AR , Windsor JA . Early nasogastric tube feeding versus nil per os in mild to moderate acute pancreatitis: a randomized controlled trial . Clin Nutr . 2013 ; 32 ( 5 ): 697 - 703 . doi: 10.1016/j.clnu.2012.12.011
Arvanitakis M , Ockenga J , Bezmarevic M , et al. ESPEN guideline on clinical nutrition in acute and chronic pancreatitis . Clin Nutr . 2020 ; 39 ( 3 ): 612 - 631 . doi: 10.1016/j.clnu.2020.01.004
Crockett SD , Wani S , Gardner TB , et al. ; American Gastroenterological Association Institute Clinical Guidelines Committee. American Gastroenterological Association Institute guideline on initial management of acute pancreatitis . Gastroenterology . 2018 ; 154 ( 4 ): 1096 - 1101 . doi: 10.1053/j.gastro.2018.01.032
Canine muscle condition score (MCS) . American Animal Hospital Association . 2021 . Accessed March 15, 2024. https://www.aaha.org/globalassets/02-guidelines/2021-nutrition-and-weight-management/resourcepdfs/nutritiongl_mcs.pdf
Akol KG , Washabau RJ , Saunders HM , Hendrick MJ . Acute pancreatitis in cats with hepatic lipidosis . J Vet Intern Med . 1993 ; 7 ( 4 ): 205 - 209 . doi: 10.1111/j.1939-1676.1993.tb01008.x
Haig TH . Experimental pancreatitis intensified by a high fat diet . Surg Gynecol Obs . 1970 ; 131 ( 5 ): 914 - 918 .
Lindsay S , Entenman C , Chaikoff I . Pancreatitis accompanying hepatic disease in dogs fed a high fat, low protein diet . Arch Pathol . 1948 ; 45 ; 635 - 638 .
Patterson E , Munana K , Kirk C , et al. Results of a ketogenic food trial for dogs with idiopathic epilepsy . J Vet Intern Med . 2005 ; 19 ( 30 ): 421 . 2005 ACVIM Forum research abstract 80
Wingert AM , Murray OA , Lulich JP , Hoelmer AM , Merkel LK , Furrow E . Efficacy of medical dissolution for suspected struvite cystoliths in dogs . J Vet Intern Med . 2021 ; 35 ( 5 ): 2287 - 2295 . doi: 10.1111/jvim.16252
James FE , Mansfield CS , Steiner JM , Williams DA , Robertson ID . Pancreatic response in healthy dogs fed diets of various fat compositions . Am J Vet Res . 2009 ; 70 ( 5 ): 614 - 618 . doi: 10.2460/ajvr.70.5.614
Forman MA , Steiner JM , Armstrong PJ , et al. ACVIM consensus statement on pancreatitis in cats . J Vet Intern Med . 2021 ; 35 ( 2 ): 703 - 723 . doi: 10.1111/jvim.16053
Backus RC , Rosenquist GL , Rogers QR , Calam J , Morris JG . Elevation of plasma cholecystokinin (CCK) immunoreactivity by fat, protein, and amino acids in the cat, a carnivore . Regul Pept . 1995 ; 57 ( 2 ): 123 - 131 . doi: 10.1016/0167-0115(95)00027-9
Moriyasu M , Lee YL , Lee KY , Chang TM , Chey WY . Effect of digested protein on pancreatic exocrine secretion and gut hormone release in the dog . Pancreas . 1994 ; 9 ( 1 ): 129 - 133 . doi: 10.1097/00006676-199401000-00019
Backus RC , Howard KA , Rogers QR . The potency of dietary amino acids in elevating plasma cholecystokinin immunoreactivity in cats is related to amino acid hydrophobicity . Regul Pept . 1997 ; 72 ( 1 ): 31 - 40 . doi: 10.1016/S0167-0115(97)01032-X
Moran TH , Kinzig KP . Gastrointestinal satiety signals II: cholecystokinin . Am J Physiol Gastrointest Liver Physiol . 2004 ; 286 ( 2 ): G183 - G188 . doi: 10.1152/ajpgi.00434.2003
Liddle RA , Goldfine ID , Rosen MS , Taplitz RA , Williams JA . Cholecystokinin bioactivity in human plasma: molecular forms, responses to feeding, and relationship to gallbladder contraction . J Clin Invest . 1985 ; 75 ( 4 ): 1144 - 1152 . doi: 10.1172/JCI111809
Moreno AA , Parker VJ , Winston JA , Rudinsky AJ . Dietary fiber aids in the management of canine and feline gastrointestinal disease . J Am Vet Med Assoc . 2022 ; 260 ( suppl 3 ): S33 - S45 . doi: 10.2460/javma.22.08.0351
Havel RJ . Pathogenesis, differentiation and management of hypertriglyceridemia . Adv Intern Med . 1969 ; 15 : 117 - 154 .
Saharia P , Margolis S , Zuidema GD , Cameron JL . Acute pancreatitis with hyperlipemia: studies with an isolated perfused canine pancreas . Surgery . 1977 ; 82 ( 1 ): 60 - 67 .
Xenoulis PG , Cammarata PJ , Walzem RL , Suchodolski JS , Steiner JM . Serum triglyceride and cholesterol concentrations and lipoprotein profiles in dogs with naturally occurring pancreatitis and healthy control dogs . J Vet Intern Med . 2020 ; 34 ( 2 ): 644 - 652 . doi: 10.1111/jvim.15715
Xenoulis PG , Levinski MD , Suchodolski JS , Steiner JM . Serum triglyceride concentrations in Miniature Schnauzers with and without a history of probable pancreatitis . J Vet Intern Med . 2011 ; 25 ( 1 ): 20 - 25 . doi: 10.1111/j.1939-1676.2010.0644.x
Xenoulis PG , Cammarata PJ , Walzem RL , Suchodolski JS , Steiner JM . Effect of a low-fat diet on serum triglyceride and cholesterol concentrations and lipoprotein profiles in Miniature Schnauzers with hypertriglyceridemia . J Vet Intern Med . 2020 ; 34 ( 6 ): 2605 - 2616 . doi: 10.1111/jvim.15880
Miceli DD , Vidal VP , Blatter MFC , Pignataro OP , Castillo VA . Fenofibrate treatment for severe hypertriglyceridemia in dogs . Domest Anim Endocrinol . 2021 ; 74 : 106578 . doi: 10.1016/j.domaniend.2020.106578
Kontostathi M , Isou S , Mostratos D , et al. Influence of omega-3 fatty acid-rich fish oils on hyperlipidemia: effect of eel, sardine, trout, and cod oils on hyperlipidemic mice . J Med Food . 2021 ; 24 ( 7 ): 749 - 755 . doi: 10.1089/jmf.2020.0114
Skulas-Ray AC , Wilson PWF , Harris WS , et al. Omega-3 fatty acids for the management of hypertriglyceridemia: a science advisory from the American Heart Association . Circulation . 2019 ; 140 ( 12 ): e673 - e691 . doi: 10.1161/CIR.0000000000000709
de Albuquerque P , De Marco V , Vendramini THA , et al. Supplementation of omega-3 and dietary factors can influence the cholesterolemia and triglyceridemia in hyperlipidemic Schnauzer dogs: a preliminary report . PLoS One . 2021 ; 16 ( 10 ): e0258058 . doi: 10.1371/journal.pone.0258058
Bauer JE . The essential nature of dietary omega-3 fatty acids in dogs . J Am Vet Med Assoc . 2016 ; 249 ( 11 ): 1267 - 1272 . doi: 10.2460/javma.249.11.1267
Zhang Y , Kirk CA , Tolbert MK , et al. Impact of fatty acid composition on markers of exocrine pancreatic stimulation in dogs . PLoS One . 2023 ; 18 ( 8 ): e0290555 . doi: 10.1371/journal.pone.0290555
Lem KY , Fosgate GT , Norby B , Steiner JM . Associations between dietary factors and pancreatitis in dogs . J Am Vet Med Assoc . 2008 ; 233 ( 9 ): 1425 - 1431 . doi: 10.2460/javma.233.9.1425
Allenspach K , Wieland B , Gröne A , Gaschen F . Chronic enteropathies in dogs: evaluation of risk factors for negative outcome . J Vet Intern Med . 2007 ; 21 ( 4 ): 700 - 708 . doi: 10.1111/j.1939-1676.2007.tb03011.x
Kathrani A . Dietary and nutritional approaches to the management of chronic enteropathy in dogs and cats . Vet Clin North Am Small Anim Pract . 2021 ; 51 ( 1 ): 123 - 136 . doi: 10.1016/j.cvsm.2020.09.005
Leib MS . Treatment of chronic idiopathic large-bowel diarrhea in dogs with a highly digestible diet and soluble fiber: a retrospective review of 37 cases . J Vet Intern Med . 2000 ; 14 ( 1 ): 27 - 32 .
Lecoindre P , Gaschen FP . Chronic idiopathic large bowel diarrhea in the dog . Vet Clin North Am Small Anim Pract . 2011 ; 41 ( 2 ): 447 - 456 . doi: 10.1016/j.cvsm.2011.02.004
Segarra S , Martínez-Subiela S , Cerdà-Cuéllar M , et al. Oral chondroitin sulfate and prebiotics for the treatment of canine inflammatory bowel disease: a randomized, controlled clinical trial . BMC Vet Res . 2016 ; 12 ( 1 ): 49 . doi: 10.1186/s12917-016-0676-x
Rossi G , Cerquetella M , Gavazza A , et al. Rapid resolution of large bowel diarrhea after the administration of a combination of a high-fiber diet and a probiotic mixture in 30 dogs . Vet Sci . 2020 ; 7 ( 1 ): 7 . doi: 10.3390/vetsci7010021
Dennis JS , Kruger JM , Mullaney TP . Lymphocytic/plasmacytic colitis in cats: 14 cases (1985-1990) . J Am Vet Med Assoc . 1993 ; 202 ( 2 ): 313 - 318 . doi: 10.2460/javma.1993.202.02.313
Ramakrishna BS , Mathan M , Mathan VI . Alteration of colonic absorption by long-chain unsaturated fatty acids: influence of hydroxylation and degree of unsaturation . Scand J Gastroenterol . 1994 ; 29 ( 1 ): 54 - 58 . doi: 10.3109/00365529409090437
Laflamme DP , Xu H , Long GM . Effect of diets differing in fat content on chronic diarrhea in cats . J Vet Intern Med . 2011 ; 25 ( 2 ): 230 - 235 . doi: 10.1111/j.1939-1676.2010.0665.x
Weiss DJ , Gagne JM , Armstrong PJ . Relationship between inflammatory hepatic disease and inflammatory bowel disease, pancreatitis, and nephritis in cats . J Am Vet Med Assoc . 1996 ; 209 ( 6 ): 1114 - 1116 . doi: 10.2460/javma.1996.209.06.1114
Callahan Clark JE , Haddad JL , Brown DC , Morgan MJ , Van Winkle TJ , Rondeau MP . Feline cholangitis: a necropsy study of 44 cats (1986-2008) . J Feline Med Surg . 2011 ; 13 ( 8 ): 570 - 576 . doi: 10.1016/j.jfms.2011.05.002
Fragkou FC , Adamama-Moraitou KK , Poutahidis T , et al. Prevalence and clinicopathological features of triaditis in a prospective case series of symptomatic and asymptomatic cats . J Vet Intern Med . 2016 ; 30 ( 4 ): 1031 - 1045 . doi: 10.1111/jvim.14356
Simpson KW . Pancreatitis and triaditis in cats: causes and treatment . J Small Anim Pract . 2015 ; 56 ( 1 ): 40 - 49 . doi: 10.1111/jsap.12313
Hill RC , Van Winkle TJ . Acute necrotizing pancreatitis and acute suppurative pancreatitis in the cat: a retrospective study of 40 cases (1976-1989) . J Vet Intern Med . 1993 ; 7 ( 1 ): 25 - 33 . doi: 10.1111/j.1939-1676.1993.tb03165.x
Ferreri JA , Hardam E , Kimmel SE , et al. Clinical differentiation of acute necrotizing from chronic nonsuppurative pancreatitis in cats: 63 cases (1996-2001) . J Am Vet Med Assoc . 2003 ; 223 ( 4 ): 469 - 474 . doi: 10.2460/javma.2003.223.469
Xenoulis PG , Suchodolski JS , Steiner JM . Chronic pancreatitis in dogs and cats . Compend Contin Educ Vet . 2008 ; 30 ( 3 ): 166 - 180 .
Davenport D , Remillard R , Simpson K . Exocrine pancreatic insufficiency . In: Hand M , Thatcher C , Remillard R , Roudebush P , Novotny B , eds. Small Animal Clinical Nutrition . 5th ed . Mark Morris Institute ; 2010 .
Xenoulis PG , Zoran DL , Fosgate GT , Suchodolski JS , Steiner JM . Feline exocrine pancreatic insufficiency: a retrospective study of 150 cases . J Vet Intern Med . 2016 ; 30 ( 6 ): 1790 - 1797 . doi: 10.1111/jvim.14560
Westermarck E , Wiberg M , Junttila J . Role of feeding in the treatment of dogs with pancreatic degenerative atrophy . Acta Vet Scand . 1990 ; 31 ( 3 ): 325 - 331 . doi: 10.1186/BF03547544
Pidgeon G . Effect of diet on exocrine pancreatic insufficiency in dogs . J Am Vet Med Assoc . 1982 ; 181 ( 3 ): 232 - 235 .
Isaksson G , Lundquist I , Ihse I . Effect of dietary fiber on pancreatic enzyme activity in vitro . Gastroenterology . 1982 ; 82 ( 5 pt 1 ): 918 - 924 . doi: 10.1016/S0016-5085(82)80256-4
Balaghi M , Wagner C . Folate deficiency inhibits pancreatic amylase secretion in rats . Am J Clin Nutr . 1995 ; 61 ( 1 ): 90 - 96 . doi: 10.1093/ajcn/61.1.90
Barnes A , Gates K , Kuntz J . Fat-soluble vitamin deficiency and subsequent coagulopathy in a cat with exocrine pancreatic insufficiency . Vet Rec Case Rep . 2020 ; 8 ( 1 ): e001019 . doi: 10.1136/vetreccr-2019-001019
Ross SJ , Osborne CA , Kirk CA , Lowry SR , Koehler LA , Polzin DJ . Clinical evaluation of dietary modification for treatment of spontaneous chronic kidney disease in cats . J Am Vet Med Assoc . 2006 ; 229 ( 6 ): 949 - 957 . doi: 10.2460/javma.229.6.949
Jacob F , Polzin DJ , Osborne CA , et al. Clinical evaluation of dietary modification for treatment of spontaneous chronic renal failure in dogs . J Am Vet Med Assoc . 2002 ; 220 ( 8 ): 1163 - 1170 . doi: 10.2460/javma.2002.220.1163
Parker VJ . Nutritional management for dogs and cats with chronic kidney disease . Vet Clin North Am Small Anim Pract . 2021 ; 51 ( 3 ): 685 - 710 . doi: 10.1016/j.cvsm.2021.01.007
Parker VJ , Hill RC . Nutritional management of cats and dogs with diabetes mellitus . Vet Clin North Am Small Anim Pract . 2023 ; 53 ( 3 ): 657 - 674 . doi: 10.1016/j.cvsm.2023.01.007
Mansfield CS , James FE , Steiner JM , Suchodolski JS , Robertson ID , Hosgood G . A pilot study to assess tolerability of early enteral nutrition via esophagostomy tube feeding in dogs with severe acute pancreatitis . J Vet Intern Med . 2011 ; 25 ( 3 ): 419 - 425 . doi: 10.1111/j.1939-1676.2011.0703.x
Klaus JA , Rudloff E , Kirby R . Nasogastric tube feeding in cats with suspected acute pancreatitis: 55 cases (2001-2006) . J Vet Emerg Crit Care (San Antonio) . 2009 ; 19 ( 4 ): 337 - 346 . doi: 10.1111/j.1476-4431.2009.00438.x
Harris JP , Parnell NK , Griffith EH , Saker KE . Retrospective evaluation of the impact of early enteral nutrition on clinical outcomes in dogs with pancreatitis: 34 cases (2010-2013) . J Vet Emerg Crit Care (San Antonio) . 2017 ; 27 ( 4 ): 425 - 433 . doi: 10.1111/vec.12612
Chan DL , Freeman LM . Nutrition in critical illness . Vet Clin North Am Small Anim Pract . 2006 ; 36 ( 6 ): 1225 - 1241 , v-vi. v-vi. doi: 10.1016/j.cvsm.2006.08.009
Saker K , Remillard R . Critical care nutrition and enteral-assisted feeding . In: Hand M , Thatcher C , Remillard R , Roudebush P , Novotny B , eds. Small Animal Clinical Nutrition . 5th ed . Mark Morris Institute ; 2010 : 439 - 476 .
Morris JG , Rogers QR . Ammonia intoxication in the near-adult cat as a result of a dietary deficiency of arginine . Science . 1978 ; 199 ( 4327 ): 431 - 432 . doi: 10.1126/science.619464
Qin HL , Su ZD , Hu LG , Ding ZX , Lin QT . Effect of parenteral and early intrajejunal nutrition on pancreatic digestive enzyme synthesis, storage and discharge in dog models of acute pancreatitis . World J Gastroenterol . 2007 ; 13 ( 7 ): 1123 - 1128 . doi: 10.3748/wjg.v13.i7.1123
Thompson LJ , Seshadri R , Raffe MR . Characteristics and outcomes in surgical management of severe acute pancreatitis: 37 dogs (2001-2007) . J Vet Emerg Crit Care (San Antonio) . 2009 ; 19 ( 2 ): 165 - 173 . doi: 10.1111/j.1476-4431.2009.00401.x
Son TT , Thompson L , Serrano S , Seshadri R . Surgical intervention in the management of severe acute pancreatitis in cats: 8 cases (2003-2007) . J Vet Emerg Crit Care (San Antonio) . 2010 ; 20 ( 4 ): 426 - 435 . doi: 10.1111/j.1476-4431.2010.00554.x
Zhu Y , Yin H , Zhang R , Ye X , Wei J . Nasogastric nutrition versus nasojejunal nutrition in patients with severe acute pancreatitis: a meta-analysis of randomized controlled trials . Gastroenterol Res Pract . 2016 ; 2016 : 6430632 . doi: 10.1155/2016/6430632
Chan DL , Freeman LM , Labato MA , Rush JE . Retrospective evaluation of partial parenteral nutrition in dogs and cats . J Vet Intern Med . 2002 ; 16 ( 4 ): 440 - 445 . doi: 10.1892/0891-6640(2002)016<0440:reoppn>2.3.co;2
- Supplementary Material S1 (PDF 108 KB)
- Supplementary Material S2 (PDF 145 KB)
- Supplementary Material S3 (PDF 176 KB)
- Supplementary Material S4 (PDF 115 KB)
- PubMed Citation
- Article by Harry Cridge
- Article by Valerie J. Parker
- Article by Aarti Kathrani
- Similar articles in PubMed
Follow JAVMA
Follow AJVR
Follow the AVMA
Subscribe to newsletters
© 2024 American Veterinary Medical Association. All rights reserved.
Powered by KGL PubFactory
- [66.249.64.20|185.66.14.133]
- 185.66.14.133
Character limit 500 /500

IMAGES
VIDEO
COMMENTS
The 3 different diets were low-carb, low-fat, and very-low-fat vegan. The weight gain over 21 days was 1.3 kg for low-carb, 7.1 kg for low-fat, and 4.7 kg for very-low-fat vegan. Summary: In this n-of-1 study, consuming 5800 Calories/day of 3 different diets for 21 days did not lead to the same amount of weight gain.
Recent findings This study reports a case study of an individual who ate 5800 Calories per day of 3 different diets for 21 days at a time. The 3 different diets were low-carb, low-fat, and very-low-fat vegan. The weight gain over 21 days was 1.3 kg for low-carb, 7.1 kg for low-fat, and 4.7 kg for very-low-fat vegan. Summary In this n-of-1 study, consuming 5800 Calories/day of 3 different diets ...
Recent findings: This study reports a case study of an individual who ate 5800 Calories per day of 3 different diets for 21 days at a time. The 3 different diets were low-carb, low-fat, and very ...
To our knowledge, there has never been an overfeeding study comparing the effects of multiple diets. Recent findings This study reports a case study of an individual who ate 5800 Calories per day of 3 different diets for 21 days at a time. The 3 different diets were low-carb, low-fat, and very-low-fat vegan. The weight gain over 21 days was 1.3 ...
To our knowledge, there has never been an overfeeding study comparing the effects of multiple diets. Recent findings This study reports a case study of an individual who ate 5800 Calories per day of 3 different diets for 21 days at a time. The 3 different diets were low-carb, low-fat, and very-low-fat vegan. The weight gain over 21 days was 1.3 ...
To our knowledge, there has never been an overfeeding study comparing the effects of multiple diets. RECENT FINDINGS: This study reports a case study of an individual who ate 5800 Calories per day of 3 different diets for 21 days at a time. The 3 different diets were low-carb, low-fat, and very-low-fat vegan. The weight gain over 21 days was 1. ...
The studies by Antonio et al. collectively suggest that a high-protein diet may reduce FM if there is an alteration in the training regimen; however, changes in FFM are not different between a 2.6 and 3.3 g/kg/d protein intake suggesting there may be an upper limit to protein intake vis a vis gains in FFM.
To our knowledge, there has never been an overfeeding study comparing the effects of multiple diets. Recent findings This study reports a case study of an individual who ate 5800 Calories per day of 3 different diets for 21 days at a time. The 3 different diets were low-carb, low-fat, and very-low-fat vegan. The weight gain over 21 days was 1.3 ...
A case study of overfeeding 3 different diets. Feltham, Sam; Westman, Eric C. Current Opinion in Endocrinology & Diabetes and Obesity. 28(5):446-452, October 2021. Abstract. Favorite; PDF; Permissions Open. Nutritional ketosis is well-tolerated, even in type 1 diabetes: the ZeroFive100 Project; a proof-of-concept study ...
A case study of overfeeding 3 different diets. Sam Feltham, Eric C. Westman. Quality or quantity of food has been at the heart of the diet debate for decades and will seemingly continue for many to come unless tightly controlled studies are conducted. To our knowledge, there has never been an overfeeding study comparing the effects of multiple ...
Abstract. This systematic review has examined more than 300 original papers dealing with the biology of overfeeding. Studies have varied from 1 day to 6 months. Overfeeding produced weight gain in adolescents, adult men and women and in older men. In longer term studies, there was a clear and highly significant relationship between energy ...
Overfeeding experiments, in which we impose short-term positive energy balance, help unravel the cellular, physiological and behavioural adaptations to nutrient excess. These studies mimic longer ...
This systematic review has examined more than 300 original papers dealing with the biology of overfeeding. Studies have varied from 1 day to 6 months. ... Growth hormone, in contrast, was rapidly suppressed. Changes in plasma lipids were influenced by diet, exercise and the magnitude of weight gain. Adipose tissue and skeletal muscle morphology ...
This study reports a case study of an individual who ate 5800 Calories per day of 3 different diets for 21 days at a time. The 3 different diets were low-carb, low-fat, and very-low-fat vegan. The weight gain over 21 days was 1.3 kg for low-carb, 7.1 kg for low-fat, and 4.7 kg for very-low-fat vegan. Summary In this n-of-1 study, consuming 5800 ...
This review summarises insight gained from overfeeding studies regarding susceptibility to obesity and related complications with nutrient excess. International Journal of Obesity (2017) 41, 853 ...
This systematic review has examined more than 300 original papers dealing with the biology of overfeeding. Studies have varied from 1 day to 6 months. Overfeeding produced weight gain in ...
A case study of overfeeding 3 different diets. Journal Article Curr Opin Endocrinol Diabetes Obes · October 1, 2021 PURPOSE OF REVIEW: Quality or quantity of food has been at the heart of the diet debate for decades and will seemingly continue for many to come unless tightly controlled studies are conducted. To our knowledge, there has never ...
Although controlled under- and overfeeding studies published so far provide a sound basis to model weight changes, there is a need for re-review because of the following: (a) different methods had been used to assess body composition and there is need to address their limitations; (b) there was a considerable variance in the study protocols without taking into account the dynamics of weight ...
Overfeeding triggers homeostatic compensatory mechanisms that counteract weight gain. Here, we show that both lean and diet-induced obese (DIO) male mice exhibit a potent and prolonged inhibition ...
A case study of overfeeding 3 different diets. Article. Aug 2021; Sam Feltham; Eric C. Westman; Purpose of study: Quality or quantity of food has been at the heart of the diet debate for decades ...
Compared to investigations on hypocaloric diets, the effects of chronic overfeeding have been less studied. It has been posited that consuming calories in excess of daily caloric requirements will result in a gain in body weight and in particular fat mass regardless of which macronutrient(s) are consumed. However, recent evidence suggests that ...
1 Model and process for nutrition and dietetic practice, 3 2 Nutrition care process terminology (NCPT), 8 3 Record keeping, 12 4 Assessment, 16 PART II Case studies 1 Veganism, 25 2 Older person - ethical dilemma, 28 3 Older person, 31 4 Learning disabilities: Prader-Willi syndrome, 34 5 Freelance practice, 39 6 Public health - weight ...
Data from a case series of 24 individuals who underwent similar protocols similarly found that overfeeding on a ketogenic diet decreased LDL-C. Summary . This n = 1 study and associated case series provide data that short-term overfeeding can lower LDL-C in the context of carbohydrate restriction.
Observational studies have associated a cumulative protein and energy deficit with impaired outcome of critical illness [2, 3, 11].However, the results of these studies might also be explained by feeding intolerance as a marker of severity of illness [2, 3, 12].In the last decade, several large RCTs have addressed the timing, route and dosage of medical nutrition therapy in critically ill ...
There are hundreds, probably thousands, of different substances that can act as antioxidants. The most familiar ones are vitamin C, vitamin E, beta-carotene, and other related carotenoids, along with the minerals selenium and manganese. They're joined by glutathione, coenzyme Q10, lipoic acid, flavonoids, phenols, polyphenols, phytoestrogens ...
Participants in a National Institutes of Health study who ate a diet high in ultra-processed food ate around 500 calories more a day, and gained an average of two pounds over the 28-day study ...
The aims of this study were to investigate the antioxidative, anti-inflammatory, and lipid metabolism-regulating effects of berberine against high-fat diet (HFD)-induced liver damage and to clarify the underlying molecular mechanisms. Tilapia were fed diets containing two doses of berberine (50 and 100 mg/kg diet) alongside high fat for 60 days.
Prolonged fasting triggers a stress response within the human body. Our objective was to investigate the impact of prolonged fasting, in conjunction with stress, on kynurenine pathway metabolites.
Substantial weight loss in people living with type 2 diabetes (T2D) can reduce the need for glucose-lowering medications while concurrently lowering glycemia below the diagnostic threshold for the disease. Furthermore, weight-loss interventions have also been demonstrated to improve aspects of underlying T2D pathophysiology related to ectopic fat in the liver and pancreatic beta-cell function.
Abstract Nutrition is considered a key part of the management of pancreatitis in dogs and cats. While limited prospective research exists, experimental studies, retrospective studies, and anecdote allow for formulation of nutritional guidelines. Historically, fat has been considered the key nutrient of interest in pancreatitis; however, other nutrients and dietary factors, including energy ...