- Search Menu
- Advance Articles
- Supplements
- Special Series
- Breast Cancer
- Cancer Diagnostics and Molecular Pathology
- Community Outreach
- Endocrinology
- Gastrointestinal Cancer
- Genitourinary Cancer
- Geriatric Oncology
- Global Health and Cancer
- Gynecologic Oncology
- Head and Neck Cancers
- Health Outcomes and Economics of Cancer Care
- Hematologic Malignancies
- Hepatobiliary
- Immuno-Oncology
- Lung Cancer
- Medical Ethics
- Melanoma and Cutaneous Malignancies
- Neuro-Oncology
- New Drug Development and Clinical Pharmacology
- Pediatric Oncology
- Radiation Oncology
- Browse content in Regulatory Issues
- Regulatory Issues: FDA
- Regulatory Issues: EMA
- Symptom Management and Supportive Care
- Author Guidelines
- Submission Site
- Why Publish With Us?
- Open Access Options
- Self-Archiving Policy
- Young Investigator Award
- Advertising & Corporate Services
- Reprints, ePrints, Supplements
- About The Oncologist
- About the Society for Translational Oncology
- Editorial Board
- Discussions with Don S. Dizon
- Journals on Oxford Academic
- Books on Oxford Academic


Article Contents
I ntroduction, e pidemiology and r isk f actors for t riple ‐n egative b reast c ancer, l imitations of c urrent t reatment s trategies for t riple ‐n egative b reast c ancer, p latinum s alts in t riple ‐n egative b reast c ancer, b evacizumab, egfr i nhibitors, o ther p otential t herapeutic t argets in t riple ‐n egative b reast c ancer, p oly (adp‐ ribose ) p olymerase i nhibitors, a ndrogen r eceptor t argeted t herapy, a ddressing c urrent u nmet n eeds in c linical p ractice, c onclusions, r eferences.
- < Previous
Triple‐Negative Breast Cancer: An Unmet Medical Need
Disclosures: Clifford A. Hudis: Consultant/advisory role : Genentech; Research funding/contracted research : Onyx, Merck; Luca Gianni: None.
The content of this article has been reviewed by independent peer reviewers to ensure that it is balanced, objective, and free from commercial bias. No financial relationships relevant to the content of this article have been disclosed by the independent peer reviewers.
- Article contents
- Figures & tables
- Supplementary Data
Clifford A. Hudis, Luca Gianni, Triple‐Negative Breast Cancer: An Unmet Medical Need, The Oncologist , Volume 16, Issue S1, January 2011, Pages 1–11, https://doi.org/10.1634/theoncologist.2011-S1-01
- Permissions Icon Permissions
Triple‐negative breast cancer, characterized by tumors that do not express estrogen receptor (ER), progesterone receptor (PR), or HER‐2 genes, represents an important clinical challenge because these cancers do not respond to endocrine therapy or other available targeted agents. The metastatic potential in triple‐negative breast cancer is similar to that of other breast cancer subtypes, but these tumors are associated with a shorter median time to relapse and death. One important goal is therefore the identification of prognostic factors and markers to reliably select high and low risk subsets of patients with triple‐negative disease for different treatment approaches of subtypes with differential responsiveness to specific agents. However, a reliable prognostic marker has been elusive, and markers have been inconsistently useful. For example, epidermal growth factor receptor (EGFR) has been studied, but there is still a lack of agreement on a standard assay or cutoff for EGFR expression levels with respect to prognosis. Similarly, because triple‐negative status is sometimes used as a surrogate for basal‐like breast cancer, specific basal markers have been explored. Indeed, trials designed to accrue patients with basal‐like breast cancer using ER/PR and HER‐2 negativity may provide only an approximation of the triple‐negative population and are sometimes reanalyzed using more specific indicators like CK 5/6, EGFR status, and others, again marred by discordances. Chemotherapy remains the mainstay of treatment of triple‐negative breast cancer, but important limitations still need to be overcome in the next few years if any significant clinical strides are to be made. Current treatment strategies for triple‐negative disease include anthracyclines, taxanes, ixabepilone, platinum agents, and biologic agents. More recently, EGFR inhibition has been proposed as a therapeutic mechanism in triple‐negative breast cancer, again with mixed results. Agents that target poly(ADP‐ribose) polymerase and androgen receptors have also been proposed in these patients or subsets of them, and ongoing trials should result in definitive guidance with respect to the value of these agents in triple‐negative disease.
Triple‐negative breast cancer is clearly a distinct clinical subtype, from the perspective of both ER and HER‐2 expression, but further subclassification is needed. At present, there is not a clear, proven effective single agent that targets a defining vulnerability in triple‐negative breast cancer. This article will review the clinical problem of triple‐negative disease, potential prognostic factors, demonstrated efficacy of currently available therapeutic options, and new potential therapies.
Although a good deal is known about available and some experimental agents in triple‐negative breast cancer, a number of important limitations still need to be overcome in the next few years if any significant clinical strides are to be made. This discussion will focus on basal‐like breast cancer and triple‐negative breast cancer, understanding that there is a large degree of overlap between these two terms.
Higher rates of triple‐negative breast cancer have been observed in women who are younger, which may be associated with a greater likelihood of BRCA1 expression. Women of African or Hispanic ancestry have been shown to have higher rates of triple‐negative breast cancer, as have women in lower socioeconomic groups. It is therefore important to be somewhat skeptical of a few superficial phenotypic characteristics and genes being associated globally with a specific subtype of a malignancy like breast cancer, because some of the incidence may be related to the impact of lower socioeconomic status. A lower proportion of triple‐negative breast cancers are discovered by mammography, which is possibly related to the age distribution of these patients [ 1 – 4 ].
From an epidemiologic perspective, the known risk factors for triple‐negative disease are modest, suggesting few clear interventions. From a purely histologic point of view, triple‐negative breast cancer consists of a collection of subtypes, with some tumors, such as the secretory or adenoid cystic tumors, being relatively less aggressive, even though they are triple‐negative, and others being associated with a rapidly progressive course ( Fig. 1 ).

Triple‐negative breast cancer: Range of histology.
The natural history of triple‐negative breast cancer has been redefined over the last few years, and it is remarkable how many different clinical data sets provide similar curves. Luminal A metastatic hormone receptor positive breast cancer typically causes late bone metastases, whereas triple‐negative breast cancer is more likely to cause early visceral metastases. Fundamentally, in the early‐stage setting, triple‐negative breast cancer is associated with earlier versus later events, as well as a shorter period from the time of recurrence until death ( Fig. 2 ) [ 5 ].

Triple‐negative breast cancer: Recurrence and survival. From Kim K, Lee E, Lee J et al.; Korea Breast Cancer Society. Clinicopathologic signature of TNBC patients with good prognosis. Paper presented at: San Antonio Breast Cancer Symposium (SABCS) 2009; December 15, 2009; San Antonio, Texas; abstract 4065; doi: 10.1158/0008‐5472.SABCS‐09‐4065, with permission.
The metastatic potential of all subtypes of breast cancers is ultimately similar, but the growth rates and tumor distributions vary so that the natural history and clinical course can appear divergent, especially over the short run. Once a metastatic triple‐negative breast cancer is present, there is a much shorter median time from relapse to death [ 6 ]. This represents a key challenge, as clinicians try to palliate an incurable disease and extend life when possible. Although prognostic markers indicative of poor outcome have been identified, these factors come up in any series of metastatic breast cancers, and some of them are not particularly well categorized. For example, as already described, there is still no agreement on standard assay or expression levels for epidermal growth factor receptor (EGFR). Nevertheless, this is often listed as a poor prognostic factor in triple‐negative disease.
Current treatment strategies include many chemotherapy agents, such as the anthracyclines, taxanes, ixabepilone, and platinum agents, as well as selected biologic agents and possibly anti‐EGFR drugs.
Platinum therapy in particular requires close scrutiny, because preclinical evidence suggests that it may be especially useful in triple‐negative breast cancer and BRCA mutation‐associated malignancy in particular. However, assessing its effectiveness is challenging because focused trials have not been performed. Notably, triple‐negative breast tumors can have higher response rates to a variety of chemotherapy agents despite being associated with poorer outcomes. This may limit the utility of response rates as indicators of superiority.
A related challenge, from a drug approval or regulatory perspective, is utility of the widely accepted surrogate endpoint of progression‐free survival (PFS). It has been demonstrated that regimens based on anthracyclines or taxanes, such as the taxane‐fluorouracil‐doxorubicin‐cyclophosphamide (T‐FAC) regimen used at MD Anderson or doxorubicin‐cyclophosphamide‐taxane used in the National Surgical Adjuvant Breast and Bowel Project (NSABP) trials, in relatively small series of patients treated in the preoperative setting, are effective with high in‐breast response rates [ 7 , 8 ]. In the triple‐negative breast cancers, as opposed to especially the luminal As or Bs, there is a markedly higher response rate. However, despite this higher response rate, this subtype has a far shorter disease‐free survival and overall survival (OS). If accrual to a trial is not limited to one subtype of breast cancer, clinicians should exercise caution when using in‐breast response as a surrogate for outcomes. There is no doubt that, in a defined subset, an in‐breast response yields a better disease‐free and overall survival, but if the patient population is not selected appropriately, one may observe a paradox of high in‐breast activity and worse overall outcomes, compared to other groups.
For example, a larger T‐FAC series compared outcomes in patients with triple‐negative and non–triple‐negative breast cancer. This study demonstrated a paradoxical doubling of the pathological complete response (CR) rate but a shortening of both PFS and OS [ 9 ].
A meta‐analysis by Di Leo et al., as well as smaller phase II and phase III trials with anthracyclines, have shown variable results for individual agents and regimens in this subtype of breast cancer ( Table 1 ) [ 10 – 12 ]. From these studies, we can say that these drugs have activity in triple‐negative breast cancer but their relative merit, compared to other available agents, cannot be established.
Anthracyclines for triple‐negative breast cancer

Continuing to focus on the taxanes, Hayes and colleagues retrospectively studied a subset of patients enrolled in the Cancer and Leukemia Group B (CALGB) 9344 study for whom paraffin samples were available. Patients were classified into one of four groups, as shown in Figure 3 . Critically, the largest group is the HER‐2‐negative, estrogen receptor (ER)‐positive cohort, and despite the overall positive findings in the 9344 trial with the addition of the paclitaxel, that subgroup does not appear to get a large benefit. However, clinicians should note that these results have not been uniformly consistent across other trials, such as those from the GEICAM (Grupo Español de Investigación del Cáncer de Mama) investigators. Turning specifically to triple‐negative disease, as shown in Figure 3 , there is a large benefit for paclitaxel. These data and others are consistent with the hypothesis that the taxanes are effective in triple‐negative breast cancer. Paclitaxel was also useful in HER‐2 positive breast cancer, regardless of receptor status, in the pre‐trastuzumab era ( Fig. 3 ) [ 13 ].

C9344 disease‐free survival for paclitaxel by ER and HER‐2 status. From Hayes DF, Thor AD, Dressler LG et al. HER‐2 and response to paclitaxel in node‐positive breast cancer. N Engl J Med 2007;357:1496–1506, with permission. Copyright © 2007 Massachusetts Medical Society. All rights reserved.
Other studies have investigated the use of adjuvant anthracycline plus taxane in triple‐negative breast cancer ( Fig. 4 A). The Breast Cancer International Research Group (BCIRG) 001 trial compared docetaxel‐doxorubicin‐cyclophosphamide versus fluorouracil‐doxorubicin‐taxane (FAC). The addition of the taxane yielded an advantage in the triple‐negative cohort, as was true for the overall trial [ 14 ]. In a slightly more difficult‐to‐interpret trial that investigated additional cycles of paclitaxel instead of cyclophosphamide, the addition of more, versus less, paclitaxel was associated with a benefit in the triple‐negative cohort ( Fig. 4 B) [ 15 ]. These three data sets all consistently suggest that, in triple‐negative disease, there is a benefit for taxanes.

Adjuvant anthracycline plus taxane for triple‐negative breast cancer. (A) From Hugh J, Hanson J, Cheang MC et al. Breast cancer subtypes and response to docetaxel in node‐positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol 2009;27:1168–1176. Reprinted with permission. © 2009 American Society of Clinical Oncology. All rights reserved. (B) From Loesch DM, Greco F, O'Shaughnessy J et al. A randomized, multicenter phase III trial comparing doxorubicin + cyclophosphamide followed by paclitaxel or doxorubicin + paclitaxel followed by weekly paclitaxel as adjuvant therapy for high‐risk breast cancer. J Clin Oncol 2007;25(suppl 18);abstract 517. Reprinted with permission. © 2007 American Society of Clinical Oncology. All rights reserved.
A newer agent, ixabepilone, has been evaluated in subsets of patients with triple‐negative metastatic breast cancer, in phase II and phase III studies [ 16 , 17 ]. Like the other agents discussed, there is evidence of activity, but it is difficult to assess the relative value of this drug compared with the others in this cohort beyond stating simply that it is active ( Table 2 ).
Ixabepilone for triple‐negative breast cancer

A key issue is the role of platinum salts because of their specific mechanism of action, in that they cause DNA cross‐link strand breaks. In cells that lack homologous repair, such as BRCA mutants, and possibly in BRCA‐deficient cells, this could be a particularly effective treatment approach. However, determining whether they actually have clinically distinct activity in triple‐negative disease has been challenging.
Although platinum salts have been investigated for a century and a half, clinical trials with platinum salts in cancer did not begin until the early 1970s. The FDA approval was specifically in ovarian and testicular cancer in 1978. Then in 1989, replacing cisplatin in many centers, carboplatin became available. Although it too forms DNA cross‐breaks, it appears to be cross‐resistant in some cases. A number of platinum agents were subsequently developed in an attempt to provide a greater therapeutic index, but randomized data to address the role of platinums have been limited. A rigorous meta‐analysis of chemotherapy agents performed in 1998 by Fossati and colleagues included one randomized trial with a platinum agent [ 18 ].
The available data with platinum agents is further confounded by the inclusion of asymmetric comparator arms. For instance, one study compared cisplatin plus doxorubicin versus a non–doxorubicin‐containing regimen, which also omitted the platinum. In this trial, the combination of these two active drugs—platinum and doxorubicin—was actually associated with a reduction in benefit. Another small, randomized trial compared epirubicin alone versus epirubicin plus cisplatin in metastatic disease and showed at least a borderline advantage for the addition of platinum in a completely unselected cohort of 139 randomized patients [ 19 ]. More recently, there have been a series of phase II studies, some of them modest in size and others reported retrospectively in the preoperative or metastatic setting, using both carboplatin and cisplatin, and also confounded by the addition of other agents. These data continue to show pathologic complete response rates in the range seen in other clinical trials with non–platin‐containing regimens ( Table 3 ) [ 20 – 25 ].
Platinum agents for triple‐negative breast cancer

One phase II study ( NCT00148694 ) in particular received attention, because it was prospectively conducted and enrolled a specifically defined cohort of triple‐negative patients. In the neoadjuvant setting at Dana Farber Cancer Institute, 14 of 28 patients who were given single‐agent cisplatin responded including six CRs. However, there was a relatively high incidence of BRCA mutation carriers in this patient population. When two patients who were known to be BRCA mutation carriers were excluded from the analysis, leaving behind the patients who were known not to have those mutations, the CR rate was 4 patients of 26. That is a 15% CR rate to a single agent in a prospectively conducted study at a large cancer center, which may be viewed as impressive results [ 22 ].
However, in comparison, a non–platinum‐containing regimen reported in 2002, looking at neoadjuvant cyclophosphamide‐vincristine‐doxorubicin‐prednisolone (CVAP) followed after four cycles by randomization in the responders to docetaxel or more of the CVAP, showed a 15% increment in the CR rate with the taxane [ 26 ]. This suggests that CRs in triple‐negative breast cancer, apart from the BRCA mutation subset, may not be particularly greater with platinums than they are with other regimens.
Similar results have been seen in trials conducted at MD Anderson, where the CR rate was 27% for the paclitaxel‐FAC regimen [ 23 ]. Importantly, these data included the diluting effects of a large number of patients who had ER positive disease. Consequently, there is no clear answer in the unselected triple‐negative population as to whether or not the platinums represent a special agent. This is a question that still remains under careful study, as will be discussed.
The clinical impact of bevacizumab is complicated for all breast cancers and no clearer for triple‐negative disease. Across all of the reported prospective clinical trials of bevacizumab, there is no clear signal that this anti‐angiogenic agent has any special properties in the triple‐negative cohort compared with the broader population ( Table 4 ) [ 27 , 28 ].
Bevacizumab for triple‐negative breast cancer

The potential of EGFR inhibition in breast cancer has been another interesting story. Modi et al. investigated a regimen of cetuximab plus paclitaxel, and then cetuximab plus doxorubicin, in breast cancer. The cetuximab caused an unacceptable acneiform rash for women with breast cancer and did not appear to have particular activity [ 29 ]. In a prospective phase II study by the Translational Breast Cancer Research Consortium (TBCRC), single‐agent cetuximab was evaluated alone or in combination with carboplatin. The single‐agent cetuximab was deemed not worthy of further study in this trial, and the combination was deemed possibly worthy of further study. However, this was simply a comparison of a regimen with or without a platinum agent, and whether or not the anti‐EGFR antibody was contributing at all is difficult to determine [ 30 ]. In contrast, another phase II trial was conducted of irinotecan plus carboplatin, alone or in combination with cetuximab. In the triple‐negative cohort, an increase in activity was observed with irinotecan ( Table 5 ) [ 31 ]. Additional trials are ongoing. Very recently, another randomized phase II study showed modest activity [ 32 ].
Epidermal growth factor receptor inhibition for triple‐negative breast cancer: Efficacy data from phase II trials

A variety of other potential targets have been incompletely validated in triple‐negative breast cancer [ 33 ]. Particular attention should be paid to a number of the vascular endothelial growth factor receptor inhibitors [ 34 ], dasatinib (a Src kinase inhibitor) [ 35 ], and checkpoint kinase 1 inhibitors currently under development, among many others ( Table 6 ).
Other targets for triple‐negative breast cancer

Although the poly(ADP‐ribose) polymerase (PARP) inhibitor story will be thoroughly addressed in other sections of this supplement, it is important to highlight the rapidity of translation from preclinical experiments [ 36 , 37 ] into a meaningful clinical advance. On the basis of initial findings, preclinical investigators proposed that a PARP inhibitor would be especially useful as a single agent, or in combination regimens in patients with BRCA mutations.
A phase II study was conducted with olaparib, an oral PARP inhibitor, in BRCA‐deficient metastatic breast cancer [ 38 ]. The patient cohort included those with metastatic disease at multiple sites, extensively pretreated for a median of three prior systemic chemotherapy regimens, having BRCA1 or BRCA2 mutations or an overwhelming family history consistent and suggestive of that mutation. Patients were treated with olaparib 100 mg twice daily (bid) or 400 mg bid, on the basis of preclinical data suggesting that the lower dose would be sufficient to achieve a therapeutic serum level for inhibition of the target.
This may be a bit of a peril as investigators move away from dosing in drug development trials based on maximum tolerated dose and toward known target inhibition. In 27 patients, the 100‐mg bid dose (which was believed to be effective at target inhibition) achieved a 22% response rate, an impressive result in the setting of salvage therapy for these patients. However, the higher dose of 400 mg bid resulted in an apparent almost doubling of response rate to 41%. This high response rate with a single‐agent, relatively nontoxic oral therapy in an extensively pretreated patient population was remarkable. Both complete and partial response rates were significant, and the time to progression was ∼2 months longer in this nonrandomized intrastudy comparison. These findings led to investigators dropping the 100‐mg dose arm and allowing some of the patients to cross over to the higher dose. The toxicity associated with this single agent was modest, with grade 3 or 4 toxicity rates reported in single‐digit percentages [ 38 ].
Recent research focusing on the androgen receptor (AR) is an example of taking an old story and making it new. One investigation processed 99 specimens through tissue microarray (TMA), with cluster analysis. In Figure 5 , ER positive cancers are shown in blue and ER negative cancers are shown in red. In a small sliver of the specimens shown on the left, a number of the ER negatives, by cluster analysis, were grouped with the ER positives. While determining the reasons for these results using principal component analysis, the investigators found that there was a series of additional genes that were upregulated here that were similar to what is seen in ER positive breast cancer. Investigators then painstakingly went back through these specimens and identified by mRNA and repeat immunohistochemistry that they lacked ER [ 39 ].

Unsupervised cluster analysis of 99 primary breast carcinomas. Reprinted by permission from Macmillan Publishers Ltd: Oncogene. Doane AS, Danso M, Lal P et al. An estrogen receptor‐negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene 2006;25:3994–4008, copyright 2006.
In further analyses of these ER negative tumors, investigators noted that what actually defined them is that, despite the lack of ER and progesterone receptor (PR) negativity, this downstream expression analysis suggested that they were, in fact, similar to ER positives. However, these patients exhibited AR expression as opposed to ER. [ 39 ]. The role of AR has therefore been rediscovered, in this regard. Although it has been known for some time that AR is widely expressed in breast cancer, it has not been a focus of interest in triple‐negative breast cancer until recently.
Although the incidence of AR positivity is lower in triple‐negative disease than in ER positives, it is perhaps more important, because there are few proven, effective, nontoxic therapies as discussed above for these patients. To transition these initial findings into a clinically meaningful result, a phase II trial ( NCT00468715 ) is now underway in the Translational Breast Cancer Consortium with bicalutamide, an AR antagonist and widely available prostate cancer drug, in triple‐negative breast cancer. The trial aims to accrue 28 patients who are triple‐negative but AR positive. A challenge has been the rarity of AR positivity in the clinic, estimated to be 20% in triple‐negative disease in the TMA previously discussed [ 39 ]. In anecdotal clinical practice, the rate has been closer to 10% among triple‐negative patients.
The challenge in using AR targeted drugs is that, if triple‐negative breast cancer represents 20% of the disease, and if 10% of that is AR positive, then this represents only 2% of overall breast cancer cases. If the AR was a new target, and a new drug was under development, substantial business and regulatory challenges would exist in demonstrating efficacy and moving forward.
Clinicians have been challenged with a lack of guidelines that specifically address the management of patients with triple‐negative disease. Although the St. Gallen guidelines briefly mention triple‐negative disease, the National Comprehensive Cancer Network (NCCN) is nonspecific in terms of drugs and agents but includes triple‐negative disease in its overall guidelines. Guidelines from the American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) are also nondirective on the subject of managing triple‐negative breast cancer.
There are, therefore, a variety of ongoing prospective trials to explore a range of therapeutic options for these patients, and more than 61 trials are currently listed at http://clinicaltrials.gov . To highlight a few current clinical trials, the BEATRICE study is testing the activity of bevacizumab, and there are trials in the metastatic setting looking at various chemotherapy combinations, as well as neoadjuvant studies, including one in the CALGB that is prospectively evaluating the roles of carboplatin and bevacizumab as well, with correlative signs ( Table 7 ).
Questions being asked in ongoing trials

Triple‐negative breast cancer is clearly a distinct subtype, from the perspective of both ER and HER‐2, and there may yet be further distinct subclassifications. This disease presentation clearly represents an important clinical challenge. Triple‐negative breast cancer is also a surrogate of basal‐like breast cancer. Therefore, trials designed to accrue patients with basal‐like breast cancer using ER/PR and HER‐2 negativity provide an approximation of the triple‐negative population, but, as described in the introduction, there is some discordance, including some HER‐2 positives and some ER positives among the basals. At present, there is not a clear, proven effective single agent that targets a driving vulnerability in triple‐negative breast cancer. However, there are a number of potential therapies currently under investigation that may eventually improve outcomes in these patients.
Fulford LG , Easton DF , Reis‐Filho JS et al. Specific morphological features predictive for the basal phenotype in grade 3 invasive ductal carcinoma of breast Histopathology 2006 ; 49 1 22 – 34
Google Scholar
Livasy CA , Karaca G , Nanda R et al. Phenotypic evaluation of the basal‐like subtype of invasive breast carcinoma Mod Pathol 2006 ; 19 2 264 – 271
Bauer KR , Brown M , Cress RD et al. Descriptive analysis of estrogen receptor (ER)‐negative, progesterone receptor (PR)‐negative, and HER‐2‐negative invasive breast cancer, the so‐called triple‐negative phenotype: a population‐based study from the California cancer Registry Cancer 2007 ; 109 9 1721 – 1728
Carey LA , Perou CM , Livasy CA et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study JAMA 2006 ; 295 21 2492 – 2502
Kim K , Lee E , Lee J . Korea Breast Cancer Society. Clinicopathologic Signature of TNBC Patients with Good Prognosis , San Antonio, Texas : Paper presented at: San Antonio Breast Cancer Symposium (SABCS) , December 15, 2009 . abstract 4065; doi: 10.1158/0008‐5472.SABCS‐09‐4065 .
Google Preview
Dent R , Trudeau M , Pritchard KI et al. Triple‐negative breast cancer: clinical features and patterns of recurrence Clin Cancer Res 2007 ; 13 15 Pt 1 4429 – 4434
Rouzier R , Perou CM , Symmans WF et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy Clin Cancer Res 2005 ; 11 16 5678 – 5685
Carey LA , Dees EC , Sawyer L et al. The triple‐negative paradox: primary tumor chemosensitivity of breast cancer subtypes Clin Cancer Res 2007 ; 13 8 2329 – 2334
Liedtke C , Mazouni C , Hess KR et al. Response to neoadjuvant therapy and long‐term survival in patients with triple‐negative breast cancer J Clin Oncol 2008 ; 26 8 1275 – 1281
Di Leo A , Isola J , Piette F et al. A meta‐analysis of phase III trials evaluating the predictive value of HER‐2 and topoisomerase II alpha in early breast cancer patients treated with CMF or anthracycline‐based adjuvant therapy Breast Cancer Res Treat 2008 ; 107 : 24
Bidard FC , Matthieu MC , Chollet P et al. p53 status and efficacy of primary anthracyclines/alkylating agent‐based regimen according to breast cancer molecular classes Ann Oncol 2008 ; 19 : 1261 – 1265
Gluz O , Nitz UA , Harbeck N et al. Triple‐negative high‐risk breast cancer derives particular benefit from dose intensification of adjuvant chemotherapy: Results of WSG AM‐01 trial Ann Oncol 2008 ; 19 : 861 – 870
Hayes DF , Thor AD , Dressler LG et al. HER‐2 and response to paclitaxel in node‐positive breast cancer N Engl J Med 2007 ; 357 15 1496 – 1506
Hugh J , Hanson J , Cheang MC et al. Breast cancer subtypes and response to docetaxel in node‐positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial J Clin Oncol 2009 ; 27 8 1168 – 1176
Loesch DM , Greco F , O'Shaughnessy J et al. A randomized, multicenter phase III trial comparing doxorubicin + cyclophosphamide followed by paclitaxel or doxorubicin + paclitaxel followed by weekly paclitaxel as adjuvant therapy for high‐risk breast cancer J Clin Oncol 2007 ; 25 suppl 18 . abstract 517.
Pivot XB , Li RK , Thomas ES et al. Activity of ixabepilone in oestrogen receptor‐negative and oestrogen receptor‐progesterone receptor‐human epidermal growth factor receptor 2‐negative metastatic breast cancer Eur J Cancer 2009 ; 45 17 2940 – 2946
Baselga J , Zambetti M , Llombart‐Cussac A et al. Phase II genomics study of ixabepilone as neoadjuvant treatment for breast cancer J Clin Oncol 2009 ; 27 4 526 – 534
Fossati R , Confalonieri C , Torri V et al. Cytotoxic and hormonal treatment for metastatic breast cancer: a systematic review of published randomized trials involving 31,510 women J Clin Oncol 1998 ; 16 10 3439 – 3460
Nielsen D , Dombernowsky P , Larsen SK et al. Epirubicin or epirubicin and cisplatin as first‐line therapy in advanced breast cancer. A phase III study Cancer Chemother Pharmacol 2000 ; 46 6 459 – 466
Sikov WM , Dizon DS , Strenger R et al. Frequent pathologic complete responses in aggressive stages II to III breast cancers with every‐4‐week carboplatin and weekly paclitaxel with or without trastuzumab: a Brown University Oncology Group Study J Clin Oncol 2009 ; 27 28 4693 – 4700
Torrisi R , Balduzzi A , Ghisini R et al. Tailored preoperative treatment of locally advanced triple‐negative (hormone receptor negative and HER‐2 negative) breast cancer with epirubicin, cisplatin, and infusional fluorouracil followed by weekly paclitaxel Cancer Chemother Pharmacol 2008 ; 62 4 667 – 672
Silver DP , Richardson AL , Eklund AC et al. Efficacy of neoadjuvant Cisplatin in triple‐negative breast cancer J Clin Oncol 2010 ; 28 7 1145 – 1153
Buzdar AU , Singletary SE , Theriault RL et al. Prospective evaluation of paclitaxel versus combination chemotherapy with fluorouracil, doxorubicin, and cyclophosphamide as neoadjuvant therapy in patients with operable breast cancer J Clin Oncol 1999 ; 17 : 3412 – 3417
Leone JP , Guardiola V , Venkatraman A et al. Neoadjuvant platinum‐based chemotherapy (CT) for triple‐negative locally advanced breast cancer (LABC): Retrospective analysis of 125 patients J Clin Oncol 2009 ; 27 suppl 15 . abstract 625.
Yi S , Uhm J , Cho E et al. Clinical outcomes of metastatic breast cancer patients with triple‐negative phenotype who received platinum‐containing chemotherapy J Clin Oncol 2008 ; 26 suppl 15 . abstract 1008.
Smith IC , Heys SD , Hutcheon AW et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel J Clin Oncol 2002 ; 20 : 1456 – 1466
Thomssen , et al. First‐Line Bevacizumab Combination Therapy in Triple‐Negative Locally Recurrent/Metastatic Breast Cancer: Subpopulation Analysis of Study MO19391 in >2000 Patients , San Antonio, Texas : Paper presented at: 2009 San Antonio Breast Cancer Symposium , December 13, 2009 . abstract 6093.
O'Shaughnessy J , Dieras V , Glaspy J , et al. Comparison of Subgroup Analyses of PFS from Three Phase III Studies of Bevacizumab in Combination with Chemotherapy in Patients with HER‐2‐Negative Metastatic Breast Cancer , San Antonio, Texas : Paper presented at: 2009 San Antonio Breast Cancer Symposium , December 10, 2009 . abstract 207.
Modi S , D'Andrea G , Norton L et al. A phase I study of cetuximab/paclitaxel in patients with advanced‐stage breast cancer Clin Breast Cancer 2006 ; 7 : 270 – 277
Carey LA , Rugo HS , Marcom PK et al. TBCRC 001: EGFR inhibition with cetuximab added to carboplatin in metastatic triple‐negative (basal‐like) breast cancer J Clin Oncol 2008 ; 26 May 20 suppl . abstract 1009.
O'Shaughnessy J , Weckstein DJ , Vukelja SJ , et al. Preliminary results of a randomized phase II study of weekly irinotecan/carboplatin with or without cetuximab in patients with metastatic breast cancer , San Antonio, Texas : Paper presented at: 2007 San Antonio Breast Cancer Symposium , December 14, 2007 . abstract 308.
Baselga J et al. The addition of cetuximab to cisplatin increases overall response rate and progression‐free survival in metastatic triple‐negative breast cancer: results of a randomized phase II study (BALI‐1) ESMO 2010 135 . Program Book.
Tan AR , Swain SM Therapeutic strategies for triple‐negative breast cancer Cancer J 2008 ; 14 6 343 – 351
Burstein HJ , Elias AD , Rugo HS et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane J Clin Oncol 2008 ; 26 11 1810 – 1816
Finn RS , Dering J , Ginther C et al. Dasatinib, an orally active small molecule inhibitor of both the src and abl kinases, selectively inhibits growth of basal‐type/“triple‐negative” breast cancer cell lines growing in vitro Breast Cancer Res Treat 2007 ; 105 3 319 – 326
Bryant HE , Schultz N , Thomas HD et al. Specific killing of BRCA2‐deficient tumours with inhibitors of poly(ADP‐ribose) polymerase Nature 2005 ; 434 7035 913 – 917
Farmer H , McCabe N , Lord CJ et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy Nature 2005 ; 434 7035 917 – 921
Tutt A , Robson M , Garber JE et al. Oral poly(ADP‐ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof‐of‐concept trial Lancet 2010 ; 376 : 235 – 244
Doane AS , Danso M , Lal P et al. An estrogen receptor‐negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen Oncogene 2006 ; 25 28 3994 – 4008
Author notes
Email alerts, citing articles via.
- Advertising and Corporate Services
- Journals Career Network
Affiliations

- Online ISSN 1549-490X
- Print ISSN 1083-7159
- Copyright © 2024 Oxford University Press
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Triple-negative breast cancer: recent treatment advances
Affiliations.
- 1 Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia.
- 2 Sir Peter MacCallum Department of Oncology, University of Melbourne, Melbourne, Victoria, Australia.
- PMID: 31448088
- PMCID: PMC6681627
- DOI: 10.12688/f1000research.18888.1
Triple-negative breast cancer (TNBC) is a breast cancer subtype renowned for its capacity to affect younger women, metastasise early despite optimal adjuvant treatment and carry a poor prognosis. Neoadjuvant therapy has focused on combinations of systemic agents to optimise pathological complete response. Treatment algorithms now guide the management of patients with or without residual disease, but metastatic TNBC continues to harbour a poor prognosis. Innovative, multi-drug combination systemic therapies in the neoadjuvant and adjuvant settings have led to significant improvements in outcomes, particularly over the past decade. Recently published advances in the treatment of metastatic TNBC have shown impressive results with poly (ADP-ribose) polymerase (PARP) inhibitors and immunotherapy agents. Immunotherapy agents in combination with traditional systemic chemotherapy have been shown to alter the natural history of this devastating condition, particularly in patients whose tumours are positive for programmed cell death ligand 1 (PD-L1).
Keywords: Immunotherapy; Triple negative breast cancer.
Publication types
- Research Support, Non-U.S. Gov't
- Immunotherapy*
- Neoadjuvant Therapy
- Triple Negative Breast Neoplasms* / therapy
Grants and funding

- Adolescent and Young Adult Cancer
- Bile Duct Cancer
- Bladder Cancer
- Brain Cancer
- Breast Cancer
- Cervical Cancer
- Childhood Cancer
- Colorectal Cancer
- Endometrial Cancer
- Esophageal Cancer
- Head and Neck Cancer
- Kidney Cancer
- Liver Cancer
- Lung Cancer
- Mouth Cancer
- Mesothelioma
- Multiple Myeloma
- Neuroendocrine Tumors
- Ovarian Cancer
- Pancreatic Cancer
- Prostate Cancer
- Skin Cancer/Melanoma
- Stomach Cancer
- Testicular Cancer
- Throat Cancer
- Thyroid Cancer
- Prevention and Screening
- Diagnosis and Treatment
- Research and Clinical Trials
- Survivorship

Request an appointment at Mayo Clinic

New study finds triple-negative breast cancer tumors with an increase in immune cells have lower risk of recurrence after surgery
Share this:.
By Kelley Luckstein
A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
TNBC is a breast cancer subtype that does not respond to drugs that target the estrogen receptor or the HER2 protein. It grows rapidly, is more likely to spread beyond the breast before diagnosis and is more likely to recur than other breast cancers. TNBC represents about 15% of all breast cancers and is more common in younger people and in women of African American, Hispanic and Indian descent. Immune cells, also known as tumor-infiltrating lymphocytes, or TILs, are naturally existing immune system cells that can move from the bloodstream into a tumor and can recognize and destroy cancer cells.

"This is an important finding because it highlights that the abundance of TILs in breast tissue is a prognostic biomarker in people with early-stage triple-negative breast cancer, even when chemotherapy is not administered," says Roberto Leon-Ferre, M.D. , a breast medical oncologist at Mayo Clinic Comprehensive Cancer Center and first author of the study. "The study's findings may inspire future clinical trials to explore whether patients with a favorable prognosis (high TILs) can avoid intensive chemotherapy regimens."
"This meta-analysis confirms robustly the prognostic value of TILs that we have previously reported in TNBC patients treated with chemotherapy and expands it to patients treated without chemotherapy," says Sarah Flora Jonas, Ph.D., a statistician at Gustave Roussy and co-first author of the study. "Future studies may allow the use of this biomarker along with standard clinicopathological factors to inform treatment decisions in TNBC patients."
"Of interest, the first report suggesting that an increased number of immune cells being associated with better prognosis in breast cancer patients was described by doctors at Mayo Clinic more than 100 years ago," says Roberto Salgado, M.D., co-chair of the International Immuno-Oncology Biomarker Working Group; co-lead of the study; and pathologist from the Peter MacCallum Cancer Centre, Melbourne, Australia, and ZAS Hospitals, Antwerp, Belgium. "It took a global effort and a century later to reexamine this biomarker and bring it closer to application in patient care."

"TILs are not currently measured or reported in the routine examination of tissue samples of breast cancer," says co-senior author, Matthew Goetz, M.D. , a medical oncologist at Mayo Clinic Comprehensive Cancer Center and the Erivan K. Haub Family Professor of Cancer Research Honoring Richard F. Emslander, M.D. "While prior studies have focused on measuring TILs in people treated with chemotherapy, this is the largest study to comprehensively demonstrate that the presence of TILs influences the natural behavior of breast cancer in people who have surgery and/or radiation with no additional medical treatment."
For this study, Mayo Clinic and Gustave Roussy researchers, in collaboration with the International Immuno-Oncology Biomarker Working Group, led 11 additional groups to collect data on 1,966 participants with early-stage TNBC who only underwent surgery with or without radiation therapy but did not receive chemotherapy. The participants had been followed for a median of 18 years. The results showed that higher levels of TILs in breast cancer tissue were associated with lower recurrence rates among participants with early-stage TNBC.
"Five years after surgery, 95% of participants with small tumors, stage 1 TNBC, and whose tumors had high TILs were alive, compared to 82% of patients whose tumors had low TILs. Importantly, the breast cancer recurrence rate was significantly lower among patients whose tumors had high TILs," says co-senior author, Stefan Michiels, Ph.D. , head of Oncostat team, Gustave Roussy, Inserm U1018, University Paris-Saclay. "With nearly 2,000 participants involved in the study, we have now assembled the largest international cohort across three continents of people with TNBC in which the primary treatment was surgery without chemotherapy."
"The results of this study could lead to a recommendation to include TILs in the pathology reports of early-stage TNBC worldwide, as it has the potential to inform clinicians and patients when they discuss treatment options," says Dr. Salgado.
Furthermore, this biomarker would only require a visual evaluation by a pathologist looking through a microscope, meaning there are no additional costs associated with identifying the presence of immune cells. This could be particularly beneficial to regions with limited resources, adds Dr. Leon-Ferre.
Most people with early-stage TNBC undergo chemotherapy either before or after surgery, including people with stage 1 breast cancer. Most people receive multiple chemotherapy drugs in combination, which can cause significant side effects. Currently, the main factors considered to determine the course of chemotherapy treatment for each person are the tumor size and whether the cancer has spread to the lymph nodes. However, the authors identified that the number of TILs further influences the risk of future recurrence.
The researchers plan to evaluate TILs as biomarkers in prospective clinical trials evaluating chemotherapy selection based on TIL levels. Ongoing efforts to conduct additional research with other potential biomarkers are underway.
For a complete list of authors, disclosures and funding, see the full paper here .
Learn more about breast cancer and find a clinical trial at Mayo Clinic.
Join the Breast Cancer Support Group on Mayo Clinic Connect , an online community moderated by Mayo Clinic for patients and caregivers.
Also, read these articles:
- Understanding triple-negative breast cancer and its treatment
- 17-gene signature linked to remission after triple-negative breast cancer treatment
A version of this article was originally published as a press release on the Mayo Clinic News Network .
Related Posts

Dr. Maria Linnaus discusses the link between obesity and cancer risk and how bariatric surgery may reduce that risk.

Dr. Dawn Mussallem, a Mayo Clinic lifestyle medicine expert, says consuming soy products in moderation can be beneficial.

Dr. Jesse Bracamonte discusses the importance of cancer screenings as well as preventive screenings for diabetes and cardiovascular disease.
UNIVERSITY OF ILLINOIS URBANA-CHAMPAIGN

- Wang Lab Develops ‘Drug Depot’: A Novel Technology for Prevention of Post-Surgical Triple-Negative Breast Cancer Recurrence
May 14, 2024 | Cancer Center News
Cancer Center at Illinois (CCIL) member Hua Wang , professor of materials science and engineering, published new research in Materials Today Bio demonstrating the development of a novel technology to prevent post-surgical recurrence of breast cancer, especially triple-negative breast cancer (TNBC).
An estimated 310,000 American women will be diagnosed with breast cancer in 2024, according to the American Cancer Society. TNBC accounts for as many as 15% of these diagnoses and is statistically more common in women under age 40, Black women, or women with a BRCA1 mutation. Lumpectomy followed by adjuvant chemotherapy or radiation therapy is the mainstream clinical treatment, and yet TNBC patients experience high rates of post-surgical cancer recurrence.
The underlying challenge for researchers and clinicians is the ability to deliver anticancer drugs to the tumor resection site whenever needed after surgery.

Left to right: Yang Bo and Hua Wang
To address this fundamental problem, the Wang lab designed an innovative method with promise to reduce high cancer recurrence rates after surgical resection. Considering the predominant clinical practice of grafting autologous fat tissue for breast reconstruction, Wang’s lab developed a fat tissue-based “drug depot” to catch anticancer therapeutic “cargo” from the bloodstream, which then gradually releasess to neighboring cells. This adaptive technology is novel in its methodology and practical in leveraging the existing surgical reconstruction process.
“This research project is more personal, motivated by personal experience of someone close to me who suffered from breast cancer. Surgical resection and the concern about potential cancer recurrence is a difficult experience for women with breast cancer,” said Wang. “We wanted to find a solution that would limit further complications and cancer recurrence. After the conversations with the breast cancer oncologists, we asked ourselves, ‘How can we find a strategy to label the fat tissue used for the breast reconstruction and convert it into a targetable drug depot?’ We wanted to adapt our strategy to clinical practice and leverage the strengths of our lab’s existing chemical tagging technology. We’ve been using this technology for quite some time, and its proven efficacy seemed appropriate for this common breast cancer problem.”
Wang’s “depot and cargo method” introduces their lab’s unique click chemistry, metabolic glycan labeling technology into the breast cancer reconstruction procedure. Metabolic labeling of primary cells, as demonstrated by Wang’s team, is not currently reported in research methods. The team demonstrated that azido-labeled primary adipocytes, upon grafting to the tumor resection site, can act as a “drug depot,” capturing circulating dibenzocyclooctyne (DBCO) drugs in vivo via efficient click chemistry. Using a linkage between DBCO and the drug, the conjugated drugs (or “cargo”) can be gradually released from adipocyte membranes to affect neighboring residual tumor cells for the improved prevention of tumor recurrence and metastasis in a 4T1 TNBC model. This targeting approach holds promise for wide application to other cancer therapeutics.
First author of the paper Yang Bo, who now works as a postdoctoral researcher at the University of Washington, said of the discoveries in this research: “It was intriguing to discover that metabolically inert primary adipocytes could be labeled with azido groups by unnatural sugars. This key feature makes labeled adipocyte a future platform for post-operative targeted treatment of residual cancer cells. Knowing that our targetable drug depot holds promise for post-surgical cancer therapeutics is very encouraging. This project has inspired me to dig deeper into the clinically relevant techniques of my future research endeavors that might improve the efficacy of cancer-targeted treatments.”
Editor’s notes:
Hua Wang is an Assistant Professor of Materials Science and Engineering and is an affiliate of the Department of Bioengineering, the Materials Research Laboratory, the Beckman Institute, the Carle College of Medicine, and the Carl R. Woese Institute for Genomic Biology.
To contact Hua Wang, email [email protected]
The paper “Primary adipocytes as targetable drug depot to prevent post-surgical cancer recurrence” is available online . doi.org/10.1016/j.mtbio.2024.101020
This story was written by Jonathan King, CCIL Communications Specialist
Recent Posts
- New Photonic Crystal Approach can Enable Sensitive and Affordable Detection of Biomarkers
- CCIL Personnel Expands in 2024
- CCIL Members Awarded DOD Funding to Pursue Kidney Cancer Immunotherapy
- CEPaCT Meeting Explores Efforts to Study and Regulate Forever Chemicals
- February 2024
- January 2024
- December 2023
- November 2023
- October 2023
- September 2023
- August 2023
- February 2023
- January 2023
- December 2022
- November 2022
- October 2022
- September 2022
- August 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- February 2020
- January 2020
- December 2019
- November 2019
- October 2019
- September 2019
- August 2019
- February 2019
- January 2019
- October 2018
- August 2018
- February 2018
- January 2018
- December 2017
- November 2017
- October 2017
- August 2017
- December 2016
- Administration
- Cancer Center News
- Cancer Scholars Program
- CCIL Ambassadors
- CCIL Shared Resources
- Honors and Awards
- Student Spotlight
- TiMe Program

More research supports androgen treatment for breast cancer

Photo credit: bymuratdeniz
A study by researchers from the University of Adelaide has provided new insight into the fight against breast cancer.
The laboratory-based study was the work of co-senior authors Associate Professor Theresa Hickey and Dr Amy Dwyer together with Professor Wayne Tilley of the Dame Roma Mitchell Cancer Research Laboratories, in collaboration with researchers at Cancer Research UK (CRUK), Cambridge Institute, University of Cambridge (UK) and the Imperial College of London.
“Our study employed a relatively new technology developed by the CRUK team, which was used to identify GATA3 (a transcription factor critical for the embryonic development of various tissues) as an important interacting partner of the androgen receptor in breast cancer,” said Associate Professor Hickey.
The research, published in the journal Genome Biology , found that when the androgen receptor interacted with GATA3, it stimulated breast cancer cells to become more functionally mature.
“This study revealed an important means by which androgen receptor activity exerts anti-cancer activity in breast cancer,” said Associate Professor Hickey.
“Discovering how the androgen receptor exerts anti-cancer activity in the breast is important because the opposite happens in the prostate where androgen receptor activity promotes cancer.”
The finding supports work by the Dame Roma Mitchell Cancer Research Laboratory threesome, with Professor Tilley as senior author, published in Lancet Oncology in February. That clinical study found that the androgen receptor stimulating drug enobosarm was effective against estrogen receptor-positive breast cancer, which constitutes up to 80 per cent of all cases of this disease.
“Information from the GATA3 study supports the use of androgen receptor stimulating drugs for treatment of estrogen receptor positive breast cancer (as reported in the recent Lancet Oncology paper) and provides laboratory evidence to support this therapeutic strategy for other subtypes of disease that are not driven by the estrogen receptor." Associate Professor Theresa Hickey, University of Adelaide
“This includes the triple negative subtype of breast cancer," she said.
“Drugs that stimulate the androgen receptor are not yet part of mainstream treatment for any type of breast cancer but is gaining momentum for the treatment of estrogen receptor positive disease.
“The GATA3 study provides evidence that this new therapeutic strategy will work by providing an explanation for how it works.”
Associate Professor Hickey said she expected more developments to come from the study.
“While the current study focused on interaction between the androgen receptor and GATA3, the new technology we used to identify this interaction revealed many other factors that interact with the androgen receptor in breast cancer cells,” she said.
“We are currently investigating the importance of those other factors in mediating androgen receptor activity in breast cancer.”
- Previous page
- Open access
- Published: 12 May 2024
Ternary heterostructure-driven photoinduced electron-hole separation enhanced oxidative stress for triple-negative breast cancer therapy
- Shuqing Dong 1 , 2 , 3 na1 ,
- Yuqi Huang 2 na1 ,
- Hanrong Yan 2 ,
- Huarong Tan 2 ,
- Liying Fan 2 , 3 ,
- Minghao Chao 2 ,
- Yiping Ren 2 ,
- Ming Guan 1 ,
- Jiaxin Zhang 3 ,
- Zhao Liu 3 &
- Fenglei Gao 1 , 2
Journal of Nanobiotechnology volume 22 , Article number: 240 ( 2024 ) Cite this article
71 Accesses
Metrics details
Zinc oxide nanoparticles (ZnO NPs) stand as among the most significant metal oxide nanoparticles in trigger the formation of reactive oxygen species (ROS) and induce apoptosis. Nevertheless, the utilization of ZnO NPs has been limited by the shallowness of short-wavelength light and the constrained production of ROS. To overcome these limitations, a strategy involves achieving a red shift towards the near-infrared (NIR) light spectrum, promoting the separation and restraining the recombination of electron-hole (e − -h + ) pairs. Herein, the hybrid plasmonic system Au@ZnO (AZ) with graphene quantum dots (GQDs) doping (AZG) nano heterostructures is rationally designed for optimal NIR-driven cancer treatment. Significantly, a multifold increase in ROS generation can be achieved through the following creative initiatives: (i) plasmonic Au nanorods expands the photocatalytic capabilities of AZG into the NIR domain, offering a foundation for NIR-induced ROS generation for clinical utilization; (ii) elaborate design of mesoporous core-shell AZ structures facilitates the redistribution of electron-hole pairs; (iii) the incorporation GQDs in mesoporous structure could efficiently restrain the recombination of the e − -h + pairs; (iv) Modification of hyaluronic acid (HA) can enhance CD44 receptor mediated targeted triple-negative breast cancer (TNBC). In addition, the introduced Au NRs present as catalysts for enhancing photothermal therapy (PTT), effectively inducing apoptosis in tumor cells. The resulting HA-modified AZG (AZGH) exhibits efficient hot electron injection and e − -h + separation, affording unparalleled convenience for ROS production and enabling NIR-induced PDT for the cancer treanment. As a result, our well-designed mesoporous core-shell AZGH hybrid as photosensitizers can exhibit excellent PDT efficacy.
Introduction
Within mitochondria and peroxisomes, reactive oxygen species (ROS) have assumed pivotal roles in numerous signaling pathways, finely orchestrating physiological and pathological functions [ 1 , 2 ]. In cancerous tissues, ROS exhibit a proximate correlation with tumorigenesis and facilitates the progression of tumors; however, it has been experimentally demonstrated that elevated ROS levels manifest cytotoxic effects, leading to the demise of neoplastic cells [ 3 , 4 ]. This heightened ROS functionality primarily arises from oncogenic receptor activity. Excessive ROS levels can instigate irrevocable harm to intracellular constituents, encompassing organelles and the cytoskeleton, ultimately culminating in the demise of tumor cells [ 5 , 6 ]. Non-invasive therapeutic approaches, notably the field of photodynamic therapy (PDT), have garnered significant attention, primarily due to their capacity for precise spatiotemporal control and their minimal propensity for adverse effects, as extensively studied [ 7 , 8 , 9 ]. In the realm of nanomaterials, such as quantum dots (QDs) [ 10 , 11 ], zinc oxide nanoparticles (ZnO NPs) [ 12 ], silicon (Si), and titanium dioxide (TiO 2 ) [ 13 , 14 ], their potential as nano photosensitizers has attracted interest for their remarkable photodegradation resistance.
ZnO NPs represent essential semiconductor materials possessing intriguing photoresponsive characteristics [ 15 ]. Over time, ZnO-based NPs have exhibited considerable potential in the realm of PDT owing to their biocompatibility and remarkable ability to generate tumor-destructive ROS via mild photodynamic activation [ 16 , 17 , 18 , 19 ]. In the realm of oncological research, ZnO NPs manifest a distinct proclivity for inducing cellular toxicity specific to cancer cells. This propensity is attributed to their remarkable capacity to engender ROS and disrupt the structural integrity of the mitochondrial membrane [ 20 , 21 , 22 , 23 ]. While the potential of ZnO NPs in precisely targeting malignant cells has garnered considerable attention, it remains imperative to acknowledge the extant challenges that confront their practical application. These challenges encompass the swift recombination of e − -h + pairs, which necessitates UV light for activation [ 24 ]. In general, accelerated charge recombination often leads to a constrained generation of ROS, thereby hindering the efficacy of PDT. The limited responsiveness of ZnO NPs exclusively to UV radiation serves to constrain their utility in the treatment of deeply situated tumors [ 25 ]. This constraint stems from the inherent incapacity of UV light to effectively permeate deep tissue regions, as it is predominantly absorbed by the adjacent biological milieu.
Plasmonic metallic nanostructures, as exemplified by gold nanorods (Au NRs), offer an avenue for tailored enhancement of photocatalytic performance in semiconductors, particularly within the visible and NIR spectral regions, thereby augmenting the scope for clinical applications by extending the depth of penetration [ 26 , 27 , 28 ]. Consequently, a plethora of methodologies have been devised to amalgamate the distinctive surface plasmon resonance (SPR) inherent to Au NRs with ZnO (Zhou et al., 2021), employing core-shell architectures and coating techniques [ 29 , 30 ]. Moreover, amalgamating plasmonic Au NRs with ZnO within a precisely engineered core-shell nanoarchitecture holds the promise of enhancing charge carrier excitation and transfer, culminating in a substantial enhancement in the efficacy of photocatalytic procedures. Nevertheless, the ROS yield of this composite system, denoted as Au@ZnO (AZ), remained constrained. The conventional core-shell configuration and coating approach fail to effectively segregate the spatial distribution of energetic e − -h + pairs [ 31 ].
On the other hand, graphene’s incorporation as a co-catalyst has sparked considerable interest due to its distinct attributes, including high thermal conductivity, exceptional charge carrier mobility, expansive surface area, and mechanical stability [ 32 , 33 ]. As a co-catalyst, graphene offers notable advantages: it provides a robust scaffold for anchoring finely dispersed metallic or oxide nanoparticles, acts as a highly conductive matrix for efficient electrical contact, facilitates electron transfer from the semiconductor’s conduction band, enhancing charge separation efficiency, and serves as a co-catalyst for ROS generation, courtesy of its extensive surface area and electron mobility [ 34 , 35 ]. Hence, to enable NIR radiation-triggered PDT, the design of graphene doping strategies becomes crucial. These strategies must effectively extend the separation of hot e − -h + pairs, ultimately promoting increased ROS production.
Photothermal therapy (PTT), a highly promising approach for cancer treatment, has garnered significant attention due to its favorable treatment outcomes. PTT agents, concentrated within tumors, can effectively engage with external laser sources, generating localized heat to eradicate tumors while minimizing damage to adjacent healthy tissue [ 36 ]. Au NRs, recognized as exemplary PTT agents, exhibit robust light absorption in the NIR region, showcasing remarkable therapeutic efficacy in PTT applications.
In this study, we propose a novel approach utilizing GQDs incorporated within mesoporous AZ NPs, further functionalized with hyaluronic acid (HA). Within this framework, plasmonic Au NRs serve as an energy source, effectively generating ROS at the tumor site. Moreover, the introduced Au NRs emerge as highly promising catalysts for facilitating PTT, a modality demonstrated to proficiently kill tumor cells through the induction of apoptosis. ZnO, known for its capability to accept high-energy electrons, complements this system. Furthermore, the introduction of GQDs as dopants acts as a barrier, effectively preventing the rapid recombination of e − -h + pairs. Consequently, our designed Au@ZnO@GQDs/HA (AZGH) NPs exhibit the potential to significantly enhance PDT efficiency by mitigating e − -h + pairs recombination, all while offering an approach for the therapy associated with triple-negative breast cancer (TNBC). This innovative design we present here represents a promising avenue to elevate the efficacy of PDT, thus contributing to its broader clinical application (Scheme 1 ).
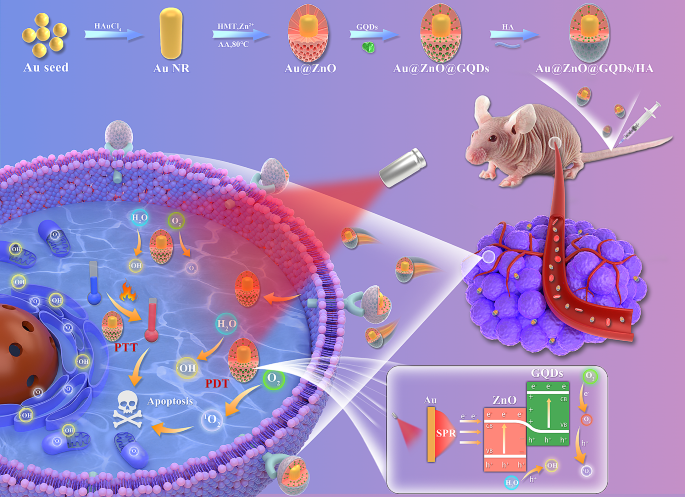
Schematic diagram of the synthesis of AZGH NPs and the therapeutic process of TNBC
Experimental
Synthesis of aunr@zno.
AuNR@ZnO core/shell nanoparticles involved the the Zn 2+ precursors hydrolysis in an alkaline milieu [ 37 ]. In brief, a solution was created by gently combining 24 mM CTAB, 12 mM AA, 24 mM freshly prepared Zn(NO 3 ) 2 , and 24 mM HMT, each in equal parts, which served as the growth solution. Subsequently, 4.0 mL CTAB-stabilized Au NRs underwent two washes via centrifugation employing deionized water. Following this, the aforementioned growth medium (12.0 mL) was added to a specific quantity of Au NRs, confined within a glass receptacle. The Zn 2+ /Au NRs volume ratio was maintained at 3:7. The pH of the resulting amalgam was titrated to 9.0 through the addition of a 0.1 M NaOH solution. The amalgamation was subsequently exposed to an 80 ℃ convection oven, where it remained tranquil for a duration of 6 h. Finally, the synthesized AuNR@ZnO nanoparticles were purified through a process of washing with deionized water and centrifugation at 8000 rpm.
Synthesis of AuNR@ZnO@GQDs
In the as-synthesized AuNR@ZnO, immersion took place within a specified volume of GQDs stock solution, accompanied by vigorous magnetic agitation. The resulting blend was stirred for a period of 5 min, followed by centrifugation at a velocity of 8000 revolutions per min for a period of 300 s.
Synthesis of AuNR@ZnO@GQDs-HA
A 25 mL aliquot of deionized water was meticulously introduced to HA (100 mg), with the HA achieving complete solubilization via ultrasonic treatment. Subsequently, the resulting AuNR@ZnO@GQDs composite was introduced into the HA solution and subjected to vigorous agitation under ambient conditions for a duration of 24 h. Following this agitation period, the ultimate product was acquired through centrifugation [ 38 ].
1 O 2 detection in vitro
To substantiate the production of 1 O 2 , the singlet oxygen sensor green (SOSG) probe had been employed. 2 mL AZGH suspension (100 μg mL − 1 ) was amalgamated within a 2 μM pre-prepared stock solution and subsequently subjected to irradiation using an 808 nm laser over varying time intervals. The resulting time-dependent fluorescence, emanating from the oxidation of SOSG, was qualitatively evaluated by means of a fluorescence microplate reader.
•OH generation in vitro
To elucidate the generation of •OH species facilitated by AZGH nanoparticles, we harnessed methylene blue (MB) as a reagent. The oxidative prowess of •OH was employed to prompt the alteration in absorption at 665 nm in MB. 2 mL aliquot of AZGH (100 μg mL − 1 ) was conjoined within a buffer solution containing MB (10 μg mL − 1 ), subjected to irradiation using an 808 nm laser with variable exposure durations. Subsequently, the reaction transpired under constant conditions at 37 °C. To probe the optical properties of the solution, an absorbance measurement was performed at a specific wavelength of 665 nm.
•OH and 1 O 2 evaluation by ESR
At ambient temperature, the generation of •OH and 1 O 2 species was efficiently trapped through the utilization of an ESR instrument. Specifically, the quantification of 1 O 2 and •OH was judiciously accomplished by employing TEMP and DMPO. To initiate the experiments, a combination of 50 mL of AZ solution (100 μg mL − 1 ), 10 mL each of DMPO and TEMP was meticulously prepared. The same rigorous protocol was consistently followed for AZG (100 μg mL − 1 ). The reaction mixture was contained within a quartz capillary, rendering its suitability for subsequent analysis. For all NIR groups, the compound underwent exposure to an 808 nm laser operating at 1.0 W cm − 2 for a duration of 10 min prior to detection.
Intracellular uptake analysis
In order to scrutinize the intracellular uptake of AZGH, a Petri dish (35 mm) was used to plate 4T1 cells (positive CD44 expression) and L929 cells (negative CD44 expression), with each dish containing either 5 × 10 4 cells through a 12 h incubation period. Subsequently, these cells were exposed to a culture medium consisting of 1.5 mL of RPMI-1640 solution, incorporating either 100 μg mL − 1 of AZGH or AZG. The incubation was conducted for durations of 1, 4, and 8 h at a constant temperature, respectively. Following this exposure, the cells underwent a triple wash with PBS and were subjected to nuclear staining with DAPI for a duration of 5 min, after which they were imaged using a CLSM.
Intracellular ROS detection
Five experimental groups, namely the control group (PBS), NIRgroup, AZ + NIR group, AZG + NIR group, and AZGH + NIR group, were established to investigate and compare the in vitro efficiency of ROS production. The intracellular ROS detection was systematically executed via CLSM, and DCFH-DA was greatly employed to facilitate this endeavor as a ROS fluorescent marker probe. 4T1 cells were subjected to an incubation period with disparate reagents in tandem with DCFH-DA (20 μM) for a stipulated temporal span of 40 min. Consecutively, the cells were subjected to irradiation via an 808 nm laser (10 min, 1 W cm − 2 ). ROS generation was observed and documented via the application of confocal laser scanning microscopy and flow cytometer.
In vitro anticancer efficacy
4T1 cells were plated in 96-well microplates and left incubate overnight. Subsequent treatments encompassed different regimens, including (1) Control (PBS), (2) NIR, (3) AZ + NIR, (4) AZG + NIR, and (5) AZGH + NIR, and the CCK-8 assay, as described previously, was employed to assess cell viability. In order to take stock of treatment effect of these diverse therapies, Calcein-AM and PI were subjected to co-staining with cells, for a duration of 50 min. Post-staining, the cells underwent triple rinsing with PBS and were subsequently visualized through the utilization of an inverted fluorescence microscope. Moreover, to quantify cellular apoptosis, flow cytometry was employed. Following 48 h incubation period, cell cultures were cleaned with PBS, followed by a 15 min staining procedure with Annexin V/propidium iodide. Ultimately, the cell apoptosis was detected by flow cytometry, Ultimately, flow cytometry was employed to detect cell apoptosis. After that, the resultant data was subjected to analysis using FlowJo software.
In the context of in vivo research, Balb/c nude mice, approximately 5 weeks old, were procured from Gempharmatech Co., Ltd. All animal-handling protocols abided by the ethical and scientific criteria stipulated and endorsed by the Experimental Animal Ethics Committee of Xuzhou Medical University. The construction of the 4T1 female nude mice tumor model was executed by injecting on the right shoulder subcutaneous by means of approximately 2 × 10 6 4T1 cells, inclusived the 120 μL PBS. Subsequently, these mice were incorporated into the ensuing experimental protocols, once the volume (computing method: (length×(width) 2 )/2) attained approximated 100 mm 3 .
Hemolysis assay
In order to ascertain the potential hemolytic effects of AZGH NPs, we introduced a 0.3 mL erythrocyte suspension from Balb/C nude mice into three distinct solutions. These solutions inclusived 1 mL deionized water, serving as the positive control, and 1 mL PBS, representing the negative control. Additionally, separate AZGH NPs dispersions were provided for varying concentrations, ranging from 12.5, 25, 50, 75, and 100 μg mL − 1 . Subsequently, these mixtures were subjected to an incubation period of 12 h at 37 °C, after which the absorbance of each specimen was assessed at a wavelength of 540 nm.
In vivo laser induced thermal imaging
IRT, standing for infrared thermal imaging, was adopted through the utilization of an IR thermal camera, while employing an excitation laser tuned to a wavelength of 808 nm. Upon the attainment of a tumor size of approximately 100 mm³, the tumor sites encompassed three distinct cohorts, for instance, (1) mice injected solely with PBS; (2) mice subjected to intravenous administration of AZG (100 μg mL − 1 ); (3) mice receiving intravenous injection of AZGH (100 μg mL − 1 ). Subsequent to that, the location of tumor was exposed to NIR laser irradiation (1.0 W cm − 2 , 10 min). Real-time imaging and data recording were carried out at various time points.
In vivo fluorescence imaging
Upon the attainment of a tumor size of approximately 100 mm³, AZGH NPs (100 ml, 5 mg kg − 1 ) were administered via tail injection to BALB/c nude mice. Subsequently, the IVIS Lumina S5 Imaging System was used to perform whole-body fluorescent imaging in vivo at 2, 4, 8, 16, and 24 h intervals subsequent to injection. Post 48 h following the injection, the mice were euthanized in accordance with ethical guidelines. Both tumors and vital organs were excised for the purpose of in vivo fluorescence imaging to scrutinize the distribution of nanoparticles within the tissues.
In vivo anticancer treatment performance
Upon reaching a tumor volume of approximately 100 mm³, the therapeutic potential of multimodal oncotherapy was investigated. In accordance with the experimental design, mice were apportioned into one of five cohorts ( n = 4) at random, as delineated below: (1) Control (PBS); (2) NIR; (3) AZ + NIR; (4) AZG + NIR; (5) AZGH + NIR. A uniform dosage of the test agents (100 μg mL -1 , 200 μL) was administered to all mice via the tail vein. Following a 24 h post-injection interval, each cohort underwent irradiation employing NIR laser, delivering a radiant power of 1.0 W cm -2 ; for a duration of 10 min. The progression of the treatment protocol was meticulously monitored through daily assessments of mice weights, dimensions, and tumor volumes throughout the duration of the study. Upon conclusion of the treatment regimen, marking the 14th day, all mice were dealt by the humane euthanization. Subsequently, the harvested tumors underwent a comprehensive assessment, encompassing photography, weight measurement, and thereafter, histological inspection through the application of hematoxylin and eosin (H&E) staining.
Statistical analysis
All experimental results were presented in the form of mean values ± S.D. Statistical comparisons were conducted using a one-way analysis of variance (ANOVA). *** p < 0.001, ** p < 0.01, or * p < 0.05.
Results and discussion
Preparation and characterization of the azgh nps.
Mesoporous core-shell heterostructures of AZGH were successfully fabricated employing a systematic four-step technique. Precisely, uniform Au NRs were synthesized with an average aspect ratio of approximately 7:1 (Figure S1 A) [ 39 ]. AZ were synthesized through an ascorbic acid-assisted growth approach, building upon previous design with certain refinements [ 37 ]. Following this, uniform AZ core-shell nanostructures was achieved by means of the deposition of a mesoporous ZnO layer around the Au NRs (Figure S1 B). Figure S1 C shows the typical HRTEM image of GQDs. The features of GQDs were elucidated in Figure S2 . UV illumination at 365 nm, GQDs suspended in water exhibit vibrant green fluorescence [ 40 ]. The PL spectra of GQDs exhibit a gradual increase at 515 nm, whenthe concentration varies within the range of 12.5 to 200 μg mL − 1 . It is worth noting that the PL spectra of AZGH reveal analogous emission peaks to those observed with GQDs, providing initial evidence substantiating the successful doping of GQDs into AZGH (Fig. 1 F). Subsequently, the formation of AZG ensued through the rapid injection of a well-suited mixture of GQDs into the mesoporous core-shell nanostructures of AZ at ambient temperature (Fig. 1 A). The final step involved the loading of the targeting agent HA onto AZG to yield AZGH NPs, which exhibited a distinctive elliptical configuration, as showed by the TEM images (Fig. 1 D), boasting an average diameter of around 180 nm (Fig. 1 B). The AZGH NPs achieve homogeneous dispersion under physiological conditions, maintaining their average size virtually unaltered over a 24 h duration (Figure S3 ), which is of paramount importance for biological applications. Initial validation of AZGH NPs were investigated utilizing UV–vis spectroscopy (Fig. 1 E). The absorption peak pertaining to Au nanorods is conspicuously situated at approximately 790 nm. Notably, the UV-vis extinction spectra of AZ NPs reveal a prominent redshift in the longitudinal SPR bands when compared to their Au NRs counterparts. A discernible peak emerged at 352 nm, signifying that the surface of the Au NRs was successfully encased with the formation of a ZnO shell structure. The UV-vis spectra of AZG NPs exhibit a prominent peak at around 280 nm, a distinctive attribute attributed to the presence of GQDs. The investigation of the electronic structure and chemical composition about AZG NPs was undertaken through the application of XPS. Presented in Fig. 1 I were the high-resolution XPS spectra delineating the core levels of Au (4f), Zn (2p), C (1s), and O (1s) species encompassed within the AZG NPs, while AZ NPs were Presented in Fig. 1 H. Additionally, Figure S4 A showcases the four core levels of both Au and ZnO. Notably, the prominent spectral features were discerned at binding energies of 88.8 and 86.7 electronvolts (eV), which amount to the core levels of the Au 4f 7/2 and Au 4f 5/2 states, denoting a zero valence state (Au 0 ) [ 41 ]. Conversely, the distinctive features at elevated binding energies, namely 92.2 and 90.3 eV, ascribed to the peaks of the Zn 3p 3/2 and Zn 3p 1/2 core levels, signifying a valence state of Zn 2+ [ 42 ]. In Figure S4 B, a pair of distinctive features manifests itself at binding energies of 1045.0 and 1021.9 eV, representating Zn 2p 3/2 and Zn 2p 1/2 states, respectively [ 43 ]. Furthermore, a couple of appreciably heightened signal, notably observed in the C = O and C-O-C domains, within the AZG NPs hints at the binding of C in graphene and O in ZnO (Figure S4 C). Notably, the binding energies pertaining to O1 at 531.3 eV, exhibit an overlap and are attributed to the presence of C = O and C-O chemical bonds, whereas O2 and O3, residing within the 532.1-533.1 eV range, are ascribed to the existence of C-OH [ 44 ]. Collectively, those compelling body of consequence further demonstrates that C element from GQDs is conjoined with O element from ZnO (Figure S4 D). To analyze the composition of the AZGH NPs, an elemental mapping of AZGH NPs was conducted. The findings unequivocally reveal that the AZGH NPs predominantly comprise elements from the Au, O, and Zn components (Fig. 1 G and Figure S5 ). Fourier transform infrared spectroscopy (FT-IR) further corroborated the successful synthesis of AZGH NPs. As shown in Figure S6 , the absorption peaks at approximately 3350 cm − 1 were assigned to O − H and N − H stretching vibrations from HA. The usual glycosyl peaks in the HA structure (1150 cm − 1 ) also indicates the presence of HA. Additionally, in comparison with AZG NPs, the zeta potentials of AZGH NPs exhibited negativity, thus confirming the effective loading of HA onto their surface (Fig. 1 C).
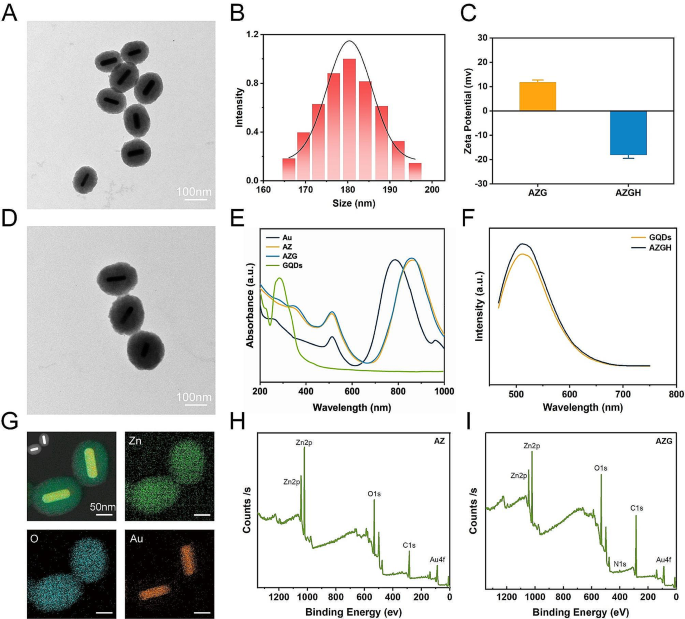
Establishment and characterization of AZGH NPs. ( A ) TEM image of AZ NPs (Scale bar: 100 nm). ( B ) The diameters distribution curve of the AZGH NPs. ( C ) Zeta potential of the AZG and AZGH NPs. ( D ) TEM image of AZGH NPs (Scale bar: 100 nm). ( E ) UV-vis spectroscopy of Au, AZ, AZG and GQDs NPs. ( F ) The PL spectra of GQDs and AZGH NPs. ( G ) EDX element mapping of AZGH NPs. ( H ) XPS spectrum of AZ NPs. ( H ) XPS spectrum of AZG NPs
ROS generation properties and photothermal properties
The crucial role of GQDs within the context of AZGH NPs involves facilitating the transfer of photoexcited electrons from Au NRs upon exposure to NIR radiation to ZnO. This efficient electron transfer process effectively impedes the recombination of e − -h + pairs, leading to the substantial generation of ROS, including •OH and 1 O 2 (Fig. 2 A). Photoluminescence (PL) emission spectroscopy stands as a potent tool for elucidating the radiative recombination dynamics of photoexcited carriers within photocatalysts. Broadly speaking, a diminished recombination rate of photoexcited electron-hole pairs corresponds to a lower PL emission intensity. In this context, we conducted PL spectral analyses on both AZ and AZG. As depicted in Figure S7 , the PL intensity of AZG experiences a noteworthy reduction compared to that of AZ. This observation implies that GQDs effectively impede the recombination of photoexcited e − -h + pairs in AZ, consequently contributing to a further enhancement in photocatalytic performance. The ROS generation was investigated via electron spin resonance (ESR) spectroscopy, while 5,5-dimethyl-1-pyrroline N-oxide (DMPO) functions as a •OH trapping agent, and 2,2,6,6-tetramethyl-4-piperidine (TEMP) serves as a 1 O 2 trapping agent [ 45 ]. As depicted in Fig. 2 B, “AZ + NIR” yielded a representative 1:1:1 triplet ESR signal, thereby confirming the generation of TEMP/ 1 O 2 adduct. The other way around, the “AZG + NIR” group exhibited a more robust ESR signal intensity, suggesting enhanced 1 O 2 generation possibly attributable to the involvement of GQDs. Similarly, the more businesslike generation of •OH was observed in “AZG + NIR”, showing the highest ESR signal amplitude associated with the DMPO/•OH adduct, displaying a typical 1:2:2:1 quartet ratio (Fig. 2 C). Moreover, 1 O 2 production of AZG NPs at different time points ranging from 0 to 10 min were monitored under NIR irradiation. Upon prolonged NIR exposure, a notable augmentation in the absorption of SOSG was observed, signifying the generation of 1 O 2 during the course of the experiment (Fig. 2 D) [ 46 ]. The production of •OH by AZG NPs was found to be contingent upon the duration of NIR exposure. As anticipated, the emblematic absorbance peak corresponding to the MB at 664 nm exhibited a decrement along with the extension to the duration of NIR exposure (Fig. 2 E) [ 47 ]. Subsequently, the assessment of •OH generation was conducted employing 3,3′,5,5′-tetramethylbenzidine (TMB) as the sensor. Initially, TMB underwent oxidation, resulting in the formation of blue oxTMB, which was quantified through spectroscopic analysis. Remarkably, a progressive elevation in the concentration of AZGH NPs led to a corresponding increase in •OH generation (Figure S8 ). In light of the observation that a pronounced absorption peak within the NIR spectra was manifested in the UV − vis − NIR absorption spectrum of AZG NPs (Fig. 1 E), AZGH NPs was designated as a potential NIR photothermal agent. In order to picture the temperature elevation about AZG NPs solution across different concentrations under 808 nm NIR irradiation, infrared thermal imaging techniques were employed (Figure S9 ). The photothermal heating response subsequently validated the degree of temperature elevation hinging upon the concentration of AZGH NPs, the power intension, and the duration of NIR irradiation (Fig. 2 F and G). Importantly, PTT was realized by the time of the temperature raised to ∼ 45 °C, at which point the concentration was 75 μg mL − 1 and the power density was 1 W cm − 2 . Furthermore, as exemplified in Fig. 2 H, the photothermal stabilizing power of the suspension of AZGH NPs (75 μg mL − 1 ) was demonstrated. Notably, even after subjecting the AZGH NPs to 4 cycles of repetitive NIR laser irradiation, the peak temperature remained consistent. These results collectively affirmed the exceptional capability of AZGH NPs in efficiently absorbing and converting NIR light into thermal energy, thereby offering promising prospects for effective PTT treatment (Fig. 2 I).
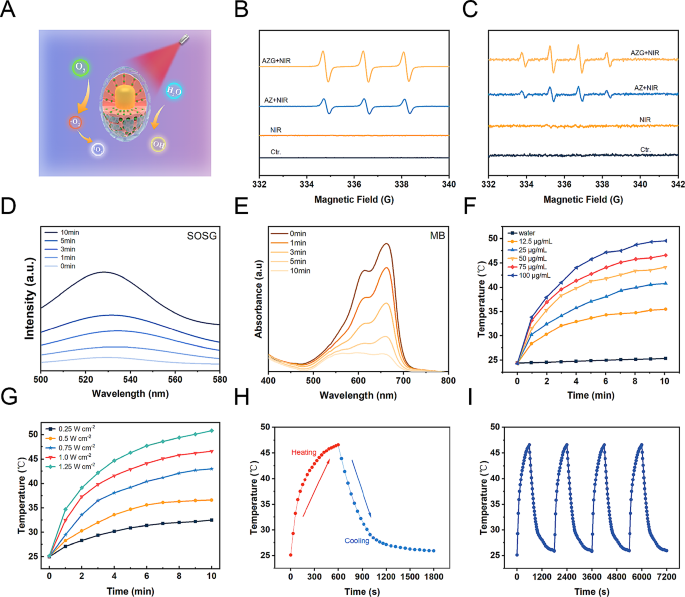
Illustration of ROS generation and o photothermal effect in AZGH NPs. ( A ) A schematic illustration of ROS generation. ( B ) ESR spectra of 1 O 2 trapped by TEMP under different groups. ( C ) ESR spectra of •OH trapped by DMPO under different groups. ( D ) Generation of 1 O 2 at different times measured using SOSG as the probe. ( E ) Production of •OH at different times using MB as the probe. ( F ) Photothermal curves of AZGH NPs aqueous solutions at various concentrations (0, 12.5, 25, 50, 75, and 100 μg mL − 1 ) under the NIR irradiation (808 nm, 1.0 W cm − 2 ) for 10 min. ( G ) Photothermal curves of 75 μg mL − 1 of aqueous AZGH NPs irradiated with NIR laser (808 nm, 0.25, 0.5, 0.75, 1.0, and 1.25 W cm − 2 ) for 10 min. ( H ) Heating and cooling curve of an aqueous dispersion of AZGH NPs at 75 μg mL − 1 concentration under 808 nm laser irradiation (1.0 W cm − 2 ). ( I ) Recycling-heating profiles of AZGH NPs aqueous solution at 75 μg mL − 1 concentration after 808 nm laser irradiation at 1.0 W cm − 2 for four laser on/off cycles
Safety properties, tumor uptake and ROS production in 4T1 cells
In order to furnish a more thorough assessment of biocompatibility, a CCK-8 evaluation was conducted to elucidate the physiological toxicity about AZGH NPs. The toxic effects of AZGH NPs were assessed by incubating them with 4T1 cells at varying concentrations ranging from 0 to 200 μg mL − 1 over a 48 h time frame. Progressively with increasing the concentrations of AZG NPs, the 4T1 cells activity decreased gradually. Notably, there was no significant decrease in 4T1 cells activity as the concentrations of AZGH NPs increased. At a concentration of 200 μg mL − 1 , the cellular viability of cells cultured with AZG NPs was only 80%, whereas the cell survival rate increased by 12% in cultures with AZGH NPs. This observation can be attributed to the enhanced biocompatibility of HA (Fig. 3 A) [ 48 , 49 ]. Subsequently, 4T1 and L929 cells underwent incubation with AZGH NPs across various concentrations and for different durations to further establish their biocompatibility. Remarkably, both cell types exhibited virtually no cytotoxicity (Fig. 3 B and C). To further corroborate the nontoxicity of AZGH NPs, flow cytometry was conducted employing Annexin V-FITC/PI staining to cell apoptosis analysis (Fig. 3 D). The intracellular endocytosis of AZGH NPs was undertaken as a preliminary step, for the sake of cellular level therapeutic mechanism. Primarily, both AZG NPs and AZGH NPs were labeled with Cy5.5. Subsequently, a series of incubation periods, spanning 1, 4, and 8 h, were employed for treating 4T1 or L929 cells with AZG-Cy5.5 and AZGH-Cy5.5. The resulting fluorescence signal within the cells was then captured using a laser scanning confocal microscope. Compared to the 4T1 cells exposed to AZG-Cy5.5, a robust red fluorescence signal was discerned in those subjected to AZGH-Cy5.5 treatment over the course of time, signifying a heightened uptake of AZGH NPs (Fig. 3 E). Furthermore, L929 cells exhibiting diminished CD44 expression demonstrated a notably diminished fluorescence intensity on their cell surfaces compared to the temporal progression observed in 4T1 cells (Figure S10 ). In order to ascertain that AZGH NPs can produce ROS efficiently, DCFH-DA known as a fluorescent redox probe was utilized to detect ROS in cell experiments. As a cell permeability tracer, DCFH-DA can be oxidized instantaneously upon interaction with ROS to yield extremely high flourescent 2’, 7’-dichlorofluorescein (DCF) molecules [ 50 ]. Upon exposure to NIR irradiation, AZ NPs incited a discernible level of ROS production. In comparison, AZG NPs exhibited an augmented ROS generation, as evidenced by the intensified DCF fluorescence, a phenomenon explicable by the synergistic catalytic activity augmentation facilitated by the introduction of GQDs. Notably, the zenith of ROS accumulation was achieved with AZGH NPs, and this heightened ROS production was attributed to the enhanced targeting of 4T1 cells (Fig. 3 F). Moreover, intracellular ROS levels were evaluated through flow cytometry analysis, aligning with the heightened ROS levels observed in DCFH-DA analysis (Figure S11 ). Consequently, the efficacy of AZGH NPs in elevating intracellular ROS levels in 4T1 cells had been established.
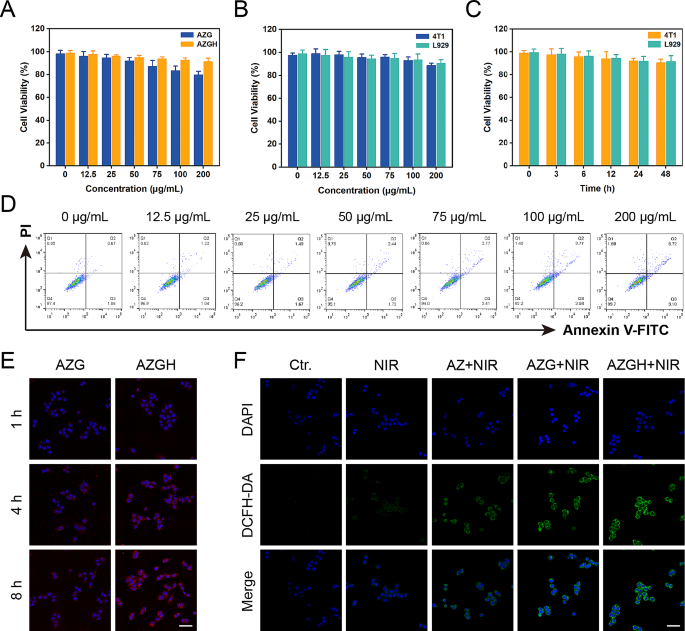
Safety properties, tumor uptake and ROS-generation properties in 4T1 cells. ( A ) Cell viability of 4T1 cells incubated with different concentrations of AZG and AZGH NPs measured by the CCK-8 assay. ( B ) Cell viability of 4T1 and L929 cells after 24 h of co-incubation with different AZGH NPs concentrations. ( C ) Cell viability of 4T1 and L929 cells co-incubation with different incubation times of AZGH NPs. ( D ) Apoptosis/necrosis of 4T1 cells after co-incubation with various dosages of AZGH NPs. ( E ) Fluorescence images of 4T1 cells co-incubation with AZG or AZGH NPs after 1, 4 and 8 h intervention (Scale bar is 50 μm). ( F ) Confocal pictures of 4T1 cells incubated with DCFH-DA after various conditions (Scale bar is 50 μm)

In vitro cellular therapy of the enhanced PDT
Afterwards, the investigation of the tumor-specific cytotoxicity induced by AZGH nanoparticles was carried out through a differential fluorescent staining targeting live and deceased cellular populations (Fig. 4 A). Within the NIR-treated group, the discernible absence of significant cellular detriment to 4T1 cells was unequivocally established, as substantiated by the prevalent presence of green fluorescent signals within the observational field. In stark contrast, the AZ + NIR group was discerned the sporadic emergence of red fluorescence, meanwhile the AZG + NIR team was indicative of moderate cellular injury discernible. Notably, the AZGH + NIR group exhibited a more pronounced cytotoxic effect. Remarkably, AZGH NPs displayed the most remarkable cellular destruction, attributable to the hybrid plasmonic nano heterostructures that achieved maximum ROS generation. The influence of AZGH NPs was further assessed by a CCK-8 assay. Specifically, AZ, AZG, and AZGH NPs, each at an equivalent concentration, were individually co-incubated with 4T1 cells for 4 h. Subsequently, cells from distinct groups underwent NIR irradiation and were further incubated for 24 h to conduct the CCK-8 experiment for therapeutic evaluation. As depicted in Fig. 4 B, pure PBS and NIR irradiation alone exhibited no detrimental effects on the cells, but significant cytotoxicity became evident by the way of the cell subjected to the AZG + NIR group It is reasonable to infer that the combination of AZGH and NIR irradiation manifests the most profound cytotoxicity. In light of the pivotal role of mitochondria in mediating metabolic dysfunction-related cellular injury, an assessment of mitochondrial status was undertaken through the evaluation of mitochondrial membrane potential (MMP) via the utilization of the cationic carbocyanine JC-1 dye [ 51 ]. Under the presence of NIR irradiation, AZ NPs induced a modest level of mitochondrial dysfunction, attributed to a limited production of ROS. On the other hand, heightened green fluorescent intensity further confirmed that AZG NPs caused a more substantial degree of mitochondrial damage. Predictably, the most extensive disruption of mitochondrial function was elicited by AZGH NPs, closely aligning with the outcomes of the CCK-8 assay (Fig. 4 C). Ultimately, a quantitative evaluation of mitochondrial condition was performed by the calculation of the mean fluorescence intensity (MFI) for both the monomeric form (Fig. 4 D) and the aggregate form (Fig. 4 E). Moreover, the therapeutic efficacy was also validated through precise flow cytometry apoptosis analysis employing the established Annexin V–FITC/PI dual staining methodology. Notably, the findings underscored that AZGH in conjunction with NIR irradiation exhibited the most pronounced therapeutic outcome (Fig. 4 F).
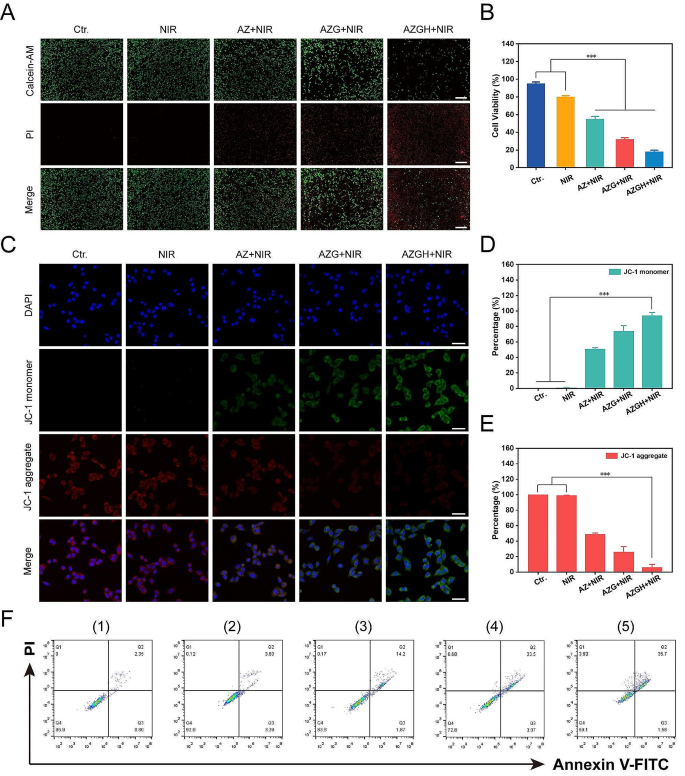
The combined treatment efficacy of AZGH NPs in 4T1 cells. ( A ) 4T1 cells dyed with AM/PI calcein in fluorescence microscope images following different treatments (Scale bar is 100 μm); ( B ) Cell viability of 4T1 cells treated with Control, NIR, AZ, AZG and AZGH NPs. ( C ) The confocal images of 4T1 cells that were treated with JC-1 staining (Scale bar is 50 μm). Fluorescence intensity analysis of monomeric form ( D ) and aggregate form E ). F ) The Annexin V-FITC and PI staining assays were performed using flow cytometry after various treatments. (1) control (PBS); (2) NIR; (3) AZ + NIR; (4) AZG + NIR; (5) AZGH + NIR
In vivo biological safety evaluation, targeting effects and phototherapeutic efficacy
In anticipation of in vivo investigation into the antitumoral efficacy, a comprehensive assessment of systemic toxicity was executed to validate the biosafety. Initially, a hemolytic assay was employed, wherein AZGH NPs at elevated concentrations of up to 100 mg mL − 1 exhibited a low hemolytic rate of less than 5%, thereby affirming their remarkable hemocompatibility (Figure S12 ). Subsequently, an acute toxicity examination was conducted via intravenous administration of three various dosages of AZGH NPs (50, 100, and 200 mg kg − 1 ) in mice. Upon the 14th day post-intravenous administration, the outcomes stemming from blood biochemistry assays demonstrated unaltered hepatic and renal function indices. Notably, the activities of ALT and AST, alongside BUN and CREA levels, exhibited no discernible deviations in the mice treated with AZGH NPs, as juxtaposed to the control counterpart (Figure S13 ). Additionally, in the blood routine examination, WBCs, RBCs, HGB, and PLT all remained within the normal range, irrespective of the various doses of AZGH NPs (Fig. 5 A-D and S14 ). In order to facilitate a comprehensive observation of the biological distribution about AZG NPs and AZGH NPs in 4T1 tumor-bearing mice prior to initiation of in vivo therapy, we made use of a fluorescence imaging technique in vivo and ex vivo. As the temporal progression unfolded, both AZG NPs and AZGH NPs displayed a progressive increase in their respective fluorescence intensities. At the 24 h mark post-injection, AZG NPs retained a relatively weak fluorescence, primarily on account of the enhanced permeability and retention (EPR) effect. Conversely, AZGH NPs manifested a heightened fluorescence intensity surpassing that of AZG NPs, signifying a greater accumulation of AZGH NPs within the tumors due to their specific HA-targeting effect (Fig. 5 E). In order to gain deeper insights into the metabolism of AZG NPs and AZGH NPs within the mice organs, an examination was conducted on the fluorescence signal present in key organs, including the heart, liver, spleen, lung, kidney, and tumor, 12 h after injection. The presence of fluorescence signal within the tumors suggested a substantial accumulation of AZGH NPs at the tumor sites, thereby augmenting the potential for subsequent therapy (Fig. 5 F). Subsequently, in order to more intuitively understand the temperature variations in different nanoparticles after laser irradiation in vivo, we employed infrared thermal imager to capture photothermal image technology for monitoring. The protracted duration of irradiation yielded a discernible augmentation in the PTI signals localized at the tumor site, thereby signifying a substantial elevation in temperature. After 10 min laser irradiation, a rapid elevation in temperature was distinctly observed in the tumor site of the murine model, reaching ∼ 49 °C in the AZG NPs and ∼ 44 °C with AZGH NPs (Fig. 5 G and S15 ). Notably, the cellular membrane of 4T1 cells was adorned by an abundance of CD44 receptors, thereby elucidating the conspicuous thermal disparity discernible at the tumor locus across the two aforementioned NPs. This receptor-rich condition substantially expedited the intracellular uptake of AZGH NPs, resulting in a substantial enhancement of active targeting. Distinct 3D photothermal images were obtained for each group, depicting temperature fluctuations (Fig. 5 H). In conclusion, the excellence of PT capacity in AZGH NPs was unequivocally validated.
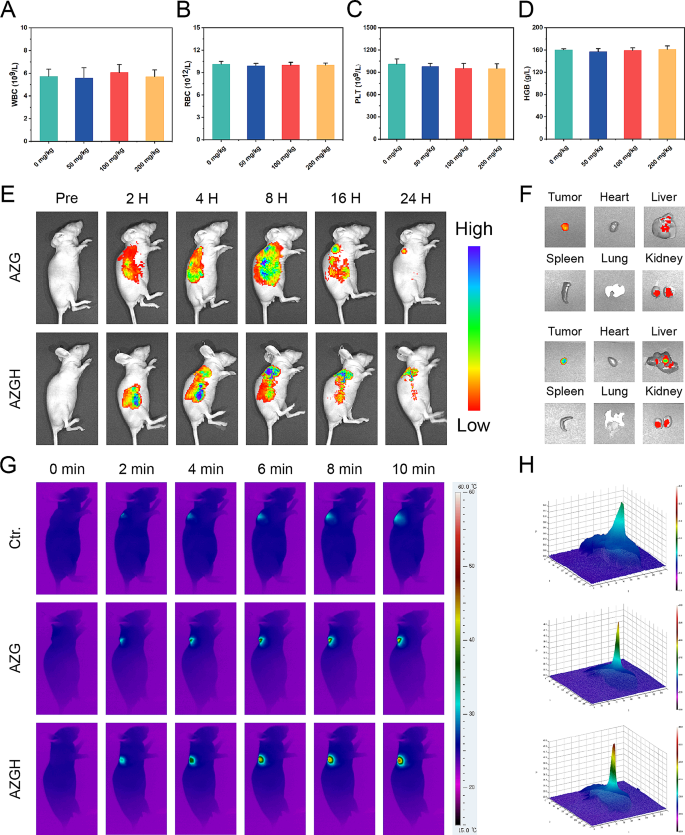
The acute toxicity, targeting effects and phototherapeutic efficacy of AZGH NPs in vivo on Balb/C nude mice. A - D ) Blood routine examination on day 14 following i.v. injection of AZGH NPs at various dosages (0, 50, 100, and 200 mg kg − 1 ). E ) Real-time fluorescence images captured in 4T1 tumor-bearing mice following intravenous injection of Cy5 labeled AZG NPs and AZGH NPs. F ) Fluorescence pictures of the tumor and key organs were taken at the middle stage of the examination. G ) After 24 h of tail vein injection of PBS, AZG or AZGH, the mice were irradiated using a NIR laser for 10 min (NIR: 808 nm, 1.0 W cm − 2 ) and photothermal images within the tumor region were subsequently recorded. H ) The corresponding 3D photothermal images of the tumor areas of the mice were recorded after 10 min
Antitumor effect in vivo
Motivated by the remarkable cytotoxicity exhibited at the cell-based assays, the therapeutic effectiveness of AZGH NPs in inhibiting tumor growth was exhaustively evaluated in murine model harboring 4T1 tumor. To this end, the murine model was initially injected with AZGH NPs (equivalent AZ and AZG content) through the tail vein, followed by NIR irradiation directed towards the lesion site 24 h post injection (Fig. 6 A). In comparison to the PBS control group, it became evident that NIR irradiation had little impact on altering tumor growth, signifying a marginal impact of exogenous stimulation throughout the treatment regimen. Conversely, the expansion of tumors was partly impeded through the application of AZ + NIR and AZG + NIR, owing to the manifestation of their cytotoxic PDT effects. Remarkably, the AZGH + NIR group showed the most fascinating suppression impact on tumor growth after a span of 14 days post inoculation, displaying a remarkably advanced tumor inhibition rate of 94.2%, a phenomenon likely ascribed to the enhanced ROS generation induce 4T1 cells apoptosis (Fig. 6 B). Furthermore, our findings demonstrated that the survival rates of mice subjected to AZGH NPs injection surpassed 75% upon exposure to NIR irradiation. However, all mice treated with NIR only died after 20 days, which indicated the remarkable anti-tumor efficiency of AZGH NPs under NIR irradiation (Fig. 6 D). Upon the conclusion of the therapeutic intervention, the potent eradication of tumor by AZGH NPs was additionally corroborated through the quantitative assessment of solid tumors from dissected mice (Fig. 6 B and S16 ). Concurrently, the mice body weight exhibited no significant fluctuations throughout the treatment duration, indicating a negligible deleterious impact of said therapeutic regimen on murine metabolic homeostasis (Fig. 6 C). Histological examination employing hematoxylin and eosin (H&E) stained sections of vital organs, including the heart, liver, spleen, lung, and kidney derived from the entire cohort of mice, revealed an absence of any discernible pathological anomalies, thereby underscoring the remarkable biocompatibility of AZ, AZG, and AZGH NPs (Fig. 6 E and S17 ). Finally, to elucidate the in vivo tumor inhibition mechanism of the AZGH nanase combined with NIR irradiation strategy, we conducted TUNEL and ROS staining on diverse sets of tissue sections (Fig. 6 F and G). Employing the TUNEL technique to visualize processed DNA fragments, we observed that AZGH nanase, when coupled with NIR irradiation, induced apoptosis and denucleation in the majority of tumor cells, underscoring the substantial DNA damage inflicted (Fig. 6 F). DHE fluorescence demonstrated a noteworthy increase in ROS levels within tumor tissues upon simultaneous exposure to AZGH NCs and NIR (Fig. 6 G). These findings collectively suggest that AZGH NRs exert a tumor growth inhibitory effect on breast cancer without causing significant harm to vital organs during treatment.
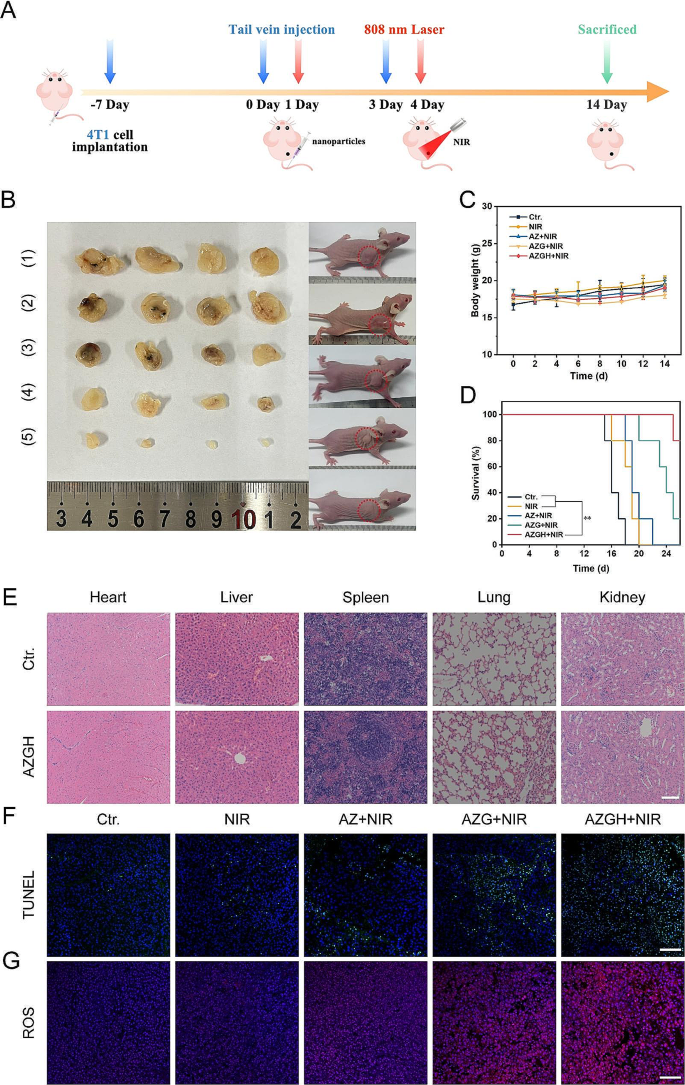
In vivo antitumor efficacy in 4T1 tumor-bearing mice. ( A ) Schematic illustration and timetable for the cancer treatment. ( B ) Photographs of the mice and tumors following the various groups. (1) control (PBS); (2) NIR; (3) AZ + NIR; (4) AZG + NIR; (5) AZGH + NIR. Body weight curves C ), and curves of survival D ) in mice given therapy. E ) H&E staining of the key organs slices in Ctr. and AZGH groups (Scale bar is 100 μm). F ) TUNEL staining of the tumor slices in different groups (Scale bar is 100 μm). G ) ROS staining of the tumor slices in different groups (Scale bar is 100 μm)
Conclusions
In brief, to demonstrate the feasibility and effectiveness of synchronous PDT and PTT treatments for TNBC, we synthesized AZGH NRs possessing good biocompatibility. The nano heterostructures of AZGH is established through the integration of GQDs into AZ core-shell structure and successfully exploited in advancing tumor elimination via a ROS generation mechanism. Comparative analysis with AZ NPs reveals that the as-synthesized AZG NPs heterostructure enhances ROS production when subjected to NIR actuation. This augmented efficiency in generating 1 O 2 and •OH is attributed to the rapid separation of e − -h + pairs. Moreover, the decorated HA can target 4T1 cells and increase the content of AZGH decomposition in 4T1 cells, and the photocatalytic activity effectively play the role in combination therapy. Meanwhile, Au NRs, acknowledged as exemplary PTT agents, demonstrate pronounced light absorption within the NIR spectrum, efficiently instigating apoptosis in tumor cells. The results have demonstrated that the designed AZGH NPs has potent ability to eradicate tumor subjected to NIR irradiation. This study introduces a promising approach to design an efficient photosensitizer for enhancing photocatalysis therapy through the facilitation of electrons transfer for clinical application.
Data availability
No datasets were generated or analysed during the current study.
Sies H, Belousov VV, Chandel NS, Davies MJ, Jones DP, Mann GE, et al. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat Rev Mol Cell Biol. 2022;23:499–515.
Article CAS PubMed Google Scholar
Shao F, Han J, Tian Z, Wang Z, Liu S, Wu Y. Synergistic ROS generation and directional overloading of endogenous calcium induce mitochondrial dysfunction in living cells. Biomaterials. 2023;301:122284.
Wu L, Jin Y, Zhao X, Tang K, Zhao Y, Tong L, et al. Tumor aerobic glycolysis confers immune evasion through modulating sensitivity to T cell-mediated bystander killing via TNF-α. Cell Metab. 2023;35:1580–e15969.
Tong L, Chuang CC, Wu S, Zuo L. Reactive oxygen species in redox cancer therapy. Cancer Lett. 2015;367:18–25.
Xia J, Hu C, Ji Y, Wang M, Jin Y, Ye L, et al. Copper-loaded nanoheterojunction enables superb orthotopic osteosarcoma therapy via oxidative stress and cell cuproptosis. ACS Nano. 2023;17:21134–52.
Article PubMed Google Scholar
Li K, Xu K, Liu S, He Y, Tan M, Mao Y, et al. All-in-one engineering multifunctional nanoplatforms for sensitizing tumor low-temperature photothermal therapy in vivo. ACS Nano. 2023;17:20218–36.
Ding F, Liu J, Ai K, Xu C, Mao X, Liu Z et al. Simultaneous activation of pyroptosis and cGAS-STING pathway with epigenetic/ photodynamic nanotheranostic for enhanced tumor photoimmunotherapy. Adv Mater. 2023;e2306419.
Palanikumar L, Kalmouni M, Houhou T, Abdullah O, Ali L, Pasricha R, et al. pH-responsive upconversion mesoporous silica nanospheres for combined multimodal diagnostic imaging and targeted photodynamic and photothermal cancer therapy. ACS Nano. 2023;17:18979–99.
Article CAS PubMed PubMed Central Google Scholar
Liu B, Jiao J, Xu W, Zhang M, Cui P, Guo Z, et al. Highly efficient far-red/NIR-absorbing neutral ir(III) complex micelles for potent photodynamic/photothermal therapy. Adv Mater. 2021;33:e2100795.
Jiang Y, Weiss EA. Colloidal quantum dots as photocatalysts for triplet excited state reactions of organic molecules. J Am Chem Soc. 2020;142:15219–29.
Chen LL, Zhao L, Wang ZG, Liu SL, Pang DW. Near-infrared-II quantum dots for in vivo imaging and cancer therapy. Small. 2022;18:e2104567.
Sivakumar P, Lee M, Kim YS, Shim MS. Photo-triggered antibacterial and anticancer activities of zinc oxide nanoparticles. J Mater Chem B. 2018;6:4852–71.
Guo Q, Zhou C, Ma Z, Yang X. Fundamentals of TiO 2 photocatalysis: concepts, mechanisms, and challenges. Adv Mater. 2019;31:e1901997.
Yang C, Zhu Y, Li D, Liu Y, Guan C, Man X, et al. Red phosphorus decorated TiO 2 nanorod mediated photodynamic and photothermal therapy for renal cell carcinoma. Small. 2021;17:e2101837.
Fatima H, Jin ZY, Shao Z, Chen XJ. Recent advances in ZnO-based photosensitizers: synthesis, modification, and applications in photodynamic cancer therapy. J Colloid Interface Sci. 2022;621:440–63.
Li J, Wang C, Wu S, Cui Z, Zheng Y, Li Z, et al. Superlattice nanofilm on a touchscreen for photoexcited bacteria and virus killing by tuning electronic defects in the heterointerface. Adv Mater. 2023;35:e2300380.
Ye DX, Ma YY, Zhao W, Cao H-M, Kong JL, Xiong HM, et al. ZnO-based nanoplatforms for labeling and treatment of mouse tumors without detectable toxic side effects. ACS Nano. 2016;10:4294–300.
Jin Y, Wang C, Xia Z, Niu P, Li Y, Miao W. Photodynamic chitosan sponges with dual instant and enduring bactericidal potency for treating skin abscesses. Carbohydr Polym. 2023;306:120589.
Sirelkhatim A, Mahmud S, Seeni A, Kaus NHM, Ann LC, Bakhori SKM, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nanomicro Lett. 2015;7:219–42.
CAS PubMed Google Scholar
Singh A, Kumar P, Sarkar N, Kaushik M. Influence of green synthesized zinc oxide nanoparticles on molecular interaction and comparative binding of azure dye with chymotrypsin: novel nano-conjugate for cancer phototherapy. Pharmaceutics. 2022;15:74.
Article PubMed PubMed Central Google Scholar
Anjum S, Hashim M, Malik SA, Khan M, Lorenzo JM, Abbasi BH, et al. Recent advances in zinc oxide nanoparticles (ZnO NPs) for cancer diagnosis, target drug delivery, and treatment. Cancers (Basel). 2021;13:4570.
Kundu M, Sadhukhan P, Ghosh N, Chatterjee S, Manna P, Das J, et al. pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized ZnO nanoparticles for breast cancer therapy. J Adv Res. 2019;18:161–72.
Lin LS, Wang JF, Song J, Liu Y, Zhu G, Dai Y, et al. Cooperation of endogenous and exogenous reactive oxygen species induced by zinc peroxide nanoparticles to enhance oxidative stress-based cancer therapy. Theranostics. 2019;9:7200–9.
Annam Renita A, Sathish S, Kumar PS, Prabu D, Manikandan N, Mohamed Iqbal A, et al. Emerging aspects of metal ions-doped zinc oxide photocatalysts in degradation of organic dyes and pharmaceutical pollutants - a review. J Environ Manage. 2023;344:118614.
Singh K, Neha, Kumar M, Singh H, Singh G. ZnO NPs: photocatalytic potential, mechanistic insights, favorable parameters and challenges. Materials Today: Proceedings. 2023;03:002.
Zhu H, Yuan X, Yao Q, Xie J. Shining photocatalysis by gold-based nanomaterials. Nano Energy. 2021;88:106306.
Article CAS Google Scholar
Wang D, Wang H, Ji L, Xu M, Bai B, Wan X, et al. Hybrid plasmonic nanodumbbells engineering for multi-intensified second near-infrared light induced photodynamic therapy. ACS Nano. 2021;15:8694–705.
Liu D, Xue C. Plasmonic coupling architectures for enhanced photocatalysis. Adv Mater. 2021;33:e2005738.
Zhou N, Yan R, Wang X, Fu J, Zhang J, Li Y, et al. Tunable thickness of mesoporous ZnO-coated metal nanoparticles for enhanced visible-light driven photoelectrochemical water splitting. Chemosphere. 2021;273:129679.
Zhou N, Zhu H, Li S, Yang J, Zhao T, Li Y, et al. Au nanorod/ZnO core–shell nanoparticles as nano-photosensitizers for near-infrared light-induced singlet oxygen generation. J Phys Chem C. 2018;122:7824–30.
Zhang Y, Lei P, Zhu X, Zhang Y. Full shell coating or cation exchange enhances luminescence. Nat Commun. 2021;12:6178.
Li Q, Li X, Wageh S, Al-Ghamdi AA, Yu J. CdS/graphene nanocomposite photocatalysts. Adv Energy Mater. 2015;5:1500010.
Article Google Scholar
Jayaramulu K, Mukherjee S, Morales DM, Dubal DP, Nanjundan AK, Schneemann A, et al. Graphene-based metal-organic framework hybrids for applications in catalysis, environmental, and energy technologies. Chem Rev. 2022;122:17241–338.
Wang F, Wang B, You W, Chen G, You Y-Z. Integrating au and ZnO nanoparticles onto graphene nanosheet for enhanced sonodynamic therapy. Nano Res. 2022;15:9223–33.
Machín A, Arango JC, Fontánez K, Cotto M, Duconge J, Soto-Vázquez L, et al. Biomimetic catalysts based on Au@ZnO-graphene composites for the generation of hydrogen by water splitting. Biomimetics (Basel). 2020;5:39.
Xie X, Gao W, Hao J, Wu J, Cai X, Zheng Y. Self-synergistic effect of prussian blue nanoparticles for cancer therapy: driving photothermal therapy and reducing hyperthermia-induced side effects. J Nanobiotechnol. 2021;19:126.
Yang Y, Han S, Zhou G, Zhang L, Li X, Zou C, et al. Ascorbic-acid-assisted growth of high quality M@ZnO: a growth mechanism and kinetics study. Nanoscale. 2013;5:11808–19.
Dong S, Bi Y, Sun X, Zhao Y, Sun R, Hao F, et al. Dual-loaded liposomes tagged with hyaluronic acid have synergistic effects in triple-negative breast cancer. Small. 2022;18:e2107690.
Ye X, Gao Y, Chen J, Reifsnyder DC, Zheng C, Murray CB. Seeded growth of monodisperse gold nanorods using bromide-free surfactant mixtures. Nano Lett. 2013;13:2163–71.
Chung S, Revia RA, Zhang M. Graphene quantum dots and their applications in bioimaging, biosensing, and therapy. Adv Mater. 2021;33:e1904362.
Casaletto MP, Longo A, Martorana A, Prestianni A, Venezia AM. XPS study of supported gold catalysts: the role of Au 0 and Au +δ species as active sites. Surf Interface Anal. 2006;38:215–8.
Chen PK, Lee GJ, Davies SH, Masten SJ, Amutha R, Wu JJ. Hydrothermal synthesis of coral-like Au/ZnO catalyst and photocatalytic degradation of orange II dye. Mater Res Bull. 2013;48:2375–82.
Wang Y, Zhang S, Wu X. Green light luminescence from ZnO/dodecylamine mesolamellar nanocomposites synthesized by self-assembly. Eur J Inorg Chem. 2005;2005:727–31.
Zhao Z, Fang F, Wu J, Tong X, Zhou Y, Lv Z, et al. Interfacial chemical effects of amorphous zinc oxide/graphene. Mater (Basel). 2021;14:2481.
Zhang L, Hu C, Sun M, Ding X, Cheng H, Duan S et al. BODIPY-functionalized natural polymer coatings for multimodal therapy of drug‐resistant bacterial infection. Adv Sci. 2023;10.
Wei Z, Zou H, Liu G, Song C, Tang C, Chen S, et al. Peroxidase-mimicking evodiamine/indocyanine green nanoliposomes for multimodal imaging-guided theranostics for oral squamous cell carcinoma. Bioactive Mater. 2021;6:2144–57.
He T, Jiang C, He J, Zhang Y, He G, Wu J, et al. Manganese-dioxide-coating-instructed plasmonic modulation of gold nanorods for activatable duplex-imaging-guided NIR-II photothermal-chemodynamic therapy. Adv Mater. 2021;33:e2008540.
Borhaninia M, Zahiri M, Abnous K, Taghdisi SM, Ramezani M, Alibolandi M. Self-targeted hyaluronic acid-b-poly (β-amino ester) pH-switchable polymersome for guided doxorubicin delivery to metastatic breast cancer. Int J Biol Macromol. 2023;248:125882.
Wu S, Zhang K, Liang Y, Wei Y, An J, Wang Y, et al. Nano-enabled tumor systematic energy exhaustion via zinc (II) interference mediated glycolysis inhibition and specific GLUT1 depletion. Adv Sci (Weinh). 2022;9:e2103534.
Huang L, Nie T, Jiang L, Chen Y, Zhou Y, Cai X, et al. Acidity-biodegradable iridium-coordinated nanosheets for amplified ferroptotic cell death through multiple regulatory pathways. Adv Healthc Mater. 2023;12:e2202562.
Zheng P, Ding B, Zhu G, Li C, Lin J. Biodegradable ca 2+ nanomodulators activate pyroptosis through mitochondrial Ca 2+ overload for cancer immunotherapy. Angew Chem Int Ed Engl. 2022;61:e202204904.
Download references
Acknowledgements
The authors thank the Public Experimental Research Center of Xuzhou Medical University.
This work was supported by the Natural Scientific Foundation of China (NNSFC) Project (22174123), the Innovation Group Project of Shanghai Municipal Health Commission (2019CXJQ03), Jiangsu Outstanding Youth Fund (BK20220062), Key University Science Research Project of Jiangsu Province (21KJA350003), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_2665, KYCX23_2932, KYCX22_2951).
Author information
Shuqing Dong and Yuqi Huang contributed equally to this work.
Authors and Affiliations
Department of Laboratory Medicine, Shanghai Medical College, Huashan Hospital, Fudan University, Shanghai, 200040, China
Shuqing Dong, Ming Guan & Fenglei Gao
Jiangsu Key Laboratory of New Drug Research and Clinical Pharmacy, Xuzhou Medical University, Xuzhou, 221004, China
Shuqing Dong, Yuqi Huang, Hanrong Yan, Huarong Tan, Liying Fan, Minghao Chao, Yiping Ren & Fenglei Gao
Department of Thyroid and Breast Surgery, Affiliated Hospital of Xuzhou Medical University, Xuzhou, 221004, China
Shuqing Dong, Liying Fan, Jiaxin Zhang & Zhao Liu
You can also search for this author in PubMed Google Scholar
Contributions
Shuqing Dong: All the experiment and Original draft preparation. Yuqi Huang: Synthesis of AuNR@ZnO@GQDs. Hanrong Yan: Intracellular uptake analysis. Huarong Tan: Reviewing and Editing. Liying Fan: 1O2 detection in vitro. Minghao Chao: •OH generation in vitro. Yiping Ren: •OH and 1O2 evaluation by ESR. Ming Guan: Revised the paper. Jiaxin Zhang: Supervision and Revised the paper. Zhao Liu: Supervision and Revised the paper. Fenglei Gao: Supervision and Revised the paper.
Corresponding authors
Correspondence to Ming Guan , Jiaxin Zhang , Zhao Liu or Fenglei Gao .
Ethics declarations
Ethics approval and consent to participate.
All animal experiments were approved by the Animal Protection and Ethics Committee of Xuzhou Medical University. The ethical code of animal study is 202210S023.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12951_2024_2530_MOESM1_ESM.doc
Supplementary Material 1: Materials, Apparatus, Synthesis of Au NRs, Characterization of AuNR@ZnO@GQDs-HA, Photothermal performance in vitro, Toxicity and safety studies in vitro, Mitochondrial integrity assay
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Dong, S., Huang, Y., Yan, H. et al. Ternary heterostructure-driven photoinduced electron-hole separation enhanced oxidative stress for triple-negative breast cancer therapy. J Nanobiotechnol 22 , 240 (2024). https://doi.org/10.1186/s12951-024-02530-4
Download citation
Received : 07 December 2023
Accepted : 03 May 2024
Published : 12 May 2024
DOI : https://doi.org/10.1186/s12951-024-02530-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Plasmon resonance
- Oxidative stress
- Ternary heterostructures
- e − -h + pairs
Journal of Nanobiotechnology
ISSN: 1477-3155
- Submission enquiries: [email protected]
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Breast Cancer (Dove Med Press)
Triple-negative breast cancer: treatment challenges and solutions
Joëlle collignon.
1 Medical Oncology Department, CHU Sart Tilman Liege, Domaine Universitaire du Sart Tilman, Liege, Belgium
Laurence Lousberg
Hélène schroeder, guy jerusalem.
2 University of Liege, Liege, Belgium
Triple-negative breast cancers (TNBCs) are defined by the absence of estrogen and progesterone receptors and the absence of HER2 overexpression. These cancers represent a heterogeneous breast cancer subtype with a poor prognosis. Few systemic treatment options exist besides the use of chemotherapy (CT). The heterogeneity of the disease has limited the successful development of targeted therapy in unselected patient populations. Currently, there are no approved targeted therapies for TNBC. However, intense research is ongoing to identify specific targets and develop additional and better systemic treatment options. Standard adjuvant and neoadjuvant regimens include anthracyclines, cyclophosphamide, and taxanes. Platinum-based CT has been proposed as another CT option of interest in TNBC. We review the role of this therapy in general, and particularly in patients carrying BRCA germ-line mutations. Available data concerning the role of platinum-based CT in TNBC were acquired primarily in the neoadjuvant setting. The routine use of platinum-based CT is not yet recommended by available guidelines. Many studies have reported the molecular characterization of TNBCs. Several actionable targets have been identified. Novel therapeutic strategies are currently being tested in clinical trials based on promising results observed in preclinical studies. These targets include androgen receptor, EGFR, PARP, FGFR, and the angiogenic pathway. We review the recent data on experimental drugs in this field. We also discuss the recent data concerning immunologic checkpoint inhibitors.
Introduction
In 2012, 1.7 million women worldwide were diagnosed with breast cancer (BC), and 521,900 women died from it. 1 These statistics include all subtypes of BC, but it is well known that BC is not a homogeneous disease. Four major intrinsic subtypes have been identified by genomic studies: the luminal subtypes A and B, which express hormone receptor-related genes, basal-like (BL) BC, and HER2-positive BC. 2 , 3
Triple-negative BC (TNBC) is a heterogeneous group characterized by the lack of expression of hormonal receptors and the absence of HER2 overexpression. The definition of negative estrogen receptor (ER) status by immunohistochemistry (IHC) is not concordant in the literature, with some definitions considering ER expression to be significant only if at least 10% of tumor cells express the receptors. However, the St Gallen guidelines, 4 the American Society of Clinical Oncology, 5 and the American College of Pathology 5 have defined TNBC as BC with less than 1% of tumor cells expressing the ER and progesterone receptors via IHC.
TNBC represents approximately 15% of all BCs and is characterized by shorter overall survival and an early peak of distant recurrences at 3 years after diagnosis. The majority of deaths occur in the first 5 years following initial diagnosis. Late tumor recurrences are unusual with this BC subtype and recurrences generally are not observed after 8 years. 6 TNBC has an aggressive clinical behavior, with a higher risk of both local and distant relapses that frequently present as visceral and/or brain metastases. 7 , 8 TNBCs are frequently assimilated into the intrinsic subgroup of BCs that have been described in microarray-based expression profiling research as the BL molecular phenotype. 2 However, not all TNBCs display a BL molecular phenotype on gene expression arrays. Indeed, 75% to 80% of TNBCs are actually BL cancers. Some markers that have been identified by IHC in tumor cells are also found in normal basal/myoepithelial cells of the breast, including high-molecular-weight basal cytokeratin 5/6 (CK5/6), CK14, B crystallin, CK17, epidermal growth factor receptor (EGFR) HER1, caveolin 1/2 (CAV1/2), vimentin, fascin, c-Kit, and P-cadherin. TNBC is less likely to express epithelial markers such as E-cadherin. 9 – 11 Similarly, not all BL BCs are TNBC: up to 54% of cancers in the BL subgroup do not have a TN phenotype on IHC. 11 , 12 Indeed, some BL cancers express ER or overexpress HER2. Currently, there is no international definition of TNBC/BL cancer, but the most appropriate approach to use in the absence of access to molecular profiling is a panel of four antibodies (ER, HER2, CK5/6, and EGFR HER1), which can best characterize BL tumors based on IHC criteria. 9
In a large population-based study from the California Cancer Registry, TNBCs were significantly more frequent in women under the age of 40 years. 13 TNBCs are also more frequent in women who are germ-line BRCA1 / 2 mutation carriers and in women of black race or Hispanic ethnicity. 14 Up to 19.5% of patients suffering from TNBC present BRCA1 / 2 mutations. 15 , 16 For this reason, it is recommended to test all women under the age of 60 years suffering from TNBC for BRCA1 / 2 mutations (National Comprehensive Cancer Network guidelines). 17
Currently, only chemotherapy (CT) is routinely used as systemic treatment in patients with TNBC, although in some countries bevacizumab is still added to CT in advanced BC (ABC), even in the absence of any demonstrated overall survival benefit. However, intense research is ongoing to identify actionable targets. A large number of clinical trials are ongoing that aim to improve current treatment outcomes. Better knowledge of the biology of this BC subgroup will allow us to evaluate new specific treatment approaches dedicated to this hard-to-treat disease.
We first review the available data on molecular heterogeneity and BRCA1 -associated TNBC/BL BC. Thereafter, we discuss the current treatment options and some promising new treatment approaches that include targeted treatments.
Understanding TNBC heterogeneity
Before molecular profiling confirmed the important heterogeneity in the biology of TNBC, clinical data had already indicated the existence of heterogeneous treatment responses and long-term outcomes. Some patients respond very well to neoadjuvant CT and present a pathologic complete response (pCR) at the time of surgery. Other patients present no response to neoadjuvant CT and suffer from early relapse after surgery. 6 , 18 , 19 Unfortunately, predictive factors that allow the identification of patients who will present a pCR and those who will not benefit from CT at the time of diagnosis do not exist.
The vast majority of TNBCs are high-grade invasive ductal carcinomas, but some rare cases are histologically different, such as adenoid cystic carcinoma, secretory carcinoma, medullary carcinoma, and metaplastic carcinoma. The prognosis depends on the TNBC pathological subtype. 20 – 22
The Cancer Genome Atlas Research Network used six methods to analyze primary BCs: genomic DNA copy-number arrays, DNA methylation, exome sequencing, messenger-RNA arrays, microRNA sequencing, and reverse-phase protein arrays. 23 Only in three genes did somatic mutations occur at a frequency higher than 10% across all BCs: TP53 , PIK3CA , and GATA3 . Specific mutations are more frequent in some BC subtypes. In TNBC/BL cancers, the most frequent findings were the loss of TP53 , RB1 , BRCA1 , and PIK3CA . 23 Known drivers, such as P53 , PIK3CA , and PTEN , have the highest clonal frequencies, but at the time of diagnosis, some patients present low clonality, while others have a more extensive clonal evolution, illustrating further important heterogeneity in TNBC. 24
Subtyping TNBC: clinical implications
More recently, gene expression profiling of 587 TNBCs identified six different subtypes: BL1 and BL2, an immunomodulatory (IM) subtype, a mesenchymal subtype, a mesenchymal stem like (MSL) subtype, and a luminal androgen receptor (LAR) subtype. 25 The strengths of this study were to identify further the molecular drivers in corresponding cell-line models to provide preclinical platforms for the development of effective therapies ( Table 1 ). For example, the authors showed that BL1 lines were the most sensitive to cisplatin and that the mesenchymal and MSL lines were most sensitive to the Abl/Src inhibitor dasatinib. 20
Genomic TNBC subtypes and potential therapeutic targets
Note: Data from Lehmann et al. 25
Abbreviations: BC, breast cancer; BL, basal-like; CSC, cancer stem cells; DNA, deoxyribonucleic acid; EGFR, epidermal growth factor receptor; EMT, epithelial-mesenchymal-transition; IGF-1R, insulin-like growth factor I receptor; IL, interleukin; IM, immunomodulatory; LAR, luminal androgen receptor; M, mesenchymal-like; MET, hepatocyte growth factor; mTOR, mammalian target of rapamycin; MSL, mesenchymal stem-like; NGF, nerve growth factor; PARP, poly ADP ribose polymerase; PD1, programmed cell death 1; PDL1, programmed death-ligand 1; PI3K, phosphatidylinositol 3-kinase; TNBC, triple negative breast cancer.
The same group used the intrinsic subtype tool to examine the composition of each TNBC subtype. The authors showed that all TNBC subtypes except MSL and LAR were composed primarily of the BL intrinsic subtype (BL1 [99%], BL2 [95%], IM [84%] and mesenchymal [97%]). The LAR subtype is classified as HER2 (74%) and luminal B (14%), and the MSL subtype includes BL (50%), normal-like (28%), and luminal B (14%). 20
Other gene expression analyses have also defined a claudin-low tumor subtype. 26 Molecular characterization showed that these tumors are enriched in epithelial-to-mesenchymal transition, immune system response, and stem-like features, but show low expression of luminal and proliferation-associated genes. 27
Another group used genomic profiling of 198 TNBCs to identify four TNBC subtypes: LAR, mesenchymal, BL immunosuppressed, and BL immunoactivated. 28 If compared with the results reported by Lehmann et al, 25 LAR and mesenchymal tumors fall into the LAR and MSL subtypes.
The comprehensive analysis of TNBC can lead to the improved selection of study populations that have the highest probability of responding to specific treatments. For example, TNBC molecular subtypes may respond differently to CT. The clinical validity of the genomic classification was indeed confirmed by a retrospective analysis of the response to neoadjuvant CT. The overall pCR in this study was 28%. In the BL1 subtype, the pCR was the highest (51%), 29 in comparison to 0 in the BL2 subtype and 10% in the LAR subtype, clearly showing the need to develop alternative treatments for some subgroups.
In the future, the evaluation of heterogeneity in TNBC and subtyping may lead to different therapeutic strategies. Potential targets and approaches include DNA damage and repair, immunomodulation, hormone receptor modulation, and signaling pathway inhibition.
Prospective trials will help us better understand the role of subtyping in the prediction of not only pCR but also long-term patient outcomes. New therapeutic strategies are needed for subgroups with the poorest therapeutic responses to standard CT.
DNA-damaging chemotherapy and DNA repair targets
Brca1 mutation and “brcaness”.
Many external or internal agents, such as ultraviolet light, ionizing radiation, CT, and chemical substances or products of normal cellular metabolism, including oxidation and hydrolysis, affect double-stranded DNA. DNA repair mechanisms are important for maintaining the stability and integrity of the genome, and include nucleotide- and base-excision repair, homologous recombination, end joining, mismatch repair, and telomere metabolism. 30 , 31 Inherited defects in one of these important genes can lead to cancer, as observed in the BRCA1 / 2 syndrome. DNA repair mechanisms are classified as the repair of single- or double-stranded damage. BRCA1 and BRCA2 are important proteins in the homologous recombination process when damage leads to breaks in both DNA strands. The proteins have also been implicated in other fundamental cellular processes, such as cell cycle control and transcription. 32
BC in BRCA1 germ-line mutation carriers most often displays a TN phenotype, as indicated by IHC and genomic studies. 33 Due to the similarity between sporadic TNBC and familial BRCA1 cancers, the concept of BRCAness has been developed. 34 – 36 In sporadic cancers, BRCA1 is inactivated by an epigenetic mechanism: the aberrant methylation of cytosine residues in CpG dinucleotides. 37 Aberrant methylation of the BRCA1 promoter is found in 11%–14% of sporadic BCs. 35 , 38 In contrast, BRCA2 tumors lack a clear pathological phenotype.
Knowledge of DNA repair mechanism defects leads to some specific treatment approaches in TNBC. These tumors present potentially higher sensitivity to DNA-damaging agents, such as platinum salts. The concept of “synthetic lethality” is also tested in the clinic, with the development of drugs (poly ADP-ribose polymerase [PARP] inhibitors) that target single-stranded DNA repair when homologous recombination is defective in BRCA -mutant tumors or in BRCAness tumors. 39 Several studies have attempted to find a biomarker of homologous recombination deficiency (HRD) with the aim of better predicting responders to PARP inhibitors and DNA-damaging chemotherapies. 40 – 42
Platinum-based chemotherapy
Metastatic tnbc.
The use of platinum compounds in metastatic BC was evaluated many years ago. Objective responses have been reported in ABC. 43 Some retrospective analyses have also suggested the occurrence of increased survival with platinum-based CT in patients with advanced TNBC. 44 , 45
A prospective Phase II study showed activity of platinum agents, including cisplatin (75 mg/m 2 /3 weeks) and carboplatin (area under the curve 6/3 weeks), in patients with metastatic TNBC, especially in patients with germ-line BRCA1 / 2 (gBRCA1/2) mutations. 46 A total of 86 patients were enrolled. The overall response rate was 25.6%, but in patients with germ-line BRCA1 / 2 mutations, the response rate increased to 54.5%. Interestingly, using a measure of DNA repair function, those authors also identified patients without mutations who had the potential to benefit from platinum therapy. They used two HRD assays to characterize BRCA -like genomic instability: the HRD large-scale state transition assay and the HRD loss of heterozygosity assay. They observed that patients who presented higher values in these assays also responded better to platinum-based treatments, even in the absence of germ-line mutations. 46
A prospective randomized trial comparing docetaxel with carboplatin in patients suffering from TNBC was presented at the 2014 San Antonio Breast Cancer Symposium (SABCS). 47 Those authors observed similar results for unselected TNBC, but patients with BRCA1 / 2 mutations experienced a significantly higher response rate and improved progression-free survival (PFS) when receiving carboplatin in comparison to docetaxel.
The rate of pCR after anthracycline- and taxane-based neoadjuvant CT is higher in TNBC (±30%) than in luminal BC. In addition, patients presenting pCR after neoadjuvant CT generally have a better prognosis compared to patients who do not present pCR at the time of surgery. 48 , 49 Unfortunately, patients suffering from TNBC who present residual disease after neoadjuvant CT have very poor outcomes. New therapies should be evaluated in patients who present residual disease after neoadjuvant CT. Retrospective research has suggested improved outcomes in terms of pCR when cisplatin is added to the neoadjuvant treatment. Byrski et al 50 published a retro spective analysis of 6,903 patients, including 102 patients with the germ-line BRCA1 mutation. The highest pCR rate was reported in germ-line BRCA1 -mutation carriers who received neoadjuvant cisplatin therapy: 24% of the BRCA1 -mutation carriers had pCR, but in the subgroup that received cisplatin, a much higher rate of 83% pCR rate was observed. 50
Five randomized studies evaluated the addition of carboplatin to standard neoadjuvant therapy 51 – 55 ( Table 2 ). Experimental arm 1 from the I-SPY 2 trial showed an increased rate of pCR in patients with TNBC if standard CT (paclitaxel/anthracycline–cyclophosphamide) was combined with carboplatin and the PARP inhibitor veliparib. A confirmatory Phase III trial is underway. 55 The GeparSixto 52 and CALGB 40603 53 trials showed increased rates of pCR with carboplatin, but toxicities were also more frequent and more significant if carboplatin was added. More dose reductions and early study discontinuations occurred due to these toxicities. The incidence of grade 3–4 hematological toxicities almost doubled. Data on late toxicities are missing, because the median follow-up period was only 3 years. Recently, at the 2015 SABCS, improved overall survival for patients receiving carboplatin was reported in the GeparSixto trial. 56 A subsequent Phase III trial evaluating two dose-dense regimens is recruiting participants (GeparOcto, {"type":"clinical-trial","attrs":{"text":"NCT02125344","term_id":"NCT02125344"}} NCT02125344 ). 57 In contrast, in the CALGB 40603 trial, despite significantly higher pCR rates, neither carboplatin nor bevacizumab showed improved event-free or overall survival. In addition, not all Phase II trials evaluating the use of carboplatin have shown improvements in pCR. 54
Carboplatin-based chemotherapy in neoadjuvant treatment: randomized Phase II results
Abbreviations: AUC, area under curve; EFS, event-free survival; is, in situ; pCR, pathologic complete response; RFS, relapse-free survival; TNBC, triple-negative breast cancer.
Currently, platinum compounds are not included in the guidelines for the treatment of early TNBC, but their role should be discussed in some specific cases, such as patients with a higher risk of relapse or requiring rapid disease control. Some recommend the use of carboplatin only for patients with known BRCA mutations, 58 but the available data are conflicting, as illustrated by the GeparSixto trial, which showed better outcomes when using carboplatin in patients with TNBC, independently of germ-line BRCA status. Interestingly, the GeparQuinto trial showed a statistically higher pCR rate with a classical sequential anthracycline–taxane regimen and a trend for better disease-free survival (hazard ratio 0.64, P =0.06) in the subgroup of patients with BRCA mutations (82 of 471 patients). 59 These results suggest higher chemosensitivity and better prognosis in patients with germ-line BRCA mutations, even without platinum compounds. Participation in clinical trials is recommended in the neoadjuvant and postneoadjuvant setting in the absence of pCR to better define the optimal standard systemic therapy for TNBC ( Table 3 ).
Trials in progress in the neoadjuvant and adjuvant settings for TNBC
Abbreviations: AC, doxorubicin + cyclophosphamide; CR, complete response; CRR, clinical response rate; DFS, disease-free survival; EC, epirubicin–cyclophosphamide; EFS, event-free survival; ETC, epirubicin–paclitaxel–cyclophosphamide; HRD, homologous recombination deficiency; IDFS, invasive DFS; PARP, poly ADP ribose polymerase; P, paclitaxel; pCR, pathologic complete response; PM(Cb), paclitaxel–Myocet (carboplatin); TNBC, triple-negative breast cancer.
The potential role of PARP inhibitors in TNBC
Based on the synthetic lethality concept, PARP inhibitors were developed for the treatment of cancers with specific DNA-repair deficits, such as TNBC with BRCA1 / 2 mutations and BRCAness TNBC. 35 The first drug of this class, olaparib, was approved by the US Food and Drug Administration in December 2014 as a single-agent treatment for patients with deleterious or suspected deleterious germ-line BRCA -mutated advanced ovarian cancer who were treated with three or more prior lines of CT. In BC, several Phase I and II trials have shown antitumor activity in BRCA -mutated patients ( Table 4 ). 60 – 63 In the proof-of-concept Phase II trial, 61 Tutt et al 61 reported on two cohorts of patients with BRCA1 / 2 -mutated ABC. In the first cohort (27 patients), which was assigned 400 mg twice daily, the objective response rate was 41% compared to 22% in the cohort (27 patients) treated with a lower dose (100 mg twice daily). Toxicities were mild, and included nausea and fatigue. Clinical trials are ongoing in patients with high-risk BRCA -mutated primary BC in the neoadjuvant and adjuvant settings. 64
Results of trials with PARP inhibitors in triple-negative breast cancer
Abbreviation: TNBC, triple-negative breast cancer.
Androgen receptor and TNBC
The LAR subtype is characterized by luminal gene expression and is driven by the AR. 25 The AR is expressed in normal and malignant breast tissue, and its prevalence is variable according to the subtype of BC. Approximately 10%–15% of TNBCs express the AR. 65 , 66 The LAR subtype demonstrates some similarities with the apocrine subtype. Indeed, in this histologic subtype, the gene expression profile is highly correlated with the LAR subtype. These findings indicate that the LAR subtype includes BCs with apocrine histology. 67 , 68 The function of the AR is less well understood in BC than in prostate cancer. In a paper by Doane et al, 69 a cell line that recapitulated the molecular profile of the LAR subtype was identified. This cell line was used in preclinical models, and androgen-dependent growth was demonstrated in an estrogen-independent manner. This growth was inhibited by an AR antagonist (flutamide). This study was the first proof of concept for androgen blockade in the LAR subtype. 67 , 69
At present, results from two Phase II studies have been presented and showed a benefit for androgen blockade in this LAR subtype. The first study was conducted by Gucalp et al. 70 That was a multicenter Phase II study that investigated the use of bicalutamide at a dose of 150 mg daily in AR-positive, ER- and PgR-negative metastatic BC (26 patients). The majority of patients were HER2-negative. The AR was expressed in 12% of patients with ER/PgR-negative BC. This study showed a clinical benefit rate (CBR; = CR + partial response [PR] + stable disease >6 months) of 19% for bicalutamide. The median PFS was 12 weeks (comparable to single-agent or combination CT in TNBC). The treatment was well tolerated, with the most common side effects including fatigue, hot flashes, limb edema, and transaminase elevations. 70
The second study evaluated the activity of the next-generation antiandrogen enzalutamide in advanced AR-positive TNBC. That study was a multicenter Phase II trial conducted in two stages. In stage 1, 26 patients were evaluated for the primary end point of the CBR at 16 weeks (CBR 16 ; = CR + PR + stable disease at 16 weeks). These patients received enzalutamide at a dose of 160 mg orally daily. The stage 1 result was a CBR 16 of 42% (95% confidence interval 24%–62%), including one CR and one PR. 71 For the stage 2 study, 165 patients were screened, and 75 patients had AR IHC ≥10% and more than one postbaseline evaluation. Patients with TNBC had a median of one line of prior therapy. The data were presented at the American Society of Clinical Oncology’s 2015 meeting and showed a CBR 16 of 35% and a median PFS of 14.7 weeks. 72 Because of these results, interest in androgen blockade therapy in the LAR subtype is growing. A number of different trials are in the recruitment stage or are waiting for results ( Table 5 ).
First results with antiandrogen therapy in TNBC and studies in progress
Abbreviations: BC, breast cancer; CBR, clinical benefit rate; CBR 16 , clinical benefit rate at 16; CBR 24 , clinical benefit rate at 24; CR, complete response; mTNBC, metastatic triple-negative breast cancer; PFS, progression-free survival; PR, partial response; TNBC, triple-negative breast cancer; AR, androgen receptor; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR-, progesterone receptor negative.
Blocking the AR pathway is a promising approach in the treatment of metastatic patients with the LAR subtype of TNBC. As an example, we would like to mention an isolated clinical case in which a heavily pretreated woman with metastatic TNBC and AR expression achieved a complete clinical response after 4 months of treatment with the AR antagonist bicalutamide. 73 Although this new targeted therapy is promis ing, more data are needed before it can be considered as a new validated treatment option. Future challenges related to the LAR subtype include understanding the role of the AR in tumorigenesis, understanding the escape mechanisms in AR-directed therapy, and discovering predictive biomarkers. 74
Immune subtype and role of immunotherapy
BC was not previously considered to be an “immunogenic” malignancy. Nevertheless, accumulating evidence indicates the prognostic and predictive values of tumor-infiltrating lymphocytes (TILs) in BC. 75 , 76 The degree of immune infiltration differs among BC subtypes. TIL levels are significantly higher in TNBC and HER2-positive BC. 77 – 79 Hormone receptor-positive disease is the subtype associated with the least robust number of TILs. Recently, several studies confirmed the prognostic value of TILs in TNBC. The lymphocyte-predominant BC subtype, which contains high levels of TILs (>50%), is associated with improved disease-free and overall survival and pCR in the neoadjuvant setting. 80 – 82
These findings suggest that immunomodulation could represent a new approach in the treatment of these aggressive BC subtypes. 79 Our current understanding suggests that the immunogenic potential of TNBC is derived at least in part from its genetic instability and high mutation rate. Tumors from patients with TNBC are more likely than tumors from patients with other subtypes to exhibit chromosomal instability and potential mutations. 83
TNBC is the subtype that is most frequently associated with TILs, but only a minority of TNBCs demonstrate a high number of TILs, suggesting that IM therapy could be necessary to promote immunorecognition and increase the adaptive immune infiltrate to levels adequate for a survival benefit in the majority of patients with this BC subtype. Patients with high levels of TILs at the time of diagnosis might benefit from the use of drugs that can enhance antitumoral immune responses.
Monoclonal antibodies have been developed to block specific immune-checkpoint proteins. Some of these antibodies have already been approved by the Food and Drug Administration for the treatment of metastatic melanoma. Three categories of these antibodies exist: antibodies that block CTLA4, PD1, or PDL1.
CTLA4 was the first immune checkpoint receptor to be targeted clinically. Two antibodies are known: ipilimumab and tremelimumab. Normally, after T-cell activation, CTLA4 is upregulated on the plasma membrane, where its function is to downregulate T-cell function through a variety of mechanisms, including preventing costimulation via CD28 and its ligand – B7. CTLA4 plays an essential role in maintaining normal immunologic homeostasis. Ipilimumab blocks CTLA4, and does not allow the T cell to interact with the receptor via CD28 on its cell surface. 84
PD1 is also a negative regulator of T-cell activity that limits the activity of T cells at a variety of stages of the immune response when it interacts with its two ligands: PDL1 and PDL2. Unlike CTLA4, which is primarily believed to regulate immune responses early in T-cell activation, PD1 is primarily believed to inhibit effector T-cell activity in the effector phase within tissues and tumors.
Targeting PDL1 is a similarly promising approach to targeting PD1. However, targeting PDL1 may result in different biologic effects than targeting PD1. In addition to binding PD1, PDL1 is also believed to exert negative signals on T cells by interacting with B7. PDL1-blocking antibodies prevent this interaction, but PD1-blocking antibodies do not. Another slight difference is that PDL1 antibodies do not prevent PD1 from interacting with PDL2, although the effect of this interaction remains unknown. Nivolumab and pembrolizumab are antibodies that block PD1 on the surface of T cells and prevent those T cells from interacting with PDL1. Monoclonal antibodies that block PDL1 are being evaluated in clinical trials. 84 , 85
Investigations evaluating the presence of PD1 on TILs and PDL1 on tumor cells in BC found that immune checkpoint proteins are upregulated in many BCs, particularly the TN subtype. The reported incidence of expression is highly variable. TILs expressing PD1 appear to be found more frequently in the TN subtype than in the other subtypes. In addition, the same pattern appears to occur for PDL1. These data support the study of immune checkpoint inhibitors in TNBC. 86 , 87
Clinical trials of immunotherapy in TNBC
One of the first completed clinical trials of a PD1 monoclonal antibody (pembrolizumab) in TNBC was reported at the 2014 SABCS by Nanda et al. 88 That was a Phase IB study that enrolled 32 patients with TNBC who had recurrent or metastatic disease (47% of whom had had more than three lines of previous CT). The participants were all PDL1-positive. Pembrolizumab was administered intravenously at a dose of 10 mg/kg every 2 weeks, and treatment could continue indefinitely as long as the patients were stable and their disease was not clearly progressing, as assessed by Response Evaluation Criteria in Solid Tumors version 1.1 every 8 weeks. Treatment with PD1 blockade was tolerable, with 56% of patients reporting an adverse event, but only 16% reporting grade 3–5 toxicity. Toxicity was essentially low-grade joint and muscle pain, fatigue, and nausea. One treatment-related death was caused by disseminated intravascular coagulation. Overall, 18.5% of 27 evaluable patients responded to pembrolizumab, with one (4%) CR, four (15%) PRs, and seven patients (26%) with stable disease. The median time to response was 18 weeks. The median PFS was just under 2 months. Three patients remained on pembrolizumab for at least 11 months. 88
At the same meeting, initial data from a Phase I study of an anti-PDL1 monoclonal antibody (MPDL3280A, atezolizumab) in metastatic TNBC were also reported. Emens et al 89 showed results from 12 patients with PDL1-positive disease. Grade 3–4 toxicities occurred in 8% of patients. Although immune-related adverse events have been reported with the use of immune checkpoint inhibitor agents, only one patient in this study demonstrated grade 2 pyrexia that was potentially attributable to immune activation. In general, immune-related adverse events occurred in a minority of patients. There were no toxicity-related deaths. Although over 90% of patients had been previously treated with more than two prior regimens and one-third of those enrolled had visceral metastases, the overall response rate was 33% in the nine patients who were evaluable for efficacy (one CR and two PRs). All responses were seen within the first 6 weeks of treatment. 89
Recently, data presented at SABCS 2015 encouraged the evaluation of another anti-PDL1 agent (avelumab). The trial showed promising results in a subgroup of TNBC. For those patients with TNBC and PDL1 expression on immune cells, the clinical response with avelumab was 44.4% versus 2.6% in the absence of expression. 90
All of these preliminary results are promising for the use of immuno-oncology agents in TNBC. The future challenge will be the identification of tumoral immune microenvironments that improve prognosis in BC. In this way, we hope to promote efficacious antitumoral immunity for all BCs. For example, in TNBC, controlling tumor growth with conventional chemotherapies in combination with immune checkpoint inhibitors could increase response rates. 82 , 91 For patients with limited T-cell infiltration, vaccine priming before or concurrent with immune checkpoint inhibitors may also result in additional clinical benefits. Many studies of further immunotherapy in BC are ongoing or planned. We hope that the best is still to come with respect to this therapy in the field of TNBC ( Table 6 ). 92
Immunotherapy trials in breast cancer
Abbreviations: DLT, dose-limiting toxicity; Nab, nanoparticle albumin-bound; ORR, objective response rate; OS, overall survival; pCR, pathologic complete response; PFS, progression-free survival; SUV, standardized uptake value; PK, pharmacokinetic; TNBC, triple-negative breast cancer; CTLA4, cytotoxic T-lymphocyte-associated protein 4; PD1, programmed cell death 1; PDL1, programmed death-ligand 1.
Growth factor overexpression in TNBC
Different growth factors are overexpressed in TNBC, such as vascular endothelial growth factor (VEGF) and EGFR. Targeting these pathways showed only limited activity in unselected TNBC. For the VEGF pathway, different trials in patients with ABC and in the adjuvant setting did not show any benefit in overall survival, even in trials dedicated to TNBC, such as the BEATRICE trial. 93 Similarly, for the EGFR pathway, the results have been disappointing. EGFR is overexpressed in more than 50% of TNBCs, but the rate of mutation is low (10%) and is found only in Asian populations. 94 Trials with anti-EGFR therapies have suggested that EGFR overexpression is not correlated with the activity of anti-EGFR agents in TNBC. 95 , 96 FGFRs may be a better target. FGFRs are expressed on many different cells, and regulate cell growth, survival, migration, and differentiation. In many cancer types, FGF signaling is implicated in oncogenic behavior. Targeting this pathway is a current area of drug development, not only primarily with tyrosine-kinase inhibitors but also with monoclonal antibodies that target FGFs/FGFRs and the FGF-ligand trap. 97 , 98 In BC, only 9% of tumors have FGFR1 amplification and 4% have FGFR2 amplification. 23 , 99 These tumors represent a very small population, but FGFRs could be an interesting target. Participation in clinical trials is crucial for improved evaluation of the potential of this new treatment strategy according to specific FGFR alterations. 100
Conclusion and perspectives
Progress in the treatment of TNBC remains an important challenge. In clinical practice, we still use standard CT (anthracyclines and taxanes). Some data in favor of the use of platinum-based CT in TNBC are now available, particularly in BRCA -mutation carriers. Clinical research is focused on two main axes in the neoadjuvant setting: how to increase pCR and how to improve outcomes in patients with residual disease. New targeted treatments and immunotherapeutic drugs are under development. The challenge is to drive studies on more selected patient populations due to the importance of heterogeneity in TNBC. The most promising new approaches include immunotherapy with checkpoint inhibitors, PARP inhibitors, and AR inhibitors. Furthermore, active research to discover additional specific targets in TNBC is ongoing. 101
GJ has received honoraria from Amgen, Pfizer, Novartis, Roche, and Celgene, and research grants from Novartis, Roche, and MSD. The other authors report no conflicts of interest in this work.

COMMENTS
Triple-negative breast cancer (TNBC) is an aggressive type of cancer but lacks targeted therapy methods such as hormone therapy due to the low expression of three primary receptors (ER, PR, and HER2). ... Proceedings of the American Association for Cancer Research Annual Meeting 2019; Atlanta, GA, USA. 29 March-3 April 2019; p. 121. ...
Triple-negative breast cancer (TNBC) is a kind of breast cancer that lacks estrogen, progesterone, and human epidermal growth factor receptor 2. This cancer is responsible for more than 15-20% of all breast cancers and is of particular research interest as it is therapeutically challenging mainly because of its low response to therapeutics ...
Triple-negative breast cancer (TNBC), a specific subtype of breast cancer that does not express estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER-2), has clinical features that include high invasiveness, high metastatic potential, proneness to relapse, and poor prognosis. Because TNBC tumors lack ER, PR, and HER2 expression, they are not ...
Introduction. Triple negative breast cancer (TNBC), which accounts for 15-20% of incident breast cancers, is the only breast cancer (BC) subtype that lacks targeted treatments. Using clinical ...
Triple negative is a term applied to breast cancers that do not meaningfully express the estrogen or progesterone hormone receptors or overexpress the human epidermal growth factor receptor 2 tyrosine kinase. At present, the only proven method for systemic management of triple-negative breast cancer for both early-stage and metastatic settings is cytotoxic chemotherapy. Here, we provide a ...
Triple negative breast cancer (TNBC) has long been a challenging disease to treat due to its aggressive behavior and the lack of actionable targets 1.It is commonly diagnosed at a younger age ...
Introduction. Breast cancer continues to be the second cause of death in women worldwide. 1 Triple-negative breast cancer (TNBC) is defined by the lack of expression of estrogen (ER), progesterone (PR) and HER2 receptors. TNBC represents approximately 10-15% of all diagnosed breast cancers. 2 The pattern of metastatic spread in TNBC is different from the other breast cancer subtypes with a ...
Molecular subtyping of triple-negative breast cancer (TNBC) is essential for understanding the mechanisms and discovering actionable targets of this highly heterogeneous type of breast cancer. We ...
Triple negative breast cancer (TNBC) continues to be the subtype of breast cancer with the highest rates of recurrence and mortality. The lack of expression of targetable proteins such as the estrogen receptor and absence of HER2 amplification have made relying on cytotoxic chemotherapy necessary for decades. In the operable setting, efforts to improve outcomes have focused on escalation of ...
Triple-negative breast cancer (TNBC) cells are deficient in estrogen, progesterone and ERBB2 receptor expression, presenting a particularly challenging therapeutic target due to their highly invasive nature and relatively low response to therapeutics. There is an absence of specific treatment strategies for this tumor subgroup, and hence TNBC ...
Abstract. Triple-negative breast cancer (TNBC), a specific subtype of breast cancer that does not express estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER-2), has clinical features that include high invasiveness, high metastatic potential, proneness to relapse, and poor prognosis.
Triple-negative breast cancer (TNBC) is an aggressive breast type of cancer with no expression of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2). ... Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a ...
Triple Negative Breast Cancer (TNBC) is a subtype of breast cancer that based on immunohistochemistry (IHC) is estrogens receptor (ER) negative ... U54 RR02613, 5P20RR11104 and NIHMD research endowment grant 2S21MD000101, U54 CA118638 and ING foundation grant to V.N.R. References. 1. Perou CM. Molecular stratification of triple-negative breast ...
Triple-negative breast cancer (TNBC) is defined as a Breast cancer is the most common malignancy in type of breast cancer with negative expression of estro-women. The 2012 global cancer statistics showed that gen (ER), progesterone (PR), and human epidermal there were approximately 1.7 million women diagnosed growth factor receptor-2 (HER2) [3].
Triple-negative breast cancer (TNBC) is a biologically and clinically heterogeneous disease, which has long been considered to present a major unmet need due to its aggressive behaviour and poor ...
Triple‐negative breast cancer, characterized by tumors that do not express estrogen receptor, progesterone receptor, or HER‐2 genes, represents an importan ... In a prospective phase II study by the Translational Breast Cancer Research Consortium (TBCRC), single‐agent cetuximab was evaluated alone or in combination with carboplatin ...
Triple-negative breast cancer (TNBC) is a breast cancer subtype renowned for its capacity to affect younger women, metastasise early despite optimal adjuvant treatment and carry a poor prognosis. Neoadjuvant therapy has focused on combinations of systemic agents to optimise pathological complete response. Treatment algorithms now guide the ...
By Kelley Luckstein. A new multicenter, international study suggests that people who have early-stage triple-negative breast cancer (TNBC) and high levels of immune cells within their tumors may have a lower risk of recurrence and better survival rates even when not treated with chemotherapy. The study was published today in the Journal of American Medical Association (JAMA).
Advancements in cell culturing techniques have allowed the development of three-dimensional (3D) cell culture models sourced directly from patients' tissues and tumors, faithfully replicating the native tissue environment. These models provide a more clinically relevant platform for studying disease progression and treatment responses compared to traditional two-dimensional (2D) models ...
Cancer Center at Illinois (CCIL) member Hua Wang, professor of materials science and engineering, published new research in Materials Today Bio demonstrating the development of a novel technology to prevent post-surgical recurrence of breast cancer, especially triple-negative breast cancer (TNBC).. An estimated 310,000 American women will be diagnosed with breast cancer in 2024, according to ...
Triple negative breast cancer (TNBC) is characterized by the absence of estrogen receptor (ER) and progesterone receptor (PR), as well as human epidermal growth factor receptor-2 (Her2) . Recently when referring to TNBC, the terms basal-like breast cancer (BC) and claudin-low BC should be mentioned.
Elderly triple-negative breast cancer patients displayed obvious lower 5-year survival rate compared to reference group, even though they have better grade stage, minor tumor, less lymph node metastasis, and higher rate of metastasis at diagnosis, which must contribute to their poor outcome.
Using their immuno-oncology (IO) database, the Cancer Research Institute (CRI) explored the number and type of IO agents being developed for use in breast cancer, specifically TNBC (Fig. 1a) 26 ...
The research, published in the journal Genome Biology, found that when the androgen receptor interacted with GATA3, it stimulated breast cancer cells to become more functionally mature. "This study revealed an important means by which androgen receptor activity exerts anti-cancer activity in breast cancer," said Associate Professor Hickey ...
The keywords included "triple negative breast cancer", "metastatic triple negative breast cancer", "treatment", and "guidelines". The inclusion criteria for evaluation included publications that involved only basic research, clinical research, and translational research papers written in English.
Based on 9MW2821's current ongoing monotherapy clinical data, in 20 subjects with locally advanced or metastatic triple-negative breast cancer treated with the 1.25 mg/kg dose and evaluable for ...
RESEARCH AGENDA FOR THE NEXT DECADE. The introduction of immunotherapy marks a revolution in the treatment of early-stage TNBC. KEYNOTE-522 has shown that, by unleashing anti-cancer immune ...
Zinc oxide nanoparticles (ZnO NPs) stand as among the most significant metal oxide nanoparticles in trigger the formation of reactive oxygen species (ROS) and induce apoptosis. Nevertheless, the utilization of ZnO NPs has been limited by the shallowness of short-wavelength light and the constrained production of ROS. To overcome these limitations, a strategy involves achieving a red shift ...
Triple-negative breast cancers (TNBCs) are defined by the absence of estrogen and progesterone receptors and the absence of HER2 overexpression. These cancers represent a heterogeneous breast cancer subtype with a poor prognosis. Few systemic treatment options exist besides the use of chemotherapy (CT). The heterogeneity of the disease has ...