

What’s the latest on GMOs and gene-edited foods – and what are the concerns? An expert explains
Research Fellow, Centre for Crop Science, The University of Queensland
Disclosure statement
Karen Massel does not work for, consult, own shares in or receive funding from any company or organisation that would benefit from this article, and has disclosed no relevant affiliations beyond their academic appointment.
University of Queensland provides funding as a member of The Conversation AU.
View all partners
Advances in genetic engineering have given rise to an era of foods – including genetically modified organisms (GMOs) and gene-edited foods – that promise to revolutionise the way we eat.
Critics argue these foods could pose risks to human health and the environment. Proponents point to their potential for enhancing yields, reducing food waste, and even combating climate change.
What are GMOs and gene-edited foods? And how are they shaping the future of our food systems?
GMOs and gene-edited foods aren’t the same
GMOs are organisms whose genetic material has been artificially altered by inserting a piece of foreign DNA. This DNA may be synthetic in origin or sourced from other organisms.
Gene editing involves making precise changes to an organism’s genome without the integration of foreign DNA elements. Using techniques such as CRISPR/Cas, scientists make precise “cuts” in the DNA to create new genetic variation. Unlike with GMOs, this introduces only minor modifications, which are indistinguishable from natural mutations.
Although GMOs and gene-edited foods have been in circulation for almost three decades, research in this space continues to deliver breakthroughs. These technologies are being applied to provide a range of benefits, from improved nutrition in food, to reduced food waste and increased crop tolerance against climate stresses.
Read more: What is CRISPR, the gene editing technology that won the Chemistry Nobel prize?
What are the concerns?
The major criticisms of GMOs are related to the overuse of specific herbicides.
GMOs are mainly used to produce crops that are herbicide-resistant or produce pesticides. Farmers can then use herbicides on those crops to control weeds more effectively, without the plants themselves dying. This leads to higher yields on less land, and often with less chemicals used overall.
However, these crops rely on the use of said lab-made chemicals . And although the government regulates them, ethical and safety debates continue. People raise concerns over potential long-term health impacts, impacts on biodiversity and ecosystems, and the increased corporate control over agriculture.
Concerns generally aren’t related to the actual manipulation of the plants’ DNA.
Is genetic modification itself unsafe?
When it comes to the food we eat, how much do we really know about its DNA? Even among experts with genome-sequencing information, most have only one or a few sequenced “reference” varieties, and these often aren’t the same as the plants we eat.
The fact is, we don’t really understand the genomes of many plants and animals we eat. So there’s no reason to suggest tweaking their gene sequences will make consumption harmful. Moreover, there’s currently no evidence regulator-approved GMOs or gene-edited foods aren’t safe for human consumption.
In regards to food safety, one valid concern would be the potential creation of new allergens: proteins within the crop the body recognises and creates an immune response to.
But it’s important to remember many foods we eat are already allergenic. Common examples include wheat, peanuts, soy, milk and eggs. Some common foods are even toxic if consumed in large quantities or without appropriate preparation, such as rhubarb leaves, raw cassava, raw kidney beans and raw cashews.
Ironically, researchers are using gene editing to work towards eliminating proteins that cause allergies and intolerances. Gluten-free wheat is one example.
GMOs and gene-edited foods are widespread
Due to inconsistent rules about labelling GMOs and gene-edited foods around the world, many consumers may not realise they’re already eating them.
For example, the most widely used enzyme in cheese-making, rennet , is produced from a GMO bacterium. GMO microbial rennet produces a specific enzyme called chymosin, which helps coagulate milk and form curds. Historically, chymosin was extracted from young cow stomachs, but in the 1990s scientists managed to genetically engineer a bacterium to synthesise it.
GMOs and gene-edited cereal and oilseed products are also widely used in stockfeeds. There is ongoing research to improve feed through enhanced nutrition , and produce crops that will decrease methane emissions from cattle .
When it comes to modifying animals themselves, ethical considerations must be balanced alongside potential benefits.
In Australia, about 70% of cattle are genetically polled (hornless). Having polled cows improves meat quality through less injury to meat, and is considered better for animal welfare. In the US, fast-growing genetically modified salmon has been approved for consumption.
In a horticultural context, the genetically modified rainbow papaya stands out. It was developed in the late 1990s in response to a ringspot virus outbreak that nearly wiped out the global papaya industry. Researchers created the virus-resistant “transgenic” papaya, which now makes up a significant proportion of papayas consumed.

In terms of boosting nutritional content, “ golden rice ” biofortified with Vitamin A (GMO) is being cultivated in the Philippines, as are tomatoes biofortified with Vitamin D (GE) in the United Kingdom, and GABA-enriched tomatoes (GE) in Japan.
Research is also being done to create non-browning mushrooms , apples and potatoes. A simple gene edit can help inhibit the browning oxidation reaction, leading to a longer shelf-life and less food waste.
Regulation in Australia and New Zealand
So why don’t you see non-browning mushrooms at your local supermarket?
In Australia, the Office of the Gene Technology Regulator regulates GMOs. It has approved four GMO crops for cultivation: cotton, canola, safflower and Indian mustard. However, many more are imported for food ingredients (including modified soy, cottonseed oil, corn and sugar beet) and stockfeed (canola, maize and soy).
Gene-edited food crops can be cultivated without any regulatory restrictions or labelling in Australia. The Gene Technology Act 2000 deregulated these products in 2019.
On the other hand, New Zealand’s Environmental Protection Authority has maintained regulatory restrictions on both gene-edited foods and GMOs. Divergent definitions have led the bi-national agency Food Standards Australia New Zealand (FSANZ) to adopt a cautious approach, regulating gene-edited foods and feeds as GMOs.
The lack of alignment in definitions in Australian has confused producers and consumers alike. FSANZ has said it will continue to monitor developments in gene-editing technology, and will consider reviewing its regulatory approach.
Responsible research
Both GMOs and gene-edited foods offer great promise. Of course there are valid concerns, such as the potential to create new allergens, unintended consequences for ecosystems, and growing corporate control over food. But these can be addressed through responsible research and regulatory frameworks.
Ultimately, the development of future foods must be guided by a commitment to sustainability, social justice and scientific rigour.
Correction: This article previously said the transgenic rainbow papaya made up the majority of papayas consumed worldwide. This was incorrect and the wording has been amended.
- Genetically Modified Organisms
- Genetically modified crops
- Genetically modified
- GMO labelling
- gene-editing technology
- Genetically engineered food
- Gene-editing

Lecturer / Senior Lecturer - Marketing

Communications and Engagement Officer, Corporate Finance Property and Sustainability

Assistant Editor - 1 year cadetship

Executive Dean, Faculty of Health

Lecturer/Senior Lecturer, Earth System Science (School of Science)
- Open access
- Published: 13 January 2022
Evaluation of adverse effects/events of genetically modified food consumption: a systematic review of animal and human studies
- Chen Shen 1 ,
- Xiang-Chang Yin 2 ,
- Bo-Yang Jiao 3 ,
- Jing Li 4 ,
- Peng Jia 5 ,
- Xiao-Wen Zhang 1 ,
- Xue-Hao Cheng 6 ,
- Jian-Xin Ren 6 ,
- Hui-Di Lan 7 ,
- Wen-Bin Hou 1 ,
- Min Fang 1 ,
- Yu-Tong Fei 1 ,
- Nicola Robinson 1 , 8 &
- Jian-Ping Liu ORCID: orcid.org/0000-0002-0320-061X 1 , 9
Environmental Sciences Europe volume 34 , Article number: 8 ( 2022 ) Cite this article
44k Accesses
15 Citations
67 Altmetric
Metrics details
A systematic review of animal and human studies was conducted on genetically modified (GM) food consumption to assess its safety in terms of adverse effects/events to inform public concerns and future research.
Seven electronic databases were searched from January 1st 1983 till July 11th 2020 for in vivo, animal and human studies on the incidence of adverse effects/events of GM products consumption. Two authors independently identified eligible studies, assessed the study quality, and extracted data on the name of the periodical, author and affiliation, literature type, the theme of the study, publication year, funding, sample size, target population characteristics, type of the intervention/exposure, outcomes and outcome measures, and details of adverse effects/events. We used the Chi-square test to compare the adverse event reporting rates in articles funded by industry funding, government funding or unfunded articles.
One crossover trial in humans and 203 animal studies from 179 articles met the inclusion criteria. The study quality was all assessed as being unclear or having a high risk of bias. Minor illnesses were reported in the human trial. Among the 204 studies, 59.46% of adverse events (22 of 37) were serious adverse events from 16 animal studies (7.84%). No significant differences were found in the adverse event reporting rates either between industry and government funding ( χ 2 = 2.286, P = 0.131), industry and non-industry funding ( χ 2 = 1.761, P = 0.185) or funded and non-funded articles ( χ 2 = 0.491, P = 0.483). We finally identified 21 GM food-related adverse events involving 7 GM events (NK603 × MON810 maize, GTS 40-3-2 soybean, NK603 maize, MON863 maize, MON810 maize, MON863 × MON810 × NK603 maize and GM Shanyou 63 rice), which had all been on regulatory approval in some countries/regions.
Serious adverse events of GM consumption include mortality, tumour or cancer, significant low fertility, decreased learning and reaction abilities, and some organ abnormalities. Further clinical trials and long-term cohort studies in human populations, especially on GM food-related adverse events and the corresponding GM events, are still warranted. It suggests the necessity of labelling GM food so that consumers can make their own choice.
Introduction
Genetic modification is defined as introducing transgene(s) with desired traits into the recipient organism’s genome by recombinant deoxyribonucleic acid (DNA) technology, and therefore it does not occur naturally [ 1 , 2 , 3 ]. Genetically modified (GM) crops are thought to address food security, sustainability and climate change solutions by improving crop yields, conserving biodiversity, providing a better environment in terms of the insect-resistant and herbicide-tolerant traits, reducing CO 2 emissions and helping alleviate poverty through uplifting the economic situation [ 4 ]. Insect-resistant and herbicide-tolerant traits were first introduced into four types of crop, canola, cotton, maize and soybeans, at the beginning of GM production [ 5 ]. At present, the mainstream characteristics of new crops still pursue higher-yielding, more nutritious, pest- and disease-resistant and climate-smart to meet future demand for a yield increase of major crops such as wheat, rice and corn, due to the growing population [ 6 ].
Since 1996, the first year of commercialization of GM crops, 70 countries had adopted GM crops until 2018, including 26 countries that cumulatively planted 2.5 billion hectares of GM crops and an additional 44 countries that imported GM crops. During the 27 years (1992 to 2018), 4349 approvals for 387 GM events from 27 GM crops were granted by 70 countries involving 2063 for food (when the direct consumers are mainly humans), 1461 for feed (the products only intended for animal consumption) use and 825 for environmental release or cultivation [ 4 , 7 ]. The major agricultural product exporting countries like the U.S.A., Brazil and Argentina show over 90% adoption of biotech crops [ 4 ]. For GM animal products, biotech salmon, considered to be the first genetically engineered animal for human consumption, was approved by the United States Department of Agriculture and Food & Drug Administration in 2015 [ 8 ]. In addition, it is illegal to grow major GM food crops in China while there are substantial investments in biotechnology research and GM maize, soybeans, and canola are allowed to import and eat [ 9 ].
Genetically modified food, however, is an example of the controversial relation between the inherent uncertainty of the scientific approach and the need of consumers to use products resulting from scientific developments thought to be safe [ 10 ]. Significant health risks have not been reported in peer-reviewed studies on GM food safety/security, which may cause some publication bias [ 11 ] but with a few exceptions, like the most famous “Monarch Butterfly controversy” [ 12 ], "Pusztai case" [ 13 ] and the "Séralini case" [ 14 ]. Unexpected effects of GM crops were reported in these studies, occupying an important place in the pages of scientific journals. Nevertheless, the above controversies severely impacted the public image, leading to full or partial bans in 38 countries including the European Union [ 15 ].
The complexity of risk evaluation is shown in these conflicting results, and concerns about the citizen-consumers have been raised against GM food [ 10 ]. Of most concern, aroused from the controversial events and some research results, is the potential of carcinogenesis, teratogenesis [ 16 ], lethal effects and adverse influences on fertility. GM agriculture is now widely discussed in both positive and negative frames and currently serves as a hotbed of debate in the public and policymakers. Although there are some reports and evidence from human and animal studies on the potential health effects of GM food/feed, the evidence is not conclusive and public concerns have not been resolved.
We aimed to conduct a systematic review of animal and human studies on GM food consumption to assess its safety in terms of adverse effects/events to inform public concerns and future research.
This study was a systematic review of previously published studies, conducted and reported in adherence with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [ 17 ] guideline.
Search strategy
China National Knowledge Infrastructure (CNKI), Wanfang, VIP Database, Chinese Biomedical Database (SinoMed), PubMed, the Cochrane Library and Embase databases were searched from January, 1st, 1983 till July, 11th, 2020, using a predefined search strategy (Additional file 1 : Appendix S1). Reference lists of retrieved articles were also searched.
Eligibility criteria
Based on the evidence pyramid proposed by the Medical Center of State University of New York in 2001, we determined the type of research we included in the study. For a comprehensive evaluation of the literature, all in vivo animal studies and human studies (cross-sectional studies, case reports, case series, case–control studies, case–crossover studies, cohort studies, controlled clinical trials, including randomized trials, quasi-randomized trials and non-randomized trials) in multiple languages were included. Animal studies in all fields were included, that is, they could be clinical, agricultural and animal husbandry, veterinary medicine, life sciences, etc. Field studies were excluded.
The study population in animal studies was applied with inclusion criteria based on the categorization approach that highlights the actual use of them: laboratory animals and economical animals (livestock and aquatilia) were included, with no prespecified limitations on age, population, species/races, health status or others. Interventions/exposures of the genetically modified animal/plant/microorganism products included for animal/human ingestion referred to GM food, GM food ingredients and GM feed, regardless of their dosage or duration. The GM strain (line) and GM event were not limited. There was no restriction on whether controls were or were not included. The studies were excluded if they focused on the effects of GM food/feed on secondary or multilevel consumers in the food chain where GM food/feed was only consumed by primary consumers in the predator relationships. For instance, if non-GM fishes were fed with diet containing GM ingredients and then the fish was fed to the experimental cats, the study was excluded.
Outcomes focused on the incidence of adverse effects or adverse events in GM food/feed consumption, including primary outcomes on carcinogenesis, teratogenesis, lethal effect (all-cause mortality) and reproduction and secondary outcomes on other biomarkers were included. Toxicity studies of general toxicity studies (acute, sub-acute, sub-chronic, chronic and carcinogenicity toxicity studies) and specific toxicity studies (genotoxicity, reproductive and developmental toxicity, immunotoxicity and other toxicology studies) were included. Mortality in pups before weaning was considered as an outcome of reproductive toxicity but not as a lethal effect. Outcomes of adverse events in laboratory testing would not be included only when they could indicate tissue or organ toxicity. Outcomes of adverse events in breeding performance in animal husbandry studies, which focused on the economic benefits of the animal products, were included and these indicators were regarded as reproduction biomarkers in this research.
Outcomes of adverse events on growth performance, carcass traits, meat and fur production performance and meat quality for economic benefit evaluation of live stocks were excluded, of which the indicators included final body weight, weight gain, feed to gain ratio, half-eviscerated weight, eviscerated weight, percentage of eviscerated yield and muscle lean meat, sebum rate in some parts of the body, etc. Studies on the insecticidal effect of insect-resistant GM feed and outcomes of adverse events in gene fragments residual in the digestive tract were excluded. Besides, duplicate publications, studies with duplicate statistics, or references devoid of necessary information of participants, sample size, interventions/exposures or results were excluded.
Study selection and data extraction
Titles and abstracts of the retrieved articles were reviewed by 6 researchers in pair (C Shen, XC Yin, BY Jiao, J Peng, YZ Li, XH Cheng). 6 authors (C Shen, XC Yin, BY Jiao, JX Ren, J Li and XW Zhang) independently reviewed the full texts to identify the studies meeting eligibility criteria and then 8 researchers in pair (C Shen, XC Yin, BY Jiao, J Li, P Jia, XW Zhang, XH Cheng and JX Ren) independently extracted data from the included studies according to a predesignated extraction table. The discrepancies were resolved through consensus and if necessary, arbitrated by another author (JP Liu).
We extracted the name of the periodical, author and affiliation, literature type, the theme of the study, publication year, funding, sample size, target population characteristics, type of the intervention/exposure, outcomes and outcome measures. For those studies in which adverse effects/events occurred, details of interventions/exposures and control conditions (if any), dosage, duration, number of the generation, and the results were extracted.
Quality assessment
The methodological quality for animal studies was assessed, using criteria from the SYRCLE’s risk of bias tool for animal studies. The quality of animal studies was categorized into low risk of bias, unclear risk of bias, or high risk of bias according to the risk for each important outcome within included studies, including the adequacy of generation of the sequence generation, baseline characteristics, allocation concealment, random housing, blinding (performance bias), random outcome assessment, blinding (detection bias), incomplete outcome data, selective outcome reporting, or other sources of bias. The judgment of other risk of bias was based on whether there were contamination (pooling drugs), inappropriate influence of funders, unit of analysis errors, design-specific risks of bias or new animals added to the control and experimental groups to replace drop-outs from the original population.
Statistical synthesis and analyses
Statistical analyses were carried out using Microsoft Excel 2016 and SPSS 20.0. The findings were reported mainly in two parts, characteristics of the included studies and detailed information on the studies in which adverse effects/events occurred. Initially, descriptive statistics, frequencies, and percentages were calculated to summarize the data. Subsequently, studies that evaluated similar populations, interventions, controls (if any) and outcomes were pooled using a random-effects meta-analysis, and data from other studies were presented in tables and described in a narrative summary. The incidence of adverse events reported in articles funded by industry funding, government funding or unfunded articles were, respectively, counted and the Chi-square test was used for the comparisons.
Besides, we figured the incidence of serious adverse events (SAEs) by percentage. With reference to the Food and Drug Administration’s definition [ 18 ], our study defined SAEs as death, life-threatening, hospitalization (initial or prolonged), disability or permanent change, disruption, impairment or damage in a body function or structure (including cancer or tumour), in physical activities or quality of life, congenital anomaly or birth defect in the newborn child or pups, infertility or significant low in the number of deliveries or live birth rate than the non-GM commercial, conventional or blank controls, and an event resulting in intervention/treatment to prevent permanent impairment, damage or to prevent one of the other outcomes.
Meanwhile, the adverse events which cannot be ruled out that it has nothing to do with GM food (hereinafter abbreviated as GM food-related adverse events) were identified and the percentages under each outcome were calculated.
Description of studies
The flow diagram of the literature selection is shown in Fig. 1 . A total of 9668 records were identified, including 9584 from the initial search through seven databases and 84 from other sources. After removal of duplicates and exclusion of references by reading titles and abstracts, 455 full-text articles were screened and 276 references were excluded with reasons (seen in the flow chart). Finally, 204 studies from 179 articles [ 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 ] (153 journal articles, 22 dissertations, 3 conference proceedings and 1 unpublished report) were included in data synthesis, since there were more than one study conducted in each of the 2 included dissertations [ 107 , 127 ], 11 journal articles [ 19 , 33 , 35 , 63 , 67 , 88 , 102 , 118 , 132 , 172 , 184 ] and 1 unpublished report [ 32 ]. The included studies were of 203 in vivo animal studies and 1 crossover trial [ 97 ] in humans.
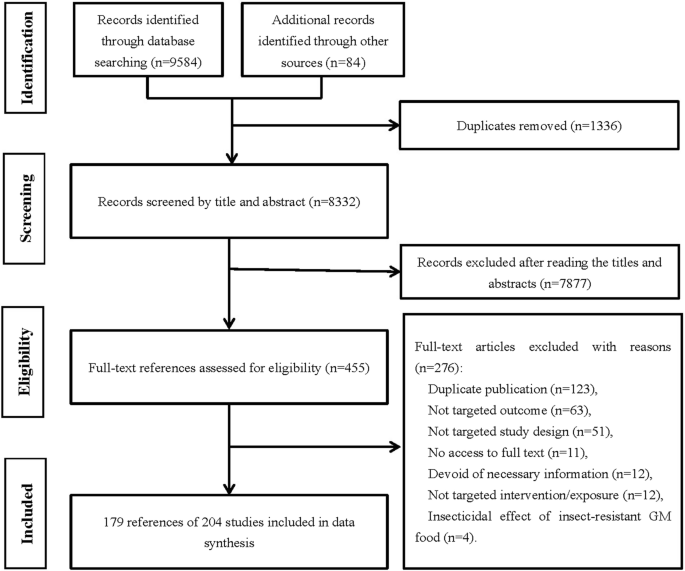
The flow of literature search and selection of studies on the safety of GM food
Study characteristics
Of the 179 included articles, 94 were in English [ 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 ], 83 were published in Chinese [ 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 , 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 ], and 2 in Japanese [ 196 , 197 ]. The earliest included reference dated back to 1998 [ 153 ] (shown in Fig. 2 ), after which the remaining articles were distributed from 2000 to 2020 (45 articles in the 2000s, while 131 in the 2010s and 2 in the 2020s). The year 2012 witnessed the largest volume of publication (n = 26 articles, 14.53%). For funding sources or sponsors (Additional file 1 : Appendix S2), in addition to 57 articles not mentioning the funding/sponsor (hereinafter as non-funded articles), there were 116 articles (64.8% of the 179 articles) supported by 56 kinds of government funding from 12 countries/government organizations and, still, 9 articles (5.03%) by 10 kinds of industry/institute funding sources/sponsors from 4 countries (America, Australia, French and German). Among them, 3 articles [ 29 , 62 , 74 ] claimed to have been funded or sponsored by both government and industry. China had undertaken the most government/school-level funding projects (39 of 56 projects, 69.64%).
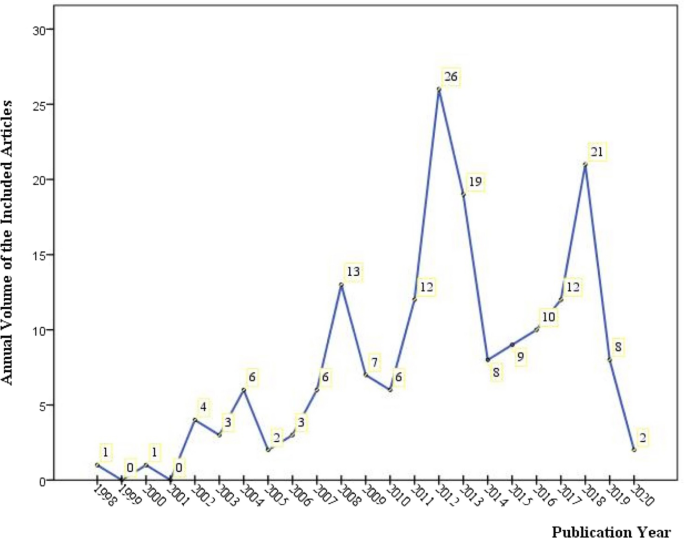
The publications (number of articles) on the safety of GM food by year
The periodicals that have published more than 5 included articles were Food and Chemical Toxicology (published 25 included articles), EFSA Journal (13), Regulatory Toxicology and Pharmacology (9), Journal of Hygiene Research (9) and Chinese Journal of Food Hygiene (8). 11 of 13 authors, who have published ten or more included studies, were from European Food Safety Authority and published 12 included articles as co-authors. They were Christina Tlustos (published 12 included articles), Claudia Bolognesi (12), Konrad Grob (12), Vittorio Silano (12), Andre Penninks (11), Gilles Riviere (11), Holger Zorn (11), Karl-Heinz Engel (11), Yi Liu (11), Natalia Kovalkovicova (10), Sirpa Karenlampi (10). In addition to the above 12 articles, the top 3 of the 11 authors who published five or more included studies was Yang Xiao-Guang (from Chinese Center for Disease Control and Prevention, published 11 included articles), Wang Jing (from Tianjin Centre for Disease Control and Prevention, published 10 included articles) and Zhuo Qin (from Chinese Center for Disease Control and Prevention, published 7 included articles). The top 5 affiliations which published included articles were Chinese Center for Disease Control and Prevention (published 16 included articles), Tianjin Centre for Disease Control and Prevention (12), European Food Safety Authority (12), National Chung Hsing University (10), International Rice Research Institute (9).
Of the 204 included studies, one was a double-blind crossover trial ( n = 36) in humans and the others were all animal studies. Individual sample sizes of the total 54,392 study population ranged from 4 (cats) [ 153 ] to 21,000 (Atlantic salmon) [ 23 ]. The studies involved 14 different kinds of animals (see Table 1 ). Apart from the most commonly used rats/mice (in 160 studies, 78.82%), pigs and chicks were two of the most extensively studied animals (in 23 studies, 11.33%). For themes of the 178 included animal studies, 158 were on clinical and 20 were on agricultural and animal husbandry. For the ones on clinical, 117 were on general toxicity (8 on acute, 6 sub-acute, 84 sub-chronic, 16 chronic toxicity, and still 3 on both acute, sub-acute and sub-chronic toxicity), 35 on specific toxicity (15 on reproductive and developmental toxicity, 16 on immunotoxicity, 3 on teratogenic effect and 1 on mutagenicity), 3 on allergenicity, 1 on learning and memory ability, 1 on athletic ability and 1 on both sub-chronic toxicity and allergenicity.
For interventions/exposures, 31 kinds of GM food were identified, including 18 kinds of GM plant food, 7 kinds of GM animal food and 6 kinds of GM microorganism food. Each included study covered one intervention/exposure, except for one study, Chen [ 29 ], that involved two kinds of GM products (sweet pepper and tomato) modified with the same gene (coat protein gene of cucumber mosaic virus), respectively, in two experimental groups. Maize, rice and soybean were the three most popular kinds of GM plant food (taken 79.38%) in research while milk/milk powder and animal-derived protein occupied the top two in GM animal food (56.25%). As for GM microorganism products, 5 kinds of food/feed enzyme derived from 5 different kinds of GM fungi or bacteria as well as 1 kind of microorganism-derived protein were among included studies.
Methodological quality of the animal studies
According to our predefined quality assessment criteria, all of the studies were identified as being unclear or having a high risk of bias (Fig. 3 ). None of the studies were reported to blind researchers from knowing which intervention each animal received. None of the studies reported prior sample-size calculation, 31 studies (15.27%) described wrong randomization procedures or did not mention the method of “randomization”, and 12 studies (5.91%) did not report adequate allocation concealment. 28 studies (13.79%) described that the groups were similar at baseline and 76 studies (37.44%) claimed that the housing conditions of animals from the various experimental groups were identical. 10 studies (4.93%) described randomly pick an animal during outcome assessment while 7 studies (3.45%) failed to select animals at random for outcome assessment. 88 studies (43.35%) completely used objective outcome indicators for outcome measurement. 185 studies (91.13%) reported consistent outcomes in the method and result sections while 5 studies did not, but none of the study protocols were available.
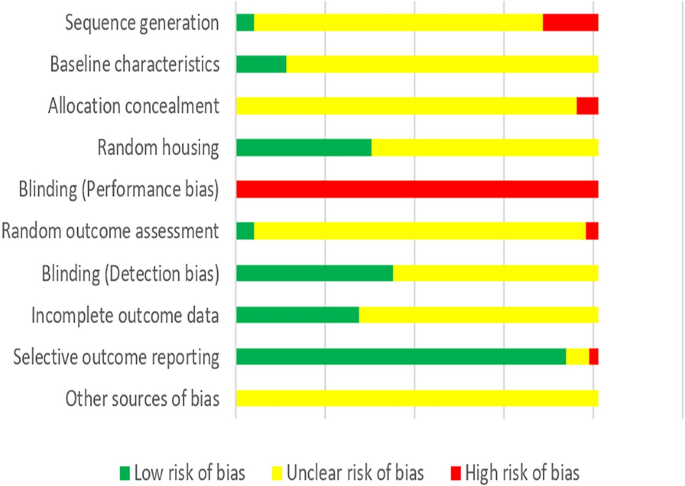
Risk of bias of the included animal studies
Incidence of adverse events/effects
No meta-analysis was conducted due to the significant heterogeneity of the primary studies. Among the 204 studies, a total of 29 studies (14.22%) from 23 articles reported 37 adverse events, involving 13 on mortality, 6 on reproductive toxicity, 3 on carcinogenesis and 15 on other biomarkers (including one human trial). It is worth noting that when, in one study, there were multiple aspects of adverse events on “other biomarkers”, we recorded it as 1 adverse event. Then, 22 serious adverse events (59.46% of adverse events) were identified in 16 studies (7.84% of the included studies and 55.17% of the studies reporting adverse events, marked in the tables with double asterisks). The SAEs mainly rested on mortality (13 studies), tumour or cancer (3), significant low in the number of pup deliveries (2), decreased learning and reaction abilities (1), severe stomach inflammation (1), intestinal adenoma lesions (1), and other pathology abnormalities (1) as hypertrophies and hyperplasia in mammary glands and pituitary, liver congestions and necrosis as well as severe chronic progressive nephropathies.
The incidence of adverse events reporting in government funding, industry funding and non-funded articles were 10.34% (12 of 116), 33.33% (3 of 9) and 15.79% (9 of 57), respectively. When comparing the adverse event reporting rates using the Chi-square test, we found that there were no significant differences either between industry funding and government funding ( χ 2 = 2.286, P = 0.131), industry funding and non-industry funding ( χ 2 = 1.761, P = 0.185) or funded and non-funded articles ( χ 2 = 0.491, P = 0.483).
Incidence of adverse events/effects in human trial
As for the human trial [ 97 ], shown in Table 2 , a randomized double-blind crossover design was conducted for acute consumption of two single breakfasts, with a 14-day washout period, containing either seed oil generated from transgenic Camelina sativa plants or commercially blended fish oil. 36 healthy people were randomly allocated into two groups and venous blood samples were collected after the postprandial session, 8 h after each meal. No follow-up was reported. No major adverse symptoms or health effects were reported but some unrelated minor illnesses for the 72 postprandial sessions from 36 participants, such as minor upper respiratory tract infections (2.78%), minor nose bleed (1.39%), pyelonephritis (1.39%) and headaches (8.33%).
Incidence of adverse events/effects in animal studies
For the 203 animal studies, 28 studies (13.79%) from 22 articles reported 36 adverse events, including 13 on mortality (Table 3 , 36.11%), 6 on reproductive toxicity ( Table 4 , 16.67%), 3 on carcinogenesis (Table 5 , 8.33%) and 14 on other biomarkers (Additional file 1 : Appendix S3, 38.89%).
All causes of death were included in this analysis and 11 of the 13 studies claimed that the mortality was not significantly different between the groups or had nothing to do with GM food. One study (Ermakova [ 37 ]) reported higher pup mortality in the Roundup-Ready soya (40.3.2 line) group compared with the controls. In Séralini [ 74 ] , the general cause of death was large mammary tumours in females and other organ problems in males. Besides, rats in the Roundup-tolerant GM NK603 maize groups were 2–3 times more likely to die than controls, and more rapidly.
With respect to effects on reproduction, 5 animal feeding studies were reported to trigger reproductive toxicity but one study (Cisterna [ 31 ]) claimed to have no substantial impact on fertility. The reproductive toxicity manifested in the significant low in the number of deliveries, survival rate (from birth to weaning), litter weight, litter size and weight of some organs in the pups. For example, in Ermakova I 2005, the rats fed with Roundup-Ready soya had a 55.6% pup mortality rate during lactation periods compared to 9% in the control of traditional soya and 6.8% in the reference group. The pups kept dying during the lactation period while pups from the control group only died during the first week. Cyran N 2008 a and Cyran N 2008 c [ 32 ] were two rat feeding studies reported in one article, both given NK603 × MON810 maize. A multi-generation study was conducted as Cyran N 2008 a while Cyran N 2008 c did a continuous breeding study. Both of them indicated that fewer sum of pups was born and weaned in the GM groups. Pup losses, in Cyran N 2008 a, overall generations were about twice as many pups lost as compared to the control group (14.59% vs 7.4%) but was not significantly different and significantly lower litter weight was also reported in Cyran N 2008 c.
Three mouse/rat feeding studies reported triggering cancers/tumours when Tang [ 156 ] attributed the incidence of the tumour to the elder age of rats. Séralini 2014 (on Roundup-tolerant GM maize) found that females in the treatment groups almost always developed large mammary tumours more often than and controls. As for males, 4 times larger palpable tumours than controls were presented which emerged up to 600 days earlier. Cyran 2008 b [ 32 ] revealed a life term study where mice in the three groups were fed with transgenic maize NK603xMON810 (from 33.0% in the diet), control isoline diet and GM-free Austrian corn reference diet, respectively. The survival rate was not significantly different while cancer (leucosis) was the common cause of death.
GM food-related adverse events
Among the 37 adverse events reported, 16 of them claimed to have nothing to do with GM food, while the rest 21 (from 17 studies) did not, still leaving the question open. The GM food-related adverse events existed in mortality (2 studies), reproductive toxicity (5), carcinogenesis (2), and other biomarkers (12).
By gathering evidence, we identified 3 kinds of GM food associated with adverse events, GM soybean, GM maize as well as GM rice. For the 17 studies involved in the GM food-related adverse events, 4 studies were absent of information on the GM event of their test substance and the remainder concentrated on 7 GM events (3 studies on NK603 × MON810 maize, 2 on GTS 40-3-2 soybean, 2 on NK603 maize, 2 on MON863 maize, 2 on MON810 maize, 1 on maize mixed with MON863 × MON810 × NK603, NK603 × MON810 and NK603 and 1 on GM Shanyou 63 rice). When searching in the GM Approval Database on the ISAAA website, we found that all of the first 6 GM events listed, all developed by Monsanto Company, had been on regulatory approval for food, feed and cultivation in multiple countries/regions, including the European Union. GM -39 Shanyou 63 was developed in China and given approval for food, feed, and cultivation only by China in 2009.
Summary of findings
We included 203 in vivo animal studies and 1 human trial, and all of the studies were identified as being unclear or having a high risk of bias. Overall, we reported two main findings. First, we identified 37 adverse events for GM food consumption while 22 of them (59.46%) were serious adverse events extracted from 16 animal studies (7.84%). SAEs were mortality, tumour or cancer, significantly low in the number of pup deliveries, decreased learning and reaction abilities, severe stomach inflammation, intestinal adenoma lesions, and other pathological abnormalities in the mammary glands, pituitary, liver and kidney.
Second, there were 21 GM food-related adverse events indicating that GM food may have effects on increased mortality (2 studies), reproductive toxicity (5 studies), which referred to significantly low fertility in parental generation and low survival rate, litter weight, litter size and weight of some organs in the pups, carcinogenesis (2 studies) and other biomarkers (12 studies). The effect-related GM food included 7 GM events (NK603 × MON810 maize, GTS 40-3-2 soybean, NK603 maize, MON863 maize, MON810 maize, MON863 × MON810 × NK603 maize and GM Shanyou 63 rice), which had all been on regulatory approval for food, feed and cultivation in some countries/regions.
Agreements and disagreements with other reviews
To our knowledge, there have been 3 previous systematic reviews (SRs) [ 198 , 199 , 200 ] and 6 conventional reviews [ 16 , 201 , 202 , 203 , 204 , 205 ] addressing similar research questions on the unexpected effects of GM food consumption. Keshani et al. [ 198 ], searching in 4 English databases, included experimental studies on GM crops’ potential effects on sperm parameters. The study finally included 7 rat feeding studies, which were all identified in our study, and indicated no harm to GM plants consumers. Edge et al. [ 199 ] addressed 30 review questions for including human studies, published in recent 20 years (1994–2014), on health effects of genetically engineered (GE) food crops, but found no human study on 25 questions. The remaining 5 questions, related to allergenicity and nutrient adequacy, were answered based on 21 human studies. The human studies were all excluded in our research because of no direct ingestion of GE food in the allergenicity assessment studies or no targeted outcomes in the nutrient assessment trial. To illustrate, the above-mentioned nutrient assessment clinical trial evaluated the effect of carrots containing twofold higher calcium content on calcium absorption and we thought it was not on outcome related to adverse events/effects. The conclusion of the research also supported that there were no clear adverse health effects associated with the consumption of GE food. Moreover, Dunn et al. [ 200 ] included both human and animal studies for examining the allergenicity of GM organisms and finally found 34 human studies and 49 animal studies eligible. In addition to 32 human studies which involved human serum for IgE binding or inhibition studies and not direct consumption of GM product, the rest 2 [ 206 , 207 ]studies were on actual ingestion of a GM food. However, they were not included in our research because of not targeted study type and unrelated outcomes. The conclusion agreed with the first two SRs that GM foods did not appear to be more allergenic than their conventional counterparts.
As for conventional reviews, Domingo showed special attention to the safety of GM food and published four literature reviews in 2000 [ 203 ], 2007 [ 204 ], 2011 [ 205 ] and 2016 [ 16 ]. Domingo searched two databases, PubMed and Scopus, to assess adverse/toxic effects of GM plants. In the latest updated review, he addressed the conclusion that GM soybeans, rice, corn/maize and wheat would be as safe as the parental species of these plants. However, our results may not be consistent with Domingo’s conclusion: we focus on a summarization of adverse events for GM food consumption through a systematic search in 7 databases; we identified 37 adverse events, 22 serious adverse events and 21 GM food-related adverse events; GM maize, soybean and rice with some specific GM events were all related to GM food-related adverse events. In addition, Domingo found a notable advance of studies published in scientific journals by biotechnology companies. Coincidentally, we did a Chi-square test to compare the adverse event reporting rates and found no significant differences between industry funding, government funding and non-funded articles. Besides, our systematic review validated Domingo’s findings that some GM plants were studied scarcely in recent years including GM potatoes discussed in the controversy of Pusztai case.
Strengths and limitations
In this review, a systematic search of major databases was conducted to identify all available studies in all languages on the adverse effects/events of GM food consumption. To make the inclusion and data synthesis comprehensive, both in vivo human and animal studies in all fields were included, with no limitations on the type of participant, type of intervention/exposure or whether control was included. The terms used for searching, containing all kinds of names of GM food, were based on a basic search on the internet by the researchers and the list was perfected as much as possible. With respect to additional searching, we went through multifarious news which reported controversy of GM food and thus we identified several hot studies by following the clue. In order to trace the potential conflicts of interest, we performed a Chi-square test for comparing adverse events report rates in articles funded by industry funding, government funding or unfunded articles, but found no statistical significance. Nevertheless, it was hard to conduct a quantitative data synthesis for the effects of GM food consumption on the adverse events because of the significant heterogeneity of the primary studies.
There are several limitations in this review. The methodological quality of the included studies is generally poor, which indicates a high or unclear risk of bias resulting from insufficient reporting of methodological components in the studies. Methodological quality may not be fully reflected based solely on the reporting of the manuscript. There were unclear descriptions of randomization procedures and a lack of blinding in all of the studies, which may have created potential performance biases and detection biases, as researchers might have been aware of the effects of interventions. The ability to perform meta-analysis was limited because of the heterogeneity of the participants, interventions (GM food in various GM events), comparisons, feeding doses, administration time, other exposure factors, and the variance of composite outcome measures used in the 204 included studies. When we did the manual search, we found that related publications were retracted sometimes, under the name of inadequate experimental designs or statistical analysis. For example, Séralini 2012 was retracted by Food and Chemical Toxicology , but subsequently republished in another journal [ 14 , 74 ]. This indicates that it was hard for us to find the original full-text papers of the retracted publications and articles provided by databases still have some unavoidable publication bias. The retraction on controversial researches may also cause the controversy for the public to doubt the reality of the studies published and to concern the safety of GM food. In addition, the lack of human studies is another key limitation of this research. As for the searching strategy, we did not include publication types as newspaper articles and comments. This was thought to be a limitation of this research because these sources may give us clues of related researches and can help us to do a manual search comprehensively. It is also an implication for future systematic reviews.
Implications for research
Future research should be conducted in humans, especially observational cohort studies. High-quality animal studies according to the ARRIVE reporting standard focusing on reproductive toxicity and carcinogenesis are still needed. Trials or studies should be registered prospectively, and be accessible. Furthermore, to address public concerns, future studies should focus on SAEs and GM food-related adverse events reported in this research such as NK603 maize, MON863 maize and MON810 maize. Meanwhile, some implications of findings still could be explored such as how GM food affects people’s eating habits, labelling of GM food and public choice. Some of the included studies conducted an intergenerational or multigenerational evaluation of the safety of GM food, but only two studies (Cyran N 2008 a and Cyran N 2008 c) in one article reported adverse events related to fertility. The differences in the results may be due to different interventions/exposures (GM food in certain GM events), laboratory animals, intervention/exposure time, experiment environment, etc. Therefore, it is necessary for subsequent studies to start with intergenerational or multigenerational research to verify the safety of GM food in terms of study design.
Serious adverse events accounted for 59.46% of the total 37 identified adverse events of GM consumption, which include: mortality, tumour or cancer, significantly lower number of pup deliveries, decreased learning and reaction abilities, and organ abnormalities in the stomach, intestinal adenoma, mammary glands, pituitary, liver and kidney. The interventions/exposures in the adverse event related studies emphasized on GM soybean, maize and rice in specific GM events. Animal studies occupy the lowest hierarchy of evidence, and there are flaws in study design and is not convincing enough. The evidence on the effect of GM consumption on humans is still insufficient. Further clinical trials and long-term cohort studies in human populations, especially on GM food-related adverse events and the corresponding GM events, are still warranted. It is better to prove the safety before they are approved for food consumption and it also suggests the necessity of labelling on GM food so that consumers can make their own choice.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
Genetically modified
Deoxyribonucleic acid
China National Knowledge Infrastructure
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Serious adverse event
Camelina sativa Seed oil
Blended fish oil
Body weight
Systematic reviews
Genetically engineered
Hundleby PA, Harwood WA (2019) Impacts of the EU GMO regulatory framework for plant genome editing. Food Energy Secur. https://doi.org/10.1002/fes3.161
Article Google Scholar
Sprink T, Eriksson D, Schiemann J et al (2016) Regulatory hurdles for genome editing: process- vs. product-based approaches in different regulatory contexts. Plant Cell Rep 35:1493–1506. https://doi.org/10.1007/s00299-016-1990-2
Article CAS Google Scholar
Georges F, Ray H (2017) Genome editing of crops: a renewed opportunity for food security. GM Crops Food 8:1–12. https://doi.org/10.1080/21645698.2016.1270489
ISAAA (2018) Global status of commercialized biotech/GM Crops in 2018: biotech crops continue to help meet the challenges of increased population and climate change. ISAAA Brief No. 54 [Online]. http://www.isaaa.org/resources/publications/briefs/54/default.asp . Accessed 17 July 2020
Andrew J, Ismail NW, Djama M (2018) An overview of genetically modified crop governance, issues and challenges in Malaysia. J Sci Food Agric 98:12–17. https://doi.org/10.1002/jsfa.8666
Hickey LT, Hafeez AN, Robinson H et al (2019) Breeding crops to feed 10 billion. Nat Biotechnol 37(7):744–754. https://doi.org/10.1038/s41587-019-0152-9
Giraldo PA, Shinozuka H, Spangenberg GC et al (2019) Safety assessment of genetically modified feed: is there any difference from food? Front Plant Sci 10:1592. https://doi.org/10.3389/fpls.2019.01592
ISAAA (2016) Global status of commercialized biotech/GM crops: 2016. ISAAA Brief No. 52 [Online]. http://www.isaaa.org/resources/publications/briefs/52/default.asp . Accessed 17 July 2020
Pray C, Huang J, Hu R, Deng H et al (2018) Prospects for cultivation of genetically engineered food crops in China. Global Food Secur Agric Policy Econ Environ 16:133–137. https://doi.org/10.1016/j.gfs.2018.01.003
Martinelli L, Karbarz M, Siipi H (2013) Science, safety, and trust: the case of transgenic food. Croat Med J 54:91–96. https://doi.org/10.3325/cmj.2013.54.91
Klümper W, Qaim M (2014) A meta-analysis of the impacts of genetically modified crops. PLoS ONE. https://doi.org/10.1371/journal.pone.0111629
Losey JE, Rayor LS, Carter ME (1999) Transgenic pollen harms monarch larvae. Nature 399:214. https://doi.org/10.1038/20338
Ewen SW, Pusztai A (1999) Effect of diets containing genetically modified potatoes expressing Galanthus nivalis lectin on rat small intestine. Lancet 354:1353–1354. https://doi.org/10.1016/S0140-6736(98)05860-7
Séralini GE, Clair E, Mesnage R et al (2012) Long term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize [retracted in: Food Chem Toxicol. 2014 Jan;63:244]. Food Chem Toxicol 50(11):4221–4231. https://doi.org/10.1016/j.fct.2012.08.005
Genetic Literacy Project (2017) Where are GMOs grown and banned? [Online]. https://gmo.geneticliteracyproject.org/FAQ/where-are-gmos-grownand-banned/ . Accessed 5 Sept 2020
Domingo JL (2016) Safety assessment of GM plants: an updated review of the scientific literature. Food Chem Toxicol 95:12–18. https://doi.org/10.1016/j.fct.2016.06.013 ( Epub 2016 Jun 16 )
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. BMJ. https://doi.org/10.1136/bmj.b2535
FDA (2016) What is a serious adverse event? [Online]. https://www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-event . Accessed 28 Sept 2020
Al-Harbi A, Lary S, Edwards MG et al (2019) A proteomic-based approach to study underlying molecular responses of the small intestine of Wistar rats to genetically modified corn (MON810). Transgenic Res 28(5–6):479–498. https://doi.org/10.1007/s11248-019-00157-y
Appenzeller LM, Munley SM, Hoban D et al (2009) Subchronic feeding study of grain from herbicide-tolerant maize DP-Ø9814Ø-6 in Sprague-Dawley rats. Food Chem Toxicol 47:2269–2280. https://doi.org/10.1016/j.fct.2009.06.014
Bai H, Wang Z, Hu R et al (2015) A 90-day toxicology study of meat from genetically modified sheep overexpressing TLR4 in Sprague-Dawley rats. PLoS ONE. https://doi.org/10.1371/journal.pone.0121636
Bakke-Mckellep AM, Koppang EO, Gunnes G et al (2007) Histological, digestive, metabolic, hormonal and some immune factor responses in Atlantic salmon, Salmo salar L, fed genetically modified soybeans. J Fish Dis 30:65–79. https://doi.org/10.1111/j.1365-2761.2007.00782.x
Bakke-McKellep AM, Sanden M, Danieli A et al (2008) Atlantic salmon ( Salmo salar L.) parr fed genetically modified soybeans and maize: histological, digestive, metabolic, and immunological investigations. Res Vet Sci 84:395–408. https://doi.org/10.1016/j.rvsc.2007.06.008
Buzoianu SG, Walsh MC, Rea MC et al (2012) Effect of feeding genetically modified Bt MON810 maize to ∼ 40-day-old pigs for 110 days on growth and health indicators. Animal 6:1609–1619. https://doi.org/10.1017/S1751731112000249
Buzoianu SG, Walsh MC, Rea MC et al (2012) Effects of feeding Bt maize to sows during gestation and lactation on maternal and offspring immunity and fate of transgenic material. PLoS ONE. https://doi.org/10.1371/journal.pone.0047851
Carman JA, Vlieger HR, Ver Steeg LJ et al (2013) A long-term toxicology study on pigs fed a combined genetically modified (GM) soy and GM maize diet. J Organic Systems 1:1–12
Google Scholar
Chen X, Gao MQ, Liang D et al (2017) Safety assessment of genetically modified milk containing human beta-defensin-3 on rats by a 90-day feeding study. Food Chem Toxicol 100:34–41. https://doi.org/10.1016/j.fct.2016.12.012
Chen YN, Hwang WZ, Fang TJ et al (2011) The impact of transgenic papaya (TPY10-4) fruit supplementation on immune responses in ovalbumin-sensitised mice. J Sci Food Agric 91:539–546. https://doi.org/10.1002/jsfa.4218
Chen ZL, Gu H, Li Y et al (2003) Safety assessment for genetically modified sweet pepper and tomato. Toxicology 188:297–307. https://doi.org/10.1016/s0300-483x(03)00111-2
Chukwudebe A, Privalle L, Reed A et al (2012) Health and nutritional status of Wistar rats following subchronic exposure to CV127 soybeans. Food Chem Toxicol 50:956–971. https://doi.org/10.1016/j.fct.2011.11.034
Cisterna B, Flach F, Vecchio L et al (2008) Can a genetically-modified organism-containing diet influence embryo development? A preliminary study on pre-implantation mouse embryos. Eur J Histochem 52:263–267. https://doi.org/10.4081/1226
Cyran N, Gülly C, Handl S et al (2008) Biological effects of transgenic maize NK603xMON810 fed in long term reproduction studies in mice. FiBL, Wien
de Vendômois JS, Roullier F, Cellier D et al (2009) A comparison of the effects of three GM corn varieties on mammalian health. Int J Biol Sci 5:706–726. https://doi.org/10.7150/ijbs.5.706
Delaney B, Appenzeller LM, Munley SM et al (2008) Subchronic feeding study of high oleic acid soybeans (Event DP-3Ø5423-1) in Sprague-Dawley rats. Food Chem Toxicol 46:3808–3817. https://doi.org/10.1016/j.fct.2008.10.003
EFSA Panel on Genetically Modified Organisms (GMO), Naegeli H, Birch AN et al (2018) Assessment of genetically modified soybean MON 87751 for food and feed uses under Regulation (EC) No 1829/2003 (application EFSA-GMO-NL-2014-121). EFSA J 16(8):e05346. https://doi.org/10.2903/j.efsa.2018.5346
El-Shamei ZS, Gab-Alla AA, Shatta AA (2012) Histopathological changes in some organs of male rats fed on genetically modified corn (Ajeeb YG). Am J Sci 10(8):684–696
Ermakova I (2005) Influence of genetically modified soya on the birth-weight and survival of rat pups. In: Epigenetics, transgenic plants& risk assessment.
Gao MQ, Zhang R, Yang Y et al (2018) A subchronic feeding safety evaluation of transgenic milk containing human β-defensin 3 on reproductive system of C57BL/6J mouse. Food Chem Toxicol 115:198–204. https://doi.org/10.1016/j.fct.2018.03.007
Gu J, Krogdahl Å, Sissener NH et al (2013) Effects of oral Bt-maize (MON810) exposure on growth and health parameters in normal and sensitised Atlantic salmon, Salmo salar L. Br J Nutr 109:1408–1423. https://doi.org/10.1017/S000711451200325X
Guo QY, He LX, Zhu H et al (2015) Effects of 90-day feeding of transgenic maize BT799 on the reproductive system in male Wistar rats. Int J Environ Res Public Health 12:15309–15320. https://doi.org/10.3390/ijerph121214986
Hammond B, Dudek R, Lemen J et al (2004) Results of a 13 week safety assurance study with rats fed grain from glyphosate tolerant corn. Food Chem Toxicol 42:1003–1014. https://doi.org/10.1016/j.fct.2004.02.013
Hammond B, Lemen J, Dudek R et al (2006) Results of a 90-day safety assurance study with rats fed grain from corn rootworm-protected corn. Food Chem Toxicol 44:147–160. https://doi.org/10.1016/j.fct.2005.06.008
He XY, Tang MZ, Luo YB et al (2009) A 90-day toxicology study of transgenic lysine-rich maize grain (Y642) in Sprague-Dawley rats. Food Chem Toxicol 47:425–432. https://doi.org/10.1016/j.fct.2008.11.032
Healy C, Hammond B, Kirkpatrick J (2008) Results of a 13-week safety assurance study with rats fed grain from corn rootworm-protected, glyphosate-tolerant MON 88017 corn. Food Chem Toxicol 46:2517–2524. https://doi.org/10.1016/j.fct.2008.04.005
Ibrahim MA, Okasha EF (2016) Effect of genetically modified corn on the jejunal mucosa of adult male albino rat. Exp Toxicol Pathol 68:579–588. https://doi.org/10.1016/j.etp.2016.10.001
Kiliç A, Akay MT (2008) A three generation study with genetically modified Bt corn in rats: Biochemical and histopathological investigation. Food Chem Toxicol 46:1164–1170. https://doi.org/10.1016/j.fct.2007.11.016
Kiliçgün H, Gürsul C, Sunar M et al (2013) The comparative effects of genetically modified maize and conventional maize on rats. J Clin Anal Med 4:136–139
Lee NJ, Yang BC, Hwang JS et al (2010) Effects of cloned-cattle meat diet on reproductive parameters in pregnant rabbits. Food Chem Toxicol 48:871–876. https://doi.org/10.1016/j.fct.2009.12.025
Lee NJ, Yang BC, Im GS et al (2013) No long-term feeding toxicities on the health status in rats fed with cloned Korean native beef cattle (Hanwoo) meat. Toxicol Pathol 41:872–879. https://doi.org/10.1177/0192623312470762
Lin HT, Lee WC, Tsai YT et al (2016) Subchronic immunotoxicity assessment of genetically modified virus-resistant papaya in rats. J Agric Food Chem 64:5935–5940. https://doi.org/10.1021/acs.jafc.6b02242
Liu P, He X, Chen D et al (2012) A 90-day subchronic feeding study of genetically modified maize expressing Cry1Ac-M protein in Sprague-Dawley rats. Food Chem Toxicol 50:3215–3221. https://doi.org/10.1016/j.fct.2012.06.009
Liu Q, Yang W, Li M et al (2017) Effects of 60-week feeding diet containing Bt rice expressing the Cry1Ab protein on the offspring of inbred Wuzhishan Pigs fed the same diet. J Agric Food Chem 65:10300–10309. https://doi.org/10.1021/acs.jafc.7b04067
Liu S, Li CX, Feng XL et al (2013) Safety assessment of meat from transgenic cattle by 90-day feeding study in rats. Food Chem Toxicol 57:314–321. https://doi.org/10.1016/j.fct.2013.04.00
Liu S, Liu HB, Wang HL et al (2019) Evaluation of behavioral profiles in mice fed with milk supplemented diets derived from human lactoferrin gene-modified cows. Regul Toxicol Pharmacol 104:133–140. https://doi.org/10.1016/j.yrtph.2019.03.008
Liu Y, Zhang S, Zhou Q et al (2020) Subchronic feeding toxicity studies of drought-tolerant transgenic wheat MGX11-10 in Wistar Han RCC rats. Food Chem Toxicol 137:111129. https://doi.org/10.1016/j.fct.2020.111129
MacKenzie SA, Lamb I, Schmidt J et al (2007) Thirteen week feeding study with transgenic maize grain containing event DAS-Ø15Ø7-1 in Sprague-Dawley rats. Food Chem Toxicol 45:551–562. https://doi.org/10.1016/j.fct.2006.09.016
Malatesta M, Boraldi F, Annovi G et al (2008) A long-term study on female mice fed on a genetically modified soybean: effects on liver ageing. Histochem Cell Biol 130(5):967–977. https://doi.org/10.1007/s00418-008-0476-x
Malatesta M, Caporaloni C, Gavaudan S et al (2002) Ultrastructural morphometrical and immunocytochemical analyses of hepatocyte nuclei from mice fed on genetically modified soybean [published correction appears in Cell Struct Funct. 2002 Oct;27(5):399]. Cell Struct Funct 27(4):173–180. https://doi.org/10.1247/csf.27.173
Malatesta M, Caporaloni C, Rossi L et al (2002) Ultrastructural analysis of pancreatic acinar cells from mice fed on genetically modified soybean. J Anat 201(5):409–415. https://doi.org/10.1046/j.0021-8782.2002.00103.x
Mao J, Sun X, Cheng JH et al (2016) A 52-week safety study in cynomolgus macaques for genetically modified rice expressing Cry1Ab/1Ac protein. Food Chem Toxicol 95:1–11. https://doi.org/10.1016/j.fct.2016.06.015
Nouri-Ellouz O, Zeghal N, Makni S et al (2015) New food from a potato somatic hybrid: nutritional equivalence and safety assessment by a feeding study on rats. J Sci Food Agric 95(9):1911–1917. https://doi.org/10.1002/jsfa.6898
Oliva N, Florida Cueto-Reaño M, Trijatmiko KR et al (2020) Molecular characterization and safety assessment of biofortified provitamin A rice. Sci Rep 10(1):1376. https://doi.org/10.1038/s41598-020-57669-5
Papineni S, Golden RM, Thomas J (2017) The aryloxyalkanoate dioxygenase-12 (AAD-12) protein is not acutely toxic in mice[J]. Food Chem Toxicol 110:200–203. https://doi.org/10.1016/j.fct.2017.10.036
Papineni S, Murray JA, Ricardo E et al (2017) Evaluation of the safety of a genetically modified DAS-444Ø6-6 soybean meal and hulls in a 90-day dietary toxicity study in rats. Food Chem Toxicol 109(Pt 1):245–252. https://doi.org/10.1016/j.fct.2017.08.048
Papineni S, Passage JK, Ekmay RD et al (2018) Evaluation of 30% DAS-444Ø6-6 soybean meal in a subchronic rat toxicity study. Regul Toxicol Pharmacol 94:57–69. https://doi.org/10.1016/j.yrtph.2018.01.005
Poulsen M, Kroghsbo S, Schrøder M et al (2007) A 90-day safety study in Wistar rats fed genetically modified rice expressing snowdrop lectin Galanthus nivalis (GNA). Food Chem Toxicol 45(3):350–363. https://doi.org/10.1016/j.fct.2006.09.002
Qian ZY, Zhang SJ, Zhang L et al (2018) Subchronic toxicity study in rats evaluating genetically modified DAS-81419-2 soybean. Regul Toxicol Pharmacol 96:48–56. https://doi.org/10.1016/j.yrtph.2018.04.019
Qian ZY, Bultman J, Papineni S et al (2018) Safety evaluation of DAS-44406-6 soybeans in Wistar rats. Regul Toxicol Pharmacol 92:152–164. https://doi.org/10.1016/j.yrtph.2017.11.016
Tudisco R, Calabrò S, Cutrignelli MI et al (2015) Genetically modified soybean in a goat diet: Influence on kid performance. Small Rumin Res 126:67–74. https://doi.org/10.1016/j.smallrumres.2015.01.023
Richards HA, Han CT, Hopkins RG et al (2003) Safety assessment of recombinant green fluorescent protein orally administered to weaned rats. J Nutr 133(6):1909–1912. https://doi.org/10.1093/jn/133.6.1909
Sanden M, Ornsrud R, Sissener NH et al (2013) Cross-generational feeding of Bt ( Bacillus thuringiensis )-maize to zebrafish ( Danio rerio ) showed no adverse effects on the parental or offspring generations. Br J Nutr 110(12):2222–2233. https://doi.org/10.1017/S0007114513001748
Schrøder M, Poulsen M, Wilcks A et al (2007) A 90-day safety study of genetically modified rice expressing Cry1Ab protein ( Bacillus thuringiensis toxin) in Wistar rats. Food Chem Toxicol 45(3):339–349. https://doi.org/10.1016/j.fct.2006.09.001
Séralini GE, Cellier D, de Vendomois JS (2007) New analysis of a rat feeding study with a genetically modified maize reveals signs of hepatorenal toxicity. Arch Environ Contam Toxicol 52(4):596–602. https://doi.org/10.1007/s00244-006-0149-5
Séralini GE, Clair E, Mesnage R et al (2014) Republished study: long-term toxicity of a roundup herbicide and a roundup-tolerant genetically modified maize. Environ Sci Eur 26(1):14. https://doi.org/10.1186/s12302-014-0014-5
Sheng Y, Qi X, Liu Y et al (2014) Subchronic toxicity study in vivo and allergenicity study in vitro for genetically modified rice that expresses pharmaceutical protein (human serum albumin). Food Chem Toxicol 72:242–246. https://doi.org/10.1016/j.fct.2014.07.030
EFSA Panel on Food Contact Materials, Enzymes, Processing Aids (EFSA CEP Panel) et al (2019) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Bacillus licheniformis (strain NZYM-CE). EFSA J 17(4):e05685. https://doi.org/10.2903/j.efsa.2019.5685 ( Published 2019 Apr 30 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Bacillus subtilis (strain LMG S-24584). EFSA J 16(10):e05447. https://doi.org/10.2903/j.efsa.2018.5447 ( Published 2018 September 27 )
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP), Silano V et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Aspergillus oryzae (strain NZYM-FA). EFSA J 16(11):e05480. https://doi.org/10.2903/j.efsa.2018.5480 ( Published 2018 Nov 16 )
EFSA Panel on Food Contact Materials, Enzymes and Processing Aids (CEP), Silano V et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Trichoderma reesei (strain DP-Nzd22). EFSA J 16(11):e05479. https://doi.org/10.2903/j.efsa.2018.5479 ( Published 2018 Nov 30 )
EFSA Panel on Food Contact Materials, Enzymes, Processing Aids (CEP) et al (2019) Safety evaluation of the food enzyme pullulanase from a genetically modified Bacillus licheniformis (strain DP-Dzp39). EFSA J 17(1):e05554. https://doi.org/10.2903/j.efsa.2019.5554 ( Published 2019 Jan 10 )
EFSA Panel on Food Contact Materials, Enzymes, Processing Aids (CEP) et al (2018) Safety evaluation of the food enzyme α-amylase from a genetically modified Aspergillus niger (strain NZYM-MC). EFSA J 16(10):e05451. https://doi.org/10.2903/j.efsa.2018.5451 ( Published 2018 Oct 31 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme alpha-amylase from a genetically modified Bacillus licheniformis (strain NZYM-AN). EFSA J 16(7):e05317. https://doi.org/10.2903/j.efsa.2018.5317 ( Published 2018 Jul 6 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme glucose oxidase from a genetically modified Aspergillus oryzae (strain NZYM-KP). EFSA J 16(7):e05319. https://doi.org/10.2903/j.efsa.2018.5319 ( Published 2018 Jul 6 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme endo-1,4-β-xylanase from a genetically modified Aspergillus niger (strain XEA). EFSA J 16(4):e05228. https://doi.org/10.2903/j.efsa.2018.5228 ( Published 2018 Apr 27 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme aqualysin 1 from a genetically modified Bacillus subtilis (strain LMGS 25520). EFSA J 16(5):e05170. https://doi.org/10.2903/j.efsa.2018.5170 ( Published 2018 May 2 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of the food enzyme xylanase from a genetically modified Bacillus subtilis strain TD160(229). EFSA J 16(1):e05008. https://doi.org/10.2903/j.efsa.2018.5008 ( Published 2018 Jan 22 )
EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF) et al (2018) Safety evaluation of food enzyme xylanase from a genetically modified Bacillus subtilis (strain LMG S-27588). EFSA J 16(5):e05169. https://doi.org/10.2903/j.efsa.2018.5169 ( Published 2018 May 2 )
Talyn B, Lemon R, Badoella M et al (2019) Roundup ®, but not roundup-ready ® corn, increases mortality of drosophila melanogaster. Toxics. https://doi.org/10.3390/toxics7030038
Tang M, Xie T, Cheng W et al (2012) A 90-day safety study of genetically modified rice expressing rhIGF-1 protein in C57BL/6J rats [published correction appears in Transgenic Res. 2012 Aug;21(4):927]. Transgenic Res 21(3):499–510. https://doi.org/10.1007/s11248-011-9550-6
Tang X, Han F, Zhao K et al (2012) A 90-day dietary toxicity study of genetically modified rice T1C–1 expressing Cry1C protein in Sprague Dawley rats. PLoS ONE 7(12):e52507. https://doi.org/10.1371/journal.pone.0052507
Teshima R, Watanabe T, Okunuki H et al (2002) Effect of subchronic feeding of genetically modified corn (CBH351) on immune system in BN rats and B10A mice. Shokuhin Eiseigaku Zasshi 43(5):273–279. https://doi.org/10.3358/shokueishi.43.273
Trabalza Marinucci M, Brandi G, Rondini C (2008) A three-year longitudinal study on the effects of a diet containing genetically modified Bt176 maize on the health status and performance of sheep. Livest Sci 2008(113):178–190
Walsh MC, Buzoianu SG, Gardiner GE et al (2011) Fate of transgenic DNA from orally administered Bt MON810 maize and effects on immune response and growth in pigs. PLoS ONE 6(11):e27177. https://doi.org/10.1371/journal.pone.0027177
Walsh MC, Buzoianu SG, Gardiner GE et al (2012) Effects of short-term feeding of Bt MON810 maize on growth performance, organ morphology and function in pigs. Br J Nutr 107(3):364–371. https://doi.org/10.1017/S0007114511003011
Walsh MC, Buzoianu SG, Rea MC et al (2012) Effects of feeding Bt MON810 maize to pigs for 110 days on peripheral immune response and digestive fate of the cry1Ab gene and truncated Bt toxin. PLoS ONE 7(5):e36141. https://doi.org/10.1371/journal.pone.0036141
Wang X, He X, Zou S et al (2016) A subchronic feeding study of dicamba-tolerant soybean with the dmo gene in Sprague-Dawley rats. Regul Toxicol Pharmacol 77:134–142. https://doi.org/10.1016/j.yrtph.2016.02.001
West AL, Miles EA, Lillycrop KA et al (2019) Postprandial incorporation of EPA and DHA from transgenic Camelina sativa oil into blood lipids is equivalent to that from fish oil in healthy humans. Br J Nutr 121(11):1235–1246. https://doi.org/10.1017/S0007114519000825
Wu Y, Xu Y, Du Y et al (2017) Dietary safety assessment of genetically modified rice EH rich in β-carotene. Regul Toxicol Pharmacol 88:66–71. https://doi.org/10.1016/j.yrtph.2017.05.019
Xiao GJ, Jiang SW, Qian LL et al (2016) A 90-day feeding study in rats to assess the safety of genetically engineered pork. PLoS ONE 11(11):e0165843. https://doi.org/10.1371/journal.pone.0165843
Xie Z, Zou S, Xu W et al (2018) No subchronic toxicity of multiple herbicide-resistant soybean FG72 in Sprague-Dawley rats by 90-days feeding study. Regul Toxicol Pharmacol 94:299–305. https://doi.org/10.1016/j.yrtph.2018.02.004
Yang L, Sun Y, Wang Y et al (2014) Effects of dietary transgenic poplar leaf pellets on performance and tissues in rabbits. J Sci Food Agric 94(6):1163–1167. https://doi.org/10.1002/jsfa.6388
Yen GC, Lin HT, Cheng YH et al (2011) Food safety evaluation of papaya fruits resistant to papaya ring spot virus. J Food Drug Anal 19(2):269–377
CAS Google Scholar
Yong L, Liu YM, Jia XD et al (2012) Subchronic toxicity study of GH transgenic carp. Food Chem Toxicol 50(11):3920–3926. https://doi.org/10.1016/j.fct.2012.07.064
Zeljenková D, Aláčová R, Ondrejková J et al (2016) One-year oral toxicity study on a genetically modified maize MON810 variety in Wistar Han RCC rats (EU 7th Framework Programme project GRACE). Arch Toxicol 90(10):2531–2562. https://doi.org/10.1007/s00204-016-1798-4
Zeljenková D, Ambrušová K, Bartušová M et al (2014) Ninety-day oral toxicity studies on two genetically modified maize MON810 varieties in Wistar Han RCC rats (EU 7th Framework Programme project GRACE). Arch Toxicol 88(12):2289–2314. https://doi.org/10.1007/s00204-014-1374-8
Zhou C, Wang JW, Huang KL et al (2011) A 90-day safety study in Sprague-Dawley rats fed milk powder containing recombinant human lactoferrin (rhLF) derived from transgenic cloned cattle. Drug Chem Toxicol 34(4):359–368. https://doi.org/10.3109/01480545.2010.542465
Zhou XH, Dong Y, Xiao X et al (2011) A 90-day toxicology study of high-amylose transgenic rice grain in Sprague-Dawley rats. Food Chem Toxicol 49:3112–3118. https://doi.org/10.1016/j.fct.2011.09.024
Zhu HJ, Chen Y, Li YH et al (2015) A 90 day safety assessment of genetically modified rice expressing Cry1Ab/1Ac protein using an aquatic animal model [published correction appears in J Agric Food Chem. 2015 Aug 26;63(33):7462]. J Agric Food Chem 63(14):3627–3633. https://doi.org/10.1021/jf5055547
Zhu Y, He X, Luo Y et al (2013) A 90-day feeding study of glyphosate-tolerant maize with the G2-aroA gene in Sprague-Dawley rats. Food Chem Toxicol 51:280–287. https://doi.org/10.1016/j.fct.2012.09.008
Zou S, Tang M, He X et al (2015) A 90-day subchronic study of rats fed lean pork from genetically modified pigs with muscle-specific expression of recombinant follistatin. Regul Toxicol Pharmacol 73(2):620–628. https://doi.org/10.1016/j.yrtph.2015.09.009
Dong SS, Zhang DN, Zhang ZH et al (2019) Ecotoxicological effects of transgenic mCry1Ac maize (BT799) on zebrafish. Ying Yong Sheng Tai Xue Bao 30(8):2845–2853. https://doi.org/10.13287/j.1001-9332.201908.031
Hu Y, Piao J, Yang X et al (2012) Nutritional components and sub-chronic toxicity of genetically modified rice expressing human lactoferrin. Wei Sheng Yan Jiu 41(1):6–12. https://doi.org/10.2166/wst.2012.090
Bai H (2015) Bio-Safety assessment of transgenic sheep overexpressing oTLR4. PhD Thesis, China Agricultural University, Beijing, China
Bao ZK (2016) Safety assessment of GH-Transgenic dairy goats. MS Thesis, Nanjing Agricultural University, Nanjing, China
Cao ZH (2014) Safety assessment of transgenic Bt rice in growing pig diet. MS Thesis, Henan University of Science and Technology, Henan, China
Chen XP, Zhuo Q, Piao JH et al (2004) Immunotoxicologic assessment of transgenetic rice. J Hyg Res 33(1):77–80
Chu HH, Si QQ, Xu Y et al (2016) Effect of genetically modified soybean meal on the immune function, intestinal digestive enzyme activities and serum biochemical indexes of growing pigs. J Qingdao Agric Univ Nat Sci 33(02):119–123. https://doi.org/10.3969/J.ISSN.1674-148X.2016.02.008
Dai YN, Yang XZ, Liu Y et al (2018) Acute oral toxicity and 90 days feeding test of recombinant human lactoferrin. J Hyg Res 47(02):286–311
Du HF (2006) Safety assessment of transgenic rice used in broiler diet. PhD Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Feng XL, Wang HL, Li CX et al (2017) Safety assessment of meat from transgenic cattle by 90-day feeding study in rats. J Hyg Res 29(1):19–25. https://doi.org/10.13590/j.cjfh.2017.01.005
Feng YQ, Hu J, Zhi Y et al (2013) The effect of exposure to transgenic Bt rice on the immune system of parental female rats. Chin J Food Hyg 25(4):298–302
Feng YQ, Wang EH, Zhi Y et al (2013) The effect of exposure to transgenic Bt rice on reproductive system of male offspring rats. Chin J Food Hyg 25(02):113–117
Guo MF (2018) A three generation study to evaluate reproductive and neurodevelopmental toxicity of genetically modified maize with Cry1Ab and epsps genes in SD rats. MS Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Hu J (2013) Establish of a rat model for development immunotoxicity and its application in safety evaluation of transgenic CryAb/Ac Rice. MS Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Huang Q, Xu HB, Gao F et al (2009) Anaphylactic reactions in WZS minipig orally induced by glycinin. J Hyg Res 38(05):531–534
Jia XD, Li N, Wang W et al (2005) Assessment of allergenicity of genetically modified rice S86 by BN rat model. Chin J Food Hyg 01:7–9. https://doi.org/10.13590/j.cjfh.2005.01.003
Li M (2012) Safety evaluation of recombinant herbicide-resistant protein AROa-CC-M and transgenic insect-resistant maize BT-799. MS Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Li M, Piao JH, Yang XG (2010) Subchronic toxicity test of genetically modified rice with double antisense starch-branching enzyme gene. J Hyg Res 39(04):436–443
Li R, Wang J, Jiang WL et al (2012) Effects of rice genetically modified with HJC-1 and G6-EPSPS genes on immunological parameters in Wuzhishan minipigs. J Environ Health 29(08):689–692. https://doi.org/10.16241/j.cnki.1001-5914.2012.08.017
Li YH, Piao JH, Chen XP et al (2004) Immunotoxicologic assessment on transgenic rice. China Public Health 20(04):20–22
Li YH, Piao JH, Zhuo Q et al (2004) Study on the teratogenicity effects of genetically modified rice with Xa21 on rats. J Hyg Res 33(06):710–712
Liang LQ, Wang J, Cao XC et al (2010) Toxicity analysis of common carp transferred salmon growth hormone gene. Food Sci 31(05):261–265
Liu HT, Wang CR, Liu L et al (2018) Acute toxicity of fresh leaves of insect resistant transgenic populus nigra to mice. J Anhui Agric Sci 46(01):94–136. https://doi.org/10.13989/j.cnki.0517-6611.2018.01.028
Liu HL, Wang J, Zeng Q et al (2012) Effects of rapeseed genetically modified with bar gene on immunological indicators in WZS minipigs. J Environ Health 29(11):977–980. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.020
Liu SS, Tan JZ, Sun Z et al (2011) Effects of glyphosate—resistant soybean meal on immune function of AA broilers. Chin J Anim Sci 47(13):41–46
Liu S, Wang XD, Feng XL et al (2013) Twenty-eight days feeding study on human lactoferrin expressed by cattle mammary bioreactor in mice. Chin J Public Health 29(02):230–232
Liu YH, Jiang SQ, Zhang J et al (2018) Subchronic toxicity of genetically modified Herbicide-resistant maize MON87427 with Cp4epsps gene in Wistar rats. J Public Health Prevent Med 29(06):17–20. https://doi.org/10.3969/j.issn.1006-2483.2018.06.004
Liu YF, Liu WH, He L et al (2008) Acute toxicity and mutagenic effect of transgenic rice on mice. J Hunan Univ Sci Technol Nat Sci Ed 23(04):111–117
Liu YF, Liu WH, He L et al (2008) Effects of resistant insects transgenic hybrid rice 21S/MSB on behavior and physiology of SD rats. Life Sci Res 12(03):257–261. https://doi.org/10.16605/j.cnki.1007-7847.2008.03.013
Lu MJ, Li F, Zhou GL et al (2008) Assessment of an anti-LeETR1 genetically modified tomato on reproductive-development toxicity and transferability of the transgene. J Toxicol 22(04):272–274. https://doi.org/10.16421/j.cnki.1002-3127.2008.04.006
Lu CB, Lin ZB, Zhang Y et al (2016) Effects of glyphosate-resistant transgenic soybean on physical enginery of male mice. Acta Agriculturae Zhejiangensis 28(07):1115–1120. https://doi.org/10.3969/j.issn.1004-1524.2016.07.04
Lu CB, Yang DY, Gao Z et al (2012) Safety assessment of reproductive system in male mice fed with genetically modified soybeans. J Yangzhou Univ Agric Life Sci Ed 33(01):23–27. https://doi.org/10.16872/j.cnki.1671-4652.2012.01.006
Lu CB, Zhang Y, Chen BH et al (2017) Effects of glyphosate-resistant transgenic soybean on in vitro fertilization of male mice with reproductive damage. Acta Agriculturae Zhejiangensis 29(06):910–916. https://doi.org/10.3969/j.issn.1004-1524.2017.06.08
Lu CB, Zhou W, Liu B et al (2013) Effects of transgenic soybean on reproductive system in male mice. Soybean Sci 32(01):119–123
Lv L, Guo J, Li SF et al (2013) Effects of transphytase gene maize on organ development and pathological changes of broilers. Chin J Anim Sci 49(05):31–34
Ma BT, Yuan XY, Wang XD et al (2017) Animal experiment of recombinant human lactoferrin based on the 28 days repeated oral toxicity. J Hyg Res 46(03):443–454
Ma YM, Wang J, Jiang WL et al (2012) Subchronic oral toxicity of genetically modified cottonseed with FBP7-iaaM gene in rats. J Environ Health 29(11):1001–1007. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.024
Qi XZ, Wang J, Zhou C et al (2010) Effect of transferred human lactoferrin milk powder on serum iron and ferritin in rats. Food Sci 31(23):340–343
Qin HF (2012) Safety assessment of rice genetically modified with Cry1Ac and sck feeding studies on broilers. PhD Thesis, Chinese Academy of Agricultural Sciences, Beijing, China.
Qiu ZL, Sun N, Wang J et al (2011) Sub-Chronic toxicity study of transgenic cottonseed in SD rats. Progr Mod Biomed 11(12):2215–2220. https://doi.org/10.13241/j.cnki.pmb.2011.12.010
Song LS, Gao GQ, Wei ZY et al (2017) Effects of Fat1 transgenic milk on the health and reproductive ability of mice. Lab Anim Sci 34(03):28–37
Song Y (2013) Establishment of immunotoxicity screening system in rodents and its application in immunotoxicity evaluation of pesticides and genetically modified foods. PhD Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Sun XW, Liang LQ, Yan XC et al (1998) Research on transgenic carp as food. High Technol Lett 03:3–5
Sun Z, Liu SS, Tan JZ et al (2011) Effects of glyphosate—resistant soybean meal on immune function of AA broilers. Chin J Anim Sci 23:836–841
Tan JZ (2011) The feed safety assessment of Glyphosate-Tolerant soybean meal in broilers. MS Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Tang XQ, Wang YF, Pei LJ et al (2019) Long-term toxicity study on transgenic rice T2A–1 with cry2A* gene. Chin J Food Hyg 31(6):510–516. https://doi.org/10.13590/j.jfh.2019.06.002
Tao R (2008) Detection of transgenic ingredients in feed and primary assessment on the safety of aquatic livestock fed transgenic soybean. MS Thesis, Chinese Academy of Sciences, Shandong, China
Wang EH (2014) A study to access effects of transgenic Cry1Ab/Ac Rice TT51 on reproductive and neural development in rats. PhD Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Wang EH, Yu Z, Fang HQ et al (2013) Effect of transgenic Bt rice TT51 on early physiological and neurological development of rats offspring. Chin J Food Hyg 25(06):485–488. https://doi.org/10.13590/j.cjfh.2013.06.008
Wang HM, Yin JY, Zhai WS, et al (2015) Toxic pathology of cynomolgus monkeys fed transgenic rice for 52 weeks. In: The 7th National Toxicology Conference of China Toxicology Society and the 8th Hubei Science and Technology Forum, Wuhan, China, pp 442–443
Wang J, Jiang WL, Wang XJ et al (2002) Toxicological safety evaluation of transgenic T5 line pepper. Chin J Urban Rural Ind Hyg 01:46
Wang J, Jiang WL, Wang Y et al (2012) Subacute oral toxicity of recombinant human lactoferrin from transgenic cows in rats on 28d. J Toxicol 26(05):393–397
Wang J, Li R, Liu HL (2012) Assessment of allergenicity of rice genetically modified with HJC-1 and G6-EPSPS genes by BN rats model. J Environ Health 29(11):967–970. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.014
Wang J, Liu HL, Zeng Q et al (2012) Subacute toxicity of rapeseed genetically modified with bar gene in WZS minipigs. J Environ Health 29(11):980–984. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.021
Wang J, Zhou C, Che HL et al (2010) Study on sub-chronic toxicity of powered milk containing transgenic lactoferrin on SD rats. Progr Mod Biomed 10(15):2809–2813. https://doi.org/10.13241/j.cnki.pmb.2010.15.002
Wang RY (2017) The effect of genetically modified feed on structure of mice testes. Livestock Poult Ind 28(06):6–7. https://doi.org/10.19567/j.cnki.1008-0414.2017.06.004
Wang R, Hu YC, Li M et al (2017) Study on the subchronic toxicity of transgenic DBN9978 herbicide resistant maize to rats. Food Nutr China 23(06):12–17
Wang XJ, Wang J, Liu HL et al (2012) Subchronic toxicity test of rice containing transgenic HJC-1 and G6-EPSPS in rats. J Environ Health 29(11):970–976. https://doi.org/10.16241/j.cnki.1001-5914.2012.11.019
Wang Y (2011) Food safety assessment of genetically modified milk with human lactoferrin gene. MS Thesis, Tianjin Medical University, Tianjin, China
Wang Y, Lai WQ, Chen JG et al (2000) Toxicity of anti-herbicide gene (BAR) transgenic rice. J Hyg Res 29(03):141–142
Wu JH (2013) The nutritional, edible safety and efficacy assessment of genetically modified rice with human lactoferrin gene and its purified protein and the nutritional assessment of genetically modified wheat expressing GmDREB/TaDREB4 genes with drought-resistance. Ph.D. Thesis, Chinese Center for Disease Control and Prevention, Beijing, China
Wu P, Su YL, Zhang J et al (2003) Safety evaluation of transgenic tomato against Cucumber Mosaic Virus. J Capital Univ Med Sci 24(03):254–258
Xu YJ (2012) The forage safety assessment of genetically modified organism corn to weaning piglets. M.S. Thesis, Fujian Agriculture and Forestry University, Fujian, China
Yu T, Liu Y, Wang JW et al (2017) Teratogenic test of recombinant human lactoferrin in rats. J Toxicol 31(03):247–250
Yuan JQ (2015) The detection of the transgenic GTS40-3-2 related genes and their products of livestock products sold and toxicology study of Sprague-Dawley rats ( Rattus norvegicus ). Ph.D. Thesis, Shanxi Agricultural University, Shanxi, China
Zhang L, Cheng C, He N et al (2011) Study on subchronic toxicity of transgenic soybean with high oleic acid on rats. J Toxicol 25(05):391–394. https://doi.org/10.16421/j.cnki.1002-3127.2011.05.012
Zhang L, Wang J, Jiang SQ et al (2016) Subchronic toxicity of genetically modified corn with Cry1Ab/Cry2Aj and G10evo (EPSPS) genes in rats. J Environ Health 33(07):585–589
Zhang LL (2018) The Unintended Effects of Long-Term Intergenerational Feeding Transgenic Maize Diets to Pure Line White Leghorn Chickens on the Intestinal Health. M.S. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China
Zhang M, Zhuo Q, Tian Y et al (2012) Study on chronic toxicity of genetically modified rice expressing human lactoferrin. Chin J Food Hyg 24(06):391–394. https://doi.org/10.13590/j.cjfh.2012.06.008
Zhang Q (2014) Production of GH transgenic goat by somatic cell nuclear transfer. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China
Zhang WP, Li YY, Wang WG et al (2009) Detection of mutagenicity of chitinase and -1, 3 dextran gene in maize. Chin Remed Clin 9(05):405–407
Zhang ZY, Liu LJ, Zhang L et al (2010) Subchronic toxicity of Bt transgenic rice to mice. J Toxicol 24(02):126–129. https://doi.org/10.16421/j.cnki.1002-3127.2010.02.016
Zhao L, Zhang L, Zhang YY et al (2009) Immunotoxicological evaluation of transgenic soybean oil. China Health Care Nutr 11:5
Zhi Y, Liu HB, Di GY et al (2013) Genetic toxicity of transgenic human α-lactalbumin powdered milk. Carcinogen Teratogen Mutagen 25(02):124–133. https://doi.org/10.3969/j.issn.1004-616x.2013.02.010
Zhi Y, Liu HB, Di GY et al (2011) Study on sub-chronic toxicity of powered milk containing transgenic human α-lactalbumin. J Hyg Res 40(04):426–430. https://doi.org/10.19813/j.cnki.weishengyanjiu.2011.04.004
Zhong F (2013) Subchronic feeding study of transgenic BADH alfalfa on rabbits. M.S. Thesis, Shandong Agricultural University, Shandong, China
Zhou GL, Lu MJ, Chen YX et al (2007) Antisense LeETR1 transgenic tomato rats were fed for 4 weeks. J Toxicol 21(02):160–161
Zhou H (2012) Safety aeeseement of the phytase transgenic corn in the broiler diet. M.S. Thesis, Fujian Agriculture and Forestry University, Fujian, China
Zhou LG (2009) Research of high lysine transgenic paddy on broiler feed security. M.S. Thesis, Yangzhou University, Jiangsu, China
Zhou WL, Yang XF, Ping A et al (2014) Effects of transgenic Dwarf-mosaic-resistant maize on learning and memory abilities of rats. J Shanxi Agric Univ Nat Sci Ed 34(02):129–131. https://doi.org/10.13842/j.cnki.issn1671-8151.2014.02.006
Zhou XH (2012) Toxicological studies on the food safety of two transgenic rice. Ph.D. Thesis, Jiangsu University, Jiangsu, China
Zhou ZW, Wang DZ, Shen H et al (2012) Comprehensive evaluation on functions & safety of imported GM soybean using BDI-GS system. Soybean Sci 31(05):822–826
Zhu H, Zhu LY, Guo JY et al (2014) Safety evaluation of BT-799 corn on Wistar rats. Food Nutr China 20(09):63–67
Zhuo Q, Chen XP, Piao JH et al (2004) Experimental study on converting cowpea trypsin inhibitor rice for 90 days. J Hyg Res 33(02):176–179
Zhuo Q, Chen XP, Piao JH et al (2004) Study on teratogenic effect of cowpea trypsin inhibitor rice. J Hyg Res 33(01):74–77
Sakamoto Y, Tada Y, Fukumori N et al (2008) A 104-week feeding study of genetically modified soybeans in F344 rats. Shokuhin Eiseigaku Zasshi 49(4):272–282. https://doi.org/10.3358/shokueishi.49.272
Sakamoto Y, Tada Y, Fukumori N et al (2007) A 52-week feeding study of genetically modified soybeans in F344 rats. Shokuhin Eiseigaku Zasshi 48(3):41–50. https://doi.org/10.3358/shokueishi.48.41
Keshani P, Sharifi MH, Heydari MR et al (2020) The effect of genetically modified food on infertility indices: a systematic review study. Sci World J. https://doi.org/10.1155/2020/1424789
Edge MS, Kunkel ME, Schmidt J et al (2018) Evidence analysis library systematic review on advanced technology in food production. J Acad Nutr Diet 118(6):1106–1127. https://doi.org/10.1016/j.jand.2017.08.005
Dunn SE, Vicini JL, Glenn KC et al (2017) The allergenicity of genetically modified foods from genetically engineered crops: a narrative and systematic review. Ann Allergy Asthma Immunol 119(3):214–222. https://doi.org/10.1016/j.anai.2017.07.010
de Vos CJ, Swanenburg M (2018) Health effects of feeding genetically modified (GM) crops to livestock animals: a review. Food Chem Toxicol 117:3–12. https://doi.org/10.1016/j.fct.2017.08.031
Ricroch AE, Boisron A, Kuntz M (2014) Looking back at safety assessment of GM food/feed: an exhaustive review of 90-day animal feeding studies. Int J Biotechnology 13(4):230–256
Domingo Roig JL, Gómez Arnáiz M (2000) Riesgos sobre la salud de los alimentos modificados genéticamente: una revisión bibliográfica [Health risks of genetically modified foods: a literature review]. Revista espanola de salud publica 74(3):255–261
Domingo JL (2007) Toxicity studies of genetically modified plants: a review of the published literature. Crit Rev Food Sci Nutr 47(8):721–733. https://doi.org/10.1080/10408390601177670
Domingo JL, Giné Bordonaba J (2011) A literature review on the safety assessment of genetically modified plants. Environ Int 37(4):734–742. https://doi.org/10.1016/j.envint.2011.01.003
Teshima R, Watanabe T, Okunuki H et al (2002) Effect of subchronic feeding of genetically modified corn (CBH351) on immune system in BN rats and B10A mice. J Food Hyg Soc Jpn 43:273–279
Dubois AEJ, Pagliarani G, Brouwer RM et al (2015) First successful reduction of clinical allergenicity of food by genetic modification: Mal d 1-silenced apples cause fewer allergy symptoms than the wild-type cultivar. Allergy 70:1406–1412
Download references
Acknowledgements
We appreciate Yi-Zhen Li for participating in screening the titles and abstracts .
This work was supported by Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-C-202006). Prof. Nicola Robinson (visiting professor of Beijing University of Chinese Medicine) was funded by the International development and capacity enhancement of evidence-based Chinese medicine Project, Ministry of Science and Technology of the People's Republic of China, G20200001187.
Author information
Authors and affiliations.
Centre for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, 11 Bei San Huan Dong Lu, Chaoyang District, Beijing, 100029, China
Chen Shen, Xiao-Wen Zhang, Wen-Bin Hou, Min Fang, Xun Li, Yu-Tong Fei, Nicola Robinson & Jian-Ping Liu
Beijing Institute of Radiation Medicine, Beijing, China
Xiang-Chang Yin
School of Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Bo-Yang Jiao
Beijing Key Laboratory of the Innovative Development of Functional Staple and the Nutritional Intervention for Chronic Disease, China National Research Institute of Food & Fermentation Industries Co,. Ltd, Beijing, China
School of Life Sciences, Beijing University of Chinese Medicine, Beijing, China
School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, China
Xue-Hao Cheng & Jian-Xin Ren
Ruikang Hospital, Guangxi University of Chinese Medicine, Guangxi, China
School of Health and Social Care, London South Bank University, London, SE1 OAA, UK
Nicola Robinson
Institute for Excellence in Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
Jian-Ping Liu
You can also search for this author in PubMed Google Scholar
Contributions
All work was done by the authors. JPL and YTF conceived the study and revised the manuscript. CS contributed to data searching, screening and extraction, analysis of the data, drafted and revised the paper and approved the final version to be submitted. XCY, BYJ, JP, XHC, JXR, JL, XWZ, HDL, WBH and MF participated in identifying or screening the titles, abstracts and full-text screening and data extraction. XL, NR and JPL advised on the analysis of the data and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Correspondence to Jian-Ping Liu .
Ethics declarations
Ethics approval and consent to participate.
Not applicable.
Consent for publication
Competing interests.
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix s1..
Search strategy applied in English language databases. Appendix S2. Funding sources or sponsors. Appendix S3. Adverse events/effects—other biomarkers.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Shen, C., Yin, XC., Jiao, BY. et al. Evaluation of adverse effects/events of genetically modified food consumption: a systematic review of animal and human studies. Environ Sci Eur 34 , 8 (2022). https://doi.org/10.1186/s12302-021-00578-9
Download citation
Received : 07 July 2021
Accepted : 11 December 2021
Published : 13 January 2022
DOI : https://doi.org/10.1186/s12302-021-00578-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Adverse event
- Genetically modified food
- Evidence-based medicine
- Systematic review
September 1, 2013
13 min read
The Truth about Genetically Modified Food
Proponents of genetically modified crops say the technology is the only way to feed a warming, increasingly populous world. Critics say we tamper with nature at our peril. Who is right?
By David H. Freedman
Robert Goldberg sags into his desk chair and gestures at the air. “Frankenstein monsters, things crawling out of the lab,” he says. “This the most depressing thing I've ever dealt with.”
Goldberg, a plant molecular biologist at the University of California, Los Angeles, is not battling psychosis. He is expressing despair at the relentless need to confront what he sees as bogus fears over the health risks of genetically modified (GM) crops. Particularly frustrating to him, he says, is that this debate should have ended decades ago, when researchers produced a stream of exonerating evidence: “Today we're facing the same objections we faced 40 years ago.”
Across campus, David Williams, a cellular biologist who specializes in vision, has the opposite complaint. “A lot of naive science has been involved in pushing this technology,” he says. “Thirty years ago we didn't know that when you throw any gene into a different genome, the genome reacts to it. But now anyone in this field knows the genome is not a static environment. Inserted genes can be transformed by several different means, and it can happen generations later.” The result, he insists, could very well be potentially toxic plants slipping through testing.
On supporting science journalism
If you're enjoying this article, consider supporting our award-winning journalism by subscribing . By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Williams concedes that he is among a tiny minority of biologists raising sharp questions about the safety of GM crops. But he says this is only because the field of plant molecular biology is protecting its interests. Funding, much of it from the companies that sell GM seeds, heavily favors researchers who are exploring ways to further the use of genetic modification in agriculture. He says that biologists who point out health or other risks associated with GM crops—who merely report or defend experimental findings that imply there may be risks—find themselves the focus of vicious attacks on their credibility, which leads scientists who see problems with GM foods to keep quiet.
Whether Williams is right or wrong, one thing is undeniable: despite overwhelming evidence that GM crops are safe to eat, the debate over their use continues to rage, and in some parts of the world, it is growing ever louder. Skeptics would argue that this contentiousness is a good thing—that we cannot be too cautious when tinkering with the genetic basis of the world's food supply. To researchers such as Goldberg, however, the persistence of fears about GM foods is nothing short of exasperating. “In spite of hundreds of millions of genetic experiments involving every type of organism on earth,” he says, “and people eating billions of meals without a problem, we've gone back to being ignorant.”
So who is right: advocates of GM or critics? When we look carefully at the evidence for both sides and weigh the risks and benefits, we find a surprisingly clear path out of this dilemma.
Benefits and worries
The bulk of the science on GM safety points in one direction. Take it from David Zilberman, a U.C. Berkeley agricultural and environmental economist and one of the few researchers considered credible by both agricultural chemical companies and their critics. He argues that the benefits of GM crops greatly outweigh the health risks, which so far remain theoretical. The use of GM crops “has lowered the price of food,” Zilberman says. “It has increased farmer safety by allowing them to use less pesticide. It has raised the output of corn, cotton and soy by 20 to 30 percent, allowing some people to survive who would not have without it. If it were more widely adopted around the world, the price [of food] would go lower, and fewer people would die of hunger.”
In the future, Zilberman says, those advantages will become all the more significant. The United Nations Food and Agriculture Organization estimates that the world will have to grow 70 percent more food by 2050 just to keep up with population growth. Climate change will make much of the world's arable land more difficult to farm. GM crops, Zilberman says, could produce higher yields, grow in dry and salty land, withstand high and low temperatures, and tolerate insects, disease and herbicides.
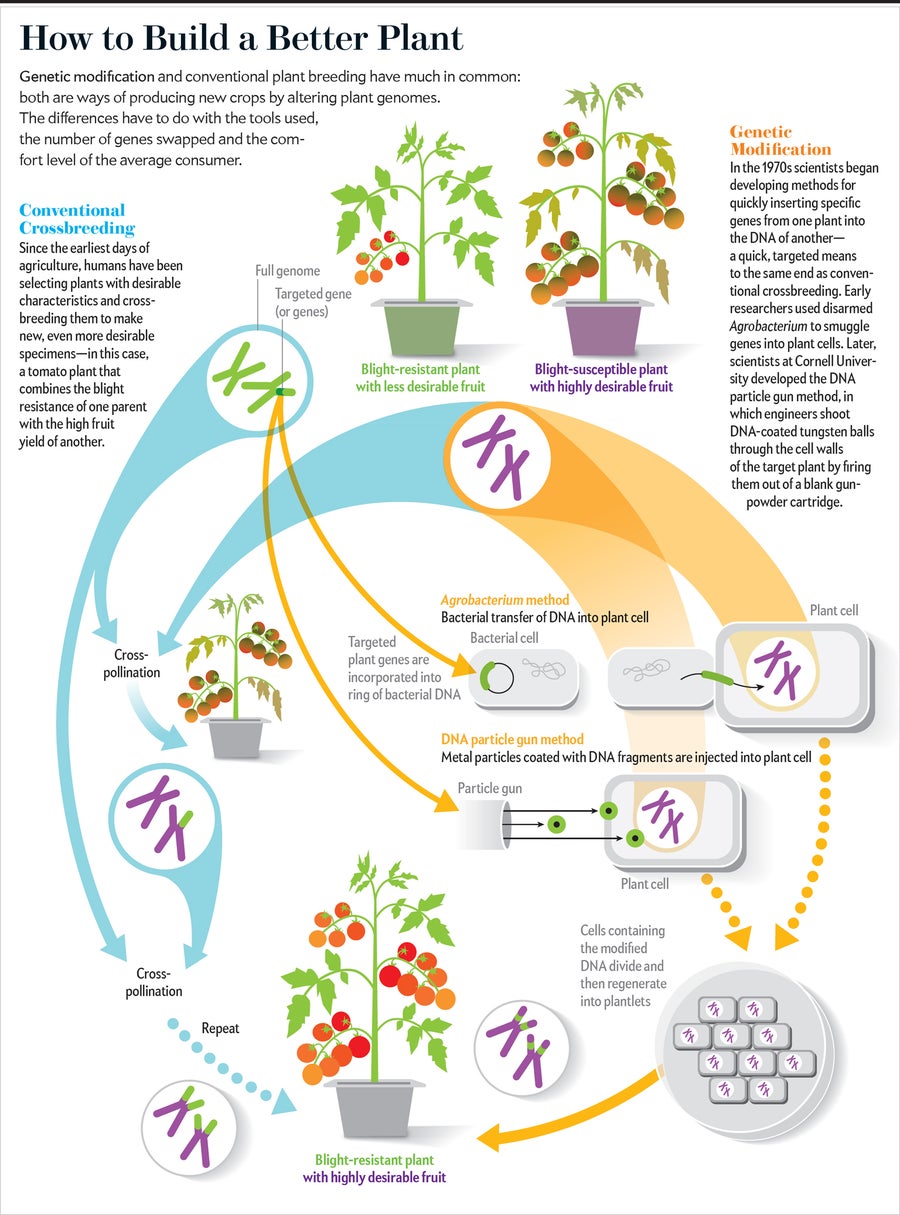
Credit: Jen Christiansen
Despite such promise, much of the world has been busy banning, restricting and otherwise shunning GM foods. Nearly all the corn and soybeans grown in the U.S. are genetically modified, but only two GM crops, Monsanto's MON810 maize and BASF's Amflora potato, are accepted in the European Union. Ten E.U. nations have banned MON810, and although BASF withdrew Amflora from the market in 2012, four E.U. nations have taken the trouble to ban that, too. Approval of a few new GM corn strains has been proposed there, but so far it has been repeatedly and soundly voted down. Throughout Asia, including in India and China, governments have yet to approve most GM crops, including an insect-resistant rice that produces higher yields with less pesticide. In Africa, where millions go hungry, several nations have refused to import GM foods in spite of their lower costs (the result of higher yields and a reduced need for water and pesticides). Kenya has banned them altogether amid widespread malnutrition. No country has definite plans to grow Golden Rice, a crop engineered to deliver more vitamin A than spinach (rice normally has no vitamin A), even though vitamin A deficiency causes more than one million deaths annually and half a million cases of irreversible blindness in the developing world.
Globally, only a tenth of the world's cropland includes GM plants. Four countries—the U.S., Canada, Brazil and Argentina—grow 90 percent of the planet's GM crops. Other Latin American countries are pushing away from the plants. And even in the U.S., voices decrying genetically modified foods are becoming louder. In 2016 the U.S. federal government passed a law requiring labeling of GM ingredients in food products, replacing GM-labeling laws in force or proposed in several dozen states.
The fear fueling all this activity has a long history. The public has been worried about the safety of GM foods since scientists at the University of Washington developed the first genetically modified tobacco plants in the 1970s. In the mid-1990s, when the first GM crops reached the market, Greenpeace, the Sierra Club, Ralph Nader, Prince Charles and a number of celebrity chefs took highly visible stands against them. Consumers in Europe became particularly alarmed: a survey conducted in 1997, for example, found that 69 percent of the Austrian public saw serious risks in GM foods, compared with only 14 percent of Americans.
In Europe, skepticism about GM foods has long been bundled with other concerns, such as a resentment of American agribusiness. Whatever it is based on, however, the European attitude reverberates across the world, influencing policy in countries where GM crops could have tremendous benefits. “In Africa, they don't care what us savages in America are doing,” Zilberman says. “They look to Europe and see countries there rejecting GM, so they don't use it.” Forces fighting genetic modification in Europe have rallied support for “the precautionary principle,” which holds that given the kind of catastrophe that would emerge from loosing a toxic, invasive GM crop on the world, GM efforts should be shut down until the technology is proved absolutely safe.
But as medical researchers know, nothing can really be “proved safe.” One can only fail to turn up significant risk after trying hard to find it—as is the case with GM crops.
A clean record
The human race has been selectively breeding crops, thus altering plants' genomes, for millennia. Ordinary wheat has long been strictly a human-engineered plant; it could not exist outside of farms, because its seeds do not scatter. For some 60 years scientists have been using “mutagenic” techniques to scramble the DNA of plants with radiation and chemicals, creating strains of wheat, rice, peanuts and pears that have become agricultural mainstays. The practice has inspired little objection from scientists or the public and has caused no known health problems.
The difference is that selective breeding or mutagenic techniques tend to result in large swaths of genes being swapped or altered. GM technology, in contrast, enables scientists to insert into a plant's genome a single gene (or a few of them) from another species of plant or even from a bacterium, virus or animal. Supporters argue that this precision makes the technology much less likely to produce surprises. Most plant molecular biologists also say that in the highly unlikely case that an unexpected health threat emerged from a new GM plant, scientists would quickly identify and eliminate it. “We know where the gene goes and can measure the activity of every single gene around it,” Goldberg says. “We can show exactly which changes occur and which don't.”
And although it might seem creepy to add virus DNA to a plant, doing so is, in fact, no big deal, proponents say. Viruses have been inserting their DNA into the genomes of crops, as well as humans and all other organisms, for millions of years. They often deliver the genes of other species while they are at it, which is why our own genome is loaded with genetic sequences that originated in viruses and nonhuman species. “When GM critics say that genes don't cross the species barrier in nature, that's just simple ignorance,” says Alan McHughen, a plant molecular geneticist at U.C. Riverside. Pea aphids contain fungi genes. Triticale is a century-plus-old hybrid of wheat and rye found in some flours and breakfast cereals. Wheat itself, for that matter, is a cross-species hybrid. “Mother Nature does it all the time, and so do conventional plant breeders,” McHughen says.
Could eating plants with altered genes allow new DNA to work its way into our own? It is possible but hugely improbable. Scientists have never found genetic material that could survive a trip through the human gut and make it into cells. Besides, we are routinely exposed to—and even consume—the viruses and bacteria whose genes end up in GM foods. The bacterium Bacillus thuringiensis , for example, which produces proteins fatal to insects, is sometimes enlisted as a natural pesticide in organic farming. “We've been eating this stuff for thousands of years,” Goldberg says.
In any case, proponents say, people have consumed as many as trillions of meals containing genetically modified ingredients over the past few decades. Not a single verified case of illness has ever been attributed to the genetic alterations. Mark Lynas, a prominent anti-GM activist who in 2013 publicly switched to strongly supporting the technology, has pointed out that every single news-making food disaster on record has been attributed to non-GM crops, such as the Escherichia coli –infected organic bean sprouts that killed 53 people in Europe in 2011.
Critics often disparage U.S. research on the safety of genetically modified foods, which is often funded or even conducted by GM companies, such as Monsanto. But much research on the subject comes from the European Commission, the administrative body of the E.U., which cannot be so easily dismissed as an industry tool. The European Commission has funded 130 research projects, carried out by more than 500 independent teams, on the safety of GM crops. None of those studies found any special risks from GM crops.
Plenty of other credible groups have arrived at the same conclusion. Gregory Jaffe, director of biotechnology at the Center for Science in the Public Interest, a science-based consumer-watchdog group in Washington, D.C., takes pains to note that the center has no official stance, pro or con, with regard to genetically modifying food plants. Yet Jaffe insists the scientific record is clear. “Current GM crops are safe to eat and can be grown safely in the environment,” he says. The American Association for the Advancement of Science, the American Medical Association and the National Academy of Sciences have all unreservedly backed GM crops. The U.S. Food and Drug Administration, along with its counterparts in several other countries, has repeatedly reviewed large bodies of research and concluded that GM crops pose no unique health threats. Dozens of review studies carried out by academic researchers have backed that view.
Opponents of genetically modified foods point to a handful of studies indicating possible safety problems. But reviewers have dismantled almost all of those reports. For example, a 1998 study by plant biochemist Árpád Pusztai, then at the Rowett Institute in Scotland, found that rats fed a GM potato suffered from stunted growth and immune system–related changes. But the potato was not intended for human consumption—it was, in fact, designed to be toxic for research purposes. The Rowett Institute later deemed the experiment so sloppy that it refuted the findings and charged Pusztai with misconduct.
Similar stories abound. Most recently, a team led by Gilles-Éric Séralini, a researcher at the University of Caen Lower Normandy in France, found that rats eating a common type of GM corn contracted cancer at an alarmingly high rate. But Séralini has long been an anti-GM campaigner, and critics charged that in his study, he relied on a strain of rat that too easily develops tumors, did not use enough rats, did not include proper control groups and failed to report many details of the experiment, including how the analysis was performed. After a review, the European Food Safety Authority dismissed the study's findings. Several other European agencies came to the same conclusion. “If GM corn were that toxic, someone would have noticed by now,” McHughen says. “Séralini has been refuted by everyone who has cared to comment.”
Some scientists say the objections to GM food stem from politics rather than science—that they are motivated by an objection to large multinational corporations having enormous influence over the food supply; invoking risks from genetic modification just provides a convenient way of whipping up the masses against industrial agriculture. “This has nothing to do with science,” Goldberg says. “It's about ideology.” Former anti-GM activist Lynas agrees. He has gone as far as labeling the anti-GM crowd “explicitly an antiscience movement.”
Persistent doubts
Not all objections to genetically modified foods are so easily dismissed, however. Long-term health effects can be subtle and nearly impossible to link to specific changes in the environment. Scientists have long believed that Alzheimer's disease and many cancers have environmental components, but few would argue we have identified all of them.
And opponents say that it is not true that the GM process is less likely to cause problems simply because fewer, more clearly identified genes are replaced. David Schubert, an Alzheimer's researcher who heads the Cellular Neurobiology Laboratory at the Salk Institute for Biological Studies in La Jolla, Calif., asserts that a single, well-characterized gene can still settle in the target plant's genome in many different ways. “It can go in forward, backward, at different locations, in multiple copies, and they all do different things,” he says. And as U.C.L.A.'s Williams notes, a genome often continues to change in the successive generations after the insertion, leaving it with a different arrangement than the one intended and initially tested. There is also the phenomenon of “insertional mutagenesis,” Williams adds, in which the insertion of a gene ends up quieting the activity of nearby genes.
True, the number of genes affected in a GM plant most likely will be far, far smaller than in conventional breeding techniques. Yet opponents maintain that because the wholesale swapping or alteration of entire packages of genes is a natural process that has been happening in plants for half a billion years, it tends to produce few scary surprises today. Changing a single gene, on the other hand, might turn out to be a more subversive action, with unexpected ripple effects, including the production of new proteins that might be toxins or allergens.
Opponents also point out that the kinds of alterations caused by the insertion of genes from other species might be more impactful, more complex or more subtle than those caused by the intraspecies gene swapping of conventional breeding. And just because there is no evidence to date that genetic material from an altered crop can make it into the genome of people who eat it does not mean such a transfer will never happen—or that it has not already happened and we have yet to spot it. These changes might be difficult to catch; their impact on the production of proteins might not even turn up in testing. “You'd certainly find out if the result is that the plant doesn't grow very well,” Williams says. “But will you find the change if it results in the production of proteins with long-term effects on the health of the people eating it?”
It is also true that many pro-GM scientists in the field are unduly harsh—even unscientific—in their treatment of critics. GM proponents sometimes lump every scientist who raises safety questions together with activists and discredited researchers. And even Séralini, the scientist behind the study that found high cancer rates for GM-fed rats, has his defenders. Most of them are nonscientists, or retired researchers from obscure institutions, or nonbiologist scientists, but the Salk Institute's Schubert also insists the study was unfairly dismissed. He says that as someone who runs drug-safety studies, he is well versed on what constitutes a good-quality animal toxicology study and that Séralini's makes the grade. He insists that the breed of rat in the study is commonly used in respected drug studies, typically in numbers no greater than in Séralini's study; that the methodology was standard; and that the details of the data analysis are irrelevant because the results were so striking.
Schubert joins Williams as one of a handful of biologists from respected institutions who are willing to sharply challenge the GM-foods-are-safe majority. Both charge that more scientists would speak up against genetic modification if doing so did not invariably lead to being excoriated in journals and the media. These attacks, they argue, are motivated by the fear that airing doubts could lead to less funding for the field. Says Williams: “Whether it's conscious or not, it's in their interest to promote this field, and they're not objective.”
Both scientists say that after publishing comments in respected journals questioning the safety of GM foods, they became the victims of coordinated attacks on their reputations. Schubert even charges that researchers who turn up results that might raise safety questions avoid publishing their findings out of fear of repercussions. “If it doesn't come out the right way,” he says, “you're going to get trashed.”
There is evidence to support that charge. In 2009 Nature detailed the backlash to a reasonably solid study published in the Proceedings of the National Academy of Sciences USA by researchers from Loyola University Chicago and the University of Notre Dame. The paper showed that GM corn seemed to be finding its way from farms into nearby streams and that it might pose a risk to some insects there because, according to the researchers' lab studies, caddis flies appeared to suffer on diets of pollen from GM corn. Many scientists immediately attacked the study, some of them suggesting the researchers were sloppy to the point of misconduct.
A way forward
There is a middle ground in this debate. Many moderate voices call for continuing the distribution of GM foods while maintaining or even stepping up safety testing on new GM crops. They advocate keeping a close eye on the health and environmental impact of existing ones. But they do not single out GM crops for special scrutiny, the Center for Science in the Public Interest's Jaffe notes: all crops could use more testing. “We should be doing a better job with food oversight altogether,” he says.
Even Schubert agrees. In spite of his concerns, he believes future GM crops can be introduced safely if testing is improved. “Ninety percent of the scientists I talk to assume that new GM plants are safety-tested the same way new drugs are by the FDA,” he says. “They absolutely aren't, and they absolutely should be.”
Stepped-up testing would pose a burden for GM researchers, and it could slow down the introduction of new crops. “Even under the current testing standards for GM crops, most conventionally bred crops wouldn't have made it to market,” McHughen says. “What's going to happen if we become even more strict?”
That is a fair question. But with governments and consumers increasingly coming down against GM crops altogether, additional testing may be the compromise that enables the human race to benefit from those crops' significant advantages.
David H. Freedman is a journalist who has been covering science, business and technology for more than 30 years.

share this!
May 8, 2023
This article has been reviewed according to Science X's editorial process and policies . Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
written by researcher(s)
What's the latest on GMOs and gene-edited foods—and what are the concerns? An expert explains
by Karen Massel, The Conversation

Advances in genetic engineering have given rise to an era of foods—including genetically modified organisms (GMOs) and gene-edited foods—that promise to revolutionize the way we eat.
Critics argue these foods could pose risks to human health and the environment. Proponents point to their potential for enhancing yields, reducing food waste , and even combating climate change.
What are GMOs and gene-edited foods? And how are they shaping the future of our food systems?
GMOs and gene-edited foods aren't the same
GMOs are organisms whose genetic material has been artificially altered by inserting a piece of foreign DNA. This DNA may be synthetic in origin or sourced from other organisms.
Gene editing involves making precise changes to an organism's genome without the integration of foreign DNA elements. Using techniques such as CRISPR/Cas, scientists make precise "cuts" in the DNA to create new genetic variation. Unlike with GMOs, this introduces only minor modifications, which are indistinguishable from natural mutations.
Although GMOs and gene-edited foods have been in circulation for almost three decades, research in this space continues to deliver breakthroughs. These technologies are being applied to provide a range of benefits, from improved nutrition in food, to reduced food waste and increased crop tolerance against climate stresses.
What are the concerns?
The major criticisms of GMOs are related to the overuse of specific herbicides.
GMOs are mainly used to produce crops that are herbicide-resistant or produce pesticides. Farmers can then use herbicides on those crops to control weeds more effectively, without the plants themselves dying. This leads to higher yields on less land, and often with less chemicals used overall.
However, these crops rely on the use of said lab-made chemicals . And although the government regulates them, ethical and safety debates continue. People raise concerns over potential long-term health impacts, impacts on biodiversity and ecosystems, and the increased corporate control over agriculture.
Concerns generally aren't related to the actual manipulation of the plants' DNA.
Is genetic modification itself unsafe?
When it comes to the food we eat, how much do we really know about its DNA? Even among experts with genome-sequencing information, most have only one or a few sequenced "reference" varieties, and these often aren't the same as the plants we eat.
The fact is, we don't really understand the genomes of many plants and animals we eat. So there's no reason to suggest tweaking their gene sequences will make consumption harmful. Moreover, there's currently no evidence regulator-approved GMOs or gene-edited foods aren't safe for human consumption.
In regards to food safety, one valid concern would be the potential creation of new allergens: proteins within the crop the body recognizes and creates an immune response to.
But it's important to remember many foods we eat are already allergenic. Common examples include wheat, peanuts, soy, milk and eggs. Some common foods are even toxic if consumed in large quantities or without appropriate preparation, such as rhubarb leaves, raw cassava, raw kidney beans and raw cashews.
Ironically, researchers are using gene editing to work towards eliminating proteins that cause allergies and intolerances. Gluten-free wheat is one example.
GMOs and gene-edited foods are widespread
Due to inconsistent rules about labeling GMOs and gene-edited foods around the world, many consumers may not realize they're already eating them.
For example, the most widely used enzyme in cheese-making, rennet , is produced from a GMO bacterium. GMO microbial rennet produces a specific enzyme called chymosin, which helps coagulate milk and form curds. Historically, chymosin was extracted from young cow stomachs, but in the 1990s scientists managed to genetically engineer a bacterium to synthesize it.
GMOs and gene-edited cereal and oilseed products are also widely used in stockfeeds. There is ongoing research to improve feed through enhanced nutrition , and produce crops that will decrease methane emissions from cattle .
When it comes to modifying animals themselves, ethical considerations must be balanced alongside potential benefits.
In Australia, about 70% of cattle are genetically polled (hornless). Having polled cows improves meat quality through less injury to meat, and is considered better for animal welfare. In the US, fast-growing genetically modified salmon has been approved for consumption.
In a horticultural context, the genetically modified rainbow papaya stands out. It was developed in the late 1990s in response to a ringspot virus outbreak that nearly wiped out the global papaya industry. Researchers created the virus-resistant "transgenic" papaya, which now makes up the majority of papayas consumed worldwide.
In terms of boosting nutritional content, " golden rice " biofortified with Vitamin A (GMO) is being cultivated in the Philippines, as are tomatoes biofortified with Vitamin D (GE) in the United Kingdom, and GABA-enriched tomatoes (GE) in Japan.
Research is also being done to create non-browning mushrooms , apples and potatoes. A simple gene edit can help inhibit the browning oxidation reaction, leading to a longer shelf-life and less food waste.
Regulation in Australia and New Zealand
So why don't you see non-browning mushrooms at your local supermarket?
In Australia, the Office of the Gene Technology Regulator regulates GMOs. It has approved four GMO crops for cultivation: cotton, canola, safflower and Indian mustard. However, many more are imported for food ingredients (including modified soy, cottonseed oil, corn and sugar beet) and stockfeed (canola, maize and soy).
Gene-edited foods can be cultivated without any regulatory restrictions or labeling in Australia. The Gene Technology Act 2000 deregulated these products in 2019.
On the other hand, New Zealand's Environmental Protection Authority has maintained regulatory restrictions on both gene-edited foods and GMOs. Divergent definitions have led the bi-national agency Food Standards Australia New Zealand (FSANZ) to adopt a cautious approach, regulating gene-edited foods and feeds as GMOs.
The lack of alignment in definitions in Australian has confused producers and consumers alike. FSANZ has said it will continue to monitor developments in gene-editing technology, and will consider reviewing its regulatory approach.
Responsible research
Both GMOs and gene-edited foods offer great promise. Of course there are valid concerns, such as the potential to create new allergens, unintended consequences for ecosystems, and growing corporate control over food. But these can be addressed through responsible research and regulatory frameworks.
Ultimately, the development of future foods must be guided by a commitment to sustainability, social justice and scientific rigour.
Provided by The Conversation
Explore further
Feedback to editors
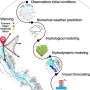
Research team develops an impact-based forecasting system for improved early flood warning
4 minutes ago

Centromere research yields new insights into the mechanisms of chromosome segregation errors
7 minutes ago
Biohybrid robot made from flour and oats could act as a biodegradable vector for reforestation

Island birds more adaptable than previously thought

Scientists discover 'weird' statistics of electrons ejected by intense quantum light
New gel breaks down alcohol in the body
2 hours ago

Discovery of biomarkers in space—conditions on Saturn's moon Enceladus simulated in the laboratory

Researchers uncover mechanism for short-distance vesicle movements

Two-year study shows some varieties of annual flowers have a place in pollinator-friendly gardens

The secret to mimicking natural faults? Plexiglass and Teflon
Relevant physicsforums posts, a brief biography of dr virgina apgar, creator of the baby apgar test.
May 12, 2024
Who chooses official designations for individual dolphins, such as FB15, F153, F286?
May 9, 2024
Is it usual for vaccine injection site to hurt again during infection?
May 8, 2024
The Cass Report (UK)
May 1, 2024
Is 5 milliamps at 240 volts dangerous?
Apr 29, 2024
Major Evolution in Action
Apr 22, 2024
More from Biology and Medical
Related Stories

Gene-edited food quietly arrives in restaurant cooking oil
Mar 12, 2019

Who trusts gene-edited foods? New study gauges public acceptance
Jun 28, 2022

A mammoth meatball hints at a future of exotic lab-grown meats, but the reality may be far more boring
Apr 6, 2023

Clearer distinction needed between GMOs and genome-edited organisms
Jan 24, 2022

GMO skeptics still distrust big agriculture's climate pitch
Nov 13, 2022

Video: How GMOs are, or are not, regulated
May 18, 2017
Recommended for you

Long-term study finds organic farming leads to adaptations in the genetic material in plants


Angling fish for food: Study finds recreational fishing accounts for 11% of reported harvest in inland fisheries
3 hours ago

Researchers breed tomato plants that contain the complete genetic material of both parent plants
4 hours ago

Genetic study of cauliflower reveals its evolutionary history
May 10, 2024

Scientists unlock key to breeding 'carbon gobbling' plants with a major appetite

Changes in pig farming in the 20th century spread antibiotic-resistant Salmonella around the world, finds study
Let us know if there is a problem with our content.
Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use our contact form . For general feedback, use the public comments section below (please adhere to guidelines ).
Please select the most appropriate category to facilitate processing of your request
Thank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.
E-mail the story
Your email address is used only to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form.
Newsletter sign up
Get weekly and/or daily updates delivered to your inbox. You can unsubscribe at any time and we'll never share your details to third parties.
More information Privacy policy
Donate and enjoy an ad-free experience
We keep our content available to everyone. Consider supporting Science X's mission by getting a premium account.
E-mail newsletter
Numbers, Facts and Trends Shaping Your World
Read our research on:
Full Topic List
Regions & Countries
- Publications
- Our Methods
- Short Reads
- Tools & Resources
Read Our Research On:
Many publics around world doubt safety of genetically modified foods

Concern about genetically modified foods is widespread globally, with about half of people in 20 publics around the world saying these foods are unsafe to eat, according to a Pew Research Center survey conducted between October 2019 and March 2020.
As growth in the world’s population increases demand on the global food supply, nations have debated the role of genetically engineered or genetically modified foods. Advocates see such foods as one route to meeting food production demands.
This analysis is part of a study focused on understanding public opinion across a range of science-related issues. The data reported here comes from a survey conducted in 20 publics across Europe, Russia, the Americas and the Asia-Pacific region from October 2019 to March 2020. The surveys were conducted by face-to-face interviews in Russia, Poland, the Czech Republic, India and Brazil. In all other places, the surveys were conducted by telephone. All surveys were conducted with representative samples of adults ages 18 and older in each survey public.
Here are the questions used for this report, along with responses, and the survey methodology .
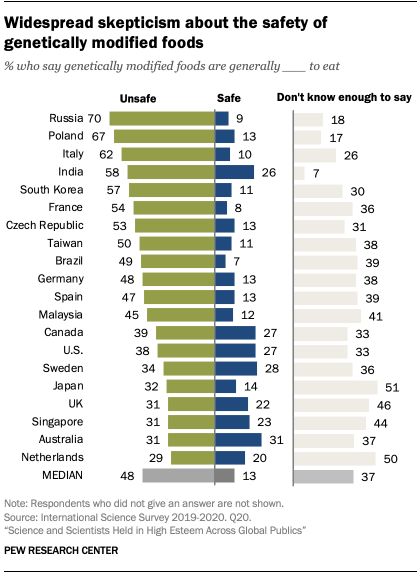
A 20-public median of 48% say genetically modified, or GM, foods are unsafe to eat, while a much smaller median of 13% say GM foods are safe. The survey included an option for people with limited familiarity about GM foods to indicate this; a median of 37% say they don’t know enough to offer a view about the safety of GM foods.
Majorities in places such as Russia (70%), Italy (62%), India (58%) and South Korea (57%) view GM foods as generally unsafe to eat. The balance of opinion tilts negative even in places where sizable shares say they don’t know enough about GM foods to offer a view. For example, 47% of Spaniards say GM foods are unsafe, while just 13% say they are safe to eat. Australia is the only place surveyed where at least as many view GM foods as safe as view them to be unsafe (31% to 31%).
The introduction of genetically modified crops and other developments in biotechnology have dramatically changed agriculture and food production in many parts of the world in recent decades. Some worry about possible health implications from these new practices, though that view is at odds with scientific consensus. A 2016 report from the National Academies of Science, Engineering and Medicine highlighted a consensus among scientific experts in the United States that GM foods are safe. In 2019, an expert panel in Japan came to the same conclusion.
Still, regulations of GM foods are dramatically different around the world. Many European countries, such as France and Germany , have banned growing GM crops. The U.S. and Brazil generally have more favorable regulations for GM crops and are among the world’s largest producers of such crops.
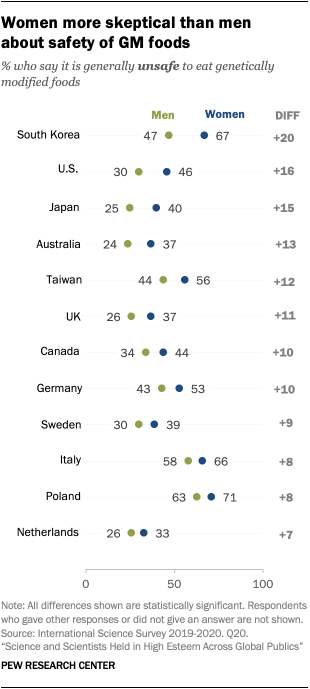
On balance, men and women generally view GM foods as being unsafe rather than safe. Women are especially likely to express concern about the safety of GM foods, however.
In 12 of the 20 publics surveyed, larger shares of women than men describe GM foods as unsafe to eat. For instance, women are at least 10 points more likely than men to see GM foods as unsafe in South Korea (20 points), the U.S. (16 points) and UK (11 points).
The gender gap in the U.S. is in line with previous Center research that found American women are more likely than men to say GM foods are worse for one’s health than non-GM foods (58% to 42% in 2019).
In most places, both those with higher and lower levels of education tend to see genetically modified foods as unsafe to eat. However, people with more education, and specifically those who have completed at least three science courses during their secondary or tertiary schooling, are more inclined to see GM foods as safe. For example, in the Netherlands, 27% of those with at least some postsecondary education who completed two or fewer science courses consider GM foods to be safe, while half of those who completed at least three science courses say the same.
Note: Here are the questions used for this report, along with responses, and the survey methodology .
- Food Science
- Medicine & Health

Brian Kennedy is a senior researcher focusing on science and society research at Pew Research Center .
Cary Lynne Thigpen is a former research assistant focusing on science and society .
How Americans View Weight-Loss Drugs and Their Potential Impact on Obesity in the U.S.
Science and scientists held in high esteem across global publics, about half of u.s. adults are wary of health effects of genetically modified foods, but many also see advantages, findings at a glance: nutrition research scientists, findings at a glance: dietitians, most popular.
1615 L St. NW, Suite 800 Washington, DC 20036 USA (+1) 202-419-4300 | Main (+1) 202-857-8562 | Fax (+1) 202-419-4372 | Media Inquiries
Research Topics
- Age & Generations
- Coronavirus (COVID-19)
- Economy & Work
- Family & Relationships
- Gender & LGBTQ
- Immigration & Migration
- International Affairs
- Internet & Technology
- Methodological Research
- News Habits & Media
- Non-U.S. Governments
- Other Topics
- Politics & Policy
- Race & Ethnicity
- Email Newsletters
ABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan fact tank that informs the public about the issues, attitudes and trends shaping the world. It conducts public opinion polling, demographic research, media content analysis and other empirical social science research. Pew Research Center does not take policy positions. It is a subsidiary of The Pew Charitable Trusts .
Copyright 2024 Pew Research Center
- Skip to main content
- Skip to FDA Search
- Skip to in this section menu
- Skip to footer links

The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
U.S. Food and Drug Administration
- Search
- Menu
- Resources for You (Food)
- Agricultural Biotechnology
How GMO Crops Impact Our World
Feed Your Mind Main Page
en Español (Spanish)
Many people wonder what impacts GMO crops have on our world. “GMO” (genetically modified organism) is the common term consumers and popular media use to describe a plant, animal, or microorganism that has had its genetic material (DNA) changed using technology that generally involves the specific modification of DNA, including the transfer of specific DNA from one organism to another. Scientists often refer to this process as genetic engineering . Since the first genetically engineered crops, or GMOs, for sale to consumers were planted in the 1990s, researchers have tracked their impacts on and off the farm.
Why do farmers use GMO crops?
Most of the GMO crops grown today were developed to help farmers prevent crop loss. The three most common traits found in GMO crops are:
- Resistance to insect damage
- Tolerance to herbicides
- Resistance to plant viruses
For GMO crops that are resistant to insect damage, farmers can apply fewer spray pesticides to protect the crops. GMO crops that are tolerant to herbicides help farmers control weeds without damaging the crops. When farmers use these herbicide-tolerant crops they do not need to till the soil, which they normally do to get rid of weeds. This no-till planting helps to maintain soil health and lower fuel and labor use. Taken together, studies have shown positive economic and environmental impacts.
The GMO papaya, called the Rainbow papaya , is an example of a GMO crop developed to be resistant to a virus. When the ringspot virus threatened the Hawaii papaya industry and the livelihoods of Hawaiian papaya farmers, plant scientists developed the ringspot virus-resistant Rainbow papaya. The Rainbow papaya was commercially planted in 1998, and today it is grown all over Hawaii and exported to Japan.
Learn more on Why Do Farmers in the U.S. Grow GMO Crops?
Do GMOs have impacts beyond the farm?
The most common GMO crops were developed to address the needs of farmers, but in turn they can help foods become more accessible and affordable for consumers. Some GMO crops were developed specifically to benefit consumers. For example, a GMO soybean that is used to create a healthier oil is commercially grown and available. GMO apples that do not brown when cut are now available for sale and may help reduce food waste. Plant scientists continue to develop GMO crops that they hope will benefit consumers.
Learn more about GMOs and the Environment .
Do GMOs have impacts outside the United States?
GMOs also impact the lives of farmers in other parts of the world. The U.S. Agency for International Development (USAID) is working with partner countries to use genetic engineering to improve staple crops, the basic foods that make up a large portion of people’s diets. For example, a GMO eggplant developed to be insect resistant has been slowly released to farmers in Bangladesh since 2014. Farmers who grow GMO eggplants are earning more and have less exposure to pesticides. USAID is also working with partner countries in Africa and elsewhere on several staple crops, such as virus-resistant cassava , insect-resistant cowpea , and blight-resistant potato .
Learn more about GMO Crops and Humanitarian Reasons for Development and GMOs Outside the U.S .
How GMOs Are Regulated in the United States
Science and History of GMOs and Other Food Modification Processes
GMO Crops, Animal Food, and Beyond
www.fda.gov/feedyourmind
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 05 June 2018
Public perception of genetically-modified (GM) food: A Nationwide Chinese Consumer Study
- Kai Cui 1 , 2 &
- Sharon P. Shoemaker 1
npj Science of Food volume 2 , Article number: 10 ( 2018 ) Cite this article
93k Accesses
128 Citations
109 Altmetric
Metrics details
- Agriculture
- Environmental biotechnology
After more than 25 years of research and development on the genetic modification of a wide range of crops for food and fodder, China has reached a decision point as to whether it should accept, reject, or go slow with the use of genetically modified (GM) technology to produce the food and feed needed to sustain its population growth and economic renaissance. Here, we report a consumer survey on GM food that includes input from all provinces in China. Chinese consumers were surveyed for their awareness, knowledge, and opinion on GM food. The survey resulted in 11.9, 41.4, and 46.7% of respondents having a positive, neutral, or negative view on GM food, respectively. A minority of respondents (11.7%) claimed they understood the basic principles of GM technology, while most were either “neutral” or “unfamiliar with GM technology”. Most respondents (69.3%) obtained their information on GM food through the Internet and 64.3% of respondents thought that media coverage was predominately negative on GM food. The reasons given by consumers in favor of, or against, the use of GM food, were complex, as seen by the response of 13.8% of respondents who felt GM technology was a form of bioterrorism targeted at China. China’s Ministry of Agriculture and the science community generally expressed a positive attitude toward GM food, but the percentage of respondents that trusted the government and scientists was only 11.7 and 23.2%, respectively. Post-survey comments of respondents made suggestions on how the industrialization of GM technology might impact the future of China’s food supply and value chains. Finally, the impact of emerging technologies like genome editing and genome-edited organisms (GEOs) on the GM food debate is discussed.
Similar content being viewed by others

The Chinese public’s awareness and attitudes toward genetically modified foods with different labeling

Expert and public perceptions of gene-edited crops: attitude changes in relation to scientific knowledge
Consumer acceptance of novel food technologies
Introduction.
Genetically modified (GM) technology is a highly controversial topic for today’s global food consumer. The commercial development of GM crops began in 1996 with GM corn and has expanded every year with the cultivation of GM crops. In 2016, global land use for GM crops reached 185.1 million hectors. 1 Although GM foods had helped sustain the nutritional needs of human beings and farm animals and mounting evidence showed that GM foods were substantially equivalent to traditionally bred food sources, it has also sparked fierce debate about its safety. This has generated worldwide interest in finding a common and harmonious narrative to deal with new opportunities and challenges of biotechnology. A recent review of public perceptions of animal biotechnology, 2 provides an excellent context for understanding public knowledge, attitudes, and perception of GM Food in China.
China comprises 20% of the world’s population, 25% of the world’s grain output, 7% of the world’s arable land, and 35% of the world’s use of agricultural chemicals. 3 Consequently, China faces risks to its food security and pollution of the environment. The government has invested heavily in research and development of technologies to improve quality and increase the output of its foodstuffs, especially grains. GM technology provides a such feasible approach 4 , 5 to realize these goals. As the complexity of the GM issue mounts, the controversy surrounding GM food has moved farther away from science. While China’s president calls for its scientists to “boldly research and innovate [and] dominate the high points of GMO techniques”, 6 the people of China are largely opposed to GMO foods, but are not sure why. 7 Thus, this nationwide survey on the current Chinese public perception of GM food should be helpful to policy-makers, technology developers, as well as to consumers.
Consumer attitudes about GM food are complex and interwoven with the consumer’s knowledge of the science, lifestyle and public perception. Since 2002, surveys have been conducted in China on public acceptance of GM food from the perspective of consumer behavior, such as intent to purchase, presence of GM markers, and sensitivity to price point 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 (Table 1 ). There has been a general lack of fundamental studies on the public’s scientific perception and policy interpretation of GM food. Moreover, the scope of previous surveys has been limited to a few of the largest cities in developed areas of China, with little or no coverage of rural areas. In all cases, the number of respondents in most of these earlier surveys was less than 1000. This study summarizes the status of GM food in China and provides the results of questionnaires that surveyed consumers from every province on their knowledge level, present attitudes, and future thoughts of GM food in China. A statistically relevant sample size of 2063 questionnaires were satisfactorily completed. The findings in this survey provide insight into Chinese consumers and offer a possible path for “smart” industrialization of GM technologies in China.
General consumer attitudes of GM food
The first six questions of the survey asked about the respondent’s background, followed by 18 questions that addressed their awareness, knowledge, and opinion on GM Foods. The seventh question asked, “In general, will you support GM food?” The percentage of those who supported, opposed or were neutral were 11.9, 41.4, and 46.7%, respectively. These results suggest that the overall attitude of the Chinese consumer is cautious of GM food.
GM technology was first introduced in the pharmaceutical industry and then applied to agriculture. Did the public’s skepticism originate from GM food safety or GM technology itself? Question #8 was designed to address this question. “If GM technology is applied in medical area to produce medicine, such as insulin and hepatitis B vaccine, what is your opinion?” The percentage of those who supported, opposed or were neutral to GM pharmaceuticals was 46.8, 12.8, and 40.4%, respectively. Support for GM pharmaceuticals was higher than that found for GM food and again, there were many in the neutral category. This result suggests that some respondents were against GM food but not against GM technology. Still, there were 12.8% of respondents that took a negative view about GM pharmaceuticals, although they may not have known that the insulin and hepatitis B vaccine widely used today are GM-derived pharmaceuticals.
Since 2002, the year when China implemented legislation mandating the labeling of GM food products, numerous surveys in China were carried out to gain insight into the public’s attitude to GM food. The results from these early surveys were compared to the results of the present survey (Table 1 ). Significant differences were found between the surveys, likely due, in part, to differences in the number of respondents, where they resided, and when the surveys were conducted. The results were also difficult to interpret because of differences in content of each survey and in the respondents. The respondents in the surveys represented the public, media, private enterprise and government. Overall, the trends were interesting even with this inherent variability, and reflected consumer preferences about GM food. The ratio of “support” vs. “oppose” GM food was used as a measure to compare the different surveys (Table 1 ). This measure suggests an interesting trend in that the ratios before 2012 were larger than 1.0 (with one exception) and thereafter, were less than 1.0. The survey reported here gave the lowest ratio, 0.29. In summary, the initial positive attitude towards GM food in 2002 generally decreased in subsequent years.
To gain further insight into consumer attitudes toward GM food among the respondents, six factors were selected as research variables. As shown in Table 2 , respondent’s attitudes towards GM food were correlated to their age, sampling location, educational level, major in college and income. A negative attitude toward GM food was more frequent among those respondents born before 1969 (59.3%). The public-sector group from Western China reported 51.3% against GM food, compared to 29.7% from those located in the center and in northeastern China. The percentage of those respondents with college degrees who supported GM food was 9.5%, which was the lowest number relative to any other group. The percentage of respondents with a positive attitude was higher for those with a science background (14.1%) compared to those with a liberal arts background (7.5%). The percentage of respondents with a negative attitude was higher (51.6%) with those who reported an annual household income above one million Chinese Yuan (RMB), compared to those with an annual household income below 80,000 RMB (34.2%). Gender was not found to be a factor in shaping attitudes towards GM food.
We further queried the state of Chinese public opinions on GM food and determined the main reasons for the either their support (Question #9) or opposition-against (Question #10) to GM food, from what was known previously. The statistical results showed that the total number of “support” and “oppose” was 3248 and 4751, respectively. This demonstrates again that the public is cautious about GM food. The relative percentage of choice, “frequency” (defined as the number in support or against divided by the total number in the respective area) is listed in Table 3 .
GM technology is potentially a paradigm shift for farmers in developing countries and is an important tool in the toolbox for addressing global challenges, such as persistent poverty, climate change, and the challenge of feeding 9.7 billion people by 2050. Some studies suggested that efforts to change consumer perception about GM food should address risk perception factors and promote the beneficial effects of biotech crops. 24 As a nonpartisan, nonprofit organization, Intelligence Squared U.S held a TV debate on December 4, 2014 on whether the world is better off with or without GM food. The discussion was whether GM food is safe, how it impacts the environment and can it improve food security). Both the positive and negative sides had experts debating for or against GM food. Among the attendees who were present, the percentages in favor or against “genetically modified food” were 32 and 30%, respectively, before the debate, but this changed to 60 and 31%, respectively, after 100 min of debating the topic. This result suggests that efforts to change public perception about GM food should address risk perception factors and promote the beneficial effects of biotech crops. It should be noted that some opponents of GM food have started to rethink their prior attitudes about GM food. 25 On the other hand, some research suggested that many opponents are evidence-insensitive and will not be influenced by arguments about risks vs. benefits. 26 Food Evolution, a 2017 documentary film directed by Scott Hamilton Kennedy and sponsored by the Institute of Food Technologists (IFT) vividly illustrated the polarizing worldwide debate, “for and against” GM food. Its fact based, story telling narrative delivered a powerful educational message on new technologies and the process of acceptance by consumers. People involved in the making of the film tried to encourage audiences to think critically and reexamine their information sources and beliefs regarding GM food.
Factors shaping public perception of GM food
How much did the public know about GM technologies? Some earlier studies 12 , 17 , 27 , 28 , 29 based their conclusions on individual and subjective questioning, and only asked the respondents: “Do you know GM technologies?” The authors in this study agree with Hallman 30 that the self-reported awareness of GM does not necessarily mean respondents understand the principles and purpose of GM food. Thus, Question #11 was asked in this survey: “Do you know the principle of GMO such as introducing foreign genes, genetic recombination and gene expression? “
The result of our survey showed only 11.7% of the respondents self-reported that they were familiar with the general scientific principles of GM technology, contrasted to 49.5 and 38.8% saying they know something and nothing, respectively, about the subject. In the absence of sufficient understanding of biotechnology, the public’s attitude towards GM food safety can be misleading. Thus, we carried out a correlation analysis between the public’s perception (Question #11) and attitudes towards GM technology (Question #7). The results are given in Table 4 .
The design of this questionnaire was based on the following hypothesis: The opinion of consumers to GM food will be related to their knowledge of GM food. This was confirmed in this survey. There were positive correlations between “know a lot” and “support”, “know nothing” and “oppose”. At the same time, there were negative correlations between “know a lot” and “oppose”, “know nothing” and “support”. The lower the understanding of GM technology, the more hesitant the respondents were to accept GM food. These results also highlight the influence and importance of studies on the public perception of science in China.
Chinese food safety scandals have been a growing concern for Chinese consumers in recent years. The incidences of illegal “gutter oil” used in cooking, pesticide residue contamination, use of feed additives and polluted water along the food chain are common problems and even with proper regulatory oversight, the risk for criminal activity is ever present. The consumers in China, as well as consumers in other parts of the world, are increasingly risk adverse and seek out “clean, natural food”. Thus, the perceived risk of GM food was heightened because of these scandals, even though perceived risk of GM food is mostly based in perception rather than in practice. How deeply does the Chinese public think about the safety of GM food? Question #12 was asked to reflect this: “Compared to other food safety issues in China, such as illegal cooking oil, pesticide residue, feed additive and water pollution, your concerns on the safety of GM foods are?” The result illustrated that 20% of respondents thought the safety issue of GM food was more severe than other issues compared 31.8% of respondents thought “nearly the same”, 22.5% of respondents thought “not as severe” and 25.7% of respondents “have no idea”. These results mean that more than half of the respondents were concerned about the safety of GM food, of which 20% were deeply concerned, above and beyond any other food issue facing China.
Source of information on GM foods
The respondents were asked, “Have you actively searched for information on GMO’s using web search, reading books and verbal inquiries after graduation?” (Question #13). The result showed that 38.7% chose “yes”, compared 36.2% who chose “No, but I really care about GMO”, and lastly, 25.2% who chose “No, I don’t care about GMO”. When asked, “How do you acquire information on GM Food?” (Question #14), the result showed that 69.3% of respondents acquire information from the Internet as compared to 45.3% from television, 27.8% from books and periodicals, 22.8% from communication from relatives and friends, 22.4% from learning at school and 9.6% from public lectures. It is well known that GM food is a complex issue, and information from the Internet is often unverified and inaccurate. Thus, there is an urgent need in China to educate the public on GM technology and GM food by providing balanced, evidence-based perspectives of the technology to consumers through presentations, written materials, documentaries and educational courses that are made widely available through various media. The government can play a key leadership role by supporting educational programs, particularly targeting young people. It also crucial to put in place safeguards and the communication needed to ensure to the public that GM foods are thoroughly tested and regarded as safe. Regulatory groups worldwide must demonstrate their ability to ensure the safety of “new” foods and food ingredients, in a harmonious and transparent manner. Another question (#15) asked was, “Based on your experience, you have found that the media reports and Internet rumors about GM Food generally tend to be?” The results showed that respondents answered the question of media atmosphere as negative (64.3%), positive (11.5%) or neutral (24.2%).
Other studies have shown that the public tends to build upon its negative impression of GM food even in the face of positive information. 31 , 32 The lack of understanding of the principles and benefits of GM technology, make the general population more susceptible to negative media reports. The debate around GM food has become increasingly one-sided in recent years, with activists spreading misinformation via social media about the human health dangers of GM food as well as the negative environmental impact of GM crops on transitional agricultural eco-systems. Additional negative information on social media had a great impact, driving down the willingness to accept GM food. This led to food-centered non-governmental organizations (NGO’s) directing their attention to generating debates, educational packages and other formats to reach out to the general public (e.g., work of US based Farmer’s and Rancher’s Association and IFT). Research supported by the Chinese Academy of Social Sciences showed that rumors about food security accounted for 45% of all Internet rumors which severely influenced the public’s trust. 33 Our study also attempted to probe into the public attitudes toward rumors about GM food on the Internet. For example, in China, rice is the main staple food for 60% of its people, and hybrid rice accounts for about half the planting area of rice. Rumors were spread that hybrid rice is a GM crop. Through self-interest, some non-GMO food producers condemned GM food with malicious gossip and misplaced nationalism, fomenting the notion that GM technology originated in the U.S. as a form of bioterrorism against China. What did the public think about this? (Question #16, 17 and 18). The result (Table 5 ) showed that 15.8% of respondents think that hybrid rice is one kind of GM crop, 25% of respondents think that there is unfair business competition with GM food, 13.8% of respondents agree that GM technology maybe considered as bioterrorism to China. These results pointed to an underlying problem that the debate on GM food in China has deteriorated. It is worth mentioning, however, that more than half of the respondents (54.4%) believed that debate on GM food should be based on science. This is the basis for why the debate about GM food should be based on scientific evidence.
Since the GM food debate should be evidence-based, the public needs to put more trust in scientific explanations and research data that can be understood by the average consumer. Many scientists including 110 Nobel Prize winners openly support GMO technology in the recent years. The 2016 Report 34 issued by the U.S. National Academies of Sciences, Engineering, and Medicine found “no substantiated evidence of a difference in risks to human health between currently commercialized genetically engineered (GE) crops and conventionally bred crops.” What do the American public think about the above report? A survey carried out by University of Pennsylvania 35 showed that only 22% of those surveyed agreed that scientists have not found any risks to human health from eating GM foods, while 48% of the people disagreed with that statement. What is the situation in China? The result (Question #19) showed that 23.2% of the respondents chose to “believe in biologist’s opinion” compared to 45.5% who chose to “do not trust biologist’s opinion” and 31.3% who chose to “have no idea about this.” This result reflects that scientists are “under suspicion” on the issue of GM food both in China and the US. The film, Food Evolution, and other educational materials are helping to change this viewpoint. “What is the most important information that the public wants to know about GM food?” We asked this question (#20) in the survey. The result (Table 6 ) showed that more than two out of three respondents (68.9%) wanted to know more about the safety of GM food.
Public perception and attitude to policy
The Dean and Shepherd study 36 found that participants’ perceptions of risk lessened when governmental agencies presented a consistent message to the public. China’s Ministry of Agriculture claimed in 2016 that there is no substantiated evidence showing that genetically modified foods are unsafe during the past 20 years of commercial cultivation. But according to our survey (Question #21), only 11.7% of respondents thought that the government’s statement was an “authoritative interpretation”, compared 10.9% who chose “that is concealing the truth” and 77.4% who chose “No evidence now does not mean no evidence in the future. We should still be cautious to GM foods.” To a certain extent this result demonstrates that the public does not consider the government as a credible source of information on the issue of GM food.
Question #22 addressed the following, “What kind of GM crops were approved by the government to cultivate and produce in China?” Seven options were provided, including corn, rice, wheat, soybean, cotton, rape, and papaya. Only GM cotton and GM papaya have been approved for commercial cultivation in China. According to our survey, disappointingly few, only 1.2% of respondents chose the right answers. Apparently, government sources of information on GM crops has not been effective in educating the Chinese public about GM food.
In Question #23, the respondents were asked “What do you think of the force of government supervision for the production and import of GM food?” The result showed that 47.1% of respondents felt that the government should “strengthen supervision force, it is best to totally ban the GM foods”, compared that 43.3% felt “supervision force is appropriate” and 9.6% felt “supervision force is too tight.”
“The Chinese Ministry of Agriculture claimed that GM crops and GM food are advanced technologies that can serve as the foundation of a new industrial sector with broad implications for human health and wellbeing. As a large agricultural county, China should have a place for transgenic (GMO) technologies. What do you think about this?” (Question #24) The result showed that only 28.8% of respondents “support” this policy, compared 18.9% that chose “opposed” and 52.3% that chose “neutral”. In the face of widespread suspicion and misinformation about GM foods, more effort is needed to gain the confidence, trust and support from the public domain.
GM crops and the foods derived from them are considered the most immediate solution to alleviate global hunger and malnutrition. The benefits of GM crops such as greater productivity, reduced need for pesticides and herbicides, increased economic benefits for large and small farmers alike, have been extensively reviewed. 37 However, public attitudes toward GM food from country to country in different regions of the world continue to vary. The recent review by Van Eenennaam and Young 2 gives an excellent summary of the complexity of surveying and interpreting global public opinion on GM foods. In short, the authors noted the negative view of GM food in Europe, was exacerbated by the bovine spongiform encephalopathy (BSE) crisis first in the late 1980s and again in the 1990s. It was thought that GM technology might be used to mask the effects of poor housing of animals, not to mention the sense of supporting global agro-business rather than smaller family farms which are typical in Europe. In contrast, the United States, Canada and some Latin American countries (namely Brazil and Argentina) have widely adopted GM crops. Brazil is the second only to the United States in the land used for GM food crops. A review of acceptance, policies and actions in the African countries illustrated the complex and myriad issues that slow the adoption of GM food, thereby deleteriously impacting African countries. 38 Though the progress is slow, there seems to be a new receptiveness for GM food amongst some of the African countries. It is interesting to note that a study in Africa in 2005, showed that of the 7000 people surveyed, 80% did not know the meaning of the word “biotechnology”. 2 In Asian countries, it has been noted that China’s initial lead position in GM food has slowed over time due to global resistance 39 to GM food. However, signs of acceptance of GM food in China are encouraging. 40 , 41 Finally, Van Eenennaam and Young 2 compared China with other Asia countries (India, The Philippines) where bans on GM foods or vandalism on GM crops have occurred. On the other hand, Bangladesh has successfully adopted insect-resistant GM eggplant and has become a success story for the adoption of GM crops. 2 , 42
In our analysis, public attitudes toward GM food continue to swing widely across China from opposition to acceptance. On one side, some socialistic organic farmers, environmentalists and NGO’s have questioned the security of GM food, with some even calling for a ban on growing most GM crops. On the other side, agricultural specialists and biotech industry representatives highlight the benefits of GM technology to concerned consumers. The survey reported here was intended to be very broad in the type and range of questions asked. The authors plan to follow up with a more focused survey on safety issues related to GM food. Transparent and harmonious regulatory oversight is helping to further ensure the safety of GM technology and GM food but this must be understood and agreed by consumers as well as scientists. We should not expect, however, any convergence of opinions in the very near future. Based on the results of this study, suggestions about the future industrialization of GM technologies and GM food in China are presented as follows.
Strengthen communication to the public, making order out of confusion
Chinese consumers, in general, were found to be unfamiliar with GM technologies and the benefits they provide. They were also skeptical of scientists and the government on the topic of GMO, GM technologies and GM food. Fortunately, there is consensus in the public domain that more discussion on GMO and GM technologies is needed to better understand the scientific and social implications of GM food. Accordingly, public lectures and other educational formats need to be expanded in China to help the public develop evidence-based attitudes about GM foods. Until public doubts about GM food are addressed in a balanced and evidence-based manner, it will be difficult for China to develop sound policies and programs that will benefit the agribusiness industry and consumers. All forms of the media in China should be encouraged to incorporate scientific facts in their reporting and to discourage exaggerated reports and “fake” news. There should be a constructive vision and plan for building a future society that includes rational attitudes and a foundation for a food secure global society with adequate safety safeguards in place.
Government work should transform passivity into initiatives
China’s central government recently issued a document calling for more research, development and supervision of agricultural GMO and GM technologies, and the careful promotion of GM food that is safe, affordable, and healthy. From the result of the surveys taken in recent years, it was found that the percentage of respondents who opposed GM food is on the rise, and significant effort is needed to overcome that trend. The issue of GM food is very sensitive in China, GM policies have wavered among concerns over the bio-safety debate and development goals, such as food security, poverty reduction and the approval of transgenic commercial planting that was brought to a halt in recent years. In the long run, GM policies will influence the international competitiveness of the seed industry and agricultural development in China. As mentioned above, the safety of GM food should be based on science, and a modern society should not judge the safety of one kind of food by the way of a referendum. The government should enhance communications with the public and strive for the understanding and support of the public for China’s GMO policy.
Respect public opinion, improve gradually
Throughout history, many innovations have experienced both headwinds and tailwinds before being accepted by society. There is a persistent gap between expert knowledge of scientific issues and public perception of these issues. The conclusion of natural sciences usually is only truth, although the culture and attitudes can be diversified, being influenced by religious beliefs and/or political parties. Differences in public opinion towards GMO, GM technologies, and GM food should be respected. What is needed is government leadership in constructing a transparent system for evaluation of these technologies for commercial use while, at the same time, upholding the public’s right to have a choice by labeling GM food products. This will enable the public to make their own choices about GM food.
Lurking in the background, however, are new technologies that can produce genetic modifications in plants and animals in ways that are different and more precise that traditional GM technologies. The CRISPR-Cas9 genome editing technology 43 together with new signal DNA base editing 44 and RNA base editing 45 are currently revolutionizing the fields of agriculture, medicine and basic research. Unlike the traditional GM technology that adds foreign DNA to the recipient organism as part of the process, genome-editing, and base-editing simply switch out mutated or otherwise undesirable DNA bases that detract from the overall fitness, productivity, quality and usefulness of the organism, in question. Regulatory policies in the United States were written nearly 30 years ago and do not address the safety of genome-edited or base-edited organisms (GEOs). Currently, regulatory agencies are declaring these “edited” organisms and foods as safe and they are exempt from testing and labeling requirements. GM technology opponents have already spoken out against these forms of genetic modification and now that public must make their voices heard.
Only time will tell if foods derived from GM technology or genome-edited and base-edited organisms will be the best solution to achieving food safety, security, and sustainability. At least for GM foods, the lack of any documented adverse effects is encouraging. With the improvement of the scientific literacy, the debate about GM food should return to a rational one and one that will shape the future Chinese society.
Questionnaire development
The initial design, order and questions used in this questionnaire were based on both past information 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 and input from 40 interviewees, representing consumers, agricultural officials, seed companies, farmers, biologists, and sociologists. From this input, 28 questions were generated as a pre-survey test to address the public perception of GM Food. The pre-survey was carried out in March 2016 with 100 respondents. Based on their feedback, the questionnaire was refined further into the final survey of 24 questions used in this study. The goal was to gain insight into the following four questions through this survey:
In general, what are consumer’s attitudes to GM food in China?
How does public perception of GM food correlate to the science behind GM food?
What is their source of information on GM foods and how does this source influence their perception?
How does the public’s perception and attitude correlate to policy?
The survey was designed to offer a range of questions to determine the respondent’s demographics, educational level, knowledge of GM food. The survey was conducted in both public and private meeting rooms between May 2016 and October 2016. The questionnaires were distributed altogether in 38 different venues. All questionnaires were handed out to individuals and collected after 10 min by Dr. Kai Cui.
Participants
A summary of the participants in the survey is given in Table 2 . They were all Chinese citizens over the age of 15, from 193 cities and, in total, included representation from all 31 provinces in China.
Approach to distribution
The questionnaires were distributed as part of a course on investment and finance. The course was conducted by the sole instructor, Dr. Kai Cui. After the course participants became familiar with the instructor (1–2 days) and understood the purpose of the course, they were administered the questionnaires. While instructing the course, students were asked to fill out a questionnaire to give their opinions on the level of understanding of GM technology in China from a consumer’s perspective. A total of 2200 questionnaires were distributed during this 6-month period with 2063 questionnaires satisfactorily completed.
Statistical analysis
Analysis of the survey results was done using the software program package - Statistical Product and Service Solutions (SPSS)19.0.
Data availability statement
A sample of the questionnaire. translated into English, is available in supplementary information at npj: Science of Food’s website. The completed 2063 questionnaires and the resulting database for the statistical analyses are in mandarin are not publicly available but can be made available from the corresponding author on reasonable request.
James, C. Global developing trends in biotechnology/genetically modified crop in 2015. China Biotechnol. 36 , 1–11 (2016).
Google Scholar
Van Eenennaam, A., & Young, A. E. in Animal Biotechnology 2: eEmerging breeding technologies (eds Niemann, H. & Wrenzycki, C.) Ch. 13 (Springer, in press).
Cui, K. & Shoemaker, S. P. A look at food security in China. npj Sci. Food 2 , 4 (2018).
Article Google Scholar
Carter, C. A., Zhong, F. & Zhu, J. Advances in Chinese agriculture and its global implications. Appl. Econ. Pers. Policy 34 , 1–36 (2012).
Wang, Q. China’s scientists must engage the public on GM. Nature 519 , 7 (2015).
Article CAS Google Scholar
Roberts, D. & Bjerga, A. China does an about-face on GMOs. Bloom. News . http://www.bloomberg.com/news/articles/2015-05-21/china-does-an-about-face-on-gmos (2015).
Prakash, C. S. GM crops in the media. GM Crops & Food 6 , 63–68 (2015).
Huang, J. Awareness, acceptance and willingness to buy genetically modified food in urban China. China Soft Sci. 2 , 61–67 (2006).
Liu, Z., Wang, C., Li, N., Zhang, J. & Zhang, K. Investigation and analysis for Jinan consumers’ recognition to genetically modified food. Rev. China Agric. Sci. Technol. 1 , 52–58 (2007).
Zhou, M. & Liu, Q. Investigation for Changsha consumers’ recognition and attitude to genetically modified food. Consum. Econ. 3 , 51–53 (2009).
Fan, L., Wei, W. & Zhu, Z. Investigation and thinking for consumers’ recognition to GMfood. Chin. Agric. Sci. Bull. 20 , 80–85 (2010).
Shen, J., Yan, M., Tian, Z. & Zhu, X. The survey on consumer perception about GM food in Nanjing City. J. Anhui Agric. Sci. 39 , 10909–10912 (2011).
Li, P. Consumer awareness and acceptance of GM foods in Guangzou city. Mark. Mod. 19 , 70–72 (2010).
Feng, L., Qi, Z., Tian, Y. & Zhou, H. Analysis on the impact factors of consumers’ purchase intention of GMfood. J. China Agric. Univ. 3 , 7–14 (2012).
Wu, W. Consumer awareness of genetically modified foods and consumer attitude survey analysis of the situation. J. Southwest Univ. Natl 5 , 771–775 (2011).
Xue, X. Investigation for Hangzhou urban population’s recognition and attitude to GM Food. Mod. Prop. Mgmt. 1 , 84–85 (2012).
Ruan, J., Chen, Chen, L., Guo, S. & La, W. Investigation and analysis of consumer recognition of genetically modified foods and transgenic labeling—a case study of Shenzhen city. Mod. Food Sci. Technol . 4 , 848–852 (2013).
Zheng, K., Wende, Chen & Jiayin., Xu Investigation and analysis for Chengdu consumers’ recognition to genetically modified food. J. Anhui Agric. Sci. 33 , 12966–12968 (2013).
Zhang, Y., Zheng, Z. & Gao, Y. Consumer perception and acceptance of GMfood. China Rural Surv. 6 , 49–61 (2015).
Li, Q., Wang, Q., Liu, Y., Ma, L. & Ma, M. Analysis of the perception and purchase of GM food in Anhui Province. Chin. Agric. Sci. Bull. 35 , 116–121 (2015).
Zhang, X., Liu, X. & Deng, M. GMfood: a study of Chinese public’s recognition and attitude. J. Anhui Agric. Sci. 20 , 6783–6786 (2014).
Guo, L. An investigation on cognition attitudes of the consumers towards GM foods in Zhuzhou. Mod. Food 21 , 12–15 (2015).
Meng, L., Yang, L. & Cheng, J. Survey of consumer recognition of GM food in Shanxi province. Food Saf. Guide 24 , 55–57 (2016).
Evans, E. A., & Ballen, F. H. A synopsis of US consumer perception of genetically modified crops. Univ. Florida IFAS Extension - Document FE934 . http://edis.ifas.ufl.edu/fe934 (2016).
Barrows, G., Sexton, S. & Zilberman, D. Agricultural biotechnology: the promise and prospects of genetically modified crops. J. Econ. Perspect. 1 , 99–120 (2014).
Scott, S. E., Inbar, Y. & Rozin, P. Evidence for absolute moral opposition to genetically modified food in the United States. Perspect. Psychol. Sci. 11 , 315–324 (2016).
Wang, Y. Investigation and analysis for consumers’ recognition to genetically modified food. Environ. Prot. 3 , 46–52 (2005).
Shi, S. Survey of consumer recognition of GM food in Wenzhou City. Mark. Mod. 30 , 9–10 (2015).
Tang, Y., Zuo, C., Zhao, S., Li, X. & Wang, X. Analyses of public GM food acceptance in Xian City. J. Anhui Agric. Sci. 34 , 267–270 (2015).
Hallman, W. K, HebdenW. C. & AquinoH. L. Public Perception of Genetically Modified Foods: A National Study of American Knowledge and Opinion. (Food Policy Institute, Rutgers University: New Brunswick, 2003). Report Number RR-1003-1004.
Ho, P. & Vermeer, E. B. Food safety concerns and biotechnology: consumers’ attitudes to genetically modified products in urban China. AgbioForum 4 , 158–175 (2004).
Zheng, Z. The effects of information on consumer behavior: citing GM rice. J. World Econ. 9 , 146–167 (2015).
Hu, Y. Why Rumors of Food Security have the Market? Beijing Youth Daily . (2016).
National Academies of Sciences, Engineering, and Medicine. Genetically Engineered Crops: Experiences and Prospect . (The National Academies Press, 2016) https://doi.org/10.17226/23395 .
Jamieson K. H. and Winneg, K., Annenberg Science Knowledge Survey: Americans support GMO food labels but don’t know much about safety of GM foods. (2016) https://www.annenbergpublicpolicycenter.org/americans-support-gmo-food-labels-but-dont-know-much-about-safety-of-genetically-modified-foods/ .
Dean, M. & Shepherd, R. Effects from sources in conflict and in consensus on perceptions of genetically modified food. Food Qual. Prefer. 18 , 460–469 (2007).
Qaim, M. Benefits of genetically modified crops for the poor: household income, nutrition and health. New Biotechnol. 27 , 552–557 (2010).
Wesseler, J., Smart, R. D., Thomson, J. & Ziberman, D. Foregone benefits of important food crop improvements in Sub-Saharan Africa. PLoS One 12 , e0181353 (2017).
Jayaranman, K. & Jia, H. GM phobia spreads in South Asia. Natl Biotechnol. 30 , 1017–1019 (2012).
Li, R., Wang, Q. & McHughen, A. Chinese government reaffirms backing for GM products. Natl Biotechnol. 33 , 1029 (2015).
Vazquez–Salat, N. & Houdebine, L. Will GM animals follow the GM planet fate? Transgen. Res. 22 , 5–13 (2013).
ISAAA. Global Status of Commercialized Biotech GM Crops: 2016 . (International Service for the Acquisition of Agri-Biotech Applications, Ithaca, NY, 2016).
Carroll, D. Genome editing: past, present and future. Yale J. Biol. Med. 90 , 663–659 (2017).
Gaudelli, N. M. et al. Programmable base editing of A.T to G.C in genomic DNA without DNA cleavage. Nature 551 , 464–471 (2017).
Cox, D. B. T. et al. RNA editing with CRISPR-Cas13. Science 358 , 1019–1027 (2017).
Download references
Acknowledgements
Project supported by the National Natural Science Foundation of China (Grant No. 7157317). The corresponding author would like to express the gratitude to Hui Meng (Professor of Eastern China Normal University), Dr. Xiaojun Lv (Associate Professor of Shanghai Jiaotong University) and Dr. Yan Liu (Associate Professor of Indiana University) for their suggestions in the design of the questionnaire and also acknowledge Beina Zhang and Yongyong Yang (Master students of Shanghai Normal University) for their support in data analysis. The co-author would like to gratefully acknowledge Professors Raymond Rodriguez, Professor Alison Van Eeneenaam and Christine Bruhn from the University of California, Davis, for their editorial assistance in the preparation of this manuscript. Project supported by the National Natural Science Foundation of China (Grant No. 71573173).
Author information
Authors and affiliations.
California Institute of Food and Agricultural Research, University of California Davis, Davis, USA
Kai Cui & Sharon P. Shoemaker
Antai College of Economics and Management, Shanghai Jiao Tong University, Shanghai Shi, China
You can also search for this author in PubMed Google Scholar
Contributions
Dr. Kai Cui, corresponding author, designed the questionnaire and delivered it to groups he met with in China. He secured the help for the statistical evaluation of the respondents in the survey. Dr. Sharon Shoemaker provided advice and collaboration in the fundamentals and consumer attitudes of GM technology. She was Dr. Cui’s mentor while he was at the California Institute of Food and Agricultural Research (CIFAR), UC Davis, and she provided basic understanding on the topic of GM Food and biotechnology, in general. She also contributed to the writing and editing of the manuscript in English.
Corresponding author
Correspondence to Kai Cui .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary material, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cui, K., Shoemaker, S.P. Public perception of genetically-modified (GM) food: A Nationwide Chinese Consumer Study. npj Sci Food 2 , 10 (2018). https://doi.org/10.1038/s41538-018-0018-4
Download citation
Received : 17 August 2017
Revised : 28 January 2018
Accepted : 09 April 2018
Published : 05 June 2018
DOI : https://doi.org/10.1038/s41538-018-0018-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Assessing knowledge and willingness to use genetically modified crops in uganda.
- Abubakar Sadik Mustafa
- Jamilu E. Ssenku
- Godwin Anywar
Agriculture & Food Security (2023)
From present to prosperity: assessing the current status and envisioning opportunities in the industrial-scale cultivation of Haematococcus pluvialis for astaxanthin production
- Thilini U. Ariyadasa
- Bavatharny Thevarajah
- Wanni Arachchige Jalitha Wasath
Phytochemistry Reviews (2023)
The decision to buy genetically modified foods in China: what makes the difference?
- Weizhuo Wang
- Christopher Gan
- Quang Thi Thieu Nguyen
Environment, Development and Sustainability (2023)
Information seeking about genetically modified foods: readability of online information
- Lalitha Samuel
- Sawyer I. Basch
- Joseph Fera
Journal of Consumer Protection and Food Safety (2023)
Genetic modification strategies for enhancing plant resilience to abiotic stresses in the context of climate change
- Amman KhokharVoytas
- Muhammad Shahbaz
- Manal Abdullah AlShaqhaa
Functional & Integrative Genomics (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
Our websites may use cookies to personalize and enhance your experience. By continuing without changing your cookie settings, you agree to this collection. For more information, please see our University Websites Privacy Notice .
College of Agriculture, Health and Natural Resources
Science of GMOs
Gmos and food safety.

Food products which are developed via Genetically Modified Organisms (GMOs) are widely distributed in the U.S. food supply. In addition, new products are being approved for use as techniques are developed and new uses for GMOs are considered. Biotech/GMO derived foods currently in use include corn used in milled corn products; soy used in milled soy products such as soybean oil and textured soy protein; animal feeds; and sugar beets. In addition, the Arctic apple (resists browning) and GMO Salmon have been recently approved as “safe” by the U.S. Food and Drug Administration (FDA).
The safety of Genetically Modified Organisms (GMO) has been debated almost since the phrase was attributed to the development of antibiotic resistant tobacco in 1983. Consumers continue to be concerned about both the food safety and the nutritional equivalence of GMO foods. In a 2015 Pew Research Center survey of consumers, 57% of adults believe that eating GMO foods is unsafe, while 37% say they believe it is generally safe.
Yet, science continues to suggest that there is no substantiated evidence that GMO foods are less safe than non-GMO derived food products. A 2016 report from the National Academies of Science, Genetically Engineered Crops: Experiences and Prospects discusses effects on human health. Claims regarding human health and safety of GMO foods included increased risks from cancers, kidney disease, obesity, celiac disease, diabetes and allergies. When comparing rates of these conditions in the United States, where GMOs are ubiquitous in the food supply to the United Kingdom, where essentially no GMOs are consumed, there were no significant differences.
That said, it must be emphasized that science is ever changing, the science of genetic engineering is relatively young, and there is still uncertainty around long term effects. Absolute safety cannot be guaranteed for any specific food. However, companies do withdraw products from consideration that are determined to be unsafe. In 1996, researchers found that an allergen from Brazil nuts continued to be allergenic when transferred into a GMO soybean. That soybean was never approved. This experience resulted in policies that proteins that have ever been suspected of being or shown to be an allergen should never be introduced in GMO crops.
The FDA developed its Plant Biotechnology Consultation Program in the 1990’s to cooperatively work with GE (genetically engineered) plant developers to help make sure that foods made from their new GE plant varieties are safe and meet all regulations. FDA evaluates the safety of food produced by new GE crops before it enters the market. This is a voluntary program and FDA has evaluated 150 genetically engineered foods since the program began. These evaluations are publicly available on the FDA website, www.fda.gov .
By Diane Hirsch, Extension Educator Emerita, UConn Extension
Published October 18, 2017
Should our future food be genetically engineered?
Genetically modified crops can help cut carbon emissions, research shows — but they still face major hurdles.

The Philippines Department of Agriculture has a vision: to become the first country to allow the commercial production of golden rice , a 20-year-old genetically modified crop that could prevent hundreds of thousands of cases of childhood blindness around the world.
But the country’s appeals court came to a very different decision last month. The court banned cultivating the crop, named for the yellow color that comes from the addition of Vitamin A , as well as a genetically modified eggplant.
“This decision is a monumental win for Filipino farmers and Filipino people who have for decades stood up against genetically modified (GM) crops,” Wilhelmina Pelegrina, a Southeast Asia campaigner for Greenpeace, an advocacy group that has opposed genetically modified crops for decades, said in a statement .
While genetically modified crops may still provoke fear and uncertainty, some scientists argue that not only can they help to alleviate human health concerns, but they might also be able to help fight climate change. And as new tools like CRISPR , which can make targeted cuts in DNA, gain traction, genetic food engineering could be on the cusp of a quantum leap.
“It’s all political,” Stuart Smyth, a professor of agricultural and resource economics at the University of Saskatchewan, said of the Philippines decision. “It’s not based on science.”
Genetically modified crops are ones that have had genetic material inserted from another species of organism. For example, the first genetically modified food product — a tomato introduced to the public in 1994 as the “Flavr Savr” — had two genes added. One conferred antibiotic resistance, and another gave the tomato a longer shelf life. (The company manufacturing the Flavr Savr, Calgene, had to cease production in 1997 because of rising costs.)
Today, there are only a few genetically modified crops in production, but those that exist are widely grown. In the United States, 94 percent of all soybeans, 96 percent of all cotton and 92 percent of all corn was genetically modified as of 2020, according to the Food and Drug Administration . These crops became popular because of their ability to withstand glyphosate, a key ingredient in the herbicide known as “Roundup.” Other countries that grow genetically modified crops widely include Canada, Brazil and India.
No major scientific research has found that genetically modified crops cause health problems in humans. In a 400-plus-page report published in 2016, the National Academies of Science found that “no substantiated evidence that foods from GE [genetically engineered] crops were less safe than foods from non-GE crops.” The report urged analysis of such foods by the traits that they include, rather than how they were created.
Yet engineered crops remain unpopular. According to a Pew Research Center poll from 2020, 38 percent of Americans believe genetically modified crops are unsafe, compared with 27 percent who believe they are safe. Thanks to a law passed by Congress in 2016, foods in the United States are required to be labeled as bioengineered if they involved genetic engineering beyond what could be accomplished with conventional breeding techniques. One analysis showed that consumers are willing to pay 20 percent more to avoid GM foods.
At the same time, a small but growing body of research has argued that GM foods could play a significant role in cutting carbon emissions. In a study published last year, researchers at the University of Bonn in Germany and the Berkeley, Calif.-based Breakthrough Institute found that widespread use of these crops in Europe could cut the agricultural sector’s emissions by 7.5 percent.
Another study found that the use of GM crops globally saves around 23 million metric tons of carbon dioxide every year — equal to removing around half of all vehicles from roads in the United Kingdom.
There are two primary ways genetically engineered crops could cut carbon emissions.
First, they can be more productive, creating higher yields for farmers and allowing them to grow more food on less land. One global analysis found that GM crops on average lead to a 22 percent increase in yields. At the same time, one-third of all emissions from agriculture are from deforestation and the destruction of other natural areas — as farmers expand and grow more crops, they cut down trees that are storing CO2 in their trunks and leaves.
If farmers can grow their crops on less land, less forest is converted into farmland, allowing trees and landscapes to store more carbon. “That decrease in deforestation is the big reason why yield increases cut emissions,” said Emma Kovak, a senior food and agriculture analyst for the Breakthrough Institute.
Other scientists say crops with herbicide resistance can require less tilling. “Every time soil is tilled, it releases carbon back into the atmosphere,” Smyth said. Herbicide-resistant corn, for example, can endure being sprayed by weed-killing agents, preventing farmers from having to till the land to remove weeds.
But the environmental community is split. Some activists say focusing on climate change obscures the real problem with genetically modified crops: the role of big corporations in controlling food production.
“We see GMOs as a tool of the major corporations that already have a stranglehold on our food system,” said Amanda Starbuck, research director at Food and Water Watch. Many genetically modified crops, Starbuck says, go toward feeding animals for meat production — and improvements in yield won’t change the fact that humans need to move away from eating so much meat. “We need to move to significantly reduce that consumption,” she added.
Research into alleviating climate change with genetically modified crops has just begun. “On a scale of one to 100, I’d say it’s single digits,” Smyth said. Scientists say they need more analysis of how GM crops change land use and carbon sequestration, and studies that take place over longer periods of time.
But even in areas where the science is relatively settled, genetically engineered foods have struggled to gain acceptance. Golden rice was developed in 1999 by a Swiss scientist; it was intended to combat the estimated 250,000 to 500,000 children every year who go blind from Vitamin A deficiency. More than two decades later, however, the crop has not entered widespread cultivation, thanks in part to regulatory battles in Asia and resistance from environmentalists.
In its decision to ban genetically modified crops, the appeals court cited a Philippines legal principle granting the right to a healthy environment.
For opponents of genetically engineered crops, that is a victory; for some scientists, it is a missed opportunity. “It’s sad that something someone developed in the 1980s to solve a problem — a really bad problem, children going blind — is still relevant,” Kovak said.
And while the battle lines around genetically modified crops have been set for decades, new technologies may shake things up. Gene-editing tools like CRISPR allow scientists to make tweaks, deletions or changes in a genome without inserting genes from another species. Researchers are already working on gene-edited crops that could speed up photosynthesis and increase crop yields.
Changing a genome without adding a component from another species could be more palatable to consumers — but some environmental groups believe it is just a way to rebrand the same type of work.
“Industry could say, ‘Well, it’s not GMO. It’s gene-edited,’” Starbuck said. “It’s just another smokescreen.”
The shift could also complicate existing regulations, which have been tied to older definitions of genetic modification.
“It’s frustrating,” Smyth said. “We need to make all of these changes to cut carbon emissions. But how are we supposed to meet the Paris accord with one hand tied behind our back?”
More on climate change
Understanding our climate: Global warming is a real phenomenon , and weather disasters are undeniably linked to it . As temperatures rise, heat waves are more often sweeping the globe — and parts of the world are becoming too hot to survive .
What can be done? The Post is tracking a variety of climate solutions , as well as the Biden administration’s actions on environmental issues . It can feel overwhelming facing the impacts of climate change, but there are ways to cope with climate anxiety .
Inventive solutions: Some people have built off-the-grid homes from trash to stand up to a changing climate. As seas rise, others are exploring how to harness marine energy .
What about your role in climate change? Our climate coach Michael J. Coren is answering questions about environmental choices in our everyday lives. Submit yours here. You can also sign up for our Climate Coach newsletter .

- Skip to main content
- Keyboard shortcuts for audio player
Your Health
- Treatments & Tests
- Health Inc.
- Public Health
A mix-up over bioengineered tomato seeds sparked fears about spread of GMO crops
Sasa Woodruff

The Purple Galaxy Tomato splashed across the cover of this season's Baker Creek Heirloom Seeds catalog: a closeup of a blackish-purple tomato speckled with tiny pink dots. Next to it, sits a sliced open fruit, revealing deep fuchsia seeds and flesh.
"This beauty is believed to be the first — and the purplest — non-GMO purple tomato in the universe!" read the catalog copy.
Only problem? The seeds actually may have been a GMO variety, the recently released Purple Tomato, created using genes from a snapdragon flower by Norfolk Healthy Produce.
The mix-up has caused consternation for the heirloom seed company that prides itself on offering rare and organic varieties and takes a firm stance against GMO crops. And it's triggered debate about biodiversity and what can happen with GMO seeds when they begin to spread.
When news of a non-GMO purple-fleshed tomato variety first started circulating on social media last fall, some scientists and tomato enthusiasts weren't so sure.

Shots - Health News
Gardeners can now grow a genetically modified purple tomato made with snapdragon dna.
"I had discussions with colleagues about it, and all of us just looked at it and said, well, that's the GMO tomato," says David Francis, a professor of horticulture and crop science at the Ohio State University who specializes in tomato breeding and genetics.
Traditional plant breeders to date have not been able to create a purple-fleshed tomato with cross pollination. Purple skin, yes? Purple flesh, not so much.
But using recombinant DNA technology, scientists in the United Kingdom had developed a purple-fleshed tomato high in antioxidants. It was recently approved for sale and consumption in the United States.

A caprese salad prepared with Norfolk's Purple Tomato. The tomato was created using genes from a snapdragon. Norfolk Plant Sciences hide caption
A caprese salad prepared with Norfolk's Purple Tomato. The tomato was created using genes from a snapdragon.
After Nathan Pumplin, CEO of Norfolk Healthy Produce, saw Instagram videos of the heirloom seed company's Purple Galaxy tomato, he contacted Baker Creek. And here's where the story gets murky.
John Brazaitis, general manager of Baker Creek Heirloom Seeds says their seeds were developed by a hobby breeder in France where growing GMOs is banned. Brazaitis says they tested for NPTII, a common marker for GMOs, but didn't specifically test for the snapdragon genes.
After some correspondence and disagreement about the testing, Baker Creek pulled the seeds from its collection and destroyed its stock.
The seed company refused to say whether or not the seeds were GMO and wrote in a statement : "After repeated testing, we are unable to conclusively establish that the Purple Galaxy does not contain any genes that have been genetically modified."
Pumplin wouldn't say definitively either, but their website says this : "We are told that laboratory testing determined that it is, in fact, bioengineered (GMO). This result supports the fact that the only reported way to produce a purple-fleshed tomato rich in anthocyanin antioxidants is with Norfolk's patented technology."
But the next mystery is one that's harder to answer: How could seeds get from a closed lab in the United Kingdom to a hobby gardener in France?
"I don't think it's a runaway train. You could easily argue that Baker Creek has it in their catalog because somebody misappropriated it and didn't do their due diligence," Ohio State's Francis says, "Whether that was just incompetence or a mistake, who knows?"
Francis says this isn't a case of the modified tomato genes escaping into the wild from a UK lab and traveling by wind across the English Channel to France because tomatoes don't spread like dandelions, purslane or ivy.
"For the same reason that regular tomatoes don't become weeds," he says, "They just don't have the characteristics that allow them to compete well in a crowded environment."
Francis says humans were most certainly involved. The GMO Purple Tomato was in development for 20 years, which means access to plant materials was long and sustained.
"Maybe it's a collaborator in France had some and their technician took it, and then their technician gave it to a friend who knows, right?" he says, "Somebody took it and said, hey, I'm going to play with this."
This isn't the first time a genetically engineered plant ended up with unwitting producers or consumers. In 1987, a German lab created an orange petunia by inserting a maize gene. It was never released to the public, but almost 30 years later, it was found in Finland, again almost certainly from someone illicitly breeding them. The culprit plants were all over Europe and the United States, not growing in the wild, but in gardens, parks and train stations.
Most of Europe has a GMO ban, so government agencies asked growers to destroy the orange varieties. When the USDA asked for a recall in 2017 , there were nine varieties growers had to destroy with names like Trilogy Mango, Petunia Salmon Ray or Sweetunia Orange Flash. The USDA approved the orange petunia for sale in 2021 .
Even if the GMO purple tomato seeds were not spreading in the wild, Baker Creek's Brazaitis is concerned that GM seeds could show up in surprising places and growers won't know if they have GM seeds or not.
"It's going to happen again and again as we see more GM crops come to market for consumers," says Brazaitis.
Baker Creek's Brazaitis says the whole experience of pulling the seed from their collection was very painful and worries about the long-term implications.
"We were absolutely over the moon about finding this really unique variety," Brazaitis says, "The comedown from that has been really hard. We never thought we'd be facing a GMO issue with tomatoes."
Pumplin says that USDA evaluated their tomato (as it does for all approved GM crops) to make sure it was unlikely to start spreading like a weed. "There is nothing in the purple tomato that would make it overtake other tomato populations," says Pumplin.
Tomatoes have about 35,000 genes and Pumplin points out the Purple Tomato has only two extra from a snapdragon. Tomatoes are self pollinating, which means pollination is contained within the flower and the risk of gene spread is very low.
Still, Brazaitis worries that GM varieties of plants could take over. "If we lose the biodiversity in our plant world, these varieties no longer exist and you're entirely dependent on things like GMOs to provide food," he says.
He says maintaining heirloom varieties is important because they're constantly adapting to new environments. USDA organic certified products don't allow GM varieties.
Francis argues that biodiversity is thriving in the tomato world.
"Some of the research that my group has done on tomatoes shows pretty conclusively that contemporary tomatoes, what we're using today, are more genetically diverse than the heirloom tomatoes of old," Francis says.
One of the main reasons is wild tomato genes have been pulled in and crossed for disease resistance and nutritional content is actually widening the gene pool of our food.
Correction April 28, 2024
In an earlier version of this story the name of Norfolk Healthy Produce CEO Nathan Pumplin was misspelled as Pumpkin.
- organic food
- purple tomato
- Reference Manager
- Simple TEXT file
People also looked at
Review article, modernizing and harmonizing regulatory data requirements for genetically modified crops—perspectives from a workshop.

- 1 Corteva™ Agriscience, Indianapolis, IN, United States
- 2 CropLife International, Arlington, VA, United States
- 3 BASF Corporation, Research Triangle Park, NC, United States
- 4 Corteva™ Agriscience, Johnston, IA, United States
- 5 Syngenta Seeds LLC, Research Triangle Park, NC, United States
- 6 Bayer Crop Science, Chesterfield, MO, United States
Genetically modified (GM) crops that have been engineered to express transgenes have been in commercial use since 1995 and are annually grown on 200 million hectares globally. These crops have provided documented benefits to food security, rural economies, and the environment, with no substantiated case of food, feed, or environmental harm attributable to cultivation or consumption. Despite this extensive history of advantages and safety, the level of regulatory scrutiny has continually increased, placing undue burdens on regulators, developers, and society, while reinforcing consumer distrust of the technology. CropLife International held a workshop at the 16th International Society of Biosafety Research (ISBR) Symposium to examine the scientific basis for modernizing global regulatory frameworks for GM crops. Participants represented a spectrum of global stakeholders, including academic researchers, GM crop developers, regulatory consultants, and regulators. Concurrently examining the considerations of food and feed safety, along with environmental safety, for GM crops, the workshop presented recommendations for a core set of data that should always be considered, and supplementary (i.e., conditional) data that would be warranted only on a case-by-case basis to address specific plausible hypotheses of harm. Then, using a case-study involving a hypothetical GM maize event expressing two familiar traits (insect protection and herbicide tolerance), participants were asked to consider these recommendations and discuss if any additional data might be warranted to support a science-based risk assessment or for regulatory decision-making. The discussions during the workshop highlighted that the set of data to address the food, feed, and environmental safety of the hypothetical GM maize, in relation to a conventional comparator, could be modernized compared to current global regulatory requirements. If these scientific approaches to modernize data packages for GM crop regulation were adopted globally, GM crops could be commercialized in a more timely manner, thereby enabling development of more diverse GM traits to benefit growers, consumers, and the environment.
1 Introduction
Genetically modified (GM) crops that have been engineered to express transgenes have been commercially cultivated since 1995 and are annually grown on 200 million hectares globally. These crops have delivered important societal benefits, such as increased crop yields, resilience to adverse growing conditions, reduced tillage leading to improved soil health, reduction in the need for crop protection inputs, preservation of natural resources, and improved rural economies ( Klümper and Qaim, 2014 ; Dively et al., 2018 ; Zilberman et al., 2018 ; Smyth, 2020 ; Ala-Kokko et al., 2021 ; Macall et al., 2021 ; Peshin et al., 2021 ; Brookes, 2022a ; Brookes, 2022b ; Brookes, 2022c ). These benefits have led to rapid adoption of GM technology for agricultural production, including 80% of global cotton and 73% of global soybean. One-third of global maize production includes GM traits for herbicide tolerance, insect protection, or both ( AgbioInvestor, 2023 ). GM traits have been introduced in other row crops such as oilseed rape, sugar beet, and alfalfa and, at a smaller scale, in specialty crops such as apples, eggplant, squash and potatoes ( ISAAA, 2020 ). Hundreds of studies have been conducted to assess the safety of GM crops, and there have been no substantiated cases of resulting harm to people or livestock that consume GM crops or to the environment in which they are grown ( European Commission, 2010 ; Snell et al., 2012 ; Van Eenennaam and Young, 2014 ; NASEM, 2016 ).
Despite this track record of safety and benefits, regulatory data requirements for approval and commercialization of GM crops have continued to grow globally. GM technology is primarily limited to major global crops, like maize and soybean, and to major input traits, such as insect protection and herbicide tolerance. While there are many efforts underway to use GM technology for other traits and to improve minor crops, especially for small holders in the developing world ( David, 2009 ; Shelton, 2021 ; Woodruff, 2024 ), securing the regulatory approvals to enable cultivation and avoid potential trade disruptions can present often insurmountable challenges to commercialization. Only a few large multinational developers can afford the US$115 million cost and also persist for the 16 years that it currently takes, on average, to bring a new trait to the global market. More than one-third of those costs, and more than one-half of that time, are taken by the regulatory process ( AgbioInvestor, 2022 ). These extensive and complex regulatory systems also mean that governments must invest significant resources in developing and maintaining regulatory bodies staffed with sufficient people and expertise, creating a burden on taxpayers and society. Countries that cannot afford such an investment are missing out on the benefits of GM crops.
CropLife International and its member companies that develop GM crops (BASF, Bayer Crop Science, Corteva™ Agriscience, and Syngenta) have proposed a modernized regulatory framework and streamlining of data requirements for GM crops that is based on scientific rationale and builds on the 25 years of experience with the technology, and the history of its safe use ( Mathesius et al., 2020 ; Anderson et al., 2021 ; Bachman et al., 2021 ; Brune et al., 2021 ; Goodwin et al., 2021 ; McClain et al., 2021 ; Roper et al., 2021 ; Waters et al., 2021 ). The development of the proposed framework was motivated and guided by considering four key questions. 1) Are today’s regulations for GM crop approvals risk-proportionate? 2) Do today’s data requirements act as an unnecessary barrier to beneficial innovation? 3) How can knowledge and experience accumulated over the last 25 years inform modernization of regulations? 4) Can data requirements be streamlined and harmonized across countries and authorities? These questions were used to guide the determination of the types of data that are necessary to ensure GM crops are developed and deployed without increased risks for food and feed safety or the environment compared to conventional crops. Under this framework, core data, which are important for the problem formulation step of the risk assessment of the GM crop, were identified. The core data are used for problem formulation to identify plausible cause-and-effect hypotheses of harm from the GM crop. Depending upon the outcome of the problem formulation for a specific crop by trait combination, additional supplementary (i.e., conditional) studies may be needed, on a case-by-case basis, to analyze any plausible risk identified. Figure 1A outlines proposed core and supplemental studies for a Food and Feed Safety Assessment; Figure 1B outlines proposed core and supplemental studies for an Environmental Risk Assessment. CropLife International took an approach that is consistent with principles of risk assessment such that the proposed data requirements can fully inform decision-making by a regulatory agency, without the extraneous data present in many current regulatory submissions that does not meaningfully contribute to the risk assessment of the GM crop.
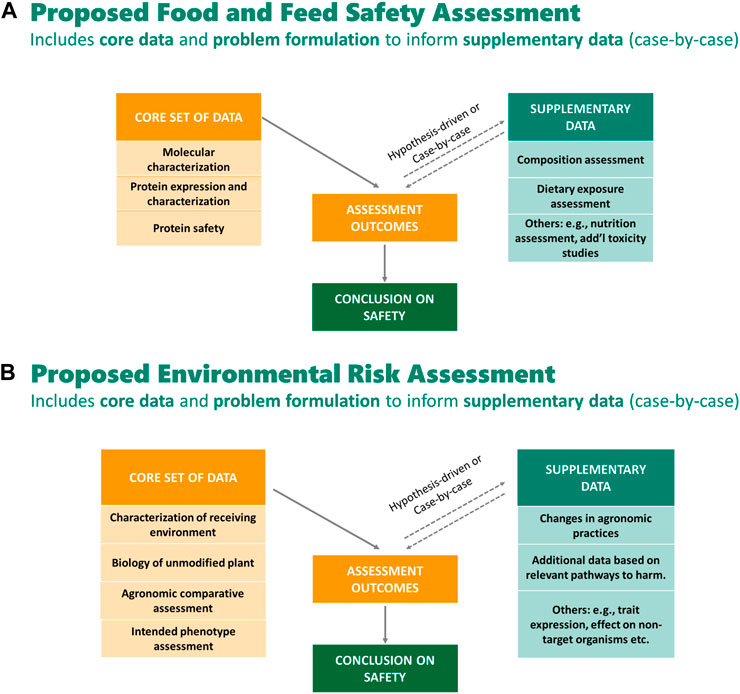
Figure 1 . (A) proposes a set of data recommended for a science-based food and feed safety assessment for a typical GM crop and considers as core studies: basic molecular characterization, protein characterization and expression, and protein safety (i.e., history of safe use of the protein and source organism and bioinformatics to identify potential toxins and allergens). The outcomes of these core data are used to inform the problem formulation step and decide, on a case-by-case basis which, if any, supplementary studies are needed to make a conclusion on safety ( Brune et al., 2021 ; Waters et al., 2021 ). (A) is adapted from Brune et al., 2021 and Waters et al., 2021 . (B) proposes a set of data recommended for a science-based environmental risk assessment for a typical GM crop and considers as data: understanding the receiving environment and the basic biology of the unmodified plant; assessing the agronomic similarity of the GM crop to its conventional counterparts (i.e., agronomic comparative assessment); and understanding the intended trait of the GM plant and assessment of how the intended trait may lead to environmental harm. The core data should be used first to inform the problem formulation. If a conclusion cannot be made about the pathway to harm using the core data, additional case-by-case hypothesis-driven supplementary studies should be considered ( Anderson et al., 2021 ).
To further examine whether CropLife International’s proposed modernized data requirements are sufficient for food and feed safety assessments and for environmental risk assessments, a workshop was held at the 16th International Society of Biosafety Research (ISBR) Symposium (St. Louis, USA) in 2023. Using a case study of a hypothetical GM maize event containing two familiar transgenic traits (herbicide resistance and insect protection). The workshop participants were charged with considering whether the proposed data in the case study are scientifically both necessary and sufficient to determine the food, feed and environmental safety of the hypothetical GM crop.: CropLife International member representatives that served as moderators during the workshop authored this publication to report the outcomes and summarize the discussions that took place among the participants. The participants varied in their backgrounds and prior experience with risk assessment and included individuals from regulatory agencies, technology developers, consultant groups, and academia. A wide range of geographical areas were represented.
2 Case study description
For the case study, a hypothetical GM maize event was presented to the workshop participants for evaluation. The hypothetical event was intentionally simple for this exercise (i.e., a familiar crop with traits that are similar to many transgenic events that have already been reviewed and approved by regulatory agencies globally, with several in commercial production for many years), which enabled the participants to analyze in greater depth the need for data that is routinely submitted but may not contribute to the safety assessment. More specifically, a maize ( Zea mays ) event containing a single insertion encoding for two proteins from a single T-DNA introduced using standard disarmed Agrobacterium tumefaciens-based transformation was described. The two hypothetical traits provide protection against lepidopteran pests and tolerance to treatment with glyphosate herbicide, using a hypothetical Cry1 protein from Bacillus thuringiensis ( Bt ) and a hypothetical EPSPS protein variant isolated from maize, respectively. The workshop participants were asked to separately consider a food and feed safety assessment or an environmental risk assessment for this same hypothetical GM maize event. Additional distinctions between the presentation of the case study for the different assessments are outlined below.
2.1 Food and feed safety assessment
For the Food and Feed Safety Assessment, the results from hypothetical evaluations of core data on the characterization and safety assessment of the event were provided (summarized in Table 1 ). Throughout this paper, the term ‘data’ refers to both the results of experiments or studies as well as information gathered from literature reviews, consensus documents and other similar sources. As described in Waters et al. (2021) , the core data for a food and feed safety assessment are: 1) molecular characterization, 2) protein characterization, and 3) protein safety (allergenicity and toxicity). The results of the molecular characterization demonstrated that there was an insertion of a single T-DNA sequence into the maize genomic DNA without any vector backbone sequences. There were no changes in the intended protein coding sequence and constitutive expression of both proteins were driven by familiar promoter elements (35S from cauliflower mosaic virus and ubiquitin promoter from Zea mays , respectively). Finally, the inserted DNA and the traits were indicated as being stable over three generations. The protein characterization data given to participants indicated that the molecular weight and amino acid sequence were as expected for both proteins. The function of the hypothetical Cry1 protein was established as having activity limited to target lepidopteran pest species, with no activity against other insect orders. Field tolerance to glyphosate from the hypothetical EPSPS protein variant was also as expected. The protein safety data indicated that both proteins are similar to proteins that have a history of safe use for food and feed; neither EPSPS proteins nor Cry proteins have any known toxicity or allergenicity concerns. Bioinformatics analysis comparing the amino acid sequences of both hypothetical proteins to a protein database also demonstrated that neither protein is related to any protein of toxicological concern nor related to any allergens in the qualified allergen database.
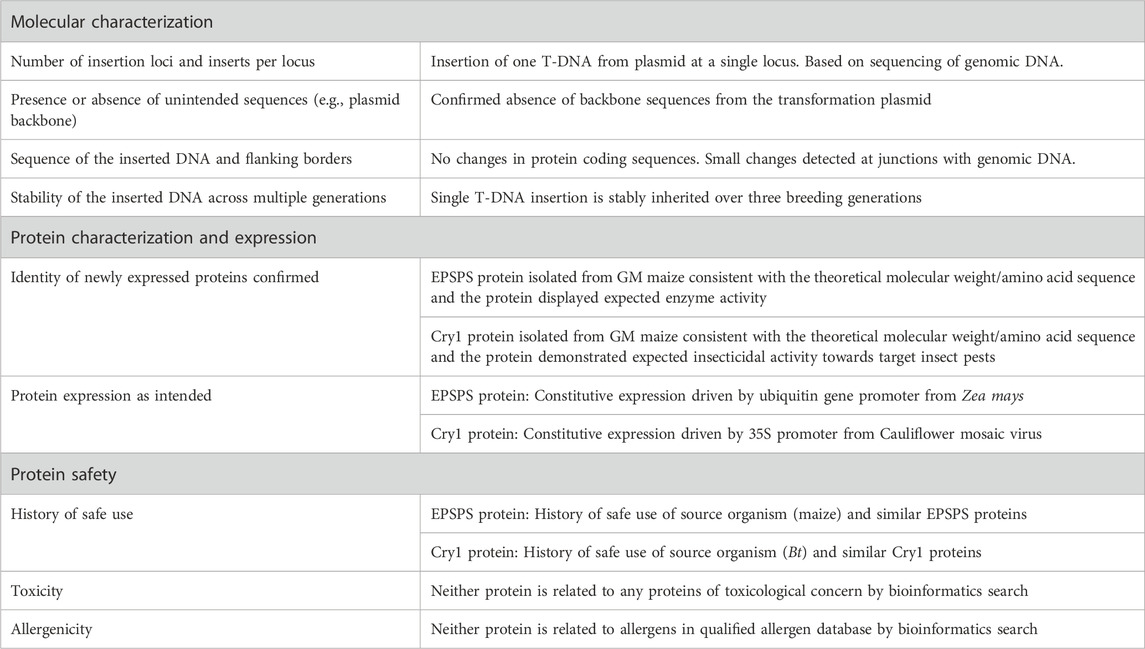
Table 1 . Summary of food and feed safety assessment core data of the hypothetical GM maize.
A familiar crop with familiar traits and minimal genetic disruptions was used for the workshop to promote discussion of what data is really needed to establish the food and feed safety of a GM crop event. It was also noted to workshop participants that extensive protein expression data in the plant was not obtained, nor was detailed proximate or nutrient composition data included. Further, while it was established that bioinformatics confirmed no homology to known allergens or toxins, no exposure assessments, no animal feeding studies, or other more direct assessments of potential for harm from the hypothetical event were included. As presented, the case study stated that considering 1) the assessment from the core data, 2) the familiarity of the crop and traits, and 3) the lack of direct interaction with other metabolic pathways of the plant, there was no hypothesis of food and/or feed safety risks for the new GM maize crop, and therefore additional supplementary data are not warranted to establish food and feed safety, in accordance with the approach established in Brune et al. (2021) , McClain et al. (2021) and Roper et al. (2021) .
2.2 Environmental risk assessment
For Environmental Risk Assessment (ERA), the intention of the case study was to model how problem formulation and core data should be leveraged to inform ERA of a GM crop for cultivation safety. Problem formulation is a process used in the ERA to develop plausible pathways to harm resulting from cultivation of the GM crop. Problem formulation first considers core data, then considers other data on a case-by-case basis if it is deemed necessary to inform the risk assessment. For ERA, core data includes information related to the receiving environment, description of basic biology of the unmodified plant, assessment of the agronomic similarity of the GM crop to its conventional counterparts, and characterization of the intended traits of the GM crop (summarized in Table 2 ). For the purpose of the case study, the protection goal was broadly stated as protection of biodiversity, specifically protection of beneficial or charismatic species. For the purposes of the workshop, the core characteristics of the event as described for the food and feed assessment were considered the same (e.g., molecular features), with additional information focused on agronomic and environmental aspects provided to guide the ERA discussion.
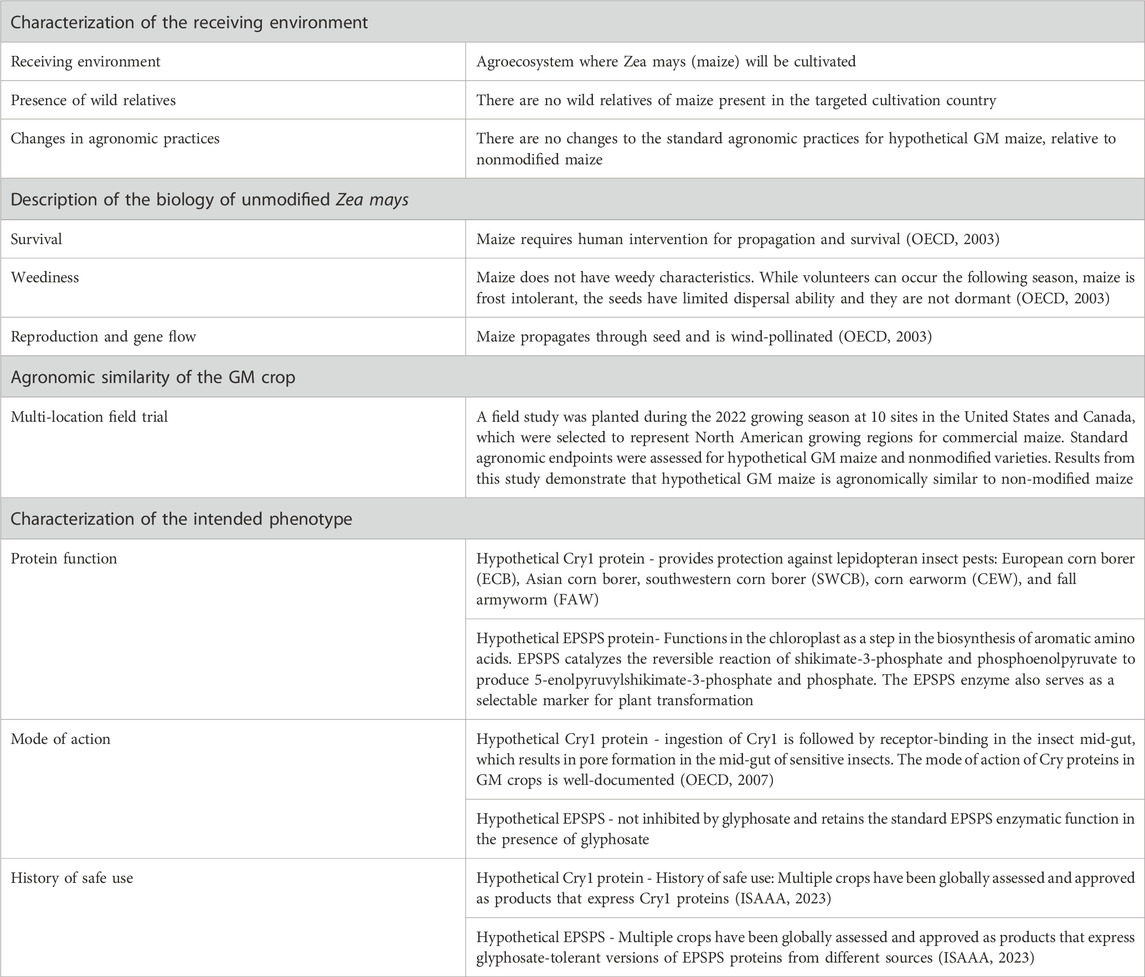
Table 2 . Summary of environmental risk assessment core data.
The participants were presented with the following set of core data (summarized in Table 2 ) and were asked to consider if a plausible pathway to harm could be developed related to weediness, invasiveness, gene flow to wild relatives or hazard to non-target organisms: 1) assessment of the receiving environment indicating no wild relatives of maize present in the cultivation country and no changes to the standard agronomic practices relative to non-modified maize; 2) assessment of the basic biology of maize, using consensus documents, demonstrating non-modified maize has no weediness characteristics and requires human intervention for propagation and survival; 3) multilocation field trial data demonstrating hypothetical maize was agronomically similar to non-modified maize; and 4) assessment of the intended phenotype (i.e., insect protection and herbicide tolerant traits are not intended to increase fitness or survival in the environment).
Based on the core data assessed, the case study proposed that there are no plausible hypotheses for how cultivation of the hypothetical maize could result in environmental harm related to weediness, invasiveness, and gene flow to wild relatives. Thus, additional data will not further contribute to meaningful assessment of environmental safety. However, the case study proposed that a plausible pathway to harm to non-target organisms could be developed based on the intended insect protection phenotype. The hypothetical Cry1 protein was presented as providing protection against specific lepidopteran insect pests (European corn borer, Asian corn borer, Southwestern corn borer, corn earworm, and fall armyworm).
The mode of action of Cry proteins in GM crops is well-documented ( Bravo et al., 2007 ; OECD, 2007 ). In this case study, additional supplemental protein expression data and non-target organism hazard data were provided to the participants, and they were asked to consider if additional plausible pathways to harm could be developed. The set of supplemental data (summarized in Table 3 ) was as follows: 1) multilocation field trial data measuring the concentration of the hypothetical Cry1 protein in several plant tissues to inform exposure assessment; 2) an exposure assessment for different non-target organisms to consider the likelihood and magnitude of exposure to the hypothetical Cry1 protein; and 3) results of non-target organism Tier I hazard studies for several surrogate species representing different taxonomic orders (e.g., ladybird beetle, a soil dwelling organism, and a non-target predator) conducted with the Cry1 protein in the diet.
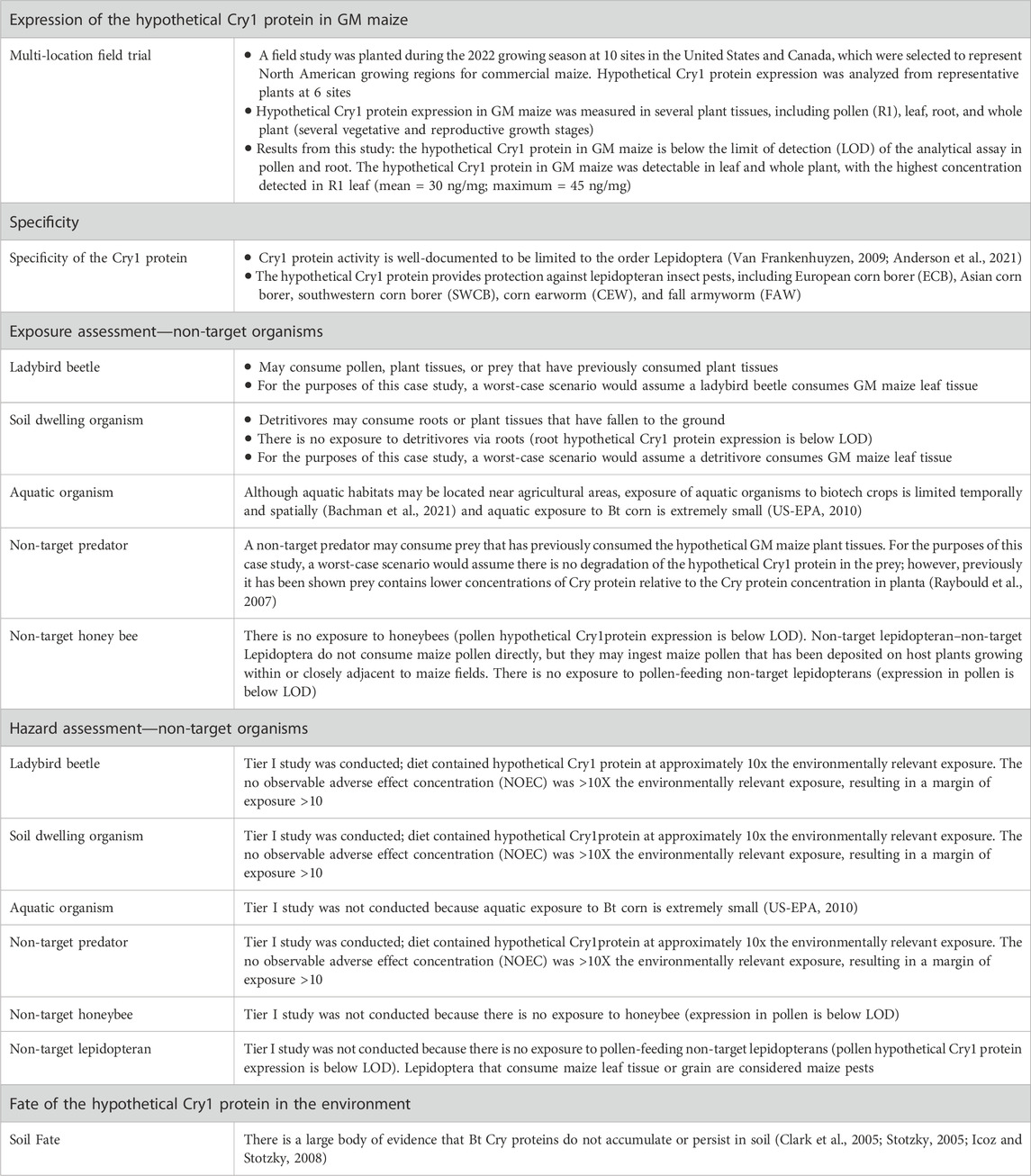
Table 3 . Summary of environmental risk assessment supplementary data.
The multilocation field trial data showed that the Cry1 protein was only detectable (above the limit of detection) in the leaf and whole plant, with the highest concentration found in R1 leaf. The protein was below the limit of detection of the analytical assay in pollen and root. Based on the tissue expression, the exposure assessment concluded that since there is no expression of the Cry1 protein in pollen, there would be no route of exposure to non-target pollen feeding organisms (e.g., honeybee). Finally, the Tier I hazard studies indicated that no hazard was observed at concentrations that exceeded >10x the expected environmental concentration.
Usually, the assessment of adverse effects in non-target organisms follows a tiered approach that starts with laboratory studies at levels that exceed worst-case exposure conditions ( Romeis et al., 2011 ). Tier I laboratory studies with non-target organisms are typically conducted using at least 10X the worst-case expected environmental concentration. In this case, the results of the hypothetical Tier I dietary studies indicated no hazard (i.e., adverse effects) at concentrations that exceeded 10x the worst-case expected environmental concentration, and thus a conclusion that evidence is sufficient without conducting additional hazard testing was indicated. Based on data from the exposure assessment and non-target hazard assessment studies, the case study proposed that there were no plausible pathways to harm to non-target organisms due to lack of exposure and/or lack of risk because there were no adverse effects at concentrations that exceeded 10X the worst-case expected environmental concentration. Participants were asked to consider whether they agreed with the conclusions proposed by the case study based on core data and additional supplementary data related to protein expression, non-target organism exposure, and non-target organism hazard.
Additional information such as molecular data to confirm that the insert is an intact single copy, stable across generations, and that there is no insertion of DNA from the plasmid backbone were not provided in the ERA case study. These additional data for product characterization have historically been submitted to regulators as part of cultivation applications, but they are not directly relevant to ERA ( Anderson et al., 2021 ).
3 Learnings from breakout group discussions
After participants attended the introductory presentation session of the workshop, they were distributed into smaller discussion groups of approximately 10 people, with CropLife International member representatives serving as moderators. Each participant had the opportunity to choose either the Food and Feed Safety Assessment or the Environmental Risk Assessment, depending on their respective areas of interest.
The goal of the smaller group discussion sessions was to allow participants to go into deeper conversations about the proposed modernized paradigm for a risk assessment of a GM crop. Discussions were aided by a distribution of a printed booklet that included a description of the hypothetical GM maize event and the data collected, and that outlined the key concepts of using the core data for a Food and Feed Safety Assessment and Environmental Risk Assessment. Moderators provided some time for the participants to review the information and then introduced the case study by giving a brief overview of the information provided in each data section of the case study. Participants were encouraged to provide feedback and to bring up questions and/or comments about topics/elements of the case study that they considered not sufficiently covered by the data provided. They were also asked to complete a worksheet allowing for comments on the specific steps of the assessment process.
Discussions during this small group session were productive and highly informative. Overall, the participants were engaged, willing to discuss, and mostly supportive of the general assessment framework of primarily using core data and only using further assessments on a case-by-case basis.
A summary of key points from the breakout group discussions is shared below. This section is not intended to be a complete summary of the discussion, rather the authors have captured points of interest with an emphasis on points that are worth considering for future workshops and discussions on this topic.
3.1 Food and feed safety assessment
In the small group session, participants were asked to consider 1) the assessment from the core studies (see Table 1 ), 2) the familiarity of the crop and traits, and 3) the lack of direct interaction with other metabolic pathways of the plant, and then decide whether there was a hypothesis of food and/or feed safety risks for the new GM maize crop. Because of these considerations, the position for the case-study was that, for the hypothetical event, additional supplemental studies are not warranted to establish food and feed safety, and the participants discussed whether they agreed with this position.
Below are some key feedback and questions captured during the workshop regarding the proposed approach for the assessment of Food and Feed Safety of the hypothetical GM maize event.
3.1.1 Molecular characterization (transformation method, transformation construct, DNA insert characterization)
Overall, the participants agreed that the proposed molecular characterization core data is aligned with what is currently provided and that the information was sufficient to inform a food and feed safety assessment. One potential exception to the core data package that was discussed is data demonstrating that the insert is stable over at least three generations. The participants suggested that this study could be considered as supplemental, and not necessarily required as part of the core data package, if the insert is demonstrated to be inserted into the chromosome and is not interrupting endogenous genes or regulatory elements, and there is no other reason to expect that the insert might be unstable (e.g., insertion site near a transposon). There was some discussion that three generations of data may not be considered enough by all regulatory agencies and that additional generations could be required for polyploid crop species. Additionally, participants raised questions about Agrobacterium transformation not being targeted and discussed providing data on whether any internal genes were modified. It was also noted by workshop participants that the use of Next-Generation Sequencing (NGS) to characterize the insert is not yet accepted by all regulatory agencies, but also there was recognition of the utility of NGS to provide a more comprehensive characterization of the insert and the insertion site compared to traditional methods (e.g., Southern blots).
3.1.2 Protein characterization (molecular weight, protein sequence confirmation, protein function)
Participants agreed that the protein characterization information was sufficient to inform the food and feed safety assessment, with some discussions around whether a registrant would always be able to provide what is required, as some proteins may be more challenging to characterize (e.g., difficulties in isolating the proteins in an active form, generating specific antibodies, or generating SDS-PAGE and Western blot data). A question was also raised on maize codon optimization and if the protein would still be considered the same as the native version. Future workshops can reinforce that maize codon optimization of the GM trait gene does not alter the trait protein sequence. Thus, it should not change the safety profile of the protein if there is no change to the amino acid sequence. Discussion also occurred regarding familiarity with promoters and the relationship to expression levels. The participants discussed if there might be a need to better understand the protein expression levels for unfamiliar promoters and also if increased expression levels might raise a concern of potentially increased allergenicity risk.
3.1.3 Protein safety/toxicology/allergenicity (background, source, history of safe use, bioinformatics)
Participants agreed that the EPSPS protein information for safety was sufficient to inform the food and feed risk assessments, but questions were raised about Cry proteins around digestibility and heat stability. There was also discussion regarding how similar a protein would need to be to a known protein to be considered familiar. Additionally, concerns were raised in the small group discussion on the limited protein expression data provided in the case study as it related to an exposure assessment. In response, the moderators noted that an exposure assessment is not necessary, because no hazard was identified from the proteins. However, when a hazard is identified, then protein expression levels are needed to enable assessment of potential exposure ( Brune et al., 2021 ).
3.1.4 Additional information needed to determine event safety
It was stated by one participant that if there was a disruption of a native gene, then composition data could be requested. Discussion also occurred regarding the concept of History of Safe Use (HOSU), and the amount of data, time and similarity (e.g., consideration of minor protein sequence differences) needed to establish something as having sufficient familiarity to be considered safe without additional data. One participant suggested that protein sequence data would be needed to demonstrate a HOSU and could be useful in determining the activity of the protein.
3.1.5 General feedback for food and feed safety assessment
Although participants generally agreed that the case study with a familiar crop and familiar traits is a good starting point for the discussions, several suggestions were made for further discussions to also provide a case study on an unfamiliar event or protein, to lay out how each study informs the safety assessment, to provide more on the problem formulation process, and to provide more graphics and to use examples. Discussion also occurred around the challenges of communicating and making changes to the currently provided data in regulatory applications. On this topic, proposals from participants included suggestions to emphasize more the end goal of getting needed products on the market sooner with less regulatory burden for all stakeholders and to publish more data prior to submission of the application in the scientific literature, and to be ready to provide additional data upon request.
3.2 Environmental risk assessment
After introducing the case study, the CropLife International moderator described a list (provided with the case study) of the specific potential pathways to harm that are relevant to the cultivation of the hypothetical maize event. Additionally, an explanation for how the core data can be used to sufficiently assess environmental risk was provided. For plausible pathways to harm that may not be sufficiently addressed by the core data (i.e., potential harm to non-target organisms), another list of potential pathways to harm that are specific to non-target organism (NTO) exposure was also presented.
Below are some key feedback and questions captured during the workshop regarding the proposed approach for the Environmental Risk Assessment of the hypothetical GM maize event.
3.2.1 Weediness potential
There was an overall consensus among the workshop groups that weediness can be adequately assessed using only core data. Participants agreed that there is not a plausible pathway to harm in the case study since maize is highly domesticated and volunteers will not survive without human intervention and management. One group discussed questions around the potential for dormancy, which may be a weediness trait, and whether it can be assessed in the core data (multilocation field trial; Table 2 ). It was concluded within the groups that the similarity in agronomic characteristics between the GM maize event and the non-GM maize in the case study core data is sufficient to show that there is a highly unlikely risk of weediness potential. This follows the principle of placing risk in the context of current practice (i.e., that the modified maize will have no greater risk than that of cultivation of the non-modified maize) ( Raybould and MacDonald, 2018 ). However, one workshop group had unresolved discussions on whether a difference in agronomic performance between different geographical regions may result in differences in the risk assessment and what specific agronomic elements are the most relevant to consider. Some participants in this group proposed scenarios in which the agronomic data generated in field trials performed outside of the cultivation country may not sufficiently represent the agronomic outcomes of field trials performed within the cultivation country.
3.2.2 Gene flow potential to wild relatives
There was general consensus that there is no environmental safety concern of gene flow in the case study based on the core data because there were no wild relatives present in the hypothetical cultivating environment. There was some interest from participants in further exploring how the risk assessment and data requirements will change if the cultivation environment did contain wild relatives. Also, there was some discussion on the threshold of relatedness between the GM maize and a wild relative species that constitutes a safety concern in terms of gene flow. Ultimately, there was additional consensus that product registrants should demonstrate that there are no wild relative species that are reproductively compatible with GM maize (regardless of species relatedness) to position that there is no gene flow concern. Alternatively, if there are wild relative species in the area of cultivation an assessment of the likelihood and consequences of trait introgression into the wild relative population may be warranted based on a problem formulation approach ( Anderson et al., 2021 ). Participants generally stressed the importance of citing published literature (e.g., accepted consensus references on crop-specific biology) as part of the core data to support the environmental risk assessment. Although it was acknowledged that gene flow will not likely occur between GM maize and wild relatives in the case study example, there was some discussion around whether gene flow may occur between the GM maize and adjacent local non-GM maize varieties and negatively impact crop integrity and biodiversity. The case study focused on assessing plausible pathways to harm related to gene flow between GM maize and sexually compatible weedy relatives. Future workshops can address concerns that were raised about coexistence of GM and non-GM cropping systems. Such a workshop may have to distinguish between environmental risks and market or socio-political concerns. For example, countries that have landrace populations for which the genetic make-up per se is a protection goal may have societal concerns about coexistence (for example, there could be changes the genetic identity of the landrace).
3.2.3 Plausible pathways to harm for non-target organisms (NTO)
All groups aligned that the only plausible pathway to harm from the case study that could not be sufficiently addressed with core data alone was the potential for harm to NTOs from potential exposure to the hypothetical Cry1 protein ( Table 2 ). Participants discussed the plausible pathways to harm that are specific to NTOs. There was general agreement that no additional data was needed to assess the potential for the EPSPS protein conferring the herbicide tolerance trait to cause harm to NTOs. However, participants acknowledged that public perception of herbicide tolerance traits could influence regulatory decisions and may need to be considered when determining the registrability of a GM crop. Such perceptions are not reflective of an actual risk, and the additional data generated do not inform the science-based risk assessment. For other pathways to harm, there was consensus that if there was either no hazard or no detectable exposure, then there is low risk to NTOs. For example, honeybees that may directly consume maize pollen and NTO lepidopterans that may indirectly consume maize pollen that drifts onto their host plants should have low risk in the ERA case study since the GM maize event has expression less than the limit of detection (LOD) of the insecticidal protein in pollen tissue ( Table 2 ). It was generally accepted by workshop participants that if expression of the insecticidal protein is <LOD in tissues that might be consumed by an NTO, further toxicity testing to determine hazard is not warranted.
Participants were also mostly aligned that aquatic environments generally experience minimal exposure to GM crop tissue and so additional toxicity testing is not needed for aquatic NTO species in most situations. However, some participants expressed uncertainty on whether this may be an issue if GM crops are cultivated very close to aquatic environments, which may affect exposure levels to NTO aquatic species. For NTO species where there is a plausible pathway to harm, all groups agreed that further data (exposure assessment or NTO Tier I laboratory testing) might be needed. Some discussions among participants regarding appropriate surrogate species to use for NTO testing and to what extent test species need to match those found in the cultivation regions were not resolved in the workshop. There was some additional discussion around the large body of scientific literature describing the surrogate species concept for testing Cry proteins and other types of plant incorporated protectants (e.g., Romeis, et al., 2011 ; Romeis et al., 2013 ; Bachman et al., 2021 ). While the terms “focal species” and “indicator species” were not discussed directly as part of the workshop, understanding protection goals and selecting appropriate surrogate species or indicator species to inform the science-based assessment of risk is an important consideration ( Rose, 2007 ; Roberts et al., 2020 ). Despite the lack of consensus on species selection, there was clear alignment among participants that NTO species representatives should only be tested if there is a valid hypothesis that there is a plausible pathway to harm for that specific organism type. For this reason, NTO studies should only be conducted when hypothesis-driven ( Figure 1B ).
3.2.4 General feedback and future considerations for ERA
Although participants agreed that a generic ERA case study is a good starting place, participants indicated that future workshops using a modified case study tailored for specific geographical regions will be even more helpful. As different countries have different sets of questions and concerns from local regulatory agencies, using more country-specific scenarios and less familiar pest-control traits in a case study may be more directly relevant in that region.
Related to gene flow, there was not a consensus about potential for harm in small team discussions. Future workshops would benefit from guided discussion to help develop problem formulation for gene flow. For example, it could be established as a baseline that for gene flow to occur naturally in the environment, and when assessing the potential for harm from gene flow between GM maize and local maize varieties, it should be compared to potential for harm from gene flow of non-GM maize and local maize varieties ( OECD, 2023 ). Furthermore, future workshops can reinforce that if gene flow to local maize varieties is a relevant concern for a specific cultivation country, then there is a large body of literature to leverage to assess if additional data is needed to inform the risk assessment (See OECD, 2023 Annex B for recent review) such a workshop would need to distinguish between the true environmental impact and concerns related to trade or economic issues.
Also, there were productive discussions on the topic of data transportability. Participants generally accepted the concept of transportability for lab study data. However, due to a lack of time for discussion, some unresolved questions remained regarding the transportability of field study data. Future workshops will benefit from guided discussion to help explain the principle of data transportability. An underlying principle of data transportability is that if no biologically relevant differences between a GM crop and its conventional counterparts are observed in one country or region, data from these studies can be used to inform the risk assessment in another country, regardless of agroclimatic zone ( Bachman et al., 2021 ). Following the recommendations for modernizing global regulatory frameworks for GM crops, additional agronomic data should only be collected in the local environment if there are plausible pathways for harm that cannot be fully informed by the core data.
Furthermore, there was some interest from participants in discussing how the proposed risk assessment paradigm might apply to combined GM products (i.e., breeding stacks), yield and stress traits (e.g., drought resistance), and streamlining of import registrations.
One topic that generated discussion across groups was the value of product characterization data in an environmental risk assessment. In the proposed modernized regulatory framework ( Anderson et al., 2021 ), underlying characterization data for the GM event are not regarded as core to the regulatory assessments (such as molecular data to confirm that the insert is an intact single copy, stable across generations, and that there is no plasmid backbone DNA). Although these data do not directly inform the ERA ( Anderson et al., 2021 ), it was discussed that an understanding of the characteristics of the GM product provides foundational information that enables the regulatory assessments to focus on the intended introduced trait during the problem formulation stage. Therefore, it was proposed to consider including, as part of the modernized ERA framework, a set of foundational information and data from the characterization of the GM event that confirms that (1) the intended gene sequence was inserted and functions as intended, as well as the number of such insertions; (2) the plants produce the intended newly expressed protein (NEP); (3) the intended phenotype is achieved.
4 Key considerations and takeaways from the workshop
The case study for the workshop considered a single event, albeit one that contained genetic material encoding for two proteins leading to two distinct traits (herbicide tolerance and insect protection). However, the majority of commercialized products contain multiple GM events that are combined through conventional breeding (also known as stacked trait products). The typical regulatory process first assesses all single events, before applying regulatory processes, if any, to the stacked trait products. In this sense, the case study used for the workshop reflected a realistic scenario in which regulators assess a single event regardless of whether the event will be commercialized as a single event or as a stacked trait product.
Regulatory processes for stacked trait products vary globally, with many countries recognizing the long, safe history of conventional breeding and not requiring additional assessment once all the single events are approved. It is the position of CropLife International that additional safety assessment of a stacked trait product produced by conventional breeding should not be required unless there is a plausible and testable hypothesis for interaction of the traits ( Goodwin et al., 2021 ). This case study did not address stacked trait products however, further iterations could include consideration of stacked trait products and how to evaluate possible interaction of traits.
The workshop was convened to explore the proposed modernized data requirements for regulatory assessments of GM crops ( Anderson et al., 2021 ; Waters et al., 2021 ). The participants were charged with considering whether currently implemented regulations for GM crops are risk-proportionate or whether they create an unwarranted barrier to the introduction of new traits. The organizers presented a position that knowledge and experience from 25 years of research and development could inform regulatory modernization and that streamlined data requirements could advance harmonization across countries and authorities.
Overall, considering the case study discussed, the participants at the workshop found the proposed modernized data requirements generally to be necessary and sufficient for decision making to support the safe commercial introduction of a new GM crop. There was a clear consensus that some of the current data requirements are no longer routinely warranted for familiar traits such as that discussed in the case study, given the track record of GM crops not presenting unexpected or unintended effects on food or feed safety or environmental risk relative to their conventional counterparts. Participants appreciated the benefit of harmonized hypothesis-based risk assessments to enable future deployment of GM crops that can address emerging agricultural challenges associated with increasing demand for affordable healthy food and changing agricultural environments. The points discussed in this publication will be used to further clarify recommendations for supplementary case-by-case data and guide the development of future, more targeted workshops and related discussions. In particular, applying the proposed framework to traits and crops with which there is less familiarity and established HOSU than those used in the case study may be associated with greater uncertainty in the foundational information of the GM event. Additional case studies involving less familiar traits and different crops should be used to further test the robustness of the modernized regulatory framework.
The workshop focused on what data was scientifically necessary and sufficient to make a conclusion on the food, feed and environmental safety of the GM crop. However, several participants noted that certain data not included in the case study was either required in their jurisdiction or routinely submitted by applicants. While it was beyond the scope of this workshop, future targeted workshops or symposia could address the extent to which regulatory authorities have the flexibility to decide, on a case-by-case basis, what data is necessary to make a conclusion on safety. In some jurisdictions the recommendations of the modernization project could be implemented by applicants by including a scientific rationale in their submission for why a specific study is not necessary. In other cases, changes to laws, regulations, or written guidance would be needed to implement these recommendations.
The case study for the first workshop, as described in this publication, was a valuable tool to foster discussion about science-based data requirements for the assessment of GM crops. If these scientific approaches to modernize data packages for GM crop regulation were adopted globally, delays to the commercialization of GM crops could be reduced, thereby allowing farmers access to new GM traits that will benefit not just growers, but consumers and the environment as well. For more information on the case study used in the workshop, or if there is interest in hosting a similar workshop, please contact the corresponding author.
Author contributions
NS: Conceptualization, Writing–original draft, Writing–review and editing. AS: Conceptualization, Writing–original draft, Writing–review and editing. JS: Writing–original draft, Writing–review and editing. JA: Writing–original draft, Writing–review and editing. MH: Writing–original draft, Writing–review and editing. DM: Writing–original draft, Writing–review and editing. CM: Writing–original draft, Writing–review and editing. MS: Writing–original draft, Writing–review and editing. SS: Writing–original draft, Writing–review and editing. EU-W: Writing–original draft, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. CropLife International supported the open access publication of this work. The funder was not involved in the writing of this article, or the decision to submit it for publication.
Conflict of interest
Authors NS, JA, and CM were employed by Corteva™ Agriscience. Author AS was employed by CropLife International. Authors JS and MS were employed by BASF Corporation. Authors MH and SS were employed by Syngenta Seeds LLC. Authors DM and E-UW were employed by Bayer Crop Science. BASF Corporation, Bayer Crop Science, Corteva™ Agriscience, and Syngenta Seeds LLC are commercial developers of GM crops.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AgbioInvestor (2022). Time and cost to develop a new GM trait. Available at: https://agbioinvestor.com/wp-content/uploads/2022/05/AgbioInvestor-Time-and-Cost-to-Develop-a-New-GM-Trait.pdf (Accessed November 18, 2023).
Google Scholar
AgbioInvestor (2023). Global GM crop area review. Available at: https://gm.agbioinvestor.com/downloads (Accessed November 29, 2023).
Ala-Kokko, K., Lanier Nalley, L., Shew, A. M., Tack, J. B., Chaminuka, P., Matlock, M. D., et al. (2021). Economic and ecosystem impacts of GM maize in South Africa. Glob. Food Secur. 29, 100544. doi:10.1016/j.gfs.2021.100544
CrossRef Full Text | Google Scholar
Anderson, J., Bachman, P. M., Burns, A., Chakravarthy, S., Goodwin, L., Privalle, L., et al. (2021). Streamlining data requirements for the environmental risk assessment of genetically modified (GM) crops for cultivation approvals. J. Regul. Sci. 9 (1), 26–37. doi:10.21423/jrs-v09i1anderson
Bachman, P. M., Anderson, J., Burns, A., Chakravarthy, S., Goodwin, L., Privalle, L., et al. (2021). Data transportability for studies performed to support an environmental risk assessment for genetically modified (GM) crops. J. Regul. Sci. 9 (1), 38–44. doi:10.21423/jrs-v09i1bachman
Bravo, A., Gill, S. S., and Soberón, M. (2007). Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49 (4), 423–435. doi:10.1016/j.toxicon.2006.11.022
PubMed Abstract | CrossRef Full Text | Google Scholar
Brookes, G. (2022a). Farm income and production impacts from the use of genetically modified (GM) crop technology 1996-2020. Gm. Crops Food 13 (1), 171–195. doi:10.1080/21645698.2022.2105626
Brookes, G. (2022b). Genetically modified (GM) crop use 1996–2020: environmental impacts associated with pesticide use change. Gm. Crops Food 13 (1), 262–289. doi:10.1080/21645698.2022.2118497
Brookes, G. (2022c). Genetically modified (GM) crop use 1996–2020: impacts on carbon emissions. Gm. Crops Food 13 (1), 242–261. doi:10.1080/21645698.2022.2118495
Brune, P., Chakravarthy, S., Graser, G., Mathesius, C. A., McClain, S., Petrick, J. S., et al. (2021). Core and supplementary studies to assess the safety of genetically modified (GM) plants used for food and feed. J. Regul. Sci. 9 (1), 45–60. doi:10.21423/jrs-v09i1brune
Clark, B. W., Phillips, T. A., and Coats, J. R. (2005). Environmental fate and effects of Bacillus thuringiensis (bt) proteins from transgenic crops: a review. J. Agric. Food Chem. 53 (12), 4643–4653. doi:10.1021/jf040442k
David, M. A. (2009). GAIN report: Nigeria agricultural biotechnology annual report. Available at: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=AGRICULTURAL%20BIOTECHNOLOGY%20ANNUAL_Lagos_Nigeria_8-3-2009 (Accessed February 12, 2024).
Dively, G. P., Venugopal, P. D., Bean, D., Whalen, J., Holmstrom, K., Kuhar, T. P., et al. (2018). Regional pest suppression associated with widespread Bt maize adoption benefits vegetable growers. Proc. Natl. Acad. Sci. 115 (13), 3320–3325. doi:10.1073/pnas.1720692115
European Commission, Directorate-General for Research and Innovation (2010). A decade of EU-funded GMO research (2001-2010) . Brussels: European Commission . doi:10.2777/97784
Goodwin, L., Hunst, P., Burzio, L. A., Rowe, L., Money, S., and Chakravarthy, S. (2021). Stacked trait products are as safe as non-genetically modified (GM) products developed by conventional breeding practices. J. Regul. Sci. 9 (1), 22–25. doi:10.21423/jrs-v09i1goodwin
Icoz, I., and Stotzky, G. (2008). Fate and effects of insect-resistant Bt crops in soil ecosystems. Soil Biol. Biochem. 40 (3), 559–586. doi:10.1016/j.soilbio.2007.11.002
International Service for the Acquisition of Agri-biotech Applications (ISAAA) (2020). Global status of commercialized biotech/GM crops in 2019 (brief 55). Available at: https://www.isaaa.org/resources/publications/briefs/55/executivesummary/default.asp .
International Service for the Acquisition of Agri-biotech Applications (ISAAA) (2023). ISAAA’s GM approval database. Available at: https://www.isaaa.org/gmapprovaldatabase (Accessed September 14, 2023).
Klümper, W., and Qaim, M. (2014). A meta-analysis of the impacts of genetically modified crops. PLoS ONE 9 (11), e111629. doi:10.1371/journal.pone.0111629
Macall, D. M., Trabanino, C. R., Soto, A. H., and Smyth, S. J. (2020). Genetically modified maize impacts in Honduras: production and social issues. Transgenic Res. 29 (5), 575–586. doi:10.1007/s11248-020-00221-y
Mathesius, C. A., Sauve-Ciencewicki, A., Anderson, J., Cleveland, C., Fleming, C., Frierdich, G. E., et al. (2020). Recommendations for assessing human dietary exposure to newly expressed proteins in genetically modified crops. J. Regul. Sci. 8, 1–12. doi:10.21423/JRS-V08MATHESIUS
McClain, S., Herman, R. A., Islamovic, E., Ranjan, R., Silvanovich, A., Song, P., et al. (2021). Allergy risk assessment for newly expressed proteins (NEPs) in genetically modified (GM) plants. J. Regul. Sci. 9 (1), 67–75. doi:10.21423/jrs-v09i1mcclain
National Academies of Sciences, Engineering, and Medicine (2016). Genetically engineered crops: experiences and prospects . Washington, DC, USA: The National Academies Press US . doi:10.17226/23395
OECD (2003). Consensus document on the biology of Zea mays subsp. mays (maize) . ENV/JM/MONO(2007)14. Paris, France: Organisation for Economic Cooperative Development . Available at: https://one.oecd.org/document/env/jm/mono(2003)11/en/pdf .
OECD (2007). Consensus document on safety information on transgenic plants expressing Bacillus thuringiensis - derived insect control protein . ENV/JM/MONO(2003)11. Paris, France: Organisation for Economic Cooperative Development . Available at: https://one.oecd.org/document/env/jm/mono(2007)14/en/pdf .
OECD (2023). Consensus document on environmental considerations for the release of transgenic plants, harmonisation of regulatory oversight in biotechnology . ENV/CBC/MONO(2023)30. Paris, France: Organisation for Economic Cooperative Development . doi:10.1787/62ed0e04-en
Peshin, R., Hansra, B. S., Singh, K., Nanda, R., Sharma, R., Yangsdon, S., et al. (2021). Long-term impact of Bt cotton: an empirical evidence from North India. J. Clean. Prod. 312, 127575. doi:10.1016/j.jclepro.2021.127575
Raybould, A., and Macdonald, P. (2018). Policy-led comparative environmental risk assessment of genetically modified crops: testing for increased risk rather than profiling phenotypes leads to predictable and transparent decision-making. Front. Bioeng. Biotechnol. 6, 43. doi:10.3389/fbioe.2018.00043
Raybould, A., Stacey, D., Vlachos, D., Graser, G., Li, X., and Joseph, R. (2007). Non-target organism risk assessment of MIR604 maize expressing mCry3A for control of corn rootworm. J. Appl. Entomology 131 (6), 391–399. doi:10.1111/j.1439-0418.2007.01200.x
Roberts, A., Boeckman, C. J., Mühl, M., Romeis, J., Teem, J. L., Valicente, F. H., et al. (2020). Sublethal endpoints in non-target organism testing for insect-active GE crops. Front. Bioeng. Biotechnol. 8, 556. doi:10.3389/fbioe.2020.00556
Romeis, J., Hellmich, R., Candolfi, M., Carstens, K., De Schrijver, A., Gatehouse, A., et al. (2011). Recommendations for the design of laboratory studies on non-target arthropods for risk assessment of genetically engineered plants. Transgenic Res. 20 (1), 1–22. doi:10.1007/s11248-010-9446-x
Romeis, J., McLean, M. A., and Shelton, A. M. (2013). When bad science makes good headlines: bt maize and regulatory bans. Nat. Biotech. 31 (5), 386–387. doi:10.1038/nbt.2578
Roper, J., Lipscomb, E. A., Petrick, J. S., Ranjan, R., Sauve-Ciencewicki, A., and Goodwin, L. (2021). Toxicological assessment of newly expressed proteins (NEPs) in genetically modified (GM) plants. J. Regul. Sci. 9 (1), 61–66. doi:10.21423/jrs-v09i1roper
Rose, R. I. (2007). White paper on tier-based testing for the effects of proteinaceous insecticidal plant-incorporated protectants on NonTarget arthropods for regulatory risk assessments. Available at: https://www.epa.gov/sites/default/files/2015-09/documents/tier-based-testing.pdf (Accessed April 2, 2024).
Shelton, A. M. (2021). Bt eggplant: a personal account of using biotechnology to improve the lives of resource-poor farmers. Am. Entomologist 67 (3), 52–59. doi:10.1093/ae/tmab036
Smyth, S. J. (2020). The human health benefits from GM crops. Plant Biotechnol. J. 18 (4), 887–888. doi:10.1111/pbi.13261
Snell, C., Bernheim, A., Bergé, J.-B., Kuntz, M., Pascal, G., Paris, A., et al. (2012). Assessment of the health impact of GM plant diets in long-term and multigenerational animal feeding trials: a literature review. Food Chem. Toxicol. 50 (3), 1134–1148. doi:10.1016/j.fct.2011.11.048
Stotzky, G. (2005). Persistence and biological activity in soil of the insecticidal proteins from Bacillus thuringiensis, especially from transgenic plants. Plant Soil 266 (1), 77–89. doi:10.1007/s11104-005-5945-6
U.S. Environmental Protection Agency (US EPA) (2010). Bacillus thurigiensis Cry3Bb1 corn biopesticides registration action document. Available at: https://www3.epa.gov/pesticides/chem_search/reg_actions/pip/cry3bb1-brad.pdf .
Van Eenennaam, A. L., and Young, A. E. (2014). Prevalence and impacts of genetically engineered feedstuffs on livestock populations. J. Animal Sci. 92 (11), 4255–4278. doi:10.2527/jas.2014-8124
van Frankenhuyzen, K. (2009). Insecticidal activity of Bacillus thuringiensis crystal proteins. J. Invertebr. Pathol. 101 (1), 1–16. doi:10.1016/j.jip.2009.02.009
Waters, S., Ramos, A., Henderson Culler, A., Hunst, P., Zeph, L., Gast, R., et al. (2021). Recommendations for science-based safety assessment of genetically modified (GM) plants for food and feed uses. J. Regul. Sci. 9 (1), 16–21. doi:10.21423/JRS-V09I1WATERS
Woodruff, S. (2024). Gardeners can now grow a genetically modified purple tomato made with snapdragon DNA. Available at: https://www.npr.org/sections/health-shots/2024/02/06/1228868005/purple-tomato-gmo-gardeners .
Zilberman, D., Holland, T. G., and Trilnick, I. (2018). Agricultural GMOs—what we know and where scientists disagree. Sustainability 10 (5), 1514. doi:10.3390/su10051514
Keywords: genetically modified (GM), regulation, food and feed, safety assessment, environmental risk assessment (ERA), problem formulation, cultivation, data requirements
Citation: Storer NP, Simmons AR, Sottosanto J, Anderson JA, Huang MH, Mahadeo D, Mathesius CA, Sanches da Rocha M, Song S and Urbanczyk-Wochniak E (2024) Modernizing and harmonizing regulatory data requirements for genetically modified crops—perspectives from a workshop. Front. Bioeng. Biotechnol. 12:1394704. doi: 10.3389/fbioe.2024.1394704
Received: 02 March 2024; Accepted: 12 April 2024; Published: 10 May 2024.
Reviewed by:
Copyright © 2024 Storer, Simmons, Sottosanto, Anderson, Huang, Mahadeo, Mathesius, Sanches da Rocha, Song and Urbanczyk-Wochniak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abigail R. Simmons, [email protected]
This article is part of the Research Topic
Advancing Science in Support of Sustainable Bio-Innovation: 16th ISBR Symposium

IMAGES
VIDEO
COMMENTS
Quantification of genetically modified organisms (GMOs) in foods. Testing on GMOs in food and feed is routinely done using molecular techniques like DNA microarrays or qPCR. These tests are based on screening genetic elements like p35S, tNos, pat, or bar or event specific markers for the official GMOs like Mon810, Bt11, or GT73.
A predictive study conducted by the International Food Policy Research Institute indicated that by 2050, ... The relevance of gene transfer to the safety of food and feed derived from genetically modified (GM) plants. Food and Chemical Toxicology, 42 (7), 1127-1156. 10.1016/j.fct.2004.02.001 ...
1990s: The first wave of GMO produce created through genetic engineering becomes available to consumers: summer squash, soybeans, cotton, corn, papayas, tomatoes, potatoes, and canola. Not all are ...
A series of extensive and long-term research has shown that the benefits of growing genetically modified crops in the fight against global food shortages and hunger have been significant. The steady increase in the global population has led researchers to focus on the benefits of developing genetically modified products, rather than the ...
Research shows that foods like eggs, dairy products, and meat that come from animals that eat GMO food are equal in nutritional value, safety, and quality to foods made from animals that eat only ...
The term "genetic modified organisms (GMO)" has become a controversial topic as its benefits for both food producers and consumers are companied by potential biomedical risks and environmental side effects. Increasing concerns from the public about GMO, particularly in the form of genetic modified (GM) foods, are aimed at the short- and ...
An expert explains. Advances in genetic engineering have given rise to an era of foods - including genetically modified organisms (GMOs) and gene-edited foods - that promise to revolutionise ...
A systematic review of animal and human studies was conducted on genetically modified (GM) food consumption to assess its safety in terms of adverse effects/events to inform public concerns and future research. Seven electronic databases were searched from January 1st 1983 till July 11th 2020 for in vivo, animal and human studies on the incidence of adverse effects/events of GM products ...
Genetically modified (GM) foods contain at least one ingredient coming from a plant with an altered genetic composition. 16 Genetic modification, also known as genetic engineering, often introduces new, desirable characteristics to plants, such as greater resistance to pests. Many U.S. crops are grown using genetically engineered seeds, including a large share of the soybean, corn, cotton and ...
In the United States, France and Germany, as peoples' opposition to genetically modified (GM) foods becomes more extreme, their self-rated understanding of genetic modification increases, but ...
Nearly all the corn and soybeans grown in the U.S. are genetically modified, but only two GM crops, Monsanto's MON810 maize and BASF's Amflora potato, are accepted in the European Union. Ten E.U ...
Responsible research. Both GMOs and gene-edited foods offer great promise. Of course there are valid concerns, such as the potential to create new allergens, unintended consequences for ecosystems ...
After genetically modified foods were introduced in the United States a few decades ago, people independently reported toxic effects caused by GMOs. ... To directly test the ability of a GMO to cause mutations, a research group from the National Laboratory of Protein Engineering and Plant Genetic Engineering in Beijing, China applied the Ames ...
In this chapter, the committee examines the evidence that substantiates or negates specific hypotheses and claims about the health risks and benefits associated with foods derived from genetically engineered (GE) crops. There are many reviews and official statements about the safety of foods from GE crops (for example, see Box 5-1), but to conduct a fresh examination of the evidence, the ...
In the late 1980s - early 1990s, the results of decades of molecular research reached the public domain. Until that time, consumers were generally not very aware of the potential of this research. In the case of food, consumers started to wonder about safety because they perceive that modern biotechnology is leading to the creation of new ...
Concern about genetically modified foods is widespread globally, with about half of people in 20 publics around the world saying these foods are unsafe to eat, according to a Pew Research Center survey conducted between October 2019 and March 2020.. As growth in the world's population increases demand on the global food supply, nations have debated the role of genetically engineered or ...
The three most common traits found in GMO crops are: Resistance to insect damage. Tolerance to herbicides. Resistance to plant viruses. For GMO crops that are resistant to insect damage, farmers ...
The pharmaceutical industry is another frontier for the use of GMOs. In 1986, human growth hormone was the first protein pharmaceutical made in plants (Barta et al., 1986), and in 1989, the first ...
While China's president calls for its scientists to "boldly research and innovate [and] dominate the high points of GMO techniques", 6 the people of China are largely opposed to GMO foods ...
In a 2015 Pew Research Center survey of consumers, 57% of adults believe that eating GMO foods is unsafe, while 37% say they believe it is generally safe. Yet, science continues to suggest that there is no substantiated evidence that GMO foods are less safe than non-GMO derived food products. A 2016 report from the National Academies of Science ...
Genetically modified crops can help cut carbon emissions, research shows — but they still face major hurdles. Workers inspect fully grown rice varieties at the International Rice Research ...
A mix-up over bioengineered tomato seeds sparked fears about spread of GMO crops. The Purple Galaxy Tomato splashed across the cover of this season's Baker Creek Heirloom Seeds catalog: a closeup ...
Genetically modified (GM) crops that have been engineered to express transgenes have been in commercial use since 1995 and are annually grown on 200 million hectares globally. These crops have provided documented benefits to food security, rural economies, and the environment, with no substantiated case of food, feed, or environmental harm attributable to cultivation or consumption.
Genetically modified crops have made significant contributions to address the United Nations Sustainable Development Goals, in particular goals 1 (reducing poverty) and 2 (reducing hunger). While increased yields have contributed to higher household incomes, which reduce poverty, the increased yields have also enhanced household food security.
Genetically modified organisms (GMOs) and organic farming have historically been regarded as opposing forces in agricultural practices. While GMOs utilize genetic engineering to introduce specific traits into plants, such as resistance to pests or herbicides, organic farming practices prioritize natural processes and generally prohibit the use of GMOs.