Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 15 February 2024

Validation of portable in-clinic video-based gait analysis for prosthesis users
- Anthony Cimorelli 1 ,
- Ankit Patel 2 , 3 ,
- Tasos Karakostas 1 , 4 &
- R. James Cotton ORCID: orcid.org/0000-0001-5714-1400 1 , 4
Scientific Reports volume 14 , Article number: 3840 ( 2024 ) Cite this article
920 Accesses
1 Citations
1 Altmetric
Metrics details
- Biomedical engineering
- Bone quality and biomechanics
- Outcomes research
Despite the common focus of gait in rehabilitation, there are few tools that allow quantitatively characterizing gait in the clinic. We recently described an algorithm, trained on a large dataset from our clinical gait analysis laboratory, which produces accurate cycle-by-cycle estimates of spatiotemporal gait parameters including step timing and walking velocity. Here, we demonstrate this system generalizes well to clinical care with a validation study on prosthetic users seen in therapy and outpatient clinics. Specifically, estimated walking velocity was similar to annotated 10-m walking velocities, and cadence and foot contact times closely mirrored our wearable sensor measurements. Additionally, we found that a 2D keypoint detector pretrained on largely able-bodied individuals struggles to localize prosthetic joints, particularly for those individuals with more proximal or bilateral amputations, but after training a prosthetic-specific joint detector video-based gait analysis also works on these individuals. Further work is required to validate the other outputs from our algorithm including sagittal plane joint angles and step length. Code for the gait transformer and the trained weights are available at https://github.com/peabody124/GaitTransformer .
Similar content being viewed by others

The Toronto older adults gait archive: video and 3D inertial motion capture data of older adults’ walking

Algorithm based on one monocular video delivers highly valid and reliable gait parameters
Deep neural networks enable quantitative movement analysis using single-camera videos
Introduction.
Gait impairments are a common target for rehabilitation. The most widely used outcome measures are the 10-m walk test or 6-min walk test which measure walking speed and endurance 1 , but do not capture more detailed walking biomechanics. Alternatively, a motion analysis laboratory uses optical motion capture and force plates to obtain precise estimates of joint kinematics and kinetics and compute temporal and spatiotemporal gait parameters 2 . However, the cost, time, and equipment required for formal gait analysis preclude performing it frequently, during clinical encounters, or outside the laboratory. There is a substantial need for clinically-usable tools that fill the gap between formal gait assessment and performance-based outcome measures to enable routine, quantitative characterization of gait and its associated impairments. These tools would enable more sensitive outcome measures to follow patients’ progress with therapy as well as to better power research to improve interventions. It would also enable routine screening of gait parameters in the clinic. This has many potential applications, such as allowing early detection of gait parameters associated with a risk of falling 3 , which could then enable earlier interventions with fall prevention strategies.
Given the importance of analyzing gait and mobility, numerous approaches have been explored including wearable sensors and video analysis. Many different types of wearable sensors and algorithms have been described ranging from wrist devices that estimate step counts to placing numerous sensors over the body to estimate more complete kinematics 4 , 5 , 6 . An advantage of wearables is they can enable ubiquitous monitoring of activity throughout the day 7 . However, they can often require extensive and time-consuming calibration, require complicated and often proprietary algorithms to extract the relevant information, and have a wide range of reported accuracies 8 .
In recent years, human pose estimation using deep learning has seen an extremely rapid advance yielding numerous approaches that can estimate 3D joint locations and pose 9 , 10 , 11 . However, the performance measure for the majority of this computer vision research is the accuracy of estimating 3D joint locations and not biomechanically motivated kinematics or gait parameters. Using multiple cameras, joint locations can be triangulated in 3D to produce more accurate estimates and these systems have been validated on numerous aspects of gait 12 , 13 , 14 , 15 , 16 , 17 , 18 . In general, the need for multiple cameras makes these systems less portable and amenable for use in a clinic, although OpenCap has shown it is possible using only a computer and two smartphones with calibrated positions 19 . Approaches have been developed that can analyze cycle-by-cycle gait from monocular video 20 , 21 , but they have not been validated on data acquired in the clinic or on clinical populations. Alternatively, other approaches train a neural network to directly map a sequence of 2D keypoints to average gait parameters that have been tested on clinical populations but do not enable analyzing individual gait cycles 22 , 23 .
We developed an algorithm, the Gait Transformer, trained on a large clinical gait laboratory dataset of paired videos and motion capture data 24 . This Gait Transformer decomposes Human Pose Estimation (HPE) trajectories of walking into individual gait cycles to produce accurate estimates of gait event timing and walking velocity, when tested on that dataset . However, artificial intelligence (AI) algorithms can be sensitive to changes in the data distribution. In this case, there are numerous differences between videos of gait collected in the real world or clinic and those from the gait laboratory. Thus, validating how this algorithm generalizes to data collected in clinical settings—the primary goal—is critical to enabling its use. The goals of this work include: (1) describe our combined system including smartphone application, wearable sensors, Pose Pipeline 25 and Gait Transformer 24 for clinical gait analysis, (2) validate the performance of this system on data acquired in the clinic, (3) identify under which conditions it performs well and when it is less reliable.
We performed this validation on prosthesis users and selected this population for several reasons. From a technical perspective, this is a challenging test of this system as the limbs of prosthesis users often appear visually different than able-bodied individuals, and it was previously unknown if pretrained HPE algorithms will generalize to prosthesis users. In addition, some prosthesis users walk with significant gait deviations compared to able-bodied individuals 26 , which further challenges the Gait Transformer. From a clinical perspective, we expect that routine access to video-based gait analysis would enable better outcomes to monitor improvements in walking with therapy or with adjustments to prosthetic components, but this will ultimately need to be empirically validated through clinical trials. Previous studies have demonstrated that lower limb prosthesis users saw an improvement in various gait parameters including walking speed, distance walked, spatiotemporal measures and biomechanics with specialized therapeutic interventions 27 , 28 . However, these studies used performance-based outcome measures and those that analyzed biomechanics were limited to laboratory settings over level ground. The ability to perform routine, quantitative gait analysis could identify improvements in the quality of walking during therapy, such as increased symmetry and time spent in single stance on the prosthetic limb. This increased level of detail for analyzing gait may allow for more sensitive outcome measurements to demonstrate how an individual’s gait continues to improve when their walking velocity plateaus. This could enable physical therapists to demonstrate to insurance agencies that patients are still making progress and justify additional sessions. Or, more sensitive measures could provide data for prosthetists to show advanced components also improve quality of walking. As prosthetic components continue to advance, it is becoming increasingly difficult to get high-end prosthetic components covered by payers and there is a need to develop systems that can quantify prosthetic gait in real-world settings 29 . Due to the many limitations associated with current gait analysis systems, prosthetists typically rely on observational gait analysis when performing dynamic alignment of a prosthetic device 30 . Therefore, having access to routine quantitative gait analysis could assist prosthetists with dynamic alignment during clinical visits.
Data collection
Mobile acquisition and wearable sensors.
Video and sensor data is acquired on an Android smartphone using a custom app to synchronize recording from both modalities (Fig. 1 ). The mobile phone is mounted on a 3-axis gimbal (DJI Osmo Mobile 2) to improve the video stability when following subjects during ambulation. The video was obtained in portrait orientation at 1080 × 1920 resolution at 30 frames per second.
The wearable sensors are a custom design and have previously been described 31 , 32 . For the purpose of this study, the wearable sensor data was used solely for validation. The sensors stream data from the IMU to the smartphone over Bluetooth Low-Energy (BLE). The IMU is an ICM-20948 and outputs 3-axis accelerometer and gyroscope data at 562.5 Hz. Magnetometer data is available but is not used in this study and we typically do not stream it in order to optimize the BLE bandwidth. The sensors can also acquire two channels of EMG data, but this was not used in these experiments. Prior to experiments, the magnetometer is calibrated in the location where data will be collected by rotating around each of the axes and accounting for hard and soft iron distortions. IMU data is fused on the sensor using a complementary filter to compute the 3D orientation, and the estimated orientation is streamed over BLE. Compared to our prior works using silicone-encapsulated wearable sensors attached to the skin, in this work the sensors were placed in a 3D-printed case with Velcro \(\circledR\) on the outside for attachment to the body.
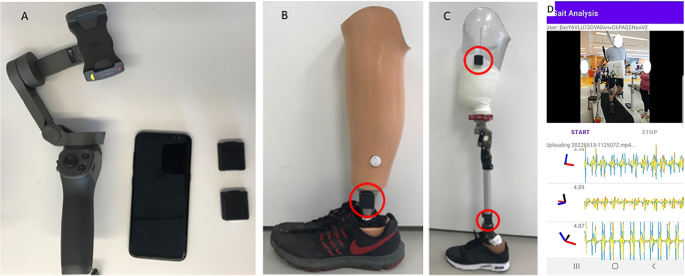
( A ) Android cell phone, two wearable sensors and gimbal. ( B ) One sensor placed on the shank of a definitive transtibial prosthesis with adhesive Velcro \(\circledR\) . ( C ) Two sensors placed on the shank/thigh of a diagnostic transfemoral. ( D ) Screenshot from gait analysis app, with video in the top panel and sensor data in the bottom panel.
Clinical population and data annotation
This study was approved by the Northwestern University Institutional Review Board. All methods were performed in accordance with the relevant guidelines and regulations. All participants gave written consent. Participants shown in images in this manuscript gave additional consent for the use of their images and videos in scientific publications and their faces were masked. Video of gait and other activities was obtained from a convenience sample of lower-limb prosthesis users seen in an outpatient prosthetics clinic or participating in outpatient physical therapy at Shirley Ryan AbilityLab. For each subject, we recorded their age, height, level, and (bi)laterality of amputation, the etiology of the amputation, the type of prosthetic components, and their Medicare Functional Classification Level (K-level). Walking data was collected in either prosthetic or therapy clinics. In the case of therapy, videos were obtained as participants performed their usual therapy.
Sensors were placed on both the shank and thigh of the prosthetic limb(s), with up to four sensors used in the case of individuals with bilateral amputations. They were attached either with double-sided Velcro \(\circledR\) attached to the prosthetic pylon, and socket (for transfemoral amputees) or with a Velcro \(\circledR\) strap around the thigh. They were placed laterally, with the IMU Z axis pointed laterally and the X axis pointed down. In this work, only the data from the shank IMU was used to detect the prosthetic limb swinging.
After data collection was complete, videos were annotated with the following categories by the author: the activity being performed (e.g., overground walking, treadmill walking, running, other therapeutic tasks), the view (frontal, sagittal, or a mixture), and the subjective accuracy of keypoint tracking of the prosthetic limb with a 3-point Likert scale ranging from 1 (poor) to 3 (good). Specifically 1 indicated the keypoints do not locate the prosthetic joints and tracking is frequently inaccurate, 2 indicated they locate the joint but are intermittently inaccurate, and 3 indicates the locate the joint well throughout the video. The individual performing the manual annotation was the main author and they were blinded to the keypoint confidence predictions prior to annotation. Whether the prostheses were visible or occluded by clothing was also annotated for each video. Time points when the participant entered and exited 10-m areas indicated by tape on the ground were also recorded to compute ground truth velocity.
Velocity annotation
Tape was placed on the ground at 10-m spacing in locations where subjects would typically walk such as the hallway in the prosthetic clinic and in multiple locations in the therapy gyms. The start and end times when subjects completed a straight overground walk between these markers were retrospectively annotated. This provided both ground truth measurements of their walking velocity and identified specific video segments where participants were walking, as the collected data contained a mixture of activities.
In this work, we focus our analysis on video segments where individuals are performing above-ground walking in a forward direction during the time window where they were walking between the two pieces of tape, which we refer to as timed walking segments. We focus on the 10-m annotation periods both to determine the accuracy of the system when computing gait speed and because these were segments where individuals were known to be walking and were in view of the camera.
Data processing
The input to the gait transformer is a series of 3D keypoint locations. To obtain these, the video was processed with PosePipe 25 , a human pose estimation pipeline based on DataJoint 33 that simplifies running cutting-edge HPE algorithms on video. The steps used in the pipeline include (1) a tracking algorithm 34 to compute bounding box tracks for all people in the scene followed by (2) manually annotating the bounding box for the subject of interest undergoing gait analysis. (3) Then we perform top-down 2D keypoints detection in each frame using the MMPose toolbox 35 , specifically using an HRNet 36 trained on the COCO dataset 37 using distribution aware relative keypoints 38 , (4) the 2D keypoint trajectories are then lifted to 3D joint locations 39 .
We also used DeepLabCut (DLC) 40 to train a custom 2D keypoint detector for a subset of prosthesis users with videos where keypoint detection was performing poorly. This is completed by manually annotating both intact and prosthetic hip, knee and ankle joints. We computed the 2D keypoints on those same videos with this model and replaced any prosthetic joints computed by MMPose with the estimates from DLC. This corrected set of keypoints was then passed to the lifting step and then to the gait transformer.
We flagged any frames as clipped whenever any of the keypoints of the leg came within 10 pixels of the edge of the screen.
Gait transformer
The sequence of 3D joint location was mapped onto the relative timing of four gait events (right and left foot contact and toe off) as well as the pelvis velocity using the Gait Transformer 24 . This is trained on a large dataset of walking videos with synchronous marker-based motion capture and force plate data from our clinical gait laboratory. For training, data was aligned in the sagittal direction using the medial orientation of the pelvis. It also outputs sagittal plane joint kinematics including foot position relative to the pelvis, foot velocity, and hip and knee angles, all of which we do not focus on in this work. We refer the reader to the 24 for details of the architecture and training of the gait transformer. Compared to that work, we retrained the gait transformer and excluded bilateral elbows and wrists as we found occasionally the use of assistive devices (i.e., canes or crutches) in a less common pattern would trigger false detection of steps.
The Gait Transformer was applied to the lifted 3D joint trajectories. Training samples from the gait laboratory are typically only a few gait cycles long and we found that it did not generalize to inference on much longer sequences. Videos acquired in the clinic were much longer than a few gait cycles, so we applied it on a sliding window of 90 frames (3 s), which covers at least one complete stride for the majority of subjects. For each position of the sliding window, we used the middle output other than the beginning and end where we used the corresponding half of the sequence to pad the output.
Sensor processing
In order to validate the accuracy of the gait transformer event timing of the prosthetic limb, we used gyroscope data from the wearable sensor on the prosthetic shank to detect the prosthetic-limb swing phase. Sensor data is timestamped to the smartphone time. Gyroscope data were sampled at a nominal sampling rate of 562.5 Hz. The Android system time of each Bluetooth packet is also stored and linear regression is used to calibrate the sensor timebase against the Android time, typically with an updated sampling rate of 1–2 Hz different than the nominal value.
We also noted that the video start timestamp showed some latency compared to the sensor timestamps. This has been resolved in more recent versions of our smartphone application with an API that acquires a more precise, per-frame timestamp. We used the hip and knee sagittal plane angles from the gait transformer to adjust for this timing error by finding the offset that minimized the mean squared error between the gyroscope on the shank and the change in that angle computed from the gait transformer outputs. This was typically around 170 ms.
We detected a prosthetic limb swing from the gyroscope on the prosthetic shank. With sensors placed on the lateral side of the shank, the z-axis is roughly aligned to be perpendicular to the sagittal plane. We applied an 8th-order low-pass Chebyshev filter to the gyroscope with a cutoff of 35 Hz. All negative values were zeroed out and a median filter of 360 ms was applied to deglitch a few strides where participants caught their toe and a brief reversal was seen in the gyroscope midtrace. These deglitched positive segments were identified as swings with the time the sign became negative identified as the start and end of swing periods.
Sensor versus video cadence
We compared the cadence estimated with the gait transformer over 10-m walking segments to that computed from the sensors. We computed the cadence from video by averaging \(\dot{\omega }\) over the timed walking segment and converting from strides in rad/s to steps/min (i.e. \(c_v=\frac{120}{2\pi (t_e-t_s)}\int _{t_s}^{t_e} \dot{\omega }(t) \, \partial t\,\) ). We computed cadence from the sensors using the average stride time of the prosthetic limb side over the steps in the time window: \(c_s=120 / \frac{s_j-s_i}{j-i}\) , where \(i\) and \(j\) index the first and last sensor swing time, respective, that fall within the timed walking period.
Matching video and sensor foot contacts
We also compared the time when the end of swing from the gyroscopes to the foot contact time from the gait transformer. This analysis only reflects a bound, because detecting when the prosthetic shank stops rotating forward in swing (i.e., when the gyroscope swaps signs) approximates the end of swing time but is not the actual time the foot contacts the ground and will typically occur slightly before true foot contact (Fig. 2 ). For each walking segment, we computed the offset that produced the closest matches between the end of prosthetic leg swings detected by the sensors and the time of prosthetic foot contact estimated from the Gait Transformer and Kalman filter (typically about 100ms). After this we measured the foot contact detection accuracy as the residual timing error between the offset sensor times and the detected video times. We also measured the fraction of events that were detected with a window of 500 ms.
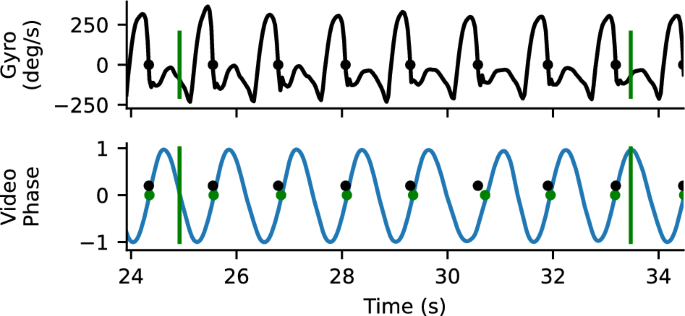
Example gyro and video timing information. Top trace is the z-component of the gyroscope mounted on the tibial shank. The time point where the gyroscope goes from positive to negative (where heel stops rotating forward relative to knee) is identified as a close proxy for foot contact. Bottom plot shows the sin component of the quadrature output for heel strike, with the positively directed zero crossing marker. There is a close correspondence between detected foot contacts from the gyro (blue) and the video (yellow). Vertical bars correspond to the annotated boundaries of the 10-m walk test.
Participant demographics
From 19 participants, we annotated 231 timed walking segments during level walking, with 79 in the frontal plane, 67 in the sagittal plane and the remainder either oblique or changing. When restricted to only the frontal plane, timed walking segments were obtained from a total of 16 participants (Table 1 ).
Usability of the system
Our system made it easy to collect gait data in clinical situations, including physical therapy and prosthetic appointments. Wearable sensors took less than 30 seconds to apply and remove and would require no setup time if not using sensors for validation studies. The sensors connect to the cell phone through our Android app, so acquiring data is as easy as pressing the video record button in our app. Currently, running the analysis pipeline requires technical skills, but we hope to fully automate this in the future. Data was easily and routinely obtained from subjects in both therapy and prosthetic appointments. This was particularly true for the frontal view, but obtaining clear sagittal views in hallways was often challenging due to space limitations. No subjects withdrew from the study. Example visualizations from the system are shown in Figs. 3 and 4 .
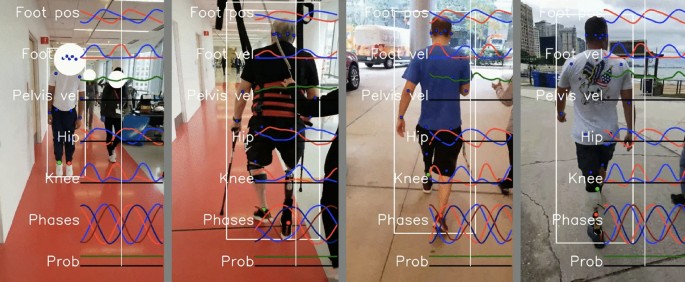
Example visualizations from the gait transformer. Keypoints on the ankle become colored red when in contact with the ground. Overlaid traces show the foot position, foot velocity, pelvis velocity, hip and knee angles, and the quadrature encoded gait timing.
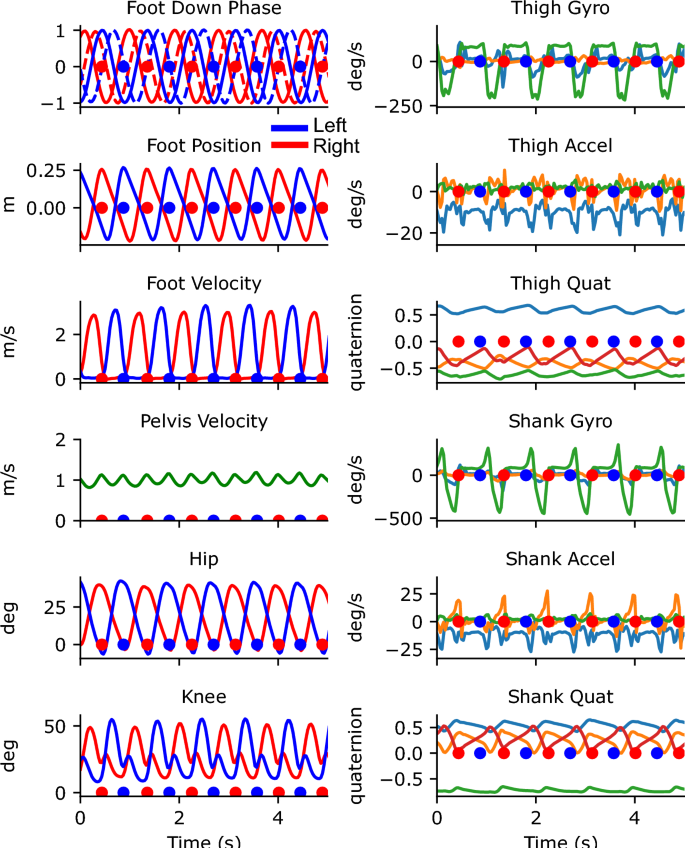
Traces from 5 s of walking from the portable system. The left column shows the outputs from the Gait Transformer. Traces from the left leg are shown in blue and from the right leg are shown in red. The additional dashed traces in the foot-down plot correspond to the phase-shifted quadrature encoding. The right column shows the raw sensor data from the thigh and shank. For gyro and accel plots, the three colors correspond to the x, y, and z IMU axis. For the quaternion plots, there are four colors for the individual quaternion components. Blue and red dots indicate the foot down events on the left and right, respectively.
2D keypoint accuracy
Our 2D keypoint detection used an MMPose model, which is trained on the COCO dataset and primarily contains able-bodied individuals. We found that it frequently generalized to prosthesis users, but not always, and that quality of 2D keypoint tracking varied across subjects. In some cases, the detector would eschew localizing the prosthetic joint of the subject of interest and track the corresponding joint of a nearby therapist. While 2D keypoint detectors, including MMPose, output a per-joint confidence estimate, this has not been systematically tested for prosthesis users. For each video, we manually annotated our subjective estimate of tracking quality from 1 (poor) to 3 (good). For each timed walking segment, we also computed the average confidence that MMPose reported for the prosthetic ankle. As the 2D keypoint algorithms have primarily been trained on able-bodied individuals, we first wanted to analyze the accuracy of the keypoint confidence values on prosthetic limbs compared to manual annotation. Figure 5 a shows the histogram of ankle keypoint qualities, which demonstrates that the confidence estimates from the keypoint detector align with our manual annotation. In fact, the interquartile ranges of ankle confidence conditioned on each of our annotated qualities were nearly non-overlapping (IQR for keypoint quality 1: [0.44,0.60]; 2: [0.59,0.80], 3: [0.77,0.87]).
After validating that the ankle joint confidence is valid in prosthesis users, we then conditioned on the prosthetic level and whether clothing was covering the prosthesis or not, which revealed two trends (Fig. 5 b). First, ankle tracking was worse for bilateral prosthesis users compared to unilateral prosthesis users. Secondly, among unilateral prosthesis users, ankle tracking was worse for those with higher levels of amputation. Ankle tracking was particularly poor in the case of a prosthesis user participant with a bilateral transfemoral amputation and another with a hip disarticulation.
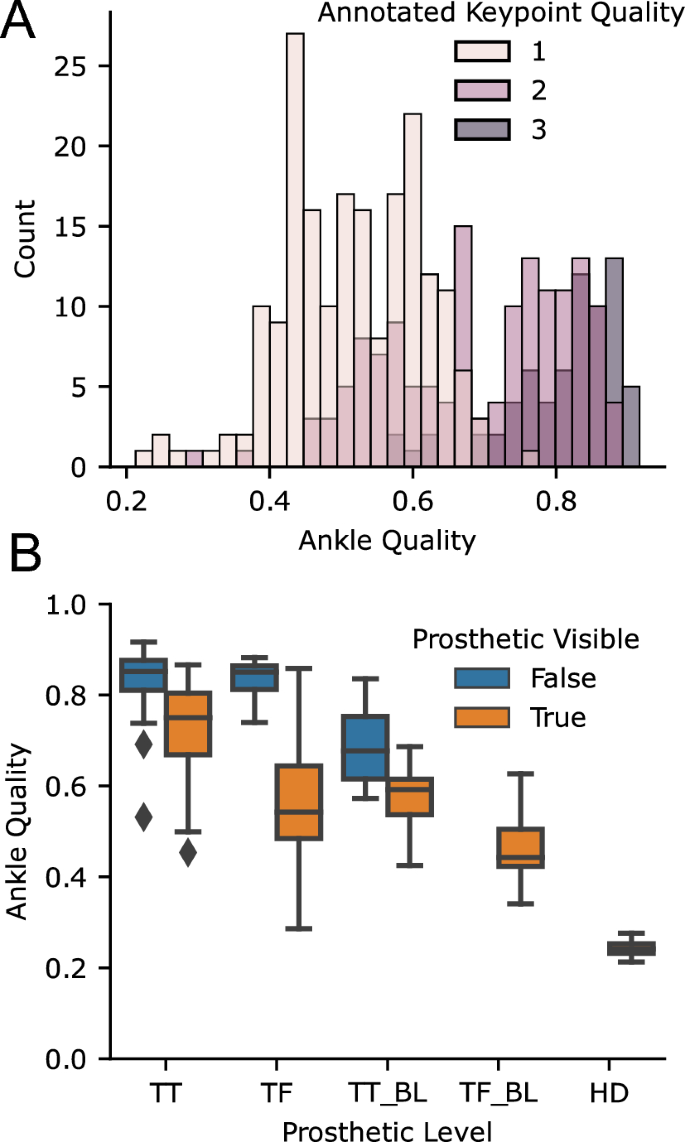
Accuracy of ankle keypoints for prosthesis users with off-the-shelf 2D keypoint detection algorithm. ( A ) shows the histogram of average ankle qualities for videos stratified by manual annotation of quality (with 3 being the best), showing that the estimated quality corresponded with our annotation. The ( B ) shows the average ankle quality stratified by prosthetic level and whether the prostheses were covered by clothing. TT transtibial, TF transfemoral, HD hip-disarticulation, BL bilateral.
In comparison to the MMPose model trained on COCO 37 , our DLC model that was specifically trained on our prosthetic user was able to perform much better, Fig. 6 . Note that we did not test the generalization of this model to new users, and all prosthesis users we analyzed with our DLC model had 20 frames manually annotated and were included in the training dataset.
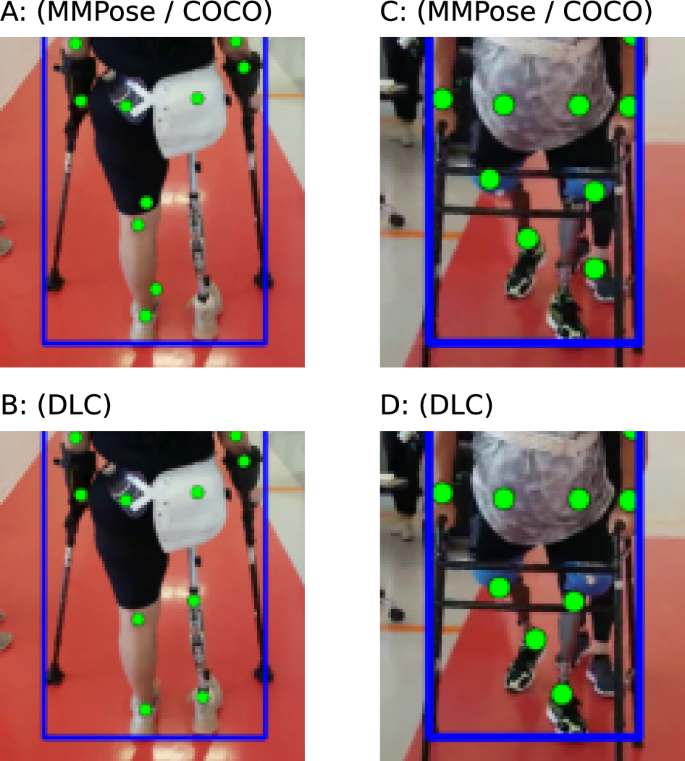
Examples of poor keypoint detection using pretrained algorithm (top row) that were corrected by training with DLC (bottom row).
Viewpoint sensitivity and clipping
We also classified segments as clipped or not if any of the 2D keypoints of the leg hit the edge of view on more than 1% of the frames, because we found the transformer was sensitive to the errors this produced. This occurred in none of the 79 frontal views, in 16 of the 67 sagittal views, and in 7 of the 85 mixed or oblique views. This was due to the somewhat limited space in clinical settings, where in hallways it can be hard to track far enough to the side of a person of interest to frame them with room for error.
Velocity and cadence accuracy
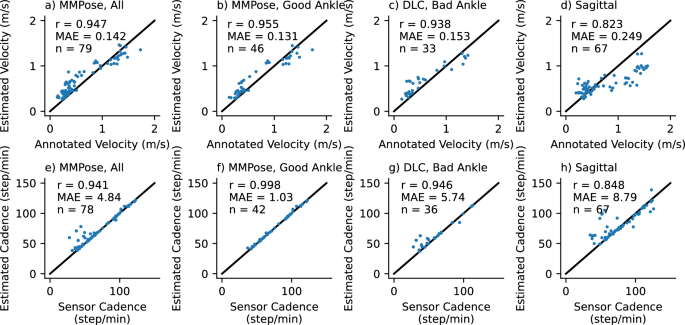
Accuracy of gait transformer for velocity (top row) and cadence (bottom row) under different conditions (columns). Text insets note the correlation coefficient (r), mean absolute error (MAE), and number of walking segments (n). The first column is all videos in the frontal plane. The second column is those where the average ankle detection quality was greater than 0.7. The third column is the excluded segments reprocessed with DLC. The last are videos acquired in the sagittal plane.
We compared the velocity estimated from the video acquired in the frontal plane with the gait transformer to the velocity computed from the manually annotated times as participants walked over a 10-meter interval between the tape on the ground. We found the gait transformer velocity for videos acquired in the frontal plane was quite accurate compared to ground truth, with a correlation (r) of 0.95 and a mean absolute error (MAE) of 0.14 m/s (Fig. 7 a). We also found that the cadence from the gait transformer was a close fit to the sensor data with an MAE of 4.8 steps/min, with most of this error coming from outliers (Fig. 7 e).
We repeated this analysis, excluding segments where the average prosthetic ankle quality was less than 0.7 (Fig. 7 b,f). Notably, we found this removed almost all the error estimating cadence (r = 0.998, MAE = 1.0). The accuracy of the estimated velocity also improved slightly (MAE = 0.13 m/s). We then used our custom prosthetic keypoint detector trained in DLC to replace the prosthetic joints in the excluded segments (Fig. 7 c,g). We found this improved cadence detection for most sessions, but in particular, there were still several outliers for one individual with a slower cadence compared to other individuals.
We found the performance was quite poor when tested on video acquired in the sagittal plane (Fig. 7 d,h). This was relatively unsurprising since the gait transformer is trained on video acquired in the frontal plane. We attempted to improve the viewpoint invariance by augmenting the training process by randomly rotating the lifted 3D keypoints. We found that while it resulted in a tighter correlation with the outputs, both in the gait laboratory validation dataset and on the prosthetic data it resulted in a bias that underestimated velocity at faster speeds.
Step time accuracy
Over the frontal view timed walking segments, we detected and matched 889 prosthetic foot contact events and were unable to detect only 12 of these events. For each walking segment, we also computed the mean absolute error of the residuals. The average of this over all sessions was 72 ms. For sessions with good ankle tracking, we detected 490 foot contact events and missed only 1 event, with an average error over sessions of 45 ms.
Previously, we trained an algorithm for video-based gait analysis on a large clinical gait laboratory dataset of paired videos and motion capture data 24 . While the algorithm validated well on this dataset, there are differences between the training data and the intended application of the algorithm that could result in poor generalization. These include recording portrait videos while walking with the patient through a therapy clinic where other people are present. Our training dataset also had a predominance of children, who are most commonly analyzed in these laboratories. For any clinical application of AI, it is critical to evaluate the external validity (or out-of-domain generalization in machine learning parlance) for the intended use case.
In this study, we evaluated the performance of our gait transformer when tested on prosthesis users walking in therapy or outpatient clinic. This was a powerful stress test for our algorithm, as there are several properties of prosthesis users that might cause a failure to generalize including the visual appearance of prostheses and prosthetic gait patterns. This study also highlighted the power of an interpretable pipeline, with understandable features at multiple stages, such as 2D keypoint detection accuracy or failures to detect a single step. In development, this aspect of the pipeline was extremely important as it enables identifying and alleviating points of failure throughout the different stages of the pipeline to ensure we can trust the outputs from the transformer and make adjustments when necessary to improve accuracy.
Large datasets are a critical driving force of AI algorithms and have enabled impressive advances in HPE in recent years. However, public HPE datasets, like COCO 37 , primarily contain able-bodied individuals. Perhaps unsurprisingly, we found that a 2D keypoint detector trained on COCO did a poor job detecting the ankles of some prosthesis users. This was most pronounced with more proximal or bilateral amputations. This is most likely due the greater visual difference between these limbs and joints compared to the able-bodied individuals in the dataset, resulting in worse out-of-domain generalization of the algorithms. In these cases, tools like DLC 40 make it relatively easy to train a custom keypoint detector. However, using DLC is still labor intensive as it requires manual annotation of each video. Future work will look to improve 2D keypoint detection for prosthetic limbs using self-supervised-learning. Towards this goal, we recently developed a markerless motion capture system and validated it on prosthesis users 41 , 42 Importantly, this work also speaks to the need for more work on AI fairness for people with disabilities 43 , 44 .
When we removed the videos with poor ankle tracking quality and only included videos where the 2D keypoints were accurately localized, we found that our algorithm performed well on videos of prosthesis users for video acquired in the frontal plane. Specifically, we had a mean absolute error for the velocity of 0.13 m/s and for the cadence of 1 step/min. Whether this is sufficiently accurate ultimately depends on the clinical question. One study found the minimally clinically important difference for walking velocity of prosthesis users as 0.21 m/s 45 , which is greater than our algorithm. However, for older adults, it has been suggested a small meaningful change in walking speed is 0.05 m/s 46 . Our results also do not indicate whether analyzing longer segments of walking would reduce the error, or whether this arises from a bias for given individuals that would persist over longer recordings.
The requirement to record video in the frontal plane to obtain accurate results is a limitation of our current approach. However, given the difficulties obtaining good videos in sagittal planes while walking in hallways and therapy gyms with other people present, it is also the most convenient approach for its intended use setting. An argument for sagittal videos is that they should enable more accurate estimates of many important sagittal plane kinematics. Our system outputs many of these and performs well on the training data but testing the external validity of these outputs is important future work. Specific to prosthesis users, testing the external validity of joint kinematics will be an area of challenge as prosthetic components attempt to mimic anatomic motion but do not move in an exact manner as able-bodied joints. As the gait transformer was primarily trained on individuals with intact limbs, this may affect the ability to accurately estimate prosthetic joint kinematics.In this study we validated the accuracy of the gait transformer’s velocity, cadence and step timing measures as these were measures we could easily validate with wearable sensors in the clinic. However, our long-term goal is to develop a system that can accurately output several spatiotemporal and kinematic gait parameters to allow clinicians to further quantify the quality of gait. Further validation to determine the external validity of our portable gait analysis system to measure other spatiotemporal and kinematic gait parameters in prosthesis users is a high priority, which will leverage our recently developed markerless motion capture system 41 , 42 .
Several other groups have looked at the ability to estimate gait parameters from monocular video. Stenum and colleagues analyzed sagittal videos from a dataset 47 of 32 healthy individuals walking down a walkway a fixed distance from a mounted camera using OpenPose, and showed they could accurately estimate gait event timing, sagittal plane joint angles, step length and walking velocity 20 . A pipeline similar to ours using 2D keypoints followed by lifted 3D joints was used with height-informed skeletal refinement prior to extracting gait parameters of healthy individuals walked towards a stationary camera and produced accurate estimates of step timing, length, and walking velocity 21 . Similarly to our work, both of these computed features on individual gait cycles, but in comparison, they were not externally validated on clinical populations or on video acquired in the community or clinic. A neural network can also be trained to directly map 2D keypoint trajectories from the video onto average gait parameters, and such an approach has been used on gait laboratory data from children 22 and subsequently we showed a similar approach with stroke survivors 23 . However, this approach does not allow for examining the cycle-by-cycle variability of gait parameters. Finally, by triangulating keypoints detected from multiple cameras it is possible to estimate more accurate 3D keypoint locations and perform inverse kinematics to fit biomechanical models to them 16 , 17 , 41 , 42 . OpenCap is a particularly portable version of this that only requires two iPhones 19 .
There are several future directions that we anticipate will improve this system. One is better fusion with additional modalities of data, including the camera depth channel and inertial measurements from the wearable sensors. In this work, we have focused purely on monocular video as this is the most widely available modality. We are also enthusiastic about integrating physics-based modeling in the inference process 48 , 49 , 50 , 51 and ways to combine this with self-supervised learning 52 . It is also important to make this system easier to use in a higher-throughput manner. One need is to automate the annotation step to robustly identify the subject of interest. A potential solution is through the use of QR codes placed on the participant during data collection which would allow the computer vision to identify the person of interest without requiring manual annotation. In this work, we used the periods where 10-meter walk tests were annotated to select what to analyze, but this is only a small fraction of the data we acquired. Utilizing activity recognition to identify when the subject is walking, using the detected pose to identify viewpoint, and the Kalman error to determine when walking is being reliably tracked could help automate analysis of the remainder of the data. We recently developed a 3D lifting algorithm that produces calibrated confidence estimates of the joint locations, and integrating this could also help determine the trustworthiness of outputs 53 . Finally, there are many other clinically meaningful gait parameters available in our dataset that we could train the gait transformer to output and validate, including step width and center of mass.
Data availability
The raw video datasets generated and/or analysed during the current study are not publicly available due to videos from clinical settings with faces visible. The 2D and 3D keypoint trajectories and the annotated velocities and cadences will also be made available from the corresponding author on reasonable request. Code for the gait transformer and the trained weights are available at https://github.com/peabody124/GaitTransformer .
Moore, J. L. et al. A core set of outcome measures for adults with neurologic conditions undergoing rehabilitation: A clinical practice guideline. J. Neurol. Phys. Ther. 42 , 174–220. https://doi.org/10.1097/NPT.0000000000000229 (2018).
Article PubMed PubMed Central Google Scholar
Richard, J., Levine, D. & Whittle, M. Whittle’s Gait Analysis 5th edn. (Elsevier, 2012).
Hamacher, D., Singh, N. B., Van Dieën, J. H., Heller, M. O. & Taylor, W. R. Kinematic measures for assessing gait stability in elderly individuals: A systematic review. J. R. Soc. 8 , 1682–1698. https://doi.org/10.1098/rsif.2011.0416 (2011).
Article CAS Google Scholar
Prasanth, H. et al. Wearable sensor-based real-time gait detection: A systematic review. Sensors 21 , 2727. https://doi.org/10.3390/s21082727 (2021).
Article PubMed PubMed Central ADS Google Scholar
Weygers, I. et al. Inertial sensor-based lower limb joint kinematics: A methodological systematic review. Sensors 20 , 673. https://doi.org/10.3390/s20030673 (2020).
Picerno, P. 25 years of lower limb joint kinematics by using inertial and magnetic sensors: A review of methodological approaches. Gait Posture 51 , 239–246. https://doi.org/10.1016/j.gaitpost.2016.11.008 (2017).
Article PubMed Google Scholar
Rast, F. M. & Labruyère, R. Systematic review on the application of wearable inertial sensors to quantify everyday life motor activity in people with mobility impairments. J. NeuroEng. Rehabil. 17 , 148. https://doi.org/10.1186/s12984-020-00779-y (2020).
Klöpfer-Krämer, I. et al. Gait analysis: Available platforms for outcome assessment. Injury 51 (Suppl 2), S90–S96 (2020).
Zheng, C. et al. Deep Learning-Based Human Pose Estimation: A Survey (2020).
Liu, W. & Mei, T. Recent advances of monocular 2D and 3D human pose estimation: A deep learning perspective. ACM Comput. Surv. 2022 , 3524497. https://doi.org/10.1145/3524497 (2022).
Article Google Scholar
Muhammad, Z.-U.-D., Huang, Z. & Khan, R. A review of 3D human body pose estimation and mesh recovery. Digit. Signal Process. 128 , 103628. https://doi.org/10.1016/j.dsp.2022.103628 (2022).
Nakano, N. et al. Evaluation of 3D markerless motion capture accuracy using OpenPose with multiple video cameras. Front. Sports Active Living 2 , 50. https://doi.org/10.3389/fspor.2020.00050 (2020).
Pagnon, D., Domalain, M. & Reveret, L. Pose2Sim: An end-to-end workflow for 3D markerless sports kinematics—Part 1: Robustness. Sensors 21 , 6530. https://doi.org/10.3390/s21196530 (2021).
Needham, L. et al. The development and evaluation of a fully automated markerless motion capture workflow. J. Biomech. 144 , 111338. https://doi.org/10.1016/j.jbiomech.2022.111338 (2022).
Kanko, R. M., Laende, E., Selbie, W. S. & Deluzio, K. J. Inter-session repeatability of markerless motion capture gait kinematics. J. Biomech. 121 , 110422. https://doi.org/10.1016/j.jbiomech.2021.110422 (2021).
Kanko, R. M. et al. Assessment of spatiotemporal gait parameters using a deep learning algorithm-based markerless motion capture system. J. Biomech. 122 , 110414. https://doi.org/10.1016/j.jbiomech.2021.110414 (2021).
Kanko, R., Laende, E., Davis, E., Selbie, W. S. & Deluzio, K. J. Concurrent assessment of gait kinematics using marker-based and markerless motion capture. J. Biomech. https://doi.org/10.1101/2020.12.10.420075 (2020).
McGuirk, T. E., Perry, E. S., Sihanath, W. B., Riazati, S. & Patten, C. Feasibility of Markerless motion capture for three-dimensional gait assessment in community settings. Front. Hum. Neurosci. 16 , 867485 (2022).
Uhlrich, S. D. et al. OpenCap: 3D human movement dynamics from smartphone videos. PLoS Comput. Biol. 19 , e1011462. https://doi.org/10.1101/2022.07.07.499061 (2022).
Stenum, J., Rossi, C. & Roemmich, R. T. Two-dimensional video-based analysis of human gait using pose estimation. PLOS Comput. Biol. 17 , e1008935. https://doi.org/10.1371/journal.pcbi.1008935 (2021).
Article CAS PubMed PubMed Central ADS Google Scholar
Azhand, A., Rabe, S., Müller, S., Sattler, I. & Heimann-Steinert, A. Algorithm based on one monocular video delivers highly valid and reliable gait parameters. Sci. Rep. 11 , 14065. https://doi.org/10.1038/s41598-021-93530-z (2021).
Kidziński, Ł et al. Deep neural networks enable quantitative movement analysis using single-camera videos. Nat. Commun. 11 , 1–10. https://doi.org/10.1038/s41467-020-17807-z (2020).
Lonini, L. et al. Video-based pose estimation for gait analysis in stroke survivors during clinical assessments: A proof-of-concept study. Digit. Biomark. 6 , 9–18. https://doi.org/10.1159/000520732 (2022).
Cotton, R. J., McClerklin, E., Cimorelli, A., Patel, A. & Karakostas, T. Transforming Gait: Video-Based Spatiotemporal Gait Analysis. In 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC) 115–120. https://doi.org/10.1109/EMBC48229.2022.9871036 (2022).
Cotton, R. J. PosePipe: Open-Source Human Pose Estimation Pipeline for Clinical Research. arXiv:2203.08792 [cs, q-bio] (2022).
Krajbich, J. I., Pinzur, M. S., Potter, B. K. & Stevens, P. M. Atlas of Amputations and Limb Deficiencies: Surgical, Prosthetic, and Rehabilitation Principles (American Academy of Orthopaedic Surgeons, 2016). arXiv:NkN2xwEACAAJ .
Wong, C. K. et al. Exercise programs to improve gait performance in people with lower limb amputation: A systematic review. Prosthet. Orthot. Int. 40 , 8–17 (2016).
Article PubMed ADS Google Scholar
Highsmith, M. J. et al. Gait training interventions for lower extremity amputees: A systematic literature review. Technol. Innov. 18 , 99–113 (2016).
Gard, S. A. Use of quantitative gait analysis for the evaluation of prosthetic walking performance. J. Prosthet. Orthot. https://doi.org/10.1097/00008526-200601001-00011 (2006).
Brinkmann, P. M. & Stevens, J. T. Clinical Considerations of Observational Gait Analysis. In Atlas of Amputations and Limb Deficiencies Surgical, Prosthetic, and Rehabilitation Principles 81–95 (2016).
Cotton, R. J. & Rogers, J. Wearable Monitoring of Joint Angle and Muscle Activity. In 2019 IEEE 16th International Conference on Rehabilitation Robotics (ICORR) 258–263. https://doi.org/10.1109/ICORR.2019.8779538 (IEEE, 2019).
Cotton, R. J. Kinematic Tracking of rehabilitation patients with markerless pose estimation fused with wearable inertial sensors. IEEE 15th International Conference on Automatic Face & Gesture Recognition (2020).
Yatsenko, D. et al. DataJoint: Managing big scientific data using MATLAB or Python . https://doi.org/10.1101/031658 (2015).
Wojke, N., Bewley, A. & Paulus, D. Simple Online and Realtime Tracking with a Deep Association Metric . arXiv:1703.07402 [cs]. arXiv:1703.07402 (2017).
Contributors, M. OpenMMLab Pose Estimation Toolbox and Benchmark (2020).
Sun, K., Xiao, B., Liu, D. & Wang, J. Deep high-resolution representation learning for human pose estimation. Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition 5686–5696. https://doi.org/10.1109/CVPR.2019.00584 (2019).
Lin, T.-Y. et al. Microsoft COCO: Common Objects in Context. In Computer Vision - ECCV 2014 Lecture Notes in Computer Science (eds Fleet, D. et al. ) 740–755 (Springer International Publishing, 2014).
Zhang, F., Zhu, X., Dai, H., Ye, M. & Zhu, C. Distribution-Aware Coordinate Representation for Human Pose Estimation. In 2020 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR) 7091–7100. https://doi.org/10.1109/CVPR42600.2020.00712 (2020).
Liu, J., Rojas, J., Liang, Z., Li, Y. & Guan, Y. A Graph Attention Spatio-temporal Convolutional Network for 3D Human Pose Estimation in Video (2020). arXiv:2003.14179 .
Mathis, A. et al. DeepLabCut: Markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 2018 , 1. https://doi.org/10.1038/s41593-018-0209-y (2018).
Cotton, R. J. et al. Markerless Motion Capture and Biomechanical Analysis Pipeline. In IEEE International Consortium for Rehabilitation Robotics , (arXiv). arXiv:2303.10654 .
Cotton, R. J. et al. Improved Trajectory Reconstruction for Markerless Pose Estimation. In 45th Annual International Conference of the IEEE Engineering in Medicine and Biology Society . arXiv:2303.02413 .
Guo, A., Kamar, E., Vaughan, J. W., Wallach, H. & Morris, M. R. Toward Fairness in AI for People with Disabilities: A Research Roadmap . arXiv (2019).
Trewin, S. AI Fairness for People with Disabilities: Point of View . arXiv:1811.10670 [cs] (2018).
Carse, B., Scott, H., Davie-Smith, F., Brady, L. & Colvin, J. Minimal clinically important difference in walking velocity, gait profile score and two minute walk test for individuals with lower limb amputation. Gait Posture 88 , 221–224. https://doi.org/10.1016/j.gaitpost.2021.06.001 (2021).
Perera, S., Mody, S. H., Woodman, R. C. & Studenski, S. A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 54 , 743–749. https://doi.org/10.1111/j.1532-5415.2006.00701.x (2006).
Kwolek, B. et al. Calibrated and synchronized multi-view video and motion capture dataset for evaluation of gait recognition. Multimed. Tools Appl. 78 , 32437–32465. https://doi.org/10.1007/s11042-019-07945-y (2019).
Yuan, Y., Wei, S.-E., Simon, T., Kitani, K. & Saragih, J. SimPoE: Simulated character control for 3D human pose estimation. In 2021 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR) 7155–7165, https://doi.org/10.1109/CVPR46437.2021.00708 (2021).
Xie, K. et al. Physics-based Human Motion Estimation and Synthesis from Videos . arXiv:2109.09913 [cs] (2021).
Shimada, S., Golyanik, V., Xu, W. & Theobalt, C. PhysCap: Physically Plausible Monocular 3D Motion Capture in Real Time . arXiv:2008.08880 [cs] (2020).
Shi, M. et al. MotioNet: 3D Human motion reconstruction from monocular video with skeleton consistency. ACM Trans. Graph. 40 , 1–15 (2020).
Gong, K. et al. PoseTriplet: Co-evolving 3D Human Pose Estimation, Imitation, and Hallucination under Self-supervision . arXiv:2203.15625 (2022).
Pierzchlewicz, P. A., Cotton, R. J., Bashiri, M. & Sinz, F. H. Multi-hypothesis 3D human pose estimation metrics favor miscalibrated distributions . arXiv:2210.11179 (2022).
Download references
Acknowledgements
This work was generously supported by the Research Accelerator Program of the Shirley Ryan AbilityLab. We would like to thank Emoonah McClerklin for help with data collection.
Author information
Authors and affiliations.
Shirley Ryan AbilityLab, Chicago, USA
Anthony Cimorelli, Tasos Karakostas & R. James Cotton
Department of Neuroscience, Baylor College of Medicine, Houston, USA
Ankit Patel
Department of Electrical & Computer Engineering, Rice University, Houston, USA
Department of Physical Medicine and Rehabilitation, Northwestern University, Evanston, USA
Tasos Karakostas & R. James Cotton
You can also search for this author in PubMed Google Scholar
Contributions
R.J.C. and A.C. designed the study, collected the data and analyzed the data. T.K. and A.P. provided guidance on the development and validation on the gait transformer algorithm. T.K. provided the dataset for training the gait tranformer algorithm and R.J.C. trained it on this data. All authors were involved in the preparation of the manuscript.
Corresponding author
Correspondence to R. James Cotton .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Cimorelli, A., Patel, A., Karakostas, T. et al. Validation of portable in-clinic video-based gait analysis for prosthesis users. Sci Rep 14 , 3840 (2024). https://doi.org/10.1038/s41598-024-53217-7
Download citation
Received : 17 January 2023
Accepted : 30 January 2024
Published : 15 February 2024
DOI : https://doi.org/10.1038/s41598-024-53217-7
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Clinical Gait Analysis and Musculoskeletal Modeling
- First Online: 24 December 2013
Cite this chapter

- Karelia Tecante 4 ,
- Frank Seehaus 4 ,
- Bastian Welke 4 ,
- Gavin Olender 4 ,
- Michael Schwarze 4 ,
- Sean Lynch 4 &
- Christoph Hurschler 4
1732 Accesses
1 Citations
Gait analysis goal is to investigate the mechanics of the muscle, the relationships between muscles and bones and the motions of joints. However, for a deeper analysis of the internal force acting on the human body research has focused on multi-body modeling and simulation. The aim is to integrate the elements of the musculoskeletal system and the joint mechanics in order to better understand what has been learned through in vivo and in vitro experiments. This chapter presents a general overview of musculoskeletal modeling and simulation in the clinical gait area.
This is a preview of subscription content, log in via an institution to check access.
Access this chapter
- Available as EPUB and PDF
- Read on any device
- Instant download
- Own it forever
- Compact, lightweight edition
- Dispatched in 3 to 5 business days
- Free shipping worldwide - see info
- Durable hardcover edition
Tax calculation will be finalised at checkout
Purchases are for personal use only
Institutional subscriptions
Similar content being viewed by others

A review of simulation methods for human movement dynamics with emphasis on gait

On the Modeling of Biomechanical Systems for Human Movement Analysis: A Narrative Review

Objectifying Treatment Outcomes Using Musculoskeletal Modelling-Based Simulations of Motion
Shihab, A., & Moataz, E. (2011). Development and validation of a three-dimensional biomechanical model of the lower extremity. In V. Klika (Ed.), Theoretical biomechanics . ISBN: 978-953-307-851-9, InTech, DOI: 10.5772/24156 .
Erdemir, A., Scott M., Walter H., van den Bogert, A. J., et al. (2007). Model-based estimation of muscle forces exerted during movements. Clinical Biomechanics (Bristol, Avon) , 22 (2), 131–154.
Google Scholar
Schwarze, M., Hurschler, C., Seehaus, F., Oehler, S., & Welke, B. (2013). Loads on the prosthesis-socket interface of above-knee amputees during normal gait: Validation of a multi-body simulation. Journal of Biomechanics , 46 (6), 1201–1206.
Welke, B., Schwarze, M., Hurschler, C., Calliess, T., & Seehaus, F. (2013). Multi-body simulation of various falling scenarios for determining resulting loads at the prosthesis interface of transfemoral amputees with osseointegrated fixation. Journal of Orthopaedic Research , 31 (7), 1123–1129.
Simon, S. R. (2004). Quantification of human motion: Gait analysis-benefits and limitations to its application to clinical problems. Journal of Biomechanics , 37 (12), 1869–1880.
Hausdorff, J. M., Merit, E. C., Renée F., Jeanne Y. W., & Ary L. G. (1998). Gait variability and basal ganglia disorders: Stride-to-stride variations of gait cycle timing in parkinson’s disease and Huntington’s disease. Movement Disorders , 13 (3), 428–437.
Hausdorff, J. M., Apinya, L., Merit, E. C., Amie, L. P., David, K., Ary, L. G., et al. (2000). Dynamic markers of altered gait rhythm in amyotrophic lateral sclerosis. Journal of Applied Physiology , 88 (6), 2045–2053.
Hausdorff, J. M., Schaafsma, J. D., Balash, Y., Bartels, A. L., Gurevich, T., & Giladi, N. (2003). Impaired regulation of stride variability in Parkinson’s disease subjects with freezing of gait. Experimental Brain Research , 149 (2), 187–194.
Bowen, J. D., & Gerry S. M. B. A. (2010). Gait Assessment. In The hip and pelvis in sports medicine and primary care (pp. 71–86). New York: Springer.
Klets, O., Riad, J. & Gutierrez-Farewik, E. M. (2010). Personalized musculoskeletal modeling of lower extremities based on magnetic resonance imaging data of 15 patients with hemiplegic cerebral palsy, IUTAM Symposium on human movement analysis and simulation, 2010 September 13th–15th, Belgium: Leuven.
Klets, O. (2011). Subject-specific musculoskeletal modeling of the lower extremities in persons with unilateral cerebral palsy. Licentiate dissertation. Stockholm: KTH Royal Institute of Technology.
Delp, S. L., & Loan, J. P. (1995). A graphics-based software system to develop and analyze models of musculoskeletal structures. Computers in Biology and Medicine , 25 (1), 21–34.
Arnold, E. M., Samuel, R. W., Richard L. L., & Scott L. D, (2010). A model of the lower limb for analysis of human movement. Annals of Biomedical Engineering , 38 (2), 269–279.
Delp, S. L., Loan, J. P., Melissa, G. H., Felix E. Z., Eric L. T., & Joseph M. R. (1990). An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Transactions on Biomedical Engineering , 37 (8), 757–767.
Lenaerts, G., Ward, B., Frederik, G., Michiel, M., Arthur, S., Van der Perre, G., et al. (2009). Subject-specific hip geometry and hip joint centre location affects calculated contact forces at the hip during gait. Journal of Biomechanics , 42 (9), 1246–1251.
Correa, T. A., Baker, R., & Pandy, M. G. (2011). Accuracy of generic musculoskeletal models in predicting the functional roles of muscles in human gait. Journal of Biomechanics , 44 (11), 2096–2105.
Article Google Scholar
Cuypers, R., Tang, Z., Luther, W., & Pauli, J. (2008). Efficient and accurate femur reconstruction using model-based segmentation and superquadric shapes. Proceedings of the Fourth IASTED International Conference , 619 (007), 99.
Brunner, G., Nambi, V., Yang, E., Kumar, A., Virani, S. S., Kougias, P., et al. (2011). Automatic quantification of muscle volumes in magnetic resonance imaging scans of the lower extremities. Magnetic Resonance Imaging , 29 (8), 1065–1075.
Schmid, J., Kim, J., & Magnenat-Thalmann, N. (2011). Extreme leg motion analysis of professional ballet dancers via MRI segmentation of multiple leg postures. International Journal of Computer Assisted Radiology and Surgery , 6 (1), 47–57.
Schmid, J., Sandholm, A., Chung, F., Thalmann, D., Delingette, H., & Magnenat-Thalmann, N. (2009). Musculoskeletal simulation model generation from MRI data sets and motion capture data. In Recent advances in the 3D physiological human (pp. 3–19). Berlin: Springer.
Teran, J., Sifakis, E., Blemker, S. S., Ng-Thow-Hing, V., Lau, C., & Fedkiw, R. (2005). Creating and simulating skeletal muscle from the visible human data set. IEEE Transactions on Visualization and Computer Graphics , 11 (3), 317–328.
Nikravesh, PE. (1998). Computer-aided analysis of mechanical systems, Prentice-Hall Inc, NJ: Englewood Cliff.
Seireg, A., & Arvikar, R. J. (1973). A mathematical model for evaluation of forces in lower extremeties of the musculo-skeletal system. Journal of Biomechanics , 6 (3), 313–326.
Raikova, R. (1992). A general approach for modelling and mathematical investigation of the human upper limb. Journal of Biomechanics , 25 (8), 857–867.
Jensen, R. H., & Davy, D. T. (1975). An investigation of muscle lines of action about the hip: a centroid line approach vs the straight line approach. Journal of Biomechanics , 8 (2), 103–110.
Blemker, S. S., & Delp, S. L. (2005). Three-dimensional representation of complex muscle architectures and geometries. Annals of Biomedical Engineering , 33 (5), 661–673.
Van der Helm, F. C., Veeger, H. E., Pronk, G. M., Van der Woude, L. H., & Rozendal, R. H. (1992). Geometry parameters for musculoskeletal modelling of the shoulder system. Journal of Biomechanics , 25 (2), 129–144.
Arnold, A. S., Salinas, S., Asakawa, D. J., & Delp, S. L. (2000). Accuracy of muscle moment arms estimated from MRI-based musculoskeletal models of the lower extremity. Computer Aided Surgery , 5 (2), 108–119.
Garner, B. A., & Pandy, M. G. (2000). The Obstacle-set method for representing muscle paths in musculoskeletal models. Computer Methods in Biomechanics and Biomedical Engineering , 3 (1), 1–30. doi: 10.1080/10255840008915251 .
Lieber, R. L., & Fridén, J. (2000). Functional and clinical significance of skeletal muscle architecture. Muscle and Nerve , 23 (11), 1647–1666.
Hill, A. V. (1938). The heat of shortening and the dynamic constants of muscle. Proceedings of the Royal Society , B126 , 136–195.
Zajac, F. E. (1989). Muscle and tendon: Properties, models, scaling, and application to biomechanics and motor control. Critical Reviews in Biomedical Engineering , 17 (4), 359.
Blemker, S. S., Asakawa, D. S., Gold, G. E., & Delp, S. L. (2007). Image-based musculoskeletal modeling: Applications, advances, and future opportunities. Journal of Magnetic Resonance Imaging , 25 (2), 441–451.
Veeger, D. H. (2011). “What if”: The use of biomechanical models for understanding and treating upper extremity musculoskeletal disorders. Manual Therapy , 16 (1), 48–50.
Stewart, C., & Shortland, A. P. (2010). The biomechanics of pathological gait-from muscle to movement. Acta of Bioengineering and Biomechanics , 12 (3), 3–12.
Anderson, A. E., Ellis, B. J., & Weiss, J. A. (2007). Verification, validation and sensitivity studies in computational biomechanics. Computer Methods in Biomechanics and Biomedical Engineering , 10 (3), 171–184.
Leardini, A., Chiari, L., Croce, U. D., Cappozzo, A., et al. (2005). Human movement analysis using stereophotogrammetry. Part 3. Soft tissue artifact assessment and compensation. Gait and Posture , 21 (2), 212.
Lu, T. W., & O’connor, J. J. (1999). Bone position estimation from skin marker coordinates using global optimisation with joint constraints. Journal of biomechanics , 32 (2), 129–134.
Högfors, C., Peterson, B., Sigholm, G., & Herberts, P. (1991). Biomechanical model of the human shoulder joint–II. The shoulder rhythm. Journal of Biomechanics , 24 (8), 699–709.
Komistek, R. D., Kane, T. R., Mahfouz, M., Ochoa, J. A., & Dennis, D. A. (2005). Knee mechanics: A review of past and present techniques to determine in vivo loads. Journal of Biomechanics , 38 , 215–228.
Anderst, W., Zauel, R., Bishop, J., Demps, E., & Tashman, S. (2009). Validation of three-dimensional model-based tiobio-femoral tracking during running. Medical Engineering and Physics , 31 , 10–16.
Nester, C. J., et al. (2009). Lessons from dynamic cadaver and invasive bone pin studies: Do we know how the foot really moves during gait. Journal of Foot and Ankle Research , 2 , 18.
Hurschler, C., Wülker, N., Windhagen, H., Hellmers, N., & Plumhoff, P. (2004). Evaluation of the lag sign tests for external rotator function of the shoulder. Journal of Shoulder and Elbow Surgery , 13 (3), 298–304.
Elias, J. J., Kirkpatrick, M. S., Saranathan, A., Mani, S., Smith, L. G., & Tanaka, M. J. (2011). Hamstrings loading contributes to lateral patellofemoral malalignment and elevated cartilage pressures: An in vitro study. Clinical Biomechanics , 26 (8), 841–846.
Wünschel, M., Leichtle, U., Obloh, C., Wülker, N., & Müller, O. (2011). The effect of different quadriceps loading patterns on tibiofemoral joint kinematics and patellofemoral contact pressure during simulated partial weight-bearing knee flexion. Knee Surgery, Sports Traumatology, Arthroscopy , 19 (7), 1099–1106.
Wülker, N., Hurschler, C., & Emmerich, J. (2003). In vitro simulation of stance phase gait part II: Simulated anterior tibial tendon dysfunction and potential compensation. Foot and Ankle International , 24 , 623–629.
Wünschel, M., Leichtle, U., Lo, J., Wülker, N., & Müller, O. (2012). Differences in tibiofemoral kinematics between the unloaded robotic passive path and a weightbearing knee simulator. Orthopedic Reviews , 4 (1), e2. doi: 10.4081/or.2012.e2 .
Davoodi, Rahman, & Gerald, E. (2002). A software tool for faster development of complex models of musculoskeletal systems and sensorimotor controllers in simulinkTM. Journal of Applied Biomechanics , 18 (4), 357–365.
Delp, S. L., Frank, C., Chand, T., & Darryl, G. (2007). OpenSim: Open-source software to create and analyze dynamic simulations of movement. IEEE Transactions on Biomedical Engineering , 54 (11), 1940–1950.
Mansouri, M., & Reinbolt, J. A. (2012). A platform for dynamic simulation and control of movement based on OpenSim and MATLAB. Journal of Biomechanics , 45 (8), 1517–1521.
Higginson, J. S., Zajac, F. E., Neptune, R. R., Kautz, S. A., & Delp, S. L. (2006). Muscle contributions to support during gait in an individual with post-stroke hemiparesis. Journal of Biomechanics , 39 (10), 1769–1777.
Jonkers, I., Stewart, C., Desloovere, K., Molenaers, G., & Spaepen, A. (2006). Musculo-tendon length and lengthening velocity of rectus femoris in stiff knee gait. Gait and Posture , 23 (2), 222–229.
Crabtree, C. A., & Jill, S. (2009). Modeling neuromuscular effects of ankle foot orthoses (AFOs) in computer simulations of gait. Gait and Posture , 29 (1), 65–70.
Nair, P. M., Rooney, K. L. Kautz, S. A. Behrman, A. L. et al. (2010). Stepping with an ankle foot orthosis re-examined: A mechanical perspective for clinical decision making. Clinical Biomechanics (Bristol, Avon) , 25 (6), 618.
Sherman, M. A., & Seth, A. (2011). Simbody: Multibody dynamics for biomedical research. Procedia IUTAM , 2 , 241–261.
Silverman, A. K., & Neptune, R. R. (2010). Individual muscle function in below Knee amputee walking. In Conference Proceedings of the Annual Meeting of the American Society , p. 166.
Fey, N. P., Klute, G. K., & Neptune, R. R. (2013). Altering prosthetic foot stiffness influences foot and muscle function during below-knee amputee walking: A modeling and simulation analysis. Journal of Biomechanics , 46 (4), 637–644.
Worsley, P., Stokes, M., & Taylor, M. (2011). Predicted knee kinematics and kinetics during functional activities using motion capture and musculoskeletal modelling in healthy older people. Gait and Posture , 33 (2), 268–273.
Michiel, O., Telfezr, S., Tørholm, S., Carbes, S., van Rhijn, L., Ross, M. et al. (2011). Generation of subject-specific, dynamic, multisegment ankle and foot models to improve orthotic design: A feasibility study. BMC Musculoskeletal Disorders , 12 (1), 256.
Nolte, D., Andersen, M. S., Rasmussen, J., & Al-Munajjed, A. (2013). Development of a patient-specific musculoskeletal model of a healthy knee to analyze hard and soft tissue loading. In 21th Annual Symposium on Computational Methods in Orthoopeadic Biomechanics .
Alkjær, T., Wieland, M. R., Andersen, M. S., Simonsen, E. B., & John, R. (2012). Computational modeling of a forward lunge: Towards a better understanding of the function of the cruciate ligaments. Journal of Anatomy , 221 (6), 590–597.
Ali, Nicholas, Michael Skipper Andersen, John Rasmussen, D Gordon E Robertson, and Gholamreza Rouhi. 2013. “The application of musculoskeletal modeling to investigate gender bias in non-contact ACL injury rate during single-leg landings”. Computer methods in biomechanics and biomedical engineering (ahead-of-print): 1–15.
Cao, E., Inoue, Y. Liu, T. & Shibata, K. (2012). Estimation of lower limb muscle forces during human sit-to-stand process with a rehabilitation training system. In 2012 IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI) , pp. 1016–1019.
Lemieux, P. O., Tétreault, P. Hagemeister, N. & Nuño, N. (2012). Influence of prosthetic humeral head size and medial offset on the mechanics of the shoulder with cuff tear arthropathy: A numerical study. Journal of Biomechanics , 46 (3), 806–812.
Jackson, M., Sylvestre, É. Bleau, J. Allard, P & Begon, M. (2012). Estimating optimal shoulder immobilization postures following surgical repair of massive rotator cuff tears. Journal of Biomechanics , 46 (1), 179–182.
Galibarov, P. E., Dendorfer, S., & Rasmussen, J. (2011). Two computational models of the lumbar spine: Comparison and validation . Long Beach, CA: ORS—Orthopaedic Research Society.
Han, K. S., Zander, T., Taylor, W. R., & Rohlmann, A. (2012). An enhanced and validated generic thoraco-lumbar spine model for prediction of muscle forces. Medical Engineering and Physics , 34 (6), 709–716.
Ulrey, B. L., & Fathallah, F. A. (2012). Subject-specific, whole-body models of the stooped posture with a personal weight transfer device. Journal of Electromyography and Kinesiology .
Lundberg, H. J., Foucher, K. C., Andriacchi, T. P., & Wimmer, M. A. (2012). Direct comparison of measured and calculated total knee replacement force envelopes during walking in the presence of normal and abnormal gait patterns. Journal of Biomechanics , 45 (6), 990–996.
Taddei, F., Martelli, S., Valente, G., Leardini, A., Benedetti, M, G., Manfrini, M., et al. (2012). Femoral loads during gait in a patient with massive skeletal reconstruction. Clinical Biomechanics , 27 (3), 273–280.
Donnelly, C. J., & Lloyd, D. G. (2012). Optimizing whole-body kinematics to minimize valgus knee loading during sidestepping: Implications for ACL injury risk. Journal of Biomechanics , 45 (8), 1491–1497.
Crossley, K. M, Dorn, T. W. Ozturk, H., van den Noort, J., Schache, A. G., & Pandy, M. G. (2012). Altered hip muscle forces during gait in people with patellofemoral osteoarthritis. steoarthritis and Cartilage .
Mansouri, M., Clark, A. E., & Reinbolt, J. A. (2012). The use of a platform for dynamic simulation of movement: Application to balance recovery. Proceedings of the American Society of Biomechanics , Gainesville, FL. Aug. 2012 (Available at: http://works.bepress.com/jeffrey_reinbolt/57 ).
Branemark, R., Branemark, P. I., Rydevik, B., & Myers, R. R. (2001). Osseointegration in skeletal reconstruction and rehabilitation: A review. Journal of rehabilitation research and development , 38 (2), 175–182.
Brånemark, P. I. (1983). Osseointegration and its experimental background. The Journal of prosthetic dentistry , 50 (3), 399–410.
Hagberg, K., Branemark, R., Guntorberg, B., et al. (2008). Osseointegrated trans-femoral amputation prostheses: Pro-spective results of general and condition-specific quality of life in 18 patients at 2-year follow-up. Prosthetics and Orthotics International , 32 , 29–41.
Grundei, H., Von Stein, T., Schulte-Bockhof, D., Kausch, C., Gollwitzer, H., & Gradinger, R. (2009). Die Endo-Exo-Femurprothese—Update eines Versorgungskonzeptes zur Rehabilitation von Oberschenkelamputierten. Orthopädie-Technik , 12 , 143–149.
Kadaba, M. P., Ramakrishnan, H. K., & Wootten, M. E. (1990). Measurement of lower extremity kinematics during level walking. Journal of Orthopaedic Research?: official publication of the Orthopaedic Research Society , 8 (3), 383–92. doi:10.1002/jor.1100080310.
Vicon Plug-in Gait Product Guide–Foundation Notes Revision 2.0 March 2010 For use with Plug-in Gait Version 2.0 in Vicon Nexus, 2010.
Tomaszewski, P. K., Verdonschot, N., Bulstra, S. K., & Verkerke, G. J. (2010). A comparative finite-element analysis of bone failure and load transfer of osseointegrated prostheses fixations. Annals of Biomedical Engineering , 38 (7), 2418–2427.
Download references
Acknowledgments
This study was funded by the German Federal Ministry of Education and Research (BMBF AZ: 01EZ0775). The authors like to thank TU Berlin and Otto Bock Healthcare GmbH, Duderstadt, Germany for cooperation in TExoPro and EU Marie Curie Actions-Marie Curie Research Training Networks/ Multi-scale Biological Modalities for Physiological Human articulation 289897 (FP7-PEOPLE-2011-ITN) for their funding
Author information
Authors and affiliations.
Laboratory for Biomechanics and Biomaterials (LBB) Orthopaedic Department, Hannover Medical School (MHH), Hannover, Germany
Karelia Tecante, Frank Seehaus, Bastian Welke, Gavin Olender, Michael Schwarze, Sean Lynch & Christoph Hurschler
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Karelia Tecante .
Editor information
Editors and affiliations.
Centre Universitaire Informatique, MIRALab - University of Geneva, Carouge, Geneve, Switzerland
Nadia Magnenat-Thalmann
Nuclear Medicine Division, University Hospital of Geneva, Geneva, Switzerland
Osman Ratib
Hon Fai Choi
Rights and permissions
Reprints and permissions
Copyright information
© 2014 Springer-Verlag London
About this chapter
Tecante, K. et al. (2014). Clinical Gait Analysis and Musculoskeletal Modeling. In: Magnenat-Thalmann, N., Ratib, O., Choi, H. (eds) 3D Multiscale Physiological Human. Springer, London. https://doi.org/10.1007/978-1-4471-6275-9_7
Download citation
DOI : https://doi.org/10.1007/978-1-4471-6275-9_7
Published : 24 December 2013
Publisher Name : Springer, London
Print ISBN : 978-1-4471-6274-2
Online ISBN : 978-1-4471-6275-9
eBook Packages : Computer Science Computer Science (R0)
Share this chapter
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Publish with us
Policies and ethics
- Find a journal
- Track your research
- Open access
- Published: 02 March 2006
Gait analysis methods in rehabilitation
- Richard Baker 1 , 2 , 3 , 4
Journal of NeuroEngineering and Rehabilitation volume 3 , Article number: 4 ( 2006 ) Cite this article
76k Accesses
309 Citations
Metrics details
Introduction
Brand's four reasons for clinical tests and his analysis of the characteristics of valid biomechanical tests for use in orthopaedics are taken as a basis for determining what methodologies are required for gait analysis in a clinical rehabilitation context.
Measurement methods in clinical gait analysis
The state of the art of optical systems capable of measuring the positions of retro-reflective markers placed on the skin is sufficiently advanced that they are probably no longer a significant source of error in clinical gait analysis. Determining the anthropometry of the subject and compensating for soft tissue movement in relation to the under-lying bones are now the principal problems. Techniques for using functional tests to determine joint centres and axes of rotation are starting to be used successfully. Probably the last great challenge for optical systems is in using computational techniques to compensate for soft tissue measurements. In the long term future it is possible that direct imaging of bones and joints in three dimensions (using MRI or fluoroscopy) may replace marker based systems.
Methods for interpreting gait analysis data
There is still not an accepted general theory of why we walk the way we do. In the absence of this, many explanations of walking address the mechanisms by which specific movements are achieved by particular muscles. A whole new methodology is developing to determine the functions of individual muscles. This needs further development and validation. A particular requirement is for subject specific models incorporating 3-dimensional imaging data of the musculo-skeletal anatomy with kinematic and kinetic data.
Methods for understanding the effects of intervention
Clinical gait analysis is extremely limited if it does not allow clinicians to choose between alternative possible interventions or to predict outcomes. This can be achieved either by rigorously planned clinical trials or using theoretical models. The evidence base is generally poor partly because of the limited number of prospective clinical trials that have been completed and more such studies are essential. Very recent work has started to show the potential of using models of the mechanisms by which people with pathology walk in order to simulate different potential interventions. The development of these models offers considerable promise for new clinical applications of gait analysis.
For the purposes of this paper gait analysis will be assumed to refer to the instrumented measurement of the movement patterns that make up walking and the associated interpretation of these. The core of most contemporary gait analysis is the measurement of joint kinematics and kinetics. Other measurements regularly made are electromyography (EMG), oxygen consumption and foot pressures. A systematic physical examination of the patient is usually conducted as part of a gait analysis.
Rehabilitation is a clinical discipline and this paper will thus concentrate on clinical gait analysis. Richard Brand [ 1 , 2 ] proposed four reasons for performing any clinical test (see Table 1 ). The third of these might actually be taken as a definition of the word clinical i.e. a clinical test is one conducted in order to select from among different management options for a patient (including the possibility of not intervening).
Much contemporary gait analysis is done for the purpose of clinical research . This differs from clinical testing in that the reason is not to make clinical decisions for the individual patient, but to learn about a condition affecting a group of patients or the effect of an intervention. It is important to remember that the criteria for valid clinical research may not be the same as those for valid clinical testing. For example if a measurement made on a patient cannot be relied upon because of random errors then that measurement will not be useful for clinical purposes. By increasing the number of patients in a sample however, even measurements with quite large random errors can result in meaningful conclusions in clinical research. This paper will focus on gait analysis for clinical use. It will also focus on methodology rather than areas of clinical application.
Brand's [ 1 , 2 ] other three possible reasons for performing any clinical test are to distinguish between disease entities (diagnosis), to determine the severity, extent or nature of a disease or injury (assessment), and to predict outcomes of intervention (or the absence of intervention). The monitoring of the progress of a patient's condition either following intervention or in its absence might be regarded as an additional reason. This modification of Brand's approach is summarised in Table 2 .
Brand went on to propose a number of criteria for assessing the usefulness of biomechanical measurements in general which, with some modification, can be used as criteria for the usefulness of all clinical gait analysis. These are listed in Table 3 . The first requirement of any clinical measurement is that it should characterise the patient, that is if the patient attends on two separate occasions, between which his or her condition might be considered as stable, the measurements taken should be similar. This requires that the measurement technique itself is repeatable but also that the quantity being measured is stable and independent of factors such as mood, motivation or pain. Measurements can be repeatable and stable without necessarily being accurate (representative of a specific physical quantity). Such tests can be clinically useful but will be much easier to interpret if they are also accurate. In an era of evidence based clinical practice it is essential that any measurement techniques are appropriately validated which must include assessments of both their repeatability and accuracy.
In order to perform a diagnostic function it is necessary for measurements to be able to distinguish normal from abnormal patterns of movement and also between the characteristics of one disease entity and another. There are two aspects to this. The first is having measurement systems capable of working to adequate precision. The second is a knowledge of what characterises normal walking or a particular disease entity.
The requirement for patient assessment pre-supposes that a diagnosis does not give sufficient information to determine the most appropriate management for a patient and that measuring the precise characteristics of a patient's condition are essential for this. Measurements thus have to be sufficiently precise to reveal clinically important differences between patients with the same diagnosis. For monitoring purposes measurements need to be sufficiently precise to be able to determine whether a patient's condition is stable, improving or deteriorating.
Brand suggested that the measurement technique should not affect the function it is measuring. The walking performed in a gait analysis laboratory however, with the patient concentrating on what they are doing in an idealised environment, is not necessarily representative of their normal walking. At the very least this must be taken into account when interpreting results.
Gait analysis should reveal information that is useful to the clinician and this will generally require that results are reported in terms analogous to accepted clinical concepts. It must be cost-effective, that is the benefit of performing the test must be worth the cost. This balance need not necessarily be determined in purely financial terms but the financial cost of gait analysis is a significant factor. Finally there is no point doing any clinical test if the results could be obtained sufficiently well by simply observing the patient
The information obtained by assessing the patient is that used for selecting management options. This process does not, therefore, make further demands on the measurement systems but does require an understanding of how the patient's condition is likely to be affected by an intervention (or none) to a level sufficient to determine which options are preferable. Prediction of outcomes takes this one stage further to being able to determine not only which management option is best but also how the patient will be after that intervention.
This sequential analysis of the four potential purposes of clinical tests reveals a progression from just requiring reliable and precise measurements to the additional requirement of having an understanding of how such information is incorporated into clinical practice. The state of the art is that the measurement component of gait analysis can reasonably be described as an objective process whereas the interpretation component is predominantly subjective.
Making the interpretive component more objective can be achieved in two ways. The first is to develop a general theory of how people walk whether they have recognised pathology or not. As long ago as 1982 Cappozzo lamented, "The approaches to clinical gait analysis and evaluation are not supported by general theories" [ 3 ] and despite over 20 years of intense activity this is still a reasonable summary of the state of the art. The second approach, which must operate in the absence of the former, is to conduct clinical research to ascertain the outcome of particular interventions on groups of patients characterised by certain measurements. Most of the knowledge base used in the interpretive component of gait analysis comes from such studies. It is because there are relatively few studies available to base such interpretations on that the subjective element of interpretation is necessary in contemporary clinical gait analysis.
Modern clinical gait analysis traces its origins back to the early 1980s with the opening of the laboratory developed by the United Technologies Corporation at Newington, Connecticut and those provided with equipment by Oxford Dynamics (later to become Oxford Metrics) in Boston, Glasgow and Dundee. Retro-reflective markers were placed on the skin in relation to bony landmarks. These were illuminated stroboscopically and detected by modified video cameras. If two or more cameras detect a marker and the position and orientation of these cameras are known then it is possible to detect the three-dimensional position of that marker [ 4 ].
Whilst the basic principles remain the same as the earliest systems, the speed, accuracy and reliability has advanced beyond all recognition. It is not uncommon now to find clinical systems using 8, 10 or more cameras functioning at over 100 Hz and capable of detecting reliably the presence of many tens of markers of between 9 and 25 mm diameter. Calibration of the systems (the determination of the position, orientation and optical and electronic characteristics of the cameras) can generally be accomplished in less than a minute. Marker positions from clinical trials can be reconstructed and markers labelled automatically in real time (although this feature is often not essential for clinical studies). The determination of the accuracy of such systems is now generally limited by the accuracy of any alternative means to determine marker position and can be taken to be of the order of 1 mm. This is probably an order of magnitude smaller than other sources of error in determining joint kinematics and kinetics. This particular measurement technology has thus reached a mature state of development that, whilst advances will almost certainly continue, already probably delivers all that is required by conventional gait analysis [ 5 ].
The same cannot be said of the computer models used to derive joint kinematics and kinetics from the marker position data supplied by the measurement hardware. Almost all commercially available clinical systems use some variant of the Conventional Gait Model [ 6 ] which has been referred to as the Newington, Gage, Davis [ 7 ], Helen Hayes, Kadaba [ 8 , 9 ] or Vicon Clinical Manager (VCM) model. This was developed using the minimum number of markers possible to determine 3-dimensional kinematics and kinetics [ 10 , 11 ] of the lower limb at a time when measurement systems were only capable of detecting a handful of markers. It assumes three degree of freedom joints for the hip and knee and a two degree of freedom joint at the ankle. The model is hierarchical requiring the proximal segments to have been detected in order that distal segments can be defined and incorporates regression equations to determine the position of the hip joint centre with respect to pelvic markers. Kinetics are determined using an inverse dynamics approach which generally requires considerable filtering to give any useful signals. An alternative system the Cleveland Clinic Model based around a cluster of markers on a rigid base attached to each segment is the only other widely used model. Unfortunately documentation of this model in the scientific literature is very poor.
The problem of limited repeatability
The primary problem of current measurement technology is that of reliability in routine clinical use. Several studies have now been reported in which a single subject has been analysed in a number of different laboratories [ 12 – 14 ]. These have shown a degree of variability between sites that would appear to be sufficient to undermine clinical applications. In retrospect, the original studies of the reliability are flawed. There was no such study of the Davis implementation of the model and the statistics used by Kadaba et al [ 8 , 9 ] to report reliability of their implementation probably acted to mask deficiencies. In particular, use of relative measures of reliability such as the coefficient of multiple correlation (CMC) makes interpretation of findings difficult. Almost all reliability studies have been done on subjects without pathology where marker placement is reasonably straightforward. Reliability for clinical populations is rarely reported in the literature and is almost certainly inferior.
At least one recent study has shown that it is possible to get levels of reliability sufficient to justify the continued clinical use of gait analysis within a single centre [ 15 ]. Too few centres however are providing evidence to establish that this is the rule rather than the exception.
Whilst not the most exciting field of research, a very real need of clinical gait analysis is for the development of techniques for establishing the reliability of measurement techniques and of methods of quality assurance that will ensure that the very highest standards of reliability are achieved in routine clinical practice .
Source of error: Model calibration
There are two principal sources of error. The first is the difficulty determining the anthropometry of the individual subject (known as model calibration ). This has two aspects, placing markers accurately with respect to specific anatomical landmarks and determining the location of the joint centres (and other anatomical features) in relation to these markers. Failure to place markers accurately is probably the single greatest contributor to measurement variability in contemporary clinical gait analysis. This is partly a matter of appropriate staff training and quality assurance but at least as important, and more fundamental, is the problem that many of the landmarks used to guide marker placement are not themselves particularly well defined in patients with certain conditions [ 16 ]. Even when bony landmarks are sharply defined an increasing number of patients have a considerable thickness of subcutaneous fat that makes palpation difficult.
The Conventional Gait Model uses regression equations to determine the position of the hip joint centre in relation to the pelvis. Both Bell's [ 17 – 19 ] and Davis' [ 7 ] equations are commonly used and there is now good evidence that neither is satisfactory in healthy adults [ 20 ]. There have still been no published studies of whether either is valid for healthy children. Children with orthopaedic conditions including cerebral palsy may often have dysplasia of the hip or deformity of the pelvis, and it is exceedingly unlikely that any form of regression equation could be used in these patients to determine hip joint position.
Methods for moving away from anatomical landmarks and regressions equations for determining joint centres have been around for nearly a decade, the process being known as anatomical calibration [ 21 ]. They rely on calibration movements to be performed before capturing walking data and some form of fitting of the measured marker positions to an underlying model of how the body moves. The simplest example is probably the determination of the hip joint centre. It is assumed that the hip joint moves as a ball and socket joint about centre of rotation fixed in the pelvis. Any marker on the femur would thus be expected to describe a path on the surface of a sphere centred on the hip joint centre when the hip joint is moving. A least squares fit of the measured data to such a sphere allows the location of that joint centre to be determined [ 20 , 22 ]. Similar approaches are applicable to determine that axis of the knee joint which for this purpose has often been assumed to be a simple hinge joint.
Various approaches to fitting data to an underlying model have been attempted and many seem to give reasonable results [ 20 , 22 – 28 ]. Such techniques have not so far been widely accepted into clinical practice probably because there is a perception that such calibration trials are too difficult for patients to execute. At least one clinical lab however has now committed itself to implementing such techniques into routine practice and has reported failure to perform test adequately in only one of over 700 patients tested so far.
Further studies are needed to confirm these studies and to identify which of the range of available optimisation techniques is the best suited to clinical applications. Comprehensive reliability studies are again needed to demonstrate the advantages of using such models over the conventional model .
Sources of error: Soft tissue artefact
The second source of error is the degree of movement of the skin, muscle and other soft tissues in relation to the bones that occurs during walking. This is perhaps most marked in relation to the rotational profile of the hip. Lamoreux [ 29 ], as far back as 1991, reported that with optimal placement of thigh wands only 65% of transverse plane hip joint rotation was detected and that with poor placement this could be as little as 35%.
The problem of skin and other soft tissue movement is more problematic than that of model calibration. Lu and O'Connor where the first to propose fitting a model of how the body is expected to move to marker co-ordinate data [ 30 ] using an optimisation approach. This model uses a least squares fit, similar to some of the techniques described above for model calibration, and thus makes no assumptions about the nature of the soft tissue movement. Other similar models have now been made commercially available [ 26 ]. More recent studies have started to try to map out the movement of markers with respect to the underlying bones [ 31 , 32 ]. If such movement can be characterised as a function of joint angle then, in principle, this knowledge could be built into a model to allow such movements to be compensated for. Such mapping is only likely to be useful if it can be shown that soft tissue movement is consistent across a range of subjects and activities. It is not clear at present whether these conditions are satisfied. A particular problem in regard to mapping soft tissue movement is that of defining what the "true" movement of the bones is. In the absence of any gold standard a variety of assumptions are being used most of which have serious limitations.
Significant work is needed in this area. A gold standard method for determining joint movement is required.
Maps of soft-tissue movement as a function of joint angle are required and work done to establish how these vary from individual to individual and from task to task.
Marker sets need to be defined based on the optimum placement of markers given knowledge of the soft-tissue displacements.
Finally it is possible that knowledge of likely soft-tissue displacement could be built into the optimisation algorithms allowing for better estimates of the movements of the underlying skeleton.
The development of a gold standard method for determining joint movement will probably require a move away from skin-mounted markers (or other sensors). Once such technology is available however it is quite possible that this will supersede the presently available systems. The cost of any such new systems however is likely to prohibit ready clinical availability in the foreseeable future.
There has been some work done on markerless optical methods. By placing a number of video cameras around a subject and tracing the silhouette of the walking subject on each it is possible to generate a 3-dimentional silhouette of that subject. This has already been achieved but the next step of using such a silhouette to determine the co-ordinate systems associated with the moving body segments has not yet been satisfactorily achieved.
It is possible that the problem of skin movement can only be satisfactorily addressed by making direct measurements of bone position. It is now possible to take 3-dimensional images of bones (and muscles) using MRI but only within a very restricted capture volume [ 33 – 35 ]. The image processing problem of automatically determining a bone embedded axis system from such images has yet to be solved satisfactorily. Similarly both uniplanar and biplanar cine fluoroscopy [ 36 – 40 ] has been used to detect the 3-dimensional movement of the internal knee prostheses during a variety of movements. This is possible because a knowledge of the exact size and shape of the prosthetic components and their opacity to x-rays greatly simplifies the image processing problem. Using similar techniques to determine the movement of joints has also been reported [ 41 – 43 ]
Using 3-dimensional imaging techniques to directly determine bone movements during walking either as a technology with potential clinical applicability or for use as a gold reference standard from which to improve the implementation of conventional marker based technologies is one of the greatest challenges in this area.
Methods for interpreting clinical gait analysis data
The second element of clinical gait analysis is the interpretation of data. Conventions for describing 3-dimensional joint kinematics and kinetics are well formulated. Many laboratories are augmenting conventional kinematics and kinetics with muscle length and, less commonly, moment arm graphs. Normal patterns of movement as represented by these data are now generally fairly well understood by clinical specialists although there is actually very little normative data published in the peer-reviewed literature. Similarly, many abnormal patterns of movement are quite widely recognised by clinicians but there few published attempts at formal classification of these [ 44 – 46 ]. Many clinicians have learnt to associate particular abnormal patterns in particular patient groups with particular impairments of body structure and function. Intervention based on such an understanding often leads to a normalisation of gait patterns at subsequent assessments (e.g. [ 47 – 55 ]). It is on this basis that clinical gait analysis operates at present.
Despite the widespread acceptance of many of these conventions there are still problems. Baker [ 56 ] demonstrated that the Euler sequence used to calculate pelvic angles gives rise to data that can be mis-leading to clinicians and proposed an alternative to correct this which is yet to be adopted widely within clinical analysis. Methods for interpreting angles in three dimensions, either in terms of Euler/Cardan rotations or the Grood and Suntay convention [ 57 , 58 ] are not well understood either by clinicians or many bioengineers. A recent attempt to standardise the reporting of joint angles [ 59 ] proposed a different convention to that of the Conventional Gait Model and the continuing debate as to which is preferable illustrates this confusion [ 60 , 61 ]. Joint moments are generally reported with reference to orthogonal axis systems fixed in the distal (Conventional Gait Model) or proximal segments (or occasionally the laboratory axis system). These differ significantly depending on the axis system chosen [ 6 , 62 ] yet there has been no debate about which if any is preferable. Reporting moments about orthogonal axis systems and joint rotations about non-orthogonal ones leads to difficulties in relating the moments to the changes in joint angles to which they are related. The use of muscle moment arms will be discussed further below but it is interesting that there is no straightforward definition of the meaning of the term moment arm in three dimensions [ 63 ] and it is often not clear how such data should be interpreted.
A consistent, comprehensive and clear method for describing joint kinematics and kinetics in three dimensions would be of immense benefit for the clinical gait analysis community.
Perhaps the most important limitation of our present understanding of human walking, however, is that it is primarily descriptive. We know what happens rather than why it happens. Many in the clinical gait analysis community regard kinematics as descriptive but contend that kinetics explain movement patterns. This is almost certainly misguided. Kinetics are simply another set of measurements and can thus only be descriptive.
There have been various attempts at establishing a theory of walking but none is particularly convincing. Saunders, Inman and Eberhart's determinants of normal walking [ 64 ] are perhaps the best known of these. Recent publications however have questioned how the detail of these reflects experimental data [ 65 – 70 ]. Gage [ 71 , 72 ] based his pre-requisites of gait on earlier work by Perry [ 73 ] but these are best regarded as pointers to where particular patients are deficient rather than explanations of how they are achieving walking with or without pathology.
Perhaps the closest we have come so far to understanding why we walk the way we do has come from the work of Pandy and Anderson [ 74 , 75 ]. They have shown that it is possible to construct a mathematical simulation of muscle function during normal walking based on the assumption that the total consumption of energy per unit distance walked is minimised. The authors, however, commented that the model seems more dependent on the boundary conditions imposed than on the nature of the optimisation function. Further, because of the complex nature of the optimisation process driving the model it is still difficult to explain how the precise characteristics of any particular feature of the walking pattern affect the overall calculation of energy expenditure. So far such a model has only been constructed for normal walking.
An obvious challenge in the emerging field of computational biomechanics is to apply similar techniques to model walking with particular forms of pathology.
Conceptually, modifying such models to incorporate a specific abnormality of the musculo-skeletal anatomy such as a leg length discrepancy or contracture of a particular muscle is reasonably straightforward. It is much less certain whether such techniques can be applied at all to patients with neuromuscular pathology who are most frequently seen by clinical gait analysis services. Optimisation techniques assume that movements are controlled in such a way that a specific control function is minimised. In many neuromuscular conditions (Cerebral Palsy, Parkinson's disease, adult hemiplegia) the problem is one of a loss of central control and this would appear to invalidate any techniques modelling human movement as an optimised process.
If such models are developed it will be interesting to see whether they give any insights into the clinical management of patients. Further it will be interesting to see whether their use leads to an understanding of why we walk the way we do which can be formulated as theories that are applicable without the use of such complex models.
Perhaps the greatest challenge in clinical gait analysis is still to answer the question. "Why do we walk the way we do and why don't our patients?".
Whilst the answer to this question still seems as far away as ever, significant advances have been made over recent years in understanding the mechanisms by which we walk particularly in the way that muscles act. For many years it was assumed that a muscle's anatomical position determines how it acts. It was assumed for example that the action of the hamstrings, passing behind the knee, was always to flex the knee. It is only comparatively recently that biomechanists have come to appreciate that any individual muscle has an effect on all the segments of the body and that in some circumstances this may result in a muscle having an action different to its anatomical function [ 76 – 81 ]. It is now fairly well accepted, for example, that the hamstrings functions as a knee extensor during early stance in normal walking because its effect in extending the hip has a secondary tendency to extend the knee which is greater than its direct effect as an anatomical knee flexor [ 82 ].
Such work depends on knowing the joint kinematics and kinetics and inertial properties of the body segments. These can be used to estimate the forces in individual muscles [ 81 – 83 ]. This is an indeterminate problem so is dependent on an optimisation approach (and the validity of this in neuromuscular pathology is questioned in the same way as that of the simulations described above). Once the muscle forces are known forward modelling can be used to determine the effect that a given muscle is having on any segment (or joint) of the body. Until very recently the first part of this problem, the estimation of muscle forces had not been achieved which limited the application of the second part, the forward modelling to data obtained from the simulations described above [ 74 , 75 ]. Recently methods have been develop to estimate the muscle forces required to generate measured joint kinematics and ground reaction forces and have been used both to understand the function of individual muscles during pathological gait and predict the effect of interventions [ 84 , 85 ]. These have been based on scaled models of the adult musculo-skeletal anatomy.
A further area of challenge is in using 3-dimensional imaging techniques to model musculo-skeletal deformities to allow the generation of patient-specific models of walking.
There is also considerable debate at present about the validity of these techniques (the simulations, the estimations of muscle forces and the forward modelling). Whilst the general principles are sound the techniques are known to be extremely sensitive to certain aspects of their implementation (and may be sensitive to many more). For example the forward modelling in particular is sensitive to how the interaction between the foot and the floor is modelled with there being no clear consensus as to the most appropriate method for this [ 74 , 77 , 81 ].
Implementation of these models must be based on robust techniques being developed to validate models, the first step of this is in rigorous analysis of the sensitivity of models to the assumptions on which they are based.
Methods for understanding the effect of intervention
Understanding how to interpret clinical gait analysis data is not itself sufficient to allow selection from amongst treatment options (Table 1 ). For this it is also necessary to know what effect the available interventions are likely to have on someone's walking pattern. If we had a general theory of walking then it might be possible to develop a theoretical basis for considering the effect of any intervention. For patients whose walking could be modelled using a simulation based on specific musculo-skeletal abnormalities it might be possible to use similar simulations to model what might happen if partial correction of those abnormalities were attempted (obviously full correction would restore normal walking!). The author is unaware of any published work at this level at present.
There are then two methods for understanding the effects of intervention in these patients; clinical research to establish what the actual effect of a given intervention is or using knowledge of the mechanisms of walking to predict the effect of modifying the characteristics of the musculo-skeletal anatomy.
By far the most common approach to date has been the use of clinical research – the comparison of gait patterns before and after a particular intervention [ 47 – 55 , 86 – 88 ]. Even so there have been comparatively few studies that have given conclusive findings. Many studies which claim to have done so have quite serious methodological flaws. This is particularly true of research into orthopaedic surgery for children with CP where researchers have used retrospective audits of clinical practice to try and answer specific questions. Many of these studies attempt to make inferences about individual procedures which have only ever been performed as part of a multi-level surgical package [ 47 , 49 – 51 , 54 , 55 ]. It is impossible to tell from these studies which effects are due to the particular procedure being considered and which are due to the overall package. Several studies have attempted to separate out those effects by dividing patients into those who have and those who have not had a particular procedure as part of the overall package of surgery and use methods to compare groups similar to those that would be used for a randomised clinical trial [ 47 , 54 ]. The validity of this approach is questionable, however, because generally the two groups of patients were not similar to start with. Those that had the procedure had it because it was considered that the patient needed it and vice versa. Comparison of the two groups to give insight into the effect of the procedure is thus invalid.
Perhaps the most challenging field of research for clinical gait analysis is in the design and conduct of prospective clinical trials to ascertain the effects of specific treatments on specific patient groups.
An alternative to the use of clinical trials is to use knowledge of the mechanisms of walking as a basis for modelling the effect of changing that mechanism. Reports of such studies are now starting to emerge. For example Arnold et al. [ 84 ] have reported a subject specific model of a cerebral palsy patient with a stiff knee gait and used it to predict the effect of three different potential interventions. These indicated a preferable intervention and the post-intervention gait data showed at least qualitative agreement with the theoretical predictions.
Application of such techniques to a wider range of clinical problems represents another exciting sphere of research in clinical gait analysis. It may well be that such techniques are limited to the fairly narrow range of interventions that are based on correction of the mechanisms for very specific aspects of walking but identifying the range of potential applications will be an important part of this process .
Brand RA: Can Biomechanics contribute to clinical orthopaedic assessments. Iowa Orthopaedic Journal 1987, 9: 61-64.
Google Scholar
Brand RA, Crowninshield RD: Comment on criteria for patient evaluation tools. Journal of Biomechanics 1981, 14: 655. 10.1016/0021-9290(81)90093-2
Article CAS PubMed Google Scholar
Cappozzo A: Considerations on clinical gait evaluation. Journal of Biomechanics 1983, 16: 302. 10.1016/0021-9290(83)90202-6
Cappozzo A, Della Croce U, Leardini A, Chiari L: Human movement analysis using stereophotogrammetry. Part 1: theoretical background. Gait and Posture 2005, 21: 186-196.
PubMed Google Scholar
Chiari L, Della Croce U, Leardini A, Cappozzo A: Human movement analysis using stereophotogrammetry. Part 2: instrumental errors. Gait and Posture 2005, 21: 197-211. 10.1016/j.gaitpost.2004.04.004
Article PubMed Google Scholar
Baker R, Rodda J: All you ever wanted to know about the conventional gait model but were afraid to ask. Melbourne, Women and Children's Health; 2003.
Davis RB, Ounpuu S, Tyburski D, Gage JR: A gait analysis data collection and reduction technique. Human Movement Science 1991, 10: 575-587. 10.1016/0167-9457(91)90046-Z
Article Google Scholar
Kadaba MP, Ramakrishnan HK, Wootten ME: Measurement of lower extremity kinematics during level walking. Journal of Orthopaedic Research 1990, 8: 383-391. 10.1002/jor.1100080310
Kadaba MP, Ramakrishnan HK, Wootten ME, Gainey J, Gorton G, Cochran GVB: Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. Journal of Orthopaedic Research 1989, 7: 849-860. 10.1002/jor.1100070611
Ounpuu S, Gage JR, Davis RB: Three-dimensional lower extremity joint kinetics in normal pediatric gait. Journal of Pediatric Orthopaedics 1991, 11: 341-349.
Ounpuu O, Davis RB, Deluca PA: Joint kinetics: Methods, interpretation and treatment decision-making in children with cerebral palsy and myelomeningocele. Gait and Posture 1996, 4: 62-78. 10.1016/0966-6362(95)01044-0
Noonan KJ, Halliday S, Browne R, O'Brien S, Kayes K, J F: Inter-observer variability of gait analysis in patients with cerebral palsy. Journal of Pediatric Orthopaedics 2003, 23: 279-287. 10.1097/00004694-200305000-00001
Gorton G, Hebert D, Goode B: Assessment of the kinematic variability between 12 Shriners motion analysis laboratories. Gait and Posture 2001, 13: 247.
Gorton G, Hebert D, Goode B: Assessment of kinematic variability between 12 Shriners motion analysis laboratories part 2: Short term follow up. Gait and Posture 2002, 16 (suppl 1): S65-66.
Schwartz MH, Trost JP, Wervey RA: Measurement and management of errors in quantitative gait data. Gait and Posture 2004, 20: 196-203. 10.1016/j.gaitpost.2003.09.011
Della Croce U, Leardini A, Chiari L, Cappozzo A: Human movement analysis using stereophotogrammetry. Part 4: assessment of anatomical landmark misplacement and its effects on joint kinematics. Gait and Posture 2005, 21: 226-237. 10.1016/j.gaitpost.2004.05.003
Bell AL, Brand RA, Pedersen DR: Prediction of hip joint center location from external landmark: ; Atlanta, Georgia. ; 1988:212.
Bell AL: A comparison of the accuracy of several hip centre location prediction methods. Journal of Biomechanics 1990, 23: 617-621. 10.1016/0021-9290(90)90054-7
Bell AL, Brand RA, Pedersen DR: Prediction of hip joint centre location from external landmarks. Human Movement Science 1989, 8: 3-16. 10.1016/0167-9457(89)90020-1
Leardini A, Cappozzo A, Catani F, Toksvig-Larsen S, Petitto A, Sforza V, Cassanelli G, Giannini S: Validation of a functional method for the estimation of hip joint centre location. Journal of Biomechanics 1999, 32: 99-103. 10.1016/S0021-9290(98)00148-1
Cappozzo A, Catani F, Della Croce U, Leardini A: Position and orientation in space of bones during movement: anatomoical frame definition and determination. Clinical Biomechanics 1995, 10: 171-178. 10.1016/0268-0033(95)91394-T
Piazza SJ, Okita N, Cavanagh PR: Accuracy of the functional method of hip joint center location: effects of limited motion and varied implementation. Journal of Biomechanics 2001, 34: 967-973. 10.1016/S0021-9290(01)00052-5
Hicks JL, Richards JG: Clinical applicability of using spherical fitting to find hip joint centers. Gait and Posture 2005, 22: 138-145. 10.1016/j.gaitpost.2004.08.004
Piazza SJ, Erdemir A, Okita N, Cavanagh PR: Assessment of the functional method of hip joint center location subject to reduced range of hip motion. Journal of Biomechanics 2004, 37: 349-356. 10.1016/S0021-9290(03)00288-4
Camomilla V, Cereatti A, Vannozzi G, Cappozzo A: An optimised protocol for hip joint centre determination using the functional method. Journal of Biomechanics 2005, In press, available on-line.
Charlton IW, Tate P, Smyth P, Roren L: Repeatability of an optimised lower body model. Gait and Posture 2004, 20: 213-221. 10.1016/j.gaitpost.2003.09.004
Schwartz MH, Rozumalski A: A new method for estimating joint parameters from motion data. Journal of Biomechanics 2005, 38: 107-116.
Reinbolt JA, Schutte JF, Fregly BJ, Koh BI, Haftka RT, George AD, Mitchell KH: Determination of patient-specific multi-joint kinematic models through two-level optimization. Journal of Biomechanics 2005, 38: 621-626. 10.1016/j.jbiomech.2004.03.031
Lamoreux LW: Errors in thigh axial rotation measurements using skin mounted markers. 1991, 372-373.
Lu TW, O'Connor JJ: Bone position estimation from skin marker co-ordinates using global optimisatoin with joint constraints. Journal of Biomechanics 1999, 32: 129-134. 10.1016/S0021-9290(98)00158-4
Alexander EJ, Andriacchi TP: Correcting for deformation in skin-based marker systems. Journal of Biomechanics 2001, 34: 355-361. 10.1016/S0021-9290(00)00192-5
Leardini A, Chiari L, Della Croce U, Cappozzo A: Human movement analysis using stereophotogrammetry. Part 3. Soft tissue artifact assessment and compensation. Gait and Posture 2005, 21: 212-225. 10.1016/j.gaitpost.2004.05.002
Asakawa DS, Pappas GP, Blemker SS, Drace JE, Delp SL: Cine phase-contrast magnetic resonance imaging as a tool for quantification of skeletal muscle motion. Seminars on Musculoskelet Radiology 2003, 7: 287-295. 10.1055/s-2004-815676
Rebmann AJ, Sheehan FT: Precise 3D skeletal kinematics using fast phase contrast magnetic resonance imaging. Journal of Magnetic Resonance Imaging 2003, 17: 206-213. 10.1002/jmri.10253
Barrance PJ, Williams GN, Novotny JE, Buchanan TS: A method for measurement of joint kinematics in vivo by registration of 3-D geometric models with cine phase contrast magnetic resonance imaging data. Journal of Biomechanical Engineering 2005, 127: 829-837. 10.1115/1.1992524
Banks S, Bellemans J, Nozaki H, Whiteside LA, Harman M, Hodge WA: Knee motions during maximum flexion in fixed and mobile-bearing arthroplasties. Clinical Orthopaedics and Related Research 2003, 131-138. 10.1097/01.blo.0000063121.39522.19
Banks SA, Fregly BJ, Boniforti F, Reinschmidt C, Romagnoli S: Comparing in vivo kinematics of unicondylar and bi-unicondylar knee replacements. Knee Surg Sports Traumatol Arthrosc 2005, 13: 551-556. 10.1007/s00167-004-0565-x
Banks SA, Hodge WA: Implant design affects knee arthroplasty kinematics during stair-stepping. Clinical Orthopaedics and Related Research 2004, 187-193. 10.1097/01.blo.0000138956.04316.ac
Banks SA, Hodge WA: 2003 Hap Paul Award Paper of the International Society for Technology in Arthroplasty. Design and activity dependence of kinematics in fixed and mobile-bearing knee arthroplasties. Journal of Arthroplasty 2004, 19: 809-816. 10.1016/j.arth.2004.04.011
Stagni R, Fantozzi S, Cappello A, Leardini A: Quantification of soft tissue artefact in motion analysis by combining 3D fluoroscopy and stereophotogrammetry: a study on two subjects. Clinical Biomechanics 2005, 20: 320-329. 10.1016/j.clinbiomech.2004.11.012
Fregly BJ, Rahman HA, Banks SA: Theoretical accuracy of model-based shape matching for measuring natural knee kinematics with single-plane fluoroscopy. Journal of Biomechanical Engineering 2005, 127: 692-699. 10.1115/1.1933949
Article PubMed Central PubMed Google Scholar
Li G, DeFrate LE, Park SE, Gill TJ, Rubash HE: In vivo articular cartilage contact kinematics of the knee: an investigation using dual-orthogonal fluoroscopy and magnetic resonance image-based computer models. American Journal of Sports Medicine 2005, 33: 102-107. 10.1177/0363546504265577
Li G, Wuerz TH, DeFrate LE: Feasibility of using orthogonal fluoroscopic images to measure in vivo joint kinematics. Journal of Biomechanical Engineering 2004, 126: 314-318. 10.1115/1.1691448
Rodda JM, Graham HK, Carson L, Galea MP, Wolfe R: Sagittal gait patterns in spastic diplegia. Journal of Bone and Joint Surgery 2004, 86: 251-258. 10.1302/0301-620X.86B2.13878
Winters TF, Gage JR, Hicks R: Gait patterns in spastic hemiplegia in children and young adults. Journal of Bone and Joint Surgery 1987, 69a: 437-441.
Hullin MG, Robb JE, Loudon IR: Gait patterns in children with hemiplegic spastic cerebral palsy. Journal of Pediatric Orthopaedics 1996, 5: 547-251.
Novacheck TF, Trost JP, Schwartz MH: Intramuscular psoas lengthening improves dynamic hip function in children with cerebral palsy. Journal of Pediatric Orthopaedics 2002, 22: 158-164. 10.1097/00004694-200203000-00004
Rose SA, DeLuca PA, Davis RBIII, Ounpuu S, Gage JR: Kinematic and kinetic evaluation of the ankle after lengthening of the gastrocnemuis fascia in children with cerebral palsy. Journal of Pediatric Orthopaedics 1993, 13: 727-732.
Ounpuu S, DeLuca P, Davis R, Romness M: Long-term effects of femoral derotation osteotomies: an evaluation using three-dimensional gait analysis. Journal of Pediatric Orthopaedics 2002, 22: 139-145. 10.1097/00004694-200203000-00001
Ounpuu S, Muik E, Davis RB, Gage JR, DeLuca PA: Rectus femoris surgery in children with cerebral palsy. Part II: A comparison between the effect of transfer and release of the distal rectus femoris on knee motion. Journal of Pediatric Orthopaedics 1993, 13: 331-335.
Ounpuu S, Muik E, Davis RB, Gage JR, DeLuca PA: Rectus femoris surgery in children with cerebral palsy. Part I: The effect of rectus femoris transfer location on knee motion. Journal of Pediatric Orthopaedics 1993, 13: 325-330.
Pirpiris M, Trivett A, Baker R, Rodda J, Nattrass GR, Graham HK: Femoral derotation osteotomy in spastic diplegia. Proximal or distal? Journal of Bone and Joint Surgery 2003, 85: 265-272. 10.1302/0301-620X.85B2.13342
Pirpiris M: Single event multi-level surgery in spastic diplegia: comprehensive outcome analysis. In Department of Paediatrics . , University of Melbourne; 2002.
DeLuca P, Ounpuu O, Davis RB, Walsh J: Effect of hamstrings and psoas lengthening on pelvic tilt in patients with spastic diplegic cerebral palsy. Journal of Pediatric Orthopaedics 1998, 18: 712-718. 10.1097/00004694-199811000-00004
CAS PubMed Google Scholar
Gage J, Perry J, Hicks R, Koop S, Wernt J: Rectus femoris transfer to improve knee function of children with cerebral palsy. Developmental Medicine and Child Neurology 1987, 29: 159-166.
Baker R: Pelvic angles: a mathematically rigorous definition which is consistent with a conventional clinical understanding of the terms. Gait and Posture 2001, 13: 1-6. 10.1016/S0966-6362(00)00083-7
Grood ES, Suntay WJ: A joint coordinate system for the clinical description of three-dimensional motions: Application to the knee. Transactions of the ASME, Journal of Biomechanical Engineering 1983, 105: 136-143.
Article CAS Google Scholar
Chao EYS: Justification of triaxial goniometer for the measurement of joint rotation. Journal of Biomechanics 1980, 13: 989-1006. 10.1016/0021-9290(80)90044-5
Wu G, van der Helm FC, Veeger HE, Makhsous M, Van Roy P, Anglin C, Nagels J, Karduna AR, McQuade K, Wang X, Werner FW, Buchholz B: ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. Journal of Biomechanics 2005, 38: 981-992. 10.1016/j.jbiomech.2004.05.042
Baker R: ISB recommendation on definition of joint coordinate systems for the reporting of human joint motion-part I: ankle, hip and spine. J Biomech 2003, 36: 300-2; author reply 303-4. 10.1016/S0021-9290(02)00336-6
Schache A, Baker R, Vaughan C: Differences in lower limb transverse plane joint moments during gait when expressed in two alternative reference frames. Journal of Biomechanics 2006., In press:
Pandy MG: Moment arm of a muscle force. Exercise and Sports Science Reviews 1999, 27: 79-118.
Saunders JBDM, Inman VT, Eberhart HD: The major determinants in normal and pathological gait. Journal of Bone and Joint Surgery 1953, 35A: 543-728.
Ortega J, Farley C: Minimising vertical excursion of centre of mass movement does not reduce metabolic cost in walking: ; Toledo, OH. ; 2003.
Gard SA, Childress DS: The effect of pelvic list on the vertical displacement of the trunk during normal walking. Gait and Posture 1997, 5: 233-238. 10.1016/S0966-6362(96)01089-2
Gard SA, Childress DS: The influence of stance-phase knee flexion on the vertical displacement of the trunk during normal walking. Archives of Physical Medicine and Rehabilitation 1999, 80: 26-32. 10.1016/S0003-9993(99)90303-9
Gard SA, Childress DS: What determins the vertical displacement of the body during normal walking? Journal of Prosthetics and Orthotics 2001, 13: 64-67. 10.1097/00008526-200109000-00009
Kerrigan DC, Riley PO, Lelas J, Della Croce U: Quantification of pelvic rotation as a determinant of gait. Archives of Physical Medicine and Rehabilitation 2001, 82: 217-220. 10.1053/apmr.2001.18063
Kerrigan DC, Della Croce U, Marciello M, Riley PO: A refined view of the determinants of gait: significance of heel rise. Archives of Physical Medicine and Rehabilitation 2000, 81: 1077-1080. 10.1053/apmr.2000.6306
Gage JR: Gait Analysis in Cerebral Palsy. Oxford, Mac Keith Press; 1991.
Gage JR: The treatment of gait problems in cerebral palsy. London, Mac Keith Press; 2004.
Perry J: Normal and pathological gait. In Atlas of orthotics . Edited by: Bunch WH. St Louis, CV Mosby; 1985:76-111.
Anderson FC, Pandy MG: Dynamic optimization of human walking. Journal of Biomechanical Engineering 2001, 123: 381-390. 10.1115/1.1392310
Anderson FC, Ziegler JM, Pandy MG, Whalen RT: Application of high-performance computing to numerical simulation of human movement. Journal of Biomechanical Engineering 1995, 117: 155-157. 10.1115/1.2792264
Zajac FE, Neptune RR, Kautz SA: Biomechanics and muscle contraction of human walking: Part I: Introduction to concepts, power transfer, dynamics and simulations. Gait and Posture 2002, 16: 215-232. 10.1016/S0966-6362(02)00068-1
Zajac FE, Neptune RR, Kautz SA: Biomechanics and muscle co-ordination of human walking: Part II: Lessons from dynamical simulations and clinical implications. Gait and Posture 2003, 17: 1-17. 10.1016/S0966-6362(02)00069-3
Neptune RR, Kautz SA, Zajac FE: Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. Journal of Biomechanics 2001, 34: 1387-1398. 10.1016/S0021-9290(01)00105-1
Kepple T, Siegel K, Stanhope S: Relative contributions of the lower extremity joint moments to forward progression and support during gait. Gait and Posture 1997, 6: 1-8. 10.1016/S0966-6362(96)01094-6
Anderson FC, Pandy MG: Static and dynamic optimization solutions for gait are practically equivalent. Journal of Biomechanics 2001, 34: 153-161. 10.1016/S0021-9290(00)00155-X
Anderson FC, Pandy MG: Individual muscle contributions to support in normal walking. Gait and Posture 2003, 17: 159-169. 10.1016/S0966-6362(02)00073-5
Arnold AS, Anderson FC, Pandy MG, Delp SL: Muscular contributions to hip and knee extension during the single limb stance phase of normal gait: a framework for investigating the causes of crouch gait. Journal of Biomechanics 2005, 38: 2181-2189. 10.1016/j.jbiomech.2004.09.036
Anderson FC, Goldberg SR, Pandy MG, Delp SL: Contributions of muscle forces and toe-off kinematics to peak knee flexion during the swing phase of normal gait: an induced position analysis. Journal of Biomechanics 2004, 37: 731-737. 10.1016/j.jbiomech.2003.09.018
Arnold AS, Anderson FC, Liu M, Goldstein S, Thelen D, Ounpuu S, Delp SL: Biomechanical efficacy of treatments for stiff-knee gait: a simulation-based case study: ; Portland, Oregon, USA. ; 2005.
Liu M, Arnold AS, Goldberg SR, Anderson FC, Thelen , Ounpuu S, Delp SL: Quadriceps force in stance limits knee flexion in swing: insight from a subject specific simulation of stiff-knee gait: ; Portland, Oregon, USA. ; 2005.
Baker RJ, Jasinski M, Maciag-Tymecka I, Michalowska-Mrozek J, Bonikwski M, Carr LJ, MacLean J, Lin JP, Lynch B, Theologis T, Wendorff J, Eunson P, Cosgrove A: Botulinum toxin treatment of spasticity in diplegic cerebral palsy: a randomized, double-blind, placebo-controlled, dose-ranging study. Developmental Medicine and Child Neurology 2002, 44: 666-675. 10.1017/S0012162201002730
Eames NWA, Baker R, Hill N, Graham HK, Taylor T, Cosgrove A: The effect of botulinum toxin A on gastrocnemius length: magnitude and duration of response. Developmental Medicine and Child Neurology 1999, 41: 226-232. 10.1017/S0012162299000493
Saraph V, Zwick E, Zwick G, Steinwender C, Steinwender G, Linhart W: Multilevel surgery in spastic diplegia: evaluation by physical examination and gait analysis in 25 children. Journal of Pediatric Orthopaedics 2002, 22: 150-157. 10.1097/00004694-200203000-00003
Saraph V, Zwick E, Auner C, Schneider F, Steinwender G, Linhart W: Gait improvement surgery in diplegic children: How long do improvements last? Journal of Pediatric Orthopaedics 2005, 25: 263-267. 10.1097/01.bpo.0000151053.16615.86
Download references
Author information
Authors and affiliations.
Hugh Williamson Gait Analysis Service, Royal Children's Hospital, Parkville, Victoria, Australia
Richard Baker
Gait CCRE, Murdoch Children's Research Institute, Parkville, Victoria, Australia
Department of Mechanical and Manufacturing Engineering, University of Melbourne, Parkville, Australia
Musculoskeletal Research Centre, La Trobe University, Bundoora, Victoria, Australia
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to Richard Baker .
Additional information
Competing interests.
The author has received research funding from Oxford Metrics Plc (Oxford, UK)
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and permissions
About this article
Cite this article.
Baker, R. Gait analysis methods in rehabilitation. J NeuroEngineering Rehabil 3 , 4 (2006). https://doi.org/10.1186/1743-0003-3-4
Download citation
Received : 29 April 2005
Accepted : 02 March 2006
Published : 02 March 2006
DOI : https://doi.org/10.1186/1743-0003-3-4
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cerebral Palsy
- Gait Analysis
- Normal Walking
- Joint Kinematic
- Joint Centre
Journal of NeuroEngineering and Rehabilitation
ISSN: 1743-0003
- Submission enquiries: [email protected]
- Reference Manager
- Simple TEXT file
People also looked at
Review article, present and future of gait assessment in clinical practice: towards the application of novel trends and technologies.

- 1 Mechanical Engineering Department, Khalifa University, Abu Dhabi, United Arab Emirates
- 2 School of Mechanical and Aerospace Engineering, Monash University, Clayton Campus, Melbourne, Australia
- 3 Weill Cornell Medicine, New York City, NY, United States
- 4 Biomedical Engineering Department, Khalifa University, Abu Dhabi, United Arab Emirates
- 5 Health Engineering Innovation Center, Khalifa University, Abu Dhabi, United Arab Emirates
Background: Despite being available for more than three decades, quantitative gait analysis remains largely associated with research institutions and not well leveraged in clinical settings. This is mostly due to the high cost/cumbersome equipment and complex protocols and data management/analysis associated with traditional gait labs, as well as the diverse training/experience and preference of clinical teams. Observational gait and qualitative scales continue to be predominantly used in clinics despite evidence of less efficacy of quantifying gait.
Research objective: This study provides a scoping review of the status of clinical gait assessment, including shedding light on common gait pathologies, clinical parameters, indices, and scales. We also highlight novel state-of-the-art gait characterization and analysis approaches and the integration of commercially available wearable tools and technology and AI-driven computational platforms.
Methods: A comprehensive literature search was conducted within PubMed, Web of Science, Medline, and ScienceDirect for all articles published until December 2021 using a set of keywords, including normal and pathological gait, gait parameters, gait assessment, gait analysis, wearable systems, inertial measurement units, accelerometer, gyroscope, magnetometer, insole sensors, electromyography sensors. Original articles that met the selection criteria were included.
Results and significance: Clinical gait analysis remains highly observational and is hence subjective and largely influenced by the observer's background and experience. Quantitative Instrumented gait analysis (IGA) has the capability of providing clinicians with accurate and reliable gait data for diagnosis and monitoring but is limited in clinical applicability mainly due to logistics. Rapidly emerging smart wearable technology, multi-modality, and sensor fusion approaches, as well as AI-driven computational platforms are increasingly commanding greater attention in gait assessment. These tools promise a paradigm shift in the quantification of gait in the clinic and beyond. On the other hand, standardization of clinical protocols and ensuring their feasibility to map the complex features of human gait and represent them meaningfully remain critical challenges.
1. Introduction
Changes in the signature of gait, or the unique sequential walking pattern in humans, reveal key information about the status and progression of numerous underlying health challenges, from neurological and musculoskeletal conditions to cardiovascular and metabolic disease, and to ageing-associated ambulatory dysfunction and trauma. Accurate reliable identification of gait patterns and characteristics in clinical settings, as well as monitoring and evaluating them over time, enable effective tailored treatment, inform predictive outcome assessment, and an allow for an overall better practice of precision medicine.
In clinical gait assessment, both a person's “ability” to walk and “how” the individual walks are highly relevant. The walking ability of a person is typically based on two main aspects: how far can an individual walk and what is his/her tolerance level ( 1 ). For example, for post stroke gait assessment, 3-, 6-, or 10 min walk tests are used, in addition to Functional Ambulation Category (FAC), Short Physical Performance Battery (SPPB), and/or Motor Assessment Scale (MAS). Other clinical subjective assessment scales include the Unified Parkinson Disease Rating Scale (UPDRS) the Scale for the Rating and Assessment of Ataxia (SARA)], the Alzheimer's Disease Assessment Scale (ADAS), the Expanded Disability Status Scale (EDSS) the High-level MobilitARTIy Assessment Tool (HiMAT), and the Dynamic Gait Index ( 2 ). The quality of gait or “how” the person walks, on the other hand, highly depends on the quantification of gait patterns and accurate identification of specific gait characteristics. Despite evidence of the advantages of quantitative instrumented gait analysis (IGA) in clinical practice and recommendations by the National Institute for Health and Clinical Excellence (NICE) ( 3 ) identifying IGA is the preferrable choice for “gait-improving surgery”, it remains not well leveraged in clinical settings due to the high cost/cumbersome equipment and complex protocols/data analysis associated with traditional gait labs, as well as diverse training, experience and preference of clinical teams ( 3 – 5 ). Moreover, the use of IGA by allied health professionals, such as physiotherapists, occupational therapists and orthotists, and training also remain non standardized and limited ( 5 – 7 ).
Observational gait analysis continues to be popular among clinicians due to its inherent simplicity, availability, and low cost ( 8 ). On the other hand, the validity, reliability, specificity, and responsiveness ( 9 , 10 ) of these qualitative methods are controversial and increasingly being questioned ( 6 ). Furthermore, there is evidence to suggest that subjective clinical assessment scales may not be sensitive to disease severity and specific characteristics and may limit understanding of underlying disease mechanisms, hence adversely impacting optimal treatment ( 11 ). Examples of such scales include Multiple Sclerosis (MS), where subjective measures, such as the Expanded Disability Status Scale (EDSS), the Multiple Sclerosis Severity Scale (MSSS), Multiple Sclerosis Walking Scale (MSWS), and Multiple Sclerosis Functional Composite (MSFC), continue to be widely used in clinical practice. These scales have been criticized for lack of sensitivity ( 12 ), high interrater variability ( 13 ), as well as being prone to practice effects and variability ( 14 , 15 ). Similarly, clinical assessment of Parkinson's disease (PD) using the Unified Parkinson's Disease Rating Scale (UPDRS) is subjective and largely dependent on the expertise and experience of the clinicians, as well as the severity of the disease ( 16 ). In Stroke patients, assessment tests such as Functional Ambulation Category (FAC), Short Physical Performance Battery (SPPB), and/or Motor Assessment Scale (MAS) are typically employed, along with qualitative observational/visual gait analysis (using naked eye or video images). Nevertheless, the validity, reliability, specificity, and responsiveness of these qualitative methods are questioned ( 9 ), and although they may be useful for the rudimentary evaluation of some gait parameters, they are not adequate for analyzing the multifaceted aspects of gait variability and complexity ( 17 ).
Instrumented gait analysis (IGA), which can provide accurate and precise quantitative measurement of gait patterns and characteristics, has long been the gold standard for gait assessment in research practice ( 18 ). IGA generally refers to the use of instrumentation to capture and analyze a variety of human gait parameters (spatiotemporal, kinematic, and kinetic measures). Traditional IGA systems include motion capture systems, and force plates, instrumented walkways, and treadmills, while more recent systems comprise of miniaturized wearable sensing system, computational platforms and modalities ( 18 ). Literature on the clinical applicability and efficacy of IGA indicates that IGA-based quantitative assessment can improve the diagnosis, outcome prediction, and rehabilitation of various gait impairments as compared to conventional observational scales and techniques for gait dysfunction in a wide spectrum of diseases including MS, PD, Stroke, and Cerebral Palsy ( 9 – 13 ). A recent review on the clinical efficacy of IGA confirms that there is strong evidence that 3-D gait analysis, or 3DGA; has the potential to alter and reinforce treatment decisions; increases confidence in treatment planning and agreement among clinicians; can better identify diagnostic groups and expected treatment outcomes; and overall can improve patient outcomes if recommendations are followed ( 19 ).
Emerging at an unprecedented rate, wearable sensing systems and associated computational modalities are rapidly transforming the quality and accessibility of healthcare, spanning multiple applications from neurology and orthopedics to cardiovascular, metabolic, and mental health. Magnetic (e.g., magnetometers), inertial measurement (e.g., accelerometers and gyroscopes), and force sensors (e.g., insole foot pressure) nowadays offer unprecedented data capture opportunities that can overcome limitations of non-wearable devices due to their low-cost, less setup-time and complexity, lightweight, and portability, making them ideal for out-of-lab and continuous monitoring in the clinic and beyond ( 20 ). Magneto-inertial measurement units (MIMUs), in conjunction with force pressure sensors, have the capability of capturing spatiotemporal, kinematic, and kinetic gait data ( 2 ) rendering the concept of a mobile gait lab a reality. Such labs can inherently overcome the limitations of IGA traditional labs, providing less costly and cumbersome tools with potential for gait assessment in natural environments (clinics, homes, sports arenas, etc.), user friendly interfaces, and the opportunity to provide continuous real-time feedback to clinicians and patients, as well as tele rehabilitation capabilities. In addition, wearable systems allow for easy synchronization with other physiological measurement systems, including EMG, ECG, and EEG, towards the acquisition of invaluable multimodal continuous physiological data in various settings.
This scoping review aims to provide a summary of the current state of clinical gait assessment, including shedding light on gait pathologies and clinical indices and scales, as well as a roadmap for the development of future gait mobile labs- highlighting the clinical validity and reliability of the latest devices and data interpretation algorithms. The word novel in the title of this review reflects recent emergence/implementation of the technologies reviewed and/or recent commercialization. This includes wearable technologies, as well as AI-driven computational platforms. The remainder of the review is structured as follows: Section 2 describes the adopted methodology, including the approach, search strategy and selection criteria. Section 3 details clinical gait pathologies, relevant parameters, as well as current clinical gait assessment tools, scales, and indices, while Section 4 presents gait assessment technologies applicable to clinical settings, including state-of-the-art imaging techniques and wearable technologies, algorithms, and novel AI-driven computational platforms. Section 5 deliberates on the concept of a mobile gait lab for clinical applications. Section 6 highlights the limitations, while Section 7 presents the conclusive remarks and future work.
This review is aimed at summarizing various clinical gait pathologies and associated parameters, applicable gait analysis techniques and gait indices, and the latest trends in wearables systems and algorithms. To address this broader research objective, the authors adopted a scoping review approach rather than a systematic review approach. As reported in ( 21 ), scoping reviews are ideal for addressing a broader scope with a more expansive inclusion criterion.
2.1. Search criteria
A keyword search was performed in PubMed, Web of Science, Medline, and ScienceDirect databases, using a combination of search terms from the following groups: 1. (normal gait OR pathological gait OR gait parameters OR gait indices), 2. (gait assessment OR gait analysis), 3. (wearable systems OR wearable algorithms), 4. (inertial measurement units OR accelerometer OR gyroscope OR magnetometer OR insole sensors OR electromyography sensors). No limit for the year of publication was set, however, the search was last updated in December 2021. Only articles written in the English language were considered in this review. In addition, the reference list of the included articles was checked to identify additional relevant publications meeting the inclusion criteria. The literature search and data extraction were carried out independently by two authors (AAH, DMM) and any inconsistencies and disagreements discrepancies were resolved through following discussions with the other authors (NA, MER, KK).
This scoping review included original published works and review articles which met the following inclusion/exclusion criteria: (i) studies addressing various gait disorders and associated gait parameters, (ii) studies focusing on instrumented gait analysis techniques and gait indices, (iii) studies evaluating the use, validity, and reliability of wearable-based gait measurement devices/systems for measuring gait events, and evaluating and assessing gait dysfunction, (iv) studies concerning the applicability of sensor fusion techniques and algorithms applicable for wearable-based systems with application to gait analysis. The title and/or abstract of the studies were initially screened for suitability. The full-text articles meeting the inclusion criteria were obtained for data extraction and synthesis. A flowchart explaining the same is shown in Figure 1 .
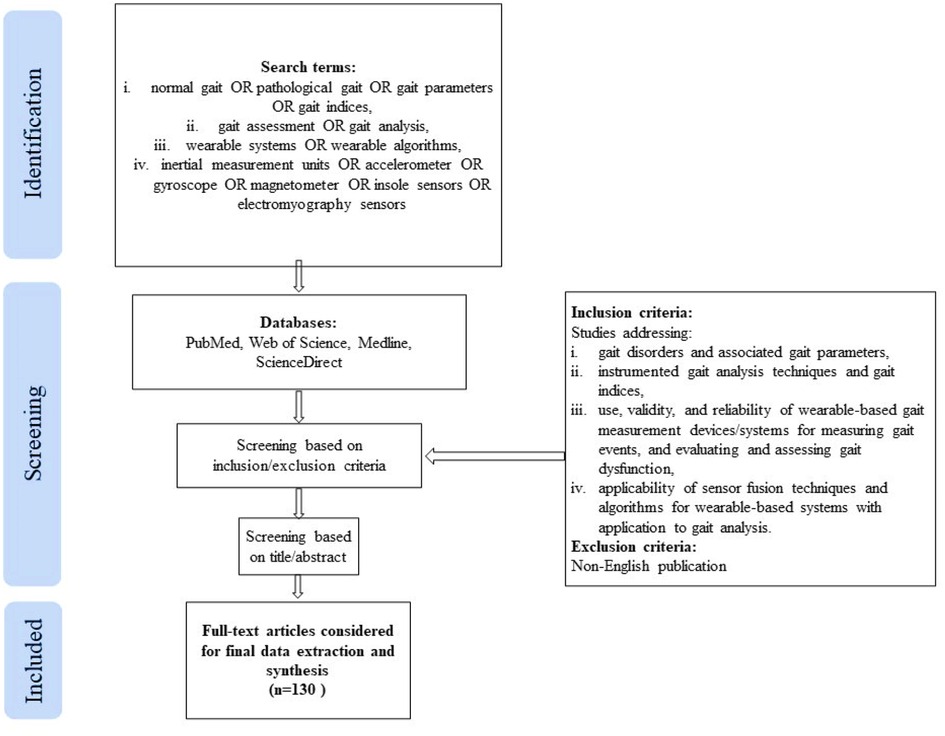
Figure 1 . Publication selection process.
3. Clinical gait pathologies and parameters
3.1. normal gait cycle and parameters.
Normal gait can be defined as a series of rhythmic, systematic, and coordinated movements of the limbs and trunk that results in the forward advancement of the body's center of mass ( 22 ). A result of intricate dynamic interactions between the central nervous system and feedback mechanisms ( 23 ), walking is characterized by individual gait cycles and functional phases ( Figure 2 ). A gait cycle consists of two main phases, stance, and swing, which are further divided into five and three functional phases, respectively. The stance phase corresponds to the duration between heel strike and toe-off of the same foot, constituting approximately 60% of the gait cycle. The swing phase begins with toe-off and ends with heel contact of the same foot and occupies 40% of the cycle. As each functional phase contributes to successfully accomplishing the goal of walking, healthy gait involves cyclic and complementary movements of the limbs under control. It is characterized by stance stability; toe clearance during the swing; pre-positioning at swing; sufficient step length; as well as mechanical and metabolic efficiency ( 24 ). Table 1 provides gait parameter ranges based on studies on healthy adults. Determining an appropriate normal range for many of the features is highly challenging as individuals exhibit a wide range of gait patterns across different age groups and gender ( 17 ).
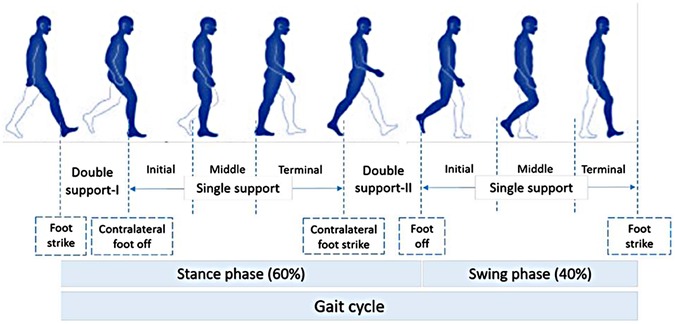
Figure 2 . Normal gait cycle (adapted from 1 ).

Table 1 . Gait parameters for healthy individuals ( 1 ).
3.2. Gait parameters associated with pathology
Gait disorders are typically associated with deficits in the brain, spinal cord, peripheral nerves, muscles, joints, or bones. Some medical conditions leading to pathological gait include but not limited to muscular dystrophy, myelodysplasia, cerebral palsy, arthritis, osteoarthritis, head injury, lower limb amputation, multiple sclerosis, rheumatoid, spinal cord injury, parkinsonism, and stroke ( 25 ).
In neuromuscular conditions, the loss of central control affects the motion. In general, patients walk slower than healthy individuals and with compromised spatiotemporal, kinematic, and kinetic parameters. In older adults, a walking speed decline of 0.7% per year is observed, along with significant changes in cadence and step length. The aging population also exhibits lower knee extension at heel-strike and knee flexion during the swing phase ( 23 , 26 ). The following subsections describe some of the most common gait disorders and associated pathological parameters. The associated impacted parameters are summarized in Table 2 .

Table 2 . Effect of pathology on gait disorders.
3.2.1. Neurological gait disorders in elderly people
Gait ailments associated with aging lead to reduction in the quality of life and increased morbidity and mortality. Elderly patients exhibit complex gait disorders, and their dual task ability deteriorates due to a decline in their central resources ( 23 , 26 ).
Specific gait dysfunction noted in the elderly population are summarized as follows:
3.2.1.1. Hypokinetic-rigid gait disorders
Shuffling with a reduced step height and stride length characterizes hypokinetic gait disorder ( 27 ). Reduced arm swing with slow turning movements is also present in isolation. Festination, when patients use rapid small steps to maintain the feet beneath the forward moving trunk, is also observed. Ataxic elements include broad stance width and an increased variability in timing and amplitude of steps ( 27 ). Gait associated with underlying diseases, such as Parkinson's disease, cerebrovascular disease, and ventricular widening, is classified within hypokinetic-rigid gait disorders ( 27 , 28 ).
3.2.1.2. Cautious and careless gait
Defined as gait during which people move slowly with a wider base, and shorter stride, with minimal trunk movement, while the knees and elbows are bent. Whereas careless gait is when patients appear overly confident and walk insensitively fast. Careless gait is due to confusion and delirium associated with old age ( 27 ).
3.2.1.3. Dyskinetic gait or involuntary movements
Patients with post-anoxic encephalopathy exhibit bouncing gait and stance. This is also observed in patients with Parkinson's disease-causing excessive trunk movements contributing to falls. Several dystonic patients are reported to walk on their toes ( 27 , 28 ).
3.21.1.4. Psychogenic gait disorders
Gait dysfunction is common in elderly people due to adverse effects of drugs leading to extrapyramidal side-effects, sedation, orthostatic hypotension, behavioral abnormalities, or ataxia ( 27 , 28 ).
3.2.1.5. Fluctuating or episodic gait disorders
Elderly people often exhibit fluctuating or episodic gait disorder after exercise due to fatigue, and it might be an indication of underlying vascular or neurogenic limping. Freezing gait is part of hypokinetic-rigid syndrome ( 27 , 28 ).
3.2.2. Gait disorders in Parkinson's disease
PD is a neurological disorder which leads to cognition, where gait impairment deteriorates with disease progression, increasing reliance on cognition to control gait. Due to cognitive impairment with PD, the ability to compensate for gait disorders diminishes, leading to further gait impairment. PD is characterized by deficit in amplitude and gait speed, along with increased gait variability ( 29 ).
3.2.3. Gait in diabetic peripheral neuropathy
Neuropathy of motor, sensory, and autonomic components of the nervous system are one of the many complications of Type II Diabetes (T2D). An intact central and peripheral nervous system are essential to initiate and control healthy gait, along with sufficient muscle strength, bone, and joint movements in complete range for normal locomotion. Patients diagnosed with T2D take extra steps when walking in straight paths and during turns, along with an overall reduction in walking speed, step length, cadence, and fewer acceleration patterns as compared to age-matched healthy controls. Joint range of motion is also altered in T2D, where patients with diabetic peripheral neuropathy exhibit a reduced range of motion at the ankle joint in dorsi and plantar flexion and a reduced flexion and extension range of motion at the knee joint in both, as compared to non-diabetic people ( 24 ).
3.2.4. Post stroke gait
Hemiplegia after stroke contributes to significant reduction in gait performance. In stroke survivors, function of the cerebral cortex is usually impaired, whilst that of spinal cord is preserved. Dysfunction is typically demonstrated by a marked asymmetrical deficit. Decreased walking speed and cadence, in addition to longer gait cycle and double limb support as compared to healthy individuals. For hemiplegic stroke survivors, a reduced peak extension of the hip joint in late stance, varying peak lateral pelvis displacement, knee flexion and decreased plantarflexion of ankle at toe off are reported. The GRF (Ground Reaction Force) pattern is characterized as asymmetric, along with decreased amplitude of joint moments, at the lower limb joints on the paretic side ( 30 ).
3.2.5. Total hip arthroplasty (THA)
Large deficits in gait speed ( 31 ), stride length ( 32 , 33 ), sagittal hip range of motion ( 32 , 33 ), hip abduction moment-coronal plane ( 31 ), and negligible changes in transverse plane hip range of motion ( 31 ), deficiency in single limb support time ( 31 ), are reported in patients post THA as compared to healthy controls. Peak hip extension is typically reduced, whereas peak hip flexion remains similar as compared to controls. In addition, peak hip abduction moment is reduced along with peak hip external rotation moment ( 34 ).
3.3. Clinical gait assessment measures and indices
The use of observational gait analysis and subjective rating sales continues to be widespread in clinical settings, both as a diagnostic tool and as a prognostic measure, as previously mentioned. Although these techniques can be useful for the initial rudimentary evaluation of some gait parameters, the validity, reliability, specificity, and responsiveness of these qualitative methods are highly questionable. Researchers have therefore proposed various pathology-specific gait indices and summary measures ( 35 ) based on commercially available technologies with accepted levels of accuracy Table 3 . summarizes the current clinical gait summary measures, discrete and continuous gait indices, and non-linear approaches reported in literature, along with advantages and disadvantages.

Table 3 . Clinical gait measures and indices ( 123 , 124 ).
4. Gait assessment technologies applicable to clinical settings
In the past couple of decades, remarkable technological advancement has been witnessed in the field of gait assessment and analysis, particularly in gait assessment technology. Instrumented walkways, both portable and non-portable, became a good alternative to complicated, bulky and non-portable traditional gait labs. These systems (for example the Walkway and StrideWay from Tekscan Inc., Boston, United States) are now widely used in research and to a limited extent in clinical practice. They typically include low-profile floor walkway systems equipped with grids of embedded sensors below the surface, which record foot-strike patterns as a function of time and space as an individual walks across the platform, and dedicated software which computes the various spatiotemporal gait measures Although these instrumented mats involve less setup time and are generally simple to operate as compared to traditional IGA labs, they are expensive, restrictive to specific operational environment to over-ground trials ( 36 , 37 ).
Marker-based optical motion capture (Mocap) is another rapidly emerging technology effective for obtaining 3D kinematic movement data. Passive Mocap systems [e.g., Vicon (Vicon Motion Systems Ltd, Oxford, United Kingdom) and ELITE optoelectronic system (BTS S. p .A., Milano, Italy)], include retro-reflective markers (that reflect the light emitted by high-resolution infrared cameras) attached to specific anatomic landmarks. The location of the marker is identified by decoding the camera images. Here, the markers must be calibrated for identification before the recording session commences. Active Mocap systems (e.g., Optotrak motion capture system; Northern Digital Inc., Waterloo, Canada), on the other hand, use light-emitting diode (LED) markers (reflect their own light powered by a battery), which are automatically identified ( 38 , 39 ). In the context of clinical relevance, although such systems yield extremely accurate reliable data, operational factors including infrastructure, non-portability, high cost, additional time required for initial set-up and calibration, operational complexity, and restrictions to indoor setup impose hurdles to their functional deployment in clinics and rehabilitation centers ( 84 ). Recently, more portable cost-effective alternatives, such as Microsoft Kinect (based on a depth sensor-based markerless motion capture solution) became the application of choice ( 40 ).
Optoelectronic systems (e.g., Optogait®, Microgate, Italy) have also been used to capture spatiotemporal gait parameters. These mainly consist of a transmitting and a receiving bar containing an infrared light. Interruptions of the communication between the emitter and receiver are detected by the system to calculate the various gait parameters ( 41 ).
An evolution in the measurement of gait kinetic parameters can also be witnessed in the last two decades. These parameters include ground reaction forces, and intersegmental joint reaction forces, moments, and powers. Instrumented walkways offer dynamic plantar pressure mapping but are expensive and do not provide joint kinetic data. Force plates are also used in various gait analysis studies ( 38 , 39 , 42 ). These are able to provide intersegmental joint reaction forces by using the ground reaction forces measured along with inverse dynamics models (Winters book) Chen et al. ( 93 ) developed a novel remote sensing technology called “Electrostatic Field Sensing (EFS)” for measuring human gait including stepping, walking, and running, and further extended the work to post-stroke gait. This technology is credited with several advantages, such as being non-contact, affordable, and allows long-time monitoring ( 43 ). Shoe insole systems represent another category of gait quantification tools and techniques. These systems are designed to allow for the recording of both dynamic plantar pressure and spatiotemporal data. F-scan (Tekscan Inc., Boston, United States) is an ultra-thin in-shoe pressure measurement system utilizing Force-Sensitive Resistive films (FSR) technology ( 44 ).
The characteristics of different measurement systems applicable to clinical settings are summarized in Table 4 , and the pros and cons of these systems are listed in Table 5 .
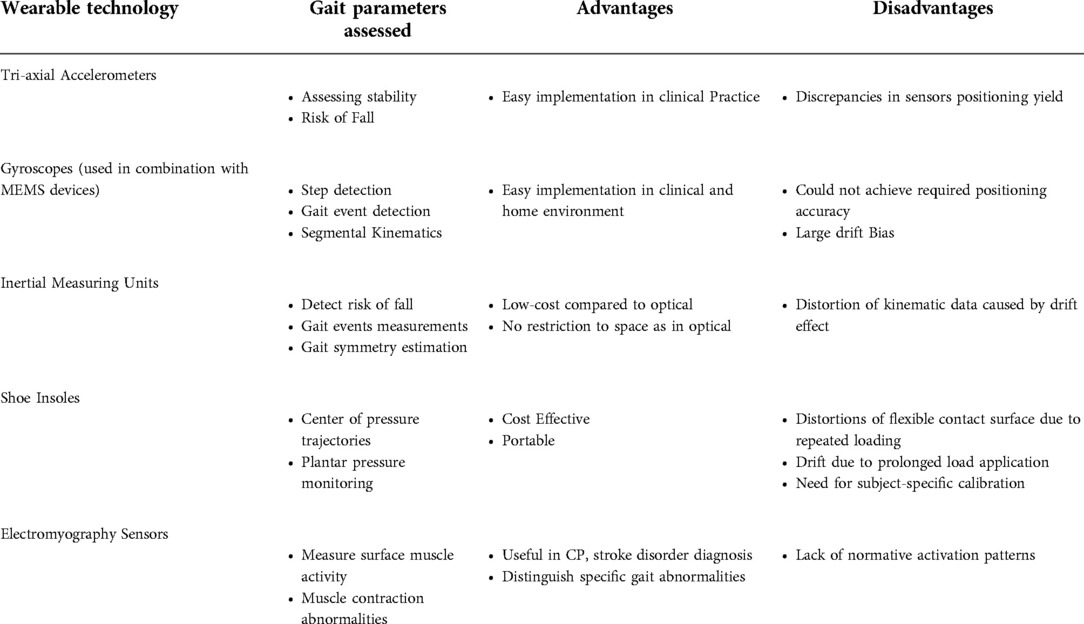
Table 4 . Portable wearable gait assessment tools.
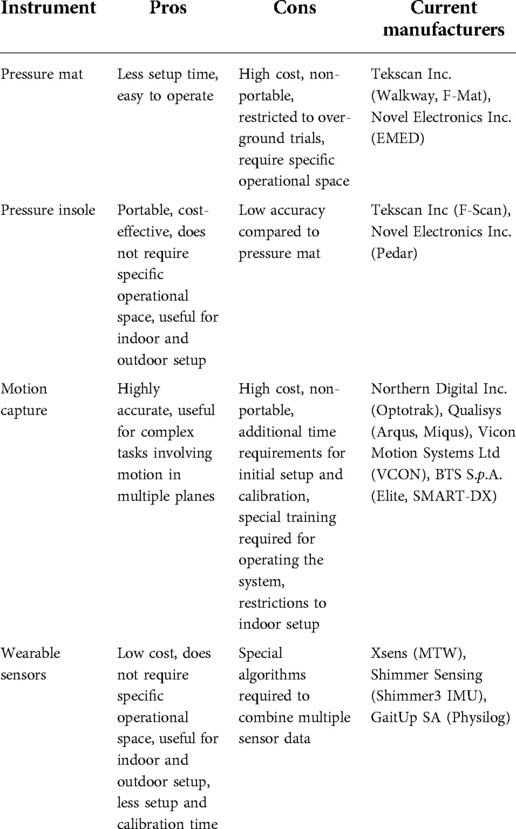
Table 5 . Pros and cons of different IGA systems.
Computational pipeline using computer vision techniques has been proposed as an ecological and precise method to quantify gait in children with neurodevelopmental disorders, along with the pose estimation software to obtain whole-body gait synchrony and balance ( 45 ). Speed, arm swing, postural control, and smoothness (or roughness) of movement features of gait for Parkinson's patients were extracted using videos processed by ordinal random forest classification model. Significant correlation between clinician labels and model estimates was reported, which provides gait impairment severity assessment in Parkinson's disease using single patient video, thereby reducing the need for sophisticated gait equipment ( 46 ). Computer vision-based gait assessment tools promise frequent gait monitoring using minimal resources ( 46 ). Deep learning to detect human subject in 2D images and then combining 3D sensing data to measure gait features has proven to be more robust than depth cameras in gait parameter acquisition ( 47 ).
4.1. Imaging techniques for gait assessment
As previously mentioned, marker-based optoelectronic systems are currently the most widely used systems in IGA among both research and clinical communities. On the other hand, one of the main sources of error inherent to these systems is the degree of movement of the skin, muscle, and other soft tissues, or the so- called soft tissue artifacts (STA), under the markers in relation to bony landmarks, hence violating the rigid body assumption underlying these methods ( 48 , 49 ). Moreover, STA varies by marker location in a unique and unpredictable manner, particularly during dynamic activities, which can make it unreliable for clinical applications ( 50 ).
Although not yet widespread in biomechanics, computer vision based markerless gait assessment methods offer a promising tool for gait assessment in research, as well clinical and sports biomechanics applications. By leveraging modern technologies, such as improved solvers, advanced image features and modern machine learning, markerless vision-based systems can reduce the required number of cameras, incorporating moving cameras, increasing the number of tracked individuals, and offering robust detection and fitting in diverse environments. On the other hand, issues such as accuracy and field-based feasibility remain to be addressed ( 51 ).
Three-dimensional imaging techniques have been successfully used to directly determine bone movements during walking as a gold reference standard to validate/improve current motion capture techniques ( 54 ). For example, researchers have resorted to quantifying STA by comparing with reference 3D kinematics of bone reconstructed from fluoroscopy-based tracking ( 53 ). Fluoroscopy has also emerged as a means for tracking position and orientation of underlying skeletal anatomy of the foot/ankle ( 54 ). Although single plane fluoroscopy yielded large errors when used to evaluate the accuracy of multi-segment foot models ( 49 ), dual fluoroscopy (DF) was found reliable and is considered as the current reference standard to compare joint angles ( 55 ). Combined with 2D/3D registration, video-fluoroscopy allows for accurate quantification of 3D joint motion free of STA ( 56 ). High-speed dual fluoroscopy (DF) has been reported to measure in-vivo bone motion of the foot and ankle with sub-millimeter and sub-degree errors ( 57 ). DF has also been used to evaluate multi-segment foot models and reported good agreement between DF and skin-marker data for the first metatarsal and sagittal plane measurements of the longitudinal arch ( 48 ).
Various researchers investigated the use of DF for clinical applications . In-vivo dual fluoroscopy was used to quantify the hip joint kinematics of patients with Femoroactabular impingement syndrome (FAIS) relative to asymptomatic, morphologically normal control participants during standing, level walking, incline walking and an unweighted functional activity. The kinematic position of the hip joint was obtained by registering projections of 3D computed Tomography models with DF images ( 58 ). Knee kinematic profiles were also obtained using 3D video-fluoroscopy and compared to actual and nominal flexion-extension, internal-external rotations, and antero-posterior translations profiles with optical mocap during stair climbing ( 59 ). Joint function for total talonavicular replacement after a complex articular fracture was evaluated using a full body gait analysis and 3D joint kinematics based on single-plane fluoroscopy ( 60 ). The 3D video fluoroscopic analysis was performed to assess joint motion of the replaced ankle ( 60 ). DF and CT imaging techniques were both employed to calculate in-vivo hip kinematics, along with model-based tracking, to compare the effect of different coordinate systems ( 61 ). Since marker-based systems are unable to accurately analyze talocrural or subtalar motion because the talus lacks palpable landmarks to place external markers ( 54 ), digitized video fluoroscopy was reportedly used to determine the sagittal plane motion of the medial longitudinal arch during dynamic gait ( 62 ). Characteristics of knee joint motion were also analyzed in 6DOF during treadmill walking using a dual fluoroscopy imaging system at different speeds ( 63 ).
DF uses anatomical landmarks visible on 3D CT reconstructions which substantially reduces errors due to STA ( 58 ). Computed tomography (CT) scans of participants are usually needed in DF to determine bone position from the DF images. Single plane fluoroscopy is restricted to 2D motion capture, while using a second FS allows for a full 3D analysis although a single gantry system has lower radiation than the biplane system with reported ionizing radiation levels of 10 µSv per trial ( 54 ). Stationary image intensifiers and static systems have a restricted field of view limiting their application to highly restricted movements ( 56 ). Moving fluoroscopes, consisting of a fluoroscopic unit mounted on a moving trolley which moves with the subject and is controlled by wire sensors to ensure that it remains in the field of view of the image intensifier ( 56 ), provide an enhanced field of view ideal for dynamic scenarios and moving joints.
Fluoroscopic systems designed for precise capture of bone movement and joint kinematics, unlike optical or inertial systems, are not yet commercially available, generally requiring in-house instrumentation and further performance evaluation. The evaluation would typically include determining the resolution of the hardware imaging chain, assessing how the hardware and software reduce or eliminate various distortions, and measuring static and dynamic accuracies and precisions based on precisely known motions and positions ( 64 ). Image quality is a major determinant of error in fluoroscopic applications ( 62 ). Pulse imaging of fluoroscopes, such as pulse width, limits image quality at a given frame rate. Increasing the pulse rate, which is function of pulse width, may add to radiation exposure, leading to an important tradeoff consideration between image quality and radiation exposure ( 63 ). Moving video-fluoroscopes reported lower gait velocity, step length, and cadence as compared to control conditions, indicating altered time distance parameters towards those of slow walking ( 56 ). So far, dynamic MRI used to define in- vivo talocrural and subtalar kinematics ( 65 ) does not allow data collection during normal gait.
Continued multidisciplinary collaborative efforts among biomechanists, imaging and computer vision experts, and clinicians are essential for fully leveraging these highly promising techniques in clinical applications.
4.2. Portable wearable systems for gait assessment
Wearable technology – the use of body-worn sensors to measure the characteristics of human locomotion, has recently emerged as an efficient, convenient, and most importantly, inexpensive option to quantitative gait analysis for both clinical and research-based applications ( Figure 3 ). In general, it uses individual sensor elements, such as accelerometers, gyroscopes, magneto resistive sensors, force/pressure sensors, goniometers, inclinometers, and electromyographic (EMG) sensors, or combined as an inertial measurement unit (IMU) ( 66 ). In comparison to conventional counterparts (e.g., walkway and camera based Mocap), wearable sensing enables continuous gait monitoring (> 2 h) outside the lab or clinic, allowing for replication of natural patterns of walking. Moreover, gait patterns over an ample distance could be measured as opposed to limited walking distance in a lab-based setting.
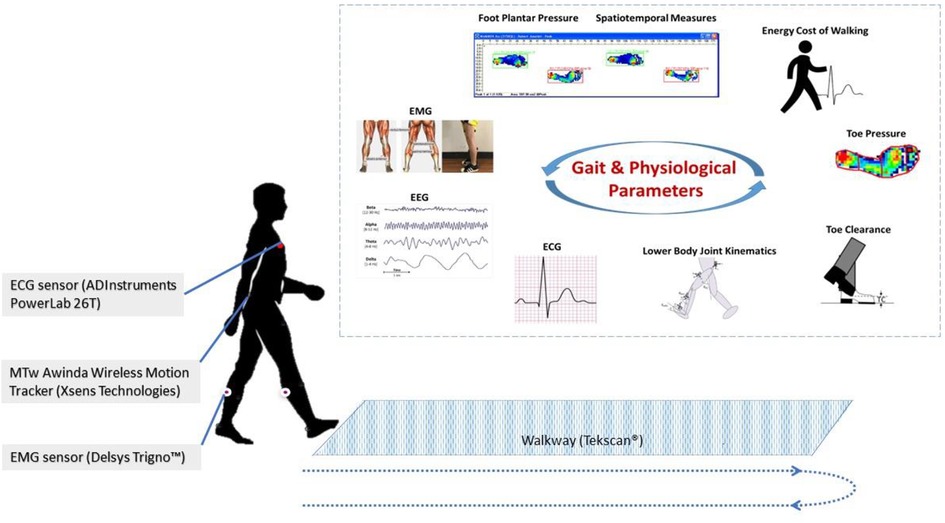
Figure 3 . Wearable gait lab
Accelerometers are often used in gait analysis for assessing stability and risk of fall. In a study which used a single tri-axial accelerometer mounted on the sacrum to analyze the risk of fall among 80 participants, accelerometry-based techniques were found to be able to detect subjects with increased risk of fall by employing appropriate machine learning techniques ( 66 ). In ( 67 ), a 3D accelerometer attached to the lower back was used for stability assessment of older adults. The applicability of a single accelerometer, worn on the back was further examined in ( 68 ), which highlights promising results for implementation in routine clinical practices. Considerable work has also been carried out to assess the consistency of gait characteristics obtained from accelerometers, where discrepancies in sensors positioning yield to critical errors ( 69 ). Furthermore, in ( 70 ), the authors have provided a comprehensive review on the use of accelerometry-based gait analysis techniques and their application to clinical settings.
Gyroscopes are also increasingly employed for gait studies. These devices measure angular velocity and are often combined with accelerometers and other micro-electromechanical systems (MEMS) devices to enhance performance through sensor fusion techniques. They have found applications in step detection, gait event detection, segmental kinematics, and more. For instance, a single gyroscope placed in the instep of the foot was successfully used to detect gait events, including heel strike, foot flat, heel off, and toe-off ( 71 ). Another study involved two gyroscopes, mounted on the lower left and right side of the waist to calculate walking steps and step length ( 72 ).
Magnetometers measure the magnetic field direction and intensity at a specific point. In combination with other inertial sensors (accelerometers and gyroscopes), they form a so-called inertial measurement unit (IMU), which can produce a drift-free estimation of gait parameters ( 73 ). Sophisticated commercialized IMUs (Physiolog 5 IMU, Gait Up, Switzerland, MTw Awinda, Xsens Technologies B.V., Netherlands), as well as in-house developed systems, were equally used for gait studies ( 74 ). In the context of human motion analysis, IMUs are employed for several possible goals, for example, to estimate the joint angles ( 74 ), to detect the risk of fall in an elderly population, long term monitoring of activities and symptoms ( 75 ), measurement of gait events, spatiotemporal parameters ( 76 , 77 , 78 ), ground reaction forces and moments ( 79 ), and estimation of gait symmetry ( 80 ). Mariani et al. (2010) used IMUs to measure foot kinematics in a study involving both young and elderly and reported the suitability of the system to clinical practice ( 81 ). Parisi et al. developed a low-cost system with a single IMU attached to the lower trunk to examine the gait characteristics of both hemiparetic and normal control subjects through measurement of spatiotemporal parameters, which showed excellent correlation with the parameters obtained from a standard reference system ( 78 ).
Insole systems for gait measurement and analysis represent a major category, which is cost-effective, portable, and applicable for both indoor and outdoor settings. Over the years, various technologies were developed ( 82 ), tested, and commercialized. These include capacitive sensors (Pedar system, Novel GmbH, Germany) ( 83 ), force-sensing resistors (FSR) (F-Scan, Tekscan Inc., United States) ( 84 ), and piezoresistive sensors (FlexiForce system, Tekscan, United States and ParoTec system, Paromed, Germany) ( 82 ). Researchers have adopted different approaches about the design, fabrication, and applications of insole systems. Both prefabricated and in-house fabricated insole systems have been tested for healthy and pathological gait ( 85 , 86 ). Some studies have also integrated inertial measurement units (IMU) with shoe insoles to enhance their capabilities. Despite the fact that these shoe-based systems have successfully been used for various gait analysis applications, they suffer from some drawbacks, such as (i) distortion of the flexible contact surface due to repeated loading, which leads to changes in the sensor response, (ii) drift in the output due to prolonged load application that causes heat inside the shoe, and (iii) need for subject-specific calibration that may alter accuracy ( 87 ). Mancinelli et al. (2012) presented ActiveGait – a novel sensorized shoe system for real-time monitoring of gait deviations associated with Cerebral Palsy in children. They reported that the severity of gait deviations can be estimated with an accuracy greater than 80% using the features derived from the center of pressure trajectories gathered from the shoe system ( 88 ). In ( 87 ), the authors designed a novel flexible foot insole system using an optoelectronic sensing technology for monitoring plantar pressure deviations in real-time. The system consists of an array of 64 sensing elements and onboard electronics for signal processing and transmission. Experimental validation was conducted on healthy subjects while walking at self-selected slow and normal speed. A commercial force plate (AMTI, Watertown, United States) was used as a reference system for benchmarking. Jagos et al. (2017), on the other hand, developed the eSHOE, which consists of four FSR sensors, a three-axis accelerometer, and a three-axis gyroscope, and reported good agreement with the gait parameters obtained from the GAITRite mat ( 89 ). Various other studies have also examined the applicability of shoe-based systems for gait analysis ( 85 , 90 – 92 ).
Another class of sensors that found major applications in gait studies is electromyography (EMG) sensors. Surface EMG is a non-invasive technique used to measure muscle activity. In ( 93 ), Lee et al. proposed a method using EMG signals to obtain biometrics from gait for personal identification methods. Another study adopted EMG techniques to understand the co-contraction patterns of thigh muscle during free walking using surface EMG ( 94 ). These research efforts emphasize the importance of wearable sensors in the study of human gait. The wearable systems discussed in this section are summarized in Table 6 .
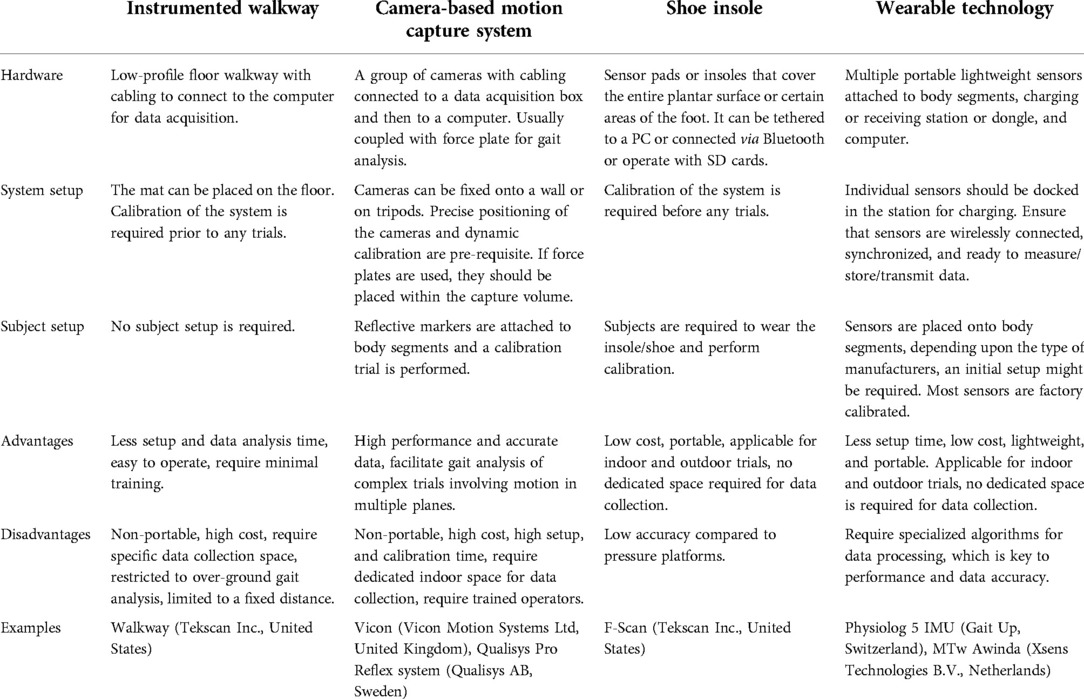
Table 6 . Instrumented gait analysis (IGA) systems and their features.
Although emerging new wearable technologies promise to enhance gait assessment and rehabilitation, there is limited research on the use of wearable technology to assess gait and mobility and its efficacy in clinical settings. According to a recently published review by Peters et. al. on the use of wearable technology to assess gait and mobility in stroke patients ( 95 ), most of the available studies are intervention studies conducted in laboratory settings that have used sensors to investigate change in cadence, step time variability, and gait speed. As wearable technologies continue to progress in affordability and accessibility, it is expected that such technologies would enable the gathering of movement-related data in “real-world” and various clinical settings. Importantly, these researchers indicated that so far only a limited number of studies examined reliability and validity of existing wearable devices, highlighting the need for more studies to examine psychometric and other properties when collecting gait and mobility information to determine which wearable technologies are most effective. Another recent review on the evaluation of the use of wearables in PD also indicates that novel technologies and wearables have the potential to enable early or differential diagnosis of PD, monitoring of motion state, prevention, or reduction of off-stage status, and assessing of movement complications. On the other hand, more research is required for the validation and the identification of more accurate markers of PD progression ( 96 ). Importantly, these authors warn that wearable devices may not be appropriate in cases of severe motor impairment, off-stage state, cognitive impairment, and for elderly patients and that further research is required for clinical validation.

4.3. Wearable-based gait computational algorithms
Besides sensor technology, sensor fusion algorithms play a critical role in predicting the accuracy/precision of these wearable-based systems. Most of the research has focused mainly on gait feature detection, daily physical activity monitoring, and gait data classification targeting disease diagnosis and user recognition. These algorithms are based on different data mining and AI technology, including machine learning, fuzzy computing, wavelet transforms, genetic algorithms, and data fusions. Alaqtash et al. ( 97 ) developed an intelligent fuzzy computational algorithm for characterizing gait in healthy, as well as impaired subjects. McCamley et al. established a method to calculate initial and final contact of gait using continuous wavelet transforms, employing waist-mounted inertial sensors ( 98 ). Another study cited the use of a single accelerometer mounted at the lower trunk and a corresponding algorithm to identify gait spatiotemporal parameters ( 68 ). A real-time gait event detection algorithm was proposed in ( 99 ) making use of adaptive decision rules. Further in ( 100 ), an original signal processing algorithm is developed to extract heel strike, toe strike, heel-off, and toe-off from an accelerometer positioned on the feet.
A novel gyroscope only (GO) algorithm was proposed in ( 101 ) to calculate knee angle through the integration of gyroscope-derived knee angular velocity. A zero-angle update algorithm was implemented to eliminate drift in the integral value. In addition, published work on noise-zero crossing (NZC) gait phase algorithm was also adapted. This method is applicable for continuous monitoring of gait data. Nukala et al. used support vector machines (SVM), KNN, binary decision trees (BDT), and backpropagation artificial neural network (BP-ANN) to classify the gait of patients from normal subjects, where features extracted from raw signals from gyroscopes and accelerometers were used as inputs. This study reported the highest overall classification accuracy of 100% with BP-ANN, 98% with SVM, 96% with KNN, and 94% with BDT ( 102 ).
Li et al. proposed DTW algorithm, sample entropy method, and empirical mode decomposition to calculate 3 main gait features of post-stroke subjects: symmetry, complexity character, and stepping stability. A k-nearest neighbor (KNN) classifier trained on the acquired features showed a promising result (area under the curve (AUC) of 0.94), which suggests the feasibility of such techniques to automatic gait analysis systems ( 43 ). Rastegari et al. employed a feature selection technique called maximum information gain minimum correlation (MIGMC) to extract gait data of subjects with Parkinson's Disease ( 103 ). The performance of several machine learning classifiers, including Support Vector Machines, Random Forest, AdaBoost, Bagging, and Naïve Bayes were also assessed to test the power of the feature set obtained.
The use of novel computational platforms, including Machine Learning, Support Vector Machine, and Neural Network approaches, are increasingly commanding greater attention in gait and rehabilitation research. Although their use in clinical settings are not yet well leveraged, these tools promise a paradigm shift in stroke gait quantification and rehabilitation, as they provide means for acquiring, storing and analyzing multifactorial complex gait data, while capturing its non-linear dynamic variability and offering the invaluable benefits of predictive analytics ( 1 ). A recent review article discussed the potential value of ML in gait analysis towards quantification and rehabilitation ( 104 ). The authors concluded that further evidence is required although preliminary data demonstrates that the control strategies for gait rehabilitation benefit from reinforcement learning and (deep) neural-networks due to their ability to capture participants' variability. This review paper demonstrated the success of ML techniques in detecting gait disorders, predicting rehabilitation length, and control of rehabilitation devices. Further work is needed for verification in clinical settings.
4.4. Data-driven gait rehabilitation in clinical settings
Quantitative gait assessment is invaluable towards disease-specific and patient specific rehabilitation/therapeutic interventions. Spatiotemporal, kinematic, and kinetic parameters obtained during instrumented gait assessment can help clinicians benchmark, devise strategies, and evaluate the effect of various rehabilitation interventions. Gait disorders not only affect these parameters, and patterns and time spent in the various gait phases, but can also highly impact gait symmetry, and regularity, depending on the disease and severity ( 105 ). Increasing evidence supports a data-driven physical rehabilitation approach to the treatment of functional gait disturbance ( 106 ). There are multiple examples in literature on the effective use of quantitative gait measures towards more effective data-driven rehabilitation. A recent review by Biase et al. ( 107 ) studied the most relevant technologies used to evaluate gait features and the associated algorithms that have shown promise to aid diagnosis and symptom monitoring towards rehabilitation in Parkinson's disease (PD) patients. They reported physical kinematic features of pitch, roll and yaw rotations of the foot during walking, based on which feature extraction and classification techniques, such as principal component analysis (PCA) and support vector machines (SVM) method were used to classify the PD patients. They also used gait features, including step duration, rise and fall gradients of the swing phase, as well as standard deviation of the minima as quantitative measures, for benchmarking and monitoring PD motor status during rehabilitation. Interestingly, this review sheds light on need to change the evaluated gait features as a function of disease progression. Another study was Pistacchi et al. ( 108 ) suggested spatiotemporal gait parameters, such as speed and step length, where reduced step length seems to be a specific feature of Parkinson's disease gait particularly in early disease stages. On the other hand, asymmetry, step shuffling, double-limb support and increased cadence are more common in mild to moderate stages, while advanced stages are more frequent freezing of gait (FOG) and motor blocks, reduced balance and postural control, motor fluctuations and dyskinesia ( 109 ). Researchers have also investigated the evaluation of ambulatory systems for gait analysis post hip replacement ( 110 ). They found gait characteristics such as stride length and velocity, as well as thigh and shank rotations different from healthy individuals and recommended their use to monitor post-surgical rehabilitation efficacy. Spatiotemporal gait parameters, such as step length, width and cadence have been used ( 111 ) to assess the effect of swing resistance and assistance rehabilitation on gait symmetry in hemiplegic patients. Investigators have also studied whether specific variables measured routinely at a rehabilitation center were predictors of gait performance of hemiparetic stroke patients ( 112 ). They found that motor control and balance were the best predictors of gait performance. A recent review article on assessment methods of post stroke gait suggests that multiple spatiotemporal, kinematic, and kinetic parameters can be useful in diagnosing post-stroke gait dysfunction and as quantitative measures to evaluate rehabilitation outcomes ( 1 ). Spatiotemporal characteristics of post-stroke gait include reduced step or stride length, increased step length on the hemiparetic side, wider base of support, greater toe-out angle, reduced walking speed and cadence. Stride time, stance period on both lower limb, and double support time are also increased, in addition to less time in stance and more time in swing phase for the paretic side, as well as asymmetries in spatial and temporal factors. Kinematic parameters associated with hemiplegic gait (reduced mean peak extension of the hip joint in late stance, alterations in the lateral displacement of the pelvis and flexion of the knee, and decreased plantarflexion of the ankle at toe-off, in addition to a significant decrease in peak hip and knee flexion during the swing phase, reduced knee extension prior to initial contact, as well as decreased ankle dorsiflexion during swing), and kinetic parameters (asymmetric patterns, as well as decreased amplitudes of the joint moments and joint powers at the hip, knee, and ankle joints on the paretic side) can be used as quantitative means to design and evaluate effective rehabilitation ( 113 – 115 ). IGA has also been successfully used to quantify and improve gait dysfunction associated with ageing and assess the risk of falling ( 116 ). Spatiotemporal gait parameters such as velocity, swing time, stride length, stride time- and double support time variability, as well as heel strike and toe off angles, and foot clearance, have been suggested as plausible indicative quantitative measures ( 116 ) to assess the risk of falling in elderly subjects. Inertial sensor-equipped shoes additionally provided heel strike and toe off angles, and foot clearance ( 116 ). The study ( 117 ) summarizes that multi-component exercise therapy which consisted of strength, ROM exercise, balance, flexibility and stretching exercises, circuit exercise training, and gait training was found to enhance gait function for individuals suffering with diabetic peripheral neuropathy compared to control groups using spatiotemporal gait parameters like velocity, cadence, step length, step time, double support time, stride length, stride time, ankle ROM. Gait assessment has potential to develop patient training paradigms for overcoming gait disorders ( 111 ).
5. Mobile gait lab for clinical applications and beyond
In recent decades, the healthcare field has witnessed a tremendous interest in the use of wearable sensing modalities and AI-driven data management/analysis techniques for patient diagnosis, monitoring, and rehabilitation. The portability, lightweight, ease of use, and high-power efficiency are some of the factors that promote applicability to a clinical platform.
There are few examples in literature demonstrating the potential success of using wearable-based systems for gait assessment in clinical settings. Prajapati et al. assessed the walking activity of inpatients with subacute stroke using commercial accelerometers attached above the ankle. They found that the walking bouts were shorter in duration and gait was more asymmetric ( 118 ). Studies have established test-retest reliability and accuracy of different sensor technologies; however, further validation trials are recommended prior to any clinical use. Hsu et al. assessed the test-retest reliability of an accelerometer-based system with infrared assist for measuring spatiotemporal parameters, including walking speed, step length, and cadence, as well as trunk control parameters, including gait symmetry, gait regularity, acceleration root mean square, and acceleration root mean square ratio of healthy subjects in hospital ( 119 ). This study showed excellent test-retest reliability of the parameters considered, and thus highlighting the reliability of an infrared assisted, trunk accelerometer-based device for clinical gait analysis. Another study investigated the concurrent validity and test-retest reliability of gait parameters (cadence, gait velocity, step time, step length, step time variability, and step time asymmetry) acquired from elderly subjects, using a tri-axial accelerometer attached to the center of body mass ( 120 ). In comparison to a reference GAITRite system, the acquired parameters showed good validity and reliability. Poitras et al. performed a systematic review of 42 studies assessing the reliability and validity of wearable sensors, specifically, IMUs, for quantifying the joint motion ( 121 ). Evidence suggests that IMU could be an alternative solution to an expensive motion capture system, as it shows good validity for lower-limb analysis involving fewer complex tasks. However, more work is needed to draw a better conclusion with regards to its reliability, as well as to standardize the protocol to get more accurate data in a clinical setting. Importantly, additional research efforts are needed to examine the responsiveness of wearables in free-living conditions in hospital settings.
6. Limitations
This review aimed to summarize available published work on the present and future of gait analysis in clinical settings. The focus was to highlight current systems, scales, and indices, as well as recent technology-driven gait characterization and analysis approaches and their applicability to clinical settings. Within this context, pathological gait associated with different disease, as well as ageing was briefly discussed. As such, this article may have not covered the complete spectrum of gait pathologies and associated parameters. A scoping (non-systematic) search methodology was selected to broaden the scope and integration of the three main aspects of focus (gait pathology, clinical assessment, recent tools, and technologies). In addition, we do not recommend any specific protocol over the other, as most of the papers incorporate different inclusion/exclusion criteria for subject selection, as well as different sampling sizes, which may render comparisons unrealistic.
7. Conclusive remarks and future work
This scoping review aimed to shed light on the status of gait assessment in clinical settings, as well as the state-of-the-art emerging tools and technologies and their potential clinical applicability. Clinical gait analysis continues to rely mainly on observational gait and quantitative scales and is hence subjective and suffers from variability and the lack of sensitivity influenced by the observer's background and experience. Based on the reviewed literature, quantitative IGA-based gait analysis, commonly used in research labs, has the capability of providing clinicians with accurate and reliable gait data for informed diagnosis and continuous monitoring. On the other hand, several factors, including high cost and infrastructure challenges; data variability, complexity, and multidimensionality; lack of sufficient knowledge and standardized training in clinical environments; and time constraints, continue to limit its wide-spread deployment. Rapidly emerging smart wearable technology and AI, including Machine Learning, Support Vector Machine, and Neural Network approaches, are increasingly playing a bigger role in gait assessment. Although their use in clinical settings is not yet well leveraged, these tools promise an unprecedented paradigm shift in the quantification of gait in the clinic and beyond, as they provide means for acquiring, storing, and analyzing multifactorial complex gait data, while capturing its non-linear dynamic variability and offering the invaluable benefits of predictive analytics.
Researchers are also paying increased attention to multisource and multi-modality sensor fusion approaches, which can further add value by integrating the output of multiple sensors to capture the complexity and variability of gait. Multimodality sensor fusion also allows for simultaneous monitoring of various physiological signals during locomotion, such as EMG, ECG, and EEG, where fusing these with various gait measures (spatiotemporal, kinematic, and kinetic) can shed light on underlying health conditions and disease etiology towards better informed outcome prediction and clinical decisions. As the volume of data from the variety of sensors, including electroencephalography, electro-oculography, electro-cardiography, and electromyography, motion capture and force sensors data, substantially increases, more AI-driven sophisticated data management and modeling are needed to quantify and interpret complex network AI/NN models. Models which include static and dynamic features, combined with sophisticated data reduction and individualized feature selection of the most relevant gait characteristics are needed to close the loop for this paradigm shift. Future work is warranted on a multidisciplinary level: to validate the clinical applicability and integration of the various sensing modalities, to ensure proper synchronization of the various systems for accurate continuous real-time monitoring, to develop and validate fast and reliable computational platforms, and to implement modular user-friendly interfaces easy to use in any environment.
In summary, instrumented gait analysis is a well-established tool for the quantitative assessment of gait dysfunction which could be effectively used for functional diagnosis, treatment/surgery/rehabilitation/planning, and progression monitoring for a wide spectrum of disease. The literature indicates that recent advancement in wearable technology and computationally advanced data analytics, including AI, can overcome the challenges of traditional gait labs, allowing for less costly, portable, and relatively simple gait testing protocols in clinical settings, as well as user-friendly data management, analysis, and interpretation computational platforms. On the other hand, the development of clinically driven standardized methodology and procedures is of paramount significance and remains largely unaddressed. These standardized practices should not only focus on quantitative gait diagnosis but should also incorporate sophisticated objective measures and 3-D dynamic gait profiles and markers for monitoring progress and outcome prediction and evaluation. Proper gait protocols should be devised and leveraged towards identifying gait characteristics that could be effectively used as early disease diagnostic markers. Importantly, training clinical teams at various levels, from doctors and surgeons to physiotherapists and other allied health professionals, on properly using these novel assessment and computational tools is equally important and warrants an equally rapid paradigm shift in training and practice in clinical settings towards patient-specific precise medicine.
Author contributions
AAH, MR and KK conceived the idea. AAH, DMM, KK and MR formulated the objective for this review. AAH designed the search strategy, conducted abstract screening and full text review, extracted the data, and drafted the manuscript. KK, NA, DMM, and AAH contributed to writing the manuscript. DMM and NA performed a part of the literature survey, including abstract screening, full text review, and data extraction. MR, and KK provided significant guidance on the content of the manuscript, overall supervision, and critical feedback. All authors contributed to the article and approved the submitted version.
This publication is based upon work supported by the HEIC at Khalifa University of Science and Technology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mohan DM, Khandoker AH, Wasti SA, Ismail Ibrahim Ismail Alali S, Jelinek HF, Khalaf K. Assessment methods of post-stroke gait: a scoping review of technology-driven approaches to gait characterization and analysis. Front Neurol . (2021) 12:650024. doi: 10.3389/fneur.2021.650024
PubMed Abstract | CrossRef Full Text | Google Scholar
2. Celik Y, Stuart S, Woo WL, Godfrey A. Gait analysis in neurological populations: progression in the use of wearables. Med Eng Phys . (2021) 87:9–29. doi: 10.1016/j.medengphy.2020.11.005
3. Rathinam C, Bateman A, Peirson J, Skinner J. Observational gait assessment tools in paediatrics–a systematic review. Gait Posture . (2014) 40:279–85. S0966-6362(14)00478-0 24798609
PubMed Abstract | Google Scholar
4. Eastlack ME, Arvidson J, Snyder-Mackler L, Danoff JV, McGarvey CL. Interrater reliability of videotaped observational gait-analysis assessments. Phys Ther . (1991) 71:465–72. doi: 10.1093/ptj/71.6.465
5. Hebda-Boon A, Zhang B, Amankwah A, Shortland AP, Morrissey D. Clinicians’ experiences of instrumented gait analysis in management of patients with cerebral palsy: a qualitative study. Phys Occup Ther Pediatr . (2022) 42(4):403–15. doi: 10.1080/01942638.2022.2037808
6. Toro B, Nester C, Farren P. A review of observational gait assessment in clinical practice. null . (2003) 19:137–49. doi: 10.1080/09593980307964
CrossRef Full Text | Google Scholar
7. Franki I, Desloovere K, De Cat J, Feys H, Molenaers G, Calders P, et al. The evidence-base for basic physical therapy techniques targeting lower limb function in children with cerebral palsy: a systematic review using the international classification of functioning, disability and health as a conceptual framework. J Rehabil Med . (2012) 44:385–95. doi: 10.2340/16501977-0983
8. Wallmann HW. Introduction to observational gait analysis. Home Health Care Manag Pract . (2009) 22:66–8. doi: 10.1177/1084822309343277
9. Ferrarello F, Bianchi VA, Baccini M, Rubbieri G, Mossello E, Cavallini MC, et al. Tools for observational gait analysis in patients with stroke: a systematic review. Phys Ther . (2013) 93:1673–85. doi: 10.2522/ptj.20120344
10. Turani N, Kemiksizoğlu A, Karataş M, Ozker R. Assessment of hemiplegic gait using the Wisconsin gait scale. Scand J Caring Sci . (2004) 18:103–8. doi: 10.1111/j.1471-6712.2004.00262.x
11. Dasgupta P, VanSwearingen J, Godfrey A, Redfern M, Montero-Odasso M, Sejdic E. Acceleration gait measures as proxies for motor skill of walking: a narrative review. IEEE Trans Neural Syst Rehabil Eng . (2021) 29:249–61. doi: 10.1109/TNSRE.2020.3044260
12. Noseworthy JH. Clinical scoring methods for multiple sclerosis. Ann Neurol . (1994) 36:S80–5. doi: 10.1002/ana.410360718
13. Noseworthy JH, Vandervoort MK, Wong CJ, Ebers GC. Interrater variability with the expanded disability Status scale (EDSS) and functional systems (FS) in a multiple sclerosis clinical trial. The Canadian cooperation MS study group. Neurol . (1990) 40:971–5. doi: 10.1212/wnl.40.6.971
14. Roxburgh RH, Seaman SR, Masterman T, Hensiek AE, Sawcer SJ, Vukusic S, et al. Multiple sclerosis severity score: using disability and disease duration to rate disease severity. Neurol . (2005) 64:1144–51. doi: 10.1212/01.WNL.0000156155.19270.F8
15. Vienne-Jumeau A, Quijoux F, Vidal PP, Ricard D. Value of gait analysis for measuring disease severity using inertial sensors in patients with multiple sclerosis: protocol for a systematic review and meta-analysis. Syst Rev . (2019) 8:15. doi: 10.1186/s13643-018-0918-z
16. Balaji E, Brindha D, Elumalai VK, Umesh K. Data-driven gait analysis for diagnosis and severity rating of Parkinson's Disease. Med Eng Phys . (2021) 91:54–64. S1350-4533(21)00028-X 34074466
17. Mohan DM, Khandoker AH, Wasti SA, Ismail Ibrahim Ismail Alali S, Jelinek HF, Khalaf K. Assessment methods of post-stroke gait: a scoping review of technology-driven approaches to gait characterization and Analysis. Front Neurol . (2021) 12:650024. doi: 10.3389/fneur.2021.650024
18. Cappozzo A. Gait analysis methodology. Hum Mov Sci . (1984) 3:27–50. doi: 10.1016/0167-9457(84)90004-6
19. Wren TAL, Tucker CA, Rethlefsen SA, Gorton GE 3rd, Õunpuu S. Clinical efficacy of instrumented gait analysis: systematic review 2020 update. Gait Posture . (2020) 80:274–9. S0966-6362(20)30181-8 32563727
20. Saboor A, Kask T, Kuusik A, Alam MM, Le Moullec Y, Niazi IK, et al. Latest research trends in gait analysis using wearable sensors and machine learning: a systematic review. IEEE Access . (2020) 8:167830–64. doi: 10.1109/ACCESS.2020.3022818
21. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol . (2018) 18:143. doi: 10.1186/s12874-018-0611-x
22. Winter DA. The biomechanics and motor control of humna gait: Normal, elderly and pathological . Waterloo: Ont.: Waterloo Biomechanics (1991).
23. Ali A, Sundaraj K, Ahmad B, Ahamed N, Islam A. Gait disorder rehabilitation using vision and non-vision based sensors: a systematic review. Bosnian Journal of Basic Medical Sciences . (2012) 12:193–202. doi: 10.17305/bjbms.2012.2484
24. Alam U, Riley DR, Jugdey RS, Azmi SS, Rajbhandari SS, D’août K, et al. Diabetic neuropathy and gait: a review. Diabetes Ther . (2017) 8:1253. doi: 10.1007/s13300-017-0295-y
25. Whittle MW. Clinical gait analysis: a review. Hum Mov Sci . (1996) 15:369–87. doi: 10.1016/0167-9457(96)00006-1
26. Fukuchi CA, Fukuchi RK, Duarte M. Effects of walking speed on gait biomechanics in healthy participants: a systematic review and meta-analysis. Syst Rev . (2019) 8:153. doi: 10.1186/s13643-019-1063-z
27. Snijders AH, van de Warrenburg BP, Giladi N, Bloem BR. Neurological gait disorders in elderly people: clinical approach and classification. Lancet Neurol . (2007) 6:63–74. S1474-4422(06)70678-0 [pii].17166803
28. Snijders AH, van de Warrenburg BP, Giladi N, Bloem BR. Neurological gait disorders in elderly people: clinical approach and classifi cation. Lancet Neurol . (2007) 6(1):63–74. doi: 10.1016/S1474-4422(06)70678-0
29. Intzandt B, Beck EN, Silveira CRA. The effects of exercise on cognition and gait in Parkinson's Disease: a scoping review. Neurosci Biobehav Rev . (2018) 95:136–69. doi: 10.1016/j.neubiorev.2018.09.018
30. Belda-Lois J, Mena-del Horno S, Bermejo-Bosch I, Moreno JC, Pons JL, Farina D, et al. Rehabilitation of gait after stroke: a review towards a top-down approach. J Neuroeng Rehabil . (2011) 8:66. doi: 10.1186/1743-0003-8-66
31. Bahl JS, Nelson MJ, Taylor M, Solomon LB, Arnold JB, Thewlis D. Biomechanical changes and recovery of gait function after total hip arthroplasty for osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage . (2018) 26:847–63. S1063-4584(18)31013-6 [pii].29474993
32. Ewen AM, Stewart S, St Clair Gibson A, Kashyap SN, Caplan N. Post-operative gait analysis in total hip replacement patients—a review of current literature and meta-analysis. Gait Posture . (2012) 36:1–6. doi: 10.1016/j.gaitpost.2011.12.024
33. Kolk S, Minten MJ, van Bon GE, Rijnen WH, Geurts AC, Verdonschot N, et al. Gait and gait-related activities of daily living after total hip arthroplasty: a systematic review. Clin Biomech (Bristol, Avon) . (2014) 29:705–18. S0268-0033(14)00127-2 [pii].24951319
34. Kolk S, Minten MJM, van Bon GEA, Rijnen WH, Geurts ACH, Verdonschot N, et al. Gait and gait-related activities of daily living after total hip arthroplasty: a systematic review. Clin Biomech . (2014) 29:705–18. doi: 10.1016/j.clinbiomech.2014.05.008
35. Ladha C, Din S, Nazarpour K, Hickey A, Morris R, Catt M, et al. Toward a low-cost gait analysis system for clinical and free-living assessment. Annu Int Conf IEEE Eng Med Biol Soc . (2016) 2016:1874–7. doi: 10.1109/EMBC.2016.7591086. PMID: 28268692
36. Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture . (2010) 31:241–6. doi: 10.1016/j.gaitpost.2009.10.014
37. Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Changes in gait symmetry and velocity after stroke: a cross-sectional study from weeks to years after stroke. Neurorehabil Neural Repair . (2010) 24:783–90. doi: 10.1177/1545968310372091
38. Laudanski A, Brouwer B, Li Q. Measurement of lower limb joint kinematics using inertial sensors during stair ascent and descent in healthy older adults and stroke survivors. J Healthc Eng . (2013) 4:555–76. doi: 10.1260/2040-2295.4.4.555
39. Rastegarpanah A, Scone T, Saadat M, Rastegarpanah M, Taylor SJ, Sadeghein N. Targeting effect on gait parameters in healthy individuals and post-stroke hemiparetic individuals. J Rehabil Assist Technol Eng . (2018) 5:2055668318766710. doi: 10.1177/2055668318766710
40. Latorre J, Colomer C, Alcañiz M, Llorens R. Gait analysis with the kinect v2: normative study with healthy individuals and comprehensive study of its sensitivity, validity, and reliability in individuals with stroke. J Neuroeng Rehabil . (2019) 16:97. doi: 10.1186/s12984-019-0568-y
41. Iosa M, Bini F, Marinozzi F, Fusco A, Morone G, Koch G, et al. Stability and harmony of gait in patients with subacute stroke. J Med Biol Eng . (2016) 36:635–43. doi: 10.1007/s40846-016-0178-0 [pii].27853414
42. Sousa ASP, Silva A, Santos R, Sousa F, Tavares JMRS. Interlimb coordination during the stance phase of gait in subjects with stroke. Arch Phys Med Rehabil . (2013) 94:2515–22. S0003-9993(13)00537-6 [pii].23871877
43. Li M, Tian S, Sun L, Chen X. Gait analysis for post-stroke hemiparetic patient by multi-features fusion method. Sens (Basel) . (2019) 19(7):1737. doi: 10.3390/s19071737
44. Krishnan V, Khoo I, Marayong P, DeMars K, Cormack J. Gait training in chronic stroke using walk-even feedback device: a pilot study. Neurosci J . (2016) 2016:1–8. doi: 10.1155/2016/6808319
45. Ardalan A, Yamane N, Rao AK, Montes J, Goldman S. Analysis of gait synchrony and balance in neurodevelopmental disorders using computer vision techniques. Health Informatics J . (2021) 27:14604582211055650. doi: 10.1177/14604582211055650
46. Rupprechter S, Morinan G, Peng Y, Foltynie T, Sibley K, Weil RS, et al. A clinically interpretable computer-vision based method for quantifying gait in Parkinson's Disease. Sens (Basel) . (2021) 21:5437. doi: 10.3390/s21165437
47. Li Y, Zhang P, Zhang Y, Miyazaki K. 2019 41st annual international conference of the IEEE engineering in medicine and biology society (EMBC). Gait analysis using stereo camera in daily environment (2019). p. 1471–5
48. Kessler SE, Rainbow MJ, Lichtwark GA, Cresswell AG, D'Andrea SE, Konow N, et al. A direct comparison of biplanar videoradiography and optical motion capture for foot and ankle kinematics. Front Bioeng Biotechnol . (2019) 7:199. doi: 10.3389/fbioe.2019.00199
49. Tranberg R, Karlsson D. The relative skin movement of the foot: a 2-D roentgen photogrammetry study. Clin Biomech (Bristol, Avon) . (1998) 13:71–6. S0268003397000521 [pii].11415773
50. Nester C, Jones RK, Liu A, Howard D, Lundberg A, Arndt A, et al. Foot kinematics during walking measured using bone and surface mounted markers. J Biomech . (2007) 40:3412–23. doi: 10.1016/j.jbiomech.2007.05.019
51. Colyer SL, Evans M, Cosker DP, Salo AIT. A review of the evolution of vision-based motion analysis and the integration of advanced computer vision methods towards developing a markerless system. Sports Med Open . (2018) 4:24. doi: 10.1186/s40798-018-0139-y
52. Baker R. Gait analysis methods in rehabilitation. J Neuroeng Rehabil . (2006) 3:4. doi: 10.1186/1743-0003-3-4
53. Stagni R, Fantozzi S, Cappello A, Leardini A. Quantification of soft tissue artefact in motion analysis by combining 3D fluoroscopy and stereophotogrammetry: a study on two subjects. Clin Biomech (Bristol, Avon) . (2005) 20:320–9. doi: 10.1016/j.clinbiomech.2004.11.012
54. McHenry BD, Exten E, Long JT, Harris GF. Sagittal fluoroscopy for the assessment of hindfoot kinematics. J Biomech Eng . (2016) 138:4032445. doi: 10.1115/1.4032445
55. Roach KE, Foreman KB, MacWilliams BA, Karpos K, Nichols J, Anderson AE. The modified shriners hospitals for children Greenville (mSHCG) multi-segment foot model provides clinically acceptable measurements of ankle and midfoot angles: a dual fluoroscopy study. Gait Posture . (2021) 85:258–65. doi: 10.1016/j.gaitpost.2021.02.004
56. Hitz M, Schütz P, Angst M, Taylor WR, List R. Influence of the moving fluoroscope on gait patterns. PLoS One . (2018) 13:e0200608. doi: 10.1371/journal.pone.0200608
57. Roach KE, Wang B, Kapron AL, Fiorentino NM, Saltzman CL, Bo Foreman K, et al. In vivo kinematics of the tibiotalar and subtalar joints in asymptomatic subjects: a high-speed dual fluoroscopy study. J Biomech Eng . (2016) 138:910061. doi: 10.1115/1.4034263
58. Atkins PR, Fiorentino NM, Hartle JA, Aoki SK, Peters CL, Foreman KB, et al. In vivo pelvic and hip joint kinematics in patients with cam femoroacetabular impingement syndrome: a dual fluoroscopy study. J Orthop Res . (2020) 38:823–33. doi: 10.1002/jor.24509
59. Battaglia S, Belvedere C, Jaber SA, Affatato S, D'Angeli V, Leardini A. A new protocol from real joint motion data for wear simulation in total knee arthroplasty: stair climbing. Med Eng Phys . (2014) 36:1605–10. doi: 10.1016/j.medengphy.2014.08.010
60. Belvedere C, Cadossi M, Mazzotti A, Giannini S, Leardini A. Fluoroscopic and gait analyses for the functional performance of a custom-made total talonavicular replacement. J Foot Ankle Surg . (2017) 56:836–44. doi: 10.1053/j.jfas.2017.02.004
61. Uemura K, Atkins PR, Anderson AE. The effect of using different coordinate systems on in-vivo hip angles can be estimated from computed tomography images. J Biomech . (2019) 95:109318. doi: 10.1016/j.jbiomech.2019.109318
62. Wearing SC, Urry S, Perlman P, Smeathers J, Dubois P. Sagittal plane motion of the human arch during gait: a videofluoroscopic analysis. Foot Ankle Int . (1998) 19:738–42. doi: 10.1177/107110079801901105
63. Li G, Kozanek M, Hosseini A, Liu F, Van de Velde SK, Rubash HE. New fluoroscopic imaging technique for investigation of 6DOF knee kinematics during treadmill gait. J Orthop Surg Res . (2009) 4:6. doi: 10.1186/1749-799X-4-6
64. Iaquinto JM, Tsai R, Haynor DR, Fassbind MJ, Sangeorzan BJ, Ledoux WR. Marker-based validation of a biplane fluoroscopy system for quantifying foot kinematics. Med Eng Phys . (2014) 36:391–6. doi: 10.1016/j.medengphy.2013.08.013
65. Sheehan FT, Seisler AR, Siegel KL. In vivo talocrural and subtalar kinematics: a non-invasive 3D dynamic MRI study. Foot Ankle Int . (2007) 28:323–35. doi: 10.3113/FAI.2007.0323
66. Tao W, Liu T, Zheng R, Feng H. Gait analysis using wearable sensors. Sens (Basel, Switzerland) . (2012) 12:2255–83. doi: 10.3390/s120202255
67. Ihlen EAF, Weiss A, Beck Y, Helbostad JL, Hausdorff JM. A comparison study of local dynamic stability measures of daily life walking in older adult community-dwelling fallers and non-fallers. J Biomech . (2016) 49:1498–503. doi: 10.1016/j.jbiomech.2016.03.019
68. Bugané F, Benedetti MG, Casadio G, Attala S, Biagi F, Manca M, et al. Estimation of spatial-temporal gait parameters in level walking based on a single accelerometer: validation on Normal subjects by standard gait analysis. Comput Methods Programs Biomed . (2012) 108:129–37. doi: 10.1016/j.cmpb.2012.02.003
69. Rispens SM, Pijnappels M, van Schooten KS, Beek PJ, Daffertshofer A, van Dieën JH. Consistency of gait characteristics as determined from acceleration data collected at different trunk locations. Gait Posture . (2014) 40:187–92. doi: 10.1016/j.gaitpost.2014.03.182
70. Jarchi D, Pope J, Lee TKM, Tamjidi L, Mirzaei A, Sanei S. A review on accelerometry-based gait analysis and emerging clinical applications. IEEE Rev Biomed Eng . (2018) 11:177–94. doi: 10.1109/RBME.2018.2807182
71. Felix P, Figueiredo J, Santos C, Moreno J. ADAPTIVE REAL-TIME TOOL FOR HUMAN GAIT EVENT DETECTION USING A WEARABLE GYROSCOPE. (2017). 653 p.
72. Nasr A, Nadeem T. A Novel Technique for Gait Analysis Using Two Waist Mounted Gyroscopes. In: Anonymous - 2019 IEEE Global Communications Conference (GLOBECOM); (2019). p. 1-6.
73. Kun L, Inoue Y, Shibata K, Enguo C. Ambulatory estimation of knee-joint kinematics in anatomical coordinate system using accelerometers and magnetometers. IEEE Trans on Biomed Eng . (2011) 58:435–42. doi: 10.1109/TBME.2010.2089454
74. Rodríguez-Martín D, Pérez-López C, Samà A, Cabestany J, Català A. A wearable inertial measurement unit for long-term monitoring in the dependency care area. Sens . (2013) 13(10):14079–104. doi: 10.3390/s131014079
75. Seel T, Raisch J, Schauer T. IMU-Based Joint angle measurement for gait analysis. Sens . (2014) 14(4):6891–909. doi: 10.3390/s140406891
76. Teufl W, Lorenz M, Miezal M, Taetz B, Fröhlich M, Bleser G. Towards inertial sensor based Mobile gait analysis: event-detection and spatio-temporal parameters. Sens . (2019) 19(1):38. doi: 10.3390/s19010038
77. Hwang T-H, Reh J, Effenberg AO, Blume H. Real-Time gait analysis using a single head-worn inertial measurement unit. IEEE Trans on Cons Elec . (2018) 64:240–8. doi: 10.1109/TCE.2018.2843289
78. Parisi F, Ferrari G, Baricich A, D'Innocenzo M, Cisari C, Mauro A. Accurate gait analysis in post-stroke patients using a single inertial measurement unit. In: Anonymous - 2016 IEEE 13th International Conference on Wearable and Implantable Body Sensor Networks (BSN); (2016). p. 335-340).
79. Karatsidis A, Bellusci G, Schepers HM, De Zee M, Andersen MS, Veltink PH. Estimation of ground reaction forces and moments during gait using only inertial motion capture. Senss . (2017) 17(1):75. doi: 10.3390/s17010075
80. Sant’ Anna A, Wickström N, Eklund H, Zügner R, Tranberg R. Assessment of gait symmetry and gait normality using inertial sensors: in-lab and in-situ evaluation . Berlin, Heidelberg: Springer (2013).
81. Mariani B, Hoskovec C, Rochat S, Büla C, Penders J, Aminian K. 3D Gait assessment in young and elderly subjects using foot-worn inertial sensors. J Biomech . (2010) 43:2999–3006. doi: 10.1016/j.jbiomech.2010.07.003
82. Razak AHA, Zayegh A, Begg RK, Wahab Y. Foot plantar pressure measurement system: a review. Sens (Basel, Switzerland) . (2012) 12:9884–912. doi: 10.3390/s120709884
83. Novel® Pedar® System [Internet]. []. Available at: https://www.novel.de/products/pedar/
84. Tekscan® F-Scan® System.
85. Leitch KM, Birmingham TB, Jones IC, Giffin JR, Jenkyn TR. In-shoe plantar pressure measurements for patients with knee osteoarthritis: reliability and effects of lateral heel wedges. Gait Posture . (2011) 34:391–6. doi: 10.1016/j.gaitpost.2011.06.008
86. Malvade PS, Joshi AK, Madhe SP. In-sole Shoe Foot Pressure Monitoring for Gait Analysis. In: Anonymous - 2017 International Conference on Computing, Communication, Control and Automation (ICCUBEA); (2017). p. 1-4.
87. Crea S, Donati M, De Rossi S, Marco Maria Oddo CM, Vitiello N. A wireless flexible sensorized insole for gait analysis. Sens (Basel, Switzerland) . (2014) 14:1073–93. doi: 10.3390/s140101073
88. Mancinelli C, Patel S, Deming LC, Nimec D, Chu JJ, Beckwith J, et al. A novel sensorized shoe system to classify gait severity in children with cerebral palsy. Annu Int Conf IEEE Eng Med Biol Soc . (2012) 2012:5010–3. doi: 10.1109/EMBC.2012.6347118
89. Jagos H, Pils K, Haller M, Wassermann C, Chhatwal C, Rafolt D, et al. Mobile gait analysis via eSHOEs instrumented shoe insoles: a pilot study for validation against the gold standard GAITRite(®). J Med Eng Technol . (2017) 41:375–86. doi: 10.1080/03091902.2017.1320434
90. Arafsha F, Hanna C, Aboualmagd A, Fraser S, El Saddik A. Instrumented wireless SmartInsole system for Mobile gait analysis: a validation pilot study with tekscan strideway. J Sens and Actuator Netw . (2018) 7(3):36. doi: 10.3390/jsan7030036
91. Nagano H, Begg RK. Shoe-Insole technology for injury prevention in walking. Sens (Basel) . (2018) 18:1468. doi: 10.3390/s18051468
92. Lin F, Wang A, Zhuang Y, Tomita MR, Xu W. Smart insole: a wearable sensor device for unobtrusive gait monitoring in daily life. IEEE Trans on Indust Informat . (2016) 12:2281–91. doi: 10.1109/TII.2016.2585643
93. Lee M, Ryu J, Youn I. Biometric personal identification based on gait analysis using surface EMG signals. In: Anonymous - 2017 2nd IEEE International Conference on Computational Intelligence and Applications (ICCIA); (2017). p. 318-321).
94. Strazza A, Mengarelli A, Fioretti S, Burattini L, Agostini V, Knaflitz M, et al. Surface-EMG analysis for the quantification of thigh muscle dynamic co-contractions during Normal gait. Gait Posture . (2017) 51:228–33. doi: 10.1016/j.gaitpost.2016.11.003
95. Peters D, O’Brien E, Kamrud K, Roberts S, Rooney T, Thibodeau K, et al. Utilization of wearable technology to assess gait and mobility post-stroke: a systematic review. J Neuroeng Rehabil . (2021) 18(1):67. doi: 10.1186/s12984-021-00863-x
96. Lu R, Xu Y, Li X, Fan Y, Zeng W, Tan Y, et al. Evaluation of wearable sensor devices in Parkinson's Disease: a review of current Status and future prospects. Parkinson's Disease . (2020) 2020:4693019. doi: 10.1155/2020/4693019
97. Alaqtash M, Yu H, Brower R, Abdelgawad A, Sarkodie-Gyan T. Application of wearable sensors for human gait analysis using fuzzy computational algorithm. Eng Appl Artif Intell . (2011) 24:1018–25. doi: 10.1016/j.engappai.2011.04.010
98. McCamley J, Donati M, Grimpampi E, Mazzà C. An enhanced estimate of initial contact and final contact instants of time using lower trunk inertial sensor data. Gait Posture . (2012) 36:316–8. doi: 10.1016/j.gaitpost.2012.02.019
99. Felix P, Figueiredo J, Santos C, Moreno J. ADAPTIVE REAL-TIME TOOL FOR HUMAN GAIT EVENT DETECTION USING A WEARABLE GYROSCOPE. (2017). 653 p).
100. Boutaayamou M, Schwartz C, Stamatakis J, Denoël V, Maquet D, Forthomme B, et al. Development and validation of an accelerometer-based method for quantifying gait events. Med Eng Phys . (2015) 37:226–32. doi: 10.1016/j.medengphy.2015.01.001
101. Allseits E, Kim KJ, Bennett C, Gailey R, Gaunaurd I, Agrawal V. A novel method for estimating knee angle using two leg-mounted gyroscopes for continuous monitoring with Mobile health devices. Sens (Basel) . (2018) 18:2759. doi: 10.3390/s18092759
102. Nukala BT, Nakano T, Rodriguez A, Tsay J, Lopez J, Nguyen TQ, et al. Real-Time classification of patients with balance disorders vs. Normal subjects using a low-cost small wireless wearable gait sensor. Biosens . (2016) 6(4):58. doi: 10.3390/bios6040058
103. Rastegari E, Azizian S, Ali H. Machine Learning and Similarity Network Approaches to Support Automatic Classification of Parkinson's Diseases Using Accelerometer-based Gait Analysis. (2019).).
104. Khera P, Kumar N. Role of machine learning in gait analysis: a review. J Med Eng Technol . (2020) 44:441–67. doi: 10.1080/03091902.2020.1822940
105. Zhao H, Wang Z, Qiu S, Shen Y, Wang J. IMU-based gait analysis for rehabilitation assessment of patients with gait disorders . In: Anonymous (2017). 622–6.
106. Butz C, Iske C, Truba N, Trott K. Treatment of functional gait abnormality in a rehabilitation setting: emphasizing the physical interventions for treating the whole child. Innov Clin Neurosci . (2019) 16:18–21.31832259
107. di Biase L, Di Santo A, Caminiti ML, De Liso A, Shah SA, Ricci L, et al. Gait analysis in Parkinson's Disease: an overview of the most accurate markers for diagnosis and symptoms monitoring. Sens (Basel) . (2020) 20:3529. doi: 10.3390/s20123529
108. Pistacchi M, Gioulis M, Sanson F, De Giovannini E, Filippi G, Rossetto F, et al. Gait analysis and clinical correlations in early Parkinson's Disease. Funct Neurol . (2017) 32:28–34. doi: 10.11138/FNeur/2017.32.1.028
109. Mirelman A, Bonato P, Camicioli R, Ellis TD, Giladi N, Hamilton JL, et al. Gait impairments in Parkinson's Disease. Lancet Neurol . (2019) 18:697–708. doi: 10.1016/S1474-4422(19)30044-4
110. Aminian K, Trevisan C, Najafi B, Dejnabadi H, Frigo C, Pavan E, et al. Evaluation of an ambulatory system for gait analysis in hip osteoarthritis and after total hip replacement. Gait Posture . (2004) 20:102–7. doi: 10.1016/S0966-6362(03)00093-6
111. Yen SC, Schmit BD, Wu M. Using swing resistance and assistance to improve gait symmetry in individuals post-stroke. Hum Mov Sci . (2015) 42:212–24. doi: 10.1016/j.humov.2015.05.010
112. Bohannon RW. Gait performance of hemiparetic stroke patients: selected variables. Arch Phys Med Rehabil . (1987) 68:777–81.3675175
113. Nadeau S, Betschart M, Bethoux F. Gait analysis for poststroke rehabilitation: the relevance of biomechanical analysis and the impact of gait speed. Phys Med Rehabil Clin N Am . (2013) 24:265–76. doi: 10.1016/j.pmr.2012.11.007
114. Moseley A, Wales A, Herbert R, Schurr K, Moore S. Observation and analysis of hemiplegic gait: stance phase. Aust J Physiother . (1993) 39:259–67. doi: 10.1016/S0004-9514(14)60486-4
115. von Schroeder HP, Coutts RD, Lyden PD, Billings E Jr, Nickel VL. Gait parameters following stroke: a practical assessment. J Rehabil Res Dev . (1995) 32:25–31.7760264
116. Schülein S, Barth J, Rampp A, Rupprecht R, Eskofier BM, Winkler J, et al. Instrumented gait analysis: a measure of gait improvement by a wheeled walker in hospitalized geriatric patients. J Neuroeng Rehabil . (2017) 14:18. doi: 10.1186/s12984-017-0228-z
117. Melese H, Alamer A, Hailu Temesgen M, Kahsay G. Effectiveness of exercise therapy on gait function in diabetic peripheral neuropathy patients: a systematic review of randomized controlled trials. Diabetes Metab Syndr Obes . (2020) 13:2753–64. doi: 10.2147/DMSO.S261175
118. Prajapati SK, Gage WH, Brooks D, Black SE, McIlroy WE. A novel approach to ambulatory monitoring: investigation into the quantity and control of everyday walking in patients with subacute stroke. Neurorehabil Neural Repair . (2011) 25:6–14. doi: 10.1177/1545968310374189
119. Hsu C, Tsai Y, Yau C, Shie H, Wu C. Test-Retest reliability of an automated infrared-assisted trunk accelerometer-based gait analysis system. Sens . (2016) 16(8):1156. doi: 10.3390/s16081156
120. Byun S, Han JW, Kim TH, Kim KW. Test-Retest reliability and concurrent validity of a single tri-axial accelerometer-based gait analysis in older adults with Normal cognition. PLoS One . (2016) 11:e0158956. doi: 10.1371/journal.pone.0158956
121. Poitras I, Dupuis F, Bielmann M, Campeau-Lecours A, Mercier C, Bouyer LJ, et al. Validity and reliability of wearable sensors for joint angle estimation: a systematic review. Sens . (2019) 19(7):1555. doi: 10.3390/s19071555
122. Ro DH, Lee J, Lee J, Park JY, Han HS, Lee MC. Effects of knee osteoarthritis on hip and ankle gait mechanics. Adv Orthop . (2019) 2019:9757369. doi: 10.1155/2019/9757369
123. Viteckova S, Kutilek P, Svoboda Z, Krupicka R, Kauler J, Szabo Z. Gait symmetry measures: a review of current and prospective methods. Biomed Signal Process Control . (2018) 42:89–100. doi: 10.1016/j.bspc.2018.01.013
124. Cimolin V, Galli M. Summary measures for clinical gait analysis: a literature review. Gait Posture . (2014) 39:1005–10. doi: 10.1016/j.gaitpost.2014.02.001
Keywords: clinical gait assessment, gait technologies, gait measures, mobile gait lab, gait pathologies
Citation: Hulleck AA, Menoth Mohan D, Abdallah N, El Rich M and Khalaf K (2022) Present and future of gait assessment in clinical practice: Towards the application of novel trends and technologies. Front. Med. Technol. 4:901331. doi: 10.3389/fmedt.2022.901331
Received: 21 March 2022; Accepted: 17 November 2022; Published: 16 December 2022.
Reviewed by:
© 2022 Hulleck, Menoth Mohan, Abdallah, El Rich and Khalaf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Kinda Khalaf [email protected]
Specialty Section: This article was submitted to Diagnostic and Therapeutic Devices, a section of the journal Frontiers in Medical Technology
This article is part of the Research Topic
Insights in Diagnostic and Therapeutic Devices
Clinical Gait Analysis: Characterizing Normal Gait and Pathological Deviations Due to Neurological Diseases
Affiliations.
- 1 SAMOVAR, Télécom SudParis, Institut Polytechnique de Paris, 9 Rue Charles Fourier, 91011 Evry, France.
- 2 Movement Analysis Laboratory, UGECAM Ile-de-France, 77170 Coubert, France.
- 3 Informatique, Bio-Informatique et Systèmes Complexes (IBISC) EA 4526, Université Paris-Saclay, 91020 Evry, France.
- PMID: 37514861
- PMCID: PMC10386217
- DOI: 10.3390/s23146566
This study addresses the characterization of normal gait and pathological deviations induced by neurological diseases, considering knee angular kinematics in the sagittal plane. We propose an unsupervised approach based on Dynamic Time Warping (DTW) to identify different normal gait profiles (NGPs) corresponding to real cycles representing the overall behavior of healthy subjects, instead of considering an average reference, as done in the literature. The obtained NGPs are then used to measure the deviations of pathological gait cycles from normal gait with DTW. Hierarchical Clustering is applied to stratify deviations into clusters. Results show that three NGPs are necessary to finely characterize the heterogeneity of normal gait and accurately quantify pathological deviations. In particular, we automatically identify which lower limb is affected for Hemiplegic patients and characterize the severity of motor impairment for Paraplegic patients. Concerning Tetraplegic patients, different profiles appear in terms of impairment severity. These promising results are obtained by considering the raw description of gait signals. Indeed, we have shown that normalizing signals removes the temporal properties of signals, inducing a loss of dynamic information that is crucial for accurately measuring pathological deviations. Our methodology could be exploited to quantify the impact of therapies on gait rehabilitation.
Keywords: 3D gait deviation; Dynamic Time Warping; clinical gait analysis; neurological diseases; normal gait characterization; unsupervised machine learning.
- Biomechanical Phenomena
- Lower Extremity
- Nervous System Diseases*
Grants and funding
- Study Protocol
- Open access
- Published: 14 May 2024
Can on-line gait training improve clinical practice? Study protocol for feasibility randomised controlled trial of an on-line educational intervention to improve clinician’s gait-related decision-making in ambulant children and young people with cerebral palsy
- Anna Hebda-Boon ORCID: orcid.org/0000-0002-7091-2828 1 ,
- Adam P. Shortland 2 ,
- Aleksandra Birn-Jeffery 3 &
- Dylan Morrissey 1 , 4
Pilot and Feasibility Studies volume 10 , Article number: 76 ( 2024 ) Cite this article
77 Accesses
Metrics details
Instrumented gait analysis (IGA) is an assessment and research tool with proven impacts on clinical decision-making for the management of ambulant children and young people with cerebral palsy (CYPwCP) but is underused and variably understood by relevant clinicians. Clinicians’ difficulties in gaining expertise and confidence in using IGA are multifactorial and related to access for clinical decision-making, limited training opportunities and inability to translate this training into clinical practice.
The primary aim of this study is to test the feasibility of an educational intervention to advance clinicians’ application of gait analysis in CYPwCP, to inform a definitive trial. The secondary aim is to measure the effect that appropriate IGA training has on physiotherapists’ knowledge, skills, confidence and behaviours. This will be a two-arm feasibility randomised controlled trial with an experimental and control group. The 6-week on-line intervention uses a multicomponent approach grounded in behavioural change techniques. A repeated measures design will be adopted, whereby participants will complete outcome measures at baseline, immediately after the intervention and at 4 months. The primary outcome measures (trial feasibility-related outcomes) are recruitment and engagement. The secondary outcome measures (trial research-related outcomes) are knowledge, skills, confidence and practice change. Outcome measures will be collected via online questionnaires and during observed skill assessments. Analysis of data will use descriptive statistics, two-way mixed ANOVA model and qualitative content analysis.
This study will determine feasibility of the definitive randomised control trial of educational intervention delivered to advance clinicians’ application of gait analysis in CYPwCP. This study offers the shift in emphasis from regarding IGA as a tool to a focus on clinicians’ requirements for access, training and a well-defined role to optimise utilisation of IGA. The impact of this should be better engagement with IGA and clinical practice change. This study will contribute to a body of educational research into clinical education of healthcare professionals and IGA training offering insight into high levels of evaluation evidence including clinical behaviour change.
Trial registration
Protocol has been registered with the Open Science Framework (osf.io/nweq6) in June 2023.
Peer Review reports
The National Institute for Clinical Excellence (NICE) refers to instrumented gait analysis (IGA) assessment as a preferable choice prior to gait-improving orthopaedic surgery [ 1 ]. The impact of IGA on decision-making in treatment planning and treatment outcomes for ambulant CYPwCP has been broadly debated in the literature particularly in areas of orthopaedic decision-making [ 2 , 3 , 4 , 5 ] and individually tailored nonsurgical treatments [ 6 , 7 ]. Generally, single event multilevel surgeries (SEMLS) are performed after IGA is conducted as the IGA results can help to determine which specific soft-tissue or bony surgical procedures should be performed [ 8 ]. Furthermore, studies show that use of IGA for treatment decision-making has potential to improve patient outcomes — authors indicate the positive gait-related outcomes and improvement in gait parameters when treatment matches IGA recommendations [ 9 , 10 , 11 ]. Despite this, more standardised access pathways for CYPwCP to IGA are yet to be established [ 7 , 12 ], and access to the IGA for other professionals involved in gait management such as physiotherapists or orthotists and their formal IGA education remains limited [ 13 ]. As a science, gait analysis brings a wide spectrum of knowledge and skills, making it hard to educate and successfully integrate it into undergraduate curricula [ 14 ]. Clinicians’ difficulties in gaining expertise and confidence in using IGA are multifactorial and can be related to lack of IGA access for clinical decision-making, limited training opportunities and inability to translate this training into clinical practice [ 15 ].
According to research, clinician-centred factors such as IGA training and affiliation to IGA laboratory [ 16 ] are shown to influence engagement with IGA-derived recommendations and may therefore impact on patient outcomes [ 17 ].
This indicates a required shift in emphasis from regarding IGA as a tool providing 3rd party recommendations to a focus on clinicians’ requirements for access, training and a well-defined role to optimise utilisation of IGA [ 17 ]. This is essential to address in order to improve inequity of access and patient outcomes. Findings of our previous research [ 15 , 17 ] provided context for the design and delivery of a feasibility randomised controlled trial (RCT) of an educational intervention to improve clinicians’ engagement with the IGA.
Study aims and objectives
The primary aim of this study is to determine the feasibility of an educational intervention to advance clinicians’ application of instrumented gait analysis in children and young people with cerebral palsy, to inform the design of a full trial. Objectives are as follows:
To establish the feasibility of a future randomised controlled trial of educational intervention.
Assess the rate of participant enrolment, retention and compliance with intervention.
Assess whether the inclusion and exclusion criteria for participants are appropriate.
Assess whether the duration of intervention is appropriate.
Assess whether intervention delivery in a virtual learning environment is feasible and acceptable.
Explore if the outcome measures are appropriate for the study aims.
Define the sample size for a definitive trial.
Explore the fidelity of intervention delivery.
Further understand the barriers and facilitators of the intervention.
The secondary aim is to measure the effect that appropriate IGA training and its delivery has on physiotherapists’ knowledge, skills and attitudes.
This feasibility trial protocol follows the SPIRIT statement on defining standard protocol items for clinical trials and its checklist [ 18 ] and the CONSORT statement extension to randomised pilot and feasibility trials and its checklist [ 19 ].
Trial design
This will be a two-arm feasibility randomised controlled trial with an experimental and control group. The 6-week on-line intervention delivered as part of the trial is a stand-alone, post-graduate level educational course called Virtual Gait Analysis Course for Paediatric Physiotherapists (VGAPP). Eligible physiotherapists who consent to take part in the study will be randomly allocated into experimental and control groups. A repeated measures design will be adopted, whereby participants will complete outcome measures at baseline, immediately after the intervention, and at 4 months. This will include collection of feedback as part of a full process evaluation.
The trial will be determined feasible if a priori set criteria based on primary outcome measures and included in the process evaluation will be achieved at or above agreed levels (see the ‘ Outcome measures ’ section of ‘ Methods ’). After conducting and reviewing outcomes of the full evaluation process, the decision about delivery of the definitive trial will be made.
Figure 1 shows the study flow diagram, and Table 1 indicates the schedule of enrolment, intervention, and outcome measures [ 18 ].
Study flow diagram
Participants
Study setting.
This study will be conducted virtually using Queen Mary University of London (QMUL) virtual learning environment (VLE), online questionnaires (SurveyMonkey), and Microsoft Teams, eradicating the need for participants to travel, reducing both cost and participants’ time. Participating clinicians will be working in a variety of settings (acute and community, special schools, both national health service and private settings) within the UK, where the data will be collected. Each participant’s data will be collected under their unique student number. To ensure anonymity, once data collection is complete, student numbers will be additionally coded.
Eligibility criteria
The aim of the inclusion and exclusion criteria is to ensure that participants are actively involved in assessment and treatment of ambulant CYPwCP and have currently available opportunities to apply the taught knowledge and skills in their workplace. The eligibility criteria were reviewed during the stakeholder focus groups including both clinicians and educators. Focus groups found inclusion and exclusion criteria appropriate for the feasibility trial (see Supplementary material).
Inclusion criteria are as follows:
18 years of age or older
Physiotherapists currently providing assessment and treatment to ambulant children and young people with cerebral palsy
Practicing within the UK (any National Health Service or private practice setting)
Exclusion criteria are as follows:
Outside of UK
Not currently employed as physiotherapist or on a career break
In rotational posts, where they could rotate to specialty not managing ambulant CYPwCP
Intervention
Design and refinement.
This educational intervention uses a multicomponent approach grounded in behavioural change techniques (BCTs). The overall aim of the intervention is to improve gait-related clinical practice.
Intervention (VGAPP) will be delivered via QMUL VLE and will comprise of pre-course resources and a 6-week course. Content of the VGAPP course has been developed based on evidence from the scientific literature, current best practice and informed by the scoping review [ 17 ], qualitative study [ 15 ] and results from a national survey of paediatric physiotherapists in the UK (unpublished, in review). Stakeholder engagement has been integral to the research and intervention design, delivery and evaluation process and included Patient and Families (PPI-A) interviews and Clinicians and Educators Focus Groups (PPI-B) (Fig. 2 ). PPI-A included children, young people and their families who have a lived experience of cerebral palsy and received IGA as part of management of their condition. PPI-A was involved in the design of intervention prior to involving clinicians in order to ensure that the project is centred around the needs of patients and to ensure that the practice behaviour change, and transfer of knowledge will directly benefit patients and their families. Themes, subthemes and illustrative quotes from patients and parents’ interviews and changes applied to the intervention and evaluation content are available in the Supplementary Table 1 . PPI-B were representatives from all UK nations, with a variety of paediatric physiotherapy specialisms, experience levels and from different work settings, thus providing invaluable insight and the opportunity for further refinement of the intervention design (in the areas of recruitment, eligibility criteria, sample size, control group intervention), content, delivery and evaluation methods. Themes, subthemes, and illustrative quotes from clinicians and educators focus groups and changes applied to intervention and evaluation content are available in the Supplementary Table 2 .

Stakeholder engagement
Through this process, several changes were implemented to the intervention content and assessment process in areas of communication, patient/family perspectives, orthotics, and linking elements of gait-related practice to the ICF domains. A detailed PPI involvement report, including the educational intervention refinement process is available from the corresponding author on reasonable request.
Pre-intervention resources
Pre-intervention resources will include the pre-course manual, ‘meet and greet’ forum and the reading list. Participants will be able to complete a self-diagnostic tool to identify and reflect on their current IGA engagement and barriers to confident gait-related practice.
Intervention components
The intervention will be a stand-alone, post-graduate level educational event delivered fully on-line. It will employ the delivery of weekly on-line plenary sessions incorporating active learning — synchronous on-line problem-based learning sessions and seminars integrating elements of experimental learning within the learning community. These sessions will be delivered by experienced educators and clinicians working in the instrumented gait analysis laboratories, with a track record of delivering education within the field of gait analysis and paediatric neurodisability. Educators will be approached via email by the lead researcher. Content of the intervention will encompass an array of gait analysis methods and an overview of equipment currently used in the clinical practice. This will include but will not be limited to clinical outcome measures, measurement software, videography techniques and setup, 3-dimensional motion laboratory equipment, and laboratory setup (examples of Vicon and Codamotion Systems). Intervention will comprise of weekly tasks (asynchronous) to facilitate revision and application in practice and formative assessment/feedback opportunities (short knowledge quizzes, open questions within the discussion forum) to support learning autonomy and facilitate participant’s recall and self-regulation. Table 2 provides an indicative number of hours for each activity to give an overall picture of the workload a participant would be expected to undertake.
The intended learning outcomes (ILOs) have been designed and benchmarked against the QAA Statements Physiotherapy (2001) Academic Content.
Academic content is as follows:
Demonstrates an understanding of the interdisciplinary knowledge that underpins gait analysis practice including elements of human anatomy, biomechanics, and gross motor development: C1
Demonstrates an understanding of the principles of typical gait pattern and how movement patterns are likely to be affected by some of the childhood diseases: C1
Demonstrates an understanding of the available measurement technologies and the principles on which they are based: C1
Disciplinary skills are as follows:
Applies variety of gait assessment methods in context of own practice and service delivery: B1 and C2
Uses the gait analysis outputs in clinical practice to aid treatment decision-making and measurement — in line with clinical reasoning paradigms and evidence-based practice: A1, B1, and C2
Communicates assessment findings and gait-related decision-making effectively with multidisciplinary team, patients, and families: A2, A3, B2, and C2
Attributes are as follows:
Cultivates an individualised, patient-centred approach to assessment and treatment planning: B2
Reflects on own practice to identify the needs within own role and wider aspects of service delivery: A3, A4, and B2 (health and social care equivalent B4)
Demonstrates a creative drive to implement the knowledge and skills, improve own practice, and support development of others: A3, A4, and B2 (health and social care equivalent B3)
Behaviour change techniques (BCT)
Utilisation of the BCT taxonomy [ 20 ] will support refinement of the targeted behaviours. It will also support the process evaluation analysis to gain understanding of how the change is expected to take place [ 21 ] and related barriers and facilitators of implementing the feasibility trial. To support knowledge transfer, several behaviour change techniques will be used in the intervention content.
Prior to the course, participants will gain access to a diagnostic session to identify potential internal and/or external barriers to their gait-related practice. They will be encouraged to set their personal and service goals and will be supported in making plans for delivery. Participants will share their plans and progress as part of the evaluation process.
A variety of synchronous (problem-based learning sessions) and asynchronous resources (lectures, reading links and podcasts) will incorporate instruction on how to perform new or refined gait-related practice behaviours. These resources will also support shaping of the participant’s knowledge through instruction and demonstration on how to perform the behaviours and setting clinically oriented practical tasks focusing on the behaviour. Throughout the course, participants will be provided strategies to support behaviours through associations such as regular prompts and cues, ideas on restructuring of their clinical environment to improve their gait assessment quality and techniques, or through objects which could be added into their environment (such as outcome measure templates — digital and/or printed). A virtual learning community, created through group chats and discussion forums, will aim to support emergence of the identity associated with changed behaviours.
Figure 3 outlines the simplified logic model of the study.
Feasibility RCT study logic model
Control group intervention
To compare the effects of the intervention against usual practice, participants allocated into the control group will be asked to continue with their usual practice. At the point of enrolment, the control group will gain access to the virtual learning environment and receive basic orientation resources, but no training or guidance will be offered during this time. Participants in the control group will be asked to complete the same measurements as those in the intervention group and at the same timepoints (Table 1 ). The control group will be offered the full intervention after the completion of the third round of assessments. Provision of educational content and its timing in the control group were reviewed during the stakeholder focus groups including of clinicians and educators.
Outcome measures
Outcome measures were grouped as primary outcome measures (trial feasibility-related outcomes) and secondary outcome measures (trial research-related outcomes) collated in Table 3 .
Recruitment will be determined as feasible if study is able to recruit 24 participants within 4 months [ 22 , 23 ]. Retention rates will be considered at two stages: (1) from expression of interest to consent — it will be deemed feasible if greater than 50%, and (2) from consent to course completion — it will be deemed feasible if greater than 75% [ 24 , 25 ]. Additionally, engagement (participants’ interactions with an online system) data will be collected during intervention via the analytics tools in the QMUL Virtual Learning Environment which log the detail of activity access, time, and completion for each component. These analytic tools are part of the general-purpose dashboard and provide an algorithmic representation of student online behaviours based on whether the behaviour occurred and for how long, rather than quality of these behaviours. Previous studies show that these analytics have been positively correlated with student performance [ 26 , 27 , 28 ]. It will be deemed feasible if the average proportion of completed learning sessions and tasks will be ≥ 66%.
Secondary outcomes are as follows: knowledge, skills, attitudes, and satisfaction will be collected via online questionnaires (SurveyMonkey) and during skill tests (OSCE). Knowledge, skills, and attitudes will be collected at three timepoints (Table 1 ).
Baseline (pre-intervention)
Questionnaire including background (demographics, current gait analysis practice, access to IGA equipment, barriers to gait analysis practice), attitudes (reasons for joining the study, anticipated changes in practice after the intervention, beliefs), confidence (self-rated), and knowledge (self-rated and multiple-choice question test)
Objective structured clinical examination (OSCE) of a patient case: Assessment will be delivered on-line, recorded and scored against a standardised scoring sheet including gait-related clinical reasoning and treatment planning based on evidence and findings, problem-solving, systematicity of approach, ability to link various types of gait-related information, confidence in engagement with gait data, analysis of gait graphs, communication (including use of gait-related terminology, providing lay explanations to a parent), and implementation of biopsychosocial model or ICF to decision-making
Post intervention (immediately after 6-week intervention)
Questionnaire including attitudes (planning practice change, implemented practice change, beliefs), confidence (self-rated), knowledge (self-rated and multiple-choice question test), and satisfaction (experimental group only)
OSCE of a different patient case (scored against the same criteria as at baseline)
Re-test (4-month post-intervention)
Questionnaire attitudes (planning practice change, implemented practice change, beliefs), confidence (self-rated), and knowledge (self-rated and multiple-choice question test)
Knowledge and skills retention as well as attitudes will be measured between timepoints, with a focus on changes between baseline and immediately post intervention and at 4-month follow-up. Satisfaction questionnaire will contain 28 items, each assessed on a 5-point Likert scale, related to the relevance and scientific quality of the content, the educational structure, and delivery. Satisfaction feedback will be collected immediately after intervention delivery (experimental group).
Sample size
Considering the study objectives and recommendations, the target sample size will be of a minimum 12 participants per trial arm; therefore, a minimum of 24 in total is anticipated. Guidance from the National Institute for Health Research (NIHR) indicates that a sample size of 30 is appropriate to answer the questions posed by a feasibility trial [ 23 ]. A lower number of participants will be better suited for an educational intervention for clinicians — it will ensure delivery of a high-quality learning experience and allow for active engagement with tutors during problem-based learning within the experimental group. Furthermore, the stakeholder focus groups including of clinicians and educators reviewed the proposed sample size and reported it as appropriate for the feasibility trial.
Recruitment
Participants will be recruited via the largest national paediatric physiotherapy network (Association of Chartered Paediatric Physiotherapists) using bulletins, social media, and targeted emails to team leads across the UK. The advertisement will provide general information about the intervention and the research study together with inclusion and exclusion criteria. Upon expression of interest, participants will be screened against eligibility criteria, and the participant’s information sheet and consent form will be sent to prospective participants via email. Participants will return signed consent forms electronically to the research lead. In line with advice from the Clinician and Educator Focus Group (PPI-B), the recruitment of study participants will commence early to ensure that participants are able to make suitable arrangements in the workplace, such as request study leave and ‘block time’ to attend synchronous sessions etc.
Participant timeline
Time schedule of enrolment, intervention, and assessments is presented in Table 1 . After the eligibility criteria screen and receipt of their written informed consent, 24 participants will be enrolled to the study. After random allocation to the trial arms, participants will receive access to the password-protected online platform hosted by Queen Mary University of London. All participants will be asked to complete the baseline assessment including the questionnaire (background, attitudes, knowledge, and skills) and objective structured clinical examination (OSCE) of a patient case (assessment will be delivered on-line, recorded, and scored against standardised scoring sheet). After completing the baseline assessment, participants assigned to the experimental arm will gain access to the pre-course learning resources (6 weeks prior to start of the course). The experimental group will commence the 6 week blocks of intervention including pre-recorded resources, problem-based learning tasks, discussion forums, and live sessions. At 6 weeks, participants from both arms will be asked to complete the second assessment including the questionnaire (attitudes, knowledge and skills, and satisfaction scores in experimental group only) and the second OSCE of a patient case. Four months after the intervention, participants in both trial arms will be asked to complete the third assessment including the questionnaire (attitudes, knowledge, and skills) and the OSCE of a patient case. Once all the data is collected, participants in the control group will gain access to the prereading resources and start the 6-weekly intervention sessions.
Assignment of intervention
Allocation, concealment mechanism, and implementation.
Participants who meet the inclusion criteria and return the consent form will be assigned an ID number in the Microsoft Excel spreadsheet. Participants will be assigned to groups randomly. In case there are more eligible physiotherapists than spaces, participants will be chosen by the number generation software which will be used in the allocation process. This will be conducted by an external person not related to the study or the research team. To avoid contamination, participants from the same healthcare trusts will be randomised to the same group.
Information about group randomisation will be provided in the participant’s information sheet. Participants in this study will not be blinded to the group allocation or deceived. This was discussed in the stakeholder focus groups who agreed that in the context of clinical practice, deceiving participants could mean a loss of their study/annual leave if pre-booked specifically to attend the intervention as well as potential cancelations of clinics in the control group. Participants will be informed about their allocation at the time of receiving instructions with the QMUL VLE platform access. At this time, the control group will be informed about timings of gaining their access to the full intervention and all resources provided to the experimental group after final assessments are completed. Participants will be informed that they are free to withdraw at any time without needing to provide a reason and with no penalties or detrimental effects.
Data collection, management, and analysis
In line with accepted practice for feasibility studies, no power analysis will be conducted, and all analyses will be exploratory only [ 29 ]. Data analysis will be performed after the last trial participant has completed final assessments (outcomes at 4 months post intervention). Data will be managed initially in Microsoft Excel software and analysed using IBM SPSS statistics software. Table 3 provides a summary of outcome measures, hypotheses, and analysis planned in the study.
Data management and research governance
A baseline table (descriptive statistics and frequencies) will compare the demographic and clinical characteristics including gender, age, experience, education, practice setting, contract type, study leave availability to participate in intervention, access to equipment, and gait analysis training. The primary outcomes will be reported using descriptive statistics. The quantitative variables will be presented as means and standard deviations.
A preliminary analysis of between-group differences will be conducted to determine the range of potential effect sizes from repeated measures ANOVA. Feasibility outcomes will be presented as number of participants meeting the a priori definitions. Kendall’s tau-b ( τ b ) correlation coefficient will be used to measure of the strength and direction of association that exists between two variables measured on at least an ordinal scale. To explore the extent and patterns of missing outcome data, we will report the proportion of missing values per item and the proportion of participants who will complete all items on the questionnaires. The proportion of missing data will also be reported for the other key outcomes and compared between the participants from intervention and control groups.
Qualitative data will be analysed according to the framework approach [ 30 ], a realist approach located within an interpretivist frame. The opinions and experiences of participants will be explored to understand any barriers and facilitators related to running of the educational intervention. During active familiarisation, the textual data will be coded, and codes will be organised into themes and subthemes to construct a thematic framework to aid indexing. To ensure rigour and consistency, the analysis process will undergo investigator triangulation. In this process, different observers, examiners, and analysts will compare and check data collection and/or interpretation [ 30 , 31 ]. Qualitative data will be presented as quotes and descriptive summaries.
Process evaluation and implementation outcomes
The process evaluation has been informed by Medical Research Council guidance on process evaluation of complex interventions [ 32 , 33 ] and the Implementation Outcome Framework (IOF) [ 34 ]. Proctor et al. described eight implementation outcomes in the IOF: acceptability, adoption, appropriateness, feasibility, fidelity, implementation cost, penetration (or coverage), and sustainability. Each of these implementation outcomes aligns with important considerations for trial design and implementation; however, the ‘adoption’ outcome does not directly align with process evaluation of our current feasibility trial design and delivery, as it is not offered by other educational providers. Therefore, seven out of eight implementation outcomes will be included in this process evaluation. Acceptability of the intervention and of the assessments will include data on the duration, content, and delivery methods (including satisfaction scores). Synthesis of satisfaction scores, feedback, and reports on participants’ logistics related to taking part in the trial (protected study time, ensuring opportunities in practice, assessment burden) will be carried out. The findings will be supplemented with observations made by the researchers, educators, administrative staff, and examiners throughout the implementation of the intervention. Collectively, these will provide information on the acceptability of the trial measurements and the intervention. Feasibility measures will include participant recruitment rate, retention, and engagement thresholds as described in the ‘ Methods ’ section. The process evaluation will include analysis of proportion of eligible participants being offered trial and, if possible, proportion of participants in the population represented by eligibility criteria (coverage).
Baseline comparisons will be conducted to detect any substantial differences between participants recruited from the control and intervention arms. Sample size and anticipated effect size defined for the definitive trial will be reviewed and assessed for feasibility. Participant withdrawals and number of participants lost to follow-up (and where possible reasons and participants’ key baseline characteristics) will be analysed. The study protocol adherence will be reviewed within the research team. Fidelity to the trial protocol including follow-up, dosage of the intervention, crossover between study arms, and adherence to intervention delivery plan will be assessed against study protocol and participant timelines. Any changes to the protocol will be reported.
Furthermore, appropriateness of the trial design for the trial aim, inclusion and exclusion criteria, outcome measures, and intervention components will undergo an exploratory analysis of participants’ outcomes, engagement with content, and assessments, together with qualitative analysis of participants and educators’ feedback. Sustained participant interest throughout the trial period and sustained staffing levels to deliver and facilitate participants’ learning journey during intervention will be explored to inform the sustainability criteria for the definitive trial. The implementation cost analysis will be explored with the aim to inform the design of a full cost-utility analysis alongside a future definitive trial. Implementation cost will include the cost of administration involved in running the trial and cost related to production and delivery of the intervention and assessment components — such as speaker fees, and OSCE examiners and moderators will be reviewed.
In addition, the COM-B model and the behaviour change techniques taxonomy (BCTT) [ 35 ], widely used frameworks in behaviour change and implementation research, will support the process evaluation analysis and an in-depth exploration of the barriers and facilitators of implementing the feasibility trial.
This article describes the protocol of a study evaluating the feasibility of conducting definitive RCT of the educational intervention for paediatric physiotherapists working with ambulant CYPwCP in the UK. This feasibility study was designed to assess predefined criteria related to the evaluation design (such as reducing uncertainty around recruitment, retention, choice of outcomes, analysis) and the intervention (its content and delivery, acceptability, adherence, cost-effectiveness, etc.) in line with the current guidance [ 32 , 33 ].
The educational intervention planned for this trial intends to integrate the complexity of knowledge, skills within the realities of clinicians’ practice to support knowledge translation to influence the practice behaviour change. Due to its complexity, the design of the study was preceded by in-depth research studies of the intervention’s context and implementation factors within the clinical practice reality of paediatric physiotherapists. This included close collaboration with stakeholders — patients and their families, clinicians, and clinical educators [ 33 ].
The need for gait analysis training was clearly identified in previous study of physiotherapists in the UK [ 13 ]. Despite extensive gait-related practice [ 36 , 37 ], evidence of how paediatric physiotherapists engage with instrumentation or access the IGA training is sparse. There are currently many gait-related courses available world-wide delivered by a variety of providers specifically targeting this clinical group (CMAS workshop 2023). Although there is a rich training offer, the impact of training on skills and behaviour, evaluation of needs, and barriers to knowledge transfer are not addressed in the current literature showing an evidence gap (CMAS 2023 education workshop). The impact of existing educational interventions is rarely reported [ 38 , 39 ] and concerns low levels of evaluation evidence, omitting evaluation clinical behaviour change or organisational impact. Our previous studies show that transfer of gait-related knowledge from the classroom to the clinic room also poses challenges to clinicians at different levels of practice expertise [ 15 ]. The lack of institutional resources (financial, such as availability of funding for staff’s training or limited study leave), spatial and temporal to promote implementation of new procedural skills and motivation to engage with learning, may also influence low uptake of professional training.
One of the main challenges will be associated with possible low uptake in the study and high drop-out rate. High work pressures and limited time to study may result in reduced opportunity or willingness to participate in the intervention and multiple assessments.
Limitations
Participants in this trial will not be blinded to allocation. After discussions within the research team and stakeholder focus groups, it was decided that if a participant secures study leave to take part in the 6-week intervention (potentially taking time off clinical work which may lead to cancellation of clinics) and would not receive the intervention due to allocation to the control group — this may result in loss of study leave and could have a potentially negative impact on the patient’s care by added waiting time.
The intervention lead is a paediatric physiotherapist experienced in gait-related practice which may be a source of potential bias. To mitigate this risk, multiple educators and clinical experts will be appointed to co-deliver the intervention, and additional examiners and moderators will be blinded to participants’ allocation. The intervention lead will keep a reflective diary and will have access to de-brief meetings within the research team [ 40 ]. Involvement of a considerable number of experts co-delivering the content of the intervention may pose risk to intervention integrity. To mitigate this risk, the intervention lead will be providing detailed 1:1 briefing about the study, targeted behaviours, session aims, and ILOs.
Generalisability
A relatively small sample planned for this feasibility study may pose questions regarding the applicability of findings to the future definitive trial and other studies. To ensure that the feasibility sample is representative of the UK paediatric physiotherapists, the study will be broadly advertised to reach therapists in all four UK countries and across the healthcare sectors.
Despite the extensive context research, a wide array of primary and secondary outcome measures planned to be used in the process evaluation, there may be factors influential to the trial but not be captured by the feasibility testing. Use of MRC guidance on process evaluation of complex interventions [ 32 , 33 ] and the IOF [ 34 ] will ensure thorough investigation of the change mechanisms and how the effects will occur [ 32 , 41 ]. Furthermore, the COM-B model and BCTT [ 35 ] are useful tools to characterise the targeted behaviours and content of educational interventions focused on continuing professional development in healthcare [ 42 ]. These were used throughout design of the study and will support the process evaluation to further advance understanding of their mechanisms of action.
With the detailed planning of this protocol and careful consideration of challenges and limitations, this study will offer essential preliminary data about the feasibility of implementing the VGAPP intervention to improve gait-related practice of paediatric physiotherapists in the UK. Study findings will provide a comprehensive understanding of whether a full randomised control trial is viable and identify any areas which could be enhanced. Furthermore, this study will contribute to a body of educational research into clinical training of healthcare professionals and IGA training.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Abbreviations
Behaviour change techniques taxonomy
Clinical Movement Analysis Society
Capability, opportunity, motivation, behaviour model
Consolidated Standards of Reporting Trials for Pilot and Feasibility trials
Children and young people with cerebral palsy
International Classification of Functioning, Disability and Health
- Instrumented gait analysis
Intended learning outcomes
Implementation Outcome Framework
Medical Research Council
National Institute for Clinical Excellence
Objective structured clinical examination
Patient and public involvement
Standard Protocol Items: Recommendations for Interventional Trials
Virtual gait analysis course for paediatric physiotherapists
Virtual learning environment
NICE. Clinical guideline [CG145] Spasticity in under 19s: management. https://www.nice.org.uk/guidance/cg145/chapter/1-guidance2016 .
Wren TA, Lening C, Rethlefsen SA, Kay RM. Impact of gait analysis on correction of excessive hip internal rotation in ambulatory children with cerebral palsy: a randomized controlled trial. Dev Med Child Neurol. 2013;55(10):919–25.
Article PubMed Google Scholar
Theologis T, Stebbins J. The use of gait analysis in the treatment of pediatric foot and ankle disorders. Foot Ankle Clin. 2010;15(2):365–82.
Kay RM, Wren TA, Bowen RE, Otsuka NY, Scaduto AA, Chan LS, et al. Influence of gait analysis on decision-making for lower extremity surgery. Dev Med Child Neurol. 2009;51:1.
Google Scholar
Lofterod B, Terjesen T, Skaaret I, Huse AB, Jahnsen R. Preoperative gait analysis has a substantial effect on orthopedic decision making in children with cerebral palsy - comparison between clinical evaluation and gait analysis in 60 patients. Acta Orthop. 2007;78(1):74–80.
Franki I, De Cat J, Deschepper E, Molenaers G, Desloovere K, Himpens E, et al. A clinical decision framework for the identification of main problems and treatment goals for ambulant children with bilateral spastic cerebral palsy. Res Dev Disabil. 2014;35(5):1160–76.
Rasmussen HM, Pedersen NW, Overgaard S, Hansen LK, Dunkhase-Heinl U, Petkov Y, et al. Gait analysis for individually tailored interdisciplinary interventions in children with cerebral palsy: a randomized controlled trial. Dev Med Child Neurol. 2019;61(10):1189–95.
McGinley J, Dobson F, Ganeshalingham R, Shore B, Rutz E, Graham HK. A systematic review of single event multilevel surgery for children with cerebral palsy. Dev Med Child Neurol. 2012;54:54–5.
Article Google Scholar
Wren T, Otsuka N, Bowen R, Scaduto A, Chan L, Dennis S, et al. Outcomes of lower extremity orthopaedic surgery in ambulatory children with cerebral palsy with and without gait analysis: results of a randomised controlled trial. Gait Posture. 2013;38:236–41.
Gough M, Shortland AP. Can clinical gait analysis guide the management of ambulant children with bilateral spastic cerebral palsy? J Pediatr Orthop. 2008;28(8):879–83.
Chang FM, Seidl AJ, Muthusamy K, Meininger AK, Carollo JJ. Effectiveness of instrumented gait analysis in children with cerebral palsy–comparison of outcomes. J Pediatr Orthop. 2006;26(5):612–6.
Gaston MS. CPIPS: musculoskeletal and hip surveillance for children with cerebral palsy. Paediatr Child Health. 2019;29(11):489–94.
Toro B, Nester C, Farren P. The status of gait assessment among physiotherapists in the United Kingdom. Arch Phys Med Rehab. 2003;84(12):1878–84.
Baker R, Esquenazi A, Benedetti MG, Desloovere K. Gait analysis: clinical facts. Eur J Phys Rehabil Med. 2016;52(4):560–74.
PubMed Google Scholar
Hebda-Boon A, Zhang B, Amankwah A, Shortland AP, Morrissey D. Clinicians’ experiences of instrumented gait analysis in management of patients with cerebral palsy: a qualitative study. Phys Occup Ther Pediatr. 2022;42:1–13.
Wren TA, Elihu KJ, Mansour S, Rethlefsen SA, Ryan DD, Smith ML, et al. Differences in implementation of gait analysis recommendations based on affiliation with a gait laboratory. Gait Posture. 2013;37(2):206–9.
Hebda-Boon A, Tan XL, Tillmann R, Shortland AP, Firth GB, Morrissey D. The impact of instrumented gait analysis on decision-making in the interprofessional management of cerebral palsy: a scoping review. Eur J Paediatr Neurol. 2023;42:60–70.
Chan AW, Tetzlaff JM, Altman DG, Dickersin K, Moher D. SPIRIT 2013: new guidance for content of clinical trial protocols. Lancet. 2013;381(9861):91–2.
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. Pilot Feasibil Stud. 2016;2:64.
Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46(1):81–95.
Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ. 2000;321(7262):694–6.
Article CAS PubMed PubMed Central Google Scholar
Jacques RM, Ahmed R, Harper J, Ranjan A, Saeed I, Simpson RM, et al. Recruitment, consent and retention of participants in randomised controlled trials: a review of trials published in the National Institute for Health Research (NIHR) Journals Library (1997–2020). BMJ Open. 2022;12(2):e059230.
Article PubMed PubMed Central Google Scholar
Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract. 2004;10(2):307–12.
Trivedi RB, Szarka JG, Beaver K, Brousseau K, Nevins E, Yancy WS Jr, et al. Recruitment and retention rates in behavioral trials involving patients and a support person: a systematic review. Contemp Clin Trials. 2013;36(1):307–18.
Poongothai S, Anjana RM, Aarthy R, Unnikrishnan R, Narayan KMV, Ali MK, et al. Strategies for participant retention in long term clinical trials: a participant -centric approaches. Perspect Clin Res. 2023;14(1):3–9.
Jayaprakash SM, Moody EW, Lauría EJM, Regan JR, Baron JD. Early alert of academically at-risk students: an open source analytics initiative. J Learn Analytics. 2014;1(1):6–47.
Macfadyen L, Dawson S. Numbers are not enough. Why e-learning analytics failed to inform an institutional strategic plan. Educational Technology & Society. 2012;15(3):149–63.
Atif A Froissard C, Liu DY, Richards D. Validating the effectiveness of the moodle engagement analytics plugin to predict student academic performance. Americas Conference on Information Systems. in 21st Americas Conference on Information Systems, AMCIS 2015 (pp 1-10).
Teresi JA, Yu X, Stewart AL, Hays RD. Guidelines for designing and evaluating feasibility pilot studies. Med Care. 2022;60(1):95–103.
Ritchie J. Qualitative research practice: a guide for social science students and researchers. 2nd ed. London: Sage; 2014.
Elliott R, Fischer CT, Rennie DL. Evolving guidelines for publication of qualitative research studies in psychology and related fields. Br J Clin Psychol. 1999;38(3):215–29.
Article CAS PubMed Google Scholar
Moore GF, Audrey S, Barker M, Bond L, Bonell C, Hardeman W, et al. Process evaluation of complex interventions: medical research council guidance. BMJ. 2015;350:h1258.
Skivington K, Matthews L, Simpson SA, Craig P, Baird J, Blazeby JM, et al. Framework for the development and evaluation of complex interventions: gap analysis, workshop and consultation-informed update. Health Technol Assess. 2021;25(57):1–132.
Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76.
Michie S, Atkins L, West R. The behaviour change wheel. A guide to designing interventions. UK London.: Silverback publishing; 2014.
Franki I, Desloovere K, De Cat J, Feys H, Molenaers G, Calders P, et al. The evidence-base for basic physical therapy techniques targeting lower limb function in children with cerebral palsy: a systematic review using the international classification of functioning, disability and health as a conceptual framework. J Rehabil Med. 2012;44(5):385–95.
Rapson R, Latour JM, Marsden J, Hughes H, Carter B. Defining usual physiotherapy care in ambulant children with cerebral palsy in the United Kingdom: a mixed methods consensus study. Child Care Health Dev. 2022;48(5):708–23.
Malone JB, Burns JD, Belthur MV, Karlen JW. Motion laboratory gait analysis and orthopedic resident education: preliminary results. J Pediatr Orthop B. 2022;31(1):e65–8.
Baskwill A, Belli P, Keller L. Evaluation of a gait assessment module using 3D Motion capture technology. Int J Ther Massage Bodywork. 2017;10(1):3–9.
PubMed PubMed Central Google Scholar
Korstjens I, Moser A. Series: practical guidance to qualitative research. Part 4: Trustworthiness and publishing. Eur J Gen Pract. 2018;24(1):120–4.
O’Cathain A, Croot L, Duncan E, Rousseau N, Sworn K, Turner KM, et al. Guidance on how to develop complex interventions to improve health and healthcare. BMJ Open. 2019;9(8):e029954.
Konnyu KJ, McCleary N, Presseau J, Ivers NM, Grimshaw JM. Behavior change techniques in continuing professional development. J Contin Educ Health Prof. 2020;40(4):268–73.
Download references
This study is funded by Private Physiotherapy Education Fund grant.
Author information
Authors and affiliations.
Sport and Exercise Medicine, Barts and the London School of Medicine and Dentistry, Queen Mary University of London, London, UK
Anna Hebda-Boon & Dylan Morrissey
School of Biomedical Engineering and Imaging Science, King’s College London, London, UK
Adam P. Shortland
School of Sport, Rehabilitation and Exercises Sciences, University of Essex, Essex, UK
Aleksandra Birn-Jeffery
Physiotherapy Department, Barts Health NHS Trust, London, UK
Dylan Morrissey
You can also search for this author in PubMed Google Scholar
Contributions
All authors made substantial contributions to the conception, design, data acquisition, analysis, and delivery of the protocol. All authors contributed to manuscript preparation.
Corresponding author
Correspondence to Anna Hebda-Boon .
Ethics declarations
Ethics approval and consent to participate.
The project has been granted ethical approval of the Queen Mary Ethics of Research Committee (reference number: QMERC20.531).
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary material 1., supplementary material 2., rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Hebda-Boon, A., Shortland, A.P., Birn-Jeffery, A. et al. Can on-line gait training improve clinical practice? Study protocol for feasibility randomised controlled trial of an on-line educational intervention to improve clinician’s gait-related decision-making in ambulant children and young people with cerebral palsy. Pilot Feasibility Stud 10 , 76 (2024). https://doi.org/10.1186/s40814-024-01477-5
Download citation
Received : 09 August 2023
Accepted : 12 March 2024
Published : 14 May 2024
DOI : https://doi.org/10.1186/s40814-024-01477-5
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Gait analysis
- Cerebral palsy
- Physiotherapy
- Paediatric physiotherapy
- Educational intervention
- Clinical education
- Randomised controlled trial
Pilot and Feasibility Studies
ISSN: 2055-5784
- Submission enquiries: Access here and click Contact Us
- General enquiries: [email protected]
- Quick Links
Tools & Resources
- Events Calendar
- Strauss Health Sciences Library
- Department A-Z Directory
- Campus Directory
- Faculty & Staff Resources
- Supporter & Alumni Resources
- Student Resources
- Mental Health Resources
- University Policies
CU Campuses
Cu anschutz medical campus.
- CU Colorado Springs
- School of Dental Medicine
- Graduate School
- School of Medicine
- College of Nursing
- Skaggs School of Pharmacy and Pharmaceutical Sciences
- Colorado School of Public Health
Department of Orthopedics
Center for gait and movement analysis (cgma).

James Carollo, PhD, PE

The Center for Gait and Movement Analysis
Children's Hospital Colorado's Center for Gait and Movement Analysis holds the distinction of being the sole accredited clinical gait and movement analysis laboratory in its region, standing as one of just 14 accredited gait labs across the nation.
Publications
Our work in practice, surgical outcomes in cerebral palsy, nonsurgical interventions in cerebral palsy, quality of life in individuals with cerebral palsy, methodological advances in cerebral palsy , applications of motion capture beyond cerebral palsy and collaborations, 2014-15 active projects.
1. Determining the Natural History of the Femoral Physeal Angle in Normal Children During Development
Collaborators: Chang FM, Miller NH, Kolnik A, Carry P, Merritt C, Pan Z & Davis J
2. Outcomes of Varus Derotational Osteotomies for Hip Dysplasia in Children with Cerebral Palsy and Predictors for Re-subluxation
Collaborators: May A, Chang FM, Novais E, Faulk LW, Miller NH, Pan Z, Davies K, Kark J, Ma J
3. Acetabular Remodeling After a Varus Derotational Osteotomy (VDRO) in Children with Cerebral Palsy
Collaborators: Chang FM, Novais E, Pan Z, Ma J, Ingram JD
4. The Relationship Between Hip Disease and Scoliosis in Children with Cerebral Palsy
Collaborators: Garg S, Chang FM, Miller NH
5. Results of Patellar Advancement Procedures for the Treatment of Crouch Gait in Patients with Cerebral Palsy (Collaboration with MRC)
Collaborators: Chen Q, Rhodes J, Hotchkiss M, Robertson D, Pritchard B, Frickman A
6. Effective of Age at Surgery on Distal Femoral Remodeling Following Extension Osteotomies in Children with Cerebral Palsy
Collaborators: Rhodes J, Roy D, Pan Z, Chang F, Pritchard B, Frickman A
7. Retrospective Comparison of Allograft versus Bovine Xenograft in Evan’s Calcaneal Osteotomy for Planovalgus Foot Deformity in Cerebral Palsy
Collaborators: Chang FM, Mansour A, Davies K, Pritchard B, Miller N
8. Comparison of Graft Materials Used in the Evans Calcaneal Lengthening in Children with Cerebral Palsy
Collaborators: Chang FM, Rhodes J, Miller N, Davies K, Pritchard B, Autruong P
9. Rectus Femoris Manuscript
Collaborators: Rhodes J, Carollo JJ, Chang FM, Friesen R, Pritchard B
10. Correction of Ankle Valgus in Children Using a Transphyseal Medial Malleolar Screw
Collaborators: Chang FM, Hoversten L, Ma J, Novais E
11. Clinical Outcomes and Biomechanical Assessment of Pes Planovalgus in Children with Cerebral Palsy
Collaborators: Chang FM, Look N, Carollo J, Ma J, Autruong P
12. A Comparison of Treatment Methods for Femur Fractures in Patients Affected by Cerebral Palsy
Collaborators: Chang FM, Tulk K, Autruong P
13. Timing of intensive strengthening and immobilization following Strayer, Percutaneous TAL and Open TAL procedures in patients with hemiplegic cerebral palsy
Collaborators: Rhodes JT, Harris N, Hutchinson B, Frickman A
14. Biomechanical Assessment of Three Patellar Advancement Procedures
Collaborators: Carollo J, Seidl A, Rhodes J, Baldini T
15. Upper extremity kinematics in hemiplegic cerebral palsy before and after wrist tendon transfer
Collaborators: Mayo M, Scott F, Faussett M, Carollo J
16. Evaluation of Upper Limb Motor Control in Children with Hemiplegic Cerebral Palsy after Combined Botulinum Toxin – A Injections and Constraint-Induced Movement Therapy: A Pilot Study
Collaborators: Oleszek J, Valvano J, Kenyon P, Denniston N.
17. Effect of Adapted Skiing and Snowboarding on the Motor Function and Endurance of Children with Physical Disabilities
Collaborators: Chang FM, Davies K, Valvano J, Kanai S, Faulk W, Pritchard B, Carry P Carollo JJ, Ma J
18. Musculoskeletal Simulation Guided Therapy for Children with Cerebral Palsy
Collaborators: Carollo J, Silverman A, UNMC, Omaha, NE
Quality of life and health outcomes
19. Walking and its Effect on Health and Function in Inpiduals with Cerebral Palsy as they Transition to Adulthood: A Health Outcomes Study
Collaborators: Carollo JJ, Hein P, Valvano J, Bodkin A
20. Cerebral Palsy Transition to Adulthood: Systematic Review
Collaborators: Robertson DM, Heyn P, Carollo JJ, Valvano J
21. Effects of Adapted Skiing and Snowboarding On Quality Of Life of Children With Physical Disabilities
Collaborators: Chang FM, Davies K, Hotchkiss M, Prichard B, Ma J, Autruong P
Methodological advances in cerebral palsy
22. Changes in Coordination and Functional Outcomes after the Rectus Femoris Transfer Procedure in Children with Spastic Cerebral Palsy
Collaborators: Valvano J, Worster K, Carollo JJ, Pan Z, Davies K, Ma J
23. Apparent Equinus Gait in Children with Cerebral Palsy, A Quantitative Analysis
Collaborators: Rhodes J, Halgrimson W, Davies K, Sinha, Carollo JJ, Pritchard B, Frickman A
24. An Analysis of Leg Length Discrepancies in Children with Hemiplegic Cerebral Palsy
Collaborators: Rhodes J, Chang FM, Pritchard B, Dudevoir M, Frickman A
25. Inter-Segmental Coordination and Ankle-foot Orthoses during Gait by Children with Spastic Cerebral Palsy
Collaborators: Carollo JJ, Valvano, J, Worster K, Robertson D
26. The Effect of Pelvic Positioning on Radiographic Measurements Used in Treating Hip Dysplasia in Children with Cerebral Palsy
Collaborators: Carollo JJ, Chang FM, Novais E, Robertson DM, Ma J, Ingram JD, Autruong P
27. Data Base Setup For Developing Gait-Cycle Indexed Gait Performance Score
Collaborators: Pan Z, Carollo JJ, Denniston N, Worster K
28. Coordination Dynamics of Walking – Dissertation
Collaborators: Worster K, Carollo J, Valvano J
29. IMove: Instrumented Movement Analysis to Quanitfy Gait in Cerebral Palsy
Collaborators: Carollo J, Bodkin A, Robertson D, Frickman A
30. Gait Deviation Index Representative Gait Cycle Selection in Cerebral Palsy
Collaborators: Sauer C, Carollo J
31. Impact of Spinal Fusion Construct with Sacropelvic Fixation on Gait Dynamics
Collaborators: Carollo JJ, Erikson MA, Miller NH, Nicklas T, Hotchkiss M, Hogy S, Robertson DM
32. Impact of VEPTR (Vertical Expandable Prosthetic Titanium Rib) Surgery on Chest Wall Dynamics
Collaborators: Erickson MA, Carollo JJ, Nicklas T, Miller NH, Nguyen T, Hotchkiss M, Robertson D
33. Is There a Correlation Between Clinical and Radiographic Measures in Scoliosis?
Collaborators: Garg S, Carollo JJ, Robertson DM, Niswander C
34. Manual Task Analysis Discerns Limitations in Motor Control in Children with Down Syndrome
Collaborators: Valvano J, Worster K, Denniston N, Winders P, Hogy S, Carollo JJ, Ma J
35. An Investigation in Validating a Six Degree of Freedom Knee Model with Applications in Total Knee Replacement Stability in Downhill Walking
Collaborators: Cucuel C, Carollo JJ
36. Stability as an Assessment Tool for ACL Repair Comparison
Collaborators: Burnim K, Carollo JJ, Albright J, Terhune E, Sochanska A
37. Anterior Knee Pain in Adolescents (Collaboration with MRC)
Collaborators: Provance A, Carry P, James D, Kanai S, Coel R, Mooney R
38. Evaluating the Functional Muscle Units in Van Ness Rotationplasties
Collaborators: Heare T, Silverman A, Donaldson N, Mooney R, Carollo JJ, Kanai S, Hogy S, Wylie E, Beebe C
39. Lower limb Alignment and Biomechanics after Derotational Femoral Osteotomy
Collaborators: Rhodes JT, Mei-dan O, Kanai S, Frickman A
40.Validity and Reliability of 2D versus 3D gait analysis for kinematic measures during running gait
Collaborators: Nagle K, Rhodes J, Kanai S, Sremba M, Frickman A
Featured Research:
Cerebral Palsy Adult Transition Study
Contact the Center for Gait and Movement Analysis
For appointments at Anschutz Medical Campus or South Campus, call 720-777-5805 .
All appointments for the Gait Lab require a referral from a patient’s primary doctor. The doctor must complete the gait analysis referral form. Download the Gait Analysis Referral Form.
Orthopedics (SOM)
CU Anschutz
Academic Office One
12631 East 17th Avenue
Aurora, CO 80045
720-848-1900
- Anschutz Medical Campus
- Adult Reconstruction
- Upper Extremity
- Foot & Ankle
- Sports Medicine
- Orthopedic Oncology
- Make an Appointment
- Pediatric Orthopedics
- Trauma & Fracture
- Find a Doctor
- Patient Care
- News & Media
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Integrating a gait analysis test in hospital rehabilitation: A service design approach
Javier marín.
1 IDERGO (Research and Development in Ergonomics) Research Group, I3A (Aragon Institute of Engineering Research), University of Zaragoza, Zaragoza, Spain
2 Department of Design and Manufacturing Engineering, University of Zaragoza, Zaragoza, Spain
Teresa Blanco
3 HOWLab (Human Openware Research Lab) Research Group, I3A, University of Zaragoza, Zaragoza, Spain
4 GeoSpatiumLab, S.L. Zaragoza, Spain
José J. Marín
Alejandro moreno.
5 Department of Health and Sports Sciences, University of Zaragoza, Zaragoza, Spain
Elena Martitegui
6 Rehabilitation and Physical Medicine Service, HUMS (Miguel Servet University Hospital), Zaragoza, Spain
Juan C. Aragüés
Associated data.
All relevant data are within the manuscript and its Supporting Information files.
Gait analysis with motion capture (MoCap) during rehabilitation can provide objective information to facilitate treatment decision making. However, designing a test to be integrated into healthcare services requires considering multiple design factors. The difficulty of integrating a ‘micro-service’ (gait test) within a ‘macro-service’ (healthcare service) has received little attention in the gait analysis literature. It is a challenge that goes beyond the gait analysis case study because service design methods commonly focus on the entire service design (macro-level).
This study aims to extract design considerations and generate guidelines to integrate MoCap technology for gait analysis in the hospital rehabilitation setting. Specifically, the aim is to design a gait test to assess the response of the applied treatments through pre- and post-measurement sessions.
We focused on patients with spasticity who received botulinum toxin treatment. A qualitative research design was used to investigate the integration of a gait analysis system based on inertial measurement units in a rehabilitation service at a reference hospital. The methodological approach was based on contrasted methodologies from the service design field, which materialise through observation techniques (during system use), semi-structured interviews, and workshops with healthcare professionals (13 patients, 10 ‘proxies’, and 6 doctors).
The analysis resulted in six themes: (1) patients’ understanding, (2) guiding the gait tests, (3) which professionals guide the gait tests, (4) gait test reports, (5) requesting gait tests (doctors and test guide communication), and the (6) conceptual design of the service with the gait test.
Conclusions
The extracted design considerations and guidelines increase the applicability and usefulness of the gait analysis technology, improving the link between technologists and healthcare professionals. The proposed methodological approach can also be useful for service design teams that deal with the integration of one service into another.
Although we are not conscious of its complexity, gait is a complex activity for human beings. It requires high motor control, and its pathologies have a harmful effect on personal autonomy and daily life activities [ 1 ]. Gait analysis with motion capture (MoCap) technology in the usual clinical practice is called ‘clinical gait analysis’ [ 2 ]; it is considered an important measurement in the rehabilitation field, where decision making on numerous treatments and interventions can benefit from objective information on the patient’s walking pattern [ 3 – 7 ]. Clinical gait analysis is especially important in the treatment of hemiparetic individuals after a stroke (or other causes), because they experience numerous impairments in walking skills that are reflected in the gait pattern [ 8 ].
In this regard, clinical gait analysis based on MoCap technology can be defined as the instrumented measurement of movement patterns that comprise walking and the associated interpretation of these patterns[ 2 ]. A MoCap instrumentation that is extensively used in biomedical research is optical technology (gold standard), which tracks the position of reflective surface markers with infrared cameras. Another instrumentation is technology based on inertial measurement units (IMU), which are electronic devices that measure rotations (rotation matrices, Euler angles, quaternions, etc.) by processing the signal of embedded sensors (accelerometers, gyroscopes, and magnetometers) [ 9 – 13 ]. Regarding their differences, optical technology is more accurate than IMU technology, which could present drift errors. However, it requires a camera infrastructure and sometimes presents shadowing problems [ 14 ]. In contrast, the IMU technology is more economical and portable and has been recently used in wearable technology [ 15 ], which could encourage cloud data processing and information exchange in the context of the Internet of things [ 16 ].
MoCap gait analysis is widely used in clinical research; however, certain factors have prevented the spread of this technology in hospital rehabilitation [ 17 , 18 ]. Nowadays, gait evaluation relies mostly on observational criteria. These systems involve high technical complexity, requiring the application of a strict protocol for accurate and repeatable measurements [ 2 , 19 , 20 ]. The massive amount of information they provide requires complex processing methods [ 8 , 21 , 22 ]. Currently, the professionals involved in this type of analysis must be highly qualified [ 2 ], which conflicts with the present needs for simplicity, usability, and intuitiveness [ 23 ].
Many of these factors have been identified by the cited researchers; however, despite being relevant, the difficulty of the ‘servitisation’ of gait analysis has received little attention in the literature [ 15 ]. Because of the challenges that servitisation poses, it should be added as an additional research objective.
Designing an integrated test in the biomedical field is particularly complex because multiple stakeholders have different needs. Solutions that involve all users (patients, doctors, therapists, etc.) in the context of the technology are required to make it useful, cost-effective, and truly usable [ 24 ]. This includes family members and people close to patients (proxies), who are especially affected by the situation that the patient experiences [ 25 , 26 ]. Therefore, we address a problem that goes beyond the development of the technology by investigating how to apply a certain technology (gait analysis) to a specific context (hospital rehabilitation services).
If we assume that the design object is a service and not only the technology that measures movement, the use of specific service design techniques [ 27 , 28 ] will allow us to predict failures, extract critical points, and consider the intangible and contextual part of the product [ 15 ]. These methods are aligned with the patient-centred care (PCC) philosophy, which is a priority in the healthcare field [ 29 ] and shares its philosophy with human-centred design (HCD) [ 30 ]. Despite the uncertainty in health services (timing, diagnoses, resources per patient, etc.), these methods are relevant for professionals to know exactly what their roles are and to work in a more optimal and orderly manner. To achieve this, the involvement of users in the design process is key [ 31 ].
However, these methods, although highly flexible, are commonly focused on the entire service design [ 27 , 30 ]. Service design methods and the theoretical basis of service-dominant logic [ 32 ] aim to guide service innovations and are focused on the perspective of the whole company. Wetter et al. [ 33 ] identified this situation as a ‘macro-level’ approach and asserted that, in some cases, it is not clear how to act at a more operational level with a ‘micro-level’ approach or how to guide specific actions and projects to favour service innovations. Thus, contributions are needed to connect design methods with real situations and problems, producing pragmatic, empirical, and micro-level approaches.
Based on this terminology, we face a scenario in which we incorporate what we call a ‘micro-service’ (a gait analysis test) within a more complex ‘macro-service’ (rehabilitation services). In terms of service design, this situation has particularities and implications that are essential to consider. How can the gait test be included in the hospital as an additional medical test? What additional materials must be designed, developed, or adapted for the gait test? How do the patient’s capabilities influence the design and guidance of the gait test? Which professionals will guide it? Is there a lack of certain professionals in the macro-service? In this regard, qualitative studies can provide a meaningful view of the perspectives, opinions, and priorities of the users (patients, healthcare professionals, and proxies) and reveal the underlying conceptual structure of their existing and/or desired interactions [ 34 – 36 ].
This article qualitatively evaluates a MoCap gait analysis system during its integration in a hospital rehabilitation environment, constructing the research through design methods. We focus on a specific case study of neurological patients with spasticity in the lower limbs who are treated with botulinum toxin. Thus, we present design considerations and guidelines for the improvement and adaptation of the system, which can be extrapolated to other scenarios at both the service design and hospital environment levels. In the results section, the guidelines are structured into six main themes. Finally, in the discussion section, we discuss the advantages and benefits that an integrated gait analysis test would introduce in rehabilitation services.
A qualitative research design was used to provide design considerations and guidelines to integrate the MoCap technology for gait analysis in a hospital rehabilitation service. The methodological approach was based on contrasted methodologies from the field of service design, which were materialised through observation techniques (during system use), semi-structured interviews, and workshops with healthcare professionals. A sample of 29 participants was analysed (13 patients, 10 ‘proxies’, and 6 doctors). The reporting of the results follows the Consolidated Criteria for Reporting Qualitative Research (COREQ) statement [ 37 ] ( S1 Table ).
Paradigmatic position
Within the paradigms ‘that guide disciplined research’ [ 38 ], the paradigmatic position of this research is in the field of interpretivism (aligned with qualitative research). In interpretivism, ‘reality’ is constructed in people’s minds and can be clearly understood through an interactive dialogue between the researcher and participant [ 39 ]. The interpretivism paradigm and the qualitative methods are naturally closer than quantitative methods to design practice [RW.ERROR—Unable to find reference:453]. According to Blanco et al. [ 40 ], not considering the qualitative perspective of end users could lead to suboptimal solutions in product and service designs. In our case, the research question of this study is concerned with providing an understanding of how to integrate MoCap gait analysis technology into the rehabilitation field according to the end user’s knowledge, experience, and expectations.
The experimental study was conducted after the formal approval of the local ethical committee (Bioethics Committee of Aragón Spain, CEICA; Act No. 12/2018). The study was conducted in accordance with relevant ethical guidelines, including a verbal explanation and written informed consent from the participants.
The gait analysis system
The MoCap system used in this study was the Move-Human Sensors system developed by the IDERGO research group, which includes a module for gait cycle analysis [ 15 , 41 ]. The first version of the system was implemented in 2007. Since then, it has been used in numerous public and private projects both in hospitals (healthcare field) and companies (ergonomics field). The system has been incorporating the concerns of the professionals involved in the projects (engineers, doctors, ergonomists, prevention technicians, etc.).
The measurement validity of the MoCap system is largely determined by the accuracy of the sensors it uses. In this case, it is based on wireless IMUs, specifically, the NGIMUs devices [ 42 ], which are placed on the patient’s body with elastic bands. The NGIMU sensors are calibrated by the manufacturer. They filter and process the signal internally to directly send the rotation information. The NGIMU sensors have been used and assessed in numerous publications, which have guaranteed their accuracy, as can be found on the manufacturer’s website [ 43 ].
The gait test of this study is aimed to be used as a medical test based on pre- and post-measurement sessions for the applied rehabilitation treatments. The generated reports show the changes that have occurred in each patient’s gait between the pre- and post-sessions. These reports can be used to make decisions about the treatments (continue treatment, change to another, increase the intensity, etc.). In this regard, it should be noted that this study does not focus on the gait report, how it is designed, or what information it contains. Instead, we present a more global perspective of the service design. However, because the gait report design is a relevant research issue, we have considered it in the fieldwork to extract participants’ expectations and research opportunities.
Hospital setting
The research setting was the Rehabilitation and Physical Medicine Service of the Miguel Servet University Hospital (HUMS) of Zaragoza, Spain, a reference hospital in the country. This service provides rehabilitative assistance to return the highest degree of functional capacity and independence to the patient as possible, favouring family, social, and work reinsertion. It is organised into areas of hospitalisation, outpatient consultation, and therapy (physiotherapy, occupational therapy, hydrotherapy, electrotherapy, and speech therapy).
Pathology and treatment
We were focused on evaluating the gait of patients with spasticity when they receive treatment with botulinum toxin. The choice of these patients makes it possible to extrapolate the results to other patients with more favourable physical or cognitive conditions. Spasticity is a symptom that affects a large group of patients after suffering from neurological damage. Negative effects include pain, decreased mobility, contractures, and muscle spasms, which can interfere with daily life activities and sleep to a greater or lesser degree. The causes are diverse; some of the best-known causes are stroke [ 44 ], multiple sclerosis [ 45 ], post-traumatic brain damage [ 44 ], spinal cord injury [ 46 ], cerebral palsy, amyotrophic lateral sclerosis, and polyradiculitis. In HUMS, more than 850 patients were admitted with stroke pathology in 2017, and 75% of these cases required attention and subsequent follow-up.
The botulinum toxin treatment aims to treat focal spasticity via muscle infiltration with reversible paralytic action after 4 to 6 months [ 44 , 47 , 48 ]. Although there are other possible treatments for spasticity, for this study, the observed efficacy, personalised patient needs (dose, muscles to be inoculated, etc.), relatively high cost, and widespread use of the treatment justify the treatment choice. The gait test can aid the doctor in decisions [ 49 ] regarding (1) continuing to apply the treatment, (2) detecting whether the infiltrated muscles are adequate, and (3) maintaining or modifying the dose.
Study design
Discover, define, design, and develop are the most common phases of service model development [ 28 ], constituting the global structure and philosophy that we follow. However, in this paper, as claimed in the literature [ 33 ], we delved into the specific methods related to our case study, providing the methodology design, reasoning, and how the specific methods were used and applied. Consistently, we contextualised the study design through a theoretical framework based on methods endorsed by the scientific community.
Our scenario started from an existing gait test based on IMU technology. In this context, we proposed a methodological approach to qualitatively assess three dimensions of the test, which gave rise to our research phases: (1) the user-product proximity effect, (2) the effect on and value in the service, and (3) user interactions. The theoretical framework of this approach was based on the following contrasted methodologies from the field of service design and HCD (which, as we have seen, is related to the PCC philosophy):
- The general vision was based on the Octopus methodology by Marin et al. [ 15 ], which aims to define design specifications to create MoCap devices from three points of view: product, software (information analysis), and service. As the design object is a service, this study focused on this approach.

- Finally, Phase 3 was based on Community, which Blanco [ 26 ] proposed as a methodology for the design of complex services with interrelationships between multiple users. Here, it was adapted to a workshop format for professionals from the health sector.
Table 1 shows the construction of the research methodology. Three assessment dimensions (phases) led to concrete research questions, which were answered with different user profiles (participants) through observation techniques, semi-structured interviews, and workshops.
Regarding the participants mentioned in Table 1 , the relationship established with the doctors was possibly due to previous meetings in which they showed a shared interest in integrating a gait analysis system into their service. A relationship was established with the patients with the six doctors in Table 1 , who conducted the patient rehabilitation and recruited them face-to-face during the consultation. The interest in improving rehabilitation through gait analysis was communicated to the patients and proxies. No one refused to participate in the study.
The sample size of patients and proxies (phase 1) was determined by the concept of saturation , which was defined by Glaser and Strauss [ 50 ] and has been widely used in qualitative research. Saturation has been reached when adding more participants to the study does not generate additional insight or information. In this way, the measurement sessions were repeated until saturation was reached with 13 patients and 10 proxies. The sample size of doctors was six (Phases 1, 2, and 3), which corresponds to the number of physicians involved in the analysed rehabilitation service and the applied treatment.
In the following sections, each phase of the methodology is explained in depth, including the specific research objectives, participants, and study design.
Phase 1: User-product proximity effect
Phase 1 aims to learn from observing the use of the MoCap system in its context. The strategy in this step was to perform the gait test in the rehabilitation service, carrying out an observation focused on understanding the effect on the involved actors (patients, proxies, and professionals).
There were 26 observation sessions carried out with 29 users of different profiles: the patients ( n = 13) who performed the test, the proxies ( n = 10) who (in some cases) accompanied the patients and observed the test from nearby, and the doctors of the service ( n = 5 rehabilitation specialist doctors and 1 resident doctor) who were free to observe the gait test and talk with the patients or proxies. Each of the 13 patients with spasticity (7 men and 6 women; average age = 45.9 ± 19.8 years) underwent the gait test twice. The first test was performed a few minutes before receiving the botulinum toxin treatment, and a follow-up was performed a month later.
The patients had been diagnosed by the rehabilitation service of the hospital in the evaluation consultation and were selected for the study by meeting the following inclusion criteria:
- The patient can walk autonomously.
- The patient presents a dynamic or reducible contracture that alters motor function.
- A reduction in spasticity is expected to lead to a functional improvement with the treatment, according to the doctor’s previous experience.
- The patient has the possibility of receiving periodic controls to learn patterns of movement at home or complementary physiotherapy treatment.
- The patient is included in the indications of the technical sheet approved by the Spanish Agency of Medicines.
Table 2 includes the patient’s characteristics. The gait speed at the beginning of the patient’s evaluation has been included because it is an important indicator to represent the general state of health and is related to impairment, functionality, mobility, independence, autonomy, and comorbidity levels [ 51 , 52 ].
According to the statement terminology of the COREQ [ 37 ], the sampling method was purposive in the case of the patients because they met the inclusion criteria and were of diverse ages and disease levels, according to the doctor’s criteria. Proxies were recruited through the snowball method because they were dependent on the patient section. Doctors were also purposively sampled because they represented different profiles (two heads, three specialists, and a resident doctor).
The gait test was carried out using two ‘test guides’, one engineer (JM), who managed the computer, and a physiotherapist (AM), who guided the patients and interacted with them. In each measurement session, the patients were instrumented with the MoCap sensors ( Fig 2 ) and walked naturally 6 m in a straight line at a self-selected speed. When the distance was completed, the patient turned around and walked back in the opposite direction. This operation was repeated to measure up to 25 strides. Only strides in a straight line were used, discarding any turns and start and stop zones. The duration of each test was 20 to 25 minutes.

The individual in this picture has given written informed consent (as outlined in the PloS consent form) to publish these case details.
The observations during the test were carried out by the test guides. It was established that observers should focus on the following factors or dimensions: (1) physical and cognitive abilities (patients), (2) motivation (patients), (3) concentration (patients), (4) reactions to the test and technology including what they said, what they did, and how they responded (patients, relatives, and doctors), and (5) operational and technical problems (test guides). Field notes were made during the observation. Audio and visual recordings were not used because the pre-defined observation dimensions were considered enough to address the research objectives.
Phase 2: Effect and value in the service
Designing a system that aims to advise professionals in the rehabilitation process requires going beyond what happens only in the test session. Phase 2 evaluates the effect and value of the micro-service within the HUMS macro-service, seeking to obtain an overview of the general path followed by the patient according to the diagnostic and care decisions.
Regarding the participants involved in this phase, owing to their deep understanding of service performance, the participants were two doctors who were familiar with the gait test: (1) the head of the neurological rehabilitation section and (2) the head of the rehabilitation service.
First, a face-to-face semi-structured interview with the user (1) was conducted, addressing the issues collected in Table 3 . This allowed us to develop the first version of a graphic map that represents the flow of clinical decisions of the service with the new test introduced in it.
Following a ‘combination’ strategy [ 40 ], in the second session, a workshop was held with both users (1 and 2) to evaluate the map of the service proposed after the first interview. The users had the opportunity to redefine and improve it to ensure that it reflected the entire process. The duration of each session was 40 minutes. They were pilot tested and conducted by JM and AM who recorded the audio.
Phase 3: User interactions
We assume that we are facing a complex and multi-user information network. For this reason, Phase 3 aims to define the information flows (connections) that must exist between the macro-service users for an adequate implementation of the gait test. These connections should allow the test implementation and should cover the relational needs of the Community methodology [ 17 ], improving the user experience, acceptance, and clinical effectiveness of the new gait test.
A workshop was held twice in succession with six doctors from the rehabilitation service, as shown in Table 1 , first with three doctors and later with another three others. They were pilot tested and conducted by JM and AM. The duration of each workshop was 100 minutes, and the audio was recorded.
The workshop challenge was ‘How could we develop a useful gait test for the rehabilitation service?’. The main task involved collaboratively drawing the users’ relational needs by connecting user groups using arrows (unidirectional or bidirectional) that represent information exchanges and/or interactions. To facilitate the process, pre-designed cards with icons of the involved professionals had been prepared. Initially, the organisers structured some user groups and connections as an example. The schemes created by the doctors during the sessions are shown in Fig 3 . In the second workshop, to reach a consensus between both workshops, following a ‘combination’ strategy [ 40 ], the researchers inquired about the differences from the map developed in the first session.

Data analysis
Observational notes of Phase 1 and the audios of Phases 2 and 3 were transcribed and coded using the thematic analysis approach [ 53 ]. The full transcription was read several times separately by JM, JJM, and TB to identify differences and similarities of the content. Similar content was underlined in the same colour, and a descriptive concept (category) was assigned to each colour. Afterwards, the researchers discussed their reflections. Once a consensus was reached, the latent content and implicit messages of each category were described in the results section. According to the COREQ statement [ 37 ], the identified categories were derived from the data (i.e. they are inductive). To improve the understanding of the maps from Phases 2 and 3, they were laid out as simply and perceptibly as possible. It was necessary to hear the audios repeatedly to include all the comments in the maps, not just the handwritten information. Microsoft Word and Excel were used to manage the data, and Illustrator was used to create the figures.
Trustworthiness
To achieve scientific rigour in qualitative research, Guba and Lincoln [ 38 ] proposed including a section about the trustworthiness of the interpretations. An integral aspect of trustworthiness is maintaining a detailed audit trail. Thus, we recorded our reflective memos and analysis decisions throughout the study. Additionally, in this paper, we provide clear and thick descriptions of the context, data collection, and analysis process, which favour the reproducibility of the study. To consider different perspectives and avoid bias, members of the research team represented different professions, and three of them independently identified and agreed on the categories presented in the results section. Finally, the resulting maps from Phases 2 and 3 reduced the subjective nature of the paper because they are tangible and factual objects proposed by the participants, who constitute an additional perspective as experts on their own experience in the environment [ 31 ].
From the field research, design considerations and guidelines have been obtained and grouped in the following sections: (1) patients’ understanding, (2) guiding the gait test, (3) which professionals guide the gait tests, (4) gait test reports, (5) requesting gait tests (doctors and test guide communication), and the (6) conceptual design of the service with the gait test.
Patients’ understanding
The extreme diversity of the patients is evidenced. One of the most repeated phrases among doctors is ‘each patient is a whole world’ on a physical and cognitive level and in terms of the care that each one requires.
- The accessibility level of the gait test should be maximised .
Most patients receive other types of therapy than just those received in the hospital, such as therapies in elderly centres, associations, or private centres: ‘I've been going to rehabilitation since I can remember’. They are aware of their limitations and are realistic about their situation: ‘You can notice evolution for a while, but then you stabilise at a certain level’. ‘In winter, I can feel my muscles [are] more contracted’. However, we also observe how doctors try to lower the expectations that the proxies sometimes have with botulinum toxin therapy. They indicated that rehabilitation objectives that are too high or optimistic can lead to frustration and disinterest in the rehabilitation process.
- It should be assumed that not all patients will be able to recover their previous functional capacity. They usually know their own limitations. We should take this into account and be honest in relation to their chances of recovery .
Those who have decided to participate in this study go to rehabilitation voluntarily and have a clear motivation to recover; however, the degree and origin of this motivation are very diverse. Some of them transmit their willpower with their daily habits, ‘every morning I walk around the block’. ‘I am always trying to improve, stretching and moving, for example, while I cook or while I dress’. Those who are older show that they have recovered the motivation they had lost: ‘Recently, we went to the neurologist, and he explained the treatment to us. We have been encouraged to try. We had been disconnected from this world for many years’. In the youngest patients, motivation, strength, and interest usually come from family members, who are the protagonists in the interactions with the test guides and express their curiosity regarding new treatments and techniques.
- The motivation of the patients and proxies should be exploited and maximised as much as possible. The gait test should become a motivating and engaging element whether the results are positive or negative .
Guiding the gait test
How and who performs the test can be keys to its success. The test guides should ensure that patients walk with their usual pattern. In Fig 4 , we see the movement curve irregularities of (a) a patient who is not accustomed to walking in the test and (b) those of the same patient who became accustomed to walking in the test after crossing the corridor two or three times.

Femur angle with respect to the hip in the sagittal plane. (a) Before and (b) after the patient adapted to walking using the usual pattern.
As a general rule, the concentration level of the patients during the test was high. They were previously silent, struggling, and looking at the ground as they walked. Even those with limited cognitive and communicative ability understood the explanations and satisfactorily executed the test guide instructions. However, there are factors that affect the concentration and are necessary to consider to accelerate the tests and avoid incidents that would require discarding the results.
The patient’s concentration is improved with a calm and constant environment without sudden noises, foot traffic, or conversations. Patients can sometimes lose concentration, become scared, and even stop walking when someone suddenly enters the room, when there is a slam, or when staff or family members converse, more so if the conversation deals with issues related to the patient. To create this environment, the test guides must empathise with the patient and their relatives, using appropriate language with clear, concrete, and predictable instructions, while avoiding technical terminology (e.g. using anatomical calibration functions: ‘Now I need you to be very still, like in a picture’).
- The role of the test guides is especially relevant. They should be able to extract the usual walking pattern of the patients. Access should be restricted to the room to achieve a calm and favourable environment during the performance of the test .
During the tests, the patients displayed different emotions and reactions from showing fear of pain to satisfaction or gratitude. Considering these reactions can provide value from the user-experience point of view. Below, the reactions are sorted in the order of appearance along the process.
- Mistrust, does it hurt? One of the first reactions and doubts that arose in both patients and family members was regarding whether the test was painful. They worried about whether the devices give electric shocks or punctures. Many of them had suffered from various painful treatments, and these doubts could be a reason for the initial rejection.
- Arriving late, anxiety . Some were nervous about being late for the tests, either due to not finding the room, transportation difficulties, or the schedules of the relatives who accompanied them. The doctors indicated that sometimes patients present anxiety for these reasons, and they must spend the first minutes of the consultation session reassuring them. Nonetheless, this was not the case in this study.
- Fatigue . Some patients, owing to their physical condition and especially their age, asked to rest as soon as they reached the room. In these cases, the sensors were placed and removed while they were sitting.
- Feeling observed . Once we placed the sensors, we saw how the patients joked with their relatives. Others, especially the younger ones, presented a certain shyness and appeared to feel uncomfortable, exposed, and observed.
- Gratitude . At the end of the test, gratitude reactions often arose: ‘It is a very difficult disease. It is comforting to see how people are working on this. We will help with everything that we can’. It is important that they feel they are part of the technological evolution in this field.
- Bond of trust in the second session . As a general rule, on the second day, the patients were more confident. They interacted more and had a greater link with the test guides. The most noticeable effect occurred in the young patients, who, as mentioned, were uncomfortable and shy on the first day.
- The test guides should explain the test properly and act effectively towards the different reactions that patients may present. Behaviour guidelines for test guides should be established .
Which professionals should guide the gait tests?
Due to the test characteristics, doctors concluded that two people are needed to run the test: one to guide the patient (place and remove sensors and give instructions to the patient) and another to operate the computer. Different health professionals have adequate training and capacity (doctors, nurses, physiotherapists, occupational therapists, etc.). According to healthcare professionals, in our environment, the test would be carried out by the nursing section: ‘Nursing is accustomed to doing this type of test; it falls within their competence’. ‘Certainly, there are tests performed by a doctor, for example, an endoscopy, but in that case, you are getting inside a human being. This test has nothing to do with that’.
Likewise, tests could also be performed by physiotherapists. These professionals are the most interested (along with doctors) in obtaining objective information on the gait pattern because they also apply therapies aimed at rehabilitating walking; however, the availability of these professionals is reduced, at least at HUMS. The final decision rests on the service head, on the professional availability of each hospital, and on the derived costs.
- The test should be conducted by two trained healthcare professionals. It would be feasible for nurses or physiotherapists to conduct the test. The decision depends on each service .
Gait test reports
Doctors have a negative perception of the results provided by the test. They consider the test to be far from their area of knowledge and difficult to interpret, requiring a high learning curve. They called it ‘the test of the engineers’, in some cases, showing concern and anxiety regarding who would interpret the data and how much time would be necessary to do so.
- The gait analysis test should provide a brief and easily interpreted report. Results must be communicated to patients in an understandable and personalised way to make more consensual decisions .
Defining the content and design of the test report is one of the challenges that must be addressed. According to the doctors’ opinion, the first test should serve to assess the functional state of the patient and, together with the rest of the information and inputs (clinical exploration, interview with the patient, clinical history, etc.), establish the rehabilitation objectives. Regarding the second test, they affirm that it should measure the treatment effects and allow the assessment of the achievement of the initial objectives: ‘We could try to assess if the patient has improved above what is expected, at what is expected, or below what is expected’.
- Future research should serve to detect the most representative and useful information for clinical decision making .
- With the first test, the rehabilitation objectives are established. With the second test, whether the treatment effects were as expected is assessed .
A general statement was made that the test report results should be available in digital format for consultation between the professionals involved in a patient’s rehabilitation. Consequently, the report should be uploaded and incorporated into the patient’s electronic medical record. In this regard, HUMS has its own intranet and another at the regional level. In our research, we refer to the electronic medical record without differentiating between these two. In this sense, the communication vehicle between doctors and therapists is the electronic rehabilitation plan, which allows prescribing treatments and tracking the patient by the involved physicians (renamed the electronic monitoring plan once the treatment begins). Uploading the gait test report to the electronic medical record would improve the doctor-therapist communication so they could share considerations about the results through the electronic rehabilitation plan and take more consensual decisions based on objective information.
- The doctor should be able to upload the gait test report in digital format to the electronic medical record .
Requesting gait tests: Doctors and test guide communication
Doctors agreed that it is not possible to run the test during consultation time. According to them, ‘it is the same as an X-ray or a blood test; I prescribe it, and I get the results back’. One of the questions that emerged in the workshops was how a doctor could request a test from the consultation. In this regard, doctors proposed the creation of a new agenda called the ‘gait test agenda’ with specific schedules and professionals assigned that would be shared between doctors and test guides and completed in the consultation.
- To cite prescribe patients to perform the test, it is proposed to create an electronic ‘gait test agenda’ shared between doctors and test guides .
Conceptual design of the service with the gait test
Based on the specifications obtained in the previous sections and according to the specific outputs of Phases 2 and 3 of the methodology ( Table 1 ), two schemes are presented that represent a conceptual proposal of the rehabilitation service that includes the new gait test in its operation. The first scheme, called the actuation flowchart ( Fig 5 ), shows the circuit of decisions that physicians must face during the spasticity rehabilitation process. The figure shows two different areas shaded in grey: consultation and intervention. A patient will go through both areas iteratively as many times as necessary. It also shows the gait test, which is accessed through two entryways:

Figure elaborated by authors. Icons made by Freepick, Pause08, Pixel perfect and Srip from www.flaticon.com.
- First gait entry . The test can be prescribed from a consultation in which the doctor considers that further information is needed. In this case, the test would have a diagnostic purpose. This option is parallel to the current possibility of requesting information from other services (e.g. an interconsultation request to obtain a psychological report).
- Second gait entry . It is possible to incorporate the gait tests during the intervention process, as long as it is planned from the consultation. In this case, the test would have a monitoring function. This option, based on pre- and post-treatment measurement sessions, is the one planned (methods section) to assist clinicians during the rehabilitation process.
Note that an item drawn with an empty circle indicates that the patient will undergo the process only if it is prescribed by the responsible physician (e.g. a patient may receive therapy sessions but not receive medical and nursing treatment sessions).
The second scheme, developed following Community [ 26 ], represents the connections between the user profiles that interact with the gait test and its results ( Fig 6 ; arrows from 1 to 7). Dark arrows represent the information flows that need to be designed and implemented. Light coloured lines represent the macro-service connections, which our design will not influence, but it will have a certain influence on the gait test integration. The represented users are grouped according to the ‘network spaces’, areas of conceptual interaction with different objectives and themes where users communicate and interactions can take place (e.g. gait test session, management, etc.). We see how a user can be present in several ‘network spaces’.

Figure elaborated by authors. Icons made by Freepik, Smartline, Mynamepong, Pause08 and dDara from www.flaticon.com.
Likewise, in the scheme, the materials to be developed are established (graphic documents, electronic agenda, cloud, etc.), which are also called ‘touch points’, that is, tangible or intangible elements that are in contact with the users. In line with the theory of service design, two conceptual interaction areas are also included, ‘backstage’ and ‘onstage’ [ 54 ]. Backstage is the invisible part for the user, where the processes that articulate the services take place. Onstage is the visible part that encompasses the target users.
Note that ‘developers’ have been included as temporary participants in the process because, although their permanent presence could be useful, their role during the first stages will be to process the information manually. Once the project evolves, and it is decided which information is the most adequate to facilitate the clinical evaluation, this process can be automated, and these users can be eliminated.
This study highlights an underlying need for MoCap gait analysis technology. Although it can generate individual objective outcome measures in usual clinical practice [ 55 ], further research is required for its effective and useful clinical application. In this framework, we apply a research methodology that starts from a specific concept: to design a gait test integrated into the hospital setting that provides objective information to support decision making regarding the rehabilitation process. We focus on the case study of neurological patients with disorders derived from spasticity who receive treatment with botulinum toxin. We have conducted a field investigation introducing a gait analysis system into the HUMS environment and evaluated it through observation, interviews, and workshops. As a result, design considerations have been obtained that can be useful for design teams that face these types of challenges in the future. Among these considerations, the micro-service (gait test) integrated into the macro-service (hospital rehabilitation service) has been conceptually defined through two infographics.
We assume that the proposed concept involves an extra effort for hospitals (economic and resource investment), for professionals (more workload and learning), and for patients and proxies (more visits to the hospital). However, the gait test would provide a substantial improvement in the quality of care, encouraging professional development and collaborative research among physicians. Likewise, the system requirements are relatively simple. With a short test duration and a short preparation time (20–25 min per patient), it does not require an exclusively dedicated room. For patients, it is not an invasive test, and it does not entail visiting centres other than the hospital where they usually receive treatments. The advantages and benefits that it would bring in rehabilitation services are described below:
- Sustain decisions at a clinical level . Having a test that provides objective data on the patient therapy response allows more precision in the decision-making process, including the option of not further intervening.
- Sustain decisions at the administrative and social levels . Inevitably, physicians are under pressure that comes with human responsibility, schedules, efficiency requirements, legal disputes, or limitations in resources.
- Improve treatment profitability . Some treatments entail significant costs to the public or private health entities involved, as in the case of treatment with botulinum toxin. The gait test can avoid applying those treatments that do not provide positive effects to certain patients.
- Facilitate communication between physicians . The test could unify gait evaluation and enhance the transmission of knowledge among health professionals.
- Positive feedback for the therapist . Objective information allows the clinical plan to be personalised for each patient, establishing more realistic objectives and generating a theoretical basis that could improve their evolution.
- Positive feedback for the patient . It may involve extra motivation for patients, encouraging confidence in the treatments and a greater perception of healthcare safety and trust.
- Improve medical and patient communication . Properly presented information can establish a doctor-patient communication pathway, improving the reasoning of patients (and relatives) and improving treatment decision making.
- Feed databases . Collecting gait information may have a practical effect to create predictions for new patients and facilitate diagnoses through machine-learning techniques.
- Enhance research among physicians . It can improve the collaborative research work among physicians, allowing the validation of new or modified treatments. Data from the database can be filtered for each investigation.
The mentioned benefits coincide with some concepts that McHorney and Tarlov [ 56 ] generically described for the healthcare field sector. They provide ways in which data from individual people in healthcare can be used, including to describe a patient’s health state, to monitor disease progression, or to standardize interactions between health care practitioners and patients [ 56 ]. In this regard, Cella et al. [ 57 ] summarised the concepts from McHorney and Tarlov [ 56 ] into two possible perspectives or uses that have similarities with the two gait test entries that we propose in Fig 5 . The first use (similar to the first gait entry) is clinical decision making under uncertainty: ‘estimating the likelihood that a patient will profit from a given intervention’. The second use (similar to the second gait entry) is clinical evaluation: ‘examining whether a given treatment makes a meaningful difference for an individual patient’.
Beyond the benefits that MoCap gait analysis would bring in rehabilitation services, this paper has two main strengths or contributions. First, it provides design considerations and guidelines to integrate a gait analysis test based on MoCap technology in the rehabilitation hospital setting, which is a novel contribution and can encourage the use of this technology in this field. In this regard, Martin et al. [ 58 ] encouraged researchers to realise studies that propose design recommendations based on HCD, especially in scenarios where users (in our case patients) are heterogeneous (as we see in patients with hemiplegia). This reasoning supports the presented results.
The second strength is proposing a reproducible methodological approach to integrate a ‘micro-service’ (gait test) within a ‘macro-service’ (rehabilitation service), which advances service design knowledge and may be applied to other case studies or other technologies. This methodological approach had great acceptance among physicians. They ensured that the sessions where they participated were useful, practical, and different from the usual way of working: ‘I see these types of sessions and workshops as essential. It is useful to clean up many useless connections and make them simpler. I like it [the map]; it’s clean and illustrative’.
The importance of properly designing the rehabilitation service is manifested in numerous investigations that seek to assess the patient’s experience (e.g. [ 59 , 60 ]). In this context, service design and the theoretical basis of service-dominant logic [ 32 ] have great importance. They allow one to address stakeholder’s needs and strengthen professional relationships. However, as Han et al. [ 28 ] indicated, it is necessary to consider the specific needs of the clinical environment when applying service design techniques. This is translated to our specific case study in the necessity of integrating a ‘micro-service’ (gait test) within a more complex ‘macro-service’ (rehabilitation service). The scenario of the micro-service integration has particularities that differentiate it from a mere application of service design methods, which concern the design of the service as a whole. In return, we focus our design on ‘one link in the chain’, a new gait test in a hospital rehabilitation service. According to Wetter et al. [ 33 ], this contributes to improving the theoretical framework of the service design because it is not actually clear how to act at a ‘micro-level’ approach to connect design methods with real situations and favour service innovations.
Additionally, certain weaknesses or limitations have been identified in this study. It should be noted that we focus on a relatively specific case (gait tests for patients with spasticity in a particular rehabilitation service) and on a limited sample of professionals who know the service and the treatment used. Thus, if one intends to integrate this or another similar system into another hospital, it should be considered that other centres may be different at the organisational level. Some concepts (mentioned throughout the results section), such as agendas, the involved personnel, the rehabilitation plan, or the patients under study, may be different; thus, certain sections may be adapted to the particular case. In this regard, it may be useful to rely on the research methodology presented, especially in Phases 2 and 3.
Another limitation concerns the (1) gait report (including processing backwards) and the (2) measurement validity of the MoCap system. Although they are not part of the objectives of this study, they are key questions for the system design to achieve successful integration in a hospital. Both topics have been extensively discussed in the literature, and we encourage further investigation. In this sense, this paper is focused on the service design, which complements and strengthens this area of research.
Additionally, it should be noted that this study is interpretivist and the research techniques used are qualitative. This intrinsically implies that the participants are those who experience, process, and label the ‘reality’ under investigation and transmit it to the researcher [ 39 ]. To do this, the participants rely on their individual experience, memories, and expectations [ 38 ]. This prevents the total objectivity of the study and complete neutrality.
In summary, although we focused on a relatively specific case (gait tests for patients with spasticity in a particular rehabilitation service), the proposed design recommendations are partially generalisable to other treatments or health centres. Additionally, the proposed methodology can be useful and reproducible to obtain design considerations to integrate a micro-service into a macro-service.
Nowadays, healthcare technology is developing more rapidly and efficiently and requires fewer resources. This research contributes to improving the application of this type of system and other Internet of things devices [ 16 ] that can be developed in the healthcare field of ‘smart health’ [ 61 , 62 ]. It is expected that future health devices (micro-services) could be more useful to hospital services (macro-services) by maintaining their use over time and improving their acceptance.
We conducted a field investigation introducing a gait analysis system into a hospital rehabilitation environment and evaluated it through observation, interviews, and workshops. We focused on patients with spasticity who received treatment with botulinum toxin. The main conclusion is that integrating a gait test into hospital rehabilitation services is beneficial for physicians, patients, and proxies, but to make it really applicable and useful, some design specifications should be considered. The design specifications and the methods applied to obtain them can be useful for both technology developers and healthcare professionals who seek to improve the quality of healthcare services.
Supporting information
Acknowledgments.
The authors would like to acknowledge and thank Dr Nicolas Rivas, Dr Ricardo Jariod, Dr Inmaculada Salcedo, and Dr M. Teresa Zarraluqui for their suggestions, involvement in the project, and their interest. Likewise, the authors would like to thank all participants who voluntarily participated in this study.
Funding Statement
The authors received no specific funding for this work.
Data Availability

IMAGES
VIDEO
COMMENTS
Clinical gait analysis remains highly observational and is hence subjective and largely influenced by the observer's background and experience. ... has recently emerged as an efficient, convenient, and most importantly, inexpensive option to quantitative gait analysis for both clinical and research-based applications (Figure 3). In general, it ...
Clinical gait analysis has taken great leaps forward over the past five decades. A gait assessment in 1973 looked very different from today. The equipment was larger and more cumbersome, the set-up time took longer, the collection and analysis of the data were laborious, and the output far sparser.
Interest in video-based, markerless gait analysis has accelerated rapidly. Previous studies have used various approaches to move quantitative clinical gait analysis outside of the laboratory or research center and directly into the home or clinic [5,6,13-15,17]. Here, we aimed to develop a single approach that addressed several outstanding ...
1. Introduction. The appropriate role of gait analysis in clinical care remains controversial. Proponents argue that gait analysis provides important information needed to optimize the care of patients with complex walking problems [1].Opponents counter that, although gait analysis is a useful tool for research, as a clinical tool it adds unnecessary cost without providing any proven benefits ...
Clinical observational analysis of gait alone seems to be not enough to establish an accurate "diagnosis" of gait abnormalities, which differs among the ND etiologies. ... Data collected from these devices could improve the quality of gait assessment and the strength of research results in the neurorehabilitation field. Thus, collaborations ...
To perform survey on research work in gait analysis. ... Clinical Gait Analysis can be defined as a technique that deals with diagnosing hidden impairments and can affect gait patterns. Fig. 5 shows the gait abnormalities. Table 5 shows a different type of gait abnormalities. Healthcare practitioners can apply the gait segmentation technique in ...
Clinical Gait Analysis tackles the characterization of pathologies through quantitative measures computed on gait signals. ... In addition, our research has focused primarily on the knee joint. It is necessary to consider other joints, such as the hip and ankle, in order to attain a more complete understanding of the biomechanics involved in ...
When brain damage occurs, gait and balance are often impaired. Evaluation of the gait cycle, therefore, has a pivotal role during the rehabilitation path of subjects who suffer from neurological disorders. Gait analysis can be performed through laboratory systems, non-wearable sensors (NWS), and/or wearable sensors (WS). Using these tools, physiotherapists and neurologists have more objective ...
Gait is a complex, whole-body movement, as each subject has its own biological characteristics, and a deviation in the patterns of walking can be an indication of various diseases. 1-3 Studying and analyzing gait in a medical context can contribute to the diagnosis of pathologies that affect gait. 4,5 However, marker-based gait analysis requires a dedicated gait lab with specialized equipment ...
Background: This paper updates our 2011 systematic review on the clinical efficacy of three-dimensional instrumented gait analysis (3DGA). Research question: What is the current evidence base pertaining to the clinical efficacy of 3DGA? Methods: We identified English language articles published from September 2009 to October 2019 reporting primary research that used typical motion analysis ...
Instrumented gait analysis (IGA) is an assessment and research tool with proven impacts on clinical decision-making for the management of ambulant children and young people with cerebral palsy (CYPwCP) but is underused and variably understood by relevant clinicians. Clinicians' difficulties in gaining expertise and confidence in using IGA are multifactorial and related to access for clinical ...
Previously, we trained an algorithm for video-based gait analysis on a large clinical gait laboratory dataset of paired videos and motion capture data 24. While the algorithm validated well on ...
There is a large volume of literature on the research use of gait analysis, but evidence on its clinical routine use supports a favorable cost-benefit ratio in a limited number of conditions. Initially gait analysis was introduced to clinical practice to improve the management of children with cerebral palsy.
Abstract. Gait analysis can be considered a backbone of modern clinical locomotion biomechanics. Basic methodology, integrating kinematic, kinetic and myoelectric measurements in course of a subject's walking and subsequent interpretation of results is summarized and illustrated mainly with findings from our Zagreb and Salerno situated ...
Abstract. When brain damage occurs, gait and balance are often impaired. Evaluation of the gait cycle, therefore, has a pivotal role during the rehabilitation path of subjects who suffer from neurological disorders. Gait analysis can be performed through laboratory systems, non-wearable sensors (NWS), and/or wearable sensors (WS).
Clinical gait analysis is a reliable tool to predict fall onsets. ... This paper also explores the pose estimation techniques for clinical gait analysis that open future research directions in ...
changes in gait that occur in response to a change in speed. Here, we present a novel, versatile approach for performing clinical gait analysis using only simple digital videos. First, we developed and tested a novel workflow that performs a gait analysis using frontal plane recordings of a person walking either away from or toward the camera ...
Abstract. Gait analysis goal is to investigate the mechanics of the muscle, the relationships between muscles and bones and the motions of joints. However, for a deeper analysis of the internal force acting on the human body research has focused on multi-body modeling and simulation. The aim is to integrate the elements of the musculoskeletal ...
Brand's four reasons for clinical tests and his analysis of the characteristics of valid biomechanical tests for use in orthopaedics are taken as a basis for determining what methodologies are required for gait analysis in a clinical rehabilitation context. The state of the art of optical systems capable of measuring the positions of retro-reflective markers placed on the skin is sufficiently ...
BackgroundDespite being available for more than three decades, quantitative gait analysis remains largely associated with research institutions and not well leveraged in clinical settings. This is mostly due to the high cost/cumbersome equipment and complex protocols and data management/analysis associated with traditional gait labs, as well as the diverse training/experience and preference of ...
This study addresses the characterization of normal gait and pathological deviations induced by neurological diseases, considering knee angular kinematics in the sagittal plane. We propose an unsupervised approach based on Dynamic Time Warping (DTW) to identify different normal gait profiles (NGPs) …
Instrumented gait analysis (IGA) is an assessment and research tool with proven impacts on clinical decision-making for the management of ambulant children and young people with cerebral palsy (CYPwCP) but is underused and variably understood by relevant clinicians. Clinicians' difficulties in gaining expertise and confidence in using IGA are multifactorial and related to access for clinical ...
Children's Hospital Colorado's Center for Gait and Movement Analysis holds the distinction of being the sole accredited clinical gait and movement analysis laboratory in its region, standing as one of just 14 accredited gait labs across the nation. ... Our research methods have expanded beyond motion analysis techniques to include measures of ...
Gait analysis serves as a critical diagnostic tool for identifying neurologic and musculoskeletal damage. Traditional manual analysis of motion data, however, is labor-intensive and heavily reliant on the expertise and judgment of the therapist. ... The absence of data is a big obstacle in clinical gait research as privacy protocols necessitate ...
Trends clearly point to more research focusing on the development of wearable gait analysis systems, such as the instrumented insole developed by Howell et al. , who demonstrated that the insole results for ground reaction force and ankle moment highly correlated with data collected from a clinical motion analysis laboratory (all >0.95) for all ...
Annals of Clinical and Translational Neurology is an ANA journal publishing original research and reviews on the mechanisms and treatments of diseases of the nervous system. Abstract Objective Voluntary upper limb movements are an ecologically important yet insufficiently explored digital-motor outcome domain for trials in degenerative ataxia.
Description. Clinical Gait Analysis takes a fresh look at the study of human gait. Using an easy-to-read writing style, the author reviews the biomechanical principles, techniques and clinical approach to the assessment of walking disorders. Both theory and practice are brought into focus, and currently contentious issues are highlighted to ...
Background: Flatfeet in children are common, causing concern for parents due to potential symptoms. Technological advances, like 3D foot kinematic analysis, have revolutionized assessment. This review examined 3D assessments in paediatric idiopathic flexible flat feet (FFF). Methods: Searches focused on paediatric idiopathic FFF in PubMed, Web of Science, and SCOPUS. Inclusion criteria ...
In this regard, clinical gait analysis based on MoCap technology can be defined as the instrumented measurement of movement patterns that comprise walking and the associated interpretation of these patterns. A MoCap instrumentation that is extensively used in biomedical research is optical technology (gold standard), which tracks the position ...