Breast Cancer Research


Breast Cancer Risk Factors
Breast Cancer Research is presenting our Retrospective Collection on "Breast Cancer Risk Factors." Celebrating 'Breast Cancer Awareness Month (1 October- 31 October)', with this Collection, we aim to gain valuable insights into the multifaceted aspects of breast cancer risk to promote awareness, prevention, and early detection.
NEW CROSS-JOURNAL COLLECTIONS Find out more by clicking the links below:
Artif icial Intelligence in Breast Imaging PDGFB in Br east Cancer Initiation,Progression, and Metastasis

Aims and scope
- Most accessed
Inflammation at diagnosis and cognitive impairment two years later in breast cancer patients from the Canto-Cog study
Authors: Mylène Duivon, Justine Lequesne, Antonio Di Meglio, Caroline Pradon, Ines Vaz-Luis, Anne-Laure Martin, Sibille Everhard, Sophie Broutin, Olivier Rigal, Chayma Bousrih, Christelle Lévy, Florence Lerebours, Marie Lange and Florence Joly
Increased expression of REG3A promotes tumorigenic behavior in triple negative breast cancer cells
Authors: Xiaoxia Jin, Shuyun Yang, Xiaoyun Lu, Xudong Chen and Wencheng Dai
Alpha-6 integrin deletion delays the formation of Brca1/p53-deficient basal-like breast tumors by restricting luminal progenitor cell expansion
Authors: Marisa M. Faraldo, Mathilde Romagnoli, Loane Wallon, Pierre Dubus, Marie-Ange Deugnier and Silvia Fre
Deep learning-based risk stratification of preoperative breast biopsies using digital whole slide images
Authors: Constance Boissin, Yinxi Wang, Abhinav Sharma, Philippe Weitz, Emelie Karlsson, Stephanie Robertson, Johan Hartman and Mattias Rantalainen
Unraveling malignant phenotype of peritumoral tissue: transcriptomic insights into early-stage breast cancer
Authors: Pere Miquel Morla-Barcelo, David Laguna-Macarrilla, Octavi Cordoba, Gabriel Matheu, Jordi Oliver, Pilar Roca, Mercedes Nadal-Serrano and Jorge Sastre-Serra
Most recent articles RSS
View all articles
Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib
Authors: Nusayba Bagegni, Shana Thomas, Ning Liu, Jingqin Luo, Jeremy Hoog, Donald W. Northfelt, Matthew P. Goetz, Andres Forero, Mattias Bergqvist, Jakob Karen, Magnus Neumüller, Edward M. Suh, Zhanfang Guo, Kiran Vij, Souzan Sanati, Matthew Ellis…
Choosing the right cell line for breast cancer research
Authors: Deborah L Holliday and Valerie Speirs
Breast asymmetry and predisposition to breast cancer
Authors: Diane Scutt, Gillian A Lancaster and John T Manning
Triple-negative breast cancer molecular subtyping and treatment progress
Authors: Li Yin, Jiang-Jie Duan, Xiu-Wu Bian and Shi-cang Yu
Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer
Authors: Suzanne A Eccles, Eric O Aboagye, Simak Ali, Annie S Anderson, Jo Armes, Fedor Berditchevski, Jeremy P Blaydes, Keith Brennan, Nicola J Brown, Helen E Bryant, Nigel J Bundred, Joy M Burchell, Anna M Campbell, Jason S Carroll, Robert B Clarke, Charlotte E Coles…
Most accessed articles RSS

Editor-in-Chief
Lewis Chodosh , University of Pennsylvania, USA

Trending in the Media
Click here to see the most popular articles published in Breast Cancer Research in the past three months.

BCR's 20th Anniversary
20 years ago Breast Cancer Research published its first articles with BMC. Well-respected in the field, the journal has continually placed in the first quartile of the ‘Oncology’ category of Journal Citation Reports. Over the past decade, Breast Cancer Research (BCR) has also become the highest ranked breast cancer focused title in the field.
Look back at the journal’s milestone achievements and article highlights .

Featured Review - Artificial intelligence in mammographic phenotyping of breast cancer risk: a narrative review
In this review, we provide a useful reference for AI researchers investigating image-based breast cancer risk assessment while indicating key priorities and challenges that, if properly addressed, could accelerate the implementation of AI-assisted risk stratification to future refine and individualize breast cancer screening strategies.
Springer Nature Oncology Portfolio
Discover the range of academic oncology titles at Springer Nature here .

Management of patients with advanced-stage HER2-positive breast cancer: current evidence and future perspectives
Towards personalized treatment for early stage HER2-positive breast cancer
Targeted therapeutic options and future perspectives for HER2-positive breast cancer
Introduction.
Innovations in pathology, molecular biology and drug development have enabled HER2-positive breast cancer (BC), a historically aggressive subtype, to become one with impressive outcomes. The field was energized in 1978 when the first tyrosine kinase, epidermal growth factor receptor (EGFR), was discovered followed by the identification of the neu or HER2 (also known as ERBB2 ) gene in 1984 1 , 2 . The discovery that amplification or overexpression of HER2 was associated with extremely poor survival in BC ultimately led to the development of a monoclonal antibody (mAb) to HER2, trastuzumab 3 (Box 1 ).
Until this point, triple-negative and HER2-overexpressing disease were widely regarded as the most aggressive BC histologies, with unfavourable prognoses. Advanced BC was considered incurable, and treatment was purely palliative. However, newer and novel therapeutic strategies have led to markedly improved survival outcomes. The dependence of the tumour on HER2, coupled with effective HER2-targeted drugs such as trastuzumab, pertuzumab and most recently, tucatinib and trastuzumab deruxtecan (T-DXd), have contributed to these survival improvements in patients with HER2-positive (HER2 + ) BC 4 . Currently, survival rates exceed 90% in HER2 + early breast cancer (EBC) treated with chemotherapy and dual antibody therapy 5 . More than half of patients with metastatic HER2 + disease are diagnosed de novo, further demonstrating that most patients presenting with early disease are cured 6 .
However, despite this success, metastatic HER2 + tumours inevitably develop resistance, leading to disease progression. As such, the goal of therapy in HER2 + BC is to expand the number of patients cured in the early setting and prevent recurrence. In those HER2 + cancers that do present with de novo stage IV disease or ultimately recur, development of novel therapies is needed as these tumours continue to be dependent on HER2 signalling. Therefore, extensive research is ongoing in the preclinical, translational and clinical arenas to develop original and more potent therapies for this exceptionally sensitive target, HER2.
Advances in targeting HER2 include further exploitation of antibody–drug conjugates (ADCs), altering the linkers, payload or antibody scaffold to optimize efficacy 7 , 8 . Another approach is the development of bispecific antibodies, which use binding of two different HER2 epitopes to maximize efficacy 9 . As immunotherapy has shown benefit in triple-negative breast cancer (TNBC), attempts to mobilize the immune system in HER2 + disease are also ongoing. Immunotherapy is being approached from various angles including administering checkpoint inhibitors, linking effector T cells to HER2 antibodies, cellular therapy and vaccines 9 , 10 , 11 , 12 .
This Review summarizes the successful therapeutic approaches approved for treatment of HER2 + BC, which are essential to understanding ensuing developments, exploring the potential areas of drug resistance that are the foundation for future drug development. A survey of the landscape of platforms being used to maximize HER2-targeted therapeutic efficacy are enumerated, which includes mAbs, tyrosine kinase inhibitors (TKIs), ADCs, bispecific antibodies, immune system targeting agents, cellular therapy and targeted protein degraders. As HER2 is such a sensitive target, continued investigation to advance therapeutic benefit will undoubtedly lead to improvements in survival.
Box 1 History of HER2 receptor biology
In 1962, Stanley Cohen discovered the protein responsible for incisor and eyelid opening in mice, termed epidermal growth factor (EGF), thus beginning the journey from bench to bedside for HER2-targeted therapy 237 , 238 . Demonstration that the EGF receptor (EGFR) formed complexes after EGF binding was postulated to be the initial step for cell growth 1 . Subsequently, in 1986 Cohen and Rita Levi-Montalcini were jointly awarded the Nobel Prize in Physiology or Medicine for the discovery of growth factors (see Related links: https://www.nobelprize.org/prizes/medicine/1986/cohen/lecture/ ). The description and discovery of oncogenes, genes that transformed cells into tumour cells, was begun in the mid-1970s by Harold Varmus and Michael Bishop who also received the Nobel Prize in Physiology or Medicine for their discovery that retroviruses obtained cellular genes from the host (that is, oncogenes; see Related links: https://www.nobelprize.org/prizes/medicine/1989/varmus/lecture/ ; https://www.nobelprize.org/prizes/medicine/1989/press-release/ ). The intersection of two scientific fields generated knowledge that there was homology between oncogenes and growth factor receptors.
ERBB , consisting of two parts, v- erbA and v- erbB , was initially described in 1935 in an avian erythroblastosis retrovirus 239 . v- erbB was discovered to be transforming, hence an oncogene, while v- erb A was not 240 . Eventually, erythroblastic leukaemia viral oncogene homologue 2 (v-erbB2) was found to be closely homologous to EGFR 241 . Moreover, it was thought that EGFR was acquired from the c- erbB2 oncogene. Mouse and non-mouse cell lines were reported to be transformed by neuroblastoma, glioma and carcinoma DNA (later named neu ) from malignant rat or mouse cell lines 242 , 243 . A 185,000-dalton protein was found to induce the transformation by neu ; the neu gene was homologous to erb-B , and p185 was related to EGFR 2 , 244 , 245 . The sequence for EGFR published in 1984 established that it was similar to v- erbB2 (refs. 241 , 246 ).
Sequencing studies revealed that the tyrosine kinase receptor named HER2 had extensive homology to neu , and both were located on chromosome 17 (ref. 247 ). HER2 and neu had different sequences but were closely related to the EGFR gene, located on chromosome 7 (ref. 247 ). Thus, HER2 and neu were determined to be homologous with ERBB2 but different from EGFR (HER1). Eventually two other members of the HER family were described: HER3 on chromosome 12 and HER4 on chromosome 2 (refs. 248 , 249 ). The tyrosine-binding domains of all but HER3 (which has no catalytic tyrosine kinase activity) are similar. A monoclonal antibody (mAb) to p185 in neu -transformed cell lines was subsequently shown to revert some of the characteristics to a non-transformed phenotype and inhibit tumour growth in mice 250 , 251 , 252 . This work spearheaded the concept of HER2-targeted therapy.
Ensuing research determined that only gene amplification with resultant overexpression of protein HER2 was needed for cellular transformation 253 , 254 . Overexpression of HER2 was found to occur in human breast tumours, and HER2 signalling and transforming functions leading to growth were associated with a poor prognosis 3 , 255 . This work led the way to the development of a mAb to target the HER2 receptor in human breast cancer: a murine mAb to HER2, m4D5, generated to p185 HER2 that decreased cell proliferation, spurred the development of a humanized mAb to HER2, humAb4D5-8, eventually named trastuzumab or Herceptin 256 , 257 (Fig. 1 ). Phase I and II clinical studies demonstrating activity of trastuzumab were followed by a phase III registrational study that led to the approval of trastuzumab in 1998 by the FDA for patients with HER2 + metastatic breast cancer (MBC) 258 , 259 .
Current standard of care for HER2 + BC
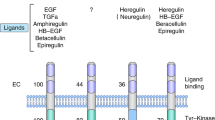
As the understanding of HER2 biology has evolved (Box 1 ), so has the development of agents that target HER2. HER receptors contain an extracellular ligand-binding domain, a transmembrane domain, and an intracellular tyrosine kinase domain. Ligand binding to the HER proteins results in homodimerization or heterodimerization of these receptors, leading to activation of downstream signalling pathways that promote cell division and growth and inhibit apoptosis 13 . There is no known ligand for HER2, but it is a preferred dimerization partner for the other HER proteins, especially HER3 (ref. 14 ). HER2 overexpression or amplification leads to ligand-independent dimerization and abnormal signalling in addition to increased signalling through ligand-dependent heterodimerization 13 . The efficacy of HER2-targeted agents is most prominent in these ‘HER2-positive’ tumours.
The definition of HER2 positivity according to American Society of Clinical Oncology–College of American Pathologists (ASCO–CAP) guidelines, includes tumours that have 3+ positive staining by immunohistochemistry (IHC) in ≥10% of tumour cells, or HER2 gene amplification detected by fluorescence in situ hybridization (FISH) 15 , 16 (Box 2 ). Recent research has identified a subset of patients with ‘HER2-low’ (HER2 low ) BC that is responsive to novel HER2-targeted ADCs 17 . HER2 low is defined as HER2 IHC 1+ by itself or 2+ in the absence of HER2 gene amplification by ISH (in situ hybridization). The cut-off for the level of HER2 expression by IHC is only a crude estimation of those who may actually benefit from anti-HER2 therapies. With the introduction of the new HER2 low definition, additional diagnostic tools may need to be considered.
Since the initial approval of trastuzumab for HER2 + BC, multiple agents exhibiting various mechanisms of action and safety profiles have been approved for the treatment of early-stage and metastatic disease (Fig. 1 and Box 3 ). Below, agents that have been approved by regulatory agencies are briefly described and the advantages and limitations of each strategy are summarized.
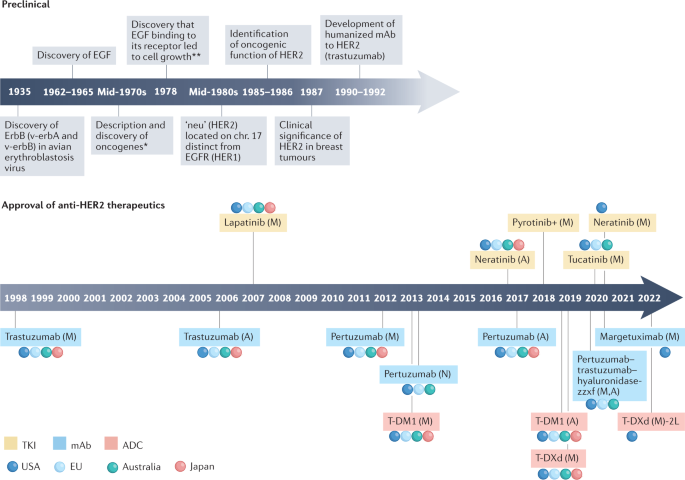
Timeline of preclinical discovery milestones for HER2 biology and regulatory approval for anti-HER2 therapies. A, adjuvant setting; M, metastatic setting; N, neoadjuvant setting; +, approved in China only; * , M. Bishop and H. Varmus awarded Nobel Prize in 1989 for this discovery; ** , S. Cohen and R. Levi-Montalcini awarded Nobel Prize in 1986 for discovery of growth factors and their receptors.
Box 2 HER2 diagnostics
ERBB2 , the gene that encodes HER2, is located on chromosome 17q21. HER2 acts as an oncogene, and its amplification results in overexpression of the HER2 protein, a transmembrane receptor kinase. This abnormal expression leads to a cascade of constitutive activation of downstream signalling pathways that promote uncontrolled tumour cell proliferation. HER2 expression is associated with poor prognosis, including early recurrence and metastatic disease in breast cancer 3 , 260 , 261 . HER2 overexpression is also predictive of response to several HER2-targeted therapies, including monoclonal antibodies (mAbs) such as trastuzumab and pertuzumab, tyrosine kinase inhibitors (TKIs) — lapatinib, tucatinib — as well as antibody–drug conjugates (ADCs) such as ado-trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan. Given the significance of HER2 status for prognosis and clinical decision-making for treatment, accurate assessment of this biomarker is crucial.
Standard methods of HER2 testing include fluorescence in situ hybridization (FISH)/ISH, which quantifies the HER2 gene copy number, and immunohistochemistry (IHC), which measures HER2 expression on the cell surface. IHC quantifies the HER2 expressed on the cell surface using membranous staining, whereas FISH reports the level of amplification of the HER2 gene. Typically, equivocal results with IHC are confirmed by FISH. The results using current testing methods are not unambiguous, complicating clinical decisions regarding use of anti-HER2 therapy. Standards regarding the test used and timing differ from institution to institution.
There are two FDA-approved HER2 IHC tests: HercepTes (Dako) and Pathway (Ventana Medical Systems). These tests use IHC staining of the HER2 protein with a pathologist scoring the extent of staining as 0, 1+, 2+ or 3+. The reliability of the results from IHC is influenced by pre-analytical variables, including sample handling, fixation and storage, as well as staining 262 , 263 . The subjective nature of the pathologist’s interpretation also gives rise to variability in the IHC results, as does the lack of reproducibility across laboratories 264 , 265 . A big challenge in assessment of HER2 is with samples scored as IHC 2+; the variation across labs for IHC 2+ was fivefold higher than in samples scored as 3+ in both breast and gastric cancers 266 .
The FDA-approved FISH assays include PathVysion (Abbott), INFORM (Ventana Medical Systems) and PharmDx (Dako). Dual probe assays report the ratio of HER2 to CEP17 (centromere 17), whereas single-probe assays give a direct HER2 copy number. CEP17 serves as an internal control, something that is lacking in IHC. Although rare, polysomy of chromosome 17 may lead to false negative results for HER2 amplification 267 , 268 . Some drawbacks with FISH are that it is more expensive, technically more challenging, and time consuming. However, FISH is associated with less inter-observer variability, is considered quite accurate and produces equivocal results in only ~5% of cases 269 .
The American Society of Clinical Oncology–College of American Pathologists (ASCO–CAP) guidelines have continued to evolve to improve the accuracy and clinical utility of HER2 testing by providing specific algorithms 15 , 16 . In parallel with existing techniques, novel methods to quantify HER2 levels or standardize procedures are being investigated, including quantitative assays to measure HER2 protein expression at the single cell level, automated scoring of HER2 FISH, microRNA signatures in primary tumour tissue as a prognostic/predictive tool, using a mass spectrometry system to measure HER2 at attomols mm 2 (refs. 270 , 271 , 272 , 273 ). Furthermore, gene expression-based tools for prognostic and predictive purposes are also under evaluation in HER2 + breast cancer, although none of these has yet reached mainstream use 274 , 275 .
Recent research has defined a ‘HER2-low’ phenotype based on IHC (HER2 IHC 1+ or 2+ and ISH negative), which defines a group that responds to trastuzumab deruxtecan. This agent has just received FDA approval in this patient population 17 , 276 . A recent analysis using current standard HER2 assays coupled with CAP data from 1,400 labs worldwide revealed poor agreement in evaluation of HER2 0 and 1+ cases; similar results were seen with a separate Yale cohort 277 . These inaccuracies in the real world underscore the urgency to develop more sensitive HER2 diagnostic assays to ensure that eligible patients are not deprived of effective therapies.
Box 3 Toxicities with HER2-targeted therapies
Although highly effective in disease control and improving survival, approved anti-HER2 therapies are not without potential adverse events (AEs), some of which require careful monitoring.
Cardiotoxicity. Cardiac dysfunction with trastuzumab is an AE of concern in the metastatic breast cancer (MBC) and early breast cancer settings, particularly when given in combination with anthracyclines 258 , 278 . Dual HER2-targeted therapy with pertuzumab and trastuzumab in HER2 + MBC has not been shown to exacerbate cardiotoxicity or lead to increased cardiac events after long-term follow-up in the adjuvant setting 279 , 280 .
Trastuzumab-induced cardiotoxicity is usually asymptomatic, not related to cumulative dose and largely reversible.
Numerous measurements of left ventricular ejection fraction (LVEF) during trastuzumab therapy may lead to false positive elevations 281 .
Increased rates of cardiotoxicity have not been observed in long-term follow-up 278 , 282 .
The FDA recommends baseline LVEF evaluation before initiating trastuzumab.
Cardiac monitoring strategies have been developed by the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) 283 , 284 .
Gastrointestinal toxicity. Gastrointestinal toxicities and rash are frequently observed with tyrosine kinase inhibitors (TKIs), primarily due to epidermal growth factor receptor (EGFR) targeting.
Diarrhoea is more frequent and severe with neratinib and pyrotinib than other TKIs (especially relevant in the adjuvant setting where adherence to therapy may be compromised).
The addition of budesonide or colestipol to loperamide prophylaxis may decrease neratinib discontinuation due to diarrhoea 285 .
Starting with a lower dose of neratinib and working up to the 240 mg daily dose over 2 weeks may improve tolerability.
The higher specificity of tucatinib for HER2 over EGFR has led to less severe gastrointestinal effects, rash and skin toxicity 72 .
Liver toxicity. Elevated liver enzymes are commonly reported with ado-trastuzumab emtansine (T-DM1) and tucatinib 72 , 286 , 287 .
Dose interruptions and adjustments are primary management.
Careful monitoring is recommended, and concurrent treatment with strong or moderate CYP3A inhibitors should be avoided.
Thrombocytopenia. Thrombocytopenia has occurred with T-DM1 and is attributed to the DM1-induced impairment of megakaryocyte differentiation 288 .
Mitigation strategies include dose interruptions and dose modifications.
Interstitial lung disease. Interstitial lung disease (ILD) has been attributed to trastuzumab deruxtecan (T-DXd) and was first reported in 13.6% of patients in the DESTINY-Breast01 trial 84 . One patient had a grade 3 event, and four deaths were attributed to ILD.
Most ILD events were grade 1/2 and occurred in the first 12 months, with declining risk thereafter 289 .
Increased awareness coupled with guidelines for interrupting therapy and prompt treatment improved ILD (no grade 4/5 events and <1% grade 3 events) 84 , 85 , 290 .
The unique AE profiles of anti-HER2 therapies enable customized treatment based on patients’ comorbidities. Because some AEs may be exacerbated in combination with chemotherapy, awareness and careful monitoring with implementation of mitigation strategies in the event of an AE will enable maximal treatment benefit with minimized toxicity.
Monoclonal antibodies
Trastuzumab was the first humanized mAb developed to HER2 that achieved remarkable success in the treatment of HER2 + BC. Trastuzumab binds to the extracellular domain (ECD) of HER2, suppresses intracellular HER2 signalling pathways, inhibits cell cycle arrest and mediates antibody-dependent cell-mediated cytotoxicity (ADCC) 18 . Preclinical data demonstrating synergy between cytotoxic agents and trastuzumab paved the way for clinical trial designs with chemotherapy combinations for treatment of HER2-overexpressing metastatic breast cancer (MBC) and subsequently in the adjuvant setting (Supplementary Table 1 ) 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 . All these trials demonstrated favourable outcomes with trastuzumab and chemotherapy, leading to swift approvals from the regulatory agencies in the metastatic and curative settings (Fig. 1 ).
With these successes, trastuzumab became firmly established as the treatment of choice for patients with HER2 + BC, and HER2 drug development that would revolutionize the outcome for patients facing this disease commenced.
However, although trastuzumab improves responses and outcomes, a substantial number of patients develop therapeutic resistance and disease relapse 27 . Early studies showed that antibodies targeting multiple domains in HER2 can exert synergistic antitumour effects 28 . Consequently, a second humanized anti-HER2 mAb, pertuzumab, was developed. Unlike trastuzumab, which binds to ECD IV of HER2, pertuzumab binds to ECD II, preventing HER2 heterodimerization with HER1, HER3 and HER4, blocking downstream tumour signalling 29 . Trastuzumab is more effective at inhibiting cell growth in the absence of HER3 ligand 30 , 31 . These complementary mechanisms of action and the effect of the two agents on immune system-mediated antitumour activity via ADCC and/or complement-mediated cytotoxicity (CDC), suggested that combination therapy could be synergistic 32 , 33 , 34 .
Trials that combined these two mAbs with chemotherapy as treatment for HER2 + BC in the metastatic, adjuvant and neoadjuvant settings demonstrated better outcomes than trastuzumab and chemotherapy combinations, leading to FDA approval of pertuzumab in these settings 5 , 35 , 36 , 37 (Supplementary Table 1 ). These trials changed the course of disease for patients with HER2 + BC and improved survival.
The efficacy of trastuzumab, in part, is dependent on ADCC mediated through its Fc domain . Patients whose immune effector cells ( natural killer (NK) cells or dendritic cells (DCs)) can bind more tightly to the Fc domain have stronger responses to trastuzumab 38 , 39 . A novel anti-HER2 IgG1 mAb, margetuximab, has an engineered Fc domain to increase the affinity for the activating Fcγ receptor (CD16A) and to decrease the affinity for the inhibitory Fcγ receptor (CD32B) expressed on immune effector cells. The optimized Fc domain confers enhanced ADCC activity against HER2 + tumours and more potently stimulates ADCC than trastuzumab or pertuzumab in vitro 40 . The FDA granted regulatory approval for margetuximab plus chemotherapy for the treatment of HER2 + MBC on the basis of the results from the SOPHIA study, adding another drug to the HER2 armamentarium 41 (Supplementary Table 1 ).
Trastuzumab biosimilars
Unlike novel synthetic HER2-targeting antibodies, trastuzumab biosimilars are biologic agents created from living cells and have similar pharmacokinetic and pharmacodynamic properties to the original product. They may have minor differences in clinically inactive components from the original biologic medication, but there are no clinically meaningful differences between the two with respect to safety, purity and potency (see Related links: https://www.fda.gov/media/82647/download ). There are currently five trastuzumab biosimilars approved by the FDA for US markets, with additional agents in development (see Related links: https://www.fda.gov/drugs/biosimilars/biosimilar-productinformation ). Trastuzumab-dkst (MYL1401O) was the first trastuzumab biosimilar to receive FDA approval (in 2017) for patients with HER2-overexpressing BC and gastrointestinal (GI) cancers. Subsequent approvals included trastuzumab-pkrb (CT-P6) for HER2 + BC (December 2018); trastuzumab-dttb (SB3) (January 2019), trastuzumab-qyyp (PF-05280014; March 2019) and trastuzumab-anns (ABP980) for HER2 + breast and GI tumours (June 2019). Although clinical guidelines support biosimilars, their adoption in clinical practice has been slow, hampered by physician and commercial payer awareness, perceptions and preferences.
Formulations of monoclonal antibodies
In an effort to conserve resources and reduce the burden of intravenous (i.v.) infusions to patients, a subcutaneous version of trastuzumab (trastuzumab–hyaluronidase-oysk) was developed and validated in clinical trials and approved in the EU in 2013 and in the USA in 2019 42 . Pertuzumab–trastuzumab–hyaluronidase-zzxf, a subcutaneous formulation of a fixed dose formulation of trastuzumab and pertuzumab demonstrated safety and non-inferiority in pathological complete response (pCR) versus corresponding i.v. versions in patients with EBC and was granted FDA approval in the adjuvant and metastatic settings in 2020 43 .
Tyrosine kinase inhibitors
TKIs are small molecules that target the intracellular catalytic kinase domain of HER2, competing with ATP, blocking phosphorylation and activating downstream signalling cascades. Lapatinib, an oral, 4-anilinoquinazoline TKI derivative, is a reversible inhibitor of EGFR (also known as HER1) and HER2, with activity in HER2-driven tumours that are insensitive to trastuzumab 44 . Lapatinib overcomes trastuzumab resistance mediated by insulin-like growth factor 1 receptor (IGF1R) upregulation by blocking the crosstalk between HER2 and IGF1R 45 . Cell lines and xenografts expressing the p95 HER2 variant that lack the trastuzumab-binding ECD were susceptible to lapatinib, presumably because it targets the intracellular kinase domain of HER2 (ref. 46 ). In vitro studies showed that pTEN-deficient, trastuzumab-resistant HER2 + BC cell lines remained sensitive to lapatinib 47 . These and other data supported the clinical development of lapatinib in trastuzumab-resistant BC 48 . Lapatinib in combination with capecitabine was superior to capecitabine alone in trastuzumab-treated HER2 + MBC, and the lapatinib–letrozole doublet was more efficacious than letrozole alone in hormone receptor-positive (HR + )/HER2 + MBC, leading to regulatory approval of these combinations and offering an alternative to HER2-targeting antibody combinations for these patients 49 , 50 , 51 (Supplementary Table 1 ). Several trials were conducted with lapatinib in HER2 + EBC, the details of which are succinctly summarized in a recent review 52 .
A significant sanctuary for recurrent HER2 + disease is the central nervous system (CNS), and up to 50% of patients with HER2 + BC develop brain metastases 53 , 54 , 55 . Moreover, the blood–tumour barrier (BTB), which evolves from the blood–brain barrier (BBB), regulates drug distribution to brain metastases, posing a clinical challenge 56 . It has also been suggested that the large size of the HER2 antibodies (for example, trastuzumab) prevents penetration of the BBB and efficacy in the CNS. Lapatinib, by virtue of its small size and potent anti-HER2 activity, found a niche in this arena. Lapatinib monotherapy and combination therapy demonstrated lower rates of CNS progression and responses in the CNS in HER2 + MBC, including in patients with previously untreated brain metastases 49 , 57 , 58 . These data provided the impetus for further refinements in next-generation TKIs to tackle the growing problem of brain metastases associated with HER2 + MBC.
In contrast to lapatinib, neratinib (HKI-272) is an irreversible pan-HER TKI that targets EGFR, HER2 and HER4 (ref. 59 ). Neratinib inhibits growth in trastuzumab-resistant cell lines and is synergistic with trastuzumab 60 , 61 . A unique feature of neratinib is its activity in cell lines with somatic HER2 mutations in the absence of HER2 amplification, suggesting that it can overcome possible resistance to other anti-HER2 therapies 61 . The co-occurrence of somatic HER2 mutations and HER2 amplification has also been observed, and a prevalence of 7.1% was reported in patients with HER2 + MBC, all of whom had poor response to dual anti-HER2 antibody therapy 62 . Durable tumour shrinkage was seen with neratinib, but not with lapatinib or trastuzumab, in animal models with concomitant HER2 mutation and amplification, and similar efficacy with neratinib was also evident in the clinical setting 62 .
Treatment with neratinib after standard adjuvant trastuzumab improved invasive disease-free survival (iDFS) rates in HER2 + BC, especially in patients with HR + tumours 63 , 64 (Supplementary Table 1 ). Similarly to lapatinib, the neratinib–capecitabine combination improved outcomes in HER2 + MBC, earning a nod from the FDA as third-line therapy 65 (Supplementary Table 1 ). Given the rapid development of newer HER2-targeted therapies, including novel TKIs with less toxicity and higher efficacy, the role of the neratinib–capecitabine combination appears limited.
Pyrotinib, another oral, irreversible pan-HER TKI that targets EGFR, HER2 and HER4, has been approved by the Chinese regulatory authority in combination with capecitabine in HER2 + MBC treated with prior trastuzumab and taxane 66 (Supplementary Table 1 ). Pyrotinib is similar to the other pan-HER2 TKIs, with diarrhoea as its most common toxicity 66 . Several trials with pyrotinib are ongoing in breast and other cancers, but it is unclear whether its use in HER2 + MBC will expand to other countries.
Tucatinib is a potent, oral, HER2-specific TKI with >1,000-fold greater potency for HER2 than EGFR 67 . Tucatinib demonstrated CNS penetration in intracranial xenograft models and was superior to lapatinib in preclinical studies 68 , 69 , 70 , 71 . The pivotal HER2CLIMB trial demonstrated superiority of the tucatinib plus capecitabine–trastuzumab combination in extending progression-free survival (PFS) and overall survival (OS) in HER2 + MBC previously treated with anti-HER2 therapies 72 , 73 (Supplementary Table 1 ). The innovative design of the trial allowed patients with stable as well as active brain metastases, and HER2CLIMB is the first randomized trial to demonstrate clinically meaningful benefits in patients with HER2 + brain metastases, marking an important milestone in the treatment of HER2 + BC. Tucatinib was FDA approved in April 2020 for the treatment of HER2 + MBC, including patients with CNS metastases. Ongoing trials with tucatinib include a maintenance trial in first-line HER2 + MBC with the goal of delaying CNS progression ( NCT05132582 ), an adjuvant study for patients with residual disease following neoadjuvant chemotherapy ( NCT04457596 ) and the neoadjuvant I-SPY 2 trial ( NCT01042379 ) (see Related links).
Antibody–drug conjugates
ADCs were designed to channel the cytotoxic effects of chemotherapy to specific tumour cells. ADCs contain a tumour-targeting antibody covalently bound to a cytotoxic drug (payload) via a synthetic linker 74 . The ADC is directed to cancer cells expressing the target (for example, HER2) on the cell surface, followed by internalization of the ADC and release of the cytotoxic payload, resulting in tumour cell death. Cleavable linkers in ADCs enable release of the cytotoxic payload from the target cell to the extracellular space, leading to destruction of surrounding cancer cells that may not have high target protein expression. This bystander effect further enhances the efficacy of ADCs against tumour cells 75 .
Ado-trastuzumab emtansine (T-DM1), the first anti-HER2 ADC to be developed, is composed of trastuzumab connected via a stable linker to DM1, a maytansine derivative with a drug-to antibody ratio (DAR) of ~3.5. T-DM1 caused mitotic disruption and apoptosis in HER2-overexpressing cell lines regardless of their sensitivity to trastuzumab and lapatinib 76 , 77 . T-DM1 prolonged PFS and OS in patients with HER2 + MBC compared with current standard of care in large, randomized trials, validating HER2 overexpression as a target in trastuzumab-resistant BC and showcasing the efficacy of ADCs in this setting. These results led to FDA approval of T-DM1 for HER2 + MBC (Fig. 1 and Supplementary Table 1 ). Although the attempt to replace trastuzumab with T-DM1 as part of the dual anti-HER2 (neo)adjuvant regimens was not successful, T-DM1 reduced the risk of recurrence in patients with HER2 + EBC without upfront pCR after neoadjuvant chemotherapy 78 , 79 , 80 (Fig. 1 and Supplementary Table 1 ). T-DM1 also demonstrated activity in a subset of patients with HER2 + MBC and brain metastases in the KAMILLA study 81 .
T-DXd is a HER2 ADC comprising a humanized HER2 antibody with the same sequence as trastuzumab conjugated to deruxtecan (DXd); T-DXd has a DAR of 8 and exhibits the enhanced features of DXd. The novel DXd ADC technology consists of a cleavable tetrapeptide-based linker, a self-immolative amino methylene spacer and a novel topoisomerase inhibitor payload derivative of exatecan (DS-8951) 82 . The linker of DXd is selectively cleaved by cathepsins, which are upregulated in tumours, releasing the payload preferentially inside cancer cells. This feature, coupled with the short half-life of DXd in vivo, limits systemic exposure of the cytotoxic agent, with the goal of reducing toxicity. The high membrane permeability of DXd enables local bystander effects, leading to the death of tumour cells in the tumour microenvironment (TME) 82 .
Single-agent T-DXd demonstrated impressive antitumour activity in refractory HER2 + MBC and an unprecedented improvement in PFS when compared head to head with T-DM1 in second-line HER2 + MBC leading to its FDA approval in these patient populations 83 , 84 , 85 , 86 (Fig. 1 and Supplementary Table 1 ). T-DXd has also shown encouraging activity in BC brain metastases, in DESTINY-Breast01 (ref. 87 ). DEBBRAH is evaluating T-DXd in patients with HER2 + or HER2 low MBC with brain metastases and/or leptomeningeal metastases; preliminary data are promising 88 .
A unique feature of T-DXd is its ability to target HER2 low MBC as evidenced by activity in this subset of patients in a phase I trial 83 . This remarkable efficacy appears multifactorial based on enhanced features of T-DXd compared with T-DM1, the ability to deliver a higher dose and the bystander effect tackling intratumour HER2 heterogeneity. T-DXd demonstrated statistically significant and clinically meaningful improvements in PFS and OS versus treatment of physician’s choice (TPC) chemotherapy in the phase III DESTINY-Breast04 trial in HER2 low MBC 17 . These results are likely to redefine nomenclature around HER2 expression and what is considered actionable in terms of HER2 expression. The FDA recently approved the use of T-DXd for patients with HER2 low MBC on the basis of data from the DESTINY-Breast04 study (see Related links: https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-her2-low-breast-cancer ).
Recent results from the DAISY trial showed that T-DXd antitumour activity was associated with level of HER2 expression in HER2 + MBC 89 . Interestingly, a high percentage of HER2 IHC 0 cells in the tumour and their spatial distribution relative to HER2-overexpressing cells were associated with a decreased response to T-DXd. This highlights the need to develop more sensitive methods for detection of HER2 expression and novel technologies to assess heterogeneous HER2 expression within the tumour.
Mechanisms of HER2-targeted resistance
Resistance to anti-HER2 therapies may occur via multiple mechanisms, some of which appear to be shared between different agents. A common reason for trastuzumab treatment failure is incomplete inhibition of the HER family of receptors, which can be overcome by dual HER2-targeted therapy or ADCs with potent payloads that have activity even with lower HER2 expression. Effective inhibition of HER2 may also be thwarted by the emergence of HER2-activating mutations. Other resistance mechanisms include generation of p95 HER2 , a truncated form of HER2 that lacks the ECD that is recognized by anti-HER2 antibodies and Δ16HER2, a splice variant lacking the ECD encoded by exon 16, which leads to stabilization of homodimers and constitutive activation of downstream signalling. Figure 2 depicts selected mechanisms of HER2-targeted resistance.
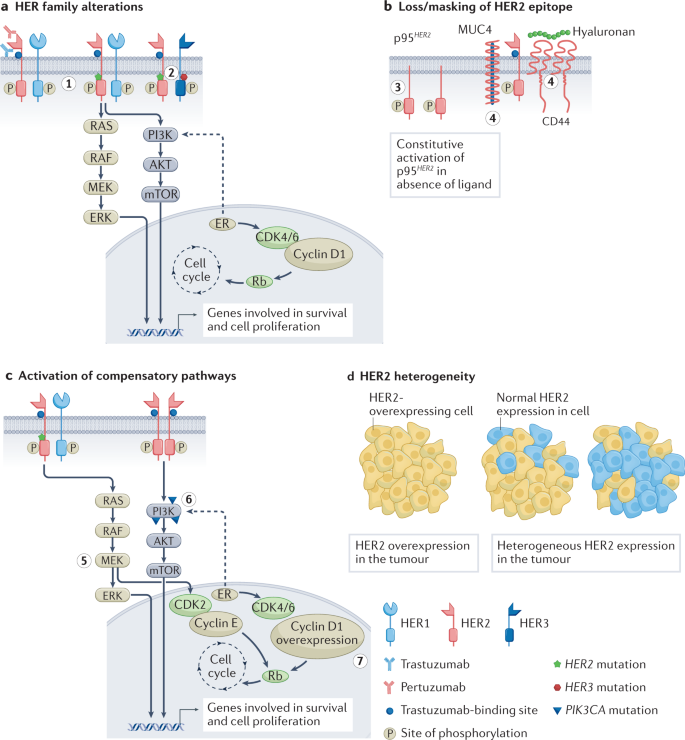
a , Mutations and/or alterations in the HER family of receptors that lead to activation of downstream signalling pathways. (1) Mutations in HER2 leading to P13K–AKT and RAS–MAPK pathway activation. (2) Co-occuring mutations in HER2 and HER3 leading to PI3K–AKT pathway activation. b , Loss of HER2 extracellular domain in cells overexpressing p95 HER2 receptor. Masking of the trastuzumab-binding site on HER2 owing to overexpression of mucin 4 (MUC4) and CD44–polymeric hyaluronan complex. (3) p95 HER2 overexpression. (4) MUC4 overexpression and CD44–polymeric hyaluronan complex. c , Activation of compensatory pathways. (5) Mutations in HER2 promote MEK–ERK signalling, which activates CDK2 kinase. (6) PIK3CA mutations lead to P13K–AKT pathway activation. (7) Cyclin D1 gene overexpression leads to resistance to anti-HER2 therapies. d , Heterogeneous expression of the HER2 receptor in tumours leads to decreased sensitivity to HER2-targeted therapies that are dependent on overexpression of HER2. ER, oestrogen receptor.
HER family alterations
HER2 mutations can drive BC growth even in the absence of HER2 overexpression or amplification. The frequency of HER2 mutations in BC is ~3%. HER2(L755S) is the most common alteration associated with lapatinib resistance in MBC treated with prior trastuzumab 90 , 91 . This activating HER2 mutation has also conferred resistance to dual blockade by trastuzumab and pertuzumab and reduced sensitivity to T-DM1. Second-generation TKIs (for example, afatinib and neratinib) can overcome treatment resistance in BC models, suggesting that they may be therapeutic alternatives for treatment of patients with HER2 + tumours harbouring the HER2 L755S mutation 92 . Neratinib was shown to be active against HER2 mutant/HER2-non-amplified MBC in the SUMMIT basket trial 93 .
HER2 mutations frequently co-occur with HER3 mutations, and cancers with both of these mutations respond poorly to neratinib. The HER3 E928G kinase domain mutation has been shown to enhance the affinity of HER2/HER3 and reduce binding of HER2 to neratinib 94 . Co-expression of HER2 and HER3 mutations leads to enhanced downstream PI3K–AKT pathways and resistance to neratinib. Thus, combined anti-HER2 and PI3K inhibition with a PI3Kα inhibitor such as alpelisib may be a promising strategy to overcome HER2 resistance due to co-occurring HER2 and HER3 mutations.
The generation of tucatinib-resistant BC cell lines revealed significant phosphorylation of HER2 receptors and reactivation of downstream signalling pathways, unlike the partial reactivation of HER signalling seen in lapatinib- and neratinib-resistant models 95 . Acquired resistance to tucatinib was dependent on amplified EGFR signalling and could be overcome by a combination of gefitinib and tucatinib or pan-HER TKIs, such as neratinib, pyrotinib or poziotinib. Using organoids derived from xenograft tumours of HER2 + BC resistant models, neratinib-resistant models were shown to be cross-resistant to other single-agent HER2 TKIs, but the presence of co-occurring HER2 and PIK3CA mutations suggested susceptibility to combination with a PI3K or AKT inhibitor 96 .
Loss or masking of HER2 epitope
The presence of p95 HER2 has been associated with poor outcomes in patients with HER2 + EBC, and patients with MBC overexpressing p95 HER2 had lower response rates to trastuzumab than those expressing full-length HER2 (ref. 97 ). Chemotherapy sensitized p95 HER2 /611CTF xenografts to trastuzumab, presumably via HER2 stabilization induced by chemotherapy 98 . Lapatinib inhibited p95 HER2 phosphorylation in cell lines, reducing downstream activation of AKT and MAPK and inhibiting cell growth 46 . Retrospective analyses from clinical trials with lapatinib noted that the presence of p95 HER2 had no influence on lapatinib efficacy 99 . Similarly, in the CHER-LOB neoadjuvant study, which randomized patients to receive trastuzumab, lapatinib or their combination, p95 HER2 expression was not predictive of outcome nor did it predict for sensitivity to either anti-HER2 agent 100 . Therefore, the role of p95 HER2 as a biomarker of resistance or sensitivity remains to be confirmed.
Overexpression of mucin 4 (MUC4) and the CD44–hyaluronan polymer complex interferes with trastuzumab binding by masking the HER2 epitope and activating HER2. Increased MUC4 expression in oestrogen receptor-positive (ER + )/HER2 + tumours leads to reduced trastuzumab-binding sites 101 . Soluble TNF upregulated MUC4 expression, resulting in trastuzumab resistance, and combining a soluble TNF inhibitor with trastuzumab prevented tumour growth in preclinical models 102 . Recent data also show that MUC4 expression results in an immunosuppressive TME in HER2 + BC and emphasize the role of tumour-infiltrating macrophages in mounting an antitumour response 103 . INB03, a second-generation TNF inhibitor that increases antitumour macrophage phagocytosis and increases lymphocyte function in the TME, is being considered for evaluation in clinical trials.
Activation of compensatory pathways
The activation of compensatory signalling pathways to overcome effects of trastuzumab treatment has been a subject of extensive exploration. Nearly 25–50% of BCs harbour PIK3CA mutations, with enrichment in HR + (~35%) and HER2 + (~25%) subtypes. PIK3CA mutations are associated with reduced pCR rates to neoadjuvant anti-HER2 therapies and decreased efficacy with trastuzumab or pertuzumab in HER2 + MBC 104 , 105 . Loss of PTEN (a key tumour suppressor), which leads to hyperactivation of the PI3K pathway, has also been observed in trastuzumab-resistant tumours 106 , 107 . Development of PI3K and mTOR inhibitors offered hope of a therapeutic option, but early trials with buparlisib (a pan-PI3K inhibitor) in the neoadjuvant setting did not yield the desired outcomes 108 . The BOLERO-1 and BOLERO-3 trials evaluated the addition of the mTOR inhibitor everolimus to trastuzumab plus chemotherapy in first-line and trastuzumab-resistant HER2 + MBC settings, respectively. Biomarker analysis from the pooled study populations indicated PI3K–AKT–mTOR pathway aberrations in approximately 40% of tumours, with everolimus treatment leading to a consistent benefit in these patients versus patients with tumours not exhibiting the aberrations 109 . Although these results suggested proof of concept, the triplet combinations led to increased toxicity. The advent of isoform-specific PI3K inhibitors such as alpelisib has led to a maintenance study of triplet alpelisib-trastuzumab-pertuzumab in patients with PIK3CA-mutant HER2 + MBC ( NCT04208178 ; see Related links).
Bidirectional crosstalk exists between the HER2 and ER pathways, and preclinical studies have demonstrated a role for ER signalling in promoting resistance to anti-HER2 therapies 110 , 111 . Clinical evidence also shows that ER pathway activation offers an escape from HER2 inhibition, and concomitant inhibition of both ER and HER2 signalling may be necessary as demonstrated in trials of ER + /HER2 + BC 51 , 112 , 113 , 114 . The cyclin D1–CDK4/6 axis also has a role in resistance to anti-HER2 therapies 115 . CDK4/6 inhibitors appear to be synergistic with trastuzumab and/or lapatinib in inhibiting growth of HER2 + cell lines 116 , 117 . This was evidenced in the clinical setting in which dual inhibition of CDK4/6 and HER2 led to improved outcomes in monarcHER and PATRICIA trials in heavily pretreated HR + /HER2 + MBC 118 , 119 . The PATINA trial is evaluating whether addition of palbociclib to front-line trastuzumab, pertuzumab and taxane plus endocrine therapy (ET) in HR + /HER2 + MBC will delay the onset of therapeutic resistance ( NCT02947685 ; see Related links). Preclinical and clinical data corroborate cyclin D1-mediated resistance to HER2-targeted therapies, and CDK4/6 inhibitors can overcome this resistance. Cyclin D1 overexpression correlated with lower pCR rates in patients receiving neoadjuvant trastuzumab plus chemotherapy 117 . In the Na-PHER2 study, trastuzumab and pertuzumab given with fulvestrant and palbociclib neoadjuvantly to patients with ER + /HER2 + BC, led to a significant Ki67 reduction after 2 weeks of therapy, clinical complete response (CR) in 50% of patients and a 27% pCR rate (breast and axilla) 120 . These data suggest that a combination blocking HER2 and ER and CDK4/6–cyclin D1 activation offers a chemotherapy-free alternative for treatment of HR + /HER2 + BC. An ongoing trial is evaluating trastuzumab plus palbociclib with or without letrozole in HER2 + and ER +/− MBC ( NCT02448420 ; see Related links).
Genomic sequencing analysis of 733 HER2-amplified primary and metastatic breast tumours revealed significant enrichment of mutations that activate RAS–MAPK signalling in advanced tumours treated with prior anti-HER2 therapies 121 . These mutations, including NF1 and HER2 activating mutations, contribute to resistance to tucatinib and neratinib. The resistant tumours were highly sensitive to MEK–ERK inhibition, with susceptibility due to MEK-dependent activation of CDK2 kinase, thus offering the possibility of overcoming HER2 resistance with MEK–ERK inhibitors.
HER2 heterogeneity
HER2 heterogeneity — the variable expression of HER2 across the tumour — is another potential source of resistance to HER2-targeted therapies. HER2 heterogeneity, defined as HER2 positivity by FISH in 5–50% of tumour cells, or an area of tumour that tested HER2-negative (HER2 − ) in multiple core biopsies, was found in 10% of patients in a phase II trial of neoadjuvant T-DM1 plus pertuzumab 122 . A significant association was found between HER2 heterogeneity and lack of pCR following dual HER2-targeted therapy; none of the patients with HER2 heterogeneity achieved a pCR, whereas 55% of patients not classified as HER2 heterogeneous had a pCR. T-DXd demonstrated significant activity in HER2 low MBC, significantly improving PFS and OS, and this attribute may also enable T-DXd to overcome resistance due to heterogeneous expression of HER2, which could potentially become even more important in the early setting for patients with tumour heterogeneity 17 .
Host and tumour immunity
ADCC is a key mechanism mediating the antitumour activity of trastuzumab, and it can be hampered by an immunosuppressive TME. NK cells have an important role in antitumour immunity, and their activity is regulated by careful modulation of inhibitory and activating receptor signalling 123 . Tumour cells expressing high levels of HLA class I molecules can inhibit NK cells through the engagement of killer cell immunoglobulin-like receptors (KIRs). HLA-G was shown to desensitize BC cells to trastuzumab by binding to the NK cell receptor KIR2DL4, and blocking this HLA-G–KIR2DL4 signalling made HER2 + BC susceptible to trastuzumab treatment in vivo 123 . Moreover, trastuzumab increased the production of TGFβ and interferon-γ (IFN-γ) by BC cells and NK cells, respectively. TGFβ induced PD1 expression on NK cells, and PD1 blockade significantly increased cytotoxicity of NK cells. Accordingly, combined blockade of HLA-G and PDL1/PD1 may be necessary for effective treatment of trastuzumab-resistant BC.
Trastuzumab can also engage Fcγ receptors on macrophages to promote antibody-dependent cellular phagocytosis (ADCP), which contributes to its antitumour efficacy. Magrolimab, a humanized mAb that targets CD47 was studied to combat trastuzumab resistance by activating ADCP 124 . CD47 is a protein that acts as a ‘don’t eat me’ signal via its interaction with signal regulatory protein-α (SIRPα) on macrophages to inhibit phagocytosis. CD47 has been shown to be upregulated in HER2 + BC 125 . The combination of magrolimab and trastuzumab was found to eliminate HER2 + BC cells with increased efficacy due to enhancement of ADCP by macrophages, even in HER2 + BC resistant to ADCC 124 . This offers a novel therapeutic approach to treat trastuzumab-sensitive or trastuzumab-resistant HER2 + BC, provided the trastuzumab-binding epitope on HER2 is accessible.
Other potential mechanisms of resistance
Several other mechanisms of anti-HER2 therapy resistance have recently been elucidated. A study modelling resistance of HER2 + PIK3CA-mutant BC using two patient-derived xenografts, one resistant to paclitaxel and T-DM1 and the other insensitive to T-DM1 and pertuzumab, demonstrated that alveolar epithelial and fibroblastic reticular as well as lymphatic vessel endothelial hyaluronan receptor 1-positive (Lyve1 + ) macrophages may be putative drivers of therapeutic resistance 126 . These intriguing findings require further studies comparing data from transcriptome and exome profiling from trials with anti-HER2 therapies. Preclinical studies hint that abnormal transit of T-DM1 through the endosomal maturation pathway may be responsible for resistance to T-DM1 treatment, but this has not been validated or studied in clinical trials 127 .
Three novel markers, RAC1, CDK12 and VTCN1, have been found to correlate with response to lapatinib, neratinib and tucatinib in a study that compared the TKI anti-proliferative effects using a 115-cancer cell line panel to identify novel markers of TKI response and/or resistance markers 128 . Prior data have implicated these genes in resistance to anti-HER2 therapies or immunotherapy 129 , 130 , 131 . Hence, combinations of HER2 TKIs and CDK12 and RAC1 inhibitors may offer a therapeutic strategy in high CDK12- or RAC1-expressing HER2 + BC.
Next-generation therapies for HER2 + BC
The ever-changing face of cancer and its ability to evade existing therapies have underscored the need for continued development of therapeutics based on existing and/or novel platforms and for uncovering new vulnerabilities in resistant tumours. Antibodies targeting alternative HER2 domains or other HER family members have been explored with the goal of achieving a more complete blockade of HER2 and dampening the effects on downstream signalling pathways. Novel ADCs carrying different payloads to avoid cross-resistance to existing therapies or new linkers to offset off-target toxicity are also being actively pursued.
Disruption of HER2–HER3 dimerization is important for HER2-driven signalling and is targeted effectively by pertuzumab 132 . The positive results from pertuzumab trials supported a strategy of targeting HER3, which has a crucial role in HER2-mediated tumorigenesis. HER3 is unique compared with other HER family members as it is defined by the absence of a functional kinase domain and thus any catalytic activity. HER3 is the preferred dimerization partner for HER2, and HER2–HER3 dimerization leads to oncogenic activation of the PI3K signalling pathway, mediating resistance to HER2-targeted therapy 133 . Several HER3-targeted antibodies have been evaluated in the past decade, mainly targeting the ECD of HER3 (for example, seribantumab and patritumab) and some with modifications to improve ADCC (for example, lumretuzumab, TrasGex) or trap HER3 in an inactive conformation (elgemtumab). Although these drugs have shown some promising preliminary activity, most are no longer in clinical development for HER2 + BC given the high bar of efficacy set by standard-of-care anti-HER2 therapies 133 , 134 , 135 , 136 , 137 , 138 , 139 . Now, there is greater focus on novel ADCs that target HER2 or HER3. Additionally, bispecific antibodies that target multiple epitopes of HER2, or HER2 and HER3 together in one molecule, are under clinical investigation and are discussed in the next section.
ADCs have successfully combined the antitumoural features of cytotoxics and HER2 antibodies into a single pharmacological entity that has greater efficacy than the sum of its parts 7 , 8 . In addition to the ability of ADCs to hone in on the cells expressing the target protein and cause tumour cell lysis, the membrane-permeable payload can diffuse into the surrounding tumour milieu, inducing the bystander effect. This feature enables activity against tumours with low or heterogeneous target expression, thus expanding the pool of susceptible tumour cells. Furthermore, the ADCC of the Fc fragment of the antibody may also contribute to antitumour efficacy. HER2 ADCs can also retain trastuzumab-mediated activity such as inhibiting the HER2 dimerization and suppression of downstream signalling. There are encouraging data against HER2 + brain metastases with these HER2 ADCs 81 , 87 , 140 . Coupled with their demonstrated activity in HER2 low MBC, further evaluation of these ADCs is warranted in this high-risk patient population 17 .
Given the success of T-DM1 and T-DXd, there are more than a dozen HER2-targeted ADCs now in clinical development, with the aim of improving therapeutic index and efficacy. These ADCs differ from the approved agents in cytotoxic payload, DAR, linker or the HER2 epitope targeted. The development of linkers for ADCs has been a very important area of investigation and has been recently reviewed 141 . Several ADCs currently in development for HER2 + BC are listed in Table 1 , and some of these are discussed in further detail below.
Trastuzumab duocarmycin (SYD985)
Trastuzumab duocarmycin (SYD985) is a HER2-targeted ADC based on trastuzumab with a cleavable linker duocarmycin (vc-seco-DUBA) payload. The novel payload is an active toxin (DUBA) that alkylates DNA, causing DNA damage in both dividing and non-dividing cells. The protease-cleavable linker, and subsequent release of the payload into the TME by diffusion, promotes a bystander effect that allows activity in tumour cells with low HER2 expression 142 . In the phase III TULIP study of SYD985 versus physician’s choice of chemotherapy plus anti-HER2 therapy in patients with HER2 + MBC who had received two or more lines of MBC therapy, SYD985 was associated with a significant improvement in PFS 143 , 144 . Ocular toxicity was the most common adverse event (AE) reported, and as for other HER2 ADCs, interstitial lung disease (ILD)/pneumonitis was also observed in a small percentage of patients. A biologics licence application for SYD985 has been recently submitted to the FDA (see Related links: https://go.nature.com/3VZlL4a ), and it remains to be seen how it may integrate into the standard of care with T-DXd and tucatinib.
ARX788 is a next-generation HER2 ADC created using site-specific oxime conjugation technology and a non-cleavable linker designed for homogeneity and chemical stability 145 . It also employs a highly hydrophilic payload (AS269, synthetic dolastatin) with limited cell permeability, unlike other ADCs that use highly permeable payloads to elicit a bystander killing effect. Preclinical data with ARX788 demonstrated activity in HER2 + tumours, T-DM1-resistant BCs, and HER2 low tumours 145 . Promising efficacy data and low systemic toxicity (due to the stable linker) were reported from a phase I trial, and a phase II randomized trial is ongoing ( NCT04829604 ; see Related links) 146 .
Disitamab vedotin (RC48-ADC)
Disitamab vedotin (RC48-ADC) comprises the humanized anti-HER2 antibody hertuzumab coupled via a cleavable linker to the cytotoxic agent monomethyl auristatin E (MMAE). Disitamab targets different epitopes of the HER2 receptor and has better molecular affinity for HER2 than trastuzumab 147 . The linker in disitamab vedotin uses random coupling of cysteine, which is more homogeneous than lysine. Furthermore, this agent has better endocytosis, which is independent of V-ATPase activity, and has no lysosomal resistance 147 . Preclinical data demonstrated good activity in HER2-overexpressing cancer cells and a robust bystander effect targeting neighbouring tumour cells 147 . Multiple clinical trials evaluating disitamab vedotin are ongoing in solid tumours including MBC and gastric cancer. Disitamab vedotin has already received regulatory approval in China for treatment of HER2 + gastric cancer and urothelial cancer 147 , 148 .
Zanidatamab zovodotin (ZW49)
Zanidatamab zovodotin (ZW49) is a bispecific HER2-targeted ADC combining the unique design of zanidatamab (ZW25) (binds ECDs II and IV of HER2) with a cytotoxic and cleavable linker. The biparatopic antibody of ZW49 demonstrated lysosomal trafficking and superior internalization relative to a HER2-targeted monospecific ADC 149 . Preclinical data indicated efficacy in HER2 low - and HER2 high -expressing models and also in a brain metastasis model 149 . ZW49 is being evaluated in a phase I trial in patients with metastatic HER2-expressing cancers that have progressed following standard therapies, including HER2-targeted agents ( NCT03821233 ; see Related links).
In an effort to improve the potency of HER2-targeting ADCs, a pertuzumab-based ADC with lower affinity for HER2 at acidic endosomal pH was developed 150 . Engineering the ADC to confer endosomal dissociation from its target is expected to enable payload entry into lysosomes and recycling of unbound target. This engineered HER2 ADC variant (referred to as an ALTA-ADC) demonstrated increased lysosomal delivery and cytotoxicity even on tumour cells with intermediate levels of HER2 expression, and higher efficacy in xenograft models in mice compared with T-DM1. Furthermore, the ability of the ALTA-ADCs to achieve a therapeutic effect at lower doses may help to overcome the dose-limiting toxicities for other tumour targets.
Targeted thorium-227 conjugates
Targeted α-therapy aims to deliver α-particle-emitting radionuclides selectively to cancer cells in the TME. These α-particles are highly cytotoxic, inducing difficult-to-repair, clustered double strand DNA breaks, leading to cell death 151 . Targeted thorium-227 conjugates (TTCs) have been generated using efficient chelators that connect the α-particle-emitting radionuclide to an antibody to a target expressed on tumours 151 . Exposure of cancer cells to TTCs releases markers of danger-associated molecular patterns (DAMPs), which are upregulated by dying cells to alert the immune system and to initiate immunogenic cell death 152 . The synthetic lethal effect of the combination of TTCs with other DNA-damaging agents was demonstrated using colorectal cancer xenografts 153 . Combination of a HER2 TTC with olaparib resulted in complete growth inhibition in a human DLD-1 BRCA2 –/– xenograft. A first-in-human (FIH) study has been initiated with a HER2 TTC in patients with metastatic breast or gastric cancer ( NCT04147819 ; see Related links).
Targeting the intracellular kinase domain of HER2 using small-molecule inhibitors continues to be investigated with next-generation TKIs. This class of compounds is attractive owing to their unique ability to cross the BBB and BTB although some compounds pose a challenge owing to promiscuous activity and EGFR side effects of rash and diarrhoea. Selected new TKIs in development are listed in Table 2 .
DZD1516 was designed as an oral, reversible and selective HER2 kinase that has full BBB penetration. It demonstrated tumour regressions in xenograft mouse models including subcutaneous, brain metastasis and leptomeningeal metastasis models. Phase I pharmacokinetic data supported once-daily dosing. DZD1516 was also well tolerated, with most AEs being grade 1 events 154 . Interestingly, diarrhoea was not noted as an AE in this study, distinguishing it from most of the other HER2 TKIs studied.
Oncogenic mutations in HER2 have been identified in multiple solid tumours including BC, and most of these occur at allosteric sites outside the ATP-binding site of HER2. BDTX-189 is an oral, ATP-competitive, irreversible, small-molecule inhibitor of EGFR/HER2 alterations and HER2 wild type, designed to spare EGFR wild type to minimize toxicity 155 . BDTX-189 demonstrated potent, sustained inactivation of multiple allosteric ERBB mutants in vivo. A phase I trial in advanced solid tumours harbouring specific allosteric HER2 or HER3 mutations or other EGFR/HER2 alterations is ongoing. Early data show activity with BDTX-189 in HER2-amplified tumours and in patients with non-small-cell lung cancer (NSCLC) with EGFR and HER2 exon 20 insertions 155 .
In general, it appears that the focus of newer HER2 TKIs has shifted from HER2 + BC to solid tumours harbouring HER2 point mutations or alterations given that the frequency of these alterations varies from 1% to 2% in BC and up to 5–10% in other cancers (for example, stomach and bladder cancers) 156 . Preliminary activity of poziotinib in HER2 exon 20 mutant NSCLC has been reported, and there is an ongoing basket trial with neratinib in solid tumours with HER2 mutations ( NCT01953926 ; see Related links) 157 .
Bispecific antibodies
Advances in antibody biology and engineering have led to the development of bispecific antibodies that contain two binding sites directed against two separate antigens or conversely, can target two separate epitopes on the same antigen. Great diversity is possible with this format as bispecifics can also target an antigen with one binding site, and the other site can be an immune target that could elicit a synergistic effect. Key examples of bispecific antibodies currently in development are discussed below.
Zanidatamab (ZW25)
Zanidatamab (ZW25) is a humanized, bispecific, IgG1 antibody directed against the ECD IV and the dimerization domain (ECD II) of HER2, the same domains as are targeted by trastuzumab and pertuzumab, respectively. Unlike trastuzumab, where each receptor can be bound by only one mAb, zanidatamab promotes receptor clustering whereby each HER2 receptor can be targeted by two zanidatamab antibodies. Hence, treatment with zanidatamab leads to enhanced HER2 internalization, downregulation and potent effector-function-mediated cytotoxicity 158 . Zanidatamab showed promising antitumour activity as monotherapy or in combination with chemotherapy in patients with advanced HER2-expressing cancers that had progressed on anti-HER2 therapies 159 , 160 . Zanidatamab in combination with palbociclib and fulvestrant is currently under evaluation in HR + /HER2 + MBC ( NCT04224272 ; see Related links), and zanidatamab in combination with ALX148 (a CD47 blocker) is being investigated in HER2 high and HER2 low BC ( NCT05027139 ; see Related links). A neoadjuvant pilot trial with single-agent zanidatamab is also being planned in HER2 + EBC ( NCT05035836 ; see Related links).
Zenocutuzumab (MCLA-128)
Another bispecific, humanized IgG1 antibody that is under investigation is zenocutuzumab (MCLA-128), which acts via two independent mechanisms of action: inhibition of HER2–HER3 signalling and elimination of tumour cells via ADCC. MCLA-128 functions via a ‘dock and block’ mechanism whereby one arm of the antibody binds HER2 domain I and optimally positions the anti-HER3 arm to block the ligand–HER3 receptor interaction, preventing HER2–HER3 dimerization and activation of downstream signalling 161 . Zenocutuzumab in combination with trastuzumab and vinorelbine demonstrated a 35% clinical benefit rate at 6 months in patients with HER2 + MBC who had progressed on prior anti-HER2 therapy including T-DM1 (ref. 162 ). Further development of zenocutuzumab in HER2 + BC is uncertain; however, its ability to bind HER2 and block NRG1 fusion protein binding and subsequent HER2–HER3 dimerization is being actively explored in NRG1 fusion-positive cancers ( NCT02912949 ; see Related links).
KN026 is a bispecific antibody that targets two distinct epitopes on HER2 (domains II and IV) leading to dual HER2 signal blockade, presumably by causing HER2 to aggregate on the cell surface and endocytose. Results of a FIH trial of KN026 in heavily pretreated patients with HER2 + MBC showed a 28% objective response rate (ORR) and a median PFS of 6.8 months 163 . Translational research suggested that patients with co-amplification of CDK12 and HER2 had better responses to KN026 than patients without the co-amplification. On the basis of these results, additional trials are ongoing or planned with KN026 in HER2 + BC ( NCT04521179 , NCT04881929 , NCT04778982 ; see Related links).
Targeted protein degraders
Targeted protein degradation is being explored as an alternative strategy in cancer, whereby the natural protein degradation system is co-opted for therapeutic purposes. Recently developed novel molecules called proteolysis-targeting chimeras (PROTACs) are heterobifunctional molecules with two ligands joined by a linker. One ligand binds to the ‘protein of interest’ (POI) and the second ligand binds to an E3 ubiquitin ligase. Simultaneous binding of the PROTAC to the POI and ligase induces ubiquitylation of the POI and its degradation by the ubiquitin–proteasome system, followed by regeneration of the PROTAC to tackle another copy of the target 164 . PROTACs are unique because they exhibit a catalyst-type mechanism of action, unlike classic inhibitors which have a one-to-one relationship with the target protein. Two PROTACs, one targeting ER (ARV-471) and the other targeting the androgen receptor (AR) (ARV-110) have demonstrated clinical efficacy; however, these are not tissue specific because they use E3 ligases that have a broad expression profile. In an effort to optimize the therapeutic window and potentially minimize side effects of broad-spectrum PROTACs, an antibody–PROTAC conjugate that specifically targets HER2-expressing cells was developed 165 . This trastuzumab–PROTAC conjugate cages E3 ligase-directed degrader activity with an antibody linker that can be hydrolysed after antibody–PROTAC internalization, releasing the active PROTAC that induces catalytic protein degradation. Studies of a trastuzumab–BRD4 degrader conjugate demonstrated that it selectively targets BRD4 for degradation only in HER2-overexpressing BC cell lines, but not in HER2-negative cell lines 165 . This novel antibody–PROTAC strategy combines the catalytic potency of PROTACs with the tissue specificity of ADCs, enabling the development of new molecules that can target degradation of specific molecules in selected tissues.
An emerging technology aims to selectively degrade HER2-expressing cells by coupling targeted protein degraders to a HER2-specific antibody, generating an antibody neodegrader conjugate (AnDC). Conjugating the protein degrader to the HER2 antibody directs the degrader specifically to the cytosol of the target cells. ORM-5029 is designed to deliver catalytic GSPT1 protein degrader (SMol006) to HER2-expressing tumours via antibody targeting (pertuzumab). Once the antibody and degrader enter the HER2 + tumour cell by endocytosis, the antibody is degraded in the lysosome, releasing the free degrader, which binds to GSPT1 in the cytosol. The natural protein degradation system of the cell is then harnessed to destroy GSPT1, leading to cancer cell death. ORM-5029 displayed in vitro and in vivo efficacy that was comparable to that of other GSPT1 degraders and approved ADCs 166 .
Harnessing the immune system
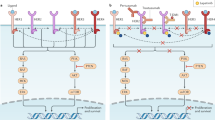
In addition to directly targeting the oncogenic driver HER2 and inhibiting its function, exploiting the innate and adaptive immune system to tackle proliferating cancer cells is an area of promise and active investigation in HER2 + BC. Some of these strategies are outlined below and in Table 3 and Fig. 3 .
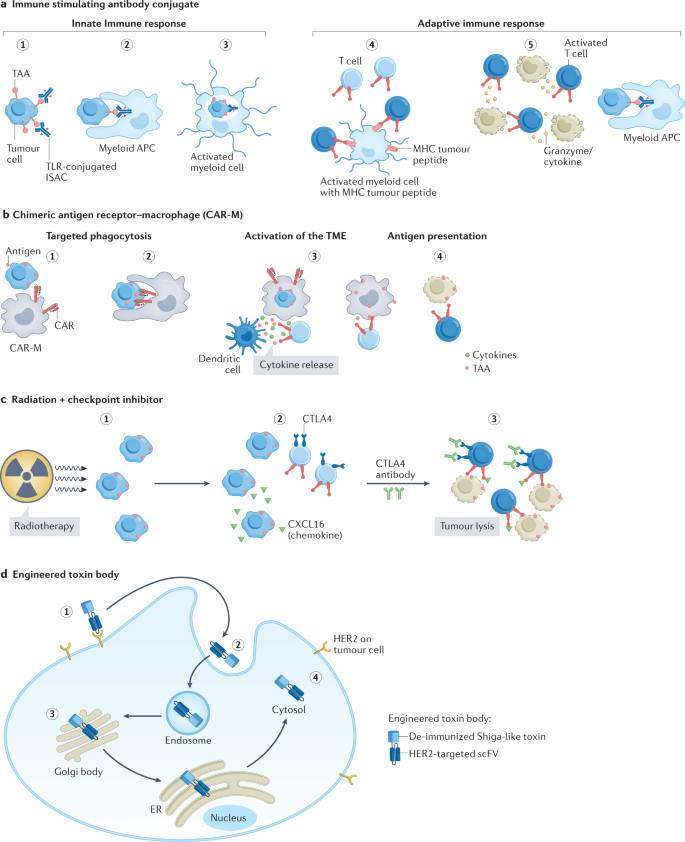
a , Immune-stimulating antibody conjugate (ISAC). (1) ISAC binds to cognate tumour-associated antigen (TAA). (2) Fc receptor-dependent phagocytosis of the tumour cell by myeloid antigen-presenting cell (APC). (3) Toll-like receptor (TLR)-mediated activation of the myeloid cells leads to chemokine and/or cytokine secretion and enhanced antigen presentation. (4) T cell priming by expression of major histocompatibility complex (MHC)–tumour peptide on myeloid cells and expansion of activated T cells. (5) Chemokines attract immune effector cells. Increased myeloid APC phagocytosis. Migration of activated T cells to the tumour and killing of tumour cells. b , Chimeric antigen receptor–macrophage (CAR-M). (1) Targeting of CAR-M to tumour cell expressing the antigen leads to its activation. (2) Phagocytosis of the tumour cell by CAR-M. (3) CAR-M activates the tumour microenvironment (TME) and primes T cells. (4) Primed T cells induce antitumour immune response. c , Radiation plus checkpoint inhibitor. (1) Exposure to radiation results in release of chemokines from tumour cells. (2) Migration of CXC chemokine receptor type 6 (CXCR6)-expressing T cells attracted by chemokines to tumour. (3) Addition of anti-CTLA4 antibody helps to neutralize the CTLA4-mediated inhibition of T cell activation. Activated T cells cause tumour cell lysis. d , (1) HER2-engineered toxin body (ETB) binds to HER2 receptor, followed by (2) forced internalization, (3) intracellular self-routing and (4) ribosome inactivation.
Combinations with checkpoint inhibitors
Immune checkpoint inhibitors (CPIs) have transformed the treatment landscape of solid tumours (for example, melanoma and lung cancer) by inducing the immune system to attack cancer cells, resulting in durable tumour regression and prolonged survival. However, success with these agents in BC has been limited compared with other tumour histologies. There have been no trials in metastatic disease showing benefit outside of the 30–40% of metastatic TNBCs that express PDL1 in the first-line setting. CPI benefits in neoadjuvant TNBC have been broader, irrespective of PDL1 status. Disappointingly, the efficacy of CPI in HER2 + BC has been modest despite high levels of PDL1 expression. Levels of tumour-infiltrating lymphocytes (TILs) in primary HER2 + breast tumours are on par with that in TNBC, indicating a potential for leveraging the immune system 11 . Furthermore, the immune-mediated ADCC mechanism of trastuzumab and pertuzumab suggests that combination immunotherapies may be effective 167 , 168 . Combinations of HER2-targeted agents with CPI have demonstrated preliminary antitumour activity in phase I trials, and T-DXd is being investigated in combination with pembrolizumab ( NCT04042701 ; see Related links) 169 , 170 . In the KATE2 randomized phase II trial of T-DM1 with atezolizumab or placebo in previously treated HER2 + MBC, the addition of atezolizumab did not improve PFS relative to T-DM1 alone 171 . However, this was a later-line setting, and we have learned from TNBC that both setting and line may have profound implications for the activity of immunotherapy in BC. On the basis of the trend to improvement in the PDL1 + subset in KATE2, the phase III KATE3 trial is enrolling patients with PDL1 + HER2 + MBC to receive T-DM1 plus atezolizumab or placebo ( NCT04740918 ; see Related links). Further investigations of anti-HER2 therapies in combination with CPI in earlier lines of treatment are ongoing ( NCT03199885 , NCT04538742 ; see Related links). The underlying complexity of tumour–immune system interactions may limit the efficacy of targeting a single immune checkpoint in isolation. Hence, combination strategies that simultaneously hinder multiple checkpoints or those that invoke lasting immunological memory may be more effective and may also counter the development of resistance.
Radiation therapy (RT), a common modality for BC treatment, has an immunostimulatory effect by altering the TME, exposing tumour antigen and inducing anti-inflammatory responses 172 . Hence, addition of RT to CPI may enhance responses (Fig. 3 ). Results from a trial of whole-brain radiation therapy (WBRT) and concurrent CTLA4-mediated immune modulation with tremelimumab plus or minus trastuzumab in patients with BC with brain metastases, demonstrated a 12-week non-CNS disease control rate (primary end point) of 10% in patients with HER2 − MBC and 33% in patients with HER2 + MBC 173 . Tremelimumab plus durvalumab (anti-PD1 antibody) with brain RT has also been evaluated in MBC including in HER2 + disease ( NCT02563925 ; see Related links). Most ongoing trials exploring RT plus CPI are restricted to patients with HER2 − or TNBC disease, although there is one trial that aims to combine pembrolizumab with single-fraction radiation boost to enhance efficacy in operable BC including HER2 + disease ( NCT04454528 ; see Related links).
Bispecific engagers
The poor activity of immunotherapy in BC is attributed to the immunosuppressive TME after chemotherapy, potentially due to loss of the MHC class I molecules on metastatic tumours leading to reduced or delayed recovery of the T cell repertoire clones that target BC-specific antigens 174 . Novel bispecific antibody (BsAb) formats can overcome this barrier by simultaneously binding a tumour-specific antigen and an immune cell to cause tumour cell death. HER2 is a common antigen targeted by these BsAbs. Bispecific T cell engagers (BiTEs) redirect T cells to target HER2-expressing tumour cells using a BsAb directed against CD3 and HER2 (Fig. 4 ). The advantage of the BiTE approach is that T cell activation is independent of antigen specificity, and a large fraction of the T cells are activated. Bispecific killer cell engagers (BiKEs) bind to CD16 on natural killer (NK)/monocytic cells and HER2 on tumour cells to eradicate HER2-expressing cancer cells.
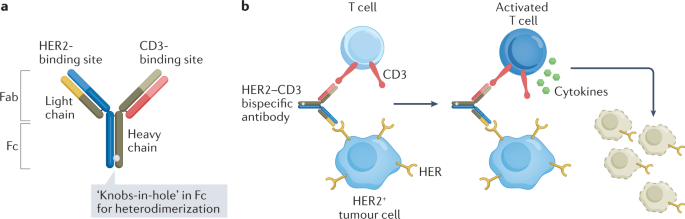
a , Structure of HER2–CD3 bispecific antibody with ‘knobs-in-hole’ technology. b , Mechanism of action of a HER2–CD3 bispecific antibody. Step 1: binding of HER2–CD3 bispecific antibody to HER2 on tumour cell and CD3 on T cell. Step 2: activation of T cell causing release of cytokines (TNF and interferon-γ (IFNγ)). Step 3: tumour cell lysis.
An early candidate, 2B1, was a murine-derived HER2 with a Fcγ bispecific antibody that activated NK cells against HER2-expressing tumour cells. Although 2B1 did not demonstrate antitumour activity in a phase I trial, there was evidence of immune activation in 10 of 20 patients 175 . These findings contributed to a better understanding of the mechanism by which antibody-induced ADCC may exert antitumour activity.
Innovations led to the ‘knobs-in-hole’ technology, which involves replacing a smaller amino acid with a larger amino acid (T336Y) in the CH3 region of an antibody chain to form a ‘knobs’ structure and at the same time substituting a larger amino acid in the other chain with a smaller amino acid to form a ‘hole’ structure (Y407T). This technology, which enables Fc heterodimerization, results in a BsAb that is more stable, has favourable pharmacokinetics and can be produced with high quality and reproducibility 176 . M-802 and runimotamab (BTRC4017A) are examples of bispecifics that use this technology that are under evaluation in clinical trials (Table 3 and Fig. 4 ; NCT04501770 , NCT03448042 ; see Related links). An alternative approach is to use antibodies directed against specific receptors expressed on T cells or NK cells (for example, CD137, NKG2A) in combination with HER2-targeted therapies to enhance antitumour activity. Monalizumab is an antibody that blocks the interaction between the inhibitory checkpoint receptor NKG2A expressed on NK cells and CD8 + T cells and HLA-E, which is overexpressed on malignant tumours 177 . Blocking this inhibitory interaction between NKG2A and HLA-E overcomes tumour resistance to NK cells. Since monalizumab can act simultaneously on both NK cells and T cells as well as tumour-infiltrating immune cells, it can enhance the cytotoxic potential of therapeutic antibodies such as trastuzumab and as such, is being investigated in the phase II MIMOSA trial in patients with HER2 + MBC ( NCT04307329 ; see Related links). CD137 is a costimulatory immune receptor expressed on activated T and B cells and NK cells and a potential target for cancer immunotherapy as it is expressed on TILs. Upon activation, it promotes increased proliferation, cytokine production and cell lysis by T and NK cells 178 , 179 .
Other bispecific antibodies
A different approach combining anti-HER2 and PDL1 antibodies into one BsAb (IBI315) was employed to redirect anti-PDL1 response to HER2-expressing tumour cells. Results of a phase I dose-escalation trial in patients with advanced solid tumours treated with various dose levels of IBI315 showed a 20% ORR (see Innovent press release in Related links: https://go.nature.com/3f91toe ). Theoretically, this may be a very clean way to use immunotherapy: effectively, targeting just the HER2-expressing cancer cells without as many off-target effects.
PRS-343 (cinrebafusp alfa) (Table 3 ) is a novel bispecific fusion protein that combines a 4-1BB (CD137)-targeting anticalin protein and a modified version of trastuzumab. The HER2-targeting moiety localizes the drug to the HER2-expressing cells in the TME and facilitates 4-1BB cross-linking, which ameliorates T cell exhaustion and enables T cell expansion and eventual tumour regression. In a phase I study in patients with HER2-expressing solid tumours, PRS-343 demonstrated activity as monotherapy and in combination with atezolizumab 180 .
Another BsAb innovation is the generation of patient-derived activated T cells (ATCs) armed with an anti-CD3–anti-HER2 bispecific antibody (HER2Bi), meaning these T cells are coated with the BsAb. Essentially, polyclonal T cell populations expanded and activated ex-vivo can be armed with a HER2–CD3 bispecific antibody to specifically recognize and kill HER2-expressing tumour cells in vivo. Arming ATCs with HER2Bi redirects the non-MHC-restricted cytotoxicity of ATCs to a HER2-specific target. A phase I trial that evaluated HER2Bi–aATCs administered with IL-2 and granulocyte–macrophage colony-stimulating factor (GM-CSF) in 23 patients with MBC demonstrated preliminary efficacy, and no dose-limiting toxicities (DLTs) were observed 181 .
Immune-stimulating antibody conjugates
Immune-stimulating antibody conjugates (ISACs) covalently attach immune stimulants to tumour-specific antibodies such as trastuzumab and can trigger target tumour-dependent activation of the innate and adaptive immune systems to eradicate tumours 182 (Fig. 3 ).
Toll-like receptors (TLRs) are important components that initiate innate immunity. These receptors also bridge innate and adaptive immunity 183 . BDC-1001 is an example of an ISAC that consists of a trastuzumab biosimilar conjugated to a TLR7/8 agonist with a non-cleavable linker. BDC-1001 employs a three-pronged approach — direct antitumour effects mediated by trastuzumab, localized phagocytosis and elimination of HER2-expressing tumour cells by the immune-stimulating TLR7/8 molecules that activate the myeloid antigen-presenting cells (APCs), that is, macrophages and DCs. This results in cytotoxicity and subsequent processing and presentation of neoantigens that stimulates T cell-mediated durable immunity. The advantage with this construct is that TLR7/8 activation occurs only after internalization into the APCs, thus mitigating the risk of nonspecific immune activation. In essence, these ISACs convert the TME from ‘cold’ to ‘hot’ by localized stimulation of the immune system. Additional agents conjugating trastuzumab or biosimilar to TLR7/8 that are in development are listed in Table 3 .
Engineered toxin bodies
Engineered toxin bodies (ETBs) are novel immunotoxins that combine the specificity of antibodies with the potent mechanism of bacterial toxin cell destruction. MT-5111 (Table 3 ), an example of a de-immunized ETB fused to a HER2 antibody, has a novel mechanism of action that induces direct cell-kill via enzymatic and permanent ribosome destruction, thus potentially bypassing resistance mechanisms that exist for HER2 TKIs, ADCs or antibody modalities (Fig. 3 ). Furthermore, MT-5111 binds to an epitope on HER2 that is distinct from that of trastuzumab and pertuzumab, enabling combination with existing HER2-targeting agents 184 . Preliminary results of a phase I trial of MT-5111 in HER2-expressing solid tumours showed stable disease as best response, no DLTs and maximum tolerated dose not yet reached 185 . An expansion cohort for patients with MBC is ongoing.
CAR-M and CAR-NK therapies
Chimeric antigen receptors (CARs), engineered molecules that combine the specificity of antibodies with the downstream signalling of T cells, represent a key class of cellular immunotherapies. Although FDA-approved CAR-T therapies are available for haematological malignancies, their application to solid tumours has been hindered by the need for active trafficking and penetration of T cells into the TME. As such, alternative immune cells such as human macrophages have been genetically engineered with CARs (CAR-M) to redirect their phagocytic activity against solid tumours 186 (Fig. 3 ). A single infusion of these CAR-Ms was able to shrink tumours and improve OS in solid tumour xenograft mouse models 10 . Furthermore, the CAR-Ms converted bystander immunosuppressive M2 macrophages to pro-inflammatory M1 macrophages, upregulated the APC machinery and reprogrammed the TME by activating immature DCs and recruiting activated CD8 + T cells to the tumour sites, thus amplifying the antitumour response.
CT-0508 is a cell product comprising autologous peripheral blood monocyte-derived macrophages that are transduced with an adenoviral vector containing an anti-HER2 CAR-M and locked into a pro-inflammatory M1 phenotype. This agent is being investigated in an ongoing FIH trial in patients with HER2-overexpressing solid tumours, and early data demonstrate good tolerability 187 . Correlative analyses demonstrated evidence of broad reprogramming of the TME as well as intratumoural T cell expansion and activation with altered peripheral T cell repertoire.
CAR-engineered NK cells are superior to CAR-T cells; they appear to be safer because they do not induce cytokine release syndrome (CRS) and they engage multiple mechanisms to promote cytotoxicity 188 . They also lend themselves to ‘off-the shelf’ manufacturing, that is, they can be generated from peripheral blood cells or from stem cell sources or NK-92 cell lines instead of using a patient’s own immune cells 188 . The CAR-NK cells represent receptors against tumour-associated antigens (TAAs) and redirect the effector NK cells to target specific tumours. CAT-179 is an off-the-shelf CAR-NK cell therapy in development, with an optimized CAR that targets HER2, an IL-15 cytokine that enables enhanced and sustained NK cell activity and a TME switch to counteract the immunosuppressive TGFβ signal in the TME.
Cancer vaccines targeting HER2
Cancer vaccines are designed to kindle the patient’s own immune system to identify and kill tumour cells by stimulating CD8 + and CD4 + T cell responses to tumour-specific antigens 189 . Several platforms have been used to develop cancer vaccines, including HER2-specific vaccines. These range from simple peptide vaccines to the more complex autologous or allogenic cell-based vaccines summarized in Table 4 .
A key consideration regarding cancer vaccines is the treatment setting. It is well recognized that disease burden and the immunosuppressive TME in metastatic disease may limit T cell activity. Vaccines may be more successful in a setting of very low disease burden, such as post-operative EBC as well as the pre-malignant ductal carcinoma in situ (DCIS) setting 12 , 190 . Furthermore, combinations with CPIs or chemotherapy and targeted agents may enhance vaccine efficacy in the (neo)adjuvant setting and potentially help to overcome the suppressive TME in the advanced disease setting 191 .
Peptide vaccines
These may be the most common vaccine design and have several advantages over other vaccine types: production is very easy, more cost-effective and easier to administer with relatively few side effects. Peptide vaccines using various domains of the HER2 protein have been evaluated in patients with BC. E75 is a nine-amino-acid immunogenic peptide derived from the extracellular domain of HER2 and is expressed in 60–75% of the population. Nelipepimut-S/NeuVax is an MHC class I vaccine that consists of E75 combined with an immunoadjuvant GM-CSF 192 . A disadvantage is that by being more specific, their T cell immunological responses may be more limited in terms of cell type and may not stimulate T helper cells and B cells sufficiently.
Protein-based vaccines
These offer an advantage over peptide vaccines as they contain both HLA class I and II epitopes and hence are not subject to HLA restrictions. Moreover, they can significantly activate T cells, leading to an enhanced immune response and better T cell activation. Although this approach has not been explored extensively, early trials using a fragment from the intracellular domain (ICD) of HER2 was shown to be well tolerated, and patients developed HER2 ICD-specific T cell immunity 193 . A phase I trial evaluating dHER2 vaccine, a recombinant protein comprising the ECD and a fragment of the ICD combined with the adjuvant AS15, combined with lapatinib in 12 patients with trastuzumab-refractory HER2 + MBC demonstrated anti-HER2 antibody induction in all patients. HER2-specific T cells were detected in one patient, and importantly, there were no signals of cardiotoxicity 194 .
Cell-based vaccines
These can be derived from the patient’s own tumour cells to generate autologous vaccines or alternatively, allogeneic tumour cell lines can be used to develop cell-based vaccines. Allogeneic vaccines offer an alternative because they can be obtained from established cancer cell lines that express specific TAAs. Initial data with an allogeneic HER2–GM-CSF vaccine given with cyclophosphamide and weekly trastuzumab appear promising, warranting further studies 195 (Table 4 ). One disadvantage of autologous vaccines is variability in the vaccine and a lengthy manufacturing process.
Dendritic cells
DCs are potent modulators of the immune response and are specialized APCs that can stimulate naive T lymphocytes while generating memory T lymphocytes. The DC vaccine platform allows for antigen processing by the patient’s own immune system, eliciting immunological responses against multiple epitopes on the target, versus a single epitope approach with an antibody or a small-molecule inhibitor. Preliminary studies using DC vaccines have been conducted in patients with invasive BC and even DCIS; the latter hinting at the potential for possible development of a vaccine for prevention of BC 196 , 197 , 198 , 199 . Although DC vaccines are a promising individualized strategy, the vaccine manufacturing process is complex and will need to be streamlined to make it more accessible. Exploration of allogeneic or artificial APCs should be pursued to overcome this limitation.
Recombinant DNA- or virus-based vaccines
These can be used to deliver tumour-specific antigens and generate antigen-specific cellular and humoral immune responses. DNA-based vaccines are an easy and practical approach given their simplicity, safety and cost effectiveness. Multiple preclinical studies have attested to the efficacy of this strategy. Clinical trials with plasmid-based vaccines are underway in patients with HER2 + EBC with the goal of evaluating the immunogenicity of these vaccines (Table 4 ). The natural immunogenic nature of viruses is an inherent advantage. Unlike DNA-based vaccines, TAAs are presented along with viral antigens, potentially leading to enhanced and long-lasting immune T cell responses 200 . Preliminary results from a phase I trial with a novel alphaviral vector encoding a self-amplifying replicon RNA encoding HER2 segment (VRP-HER2) have been encouraging 201 . Subsequent preclinical studies have shown that only vaccines targeting true oncogenic drivers of HER2 elicited significant antitumour responses, and long-term tumour control was observed only in combination with a CPI. This has led to a phase II study evaluating a combination of VRP-HER2 with pembrolizumab (Table 4 ; NCT03632941 ; see Related links).
Despite being inherently well tolerated and inducing immunogenicity and long-term survival in select patients, cancer vaccines have not demonstrated efficacy in randomized phase II or III trials 202 , 203 . Novel delivery vehicles such as lipid nanoparticles, virus-like particles, polymeric and non-degradable particles are being explored for cancer vaccines 191 , 204 . Previous iterations of vaccines have focused on using TAAs over neoantigens; the latter arise from mutations in the tumour cells owing to their inherent genetic instability, whereas the former are non-mutated antigens, which may explain the lacklustre efficacy observed with vaccine therapies. Neoantigens are able to induce T cell responses comparable to that found in antiviral T cells. New bioinformatic tools for neoantigen prediction have been developed with promising results, which may spur further development of personalized, neoantigen-based therapeutic cancer vaccines including for BC 205 , 206 . The success of the coronavirus disease 2019 (COVID-19) mRNA vaccines has breathed new life into exploring this technology for cancer, and trials with mRNA-based vaccines against solid tumours are in development.
Conclusions and future directions
The remarkable journey of discovery of HER2 as an oncogene, biomarker and target for treatment of a very aggressive form of BC has led to an unprecedented improvement in survival. This success results from the exquisite response of the HER2 receptor to HER2-targeted therapy, which is maintained even after multiple lines of treatment. The huge interest in further development and drug discovery for this focused group of BCs is evidenced by the 1,922 clinical trials for HER2 + BC currently listed in Clinical Trials.gov (see Related links: https://clinicaltrials.gov/ct2/results?cond=HER2-positive+Breast+Cancer&term=&cntry=&state=&city=&dist= ). This success and continued enthusiasm truly represent an enormous effort and cooperation between many basic and clinical scientists to improve the lives of patients. The bridging of drug development from academia to industry and vice versa is a crucial element of the process.
Several pathways have been successfully targeted, and there are various mechanisms of resistance that can be explored to potentially cure HER2 + MBC. The recent update of the hallmarks of cancer adding ‘enabling characteristics’ serves to remind us of the constant discoveries in the area of cancer research and the dynamics of thought regarding the carcinogenic process 207 . There are many fascinating fields of research that are being explored, such as harnessing the microbiome and continued exploration of the role of the gut in immunotherapy as one example 208 . In addition, many tools have been discovered and flourished to enhance drug development, such as artificial intelligence, CRISPR–Cas9, single-cell sequencing, spatial transcriptomics, spatial proteomics and theranostics ; the list keeps growing 209 , 210 , 211 , 212 , 213 , 214 , 215 . Recent drug constructs such as the ADCs and upcoming bispecifics will continue to improve our ability to safely target HER2 + cells, while limiting AEs for our patients. Building on the past accomplishments in treatment with HER2-targeted therapy, the investigation of these newer concepts and use of these methods will ultimately lead to continued advances.
Carpenter, G., King, L. Jr & Cohen, S. Epidermal growth factor stimulates phosphorylation in membrane preparations in vitro. Nature 276 , 409–410 (1978).
Article CAS Google Scholar
Schechter, A. L. et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature 312 , 513–516 (1984).
Slamon, D. J. et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235 , 177–182 (1987). This work demonstrated that HER2 amplification is a prognostic factor and predictive of outcomes in breast cancer .
Giordano, S. H. et al. Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO guideline update. J. Clin. Oncol. 40 , 2612–2635 (2022).
von Minckwitz, G. et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 377 , 122–131 (2017).
Article Google Scholar
Tripathy, D. et al. De novo versus recurrent HER2-positive metastatic breast cancer: patient characteristics, treatment, and survival from the SystHERs registry. Oncologist 25 , e214–e222 (2020).
Kostova, V., Désos, P., Starck, J.-B. & Kotschy, A. The chemistry behind ADCs. Pharmaceuticals 14 , 442 (2021).
Fu, Z., Li, S., Han, S., Shi, C. & Zhang, Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal. Transduct. Target. Ther. 71 , 93 (2022).
Roussos Torres, E. T. & Emens, L. A. Emerging combination immunotherapy strategies for breast cancer: dual immune checkpoint modulation, antibody-drug conjugates and bispecific antibodies. Breast Cancer Res. Treat. 191 , 291–302 (2022).
Klichinsky, M. et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat. Biotechnol. 38 , 947–953 (2020).
Savas, P. et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat. Rev. Clin. Oncol. 13 , 228–241 (2016).
Disis, M. L. & Cecil, D. L. Breast cancer vaccines for treatment and prevention. Breast Cancer Res. Treat. 191 , 481–489 (2022).
Tebbutt, N., Pedersen, M. W. & Johns, T. G. Targeting the ERBB family in cancer: couples therapy. Nat. Rev. Cancer 13 , 663e73 (2013).
Baselga, J. & Swain, S. M. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat. Rev. Cancer 9 , 463–475 (2009).
Wolff, A. C. et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 31 , 3997–4013 (2013).
Wolff, A. C. et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J. Clin. Oncol. 36 , 2105–2122 (2018). Important guidelines for interpretation of HER2 testing results from IHC and FISH.
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N. Engl. J. Med. 387 , 9–20 (2022). A phase III trial that demonstrated the efficacy of the anti-HER2 ADC, trastuzumab deruxtecan, in HER2-low metastatic breast cancer.
Baselga, J. Treatment of HER2-overexpressing breast cancer. Ann. Oncol. 21 , vii36–vii40 (2010).
Baselga, J., Norton, L., Albanell, J., Kim, Y. M. & Mendelsohn, J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 58 , 2825–2831 (1998).
CAS Google Scholar
Pegram, M. et al. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 18 , 2241–2251 (1999).
Pietras, R. J. et al. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene 9 , 1829–1838 (1994).
Pietras, R. J., Pegram, M. D., Finn, R. S., Maneval, D. A. & Slamon, D. J. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene 17 , 2235–2249 (1998).
Piccart-Gebhart, M. J. et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 353 , 1659–1672 (2005).
Romond, E. H. et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 353 , 1673–1684 (2005).
Slamon, D. et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 365 , 1273–1283 (2011).
Moja, L. et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst. Rev. 2012 , CD006243 (2012). A review of ~12,000 patients with breast cancer enrolled in randomized controlled trials evaluating trastuzumab alone or in combination with chemotherapy versus no treatment or standard chemotherapy alone as adjuvant therapy for breast cancer.
Google Scholar
Nahta, R. & Esteva, F. J. Herceptin: mechanisms of action and resistance. Cancer Lett. 232 , 123–138 (2006).
Drebin, J. A., Link, V. C. & Greene, M. I. Monoclonal antibodies reactive with distinct domains of the neu oncogene-encoded p185 molecule exert synergistic anti-tumor effects in vivo. Oncogene 2 , 273–277 (1988).
Ishii, K., Morii, N. & Yamashiro, H. Pertuzumab in the treatment of HER2-positive breast cancer: 671 an evidence-based review of its safety, efficacy, and place in therapy. Core Evid. 14 , 51–70 (2019).
Agus, D. B. et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2 , 127–137 (2002).
Lee-Hoeflich, S. T. et al. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 68 , 5878–5887 (2008).
Nahta, R., Hung, M. C. & Esteva, F. J. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 64 , 2343–2346 (2004).
Scheuer, W. et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 69 , 9330–9336 (2009).
Mamidi, S., Cinci, M., Hasmann, M., Fehring, V. & Kirschfink, M. Lipoplex mediated silencing of membrane regulators (CD46, CD55 and CD59) enhances complement-dependent anti-tumor activity of trastuzumab and pertuzumab. Mol. Oncol. 7 , 580–594 (2013).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 372 , 724–734 (2015). The first randomized phase III trial to demonstrate superiority of dual HER2 therapy of pertuzumab and trastuzumab in HER2 + metastatic breast cancer .
Gianni, L. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 13 , 25–32 (2012).
Schneeweiss, A. et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 24 , 2278–2284 (2013).
Musolino, A. et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu positive metastatic breast cancer. J. Clin. Oncol. 26 , 1789–1796 (2008).
Nordstrom, J. L. et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcγ receptor binding properties. Breast Cancer Res. 13 , R123 (2011).
Liu, L., Yang, Y. & Burns, R. Margetuximab mediates greater Fc-dependent anti-tumor activities than trastuzumab or pertuzumab in vitro. Cancer Res. 79 (Suppl. 13), Abstr. 1538 (2019).
Rugo, H. S. et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 7 , 573–584 (2021).
Jackisch, C. et al. Subcutaneous vs intravenous trastuzumab for patients with ERBB2-positive early breast cancer: final analysis of the HannaH phase 3 randomized clinical trial. JAMA Oncol. 5 , e190339 (2019).
Tan, A. R. et al. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): a randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 22 , 85–97 (2021).
Konecny, G. E. et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2- overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 66 , 1630–1639 (2006).
Nahta, R., Yuan, L. X., Du, Y. & Esteva, F. J. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: effects on insulin-like growth factor I signaling. Mol. Cancer Ther. 6 , 667–674 (2007).
Scaltriti, M. et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J. Natl Cancer Inst. 99 , 628–638 (2007).
Xia, W. et al. Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Cancer Res. 67 , 1170–1175 (2007).
O’Brien, N. A. et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol. Cancer Ther. 9 , 1489–1502 (2010).
Geyer, C. E. et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N. Engl. J. Med. 355 , 2733–2743 (2006). The first trial to demonstrate efficacy of a HER2 TKI + chemotherapy in trastuzumab-resistant HER2 + MBC .
Cameron, D. et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: updated efficacy and biomarker analyses. Breast Cancer Res. Treat. 112 , 533–543 (2008).
Johnston, S. et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 27 , 5538–5546 (2009).
Schlam, I. & Swain, S. M. HER2-positive breast cancer and tyrosine kinase inhibitors: the time is now. NPJ Breast Cancer 7 , 56 (2021).
Lin, N. U. & Winer, E. P. Brain metastases: the HER2 paradigm. Clin. Cancer Res. 13 , 1648–1655 (2007). A review that highlights the increase in brain metastases in patients with HER2 + MBC, the probable causes of this increase, and the development of novel treatments .
Pestalozzi, B. C. et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG). Ann. Oncol. 17 , 935–944 (2006).
Clayton, A. J. et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br. J. Cancer 91 , 639–643 (2004).
Steeg, P. S. The blood–tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 18 , 696–714 (2022).
Lin, N. U. et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin. Cancer Res. 15 , 1452–1459 (2009). The first prospective multicentre study for patients with HER2 + MBC and brain metastases that demonstrated the modest activity of lapatinib in this group of patients .
Bachelot, T. et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 14 , 64–71 (2013).
Collins, D. M. et al. Preclinical characteristics of the irreversible pan-HER kinase inhibitor neratinib compared with lapatinib: implications for the treatment of HER2-positive and HER2-mutated breast cancer. Cancers 11 , 737 (2019).
Rabindran, S. K. et al. Antitumor activity of HKI-272, an orally active, irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 64 , 3958–3965 (2004).
Canonici, A. et al. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget 4 , 1592–1605 (2013).
Cocco, E. et al. Neratinib is effective in breast tumors bearing both amplification and mutation of ERBB2 (HER2). Sci. Signal. 11 , eaat9773 (2018).
Chan, A. et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 17 , 367–377 (2016).
Chan, A. et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the phase III ExteNET trial. Clin. Breast Cancer 21 , 80–91.e7 (2021).
Saura, C. et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥2 HER2-directed regimens: phase III NALA Trial. J. Clin. Oncol. 38 , 3138–3149 (2020).
Xu, B. et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 22 , 351–360 (2021).
Kulukian, A. et al. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol. Cancer Ther. 19 , 976–987 (2020).
Dinkel, V. et al. ARRY-380, a potent, small molecule inhibitor of ErbB2, increases survival in intracranial ErbB2+ xenograft models in mice. Cancer Res. 72 , 852 (2012).
Olson, D. J. et al. Preclinical characterization of tucatinib in HER2-amplified xenograft and CNS implanted tumors. Cancer Res. 80 (Suppl. 16), Abstr. 1962 (2020).
Stringer-Reasor, E. M. et al. Pharmacokinetic (PK) analyses in CSF and plasma from TBCRC049, an ongoing trial to assess the safety and efficacy of the combination of tucatinib, trastuzumab and capecitabine for the treatment of leptomeningeal metastasis (LM) in HER2 positive breast cancer. J. Clin. Oncol. 39 , 1044 (2021).
O’Brien, N. A. et al. The small molecule inhibitor of HER2, tucatinib, has potent and highly selective activity in preclinical modes of HER2-driven cancer. Cancer Res. 79 (Suppl. 4), Abstr. P6-17-11 (2019).
Murthy, R. K. et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 382 , 597–609 (2020). The first randomized trial in HER2 + MBC that enrolled patients with active brain metastases and demonstrated improved outcomes with tucatinib + trastuzumab + capecitabine in this patient population .
Hamilton, E. et al. Tucatinib vs placebo in combination with trastuzumab and capecitabine for patients with locally advanced unresectable or HER2-positive metastatic breast cancer (HER2CLIMB): Outcomes by hormone receptor status. Cancer Res. 81 (Suppl. 4), Abstr. PD3-08 (2021).
Beck, A., Goetsch, L., Dumontet, C. & Corvaia, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug. Discov. 16 , 315–337 (2017).
Staudacher, A. H. & Brown, M. P. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br. J. Cancer 117 , 1736–1742 (2017).
Lewis Phillips, G. D. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 68 , 9280–9290 (2008).
Junttila, T. T., Li, G., Parsons, K., Phillips, G. L. & Sliwkowski, M. X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res. Treat. 128 , 347–356 (2011).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380 , 617–628 (2019).
Hurvitz, S. A. et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 19 , 115–126 (2018).
Krop, I. E. et al. Trastuzumab emtansine plus pertuzumab versus taxane plus trastuzumab plus pertuzumab after anthracycline for high-risk human epidermal growth factor receptor 2-positive early breast cancer: the phase III KAITLIN study. J. Clin. Oncol. 40 , 438–448 (2022).
Montemurro, F. et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann. Oncol. 31 , 1350–1358 (2020).
Yver, A., Agatsuma, T. & Soria, J. C. The art of innovation: clinical development of trastuzumab deruxtecan and redefining how antibody-drug conjugates target HER2-positive cancers. Ann. Oncol. 31 , 430–434 (2020).
Tamura, K. et al. Trastuzumab deruxtecan (DS-8201a) in patients with advanced HER2-positive breast cancer previously treated with trastuzumab emtansine: a dose-expansion, phase 1 study. Lancet Oncol. 20 , 816–826 (2019).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382 , 610–621 (2020).
Cortés, J. et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386 , 1143–1154 (2022).
Swain, S. M. et al. Multidisciplinary clinical guidance on trastuzumab deruxtecan (T-DXd)–related interstitial lung disease/pneumonitis — focus on proactive monitoring, diagnosis, and management. Cancer Treat. Rev. 106 , 102378 (2022).
Jerusalem, G. H. M. et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2+ metastatic breast cancer with brain metastases: a subgroup analysis of the DESTINY-Breast01 trial. J. Clin. Oncol. 39 , 526 (2021).
Pérez-García, J. M. et al. Trastuzumab deruxtecan in patients with central nervous system involvement from HER2-positive breast cancer: the DEBBRAH trial. Neuro-oncol. https://doi.org/10.1093/neuonc/noac144 (2022).
Mosele, M. F. et al. Unraveling the mechanism of action and resistance to trastuzumab deruxtecan (T-DXd): biomarker analyses from patients from DAISY trial. Ann. Oncol. 33 , S123 (2022).
Kancha, R. K. et al. Differential sensitivity of ERBB2 kinase domain mutations towards lapatinib. PLoS ONE 6 , e26760 (2011).
Wagle, N. et al. Whole exome sequencing (WES) of HER2+ metastatic breast cancer (MBC) from patients with or without prior trastuzumab (T): a correlative analysis of TBCRC003. Cancer Res. 75 (Suppl. 9), Abstr. PD3-5 (2015).
Xu, X. et al. HER2 reactivation through acquisition of the HER2 L755S mutation as a mechanism of acquired resistance to HER2-targeted therapy in HER2(+) breast cancer. Clin. Cancer Res. 23 , 5123–5134 (2017).
Hyman, D. M. et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature 554 , 189–194 (2018). Results from the SUMMIT basket trial which demonstrated the clinical actionability of HER2 and HER3 mutations.
Hanker, A. B. et al. Co-occurring gain-of-function mutations in HER2 and HER3 modulate HER2/HER3 activation, oncogenesis, and HER2 inhibitor sensitivity. Cancer Cell 39 , 1099–1114.e8 (2021).
Veeraraghavan J. et al. Acquired resistance to tucatinib is associated with EGFR amplification in HER2+ breast cancer (BC) models and can be overcome by a more complete blockade of HER receptor layer. Cancer Res. 82 (Suppl. 4), Abstr. PD8-06 (2022).
Bose, S. et al. Resistance to next generation tyrosine kinase inhibitors (TKIs) in HER2-positive breast cancer (BC): role of HER and PIK3CA mutations and development of new treatment strategies and study models. Cancer Res. 82 (Suppl. 4), Abstr. P4-01-01 (2022).
Derakhshani, A. et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J. Cell Physiol. 235 , 3142–3156 (2020).
de Melo Gagliato, D., Jardim, D. L. F., Marchesi, M. S. P. & Hortobagyi, G. N. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget 7 , 64431–64446 (2016).
Scaltriti, M. et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2–positive breast tumors coexpressing the truncated p95HER2 receptor. Clin. Cancer Res. 16 , 2688–2695 (2010).
Guarneri, V. et al. Prospective biomarker analysis of the randomized CHER-LOB study evaluating the dual anti-HER2 treatment with trastuzumab and lapatinib plus chemotherapy as neoadjuvant therapy for HER2-positive breast cancer. Oncologist 20 , 1001–1010 (2015).
Miranda, F., Prazeres, H., Mendes, F., Martins, D. & Schmitt, F. Resistance to endocrine therapy in HR + and/or HER2 + breast cancer: the most promising predictive biomarkers. Mol. Biol. Rep. 49 , 717–733 (2022).
Schillaci, R. et al. Neutralizing soluble tumor necrosis factor alpha overcomes trastuzumab-resistant breast cancer immune evasion by downregulating mucin 4, improving NK cell function and decreasing myeloid-derived suppressor cells in tumor microenvironment. Cancer Res. 79 (Suppl. 4), Abstr. P6-20-14 (2019).
Schillaci, R. et al. Mucin 4 expression in high risk breast cancer: predicting and overcoming resistance to immunotherapy. Cancer Res. 82 (Suppl. 4), Abstr. P5-13-32 (2022).
Loibl, S. et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann. Oncol. 27 , 1519–1525 (2016).
Baselga, J. et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J. Clin. Oncol. 32 , 3753–3761 (2014).
Chandarlapaty, S. et al. Frequent mutational activation of the PI3K-AKT pathway in trastuzumab-resistant breast cancer. Clin. Cancer Res 18 , 6784–6791 (2012).
Rimawi, M. F., De Angelis, C. & Schiff, R. Resistance to anti-HER2 therapies in breast cancer. Am. Soc. Clin. Oncol. Ed. Book 35 , e157–e164 (2015).
Loibl, S. et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer: a randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE). Eur. J. Cancer 85 , 133–145 (2017).
André, F. et al. Molecular alterations and everolimus efficacy in human epidermal growth factor receptor 2-overexpressing metastatic breast cancers: combined exploratory biomarker analysis from BOLERO-1 and BOLERO-3. J. Clin. Oncol. 34 , 2115–2124 (2016).
Xia, W. et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc. Natl Acad. Sci. USA 103 , 7795–7800 (2006).
Wang, Y. C. et al. Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers-role of estrogen receptor and HER2 reactivation. Breast Cancer Res. 13 , R121 (2011).
Vaz-Luis, I., Winer, E. P. & Lin, N. U. Human epidermal growth factor receptor-2-positive breast cancer: does estrogen receptor status define two distinct subtypes? Ann. Oncol. 24 , 283–291 (2013).
Kaufman, B. et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized phase III TAnDEM study. J. Clin. Oncol. 27 , 5529–5537 (2009).
Arpino, G. et al. Final analysis of PERTAIN: a randomized, two-arm, open-label, multicenter phase II trial assessing the efficacy and safety of first-line pertuzumab given in combination with trastuzumab plus an aromatase inhibitor in patients with HER2-positive and hormone receptor-positive metastatic or locally advanced breast cancer. Cancer Res. 81 (Suppl. 4), Abstr. PD3-02 (2021).
Vernieri, C. et al. Resistance mechanisms to anti-HER2 therapies in HER2-positive breast cancer: current knowledge, new research directions and therapeutic perspectives. Crit. Rev. Oncol. Hematol. 139 , 53–66 (2019).
Finn, R. S. et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 11 , R77 (2009).
Goel, S. et al. Overcoming therapeutic resistance in HER2-positive breast cancers with CDK4/6 inhibitors. Cancer Cell 29 , 255–269 (2016).
Tolaney, S. M. et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemotherapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): a randomised, open-label, phase 2 trial. Lancet Oncol. 21 , 763–775 (2020).
Ciruelos, E. et al. Palbociclib and trastuzumab in HER2-positive advanced breast cancer: results from the phase II SOLTI-1303 PATRICIA trial. Clin. Cancer Res. 26 , 5820–5829 (2020).
Gianni, L. et al. Neoadjuvant treatment with trastuzumab and pertuzumab plus palbociclib and fulvestrant in HER2-positive, ER-positive breast cancer (NA-PHER2): an exploratory, open-label, phase 2 study. Lancet Oncol. 19 , 249–256 (2018).
Smith, A. E. et al. HER2 + breast cancers evade anti-HER2 therapy via a switch in driver pathway. Nat. Commun. 12 , 6667 (2021).
Metzger Filho, O. et al. Impact of HER2 heterogeneity on treatment response of early-stage HER2-positive breast cancer: phase II neoadjuvant clinical trial of T-DM1 combined with pertuzumab. Cancer Discov. 11 , 2474–2487 (2021). A prospective trial evaluating heterogenous HER2 expression in tumours, and its impact on response to anti-HER2 therapy.
Zheng, G. et al. Interaction between HLA-G and NK cell receptor KIR2DL4 orchestrates HER2-positive breast cancer resistance to trastuzumab. Signal. Transduct. Target. Ther. 6 , 236 (2021).
Upton, R. et al. Combining CD47 blockade with trastuzumab eliminates HER2-positive breast cancer cells and overcomes trastuzumab tolerance. Proc. Natl Acad. Sci. USA 118 , e2026849118 (2021).
Betancur, P. A. et al. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat. Commun. 8 , 14802 (2017).
Janiszewska, M. et al. The impact of tumor epithelial and microenvironmental heterogeneity on treatment responses in HER2+ breast cancer. JCI Insight 6 , e147617 (2021).
Hunter, F. W. et al. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br. J. Cancer 122 , 603–612 (2020).
Conlon, N. T. et al. Comparative analysis of drug response and gene profiling of HER2-targeted tyrosine kinase inhibitors. Br. J. Cancer 124 , 1249–1259 (2021).
Kim, J. Y. et al. Immune signature of metastatic breast cancer: Identifying predictive markers of immunotherapy response. Oncotarget 8 , 47400–47411 (2017).
Choi, H. et al. CDK 12 drives breast tumor initiation and trastuzumab resistance via WNT and IRS 1‐ErbB‐PI 3K signaling. EMBO Rep. 20 , e48058 (2019).
Zhao, Y., Wang, Z., Jiang, Y. & Yang, C. Inactivation of Rac1 reduces trastuzumab resistance in PTEN deficient and insulin-like growth factor I receptor overexpressing human breast cancer SKBR3 cells. Cancer Lett. 313 , 54–63 (2011).
Holbro, T. et al. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc. Natl Acad. Sci. USA 100 , 8933–8938 (2003).
Reynolds, K. L. A phase I open-label dose-escalation study of the anti-HER3 monoclonal antibody LJM716 in patients with advanced squamous cell carcinoma of the esophagus or head and neck and HER2-overexpressing breast or gastric cancer. BMC Cancer 17 , 646 (2017).
Saeki, T. & Mukai, H. Phase I study of HER3 targeted antibody patritumab in combination with trastuzumab and paclitaxel in patients with HER2-overexpressing metastatic breast cancer (MBC). J. Clin. Oncol. 33 , 584 (2015).
Helsten, T. L. et al. Evaluation of patritumab/paclitaxel/trastuzumab over standard paclitaxel/trastuzumab in early stage, high-risk HER2 positive breast cancer: results from the neoadjuvant I-SPY 2 trial. Cancer Res. 80 (Suppl. 4) Abstr. P3-11-02 (2022).
Meulendijks, D. et al. First-in-human phase I study of lumretuzumab, a glycoengineered humanized anti-HER3 monoclonal antibody, in patients with metastatic or advanced HER3-positive solid tumors. Clin. Cancer Res. 22 , 877–885 (2016).
Schneeweiss, A. et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Invest. New Drugs 36 , 848–859 (2018).
Im, S.-A. et al. A phase 1 dose-escalation study of anti-HER3 monoclonal antibody LJM716 in combination with trastuzumab in patients with HER2-overexpressing metastatic breast or gastric cancer. J. Clin. Oncol. 32 , 2519 (2014).
Fielder, W. et al. Phase I study of TrasGEX, a glycooptimised anti-HER2 monoclonal antibody, in patients with HER2positive solid tumours. ESMO Open 3 , e000381 (2018).
Bartsch, R. et al. Trastuzumab-deruxtecan (T-DXd) in HER2-positive breast cancer patients (pts) with active brain metastases: primary outcome analysis from the TUXEDO-1 trial. Ann. Oncol. 33 , S198 (2022).
Su, Z. et al. Antibody-drug conjugates: recent advances in linker chemistry. Acta Pharmaceutica Sin. B 11 , 3889–3907 (2021).
van der Lee, M. M. et al. The preclinical profile of the duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol. Cancer Ther. 14 , 692–703 (2015).
Banerji, U. et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 20 , 1124–1135 (2019).
Saura Manich, C. et al. Primary outcome of the phase III SYD985.002/TULIP trial comparing [vic-]trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann. Oncol. 32 , S1283–S1346 (2021).
Skidmore, L. et al. ARX788, a site-specific anti-HER2 antibody-drug conjugate, demonstrates potent and selective activity in HER2-low and T-DM1-resistant breast and gastric cancers. Mol. Cancer Ther. 19 , 1833–1843 (2020).
Zhang, J. et al. Phase I trial of a novel anti-HER2 antibody-drug conjugate, ARX788, for the treatment of HER2-positive metastatic breast cancer. Clin. Cancer Res. 28 , 4212–4221 (2022).
Shi, F. et al. Disitamab vedotin: a novel antibody-drug conjugates for cancer therapy. Drug. Deliv. 29 , 1335–1344 (2022).
Deeks, E. D. Disitamab vedotin: first approval. Drugs 81 , 1929–1935 (2021).
Hamblett, K. J. et al. ZW49, a HER2-targeted biparatopic antibody drug conjugate for the treatment of HER2-expressing cancers. Cancer Res. 79 (Suppl. 4), Abstr. P6-17-13 (2019).
Kang, J. C. et al. Engineering a HER2-specific antibody–drug conjugate to increase lysosomal delivery and therapeutic efficacy. Nat. Biotechnol. 37 , 523–526 (2019).
Hagemann, U. B. et al. Advances in precision oncology: targeted thorium-227 conjugates as a new modality in targeted alpha therapy. Cancer Biother. Radiopharm. 35 , 497–510 (2020).
Hagemann, U. B. et al. Mesothelin-targeted thorium-227 conjugate (MSLN-TTC): preclinical evaluation of a new targeted alpha therapy for mesothelin-positive cancers. Clin. Cancer Res. 25 , 4723–4734 (2019).
Wickstroem, K. et al. Preclinical combination studies of an FGFR2 targeted thorium-227 conjugate and the ATR inhibitor BAY 1895344. Int. J. Radiat. Oncol. Biol. Phys. 105 , 410–422 (2019).
Zhang, J. et al. Preclinical and early clinical safety and pharmacokinetics data of DZD1516, an BBB-penetrant selective HER2 inhibitor for the treatment of HER2 positive metastatic breast cancer. Cancer Res. 82 (Suppl. 4), Abstr. P2-13-43 (2022).
Schram, A. M. et al. Safety and preliminary efficacy from the phase 1 portion of MasterKey-01: a first-in-human dose-escalation study to determine the recommended phase 2 dose (RP2D), pharmacokinetics (PK) and preliminary antitumor activity of BDTX-189, an inhibitor of allosteric ErbB mutations, in patients (pts) with advanced solid malignancies. J. Clin. Oncol. 39 , 3086 (2021).
Connell, C. M. & Doherty, G. J. Activating HER2 mutations as emerging targets in multiple solid cancers. ESMO Open 2 , e000279 (2017).
Elamin, Y. Y. et al. Poziotinib for patients with HER2 exon 20 mutant non–small-cell lung cancer: results from a phase II trial. J. Clin. Oncol. 40 , 702–709 (2022).
Weisser, N. E. et al. The bispecific antibody zanidatamab’s (ZW25’s) unique mechanisms of action and durable anti-tumor activity in HER2-expressing cancers. Cancer Res. 81 , 1005 (2021).
Meric-Bernstam, F. et al. Safety, anti-tumor activity, and biomarker results of the HER2-targeted bispecific antibody ZW25 in HER2-expressing solid tumors. Ann. Oncol. 30 , V159–v193 (2019).
Bedard, P. L. et al. Zanidatamab (ZW25), a HER2-targeted bispecific antibody, in combination with chemotherapy (chemo) for HER2-positive breast cancer (BC): results from a phase 1 study. Cancer Res. 82 (Suppl. 4), Abstr. P2-13-07 (2022).
Geuijen, C. A. et al. Unbiased combinatorial screening identifies a bispecific IgG1 that potently inhibits HER3 signaling via HER2-guided ligand blockade. Cancer Cell 33 , 922–936 (2018).
Hamilton, E. P. et al. Clinical activity of MCLA-128 (zenocutuzumab), trastuzumab, and vinorelbine in HER2 amplified metastatic breast cancer (MBC) patients (pts) who had progressed on anti-HER2 ADCs. J. Clin. Oncol. 38 , 3093 (2020).
Zhang, J. et al. First-in-human HER2-targeted bispecific antibody KN026 for the treatment of patients with HER2-positive metastatic breast cancer: results from a phase I study. Clin. Cancer Res. 28 , 618–628 (2022).
Békés, M., Langley, D. R. & Crews, C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug. Discov. 21 , 181–200 (2022).
Maneiro, M. et al. Antibody–PROTAC conjugates enable HER2-dependent targeted protein degradation of BRD4. ACS Chem. Biol. 15 , 1306–1312 (2020).
Palacino, J. et al. ORM-5029: A first-in-class targeted protein degradation therapy using antibody neodegrader conjugate (AnDC) for HER2-expressing breast cancer. Cancer Res. 82 (Suppl. 4), Abstr. 3933 (2022).
Stagg, J. et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl Acad. Sci. USA 108 , 7142–7247 (2011).
Varchetta, S. et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer. Cancer Res. 67 , 11991–11999 (2007).
Loi, S. et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): a single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 20 , 371–382 (2019).
Hamilton, E. et al. Trastuzumab deruxtecan (T-DXd; DS-8201) with nivolumab in patients with HER2-expressing, advanced breast cancer: a 2-part, phase 1b, multicenter, open-label study. Cancer Res. 81 (Suppl. 4), PD3-07 (2021).
Emens, L. A. et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): a phase 2, multicentre, randomized, double-blind trial. Lancet Oncol. 21 , 1283–1295 (2020).
Nguyen, A. T., Shiao, S. L. & McArthur, H. L. Advances in combining radiation and immunotherapy in breast cancer. Clin. Breast Cancer 21 , 143–152 (2021).
Page, D. B. et al. Brain radiotherapy, tremelimumab-mediated CTLA-4-directed blockade +/− trastuzumab in patients with breast cancer brain metastases. NPJ Breast Cancer 8 , 50 (2022).
Messaoudene, M. et al. T-cell bispecific antibodies in node-positive breast cancer: novel therapeutic avenue for MHC class I loss variants. Ann. Oncol. 30 , 934–944 (2019).
Borghaei, H. et al. Induction of adaptive anti-HER2/neu immune responses in a phase 1B/2 trial of 2B1 bispecific murine monoclonal antibody in metastatic breast cancer (E3194): a trial coordinated by the eastern cooperative oncology group. J. Immunother. 30 , 455–467 (2007).
Yu, S. et al. A novel asymmetrical anti-HER2/CD3 bispecific antibody exhibits potent cytotoxicity for HER2-positive tumor cells. J. Exp. Clin. Cancer Res. 38 , 355 (2019).
Mandó, P., Rivero, S. G., Rizzo, M. M., Pinkasz, M. & Levy, E. M. Targeting ADCC: a different approach to HER2 breast cancer in the immunotherapy era. Breast 60 , 15–25 (2021). A review summarizing the immunological mechanisms of antibody-mediated cellular cytotoxicity induced by anti-HER2 therapies, and novel strategies in drug development in this area.
Melero, I. et al. Amplification of tumor immunity by gene transfer of the co-stimulatory 4-1BB ligand: synergy with the CD28 co-stimulatory pathway. Eur. J. Immunol. 28 , 1116–1121 (1998).
Melero, I., Murillo, O., Dubrot, J., Hervas-Stubbs, S. & Perez-Gracia, J. L. Multi-layered action mechanisms of CD137 (4-1BB)-targeted immunotherapies. Trends Pharmacol. Sci. 29 , 383–390 (2008).
Ku, G. et al. A phase I dose escalation study of PRS-343, a HER2/4-1BB bispecific molecule, in patients with HER2-positive malignancies. Ann. Oncol. 31 , S462–S463 (2020).
Lum, L. G. et al. Targeted T-cell therapy in stage IV breast cancer: a phase I clinical trial. Clin. Cancer Res. 21 , 2305–2314 (2015).
Le Blanc, H. et al. Systemically administered HER2-targeted ISACs provoke a rapid, local response that engages the innate and adaptive arms of the immune system to eradicate tumors in preclinical models. J. Immunother. Cancer 8 (Suppl. 3), Abstr. 605 (2020).
Fitzgerald, K. A. & Kagan, J. C. Toll-like receptors and the control of immunity. Cell 180 , 1044–1066 (2020).
Wainberg, Z. A. et al. Phase 1 study of the novel immunotoxin MT-5111 in patients with HER-2+ tumors. Cancer Res. 81 , CT130 (2021).
Van Tine, B. et al. Interim results of a phase 1 study of the novel immunotoxin MT-5111 in patients with HER2+ tumors. Cancer Res. 8 (Suppl. 4), Abstr. P2-13-45 (2022).
Sloas, C., Gill, S. & Klichinsky, M. Engineered CAR-macrophages as adoptive immunotherapies for solid tumors. Front. Immunol. 12 , 783305 (2021).
Reiss, K. et al. A phase 1 first in human study of adenovirally transduced anti-HER2 CAR macrophages in subjects with HER2 overexpressing solid tumors: preliminary safety, pharmacokinetics, and TME reprogramming data. J. Immunother. Cancer 9 (Suppl. 2), Abstr. 951 (2021).
Marofi, F. et al. CAR-NK cell: a new paradigm in tumor immunotherapy. Front. Oncol. 11 , 673276 (2021).
Solinas, C., Aiello, M., Migliori, E., Willard-Gallo, K. & Emens, L. A. Breast cancer vaccines: heeding the lessons of the past to guide a path forward. Cancer Treat. Rev. 84 , 101947 (2020). A comprehensive review of breast cancer vaccines.
Sharma, A. et al. HER-2 pulsed dendritic cell vaccine can eliminate HER-2 expression and impact ductal carcinoma in situ. Cancer 118 , 4354–4362 (2012).
Antonarelli, G. et al. Therapeutic cancer vaccines revamping: technology advancements and pitfalls. Ann. Oncol. 32 , 1537–1551 (2021).
Clifton, G. T., Peoples, G. E. & Mittendorf, E. A. The development and use of the E75 (HER2 369–377) peptide vaccine. Future Oncol. 12 , 1321–1329 (2016).
Disis, M. L. et al. Effect of dose on immune response in patients vaccinated with an her-2/neu intracellular domain protein–based vaccine. J. Clin. Oncol. 22 , 1916–1925 (2004).
Hamilton, E. et al. Phase 1 clinical trial of HER2-specific immunotherapy with concomitant HER2 kinase inhibition [corrected]. J. Transl. Med. 10 , 28 (2012).
Chen, G. et al. A feasibility study of cyclophosphamide, trastuzumab, and an allogeneic GM-CSF-secreting breast tumor vaccine for HER2+ metastatic breast cancer. Cancer Immunol. Res. 2 , 949–961 (2014).
Morse, M. A. et al. Long term disease-free survival and T cell and antibody responses in women with high-risk Her2+ breast cancer following vaccination against Her2. J. Transl. Med. 5 , 42 (2007).
Maeng, H. M. et al. Phase I clinical trial of an autologous dendritic cell vaccine against HER2 shows safety and preliminary clinical efficacy. Front. Oncol. 11 , 789078 (2021).
Lowenfeld, L. et al. Dendritic cell vaccination enhances immune responses and induces regression of HER2 pos DCIS independent of route: results of randomized selection design trial. Clin. Cancer Res. 23 , 2961–2971 (2017).
Burke, E. E., Kodumudi, K., Ramamoorthi, G. & Czerniecki, B. J. Vaccine therapies for breast cancer. Surg. Oncol. Clin. N. Am. 28 , 353–367 (2019).
Shanmugaraj, B. et al. Bacterial and viral vectors as vaccine delivery vehicles for breast cancer therapy. Life Sci. 250 , 117550 (2020).
Crosby, E. J. et al. Vaccine-induced memory CD8+ T cells provide clinical benefit in HER2 expressing breast cancer: a mouse to human translational study. Clin. Cancer Res. 25 , 2725–2736 (2019).
Mittendorf, E. A. et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide AE37 vaccine in breast cancer patients to prevent recurrence. Ann. Oncol. 27 , 1241–1248 (2016).
Mittendorf, E. A. et al. Efficacy and safety analysis of nelipepimut-S vaccine to prevent breast cancer recurrence: a randomized, multicenter, phase III clinical trial. Clin. Cancer Res. 25 , 4248–4254 (2019).
Caldeira, J. C. et al. Virus-like particles as an immunogenic platform for cancer vaccines. Viruses 12 , 488 (2020).
Dafni, U. et al. Efficacy of cancer vaccines in selected gynaecological breast and ovarian cancers: a 20-year systematic review and meta-analysis. Eur. J. Cancer 142 , 63–82 (2021).
Blass, E. & Ott, P. A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 18 , 215–229 (2021).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12 , 31–46 (2022). An updated review discussing novel facets of cancer and proposing emerging hallmarks of cancer and enabling characteristics.
Park, E. M. et al. Targeting the gut and tumor microbiota in cancer. Nat. Med. 28 , 690–703 (2022).
Liang, G. et al. The emerging roles of artificial intelligence in cancer drug development and precision therapy. Biomed. Pharmacother. 128 , 110255 (2020).
Jinek, M. et al. Programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337 , 816–821 (2012).
Ran, F. A. et al. Genome engineering using the CRISPR–Cas9 system. Nat. Protoc. 8 , 2281–2308 (2013).
Tang, X. et al. The single-cell sequencing: new developments and medical applications. Cell Biosci. 9 , 53 (2019).
Andersson, A. et al. Spatial deconvolution of HER2-positive breast cancer delineates tumor-associated cell type interactions. Nat. Commun. 12 , 6012 (2021).
McNamara, K. L. et al. Spatial proteomic characterization of HER2-positive breast tumors through neoadjuvant therapy predicts response. Nat. Cancer 2 , 400–413 (2021).
Vahidfar, N. et al. Theranostic advances in breast cancer in nuclear medicine. Int. J. Mol. Sci. 22 , 4597 (2021).
Xu, Y. et al. Phase I study of the recombinant humanized anti-HER2 monoclonal antibody–MMAE conjugate RC48-ADC in patients with HER2-positive advanced solid tumors. Gastric Cancer 24 , 913–925 (2021).
Hu, X. et al. Phase I study of A166 in patients with HER2-expressing locally advanced or metastatic solid tumors. J. Clin. Oncol. 39 , 1024 (2021).
Park, Y. H. et al. First-in-human phase I study of ALT-P7, a HER2-targeting antibody-drug conjugate in patients with HER2-positive advanced breast cancer. J. Clin. Oncol. 38 , 3551 (2020).
Hurvitz, S. et al. Safety and unique pharmacokinetic profile of ARX788, a site-specific ADC, in heavily pretreated patients with HER2-overexpresing solid tumors: Results from two phase 1 clinical trials. J. Clin. Oncol. 39 , 1038 (2021).
Zhang, X. et al. Novel development strategies and challenges for anti-Her2 antibody-drug conjugates. Antib. Ther. 5 , 18–29 (2022).
Fasching, P. A. Invited discussant LBA1. ESMO Breast Cancer Congress (4 May 2022); https://oncologypro.esmo.org/meeting-resources/esmo-breast-cancer-congress/invited-discussant-lba1
Jhaveri, K. et al. Preliminary results from a phase 1 study using the bispecific, human epidermal growth factor 2 (HER2)-targeting antibody-drug conjugate (ADC) zanidatamab zovodotin (ZW49) in solid cancers. Ann. Oncol. 33 (Suppl. 7), S197–S224 (2022).
Macpherson, I. R. et al. A phase I/II study of epertinib plus trastuzumab with or without chemotherapy in patients with HER2-positive metastatic breast cancer. Breast Cancer Res. 22 , 1 (2019).
Brufsky, A. et al. A phase 2 study of poziotinib in patients with HER2-positive metastatic breast cancer heavily pre-treated with HER2-targeted therapy. Cancer Res. 81 (Suppl. 4), Abstr. PD1-07 (2021).
Lopez-Albaitero, A. et al. Overcoming resistance to HER2-targeted therapy with a novel HER2/CD3 bispecific antibody. Oncoimmunology 6 , e1267891 (2017).
Ruiz, I. R. et al. p95HER2-T cell bispecific antibody for breast cancer treatment. Sci. Transl. Med. 10 , eaat1445 (2018).
Oberg, H. H. et al. Tribody [(HER2) 2 xCD16] is more effective than trastuzumab in enhancing γδ T cell and natural killer cell cytotoxicity against HER2-expressing cancer cells. Front. Immunol. 9 , 814 (2018).
Mittal, D. et al. CD96 is an immune checkpoint that regulates CD8+ T-cell antitumor function. Cancer Immunol. Res. 7 , 559–571 (2019).
Sha, W. et al. SAR443216, a novel trispecific T cell engager with potent T cell-dependent cytotoxicity for HER2-low tumors. Cancer Res. 81 (Suppl. 13), Abstr. 1825 (2021).
Sharma, M. R. et al. Preliminary results from a phase I/II study of BDC-1001, a novel HER2 targeting TLR7/8 immune-stimulating antibody conjugate (ISAC), in patients (pts) with advanced HER2-expressing solid tumors. Ann. Oncol. 32 , S1453–S1454 (2021).
Janku, F. et al. 378 A first in-human, multicenter, open-label, dose-finding phase 1 study of the immune stimulator antibody conjugate NJH395 in patients with nonbreast HER2+ advanced malignancies. J. Immunother. Cancer 8 (Suppl. 3), A230 (2020).
Klemper, S. J. et al. 209P Interim results of a phase I/Ib study of SBT6050 monotherapy and pembrolizumab combination in patients with advanced HER2-expressing or amplified solid tumors. Ann. Oncol. 32 (Suppl. 5), S450 (2021).
Mittendorf, E. A. et al. Primary analysis of a prospective, randomized, single-blinded phase II trial evaluating the HER2 peptide GP2 vaccine in breast cancer patients to prevent recurrence. Oncotarget 7 , 66192–66201 (2016).
Bekaii-Saab, T. et al. Phase I immunotherapy trial with two chimeric HER-2 B-cell peptide vaccines emulsified in montanide ISA 720VG and nor-MDP adjuvant in patients with advanced solid tumors. Clin. Cancer Res. 25 , 3495–3507 (2019).
Emens, L. A. et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J. Clin. Oncol. 27 , 5911–5918 (2009).
Disis, M. L. et al. A phase I trial of the safety and immunogenicity of a DNA-based vaccine encoding the HER2/neu (HER2) intracellular domain in subjects with HER2+ breast cancer. J. Clin. Oncol. 32 , 616 (2014).
Cohen, S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J. Biol. Chem. 237 , 1555–1562 (1962).
Cohen, S. The stimulation of epidermal proliferation by a specific protein (EGF). Dev. Biol. 12 , 394–407 (1965).
Engelbreth-Holm, J. & Meyer, A. R. Variations in the percentage of takes in 3 strains of chicken leukosis. Acta Pathol. Microbiol. Scand. 12 , 366–377 (1935).
Yamamoto, T. et al. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell 35 , 71–78 (1983).
Downward, J. et al. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature 307 , 521–527 (1984).
Shih, C., Shilo, B. Z., Goldfarb, M. P., Dannenberg, A. & Weinberg, R. A. Passage of phenotypes of chemically transformed cells via transfection of DNA and chromatin. Proc. Natl Acad. Sci. USA 76 , 5714–5718 (1979).
Shih, C., Padhy, L. C., Murray, M. & Weinberg, R. A. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature 290 , 261–264 (1981).
Padhy, L. C., Shih, C., Dowing, D., Finkelstein, R. & Weinberg, R. A. Identification of a phosphoprotein specifically induced by the transforming DNA of rat neuroblastomas. Cell 28 , 865–871 (1982).
King, C. R., Kraus, M. H. & Aaronson, S. A. Amplification of a novel v-erbB-related gene in a human mammary carcinoma. Science 229 , 974–976 (1985).
Ullrich, A. et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 309 , 418–425 (1984).
Coussens, L. et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science 230 , 1132–1139 (1985).
Kraus, M. H., Issing, W., Miki, T., Popescu, N. C. & Aaronson, S. A. Isolation and characterization of ERBB3, a third member of the ERBB/epidermal growth factor receptor family: evidence for overexpression in a subset of human mammary tumors. Proc. Natl Acad. Sci. USA 86 , 9193–9197 (1989).
Plowman, G. D. et al. Ligand-specific activation of HER4/p180erbB4, a fourth member of the epidermal growth factor receptor family. Proc. Natl Acad. Sci. USA 90 , 1746–1750 (1993).
Drebin, J. A., Stern, D. F., Link, V. C., Weinberg, R. A. & Greene, M. I. Monoclonal antibodies identify a cell-surface antigen associated with an activated cellular oncogene. Nature 312 , 545–548 (1984).
Drebin, J. A., Link, V. C., Stern, D. F., Weinberg, R. A. & Greene, M. I. Down-modulation of an oncogene protein product and reversion of the transformed phenotype by monoclonal antibodies. Cell 41 , 697–706 (1985).
Drebin, J. A., Link, V. C., Weinberg, R. A. & Greene, M. I. Inhibition of tumor growth by a monoclonal antibody reactive with an oncogene-encoded tumor antigen. Proc. Natl Acad. Sci. USA 83 , 9129–9133 (1986).
Di Fiore, P. P. et al. erbB-2 is a potent oncogene when overexpressed in NIH/3T3 cells. Science 237 , 178–182 (1987).
Hudziak, R. M., Schlessinger, J. & Ullrich, A. Increased expression of the putative growth factor receptor p185 HER2 causes transformation and tumorigenesis of NIH 3T3 cells. Proc. Natl Acad. Sci. USA 84 , 7159–7163 (1989).
Slamon, D. J. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244 , 707–712 (1989).
Fendly, B. M. et al. Characterization of murine monoclonal antibodies reactive to either the human epidermal growth factor receptor or HER2/neu gene product. Cancer Res. 50 , 1550–1558 (1990).
Carter, P. Humanization of an anti-p185 HER2 antibody for human cancer therapy. Proc. Natl Acad. Sci. USA 89 , 4285–4289 (1992).
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344 , 783–792 (2001). The first publication to demonstrate the efficacy of trastuzumab + chemotherapy in HER2-overexpressing metastatic breast cancer.
Baselga, J. Phase I and II clinical trials of trastuzumab. Ann. Oncol. 12 , S49–S55 (2001).
Payne, S. J., Bowen, R. L., Jones, J. L. & Wells, C. A. Predictive markers in breast cancer–the present. Histopathology 52 , 82–90 (2008).
Chia, S. et al. Human epidermal growth factor receptor 2 overexpression as a prognostic factor in a large tissue microarray series of node-negative breast cancers. J. Clin. Oncol. 26 , 5697–5704 (2008).
Fritzsche, F. R. et al. Tissue pretreatment with formic acid might lower HercepTest scores in breast cancer. Diagn. Mol. Pathol. 15 , 237–242 (2006).
Siddiqui, S. & Rimm, D. L. Pre-analytic variables and phospho-specific antibodies: the Achilles heel of immunohistochemistry. Breast Cancer Res. 12 , 113 (2010).
Press, M. F. et al. Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin. Cancer Res. 11 , 6598–6607 (2005).
Schnitt, S. J. & Jacobs, T. W. Current status of HER2 testing: caught between a rock and a hard place. Am. J. Clin. Pathol. 116 , 806–810 (2001).
Choritz, H., Busche, G. & Kreipe, H. Quality assessment of HER2 testing by monitoring of positivity rates. Virchows Arch. 459 , 283–289 (2011).
Yeh, I. T. et al. Clinical validation of an array CGH test for HER2 status in breast cancer reveals that polysomy 17 is a rare event. Mod. Pathol. 22 , 1169–1175 (2009).
Tse, C. H. et al. Determining true HER2 gene status in breast cancers with polysomy by using alternative chromosome 17 reference genes: implications for anti-HER2 targeted therapy. J. Clin. Oncol. 29 , 4168–4174 (2011).
Ballinger, T. J., Sanders, M. E. & Abramson, V. G. Current HER2 testing recommendations and clinical relevance as a predictor of response to targeted therapy. Clin. Breast Cancer 15 , 171–180 (2015).
Onsum, M. D. et al. Single-cell quantitative HER2 measurement identifies heterogeneity and distinct subgroups within traditionally defined HER2-positive patients. Am. J. Pathol. 183 , 1446–1460 (2013).
Gu, J., Tang, Z., Chen, H., Sfamenos, S. & Geiersbach, K. B. HER2 FISH for breast cancer: advances in quantitative image analysis and automation. OBM Genetics 4 , 109 (2020).
Pizzamiglio, S. et al. What if the future of HER2-positive breast cancer patients was written in miRNAs? An exploratory analysis from NeoALTTO study. Cancer Med. 11 , 332–339 (2022).
Moutafi, M. et al. Quantitative measurement of HER2 expression to subclassify ERBB2 unamplified breast cancer. Lab. Invest. 102 , 1101–1108 (2022).
Prat, A. et al. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine 75 , 103801 (2022).
Triulzi, T. et al. The TRAR gene classifier to predict response to neoadjuvant therapy in HER2-positive and ER-positive breast cancer patients: an explorative analysis from the NeoSphere trial. Mol. Oncol. 16 , 2355–2366 (2022).
FDA Approves Fam-Trastuzumab Deruxtecan-Nxki for HER2-Low Breast Cancer (US Food and Drug Administration, 2022); https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-low-breast-cancer
Fernandez, A. I. et al. Examination of low ERBB2 protein expression in breast cancer tissue. JAMA Oncol. 8 , 1–4 (2022).
Slamon, D. J. et al. Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. Cancer Res. 76 , S5–S04 (2016).
Swain, S. M. et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 21 , 519–530 (2020).
Piccart, M. et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years’ follow-up. J. Clin. Oncol. 39 , 1448–1457 (2021).
Ewer, M. S. & Ewer, S. M. Trastuzumab cardiotoxicity after anthracycline exposure constitutes a complex and clinically important entity. JACC Heartfail. 7 , 805–807 (2019).
Cameron, D. et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 389 , 1195–1205 (2017).
Armenian, S. H. et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 35 , 893–911 (2017).
Curigliano, G. et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 31 , 171–190 (2020).
Barcenas, C. H. et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: the CONTROL trial. Ann. Oncol. 31 , 1223–1230 (2020).
Diéras, V. et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 18 , 732–742 (2017).
Krop, I. E. et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 18 , 743–754 (2017).
Uppal, H. et al. Potential mechanisms for thrombocytopenia development with trastuzumab emtansine (T-DM1). Clin. Cancer Res. 21 , 123–133 (2015).
Powell, C. A. et al. Pooled analysis of drug-related interstitial lung disease and/or pneumonitis in nine trastuzumab deruxtecan monotherapy studies. ESMO Open 7 , 100554 (2022).
Hamilton, E. P. et al. Trastuzumab deruxtecan (T-DXd) versus trastuzumab emtansine (T-DM1) in patients (pts) with HER2-positive (HER2+) unresectable and/or metastatic breast cancer (mBC): safety follow-up of the randomized, phase 3 study DESTINY-Breast03. J. Clin. Oncol. 40 (Suppl. 16), Abstr. 1000 (2022).
Download references
Acknowledgements
The authors thank C. Gilmore, of Phillips Gilmore Oncology Communications, Inc., for editorial assistance with manuscript preparation. Financial support was provided by Breast Cancer Research Foundation.
Author information
Authors and affiliations.
Department of Medicine, Georgetown Lombardi Comprehensive Cancer Center and MedStar Health, Washington, DC, USA
Sandra M. Swain
Sarah Cannon Research Institute, Nashville, TN, USA
Mythili Shastry & Erika Hamilton
Tennessee Oncology, Nashville, TN, USA
Erika Hamilton
You can also search for this author in PubMed Google Scholar
Contributions
All authors contributed to the design, research and writing of the manuscript.
Corresponding author
Correspondence to Sandra M. Swain .
Ethics declarations
Competing interests.
M.S. reports no conflict of interest. E.H.’s institution has received research funding from the following: AbbVie, Acerta Pharma, Accutar Biotechnology, ADC Therapeutics, AKESOBIO Australia, Amgen, Aravive, ArQule, Artios, AtlasMedx, Bliss BioPharmaceuticals, Cascadian Therapeutics, Clovis, Compugen, Cullen-Florentine, Curis, Dana Farber Cancer Institute, Duality Biologics, eFFECTOR Therapeutics, Ellipses Pharma, Elucida Oncology, EMD Serono, Fochon, FujiFilm, G1 Therapeutics, H3 Biomedicine, Harpoon, Hutchinson MediPharma, Immunogen, Immunomedics, Incyte, Infinity Pharmaceuticals, InventisBio, Jacobio, Karyopharm, Leap Therapeutics, Lycera, Mabspace Biosciences, Macrogenics, MedImmune, Merus, Millennium, Molecular Templates, Myraid Genetic Laboratories, Nucana, Olema, OncoMed, Onconova Therapeutics, ORIC Pharmaceuticals, Orinove, PharmaMar, Pieris Pharmaceuticals, Pionyr Immunotherapeutics, Plexxikon, Radius Health, Regeneron, Repertoire Immune Medicine, Rgenix, Sermonix Pharmaceuticals, Shattuck Labs, StemCentRx, Sutro, Syndax, Syros, Taiho, TapImmune, Tesaro, Tolmar, Torque Therapeutics, Treadwell Therapeutics, Verastem, Vincerx Pharma, Zenith Epigenetics and Zymeworks. E.H.’s institution has received consulting fees from the following: Arcus, Eisai, Greenwich Lifesciences, H3 Biomedicine, iTeos, Janssen, Loxo, Orum Therapeutics, Propella Therapeutics and Puma Biotechnology; and her institution has received research funding and consulting fees from the following: Arvinas, Black Diamond, Boehringer Ingelheim, CytomX, Dantari, Deciphera, Lilly, Merck, Mersana, Novartis, Pfizer, Relay Therapeutics, Roche/Genentech, SeaGen, Silverback. S.M.S. reports grants or contracts from Genentech/Roche, Kailos, Genetics, BCRF; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Genentech/Roche, Daiichi Sankyo; support for attending meetings and/or travel from Genentech/Roche (travel 11/2019), Daiichi Sankyo (travel 9/2022) and Sanofi (travel 9/2022); participation on a Data Safety Monitoring Board for AstraZeneca; participation in an advisory board for AstraZeneca, Daiichi Sankyo, Exact Sciences, Biotheranostics, Natera, Merck, Silverback Therapeutics, Athenex, Lilly, Aventis; and participation in a Scientific Advisory Board for Inivata. S.M.S. reports leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid: NSABP Vice Chairman; CCF, ASCO Director; and third party writing support from Genentech/Roche and AstraZeneca.
Peer review
Peer review information.
Nature Reviews Drug Discovery thanks Jamuna Veeraraghavan, who co-reviewed with Rachel Schiff, Alvin Wong and the other, anonymous reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Bishop, J.M. Retroviruses and oncogenes II. Nobel lecture (1989): https://www.nobelprize.org/prizes/medicine/1989/press-release/ .
Byondis B. V. FDA Accepts Byondis’ Biologics License Application for [Vic-] Trastuzumab Duocarmazine (SYD985) in HER2-Positive Metastatic Breast Cancer. Cision PR Newswire: https://go.nature.com/3VZlL4a (2022).
Cohen, S. Epidermal growth factor. Nobel lecture (1986): https://www.nobelprize.org/prizes/medicine/1986/cohen/lecture/ .
Innovent Biologics. Innovent Releases Preliminary Results of the Phase Ia Dose-Escalation study of IBI315 (Anti-Her2/PD-1 Bispecific Antibody) in Patients with Advanced Solid Tumors at CSCO Annual Meeting 2021. Cision PR Newswire: https://go.nature.com/3f91toe (2021).
Levi-Montalcini, R. The nerve growth factor: thirty-five years later. Nobel lecture (1986): https://www.nobelprize.org/prizes/medicine/1986/levi-montalcini/lecture/ .
US Food and Drug Administration: https://www.fda.gov/media/82647/download (2015).
US Food and Drug Administration: https://www.fda.gov/drugs/biosimilars/biosimilar-productinformation (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT05132582 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04457596 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT01042379 (2022).
US Food and Drug Administration: https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-her2-low-breast-cancer .
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04208178 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT02947685 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT02448420 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04829604 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT03821233 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT01953926 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04224272 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT05027139 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT05035836 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT02912949 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04521179 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04881929 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04778982 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04042701 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04740918 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT03199885 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04538742 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT02563925 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04454528 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04501770 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT03448042 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04307329 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT03632941 (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/results?cond=HER2-positive+Breast+Cancer&term=&cntry=&state=&city=&dist (2022).
US National Library of Medicine. ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT04147819?term=NCT04147819&draw=2&rank=1 (2022).
Varmus, H.E. Retroviruses and oncogenes I. Nobel lecture (1989): https://www.nobelprize.org/prizes/medicine/1989/varmus/lecture/ .
Supplementary information
Supplementary information.
(ADCC). An immune mechanism through which Fc receptor-bearing cytotoxic effector cells can recognize and kill antibody-coated target cells that express tumour- or pathogen-derived antigens on their surface.
(ADCP). An immunological mechanism of elimination whereby tumour cells are targeted with monoclonal antibodies to promote their clearance from the body by phagocytic immune cells such as macrophages.
A biologic product that is highly similar to and has no clinically meaningful differences from an existing FDA-approved reference product
(CDC). The mechanism by which antibody-coated target cells recruit and activate components of the complement cascade, leading to the formation of a membrane attack complex (MAC) on the cell surface and subsequent cell lysis.
Cancer that is already metastatic or stage IV at the time of diagnosis
A portion of a protein or antigen that is recognized by the immune system (specifically by antibodies).
The fragment crystallizable or the constant fragment region of the antibody, which is the tail region of the antibody that interacts with the cell surface receptors (Fc receptors) and some proteins of the complement system. This property enables antibodies to activate the immune system.
A type of immune cell that has granules (small particles) with enzymes that can kill tumour cells or cells infected with a virus. A NK cell is a type of white blood cell.
(PROTACs). Protein degraders that use the cell’s own waste disposal machinery to eliminate a target protein. PROTACs can simultaneously bind to a target protein and an E3 ligase protein, which then ubiquitylates the target, marking it for proteasomal degradation.
A type of white blood cell. T cells or T lymphocytes are part of the immune system and develop from stem cells in the bone marrow. They help protect the body from infection and may help fight cancer.
A combination of the terms therapeutics and diagnostics. It is used to describe the combination of using one radioactive drug to identify (diagnose) and a second radioactive drug to deliver therapy to treat the main tumour and any metastatic tumours.
(TILs). A type of immune cell that has moved from the blood into a tumour. TILs can recognize and kill cancer cells.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Swain, S.M., Shastry, M. & Hamilton, E. Targeting HER2-positive breast cancer: advances and future directions. Nat Rev Drug Discov 22 , 101–126 (2023). https://doi.org/10.1038/s41573-022-00579-0
Download citation
Accepted : 21 September 2022
Published : 07 November 2022
Issue Date : February 2023
DOI : https://doi.org/10.1038/s41573-022-00579-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Comprehensive review of crispr-based gene editing: mechanisms, challenges, and applications in cancer therapy.
- Mohammad Chehelgerdi
- Matin Chehelgerdi
- Abbas Mokhtari-Farsani
Molecular Cancer (2024)
Combined inhibition of HER2 and VEGFR synergistically improves therapeutic efficacy via PI3K-AKT pathway in advanced ovarian cancer
- Shuyong Zhang
Journal of Experimental & Clinical Cancer Research (2024)
Folic acid-functionalized PEGylated niosomes co-encapsulated cisplatin and doxoribicin exhibit enhanced anticancer efficacy
- Mona Safari Sharafshadeh
- Farzaneh Tafvizi
- Somayeh Ehtesham
Cancer Nanotechnology (2024)
Combined therapy of dabrafenib and an anti-HER2 antibody–drug conjugate for advanced BRAF-mutant melanoma
Cellular & Molecular Biology Letters (2024)
CDK7 in breast cancer: mechanisms of action and therapeutic potential
Cell Communication and Signaling (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Cancer newsletter — what matters in cancer research, free to your inbox weekly.
Revolutionizing Breast Cancer Treatment: Harnessing the Power of Artificial Intelligence in Overcoming Drug Resistance
- First Online: 04 June 2024
Cite this chapter

- Zilungile Mkhize-Kwitshana 2 , 3 ,
- Pragalathan Naidoo 2 , 3 ,
- Zamathombeni Duma 2 , 3 ,
- Kamal S. Saini 4 , 5 &
- Zodwa Dlamini 6
Drug resistance is one of the major challenges in the treatment of breast cancer (BC) and contributes to its high mortality rate. Detecting breast cancer at an early stage, before the cells have formed subpopulations which can reduce heterogeneous molecular features, could mitigate the development of drug resistance, and increase the efficacy of treatment. Several artificial intelligence (AI)-based models and tools that are integrated with multi-omics data (genomics, epigenetics, proteomics, and metabolomics) are available for drug discovery, drug design using AI-based database repositories and de novo drug design through AI-based algorithms, including deep reinforcement learning, variational auto-encoders, recurrent neural network, and generative adversarial network. As more data becomes available, the capacity of these algorithms improves to make them iteratively more accurate, timely, and precise. By integrating AI into predictive models, healthcare professionals can gain valuable insights into the mechanisms of drug resistance and develop personalized treatment strategies to overcome treatment failure in BC. This chapter highlights the revolutionary impact of AI in addressing early detection of BC, drug discovery and design, overcoming drug resistance, improving treatment outcomes, and paving the way for precision medicine in BC.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as PDF
- Read on any device
- Instant download
- Own it forever
- Available as EPUB and PDF
- Durable hardcover edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Abbasi, M., Santos, B. P., Pereira, T. C., Sofia, R., Monteiro, N. R. C., Simões, C. J. V., Brito, R. M. M., Ribeiro, B., Oliveira, J. L., & Arrais, J. P. (2022). Designing optimized drug candidates with generative adversarial network. Journal of Cheminformatics, 14 , 40.
Article PubMed PubMed Central Google Scholar
Ahmad, A. (2023). Pathways to breast cancer recurrence. ISRN Oncology, 2013 , 290568.
Google Scholar
Arnold, M., Morgan, E., Rumgay, H., Mafra, A., Singh, D., Laversanne, M., Vignat, J., Gralow, J. R., Cardoso, F., Siesling, S., & Soerjomataram, I. (2022). Current and future burden of breast cancer: Global statistics for 2020 and 2040. The Breast: Official Journal of the European Society of Mastology, 66 , 15–23. https://doi.org/10.1016/j.breast.2022.08.010
Article PubMed Google Scholar
Barretina, J., Caponigro, G., Stransky, N., Venkatesan, K., Margolin, A. A., Kim, S., et al. (2012). The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature, 483 , 603–607.
Article CAS PubMed PubMed Central Google Scholar
Beck, J. T., Rammage, M., Jackson, G. P., Preininger, A. M., Dankwa-Mullan, I., Roebuck, M. C., Torres, A., Holtzen, H., Coverdill, S. E., Williamson, M. P., Chau, Q., Rhee, K., & Vinegra, M. (2020). Artificial intelligence tool for optimizing eligibility screening for clinical trials in a large community cancer center. JCO Clinical Cancer Informatics, 4 , 50–59. https://doi.org/10.1200/CCI.19.00079
Bomane, A., Gonçalves, A., & Ballester, P. J. (2019). Paclitaxel response can be predicted with interpretable multi-variate classifiers exploiting DNA-methylation and miRNA data. Frontiers in Genetics, 25 (10), 1041. https://doi.org/10.3389/fgene.2019.01041
Article CAS Google Scholar
Born, J., Manica, M., Oskooei, A., Cadow, J., Markert, G., & Rodríguez Martínez, M. (2021). PaccMann(RL): De novo generation of hit-like anticancer molecules from transcriptomic data via reinforcement learning. IScience, 24 , 102269.
Bray, F., Ren, J. S., Masuyer, E., & Ferlay, J. (2013). Global estimates of cancer prevalence for 27 sites in the adult population in 2008. International Journal of Cancer, 132 (5), 1133–1145. https://doi.org/10.1002/ijc.27711
Article CAS PubMed Google Scholar
Broeders, M., Moss, S., Nyström, L., Njor, S., Jonsson, H., Paap, E., Massat, N., Duffy, S., Lynge, E., Paci, E., & EUROSCREEN Working Group. (2012). The impact of mammographic screening on breast cancer mortality in Europe: A review of observational studies. Journal of Medical Screening, 19 (1), 14–25. https://doi.org/10.1258/jms.2012.012078
Casparie, M., Tiebosch, A. T., Burger, G., Blauwgeers, H., Van De Pol, A., Van Krieken, J. H., & Meijer, G. A. (2007). Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cellular Oncology, 29 , 19–24.
CAS PubMed PubMed Central Google Scholar
Chakraborty, S., Hosen, M. I., Ahmed, M., & Shekhar, H. U. (2018). Onco-multi-OMICS approach: A new frontier in cancer research. BioMed Research International, 2018 , 9836256.
Chica-Parrado, M. R., Godoy-Ortiz, A., Jiménez, B., Ribelles, N., Barragan, I., & Alba, E. (2020). Resistance to neoadjuvant treatment in breast cancer: Clinicopathological and molecular predictors. Cancers, 12 , 2012.
Christensen, J. (2023). AI-supported mammogram screening increases breast cancer detection by 20%. Retrieved August 23, 2023, from https://edition.cnn.com/2023/08/01/health/ai-breast-cancer-detection/index.html#
Conner-Simons, A., & Gordon, R. (2019). Using AI to predict breast cancer and personalize care MIT/MGH’s image-based deep learning model can predict breast cancer up to five years in advance, CSAIL. Retrieved August 23, 2023, from https://news.mit.edu/2019/using-ai-predict-breast-cancer-and-personalize-care-0507
Consortium GO. (2004). The gene ontology (GO) database and informatics resource. Nucleic Acids Research, 32 , D258–D261.
Article Google Scholar
Corti, C., Cobanaj, M., Marian, F., Dee, E. C., Lloyd, M. R., Marcu, S., Dombrovschi, A., Biondetti, G. P., Batalini, F., Celi, L. A., & Curigliano, G. (2022). Artificial intelligence for prediction of treatment outcomes in breast cancer: Systematic review of design, reporting standards, and bias. Cancer Treatment Reviews, 108 , 102410.
Darby, S., Davies, C., & Paul McGale, P. (2005). The early breast cancer Trialists’ collaborative group: A brief history of results to date. In Y. Dodge & N. Wermuth (Eds.), Celebrating statistics’ AC davison . Oxford University Press.
DePolo, J. (2023). AI-supported mammogram reading detects 20% more cancers. Written. Retrieved August 23, 2023, from https://www.breastcancer.org/research-news/ai-mammogram-reading
Ding, Z., Zu, S., & Gu, J. (2016). Evaluating the molecule-based prediction of clinical drug responses in cancer. Bioinformatics, 32 (19), 2891–2895. https://doi.org/10.1093/bioinformatics/btw344
Edgar, R., Domrachev, M., & Lash, A. E. (2002). Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Research, 30 , 207–210.
Emran, T. B., Shahriar, A., Mahmud, A. R., Rahman, T., Abir, M. H., Faijanur-Rob Siddiquee, M., Ahmed, H., Rahman, N., Nainu, F., Wahyudin, E., Mitra, S., Dhama, K., Habiballah, M. M., Haque, S., Islam, A., & Hassan, M. M. (2022). Multidrug resistance in cancer: Understanding molecular mechanisms, immunoprevention and therapeutic approaches. Frontiers in Oncology, 12 , 1–38. https://doi.org/10.3389/fonc.2022.891652
Evans, K. K., Birdwell, R. L., & Wolfe, J. M. (2013). If you don’t find it often, you often don’t find it: Why some cancers are missed in breast cancer screening. PLoS One, 8 (5), e64366.
Fang, Y., Pan, X., & Shen, H.-B. (2023). De novo drug design by iterative multiobjective deep reinforcement learning with graph-based molecular quality assessment. Bioinformatics, 39 , 1–10.
Feinberg, E. N., Sur, D., Wu, Z., Husic, B. E., Mai, H., Li, Y., Sun, S., Yang, J., Ramsundar, B., & Pande, V. S. (2018). PotentialNet for molecular property prediction. ACS Central Science, 4 , 1520–1530.
Forbes, S. A., Bindal, N., Bamford, S., Cole, C., Kok, C. Y., Beare, D., Jia, M., Shepherd, R., Leung, K., Menzies, A., Teague, J. W., Campbell, P. J., Stratton, M. R., & Futreal, P. A. (2011). COSMIC: Mining complete cancer genomes in the catalogue of somatic mutations in cancer. Nucleic Acids Research, 39 , D945–D950.
Fradkin, P., Young, A., Atanackovic, L., Frey, B., Lee, L. J., & Wang, B. (2022). A graph neural network approach for molecule carcinogenicity prediction. Bioinformatics, 38 , i84–i91.
Gaulton, A., Bellis, L. J., Bento, A. P., Chambers, J., Davies, M., Hersey, A., Light, Y., Mcglinchey, S., Michalovich, D., Al-Lazikani, B., & Overington, J. P. (2012). ChEMBL: A large-scale bioactivity database for drug discovery. Nucleic Acids Research, 40 , D1100–D1107.
Gehrmann, M., Schmidt, M., Brase, J. C., Roos, P., & Hengstler, J. G. (2008). Prediction of paclitaxel resistance in breast cancer: Is CYP1B1*3 a new factor of influence? Pharmacogenomics, 9 , 969–974.
Goel, M., Raghunathan, S., Laghuvarapu, S., & Priyakumar, U. D. (2021). MoleGuLAR: Molecule generation using reinforcement learning with alternating rewards. Journal of Chemical Information and Modeling, 61 , 5815–5826.
Goldenberg, S. L., Nir, G., & Salcudean, S. E. (2019). A new era: Artificial intelligence and machine learning in prostate cancer. Nature Reviews Urology, 16 (7), 391–403.
Goodman, L. S., Wintrobe, M. M., Dameshek, W., Goodman, M. J., Gilman, A., & McLennan, M. T. (1946). Use of methyl-Bis (Beta-Chloroethyl)amine hydrochloride and Tris(Beta-Chloroethyl)amine hydrochloride for Hodgkin’s disease, Lymphosarcoma, leukemia and certain allied and miscellaneous disorders. Journal of the American Medical Association, 132 , 126–132.
Gore, J. C. (2020). Artificial intelligence in medical imaging. Magnetic Resonance Imaging, 68 , A1–A4.
Grisoni, F., Moret, M., Lingwood, R., & Schneider, G. (2020). Bidirectional molecule generation with recurrent neural networks. Journal of Chemical Information and Modeling, 60 , 1175–1183.
Gromski, P. S., Granda, J. M., & Cronin, L. (2020). Universal chemical synthesis and discovery with ‘the Chemputer’. Trends in Chemistry, 2 , 4–12.
Hamosh, A., Scott, A. F., Amberger, J. S., Bocchini, C. A., & Mckusick, V. A. (2005). Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Research, 33 , D514–D517.
ICGC/TCGA Pan-Cancer Analysis of Whole Genomes Consortium. (2020). Pan-cancer analysis of whole genomes. Nature, 578 (7793), 82–93.
Independent UK Panel on Breast Cancer Screening. (2012). The benefits and harms of breast cancer screening: An independent review. Lancet, 380 (9855), 1778–1786.
Irwin, J. J., Tang, K. G., Young, J., Dandarchuluun, C., Wong, B. R., Khurelbaatar, M., Moroz, Y. S., Mayfield, J., & Sayle, R. A. (2020). ZINC20—A free ultralarge-scale chemical database for ligand discovery. Journal of Chemical Information and Modeling, 60 , 6065–6073.
Kanehisa, M., & Goto, S. (2000). KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Research, 28 , 27–30.
Khan, A., Ali, L., & Wei, D. Q. (2022). Editorial: Breast cancer resistance, biomarkers and therapeutics development in the era of artificial intelligence. Frontiers in Molecular Biosciences, 9 , 1034990.
Koh, D. M., Papanikolaou, N., Bick, U., Illing, R., Kahn, C. E., Jr., Kalpathi-Cramer, J., Matos, C., Martí-Bonmatí, L., Miles, A., Mun, S. K., Napel, S., Rockall, A., Sala, E., Strickland, N., & Prior, F. (2022). Artificial intelligence and machine learning in cancer imaging. Communications Medicine (Lond), 2 , 133.
Koscielny, S. (2010). Why most gene expression signatures of tumors have not been useful in the clinic. Science Translational Medicine, 2 , 14ps2.
Lauritzen, A. D., Rodríguez-Ruiz, A., von Euler-Chelpin, M. C., Lynge, E., Vejborg, I., Nielsen, M., Karssemeijer, N., & Lillholm, M. (2022). An artificial intelligence-based mammography screening protocol for breast cancer: Outcome and radiologist workload. Radiology, 304 (1), 41–49.
Leventakos, K., Helgeson, J., Mansfield, A. S., Deering, E., Schwecke, A., Adjei, A., Molina, J., Hocum, C., Halfdanarson, T., Marks, R., Parikh, K., Pomerleau, K., Coverdill, S., Rammage, M., & Haddad, T. (2019). Implementation of artificial intelligence (AI) for lung cancer clinical trial matching in a tertiary cancer center. Annals of Oncology, 30 (Suppl 2), ii74.
Liang, G., Fan, W., Luo, H., & Zhu, X. (2020). The emerging roles of artificial intelligence in cancer drug development and precision therapy. Biomedicine & Pharmacotherapy, 128 , 110255.
Liao, J., Li, X., Gan, Y., Han, S., Rong, P., Wang, W., Li, W., & Zhou, L. (2022). Artificial intelligence assists precision medicine in cancer treatment. Frontiers in Oncology, 12 , 998222.
Lind, A. P., & Anderson, P. C. (2019). Predicting drug activity against cancer cells by random forest models based on minimal genomic information and chemical properties. PLoS One, 14 (7), e0219774.
Ma, C. X., & Ellis, M. J. (2013). The cancer genome atlas: Clinical applications for breast cancer. Oncology (Williston Park), 27 (1263–1269), 1274–1279.
MacEachern, S. J., & Forkert, N. D. (2021). Machine learning for precision medicine. Genome, 64 (4), 416–425.
Mayr, A., Klambauer, G., Unterthiner, T., & Hochreiter, S. (2016). DeepTox: Toxicity prediction using deep learning. Frontiers in Environmental Science, 3 , 80.
Maziarka, Ł., Pocha, A., Kaczmarczyk, J., Rataj, K., Danel, T., & Warchoł, M. (2020). Mol-CycleGAN: A generative model for molecular optimization. Journal of Cheminformatics, 12 , 2.
Mazo, C., Aura, C., Rahman, A., Gallagher, W. M., & Mooney, C. (2022). Application of artificial intelligence techniques to predict risk of recurrence of breast cancer: A systematic review. Journal of Personalized Medicine, 12 (9), 1496.
Nguyen, L. C., Naulaerts, S., Bruna, A., Ghislat, G., & Ballester, P. J. (2021). Predicting cancer drug response in vivo by learning an optimal feature selection of tumour molecular profiles. Biomedicine, 9 (10), 1319.
Nordenskjöld, B., & Rutqvist, L. E. (2002). Long-term effects of mammography screening: Updated overview of the Swedish randomised trials. Lancet, 359 (9310), 909–919.
Ogunleye, A. Z., Piyawajanusorn, C., Gonçalves, A., Ghislat, G., & Ballester, P. J. (2022). Interpretable machine learning models to predict the resistance of breast cancer patients to doxorubicin from their microRNA profiles. Advanced Science (Weinh), 9 (24), e2201501.
Paul, D., Sanap, G., Shenoy, S., Kalyane, D., Kalia, K., & Tekade, R. K. (2021). Artificial intelligence in drug discovery and development. Drug Discovery Today, 26 , 80–93.
Pinheiro, G. A., Mucelini, J., Soares, M. D., Prati, R. C., Da Silva, J. L. F., & Quiles, M. G. (2020). Machine learning prediction of nine molecular properties based on the SMILES representation of the QM9 quantum-chemistry dataset. The Journal of Physical Chemistry A, 124 , 9854–9866.
Popova, M., Isayev, O., & Tropsha, A. (2018). Deep reinforcement learning for de novo drug design. Science. Advances, 4 , eaap7885.
CAS Google Scholar
Prihantono, F. M. (2021). Breast cancer resistance to chemotherapy: When should we suspect it and how can we prevent it? Annals of Medicine and Surgery (Lond), 70 , 102793.
Article CAS PubMed Central Google Scholar
Ramsundar, B. (2018). Molecular machine learning with DeepChem (Doctoral dissertation, Stanford University).
Ren, F., Ding, X., Zheng, M., Korzinkin, M., Cai, X., Zhu, W., Mantsyzov, A., Aliper, A., Aladinskiy, V., Cao, Z., Kong, S., Long, X., Man Liu, B. H., Liu, Y., Naumov, V., Shneyderman, A., Ozerov, I. V., Wang, J., Pun, F. W., Polykovskiy, D. A., Sun, C., Levitt, M., Aspuru-Guzik, A., & Zhavoronkov, A. (2023). Alpha fold accelerates artificial intelligence powered drug discovery: Efficient discovery of a novel CDK20 small molecule inhibitor. Chemical Science, 14 , 1443–1452.
Samanta, B., De, A., Jana, G., Gómez, V., Chattaraj, P. K., Ganguly, N., & Gomez-Rodriguez, M. (2020). Nevae: A deep generative model for molecular graphs. The Journal of Machine Learning Research, 21 , 4556–4588.
Stork, C., Wagner, J., Friedrich, N. O., De Bruyn, K. C., Šícho, M., & Kirchmair, J. (2018). Hit dexter: A machine-learning model for the prediction of frequent hitters. ChemMedChem, 13 , 564–571.
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71 (3), 209–249.
PubMed Google Scholar
Szklarczyk, D., Gable, A. L., Nastou, K. C., Lyon, D., Kirsch, R., Pyysalo, S., Doncheva, N. T., Legeay, M., Fang, T., Bork, P., Jensen, L. J., & Von Mering, C. (2021). The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Research, 49 , D605–D612.
Tai, C. A., Hodzic, N., Flanagan, N., Gunraj, H., & Wong, A. (2023). Cancer-net BCa: Breast cancer pathologic complete response prediction using volumetric deep radiomic features from synthetic correlated diffusion imaging. Submitted to arXiv.
The ENCODE Project Consortium. (2012). An integrated encyclopedia of DNA elements in the human genome. Nature, 489 , 57–74.
Article PubMed Central Google Scholar
The Institute of cancer Research. (2023). New AI drug discovery collaboration aims to design new precision cancer drugs. Retrieved August 24, 2023, from https://www.icr.ac.uk/news-archive/new-ai-drug-discovery-collaboration-aims-to-design-new-precision-cancer-drugs
Torre, L. A., Siegel, R. L., Ward, E. M., & Jemal, A. (2016). Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiology, Biomarkers & Prevention, 25 (1), 16–27.
Vamathevan, J., Clark, D., Czodrowski, P., Dunham, I., Ferran, E., Lee, G., Li, B., Madabhushi, A., Shah, P., Spitzer, M., & Zhao, S. (2019). Applications of machine learning in drug discovery and development. Nature Reviews Drug Discovery, 18 , 463–477.
Verghese, G., Li, M., Liu, F., Lohan, A., Kurian, N. C., Meena, S., Gazinska, P., Shah, A., Oozeer, A., Chan, T., Opdam, M., Linn, S., Gillett, C., Alberts, E., Hardiman, T., Jones, S., Thavaraj, S., Jones, J. L., Salgado, R., Pinder, S. E., Rane, S., Sethi, A., & Grigoriadis, A. (2023). Multiscale deep learning framework captures systemic immune features in lymph nodes predictive of triple negative breast cancer outcome in large-scale studies. The Journal of Pathology, 260 (4), 376–389.
Wang, C., & Zhang, Y. (2017). Improving scoring-docking-screening powers of protein-ligand scoring functions using random forest. Journal of Computational Chemistry, 38 , 169–177.
Wang, L., McLeod, H. L., & Weinshilboum, R. M. (2011). Genomics and drug response. New England Journal of Medicine, 364 (12), 1144–1153.
Wang, L., Song, Y., Wang, H., Zhang, X., Wang, M., He, J., Li, S., Zhang, L., Li, K., & Cao, L. (2023). Advances of artificial intelligence in anti-cancer drug design: A review of the past decade. Pharmaceuticals, 16 , 253.
Wang, X., Zhang, H., & Chen, X. (2019). Drug resistance and combating drug resistance in cancer. Cancer Drug Resistance, 2 (2), 141–160.
PubMed PubMed Central Google Scholar
Wang, Y., Wang, Z., Xu, J., Li, J., Li, S., Zhang, M., & Yang, D. (2018). Systematic identification of non-coding pharmacogenomic landscape in cancer. Nature Communications, 9 (1), 3192.
Wang, Y., Xiao, J., Suzek TO, Zhang, J., Wang, J., & Bryant, S. H. (2009). PubChem: A public information system for analyzing bioactivities of small molecules. Nucleic Acids Research, 37 , W623–W633.
Wang, Y., Zhang, S., Li, F., Zhou, Y., Zhang, Y., Wang, Z., Zhang, R., Zhu, J., Ren, Y., Tan, Y., Qin, C., Li, Y., Li, X., Chen, Y., & Zhu, F. (2019). Therapeutic target database 2020: Enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Research, 48 , D1031–D1041.
PubMed Central Google Scholar
Wishart, D. S., Feunang, Y. D., Guo, A. C., Lo, E. J., Marcu, A., Grant, J. R., Sajed, T., Johnson, D., Li, C., Sayeeda, Z., Assempour, N., Iynkkaran, I., Liu, Y., Maciejewski, A., Gale, N., Wilson, A., Chin, L., Cummings, R., Le, D., Pon, A., Knox, C., & Wilson, M. (2018). DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Research, 46 , D1074–D1082.
Xu, Y. (2022). Deep neural networks for QSAR. Methods in Molecular Biology, 2390 , 233–260.
Yersal, O., & Barutca, S. (2014). Biological subtypes of breast cancer: Prognostic and therapeutic implications. World Journal of Clinical Oncology, 5 , 412–424.
You, Y., Lai, X., Pan, Y., Zheng, H., Vera, J., Liu, S., Deng, S., & Zhang, L. (2022). Artificial intelligence in cancer target identification and drug discovery. Signal Transduction and Targeted Therapy, 7 , 156.
Zeng, L., Liu, L., Chen, D., Lu, H., Xue, Y., Bi, H., & Yang, W. (2023). The innovative model based on artificial intelligence algorithms to predict recurrence risk of patients with postoperative breast cancer. Frontiers in Oncology, 13 , 1117420.
Zhou, J., Sun, H., Wang, Z., Cong, W., Wang, J., Zeng, M., et al. (2020). Guidelines for the diagnosis and treatment of hepatocellular carcinoma (2019 edition). Liver Cancer, 9 (6), 682–720.
Download references

Author information
Authors and affiliations.
Department of Medical Microbiology, School of Laboratory Medicine & Medical Sciences, Medical School Campus, College of Health Sciences, University of KwaZulu-Natal-Natal, Durban, South Africa
Zilungile Mkhize-Kwitshana, Pragalathan Naidoo & Zamathombeni Duma
SAMRC Research Capacity Development Division, South African Medical Research Council, Tygerberg, South Africa
Fortrea Inc., Durham, USA
Kamal S. Saini
Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK
SAMRC Precision Oncology Research Unit (PORU), DSI/NRF SARChI Chair in Precision Oncology and Cancer Prevention (POCP), Pan African Cancer Research Institute (PACRI), University of Pretoria, Pretoria, South Africa
Zodwa Dlamini
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Zilungile Mkhize-Kwitshana .
Editor information
Editors and affiliations.
SAMRC Precision Oncology Research Unit (PORU), DSI/NRF SARChI Chair in Precision Oncology and Cancer Prevention (POCP) Pan African Cancer Research Institute (PACRI), University of Pretoria, Pretoria, South Africa
Rights and permissions
Reprints and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Mkhize-Kwitshana, Z., Naidoo, P., Duma, Z., Saini, K.S., Dlamini, Z. (2024). Revolutionizing Breast Cancer Treatment: Harnessing the Power of Artificial Intelligence in Overcoming Drug Resistance. In: Dlamini, Z. (eds) Overcoming Breast Cancer Therapy Resistance. Springer, Cham. https://doi.org/10.1007/978-3-031-52860-6_10
Download citation
DOI : https://doi.org/10.1007/978-3-031-52860-6_10
Published : 04 June 2024
Publisher Name : Springer, Cham
Print ISBN : 978-3-031-52859-0
Online ISBN : 978-3-031-52860-6
eBook Packages : Biomedical and Life Sciences Biomedical and Life Sciences (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Innovations in the Integrated Management of Breast Cancer
Breast cancer is commonly acknowledged as an international priority in healthcare. To date, it is the most common cancer in women worldwide and demographic trends show a steady increase in incidence.
Over the years, increasing efforts and resources have been devoted to a meticulous analysis of risk factors, diagnostic tools and treatment strategies in order to enhance every step of breast cancer management.
Researchers and clinicians strive in search of an optimized, systematic strategy in the diagnosis and treatment of this disease. This effort has led to the creation of the “breast unit model”, which is today considered a gold standard to ensure optimal clinical services centered on patients and based on research through multidisciplinary and integrated management [ 1 ]. This approach, involving surgical, radiation and medical oncology, allows the optimization of oncological and cosmetic outcomes and the prolonged survival and improvement of patient quality of life; the integrated treatment is tailored to each patient and based on clinical examination, patient status, disease staging, biologic phenotype such as hormone receptor status and human epidermal growth factor receptor 2 (HER2) overexpression, and patient preferences. The decision-making process in the management of breast cancer includes a detailed discussion with the patient about the risks and benefits associated with the selected treatment.
This Special Issue highlights many recent innovations in the integrated management of breast cancer, their potential advantages and the many open issues that still wait to be properly defined and addressed. The authors’ interests span every aspect of breast cancer care: from early breast cancer to metastatic patients, and from surgical assessment to artificial intelligence application in data collection.
Cancer biology is addressed in two pre-clinical studies analyzing breast tissue samples. Santandrea et al. focus on hormone receptor expression in normal breast tissue, in search of a pattern that could favor the development of a breast tumor [ 2 ], while a study by Fuso et al. examines breast cancer patients treated with neoadjuvant chemotherapy in search of a miRNA expression associated with survival, and therefore acting as a predictive biomarker in women affected by early breast cancer [ 3 ].
An accurate and comprehensive preoperative assessment is crucial in order to prepare the patients for surgery, and breast cancer care still holds many issues waiting to be fine-tuned. Nonpalpable lesions can compromise and delay an otherwise smooth operation, and the surgeon should be well-prepared with potential solutions to this common problem. This Special Issue offers a review of current image-guided techniques, highlighting the benefits and controversies of each method [ 4 ]. Radiology is also tackled in a study focusing on the best imaging technique to assess patients scheduled to receive breast reconstruction via a DIEP flap, and the researchers advocate conventional CT as an alternative to the traditional but costly CT angiography [ 5 ].
During the last decade, the goal in surgery has been to make procedures less and less invasive. Much like breast surgery, which has witnessed a gradual diffusion of breast conserving techniques, axillary surgery has also evolved in an increasingly conservative manner. Where previous surgical approaches tended to favor axillary dissection at all costs, the introduction of sentinel lymph node biopsy (SLNB) has led to the preservation of non-pathological axillary lymph node tissue, and once frequent complications such as post-operative lymphedema have greatly diminished in recent years [ 6 ]. In this Special Issue we explore the possibilities of a further evolution in axillary surgery, where treatment with sole SLNB could be extended to include patients downstaged to ycN0 by neoadjuvant chemotherapy [ 7 ].
When a breast-conserving approach cannot guarantee both adequate local control and a good aesthetic result, the surgeon has to perform a mastectomy. Innovative surgical procedures called “conservative mastectomies” with immediate prepectoral implant reconstruction have been introduced in order to obtain more favorable aesthetic outcomes and avoid problems caused by manipulation of the pectoralis major muscle, such as breast animation deformity, postoperative pain and injury-induced muscular deficit [ 8 ].
The primary goal of management in metastatic disease is the alleviation of symptoms, maintenance or improvement in quality of life and prolongation of survival despite possible treatment toxicity. Patients with metastatic disease receive systemic medical treatments including endocrine therapy, chemotherapy, biologic therapies, targeted and immunotherapy and supportive care measures. However, a subset of patients may benefit from a specific loco-regional treatment [ 9 ]: oligometastatic disease has been the object of particular interest because of the possibility to aim for a long-term remission in these patients, and once-discarded options such as liver metastasectomy have been shown to be a possible therapeutic option in selected patients [ 10 , 11 ].
The benefits of a multimodal prehabilitation model are emerging in recent studies, as in this framework patients may be more receptive to health behavior changes in a structured support network. Di Leone et al. shed light on a possible personalized prehabilitation model to enhance patient care in the neoadjuvant setting, which allows each patient to receive the attention of every required specialist in a set frame of time [ 12 , 13 ]. For example, elderly patients can greatly benefit from a preoperative geriatric assessment in order to avoid negative outcomes deriving from otherwise unknown syndromes such as severe sarcopenia [ 14 ]. On the other hand, younger women with a new, unexpected diagnosis of breast cancer may face issues related to sexuality and fertility, and studies addressing the impact of treatment on ovarian reserve are paramount to better understand the mechanisms leading to early menopause and subsequent infertility. The clinician’s primary objective is to offer a timely oncofertility service, in order to preserve the opportunity for family planning without delaying chemotherapy [ 15 ]. Similar strategies must be adopted when confronting pregnancy-associated breast cancer, a rare occurrence that nonetheless threatens the wellbeing of both mother and fetus [ 16 ].
Finally, the last few years have seen the creation of new artificial intelligence technologies with the potential to radically change the modern management of breast cancer. Research itself is a viable candidate for the coming high-tech revolution: today, protocol development can be promoted, patient enrollment can be enhanced by a patient-trial matching made possible by the growing diffusion of electronic health records, and patient parameters and adherence to trials can be monitored in real-time by a variety of wearable devices. This Issue witnesses the transformation, thanks to the contribution of authors active in the field of real-world data: Cesario et al. describe the development of a digital research assistant that manages patient enrollment in trials with the employment of an artificial intelligence algorithm [ 17 ], while Marazzi et al. exploit text mining to successfully extract data from heterogeneous sources and to generate clinical evidence [ 18 , 19 ].
This Special Issue finds its place in the modern panorama of breast care by promoting a modern, holistic approach to breast disease and encouraging clinicians to tailor patient treatment. The development of appropriate clinical pathways, with a multidisciplinary and standardized approach, is essential for successful, well-rounded treatment in the era of personalized medicine.
Author Contributions
Conceptualization/original draft preparation A.M.S. and E.J.M.; Review and editing/supervision G.F.; final draft conceptualization and approval R.M. All authors have read and agreed to the published version of the manuscript.
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Conflicts of interest.
The authors declare no conflict of interest.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
- IT’S ADVANCING OUR UNDERSTANDING OF BREAST CANCER
- IT’S SAVING LIVES, IMPROVING OUTCOMES
- IT’S LEADING TO PREVENTION & A CURE
- RESEARCH IS THE REASON STORIES
- Our Approach
- The Ground We’ve Gained
- Areas of Focus
- Meet Our Researchers
- Collaborative Initiatives
- Start Your Fundraiser
- Make a planned gift
- Game for BCRF
- Other Ways to Give
- Become a Partner
- Find an event
Our History
- Board of Directors
- Scientific Advisors
- Corporate Partners
- Affiliate Organizations
- Major Donors
- Blog: The Progress Report
- Podcasts: Investigating Breast Cancer
- Video Series: Behind the Breakthroughs
- Stories: Research is the reason
- BCRF Publications
- Research is the reason

Since our founding in 1993 by Evelyn H. Lauder, BCRF has raised more than a billion dollars to fuel its mission to be the end of breast cancer. Through a unique and streamlined grants program, we seek out the brightest minds in science and medicine and give them the necessary resources to pursue their best ideas. This enables researchers to make discoveries and design new approaches to address all aspects of breast cancer—and do so in record time.
In 2023-2024, BCRF will award $60.2 million in annual grants to more than 250 scientists from top universities and medical institutions around the globe. In addition, BCRF has established the Evelyn H. Lauder Founder’s Fund , a multi-year international program to unravel the biology of metastasis. It is the largest privately funded project exclusively focused on metastasis in the world.
The thousands of women and men suffering from breast cancer today depend on us. No institution can conquer this disease alone. Together, we can.
BCRF Mission
Our mission is to prevent and cure breast cancer by advancing the world’s most promising research.
BCRF is recognized as one of the most financially efficient nonprofits in the country. We are the highest-rated breast cancer research nonprofit in the U.S.
Frequently Asked Questions
Find answers to the most commonly asked questions about BCRF, including ways to give.

It all started in 1993 at Evelyn Lauder’s kitchen table. Over a cup of coffee, Mrs. Lauder and her dear friend Dr. Larry Norton began a conversation that would change breast cancer history. Recognizing the power of research and its potential to change the lives of millions of women and men worldwide, they realized that a new approach was critical to tackle breast cancer.
Day in and day out, BCRF staff work to end breast cancer.
BOARD OF DIRECTORS
These experts in their fields lend their leadership to BCRF.
SCIENTIFIC ADVISORS
This board of world-renowned investigators shapes our grants program.
MAJOR DONORS
Thanks to their generosity, BCRF can support more than 250 investigators worldwide.
When you give to BCRF, you're funding critical hours in the lab. More time for research means longer, healthier lives for the ones we love.

Get The Latest
Connect with us.
Please remember BCRF in your will planning. Learn More
Breast Cancer Research Foundation 28 West 44th Street, Suite 609, New York, NY 10036
General Office: 646-497-2600 | Toll Free: 1-866-346-3228 [email protected] | BCRF is a 501 (c)(3) | EIN: 13-3727250
- Privacy Policy
- About Breast Cancer
- Find Support
- Get Involved
- Free Resources
- Mammogram Pledge
- Wall of Support
- In The News
- Recursos en Espa ñ ol
- National Mammography Program
- Patient Navigator Program
- Metastatic Retreats
- Support Group
- Breast Health Education
- Breast Cancer Awareness in the Workplace
Breast Cancer Research
Research philosophy.
NBCF’s mission is to help now and inspire hope to those affected by breast cancer through early detection, education, and support services. This distinguishes NBCF from other breast cancer charities . While we fully support investments in research for a cure or new life-extending therapies, we are focused on helping the patient diagnosed today. Patients diagnosed today need life-saving interventions now, and many of these patients face barriers like cost, fear, and misinformation, and simply need navigation. Research that leads to a future medical breakthrough won’t help now and will be too late for many of these patients.
Since our start in 1991, we have invested millions into research to improve early detection, diagnostics, and improvements to current therapies. While some of our funded projects have yielded impactful results, the overall investment is a drop in the bucket.
National Institutes of Health invests over $40 billion each year to medical research. Other major cancer nonprofits add hundreds of millions to that number. Meanwhile, the overall cost of cancer care in the U.S. is over $150 billion a year. While every cancer patient wants a cure, there is a bigger problem cancer patients face today – navigating cost of care and the complexities of the cancer care system.
History of Our Research Projects
NBCF was founded in 1991 with the mission to help women now. Since that time, NBCF has funded over $7 million in research projects. This investment led to the following discoveries:
- Discovery of a homologue gene of HER2 which led to the development of Herceptin.
- Founding member of Worldwide Innovative Network (WIN), created to accelerate the pace and reduce the cost of translating novel cancer treatments to the bedside by developing and applying, through worldwide clinical trials and research projects, the most promising advances in genomic-based cancer research.
- Launch of WINTHER Trial, which aimed to expand precision oncology to patients with advanced solid tumors that progressed after treatment with standard therapies.
- MD Anderson’s Breast Cancer Moon Shots Program – researching novel genetic markers to identify new plans of attack and improve triple negative breast cancer patient outcomes.
NBCF also funded breakthroughs in breast cancer early detection and patient navigation programs at Cleveland Clinic, UC San Francisco, and C-Change.
Our Research Now
NBCF supports research projects to study and improve existing programs in order to help improve quality of life for metastatic breast cancer patients and their caregivers as well as increase access to knowledge, resources, and training for patient navigators. NBCF funds the following research projects:
Metastatic Breast Cancer Retreat Research
- Research partners: Saint Luke’s Cancer Institute & Koontz Center for Advanced Breast Cancer
- Quantitative analysis
- Qualitative analysis
Patient Navigation Research
- Research partners: Academy of Oncology Nurse & Patient Navigators
- Multisite study of patient navigation programs
- Creation of navigation metrics toolkit
Metastatic Breast Cancer Retreats
Study 1 : outcomes from a metastatic breast cancer retreat for patients and caregivers: improvements in gratitude and personal meaning .
Larson, C., Harry, K. M., Geske, S. J., Metsker, J., Miller, M., Eyler, J., Adams, H., & Pluard, T. J. (2020, March). Outcomes from a Metastatic Breast Cancer Retreat for Patients and Caregivers: Improvements in Gratitude and Personal Meaning . Virtual poster presented at the annual conference of the American Psychosocial Oncology Society.
Saint Luke’s Cancer Institute & Koontz Center for Advanced Breast Cancer
Background/Purpose
Lillie Shockney’s “A Journey of Courage and Hope” three-day retreat protocol is designed to address the specific psychosocial needs of women with metastatic breast cancer (MBC) and their caregivers. Over the course of three days, patients and caregivers participated in guided activities that supported the medical, spiritual, psychological, and relational challenges of MBC. Thus, this study investigated how the three-day psychosocial retreat affected self-reported measures of gratitude, personal meaning, and emotional intimacy.
Study 2 : Experiences of Metastatic Breast Cancer Retreat: A Qualitative Analysis Comparing Patients and Their Caregivers
Carly Larson, M.A.; Savannah Geske, Ph.D., Janie Metsker, RN BSN CN-BN; Monty Miller, LCSW; Jake Eyler, MDiv., BCC
Saint Luke’s Hospital Koontz Center for Advanced Breast Cancer
Over the course of two years, Saint Luke’s Cancer Institute hosted three weekend-long therapeutic retreats for women with metastatic breast cancer and their significant others. The three-day long program was based on Lillie Shockney’s, A Journey of Courage and Hope retreat protocol. At the end of each retreat, all participants completed open-ended survey questions about their experience. This quality improvement research project reviewed the responses in order to improve and enhance the retreat curriculum to best serve both patients and their caregivers.
Patient Navigation
National evidence-based oncology navigation metrics: multisite exploratory study to demonstrate value and sustainability of navigation programs.
Over the past 3 years, a dedicated task force comprised of the Academy of Oncology Nurse & Patient Navigators ( AONN+ ) leadership and members, in collaboration with the American Cancer Society and Chartis Oncology Solutions, has been involved in the extensive exploratory multisite study to demonstrate the value and sustainability of navigation programs.
The purpose of the study is to (1) assess the reliability and validity of 10 key metrics selected from the list of 35 developed by AONN+, and (2) gain insight into the barriers and challenges navigation programs encounter during the implementation of navigation metrics. Harnessing the power of this information to create best practices will elevate navigation and garner industry support for advancing patient-centered care delivery.
The result of those efforts is the Navigation Metrics Toolkit . This new resource will be an invaluable aid to navigators, oncology program administrators, healthcare executives, and other clinicians who are linked to navigation in creating transformative patient care and defining oncology navigation professional practice.
Help support women in need
Donations are always appreciated, but there are lots of great ways to get involved.
We use cookies on our website to personalize your experience and improve our efforts. By continuing, you agree to the terms of our Privacy & Cookies Policies.
- See us on facebook
- See us on twitter
- See us on youtube
- See us on linkedin
- See us on instagram
Gene variants foretell the biology of future breast cancers in Stanford Medicine study
In a finding that vastly expands the understanding of tumor evolution, researchers discover genetic biomarkers that can predict the breast cancer subtype a patient is likely to develop.
May 30, 2024 - By Krista Conger

Stanford Medicine researchers found that inherited gene sequences can predict what type of breast cancer a patient is likely to develop, along with how aggressive that cancer may be. Emily Moskal
A Stanford Medicine study of thousands of breast cancers has found that the gene sequences we inherit at conception are powerful predictors of the breast cancer type we might develop decades later and how deadly it might be.
The study challenges the dogma that most cancers arise as the result of random mutations that accumulate during our lifetimes. Instead, it points to the active involvement of gene sequences we inherit from our parents — what’s known as your germline genome — in determining whether cells bearing potential cancer-causing mutations are recognized and eliminated by the immune system or skitter under the radar to become nascent cancers.
“Apart from a few highly penetrant genes that confer significant cancer risk, the role of hereditary factors remains poorly understood, and most malignancies are assumed to result from random errors during cell division or bad luck,” said Christina Curtis , PhD, the RZ Cao Professor of Medicine and a professor of genetics and of biomedical data science. “This would imply that tumor initiation is random, but that is not what we observe. Rather, we find that the path to tumor development is constrained by hereditary factors and immunity. This new result unearths a new class of biomarkers to forecast tumor progression and an entirely new way of understanding breast cancer origins.”
Curtis is the senior author of the study, which will be published May 31 in Science . Postdoctoral scholar Kathleen Houlahan , PhD, is the lead author of the research.
“Back in 2015, we had posited that some tumors are ‘born to be bad’ — meaning that their malignant and even metastatic potential is determined early in the disease course,” Curtis said. “We and others have since corroborated this finding across multiple tumors, but these findings cast a whole new light on just how early this happens.”
A new take on cancer’s origin
The study, which gives a nuanced and powerful new understanding of the interplay between newly arisen cancer cells and the immune system, is likely to help researchers and clinicians better predict and combat breast tumors.
Currently, only a few high-profile cancer-associated mutations in genes are regularly used to predict cancers, but these account for a small minority of cases. Those include BRCA1 and BRCA2, which occur in about one of every 500 women and confer an increased risk of breast or ovarian cancer, and rarer mutations in a gene called TP53 that causes a disease called Li Fraumeni syndrome, which predisposes to childhood and adult-onset tumors.

Christina Curtis
The findings suggest there are tens or hundreds of additional gene variants — identifiable in healthy people — that through interactions with the immune system pull the strings that determine why some people remain cancer-free throughout their lives.
“Our findings not only explain which subtype of breast cancer an individual is likely to develop,” Houlahan said, “but they also hint at how aggressive and prone to metastasizing that subtype will be. Beyond that, we speculate that these inherited variants may influence a person’s risk of developing breast cancer. However, future studies will be needed to examine this.”
The genes we inherit from our parents are known as our germline genome. They’re mirrors of our parents’ genetic makeup, and they can vary among people in small ways that give some of us blue eyes, brown hair or type O blood. Some inherited genes include mutations that confer increased cancer risk from the get-go, such as BRCA1, BRCA2 and TP53.
In contrast, most cancer-associated genes are part of what’s known as our somatic genome. As we live our lives, our cells divide and die in the tens of millions. Each time the DNA in a cell is copied, mistakes happen and mutations can accumulate. DNA in tumors is often compared with the germline genomes in blood or normal tissues in an individual to pinpoint which changes likely led to the cell’s cancerous transformation.
Classifying breast cancers
In 2012, Curtis began a deep dive — assisted by machine learning — into the types of somatic mutations that occur in thousands of breast cancers. She was eventually able to categorize the disease into 11 subtypes with varying prognoses and risk of recurrence, finding that four of the 11 groups were significantly more likely to recur even 10 or 20 years after diagnosis — critical information for clinicians making treatment decisions and discussing long-term prognoses with their patients.
Prior studies had shown that people with inherited BRCA1 mutations tend to develop a subtype of breast cancer known as triple negative breast cancer. This correlation implies some behind-the-scenes shenanigans by the germline genome that affects what subtype of breast cancer someone might develop.
“We wanted to understand how inherited DNA might sculpt how a tumor evolves,” Houlahan said. To do so, they took a close look at the immune system.
It’s a quirk of biology that even healthy cells routinely decorate their outer membranes with small chunks of the proteins they have bobbing in their cytoplasm — an outward display that reflects their inner style.

Kathleen Houlahan
The foundations for this display are what’s known as HLA proteins, and they are highly variable among individuals. Like fashion police, immune cells called T cells prowl the body looking for any suspicious or overly flashy bling (called epitopes) that might signal something is amiss inside the cell. A cell infected with a virus will display bits of viral proteins; a sick or cancerous cell will adorn itself with abnormal proteins. These faux pas trigger the T cells to destroy the offenders.
Houlahan and Curtis decided to focus on oncogenes, normal genes that, when mutated, can free a cell from regulatory pathways meant to keep it on the straight and narrow. Often, these mutations take the form of multiple copies of the normal gene, arranged nose to tail along the DNA — the result of a kind of genomic stutter called amplification. Amplifications in specific oncogenes drive different cancer pathways and were used to differentiate one breast cancer subtype from another in Curtis’ original studies.
The importance of bling
The researchers wondered whether highly recognizable epitopes would be more likely to attract T cells’ attention than other, more modest displays (think golf-ball-sized, dangly turquoise earrings versus a simple silver stud). If so, a cell that had inherited a flashy version of an oncogene might be less able to pull off its amplification without alerting the immune system than a cell with a more modest version of the same gene. (One pair of overly gaudy turquoise earrings can be excused; five pairs might cause a patrolling fashionista T cell to switch from tutting to terminating.)
The researchers studied nearly 6,000 breast tumors spanning various stages of disease to learn whether the subtype of each tumor correlated with the patients’ germline oncogene sequences. They found that people who had inherited an oncogene with a high germline epitope burden (read: lots of bling) — and an HLA type that can display that epitope prominently — were significantly less likely to develop breast cancer subtypes in which that oncogene is amplified.
There was a surprise, though. The researchers found that cancers with a large germline epitope burden that manage to escape the roving immune cells early in their development tended to be more aggressive and have a poorer prognosis than their more subdued peers.
“At the early, pre-invasive stage, a high germline epitope burden is protective against cancer,” Houlahan said. “But once it’s been forced to wrestle with the immune system and come up with mechanisms to overcome it, tumors with high germline epitope burden are more aggressive and prone to metastasis. The pattern flips during tumor progression.”
“Basically, there is a tug of war between tumor and immune cells,” Curtis said. “In the preinvasive setting, the nascent tumor may initially be more susceptible to immune surveillance and destruction. Indeed, many tumors are likely eliminated in this manner and go unnoticed. However, the immune system does not always win. Some tumor cells may not be eliminated and those that persist develop ways to evade immune recognition and destruction. Our findings shed light on this opaque process and may inform the optimal timing of therapeutic intervention, as well as how to make an immunologically cold tumor become hot, rendering it more sensitive to therapy.”
The researchers envision a future when the germline genome is used to further stratify the 11 breast cancer subtypes identified by Curtis to guide treatment decisions and improve prognoses and monitoring for recurrence. The study’s findings may also give additional clues in the hunt for personalized cancer immunotherapies and may enable clinicians to one day predict a healthy person’s risk of developing an invasive breast cancer from a simple blood sample.
“We started with a bold hypothesis,” Curtis said. “The field had not thought about tumor origins and evolution in this way. We’re examining other cancers through this new lens of hereditary and acquired factors and tumor-immune co-evolution.”
The study was funded by the National Institutes of Health (grants DP1-CA238296 and U54CA261719), the Canadian Institutes of Health Research and the Chan Zuckerberg Biohub.

About Stanford Medicine
Stanford Medicine is an integrated academic health system comprising the Stanford School of Medicine and adult and pediatric health care delivery systems. Together, they harness the full potential of biomedicine through collaborative research, education and clinical care for patients. For more information, please visit med.stanford.edu .
Hope amid crisis
Psychiatry’s new frontiers

- U.S. Department of Health & Human Services

- Virtual Tour
- Staff Directory
- En Español
You are here
Nih research matters.
June 4, 2024
Gene variants and breast cancer risk in Black women
At a glance.
- In the largest study of its kind, researchers identified genetic variants that appear to boost breast cancer risk among females of African ancestry.
- The findings could help improve risk prediction in this population and lead to more targeted therapies and prevention strategies.

Breast cancer is the most often diagnosed cancer in many parts of the world, including the U.S. More than 310,000 new cases are expected nationwide this year.
Black women tend to develop breast cancer at a younger age than White women. Black women are also more likely than Whites to die from the disease, and they are twice as likely to develop an aggressive subtype called triple-negative breast cancer. But despite the increased risks faced by women of African descent, most large-scale genetic studies of breast cancer to date have focused on women of European ancestry.
To better understand their unique genetic risks, a research team led by Dr. Wei Zheng of Vanderbilt University analyzed genetic data from over 40,000 females of African descent. About 18,000 had been diagnosed with breast cancer. The data were gathered as part of the NIH-funded African Ancestry Breast Cancer Genetic consortium, which combined data from 26 studies. Most participants (85%) were African Americans. The rest were from Barbados or Africa.
The researchers conducted a genome-wide association study (GWAS) to look for genetic variants that are found more often in participants with breast cancer than in those without. This is believed to be the largest GWAS study to date of breast cancer in this population. Results were reported in Nature Genetics on May 13, 2024.
The analysis pinpointed 12 genetic regions, or loci, associated with breast cancer. Three of these loci were linked to the aggressive triple-negative cancer. About 8% of the women carried two genetic copies of risk variants in all three of these loci. Such women, the researchers found, were 4.2 times more likely to be diagnosed with triple-negative breast cancer than women who had only one or no copies of the variants.
Because this type of cancer lacks specific cell receptors often seen with breast cancer (like estrogen or HER2 receptors), there are fewer targeted options for treatment. These findings may help researchers identify new treatment targets.
The researchers also confirmed many breast cancer risk variants that were found earlier in other populations. And they identified an uncommon risk variant in the gene ARHGEF38 , which had been previously linked to aggressive prostate and lung cancers.
The scientists used their findings to create polygenic risk scores (PRS) for breast cancer risk in females of African descent. PRS use genomic data to gauge the chance that a person will develop a certain medical condition. PRS created previously, using results from other populations, tend to perform poorly at predicting breast cancer risk for Black women. The new PRS, based on genomic data from African descendants, outperformed previous PRS at predicting breast cancer risk in this population.
The findings and data could lead to improved detection of breast cancer in this at-risk population and provide clues for potential treatment targets. Studies with even larger, more diverse populations will be needed to further improve the prediction of breast cancer risk.
“We have worked with researchers from more than 15 institutions in the U.S. and Africa to establish this large genetic consortium,” Zheng says. “Data put together in this consortium have been and will continue to be used by researchers around the world.”
—by Vicki Contie
Related Links
- Human Pangenome Boosts Accuracy and Reflects Diversity
- Technique May Improve Detection of Breast Tumors
- Test Predicts Whether Chemotherapy Will Help Early-Stage Breast Cancer Patients
- Advances in Breast Cancer: Screening and Treatment Get Personal
- Breast Cancer
- Polygenic Risk Scores
References: Genome-wide association analyses of breast cancer in women of African ancestry identify new susceptibility loci and improve risk prediction. Jia G, Ping J, Guo X, Yang Y, Tao R, Li B, Ambs S, Barnard ME, Chen Y, Garcia-Closas M, Gu J, Hu JJ, Huo D, John EM, Li CI, Li JL, Nathanson KL, Nemesure B, Olopade OI, Pal T, Press MF, Sanderson M, Sandler DP, Shu XO, Troester MA, Yao S, Adejumo PO, Ahearn T, Brewster AM, Hennis AJM, Makumbi T, Ndom P, O'Brien KM, Olshan AF, Oluwasanu MM, Reid S, Butler EN, Huang M, Ntekim A, Qian H, Zhang H, Ambrosone CB, Cai Q, Long J, Palmer JR, Haiman CA, Zheng W. Nat Genet. 2024 May;56(5):819-826. doi: 10.1038/s41588-024-01736-4. Epub 2024 May 13. PMID: 38741014.
Funding: NIH’s National Cancer Institute (NCI).
Connect with Us
- More Social Media from NIH
Cochrane Breast Cancer
Top 10 breast cancer topics needing a cochrane systematic review.

Deciding which research topics to focus on in medicine and health depends on many factors. These factors can include the currency of a topic, feedback from people providing or receiving care, and the priorities of funders.
In late 2019, the Cochrane Breast Cancer Group (part of Cochrane’s Cancer Network) conducted a formal priority-setting exercise to help decide which review topics were most needed in the Cochrane Library. The Group did this by circulating a survey listing 25 new or existing review topics to a diverse group of individuals who are part of the international breast cancer community. The survey asked individuals to rank their top 10 topics from the list. Read details about the aims and methods used for this priority-setting exercise, which adhered to the standards outlined in Cochrane’s priority setting guidance note .
What were the top 10 review topics?
Read about the ranking of the 25 new or existing review topics .
What is next?
Support to author teams For the top 10 topics, the Cochrane Breast Cancer Group will prioritise these topics during the editorial and peer-review process.
For all breast cancer review topics registered with Cochrane, the Cochrane Breast Cancer Group continues to work on these topics with author teams as these remain important topics. There will be no noticeable change in the support provided to author teams.
Future topics The Cochrane Breast Cancer Group is open to receiving new topic ideas. If you have suggestions for new topics that are not currently covered in the Cochrane Library, please send your idea to [email protected] .
Repeating this priority-setting exercise The priority-setting exercise may be repeated every 3 years, depending on resources.
Who responded to the survey?
The survey was circulated to over 800 individuals. Of the 199 people who responded, 90 people (45%) provided complete responses. The respondents were doctors (59%), researchers (18%) and people who had received treatment or currently receiving treatment for breast cancer (14%). Most respondents were from the UK, followed by the USA, Argentina, and India.
How did we calculate the ranking for each review topic?
The average ranking was calculated for each topic. This method is commonly used to determine ranking scores from surveys. This approach considers the number of counts for each ranking on a topic, the weighting of each rank (where a ranking of 1 gets the most weight) and the total number of counts.
[Cover image: foliage of the Yew tree. Taxanes, a class of chemotherapy drugs, were originally derived from the Yew tree]
Your Account
Manage your account, subscriptions and profile.
MyKomen Health
ShareForCures
In Your Community
In Your Community
View resources and events in your local community.
Change your location:

Susan G. Komen®
One moment can change everything.
What’s New in Breast Cancer
This section gives an overview of new breast cancer treatment breakthroughs and recent developments in research that are fueling new ways to assess risk, and prevent, detect, diagnose and treat breast cancer. Advances in breast cancer care are evaluated through a rigorous process that includes clinical trials and regulatory approvals before being considered standards of care and included in breast cancer care guidelines. Komen’s research team monitors the rapidly evolving breast cancer landscape, and here we will highlight new breast cancer treatment breakthroughs, innovations in technology or key advances that may be added or are new to guidelines. We will share these research advancements to empower patients with knowledge to help them make informed decisions with their doctors.
Use these links to jump to the topics below.
- Emerging Areas in Metastatic Breast Cancer Treatment
- Clinical Trials
Treatments and Drugs
For patients, new treatments can mean more options and more hope. Researchers are working to develop new breast cancer treatment breakthroughs, such as more effective drugs that will specifically target breast cancer cells, minimize side effects and prevent breast cancer cells from coming back. While some treatments increase the effectiveness of existing drugs, others may offer new, innovative strategies for attacking tumor cells.
As of August 2023, the following new treatments and drugs are currently in clinical trials and have not yet received FDA approval:
- A new antibody-drug conjugate called datopotamab deruxtecan (Dato-DXd) is currently being evaluated in three Phase 3 clinical trials for advanced estrogen receptor-positive (ER+) [2] breast cancer, metastatic triple negative [ 3 ] breast cancer and early triple negative [ 4 ] breast cancer (TNBC). Dato-DXd specifically targets a protein called TROP2, a biomarker that can be used to target cancer cells instead of healthy cells. Another TROP2-targeting therapy called sacituzumab govitecan has already been approved for TNBC and estrogen-receptor-positive breast cancer. Dato-DXd uses a different chemotherapy drug and delivery system compared to sacituzumab govitecan.
- HER2 is a common treatment target for breast cancer. This new drug targets HER3, a biomarker related to HER2, which is associated with poor breast cancer outcomes. About 10% to 20% of newly diagnosed breast cancers are HER2-positive. At the 2023 American Society for Clinical Oncology (ASCO) Annual Meeting, researchers announced positive results for a Phase 2 clinical trial studying HER3-DXd, a new HER3-targeting antibody-drug conjugate for people with metastatic breast cancer . [ 1 ]. While the study found that 35% of patients responded positively to HER3-DXd, researchers will continue to evaluate which patients could benefit most from this drug through future Phase 3 clinical trials.
- CDK4/6 inhibitors are commonly used to treat estrogen receptor-positive breast cancer, but a new CDK4/6 inhibitor called trilaciclib is being tested to treat TNBC. Results from a Phase 2 clinical trial showed that trilaciclib improved outcomes for people with advanced TNBC, and the drug is currently being evaluated in the Phase 3 PRESERVE 2 clinical trial [ 5 ]. Researchers believe that unlike currently available CDK4/6 inhibitors, trilaciclib may improve response to immunotherapy and mitigate some of the side effects of chemotherapy . If this clinical trial is successful, this would be the first CDK4/6 inhibitor approved for people with TNBC.
New and improved technologies may be able to increase the speed and accuracy of detecting, diagnosing or monitoring breast cancer for progression and response to treatment.
- Doctors may use PET scans, or positron emission tomography, to scan for evidence that breast cancer has spread or metastasized. Once breast cancer has spread, the metastases may have evolved to a different type of breast cancer than the original tumor. These differences mean the metastases and the original tumor may not respond to the same treatments. A diagnostic imaging agent called Cerianna (fluoroestradiol F-18 or FES PET) allows doctors to use PET scans to learn if estrogen receptors are present in metastatic lesions. If a person has metastatic lesions that are estrogen receptor-positive, they may respond well to hormone therapy. This agent was recently incorporated in the National Comprehensive Cancer Network (NCCN) guidelines [ 6 ] as an option for some people with metastatic or recurrent estrogen receptor-positive breast cancer to consider [ 7 ].
- Ovarian suppression increases the effectiveness of hormone therapy in some premenopausal women but comes with additional side effects that can affect quality of life. A study presented at the 2022 San Antonio Breast Cancer Symposium [ 8 ] suggests that the Breast Cancer Index , a tumor profiling test that looks at genes to predict how likely a cancer is to metastasize, may be able to identify premenopausal women that would benefit most from ovarian suppression. This test would give doctors a new tool to personalize treatment for premenopausal women with estrogen receptor-positive breast cancer. More data are needed to confirm these results.
- Doctors are getting closer to identifying which patients with early HER2-positive breast cancer can safely avoid chemotherapy by using the HER2DX genomic test. HER2DX is the first test specifically designed to identify HER2-positive patients at high and low risk for recurrence . For some people, being able to avoid chemotherapy without comprising long-term outcomes will lead to a better quality of life.

Research can take decades to reach the bedside, but what discoveries are just around the corner for patients? Susan G. Komen shares all of this and more through Breast Cancer Breakthroughs, a virtual education series focusing on the new science and technology advancements that are poised to make a difference for patients in the near future. Sign up for Breast Cancer Breakthroughs to never miss an episode.

Kimberly’s Story: Finding Joy in the Midst of a Metastatic Breast Cancer Diagnosis
After Kimberly Reinika’s mother passed away in 2019 from ovarian cancer, she worried that it would ultimately take her life, too. “That was the cancer I was checking for,” she said.
Approaches to Care
With knowledge gained from clinical trials, researchers are seeking new ways to improve patient outcomes while using existing drugs. Some new breast cancer treatment breakthroughs are the result of combining certain drugs, finding which patients can skip certain elements of treatment or changing the order of their treatments to maximize effectiveness or minimize side effects.
- Patients with early estrogen receptor-positive breast cancer generally have a good prognosis, but some people have a higher risk of recurrence for as long as 20 years. Researchers are seeking new strategies to reduce this risk of recurrence. CDK4/6 inhibitors are used to treat advanced breast cancer, but the Phase 3 NATALEE clinical trial, presented at the 2023 American Society of Clinical Oncology Annual Meeting [ 9 ], found that using the CDK4/6 inhibitor ribociclib for two years in the adjuvant setting reduced the risk of recurrence for people with estrogen receptor-positive breast cancer.
- Inflammatory breast cancer is difficult to diagnose because its symptoms often mimic infections. Additionally, because some medical professionals don’t see it often, they may lack experience in recognizing and treating inflammatory breast cancer. In partnership with the Inflammatory Breast Cancer Research Foundation and the Milburn Foundation, Susan G. Komen launched a first-of-its kind diagnostic tool for inflammatory breast cancer. Through this scoring system, the tool considers the defining features of inflammatory breast cancer and provides data that can help providers accurately determine whether a person has inflammatory breast cancer. The goal of this tool is to increase the accuracy of diagnosing inflammatory breast cancer so that people will receive the appropriate care they need to treat this aggressive disease.
- Immunotherapy targets the immune system to help the body fight off tumors. Immunotherapy is currently only available for some patients with triple negative breast cancer, but researchers are aiming to bring this cutting edge therapy to more people. In a recent announcement [ 10 ], positive results were announced for a clinical trial that evaluated the immunotherapy drug pembrolizumab in patients with early estrogen receptor-positive breast cancer. Komen will be closely monitoring the results of this study at upcoming scientific conferences and hopes to see more promising data suggesting that a new treatment option may soon be available for patients with early estrogen receptor-positive breast cancer.
- Clinical trials are often designed using the maximum tolerated dose of a drug. However, many drugs may give the same effect with a smaller dose that results in fewer side effects for the patient. The X-7/7 clinical trial, which was presented at the 2023 ASCO Annual Meeting, tested the impact of a new treatment schedule for the chemotherapy drug capecitabine to treat metastatic breast cancer. Researchers found that people who took a higher dose of capecitabine over fewer days had fewer side effects and were able to remain on their treatment longer compared to the standard regimen. This new approach can improve the quality of life for those living with metastatic breast cancer without compromising the effectiveness of their treatments.
Komen will be closely monitoring the results of these studies and more at upcoming scientific conferences and hopes to see more promising data regarding new ways to prevent, detect, diagnose and treat breast cancer.

It Looks Promising: Uncovering New Possibilities in Breast Cancer Prevention
Is breast cancer prevention possible? Komen Scientific Advisory Board Member Dr. Kornelia Polyak is exploring a new strategy to identify and eliminate cell precursors from which tumors can grow.

Help discover cures to breast cancer, faster. New treatment breakthroughs for breast cancer come from researchers learning from people who have breast cancer, but our current data sources only represent a small portion of the breast cancer community. Help us discover the cures to breast cancer, faster, by joining ShareForCures.
What’s New in Breast Cancer References
- Hamilton, E. P., et al. (2023). “A phase 2 study of HER3-DXd in patients (pts) with metastatic breast cancer (MBC).” Journal of Clinical Oncology 41(16_suppl): 1004-1004. https://meetings.asco.org/abstracts-presentations/219699
- https://classic.clinicaltrials.gov/ct2/show/NCT05104866
- https://clinicaltrials.gov/study/NCT05374512
- https://classic.clinicaltrials.gov/ct2/show/NCT05629585
- https://classic.clinicaltrials.gov/ct2/show/NCT04799249
- https://www.gehealthcare.com/about/newsroom/press-releases/ge-healthcare-announces-fes-pet-imaging-recommendation-in-nccn-clinical-practice-guidelines-in-oncology-nccn-guidelines
- https://www.nccn.org/patients/guidelines/content/PDF/breast-invasive-patient.pdf (page 16)
- https://www.sabcs.org/Portals/SABCS2016/2022%20SABCS/SABCS%202022%20Abstract%20Report.pdf?ver=2022-12-08-111637-860
- Stroyakovskiy, D., et al. (2023). “Ribociclib and endocrine therapy as adjuvant treatment in patients with HR+/HER2- early breast cancer: Primary results from the phase III NATALEE trial.” Journal of Clinical Oncology 41(17_suppl): LBA500-LBA500.
- https://www.merck.com/news/merck-announces-phase-3-keynote-756-trial-met-primary-endpoint-of-pathological-complete-response-pcr-rate-in-patients-with-high-risk-early-stage-er-her2-breast-cancer/
TOOLS & RESOURCES
NEED HELP OR MORE INFORMATION?
1-877 GO KOMEN (1-877-465-6636)
Educational Resources
Komen Financial Assistance Program
Select "Patients / Caregivers / Public" or "Researchers / Professionals" to filter your results. To further refine your search, toggle appropriate sections on or off.
Home > Cancer Researchers / Other Health Care Professionals > Research Funding > Grants > AACR-AstraZeneca Breast Cancer Research Fellowship for Endocrine Therapy Research
- Current Funding Opportunities
- Upcoming Funding Opportunities
- Information for Current Grantees
- Research Training and Fellowships
- Career Development Awards
- Independent Research Grants
- Funding Partnerships
- Stand Up To Cancer
- Research Funding Impact
AACR-AstraZeneca Breast Cancer Research Fellowship for Endocrine Therapy Research
The AACR-AstraZeneca Breast Cancer Fellowship for Endocrine Therapy Research represent a joint effort to encourage and support postdoctoral or clinical research fellows to conduct breast cancer research and to establish a successful career path in this field. Proposed research projects are restricted to basic, clinical, translational or epidemiological projects that substantially advance the field of endocrine therapy in breast cancer.
These fellowships provide two-year grants of $130,000 to support the salary and benefits of the fellow while working on mentored breast cancer research. A partial amount of funds may be designated for non-personnel expenses, such as research/laboratory supplies, equipment, publication charges for manuscripts that pertain directly to the funded project, and other research expenses. Indirect costs are not permitted.
Download the Program Guidelines and Application Instructions here .
Research projects may be in basic, translational, clinical, or population sciences research and must have direct applicability and relevance to the understanding and use of endocrine therapy in breast cancer.
Applicants must have a doctoral degree (including PhD, MD, MD/PhD, or equivalent) in a related field and not currently be a candidate for a further doctoral degree.
At the start of the grant term on December 1, 2024, applicants must:
- If eligibility is based on a future position, the position must be confirmed at the time of application and CANNOT be contingent upon receiving this grant.
- If the future position is at a different institution than the applicant’s current institution, the applicant must contact AACR’s Research and Grants Administration Department (AACR’s RGA) at [email protected] before submitting their application for information on additional verification materials/signatures that may be required.
- Applicants with a medical degree must have completed their most recent doctoral degree or medical residency - whichever date is later - within the past five years.
- Work under the auspices of a mentor at an academic, medical, or research institution anywhere in the world.
There are no citizenship or geographic requirements. However, by submitting an application for this grant, an applicant applying from an institution located in a country in which they are not a citizen or a permanent resident assures that the visa status will provide sufficient time to complete the project and grant term at the institution from which they applied.
Applicants must be AACR members in good standing (dues paid for the current year). Annual dues are not required for early-career researchers interested in Associate membership. Please be informed that AACR offers reduced membership due rates for applicants from countries with emerging economies based on the World Bank listing. Nonmembers interested in this grant opportunity must submit a satisfactory application for AACR membership by the application deadline. Nonmembers can apply for membership online . Please review the Membership Categories for the category that best fits your qualifications. Nonmembers must obtain a statement of support from a nominator who is an Active AACR member in good standing (dues paid for the current year). Nonmembers requiring assistance with finding a nominator may email [email protected] and include your geographical location to receive a list of members in your area to contact for nominations. For more information check the membership FAQ . Members can renew their membership through myAACR . You will need your logon (email address) and password to access the member portal. If you require assistance logging in, please submit a help form . Lapsed members must be members in good standing by the application deadline and should contact the Membership Department at [email protected] for assistance.
The AACR requires applicants to submit an online application by 1:00 p.m. U.S. Eastern Time on Thursday, August 15, 2024, using the ProposalCentral website at https://ProposalCentral.com.
- The Progression of Cancer
- Research Funding, Prestigious Research Grants
- Professional Development Opportunities
Confluence Project
The Confluence project will develop a large research resource to uncover breast cancer genetics through genome-wide association studies (GWAS). The resource will include at least 300,000 breast cancer cases and 300,000 controls of different races/ethnicities. This will be accomplished by the confluence of existing GWAS and new genome-wide genotyping data to be generated through this project.
Broad scientific aims that can be addressed through this resource include:
- To discover susceptibility loci and advance knowledge of etiology of breast cancer overall and by subtypes.
- To develop polygenic risk scores and integrate them with known risk factors for personalized risk assessment for breast cancer overall and by subtypes.
- To discover loci for breast cancer prognosis, long-term survival, response to treatment, and second breast cancer.
For more information, please see the Confluence Data Platform .
Please contact the project manager, Thomas Ahearn, at [email protected]
Confluence is supported by NCI Intramural Research funds.
Artemis Project®
What is nbcc's artemis project®.
Originally created as the research component of NBCC’s Breast Cancer Deadline 2020 ® initiative, the Artemis Project ® is an advocate-led collaboration of researchers and advocates that develops and implements research action plans to address overarching issues in breast cancer. The Artemis Project employs an innovative, mission-driven approach to strategic summits, catalytic workshops, and collaborative efforts of various stakeholders focusing on two areas:
- Primary Prevention: How do we stop people from getting breast cancer in the first place?
- Prevention of Metastasis: How do we stop people from dying of breast cancer?
Artemis Project participants are scientists, clinicians, advocates, and other stakeholders, who interact through an infrastructure maintained by NBCC that allows collaborations to thrive and progress rapidly. The first Artemis Project, begun in 2011, focused on primary prevention. A strategic plan for the development of an Artemis preventive vaccine was developed and is being implemented. Initial seed grants were awarded to identify vaccine targets and begin pre-clinical work, and regular meetings occur to assess progress and readjust plans. In 2020, NBCC’s plan was accepted by the NCI PREVENT program to help advance a Phase 1 clinical trial.
Artemis Project participants continue to discuss other primary prevention topics, such as exploring aspects of the microbiome and risk stratification.
In 2014, NBCC launched the first annual meeting for the Artemis Project for the Prevention of Metastasis. The initial focus has been understanding dormant disseminated tumor cells (DTCs). We know that DTCs can “wake up” and result in distant recurrence in some individuals as many as 20 to 30 years after their initial breast cancer diagnosis. Key questions addressed at Artemis include how we intervene and prevent these delayed and distant recurrences, either by targeting and killing them or by keeping them in a dormant state. Early Artemis seed grants have demonstrated some of the mechanisms by which DTCs evade the immune system. One focus of Artemis is on identifying unique cell surface architecture that might be targetable. Another area of study is on the microenvironment that DTCs persist in and how that influences dormancy. More recently, Artemis members have also focused on other novel mechanisms for preventing metastasis.
Since 2010, Artemis Project members have become a well-integrated group, collaborating throughout the year on many of the ideas stemming from the annual Artemis meetings.
- 2010 : Launched an Advocate-Led Research Initiative, the Artemis Project.
- 2012 : NBCC Begins to Award Seed Grants through the generous support of the Breast Cancer Fund of National Philanthropic Trust – Read more about the Artemis Project seed grants here .
- 2012: NBCC Awards Seed Grant to Identify Possible Vaccine Targets for Preventive Vaccine – to Dr. Paul Spellman and Dr. Joe Gray of Oregon Health and Science University, to identify possible vaccine targets using existing and developing human genomic data within different breast cancer subtypes. The analysis generated a prioritized list of potential breast cancer-specific targets to be considered for incorporation into a preventive vaccine.
- 2013 : NBCC Awards Seed Grant to Investigate Candidate Viral Causes of Breast Cancer – to Dr. Paul Ewald, Professor of Biology and Director, Program on Disease Evolution at the University of Louisville, and Dr. Vladimir Belyi, Assistant Professor at the Cancer Institute of New Jersey, Robert Wood Johnson Medical School. The researchers took a systematic look through two sets of breast cancer genomes for evidence of infectious agents.
- 2013: NBCC Awards Seed Grant to Identify Possible Vaccine Targets in DCIS Samples – to Dr. Gregory Hannon, Professor and HHMI Investigator at Cold Spring Harbor Laboratory, and Dr. H. Kim Lyerly, George Barth Geller Professor of Cancer Research, Duke University School of Medicine. To look for vaccine targets in DCIS samples and to evaluate the biology of human ductal carcinoma in situ (DCIS) through sequencing (RNAseq).
- 2016 : Pre-clinical Work begins for Artemis Project Preventive Vaccine – Keith Knutson, Professor of Immunology, College of Medicine, Mayo Clinic, began pre-clinical work on the Artemis Project preventive vaccine. These efforts have resulted in a preventive vaccine development plan presented to the Food and Drug Administration in 2018, with plans for a Phase I safety trial in 2022.
- 2017 : NBCC Awards Seed Grant to Investigate Adaptive Immune Recognition of Dormant Disseminated Tumor Cells – to Dr. Cyrus Ghajar, Director, Laboratory for the Study of Metastatic Microenvironments, Fred Hutchinson Cancer Research Center, and Dr. H. Kim Lyerly, George Barth Geller Professor of Cancer Research, Duke University School of Medicine, to determine whether the adaptive immune system can recognize and kill dormant disseminated tumor cells (DTCs), and if not, which aspect of DTC biology should be targeted to enhance T cell recognition.
- 2017: Launched DNA.Land to Develop Large-Scale Genetic Database for Breast Cancer Research – NBCC partnered with the New York Genome Center to develop a large-scale resource to study breast cancer. The DNA.Land project, supported by an Artemis Project seed grant, asked women and men who participated in genealogy tests to answer questions about breast cancer, including family history. This genomic data, along with answers from the breast cancer questionnaire, developed by NBCC-trained advocates and researchers, will be used to develop a large-scale database that researchers can use to identify genetic variants that impact the risk and recurrence of the disease.
- 2020 : Artemis Project Preventive Vaccine Awarded Contract with NCI PREVENT Program – NBCC’s Artemis Project Preventive Vaccine was awarded a contract with the National Cancer Institute (NCI) as part of its competitive PREVENT Cancer Preclinical Drug Development Program. The PREVENT program is a peer-reviewed agent development program designed to support the pre-clinical development of innovative interventions and biomarkers for cancer prevention and interception towards clinical trials.
- 2020: Independent Third-Party Assessment Published on NBCC’s Work Over the Deadline 2020 Campaign – While we knew the Deadline 2020 Campaign was powerful on many levels, we thought it would be beneficial to have a Third-Party Assessment of our work. The assessment confirmed the distinctive role NBCC plays in the breast cancer community: NBCC’s systematic understanding of research and development – including the connections among policy, scientific research, patient outcomes, and institutional structures – makes NBCC and its impact unique within the field of breast cancer research and advocacy.
Artemis Project Meeting Reports
Learn more about Artemis collaborators’ bold ideas and work plans on novel approaches for preventing breast cancer and preventing metastasis.
- Report from the 2023 Artemis Project® meeting
- Report from the 2022 Artemis Project® meeting
- Report from the 2020 Artemis Project® meeting
- Report from the 2019 Artemis Project® meeting (March 8-11, 2019) Vaccine Landscape (Part 1) Vaccine Landscape (Part 2)
- Report from the 2018 Artemis Project ® meeting (March 9-12, 2018)
- Report from the 2017 Artemis Project® meeting (March 10-13, 2017)
- Report from the 2016 Artemis Project ® meeting (March 11-14, 2016)
- Artemis General Overview & Update (October 2015)
- Report from the second Artemis Project ® Annual Prevention of Metastasis meeting (March 6-8, 2015)
- Report from the fifth Artemis Project ® for a Preventive Vaccine meeting (March 6-8, 2015)
- Report from the first Artemis Project ® Annual Prevention of Metastasis meeting (March 10-11, 2014)
- Report from the fourth Artemis Project ® for a Preventive Vaccine meeting (March 7-10, 2014)
- National Breast Cancer Coalition Awards Grant to Look for Vaccine Targets in DCIS Samples (November 6, 2013)
- Report from the first meeting on Tumor Dormancy (June 10-11, 2013)
- Report from the third Artemis Project ® for a Preventive Vaccine meeting (March 8-11, 2013)
- National Breast Cancer Coalition Awards Additional Seed Grant for Preventive Breast Cancer Vaccine (February 6, 2013)
- National Breast Cancer Coalition Awards Seed Grant for Preventative Breast Cancer Vaccine (October 9, 2012)
- Report from the second Artemis Project ® for a Preventive Vaccine meeting (March 3-5, 2012)
- Report from the first Artemis Project ® for a Preventive Vaccine meeting (April 9-11, 2011)
- The Artemis Project ® Plan to Develop a Breast Cancer Preventive Vaccine: Identification of Targets & Immune System Variations (December 2011, Prepared by SAIC for NBCC)

Leading Change in Cancer Clinical Research, Because Our Patients Can’t Wait
May 31, 2024 , by W. Kimryn Rathmell, M.D., Ph.D., and Shaalan Beg, M.D.

Greater use of technologies that can increase participation in cancer clinical trials is just one of the innovations that can help overcome some of the bottlenecks holding up progress in clinical research.
Thanks to advances in technology, data science, and infrastructure, the pace of discovery and innovation in cancer research has accelerated, producing an impressive range of potential new treatments and other interventions that are being tested in clinical studies . The extent of the innovative ideas that might help people live longer, improve our ability to detect cancer early, or otherwise transform care is staggering.
Our understanding of tumor biology is also evolving, and those gains in knowledge are being translated into the continued discovery of targets for potential interventions and the development of novel types of treatments. Some of these therapies are producing unprecedented clinical responses in studies, including in traditionally difficult-to-treat cancers.
These advances have contributed to a record number of Food and Drug Administration (FDA) approvals in recent years with, arguably, the most notable approvals being those for drugs that can be used for any cancer, regardless of where it is in the body .
In some instances, the activity of new agents has been so profound that clinical investigators are having to rethink their criteria for implementation in patient care and their definitions of treatment response.
For example, although HER2 has been a known therapeutic target in breast cancer for many decades, the new antibody-drug conjugates (ADCs) that target HER2 have proven to be vastly more effective than the original HER2-targeted therapies. This has forced researchers to rethink fundamental questions about how these ADCs are used in patient care: Can they be effective in people whose tumors have lower expression of HER2 than we previously thought was needed ? And, if so, do we need to redefine how we classify HER2-positive cancer?
As more innovative therapies like ADCs hit the clinic at a far more rapid cadence than ever before, the research community is being inundated with such fundamentally important questions.
However, the remarkable progress we're experiencing with novel new therapies is tempered by a critical bottleneck: the clinical research infrastructure can’t be expected to keep pace in this new landscape.
Currently, many studies struggle to enroll enough participants. At the same time, there are patients who don’t have ready access to studies from which they might benefit. Furthermore, ideas researchers have today for studies of innovative new interventions might not come to fruition for 2 or 3 years, or even longer—years that people with cancer don’t have.
The key to overcoming this bottleneck is to invite innovation to help reshape our clinical trials infrastructure. And here’s how we plan to accomplish that.
Testing Innovation in Cancer Clinical Trials
A transformation in cancer clinical research is already underway. That transformation has been led in part by the success of novel precision oncology approaches, such as those tested in the NCI-MATCH trial .
This innovative study ushered in novel ways of recruiting participants and involving oncologists at centers big and small. And NCI-MATCH has spawned several successor studies that are incorporating and building on its innovations and achievements.
An innovation that emerged from the COVID pandemic was the increase of remote work, even in the clinical trials domain. Indeed, staffing shortages have caused participation in NCI-funded trials to decline. In response, NCI is piloting a Virtual Clinical Trials Office to offer remote support staff to participating study sites. This support staff includes research nurses, clinical research associates, and data specialists, all of whom will help NCI-Designated Cancer Centers and community practices engaged in clinical research activities.
Such technology-enabled services can allow us to reimagine how clinical trials are designed and run. This includes developing technologies and processes for remotely identifying clinical trial participants, shipping medications to participants at home, having imaging performed in the health care settings where our patients live, and empowering local physicians to participate in clinical trials.
We also need mechanisms to test and implement innovations in designing and conducting clinical studies.
The Pragmatica-Lung Cancer Treatment Trial , an innovative phase 3 study launched by NCI’s National Clinical Trials Network (NCTN) , was designed to be easy to launch, enroll participants, and interpret its results.
NCI recently established Clinical Trials Innovation Unit (CTIU) to pressure test a variety of innovations. The CTIU, which includes leadership from FDA and NCTN, is already working on future innovations, including those that will streamline data collection and apply novel approaches to clinical studies, all with the goal of making them less burdensome to run and easier for patients to participate.
Data-Driven Solutions
The era of data-driven health care is here, providing still more opportunities to transform cancer clinical research.
The emergence of artificial intelligence (AI) solutions, large language models, and informatics brings real potential for wholesale changes in how we match patients to clinical studies, assess side effects, and monitor events like disease progression.
Recognizing this potential, NCI is offering funding opportunities and other resources that will fuel the development of AI tools for clinical research, allow us to carefully test their usefulness, and ultimately deploy them across the oncology community.
Creating Partnerships and Expanding Health Equity
To be sure, none of this will be, or can be, done by NCI alone. All these innovations require partnerships. We will increase our engagement with partners in the public- and private-sectors, including other government agencies and nonprofits.
That includes high-level engagement with the Office of the National Coordinator for Health Information Technology (ONC), with input from FDA, Centers for Medicare & Medicaid Services, and Centers for Disease Control and Prevention.

Dr. W. Kimryn Rathmell, M.D., Ph.D.
NCI Director
One example of such a partnership is the USCDI+ Cancer program . Conducted under the auspices of the ONC, this program will further the aims of the White House's reignited Cancer Moonshot SM by encouraging the adoption and utilization of interoperable cancer health IT standards, providing resources to support cancer-specific use cases, and promoting alignment between federal partners.
And just as importantly, the new partnerships we create must include those with patients, advocates, and communities in ways we have never considered before.
A central feature of this community engagement must involve intentional efforts to expand health equity, to create study designs that are inclusive and culturally appropriate. Far too many marginalized communities and populations today are further harmed by studies that fail to provide findings that apply to their unique situations and needs.
Very importantly, the future will require educating our next generation of clinical investigators and empowering them with the tools that enable new ways of managing clinical studies. By supporting initiatives spearheaded by FDA and professional groups like the American Society of Clinical Oncology, NCI is making it easier for community oncologists to participate in clinical trials and helping clarify previously misunderstood regulatory requirements.
These efforts must also ensure that we have a clinical research workforce that is representative of the people it is intended to serve. Far too many structural barriers have prevented this from taking place in the past, and it’s time for that to change.
Expanding our capacity doesn’t mean doing more of the same, it means challenging ourselves to work differently. This will let us move forward to a new state, one in which clinical research is integrated in everyday practice. It is only with more strategic partnerships and increased inclusivity that we can open the doors to seeing clinical investigation in new ways, with new standards for success.
A Collaborative Effort

Shaalan Beg, M.D.
Senior Advisor for Clinical Research
To make the kind of progress we all desire, we have to recognize that our clinical studies system needs to evolve.
There was a time when taking years to design, launch, and complete a clinical trial was acceptable. It isn’t acceptable anymore. We are in an era where we have the tools and the research talent to make far more rapid progress than we have in the past.
And we can do that by engaging with many different communities and stakeholders in unique and dynamic ways—making them partners in our effort to end cancer as we know it.
Together, our task is to capitalize on this work so we can move faster and enable cutting-edge research that benefits as many people as possible.
We also know that there are more good ideas in this space, and part of this transformation includes grass roots efforts to drive systemic change. So, we encourage you to share your ideas on how we can transform clinical research. Because achieving this goal can’t be done by any one group alone. We are all in this together.
Featured Posts
March 27, 2024, by Edward Winstead
March 21, 2024, by Elia Ben-Ari
March 5, 2024, by Carmen Phillips
- Biology of Cancer
- Cancer Risk
- Childhood Cancer
- Clinical Trial Results
- Disparities
- FDA Approvals
- Global Health
- Leadership & Expert Views
- Screening & Early Detection
- Survivorship & Supportive Care
- February (6)
- January (6)
- December (7)
- November (6)
- October (7)
- September (7)
- February (7)
- November (7)
- October (5)
- September (6)
- November (4)
- September (9)
- February (5)
- October (8)
- January (7)
- December (6)
- September (8)
- February (9)
- December (9)
- November (9)
- October (9)
- September (11)
- February (11)
- January (10)

The Breast Cancer Project

One out of eight women will get breast cancer in their lifetime, and every hour nearly five women die from this disease.
Given the critical and all too common occurrence of this form of cancer, the NCC created the Breast Cancer Project to earmark funds specifically for basic research into the causes and potential treatments and cures for this disease.
Our grant to Duke University Medical Center in Durham, NC, funds research into breast cancer genetics and is geared to making existing approaches more effective in the prevention, detection and treatment of breast cancer. This project addresses issues at the cutting edge of cancer immunotherapy.
Current Grants and Renewals
National Cancer Center has been proud to award grants to many fine research organizations. We know that a cure lies in research and we are committed to supporting as many research projects as we can.
Xiaowei Wu, Ph.D.
Dana-Farber Cancer Institute, Boston MA
PROJECT: Targeting residual cancer cells upon CDK4/6 inhibition
Yi Shi, Ph.D.
Johns Hopkins School of Medicine, Baltimore MD
PROJECT: Intratumoral hypoxia promotes metastatic liver-organotropism in breast cancer
Youngbin Cho, Ph.D.
University of Pittsburgh, Pittsburgh PA
PROJECT: Myosin II as a target for suppressing tumor-associated macrophage recruitment in triple-negative breast cancer
Past Beneficiaries
Chiwei xu, ph.d..
The Rockefeller Univ, New York, NY
PROJECT: Crosstalk between epithelial stem cells and peripheral nerves in cancer
Ibtehaj Naqvi, M.D., Ph.D.
Duke University, Durham, NC
PROJECT: Mitigating inflammation using nucleic acid scavengers to prevent breast cancer metastasis
David Frankhouser, Ph.D.
Beckman Inst, City of Hope, CA
PROJECT: Detecting Neovascularity in MRI to identify and predict breast cancer
Siang-Boon Koh, Ph.D.
Massachusetts General Hospital Cancer Center
PROJECT: Synergistic targeting of DNA damage response and EMT pathways to reverse RASAL2-driven chemoresistance
Polina Vaitsenfeld, Ph.D.
The Rockefeller University, NY, NY
PROJECT: Enhancing antibody-mediated immunity against tumor-associated carbohydrate antigens 2019-2020
Michael C. Brown Ph.D.
Duke University
PROJECT: Cancer Immunotherapy through intratumoral activation of recall responses 2018-2017
Andrej Gorbatenko
Icahn School of Medicine at Mt Sinai, NY
PROJECT: Novel targeted therapy of inflammatory breast cancer
Laura Saucedo-Cuevas, Ph.D.
PROJECT : Novel Targeted Therapy of Inflammatory Breast Cancer 2017
Yuntao Mao, Ph.D.
Harvard University
PROJECT : Role of Long Non-coding RNAs in Breast Cancer 2011-2012: 2 year fellowship
2023 New and Renewed Research Grants
2023 grants, total grand funding to date.
Stanford study suggests new ways of predicting, treating breast cancer through genes

A mammography patient receives a breast screening with the Hologic Selenia Dimension digital mammography machine at NuHealth Nassau University Medical Center. A new study says how the genes we are born with interact with our immune systems can set the stage for development of breast cancer. Credit: Chris Ware
A new study has found that the genes people are born with — and how they interact with the immune system — may have a more significant role in predicting who will develop breast cancer than previously believed.
The research out of Stanford University points to a novel way to identify the risk of the disease, along with its potential progression and treatment options, the study author says.
There are two widely known genes that have significant effects on a person's increased risk of breast cancer — BRCA 1 and BRCA 2. But this new study, to be published Friday in the journal Science, finds there are potentially many other gene variants that are weaker, but can still impact how the disease develops, said the study's senior author, Christina Curtis, the RZ Cao professor of medicine, and professor of genetics and biomedical science at Stanford Medicine.
A news release about the study, which includes the work of lead author of the research, Kathleen Houlahan, said it “challenges the dogma that most cancers arise as a result of random mutations.”
WHAT TO KNOW
- A new Stanford Medicine study found that inherited genes, and how they interact with the immune system , may play a larger role in the development of breast cancers than previously thought.
- While there are two widely known genes – BRCA 1 and BRCA 2 – the Stanford study found many more gene variants that can still impact the development of the disease.
- The study suggests there may be a new way to identify the risk of breast cancer and treatment options.
Instead, it said, the research showed “the active involvement of gene sequences we inherit from our parents — what's known as your germline genome — in determining whether cells bearing potential cancer-causing mutations are recognized and eliminated by the immune system or skitter under the radar to become nascent cancers.”
Get the latest stories every week about health and wellness, covering topics from medicine and mental health to updates on the coronavirus and new research.
By clicking Sign up, you agree to our privacy policy .
Curtis, who also directs Artificial Intelligence and Cancer Genomics at Stanford, said in an interview this week with Newsday: "There’s a key interplay with the immune system in whether a patient develops the cancer, and that’s a new way of thinking about this....If the immune system sees [early tumor cells] and can eradicate them, the cancer cells are gone. The individual may not develop the cancer in that instant.”
The study identified “a whole new host of genes that can help us stratify risk. We want to use that information to look across individuals to say 'Are you more at risk than somebody else? Is your risk higher?' " Curtis said. "A goal of this work now is to say can we separate people who are at higher risk of developing an invasive breast cancer. This information can help personalize treatment.”
Jeff Boyd, VP and chief scientific officer of Northwell Health’s Cancer Institute and director of its Center for Genomic Medicine, said his review of the news release summary presented “a novel concept.”
“This paper represents the intersection of genetics with immunology,” Boyd said, “which is the primary thing that makes this a tour de force paper that’s setting a precedent. It’s unique … They've come up with a novel paradigm … I think the take home message is this represents the intersection of the human immune system with genetic susceptibility with breast cancer.
Boyd said there are clinical implications. “Inherited germ line variants can be measured from blood, this represents a low cost biomarker, [that is] minimally invasive.” He said a blood sample can be used, as opposed to a biopsy, “to determine which type of breast cancer you’re susceptible for. There are multiple types of breast cancer with different prognoses. So this is very important.”
Dr. Brian O’Hea, Chief of Breast Surgery at Stony Brook Medicine and Director of the Carol M. Baldwin Breast Care Center, said, based on reports on the study which was not yet available, “I’m hopeful someday we can use this information to repair and replace defective genes and prevent breast cancer. That’s what it means to me.”
Breast cancer is the second-most common cancer among women in the United States, according to the Centers for Disease Control and Prevention, topped only by some types of skin cancer.
More than 43,100 women died of breast cancer in 2023, according to estimates quoted by the U.S. Preventive Services Task Force. Female breast cancer rates vary around the region, with Long Island's higher than New York City and the state.
The Nassau County rate of breast cancer is 145.9 per 100,000 and Suffolk County is 139.9, according to the New York State Cancer Registry. New York City's rate is 126.1 and the state's rate is 134 per 100,000.

MTA congestion pricing delayed ... NYPD shooter held without bail ... 12-year-old high school grad
Get more on these and other NewsdayTV stories
Most Popular
Latest videos.
Unlimited Digital Access Only 25¢ for 5 months
Together we are beating cancer
- Cancer types
- Breast cancer
- Bowel cancer
- Lung cancer
- Prostate cancer
- Cancers in general
- Clinical trials
- Causes of cancer
- Coping with cancer
- Managing symptoms and side effects
- Mental health and cancer
- Money and travel
- Death and dying
- Cancer Chat forum
- Health Professionals
- Cancer Statistics
- Cancer Screening
- Learning and Support
- NICE suspected cancer referral guidelines
- Make a donation
- By cancer type
- Leave a legacy gift
- Donate in Memory
- Find an event
- Race for Life
- Charity runs
- Charity walks
- Search events
- Relay for Life
- Volunteer in our shops
- Help at an event
- Help us raise money
- Campaign for us
- Do your own fundraising
- Fundraising ideas
- Get a fundraising pack
- Return fundraising money
- Fundraise by cancer type
- Set up a Cancer Research UK Giving Page
- Find a shop or superstore
- Become a partner
- Cancer Research UK for Children & Young People
- Our Play Your Part campaign
- Brain tumours
- Skin cancer
- All cancer types
- By cancer topic
- New treatments
- Cancer biology
- Cancer drugs
- All cancer subjects
- All locations
- By Researcher
- Professor Duncan Baird
- Professor Fran Balkwill
- Professor Andrew Biankin
- See all researchers
- Our achievements timeline
- Our research strategy
- Involving animals in research
- Research opportunities
- For discovery researchers
- For clinical researchers
- For population researchers
- In drug discovery & development
- In early detection & diagnosis
- For students & postdocs
- Our funding schemes
- Career Development Fellowship
- Discovery Programme Awards
- Clinical Trial Award
- Biology to Prevention Award
- View all schemes and deadlines
- Applying for funding
- Start your application online
- How to make a successful applicant
- Funding committees
- Successful applicant case studies
- How we deliver research
- Our research infrastructure
- Events and conferences
- Our research partnerships
- Facts & figures about our funding
- Develop your research career
- Recently funded awards
- Manage your research grant
- Notify us of new publications
- Find a shop
- Volunteer in a shop
- Donate goods to a shop
- Our superstores
- Shop online
- Wedding favours
- Cancer Care
- Flower Shop
- Our eBay store
- Shoes and boots
- Bags and purses
- We beat cancer
- We fundraise
- We develop policy
- Our global role
- Our organisation
- Our strategy
- Our Trustees
- CEO and Executive Board
- How we spend your money
- Early careers
- Your development
Cancer News
- For Researchers
- For Supporters
- Press office
- Publications
- Update your contact preferences
- About cancer
- Get involved
- Our research
- Funding for researchers
The latest news, analysis and opinion from Cancer Research UK
- Science & Technology
- Health & Medicine
- Personal Stories
- Policy & Insight
- Charity News
- Science & Technology
- Health & Medicine
ASCO 2024: data on weight loss drugs and a melanoma vaccine, a test that can predict breast cancer relapse, and more

2 June 2024
We had research experts on the ground in Chicago at the 2024 American Society of Clinical Oncology (ASCO) annual meeting, the world’s biggest cancer conference. They’ve helped put together this guide to some of the most important announcements.
The studies discussed here aren’t over yet, so we don’t have full peer-reviewed papers explaining all the data and methods behind the findings. It’s best to think of them as waypoints in a continuing journey. They tell us where cancer research could be heading over the coming years, not where cancer prevention or cancer treatment is right now.
Signs that weight loss drugs could help prevent cancers linked to obesity
Headline-making weight loss drugs like Ozempic and Wegovy could also lower people’s risk of developing cancers linked to obesity, according to US researchers.
“The link between obesity and multiple cancers is proven,” said Professor Charles Swanton, our chief clinician, who has been representing us at ASCO. “While these are effective drugs to manage weight loss, it’s still early days in our understanding of whether they can reduce people’s risk of cancer.”
Ozempic and Wegovy are the best-known examples of drugs called GLP-1 receptor agonists (GLP-1 RAs), which mimic the action of GLP-1, a hormone made by the small intestine. These drugs were originally used to treat type 2 diabetes, but they can also support weight loss by making people feel more full.
Researchers at Case Western Reserve University in Ohio found patients taking the drugs were 19% less likely to develop any of the 13 cancers we know are linked to being overweight. These include ovarian, liver, colorectal, pancreatic, bowel and breast cancer.
“This large, retrospective study suggests a link between weight loss drugs and a reduced risk of obesity related cancer after just one year, but more research is needed to prove this association,” explained Swanton.
Two other studies presented at ASCO suggest that GLP-1 RAs could reduce the risk of breast cancer returning after treatment. Those are exciting signs, but we still don’t know exactly what they mean.
“Well-designed prospective trials with randomised data will provide more clarity on the potential and safety of weight loss drugs to lower people’s risk of cancer,” Swanton said.
A test to predict if breast cancer will return
ASCO also brought news of another tool we might be able to use to tackle breast cancer relapse. A DNA blood test could tell doctors whether the disease will return after surgery, according to results from a proof-of-principle study.
The test works by picking out tiny amounts of cancer-specific DNA (called circulating tumour DNA, or ctDNA) that can escape from a tumour and persist in the blood even after initial treatment.
Although ctDNA can’t be picked up by the scans and tissue samples (biopsies) doctors currently use to look for cancer, it raises the risk of disease returning.
A team led from the Institute of Cancer Research in London have found that the NeXT Personal liquid biopsy test can show whether ctDNA remains after surgery and use that information to predict when breast cancer will relapse.
In a 76-patient study, the new test used ctDNA to correctly identify all 10 women whose breast cancers went to on to relapse – long before these returning cancers became visible on scans. The data released so far also suggests there were no false-negatives: no one the test said was ctDNA-free has seen their cancer return.
Researchers think the test could be used to show which women need preventive therapy after surgery and which can be spared further treatment. The next step is to study how well that works in practice across bigger patient groups.
“Early detection is key to improving cancer survival, so it’s positive that this study lays the foundation for a blood test that predicts the return of cancer in people who have had early breast cancer surgery,” said Dr Catherine Elliot, our director of research.
“Researchers measured circulating tumour DNA (ctDNA) to help find cancer cells that remain after surgery. The results show a strong correlation between ctDNA detection and relapse, with cancer returning around one year after detection.
“Results like this demonstrate how liquid biopsy technology can be used to inform kinder, more targeted treatment for patients based on their risk of recurrence.”
Cancer Research UK at ASCO
We’re making our own headlines at ASCO: we directly funded this study into a saliva test that can help diagnose prostate cancer sooner .
Melanoma cancer vaccine results “extremely impressive”
The first vaccine treatment for the form of skin cancer usually caused by sun damage could halve the risk of patients dying or the disease returning, according to early trial results.
Researchers led by a team at New York University combined a vaccine using the same mRNA technology as many COVID jabs with an established immunotherapy called pembrolizumab. It was given to patients after they had surgery to remove stage 3 or stage 4 melanoma.
After three years, 75% of those who had the vaccine and pembrolizumab were still cancer-free, compared to 56% of people who only received pembrolizumab.
Iain Foulkes, our executive director of research and innovation explained that the results “show positive signs of the mRNA vaccine’s long-lasting effectiveness”, although larger and longer trials are still needed.
Unlike traditional vaccines, which are used to prevent disease, cancer vaccines are used to treat cancers. The vaccine used in this trial, mRNA-4157 (created by Moderna), is different for every patient. Scientists personalise it to help people’s immune system identify their specific cancer cells. Pembrolizumab, a type of immunotherapy called a checkpoint inhibitor , supports this by effectively taking the brakes off of immune cells called T cells.
Speaking to journalists at ASCO, Swanton called the results “extremely impressive”.
“The new vaccine approach is another piece of the puzzle that will allow more patients to be cured, hopefully, or fewer patients to suffer disease relapse. Ultimately it will contribute to survival rates improving continually over the next decades and more.”
Melanoma rates are at an all time high in the UK , having increased by a third over the past decade.
Earlier in ASCO, NHS England announced it had treated its first patient with a personalised vaccine against their bowel cancer in a Cancer Vaccine Launch Pad trial .
Immunotherapy before surgery clears 10 times more bowel cancers with a specific mutation than chemo
On its own, pembrolizumab is much more effective than chemotherapy for people whose bowel cancers have a particular genetic profile, according to interim results from the phase 2 NEOPRISM-CRC trial.
Pembrolizumab, also known by the brand name Keytruda, is already used to treat non-small cell lung cancer, Hodgkin lymphoma and bladder cancer, as well as melanoma. A research team led from University College London (UCL) tested it before surgery in stage two and three bowel cancer patients with a mismatch repair (MMR) deficiency that limits cancer cells’ ability to fix DNA damage.
The results presented at ASCO indicate that more than half of the 32 people on the trial treated with pembrolizumab prior to surgery had no signs of cancer after their operations. Other studies have shown that the same is true for just 4% of people treated with chemotherapy.
The approach also meant that patients didn’t need follow-up chemotherapy after their operations, protecting them from potentially difficult side effects.
“This small single arm study adds to established evidence that checkpoint inhibitor drugs help to treat bowel cancer before surgery by activating the immune system’s anti-cancer functions in patients with a specific DNA repair abnormality,” explained Swanton.
In total, around 10-15% of patients with stage two or three bowel cancer have MMR deficient cancer cells. That suggests the treatment could one day be an option for around 2,000-3,000 people in the UK every year.
As the trial continues, researchers based at the Cancer Research UK and UCL Cancer Trials Centre will assess whether patients treated with pembrolizumab remain cancer free over a longer period of time.
“More work needs to be done to assess pembrolizumab before it could be considered standard treatment, but given the quality of the outcomes in this trial I think it’s possible that we could see it in the clinic within a couple of years if subsequent trials are similarly successful,” said Dr Marnix Jansen, a clinician and scientist working from the UCL Cancer Institute and University College London Hospital.
Lorlatinib stops some lung cancers for longer than any other drug
Remarkable results from the CROWN trial suggest a targeted drug called lorlatinib could stop a form of advanced non-small cell lung cancer “in its tracks”.
CROWN’s researchers are comparing lorlatinib to a similar drug called crizotinib as treatments for the 3% to 5% of non-small cell lung cancers with ALK (anaplastic lymphoma kinase) mutations. So far, the trial has shown that lorlatinib can stop these advanced cancers from growing or spreading for longer than any lung cancer drug in history.
“Showcasing the power of cancer growth blocker drugs , this study could present us with an effective way of stopping cancer in its tracks and preventing it from spreading to the brain,” explained Swanton, who leads our flagship lung cancer study, TRACERx.
“The groundbreaking results show that over half of the patients who took lorlatinib did not suffer a progression in their disease after five years. In contrast, over half of the patients who took crizotinib experienced disease progression after just nine months.”
Tell us what you think
Leave a reply cancel reply.
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Read our comment policy .
Highlighted content
Thousands of nhs patients to access trials of personalised cancer 'vaccines', sarah harding's legacy: finding women who may have higher breast cancer risks, at-home saliva test could help diagnose prostate cancer sooner, general election 2024: what does this mean for the tobacco and vapes bill, skin cancer cases reach all-time high, an animal's guide to staying safe in the sun, hpv vaccine slashes cervical cancer rates across society, one to one with penny: volunteering special 1 - that cancer conversation podcast, the ‘mystery’ culprit causing kidney cancer worldwide, related topics.
- Cancer in the news

IMAGES
VIDEO
COMMENTS
Posted: January 20, 2023. Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
The Confluence Project, from NCI's Division of Cancer Epidemiology and Genetics (DCEG), is developing a research resource that includes data from thousands of breast cancer patients and controls of different races and ethnicities. This resource will be used to identify genes that are associated with breast cancer risk, prognosis, subtypes ...
The Breast Cancer Research Foundation is dedicated to ending breast cancer by advancing the world's most promising research. This year, BCRF is the largest private funder of breast cancer research—and metastatic breast cancer research—worldwide and is the highest-rated breast cancer research organization in the country. Learn More Donate.
Breast Cancer Research is an international, peer-reviewed online journal, publishing original research, reviews, editorials and reports. Open access research articles of exceptional interest are published in all areas of biology and medicine relevant to breast cancer, including normal mammary gland biology, with special emphasis on the genetic, biochemical, and cellular basis of breast cancer.
BCRF announces research projects for 2023-2024. New York, NY - Sept 27, 2023 - The Breast Cancer Research Foundation (BCRF) announced its $60.2 million commitment to fund breast cancer research in 2023-2024, supporting more than 250 scientists at leading academic and medical institutions across 14 countries. BCRF-funded research spans the entire spectrum of the disease—from studying the ...
Vision - A world without breast cancer. The BCRP challenges the scientific community to design research that will address the urgency of ending breast cancer. Specifically, the BCRP seeks to accelerate high-impact research with clinical relevance, encourage innovation and stimulate creativity, and facilitate productive collaborations.
The discovery of the monoclonal antibody trastuzumab almost 25 years ago revolutionized treatment and drug development for HER2+ breast cancer. Here, Swain et al. review the current standard of ...
Breast Cancer Research Highlights. The American Cancer Society (ACS) helps people with breast cancer in every community. Our research programs have played a role in many of the prevention, screening, and treatment advances that save lives from breast cancer today. And, we continue to fund research to help save even more lives in the future.
Over 2 million new cases of breast cancer (BC) are diagnosed globally each year (Sung et al., 2021 Globocan), and their treatment is based on the expression of cell receptors such as human epidermal growth factor receptor-2 (HER-2), estrogen receptor (ER), and several other clinical and molecular features (Yersal & Barutca, 2014).The nature of BC is itself complex and so are the broad ...
Breast cancer is commonly acknowledged as an international priority in healthcare. To date, it is the most common cancer in women worldwide and demographic trends show a steady increase in incidence. Over the years, increasing efforts and resources have been devoted to a meticulous analysis of risk factors, diagnostic tools and treatment ...
The Breast Cancer Research Foundation is a nonprofit organization committed to achieving prevention and a cure for breast cancer. We provide critical funding for cancer research worldwide to fuel advances in tumor biology, genetics, prevention, treatment, metastasis and survivorship. ... It is the largest privately funded project exclusively ...
Our Research Now. NBCF supports research projects to study and improve existing programs in order to help improve quality of life for metastatic breast cancer patients and their caregivers as well as increase access to knowledge, resources, and training for patient navigators. NBCF funds the following research projects:
This new result unearths a new class of biomarkers to forecast tumor progression and an entirely new way of understanding breast cancer origins." Curtis is the senior author of the study, which will be published May 31 in Science. Postdoctoral scholar Kathleen Houlahan, PhD, is the lead author of the research.
Possible environmental causes of breast cancer have also received more attention in recent years. While much of the science on this topic is still in its earliest stages, this is an area of active research. Breast cancer prevention. Researchers are looking for ways to help reduce breast cancer risk, especially for women who are at high risk.
Black women tend to develop breast cancer at a younger age than White women. Black women are also more likely than Whites to die from the disease, and they are twice as likely to develop an aggressive subtype called triple-negative breast cancer. But despite the increased risks faced by women of African descent, most large-scale genetic studies ...
PDF | Breast cancer is the most frequent malignancy in women worldwide and is curable in ~70-80% of patients with early-stage, non-metastatic disease.... | Find, read and cite all the research you ...
The Breast Cancer Now Catalyst Programme promotes innovation and excellence in breast cancer research, through collaboration in the UK and across Europe. Right now, we're funding over 80 cutting-edge projects worth just over £29 million to discover how we can prevent breast cancer, save lives, and help people to live well with and beyond the ...
Deciding which research topics to focus on in medicine and health depends on many factors. These factors can include the currency of a topic, feedback from people providing or receiving care, and the priorities of funders. In late 2019, the Cochrane Breast Cancer Group (part of Cochrane's Cancer Network) conducted a formal priority-setting ...
HER2 is a common treatment target for breast cancer. This new drug targets HER3, a biomarker related to HER2, which is associated with poor breast cancer outcomes. About 10% to 20% of newly diagnosed breast cancers are HER2-positive. At the 2023 American Society for Clinical Oncology (ASCO) Annual Meeting, researchers announced positive results ...
Proposed research projects are restricted to basic, clinical, translational or epidemiological projects that substantially advance the field of endocrine therapy in breast cancer. These fellowships provide two-year grants of $130,000 to support the salary and benefits of the fellow while working on mentored breast cancer research.
The Confluence project will develop a large research resource to uncover breast cancer genetics through genome-wide association studies (GWAS). The resource will include at least 300,000 breast cancer cases and 300,000 controls of different races/ethnicities. This will be accomplished by the confluence of existing GWAS and new genome-wide ...
Originally created as the research component of NBCC's Breast Cancer Deadline 2020® initiative, the Artemis Project® is an advocate-led collaboration of researchers and advocates that develops and implements research action plans to address overarching issues in breast cancer. The Artemis Project employs an innovative, mission-driven ...
The Metastatic Breast Cancer Project is a groundbreaking initiative that empowers patients to share their genomic data and medical records with researchers, to accelerate the discovery of new treatments for metastatic breast cancer. Learn how you can join and contribute to this patient-driven research.
Get in touch. If you're a member of the Generations Study and want to contact the study team, please phone 020 8722 4469 or email [email protected]. The Generations Study is a major study into the causes of breast cancer following more than 113,000 women in the UK for 40 years. Find out more.
In 2020, breast cancer made up 11.7% of all new cancer cases globally, followed by lung cancer (11.4%), colorectal cancer (10.0%), prostate cancer (7.3%), and stomach cancer (5.6%). The highest incidence rates of breast cancer (new cases per population) occurred in women in high-income countries, such as those in North America (~90 new cases ...
Diet and breast cancer - A recent study connected eating the Mediterranean Diet to lower risk. And previous research has pointed to carotenoids - the family of orange- and red-colored phytonutrients in such foods as carrots and tomatoes - linking to lower risk of breast cancers. Carotenoid-rich foods and the Mediterranean Diet are packed ...
Thanks to advances in technology, data science, and infrastructure, the pace of discovery and innovation in cancer research has accelerated, producing an impressive range of potential new treatments and other interventions that are being tested in clinical studies.The extent of the innovative ideas that might help people live longer, improve our ability to detect cancer early, or otherwise ...
The Breast Cancer Project. One out of eight women will get breast cancer in their lifetime, and every hour nearly five women die from this disease. Given the critical and all too common occurrence of this form of cancer, the NCC created the Breast Cancer Project to earmark funds specifically for basic research into the causes and potential ...
The Nassau County rate of breast cancer is 145.9 per 100,000 and Suffolk County is 139.9, according to the New York State Cancer Registry. New York City's rate is 126.1 and the state's rate is 134 ...
An 'ultra-sensitive' blood test can tell doctors whether breast cancer will return after surgery, according to trial results presented at ASCO on the 1st of June. ... As the trial continues, researchers based at the Cancer Research UK and UCL Cancer Trials Centre will assess whether patients remain cancer free over a longer period of time.